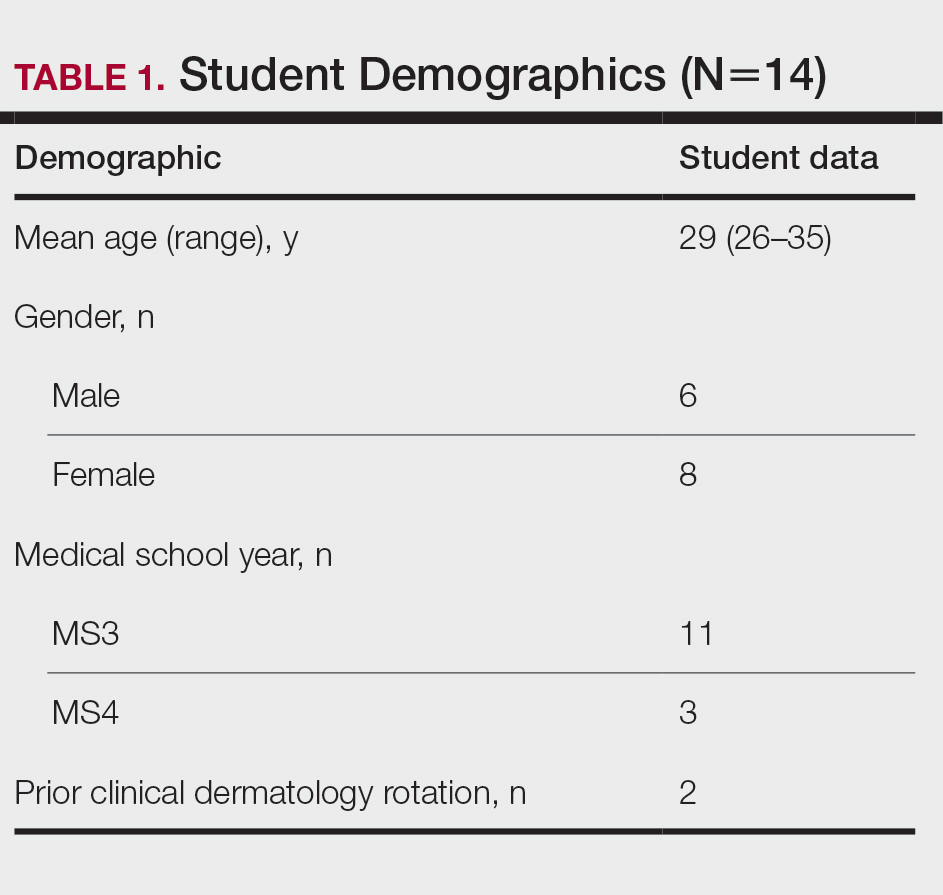
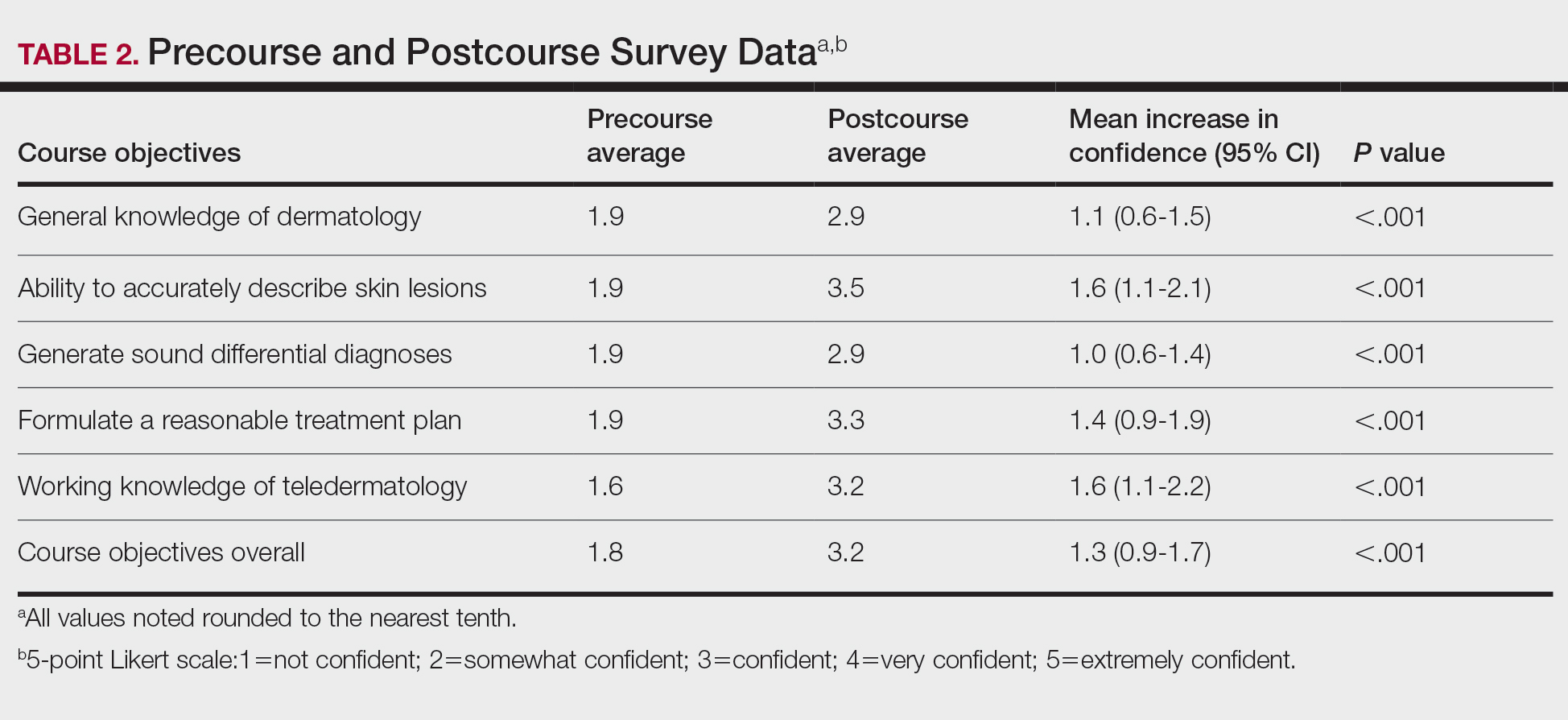
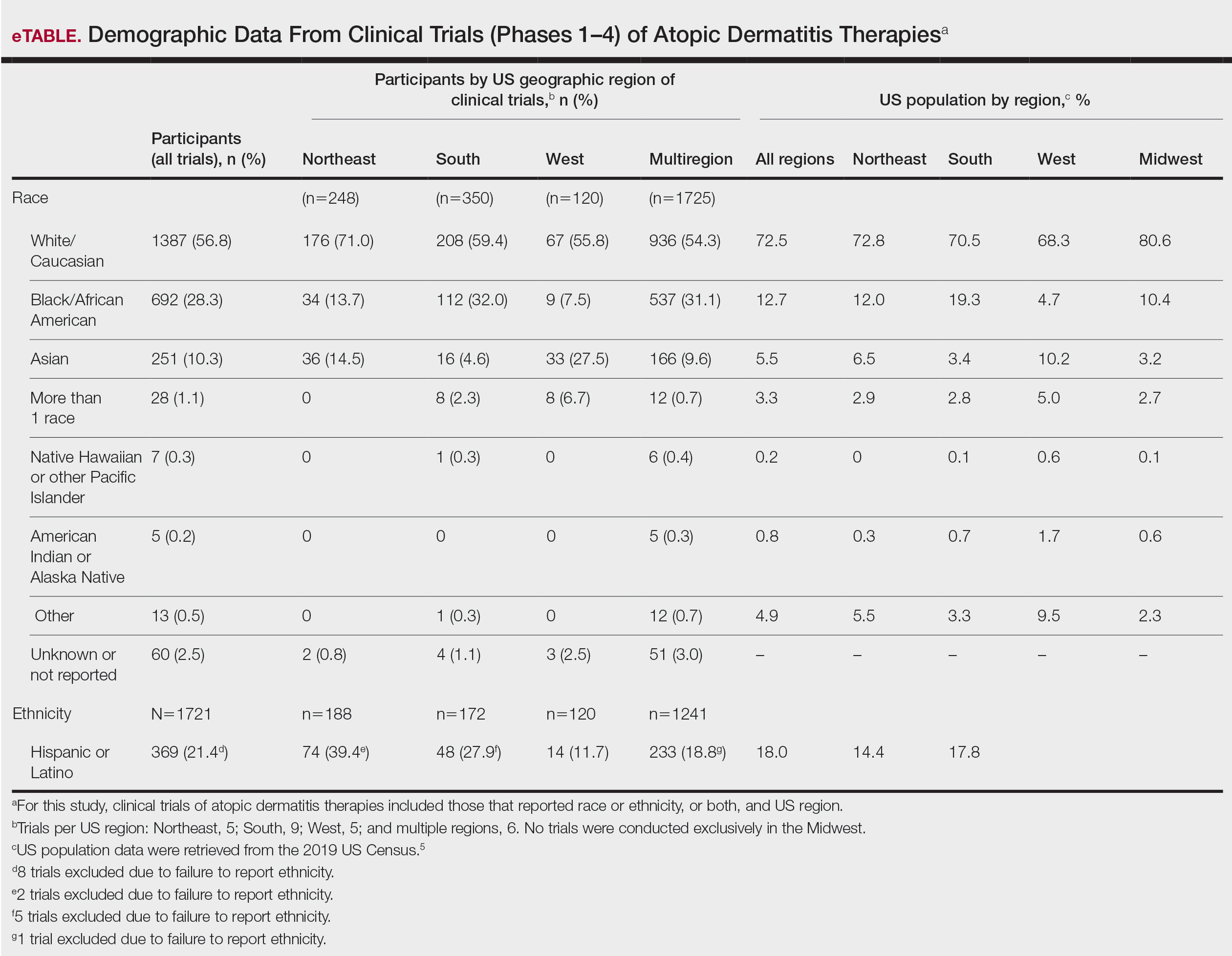
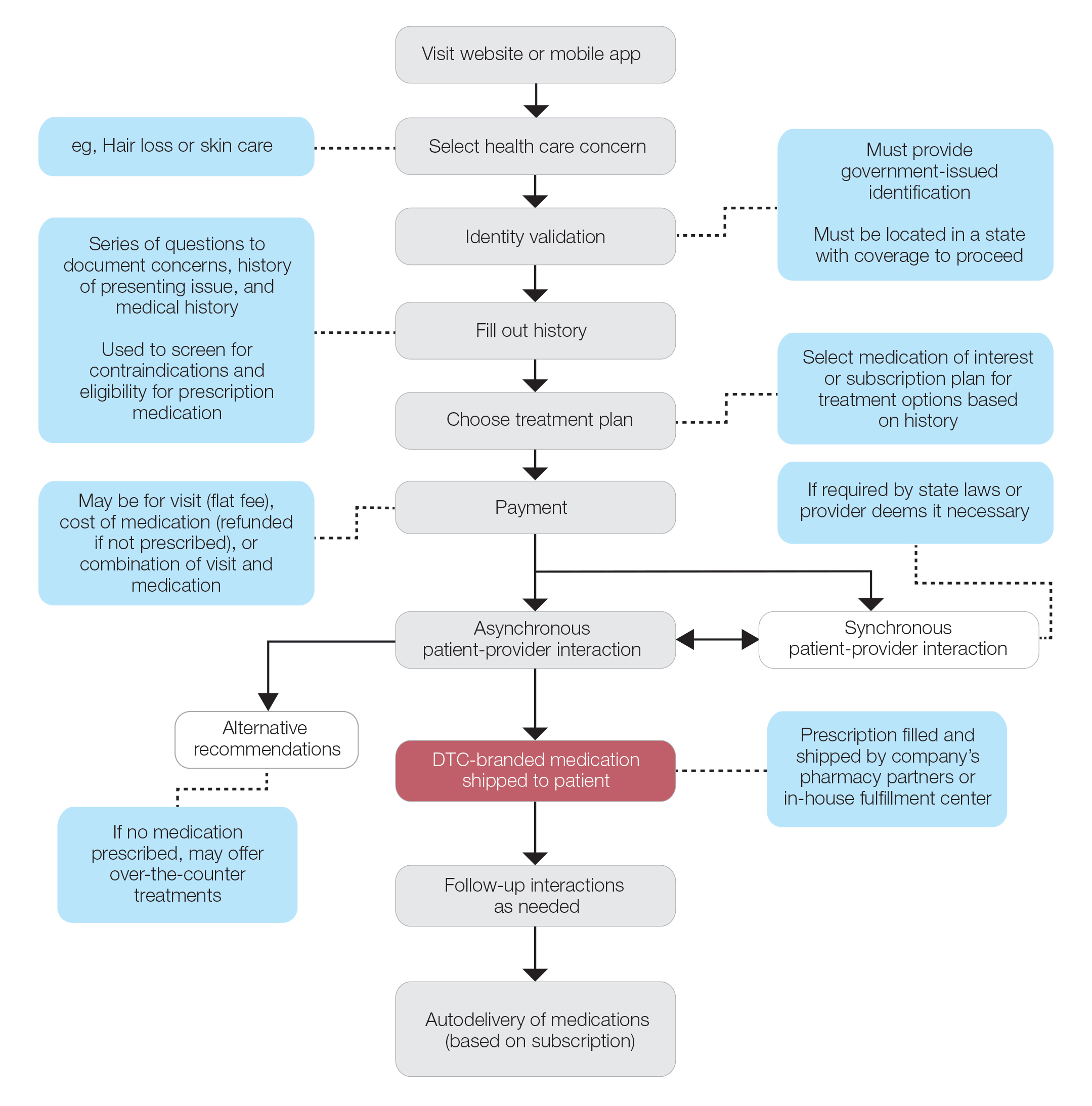
User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Blistering Lesions in a Newborn
The Diagnosis: Epidermolysis Bullosa
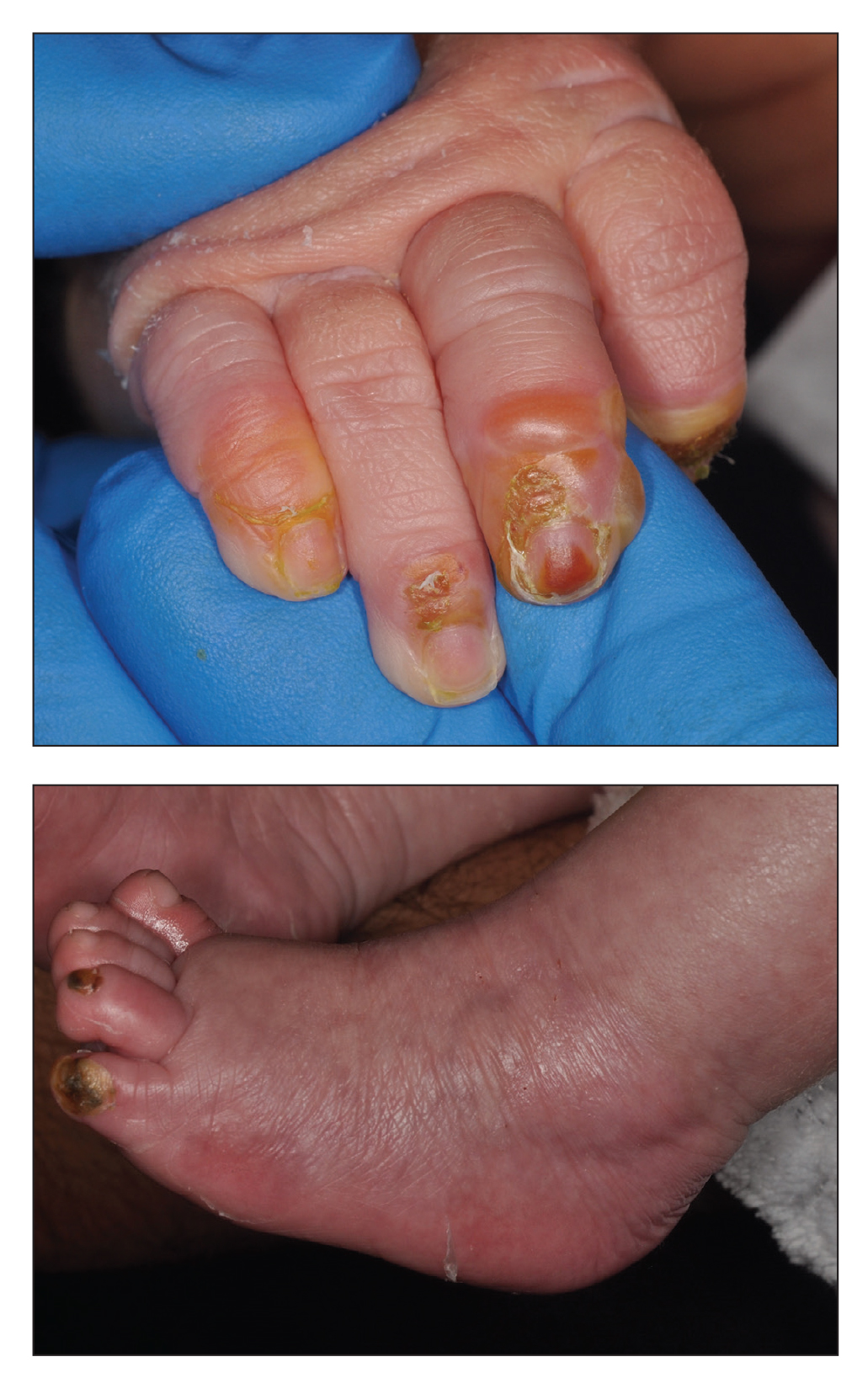
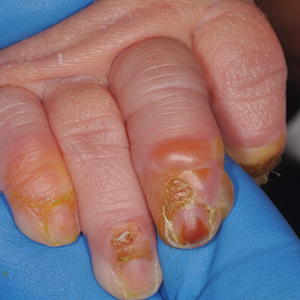
Our patient was found to have epidermolysis bullosa (EB), a rare genetic disease in which the superficial layers of the skin separate to form vesicles or bullae due to a mutation in the keratin 14 gene, KRT14. Separation of the skin occurs due to cleavage of various proteins that connect the epidermis to the dermis. A genetic mutation in KRT14, one of the more common genetic mutations associated with EB, results in cleavage at the basal epidermal protein keratin 14. The skin of individuals with EB typically is fragile and cannot tolerate friction or manipulation due to the risk for new bullae formation.1 Epidermolysis bullosa is rare, affecting approximately 20 children per 1 million births in the United States, and is not commonly seen by most general adult dermatologists.2
In our patient, the differential diagnoses included staphylococcal scalded skin syndrome (SSSS), Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), herpes simplex virus (HSV), and bullous pemphigoid (BP). Symptoms of SSSS can range from mild and localized to full-body exfoliation of the skin. Although SSSS can resemble other bullous disorders, its etiology arises from the Staphylococcus exotoxin targeting desmoglein in the stratum granulosum— the layer of the epidermis between the stratum corneum and stratum spinosum.3 Lesions start on the face, neck, and body folds, which was consistent with our patient’s presentation. However, bullae continued to develop in our patient despite antibiotic therapy, which reduced the likelihood of SSSS. Stevens-Johnson syndrome/toxic epidermal necrolysis develops rapidly and often involves the mucosa, which our patient initially did not have. In children, SJS/TEN can develop secondary to infection, whereas in adults it more commonly is associated with medication administration.4 Although the mother tested negative for HSV, the infant was started on acyclovir, which ultimately was discontinued due to low clinical suspicion. The clinical presentation of HSV (ie, clustered vesicles) was not consistent with our patient’s presentation. Bullous pemphigoid is a subepithelial blistering disease seen in older adults. Tense, fluidfilled blisters primarily are seen on the trunk and flexures. Although infantile BP can occur, it usually does not present in the neonatal period but rather at approximately 3 to 5 months of age.5
High clinical suspicion for EB due to the common characteristics of bullae location and formation following skin manipulation led to genetic testing in our patient. Mild forms of EB simplex typically appear on the upper and lower extremities with sparing of the trunk. In more severe cases of EB simplex, truncal and mucosal involvement may occur.6 In our case, the infant had a classic distribution of arm and leg blisters with truncal sparing. Epidermolysis bullosa may not be diagnosed in the neonatal period because of its similarities to other more common diseases, such as HSV or bullous impetigo, or other genetic blistering diseases, such as epidermolytic ichthyosis and incontinentia pigmenti.6
Epidermolysis bullosa can be inherited in an autosomal-dominant or autosomal-recessive fashion or with de novo mutations and is classified based on the location of cleavage in the skin. The 4 classical subtypes— simplex, junctional, dystrophic, and Kindler—have now been further subclassified. Epidermolysis bullosa simplex (intraepidermal split) is now separated into basal and suprabasal, with further subclassification including the distribution of blisters (generalized or localized) and the severity of cutaneous or extracutaneous involvement.7
In our case, the infant was found to have intraepidermal EB (simplex) due to a KRT14 mutation (missense mutation).6KRT14 (17q21.2) and KRT5 (12q13.3) are the 2 most common mutations causing cleavage at the basal intraepidermal layer. Thickening of the palms, soles, and nails can be seen; however, blisters heal well without scarring, as seen in our patient. Junctional EB due to cleavage at the intralamina lucida often involves mutations in laminin 332, plectin, and α6β4 integrin. Infants with junctional EB often die from severe infection, dehydration, or malnutrition due to mucosal involvement. Dystrophic EB occurs due to a collagen VII mutation in the dermis, leading to blisters at the sublamina densa and more severe symptoms in the recessive form.7
Newborn management for infants with EB differs from normal newborn care due to increased skin fragility with physical manipulation. Minimal skin manipulation and proper wound care are essential from the first day of life. For new bullae formation, bullae should be ruptured with a needle at the base of the blister and drained. The remaining skin overlying the wound should remain in place as a natural wound barrier. Patients with EB should not have tape or adhesive bandages applied directly to the skin. Instead, nonadhesive dressings can be placed directly on wounds and covered in soft wraps circumferentially. Dressings can be taped together without involving the skin. The cost for supplies for families to manage bullae is expensive. Fortunately, there are resources available for supplies and support for families, including the EB Research Partnership (https://www.ebresearch.org/) and DEBRA of America (https://www.debra.org/).
Currently, there is no cure for EB. Current treatment involves wound care, prevention, and symptomatic relief. Prevention includes avoiding activities that may result in increased friction of the skin and ensuring careful manipulation. Children with EB may have pain or itching from their blisters, which can be treated with oral acetaminophen or ibuprofen and diphenhydramine, respectively. Other complications of EB include anemia, dehydration, constipation, infection, and malnutrition. In more severe forms of EB, complications including eye problems, mucosal strictures, and skin cancer may occur.8 Future treatment directions include gene therapy, bone marrow transplantation, protein replacement therapies, and cell-based therapies. Prognosis for infants with EB due to KRT14 mutation is good, as it is a milder subtype of EB with a full life expectancy and improvement of blistering skin with age. The most at-risk time for early death is during infancy due to increased risk for infection.8 In this case, our patient showed full healing with no scar formation, which suggested a reassuring prognosis.
- Fine JD, Bruckner-Tuderman L, Eady RAJ, et al. Inherited epidermolysis bullosa: updated recommendations on diagnosis and classification. J Am Acad Dermatol. 2014;70:1103-1126.
- Wolff K, Johnson RA, Saavedra AP, et al. Hereditary epidermolysis bullosa. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 8th ed. McGraw-Hill Education; 2017:94-99.
- Ross A, Shoff HW. Staphylococcus scalded skin syndrome. In: StatPearls. StatPearls Publishing; 2020:1-20.
- Alerhand S, Cassella C, Koyfman A. Steven-Johnson syndrome and toxic epidermal necrolysis in the pediatric population. Pediatr Emerg Care. 2016;32:472-476.
- Schwieger-Briel A, Moellmann C, Mattulat B, et al. Bullous pemphigoid in infants: characteristics, diagnosis and treatment. Orphanet J Rare Dis. 2014;9:185.
- Gonzalez ME. Evaluation and treatment of the newborn with epidermolysis bullosa. Semin Perinatol. 2013;37:32-39.
- Has C, Bauer JW, Bodemer C, et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol. 2020;183:614-627.
- Watkins J. Diagnosis, treatment and management of epidermolysis bullosa. Br J Nurs. 2016;25:428-431.
The Diagnosis: Epidermolysis Bullosa
Our patient was found to have epidermolysis bullosa (EB), a rare genetic disease in which the superficial layers of the skin separate to form vesicles or bullae due to a mutation in the keratin 14 gene, KRT14. Separation of the skin occurs due to cleavage of various proteins that connect the epidermis to the dermis. A genetic mutation in KRT14, one of the more common genetic mutations associated with EB, results in cleavage at the basal epidermal protein keratin 14. The skin of individuals with EB typically is fragile and cannot tolerate friction or manipulation due to the risk for new bullae formation.1 Epidermolysis bullosa is rare, affecting approximately 20 children per 1 million births in the United States, and is not commonly seen by most general adult dermatologists.2
In our patient, the differential diagnoses included staphylococcal scalded skin syndrome (SSSS), Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), herpes simplex virus (HSV), and bullous pemphigoid (BP). Symptoms of SSSS can range from mild and localized to full-body exfoliation of the skin. Although SSSS can resemble other bullous disorders, its etiology arises from the Staphylococcus exotoxin targeting desmoglein in the stratum granulosum— the layer of the epidermis between the stratum corneum and stratum spinosum.3 Lesions start on the face, neck, and body folds, which was consistent with our patient’s presentation. However, bullae continued to develop in our patient despite antibiotic therapy, which reduced the likelihood of SSSS. Stevens-Johnson syndrome/toxic epidermal necrolysis develops rapidly and often involves the mucosa, which our patient initially did not have. In children, SJS/TEN can develop secondary to infection, whereas in adults it more commonly is associated with medication administration.4 Although the mother tested negative for HSV, the infant was started on acyclovir, which ultimately was discontinued due to low clinical suspicion. The clinical presentation of HSV (ie, clustered vesicles) was not consistent with our patient’s presentation. Bullous pemphigoid is a subepithelial blistering disease seen in older adults. Tense, fluidfilled blisters primarily are seen on the trunk and flexures. Although infantile BP can occur, it usually does not present in the neonatal period but rather at approximately 3 to 5 months of age.5
High clinical suspicion for EB due to the common characteristics of bullae location and formation following skin manipulation led to genetic testing in our patient. Mild forms of EB simplex typically appear on the upper and lower extremities with sparing of the trunk. In more severe cases of EB simplex, truncal and mucosal involvement may occur.6 In our case, the infant had a classic distribution of arm and leg blisters with truncal sparing. Epidermolysis bullosa may not be diagnosed in the neonatal period because of its similarities to other more common diseases, such as HSV or bullous impetigo, or other genetic blistering diseases, such as epidermolytic ichthyosis and incontinentia pigmenti.6
Epidermolysis bullosa can be inherited in an autosomal-dominant or autosomal-recessive fashion or with de novo mutations and is classified based on the location of cleavage in the skin. The 4 classical subtypes— simplex, junctional, dystrophic, and Kindler—have now been further subclassified. Epidermolysis bullosa simplex (intraepidermal split) is now separated into basal and suprabasal, with further subclassification including the distribution of blisters (generalized or localized) and the severity of cutaneous or extracutaneous involvement.7
In our case, the infant was found to have intraepidermal EB (simplex) due to a KRT14 mutation (missense mutation).6KRT14 (17q21.2) and KRT5 (12q13.3) are the 2 most common mutations causing cleavage at the basal intraepidermal layer. Thickening of the palms, soles, and nails can be seen; however, blisters heal well without scarring, as seen in our patient. Junctional EB due to cleavage at the intralamina lucida often involves mutations in laminin 332, plectin, and α6β4 integrin. Infants with junctional EB often die from severe infection, dehydration, or malnutrition due to mucosal involvement. Dystrophic EB occurs due to a collagen VII mutation in the dermis, leading to blisters at the sublamina densa and more severe symptoms in the recessive form.7
Newborn management for infants with EB differs from normal newborn care due to increased skin fragility with physical manipulation. Minimal skin manipulation and proper wound care are essential from the first day of life. For new bullae formation, bullae should be ruptured with a needle at the base of the blister and drained. The remaining skin overlying the wound should remain in place as a natural wound barrier. Patients with EB should not have tape or adhesive bandages applied directly to the skin. Instead, nonadhesive dressings can be placed directly on wounds and covered in soft wraps circumferentially. Dressings can be taped together without involving the skin. The cost for supplies for families to manage bullae is expensive. Fortunately, there are resources available for supplies and support for families, including the EB Research Partnership (https://www.ebresearch.org/) and DEBRA of America (https://www.debra.org/).
Currently, there is no cure for EB. Current treatment involves wound care, prevention, and symptomatic relief. Prevention includes avoiding activities that may result in increased friction of the skin and ensuring careful manipulation. Children with EB may have pain or itching from their blisters, which can be treated with oral acetaminophen or ibuprofen and diphenhydramine, respectively. Other complications of EB include anemia, dehydration, constipation, infection, and malnutrition. In more severe forms of EB, complications including eye problems, mucosal strictures, and skin cancer may occur.8 Future treatment directions include gene therapy, bone marrow transplantation, protein replacement therapies, and cell-based therapies. Prognosis for infants with EB due to KRT14 mutation is good, as it is a milder subtype of EB with a full life expectancy and improvement of blistering skin with age. The most at-risk time for early death is during infancy due to increased risk for infection.8 In this case, our patient showed full healing with no scar formation, which suggested a reassuring prognosis.
The Diagnosis: Epidermolysis Bullosa
Our patient was found to have epidermolysis bullosa (EB), a rare genetic disease in which the superficial layers of the skin separate to form vesicles or bullae due to a mutation in the keratin 14 gene, KRT14. Separation of the skin occurs due to cleavage of various proteins that connect the epidermis to the dermis. A genetic mutation in KRT14, one of the more common genetic mutations associated with EB, results in cleavage at the basal epidermal protein keratin 14. The skin of individuals with EB typically is fragile and cannot tolerate friction or manipulation due to the risk for new bullae formation.1 Epidermolysis bullosa is rare, affecting approximately 20 children per 1 million births in the United States, and is not commonly seen by most general adult dermatologists.2
In our patient, the differential diagnoses included staphylococcal scalded skin syndrome (SSSS), Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), herpes simplex virus (HSV), and bullous pemphigoid (BP). Symptoms of SSSS can range from mild and localized to full-body exfoliation of the skin. Although SSSS can resemble other bullous disorders, its etiology arises from the Staphylococcus exotoxin targeting desmoglein in the stratum granulosum— the layer of the epidermis between the stratum corneum and stratum spinosum.3 Lesions start on the face, neck, and body folds, which was consistent with our patient’s presentation. However, bullae continued to develop in our patient despite antibiotic therapy, which reduced the likelihood of SSSS. Stevens-Johnson syndrome/toxic epidermal necrolysis develops rapidly and often involves the mucosa, which our patient initially did not have. In children, SJS/TEN can develop secondary to infection, whereas in adults it more commonly is associated with medication administration.4 Although the mother tested negative for HSV, the infant was started on acyclovir, which ultimately was discontinued due to low clinical suspicion. The clinical presentation of HSV (ie, clustered vesicles) was not consistent with our patient’s presentation. Bullous pemphigoid is a subepithelial blistering disease seen in older adults. Tense, fluidfilled blisters primarily are seen on the trunk and flexures. Although infantile BP can occur, it usually does not present in the neonatal period but rather at approximately 3 to 5 months of age.5
High clinical suspicion for EB due to the common characteristics of bullae location and formation following skin manipulation led to genetic testing in our patient. Mild forms of EB simplex typically appear on the upper and lower extremities with sparing of the trunk. In more severe cases of EB simplex, truncal and mucosal involvement may occur.6 In our case, the infant had a classic distribution of arm and leg blisters with truncal sparing. Epidermolysis bullosa may not be diagnosed in the neonatal period because of its similarities to other more common diseases, such as HSV or bullous impetigo, or other genetic blistering diseases, such as epidermolytic ichthyosis and incontinentia pigmenti.6
Epidermolysis bullosa can be inherited in an autosomal-dominant or autosomal-recessive fashion or with de novo mutations and is classified based on the location of cleavage in the skin. The 4 classical subtypes— simplex, junctional, dystrophic, and Kindler—have now been further subclassified. Epidermolysis bullosa simplex (intraepidermal split) is now separated into basal and suprabasal, with further subclassification including the distribution of blisters (generalized or localized) and the severity of cutaneous or extracutaneous involvement.7
In our case, the infant was found to have intraepidermal EB (simplex) due to a KRT14 mutation (missense mutation).6KRT14 (17q21.2) and KRT5 (12q13.3) are the 2 most common mutations causing cleavage at the basal intraepidermal layer. Thickening of the palms, soles, and nails can be seen; however, blisters heal well without scarring, as seen in our patient. Junctional EB due to cleavage at the intralamina lucida often involves mutations in laminin 332, plectin, and α6β4 integrin. Infants with junctional EB often die from severe infection, dehydration, or malnutrition due to mucosal involvement. Dystrophic EB occurs due to a collagen VII mutation in the dermis, leading to blisters at the sublamina densa and more severe symptoms in the recessive form.7
Newborn management for infants with EB differs from normal newborn care due to increased skin fragility with physical manipulation. Minimal skin manipulation and proper wound care are essential from the first day of life. For new bullae formation, bullae should be ruptured with a needle at the base of the blister and drained. The remaining skin overlying the wound should remain in place as a natural wound barrier. Patients with EB should not have tape or adhesive bandages applied directly to the skin. Instead, nonadhesive dressings can be placed directly on wounds and covered in soft wraps circumferentially. Dressings can be taped together without involving the skin. The cost for supplies for families to manage bullae is expensive. Fortunately, there are resources available for supplies and support for families, including the EB Research Partnership (https://www.ebresearch.org/) and DEBRA of America (https://www.debra.org/).
Currently, there is no cure for EB. Current treatment involves wound care, prevention, and symptomatic relief. Prevention includes avoiding activities that may result in increased friction of the skin and ensuring careful manipulation. Children with EB may have pain or itching from their blisters, which can be treated with oral acetaminophen or ibuprofen and diphenhydramine, respectively. Other complications of EB include anemia, dehydration, constipation, infection, and malnutrition. In more severe forms of EB, complications including eye problems, mucosal strictures, and skin cancer may occur.8 Future treatment directions include gene therapy, bone marrow transplantation, protein replacement therapies, and cell-based therapies. Prognosis for infants with EB due to KRT14 mutation is good, as it is a milder subtype of EB with a full life expectancy and improvement of blistering skin with age. The most at-risk time for early death is during infancy due to increased risk for infection.8 In this case, our patient showed full healing with no scar formation, which suggested a reassuring prognosis.
- Fine JD, Bruckner-Tuderman L, Eady RAJ, et al. Inherited epidermolysis bullosa: updated recommendations on diagnosis and classification. J Am Acad Dermatol. 2014;70:1103-1126.
- Wolff K, Johnson RA, Saavedra AP, et al. Hereditary epidermolysis bullosa. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 8th ed. McGraw-Hill Education; 2017:94-99.
- Ross A, Shoff HW. Staphylococcus scalded skin syndrome. In: StatPearls. StatPearls Publishing; 2020:1-20.
- Alerhand S, Cassella C, Koyfman A. Steven-Johnson syndrome and toxic epidermal necrolysis in the pediatric population. Pediatr Emerg Care. 2016;32:472-476.
- Schwieger-Briel A, Moellmann C, Mattulat B, et al. Bullous pemphigoid in infants: characteristics, diagnosis and treatment. Orphanet J Rare Dis. 2014;9:185.
- Gonzalez ME. Evaluation and treatment of the newborn with epidermolysis bullosa. Semin Perinatol. 2013;37:32-39.
- Has C, Bauer JW, Bodemer C, et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol. 2020;183:614-627.
- Watkins J. Diagnosis, treatment and management of epidermolysis bullosa. Br J Nurs. 2016;25:428-431.
- Fine JD, Bruckner-Tuderman L, Eady RAJ, et al. Inherited epidermolysis bullosa: updated recommendations on diagnosis and classification. J Am Acad Dermatol. 2014;70:1103-1126.
- Wolff K, Johnson RA, Saavedra AP, et al. Hereditary epidermolysis bullosa. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 8th ed. McGraw-Hill Education; 2017:94-99.
- Ross A, Shoff HW. Staphylococcus scalded skin syndrome. In: StatPearls. StatPearls Publishing; 2020:1-20.
- Alerhand S, Cassella C, Koyfman A. Steven-Johnson syndrome and toxic epidermal necrolysis in the pediatric population. Pediatr Emerg Care. 2016;32:472-476.
- Schwieger-Briel A, Moellmann C, Mattulat B, et al. Bullous pemphigoid in infants: characteristics, diagnosis and treatment. Orphanet J Rare Dis. 2014;9:185.
- Gonzalez ME. Evaluation and treatment of the newborn with epidermolysis bullosa. Semin Perinatol. 2013;37:32-39.
- Has C, Bauer JW, Bodemer C, et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol. 2020;183:614-627.
- Watkins J. Diagnosis, treatment and management of epidermolysis bullosa. Br J Nurs. 2016;25:428-431.
