User login
Treating psoriasis with biologics: Recommendations from an expert
LAS VEGAS – If you’re considering adding biologics for psoriasis to your clinical practice, dermatologist Kristina C. Duffin, MD, has some advice: Don’t expect to just use one drug, focus on comorbidities, and embrace strategies to bypass the potential obstacle of prior-authorization approvals.
Here are some tips from Dr. Duffin, who spoke at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar:
- Don’t expect a one-size-fits-all medication. “There is no one, single go-to drug,” said Dr. Duffin, who is cochair of the department of dermatology at the University of Utah, Salt Lake City. “Maybe someday, we will have a biological personalized medicine marker to say this is the right drug, but for now we don’t.” More than 10 biologics are available to treat psoriasis, she said, and more are in the pipeline.
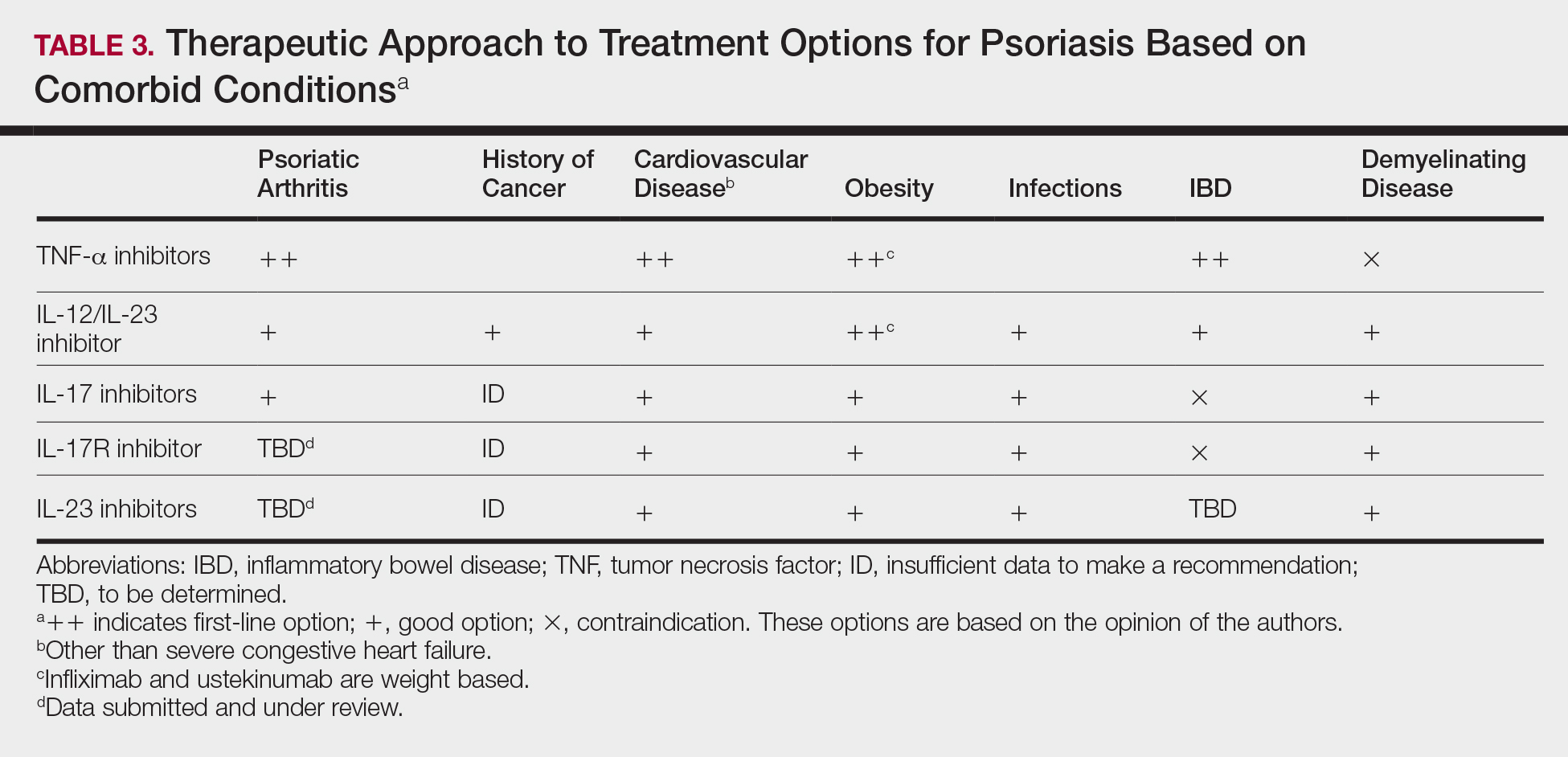
- Pay close attention to comorbidities. It’s important to “have a good grasp” of a patient’s comorbidities, which can help focus the choice of a biologic, Dr. Duffin said. She recommends starting with an anti–tumor necrosis factor (TNF) agents for patients with psoriatic arthritis. For patients with Crohn’s disease, she recommends anti-TNF (adalimumab, infliximab) and anti-interleukin–12/23 or anti-IL-23 agents (ustekinumab). Anti-TNF agents should be avoided in patients with multiple sclerosis, and anti-IL-17 agents shouldn’t be given to patients with recurrent candidiasis, she noted.
- Encourage patients to make prompt decisions. Dr. Duffin sits down with patients to discuss various biologic options, and she goes over information in handouts. She also focuses on their needs: “Are they interested in getting better fast? Do they want to be clear for their wedding in a month?” She prefers to not let patients go home to think about what they’d like to do. Instead, she advises patients to make choices while at the office visit.
- Order lab tests and be careful about vaccines. Dr. Duffin orders the following tests for all patients who are starting on biologics: CBC, comprehensive metabolic panel, hepatitis B and C, and tuberculosis. She orders HIV, Hba1c and lipid tests, if appropriate. She prefers that patients treated with biologics avoid live vaccines. She suggests other vaccines, if indicated, such as seasonal influenza and pneumonia vaccines, and for those aged 50 years and older, herpes zoster vaccine. She urges patients to call the office if they have an infection or need surgery because they may need to discuss putting a temporary hold on the biologics.
- Understand how to navigate formularies.“Getting drugs approved for patients with Medicare is a challenge,” Dr. Duffin said. It’s helpful to understand how insurers handle specific psoriasis drugs so you can choose one that’s likely to be covered if you’re unsure which one is best. The website www.covermymeds.com allows physicians to easily check insurer formularies, free of charge, she said.
- Documentation is crucial when you’re dealing with an insurer. Document body surface area, Psoriasis Area Severity Index scores, or physician global assessment measures, she advised. An app provided by the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis, is a helpful in determining these measurements, she said. Also include information about failed treatments and the rationale behind why you chose a specific treatment, she said. “If denial happens, get the details,” she said. This may turn up a clerical error on the insurer’s part that incorrectly led to a denial.
- Escalate challenges to drug denials. If the preferred treatment is denied, one option is to appeal the denial. As a resource, Dr. Duffin pointed to sample letters for appealing denials for physicians and patients on the websites for the American Academy of Dermatology and the National Psoriasis Foundation. Ask for a limited 6-month approval, she said, or have the patient write a letter to the insurer using one of the sample letter templates. Another option is to ask the insurer for a “peer-to-peer” review, she said. “Sometimes it’s really hard for insurance company folks to say no to you if you have a really good story,” she commented.
- Help your patients get financial assistance. Almost every biologic manufacturer has a patient assistance plan, which can also help with deductibles and copays, Dr. Duffin said.
Dr. Duffin discloses consulting for AbbVie, Amgen, Celgene, Janssen, Lilly, Novartis, Pfizer, and Sienna. She has received grant/contracted research support from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Janssen, Novartis, Pfizer, Sienna, Stiefel, and UCB.
SDEF and this news organization are owned by the same parent company.
LAS VEGAS – If you’re considering adding biologics for psoriasis to your clinical practice, dermatologist Kristina C. Duffin, MD, has some advice: Don’t expect to just use one drug, focus on comorbidities, and embrace strategies to bypass the potential obstacle of prior-authorization approvals.
Here are some tips from Dr. Duffin, who spoke at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar:
- Don’t expect a one-size-fits-all medication. “There is no one, single go-to drug,” said Dr. Duffin, who is cochair of the department of dermatology at the University of Utah, Salt Lake City. “Maybe someday, we will have a biological personalized medicine marker to say this is the right drug, but for now we don’t.” More than 10 biologics are available to treat psoriasis, she said, and more are in the pipeline.
- Pay close attention to comorbidities. It’s important to “have a good grasp” of a patient’s comorbidities, which can help focus the choice of a biologic, Dr. Duffin said. She recommends starting with an anti–tumor necrosis factor (TNF) agents for patients with psoriatic arthritis. For patients with Crohn’s disease, she recommends anti-TNF (adalimumab, infliximab) and anti-interleukin–12/23 or anti-IL-23 agents (ustekinumab). Anti-TNF agents should be avoided in patients with multiple sclerosis, and anti-IL-17 agents shouldn’t be given to patients with recurrent candidiasis, she noted.
- Encourage patients to make prompt decisions. Dr. Duffin sits down with patients to discuss various biologic options, and she goes over information in handouts. She also focuses on their needs: “Are they interested in getting better fast? Do they want to be clear for their wedding in a month?” She prefers to not let patients go home to think about what they’d like to do. Instead, she advises patients to make choices while at the office visit.
- Order lab tests and be careful about vaccines. Dr. Duffin orders the following tests for all patients who are starting on biologics: CBC, comprehensive metabolic panel, hepatitis B and C, and tuberculosis. She orders HIV, Hba1c and lipid tests, if appropriate. She prefers that patients treated with biologics avoid live vaccines. She suggests other vaccines, if indicated, such as seasonal influenza and pneumonia vaccines, and for those aged 50 years and older, herpes zoster vaccine. She urges patients to call the office if they have an infection or need surgery because they may need to discuss putting a temporary hold on the biologics.
- Understand how to navigate formularies.“Getting drugs approved for patients with Medicare is a challenge,” Dr. Duffin said. It’s helpful to understand how insurers handle specific psoriasis drugs so you can choose one that’s likely to be covered if you’re unsure which one is best. The website www.covermymeds.com allows physicians to easily check insurer formularies, free of charge, she said.
- Documentation is crucial when you’re dealing with an insurer. Document body surface area, Psoriasis Area Severity Index scores, or physician global assessment measures, she advised. An app provided by the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis, is a helpful in determining these measurements, she said. Also include information about failed treatments and the rationale behind why you chose a specific treatment, she said. “If denial happens, get the details,” she said. This may turn up a clerical error on the insurer’s part that incorrectly led to a denial.
- Escalate challenges to drug denials. If the preferred treatment is denied, one option is to appeal the denial. As a resource, Dr. Duffin pointed to sample letters for appealing denials for physicians and patients on the websites for the American Academy of Dermatology and the National Psoriasis Foundation. Ask for a limited 6-month approval, she said, or have the patient write a letter to the insurer using one of the sample letter templates. Another option is to ask the insurer for a “peer-to-peer” review, she said. “Sometimes it’s really hard for insurance company folks to say no to you if you have a really good story,” she commented.
- Help your patients get financial assistance. Almost every biologic manufacturer has a patient assistance plan, which can also help with deductibles and copays, Dr. Duffin said.
Dr. Duffin discloses consulting for AbbVie, Amgen, Celgene, Janssen, Lilly, Novartis, Pfizer, and Sienna. She has received grant/contracted research support from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Janssen, Novartis, Pfizer, Sienna, Stiefel, and UCB.
SDEF and this news organization are owned by the same parent company.
LAS VEGAS – If you’re considering adding biologics for psoriasis to your clinical practice, dermatologist Kristina C. Duffin, MD, has some advice: Don’t expect to just use one drug, focus on comorbidities, and embrace strategies to bypass the potential obstacle of prior-authorization approvals.
Here are some tips from Dr. Duffin, who spoke at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar:
- Don’t expect a one-size-fits-all medication. “There is no one, single go-to drug,” said Dr. Duffin, who is cochair of the department of dermatology at the University of Utah, Salt Lake City. “Maybe someday, we will have a biological personalized medicine marker to say this is the right drug, but for now we don’t.” More than 10 biologics are available to treat psoriasis, she said, and more are in the pipeline.
- Pay close attention to comorbidities. It’s important to “have a good grasp” of a patient’s comorbidities, which can help focus the choice of a biologic, Dr. Duffin said. She recommends starting with an anti–tumor necrosis factor (TNF) agents for patients with psoriatic arthritis. For patients with Crohn’s disease, she recommends anti-TNF (adalimumab, infliximab) and anti-interleukin–12/23 or anti-IL-23 agents (ustekinumab). Anti-TNF agents should be avoided in patients with multiple sclerosis, and anti-IL-17 agents shouldn’t be given to patients with recurrent candidiasis, she noted.
- Encourage patients to make prompt decisions. Dr. Duffin sits down with patients to discuss various biologic options, and she goes over information in handouts. She also focuses on their needs: “Are they interested in getting better fast? Do they want to be clear for their wedding in a month?” She prefers to not let patients go home to think about what they’d like to do. Instead, she advises patients to make choices while at the office visit.
- Order lab tests and be careful about vaccines. Dr. Duffin orders the following tests for all patients who are starting on biologics: CBC, comprehensive metabolic panel, hepatitis B and C, and tuberculosis. She orders HIV, Hba1c and lipid tests, if appropriate. She prefers that patients treated with biologics avoid live vaccines. She suggests other vaccines, if indicated, such as seasonal influenza and pneumonia vaccines, and for those aged 50 years and older, herpes zoster vaccine. She urges patients to call the office if they have an infection or need surgery because they may need to discuss putting a temporary hold on the biologics.
- Understand how to navigate formularies.“Getting drugs approved for patients with Medicare is a challenge,” Dr. Duffin said. It’s helpful to understand how insurers handle specific psoriasis drugs so you can choose one that’s likely to be covered if you’re unsure which one is best. The website www.covermymeds.com allows physicians to easily check insurer formularies, free of charge, she said.
- Documentation is crucial when you’re dealing with an insurer. Document body surface area, Psoriasis Area Severity Index scores, or physician global assessment measures, she advised. An app provided by the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis, is a helpful in determining these measurements, she said. Also include information about failed treatments and the rationale behind why you chose a specific treatment, she said. “If denial happens, get the details,” she said. This may turn up a clerical error on the insurer’s part that incorrectly led to a denial.
- Escalate challenges to drug denials. If the preferred treatment is denied, one option is to appeal the denial. As a resource, Dr. Duffin pointed to sample letters for appealing denials for physicians and patients on the websites for the American Academy of Dermatology and the National Psoriasis Foundation. Ask for a limited 6-month approval, she said, or have the patient write a letter to the insurer using one of the sample letter templates. Another option is to ask the insurer for a “peer-to-peer” review, she said. “Sometimes it’s really hard for insurance company folks to say no to you if you have a really good story,” she commented.
- Help your patients get financial assistance. Almost every biologic manufacturer has a patient assistance plan, which can also help with deductibles and copays, Dr. Duffin said.
Dr. Duffin discloses consulting for AbbVie, Amgen, Celgene, Janssen, Lilly, Novartis, Pfizer, and Sienna. She has received grant/contracted research support from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Janssen, Novartis, Pfizer, Sienna, Stiefel, and UCB.
SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
Comorbidities are important in psoriasis care
LAS VEGAS – Pay attention to comorbidities in your psoriasis patients because there may not be anyone else doing so.
“Many of our patients don’t have primary care physicians; many are untreated for psoriasis. They come to a clinical trial to get treated – some of them may not have insurance – so it is important for us to watch for these comorbidities,” Kristina C. Duffin, MD, said at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
Yet, that does not seem to be happening consistently, according to Dr. Duffin, of the department of dermatology at the University of Utah, Salt Lake City. One in five dermatologists admitted to never screening or referring their psoriasis patients for management of cardiovascular risks in a 2015 survey (J Am Acad Dermatol. 2015 doi: 10.1016/j.jaad.2015.07.029).
Often patients at the start of biologic therapy are counseled about the risk for developing tuberculosis, yet the lifetime risk for doing so in the United States is 0.3%. Similarly, patients are often counseled on the risk for developing lymphoma, even though the excess risk for developing lymphoma that can be attributed to psoriasis treatment is 7.9 per 100,000 psoriasis patients per year. That screening seems to be driven by warnings issued in direct-to-consumer advertising, Dr. Duffin suggested.
“Although psoriasis patients have an increased relative risk of lymphoma, the absolute risk attributable to psoriasis is low,” Dr. Duffin pointed out.
Some of the comorbidities she advised dermatologists to watch for are described below.
Psoriatic arthritis
Psoriatic arthritis is the most important psoriasis comorbidity, Dr. Duffin said. Between 20% and 30% of psoriasis patients will develop psoriatic arthritis.
In a study of 1,511 patients in 48 centers in Germany, 21% of psoriasis patients were diagnosed with psoriatic arthritis and of those, more than 95% had active arthritis and 53% had five or more affected joints (Br J Dermatol. 2009;160[5]:1040-7).
The GRAPPA app is an easy, free screening tool for psoriatic arthritis; patients who score 3 or more out of 5 items on the psoriasis epidemiology screening tool (PEST) are deemed positive for psoriatic arthritis, Dr. Duffin noted.
Cardiovascular disease
Psoriasis patients are at increased risk of myocardial infarction, stroke, cardiovascular death, diabetes, and chronic kidney disease, Dr. Duffin said. In fact, CV risk from severe psoriasis is similar to the risk conferred by diabetes.
She added that there is epidemiologic evidence for CV risk modification with several of the biologics approved for psoriasis.
Hypertension
Hypertension is prevalent and more severe in psoriasis patients, Dr. Duffin said, citing a 2011 case-control study of electronic medical records at the University of California, Davis. Psoriasis patients with hypertension were 5 times more likely than patients without psoriasis to be on one antihypertensive medication, 9.5 times more likely to be on two, and almost 20 times more likely to be on four antihypertensive medications (PLoS One. 2011 Mar 29;6[3]:e18227. doi: 10.1371/journal.pone.0018227).
Importantly, few primary care physicians and cardiologists are aware of the increased risk for hypertension in psoriasis patients.
Less than half (45%) of primary care physicians and 57% of cardiologists reported they were aware that psoriasis was associated with worse cardiovascular outcome, and only 43% of physicians reported screening psoriasis patients for hypertension starting at age 20 years, according to a 2012 survey of 251 physicians (J Am Acad Dermatol. 2012 Sep;67[3]:357-62).
Dr. Duffin called on dermatologists to ensure that the primary care physicians they work with understand these increased risks.
“Commit to including a comment in consultation letters or letters back to primary care physicians that talks about the cardiovascular risks of the disease,” she said.
Dr. Duffin reported that she is a consultant and has received grant or contracted research support for many companies that manufacture dermatologic therapies.
SDEF and this news organization are owned by the same parent company.
LAS VEGAS – Pay attention to comorbidities in your psoriasis patients because there may not be anyone else doing so.
“Many of our patients don’t have primary care physicians; many are untreated for psoriasis. They come to a clinical trial to get treated – some of them may not have insurance – so it is important for us to watch for these comorbidities,” Kristina C. Duffin, MD, said at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
Yet, that does not seem to be happening consistently, according to Dr. Duffin, of the department of dermatology at the University of Utah, Salt Lake City. One in five dermatologists admitted to never screening or referring their psoriasis patients for management of cardiovascular risks in a 2015 survey (J Am Acad Dermatol. 2015 doi: 10.1016/j.jaad.2015.07.029).
Often patients at the start of biologic therapy are counseled about the risk for developing tuberculosis, yet the lifetime risk for doing so in the United States is 0.3%. Similarly, patients are often counseled on the risk for developing lymphoma, even though the excess risk for developing lymphoma that can be attributed to psoriasis treatment is 7.9 per 100,000 psoriasis patients per year. That screening seems to be driven by warnings issued in direct-to-consumer advertising, Dr. Duffin suggested.
“Although psoriasis patients have an increased relative risk of lymphoma, the absolute risk attributable to psoriasis is low,” Dr. Duffin pointed out.
Some of the comorbidities she advised dermatologists to watch for are described below.
Psoriatic arthritis
Psoriatic arthritis is the most important psoriasis comorbidity, Dr. Duffin said. Between 20% and 30% of psoriasis patients will develop psoriatic arthritis.
In a study of 1,511 patients in 48 centers in Germany, 21% of psoriasis patients were diagnosed with psoriatic arthritis and of those, more than 95% had active arthritis and 53% had five or more affected joints (Br J Dermatol. 2009;160[5]:1040-7).
The GRAPPA app is an easy, free screening tool for psoriatic arthritis; patients who score 3 or more out of 5 items on the psoriasis epidemiology screening tool (PEST) are deemed positive for psoriatic arthritis, Dr. Duffin noted.
Cardiovascular disease
Psoriasis patients are at increased risk of myocardial infarction, stroke, cardiovascular death, diabetes, and chronic kidney disease, Dr. Duffin said. In fact, CV risk from severe psoriasis is similar to the risk conferred by diabetes.
She added that there is epidemiologic evidence for CV risk modification with several of the biologics approved for psoriasis.
Hypertension
Hypertension is prevalent and more severe in psoriasis patients, Dr. Duffin said, citing a 2011 case-control study of electronic medical records at the University of California, Davis. Psoriasis patients with hypertension were 5 times more likely than patients without psoriasis to be on one antihypertensive medication, 9.5 times more likely to be on two, and almost 20 times more likely to be on four antihypertensive medications (PLoS One. 2011 Mar 29;6[3]:e18227. doi: 10.1371/journal.pone.0018227).
Importantly, few primary care physicians and cardiologists are aware of the increased risk for hypertension in psoriasis patients.
Less than half (45%) of primary care physicians and 57% of cardiologists reported they were aware that psoriasis was associated with worse cardiovascular outcome, and only 43% of physicians reported screening psoriasis patients for hypertension starting at age 20 years, according to a 2012 survey of 251 physicians (J Am Acad Dermatol. 2012 Sep;67[3]:357-62).
Dr. Duffin called on dermatologists to ensure that the primary care physicians they work with understand these increased risks.
“Commit to including a comment in consultation letters or letters back to primary care physicians that talks about the cardiovascular risks of the disease,” she said.
Dr. Duffin reported that she is a consultant and has received grant or contracted research support for many companies that manufacture dermatologic therapies.
SDEF and this news organization are owned by the same parent company.
LAS VEGAS – Pay attention to comorbidities in your psoriasis patients because there may not be anyone else doing so.
“Many of our patients don’t have primary care physicians; many are untreated for psoriasis. They come to a clinical trial to get treated – some of them may not have insurance – so it is important for us to watch for these comorbidities,” Kristina C. Duffin, MD, said at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
Yet, that does not seem to be happening consistently, according to Dr. Duffin, of the department of dermatology at the University of Utah, Salt Lake City. One in five dermatologists admitted to never screening or referring their psoriasis patients for management of cardiovascular risks in a 2015 survey (J Am Acad Dermatol. 2015 doi: 10.1016/j.jaad.2015.07.029).
Often patients at the start of biologic therapy are counseled about the risk for developing tuberculosis, yet the lifetime risk for doing so in the United States is 0.3%. Similarly, patients are often counseled on the risk for developing lymphoma, even though the excess risk for developing lymphoma that can be attributed to psoriasis treatment is 7.9 per 100,000 psoriasis patients per year. That screening seems to be driven by warnings issued in direct-to-consumer advertising, Dr. Duffin suggested.
“Although psoriasis patients have an increased relative risk of lymphoma, the absolute risk attributable to psoriasis is low,” Dr. Duffin pointed out.
Some of the comorbidities she advised dermatologists to watch for are described below.
Psoriatic arthritis
Psoriatic arthritis is the most important psoriasis comorbidity, Dr. Duffin said. Between 20% and 30% of psoriasis patients will develop psoriatic arthritis.
In a study of 1,511 patients in 48 centers in Germany, 21% of psoriasis patients were diagnosed with psoriatic arthritis and of those, more than 95% had active arthritis and 53% had five or more affected joints (Br J Dermatol. 2009;160[5]:1040-7).
The GRAPPA app is an easy, free screening tool for psoriatic arthritis; patients who score 3 or more out of 5 items on the psoriasis epidemiology screening tool (PEST) are deemed positive for psoriatic arthritis, Dr. Duffin noted.
Cardiovascular disease
Psoriasis patients are at increased risk of myocardial infarction, stroke, cardiovascular death, diabetes, and chronic kidney disease, Dr. Duffin said. In fact, CV risk from severe psoriasis is similar to the risk conferred by diabetes.
She added that there is epidemiologic evidence for CV risk modification with several of the biologics approved for psoriasis.
Hypertension
Hypertension is prevalent and more severe in psoriasis patients, Dr. Duffin said, citing a 2011 case-control study of electronic medical records at the University of California, Davis. Psoriasis patients with hypertension were 5 times more likely than patients without psoriasis to be on one antihypertensive medication, 9.5 times more likely to be on two, and almost 20 times more likely to be on four antihypertensive medications (PLoS One. 2011 Mar 29;6[3]:e18227. doi: 10.1371/journal.pone.0018227).
Importantly, few primary care physicians and cardiologists are aware of the increased risk for hypertension in psoriasis patients.
Less than half (45%) of primary care physicians and 57% of cardiologists reported they were aware that psoriasis was associated with worse cardiovascular outcome, and only 43% of physicians reported screening psoriasis patients for hypertension starting at age 20 years, according to a 2012 survey of 251 physicians (J Am Acad Dermatol. 2012 Sep;67[3]:357-62).
Dr. Duffin called on dermatologists to ensure that the primary care physicians they work with understand these increased risks.
“Commit to including a comment in consultation letters or letters back to primary care physicians that talks about the cardiovascular risks of the disease,” she said.
Dr. Duffin reported that she is a consultant and has received grant or contracted research support for many companies that manufacture dermatologic therapies.
SDEF and this news organization are owned by the same parent company.
REPORTING FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
Debunking Psoriasis Myths: Remove Psoriasis Scales Gently
Myth: Pick Psoriasis Scales to Remove Them
Patients may be inclined to pick psoriasis scales that appear in noticeable areas or on the scalp. However, they should be counseled to avoid this practice, which could cause an infection. Instead, Dr. Steven Feldman (Winston-Salem, North Carolina) suggests putting on an ointment or oil-like medication to soften the scale. “Almost any kind of moisturizer will change the reflective properties of the scale so that you don’t see the scale,” he advised. He also suggested descaling agents such as topical salicylic acid or lactic acid. His patient education video is available on the American Academy of Dermatology website should you wish to direct your patients to it.
Because salicylic acid is a keratolytic (or peeling agent), it works by causing the outer layer of skin to shed. When applied topically, it helps to soften and lift psoriasis scales. Coal tar over-the-counter products also can be used for the same purpose. The over-the-counter product guide from the National Psoriasis Foundation is a valuable resource to share with patients.
Expert Commentary
I agree that it is very important to treat scale very gently. In addition to risk for infection, picking and traumatizing scale can lead to worsening of the psoriasis. This is known as the Koebner phenomenon. The phenomenon was first described by Heinrich Koebner in 1876 as the formation of psoriatic lesions in uninvolved skin of patients with psoriasis after cutaneous trauma. This isomorphic phenomenon is now known to involve numerous diseases, among them vitiligo, lichen planus, and Darier disease.
—Jeffrey M. Weinberg, MD (New York, New York)
Feldman S. How should I remove psoriasis scale? American Academy of Dermatology website. https://www.aad.org/public/diseases/scaly-skin/psoriasis/tips-for-managing-psoriasis/how-should-i-remove-psoriasis-scale. Accessed October 31, 2018.
National Psoriasis Foundation. Over-the-counter products. https://www.aad.org/public/diseases/scaly-skin/psoriasis/tips-for-managing-psoriasis/how-should-i-remove-psoriasis-scale. Published June 2017. Accessed October 31, 2018.
Sagi L, Trau H. The Koebner phenomenon. Clin Dermatol. 2011;29:231-236.
Myth: Pick Psoriasis Scales to Remove Them
Patients may be inclined to pick psoriasis scales that appear in noticeable areas or on the scalp. However, they should be counseled to avoid this practice, which could cause an infection. Instead, Dr. Steven Feldman (Winston-Salem, North Carolina) suggests putting on an ointment or oil-like medication to soften the scale. “Almost any kind of moisturizer will change the reflective properties of the scale so that you don’t see the scale,” he advised. He also suggested descaling agents such as topical salicylic acid or lactic acid. His patient education video is available on the American Academy of Dermatology website should you wish to direct your patients to it.
Because salicylic acid is a keratolytic (or peeling agent), it works by causing the outer layer of skin to shed. When applied topically, it helps to soften and lift psoriasis scales. Coal tar over-the-counter products also can be used for the same purpose. The over-the-counter product guide from the National Psoriasis Foundation is a valuable resource to share with patients.
Expert Commentary
I agree that it is very important to treat scale very gently. In addition to risk for infection, picking and traumatizing scale can lead to worsening of the psoriasis. This is known as the Koebner phenomenon. The phenomenon was first described by Heinrich Koebner in 1876 as the formation of psoriatic lesions in uninvolved skin of patients with psoriasis after cutaneous trauma. This isomorphic phenomenon is now known to involve numerous diseases, among them vitiligo, lichen planus, and Darier disease.
—Jeffrey M. Weinberg, MD (New York, New York)
Myth: Pick Psoriasis Scales to Remove Them
Patients may be inclined to pick psoriasis scales that appear in noticeable areas or on the scalp. However, they should be counseled to avoid this practice, which could cause an infection. Instead, Dr. Steven Feldman (Winston-Salem, North Carolina) suggests putting on an ointment or oil-like medication to soften the scale. “Almost any kind of moisturizer will change the reflective properties of the scale so that you don’t see the scale,” he advised. He also suggested descaling agents such as topical salicylic acid or lactic acid. His patient education video is available on the American Academy of Dermatology website should you wish to direct your patients to it.
Because salicylic acid is a keratolytic (or peeling agent), it works by causing the outer layer of skin to shed. When applied topically, it helps to soften and lift psoriasis scales. Coal tar over-the-counter products also can be used for the same purpose. The over-the-counter product guide from the National Psoriasis Foundation is a valuable resource to share with patients.
Expert Commentary
I agree that it is very important to treat scale very gently. In addition to risk for infection, picking and traumatizing scale can lead to worsening of the psoriasis. This is known as the Koebner phenomenon. The phenomenon was first described by Heinrich Koebner in 1876 as the formation of psoriatic lesions in uninvolved skin of patients with psoriasis after cutaneous trauma. This isomorphic phenomenon is now known to involve numerous diseases, among them vitiligo, lichen planus, and Darier disease.
—Jeffrey M. Weinberg, MD (New York, New York)
Feldman S. How should I remove psoriasis scale? American Academy of Dermatology website. https://www.aad.org/public/diseases/scaly-skin/psoriasis/tips-for-managing-psoriasis/how-should-i-remove-psoriasis-scale. Accessed October 31, 2018.
National Psoriasis Foundation. Over-the-counter products. https://www.aad.org/public/diseases/scaly-skin/psoriasis/tips-for-managing-psoriasis/how-should-i-remove-psoriasis-scale. Published June 2017. Accessed October 31, 2018.
