User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
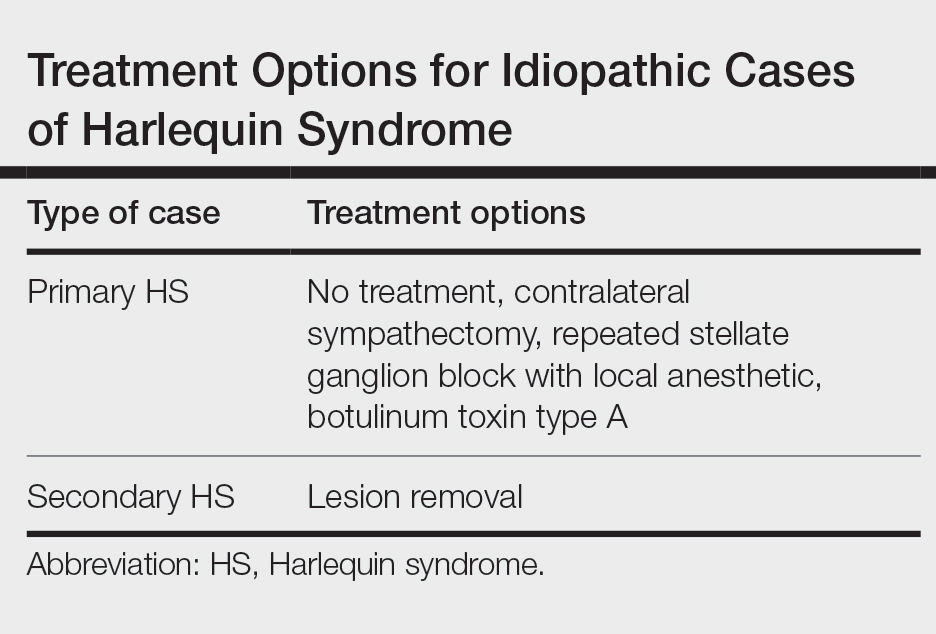
Surgical Planning for Mohs Defect Reconstruction in the Digital Age
Practice Gap
An essential part of training for a micrographic surgery and dermatologic oncology fellowship and scope of practice involves planning and execution of reconstructive surgery for Mohs defects. Recently, a surgical pearl presented by Rickstrew and colleagues1 highlighted the use of different colored surgical marking pens and their benefit in a trainee-based environment.
Delineating multiple options for reconstruction with different colored markers on live patients allows fellows in-training to participate in surgical planning but introduces more markings or drawings that need to be wiped off during or after surgery, potentially prolonging operative time. Furthermore, the Rickstrew approach has the potential to (1) cause unnecessary emotional distress for the patient during surgical planning and (2) add to the cost of surgery with the purchase of various colors of surgical markers.
Technique
To improve patient experience and trainee education, we propose fine-tuning the colored marker approach by utilizing a digital drawing program for surgical planning prior to the procedure. We recommend Snip & Sketch—a free, readily accessible digital annotating application that runs on the Microsoft Windows 10 operating system (https://www.microsoft.com/en-us/p/snip-sketch/9mz95kl8mr0l#activetab=pivot:overviewtab)—to mark up screenshot photographs of postoperative Mohs defects from the electronic medical record.
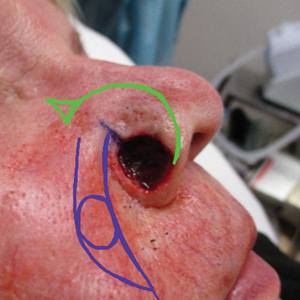
Using Snip & Sketch, the fellow in-training can then use, for example, a green “digital pen” to draw on the captured image and plan their surgical repairs (Figure 1) without input from the attending physician. Different colored pens can be used to highlight nerves, vessels, relaxed skin tension lines, and tension vectors associated with flap movement.
Subsequently, the attending physician, using a different color digital pen—say, blue—can design alternative reconstructive options (Figure 1). Suture lines also can be drawn to outline the predicted appearance of surgical scars (Figure 2).
Then, the attending physician and fellow in-training brainstorm and discuss the advantages and disadvantages of each reconstructive option to determine the optimal approach to repairing the Mohs defect.
Advantages and Disadvantages
The main advantage of using a digital drawing program is that it is time-saving and cost-efficient. Digital planning also spares the patient undue anxiety from listening to the discussion on each repair option.
The primary downside of digital surgical planning is that it is 2-dimensional, thus providing an incomplete representation of a 3-dimensional cutaneous structure. In addition, skin laxity, flap mobility, and free-margin distortion cannot be fully appreciated on a 2-dimensional image.
Despite these drawbacks, digital surgical planning provides trainees with an active learning experience through a more collaborative and comprehensive discussion of reconstructive options.
Practice Implications
Active learning using an electronic device has been validated as a beneficial addition to Mohs micrographic surgery training.2 Utilizing a digitized annotating program for surgical planning increases the independence of trainees and allows immediate feedback from the attending physician. The synergy of digital technology and collaborative learning helps cultivate the next generation of confident and competent Mohs surgeons.
- Rickstrew J, Roberts E, Amarani A, et al. Different colored surgical marking pens for trainee education. J Am Acad Dermatol. 2021:S0190-9622(21)00226-7. doi:10.1016/j.jaad.2021.01.069
- Croley JA, Malone CH, Goodwin BP, et al. Mohs Surgical Reconstruction Educational Activity: a resident education tool. Adv Med Educ Pract. 2017;8:143-147. doi:10.2147/AMEP.S125454
Practice Gap
An essential part of training for a micrographic surgery and dermatologic oncology fellowship and scope of practice involves planning and execution of reconstructive surgery for Mohs defects. Recently, a surgical pearl presented by Rickstrew and colleagues1 highlighted the use of different colored surgical marking pens and their benefit in a trainee-based environment.
Delineating multiple options for reconstruction with different colored markers on live patients allows fellows in-training to participate in surgical planning but introduces more markings or drawings that need to be wiped off during or after surgery, potentially prolonging operative time. Furthermore, the Rickstrew approach has the potential to (1) cause unnecessary emotional distress for the patient during surgical planning and (2) add to the cost of surgery with the purchase of various colors of surgical markers.
Technique
To improve patient experience and trainee education, we propose fine-tuning the colored marker approach by utilizing a digital drawing program for surgical planning prior to the procedure. We recommend Snip & Sketch—a free, readily accessible digital annotating application that runs on the Microsoft Windows 10 operating system (https://www.microsoft.com/en-us/p/snip-sketch/9mz95kl8mr0l#activetab=pivot:overviewtab)—to mark up screenshot photographs of postoperative Mohs defects from the electronic medical record.
Using Snip & Sketch, the fellow in-training can then use, for example, a green “digital pen” to draw on the captured image and plan their surgical repairs (Figure 1) without input from the attending physician. Different colored pens can be used to highlight nerves, vessels, relaxed skin tension lines, and tension vectors associated with flap movement.
Subsequently, the attending physician, using a different color digital pen—say, blue—can design alternative reconstructive options (Figure 1). Suture lines also can be drawn to outline the predicted appearance of surgical scars (Figure 2).
Then, the attending physician and fellow in-training brainstorm and discuss the advantages and disadvantages of each reconstructive option to determine the optimal approach to repairing the Mohs defect.
Advantages and Disadvantages
The main advantage of using a digital drawing program is that it is time-saving and cost-efficient. Digital planning also spares the patient undue anxiety from listening to the discussion on each repair option.
The primary downside of digital surgical planning is that it is 2-dimensional, thus providing an incomplete representation of a 3-dimensional cutaneous structure. In addition, skin laxity, flap mobility, and free-margin distortion cannot be fully appreciated on a 2-dimensional image.
Despite these drawbacks, digital surgical planning provides trainees with an active learning experience through a more collaborative and comprehensive discussion of reconstructive options.
Practice Implications
Active learning using an electronic device has been validated as a beneficial addition to Mohs micrographic surgery training.2 Utilizing a digitized annotating program for surgical planning increases the independence of trainees and allows immediate feedback from the attending physician. The synergy of digital technology and collaborative learning helps cultivate the next generation of confident and competent Mohs surgeons.
Practice Gap
An essential part of training for a micrographic surgery and dermatologic oncology fellowship and scope of practice involves planning and execution of reconstructive surgery for Mohs defects. Recently, a surgical pearl presented by Rickstrew and colleagues1 highlighted the use of different colored surgical marking pens and their benefit in a trainee-based environment.
Delineating multiple options for reconstruction with different colored markers on live patients allows fellows in-training to participate in surgical planning but introduces more markings or drawings that need to be wiped off during or after surgery, potentially prolonging operative time. Furthermore, the Rickstrew approach has the potential to (1) cause unnecessary emotional distress for the patient during surgical planning and (2) add to the cost of surgery with the purchase of various colors of surgical markers.
Technique
To improve patient experience and trainee education, we propose fine-tuning the colored marker approach by utilizing a digital drawing program for surgical planning prior to the procedure. We recommend Snip & Sketch—a free, readily accessible digital annotating application that runs on the Microsoft Windows 10 operating system (https://www.microsoft.com/en-us/p/snip-sketch/9mz95kl8mr0l#activetab=pivot:overviewtab)—to mark up screenshot photographs of postoperative Mohs defects from the electronic medical record.
Using Snip & Sketch, the fellow in-training can then use, for example, a green “digital pen” to draw on the captured image and plan their surgical repairs (Figure 1) without input from the attending physician. Different colored pens can be used to highlight nerves, vessels, relaxed skin tension lines, and tension vectors associated with flap movement.
Subsequently, the attending physician, using a different color digital pen—say, blue—can design alternative reconstructive options (Figure 1). Suture lines also can be drawn to outline the predicted appearance of surgical scars (Figure 2).
Then, the attending physician and fellow in-training brainstorm and discuss the advantages and disadvantages of each reconstructive option to determine the optimal approach to repairing the Mohs defect.
Advantages and Disadvantages
The main advantage of using a digital drawing program is that it is time-saving and cost-efficient. Digital planning also spares the patient undue anxiety from listening to the discussion on each repair option.
The primary downside of digital surgical planning is that it is 2-dimensional, thus providing an incomplete representation of a 3-dimensional cutaneous structure. In addition, skin laxity, flap mobility, and free-margin distortion cannot be fully appreciated on a 2-dimensional image.
Despite these drawbacks, digital surgical planning provides trainees with an active learning experience through a more collaborative and comprehensive discussion of reconstructive options.
Practice Implications
Active learning using an electronic device has been validated as a beneficial addition to Mohs micrographic surgery training.2 Utilizing a digitized annotating program for surgical planning increases the independence of trainees and allows immediate feedback from the attending physician. The synergy of digital technology and collaborative learning helps cultivate the next generation of confident and competent Mohs surgeons.
- Rickstrew J, Roberts E, Amarani A, et al. Different colored surgical marking pens for trainee education. J Am Acad Dermatol. 2021:S0190-9622(21)00226-7. doi:10.1016/j.jaad.2021.01.069
- Croley JA, Malone CH, Goodwin BP, et al. Mohs Surgical Reconstruction Educational Activity: a resident education tool. Adv Med Educ Pract. 2017;8:143-147. doi:10.2147/AMEP.S125454
- Rickstrew J, Roberts E, Amarani A, et al. Different colored surgical marking pens for trainee education. J Am Acad Dermatol. 2021:S0190-9622(21)00226-7. doi:10.1016/j.jaad.2021.01.069
- Croley JA, Malone CH, Goodwin BP, et al. Mohs Surgical Reconstruction Educational Activity: a resident education tool. Adv Med Educ Pract. 2017;8:143-147. doi:10.2147/AMEP.S125454
How Dermatology Residents Can Best Serve the Needs of the LGBT Community
The chances are good that at least one patient you saw today could have been provided a better environment to foster your patient-physician relationship. A 2020 Gallup poll revealed that an estimated 5.6% of US adults identified as lesbian, gay, bisexual, and transgender (LGBT).1 Based on the estimated US population of 331.7 million individuals on December 3, 2020, this means that approximately 18.6 million identified as LGBT and could potentially require health care services.2 These numbers highlight the increasing need within the medical community to provide quality and accessible care to the LGBT community, and dermatologists have a role to play. They treat conditions that are apparent to the patient and others around them, attracting those that may not be motivated to see different physicians. They can not only help with skin diseases that affect all patients but also can train other physicians to screen for some dermatologic diseases that may have a higher prevalence within the LGBT community. Dermatologists have a unique opportunity to help patients better reflect themselves through both surgical and nonsurgical modalities.
Demographics and Definitions
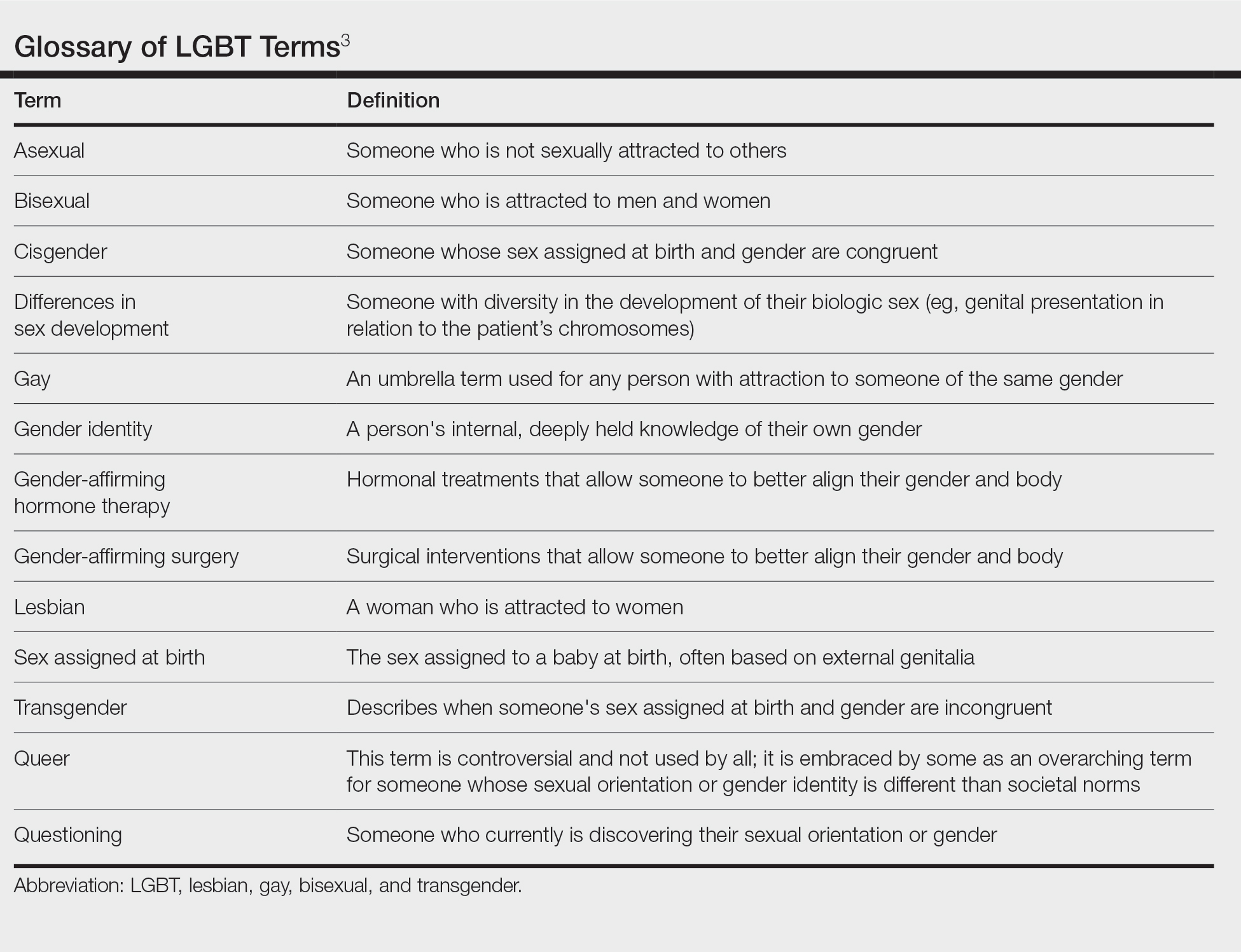
To discuss this topic effectively, it is important to define LGBT terms (Table).3 As a disclaimer, language is fluid. Despite a word or term currently being used and accepted, it quickly can become obsolete. A clinician can always do research, follow the lead of the patient, and respectfully ask questions if there is ever confusion surrounding terminology. Patients do not expect every physician they encounter to be an expert in this subject. What is most important is that patients are approached with an open mind and humility with the goal of providing optimal care.
Although the federal government now uses the term sexual and gender minorities (SGM), the more specific terms lesbian, gay, bisexual, and transgender usually are preferred.3,4 Other letters are at times added to the acronym LGBT, including Q for questioning or queer, I for intersex, and A for asexual; all of these letters are under the larger SGM umbrella. Because LGBT is the most commonly used acronym in the daily vernacular, it will be the default for this article.
A term describing sexual orientation does not necessarily describe sexual practices. A woman who identifies as straight may have sex with both men and women, and a gay man may not have sex at all. To be more descriptive regarding sexual practices, one may use the terms men who have sex with men or women who have sex with women.3 Because of this nuance, it is important to elicit a sexual history when speaking to all patients in a forward nonjudgmental manner.
The term transgender is used to describe people whose gender identity differs from the sex they were assigned at birth. Two examples of transgender individuals would be transgender women who were assigned male at birth and transgender men who were assigned female at birth. The term transgender is used in opposition to the term cisgender, which is applied to a person whose gender and sex assigned at birth align.3 When a transgender patient presents to a physician, they may want to discuss methods of gender affirmation or transitioning. These terms encompass any action a person may take to align their body or gender expression with that of the gender they identify with. This could be in the form of gender-affirming hormone therapy (ie, estrogen or testosterone treatment) or gender-affirming surgery (ie, “top” and “bottom” surgeries, in which someone surgically treats their chest or genitals, respectively).3
Creating a Safe Space
The physician is responsible for providing a safe space for patients to disclose medically pertinent information. It is then the job of the dermatologist to be cognizant of health concerns that directly affect the LGBT population and to be prepared if one of these concerns should arise. A safe space consists of both the physical location in which the patient encounter will occur and the people that will be conducting and assisting in the patient encounter. Safe spaces provide a patient with reassurance that they will receive care in a judgement-free location. To create a safe space, both the physical and interpersonal aspects must be addressed to provide an environment that strengthens the patient-physician alliance.
Dermatology residents often spend more time with patients than their attending physicians, providing them the opportunity to foster robust relationships with those served. Although they may not be able to change the physical environment, residents can advocate for patients in their departments and show solidarity in subtle ways. One way to show support for the LGBT community is to publicly display a symbol of solidarity, which could be done by wearing a symbol of support on a white coat lapel. Although there are many designs and styles to choose from, one example is the American Medical Student Association pins that combine the caduceus (a common symbol for medicine) with a rainbow design.5 Whichever symbol is chosen, this small gesture allows patients to immediately know that their physician is an ally. Residents also can encourage their department to add a rainbow flag, a pink triangle, or another symbol somewhere prominent in the check-in area that conveys a message of support.6 Many institutions require residents to perform quality improvement projects. The resident can make a substantial difference in their patients’ experiences by revising their office’s intake forms as a quality improvement project, which can be done by including a section on assigned sex at birth separate from gender.7 When inquiring about gender, in addition to “male” and “female,” a space can be left for people that do not identify with the traditional binary. When asking about sexual orientation, inclusive language options can be provided with additional space for self-identification. Finally, residents can incorporate pronouns below their name in their email signature to normalize this disclosure of information.8 These small changes can have a substantial impact on the health care experience of SGM patients.
Medical Problems Encountered
The previously described changes can be implemented by residents to provide better care to SGM patients, a group usually considered to be more burdened by physical and psychological diseases.9 Furthermore, dermatologists can provide care for these patients in ways that other physicians cannot. There are special considerations for LGBT patients, as some dermatologic conditions may be more common in this patient population.
Prior studies have shown that men who have sex with men have a higher rate of HIV and other sexually transmitted infections, methicillin-resistant Staphylococcus aureus skin infections, and potentially nonmelanoma skin cancer.10-14 Transgender women also have been found to have higher rates of HIV, in addition to a higher incidence of anal human papillomavirus.15,16 Women who have sex with women have been shown to see physicians less frequently and to be less up to date on their pertinent cancer-related screenings.10,17 Although these associations should not dictate the patient encounter, awareness of them will lead to better patient care. Such awareness also can provide further motivation for dermatologists to discuss safe sexual practices, potential initiation of pre-exposure prophylactic antiretroviral therapy, sun-protective practices, and the importance of following up with a primary physician for examinations and age-specific cancer screening.
Transgender patients may present with unique dermatologic concerns. For transgender male patients, testosterone therapy can cause acne breakouts and androgenetic alopecia. Usually considered worse during the start of treatment, hormone-related acne can be managed with topical retinoids, topical and oral antibiotics, and isotretinoin (if severe).18,19 The iPLEDGE system necessary for prescribing isotretinoin to patients in the United States recently has changed its language to “patients who can get pregnant” and “patients who cannot get pregnant,” following urging by the medical community for inclusivity and progress.20,21 This change creates an inclusive space where registration is no longer centered around gender and instead focuses on the presence of anatomy. Although androgenetic alopecia is a side effect of hormone therapy, it may not be unwanted.18 Discussion about patient desires is important. If the alopecia is unwanted, the Endocrine Society recommends treating cisgender and transgender patients the same in terms of treatment modalities.22
Transgender female patients also can experience dermatologic manifestations of gender-affirming hormone therapy. Melasma may develop secondary to estrogen replacement and can be treated with topical bleaching creams, lasers, and phototherapy.23 Hair removal may be pursued for patients with refractory unwanted body hair, with laser hair removal being the most commonly pursued treatment. Patients also may desire cosmetic procedures, such as botulinum toxin or fillers, to augment their physical appearance.24 Providing these services to patients may allow them to better express themselves and live authentically.
Final Thoughts
There is no way to summarize the experience of everyone within a community. Each person has different thoughts, values, and goals. It also is impossible to encompass every topic that is important for SGM patients. The goal of this article is to empower clinicians to be comfortable discussing issues related to sexuality and gender while also offering resources to learn more, allowing optimal care to be provided to this population. Thus, this article is not comprehensive. There are articles to provide further resources and education, such as the continuing medical education series by Yeung et al10,25 in the Journal of the American Academy of Dermatology, as well as organizations within medicine, such as the GLMA: Health Professionals Advancing LGBTQ Equality (https://www.glma.org/), and in dermatology, such as GALDA, the Gay and Lesbian Dermatology Association (https://www.glderm.org/). By providing a safe space for our patients and learning about specific health-related risk factors, dermatologists can provide the best possible care to the LGBT community.
Acknowledgments—I thank Warren R. Heymann, MD (Camden, New Jersey), and Howa Yeung, MD, MSc (Atlanta, Georgia), for their guidance and mentorship in the creation of this article.