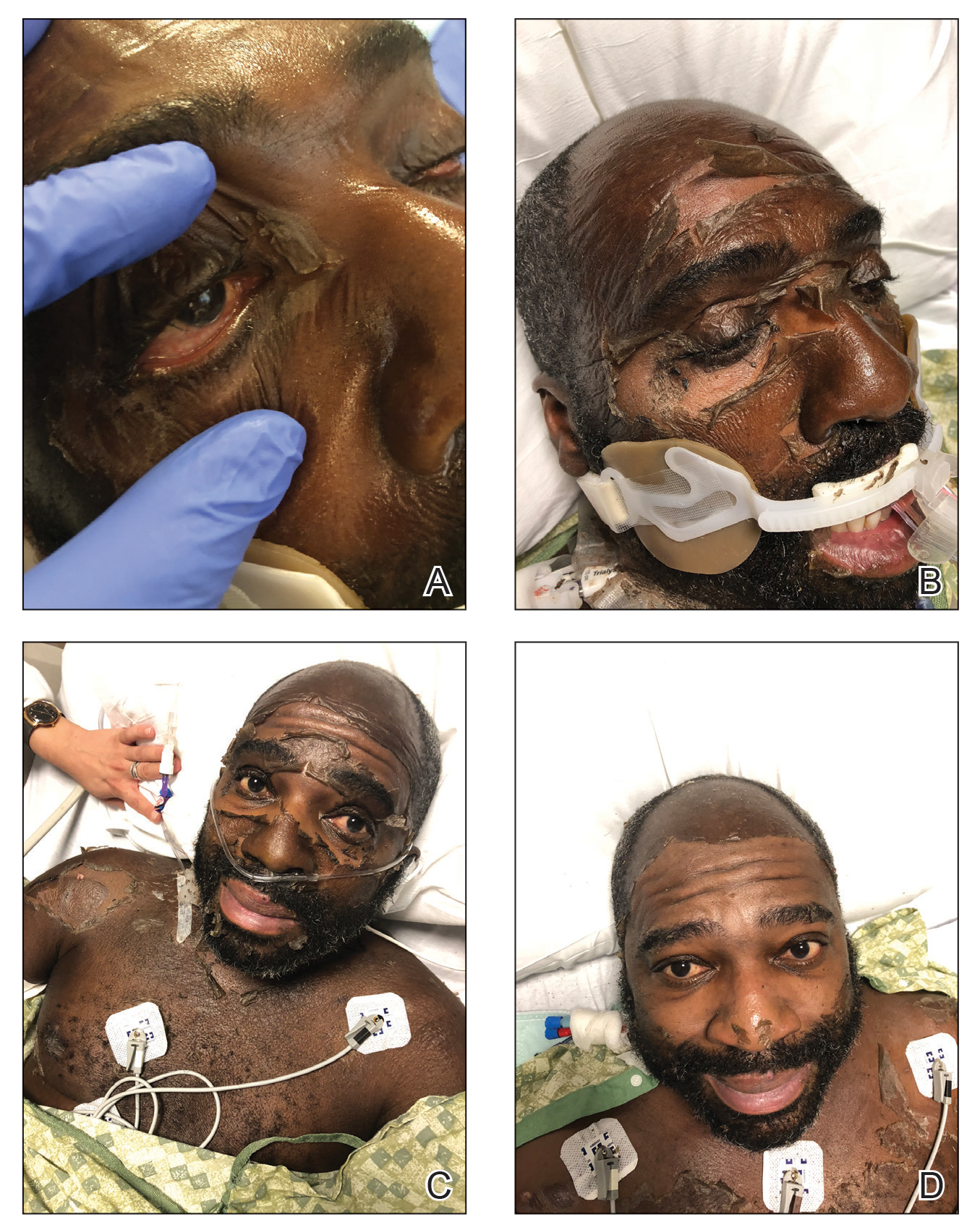
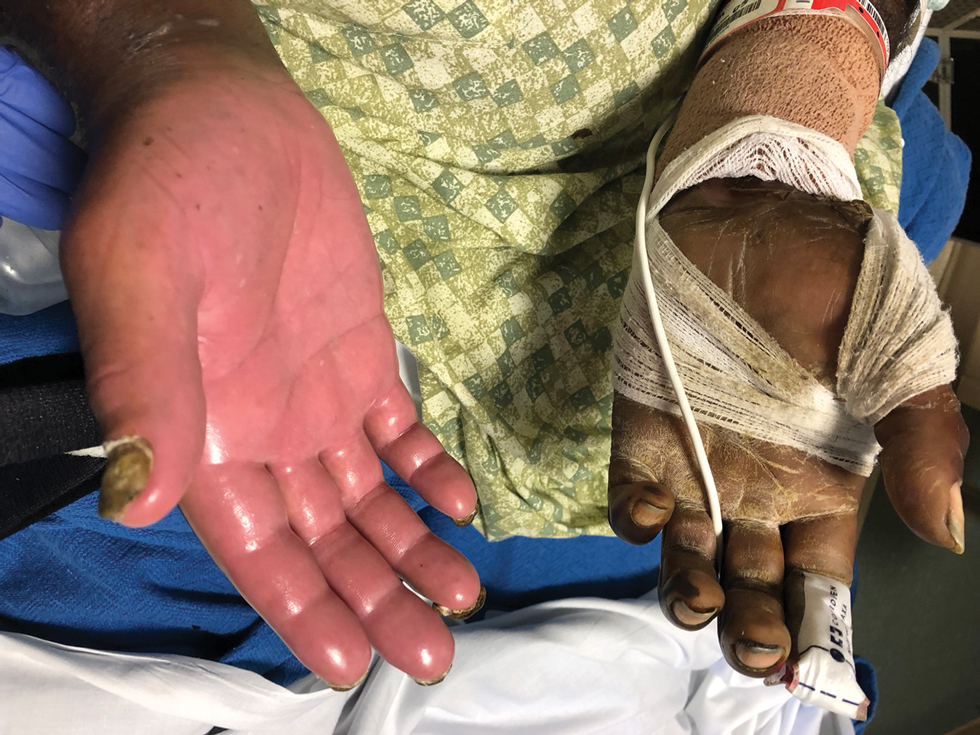
A 4-day-old infant boy presented with blisters on the skin. He was born at 36 weeks’ gestation by cesarean delivery to a nulliparous mother who received appropriate prenatal care. On day 2 of life, the patient developed bullae with breakdown of the skin on the bilateral heels and on the skin surrounding intravenous injection sites. Similar blisters subsequently developed on the fingers (top), thighs, groin, and toes (bottom), sparing the oral mucosa and trunk. He remained afebrile and stable and was started on ampicillin, gentamicin, and acyclovir with continued development of blisters. Two weeks later he developed painful ulcers on the tongue that bled upon scraping.
Violaceous Nodules on the Lower Leg
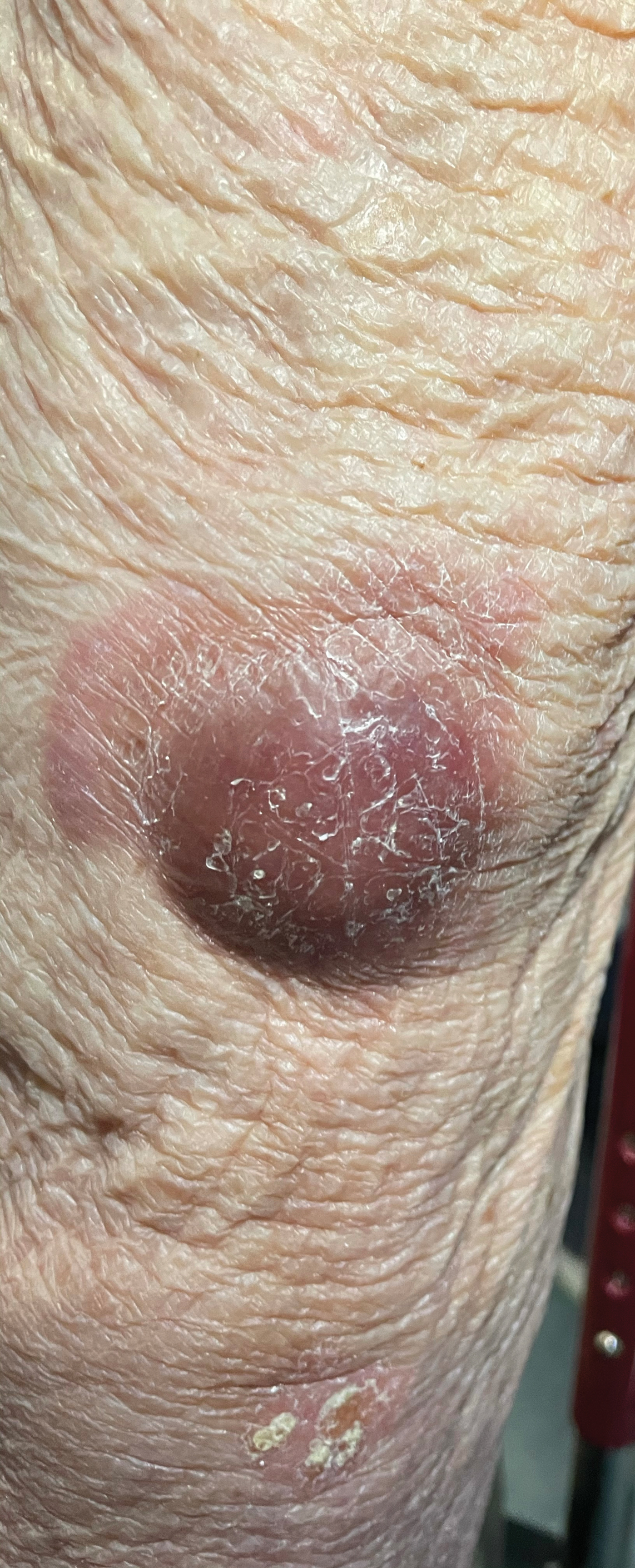
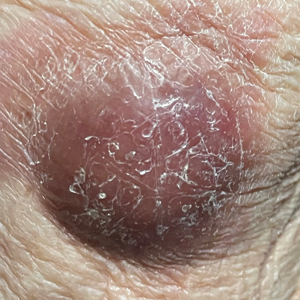
The Diagnosis: Cutaneous B-cell Lymphoma
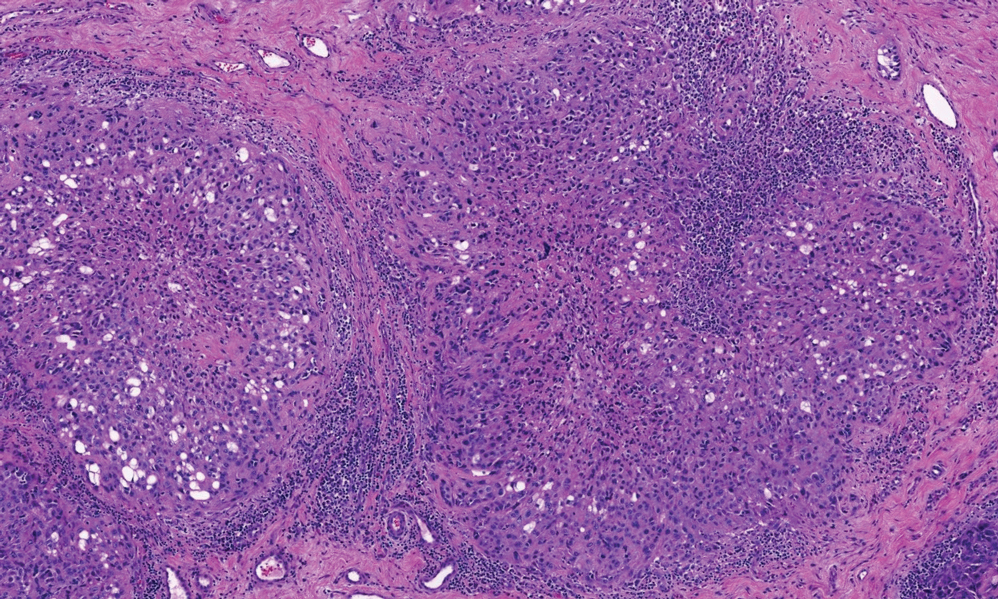
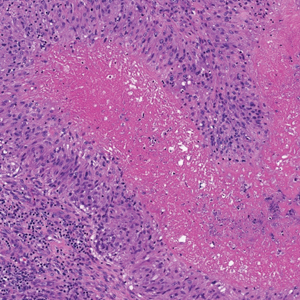
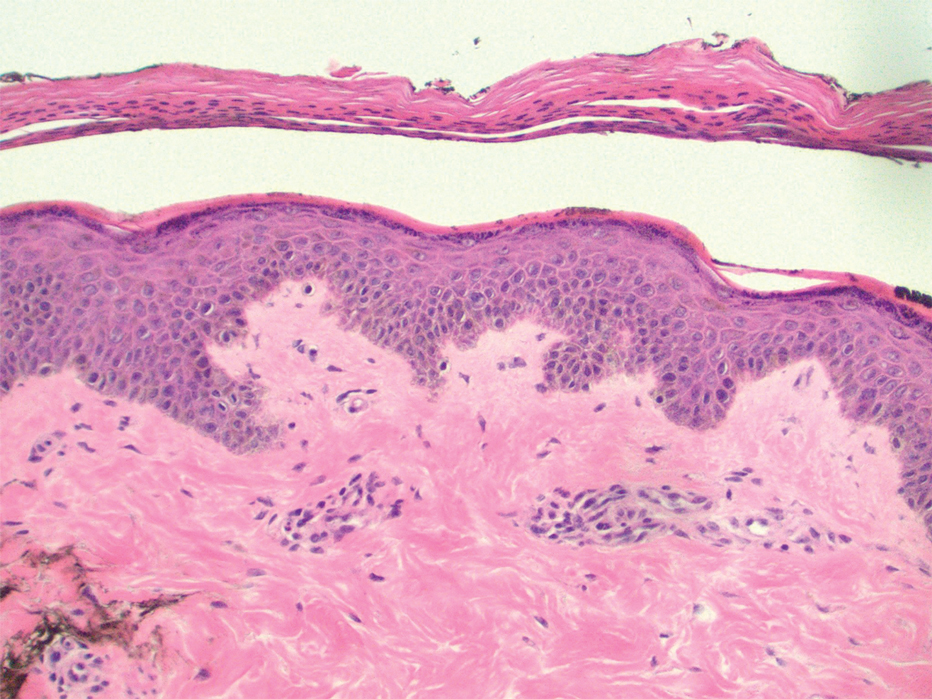
Shave biopsies of 3 lesions revealed a dense, diffuse, atypical lymphoid infiltrate occupying the entirety of the dermis and obscuring the dermoepidermal junction. The infiltrate consisted predominantly of largesized lymphoid cells with fine chromatin and conspicuous nucleoli (Figure). Immunohistochemistry was positive for CD45 and CD20, indicating B-cell lineage. Bcl-2, multiple myeloma oncogene 1, and forkhead box protein P1 also were expressed in the vast majority of lesional cells, distinguishing the lesion from other forms of cutaneous B-cell lymphomas.1 These findings were consistent with large B-cell lymphoma with a high proliferation index, consistent with primary cutaneous diffuse large B-cell lymphoma, leg type, which often presents on the lower leg.2 The patient had a negative systemic workup including bone marrow biopsy. He was started on the R-CEOP (rituximab, cyclophosphamide, etoposide, vincristine, prednisone) chemotherapy regimen.
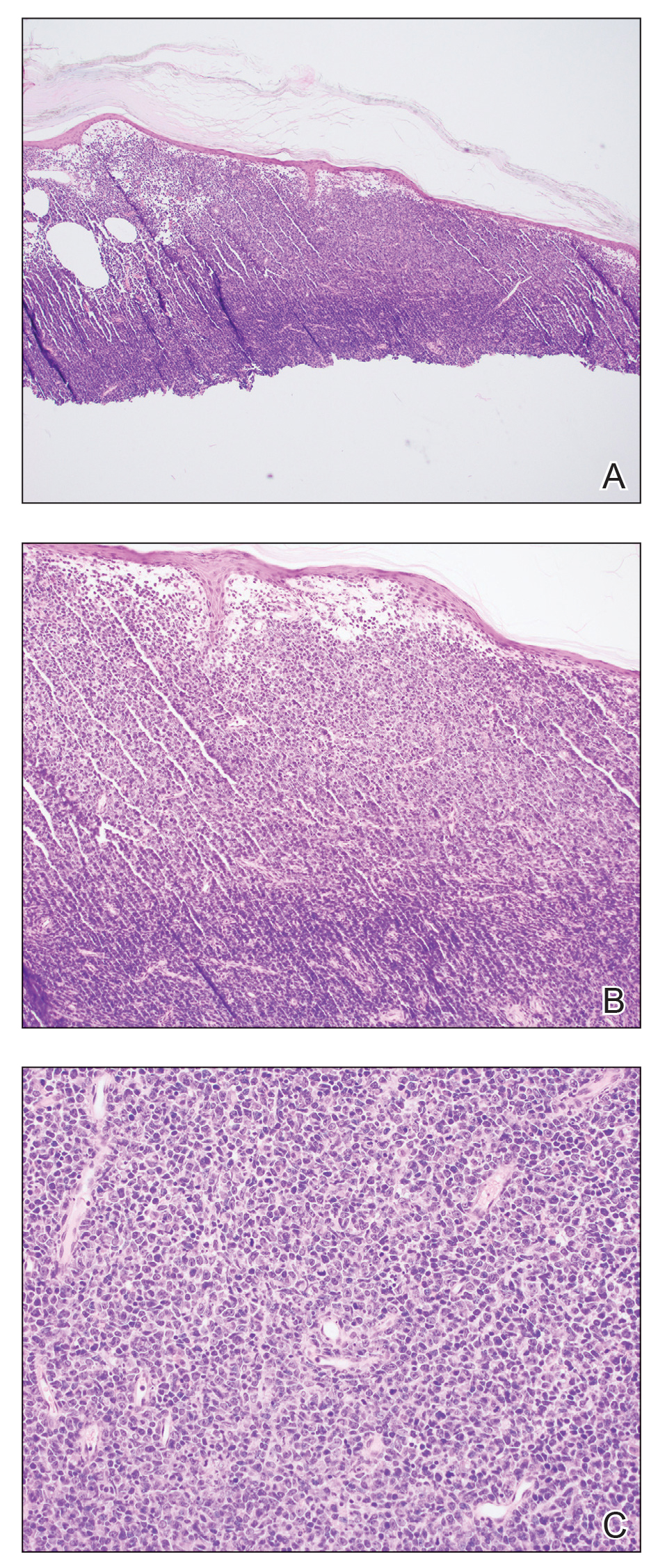
Primary cutaneous diffuse large B-cell lymphoma, leg type, is an intermediately aggressive and rare form of B-cell lymphoma with a poor prognosis that primarily affects elderly female patients. Primary cutaneous diffuse large B-cell lymphoma, leg type, accounts for only 1% to 3% of cutaneous lymphomas and approximately 10% to 20% of primary cutaneous B-cell lymphomas.2 It typically presents as multiple red-brown or bluish nodules on the lower extremities or trunk. Presentation as a solitary nodule also is possible.1,2 Histologic analysis of primary cutaneous diffuse large B-cell lymphoma, leg type, reveals large cells with round nuclei (immunoblasts and centroblasts), and the immunohistochemical profile shows strong Bcl-2 expression often accompanied by the multiple myeloma oncogene 1 protein.3 The 5-year survival rate is approximately 50%, which is lower than other types of primary cutaneous B-cell lymphomas, and the progression of disease is characterized by frequent relapses and involvement of extracutaneous regions such as the lymph nodes, bone marrow, and central nervous system.1,2,4 Patients with multiple tumors on the leg have a particularly poor prognosis; in particular, having 1 or more lesions on the leg results in a 43% 3-year survival rate while having multiple lesions has a 36% 3-year survival rate compared with a 77% 3-year survival rate for patients with the non–leg subtype or a single lesion.3 Treatment with rituximab has been shown to be effective in at least short-term control of the disease, and the R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) regimen is the standard of treatment.3,4
Primary cutaneous diffuse large B-cell lymphoma, leg type, can mimic multiple other cutaneous presentations of disease. Myeloid sarcoma (leukemia cutis) is a rare condition that presents as an extramedullary tumor often simultaneously with the onset or relapse of acute myeloid leukemia.5 Our patient had no history of leukemia, but myeloid sarcoma may predate acute myeloid leukemia in about a quarter of cases.5 It most commonly presents histologically as a diffuse dermal infiltrate that splays between collagen bundles and often is associated with an overlying Grenz zone. A nodular, or perivascular and periadnexal, pattern also may be seen. Upon closer inspection, the infiltrate is composed of immature myeloid cells (blasts) with background inflammation occasionally containing eosinophils. The immunohistochemical profile varies depending on the type of differentiation and degree of maturity of the cells. The histologic findings in our patient were inconsistent with myeloid sarcoma.
Erythema elevatum diutinum (EED) usually presents as dark red, brown, or violaceous papules or plaques and often is found on the extensor surfaces. It often is associated with hematologic abnormalities as well as recurrent bacterial or viral infections.6 Histologically, EED initially manifests as leukocytoclastic vasculitis with a mixed inflammatory infiltrate typically featuring an abundance of neutrophils, making this condition unlikely in this case. As the lesion progresses, fibrosis and scarring ensue as inflammation wanes. The fibrosis often is described as having an onion skin–like pattern, which is characteristic of established EED lesions. Our patient had no history of vasculitis, and the histologic findings were inconsistent with EED.
Angiosarcoma can present as a central nodule surrounded by an erythematous plaque. Although potentially clinically similar to primary cutaneous diffuse large B-cell lymphoma, leg type, angiosarcoma was unlikely in this case because of an absence of lymphedema and no history of radiation to the leg, both of which are key historical features of angiosarcoma.7 Additionally, the histology of cutaneous angiosarcoma is marked by vascular proliferation, which was not seen in the lesion biopsied in our patient. The histology of angiosarcoma is that of an atypical vascular proliferation, and a hallmark feature is infiltration between collagen, often referred to as giving the appearance of dissection between collagen bundles. The degree of atypia can vary widely, and epithelioid variants exist, producing a potential diagnostic pitfall. Lesional cells are positive for vascular markers, which can be used for confirmation of the endothelial lineage.
Sarcoidosis is notorious for its mimicry, which can be the case both clinically and histologically. Characteristic pathology of sarcoidosis is that of well-formed epithelioid granulomas with minimal associated inflammation and lack of caseating necrosis. Our patient had no known history of systemic sarcoidosis, and the pathologic features of noncaseating granulomas were not present. As a diagnosis of exclusion, correlation with special stains and culture studies is necessary to exclude an infectious process. The differential diagnosis for sarcoidal granulomatous dermatitis also includes foreign body reaction, inflammatory bowel disease, and granulomatous cheilitis, among others.
- Athalye L, Nami N, Shitabata P. A rare case of primary cutaneous diffuse large B-cell lymphoma, leg type. Cutis. 2018;102:E31-E34.
- Sokol L, Naghashpour M, Glass LF. Primary cutaneous B-cell lymphomas: recent advances in diagnosis and management. Cancer Control. 2012;19:236-244. doi:10.1177/107327481201900308
- Grange F, Beylot-Barry M, Courville P, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type: clinicopathologic features and prognostic analysis in 60 cases. Arch Dermatol. 2007;143:1144-1150. doi:10.1001/archderm.143.9.1144
- Patsatsi A, Kyriakou A, Karavasilis V, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type, with multiple local relapses: case presentation and brief review of literature. Hippokratia. 2013;17:174-176.
- Avni B, Koren-Michowitz M. Myeloid sarcoma: current approach and therapeutic options. Ther Adv Hematol. 2011;2:309-316.
- Yiannias JA, el-Azhary RA, Gibson LE. Erythema elevatum diutinum: a clinical and histopathologic study of 13 patients. J Am Acad Dermatol. 1992;26:38-44.
- Scholtz J, Mishra MM, Simman R. Cutaneous angiosarcoma of the lower leg. Cutis. 2018;102:E8-E11.
The Diagnosis: Cutaneous B-cell Lymphoma
Shave biopsies of 3 lesions revealed a dense, diffuse, atypical lymphoid infiltrate occupying the entirety of the dermis and obscuring the dermoepidermal junction. The infiltrate consisted predominantly of largesized lymphoid cells with fine chromatin and conspicuous nucleoli (Figure). Immunohistochemistry was positive for CD45 and CD20, indicating B-cell lineage. Bcl-2, multiple myeloma oncogene 1, and forkhead box protein P1 also were expressed in the vast majority of lesional cells, distinguishing the lesion from other forms of cutaneous B-cell lymphomas.1 These findings were consistent with large B-cell lymphoma with a high proliferation index, consistent with primary cutaneous diffuse large B-cell lymphoma, leg type, which often presents on the lower leg.2 The patient had a negative systemic workup including bone marrow biopsy. He was started on the R-CEOP (rituximab, cyclophosphamide, etoposide, vincristine, prednisone) chemotherapy regimen.

Primary cutaneous diffuse large B-cell lymphoma, leg type, is an intermediately aggressive and rare form of B-cell lymphoma with a poor prognosis that primarily affects elderly female patients. Primary cutaneous diffuse large B-cell lymphoma, leg type, accounts for only 1% to 3% of cutaneous lymphomas and approximately 10% to 20% of primary cutaneous B-cell lymphomas.2 It typically presents as multiple red-brown or bluish nodules on the lower extremities or trunk. Presentation as a solitary nodule also is possible.1,2 Histologic analysis of primary cutaneous diffuse large B-cell lymphoma, leg type, reveals large cells with round nuclei (immunoblasts and centroblasts), and the immunohistochemical profile shows strong Bcl-2 expression often accompanied by the multiple myeloma oncogene 1 protein.3 The 5-year survival rate is approximately 50%, which is lower than other types of primary cutaneous B-cell lymphomas, and the progression of disease is characterized by frequent relapses and involvement of extracutaneous regions such as the lymph nodes, bone marrow, and central nervous system.1,2,4 Patients with multiple tumors on the leg have a particularly poor prognosis; in particular, having 1 or more lesions on the leg results in a 43% 3-year survival rate while having multiple lesions has a 36% 3-year survival rate compared with a 77% 3-year survival rate for patients with the non–leg subtype or a single lesion.3 Treatment with rituximab has been shown to be effective in at least short-term control of the disease, and the R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) regimen is the standard of treatment.3,4
Primary cutaneous diffuse large B-cell lymphoma, leg type, can mimic multiple other cutaneous presentations of disease. Myeloid sarcoma (leukemia cutis) is a rare condition that presents as an extramedullary tumor often simultaneously with the onset or relapse of acute myeloid leukemia.5 Our patient had no history of leukemia, but myeloid sarcoma may predate acute myeloid leukemia in about a quarter of cases.5 It most commonly presents histologically as a diffuse dermal infiltrate that splays between collagen bundles and often is associated with an overlying Grenz zone. A nodular, or perivascular and periadnexal, pattern also may be seen. Upon closer inspection, the infiltrate is composed of immature myeloid cells (blasts) with background inflammation occasionally containing eosinophils. The immunohistochemical profile varies depending on the type of differentiation and degree of maturity of the cells. The histologic findings in our patient were inconsistent with myeloid sarcoma.
Erythema elevatum diutinum (EED) usually presents as dark red, brown, or violaceous papules or plaques and often is found on the extensor surfaces. It often is associated with hematologic abnormalities as well as recurrent bacterial or viral infections.6 Histologically, EED initially manifests as leukocytoclastic vasculitis with a mixed inflammatory infiltrate typically featuring an abundance of neutrophils, making this condition unlikely in this case. As the lesion progresses, fibrosis and scarring ensue as inflammation wanes. The fibrosis often is described as having an onion skin–like pattern, which is characteristic of established EED lesions. Our patient had no history of vasculitis, and the histologic findings were inconsistent with EED.
Angiosarcoma can present as a central nodule surrounded by an erythematous plaque. Although potentially clinically similar to primary cutaneous diffuse large B-cell lymphoma, leg type, angiosarcoma was unlikely in this case because of an absence of lymphedema and no history of radiation to the leg, both of which are key historical features of angiosarcoma.7 Additionally, the histology of cutaneous angiosarcoma is marked by vascular proliferation, which was not seen in the lesion biopsied in our patient. The histology of angiosarcoma is that of an atypical vascular proliferation, and a hallmark feature is infiltration between collagen, often referred to as giving the appearance of dissection between collagen bundles. The degree of atypia can vary widely, and epithelioid variants exist, producing a potential diagnostic pitfall. Lesional cells are positive for vascular markers, which can be used for confirmation of the endothelial lineage.
Sarcoidosis is notorious for its mimicry, which can be the case both clinically and histologically. Characteristic pathology of sarcoidosis is that of well-formed epithelioid granulomas with minimal associated inflammation and lack of caseating necrosis. Our patient had no known history of systemic sarcoidosis, and the pathologic features of noncaseating granulomas were not present. As a diagnosis of exclusion, correlation with special stains and culture studies is necessary to exclude an infectious process. The differential diagnosis for sarcoidal granulomatous dermatitis also includes foreign body reaction, inflammatory bowel disease, and granulomatous cheilitis, among others.
The Diagnosis: Cutaneous B-cell Lymphoma
Shave biopsies of 3 lesions revealed a dense, diffuse, atypical lymphoid infiltrate occupying the entirety of the dermis and obscuring the dermoepidermal junction. The infiltrate consisted predominantly of largesized lymphoid cells with fine chromatin and conspicuous nucleoli (Figure). Immunohistochemistry was positive for CD45 and CD20, indicating B-cell lineage. Bcl-2, multiple myeloma oncogene 1, and forkhead box protein P1 also were expressed in the vast majority of lesional cells, distinguishing the lesion from other forms of cutaneous B-cell lymphomas.1 These findings were consistent with large B-cell lymphoma with a high proliferation index, consistent with primary cutaneous diffuse large B-cell lymphoma, leg type, which often presents on the lower leg.2 The patient had a negative systemic workup including bone marrow biopsy. He was started on the R-CEOP (rituximab, cyclophosphamide, etoposide, vincristine, prednisone) chemotherapy regimen.

Primary cutaneous diffuse large B-cell lymphoma, leg type, is an intermediately aggressive and rare form of B-cell lymphoma with a poor prognosis that primarily affects elderly female patients. Primary cutaneous diffuse large B-cell lymphoma, leg type, accounts for only 1% to 3% of cutaneous lymphomas and approximately 10% to 20% of primary cutaneous B-cell lymphomas.2 It typically presents as multiple red-brown or bluish nodules on the lower extremities or trunk. Presentation as a solitary nodule also is possible.1,2 Histologic analysis of primary cutaneous diffuse large B-cell lymphoma, leg type, reveals large cells with round nuclei (immunoblasts and centroblasts), and the immunohistochemical profile shows strong Bcl-2 expression often accompanied by the multiple myeloma oncogene 1 protein.3 The 5-year survival rate is approximately 50%, which is lower than other types of primary cutaneous B-cell lymphomas, and the progression of disease is characterized by frequent relapses and involvement of extracutaneous regions such as the lymph nodes, bone marrow, and central nervous system.1,2,4 Patients with multiple tumors on the leg have a particularly poor prognosis; in particular, having 1 or more lesions on the leg results in a 43% 3-year survival rate while having multiple lesions has a 36% 3-year survival rate compared with a 77% 3-year survival rate for patients with the non–leg subtype or a single lesion.3 Treatment with rituximab has been shown to be effective in at least short-term control of the disease, and the R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) regimen is the standard of treatment.3,4
Primary cutaneous diffuse large B-cell lymphoma, leg type, can mimic multiple other cutaneous presentations of disease. Myeloid sarcoma (leukemia cutis) is a rare condition that presents as an extramedullary tumor often simultaneously with the onset or relapse of acute myeloid leukemia.5 Our patient had no history of leukemia, but myeloid sarcoma may predate acute myeloid leukemia in about a quarter of cases.5 It most commonly presents histologically as a diffuse dermal infiltrate that splays between collagen bundles and often is associated with an overlying Grenz zone. A nodular, or perivascular and periadnexal, pattern also may be seen. Upon closer inspection, the infiltrate is composed of immature myeloid cells (blasts) with background inflammation occasionally containing eosinophils. The immunohistochemical profile varies depending on the type of differentiation and degree of maturity of the cells. The histologic findings in our patient were inconsistent with myeloid sarcoma.
Erythema elevatum diutinum (EED) usually presents as dark red, brown, or violaceous papules or plaques and often is found on the extensor surfaces. It often is associated with hematologic abnormalities as well as recurrent bacterial or viral infections.6 Histologically, EED initially manifests as leukocytoclastic vasculitis with a mixed inflammatory infiltrate typically featuring an abundance of neutrophils, making this condition unlikely in this case. As the lesion progresses, fibrosis and scarring ensue as inflammation wanes. The fibrosis often is described as having an onion skin–like pattern, which is characteristic of established EED lesions. Our patient had no history of vasculitis, and the histologic findings were inconsistent with EED.
Angiosarcoma can present as a central nodule surrounded by an erythematous plaque. Although potentially clinically similar to primary cutaneous diffuse large B-cell lymphoma, leg type, angiosarcoma was unlikely in this case because of an absence of lymphedema and no history of radiation to the leg, both of which are key historical features of angiosarcoma.7 Additionally, the histology of cutaneous angiosarcoma is marked by vascular proliferation, which was not seen in the lesion biopsied in our patient. The histology of angiosarcoma is that of an atypical vascular proliferation, and a hallmark feature is infiltration between collagen, often referred to as giving the appearance of dissection between collagen bundles. The degree of atypia can vary widely, and epithelioid variants exist, producing a potential diagnostic pitfall. Lesional cells are positive for vascular markers, which can be used for confirmation of the endothelial lineage.
Sarcoidosis is notorious for its mimicry, which can be the case both clinically and histologically. Characteristic pathology of sarcoidosis is that of well-formed epithelioid granulomas with minimal associated inflammation and lack of caseating necrosis. Our patient had no known history of systemic sarcoidosis, and the pathologic features of noncaseating granulomas were not present. As a diagnosis of exclusion, correlation with special stains and culture studies is necessary to exclude an infectious process. The differential diagnosis for sarcoidal granulomatous dermatitis also includes foreign body reaction, inflammatory bowel disease, and granulomatous cheilitis, among others.
- Athalye L, Nami N, Shitabata P. A rare case of primary cutaneous diffuse large B-cell lymphoma, leg type. Cutis. 2018;102:E31-E34.
- Sokol L, Naghashpour M, Glass LF. Primary cutaneous B-cell lymphomas: recent advances in diagnosis and management. Cancer Control. 2012;19:236-244. doi:10.1177/107327481201900308
- Grange F, Beylot-Barry M, Courville P, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type: clinicopathologic features and prognostic analysis in 60 cases. Arch Dermatol. 2007;143:1144-1150. doi:10.1001/archderm.143.9.1144
- Patsatsi A, Kyriakou A, Karavasilis V, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type, with multiple local relapses: case presentation and brief review of literature. Hippokratia. 2013;17:174-176.
- Avni B, Koren-Michowitz M. Myeloid sarcoma: current approach and therapeutic options. Ther Adv Hematol. 2011;2:309-316.
- Yiannias JA, el-Azhary RA, Gibson LE. Erythema elevatum diutinum: a clinical and histopathologic study of 13 patients. J Am Acad Dermatol. 1992;26:38-44.
- Scholtz J, Mishra MM, Simman R. Cutaneous angiosarcoma of the lower leg. Cutis. 2018;102:E8-E11.
- Athalye L, Nami N, Shitabata P. A rare case of primary cutaneous diffuse large B-cell lymphoma, leg type. Cutis. 2018;102:E31-E34.
- Sokol L, Naghashpour M, Glass LF. Primary cutaneous B-cell lymphomas: recent advances in diagnosis and management. Cancer Control. 2012;19:236-244. doi:10.1177/107327481201900308
- Grange F, Beylot-Barry M, Courville P, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type: clinicopathologic features and prognostic analysis in 60 cases. Arch Dermatol. 2007;143:1144-1150. doi:10.1001/archderm.143.9.1144
- Patsatsi A, Kyriakou A, Karavasilis V, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type, with multiple local relapses: case presentation and brief review of literature. Hippokratia. 2013;17:174-176.
- Avni B, Koren-Michowitz M. Myeloid sarcoma: current approach and therapeutic options. Ther Adv Hematol. 2011;2:309-316.
- Yiannias JA, el-Azhary RA, Gibson LE. Erythema elevatum diutinum: a clinical and histopathologic study of 13 patients. J Am Acad Dermatol. 1992;26:38-44.
- Scholtz J, Mishra MM, Simman R. Cutaneous angiosarcoma of the lower leg. Cutis. 2018;102:E8-E11.
A 79-year-old man presented to the dermatology clinic with 4 enlarging, asymptomatic, violaceous, desquamating nodules on the left pretibial region and calf of 3 months’ duration. He denied any constitutional symptoms such as night sweats or weight loss. His medical history included a malignant melanoma on the left ear that was excised 5 years prior. He also had a history of peripheral edema, hypertension, and rheumatoid arthritis, as well as a 50-pack-year history of smoking. Physical examination revealed 2 large nodules measuring 3.0×3.0 cm each and 2 smaller nodules measuring 1.0×1.0 cm each. There was no appreciable lymphadenopathy.
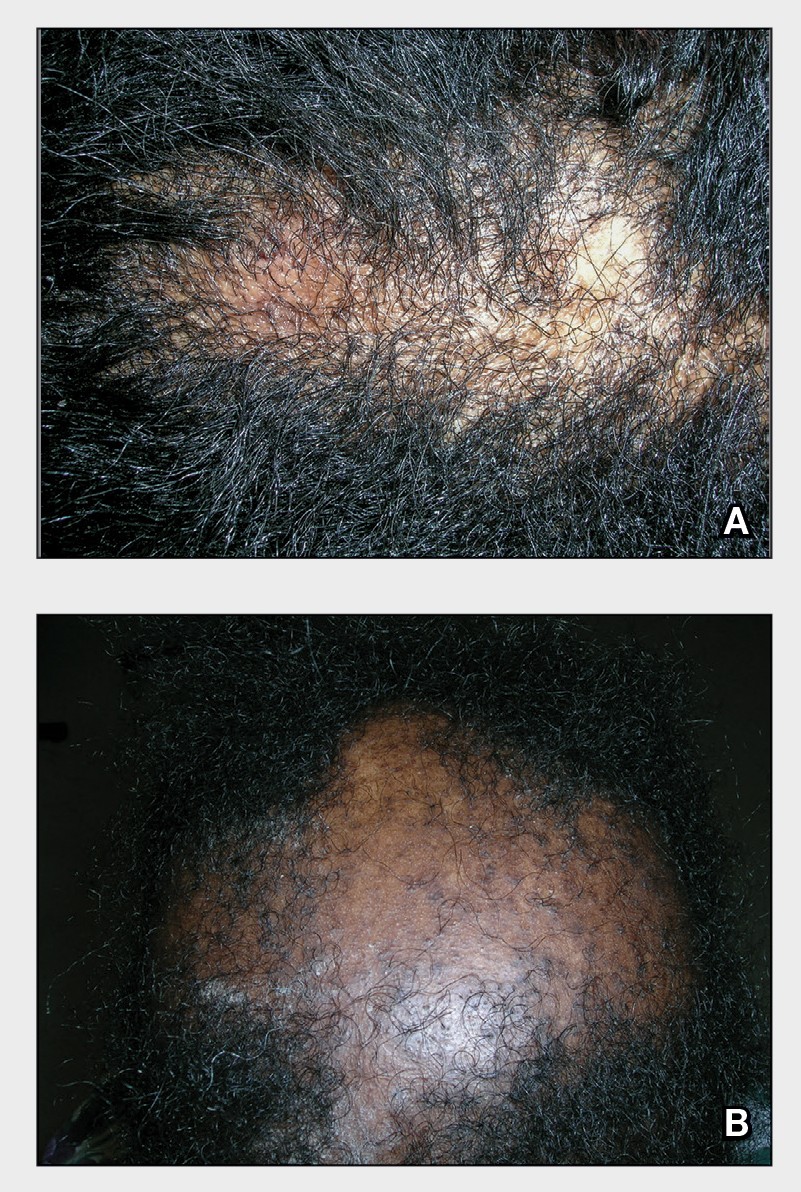
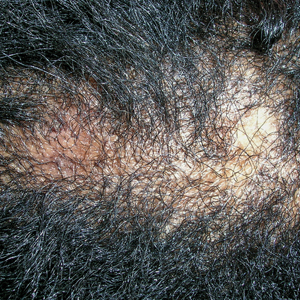
Central Centrifugal Cicatricial Alopecia
THE PRESENTATION
A Early central centrifugal cicatricial alopecia with a small central patch of hair loss in a 45-year-old Black woman.
B Late central centrifugal cicatricial alopecia with a large central patch of hair loss in a 43-year-old Black woman.
Scarring alopecia is a collection of hair loss disorders including chronic cutaneous lupus erythematosus (discoid lupus), lichen planopilaris, dissecting cellulitis, acne keloidalis, and central centrifugal cicatricial alopecia (CCCA).1 Central centrifugal cicatricial alopecia (formerly hot comb alopecia or follicular degeneration syndrome) is a progressive, scarring, inflammatory alopecia and represents the most common form of scarring alopecia in women of African descent. It results in permanent destruction of hair follicles.