Sagi L, Trau H. The Koebner phenomenon. Clin Dermatol. 2011;29:231-236.
Feldman S. How should I remove psoriasis scale? American Academy of Dermatology website. https://www.aad.org/public/diseases/scaly-skin/psoriasis/tips-for-managing-psoriasis/how-should-i-remove-psoriasis-scale. Accessed October 31, 2018.
National Psoriasis Foundation. Over-the-counter products. https://www.aad.org/public/diseases/scaly-skin/psoriasis/tips-for-managing-psoriasis/how-should-i-remove-psoriasis-scale. Published June 2017. Accessed October 31, 2018.
Sagi L, Trau H. The Koebner phenomenon. Clin Dermatol. 2011;29:231-236.
Over one-third of psoriasis patients have PsA
Over one-third of psoriasis patients have PsA
About two-thirds of patients with psoriasis in a national registry also had psoriatic arthritis (PsA) and/or psoriasis in at least one challenging-to-treat (CTT) area, and one-quarter had both, according to Kristina Callis Duffin, MD, of the University of Utah, Salt Lake City, and her associates.
Their analysis included 2,042 psoriasis patients who were enrolled in the Corrona Psoriasis Registry between April 2015 and May 2018 and initiated biologic treatment during that time. The mean age was 49.6 years, 80% of the patients were white, and 51% were obese. Mean disease duration was 19.9 years and 89.2% of the patients had moderate to severe disease. CTT areas include the scalp, nails, and palmoplantar areas.
A total of 784 people in the cohort (38.4%) had PsA, 778 (38.1%) had scalp psoriasis, 326 (16.0%) had nail psoriasis, 223 (10.9%) had palmoplantar psoriasis, and 535 (26.2%) had both PsA and psoriasis in at least two CTT areas. The most common combinations were PsA plus scalp psoriasis and PsA plus nail and scalp psoriasis.
“These results indicate a need to further characterize patients with psoriasis who have PsA and CTT areas and evaluate the impact of these factors to better understand their treatment needs,” the investigators noted.
The Corrona registry has been supported by numerous pharmaceutical companies, and the study authors reported numerous financial relationships with industry; two authors are Novartis employees.
Secukinumab effective for slowing radiographic progression in active PsA
Treatment with secukinumab significantly reduced radiographic progression in patients with active PsA, according to Désirée van der Heijde, MD, PhD, professor of rheumatology at Leiden University Medical Center, and her associates.
The results come from an analysis of the FUTURE 5 trial, a study of 996 patients with active PsA despite previous NSAID treatment, disease-modifying antirheumatic drug treatment, or anti–tumor necrosis factor (TNF) therapy. Patients were randomized to receive 300 mg subcutaneous secukinumab with loading dose, 150 mg secukinumab with loading dose, 150 mg secukinumab without loading dose, or placebo, at baseline; weeks 1, 2, 3, and 4; then every 4 weeks.
After 24 weeks, the mean change in van der Heijde–modified Total Sharp Score for PsA was 0.08 for the 300-mg secukinumab group (P less than .01), 0.17 for the 150-mg secukinumab with loading dose group (P less than .05), a reduction of 0.09 for the 150-mg secukinumab without loading dose group (P less than .01), and 0.50 for the placebo group. Lower radiographic progression was seen regardless of prior anti-TNF or concomitant methotrexate treatment.
The study was funded by Novartis. The study authors reported financial disclosures with numerous companies; five authors are Novartis employees.
Tildrakizumab sustains efficacy in plaque psoriasis treatment after 1 year
Nearly all patients receiving the interleukin-23 inhibitor tildrakizumab for the treatment of moderate to severe plaque psoriasis maintained or improved their Psoriasis Area and Severity Index (PASI) response rate after 52 weeks of treatment, compared with their response after 28 weeks.
The analysis, conducted by Boni E. Elewski, MD, of the University of Alabama at Birmingham, and her associates, included 352 patients who received 100 mg tildrakizumab and 313 who received 200 mg tildrakizumab. Treatment was received at baseline, at 4 weeks, and then every 12 weeks afterward.
At week 28, the proportions of patients achieving PASI 100, PASI 90-99, PASI 75-89, and PASI 50-74 at week 28 were 25.9%, 38.4%, 25.3%, and 10.5%, respectively, among those treated with the 100-mg dose. The proportions were 24.6%, 24.3%, 19.5%, and 31.6%, respectively, among those treated with the 200-mg dose.
In patients who achieved at least PASI 90 on either dose at week 28, 88.9%-89.4% maintained that response at week 52. For patients with PASI 75-89, 39.3%-40.4% maintained that response and 33.7%-41.0% achieved a PASI 90 response. At week 52, in patients with PASI 50-74, 20.2%-29.7% achieved at least a PASI 90, 52.5%-64.9% achieved PASI 75, and only 2.6% of patients on either dose had fallen below PASI 50.
Four study authors reported being clinical investigators on studies sponsored by Merck and Sun Pharmaceuticals; five authors are employees of Sun Pharmaceuticals.
Halobetasol/tazarotene combination most effective for plaque psoriasis treatment
A fixed combination of halobetasol propionate 0.01% and tazarotene 0.045% lotion provided a synergistic effect over either component on its own for the treatment of plaque psoriasis, according to Leon H. Kircik, MD, of Indiana University, Indianapolis, and his associates.
The investigators performed a post hoc analysis of 212 patients with moderate to severe plaque psoriasis randomized to receive either the halobetasol/tazarotene combination, halobetasol only, tazarotene only, or vehicle only for 8 weeks, with follow-up at 12 weeks. Treatment success was based on the proportion of patients who achieved at least a 2-grade improvement in the Investigator Global Assessment (IGA) score, IGA scores of “clear” or “almost clear,” and percent change from baseline in IGA multiplied by Body Surface Area (BSA) composite score (IGAxBSA). “Synergy was calculated by summing up the contribution of the individual active ingredients (HP and TAZ) to overall efficacy and comparing to the efficacy achieved with HP/TAZ lotion relative to vehicle,” the authors explained.
Relative to vehicle, treatment success for halobetasol/tazarotene after 8 weeks was 42.8%, 23.6% for halobetasol alone, and 9.0% for tazarotene alone. After 12 weeks, the difference was 31.3%, 14.1%, and 5.9%, respectively. The percent change in IGAxBSA scores from baseline after 8 weeks, relative to vehicle, were 51.6%, 37.3%, and 3.3%, respectively. After 12 weeks, the change was 47.3%, 25.7%, and 8.6%, respectively.
After 8 weeks, the synergy ratio for treatment success and IGAxBSA scores for the halobetasol/tazarotene combination was 1.3. After 12 weeks, the synergy ratio for treatment success was 1.6 and the ratio for IGAxBSA scores was 1.4.
“By combining two agents into one once-daily formulation, this novel formulation reduces the number of product applications and may help patient adherence,” the study authors noted.
Dr. Kircik reported serving as a consultant and investigator for Valeant Pharmaceuticals. One study author is an employee of Bausch Health and Ortho Dermatologics, and another is an employee of Dow Pharmaceutical Sciences (a division of Valeant).
Brodalumab demonstrates low immunogenicity in moderate to severe psoriasis
The immunogenicity of brodalumab in patients with moderate to severe plaque psoriasis was low and did not compromise the efficacy or safety profile of the drug, according to Kristian Reich, MD, of Dermatologikum Berlin and SCIderm Research Institute in Hamburg, Germany, and his associates.
Data from a 12-week, phase 2 trial with a 352-week, open-label extension and three 52-week phase 3 trials were included in the analysis. Antidrug antibodies (ADAs) were tested, and positive samples were further analyzed for neutralizing ADAs by a cell-based assay.
Out of the 4,461 patients who received brodalumab, 122 (2.7%) were positive for ADAs after starting brodalumab. The incidence rate ranged from 1.9% to 3.4% between all dosing groups (140 mg, 210 mg, variable dosing, and 210 mg of brodalumab after ustekinumab). In 58 (1.4%) of patients, ADAs were transient. No patients had neutralizing ADAs, and no evidence of altered pharmacokinetics, loss of efficacy, or changes in the safety profile of brodalumab in subjects positive for ADAs was seen.
No significant difference was seen in the incidence rate of hypersensitivity or injection site reactions in brodalumab, compared with placebo or ustekinumab. The most common injection site reactions were injection site pain, erythema, and bruising.
The study was supported by Amgen. The study authors reported numerous disclosures. Two authors are employees of Leo Pharma, one author is a former employee of the company.
Secukinumab improves patient-reported outcomes in CTT psoriasis
Treatment with secukinumab significantly improved patient-reported outcomes such as fatigue, itch, pain, and quality of life measures in patients with CTT psoriasis after 6 months, according to Jerry Bagel, MD, of the Psoriasis Treatment Center of Central New Jersey, East Windsor, and his associates.
A total of 68 patients with psoriasis localized to at least one CTT area who were enrolled in the Corrona Psoriasis Registry from April 15, 2015, through May 10, 2018, and were receiving secukinumab for the entirety of the 6-month study period were included in the analysis. Patient-reported outcomes included in the analysis were fatigue, itch, pain, Dermatology Quality of Life Index (DLQI) score, and Work Productivity and Activity Impairment (WPAI) scale.
The mean age at enrollment was 51.2 years and almost 80% of patients were white. Mean psoriasis duration was 21.8 years and nearly half had PsA.
Visual analog scale scores improved over baseline for fatigue (mean, 23.2 vs. 33.2; P = .01), itch (20.9 vs. 49.6; P less than .0001), and pain (12.1 vs. 33.8; P less than .0001). DLQI scores also improved (2.9 vs. 8.1; P less than .0001), and the proportion of patients who reported that psoriasis had at least a moderate effect on their life was reduced after 6 months (22.1% vs. 59.7%; P less than .0001).
Based on WPAI results, patients experienced significant improvements in the percentage of daily activities impaired (mean, 9.5% vs 17.5%; P = .0075); of the 42 patients who were employed, both impairment percentage (3.7% vs. 11.2%; P = .0148) and percentage of work hours affected (4.9% vs. 11.9%; P = .0486) were reduced from baseline.
“These results are consistent with previous reports from secukinumab clinical trials; however, additional real-world studies are needed to evaluate the long-term effectiveness of secukinumab for improving [patient-reported outcomes] in patients with psoriasis in CTT areas,” the authors noted.
The Corrona registry has been supported by numerous pharmaceutical companies, and several study authors reported various disclosures with industry. Two authors are Novartis employees. The study was supported by Novartis; the company participated in the interpretation of data and review and approval of the abstract.
These posters were presented at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. SDEF and this news organization are owned by the same parent company.
Over one-third of psoriasis patients have PsA
About two-thirds of patients with psoriasis in a national registry also had psoriatic arthritis (PsA) and/or psoriasis in at least one challenging-to-treat (CTT) area, and one-quarter had both, according to Kristina Callis Duffin, MD, of the University of Utah, Salt Lake City, and her associates.
Their analysis included 2,042 psoriasis patients who were enrolled in the Corrona Psoriasis Registry between April 2015 and May 2018 and initiated biologic treatment during that time. The mean age was 49.6 years, 80% of the patients were white, and 51% were obese. Mean disease duration was 19.9 years and 89.2% of the patients had moderate to severe disease. CTT areas include the scalp, nails, and palmoplantar areas.
A total of 784 people in the cohort (38.4%) had PsA, 778 (38.1%) had scalp psoriasis, 326 (16.0%) had nail psoriasis, 223 (10.9%) had palmoplantar psoriasis, and 535 (26.2%) had both PsA and psoriasis in at least two CTT areas. The most common combinations were PsA plus scalp psoriasis and PsA plus nail and scalp psoriasis.
“These results indicate a need to further characterize patients with psoriasis who have PsA and CTT areas and evaluate the impact of these factors to better understand their treatment needs,” the investigators noted.
The Corrona registry has been supported by numerous pharmaceutical companies, and the study authors reported numerous financial relationships with industry; two authors are Novartis employees.
Secukinumab effective for slowing radiographic progression in active PsA
Treatment with secukinumab significantly reduced radiographic progression in patients with active PsA, according to Désirée van der Heijde, MD, PhD, professor of rheumatology at Leiden University Medical Center, and her associates.
The results come from an analysis of the FUTURE 5 trial, a study of 996 patients with active PsA despite previous NSAID treatment, disease-modifying antirheumatic drug treatment, or anti–tumor necrosis factor (TNF) therapy. Patients were randomized to receive 300 mg subcutaneous secukinumab with loading dose, 150 mg secukinumab with loading dose, 150 mg secukinumab without loading dose, or placebo, at baseline; weeks 1, 2, 3, and 4; then every 4 weeks.
After 24 weeks, the mean change in van der Heijde–modified Total Sharp Score for PsA was 0.08 for the 300-mg secukinumab group (P less than .01), 0.17 for the 150-mg secukinumab with loading dose group (P less than .05), a reduction of 0.09 for the 150-mg secukinumab without loading dose group (P less than .01), and 0.50 for the placebo group. Lower radiographic progression was seen regardless of prior anti-TNF or concomitant methotrexate treatment.
The study was funded by Novartis. The study authors reported financial disclosures with numerous companies; five authors are Novartis employees.
Tildrakizumab sustains efficacy in plaque psoriasis treatment after 1 year
Nearly all patients receiving the interleukin-23 inhibitor tildrakizumab for the treatment of moderate to severe plaque psoriasis maintained or improved their Psoriasis Area and Severity Index (PASI) response rate after 52 weeks of treatment, compared with their response after 28 weeks.
The analysis, conducted by Boni E. Elewski, MD, of the University of Alabama at Birmingham, and her associates, included 352 patients who received 100 mg tildrakizumab and 313 who received 200 mg tildrakizumab. Treatment was received at baseline, at 4 weeks, and then every 12 weeks afterward.
At week 28, the proportions of patients achieving PASI 100, PASI 90-99, PASI 75-89, and PASI 50-74 at week 28 were 25.9%, 38.4%, 25.3%, and 10.5%, respectively, among those treated with the 100-mg dose. The proportions were 24.6%, 24.3%, 19.5%, and 31.6%, respectively, among those treated with the 200-mg dose.
In patients who achieved at least PASI 90 on either dose at week 28, 88.9%-89.4% maintained that response at week 52. For patients with PASI 75-89, 39.3%-40.4% maintained that response and 33.7%-41.0% achieved a PASI 90 response. At week 52, in patients with PASI 50-74, 20.2%-29.7% achieved at least a PASI 90, 52.5%-64.9% achieved PASI 75, and only 2.6% of patients on either dose had fallen below PASI 50.
Four study authors reported being clinical investigators on studies sponsored by Merck and Sun Pharmaceuticals; five authors are employees of Sun Pharmaceuticals.
Halobetasol/tazarotene combination most effective for plaque psoriasis treatment
A fixed combination of halobetasol propionate 0.01% and tazarotene 0.045% lotion provided a synergistic effect over either component on its own for the treatment of plaque psoriasis, according to Leon H. Kircik, MD, of Indiana University, Indianapolis, and his associates.
The investigators performed a post hoc analysis of 212 patients with moderate to severe plaque psoriasis randomized to receive either the halobetasol/tazarotene combination, halobetasol only, tazarotene only, or vehicle only for 8 weeks, with follow-up at 12 weeks. Treatment success was based on the proportion of patients who achieved at least a 2-grade improvement in the Investigator Global Assessment (IGA) score, IGA scores of “clear” or “almost clear,” and percent change from baseline in IGA multiplied by Body Surface Area (BSA) composite score (IGAxBSA). “Synergy was calculated by summing up the contribution of the individual active ingredients (HP and TAZ) to overall efficacy and comparing to the efficacy achieved with HP/TAZ lotion relative to vehicle,” the authors explained.
Relative to vehicle, treatment success for halobetasol/tazarotene after 8 weeks was 42.8%, 23.6% for halobetasol alone, and 9.0% for tazarotene alone. After 12 weeks, the difference was 31.3%, 14.1%, and 5.9%, respectively. The percent change in IGAxBSA scores from baseline after 8 weeks, relative to vehicle, were 51.6%, 37.3%, and 3.3%, respectively. After 12 weeks, the change was 47.3%, 25.7%, and 8.6%, respectively.
After 8 weeks, the synergy ratio for treatment success and IGAxBSA scores for the halobetasol/tazarotene combination was 1.3. After 12 weeks, the synergy ratio for treatment success was 1.6 and the ratio for IGAxBSA scores was 1.4.
“By combining two agents into one once-daily formulation, this novel formulation reduces the number of product applications and may help patient adherence,” the study authors noted.
Dr. Kircik reported serving as a consultant and investigator for Valeant Pharmaceuticals. One study author is an employee of Bausch Health and Ortho Dermatologics, and another is an employee of Dow Pharmaceutical Sciences (a division of Valeant).
Brodalumab demonstrates low immunogenicity in moderate to severe psoriasis
The immunogenicity of brodalumab in patients with moderate to severe plaque psoriasis was low and did not compromise the efficacy or safety profile of the drug, according to Kristian Reich, MD, of Dermatologikum Berlin and SCIderm Research Institute in Hamburg, Germany, and his associates.
Data from a 12-week, phase 2 trial with a 352-week, open-label extension and three 52-week phase 3 trials were included in the analysis. Antidrug antibodies (ADAs) were tested, and positive samples were further analyzed for neutralizing ADAs by a cell-based assay.
Out of the 4,461 patients who received brodalumab, 122 (2.7%) were positive for ADAs after starting brodalumab. The incidence rate ranged from 1.9% to 3.4% between all dosing groups (140 mg, 210 mg, variable dosing, and 210 mg of brodalumab after ustekinumab). In 58 (1.4%) of patients, ADAs were transient. No patients had neutralizing ADAs, and no evidence of altered pharmacokinetics, loss of efficacy, or changes in the safety profile of brodalumab in subjects positive for ADAs was seen.
No significant difference was seen in the incidence rate of hypersensitivity or injection site reactions in brodalumab, compared with placebo or ustekinumab. The most common injection site reactions were injection site pain, erythema, and bruising.
The study was supported by Amgen. The study authors reported numerous disclosures. Two authors are employees of Leo Pharma, one author is a former employee of the company.
Secukinumab improves patient-reported outcomes in CTT psoriasis
Treatment with secukinumab significantly improved patient-reported outcomes such as fatigue, itch, pain, and quality of life measures in patients with CTT psoriasis after 6 months, according to Jerry Bagel, MD, of the Psoriasis Treatment Center of Central New Jersey, East Windsor, and his associates.
A total of 68 patients with psoriasis localized to at least one CTT area who were enrolled in the Corrona Psoriasis Registry from April 15, 2015, through May 10, 2018, and were receiving secukinumab for the entirety of the 6-month study period were included in the analysis. Patient-reported outcomes included in the analysis were fatigue, itch, pain, Dermatology Quality of Life Index (DLQI) score, and Work Productivity and Activity Impairment (WPAI) scale.
The mean age at enrollment was 51.2 years and almost 80% of patients were white. Mean psoriasis duration was 21.8 years and nearly half had PsA.
Visual analog scale scores improved over baseline for fatigue (mean, 23.2 vs. 33.2; P = .01), itch (20.9 vs. 49.6; P less than .0001), and pain (12.1 vs. 33.8; P less than .0001). DLQI scores also improved (2.9 vs. 8.1; P less than .0001), and the proportion of patients who reported that psoriasis had at least a moderate effect on their life was reduced after 6 months (22.1% vs. 59.7%; P less than .0001).
Based on WPAI results, patients experienced significant improvements in the percentage of daily activities impaired (mean, 9.5% vs 17.5%; P = .0075); of the 42 patients who were employed, both impairment percentage (3.7% vs. 11.2%; P = .0148) and percentage of work hours affected (4.9% vs. 11.9%; P = .0486) were reduced from baseline.
“These results are consistent with previous reports from secukinumab clinical trials; however, additional real-world studies are needed to evaluate the long-term effectiveness of secukinumab for improving [patient-reported outcomes] in patients with psoriasis in CTT areas,” the authors noted.
The Corrona registry has been supported by numerous pharmaceutical companies, and several study authors reported various disclosures with industry. Two authors are Novartis employees. The study was supported by Novartis; the company participated in the interpretation of data and review and approval of the abstract.
These posters were presented at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. SDEF and this news organization are owned by the same parent company.
Over one-third of psoriasis patients have PsA
About two-thirds of patients with psoriasis in a national registry also had psoriatic arthritis (PsA) and/or psoriasis in at least one challenging-to-treat (CTT) area, and one-quarter had both, according to Kristina Callis Duffin, MD, of the University of Utah, Salt Lake City, and her associates.
Their analysis included 2,042 psoriasis patients who were enrolled in the Corrona Psoriasis Registry between April 2015 and May 2018 and initiated biologic treatment during that time. The mean age was 49.6 years, 80% of the patients were white, and 51% were obese. Mean disease duration was 19.9 years and 89.2% of the patients had moderate to severe disease. CTT areas include the scalp, nails, and palmoplantar areas.
A total of 784 people in the cohort (38.4%) had PsA, 778 (38.1%) had scalp psoriasis, 326 (16.0%) had nail psoriasis, 223 (10.9%) had palmoplantar psoriasis, and 535 (26.2%) had both PsA and psoriasis in at least two CTT areas. The most common combinations were PsA plus scalp psoriasis and PsA plus nail and scalp psoriasis.
“These results indicate a need to further characterize patients with psoriasis who have PsA and CTT areas and evaluate the impact of these factors to better understand their treatment needs,” the investigators noted.
The Corrona registry has been supported by numerous pharmaceutical companies, and the study authors reported numerous financial relationships with industry; two authors are Novartis employees.
Secukinumab effective for slowing radiographic progression in active PsA
Treatment with secukinumab significantly reduced radiographic progression in patients with active PsA, according to Désirée van der Heijde, MD, PhD, professor of rheumatology at Leiden University Medical Center, and her associates.
The results come from an analysis of the FUTURE 5 trial, a study of 996 patients with active PsA despite previous NSAID treatment, disease-modifying antirheumatic drug treatment, or anti–tumor necrosis factor (TNF) therapy. Patients were randomized to receive 300 mg subcutaneous secukinumab with loading dose, 150 mg secukinumab with loading dose, 150 mg secukinumab without loading dose, or placebo, at baseline; weeks 1, 2, 3, and 4; then every 4 weeks.
After 24 weeks, the mean change in van der Heijde–modified Total Sharp Score for PsA was 0.08 for the 300-mg secukinumab group (P less than .01), 0.17 for the 150-mg secukinumab with loading dose group (P less than .05), a reduction of 0.09 for the 150-mg secukinumab without loading dose group (P less than .01), and 0.50 for the placebo group. Lower radiographic progression was seen regardless of prior anti-TNF or concomitant methotrexate treatment.
The study was funded by Novartis. The study authors reported financial disclosures with numerous companies; five authors are Novartis employees.
Tildrakizumab sustains efficacy in plaque psoriasis treatment after 1 year
Nearly all patients receiving the interleukin-23 inhibitor tildrakizumab for the treatment of moderate to severe plaque psoriasis maintained or improved their Psoriasis Area and Severity Index (PASI) response rate after 52 weeks of treatment, compared with their response after 28 weeks.
The analysis, conducted by Boni E. Elewski, MD, of the University of Alabama at Birmingham, and her associates, included 352 patients who received 100 mg tildrakizumab and 313 who received 200 mg tildrakizumab. Treatment was received at baseline, at 4 weeks, and then every 12 weeks afterward.
At week 28, the proportions of patients achieving PASI 100, PASI 90-99, PASI 75-89, and PASI 50-74 at week 28 were 25.9%, 38.4%, 25.3%, and 10.5%, respectively, among those treated with the 100-mg dose. The proportions were 24.6%, 24.3%, 19.5%, and 31.6%, respectively, among those treated with the 200-mg dose.
In patients who achieved at least PASI 90 on either dose at week 28, 88.9%-89.4% maintained that response at week 52. For patients with PASI 75-89, 39.3%-40.4% maintained that response and 33.7%-41.0% achieved a PASI 90 response. At week 52, in patients with PASI 50-74, 20.2%-29.7% achieved at least a PASI 90, 52.5%-64.9% achieved PASI 75, and only 2.6% of patients on either dose had fallen below PASI 50.
Four study authors reported being clinical investigators on studies sponsored by Merck and Sun Pharmaceuticals; five authors are employees of Sun Pharmaceuticals.
Halobetasol/tazarotene combination most effective for plaque psoriasis treatment
A fixed combination of halobetasol propionate 0.01% and tazarotene 0.045% lotion provided a synergistic effect over either component on its own for the treatment of plaque psoriasis, according to Leon H. Kircik, MD, of Indiana University, Indianapolis, and his associates.
The investigators performed a post hoc analysis of 212 patients with moderate to severe plaque psoriasis randomized to receive either the halobetasol/tazarotene combination, halobetasol only, tazarotene only, or vehicle only for 8 weeks, with follow-up at 12 weeks. Treatment success was based on the proportion of patients who achieved at least a 2-grade improvement in the Investigator Global Assessment (IGA) score, IGA scores of “clear” or “almost clear,” and percent change from baseline in IGA multiplied by Body Surface Area (BSA) composite score (IGAxBSA). “Synergy was calculated by summing up the contribution of the individual active ingredients (HP and TAZ) to overall efficacy and comparing to the efficacy achieved with HP/TAZ lotion relative to vehicle,” the authors explained.
Relative to vehicle, treatment success for halobetasol/tazarotene after 8 weeks was 42.8%, 23.6% for halobetasol alone, and 9.0% for tazarotene alone. After 12 weeks, the difference was 31.3%, 14.1%, and 5.9%, respectively. The percent change in IGAxBSA scores from baseline after 8 weeks, relative to vehicle, were 51.6%, 37.3%, and 3.3%, respectively. After 12 weeks, the change was 47.3%, 25.7%, and 8.6%, respectively.
After 8 weeks, the synergy ratio for treatment success and IGAxBSA scores for the halobetasol/tazarotene combination was 1.3. After 12 weeks, the synergy ratio for treatment success was 1.6 and the ratio for IGAxBSA scores was 1.4.
“By combining two agents into one once-daily formulation, this novel formulation reduces the number of product applications and may help patient adherence,” the study authors noted.
Dr. Kircik reported serving as a consultant and investigator for Valeant Pharmaceuticals. One study author is an employee of Bausch Health and Ortho Dermatologics, and another is an employee of Dow Pharmaceutical Sciences (a division of Valeant).
Brodalumab demonstrates low immunogenicity in moderate to severe psoriasis
The immunogenicity of brodalumab in patients with moderate to severe plaque psoriasis was low and did not compromise the efficacy or safety profile of the drug, according to Kristian Reich, MD, of Dermatologikum Berlin and SCIderm Research Institute in Hamburg, Germany, and his associates.
Data from a 12-week, phase 2 trial with a 352-week, open-label extension and three 52-week phase 3 trials were included in the analysis. Antidrug antibodies (ADAs) were tested, and positive samples were further analyzed for neutralizing ADAs by a cell-based assay.
Out of the 4,461 patients who received brodalumab, 122 (2.7%) were positive for ADAs after starting brodalumab. The incidence rate ranged from 1.9% to 3.4% between all dosing groups (140 mg, 210 mg, variable dosing, and 210 mg of brodalumab after ustekinumab). In 58 (1.4%) of patients, ADAs were transient. No patients had neutralizing ADAs, and no evidence of altered pharmacokinetics, loss of efficacy, or changes in the safety profile of brodalumab in subjects positive for ADAs was seen.
No significant difference was seen in the incidence rate of hypersensitivity or injection site reactions in brodalumab, compared with placebo or ustekinumab. The most common injection site reactions were injection site pain, erythema, and bruising.
The study was supported by Amgen. The study authors reported numerous disclosures. Two authors are employees of Leo Pharma, one author is a former employee of the company.
Secukinumab improves patient-reported outcomes in CTT psoriasis
Treatment with secukinumab significantly improved patient-reported outcomes such as fatigue, itch, pain, and quality of life measures in patients with CTT psoriasis after 6 months, according to Jerry Bagel, MD, of the Psoriasis Treatment Center of Central New Jersey, East Windsor, and his associates.
A total of 68 patients with psoriasis localized to at least one CTT area who were enrolled in the Corrona Psoriasis Registry from April 15, 2015, through May 10, 2018, and were receiving secukinumab for the entirety of the 6-month study period were included in the analysis. Patient-reported outcomes included in the analysis were fatigue, itch, pain, Dermatology Quality of Life Index (DLQI) score, and Work Productivity and Activity Impairment (WPAI) scale.
The mean age at enrollment was 51.2 years and almost 80% of patients were white. Mean psoriasis duration was 21.8 years and nearly half had PsA.
Visual analog scale scores improved over baseline for fatigue (mean, 23.2 vs. 33.2; P = .01), itch (20.9 vs. 49.6; P less than .0001), and pain (12.1 vs. 33.8; P less than .0001). DLQI scores also improved (2.9 vs. 8.1; P less than .0001), and the proportion of patients who reported that psoriasis had at least a moderate effect on their life was reduced after 6 months (22.1% vs. 59.7%; P less than .0001).
Based on WPAI results, patients experienced significant improvements in the percentage of daily activities impaired (mean, 9.5% vs 17.5%; P = .0075); of the 42 patients who were employed, both impairment percentage (3.7% vs. 11.2%; P = .0148) and percentage of work hours affected (4.9% vs. 11.9%; P = .0486) were reduced from baseline.
“These results are consistent with previous reports from secukinumab clinical trials; however, additional real-world studies are needed to evaluate the long-term effectiveness of secukinumab for improving [patient-reported outcomes] in patients with psoriasis in CTT areas,” the authors noted.
The Corrona registry has been supported by numerous pharmaceutical companies, and several study authors reported various disclosures with industry. Two authors are Novartis employees. The study was supported by Novartis; the company participated in the interpretation of data and review and approval of the abstract.
These posters were presented at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. SDEF and this news organization are owned by the same parent company.
FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
FDA approves adalimumab biosimilar Hyrimoz
The Food and Drug Administration has approved the adalimumab biosimilar Hyrimoz (adalimumab-adaz) for a variety of conditions, according to Sandoz, the drug’s manufacturer and a division of Novartis.
FDA approval for Hyrimoz is based on a randomized, double-blind, three-arm, parallel biosimilarity study that demonstrated equivalence for all primary pharmacokinetic parameters, according to the press release. A second study confirmed these results in patients with moderate to severe plaque psoriasis, with Hyrimoz having a safety profile similar to that of adalimumab. Hyrimoz was approved in Europe in July 2018.
Hyrimoz has been approved to treat rheumatoid arthritis, juvenile idiopathic arthritis in patients aged 4 years and older, psoriatic arthritis, ankylosing spondylitis, adult Crohn’s disease, ulcerative colitis, and plaque psoriasis. The most common adverse events associated with the drug, according to the label, are infections, injection site reactions, headache, and rash.
Hyrimoz is the third adalimumab biosimilar approved by the FDA.