- Jones JM. LGBT identification rises to 5.6% in latest U.S. estimate. Gallup website. Published February 24, 2021. Accessed March 22, 2022. https://news.gallup.com/poll/329708/lgbt-identification-rises-latest-estimate.aspx
- U.S. and world population clock. US Census Bureau website. Accessed March 22, 2022. https://www.census.gov/popclock/
- National LGBTQIA+ Health Education Center. LGBTQIA+ glossary of terms for health care teams. Published February 2, 2022. Accessed April 11, 2022. https://www.lgbtqiahealtheducation.org/wp-content/uploads/2020/02/Glossary-2022.02.22-1.pdf
- National Institutes of Health Sexual and Gender Minority Research Coordinating Committee. NIH FY 2016-2020 strategic plan to advance research on the health and well-being of sexual and gender minorities. NIH website. Accessed March 23, 2022. https://www.edi.nih.gov/sites/default/files/EDI_Public_files/sgm-strategic-plan.pdf
- Caduceus pin—rainbow. American Medical Student Association website. Accessed March 23, 2022. https://www.amsa.org/member-center/store/Caduceus-Pin-Rainbow-p67375123
- 10 tips for caring for LGBTQIA+ patients. Nurse.org website. Accessed March 23, 2022. https://nurse.org/articles/culturally-competent-healthcare-for-LGBTQ-patients/
- Cartron AM, Raiciulescu S, Trinidad JC. Culturally competent care for LGBT patients in dermatology clinics. J Drugs Dermatol. 2020;19:786-787.
- Wareham J. Should you put pronouns in email signatures and social media bios? Forbes website. Published Dec 30, 2019. Accessed March 23, 2022. https://www.forbes.com/sites/jamiewareham/2020/12/30/should-you-put-pronouns-in-email-signatures-and-social-media-bios/?sh=5b74f1246320
- Hafeez H, Zeshan M, Tahir MA, et al. Healthcare disparities among lesbian, gay, bisexual, and transgender youth: a literature review. Cureus. 2017;9:E1184.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons. part II. epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Centers for Disease Control and Prevention. CDC fact sheet: HIV among gay and bisexual men. CDC website. Accessed April 14, 2022. https://www.cdc.gov/nchhstp/newsroom/docs/factsheets/cdc-msm-508.pdf
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2016. CDC website. Accessed April 14, 2022. https://www.cdc.gov/std/stats16/CDC_2016_STDS_Report-for508WebSep21_2017_1644.pdf
- Galindo GR, Casey AJ, Yeung A, et al. Community associated methicillin resistant Staphylococcus aureus among New York City men who have sex with men: qualitative research findings and implications for public health practice. J Community Health. 2012;37:458-467.
- Blashill AJ. Indoor tanning and skin cancer risk among diverse US youth: results from a national sample. JAMA Dermatol. 2017;153:344-345.
- Herbst JH, Jacobs ED, Finlayson TJ, et al. Estimating HIV prevalence and risk behaviors of transgender persons in the United States: a systematic review. AIDS Behav. 2008;12:1-17.
- Uaamnuichai S, Panyakhamlerd K, Suwan A, et al. Neovaginal and anal high-risk human papillomavirus DNA among Thai transgender women in gender health clinics. Sex Transm Dis. 2021;48:547-549.
- Valanis BG, Bowen DJ, Bassford T, et al. Sexual orientation and health: comparisons in the women’s health initiative sample. Arch Fam Med. 2000;9:843-853.
- Wierckx K, Van de Peer F, Verhaeghe E, et al. Short- and long-term clinical skin effects of testosterone treatment in trans men. J Sex Med. 2014;11:222-229.
- Turrion-Merino L, Urech-Garcia-de-la-Vega M, Miguel-Gomez L, et al. Severe acne in female-to-male transgender patients. JAMA Dermatol. 2015;151:1260-1261.
- Questions and answers on the iPLEDGE REMS. US Food and Drug Administration website. Published October 12, 2021. Accessed March 23, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/questions-and-answers-ipledge-rems#:~:text=The%20modification%20will%20become%20effective,verify%20authorization%20to%20dispense%20isotretinoin
- Gao JL, Thoreson N, Dommasch ED. Navigating iPLEDGE enrollment for transgender and gender diverse patients: a guide for providing culturally competent care. J Am Acad Dermatol. 2021;85:790-791.
- Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2017;102:3869-3903.
- Garcia-Rodriguez L, Spiegel JH. Melasma in a transgender woman. Am J Otolaryngol. 2018;39:788-790.
- Ginsberg BA, Calderon M, Seminara NM, et al. A potential role for the dermatologist in the physical transformation of transgender people: a survey of attitudes and practices within the transgender community.J Am Acad Dermatol. 2016;74:303-308.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian,gay, bisexual, and transgender persons. part I. terminology, demographics, health disparities, and approaches to care. J Am Acad Dermatol. 2019;80:581-589.
The chances are good that at least one patient you saw today could have been provided a better environment to foster your patient-physician relationship. A 2020 Gallup poll revealed that an estimated 5.6% of US adults identified as lesbian, gay, bisexual, and transgender (LGBT).1 Based on the estimated US population of 331.7 million individuals on December 3, 2020, this means that approximately 18.6 million identified as LGBT and could potentially require health care services.2 These numbers highlight the increasing need within the medical community to provide quality and accessible care to the LGBT community, and dermatologists have a role to play. They treat conditions that are apparent to the patient and others around them, attracting those that may not be motivated to see different physicians. They can not only help with skin diseases that affect all patients but also can train other physicians to screen for some dermatologic diseases that may have a higher prevalence within the LGBT community. Dermatologists have a unique opportunity to help patients better reflect themselves through both surgical and nonsurgical modalities.
Demographics and Definitions
To discuss this topic effectively, it is important to define LGBT terms (Table).3 As a disclaimer, language is fluid. Despite a word or term currently being used and accepted, it quickly can become obsolete. A clinician can always do research, follow the lead of the patient, and respectfully ask questions if there is ever confusion surrounding terminology. Patients do not expect every physician they encounter to be an expert in this subject. What is most important is that patients are approached with an open mind and humility with the goal of providing optimal care.
Although the federal government now uses the term sexual and gender minorities (SGM), the more specific terms lesbian, gay, bisexual, and transgender usually are preferred.3,4 Other letters are at times added to the acronym LGBT, including Q for questioning or queer, I for intersex, and A for asexual; all of these letters are under the larger SGM umbrella. Because LGBT is the most commonly used acronym in the daily vernacular, it will be the default for this article.
A term describing sexual orientation does not necessarily describe sexual practices. A woman who identifies as straight may have sex with both men and women, and a gay man may not have sex at all. To be more descriptive regarding sexual practices, one may use the terms men who have sex with men or women who have sex with women.3 Because of this nuance, it is important to elicit a sexual history when speaking to all patients in a forward nonjudgmental manner.
The term transgender is used to describe people whose gender identity differs from the sex they were assigned at birth. Two examples of transgender individuals would be transgender women who were assigned male at birth and transgender men who were assigned female at birth. The term transgender is used in opposition to the term cisgender, which is applied to a person whose gender and sex assigned at birth align.3 When a transgender patient presents to a physician, they may want to discuss methods of gender affirmation or transitioning. These terms encompass any action a person may take to align their body or gender expression with that of the gender they identify with. This could be in the form of gender-affirming hormone therapy (ie, estrogen or testosterone treatment) or gender-affirming surgery (ie, “top” and “bottom” surgeries, in which someone surgically treats their chest or genitals, respectively).3
Creating a Safe Space
The physician is responsible for providing a safe space for patients to disclose medically pertinent information. It is then the job of the dermatologist to be cognizant of health concerns that directly affect the LGBT population and to be prepared if one of these concerns should arise. A safe space consists of both the physical location in which the patient encounter will occur and the people that will be conducting and assisting in the patient encounter. Safe spaces provide a patient with reassurance that they will receive care in a judgement-free location. To create a safe space, both the physical and interpersonal aspects must be addressed to provide an environment that strengthens the patient-physician alliance.
Dermatology residents often spend more time with patients than their attending physicians, providing them the opportunity to foster robust relationships with those served. Although they may not be able to change the physical environment, residents can advocate for patients in their departments and show solidarity in subtle ways. One way to show support for the LGBT community is to publicly display a symbol of solidarity, which could be done by wearing a symbol of support on a white coat lapel. Although there are many designs and styles to choose from, one example is the American Medical Student Association pins that combine the caduceus (a common symbol for medicine) with a rainbow design.5 Whichever symbol is chosen, this small gesture allows patients to immediately know that their physician is an ally. Residents also can encourage their department to add a rainbow flag, a pink triangle, or another symbol somewhere prominent in the check-in area that conveys a message of support.6 Many institutions require residents to perform quality improvement projects. The resident can make a substantial difference in their patients’ experiences by revising their office’s intake forms as a quality improvement project, which can be done by including a section on assigned sex at birth separate from gender.7 When inquiring about gender, in addition to “male” and “female,” a space can be left for people that do not identify with the traditional binary. When asking about sexual orientation, inclusive language options can be provided with additional space for self-identification. Finally, residents can incorporate pronouns below their name in their email signature to normalize this disclosure of information.8 These small changes can have a substantial impact on the health care experience of SGM patients.
Medical Problems Encountered
The previously described changes can be implemented by residents to provide better care to SGM patients, a group usually considered to be more burdened by physical and psychological diseases.9 Furthermore, dermatologists can provide care for these patients in ways that other physicians cannot. There are special considerations for LGBT patients, as some dermatologic conditions may be more common in this patient population.
Prior studies have shown that men who have sex with men have a higher rate of HIV and other sexually transmitted infections, methicillin-resistant Staphylococcus aureus skin infections, and potentially nonmelanoma skin cancer.10-14 Transgender women also have been found to have higher rates of HIV, in addition to a higher incidence of anal human papillomavirus.15,16 Women who have sex with women have been shown to see physicians less frequently and to be less up to date on their pertinent cancer-related screenings.10,17 Although these associations should not dictate the patient encounter, awareness of them will lead to better patient care. Such awareness also can provide further motivation for dermatologists to discuss safe sexual practices, potential initiation of pre-exposure prophylactic antiretroviral therapy, sun-protective practices, and the importance of following up with a primary physician for examinations and age-specific cancer screening.
Transgender patients may present with unique dermatologic concerns. For transgender male patients, testosterone therapy can cause acne breakouts and androgenetic alopecia. Usually considered worse during the start of treatment, hormone-related acne can be managed with topical retinoids, topical and oral antibiotics, and isotretinoin (if severe).18,19 The iPLEDGE system necessary for prescribing isotretinoin to patients in the United States recently has changed its language to “patients who can get pregnant” and “patients who cannot get pregnant,” following urging by the medical community for inclusivity and progress.20,21 This change creates an inclusive space where registration is no longer centered around gender and instead focuses on the presence of anatomy. Although androgenetic alopecia is a side effect of hormone therapy, it may not be unwanted.18 Discussion about patient desires is important. If the alopecia is unwanted, the Endocrine Society recommends treating cisgender and transgender patients the same in terms of treatment modalities.22
Transgender female patients also can experience dermatologic manifestations of gender-affirming hormone therapy. Melasma may develop secondary to estrogen replacement and can be treated with topical bleaching creams, lasers, and phototherapy.23 Hair removal may be pursued for patients with refractory unwanted body hair, with laser hair removal being the most commonly pursued treatment. Patients also may desire cosmetic procedures, such as botulinum toxin or fillers, to augment their physical appearance.24 Providing these services to patients may allow them to better express themselves and live authentically.
Final Thoughts
There is no way to summarize the experience of everyone within a community. Each person has different thoughts, values, and goals. It also is impossible to encompass every topic that is important for SGM patients. The goal of this article is to empower clinicians to be comfortable discussing issues related to sexuality and gender while also offering resources to learn more, allowing optimal care to be provided to this population. Thus, this article is not comprehensive. There are articles to provide further resources and education, such as the continuing medical education series by Yeung et al10,25 in the Journal of the American Academy of Dermatology, as well as organizations within medicine, such as the GLMA: Health Professionals Advancing LGBTQ Equality (https://www.glma.org/), and in dermatology, such as GALDA, the Gay and Lesbian Dermatology Association (https://www.glderm.org/). By providing a safe space for our patients and learning about specific health-related risk factors, dermatologists can provide the best possible care to the LGBT community.
Acknowledgments—I thank Warren R. Heymann, MD (Camden, New Jersey), and Howa Yeung, MD, MSc (Atlanta, Georgia), for their guidance and mentorship in the creation of this article.
The chances are good that at least one patient you saw today could have been provided a better environment to foster your patient-physician relationship. A 2020 Gallup poll revealed that an estimated 5.6% of US adults identified as lesbian, gay, bisexual, and transgender (LGBT).1 Based on the estimated US population of 331.7 million individuals on December 3, 2020, this means that approximately 18.6 million identified as LGBT and could potentially require health care services.2 These numbers highlight the increasing need within the medical community to provide quality and accessible care to the LGBT community, and dermatologists have a role to play. They treat conditions that are apparent to the patient and others around them, attracting those that may not be motivated to see different physicians. They can not only help with skin diseases that affect all patients but also can train other physicians to screen for some dermatologic diseases that may have a higher prevalence within the LGBT community. Dermatologists have a unique opportunity to help patients better reflect themselves through both surgical and nonsurgical modalities.
Demographics and Definitions
To discuss this topic effectively, it is important to define LGBT terms (Table).3 As a disclaimer, language is fluid. Despite a word or term currently being used and accepted, it quickly can become obsolete. A clinician can always do research, follow the lead of the patient, and respectfully ask questions if there is ever confusion surrounding terminology. Patients do not expect every physician they encounter to be an expert in this subject. What is most important is that patients are approached with an open mind and humility with the goal of providing optimal care.
Although the federal government now uses the term sexual and gender minorities (SGM), the more specific terms lesbian, gay, bisexual, and transgender usually are preferred.3,4 Other letters are at times added to the acronym LGBT, including Q for questioning or queer, I for intersex, and A for asexual; all of these letters are under the larger SGM umbrella. Because LGBT is the most commonly used acronym in the daily vernacular, it will be the default for this article.
A term describing sexual orientation does not necessarily describe sexual practices. A woman who identifies as straight may have sex with both men and women, and a gay man may not have sex at all. To be more descriptive regarding sexual practices, one may use the terms men who have sex with men or women who have sex with women.3 Because of this nuance, it is important to elicit a sexual history when speaking to all patients in a forward nonjudgmental manner.
The term transgender is used to describe people whose gender identity differs from the sex they were assigned at birth. Two examples of transgender individuals would be transgender women who were assigned male at birth and transgender men who were assigned female at birth. The term transgender is used in opposition to the term cisgender, which is applied to a person whose gender and sex assigned at birth align.3 When a transgender patient presents to a physician, they may want to discuss methods of gender affirmation or transitioning. These terms encompass any action a person may take to align their body or gender expression with that of the gender they identify with. This could be in the form of gender-affirming hormone therapy (ie, estrogen or testosterone treatment) or gender-affirming surgery (ie, “top” and “bottom” surgeries, in which someone surgically treats their chest or genitals, respectively).3
Creating a Safe Space
The physician is responsible for providing a safe space for patients to disclose medically pertinent information. It is then the job of the dermatologist to be cognizant of health concerns that directly affect the LGBT population and to be prepared if one of these concerns should arise. A safe space consists of both the physical location in which the patient encounter will occur and the people that will be conducting and assisting in the patient encounter. Safe spaces provide a patient with reassurance that they will receive care in a judgement-free location. To create a safe space, both the physical and interpersonal aspects must be addressed to provide an environment that strengthens the patient-physician alliance.
Dermatology residents often spend more time with patients than their attending physicians, providing them the opportunity to foster robust relationships with those served. Although they may not be able to change the physical environment, residents can advocate for patients in their departments and show solidarity in subtle ways. One way to show support for the LGBT community is to publicly display a symbol of solidarity, which could be done by wearing a symbol of support on a white coat lapel. Although there are many designs and styles to choose from, one example is the American Medical Student Association pins that combine the caduceus (a common symbol for medicine) with a rainbow design.5 Whichever symbol is chosen, this small gesture allows patients to immediately know that their physician is an ally. Residents also can encourage their department to add a rainbow flag, a pink triangle, or another symbol somewhere prominent in the check-in area that conveys a message of support.6 Many institutions require residents to perform quality improvement projects. The resident can make a substantial difference in their patients’ experiences by revising their office’s intake forms as a quality improvement project, which can be done by including a section on assigned sex at birth separate from gender.7 When inquiring about gender, in addition to “male” and “female,” a space can be left for people that do not identify with the traditional binary. When asking about sexual orientation, inclusive language options can be provided with additional space for self-identification. Finally, residents can incorporate pronouns below their name in their email signature to normalize this disclosure of information.8 These small changes can have a substantial impact on the health care experience of SGM patients.
Medical Problems Encountered
The previously described changes can be implemented by residents to provide better care to SGM patients, a group usually considered to be more burdened by physical and psychological diseases.9 Furthermore, dermatologists can provide care for these patients in ways that other physicians cannot. There are special considerations for LGBT patients, as some dermatologic conditions may be more common in this patient population.
Prior studies have shown that men who have sex with men have a higher rate of HIV and other sexually transmitted infections, methicillin-resistant Staphylococcus aureus skin infections, and potentially nonmelanoma skin cancer.10-14 Transgender women also have been found to have higher rates of HIV, in addition to a higher incidence of anal human papillomavirus.15,16 Women who have sex with women have been shown to see physicians less frequently and to be less up to date on their pertinent cancer-related screenings.10,17 Although these associations should not dictate the patient encounter, awareness of them will lead to better patient care. Such awareness also can provide further motivation for dermatologists to discuss safe sexual practices, potential initiation of pre-exposure prophylactic antiretroviral therapy, sun-protective practices, and the importance of following up with a primary physician for examinations and age-specific cancer screening.
Transgender patients may present with unique dermatologic concerns. For transgender male patients, testosterone therapy can cause acne breakouts and androgenetic alopecia. Usually considered worse during the start of treatment, hormone-related acne can be managed with topical retinoids, topical and oral antibiotics, and isotretinoin (if severe).18,19 The iPLEDGE system necessary for prescribing isotretinoin to patients in the United States recently has changed its language to “patients who can get pregnant” and “patients who cannot get pregnant,” following urging by the medical community for inclusivity and progress.20,21 This change creates an inclusive space where registration is no longer centered around gender and instead focuses on the presence of anatomy. Although androgenetic alopecia is a side effect of hormone therapy, it may not be unwanted.18 Discussion about patient desires is important. If the alopecia is unwanted, the Endocrine Society recommends treating cisgender and transgender patients the same in terms of treatment modalities.22
Transgender female patients also can experience dermatologic manifestations of gender-affirming hormone therapy. Melasma may develop secondary to estrogen replacement and can be treated with topical bleaching creams, lasers, and phototherapy.23 Hair removal may be pursued for patients with refractory unwanted body hair, with laser hair removal being the most commonly pursued treatment. Patients also may desire cosmetic procedures, such as botulinum toxin or fillers, to augment their physical appearance.24 Providing these services to patients may allow them to better express themselves and live authentically.
Final Thoughts
There is no way to summarize the experience of everyone within a community. Each person has different thoughts, values, and goals. It also is impossible to encompass every topic that is important for SGM patients. The goal of this article is to empower clinicians to be comfortable discussing issues related to sexuality and gender while also offering resources to learn more, allowing optimal care to be provided to this population. Thus, this article is not comprehensive. There are articles to provide further resources and education, such as the continuing medical education series by Yeung et al10,25 in the Journal of the American Academy of Dermatology, as well as organizations within medicine, such as the GLMA: Health Professionals Advancing LGBTQ Equality (https://www.glma.org/), and in dermatology, such as GALDA, the Gay and Lesbian Dermatology Association (https://www.glderm.org/). By providing a safe space for our patients and learning about specific health-related risk factors, dermatologists can provide the best possible care to the LGBT community.
Acknowledgments—I thank Warren R. Heymann, MD (Camden, New Jersey), and Howa Yeung, MD, MSc (Atlanta, Georgia), for their guidance and mentorship in the creation of this article.
- Jones JM. LGBT identification rises to 5.6% in latest U.S. estimate. Gallup website. Published February 24, 2021. Accessed March 22, 2022. https://news.gallup.com/poll/329708/lgbt-identification-rises-latest-estimate.aspx
- U.S. and world population clock. US Census Bureau website. Accessed March 22, 2022. https://www.census.gov/popclock/
- National LGBTQIA+ Health Education Center. LGBTQIA+ glossary of terms for health care teams. Published February 2, 2022. Accessed April 11, 2022. https://www.lgbtqiahealtheducation.org/wp-content/uploads/2020/02/Glossary-2022.02.22-1.pdf
- National Institutes of Health Sexual and Gender Minority Research Coordinating Committee. NIH FY 2016-2020 strategic plan to advance research on the health and well-being of sexual and gender minorities. NIH website. Accessed March 23, 2022. https://www.edi.nih.gov/sites/default/files/EDI_Public_files/sgm-strategic-plan.pdf
- Caduceus pin—rainbow. American Medical Student Association website. Accessed March 23, 2022. https://www.amsa.org/member-center/store/Caduceus-Pin-Rainbow-p67375123
- 10 tips for caring for LGBTQIA+ patients. Nurse.org website. Accessed March 23, 2022. https://nurse.org/articles/culturally-competent-healthcare-for-LGBTQ-patients/
- Cartron AM, Raiciulescu S, Trinidad JC. Culturally competent care for LGBT patients in dermatology clinics. J Drugs Dermatol. 2020;19:786-787.
- Wareham J. Should you put pronouns in email signatures and social media bios? Forbes website. Published Dec 30, 2019. Accessed March 23, 2022. https://www.forbes.com/sites/jamiewareham/2020/12/30/should-you-put-pronouns-in-email-signatures-and-social-media-bios/?sh=5b74f1246320
- Hafeez H, Zeshan M, Tahir MA, et al. Healthcare disparities among lesbian, gay, bisexual, and transgender youth: a literature review. Cureus. 2017;9:E1184.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons. part II. epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Centers for Disease Control and Prevention. CDC fact sheet: HIV among gay and bisexual men. CDC website. Accessed April 14, 2022. https://www.cdc.gov/nchhstp/newsroom/docs/factsheets/cdc-msm-508.pdf
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2016. CDC website. Accessed April 14, 2022. https://www.cdc.gov/std/stats16/CDC_2016_STDS_Report-for508WebSep21_2017_1644.pdf
- Galindo GR, Casey AJ, Yeung A, et al. Community associated methicillin resistant Staphylococcus aureus among New York City men who have sex with men: qualitative research findings and implications for public health practice. J Community Health. 2012;37:458-467.
- Blashill AJ. Indoor tanning and skin cancer risk among diverse US youth: results from a national sample. JAMA Dermatol. 2017;153:344-345.
- Herbst JH, Jacobs ED, Finlayson TJ, et al. Estimating HIV prevalence and risk behaviors of transgender persons in the United States: a systematic review. AIDS Behav. 2008;12:1-17.
- Uaamnuichai S, Panyakhamlerd K, Suwan A, et al. Neovaginal and anal high-risk human papillomavirus DNA among Thai transgender women in gender health clinics. Sex Transm Dis. 2021;48:547-549.
- Valanis BG, Bowen DJ, Bassford T, et al. Sexual orientation and health: comparisons in the women’s health initiative sample. Arch Fam Med. 2000;9:843-853.
- Wierckx K, Van de Peer F, Verhaeghe E, et al. Short- and long-term clinical skin effects of testosterone treatment in trans men. J Sex Med. 2014;11:222-229.
- Turrion-Merino L, Urech-Garcia-de-la-Vega M, Miguel-Gomez L, et al. Severe acne in female-to-male transgender patients. JAMA Dermatol. 2015;151:1260-1261.
