User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Acral Papulovesicular Eruption in a Soldier Following Smallpox Vaccination
Following the attacks of September 11, 2001, heightened concerns over bioterrorism and the potential use of smallpox as a biological weapon made smallpox vaccination a critical component of military readiness. Therefore, the US Military resumed its smallpox vaccination program in 2002 using the first-generation smallpox vaccine (Dryvax, Wyeth Pharmaceuticals), a live vaccinia virus vaccine created in the late 19th century. This vaccine was developed by pooling vaccinia strains from the skin of infected cows1 and had previously been used during the worldwide vaccination campaign in the 1970s. Dryvax was associated with various cardiac and cutaneous complications, from benign hypersensitivity reactions to life-threatening eczema vaccinatum and progressive vaccinia.
Due to concerns that the remaining supply of Dryvax was insufficient to vaccinate the US population in the case of a bioterrorism attack, investigators developed the second-generation smallpox vaccine (ACAM2000, Sanofi Pasteur Biologics Co) using advances in vaccine technology.2 ACAM2000 is a plaque-purified isolate of vaccinia virus propagated in cell culture, thereby reducing contaminants and lot-to-lot variation.1 Clinical trials demonstrated comparable immunogenicity and frequency of adverse events compared with Dryvax,2 and ACAM2000 replaced Dryvax in 2008. However, these trials focused on serious adverse events, such as cardiac complications and postvaccinal encephalitis, with less specific characterization and description of cutaneous eruptions.3
Since 2008, there have been few reports of cutaneous adverse reactions following vaccination with ACAM2000. Beachkofsky et al4 described 7 cases of papulovesicular eruptions and 1 case of generalized vaccinia. Freeman and Lenz5 described 4 cases of papulovesicular eruptions, and there has been 1 case of progressive vaccinia reported in a soldier with newly diagnosed acute myelogenous leukemia.6 Kramer7 described a patient with multiple vesiculopustular lesions secondary to autoinoculation. The distinct pruritic acral papulovesicular eruptions following ACAM2000 vaccination have occurred in healthy military service members at different locations since the introduction of ACAM2000. We describe an additional case of this unique cutaneous eruption, followed by a review of previously described cutaneous adverse events associated with smallpox vaccination.
Case Report
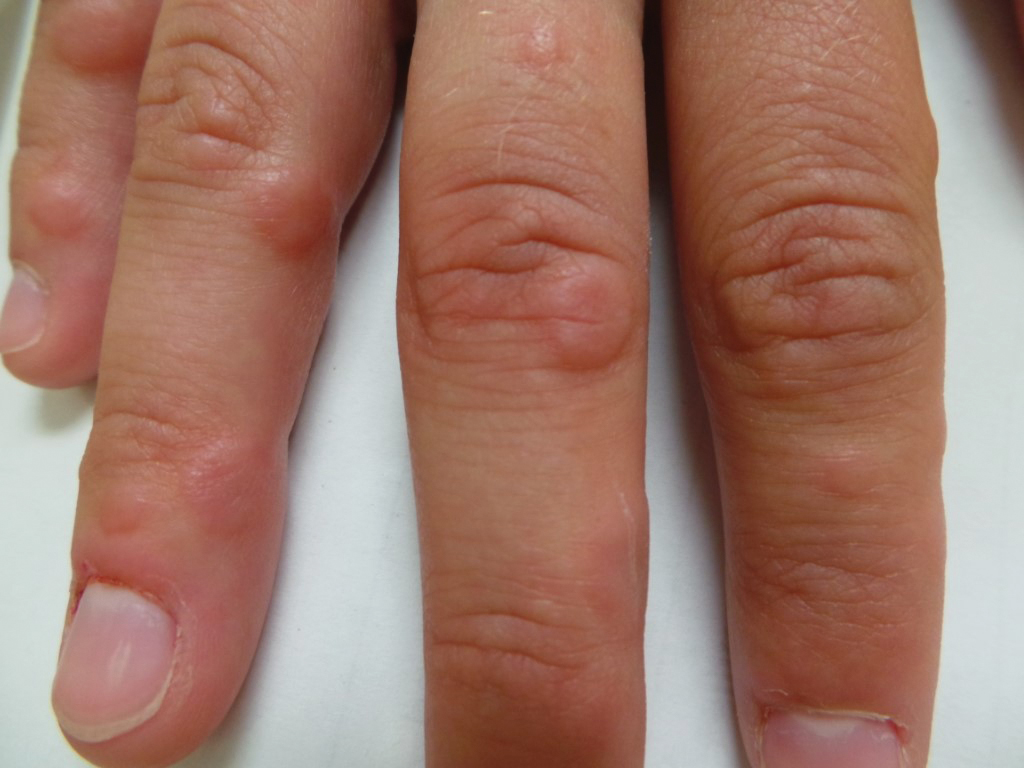
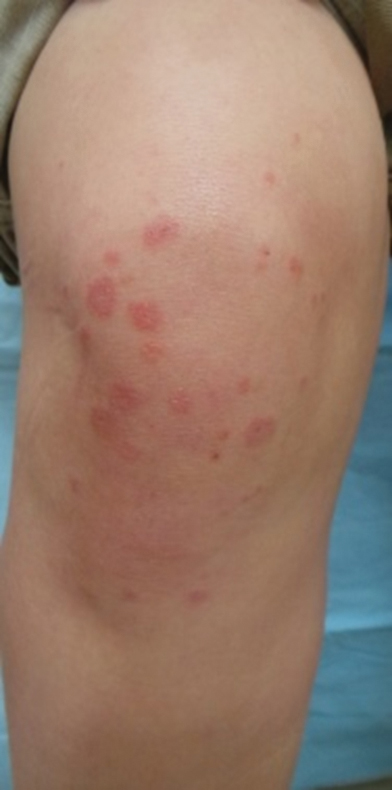
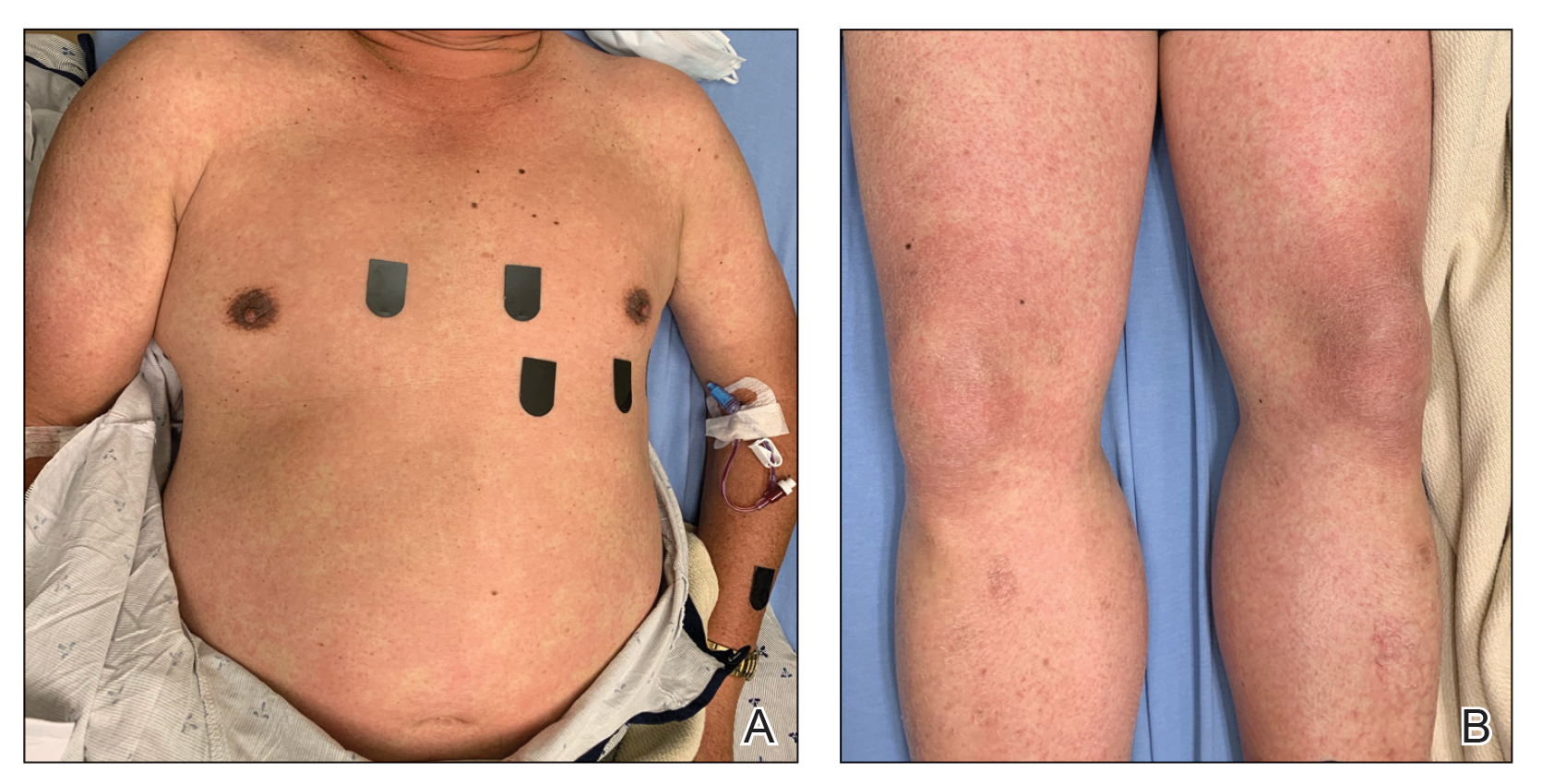
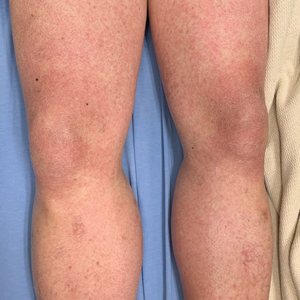
A 21-year-old female soldier who was otherwise healthy presented to the dermatology clinic with a pruritic papular eruption involving the upper and lower extremities of 1 week’s duration. The lesions first appeared 8 days after she received the ACAM2000 vaccine. She received no other concurrent vaccines, had no history of atopic dermatitis, and had no systemic symptoms. Physical examination revealed numerous erythematous indurated papules involving the dorsolateral hands and fingers, as well as the extensor surfaces of the elbows, knees, and thighs (Figures 1 and 2). Based on the clinical presentation, the differential diagnosis included lichen planus, verruca plana, dyshidrotic eczema, and smallpox vaccine reaction. Erythema multiforme was considered; however, the absence of palmoplantar involvement and typical targetoid lesions made this diagnosis less likely.
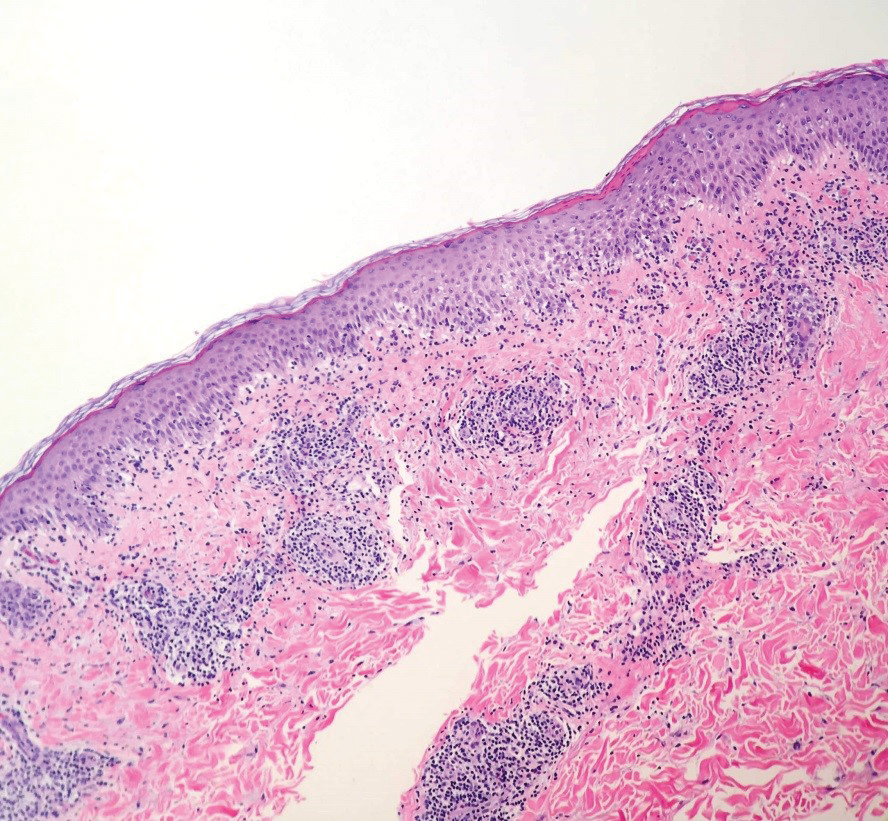
Biopsies of lesions on the arm and thigh were performed. Histologic findings revealed interface and spongiotic dermatitis with scattered necrotic keratinocytes and extravasated erythrocytes (Figure 3). There was no evidence of viral cytopathic effects. Similar clinical and histologic findings have been reported in the literature as acral papulovesicular eruptions following smallpox vaccination or papular spongiotic dermatitis of smallpox vaccination.8 The presence of eosinophils was not conspicuous in the current case and was only a notable finding in 1 of 2 cases previously described by Gaertner et al.8 This may simply be due to an idiosyncratic drug reaction. Furthermore, in the cases described by Beachkofsky et al,4 there were essentially 2 histologic groups. The first group demonstrated a dermal hypersensitivity-type reaction, and the second group demonstrated a lymphocytic capillaritis.
Based on these findings, the patient was diagnosed with an acral papulovesicular eruption following smallpox vaccination. Of note, the patient’s presentation was not consistent with other described smallpox vaccine reactions, which included eczema vaccinatum, autoinoculation, generalized vaccinia, and progressive vaccinia. The patient was treated supportively with triamcinolone acetonide cream 0.1%, cool compresses, and oral diphenhydramine as needed for pruritus. The lesions notably improved within the first week of treatment.
Comment
Reported cases of acral papulovesicular eruption4-6 demonstrated an onset of cutaneous symptoms an average of 14 days following vaccination (range, 8–18 days postvaccination). Lesions were benign and self-limited in all cases, with resolution within an average of 25 days (range, 7–71 days). All patients were active-duty military adults with a mean age of 24 years. Supportive treatment varied from topical steroids and oral antihistamines to tapering oral prednisone doses. Of note, all previously reported cases of this reaction occurred in patients who also had received other concurrent or near-concurrent vaccines, including anthrax, hepatitis B, influenza, and typhoid. Our patient represents a unique case of a papulovesicular eruption following smallpox vaccination with no history of concurrent vaccines.
Since the 1970s, smallpox vaccination has been associated with numerous cutaneous reactions, most of which have been reported with the first-generation Dryvax. Minor local reactions occurred in approximately 2% to 6% of vaccinees in clinical trials.9 These reactions included local edema involving the upper arm, satellite lesions within 2.5 cm of the vaccination site, local lymphadenopathy, intense inflammation or viral cellulitis surrounding the inoculation site, and viral lymphangitis tracking to axillary lymph nodes. In clinical trials, these reactions were self-limited and required only symptomatic treatment.9
Autoinoculation is another cutaneous reaction that can occur because Dryvax and ACAM2000 both contain live-attenuated replicating vaccinia virus. Accidental implantation may occur when the high titers of virus present at the vaccine site are subsequently transferred to other sites, especially abnormal mucosa or skin, resulting in an additional primary inoculation site.10
Eczema vaccinatum is a potentially life-threatening reaction that may occur in patients with disruptive skin disorders, such as atopic dermatitis. These patients are at risk for massive confluent vaccinia infection of the skin.10 In patients with atopic dermatitis, the virus rapidly disseminates due to both skin barrier dysfunction and impaired immunomodulation, resulting in large confluent skin lesions and the potential for viremia, septic shock, and death.10,11 Mortality from eczema vaccinatum may be reduced by administration of vaccinia immune globulin.10
The vaccinia virus also may spread hematogenously in healthy individuals,10 resulting in a benign reaction called generalized vaccinia. These patients develop pustules on areas of the skin other than the vaccination site. Although typically benign and self-limited, Beachkofsky et al4 described a case of generalized vaccinia in a healthy 34-year-old man resulting in a rapidly progressive vesiculopustular eruption with associated fever and pancytopenia. The patient made a complete recovery over the course of the following month.4
Alternatively, progressive vaccinia is a severe complication of smallpox vaccination seen in patients with impaired cell-mediated immunity. It also is known as vaccinia gangrenosum or vaccinia necrosum. These patients develop expanding ulcers due to exaggerated viral replication and cell-to-cell spread of the vaccinia virus.10,11 Hematogenous spread may result in viral implantation at distant sites of the body. This disease slowly progresses over weeks to months, and it often is resistant to treatment and fatal in patients with severe T-cell deficiency.10
Acral papulovesicular eruption is a distinct cutaneous adverse event following smallpox vaccination. Although further research is needed to discern the pathogenesis of this reaction, it is benign and self-limited, and patients have fully recovered with supportive care. In addition, a modified vaccinia Ankara vaccine (Bavarian Nordic) was approved by the US Food and Drug Administration in 2019.12,13 It is a nonreplicating attenuated viral vaccine that had fewer adverse events compared to ACAM2000 in clinical trials.13 To date, papulovesicular eruptions have not been reported following vaccination with the modified vaccinia Ankara vaccine; however, continued monitoring will help to further characterize any cutaneous reactions to this newer vaccine.
- Nalca A, Zumbrun EE. ACAM2000: the new smallpox vaccine for United States Strategic National Stockpile. Drug Des Devel Ther. 2010;4:71-79.
- Monath TP, Caldwell JR, Mundt W, et al. ACAM2000 clonal Vero cell culture vaccinia virus (New York City Board of Health strain)—a second-generation smallpox vaccine for biological defense. Int J Infect Dis. 2004;8:S31-S44.
- Thomas TN, Reef S, Neff L, et al. A review of the smallpox vaccine adverse events active surveillance system. Clin Infect Dis. 2008;46:S212-S220.
- Beachkofsky TM, Carrizales SC, Bidinger JJ, et al. Adverse events following smallpox vaccination with ACAM2000 in a military population. Arch Dermatol. 2010;146:656-661.
- Freeman R, Lenz B. Cutaneous reactions associated with ACAM2000 smallpox vaccination in a deploying U.S. Army unit. Mil Med. 2015;180:E152-E156.
- Centers for Disease Control and Prevention. Progressive vaccinia in a military smallpox vaccinee—United States, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:532-536.
- Kramer TR. Post–smallpox vaccination skin eruption in a marine. Mil Med. 2018;183:E649-E653.
- Gaertner EM, Groo S, Kim J. Papular spongiotic dermatitis of smallpox vaccination: report of 2 cases with review of the literature. Arch Pathol Lab Med. 2004;128:1173-1175.
- Fulginiti VA, Papier A, Lane JM, et al. Smallpox vaccination: a review, part I. background, vaccination technique, normal vaccination and revaccination, and expected normal reactions. Clin Infect Dis. 2003;37:241-250.
- Fulginiti VA, Papier A, Lane JM, et al. Smallpox vaccination: a review, part II. adverse events. Clin Infect Dis. 2003;37:251-271.
- Bray M. Understanding smallpox vaccination. J Infect Dis. 2011;203:1037-1039.
- Greenberg RN, Hay CM, Stapleton JT, et al. A randomized, double-blind, placebo-controlled phase II trial investigating the safety and immunogenicity of modified vaccinia ankara smallpox vaccine (MVA-BN®) in 56-80-year-old subjects. PLoS One. 2016;11:E0157335.
- Pittman PR, Hahn M, Lee HS, et al. Phase 3 efficacy trial of modified vaccinia Ankara as a vaccine against smallpox. N Engl J Med. 2019;381:1897-1908.
Following the attacks of September 11, 2001, heightened concerns over bioterrorism and the potential use of smallpox as a biological weapon made smallpox vaccination a critical component of military readiness. Therefore, the US Military resumed its smallpox vaccination program in 2002 using the first-generation smallpox vaccine (Dryvax, Wyeth Pharmaceuticals), a live vaccinia virus vaccine created in the late 19th century. This vaccine was developed by pooling vaccinia strains from the skin of infected cows1 and had previously been used during the worldwide vaccination campaign in the 1970s. Dryvax was associated with various cardiac and cutaneous complications, from benign hypersensitivity reactions to life-threatening eczema vaccinatum and progressive vaccinia.
Due to concerns that the remaining supply of Dryvax was insufficient to vaccinate the US population in the case of a bioterrorism attack, investigators developed the second-generation smallpox vaccine (ACAM2000, Sanofi Pasteur Biologics Co) using advances in vaccine technology.2 ACAM2000 is a plaque-purified isolate of vaccinia virus propagated in cell culture, thereby reducing contaminants and lot-to-lot variation.1 Clinical trials demonstrated comparable immunogenicity and frequency of adverse events compared with Dryvax,2 and ACAM2000 replaced Dryvax in 2008. However, these trials focused on serious adverse events, such as cardiac complications and postvaccinal encephalitis, with less specific characterization and description of cutaneous eruptions.3
Since 2008, there have been few reports of cutaneous adverse reactions following vaccination with ACAM2000. Beachkofsky et al4 described 7 cases of papulovesicular eruptions and 1 case of generalized vaccinia. Freeman and Lenz5 described 4 cases of papulovesicular eruptions, and there has been 1 case of progressive vaccinia reported in a soldier with newly diagnosed acute myelogenous leukemia.6 Kramer7 described a patient with multiple vesiculopustular lesions secondary to autoinoculation. The distinct pruritic acral papulovesicular eruptions following ACAM2000 vaccination have occurred in healthy military service members at different locations since the introduction of ACAM2000. We describe an additional case of this unique cutaneous eruption, followed by a review of previously described cutaneous adverse events associated with smallpox vaccination.
Case Report
A 21-year-old female soldier who was otherwise healthy presented to the dermatology clinic with a pruritic papular eruption involving the upper and lower extremities of 1 week’s duration. The lesions first appeared 8 days after she received the ACAM2000 vaccine. She received no other concurrent vaccines, had no history of atopic dermatitis, and had no systemic symptoms. Physical examination revealed numerous erythematous indurated papules involving the dorsolateral hands and fingers, as well as the extensor surfaces of the elbows, knees, and thighs (Figures 1 and 2). Based on the clinical presentation, the differential diagnosis included lichen planus, verruca plana, dyshidrotic eczema, and smallpox vaccine reaction. Erythema multiforme was considered; however, the absence of palmoplantar involvement and typical targetoid lesions made this diagnosis less likely.
Biopsies of lesions on the arm and thigh were performed. Histologic findings revealed interface and spongiotic dermatitis with scattered necrotic keratinocytes and extravasated erythrocytes (Figure 3). There was no evidence of viral cytopathic effects. Similar clinical and histologic findings have been reported in the literature as acral papulovesicular eruptions following smallpox vaccination or papular spongiotic dermatitis of smallpox vaccination.8 The presence of eosinophils was not conspicuous in the current case and was only a notable finding in 1 of 2 cases previously described by Gaertner et al.8 This may simply be due to an idiosyncratic drug reaction. Furthermore, in the cases described by Beachkofsky et al,4 there were essentially 2 histologic groups. The first group demonstrated a dermal hypersensitivity-type reaction, and the second group demonstrated a lymphocytic capillaritis.
Based on these findings, the patient was diagnosed with an acral papulovesicular eruption following smallpox vaccination. Of note, the patient’s presentation was not consistent with other described smallpox vaccine reactions, which included eczema vaccinatum, autoinoculation, generalized vaccinia, and progressive vaccinia. The patient was treated supportively with triamcinolone acetonide cream 0.1%, cool compresses, and oral diphenhydramine as needed for pruritus. The lesions notably improved within the first week of treatment.
Comment
Reported cases of acral papulovesicular eruption4-6 demonstrated an onset of cutaneous symptoms an average of 14 days following vaccination (range, 8–18 days postvaccination). Lesions were benign and self-limited in all cases, with resolution within an average of 25 days (range, 7–71 days). All patients were active-duty military adults with a mean age of 24 years. Supportive treatment varied from topical steroids and oral antihistamines to tapering oral prednisone doses. Of note, all previously reported cases of this reaction occurred in patients who also had received other concurrent or near-concurrent vaccines, including anthrax, hepatitis B, influenza, and typhoid. Our patient represents a unique case of a papulovesicular eruption following smallpox vaccination with no history of concurrent vaccines.
Since the 1970s, smallpox vaccination has been associated with numerous cutaneous reactions, most of which have been reported with the first-generation Dryvax. Minor local reactions occurred in approximately 2% to 6% of vaccinees in clinical trials.9 These reactions included local edema involving the upper arm, satellite lesions within 2.5 cm of the vaccination site, local lymphadenopathy, intense inflammation or viral cellulitis surrounding the inoculation site, and viral lymphangitis tracking to axillary lymph nodes. In clinical trials, these reactions were self-limited and required only symptomatic treatment.9
Autoinoculation is another cutaneous reaction that can occur because Dryvax and ACAM2000 both contain live-attenuated replicating vaccinia virus. Accidental implantation may occur when the high titers of virus present at the vaccine site are subsequently transferred to other sites, especially abnormal mucosa or skin, resulting in an additional primary inoculation site.10
Eczema vaccinatum is a potentially life-threatening reaction that may occur in patients with disruptive skin disorders, such as atopic dermatitis. These patients are at risk for massive confluent vaccinia infection of the skin.10 In patients with atopic dermatitis, the virus rapidly disseminates due to both skin barrier dysfunction and impaired immunomodulation, resulting in large confluent skin lesions and the potential for viremia, septic shock, and death.10,11 Mortality from eczema vaccinatum may be reduced by administration of vaccinia immune globulin.10
The vaccinia virus also may spread hematogenously in healthy individuals,10 resulting in a benign reaction called generalized vaccinia. These patients develop pustules on areas of the skin other than the vaccination site. Although typically benign and self-limited, Beachkofsky et al4 described a case of generalized vaccinia in a healthy 34-year-old man resulting in a rapidly progressive vesiculopustular eruption with associated fever and pancytopenia. The patient made a complete recovery over the course of the following month.4
Alternatively, progressive vaccinia is a severe complication of smallpox vaccination seen in patients with impaired cell-mediated immunity. It also is known as vaccinia gangrenosum or vaccinia necrosum. These patients develop expanding ulcers due to exaggerated viral replication and cell-to-cell spread of the vaccinia virus.10,11 Hematogenous spread may result in viral implantation at distant sites of the body. This disease slowly progresses over weeks to months, and it often is resistant to treatment and fatal in patients with severe T-cell deficiency.10
Acral papulovesicular eruption is a distinct cutaneous adverse event following smallpox vaccination. Although further research is needed to discern the pathogenesis of this reaction, it is benign and self-limited, and patients have fully recovered with supportive care. In addition, a modified vaccinia Ankara vaccine (Bavarian Nordic) was approved by the US Food and Drug Administration in 2019.12,13 It is a nonreplicating attenuated viral vaccine that had fewer adverse events compared to ACAM2000 in clinical trials.13 To date, papulovesicular eruptions have not been reported following vaccination with the modified vaccinia Ankara vaccine; however, continued monitoring will help to further characterize any cutaneous reactions to this newer vaccine.
Following the attacks of September 11, 2001, heightened concerns over bioterrorism and the potential use of smallpox as a biological weapon made smallpox vaccination a critical component of military readiness. Therefore, the US Military resumed its smallpox vaccination program in 2002 using the first-generation smallpox vaccine (Dryvax, Wyeth Pharmaceuticals), a live vaccinia virus vaccine created in the late 19th century. This vaccine was developed by pooling vaccinia strains from the skin of infected cows1 and had previously been used during the worldwide vaccination campaign in the 1970s. Dryvax was associated with various cardiac and cutaneous complications, from benign hypersensitivity reactions to life-threatening eczema vaccinatum and progressive vaccinia.
Due to concerns that the remaining supply of Dryvax was insufficient to vaccinate the US population in the case of a bioterrorism attack, investigators developed the second-generation smallpox vaccine (ACAM2000, Sanofi Pasteur Biologics Co) using advances in vaccine technology.2 ACAM2000 is a plaque-purified isolate of vaccinia virus propagated in cell culture, thereby reducing contaminants and lot-to-lot variation.1 Clinical trials demonstrated comparable immunogenicity and frequency of adverse events compared with Dryvax,2 and ACAM2000 replaced Dryvax in 2008. However, these trials focused on serious adverse events, such as cardiac complications and postvaccinal encephalitis, with less specific characterization and description of cutaneous eruptions.3
Since 2008, there have been few reports of cutaneous adverse reactions following vaccination with ACAM2000. Beachkofsky et al4 described 7 cases of papulovesicular eruptions and 1 case of generalized vaccinia. Freeman and Lenz5 described 4 cases of papulovesicular eruptions, and there has been 1 case of progressive vaccinia reported in a soldier with newly diagnosed acute myelogenous leukemia.6 Kramer7 described a patient with multiple vesiculopustular lesions secondary to autoinoculation. The distinct pruritic acral papulovesicular eruptions following ACAM2000 vaccination have occurred in healthy military service members at different locations since the introduction of ACAM2000. We describe an additional case of this unique cutaneous eruption, followed by a review of previously described cutaneous adverse events associated with smallpox vaccination.
Case Report
A 21-year-old female soldier who was otherwise healthy presented to the dermatology clinic with a pruritic papular eruption involving the upper and lower extremities of 1 week’s duration. The lesions first appeared 8 days after she received the ACAM2000 vaccine. She received no other concurrent vaccines, had no history of atopic dermatitis, and had no systemic symptoms. Physical examination revealed numerous erythematous indurated papules involving the dorsolateral hands and fingers, as well as the extensor surfaces of the elbows, knees, and thighs (Figures 1 and 2). Based on the clinical presentation, the differential diagnosis included lichen planus, verruca plana, dyshidrotic eczema, and smallpox vaccine reaction. Erythema multiforme was considered; however, the absence of palmoplantar involvement and typical targetoid lesions made this diagnosis less likely.
Biopsies of lesions on the arm and thigh were performed. Histologic findings revealed interface and spongiotic dermatitis with scattered necrotic keratinocytes and extravasated erythrocytes (Figure 3). There was no evidence of viral cytopathic effects. Similar clinical and histologic findings have been reported in the literature as acral papulovesicular eruptions following smallpox vaccination or papular spongiotic dermatitis of smallpox vaccination.8 The presence of eosinophils was not conspicuous in the current case and was only a notable finding in 1 of 2 cases previously described by Gaertner et al.8 This may simply be due to an idiosyncratic drug reaction. Furthermore, in the cases described by Beachkofsky et al,4 there were essentially 2 histologic groups. The first group demonstrated a dermal hypersensitivity-type reaction, and the second group demonstrated a lymphocytic capillaritis.
Based on these findings, the patient was diagnosed with an acral papulovesicular eruption following smallpox vaccination. Of note, the patient’s presentation was not consistent with other described smallpox vaccine reactions, which included eczema vaccinatum, autoinoculation, generalized vaccinia, and progressive vaccinia. The patient was treated supportively with triamcinolone acetonide cream 0.1%, cool compresses, and oral diphenhydramine as needed for pruritus. The lesions notably improved within the first week of treatment.
Comment
Reported cases of acral papulovesicular eruption4-6 demonstrated an onset of cutaneous symptoms an average of 14 days following vaccination (range, 8–18 days postvaccination). Lesions were benign and self-limited in all cases, with resolution within an average of 25 days (range, 7–71 days). All patients were active-duty military adults with a mean age of 24 years. Supportive treatment varied from topical steroids and oral antihistamines to tapering oral prednisone doses. Of note, all previously reported cases of this reaction occurred in patients who also had received other concurrent or near-concurrent vaccines, including anthrax, hepatitis B, influenza, and typhoid. Our patient represents a unique case of a papulovesicular eruption following smallpox vaccination with no history of concurrent vaccines.
Since the 1970s, smallpox vaccination has been associated with numerous cutaneous reactions, most of which have been reported with the first-generation Dryvax. Minor local reactions occurred in approximately 2% to 6% of vaccinees in clinical trials.9 These reactions included local edema involving the upper arm, satellite lesions within 2.5 cm of the vaccination site, local lymphadenopathy, intense inflammation or viral cellulitis surrounding the inoculation site, and viral lymphangitis tracking to axillary lymph nodes. In clinical trials, these reactions were self-limited and required only symptomatic treatment.9
Autoinoculation is another cutaneous reaction that can occur because Dryvax and ACAM2000 both contain live-attenuated replicating vaccinia virus. Accidental implantation may occur when the high titers of virus present at the vaccine site are subsequently transferred to other sites, especially abnormal mucosa or skin, resulting in an additional primary inoculation site.10
Eczema vaccinatum is a potentially life-threatening reaction that may occur in patients with disruptive skin disorders, such as atopic dermatitis. These patients are at risk for massive confluent vaccinia infection of the skin.10 In patients with atopic dermatitis, the virus rapidly disseminates due to both skin barrier dysfunction and impaired immunomodulation, resulting in large confluent skin lesions and the potential for viremia, septic shock, and death.10,11 Mortality from eczema vaccinatum may be reduced by administration of vaccinia immune globulin.10
The vaccinia virus also may spread hematogenously in healthy individuals,10 resulting in a benign reaction called generalized vaccinia. These patients develop pustules on areas of the skin other than the vaccination site. Although typically benign and self-limited, Beachkofsky et al4 described a case of generalized vaccinia in a healthy 34-year-old man resulting in a rapidly progressive vesiculopustular eruption with associated fever and pancytopenia. The patient made a complete recovery over the course of the following month.4
Alternatively, progressive vaccinia is a severe complication of smallpox vaccination seen in patients with impaired cell-mediated immunity. It also is known as vaccinia gangrenosum or vaccinia necrosum. These patients develop expanding ulcers due to exaggerated viral replication and cell-to-cell spread of the vaccinia virus.10,11 Hematogenous spread may result in viral implantation at distant sites of the body. This disease slowly progresses over weeks to months, and it often is resistant to treatment and fatal in patients with severe T-cell deficiency.10
Acral papulovesicular eruption is a distinct cutaneous adverse event following smallpox vaccination. Although further research is needed to discern the pathogenesis of this reaction, it is benign and self-limited, and patients have fully recovered with supportive care. In addition, a modified vaccinia Ankara vaccine (Bavarian Nordic) was approved by the US Food and Drug Administration in 2019.12,13 It is a nonreplicating attenuated viral vaccine that had fewer adverse events compared to ACAM2000 in clinical trials.13 To date, papulovesicular eruptions have not been reported following vaccination with the modified vaccinia Ankara vaccine; however, continued monitoring will help to further characterize any cutaneous reactions to this newer vaccine.
- Nalca A, Zumbrun EE. ACAM2000: the new smallpox vaccine for United States Strategic National Stockpile. Drug Des Devel Ther. 2010;4:71-79.
- Monath TP, Caldwell JR, Mundt W, et al. ACAM2000 clonal Vero cell culture vaccinia virus (New York City Board of Health strain)—a second-generation smallpox vaccine for biological defense. Int J Infect Dis. 2004;8:S31-S44.
- Thomas TN, Reef S, Neff L, et al. A review of the smallpox vaccine adverse events active surveillance system. Clin Infect Dis. 2008;46:S212-S220.
- Beachkofsky TM, Carrizales SC, Bidinger JJ, et al. Adverse events following smallpox vaccination with ACAM2000 in a military population. Arch Dermatol. 2010;146:656-661.
- Freeman R, Lenz B. Cutaneous reactions associated with ACAM2000 smallpox vaccination in a deploying U.S. Army unit. Mil Med. 2015;180:E152-E156.
- Centers for Disease Control and Prevention. Progressive vaccinia in a military smallpox vaccinee—United States, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:532-536.
- Kramer TR. Post–smallpox vaccination skin eruption in a marine. Mil Med. 2018;183:E649-E653.
- Gaertner EM, Groo S, Kim J. Papular spongiotic dermatitis of smallpox vaccination: report of 2 cases with review of the literature. Arch Pathol Lab Med. 2004;128:1173-1175.
- Fulginiti VA, Papier A, Lane JM, et al. Smallpox vaccination: a review, part I. background, vaccination technique, normal vaccination and revaccination, and expected normal reactions. Clin Infect Dis. 2003;37:241-250.
- Fulginiti VA, Papier A, Lane JM, et al. Smallpox vaccination: a review, part II. adverse events. Clin Infect Dis. 2003;37:251-271.
- Bray M. Understanding smallpox vaccination. J Infect Dis. 2011;203:1037-1039.
- Greenberg RN, Hay CM, Stapleton JT, et al. A randomized, double-blind, placebo-controlled phase II trial investigating the safety and immunogenicity of modified vaccinia ankara smallpox vaccine (MVA-BN®) in 56-80-year-old subjects. PLoS One. 2016;11:E0157335.
- Pittman PR, Hahn M, Lee HS, et al. Phase 3 efficacy trial of modified vaccinia Ankara as a vaccine against smallpox. N Engl J Med. 2019;381:1897-1908.
- Nalca A, Zumbrun EE. ACAM2000: the new smallpox vaccine for United States Strategic National Stockpile. Drug Des Devel Ther. 2010;4:71-79.
- Monath TP, Caldwell JR, Mundt W, et al. ACAM2000 clonal Vero cell culture vaccinia virus (New York City Board of Health strain)—a second-generation smallpox vaccine for biological defense. Int J Infect Dis. 2004;8:S31-S44.