“Biosimilars can help people suffering from chronic, debilitating conditions gain expanded access to important medicines that may change the outcome of their disease. With the FDA approval of Hyrimoz, Sandoz is one step closer to offering U.S. patients with autoimmune diseases the same critical access already available in Europe,” Stefan Hendriks, global head of biopharmaceuticals at Sandoz, said in the press release.
Find the full press release on the Novartis website.
The Food and Drug Administration has approved the adalimumab biosimilar Hyrimoz (adalimumab-adaz) for a variety of conditions, according to Sandoz, the drug’s manufacturer and a division of Novartis.
FDA approval for Hyrimoz is based on a randomized, double-blind, three-arm, parallel biosimilarity study that demonstrated equivalence for all primary pharmacokinetic parameters, according to the press release. A second study confirmed these results in patients with moderate to severe plaque psoriasis, with Hyrimoz having a safety profile similar to that of adalimumab. Hyrimoz was approved in Europe in July 2018.
Hyrimoz has been approved to treat rheumatoid arthritis, juvenile idiopathic arthritis in patients aged 4 years and older, psoriatic arthritis, ankylosing spondylitis, adult Crohn’s disease, ulcerative colitis, and plaque psoriasis. The most common adverse events associated with the drug, according to the label, are infections, injection site reactions, headache, and rash.
Hyrimoz is the third adalimumab biosimilar approved by the FDA.
“Biosimilars can help people suffering from chronic, debilitating conditions gain expanded access to important medicines that may change the outcome of their disease. With the FDA approval of Hyrimoz, Sandoz is one step closer to offering U.S. patients with autoimmune diseases the same critical access already available in Europe,” Stefan Hendriks, global head of biopharmaceuticals at Sandoz, said in the press release.
Find the full press release on the Novartis website.
The Food and Drug Administration has approved the adalimumab biosimilar Hyrimoz (adalimumab-adaz) for a variety of conditions, according to Sandoz, the drug’s manufacturer and a division of Novartis.
FDA approval for Hyrimoz is based on a randomized, double-blind, three-arm, parallel biosimilarity study that demonstrated equivalence for all primary pharmacokinetic parameters, according to the press release. A second study confirmed these results in patients with moderate to severe plaque psoriasis, with Hyrimoz having a safety profile similar to that of adalimumab. Hyrimoz was approved in Europe in July 2018.
Hyrimoz has been approved to treat rheumatoid arthritis, juvenile idiopathic arthritis in patients aged 4 years and older, psoriatic arthritis, ankylosing spondylitis, adult Crohn’s disease, ulcerative colitis, and plaque psoriasis. The most common adverse events associated with the drug, according to the label, are infections, injection site reactions, headache, and rash.
Hyrimoz is the third adalimumab biosimilar approved by the FDA.
“Biosimilars can help people suffering from chronic, debilitating conditions gain expanded access to important medicines that may change the outcome of their disease. With the FDA approval of Hyrimoz, Sandoz is one step closer to offering U.S. patients with autoimmune diseases the same critical access already available in Europe,” Stefan Hendriks, global head of biopharmaceuticals at Sandoz, said in the press release.
Find the full press release on the Novartis website.
Psoriasis adds to increased risk of cardiovascular procedures, surgery in patients with hypertension
compared with patients with hypertension alone.
“The results suggested that hypertensive patients with concurrent psoriasis experienced an earlier and more aggressive disease progression of hypertension, compared with general hypertensive patients,” Hsien-Yi Chiu, MD, PhD, from the department of dermatology at the National Taiwan University Hospital in Hsinchu, Taiwan, and his colleagues wrote in the Journal of Dermatology. “Thus, patients with hypertension and psoriasis should be considered for more aggressive strategies for prevention of primary [cardiovascular disease] and more intense assessments for cardiovascular interventions needed to improve [cardiovascular disease] outcome in these patients.”
They performed a nationwide cohort study of patients in the Taiwan National Health Insurance Research Database with new onset hypertension from 2005 to 2006. Those with psoriasis (4,039 patients) were matched by age and sex to patients in the database who were diagnosed with hypertension but not psoriasis; the mean follow-up was 5.62 years. Their mean age was 58 years and about 31% of the psoriasis cohort were female. They were divided into groups based on psoriasis severity (mild and severe psoriasis) and type (psoriasis with and without arthritis). Researchers noted patients with both psoriasis and hypertension also had higher rates of cerebrovascular disease, coronary heart disease, hyperlipidemia, and diabetes mellitus during the year prior to the study.
The outcome measured was having a cardiovascular procedure (percutaneous coronary intervention with/without stenting or percutaneous transluminal coronary angioplasty and transcatheter radiofrequency ablation for arrhythmia) and cardiovascular surgery (coronary artery bypass grafting and other surgery for heart valves, arrhythmia, cerebrovascular disease, peripheral vessels, and the aorta).
Patients with both psoriasis and hypertension were at an increased risk for having a cardiovascular procedure and surgery (adjusted hazard ratio, 1.28; 95% confidence interval, 1.07-1.53), compared with patients with only hypertension. The risk of this outcome was also increased among patients with severe psoriasis or psoriatic arthritis, compared with patients who had mild psoriasis (aHR, 1.22; 95% CI, 0.98-1.51) and with patients with psoriasis but not arthritis (aHR, 1.15; 95% CI, 0.84-1.58); however, the results did not reach statistical significance after adjustment, which the researchers attributed to the small subgroup size.
“Another possible explanation was that the observed increased requirement for cardiovascular procedure and surgery in patients with severe psoriasis was mediated by a complex interplay among inflammation, traditional risk factors for [cardiovascular disease], and antirheumatic drugs, which probably attenuate the effects conferred by psoriasis,” the authors wrote.
Limitations in the study included reliance on administrative claims data for psoriasis diagnosis, unavailability of some details of the cardiovascular procedures and surgery, lack of blood pressure data to identify hypertension severity, as well as unmeasured factors and confounders. Further, “comparative occurrence of a requirement for cardiovascular procedure and surgery in the two groups may be influenced by a competing risk for death,” the researchers noted.
This study was supported in part through grants by the National Taiwan University Hospital, Asia-Pacific La Roche–Posay Foundation 2014, and the Ministry of Science and Technology. Dr. Chiu is on the speaker’s bureau for AbbVie, Janssen Pharmaceuticals, Novartis, Eli Lilly and Pfizer. Another author has conducted clinical trials for or received fees for being a consultant or speaker for companies that include Abbvie, Boehringer Ingelheim, and Celgene. The remaining authors reported no relevant conflicts of interest.
SOURCE: Chiu H-Y et al. J Dermatol. 2018 Oct 16. doi: 10.1111/1346-8138.14654.
compared with patients with hypertension alone.
“The results suggested that hypertensive patients with concurrent psoriasis experienced an earlier and more aggressive disease progression of hypertension, compared with general hypertensive patients,” Hsien-Yi Chiu, MD, PhD, from the department of dermatology at the National Taiwan University Hospital in Hsinchu, Taiwan, and his colleagues wrote in the Journal of Dermatology. “Thus, patients with hypertension and psoriasis should be considered for more aggressive strategies for prevention of primary [cardiovascular disease] and more intense assessments for cardiovascular interventions needed to improve [cardiovascular disease] outcome in these patients.”
They performed a nationwide cohort study of patients in the Taiwan National Health Insurance Research Database with new onset hypertension from 2005 to 2006. Those with psoriasis (4,039 patients) were matched by age and sex to patients in the database who were diagnosed with hypertension but not psoriasis; the mean follow-up was 5.62 years. Their mean age was 58 years and about 31% of the psoriasis cohort were female. They were divided into groups based on psoriasis severity (mild and severe psoriasis) and type (psoriasis with and without arthritis). Researchers noted patients with both psoriasis and hypertension also had higher rates of cerebrovascular disease, coronary heart disease, hyperlipidemia, and diabetes mellitus during the year prior to the study.
The outcome measured was having a cardiovascular procedure (percutaneous coronary intervention with/without stenting or percutaneous transluminal coronary angioplasty and transcatheter radiofrequency ablation for arrhythmia) and cardiovascular surgery (coronary artery bypass grafting and other surgery for heart valves, arrhythmia, cerebrovascular disease, peripheral vessels, and the aorta).
Patients with both psoriasis and hypertension were at an increased risk for having a cardiovascular procedure and surgery (adjusted hazard ratio, 1.28; 95% confidence interval, 1.07-1.53), compared with patients with only hypertension. The risk of this outcome was also increased among patients with severe psoriasis or psoriatic arthritis, compared with patients who had mild psoriasis (aHR, 1.22; 95% CI, 0.98-1.51) and with patients with psoriasis but not arthritis (aHR, 1.15; 95% CI, 0.84-1.58); however, the results did not reach statistical significance after adjustment, which the researchers attributed to the small subgroup size.
“Another possible explanation was that the observed increased requirement for cardiovascular procedure and surgery in patients with severe psoriasis was mediated by a complex interplay among inflammation, traditional risk factors for [cardiovascular disease], and antirheumatic drugs, which probably attenuate the effects conferred by psoriasis,” the authors wrote.
Limitations in the study included reliance on administrative claims data for psoriasis diagnosis, unavailability of some details of the cardiovascular procedures and surgery, lack of blood pressure data to identify hypertension severity, as well as unmeasured factors and confounders. Further, “comparative occurrence of a requirement for cardiovascular procedure and surgery in the two groups may be influenced by a competing risk for death,” the researchers noted.
This study was supported in part through grants by the National Taiwan University Hospital, Asia-Pacific La Roche–Posay Foundation 2014, and the Ministry of Science and Technology. Dr. Chiu is on the speaker’s bureau for AbbVie, Janssen Pharmaceuticals, Novartis, Eli Lilly and Pfizer. Another author has conducted clinical trials for or received fees for being a consultant or speaker for companies that include Abbvie, Boehringer Ingelheim, and Celgene. The remaining authors reported no relevant conflicts of interest.
SOURCE: Chiu H-Y et al. J Dermatol. 2018 Oct 16. doi: 10.1111/1346-8138.14654.
compared with patients with hypertension alone.
“The results suggested that hypertensive patients with concurrent psoriasis experienced an earlier and more aggressive disease progression of hypertension, compared with general hypertensive patients,” Hsien-Yi Chiu, MD, PhD, from the department of dermatology at the National Taiwan University Hospital in Hsinchu, Taiwan, and his colleagues wrote in the Journal of Dermatology. “Thus, patients with hypertension and psoriasis should be considered for more aggressive strategies for prevention of primary [cardiovascular disease] and more intense assessments for cardiovascular interventions needed to improve [cardiovascular disease] outcome in these patients.”
They performed a nationwide cohort study of patients in the Taiwan National Health Insurance Research Database with new onset hypertension from 2005 to 2006. Those with psoriasis (4,039 patients) were matched by age and sex to patients in the database who were diagnosed with hypertension but not psoriasis; the mean follow-up was 5.62 years. Their mean age was 58 years and about 31% of the psoriasis cohort were female. They were divided into groups based on psoriasis severity (mild and severe psoriasis) and type (psoriasis with and without arthritis). Researchers noted patients with both psoriasis and hypertension also had higher rates of cerebrovascular disease, coronary heart disease, hyperlipidemia, and diabetes mellitus during the year prior to the study.
The outcome measured was having a cardiovascular procedure (percutaneous coronary intervention with/without stenting or percutaneous transluminal coronary angioplasty and transcatheter radiofrequency ablation for arrhythmia) and cardiovascular surgery (coronary artery bypass grafting and other surgery for heart valves, arrhythmia, cerebrovascular disease, peripheral vessels, and the aorta).
Patients with both psoriasis and hypertension were at an increased risk for having a cardiovascular procedure and surgery (adjusted hazard ratio, 1.28; 95% confidence interval, 1.07-1.53), compared with patients with only hypertension. The risk of this outcome was also increased among patients with severe psoriasis or psoriatic arthritis, compared with patients who had mild psoriasis (aHR, 1.22; 95% CI, 0.98-1.51) and with patients with psoriasis but not arthritis (aHR, 1.15; 95% CI, 0.84-1.58); however, the results did not reach statistical significance after adjustment, which the researchers attributed to the small subgroup size.
“Another possible explanation was that the observed increased requirement for cardiovascular procedure and surgery in patients with severe psoriasis was mediated by a complex interplay among inflammation, traditional risk factors for [cardiovascular disease], and antirheumatic drugs, which probably attenuate the effects conferred by psoriasis,” the authors wrote.
Limitations in the study included reliance on administrative claims data for psoriasis diagnosis, unavailability of some details of the cardiovascular procedures and surgery, lack of blood pressure data to identify hypertension severity, as well as unmeasured factors and confounders. Further, “comparative occurrence of a requirement for cardiovascular procedure and surgery in the two groups may be influenced by a competing risk for death,” the researchers noted.
This study was supported in part through grants by the National Taiwan University Hospital, Asia-Pacific La Roche–Posay Foundation 2014, and the Ministry of Science and Technology. Dr. Chiu is on the speaker’s bureau for AbbVie, Janssen Pharmaceuticals, Novartis, Eli Lilly and Pfizer. Another author has conducted clinical trials for or received fees for being a consultant or speaker for companies that include Abbvie, Boehringer Ingelheim, and Celgene. The remaining authors reported no relevant conflicts of interest.
SOURCE: Chiu H-Y et al. J Dermatol. 2018 Oct 16. doi: 10.1111/1346-8138.14654.
FROM THE JOURNAL OF DERMATOLOGY
Key clinical point: More aggressive cardiovascular disease preventive strategies should be considered in patients with hypertension who also have psoriasis.
Major finding: Patients with both psoriasis and hypertension were at an increased risk for requiring a cardiovascular procedure and surgery (adjusted hazard ratio, 1.28), compared with patients with hypertension alone.
Study details: A retrospective cohort study evaluated risk of this outcome in 4,039 patients with psoriasis and hypertension, compared with patients who had hypertension, matched for age and sex.
Disclosures: This study was supported in part through grants by the National Taiwan University Hospital Hsin-Chu Branch, Asia-Pacific La Roche–Posay Foundation 2014, and the Ministry of Science and Technology. Dr. Chiu is on the speaker’s bureau for companies including AbbVie, Novartis, and Eli Lilly. Another author has conducted clinical trials for or received fees for being a consultant or speaker for Abbvie, Boehringer Ingelheim, Celgene, Janssen Pharmaceuticals, Eli Lilly, Galderma, Novartis, and Pfizer. The other authors reported no relevant conflicts of interest.
Source: Chiu H-Y et al. J Dermatol. 2018 Oct 16. doi:10.1111/1346-8138.14654.
Psychosocial Impact of Psoriasis: A Review for Dermatology Residents
The psychosocial impact of psoriasis is a critical component of disease burden. Psoriatic patients have high rates of depression and anxiety, problems at work, and difficulties with interpersonal relationships and intimacy.1 A National Psoriasis Foundation (NPF) survey from 2003 to 2011 reported that psoriasis affects overall emotional well-being in 88% of patients and enjoyment of life in 82% of patients.2
The reasons for psychosocial burden stem from public misconceptions and disease stigma. A survey of 1005 individuals (age range, 16–64 years) about their perceptions of psoriasis revealed that 16.5% believed that psoriasis is contagious and 6.8% believed that psoriasis is related to personal hygiene.3 Fifty percent practiced discriminatory behavior toward psoriatic patients, including reluctance to shake hands (28.8%) and engage in sexual relations/intercourse (44.1%). Sixty-five percent of psoriatic patients felt their appearance is unsightly, and 73% felt self-conscious about having psoriasis.2
The psychosocial burden exists despite medical treatment of the disease. In a cross-sectional study of 1184 psoriatic patients, 70.2% had impaired quality of life (QOL) as measured by the dermatology life quality index (DLQI), even after receiving a 4-week treatment for psoriasis.4 Medical treatment of psoriasis is not enough; providers need to assess overall QOL and provide treatment and resources for these patients in addition to symptomatic management.
There have been many studies on the psychosocial burden of psoriasis, but few have focused on a dermatology resident’s role in addressing this issue. This article will review psychosocial domains—psychiatric comorbidities and social functioning including occupational functioning, interpersonal relationships, and sexual functioning— and discuss a dermatology resident’s role in assessing and addressing each of these areas.
Methods
A PubMed search of articles indexed for MEDLINE was conducted using the following terms: psoriasis, depression, anxiety, work productivity, sexual functioning, and interpersonal relationships. Selected articles covered prevalence, assessment, and management of each psychosocial domain.
Results
Psychiatric Comorbidities
Prevalence
A high prevalence of psychiatric comorbidities exists in psoriatic patients. In a study of 469,097 patients with psoriasis, depression was the third most prevalent comorbidity (17.91%), following hyperlipidemia (45.64%) and hypertension (42.19%).5 In a 10-year longitudinal, population-based, prospective cohort study, antidepressant prescriptions were twice as frequent in psoriatic patients (17.8%) compared to control (7.9%)(P<.001).6 In a meta-analysis of 98 studies investigating psoriatic patients and psychiatric comorbidities, patients with psoriasis were 1.5 times more likely to experience depression (odds ratio [OR]: 1.57; 95% CI, 1.40-1.76) and use antidepressants (OR: 4.24; 95% CI, 1.53-11.76) compared to control.7 Patients with psoriasis were more likely to attempt suicide (OR: 1.32; 95% CI, 1.14-1.54) and complete suicide (OR: 1.20; 95% CI, 1.04-1.39) compared to people without psoriasis.8 A 1-year cross-sectional study of 90 psoriatic patients reported 78.7% were diagnosed with depression and 76.7% were diagnosed with anxiety. Seventy-two percent reported both anxiety and depression, correlating with worse QOL (χ2=26.7; P<.05).9
Assessment
Psychiatric comorbidities are assessed using clinical judgment and formal screening questionnaires in research studies. Signs of depression in patients with psoriasis can manifest as poor treatment adherence and recurrent flares of psoriasis.10,11 Psoriatic patients with psychiatric comorbidities were less likely to be adherent to treatment (risk ratio: 0.35; P<.003).10 The patient health questionnaire (PHQ) 9 and generalized anxiety disorder scale (GAD) 7 are validated and reliable questionnaires. The first 2 questions in PHQ-9 and GAD-7 screen for depression and anxiety, respectively.12-14 These 2-question screens are practical in a fast-paced dermatology outpatient setting. Systematic questionnaires specifically targeting mood disorders may be more beneficial than the widely used DLQI, which may not adequately capture mood disorders. Over the course of 10 months, 607 patients with psoriasis were asked to fill out the PHQ-9, GAD-7, and DLQI. Thirty-eight percent of patients with major depressive disorder had a DLQI score lower than 10, while 46% of patients with generalized anxiety disorder had a DLQI score lower than 10.15 Other questionnaires, including the hospital anxiety and depression scale and Beck depression inventory, are valid instruments with high sensitivity but are commonly used for research purposes and may not be clinically feasible.16
Management
Dermatologists should refer patients with depression and/or anxiety to psychiatry. Interventions include pharmacologic and nonpharmacologic management. First-line therapy for depression and anxiety is a combination of selective serotonin reuptake inhibitors and cognitive behavioral therapy.17 In addition, providers can direct patients to online resources such as the NPF website, where patients with psoriasis can access information about the signs and symptoms of mood disorders and contact the patient navigation center for further help.18
Social Functioning
Occupational Prevalence
The NPF found that 92% of patients with psoriasis or psoriatic arthritis (PsA) surveyed between 2003 and 2011 cited their psoriasis as reason for unemployment.2 In a survey of 43 patients asked about social and occupational functioning using the social and occupational assessment scale, 62.5% of psoriatic patients reported distress at work and 51.1% reported decreased efficiency at work.19 A national online survey that was conducted in France and issued to patients with and without psoriasis assessed overall QOL and work productivity using the work productivity and activity impairment questionnaire for psoriasis (WPAI-PSO). Of 714 patients with psoriasis and PsA, the latter had a 57.6% decrease in work productivity over 7 days compared to 27.9% in controls (P<.05).20 Occupational impairment leads to lost wages and hinders advancement, further exacerbating the psychosocial burden of psoriasis.21
Occupational Assessment
Formal assessment of occupational function can be done with the WPAI-PSO, a 6-question valid instrument.22 Providers may look for risk factors associated with greater loss in work productivity to help identify and offer support for patients. Patients with increased severity of itching, pain, and scaling experienced a greater decrease in work productivity.21,23 Patients with PsA warrant early detection and treatment because they experience greater physical restraints that can interfere with work activities. Of the 459 psoriatic patients without a prior diagnosis of PsA who filled out the PsA screening and evaluation questionnaire, 144 (31.4%) received a score of 44 or higher and were referred to rheumatology for further evaluation with the classification criteria for PsA. Nine percent of patients failed to be screened and remained undiagnosed with PsA.24 In a study using the health assessment questionnaire to assess 400 patients with PsA, those with worse physical function due to joint pain and stiffness were less likely to remain employed (OR: 0.56; P=.02).25
Occupational Management
Identifying and coordinating symptoms of PsA between dermatology and rheumatology is beneficial for patients who experience debilitating symptoms. There are a variety of treatments available for PsA. According to the European League Against Rheumatism 2015 guidelines developed from expert opinion and systematic reviews for PsA management, there are 4 phases of treatment, with reassessment every 3 to 6 months for effectiveness of therapy.26,27 Phase I involves initiating nonsteroidal anti-inflammatory drugs with or without glucocorticoid injections. Phase II involves synthetic disease-modifying drugs, including methotrexate, leflunomide, sulfasalazine, or cyclosporine. Phase III involves adding a second synthetic disease-modifying drug or starting a biologic, such as an anti–tumor necrosis factor, IL-12/IL-23, or IL-17 inhibitor. Phase IV involves switching to a different drug in either aforementioned class.26,27 Treatment with biologics improves work productivity as assessed by WPAI-PSO for psoriasis and PsA.28-30 Encouraging patients to speak up in the workplace and request small accommodations such as timely breaks or ergonomic chairs can help patients feel more comfortable and supported in the work environment.18 Patients who felt supported at work were more likely to remain employed.25
Interpersonal Relationships Prevalence
Misinformation about psoriasis, fear of rejection, and feelings of isolation may contribute to interpersonal conflict. Patients have feelings of shame and self-consciousness that hinder them from engaging in social activities and seeking out relationships.31 Twenty-nine percent of patients feel that psoriasis has interfered with establishing relationships because of negative self-esteem associated with the disease,32 and 26.3% have experienced people avoiding physical contact.33 Family and spouses of patients with psoriasis may be secondarily affected due to economic and emotional distress. Ninety-eight percent of family members of psoriatic patients experienced emotional distress and 54% experienced the burden of care.34 In a survey of 63 relatives and partners of patients with psoriasis, 57% experienced psychological distress, including anxiety and worry over a psoriatic patient’s future.35
Interpersonal Relationships Assessment
Current available tools, including the DLQI and short form health survey, measure overall QOL, including social functioning, but may not be practical in a clinic setting. Although no quick-screening test to assess for this domain exists, providers are encouraged to ask patients about disease impact on interpersonal relationships. The family DLQI questionnaire, adapted from the DLQI, may help physicians and social workers evaluate the burden on a patient’s family members.34
Interpersonal Relationships Management
It may be difficult for providers to address problems with interpersonal relationships without accessible tools. Patients may not be accompanied by family or friends during appointments, and it is difficult to screen for these issues during visits. Providers may offer resources such as the NPF website, which provides information about support groups. It also provides tips on dating and connecting to others in the community who share similar experiences.18 Encouraging patients to seek family or couples therapy also may be beneficial. Increased social support can lead to better QOL and fewer depressive symptoms.36
Sexual Functioning Prevalence
Psoriasis affects both physical and psychological components of sexual function. Among 3485 patients with skin conditions who were surveyed about sexual function, 34% of psoriatic patients reported that psoriasis interfered with sexual functioning at least to a certain degree.37 Sexual impairment was strongly associated with depression, anxiety, and suicidal ideation; 24% of depressed patients and 20% of anxious patients experienced sexual problems a lot or very much, based on the DLQI.37 Depending on the questionnaire used, the prevalence of sexual dysfunction due to psoriasis ranged from 35.5% to 71.3%.38 In an observational cohort study of 158 participants (n=79 psoriasis patients and n=79 controls), 34.2% of patients with psoriasis experienced erectile dysfunction compared to 17.7% of controls.39 Forty-two percent of psoriatic patients with genital involvement reported dyspareunia, 32% reported worsening of genital psoriasis after intercourse, and 43% reported decreased frequency of intercourse.40
Sexual Functioning Assessment
The Skindex-29, DLQI, and psoriasis disability index are available QOL tools that include one question evaluating difficulties with sexual function. The
Sexual Functioning Management
Better disease control leads to improved sexual function, as patients experience fewer feelings of shame, anxiety, and depression, as well as improvement of physical symptoms that can interfere with sexual functioning.38,43,44 Reducing friction, warmth, and moisture, as well as avoiding tight clothing, can help those with genital psoriasis. Patients are advised to reapply topical medications after sexual intercourse. Patients also can apply makeup to disguise psoriasis and help reduce feelings of self-consciousness that can impede sexual intimacy.18
Comment
The psychosocial burden of psoriasis penetrates many facets of patient lives. Psoriasis can invoke feelings of shame and embarrassment that are worsened by the public’s misconceptions about psoriasis, resulting in serious mental health issues that can cause even greater disability. Depression and anxiety are prevalent in patients with psoriasis. The characteristic symptoms of pain and pruritus along with psychiatric comorbidities can have an underestimated impact on daily activities, including employment, interpersonal relationships, and sexual function. Such dysfunctions have serious implications toward wages, professional advancement, social support, and overall QOL.
Dermatology providers play an important role in screening for these problems through validated questionnaires and identifying risks. Simple screening questions such as the PHQ-9 can be beneficial and feasible during dermatology visits. Screening for PsA can help patients avoid problems at work. Sexual dysfunction is a sensitive topic; however, providers can use a 1-question screen from valid questionnaires and inquire about the location of lesions as opportunities to address this issue.
Interventions lead to better disease control, which concurrently improves overall QOL. These interventions depend on both patient adherence and a physician’s commitment to finding an optimal treatment regimen for each individual. Medical management; coordinating care; developing treatment plans with psychiatry, rheumatology, and primary care providers; and psychological counseling and services may be necessary and beneficial (Table). Offering accessible resources such as the NPF website helps patients access information outside the clinic when it is not feasible to address all these concerns in a single visit. Psoriasis requires more than just medical management; it requires dermatology providers to use a multidisciplinary approach to address the psychosocial aspects of the disease.
Conclusion
The psychosocial burden of psoriasis is immense. Stigma, public misconception, mental health concerns, and occupational and interpersonal difficulty are the basis of disease burden. Providers play a vital role in assessing the effect psoriasis has on different areas of patients’ lives and providing appropriate interventions and resources to reduce disease burden.
- Kimball AB, Jacobson C, Weiss S, et al. The psychosocial burden of psoriasis. Am J Clin Dermatol. 2005;6:383-392.
- Armstrong AW, Schupp C, Wu J, et al. Quality of life and work productivity impairment among psoriasis patients: findings from the National Psoriasis Foundation survey data 2003-2011. PloS One. 2012;7:e52935.
- Halioua B, Sid-Mohand D, Roussel ME, et al. Extent of misconceptions, negative prejudices and discriminatory behaviour to psoriasis patients in France. J Eur Acad Dermatol Venereol. 2016;30:650-654.
- Wolf P, Weger W, Legat F, et al. Quality of life and treatment goals in psoriasis from the patient perspective: results of an Austrian cross-sectional survey. J Dtsch Dermatol Ges. 2018;16:981-990.
- Shah K, Mellars L, Changolkar A, et al. Real-world burden of comorbidities in US patients with psoriasis. J Am Acad Dermatol. 2017;77:287-292.e4.
- Dowlatshahi EA, Wakkee M, Herings RM, et al. Increased antidepressant drug exposure in psoriasis patients: a longitudinal population-based cohort study. Acta Derm Venereol. 2013;93:544-550.
- Dowlatshahi EA, Wakkee M, Arends LR, et al. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol. 2014;134:1542-1551.
- Singh S, Taylor C, Kornmehl H, et al. Psoriasis and suicidality: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:425.e2-440.e2.
- Lakshmy S, Balasundaram S, Sarkar S, et al. A cross-sectional study of prevalence and implications of depression and anxiety in psoriasis. Indian J Psychol Med. 2015;37:434-440.
- Renzi C, Picardi A, Abeni D, et al. Association of dissatisfaction with care and psychiatric morbidity with poor treatment compliance. Arch Dermatol. 2002;138:337-342.
- Kulkarni AS, Balkrishnan R, Camacho FT, et al. Medication and health care service utilization related to depressive symptoms in older adults with psoriasis. J Drugs Dermatol. 2004;3:661-666.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
- Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
- Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41:1284-1292.
- Lamb RC, Matcham F, Turner MA, et al. Screening for anxiety and depression in people with psoriasis: a cross-sectional study in a tertiary referral setting. Br J Dermatol. 2017;176:1028-1034.
- Law M, Naughton MT, Dhar A, et al. Validation of two depression screening instruments in a sleep disorders clinic. J Clin Sleep Med. 2014;10:683-688.
- Cuijpers P, Dekker J, Hollon SD, et al. Adding psychotherapy to pharmacotherapy in the treatment of depressive disorders in adults: a meta-analysis. J Clin Psychiatry. 2009;70:1219-1229.
- National Psoriasis Foundation. Living with psoriatic arthritis. https://www.psoriasis.org/life-with-psoriatic-arthritis. Accessed September 23, 2018.
- Gaikwad R, Deshpande S, Raje S, et al. Evaluation of functional impairment in psoriasis. Indian J Dermatol Venereol Leprol. 2006;72:37-40.
- Claudepierre P, Lahfa M, Levy P, et al. The impact of psoriasis on professional life: PsoPRO, a French national survey [published online April 6, 2018]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.14986.
- Korman NJ, Zhao Y, Pike J, et al. Relationship between psoriasis severity, clinical symptoms, quality of life and work productivity among patients in the USA. Clin Exp Dermatol. 2016;41:514-521.
- Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. PharmacoEconomics. 1993;4:353-365.
- Korman NJ, Zhao Y, Pike J, et al. Increased severity of itching, pain, and scaling in psoriasis patients is associated with increased disease severity, reduced quality of life, and reduced work productivity. Dermatol Online J. 2015;21.
- Spelman L, Su JC, Fernandez-Penas P, et al. Frequency of undiagnosed psoriatic arthritis among psoriasis patients in Australian dermatology practice. J Eur Acad Dermatol Venereol. 2015;29:2184-2191.
- Tillett W, Shaddick G, Askari A, et al. Factors influencing work disability in psoriatic arthritis: first results from a large UK multicentre study. Rheumatology (Oxford). 2015;54:157-162.
- Raychaudhuri SP, Wilken R, Sukhov AC, et al. Management of psoriatic arthritis: early diagnosis, monitoring of disease severity and cutting edge therapies. J Autoimmun. 2017;76:21-37.
- Gossec L, Smolen JS, Ramiro S, et al. European League Against Rheumatism (EULAR) recommendations for the manegement of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75:499-510.
- Beroukhim K, Danesh M, Nguyen C, et al. A prospective, interventional assessment of the impact of ustekinumab treatment on psoriasis-related work productivity and activity impairment. J Dermatol Treat. 2016;27:552-555.