- Questions and answers on the iPLEDGE REMS. US Food and Drug Administration website. Published October 12, 2021. Accessed March 23, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/questions-and-answers-ipledge-rems#:~:text=The%20modification%20will%20become%20effective,verify%20authorization%20to%20dispense%20isotretinoin
- Gao JL, Thoreson N, Dommasch ED. Navigating iPLEDGE enrollment for transgender and gender diverse patients: a guide for providing culturally competent care. J Am Acad Dermatol. 2021;85:790-791.
- Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2017;102:3869-3903.
- Garcia-Rodriguez L, Spiegel JH. Melasma in a transgender woman. Am J Otolaryngol. 2018;39:788-790.
- Ginsberg BA, Calderon M, Seminara NM, et al. A potential role for the dermatologist in the physical transformation of transgender people: a survey of attitudes and practices within the transgender community.J Am Acad Dermatol. 2016;74:303-308.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian,gay, bisexual, and transgender persons. part I. terminology, demographics, health disparities, and approaches to care. J Am Acad Dermatol. 2019;80:581-589.
- Jones JM. LGBT identification rises to 5.6% in latest U.S. estimate. Gallup website. Published February 24, 2021. Accessed March 22, 2022. https://news.gallup.com/poll/329708/lgbt-identification-rises-latest-estimate.aspx
- U.S. and world population clock. US Census Bureau website. Accessed March 22, 2022. https://www.census.gov/popclock/
- National LGBTQIA+ Health Education Center. LGBTQIA+ glossary of terms for health care teams. Published February 2, 2022. Accessed April 11, 2022. https://www.lgbtqiahealtheducation.org/wp-content/uploads/2020/02/Glossary-2022.02.22-1.pdf
- National Institutes of Health Sexual and Gender Minority Research Coordinating Committee. NIH FY 2016-2020 strategic plan to advance research on the health and well-being of sexual and gender minorities. NIH website. Accessed March 23, 2022. https://www.edi.nih.gov/sites/default/files/EDI_Public_files/sgm-strategic-plan.pdf
- Caduceus pin—rainbow. American Medical Student Association website. Accessed March 23, 2022. https://www.amsa.org/member-center/store/Caduceus-Pin-Rainbow-p67375123
- 10 tips for caring for LGBTQIA+ patients. Nurse.org website. Accessed March 23, 2022. https://nurse.org/articles/culturally-competent-healthcare-for-LGBTQ-patients/
- Cartron AM, Raiciulescu S, Trinidad JC. Culturally competent care for LGBT patients in dermatology clinics. J Drugs Dermatol. 2020;19:786-787.
- Wareham J. Should you put pronouns in email signatures and social media bios? Forbes website. Published Dec 30, 2019. Accessed March 23, 2022. https://www.forbes.com/sites/jamiewareham/2020/12/30/should-you-put-pronouns-in-email-signatures-and-social-media-bios/?sh=5b74f1246320
- Hafeez H, Zeshan M, Tahir MA, et al. Healthcare disparities among lesbian, gay, bisexual, and transgender youth: a literature review. Cureus. 2017;9:E1184.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons. part II. epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Centers for Disease Control and Prevention. CDC fact sheet: HIV among gay and bisexual men. CDC website. Accessed April 14, 2022. https://www.cdc.gov/nchhstp/newsroom/docs/factsheets/cdc-msm-508.pdf
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2016. CDC website. Accessed April 14, 2022. https://www.cdc.gov/std/stats16/CDC_2016_STDS_Report-for508WebSep21_2017_1644.pdf
- Galindo GR, Casey AJ, Yeung A, et al. Community associated methicillin resistant Staphylococcus aureus among New York City men who have sex with men: qualitative research findings and implications for public health practice. J Community Health. 2012;37:458-467.
- Blashill AJ. Indoor tanning and skin cancer risk among diverse US youth: results from a national sample. JAMA Dermatol. 2017;153:344-345.
- Herbst JH, Jacobs ED, Finlayson TJ, et al. Estimating HIV prevalence and risk behaviors of transgender persons in the United States: a systematic review. AIDS Behav. 2008;12:1-17.
- Uaamnuichai S, Panyakhamlerd K, Suwan A, et al. Neovaginal and anal high-risk human papillomavirus DNA among Thai transgender women in gender health clinics. Sex Transm Dis. 2021;48:547-549.
- Valanis BG, Bowen DJ, Bassford T, et al. Sexual orientation and health: comparisons in the women’s health initiative sample. Arch Fam Med. 2000;9:843-853.
- Wierckx K, Van de Peer F, Verhaeghe E, et al. Short- and long-term clinical skin effects of testosterone treatment in trans men. J Sex Med. 2014;11:222-229.
- Turrion-Merino L, Urech-Garcia-de-la-Vega M, Miguel-Gomez L, et al. Severe acne in female-to-male transgender patients. JAMA Dermatol. 2015;151:1260-1261.
- Questions and answers on the iPLEDGE REMS. US Food and Drug Administration website. Published October 12, 2021. Accessed March 23, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/questions-and-answers-ipledge-rems#:~:text=The%20modification%20will%20become%20effective,verify%20authorization%20to%20dispense%20isotretinoin
- Gao JL, Thoreson N, Dommasch ED. Navigating iPLEDGE enrollment for transgender and gender diverse patients: a guide for providing culturally competent care. J Am Acad Dermatol. 2021;85:790-791.
- Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2017;102:3869-3903.
- Garcia-Rodriguez L, Spiegel JH. Melasma in a transgender woman. Am J Otolaryngol. 2018;39:788-790.
- Ginsberg BA, Calderon M, Seminara NM, et al. A potential role for the dermatologist in the physical transformation of transgender people: a survey of attitudes and practices within the transgender community.J Am Acad Dermatol. 2016;74:303-308.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian,gay, bisexual, and transgender persons. part I. terminology, demographics, health disparities, and approaches to care. J Am Acad Dermatol. 2019;80:581-589.
Resident Pearl
- Because of the longitudinal relationships dermatology residents make with their patients, they have a unique opportunity to provide a safe space and life-changing care to patients within the lesbian, gay, bisexual, and transgender community.
Melanoma
THE COMPARISON
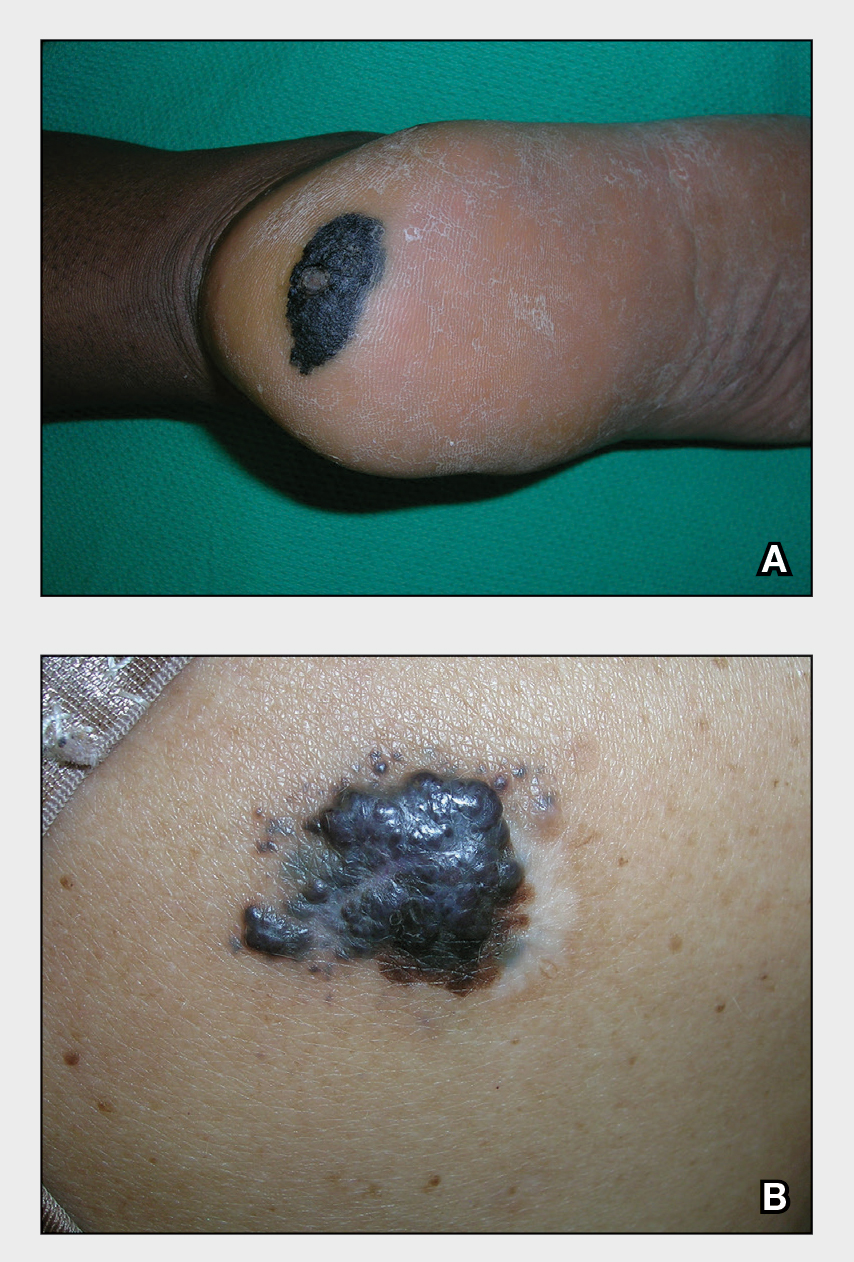
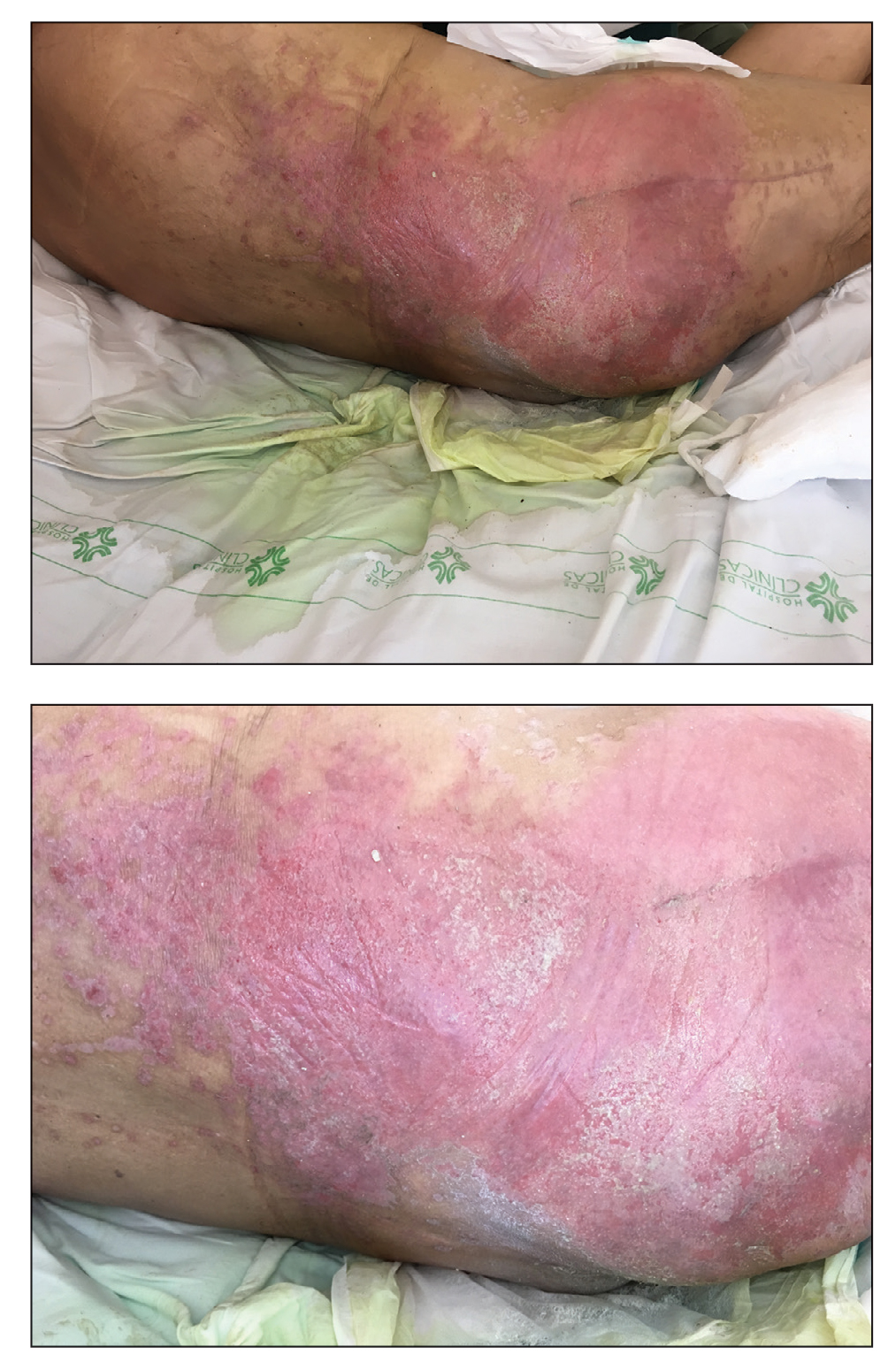
A Acral lentiginous melanoma on the sole of the foot in a 30-year-old Black woman. The depth of the lesion was 2 mm with a positive sentinel lymph node biopsy.
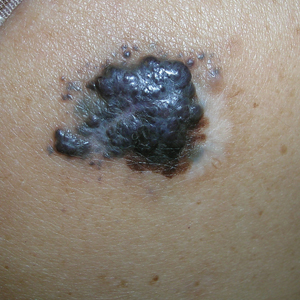
B Nodular melanoma on the shoulder of a 63-year-old Hispanic woman. The depth of the lesion was 5.5 mm with a positive sentinel lymph node biopsy.
Melanoma occurs less frequently in individuals with darker skin types than in lighter skin types but is associated with higher rates of morbidity and mortality in this patient population.1-7 In the cases shown here (A and B), both patients had advanced melanomas with large primary lesions and lymph node metastases.
Epidemiology
A systematic review by Higgins et al6 reported the following on the epidemiology of melanomas in patients with skin of color:
- African Americans have deeper tumors at the time of diagnosis, in addition to increased rates of regionally advanced and distant disease. Lesions generally are located on the lower extremities and have an increased propensity for ulceration. Acral lentiginous melanoma is the most common melanoma subtype found in African American patients.6
- In Hispanic individuals, superficial spreading melanoma is the most common melanoma subtype. Lower extremity lesions are more common relative to White individuals. Hispanic individuals have the highest rate of oral cavity melanomas across all ethnic groups.6
- In Asian individuals, acral and subungual sites are most common. Specifically, Pacific Islanders have the highest proportion of mucosal melanomas across all ethnic groups.6
Key clinical features in people with darker skin tones
Melanomas are found more often on the palms, soles, nail units, oral cavity, and mucosae.6 The melanomas have the same clinical and dermoscopic features found in individuals with lighter skin tones.
Worth noting
Factors that may contribute to the diagnosis of more advanced melanomas in racial/ethnic minorities in the United States include:
- decreased access to health care based on lack of health insurance and low socioeconomic status,
- less awareness of the risk of melanoma among patients and health care providers because melanoma is less common in persons of color, and
- lesions found in areas less likely to be seen in screening examinations, such as the soles of the feet and the oral and genital mucosae.
Health disparity highlight
- In a large US study of 96,953 patients with a diagnosis of cutaneous melanoma from 1992 to 2009, the proportion of later-stage melanoma—stages II to IV—was greater in Black patients compared to White patients.7
- Based on this same data set, White patients had the longest survival time (P<.05), followed by Hispanic (P<.05), Asian American/Native American/Pacific Islander (P<.05), and Black (P<.05) patients, respectively.7
- In Miami-Dade County, one study of 1690 melanoma cases found that 48% of Black patients had regional or distant disease at presentation compared to 22% of White patients (P=.015).5 Analysis of multiple factors found that only race was a significant predictor for late-stage melanoma (P<.001). Black patients in this study were 3 times more likely than others to be diagnosed with melanoma at a late stage (P=.07).5
- Black patients in the United States are more likely to have a delayed time from diagnosis to definitive surgery even when controlled for type of health insurance and stage of diagnosis.8
Final thoughts
Efforts are needed to overcome these disparities by:
- educating patients with skin of color and their health care providers about the risks of advanced melanoma with the goal of prevention and earlier diagnosis;
- breaking down barriers to care caused by poverty, lack of health insurance, and systemic racism; and
- eliminating factors that lead to delays from diagnosis to definitive surgery.
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37. doi:10.1016/j.jaad.2001.05.034
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914. doi:10.1001/archinte.166.17.1907
- Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California cancer registry data, 1988-93. Cancer Causes Control. 1997;8:246-252. doi:10.1023/a:1018432632528
- Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;51:1031-1032. doi:10.1016/j. jaad.2004.05.005
- Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142:704-708. doi:10.1001/archderm.142.6.704
- Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019;45:791-801. doi:10.1097/DSS.0000000000001759
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival [published online July 28, 2016]. J Am Acad Dermatol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
- Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016)[published online August 27, 2020]. J Am Acad Dermatol. 2021;84:1585-1593. doi:10.1016/j. jaad.2020.08.097
THE COMPARISON
A Acral lentiginous melanoma on the sole of the foot in a 30-year-old Black woman. The depth of the lesion was 2 mm with a positive sentinel lymph node biopsy.
B Nodular melanoma on the shoulder of a 63-year-old Hispanic woman. The depth of the lesion was 5.5 mm with a positive sentinel lymph node biopsy.
Melanoma occurs less frequently in individuals with darker skin types than in lighter skin types but is associated with higher rates of morbidity and mortality in this patient population.1-7 In the cases shown here (A and B), both patients had advanced melanomas with large primary lesions and lymph node metastases.
Epidemiology
A systematic review by Higgins et al6 reported the following on the epidemiology of melanomas in patients with skin of color:
- African Americans have deeper tumors at the time of diagnosis, in addition to increased rates of regionally advanced and distant disease. Lesions generally are located on the lower extremities and have an increased propensity for ulceration. Acral lentiginous melanoma is the most common melanoma subtype found in African American patients.6
- In Hispanic individuals, superficial spreading melanoma is the most common melanoma subtype. Lower extremity lesions are more common relative to White individuals. Hispanic individuals have the highest rate of oral cavity melanomas across all ethnic groups.6
- In Asian individuals, acral and subungual sites are most common. Specifically, Pacific Islanders have the highest proportion of mucosal melanomas across all ethnic groups.6
Key clinical features in people with darker skin tones
Melanomas are found more often on the palms, soles, nail units, oral cavity, and mucosae.6 The melanomas have the same clinical and dermoscopic features found in individuals with lighter skin tones.
Worth noting
Factors that may contribute to the diagnosis of more advanced melanomas in racial/ethnic minorities in the United States include:
- decreased access to health care based on lack of health insurance and low socioeconomic status,
- less awareness of the risk of melanoma among patients and health care providers because melanoma is less common in persons of color, and
- lesions found in areas less likely to be seen in screening examinations, such as the soles of the feet and the oral and genital mucosae.
Health disparity highlight
- In a large US study of 96,953 patients with a diagnosis of cutaneous melanoma from 1992 to 2009, the proportion of later-stage melanoma—stages II to IV—was greater in Black patients compared to White patients.7
- Based on this same data set, White patients had the longest survival time (P<.05), followed by Hispanic (P<.05), Asian American/Native American/Pacific Islander (P<.05), and Black (P<.05) patients, respectively.7
- In Miami-Dade County, one study of 1690 melanoma cases found that 48% of Black patients had regional or distant disease at presentation compared to 22% of White patients (P=.015).5 Analysis of multiple factors found that only race was a significant predictor for late-stage melanoma (P<.001). Black patients in this study were 3 times more likely than others to be diagnosed with melanoma at a late stage (P=.07).5
- Black patients in the United States are more likely to have a delayed time from diagnosis to definitive surgery even when controlled for type of health insurance and stage of diagnosis.8
Final thoughts
Efforts are needed to overcome these disparities by:
- educating patients with skin of color and their health care providers about the risks of advanced melanoma with the goal of prevention and earlier diagnosis;
- breaking down barriers to care caused by poverty, lack of health insurance, and systemic racism; and
- eliminating factors that lead to delays from diagnosis to definitive surgery.
THE COMPARISON
A Acral lentiginous melanoma on the sole of the foot in a 30-year-old Black woman. The depth of the lesion was 2 mm with a positive sentinel lymph node biopsy.
B Nodular melanoma on the shoulder of a 63-year-old Hispanic woman. The depth of the lesion was 5.5 mm with a positive sentinel lymph node biopsy.
Melanoma occurs less frequently in individuals with darker skin types than in lighter skin types but is associated with higher rates of morbidity and mortality in this patient population.1-7 In the cases shown here (A and B), both patients had advanced melanomas with large primary lesions and lymph node metastases.
Epidemiology
A systematic review by Higgins et al6 reported the following on the epidemiology of melanomas in patients with skin of color:
- African Americans have deeper tumors at the time of diagnosis, in addition to increased rates of regionally advanced and distant disease. Lesions generally are located on the lower extremities and have an increased propensity for ulceration. Acral lentiginous melanoma is the most common melanoma subtype found in African American patients.6
- In Hispanic individuals, superficial spreading melanoma is the most common melanoma subtype. Lower extremity lesions are more common relative to White individuals. Hispanic individuals have the highest rate of oral cavity melanomas across all ethnic groups.6
- In Asian individuals, acral and subungual sites are most common. Specifically, Pacific Islanders have the highest proportion of mucosal melanomas across all ethnic groups.6
Key clinical features in people with darker skin tones
Melanomas are found more often on the palms, soles, nail units, oral cavity, and mucosae.6 The melanomas have the same clinical and dermoscopic features found in individuals with lighter skin tones.
Worth noting
Factors that may contribute to the diagnosis of more advanced melanomas in racial/ethnic minorities in the United States include:
- decreased access to health care based on lack of health insurance and low socioeconomic status,
- less awareness of the risk of melanoma among patients and health care providers because melanoma is less common in persons of color, and
- lesions found in areas less likely to be seen in screening examinations, such as the soles of the feet and the oral and genital mucosae.
Health disparity highlight
- In a large US study of 96,953 patients with a diagnosis of cutaneous melanoma from 1992 to 2009, the proportion of later-stage melanoma—stages II to IV—was greater in Black patients compared to White patients.7
- Based on this same data set, White patients had the longest survival time (P<.05), followed by Hispanic (P<.05), Asian American/Native American/Pacific Islander (P<.05), and Black (P<.05) patients, respectively.7
- In Miami-Dade County, one study of 1690 melanoma cases found that 48% of Black patients had regional or distant disease at presentation compared to 22% of White patients (P=.015).5 Analysis of multiple factors found that only race was a significant predictor for late-stage melanoma (P<.001). Black patients in this study were 3 times more likely than others to be diagnosed with melanoma at a late stage (P=.07).5
- Black patients in the United States are more likely to have a delayed time from diagnosis to definitive surgery even when controlled for type of health insurance and stage of diagnosis.8
Final thoughts
Efforts are needed to overcome these disparities by:
- educating patients with skin of color and their health care providers about the risks of advanced melanoma with the goal of prevention and earlier diagnosis;
- breaking down barriers to care caused by poverty, lack of health insurance, and systemic racism; and
- eliminating factors that lead to delays from diagnosis to definitive surgery.