- Thomas TN, Reef S, Neff L, et al. A review of the smallpox vaccine adverse events active surveillance system. Clin Infect Dis. 2008;46:S212-S220.
- Beachkofsky TM, Carrizales SC, Bidinger JJ, et al. Adverse events following smallpox vaccination with ACAM2000 in a military population. Arch Dermatol. 2010;146:656-661.
- Freeman R, Lenz B. Cutaneous reactions associated with ACAM2000 smallpox vaccination in a deploying U.S. Army unit. Mil Med. 2015;180:E152-E156.
- Centers for Disease Control and Prevention. Progressive vaccinia in a military smallpox vaccinee—United States, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:532-536.
- Kramer TR. Post–smallpox vaccination skin eruption in a marine. Mil Med. 2018;183:E649-E653.
- Gaertner EM, Groo S, Kim J. Papular spongiotic dermatitis of smallpox vaccination: report of 2 cases with review of the literature. Arch Pathol Lab Med. 2004;128:1173-1175.
- Fulginiti VA, Papier A, Lane JM, et al. Smallpox vaccination: a review, part I. background, vaccination technique, normal vaccination and revaccination, and expected normal reactions. Clin Infect Dis. 2003;37:241-250.
- Fulginiti VA, Papier A, Lane JM, et al. Smallpox vaccination: a review, part II. adverse events. Clin Infect Dis. 2003;37:251-271.
- Bray M. Understanding smallpox vaccination. J Infect Dis. 2011;203:1037-1039.
- Greenberg RN, Hay CM, Stapleton JT, et al. A randomized, double-blind, placebo-controlled phase II trial investigating the safety and immunogenicity of modified vaccinia ankara smallpox vaccine (MVA-BN®) in 56-80-year-old subjects. PLoS One. 2016;11:E0157335.
- Pittman PR, Hahn M, Lee HS, et al. Phase 3 efficacy trial of modified vaccinia Ankara as a vaccine against smallpox. N Engl J Med. 2019;381:1897-1908.
Practice Points
- There are several potential cutaneous adverse reactions associated with smallpox vaccination, ranging from benign self-limited hypersensitivity reactions to life-threatening eczema vaccinatum and progressive vaccinia.
- Acral papulovesicular eruption is a distinct presentation that has been described in the US Military following vaccination with the second-generation live smallpox vaccine (ACAM2000).
Vesicular Eruption Secondary to Bites by Larval Amblyomma americanum
Case Report
A 58-year-old woman presented to the dermatology office with a widespread pruritic eruption of 3 days’ duration that started in the groin and spread to the rest of the body. No treatments had been attempted. She had no notable medical history, and she denied any recent illness, change in personal care products, or new medications or supplements. She reported a camping trip 2 weeks prior to presentation on the east end of Long Island, New York. She later learned that others on the same trip developed a similar, albeit less widespread, eruption.
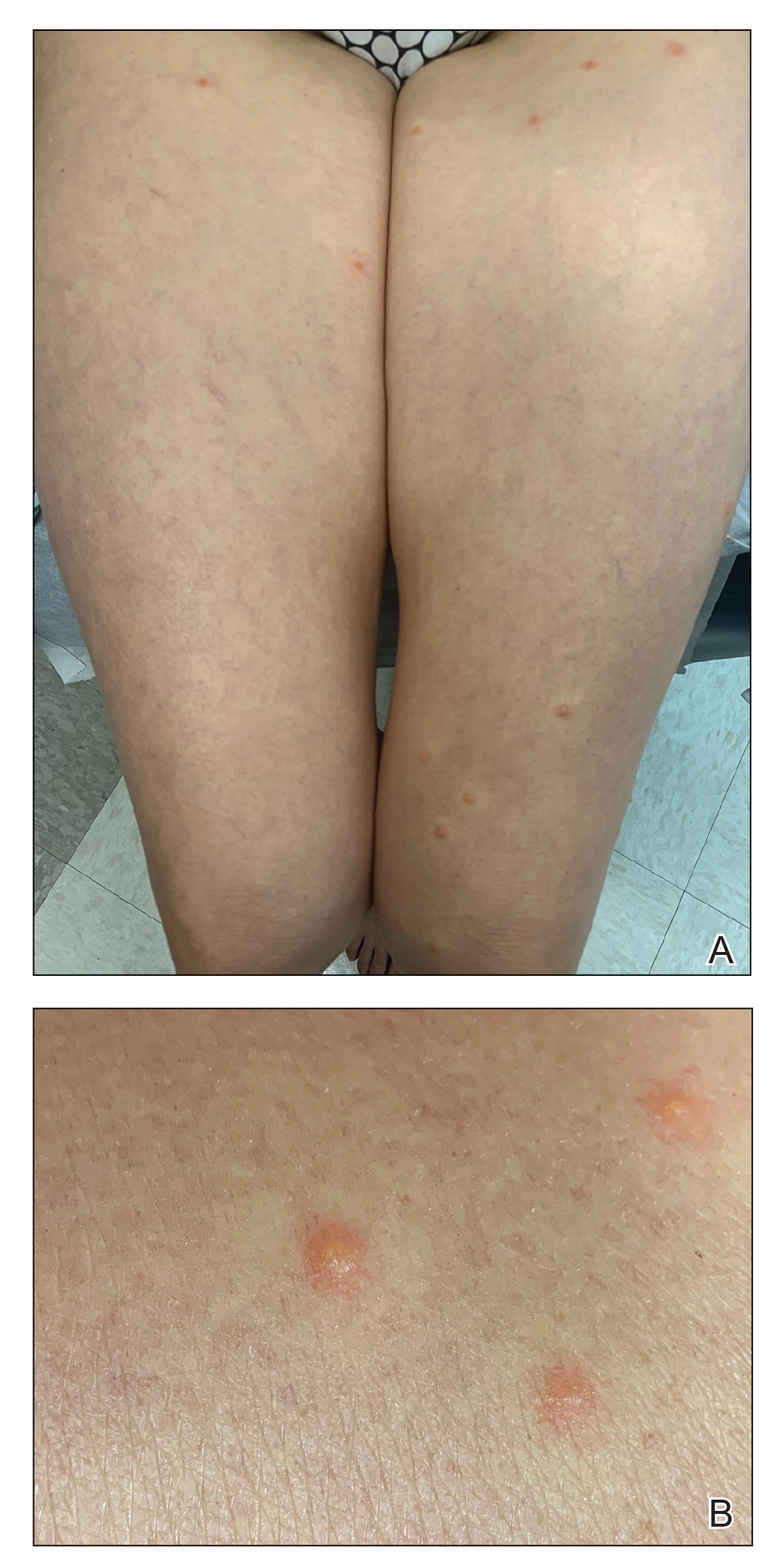
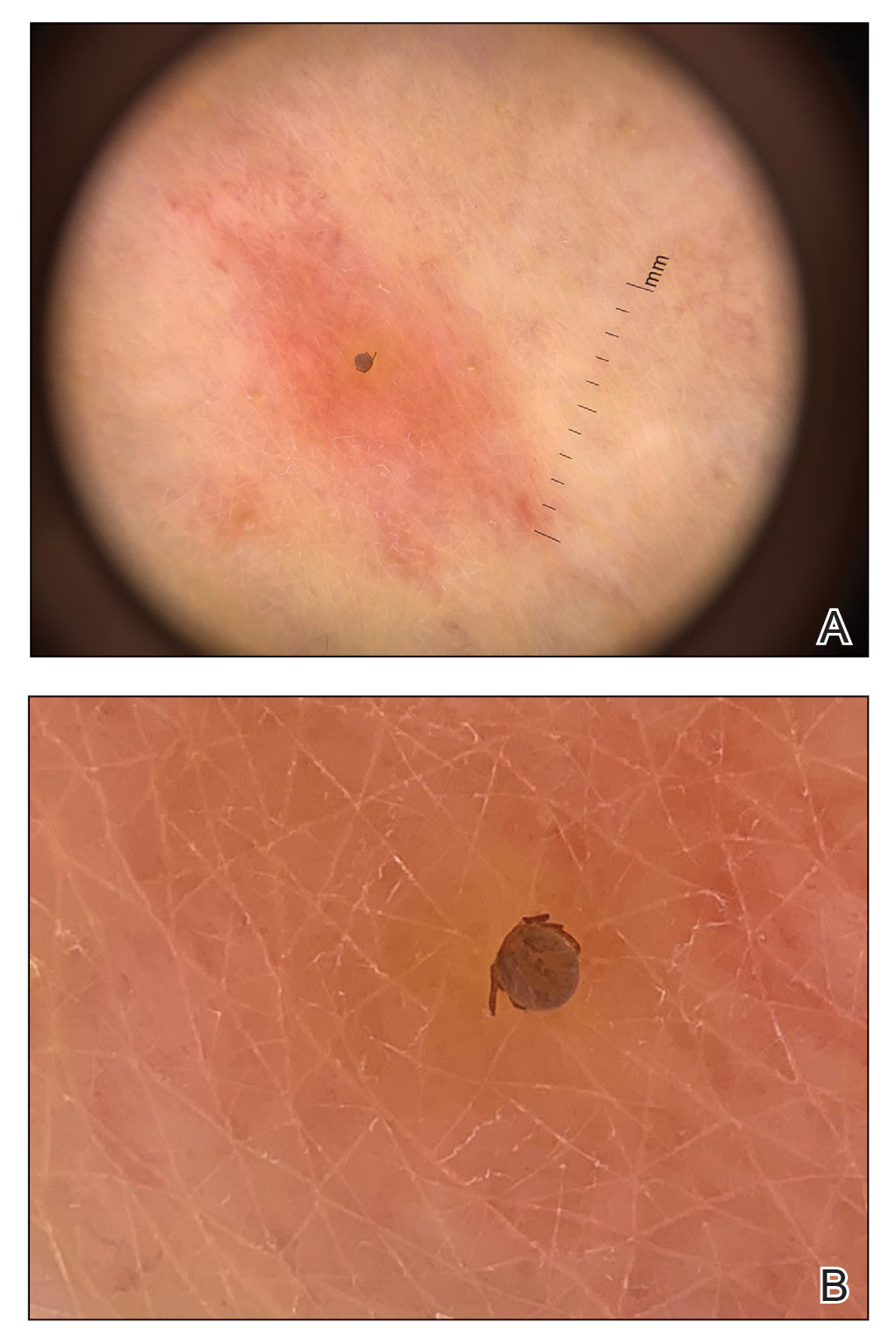
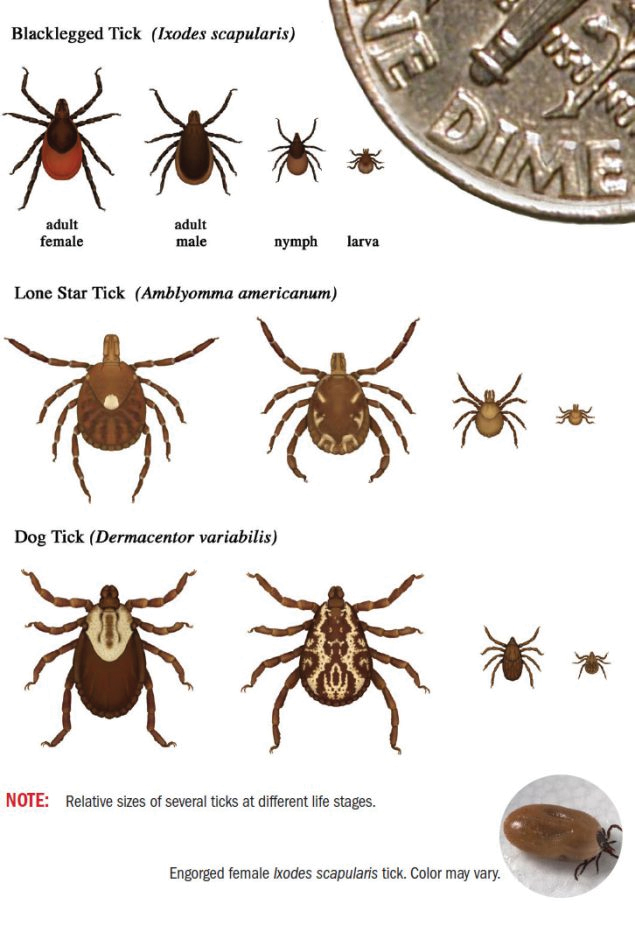
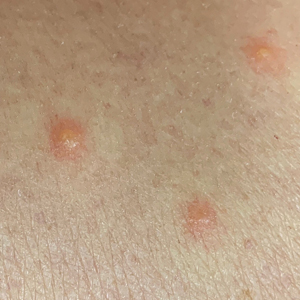
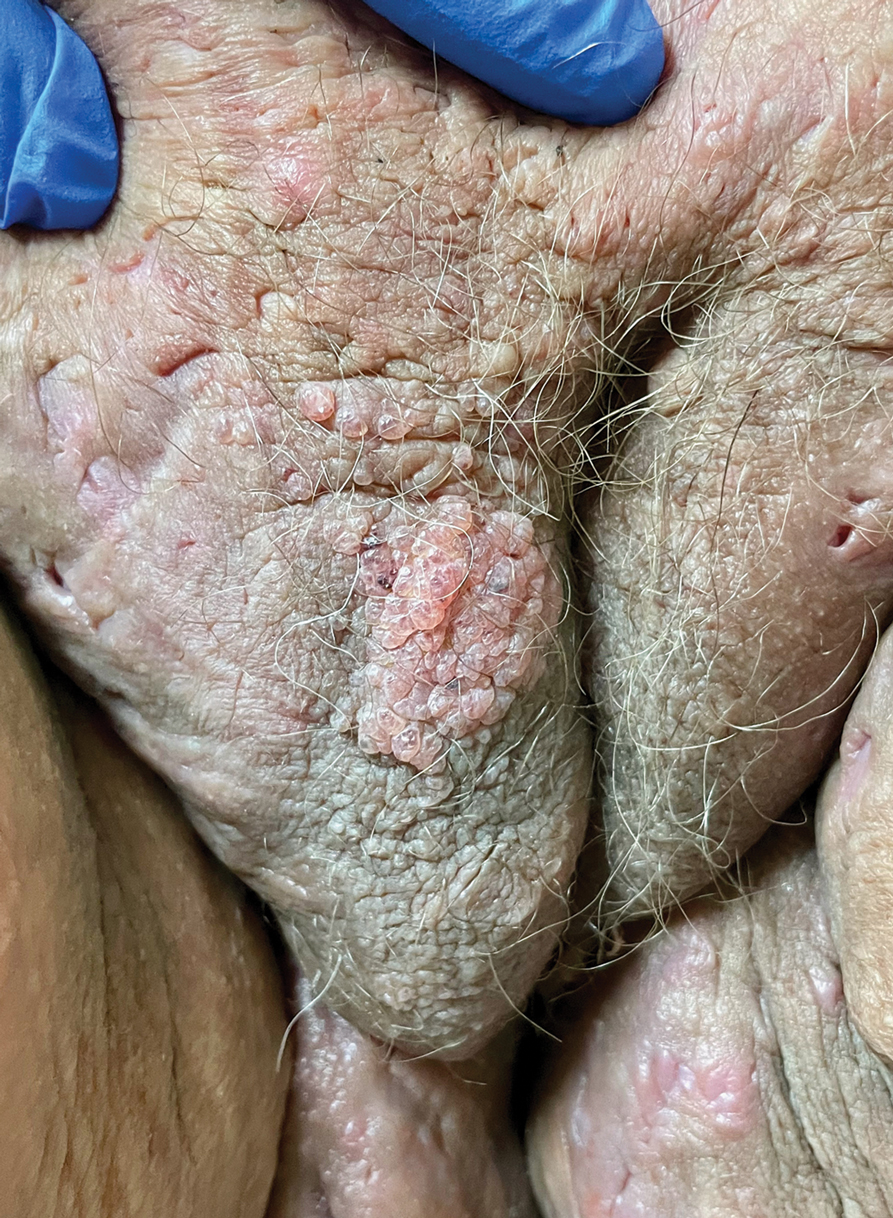
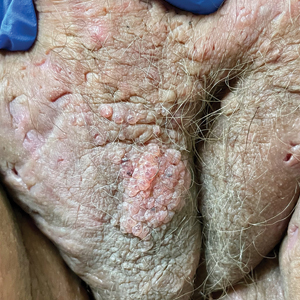
Physical examination revealed clear vesicles on the arms, legs, trunk, and pubic area (Figure 1). Dermoscopy revealed a small lone star tick larva in the center of one of the vesicles (Figure 2). The type of tick larva was identified using resources from the Centers for Disease Control and Prevention (Figure 3).1 Careful inspection revealed dark marks on various vesicles, mostly in the perineum, yielding nearly 20 larvae, which were removed with forceps. The patient was counseled to cover herself in petrolatum for 2 to 3 hours with the hope of smothering any remaining tick larvae. She was given triamcinolone cream and was encouraged to take a nonsedating antihistamine for itch. The patient was seen back in clinic 2 weeks later and the eruption had resolved.
Comment
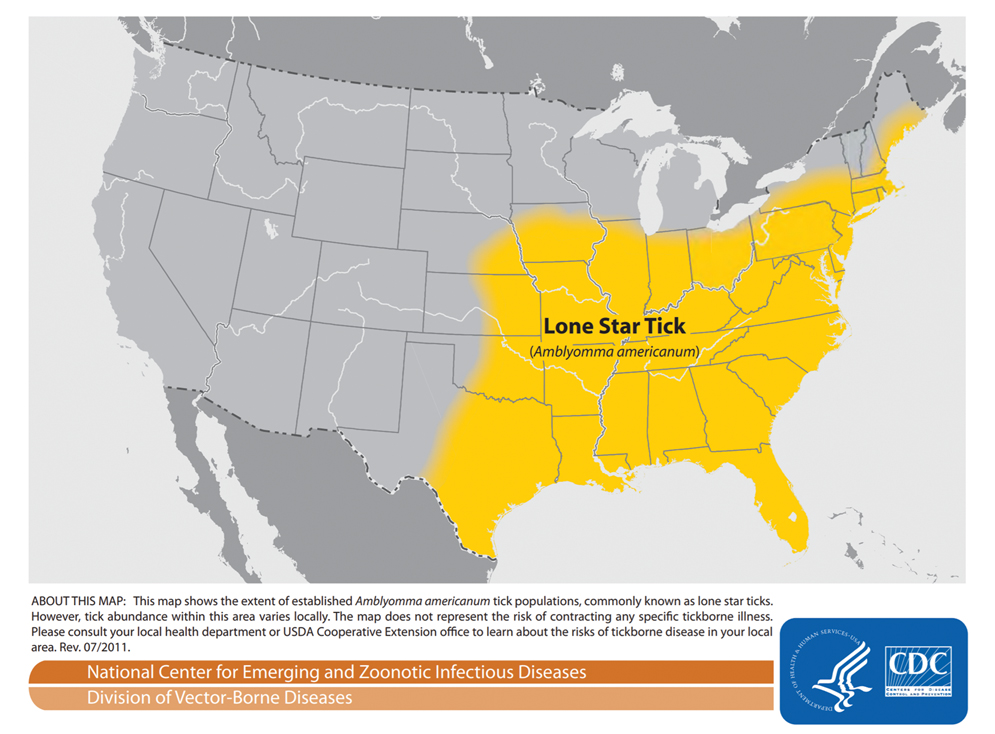
Spread of Tick-Borne Disease—Ticks and tick-borne disease are increasing major health concerns for humans, domesticated animals, and livestock. Reported cases of bacterial and protozoan tick-borne disease doubled in the United States between 2004 and 2016. Ninety percent of the nearly 60,000 cases of nationally notifiable vector-borne diseases reported in 2017 were linked to ticks.2 Geographic ranges of multiple tick species continue to expand, which is thought to be secondary to rising global temperatures, ecologic changes, reforestation, and increases in commerce and travel (Figure 4).3 Not only have warming temperatures contributed to geographic range expansion, they also may extend ticks’ active season. The lone star tick (Amblyomma americanum) is widely distributed throughout much of the eastern United States.4 The range of A americanum has expanded north in recent years from its prior core range in the southeastern United States.2 One study found that from 2006 to 2016, the vector tick species most commonly collected from humans and submitted to a tick surveillance system in New Jersey shifted from Ixodes scapularis to A americanum.5
Bites by Amblyomma Ticks—As with most hard ticks, the life cycle of A americanum lasts 2 years and includes the egg, the 6-legged larva or “seed tick,” the 8-legged immature nymph, and the 8-legged reproductively mature adult (Figure 3). Amblyomma americanum can lay several thousand eggs.2 Because our patient had numerous bites, it is plausible that she came into contact with a nest of newly hatched tick larvae. Morphogenesis from larva to nymph, then nymph to adult, requires a blood meal.6,7 The larvae emerge from eggs deposited on the ground and then crawl up low vegetation where they can easily attach to passing hosts. The tick clings to hair or clothing and waits until the host is at rest before moving to a favorable location and then bites.8 When attaching, ticks inject an anesthetic akin to lidocaine, making the bite painless. A tick may spend up to 24 hours on the host prior to biting and then feed for 2 hours to 7 days before releasing.9 For the majority of tick-borne illnesses, the tick must remain attached for 24 to 48 hours before disease is transmitted.10
All stages of
Even when the ticks do not transmit disease, tick bites can cause impressive local reactions. Uncomplicated bites can be painful and leave a puncture wound that can take 1 to 2 weeks to heal.13 Rarely, bites can cause a delayed hypersensitivity reaction including fever, pruritus, and urticaria. Granulomas can develop if a tick is improperly removed.9 Other reports describe prurigo lesions, skin hemorrhage, papular urticaria, diffuse papules, vesicles and bullae, necrotic ulcers, and patchy alopecia.14,15 A 2015 systematic controlled study of human bite reactions from A americanum demonstrated the development of itchy erythematous papules and vesicles within 48 hours of larval tick attachment to research participants. The study found tissue damage from A americanum mouthparts, and degranulating mast cells may be evident in as little as 15 minutes.16 The severity of individual skin reaction is hypothesized to depend on several variables, such as the duration of feeding, size of mouthparts, type of tick secretions, changes in secretions during feeding, and prior exposures of the host.14
Tick Removal—If patients present to clinic with ticks attached, removal can be challenging. Removal recommendations call for use of blunt forceps or tweezers. Ticks should be grasped near the skin with consistent pressure, and the tick should be pulled straight out, perpendicular to the skin. Twisting motions can cause the head to separate from the body and remain in the bite wound. Immediately following removal, the area should be cleansed with a disinfectant.10,17 After the tick is removed, some studies recommend storing the tick at −20 °C; should the patient develop disease, the tick could be sent for evaluation.6,17 If there is no clinical or serologic evidence of infection, testing for the presence of antibodies against tick-borne bacteria at presentation and at 3 and 6 weeks is not recommended due to low sensitivity, low positive predictive value, and cost. Clinicians must only observe and treat if disease occurs.17
Prevention of Tick Bites—Tick bites are best prevented by avoiding tick-infested areas; when these areas are unavoidable, tick bites may be prevented by wearing long pants with the pant legs tucked into boots. In addition, applying topical DEET (N,N-diethyl-m-toluamide) repellent to exposed skin and treating clothing with permethrin can be helpful.17 When used alone, DEET provides greater than 90% protection for up to 2.7 hours against A americanum.18 Permethrin-treated clothing alone is 79% to 100% effective at killing A americanum ticks or disabling them for several hours.19
Conclusion
Tick-borne illness is an increasingly important cause of human infectious disease. In addition to their role as a disease vector, ticks can produce primary skin disorders. This case posed a diagnostic challenge because of the unusually large number and wide distribution of bites as well as the subsequent vesicular reaction that ensued. It is important to keep tick larvae or adult tick bites in the differential when evaluating a patient to expedite tick removal and begin clinical monitoring. Recognition of A americanum larvae as a potential cause of pruritic papules may be helpful in similar cases. In addition, it is important for dermatologists to be aware of the tick species in their area.
- Centers for Disease Control and Prevention. Tick ID. Accessed February 21, 2022. https://www.cdc.gov/ticks/tickbornediseases/tickID.html
- Molaei G, Little EAH, Williams SC, et al. Bracing for the worst—range expansion of the lone star tick in the northeastern United States. N Engl J Med. 2019;381:2189-2192.
- Centers for Disease Control and Prevention, Division of Vector-Borne Diseases. Lone star tick (Amblyomma americanum). Accessed March 23, 2022. https://www.cdc.gov/ticks/maps/lone_star_tick.pdf
- Reynolds HH, Elston DM. What’s eating you? lone star tick (Amblyomma americanum). Cutis. 2017;99:111-114.
- Jordan RA, Egizi A. The growing importance of lone star ticks in a Lyme disease endemic county: passive tick surveillance in Monmouth County, NJ, 2006–2016. PLoS One. 2019;14:E0211778.
- Singh-Behl D, La Rosa SP, Tomecki KJ. Tick-borne infections. Dermatol Clin. 2003;21:237-244, v.
- Spach DH, Liles WC, Campbell GL, et al. Tick-borne diseases in the United States. N Engl J Med. 1993;329:936-947.
- Duckworth PF Jr, Hayden GF, Reed CN. Human infestation by Amblyomma americanum larvae (“seed ticks”). South Med J. 1985;78:751-753.
- Middleton DB. Tick-borne infections. what starts as a tiny bite may have a serious outcome. Postgrad Med. 1994;95:131-139.
- Moody EK, Barker RW, White JL, et al. Ticks and tick-borne diseases in Oklahoma. J Okla State Med Assoc. 1998;91:438-445.
- Jones BE. Human ‘seed tick’ infestation. Amblyomma americanum larvae. Arch Dermatol. 1981;117:812-814.
- Centers for Disease Control and Prevention. Tick bite prophylaxis. Accessed February 21, 2022. https://www.cdc.gov/ticks/tickbornediseases/tick-bite-prophylaxis.html
- Fisher EJ, Mo J, Lucky AW. Multiple pruritic papules from lone star tick larvae bites. Arch Dermatol. 2006;142:491-494.
- Krinsky WL. Dermatoses associated with the bites of mites and ticks (Arthropoda: Acari). Int J Dermatol. 1983;22:75-91.
- Yesudian P, Thambiah AS. Persistent papules after tick-bites. Dermatologica. 1973;147:214-218.
- Goddard J, Portugal JS. Cutaneous lesions due to bites by larval Amblyomma americanum ticks. JAMA Dermatol. 2015;151:1373-1375.
- Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32:897-928.
- Solberg VB, Klein TA, McPherson KR, et al. Field evaluation of DEET and a piperidine repellent (AI3-37220) against Amblyomma americanum (Acari: Ixodidae). J Med Entomol. 1995;32:870-875.
- Evans SR, Korch GW Jr, Lawson MA. Comparative field evaluation of permethrin and DEET-treated military uniforms for personal protection against ticks (Acari). J Med Entomol. 1990;27:829-834.
Case Report
A 58-year-old woman presented to the dermatology office with a widespread pruritic eruption of 3 days’ duration that started in the groin and spread to the rest of the body. No treatments had been attempted. She had no notable medical history, and she denied any recent illness, change in personal care products, or new medications or supplements. She reported a camping trip 2 weeks prior to presentation on the east end of Long Island, New York. She later learned that others on the same trip developed a similar, albeit less widespread, eruption.
Physical examination revealed clear vesicles on the arms, legs, trunk, and pubic area (Figure 1). Dermoscopy revealed a small lone star tick larva in the center of one of the vesicles (Figure 2). The type of tick larva was identified using resources from the Centers for Disease Control and Prevention (Figure 3).1 Careful inspection revealed dark marks on various vesicles, mostly in the perineum, yielding nearly 20 larvae, which were removed with forceps. The patient was counseled to cover herself in petrolatum for 2 to 3 hours with the hope of smothering any remaining tick larvae. She was given triamcinolone cream and was encouraged to take a nonsedating antihistamine for itch. The patient was seen back in clinic 2 weeks later and the eruption had resolved.
Comment
Spread of Tick-Borne Disease—Ticks and tick-borne disease are increasing major health concerns for humans, domesticated animals, and livestock. Reported cases of bacterial and protozoan tick-borne disease doubled in the United States between 2004 and 2016. Ninety percent of the nearly 60,000 cases of nationally notifiable vector-borne diseases reported in 2017 were linked to ticks.2 Geographic ranges of multiple tick species continue to expand, which is thought to be secondary to rising global temperatures, ecologic changes, reforestation, and increases in commerce and travel (Figure 4).3 Not only have warming temperatures contributed to geographic range expansion, they also may extend ticks’ active season. The lone star tick (Amblyomma americanum) is widely distributed throughout much of the eastern United States.4 The range of A americanum has expanded north in recent years from its prior core range in the southeastern United States.2 One study found that from 2006 to 2016, the vector tick species most commonly collected from humans and submitted to a tick surveillance system in New Jersey shifted from Ixodes scapularis to A americanum.5
Bites by Amblyomma Ticks—As with most hard ticks, the life cycle of A americanum lasts 2 years and includes the egg, the 6-legged larva or “seed tick,” the 8-legged immature nymph, and the 8-legged reproductively mature adult (Figure 3). Amblyomma americanum can lay several thousand eggs.2 Because our patient had numerous bites, it is plausible that she came into contact with a nest of newly hatched tick larvae. Morphogenesis from larva to nymph, then nymph to adult, requires a blood meal.6,7 The larvae emerge from eggs deposited on the ground and then crawl up low vegetation where they can easily attach to passing hosts. The tick clings to hair or clothing and waits until the host is at rest before moving to a favorable location and then bites.8 When attaching, ticks inject an anesthetic akin to lidocaine, making the bite painless. A tick may spend up to 24 hours on the host prior to biting and then feed for 2 hours to 7 days before releasing.9 For the majority of tick-borne illnesses, the tick must remain attached for 24 to 48 hours before disease is transmitted.10
All stages of
Even when the ticks do not transmit disease, tick bites can cause impressive local reactions. Uncomplicated bites can be painful and leave a puncture wound that can take 1 to 2 weeks to heal.13 Rarely, bites can cause a delayed hypersensitivity reaction including fever, pruritus, and urticaria. Granulomas can develop if a tick is improperly removed.9 Other reports describe prurigo lesions, skin hemorrhage, papular urticaria, diffuse papules, vesicles and bullae, necrotic ulcers, and patchy alopecia.14,15 A 2015 systematic controlled study of human bite reactions from A americanum demonstrated the development of itchy erythematous papules and vesicles within 48 hours of larval tick attachment to research participants. The study found tissue damage from A americanum mouthparts, and degranulating mast cells may be evident in as little as 15 minutes.16 The severity of individual skin reaction is hypothesized to depend on several variables, such as the duration of feeding, size of mouthparts, type of tick secretions, changes in secretions during feeding, and prior exposures of the host.14
Tick Removal—If patients present to clinic with ticks attached, removal can be challenging. Removal recommendations call for use of blunt forceps or tweezers. Ticks should be grasped near the skin with consistent pressure, and the tick should be pulled straight out, perpendicular to the skin. Twisting motions can cause the head to separate from the body and remain in the bite wound. Immediately following removal, the area should be cleansed with a disinfectant.10,17 After the tick is removed, some studies recommend storing the tick at −20 °C; should the patient develop disease, the tick could be sent for evaluation.6,17 If there is no clinical or serologic evidence of infection, testing for the presence of antibodies against tick-borne bacteria at presentation and at 3 and 6 weeks is not recommended due to low sensitivity, low positive predictive value, and cost. Clinicians must only observe and treat if disease occurs.17
Prevention of Tick Bites—Tick bites are best prevented by avoiding tick-infested areas; when these areas are unavoidable, tick bites may be prevented by wearing long pants with the pant legs tucked into boots. In addition, applying topical DEET (N,N-diethyl-m-toluamide) repellent to exposed skin and treating clothing with permethrin can be helpful.17 When used alone, DEET provides greater than 90% protection for up to 2.7 hours against A americanum.18 Permethrin-treated clothing alone is 79% to 100% effective at killing A americanum ticks or disabling them for several hours.19
Conclusion
Tick-borne illness is an increasingly important cause of human infectious disease. In addition to their role as a disease vector, ticks can produce primary skin disorders. This case posed a diagnostic challenge because of the unusually large number and wide distribution of bites as well as the subsequent vesicular reaction that ensued. It is important to keep tick larvae or adult tick bites in the differential when evaluating a patient to expedite tick removal and begin clinical monitoring. Recognition of A americanum larvae as a potential cause of pruritic papules may be helpful in similar cases. In addition, it is important for dermatologists to be aware of the tick species in their area.
Case Report
A 58-year-old woman presented to the dermatology office with a widespread pruritic eruption of 3 days’ duration that started in the groin and spread to the rest of the body. No treatments had been attempted. She had no notable medical history, and she denied any recent illness, change in personal care products, or new medications or supplements. She reported a camping trip 2 weeks prior to presentation on the east end of Long Island, New York. She later learned that others on the same trip developed a similar, albeit less widespread, eruption.
Physical examination revealed clear vesicles on the arms, legs, trunk, and pubic area (Figure 1). Dermoscopy revealed a small lone star tick larva in the center of one of the vesicles (Figure 2). The type of tick larva was identified using resources from the Centers for Disease Control and Prevention (Figure 3).1 Careful inspection revealed dark marks on various vesicles, mostly in the perineum, yielding nearly 20 larvae, which were removed with forceps. The patient was counseled to cover herself in petrolatum for 2 to 3 hours with the hope of smothering any remaining tick larvae. She was given triamcinolone cream and was encouraged to take a nonsedating antihistamine for itch. The patient was seen back in clinic 2 weeks later and the eruption had resolved.
Comment
Spread of Tick-Borne Disease—Ticks and tick-borne disease are increasing major health concerns for humans, domesticated animals, and livestock. Reported cases of bacterial and protozoan tick-borne disease doubled in the United States between 2004 and 2016. Ninety percent of the nearly 60,000 cases of nationally notifiable vector-borne diseases reported in 2017 were linked to ticks.2 Geographic ranges of multiple tick species continue to expand, which is thought to be secondary to rising global temperatures, ecologic changes, reforestation, and increases in commerce and travel (Figure 4).3 Not only have warming temperatures contributed to geographic range expansion, they also may extend ticks’ active season. The lone star tick (Amblyomma americanum) is widely distributed throughout much of the eastern United States.4 The range of A americanum has expanded north in recent years from its prior core range in the southeastern United States.2 One study found that from 2006 to 2016, the vector tick species most commonly collected from humans and submitted to a tick surveillance system in New Jersey shifted from Ixodes scapularis to A americanum.5
Bites by Amblyomma Ticks—As with most hard ticks, the life cycle of A americanum lasts 2 years and includes the egg, the 6-legged larva or “seed tick,” the 8-legged immature nymph, and the 8-legged reproductively mature adult (Figure 3). Amblyomma americanum can lay several thousand eggs.2 Because our patient had numerous bites, it is plausible that she came into contact with a nest of newly hatched tick larvae. Morphogenesis from larva to nymph, then nymph to adult, requires a blood meal.6,7 The larvae emerge from eggs deposited on the ground and then crawl up low vegetation where they can easily attach to passing hosts. The tick clings to hair or clothing and waits until the host is at rest before moving to a favorable location and then bites.8 When attaching, ticks inject an anesthetic akin to lidocaine, making the bite painless. A tick may spend up to 24 hours on the host prior to biting and then feed for 2 hours to 7 days before releasing.9 For the majority of tick-borne illnesses, the tick must remain attached for 24 to 48 hours before disease is transmitted.10
All stages of
Even when the ticks do not transmit disease, tick bites can cause impressive local reactions. Uncomplicated bites can be painful and leave a puncture wound that can take 1 to 2 weeks to heal.13 Rarely, bites can cause a delayed hypersensitivity reaction including fever, pruritus, and urticaria. Granulomas can develop if a tick is improperly removed.9 Other reports describe prurigo lesions, skin hemorrhage, papular urticaria, diffuse papules, vesicles and bullae, necrotic ulcers, and patchy alopecia.14,15 A 2015 systematic controlled study of human bite reactions from A americanum demonstrated the development of itchy erythematous papules and vesicles within 48 hours of larval tick attachment to research participants. The study found tissue damage from A americanum mouthparts, and degranulating mast cells may be evident in as little as 15 minutes.16 The severity of individual skin reaction is hypothesized to depend on several variables, such as the duration of feeding, size of mouthparts, type of tick secretions, changes in secretions during feeding, and prior exposures of the host.14
Tick Removal—If patients present to clinic with ticks attached, removal can be challenging. Removal recommendations call for use of blunt forceps or tweezers. Ticks should be grasped near the skin with consistent pressure, and the tick should be pulled straight out, perpendicular to the skin. Twisting motions can cause the head to separate from the body and remain in the bite wound. Immediately following removal, the area should be cleansed with a disinfectant.10,17 After the tick is removed, some studies recommend storing the tick at −20 °C; should the patient develop disease, the tick could be sent for evaluation.6,17 If there is no clinical or serologic evidence of infection, testing for the presence of antibodies against tick-borne bacteria at presentation and at 3 and 6 weeks is not recommended due to low sensitivity, low positive predictive value, and cost. Clinicians must only observe and treat if disease occurs.17
Prevention of Tick Bites—Tick bites are best prevented by avoiding tick-infested areas; when these areas are unavoidable, tick bites may be prevented by wearing long pants with the pant legs tucked into boots. In addition, applying topical DEET (N,N-diethyl-m-toluamide) repellent to exposed skin and treating clothing with permethrin can be helpful.17 When used alone, DEET provides greater than 90% protection for up to 2.7 hours against A americanum.18 Permethrin-treated clothing alone is 79% to 100% effective at killing A americanum ticks or disabling them for several hours.19
Conclusion
Tick-borne illness is an increasingly important cause of human infectious disease. In addition to their role as a disease vector, ticks can produce primary skin disorders. This case posed a diagnostic challenge because of the unusually large number and wide distribution of bites as well as the subsequent vesicular reaction that ensued. It is important to keep tick larvae or adult tick bites in the differential when evaluating a patient to expedite tick removal and begin clinical monitoring. Recognition of A americanum larvae as a potential cause of pruritic papules may be helpful in similar cases. In addition, it is important for dermatologists to be aware of the tick species in their area.