- Armstrong AW, Lynde CW, McBride SR, et al. Effect of ixekizumab treatment on work productivity for patients with moderate-to-severe plaque psoriasis: analysis of results from 3 randomized phase 3 clinical trials. JAMA Dermatol. 2016;152:661-669.
- Kimball AB, Yu AP, Signorovitch J, et al. The effects of adalimumab treatment and psoriasis severity on self-reported work productivity and activity impairment for patients with moderate to severe psoriasis. J Am Acad Dermatol. 2012;66:e67-76.
- Feldman SR, Malakouti M, Koo JY. Social impact of the burden of psoriasis: effects on patients and practice. Dermatol Online J. 2014;20.
- Reich A, Welz-Kubiak K, Rams Ł. Apprehension of the disease by patients suffering from psoriasis. Postepy Dermatol Alergol. 2014;31:289-293.
- Gupta MA, Gupta AK, Watteel GN. Perceived deprivation of social touch in psoriasis is associated with greater psychologic morbidity: an index of the stigma experience in dermatologic disorders. Cutis. 1998;61:339-342.
- Basra MK, Finlay AY. The family impact of skin diseases: the Greater Patient concept. Br J Dermatol. 2007;156:929-937.
- Eghlileb AM, Davies EE, Finlay AY. Psoriasis has a major secondary impact on the lives of family members and partners. Br J Dermatol. 2007;156:1245-1250.
- Janowski K, Steuden S, Pietrzak A, et al. Social support and adaptation to the disease in men and women with psoriasis. Arch Dermatol Res. 2012;304:421-432.
- Sampogna F, Abeni D, Gieler U, et al. Impairment of sexual life in 3,485 dermatological outpatients from a multicentre study in 13 European countries. Acta Derm Venereol. 2017;97:478-482.
- Sampogna F, Gisondi P, Tabolli S, et al. Impairment of sexual life in patients with psoriasis. Dermatology. 2007;214:144-150.
- Molina-Leyva A, Molina-Leyva I, Almodovar-Real A, et al. Prevalence and associated factors of erectile dysfunction in patients with moderate to severe psoriasis and healthy population: a comparative study considering physical and psychological factors. Arch Sex Behav. 2016;45:2047-2055.
- Ryan C, Sadlier M, De Vol E, et al. Genital psoriasis is associated with significant impairment in quality of life and sexual functioning. J Am Acad Dermatol. 2015;72:978-983.
- Labbate LA, Lare SB. Sexual dysfunction in male psychiatric outpatients: validity of the Massachusetts General Hospital Sexual Functioning Questionnaire. Psychother Psychosom. 2001;70:221-225.
- Molina-Leyva A, Almodovar-Real A, Ruiz-Carrascosa JC, et al. Distribution pattern of psoriasis affects sexual function in moderate to severe psoriasis: a prospective case series study. J Sex Med. 2014;11:2882-2889.
- Guenther L, Han C, Szapary P, et al. Impact of ustekinumab on health-related quality of life and sexual difficulties associated with psoriasis: results from two phase III clinical trials. J Eur Acad Dermatol Venereol. 2011;25:851-857.
- Guenther L, Warren RB, Cather JC, et al. Impact of ixekizumab treatment on skin-related personal relationship difficulties in moderate-to-severe psoriasis patients: 12-week results from two Phase 3 trials. J Eur Acad Dermatol Venereol. 2017;31:1867-1875.
The psychosocial impact of psoriasis is a critical component of disease burden. Psoriatic patients have high rates of depression and anxiety, problems at work, and difficulties with interpersonal relationships and intimacy.1 A National Psoriasis Foundation (NPF) survey from 2003 to 2011 reported that psoriasis affects overall emotional well-being in 88% of patients and enjoyment of life in 82% of patients.2
The reasons for psychosocial burden stem from public misconceptions and disease stigma. A survey of 1005 individuals (age range, 16–64 years) about their perceptions of psoriasis revealed that 16.5% believed that psoriasis is contagious and 6.8% believed that psoriasis is related to personal hygiene.3 Fifty percent practiced discriminatory behavior toward psoriatic patients, including reluctance to shake hands (28.8%) and engage in sexual relations/intercourse (44.1%). Sixty-five percent of psoriatic patients felt their appearance is unsightly, and 73% felt self-conscious about having psoriasis.2
The psychosocial burden exists despite medical treatment of the disease. In a cross-sectional study of 1184 psoriatic patients, 70.2% had impaired quality of life (QOL) as measured by the dermatology life quality index (DLQI), even after receiving a 4-week treatment for psoriasis.4 Medical treatment of psoriasis is not enough; providers need to assess overall QOL and provide treatment and resources for these patients in addition to symptomatic management.
There have been many studies on the psychosocial burden of psoriasis, but few have focused on a dermatology resident’s role in addressing this issue. This article will review psychosocial domains—psychiatric comorbidities and social functioning including occupational functioning, interpersonal relationships, and sexual functioning— and discuss a dermatology resident’s role in assessing and addressing each of these areas.
Methods
A PubMed search of articles indexed for MEDLINE was conducted using the following terms: psoriasis, depression, anxiety, work productivity, sexual functioning, and interpersonal relationships. Selected articles covered prevalence, assessment, and management of each psychosocial domain.
Results
Psychiatric Comorbidities
Prevalence
A high prevalence of psychiatric comorbidities exists in psoriatic patients. In a study of 469,097 patients with psoriasis, depression was the third most prevalent comorbidity (17.91%), following hyperlipidemia (45.64%) and hypertension (42.19%).5 In a 10-year longitudinal, population-based, prospective cohort study, antidepressant prescriptions were twice as frequent in psoriatic patients (17.8%) compared to control (7.9%)(P<.001).6 In a meta-analysis of 98 studies investigating psoriatic patients and psychiatric comorbidities, patients with psoriasis were 1.5 times more likely to experience depression (odds ratio [OR]: 1.57; 95% CI, 1.40-1.76) and use antidepressants (OR: 4.24; 95% CI, 1.53-11.76) compared to control.7 Patients with psoriasis were more likely to attempt suicide (OR: 1.32; 95% CI, 1.14-1.54) and complete suicide (OR: 1.20; 95% CI, 1.04-1.39) compared to people without psoriasis.8 A 1-year cross-sectional study of 90 psoriatic patients reported 78.7% were diagnosed with depression and 76.7% were diagnosed with anxiety. Seventy-two percent reported both anxiety and depression, correlating with worse QOL (χ2=26.7; P<.05).9
Assessment
Psychiatric comorbidities are assessed using clinical judgment and formal screening questionnaires in research studies. Signs of depression in patients with psoriasis can manifest as poor treatment adherence and recurrent flares of psoriasis.10,11 Psoriatic patients with psychiatric comorbidities were less likely to be adherent to treatment (risk ratio: 0.35; P<.003).10 The patient health questionnaire (PHQ) 9 and generalized anxiety disorder scale (GAD) 7 are validated and reliable questionnaires. The first 2 questions in PHQ-9 and GAD-7 screen for depression and anxiety, respectively.12-14 These 2-question screens are practical in a fast-paced dermatology outpatient setting. Systematic questionnaires specifically targeting mood disorders may be more beneficial than the widely used DLQI, which may not adequately capture mood disorders. Over the course of 10 months, 607 patients with psoriasis were asked to fill out the PHQ-9, GAD-7, and DLQI. Thirty-eight percent of patients with major depressive disorder had a DLQI score lower than 10, while 46% of patients with generalized anxiety disorder had a DLQI score lower than 10.15 Other questionnaires, including the hospital anxiety and depression scale and Beck depression inventory, are valid instruments with high sensitivity but are commonly used for research purposes and may not be clinically feasible.16
Management
Dermatologists should refer patients with depression and/or anxiety to psychiatry. Interventions include pharmacologic and nonpharmacologic management. First-line therapy for depression and anxiety is a combination of selective serotonin reuptake inhibitors and cognitive behavioral therapy.17 In addition, providers can direct patients to online resources such as the NPF website, where patients with psoriasis can access information about the signs and symptoms of mood disorders and contact the patient navigation center for further help.18
Social Functioning
Occupational Prevalence
The NPF found that 92% of patients with psoriasis or psoriatic arthritis (PsA) surveyed between 2003 and 2011 cited their psoriasis as reason for unemployment.2 In a survey of 43 patients asked about social and occupational functioning using the social and occupational assessment scale, 62.5% of psoriatic patients reported distress at work and 51.1% reported decreased efficiency at work.19 A national online survey that was conducted in France and issued to patients with and without psoriasis assessed overall QOL and work productivity using the work productivity and activity impairment questionnaire for psoriasis (WPAI-PSO). Of 714 patients with psoriasis and PsA, the latter had a 57.6% decrease in work productivity over 7 days compared to 27.9% in controls (P<.05).20 Occupational impairment leads to lost wages and hinders advancement, further exacerbating the psychosocial burden of psoriasis.21
Occupational Assessment
Formal assessment of occupational function can be done with the WPAI-PSO, a 6-question valid instrument.22 Providers may look for risk factors associated with greater loss in work productivity to help identify and offer support for patients. Patients with increased severity of itching, pain, and scaling experienced a greater decrease in work productivity.21,23 Patients with PsA warrant early detection and treatment because they experience greater physical restraints that can interfere with work activities. Of the 459 psoriatic patients without a prior diagnosis of PsA who filled out the PsA screening and evaluation questionnaire, 144 (31.4%) received a score of 44 or higher and were referred to rheumatology for further evaluation with the classification criteria for PsA. Nine percent of patients failed to be screened and remained undiagnosed with PsA.24 In a study using the health assessment questionnaire to assess 400 patients with PsA, those with worse physical function due to joint pain and stiffness were less likely to remain employed (OR: 0.56; P=.02).25
Occupational Management
Identifying and coordinating symptoms of PsA between dermatology and rheumatology is beneficial for patients who experience debilitating symptoms. There are a variety of treatments available for PsA. According to the European League Against Rheumatism 2015 guidelines developed from expert opinion and systematic reviews for PsA management, there are 4 phases of treatment, with reassessment every 3 to 6 months for effectiveness of therapy.26,27 Phase I involves initiating nonsteroidal anti-inflammatory drugs with or without glucocorticoid injections. Phase II involves synthetic disease-modifying drugs, including methotrexate, leflunomide, sulfasalazine, or cyclosporine. Phase III involves adding a second synthetic disease-modifying drug or starting a biologic, such as an anti–tumor necrosis factor, IL-12/IL-23, or IL-17 inhibitor. Phase IV involves switching to a different drug in either aforementioned class.26,27 Treatment with biologics improves work productivity as assessed by WPAI-PSO for psoriasis and PsA.28-30 Encouraging patients to speak up in the workplace and request small accommodations such as timely breaks or ergonomic chairs can help patients feel more comfortable and supported in the work environment.18 Patients who felt supported at work were more likely to remain employed.25
Interpersonal Relationships Prevalence
Misinformation about psoriasis, fear of rejection, and feelings of isolation may contribute to interpersonal conflict. Patients have feelings of shame and self-consciousness that hinder them from engaging in social activities and seeking out relationships.31 Twenty-nine percent of patients feel that psoriasis has interfered with establishing relationships because of negative self-esteem associated with the disease,32 and 26.3% have experienced people avoiding physical contact.33 Family and spouses of patients with psoriasis may be secondarily affected due to economic and emotional distress. Ninety-eight percent of family members of psoriatic patients experienced emotional distress and 54% experienced the burden of care.34 In a survey of 63 relatives and partners of patients with psoriasis, 57% experienced psychological distress, including anxiety and worry over a psoriatic patient’s future.35
Interpersonal Relationships Assessment
Current available tools, including the DLQI and short form health survey, measure overall QOL, including social functioning, but may not be practical in a clinic setting. Although no quick-screening test to assess for this domain exists, providers are encouraged to ask patients about disease impact on interpersonal relationships. The family DLQI questionnaire, adapted from the DLQI, may help physicians and social workers evaluate the burden on a patient’s family members.34
Interpersonal Relationships Management
It may be difficult for providers to address problems with interpersonal relationships without accessible tools. Patients may not be accompanied by family or friends during appointments, and it is difficult to screen for these issues during visits. Providers may offer resources such as the NPF website, which provides information about support groups. It also provides tips on dating and connecting to others in the community who share similar experiences.18 Encouraging patients to seek family or couples therapy also may be beneficial. Increased social support can lead to better QOL and fewer depressive symptoms.36
Sexual Functioning Prevalence
Psoriasis affects both physical and psychological components of sexual function. Among 3485 patients with skin conditions who were surveyed about sexual function, 34% of psoriatic patients reported that psoriasis interfered with sexual functioning at least to a certain degree.37 Sexual impairment was strongly associated with depression, anxiety, and suicidal ideation; 24% of depressed patients and 20% of anxious patients experienced sexual problems a lot or very much, based on the DLQI.37 Depending on the questionnaire used, the prevalence of sexual dysfunction due to psoriasis ranged from 35.5% to 71.3%.38 In an observational cohort study of 158 participants (n=79 psoriasis patients and n=79 controls), 34.2% of patients with psoriasis experienced erectile dysfunction compared to 17.7% of controls.39 Forty-two percent of psoriatic patients with genital involvement reported dyspareunia, 32% reported worsening of genital psoriasis after intercourse, and 43% reported decreased frequency of intercourse.40
Sexual Functioning Assessment
The Skindex-29, DLQI, and psoriasis disability index are available QOL tools that include one question evaluating difficulties with sexual function. The
Sexual Functioning Management
Better disease control leads to improved sexual function, as patients experience fewer feelings of shame, anxiety, and depression, as well as improvement of physical symptoms that can interfere with sexual functioning.38,43,44 Reducing friction, warmth, and moisture, as well as avoiding tight clothing, can help those with genital psoriasis. Patients are advised to reapply topical medications after sexual intercourse. Patients also can apply makeup to disguise psoriasis and help reduce feelings of self-consciousness that can impede sexual intimacy.18
Comment
The psychosocial burden of psoriasis penetrates many facets of patient lives. Psoriasis can invoke feelings of shame and embarrassment that are worsened by the public’s misconceptions about psoriasis, resulting in serious mental health issues that can cause even greater disability. Depression and anxiety are prevalent in patients with psoriasis. The characteristic symptoms of pain and pruritus along with psychiatric comorbidities can have an underestimated impact on daily activities, including employment, interpersonal relationships, and sexual function. Such dysfunctions have serious implications toward wages, professional advancement, social support, and overall QOL.
Dermatology providers play an important role in screening for these problems through validated questionnaires and identifying risks. Simple screening questions such as the PHQ-9 can be beneficial and feasible during dermatology visits. Screening for PsA can help patients avoid problems at work. Sexual dysfunction is a sensitive topic; however, providers can use a 1-question screen from valid questionnaires and inquire about the location of lesions as opportunities to address this issue.
Interventions lead to better disease control, which concurrently improves overall QOL. These interventions depend on both patient adherence and a physician’s commitment to finding an optimal treatment regimen for each individual. Medical management; coordinating care; developing treatment plans with psychiatry, rheumatology, and primary care providers; and psychological counseling and services may be necessary and beneficial (Table). Offering accessible resources such as the NPF website helps patients access information outside the clinic when it is not feasible to address all these concerns in a single visit. Psoriasis requires more than just medical management; it requires dermatology providers to use a multidisciplinary approach to address the psychosocial aspects of the disease.
Conclusion
The psychosocial burden of psoriasis is immense. Stigma, public misconception, mental health concerns, and occupational and interpersonal difficulty are the basis of disease burden. Providers play a vital role in assessing the effect psoriasis has on different areas of patients’ lives and providing appropriate interventions and resources to reduce disease burden.
The psychosocial impact of psoriasis is a critical component of disease burden. Psoriatic patients have high rates of depression and anxiety, problems at work, and difficulties with interpersonal relationships and intimacy.1 A National Psoriasis Foundation (NPF) survey from 2003 to 2011 reported that psoriasis affects overall emotional well-being in 88% of patients and enjoyment of life in 82% of patients.2
The reasons for psychosocial burden stem from public misconceptions and disease stigma. A survey of 1005 individuals (age range, 16–64 years) about their perceptions of psoriasis revealed that 16.5% believed that psoriasis is contagious and 6.8% believed that psoriasis is related to personal hygiene.3 Fifty percent practiced discriminatory behavior toward psoriatic patients, including reluctance to shake hands (28.8%) and engage in sexual relations/intercourse (44.1%). Sixty-five percent of psoriatic patients felt their appearance is unsightly, and 73% felt self-conscious about having psoriasis.2
The psychosocial burden exists despite medical treatment of the disease. In a cross-sectional study of 1184 psoriatic patients, 70.2% had impaired quality of life (QOL) as measured by the dermatology life quality index (DLQI), even after receiving a 4-week treatment for psoriasis.4 Medical treatment of psoriasis is not enough; providers need to assess overall QOL and provide treatment and resources for these patients in addition to symptomatic management.
There have been many studies on the psychosocial burden of psoriasis, but few have focused on a dermatology resident’s role in addressing this issue. This article will review psychosocial domains—psychiatric comorbidities and social functioning including occupational functioning, interpersonal relationships, and sexual functioning— and discuss a dermatology resident’s role in assessing and addressing each of these areas.
Methods
A PubMed search of articles indexed for MEDLINE was conducted using the following terms: psoriasis, depression, anxiety, work productivity, sexual functioning, and interpersonal relationships. Selected articles covered prevalence, assessment, and management of each psychosocial domain.
Results
Psychiatric Comorbidities
Prevalence
A high prevalence of psychiatric comorbidities exists in psoriatic patients. In a study of 469,097 patients with psoriasis, depression was the third most prevalent comorbidity (17.91%), following hyperlipidemia (45.64%) and hypertension (42.19%).5 In a 10-year longitudinal, population-based, prospective cohort study, antidepressant prescriptions were twice as frequent in psoriatic patients (17.8%) compared to control (7.9%)(P<.001).6 In a meta-analysis of 98 studies investigating psoriatic patients and psychiatric comorbidities, patients with psoriasis were 1.5 times more likely to experience depression (odds ratio [OR]: 1.57; 95% CI, 1.40-1.76) and use antidepressants (OR: 4.24; 95% CI, 1.53-11.76) compared to control.7 Patients with psoriasis were more likely to attempt suicide (OR: 1.32; 95% CI, 1.14-1.54) and complete suicide (OR: 1.20; 95% CI, 1.04-1.39) compared to people without psoriasis.8 A 1-year cross-sectional study of 90 psoriatic patients reported 78.7% were diagnosed with depression and 76.7% were diagnosed with anxiety. Seventy-two percent reported both anxiety and depression, correlating with worse QOL (χ2=26.7; P<.05).9
Assessment
Psychiatric comorbidities are assessed using clinical judgment and formal screening questionnaires in research studies. Signs of depression in patients with psoriasis can manifest as poor treatment adherence and recurrent flares of psoriasis.10,11 Psoriatic patients with psychiatric comorbidities were less likely to be adherent to treatment (risk ratio: 0.35; P<.003).10 The patient health questionnaire (PHQ) 9 and generalized anxiety disorder scale (GAD) 7 are validated and reliable questionnaires. The first 2 questions in PHQ-9 and GAD-7 screen for depression and anxiety, respectively.12-14 These 2-question screens are practical in a fast-paced dermatology outpatient setting. Systematic questionnaires specifically targeting mood disorders may be more beneficial than the widely used DLQI, which may not adequately capture mood disorders. Over the course of 10 months, 607 patients with psoriasis were asked to fill out the PHQ-9, GAD-7, and DLQI. Thirty-eight percent of patients with major depressive disorder had a DLQI score lower than 10, while 46% of patients with generalized anxiety disorder had a DLQI score lower than 10.15 Other questionnaires, including the hospital anxiety and depression scale and Beck depression inventory, are valid instruments with high sensitivity but are commonly used for research purposes and may not be clinically feasible.16
Management
Dermatologists should refer patients with depression and/or anxiety to psychiatry. Interventions include pharmacologic and nonpharmacologic management. First-line therapy for depression and anxiety is a combination of selective serotonin reuptake inhibitors and cognitive behavioral therapy.17 In addition, providers can direct patients to online resources such as the NPF website, where patients with psoriasis can access information about the signs and symptoms of mood disorders and contact the patient navigation center for further help.18
Social Functioning
Occupational Prevalence
The NPF found that 92% of patients with psoriasis or psoriatic arthritis (PsA) surveyed between 2003 and 2011 cited their psoriasis as reason for unemployment.2 In a survey of 43 patients asked about social and occupational functioning using the social and occupational assessment scale, 62.5% of psoriatic patients reported distress at work and 51.1% reported decreased efficiency at work.19 A national online survey that was conducted in France and issued to patients with and without psoriasis assessed overall QOL and work productivity using the work productivity and activity impairment questionnaire for psoriasis (WPAI-PSO). Of 714 patients with psoriasis and PsA, the latter had a 57.6% decrease in work productivity over 7 days compared to 27.9% in controls (P<.05).20 Occupational impairment leads to lost wages and hinders advancement, further exacerbating the psychosocial burden of psoriasis.21
Occupational Assessment
Formal assessment of occupational function can be done with the WPAI-PSO, a 6-question valid instrument.22 Providers may look for risk factors associated with greater loss in work productivity to help identify and offer support for patients. Patients with increased severity of itching, pain, and scaling experienced a greater decrease in work productivity.21,23 Patients with PsA warrant early detection and treatment because they experience greater physical restraints that can interfere with work activities. Of the 459 psoriatic patients without a prior diagnosis of PsA who filled out the PsA screening and evaluation questionnaire, 144 (31.4%) received a score of 44 or higher and were referred to rheumatology for further evaluation with the classification criteria for PsA. Nine percent of patients failed to be screened and remained undiagnosed with PsA.24 In a study using the health assessment questionnaire to assess 400 patients with PsA, those with worse physical function due to joint pain and stiffness were less likely to remain employed (OR: 0.56; P=.02).25
Occupational Management
Identifying and coordinating symptoms of PsA between dermatology and rheumatology is beneficial for patients who experience debilitating symptoms. There are a variety of treatments available for PsA. According to the European League Against Rheumatism 2015 guidelines developed from expert opinion and systematic reviews for PsA management, there are 4 phases of treatment, with reassessment every 3 to 6 months for effectiveness of therapy.26,27 Phase I involves initiating nonsteroidal anti-inflammatory drugs with or without glucocorticoid injections. Phase II involves synthetic disease-modifying drugs, including methotrexate, leflunomide, sulfasalazine, or cyclosporine. Phase III involves adding a second synthetic disease-modifying drug or starting a biologic, such as an anti–tumor necrosis factor, IL-12/IL-23, or IL-17 inhibitor. Phase IV involves switching to a different drug in either aforementioned class.26,27 Treatment with biologics improves work productivity as assessed by WPAI-PSO for psoriasis and PsA.28-30 Encouraging patients to speak up in the workplace and request small accommodations such as timely breaks or ergonomic chairs can help patients feel more comfortable and supported in the work environment.18 Patients who felt supported at work were more likely to remain employed.25
Interpersonal Relationships Prevalence
Misinformation about psoriasis, fear of rejection, and feelings of isolation may contribute to interpersonal conflict. Patients have feelings of shame and self-consciousness that hinder them from engaging in social activities and seeking out relationships.31 Twenty-nine percent of patients feel that psoriasis has interfered with establishing relationships because of negative self-esteem associated with the disease,32 and 26.3% have experienced people avoiding physical contact.33 Family and spouses of patients with psoriasis may be secondarily affected due to economic and emotional distress. Ninety-eight percent of family members of psoriatic patients experienced emotional distress and 54% experienced the burden of care.34 In a survey of 63 relatives and partners of patients with psoriasis, 57% experienced psychological distress, including anxiety and worry over a psoriatic patient’s future.35
Interpersonal Relationships Assessment
Current available tools, including the DLQI and short form health survey, measure overall QOL, including social functioning, but may not be practical in a clinic setting. Although no quick-screening test to assess for this domain exists, providers are encouraged to ask patients about disease impact on interpersonal relationships. The family DLQI questionnaire, adapted from the DLQI, may help physicians and social workers evaluate the burden on a patient’s family members.34
Interpersonal Relationships Management
It may be difficult for providers to address problems with interpersonal relationships without accessible tools. Patients may not be accompanied by family or friends during appointments, and it is difficult to screen for these issues during visits. Providers may offer resources such as the NPF website, which provides information about support groups. It also provides tips on dating and connecting to others in the community who share similar experiences.18 Encouraging patients to seek family or couples therapy also may be beneficial. Increased social support can lead to better QOL and fewer depressive symptoms.36
Sexual Functioning Prevalence
Psoriasis affects both physical and psychological components of sexual function. Among 3485 patients with skin conditions who were surveyed about sexual function, 34% of psoriatic patients reported that psoriasis interfered with sexual functioning at least to a certain degree.37 Sexual impairment was strongly associated with depression, anxiety, and suicidal ideation; 24% of depressed patients and 20% of anxious patients experienced sexual problems a lot or very much, based on the DLQI.37 Depending on the questionnaire used, the prevalence of sexual dysfunction due to psoriasis ranged from 35.5% to 71.3%.38 In an observational cohort study of 158 participants (n=79 psoriasis patients and n=79 controls), 34.2% of patients with psoriasis experienced erectile dysfunction compared to 17.7% of controls.39 Forty-two percent of psoriatic patients with genital involvement reported dyspareunia, 32% reported worsening of genital psoriasis after intercourse, and 43% reported decreased frequency of intercourse.40
Sexual Functioning Assessment
The Skindex-29, DLQI, and psoriasis disability index are available QOL tools that include one question evaluating difficulties with sexual function. The
Sexual Functioning Management
Better disease control leads to improved sexual function, as patients experience fewer feelings of shame, anxiety, and depression, as well as improvement of physical symptoms that can interfere with sexual functioning.38,43,44 Reducing friction, warmth, and moisture, as well as avoiding tight clothing, can help those with genital psoriasis. Patients are advised to reapply topical medications after sexual intercourse. Patients also can apply makeup to disguise psoriasis and help reduce feelings of self-consciousness that can impede sexual intimacy.18
Comment
The psychosocial burden of psoriasis penetrates many facets of patient lives. Psoriasis can invoke feelings of shame and embarrassment that are worsened by the public’s misconceptions about psoriasis, resulting in serious mental health issues that can cause even greater disability. Depression and anxiety are prevalent in patients with psoriasis. The characteristic symptoms of pain and pruritus along with psychiatric comorbidities can have an underestimated impact on daily activities, including employment, interpersonal relationships, and sexual function. Such dysfunctions have serious implications toward wages, professional advancement, social support, and overall QOL.
Dermatology providers play an important role in screening for these problems through validated questionnaires and identifying risks. Simple screening questions such as the PHQ-9 can be beneficial and feasible during dermatology visits. Screening for PsA can help patients avoid problems at work. Sexual dysfunction is a sensitive topic; however, providers can use a 1-question screen from valid questionnaires and inquire about the location of lesions as opportunities to address this issue.
Interventions lead to better disease control, which concurrently improves overall QOL. These interventions depend on both patient adherence and a physician’s commitment to finding an optimal treatment regimen for each individual. Medical management; coordinating care; developing treatment plans with psychiatry, rheumatology, and primary care providers; and psychological counseling and services may be necessary and beneficial (Table). Offering accessible resources such as the NPF website helps patients access information outside the clinic when it is not feasible to address all these concerns in a single visit. Psoriasis requires more than just medical management; it requires dermatology providers to use a multidisciplinary approach to address the psychosocial aspects of the disease.
Conclusion
The psychosocial burden of psoriasis is immense. Stigma, public misconception, mental health concerns, and occupational and interpersonal difficulty are the basis of disease burden. Providers play a vital role in assessing the effect psoriasis has on different areas of patients’ lives and providing appropriate interventions and resources to reduce disease burden.
- Kimball AB, Jacobson C, Weiss S, et al. The psychosocial burden of psoriasis. Am J Clin Dermatol. 2005;6:383-392.
- Armstrong AW, Schupp C, Wu J, et al. Quality of life and work productivity impairment among psoriasis patients: findings from the National Psoriasis Foundation survey data 2003-2011. PloS One. 2012;7:e52935.
- Halioua B, Sid-Mohand D, Roussel ME, et al. Extent of misconceptions, negative prejudices and discriminatory behaviour to psoriasis patients in France. J Eur Acad Dermatol Venereol. 2016;30:650-654.
- Wolf P, Weger W, Legat F, et al. Quality of life and treatment goals in psoriasis from the patient perspective: results of an Austrian cross-sectional survey. J Dtsch Dermatol Ges. 2018;16:981-990.
- Shah K, Mellars L, Changolkar A, et al. Real-world burden of comorbidities in US patients with psoriasis. J Am Acad Dermatol. 2017;77:287-292.e4.
- Dowlatshahi EA, Wakkee M, Herings RM, et al. Increased antidepressant drug exposure in psoriasis patients: a longitudinal population-based cohort study. Acta Derm Venereol. 2013;93:544-550.
- Dowlatshahi EA, Wakkee M, Arends LR, et al. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol. 2014;134:1542-1551.
- Singh S, Taylor C, Kornmehl H, et al. Psoriasis and suicidality: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:425.e2-440.e2.
- Lakshmy S, Balasundaram S, Sarkar S, et al. A cross-sectional study of prevalence and implications of depression and anxiety in psoriasis. Indian J Psychol Med. 2015;37:434-440.
- Renzi C, Picardi A, Abeni D, et al. Association of dissatisfaction with care and psychiatric morbidity with poor treatment compliance. Arch Dermatol. 2002;138:337-342.
- Kulkarni AS, Balkrishnan R, Camacho FT, et al. Medication and health care service utilization related to depressive symptoms in older adults with psoriasis. J Drugs Dermatol. 2004;3:661-666.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
- Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
- Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41:1284-1292.
- Lamb RC, Matcham F, Turner MA, et al. Screening for anxiety and depression in people with psoriasis: a cross-sectional study in a tertiary referral setting. Br J Dermatol. 2017;176:1028-1034.
- Law M, Naughton MT, Dhar A, et al. Validation of two depression screening instruments in a sleep disorders clinic. J Clin Sleep Med. 2014;10:683-688.
- Cuijpers P, Dekker J, Hollon SD, et al. Adding psychotherapy to pharmacotherapy in the treatment of depressive disorders in adults: a meta-analysis. J Clin Psychiatry. 2009;70:1219-1229.
- National Psoriasis Foundation. Living with psoriatic arthritis. https://www.psoriasis.org/life-with-psoriatic-arthritis. Accessed September 23, 2018.
- Gaikwad R, Deshpande S, Raje S, et al. Evaluation of functional impairment in psoriasis. Indian J Dermatol Venereol Leprol. 2006;72:37-40.
- Claudepierre P, Lahfa M, Levy P, et al. The impact of psoriasis on professional life: PsoPRO, a French national survey [published online April 6, 2018]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.14986.
- Korman NJ, Zhao Y, Pike J, et al. Relationship between psoriasis severity, clinical symptoms, quality of life and work productivity among patients in the USA. Clin Exp Dermatol. 2016;41:514-521.
- Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. PharmacoEconomics. 1993;4:353-365.