Epidemiology
Central centrifugal cicatricial alopecia predominantly affects women of African descent but also may affect men. The prevalence of CCCA in those of African descent has varied in the literature. Khumalo2 reported a prevalence of 1.2% for women younger than 50 years and 6.7% in women older than 50 years. Central centrifugal cicatricial alopecia has been reported in other ethnic groups, such as those of Asian descent.3
Historically, hair care practices that are more common in those of African descent, such as high-tension hairstyles as well as heat and chemical hair relaxers, were implicated in the development of CCCA. However, the causes of CCCA are most likely multifactorial, including family history, genetic mutations, and hair care practices.4-7PADI3 mutations likely predispose some women to CCCA. Mutations in PADI3, which encodes peptidyl arginine deiminase 3 (an enzyme that modifies proteins crucial for the formation of hair shafts), were found in some patients with CCCA.8 Moreover, other genetic defects also likely play a role.7
Key clinical features
Early recognition is key for patients with CCCA.
• Central centrifugal cicatricial alopecia begins in the central scalp (crown area, vertex) and spreads centrifugally.
• Scalp symptoms such as tenderness, pain, a tingling or crawling sensation, and itching may occur.9 Some patients may not have any symptoms at all, and hair loss may progress painlessly.
• Central hair breakage—forme fruste CCCA—may be a presenting sign of CCCA.9
• Loss of follicular ostia and mottled hypopigmented and hyperpigmented macules are common findings.6
• Central centrifugal cicatricial alopecia can be diagnosed clinically and by histopathology.
Worth noting
Patients may experience hair loss and scalp symptoms for years before seeking medical evaluation. In some cultures, hair breakage or itching on the top of the scalp may be viewed as a normal occurrence in life.
It is important to set patient expectations that CCCA is a scarring alopecia, and the initial goal often is to maintain the patient's existing hair. However, hair and areas responding to treatment should still be treated. Without any intervention, the resulting scarring from CCCA may permanently scar follicles on the entire scalp.
Due to the inflammatory nature of CCCA, potent topical corticosteroids (eg, clobetasol propionate), intralesional corticosteroids (eg, triamcinolone acetonide), and oral anti-inflammatory agents (eg, doxycycline) are utilized in the treatment of CCCA. Minoxidil is another treatment option. Adjuvant therapies such as topical metformin also have been tried.10 Importantly, treatment of CCCA may halt further permanent destruction of hair follicles, but scalp symptoms may reappear periodically and require re-treatment with anti-inflammatory agents.
Health care highlight
Thorough scalp examination and awareness of clinical features of CCCA may prompt earlier diagnosis and prevent future severe permanent alopecia. Clinicians should encourage patients with suggestive signs or symptoms of CCCA to seek care from a dermatologist.
- Sperling LC. Scarring alopecia and the dermatopathologist. J Cutan Pathol. 2001;28:333-342. doi:10.1034/j.1600-0560.2001 .280701.x
- Khumalo NP. Prevalence of central centrifugal cicatricial alopecia. Arch Dermatol. 2011;147:1453-1454. doi:10.1001/archderm.147.12.1453
- Su HJ, Cheng AY, Liu CH, et al. Primary scarring alopecia: a retrospective study of 89 patients in Taiwan [published online January 16, 2018]. J Dermatol. 2018;45:450-455. doi:10.1111 /1346-8138.14217
- Sperling LC, Cowper SE. The histopathology of primary cicatricial alopecia. Semin Cutan Med Surg. 2006;25:41-50
- Dlova NC, Forder M. Central centrifugal cicatricial alopecia: possible familial aetiology in two African families from South Africa. Int J Dermatol. 2012;51(supp 1):17-20, 20-23.
- Ogunleye TA, Quinn CR, McMichael A. Alopecia. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Dermatology for Skin of Color. McGraw Hill; 2016:253-264.
- Uitto J. Genetic susceptibility to alopecia [published online February 13, 2019]. N Engl J Med. 2019;380:873-876. doi:10.1056 /NEJMe1900042
- Malki L, Sarig O, Romano MT, et al. Variant PADI3 in central centrifugal cicatricial alopecia. N Engl J Med. 2019;380:833-841.
- Callender VD, Wright DR, Davis EC, et al. Hair breakage as a presenting sign of early or occult central centrifugal cicatricial alopecia: clinicopathologic findings in 9 patients. Arch Dermatol. 2012;148:1047-1052.
- Araoye EF, Thomas JAL, Aguh CU. Hair regrowth in 2 patients with recalcitrant central centrifugal cicatricial alopecia after use of topical metformin. JAAD Case Rep. 2020;6:106-108. doi:10.1016/j .jdcr.2019.12.008
THE PRESENTATION
A Early central centrifugal cicatricial alopecia with a small central patch of hair loss in a 45-year-old Black woman.
B Late central centrifugal cicatricial alopecia with a large central patch of hair loss in a 43-year-old Black woman.
Scarring alopecia is a collection of hair loss disorders including chronic cutaneous lupus erythematosus (discoid lupus), lichen planopilaris, dissecting cellulitis, acne keloidalis, and central centrifugal cicatricial alopecia (CCCA).1 Central centrifugal cicatricial alopecia (formerly hot comb alopecia or follicular degeneration syndrome) is a progressive, scarring, inflammatory alopecia and represents the most common form of scarring alopecia in women of African descent. It results in permanent destruction of hair follicles.
Epidemiology
Central centrifugal cicatricial alopecia predominantly affects women of African descent but also may affect men. The prevalence of CCCA in those of African descent has varied in the literature. Khumalo2 reported a prevalence of 1.2% for women younger than 50 years and 6.7% in women older than 50 years. Central centrifugal cicatricial alopecia has been reported in other ethnic groups, such as those of Asian descent.3
Historically, hair care practices that are more common in those of African descent, such as high-tension hairstyles as well as heat and chemical hair relaxers, were implicated in the development of CCCA. However, the causes of CCCA are most likely multifactorial, including family history, genetic mutations, and hair care practices.4-7PADI3 mutations likely predispose some women to CCCA. Mutations in PADI3, which encodes peptidyl arginine deiminase 3 (an enzyme that modifies proteins crucial for the formation of hair shafts), were found in some patients with CCCA.8 Moreover, other genetic defects also likely play a role.7
Key clinical features
Early recognition is key for patients with CCCA.
• Central centrifugal cicatricial alopecia begins in the central scalp (crown area, vertex) and spreads centrifugally.
• Scalp symptoms such as tenderness, pain, a tingling or crawling sensation, and itching may occur.9 Some patients may not have any symptoms at all, and hair loss may progress painlessly.
• Central hair breakage—forme fruste CCCA—may be a presenting sign of CCCA.9
• Loss of follicular ostia and mottled hypopigmented and hyperpigmented macules are common findings.6
• Central centrifugal cicatricial alopecia can be diagnosed clinically and by histopathology.
Worth noting
Patients may experience hair loss and scalp symptoms for years before seeking medical evaluation. In some cultures, hair breakage or itching on the top of the scalp may be viewed as a normal occurrence in life.
It is important to set patient expectations that CCCA is a scarring alopecia, and the initial goal often is to maintain the patient's existing hair. However, hair and areas responding to treatment should still be treated. Without any intervention, the resulting scarring from CCCA may permanently scar follicles on the entire scalp.
Due to the inflammatory nature of CCCA, potent topical corticosteroids (eg, clobetasol propionate), intralesional corticosteroids (eg, triamcinolone acetonide), and oral anti-inflammatory agents (eg, doxycycline) are utilized in the treatment of CCCA. Minoxidil is another treatment option. Adjuvant therapies such as topical metformin also have been tried.10 Importantly, treatment of CCCA may halt further permanent destruction of hair follicles, but scalp symptoms may reappear periodically and require re-treatment with anti-inflammatory agents.
Health care highlight
Thorough scalp examination and awareness of clinical features of CCCA may prompt earlier diagnosis and prevent future severe permanent alopecia. Clinicians should encourage patients with suggestive signs or symptoms of CCCA to seek care from a dermatologist.
THE PRESENTATION
A Early central centrifugal cicatricial alopecia with a small central patch of hair loss in a 45-year-old Black woman.
B Late central centrifugal cicatricial alopecia with a large central patch of hair loss in a 43-year-old Black woman.
Scarring alopecia is a collection of hair loss disorders including chronic cutaneous lupus erythematosus (discoid lupus), lichen planopilaris, dissecting cellulitis, acne keloidalis, and central centrifugal cicatricial alopecia (CCCA).1 Central centrifugal cicatricial alopecia (formerly hot comb alopecia or follicular degeneration syndrome) is a progressive, scarring, inflammatory alopecia and represents the most common form of scarring alopecia in women of African descent. It results in permanent destruction of hair follicles.
Epidemiology
Central centrifugal cicatricial alopecia predominantly affects women of African descent but also may affect men. The prevalence of CCCA in those of African descent has varied in the literature. Khumalo2 reported a prevalence of 1.2% for women younger than 50 years and 6.7% in women older than 50 years. Central centrifugal cicatricial alopecia has been reported in other ethnic groups, such as those of Asian descent.3
Historically, hair care practices that are more common in those of African descent, such as high-tension hairstyles as well as heat and chemical hair relaxers, were implicated in the development of CCCA. However, the causes of CCCA are most likely multifactorial, including family history, genetic mutations, and hair care practices.4-7PADI3 mutations likely predispose some women to CCCA. Mutations in PADI3, which encodes peptidyl arginine deiminase 3 (an enzyme that modifies proteins crucial for the formation of hair shafts), were found in some patients with CCCA.8 Moreover, other genetic defects also likely play a role.7
Key clinical features
Early recognition is key for patients with CCCA.
• Central centrifugal cicatricial alopecia begins in the central scalp (crown area, vertex) and spreads centrifugally.
• Scalp symptoms such as tenderness, pain, a tingling or crawling sensation, and itching may occur.9 Some patients may not have any symptoms at all, and hair loss may progress painlessly.
• Central hair breakage—forme fruste CCCA—may be a presenting sign of CCCA.9
• Loss of follicular ostia and mottled hypopigmented and hyperpigmented macules are common findings.6
• Central centrifugal cicatricial alopecia can be diagnosed clinically and by histopathology.
Worth noting
Patients may experience hair loss and scalp symptoms for years before seeking medical evaluation. In some cultures, hair breakage or itching on the top of the scalp may be viewed as a normal occurrence in life.
It is important to set patient expectations that CCCA is a scarring alopecia, and the initial goal often is to maintain the patient's existing hair. However, hair and areas responding to treatment should still be treated. Without any intervention, the resulting scarring from CCCA may permanently scar follicles on the entire scalp.
Due to the inflammatory nature of CCCA, potent topical corticosteroids (eg, clobetasol propionate), intralesional corticosteroids (eg, triamcinolone acetonide), and oral anti-inflammatory agents (eg, doxycycline) are utilized in the treatment of CCCA. Minoxidil is another treatment option. Adjuvant therapies such as topical metformin also have been tried.10 Importantly, treatment of CCCA may halt further permanent destruction of hair follicles, but scalp symptoms may reappear periodically and require re-treatment with anti-inflammatory agents.
Health care highlight
Thorough scalp examination and awareness of clinical features of CCCA may prompt earlier diagnosis and prevent future severe permanent alopecia. Clinicians should encourage patients with suggestive signs or symptoms of CCCA to seek care from a dermatologist.
- Sperling LC. Scarring alopecia and the dermatopathologist. J Cutan Pathol. 2001;28:333-342. doi:10.1034/j.1600-0560.2001 .280701.x
- Khumalo NP. Prevalence of central centrifugal cicatricial alopecia. Arch Dermatol. 2011;147:1453-1454. doi:10.1001/archderm.147.12.1453
- Su HJ, Cheng AY, Liu CH, et al. Primary scarring alopecia: a retrospective study of 89 patients in Taiwan [published online January 16, 2018]. J Dermatol. 2018;45:450-455. doi:10.1111 /1346-8138.14217
- Sperling LC, Cowper SE. The histopathology of primary cicatricial alopecia. Semin Cutan Med Surg. 2006;25:41-50
- Dlova NC, Forder M. Central centrifugal cicatricial alopecia: possible familial aetiology in two African families from South Africa. Int J Dermatol. 2012;51(supp 1):17-20, 20-23.
- Ogunleye TA, Quinn CR, McMichael A. Alopecia. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Dermatology for Skin of Color. McGraw Hill; 2016:253-264.
- Uitto J. Genetic susceptibility to alopecia [published online February 13, 2019]. N Engl J Med. 2019;380:873-876. doi:10.1056 /NEJMe1900042
- Malki L, Sarig O, Romano MT, et al. Variant PADI3 in central centrifugal cicatricial alopecia. N Engl J Med. 2019;380:833-841.
- Callender VD, Wright DR, Davis EC, et al. Hair breakage as a presenting sign of early or occult central centrifugal cicatricial alopecia: clinicopathologic findings in 9 patients. Arch Dermatol. 2012;148:1047-1052.
- Araoye EF, Thomas JAL, Aguh CU. Hair regrowth in 2 patients with recalcitrant central centrifugal cicatricial alopecia after use of topical metformin. JAAD Case Rep. 2020;6:106-108. doi:10.1016/j .jdcr.2019.12.008
- Sperling LC. Scarring alopecia and the dermatopathologist. J Cutan Pathol. 2001;28:333-342. doi:10.1034/j.1600-0560.2001 .280701.x
- Khumalo NP. Prevalence of central centrifugal cicatricial alopecia. Arch Dermatol. 2011;147:1453-1454. doi:10.1001/archderm.147.12.1453
- Su HJ, Cheng AY, Liu CH, et al. Primary scarring alopecia: a retrospective study of 89 patients in Taiwan [published online January 16, 2018]. J Dermatol. 2018;45:450-455. doi:10.1111 /1346-8138.14217
- Sperling LC, Cowper SE. The histopathology of primary cicatricial alopecia. Semin Cutan Med Surg. 2006;25:41-50
- Dlova NC, Forder M. Central centrifugal cicatricial alopecia: possible familial aetiology in two African families from South Africa. Int J Dermatol. 2012;51(supp 1):17-20, 20-23.
- Ogunleye TA, Quinn CR, McMichael A. Alopecia. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Dermatology for Skin of Color. McGraw Hill; 2016:253-264.
- Uitto J. Genetic susceptibility to alopecia [published online February 13, 2019]. N Engl J Med. 2019;380:873-876. doi:10.1056 /NEJMe1900042
- Malki L, Sarig O, Romano MT, et al. Variant PADI3 in central centrifugal cicatricial alopecia. N Engl J Med. 2019;380:833-841.
- Callender VD, Wright DR, Davis EC, et al. Hair breakage as a presenting sign of early or occult central centrifugal cicatricial alopecia: clinicopathologic findings in 9 patients. Arch Dermatol. 2012;148:1047-1052.
- Araoye EF, Thomas JAL, Aguh CU. Hair regrowth in 2 patients with recalcitrant central centrifugal cicatricial alopecia after use of topical metformin. JAAD Case Rep. 2020;6:106-108. doi:10.1016/j .jdcr.2019.12.008
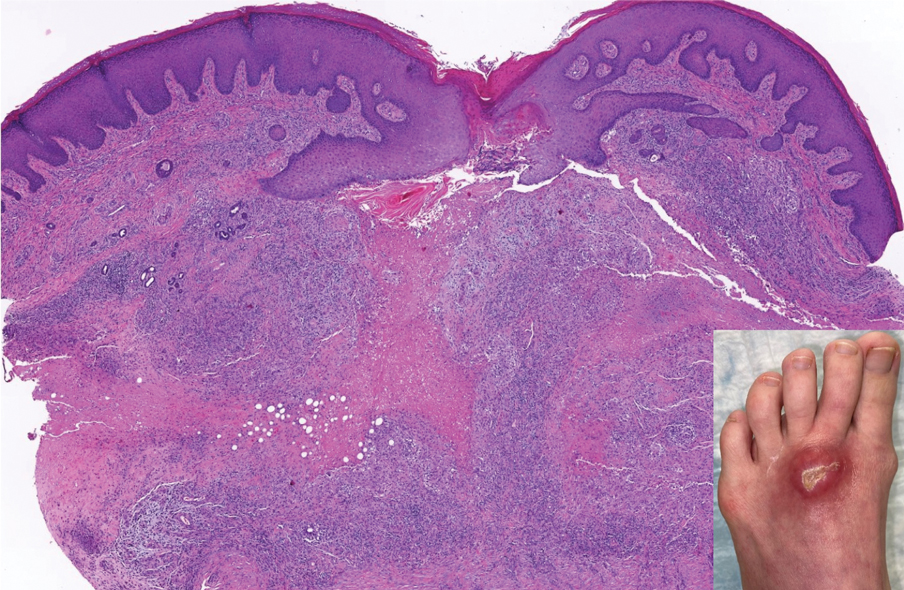
Ulcerating Nodule on the Foot
The Diagnosis: Perforating Rheumatoid Nodule
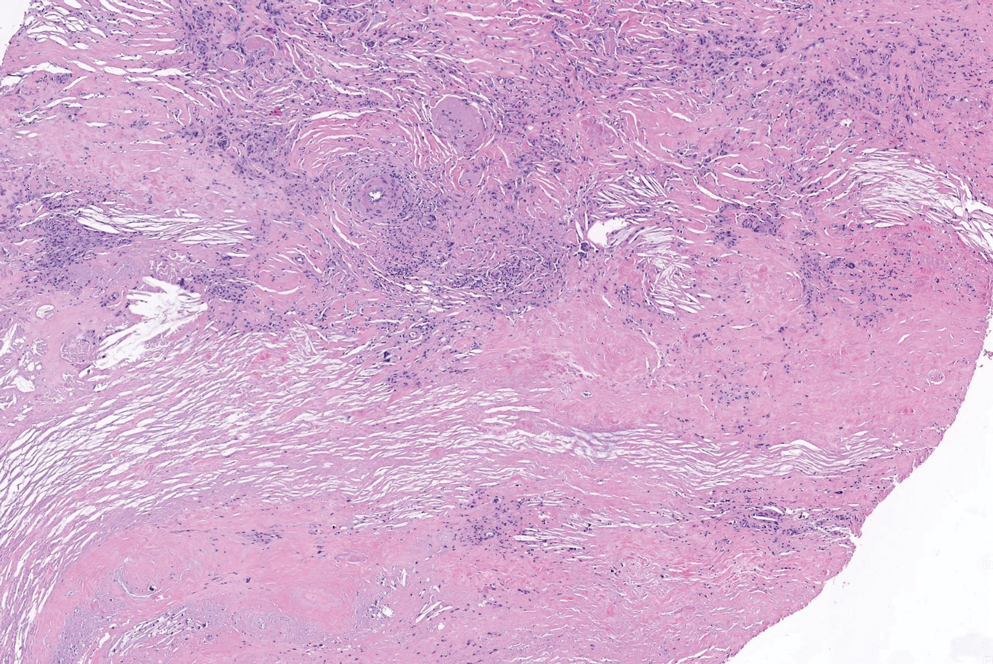
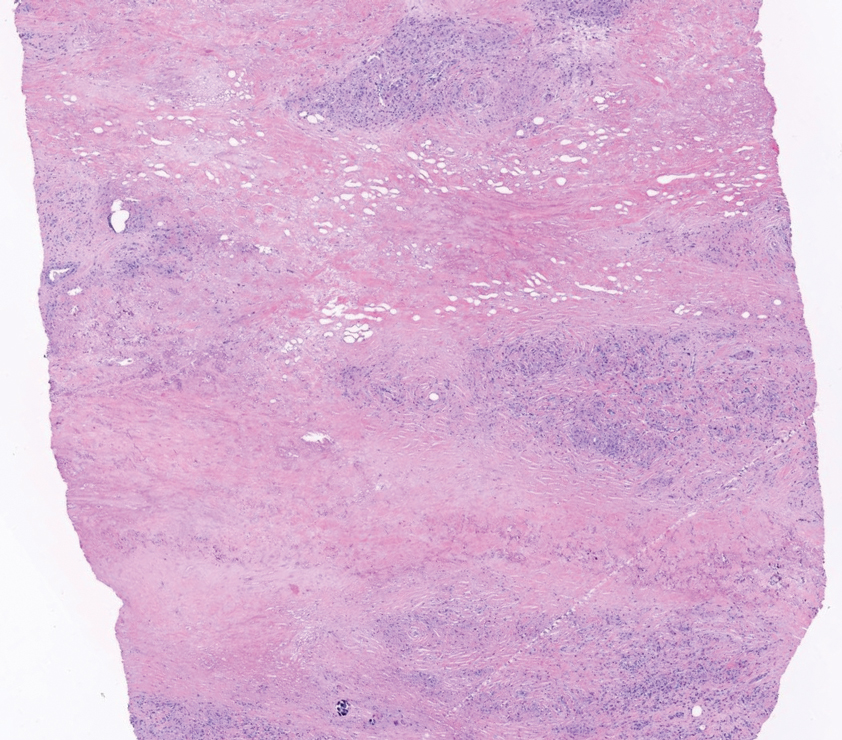
Perforating rheumatoid nodule (RN) is a variant of RN that demonstrates necrobiotic material extruding through the epidermis via the process of transepidermal elimination.1 The necrobiotic material contains fibrin and often harbors karyorrhectic debris. The pathogenesis of RN remains unclear; possible mechanisms include a small vessel vasculitis or mechanical trauma inciting a localized aggregation of inflammatory products and rheumatoid factor complexes. This induces macrophage activation, fibrin deposition, and necrosis.2 The majority of patients with RNs have detectable rheumatoid factor and anticyclic citrullinated protein in the blood.3 Rheumatoid nodules are the most common cutaneous manifestations of rheumatoid arthritis (RA) and will develop in 30% to 40% of RA patients.4,5 They typically are associated with advanced RA but may precede the onset of clinically severe RA in 5% to 10% of patients.5 Rheumatoid nodules generally range in size from 2 mm to 5 cm and are slightly more prevalent in men than in women. They present as firm painless masses typically on the extensor surfaces of the hands and olecranon process but can occur over any tendinous or ligamentlike structure.6,7 Perforating RNs are most common on areas subjected to pressure or repeated trauma, such as the sacrum.
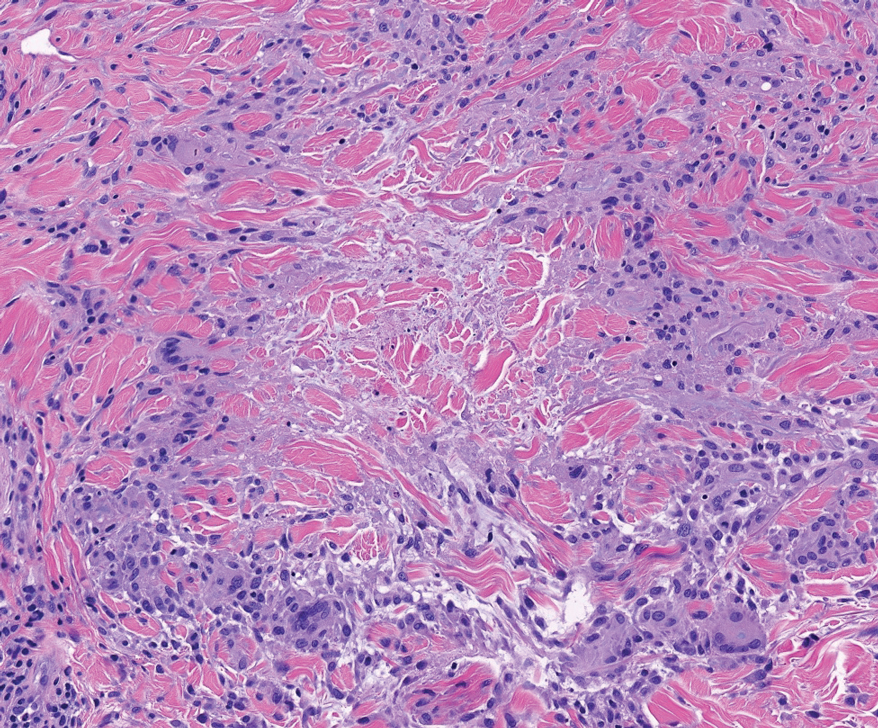
The diagnosis usually is clinical; however, in cases of diagnostic uncertainty, RN can be distinguished by its histologic appearance. Rheumatoid nodules demonstrate granulomatous palisading necrobiosis with a central zone of highly eosinophilic fibrinoid necrobiosis surrounded by palisading mononuclear cells and an outer zone of granulation tissue. There may be a mixed chronic inflammatory infiltrate predominantly composed of lymphocytes and histiocytes in the background.
Rheumatoid nodules typically do not require treatment; however, perforation is known to increase the risk for infection, and surgical excision generally is indicated for prophylaxis against infection, though nodules may recur in the excision area.1,3,8 Alternatively, disease-modifying antirheumatic drugs and intralesional corticosteroids may effectively reduce the size of RNs. The differential diagnosis for perforating RNs includes epithelioid sarcoma, perforating granuloma annulare, necrobiotic xanthogranuloma, and necrobiosis lipoidica.
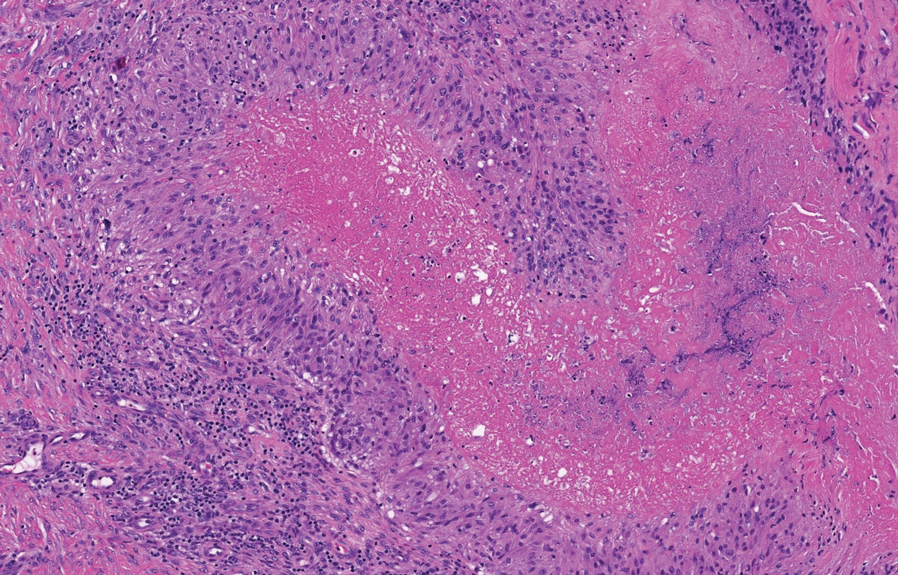
Epithelioid sarcoma is a malignant soft tissue tumor typically found on the upper extremities of adolescent or young adult males. They usually present as hard tender nodules that commonly ulcerate. Epithelioid sarcoma makes up less than 1% of soft tissue sarcomas.9 Although rare, they present a diagnostic pitfall, as the histology may mimic an inflammatory palisaded granulomatous dermatitis similar to RN and granuloma annulare, thus a high index of suspicion is required to not overlook this aggressive malignancy. Histology is typified by nodular aggregates of epithelioid cells with abundant eosinophilic cytoplasm and often with central zones of necrosis (Figure 1). Epithelioid sarcoma displays immunoreactivity to cytokeratin, CD34, and epithelial membrane antigen, but loss of integrase interactor 1 expression. Cytologic abnormalities such as pleomorphism and hyperchromatism can be helpful in distinguishing between epithelioid sarcoma and RN.
Perforating granuloma annulare is a rare subtype of granuloma annulare that presents with flesh- to red-colored papules that develop central crust or scale. Perforating granuloma annulare composes approximately 5% of granuloma annulare cases. Perforating granuloma annulare can develop on any region of the body but has an affinity for the extensor surfaces of the extremities. It most frequently occurs in young women and rarely presents as a single lesion.10 Granuloma annulare typically is not associated with joint pain, and thus it differs from most cases of RNs. Histologically, it presents with an inflammatory palisading granuloma. There may be overlying epidermal thinning or parakeratosis, which can progress to perforation and extrusion of necrobiotic material. In comparison with RN, perforating granuloma annulare displays mucin deposition in the necrobiotic zones in lieu of fibrin (Figure 2).10,11
Necrobiotic xanthogranuloma is a rare chronic form of non-Langerhans histiocytosis that characteristically presents with yellow or violaceous indurated plaques and nodules in a periorbital distribution. It often is associated with monoclonal gammopathy of IgG-κ. Lesions will ulcerate in 40% to 50% of patients.12 The mean age at presentation is in the sixth decade of life, and it is moderately predominant in females.13 Histopathology demonstrates palisading granulomatous formations with a lymphoplasmacytic infiltrate and zones of necrobiosis in the mid dermis extending into the panniculus. Characteristic histologic features that are variably present in necrobiotic xanthogranuloma but typically absent in RN include neutrophilic debris, cholesterol clefts, and Touton or foreign body giant cells (Figure 3).13
Necrobiosis lipoidica is a rare chronic granulomatous disease characterized by well-demarcated, atrophic, yellow-brown plaques on the pretibial surfaces. It typically presents in the third decade of life in women, and most cases are associated with diabetes mellitus types 1 or 2 or autoimmune conditions.14 Necrobiosis lipoidica begins as asymptomatic papules that enlarge progressively over months to years. They can become pruritic or painful and often develop ulceration. Histopathology shows horizontal zones of palisading histiocytes with intervening necrobiosis. An inflammatory infiltrate containing plasma cells also may be present (Figure 4).
- Horn RT Jr, Goette DK. Perforating rheumatoid nodule. Arch Dermatol. 1982;118:696-697.
- Tilstra JS, Lienesch DW. Rheumatoid nodules. Dermatol Clin. 2015;33:361-371. doi:10.1016/j.det.2015.03.004
- Kaye BR, Kaye RL, Bobrove A. Rheumatoid nodules. review of the spectrum of associated conditions and proposal of a new classification, with a report of four seronegative cases. Am J Med. 1984;76:279-292. doi:10.1016/0002-9343(84)90787-3
- Nyhäll-Wåhlin BM, Jacobsson LT, Petersson IF, et al; BARFOT study group. Smoking is a strong risk factor for rheumatoid nodules in early rheumatoid arthritis. Ann Rheum Dis. 2006;65:601-606. doi:10.1136/ard.2005.039172
- Turesson C, O’Fallon WM, Crowson CS, et al. Occurrence of extraarticular disease manifestations is associated with excess mortality in a community-based cohort of patients with rheumatoid arthritis. J Rheumatol. 2002;29:62-67.
- Bang S, Kim Y, Jang K, et al. Clinicopathologic features of rheumatoid nodules: a retrospective analysis. Clin Rheumatol. 2019;38:3041-3048. doi:10.1007/s10067-019-04668-1
- Chaganti S, Joshy S, Hariharan K, et al. Rheumatoid nodule presenting as Morton’s neuroma. J Orthop Traumatol. 2013;14:219-222. doi:10.1007/s10195-012-0215-x
- Sayah A, English JC 3rd. Rheumatoid arthritis: a review of the cutaneous manifestations. J Am Acad Dermatol. 2005;53:191-209; quiz 210-212. doi:10.1016/j.jaad.2004.07.023
- de Visscher SA, van Ginkel RJ, Wobbes T, et al. Epithelioid sarcoma: still an only surgically curable disease. Cancer. 2006;107:606-612. doi:10.1002/cncr.22037
- Penas PF, Jones-Caballero M, Fraga J, et al. Perforating granuloma annulare. Int J Dermatol. 1997;36:340-348. doi:10.1046 /j.1365-4362.1997.00047.x
- Gale M, Gilbert E, Blumenthal D. Isolated rheumatoid nodules: a diagnostic dilemma. Case Rep Med. 2015;2015:352352. doi:10.1155/2015/352352
- Wood AJ, Wagner MV, Abbott JJ, et al. Necrobiotic xanthogranuloma: a review of 17 cases with emphasis on clinical and pathologic correlation. Arch Dermatol. 2009;145:279-284. doi:10.1001 /archdermatol.2008.583
- Nelson CA, Zhong CS, Hashemi DA, et al. A multicenter crosssectional study and systematic review of necrobiotic xanthogranuloma with proposed diagnostic criteria. JAMA Dermatol. 2020;156:270-279. doi:10.1001/jamadermatol.2019.4221
- Sibbald C, Reid S, Alavi A. Necrobiosis lipoidica. Dermatol Clin. 2015;33:343-360. doi:10.1016/j.det.2015.03.003
The Diagnosis: Perforating Rheumatoid Nodule
Perforating rheumatoid nodule (RN) is a variant of RN that demonstrates necrobiotic material extruding through the epidermis via the process of transepidermal elimination.1 The necrobiotic material contains fibrin and often harbors karyorrhectic debris. The pathogenesis of RN remains unclear; possible mechanisms include a small vessel vasculitis or mechanical trauma inciting a localized aggregation of inflammatory products and rheumatoid factor complexes. This induces macrophage activation, fibrin deposition, and necrosis.2 The majority of patients with RNs have detectable rheumatoid factor and anticyclic citrullinated protein in the blood.3 Rheumatoid nodules are the most common cutaneous manifestations of rheumatoid arthritis (RA) and will develop in 30% to 40% of RA patients.4,5 They typically are associated with advanced RA but may precede the onset of clinically severe RA in 5% to 10% of patients.5 Rheumatoid nodules generally range in size from 2 mm to 5 cm and are slightly more prevalent in men than in women. They present as firm painless masses typically on the extensor surfaces of the hands and olecranon process but can occur over any tendinous or ligamentlike structure.6,7 Perforating RNs are most common on areas subjected to pressure or repeated trauma, such as the sacrum.
The diagnosis usually is clinical; however, in cases of diagnostic uncertainty, RN can be distinguished by its histologic appearance. Rheumatoid nodules demonstrate granulomatous palisading necrobiosis with a central zone of highly eosinophilic fibrinoid necrobiosis surrounded by palisading mononuclear cells and an outer zone of granulation tissue. There may be a mixed chronic inflammatory infiltrate predominantly composed of lymphocytes and histiocytes in the background.
Rheumatoid nodules typically do not require treatment; however, perforation is known to increase the risk for infection, and surgical excision generally is indicated for prophylaxis against infection, though nodules may recur in the excision area.1,3,8 Alternatively, disease-modifying antirheumatic drugs and intralesional corticosteroids may effectively reduce the size of RNs. The differential diagnosis for perforating RNs includes epithelioid sarcoma, perforating granuloma annulare, necrobiotic xanthogranuloma, and necrobiosis lipoidica.
Epithelioid sarcoma is a malignant soft tissue tumor typically found on the upper extremities of adolescent or young adult males. They usually present as hard tender nodules that commonly ulcerate. Epithelioid sarcoma makes up less than 1% of soft tissue sarcomas.9 Although rare, they present a diagnostic pitfall, as the histology may mimic an inflammatory palisaded granulomatous dermatitis similar to RN and granuloma annulare, thus a high index of suspicion is required to not overlook this aggressive malignancy. Histology is typified by nodular aggregates of epithelioid cells with abundant eosinophilic cytoplasm and often with central zones of necrosis (Figure 1). Epithelioid sarcoma displays immunoreactivity to cytokeratin, CD34, and epithelial membrane antigen, but loss of integrase interactor 1 expression. Cytologic abnormalities such as pleomorphism and hyperchromatism can be helpful in distinguishing between epithelioid sarcoma and RN.
Perforating granuloma annulare is a rare subtype of granuloma annulare that presents with flesh- to red-colored papules that develop central crust or scale. Perforating granuloma annulare composes approximately 5% of granuloma annulare cases. Perforating granuloma annulare can develop on any region of the body but has an affinity for the extensor surfaces of the extremities. It most frequently occurs in young women and rarely presents as a single lesion.10 Granuloma annulare typically is not associated with joint pain, and thus it differs from most cases of RNs. Histologically, it presents with an inflammatory palisading granuloma. There may be overlying epidermal thinning or parakeratosis, which can progress to perforation and extrusion of necrobiotic material. In comparison with RN, perforating granuloma annulare displays mucin deposition in the necrobiotic zones in lieu of fibrin (Figure 2).10,11
Necrobiotic xanthogranuloma is a rare chronic form of non-Langerhans histiocytosis that characteristically presents with yellow or violaceous indurated plaques and nodules in a periorbital distribution. It often is associated with monoclonal gammopathy of IgG-κ. Lesions will ulcerate in 40% to 50% of patients.12 The mean age at presentation is in the sixth decade of life, and it is moderately predominant in females.13 Histopathology demonstrates palisading granulomatous formations with a lymphoplasmacytic infiltrate and zones of necrobiosis in the mid dermis extending into the panniculus. Characteristic histologic features that are variably present in necrobiotic xanthogranuloma but typically absent in RN include neutrophilic debris, cholesterol clefts, and Touton or foreign body giant cells (Figure 3).13
Necrobiosis lipoidica is a rare chronic granulomatous disease characterized by well-demarcated, atrophic, yellow-brown plaques on the pretibial surfaces. It typically presents in the third decade of life in women, and most cases are associated with diabetes mellitus types 1 or 2 or autoimmune conditions.14 Necrobiosis lipoidica begins as asymptomatic papules that enlarge progressively over months to years. They can become pruritic or painful and often develop ulceration. Histopathology shows horizontal zones of palisading histiocytes with intervening necrobiosis. An inflammatory infiltrate containing plasma cells also may be present (Figure 4).
The Diagnosis: Perforating Rheumatoid Nodule
Perforating rheumatoid nodule (RN) is a variant of RN that demonstrates necrobiotic material extruding through the epidermis via the process of transepidermal elimination.1 The necrobiotic material contains fibrin and often harbors karyorrhectic debris. The pathogenesis of RN remains unclear; possible mechanisms include a small vessel vasculitis or mechanical trauma inciting a localized aggregation of inflammatory products and rheumatoid factor complexes. This induces macrophage activation, fibrin deposition, and necrosis.2 The majority of patients with RNs have detectable rheumatoid factor and anticyclic citrullinated protein in the blood.3 Rheumatoid nodules are the most common cutaneous manifestations of rheumatoid arthritis (RA) and will develop in 30% to 40% of RA patients.4,5 They typically are associated with advanced RA but may precede the onset of clinically severe RA in 5% to 10% of patients.5 Rheumatoid nodules generally range in size from 2 mm to 5 cm and are slightly more prevalent in men than in women. They present as firm painless masses typically on the extensor surfaces of the hands and olecranon process but can occur over any tendinous or ligamentlike structure.6,7 Perforating RNs are most common on areas subjected to pressure or repeated trauma, such as the sacrum.
The diagnosis usually is clinical; however, in cases of diagnostic uncertainty, RN can be distinguished by its histologic appearance. Rheumatoid nodules demonstrate granulomatous palisading necrobiosis with a central zone of highly eosinophilic fibrinoid necrobiosis surrounded by palisading mononuclear cells and an outer zone of granulation tissue. There may be a mixed chronic inflammatory infiltrate predominantly composed of lymphocytes and histiocytes in the background.
Rheumatoid nodules typically do not require treatment; however, perforation is known to increase the risk for infection, and surgical excision generally is indicated for prophylaxis against infection, though nodules may recur in the excision area.1,3,8 Alternatively, disease-modifying antirheumatic drugs and intralesional corticosteroids may effectively reduce the size of RNs. The differential diagnosis for perforating RNs includes epithelioid sarcoma, perforating granuloma annulare, necrobiotic xanthogranuloma, and necrobiosis lipoidica.