- Centers for Disease Control and Prevention. Tick ID. Accessed February 21, 2022. https://www.cdc.gov/ticks/tickbornediseases/tickID.html
- Molaei G, Little EAH, Williams SC, et al. Bracing for the worst—range expansion of the lone star tick in the northeastern United States. N Engl J Med. 2019;381:2189-2192.
- Centers for Disease Control and Prevention, Division of Vector-Borne Diseases. Lone star tick (Amblyomma americanum). Accessed March 23, 2022. https://www.cdc.gov/ticks/maps/lone_star_tick.pdf
- Reynolds HH, Elston DM. What’s eating you? lone star tick (Amblyomma americanum). Cutis. 2017;99:111-114.
- Jordan RA, Egizi A. The growing importance of lone star ticks in a Lyme disease endemic county: passive tick surveillance in Monmouth County, NJ, 2006–2016. PLoS One. 2019;14:E0211778.
- Singh-Behl D, La Rosa SP, Tomecki KJ. Tick-borne infections. Dermatol Clin. 2003;21:237-244, v.
- Spach DH, Liles WC, Campbell GL, et al. Tick-borne diseases in the United States. N Engl J Med. 1993;329:936-947.
- Duckworth PF Jr, Hayden GF, Reed CN. Human infestation by Amblyomma americanum larvae (“seed ticks”). South Med J. 1985;78:751-753.
- Middleton DB. Tick-borne infections. what starts as a tiny bite may have a serious outcome. Postgrad Med. 1994;95:131-139.
- Moody EK, Barker RW, White JL, et al. Ticks and tick-borne diseases in Oklahoma. J Okla State Med Assoc. 1998;91:438-445.
- Jones BE. Human ‘seed tick’ infestation. Amblyomma americanum larvae. Arch Dermatol. 1981;117:812-814.
- Centers for Disease Control and Prevention. Tick bite prophylaxis. Accessed February 21, 2022. https://www.cdc.gov/ticks/tickbornediseases/tick-bite-prophylaxis.html
- Fisher EJ, Mo J, Lucky AW. Multiple pruritic papules from lone star tick larvae bites. Arch Dermatol. 2006;142:491-494.
- Krinsky WL. Dermatoses associated with the bites of mites and ticks (Arthropoda: Acari). Int J Dermatol. 1983;22:75-91.
- Yesudian P, Thambiah AS. Persistent papules after tick-bites. Dermatologica. 1973;147:214-218.
- Goddard J, Portugal JS. Cutaneous lesions due to bites by larval Amblyomma americanum ticks. JAMA Dermatol. 2015;151:1373-1375.
- Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32:897-928.
- Solberg VB, Klein TA, McPherson KR, et al. Field evaluation of DEET and a piperidine repellent (AI3-37220) against Amblyomma americanum (Acari: Ixodidae). J Med Entomol. 1995;32:870-875.
- Evans SR, Korch GW Jr, Lawson MA. Comparative field evaluation of permethrin and DEET-treated military uniforms for personal protection against ticks (Acari). J Med Entomol. 1990;27:829-834.
- Centers for Disease Control and Prevention. Tick ID. Accessed February 21, 2022. https://www.cdc.gov/ticks/tickbornediseases/tickID.html
- Molaei G, Little EAH, Williams SC, et al. Bracing for the worst—range expansion of the lone star tick in the northeastern United States. N Engl J Med. 2019;381:2189-2192.
- Centers for Disease Control and Prevention, Division of Vector-Borne Diseases. Lone star tick (Amblyomma americanum). Accessed March 23, 2022. https://www.cdc.gov/ticks/maps/lone_star_tick.pdf
- Reynolds HH, Elston DM. What’s eating you? lone star tick (Amblyomma americanum). Cutis. 2017;99:111-114.
- Jordan RA, Egizi A. The growing importance of lone star ticks in a Lyme disease endemic county: passive tick surveillance in Monmouth County, NJ, 2006–2016. PLoS One. 2019;14:E0211778.
- Singh-Behl D, La Rosa SP, Tomecki KJ. Tick-borne infections. Dermatol Clin. 2003;21:237-244, v.
- Spach DH, Liles WC, Campbell GL, et al. Tick-borne diseases in the United States. N Engl J Med. 1993;329:936-947.
- Duckworth PF Jr, Hayden GF, Reed CN. Human infestation by Amblyomma americanum larvae (“seed ticks”). South Med J. 1985;78:751-753.
- Middleton DB. Tick-borne infections. what starts as a tiny bite may have a serious outcome. Postgrad Med. 1994;95:131-139.
- Moody EK, Barker RW, White JL, et al. Ticks and tick-borne diseases in Oklahoma. J Okla State Med Assoc. 1998;91:438-445.
- Jones BE. Human ‘seed tick’ infestation. Amblyomma americanum larvae. Arch Dermatol. 1981;117:812-814.
- Centers for Disease Control and Prevention. Tick bite prophylaxis. Accessed February 21, 2022. https://www.cdc.gov/ticks/tickbornediseases/tick-bite-prophylaxis.html
- Fisher EJ, Mo J, Lucky AW. Multiple pruritic papules from lone star tick larvae bites. Arch Dermatol. 2006;142:491-494.
- Krinsky WL. Dermatoses associated with the bites of mites and ticks (Arthropoda: Acari). Int J Dermatol. 1983;22:75-91.
- Yesudian P, Thambiah AS. Persistent papules after tick-bites. Dermatologica. 1973;147:214-218.
- Goddard J, Portugal JS. Cutaneous lesions due to bites by larval Amblyomma americanum ticks. JAMA Dermatol. 2015;151:1373-1375.
- Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32:897-928.
- Solberg VB, Klein TA, McPherson KR, et al. Field evaluation of DEET and a piperidine repellent (AI3-37220) against Amblyomma americanum (Acari: Ixodidae). J Med Entomol. 1995;32:870-875.
- Evans SR, Korch GW Jr, Lawson MA. Comparative field evaluation of permethrin and DEET-treated military uniforms for personal protection against ticks (Acari). J Med Entomol. 1990;27:829-834.
Practice Points
- The range of Amblyomma americanum has expanded north in recent years from its core range in the southeastern United States. Warming temperatures also have increased the duration of the ticks’ active season.
- Amblyomma americanum can lay several thousand eggs. A person happening upon a newly hatched nest of larval ticks could sustain a widespread vesicular eruption secondary to tick bites.
- It is important to keep larval tick infestation in the differential when evaluating a patient with a new widespread vesicular eruption to expedite prompt removal of the offending ticks and to begin clinical monitoring.
The Residency Application Process: Current and Future Landscape
Amid increasing numbers of applications, decreasing match rates, and ongoing lack of diversity in the dermatology trainee workforce, the COVID-19 pandemic introduced additional challenges to the dermatology residency application process and laid bare systemic inequities and inherent problems that must be addressed. Historically, dermatology applicants have excelled in academic metrics, such as US Medical Licensing Examination (USMLE) scores and nomination to the Alpha Omega Alpha honor society. As biases associated with these academic metrics are being elucidated, they have in turn become less available. With the upcoming change in USMLE Step 1 reporting to pass/fail only, as well as the elimination of Alpha Omega Alpha nomination for students, clinical grades, and/or class ranks at many medical schools, other elements of the application, such as volunteer experiences and research publications, may be weighed more heavily in the selection process. This may serve to exacerbate the application arms race, characterized by a steady rise in volunteer experiences, research publications, and research gap years that has already begun and likely will continue, particularly among dermatology applicants.
These issues are not unique to dermatology and are occurring across all medical specialties to varying degrees. The monetary and opportunity costs of the application process have become astronomical for both applicants and faculty. Faculty are overburdened with administrative duties related to resident recruitment and advising, and students are experiencing heightened match-related anxiety earlier and more acutely. These factors may contribute to burnout among trainees and faculty and may have deleterious effects on medical education. It is clear that transformative work must be pursued to ensure an equitable and sustainable residency application process moving forward. In this column, we review the notable work being done within dermatology and across specialties to reform the residency application process.
Coalition Recommendations
In August 2021, the Coalition for Physician Accountability (CoPA) released recommendations for comprehensive improvement of the undergraduate medical education (UME) to graduate medical education transition, which includes residency application. Of the 9 principal themes addressed, 2 focus on the residency application process: (1) equitable mission-driven application review, and (2) optimization of the application, interview, and selection processes, which relates to application volume as well as interview offers and formats.1
In the area of application review, CoPA recommends replacing all letters of recommendation with structured evaluative letters as a universal tool in the application process.1 These letters would include specialty-specific questions based on core competencies and would be completed by an evaluator who directly observed the student. Additionally, the group recommends revising the content and structure of the medical student performance evaluation to improve access to longitudinal assessment data about students. Ideally, developing UME competency outcomes to apply across learners would decrease reliance on traditional but potentially problematic application elements, such as licensing examination scores, clinical grades, and narrative evaluations.1
To optimize residency application processes, CoPA recommends exploring innovative approaches to reduce application volume and maximize applicants interviewing and matching at programs where mutual interest is high.1 Suggestions to address these issues include preference signaling, application caps, and/or additional rounds of application or matching. Standardization of the interview process also is recommended to improve equity, minimize educational disruption, and improve applicant well-being. Suggestions include the use of common interview offer and scheduling platforms, policies to govern interview offers and scheduling timelines, interview caps, and ongoing study of the impact of virtual interviews.1
Residency Application Innovations Implemented by Other Specialties
A number of specialties have developed innovations in the residency application process to improve equity and fairness as well as optimize applicant-program fit. Emergency medicine created a now widely adopted, specialty-specific standardized letter of evaluation (SLOE).2 It compares applicants across a number of measures that include personal qualities, clinical skills, and a global assessment. The SLOE is designed to assess and compare applicants across institutions rather than provide recommendations. The emergency medicine SLOE also provides useful information about the letter writer, including duration and depth of interaction with the applicant and distribution of rankings of prior applicants.2
In 2019, obstetrics and gynecology launched a standardized application and interview process, which set a specialty-wide application deadline, limited interview invitations to the number of interview positions available, encouraged coordinated release of interview offers, and allowed applicants 72 hours to respond to invitations.3 These measures were implemented to improve fairness, transparency, and applicant well-being, as well as to promote equitable distribution of interviews. Data following this launch suggested that universal offer dates reduced excessive interviewing among competitive applicants.3
Last year, otolaryngology implemented a process known as preference signaling in which applicants were able to signal up to 5 preferred programs at the time of application. A signal allowed applicants to demonstrate interest in specific programs and could be used by programs during their application review process. Most applicants opted to submit signals, and programs received 0 to 71 signals (mean, 22).4 Almost all programs received at least 1 signal. The rate of receiving an interview was significantly higher for signaled programs (58%) compared to nonsignaled programs (14%)(P<.001), indicating that preference signaling may be beneficial for both programs and applicants for interview selection.4
Residency Application Innovations Implemented by Dermatology
Over the last 2 application cycles, dermatology has implemented several innovations to the residency application process. Initial work included release of guidelines for residency programs to conduct holistic application review,5 recommendations for website updates to share program-specific information with prospective trainees,6 and informational webinars and statements to update dermatology applicants about changes to the process and to answer application-related questions.7-9
In 2020, dermatology initiated a coordinated interview invitation release in which interview offers were released on prespecified dates and applicants were given 48 hours prior to scheduling. Approximately 50% of residency programs participated in the first year, yet nearly all programs released on 1 of 2 universal dates in the current cycle. In a recent survey of dermatology applicants, nearly 90% supported coordinated release.10 Several other specialties also have incorporated universal release dates into their processes.
For the 2021-2022 application cycle, dermatology—along with internal medicine and general surgery—participated in the Association of American Medical Colleges’ pilot supplemental Electronic Residency Application Service (ERAS) application.11 The pilot was designed as a first step to updating the ERAS content by allowing students to share more information about their extracurricular, research, and clinical activities, as well as geographic and program preferences to optimize applicant-program fit. Preference signaling, similar to the otolaryngology process, was included in the supplemental application, with dermatology applicants choosing up to 3 preferred programs to signal, excluding their home programs and any programs where they completed in-person away rotations. Preliminary data suggest that the vast majority of dermatology programs and applicants participated in the supplemental application.12 Ongoing analysis of survey data from applicants, advisors, and program directors will help inform future directions. Dermatology has been an integral partner in the development, implementation, and evaluation of this pilot.
Proposed Innovations to the Application Process
Given the challenges of the current application process, there has been a long list of proposed innovations to ameliorate applicant, advisor, and program concerns.13 Many of these approaches are intended to respond to increasing costs to programs and applicants as well as the lack of equity in the process. Application caps and an early result acceptance program have both been proposed to address the ever-increasing volume of applications.14,15 Neither of these proposals has been adopted by a specialty yet, but obstetrics and gynecology stakeholders have shown broad support for an early result acceptance program, signaling a possible future pilot.16
Interview caps also have been proposed to promote more equitable distribution of interview positions.17 Ophthalmology implemented this approach in the 2021-2022 application cycle, with applicants limited to a maximum of 18 interviews.18 Data from this pilot will help determine the effect of interview caps as well as the optimal limit, which will vary by specialty.
Changes to the application content itself could better facilitate holistic review and optimize applicant-program fit. This is the principle driving the pilot supplemental ERAS application, but it also has been addressed in other specialties. Ophthalmology replaced the traditional personal statement with a shorter autobiographical statement as well as 2 short personal essay questions. Plastic surgery designed a common supplemental application, currently in its second iteration, that highlights specialty-specific information from applicants to promote holistic review and eventually reduce application costs.19
Final Thoughts
The reforms introduced and proposed by dermatology and other specialties represent initial steps to address the issues inherent to the current residency application process. Providing faculty with better tools to holistically assess applicants during the review process and increasing transparency between programs and applicants should help optimize applicant-program fit and increase diversity in the dermatology workforce. Streamlining the application process to allow students to highlight their unique qualities in a user-friendly format as well as addressing potential inequities in interview distribution and access to the application process hopefully will contribute to better outcomes for both programs and applicants. However, many of these steps are likely to create additional administrative burdens on program faculty and are unlikely to allay student fears about matching.
The underlying issue for many specialties, and particularly for dermatology, is that demand far outstrips supply. With stable numbers of residency positions and an ever-increasing number of applicants, the match rate will continue to decrease, leading to increased anxiety among those interested in pursuing dermatology. Although USMLE Step 1 scores have been shown to have racial bias20 and there are no data correlating scores with clinical performance, the elimination of a scoring system may affect the number of applicants entering dermatology with downstream effects on match rates. Heightened anxiety places increased pressure on students to choose a specialty earlier in their training and impacts the activities they pursue during medical school. Overemphasis on specialty choice and the match process can lead to higher rates of burnout among students and trainees, as students may focus on activities designed to increase their chances of matching at the expense of pursuing activities that could lead to greater engagement and passion in their careers—a key protective factor against burnout.
The goal of the residency application process is to optimize fit between candidates and programs by aligning goals, values, and learning environment. Students and programs working together as honest brokers can lead to transformative change in the process, freeing both parties to highlight their unique qualities and contributions. Programs benefit from optimal fit by being able to hone their particular mission and recruit and retain residents and faculty engaged in that mission. Residents will thrive in programs that support their learning and career goals and will ultimately be better positioned to meaningfully contribute to their chosen field in whatever capacity they choose.
Acknowledgments—The views presented in this column reflect those of the 9 elected members of the Association of Professors of Dermatology Residency Program Directors Section steering committee, all of whom are program directors at their institutions (listed in parentheses): Ammar Ahmed, MD (The University of Texas at Austin, Austin, Texas); Yolanda Helfrich, MD (University of Michigan, Ann Arbor, Michigan); Jo-Ann M. Latkowksi, MD (New York University, New York); Kiran Motaparthi, MD (University of Florida, Gainesville, Florida); Adena E. Rosenblatt, MD, PhD (The University of Chicago, Chicago, Illinois); Ilana S. Rosman, MD (Washington University, St. Louis, Missouri); Travis Vandergriff, MD (University of Texas Southwestern, Dallas, Texas); Diane Whitaker-Worth, MD (University of Connecticut, Farmington, Connecticut); Scott Worswick, MD (University of Southern California, Los Angeles, California).
- Coalition for Physician Accountability. The Coalition for Physician Accountability’s Undergraduate Medical Education–Graduate Medical Education Review Committee (UGRC): recommendations for comprehensive improvement of the UME-GME transition. Accessed March 7, 2022. https://physicianaccountability.org/wp-content/uploads/2021/08/UGRC-Coalition-Report-FINAL.pdf
- Jackson JS, Bond M, Love JN, et al. Emergency medicine standardized letter of evaluation (SLOE): findings from the new electronic SLOE format. J Grad Med Educ. 2019;11:182-186.
- Santos-Parker KS, Morgan HK, Katz NT, et al. Can standardized dates for interview offers mitigate excessive interviewing? J Surg Educ. 2021;78:1091-1096.
- Pletcher SD, Chang CWD, Thorne MC, et al. The otolaryngology residency program preference signaling experience [published online October 5, 2021]. Acad Med. doi:10.1097/ACM.0000000000004441
- Association of Professors of Dermatology. Holistic review. Accessed March 7, 2022. https://www.dermatologyprofessors.org/files/3_Holistic%20review_Oct2020.pdf
- Rosmarin D, Friedman AJ, Burkemper NM, et al. The Association of Professors of Dermatology Program Directors Task Force and Residency Program Transparency Work Group guidelines on residency program transparency. J Drugs Dermatol. 2020;19:1117-1118.
- Rosman IS, Schadt CR, Samimi SS, et al. Approaching the dermatology residency application process during a pandemic. J Am Acad Dermatol. 2020;83:E351-E352.
- Association of Professors of Dermatology. Program director resources. Accessed March 7, 2022. https://www.dermatologyprofessors.org/programdirectors_resources.php
- Brumfiel CM, Jefferson IS, Wu AG, et al. A national webinar for dermatology applicants during the COVID-19 pandemic. J Am Acad Dermatol. 2021;84:574-575.
- Brumfiel CM, Jefferson IS, Rinderknecht FA, et al. Current perspectives of and potential reforms to the dermatology residency application process: a nationwide survey of program directors and applicants. Clin Dermatol. In press.
- Association of American Medical Colleges. Supplemental ERAS application (for the ERAS 2022 cycle). Accessed March 7, 2022. https://students-residents.aamc.org/applying-residencies-eras/supplementalerasapplication
- Association of American Medical Colleges. AAMC supplemental ERAS application: key findings from the 2022 application cycle. Accessed March 11, 2022. https://www.aamc.org/media/58891/download
- Warm EJ, Kinnear B, Pereira A, et al. The residency match: escaping the prisoner’s dilemma. J Grad Med Educ. 2021;13:616-625.
- Carmody JB, Rosman IS, Carlson JC. Application fever: reviewing the causes, costs, and cures for residency application inflation. Cureus. 2021;13:E13804.
- Hammoud MM, Andrews J, Skochelak SE. Improving the residency application and selection process: an optional early result acceptance program. JAMA. 2020;323:503-504.
- Winkel AF, Morgan HK, Akingbola O, et al. Perspectives of stakeholders about an early release acceptance program to complement the residency match in obstetrics and gynecology. JAMA Netw Open. 2021;4:E2124158.
- Morgan HK, Winkel AF, Standiford T, et al. The case for capping residency interviews. J Surg Educ. 2021;78:755-762.
- Association of University Professors of Ophthalmology. 2021-22 ophthalmology residency match FAQs. Accessed March 7, 2022. https://aupo.org/sites/default/files/2021-06/Residency%20Match%20FAQs_2021.pdf
- American Council of Academic Plastic Surgeons. Applying to plastic surgery (PSCA). Accessed March 7, 2022. https://acaplasticsurgeons.org/PSCA/
- Rubright JD, Jodoin M, Barone MA. Examining demographics, prior academic performance, and United States Medical Licensing Examination Scores. Acad Med. 2019;94:364-370.
Amid increasing numbers of applications, decreasing match rates, and ongoing lack of diversity in the dermatology trainee workforce, the COVID-19 pandemic introduced additional challenges to the dermatology residency application process and laid bare systemic inequities and inherent problems that must be addressed. Historically, dermatology applicants have excelled in academic metrics, such as US Medical Licensing Examination (USMLE) scores and nomination to the Alpha Omega Alpha honor society. As biases associated with these academic metrics are being elucidated, they have in turn become less available. With the upcoming change in USMLE Step 1 reporting to pass/fail only, as well as the elimination of Alpha Omega Alpha nomination for students, clinical grades, and/or class ranks at many medical schools, other elements of the application, such as volunteer experiences and research publications, may be weighed more heavily in the selection process. This may serve to exacerbate the application arms race, characterized by a steady rise in volunteer experiences, research publications, and research gap years that has already begun and likely will continue, particularly among dermatology applicants.
These issues are not unique to dermatology and are occurring across all medical specialties to varying degrees. The monetary and opportunity costs of the application process have become astronomical for both applicants and faculty. Faculty are overburdened with administrative duties related to resident recruitment and advising, and students are experiencing heightened match-related anxiety earlier and more acutely. These factors may contribute to burnout among trainees and faculty and may have deleterious effects on medical education. It is clear that transformative work must be pursued to ensure an equitable and sustainable residency application process moving forward. In this column, we review the notable work being done within dermatology and across specialties to reform the residency application process.
Coalition Recommendations
In August 2021, the Coalition for Physician Accountability (CoPA) released recommendations for comprehensive improvement of the undergraduate medical education (UME) to graduate medical education transition, which includes residency application. Of the 9 principal themes addressed, 2 focus on the residency application process: (1) equitable mission-driven application review, and (2) optimization of the application, interview, and selection processes, which relates to application volume as well as interview offers and formats.1
In the area of application review, CoPA recommends replacing all letters of recommendation with structured evaluative letters as a universal tool in the application process.1 These letters would include specialty-specific questions based on core competencies and would be completed by an evaluator who directly observed the student. Additionally, the group recommends revising the content and structure of the medical student performance evaluation to improve access to longitudinal assessment data about students. Ideally, developing UME competency outcomes to apply across learners would decrease reliance on traditional but potentially problematic application elements, such as licensing examination scores, clinical grades, and narrative evaluations.1
To optimize residency application processes, CoPA recommends exploring innovative approaches to reduce application volume and maximize applicants interviewing and matching at programs where mutual interest is high.1 Suggestions to address these issues include preference signaling, application caps, and/or additional rounds of application or matching. Standardization of the interview process also is recommended to improve equity, minimize educational disruption, and improve applicant well-being. Suggestions include the use of common interview offer and scheduling platforms, policies to govern interview offers and scheduling timelines, interview caps, and ongoing study of the impact of virtual interviews.1
Residency Application Innovations Implemented by Other Specialties
A number of specialties have developed innovations in the residency application process to improve equity and fairness as well as optimize applicant-program fit. Emergency medicine created a now widely adopted, specialty-specific standardized letter of evaluation (SLOE).2 It compares applicants across a number of measures that include personal qualities, clinical skills, and a global assessment. The SLOE is designed to assess and compare applicants across institutions rather than provide recommendations. The emergency medicine SLOE also provides useful information about the letter writer, including duration and depth of interaction with the applicant and distribution of rankings of prior applicants.2
In 2019, obstetrics and gynecology launched a standardized application and interview process, which set a specialty-wide application deadline, limited interview invitations to the number of interview positions available, encouraged coordinated release of interview offers, and allowed applicants 72 hours to respond to invitations.3 These measures were implemented to improve fairness, transparency, and applicant well-being, as well as to promote equitable distribution of interviews. Data following this launch suggested that universal offer dates reduced excessive interviewing among competitive applicants.3
Last year, otolaryngology implemented a process known as preference signaling in which applicants were able to signal up to 5 preferred programs at the time of application. A signal allowed applicants to demonstrate interest in specific programs and could be used by programs during their application review process. Most applicants opted to submit signals, and programs received 0 to 71 signals (mean, 22).4 Almost all programs received at least 1 signal. The rate of receiving an interview was significantly higher for signaled programs (58%) compared to nonsignaled programs (14%)(P<.001), indicating that preference signaling may be beneficial for both programs and applicants for interview selection.4
Residency Application Innovations Implemented by Dermatology
Over the last 2 application cycles, dermatology has implemented several innovations to the residency application process. Initial work included release of guidelines for residency programs to conduct holistic application review,5 recommendations for website updates to share program-specific information with prospective trainees,6 and informational webinars and statements to update dermatology applicants about changes to the process and to answer application-related questions.7-9
In 2020, dermatology initiated a coordinated interview invitation release in which interview offers were released on prespecified dates and applicants were given 48 hours prior to scheduling. Approximately 50% of residency programs participated in the first year, yet nearly all programs released on 1 of 2 universal dates in the current cycle. In a recent survey of dermatology applicants, nearly 90% supported coordinated release.10 Several other specialties also have incorporated universal release dates into their processes.
For the 2021-2022 application cycle, dermatology—along with internal medicine and general surgery—participated in the Association of American Medical Colleges’ pilot supplemental Electronic Residency Application Service (ERAS) application.11 The pilot was designed as a first step to updating the ERAS content by allowing students to share more information about their extracurricular, research, and clinical activities, as well as geographic and program preferences to optimize applicant-program fit. Preference signaling, similar to the otolaryngology process, was included in the supplemental application, with dermatology applicants choosing up to 3 preferred programs to signal, excluding their home programs and any programs where they completed in-person away rotations. Preliminary data suggest that the vast majority of dermatology programs and applicants participated in the supplemental application.12 Ongoing analysis of survey data from applicants, advisors, and program directors will help inform future directions. Dermatology has been an integral partner in the development, implementation, and evaluation of this pilot.
Proposed Innovations to the Application Process
Given the challenges of the current application process, there has been a long list of proposed innovations to ameliorate applicant, advisor, and program concerns.13 Many of these approaches are intended to respond to increasing costs to programs and applicants as well as the lack of equity in the process. Application caps and an early result acceptance program have both been proposed to address the ever-increasing volume of applications.14,15 Neither of these proposals has been adopted by a specialty yet, but obstetrics and gynecology stakeholders have shown broad support for an early result acceptance program, signaling a possible future pilot.16
Interview caps also have been proposed to promote more equitable distribution of interview positions.17 Ophthalmology implemented this approach in the 2021-2022 application cycle, with applicants limited to a maximum of 18 interviews.18 Data from this pilot will help determine the effect of interview caps as well as the optimal limit, which will vary by specialty.
Changes to the application content itself could better facilitate holistic review and optimize applicant-program fit. This is the principle driving the pilot supplemental ERAS application, but it also has been addressed in other specialties. Ophthalmology replaced the traditional personal statement with a shorter autobiographical statement as well as 2 short personal essay questions. Plastic surgery designed a common supplemental application, currently in its second iteration, that highlights specialty-specific information from applicants to promote holistic review and eventually reduce application costs.19
Final Thoughts
The reforms introduced and proposed by dermatology and other specialties represent initial steps to address the issues inherent to the current residency application process. Providing faculty with better tools to holistically assess applicants during the review process and increasing transparency between programs and applicants should help optimize applicant-program fit and increase diversity in the dermatology workforce. Streamlining the application process to allow students to highlight their unique qualities in a user-friendly format as well as addressing potential inequities in interview distribution and access to the application process hopefully will contribute to better outcomes for both programs and applicants. However, many of these steps are likely to create additional administrative burdens on program faculty and are unlikely to allay student fears about matching.
The underlying issue for many specialties, and particularly for dermatology, is that demand far outstrips supply. With stable numbers of residency positions and an ever-increasing number of applicants, the match rate will continue to decrease, leading to increased anxiety among those interested in pursuing dermatology. Although USMLE Step 1 scores have been shown to have racial bias20 and there are no data correlating scores with clinical performance, the elimination of a scoring system may affect the number of applicants entering dermatology with downstream effects on match rates. Heightened anxiety places increased pressure on students to choose a specialty earlier in their training and impacts the activities they pursue during medical school. Overemphasis on specialty choice and the match process can lead to higher rates of burnout among students and trainees, as students may focus on activities designed to increase their chances of matching at the expense of pursuing activities that could lead to greater engagement and passion in their careers—a key protective factor against burnout.
The goal of the residency application process is to optimize fit between candidates and programs by aligning goals, values, and learning environment. Students and programs working together as honest brokers can lead to transformative change in the process, freeing both parties to highlight their unique qualities and contributions. Programs benefit from optimal fit by being able to hone their particular mission and recruit and retain residents and faculty engaged in that mission. Residents will thrive in programs that support their learning and career goals and will ultimately be better positioned to meaningfully contribute to their chosen field in whatever capacity they choose.
Acknowledgments—The views presented in this column reflect those of the 9 elected members of the Association of Professors of Dermatology Residency Program Directors Section steering committee, all of whom are program directors at their institutions (listed in parentheses): Ammar Ahmed, MD (The University of Texas at Austin, Austin, Texas); Yolanda Helfrich, MD (University of Michigan, Ann Arbor, Michigan); Jo-Ann M. Latkowksi, MD (New York University, New York); Kiran Motaparthi, MD (University of Florida, Gainesville, Florida); Adena E. Rosenblatt, MD, PhD (The University of Chicago, Chicago, Illinois); Ilana S. Rosman, MD (Washington University, St. Louis, Missouri); Travis Vandergriff, MD (University of Texas Southwestern, Dallas, Texas); Diane Whitaker-Worth, MD (University of Connecticut, Farmington, Connecticut); Scott Worswick, MD (University of Southern California, Los Angeles, California).
Amid increasing numbers of applications, decreasing match rates, and ongoing lack of diversity in the dermatology trainee workforce, the COVID-19 pandemic introduced additional challenges to the dermatology residency application process and laid bare systemic inequities and inherent problems that must be addressed. Historically, dermatology applicants have excelled in academic metrics, such as US Medical Licensing Examination (USMLE) scores and nomination to the Alpha Omega Alpha honor society. As biases associated with these academic metrics are being elucidated, they have in turn become less available. With the upcoming change in USMLE Step 1 reporting to pass/fail only, as well as the elimination of Alpha Omega Alpha nomination for students, clinical grades, and/or class ranks at many medical schools, other elements of the application, such as volunteer experiences and research publications, may be weighed more heavily in the selection process. This may serve to exacerbate the application arms race, characterized by a steady rise in volunteer experiences, research publications, and research gap years that has already begun and likely will continue, particularly among dermatology applicants.
These issues are not unique to dermatology and are occurring across all medical specialties to varying degrees. The monetary and opportunity costs of the application process have become astronomical for both applicants and faculty. Faculty are overburdened with administrative duties related to resident recruitment and advising, and students are experiencing heightened match-related anxiety earlier and more acutely. These factors may contribute to burnout among trainees and faculty and may have deleterious effects on medical education. It is clear that transformative work must be pursued to ensure an equitable and sustainable residency application process moving forward. In this column, we review the notable work being done within dermatology and across specialties to reform the residency application process.
Coalition Recommendations
In August 2021, the Coalition for Physician Accountability (CoPA) released recommendations for comprehensive improvement of the undergraduate medical education (UME) to graduate medical education transition, which includes residency application. Of the 9 principal themes addressed, 2 focus on the residency application process: (1) equitable mission-driven application review, and (2) optimization of the application, interview, and selection processes, which relates to application volume as well as interview offers and formats.1
In the area of application review, CoPA recommends replacing all letters of recommendation with structured evaluative letters as a universal tool in the application process.1 These letters would include specialty-specific questions based on core competencies and would be completed by an evaluator who directly observed the student. Additionally, the group recommends revising the content and structure of the medical student performance evaluation to improve access to longitudinal assessment data about students. Ideally, developing UME competency outcomes to apply across learners would decrease reliance on traditional but potentially problematic application elements, such as licensing examination scores, clinical grades, and narrative evaluations.1
To optimize residency application processes, CoPA recommends exploring innovative approaches to reduce application volume and maximize applicants interviewing and matching at programs where mutual interest is high.1 Suggestions to address these issues include preference signaling, application caps, and/or additional rounds of application or matching. Standardization of the interview process also is recommended to improve equity, minimize educational disruption, and improve applicant well-being. Suggestions include the use of common interview offer and scheduling platforms, policies to govern interview offers and scheduling timelines, interview caps, and ongoing study of the impact of virtual interviews.1
Residency Application Innovations Implemented by Other Specialties
A number of specialties have developed innovations in the residency application process to improve equity and fairness as well as optimize applicant-program fit. Emergency medicine created a now widely adopted, specialty-specific standardized letter of evaluation (SLOE).2 It compares applicants across a number of measures that include personal qualities, clinical skills, and a global assessment. The SLOE is designed to assess and compare applicants across institutions rather than provide recommendations. The emergency medicine SLOE also provides useful information about the letter writer, including duration and depth of interaction with the applicant and distribution of rankings of prior applicants.2
In 2019, obstetrics and gynecology launched a standardized application and interview process, which set a specialty-wide application deadline, limited interview invitations to the number of interview positions available, encouraged coordinated release of interview offers, and allowed applicants 72 hours to respond to invitations.3 These measures were implemented to improve fairness, transparency, and applicant well-being, as well as to promote equitable distribution of interviews. Data following this launch suggested that universal offer dates reduced excessive interviewing among competitive applicants.3
Last year, otolaryngology implemented a process known as preference signaling in which applicants were able to signal up to 5 preferred programs at the time of application. A signal allowed applicants to demonstrate interest in specific programs and could be used by programs during their application review process. Most applicants opted to submit signals, and programs received 0 to 71 signals (mean, 22).4 Almost all programs received at least 1 signal. The rate of receiving an interview was significantly higher for signaled programs (58%) compared to nonsignaled programs (14%)(P<.001), indicating that preference signaling may be beneficial for both programs and applicants for interview selection.4
Residency Application Innovations Implemented by Dermatology
Over the last 2 application cycles, dermatology has implemented several innovations to the residency application process. Initial work included release of guidelines for residency programs to conduct holistic application review,5 recommendations for website updates to share program-specific information with prospective trainees,6 and informational webinars and statements to update dermatology applicants about changes to the process and to answer application-related questions.7-9
In 2020, dermatology initiated a coordinated interview invitation release in which interview offers were released on prespecified dates and applicants were given 48 hours prior to scheduling. Approximately 50% of residency programs participated in the first year, yet nearly all programs released on 1 of 2 universal dates in the current cycle. In a recent survey of dermatology applicants, nearly 90% supported coordinated release.10 Several other specialties also have incorporated universal release dates into their processes.
For the 2021-2022 application cycle, dermatology—along with internal medicine and general surgery—participated in the Association of American Medical Colleges’ pilot supplemental Electronic Residency Application Service (ERAS) application.11 The pilot was designed as a first step to updating the ERAS content by allowing students to share more information about their extracurricular, research, and clinical activities, as well as geographic and program preferences to optimize applicant-program fit. Preference signaling, similar to the otolaryngology process, was included in the supplemental application, with dermatology applicants choosing up to 3 preferred programs to signal, excluding their home programs and any programs where they completed in-person away rotations. Preliminary data suggest that the vast majority of dermatology programs and applicants participated in the supplemental application.12 Ongoing analysis of survey data from applicants, advisors, and program directors will help inform future directions. Dermatology has been an integral partner in the development, implementation, and evaluation of this pilot.
Proposed Innovations to the Application Process
Given the challenges of the current application process, there has been a long list of proposed innovations to ameliorate applicant, advisor, and program concerns.13 Many of these approaches are intended to respond to increasing costs to programs and applicants as well as the lack of equity in the process. Application caps and an early result acceptance program have both been proposed to address the ever-increasing volume of applications.14,15 Neither of these proposals has been adopted by a specialty yet, but obstetrics and gynecology stakeholders have shown broad support for an early result acceptance program, signaling a possible future pilot.16
Interview caps also have been proposed to promote more equitable distribution of interview positions.17 Ophthalmology implemented this approach in the 2021-2022 application cycle, with applicants limited to a maximum of 18 interviews.18 Data from this pilot will help determine the effect of interview caps as well as the optimal limit, which will vary by specialty.
Changes to the application content itself could better facilitate holistic review and optimize applicant-program fit. This is the principle driving the pilot supplemental ERAS application, but it also has been addressed in other specialties. Ophthalmology replaced the traditional personal statement with a shorter autobiographical statement as well as 2 short personal essay questions. Plastic surgery designed a common supplemental application, currently in its second iteration, that highlights specialty-specific information from applicants to promote holistic review and eventually reduce application costs.19
Final Thoughts
The reforms introduced and proposed by dermatology and other specialties represent initial steps to address the issues inherent to the current residency application process. Providing faculty with better tools to holistically assess applicants during the review process and increasing transparency between programs and applicants should help optimize applicant-program fit and increase diversity in the dermatology workforce. Streamlining the application process to allow students to highlight their unique qualities in a user-friendly format as well as addressing potential inequities in interview distribution and access to the application process hopefully will contribute to better outcomes for both programs and applicants. However, many of these steps are likely to create additional administrative burdens on program faculty and are unlikely to allay student fears about matching.