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37. doi:10.1016/j.jaad.2001.05.034
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914. doi:10.1001/archinte.166.17.1907
- Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California cancer registry data, 1988-93. Cancer Causes Control. 1997;8:246-252. doi:10.1023/a:1018432632528
- Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;51:1031-1032. doi:10.1016/j. jaad.2004.05.005
- Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142:704-708. doi:10.1001/archderm.142.6.704
- Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019;45:791-801. doi:10.1097/DSS.0000000000001759
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival [published online July 28, 2016]. J Am Acad Dermatol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
- Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016)[published online August 27, 2020]. J Am Acad Dermatol. 2021;84:1585-1593. doi:10.1016/j. jaad.2020.08.097
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37. doi:10.1016/j.jaad.2001.05.034
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914. doi:10.1001/archinte.166.17.1907
- Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California cancer registry data, 1988-93. Cancer Causes Control. 1997;8:246-252. doi:10.1023/a:1018432632528
- Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;51:1031-1032. doi:10.1016/j. jaad.2004.05.005
- Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142:704-708. doi:10.1001/archderm.142.6.704
- Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019;45:791-801. doi:10.1097/DSS.0000000000001759
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival [published online July 28, 2016]. J Am Acad Dermatol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
- Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016)[published online August 27, 2020]. J Am Acad Dermatol. 2021;84:1585-1593. doi:10.1016/j. jaad.2020.08.097
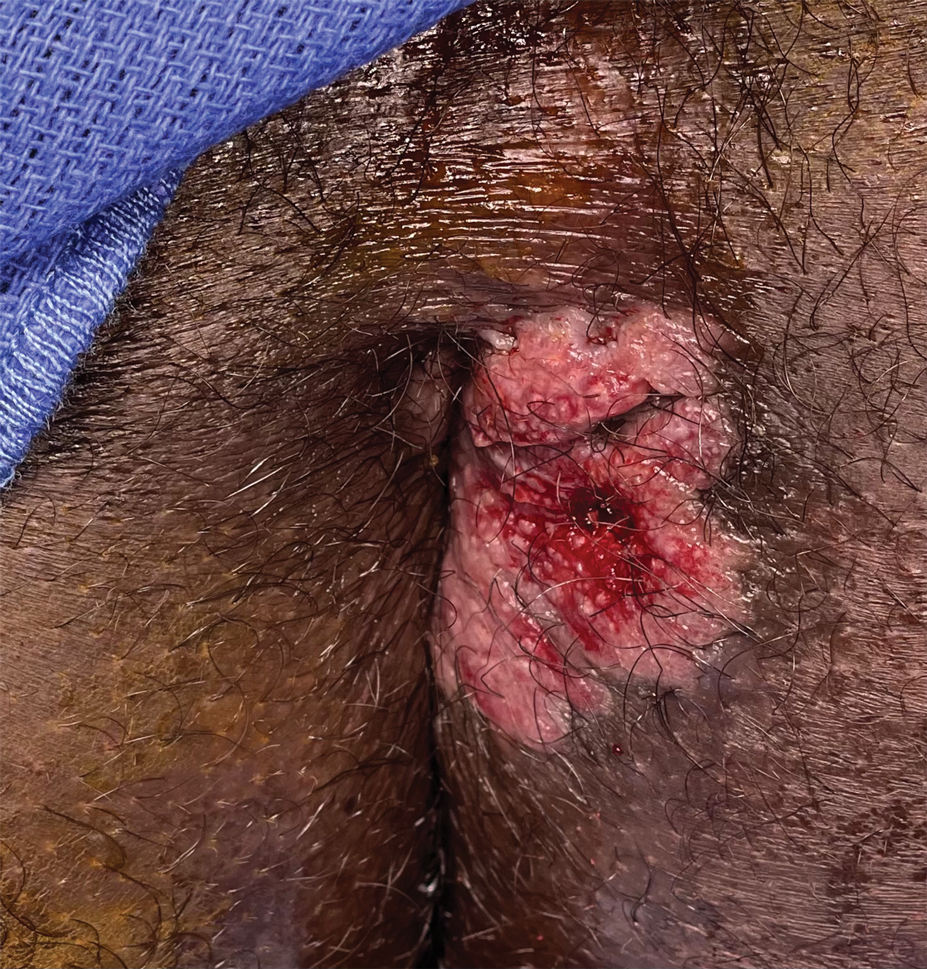
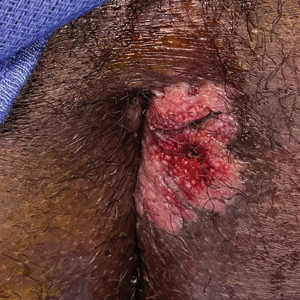
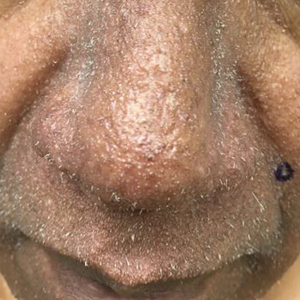
Painful Fungating Perianal Mass
The Diagnosis: Condyloma Latum
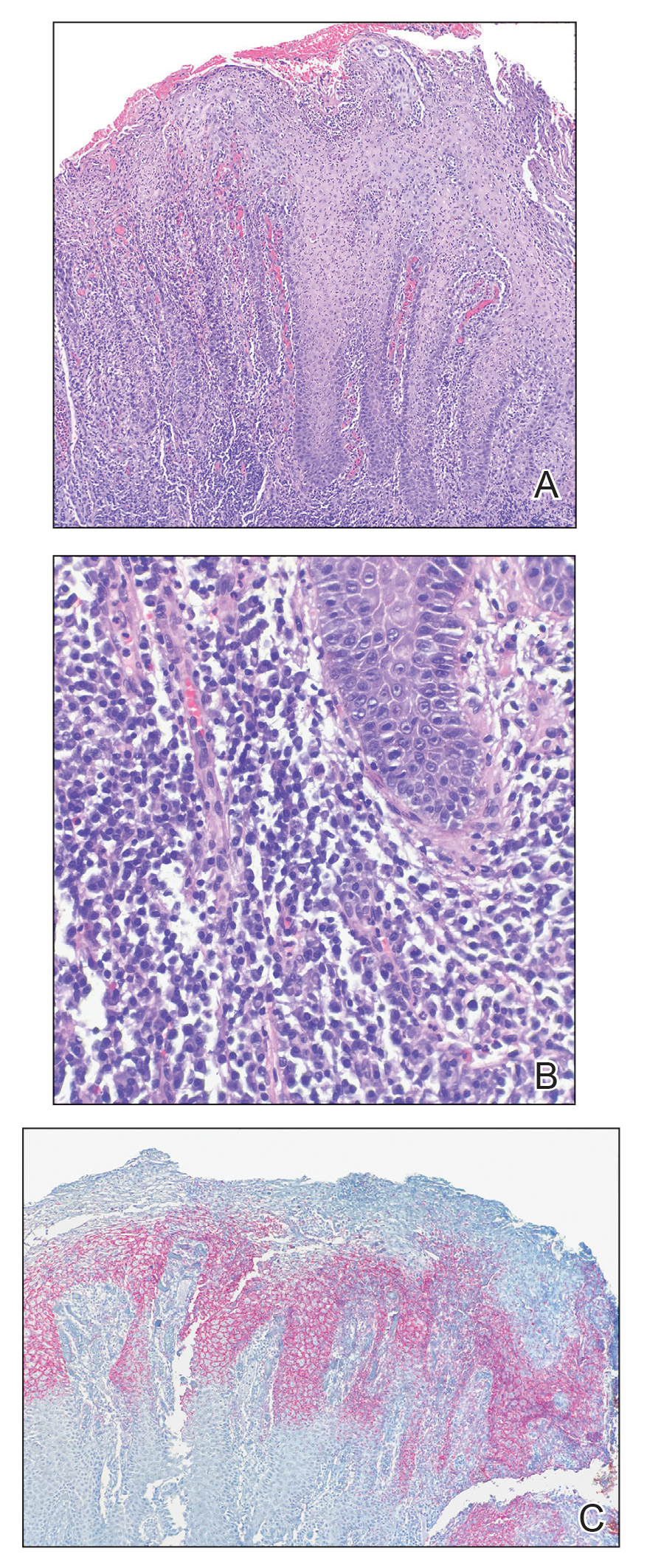
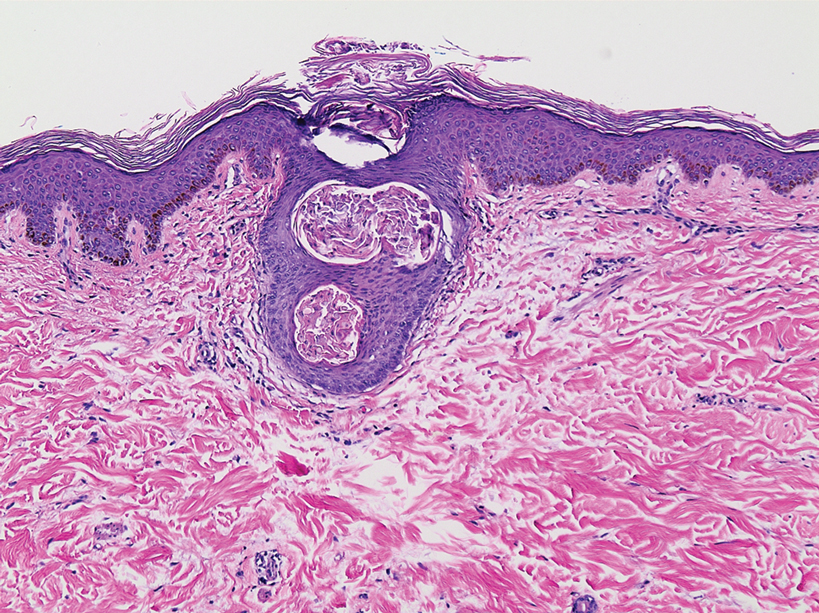
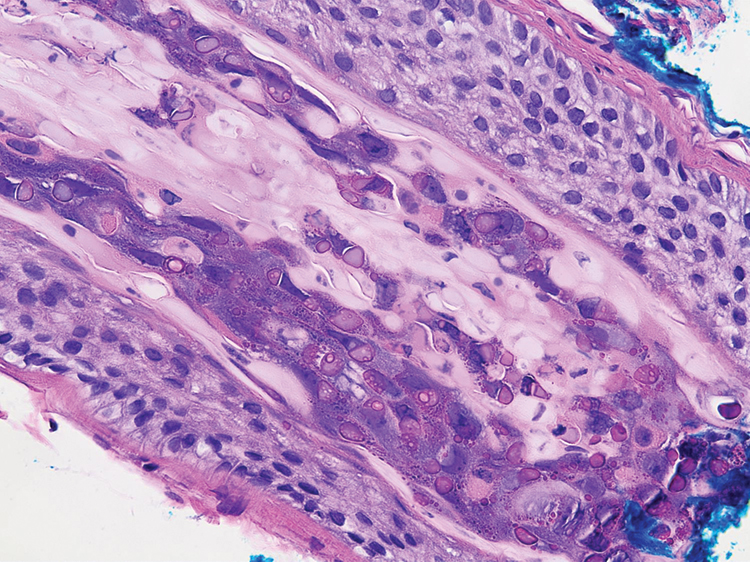
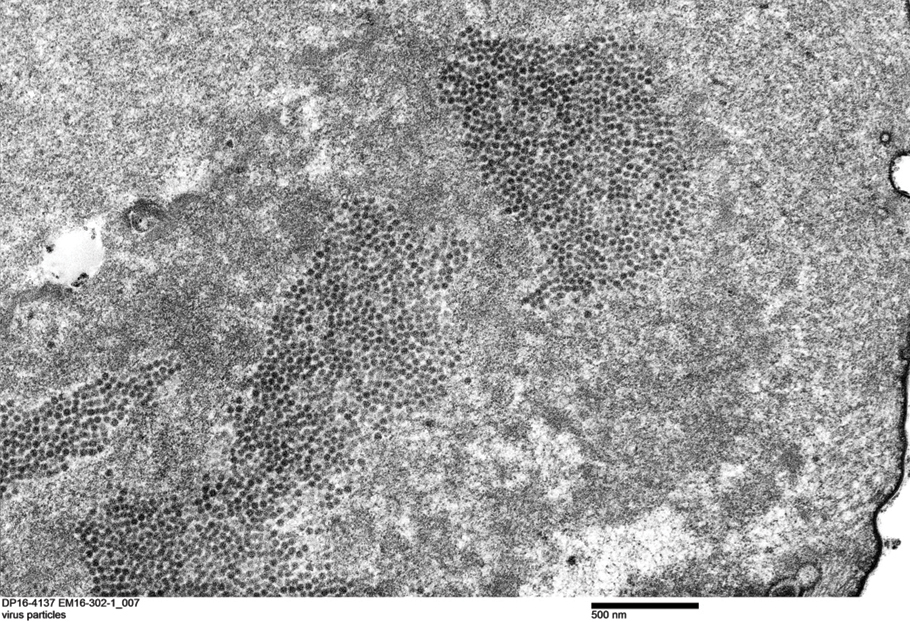
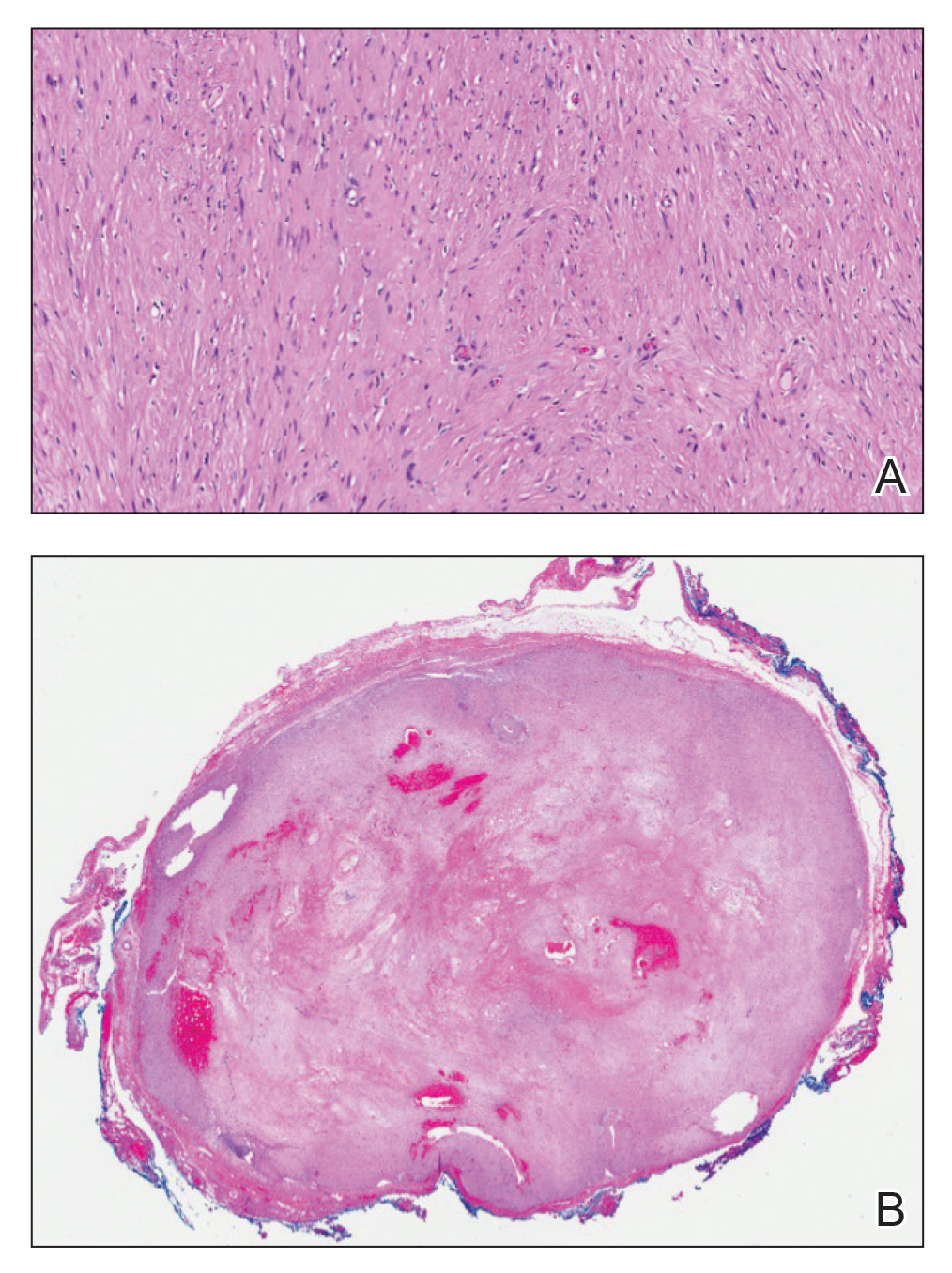
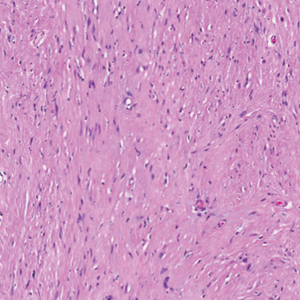
A punch biopsy of the perianal mass revealed epidermal acanthosis with elongated slender rete ridges, scattered intraepidermal neutrophils, and a dense dermal inflammatory infiltrate (Figure, A) with a prominent plasma cell component (Figure, B). A treponemal immunohistochemical stain revealed numerous coiled spirochetes concentrated in the lower epidermis (Figure, C). Serologic test results including rapid plasma reagin (titer 1:1024) and Treponema pallidum antibody were reactive, confirming the diagnosis of secondary syphilis with condyloma latum. The patient was treated with intramuscular penicillin G with resolution of the lesion 2 weeks later.
Syphilis, a sexually transmitted infection caused by the spirochete T pallidum, reached historically low rates in the United States in the early 2000s due to the widespread use of penicillin and effective public health efforts.1 However, the rates of primary and secondary syphilis infections recently have markedly increased, resulting in the current epidemic of syphilis in the United States and Europe.1,2 Its wide variety of clinical and histopathologic manifestations make recognition challenging and lend it the moniker “the great imitator.”
Secondary syphilis results from the systemic spread of T pallidum and classically is characterized by the triad of a skin rash that frequently involves the palms and soles, mucosal ulceration such as condyloma latum, and lymphadenopathy.2,3 However, condyloma latum may represent the only manifestation of secondary syphilis in a subset of patients,4 as observed in our patient.
In the 2 months prior to diagnosis, our patient was evaluated at multiple emergency departments and primary care clinics, receiving diagnoses of condyloma acuminatum, genital herpes simplex virus, hemorrhoids, and suspicion for malignancy—entities that comprise the differential diagnosis for condyloma latum.2,5 Despite some degree of overlap in patient populations, risk factors, and presentations between these diagnostic considerations, recognition of certain clinical features, in addition to histopathologic evaluation, may facilitate navigation of this differential diagnosis.
Primary and secondary syphilis infections have been predominantly observed in men, mostly men who have sex with men and/or those who are infected with HIV.1 Condyloma acuminata, genital herpes simplex virus, and chancroid also are seen in younger individuals, more commonly in those with multiple sexual partners, but show a more even gender distribution and are not restricted to those partaking in anal intercourse. The clinical presentation of condyloma latum can be differentiated by its painless, flat, smooth, and commonly hypopigmented appearance, often with associated surface erosion and a gray exudate, in contrast to condyloma acuminatum, which typically presents as nontender, flesh-colored or hyperpigmented, exophytic papules that may coalesce into plaques.2,3,6 Genital herpes simplex virus infection presents with multiple small papulovesicular lesions with ulceration, most commonly on the tip or shaft of the penis, though perianal lesions may be seen in men who have sex with men.7 Similarly, chancroid presents with painful necrotizing genital ulcers most commonly on the penis, though perianal lesions also may be seen.8 Hemorrhoids classically are seen in middle-aged adults with a history of constipation, present with rectal bleeding, and may be associated with pain in the setting of thrombosis or ulceration.9 Finally, perianal squamous cell carcinoma primarily occurs in older adults, typically in the sixth decade of life. Verrucous carcinoma most commonly arises in the oropharynx or anogenital region in sites of chronic irritation and presents as a slow-growing exophytic mass. Classic squamous cell carcinoma most commonly occurs in association with human papillomavirus infection and presents with scaly erythematous papules or plaques.10
Our case highlighted the clinical difficulty in recognizing condyloma latum, as this lesion remained undiagnosed for 2 months, and our patient presumptively was treated for multiple perianal pathologies prior to a biopsy being performed. Due to the clinical similarity of various perianal lesions, the diagnosis of condyloma latum should be considered, and serologic studies should be performed in fitting clinical contexts, especially in light of recently rising rates of syphilis infection.1,2
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Tayal S, Shaban F, Dasgupta K, et al. A case of syphilitic anal condylomata lata mimicking malignancy. Int J Surg Case Rep. 2015; 17:69-71.
- Aung PP, Wimmer DB, Lester TR, et al. Perianal condylomata lata mimicking carcinoma. J Cutan Pathol. 2022;49:209-214.
- Pourang A, Fung MA, Tartar D, et al. Condyloma lata in secondary syphilis. JAAD Case Rep. 2021;10:18-21.
- Bruins FG, van Deudekom FJ, de Vries HJ. Syphilitic condylomata lata mimicking anogenital warts. BMJ. 2015;350:h1259.
- Leslie SW, Sajjad H, Kumar S. Genital warts. In: StatPearls. StatPearls Publishing; 2021.
- Groves MJ. Genital herpes: a review. Am Fam Physician. 2016; 93:928-934.
- Irizarry L, Velasquez J, Wray AA. Chancroid. In: StatPearls. StatPearls Publishing; 2022.
- Mounsey AL, Halladay J, Sadiq TS. Hemorrhoids. Am Fam Physician. 2011;84:204-210.
- Abbass MA, Valente MA. Premalignant and malignant perianal lesions. Clin Colon Rectal Surg. 2019;32:386-393.
The Diagnosis: Condyloma Latum
A punch biopsy of the perianal mass revealed epidermal acanthosis with elongated slender rete ridges, scattered intraepidermal neutrophils, and a dense dermal inflammatory infiltrate (Figure, A) with a prominent plasma cell component (Figure, B). A treponemal immunohistochemical stain revealed numerous coiled spirochetes concentrated in the lower epidermis (Figure, C). Serologic test results including rapid plasma reagin (titer 1:1024) and Treponema pallidum antibody were reactive, confirming the diagnosis of secondary syphilis with condyloma latum. The patient was treated with intramuscular penicillin G with resolution of the lesion 2 weeks later.
Syphilis, a sexually transmitted infection caused by the spirochete T pallidum, reached historically low rates in the United States in the early 2000s due to the widespread use of penicillin and effective public health efforts.1 However, the rates of primary and secondary syphilis infections recently have markedly increased, resulting in the current epidemic of syphilis in the United States and Europe.1,2 Its wide variety of clinical and histopathologic manifestations make recognition challenging and lend it the moniker “the great imitator.”