Epithelioid sarcoma is a malignant soft tissue tumor typically found on the upper extremities of adolescent or young adult males. They usually present as hard tender nodules that commonly ulcerate. Epithelioid sarcoma makes up less than 1% of soft tissue sarcomas.9 Although rare, they present a diagnostic pitfall, as the histology may mimic an inflammatory palisaded granulomatous dermatitis similar to RN and granuloma annulare, thus a high index of suspicion is required to not overlook this aggressive malignancy. Histology is typified by nodular aggregates of epithelioid cells with abundant eosinophilic cytoplasm and often with central zones of necrosis (Figure 1). Epithelioid sarcoma displays immunoreactivity to cytokeratin, CD34, and epithelial membrane antigen, but loss of integrase interactor 1 expression. Cytologic abnormalities such as pleomorphism and hyperchromatism can be helpful in distinguishing between epithelioid sarcoma and RN.
Perforating granuloma annulare is a rare subtype of granuloma annulare that presents with flesh- to red-colored papules that develop central crust or scale. Perforating granuloma annulare composes approximately 5% of granuloma annulare cases. Perforating granuloma annulare can develop on any region of the body but has an affinity for the extensor surfaces of the extremities. It most frequently occurs in young women and rarely presents as a single lesion.10 Granuloma annulare typically is not associated with joint pain, and thus it differs from most cases of RNs. Histologically, it presents with an inflammatory palisading granuloma. There may be overlying epidermal thinning or parakeratosis, which can progress to perforation and extrusion of necrobiotic material. In comparison with RN, perforating granuloma annulare displays mucin deposition in the necrobiotic zones in lieu of fibrin (Figure 2).10,11
Necrobiotic xanthogranuloma is a rare chronic form of non-Langerhans histiocytosis that characteristically presents with yellow or violaceous indurated plaques and nodules in a periorbital distribution. It often is associated with monoclonal gammopathy of IgG-κ. Lesions will ulcerate in 40% to 50% of patients.12 The mean age at presentation is in the sixth decade of life, and it is moderately predominant in females.13 Histopathology demonstrates palisading granulomatous formations with a lymphoplasmacytic infiltrate and zones of necrobiosis in the mid dermis extending into the panniculus. Characteristic histologic features that are variably present in necrobiotic xanthogranuloma but typically absent in RN include neutrophilic debris, cholesterol clefts, and Touton or foreign body giant cells (Figure 3).13
Necrobiosis lipoidica is a rare chronic granulomatous disease characterized by well-demarcated, atrophic, yellow-brown plaques on the pretibial surfaces. It typically presents in the third decade of life in women, and most cases are associated with diabetes mellitus types 1 or 2 or autoimmune conditions.14 Necrobiosis lipoidica begins as asymptomatic papules that enlarge progressively over months to years. They can become pruritic or painful and often develop ulceration. Histopathology shows horizontal zones of palisading histiocytes with intervening necrobiosis. An inflammatory infiltrate containing plasma cells also may be present (Figure 4).
- Horn RT Jr, Goette DK. Perforating rheumatoid nodule. Arch Dermatol. 1982;118:696-697.
- Tilstra JS, Lienesch DW. Rheumatoid nodules. Dermatol Clin. 2015;33:361-371. doi:10.1016/j.det.2015.03.004
- Kaye BR, Kaye RL, Bobrove A. Rheumatoid nodules. review of the spectrum of associated conditions and proposal of a new classification, with a report of four seronegative cases. Am J Med. 1984;76:279-292. doi:10.1016/0002-9343(84)90787-3
- Nyhäll-Wåhlin BM, Jacobsson LT, Petersson IF, et al; BARFOT study group. Smoking is a strong risk factor for rheumatoid nodules in early rheumatoid arthritis. Ann Rheum Dis. 2006;65:601-606. doi:10.1136/ard.2005.039172
- Turesson C, O’Fallon WM, Crowson CS, et al. Occurrence of extraarticular disease manifestations is associated with excess mortality in a community-based cohort of patients with rheumatoid arthritis. J Rheumatol. 2002;29:62-67.
- Bang S, Kim Y, Jang K, et al. Clinicopathologic features of rheumatoid nodules: a retrospective analysis. Clin Rheumatol. 2019;38:3041-3048. doi:10.1007/s10067-019-04668-1
- Chaganti S, Joshy S, Hariharan K, et al. Rheumatoid nodule presenting as Morton’s neuroma. J Orthop Traumatol. 2013;14:219-222. doi:10.1007/s10195-012-0215-x
- Sayah A, English JC 3rd. Rheumatoid arthritis: a review of the cutaneous manifestations. J Am Acad Dermatol. 2005;53:191-209; quiz 210-212. doi:10.1016/j.jaad.2004.07.023
- de Visscher SA, van Ginkel RJ, Wobbes T, et al. Epithelioid sarcoma: still an only surgically curable disease. Cancer. 2006;107:606-612. doi:10.1002/cncr.22037
- Penas PF, Jones-Caballero M, Fraga J, et al. Perforating granuloma annulare. Int J Dermatol. 1997;36:340-348. doi:10.1046 /j.1365-4362.1997.00047.x
- Gale M, Gilbert E, Blumenthal D. Isolated rheumatoid nodules: a diagnostic dilemma. Case Rep Med. 2015;2015:352352. doi:10.1155/2015/352352
- Wood AJ, Wagner MV, Abbott JJ, et al. Necrobiotic xanthogranuloma: a review of 17 cases with emphasis on clinical and pathologic correlation. Arch Dermatol. 2009;145:279-284. doi:10.1001 /archdermatol.2008.583
- Nelson CA, Zhong CS, Hashemi DA, et al. A multicenter crosssectional study and systematic review of necrobiotic xanthogranuloma with proposed diagnostic criteria. JAMA Dermatol. 2020;156:270-279. doi:10.1001/jamadermatol.2019.4221
- Sibbald C, Reid S, Alavi A. Necrobiosis lipoidica. Dermatol Clin. 2015;33:343-360. doi:10.1016/j.det.2015.03.003
- Horn RT Jr, Goette DK. Perforating rheumatoid nodule. Arch Dermatol. 1982;118:696-697.
- Tilstra JS, Lienesch DW. Rheumatoid nodules. Dermatol Clin. 2015;33:361-371. doi:10.1016/j.det.2015.03.004
- Kaye BR, Kaye RL, Bobrove A. Rheumatoid nodules. review of the spectrum of associated conditions and proposal of a new classification, with a report of four seronegative cases. Am J Med. 1984;76:279-292. doi:10.1016/0002-9343(84)90787-3
- Nyhäll-Wåhlin BM, Jacobsson LT, Petersson IF, et al; BARFOT study group. Smoking is a strong risk factor for rheumatoid nodules in early rheumatoid arthritis. Ann Rheum Dis. 2006;65:601-606. doi:10.1136/ard.2005.039172
- Turesson C, O’Fallon WM, Crowson CS, et al. Occurrence of extraarticular disease manifestations is associated with excess mortality in a community-based cohort of patients with rheumatoid arthritis. J Rheumatol. 2002;29:62-67.
- Bang S, Kim Y, Jang K, et al. Clinicopathologic features of rheumatoid nodules: a retrospective analysis. Clin Rheumatol. 2019;38:3041-3048. doi:10.1007/s10067-019-04668-1
- Chaganti S, Joshy S, Hariharan K, et al. Rheumatoid nodule presenting as Morton’s neuroma. J Orthop Traumatol. 2013;14:219-222. doi:10.1007/s10195-012-0215-x
- Sayah A, English JC 3rd. Rheumatoid arthritis: a review of the cutaneous manifestations. J Am Acad Dermatol. 2005;53:191-209; quiz 210-212. doi:10.1016/j.jaad.2004.07.023
- de Visscher SA, van Ginkel RJ, Wobbes T, et al. Epithelioid sarcoma: still an only surgically curable disease. Cancer. 2006;107:606-612. doi:10.1002/cncr.22037
- Penas PF, Jones-Caballero M, Fraga J, et al. Perforating granuloma annulare. Int J Dermatol. 1997;36:340-348. doi:10.1046 /j.1365-4362.1997.00047.x
- Gale M, Gilbert E, Blumenthal D. Isolated rheumatoid nodules: a diagnostic dilemma. Case Rep Med. 2015;2015:352352. doi:10.1155/2015/352352
- Wood AJ, Wagner MV, Abbott JJ, et al. Necrobiotic xanthogranuloma: a review of 17 cases with emphasis on clinical and pathologic correlation. Arch Dermatol. 2009;145:279-284. doi:10.1001 /archdermatol.2008.583
- Nelson CA, Zhong CS, Hashemi DA, et al. A multicenter crosssectional study and systematic review of necrobiotic xanthogranuloma with proposed diagnostic criteria. JAMA Dermatol. 2020;156:270-279. doi:10.1001/jamadermatol.2019.4221
- Sibbald C, Reid S, Alavi A. Necrobiosis lipoidica. Dermatol Clin. 2015;33:343-360. doi:10.1016/j.det.2015.03.003
A 59-year-old woman with a history of joint pain presented with a foot nodule that developed over the course of 2 years. Physical examination revealed a firm, mobile, mildly tender, 3-cm, deep red nodule on the dorsal aspect of the left foot (top [inset]) with an overlying central epidermal defect and thick keratinaceous debris. The remainder of the physical examination was unremarkable. Empiric treatments with oral antibiotics and intralesional corticosteroids were unsuccessful. Incisional biopsy was performed for histologic review, and tissue culture studies were negative.
Developing and Measuring Effectiveness of a Distance Learning Dermatology Course: A Prospective Observational Study
Medical education has seen major changes over the last decade. The allotted time for preclinical education has decreased from 24 months to 18 months or less at most institutions, with an increased focus on content associated with health care delivery and health system science.1,2 Many schools now include at least some blended learning with online delivery of preclinical education.3 On the other hand, the clinical portion of medical education has remained largely unchanged prior to the COVID-19 pandemic, with the apprenticeship framework allowing the experienced physician to observe, mentor, and pass on practical knowledge so that the apprentice can one day gain independence after demonstrating adequate proficiency.4
With respect to dermatology education, skin disorders are in the top 5 reported reasons for visits to primary care5; however, a 2009 survey found that only 0.24% to 0.30% of medical schools’ curricula are spent on dermatology.6 Moreover, one institution found that fourth-year medical students received an average of 46.6% on a 15-item quiz designed to assess the ability to diagnose and treat common dermatologic conditions, and within that same cohort, 87.6% of students felt that they received inadequate training in dermatology during medical school.7
COVID-19 caused an unprecedented paradigm shift when medical schools throughout the country, including our own, canceled clinical rotations at the end of March 2020 to protect students and control the spread of infection. To enable clinical and preclinical learning to continue, institutions around the globe turned to either online learning or participation in telehealth as a substitute for clinical rotations.8-10 At the Uniformed Services University of the Health Sciences (Bethesda, Maryland), one of the many online clinical courses offered included a distance learning (DL) dermatology course. Herein, we describe the results of a prospective study evaluating short-term information recall and comprehension as well as students’ confidence in their ability to apply course objectives over 3 months of an online DL dermatology course.
Methods
Between April and July 2020, 14 students at the Uniformed Services University of the Health Sciences (Table 1) enrolled in 1 of 3 four-week DL dermatology classes. The students independently completed the Basic Dermatology Curriculum, a set of online modules with demonstrated efficacy from the American Academy of Dermatology, over 4 weeks.11 Additionally, students were instructed to review an hour of clinical dermatology images daily from online dermatology atlases and e-books accessed through our medical school’s virtual library. Optional Free Open Access Meducation resources also were provided. The course syllabus provided the students with clear expectations, links to the resources, and a recommended daily schedule.
An online video conferencing platform was utilized for an orientation session and 4 subsequent weekly 1.5-hour virtual meetings. The weekly DL meetings focused on a discussion of clinical images pertinent to the American Academy of Dermatology modules covered for the week. These interactive analytic sessions were referred to as Clinpic sessions. With instructor guidance, the students learned to describe images, and they provided differential diagnoses, workup, and treatments for various skin diseases. The virtual meetings included supplemental lectures detailing the use of teledermatology and laser therapy in the Military Health System and a journal review on the cutaneous manifestations of COVID-19.
A 40-question, image-based pretest and posttest utilized during clinical rotations evaluated knowledge recall and comprehension. A precourse and postcourse survey using a 5-point Likert scale (1=not confident; 5=extremely confident) assessed students’ confidence levels across course objectives: general knowledge of dermatology, working knowledge of teledermatology, ability to accurately describe skin lesions, generate sound differential diagnoses, and formulate a reasonable treatment plan. Statistical analysis was performed using free online statistical software at statskingdom.com.12
Results
All 14 student enrollees completed the precourse and postcourse tests and surveys. Pretest and posttest scores followed a normal distribution and therefore met criteria for utilization of a parametric test. The precourse test average of 67% (range, 40%–90%) improved to 84% postcourse (range, 70%–98%; P<.001; 95% CI, 11-23 by paired t test). Not surprisingly, the 2 students who had completed a dermatology rotation had higher average pretest and posttest scores (pretest, 87%; posttest, 94%). Students’ confidence with the course objectives were mostly at the somewhat confident level on the 5-point Likert scale precourse survey. By the end of the course, student survey responses increased to confident and very confident levels, corresponding to an overall improvement of 1.3 points (P<.001 by paired t test)(Table 2) when the mean of the survey results was aggregated across every question. Instructor evaluation of student performance mirrored student assessments.
Comment
The DL dermatology course succeeded in helping the enrolled students attain course objectives and offered a reasonable solution when in-person interaction was restricted. The students in the DL course made notable improvements in their dermatology knowledge and improved their communication, diagnosis, and management skills. Although a blended dermatology curriculum with e-learning combined with clinical experience has been shown to increase knowledge acquisition,13,14 our results suggest that an online-only program also can increase comprehension as well as students’ confidence in their abilities.
A major challenge for the DL course was the lack of opportunity to perform common dermatology procedures. The addition of a hands-on skin procedure module would have been a great supplement to the course but was not possible due to social distancing guidelines during the COVID-19 pandemic. The small sample size and voluntary enrollment were limitations to this study.
Conclusion
Although the traditional dermatology rotation remains the gold standard for clinical instruction, a well-organized DL teaching environment allowed for a more controlled learning experience with a broader coverage of topics to include potentially greater exposure to rare skin disorders not typically encountered in everyday practice. A DL dermatology course may serve as an enduring curriculum for those who wish to learn dermatology more broadly and are not interested in performing skin procedures or direct patient exposure (eg, those pursuing non–primary care specialties, pathology, or radiology). It also may be attractive to students who have had a prior clinical dermatology rotation and desire a different learning experience with a wide coverage of topics.
Acknowledgments—The authors thank Thomas Darling, MD, PhD (Bethesda, Maryland), for coining the term Clinpic and providing critical feedback throughout the course. The authors also thank Sorana Raiciulescu, MS (Bethesda, Maryland), for assistance with the statistical analysis.
- Emanuel EJ. The inevitable reimagining of medical education. JAMA. 2020;323:1127-1128.
- Skochelak SE, Stack SJ. Creating the medical schools of the future. Acad Med. 2017;92:16-19.
- Vallée A, Blacher J, Cariou A, et al. Blended learning compared to traditional learning in medical education: systematic review and meta-analysis. J Med Internet Res. 2020;22:E16504.
- Rangachari D, Brown LE, Kern DE, et al. Clinical coaching: evolving the apprenticeship model for modern housestaff. Med Teach. 2017;39:780-782.
- Finley CR, Chan DS, Garrison S, et al. What are the most common conditions in primary care? Can Fam Physician. 2018;64:832-840.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.e4.
- Ulman CA, Binder SB, Borges NJ. Assessment of medical students’ proficiency in dermatology: are medical students adequately prepared to diagnose and treat common dermatologic conditions in the United States? J Educ Eval Health Prof. 2015;12:18.
- Loh TY, Hsiao JL, Shi VY. COVID-19 and its effect on medical student education in dermatology. J Am Acad Dermatol. 2020;83:E163-E164.
- Hilburg R, Patel N, Ambruso S, et al. Medical education during the coronavirus disease-2019 pandemic: learning from a distance. Adv Chronic Kidney Dis. 2020;27:412-417.
- Rose S. Medical student education in the time of COVID-19. JAMA. 2020;323:2131-2132.
- McCleskey PE. Clinic teaching made easy: a prospective study of the American Academy of Dermatology core curriculum in primary care learners. J Am Acad Dermatol. 2013;69:273-279.e1.
- Paired T Test calculator. Statistics Kingdom website. Accessed February 7, 2022. http://www.statskingdom.com/160MeanT2pair.html
- Fransen F, Martens H, Nagtzaam I, et al. Use of e-learning in clinical clerkships: effects on acquisition of dermatological knowledge and learning processes. Int J Med Educ. 2018;9:11-17.
- Silva CS, Souza MB, Silva Filho RS, et al. E-learning program for medical students in dermatology. Clinics. 2011;66:619-622.
Medical education has seen major changes over the last decade. The allotted time for preclinical education has decreased from 24 months to 18 months or less at most institutions, with an increased focus on content associated with health care delivery and health system science.1,2 Many schools now include at least some blended learning with online delivery of preclinical education.3 On the other hand, the clinical portion of medical education has remained largely unchanged prior to the COVID-19 pandemic, with the apprenticeship framework allowing the experienced physician to observe, mentor, and pass on practical knowledge so that the apprentice can one day gain independence after demonstrating adequate proficiency.4
With respect to dermatology education, skin disorders are in the top 5 reported reasons for visits to primary care5; however, a 2009 survey found that only 0.24% to 0.30% of medical schools’ curricula are spent on dermatology.6 Moreover, one institution found that fourth-year medical students received an average of 46.6% on a 15-item quiz designed to assess the ability to diagnose and treat common dermatologic conditions, and within that same cohort, 87.6% of students felt that they received inadequate training in dermatology during medical school.7
COVID-19 caused an unprecedented paradigm shift when medical schools throughout the country, including our own, canceled clinical rotations at the end of March 2020 to protect students and control the spread of infection. To enable clinical and preclinical learning to continue, institutions around the globe turned to either online learning or participation in telehealth as a substitute for clinical rotations.8-10 At the Uniformed Services University of the Health Sciences (Bethesda, Maryland), one of the many online clinical courses offered included a distance learning (DL) dermatology course. Herein, we describe the results of a prospective study evaluating short-term information recall and comprehension as well as students’ confidence in their ability to apply course objectives over 3 months of an online DL dermatology course.
Methods
Between April and July 2020, 14 students at the Uniformed Services University of the Health Sciences (Table 1) enrolled in 1 of 3 four-week DL dermatology classes. The students independently completed the Basic Dermatology Curriculum, a set of online modules with demonstrated efficacy from the American Academy of Dermatology, over 4 weeks.11 Additionally, students were instructed to review an hour of clinical dermatology images daily from online dermatology atlases and e-books accessed through our medical school’s virtual library. Optional Free Open Access Meducation resources also were provided. The course syllabus provided the students with clear expectations, links to the resources, and a recommended daily schedule.
An online video conferencing platform was utilized for an orientation session and 4 subsequent weekly 1.5-hour virtual meetings. The weekly DL meetings focused on a discussion of clinical images pertinent to the American Academy of Dermatology modules covered for the week. These interactive analytic sessions were referred to as Clinpic sessions. With instructor guidance, the students learned to describe images, and they provided differential diagnoses, workup, and treatments for various skin diseases. The virtual meetings included supplemental lectures detailing the use of teledermatology and laser therapy in the Military Health System and a journal review on the cutaneous manifestations of COVID-19.
A 40-question, image-based pretest and posttest utilized during clinical rotations evaluated knowledge recall and comprehension. A precourse and postcourse survey using a 5-point Likert scale (1=not confident; 5=extremely confident) assessed students’ confidence levels across course objectives: general knowledge of dermatology, working knowledge of teledermatology, ability to accurately describe skin lesions, generate sound differential diagnoses, and formulate a reasonable treatment plan. Statistical analysis was performed using free online statistical software at statskingdom.com.12
Results
All 14 student enrollees completed the precourse and postcourse tests and surveys. Pretest and posttest scores followed a normal distribution and therefore met criteria for utilization of a parametric test. The precourse test average of 67% (range, 40%–90%) improved to 84% postcourse (range, 70%–98%; P<.001; 95% CI, 11-23 by paired t test). Not surprisingly, the 2 students who had completed a dermatology rotation had higher average pretest and posttest scores (pretest, 87%; posttest, 94%). Students’ confidence with the course objectives were mostly at the somewhat confident level on the 5-point Likert scale precourse survey. By the end of the course, student survey responses increased to confident and very confident levels, corresponding to an overall improvement of 1.3 points (P<.001 by paired t test)(Table 2) when the mean of the survey results was aggregated across every question. Instructor evaluation of student performance mirrored student assessments.
Comment
The DL dermatology course succeeded in helping the enrolled students attain course objectives and offered a reasonable solution when in-person interaction was restricted. The students in the DL course made notable improvements in their dermatology knowledge and improved their communication, diagnosis, and management skills. Although a blended dermatology curriculum with e-learning combined with clinical experience has been shown to increase knowledge acquisition,13,14 our results suggest that an online-only program also can increase comprehension as well as students’ confidence in their abilities.
A major challenge for the DL course was the lack of opportunity to perform common dermatology procedures. The addition of a hands-on skin procedure module would have been a great supplement to the course but was not possible due to social distancing guidelines during the COVID-19 pandemic. The small sample size and voluntary enrollment were limitations to this study.
Conclusion
Although the traditional dermatology rotation remains the gold standard for clinical instruction, a well-organized DL teaching environment allowed for a more controlled learning experience with a broader coverage of topics to include potentially greater exposure to rare skin disorders not typically encountered in everyday practice. A DL dermatology course may serve as an enduring curriculum for those who wish to learn dermatology more broadly and are not interested in performing skin procedures or direct patient exposure (eg, those pursuing non–primary care specialties, pathology, or radiology). It also may be attractive to students who have had a prior clinical dermatology rotation and desire a different learning experience with a wide coverage of topics.
Acknowledgments—The authors thank Thomas Darling, MD, PhD (Bethesda, Maryland), for coining the term Clinpic and providing critical feedback throughout the course. The authors also thank Sorana Raiciulescu, MS (Bethesda, Maryland), for assistance with the statistical analysis.
Medical education has seen major changes over the last decade. The allotted time for preclinical education has decreased from 24 months to 18 months or less at most institutions, with an increased focus on content associated with health care delivery and health system science.1,2 Many schools now include at least some blended learning with online delivery of preclinical education.3 On the other hand, the clinical portion of medical education has remained largely unchanged prior to the COVID-19 pandemic, with the apprenticeship framework allowing the experienced physician to observe, mentor, and pass on practical knowledge so that the apprentice can one day gain independence after demonstrating adequate proficiency.4
With respect to dermatology education, skin disorders are in the top 5 reported reasons for visits to primary care5; however, a 2009 survey found that only 0.24% to 0.30% of medical schools’ curricula are spent on dermatology.6 Moreover, one institution found that fourth-year medical students received an average of 46.6% on a 15-item quiz designed to assess the ability to diagnose and treat common dermatologic conditions, and within that same cohort, 87.6% of students felt that they received inadequate training in dermatology during medical school.7
COVID-19 caused an unprecedented paradigm shift when medical schools throughout the country, including our own, canceled clinical rotations at the end of March 2020 to protect students and control the spread of infection. To enable clinical and preclinical learning to continue, institutions around the globe turned to either online learning or participation in telehealth as a substitute for clinical rotations.8-10 At the Uniformed Services University of the Health Sciences (Bethesda, Maryland), one of the many online clinical courses offered included a distance learning (DL) dermatology course. Herein, we describe the results of a prospective study evaluating short-term information recall and comprehension as well as students’ confidence in their ability to apply course objectives over 3 months of an online DL dermatology course.
Methods
Between April and July 2020, 14 students at the Uniformed Services University of the Health Sciences (Table 1) enrolled in 1 of 3 four-week DL dermatology classes. The students independently completed the Basic Dermatology Curriculum, a set of online modules with demonstrated efficacy from the American Academy of Dermatology, over 4 weeks.11 Additionally, students were instructed to review an hour of clinical dermatology images daily from online dermatology atlases and e-books accessed through our medical school’s virtual library. Optional Free Open Access Meducation resources also were provided. The course syllabus provided the students with clear expectations, links to the resources, and a recommended daily schedule.
An online video conferencing platform was utilized for an orientation session and 4 subsequent weekly 1.5-hour virtual meetings. The weekly DL meetings focused on a discussion of clinical images pertinent to the American Academy of Dermatology modules covered for the week. These interactive analytic sessions were referred to as Clinpic sessions. With instructor guidance, the students learned to describe images, and they provided differential diagnoses, workup, and treatments for various skin diseases. The virtual meetings included supplemental lectures detailing the use of teledermatology and laser therapy in the Military Health System and a journal review on the cutaneous manifestations of COVID-19.
A 40-question, image-based pretest and posttest utilized during clinical rotations evaluated knowledge recall and comprehension. A precourse and postcourse survey using a 5-point Likert scale (1=not confident; 5=extremely confident) assessed students’ confidence levels across course objectives: general knowledge of dermatology, working knowledge of teledermatology, ability to accurately describe skin lesions, generate sound differential diagnoses, and formulate a reasonable treatment plan. Statistical analysis was performed using free online statistical software at statskingdom.com.12
Results
All 14 student enrollees completed the precourse and postcourse tests and surveys. Pretest and posttest scores followed a normal distribution and therefore met criteria for utilization of a parametric test. The precourse test average of 67% (range, 40%–90%) improved to 84% postcourse (range, 70%–98%; P<.001; 95% CI, 11-23 by paired t test). Not surprisingly, the 2 students who had completed a dermatology rotation had higher average pretest and posttest scores (pretest, 87%; posttest, 94%). Students’ confidence with the course objectives were mostly at the somewhat confident level on the 5-point Likert scale precourse survey. By the end of the course, student survey responses increased to confident and very confident levels, corresponding to an overall improvement of 1.3 points (P<.001 by paired t test)(Table 2) when the mean of the survey results was aggregated across every question. Instructor evaluation of student performance mirrored student assessments.
Comment
The DL dermatology course succeeded in helping the enrolled students attain course objectives and offered a reasonable solution when in-person interaction was restricted. The students in the DL course made notable improvements in their dermatology knowledge and improved their communication, diagnosis, and management skills. Although a blended dermatology curriculum with e-learning combined with clinical experience has been shown to increase knowledge acquisition,13,14 our results suggest that an online-only program also can increase comprehension as well as students’ confidence in their abilities.
A major challenge for the DL course was the lack of opportunity to perform common dermatology procedures. The addition of a hands-on skin procedure module would have been a great supplement to the course but was not possible due to social distancing guidelines during the COVID-19 pandemic. The small sample size and voluntary enrollment were limitations to this study.
Conclusion
Although the traditional dermatology rotation remains the gold standard for clinical instruction, a well-organized DL teaching environment allowed for a more controlled learning experience with a broader coverage of topics to include potentially greater exposure to rare skin disorders not typically encountered in everyday practice. A DL dermatology course may serve as an enduring curriculum for those who wish to learn dermatology more broadly and are not interested in performing skin procedures or direct patient exposure (eg, those pursuing non–primary care specialties, pathology, or radiology). It also may be attractive to students who have had a prior clinical dermatology rotation and desire a different learning experience with a wide coverage of topics.
Acknowledgments—The authors thank Thomas Darling, MD, PhD (Bethesda, Maryland), for coining the term Clinpic and providing critical feedback throughout the course. The authors also thank Sorana Raiciulescu, MS (Bethesda, Maryland), for assistance with the statistical analysis.
- Emanuel EJ. The inevitable reimagining of medical education. JAMA. 2020;323:1127-1128.
- Skochelak SE, Stack SJ. Creating the medical schools of the future. Acad Med. 2017;92:16-19.
- Vallée A, Blacher J, Cariou A, et al. Blended learning compared to traditional learning in medical education: systematic review and meta-analysis. J Med Internet Res. 2020;22:E16504.
- Rangachari D, Brown LE, Kern DE, et al. Clinical coaching: evolving the apprenticeship model for modern housestaff. Med Teach. 2017;39:780-782.
- Finley CR, Chan DS, Garrison S, et al. What are the most common conditions in primary care? Can Fam Physician. 2018;64:832-840.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.e4.
- Ulman CA, Binder SB, Borges NJ. Assessment of medical students’ proficiency in dermatology: are medical students adequately prepared to diagnose and treat common dermatologic conditions in the United States? J Educ Eval Health Prof. 2015;12:18.
- Loh TY, Hsiao JL, Shi VY. COVID-19 and its effect on medical student education in dermatology. J Am Acad Dermatol. 2020;83:E163-E164.
- Hilburg R, Patel N, Ambruso S, et al. Medical education during the coronavirus disease-2019 pandemic: learning from a distance. Adv Chronic Kidney Dis. 2020;27:412-417.
- Rose S. Medical student education in the time of COVID-19. JAMA. 2020;323:2131-2132.
- McCleskey PE. Clinic teaching made easy: a prospective study of the American Academy of Dermatology core curriculum in primary care learners. J Am Acad Dermatol. 2013;69:273-279.e1.
- Paired T Test calculator. Statistics Kingdom website. Accessed February 7, 2022. http://www.statskingdom.com/160MeanT2pair.html
- Fransen F, Martens H, Nagtzaam I, et al. Use of e-learning in clinical clerkships: effects on acquisition of dermatological knowledge and learning processes. Int J Med Educ. 2018;9:11-17.
- Silva CS, Souza MB, Silva Filho RS, et al. E-learning program for medical students in dermatology. Clinics. 2011;66:619-622.
- Emanuel EJ. The inevitable reimagining of medical education. JAMA. 2020;323:1127-1128.
- Skochelak SE, Stack SJ. Creating the medical schools of the future. Acad Med. 2017;92:16-19.
- Vallée A, Blacher J, Cariou A, et al. Blended learning compared to traditional learning in medical education: systematic review and meta-analysis. J Med Internet Res. 2020;22:E16504.
- Rangachari D, Brown LE, Kern DE, et al. Clinical coaching: evolving the apprenticeship model for modern housestaff. Med Teach. 2017;39:780-782.
- Finley CR, Chan DS, Garrison S, et al. What are the most common conditions in primary care? Can Fam Physician. 2018;64:832-840.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.e4.
- Ulman CA, Binder SB, Borges NJ. Assessment of medical students’ proficiency in dermatology: are medical students adequately prepared to diagnose and treat common dermatologic conditions in the United States? J Educ Eval Health Prof. 2015;12:18.
- Loh TY, Hsiao JL, Shi VY. COVID-19 and its effect on medical student education in dermatology. J Am Acad Dermatol. 2020;83:E163-E164.
- Hilburg R, Patel N, Ambruso S, et al. Medical education during the coronavirus disease-2019 pandemic: learning from a distance. Adv Chronic Kidney Dis. 2020;27:412-417.
- Rose S. Medical student education in the time of COVID-19. JAMA. 2020;323:2131-2132.
- McCleskey PE. Clinic teaching made easy: a prospective study of the American Academy of Dermatology core curriculum in primary care learners. J Am Acad Dermatol. 2013;69:273-279.e1.
- Paired T Test calculator. Statistics Kingdom website. Accessed February 7, 2022. http://www.statskingdom.com/160MeanT2pair.html
- Fransen F, Martens H, Nagtzaam I, et al. Use of e-learning in clinical clerkships: effects on acquisition of dermatological knowledge and learning processes. Int J Med Educ. 2018;9:11-17.
- Silva CS, Souza MB, Silva Filho RS, et al. E-learning program for medical students in dermatology. Clinics. 2011;66:619-622.
Practice Points
- An e-learning distance learning (DL) dermatology course can substantially improve clinically relevant skills and knowledge in dermatology.
- A DL dermatology course may serve as an alternative to clinical rotations for those who wish to learn dermatology more broadly and are not interested in performing skin procedures or direct patient exposure.
Review of Ethnoracial Representation in Clinical Trials (Phases 1 Through 4) of Atopic Dermatitis Therapies
To the Editor:
Atopic dermatitis (AD) affects an estimated 7.2% of adults and 10.7% of children in the United States; however, AD might affect different races at a varying rate.1 Compared to their European American counterparts, Asian/Pacific Islanders and African Americans are 7 and 3 times more likely, respectively, to be given a diagnosis of AD.2
Despite being disproportionately affected by AD, minority groups might be underrepresented in clinical trials of AD treatments.3 One explanation for this imbalance might be that ethnoracial representation differs across regions in the United States, perhaps in regions where clinical trials are conducted. Price et al3 investigated racial representation in clinical trials of AD globally and found that patients of color are consistently underrepresented.
Research on racial representation in clinical trials within the United States—on national and regional scales—is lacking from the current AD literature. We conducted a study to compare racial and ethnic disparities in AD clinical trials across regions of the United States.
Using the ClinicalTrials.gov database (www.clinicaltrials.gov) of the National Library of Medicine, we identified clinical trials of AD treatments (encompassing phases 1 through 4) in the United States that were completed before March 14, 2021, with the earliest data from 2013. Search terms included atopic dermatitis, with an advanced search for interventional (clinical trials) and with results.
In total, 95 completed clinical trials were identified, of which 26 (27.4%) reported ethnoracial demographic data. One trial was excluded due to misrepresentation regarding the classification of individuals who identified as more than 1 racial category. Clinical trials for systemic treatments (7 [28%]) and topical treatments (18 [72%]) were identified.
All ethnoracial data were self-reported by trial participants based on US Food and Drug Administration guidelines for racial and ethnic categorization.4 Trial participants who identified ethnically as Hispanic or Latino might have been a part of any racial group. Only 7 of the 25 included clinical trials (28%) provided ethnic demographic data (Hispanic [Latino] or non-Hispanic); 72% of trials failed to report ethnicity. Ethnic data included in our analysis came from only the 7 clinical trials that included these data. International multicenter trials that included a US site were excluded.
Ultimately, the number of trials included in our analysis was 25, comprised of 2443 participants. Data were further organized by US geographic region (Northeast, Midwest, South, West, and multiregion trials [ie, conducted in ≥2 regions]). No AD clinical trials were conducted solely in the Midwest; it was only included within multiregion trials.
Compared to their representation in the 2019 US Census, most minority groups were overrepresented in clinical trials, while White individuals were underrepresented (eTable). The percentages of our findings on representation for race are as follows (US Census data are listed in parentheses for comparison5):
- White: 56.8% (72.5%)
- Black/African American: 28.3% (12.7%)
- Asian: 10.3% (5.5%)
- Multiracial: 1.1% (3.3%)
- Native Hawaiian or other Pacific Islander: 0.3% (0.2%)
- American Indian or Alaska Native: 0.2% (0.8%)
- Other: 0.5% (4.9%).
Our findings on representation for ethnicity are as follows (US Census data is listed in parentheses for comparison5):
- Hispanic or Latino: 21.4% (18.0%)
Although representation of Black/African American and Asian participants in clinical trials was higher than their representation in US Census data and representation of White participants was lower in clinical trials than their representation in census data, equal representation among all racial and ethnic groups is still lacking. A potential explanation for this finding might be that requirements for trial inclusion selected for more minority patients, given the propensity for greater severity of AD among those racial groups.2 Another explanation might be that efforts to include minority patients in clinical trials are improving.
There were great differences in ethnoracial representation in clinical trials when regions within the United States were compared. Based on census population data by region, the West had the highest percentage (29.9%) of Hispanic or Latino residents; however, this group represented only 11.7% of participants in AD clinical trials in that region.5
The South had the greatest number of participants in AD clinical trials of any region, which was consistent with research findings on an association between severity of AD and heat.6 With a warmer climate correlating with an increased incidence of AD, it is possible that more people are willing to participate in clinical trials in the South.
The Midwest was the only region in which region-specific clinical trials were not conducted. Recent studies have shown that individuals with AD who live in the Midwest have comparatively less access to health care associated with AD treatment and are more likely to visit an emergency department because of AD than individuals in any other US region.7 This discrepancy highlights the need for increased access to resources and clinical trials focused on the treatment of AD in the Midwest.
In 1993, the National Institutes of Health Revitalization Act established a federal legislative mandate to encourage inclusion of women and people of color in clinical trials.8 During the last 2 decades, there have been improvements in ethnoracial reporting. A 2020 global study found that 81.1% of randomized controlled trials (phases 2 and 3) of AD treatments reported ethnoracial data.3
Equal representation in clinical trials allows for further investigation of the connection between race, AD severity, and treatment efficacy. Clinical trials need to have equal representation of ethnoracial categories across all regions of the United States. If one group is notably overrepresented, ethnoracial associations related to the treatment of AD might go undetected.9 Similarly, if representation is unequal, relationships of treatment efficacy within ethnoracial groups also might go undetected. None of the clinical trials that we analyzed investigated treatment efficacy by race, suggesting that there is a need for future research in this area.
It also is important to note that broad classifications of race and ethnicity are limiting and therefore overlook differences within ethnoracial categories. Although representation of minority patients in clinical trials for AD treatments is improving, we conclude that there remains a need for greater and equal representation of minority groups in clinical trials of AD treatments in the United States.
- Avena-Woods C. Overview of atopic dermatitis. Am J Manag Care. 2017;23(8 suppl):S115-S123.
- Kaufman BP, Guttman‐Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups—variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357. doi:10.1111/exd.13514
- Price KN, Krase JM, Loh TY, et al. Racial and ethnic disparities in global atopic dermatitis clinical trials. Br J Dermatol. 2020;183:378-380. doi:10.1111/bjd.18938
- Collection of race and ethnicity data in clinical trials: guidance for industry and Food and Drug Administration staff. US Food and Drug Administration; October 26, 2016. Accessed February 20, 2022. https://www.fda.gov/media/75453/download
- United States Census Bureau. 2019 Population estimates by age, sex, race and Hispanic origin. Published June 25, 2020. Accessed March 22, 2022. https://www.census.gov/newsroom/press-kits/2020/population-estimates-detailed.html
- Fleischer AB Jr. Atopic dermatitis: the relationship to temperature and seasonality in the United States. Int J Dermatol. 2019;58:465-471. doi:10.1111/ijd.14289
- Wu KK, Nguyen KB, Sandhu JK, et al. Does location matter? geographic variations in healthcare resource use for atopic dermatitis in the United States. J Dermatolog Treat. 2021;32:314-320. doi:10.1080/09546634.2019.1656796
- National Institutes of Health Revitalization Act of 1993, 42 USC 201 (1993). Accessed February 20, 2022. https://www.govinfo.gov/content/pkg/STATUTE-107/pdf/STATUTE-107-Pg122.pdf
- Hirano SA, Murray SB, Harvey VM. Reporting, representation, and subgroup analysis of race and ethnicity in published clinical trials of atopic dermatitis in the United States between 2000 and 2009. Pediatr Dermatol. 2012;29:749-755. doi:10.1111/j.1525-1470.2012.01797.x
To the Editor:
Atopic dermatitis (AD) affects an estimated 7.2% of adults and 10.7% of children in the United States; however, AD might affect different races at a varying rate.1 Compared to their European American counterparts, Asian/Pacific Islanders and African Americans are 7 and 3 times more likely, respectively, to be given a diagnosis of AD.2
Despite being disproportionately affected by AD, minority groups might be underrepresented in clinical trials of AD treatments.3 One explanation for this imbalance might be that ethnoracial representation differs across regions in the United States, perhaps in regions where clinical trials are conducted. Price et al3 investigated racial representation in clinical trials of AD globally and found that patients of color are consistently underrepresented.
Research on racial representation in clinical trials within the United States—on national and regional scales—is lacking from the current AD literature. We conducted a study to compare racial and ethnic disparities in AD clinical trials across regions of the United States.