The underlying issue for many specialties, and particularly for dermatology, is that demand far outstrips supply. With stable numbers of residency positions and an ever-increasing number of applicants, the match rate will continue to decrease, leading to increased anxiety among those interested in pursuing dermatology. Although USMLE Step 1 scores have been shown to have racial bias20 and there are no data correlating scores with clinical performance, the elimination of a scoring system may affect the number of applicants entering dermatology with downstream effects on match rates. Heightened anxiety places increased pressure on students to choose a specialty earlier in their training and impacts the activities they pursue during medical school. Overemphasis on specialty choice and the match process can lead to higher rates of burnout among students and trainees, as students may focus on activities designed to increase their chances of matching at the expense of pursuing activities that could lead to greater engagement and passion in their careers—a key protective factor against burnout.
The goal of the residency application process is to optimize fit between candidates and programs by aligning goals, values, and learning environment. Students and programs working together as honest brokers can lead to transformative change in the process, freeing both parties to highlight their unique qualities and contributions. Programs benefit from optimal fit by being able to hone their particular mission and recruit and retain residents and faculty engaged in that mission. Residents will thrive in programs that support their learning and career goals and will ultimately be better positioned to meaningfully contribute to their chosen field in whatever capacity they choose.
Acknowledgments—The views presented in this column reflect those of the 9 elected members of the Association of Professors of Dermatology Residency Program Directors Section steering committee, all of whom are program directors at their institutions (listed in parentheses): Ammar Ahmed, MD (The University of Texas at Austin, Austin, Texas); Yolanda Helfrich, MD (University of Michigan, Ann Arbor, Michigan); Jo-Ann M. Latkowksi, MD (New York University, New York); Kiran Motaparthi, MD (University of Florida, Gainesville, Florida); Adena E. Rosenblatt, MD, PhD (The University of Chicago, Chicago, Illinois); Ilana S. Rosman, MD (Washington University, St. Louis, Missouri); Travis Vandergriff, MD (University of Texas Southwestern, Dallas, Texas); Diane Whitaker-Worth, MD (University of Connecticut, Farmington, Connecticut); Scott Worswick, MD (University of Southern California, Los Angeles, California).
- Coalition for Physician Accountability. The Coalition for Physician Accountability’s Undergraduate Medical Education–Graduate Medical Education Review Committee (UGRC): recommendations for comprehensive improvement of the UME-GME transition. Accessed March 7, 2022. https://physicianaccountability.org/wp-content/uploads/2021/08/UGRC-Coalition-Report-FINAL.pdf
- Jackson JS, Bond M, Love JN, et al. Emergency medicine standardized letter of evaluation (SLOE): findings from the new electronic SLOE format. J Grad Med Educ. 2019;11:182-186.
- Santos-Parker KS, Morgan HK, Katz NT, et al. Can standardized dates for interview offers mitigate excessive interviewing? J Surg Educ. 2021;78:1091-1096.
- Pletcher SD, Chang CWD, Thorne MC, et al. The otolaryngology residency program preference signaling experience [published online October 5, 2021]. Acad Med. doi:10.1097/ACM.0000000000004441
- Association of Professors of Dermatology. Holistic review. Accessed March 7, 2022. https://www.dermatologyprofessors.org/files/3_Holistic%20review_Oct2020.pdf
- Rosmarin D, Friedman AJ, Burkemper NM, et al. The Association of Professors of Dermatology Program Directors Task Force and Residency Program Transparency Work Group guidelines on residency program transparency. J Drugs Dermatol. 2020;19:1117-1118.
- Rosman IS, Schadt CR, Samimi SS, et al. Approaching the dermatology residency application process during a pandemic. J Am Acad Dermatol. 2020;83:E351-E352.
- Association of Professors of Dermatology. Program director resources. Accessed March 7, 2022. https://www.dermatologyprofessors.org/programdirectors_resources.php
- Brumfiel CM, Jefferson IS, Wu AG, et al. A national webinar for dermatology applicants during the COVID-19 pandemic. J Am Acad Dermatol. 2021;84:574-575.
- Brumfiel CM, Jefferson IS, Rinderknecht FA, et al. Current perspectives of and potential reforms to the dermatology residency application process: a nationwide survey of program directors and applicants. Clin Dermatol. In press.
- Association of American Medical Colleges. Supplemental ERAS application (for the ERAS 2022 cycle). Accessed March 7, 2022. https://students-residents.aamc.org/applying-residencies-eras/supplementalerasapplication
- Association of American Medical Colleges. AAMC supplemental ERAS application: key findings from the 2022 application cycle. Accessed March 11, 2022. https://www.aamc.org/media/58891/download
- Warm EJ, Kinnear B, Pereira A, et al. The residency match: escaping the prisoner’s dilemma. J Grad Med Educ. 2021;13:616-625.
- Carmody JB, Rosman IS, Carlson JC. Application fever: reviewing the causes, costs, and cures for residency application inflation. Cureus. 2021;13:E13804.
- Hammoud MM, Andrews J, Skochelak SE. Improving the residency application and selection process: an optional early result acceptance program. JAMA. 2020;323:503-504.
- Winkel AF, Morgan HK, Akingbola O, et al. Perspectives of stakeholders about an early release acceptance program to complement the residency match in obstetrics and gynecology. JAMA Netw Open. 2021;4:E2124158.
- Morgan HK, Winkel AF, Standiford T, et al. The case for capping residency interviews. J Surg Educ. 2021;78:755-762.
- Association of University Professors of Ophthalmology. 2021-22 ophthalmology residency match FAQs. Accessed March 7, 2022. https://aupo.org/sites/default/files/2021-06/Residency%20Match%20FAQs_2021.pdf
- American Council of Academic Plastic Surgeons. Applying to plastic surgery (PSCA). Accessed March 7, 2022. https://acaplasticsurgeons.org/PSCA/
- Rubright JD, Jodoin M, Barone MA. Examining demographics, prior academic performance, and United States Medical Licensing Examination Scores. Acad Med. 2019;94:364-370.
- Coalition for Physician Accountability. The Coalition for Physician Accountability’s Undergraduate Medical Education–Graduate Medical Education Review Committee (UGRC): recommendations for comprehensive improvement of the UME-GME transition. Accessed March 7, 2022. https://physicianaccountability.org/wp-content/uploads/2021/08/UGRC-Coalition-Report-FINAL.pdf
- Jackson JS, Bond M, Love JN, et al. Emergency medicine standardized letter of evaluation (SLOE): findings from the new electronic SLOE format. J Grad Med Educ. 2019;11:182-186.
- Santos-Parker KS, Morgan HK, Katz NT, et al. Can standardized dates for interview offers mitigate excessive interviewing? J Surg Educ. 2021;78:1091-1096.
- Pletcher SD, Chang CWD, Thorne MC, et al. The otolaryngology residency program preference signaling experience [published online October 5, 2021]. Acad Med. doi:10.1097/ACM.0000000000004441
- Association of Professors of Dermatology. Holistic review. Accessed March 7, 2022. https://www.dermatologyprofessors.org/files/3_Holistic%20review_Oct2020.pdf
- Rosmarin D, Friedman AJ, Burkemper NM, et al. The Association of Professors of Dermatology Program Directors Task Force and Residency Program Transparency Work Group guidelines on residency program transparency. J Drugs Dermatol. 2020;19:1117-1118.
- Rosman IS, Schadt CR, Samimi SS, et al. Approaching the dermatology residency application process during a pandemic. J Am Acad Dermatol. 2020;83:E351-E352.
- Association of Professors of Dermatology. Program director resources. Accessed March 7, 2022. https://www.dermatologyprofessors.org/programdirectors_resources.php
- Brumfiel CM, Jefferson IS, Wu AG, et al. A national webinar for dermatology applicants during the COVID-19 pandemic. J Am Acad Dermatol. 2021;84:574-575.
- Brumfiel CM, Jefferson IS, Rinderknecht FA, et al. Current perspectives of and potential reforms to the dermatology residency application process: a nationwide survey of program directors and applicants. Clin Dermatol. In press.
- Association of American Medical Colleges. Supplemental ERAS application (for the ERAS 2022 cycle). Accessed March 7, 2022. https://students-residents.aamc.org/applying-residencies-eras/supplementalerasapplication
- Association of American Medical Colleges. AAMC supplemental ERAS application: key findings from the 2022 application cycle. Accessed March 11, 2022. https://www.aamc.org/media/58891/download
- Warm EJ, Kinnear B, Pereira A, et al. The residency match: escaping the prisoner’s dilemma. J Grad Med Educ. 2021;13:616-625.
- Carmody JB, Rosman IS, Carlson JC. Application fever: reviewing the causes, costs, and cures for residency application inflation. Cureus. 2021;13:E13804.
- Hammoud MM, Andrews J, Skochelak SE. Improving the residency application and selection process: an optional early result acceptance program. JAMA. 2020;323:503-504.
- Winkel AF, Morgan HK, Akingbola O, et al. Perspectives of stakeholders about an early release acceptance program to complement the residency match in obstetrics and gynecology. JAMA Netw Open. 2021;4:E2124158.
- Morgan HK, Winkel AF, Standiford T, et al. The case for capping residency interviews. J Surg Educ. 2021;78:755-762.
- Association of University Professors of Ophthalmology. 2021-22 ophthalmology residency match FAQs. Accessed March 7, 2022. https://aupo.org/sites/default/files/2021-06/Residency%20Match%20FAQs_2021.pdf
- American Council of Academic Plastic Surgeons. Applying to plastic surgery (PSCA). Accessed March 7, 2022. https://acaplasticsurgeons.org/PSCA/
- Rubright JD, Jodoin M, Barone MA. Examining demographics, prior academic performance, and United States Medical Licensing Examination Scores. Acad Med. 2019;94:364-370.
Practice Points
- Dermatology has implemented several reforms to the residency application process, including coordinated interview invitation release, mechanisms for enhanced transparency between programs and applicants, and a new common supplemental application.
- Across specialties, additional innovations to the residency application process have been implemented and proposed, including preference signaling, an early result acceptance process, and interview and application limits.
- Current efforts to improve the residency application process are ongoing with cross-specialty collaboration.
Residency Roundup: Introducing a New Partnership Between Cutis and the APD-RPDS
We are excited to announce a new partnership between Cutis and the Association of Professors of Dermatology Residency Program Directors Section (APD-RPDS). The new APD-RPDS column Residency Roundup will contain quarterly communications and submissions that we hope will facilitate greater dissemination of information that is useful to the dermatology teaching community.
The APD is a group of academic dermatologists whose membership comprises chairs, chiefs, residency and fellowship program directors, and teaching faculty. Each fall, the group convenes in Chicago, Illinois, for a 2-day meeting centered around departmental and program leadership with a focus on education. The APD-RPDS was formed in 2020 and is led by a steering committee of 9 members, including our current Chair, Ilana S. Rosman, MD (Washington University School of Medicine, St. Louis, Missouri), and Vice Chair, Jo-Ann M. Latkowski, MD (New York University, New York). Committee members are elected from and by the APD membership and must serve in program leadership at their home programs. The APD-RPDS helps plan and coordinate breakout sessions and lectures at the annual APD meeting, which typically relate to program director duties, changing policies within the American Board of Dermatology or Accreditation Council for Graduate Medical Education, ideas for future growth, and changes in our specialty and in resident education. Members of the APD-RPDS have access to the APD listserv, a valuable resource for discussing issues affecting residency training. We also have work groups led by our members, which include diversity, equity, and inclusion; resource development; communications; and the annual survey. To join the APD, the RPDS, and/or any of our workgroups, please reach out to us or visit the APD website (https://www.dermatologyprofessors.org).
We look forward to welcoming and expediently reviewing members’ submissions to the new Residency Roundup column falling into 2 principal categories within the scope of dermatologic recruitment, didactic education, and clinical training. The first category will feature novel tools, programs, and platforms to improve dermatology training through collaboration. This could entail a description of a new platform designed for sharing resources among programs and specialties to enhance learning for trainees and faculty alike. For example, if a database is created that contains prerecorded lectures pertaining to alopecia, a potential article submission might introduce the database and provide information on what topics are covered and how to access these lectures for readers worldwide. Likewise, if a new technology emerges that allows for easier collaboration among programs, a possible submission would introduce the technology and discuss its potential benefits to trainees, faculty, and practicing dermatologists.
Secondly and more commonly, we anticipate the Residency Roundup column will feature articles that delve into the critical issues and challenges currently impacting recruitment, training, and administration in dermatology residency programs. Specific topics may include but are not limited to recruitment of underrepresented in medicine applicants to dermatology, technological advances to improve teaching methods within training programs, surveys delving into the dermatology match process, and educational gaps or future directions in the specialty. The column occasionally may be used to disseminate information from our section of the APD, including consensus statements or editorials related to changes implemented in the dermatology residency application process. A prospective editorial on this subject could explore varying viewpoints of implemented and proposed changes as well as the reasons behind the changes.
Our group is collaborative, and our aim is to improve education, equity, management of program director responsibilities, and the dermatology application process for programs and applicants alike. With your input, experience, and varied perspectives, we look forward to moving the field of dermatology to a better future by working together.
We are excited to announce a new partnership between Cutis and the Association of Professors of Dermatology Residency Program Directors Section (APD-RPDS). The new APD-RPDS column Residency Roundup will contain quarterly communications and submissions that we hope will facilitate greater dissemination of information that is useful to the dermatology teaching community.
The APD is a group of academic dermatologists whose membership comprises chairs, chiefs, residency and fellowship program directors, and teaching faculty. Each fall, the group convenes in Chicago, Illinois, for a 2-day meeting centered around departmental and program leadership with a focus on education. The APD-RPDS was formed in 2020 and is led by a steering committee of 9 members, including our current Chair, Ilana S. Rosman, MD (Washington University School of Medicine, St. Louis, Missouri), and Vice Chair, Jo-Ann M. Latkowski, MD (New York University, New York). Committee members are elected from and by the APD membership and must serve in program leadership at their home programs. The APD-RPDS helps plan and coordinate breakout sessions and lectures at the annual APD meeting, which typically relate to program director duties, changing policies within the American Board of Dermatology or Accreditation Council for Graduate Medical Education, ideas for future growth, and changes in our specialty and in resident education. Members of the APD-RPDS have access to the APD listserv, a valuable resource for discussing issues affecting residency training. We also have work groups led by our members, which include diversity, equity, and inclusion; resource development; communications; and the annual survey. To join the APD, the RPDS, and/or any of our workgroups, please reach out to us or visit the APD website (https://www.dermatologyprofessors.org).
We look forward to welcoming and expediently reviewing members’ submissions to the new Residency Roundup column falling into 2 principal categories within the scope of dermatologic recruitment, didactic education, and clinical training. The first category will feature novel tools, programs, and platforms to improve dermatology training through collaboration. This could entail a description of a new platform designed for sharing resources among programs and specialties to enhance learning for trainees and faculty alike. For example, if a database is created that contains prerecorded lectures pertaining to alopecia, a potential article submission might introduce the database and provide information on what topics are covered and how to access these lectures for readers worldwide. Likewise, if a new technology emerges that allows for easier collaboration among programs, a possible submission would introduce the technology and discuss its potential benefits to trainees, faculty, and practicing dermatologists.
Secondly and more commonly, we anticipate the Residency Roundup column will feature articles that delve into the critical issues and challenges currently impacting recruitment, training, and administration in dermatology residency programs. Specific topics may include but are not limited to recruitment of underrepresented in medicine applicants to dermatology, technological advances to improve teaching methods within training programs, surveys delving into the dermatology match process, and educational gaps or future directions in the specialty. The column occasionally may be used to disseminate information from our section of the APD, including consensus statements or editorials related to changes implemented in the dermatology residency application process. A prospective editorial on this subject could explore varying viewpoints of implemented and proposed changes as well as the reasons behind the changes.
Our group is collaborative, and our aim is to improve education, equity, management of program director responsibilities, and the dermatology application process for programs and applicants alike. With your input, experience, and varied perspectives, we look forward to moving the field of dermatology to a better future by working together.
We are excited to announce a new partnership between Cutis and the Association of Professors of Dermatology Residency Program Directors Section (APD-RPDS). The new APD-RPDS column Residency Roundup will contain quarterly communications and submissions that we hope will facilitate greater dissemination of information that is useful to the dermatology teaching community.
The APD is a group of academic dermatologists whose membership comprises chairs, chiefs, residency and fellowship program directors, and teaching faculty. Each fall, the group convenes in Chicago, Illinois, for a 2-day meeting centered around departmental and program leadership with a focus on education. The APD-RPDS was formed in 2020 and is led by a steering committee of 9 members, including our current Chair, Ilana S. Rosman, MD (Washington University School of Medicine, St. Louis, Missouri), and Vice Chair, Jo-Ann M. Latkowski, MD (New York University, New York). Committee members are elected from and by the APD membership and must serve in program leadership at their home programs. The APD-RPDS helps plan and coordinate breakout sessions and lectures at the annual APD meeting, which typically relate to program director duties, changing policies within the American Board of Dermatology or Accreditation Council for Graduate Medical Education, ideas for future growth, and changes in our specialty and in resident education. Members of the APD-RPDS have access to the APD listserv, a valuable resource for discussing issues affecting residency training. We also have work groups led by our members, which include diversity, equity, and inclusion; resource development; communications; and the annual survey. To join the APD, the RPDS, and/or any of our workgroups, please reach out to us or visit the APD website (https://www.dermatologyprofessors.org).
We look forward to welcoming and expediently reviewing members’ submissions to the new Residency Roundup column falling into 2 principal categories within the scope of dermatologic recruitment, didactic education, and clinical training. The first category will feature novel tools, programs, and platforms to improve dermatology training through collaboration. This could entail a description of a new platform designed for sharing resources among programs and specialties to enhance learning for trainees and faculty alike. For example, if a database is created that contains prerecorded lectures pertaining to alopecia, a potential article submission might introduce the database and provide information on what topics are covered and how to access these lectures for readers worldwide. Likewise, if a new technology emerges that allows for easier collaboration among programs, a possible submission would introduce the technology and discuss its potential benefits to trainees, faculty, and practicing dermatologists.
Secondly and more commonly, we anticipate the Residency Roundup column will feature articles that delve into the critical issues and challenges currently impacting recruitment, training, and administration in dermatology residency programs. Specific topics may include but are not limited to recruitment of underrepresented in medicine applicants to dermatology, technological advances to improve teaching methods within training programs, surveys delving into the dermatology match process, and educational gaps or future directions in the specialty. The column occasionally may be used to disseminate information from our section of the APD, including consensus statements or editorials related to changes implemented in the dermatology residency application process. A prospective editorial on this subject could explore varying viewpoints of implemented and proposed changes as well as the reasons behind the changes.
Our group is collaborative, and our aim is to improve education, equity, management of program director responsibilities, and the dermatology application process for programs and applicants alike. With your input, experience, and varied perspectives, we look forward to moving the field of dermatology to a better future by working together.
Excoriated Papules and Plaques on the Arms and Legs
The Diagnosis: Reactive Perforating Collagenosis
Reactive perforating collagenosis (RPC) may be either acquired or inherited. It is 1 of 4 classical forms of transepithelial elimination, which also includes elastosis perforans serpiginosa (EPS) as well as perforating folliculitis and Kyrle disease. These 4 forms of transepithelial elimination share characteristics of the elimination of altered dermal components through the epidermis.1 The acquired subtype of RPC frequently occurs in patients with diabetes mellitus and end-stage renal disease,2 both present in our patient.
Clinical presentation typically shows pruritic hyperkeratotic papules with a central crater filled with crust that frequently are distributed on the extensor surfaces of the extremities, often in a linear pattern.3 The perforating papules and nodules occasionally may involve the trunk and face.4 Histopathologic examination is characterized by the elimination of altered collagen through the epidermis. Established lesions may show a cup-shaped depression of the epidermis filled with a keratin plug. The underlying dermis will show vertically oriented basophilic collagen fibers with focal extrusion through the epidermis, and elastic fibers will be absent.5 The exact pathophysiology of this disease is unknown, but it may represent a cutaneous response to superficial trauma caused by intense scratching.6
Standard treatment protocols are not well established for this condition, but some evidence shows that a combination of treatments can help ameliorate symptoms, even if they are not curative.7 Treatments without strong evidence have included a wide range of topical, systemic, and other therapies. Case series and anecdotal reports have used retinoids, corticosteroids, menthol, antibiotics, allopurinol antihistamines, cryotherapy, and lasers.8 One case was treated with a combination of narrowband UVB phototherapy and doxycycline with resolution in approximately 6 weeks.9 Other cases have been cured using triple therapy with antihistamines, topical or injected steroids, and emollients or oral antibiotics.7 Evidence shows that there may be benefit to combining multiple different treatment types that target pruritus, inflammation, and collagen damage.7,9 This disease usually cannot be cured, but it may be improved by the available treatments.
The differential diagnosis includes delusional parasitosis, EPS, perforating folliculitis, and prurigo nodularis. Delusional parasitosis also can be characterized by excoriated plaques and a sensation of parasites infesting the skin, as our patient described.10 However, it can be differentiated from RPC by the fact that it is a diagnosis of exclusion, which would not have the histopathologic findings of the elimination of collagen from the epidermis, as was demonstrated in our patient.11 Elastosis perforans serpiginosa is in the same family of perforating diseases as RPC; however, EPS typically appears in children or young adults and often is associated with other genetic disorders. Physical examination in a patient with EPS would reveal keratotic papules in a serpiginous pattern, whereas our patient had discrete lesions without any serpiginous pattern. The histopathologic appearance of EPS would reveal plugs of elastic fibers rather than collagen fibers, as was demonstrated in our patient.8 Perforating folliculitis, while also demonstrating transepithelial elimination similar to RPC, would appear as erythematous follicular papules with small central keratotic plugs and histopathologic findings of a widely dilated follicle with a mass of keratotic debris.12 Prurigo nodularis would appear as dome-shaped papulonodules with varying degrees of scale, crust, and erosion, with a histopathologic appearance of hyperplasia and thick hyperkeratosis.11
Overall, the histopathology is paramount in differentiating RPC from the alternative diagnoses, with the extrusion of collagen from the epidermis not being seen in these other conditions. The coupling of the medical history (type 2 diabetes mellitus and end-stage renal disease) with the clinical presentation and skin biopsy findings confirmed the diagnosis of RPC.
- Fei C, Wang Y, Gong Y, et al. Acquired reactive perforating collagenosis: a report of a typical case. Medicine (Baltimore). 2016;95:E4305.
- Matsui A, Nakano H, Aizu T, et al. Treatment of acquired reactive perforating collagenosis with 308‐nm excimer laser. Clin Exp Dermatol. 2016;41:820-821.
- Dey AK. Reactive perforating collagenosis: an important differential diagnosis in hemodialysis patients. Saudi J Kidney Dis Transpl. 2018;29:422-425.
- Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 9th ed. McGraw-Hill Education LLC; 2012.
- Plaza JA, Prieto VG. Inflammatory Skin Disorders. Demos Medical Publishing LLC; 2012.
- Kreuter A, Gambichler T. Acquired reactive perforating collagenosis. CMAJ. 2010;182:E184.
- Zhang X, Yang Y, Shao S. Acquired reactive perforating collagenosis: a case report and review of the literature. Medicine (Baltimore). 2020;99:E20391.
- Rapini RP. Perforating diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1690-1696.
- Gao L, Gu L, Chen Z, et al. Doxycycline combined with NB-UVB phototherapy for acquired reactive perforating collagenosis. Ther Clin Risk Manag. 2020;16:917-921.
- Bolognia JL, Schaffer JV, Duncan KO, et al. Psychocutaneous disorders. Dermatology Essentials. Elsevier; 2014:50-55. 11. Bolognia JL, Schaffer JV, Duncan KO, et al. Pruritus and dysesthesia. Dermatology Essentials. Elsevier; 2014:39-49. 12. Rubio FA, Herranz P, Robayna G, et al. Perforating folliculitis: report of a case in an HIV-infected man. J Am Acad Dermatol. 1999;40:300-302.
The Diagnosis: Reactive Perforating Collagenosis
Reactive perforating collagenosis (RPC) may be either acquired or inherited. It is 1 of 4 classical forms of transepithelial elimination, which also includes elastosis perforans serpiginosa (EPS) as well as perforating folliculitis and Kyrle disease. These 4 forms of transepithelial elimination share characteristics of the elimination of altered dermal components through the epidermis.1 The acquired subtype of RPC frequently occurs in patients with diabetes mellitus and end-stage renal disease,2 both present in our patient.
Clinical presentation typically shows pruritic hyperkeratotic papules with a central crater filled with crust that frequently are distributed on the extensor surfaces of the extremities, often in a linear pattern.3 The perforating papules and nodules occasionally may involve the trunk and face.4 Histopathologic examination is characterized by the elimination of altered collagen through the epidermis. Established lesions may show a cup-shaped depression of the epidermis filled with a keratin plug. The underlying dermis will show vertically oriented basophilic collagen fibers with focal extrusion through the epidermis, and elastic fibers will be absent.5 The exact pathophysiology of this disease is unknown, but it may represent a cutaneous response to superficial trauma caused by intense scratching.6
Standard treatment protocols are not well established for this condition, but some evidence shows that a combination of treatments can help ameliorate symptoms, even if they are not curative.7 Treatments without strong evidence have included a wide range of topical, systemic, and other therapies. Case series and anecdotal reports have used retinoids, corticosteroids, menthol, antibiotics, allopurinol antihistamines, cryotherapy, and lasers.8 One case was treated with a combination of narrowband UVB phototherapy and doxycycline with resolution in approximately 6 weeks.9 Other cases have been cured using triple therapy with antihistamines, topical or injected steroids, and emollients or oral antibiotics.7 Evidence shows that there may be benefit to combining multiple different treatment types that target pruritus, inflammation, and collagen damage.7,9 This disease usually cannot be cured, but it may be improved by the available treatments.
The differential diagnosis includes delusional parasitosis, EPS, perforating folliculitis, and prurigo nodularis. Delusional parasitosis also can be characterized by excoriated plaques and a sensation of parasites infesting the skin, as our patient described.10 However, it can be differentiated from RPC by the fact that it is a diagnosis of exclusion, which would not have the histopathologic findings of the elimination of collagen from the epidermis, as was demonstrated in our patient.11 Elastosis perforans serpiginosa is in the same family of perforating diseases as RPC; however, EPS typically appears in children or young adults and often is associated with other genetic disorders. Physical examination in a patient with EPS would reveal keratotic papules in a serpiginous pattern, whereas our patient had discrete lesions without any serpiginous pattern. The histopathologic appearance of EPS would reveal plugs of elastic fibers rather than collagen fibers, as was demonstrated in our patient.8 Perforating folliculitis, while also demonstrating transepithelial elimination similar to RPC, would appear as erythematous follicular papules with small central keratotic plugs and histopathologic findings of a widely dilated follicle with a mass of keratotic debris.12 Prurigo nodularis would appear as dome-shaped papulonodules with varying degrees of scale, crust, and erosion, with a histopathologic appearance of hyperplasia and thick hyperkeratosis.11
Overall, the histopathology is paramount in differentiating RPC from the alternative diagnoses, with the extrusion of collagen from the epidermis not being seen in these other conditions. The coupling of the medical history (type 2 diabetes mellitus and end-stage renal disease) with the clinical presentation and skin biopsy findings confirmed the diagnosis of RPC.
The Diagnosis: Reactive Perforating Collagenosis
Reactive perforating collagenosis (RPC) may be either acquired or inherited. It is 1 of 4 classical forms of transepithelial elimination, which also includes elastosis perforans serpiginosa (EPS) as well as perforating folliculitis and Kyrle disease. These 4 forms of transepithelial elimination share characteristics of the elimination of altered dermal components through the epidermis.1 The acquired subtype of RPC frequently occurs in patients with diabetes mellitus and end-stage renal disease,2 both present in our patient.
Clinical presentation typically shows pruritic hyperkeratotic papules with a central crater filled with crust that frequently are distributed on the extensor surfaces of the extremities, often in a linear pattern.3 The perforating papules and nodules occasionally may involve the trunk and face.4 Histopathologic examination is characterized by the elimination of altered collagen through the epidermis. Established lesions may show a cup-shaped depression of the epidermis filled with a keratin plug. The underlying dermis will show vertically oriented basophilic collagen fibers with focal extrusion through the epidermis, and elastic fibers will be absent.5 The exact pathophysiology of this disease is unknown, but it may represent a cutaneous response to superficial trauma caused by intense scratching.6
Standard treatment protocols are not well established for this condition, but some evidence shows that a combination of treatments can help ameliorate symptoms, even if they are not curative.7 Treatments without strong evidence have included a wide range of topical, systemic, and other therapies. Case series and anecdotal reports have used retinoids, corticosteroids, menthol, antibiotics, allopurinol antihistamines, cryotherapy, and lasers.8 One case was treated with a combination of narrowband UVB phototherapy and doxycycline with resolution in approximately 6 weeks.9 Other cases have been cured using triple therapy with antihistamines, topical or injected steroids, and emollients or oral antibiotics.7 Evidence shows that there may be benefit to combining multiple different treatment types that target pruritus, inflammation, and collagen damage.7,9 This disease usually cannot be cured, but it may be improved by the available treatments.
The differential diagnosis includes delusional parasitosis, EPS, perforating folliculitis, and prurigo nodularis. Delusional parasitosis also can be characterized by excoriated plaques and a sensation of parasites infesting the skin, as our patient described.10 However, it can be differentiated from RPC by the fact that it is a diagnosis of exclusion, which would not have the histopathologic findings of the elimination of collagen from the epidermis, as was demonstrated in our patient.11 Elastosis perforans serpiginosa is in the same family of perforating diseases as RPC; however, EPS typically appears in children or young adults and often is associated with other genetic disorders. Physical examination in a patient with EPS would reveal keratotic papules in a serpiginous pattern, whereas our patient had discrete lesions without any serpiginous pattern. The histopathologic appearance of EPS would reveal plugs of elastic fibers rather than collagen fibers, as was demonstrated in our patient.8 Perforating folliculitis, while also demonstrating transepithelial elimination similar to RPC, would appear as erythematous follicular papules with small central keratotic plugs and histopathologic findings of a widely dilated follicle with a mass of keratotic debris.12 Prurigo nodularis would appear as dome-shaped papulonodules with varying degrees of scale, crust, and erosion, with a histopathologic appearance of hyperplasia and thick hyperkeratosis.11
Overall, the histopathology is paramount in differentiating RPC from the alternative diagnoses, with the extrusion of collagen from the epidermis not being seen in these other conditions. The coupling of the medical history (type 2 diabetes mellitus and end-stage renal disease) with the clinical presentation and skin biopsy findings confirmed the diagnosis of RPC.
- Fei C, Wang Y, Gong Y, et al. Acquired reactive perforating collagenosis: a report of a typical case. Medicine (Baltimore). 2016;95:E4305.
- Matsui A, Nakano H, Aizu T, et al. Treatment of acquired reactive perforating collagenosis with 308‐nm excimer laser. Clin Exp Dermatol. 2016;41:820-821.
- Dey AK. Reactive perforating collagenosis: an important differential diagnosis in hemodialysis patients. Saudi J Kidney Dis Transpl. 2018;29:422-425.
- Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 9th ed. McGraw-Hill Education LLC; 2012.
- Plaza JA, Prieto VG. Inflammatory Skin Disorders. Demos Medical Publishing LLC; 2012.
- Kreuter A, Gambichler T. Acquired reactive perforating collagenosis. CMAJ. 2010;182:E184.
- Zhang X, Yang Y, Shao S. Acquired reactive perforating collagenosis: a case report and review of the literature. Medicine (Baltimore). 2020;99:E20391.
- Rapini RP. Perforating diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1690-1696.
- Gao L, Gu L, Chen Z, et al. Doxycycline combined with NB-UVB phototherapy for acquired reactive perforating collagenosis. Ther Clin Risk Manag. 2020;16:917-921.
- Bolognia JL, Schaffer JV, Duncan KO, et al. Psychocutaneous disorders. Dermatology Essentials. Elsevier; 2014:50-55. 11. Bolognia JL, Schaffer JV, Duncan KO, et al. Pruritus and dysesthesia. Dermatology Essentials. Elsevier; 2014:39-49. 12. Rubio FA, Herranz P, Robayna G, et al. Perforating folliculitis: report of a case in an HIV-infected man. J Am Acad Dermatol. 1999;40:300-302.
- Fei C, Wang Y, Gong Y, et al. Acquired reactive perforating collagenosis: a report of a typical case. Medicine (Baltimore). 2016;95:E4305.
- Matsui A, Nakano H, Aizu T, et al. Treatment of acquired reactive perforating collagenosis with 308‐nm excimer laser. Clin Exp Dermatol. 2016;41:820-821.
- Dey AK. Reactive perforating collagenosis: an important differential diagnosis in hemodialysis patients. Saudi J Kidney Dis Transpl. 2018;29:422-425.
- Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 9th ed. McGraw-Hill Education LLC; 2012.
- Plaza JA, Prieto VG. Inflammatory Skin Disorders. Demos Medical Publishing LLC; 2012.
- Kreuter A, Gambichler T. Acquired reactive perforating collagenosis. CMAJ. 2010;182:E184.
- Zhang X, Yang Y, Shao S. Acquired reactive perforating collagenosis: a case report and review of the literature. Medicine (Baltimore). 2020;99:E20391.
- Rapini RP. Perforating diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1690-1696.
- Gao L, Gu L, Chen Z, et al. Doxycycline combined with NB-UVB phototherapy for acquired reactive perforating collagenosis. Ther Clin Risk Manag. 2020;16:917-921.
- Bolognia JL, Schaffer JV, Duncan KO, et al. Psychocutaneous disorders. Dermatology Essentials. Elsevier; 2014:50-55. 11. Bolognia JL, Schaffer JV, Duncan KO, et al. Pruritus and dysesthesia. Dermatology Essentials. Elsevier; 2014:39-49. 12. Rubio FA, Herranz P, Robayna G, et al. Perforating folliculitis: report of a case in an HIV-infected man. J Am Acad Dermatol. 1999;40:300-302.
A 73-year-old woman presented for evaluation of a rash on the arms and legs of 3 months’ duration. The rash had developed abruptly, and she believed it was caused by bugs in the skin; her husband noted that she constantly picked at her arms and legs. She had a medical history of hypertension, type 2 diabetes mellitus, and endstage renal disease on dialysis. Physical examination revealed multiple pigmented papules and plaques, some with keratotic scale, on the lower legs (left) and arms, with greater involvement on the left arm (right). The lesions were of various sizes and shapes, some with a central keratotic core, and several lesions demonstrated erosion, excoriation, or ulceration. Histopathologic examination revealed slight attenuation of the epidermis with loss of normal rete peg architecture, alternating areas of hypergranulosis and hypogranulosis, central ulceration with inflammatory cells, and a basophilic hue to the ulcer base with sweeping up of the collagen fibers.
Verrucous Carcinoma of the Foot: A Retrospective Study of 19 Cases and Analysis of Prognostic Factors Influencing Recurrence
Verrucous carcinoma is a rare cancer with the greatest predilection for the foot. Multiple case reports with only a few large case series have been published. 1-3 Plantar verrucous carcinoma is characterized as a slowly but relentlessly enlarging warty tumor with low metastatic potential and high risk for local invasion. The tumor occurs most frequently in patients aged 60 to 70 years, predominantly in White males. 1 It often is misdiagnosed for years as an ulcer or wart that is highly resistant to therapy. Size typically ranges from 1 to 12 cm in greatest dimension. 1
The pathogenesis of plantar verrucous carcinoma remains unclear, but some contributing factors have been proposed, including trauma, chronic irritation, infection, and poor local hygiene.2 This tumor has been reported to occur in chronic foot ulcerations, particularly in the diabetic population.4 It has been proposed that abnormal expression of the p53 tumor suppressor protein and several types of human papillomavirus (HPV) may have a role in the pathogenesis of verrucous carcinoma.5
The pathologic hallmarks of this tumor include a verrucous/hyperkeratotic surface with a deeply endophytic, broad, pushing base. Tumor cells are well differentiated, and atypia is either absent or confined to 1 or 2 layers at the base of the tumor. Overt invasion at the base is lacking, except in cases with a component of conventional invasive squamous cell carcinoma. Human papillomavirus viropathic changes are classically absent.1,3 Studies of the histopathology of verrucous carcinoma have been complicated by similar entities, nomenclatural uncertainty, and variable diagnostic criteria. For example, epithelioma cuniculatum variously has been defined as being synonymous with verrucous carcinoma, a distinct clinical verrucous carcinoma subtype occurring on the soles, a histologic subtype (characterized by prominent burrowing sinuses), or a separate entity entirely.1,2,6,7 Furthermore, in the genital area, several different types of carcinomas have verruciform features but display distinct microscopic findings and outcomes from verrucous carcinoma.8
Verrucous carcinoma represents an unusual variant of squamous cell carcinoma and is treated as such. Treatments have included laser surgery; immunotherapy; retinoid therapy; and chemotherapy by oral, intralesional, or iontophoretic routes in select patients.9 Radiotherapy presents another option, though reports have described progression to aggressive squamous cell carcinoma in some cases.9 Surgery is the best course of treatment, and as more case reports have been published, a transition from radical resection to wide excision with tumor-free margins is the treatment of choice.2,3,10,11 To minimize soft-tissue deficits, Mohs micrographic surgery has been discussed as a treatment option for verrucous carcinoma.11-13
Few studies have described verrucous carcinoma recurrence, and none have systematically examined recurrence rate, risk factors, or prognosis
Methods
Patient cases were
Of the 19 cases, 16 were treated at the University of Michigan and are included in the treatment analyses. Specific attention was then paid to the cases with a clinical recurrence despite negative surgical margins. We compared the clinical and surgical differences between recurrent cases and nonrecurrent cases.