Secondary syphilis results from the systemic spread of T pallidum and classically is characterized by the triad of a skin rash that frequently involves the palms and soles, mucosal ulceration such as condyloma latum, and lymphadenopathy.2,3 However, condyloma latum may represent the only manifestation of secondary syphilis in a subset of patients,4 as observed in our patient.
In the 2 months prior to diagnosis, our patient was evaluated at multiple emergency departments and primary care clinics, receiving diagnoses of condyloma acuminatum, genital herpes simplex virus, hemorrhoids, and suspicion for malignancy—entities that comprise the differential diagnosis for condyloma latum.2,5 Despite some degree of overlap in patient populations, risk factors, and presentations between these diagnostic considerations, recognition of certain clinical features, in addition to histopathologic evaluation, may facilitate navigation of this differential diagnosis.
Primary and secondary syphilis infections have been predominantly observed in men, mostly men who have sex with men and/or those who are infected with HIV.1 Condyloma acuminata, genital herpes simplex virus, and chancroid also are seen in younger individuals, more commonly in those with multiple sexual partners, but show a more even gender distribution and are not restricted to those partaking in anal intercourse. The clinical presentation of condyloma latum can be differentiated by its painless, flat, smooth, and commonly hypopigmented appearance, often with associated surface erosion and a gray exudate, in contrast to condyloma acuminatum, which typically presents as nontender, flesh-colored or hyperpigmented, exophytic papules that may coalesce into plaques.2,3,6 Genital herpes simplex virus infection presents with multiple small papulovesicular lesions with ulceration, most commonly on the tip or shaft of the penis, though perianal lesions may be seen in men who have sex with men.7 Similarly, chancroid presents with painful necrotizing genital ulcers most commonly on the penis, though perianal lesions also may be seen.8 Hemorrhoids classically are seen in middle-aged adults with a history of constipation, present with rectal bleeding, and may be associated with pain in the setting of thrombosis or ulceration.9 Finally, perianal squamous cell carcinoma primarily occurs in older adults, typically in the sixth decade of life. Verrucous carcinoma most commonly arises in the oropharynx or anogenital region in sites of chronic irritation and presents as a slow-growing exophytic mass. Classic squamous cell carcinoma most commonly occurs in association with human papillomavirus infection and presents with scaly erythematous papules or plaques.10
Our case highlighted the clinical difficulty in recognizing condyloma latum, as this lesion remained undiagnosed for 2 months, and our patient presumptively was treated for multiple perianal pathologies prior to a biopsy being performed. Due to the clinical similarity of various perianal lesions, the diagnosis of condyloma latum should be considered, and serologic studies should be performed in fitting clinical contexts, especially in light of recently rising rates of syphilis infection.1,2
The Diagnosis: Condyloma Latum
A punch biopsy of the perianal mass revealed epidermal acanthosis with elongated slender rete ridges, scattered intraepidermal neutrophils, and a dense dermal inflammatory infiltrate (Figure, A) with a prominent plasma cell component (Figure, B). A treponemal immunohistochemical stain revealed numerous coiled spirochetes concentrated in the lower epidermis (Figure, C). Serologic test results including rapid plasma reagin (titer 1:1024) and Treponema pallidum antibody were reactive, confirming the diagnosis of secondary syphilis with condyloma latum. The patient was treated with intramuscular penicillin G with resolution of the lesion 2 weeks later.
Syphilis, a sexually transmitted infection caused by the spirochete T pallidum, reached historically low rates in the United States in the early 2000s due to the widespread use of penicillin and effective public health efforts.1 However, the rates of primary and secondary syphilis infections recently have markedly increased, resulting in the current epidemic of syphilis in the United States and Europe.1,2 Its wide variety of clinical and histopathologic manifestations make recognition challenging and lend it the moniker “the great imitator.”
Secondary syphilis results from the systemic spread of T pallidum and classically is characterized by the triad of a skin rash that frequently involves the palms and soles, mucosal ulceration such as condyloma latum, and lymphadenopathy.2,3 However, condyloma latum may represent the only manifestation of secondary syphilis in a subset of patients,4 as observed in our patient.
In the 2 months prior to diagnosis, our patient was evaluated at multiple emergency departments and primary care clinics, receiving diagnoses of condyloma acuminatum, genital herpes simplex virus, hemorrhoids, and suspicion for malignancy—entities that comprise the differential diagnosis for condyloma latum.2,5 Despite some degree of overlap in patient populations, risk factors, and presentations between these diagnostic considerations, recognition of certain clinical features, in addition to histopathologic evaluation, may facilitate navigation of this differential diagnosis.
Primary and secondary syphilis infections have been predominantly observed in men, mostly men who have sex with men and/or those who are infected with HIV.1 Condyloma acuminata, genital herpes simplex virus, and chancroid also are seen in younger individuals, more commonly in those with multiple sexual partners, but show a more even gender distribution and are not restricted to those partaking in anal intercourse. The clinical presentation of condyloma latum can be differentiated by its painless, flat, smooth, and commonly hypopigmented appearance, often with associated surface erosion and a gray exudate, in contrast to condyloma acuminatum, which typically presents as nontender, flesh-colored or hyperpigmented, exophytic papules that may coalesce into plaques.2,3,6 Genital herpes simplex virus infection presents with multiple small papulovesicular lesions with ulceration, most commonly on the tip or shaft of the penis, though perianal lesions may be seen in men who have sex with men.7 Similarly, chancroid presents with painful necrotizing genital ulcers most commonly on the penis, though perianal lesions also may be seen.8 Hemorrhoids classically are seen in middle-aged adults with a history of constipation, present with rectal bleeding, and may be associated with pain in the setting of thrombosis or ulceration.9 Finally, perianal squamous cell carcinoma primarily occurs in older adults, typically in the sixth decade of life. Verrucous carcinoma most commonly arises in the oropharynx or anogenital region in sites of chronic irritation and presents as a slow-growing exophytic mass. Classic squamous cell carcinoma most commonly occurs in association with human papillomavirus infection and presents with scaly erythematous papules or plaques.10
Our case highlighted the clinical difficulty in recognizing condyloma latum, as this lesion remained undiagnosed for 2 months, and our patient presumptively was treated for multiple perianal pathologies prior to a biopsy being performed. Due to the clinical similarity of various perianal lesions, the diagnosis of condyloma latum should be considered, and serologic studies should be performed in fitting clinical contexts, especially in light of recently rising rates of syphilis infection.1,2
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Tayal S, Shaban F, Dasgupta K, et al. A case of syphilitic anal condylomata lata mimicking malignancy. Int J Surg Case Rep. 2015; 17:69-71.
- Aung PP, Wimmer DB, Lester TR, et al. Perianal condylomata lata mimicking carcinoma. J Cutan Pathol. 2022;49:209-214.
- Pourang A, Fung MA, Tartar D, et al. Condyloma lata in secondary syphilis. JAAD Case Rep. 2021;10:18-21.
- Bruins FG, van Deudekom FJ, de Vries HJ. Syphilitic condylomata lata mimicking anogenital warts. BMJ. 2015;350:h1259.
- Leslie SW, Sajjad H, Kumar S. Genital warts. In: StatPearls. StatPearls Publishing; 2021.
- Groves MJ. Genital herpes: a review. Am Fam Physician. 2016; 93:928-934.
- Irizarry L, Velasquez J, Wray AA. Chancroid. In: StatPearls. StatPearls Publishing; 2022.
- Mounsey AL, Halladay J, Sadiq TS. Hemorrhoids. Am Fam Physician. 2011;84:204-210.
- Abbass MA, Valente MA. Premalignant and malignant perianal lesions. Clin Colon Rectal Surg. 2019;32:386-393.
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Tayal S, Shaban F, Dasgupta K, et al. A case of syphilitic anal condylomata lata mimicking malignancy. Int J Surg Case Rep. 2015; 17:69-71.
- Aung PP, Wimmer DB, Lester TR, et al. Perianal condylomata lata mimicking carcinoma. J Cutan Pathol. 2022;49:209-214.
- Pourang A, Fung MA, Tartar D, et al. Condyloma lata in secondary syphilis. JAAD Case Rep. 2021;10:18-21.
- Bruins FG, van Deudekom FJ, de Vries HJ. Syphilitic condylomata lata mimicking anogenital warts. BMJ. 2015;350:h1259.
- Leslie SW, Sajjad H, Kumar S. Genital warts. In: StatPearls. StatPearls Publishing; 2021.
- Groves MJ. Genital herpes: a review. Am Fam Physician. 2016; 93:928-934.
- Irizarry L, Velasquez J, Wray AA. Chancroid. In: StatPearls. StatPearls Publishing; 2022.
- Mounsey AL, Halladay J, Sadiq TS. Hemorrhoids. Am Fam Physician. 2011;84:204-210.
- Abbass MA, Valente MA. Premalignant and malignant perianal lesions. Clin Colon Rectal Surg. 2019;32:386-393.
A 21-year-old man presented to our clinic with rectal pain of 2 months’ duration that occurred in association with bowel movements and rectal bleeding in the setting of constipation. The patient’s symptoms had persisted despite multiple clinical encounters and treatment with sulfamethoxazole-trimethoprim, clotrimazole, valacyclovir, topical hydrocortisone and pramoxine, topical lidocaine, imiquimod, and psyllium seed. The patient denied engaging in receptive anal intercourse and had no notable medical or surgical history. Physical examination revealed a 6-cm hypopigmented fungating mass on the left gluteal cleft just external to the anal verge; there were no other abnormal findings. The patient denied any other systemic symptoms.
Facial Follicular Spicules: A Rare Cutaneous Presentation of Trichodysplasia Spinulosa
To the Editor:
A 57-year-old man with hypertension, dyslipidemia, and congestive heart failure presented with a disfiguring eruption comprised of asymptomatic papules on the face that appeared 12 months post–heart transplantation. Immunosuppressive medications included mycophenolic acid and tacrolimus ointment (FK506). The pinpoint papules spread from the central face to the ears, arms, and legs. Physical examination revealed multiple 0.5- to 1-mm flesh-colored papules over the glabella, nose, nasolabial folds, philtrum, chin, ears, arms, and legs sparing the trunk. The initial appearance of the facial rash resembled the surface of a nutmeg grater with central white spiny excrescences overlying fine papules (spinulosism)(Figure 1). In addition, eyebrow alopecia was present.
A 3-mm punch biopsy of a papule with a central spine was performed on the left thigh. Microscopic examination revealed marked dilatation of anagen hair follicles with a proliferation of haphazard inner root sheath cells replacing the follicular lumen. Hair shafts were absent, and plugged infundibula were observed (Figure 2). The inner root sheath keratinocytes were enlarged and dystrophic with deeply eosinophilic trichohyalin granules (Figure 3). The epidermis, outer root sheath epithelium, and eccrine structures were unremarkable.
Transmission electron microscopy (TEM) confirmed the presence of intranuclear viral inclusions within affected inner root sheath keratinocytes composed of nonenveloped icosahedral viral particles measuring 33 to 38 nm in diameter (Figure 4). These findings morphologically were consistent with a polyomavirus. No intracytoplasmic or extracellular viral particles were identified. The clinical history, physical examination, histopathology, and electron microscopy features strongly supported the diagnosis of trichodysplasia spinulosa (TS) despite insufficient material being retrieved for polymerase chain reaction identification.
Trichodysplasia spinulosa was first described by Haycox et al1 in 1999. The authors suggested a viral etiology. Eleven years later, TS-associated polyomavirus (TSPyV) was identified by van der Meijden et al.2 Follicular keratinocytes are the specific target for TSPyV.3 Evidence has been presented suggesting that TS is caused by a primary infection or reactivation of TSPyV in the setting of immunosuppression.4,5
Patients with TS present with papular eruptions that appear on the central face with spiny excrescences and various degrees of alopecia involving the eyebrows or eyelashes. Histopathologic features include distended hair follicles with expansion of inner root sheath cells, eosinophilic trichohyalin granules, and the absence of hair shafts. The viral protein can be verified through immunohistochemistry TSPyV VP1 staining that demonstrates co-localization with trichohyalin. Viral particles also can be visualized as 35- to 38-nm intranuclear particles with an organized crystalloid morphology on TEM.6,7 The negative polymerase chain reaction in our patient could be the result of suboptimal template DNA concentration extracted from the limited amount of tissue remaining in the block after hematoxylin and eosin staining.
The clinical differential diagnosis of central facial spinulosism includes the follicular spicules of multiple myeloma (FSMM). In fact, FSMM and TS can only be differentiated after obtaining a blood profile and bone marrow biopsy that excludes the diagnosis of FSMM. A history of immunosuppression typically suggests TS. Histopathology often is equivocal in FSMM8; however, TEM reveals viral particles (TSPyV) in TS. Transmission electron microscopy in FSMM demonstrates fibrillary structures arranged in a paracrystalline configuration with unknown significance instead of viral particles. Despite the absence of viral particles on TEM, a low mean copy number of Merkel cell polyomavirus was isolated from a patient with FSMM who responded dramatically to treatment with topical cidofovir gel 1%.8 In addition to treating the underlying multiple myeloma in FSMM, topical cidofovir gel 1% also may have a role in treatment of these patients, suggesting a possible viral rather than simply paraneoplastic etiology of FSMM. Therefore, polyomavirus infection should be considered in the initial workup of any patient with fine facial follicular spicules.
The most effective management of TS in transplant recipients is to reduce immunosuppression to the lowest level possible without jeopardizing the transplanted organ.9 In our case, reduction of immunosuppressive drugs was not possible. In fact, immunosuppression in our patient was increased following evidence of early rejection of the heart transplant. Although manual extraction of the keratin spicules resulted in considerable improvement in a similar facial eruption in a patient with pediatric pre–B-cell acute lymphoblastic leukemia developing TS,10 it is impossible to apply this approach to patients such as ours who have thousands of tiny lesions. Fortunately, custom-compounded cidofovir gel 1% applied twice daily to the patient’s face and ears for 4 weeks led to near-complete clearance at follow-up (Figure 5). Due to the high cost of the medication (approaching $700 for one tube), our patient applied this medication to the face only several times weekly with excellent improvement. Thus, it appears that it is possible to suppress this virus with topical medication alone.
Polyomavirus infection should be considered in patients presenting with fine follicular spiny papules, especially those who are immunosuppressed. The possibility of coexisting multiple myeloma should be excluded.
Acknowledgment—We sincerely thank Glenn A. Hoskins (Jackson, Mississippi), the electron microscopy technologist, for the detection of viral particles and the electron microscope photographs.
- Haycox CL, Kim S, Fleckman P, et al. Trichodysplasia spinulosa: a newly described folliculocentric viral infection in an immunocompromised host. J Investig Dermatol Symp Proc. 1999;4:268-271.
- van der Meijden E, Janssens RWA, Lauber C, et al. Discovery of a new human polyomavirus associated with trichodysplasia spinulosa in an immunocompromized patient. PLoS Pathog. 2010;6:E1001024.
- Rouanet J, Aubin F, Gaboriaud P, et al. Trichodysplasia spinulosa: a polyomavirus infection specifically targeting follicular keratinocytes in immunocompromised patients. Br J Dermatol. 2016;174:629-632.
- van der Meijden E, Kazem S, Burgers MM, et al. Seroprevalence of trichodysplasia spinulosa-associated polyomavirus. Emerg Infect Dis. 2011;17:1355-1363.
- van der Meijden E, Horváth B, Nijland M, et al. Primary polyomavirus infection, not reactivation, as the cause of trichodysplasia spinulosa in immunocompromised patients. J Infect Dis. 2017;215:1080-1084.
- Fischer MK, Kao GF, Nguyen HP, et al. Specific detection of trichodysplasia spinulosa-associated polyomavirus DNA in skin and renal allograft tissues in a patient with trichodysplasia spinulosa. Arch Dermatol. 2012;148:726-733.
- Kazem S, van der Meijden E, Feltkamp MC. The trichodysplasia spinulosa-associated polyomavirus: virological background and clinical implications. APMIS. 2013;121:770-782.
- van Boheemen S, Jones T, Muhlemann B, et al. Cidofovir gel as treatment of follicular spicules in multiple myeloma. JAMA Dermatol. 2015;151:82-84.
- DeCrescenzo AJ, Philips RC, Wilkerson MG. Trichodysplasia spinulosa: a rare complication of immunosuppression. JAAD Case Rep. 2016;2:307-309.
- Barton M, Lockhart S, Sidbury R, et al. Trichodysplasia spinulosa in a 7-year-old boy managed using physical extraction of keratin spicules. Pediatr Dermatol. 2017;34:E74-E76.
To the Editor:
A 57-year-old man with hypertension, dyslipidemia, and congestive heart failure presented with a disfiguring eruption comprised of asymptomatic papules on the face that appeared 12 months post–heart transplantation. Immunosuppressive medications included mycophenolic acid and tacrolimus ointment (FK506). The pinpoint papules spread from the central face to the ears, arms, and legs. Physical examination revealed multiple 0.5- to 1-mm flesh-colored papules over the glabella, nose, nasolabial folds, philtrum, chin, ears, arms, and legs sparing the trunk. The initial appearance of the facial rash resembled the surface of a nutmeg grater with central white spiny excrescences overlying fine papules (spinulosism)(Figure 1). In addition, eyebrow alopecia was present.
A 3-mm punch biopsy of a papule with a central spine was performed on the left thigh. Microscopic examination revealed marked dilatation of anagen hair follicles with a proliferation of haphazard inner root sheath cells replacing the follicular lumen. Hair shafts were absent, and plugged infundibula were observed (Figure 2). The inner root sheath keratinocytes were enlarged and dystrophic with deeply eosinophilic trichohyalin granules (Figure 3). The epidermis, outer root sheath epithelium, and eccrine structures were unremarkable.
Transmission electron microscopy (TEM) confirmed the presence of intranuclear viral inclusions within affected inner root sheath keratinocytes composed of nonenveloped icosahedral viral particles measuring 33 to 38 nm in diameter (Figure 4). These findings morphologically were consistent with a polyomavirus. No intracytoplasmic or extracellular viral particles were identified. The clinical history, physical examination, histopathology, and electron microscopy features strongly supported the diagnosis of trichodysplasia spinulosa (TS) despite insufficient material being retrieved for polymerase chain reaction identification.
Trichodysplasia spinulosa was first described by Haycox et al1 in 1999. The authors suggested a viral etiology. Eleven years later, TS-associated polyomavirus (TSPyV) was identified by van der Meijden et al.2 Follicular keratinocytes are the specific target for TSPyV.3 Evidence has been presented suggesting that TS is caused by a primary infection or reactivation of TSPyV in the setting of immunosuppression.4,5
Patients with TS present with papular eruptions that appear on the central face with spiny excrescences and various degrees of alopecia involving the eyebrows or eyelashes. Histopathologic features include distended hair follicles with expansion of inner root sheath cells, eosinophilic trichohyalin granules, and the absence of hair shafts. The viral protein can be verified through immunohistochemistry TSPyV VP1 staining that demonstrates co-localization with trichohyalin. Viral particles also can be visualized as 35- to 38-nm intranuclear particles with an organized crystalloid morphology on TEM.6,7 The negative polymerase chain reaction in our patient could be the result of suboptimal template DNA concentration extracted from the limited amount of tissue remaining in the block after hematoxylin and eosin staining.
The clinical differential diagnosis of central facial spinulosism includes the follicular spicules of multiple myeloma (FSMM). In fact, FSMM and TS can only be differentiated after obtaining a blood profile and bone marrow biopsy that excludes the diagnosis of FSMM. A history of immunosuppression typically suggests TS. Histopathology often is equivocal in FSMM8; however, TEM reveals viral particles (TSPyV) in TS. Transmission electron microscopy in FSMM demonstrates fibrillary structures arranged in a paracrystalline configuration with unknown significance instead of viral particles. Despite the absence of viral particles on TEM, a low mean copy number of Merkel cell polyomavirus was isolated from a patient with FSMM who responded dramatically to treatment with topical cidofovir gel 1%.8 In addition to treating the underlying multiple myeloma in FSMM, topical cidofovir gel 1% also may have a role in treatment of these patients, suggesting a possible viral rather than simply paraneoplastic etiology of FSMM. Therefore, polyomavirus infection should be considered in the initial workup of any patient with fine facial follicular spicules.
The most effective management of TS in transplant recipients is to reduce immunosuppression to the lowest level possible without jeopardizing the transplanted organ.9 In our case, reduction of immunosuppressive drugs was not possible. In fact, immunosuppression in our patient was increased following evidence of early rejection of the heart transplant. Although manual extraction of the keratin spicules resulted in considerable improvement in a similar facial eruption in a patient with pediatric pre–B-cell acute lymphoblastic leukemia developing TS,10 it is impossible to apply this approach to patients such as ours who have thousands of tiny lesions. Fortunately, custom-compounded cidofovir gel 1% applied twice daily to the patient’s face and ears for 4 weeks led to near-complete clearance at follow-up (Figure 5). Due to the high cost of the medication (approaching $700 for one tube), our patient applied this medication to the face only several times weekly with excellent improvement. Thus, it appears that it is possible to suppress this virus with topical medication alone.
Polyomavirus infection should be considered in patients presenting with fine follicular spiny papules, especially those who are immunosuppressed. The possibility of coexisting multiple myeloma should be excluded.
Acknowledgment—We sincerely thank Glenn A. Hoskins (Jackson, Mississippi), the electron microscopy technologist, for the detection of viral particles and the electron microscope photographs.
To the Editor:
A 57-year-old man with hypertension, dyslipidemia, and congestive heart failure presented with a disfiguring eruption comprised of asymptomatic papules on the face that appeared 12 months post–heart transplantation. Immunosuppressive medications included mycophenolic acid and tacrolimus ointment (FK506). The pinpoint papules spread from the central face to the ears, arms, and legs. Physical examination revealed multiple 0.5- to 1-mm flesh-colored papules over the glabella, nose, nasolabial folds, philtrum, chin, ears, arms, and legs sparing the trunk. The initial appearance of the facial rash resembled the surface of a nutmeg grater with central white spiny excrescences overlying fine papules (spinulosism)(Figure 1). In addition, eyebrow alopecia was present.
A 3-mm punch biopsy of a papule with a central spine was performed on the left thigh. Microscopic examination revealed marked dilatation of anagen hair follicles with a proliferation of haphazard inner root sheath cells replacing the follicular lumen. Hair shafts were absent, and plugged infundibula were observed (Figure 2). The inner root sheath keratinocytes were enlarged and dystrophic with deeply eosinophilic trichohyalin granules (Figure 3). The epidermis, outer root sheath epithelium, and eccrine structures were unremarkable.
Transmission electron microscopy (TEM) confirmed the presence of intranuclear viral inclusions within affected inner root sheath keratinocytes composed of nonenveloped icosahedral viral particles measuring 33 to 38 nm in diameter (Figure 4). These findings morphologically were consistent with a polyomavirus. No intracytoplasmic or extracellular viral particles were identified. The clinical history, physical examination, histopathology, and electron microscopy features strongly supported the diagnosis of trichodysplasia spinulosa (TS) despite insufficient material being retrieved for polymerase chain reaction identification.