Using the ClinicalTrials.gov database (www.clinicaltrials.gov) of the National Library of Medicine, we identified clinical trials of AD treatments (encompassing phases 1 through 4) in the United States that were completed before March 14, 2021, with the earliest data from 2013. Search terms included atopic dermatitis, with an advanced search for interventional (clinical trials) and with results.
In total, 95 completed clinical trials were identified, of which 26 (27.4%) reported ethnoracial demographic data. One trial was excluded due to misrepresentation regarding the classification of individuals who identified as more than 1 racial category. Clinical trials for systemic treatments (7 [28%]) and topical treatments (18 [72%]) were identified.
All ethnoracial data were self-reported by trial participants based on US Food and Drug Administration guidelines for racial and ethnic categorization.4 Trial participants who identified ethnically as Hispanic or Latino might have been a part of any racial group. Only 7 of the 25 included clinical trials (28%) provided ethnic demographic data (Hispanic [Latino] or non-Hispanic); 72% of trials failed to report ethnicity. Ethnic data included in our analysis came from only the 7 clinical trials that included these data. International multicenter trials that included a US site were excluded.
Ultimately, the number of trials included in our analysis was 25, comprised of 2443 participants. Data were further organized by US geographic region (Northeast, Midwest, South, West, and multiregion trials [ie, conducted in ≥2 regions]). No AD clinical trials were conducted solely in the Midwest; it was only included within multiregion trials.
Compared to their representation in the 2019 US Census, most minority groups were overrepresented in clinical trials, while White individuals were underrepresented (eTable). The percentages of our findings on representation for race are as follows (US Census data are listed in parentheses for comparison5):
- White: 56.8% (72.5%)
- Black/African American: 28.3% (12.7%)
- Asian: 10.3% (5.5%)
- Multiracial: 1.1% (3.3%)
- Native Hawaiian or other Pacific Islander: 0.3% (0.2%)
- American Indian or Alaska Native: 0.2% (0.8%)
- Other: 0.5% (4.9%).
Our findings on representation for ethnicity are as follows (US Census data is listed in parentheses for comparison5):
- Hispanic or Latino: 21.4% (18.0%)
Although representation of Black/African American and Asian participants in clinical trials was higher than their representation in US Census data and representation of White participants was lower in clinical trials than their representation in census data, equal representation among all racial and ethnic groups is still lacking. A potential explanation for this finding might be that requirements for trial inclusion selected for more minority patients, given the propensity for greater severity of AD among those racial groups.2 Another explanation might be that efforts to include minority patients in clinical trials are improving.
There were great differences in ethnoracial representation in clinical trials when regions within the United States were compared. Based on census population data by region, the West had the highest percentage (29.9%) of Hispanic or Latino residents; however, this group represented only 11.7% of participants in AD clinical trials in that region.5
The South had the greatest number of participants in AD clinical trials of any region, which was consistent with research findings on an association between severity of AD and heat.6 With a warmer climate correlating with an increased incidence of AD, it is possible that more people are willing to participate in clinical trials in the South.
The Midwest was the only region in which region-specific clinical trials were not conducted. Recent studies have shown that individuals with AD who live in the Midwest have comparatively less access to health care associated with AD treatment and are more likely to visit an emergency department because of AD than individuals in any other US region.7 This discrepancy highlights the need for increased access to resources and clinical trials focused on the treatment of AD in the Midwest.
In 1993, the National Institutes of Health Revitalization Act established a federal legislative mandate to encourage inclusion of women and people of color in clinical trials.8 During the last 2 decades, there have been improvements in ethnoracial reporting. A 2020 global study found that 81.1% of randomized controlled trials (phases 2 and 3) of AD treatments reported ethnoracial data.3
Equal representation in clinical trials allows for further investigation of the connection between race, AD severity, and treatment efficacy. Clinical trials need to have equal representation of ethnoracial categories across all regions of the United States. If one group is notably overrepresented, ethnoracial associations related to the treatment of AD might go undetected.9 Similarly, if representation is unequal, relationships of treatment efficacy within ethnoracial groups also might go undetected. None of the clinical trials that we analyzed investigated treatment efficacy by race, suggesting that there is a need for future research in this area.
It also is important to note that broad classifications of race and ethnicity are limiting and therefore overlook differences within ethnoracial categories. Although representation of minority patients in clinical trials for AD treatments is improving, we conclude that there remains a need for greater and equal representation of minority groups in clinical trials of AD treatments in the United States.
To the Editor:
Atopic dermatitis (AD) affects an estimated 7.2% of adults and 10.7% of children in the United States; however, AD might affect different races at a varying rate.1 Compared to their European American counterparts, Asian/Pacific Islanders and African Americans are 7 and 3 times more likely, respectively, to be given a diagnosis of AD.2
Despite being disproportionately affected by AD, minority groups might be underrepresented in clinical trials of AD treatments.3 One explanation for this imbalance might be that ethnoracial representation differs across regions in the United States, perhaps in regions where clinical trials are conducted. Price et al3 investigated racial representation in clinical trials of AD globally and found that patients of color are consistently underrepresented.
Research on racial representation in clinical trials within the United States—on national and regional scales—is lacking from the current AD literature. We conducted a study to compare racial and ethnic disparities in AD clinical trials across regions of the United States.
Using the ClinicalTrials.gov database (www.clinicaltrials.gov) of the National Library of Medicine, we identified clinical trials of AD treatments (encompassing phases 1 through 4) in the United States that were completed before March 14, 2021, with the earliest data from 2013. Search terms included atopic dermatitis, with an advanced search for interventional (clinical trials) and with results.
In total, 95 completed clinical trials were identified, of which 26 (27.4%) reported ethnoracial demographic data. One trial was excluded due to misrepresentation regarding the classification of individuals who identified as more than 1 racial category. Clinical trials for systemic treatments (7 [28%]) and topical treatments (18 [72%]) were identified.
All ethnoracial data were self-reported by trial participants based on US Food and Drug Administration guidelines for racial and ethnic categorization.4 Trial participants who identified ethnically as Hispanic or Latino might have been a part of any racial group. Only 7 of the 25 included clinical trials (28%) provided ethnic demographic data (Hispanic [Latino] or non-Hispanic); 72% of trials failed to report ethnicity. Ethnic data included in our analysis came from only the 7 clinical trials that included these data. International multicenter trials that included a US site were excluded.
Ultimately, the number of trials included in our analysis was 25, comprised of 2443 participants. Data were further organized by US geographic region (Northeast, Midwest, South, West, and multiregion trials [ie, conducted in ≥2 regions]). No AD clinical trials were conducted solely in the Midwest; it was only included within multiregion trials.
Compared to their representation in the 2019 US Census, most minority groups were overrepresented in clinical trials, while White individuals were underrepresented (eTable). The percentages of our findings on representation for race are as follows (US Census data are listed in parentheses for comparison5):
- White: 56.8% (72.5%)
- Black/African American: 28.3% (12.7%)
- Asian: 10.3% (5.5%)
- Multiracial: 1.1% (3.3%)
- Native Hawaiian or other Pacific Islander: 0.3% (0.2%)
- American Indian or Alaska Native: 0.2% (0.8%)
- Other: 0.5% (4.9%).
Our findings on representation for ethnicity are as follows (US Census data is listed in parentheses for comparison5):
- Hispanic or Latino: 21.4% (18.0%)
Although representation of Black/African American and Asian participants in clinical trials was higher than their representation in US Census data and representation of White participants was lower in clinical trials than their representation in census data, equal representation among all racial and ethnic groups is still lacking. A potential explanation for this finding might be that requirements for trial inclusion selected for more minority patients, given the propensity for greater severity of AD among those racial groups.2 Another explanation might be that efforts to include minority patients in clinical trials are improving.
There were great differences in ethnoracial representation in clinical trials when regions within the United States were compared. Based on census population data by region, the West had the highest percentage (29.9%) of Hispanic or Latino residents; however, this group represented only 11.7% of participants in AD clinical trials in that region.5
The South had the greatest number of participants in AD clinical trials of any region, which was consistent with research findings on an association between severity of AD and heat.6 With a warmer climate correlating with an increased incidence of AD, it is possible that more people are willing to participate in clinical trials in the South.
The Midwest was the only region in which region-specific clinical trials were not conducted. Recent studies have shown that individuals with AD who live in the Midwest have comparatively less access to health care associated with AD treatment and are more likely to visit an emergency department because of AD than individuals in any other US region.7 This discrepancy highlights the need for increased access to resources and clinical trials focused on the treatment of AD in the Midwest.
In 1993, the National Institutes of Health Revitalization Act established a federal legislative mandate to encourage inclusion of women and people of color in clinical trials.8 During the last 2 decades, there have been improvements in ethnoracial reporting. A 2020 global study found that 81.1% of randomized controlled trials (phases 2 and 3) of AD treatments reported ethnoracial data.3
Equal representation in clinical trials allows for further investigation of the connection between race, AD severity, and treatment efficacy. Clinical trials need to have equal representation of ethnoracial categories across all regions of the United States. If one group is notably overrepresented, ethnoracial associations related to the treatment of AD might go undetected.9 Similarly, if representation is unequal, relationships of treatment efficacy within ethnoracial groups also might go undetected. None of the clinical trials that we analyzed investigated treatment efficacy by race, suggesting that there is a need for future research in this area.
It also is important to note that broad classifications of race and ethnicity are limiting and therefore overlook differences within ethnoracial categories. Although representation of minority patients in clinical trials for AD treatments is improving, we conclude that there remains a need for greater and equal representation of minority groups in clinical trials of AD treatments in the United States.
- Avena-Woods C. Overview of atopic dermatitis. Am J Manag Care. 2017;23(8 suppl):S115-S123.
- Kaufman BP, Guttman‐Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups—variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357. doi:10.1111/exd.13514
- Price KN, Krase JM, Loh TY, et al. Racial and ethnic disparities in global atopic dermatitis clinical trials. Br J Dermatol. 2020;183:378-380. doi:10.1111/bjd.18938
- Collection of race and ethnicity data in clinical trials: guidance for industry and Food and Drug Administration staff. US Food and Drug Administration; October 26, 2016. Accessed February 20, 2022. https://www.fda.gov/media/75453/download
- United States Census Bureau. 2019 Population estimates by age, sex, race and Hispanic origin. Published June 25, 2020. Accessed March 22, 2022. https://www.census.gov/newsroom/press-kits/2020/population-estimates-detailed.html
- Fleischer AB Jr. Atopic dermatitis: the relationship to temperature and seasonality in the United States. Int J Dermatol. 2019;58:465-471. doi:10.1111/ijd.14289
- Wu KK, Nguyen KB, Sandhu JK, et al. Does location matter? geographic variations in healthcare resource use for atopic dermatitis in the United States. J Dermatolog Treat. 2021;32:314-320. doi:10.1080/09546634.2019.1656796
- National Institutes of Health Revitalization Act of 1993, 42 USC 201 (1993). Accessed February 20, 2022. https://www.govinfo.gov/content/pkg/STATUTE-107/pdf/STATUTE-107-Pg122.pdf
- Hirano SA, Murray SB, Harvey VM. Reporting, representation, and subgroup analysis of race and ethnicity in published clinical trials of atopic dermatitis in the United States between 2000 and 2009. Pediatr Dermatol. 2012;29:749-755. doi:10.1111/j.1525-1470.2012.01797.x
- Avena-Woods C. Overview of atopic dermatitis. Am J Manag Care. 2017;23(8 suppl):S115-S123.
- Kaufman BP, Guttman‐Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups—variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357. doi:10.1111/exd.13514
- Price KN, Krase JM, Loh TY, et al. Racial and ethnic disparities in global atopic dermatitis clinical trials. Br J Dermatol. 2020;183:378-380. doi:10.1111/bjd.18938
- Collection of race and ethnicity data in clinical trials: guidance for industry and Food and Drug Administration staff. US Food and Drug Administration; October 26, 2016. Accessed February 20, 2022. https://www.fda.gov/media/75453/download
- United States Census Bureau. 2019 Population estimates by age, sex, race and Hispanic origin. Published June 25, 2020. Accessed March 22, 2022. https://www.census.gov/newsroom/press-kits/2020/population-estimates-detailed.html
- Fleischer AB Jr. Atopic dermatitis: the relationship to temperature and seasonality in the United States. Int J Dermatol. 2019;58:465-471. doi:10.1111/ijd.14289
- Wu KK, Nguyen KB, Sandhu JK, et al. Does location matter? geographic variations in healthcare resource use for atopic dermatitis in the United States. J Dermatolog Treat. 2021;32:314-320. doi:10.1080/09546634.2019.1656796
- National Institutes of Health Revitalization Act of 1993, 42 USC 201 (1993). Accessed February 20, 2022. https://www.govinfo.gov/content/pkg/STATUTE-107/pdf/STATUTE-107-Pg122.pdf
- Hirano SA, Murray SB, Harvey VM. Reporting, representation, and subgroup analysis of race and ethnicity in published clinical trials of atopic dermatitis in the United States between 2000 and 2009. Pediatr Dermatol. 2012;29:749-755. doi:10.1111/j.1525-1470.2012.01797.x
Practice Points
- Although minority groups are disproportionally affected by atopic dermatitis (AD), they may be underrepresented in clinical trials for AD in the United States.
- Equal representation among ethnoracial groups in clinical trials is important to allow for a more thorough investigation of the efficacy of treatments for AD.
When Are Inpatient and Emergency Dermatologic Consultations Appropriate?
There are limited clinical data concerning inpatient and emergency department (ED) dermatologic consultations. The indications for these consultations vary widely, but in one study (N=271), it was found that 21% of inpatient consultations were for contact dermatitis and 10% were for drug eruptions.1 In the same study, 77% of patients who required a dermatology consultation eventually were given a different diagnosis or change in treatment after consultation. For example, of all consultations for suspected cellulitis, only 10% were confirmed after dermatology evaluation.1
Hospitalists and emergency physicians continue to struggle with the assessment of dermatologic conditions, often consulting dermatology whenever a patient has a “rash” or skin concern. Dermatology is still not emphasized in medical education and often is taught to most medical students in an abbreviated fashion, which results in physicians feeling ill-equipped to deal with any dermatologic condition—either mundane or potentially life-threatening. A study in 2016 showed that a monthly lecture series given to hospitalists over the course of 5 years did not improve diagnostic accuracy in patients who were admitted with skin manifestations.2 This further shows that there is a need for dermatologic experts in the hospital.
We need to develop better guidelines for physicians in the ED and on inpatient units to guide them on appropriate use of dermatologic consultation outside the ambulatory office and the clinic. A 2013 study showed that patients often were discharged immediately after a dermatologic consultation, furthering our hypothesis that many inpatient consultations can be delayed until after discharge.3
In an era in which medical costs are soaring and there is constant surveillance for ways to reduce costs without impairing quality of care, limiting unnecessary specialty consultations should be embraced. In 2009, $1.8 billion in Medicare claims was paid for dermatology-related admissions.3 A substantial savings to Medicare consulting fees for certain diagnoses, such as cellulitis or contact dermatitis, could be realized if patients were referred for outpatient assessment and treatment. In a study of 271 consultations, 54 patients also had a skin biopsy, which further increases dollars spent on inpatient care and is (usually) something that can be performed in the outpatient setting.1 In another study, the more common recommended treatments were topical corticosteroids and supportive educational measures for patients and hospital staff,3 which further substantiates that most dermatology consultations are not truly emergent and can wait for outpatient consultation.
In addition, we are dealing with the COVID-19 pandemic in our hospitals and EDs. Many physicians, including dermatologists, would prefer to avoid unnecessary exposure to SARS-CoV-2 on inpatient units and in the ED. It certainly would be preferable to require consultants to come in to evaluate patients only when they truly need to be seen while in the hospital.
There also is limited dermatology training in other specialties, and the dermatology team can help fill this gap with educational programs and one-on-one teaching. Hospital teams have signaled this need, but there has been limited success with multiple teaching opportunities.4
We believe that this need for inpatient dermatology services can be filled with the newer subspecialty of hospital dermatology, which is not commonly present at most hospitals; a reason why the subspecialty has not been more popular is that there are few available data in the form of randomized clinical trials that can guide inpatient dermatologists with the care of rare hospital skin diseases.5 Having a dermatologic hospitalist available might allow for patients to be seen more readily, which ultimately will save lives and health care dollars and would increase real-time teaching and education for house staff, nursing, and attendings at the bedside.
In a 2018 article,6 it was postulated that quicker diagnosis of pseudocellulitis and initiation of antibiotics to treat this condition would save the US health care system $210 million annually. We believe that pseudocellulitis would be best evaluated by inpatient dermatology teams, thereby avoiding costly dermatologic consultations, at an average rate of $138.89.6
Morbilliform drug eruptions are among the most common skin conditions seen in the hospital; approximately 95% of cases are an uncomplicated reaction to a medication or virus. Data show that many of these consultations might be unnecessary.7
Our institution (Hackensack University Medical Center, New Jersey) is a tertiary hospital that also is connected with a major cancer center. Given this connection, skin eruptions due to chemotherapy and radiation are common. The treatment of drug eruptions, graft-vs-host disease, and other oncologic or drug-related eruptions should be within the scope of practice of our hospitalists, but these cases frequently involve dermatologic consultation.
We constructed a consultation flowchart (Figure) to help guide the triage of patients in need of dermatologic evaluation by inpatient teams and possibly to avoid unnecessary consultation fees. The manner in which this—or any flowchart or teaching aid—is conveyed to hospital personnel is critical so that these tools are not perceived as patronizing or confrontational. In our flowchart, we list emergent dermatologic conditions that we believe are appropriate for dermatology consultation including erythrodermic psoriasis, bullous pemphigoid flare, and Stevens-Johnson syndrome/toxic epidermal necrolysis.
We believe that the flowchart can educate inpatient medical teams about appropriate dermatology consultation. Use of the flowchart also may decrease unnecessary consultations, which ultimately will lower health care spending overall.
- Davila M, Christenson LJ, Sontheimer RD. Epidemiology and outcomes of dermatology in-patient consultations in a Midwestern U.S. university hospital. Dermatol Online J. 2010;16:12.
- Beshay A, Liu M, Fox L, et al. Inpatient dermatology consultative programs: a continued need, tools for needs assessment for curriculum development, and a call for new methods of teaching. J Am Acad Dermatol. 2016;74:769-771. doi:10.1016/j.jaad.2015.11.017
- Hu L, Haynes H, Ferrazza D, et al. Impact of specialist consultations on inpatient admissions for dermatology-specific and related DRGs. J Gen Intern Med. 2013;28:1477-1482. doi:10.1007/s11606-013-2440-2
- Faletsky A, Han JJ, Mostaghimi A. Inpatient dermatology best practice strategies for educating and relaying findings to colleagues. Curr Dermatol Rep. 2020;9:256-260. doi:10.1007/s13671-020-00317-y
- Fox LP. Hospital dermatology, introduction. Semin Cutan Med Surg. 2017;36:1-2. doi:10.12788/j.sder.2017.015
- Li D, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543. doi:10.1001/jamadermatol.2017.6197
- Biesbroeck LK, Shinohara MM. Inpatient consultative dermatology. Med Clin North Am. 2015;99:1349-1364. doi:10.1016/j.mcna.2015.06.004
There are limited clinical data concerning inpatient and emergency department (ED) dermatologic consultations. The indications for these consultations vary widely, but in one study (N=271), it was found that 21% of inpatient consultations were for contact dermatitis and 10% were for drug eruptions.1 In the same study, 77% of patients who required a dermatology consultation eventually were given a different diagnosis or change in treatment after consultation. For example, of all consultations for suspected cellulitis, only 10% were confirmed after dermatology evaluation.1
Hospitalists and emergency physicians continue to struggle with the assessment of dermatologic conditions, often consulting dermatology whenever a patient has a “rash” or skin concern. Dermatology is still not emphasized in medical education and often is taught to most medical students in an abbreviated fashion, which results in physicians feeling ill-equipped to deal with any dermatologic condition—either mundane or potentially life-threatening. A study in 2016 showed that a monthly lecture series given to hospitalists over the course of 5 years did not improve diagnostic accuracy in patients who were admitted with skin manifestations.2 This further shows that there is a need for dermatologic experts in the hospital.
We need to develop better guidelines for physicians in the ED and on inpatient units to guide them on appropriate use of dermatologic consultation outside the ambulatory office and the clinic. A 2013 study showed that patients often were discharged immediately after a dermatologic consultation, furthering our hypothesis that many inpatient consultations can be delayed until after discharge.3
In an era in which medical costs are soaring and there is constant surveillance for ways to reduce costs without impairing quality of care, limiting unnecessary specialty consultations should be embraced. In 2009, $1.8 billion in Medicare claims was paid for dermatology-related admissions.3 A substantial savings to Medicare consulting fees for certain diagnoses, such as cellulitis or contact dermatitis, could be realized if patients were referred for outpatient assessment and treatment. In a study of 271 consultations, 54 patients also had a skin biopsy, which further increases dollars spent on inpatient care and is (usually) something that can be performed in the outpatient setting.1 In another study, the more common recommended treatments were topical corticosteroids and supportive educational measures for patients and hospital staff,3 which further substantiates that most dermatology consultations are not truly emergent and can wait for outpatient consultation.
In addition, we are dealing with the COVID-19 pandemic in our hospitals and EDs. Many physicians, including dermatologists, would prefer to avoid unnecessary exposure to SARS-CoV-2 on inpatient units and in the ED. It certainly would be preferable to require consultants to come in to evaluate patients only when they truly need to be seen while in the hospital.
There also is limited dermatology training in other specialties, and the dermatology team can help fill this gap with educational programs and one-on-one teaching. Hospital teams have signaled this need, but there has been limited success with multiple teaching opportunities.4
We believe that this need for inpatient dermatology services can be filled with the newer subspecialty of hospital dermatology, which is not commonly present at most hospitals; a reason why the subspecialty has not been more popular is that there are few available data in the form of randomized clinical trials that can guide inpatient dermatologists with the care of rare hospital skin diseases.5 Having a dermatologic hospitalist available might allow for patients to be seen more readily, which ultimately will save lives and health care dollars and would increase real-time teaching and education for house staff, nursing, and attendings at the bedside.
In a 2018 article,6 it was postulated that quicker diagnosis of pseudocellulitis and initiation of antibiotics to treat this condition would save the US health care system $210 million annually. We believe that pseudocellulitis would be best evaluated by inpatient dermatology teams, thereby avoiding costly dermatologic consultations, at an average rate of $138.89.6
Morbilliform drug eruptions are among the most common skin conditions seen in the hospital; approximately 95% of cases are an uncomplicated reaction to a medication or virus. Data show that many of these consultations might be unnecessary.7
Our institution (Hackensack University Medical Center, New Jersey) is a tertiary hospital that also is connected with a major cancer center. Given this connection, skin eruptions due to chemotherapy and radiation are common. The treatment of drug eruptions, graft-vs-host disease, and other oncologic or drug-related eruptions should be within the scope of practice of our hospitalists, but these cases frequently involve dermatologic consultation.
We constructed a consultation flowchart (Figure) to help guide the triage of patients in need of dermatologic evaluation by inpatient teams and possibly to avoid unnecessary consultation fees. The manner in which this—or any flowchart or teaching aid—is conveyed to hospital personnel is critical so that these tools are not perceived as patronizing or confrontational. In our flowchart, we list emergent dermatologic conditions that we believe are appropriate for dermatology consultation including erythrodermic psoriasis, bullous pemphigoid flare, and Stevens-Johnson syndrome/toxic epidermal necrolysis.
We believe that the flowchart can educate inpatient medical teams about appropriate dermatology consultation. Use of the flowchart also may decrease unnecessary consultations, which ultimately will lower health care spending overall.
There are limited clinical data concerning inpatient and emergency department (ED) dermatologic consultations. The indications for these consultations vary widely, but in one study (N=271), it was found that 21% of inpatient consultations were for contact dermatitis and 10% were for drug eruptions.1 In the same study, 77% of patients who required a dermatology consultation eventually were given a different diagnosis or change in treatment after consultation. For example, of all consultations for suspected cellulitis, only 10% were confirmed after dermatology evaluation.1
Hospitalists and emergency physicians continue to struggle with the assessment of dermatologic conditions, often consulting dermatology whenever a patient has a “rash” or skin concern. Dermatology is still not emphasized in medical education and often is taught to most medical students in an abbreviated fashion, which results in physicians feeling ill-equipped to deal with any dermatologic condition—either mundane or potentially life-threatening. A study in 2016 showed that a monthly lecture series given to hospitalists over the course of 5 years did not improve diagnostic accuracy in patients who were admitted with skin manifestations.2 This further shows that there is a need for dermatologic experts in the hospital.
We need to develop better guidelines for physicians in the ED and on inpatient units to guide them on appropriate use of dermatologic consultation outside the ambulatory office and the clinic. A 2013 study showed that patients often were discharged immediately after a dermatologic consultation, furthering our hypothesis that many inpatient consultations can be delayed until after discharge.3
In an era in which medical costs are soaring and there is constant surveillance for ways to reduce costs without impairing quality of care, limiting unnecessary specialty consultations should be embraced. In 2009, $1.8 billion in Medicare claims was paid for dermatology-related admissions.3 A substantial savings to Medicare consulting fees for certain diagnoses, such as cellulitis or contact dermatitis, could be realized if patients were referred for outpatient assessment and treatment. In a study of 271 consultations, 54 patients also had a skin biopsy, which further increases dollars spent on inpatient care and is (usually) something that can be performed in the outpatient setting.1 In another study, the more common recommended treatments were topical corticosteroids and supportive educational measures for patients and hospital staff,3 which further substantiates that most dermatology consultations are not truly emergent and can wait for outpatient consultation.
In addition, we are dealing with the COVID-19 pandemic in our hospitals and EDs. Many physicians, including dermatologists, would prefer to avoid unnecessary exposure to SARS-CoV-2 on inpatient units and in the ED. It certainly would be preferable to require consultants to come in to evaluate patients only when they truly need to be seen while in the hospital.
There also is limited dermatology training in other specialties, and the dermatology team can help fill this gap with educational programs and one-on-one teaching. Hospital teams have signaled this need, but there has been limited success with multiple teaching opportunities.4
We believe that this need for inpatient dermatology services can be filled with the newer subspecialty of hospital dermatology, which is not commonly present at most hospitals; a reason why the subspecialty has not been more popular is that there are few available data in the form of randomized clinical trials that can guide inpatient dermatologists with the care of rare hospital skin diseases.5 Having a dermatologic hospitalist available might allow for patients to be seen more readily, which ultimately will save lives and health care dollars and would increase real-time teaching and education for house staff, nursing, and attendings at the bedside.
In a 2018 article,6 it was postulated that quicker diagnosis of pseudocellulitis and initiation of antibiotics to treat this condition would save the US health care system $210 million annually. We believe that pseudocellulitis would be best evaluated by inpatient dermatology teams, thereby avoiding costly dermatologic consultations, at an average rate of $138.89.6
Morbilliform drug eruptions are among the most common skin conditions seen in the hospital; approximately 95% of cases are an uncomplicated reaction to a medication or virus. Data show that many of these consultations might be unnecessary.7
Our institution (Hackensack University Medical Center, New Jersey) is a tertiary hospital that also is connected with a major cancer center. Given this connection, skin eruptions due to chemotherapy and radiation are common. The treatment of drug eruptions, graft-vs-host disease, and other oncologic or drug-related eruptions should be within the scope of practice of our hospitalists, but these cases frequently involve dermatologic consultation.
We constructed a consultation flowchart (Figure) to help guide the triage of patients in need of dermatologic evaluation by inpatient teams and possibly to avoid unnecessary consultation fees. The manner in which this—or any flowchart or teaching aid—is conveyed to hospital personnel is critical so that these tools are not perceived as patronizing or confrontational. In our flowchart, we list emergent dermatologic conditions that we believe are appropriate for dermatology consultation including erythrodermic psoriasis, bullous pemphigoid flare, and Stevens-Johnson syndrome/toxic epidermal necrolysis.
We believe that the flowchart can educate inpatient medical teams about appropriate dermatology consultation. Use of the flowchart also may decrease unnecessary consultations, which ultimately will lower health care spending overall.
- Davila M, Christenson LJ, Sontheimer RD. Epidemiology and outcomes of dermatology in-patient consultations in a Midwestern U.S. university hospital. Dermatol Online J. 2010;16:12.
- Beshay A, Liu M, Fox L, et al. Inpatient dermatology consultative programs: a continued need, tools for needs assessment for curriculum development, and a call for new methods of teaching. J Am Acad Dermatol. 2016;74:769-771. doi:10.1016/j.jaad.2015.11.017
- Hu L, Haynes H, Ferrazza D, et al. Impact of specialist consultations on inpatient admissions for dermatology-specific and related DRGs. J Gen Intern Med. 2013;28:1477-1482. doi:10.1007/s11606-013-2440-2
- Faletsky A, Han JJ, Mostaghimi A. Inpatient dermatology best practice strategies for educating and relaying findings to colleagues. Curr Dermatol Rep. 2020;9:256-260. doi:10.1007/s13671-020-00317-y
- Fox LP. Hospital dermatology, introduction. Semin Cutan Med Surg. 2017;36:1-2. doi:10.12788/j.sder.2017.015
- Li D, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543. doi:10.1001/jamadermatol.2017.6197
- Biesbroeck LK, Shinohara MM. Inpatient consultative dermatology. Med Clin North Am. 2015;99:1349-1364. doi:10.1016/j.mcna.2015.06.004
- Davila M, Christenson LJ, Sontheimer RD. Epidemiology and outcomes of dermatology in-patient consultations in a Midwestern U.S. university hospital. Dermatol Online J. 2010;16:12.
- Beshay A, Liu M, Fox L, et al. Inpatient dermatology consultative programs: a continued need, tools for needs assessment for curriculum development, and a call for new methods of teaching. J Am Acad Dermatol. 2016;74:769-771. doi:10.1016/j.jaad.2015.11.017
- Hu L, Haynes H, Ferrazza D, et al. Impact of specialist consultations on inpatient admissions for dermatology-specific and related DRGs. J Gen Intern Med. 2013;28:1477-1482. doi:10.1007/s11606-013-2440-2
- Faletsky A, Han JJ, Mostaghimi A. Inpatient dermatology best practice strategies for educating and relaying findings to colleagues. Curr Dermatol Rep. 2020;9:256-260. doi:10.1007/s13671-020-00317-y
- Fox LP. Hospital dermatology, introduction. Semin Cutan Med Surg. 2017;36:1-2. doi:10.12788/j.sder.2017.015
- Li D, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543. doi:10.1001/jamadermatol.2017.6197
- Biesbroeck LK, Shinohara MM. Inpatient consultative dermatology. Med Clin North Am. 2015;99:1349-1364. doi:10.1016/j.mcna.2015.06.004
Practice Points
- Primary inpatient teams should call patients’ insurance companies to verify in-network dermatologists for eventual outpatient follow-up.
- Chronic skin problems (eg, psoriasis, hidradenitis suppurativa) are better cared for in an outpatient setting due to the necessity for follow-up reassessments.
- There remains a need to fill knowledge gaps for common inpatient dermatologic problems that do not necessitate consultations, such as morbilliform drug rash and other chronic and unchanged dermatoses.
Tinted Sunscreens: Consumer Preferences Based on Light, Medium, and Dark Skin Tones
Sunscreen formulations typically protect from UV radiation (290–400 nm), as this is a well-established cause of photodamage, photoaging, and skin cancer.1 However, sunlight also consists of visible (400–700 nm) and infrared (>700 nm) radiation.2 In fact, UV radiation only comprises 5% to 7% of the solar radiation that reaches the surface of the earth, while visible and infrared lights comprise 44% and 53%, respectively.3 Visible light (VL) is the only portion of the solar spectrum visible to the human eye; it penetrates the skin to a depth range of 90 to 750 µm compared to 1.5 to 90 µm for UV radiation.4 Visible light also may come from artificial sources such as light bulbs and digital screens. The rapidly increasing use of smartphones, tablets, laptops, and other digital screens that emit high levels of short-wavelength VL has increased concerns about the safety of these devices. Although blue light exposure from screens is small compared with the amount of exposure from the sun, there is concern about the long-term effects of excessive screen time. Recent studies have demonstrated that exposure to light emitted from electronic devices, even for as little as 1 hour, may cause reactive oxygen species generation, apoptosis, collagen degradation, and necrosis of skin cells.5 Visible light increases tyrosinase activity and induces immediate erythema in light-skinned individuals and long-lasting pigmentation in dark-skinned individuals.4,6
Sunscreens consist of chemical and mineral active ingredients that contain UV filters designed to absorb, scatter, and reflect UV photons with wavelengths up to 380 nm. Historically, traditional options do not protect against the effects induced by VL, as these sunscreens use nanosized particles that help to reduce the white appearance and result in transparency of the product.7 To block VL, the topical agent must be visible. Tinted sunscreens (TSs) are products that combine UV and VL filters. They give a colored base coverage that is achieved by incorporating a blend of black, red, and yellow iron oxides (IOs) and/or pigmentary titanium dioxide (PTD)(ie, titanium dioxide [TD] that is not nanosized). Because TSs offer an instant glow and protect the skin from both sun and artificial light, they have become increasingly popular and have been incorporated into makeup and skin care products to facilitate daily convenient use.
The purpose of this analysis was to study current available options and product factors that may influence consumer preference when choosing a TS based on the reviewer characteristics.
Methods
The keyword sunscreen was searched in the broader category of skin care products on an online supplier of sunscreens (www.sephora.com). This supplier was chosen because, unlike other sources, specific reviewer characteristics regarding underlying skin tone also were available. The search produced 161 results. For the purpose of this analysis, only facial TSs containing IO and/or PTD were included. Each sunscreen was checked by the authors, and 58 sunscreens that met the inclusion criteria were identified and further reviewed. Descriptive data, including formulation, sun protection factor (SPF), ingredient type (chemical or physical), pigments used, shades available, additional benefits, price range, rating, and user reviews, were gathered. The authors extracted these data from the product information on the website, manufacturer claims, ratings, and reviewer comments on each of the listed sunscreens.
For each product, the content of the top 10 most helpful positive and negative reviews as voted by consumers (1160 total reviews, consisting of 1 or more comments) was analyzed. Two authors (H.D.L.G. and P.V.) coded consumer-reported comments for positive and negative descriptors into the categories of cosmetic elegance, performance, skin compatibility and tolerance, tone compatibility, and affordability. Cosmetic elegance was defined as any feature associated with skin sensation (eg, greasy), color (eg, white cast), scent, ability to blend, and overall appearance of the product on the skin. Product performance included SPF, effectiveness in preventing sunburn, coverage, and finish claims (ie, matte, glow, invisible). Skin compatibility and tolerance were represented in the reviewers’ comments and reflected how the product performed in association with underlying dermatologic conditions, skin type, and if there were any side effects such as irritation or allergic reactions. Tone compatibility referred to TS color similarity with users’ skin and shades available for individual products. Affordability reflected consumers’ perceptions of the product price. Comments may be included in multiple categories (eg, a product was noted to blend well on the skin but did not provide enough coverage). Of entries, 10% (116/1160 reviews) were coded by first author (H.D.L.G.) to ensure internal validity. Reviewer characteristics were consistently available and were used to determine the top 5 recommended products for light-, medium-, and dark-skinned individuals based on the number of 5-star ratings in each group. Porcelain, fair, and light were considered light skin tones. Medium, tan, and olive were considered medium skin tones. Deep, dark, and ebony were considered dark skin tones.
Results
Sunscreen Characteristics—Among the 161 screened products, 58 met the inclusion criteria. Four types of formulations were included: lotion, cream, liquid, and powder. Twenty-nine (50%) were creams, followed by lotions (19%), liquids (28%), and powders (3%). More than 79% (46/58) of products had a reported SPF of 30 or higher. Sunscreens with an active physical ingredient—the minerals TD and/or zinc oxide (ZO)—were most common (33/58 [57%]), followed by the chemical sunscreens avobenzone, octinoxate, oxybenzone, homosalate, octisalate, and/or octocrylene active ingredients (14/58 [24%]), and a combination of chemical and physical sunscreens (11/58 [19%]). Nearly all products (55/58 [95%]) contained pigmentary IO (red, CI 77491; yellow, CI 77492; black, CI 77499). Notably, only 38% (22/58) of products had more than 1 shade. All products had additional claims associated with being hydrating, having antiaging effects, smoothing texture, minimizing the appearance of pores, softening lines, and/or promoting even skin tone. Traditional physical sunscreens (those containing TD and/or ZO) were more expensive than chemical sunscreens, with a median price of $30. The median review rating was 4.5 of 5 stars, with a median of 2300 customer reviews per product. Findings are summarized in Table 1.
Positive Features of Sunscreens—Based on an analysis of total reviews (N=1160), cosmetic elegance was the most cited positive feature associated with TS products (31%), followed by product performance (10%). Skin compatibility and tolerance (7%), tone compatibility (7%), and affordability (7%) were cited less commonly as positive features. When negative features were cited, consumers mostly noted tone incompatibility (16%) and cosmetic elegance concerns (14%). Product performance (13%) was comparatively cited as a negative feature (Table 1). Exemplary positive comments categorized in cosmetic elegance included the subthemes of rubs in well and natural glow. Exemplary negative comments in cosmetic elegance and tone compatibility categories included the subthemes patchy/dry finish and color mismatch. Table 1 illustrates these findings.
Product Recommendations—The top 5 recommendations of the best TS for each skin tone are listed in Table 2. The mean price of the recommended products was $42 for 1 to 1.9 oz. Laura Mercier Tinted Moisturizer Oil Free Natural Skin Perfector broad spectrum SPF 20 (Laura Mercier) was the top product for all 3 groups. Similarly, of 58 products available, the same 5 products—Laura Mercier Tinted Moisturizer Oil Free Natural Skin Perfector broad spectrum SPF 20, IT Cosmetics CC+ Cream with SPF 50 (IT Cosmetics, LLC), Tarte Amazonian Clay BB Tinted Moisturizer Broad Spectrum SPF 20 (Tarte Cosmetics), NARS Pure Radiant Tinted Moisturizer Broad Spectrum SPF 30 (NARS Cosmetics), and Laura Mercier Tinted Moisturizer Natural Skin Perfector broad spectrum SPF 30—were considered the best among consumers of all skin tones, with the addition of 2 different products (bareMinerals Original Liquid Mineral Foundation Broad Spectrum SPF 20 [bareMinerals] and ILIA Super Serum Skin Tint SPF 40 Foundation [ILIA Beauty]) in the dark skin group. Notably, these products were the only ones on Sephora’s website that offered up to 30 (22 on average) different shades.
Comment
Tone Compatibility—Tinted sunscreens were created to extend the range of photoprotection into the VL spectrum. The goal of TSs is to incorporate pigments that blend in with the natural skin tone, produce a glow, and have an aesthetically pleasing appearance. To accommodate a variety of skin colors, different shades can be obtained by mixing different amounts of yellow, red, and black IO with or without PTD. The pigments and reflective compounds provide color, opacity, and a natural coverage. Our qualitative analysis provides information on the lack of diversity among shades available for TS, especially for darker skin tones. Of the 58 products evaluated, 62% (32/58) only had 1 shade. In our cohort, tone compatibility was the most commonly cited negative feature. Of note, 89% of these comments were from consumers with dark skin tones, and there was a disproportional number of reviews by darker-skinned individuals compared to users with light and medium skin tones. This is of particular importance, as TSs have been shown to protect against dermatoses that disproportionally affect individuals with skin of color. When comparing sunscreen formulations containing IO with regular mineral sunscreens, Dumbuya et al3 found that IO-containing formulations significantly protected against VL-induced pigmentation compared with untreated skin or mineral sunscreen with SPF 50 or higher in individuals with Fitzpatrick skin type IV (P<.001). Similarly, Bernstein et al8 found that exposing patients with Fitzpatrick skin types III and IV to blue-violet light resulted in marked hyperpigmentation that lasted up to 3 months. Visible light elicits immediate and persistent pigment darkening in individuals with Fitzpatrick skin phototype III and above via the photo-oxidation of pre-existing melanin and de novo melanogenesis.9 Tinted sunscreens formulated with IO have been shown to aid in the treatment of melasma and prevent hyperpigmentation in individuals with Fitzpatrick skin types IV to VI.10 Patients with darker skin tones with dermatoses aggravated or induced by VL, such as melasma and postinflammatory hyperpigmentation, may seek photoprotection provided by TS but find the lack of matching shades unappealing. The dearth of shade diversity that matches all skin tones can lead to inequities and disproportionally affect those with darker skin.