Pathology was rereviewed for selected cases, including 2 cases with recurrence and matched primary, 2 cases with recurrence (for which the matched primary was unavailable for review), and 5 representative primary cases that were not complicated by recurrence. Pathology review was conducted in a blinded manner by one of the authors (P.W.H) who is a board-certified dermatopathologist for approximate depth of invasion from the granular layer, perineural invasion, bone invasion, infiltrative growth, presence of conventional squamous cell carcinoma, and margin status.
Statistical analysis was performed when appropriate using an N1 χ2 test or Student t test.
Results
Demographics and Comorbidities—The median age of the patients at the time of diagnosis was 55 years (range, 34–77 years). There were 12 males and 7 females (Table 1). Two patients were Black and 17 were White. Almost all patients had additional comorbidities including tobacco use (68%), alcohol use (47%), and diabetes (47%). Only 1 patient had an autoimmune disease and was on chronic steroids. No significant difference was found between the demographics of patients with recurrent lesions and those without recurrence.
Tumor Location and Clinical Presentation—The most common clinical presentation included a nonhealing ulceration with warty edges, pain, bleeding, and lowered mobility. In most cases, there was history of prior treatment over a duration ranging from 1 to 8 years, with a median of 5 years prior to biopsy-based diagnosis (Table 1). Six patients had a history of osteomyelitis, diagnosed by imaging or biopsy, within a year before tumor diagnosis. The size of the primary tumor ranged from 2.4 to 6 cm, with a mean of 4 cm (P=.20). The clinical presentation, time before diagnosis, and size of the tumors did not differ significantly between recurrent and nonrecurrent cases.
The tumor location for the recurrent cases differed significantly compared to nonrecurrent cases. All 5 of the patients with a recurrence presented with a tumor on the nonglabrous part of the foot. Four patients (80%) had lesions on the dorsal or lateral aspect of the great toe (P=.002), and 1 patient (20%) had a lesion on the low ankle (P=.09)(Table 1). Of the nonrecurrent cases, 1 patient (7%) presented with a tumor on the plantar surface of the great toe (P=.002), 13 patients (93%) presented with tumors on the distal plantar surface of the foot (P=.0002), and 1 patient with a plantar foot tumor (Figure 1) also had verrucous carcinoma on the thumb (Table 1 and Figure 2).
Histopathology—Available pathology slides for recurrent cases of verrucous carcinoma were reviewed alongside representative cases of verrucous carcinomas that did not progress to recurrence. The diagnosis of verrucous carcinoma was confirmed in all cases, with no evidence of conventional squamous cell carcinoma, perineural invasion, extension beyond the dermis, or bone invasion in any case. The median size of the tumors was 4.2 cm and 4 cm for nonrecurrent and recurrent specimens, respectively. Recurrences displayed a trend toward increased depth compared to primary tumors without recurrence (average depth, 5.5 mm vs 3.7 mm); however, this did not reach statistical significance (P=.24). Primary tumors that progressed to recurrence (n=2) displayed similar findings to the other cases, with invasive depths of 3.5 and 5.5 mm, and there was no evidence of conventional squamous cell carcinoma, perineural invasion, or extension beyond the dermis.
Treatment of Nonrecurrent Cases—Of the 16 total cases treated at the University of Michigan, surgery was the primary mode of therapy in every case (Tables 2 and 3). Of the 11 nonrecurrent cases, 7 patients had wide local excision with a dermal regeneration template, and delayed split-thickness graft reconstruction. Three cases had wide local excision with metatarsal resection, dermal regeneration template, and delayed skin grafting. One case had a great toe amputation
Treatment of Recurrent Cases—For the 5 patients with recurrence, surgical margins were not reported in all the cases but ranged from 0.5 to 2 cm (4/5 [80%] reported). On average, follow-up for this group of patients was 29 months, with a range of 12 to 60 months (Table 3).
The first case with a recurrence (patient 12) initially presented with a chronic calluslike growth of the medial ankle. The lesion initially was treated with wide local excision with negative margins. Reconstruction was performed in a staged fashion with use of a dermal regenerative template followed later by split-thickness skin grafting. Tumor recurrence with negative margins occurred 3 times over the next 2 years despite re-resections with negative pathologic margins. Each recurrence presented as graft breakdown and surrounding hyperkeratosis (Figure 3). After the third graft placement failed, the patient elected for a BKA. There has not been recurrence since the BKA after 5 years total follow-up from the time of primary tumor resection. Of note, this was the only patient in our cohort who was immunosuppressed and evaluated for regional nodal involvement by positron emission tomography.
Another patient with recurrence (patient 13) presented with a chronic great toe ulcer of 5 years’ duration that formed on the dorsal aspect of the great toe after a previously excised wart (Figure 4A). This patient underwent mid-proximal metatarsal amputation with 2-cm margins and subsequent skin graft. Pathologic margins were negative. Within 6 months, there was hyperkeratosis and a draining wound (Figure 4B). Biopsy results confirmed recurrent disease that was treated with re-resection, including complete metatarsal amputation with negative margins and skin graft placement. Verrucous carcinoma recurred at the edges of the graft within 8 months, and the patient elected for a BKA. In addition, this patient also presented with a verrucous carcinoma of the contralateral great toe. The tumor presented as a warty ulcer of 4 months’ duration in the setting of osteomyelitis and was resected by great toe amputation that was performed concurrently with the opposite leg BKA; there has been no recurrence. Of note, this was the only patient to have right inguinal sentinel lymph node tissue sampled and HPV testing conducted, which were negative for verrucous carcinoma and high or low strains of HPV.
Another recurrent case (patient 14) presented with a large warty lesion on the dorsal great toe positive for verrucous carcinoma. He underwent a complete great toe amputation with skin graft placement. Verrucous carcinoma recurred on the edges of the graft within 6 months, and the patient was lost to follow-up when a BKA was suggested.
The fourth recurrent case (patient 15) initially had been treated for 1 year as a viral verruca of the dorsal aspect of the great toe. He had an exophytic mass positive for verrucous carcinoma growing on the dorsal aspect of the great toe around the prior excision site. After primary wide excision with negative 1-cm margins and graft placement, the tumor was re-excised twice within the next 2 years with pathologic negative margins. The patient underwent a foot amputation due to a severe osteomyelitis infection at the reconstruction site.
The final recurrent case (patient 16) presented with a mass on the lateral great toe that initially was treated as a viral verruca (for unknown duration) that had begun to ulcerate. The patient underwent wide excision with 1-cm margins and graft placement. Final pathology was consistent with verrucous carcinoma with negative margins. Recurrence occurred within 11 months on the edge of the graft, and a great toe amputation through the metatarsal phalangeal joint was performed.
Comment
Our series of 19 cases of verrucous carcinoma adds to the limited number of reported cases in the literature. We sought to evaluate the potential risk factors for early recurrence. Consistent with prior studies, our series found verrucous carcinoma of the foot to occur most frequently in patients aged 50 to 70 years, predominantly in White men.1 These tumors grew in the setting of chronic inflammation, tissue regeneration, multiple comorbidities, and poor wound hygiene. Misdiagnosis of verrucous carcinoma often leads to ineffective treatments and local invasion of nerves, muscle, and bone tissue.9,15,16 Our case series also clearly demonstrated the diagnostic challenge verrucous carcinoma presents, with an average delay in diagnosis of 5 years; correct diagnosis often did not occur until the tumor was 4 cm in size (average) and more than 50% had chronic ulceration.
The histologic features of the tumors showed striking uniformity. Within the literature, there is confusion regarding the use of the terms verrucous carcinoma and carcinoma (epithelioma) cuniculatum and the possible pathologic differences between the two. The World Health Organization’s classification of skin tumors describes epithelioma cuniculatum as verrucous carcinoma located on the sole of the foot.7 Kubik and Rhatigan6 pointed out that carcinoma cuniculatum does not have a warty or verrucous surface, which is a defining feature of verrucous carcinoma. Multiple authors have further surmised that the deep burrowing sinus tracts of epithelioma cuniculatum are different than those seen in verrucous carcinoma formed by the undulations extending from the papillomatous and verrucous surface.1,6 We did not observe these notable pathologic differences in recurrent or nonrecurrent primary tumors or differences between primary and recurrent cases. Although our cohort was small, the findings suggest that standard histologic features do not predict aggressive behavior in verrucous carcinomas. Furthermore, our observations support a model wherein recurrence is an inherent property of certain verrucous carcinomas rather than a consequence of histologic progression to conventional squamous cell carcinoma. The lack of overt malignant features in such cases underscores the need for distinction of verrucous carcinoma from benign mimics such as viral verruca or reactive epidermal hyperplasia.
Our recurrent cases showed a greater predilection for nonplantar surfaces and the great toe (P=.002). Five of 6 cases on the nonplantar surface—1 on the ankle and 5 on the great toe—recurred despite negative pathologic margins. There was no significant difference in demographics, pathogenesis, tumor size, chronicity, phenotype, or metastatic spread in recurrent and nonrecurrent cases in our cohort.
The tumor has only been described in rare instances at extrapedal cutaneous sites including the hand, scalp, and abdomen.14,17,18 Our series did include a case of synchronous presentation with a verrucous carcinoma on the thumb. Given the rarity of this presentation, thus far there are no data supporting any atypical locations of verrucous carcinoma having greater instances of recurrence. Our recurrent cases displaying atypical location on nonglabrous skin could suggest an underlying pathologic mechanism distinct from tumors on glabrous skin and relevant to increased recurrence risk. Such a mechanism might relate to distinct genetic insults, tumor-microenvironment interactions, or field effects. There are few studies regarding physiologic differences between the plantar surface and the nonglabrous surface and how that influences cancer genesis. Within acral melanoma studies, nonglabrous skin of more sun-exposed surfaces has a higher burden of genetic insults including BRAF mutations.19 Genetic testing of verrucous carcinoma is highly limited, with abnormal expression of the p53 tumor suppressor protein and possible association with several types of HPV. Verrucous carcinoma in general has been found to contain HPV types 6 and 11, nononcogenic forms, and higher risk from HPV types 16 and 18.9,20 However, only a few cases of HPV type 16 as well as 1 case each of HPV type 2 and type 11 have been found within verrucous carcinoma of the foot.21,22 In squamous cell carcinoma of the head and neck, HPV-positive tumors have shown better response to treatment. Further investigation of HPV and genetic contributors in verrucous carcinoma is warranted.
There is notable evidence that surgical resection is the best mode of treatment of verrucous carcinoma.2,3,10,11 Our case series was treated with wide local excision, with partial metatarsal amputation or great toe amputation, in cases with bone invasion or osteomyelitis. Surgical margins were not reported in all the cases but ranged from 0.5 to 2 cm with no significant differences between the recurrent and nonrecurrent groups. After excision, closure was conducted by incorporating primary, secondary, and delayed closure techniques, along with skin grafts for larger defects. Lymph node biopsy traditionally has not been recommended due to reported low metastatic potential. In all 5 recurrent cases, the tumors recurred after multiple attempts at wide excision and greater resection of bone and tissue, with negative margins. The tumors regrew quickly, within months, on the edges of the new graft or in the middle of the graft. The sites of recurrent tumor growth would suggest regrowth in the areas of greatest tissue stress and proliferation. We recommend a low threshold for biopsy and aggressive retreatment in the setting of exophytic growth at reconstruction sites.
Recurrence is uncommon in the setting of verrucous carcinoma, with our series being the first to analyze prognostic factors.3,9,14 Our findings indicate that
- Kao GF, Graham JH, Helwig EB. Carcinoma cuniculatum (verrucous carcinoma of the skin): a clinicopathologic study of 46 cases with ultrastructural observations. Cancer. 1982;49:2395-2403.
- McKee PH, Wilkinson JD, Black M, et al. Carcinoma (epithelioma) cuniculatum: a clinic-pathologic study of nineteen cases and review of the literature. Histopathology. 1981;5:425-436.
- Penera KE, Manji KA, Craig AB, et al. Atypical presentation of verrucous carcinoma: a case study and review of the literature. Foot Ankle Spec. 2013;6:318-322.
- Rosales MA, Martin BR, Armstrong DG, et al. Verrucous hyperplasia: a common and problematic finding in the high-risk diabetic foot. J Am Podiatr Assoc. 2006:4:348-350.
- Noel JC, Peny MO, De Dobbeleer G, et al. p53 Protein overexpression in verrucous carcinoma of the skin. Dermatology. 1996;192:12-15.
- Kubik MJ, Rhatigan RM. Carcinoma cuniculatum: not a verrucous carcinoma. J Cutan Pathol. 2012;39:1083-1087
- Elder D, Massi D, Scolver R, et al. Verrucous squamous cell carcinoma. WHO Classification of Tumours (Medicine). Vol 11. 4th ed. International Agency for Research on Cancer: 2018;35-57.
- Chan MP. Verruciform and condyloma-like squamous proliferations in the anogenital region. Arch Pathol Lab Med. 2019;143:821-831
- Schwartz RA. Verrucous carcinoma of the skin and mucosa. J Am Acad Dermatol. 1995;32:1-21.
- Flynn K, Wiemer D. Treatment of an epithelioma cuniculatum plantare by local excision and a plantar skin flap. J Dermatol Surg Oncol. 1978;4:773-775.
- Spyriounis P, Tentis D, Sparveri I, et al. Plantar epithelioma cuniculatum: a case report with review of the literature. Eur J Plast Surg. 2004;27:253-256.
- Swanson NA, Taylor WB. Plantar verrucous carcinoma: literature review and treatment by the Moh’s chemosurgery technique. Arch Dermatol. 1980;116:794-797.
- Alkalay R, Alcalay J, Shiri J. Plantar verrucous carcinoma treated with Mohs micrographic surgery: a case report and literature review. J Drugs Dermatol. 2006:5:68-73.
- Kotwal M, Poflee S, Bobhate, S. Carcinoma cuniculatum at various anatomical sites. Indian J Dermatol. 2005;50:216-220.
- Nagarajan D, Chandrasekhar M, Jebakumar J, et al. Verrucous carcinoma of foot at an unusual site: lessons to be learnt. South Asian J Cancer. 2017;6:63.
- Pempinello C, Bova A, Pempinello R, et al Verrucous carcinoma of the foot with bone invasion: a case report. Case Rep Oncol Med. 2013;2013:135307.
- Vandeweyer E, Sales F, Deramaecker R. Cutaneous verrucous carcinoma. Br J Plastic Surg. 2001;54:168-170.
- Joybari A, Azadeh P, Honar B. Cutaneous verrucous carcinoma superimposed on chronically inflamed ileostomy site skin. Iran J Pathol. 2018;13:285-288.
- Davis EJ, Johnson DB, Sosman JA, et al. Melanoma: what do all the mutations mean? Cancer. 2018;124:3490-3499.
- Gissmann L, Wolnik L, Ikenberg H, et al. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci U S A. 1983;80:560-563.
- Knobler RM, Schneider S, Neumann RA, et al. DNA dot-blot hybridization implicates human papillomavirus type 11-DNA in epithelioma cuniculatum. J Med Virol. 1989;29:33-37.
- Noel JC, Peny MO, Detremmerie O, et al. Demonstration of human papillomavirus type 2 in a verrucous carcinoma of the foot. Dermatology. 1993;187:58-61.
Verrucous carcinoma is a rare cancer with the greatest predilection for the foot. Multiple case reports with only a few large case series have been published. 1-3 Plantar verrucous carcinoma is characterized as a slowly but relentlessly enlarging warty tumor with low metastatic potential and high risk for local invasion. The tumor occurs most frequently in patients aged 60 to 70 years, predominantly in White males. 1 It often is misdiagnosed for years as an ulcer or wart that is highly resistant to therapy. Size typically ranges from 1 to 12 cm in greatest dimension. 1
The pathogenesis of plantar verrucous carcinoma remains unclear, but some contributing factors have been proposed, including trauma, chronic irritation, infection, and poor local hygiene.2 This tumor has been reported to occur in chronic foot ulcerations, particularly in the diabetic population.4 It has been proposed that abnormal expression of the p53 tumor suppressor protein and several types of human papillomavirus (HPV) may have a role in the pathogenesis of verrucous carcinoma.5
The pathologic hallmarks of this tumor include a verrucous/hyperkeratotic surface with a deeply endophytic, broad, pushing base. Tumor cells are well differentiated, and atypia is either absent or confined to 1 or 2 layers at the base of the tumor. Overt invasion at the base is lacking, except in cases with a component of conventional invasive squamous cell carcinoma. Human papillomavirus viropathic changes are classically absent.1,3 Studies of the histopathology of verrucous carcinoma have been complicated by similar entities, nomenclatural uncertainty, and variable diagnostic criteria. For example, epithelioma cuniculatum variously has been defined as being synonymous with verrucous carcinoma, a distinct clinical verrucous carcinoma subtype occurring on the soles, a histologic subtype (characterized by prominent burrowing sinuses), or a separate entity entirely.1,2,6,7 Furthermore, in the genital area, several different types of carcinomas have verruciform features but display distinct microscopic findings and outcomes from verrucous carcinoma.8
Verrucous carcinoma represents an unusual variant of squamous cell carcinoma and is treated as such. Treatments have included laser surgery; immunotherapy; retinoid therapy; and chemotherapy by oral, intralesional, or iontophoretic routes in select patients.9 Radiotherapy presents another option, though reports have described progression to aggressive squamous cell carcinoma in some cases.9 Surgery is the best course of treatment, and as more case reports have been published, a transition from radical resection to wide excision with tumor-free margins is the treatment of choice.2,3,10,11 To minimize soft-tissue deficits, Mohs micrographic surgery has been discussed as a treatment option for verrucous carcinoma.11-13
Few studies have described verrucous carcinoma recurrence, and none have systematically examined recurrence rate, risk factors, or prognosis
Methods
Patient cases were
Of the 19 cases, 16 were treated at the University of Michigan and are included in the treatment analyses. Specific attention was then paid to the cases with a clinical recurrence despite negative surgical margins. We compared the clinical and surgical differences between recurrent cases and nonrecurrent cases.
Pathology was rereviewed for selected cases, including 2 cases with recurrence and matched primary, 2 cases with recurrence (for which the matched primary was unavailable for review), and 5 representative primary cases that were not complicated by recurrence. Pathology review was conducted in a blinded manner by one of the authors (P.W.H) who is a board-certified dermatopathologist for approximate depth of invasion from the granular layer, perineural invasion, bone invasion, infiltrative growth, presence of conventional squamous cell carcinoma, and margin status.
Statistical analysis was performed when appropriate using an N1 χ2 test or Student t test.
Results
Demographics and Comorbidities—The median age of the patients at the time of diagnosis was 55 years (range, 34–77 years). There were 12 males and 7 females (Table 1). Two patients were Black and 17 were White. Almost all patients had additional comorbidities including tobacco use (68%), alcohol use (47%), and diabetes (47%). Only 1 patient had an autoimmune disease and was on chronic steroids. No significant difference was found between the demographics of patients with recurrent lesions and those without recurrence.
Tumor Location and Clinical Presentation—The most common clinical presentation included a nonhealing ulceration with warty edges, pain, bleeding, and lowered mobility. In most cases, there was history of prior treatment over a duration ranging from 1 to 8 years, with a median of 5 years prior to biopsy-based diagnosis (Table 1). Six patients had a history of osteomyelitis, diagnosed by imaging or biopsy, within a year before tumor diagnosis. The size of the primary tumor ranged from 2.4 to 6 cm, with a mean of 4 cm (P=.20). The clinical presentation, time before diagnosis, and size of the tumors did not differ significantly between recurrent and nonrecurrent cases.
The tumor location for the recurrent cases differed significantly compared to nonrecurrent cases. All 5 of the patients with a recurrence presented with a tumor on the nonglabrous part of the foot. Four patients (80%) had lesions on the dorsal or lateral aspect of the great toe (P=.002), and 1 patient (20%) had a lesion on the low ankle (P=.09)(Table 1). Of the nonrecurrent cases, 1 patient (7%) presented with a tumor on the plantar surface of the great toe (P=.002), 13 patients (93%) presented with tumors on the distal plantar surface of the foot (P=.0002), and 1 patient with a plantar foot tumor (Figure 1) also had verrucous carcinoma on the thumb (Table 1 and Figure 2).
Histopathology—Available pathology slides for recurrent cases of verrucous carcinoma were reviewed alongside representative cases of verrucous carcinomas that did not progress to recurrence. The diagnosis of verrucous carcinoma was confirmed in all cases, with no evidence of conventional squamous cell carcinoma, perineural invasion, extension beyond the dermis, or bone invasion in any case. The median size of the tumors was 4.2 cm and 4 cm for nonrecurrent and recurrent specimens, respectively. Recurrences displayed a trend toward increased depth compared to primary tumors without recurrence (average depth, 5.5 mm vs 3.7 mm); however, this did not reach statistical significance (P=.24). Primary tumors that progressed to recurrence (n=2) displayed similar findings to the other cases, with invasive depths of 3.5 and 5.5 mm, and there was no evidence of conventional squamous cell carcinoma, perineural invasion, or extension beyond the dermis.
Treatment of Nonrecurrent Cases—Of the 16 total cases treated at the University of Michigan, surgery was the primary mode of therapy in every case (Tables 2 and 3). Of the 11 nonrecurrent cases, 7 patients had wide local excision with a dermal regeneration template, and delayed split-thickness graft reconstruction. Three cases had wide local excision with metatarsal resection, dermal regeneration template, and delayed skin grafting. One case had a great toe amputation
Treatment of Recurrent Cases—For the 5 patients with recurrence, surgical margins were not reported in all the cases but ranged from 0.5 to 2 cm (4/5 [80%] reported). On average, follow-up for this group of patients was 29 months, with a range of 12 to 60 months (Table 3).
The first case with a recurrence (patient 12) initially presented with a chronic calluslike growth of the medial ankle. The lesion initially was treated with wide local excision with negative margins. Reconstruction was performed in a staged fashion with use of a dermal regenerative template followed later by split-thickness skin grafting. Tumor recurrence with negative margins occurred 3 times over the next 2 years despite re-resections with negative pathologic margins. Each recurrence presented as graft breakdown and surrounding hyperkeratosis (Figure 3). After the third graft placement failed, the patient elected for a BKA. There has not been recurrence since the BKA after 5 years total follow-up from the time of primary tumor resection. Of note, this was the only patient in our cohort who was immunosuppressed and evaluated for regional nodal involvement by positron emission tomography.
Another patient with recurrence (patient 13) presented with a chronic great toe ulcer of 5 years’ duration that formed on the dorsal aspect of the great toe after a previously excised wart (Figure 4A). This patient underwent mid-proximal metatarsal amputation with 2-cm margins and subsequent skin graft. Pathologic margins were negative. Within 6 months, there was hyperkeratosis and a draining wound (Figure 4B). Biopsy results confirmed recurrent disease that was treated with re-resection, including complete metatarsal amputation with negative margins and skin graft placement. Verrucous carcinoma recurred at the edges of the graft within 8 months, and the patient elected for a BKA. In addition, this patient also presented with a verrucous carcinoma of the contralateral great toe. The tumor presented as a warty ulcer of 4 months’ duration in the setting of osteomyelitis and was resected by great toe amputation that was performed concurrently with the opposite leg BKA; there has been no recurrence. Of note, this was the only patient to have right inguinal sentinel lymph node tissue sampled and HPV testing conducted, which were negative for verrucous carcinoma and high or low strains of HPV.
Another recurrent case (patient 14) presented with a large warty lesion on the dorsal great toe positive for verrucous carcinoma. He underwent a complete great toe amputation with skin graft placement. Verrucous carcinoma recurred on the edges of the graft within 6 months, and the patient was lost to follow-up when a BKA was suggested.
The fourth recurrent case (patient 15) initially had been treated for 1 year as a viral verruca of the dorsal aspect of the great toe. He had an exophytic mass positive for verrucous carcinoma growing on the dorsal aspect of the great toe around the prior excision site. After primary wide excision with negative 1-cm margins and graft placement, the tumor was re-excised twice within the next 2 years with pathologic negative margins. The patient underwent a foot amputation due to a severe osteomyelitis infection at the reconstruction site.
The final recurrent case (patient 16) presented with a mass on the lateral great toe that initially was treated as a viral verruca (for unknown duration) that had begun to ulcerate. The patient underwent wide excision with 1-cm margins and graft placement. Final pathology was consistent with verrucous carcinoma with negative margins. Recurrence occurred within 11 months on the edge of the graft, and a great toe amputation through the metatarsal phalangeal joint was performed.
Comment
Our series of 19 cases of verrucous carcinoma adds to the limited number of reported cases in the literature. We sought to evaluate the potential risk factors for early recurrence. Consistent with prior studies, our series found verrucous carcinoma of the foot to occur most frequently in patients aged 50 to 70 years, predominantly in White men.1 These tumors grew in the setting of chronic inflammation, tissue regeneration, multiple comorbidities, and poor wound hygiene. Misdiagnosis of verrucous carcinoma often leads to ineffective treatments and local invasion of nerves, muscle, and bone tissue.9,15,16 Our case series also clearly demonstrated the diagnostic challenge verrucous carcinoma presents, with an average delay in diagnosis of 5 years; correct diagnosis often did not occur until the tumor was 4 cm in size (average) and more than 50% had chronic ulceration.
The histologic features of the tumors showed striking uniformity. Within the literature, there is confusion regarding the use of the terms verrucous carcinoma and carcinoma (epithelioma) cuniculatum and the possible pathologic differences between the two. The World Health Organization’s classification of skin tumors describes epithelioma cuniculatum as verrucous carcinoma located on the sole of the foot.7 Kubik and Rhatigan6 pointed out that carcinoma cuniculatum does not have a warty or verrucous surface, which is a defining feature of verrucous carcinoma. Multiple authors have further surmised that the deep burrowing sinus tracts of epithelioma cuniculatum are different than those seen in verrucous carcinoma formed by the undulations extending from the papillomatous and verrucous surface.1,6 We did not observe these notable pathologic differences in recurrent or nonrecurrent primary tumors or differences between primary and recurrent cases. Although our cohort was small, the findings suggest that standard histologic features do not predict aggressive behavior in verrucous carcinomas. Furthermore, our observations support a model wherein recurrence is an inherent property of certain verrucous carcinomas rather than a consequence of histologic progression to conventional squamous cell carcinoma. The lack of overt malignant features in such cases underscores the need for distinction of verrucous carcinoma from benign mimics such as viral verruca or reactive epidermal hyperplasia.
Our recurrent cases showed a greater predilection for nonplantar surfaces and the great toe (P=.002). Five of 6 cases on the nonplantar surface—1 on the ankle and 5 on the great toe—recurred despite negative pathologic margins. There was no significant difference in demographics, pathogenesis, tumor size, chronicity, phenotype, or metastatic spread in recurrent and nonrecurrent cases in our cohort.
The tumor has only been described in rare instances at extrapedal cutaneous sites including the hand, scalp, and abdomen.14,17,18 Our series did include a case of synchronous presentation with a verrucous carcinoma on the thumb. Given the rarity of this presentation, thus far there are no data supporting any atypical locations of verrucous carcinoma having greater instances of recurrence. Our recurrent cases displaying atypical location on nonglabrous skin could suggest an underlying pathologic mechanism distinct from tumors on glabrous skin and relevant to increased recurrence risk. Such a mechanism might relate to distinct genetic insults, tumor-microenvironment interactions, or field effects. There are few studies regarding physiologic differences between the plantar surface and the nonglabrous surface and how that influences cancer genesis. Within acral melanoma studies, nonglabrous skin of more sun-exposed surfaces has a higher burden of genetic insults including BRAF mutations.19 Genetic testing of verrucous carcinoma is highly limited, with abnormal expression of the p53 tumor suppressor protein and possible association with several types of HPV. Verrucous carcinoma in general has been found to contain HPV types 6 and 11, nononcogenic forms, and higher risk from HPV types 16 and 18.9,20 However, only a few cases of HPV type 16 as well as 1 case each of HPV type 2 and type 11 have been found within verrucous carcinoma of the foot.21,22 In squamous cell carcinoma of the head and neck, HPV-positive tumors have shown better response to treatment. Further investigation of HPV and genetic contributors in verrucous carcinoma is warranted.
There is notable evidence that surgical resection is the best mode of treatment of verrucous carcinoma.2,3,10,11 Our case series was treated with wide local excision, with partial metatarsal amputation or great toe amputation, in cases with bone invasion or osteomyelitis. Surgical margins were not reported in all the cases but ranged from 0.5 to 2 cm with no significant differences between the recurrent and nonrecurrent groups. After excision, closure was conducted by incorporating primary, secondary, and delayed closure techniques, along with skin grafts for larger defects. Lymph node biopsy traditionally has not been recommended due to reported low metastatic potential. In all 5 recurrent cases, the tumors recurred after multiple attempts at wide excision and greater resection of bone and tissue, with negative margins. The tumors regrew quickly, within months, on the edges of the new graft or in the middle of the graft. The sites of recurrent tumor growth would suggest regrowth in the areas of greatest tissue stress and proliferation. We recommend a low threshold for biopsy and aggressive retreatment in the setting of exophytic growth at reconstruction sites.
Recurrence is uncommon in the setting of verrucous carcinoma, with our series being the first to analyze prognostic factors.3,9,14 Our findings indicate that
Verrucous carcinoma is a rare cancer with the greatest predilection for the foot. Multiple case reports with only a few large case series have been published. 1-3 Plantar verrucous carcinoma is characterized as a slowly but relentlessly enlarging warty tumor with low metastatic potential and high risk for local invasion. The tumor occurs most frequently in patients aged 60 to 70 years, predominantly in White males. 1 It often is misdiagnosed for years as an ulcer or wart that is highly resistant to therapy. Size typically ranges from 1 to 12 cm in greatest dimension. 1
The pathogenesis of plantar verrucous carcinoma remains unclear, but some contributing factors have been proposed, including trauma, chronic irritation, infection, and poor local hygiene.2 This tumor has been reported to occur in chronic foot ulcerations, particularly in the diabetic population.4 It has been proposed that abnormal expression of the p53 tumor suppressor protein and several types of human papillomavirus (HPV) may have a role in the pathogenesis of verrucous carcinoma.5
The pathologic hallmarks of this tumor include a verrucous/hyperkeratotic surface with a deeply endophytic, broad, pushing base. Tumor cells are well differentiated, and atypia is either absent or confined to 1 or 2 layers at the base of the tumor. Overt invasion at the base is lacking, except in cases with a component of conventional invasive squamous cell carcinoma. Human papillomavirus viropathic changes are classically absent.1,3 Studies of the histopathology of verrucous carcinoma have been complicated by similar entities, nomenclatural uncertainty, and variable diagnostic criteria. For example, epithelioma cuniculatum variously has been defined as being synonymous with verrucous carcinoma, a distinct clinical verrucous carcinoma subtype occurring on the soles, a histologic subtype (characterized by prominent burrowing sinuses), or a separate entity entirely.1,2,6,7 Furthermore, in the genital area, several different types of carcinomas have verruciform features but display distinct microscopic findings and outcomes from verrucous carcinoma.8
Verrucous carcinoma represents an unusual variant of squamous cell carcinoma and is treated as such. Treatments have included laser surgery; immunotherapy; retinoid therapy; and chemotherapy by oral, intralesional, or iontophoretic routes in select patients.9 Radiotherapy presents another option, though reports have described progression to aggressive squamous cell carcinoma in some cases.9 Surgery is the best course of treatment, and as more case reports have been published, a transition from radical resection to wide excision with tumor-free margins is the treatment of choice.2,3,10,11 To minimize soft-tissue deficits, Mohs micrographic surgery has been discussed as a treatment option for verrucous carcinoma.11-13
Few studies have described verrucous carcinoma recurrence, and none have systematically examined recurrence rate, risk factors, or prognosis
Methods
Patient cases were
Of the 19 cases, 16 were treated at the University of Michigan and are included in the treatment analyses. Specific attention was then paid to the cases with a clinical recurrence despite negative surgical margins. We compared the clinical and surgical differences between recurrent cases and nonrecurrent cases.
Pathology was rereviewed for selected cases, including 2 cases with recurrence and matched primary, 2 cases with recurrence (for which the matched primary was unavailable for review), and 5 representative primary cases that were not complicated by recurrence. Pathology review was conducted in a blinded manner by one of the authors (P.W.H) who is a board-certified dermatopathologist for approximate depth of invasion from the granular layer, perineural invasion, bone invasion, infiltrative growth, presence of conventional squamous cell carcinoma, and margin status.
Statistical analysis was performed when appropriate using an N1 χ2 test or Student t test.
Results
Demographics and Comorbidities—The median age of the patients at the time of diagnosis was 55 years (range, 34–77 years). There were 12 males and 7 females (Table 1). Two patients were Black and 17 were White. Almost all patients had additional comorbidities including tobacco use (68%), alcohol use (47%), and diabetes (47%). Only 1 patient had an autoimmune disease and was on chronic steroids. No significant difference was found between the demographics of patients with recurrent lesions and those without recurrence.
Tumor Location and Clinical Presentation—The most common clinical presentation included a nonhealing ulceration with warty edges, pain, bleeding, and lowered mobility. In most cases, there was history of prior treatment over a duration ranging from 1 to 8 years, with a median of 5 years prior to biopsy-based diagnosis (Table 1). Six patients had a history of osteomyelitis, diagnosed by imaging or biopsy, within a year before tumor diagnosis. The size of the primary tumor ranged from 2.4 to 6 cm, with a mean of 4 cm (P=.20). The clinical presentation, time before diagnosis, and size of the tumors did not differ significantly between recurrent and nonrecurrent cases.
The tumor location for the recurrent cases differed significantly compared to nonrecurrent cases. All 5 of the patients with a recurrence presented with a tumor on the nonglabrous part of the foot. Four patients (80%) had lesions on the dorsal or lateral aspect of the great toe (P=.002), and 1 patient (20%) had a lesion on the low ankle (P=.09)(Table 1). Of the nonrecurrent cases, 1 patient (7%) presented with a tumor on the plantar surface of the great toe (P=.002), 13 patients (93%) presented with tumors on the distal plantar surface of the foot (P=.0002), and 1 patient with a plantar foot tumor (Figure 1) also had verrucous carcinoma on the thumb (Table 1 and Figure 2).
Histopathology—Available pathology slides for recurrent cases of verrucous carcinoma were reviewed alongside representative cases of verrucous carcinomas that did not progress to recurrence. The diagnosis of verrucous carcinoma was confirmed in all cases, with no evidence of conventional squamous cell carcinoma, perineural invasion, extension beyond the dermis, or bone invasion in any case. The median size of the tumors was 4.2 cm and 4 cm for nonrecurrent and recurrent specimens, respectively. Recurrences displayed a trend toward increased depth compared to primary tumors without recurrence (average depth, 5.5 mm vs 3.7 mm); however, this did not reach statistical significance (P=.24). Primary tumors that progressed to recurrence (n=2) displayed similar findings to the other cases, with invasive depths of 3.5 and 5.5 mm, and there was no evidence of conventional squamous cell carcinoma, perineural invasion, or extension beyond the dermis.
Treatment of Nonrecurrent Cases—Of the 16 total cases treated at the University of Michigan, surgery was the primary mode of therapy in every case (Tables 2 and 3). Of the 11 nonrecurrent cases, 7 patients had wide local excision with a dermal regeneration template, and delayed split-thickness graft reconstruction. Three cases had wide local excision with metatarsal resection, dermal regeneration template, and delayed skin grafting. One case had a great toe amputation
Treatment of Recurrent Cases—For the 5 patients with recurrence, surgical margins were not reported in all the cases but ranged from 0.5 to 2 cm (4/5 [80%] reported). On average, follow-up for this group of patients was 29 months, with a range of 12 to 60 months (Table 3).
The first case with a recurrence (patient 12) initially presented with a chronic calluslike growth of the medial ankle. The lesion initially was treated with wide local excision with negative margins. Reconstruction was performed in a staged fashion with use of a dermal regenerative template followed later by split-thickness skin grafting. Tumor recurrence with negative margins occurred 3 times over the next 2 years despite re-resections with negative pathologic margins. Each recurrence presented as graft breakdown and surrounding hyperkeratosis (Figure 3). After the third graft placement failed, the patient elected for a BKA. There has not been recurrence since the BKA after 5 years total follow-up from the time of primary tumor resection. Of note, this was the only patient in our cohort who was immunosuppressed and evaluated for regional nodal involvement by positron emission tomography.
Another patient with recurrence (patient 13) presented with a chronic great toe ulcer of 5 years’ duration that formed on the dorsal aspect of the great toe after a previously excised wart (Figure 4A). This patient underwent mid-proximal metatarsal amputation with 2-cm margins and subsequent skin graft. Pathologic margins were negative. Within 6 months, there was hyperkeratosis and a draining wound (Figure 4B). Biopsy results confirmed recurrent disease that was treated with re-resection, including complete metatarsal amputation with negative margins and skin graft placement. Verrucous carcinoma recurred at the edges of the graft within 8 months, and the patient elected for a BKA. In addition, this patient also presented with a verrucous carcinoma of the contralateral great toe. The tumor presented as a warty ulcer of 4 months’ duration in the setting of osteomyelitis and was resected by great toe amputation that was performed concurrently with the opposite leg BKA; there has been no recurrence. Of note, this was the only patient to have right inguinal sentinel lymph node tissue sampled and HPV testing conducted, which were negative for verrucous carcinoma and high or low strains of HPV.