- Korman NJ, Zhao Y, Pike J, et al. Increased severity of itching, pain, and scaling in psoriasis patients is associated with increased disease severity, reduced quality of life, and reduced work productivity. Dermatol Online J. 2015;21.
- Spelman L, Su JC, Fernandez-Penas P, et al. Frequency of undiagnosed psoriatic arthritis among psoriasis patients in Australian dermatology practice. J Eur Acad Dermatol Venereol. 2015;29:2184-2191.
- Tillett W, Shaddick G, Askari A, et al. Factors influencing work disability in psoriatic arthritis: first results from a large UK multicentre study. Rheumatology (Oxford). 2015;54:157-162.
- Raychaudhuri SP, Wilken R, Sukhov AC, et al. Management of psoriatic arthritis: early diagnosis, monitoring of disease severity and cutting edge therapies. J Autoimmun. 2017;76:21-37.
- Gossec L, Smolen JS, Ramiro S, et al. European League Against Rheumatism (EULAR) recommendations for the manegement of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75:499-510.
- Beroukhim K, Danesh M, Nguyen C, et al. A prospective, interventional assessment of the impact of ustekinumab treatment on psoriasis-related work productivity and activity impairment. J Dermatol Treat. 2016;27:552-555.
- Armstrong AW, Lynde CW, McBride SR, et al. Effect of ixekizumab treatment on work productivity for patients with moderate-to-severe plaque psoriasis: analysis of results from 3 randomized phase 3 clinical trials. JAMA Dermatol. 2016;152:661-669.
- Kimball AB, Yu AP, Signorovitch J, et al. The effects of adalimumab treatment and psoriasis severity on self-reported work productivity and activity impairment for patients with moderate to severe psoriasis. J Am Acad Dermatol. 2012;66:e67-76.
- Feldman SR, Malakouti M, Koo JY. Social impact of the burden of psoriasis: effects on patients and practice. Dermatol Online J. 2014;20.
- Reich A, Welz-Kubiak K, Rams Ł. Apprehension of the disease by patients suffering from psoriasis. Postepy Dermatol Alergol. 2014;31:289-293.
- Gupta MA, Gupta AK, Watteel GN. Perceived deprivation of social touch in psoriasis is associated with greater psychologic morbidity: an index of the stigma experience in dermatologic disorders. Cutis. 1998;61:339-342.
- Basra MK, Finlay AY. The family impact of skin diseases: the Greater Patient concept. Br J Dermatol. 2007;156:929-937.
- Eghlileb AM, Davies EE, Finlay AY. Psoriasis has a major secondary impact on the lives of family members and partners. Br J Dermatol. 2007;156:1245-1250.
- Janowski K, Steuden S, Pietrzak A, et al. Social support and adaptation to the disease in men and women with psoriasis. Arch Dermatol Res. 2012;304:421-432.
- Sampogna F, Abeni D, Gieler U, et al. Impairment of sexual life in 3,485 dermatological outpatients from a multicentre study in 13 European countries. Acta Derm Venereol. 2017;97:478-482.
- Sampogna F, Gisondi P, Tabolli S, et al. Impairment of sexual life in patients with psoriasis. Dermatology. 2007;214:144-150.
- Molina-Leyva A, Molina-Leyva I, Almodovar-Real A, et al. Prevalence and associated factors of erectile dysfunction in patients with moderate to severe psoriasis and healthy population: a comparative study considering physical and psychological factors. Arch Sex Behav. 2016;45:2047-2055.
- Ryan C, Sadlier M, De Vol E, et al. Genital psoriasis is associated with significant impairment in quality of life and sexual functioning. J Am Acad Dermatol. 2015;72:978-983.
- Labbate LA, Lare SB. Sexual dysfunction in male psychiatric outpatients: validity of the Massachusetts General Hospital Sexual Functioning Questionnaire. Psychother Psychosom. 2001;70:221-225.
- Molina-Leyva A, Almodovar-Real A, Ruiz-Carrascosa JC, et al. Distribution pattern of psoriasis affects sexual function in moderate to severe psoriasis: a prospective case series study. J Sex Med. 2014;11:2882-2889.
- Guenther L, Han C, Szapary P, et al. Impact of ustekinumab on health-related quality of life and sexual difficulties associated with psoriasis: results from two phase III clinical trials. J Eur Acad Dermatol Venereol. 2011;25:851-857.
- Guenther L, Warren RB, Cather JC, et al. Impact of ixekizumab treatment on skin-related personal relationship difficulties in moderate-to-severe psoriasis patients: 12-week results from two Phase 3 trials. J Eur Acad Dermatol Venereol. 2017;31:1867-1875.
- Kimball AB, Jacobson C, Weiss S, et al. The psychosocial burden of psoriasis. Am J Clin Dermatol. 2005;6:383-392.
- Armstrong AW, Schupp C, Wu J, et al. Quality of life and work productivity impairment among psoriasis patients: findings from the National Psoriasis Foundation survey data 2003-2011. PloS One. 2012;7:e52935.
- Halioua B, Sid-Mohand D, Roussel ME, et al. Extent of misconceptions, negative prejudices and discriminatory behaviour to psoriasis patients in France. J Eur Acad Dermatol Venereol. 2016;30:650-654.
- Wolf P, Weger W, Legat F, et al. Quality of life and treatment goals in psoriasis from the patient perspective: results of an Austrian cross-sectional survey. J Dtsch Dermatol Ges. 2018;16:981-990.
- Shah K, Mellars L, Changolkar A, et al. Real-world burden of comorbidities in US patients with psoriasis. J Am Acad Dermatol. 2017;77:287-292.e4.
- Dowlatshahi EA, Wakkee M, Herings RM, et al. Increased antidepressant drug exposure in psoriasis patients: a longitudinal population-based cohort study. Acta Derm Venereol. 2013;93:544-550.
- Dowlatshahi EA, Wakkee M, Arends LR, et al. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol. 2014;134:1542-1551.
- Singh S, Taylor C, Kornmehl H, et al. Psoriasis and suicidality: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:425.e2-440.e2.
- Lakshmy S, Balasundaram S, Sarkar S, et al. A cross-sectional study of prevalence and implications of depression and anxiety in psoriasis. Indian J Psychol Med. 2015;37:434-440.
- Renzi C, Picardi A, Abeni D, et al. Association of dissatisfaction with care and psychiatric morbidity with poor treatment compliance. Arch Dermatol. 2002;138:337-342.
- Kulkarni AS, Balkrishnan R, Camacho FT, et al. Medication and health care service utilization related to depressive symptoms in older adults with psoriasis. J Drugs Dermatol. 2004;3:661-666.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
- Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092-1097.
- Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41:1284-1292.
- Lamb RC, Matcham F, Turner MA, et al. Screening for anxiety and depression in people with psoriasis: a cross-sectional study in a tertiary referral setting. Br J Dermatol. 2017;176:1028-1034.
- Law M, Naughton MT, Dhar A, et al. Validation of two depression screening instruments in a sleep disorders clinic. J Clin Sleep Med. 2014;10:683-688.
- Cuijpers P, Dekker J, Hollon SD, et al. Adding psychotherapy to pharmacotherapy in the treatment of depressive disorders in adults: a meta-analysis. J Clin Psychiatry. 2009;70:1219-1229.
- National Psoriasis Foundation. Living with psoriatic arthritis. https://www.psoriasis.org/life-with-psoriatic-arthritis. Accessed September 23, 2018.
- Gaikwad R, Deshpande S, Raje S, et al. Evaluation of functional impairment in psoriasis. Indian J Dermatol Venereol Leprol. 2006;72:37-40.
- Claudepierre P, Lahfa M, Levy P, et al. The impact of psoriasis on professional life: PsoPRO, a French national survey [published online April 6, 2018]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.14986.
- Korman NJ, Zhao Y, Pike J, et al. Relationship between psoriasis severity, clinical symptoms, quality of life and work productivity among patients in the USA. Clin Exp Dermatol. 2016;41:514-521.
- Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. PharmacoEconomics. 1993;4:353-365.
- Korman NJ, Zhao Y, Pike J, et al. Increased severity of itching, pain, and scaling in psoriasis patients is associated with increased disease severity, reduced quality of life, and reduced work productivity. Dermatol Online J. 2015;21.
- Spelman L, Su JC, Fernandez-Penas P, et al. Frequency of undiagnosed psoriatic arthritis among psoriasis patients in Australian dermatology practice. J Eur Acad Dermatol Venereol. 2015;29:2184-2191.
- Tillett W, Shaddick G, Askari A, et al. Factors influencing work disability in psoriatic arthritis: first results from a large UK multicentre study. Rheumatology (Oxford). 2015;54:157-162.
- Raychaudhuri SP, Wilken R, Sukhov AC, et al. Management of psoriatic arthritis: early diagnosis, monitoring of disease severity and cutting edge therapies. J Autoimmun. 2017;76:21-37.
- Gossec L, Smolen JS, Ramiro S, et al. European League Against Rheumatism (EULAR) recommendations for the manegement of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75:499-510.
- Beroukhim K, Danesh M, Nguyen C, et al. A prospective, interventional assessment of the impact of ustekinumab treatment on psoriasis-related work productivity and activity impairment. J Dermatol Treat. 2016;27:552-555.
- Armstrong AW, Lynde CW, McBride SR, et al. Effect of ixekizumab treatment on work productivity for patients with moderate-to-severe plaque psoriasis: analysis of results from 3 randomized phase 3 clinical trials. JAMA Dermatol. 2016;152:661-669.
- Kimball AB, Yu AP, Signorovitch J, et al. The effects of adalimumab treatment and psoriasis severity on self-reported work productivity and activity impairment for patients with moderate to severe psoriasis. J Am Acad Dermatol. 2012;66:e67-76.
- Feldman SR, Malakouti M, Koo JY. Social impact of the burden of psoriasis: effects on patients and practice. Dermatol Online J. 2014;20.
- Reich A, Welz-Kubiak K, Rams Ł. Apprehension of the disease by patients suffering from psoriasis. Postepy Dermatol Alergol. 2014;31:289-293.
- Gupta MA, Gupta AK, Watteel GN. Perceived deprivation of social touch in psoriasis is associated with greater psychologic morbidity: an index of the stigma experience in dermatologic disorders. Cutis. 1998;61:339-342.
- Basra MK, Finlay AY. The family impact of skin diseases: the Greater Patient concept. Br J Dermatol. 2007;156:929-937.
- Eghlileb AM, Davies EE, Finlay AY. Psoriasis has a major secondary impact on the lives of family members and partners. Br J Dermatol. 2007;156:1245-1250.
- Janowski K, Steuden S, Pietrzak A, et al. Social support and adaptation to the disease in men and women with psoriasis. Arch Dermatol Res. 2012;304:421-432.
- Sampogna F, Abeni D, Gieler U, et al. Impairment of sexual life in 3,485 dermatological outpatients from a multicentre study in 13 European countries. Acta Derm Venereol. 2017;97:478-482.
- Sampogna F, Gisondi P, Tabolli S, et al. Impairment of sexual life in patients with psoriasis. Dermatology. 2007;214:144-150.
- Molina-Leyva A, Molina-Leyva I, Almodovar-Real A, et al. Prevalence and associated factors of erectile dysfunction in patients with moderate to severe psoriasis and healthy population: a comparative study considering physical and psychological factors. Arch Sex Behav. 2016;45:2047-2055.
- Ryan C, Sadlier M, De Vol E, et al. Genital psoriasis is associated with significant impairment in quality of life and sexual functioning. J Am Acad Dermatol. 2015;72:978-983.
- Labbate LA, Lare SB. Sexual dysfunction in male psychiatric outpatients: validity of the Massachusetts General Hospital Sexual Functioning Questionnaire. Psychother Psychosom. 2001;70:221-225.
- Molina-Leyva A, Almodovar-Real A, Ruiz-Carrascosa JC, et al. Distribution pattern of psoriasis affects sexual function in moderate to severe psoriasis: a prospective case series study. J Sex Med. 2014;11:2882-2889.
- Guenther L, Han C, Szapary P, et al. Impact of ustekinumab on health-related quality of life and sexual difficulties associated with psoriasis: results from two phase III clinical trials. J Eur Acad Dermatol Venereol. 2011;25:851-857.
- Guenther L, Warren RB, Cather JC, et al. Impact of ixekizumab treatment on skin-related personal relationship difficulties in moderate-to-severe psoriasis patients: 12-week results from two Phase 3 trials. J Eur Acad Dermatol Venereol. 2017;31:1867-1875.
Practice Points
- The psychosocial impact of psoriasis is an important component of the disease burden leading to reduced quality of life.
- Assessment of psychosocial dysfunction can be done through short questionnaires, asking patients directly about these issues and anticipating these problems in patients who are most vulnerable.
- Management of psychosocial impact ranges from pharmacological interventions to helpful resources such as the National Psoriasis Foundation website.
Psoriasis Risk Factors and Triggers
Psoriasis is a chronic autoimmune skin disease affecting approximately 6.7 million adults in the United States.1 Although its pathogenesis is not yet clear, risk factors and triggers provide insight into potential pathways by which psoriasis can occur. There is notable overlap between risk factors and triggers of psoriasis; perceived risk factors might, in fact, be triggers causing manifestation of disease in predisposed persons. In this review, we summarize the key factors contributing to onset and exacerbation of psoriasis. When learning to manage this chronic disease, it also may be helpful to educate patients about how these elements may affect the course of psoriasis.
Genetics
The pathogenesis of psoriasis has a strong genetic component, with approximately 70% and 20% concordance rates in monozygotic and dizygotic twins, respectively.2 Moreover, studies have shown a positive family history in approximately 35% of patients.3,4 Family-based studies have found a 50% risk of developing psoriasis in patients with 2 affected parents.5 However, the genetics of psoriasis are complex and are attributed to many different genes. Thus far, genes involving antigen presentation, T-cell receptor development and polarization, and the nuclear factor κβ (NF-κβ) pathway have been identified.6
HLA-Cw6
The most well-studied gene implicated in psoriasis is HLA-Cw6, which encodes a major histocompatibility complex class I allele supporting psoriasis as a T cell–mediated reaction to an autoantigen.6 Two potential antigens for HLA-Cw6 recently have been identified: LL-37, a cathelicidin-related antimicrobial peptide, and the A disintegrin and metalloproteinase with thrombospondin motifs-like protein 5 (ADAMTSL5), found on melanocytes and keratinocytes.7 The percentage of psoriasis patients with HLA-Cw6 ranges from 10.5% to 77.2%, with higher frequency in white individuals than in Asians.7
HLA-Cw6 manifests as specific features in psoriasis, including onset of disease before 21 years of age.8 It also is more strongly associated with guttate-type psoriasis, greater body surface area involvement, and higher incidence of Köbner phenomenon. Patients with positive HLA-Cw6 also reported worsening of psoriasis during and after throat infection.9
Caspase Recruitment Domain Family Member 14
Another gene mutation implicated in psoriasis pathogenesis is caspase recruitment domain family member 14, CARD14 (formerly PSORS2), a gene encoding a scaffolding protein important in the activation of NF-κβ.10,11 Missense CARD14 mutations cause upregulation of NF-κβ through formation of a complex with adapter protein B-cell lymphoma 10 (BCL10) and mucosa-associated lymphoid tissue lymphoma translocation protein 1 (MALT1),12 which, in turn, causes increased transcription of cytokines IL-8, C-C motif chemokine ligand 20 (CCL-20), and IL-36 gamma in the keratinocyte.13 Mutations in CARD14 alone lead to psoriasiform skin in mice through amplified activation of the IL-23/IL-17 axis.14,15 Patients with a mutation in a CARD14 variant (p.Arg820Trp) have demonstrated better response to tumor necrosis factor (TNF) inhibitors.16
Further characterization of the genetic pathogenesis of psoriasis might lead to better targeted therapies, including the possibility of MALT1 inhibitors as a treatment option.12
Infection
Streptococcus
The association between streptococcal infection and psoriasis was first documented more than 100 years ago, specifically the onset of acute guttate psoriasis.17,18 Although classically described following throat infection, psoriasis also occurs following streptococcal vulvovaginitis and perianal streptococcal infection.19,20
This type of psoriasis is typically self-limited but can recur with subsequent streptococcal infections or initiate a more chronic plaque psoriasis. Patients have a 1 in 3 risk of developing chronic psoriasis within 10 years of a single episode of acute guttate psoriasis.21 Moreover, in many patients with existing plaque psoriasis, throat infection exacerbates psoriatic symptoms.22 The mechanism of exacerbation is likely due to cross-reactivity between streptococcal M surface antigen and human keratinocytes and might also be influenced by inherited abnormalities in immune response.23-26 Therefore, tonsillectomy has been studied as a possible treatment of psoriasis but is likely helpful only in patients with exacerbations of disease that are closely associated with recurrent tonsillitis.27
Human Immunodeficiency Virus
The prevalence of psoriasis in human immunodeficiency virus (HIV) patients is similar to or greater than the general population.28 Human immunodeficiency virus infection causes new onset of psoriasis and exacerbation of existing psoriasis; severity often is correlated with worsening immune function.28,29
The clinical subtypes of psoriasis that occur most frequently with HIV include guttate, inverse, and erythrodermic, though patients may present with any subtype.28 The mechanism is puzzling because HIV is primarily mediated by helper T cell 2 (TH2) cytokines, whereas psoriasis is mainly driven by helper T cell 1 (TH1) cytokines.30 Furthermore, despite increased severity with lower CD4+ counts, treatments further lowering T-cell counts paradoxically improve symptoms.31 Current literature suggests that expansion of CD8+ memory T cells might be the primary mechanism in the exacerbation of psoriasis in HIV-mediated immunosuppression.30
Treatment of HIV-associated psoriasis presents challenges because many therapeutics cause further immunosuppression. The National Psoriasis Foundation recommends topical preparations as first-line agents for mild to moderate psoriasis.32 For moderate to severe psoriasis, retroviral agents may be effective as first-line monotherapy or when supplemented by phototherapy with UVB or psoralen plus UVA. Retinoids can be used as second-line agents.32 For cases of severe refractory psoriasis, cyclosporine, methotrexate, TNF inhibitors, or hydroxyurea can be considered. There also is evidence that apremilast is effective without risk for worsening immune function.33
Other Infections
Other bacteria associated with triggering or exacerbating psoriasis include Staphylococcus aureus and Helicobacter pylori.34,35 Fungi, such as species of the genera Malassezia and Candida, and other viruses, including papillomaviruses and retroviruses, also have been implicated.34
Medications
Numerous medications can trigger psoriasis, including lithium, nonsteroidal anti-inflammatory drugs, antimalarials, beta-blockers, and angiotensin-converting enzyme inhibitors.34 More recent literature suggests that TNF inhibitors also can paradoxically induce psoriasis in rare cases.35
Lithium
Psoriasis is the most common cutaneous adverse effect of lithium.34 It is more likely to exacerbate existing disease but also can induce onset of psoriasis; it also can cause disease that is more refractory to treatment.34,36 Current literature hypothesizes that lithium triggers psoriasis by interference of intracellular calcium channels through reduction of inositol, thereby affecting keratinocyte proliferation and differentiation.34 Lithium also inhibits glycogen synthase kinase-3 (GSK-3), a serine threonine kinase, which, in turn, induces human keratinocyte proliferation.37 However, it is unlikely lithium alone can induce psoriasis; genetic predisposition is necessary.
TNF Inhibitors
Tumor necrosis factor inhibitors such as adalimumab, etanercept, certolizumab pegol, golimumab, and infliximab are used in various inflammatory diseases, including psoriasis. Interestingly, there have been more than 200 reported cases of suspected TNF inhibitor–induced or –exacerbated psoriasis.38 This phenomenon appears to occur more frequently with infliximab and is most likely to occur in the first year of treatment of Crohn disease and rheumatoid arthritis.38 Plaque psoriasis is the most common form, but 15% to 26% of cases presented with 2 or more morphologies.38,39
Treatment options include discontinuing therapy, though many patients experience resolution while continuing treatment or switching to another TNF inhibitor.38-40 Traditional topical therapies also have been used with success.40 The pathogenesis of this phenomenon is still unclear but is thought to involve both the IL-23/helper T cell 17 (TH17) axis and dysregulation of IFN-α in the setting of TNF suppression.38
Lifestyle
Obesity is a chronic low-grade inflammatory state that can contribute to the onset of psoriasis or exacerbation of exist
The relationship between psoriasis and alcohol consumption is less clear than it is between psoriasis and obesity or smoking; greater consumption is found in psoriasis patients, but evidence is insufficient to deem alcohol a risk factor.44
Conclusion
Various factors, including genetics, infection, pharmacotherapeutic, and lifestyle, can all contribute to the induction or exacerbation of psoriasis. These factors can provide clues to the pathogenesis of psoriasis as well as help clinicians better counsel patients about their disease.
- Helmick CG, Lee-Han H, Hirsch SC, et al. Prevalence of psoriasis among adults in the U.S.: 2003-2006 and 2009-2010 National Health and Nutrition Examination Surveys. Am J Prev Med. 2014;47:37-45.
- Bowcock AM. The genetics of psoriasis and autoimmunity. Annu Rev Genomics Hum Genet. 2005;6:93-122.
- Swanbeck G, Inerot A, Martinsson T, et al. A population genetic study of psoriasis. Br J Dermatol. 1994;131:32-39.
- Kimberling W, Dobson RL. The inheritance of psoriasis. J Invest Dermatol. 1973;60:538-540.
- Gupta R, Debbaneh MG, Liao W. Genetic epidemiology of psoriasis. Curr Dermatol Rep. 2014;3:61-78.
- Harden JL, Krueger JG, Bowcock AM. The immunogenetics of psoriasis: a comprehensive review. J Autoimmun. 2015;64:66-73.
- Chen L, Tsai TF. HLA-Cw6 and psoriasis. Br J Dermatol. 2018;178:854-862.
- Enerbäck C, Martinsson T, Ineraot A, et al. Evidence that HLA-Cw6 determines early onset of psoriasis, obtained using sequence-specific primers (PCR-SSP). Acta Derm Venereol. 1997;77:273-276.
- Gudjónsson JE, Kárason A, Antonsdóttir EH, et al. HLA-Cw6-positive and HLA-Cw6-negative patients with psoriasis vulgaris have distinct clinical features. J Invest Dermatol. 2002;118:362-365.
- Tomfohrde J, Silverman A, Barnes R, et al. Gene for familial psoriasis susceptibility mapped to distal end of human chromosome 17q. Science. 1994;264:1141-1145.
- Blonska M, Lin X. NF-κB signaling pathways regulated by CARMA family of scaffold proteins. Cell Res. 2011;21:55-70.
- Van Nuffel E, Schmitt A, Afonina IS, et al. CARD14-mediated activation of paracaspase MALT1 in keratinocytes: implications for psoriasis. J Invest Dermatol. 2017;137:569-575.
- Jordan CT, Cao L, Roberson ED, et al. PSORS2 is due to mutations in CARD14. Am J Hum Genet. 2012;90:784-795.
- Wang M, Zhang S, Zheng G, et al. Gain-of-function mutation of Card14 leads to spontaneous psoriasis-like skin inflammation through enhanced keratinocyte response to IL-17A. Immunity. 2018;49:66-79.
- Mellet M, Meier B, Mohanan D, et al. CARD14 gain-of-function mutation alone is sufficient to drive IL-23/IL-17-mediated psoriasiform skin inflammation in vivo. J Invest Dermatol. 2018;138:2010-2023.
- Coto-Segura P, González-Fernández D, Batalla A, et al. Common and rare CARD14 gene variants affect the antitumour necrosis factor response among patients with psoriasis. Br J Dermatol. 2016;175:134-141.
- Winfield JM. Psoriasis as a sequel to acute inflammations of the tonsils: a clinical note. J Cutan Dis. 1916;34:441-443.
- Telfer NR, Chalmers RJG, Whale K, et al. The role of streptococcal infection in the initiation of guttate psoriasis. Arch Dermatol. 1992;128:39-42.
- Hernandez M, Simms-Cendan J, Zendell K. Guttate psoriasis following streptococcal vulvovaginitis in a five-year-old girl. J Pediatr Adolesc Gynecol. 2015;28:e127-e129.
- Herbst RA, Hoch O, Kapp A, et al. Guttate psoriasis triggered by perianal streptococcal dermatitis in a four-year-old boy. J Am Acad Dermatol. 2000;42(5, pt 2):885-887.
- Martin BA, Chalmers RJ, Telfer NR. How great is the risk of further psoriasis following a single episode of acute guttate psoriasis? Arch Dermatol. 1996;132:717-718.
- Thorleifsdottir RH, Eysteinsdóttir, Olafsson JH, et al. Throat infections are associated with exacerbation in a substantial proportion of patients with chronic plaque psoriasis. Acta Derm Venereol. 2016;96:788-791.
- McFadden J, Valdimarsson H, Fry L. Cross-reactivity between streptococcal M surface antigen and human skin. Br J Dermatol. 1991;125:443-447.
- Validmarsson H, Thorleifsdottir RH, Sigurdardottir SL, et al. Psoriasis—as an autoimmune disease caused by molecular mimicry. Trends Immunol. 2009;30:494-501.
- Muto M, Fujikara Y, Hamamoto Y, et al. Immune response to Streptococcus pyogenes and the susceptibility to psoriasis. Australas J Dermatol. 1996;37(suppl 1):S54-S55.
- Weisenseel P, Laumbacher B, Besgen P, et al. Streptococcal infection distinguishes different types of psoriasis. J Med Genet. 2002;39:767-768.
- Rachakonda TD, Dhillon JS, Florek AG, et al. Effect of tonsillectomy on psoriasis: a systematic review. J Am Acad Dermatol. 2015;72:261-275.
- Mallon E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246.
- Duvic M, Johnson TM, Rapini RP, et al. Acquired immunodeficiency syndrome-associated psoriasis and Reiter’s syndrome. Arch Dermatol. 1987;123:1622-1632.
- Fife DJ, Waller JM, Jeffes EW, et al. Unraveling the paradoxes of HIV-associated psoriasis: a review of T-cell subsets and cytokine profiles. Dermatol Online J. 2007;13:4.
- Ortonne JP, Lebwohl M, Em Griffiths C; Alefacept Clinical Study Group. Alefacept-induced decreases in circulating blood lymphocyte counts correlate with clinical response in patients with chronic plaque psoriasis. Eur J Dermatol. 2003;13:117-123.
- Menon K, Van Voorhees AS, Bebo BF Jr, et al; National Psoriasis Foundation. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Reddy SP, Shah VV, Wu JJ. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol. 2017;31:e481-e482.
- Fry L, Baker BS. Triggering psoriasis: the role of infections and medications. Clin Dermatol. 2007;25:606-615.
- Sfikakis PP, Iliopoulos A, Elezoglou A, et al. Psoriasis induced by anti-tumor necrosis factor therapy: a paradoxical adverse reaction. Arthritis Rheum. 2005;52:2513-2518.
- Yeung CK, Chan HH. Cutaneous adverse effects of lithium: epidemiology and management. Am J Clin Dermatol. 2004;5:3-8.
- Hampton PJ, Jans R, Flockhart RJ, et al. Lithium regulates keratinocyte proliferation via glycogen synthase kinase 3 and NFAT 2 (nuclear factor of activated T cells 2). J Cell Physiol. 2012;227:1529-1537.
- Brown G, Wang E, Leon A, et al. Tumor necrosis factor-α inhibitor-induced psoriasis: systematic review of clinical features, histopathological findings, and management experience. J Am Acad Dermatol. 2017;76:334-341.
- Collamer AN, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: clinical features and possible immunopathogenesis. Semin Arthritis Rheum. 2010;40:233-240.
- Collamer AN, Guerrero KT, Henning JS, et al. Psoriatic skin lesions induced by tumor antagonist therapy: a literature review and potential mechanisms of action. Arthritis Rheum. 2008;59:996-1001.
- Jensen P, Skov L. Psoriasis and obesity. Dermatology. 2016;232:633-639.
- Barrea L, Nappi F, Di Somma C, et al. Environmental risk factors in psoriasis: the point of view of the nutritionist. Int J Environ Res Public Health. 2016;13:743.
- Lee EJ, Han KD, Han JH, et al. Smoking and risk of psoriasis: a nationwide cohort study. J Am Acad Dermatol. 2017;77:573-575.
- Brenaut E, Horreau C, Pouplard C, et al. Alcohol consumption and psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2013;27(suppl 3):30-35.
Psoriasis is a chronic autoimmune skin disease affecting approximately 6.7 million adults in the United States.1 Although its pathogenesis is not yet clear, risk factors and triggers provide insight into potential pathways by which psoriasis can occur. There is notable overlap between risk factors and triggers of psoriasis; perceived risk factors might, in fact, be triggers causing manifestation of disease in predisposed persons. In this review, we summarize the key factors contributing to onset and exacerbation of psoriasis. When learning to manage this chronic disease, it also may be helpful to educate patients about how these elements may affect the course of psoriasis.