Trichodysplasia spinulosa was first described by Haycox et al1 in 1999. The authors suggested a viral etiology. Eleven years later, TS-associated polyomavirus (TSPyV) was identified by van der Meijden et al.2 Follicular keratinocytes are the specific target for TSPyV.3 Evidence has been presented suggesting that TS is caused by a primary infection or reactivation of TSPyV in the setting of immunosuppression.4,5
Patients with TS present with papular eruptions that appear on the central face with spiny excrescences and various degrees of alopecia involving the eyebrows or eyelashes. Histopathologic features include distended hair follicles with expansion of inner root sheath cells, eosinophilic trichohyalin granules, and the absence of hair shafts. The viral protein can be verified through immunohistochemistry TSPyV VP1 staining that demonstrates co-localization with trichohyalin. Viral particles also can be visualized as 35- to 38-nm intranuclear particles with an organized crystalloid morphology on TEM.6,7 The negative polymerase chain reaction in our patient could be the result of suboptimal template DNA concentration extracted from the limited amount of tissue remaining in the block after hematoxylin and eosin staining.
The clinical differential diagnosis of central facial spinulosism includes the follicular spicules of multiple myeloma (FSMM). In fact, FSMM and TS can only be differentiated after obtaining a blood profile and bone marrow biopsy that excludes the diagnosis of FSMM. A history of immunosuppression typically suggests TS. Histopathology often is equivocal in FSMM8; however, TEM reveals viral particles (TSPyV) in TS. Transmission electron microscopy in FSMM demonstrates fibrillary structures arranged in a paracrystalline configuration with unknown significance instead of viral particles. Despite the absence of viral particles on TEM, a low mean copy number of Merkel cell polyomavirus was isolated from a patient with FSMM who responded dramatically to treatment with topical cidofovir gel 1%.8 In addition to treating the underlying multiple myeloma in FSMM, topical cidofovir gel 1% also may have a role in treatment of these patients, suggesting a possible viral rather than simply paraneoplastic etiology of FSMM. Therefore, polyomavirus infection should be considered in the initial workup of any patient with fine facial follicular spicules.
The most effective management of TS in transplant recipients is to reduce immunosuppression to the lowest level possible without jeopardizing the transplanted organ.9 In our case, reduction of immunosuppressive drugs was not possible. In fact, immunosuppression in our patient was increased following evidence of early rejection of the heart transplant. Although manual extraction of the keratin spicules resulted in considerable improvement in a similar facial eruption in a patient with pediatric pre–B-cell acute lymphoblastic leukemia developing TS,10 it is impossible to apply this approach to patients such as ours who have thousands of tiny lesions. Fortunately, custom-compounded cidofovir gel 1% applied twice daily to the patient’s face and ears for 4 weeks led to near-complete clearance at follow-up (Figure 5). Due to the high cost of the medication (approaching $700 for one tube), our patient applied this medication to the face only several times weekly with excellent improvement. Thus, it appears that it is possible to suppress this virus with topical medication alone.
Polyomavirus infection should be considered in patients presenting with fine follicular spiny papules, especially those who are immunosuppressed. The possibility of coexisting multiple myeloma should be excluded.
Acknowledgment—We sincerely thank Glenn A. Hoskins (Jackson, Mississippi), the electron microscopy technologist, for the detection of viral particles and the electron microscope photographs.
- Haycox CL, Kim S, Fleckman P, et al. Trichodysplasia spinulosa: a newly described folliculocentric viral infection in an immunocompromised host. J Investig Dermatol Symp Proc. 1999;4:268-271.
- van der Meijden E, Janssens RWA, Lauber C, et al. Discovery of a new human polyomavirus associated with trichodysplasia spinulosa in an immunocompromized patient. PLoS Pathog. 2010;6:E1001024.
- Rouanet J, Aubin F, Gaboriaud P, et al. Trichodysplasia spinulosa: a polyomavirus infection specifically targeting follicular keratinocytes in immunocompromised patients. Br J Dermatol. 2016;174:629-632.
- van der Meijden E, Kazem S, Burgers MM, et al. Seroprevalence of trichodysplasia spinulosa-associated polyomavirus. Emerg Infect Dis. 2011;17:1355-1363.
- van der Meijden E, Horváth B, Nijland M, et al. Primary polyomavirus infection, not reactivation, as the cause of trichodysplasia spinulosa in immunocompromised patients. J Infect Dis. 2017;215:1080-1084.
- Fischer MK, Kao GF, Nguyen HP, et al. Specific detection of trichodysplasia spinulosa-associated polyomavirus DNA in skin and renal allograft tissues in a patient with trichodysplasia spinulosa. Arch Dermatol. 2012;148:726-733.
- Kazem S, van der Meijden E, Feltkamp MC. The trichodysplasia spinulosa-associated polyomavirus: virological background and clinical implications. APMIS. 2013;121:770-782.
- van Boheemen S, Jones T, Muhlemann B, et al. Cidofovir gel as treatment of follicular spicules in multiple myeloma. JAMA Dermatol. 2015;151:82-84.
- DeCrescenzo AJ, Philips RC, Wilkerson MG. Trichodysplasia spinulosa: a rare complication of immunosuppression. JAAD Case Rep. 2016;2:307-309.
- Barton M, Lockhart S, Sidbury R, et al. Trichodysplasia spinulosa in a 7-year-old boy managed using physical extraction of keratin spicules. Pediatr Dermatol. 2017;34:E74-E76.
- Haycox CL, Kim S, Fleckman P, et al. Trichodysplasia spinulosa: a newly described folliculocentric viral infection in an immunocompromised host. J Investig Dermatol Symp Proc. 1999;4:268-271.
- van der Meijden E, Janssens RWA, Lauber C, et al. Discovery of a new human polyomavirus associated with trichodysplasia spinulosa in an immunocompromized patient. PLoS Pathog. 2010;6:E1001024.
- Rouanet J, Aubin F, Gaboriaud P, et al. Trichodysplasia spinulosa: a polyomavirus infection specifically targeting follicular keratinocytes in immunocompromised patients. Br J Dermatol. 2016;174:629-632.
- van der Meijden E, Kazem S, Burgers MM, et al. Seroprevalence of trichodysplasia spinulosa-associated polyomavirus. Emerg Infect Dis. 2011;17:1355-1363.
- van der Meijden E, Horváth B, Nijland M, et al. Primary polyomavirus infection, not reactivation, as the cause of trichodysplasia spinulosa in immunocompromised patients. J Infect Dis. 2017;215:1080-1084.
- Fischer MK, Kao GF, Nguyen HP, et al. Specific detection of trichodysplasia spinulosa-associated polyomavirus DNA in skin and renal allograft tissues in a patient with trichodysplasia spinulosa. Arch Dermatol. 2012;148:726-733.
- Kazem S, van der Meijden E, Feltkamp MC. The trichodysplasia spinulosa-associated polyomavirus: virological background and clinical implications. APMIS. 2013;121:770-782.
- van Boheemen S, Jones T, Muhlemann B, et al. Cidofovir gel as treatment of follicular spicules in multiple myeloma. JAMA Dermatol. 2015;151:82-84.
- DeCrescenzo AJ, Philips RC, Wilkerson MG. Trichodysplasia spinulosa: a rare complication of immunosuppression. JAAD Case Rep. 2016;2:307-309.
- Barton M, Lockhart S, Sidbury R, et al. Trichodysplasia spinulosa in a 7-year-old boy managed using physical extraction of keratin spicules. Pediatr Dermatol. 2017;34:E74-E76.
Practice Points
- Trichodysplasia spinulosa (TS) is a rare skin disease caused by primary TS-associated polyomavirus (TSPyV) infecting follicular keratinocytes in immunocompromised patients.
- Trichodysplasia spinulosa typically presents with papular eruptions that appear on the central face with spiny excrescences and various degrees of alopecia involving the eyebrows or eyelashes.
- The viral protein can be verified through immunohistochemistry TSPyV major capsid protein VP1 staining or can be visualized on transmission electron microscopy.
- Follicular spicules of multiple myeloma should be ruled out before initiating treatment with cidofovir gel 1% for TS.
Erythematous Plaque on the Groin and Buttocks
The Diagnosis: Pseudomonas Pyoderma
A skin swab confirmed the presence of a ciprofloxacinsusceptible Pseudomonas aeruginosa strain. Our patient received oral ciprofloxacin 500 mg twice daily for 10 days with remarkable clinical improvement. The remaining skin lesion was successfully treated with more frequent diaper changes and the use of topical corticosteroids and emollients.
The topographical location, cutaneous morphology, clinical context, and sometimes the type of exudate are fundamental for the diagnosis of eruptions in intertriginous areas. Cutaneous Candida infections are common in these locations. They classically present as markedly erythematous plaques that occasionally are erosive, accompanied by satellite papules and pustules.1 Tinea cruris is a dermatophyte infection of the groin, proximal medial thighs, perineum, and buttocks. It usually presents as an erythematous patch that spreads centrifugally with partial central clearing and a slightly elevated, scaly border. Although candidiasis was higher on the differential, it was less likely, as our patient had a concomitant exudate inconsistent with Candida infections. Also, the lack of response to antifungal agents made hypotheses of fungal infections improbable.1
Inverse psoriasis is a variant of psoriasis identified by the development of well-demarcated, nonscaly, shiny plaques on body folds.2 Psoriasis is a chronic disease with several other cutaneous manifestations, such as nail and scalp involvement, as well as erythematous scaly plaques on the extensor surfaces of the limbs. The absence of a history of psoriasis, lack of other cutaneous manifestations, and no response to topical corticosteroids made the diagnosis of inverse psoriasis unlikely in our patient.
Erythrasma is a common superficial cutaneous infection caused by Corynebacterium minutissimum, a grampositive bacillus. It typically presents as an intertriginous eruption characterized by small erythematous to brown patches or thin plaques with fine scaling and sharp borders.3 Erythrasma displays a coral red fluorescence on Wood lamp examination that can be useful in the distinction from other causes of intertrigo.1 Although this examination had not been performed in our patient, the striking exudate made erythrasma less likely, and the culture performed on skin swab material would help to rule out this diagnosis.
Pseudomonas aeruginosa is a gram-negative strict aerobic bacillus of ubiquitous distribution with a preference for humid environments.4,5Pseudomonas aeruginosa infections were first reported in the 19th century by physicians who noticed a peculiar odorous condition that caused a blue-green discoloration on bandages. This coloration explains the species name aeruginosa which is derived from the Latin word for copper rust.4 It comes from several water-soluble pigments produced by this microorganism, the most prevalent of which are pyocyanin and pyoverdine. Pyocyanin has a greenish-blue color and is nonfluorescent, while pyoverdine is green-yellowish and fluoresces under Wood light.5 Other pigments, such as pyorubin and pyomelanin, can be produced by some Pseudomonas strains.4
Pseudomonas aeruginosa has become one of the main pathogens involved in hospital-acquired infections,6 especially in immunocompromised patients.6,7 It is a frequent cause of respiratory infections in patients with cystic fibrosis, as it is present in the airways of up to 70% of these patients in adulthood.7 Also, due to a variety of adaptive mechanisms with the development of resistance to a range of antibiotics, P aeruginosa has become a worldwide public health problem and is involved in several life-threatening nosocomial infections.7,8
Cutaneous P aeruginosa infections range from superficial to deep tissue involvement and can affect both immunocompromised and immunocompetent individuals.9 They are classified as primary when they originate directly from the skin or secondary when they occur in the context of bacteremia. Primary infections mostly are mild and often are seen in healthy individuals; they usually occur by inoculation and predominate in moist areas where skin breakdown is frequent. Secondary infections typically affect immunocompromised individuals and portend a poor prognosis.5,9
Denominated as Pseudomonas pyoderma, the superficial skin infection by P aeruginosa is described as a condition where the epidermis has a moth-eaten appearance with macerated or eroded borders.10 A blue-greenish exudate and a grape juice odor often are present. This infection usually occurs as a complication of several skin conditions such as tinea pedis, eczema, burns, wounds, and ulcers.5,10
We believe that our patient developed Pseudomonas pyoderma as a complication of diaper dermatitis. His extended hospital stay with the use of different antibiotic regimens for the treatment of several infectious complications may have contributed to the development of infection by P aeruginosa.11 Despite its great clinical relevance, there are few studies in the literature on primary skin infections caused by P aeruginosa, and clinical descriptions with images are rare. Our patient had a nonspecific noneczematous dermatitis, and the projections on the periphery of the lesion resembled the moth-eaten appearance of the classic description of Pseudomonas pyoderma.5,10 The presence of a greenish exudate should promptly raise suspicion for this entity. We believe that the presentation of this case can illustrate this finding and help physicians to recognize this infection.
- Kalra MG, Higgins KE, Kinney BS. Intertrigo and secondary skin infections. Am Fam Physician. 2014;89:569-573.
- Micali G, Verzi AE, Giuffrida G, et al. Inverse psoriasis: from diagnosis to current treatment options. Clin Cosmet Investig Dermatol. 2019; 12:953-959.
- Somerville DA. Erythrasma in normal young adults. J Med Microbiol. 1970;3:57-64.
- D’Agata E. Pseudomonas aeruginosa and other Pseudomonas species. In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Vol 2. 8th ed. Elsevier; 2015:2518-2531.
- Silvestre JF, Betlloch MI. Cutaneous manifestations due to Pseudomonas infection. Int J Dermatol. 1999;38:419-431.
- Young LS, Armstrong D. Pseudomonas aeruginosa infections. CRC Crit Rev Clin Lab Sci. 1972;3:291-347.
- Moradali MF, Ghods S, Rehm BH. Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front Cell Infect Microbiol. 2017;7:39.
- Rosenthal VD, Bat-Erdene I, Gupta D, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 45 countries for 2012-2017: device-associated module. Am J Infect Control. 2020;48:423-432.
- Wu DC, Chan WW, Metelitsa AI, et al. Pseudomonas skin infection: clinical features, epidemiology, and management. Am J Clin Dermatol. 2011;12:157-169.
- Hall JH, Callaway JL, Tindall JP, et al. Pseudomonas aeruginosa in dermatology. Arch Dermatol. 1968;97:312-324.
- Merchant S, Proudfoot EM, Quadri HN, et al. Risk factors for Pseudomonas aeruginosa infections in Asia-Pacific and consequences of inappropriate initial antimicrobial therapy: a systematic literature review and meta-analysis. J Glob Antimicrob Resist. 2018;14:33-44.
The Diagnosis: Pseudomonas Pyoderma
A skin swab confirmed the presence of a ciprofloxacinsusceptible Pseudomonas aeruginosa strain. Our patient received oral ciprofloxacin 500 mg twice daily for 10 days with remarkable clinical improvement. The remaining skin lesion was successfully treated with more frequent diaper changes and the use of topical corticosteroids and emollients.
The topographical location, cutaneous morphology, clinical context, and sometimes the type of exudate are fundamental for the diagnosis of eruptions in intertriginous areas. Cutaneous Candida infections are common in these locations. They classically present as markedly erythematous plaques that occasionally are erosive, accompanied by satellite papules and pustules.1 Tinea cruris is a dermatophyte infection of the groin, proximal medial thighs, perineum, and buttocks. It usually presents as an erythematous patch that spreads centrifugally with partial central clearing and a slightly elevated, scaly border. Although candidiasis was higher on the differential, it was less likely, as our patient had a concomitant exudate inconsistent with Candida infections. Also, the lack of response to antifungal agents made hypotheses of fungal infections improbable.1
Inverse psoriasis is a variant of psoriasis identified by the development of well-demarcated, nonscaly, shiny plaques on body folds.2 Psoriasis is a chronic disease with several other cutaneous manifestations, such as nail and scalp involvement, as well as erythematous scaly plaques on the extensor surfaces of the limbs. The absence of a history of psoriasis, lack of other cutaneous manifestations, and no response to topical corticosteroids made the diagnosis of inverse psoriasis unlikely in our patient.
Erythrasma is a common superficial cutaneous infection caused by Corynebacterium minutissimum, a grampositive bacillus. It typically presents as an intertriginous eruption characterized by small erythematous to brown patches or thin plaques with fine scaling and sharp borders.3 Erythrasma displays a coral red fluorescence on Wood lamp examination that can be useful in the distinction from other causes of intertrigo.1 Although this examination had not been performed in our patient, the striking exudate made erythrasma less likely, and the culture performed on skin swab material would help to rule out this diagnosis.
Pseudomonas aeruginosa is a gram-negative strict aerobic bacillus of ubiquitous distribution with a preference for humid environments.4,5Pseudomonas aeruginosa infections were first reported in the 19th century by physicians who noticed a peculiar odorous condition that caused a blue-green discoloration on bandages. This coloration explains the species name aeruginosa which is derived from the Latin word for copper rust.4 It comes from several water-soluble pigments produced by this microorganism, the most prevalent of which are pyocyanin and pyoverdine. Pyocyanin has a greenish-blue color and is nonfluorescent, while pyoverdine is green-yellowish and fluoresces under Wood light.5 Other pigments, such as pyorubin and pyomelanin, can be produced by some Pseudomonas strains.4
Pseudomonas aeruginosa has become one of the main pathogens involved in hospital-acquired infections,6 especially in immunocompromised patients.6,7 It is a frequent cause of respiratory infections in patients with cystic fibrosis, as it is present in the airways of up to 70% of these patients in adulthood.7 Also, due to a variety of adaptive mechanisms with the development of resistance to a range of antibiotics, P aeruginosa has become a worldwide public health problem and is involved in several life-threatening nosocomial infections.7,8
Cutaneous P aeruginosa infections range from superficial to deep tissue involvement and can affect both immunocompromised and immunocompetent individuals.9 They are classified as primary when they originate directly from the skin or secondary when they occur in the context of bacteremia. Primary infections mostly are mild and often are seen in healthy individuals; they usually occur by inoculation and predominate in moist areas where skin breakdown is frequent. Secondary infections typically affect immunocompromised individuals and portend a poor prognosis.5,9
Denominated as Pseudomonas pyoderma, the superficial skin infection by P aeruginosa is described as a condition where the epidermis has a moth-eaten appearance with macerated or eroded borders.10 A blue-greenish exudate and a grape juice odor often are present. This infection usually occurs as a complication of several skin conditions such as tinea pedis, eczema, burns, wounds, and ulcers.5,10
We believe that our patient developed Pseudomonas pyoderma as a complication of diaper dermatitis. His extended hospital stay with the use of different antibiotic regimens for the treatment of several infectious complications may have contributed to the development of infection by P aeruginosa.11 Despite its great clinical relevance, there are few studies in the literature on primary skin infections caused by P aeruginosa, and clinical descriptions with images are rare. Our patient had a nonspecific noneczematous dermatitis, and the projections on the periphery of the lesion resembled the moth-eaten appearance of the classic description of Pseudomonas pyoderma.5,10 The presence of a greenish exudate should promptly raise suspicion for this entity. We believe that the presentation of this case can illustrate this finding and help physicians to recognize this infection.
The Diagnosis: Pseudomonas Pyoderma
A skin swab confirmed the presence of a ciprofloxacinsusceptible Pseudomonas aeruginosa strain. Our patient received oral ciprofloxacin 500 mg twice daily for 10 days with remarkable clinical improvement. The remaining skin lesion was successfully treated with more frequent diaper changes and the use of topical corticosteroids and emollients.
The topographical location, cutaneous morphology, clinical context, and sometimes the type of exudate are fundamental for the diagnosis of eruptions in intertriginous areas. Cutaneous Candida infections are common in these locations. They classically present as markedly erythematous plaques that occasionally are erosive, accompanied by satellite papules and pustules.1 Tinea cruris is a dermatophyte infection of the groin, proximal medial thighs, perineum, and buttocks. It usually presents as an erythematous patch that spreads centrifugally with partial central clearing and a slightly elevated, scaly border. Although candidiasis was higher on the differential, it was less likely, as our patient had a concomitant exudate inconsistent with Candida infections. Also, the lack of response to antifungal agents made hypotheses of fungal infections improbable.1
Inverse psoriasis is a variant of psoriasis identified by the development of well-demarcated, nonscaly, shiny plaques on body folds.2 Psoriasis is a chronic disease with several other cutaneous manifestations, such as nail and scalp involvement, as well as erythematous scaly plaques on the extensor surfaces of the limbs. The absence of a history of psoriasis, lack of other cutaneous manifestations, and no response to topical corticosteroids made the diagnosis of inverse psoriasis unlikely in our patient.
Erythrasma is a common superficial cutaneous infection caused by Corynebacterium minutissimum, a grampositive bacillus. It typically presents as an intertriginous eruption characterized by small erythematous to brown patches or thin plaques with fine scaling and sharp borders.3 Erythrasma displays a coral red fluorescence on Wood lamp examination that can be useful in the distinction from other causes of intertrigo.1 Although this examination had not been performed in our patient, the striking exudate made erythrasma less likely, and the culture performed on skin swab material would help to rule out this diagnosis.
Pseudomonas aeruginosa is a gram-negative strict aerobic bacillus of ubiquitous distribution with a preference for humid environments.4,5Pseudomonas aeruginosa infections were first reported in the 19th century by physicians who noticed a peculiar odorous condition that caused a blue-green discoloration on bandages. This coloration explains the species name aeruginosa which is derived from the Latin word for copper rust.4 It comes from several water-soluble pigments produced by this microorganism, the most prevalent of which are pyocyanin and pyoverdine. Pyocyanin has a greenish-blue color and is nonfluorescent, while pyoverdine is green-yellowish and fluoresces under Wood light.5 Other pigments, such as pyorubin and pyomelanin, can be produced by some Pseudomonas strains.4
Pseudomonas aeruginosa has become one of the main pathogens involved in hospital-acquired infections,6 especially in immunocompromised patients.6,7 It is a frequent cause of respiratory infections in patients with cystic fibrosis, as it is present in the airways of up to 70% of these patients in adulthood.7 Also, due to a variety of adaptive mechanisms with the development of resistance to a range of antibiotics, P aeruginosa has become a worldwide public health problem and is involved in several life-threatening nosocomial infections.7,8
Cutaneous P aeruginosa infections range from superficial to deep tissue involvement and can affect both immunocompromised and immunocompetent individuals.9 They are classified as primary when they originate directly from the skin or secondary when they occur in the context of bacteremia. Primary infections mostly are mild and often are seen in healthy individuals; they usually occur by inoculation and predominate in moist areas where skin breakdown is frequent. Secondary infections typically affect immunocompromised individuals and portend a poor prognosis.5,9
Denominated as Pseudomonas pyoderma, the superficial skin infection by P aeruginosa is described as a condition where the epidermis has a moth-eaten appearance with macerated or eroded borders.10 A blue-greenish exudate and a grape juice odor often are present. This infection usually occurs as a complication of several skin conditions such as tinea pedis, eczema, burns, wounds, and ulcers.5,10
We believe that our patient developed Pseudomonas pyoderma as a complication of diaper dermatitis. His extended hospital stay with the use of different antibiotic regimens for the treatment of several infectious complications may have contributed to the development of infection by P aeruginosa.11 Despite its great clinical relevance, there are few studies in the literature on primary skin infections caused by P aeruginosa, and clinical descriptions with images are rare. Our patient had a nonspecific noneczematous dermatitis, and the projections on the periphery of the lesion resembled the moth-eaten appearance of the classic description of Pseudomonas pyoderma.5,10 The presence of a greenish exudate should promptly raise suspicion for this entity. We believe that the presentation of this case can illustrate this finding and help physicians to recognize this infection.
- Kalra MG, Higgins KE, Kinney BS. Intertrigo and secondary skin infections. Am Fam Physician. 2014;89:569-573.
- Micali G, Verzi AE, Giuffrida G, et al. Inverse psoriasis: from diagnosis to current treatment options. Clin Cosmet Investig Dermatol. 2019; 12:953-959.