Another recurrent case (patient 14) presented with a large warty lesion on the dorsal great toe positive for verrucous carcinoma. He underwent a complete great toe amputation with skin graft placement. Verrucous carcinoma recurred on the edges of the graft within 6 months, and the patient was lost to follow-up when a BKA was suggested.
The fourth recurrent case (patient 15) initially had been treated for 1 year as a viral verruca of the dorsal aspect of the great toe. He had an exophytic mass positive for verrucous carcinoma growing on the dorsal aspect of the great toe around the prior excision site. After primary wide excision with negative 1-cm margins and graft placement, the tumor was re-excised twice within the next 2 years with pathologic negative margins. The patient underwent a foot amputation due to a severe osteomyelitis infection at the reconstruction site.
The final recurrent case (patient 16) presented with a mass on the lateral great toe that initially was treated as a viral verruca (for unknown duration) that had begun to ulcerate. The patient underwent wide excision with 1-cm margins and graft placement. Final pathology was consistent with verrucous carcinoma with negative margins. Recurrence occurred within 11 months on the edge of the graft, and a great toe amputation through the metatarsal phalangeal joint was performed.
Comment
Our series of 19 cases of verrucous carcinoma adds to the limited number of reported cases in the literature. We sought to evaluate the potential risk factors for early recurrence. Consistent with prior studies, our series found verrucous carcinoma of the foot to occur most frequently in patients aged 50 to 70 years, predominantly in White men.1 These tumors grew in the setting of chronic inflammation, tissue regeneration, multiple comorbidities, and poor wound hygiene. Misdiagnosis of verrucous carcinoma often leads to ineffective treatments and local invasion of nerves, muscle, and bone tissue.9,15,16 Our case series also clearly demonstrated the diagnostic challenge verrucous carcinoma presents, with an average delay in diagnosis of 5 years; correct diagnosis often did not occur until the tumor was 4 cm in size (average) and more than 50% had chronic ulceration.
The histologic features of the tumors showed striking uniformity. Within the literature, there is confusion regarding the use of the terms verrucous carcinoma and carcinoma (epithelioma) cuniculatum and the possible pathologic differences between the two. The World Health Organization’s classification of skin tumors describes epithelioma cuniculatum as verrucous carcinoma located on the sole of the foot.7 Kubik and Rhatigan6 pointed out that carcinoma cuniculatum does not have a warty or verrucous surface, which is a defining feature of verrucous carcinoma. Multiple authors have further surmised that the deep burrowing sinus tracts of epithelioma cuniculatum are different than those seen in verrucous carcinoma formed by the undulations extending from the papillomatous and verrucous surface.1,6 We did not observe these notable pathologic differences in recurrent or nonrecurrent primary tumors or differences between primary and recurrent cases. Although our cohort was small, the findings suggest that standard histologic features do not predict aggressive behavior in verrucous carcinomas. Furthermore, our observations support a model wherein recurrence is an inherent property of certain verrucous carcinomas rather than a consequence of histologic progression to conventional squamous cell carcinoma. The lack of overt malignant features in such cases underscores the need for distinction of verrucous carcinoma from benign mimics such as viral verruca or reactive epidermal hyperplasia.
Our recurrent cases showed a greater predilection for nonplantar surfaces and the great toe (P=.002). Five of 6 cases on the nonplantar surface—1 on the ankle and 5 on the great toe—recurred despite negative pathologic margins. There was no significant difference in demographics, pathogenesis, tumor size, chronicity, phenotype, or metastatic spread in recurrent and nonrecurrent cases in our cohort.
The tumor has only been described in rare instances at extrapedal cutaneous sites including the hand, scalp, and abdomen.14,17,18 Our series did include a case of synchronous presentation with a verrucous carcinoma on the thumb. Given the rarity of this presentation, thus far there are no data supporting any atypical locations of verrucous carcinoma having greater instances of recurrence. Our recurrent cases displaying atypical location on nonglabrous skin could suggest an underlying pathologic mechanism distinct from tumors on glabrous skin and relevant to increased recurrence risk. Such a mechanism might relate to distinct genetic insults, tumor-microenvironment interactions, or field effects. There are few studies regarding physiologic differences between the plantar surface and the nonglabrous surface and how that influences cancer genesis. Within acral melanoma studies, nonglabrous skin of more sun-exposed surfaces has a higher burden of genetic insults including BRAF mutations.19 Genetic testing of verrucous carcinoma is highly limited, with abnormal expression of the p53 tumor suppressor protein and possible association with several types of HPV. Verrucous carcinoma in general has been found to contain HPV types 6 and 11, nononcogenic forms, and higher risk from HPV types 16 and 18.9,20 However, only a few cases of HPV type 16 as well as 1 case each of HPV type 2 and type 11 have been found within verrucous carcinoma of the foot.21,22 In squamous cell carcinoma of the head and neck, HPV-positive tumors have shown better response to treatment. Further investigation of HPV and genetic contributors in verrucous carcinoma is warranted.
There is notable evidence that surgical resection is the best mode of treatment of verrucous carcinoma.2,3,10,11 Our case series was treated with wide local excision, with partial metatarsal amputation or great toe amputation, in cases with bone invasion or osteomyelitis. Surgical margins were not reported in all the cases but ranged from 0.5 to 2 cm with no significant differences between the recurrent and nonrecurrent groups. After excision, closure was conducted by incorporating primary, secondary, and delayed closure techniques, along with skin grafts for larger defects. Lymph node biopsy traditionally has not been recommended due to reported low metastatic potential. In all 5 recurrent cases, the tumors recurred after multiple attempts at wide excision and greater resection of bone and tissue, with negative margins. The tumors regrew quickly, within months, on the edges of the new graft or in the middle of the graft. The sites of recurrent tumor growth would suggest regrowth in the areas of greatest tissue stress and proliferation. We recommend a low threshold for biopsy and aggressive retreatment in the setting of exophytic growth at reconstruction sites.
Recurrence is uncommon in the setting of verrucous carcinoma, with our series being the first to analyze prognostic factors.3,9,14 Our findings indicate that
- Kao GF, Graham JH, Helwig EB. Carcinoma cuniculatum (verrucous carcinoma of the skin): a clinicopathologic study of 46 cases with ultrastructural observations. Cancer. 1982;49:2395-2403.
- McKee PH, Wilkinson JD, Black M, et al. Carcinoma (epithelioma) cuniculatum: a clinic-pathologic study of nineteen cases and review of the literature. Histopathology. 1981;5:425-436.
- Penera KE, Manji KA, Craig AB, et al. Atypical presentation of verrucous carcinoma: a case study and review of the literature. Foot Ankle Spec. 2013;6:318-322.
- Rosales MA, Martin BR, Armstrong DG, et al. Verrucous hyperplasia: a common and problematic finding in the high-risk diabetic foot. J Am Podiatr Assoc. 2006:4:348-350.
- Noel JC, Peny MO, De Dobbeleer G, et al. p53 Protein overexpression in verrucous carcinoma of the skin. Dermatology. 1996;192:12-15.
- Kubik MJ, Rhatigan RM. Carcinoma cuniculatum: not a verrucous carcinoma. J Cutan Pathol. 2012;39:1083-1087
- Elder D, Massi D, Scolver R, et al. Verrucous squamous cell carcinoma. WHO Classification of Tumours (Medicine). Vol 11. 4th ed. International Agency for Research on Cancer: 2018;35-57.
- Chan MP. Verruciform and condyloma-like squamous proliferations in the anogenital region. Arch Pathol Lab Med. 2019;143:821-831
- Schwartz RA. Verrucous carcinoma of the skin and mucosa. J Am Acad Dermatol. 1995;32:1-21.
- Flynn K, Wiemer D. Treatment of an epithelioma cuniculatum plantare by local excision and a plantar skin flap. J Dermatol Surg Oncol. 1978;4:773-775.
- Spyriounis P, Tentis D, Sparveri I, et al. Plantar epithelioma cuniculatum: a case report with review of the literature. Eur J Plast Surg. 2004;27:253-256.
- Swanson NA, Taylor WB. Plantar verrucous carcinoma: literature review and treatment by the Moh’s chemosurgery technique. Arch Dermatol. 1980;116:794-797.
- Alkalay R, Alcalay J, Shiri J. Plantar verrucous carcinoma treated with Mohs micrographic surgery: a case report and literature review. J Drugs Dermatol. 2006:5:68-73.
- Kotwal M, Poflee S, Bobhate, S. Carcinoma cuniculatum at various anatomical sites. Indian J Dermatol. 2005;50:216-220.
- Nagarajan D, Chandrasekhar M, Jebakumar J, et al. Verrucous carcinoma of foot at an unusual site: lessons to be learnt. South Asian J Cancer. 2017;6:63.
- Pempinello C, Bova A, Pempinello R, et al Verrucous carcinoma of the foot with bone invasion: a case report. Case Rep Oncol Med. 2013;2013:135307.
- Vandeweyer E, Sales F, Deramaecker R. Cutaneous verrucous carcinoma. Br J Plastic Surg. 2001;54:168-170.
- Joybari A, Azadeh P, Honar B. Cutaneous verrucous carcinoma superimposed on chronically inflamed ileostomy site skin. Iran J Pathol. 2018;13:285-288.
- Davis EJ, Johnson DB, Sosman JA, et al. Melanoma: what do all the mutations mean? Cancer. 2018;124:3490-3499.
- Gissmann L, Wolnik L, Ikenberg H, et al. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci U S A. 1983;80:560-563.
- Knobler RM, Schneider S, Neumann RA, et al. DNA dot-blot hybridization implicates human papillomavirus type 11-DNA in epithelioma cuniculatum. J Med Virol. 1989;29:33-37.
- Noel JC, Peny MO, Detremmerie O, et al. Demonstration of human papillomavirus type 2 in a verrucous carcinoma of the foot. Dermatology. 1993;187:58-61.
- Kao GF, Graham JH, Helwig EB. Carcinoma cuniculatum (verrucous carcinoma of the skin): a clinicopathologic study of 46 cases with ultrastructural observations. Cancer. 1982;49:2395-2403.
- McKee PH, Wilkinson JD, Black M, et al. Carcinoma (epithelioma) cuniculatum: a clinic-pathologic study of nineteen cases and review of the literature. Histopathology. 1981;5:425-436.
- Penera KE, Manji KA, Craig AB, et al. Atypical presentation of verrucous carcinoma: a case study and review of the literature. Foot Ankle Spec. 2013;6:318-322.
- Rosales MA, Martin BR, Armstrong DG, et al. Verrucous hyperplasia: a common and problematic finding in the high-risk diabetic foot. J Am Podiatr Assoc. 2006:4:348-350.
- Noel JC, Peny MO, De Dobbeleer G, et al. p53 Protein overexpression in verrucous carcinoma of the skin. Dermatology. 1996;192:12-15.
- Kubik MJ, Rhatigan RM. Carcinoma cuniculatum: not a verrucous carcinoma. J Cutan Pathol. 2012;39:1083-1087
- Elder D, Massi D, Scolver R, et al. Verrucous squamous cell carcinoma. WHO Classification of Tumours (Medicine). Vol 11. 4th ed. International Agency for Research on Cancer: 2018;35-57.
- Chan MP. Verruciform and condyloma-like squamous proliferations in the anogenital region. Arch Pathol Lab Med. 2019;143:821-831
- Schwartz RA. Verrucous carcinoma of the skin and mucosa. J Am Acad Dermatol. 1995;32:1-21.
- Flynn K, Wiemer D. Treatment of an epithelioma cuniculatum plantare by local excision and a plantar skin flap. J Dermatol Surg Oncol. 1978;4:773-775.
- Spyriounis P, Tentis D, Sparveri I, et al. Plantar epithelioma cuniculatum: a case report with review of the literature. Eur J Plast Surg. 2004;27:253-256.
- Swanson NA, Taylor WB. Plantar verrucous carcinoma: literature review and treatment by the Moh’s chemosurgery technique. Arch Dermatol. 1980;116:794-797.
- Alkalay R, Alcalay J, Shiri J. Plantar verrucous carcinoma treated with Mohs micrographic surgery: a case report and literature review. J Drugs Dermatol. 2006:5:68-73.
- Kotwal M, Poflee S, Bobhate, S. Carcinoma cuniculatum at various anatomical sites. Indian J Dermatol. 2005;50:216-220.
- Nagarajan D, Chandrasekhar M, Jebakumar J, et al. Verrucous carcinoma of foot at an unusual site: lessons to be learnt. South Asian J Cancer. 2017;6:63.
- Pempinello C, Bova A, Pempinello R, et al Verrucous carcinoma of the foot with bone invasion: a case report. Case Rep Oncol Med. 2013;2013:135307.
- Vandeweyer E, Sales F, Deramaecker R. Cutaneous verrucous carcinoma. Br J Plastic Surg. 2001;54:168-170.
- Joybari A, Azadeh P, Honar B. Cutaneous verrucous carcinoma superimposed on chronically inflamed ileostomy site skin. Iran J Pathol. 2018;13:285-288.
- Davis EJ, Johnson DB, Sosman JA, et al. Melanoma: what do all the mutations mean? Cancer. 2018;124:3490-3499.
- Gissmann L, Wolnik L, Ikenberg H, et al. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci U S A. 1983;80:560-563.
- Knobler RM, Schneider S, Neumann RA, et al. DNA dot-blot hybridization implicates human papillomavirus type 11-DNA in epithelioma cuniculatum. J Med Virol. 1989;29:33-37.
- Noel JC, Peny MO, Detremmerie O, et al. Demonstration of human papillomavirus type 2 in a verrucous carcinoma of the foot. Dermatology. 1993;187:58-61.
Practice Points
- Clinicians should have a high suspicion for verrucous carcinoma in the setting of a chronic ulceration or warty lesion that is resistant to traditional treatment. Early biopsy with tissue collection of the raised ulcer borders and the deep dermis layer of warty lesions is imperative for diagnosis.
- Verrucous carcinoma originating on the nonglabrous surface of the foot may have a higher rate of recurrence often occurring within months of previous treatment. Patients presenting with nonhealing surgical sites in this area should be treated with a high level of suspicion for recurrence.
Removal of Isotretinoin Gender-Based Guidelines: Inclusivity Takes Precedence
Isotretinoin is one of the most highly regulated dermatologic medications on the market. The main reason for regulation through the US Food and Drug Administration (FDA)–managed iPLEDGE Risk Evaluation and Mitigation Strategy (REMS) is to minimize the drug’s teratogenic potential, as isotretinoin can cause profound birth defects. The program originally categorized patients into 1 of 3 categories: (1) females of reproductive potential, (2) females not of reproductive potential, and (3) males. Unless the patient commits to abstinence, the program required female patients of childbearing potential to be on 2 forms of birth control and undergo regular pregnancy testing before obtaining refills. Over the last few years, the American Academy of Dermatology Association (AADA) has been advocating for changes to the iPLEDGE system. Proposed changes have included decreasing attestation frequency for patients who cannot get pregnant, increasing contraception counseling and options, and changing enrollment guidelines to encompass all gender and sexual minorities. As of December 13, 2021, the iPLEDGE system changed enrollment categories to reflect the AADA’s wishes and rolled out gender-neutral categories for enrollment in iPLEDGE. This change will simplify and enhance patients’ experience when starting isotretinoin.
Developing Inclusive iPLEDGE Categories
In recent years, dermatologists and patients have viewed these strict gender-based categories as limiting and problematic, especially for their transgender patients and female patients of childbearing potential who exclusively engage in intercourse with cisgender females. The United States has more than 10 million LGBTQIA+ citizens and an estimated 1.4 million adults who identify as transgender individuals, rendering the previously established gender-binary iPLEDGE categories outdated.1,2
As a result, over the last few years, dermatologists, LGBTQIA+ allies, and patients have urged the FDA to create a gender-neutral registration process for iPLEDGE. With support from the AADA, the new modifications were approved for implementation and include 2 risk categories: (1) people who can get pregnant and (2) people who cannot get pregnant.3
As exciting as these changes are for the future of dermatologic practice, the actual transition to the new iPLEDGE system was described as a “failure, chaotic, and a disaster” due to additional changes made at the same time.4 The iPLEDGE system was switched to a new website administered by a different vendor and required providers to confirm each patient online by December 13, 2021. In addition, the new system required pharmacists to obtain risk management authorization via the iPLEDGE REMS website or by calling the iPLEDGE REMS center before dispensing isotretinoin. This overhaul did not work as planned, as the new website was constantly down and it was nearly impossible to reach a contact over the telephone. The complications resulted in major disruptions and delayed prescriptions for thousands of patients nationwide as well as a great disruption in workflow for physicians and pharmacists. The AADA subsequently met with the Isotretinoin Products Manufacturers Group to create workable solutions for these issues.
On January 14, 2022, the FDA posted updates regarding access to the iPLEDGE system. They have worked with the Isotretinoin Products Manufacturers Group to create workable solutions for patients and physicians while transferring the patients’ information to the new database. Their solution includes allowing physicians to send patients login links through their email to access their account instead of waiting for the call center. The majority of iPLEDGE users now have access to their accounts without issues, and the gender-neutral guidelines have been in place since the original change.
Impact of iPLEDGE Categories on Transgender Patients
These changes specifically will improve the experience of transgender men and cisgender women who are at no risk for pregnancy and could be subjected to monthly pregnancy testing when it is not medically necessary.
Consider the following patient scenario. A transgender man presents to your dermatology office seeking treatment of severe nodulocystic acne. He was placed on hormonal replacement therapy with exogenous testosterone—injections, oral pills, topical gel, topical patches, or subdermal pellets—to achieve secondary sex characteristics and promote gender congruence. The patient mentions he has been amenorrheic for several months now. He has tried many topical acne treatments as well as oral antibiotics without much benefit and is now interested in enrolling in iPLEDGE to obtain isotretinoin. With the prior iPLEDGE registration packets, how would this transgender man be classified? As a female with childbearing potential due to his retained ovaries and uterus? What if he did not endorse engaging in sexual intercourse that could result in pregnancy?
Transgender patients have unique and unmet needs that often are overlooked and prevent them from equitable, gender-affirming health care. For example, in a prospective study following 20 transgender men starting hormone replacement therapy, the percentage of patients with facial acne increased from 35% to 82% after 6 months of therapy.5 In addition, the increased psychosocial burden of acne may be especially difficult in these patients, as they already report higher rates of depression and suicidal ideation compared with their heterosexual cisgender peers.4 Further, the primary patient populations receiving isotretinoin typically are adolescents and young adults who are undergoing major physical, mental, and hormonal changes. Self-discovery and self-actualization develop over time, and our role as physicians is to advocate for all aspects of our patients’ health and eliminate barriers to optimal care.
Inclusive Language in iPLEDGE Categories
It is important to streamline access to care for all patients, and gender-affirming, culturally sensitive language is essential to building trust and understanding between patients and providers. Howa Yeung, MD, MSc, a dermatologist at Emory University (Atlanta, Georgia) who advocated for gender-neutral iPLEDGE registration, welcomes the change and stated it “will make my job easier. I no longer have to struggle between respecting the patient’s gender identity and providing medically necessary care for patients with severe acne.”3
Sanchez et al6 provided a list of structured questions providers can ask their patients to assess their risk regarding pregnancy: (1) Do you have a uterus and/or ovaries?, (2) Are you engaging in sexual intercourse with a person who has a penis?, and (3) If yes to these questions, what form(s) of birth control are you using? Providers should preface these questions with the following statement: “It is important that I ask these questions to assess your risk for becoming pregnant on this medication because isotretinoin can cause very serious birth defects.” It is important to review these questions and practice asking them so residents can operate from the same place of openness and understanding when caring for their patients.
Final Thoughts
The landscape of isotretinoin prescribing currently is changing on a day-to-day basis. As residents, it is important we stay up to date with the changes regarding our regularly dispensed medications. The main modification made to the iPLEDGE REMS system was switching the risk categories from 3 (females who can get pregnant, females who cannot get pregnant, males) to 2 (people who can get pregnant, people who cannot get pregnant). This change will make registration for iPLEDGE less complex and more inclusive for all patients. It is important for residents to stay at the forefront of these patient health issues and barriers to equal care, and this change represents a step in the right direction.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602. doi:10.1016/j.jaad.2018.02.045
- Flores AR, Herman JL, Gates GJ, et al. How many adults identify as transgender in the United States? UCLA Williams Institute website. Published June 2016. Accessed March 1, 2022. https://williamsinstitute.law.ucla.edu/publications/trans-adults-united-states/
- Doheny K. FDA OKs iPLEDGE change for gender-neutral language. Dermatology News. October 13, 2021. Accessed March 3, 2022. https://www.mdedge.com/dermatology/article/247352/acne/fda-oks-ipledge-change-gender-neutral-language/page/0/1
- Doheny K. iPLEDGE rollout described as a failure, chaotic, and a disaster. Medscape. December 16, 2021. Accessed March 1, 2022. https://www.medscape.com/viewarticle/964925?uac=423615MG
- Wierckx K, Van de Peer F, Verhaeghe E, et al. Short- and long-term clinical skin effects of testosterone treatment in trans men. J Sex Med. 2014;11:222-229.
- Sanchez DP, Brownstone N, Thibodeaux Q, et al. Prescribing isotretinoin for transgender patients: a call to action and recommendations. J Drugs Dermatol. 2021;20:106-108.
Isotretinoin is one of the most highly regulated dermatologic medications on the market. The main reason for regulation through the US Food and Drug Administration (FDA)–managed iPLEDGE Risk Evaluation and Mitigation Strategy (REMS) is to minimize the drug’s teratogenic potential, as isotretinoin can cause profound birth defects. The program originally categorized patients into 1 of 3 categories: (1) females of reproductive potential, (2) females not of reproductive potential, and (3) males. Unless the patient commits to abstinence, the program required female patients of childbearing potential to be on 2 forms of birth control and undergo regular pregnancy testing before obtaining refills. Over the last few years, the American Academy of Dermatology Association (AADA) has been advocating for changes to the iPLEDGE system. Proposed changes have included decreasing attestation frequency for patients who cannot get pregnant, increasing contraception counseling and options, and changing enrollment guidelines to encompass all gender and sexual minorities. As of December 13, 2021, the iPLEDGE system changed enrollment categories to reflect the AADA’s wishes and rolled out gender-neutral categories for enrollment in iPLEDGE. This change will simplify and enhance patients’ experience when starting isotretinoin.
Developing Inclusive iPLEDGE Categories
In recent years, dermatologists and patients have viewed these strict gender-based categories as limiting and problematic, especially for their transgender patients and female patients of childbearing potential who exclusively engage in intercourse with cisgender females. The United States has more than 10 million LGBTQIA+ citizens and an estimated 1.4 million adults who identify as transgender individuals, rendering the previously established gender-binary iPLEDGE categories outdated.1,2
As a result, over the last few years, dermatologists, LGBTQIA+ allies, and patients have urged the FDA to create a gender-neutral registration process for iPLEDGE. With support from the AADA, the new modifications were approved for implementation and include 2 risk categories: (1) people who can get pregnant and (2) people who cannot get pregnant.3
As exciting as these changes are for the future of dermatologic practice, the actual transition to the new iPLEDGE system was described as a “failure, chaotic, and a disaster” due to additional changes made at the same time.4 The iPLEDGE system was switched to a new website administered by a different vendor and required providers to confirm each patient online by December 13, 2021. In addition, the new system required pharmacists to obtain risk management authorization via the iPLEDGE REMS website or by calling the iPLEDGE REMS center before dispensing isotretinoin. This overhaul did not work as planned, as the new website was constantly down and it was nearly impossible to reach a contact over the telephone. The complications resulted in major disruptions and delayed prescriptions for thousands of patients nationwide as well as a great disruption in workflow for physicians and pharmacists. The AADA subsequently met with the Isotretinoin Products Manufacturers Group to create workable solutions for these issues.
On January 14, 2022, the FDA posted updates regarding access to the iPLEDGE system. They have worked with the Isotretinoin Products Manufacturers Group to create workable solutions for patients and physicians while transferring the patients’ information to the new database. Their solution includes allowing physicians to send patients login links through their email to access their account instead of waiting for the call center. The majority of iPLEDGE users now have access to their accounts without issues, and the gender-neutral guidelines have been in place since the original change.
Impact of iPLEDGE Categories on Transgender Patients
These changes specifically will improve the experience of transgender men and cisgender women who are at no risk for pregnancy and could be subjected to monthly pregnancy testing when it is not medically necessary.
Consider the following patient scenario. A transgender man presents to your dermatology office seeking treatment of severe nodulocystic acne. He was placed on hormonal replacement therapy with exogenous testosterone—injections, oral pills, topical gel, topical patches, or subdermal pellets—to achieve secondary sex characteristics and promote gender congruence. The patient mentions he has been amenorrheic for several months now. He has tried many topical acne treatments as well as oral antibiotics without much benefit and is now interested in enrolling in iPLEDGE to obtain isotretinoin. With the prior iPLEDGE registration packets, how would this transgender man be classified? As a female with childbearing potential due to his retained ovaries and uterus? What if he did not endorse engaging in sexual intercourse that could result in pregnancy?
Transgender patients have unique and unmet needs that often are overlooked and prevent them from equitable, gender-affirming health care. For example, in a prospective study following 20 transgender men starting hormone replacement therapy, the percentage of patients with facial acne increased from 35% to 82% after 6 months of therapy.5 In addition, the increased psychosocial burden of acne may be especially difficult in these patients, as they already report higher rates of depression and suicidal ideation compared with their heterosexual cisgender peers.4 Further, the primary patient populations receiving isotretinoin typically are adolescents and young adults who are undergoing major physical, mental, and hormonal changes. Self-discovery and self-actualization develop over time, and our role as physicians is to advocate for all aspects of our patients’ health and eliminate barriers to optimal care.
Inclusive Language in iPLEDGE Categories
It is important to streamline access to care for all patients, and gender-affirming, culturally sensitive language is essential to building trust and understanding between patients and providers. Howa Yeung, MD, MSc, a dermatologist at Emory University (Atlanta, Georgia) who advocated for gender-neutral iPLEDGE registration, welcomes the change and stated it “will make my job easier. I no longer have to struggle between respecting the patient’s gender identity and providing medically necessary care for patients with severe acne.”3
Sanchez et al6 provided a list of structured questions providers can ask their patients to assess their risk regarding pregnancy: (1) Do you have a uterus and/or ovaries?, (2) Are you engaging in sexual intercourse with a person who has a penis?, and (3) If yes to these questions, what form(s) of birth control are you using? Providers should preface these questions with the following statement: “It is important that I ask these questions to assess your risk for becoming pregnant on this medication because isotretinoin can cause very serious birth defects.” It is important to review these questions and practice asking them so residents can operate from the same place of openness and understanding when caring for their patients.
Final Thoughts
The landscape of isotretinoin prescribing currently is changing on a day-to-day basis. As residents, it is important we stay up to date with the changes regarding our regularly dispensed medications. The main modification made to the iPLEDGE REMS system was switching the risk categories from 3 (females who can get pregnant, females who cannot get pregnant, males) to 2 (people who can get pregnant, people who cannot get pregnant). This change will make registration for iPLEDGE less complex and more inclusive for all patients. It is important for residents to stay at the forefront of these patient health issues and barriers to equal care, and this change represents a step in the right direction.
Isotretinoin is one of the most highly regulated dermatologic medications on the market. The main reason for regulation through the US Food and Drug Administration (FDA)–managed iPLEDGE Risk Evaluation and Mitigation Strategy (REMS) is to minimize the drug’s teratogenic potential, as isotretinoin can cause profound birth defects. The program originally categorized patients into 1 of 3 categories: (1) females of reproductive potential, (2) females not of reproductive potential, and (3) males. Unless the patient commits to abstinence, the program required female patients of childbearing potential to be on 2 forms of birth control and undergo regular pregnancy testing before obtaining refills. Over the last few years, the American Academy of Dermatology Association (AADA) has been advocating for changes to the iPLEDGE system. Proposed changes have included decreasing attestation frequency for patients who cannot get pregnant, increasing contraception counseling and options, and changing enrollment guidelines to encompass all gender and sexual minorities. As of December 13, 2021, the iPLEDGE system changed enrollment categories to reflect the AADA’s wishes and rolled out gender-neutral categories for enrollment in iPLEDGE. This change will simplify and enhance patients’ experience when starting isotretinoin.
Developing Inclusive iPLEDGE Categories
In recent years, dermatologists and patients have viewed these strict gender-based categories as limiting and problematic, especially for their transgender patients and female patients of childbearing potential who exclusively engage in intercourse with cisgender females. The United States has more than 10 million LGBTQIA+ citizens and an estimated 1.4 million adults who identify as transgender individuals, rendering the previously established gender-binary iPLEDGE categories outdated.1,2
As a result, over the last few years, dermatologists, LGBTQIA+ allies, and patients have urged the FDA to create a gender-neutral registration process for iPLEDGE. With support from the AADA, the new modifications were approved for implementation and include 2 risk categories: (1) people who can get pregnant and (2) people who cannot get pregnant.3
As exciting as these changes are for the future of dermatologic practice, the actual transition to the new iPLEDGE system was described as a “failure, chaotic, and a disaster” due to additional changes made at the same time.4 The iPLEDGE system was switched to a new website administered by a different vendor and required providers to confirm each patient online by December 13, 2021. In addition, the new system required pharmacists to obtain risk management authorization via the iPLEDGE REMS website or by calling the iPLEDGE REMS center before dispensing isotretinoin. This overhaul did not work as planned, as the new website was constantly down and it was nearly impossible to reach a contact over the telephone. The complications resulted in major disruptions and delayed prescriptions for thousands of patients nationwide as well as a great disruption in workflow for physicians and pharmacists. The AADA subsequently met with the Isotretinoin Products Manufacturers Group to create workable solutions for these issues.
On January 14, 2022, the FDA posted updates regarding access to the iPLEDGE system. They have worked with the Isotretinoin Products Manufacturers Group to create workable solutions for patients and physicians while transferring the patients’ information to the new database. Their solution includes allowing physicians to send patients login links through their email to access their account instead of waiting for the call center. The majority of iPLEDGE users now have access to their accounts without issues, and the gender-neutral guidelines have been in place since the original change.
Impact of iPLEDGE Categories on Transgender Patients
These changes specifically will improve the experience of transgender men and cisgender women who are at no risk for pregnancy and could be subjected to monthly pregnancy testing when it is not medically necessary.
Consider the following patient scenario. A transgender man presents to your dermatology office seeking treatment of severe nodulocystic acne. He was placed on hormonal replacement therapy with exogenous testosterone—injections, oral pills, topical gel, topical patches, or subdermal pellets—to achieve secondary sex characteristics and promote gender congruence. The patient mentions he has been amenorrheic for several months now. He has tried many topical acne treatments as well as oral antibiotics without much benefit and is now interested in enrolling in iPLEDGE to obtain isotretinoin. With the prior iPLEDGE registration packets, how would this transgender man be classified? As a female with childbearing potential due to his retained ovaries and uterus? What if he did not endorse engaging in sexual intercourse that could result in pregnancy?
Transgender patients have unique and unmet needs that often are overlooked and prevent them from equitable, gender-affirming health care. For example, in a prospective study following 20 transgender men starting hormone replacement therapy, the percentage of patients with facial acne increased from 35% to 82% after 6 months of therapy.5 In addition, the increased psychosocial burden of acne may be especially difficult in these patients, as they already report higher rates of depression and suicidal ideation compared with their heterosexual cisgender peers.4 Further, the primary patient populations receiving isotretinoin typically are adolescents and young adults who are undergoing major physical, mental, and hormonal changes. Self-discovery and self-actualization develop over time, and our role as physicians is to advocate for all aspects of our patients’ health and eliminate barriers to optimal care.
Inclusive Language in iPLEDGE Categories
It is important to streamline access to care for all patients, and gender-affirming, culturally sensitive language is essential to building trust and understanding between patients and providers. Howa Yeung, MD, MSc, a dermatologist at Emory University (Atlanta, Georgia) who advocated for gender-neutral iPLEDGE registration, welcomes the change and stated it “will make my job easier. I no longer have to struggle between respecting the patient’s gender identity and providing medically necessary care for patients with severe acne.”3
Sanchez et al6 provided a list of structured questions providers can ask their patients to assess their risk regarding pregnancy: (1) Do you have a uterus and/or ovaries?, (2) Are you engaging in sexual intercourse with a person who has a penis?, and (3) If yes to these questions, what form(s) of birth control are you using? Providers should preface these questions with the following statement: “It is important that I ask these questions to assess your risk for becoming pregnant on this medication because isotretinoin can cause very serious birth defects.” It is important to review these questions and practice asking them so residents can operate from the same place of openness and understanding when caring for their patients.
Final Thoughts
The landscape of isotretinoin prescribing currently is changing on a day-to-day basis. As residents, it is important we stay up to date with the changes regarding our regularly dispensed medications. The main modification made to the iPLEDGE REMS system was switching the risk categories from 3 (females who can get pregnant, females who cannot get pregnant, males) to 2 (people who can get pregnant, people who cannot get pregnant). This change will make registration for iPLEDGE less complex and more inclusive for all patients. It is important for residents to stay at the forefront of these patient health issues and barriers to equal care, and this change represents a step in the right direction.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602. doi:10.1016/j.jaad.2018.02.045
- Flores AR, Herman JL, Gates GJ, et al. How many adults identify as transgender in the United States? UCLA Williams Institute website. Published June 2016. Accessed March 1, 2022. https://williamsinstitute.law.ucla.edu/publications/trans-adults-united-states/
- Doheny K. FDA OKs iPLEDGE change for gender-neutral language. Dermatology News. October 13, 2021. Accessed March 3, 2022. https://www.mdedge.com/dermatology/article/247352/acne/fda-oks-ipledge-change-gender-neutral-language/page/0/1
- Doheny K. iPLEDGE rollout described as a failure, chaotic, and a disaster. Medscape. December 16, 2021. Accessed March 1, 2022. https://www.medscape.com/viewarticle/964925?uac=423615MG
- Wierckx K, Van de Peer F, Verhaeghe E, et al. Short- and long-term clinical skin effects of testosterone treatment in trans men. J Sex Med. 2014;11:222-229.
- Sanchez DP, Brownstone N, Thibodeaux Q, et al. Prescribing isotretinoin for transgender patients: a call to action and recommendations. J Drugs Dermatol. 2021;20:106-108.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602. doi:10.1016/j.jaad.2018.02.045
- Flores AR, Herman JL, Gates GJ, et al. How many adults identify as transgender in the United States? UCLA Williams Institute website. Published June 2016. Accessed March 1, 2022. https://williamsinstitute.law.ucla.edu/publications/trans-adults-united-states/
- Doheny K. FDA OKs iPLEDGE change for gender-neutral language. Dermatology News. October 13, 2021. Accessed March 3, 2022. https://www.mdedge.com/dermatology/article/247352/acne/fda-oks-ipledge-change-gender-neutral-language/page/0/1
- Doheny K. iPLEDGE rollout described as a failure, chaotic, and a disaster. Medscape. December 16, 2021. Accessed March 1, 2022. https://www.medscape.com/viewarticle/964925?uac=423615MG
- Wierckx K, Van de Peer F, Verhaeghe E, et al. Short- and long-term clinical skin effects of testosterone treatment in trans men. J Sex Med. 2014;11:222-229.
- Sanchez DP, Brownstone N, Thibodeaux Q, et al. Prescribing isotretinoin for transgender patients: a call to action and recommendations. J Drugs Dermatol. 2021;20:106-108.
Resident Pearls
- Major changes in the iPLEDGE Risk Evaluation and Mitigation Strategy (REMS) system recently took place, including simplifying registration categories while making the process more inclusive for patients.
- It is important to practice culturally sensitive language when discussing subjects regarding gender identification and sexual practices. Sample questions have been provided to help familiarize practitioners with optimal ways to approach these patient encounters.
- There likely will be more changes with iPLEDGE REMS in the future as the American Academy of Dermatology Association continues to work on solutions regarding decreasing monthly qualifications for patients who cannot get pregnant and possible removal of patient attestation requirements.
Chronic Vulvar Plaque in a Patient With Severe Hidradenitis Suppurativa
The Diagnosis: Acquired Lymphangioma Circumscriptum
A skin biopsy of the plaque on the right labium majus showed a proliferation of well-formed, dilated lymphatic vessels lined by benign-appearing endothelial cells in the papillary dermis (Figure). These findings were consistent with a diagnosis of acquired lymphangioma circumscriptum (ALC) in the setting of severe hidradenitis suppurativa (HS).
Acquired lymphangioma circumscriptum (also known as acquired lymphangiectasia or secondary lymphangioma1) is a rare skin finding resulting from chronic lymphatic obstruction that leads to dilated lymphatic vessels within the dermis.2,3 There also is a distinct congenital form of lymphangioma circumscriptum caused by lymphatic malformations present at birth.2,4 Acquired lymphangioma circumscriptum of the vulva is a rare phenomenon.3 Identified causes include radiation or surgery for carcinoma, solid gynecologic tumors, lymphadenectomy, Crohn disease, and tuberculosis and other infections, all of which can disrupt normal lymphatics to cause ALC.2-4 Hidradenitis suppurativa is not a widely recognized cause of ALC; however, this phenomenon is reported in the literature. A long-standing history of severe HS complicated by lymphedema seems to precede the development of ALC in the reported cases, as in our patient.5-7
Acquired lymphangioma circumscriptum of the vulva can appear in women of all ages as frog spawn or cobblestone papules or vesicles, sometimes with a hyperkeratotic or verrucous appearance.2,4 Associated symptoms include serous drainage, edema, pruritus, and discomfort. The lesions may become eroded, which can predispose patients to secondary infections.1,2 Acquired lymphangioma circumscriptum of the vulva can be difficult to diagnose, as the time interval between the initial cause and the appearance of skin findings can be years, leading to the misdiagnosis of ALC as other similar-appearing genital skin conditions such as squamous cell carcinoma or condyloma.4,8 When misidentified as an infection, diagnosis can lead to substantial distress, abstinence from sexual activity, and unnecessary and painful treatments.