Performance—Tinted sunscreen formulations containing IO have been proven effective in protecting against high-energy VL, especially when combined synergistically with ZO.11 Kaye et al12 found that TSs containing IO and the inorganic filters TD or ZO reduced transmittance of VL more effectively than nontinted sunscreens containing TD or ZO alone or products containing organic filters. The decreased VL transmittance in the former is due to synergistic effects of the VL-scattering properties of the TD and the VL absorption properties of the IO. Similarly, Sayre et al13 demonstrated that IO was superior to TD and ZO in attenuating the transmission of VL. Bernstein et al14 found that darker shades containing higher percentages of IO increased the attenuation of VL to 98% compared with lighter shades attenuating 93%. This correlates with the results of prior studies highlighting the potential of TSs in protecting individuals with skin of color.3 In our cohort, comments regarding product performance and protection were mostly positive, claiming that consistent use reduced hyperpigmentation on the skin surface, giving the appearance of a more even skin tone.
Tolerability—Iron oxides are minerals known to be safe, gentle, and nontoxic on the surface of the skin.15 Two case reports of contact dermatitis due to IO have been reported.16,17 Within our cohort, only a few of the comments (6%) described negative product tolerance or compatibility with their skin type. However, it is more likely that these incompatibilities were due to other ingredients in the product or the individuals’ underlying dermatologic conditions.
Cosmetic Elegance—Most of the sunscreens available on the market today contain micronized forms of TD and ZO particles because they have better cosmetic acceptability.18 However, their reduced size compromises the protection provided against VL whereby the addition of IO is of vital importance. According to the RealSelf Sun Safety Report, only 11% of Americans wear sunscreen daily, and 46% never wear sunscreen.19 The most common reasons consumers reported for not wearing sunscreen included not liking how it looks on the skin, forgetting to apply it, and/or believing that application is inconvenient and time-consuming. Currently, TSs have been incorporated into daily-life products such as makeup, moisturizers, and serums, making application for users easy and convenient, decreasing the necessity of using multiple products, and offering the opportunity to choose from different presentations to make decisions for convenience and/or diverse occasions. Products containing IO blend in with the natural skin tone and have an aesthetically pleasing cosmetic appearance. In our cohort, comments regarding cosmetic elegance were highly valued and were present in multiple reviews (45%), with 69% being positive.
Affordability—In our cohort, product price was not predominantly mentioned in consumers’ reviews. However, negative comments regarding affordability were slightly higher than the positive (56% vs 44%). Notably, the mean price of our top recommendations was $42. Higher price was associated with products with a wider range of shades available. Prior studies have found similar results demonstrating that websites with recommendations on sunscreens for patients with skin of color compared with sunscreens for white or fair skin were more likely to recommend more expensive products (median, $14/oz vs $11.3/oz) despite the lower SPF level.20 According to Schneider,21 daily use of the cheapest sunscreen on the head/neck region recommended for white/pale skin ($2/oz) would lead to an annual cost of $61 compared to $182 for darker skin ($6/oz). This showcases the considerable variation in sunscreen prices for both populations that could potentiate disparities and vulnerability in the latter group.
Conclusion
Tinted sunscreens provide both functional and cosmetic benefits and are a safe, effective, and convenient way to protect against high-energy VL. This study suggests that patients with skin of color encounter difficulties in finding matching shades in TS products. These difficulties may stem from the lack of knowledge regarding dark complexions and undertones and the lack of representation of black and brown skin that has persisted in dermatology research journals and textbooks for decades.22 Our study provides important insights to help dermatologists improve their familiarity with the brands and characteristics of TSs geared to patients with all skin tones, including skin of color. Limitations include single-retailer information and inclusion of both highly and poorly rated comments with subjective data, limiting generalizability. The limited selection of shades for darker skin poses a roadblock to proper treatment and prevention. These data represent an area for improvement within the beauty industry and the dermatologic field to deliver culturally sensitive care by being knowledgeable about darker skin tones and TS formulations tailored to people with skin of color.
- McDaniel D, Farris P, Valacchi G. Atmospheric skin aging-contributors and inhibitors. J Cosmet Dermatol. 2018;17:124-137.
- Duteil L, Cardot-Leccia N, Queille-Roussel C, et al. Differences in visible light-induced pigmentation according to wavelengths: a clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014;27:822-826.
- Dumbuya H, Grimes PE, Lynch S, et al. Impact of iron-oxide containing formulations against visible light-induced skin pigmentation in skin of color individuals. J Drugs Dermatol. 2020;19:712-717.
- Lyons AB, Trullas C, Kohli I, et al. Photoprotection beyond ultraviolet radiation: a review of tinted sunscreens. J Am Acad Dermatol. 2021;84:1393-1397.
- Austin E, Huang A, Adar T, et al. Electronic device generated light increases reactive oxygen species in human fibroblasts [published online February 5, 2018]. Lasers Surg Med. doi:10.1002/lsm.22794
- Randhawa M, Seo I, Liebel F, et al. Visible light induces melanogenesis in human skin through a photoadaptive response. PLoS One. 2015;10:e0130949.
- Yeager DG, Lim HW. What’s new in photoprotection: a review of new concepts and controversies. Dermatol Clin. 2019;37:149-157.
- Bernstein EF, Sarkas HW, Boland P. Iron oxides in novel skin care formulations attenuate blue light for enhanced protection against skin damage. J Cosmet Dermatol. 2021;20:532-537.
- Duteil L, Cardot-Leccia N, Queille-Roussel C, et al. Differences in visible light-induced pigmentation according to wavelengths: a clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014;27:822-826.
- Ruvolo E, Fair M, Hutson A, et al. Photoprotection against visible light-induced pigmentation. Int J Cosmet Sci. 2018;40:589-595.
- Cohen L, Brodsky MA, Zubair R, et al. Cutaneous interaction with visible light: what do we know. J Am Acad Dermatol. 2020;S0190-9622(20)30551-X.
- Kaye ET, Levin JA, Blank IH, et al. Efficiency of opaque photoprotective agents in the visible light range. Arch Dermatol. 1991;127:351-355.
- Sayre RM, Kollias N, Roberts RL, et al. Physical sunscreens. J Soc Cosmet Chem. 1990;41:103-109.
- Bernstein EF, Sarkas HW, Boland P, et al. Beyond sun protection factor: an approach to environmental protection with novel mineral coatings in a vehicle containing a blend of skincare ingredients. J Cosmet Dermatol. 2020;19:407-415.
- MacLeman E. Why are iron oxides used? Deep Science website. February 10, 2022. Accessed March 22, 2022. https://thedermreview.com/iron-oxides-ci-77491-ci-77492-ci-77499/
- Zugerman C. Contact dermatitis to yellow iron oxide. Contact Dermatitis. 1985;13:107-109.
- Saxena M, Warshaw E, Ahmed DD. Eyelid allergic contact dermatitis to black iron oxide. Am J Contact Dermat. 2001;12:38-39.
- Smijs TG, Pavel S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: focus on their safety and effectiveness. Nanotechnol Sci Appl. 2011;4:95-112.
- 2020 RealSelf Sun Safety Report: majority of Americans don’t use sunscreen daily. Practical Dermatology. May 6, 2020. Accessed March 22, 2022. https://practicaldermatology.com/news/realself-sun-safety-report-majority-of-americans-dont-use-sunscreen-daily
- Song H, Beckles A, Salian P, et al. Sunscreen recommendations for patients with skin of color in the popular press and in the dermatology clinic. Int J Womens Dermatol. 2020;7:165-170.
- Schneider J. The teaspoon rule of applying sunscreen. Arch Dermatol. 2002;138:838-839.
- Nelson B. How dermatology is failing melanoma patients with skin of color: unanswered questions on risk and eye-opening disparities in outcomes are weighing heavily on melanoma patients with darker skin. in this article, part 1 of a 2-part series, we explore the deadly consequences of racism and inequality in cancer care. Cancer Cytopathol. 2020;128:7-8.
Sunscreen formulations typically protect from UV radiation (290–400 nm), as this is a well-established cause of photodamage, photoaging, and skin cancer.1 However, sunlight also consists of visible (400–700 nm) and infrared (>700 nm) radiation.2 In fact, UV radiation only comprises 5% to 7% of the solar radiation that reaches the surface of the earth, while visible and infrared lights comprise 44% and 53%, respectively.3 Visible light (VL) is the only portion of the solar spectrum visible to the human eye; it penetrates the skin to a depth range of 90 to 750 µm compared to 1.5 to 90 µm for UV radiation.4 Visible light also may come from artificial sources such as light bulbs and digital screens. The rapidly increasing use of smartphones, tablets, laptops, and other digital screens that emit high levels of short-wavelength VL has increased concerns about the safety of these devices. Although blue light exposure from screens is small compared with the amount of exposure from the sun, there is concern about the long-term effects of excessive screen time. Recent studies have demonstrated that exposure to light emitted from electronic devices, even for as little as 1 hour, may cause reactive oxygen species generation, apoptosis, collagen degradation, and necrosis of skin cells.5 Visible light increases tyrosinase activity and induces immediate erythema in light-skinned individuals and long-lasting pigmentation in dark-skinned individuals.4,6
Sunscreens consist of chemical and mineral active ingredients that contain UV filters designed to absorb, scatter, and reflect UV photons with wavelengths up to 380 nm. Historically, traditional options do not protect against the effects induced by VL, as these sunscreens use nanosized particles that help to reduce the white appearance and result in transparency of the product.7 To block VL, the topical agent must be visible. Tinted sunscreens (TSs) are products that combine UV and VL filters. They give a colored base coverage that is achieved by incorporating a blend of black, red, and yellow iron oxides (IOs) and/or pigmentary titanium dioxide (PTD)(ie, titanium dioxide [TD] that is not nanosized). Because TSs offer an instant glow and protect the skin from both sun and artificial light, they have become increasingly popular and have been incorporated into makeup and skin care products to facilitate daily convenient use.
The purpose of this analysis was to study current available options and product factors that may influence consumer preference when choosing a TS based on the reviewer characteristics.
Methods
The keyword sunscreen was searched in the broader category of skin care products on an online supplier of sunscreens (www.sephora.com). This supplier was chosen because, unlike other sources, specific reviewer characteristics regarding underlying skin tone also were available. The search produced 161 results. For the purpose of this analysis, only facial TSs containing IO and/or PTD were included. Each sunscreen was checked by the authors, and 58 sunscreens that met the inclusion criteria were identified and further reviewed. Descriptive data, including formulation, sun protection factor (SPF), ingredient type (chemical or physical), pigments used, shades available, additional benefits, price range, rating, and user reviews, were gathered. The authors extracted these data from the product information on the website, manufacturer claims, ratings, and reviewer comments on each of the listed sunscreens.
For each product, the content of the top 10 most helpful positive and negative reviews as voted by consumers (1160 total reviews, consisting of 1 or more comments) was analyzed. Two authors (H.D.L.G. and P.V.) coded consumer-reported comments for positive and negative descriptors into the categories of cosmetic elegance, performance, skin compatibility and tolerance, tone compatibility, and affordability. Cosmetic elegance was defined as any feature associated with skin sensation (eg, greasy), color (eg, white cast), scent, ability to blend, and overall appearance of the product on the skin. Product performance included SPF, effectiveness in preventing sunburn, coverage, and finish claims (ie, matte, glow, invisible). Skin compatibility and tolerance were represented in the reviewers’ comments and reflected how the product performed in association with underlying dermatologic conditions, skin type, and if there were any side effects such as irritation or allergic reactions. Tone compatibility referred to TS color similarity with users’ skin and shades available for individual products. Affordability reflected consumers’ perceptions of the product price. Comments may be included in multiple categories (eg, a product was noted to blend well on the skin but did not provide enough coverage). Of entries, 10% (116/1160 reviews) were coded by first author (H.D.L.G.) to ensure internal validity. Reviewer characteristics were consistently available and were used to determine the top 5 recommended products for light-, medium-, and dark-skinned individuals based on the number of 5-star ratings in each group. Porcelain, fair, and light were considered light skin tones. Medium, tan, and olive were considered medium skin tones. Deep, dark, and ebony were considered dark skin tones.
Results
Sunscreen Characteristics—Among the 161 screened products, 58 met the inclusion criteria. Four types of formulations were included: lotion, cream, liquid, and powder. Twenty-nine (50%) were creams, followed by lotions (19%), liquids (28%), and powders (3%). More than 79% (46/58) of products had a reported SPF of 30 or higher. Sunscreens with an active physical ingredient—the minerals TD and/or zinc oxide (ZO)—were most common (33/58 [57%]), followed by the chemical sunscreens avobenzone, octinoxate, oxybenzone, homosalate, octisalate, and/or octocrylene active ingredients (14/58 [24%]), and a combination of chemical and physical sunscreens (11/58 [19%]). Nearly all products (55/58 [95%]) contained pigmentary IO (red, CI 77491; yellow, CI 77492; black, CI 77499). Notably, only 38% (22/58) of products had more than 1 shade. All products had additional claims associated with being hydrating, having antiaging effects, smoothing texture, minimizing the appearance of pores, softening lines, and/or promoting even skin tone. Traditional physical sunscreens (those containing TD and/or ZO) were more expensive than chemical sunscreens, with a median price of $30. The median review rating was 4.5 of 5 stars, with a median of 2300 customer reviews per product. Findings are summarized in Table 1.
Positive Features of Sunscreens—Based on an analysis of total reviews (N=1160), cosmetic elegance was the most cited positive feature associated with TS products (31%), followed by product performance (10%). Skin compatibility and tolerance (7%), tone compatibility (7%), and affordability (7%) were cited less commonly as positive features. When negative features were cited, consumers mostly noted tone incompatibility (16%) and cosmetic elegance concerns (14%). Product performance (13%) was comparatively cited as a negative feature (Table 1). Exemplary positive comments categorized in cosmetic elegance included the subthemes of rubs in well and natural glow. Exemplary negative comments in cosmetic elegance and tone compatibility categories included the subthemes patchy/dry finish and color mismatch. Table 1 illustrates these findings.
Product Recommendations—The top 5 recommendations of the best TS for each skin tone are listed in Table 2. The mean price of the recommended products was $42 for 1 to 1.9 oz. Laura Mercier Tinted Moisturizer Oil Free Natural Skin Perfector broad spectrum SPF 20 (Laura Mercier) was the top product for all 3 groups. Similarly, of 58 products available, the same 5 products—Laura Mercier Tinted Moisturizer Oil Free Natural Skin Perfector broad spectrum SPF 20, IT Cosmetics CC+ Cream with SPF 50 (IT Cosmetics, LLC), Tarte Amazonian Clay BB Tinted Moisturizer Broad Spectrum SPF 20 (Tarte Cosmetics), NARS Pure Radiant Tinted Moisturizer Broad Spectrum SPF 30 (NARS Cosmetics), and Laura Mercier Tinted Moisturizer Natural Skin Perfector broad spectrum SPF 30—were considered the best among consumers of all skin tones, with the addition of 2 different products (bareMinerals Original Liquid Mineral Foundation Broad Spectrum SPF 20 [bareMinerals] and ILIA Super Serum Skin Tint SPF 40 Foundation [ILIA Beauty]) in the dark skin group. Notably, these products were the only ones on Sephora’s website that offered up to 30 (22 on average) different shades.
Comment
Tone Compatibility—Tinted sunscreens were created to extend the range of photoprotection into the VL spectrum. The goal of TSs is to incorporate pigments that blend in with the natural skin tone, produce a glow, and have an aesthetically pleasing appearance. To accommodate a variety of skin colors, different shades can be obtained by mixing different amounts of yellow, red, and black IO with or without PTD. The pigments and reflective compounds provide color, opacity, and a natural coverage. Our qualitative analysis provides information on the lack of diversity among shades available for TS, especially for darker skin tones. Of the 58 products evaluated, 62% (32/58) only had 1 shade. In our cohort, tone compatibility was the most commonly cited negative feature. Of note, 89% of these comments were from consumers with dark skin tones, and there was a disproportional number of reviews by darker-skinned individuals compared to users with light and medium skin tones. This is of particular importance, as TSs have been shown to protect against dermatoses that disproportionally affect individuals with skin of color. When comparing sunscreen formulations containing IO with regular mineral sunscreens, Dumbuya et al3 found that IO-containing formulations significantly protected against VL-induced pigmentation compared with untreated skin or mineral sunscreen with SPF 50 or higher in individuals with Fitzpatrick skin type IV (P<.001). Similarly, Bernstein et al8 found that exposing patients with Fitzpatrick skin types III and IV to blue-violet light resulted in marked hyperpigmentation that lasted up to 3 months. Visible light elicits immediate and persistent pigment darkening in individuals with Fitzpatrick skin phototype III and above via the photo-oxidation of pre-existing melanin and de novo melanogenesis.9 Tinted sunscreens formulated with IO have been shown to aid in the treatment of melasma and prevent hyperpigmentation in individuals with Fitzpatrick skin types IV to VI.10 Patients with darker skin tones with dermatoses aggravated or induced by VL, such as melasma and postinflammatory hyperpigmentation, may seek photoprotection provided by TS but find the lack of matching shades unappealing. The dearth of shade diversity that matches all skin tones can lead to inequities and disproportionally affect those with darker skin.
Performance—Tinted sunscreen formulations containing IO have been proven effective in protecting against high-energy VL, especially when combined synergistically with ZO.11 Kaye et al12 found that TSs containing IO and the inorganic filters TD or ZO reduced transmittance of VL more effectively than nontinted sunscreens containing TD or ZO alone or products containing organic filters. The decreased VL transmittance in the former is due to synergistic effects of the VL-scattering properties of the TD and the VL absorption properties of the IO. Similarly, Sayre et al13 demonstrated that IO was superior to TD and ZO in attenuating the transmission of VL. Bernstein et al14 found that darker shades containing higher percentages of IO increased the attenuation of VL to 98% compared with lighter shades attenuating 93%. This correlates with the results of prior studies highlighting the potential of TSs in protecting individuals with skin of color.3 In our cohort, comments regarding product performance and protection were mostly positive, claiming that consistent use reduced hyperpigmentation on the skin surface, giving the appearance of a more even skin tone.
Tolerability—Iron oxides are minerals known to be safe, gentle, and nontoxic on the surface of the skin.15 Two case reports of contact dermatitis due to IO have been reported.16,17 Within our cohort, only a few of the comments (6%) described negative product tolerance or compatibility with their skin type. However, it is more likely that these incompatibilities were due to other ingredients in the product or the individuals’ underlying dermatologic conditions.
Cosmetic Elegance—Most of the sunscreens available on the market today contain micronized forms of TD and ZO particles because they have better cosmetic acceptability.18 However, their reduced size compromises the protection provided against VL whereby the addition of IO is of vital importance. According to the RealSelf Sun Safety Report, only 11% of Americans wear sunscreen daily, and 46% never wear sunscreen.19 The most common reasons consumers reported for not wearing sunscreen included not liking how it looks on the skin, forgetting to apply it, and/or believing that application is inconvenient and time-consuming. Currently, TSs have been incorporated into daily-life products such as makeup, moisturizers, and serums, making application for users easy and convenient, decreasing the necessity of using multiple products, and offering the opportunity to choose from different presentations to make decisions for convenience and/or diverse occasions. Products containing IO blend in with the natural skin tone and have an aesthetically pleasing cosmetic appearance. In our cohort, comments regarding cosmetic elegance were highly valued and were present in multiple reviews (45%), with 69% being positive.
Affordability—In our cohort, product price was not predominantly mentioned in consumers’ reviews. However, negative comments regarding affordability were slightly higher than the positive (56% vs 44%). Notably, the mean price of our top recommendations was $42. Higher price was associated with products with a wider range of shades available. Prior studies have found similar results demonstrating that websites with recommendations on sunscreens for patients with skin of color compared with sunscreens for white or fair skin were more likely to recommend more expensive products (median, $14/oz vs $11.3/oz) despite the lower SPF level.20 According to Schneider,21 daily use of the cheapest sunscreen on the head/neck region recommended for white/pale skin ($2/oz) would lead to an annual cost of $61 compared to $182 for darker skin ($6/oz). This showcases the considerable variation in sunscreen prices for both populations that could potentiate disparities and vulnerability in the latter group.
Conclusion
Tinted sunscreens provide both functional and cosmetic benefits and are a safe, effective, and convenient way to protect against high-energy VL. This study suggests that patients with skin of color encounter difficulties in finding matching shades in TS products. These difficulties may stem from the lack of knowledge regarding dark complexions and undertones and the lack of representation of black and brown skin that has persisted in dermatology research journals and textbooks for decades.22 Our study provides important insights to help dermatologists improve their familiarity with the brands and characteristics of TSs geared to patients with all skin tones, including skin of color. Limitations include single-retailer information and inclusion of both highly and poorly rated comments with subjective data, limiting generalizability. The limited selection of shades for darker skin poses a roadblock to proper treatment and prevention. These data represent an area for improvement within the beauty industry and the dermatologic field to deliver culturally sensitive care by being knowledgeable about darker skin tones and TS formulations tailored to people with skin of color.
Sunscreen formulations typically protect from UV radiation (290–400 nm), as this is a well-established cause of photodamage, photoaging, and skin cancer.1 However, sunlight also consists of visible (400–700 nm) and infrared (>700 nm) radiation.2 In fact, UV radiation only comprises 5% to 7% of the solar radiation that reaches the surface of the earth, while visible and infrared lights comprise 44% and 53%, respectively.3 Visible light (VL) is the only portion of the solar spectrum visible to the human eye; it penetrates the skin to a depth range of 90 to 750 µm compared to 1.5 to 90 µm for UV radiation.4 Visible light also may come from artificial sources such as light bulbs and digital screens. The rapidly increasing use of smartphones, tablets, laptops, and other digital screens that emit high levels of short-wavelength VL has increased concerns about the safety of these devices. Although blue light exposure from screens is small compared with the amount of exposure from the sun, there is concern about the long-term effects of excessive screen time. Recent studies have demonstrated that exposure to light emitted from electronic devices, even for as little as 1 hour, may cause reactive oxygen species generation, apoptosis, collagen degradation, and necrosis of skin cells.5 Visible light increases tyrosinase activity and induces immediate erythema in light-skinned individuals and long-lasting pigmentation in dark-skinned individuals.4,6
Sunscreens consist of chemical and mineral active ingredients that contain UV filters designed to absorb, scatter, and reflect UV photons with wavelengths up to 380 nm. Historically, traditional options do not protect against the effects induced by VL, as these sunscreens use nanosized particles that help to reduce the white appearance and result in transparency of the product.7 To block VL, the topical agent must be visible. Tinted sunscreens (TSs) are products that combine UV and VL filters. They give a colored base coverage that is achieved by incorporating a blend of black, red, and yellow iron oxides (IOs) and/or pigmentary titanium dioxide (PTD)(ie, titanium dioxide [TD] that is not nanosized). Because TSs offer an instant glow and protect the skin from both sun and artificial light, they have become increasingly popular and have been incorporated into makeup and skin care products to facilitate daily convenient use.
The purpose of this analysis was to study current available options and product factors that may influence consumer preference when choosing a TS based on the reviewer characteristics.
Methods
The keyword sunscreen was searched in the broader category of skin care products on an online supplier of sunscreens (www.sephora.com). This supplier was chosen because, unlike other sources, specific reviewer characteristics regarding underlying skin tone also were available. The search produced 161 results. For the purpose of this analysis, only facial TSs containing IO and/or PTD were included. Each sunscreen was checked by the authors, and 58 sunscreens that met the inclusion criteria were identified and further reviewed. Descriptive data, including formulation, sun protection factor (SPF), ingredient type (chemical or physical), pigments used, shades available, additional benefits, price range, rating, and user reviews, were gathered. The authors extracted these data from the product information on the website, manufacturer claims, ratings, and reviewer comments on each of the listed sunscreens.
For each product, the content of the top 10 most helpful positive and negative reviews as voted by consumers (1160 total reviews, consisting of 1 or more comments) was analyzed. Two authors (H.D.L.G. and P.V.) coded consumer-reported comments for positive and negative descriptors into the categories of cosmetic elegance, performance, skin compatibility and tolerance, tone compatibility, and affordability. Cosmetic elegance was defined as any feature associated with skin sensation (eg, greasy), color (eg, white cast), scent, ability to blend, and overall appearance of the product on the skin. Product performance included SPF, effectiveness in preventing sunburn, coverage, and finish claims (ie, matte, glow, invisible). Skin compatibility and tolerance were represented in the reviewers’ comments and reflected how the product performed in association with underlying dermatologic conditions, skin type, and if there were any side effects such as irritation or allergic reactions. Tone compatibility referred to TS color similarity with users’ skin and shades available for individual products. Affordability reflected consumers’ perceptions of the product price. Comments may be included in multiple categories (eg, a product was noted to blend well on the skin but did not provide enough coverage). Of entries, 10% (116/1160 reviews) were coded by first author (H.D.L.G.) to ensure internal validity. Reviewer characteristics were consistently available and were used to determine the top 5 recommended products for light-, medium-, and dark-skinned individuals based on the number of 5-star ratings in each group. Porcelain, fair, and light were considered light skin tones. Medium, tan, and olive were considered medium skin tones. Deep, dark, and ebony were considered dark skin tones.
Results
Sunscreen Characteristics—Among the 161 screened products, 58 met the inclusion criteria. Four types of formulations were included: lotion, cream, liquid, and powder. Twenty-nine (50%) were creams, followed by lotions (19%), liquids (28%), and powders (3%). More than 79% (46/58) of products had a reported SPF of 30 or higher. Sunscreens with an active physical ingredient—the minerals TD and/or zinc oxide (ZO)—were most common (33/58 [57%]), followed by the chemical sunscreens avobenzone, octinoxate, oxybenzone, homosalate, octisalate, and/or octocrylene active ingredients (14/58 [24%]), and a combination of chemical and physical sunscreens (11/58 [19%]). Nearly all products (55/58 [95%]) contained pigmentary IO (red, CI 77491; yellow, CI 77492; black, CI 77499). Notably, only 38% (22/58) of products had more than 1 shade. All products had additional claims associated with being hydrating, having antiaging effects, smoothing texture, minimizing the appearance of pores, softening lines, and/or promoting even skin tone. Traditional physical sunscreens (those containing TD and/or ZO) were more expensive than chemical sunscreens, with a median price of $30. The median review rating was 4.5 of 5 stars, with a median of 2300 customer reviews per product. Findings are summarized in Table 1.
Positive Features of Sunscreens—Based on an analysis of total reviews (N=1160), cosmetic elegance was the most cited positive feature associated with TS products (31%), followed by product performance (10%). Skin compatibility and tolerance (7%), tone compatibility (7%), and affordability (7%) were cited less commonly as positive features. When negative features were cited, consumers mostly noted tone incompatibility (16%) and cosmetic elegance concerns (14%). Product performance (13%) was comparatively cited as a negative feature (Table 1). Exemplary positive comments categorized in cosmetic elegance included the subthemes of rubs in well and natural glow. Exemplary negative comments in cosmetic elegance and tone compatibility categories included the subthemes patchy/dry finish and color mismatch. Table 1 illustrates these findings.
Product Recommendations—The top 5 recommendations of the best TS for each skin tone are listed in Table 2. The mean price of the recommended products was $42 for 1 to 1.9 oz. Laura Mercier Tinted Moisturizer Oil Free Natural Skin Perfector broad spectrum SPF 20 (Laura Mercier) was the top product for all 3 groups. Similarly, of 58 products available, the same 5 products—Laura Mercier Tinted Moisturizer Oil Free Natural Skin Perfector broad spectrum SPF 20, IT Cosmetics CC+ Cream with SPF 50 (IT Cosmetics, LLC), Tarte Amazonian Clay BB Tinted Moisturizer Broad Spectrum SPF 20 (Tarte Cosmetics), NARS Pure Radiant Tinted Moisturizer Broad Spectrum SPF 30 (NARS Cosmetics), and Laura Mercier Tinted Moisturizer Natural Skin Perfector broad spectrum SPF 30—were considered the best among consumers of all skin tones, with the addition of 2 different products (bareMinerals Original Liquid Mineral Foundation Broad Spectrum SPF 20 [bareMinerals] and ILIA Super Serum Skin Tint SPF 40 Foundation [ILIA Beauty]) in the dark skin group. Notably, these products were the only ones on Sephora’s website that offered up to 30 (22 on average) different shades.
Comment
Tone Compatibility—Tinted sunscreens were created to extend the range of photoprotection into the VL spectrum. The goal of TSs is to incorporate pigments that blend in with the natural skin tone, produce a glow, and have an aesthetically pleasing appearance. To accommodate a variety of skin colors, different shades can be obtained by mixing different amounts of yellow, red, and black IO with or without PTD. The pigments and reflective compounds provide color, opacity, and a natural coverage. Our qualitative analysis provides information on the lack of diversity among shades available for TS, especially for darker skin tones. Of the 58 products evaluated, 62% (32/58) only had 1 shade. In our cohort, tone compatibility was the most commonly cited negative feature. Of note, 89% of these comments were from consumers with dark skin tones, and there was a disproportional number of reviews by darker-skinned individuals compared to users with light and medium skin tones. This is of particular importance, as TSs have been shown to protect against dermatoses that disproportionally affect individuals with skin of color. When comparing sunscreen formulations containing IO with regular mineral sunscreens, Dumbuya et al3 found that IO-containing formulations significantly protected against VL-induced pigmentation compared with untreated skin or mineral sunscreen with SPF 50 or higher in individuals with Fitzpatrick skin type IV (P<.001). Similarly, Bernstein et al8 found that exposing patients with Fitzpatrick skin types III and IV to blue-violet light resulted in marked hyperpigmentation that lasted up to 3 months. Visible light elicits immediate and persistent pigment darkening in individuals with Fitzpatrick skin phototype III and above via the photo-oxidation of pre-existing melanin and de novo melanogenesis.9 Tinted sunscreens formulated with IO have been shown to aid in the treatment of melasma and prevent hyperpigmentation in individuals with Fitzpatrick skin types IV to VI.10 Patients with darker skin tones with dermatoses aggravated or induced by VL, such as melasma and postinflammatory hyperpigmentation, may seek photoprotection provided by TS but find the lack of matching shades unappealing. The dearth of shade diversity that matches all skin tones can lead to inequities and disproportionally affect those with darker skin.
Performance—Tinted sunscreen formulations containing IO have been proven effective in protecting against high-energy VL, especially when combined synergistically with ZO.11 Kaye et al12 found that TSs containing IO and the inorganic filters TD or ZO reduced transmittance of VL more effectively than nontinted sunscreens containing TD or ZO alone or products containing organic filters. The decreased VL transmittance in the former is due to synergistic effects of the VL-scattering properties of the TD and the VL absorption properties of the IO. Similarly, Sayre et al13 demonstrated that IO was superior to TD and ZO in attenuating the transmission of VL. Bernstein et al14 found that darker shades containing higher percentages of IO increased the attenuation of VL to 98% compared with lighter shades attenuating 93%. This correlates with the results of prior studies highlighting the potential of TSs in protecting individuals with skin of color.3 In our cohort, comments regarding product performance and protection were mostly positive, claiming that consistent use reduced hyperpigmentation on the skin surface, giving the appearance of a more even skin tone.
Tolerability—Iron oxides are minerals known to be safe, gentle, and nontoxic on the surface of the skin.15 Two case reports of contact dermatitis due to IO have been reported.16,17 Within our cohort, only a few of the comments (6%) described negative product tolerance or compatibility with their skin type. However, it is more likely that these incompatibilities were due to other ingredients in the product or the individuals’ underlying dermatologic conditions.
Cosmetic Elegance—Most of the sunscreens available on the market today contain micronized forms of TD and ZO particles because they have better cosmetic acceptability.18 However, their reduced size compromises the protection provided against VL whereby the addition of IO is of vital importance. According to the RealSelf Sun Safety Report, only 11% of Americans wear sunscreen daily, and 46% never wear sunscreen.19 The most common reasons consumers reported for not wearing sunscreen included not liking how it looks on the skin, forgetting to apply it, and/or believing that application is inconvenient and time-consuming. Currently, TSs have been incorporated into daily-life products such as makeup, moisturizers, and serums, making application for users easy and convenient, decreasing the necessity of using multiple products, and offering the opportunity to choose from different presentations to make decisions for convenience and/or diverse occasions. Products containing IO blend in with the natural skin tone and have an aesthetically pleasing cosmetic appearance. In our cohort, comments regarding cosmetic elegance were highly valued and were present in multiple reviews (45%), with 69% being positive.
Affordability—In our cohort, product price was not predominantly mentioned in consumers’ reviews. However, negative comments regarding affordability were slightly higher than the positive (56% vs 44%). Notably, the mean price of our top recommendations was $42. Higher price was associated with products with a wider range of shades available. Prior studies have found similar results demonstrating that websites with recommendations on sunscreens for patients with skin of color compared with sunscreens for white or fair skin were more likely to recommend more expensive products (median, $14/oz vs $11.3/oz) despite the lower SPF level.20 According to Schneider,21 daily use of the cheapest sunscreen on the head/neck region recommended for white/pale skin ($2/oz) would lead to an annual cost of $61 compared to $182 for darker skin ($6/oz). This showcases the considerable variation in sunscreen prices for both populations that could potentiate disparities and vulnerability in the latter group.
Conclusion
Tinted sunscreens provide both functional and cosmetic benefits and are a safe, effective, and convenient way to protect against high-energy VL. This study suggests that patients with skin of color encounter difficulties in finding matching shades in TS products. These difficulties may stem from the lack of knowledge regarding dark complexions and undertones and the lack of representation of black and brown skin that has persisted in dermatology research journals and textbooks for decades.22 Our study provides important insights to help dermatologists improve their familiarity with the brands and characteristics of TSs geared to patients with all skin tones, including skin of color. Limitations include single-retailer information and inclusion of both highly and poorly rated comments with subjective data, limiting generalizability. The limited selection of shades for darker skin poses a roadblock to proper treatment and prevention. These data represent an area for improvement within the beauty industry and the dermatologic field to deliver culturally sensitive care by being knowledgeable about darker skin tones and TS formulations tailored to people with skin of color.
- McDaniel D, Farris P, Valacchi G. Atmospheric skin aging-contributors and inhibitors. J Cosmet Dermatol. 2018;17:124-137.
- Duteil L, Cardot-Leccia N, Queille-Roussel C, et al. Differences in visible light-induced pigmentation according to wavelengths: a clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014;27:822-826.
- Dumbuya H, Grimes PE, Lynch S, et al. Impact of iron-oxide containing formulations against visible light-induced skin pigmentation in skin of color individuals. J Drugs Dermatol. 2020;19:712-717.
- Lyons AB, Trullas C, Kohli I, et al. Photoprotection beyond ultraviolet radiation: a review of tinted sunscreens. J Am Acad Dermatol. 2021;84:1393-1397.
- Austin E, Huang A, Adar T, et al. Electronic device generated light increases reactive oxygen species in human fibroblasts [published online February 5, 2018]. Lasers Surg Med. doi:10.1002/lsm.22794
- Randhawa M, Seo I, Liebel F, et al. Visible light induces melanogenesis in human skin through a photoadaptive response. PLoS One. 2015;10:e0130949.
- Yeager DG, Lim HW. What’s new in photoprotection: a review of new concepts and controversies. Dermatol Clin. 2019;37:149-157.
- Bernstein EF, Sarkas HW, Boland P. Iron oxides in novel skin care formulations attenuate blue light for enhanced protection against skin damage. J Cosmet Dermatol. 2021;20:532-537.
- Duteil L, Cardot-Leccia N, Queille-Roussel C, et al. Differences in visible light-induced pigmentation according to wavelengths: a clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014;27:822-826.
- Ruvolo E, Fair M, Hutson A, et al. Photoprotection against visible light-induced pigmentation. Int J Cosmet Sci. 2018;40:589-595.
- Cohen L, Brodsky MA, Zubair R, et al. Cutaneous interaction with visible light: what do we know. J Am Acad Dermatol. 2020;S0190-9622(20)30551-X.
- Kaye ET, Levin JA, Blank IH, et al. Efficiency of opaque photoprotective agents in the visible light range. Arch Dermatol. 1991;127:351-355.
- Sayre RM, Kollias N, Roberts RL, et al. Physical sunscreens. J Soc Cosmet Chem. 1990;41:103-109.
- Bernstein EF, Sarkas HW, Boland P, et al. Beyond sun protection factor: an approach to environmental protection with novel mineral coatings in a vehicle containing a blend of skincare ingredients. J Cosmet Dermatol. 2020;19:407-415.
- MacLeman E. Why are iron oxides used? Deep Science website. February 10, 2022. Accessed March 22, 2022. https://thedermreview.com/iron-oxides-ci-77491-ci-77492-ci-77499/
- Zugerman C. Contact dermatitis to yellow iron oxide. Contact Dermatitis. 1985;13:107-109.
- Saxena M, Warshaw E, Ahmed DD. Eyelid allergic contact dermatitis to black iron oxide. Am J Contact Dermat. 2001;12:38-39.
- Smijs TG, Pavel S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: focus on their safety and effectiveness. Nanotechnol Sci Appl. 2011;4:95-112.
- 2020 RealSelf Sun Safety Report: majority of Americans don’t use sunscreen daily. Practical Dermatology. May 6, 2020. Accessed March 22, 2022. https://practicaldermatology.com/news/realself-sun-safety-report-majority-of-americans-dont-use-sunscreen-daily
- Song H, Beckles A, Salian P, et al. Sunscreen recommendations for patients with skin of color in the popular press and in the dermatology clinic. Int J Womens Dermatol. 2020;7:165-170.
- Schneider J. The teaspoon rule of applying sunscreen. Arch Dermatol. 2002;138:838-839.
- Nelson B. How dermatology is failing melanoma patients with skin of color: unanswered questions on risk and eye-opening disparities in outcomes are weighing heavily on melanoma patients with darker skin. in this article, part 1 of a 2-part series, we explore the deadly consequences of racism and inequality in cancer care. Cancer Cytopathol. 2020;128:7-8.
- McDaniel D, Farris P, Valacchi G. Atmospheric skin aging-contributors and inhibitors. J Cosmet Dermatol. 2018;17:124-137.
- Duteil L, Cardot-Leccia N, Queille-Roussel C, et al. Differences in visible light-induced pigmentation according to wavelengths: a clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014;27:822-826.
- Dumbuya H, Grimes PE, Lynch S, et al. Impact of iron-oxide containing formulations against visible light-induced skin pigmentation in skin of color individuals. J Drugs Dermatol. 2020;19:712-717.
- Lyons AB, Trullas C, Kohli I, et al. Photoprotection beyond ultraviolet radiation: a review of tinted sunscreens. J Am Acad Dermatol. 2021;84:1393-1397.