Genetics
The pathogenesis of psoriasis has a strong genetic component, with approximately 70% and 20% concordance rates in monozygotic and dizygotic twins, respectively.2 Moreover, studies have shown a positive family history in approximately 35% of patients.3,4 Family-based studies have found a 50% risk of developing psoriasis in patients with 2 affected parents.5 However, the genetics of psoriasis are complex and are attributed to many different genes. Thus far, genes involving antigen presentation, T-cell receptor development and polarization, and the nuclear factor κβ (NF-κβ) pathway have been identified.6
HLA-Cw6
The most well-studied gene implicated in psoriasis is HLA-Cw6, which encodes a major histocompatibility complex class I allele supporting psoriasis as a T cell–mediated reaction to an autoantigen.6 Two potential antigens for HLA-Cw6 recently have been identified: LL-37, a cathelicidin-related antimicrobial peptide, and the A disintegrin and metalloproteinase with thrombospondin motifs-like protein 5 (ADAMTSL5), found on melanocytes and keratinocytes.7 The percentage of psoriasis patients with HLA-Cw6 ranges from 10.5% to 77.2%, with higher frequency in white individuals than in Asians.7
HLA-Cw6 manifests as specific features in psoriasis, including onset of disease before 21 years of age.8 It also is more strongly associated with guttate-type psoriasis, greater body surface area involvement, and higher incidence of Köbner phenomenon. Patients with positive HLA-Cw6 also reported worsening of psoriasis during and after throat infection.9
Caspase Recruitment Domain Family Member 14
Another gene mutation implicated in psoriasis pathogenesis is caspase recruitment domain family member 14, CARD14 (formerly PSORS2), a gene encoding a scaffolding protein important in the activation of NF-κβ.10,11 Missense CARD14 mutations cause upregulation of NF-κβ through formation of a complex with adapter protein B-cell lymphoma 10 (BCL10) and mucosa-associated lymphoid tissue lymphoma translocation protein 1 (MALT1),12 which, in turn, causes increased transcription of cytokines IL-8, C-C motif chemokine ligand 20 (CCL-20), and IL-36 gamma in the keratinocyte.13 Mutations in CARD14 alone lead to psoriasiform skin in mice through amplified activation of the IL-23/IL-17 axis.14,15 Patients with a mutation in a CARD14 variant (p.Arg820Trp) have demonstrated better response to tumor necrosis factor (TNF) inhibitors.16
Further characterization of the genetic pathogenesis of psoriasis might lead to better targeted therapies, including the possibility of MALT1 inhibitors as a treatment option.12
Infection
Streptococcus
The association between streptococcal infection and psoriasis was first documented more than 100 years ago, specifically the onset of acute guttate psoriasis.17,18 Although classically described following throat infection, psoriasis also occurs following streptococcal vulvovaginitis and perianal streptococcal infection.19,20
This type of psoriasis is typically self-limited but can recur with subsequent streptococcal infections or initiate a more chronic plaque psoriasis. Patients have a 1 in 3 risk of developing chronic psoriasis within 10 years of a single episode of acute guttate psoriasis.21 Moreover, in many patients with existing plaque psoriasis, throat infection exacerbates psoriatic symptoms.22 The mechanism of exacerbation is likely due to cross-reactivity between streptococcal M surface antigen and human keratinocytes and might also be influenced by inherited abnormalities in immune response.23-26 Therefore, tonsillectomy has been studied as a possible treatment of psoriasis but is likely helpful only in patients with exacerbations of disease that are closely associated with recurrent tonsillitis.27
Human Immunodeficiency Virus
The prevalence of psoriasis in human immunodeficiency virus (HIV) patients is similar to or greater than the general population.28 Human immunodeficiency virus infection causes new onset of psoriasis and exacerbation of existing psoriasis; severity often is correlated with worsening immune function.28,29
The clinical subtypes of psoriasis that occur most frequently with HIV include guttate, inverse, and erythrodermic, though patients may present with any subtype.28 The mechanism is puzzling because HIV is primarily mediated by helper T cell 2 (TH2) cytokines, whereas psoriasis is mainly driven by helper T cell 1 (TH1) cytokines.30 Furthermore, despite increased severity with lower CD4+ counts, treatments further lowering T-cell counts paradoxically improve symptoms.31 Current literature suggests that expansion of CD8+ memory T cells might be the primary mechanism in the exacerbation of psoriasis in HIV-mediated immunosuppression.30
Treatment of HIV-associated psoriasis presents challenges because many therapeutics cause further immunosuppression. The National Psoriasis Foundation recommends topical preparations as first-line agents for mild to moderate psoriasis.32 For moderate to severe psoriasis, retroviral agents may be effective as first-line monotherapy or when supplemented by phototherapy with UVB or psoralen plus UVA. Retinoids can be used as second-line agents.32 For cases of severe refractory psoriasis, cyclosporine, methotrexate, TNF inhibitors, or hydroxyurea can be considered. There also is evidence that apremilast is effective without risk for worsening immune function.33
Other Infections
Other bacteria associated with triggering or exacerbating psoriasis include Staphylococcus aureus and Helicobacter pylori.34,35 Fungi, such as species of the genera Malassezia and Candida, and other viruses, including papillomaviruses and retroviruses, also have been implicated.34
Medications
Numerous medications can trigger psoriasis, including lithium, nonsteroidal anti-inflammatory drugs, antimalarials, beta-blockers, and angiotensin-converting enzyme inhibitors.34 More recent literature suggests that TNF inhibitors also can paradoxically induce psoriasis in rare cases.35
Lithium
Psoriasis is the most common cutaneous adverse effect of lithium.34 It is more likely to exacerbate existing disease but also can induce onset of psoriasis; it also can cause disease that is more refractory to treatment.34,36 Current literature hypothesizes that lithium triggers psoriasis by interference of intracellular calcium channels through reduction of inositol, thereby affecting keratinocyte proliferation and differentiation.34 Lithium also inhibits glycogen synthase kinase-3 (GSK-3), a serine threonine kinase, which, in turn, induces human keratinocyte proliferation.37 However, it is unlikely lithium alone can induce psoriasis; genetic predisposition is necessary.
TNF Inhibitors
Tumor necrosis factor inhibitors such as adalimumab, etanercept, certolizumab pegol, golimumab, and infliximab are used in various inflammatory diseases, including psoriasis. Interestingly, there have been more than 200 reported cases of suspected TNF inhibitor–induced or –exacerbated psoriasis.38 This phenomenon appears to occur more frequently with infliximab and is most likely to occur in the first year of treatment of Crohn disease and rheumatoid arthritis.38 Plaque psoriasis is the most common form, but 15% to 26% of cases presented with 2 or more morphologies.38,39
Treatment options include discontinuing therapy, though many patients experience resolution while continuing treatment or switching to another TNF inhibitor.38-40 Traditional topical therapies also have been used with success.40 The pathogenesis of this phenomenon is still unclear but is thought to involve both the IL-23/helper T cell 17 (TH17) axis and dysregulation of IFN-α in the setting of TNF suppression.38
Lifestyle
Obesity is a chronic low-grade inflammatory state that can contribute to the onset of psoriasis or exacerbation of exist
The relationship between psoriasis and alcohol consumption is less clear than it is between psoriasis and obesity or smoking; greater consumption is found in psoriasis patients, but evidence is insufficient to deem alcohol a risk factor.44
Conclusion
Various factors, including genetics, infection, pharmacotherapeutic, and lifestyle, can all contribute to the induction or exacerbation of psoriasis. These factors can provide clues to the pathogenesis of psoriasis as well as help clinicians better counsel patients about their disease.
Psoriasis is a chronic autoimmune skin disease affecting approximately 6.7 million adults in the United States.1 Although its pathogenesis is not yet clear, risk factors and triggers provide insight into potential pathways by which psoriasis can occur. There is notable overlap between risk factors and triggers of psoriasis; perceived risk factors might, in fact, be triggers causing manifestation of disease in predisposed persons. In this review, we summarize the key factors contributing to onset and exacerbation of psoriasis. When learning to manage this chronic disease, it also may be helpful to educate patients about how these elements may affect the course of psoriasis.
Genetics
The pathogenesis of psoriasis has a strong genetic component, with approximately 70% and 20% concordance rates in monozygotic and dizygotic twins, respectively.2 Moreover, studies have shown a positive family history in approximately 35% of patients.3,4 Family-based studies have found a 50% risk of developing psoriasis in patients with 2 affected parents.5 However, the genetics of psoriasis are complex and are attributed to many different genes. Thus far, genes involving antigen presentation, T-cell receptor development and polarization, and the nuclear factor κβ (NF-κβ) pathway have been identified.6
HLA-Cw6
The most well-studied gene implicated in psoriasis is HLA-Cw6, which encodes a major histocompatibility complex class I allele supporting psoriasis as a T cell–mediated reaction to an autoantigen.6 Two potential antigens for HLA-Cw6 recently have been identified: LL-37, a cathelicidin-related antimicrobial peptide, and the A disintegrin and metalloproteinase with thrombospondin motifs-like protein 5 (ADAMTSL5), found on melanocytes and keratinocytes.7 The percentage of psoriasis patients with HLA-Cw6 ranges from 10.5% to 77.2%, with higher frequency in white individuals than in Asians.7
HLA-Cw6 manifests as specific features in psoriasis, including onset of disease before 21 years of age.8 It also is more strongly associated with guttate-type psoriasis, greater body surface area involvement, and higher incidence of Köbner phenomenon. Patients with positive HLA-Cw6 also reported worsening of psoriasis during and after throat infection.9
Caspase Recruitment Domain Family Member 14
Another gene mutation implicated in psoriasis pathogenesis is caspase recruitment domain family member 14, CARD14 (formerly PSORS2), a gene encoding a scaffolding protein important in the activation of NF-κβ.10,11 Missense CARD14 mutations cause upregulation of NF-κβ through formation of a complex with adapter protein B-cell lymphoma 10 (BCL10) and mucosa-associated lymphoid tissue lymphoma translocation protein 1 (MALT1),12 which, in turn, causes increased transcription of cytokines IL-8, C-C motif chemokine ligand 20 (CCL-20), and IL-36 gamma in the keratinocyte.13 Mutations in CARD14 alone lead to psoriasiform skin in mice through amplified activation of the IL-23/IL-17 axis.14,15 Patients with a mutation in a CARD14 variant (p.Arg820Trp) have demonstrated better response to tumor necrosis factor (TNF) inhibitors.16
Further characterization of the genetic pathogenesis of psoriasis might lead to better targeted therapies, including the possibility of MALT1 inhibitors as a treatment option.12
Infection
Streptococcus
The association between streptococcal infection and psoriasis was first documented more than 100 years ago, specifically the onset of acute guttate psoriasis.17,18 Although classically described following throat infection, psoriasis also occurs following streptococcal vulvovaginitis and perianal streptococcal infection.19,20
This type of psoriasis is typically self-limited but can recur with subsequent streptococcal infections or initiate a more chronic plaque psoriasis. Patients have a 1 in 3 risk of developing chronic psoriasis within 10 years of a single episode of acute guttate psoriasis.21 Moreover, in many patients with existing plaque psoriasis, throat infection exacerbates psoriatic symptoms.22 The mechanism of exacerbation is likely due to cross-reactivity between streptococcal M surface antigen and human keratinocytes and might also be influenced by inherited abnormalities in immune response.23-26 Therefore, tonsillectomy has been studied as a possible treatment of psoriasis but is likely helpful only in patients with exacerbations of disease that are closely associated with recurrent tonsillitis.27
Human Immunodeficiency Virus
The prevalence of psoriasis in human immunodeficiency virus (HIV) patients is similar to or greater than the general population.28 Human immunodeficiency virus infection causes new onset of psoriasis and exacerbation of existing psoriasis; severity often is correlated with worsening immune function.28,29
The clinical subtypes of psoriasis that occur most frequently with HIV include guttate, inverse, and erythrodermic, though patients may present with any subtype.28 The mechanism is puzzling because HIV is primarily mediated by helper T cell 2 (TH2) cytokines, whereas psoriasis is mainly driven by helper T cell 1 (TH1) cytokines.30 Furthermore, despite increased severity with lower CD4+ counts, treatments further lowering T-cell counts paradoxically improve symptoms.31 Current literature suggests that expansion of CD8+ memory T cells might be the primary mechanism in the exacerbation of psoriasis in HIV-mediated immunosuppression.30
Treatment of HIV-associated psoriasis presents challenges because many therapeutics cause further immunosuppression. The National Psoriasis Foundation recommends topical preparations as first-line agents for mild to moderate psoriasis.32 For moderate to severe psoriasis, retroviral agents may be effective as first-line monotherapy or when supplemented by phototherapy with UVB or psoralen plus UVA. Retinoids can be used as second-line agents.32 For cases of severe refractory psoriasis, cyclosporine, methotrexate, TNF inhibitors, or hydroxyurea can be considered. There also is evidence that apremilast is effective without risk for worsening immune function.33
Other Infections
Other bacteria associated with triggering or exacerbating psoriasis include Staphylococcus aureus and Helicobacter pylori.34,35 Fungi, such as species of the genera Malassezia and Candida, and other viruses, including papillomaviruses and retroviruses, also have been implicated.34
Medications
Numerous medications can trigger psoriasis, including lithium, nonsteroidal anti-inflammatory drugs, antimalarials, beta-blockers, and angiotensin-converting enzyme inhibitors.34 More recent literature suggests that TNF inhibitors also can paradoxically induce psoriasis in rare cases.35
Lithium
Psoriasis is the most common cutaneous adverse effect of lithium.34 It is more likely to exacerbate existing disease but also can induce onset of psoriasis; it also can cause disease that is more refractory to treatment.34,36 Current literature hypothesizes that lithium triggers psoriasis by interference of intracellular calcium channels through reduction of inositol, thereby affecting keratinocyte proliferation and differentiation.34 Lithium also inhibits glycogen synthase kinase-3 (GSK-3), a serine threonine kinase, which, in turn, induces human keratinocyte proliferation.37 However, it is unlikely lithium alone can induce psoriasis; genetic predisposition is necessary.
TNF Inhibitors
Tumor necrosis factor inhibitors such as adalimumab, etanercept, certolizumab pegol, golimumab, and infliximab are used in various inflammatory diseases, including psoriasis. Interestingly, there have been more than 200 reported cases of suspected TNF inhibitor–induced or –exacerbated psoriasis.38 This phenomenon appears to occur more frequently with infliximab and is most likely to occur in the first year of treatment of Crohn disease and rheumatoid arthritis.38 Plaque psoriasis is the most common form, but 15% to 26% of cases presented with 2 or more morphologies.38,39
Treatment options include discontinuing therapy, though many patients experience resolution while continuing treatment or switching to another TNF inhibitor.38-40 Traditional topical therapies also have been used with success.40 The pathogenesis of this phenomenon is still unclear but is thought to involve both the IL-23/helper T cell 17 (TH17) axis and dysregulation of IFN-α in the setting of TNF suppression.38
Lifestyle
Obesity is a chronic low-grade inflammatory state that can contribute to the onset of psoriasis or exacerbation of exist
The relationship between psoriasis and alcohol consumption is less clear than it is between psoriasis and obesity or smoking; greater consumption is found in psoriasis patients, but evidence is insufficient to deem alcohol a risk factor.44
Conclusion
Various factors, including genetics, infection, pharmacotherapeutic, and lifestyle, can all contribute to the induction or exacerbation of psoriasis. These factors can provide clues to the pathogenesis of psoriasis as well as help clinicians better counsel patients about their disease.
- Helmick CG, Lee-Han H, Hirsch SC, et al. Prevalence of psoriasis among adults in the U.S.: 2003-2006 and 2009-2010 National Health and Nutrition Examination Surveys. Am J Prev Med. 2014;47:37-45.
- Bowcock AM. The genetics of psoriasis and autoimmunity. Annu Rev Genomics Hum Genet. 2005;6:93-122.
- Swanbeck G, Inerot A, Martinsson T, et al. A population genetic study of psoriasis. Br J Dermatol. 1994;131:32-39.
- Kimberling W, Dobson RL. The inheritance of psoriasis. J Invest Dermatol. 1973;60:538-540.
- Gupta R, Debbaneh MG, Liao W. Genetic epidemiology of psoriasis. Curr Dermatol Rep. 2014;3:61-78.
- Harden JL, Krueger JG, Bowcock AM. The immunogenetics of psoriasis: a comprehensive review. J Autoimmun. 2015;64:66-73.
- Chen L, Tsai TF. HLA-Cw6 and psoriasis. Br J Dermatol. 2018;178:854-862.
- Enerbäck C, Martinsson T, Ineraot A, et al. Evidence that HLA-Cw6 determines early onset of psoriasis, obtained using sequence-specific primers (PCR-SSP). Acta Derm Venereol. 1997;77:273-276.
- Gudjónsson JE, Kárason A, Antonsdóttir EH, et al. HLA-Cw6-positive and HLA-Cw6-negative patients with psoriasis vulgaris have distinct clinical features. J Invest Dermatol. 2002;118:362-365.
- Tomfohrde J, Silverman A, Barnes R, et al. Gene for familial psoriasis susceptibility mapped to distal end of human chromosome 17q. Science. 1994;264:1141-1145.
- Blonska M, Lin X. NF-κB signaling pathways regulated by CARMA family of scaffold proteins. Cell Res. 2011;21:55-70.
- Van Nuffel E, Schmitt A, Afonina IS, et al. CARD14-mediated activation of paracaspase MALT1 in keratinocytes: implications for psoriasis. J Invest Dermatol. 2017;137:569-575.
- Jordan CT, Cao L, Roberson ED, et al. PSORS2 is due to mutations in CARD14. Am J Hum Genet. 2012;90:784-795.
- Wang M, Zhang S, Zheng G, et al. Gain-of-function mutation of Card14 leads to spontaneous psoriasis-like skin inflammation through enhanced keratinocyte response to IL-17A. Immunity. 2018;49:66-79.
- Mellet M, Meier B, Mohanan D, et al. CARD14 gain-of-function mutation alone is sufficient to drive IL-23/IL-17-mediated psoriasiform skin inflammation in vivo. J Invest Dermatol. 2018;138:2010-2023.
- Coto-Segura P, González-Fernández D, Batalla A, et al. Common and rare CARD14 gene variants affect the antitumour necrosis factor response among patients with psoriasis. Br J Dermatol. 2016;175:134-141.
- Winfield JM. Psoriasis as a sequel to acute inflammations of the tonsils: a clinical note. J Cutan Dis. 1916;34:441-443.
- Telfer NR, Chalmers RJG, Whale K, et al. The role of streptococcal infection in the initiation of guttate psoriasis. Arch Dermatol. 1992;128:39-42.
- Hernandez M, Simms-Cendan J, Zendell K. Guttate psoriasis following streptococcal vulvovaginitis in a five-year-old girl. J Pediatr Adolesc Gynecol. 2015;28:e127-e129.
- Herbst RA, Hoch O, Kapp A, et al. Guttate psoriasis triggered by perianal streptococcal dermatitis in a four-year-old boy. J Am Acad Dermatol. 2000;42(5, pt 2):885-887.
- Martin BA, Chalmers RJ, Telfer NR. How great is the risk of further psoriasis following a single episode of acute guttate psoriasis? Arch Dermatol. 1996;132:717-718.
- Thorleifsdottir RH, Eysteinsdóttir, Olafsson JH, et al. Throat infections are associated with exacerbation in a substantial proportion of patients with chronic plaque psoriasis. Acta Derm Venereol. 2016;96:788-791.
- McFadden J, Valdimarsson H, Fry L. Cross-reactivity between streptococcal M surface antigen and human skin. Br J Dermatol. 1991;125:443-447.
- Validmarsson H, Thorleifsdottir RH, Sigurdardottir SL, et al. Psoriasis—as an autoimmune disease caused by molecular mimicry. Trends Immunol. 2009;30:494-501.
- Muto M, Fujikara Y, Hamamoto Y, et al. Immune response to Streptococcus pyogenes and the susceptibility to psoriasis. Australas J Dermatol. 1996;37(suppl 1):S54-S55.
- Weisenseel P, Laumbacher B, Besgen P, et al. Streptococcal infection distinguishes different types of psoriasis. J Med Genet. 2002;39:767-768.
- Rachakonda TD, Dhillon JS, Florek AG, et al. Effect of tonsillectomy on psoriasis: a systematic review. J Am Acad Dermatol. 2015;72:261-275.
- Mallon E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246.
- Duvic M, Johnson TM, Rapini RP, et al. Acquired immunodeficiency syndrome-associated psoriasis and Reiter’s syndrome. Arch Dermatol. 1987;123:1622-1632.
- Fife DJ, Waller JM, Jeffes EW, et al. Unraveling the paradoxes of HIV-associated psoriasis: a review of T-cell subsets and cytokine profiles. Dermatol Online J. 2007;13:4.
- Ortonne JP, Lebwohl M, Em Griffiths C; Alefacept Clinical Study Group. Alefacept-induced decreases in circulating blood lymphocyte counts correlate with clinical response in patients with chronic plaque psoriasis. Eur J Dermatol. 2003;13:117-123.
- Menon K, Van Voorhees AS, Bebo BF Jr, et al; National Psoriasis Foundation. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Reddy SP, Shah VV, Wu JJ. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol. 2017;31:e481-e482.
- Fry L, Baker BS. Triggering psoriasis: the role of infections and medications. Clin Dermatol. 2007;25:606-615.
- Sfikakis PP, Iliopoulos A, Elezoglou A, et al. Psoriasis induced by anti-tumor necrosis factor therapy: a paradoxical adverse reaction. Arthritis Rheum. 2005;52:2513-2518.
- Yeung CK, Chan HH. Cutaneous adverse effects of lithium: epidemiology and management. Am J Clin Dermatol. 2004;5:3-8.
- Hampton PJ, Jans R, Flockhart RJ, et al. Lithium regulates keratinocyte proliferation via glycogen synthase kinase 3 and NFAT 2 (nuclear factor of activated T cells 2). J Cell Physiol. 2012;227:1529-1537.
- Brown G, Wang E, Leon A, et al. Tumor necrosis factor-α inhibitor-induced psoriasis: systematic review of clinical features, histopathological findings, and management experience. J Am Acad Dermatol. 2017;76:334-341.
- Collamer AN, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: clinical features and possible immunopathogenesis. Semin Arthritis Rheum. 2010;40:233-240.
- Collamer AN, Guerrero KT, Henning JS, et al. Psoriatic skin lesions induced by tumor antagonist therapy: a literature review and potential mechanisms of action. Arthritis Rheum. 2008;59:996-1001.
- Jensen P, Skov L. Psoriasis and obesity. Dermatology. 2016;232:633-639.
- Barrea L, Nappi F, Di Somma C, et al. Environmental risk factors in psoriasis: the point of view of the nutritionist. Int J Environ Res Public Health. 2016;13:743.
- Lee EJ, Han KD, Han JH, et al. Smoking and risk of psoriasis: a nationwide cohort study. J Am Acad Dermatol. 2017;77:573-575.
- Brenaut E, Horreau C, Pouplard C, et al. Alcohol consumption and psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2013;27(suppl 3):30-35.
- Helmick CG, Lee-Han H, Hirsch SC, et al. Prevalence of psoriasis among adults in the U.S.: 2003-2006 and 2009-2010 National Health and Nutrition Examination Surveys. Am J Prev Med. 2014;47:37-45.
- Bowcock AM. The genetics of psoriasis and autoimmunity. Annu Rev Genomics Hum Genet. 2005;6:93-122.
- Swanbeck G, Inerot A, Martinsson T, et al. A population genetic study of psoriasis. Br J Dermatol. 1994;131:32-39.
- Kimberling W, Dobson RL. The inheritance of psoriasis. J Invest Dermatol. 1973;60:538-540.
- Gupta R, Debbaneh MG, Liao W. Genetic epidemiology of psoriasis. Curr Dermatol Rep. 2014;3:61-78.
- Harden JL, Krueger JG, Bowcock AM. The immunogenetics of psoriasis: a comprehensive review. J Autoimmun. 2015;64:66-73.
- Chen L, Tsai TF. HLA-Cw6 and psoriasis. Br J Dermatol. 2018;178:854-862.
- Enerbäck C, Martinsson T, Ineraot A, et al. Evidence that HLA-Cw6 determines early onset of psoriasis, obtained using sequence-specific primers (PCR-SSP). Acta Derm Venereol. 1997;77:273-276.
- Gudjónsson JE, Kárason A, Antonsdóttir EH, et al. HLA-Cw6-positive and HLA-Cw6-negative patients with psoriasis vulgaris have distinct clinical features. J Invest Dermatol. 2002;118:362-365.
- Tomfohrde J, Silverman A, Barnes R, et al. Gene for familial psoriasis susceptibility mapped to distal end of human chromosome 17q. Science. 1994;264:1141-1145.
- Blonska M, Lin X. NF-κB signaling pathways regulated by CARMA family of scaffold proteins. Cell Res. 2011;21:55-70.
- Van Nuffel E, Schmitt A, Afonina IS, et al. CARD14-mediated activation of paracaspase MALT1 in keratinocytes: implications for psoriasis. J Invest Dermatol. 2017;137:569-575.
- Jordan CT, Cao L, Roberson ED, et al. PSORS2 is due to mutations in CARD14. Am J Hum Genet. 2012;90:784-795.
- Wang M, Zhang S, Zheng G, et al. Gain-of-function mutation of Card14 leads to spontaneous psoriasis-like skin inflammation through enhanced keratinocyte response to IL-17A. Immunity. 2018;49:66-79.
- Mellet M, Meier B, Mohanan D, et al. CARD14 gain-of-function mutation alone is sufficient to drive IL-23/IL-17-mediated psoriasiform skin inflammation in vivo. J Invest Dermatol. 2018;138:2010-2023.
- Coto-Segura P, González-Fernández D, Batalla A, et al. Common and rare CARD14 gene variants affect the antitumour necrosis factor response among patients with psoriasis. Br J Dermatol. 2016;175:134-141.
- Winfield JM. Psoriasis as a sequel to acute inflammations of the tonsils: a clinical note. J Cutan Dis. 1916;34:441-443.
- Telfer NR, Chalmers RJG, Whale K, et al. The role of streptococcal infection in the initiation of guttate psoriasis. Arch Dermatol. 1992;128:39-42.
- Hernandez M, Simms-Cendan J, Zendell K. Guttate psoriasis following streptococcal vulvovaginitis in a five-year-old girl. J Pediatr Adolesc Gynecol. 2015;28:e127-e129.
- Herbst RA, Hoch O, Kapp A, et al. Guttate psoriasis triggered by perianal streptococcal dermatitis in a four-year-old boy. J Am Acad Dermatol. 2000;42(5, pt 2):885-887.
- Martin BA, Chalmers RJ, Telfer NR. How great is the risk of further psoriasis following a single episode of acute guttate psoriasis? Arch Dermatol. 1996;132:717-718.
- Thorleifsdottir RH, Eysteinsdóttir, Olafsson JH, et al. Throat infections are associated with exacerbation in a substantial proportion of patients with chronic plaque psoriasis. Acta Derm Venereol. 2016;96:788-791.
- McFadden J, Valdimarsson H, Fry L. Cross-reactivity between streptococcal M surface antigen and human skin. Br J Dermatol. 1991;125:443-447.
- Validmarsson H, Thorleifsdottir RH, Sigurdardottir SL, et al. Psoriasis—as an autoimmune disease caused by molecular mimicry. Trends Immunol. 2009;30:494-501.
- Muto M, Fujikara Y, Hamamoto Y, et al. Immune response to Streptococcus pyogenes and the susceptibility to psoriasis. Australas J Dermatol. 1996;37(suppl 1):S54-S55.
- Weisenseel P, Laumbacher B, Besgen P, et al. Streptococcal infection distinguishes different types of psoriasis. J Med Genet. 2002;39:767-768.
- Rachakonda TD, Dhillon JS, Florek AG, et al. Effect of tonsillectomy on psoriasis: a systematic review. J Am Acad Dermatol. 2015;72:261-275.
- Mallon E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246.
- Duvic M, Johnson TM, Rapini RP, et al. Acquired immunodeficiency syndrome-associated psoriasis and Reiter’s syndrome. Arch Dermatol. 1987;123:1622-1632.
- Fife DJ, Waller JM, Jeffes EW, et al. Unraveling the paradoxes of HIV-associated psoriasis: a review of T-cell subsets and cytokine profiles. Dermatol Online J. 2007;13:4.
- Ortonne JP, Lebwohl M, Em Griffiths C; Alefacept Clinical Study Group. Alefacept-induced decreases in circulating blood lymphocyte counts correlate with clinical response in patients with chronic plaque psoriasis. Eur J Dermatol. 2003;13:117-123.
- Menon K, Van Voorhees AS, Bebo BF Jr, et al; National Psoriasis Foundation. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Reddy SP, Shah VV, Wu JJ. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol. 2017;31:e481-e482.
- Fry L, Baker BS. Triggering psoriasis: the role of infections and medications. Clin Dermatol. 2007;25:606-615.
- Sfikakis PP, Iliopoulos A, Elezoglou A, et al. Psoriasis induced by anti-tumor necrosis factor therapy: a paradoxical adverse reaction. Arthritis Rheum. 2005;52:2513-2518.
- Yeung CK, Chan HH. Cutaneous adverse effects of lithium: epidemiology and management. Am J Clin Dermatol. 2004;5:3-8.
- Hampton PJ, Jans R, Flockhart RJ, et al. Lithium regulates keratinocyte proliferation via glycogen synthase kinase 3 and NFAT 2 (nuclear factor of activated T cells 2). J Cell Physiol. 2012;227:1529-1537.
- Brown G, Wang E, Leon A, et al. Tumor necrosis factor-α inhibitor-induced psoriasis: systematic review of clinical features, histopathological findings, and management experience. J Am Acad Dermatol. 2017;76:334-341.
- Collamer AN, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: clinical features and possible immunopathogenesis. Semin Arthritis Rheum. 2010;40:233-240.
- Collamer AN, Guerrero KT, Henning JS, et al. Psoriatic skin lesions induced by tumor antagonist therapy: a literature review and potential mechanisms of action. Arthritis Rheum. 2008;59:996-1001.
- Jensen P, Skov L. Psoriasis and obesity. Dermatology. 2016;232:633-639.
- Barrea L, Nappi F, Di Somma C, et al. Environmental risk factors in psoriasis: the point of view of the nutritionist. Int J Environ Res Public Health. 2016;13:743.
- Lee EJ, Han KD, Han JH, et al. Smoking and risk of psoriasis: a nationwide cohort study. J Am Acad Dermatol. 2017;77:573-575.
- Brenaut E, Horreau C, Pouplard C, et al. Alcohol consumption and psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2013;27(suppl 3):30-35.
Practice Points
- HLA-Cw6 and CARD14 are genetic factors associated with psoriasis.
- Psoriasis in the setting of human immunodeficiency virus infection may be treated with topical steroids, phototherapy, systemic retinoids, or apremilast.
- Psoriasis is a potential adverse effect in patients taking lithium or tumor necrosis factor inhibitors.
- Patients should be counseled about the role of obesity and smoking on psoriasis.
Biologic Therapy in Psoriasis: Navigating the Options
Psoriasis is a T cell–mediated inflammatory disease that manifests as erythematous scaling plaques of the skin. In recent decades, our understanding of psoriasis has transformed from a disease isolated to the skin to a systemic disease impacting the overall health of those affected.
With recent elucidation of the pathways driving psoriasis, development of targeted therapies has resulted in an influx of options to the market. Navigating the options can seem overwhelming even to the seasoned clinician. Becoming familiar with a sound treatment approach during residency will create a foundation for biologic use in psoriasis patients throughout your career. Here we offer an approach to choosing biologic treatments based on individual patient characteristics, including disease severity, comorbidities, and ultimate treatment goals.
Immune Pathogenesis
Although the pathogenesis of psoriasis is complex and outside the scope of this article, we do recommend clinicians keep in mind the current understanding of pathways involved and ways our therapies alter them. Briefly, psoriasis is a T cell–mediated disease in which IL-12 and IL-23 released by activated dendritic cells activate T helper cells including TH1, TH17, and TH22. These cells produce additional cytokines, including IFN-γ, tumor necrosis factor (TNF) α, IL-17, and IL-22, which propagate the immune response and lead to keratinocyte hyperproliferation. In general, psoriasis medications work by altering T-cell activation, effector cytokines, or cytokine receptors.
Comorbidities
A targeted approach should take into consideration the immune dysregulation shared by psoriasis and associated comorbidities (Table 1). One goal of biologic treatments is to improve comorbidities when possible. At minimum, selected treatments should not exacerbate these conditions.
Treatment Goals
Establishing treatment goals can help shape patient expectations and provide a plan for clinicians. In 2017, the National Psoriasis Foundation published a treat-to-target approach using body surface area (BSA) measurements at baseline, 3 months, and then every 6 months after starting a new treatment.12 The target response is a decrease in psoriasis to 1% or less BSA at 3 months and to maintain this response when evaluated at 6-month intervals. Alternatively, a target of 3% BSA after 3 months is satisfactory if the patient improves by 75% BSA overall. If these targets are not met after 6 months, therapeutic alternatives can be considered.12
Biologic Treatment of Psoriasis
Treatment options for patients with psoriasis depend first on disease severity. Topicals and phototherapy are first line for mild to moderate disease. For moderate to severe disease, addition of systemic agents such as methotrexate, cyclosporine, or acitretin; small-molecular-weight immunomodulators such as apremilast; or biologic medications should be considered. Current biologics available for moderate to severe plaque psoriasis target TNF-α, IL-12/IL-23, IL-23, IL-17A, or IL-17A receptor.