Skin biopsy is helpful in distinguishing ALC from other differential diagnoses such as condylomata acuminata, squamous cell carcinoma, and condyloma lata. Histopathology in ALC is notable for dilated lymphatic vessels filled with hypocellular fluid and lined with endothelial cells in the superficial dermis; the epidermis can appear hyperplastic, hyperkeratotic, or eroded.3-5,9 These lymphatic vessels stain positively for CD31 and D2-40, markers for endothelial cells and lymphatic endothelium, respectively, and negative for CD34, a marker for vascular endothelium.3,4,9 Features suggestive of condylomata acuminata such as rounded parakeratosis, hypergranulosis, and vacuolated keratinocytes9 are not present. The giant condyloma of Buschke-Löwenstein, a clinical variant of verrucous squamous cell carcinoma, also can present as a warty ulcerated papule or plaque in the genital region, but the characteristic rounded eosinophilic keratinocytes pushing down into the dermis9 are not seen in ALC. Secondary syphilis is associated with condyloma lata, which are verrucous or fleshy-appearing papules often coalescing into plaques located in the anogenital region. Pathologic features of secondary syphilis include vacuolar interface dermatitis and acanthosis with long slender rete ridges.9 Squamous cell carcinoma, which can arise from inflammation associated with long-standing HS, must be ruled out, as it is associated with a high risk of mortality in patients with HS.10
It is noteworthy to recognize the various, often confusing nomenclature used to describe cutaneous lymphatic conditions. The terms acquired lymphangioma circumscriptum, secondary lymphangioma, and lymphangiectasia are used interchangeably to describe dilated lymphatic vessels in the skin.1 The term atypical vascular lesion refers to lymphectasias of the skin of the breast due to prior radiation therapy most often used in the treatment of breast carcinoma; clinically, these present as red-brown or flesh-colored papules or telangiectatic plaques on the breast.11,12 Lymphedema also may occur alongside atypical vascular lesions, as prior radiation or surgical lymph node dissection can predispose patients to impaired lymphatic drainage.13 The lymphatic histopathologic subtype of atypical vascular lesions may appear similar to ALC; however, the vascular subtype will demonstrate collections of capillary-sized vessels and extravasated erythrocytes.11,12 Unlike ALC, the benign nature of atypical vascular lesions has been questioned, as they may be associated with a small risk for progression to angiosarcoma.11-13 It also is important to distinguish ALC from lymphangiomatosis, a generalized lymphatic anomaly that is characterized by extensive lymphatic malformations involving numerous internal organs, including the lungs and gastrointestinal tract. This condition is associated with notable morbidity and mortality.13
Although the suffix of the term lymphangioma suggests a neoplastic process, ALC is not a neoplasm and can be managed expectantly in many cases.2,3,8 However, due to cosmetic appearance, pain, discomfort, and recurrent bacterial superinfections, many patients pursue treatment. Treatment options for ALC include sclerotherapy, electrocautery, radiofrequency or carbon dioxide laser ablation, and excision, though recurrence can arise.3-5,7,8 Our patient elected to manage her asymptomatic ALC expectantly.
- Verma SB. Lymphangiectasias of the skin: victims of confusing nomenclature. Clin Exp Dermatol. 2009;34:566-569.
- Vlastos AT, Malpica A, Follen M. Lymphangioma circumscriptum of the vulva: a review of the literature. Obstet Gynecol. 2003;101:946-954.
- Chang MB, Newman CC, Davis MD, et al. Acquired lymphangiectasia (lymphangioma circumscriptum) of the vulva: clinicopathologic study of 11 patients from a single institution and 67 from the literature. Int J Dermatol. 2016;55:E482-E487.
- Stewart CJ, Chan T, Platten M. Acquired lymphangiectasia (‘lymphangioma circumscriptum’) of the vulva: a report of eight cases. Pathology. 2009;41:448-453.
- Sims SM, McLean FW, Davis JD, et al. Vulvar lymphangioma circumscriptum: a report of 3 cases, 2 associated with vulvar carcinoma and 1 with hidradenitis suppurativa. J Low Genit Tract Dis. 2010; 14:234-237.
- Moosbrugger EA, Mutasim DF. Hidradenitis suppurativa complicated by severe lymphedema and lymphangiectasias. J Am Acad Dermatol. 2011;6:1223-1224.
- Piernick DM 2nd, Mahmood SH, Daveluy S. Acquired lymphangioma circumscriptum of the genitals in an individual with chronic hidradenitis suppurativa. JAAD Case Rep. 2018;1:64-66.
- Horn LC, Kühndel K, Pawlowitsch T, et al. Acquired lymphangioma circumscriptum of the vulva mimicking genital warts. Eur J Obstet Gynecol Reprod Biol. 2005;1:118-120.
- Elston DM, Ferringer T, Ko CJ, et al. Dermatopathology. 3rd ed. Elsevier; 2019.
- Kohorst JJ, Shah KK, Hallemeier CL, et al. Squamous cell carcinoma in perineal, perianal, and gluteal hidradenitis suppurativa: experience in 12 patients. Dermatol Surg. 2019;45:519-526.
- Patton KT, Deyrup AT, Weiss SW. Atypical vascular lesions after surgery and radiation of the breast: a clinicopathologic study of 32 cases analyzing histologic heterogeneity and association with angiosarcoma. Am J Surg Pathol. 2008;32:943-950.
- Ronen S, Ivan D, Torres-Cabala CA, et al. Post-radiation vascular lesions of the breast. J Cutan Pathol. 2019;46:52-58.
- Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier; 2018.
The Diagnosis: Acquired Lymphangioma Circumscriptum
A skin biopsy of the plaque on the right labium majus showed a proliferation of well-formed, dilated lymphatic vessels lined by benign-appearing endothelial cells in the papillary dermis (Figure). These findings were consistent with a diagnosis of acquired lymphangioma circumscriptum (ALC) in the setting of severe hidradenitis suppurativa (HS).
Acquired lymphangioma circumscriptum (also known as acquired lymphangiectasia or secondary lymphangioma1) is a rare skin finding resulting from chronic lymphatic obstruction that leads to dilated lymphatic vessels within the dermis.2,3 There also is a distinct congenital form of lymphangioma circumscriptum caused by lymphatic malformations present at birth.2,4 Acquired lymphangioma circumscriptum of the vulva is a rare phenomenon.3 Identified causes include radiation or surgery for carcinoma, solid gynecologic tumors, lymphadenectomy, Crohn disease, and tuberculosis and other infections, all of which can disrupt normal lymphatics to cause ALC.2-4 Hidradenitis suppurativa is not a widely recognized cause of ALC; however, this phenomenon is reported in the literature. A long-standing history of severe HS complicated by lymphedema seems to precede the development of ALC in the reported cases, as in our patient.5-7
Acquired lymphangioma circumscriptum of the vulva can appear in women of all ages as frog spawn or cobblestone papules or vesicles, sometimes with a hyperkeratotic or verrucous appearance.2,4 Associated symptoms include serous drainage, edema, pruritus, and discomfort. The lesions may become eroded, which can predispose patients to secondary infections.1,2 Acquired lymphangioma circumscriptum of the vulva can be difficult to diagnose, as the time interval between the initial cause and the appearance of skin findings can be years, leading to the misdiagnosis of ALC as other similar-appearing genital skin conditions such as squamous cell carcinoma or condyloma.4,8 When misidentified as an infection, diagnosis can lead to substantial distress, abstinence from sexual activity, and unnecessary and painful treatments.
Skin biopsy is helpful in distinguishing ALC from other differential diagnoses such as condylomata acuminata, squamous cell carcinoma, and condyloma lata. Histopathology in ALC is notable for dilated lymphatic vessels filled with hypocellular fluid and lined with endothelial cells in the superficial dermis; the epidermis can appear hyperplastic, hyperkeratotic, or eroded.3-5,9 These lymphatic vessels stain positively for CD31 and D2-40, markers for endothelial cells and lymphatic endothelium, respectively, and negative for CD34, a marker for vascular endothelium.3,4,9 Features suggestive of condylomata acuminata such as rounded parakeratosis, hypergranulosis, and vacuolated keratinocytes9 are not present. The giant condyloma of Buschke-Löwenstein, a clinical variant of verrucous squamous cell carcinoma, also can present as a warty ulcerated papule or plaque in the genital region, but the characteristic rounded eosinophilic keratinocytes pushing down into the dermis9 are not seen in ALC. Secondary syphilis is associated with condyloma lata, which are verrucous or fleshy-appearing papules often coalescing into plaques located in the anogenital region. Pathologic features of secondary syphilis include vacuolar interface dermatitis and acanthosis with long slender rete ridges.9 Squamous cell carcinoma, which can arise from inflammation associated with long-standing HS, must be ruled out, as it is associated with a high risk of mortality in patients with HS.10
It is noteworthy to recognize the various, often confusing nomenclature used to describe cutaneous lymphatic conditions. The terms acquired lymphangioma circumscriptum, secondary lymphangioma, and lymphangiectasia are used interchangeably to describe dilated lymphatic vessels in the skin.1 The term atypical vascular lesion refers to lymphectasias of the skin of the breast due to prior radiation therapy most often used in the treatment of breast carcinoma; clinically, these present as red-brown or flesh-colored papules or telangiectatic plaques on the breast.11,12 Lymphedema also may occur alongside atypical vascular lesions, as prior radiation or surgical lymph node dissection can predispose patients to impaired lymphatic drainage.13 The lymphatic histopathologic subtype of atypical vascular lesions may appear similar to ALC; however, the vascular subtype will demonstrate collections of capillary-sized vessels and extravasated erythrocytes.11,12 Unlike ALC, the benign nature of atypical vascular lesions has been questioned, as they may be associated with a small risk for progression to angiosarcoma.11-13 It also is important to distinguish ALC from lymphangiomatosis, a generalized lymphatic anomaly that is characterized by extensive lymphatic malformations involving numerous internal organs, including the lungs and gastrointestinal tract. This condition is associated with notable morbidity and mortality.13
Although the suffix of the term lymphangioma suggests a neoplastic process, ALC is not a neoplasm and can be managed expectantly in many cases.2,3,8 However, due to cosmetic appearance, pain, discomfort, and recurrent bacterial superinfections, many patients pursue treatment. Treatment options for ALC include sclerotherapy, electrocautery, radiofrequency or carbon dioxide laser ablation, and excision, though recurrence can arise.3-5,7,8 Our patient elected to manage her asymptomatic ALC expectantly.
The Diagnosis: Acquired Lymphangioma Circumscriptum
A skin biopsy of the plaque on the right labium majus showed a proliferation of well-formed, dilated lymphatic vessels lined by benign-appearing endothelial cells in the papillary dermis (Figure). These findings were consistent with a diagnosis of acquired lymphangioma circumscriptum (ALC) in the setting of severe hidradenitis suppurativa (HS).
Acquired lymphangioma circumscriptum (also known as acquired lymphangiectasia or secondary lymphangioma1) is a rare skin finding resulting from chronic lymphatic obstruction that leads to dilated lymphatic vessels within the dermis.2,3 There also is a distinct congenital form of lymphangioma circumscriptum caused by lymphatic malformations present at birth.2,4 Acquired lymphangioma circumscriptum of the vulva is a rare phenomenon.3 Identified causes include radiation or surgery for carcinoma, solid gynecologic tumors, lymphadenectomy, Crohn disease, and tuberculosis and other infections, all of which can disrupt normal lymphatics to cause ALC.2-4 Hidradenitis suppurativa is not a widely recognized cause of ALC; however, this phenomenon is reported in the literature. A long-standing history of severe HS complicated by lymphedema seems to precede the development of ALC in the reported cases, as in our patient.5-7
Acquired lymphangioma circumscriptum of the vulva can appear in women of all ages as frog spawn or cobblestone papules or vesicles, sometimes with a hyperkeratotic or verrucous appearance.2,4 Associated symptoms include serous drainage, edema, pruritus, and discomfort. The lesions may become eroded, which can predispose patients to secondary infections.1,2 Acquired lymphangioma circumscriptum of the vulva can be difficult to diagnose, as the time interval between the initial cause and the appearance of skin findings can be years, leading to the misdiagnosis of ALC as other similar-appearing genital skin conditions such as squamous cell carcinoma or condyloma.4,8 When misidentified as an infection, diagnosis can lead to substantial distress, abstinence from sexual activity, and unnecessary and painful treatments.
Skin biopsy is helpful in distinguishing ALC from other differential diagnoses such as condylomata acuminata, squamous cell carcinoma, and condyloma lata. Histopathology in ALC is notable for dilated lymphatic vessels filled with hypocellular fluid and lined with endothelial cells in the superficial dermis; the epidermis can appear hyperplastic, hyperkeratotic, or eroded.3-5,9 These lymphatic vessels stain positively for CD31 and D2-40, markers for endothelial cells and lymphatic endothelium, respectively, and negative for CD34, a marker for vascular endothelium.3,4,9 Features suggestive of condylomata acuminata such as rounded parakeratosis, hypergranulosis, and vacuolated keratinocytes9 are not present. The giant condyloma of Buschke-Löwenstein, a clinical variant of verrucous squamous cell carcinoma, also can present as a warty ulcerated papule or plaque in the genital region, but the characteristic rounded eosinophilic keratinocytes pushing down into the dermis9 are not seen in ALC. Secondary syphilis is associated with condyloma lata, which are verrucous or fleshy-appearing papules often coalescing into plaques located in the anogenital region. Pathologic features of secondary syphilis include vacuolar interface dermatitis and acanthosis with long slender rete ridges.9 Squamous cell carcinoma, which can arise from inflammation associated with long-standing HS, must be ruled out, as it is associated with a high risk of mortality in patients with HS.10
It is noteworthy to recognize the various, often confusing nomenclature used to describe cutaneous lymphatic conditions. The terms acquired lymphangioma circumscriptum, secondary lymphangioma, and lymphangiectasia are used interchangeably to describe dilated lymphatic vessels in the skin.1 The term atypical vascular lesion refers to lymphectasias of the skin of the breast due to prior radiation therapy most often used in the treatment of breast carcinoma; clinically, these present as red-brown or flesh-colored papules or telangiectatic plaques on the breast.11,12 Lymphedema also may occur alongside atypical vascular lesions, as prior radiation or surgical lymph node dissection can predispose patients to impaired lymphatic drainage.13 The lymphatic histopathologic subtype of atypical vascular lesions may appear similar to ALC; however, the vascular subtype will demonstrate collections of capillary-sized vessels and extravasated erythrocytes.11,12 Unlike ALC, the benign nature of atypical vascular lesions has been questioned, as they may be associated with a small risk for progression to angiosarcoma.11-13 It also is important to distinguish ALC from lymphangiomatosis, a generalized lymphatic anomaly that is characterized by extensive lymphatic malformations involving numerous internal organs, including the lungs and gastrointestinal tract. This condition is associated with notable morbidity and mortality.13
Although the suffix of the term lymphangioma suggests a neoplastic process, ALC is not a neoplasm and can be managed expectantly in many cases.2,3,8 However, due to cosmetic appearance, pain, discomfort, and recurrent bacterial superinfections, many patients pursue treatment. Treatment options for ALC include sclerotherapy, electrocautery, radiofrequency or carbon dioxide laser ablation, and excision, though recurrence can arise.3-5,7,8 Our patient elected to manage her asymptomatic ALC expectantly.
- Verma SB. Lymphangiectasias of the skin: victims of confusing nomenclature. Clin Exp Dermatol. 2009;34:566-569.
- Vlastos AT, Malpica A, Follen M. Lymphangioma circumscriptum of the vulva: a review of the literature. Obstet Gynecol. 2003;101:946-954.
- Chang MB, Newman CC, Davis MD, et al. Acquired lymphangiectasia (lymphangioma circumscriptum) of the vulva: clinicopathologic study of 11 patients from a single institution and 67 from the literature. Int J Dermatol. 2016;55:E482-E487.
- Stewart CJ, Chan T, Platten M. Acquired lymphangiectasia (‘lymphangioma circumscriptum’) of the vulva: a report of eight cases. Pathology. 2009;41:448-453.
- Sims SM, McLean FW, Davis JD, et al. Vulvar lymphangioma circumscriptum: a report of 3 cases, 2 associated with vulvar carcinoma and 1 with hidradenitis suppurativa. J Low Genit Tract Dis. 2010; 14:234-237.
- Moosbrugger EA, Mutasim DF. Hidradenitis suppurativa complicated by severe lymphedema and lymphangiectasias. J Am Acad Dermatol. 2011;6:1223-1224.
- Piernick DM 2nd, Mahmood SH, Daveluy S. Acquired lymphangioma circumscriptum of the genitals in an individual with chronic hidradenitis suppurativa. JAAD Case Rep. 2018;1:64-66.
- Horn LC, Kühndel K, Pawlowitsch T, et al. Acquired lymphangioma circumscriptum of the vulva mimicking genital warts. Eur J Obstet Gynecol Reprod Biol. 2005;1:118-120.
- Elston DM, Ferringer T, Ko CJ, et al. Dermatopathology. 3rd ed. Elsevier; 2019.
- Kohorst JJ, Shah KK, Hallemeier CL, et al. Squamous cell carcinoma in perineal, perianal, and gluteal hidradenitis suppurativa: experience in 12 patients. Dermatol Surg. 2019;45:519-526.
- Patton KT, Deyrup AT, Weiss SW. Atypical vascular lesions after surgery and radiation of the breast: a clinicopathologic study of 32 cases analyzing histologic heterogeneity and association with angiosarcoma. Am J Surg Pathol. 2008;32:943-950.
- Ronen S, Ivan D, Torres-Cabala CA, et al. Post-radiation vascular lesions of the breast. J Cutan Pathol. 2019;46:52-58.
- Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier; 2018.
- Verma SB. Lymphangiectasias of the skin: victims of confusing nomenclature. Clin Exp Dermatol. 2009;34:566-569.
- Vlastos AT, Malpica A, Follen M. Lymphangioma circumscriptum of the vulva: a review of the literature. Obstet Gynecol. 2003;101:946-954.
- Chang MB, Newman CC, Davis MD, et al. Acquired lymphangiectasia (lymphangioma circumscriptum) of the vulva: clinicopathologic study of 11 patients from a single institution and 67 from the literature. Int J Dermatol. 2016;55:E482-E487.
- Stewart CJ, Chan T, Platten M. Acquired lymphangiectasia (‘lymphangioma circumscriptum’) of the vulva: a report of eight cases. Pathology. 2009;41:448-453.
- Sims SM, McLean FW, Davis JD, et al. Vulvar lymphangioma circumscriptum: a report of 3 cases, 2 associated with vulvar carcinoma and 1 with hidradenitis suppurativa. J Low Genit Tract Dis. 2010; 14:234-237.
- Moosbrugger EA, Mutasim DF. Hidradenitis suppurativa complicated by severe lymphedema and lymphangiectasias. J Am Acad Dermatol. 2011;6:1223-1224.
- Piernick DM 2nd, Mahmood SH, Daveluy S. Acquired lymphangioma circumscriptum of the genitals in an individual with chronic hidradenitis suppurativa. JAAD Case Rep. 2018;1:64-66.
- Horn LC, Kühndel K, Pawlowitsch T, et al. Acquired lymphangioma circumscriptum of the vulva mimicking genital warts. Eur J Obstet Gynecol Reprod Biol. 2005;1:118-120.
- Elston DM, Ferringer T, Ko CJ, et al. Dermatopathology. 3rd ed. Elsevier; 2019.
- Kohorst JJ, Shah KK, Hallemeier CL, et al. Squamous cell carcinoma in perineal, perianal, and gluteal hidradenitis suppurativa: experience in 12 patients. Dermatol Surg. 2019;45:519-526.
- Patton KT, Deyrup AT, Weiss SW. Atypical vascular lesions after surgery and radiation of the breast: a clinicopathologic study of 32 cases analyzing histologic heterogeneity and association with angiosarcoma. Am J Surg Pathol. 2008;32:943-950.
- Ronen S, Ivan D, Torres-Cabala CA, et al. Post-radiation vascular lesions of the breast. J Cutan Pathol. 2019;46:52-58.
- Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier; 2018.
A 38-year-old woman with long-standing severe hidradenitis suppurativa presented to our dermatology clinic with an asymptomatic, slowly enlarging growth on the right labium majus of 2 years’ duration. She also had severe persistent drainage from nodules and sinus tracts involving the abdominal pannus, inguinal folds, vulva, perineum, buttocks, and upper thighs. After treatment failure with oral antibiotics and adalimumab, her regimen included infliximab-dyyb, chronic systemic steroids, spironolactone, topical clindamycin, and benzoyl peroxide, with plans for eventual surgical intervention. Physical examination revealed the patient had numerous pink papules coalescing into a plaque on the right labium majus. She also had innumerable papulonodules, sinus tracts, and indurated scars in the inguinal folds, genitalia, and perineal region from severe hidradenitis suppurativa.
Angioimmunoblastic T-cell Lymphoma Mimicking DRESS Syndrome
Angioimmunoblastic T-cell lymphoma (AITL) is a rare and aggressive lymphoma arising from follicular T-helper cells that predominantly affects older adults and carries a 5-year overall survival rate of 32%.1 Notably, as many as 50% of AITL patients present with a skin rash in addition to the more common but nonspecific acute-onset generalized lymphadenopathy, hepatosplenomegaly, and anemia.2 At presentation, most AITL patients are already at an advanced (III/IV) stage of disease.
Formerly known as angioimmunoblastic lymphadenopathy with dysproteinemia, AITL was once considered a benign entity that carried a risk for malignant transformation. As more cases have been identified and explored, this entity has been recategorized as a frank lymphoma.3 Therefore, it is critical that AITL be diagnosed and treated as early as possible.
We present the case of a 65-year-old man with clinical features that resembled drug reaction with eosinophilia and systemic symptoms (DRESS syndrome). After extensive workup, he was found to have AITL. This atypical case highlights the importance of maintaining a flexible differential diagnosis in patients with a persistent rash that does not improve with appropriate drug withdrawal and therapy.
Case Report
A 65-year-old Filipino man whose medical history was notable for hepatitis B that had been treated with entecavir for years without issue was admitted to the internal medicine service with fever of unknown origin and malaise of approximately 6 weeks’ duration. Six days prior to admission and 5 days after completing courses of the antiviral oseltamivir phosphate and amoxicillin for an upper respiratory tract infection and sinusitis, he developed worsening of an intermittently pruritic rash of approximately 1 month's duration. The dermatology department was consulted the day of hospital admission for evaluation of the rash. Chronic home medications included entecavir, lisinopril/hydrochlorothiazide, amlodipine, atorvastatin, metformin, salsalate, and over-the-counter nonsteroidal anti-inflammatory drugs (NSAIDs) as needed.
Physical examination was notable for mild erythema and scale distributed across the entire face; mild facial edema; and a blanchable, nonconfluent, macular erythema distributed across the trunk and upper and proximal lower extremities (Figure). In addition, the patient displayed conjunctival injection, pitting edema of the hands, and bilateral cervical and inguinal lymphadenopathy.
Laboratory tests revealed mild leukocytosis (11.6×109/L, [reference range, 4.0–10.5×109/L]), anemia (hemoglobin, 125 g/L (reference range, 138–170 g/L); hematocrit, 36.9%, [reference range, 40.0%–50.0%)], eosinophilia (1.07×109/L [reference range, 0.00–0.70×109/L)], hyponatremia, hypokalemia, and a mildly elevated creatinine level. Computed tomography and full-body positron-emission tomography (PET) scans during admission demonstrated diffuse lymphadenopathy. A skin biopsy from the left chest and a left inguinal lymph node biopsy also were performed.
Despite the lack of a clear medication trigger within the usual timeline for severe cutaneous drug-induced hypersensitivity reactions, DRESS syndrome was high on the differential diagnosis at the time of the initial presentation given the diffuse morbilliform eruption with pruritus, facial edema, eosinophilia, and lymphadenopathy.
Home medications were discontinued except for amlodipine, atorvastatin, and entecavir. The patient was treated symptomatically with topical steroids because it was believed that, if the clinical presentation represented DRESS syndrome, it was a mild variant that could be treated topically.4 His case was considered mild because of a lack of confirmed organ dysfunction and a mild protracted course.
After discharge following a 3-day inpatient stay, the patient was followed in the clinic weekly for 3 weeks without considerable change in the skin or laboratory findings. Discontinuation of entecavir was discussed and approved by his hepatologist.
Posthospitalization analysis of the punch biopsy specimen from the chest performed during the patient’s hospital stay revealed a superficial and deep dermal lymphoid infiltrate comprising CD3-, CD5-, and programmed cell death protein 1–positive cells with cytologic atypia in a perivascular distribution. Analysis of the lymph node biopsy specimen performed during the hospitalization showed effacement of the nodal architecture, a polymorphous lymphoid cell population with irregular nuclear contour, and abundant clear cytoplasm associated with high endothelial venules (HEVs). Cells of interest were positive for CD3, CD4, CD2, CD5, and CD7, with a subset staining positive for programmed cell death protein 1, inducible costimulator, CD10, and chemokine (C-X-C motif) ligand (CXCL) 13. CD21 demonstrated an expanded follicular dendritic cell meshwork in association with HEVs. Polymerase chain reaction revealed a clonal T-cell population. These findings of the skin and lymph node biopsies were consistent with AITL. Subsequent bone marrow biopsy with flow cytometry showed a normal CD4:CD8 ratio in T cells and no increase in natural killer cells.
Cyclophosphamide–hydroxydaunorubicin–Oncovin–prednisone (CHOP) chemotherapy was initiated; the patient completed a total of 6 cycles. He has had near resolution of the skin findings and is considered in remission based on a PET scan performed approximately 7 months after the initial presentation.
Comment
Angioimmunoblastic T-cell lymphoma is a rare peripheral T-cell lymphoma, part of a group of aggressive neoplasms that constitute approximately 15% of peripheral T-cell lymphomas and approximately 2% of non-Hodgkin lymphomas in adults worldwide.5 Cutaneous involvement occurs in approximately half of AITL cases and can be the first manifestation of disease.2 Skin findings are largely nonspecific, ranging from simple morbilliform rashes to erythroderma, at times manifesting with purpura.
Given this variability in the presentation of AITL, early diagnosis is challenging in the absence of more specific signs and symptoms.2 It can conceivably be mistaken for common entities such as viral exanthems or drug eruptions, depending on the history and context. DRESS syndrome, a T cell-mediated, delayed type-IV hypersensitivity drug reaction can present in a manner highly similar to that of AITL, with cutaneous involvement (diffuse morbilliform rash, fever, facial edema, and generalized lymphadenopathy) and variable systemic involvement. Laboratory findings of eosinophilia, atypical lymphocytes, and thrombocytopenia also might be seen in both entities.6 Furthermore, the AITL in our patient was accompanied by electrolyte disturbances that were concerning for syndrome of inappropriate antidiuretic hormone secretion, a rare complication of patients with DRESS syndrome complicated by encephalitis.7,8
Our patient met 4 RegiSCAR criteria for DRESS syndrome, warranting high clinical suspicion for an offending drug.9 DRESS syndrome can be caused by numerous medications—most commonly anticonvulsants, sulfonamides, antibiotics, allopurinol, and NSAIDs. A review of our patient’s medication list identified NSAIDs (including salsalate), entecavir, and amoxicillin, as possible culpable medications. Notably, the only new addition to the patient’s regimen was amoxicillin, which did not fit the typical 2- to 8-week timeline for a DRESS syndrome nidus.10 Our patient’s fever began well before the antibiotic was initiated, and skin findings appeared within 1 week after the course of amoxicillin was completed. Although there is documented variability in the latency of onset of DRESS syndrome following administration of a culprit medication,11 it is critical to maintain a broad differential diagnosis to allow for further diagnostic information to be obtained, especially when the medication timeline does not align with the clinical presentation.
DRESS syndrome is far more common than AITL. Similarities in their clinical presentation pose a substantial challenge and often cause a delay in the diagnosis of AITL, which is made by excisional tissue biopsy, most commonly of a lymph node, with assessment of morphology and immunophenotyping. Histologic assessment of tissue reveals a polymorphous infiltrate of variably sized atypical lymphocytes with prominent arborizing HEVs as well as expanded populations of follicular dendritic cells that can be detected by CD21 staining. Cells express CD3 and CD4, variably express BCL6 (B-cell lymphoma 6 antigen) and CD10, and also may have partial or complete loss of expression of a subset of pan T-cell antigens (CD2, CD3, CD5, and CD7).12-18
The treatment approach to AITL mirrors that of other nodal peripheral T-cell lymphomas, including chemotherapy and consideration of autologous stem-cell transplantation. Recent prospective trials of CHOP and CHOP-like chemotherapy have reported 3-year event-free survival and overall survival rates of 50% and 68%, respectively.19 Novel chemotherapeutic targets and gene-expression profiling are being investigated as potential therapeutic avenues.20
Conclusion
DRESS syndrome and AITL can have near-identical presentations. Clinicians should maintain a high index of suspicion for AITL in patients with presumed DRESS syndrome whose rash does not improve with appropriate drug withdrawal and steroid therapy or who lack a strong offending medication history. In such cases, skin and lymph node biopsies should be performed as early as possible to evaluate for AITL and so that appropriate therapy can be initiated.
- Federico M, Rudiger T, Bellei M, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the international peripheral T-cell lymphoma project. J Clin Oncol. 2013;31:240-246. doi:10.1200/JCO.2011.37.3647
- Botros N, Cerroni L, Shawwa A, et al. Cutaneous manifestations of angioimmunoblastic T-cell lymphoma: clinical and pathological characteristics. Am J Dermatopathol. 2015;37:274-283. doi:10.1097/DAD.0000000000000144
- Sachsida-Colombo E, Barbosa Mariano LC, Bastos FQ, et al. A difficult case of angioimmunoblastic T-cell lymphoma to diagnose. Rev Bras Hematol Hemoter. 2016;38:82-85. doi:10.1016/j.bjhh.2015.11.002
- Funck-Brentano E, Duong T-A, Bouvresse S, et al. Therapeutic management of DRESS: a retrospective study of 38 cases. J Am Acad Dermatol. 2015;72:246-252. doi:10.1016/j.jaad.2014.10.032
- Lunning MA, Vose JM. Angioimmunoblastic T-cell lymphoma: the many-faced lymphoma. Blood. 2017;129:1095-1102. doi:10.1182/blood-2016-09-692541
- Sato R, Itoh M, Suzuki H, et al. Pathological findings of lymphadenopathy in drug-induced hypersensitivity syndrome (DIHS)/drug reaction with eosinophilia and systemic syndrome (DRESS): similarities with angioimmunoblastic T-cell lymphoma. Eur J Dermatol. 2017;27:201-202. doi:10.1684/ejd.2016.2954
- Osizik L, Tanriover MD, Saka E. Autoimmune limbic encephalitis and syndrome of inappropriate antidiuretic hormone secretion associated with lamotrigine-induced drug rash with eosinophilia and systemic symptoms (DRESS) syndrome. Intern Med. 2015;55:1393-1396. doi:10.2169/internalmedicine.55.6035
- Sakuma K, Kano Y, Fukuhara M, et al. Syndrome of inappropriate secretion of antidiuretic hormone associated with limbic encephalitis in a patient with drug-induced hypersensitivity syndrome. Clin Exp Dermatol. 2008;33:287-290. doi:10.1111/j.1365-2230.2007.02645.x
- Pannu AK, Saroch A. Diagnostic criteria for drug rash and eosinophilia with systemic symptoms. J Family Med Prim Care. 2017;6:693-694. doi:10.4103/2249-4863.222050
- Kardaun SH, Sekula P, Valeyrie-Allanore L, et al; RegiSCAR study group. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071-1080. doi:10.1111/bjd.12501
- Soria A, Bernier C, Veyrac G, et al. Drug reaction with eosinophilia and systemic symptoms may occur within 2 weeks of drug exposure: a retrospective study. J Am Acad Dermatol. 2020;82:606.
- Loghavi S, Wang SA, Medeiros LJ, et al. Immunophenotypic and diagnostic characterization of angioimmunoblastic T-cell lymphoma by advanced flow cytometric technology. Leuk Lymphoma. 2016;57:2804-2812. doi:10.3109/10428194.2016.1170827
- Lee S-S, R, Odenwald T, et al. Angioimmunoblastic T cell lymphoma is derived from mature T-helper cells with varying expression and loss of detectable CD4. Int J Cancer. 2003;103:12-20. doi:10.1002/ijc.10758
- Feller AC, Griesser H, Schilling CV, et al. Clonal gene rearrangement patterns correlate with immunophenotype and clinical parameters in patients with angioimmunoblastic lymphadenopathy. Am J Pathol. 1988;133:549-556.
- Swerdlow SH, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; 2008.
- Attygalle A, Al-Jehani R, Diss TC, et al. Neoplastic T cells in angioimmunoblastic T-cell lymphoma express CD10. Blood. 2002;99:627-633. doi:10.1182/blood.v99.2.627
- Mourad N, Mounier N, J, et al; Groupe d’Etude des Lymphomes de l’Adulte. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood. 2008;111:4463-4470. doi:10.1182/blood-2007-08-105759
- Marafioti T, Paterson JC, Ballabio E, et al. The inducible T-cell co-stimulator molecule is expressed on subsets of T cells and is a new marker of lymphomas of T follicular helper cell-derivation. Haematologica. 2010;95:432-439. doi:10.3324/haematol.2009.010991
- Schmitz N, L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients withT-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116:3418-3425. doi:10.1182/blood-2010-02-270785
- Moskowitz AJ. Practical treatment approach for angioimmunoblastic T-cell lymphoma. J Oncol Pract. 2019;15:137-143. doi:10.1200/JOP.18.00511
Angioimmunoblastic T-cell lymphoma (AITL) is a rare and aggressive lymphoma arising from follicular T-helper cells that predominantly affects older adults and carries a 5-year overall survival rate of 32%.1 Notably, as many as 50% of AITL patients present with a skin rash in addition to the more common but nonspecific acute-onset generalized lymphadenopathy, hepatosplenomegaly, and anemia.2 At presentation, most AITL patients are already at an advanced (III/IV) stage of disease.
Formerly known as angioimmunoblastic lymphadenopathy with dysproteinemia, AITL was once considered a benign entity that carried a risk for malignant transformation. As more cases have been identified and explored, this entity has been recategorized as a frank lymphoma.3 Therefore, it is critical that AITL be diagnosed and treated as early as possible.
We present the case of a 65-year-old man with clinical features that resembled drug reaction with eosinophilia and systemic symptoms (DRESS syndrome). After extensive workup, he was found to have AITL. This atypical case highlights the importance of maintaining a flexible differential diagnosis in patients with a persistent rash that does not improve with appropriate drug withdrawal and therapy.
Case Report
A 65-year-old Filipino man whose medical history was notable for hepatitis B that had been treated with entecavir for years without issue was admitted to the internal medicine service with fever of unknown origin and malaise of approximately 6 weeks’ duration. Six days prior to admission and 5 days after completing courses of the antiviral oseltamivir phosphate and amoxicillin for an upper respiratory tract infection and sinusitis, he developed worsening of an intermittently pruritic rash of approximately 1 month's duration. The dermatology department was consulted the day of hospital admission for evaluation of the rash. Chronic home medications included entecavir, lisinopril/hydrochlorothiazide, amlodipine, atorvastatin, metformin, salsalate, and over-the-counter nonsteroidal anti-inflammatory drugs (NSAIDs) as needed.
Physical examination was notable for mild erythema and scale distributed across the entire face; mild facial edema; and a blanchable, nonconfluent, macular erythema distributed across the trunk and upper and proximal lower extremities (Figure). In addition, the patient displayed conjunctival injection, pitting edema of the hands, and bilateral cervical and inguinal lymphadenopathy.
Laboratory tests revealed mild leukocytosis (11.6×109/L, [reference range, 4.0–10.5×109/L]), anemia (hemoglobin, 125 g/L (reference range, 138–170 g/L); hematocrit, 36.9%, [reference range, 40.0%–50.0%)], eosinophilia (1.07×109/L [reference range, 0.00–0.70×109/L)], hyponatremia, hypokalemia, and a mildly elevated creatinine level. Computed tomography and full-body positron-emission tomography (PET) scans during admission demonstrated diffuse lymphadenopathy. A skin biopsy from the left chest and a left inguinal lymph node biopsy also were performed.
Despite the lack of a clear medication trigger within the usual timeline for severe cutaneous drug-induced hypersensitivity reactions, DRESS syndrome was high on the differential diagnosis at the time of the initial presentation given the diffuse morbilliform eruption with pruritus, facial edema, eosinophilia, and lymphadenopathy.
Home medications were discontinued except for amlodipine, atorvastatin, and entecavir. The patient was treated symptomatically with topical steroids because it was believed that, if the clinical presentation represented DRESS syndrome, it was a mild variant that could be treated topically.4 His case was considered mild because of a lack of confirmed organ dysfunction and a mild protracted course.
After discharge following a 3-day inpatient stay, the patient was followed in the clinic weekly for 3 weeks without considerable change in the skin or laboratory findings. Discontinuation of entecavir was discussed and approved by his hepatologist.