- Austin E, Huang A, Adar T, et al. Electronic device generated light increases reactive oxygen species in human fibroblasts [published online February 5, 2018]. Lasers Surg Med. doi:10.1002/lsm.22794
- Randhawa M, Seo I, Liebel F, et al. Visible light induces melanogenesis in human skin through a photoadaptive response. PLoS One. 2015;10:e0130949.
- Yeager DG, Lim HW. What’s new in photoprotection: a review of new concepts and controversies. Dermatol Clin. 2019;37:149-157.
- Bernstein EF, Sarkas HW, Boland P. Iron oxides in novel skin care formulations attenuate blue light for enhanced protection against skin damage. J Cosmet Dermatol. 2021;20:532-537.
- Duteil L, Cardot-Leccia N, Queille-Roussel C, et al. Differences in visible light-induced pigmentation according to wavelengths: a clinical and histological study in comparison with UVB exposure. Pigment Cell Melanoma Res. 2014;27:822-826.
- Ruvolo E, Fair M, Hutson A, et al. Photoprotection against visible light-induced pigmentation. Int J Cosmet Sci. 2018;40:589-595.
- Cohen L, Brodsky MA, Zubair R, et al. Cutaneous interaction with visible light: what do we know. J Am Acad Dermatol. 2020;S0190-9622(20)30551-X.
- Kaye ET, Levin JA, Blank IH, et al. Efficiency of opaque photoprotective agents in the visible light range. Arch Dermatol. 1991;127:351-355.
- Sayre RM, Kollias N, Roberts RL, et al. Physical sunscreens. J Soc Cosmet Chem. 1990;41:103-109.
- Bernstein EF, Sarkas HW, Boland P, et al. Beyond sun protection factor: an approach to environmental protection with novel mineral coatings in a vehicle containing a blend of skincare ingredients. J Cosmet Dermatol. 2020;19:407-415.
- MacLeman E. Why are iron oxides used? Deep Science website. February 10, 2022. Accessed March 22, 2022. https://thedermreview.com/iron-oxides-ci-77491-ci-77492-ci-77499/
- Zugerman C. Contact dermatitis to yellow iron oxide. Contact Dermatitis. 1985;13:107-109.
- Saxena M, Warshaw E, Ahmed DD. Eyelid allergic contact dermatitis to black iron oxide. Am J Contact Dermat. 2001;12:38-39.
- Smijs TG, Pavel S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: focus on their safety and effectiveness. Nanotechnol Sci Appl. 2011;4:95-112.
- 2020 RealSelf Sun Safety Report: majority of Americans don’t use sunscreen daily. Practical Dermatology. May 6, 2020. Accessed March 22, 2022. https://practicaldermatology.com/news/realself-sun-safety-report-majority-of-americans-dont-use-sunscreen-daily
- Song H, Beckles A, Salian P, et al. Sunscreen recommendations for patients with skin of color in the popular press and in the dermatology clinic. Int J Womens Dermatol. 2020;7:165-170.
- Schneider J. The teaspoon rule of applying sunscreen. Arch Dermatol. 2002;138:838-839.
- Nelson B. How dermatology is failing melanoma patients with skin of color: unanswered questions on risk and eye-opening disparities in outcomes are weighing heavily on melanoma patients with darker skin. in this article, part 1 of a 2-part series, we explore the deadly consequences of racism and inequality in cancer care. Cancer Cytopathol. 2020;128:7-8.
Practice Points
- Visible light has been shown to increase tyrosinase activity and induce immediate erythema in light-skinned individuals and long-lasting pigmentation in dark-skinned individuals.
- The formulation of sunscreens with iron oxides and pigmentary titanium dioxide are a safe and effective way to protect against high-energy visible light, especially when combined with zinc oxide.
- Physicians should be aware of sunscreen characteristics that patients like and dislike to tailor recommendations that are appropriate for each individual to enhance adherence.
- Cosmetic elegance and tone compatibility are the most important criteria for individuals seeking tinted sunscreens.
The Molting Man: Anasarca-Induced Full-Body Desquamation
Edema blisters are a common but often underreported entity most commonly seen on the lower extremities in the setting of acute edema. 1 Reported risk factors and associations include chronic venous insufficiency, congestive heart failure, hereditary angioedema, and medications (eg, amlodipine). 1,2 We report a newly described variant that we have termed anasarca-induced desquamation in which a patient sloughed the entire cutaneous surface of the body after gaining almost 40 pounds over 5 days.
Case Report
A 50-year-old man without a home was found minimally responsive in a yard. His core body temperature was 25.5 °C. He was profoundly acidotic (pH, <6.733 [reference range, 7.35–7.45]; lactic acid, 20.5 mmol/L [reference range, 0.5–2.2 mmol/L]) at admission. His medical history was notable for diabetes mellitus, hypertension, alcohol abuse, and pulmonary embolism. The patient was resuscitated with rewarming and intravenous fluids in the setting of acute renal insufficiency. By day 5 of the hospital stay, he had a net positive intake of 21.8 L and an 18-kg (39.7-lb) weight gain.
Dermatology was consulted for skin sloughing. Physical examination revealed nonpainful desquamation of the vermilion lip, periorbital skin, right shoulder, and hips without notable mucosal changes. Two 4-mm punch biopsies of the shoulder revealed an intracorneal split with desquamation of the stratum corneum and a mild dermal lymphocytic infiltrate, consistent with exfoliation secondary to edema or staphylococcal scalded skin syndrome (Figure 1). No staphylococcal growth was noted on blood, urine, nasal, wound, and ocular cultures throughout the hospital stay.
As the patient’s anasarca improved with diuretics and continuous renal replacement therapy, the entire cutaneous surface—head to toe—underwent desquamation, including the palms and soles. He was managed with supportive skin care. The anasarca healed completely with residual hypopigmentation (Figures 2 and 3).
Comment
Anasarca-induced desquamation represents a more diffuse form of a known entity: edema blisters. Occurring most commonly in the setting of acute exacerbation of chronic venous insufficiency, edema blisters can mimic other vesiculobullous conditions, such as bullous pemphigoid and herpes zoster.3
Pathogenesis of Edema Blisters—Edema develops in the skin when the capillary filtration rate, determined by the hydrostatic and oncotic pressures of the capillaries and interstitium, exceeds venous and lymphatic drainage. The appearance of edema blisters in the acute setting likely is related to the speed at which edema develops in skin.1 Although edema blisters often are described as tense, there is a paucity of histologic data at the anatomical level of split in the skin.In our patient, desquamation was within the stratum corneum and likely multifactorial. His weight gain of nearly 40 lb, the result of intravenous instillation of fluids and low urine output, was undeniably a contributing factor. The anasarca was aggravated by hypoalbuminemia (2.1 g/dL) in the setting of known liver disease. Other possible contributing factors were hypotension, which required vasopressor therapy that led to hypoperfusion of the skin, and treatment of hypothermia, with resulting reactive vasodilation and capillary leak.
Management—Treatment of acute edema blisters is focused on the underlying cause of the edema. In a study of 13 patients with edema blisters, all had blisters on the legs that resolved with treatment, such as diuretics or compression therapy.1
Anasarca-induced desquamation is an inherently benign condition that mimics potentially fatal disorders, such as Stevens-Johnson syndrome, staphylococcal scalded skin syndrome, and toxic shock syndrome. Therefore, patients presenting with diffuse superficial desquamation should be assessed for the mucosal changes of Stevens-Johnson syndrome and a history of acute edema in the affected areas to avoid potentially harmful empiric treatments, such as corticosteroids and intravenous antibiotics.
Conclusion
Anasarca-induced desquamation represents a more diffuse form of edema blisters. This desquamation can mimic a potentially fatal rash, such as Stevens-Johnson syndrome and staphylococcal scalded skin syndrome.
- Bhushan M, Chalmers RJ, Cox NH. Acute oedema blisters: a report of 13 cases. Br J Dermatol. 2001;144:580-582. doi:10.1046/j.1365-2133.2001.04087.x
- Fabiani J, Bork K. Acute edema blisters on a skin swelling: an unusual manifestation of hereditary angioedema. Acta Derm Venereol. 2016;96:556-557. doi:10.2340/00015555-2252
- Chen SX, Cohen PR. Edema bullae mimicking disseminated herpes zoster. Cureus. 2017;9:E1780. doi:10.7759/cureus.1780
Edema blisters are a common but often underreported entity most commonly seen on the lower extremities in the setting of acute edema. 1 Reported risk factors and associations include chronic venous insufficiency, congestive heart failure, hereditary angioedema, and medications (eg, amlodipine). 1,2 We report a newly described variant that we have termed anasarca-induced desquamation in which a patient sloughed the entire cutaneous surface of the body after gaining almost 40 pounds over 5 days.
Case Report
A 50-year-old man without a home was found minimally responsive in a yard. His core body temperature was 25.5 °C. He was profoundly acidotic (pH, <6.733 [reference range, 7.35–7.45]; lactic acid, 20.5 mmol/L [reference range, 0.5–2.2 mmol/L]) at admission. His medical history was notable for diabetes mellitus, hypertension, alcohol abuse, and pulmonary embolism. The patient was resuscitated with rewarming and intravenous fluids in the setting of acute renal insufficiency. By day 5 of the hospital stay, he had a net positive intake of 21.8 L and an 18-kg (39.7-lb) weight gain.
Dermatology was consulted for skin sloughing. Physical examination revealed nonpainful desquamation of the vermilion lip, periorbital skin, right shoulder, and hips without notable mucosal changes. Two 4-mm punch biopsies of the shoulder revealed an intracorneal split with desquamation of the stratum corneum and a mild dermal lymphocytic infiltrate, consistent with exfoliation secondary to edema or staphylococcal scalded skin syndrome (Figure 1). No staphylococcal growth was noted on blood, urine, nasal, wound, and ocular cultures throughout the hospital stay.
As the patient’s anasarca improved with diuretics and continuous renal replacement therapy, the entire cutaneous surface—head to toe—underwent desquamation, including the palms and soles. He was managed with supportive skin care. The anasarca healed completely with residual hypopigmentation (Figures 2 and 3).
Comment
Anasarca-induced desquamation represents a more diffuse form of a known entity: edema blisters. Occurring most commonly in the setting of acute exacerbation of chronic venous insufficiency, edema blisters can mimic other vesiculobullous conditions, such as bullous pemphigoid and herpes zoster.3
Pathogenesis of Edema Blisters—Edema develops in the skin when the capillary filtration rate, determined by the hydrostatic and oncotic pressures of the capillaries and interstitium, exceeds venous and lymphatic drainage. The appearance of edema blisters in the acute setting likely is related to the speed at which edema develops in skin.1 Although edema blisters often are described as tense, there is a paucity of histologic data at the anatomical level of split in the skin.In our patient, desquamation was within the stratum corneum and likely multifactorial. His weight gain of nearly 40 lb, the result of intravenous instillation of fluids and low urine output, was undeniably a contributing factor. The anasarca was aggravated by hypoalbuminemia (2.1 g/dL) in the setting of known liver disease. Other possible contributing factors were hypotension, which required vasopressor therapy that led to hypoperfusion of the skin, and treatment of hypothermia, with resulting reactive vasodilation and capillary leak.
Management—Treatment of acute edema blisters is focused on the underlying cause of the edema. In a study of 13 patients with edema blisters, all had blisters on the legs that resolved with treatment, such as diuretics or compression therapy.1
Anasarca-induced desquamation is an inherently benign condition that mimics potentially fatal disorders, such as Stevens-Johnson syndrome, staphylococcal scalded skin syndrome, and toxic shock syndrome. Therefore, patients presenting with diffuse superficial desquamation should be assessed for the mucosal changes of Stevens-Johnson syndrome and a history of acute edema in the affected areas to avoid potentially harmful empiric treatments, such as corticosteroids and intravenous antibiotics.
Conclusion
Anasarca-induced desquamation represents a more diffuse form of edema blisters. This desquamation can mimic a potentially fatal rash, such as Stevens-Johnson syndrome and staphylococcal scalded skin syndrome.
Edema blisters are a common but often underreported entity most commonly seen on the lower extremities in the setting of acute edema. 1 Reported risk factors and associations include chronic venous insufficiency, congestive heart failure, hereditary angioedema, and medications (eg, amlodipine). 1,2 We report a newly described variant that we have termed anasarca-induced desquamation in which a patient sloughed the entire cutaneous surface of the body after gaining almost 40 pounds over 5 days.
Case Report
A 50-year-old man without a home was found minimally responsive in a yard. His core body temperature was 25.5 °C. He was profoundly acidotic (pH, <6.733 [reference range, 7.35–7.45]; lactic acid, 20.5 mmol/L [reference range, 0.5–2.2 mmol/L]) at admission. His medical history was notable for diabetes mellitus, hypertension, alcohol abuse, and pulmonary embolism. The patient was resuscitated with rewarming and intravenous fluids in the setting of acute renal insufficiency. By day 5 of the hospital stay, he had a net positive intake of 21.8 L and an 18-kg (39.7-lb) weight gain.
Dermatology was consulted for skin sloughing. Physical examination revealed nonpainful desquamation of the vermilion lip, periorbital skin, right shoulder, and hips without notable mucosal changes. Two 4-mm punch biopsies of the shoulder revealed an intracorneal split with desquamation of the stratum corneum and a mild dermal lymphocytic infiltrate, consistent with exfoliation secondary to edema or staphylococcal scalded skin syndrome (Figure 1). No staphylococcal growth was noted on blood, urine, nasal, wound, and ocular cultures throughout the hospital stay.
As the patient’s anasarca improved with diuretics and continuous renal replacement therapy, the entire cutaneous surface—head to toe—underwent desquamation, including the palms and soles. He was managed with supportive skin care. The anasarca healed completely with residual hypopigmentation (Figures 2 and 3).
Comment
Anasarca-induced desquamation represents a more diffuse form of a known entity: edema blisters. Occurring most commonly in the setting of acute exacerbation of chronic venous insufficiency, edema blisters can mimic other vesiculobullous conditions, such as bullous pemphigoid and herpes zoster.3
Pathogenesis of Edema Blisters—Edema develops in the skin when the capillary filtration rate, determined by the hydrostatic and oncotic pressures of the capillaries and interstitium, exceeds venous and lymphatic drainage. The appearance of edema blisters in the acute setting likely is related to the speed at which edema develops in skin.1 Although edema blisters often are described as tense, there is a paucity of histologic data at the anatomical level of split in the skin.In our patient, desquamation was within the stratum corneum and likely multifactorial. His weight gain of nearly 40 lb, the result of intravenous instillation of fluids and low urine output, was undeniably a contributing factor. The anasarca was aggravated by hypoalbuminemia (2.1 g/dL) in the setting of known liver disease. Other possible contributing factors were hypotension, which required vasopressor therapy that led to hypoperfusion of the skin, and treatment of hypothermia, with resulting reactive vasodilation and capillary leak.
Management—Treatment of acute edema blisters is focused on the underlying cause of the edema. In a study of 13 patients with edema blisters, all had blisters on the legs that resolved with treatment, such as diuretics or compression therapy.1
Anasarca-induced desquamation is an inherently benign condition that mimics potentially fatal disorders, such as Stevens-Johnson syndrome, staphylococcal scalded skin syndrome, and toxic shock syndrome. Therefore, patients presenting with diffuse superficial desquamation should be assessed for the mucosal changes of Stevens-Johnson syndrome and a history of acute edema in the affected areas to avoid potentially harmful empiric treatments, such as corticosteroids and intravenous antibiotics.
Conclusion
Anasarca-induced desquamation represents a more diffuse form of edema blisters. This desquamation can mimic a potentially fatal rash, such as Stevens-Johnson syndrome and staphylococcal scalded skin syndrome.
- Bhushan M, Chalmers RJ, Cox NH. Acute oedema blisters: a report of 13 cases. Br J Dermatol. 2001;144:580-582. doi:10.1046/j.1365-2133.2001.04087.x
- Fabiani J, Bork K. Acute edema blisters on a skin swelling: an unusual manifestation of hereditary angioedema. Acta Derm Venereol. 2016;96:556-557. doi:10.2340/00015555-2252
- Chen SX, Cohen PR. Edema bullae mimicking disseminated herpes zoster. Cureus. 2017;9:E1780. doi:10.7759/cureus.1780
- Bhushan M, Chalmers RJ, Cox NH. Acute oedema blisters: a report of 13 cases. Br J Dermatol. 2001;144:580-582. doi:10.1046/j.1365-2133.2001.04087.x
- Fabiani J, Bork K. Acute edema blisters on a skin swelling: an unusual manifestation of hereditary angioedema. Acta Derm Venereol. 2016;96:556-557. doi:10.2340/00015555-2252
- Chen SX, Cohen PR. Edema bullae mimicking disseminated herpes zoster. Cureus. 2017;9:E1780. doi:10.7759/cureus.1780
Practice Points
- The appearance of anasarca-induced desquamation can be similar to staphylococcal scalded skin syndrome and Stevens-Johnson syndrome.
- Histopathologic evaluation of this condition shows desquamation localized to the stratum corneum without epidermal necrosis.
- Careful evaluation, including bacterial culture, is required to rule out an infectious cause.
- Early diagnosis of anasarca-induced desquamation reduces the potential for providing harmful empiric treatment, such as systemic steroids and intravenous antibiotics, especially in patients known to have comorbidities.
Direct-to-Consumer Teledermatology Growth: A Review and Outlook for the Future
In recent years, direct-to-consumer (DTC) teledermatology platforms have gained popularity as telehealth business models, allowing patients to directly initiate visits with physicians and purchase medications from single platforms. A shortage of dermatologists, improved technology, drug patent expirations, and rising health care costs accelerated the growth of DTC dermatology.1 During the COVID-19 pandemic, teledermatology adoption surged due to the need to provide care while social distancing and minimizing viral exposure. These needs prompted additional federal funding and loosened regulatory provisions.2 As the userbase of these companies has grown, so have their valuations.3 Although the DTC model has attracted the attention of patients and investors, its rise provokes many questions about patients acting as consumers in health care. Indeed, DTC telemedicine offers greater autonomy and convenience for patients, but it may impact the quality of care and the nature of physician-patient relationships, perhaps making them more transactional.
Evolution of DTC in Health Care
The DTC model emphasizes individual choice and accessible health care. Although the definition has evolved, the core idea is not new.4 Over decades, pharmaceutical companies have spent billions of dollars on DTC advertising, circumventing physicians by directly reaching patients with campaigns on prescription drugs and laboratory tests and shaping public definitions of diseases.5
The DTC model of care is fundamentally different from traditional care models in that it changes the roles of the patient and physician. Whereas early telehealth models required a health care provider to initiate teleconsultations with specialists, DTC telemedicine bypasses this step (eg, the patient can consult a dermatologist without needing a primary care provider’s input first). This care can then be provided by dermatologists with whom patients may or may not have pre-established relationships.4,6
Dermatology was an early adopter of DTC telemedicine. The shortage of dermatologists in the United States created demand for increasing accessibility to dermatologic care. Additionally, the visual nature of diagnosing dermatologic disease was ideal for platforms supporting image sharing.7 Early DTC providers were primarily individual companies offering teledermatology. However, many dermatologists can now offer DTC capabilities via companies such as Amwell and Teladoc Health.8
Over the last 2 decades, start-ups such as Warby Parker (eyeglasses) and Casper (mattresses) defined the DTC industry using borrowed supply chains, cohesive branding, heavy social media marketing, and web-only retail. Scalability, lack of competition, and abundant venture capital created competition across numerous markets.9 Health care capitalized on this DTC model, creating a $700 billion market for products ranging from hearing aids to over-the-counter medications.10
Borrowing from this DTC playbook, platforms were created to offer delivery of generic prescription drugs to patients’ doorsteps. However, unlike with other products bought online, a consumer cannot simply add prescription drugs to their shopping cart and check out. In all models of American medical practice, physicians still serve as gatekeepers, providing a safeguard for patients to ensure appropriate prescription and avoid negative consequences of unnecessary drug use. This new model effectively streamlines diagnosis, prescription, and drug delivery without the patient ever having to leave home. Combining the prescribing and selling of medications (2 tasks that traditionally have been separated) potentially creates financial conflicts of interest (COIs). Additionally, high utilization of health care, including more prescriptions and visits, does not necessarily equal high quality of care. The companies stand to benefit from extra care regardless of need, and thus these models must be scrutinized for any incentives driving unnecessary care and prescriptions.
Ultimately, DTC has evolved to encompass multiple definitions in health care (Table 1). Although all models provide health care, each offers a different modality of delivery. The primary service may be the sale of prescription drugs or simply telemedicine visits. This review primarily discusses DTC pharmaceutical telemedicine platforms that sell private-label drugs and also offer telemedicine services to streamline care. However, the history, risks, and benefits discussed may apply to all models.
The DTC Landscape
Most DTC companies employ variations on a model with the same 3 main components: a triage questionnaire, telehealth services, and prescription/drug delivery (Figure). The triage questionnaire elicits a history of the patient’s presentation and medical history. Some companies may use artificial intelligence (AI) algorithms to tailor questions to patient needs. There are 2 modalities for patient-provider communication: synchronous and asynchronous. Synchronous communication entails real-time patient-physician conversations via audio only or video call. Asynchronous (or store-and-forward) communication refers to consultations provided via messaging or text-based modality, where a provider may respond to a patient within 24 hours.6 Direct-to-consumer platforms primarily use asynchronous visits (Table 2). However, some also use synchronous modalities if the provider deems it necessary or if state laws require it.
Once a provider has consulted with the patient, they can prescribe medication as needed. In certain cases, with adequate history, a prescription may be issued without a full physician visit. Furthermore, DTC companies require purchase of their custom-branded generic drugs. Prescriptions are fulfilled by the company’s pharmacy network and directly shipped to patients; few will allow patients to transfer a prescription to a pharmacy of their choice. Some platforms also sell supplements and over-the-counter medications.
Payment models vary among these companies, and most do not accept insurance (Table 2). Select models may provide free consultations and only require payment for pharmaceuticals. Others charge for consultations but reallocate payment to the cost of medication if prescribed. Another model involves flat rates for consultations and additional charges for drugs but unlimited messaging with providers for the duration of the prescription. Moreover, patients can subscribe to monthly deliveries of their medications.
Foundation of DTC
Technological advances have enabled patients to receive remote treatment from a single platform offering video calls, AI, electronic medical record interoperability, and integration of drug supply chains. Even in its simplest form, AI is increasingly used, as it allows for programs and chatbots to screen and triage patients.11 Technology also has improved at targeted mass marketing through social media platforms and search engines (eg, companies can use age, interests, location, and other parameters to target individuals likely needing acne treatment).
Drug patent expirations are a key catalyst for the rise of DTC companies, creating an attractive business model with generic drugs as the core product. Since 2008, patents for medications treating chronic conditions, such as erectile dysfunction, have expired. These patent expirations are responsible for $198 billion in projected prescription sales between 2019 and 2024.1 Thus, it follows that DTC companies have seized this opportunity to act as middlemen, taking advantage of these generic medications’ lower costs to create platforms focused on personalization and accessibility.
Rising deductibles have led patients to consider cheaper out-of-pocket alternatives that are not covered by insurance.1 For example, insurers typically do not cover finasteride treatment for conditions deemed cosmetic, such as androgenetic alopecia.12 The low cost of generic drugs creates an attractive business model for patients and investors. According to GoodRx, the average retail price for a 30-day supply of brand-name finasteride (Propecia [Merck]) is $135.92, whereas generic finasteride is $75.24.13 Direct-to-consumer pharmaceutical companies offer a 30-day supply of generic finasteride ranging from $8.33 to $30.14 The average wholesale cost for retailers is an estimated $2.31 for 30 days.15 Although profit margins on generic medications may be lower, more affordable drugs increase the size of the total market. These prescriptions are available as subscription plans, resulting in recurring revenue.
Lax US pharmaceutical marketing regulations allow direct advertising to the general public.16 In 1997, the US Food and Drug Administration allowed DTC advertisements to replace summaries of serious and common adverse effects with short statements covering important risks or referrals to other sources for complete information. In 2015, the US Food and Drug Administration guidelines preventing encouragement of self-diagnosis and self-treatment were withdrawn.5 These changes enable DTC companies to launch large advertising campaigns and to accelerate customer acquisition, as the industry often describes it, with ease.
Rapid Growth and Implications
Increasing generic drug availability and improving telemedicine capabilities have the potential to reduce costs and barriers but also have the potential for financial gain. Venture capital funds have recognized this opportunity, reflected by millions of dollars of investments, and accelerated the growth of DTC health care start-ups. For example, Ro has raised $376 million from venture capital, valuing the company at $1.5 billion.3
Direct-to-consumer companies require a heavy focus on marketing campaigns for customer acquisition. Their aesthetically pleasing websites and aggressive campaigns target specific audiences based on demographics, digital use habits, and purchasing behavior.4 Some campaigns celebrate the ease of obtaining prescriptions.17 Companies have been effective in recruiting so-called millennial and Generation Z patients, known to search the internet for remedies prior to seeking physician consultations.18 Recognizing these needs, some platforms offer guides on diseases they treat, creating effective customer-acquisition funnels. Recruitment of these technology-friendly patients has proven effective, especially given the largely positive media coverage of DTC platforms––potentially serving as a surrogate for medical credibility for patients.18
Some DTC companies also market physically; skin care ads may be strategically placed in social media feeds, or even found near mirrors in public bathrooms.19 Marketing campaigns also involve disease awareness; such efforts serve to increase diagnoses and prescribed treatments while destigmatizing diseases. Although DTC companies argue this strategy empowers patients, these marketing habits have the potential to take advantage of uninformed patients. Campaigns could potentially medicalize normal experiences and expand disease definitions resulting in overdiagnosis, overtreatment, and wasted resources.5 For example, off-label propranolol use has been advertised to attract patients who might have “nerves that come creeping before an important presentation.”17 Disease awareness campaigns also may lead people to falsely believe unproven drug benefits.5 According to studies, DTC pharmaceutical advertisements are low in informational quality and result in increased patient visits and prescriptions despite cost-effective alternatives.5,20-22
Fragmentation of the health care system is another possible complication of DTC teledermatology. These companies operate as for-profit organizations separated from the rest of the health care system, raising concerns about care coordination.8 Vital health data may not be conveyed as patients move among different providers and pharmacies. One study found DTC teledermatology rarely offered to provide medical records or facilitate a referral to a local physician.23 Such a lack of communication is concerning, as medication errors are the leading cause of avoidable harm in health care.24
Direct-to-consumer care models also seemingly redefine the physician-patient relationship by turning patients into consumers. Patient interactions may seem transactional and streamlined toward sales. For these platforms, a visit often is set up as an evaluation of a patient’s suitability for a prescription, not necessarily for the best treatment modality for the problem. These companies primarily make money through the sale of prescription drugs, creating a potential COI that may undermine the patient-physician relationship. Although some companies have made it clear that medical care and pharmaceutical sales are provided by legally separate business entities and that they do not pay physicians on commission, a conflict may still exist given the financial importance of physicians prescribing medication to the success of the business.16
Even as DTC models advertise upon expanded access and choice, the companies largely prohibit patients from choosing their own pharmacy. Instead, they encourage patients to fill prescriptions with the company’s pharmacy network by claiming lower costs compared with competitors. One DTC company, Hims, is launching a prescription-fulfillment center to further consolidate their business.17,19,25 The inherent COI of issuing and fulfilling prescriptions raises concerns of patient harm.26 For example, when Dermatology.com launched as a DTC prescription skin medication shop backed by Bausch Health Companies Inc, its model included telemedicine consultation. Although consultations were provided by RxDefine, a third party, only Dermatology.com drugs were prescribed. Given the poor quality of care and obvious financial COI, an uproar in the dermatology community and advocacy by the American Academy of Dermatology led to the shutdown of Dermatology.com’s online prescription services.26
The quality of care among DTC telemedicine platforms has been equivocal. Some studies have reported equivalent care in person and online, while others have reported poor adherence to guidelines, overuse of antibiotics, and misdiagnosis.8,23 A vital portion of the DTC experience is the history questionnaire, which is geared to diagnosis and risk assessment.25 Resneck et al23 found diagnostic quality to be adequate for simple dermatologic clinical scenarios but poor for scenarios requiring more than basic histories. Although Ro has reported leveraging data from millions of interactions to ask the right questions and streamline visits, it is still unclear whether history questionnaires are adequate.17,27 Additionally, consultations may lack sufficient counseling on adverse effects, risks, or pregnancy warnings, as well as discussions on alternative treatments and preventative care.17,23 Finally, patients often are limited in their choice of dermatologist; the lack of a fully developed relationship increases concerns of follow-up and monitoring practices. Although some DTC platforms offer unlimited interactions with physicians for the duration of a prescription, it is unknown how often these services are utilized or how adequate the quality of these interactions is. This potential for lax follow-up is especially concerning for prescriptions that autorenew on a monthly basis and could result in unnecessary overtreatment.
Postpandemic and Future Outlook
The COVID-19 pandemic dramatically impacted the use of telemedicine. To minimize COVID-19 transmission, the Centers for Medicare & Medicaid Services and private payers expanded telehealth coverage and eliminated reimbursement and licensing barriers.28 A decade’s worth of regulatory changes and consumer adoption was accelerated to weeks, resulting in telemedicine companies reaching record-high visit numbers.29 McKinsey & Company estimated that telehealth visit numbers surged 50- to 175-fold compared with pre–COVID-19 numbers. Additionally, 76% of patients were interested in future telehealth use, and 64% of providers were more comfortable using telehealth than before the pandemic.30 For their part, US dermatologists reported an increase in telemedicine use from 14.1% to 96.9% since COVID-19.31
Exactly how much DTC pharmaceutical telemedicine companies are growing is unclear, but private investments may be an indication. A record $14.7 billion was invested in the digital health sector in the first half of 2021; the majority went to telehealth companies.30 Ro, which reported $230 million in revenue in 2020 and has served 6 million visits, raised $200 milllion in July 2020 and $500 million in March 2021.32 Although post–COVID-19 health care will certainly involve increased telemedicine, the extent remains unclear, as telehealth vendors saw decreased usage upon reopening of state economies. Ultimately, the postpandemic regulatory landscape is hard to predict.30
Although COVID-19 appears to have caused rapid growth for DTC platforms, it also may have spurred competition. Telemedicine providers have given independent dermatologists and health care systems the infrastructure to implement custom DTC services.33 Although systems do not directly sell prescription drugs, the target market is essentially the same: patients looking for instant virtual dermatologic care. Therefore, sustained telemedicine services offered by traditional practices and systems may prove detrimental to DTC companies. However, unlike most telemedicine services, DTC models are less affected by certain changes in regulation since they do not rely on insurance. If regulations are tightened and reimbursements for telehealth are not attractive for dermatologists, teledermatology services may see an overall decrease. If so, patients who appreciate teledermatology may shift to using DTC platforms, even if their insurance does not cover them. Still, a nationwide survey found 56% of respondents felt an established relationship with a physician prior to a telemedicine visit is important, which may create a barrier for DTC adoption.34
Conclusion
Direct-to-consumer teledermatology represents a growing for-profit model of health care that provides patients with seemingly affordable and convenient care. However, there is potential for overtreatment, misdiagnosis, and fragmentation of health care. It will be important to monitor and evaluate the quality of care that DTC teledermatology offers and advocate for appropriate regulations and oversight. Eventually, more patients will have medications prescribed and dermatologic care administered through DTC companies. Dermatologists will benefit from this knowledge of DTC models to properly counsel patients on the risks and benefits of their use.
- Vennare J. The DTC healthcare report. Fitt Insider. September 15, 2019. Accessed February 23, 2022. https://insider.fitt.co/direct-to-consumer-healthcare-startups/
- Kannampallil T, Ma J. Digital translucence: adapting telemedicine delivery post-COVID-19. Telemed J E Health. 2020;26:1120-1122.
- Farr C. Ro, a 3-year-old online health provider, just raised a new round that values it at $1.5 billion. CNBC. July 27, 2020. Accessed February 23, 2022. https://www.cnbc.com/2020/07/27/ro-raises-200-million-at-1point5-billion-valuation-250-million-sales.html
- Elliott T, Shih J. Direct to consumer telemedicine. Curr Allergy Asthma Rep. 2019;19:1.
- Schwartz LM, Woloshin S. Medical marketing in the United States, 1997-2016. JAMA. 2019;321:80-96.
- Peart JM, Kovarik C. Direct-to-patient teledermatology practices. J Am Acad Dermatol. 2015;72:907-909.
- Coates SJ, Kvedar J, Granstein RD. Teledermatology: from historical perspective to emerging techniques of the modern era. J Am Acad Dermatol. 2015;72:563-574.
- Rheuban KS, Krupinski EA, eds. Understanding Telehealth. McGraw-Hill Education; 2017.
- Schlesinger LA, Higgins M, Roseman S. Reinventing the direct-to-consumer business model. Harvard Business Review. March 31, 2020. Accessed February 23, 2022. https://hbr.org/2020/03/reinventing-the-direct-to-consumer-business-model
- Cohen AB, Mathews SC, Dorsey ER, et al. Direct-to-consumer digital health. Lancet Digit Health. 2020;2:E163-E165.
- 6 telehealth trends for 2020. Wolters Kluwer. Published January 27, 2021. Accessed February 23, 2022. https://www.wolterskluwer.com/en/expert-insights/6-telehealth-trends-for-2020
- Jadoo SA, Lipoff JB. Prescribing to save patients money: ethical considerations. J Am Acad Dermatol. 2018;78:826-828.
- Propecia. GoodRx. Accessed February 23, 2022. https://www.goodrx.com/propecia
- Lauer A. The truth about online hair-loss treatments like Roman and Hims, according to a dermatologist. InsideHook. January 13, 2020. Accessed February 23, 2022. https://www.insidehook.com/article/grooming/men-hair-loss-treatments-dermatologist-review
- Friedman Y. Drug price trends for NDC 16729-0089. DrugPatentWatch. Accessed February 23, 2022. https://www.drugpatentwatch.com/p/drug-price/ndc/index.php?query=16729-0089
- Curtis H, Milner J. Ethical concerns with online direct-to-consumer pharmaceutical companies. J Med Ethics. 2020;46:168-171.
- Jain T, Lu RJ, Mehrotra A. Prescriptions on demand: the growth of direct-to-consumer telemedicine companies. JAMA. 2019;322:925-926.
- Shahinyan RH, Amighi A, Carey AN, et al. Direct-to-consumer internet prescription platforms overlook crucial pathology found during traditional office evaluation of young men with erectile dysfunction. Urology. 2020;143:165-172.
- Ali M. Andrew Dudum—bold strategies that propelled Hims & Hers into unicorn status. Exit Strategy with Moiz Ali. Published April 2020. Accessed February 23, 2022. https://open.spotify.com/episode/6DtaJxwZDjvZSJI88DTf24?si=b3FHQiUIQY62YjfRHmnJBQ
- Klara K, Kim J, Ross JS. Direct-to-consumer broadcast advertisements for pharmaceuticals: off-label promotion and adherence to FDA guidelines. J Gen Intern Med. 2018;33:651-658.
- Sullivan HW, Aikin KJ, Poehlman J. Communicating risk information in direct-to-consumer prescription drug television ads: a content analysis. Health Commun. 2019;34:212-219.
- Applequist J, Ball JG. An updated analysis of direct-to-consumer television advertisements for prescription drugs. Ann Fam Med. 2018;16:211-216.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Patient safety. World Health Organization. Published September 13, 2019. Accessed February 1, 2022. https://www.who.int/news-room/fact-sheets/detail/patient-safety
- Bollmeier SG, Stevenson E, Finnegan P, et al. Direct to consumer telemedicine: is healthcare from home best? Mo Med. 2020;117:303-309.
26. Court E. Bausch yanked online prescribing after dermatologist backlash. Bloomberg.com. Published March 11, 2020. Accessed September 25, 2020. https://www.bloomberg.com/news/articles/2020-03-11/bausch-yanked-online-prescribing-after-dermatologist-backlash
27. Reitano Z. The future of healthcare: how Ro helps providers treat patients 2 minutes, 2 days, 2 weeks, and 2 years at a time. Medium. Published March 4, 2019. Accessed February 1, 2022. https://medium.com/ro-co/the-future-of-healthcare-how-ro-helps-providers-treat-patients-2-mins-2-days-2-weeks-and-2-10efc0679d7
28. Lee I, Kovarik C, Tejasvi T, et al. Telehealth: helping your patients and practice survive and thrive during the COVID-19 crisis with rapid quality implementation. J Am Acad Dermatol. 2020;82:1213-1214.
29. Pifer R. “Weeks where decades happen”: telehealth 6 months into COVID-19. Healthcare Dive. Published July 27, 2020. Accessed February 23, 2022. https://www.healthcaredive.com/news/telehealth-6-months-coronavirus/581447/
30. Bestsennyy O, Gilbert G, Harris A, et al. Telehealth: a quarter-trillion-dollar post-COVID-19 reality? McKinsey & Company. Updated July 9, 2021. Accessed February 23, 2022. https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/telehealth-a-quarter-trillion-dollar-post-covid-19-reality
31. Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
32. Jennings K. Digital health startup Ro raised $500 million at $5 billion valuation. Forbes. March 22, 2021. Accessed March 29, 2022. https://www.forbes.com/sites/katiejennings/2021/03/22/digital-health-startup-ro-raised-500-million-at-5-billion-valuation/?sh=695be0e462f5
33. Hollander JE, Carr BG. Virtually perfect? telemedicine for COVID-19. N Engl J Med. 2020;382:1679-1681.
34. Welch BM, Harvey J, O’Connell NS, et al. Patient preferences for direct-to-consumer telemedicine services: a nationwide survey. BMC Health Serv Res. 2017;17:784.
In recent years, direct-to-consumer (DTC) teledermatology platforms have gained popularity as telehealth business models, allowing patients to directly initiate visits with physicians and purchase medications from single platforms. A shortage of dermatologists, improved technology, drug patent expirations, and rising health care costs accelerated the growth of DTC dermatology.1 During the COVID-19 pandemic, teledermatology adoption surged due to the need to provide care while social distancing and minimizing viral exposure. These needs prompted additional federal funding and loosened regulatory provisions.2 As the userbase of these companies has grown, so have their valuations.3 Although the DTC model has attracted the attention of patients and investors, its rise provokes many questions about patients acting as consumers in health care. Indeed, DTC telemedicine offers greater autonomy and convenience for patients, but it may impact the quality of care and the nature of physician-patient relationships, perhaps making them more transactional.