- Somerville DA. Erythrasma in normal young adults. J Med Microbiol. 1970;3:57-64.
- D’Agata E. Pseudomonas aeruginosa and other Pseudomonas species. In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Vol 2. 8th ed. Elsevier; 2015:2518-2531.
- Silvestre JF, Betlloch MI. Cutaneous manifestations due to Pseudomonas infection. Int J Dermatol. 1999;38:419-431.
- Young LS, Armstrong D. Pseudomonas aeruginosa infections. CRC Crit Rev Clin Lab Sci. 1972;3:291-347.
- Moradali MF, Ghods S, Rehm BH. Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front Cell Infect Microbiol. 2017;7:39.
- Rosenthal VD, Bat-Erdene I, Gupta D, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 45 countries for 2012-2017: device-associated module. Am J Infect Control. 2020;48:423-432.
- Wu DC, Chan WW, Metelitsa AI, et al. Pseudomonas skin infection: clinical features, epidemiology, and management. Am J Clin Dermatol. 2011;12:157-169.
- Hall JH, Callaway JL, Tindall JP, et al. Pseudomonas aeruginosa in dermatology. Arch Dermatol. 1968;97:312-324.
- Merchant S, Proudfoot EM, Quadri HN, et al. Risk factors for Pseudomonas aeruginosa infections in Asia-Pacific and consequences of inappropriate initial antimicrobial therapy: a systematic literature review and meta-analysis. J Glob Antimicrob Resist. 2018;14:33-44.
- Kalra MG, Higgins KE, Kinney BS. Intertrigo and secondary skin infections. Am Fam Physician. 2014;89:569-573.
- Micali G, Verzi AE, Giuffrida G, et al. Inverse psoriasis: from diagnosis to current treatment options. Clin Cosmet Investig Dermatol. 2019; 12:953-959.
- Somerville DA. Erythrasma in normal young adults. J Med Microbiol. 1970;3:57-64.
- D’Agata E. Pseudomonas aeruginosa and other Pseudomonas species. In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Vol 2. 8th ed. Elsevier; 2015:2518-2531.
- Silvestre JF, Betlloch MI. Cutaneous manifestations due to Pseudomonas infection. Int J Dermatol. 1999;38:419-431.
- Young LS, Armstrong D. Pseudomonas aeruginosa infections. CRC Crit Rev Clin Lab Sci. 1972;3:291-347.
- Moradali MF, Ghods S, Rehm BH. Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front Cell Infect Microbiol. 2017;7:39.
- Rosenthal VD, Bat-Erdene I, Gupta D, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 45 countries for 2012-2017: device-associated module. Am J Infect Control. 2020;48:423-432.
- Wu DC, Chan WW, Metelitsa AI, et al. Pseudomonas skin infection: clinical features, epidemiology, and management. Am J Clin Dermatol. 2011;12:157-169.
- Hall JH, Callaway JL, Tindall JP, et al. Pseudomonas aeruginosa in dermatology. Arch Dermatol. 1968;97:312-324.
- Merchant S, Proudfoot EM, Quadri HN, et al. Risk factors for Pseudomonas aeruginosa infections in Asia-Pacific and consequences of inappropriate initial antimicrobial therapy: a systematic literature review and meta-analysis. J Glob Antimicrob Resist. 2018;14:33-44.
A 68-year-old man presented with an extensive erythematous plaque of 3 weeks’ duration that started in the groin and spread to the buttocks. It was associated with pruritus and a burning sensation. He was admitted to the palliative care unit 1 year prior for the management of terminal lung cancer. Despite the use of topical corticosteroids and antifungals, the lesions gradually worsened with dissemination to the back. Physical examination revealed an erythematous macerated plaque that extended from the buttocks and groin region to the scapular area (top). Its borders had an eroded appearance with projections compatible with radial spread (bottom). A greenish exudate soaked the diaper and sheets. No other cutaneous lesions were noted.
Harlequin Syndrome: Discovery of an Ancient Schwannoma
To the Editor:
A 52-year-old man who was otherwise healthy and a long-distance runner presented with the sudden onset of diminished sweating on the left side of the body of 6 weeks’ duration. While training for a marathon, he reported that he perspired only on the right side of the body during runs of 12 to 15 miles; he observed a lack of sweating on the left side of the face, left side of the trunk, left arm, and left leg. This absence of sweating was accompanied by intense flushing on the right side of the face and trunk.
The patient did not take any medications. He reported no history of trauma and exhibited no neurologic deficits. A chest radiograph was negative. Thyroid function testing and a comprehensive metabolic panel were normal. Contrast-enhanced computed tomography of the chest and abdomen revealed a 4.3-cm soft-tissue mass in the left superior mediastinum that was superior to the aortic arch, posterior to the left subclavian artery in proximity to the sympathetic chain, and lateral to the trachea. The patient was diagnosed with Harlequin syndrome (HS).
Open thoracotomy was performed to remove the lesion. Analysis of the mass showed cystic areas, areas of hemorrhage (Figure 1A), and alternating zones of compact Antoni A spindle cells admixed with areas of less orderly Antoni B spindle cells within a hypocellular stroma (Figure 1B). Individual cells were characterized by eosinophilic cytoplasm and tapered nuclei. The mass appeared to be completely encapsulated. No mitotic figures were seen on multiple slides. The cells stained diffusely positive for S-100 proteins. At 6-month follow-up, the patient reported that he did not notice any return of normal sweating on the left side. However, the right-sided flushing had resolved.
Harlequin syndrome (also called the Harlequin sign) is a rare disorder of the sympathetic nervous system and should not be confused with lethal harlequin-type ichthyosis, an autosomal-recessive congenital disorder in which the affected newborn’s skin is hard and thickened over most of the body.1 Harlequin syndrome usually is characterized by unilateral flushing and sweating that can affect the face, trunk, and extremities.2 Physical stimuli, such as exercising (as in our patient), high body temperature, and the consumption of spicy or pungent food, or an emotional response can unmask or exacerbate symptoms of HS. The syndrome also can present with cluster headache.3 Harlequin syndrome is more common in females (66% of cases).4 Originally, the side of the face marked by increased sweating and flushing was perceived to be the pathologic side; now it is recognized that the anhidrotic side is affected by the causative pathology. The side of the face characterized by flushing might gradually darken as it compensates for lack of thermal regulation on the other side.2,5
Usually, HS is an idiopathic condition associated with localized failure of upper thoracic sympathetic chain ganglia.5 A theory is that HS is part of a spectrum of autoimmune autonomic ganglionopathy.6 Typically, the syndrome is asymptomatic at rest, but testing can reveal an underlying sympathetic lesion.7 Structural lesions have been reported as a cause of the syndrome,6 similar to our patient.
Disrupted thermoregulatory vasodilation in HS is caused by an ipsilateral lesion of the sympathetic vasodilator neurons that innervate the face. Hemifacial anhidrosis also occurs because sudomotor neurons travel within the same pathways as vasodilator neurons.4
Our patient had a posterior mediastinal ancient schwannoma to the left of the subclavian artery, lateral to the trachea, with ipsilateral anhidrosis of the forehead, cheek, chin, and torso. In the medical literature, the forehead, cheek, and chin are described as being affected in HS when the lesion is located under the bifurcation of the carotid artery.3,5 Most of the sudomotor and vasomotor fibers that innervate the face leave the spinal cord through ventral roots T2-T34 (symptomatic areas are described in Figure 2), which correlates with the hypothesis that HS results from a deficit originating in the third thoracic nerve that is caused by a peripheral lesion affecting sympathetic outflow through the third thoracic root.2 The location of our patient’s lesion supports this claim.
Harlequin syndrome can present simultaneously with ipsilateral Horner, Adie, and Ross syndromes.8 There are varying clinical presentations of Horner syndrome. Some patients with HS show autonomic ocular signs, such as miosis and ptosis, exhibiting Horner syndrome as an additional feature.5 Adie syndrome is characterized by tonic pupils with hyporeflexia and is unilateral in most cases. Ross syndrome is similar to Adie syndrome—including tonic pupils with hyporeflexia—in addition to a finding of segmental anhidrosis; it is bilateral in most cases.4
In some cases, Horner syndrome and HS originate from unilateral pharmaceutical sympathetic denervation (ie, as a consequence of paravertebral spread of local anesthetic to ipsilateral stellate ganglion).9 Facial nonflushing areas in HS typically are identical with anhidrotic areas10; Horner syndrome often is ipsilateral to the affected sympathetic region.11
Our patient exhibited secondary HS from a tumor effect; however, an underlying tumor or infarct is absent in many cases. In primary (idiopathic) cases of HS, treatment is not recommended because the syndrome is benign.10,11
If symptoms of HS cause notable social embarrassment, contralateral sympathectomy can be considered.5,12 Repeated stellate ganglion block with a local anesthetic could be a less invasive treatment option.13 When considered on a case-by-case-basis, botulinum toxin type A has been effective as a treatment of compensatory hyperhidrosis on the unaffected side.14
In cases of secondary HS, surgical removal of the lesion may alleviate symptoms, though thoracotomy in our patient to remove the schwannoma did not alleviate anhidrosis. The Table lists treatment options for primary and secondary HS.4,5,11
- Harlequin ichthyosis. MedlinePlus. National Library of Medicine [Internet]. Updated January 7, 2022. Accessed April 5, 2022. https://ghr.nlm.nih.gov/condition/harlequin-ichthyosis
- Lance JW, Drummond PD, Gandevia SC, et al. Harlequin syndrome: the sudden onset of unilateral flushing and sweating. J Neurol Neurosurg Psych. 1988;51:635-642. doi:10.1136/jnnp.51.5.635
- Lehman K, Kumar N, Vu Q, et al. Harlequin syndrome in cluster headache. Headache. 2016;56:1053-1054. doi:10.1111/head.12852
- Willaert WIM, Scheltinga MRM, Steenhuisen SF, et al. Harlequin syndrome: two new cases and a management proposal. Acta Neurol Belg. 2009;109:214-220.
- Duddy ME, Baker MR. Images in clinical medicine. Harlequin’s darker side. N Engl J Med. 2007;357:E22. doi:10.1056/NEJMicm067851
- Karam C. Harlequin syndrome in a patient with putative autoimmune autonomic ganglionopathy. Auton Neurosci. 2016;194:58-59. doi:10.1016/j.autneu.2015.12.004
- Wasner G, Maag R, Ludwig J, et al. Harlequin syndrome—one face of many etiologies. Nat Clin Pract Neurol. 2005;1:54-59. doi:10.1038/ncpneuro0040
- Guilloton L, Demarquay G, Quesnel L, et al. Dysautonomic syndrome of the face with Harlequin sign and syndrome: three new cases and a review of the literature. Rev Neurol (Paris). 2013;169:884-891. doi:10.1016/j.neurol.2013.01.628
- Burlacu CL, Buggy DJ. Coexisting Harlequin and Horner syndromes after high thoracic paravertebral anaesthesia. Br J Anaesth. 2005;95:822-824. doi:10.1093/bja/aei258
- Morrison DA, Bibby K, Woodruff G. The “Harlequin” sign and congenital Horner’s syndrome. J Neurol Neurosurg Psych. 1997;62:626-628. doi:10.1136/jnnp.62.6.626
- Bremner F, Smith S. Pupillographic findings in 39 consecutive cases of Harlequin syndrome. J Neuroophthalmol. 2008;28:171-177. doi:10.1097/WNO.0b013e318183c885
- Kaur S, Aggarwal P, Jindal N, et al. Harlequin syndrome: a mask of rare dysautonomic syndromes. Dermatol Online J. 2015;21:13030/qt3q39d7mz.
- Reddy H, Fatah S, Gulve A, et al. Novel management of Harlequin syndrome with stellate ganglion block. Br J Dermatol. 2013;169:954-956. doi:10.1111/bjd.12561
- ManhRKJV, Spitz M, Vasconcellos LF. Botulinum toxin for treatment of Harlequin syndrome. Parkinsonism Relat Disord. 2016;23:112-113. doi:10.1016/j.parkreldis.2015.11.030
To the Editor:
A 52-year-old man who was otherwise healthy and a long-distance runner presented with the sudden onset of diminished sweating on the left side of the body of 6 weeks’ duration. While training for a marathon, he reported that he perspired only on the right side of the body during runs of 12 to 15 miles; he observed a lack of sweating on the left side of the face, left side of the trunk, left arm, and left leg. This absence of sweating was accompanied by intense flushing on the right side of the face and trunk.
The patient did not take any medications. He reported no history of trauma and exhibited no neurologic deficits. A chest radiograph was negative. Thyroid function testing and a comprehensive metabolic panel were normal. Contrast-enhanced computed tomography of the chest and abdomen revealed a 4.3-cm soft-tissue mass in the left superior mediastinum that was superior to the aortic arch, posterior to the left subclavian artery in proximity to the sympathetic chain, and lateral to the trachea. The patient was diagnosed with Harlequin syndrome (HS).
Open thoracotomy was performed to remove the lesion. Analysis of the mass showed cystic areas, areas of hemorrhage (Figure 1A), and alternating zones of compact Antoni A spindle cells admixed with areas of less orderly Antoni B spindle cells within a hypocellular stroma (Figure 1B). Individual cells were characterized by eosinophilic cytoplasm and tapered nuclei. The mass appeared to be completely encapsulated. No mitotic figures were seen on multiple slides. The cells stained diffusely positive for S-100 proteins. At 6-month follow-up, the patient reported that he did not notice any return of normal sweating on the left side. However, the right-sided flushing had resolved.
Harlequin syndrome (also called the Harlequin sign) is a rare disorder of the sympathetic nervous system and should not be confused with lethal harlequin-type ichthyosis, an autosomal-recessive congenital disorder in which the affected newborn’s skin is hard and thickened over most of the body.1 Harlequin syndrome usually is characterized by unilateral flushing and sweating that can affect the face, trunk, and extremities.2 Physical stimuli, such as exercising (as in our patient), high body temperature, and the consumption of spicy or pungent food, or an emotional response can unmask or exacerbate symptoms of HS. The syndrome also can present with cluster headache.3 Harlequin syndrome is more common in females (66% of cases).4 Originally, the side of the face marked by increased sweating and flushing was perceived to be the pathologic side; now it is recognized that the anhidrotic side is affected by the causative pathology. The side of the face characterized by flushing might gradually darken as it compensates for lack of thermal regulation on the other side.2,5
Usually, HS is an idiopathic condition associated with localized failure of upper thoracic sympathetic chain ganglia.5 A theory is that HS is part of a spectrum of autoimmune autonomic ganglionopathy.6 Typically, the syndrome is asymptomatic at rest, but testing can reveal an underlying sympathetic lesion.7 Structural lesions have been reported as a cause of the syndrome,6 similar to our patient.
Disrupted thermoregulatory vasodilation in HS is caused by an ipsilateral lesion of the sympathetic vasodilator neurons that innervate the face. Hemifacial anhidrosis also occurs because sudomotor neurons travel within the same pathways as vasodilator neurons.4
Our patient had a posterior mediastinal ancient schwannoma to the left of the subclavian artery, lateral to the trachea, with ipsilateral anhidrosis of the forehead, cheek, chin, and torso. In the medical literature, the forehead, cheek, and chin are described as being affected in HS when the lesion is located under the bifurcation of the carotid artery.3,5 Most of the sudomotor and vasomotor fibers that innervate the face leave the spinal cord through ventral roots T2-T34 (symptomatic areas are described in Figure 2), which correlates with the hypothesis that HS results from a deficit originating in the third thoracic nerve that is caused by a peripheral lesion affecting sympathetic outflow through the third thoracic root.2 The location of our patient’s lesion supports this claim.
Harlequin syndrome can present simultaneously with ipsilateral Horner, Adie, and Ross syndromes.8 There are varying clinical presentations of Horner syndrome. Some patients with HS show autonomic ocular signs, such as miosis and ptosis, exhibiting Horner syndrome as an additional feature.5 Adie syndrome is characterized by tonic pupils with hyporeflexia and is unilateral in most cases. Ross syndrome is similar to Adie syndrome—including tonic pupils with hyporeflexia—in addition to a finding of segmental anhidrosis; it is bilateral in most cases.4
In some cases, Horner syndrome and HS originate from unilateral pharmaceutical sympathetic denervation (ie, as a consequence of paravertebral spread of local anesthetic to ipsilateral stellate ganglion).9 Facial nonflushing areas in HS typically are identical with anhidrotic areas10; Horner syndrome often is ipsilateral to the affected sympathetic region.11
Our patient exhibited secondary HS from a tumor effect; however, an underlying tumor or infarct is absent in many cases. In primary (idiopathic) cases of HS, treatment is not recommended because the syndrome is benign.10,11
If symptoms of HS cause notable social embarrassment, contralateral sympathectomy can be considered.5,12 Repeated stellate ganglion block with a local anesthetic could be a less invasive treatment option.13 When considered on a case-by-case-basis, botulinum toxin type A has been effective as a treatment of compensatory hyperhidrosis on the unaffected side.14
In cases of secondary HS, surgical removal of the lesion may alleviate symptoms, though thoracotomy in our patient to remove the schwannoma did not alleviate anhidrosis. The Table lists treatment options for primary and secondary HS.4,5,11
To the Editor:
A 52-year-old man who was otherwise healthy and a long-distance runner presented with the sudden onset of diminished sweating on the left side of the body of 6 weeks’ duration. While training for a marathon, he reported that he perspired only on the right side of the body during runs of 12 to 15 miles; he observed a lack of sweating on the left side of the face, left side of the trunk, left arm, and left leg. This absence of sweating was accompanied by intense flushing on the right side of the face and trunk.
The patient did not take any medications. He reported no history of trauma and exhibited no neurologic deficits. A chest radiograph was negative. Thyroid function testing and a comprehensive metabolic panel were normal. Contrast-enhanced computed tomography of the chest and abdomen revealed a 4.3-cm soft-tissue mass in the left superior mediastinum that was superior to the aortic arch, posterior to the left subclavian artery in proximity to the sympathetic chain, and lateral to the trachea. The patient was diagnosed with Harlequin syndrome (HS).
Open thoracotomy was performed to remove the lesion. Analysis of the mass showed cystic areas, areas of hemorrhage (Figure 1A), and alternating zones of compact Antoni A spindle cells admixed with areas of less orderly Antoni B spindle cells within a hypocellular stroma (Figure 1B). Individual cells were characterized by eosinophilic cytoplasm and tapered nuclei. The mass appeared to be completely encapsulated. No mitotic figures were seen on multiple slides. The cells stained diffusely positive for S-100 proteins. At 6-month follow-up, the patient reported that he did not notice any return of normal sweating on the left side. However, the right-sided flushing had resolved.
Harlequin syndrome (also called the Harlequin sign) is a rare disorder of the sympathetic nervous system and should not be confused with lethal harlequin-type ichthyosis, an autosomal-recessive congenital disorder in which the affected newborn’s skin is hard and thickened over most of the body.1 Harlequin syndrome usually is characterized by unilateral flushing and sweating that can affect the face, trunk, and extremities.2 Physical stimuli, such as exercising (as in our patient), high body temperature, and the consumption of spicy or pungent food, or an emotional response can unmask or exacerbate symptoms of HS. The syndrome also can present with cluster headache.3 Harlequin syndrome is more common in females (66% of cases).4 Originally, the side of the face marked by increased sweating and flushing was perceived to be the pathologic side; now it is recognized that the anhidrotic side is affected by the causative pathology. The side of the face characterized by flushing might gradually darken as it compensates for lack of thermal regulation on the other side.2,5
Usually, HS is an idiopathic condition associated with localized failure of upper thoracic sympathetic chain ganglia.5 A theory is that HS is part of a spectrum of autoimmune autonomic ganglionopathy.6 Typically, the syndrome is asymptomatic at rest, but testing can reveal an underlying sympathetic lesion.7 Structural lesions have been reported as a cause of the syndrome,6 similar to our patient.
Disrupted thermoregulatory vasodilation in HS is caused by an ipsilateral lesion of the sympathetic vasodilator neurons that innervate the face. Hemifacial anhidrosis also occurs because sudomotor neurons travel within the same pathways as vasodilator neurons.4
Our patient had a posterior mediastinal ancient schwannoma to the left of the subclavian artery, lateral to the trachea, with ipsilateral anhidrosis of the forehead, cheek, chin, and torso. In the medical literature, the forehead, cheek, and chin are described as being affected in HS when the lesion is located under the bifurcation of the carotid artery.3,5 Most of the sudomotor and vasomotor fibers that innervate the face leave the spinal cord through ventral roots T2-T34 (symptomatic areas are described in Figure 2), which correlates with the hypothesis that HS results from a deficit originating in the third thoracic nerve that is caused by a peripheral lesion affecting sympathetic outflow through the third thoracic root.2 The location of our patient’s lesion supports this claim.
Harlequin syndrome can present simultaneously with ipsilateral Horner, Adie, and Ross syndromes.8 There are varying clinical presentations of Horner syndrome. Some patients with HS show autonomic ocular signs, such as miosis and ptosis, exhibiting Horner syndrome as an additional feature.5 Adie syndrome is characterized by tonic pupils with hyporeflexia and is unilateral in most cases. Ross syndrome is similar to Adie syndrome—including tonic pupils with hyporeflexia—in addition to a finding of segmental anhidrosis; it is bilateral in most cases.4
In some cases, Horner syndrome and HS originate from unilateral pharmaceutical sympathetic denervation (ie, as a consequence of paravertebral spread of local anesthetic to ipsilateral stellate ganglion).9 Facial nonflushing areas in HS typically are identical with anhidrotic areas10; Horner syndrome often is ipsilateral to the affected sympathetic region.11
Our patient exhibited secondary HS from a tumor effect; however, an underlying tumor or infarct is absent in many cases. In primary (idiopathic) cases of HS, treatment is not recommended because the syndrome is benign.10,11
If symptoms of HS cause notable social embarrassment, contralateral sympathectomy can be considered.5,12 Repeated stellate ganglion block with a local anesthetic could be a less invasive treatment option.13 When considered on a case-by-case-basis, botulinum toxin type A has been effective as a treatment of compensatory hyperhidrosis on the unaffected side.14
In cases of secondary HS, surgical removal of the lesion may alleviate symptoms, though thoracotomy in our patient to remove the schwannoma did not alleviate anhidrosis. The Table lists treatment options for primary and secondary HS.4,5,11
- Harlequin ichthyosis. MedlinePlus. National Library of Medicine [Internet]. Updated January 7, 2022. Accessed April 5, 2022. https://ghr.nlm.nih.gov/condition/harlequin-ichthyosis
- Lance JW, Drummond PD, Gandevia SC, et al. Harlequin syndrome: the sudden onset of unilateral flushing and sweating. J Neurol Neurosurg Psych. 1988;51:635-642. doi:10.1136/jnnp.51.5.635
- Lehman K, Kumar N, Vu Q, et al. Harlequin syndrome in cluster headache. Headache. 2016;56:1053-1054. doi:10.1111/head.12852
- Willaert WIM, Scheltinga MRM, Steenhuisen SF, et al. Harlequin syndrome: two new cases and a management proposal. Acta Neurol Belg. 2009;109:214-220.