Posthospitalization analysis of the punch biopsy specimen from the chest performed during the patient’s hospital stay revealed a superficial and deep dermal lymphoid infiltrate comprising CD3-, CD5-, and programmed cell death protein 1–positive cells with cytologic atypia in a perivascular distribution. Analysis of the lymph node biopsy specimen performed during the hospitalization showed effacement of the nodal architecture, a polymorphous lymphoid cell population with irregular nuclear contour, and abundant clear cytoplasm associated with high endothelial venules (HEVs). Cells of interest were positive for CD3, CD4, CD2, CD5, and CD7, with a subset staining positive for programmed cell death protein 1, inducible costimulator, CD10, and chemokine (C-X-C motif) ligand (CXCL) 13. CD21 demonstrated an expanded follicular dendritic cell meshwork in association with HEVs. Polymerase chain reaction revealed a clonal T-cell population. These findings of the skin and lymph node biopsies were consistent with AITL. Subsequent bone marrow biopsy with flow cytometry showed a normal CD4:CD8 ratio in T cells and no increase in natural killer cells.
Cyclophosphamide–hydroxydaunorubicin–Oncovin–prednisone (CHOP) chemotherapy was initiated; the patient completed a total of 6 cycles. He has had near resolution of the skin findings and is considered in remission based on a PET scan performed approximately 7 months after the initial presentation.
Comment
Angioimmunoblastic T-cell lymphoma is a rare peripheral T-cell lymphoma, part of a group of aggressive neoplasms that constitute approximately 15% of peripheral T-cell lymphomas and approximately 2% of non-Hodgkin lymphomas in adults worldwide.5 Cutaneous involvement occurs in approximately half of AITL cases and can be the first manifestation of disease.2 Skin findings are largely nonspecific, ranging from simple morbilliform rashes to erythroderma, at times manifesting with purpura.
Given this variability in the presentation of AITL, early diagnosis is challenging in the absence of more specific signs and symptoms.2 It can conceivably be mistaken for common entities such as viral exanthems or drug eruptions, depending on the history and context. DRESS syndrome, a T cell-mediated, delayed type-IV hypersensitivity drug reaction can present in a manner highly similar to that of AITL, with cutaneous involvement (diffuse morbilliform rash, fever, facial edema, and generalized lymphadenopathy) and variable systemic involvement. Laboratory findings of eosinophilia, atypical lymphocytes, and thrombocytopenia also might be seen in both entities.6 Furthermore, the AITL in our patient was accompanied by electrolyte disturbances that were concerning for syndrome of inappropriate antidiuretic hormone secretion, a rare complication of patients with DRESS syndrome complicated by encephalitis.7,8
Our patient met 4 RegiSCAR criteria for DRESS syndrome, warranting high clinical suspicion for an offending drug.9 DRESS syndrome can be caused by numerous medications—most commonly anticonvulsants, sulfonamides, antibiotics, allopurinol, and NSAIDs. A review of our patient’s medication list identified NSAIDs (including salsalate), entecavir, and amoxicillin, as possible culpable medications. Notably, the only new addition to the patient’s regimen was amoxicillin, which did not fit the typical 2- to 8-week timeline for a DRESS syndrome nidus.10 Our patient’s fever began well before the antibiotic was initiated, and skin findings appeared within 1 week after the course of amoxicillin was completed. Although there is documented variability in the latency of onset of DRESS syndrome following administration of a culprit medication,11 it is critical to maintain a broad differential diagnosis to allow for further diagnostic information to be obtained, especially when the medication timeline does not align with the clinical presentation.
DRESS syndrome is far more common than AITL. Similarities in their clinical presentation pose a substantial challenge and often cause a delay in the diagnosis of AITL, which is made by excisional tissue biopsy, most commonly of a lymph node, with assessment of morphology and immunophenotyping. Histologic assessment of tissue reveals a polymorphous infiltrate of variably sized atypical lymphocytes with prominent arborizing HEVs as well as expanded populations of follicular dendritic cells that can be detected by CD21 staining. Cells express CD3 and CD4, variably express BCL6 (B-cell lymphoma 6 antigen) and CD10, and also may have partial or complete loss of expression of a subset of pan T-cell antigens (CD2, CD3, CD5, and CD7).12-18
The treatment approach to AITL mirrors that of other nodal peripheral T-cell lymphomas, including chemotherapy and consideration of autologous stem-cell transplantation. Recent prospective trials of CHOP and CHOP-like chemotherapy have reported 3-year event-free survival and overall survival rates of 50% and 68%, respectively.19 Novel chemotherapeutic targets and gene-expression profiling are being investigated as potential therapeutic avenues.20
Conclusion
DRESS syndrome and AITL can have near-identical presentations. Clinicians should maintain a high index of suspicion for AITL in patients with presumed DRESS syndrome whose rash does not improve with appropriate drug withdrawal and steroid therapy or who lack a strong offending medication history. In such cases, skin and lymph node biopsies should be performed as early as possible to evaluate for AITL and so that appropriate therapy can be initiated.
Angioimmunoblastic T-cell lymphoma (AITL) is a rare and aggressive lymphoma arising from follicular T-helper cells that predominantly affects older adults and carries a 5-year overall survival rate of 32%.1 Notably, as many as 50% of AITL patients present with a skin rash in addition to the more common but nonspecific acute-onset generalized lymphadenopathy, hepatosplenomegaly, and anemia.2 At presentation, most AITL patients are already at an advanced (III/IV) stage of disease.
Formerly known as angioimmunoblastic lymphadenopathy with dysproteinemia, AITL was once considered a benign entity that carried a risk for malignant transformation. As more cases have been identified and explored, this entity has been recategorized as a frank lymphoma.3 Therefore, it is critical that AITL be diagnosed and treated as early as possible.
We present the case of a 65-year-old man with clinical features that resembled drug reaction with eosinophilia and systemic symptoms (DRESS syndrome). After extensive workup, he was found to have AITL. This atypical case highlights the importance of maintaining a flexible differential diagnosis in patients with a persistent rash that does not improve with appropriate drug withdrawal and therapy.
Case Report
A 65-year-old Filipino man whose medical history was notable for hepatitis B that had been treated with entecavir for years without issue was admitted to the internal medicine service with fever of unknown origin and malaise of approximately 6 weeks’ duration. Six days prior to admission and 5 days after completing courses of the antiviral oseltamivir phosphate and amoxicillin for an upper respiratory tract infection and sinusitis, he developed worsening of an intermittently pruritic rash of approximately 1 month's duration. The dermatology department was consulted the day of hospital admission for evaluation of the rash. Chronic home medications included entecavir, lisinopril/hydrochlorothiazide, amlodipine, atorvastatin, metformin, salsalate, and over-the-counter nonsteroidal anti-inflammatory drugs (NSAIDs) as needed.
Physical examination was notable for mild erythema and scale distributed across the entire face; mild facial edema; and a blanchable, nonconfluent, macular erythema distributed across the trunk and upper and proximal lower extremities (Figure). In addition, the patient displayed conjunctival injection, pitting edema of the hands, and bilateral cervical and inguinal lymphadenopathy.
Laboratory tests revealed mild leukocytosis (11.6×109/L, [reference range, 4.0–10.5×109/L]), anemia (hemoglobin, 125 g/L (reference range, 138–170 g/L); hematocrit, 36.9%, [reference range, 40.0%–50.0%)], eosinophilia (1.07×109/L [reference range, 0.00–0.70×109/L)], hyponatremia, hypokalemia, and a mildly elevated creatinine level. Computed tomography and full-body positron-emission tomography (PET) scans during admission demonstrated diffuse lymphadenopathy. A skin biopsy from the left chest and a left inguinal lymph node biopsy also were performed.
Despite the lack of a clear medication trigger within the usual timeline for severe cutaneous drug-induced hypersensitivity reactions, DRESS syndrome was high on the differential diagnosis at the time of the initial presentation given the diffuse morbilliform eruption with pruritus, facial edema, eosinophilia, and lymphadenopathy.
Home medications were discontinued except for amlodipine, atorvastatin, and entecavir. The patient was treated symptomatically with topical steroids because it was believed that, if the clinical presentation represented DRESS syndrome, it was a mild variant that could be treated topically.4 His case was considered mild because of a lack of confirmed organ dysfunction and a mild protracted course.
After discharge following a 3-day inpatient stay, the patient was followed in the clinic weekly for 3 weeks without considerable change in the skin or laboratory findings. Discontinuation of entecavir was discussed and approved by his hepatologist.
Posthospitalization analysis of the punch biopsy specimen from the chest performed during the patient’s hospital stay revealed a superficial and deep dermal lymphoid infiltrate comprising CD3-, CD5-, and programmed cell death protein 1–positive cells with cytologic atypia in a perivascular distribution. Analysis of the lymph node biopsy specimen performed during the hospitalization showed effacement of the nodal architecture, a polymorphous lymphoid cell population with irregular nuclear contour, and abundant clear cytoplasm associated with high endothelial venules (HEVs). Cells of interest were positive for CD3, CD4, CD2, CD5, and CD7, with a subset staining positive for programmed cell death protein 1, inducible costimulator, CD10, and chemokine (C-X-C motif) ligand (CXCL) 13. CD21 demonstrated an expanded follicular dendritic cell meshwork in association with HEVs. Polymerase chain reaction revealed a clonal T-cell population. These findings of the skin and lymph node biopsies were consistent with AITL. Subsequent bone marrow biopsy with flow cytometry showed a normal CD4:CD8 ratio in T cells and no increase in natural killer cells.
Cyclophosphamide–hydroxydaunorubicin–Oncovin–prednisone (CHOP) chemotherapy was initiated; the patient completed a total of 6 cycles. He has had near resolution of the skin findings and is considered in remission based on a PET scan performed approximately 7 months after the initial presentation.
Comment
Angioimmunoblastic T-cell lymphoma is a rare peripheral T-cell lymphoma, part of a group of aggressive neoplasms that constitute approximately 15% of peripheral T-cell lymphomas and approximately 2% of non-Hodgkin lymphomas in adults worldwide.5 Cutaneous involvement occurs in approximately half of AITL cases and can be the first manifestation of disease.2 Skin findings are largely nonspecific, ranging from simple morbilliform rashes to erythroderma, at times manifesting with purpura.
Given this variability in the presentation of AITL, early diagnosis is challenging in the absence of more specific signs and symptoms.2 It can conceivably be mistaken for common entities such as viral exanthems or drug eruptions, depending on the history and context. DRESS syndrome, a T cell-mediated, delayed type-IV hypersensitivity drug reaction can present in a manner highly similar to that of AITL, with cutaneous involvement (diffuse morbilliform rash, fever, facial edema, and generalized lymphadenopathy) and variable systemic involvement. Laboratory findings of eosinophilia, atypical lymphocytes, and thrombocytopenia also might be seen in both entities.6 Furthermore, the AITL in our patient was accompanied by electrolyte disturbances that were concerning for syndrome of inappropriate antidiuretic hormone secretion, a rare complication of patients with DRESS syndrome complicated by encephalitis.7,8
Our patient met 4 RegiSCAR criteria for DRESS syndrome, warranting high clinical suspicion for an offending drug.9 DRESS syndrome can be caused by numerous medications—most commonly anticonvulsants, sulfonamides, antibiotics, allopurinol, and NSAIDs. A review of our patient’s medication list identified NSAIDs (including salsalate), entecavir, and amoxicillin, as possible culpable medications. Notably, the only new addition to the patient’s regimen was amoxicillin, which did not fit the typical 2- to 8-week timeline for a DRESS syndrome nidus.10 Our patient’s fever began well before the antibiotic was initiated, and skin findings appeared within 1 week after the course of amoxicillin was completed. Although there is documented variability in the latency of onset of DRESS syndrome following administration of a culprit medication,11 it is critical to maintain a broad differential diagnosis to allow for further diagnostic information to be obtained, especially when the medication timeline does not align with the clinical presentation.
DRESS syndrome is far more common than AITL. Similarities in their clinical presentation pose a substantial challenge and often cause a delay in the diagnosis of AITL, which is made by excisional tissue biopsy, most commonly of a lymph node, with assessment of morphology and immunophenotyping. Histologic assessment of tissue reveals a polymorphous infiltrate of variably sized atypical lymphocytes with prominent arborizing HEVs as well as expanded populations of follicular dendritic cells that can be detected by CD21 staining. Cells express CD3 and CD4, variably express BCL6 (B-cell lymphoma 6 antigen) and CD10, and also may have partial or complete loss of expression of a subset of pan T-cell antigens (CD2, CD3, CD5, and CD7).12-18
The treatment approach to AITL mirrors that of other nodal peripheral T-cell lymphomas, including chemotherapy and consideration of autologous stem-cell transplantation. Recent prospective trials of CHOP and CHOP-like chemotherapy have reported 3-year event-free survival and overall survival rates of 50% and 68%, respectively.19 Novel chemotherapeutic targets and gene-expression profiling are being investigated as potential therapeutic avenues.20
Conclusion
DRESS syndrome and AITL can have near-identical presentations. Clinicians should maintain a high index of suspicion for AITL in patients with presumed DRESS syndrome whose rash does not improve with appropriate drug withdrawal and steroid therapy or who lack a strong offending medication history. In such cases, skin and lymph node biopsies should be performed as early as possible to evaluate for AITL and so that appropriate therapy can be initiated.
- Federico M, Rudiger T, Bellei M, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the international peripheral T-cell lymphoma project. J Clin Oncol. 2013;31:240-246. doi:10.1200/JCO.2011.37.3647
- Botros N, Cerroni L, Shawwa A, et al. Cutaneous manifestations of angioimmunoblastic T-cell lymphoma: clinical and pathological characteristics. Am J Dermatopathol. 2015;37:274-283. doi:10.1097/DAD.0000000000000144
- Sachsida-Colombo E, Barbosa Mariano LC, Bastos FQ, et al. A difficult case of angioimmunoblastic T-cell lymphoma to diagnose. Rev Bras Hematol Hemoter. 2016;38:82-85. doi:10.1016/j.bjhh.2015.11.002
- Funck-Brentano E, Duong T-A, Bouvresse S, et al. Therapeutic management of DRESS: a retrospective study of 38 cases. J Am Acad Dermatol. 2015;72:246-252. doi:10.1016/j.jaad.2014.10.032
- Lunning MA, Vose JM. Angioimmunoblastic T-cell lymphoma: the many-faced lymphoma. Blood. 2017;129:1095-1102. doi:10.1182/blood-2016-09-692541
- Sato R, Itoh M, Suzuki H, et al. Pathological findings of lymphadenopathy in drug-induced hypersensitivity syndrome (DIHS)/drug reaction with eosinophilia and systemic syndrome (DRESS): similarities with angioimmunoblastic T-cell lymphoma. Eur J Dermatol. 2017;27:201-202. doi:10.1684/ejd.2016.2954
- Osizik L, Tanriover MD, Saka E. Autoimmune limbic encephalitis and syndrome of inappropriate antidiuretic hormone secretion associated with lamotrigine-induced drug rash with eosinophilia and systemic symptoms (DRESS) syndrome. Intern Med. 2015;55:1393-1396. doi:10.2169/internalmedicine.55.6035
- Sakuma K, Kano Y, Fukuhara M, et al. Syndrome of inappropriate secretion of antidiuretic hormone associated with limbic encephalitis in a patient with drug-induced hypersensitivity syndrome. Clin Exp Dermatol. 2008;33:287-290. doi:10.1111/j.1365-2230.2007.02645.x
- Pannu AK, Saroch A. Diagnostic criteria for drug rash and eosinophilia with systemic symptoms. J Family Med Prim Care. 2017;6:693-694. doi:10.4103/2249-4863.222050
- Kardaun SH, Sekula P, Valeyrie-Allanore L, et al; RegiSCAR study group. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071-1080. doi:10.1111/bjd.12501
- Soria A, Bernier C, Veyrac G, et al. Drug reaction with eosinophilia and systemic symptoms may occur within 2 weeks of drug exposure: a retrospective study. J Am Acad Dermatol. 2020;82:606.
- Loghavi S, Wang SA, Medeiros LJ, et al. Immunophenotypic and diagnostic characterization of angioimmunoblastic T-cell lymphoma by advanced flow cytometric technology. Leuk Lymphoma. 2016;57:2804-2812. doi:10.3109/10428194.2016.1170827
- Lee S-S, R, Odenwald T, et al. Angioimmunoblastic T cell lymphoma is derived from mature T-helper cells with varying expression and loss of detectable CD4. Int J Cancer. 2003;103:12-20. doi:10.1002/ijc.10758
- Feller AC, Griesser H, Schilling CV, et al. Clonal gene rearrangement patterns correlate with immunophenotype and clinical parameters in patients with angioimmunoblastic lymphadenopathy. Am J Pathol. 1988;133:549-556.
- Swerdlow SH, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; 2008.
- Attygalle A, Al-Jehani R, Diss TC, et al. Neoplastic T cells in angioimmunoblastic T-cell lymphoma express CD10. Blood. 2002;99:627-633. doi:10.1182/blood.v99.2.627
- Mourad N, Mounier N, J, et al; Groupe d’Etude des Lymphomes de l’Adulte. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood. 2008;111:4463-4470. doi:10.1182/blood-2007-08-105759
- Marafioti T, Paterson JC, Ballabio E, et al. The inducible T-cell co-stimulator molecule is expressed on subsets of T cells and is a new marker of lymphomas of T follicular helper cell-derivation. Haematologica. 2010;95:432-439. doi:10.3324/haematol.2009.010991
- Schmitz N, L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients withT-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116:3418-3425. doi:10.1182/blood-2010-02-270785
- Moskowitz AJ. Practical treatment approach for angioimmunoblastic T-cell lymphoma. J Oncol Pract. 2019;15:137-143. doi:10.1200/JOP.18.00511
- Federico M, Rudiger T, Bellei M, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the international peripheral T-cell lymphoma project. J Clin Oncol. 2013;31:240-246. doi:10.1200/JCO.2011.37.3647
- Botros N, Cerroni L, Shawwa A, et al. Cutaneous manifestations of angioimmunoblastic T-cell lymphoma: clinical and pathological characteristics. Am J Dermatopathol. 2015;37:274-283. doi:10.1097/DAD.0000000000000144
- Sachsida-Colombo E, Barbosa Mariano LC, Bastos FQ, et al. A difficult case of angioimmunoblastic T-cell lymphoma to diagnose. Rev Bras Hematol Hemoter. 2016;38:82-85. doi:10.1016/j.bjhh.2015.11.002
- Funck-Brentano E, Duong T-A, Bouvresse S, et al. Therapeutic management of DRESS: a retrospective study of 38 cases. J Am Acad Dermatol. 2015;72:246-252. doi:10.1016/j.jaad.2014.10.032
- Lunning MA, Vose JM. Angioimmunoblastic T-cell lymphoma: the many-faced lymphoma. Blood. 2017;129:1095-1102. doi:10.1182/blood-2016-09-692541
- Sato R, Itoh M, Suzuki H, et al. Pathological findings of lymphadenopathy in drug-induced hypersensitivity syndrome (DIHS)/drug reaction with eosinophilia and systemic syndrome (DRESS): similarities with angioimmunoblastic T-cell lymphoma. Eur J Dermatol. 2017;27:201-202. doi:10.1684/ejd.2016.2954
- Osizik L, Tanriover MD, Saka E. Autoimmune limbic encephalitis and syndrome of inappropriate antidiuretic hormone secretion associated with lamotrigine-induced drug rash with eosinophilia and systemic symptoms (DRESS) syndrome. Intern Med. 2015;55:1393-1396. doi:10.2169/internalmedicine.55.6035
- Sakuma K, Kano Y, Fukuhara M, et al. Syndrome of inappropriate secretion of antidiuretic hormone associated with limbic encephalitis in a patient with drug-induced hypersensitivity syndrome. Clin Exp Dermatol. 2008;33:287-290. doi:10.1111/j.1365-2230.2007.02645.x
- Pannu AK, Saroch A. Diagnostic criteria for drug rash and eosinophilia with systemic symptoms. J Family Med Prim Care. 2017;6:693-694. doi:10.4103/2249-4863.222050
- Kardaun SH, Sekula P, Valeyrie-Allanore L, et al; RegiSCAR study group. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071-1080. doi:10.1111/bjd.12501
- Soria A, Bernier C, Veyrac G, et al. Drug reaction with eosinophilia and systemic symptoms may occur within 2 weeks of drug exposure: a retrospective study. J Am Acad Dermatol. 2020;82:606.
- Loghavi S, Wang SA, Medeiros LJ, et al. Immunophenotypic and diagnostic characterization of angioimmunoblastic T-cell lymphoma by advanced flow cytometric technology. Leuk Lymphoma. 2016;57:2804-2812. doi:10.3109/10428194.2016.1170827
- Lee S-S, R, Odenwald T, et al. Angioimmunoblastic T cell lymphoma is derived from mature T-helper cells with varying expression and loss of detectable CD4. Int J Cancer. 2003;103:12-20. doi:10.1002/ijc.10758
- Feller AC, Griesser H, Schilling CV, et al. Clonal gene rearrangement patterns correlate with immunophenotype and clinical parameters in patients with angioimmunoblastic lymphadenopathy. Am J Pathol. 1988;133:549-556.
- Swerdlow SH, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; 2008.
- Attygalle A, Al-Jehani R, Diss TC, et al. Neoplastic T cells in angioimmunoblastic T-cell lymphoma express CD10. Blood. 2002;99:627-633. doi:10.1182/blood.v99.2.627
- Mourad N, Mounier N, J, et al; Groupe d’Etude des Lymphomes de l’Adulte. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood. 2008;111:4463-4470. doi:10.1182/blood-2007-08-105759
- Marafioti T, Paterson JC, Ballabio E, et al. The inducible T-cell co-stimulator molecule is expressed on subsets of T cells and is a new marker of lymphomas of T follicular helper cell-derivation. Haematologica. 2010;95:432-439. doi:10.3324/haematol.2009.010991
- Schmitz N, L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients withT-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116:3418-3425. doi:10.1182/blood-2010-02-270785
- Moskowitz AJ. Practical treatment approach for angioimmunoblastic T-cell lymphoma. J Oncol Pract. 2019;15:137-143. doi:10.1200/JOP.18.00511
Practice Points
- It is important to maintain a high index of suspicion for angioimmunoblastic T-cell lymphoma in older patients with a longstanding rash and no clear culprit for drug reaction with eosinophilia and systemic symptoms (DRESS syndrome).
- Consider performing a lymph node biopsy early in the course of disease in patients with presumed DRESS syndrome who do not improve with drug withdrawal and steroid therapy.
Inpatient Dermatology Consultations for Suspected Skin Cancer: A Retrospective Review
To the Editor:
Dermatologists sometimes are consulted in the inpatient setting to rule out possible skin cancer. This scenario provides an opportunity to facilitate the diagnosis and treatment of cutaneous malignancy, often in patients who might not have sought regular outpatient dermatology care. Few studies have described the outcomes of inpatient biopsies to identify skin cancer.1,2
Seeking to better understand the nature of these patient encounters, we reviewed all consultations at a medical center for which the referring physician suspected skin cancer rather than only those lesions that were biopsied by the dermatologist. We also collected data about subsequent treatment to better understand the outcomes of these patient encounters.
We conducted a retrospective review of inpatient dermatology referrals at an academic-affiliated tertiary medical center. We identified all patients who were provided with an inpatient dermatology consultation for suspected skin cancer or what was identified as a “skin lesion” between July 1, 2013, and July 1, 2019. We collected information on each patient’s sex, age at time of consultation, and race, as well as the specialty of the referring provider, lesion location, maximum diameter of the lesion, whether a biopsy was performed, where the biopsy was performed (inpatient or outpatient setting), clinical diagnosis, histopathologic diagnosis, and subsequent treatment.
The institutional review board at Eastern Virginia Medical School (Norfolk, Virginia) approved this study, and all protocol conformed to the ethical guidelines of the Declaration of Helsinki.
Thirty-eight patients met the inclusion criteria. Their characteristics are listed in the Table. Consultations for possible skin cancer accounted for 4% (38/950) of all inpatient dermatology consultations over the study period. Outcomes of the referrals are shown in the Figure. Consultations were received from 12 different physician specialties.
In the 38 patients, 47 lesions were identified; most (66% [31/47]) were on the head and neck. Twenty of 38 patients were found to have at least 1 biopsy-confirmed cutaneous malignancy (23 total tumors). Of those 23 identified malignancies, 10 were basal cell carcinoma, 11 squamous cell carcinoma, 1 malignant melanoma, and 1 anaplastic T-cell lymphoma. Of note, 17 of 23 (74%) identified cutaneous malignancies were 2.0 cm in diameter at biopsy or larger. Subsequently performed treatments for these patients included wide local excision (n=3), Mohs micrographic surgery (n=5), radiation therapy (n=3), topical fluorouracil (n=1), electrodesiccation and curettage (n=4), and chemotherapy or immunotherapy (n=2). Two patients who were diagnosed with skin cancer died of unrelated causes before treatment was completed.
In 10 of 38 patients, only nonmalignant entities were diagnosed, including seborrheic keratosis (n=6), benign melanocytic nevus (n=1), epidermal inclusion cyst (n=1), actinic keratosis (n=1), and radiation-induced necrosis (n=1). Of the 8 remaining patients, 4 were ultimately lost to follow-up before planned outpatient biopsy could be completed; 1 opted to follow up for biopsy at an unaffiliated outpatient dermatology provider. For 2 patients, the decision was made to forgo biopsy despite clinical suspicion of skin cancer because of overall poor health status, and 1 additional patient died before a planned outpatient biopsy could be performed.
In summary, approximately half of the inpatient dermatology consultations for suspected cutaneous malignancy resulted in a diagnosis of skin cancer. The patients in this population were admitted for a range of diagnoses, most unrelated to their cutaneous malignancy, suggesting that the inpatient setting offers the opportunity for physicians in a variety of specialties to help identify skin cancer that might otherwise be unaddressed and then facilitate management, whether ultimately in an inpatient or outpatient setting.
In many of these cases, it might be most appropriate to arrange subsequent outpatient dermatology follow-up after hospitalization, rather than making an inpatient consultation, as these situations usually are nonurgent and not directly related to hospitalization. However, in cases in which the lesion is directly related to admission, the lesion is advanced, there is concern for metastatic disease, or extenuating circumstances make outpatient follow-up difficult, inpatient dermatology consultation may be reasonable. There sometimes can be compelling reasons to expedite diagnosis and treatment as an inpatient.
In hospitalized, medically complex patients, in whom a new cutaneous malignancy is identified, dermatologists should discuss the situation thoughtfully with the patient, the patient’s family (when appropriate), and other physicians on the treatment team to determine the most appropriate course of action. In some cases, the most appropriate course might be to delay biopsy or treatment until the outpatient setting or to even defer further action completely when the prognosis is very limited. Consulting dermatologists must be mindful of patients’ overall medical situation in planning care for a cutaneous malignancy in these inpatient situations.
This study also highlights the surprising number of large-diameter, high-risk tumors identified in these scenarios. Limitations of this study include a relatively small sample size from a single facility that might not be representative of other practice settings and locations. Future multicenter studies could further explore the impact of inpatient dermatologic consultation on the diagnosis and management of skin cancer.
- Bauer J, Maroon M. Dermatology inpatient consultations: a retrospective study. J Am Acad Dermatol. 2010;62:518-519. doi:10.1016/j.jaad.2009.06.030
- Tsai S, Scott JF, Keller JJ, et al. Cutaneous malignancies identified in an inpatient dermatology consultation service. Br J Dermatol. 2017;177:E116-E118. doi:10.1111/bjd.15401
To the Editor:
Dermatologists sometimes are consulted in the inpatient setting to rule out possible skin cancer. This scenario provides an opportunity to facilitate the diagnosis and treatment of cutaneous malignancy, often in patients who might not have sought regular outpatient dermatology care. Few studies have described the outcomes of inpatient biopsies to identify skin cancer.1,2
Seeking to better understand the nature of these patient encounters, we reviewed all consultations at a medical center for which the referring physician suspected skin cancer rather than only those lesions that were biopsied by the dermatologist. We also collected data about subsequent treatment to better understand the outcomes of these patient encounters.
We conducted a retrospective review of inpatient dermatology referrals at an academic-affiliated tertiary medical center. We identified all patients who were provided with an inpatient dermatology consultation for suspected skin cancer or what was identified as a “skin lesion” between July 1, 2013, and July 1, 2019. We collected information on each patient’s sex, age at time of consultation, and race, as well as the specialty of the referring provider, lesion location, maximum diameter of the lesion, whether a biopsy was performed, where the biopsy was performed (inpatient or outpatient setting), clinical diagnosis, histopathologic diagnosis, and subsequent treatment.
The institutional review board at Eastern Virginia Medical School (Norfolk, Virginia) approved this study, and all protocol conformed to the ethical guidelines of the Declaration of Helsinki.
Thirty-eight patients met the inclusion criteria. Their characteristics are listed in the Table. Consultations for possible skin cancer accounted for 4% (38/950) of all inpatient dermatology consultations over the study period. Outcomes of the referrals are shown in the Figure. Consultations were received from 12 different physician specialties.
In the 38 patients, 47 lesions were identified; most (66% [31/47]) were on the head and neck. Twenty of 38 patients were found to have at least 1 biopsy-confirmed cutaneous malignancy (23 total tumors). Of those 23 identified malignancies, 10 were basal cell carcinoma, 11 squamous cell carcinoma, 1 malignant melanoma, and 1 anaplastic T-cell lymphoma. Of note, 17 of 23 (74%) identified cutaneous malignancies were 2.0 cm in diameter at biopsy or larger. Subsequently performed treatments for these patients included wide local excision (n=3), Mohs micrographic surgery (n=5), radiation therapy (n=3), topical fluorouracil (n=1), electrodesiccation and curettage (n=4), and chemotherapy or immunotherapy (n=2). Two patients who were diagnosed with skin cancer died of unrelated causes before treatment was completed.
In 10 of 38 patients, only nonmalignant entities were diagnosed, including seborrheic keratosis (n=6), benign melanocytic nevus (n=1), epidermal inclusion cyst (n=1), actinic keratosis (n=1), and radiation-induced necrosis (n=1). Of the 8 remaining patients, 4 were ultimately lost to follow-up before planned outpatient biopsy could be completed; 1 opted to follow up for biopsy at an unaffiliated outpatient dermatology provider. For 2 patients, the decision was made to forgo biopsy despite clinical suspicion of skin cancer because of overall poor health status, and 1 additional patient died before a planned outpatient biopsy could be performed.
In summary, approximately half of the inpatient dermatology consultations for suspected cutaneous malignancy resulted in a diagnosis of skin cancer. The patients in this population were admitted for a range of diagnoses, most unrelated to their cutaneous malignancy, suggesting that the inpatient setting offers the opportunity for physicians in a variety of specialties to help identify skin cancer that might otherwise be unaddressed and then facilitate management, whether ultimately in an inpatient or outpatient setting.
In many of these cases, it might be most appropriate to arrange subsequent outpatient dermatology follow-up after hospitalization, rather than making an inpatient consultation, as these situations usually are nonurgent and not directly related to hospitalization. However, in cases in which the lesion is directly related to admission, the lesion is advanced, there is concern for metastatic disease, or extenuating circumstances make outpatient follow-up difficult, inpatient dermatology consultation may be reasonable. There sometimes can be compelling reasons to expedite diagnosis and treatment as an inpatient.
In hospitalized, medically complex patients, in whom a new cutaneous malignancy is identified, dermatologists should discuss the situation thoughtfully with the patient, the patient’s family (when appropriate), and other physicians on the treatment team to determine the most appropriate course of action. In some cases, the most appropriate course might be to delay biopsy or treatment until the outpatient setting or to even defer further action completely when the prognosis is very limited. Consulting dermatologists must be mindful of patients’ overall medical situation in planning care for a cutaneous malignancy in these inpatient situations.
This study also highlights the surprising number of large-diameter, high-risk tumors identified in these scenarios. Limitations of this study include a relatively small sample size from a single facility that might not be representative of other practice settings and locations. Future multicenter studies could further explore the impact of inpatient dermatologic consultation on the diagnosis and management of skin cancer.
To the Editor:
Dermatologists sometimes are consulted in the inpatient setting to rule out possible skin cancer. This scenario provides an opportunity to facilitate the diagnosis and treatment of cutaneous malignancy, often in patients who might not have sought regular outpatient dermatology care. Few studies have described the outcomes of inpatient biopsies to identify skin cancer.1,2
Seeking to better understand the nature of these patient encounters, we reviewed all consultations at a medical center for which the referring physician suspected skin cancer rather than only those lesions that were biopsied by the dermatologist. We also collected data about subsequent treatment to better understand the outcomes of these patient encounters.
We conducted a retrospective review of inpatient dermatology referrals at an academic-affiliated tertiary medical center. We identified all patients who were provided with an inpatient dermatology consultation for suspected skin cancer or what was identified as a “skin lesion” between July 1, 2013, and July 1, 2019. We collected information on each patient’s sex, age at time of consultation, and race, as well as the specialty of the referring provider, lesion location, maximum diameter of the lesion, whether a biopsy was performed, where the biopsy was performed (inpatient or outpatient setting), clinical diagnosis, histopathologic diagnosis, and subsequent treatment.
The institutional review board at Eastern Virginia Medical School (Norfolk, Virginia) approved this study, and all protocol conformed to the ethical guidelines of the Declaration of Helsinki.
Thirty-eight patients met the inclusion criteria. Their characteristics are listed in the Table. Consultations for possible skin cancer accounted for 4% (38/950) of all inpatient dermatology consultations over the study period. Outcomes of the referrals are shown in the Figure. Consultations were received from 12 different physician specialties.
In the 38 patients, 47 lesions were identified; most (66% [31/47]) were on the head and neck. Twenty of 38 patients were found to have at least 1 biopsy-confirmed cutaneous malignancy (23 total tumors). Of those 23 identified malignancies, 10 were basal cell carcinoma, 11 squamous cell carcinoma, 1 malignant melanoma, and 1 anaplastic T-cell lymphoma. Of note, 17 of 23 (74%) identified cutaneous malignancies were 2.0 cm in diameter at biopsy or larger. Subsequently performed treatments for these patients included wide local excision (n=3), Mohs micrographic surgery (n=5), radiation therapy (n=3), topical fluorouracil (n=1), electrodesiccation and curettage (n=4), and chemotherapy or immunotherapy (n=2). Two patients who were diagnosed with skin cancer died of unrelated causes before treatment was completed.
In 10 of 38 patients, only nonmalignant entities were diagnosed, including seborrheic keratosis (n=6), benign melanocytic nevus (n=1), epidermal inclusion cyst (n=1), actinic keratosis (n=1), and radiation-induced necrosis (n=1). Of the 8 remaining patients, 4 were ultimately lost to follow-up before planned outpatient biopsy could be completed; 1 opted to follow up for biopsy at an unaffiliated outpatient dermatology provider. For 2 patients, the decision was made to forgo biopsy despite clinical suspicion of skin cancer because of overall poor health status, and 1 additional patient died before a planned outpatient biopsy could be performed.
In summary, approximately half of the inpatient dermatology consultations for suspected cutaneous malignancy resulted in a diagnosis of skin cancer. The patients in this population were admitted for a range of diagnoses, most unrelated to their cutaneous malignancy, suggesting that the inpatient setting offers the opportunity for physicians in a variety of specialties to help identify skin cancer that might otherwise be unaddressed and then facilitate management, whether ultimately in an inpatient or outpatient setting.
In many of these cases, it might be most appropriate to arrange subsequent outpatient dermatology follow-up after hospitalization, rather than making an inpatient consultation, as these situations usually are nonurgent and not directly related to hospitalization. However, in cases in which the lesion is directly related to admission, the lesion is advanced, there is concern for metastatic disease, or extenuating circumstances make outpatient follow-up difficult, inpatient dermatology consultation may be reasonable. There sometimes can be compelling reasons to expedite diagnosis and treatment as an inpatient.
In hospitalized, medically complex patients, in whom a new cutaneous malignancy is identified, dermatologists should discuss the situation thoughtfully with the patient, the patient’s family (when appropriate), and other physicians on the treatment team to determine the most appropriate course of action. In some cases, the most appropriate course might be to delay biopsy or treatment until the outpatient setting or to even defer further action completely when the prognosis is very limited. Consulting dermatologists must be mindful of patients’ overall medical situation in planning care for a cutaneous malignancy in these inpatient situations.
This study also highlights the surprising number of large-diameter, high-risk tumors identified in these scenarios. Limitations of this study include a relatively small sample size from a single facility that might not be representative of other practice settings and locations. Future multicenter studies could further explore the impact of inpatient dermatologic consultation on the diagnosis and management of skin cancer.
- Bauer J, Maroon M. Dermatology inpatient consultations: a retrospective study. J Am Acad Dermatol. 2010;62:518-519. doi:10.1016/j.jaad.2009.06.030
- Tsai S, Scott JF, Keller JJ, et al. Cutaneous malignancies identified in an inpatient dermatology consultation service. Br J Dermatol. 2017;177:E116-E118. doi:10.1111/bjd.15401
- Bauer J, Maroon M. Dermatology inpatient consultations: a retrospective study. J Am Acad Dermatol. 2010;62:518-519. doi:10.1016/j.jaad.2009.06.030
- Tsai S, Scott JF, Keller JJ, et al. Cutaneous malignancies identified in an inpatient dermatology consultation service. Br J Dermatol. 2017;177:E116-E118. doi:10.1111/bjd.15401
Practice Points
- Dermatologists who perform inpatient consultations should be prepared to be consulted for cutaneous malignancies.
- Relatively large skin tumors may be identified, often incidentally, in the inpatient population.
- Careful consideration should be involved when deciding how to diagnose and manage cutaneous malignancies identified in the inpatient setting, taking the overall medical and social context into account.