Evolution of DTC in Health Care
The DTC model emphasizes individual choice and accessible health care. Although the definition has evolved, the core idea is not new.4 Over decades, pharmaceutical companies have spent billions of dollars on DTC advertising, circumventing physicians by directly reaching patients with campaigns on prescription drugs and laboratory tests and shaping public definitions of diseases.5
The DTC model of care is fundamentally different from traditional care models in that it changes the roles of the patient and physician. Whereas early telehealth models required a health care provider to initiate teleconsultations with specialists, DTC telemedicine bypasses this step (eg, the patient can consult a dermatologist without needing a primary care provider’s input first). This care can then be provided by dermatologists with whom patients may or may not have pre-established relationships.4,6
Dermatology was an early adopter of DTC telemedicine. The shortage of dermatologists in the United States created demand for increasing accessibility to dermatologic care. Additionally, the visual nature of diagnosing dermatologic disease was ideal for platforms supporting image sharing.7 Early DTC providers were primarily individual companies offering teledermatology. However, many dermatologists can now offer DTC capabilities via companies such as Amwell and Teladoc Health.8
Over the last 2 decades, start-ups such as Warby Parker (eyeglasses) and Casper (mattresses) defined the DTC industry using borrowed supply chains, cohesive branding, heavy social media marketing, and web-only retail. Scalability, lack of competition, and abundant venture capital created competition across numerous markets.9 Health care capitalized on this DTC model, creating a $700 billion market for products ranging from hearing aids to over-the-counter medications.10
Borrowing from this DTC playbook, platforms were created to offer delivery of generic prescription drugs to patients’ doorsteps. However, unlike with other products bought online, a consumer cannot simply add prescription drugs to their shopping cart and check out. In all models of American medical practice, physicians still serve as gatekeepers, providing a safeguard for patients to ensure appropriate prescription and avoid negative consequences of unnecessary drug use. This new model effectively streamlines diagnosis, prescription, and drug delivery without the patient ever having to leave home. Combining the prescribing and selling of medications (2 tasks that traditionally have been separated) potentially creates financial conflicts of interest (COIs). Additionally, high utilization of health care, including more prescriptions and visits, does not necessarily equal high quality of care. The companies stand to benefit from extra care regardless of need, and thus these models must be scrutinized for any incentives driving unnecessary care and prescriptions.
Ultimately, DTC has evolved to encompass multiple definitions in health care (Table 1). Although all models provide health care, each offers a different modality of delivery. The primary service may be the sale of prescription drugs or simply telemedicine visits. This review primarily discusses DTC pharmaceutical telemedicine platforms that sell private-label drugs and also offer telemedicine services to streamline care. However, the history, risks, and benefits discussed may apply to all models.
The DTC Landscape
Most DTC companies employ variations on a model with the same 3 main components: a triage questionnaire, telehealth services, and prescription/drug delivery (Figure). The triage questionnaire elicits a history of the patient’s presentation and medical history. Some companies may use artificial intelligence (AI) algorithms to tailor questions to patient needs. There are 2 modalities for patient-provider communication: synchronous and asynchronous. Synchronous communication entails real-time patient-physician conversations via audio only or video call. Asynchronous (or store-and-forward) communication refers to consultations provided via messaging or text-based modality, where a provider may respond to a patient within 24 hours.6 Direct-to-consumer platforms primarily use asynchronous visits (Table 2). However, some also use synchronous modalities if the provider deems it necessary or if state laws require it.
Once a provider has consulted with the patient, they can prescribe medication as needed. In certain cases, with adequate history, a prescription may be issued without a full physician visit. Furthermore, DTC companies require purchase of their custom-branded generic drugs. Prescriptions are fulfilled by the company’s pharmacy network and directly shipped to patients; few will allow patients to transfer a prescription to a pharmacy of their choice. Some platforms also sell supplements and over-the-counter medications.
Payment models vary among these companies, and most do not accept insurance (Table 2). Select models may provide free consultations and only require payment for pharmaceuticals. Others charge for consultations but reallocate payment to the cost of medication if prescribed. Another model involves flat rates for consultations and additional charges for drugs but unlimited messaging with providers for the duration of the prescription. Moreover, patients can subscribe to monthly deliveries of their medications.
Foundation of DTC
Technological advances have enabled patients to receive remote treatment from a single platform offering video calls, AI, electronic medical record interoperability, and integration of drug supply chains. Even in its simplest form, AI is increasingly used, as it allows for programs and chatbots to screen and triage patients.11 Technology also has improved at targeted mass marketing through social media platforms and search engines (eg, companies can use age, interests, location, and other parameters to target individuals likely needing acne treatment).
Drug patent expirations are a key catalyst for the rise of DTC companies, creating an attractive business model with generic drugs as the core product. Since 2008, patents for medications treating chronic conditions, such as erectile dysfunction, have expired. These patent expirations are responsible for $198 billion in projected prescription sales between 2019 and 2024.1 Thus, it follows that DTC companies have seized this opportunity to act as middlemen, taking advantage of these generic medications’ lower costs to create platforms focused on personalization and accessibility.
Rising deductibles have led patients to consider cheaper out-of-pocket alternatives that are not covered by insurance.1 For example, insurers typically do not cover finasteride treatment for conditions deemed cosmetic, such as androgenetic alopecia.12 The low cost of generic drugs creates an attractive business model for patients and investors. According to GoodRx, the average retail price for a 30-day supply of brand-name finasteride (Propecia [Merck]) is $135.92, whereas generic finasteride is $75.24.13 Direct-to-consumer pharmaceutical companies offer a 30-day supply of generic finasteride ranging from $8.33 to $30.14 The average wholesale cost for retailers is an estimated $2.31 for 30 days.15 Although profit margins on generic medications may be lower, more affordable drugs increase the size of the total market. These prescriptions are available as subscription plans, resulting in recurring revenue.
Lax US pharmaceutical marketing regulations allow direct advertising to the general public.16 In 1997, the US Food and Drug Administration allowed DTC advertisements to replace summaries of serious and common adverse effects with short statements covering important risks or referrals to other sources for complete information. In 2015, the US Food and Drug Administration guidelines preventing encouragement of self-diagnosis and self-treatment were withdrawn.5 These changes enable DTC companies to launch large advertising campaigns and to accelerate customer acquisition, as the industry often describes it, with ease.
Rapid Growth and Implications
Increasing generic drug availability and improving telemedicine capabilities have the potential to reduce costs and barriers but also have the potential for financial gain. Venture capital funds have recognized this opportunity, reflected by millions of dollars of investments, and accelerated the growth of DTC health care start-ups. For example, Ro has raised $376 million from venture capital, valuing the company at $1.5 billion.3
Direct-to-consumer companies require a heavy focus on marketing campaigns for customer acquisition. Their aesthetically pleasing websites and aggressive campaigns target specific audiences based on demographics, digital use habits, and purchasing behavior.4 Some campaigns celebrate the ease of obtaining prescriptions.17 Companies have been effective in recruiting so-called millennial and Generation Z patients, known to search the internet for remedies prior to seeking physician consultations.18 Recognizing these needs, some platforms offer guides on diseases they treat, creating effective customer-acquisition funnels. Recruitment of these technology-friendly patients has proven effective, especially given the largely positive media coverage of DTC platforms––potentially serving as a surrogate for medical credibility for patients.18
Some DTC companies also market physically; skin care ads may be strategically placed in social media feeds, or even found near mirrors in public bathrooms.19 Marketing campaigns also involve disease awareness; such efforts serve to increase diagnoses and prescribed treatments while destigmatizing diseases. Although DTC companies argue this strategy empowers patients, these marketing habits have the potential to take advantage of uninformed patients. Campaigns could potentially medicalize normal experiences and expand disease definitions resulting in overdiagnosis, overtreatment, and wasted resources.5 For example, off-label propranolol use has been advertised to attract patients who might have “nerves that come creeping before an important presentation.”17 Disease awareness campaigns also may lead people to falsely believe unproven drug benefits.5 According to studies, DTC pharmaceutical advertisements are low in informational quality and result in increased patient visits and prescriptions despite cost-effective alternatives.5,20-22
Fragmentation of the health care system is another possible complication of DTC teledermatology. These companies operate as for-profit organizations separated from the rest of the health care system, raising concerns about care coordination.8 Vital health data may not be conveyed as patients move among different providers and pharmacies. One study found DTC teledermatology rarely offered to provide medical records or facilitate a referral to a local physician.23 Such a lack of communication is concerning, as medication errors are the leading cause of avoidable harm in health care.24
Direct-to-consumer care models also seemingly redefine the physician-patient relationship by turning patients into consumers. Patient interactions may seem transactional and streamlined toward sales. For these platforms, a visit often is set up as an evaluation of a patient’s suitability for a prescription, not necessarily for the best treatment modality for the problem. These companies primarily make money through the sale of prescription drugs, creating a potential COI that may undermine the patient-physician relationship. Although some companies have made it clear that medical care and pharmaceutical sales are provided by legally separate business entities and that they do not pay physicians on commission, a conflict may still exist given the financial importance of physicians prescribing medication to the success of the business.16
Even as DTC models advertise upon expanded access and choice, the companies largely prohibit patients from choosing their own pharmacy. Instead, they encourage patients to fill prescriptions with the company’s pharmacy network by claiming lower costs compared with competitors. One DTC company, Hims, is launching a prescription-fulfillment center to further consolidate their business.17,19,25 The inherent COI of issuing and fulfilling prescriptions raises concerns of patient harm.26 For example, when Dermatology.com launched as a DTC prescription skin medication shop backed by Bausch Health Companies Inc, its model included telemedicine consultation. Although consultations were provided by RxDefine, a third party, only Dermatology.com drugs were prescribed. Given the poor quality of care and obvious financial COI, an uproar in the dermatology community and advocacy by the American Academy of Dermatology led to the shutdown of Dermatology.com’s online prescription services.26
The quality of care among DTC telemedicine platforms has been equivocal. Some studies have reported equivalent care in person and online, while others have reported poor adherence to guidelines, overuse of antibiotics, and misdiagnosis.8,23 A vital portion of the DTC experience is the history questionnaire, which is geared to diagnosis and risk assessment.25 Resneck et al23 found diagnostic quality to be adequate for simple dermatologic clinical scenarios but poor for scenarios requiring more than basic histories. Although Ro has reported leveraging data from millions of interactions to ask the right questions and streamline visits, it is still unclear whether history questionnaires are adequate.17,27 Additionally, consultations may lack sufficient counseling on adverse effects, risks, or pregnancy warnings, as well as discussions on alternative treatments and preventative care.17,23 Finally, patients often are limited in their choice of dermatologist; the lack of a fully developed relationship increases concerns of follow-up and monitoring practices. Although some DTC platforms offer unlimited interactions with physicians for the duration of a prescription, it is unknown how often these services are utilized or how adequate the quality of these interactions is. This potential for lax follow-up is especially concerning for prescriptions that autorenew on a monthly basis and could result in unnecessary overtreatment.
Postpandemic and Future Outlook
The COVID-19 pandemic dramatically impacted the use of telemedicine. To minimize COVID-19 transmission, the Centers for Medicare & Medicaid Services and private payers expanded telehealth coverage and eliminated reimbursement and licensing barriers.28 A decade’s worth of regulatory changes and consumer adoption was accelerated to weeks, resulting in telemedicine companies reaching record-high visit numbers.29 McKinsey & Company estimated that telehealth visit numbers surged 50- to 175-fold compared with pre–COVID-19 numbers. Additionally, 76% of patients were interested in future telehealth use, and 64% of providers were more comfortable using telehealth than before the pandemic.30 For their part, US dermatologists reported an increase in telemedicine use from 14.1% to 96.9% since COVID-19.31
Exactly how much DTC pharmaceutical telemedicine companies are growing is unclear, but private investments may be an indication. A record $14.7 billion was invested in the digital health sector in the first half of 2021; the majority went to telehealth companies.30 Ro, which reported $230 million in revenue in 2020 and has served 6 million visits, raised $200 milllion in July 2020 and $500 million in March 2021.32 Although post–COVID-19 health care will certainly involve increased telemedicine, the extent remains unclear, as telehealth vendors saw decreased usage upon reopening of state economies. Ultimately, the postpandemic regulatory landscape is hard to predict.30
Although COVID-19 appears to have caused rapid growth for DTC platforms, it also may have spurred competition. Telemedicine providers have given independent dermatologists and health care systems the infrastructure to implement custom DTC services.33 Although systems do not directly sell prescription drugs, the target market is essentially the same: patients looking for instant virtual dermatologic care. Therefore, sustained telemedicine services offered by traditional practices and systems may prove detrimental to DTC companies. However, unlike most telemedicine services, DTC models are less affected by certain changes in regulation since they do not rely on insurance. If regulations are tightened and reimbursements for telehealth are not attractive for dermatologists, teledermatology services may see an overall decrease. If so, patients who appreciate teledermatology may shift to using DTC platforms, even if their insurance does not cover them. Still, a nationwide survey found 56% of respondents felt an established relationship with a physician prior to a telemedicine visit is important, which may create a barrier for DTC adoption.34
Conclusion
Direct-to-consumer teledermatology represents a growing for-profit model of health care that provides patients with seemingly affordable and convenient care. However, there is potential for overtreatment, misdiagnosis, and fragmentation of health care. It will be important to monitor and evaluate the quality of care that DTC teledermatology offers and advocate for appropriate regulations and oversight. Eventually, more patients will have medications prescribed and dermatologic care administered through DTC companies. Dermatologists will benefit from this knowledge of DTC models to properly counsel patients on the risks and benefits of their use.
In recent years, direct-to-consumer (DTC) teledermatology platforms have gained popularity as telehealth business models, allowing patients to directly initiate visits with physicians and purchase medications from single platforms. A shortage of dermatologists, improved technology, drug patent expirations, and rising health care costs accelerated the growth of DTC dermatology.1 During the COVID-19 pandemic, teledermatology adoption surged due to the need to provide care while social distancing and minimizing viral exposure. These needs prompted additional federal funding and loosened regulatory provisions.2 As the userbase of these companies has grown, so have their valuations.3 Although the DTC model has attracted the attention of patients and investors, its rise provokes many questions about patients acting as consumers in health care. Indeed, DTC telemedicine offers greater autonomy and convenience for patients, but it may impact the quality of care and the nature of physician-patient relationships, perhaps making them more transactional.
Evolution of DTC in Health Care
The DTC model emphasizes individual choice and accessible health care. Although the definition has evolved, the core idea is not new.4 Over decades, pharmaceutical companies have spent billions of dollars on DTC advertising, circumventing physicians by directly reaching patients with campaigns on prescription drugs and laboratory tests and shaping public definitions of diseases.5
The DTC model of care is fundamentally different from traditional care models in that it changes the roles of the patient and physician. Whereas early telehealth models required a health care provider to initiate teleconsultations with specialists, DTC telemedicine bypasses this step (eg, the patient can consult a dermatologist without needing a primary care provider’s input first). This care can then be provided by dermatologists with whom patients may or may not have pre-established relationships.4,6
Dermatology was an early adopter of DTC telemedicine. The shortage of dermatologists in the United States created demand for increasing accessibility to dermatologic care. Additionally, the visual nature of diagnosing dermatologic disease was ideal for platforms supporting image sharing.7 Early DTC providers were primarily individual companies offering teledermatology. However, many dermatologists can now offer DTC capabilities via companies such as Amwell and Teladoc Health.8
Over the last 2 decades, start-ups such as Warby Parker (eyeglasses) and Casper (mattresses) defined the DTC industry using borrowed supply chains, cohesive branding, heavy social media marketing, and web-only retail. Scalability, lack of competition, and abundant venture capital created competition across numerous markets.9 Health care capitalized on this DTC model, creating a $700 billion market for products ranging from hearing aids to over-the-counter medications.10
Borrowing from this DTC playbook, platforms were created to offer delivery of generic prescription drugs to patients’ doorsteps. However, unlike with other products bought online, a consumer cannot simply add prescription drugs to their shopping cart and check out. In all models of American medical practice, physicians still serve as gatekeepers, providing a safeguard for patients to ensure appropriate prescription and avoid negative consequences of unnecessary drug use. This new model effectively streamlines diagnosis, prescription, and drug delivery without the patient ever having to leave home. Combining the prescribing and selling of medications (2 tasks that traditionally have been separated) potentially creates financial conflicts of interest (COIs). Additionally, high utilization of health care, including more prescriptions and visits, does not necessarily equal high quality of care. The companies stand to benefit from extra care regardless of need, and thus these models must be scrutinized for any incentives driving unnecessary care and prescriptions.
Ultimately, DTC has evolved to encompass multiple definitions in health care (Table 1). Although all models provide health care, each offers a different modality of delivery. The primary service may be the sale of prescription drugs or simply telemedicine visits. This review primarily discusses DTC pharmaceutical telemedicine platforms that sell private-label drugs and also offer telemedicine services to streamline care. However, the history, risks, and benefits discussed may apply to all models.
The DTC Landscape
Most DTC companies employ variations on a model with the same 3 main components: a triage questionnaire, telehealth services, and prescription/drug delivery (Figure). The triage questionnaire elicits a history of the patient’s presentation and medical history. Some companies may use artificial intelligence (AI) algorithms to tailor questions to patient needs. There are 2 modalities for patient-provider communication: synchronous and asynchronous. Synchronous communication entails real-time patient-physician conversations via audio only or video call. Asynchronous (or store-and-forward) communication refers to consultations provided via messaging or text-based modality, where a provider may respond to a patient within 24 hours.6 Direct-to-consumer platforms primarily use asynchronous visits (Table 2). However, some also use synchronous modalities if the provider deems it necessary or if state laws require it.
Once a provider has consulted with the patient, they can prescribe medication as needed. In certain cases, with adequate history, a prescription may be issued without a full physician visit. Furthermore, DTC companies require purchase of their custom-branded generic drugs. Prescriptions are fulfilled by the company’s pharmacy network and directly shipped to patients; few will allow patients to transfer a prescription to a pharmacy of their choice. Some platforms also sell supplements and over-the-counter medications.
Payment models vary among these companies, and most do not accept insurance (Table 2). Select models may provide free consultations and only require payment for pharmaceuticals. Others charge for consultations but reallocate payment to the cost of medication if prescribed. Another model involves flat rates for consultations and additional charges for drugs but unlimited messaging with providers for the duration of the prescription. Moreover, patients can subscribe to monthly deliveries of their medications.
Foundation of DTC
Technological advances have enabled patients to receive remote treatment from a single platform offering video calls, AI, electronic medical record interoperability, and integration of drug supply chains. Even in its simplest form, AI is increasingly used, as it allows for programs and chatbots to screen and triage patients.11 Technology also has improved at targeted mass marketing through social media platforms and search engines (eg, companies can use age, interests, location, and other parameters to target individuals likely needing acne treatment).
Drug patent expirations are a key catalyst for the rise of DTC companies, creating an attractive business model with generic drugs as the core product. Since 2008, patents for medications treating chronic conditions, such as erectile dysfunction, have expired. These patent expirations are responsible for $198 billion in projected prescription sales between 2019 and 2024.1 Thus, it follows that DTC companies have seized this opportunity to act as middlemen, taking advantage of these generic medications’ lower costs to create platforms focused on personalization and accessibility.
Rising deductibles have led patients to consider cheaper out-of-pocket alternatives that are not covered by insurance.1 For example, insurers typically do not cover finasteride treatment for conditions deemed cosmetic, such as androgenetic alopecia.12 The low cost of generic drugs creates an attractive business model for patients and investors. According to GoodRx, the average retail price for a 30-day supply of brand-name finasteride (Propecia [Merck]) is $135.92, whereas generic finasteride is $75.24.13 Direct-to-consumer pharmaceutical companies offer a 30-day supply of generic finasteride ranging from $8.33 to $30.14 The average wholesale cost for retailers is an estimated $2.31 for 30 days.15 Although profit margins on generic medications may be lower, more affordable drugs increase the size of the total market. These prescriptions are available as subscription plans, resulting in recurring revenue.
Lax US pharmaceutical marketing regulations allow direct advertising to the general public.16 In 1997, the US Food and Drug Administration allowed DTC advertisements to replace summaries of serious and common adverse effects with short statements covering important risks or referrals to other sources for complete information. In 2015, the US Food and Drug Administration guidelines preventing encouragement of self-diagnosis and self-treatment were withdrawn.5 These changes enable DTC companies to launch large advertising campaigns and to accelerate customer acquisition, as the industry often describes it, with ease.
Rapid Growth and Implications
Increasing generic drug availability and improving telemedicine capabilities have the potential to reduce costs and barriers but also have the potential for financial gain. Venture capital funds have recognized this opportunity, reflected by millions of dollars of investments, and accelerated the growth of DTC health care start-ups. For example, Ro has raised $376 million from venture capital, valuing the company at $1.5 billion.3
Direct-to-consumer companies require a heavy focus on marketing campaigns for customer acquisition. Their aesthetically pleasing websites and aggressive campaigns target specific audiences based on demographics, digital use habits, and purchasing behavior.4 Some campaigns celebrate the ease of obtaining prescriptions.17 Companies have been effective in recruiting so-called millennial and Generation Z patients, known to search the internet for remedies prior to seeking physician consultations.18 Recognizing these needs, some platforms offer guides on diseases they treat, creating effective customer-acquisition funnels. Recruitment of these technology-friendly patients has proven effective, especially given the largely positive media coverage of DTC platforms––potentially serving as a surrogate for medical credibility for patients.18
Some DTC companies also market physically; skin care ads may be strategically placed in social media feeds, or even found near mirrors in public bathrooms.19 Marketing campaigns also involve disease awareness; such efforts serve to increase diagnoses and prescribed treatments while destigmatizing diseases. Although DTC companies argue this strategy empowers patients, these marketing habits have the potential to take advantage of uninformed patients. Campaigns could potentially medicalize normal experiences and expand disease definitions resulting in overdiagnosis, overtreatment, and wasted resources.5 For example, off-label propranolol use has been advertised to attract patients who might have “nerves that come creeping before an important presentation.”17 Disease awareness campaigns also may lead people to falsely believe unproven drug benefits.5 According to studies, DTC pharmaceutical advertisements are low in informational quality and result in increased patient visits and prescriptions despite cost-effective alternatives.5,20-22
Fragmentation of the health care system is another possible complication of DTC teledermatology. These companies operate as for-profit organizations separated from the rest of the health care system, raising concerns about care coordination.8 Vital health data may not be conveyed as patients move among different providers and pharmacies. One study found DTC teledermatology rarely offered to provide medical records or facilitate a referral to a local physician.23 Such a lack of communication is concerning, as medication errors are the leading cause of avoidable harm in health care.24
Direct-to-consumer care models also seemingly redefine the physician-patient relationship by turning patients into consumers. Patient interactions may seem transactional and streamlined toward sales. For these platforms, a visit often is set up as an evaluation of a patient’s suitability for a prescription, not necessarily for the best treatment modality for the problem. These companies primarily make money through the sale of prescription drugs, creating a potential COI that may undermine the patient-physician relationship. Although some companies have made it clear that medical care and pharmaceutical sales are provided by legally separate business entities and that they do not pay physicians on commission, a conflict may still exist given the financial importance of physicians prescribing medication to the success of the business.16
Even as DTC models advertise upon expanded access and choice, the companies largely prohibit patients from choosing their own pharmacy. Instead, they encourage patients to fill prescriptions with the company’s pharmacy network by claiming lower costs compared with competitors. One DTC company, Hims, is launching a prescription-fulfillment center to further consolidate their business.17,19,25 The inherent COI of issuing and fulfilling prescriptions raises concerns of patient harm.26 For example, when Dermatology.com launched as a DTC prescription skin medication shop backed by Bausch Health Companies Inc, its model included telemedicine consultation. Although consultations were provided by RxDefine, a third party, only Dermatology.com drugs were prescribed. Given the poor quality of care and obvious financial COI, an uproar in the dermatology community and advocacy by the American Academy of Dermatology led to the shutdown of Dermatology.com’s online prescription services.26
The quality of care among DTC telemedicine platforms has been equivocal. Some studies have reported equivalent care in person and online, while others have reported poor adherence to guidelines, overuse of antibiotics, and misdiagnosis.8,23 A vital portion of the DTC experience is the history questionnaire, which is geared to diagnosis and risk assessment.25 Resneck et al23 found diagnostic quality to be adequate for simple dermatologic clinical scenarios but poor for scenarios requiring more than basic histories. Although Ro has reported leveraging data from millions of interactions to ask the right questions and streamline visits, it is still unclear whether history questionnaires are adequate.17,27 Additionally, consultations may lack sufficient counseling on adverse effects, risks, or pregnancy warnings, as well as discussions on alternative treatments and preventative care.17,23 Finally, patients often are limited in their choice of dermatologist; the lack of a fully developed relationship increases concerns of follow-up and monitoring practices. Although some DTC platforms offer unlimited interactions with physicians for the duration of a prescription, it is unknown how often these services are utilized or how adequate the quality of these interactions is. This potential for lax follow-up is especially concerning for prescriptions that autorenew on a monthly basis and could result in unnecessary overtreatment.
Postpandemic and Future Outlook
The COVID-19 pandemic dramatically impacted the use of telemedicine. To minimize COVID-19 transmission, the Centers for Medicare & Medicaid Services and private payers expanded telehealth coverage and eliminated reimbursement and licensing barriers.28 A decade’s worth of regulatory changes and consumer adoption was accelerated to weeks, resulting in telemedicine companies reaching record-high visit numbers.29 McKinsey & Company estimated that telehealth visit numbers surged 50- to 175-fold compared with pre–COVID-19 numbers. Additionally, 76% of patients were interested in future telehealth use, and 64% of providers were more comfortable using telehealth than before the pandemic.30 For their part, US dermatologists reported an increase in telemedicine use from 14.1% to 96.9% since COVID-19.31
Exactly how much DTC pharmaceutical telemedicine companies are growing is unclear, but private investments may be an indication. A record $14.7 billion was invested in the digital health sector in the first half of 2021; the majority went to telehealth companies.30 Ro, which reported $230 million in revenue in 2020 and has served 6 million visits, raised $200 milllion in July 2020 and $500 million in March 2021.32 Although post–COVID-19 health care will certainly involve increased telemedicine, the extent remains unclear, as telehealth vendors saw decreased usage upon reopening of state economies. Ultimately, the postpandemic regulatory landscape is hard to predict.30
Although COVID-19 appears to have caused rapid growth for DTC platforms, it also may have spurred competition. Telemedicine providers have given independent dermatologists and health care systems the infrastructure to implement custom DTC services.33 Although systems do not directly sell prescription drugs, the target market is essentially the same: patients looking for instant virtual dermatologic care. Therefore, sustained telemedicine services offered by traditional practices and systems may prove detrimental to DTC companies. However, unlike most telemedicine services, DTC models are less affected by certain changes in regulation since they do not rely on insurance. If regulations are tightened and reimbursements for telehealth are not attractive for dermatologists, teledermatology services may see an overall decrease. If so, patients who appreciate teledermatology may shift to using DTC platforms, even if their insurance does not cover them. Still, a nationwide survey found 56% of respondents felt an established relationship with a physician prior to a telemedicine visit is important, which may create a barrier for DTC adoption.34
Conclusion
Direct-to-consumer teledermatology represents a growing for-profit model of health care that provides patients with seemingly affordable and convenient care. However, there is potential for overtreatment, misdiagnosis, and fragmentation of health care. It will be important to monitor and evaluate the quality of care that DTC teledermatology offers and advocate for appropriate regulations and oversight. Eventually, more patients will have medications prescribed and dermatologic care administered through DTC companies. Dermatologists will benefit from this knowledge of DTC models to properly counsel patients on the risks and benefits of their use.
- Vennare J. The DTC healthcare report. Fitt Insider. September 15, 2019. Accessed February 23, 2022. https://insider.fitt.co/direct-to-consumer-healthcare-startups/
- Kannampallil T, Ma J. Digital translucence: adapting telemedicine delivery post-COVID-19. Telemed J E Health. 2020;26:1120-1122.
- Farr C. Ro, a 3-year-old online health provider, just raised a new round that values it at $1.5 billion. CNBC. July 27, 2020. Accessed February 23, 2022. https://www.cnbc.com/2020/07/27/ro-raises-200-million-at-1point5-billion-valuation-250-million-sales.html
- Elliott T, Shih J. Direct to consumer telemedicine. Curr Allergy Asthma Rep. 2019;19:1.
- Schwartz LM, Woloshin S. Medical marketing in the United States, 1997-2016. JAMA. 2019;321:80-96.
- Peart JM, Kovarik C. Direct-to-patient teledermatology practices. J Am Acad Dermatol. 2015;72:907-909.
- Coates SJ, Kvedar J, Granstein RD. Teledermatology: from historical perspective to emerging techniques of the modern era. J Am Acad Dermatol. 2015;72:563-574.
- Rheuban KS, Krupinski EA, eds. Understanding Telehealth. McGraw-Hill Education; 2017.
- Schlesinger LA, Higgins M, Roseman S. Reinventing the direct-to-consumer business model. Harvard Business Review. March 31, 2020. Accessed February 23, 2022. https://hbr.org/2020/03/reinventing-the-direct-to-consumer-business-model
- Cohen AB, Mathews SC, Dorsey ER, et al. Direct-to-consumer digital health. Lancet Digit Health. 2020;2:E163-E165.
- 6 telehealth trends for 2020. Wolters Kluwer. Published January 27, 2021. Accessed February 23, 2022. https://www.wolterskluwer.com/en/expert-insights/6-telehealth-trends-for-2020
- Jadoo SA, Lipoff JB. Prescribing to save patients money: ethical considerations. J Am Acad Dermatol. 2018;78:826-828.
- Propecia. GoodRx. Accessed February 23, 2022. https://www.goodrx.com/propecia
- Lauer A. The truth about online hair-loss treatments like Roman and Hims, according to a dermatologist. InsideHook. January 13, 2020. Accessed February 23, 2022. https://www.insidehook.com/article/grooming/men-hair-loss-treatments-dermatologist-review
- Friedman Y. Drug price trends for NDC 16729-0089. DrugPatentWatch. Accessed February 23, 2022. https://www.drugpatentwatch.com/p/drug-price/ndc/index.php?query=16729-0089
- Curtis H, Milner J. Ethical concerns with online direct-to-consumer pharmaceutical companies. J Med Ethics. 2020;46:168-171.
- Jain T, Lu RJ, Mehrotra A. Prescriptions on demand: the growth of direct-to-consumer telemedicine companies. JAMA. 2019;322:925-926.
- Shahinyan RH, Amighi A, Carey AN, et al. Direct-to-consumer internet prescription platforms overlook crucial pathology found during traditional office evaluation of young men with erectile dysfunction. Urology. 2020;143:165-172.
- Ali M. Andrew Dudum—bold strategies that propelled Hims & Hers into unicorn status. Exit Strategy with Moiz Ali. Published April 2020. Accessed February 23, 2022. https://open.spotify.com/episode/6DtaJxwZDjvZSJI88DTf24?si=b3FHQiUIQY62YjfRHmnJBQ
- Klara K, Kim J, Ross JS. Direct-to-consumer broadcast advertisements for pharmaceuticals: off-label promotion and adherence to FDA guidelines. J Gen Intern Med. 2018;33:651-658.
- Sullivan HW, Aikin KJ, Poehlman J. Communicating risk information in direct-to-consumer prescription drug television ads: a content analysis. Health Commun. 2019;34:212-219.
- Applequist J, Ball JG. An updated analysis of direct-to-consumer television advertisements for prescription drugs. Ann Fam Med. 2018;16:211-216.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Patient safety. World Health Organization. Published September 13, 2019. Accessed February 1, 2022. https://www.who.int/news-room/fact-sheets/detail/patient-safety
- Bollmeier SG, Stevenson E, Finnegan P, et al. Direct to consumer telemedicine: is healthcare from home best? Mo Med. 2020;117:303-309.
26. Court E. Bausch yanked online prescribing after dermatologist backlash. Bloomberg.com. Published March 11, 2020. Accessed September 25, 2020. https://www.bloomberg.com/news/articles/2020-03-11/bausch-yanked-online-prescribing-after-dermatologist-backlash
27. Reitano Z. The future of healthcare: how Ro helps providers treat patients 2 minutes, 2 days, 2 weeks, and 2 years at a time. Medium. Published March 4, 2019. Accessed February 1, 2022. https://medium.com/ro-co/the-future-of-healthcare-how-ro-helps-providers-treat-patients-2-mins-2-days-2-weeks-and-2-10efc0679d7
28. Lee I, Kovarik C, Tejasvi T, et al. Telehealth: helping your patients and practice survive and thrive during the COVID-19 crisis with rapid quality implementation. J Am Acad Dermatol. 2020;82:1213-1214.
29. Pifer R. “Weeks where decades happen”: telehealth 6 months into COVID-19. Healthcare Dive. Published July 27, 2020. Accessed February 23, 2022. https://www.healthcaredive.com/news/telehealth-6-months-coronavirus/581447/
30. Bestsennyy O, Gilbert G, Harris A, et al. Telehealth: a quarter-trillion-dollar post-COVID-19 reality? McKinsey & Company. Updated July 9, 2021. Accessed February 23, 2022. https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/telehealth-a-quarter-trillion-dollar-post-covid-19-reality
31. Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
32. Jennings K. Digital health startup Ro raised $500 million at $5 billion valuation. Forbes. March 22, 2021. Accessed March 29, 2022. https://www.forbes.com/sites/katiejennings/2021/03/22/digital-health-startup-ro-raised-500-million-at-5-billion-valuation/?sh=695be0e462f5
33. Hollander JE, Carr BG. Virtually perfect? telemedicine for COVID-19. N Engl J Med. 2020;382:1679-1681.
34. Welch BM, Harvey J, O’Connell NS, et al. Patient preferences for direct-to-consumer telemedicine services: a nationwide survey. BMC Health Serv Res. 2017;17:784.
- Vennare J. The DTC healthcare report. Fitt Insider. September 15, 2019. Accessed February 23, 2022. https://insider.fitt.co/direct-to-consumer-healthcare-startups/
- Kannampallil T, Ma J. Digital translucence: adapting telemedicine delivery post-COVID-19. Telemed J E Health. 2020;26:1120-1122.
- Farr C. Ro, a 3-year-old online health provider, just raised a new round that values it at $1.5 billion. CNBC. July 27, 2020. Accessed February 23, 2022. https://www.cnbc.com/2020/07/27/ro-raises-200-million-at-1point5-billion-valuation-250-million-sales.html
- Elliott T, Shih J. Direct to consumer telemedicine. Curr Allergy Asthma Rep. 2019;19:1.
- Schwartz LM, Woloshin S. Medical marketing in the United States, 1997-2016. JAMA. 2019;321:80-96.
- Peart JM, Kovarik C. Direct-to-patient teledermatology practices. J Am Acad Dermatol. 2015;72:907-909.
- Coates SJ, Kvedar J, Granstein RD. Teledermatology: from historical perspective to emerging techniques of the modern era. J Am Acad Dermatol. 2015;72:563-574.
- Rheuban KS, Krupinski EA, eds. Understanding Telehealth. McGraw-Hill Education; 2017.
- Schlesinger LA, Higgins M, Roseman S. Reinventing the direct-to-consumer business model. Harvard Business Review. March 31, 2020. Accessed February 23, 2022. https://hbr.org/2020/03/reinventing-the-direct-to-consumer-business-model
- Cohen AB, Mathews SC, Dorsey ER, et al. Direct-to-consumer digital health. Lancet Digit Health. 2020;2:E163-E165.
- 6 telehealth trends for 2020. Wolters Kluwer. Published January 27, 2021. Accessed February 23, 2022. https://www.wolterskluwer.com/en/expert-insights/6-telehealth-trends-for-2020
- Jadoo SA, Lipoff JB. Prescribing to save patients money: ethical considerations. J Am Acad Dermatol. 2018;78:826-828.
- Propecia. GoodRx. Accessed February 23, 2022. https://www.goodrx.com/propecia
- Lauer A. The truth about online hair-loss treatments like Roman and Hims, according to a dermatologist. InsideHook. January 13, 2020. Accessed February 23, 2022. https://www.insidehook.com/article/grooming/men-hair-loss-treatments-dermatologist-review
- Friedman Y. Drug price trends for NDC 16729-0089. DrugPatentWatch. Accessed February 23, 2022. https://www.drugpatentwatch.com/p/drug-price/ndc/index.php?query=16729-0089
- Curtis H, Milner J. Ethical concerns with online direct-to-consumer pharmaceutical companies. J Med Ethics. 2020;46:168-171.
- Jain T, Lu RJ, Mehrotra A. Prescriptions on demand: the growth of direct-to-consumer telemedicine companies. JAMA. 2019;322:925-926.
- Shahinyan RH, Amighi A, Carey AN, et al. Direct-to-consumer internet prescription platforms overlook crucial pathology found during traditional office evaluation of young men with erectile dysfunction. Urology. 2020;143:165-172.
- Ali M. Andrew Dudum—bold strategies that propelled Hims & Hers into unicorn status. Exit Strategy with Moiz Ali. Published April 2020. Accessed February 23, 2022. https://open.spotify.com/episode/6DtaJxwZDjvZSJI88DTf24?si=b3FHQiUIQY62YjfRHmnJBQ
- Klara K, Kim J, Ross JS. Direct-to-consumer broadcast advertisements for pharmaceuticals: off-label promotion and adherence to FDA guidelines. J Gen Intern Med. 2018;33:651-658.
- Sullivan HW, Aikin KJ, Poehlman J. Communicating risk information in direct-to-consumer prescription drug television ads: a content analysis. Health Commun. 2019;34:212-219.
- Applequist J, Ball JG. An updated analysis of direct-to-consumer television advertisements for prescription drugs. Ann Fam Med. 2018;16:211-216.
- Resneck JS Jr, Abrouk M, Steuer M, et al. Choice, transparency, coordination, and quality among direct-to-consumer telemedicine websites and apps treating skin disease. JAMA Dermatol. 2016;152:768-775.
- Patient safety. World Health Organization. Published September 13, 2019. Accessed February 1, 2022. https://www.who.int/news-room/fact-sheets/detail/patient-safety
- Bollmeier SG, Stevenson E, Finnegan P, et al. Direct to consumer telemedicine: is healthcare from home best? Mo Med. 2020;117:303-309.
26. Court E. Bausch yanked online prescribing after dermatologist backlash. Bloomberg.com. Published March 11, 2020. Accessed September 25, 2020. https://www.bloomberg.com/news/articles/2020-03-11/bausch-yanked-online-prescribing-after-dermatologist-backlash
27. Reitano Z. The future of healthcare: how Ro helps providers treat patients 2 minutes, 2 days, 2 weeks, and 2 years at a time. Medium. Published March 4, 2019. Accessed February 1, 2022. https://medium.com/ro-co/the-future-of-healthcare-how-ro-helps-providers-treat-patients-2-mins-2-days-2-weeks-and-2-10efc0679d7
28. Lee I, Kovarik C, Tejasvi T, et al. Telehealth: helping your patients and practice survive and thrive during the COVID-19 crisis with rapid quality implementation. J Am Acad Dermatol. 2020;82:1213-1214.
29. Pifer R. “Weeks where decades happen”: telehealth 6 months into COVID-19. Healthcare Dive. Published July 27, 2020. Accessed February 23, 2022. https://www.healthcaredive.com/news/telehealth-6-months-coronavirus/581447/
30. Bestsennyy O, Gilbert G, Harris A, et al. Telehealth: a quarter-trillion-dollar post-COVID-19 reality? McKinsey & Company. Updated July 9, 2021. Accessed February 23, 2022. https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/telehealth-a-quarter-trillion-dollar-post-covid-19-reality
31. Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
32. Jennings K. Digital health startup Ro raised $500 million at $5 billion valuation. Forbes. March 22, 2021. Accessed March 29, 2022. https://www.forbes.com/sites/katiejennings/2021/03/22/digital-health-startup-ro-raised-500-million-at-5-billion-valuation/?sh=695be0e462f5
33. Hollander JE, Carr BG. Virtually perfect? telemedicine for COVID-19. N Engl J Med. 2020;382:1679-1681.
34. Welch BM, Harvey J, O’Connell NS, et al. Patient preferences for direct-to-consumer telemedicine services: a nationwide survey. BMC Health Serv Res. 2017;17:784.
Practice Points
- Direct-to-consumer (DTC) teledermatology platforms are for-profit companies that provide telemedicine visits and sell prescription drugs directly to patients.
- Although they are growing in popularity, DTC teledermatology platforms may lead to overdiagnosis, overtreatment, and fragmentation of health care. Knowledge of teledermatology will be vital to counsel patients on the risks and benefits of these platforms.