TNF-α Inhibitors
Tumor necrosis factor α inhibitors have been available for treatment of autoimmune disease for nearly 20 years. These medications block either soluble cytokine or membrane-bound cytokine. All are given as subcutaneous injections, except for infliximab, which is a weight-based infusion.
Efficacy
Tumor necrosis factor α inhibitors are the first class to demonstrate long-term efficacy and safety in both psoriasis and psoriatic arthritis (PsA). Etanercept was approved for adults with PsA in 2002 and psoriasis in 2004, and later for pediatric psoriasis (≥4 years of age) in 2016 (Table 2). Although etanercept has a sustained safety profile, the response rates are not as high as other anti–TNF-α inhibitors. Adalimumab is one of the most prescribed biologics, with a total of 10 indications at present, including PsA. Infliximab is an intravenous infusion that demonstrates a rapid and sustained response in most patients. The dose and dosing interval can be adjusted according to response. Certolizumab pegol was approved for PsA in 2013 and for psoriasis in 2018.
Tumor necrosis factor α inhibitors maintain efficacy well and work best when dosed continuously. Both neutralizing and nonneutralizing antibodies form with these agents. Neutralizing antibodies may contribute to decreased efficacy, particularly for the chimeric antibody infliximab. One approach to mitigate loss of efficacy is the short-term addition of low-dose methotrexate (eg, 7.5–15 mg weekly) for 3 to 6 months until response is recaptured.
Safety
To evaluate long-term safety, a multicenter prospective registry study (Psoriasis Longitudinal Assessment and Registry [PSOLAR]) was initiated in 2007 to follow clinical outcomes. Data through 2013 showed no significant increase in rates of infection, malignancy, or major adverse cardiovascular events in more than 12,000 patients.13
Conflicting information exists in the literature regarding risk for malignancy with TNF-α inhibitors. One recent retrospective cohort study suggested a slightly increased risk for malignancies other than nonmelanoma skin cancers in patients on TNF-α inhibitors for more than 12 months (relative risk, 1.54).14 Reports of increased risk for cutaneous squamous cell carcinomas necessitate regular skin checks.15 A potential risk for lymphoma has been noted, though having psoriasis itself imparts an increased risk for Hodgkin and cutaneous T-cell lymphoma.16
Reactivation of tuberculosis and hepatitis have been reported with TNF-α inhibition. Data suggest that infliximab may be associated with more serious infections.13
Demyelinating conditions such as multiple sclerosis have occurred de novo or worsened in patients on TNF-α inhibitors.17 Tumor necrosis factor α blockers should be avoided in patients with decompensated heart failure. Rare cases of liver enzyme elevation and cytopenia have been noted. Additionally, lupuslike syndromes, which are generally reversible upon discontinuation, have occurred in some patients.
Patient Selection
Tumor necrosis factor α inhibitors are the treatment of choice for patients with comorbid PsA. This class halts progression of joint destruction over time.18Select TNF-α inhibitors are indicated for inflammatory bowel disease (IBD) and are a preferred treatment in this patient population. Specifically, adalimumab and infliximab are approved for both Crohn disease (CD) and ulcerative colitis. Certolizumab pegol is approved for CD.
Tumor necrosis factor α is upregulated in obesity, cardiovascular disease, and atherosclerotic plaques. Evidence suggests that TNF-α blockers may lower cardiovascular risk over time.19 For patients with obesity, infliximab is a good option, as it is the only TNF-α inhibitor with weight-based dosing.
In patients with frequent infections or history of hepatitis C, etanercept has been the biologic most commonly used when no alternatives exist, in part due to its shorter half-life.
IL-12/IL-23 Inhibitor
Ustekinumab is a monoclonal antibody that binds the p40 subunit shared by IL-12 and IL-23, blocking their ability to bind receptors. IL-12 and IL-23 play a role in activating naïve T cells to become TH1 or TH17 cells, respectively.
Efficacy and Safety
Clinical trials demonstrate long-term efficacy of ustekinumab, which was approved for psoriasis in 2009, PsA in 2013, and later pediatric psoriasis (≥12 years of age) in 2017. Dosing is listed in Table 2.
Laboratory abnormalities did not arise in trials. Periodic tuberculosis screening is required. Prospective data over 5 years showed very low rates of adverse events (AEs), serious infections, malignancies, and major adverse cardiovascular events.20 Ustekinumab did not worsen or improve demyelinating disease and appears safe in this population.
Patient Selection
Ustekinumab is approved for PsA and is a good option for those who are not candidates for TNF-α and IL-17 inhibitors. Ustekinumab also is approved for CD. The dosing interval of 12 weeks makes ustekinumab convenient for patients. Two dosages exist based on the patient’s weight, offering an advantage to obese patients.
IL-17/IL-17R Inhibitors
Activated TH17 cells produce the IL-17 cytokine family, which stimulates keratinocyte proliferation and dermal inflammation. Secukinumab is a fully human monoclonal antibody, and ixekizumab is a humanized monoclonal antibody; both target IL-17A. Brodalumab targets the IL-17A receptor.
Efficacy and Safety
IL-17 inhibitors showed impressive and rapid responses in trials.21-23 The subsets of patients who responded well and continued treatment in extension trials demonstrated that these treatments maintain efficacy over time.24-26
In addition to tuberculosis reactivation, there is a small increased risk for cutaneous candidiasis with IL-17 inhibitors, which can be managed without stopping treatment. Laboratory abnormalities were limited to mild neutropenia, which was not associated with increased risk for infection.21-23 With ixekizumab, neutropenia was seen more commonly in the first 12 weeks.22
IL-17 is highly expressed in the gut mucosa, and its inhibition is thought to weaken the barrier function of the gut mucosa, promoting inflammation. As a consequence, this class is contraindicated in patients with IBD due to exacerbations of existing IBD and cases of new-onset IBD.21-23 Symptoms of diarrhea, abdominal pain, blood in stool, or nighttime stooling on review of gastrointestinal tract symptoms should prompt further evaluation.
Brodalumab has a unique warning for risk for suicidal ideation and behavior.23 Depression is more common in the psoriasis population in general; therefore, physicians should be aware of this potential comorbidity regardless of the treatment plan. Because the response rates are so impressive with brodalumab, the Risk Evaluation and Mitigation Strategy (REMS) program was established to ensure understanding of this risk so that patients can be appropriately counseled and managed.
Patient Selection
The improvement in psoriasis is rapid and may occur as early as week 2 to 3 of treatment after initiation of IL-17 inhibitors. Ixekizumab and secukinumab also are approved for PsA. Although improvement in joint disease is not as fast as with the anti-TNF inhibitors, notable improvement occurs by week 20 to 24.27
IL-23 Inhibitors
Guselkumab and tildrakizumab are the newest biologics for psoriasis, approved in 2017 and 2018, respectively. Both are monoclonal antibodies against the p19 subunit of IL-23, which blocks activation of TH17 cells.
Efficacy and Safety
Guselkumab and tildrakizumab demonstrated efficacy with minimal AEs or precautions noted thus far.28,29 Infections are again a risk, making tuberculosis testing the only recommended monitoring.
Patient Selection
Both medications offer another effective and safe option for patients with psoriasis. Similar to ustekinumab, the dosing interval of 12 weeks for tildrakizumab is ideal for patients who have needle phobia or are unable to administer their own injections.
Special Populations
Pregnancy
Antibodies cross the placenta as pregnancy progresses, with the highest rate in the third trimester. Certolizumab pegol has shown the lowest concentrations in infant serum, possibly due to its unique structure lacking the fragment crystallizable region required for passage through the placenta.30 For this reason, certolizumab pegol is a treatment of choice if biologic therapy is warranted during pregnancy.
Much of the pregnancy data for the remaining TNF-α inhibitors come from patients with rheumatoid arthritis or CD. In these populations, rates of major birth defects and miscarriages do not differ greatly from untreated women with these conditions.31 One retrospective study of unintentional pregnancies in women receiving ustekinumab showed rates of AEs similar to the general population.32
Pregnancy data for IL-17 or IL-23 inhibitors are largely limited to animal studies. One retrospective study of women exposed to secukinumab early in gestation showed no increased risk for pregnancy-related AEs.33 Discontinuation is still recommended for patients who become pregnant.
Pediatric Patients
Etanercept is approved for pediatric psoriatic patients 4 years and older. Children with juvenile idiopathic arthritis who are 2 years and older can receive etanercept. Ustekinumab is safe and effective for pediatric psoriatic patients 12 years and older, offering a second biologic option in children.
Although not approved for pediatric psoriasis, adalimumab is approved in pediatric CD (≥6 years of age) and for juvenile idiopathic arthritis (≥2 years of age). Infliximab is approved for children 6 years and older with CD or ulcerative colitis.
Monitoring
Periodic tuberculosis screening is recommended for all biologics. For patients with latent tuberculosis, biologics may be restarted after 1 month of treatment of tuberculosis.
Prior to initiation of biologics, patients should be screened for hepatitis with hepatitis B surface antigen and antibody, hepatitis B core antibody, and hepatitis C antibody. Patients at risk for human immunodeficiency virus also should be screened.
Generally, complete blood cell count and comprehensive metabolic profile are advisable prior to starting a biologic. Opinions differ on frequency of repeating laboratory work. Complete blood cell count and comprehensive metabolic profile should be monitored at least every 3 to 6 months in patients on TNF-α inhibitors, and neutrophil count should be monitored during the induction phase of IL-17 inhibitors.
All patients with psoriasis should maintain age-appropriate cancer screenings, especially those on biologics. If malignancy is discovered, biologic medication should be discontinued. Debate exists as to when therapy can be safely restarted following treatment of malignancy. Patients who are considered at low risk for recurrence may opt to restart a biologic after 5 years, or sooner if symptoms warrant.34 This decision should involve the patient’s cancer specialist.
Conclusion
Treatment choices are based on psoriasis type and severity, comorbidities, patient preferences, and drug accessibility. One approach is detailed in Table 3. As research advances the understanding of psoriasis, this field will continue to rapidly change. Knowledge of the immunopathogenesis of psoriasis and its relation to comorbidities can direct your decision-making for individual patients.
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes. 2012;2:e54.
- Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and the risk of diabetes mellitus: a systematic review and meta-analysis. JAMA Dermatol. 2013;149:84-91.
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and hypertension: a systematic review and meta-analysis of observational studies. J Hypertens. 2013;31:433-442; discussion 442-433.
- Candia R, Ruiz A, Torres-Robles R, et al. Risk of non-alcoholic fatty liver disease in patients with psoriasis: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2015;29:656-662.
- Chi CC, Tung TH, Wang J, et al. Risk of uveitis among people with psoriasis: a nationwide cohort study. JAMA Ophthalmol. 2017;135:415-422.
- Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn’s disease. J Eur Acad Dermatol Venereol. 2009;23:561-565.
- Dowlatshahi EA, Wakkee M, Arends LR, et al. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol. 2014;134:1542-1551.
- Gaeta M, Castelvecchio S, Ricci C, et al. Role of psoriasis as independent predictor of cardiovascular disease: a meta-regression analysis. Int J Cardiol. 2013;168:2282-2288.
- Ma C, Harskamp CT, Armstrong EJ, et al. The association between psoriasis and dyslipidaemia: a systematic review. Br J Dermatol. 2013;168:486-495.
- Parisi R, Webb RT, Carr MJ, et al. Alcohol-related mortality in patients with psoriasis: a population-based cohort study. JAMA Dermatol. 2017;153:1256-1262.
- Rodríguez-Zúñiga MJM, García-Perdomo HA. Systematic review and meta-analysis of the association between psoriasis and metabolic syndrome. J Am Acad Dermatol. 2017;77:657-666.e8.
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Gottlieb AB, Kalb RE, Langley RG, et al. Safety observations in 12095 patients with psoriasis enrolled in an international registry (PSOLAR): experience with infliximab and other systemic and biologic therapies. J Drugs Dermatol. 2014;13:1441-1448.
- Fiorentino D, Ho V, Lebwohl MG, et al. Risk of malignancy with systemic psoriasis treatment in the Psoriasis Longitudinal Assessment Registry. J Am Acad Dermatol. 2017;77:845-854.e5.
- van Lümig PP, Menting SP, van den Reek JM, et al. An increased risk of non-melanoma skin cancer during TNF-inhibitor treatment in psoriasis patients compared to rheumatoid arthritis patients probably relates to disease-related factors. J Eur Acad Dermatol Venereol. 2015;29:752-760.
- Gelfand JM, Berlin J, Van Voorhees A, et al. Lymphoma rates are low but increased in patients with psoriasis: results from a population-based cohort study in the United Kingdom. Arch Dermatol. 2003;139:1425-1429.
- Sicotte NL, Voskuhl RR. Onset of multiple sclerosis associated with anti-TNF therapy. Neurology. 2001;57:1885-1888.
- Finckh A, Simard JF, Duryea J, et al. The effectiveness of anti-tumor necrosis factor therapy in preventing progressive radiographic joint damage in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2006;54:54-59.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-α inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Kimball AB, Papp KA, Wasfi Y, et al. Long-term efficacy of ustekinumab in patients with moderate-to-severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J Eur Acad Dermatol Venereol. 2013;27:1535-1545.
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338.
- Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541-551.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328.
- Bissonnette R, Luger T, Thaçi D, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol. 2018;32:1507-1514.
- Leonardi C, Maari C, Philipp S, et al. Maintenance of skin clearance with ixekizumab treatment of psoriasis: three-year results from the UNCOVER-3 study. J Am Acad Dermatol. 2018;79:824-830.
- Papp K, Leonardi C, Menter A, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71:1183-1190.e1183.
- Gottlieb AB, Strand V, Kishimoto M, et al. Ixekizumab improves patient-reported outcomes up to 52 weeks in bDMARD-naïve patients with active psoriatic arthritis (SPIRIT-P1). Rheumatology (Oxford). 2018;57:1777-1788.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405-417.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390:276-288.
- Mariette X, Förger F, Abraham B, et al. Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis. 2018;77:228-233.
- Komaki F, Komaki Y, Micic D, et al. Outcome of pregnancy and neonatal complications with anti-tumor necrosis factor-α use in females with immune mediated diseases; a systematic review and meta-analysis. J Autoimmun. 2017;76:38-52.
- Götestam Skorpen C, Hoeltzenbein M, Tincani A, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016;75:795-810.
- Warren RB, Reich K, Langley RG, et al. Secukinumab in pregnancy: outcomes in psoriasis, psoriatic arthritis and ankylosing spondylitis from the global safety database [published online ahead of print June 21, 2018]. Br J Dermatol. doi:10.1111/bjd.16901.
- Elandt K, Aletaha D. Treating rheumatic patients with a malignancy. Arthritis Res Ther. 2011;13:223.
Psoriasis is a T cell–mediated inflammatory disease that manifests as erythematous scaling plaques of the skin. In recent decades, our understanding of psoriasis has transformed from a disease isolated to the skin to a systemic disease impacting the overall health of those affected.
With recent elucidation of the pathways driving psoriasis, development of targeted therapies has resulted in an influx of options to the market. Navigating the options can seem overwhelming even to the seasoned clinician. Becoming familiar with a sound treatment approach during residency will create a foundation for biologic use in psoriasis patients throughout your career. Here we offer an approach to choosing biologic treatments based on individual patient characteristics, including disease severity, comorbidities, and ultimate treatment goals.
Immune Pathogenesis
Although the pathogenesis of psoriasis is complex and outside the scope of this article, we do recommend clinicians keep in mind the current understanding of pathways involved and ways our therapies alter them. Briefly, psoriasis is a T cell–mediated disease in which IL-12 and IL-23 released by activated dendritic cells activate T helper cells including TH1, TH17, and TH22. These cells produce additional cytokines, including IFN-γ, tumor necrosis factor (TNF) α, IL-17, and IL-22, which propagate the immune response and lead to keratinocyte hyperproliferation. In general, psoriasis medications work by altering T-cell activation, effector cytokines, or cytokine receptors.
Comorbidities
A targeted approach should take into consideration the immune dysregulation shared by psoriasis and associated comorbidities (Table 1). One goal of biologic treatments is to improve comorbidities when possible. At minimum, selected treatments should not exacerbate these conditions.
Treatment Goals
Establishing treatment goals can help shape patient expectations and provide a plan for clinicians. In 2017, the National Psoriasis Foundation published a treat-to-target approach using body surface area (BSA) measurements at baseline, 3 months, and then every 6 months after starting a new treatment.12 The target response is a decrease in psoriasis to 1% or less BSA at 3 months and to maintain this response when evaluated at 6-month intervals. Alternatively, a target of 3% BSA after 3 months is satisfactory if the patient improves by 75% BSA overall. If these targets are not met after 6 months, therapeutic alternatives can be considered.12
Biologic Treatment of Psoriasis
Treatment options for patients with psoriasis depend first on disease severity. Topicals and phototherapy are first line for mild to moderate disease. For moderate to severe disease, addition of systemic agents such as methotrexate, cyclosporine, or acitretin; small-molecular-weight immunomodulators such as apremilast; or biologic medications should be considered. Current biologics available for moderate to severe plaque psoriasis target TNF-α, IL-12/IL-23, IL-23, IL-17A, or IL-17A receptor.
TNF-α Inhibitors
Tumor necrosis factor α inhibitors have been available for treatment of autoimmune disease for nearly 20 years. These medications block either soluble cytokine or membrane-bound cytokine. All are given as subcutaneous injections, except for infliximab, which is a weight-based infusion.
Efficacy
Tumor necrosis factor α inhibitors are the first class to demonstrate long-term efficacy and safety in both psoriasis and psoriatic arthritis (PsA). Etanercept was approved for adults with PsA in 2002 and psoriasis in 2004, and later for pediatric psoriasis (≥4 years of age) in 2016 (Table 2). Although etanercept has a sustained safety profile, the response rates are not as high as other anti–TNF-α inhibitors. Adalimumab is one of the most prescribed biologics, with a total of 10 indications at present, including PsA. Infliximab is an intravenous infusion that demonstrates a rapid and sustained response in most patients. The dose and dosing interval can be adjusted according to response. Certolizumab pegol was approved for PsA in 2013 and for psoriasis in 2018.
Tumor necrosis factor α inhibitors maintain efficacy well and work best when dosed continuously. Both neutralizing and nonneutralizing antibodies form with these agents. Neutralizing antibodies may contribute to decreased efficacy, particularly for the chimeric antibody infliximab. One approach to mitigate loss of efficacy is the short-term addition of low-dose methotrexate (eg, 7.5–15 mg weekly) for 3 to 6 months until response is recaptured.
Safety
To evaluate long-term safety, a multicenter prospective registry study (Psoriasis Longitudinal Assessment and Registry [PSOLAR]) was initiated in 2007 to follow clinical outcomes. Data through 2013 showed no significant increase in rates of infection, malignancy, or major adverse cardiovascular events in more than 12,000 patients.13
Conflicting information exists in the literature regarding risk for malignancy with TNF-α inhibitors. One recent retrospective cohort study suggested a slightly increased risk for malignancies other than nonmelanoma skin cancers in patients on TNF-α inhibitors for more than 12 months (relative risk, 1.54).14 Reports of increased risk for cutaneous squamous cell carcinomas necessitate regular skin checks.15 A potential risk for lymphoma has been noted, though having psoriasis itself imparts an increased risk for Hodgkin and cutaneous T-cell lymphoma.16
Reactivation of tuberculosis and hepatitis have been reported with TNF-α inhibition. Data suggest that infliximab may be associated with more serious infections.13
Demyelinating conditions such as multiple sclerosis have occurred de novo or worsened in patients on TNF-α inhibitors.17 Tumor necrosis factor α blockers should be avoided in patients with decompensated heart failure. Rare cases of liver enzyme elevation and cytopenia have been noted. Additionally, lupuslike syndromes, which are generally reversible upon discontinuation, have occurred in some patients.
Patient Selection
Tumor necrosis factor α inhibitors are the treatment of choice for patients with comorbid PsA. This class halts progression of joint destruction over time.18Select TNF-α inhibitors are indicated for inflammatory bowel disease (IBD) and are a preferred treatment in this patient population. Specifically, adalimumab and infliximab are approved for both Crohn disease (CD) and ulcerative colitis. Certolizumab pegol is approved for CD.
Tumor necrosis factor α is upregulated in obesity, cardiovascular disease, and atherosclerotic plaques. Evidence suggests that TNF-α blockers may lower cardiovascular risk over time.19 For patients with obesity, infliximab is a good option, as it is the only TNF-α inhibitor with weight-based dosing.
In patients with frequent infections or history of hepatitis C, etanercept has been the biologic most commonly used when no alternatives exist, in part due to its shorter half-life.
IL-12/IL-23 Inhibitor
Ustekinumab is a monoclonal antibody that binds the p40 subunit shared by IL-12 and IL-23, blocking their ability to bind receptors. IL-12 and IL-23 play a role in activating naïve T cells to become TH1 or TH17 cells, respectively.
Efficacy and Safety
Clinical trials demonstrate long-term efficacy of ustekinumab, which was approved for psoriasis in 2009, PsA in 2013, and later pediatric psoriasis (≥12 years of age) in 2017. Dosing is listed in Table 2.
Laboratory abnormalities did not arise in trials. Periodic tuberculosis screening is required. Prospective data over 5 years showed very low rates of adverse events (AEs), serious infections, malignancies, and major adverse cardiovascular events.20 Ustekinumab did not worsen or improve demyelinating disease and appears safe in this population.
Patient Selection
Ustekinumab is approved for PsA and is a good option for those who are not candidates for TNF-α and IL-17 inhibitors. Ustekinumab also is approved for CD. The dosing interval of 12 weeks makes ustekinumab convenient for patients. Two dosages exist based on the patient’s weight, offering an advantage to obese patients.
IL-17/IL-17R Inhibitors
Activated TH17 cells produce the IL-17 cytokine family, which stimulates keratinocyte proliferation and dermal inflammation. Secukinumab is a fully human monoclonal antibody, and ixekizumab is a humanized monoclonal antibody; both target IL-17A. Brodalumab targets the IL-17A receptor.
Efficacy and Safety
IL-17 inhibitors showed impressive and rapid responses in trials.21-23 The subsets of patients who responded well and continued treatment in extension trials demonstrated that these treatments maintain efficacy over time.24-26
In addition to tuberculosis reactivation, there is a small increased risk for cutaneous candidiasis with IL-17 inhibitors, which can be managed without stopping treatment. Laboratory abnormalities were limited to mild neutropenia, which was not associated with increased risk for infection.21-23 With ixekizumab, neutropenia was seen more commonly in the first 12 weeks.22
IL-17 is highly expressed in the gut mucosa, and its inhibition is thought to weaken the barrier function of the gut mucosa, promoting inflammation. As a consequence, this class is contraindicated in patients with IBD due to exacerbations of existing IBD and cases of new-onset IBD.21-23 Symptoms of diarrhea, abdominal pain, blood in stool, or nighttime stooling on review of gastrointestinal tract symptoms should prompt further evaluation.
Brodalumab has a unique warning for risk for suicidal ideation and behavior.23 Depression is more common in the psoriasis population in general; therefore, physicians should be aware of this potential comorbidity regardless of the treatment plan. Because the response rates are so impressive with brodalumab, the Risk Evaluation and Mitigation Strategy (REMS) program was established to ensure understanding of this risk so that patients can be appropriately counseled and managed.
Patient Selection
The improvement in psoriasis is rapid and may occur as early as week 2 to 3 of treatment after initiation of IL-17 inhibitors. Ixekizumab and secukinumab also are approved for PsA. Although improvement in joint disease is not as fast as with the anti-TNF inhibitors, notable improvement occurs by week 20 to 24.27
IL-23 Inhibitors
Guselkumab and tildrakizumab are the newest biologics for psoriasis, approved in 2017 and 2018, respectively. Both are monoclonal antibodies against the p19 subunit of IL-23, which blocks activation of TH17 cells.
Efficacy and Safety
Guselkumab and tildrakizumab demonstrated efficacy with minimal AEs or precautions noted thus far.28,29 Infections are again a risk, making tuberculosis testing the only recommended monitoring.
Patient Selection
Both medications offer another effective and safe option for patients with psoriasis. Similar to ustekinumab, the dosing interval of 12 weeks for tildrakizumab is ideal for patients who have needle phobia or are unable to administer their own injections.
Special Populations
Pregnancy
Antibodies cross the placenta as pregnancy progresses, with the highest rate in the third trimester. Certolizumab pegol has shown the lowest concentrations in infant serum, possibly due to its unique structure lacking the fragment crystallizable region required for passage through the placenta.30 For this reason, certolizumab pegol is a treatment of choice if biologic therapy is warranted during pregnancy.
Much of the pregnancy data for the remaining TNF-α inhibitors come from patients with rheumatoid arthritis or CD. In these populations, rates of major birth defects and miscarriages do not differ greatly from untreated women with these conditions.31 One retrospective study of unintentional pregnancies in women receiving ustekinumab showed rates of AEs similar to the general population.32
Pregnancy data for IL-17 or IL-23 inhibitors are largely limited to animal studies. One retrospective study of women exposed to secukinumab early in gestation showed no increased risk for pregnancy-related AEs.33 Discontinuation is still recommended for patients who become pregnant.
Pediatric Patients
Etanercept is approved for pediatric psoriatic patients 4 years and older. Children with juvenile idiopathic arthritis who are 2 years and older can receive etanercept. Ustekinumab is safe and effective for pediatric psoriatic patients 12 years and older, offering a second biologic option in children.
Although not approved for pediatric psoriasis, adalimumab is approved in pediatric CD (≥6 years of age) and for juvenile idiopathic arthritis (≥2 years of age). Infliximab is approved for children 6 years and older with CD or ulcerative colitis.
Monitoring
Periodic tuberculosis screening is recommended for all biologics. For patients with latent tuberculosis, biologics may be restarted after 1 month of treatment of tuberculosis.
Prior to initiation of biologics, patients should be screened for hepatitis with hepatitis B surface antigen and antibody, hepatitis B core antibody, and hepatitis C antibody. Patients at risk for human immunodeficiency virus also should be screened.
Generally, complete blood cell count and comprehensive metabolic profile are advisable prior to starting a biologic. Opinions differ on frequency of repeating laboratory work. Complete blood cell count and comprehensive metabolic profile should be monitored at least every 3 to 6 months in patients on TNF-α inhibitors, and neutrophil count should be monitored during the induction phase of IL-17 inhibitors.
All patients with psoriasis should maintain age-appropriate cancer screenings, especially those on biologics. If malignancy is discovered, biologic medication should be discontinued. Debate exists as to when therapy can be safely restarted following treatment of malignancy. Patients who are considered at low risk for recurrence may opt to restart a biologic after 5 years, or sooner if symptoms warrant.34 This decision should involve the patient’s cancer specialist.
Conclusion
Treatment choices are based on psoriasis type and severity, comorbidities, patient preferences, and drug accessibility. One approach is detailed in Table 3. As research advances the understanding of psoriasis, this field will continue to rapidly change. Knowledge of the immunopathogenesis of psoriasis and its relation to comorbidities can direct your decision-making for individual patients.
Psoriasis is a T cell–mediated inflammatory disease that manifests as erythematous scaling plaques of the skin. In recent decades, our understanding of psoriasis has transformed from a disease isolated to the skin to a systemic disease impacting the overall health of those affected.
With recent elucidation of the pathways driving psoriasis, development of targeted therapies has resulted in an influx of options to the market. Navigating the options can seem overwhelming even to the seasoned clinician. Becoming familiar with a sound treatment approach during residency will create a foundation for biologic use in psoriasis patients throughout your career. Here we offer an approach to choosing biologic treatments based on individual patient characteristics, including disease severity, comorbidities, and ultimate treatment goals.
Immune Pathogenesis
Although the pathogenesis of psoriasis is complex and outside the scope of this article, we do recommend clinicians keep in mind the current understanding of pathways involved and ways our therapies alter them. Briefly, psoriasis is a T cell–mediated disease in which IL-12 and IL-23 released by activated dendritic cells activate T helper cells including TH1, TH17, and TH22. These cells produce additional cytokines, including IFN-γ, tumor necrosis factor (TNF) α, IL-17, and IL-22, which propagate the immune response and lead to keratinocyte hyperproliferation. In general, psoriasis medications work by altering T-cell activation, effector cytokines, or cytokine receptors.
Comorbidities
A targeted approach should take into consideration the immune dysregulation shared by psoriasis and associated comorbidities (Table 1). One goal of biologic treatments is to improve comorbidities when possible. At minimum, selected treatments should not exacerbate these conditions.
Treatment Goals
Establishing treatment goals can help shape patient expectations and provide a plan for clinicians. In 2017, the National Psoriasis Foundation published a treat-to-target approach using body surface area (BSA) measurements at baseline, 3 months, and then every 6 months after starting a new treatment.12 The target response is a decrease in psoriasis to 1% or less BSA at 3 months and to maintain this response when evaluated at 6-month intervals. Alternatively, a target of 3% BSA after 3 months is satisfactory if the patient improves by 75% BSA overall. If these targets are not met after 6 months, therapeutic alternatives can be considered.12
Biologic Treatment of Psoriasis
Treatment options for patients with psoriasis depend first on disease severity. Topicals and phototherapy are first line for mild to moderate disease. For moderate to severe disease, addition of systemic agents such as methotrexate, cyclosporine, or acitretin; small-molecular-weight immunomodulators such as apremilast; or biologic medications should be considered. Current biologics available for moderate to severe plaque psoriasis target TNF-α, IL-12/IL-23, IL-23, IL-17A, or IL-17A receptor.
TNF-α Inhibitors
Tumor necrosis factor α inhibitors have been available for treatment of autoimmune disease for nearly 20 years. These medications block either soluble cytokine or membrane-bound cytokine. All are given as subcutaneous injections, except for infliximab, which is a weight-based infusion.
Efficacy
Tumor necrosis factor α inhibitors are the first class to demonstrate long-term efficacy and safety in both psoriasis and psoriatic arthritis (PsA). Etanercept was approved for adults with PsA in 2002 and psoriasis in 2004, and later for pediatric psoriasis (≥4 years of age) in 2016 (Table 2). Although etanercept has a sustained safety profile, the response rates are not as high as other anti–TNF-α inhibitors. Adalimumab is one of the most prescribed biologics, with a total of 10 indications at present, including PsA. Infliximab is an intravenous infusion that demonstrates a rapid and sustained response in most patients. The dose and dosing interval can be adjusted according to response. Certolizumab pegol was approved for PsA in 2013 and for psoriasis in 2018.
Tumor necrosis factor α inhibitors maintain efficacy well and work best when dosed continuously. Both neutralizing and nonneutralizing antibodies form with these agents. Neutralizing antibodies may contribute to decreased efficacy, particularly for the chimeric antibody infliximab. One approach to mitigate loss of efficacy is the short-term addition of low-dose methotrexate (eg, 7.5–15 mg weekly) for 3 to 6 months until response is recaptured.