- Duddy ME, Baker MR. Images in clinical medicine. Harlequin’s darker side. N Engl J Med. 2007;357:E22. doi:10.1056/NEJMicm067851
- Karam C. Harlequin syndrome in a patient with putative autoimmune autonomic ganglionopathy. Auton Neurosci. 2016;194:58-59. doi:10.1016/j.autneu.2015.12.004
- Wasner G, Maag R, Ludwig J, et al. Harlequin syndrome—one face of many etiologies. Nat Clin Pract Neurol. 2005;1:54-59. doi:10.1038/ncpneuro0040
- Guilloton L, Demarquay G, Quesnel L, et al. Dysautonomic syndrome of the face with Harlequin sign and syndrome: three new cases and a review of the literature. Rev Neurol (Paris). 2013;169:884-891. doi:10.1016/j.neurol.2013.01.628
- Burlacu CL, Buggy DJ. Coexisting Harlequin and Horner syndromes after high thoracic paravertebral anaesthesia. Br J Anaesth. 2005;95:822-824. doi:10.1093/bja/aei258
- Morrison DA, Bibby K, Woodruff G. The “Harlequin” sign and congenital Horner’s syndrome. J Neurol Neurosurg Psych. 1997;62:626-628. doi:10.1136/jnnp.62.6.626
- Bremner F, Smith S. Pupillographic findings in 39 consecutive cases of Harlequin syndrome. J Neuroophthalmol. 2008;28:171-177. doi:10.1097/WNO.0b013e318183c885
- Kaur S, Aggarwal P, Jindal N, et al. Harlequin syndrome: a mask of rare dysautonomic syndromes. Dermatol Online J. 2015;21:13030/qt3q39d7mz.
- Reddy H, Fatah S, Gulve A, et al. Novel management of Harlequin syndrome with stellate ganglion block. Br J Dermatol. 2013;169:954-956. doi:10.1111/bjd.12561
- ManhRKJV, Spitz M, Vasconcellos LF. Botulinum toxin for treatment of Harlequin syndrome. Parkinsonism Relat Disord. 2016;23:112-113. doi:10.1016/j.parkreldis.2015.11.030
- Harlequin ichthyosis. MedlinePlus. National Library of Medicine [Internet]. Updated January 7, 2022. Accessed April 5, 2022. https://ghr.nlm.nih.gov/condition/harlequin-ichthyosis
- Lance JW, Drummond PD, Gandevia SC, et al. Harlequin syndrome: the sudden onset of unilateral flushing and sweating. J Neurol Neurosurg Psych. 1988;51:635-642. doi:10.1136/jnnp.51.5.635
- Lehman K, Kumar N, Vu Q, et al. Harlequin syndrome in cluster headache. Headache. 2016;56:1053-1054. doi:10.1111/head.12852
- Willaert WIM, Scheltinga MRM, Steenhuisen SF, et al. Harlequin syndrome: two new cases and a management proposal. Acta Neurol Belg. 2009;109:214-220.
- Duddy ME, Baker MR. Images in clinical medicine. Harlequin’s darker side. N Engl J Med. 2007;357:E22. doi:10.1056/NEJMicm067851
- Karam C. Harlequin syndrome in a patient with putative autoimmune autonomic ganglionopathy. Auton Neurosci. 2016;194:58-59. doi:10.1016/j.autneu.2015.12.004
- Wasner G, Maag R, Ludwig J, et al. Harlequin syndrome—one face of many etiologies. Nat Clin Pract Neurol. 2005;1:54-59. doi:10.1038/ncpneuro0040
- Guilloton L, Demarquay G, Quesnel L, et al. Dysautonomic syndrome of the face with Harlequin sign and syndrome: three new cases and a review of the literature. Rev Neurol (Paris). 2013;169:884-891. doi:10.1016/j.neurol.2013.01.628
- Burlacu CL, Buggy DJ. Coexisting Harlequin and Horner syndromes after high thoracic paravertebral anaesthesia. Br J Anaesth. 2005;95:822-824. doi:10.1093/bja/aei258
- Morrison DA, Bibby K, Woodruff G. The “Harlequin” sign and congenital Horner’s syndrome. J Neurol Neurosurg Psych. 1997;62:626-628. doi:10.1136/jnnp.62.6.626
- Bremner F, Smith S. Pupillographic findings in 39 consecutive cases of Harlequin syndrome. J Neuroophthalmol. 2008;28:171-177. doi:10.1097/WNO.0b013e318183c885
- Kaur S, Aggarwal P, Jindal N, et al. Harlequin syndrome: a mask of rare dysautonomic syndromes. Dermatol Online J. 2015;21:13030/qt3q39d7mz.
- Reddy H, Fatah S, Gulve A, et al. Novel management of Harlequin syndrome with stellate ganglion block. Br J Dermatol. 2013;169:954-956. doi:10.1111/bjd.12561
- ManhRKJV, Spitz M, Vasconcellos LF. Botulinum toxin for treatment of Harlequin syndrome. Parkinsonism Relat Disord. 2016;23:112-113. doi:10.1016/j.parkreldis.2015.11.030
Practice Points
- Harlequin syndrome is a rare disorder of the sympathetic nervous system that is characterized by unilateral flushing and sweating that can affect the face, trunk, and extremities.
- Secondary causes can be from schwannomas in the cervical chain ganglion.
Granuloma Faciale in Woman With Levamisole-Induced Vasculitis
To the Editor:
A 53-year-old Hispanic woman presented to our dermatology clinic for evaluation of an expanding plaque on the right cheek of 2 months’ duration. The patient stated the plaque began as a pimple, which she picked with subsequent spread laterally across the cheek. The area was intermittently tender, but she denied tingling, burning, or pruritus of the site. She had been treated with doxycycline and amoxicillin–clavulanic acid prior to presentation without improvement. She had a history of levamisole-induced vasculitis approximately 6 months prior. A review of systems was notable for diffuse joint pain. The patient denied tobacco, alcohol, or illicit drug use in the preceding 3 months and denied any changes in her medications or in health within the last year.
Physical examination revealed a well-appearing, alert, and afebrile patient with a pink, well-demarcated plaque on the right cheek (Figure 1). The borders of the plaque were indurated, and the lateral aspect of the plaque was eroded secondary to digital manipulation by the patient. She had no cervical lymphadenopathy. There were no other abnormal cutaneous findings.
There is a broad differential diagnosis for a pink expanding plaque on the face, which requires histopathologic correlation for correct diagnosis. Three broad categories in the differential are infectious (eg, bacterial, fungal), medication related (eg, fixed drug eruption), and granulomatous (eg, granuloma faciale [GF], sarcoidosis, tumid lupus, leprosy, granulomatous rosacea). A biopsy of the lesion revealed a mixed inflammatory cell dermal infiltrate with perivascular accentuation and intense vasculitis that was consistent with GF (Figure 2). Gomori methenamine-silver, periodic acid–Schiff, Fite-Faraco, acid-fast bacilli, and Gram staining were negative for organisms. Tissue cultures were negative for bacterial, mycobacterial, and fungal etiology. The patient was started on high-potency topical steroids with a 50% improvement in the appearance of the skin lesion at 1-month follow-up.
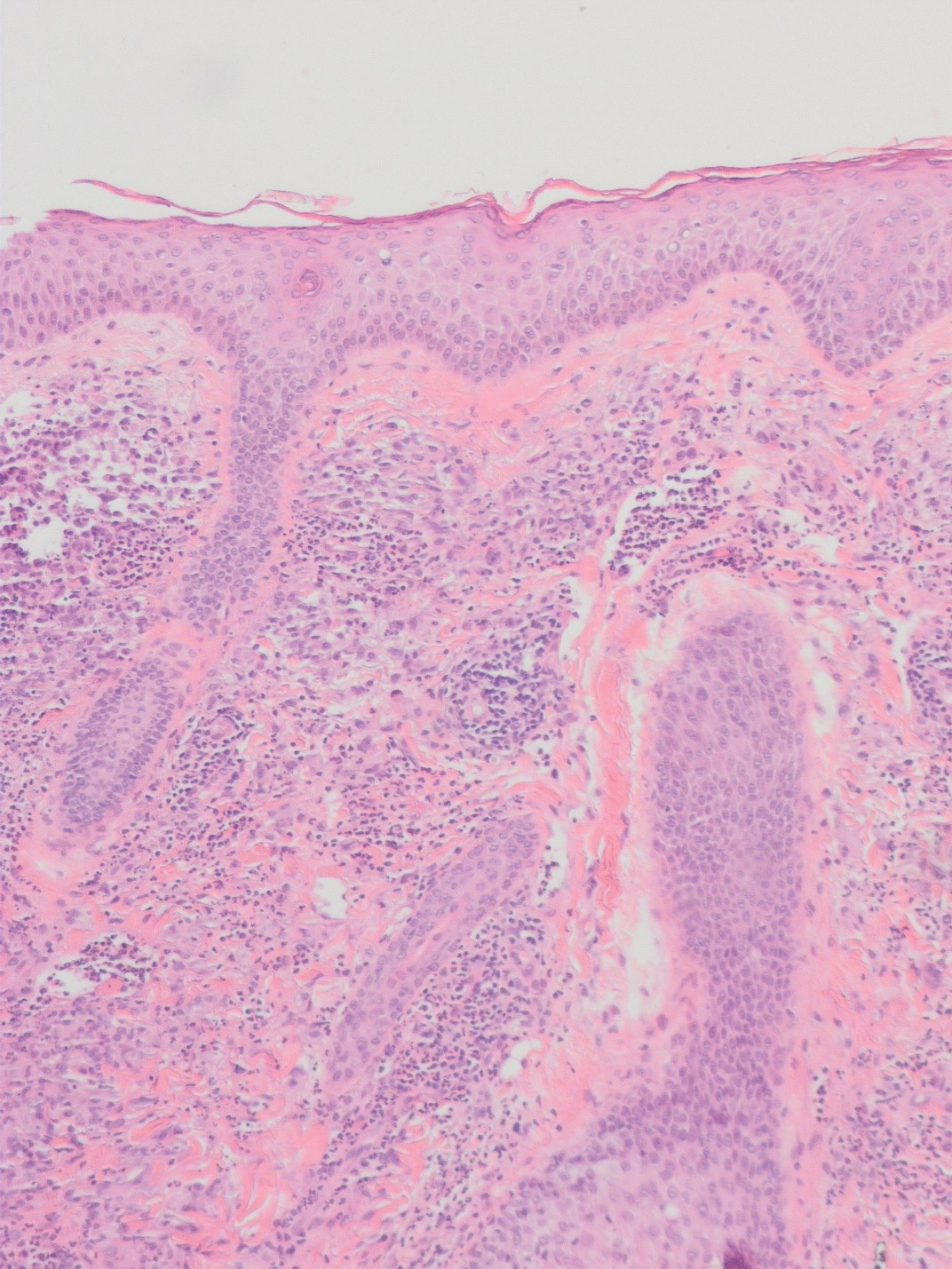
Granuloma faciale is a rare chronic inflammatory dermatosis with a predilection for the face that is difficult to diagnose and treat. The diagnosis is based on clinical and histologic findings, and it typically presents as single or multiple, well-demarcated, red-brown nodules, papules, or plaques that range from several millimeters to centimeters in diameter.1,2 Extrafacial lesions may be seen.3 Granuloma faciale usually is asymptomatic but occasionally has associated pruritus and rarely ulceration. The prevalence and pathophysiology of GF is not well defined; however, GF more commonly is reported in middle-aged White males.1
Histologic examination of GF reveals a mixed inflammatory cellular infiltrate in the upper dermis. A grenz zone, which is a narrow area of the papillary dermis uninvolved by the underlying pathology, may be seen.1 Contrary to the name, granulomas are not found histologically. Rather, vascular changes or damage frequently are present and may indicate a small vessel vasculitis pathologic mechanism. Granuloma faciale also has been associated with follicular ostia accentuation and telangiectases.4
Many cases of GF have been misdiagnosed as sarcoidosis, lymphoma, lupus, and basal cell carcinoma.1 In addition, GF shares many clinical and histologic features with erythema elevatum diutinum (EED). However, the defining features that suggest EED over GF is that EED has a predilection for the skin overlying the joints. Histopathologically, EED displays granulomas and fibrosis with few eosinophils.5,6
The variable response of GF to treatments and lack of efficacy data have contributed to the complexity and uncertainty of managing GF. The current first-line therapies are topical tacrolimus,7 cryotherapy,8 or corticosteroid therapy.9
- Ortonne N, Wechsler J, Bagot M, et al. Granuloma faciale: a clinicopathologic study of 66 patients. J Am Acad Dermatol. 2005;53:1002-1009.
- Marcoval J, Moreno A, Peyr J. Granuloma faciale: a clinicopathological study of 11 cases. J Am Acad Dermatol. 2004;51:269-273.
- Nasiri S, Rahimi H, Farnaghi A, et al. Granuloma faciale with disseminated extra facial lesions. Dermatol Online J. 2010;16:5.
- Roustan G, Sánchez Yus E, Salas C, et al. Granuloma faciale with extrafacial lesions. Dermatology. 1999;198:79-82.
- LeBoit PE. Granuloma faciale: a diagnosis deserving of dignity. Am J Dermatopathol. 2002;24:440-443.
- Ziemer M, Koehler MJ, Weyers W. Erythema elevatum diutinum: a chronic leukocytoclastic vasculitis microscopically indistinguishable from granuloma faciale? J Cutan Pathol. 2011;38:876-883.
- Cecchi R, Pavesi M, Bartoli L, et al. Topical tacrolimus in the treatment of granuloma faciale. Int J Dermatol. 2010;49:1463-1465.
- Panagiotopoulos A, Anyfantakis V, Rallis E, et al. Assessment of the efficacy of cryosurgery in the treatment of granuloma faciale. Br J Dermatol. 2006;154:357-360.
- Radin DA, Mehregan DR. Granuloma faciale: distribution of the lesions and review of the literature. Cutis. 2003;72:213-219.
To the Editor:
A 53-year-old Hispanic woman presented to our dermatology clinic for evaluation of an expanding plaque on the right cheek of 2 months’ duration. The patient stated the plaque began as a pimple, which she picked with subsequent spread laterally across the cheek. The area was intermittently tender, but she denied tingling, burning, or pruritus of the site. She had been treated with doxycycline and amoxicillin–clavulanic acid prior to presentation without improvement. She had a history of levamisole-induced vasculitis approximately 6 months prior. A review of systems was notable for diffuse joint pain. The patient denied tobacco, alcohol, or illicit drug use in the preceding 3 months and denied any changes in her medications or in health within the last year.
Physical examination revealed a well-appearing, alert, and afebrile patient with a pink, well-demarcated plaque on the right cheek (Figure 1). The borders of the plaque were indurated, and the lateral aspect of the plaque was eroded secondary to digital manipulation by the patient. She had no cervical lymphadenopathy. There were no other abnormal cutaneous findings.

There is a broad differential diagnosis for a pink expanding plaque on the face, which requires histopathologic correlation for correct diagnosis. Three broad categories in the differential are infectious (eg, bacterial, fungal), medication related (eg, fixed drug eruption), and granulomatous (eg, granuloma faciale [GF], sarcoidosis, tumid lupus, leprosy, granulomatous rosacea). A biopsy of the lesion revealed a mixed inflammatory cell dermal infiltrate with perivascular accentuation and intense vasculitis that was consistent with GF (Figure 2). Gomori methenamine-silver, periodic acid–Schiff, Fite-Faraco, acid-fast bacilli, and Gram staining were negative for organisms. Tissue cultures were negative for bacterial, mycobacterial, and fungal etiology. The patient was started on high-potency topical steroids with a 50% improvement in the appearance of the skin lesion at 1-month follow-up.

Granuloma faciale is a rare chronic inflammatory dermatosis with a predilection for the face that is difficult to diagnose and treat. The diagnosis is based on clinical and histologic findings, and it typically presents as single or multiple, well-demarcated, red-brown nodules, papules, or plaques that range from several millimeters to centimeters in diameter.1,2 Extrafacial lesions may be seen.3 Granuloma faciale usually is asymptomatic but occasionally has associated pruritus and rarely ulceration. The prevalence and pathophysiology of GF is not well defined; however, GF more commonly is reported in middle-aged White males.1
Histologic examination of GF reveals a mixed inflammatory cellular infiltrate in the upper dermis. A grenz zone, which is a narrow area of the papillary dermis uninvolved by the underlying pathology, may be seen.1 Contrary to the name, granulomas are not found histologically. Rather, vascular changes or damage frequently are present and may indicate a small vessel vasculitis pathologic mechanism. Granuloma faciale also has been associated with follicular ostia accentuation and telangiectases.4
Many cases of GF have been misdiagnosed as sarcoidosis, lymphoma, lupus, and basal cell carcinoma.1 In addition, GF shares many clinical and histologic features with erythema elevatum diutinum (EED). However, the defining features that suggest EED over GF is that EED has a predilection for the skin overlying the joints. Histopathologically, EED displays granulomas and fibrosis with few eosinophils.5,6
The variable response of GF to treatments and lack of efficacy data have contributed to the complexity and uncertainty of managing GF. The current first-line therapies are topical tacrolimus,7 cryotherapy,8 or corticosteroid therapy.9
To the Editor:
A 53-year-old Hispanic woman presented to our dermatology clinic for evaluation of an expanding plaque on the right cheek of 2 months’ duration. The patient stated the plaque began as a pimple, which she picked with subsequent spread laterally across the cheek. The area was intermittently tender, but she denied tingling, burning, or pruritus of the site. She had been treated with doxycycline and amoxicillin–clavulanic acid prior to presentation without improvement. She had a history of levamisole-induced vasculitis approximately 6 months prior. A review of systems was notable for diffuse joint pain. The patient denied tobacco, alcohol, or illicit drug use in the preceding 3 months and denied any changes in her medications or in health within the last year.
Physical examination revealed a well-appearing, alert, and afebrile patient with a pink, well-demarcated plaque on the right cheek (Figure 1). The borders of the plaque were indurated, and the lateral aspect of the plaque was eroded secondary to digital manipulation by the patient. She had no cervical lymphadenopathy. There were no other abnormal cutaneous findings.

There is a broad differential diagnosis for a pink expanding plaque on the face, which requires histopathologic correlation for correct diagnosis. Three broad categories in the differential are infectious (eg, bacterial, fungal), medication related (eg, fixed drug eruption), and granulomatous (eg, granuloma faciale [GF], sarcoidosis, tumid lupus, leprosy, granulomatous rosacea). A biopsy of the lesion revealed a mixed inflammatory cell dermal infiltrate with perivascular accentuation and intense vasculitis that was consistent with GF (Figure 2). Gomori methenamine-silver, periodic acid–Schiff, Fite-Faraco, acid-fast bacilli, and Gram staining were negative for organisms. Tissue cultures were negative for bacterial, mycobacterial, and fungal etiology. The patient was started on high-potency topical steroids with a 50% improvement in the appearance of the skin lesion at 1-month follow-up.

Granuloma faciale is a rare chronic inflammatory dermatosis with a predilection for the face that is difficult to diagnose and treat. The diagnosis is based on clinical and histologic findings, and it typically presents as single or multiple, well-demarcated, red-brown nodules, papules, or plaques that range from several millimeters to centimeters in diameter.1,2 Extrafacial lesions may be seen.3 Granuloma faciale usually is asymptomatic but occasionally has associated pruritus and rarely ulceration. The prevalence and pathophysiology of GF is not well defined; however, GF more commonly is reported in middle-aged White males.1
Histologic examination of GF reveals a mixed inflammatory cellular infiltrate in the upper dermis. A grenz zone, which is a narrow area of the papillary dermis uninvolved by the underlying pathology, may be seen.1 Contrary to the name, granulomas are not found histologically. Rather, vascular changes or damage frequently are present and may indicate a small vessel vasculitis pathologic mechanism. Granuloma faciale also has been associated with follicular ostia accentuation and telangiectases.4
Many cases of GF have been misdiagnosed as sarcoidosis, lymphoma, lupus, and basal cell carcinoma.1 In addition, GF shares many clinical and histologic features with erythema elevatum diutinum (EED). However, the defining features that suggest EED over GF is that EED has a predilection for the skin overlying the joints. Histopathologically, EED displays granulomas and fibrosis with few eosinophils.5,6
The variable response of GF to treatments and lack of efficacy data have contributed to the complexity and uncertainty of managing GF. The current first-line therapies are topical tacrolimus,7 cryotherapy,8 or corticosteroid therapy.9
- Ortonne N, Wechsler J, Bagot M, et al. Granuloma faciale: a clinicopathologic study of 66 patients. J Am Acad Dermatol. 2005;53:1002-1009.
- Marcoval J, Moreno A, Peyr J. Granuloma faciale: a clinicopathological study of 11 cases. J Am Acad Dermatol. 2004;51:269-273.
- Nasiri S, Rahimi H, Farnaghi A, et al. Granuloma faciale with disseminated extra facial lesions. Dermatol Online J. 2010;16:5.
- Roustan G, Sánchez Yus E, Salas C, et al. Granuloma faciale with extrafacial lesions. Dermatology. 1999;198:79-82.
- LeBoit PE. Granuloma faciale: a diagnosis deserving of dignity. Am J Dermatopathol. 2002;24:440-443.
- Ziemer M, Koehler MJ, Weyers W. Erythema elevatum diutinum: a chronic leukocytoclastic vasculitis microscopically indistinguishable from granuloma faciale? J Cutan Pathol. 2011;38:876-883.
- Cecchi R, Pavesi M, Bartoli L, et al. Topical tacrolimus in the treatment of granuloma faciale. Int J Dermatol. 2010;49:1463-1465.
- Panagiotopoulos A, Anyfantakis V, Rallis E, et al. Assessment of the efficacy of cryosurgery in the treatment of granuloma faciale. Br J Dermatol. 2006;154:357-360.
- Radin DA, Mehregan DR. Granuloma faciale: distribution of the lesions and review of the literature. Cutis. 2003;72:213-219.
- Ortonne N, Wechsler J, Bagot M, et al. Granuloma faciale: a clinicopathologic study of 66 patients. J Am Acad Dermatol. 2005;53:1002-1009.
- Marcoval J, Moreno A, Peyr J. Granuloma faciale: a clinicopathological study of 11 cases. J Am Acad Dermatol. 2004;51:269-273.
- Nasiri S, Rahimi H, Farnaghi A, et al. Granuloma faciale with disseminated extra facial lesions. Dermatol Online J. 2010;16:5.
- Roustan G, Sánchez Yus E, Salas C, et al. Granuloma faciale with extrafacial lesions. Dermatology. 1999;198:79-82.
- LeBoit PE. Granuloma faciale: a diagnosis deserving of dignity. Am J Dermatopathol. 2002;24:440-443.
- Ziemer M, Koehler MJ, Weyers W. Erythema elevatum diutinum: a chronic leukocytoclastic vasculitis microscopically indistinguishable from granuloma faciale? J Cutan Pathol. 2011;38:876-883.
- Cecchi R, Pavesi M, Bartoli L, et al. Topical tacrolimus in the treatment of granuloma faciale. Int J Dermatol. 2010;49:1463-1465.
- Panagiotopoulos A, Anyfantakis V, Rallis E, et al. Assessment of the efficacy of cryosurgery in the treatment of granuloma faciale. Br J Dermatol. 2006;154:357-360.
- Radin DA, Mehregan DR. Granuloma faciale: distribution of the lesions and review of the literature. Cutis. 2003;72:213-219.
Practice Points
- Granuloma faciale is a benign dermal process presenting with a red-brown plaque on the face of adults that typically is not ulcerated unless physically manipulated.
- Skin biopsy often is required for correct diagnosis.
- Granuloma faciale does not resolve spontaneously and tends to be chronic.