Safety
To evaluate long-term safety, a multicenter prospective registry study (Psoriasis Longitudinal Assessment and Registry [PSOLAR]) was initiated in 2007 to follow clinical outcomes. Data through 2013 showed no significant increase in rates of infection, malignancy, or major adverse cardiovascular events in more than 12,000 patients.13
Conflicting information exists in the literature regarding risk for malignancy with TNF-α inhibitors. One recent retrospective cohort study suggested a slightly increased risk for malignancies other than nonmelanoma skin cancers in patients on TNF-α inhibitors for more than 12 months (relative risk, 1.54).14 Reports of increased risk for cutaneous squamous cell carcinomas necessitate regular skin checks.15 A potential risk for lymphoma has been noted, though having psoriasis itself imparts an increased risk for Hodgkin and cutaneous T-cell lymphoma.16
Reactivation of tuberculosis and hepatitis have been reported with TNF-α inhibition. Data suggest that infliximab may be associated with more serious infections.13
Demyelinating conditions such as multiple sclerosis have occurred de novo or worsened in patients on TNF-α inhibitors.17 Tumor necrosis factor α blockers should be avoided in patients with decompensated heart failure. Rare cases of liver enzyme elevation and cytopenia have been noted. Additionally, lupuslike syndromes, which are generally reversible upon discontinuation, have occurred in some patients.
Patient Selection
Tumor necrosis factor α inhibitors are the treatment of choice for patients with comorbid PsA. This class halts progression of joint destruction over time.18Select TNF-α inhibitors are indicated for inflammatory bowel disease (IBD) and are a preferred treatment in this patient population. Specifically, adalimumab and infliximab are approved for both Crohn disease (CD) and ulcerative colitis. Certolizumab pegol is approved for CD.
Tumor necrosis factor α is upregulated in obesity, cardiovascular disease, and atherosclerotic plaques. Evidence suggests that TNF-α blockers may lower cardiovascular risk over time.19 For patients with obesity, infliximab is a good option, as it is the only TNF-α inhibitor with weight-based dosing.
In patients with frequent infections or history of hepatitis C, etanercept has been the biologic most commonly used when no alternatives exist, in part due to its shorter half-life.
IL-12/IL-23 Inhibitor
Ustekinumab is a monoclonal antibody that binds the p40 subunit shared by IL-12 and IL-23, blocking their ability to bind receptors. IL-12 and IL-23 play a role in activating naïve T cells to become TH1 or TH17 cells, respectively.
Efficacy and Safety
Clinical trials demonstrate long-term efficacy of ustekinumab, which was approved for psoriasis in 2009, PsA in 2013, and later pediatric psoriasis (≥12 years of age) in 2017. Dosing is listed in Table 2.
Laboratory abnormalities did not arise in trials. Periodic tuberculosis screening is required. Prospective data over 5 years showed very low rates of adverse events (AEs), serious infections, malignancies, and major adverse cardiovascular events.20 Ustekinumab did not worsen or improve demyelinating disease and appears safe in this population.
Patient Selection
Ustekinumab is approved for PsA and is a good option for those who are not candidates for TNF-α and IL-17 inhibitors. Ustekinumab also is approved for CD. The dosing interval of 12 weeks makes ustekinumab convenient for patients. Two dosages exist based on the patient’s weight, offering an advantage to obese patients.
IL-17/IL-17R Inhibitors
Activated TH17 cells produce the IL-17 cytokine family, which stimulates keratinocyte proliferation and dermal inflammation. Secukinumab is a fully human monoclonal antibody, and ixekizumab is a humanized monoclonal antibody; both target IL-17A. Brodalumab targets the IL-17A receptor.
Efficacy and Safety
IL-17 inhibitors showed impressive and rapid responses in trials.21-23 The subsets of patients who responded well and continued treatment in extension trials demonstrated that these treatments maintain efficacy over time.24-26
In addition to tuberculosis reactivation, there is a small increased risk for cutaneous candidiasis with IL-17 inhibitors, which can be managed without stopping treatment. Laboratory abnormalities were limited to mild neutropenia, which was not associated with increased risk for infection.21-23 With ixekizumab, neutropenia was seen more commonly in the first 12 weeks.22
IL-17 is highly expressed in the gut mucosa, and its inhibition is thought to weaken the barrier function of the gut mucosa, promoting inflammation. As a consequence, this class is contraindicated in patients with IBD due to exacerbations of existing IBD and cases of new-onset IBD.21-23 Symptoms of diarrhea, abdominal pain, blood in stool, or nighttime stooling on review of gastrointestinal tract symptoms should prompt further evaluation.
Brodalumab has a unique warning for risk for suicidal ideation and behavior.23 Depression is more common in the psoriasis population in general; therefore, physicians should be aware of this potential comorbidity regardless of the treatment plan. Because the response rates are so impressive with brodalumab, the Risk Evaluation and Mitigation Strategy (REMS) program was established to ensure understanding of this risk so that patients can be appropriately counseled and managed.
Patient Selection
The improvement in psoriasis is rapid and may occur as early as week 2 to 3 of treatment after initiation of IL-17 inhibitors. Ixekizumab and secukinumab also are approved for PsA. Although improvement in joint disease is not as fast as with the anti-TNF inhibitors, notable improvement occurs by week 20 to 24.27
IL-23 Inhibitors
Guselkumab and tildrakizumab are the newest biologics for psoriasis, approved in 2017 and 2018, respectively. Both are monoclonal antibodies against the p19 subunit of IL-23, which blocks activation of TH17 cells.
Efficacy and Safety
Guselkumab and tildrakizumab demonstrated efficacy with minimal AEs or precautions noted thus far.28,29 Infections are again a risk, making tuberculosis testing the only recommended monitoring.
Patient Selection
Both medications offer another effective and safe option for patients with psoriasis. Similar to ustekinumab, the dosing interval of 12 weeks for tildrakizumab is ideal for patients who have needle phobia or are unable to administer their own injections.
Special Populations
Pregnancy
Antibodies cross the placenta as pregnancy progresses, with the highest rate in the third trimester. Certolizumab pegol has shown the lowest concentrations in infant serum, possibly due to its unique structure lacking the fragment crystallizable region required for passage through the placenta.30 For this reason, certolizumab pegol is a treatment of choice if biologic therapy is warranted during pregnancy.
Much of the pregnancy data for the remaining TNF-α inhibitors come from patients with rheumatoid arthritis or CD. In these populations, rates of major birth defects and miscarriages do not differ greatly from untreated women with these conditions.31 One retrospective study of unintentional pregnancies in women receiving ustekinumab showed rates of AEs similar to the general population.32
Pregnancy data for IL-17 or IL-23 inhibitors are largely limited to animal studies. One retrospective study of women exposed to secukinumab early in gestation showed no increased risk for pregnancy-related AEs.33 Discontinuation is still recommended for patients who become pregnant.
Pediatric Patients
Etanercept is approved for pediatric psoriatic patients 4 years and older. Children with juvenile idiopathic arthritis who are 2 years and older can receive etanercept. Ustekinumab is safe and effective for pediatric psoriatic patients 12 years and older, offering a second biologic option in children.
Although not approved for pediatric psoriasis, adalimumab is approved in pediatric CD (≥6 years of age) and for juvenile idiopathic arthritis (≥2 years of age). Infliximab is approved for children 6 years and older with CD or ulcerative colitis.
Monitoring
Periodic tuberculosis screening is recommended for all biologics. For patients with latent tuberculosis, biologics may be restarted after 1 month of treatment of tuberculosis.
Prior to initiation of biologics, patients should be screened for hepatitis with hepatitis B surface antigen and antibody, hepatitis B core antibody, and hepatitis C antibody. Patients at risk for human immunodeficiency virus also should be screened.
Generally, complete blood cell count and comprehensive metabolic profile are advisable prior to starting a biologic. Opinions differ on frequency of repeating laboratory work. Complete blood cell count and comprehensive metabolic profile should be monitored at least every 3 to 6 months in patients on TNF-α inhibitors, and neutrophil count should be monitored during the induction phase of IL-17 inhibitors.
All patients with psoriasis should maintain age-appropriate cancer screenings, especially those on biologics. If malignancy is discovered, biologic medication should be discontinued. Debate exists as to when therapy can be safely restarted following treatment of malignancy. Patients who are considered at low risk for recurrence may opt to restart a biologic after 5 years, or sooner if symptoms warrant.34 This decision should involve the patient’s cancer specialist.
Conclusion
Treatment choices are based on psoriasis type and severity, comorbidities, patient preferences, and drug accessibility. One approach is detailed in Table 3. As research advances the understanding of psoriasis, this field will continue to rapidly change. Knowledge of the immunopathogenesis of psoriasis and its relation to comorbidities can direct your decision-making for individual patients.
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes. 2012;2:e54.
- Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and the risk of diabetes mellitus: a systematic review and meta-analysis. JAMA Dermatol. 2013;149:84-91.
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and hypertension: a systematic review and meta-analysis of observational studies. J Hypertens. 2013;31:433-442; discussion 442-433.
- Candia R, Ruiz A, Torres-Robles R, et al. Risk of non-alcoholic fatty liver disease in patients with psoriasis: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2015;29:656-662.
- Chi CC, Tung TH, Wang J, et al. Risk of uveitis among people with psoriasis: a nationwide cohort study. JAMA Ophthalmol. 2017;135:415-422.
- Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn’s disease. J Eur Acad Dermatol Venereol. 2009;23:561-565.
- Dowlatshahi EA, Wakkee M, Arends LR, et al. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol. 2014;134:1542-1551.
- Gaeta M, Castelvecchio S, Ricci C, et al. Role of psoriasis as independent predictor of cardiovascular disease: a meta-regression analysis. Int J Cardiol. 2013;168:2282-2288.
- Ma C, Harskamp CT, Armstrong EJ, et al. The association between psoriasis and dyslipidaemia: a systematic review. Br J Dermatol. 2013;168:486-495.
- Parisi R, Webb RT, Carr MJ, et al. Alcohol-related mortality in patients with psoriasis: a population-based cohort study. JAMA Dermatol. 2017;153:1256-1262.
- Rodríguez-Zúñiga MJM, García-Perdomo HA. Systematic review and meta-analysis of the association between psoriasis and metabolic syndrome. J Am Acad Dermatol. 2017;77:657-666.e8.
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Gottlieb AB, Kalb RE, Langley RG, et al. Safety observations in 12095 patients with psoriasis enrolled in an international registry (PSOLAR): experience with infliximab and other systemic and biologic therapies. J Drugs Dermatol. 2014;13:1441-1448.
- Fiorentino D, Ho V, Lebwohl MG, et al. Risk of malignancy with systemic psoriasis treatment in the Psoriasis Longitudinal Assessment Registry. J Am Acad Dermatol. 2017;77:845-854.e5.
- van Lümig PP, Menting SP, van den Reek JM, et al. An increased risk of non-melanoma skin cancer during TNF-inhibitor treatment in psoriasis patients compared to rheumatoid arthritis patients probably relates to disease-related factors. J Eur Acad Dermatol Venereol. 2015;29:752-760.
- Gelfand JM, Berlin J, Van Voorhees A, et al. Lymphoma rates are low but increased in patients with psoriasis: results from a population-based cohort study in the United Kingdom. Arch Dermatol. 2003;139:1425-1429.
- Sicotte NL, Voskuhl RR. Onset of multiple sclerosis associated with anti-TNF therapy. Neurology. 2001;57:1885-1888.
- Finckh A, Simard JF, Duryea J, et al. The effectiveness of anti-tumor necrosis factor therapy in preventing progressive radiographic joint damage in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2006;54:54-59.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-α inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Kimball AB, Papp KA, Wasfi Y, et al. Long-term efficacy of ustekinumab in patients with moderate-to-severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J Eur Acad Dermatol Venereol. 2013;27:1535-1545.
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338.
- Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541-551.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328.
- Bissonnette R, Luger T, Thaçi D, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol. 2018;32:1507-1514.
- Leonardi C, Maari C, Philipp S, et al. Maintenance of skin clearance with ixekizumab treatment of psoriasis: three-year results from the UNCOVER-3 study. J Am Acad Dermatol. 2018;79:824-830.
- Papp K, Leonardi C, Menter A, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71:1183-1190.e1183.
- Gottlieb AB, Strand V, Kishimoto M, et al. Ixekizumab improves patient-reported outcomes up to 52 weeks in bDMARD-naïve patients with active psoriatic arthritis (SPIRIT-P1). Rheumatology (Oxford). 2018;57:1777-1788.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405-417.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390:276-288.
- Mariette X, Förger F, Abraham B, et al. Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis. 2018;77:228-233.
- Komaki F, Komaki Y, Micic D, et al. Outcome of pregnancy and neonatal complications with anti-tumor necrosis factor-α use in females with immune mediated diseases; a systematic review and meta-analysis. J Autoimmun. 2017;76:38-52.
- Götestam Skorpen C, Hoeltzenbein M, Tincani A, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016;75:795-810.
- Warren RB, Reich K, Langley RG, et al. Secukinumab in pregnancy: outcomes in psoriasis, psoriatic arthritis and ankylosing spondylitis from the global safety database [published online ahead of print June 21, 2018]. Br J Dermatol. doi:10.1111/bjd.16901.
- Elandt K, Aletaha D. Treating rheumatic patients with a malignancy. Arthritis Res Ther. 2011;13:223.
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes. 2012;2:e54.
- Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and the risk of diabetes mellitus: a systematic review and meta-analysis. JAMA Dermatol. 2013;149:84-91.
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and hypertension: a systematic review and meta-analysis of observational studies. J Hypertens. 2013;31:433-442; discussion 442-433.
- Candia R, Ruiz A, Torres-Robles R, et al. Risk of non-alcoholic fatty liver disease in patients with psoriasis: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2015;29:656-662.
- Chi CC, Tung TH, Wang J, et al. Risk of uveitis among people with psoriasis: a nationwide cohort study. JAMA Ophthalmol. 2017;135:415-422.
- Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn’s disease. J Eur Acad Dermatol Venereol. 2009;23:561-565.
- Dowlatshahi EA, Wakkee M, Arends LR, et al. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol. 2014;134:1542-1551.
- Gaeta M, Castelvecchio S, Ricci C, et al. Role of psoriasis as independent predictor of cardiovascular disease: a meta-regression analysis. Int J Cardiol. 2013;168:2282-2288.
- Ma C, Harskamp CT, Armstrong EJ, et al. The association between psoriasis and dyslipidaemia: a systematic review. Br J Dermatol. 2013;168:486-495.
- Parisi R, Webb RT, Carr MJ, et al. Alcohol-related mortality in patients with psoriasis: a population-based cohort study. JAMA Dermatol. 2017;153:1256-1262.
- Rodríguez-Zúñiga MJM, García-Perdomo HA. Systematic review and meta-analysis of the association between psoriasis and metabolic syndrome. J Am Acad Dermatol. 2017;77:657-666.e8.
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Gottlieb AB, Kalb RE, Langley RG, et al. Safety observations in 12095 patients with psoriasis enrolled in an international registry (PSOLAR): experience with infliximab and other systemic and biologic therapies. J Drugs Dermatol. 2014;13:1441-1448.
- Fiorentino D, Ho V, Lebwohl MG, et al. Risk of malignancy with systemic psoriasis treatment in the Psoriasis Longitudinal Assessment Registry. J Am Acad Dermatol. 2017;77:845-854.e5.
- van Lümig PP, Menting SP, van den Reek JM, et al. An increased risk of non-melanoma skin cancer during TNF-inhibitor treatment in psoriasis patients compared to rheumatoid arthritis patients probably relates to disease-related factors. J Eur Acad Dermatol Venereol. 2015;29:752-760.
- Gelfand JM, Berlin J, Van Voorhees A, et al. Lymphoma rates are low but increased in patients with psoriasis: results from a population-based cohort study in the United Kingdom. Arch Dermatol. 2003;139:1425-1429.
- Sicotte NL, Voskuhl RR. Onset of multiple sclerosis associated with anti-TNF therapy. Neurology. 2001;57:1885-1888.
- Finckh A, Simard JF, Duryea J, et al. The effectiveness of anti-tumor necrosis factor therapy in preventing progressive radiographic joint damage in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2006;54:54-59.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-α inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Kimball AB, Papp KA, Wasfi Y, et al. Long-term efficacy of ustekinumab in patients with moderate-to-severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J Eur Acad Dermatol Venereol. 2013;27:1535-1545.
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338.
- Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541-551.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328.
- Bissonnette R, Luger T, Thaçi D, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol. 2018;32:1507-1514.
- Leonardi C, Maari C, Philipp S, et al. Maintenance of skin clearance with ixekizumab treatment of psoriasis: three-year results from the UNCOVER-3 study. J Am Acad Dermatol. 2018;79:824-830.
- Papp K, Leonardi C, Menter A, et al. Safety and efficacy of brodalumab for psoriasis after 120 weeks of treatment. J Am Acad Dermatol. 2014;71:1183-1190.e1183.
- Gottlieb AB, Strand V, Kishimoto M, et al. Ixekizumab improves patient-reported outcomes up to 52 weeks in bDMARD-naïve patients with active psoriatic arthritis (SPIRIT-P1). Rheumatology (Oxford). 2018;57:1777-1788.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76:405-417.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390:276-288.
- Mariette X, Förger F, Abraham B, et al. Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis. 2018;77:228-233.
- Komaki F, Komaki Y, Micic D, et al. Outcome of pregnancy and neonatal complications with anti-tumor necrosis factor-α use in females with immune mediated diseases; a systematic review and meta-analysis. J Autoimmun. 2017;76:38-52.
- Götestam Skorpen C, Hoeltzenbein M, Tincani A, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis. 2016;75:795-810.
- Warren RB, Reich K, Langley RG, et al. Secukinumab in pregnancy: outcomes in psoriasis, psoriatic arthritis and ankylosing spondylitis from the global safety database [published online ahead of print June 21, 2018]. Br J Dermatol. doi:10.1111/bjd.16901.
- Elandt K, Aletaha D. Treating rheumatic patients with a malignancy. Arthritis Res Ther. 2011;13:223.
Practice Points
- Psoriasis affects millions of Americans and is associated with a growing list of comorbidities.
- With the increasing number of biologic treatment options available, the clinician must keep in mind the immune pathways involved in psoriasis and the ways our therapies alter them.
- Consider disease severity, comorbidities, patient preferences, and drug accessibility when choosing psoriasis treatments.
Update on the Pathophysiology of Psoriasis
Increased understanding of the pathophysiology of psoriasis has been one of the driving forces in the development of new therapies. An understanding of the processes involved is important in the optimal management of the disease. The last 30 years of research and clinical practice have revolutionized our understanding of the pathogenesis of psoriasis as the dysregulation of immunity triggered by environmental and genetic stimuli. Psoriasis was originally regarded as a primary disorder of epidermal hyperproliferation. However, experimental models and clinical results from immunomodulating therapies have refined this perspective in conceptualizing psoriasis as a genetically programmed pathologic interaction among resident skin cells; infiltrating immunocytes; and a host of proinflammatory cytokines, chemokines, and growth factors produced by these immunocytes. Two populations of immunocytes and their respective signaling molecules collaborate in the pathogenesis: (1) innate immunocytes, mediated by antigen-presenting cells (APCs)(including natural killer [NK] T lymphocytes, Langerhans cells, and neutrophils), and (2) acquired or adaptive immunocytes, mediated by mature CD4+ and CD8+ T lymphocytes in the skin. Such dysregulation of immunity and subsequent inflammation is responsible for the development and perpetuation of the clinical plaques and histological inflammatory infiltrate characteristic of psoriasis.
Although psoriasis is considered to be an immune-mediated disease in which intralesional T lymphocytes and their proinflammatory signals trigger primed basal layer keratinocytes to rapidly proliferate, debate and research focus on the stimulus that incites this inflammatory process. Our current understanding considers psoriasis to be triggered by exogenous or endogenous environmental stimuli in genetically susceptible individuals. Such stimuli include group A streptococcal pharyngitis, viremia, allergic drug reactions, antimalarial drugs, lithium, beta-blockers, IFN-α, withdrawal of systemic corticosteroids, local trauma (Köbner phenomenon), and emotional stress. These stimuli correlate with the onset or flares of psoriatic lesions. Psoriasis genetics centers on susceptibility loci and corresponding candidate genes, particularly the psoriasis susceptibility (PSORS) 1 locus on the major histocompatibility complex (MHC) class I region. Current research on the pathogenesis of psoriasis examines the complex interactions among immunologic mechanisms, environmental stimuli, and genetic susceptibility. After discussing the clinical presentation and histopathologic features of psoriasis, we will review the pathophysiology of psoriasis through noteworthy developments, including serendipitous observations, reactions to therapies, clinical trials, and animal model systems that have shaped our view of the disease process. In addition to the classic skin lesions, approximately 23% of psoriasis patients develop psoriatic arthritis, with a 10-year latency after diagnosis of psoriasis.1
Principles of Immunity
The immune system, intended to protect its host from foreign invaders and unregulated cell growth, employs 2 main effector pathways—the innate and the acquired (or adaptive) immune responses—both of which contribute to the pathophysiology of psoriasis.2 Innate immunity responses occur within minutes to hours of antigen exposure but fail to develop memory for when the antigen is encountered again. However, adaptive immunity responses take days to weeks to respond after challenged with an antigen. The adaptive immune cells have the capacity to respond to a greater range of antigens and develop immunologic memory via rearrangement of antigen receptors on B and T cells. These specialized B and T cells can then be promptly mobilized and differentiated into mature effector cells that protect the host from a foreign pathogen.
Innate and adaptive immune responses are highly intertwined; they can initiate, perpetuate, and terminate the immune mechanisms responsible for inflammation. They can modify the nature of the immune response by altering the relative proportions of type 1 (TH1), type 2 (TH2), and the more recently discovered type 17 (TH17) subset of helper T cells and their respective signaling molecules. A TH1 response is essential for a cellular immunologic reaction to intracellular bacteria and viruses or cellular immunity. A TH2 response promotes IgE synthesis, eosinophilia, and mast cell maturation for extracellular parasites and helminthes as well as humoral immunity, while a TH17 response is important for cell-mediated immunity to extracellular bacteria and plays a role in autoimmunity.3 The innate and adaptive immune responses employ common effector molecules such as chemokines and cytokines, which are essential in mediating an immune response.
Implicating Dysregulation of Immunity
Our present appreciation of the pathogenesis of psoriasis is based on the history of trial-and-error therapies; serendipitous discoveries; and the current immune targeting drugs used in a variety of chronic inflammatory conditions, including rheumatoid arthritis, ankylosing spondylitis, and inflammatory bowel disease. Before the mid-1980s, research focused on the hyperproliferative epidermal cells as the primary pathology because a markedly thickened epidermis was indeed demonstrated on histologic specimens. Altered cell-cycle kinetics were thought to be the culprit behind the hyperkeratotic plaques. Thus, initial treatments centered on oncologic and antimitotic therapies used to arrest keratinocyte proliferation with agents such as arsenic, ammoniated mercury, and methotrexate.4
However, a paradigm shift from targeting epidermal keratinocytes to immunocyte populations was recognized when a patient receiving cyclosporine to prevent transplant rejection noted clearing of psoriatic lesions in the 1980s.5 Cyclosporine was observed to inhibit messenger RNA transcription of T-cell cytokines, thereby implicating immunologic dysregulation, specifically T-cell hyperactivity, in the pathogenesis of psoriasis.6 However, the concentrations of oral cyclosporine reached in the epidermis exerted direct effects on keratinocyte proliferation and lymphocyte function in these patients.7 Thus, the question was raised as to whether the keratinocytes or the lymphocytes drove the psoriatic plaques. The use of an IL-2 diphtheria toxin-fusion protein, denileukin diftitox, specific for activated T cells with high-affinity IL-2 receptors and nonreactive with keratinocytes, distinguished which cell type was responsible. This targeted T-cell toxin provided clinical and histological clearing of psoriatic plaques. Thus, T lymphocytes rather than keratinocytes were recognized as the definitive driver behind the psoriatic plaques.8
Additional studies have demonstrated that treatments that induce prolonged clearing of psoriatic lesions without continuous therapy, such as psoralen plus UVA irradiation, decreased the numbers of T cells in plaques by at least 90%.9 However, treatments that require continual therapy for satisfactory clinical results, such as cyclosporine and etretinate, simply suppress T-cell activity and proliferation.10,11 Further evidence has linked cellular immunity with the pathogenesis of psoriasis, defining it as a TH1-type disease. Natural killer T cells were shown to be involved through the use of a severe combined immunodeficient mouse model. They were injected into prepsoriatic skin grafted on immunodeficient mice, creating a psoriatic plaque with an immune response showing cytokines from TH1 cells rather than TH2 cells.12 When psoriatic plaques were treated topically with the toll-like receptor 7 agonist imiquimod, aggravation and spreading of the plaques were noted. The exacerbation of psoriasis was accompanied by an induction of lesional TH1-type interferon produced by plasmacytoid dendritic cell (DC) precursors. Plasmacytoid DCs were observed to compose up to 16% of the total dermal infiltrate in psoriatic skin lesions based on their coexpression of BDCA2 and CD123.13 Additionally, cancer patients being treated with interferon alfa experienced induction of psoriasis.14 Moreover, patients being treated for warts with intralesional interferon alfa developed psoriatic plaques in neighboring prior asymptomatic skin.15 Patients with psoriasis who were treated with interferon gamma, a TH1 cytokine type, also developed new plaques correlating with the sites of injection.16
Intralesional T Lymphocytes
Psoriatic lesions contain a host of innate immunocytes, such as APCs, NK cells, and neutrophils, as well as adaptive T cells and an inflammatory infiltrate. These cells include CD4 and CD8 subtypes in which the CD8+ cells predominate in the epidermis, while CD4+ cells show preference for the dermis.17 There are 2 groups of CD8+ cells: one group migrates to the epidermis, expressing the integrin CD103, while the other group is found in the dermis but may be headed to or from the epidermis. The CD8+ cells residing in the epidermis that express the integrin CD103 are capable of interacting with E-cadherin, which enables these cells to travel to the epidermis and bind resident cells. Immunophenotyping reveals that these mature T cells represent chiefly activated memory cells, including CD2+, CD3+, CD5+, CLA, CD28, and CD45RO+.18 Many of these cells express activation markers such as HLA-DR, CD25, and CD27, in addition to the T-cell receptor (TCR).
T-Lymphocyte Stimulation
Both mature CD4+ and CD8+ T cells can respond to the peptides presented by APCs. Although the specific antigen that these T cells are reacting to has not yet been elucidated, several antigenic stimuli have been proposed, including self-proteins, microbial pathogens, and microbial superantigens. The premise that self-reactive T lymphocytes may contribute to the disease process is derived from the molecular mimicry theory in which an exuberant immune response to a pathogen produces cross-reactivity with self-antigens.19 Considering that infections have been associated with the onset of psoriasis, this theory merits consideration. However, it also has been observed that T cells can be activated without antigens or superantigens but rather with direct contact with accessory cells.20 No single theory has clearly emerged. Researchers continue to search for the inciting stimulus that triggers the T lymphocyte and attempt to determine whether T cells are reacting to a self-derived or non–self-derived antigen.
T-Lymphocyte Signaling
T-cell signaling is a highly coordinated process in which T lymphocytes recognize antigens via presentation by mature APCs in the skin rather than the lymphoid tissues. Such APCs expose antigenic peptides via class I or II MHC molecules for which receptors are present on the T-cell surface. The antigen recognition complex at the T-cell and APC interface, in concert with a host of antigen-independent co-stimulatory signals, regulates T-cell signaling and is referred to as the immunologic synapse. The antigen presentation and network of co-stimulatory and adhesion molecules optimize T-cell activation, and dermal DCs release IL-12 and IL-23 to promote a TH1 and TH17 response, respectively. The growth factors released by these helper T cells sustain neoangiogenesis, stimulate epidermal hyperproliferation, alter epidermal differentiation, and decrease susceptibility to apoptosis that characterizes the erythematous hypertrophic scaling lesions of psoriasis.21 Furthermore, the cytokines produced from the immunologic response, such as tumor necrosis factor (TNF) α, IFN-γ, and IL-2, correspond to cytokines that are upregulated in psoriatic plaques.22
Integral components of the immunologic synapse complex include co-stimulatory signals such as CD28, CD40, CD80, and CD86, as well as adhesion molecules such as cytotoxic T-lymphocyte antigen 4 and lymphocyte function-associated antigen (LFA) 1, which possess corresponding receptors on the T cell. These molecules play a key role in T-cell signaling, as their disruption has been shown to decrease T-cell responsiveness and associated inflammation. The B7 family of molecules routinely interacts with CD28 T cells to co-stimulate T-cell activation. Cytotoxic T-lymphocyte antigen 4 immunoglobulin, an antibody on the T-cell surface, targets B7 and interferes with signaling between B7 and CD28. In psoriatic patients, this blockade was demonstrated to attenuate the T-cell response and correlated with a clinical and histological decrease in psoriasiform hyperplasia.23 Biologic therapies that disrupt the LFA-1 component of the immunologic synapse also have demonstrated efficacy in the treatment of psoriasis. Alefacept is a human LFA-3 fusion protein that binds CD2 on T cells and blocks the interaction between LFA-3 on APCs and CD2 on memory CD45RO+ T cells and induces apoptosis of such T cells. Efalizumab is a human monoclonal antibody to the CD11 chain of LFA-1 that blocks the interaction between LFA-1 on the T cell and intercellular adhesion molecule 1 on an APC or endothelial cell. Both alefacept and efalizumab, 2 formerly marketed biologic therapies, demonstrated remarkable clinical reduction of psoriatic lesions, and alefacept has been shown to produce disease remission for up to 18 months after discontinuation of therapy.24-26
NK T Cells
Natural killer T cells represent a subset of CD3+ T cells present in psoriatic plaques. Although NK T cells possess a TCR, they differ from T cells by displaying NK receptors comprised of lectin and immunoglobulin families. These cells exhibit remarkable specificity and are activated upon recognition of glycolipids presented by CD1d molecules. This process occurs in contrast to CD4+ and CD8+ T cells, which, due to their TCR diversity, respond to peptides processed by APCs and displayed on MHC molecules. Natural killer T cells can be classified into 2 subsets: (1) one group that expresses CD4 and preferentially produces TH1- versus TH2-type cytokines, and (2) another group that lacks CD4 and CD8 that only produces TH1-type cytokines. The innate immune system employs NK T cells early in the immune response because of their direct cytotoxicity and rapid production of cytokines such as IFN-γ, which promotes a TH1 inflammatory response, and IL-4, which promotes the development of TH2 cells. Excessive or dysfunctional NK T cells have been associated with autoimmune diseases such as multiple sclerosis and inflammatory bowel disease as well as allergic contact dermatitis.27-29
In psoriasis, NK T cells are located in the epidermis, closely situated to epidermal keratinocytes, which suggests a role for direct antigen presentation. Furthermore, CD1d is overexpressed throughout the epidermis of psoriatic plaques, whereas normally CD1d expression is confined to terminally differentiated keratinocytes. An in vitro study examining cytokine-based inflammation demonstrative of psoriasis treated cultured CD1d-positive keratinocytes with interferon gamma in the presence of alpha-galactosylceramide of the lectin family.30 Interferon gamma was observed to enhance keratinocyte CD1d expression, and subsequently, CD1d-positive keratinocytes were found to activate NK T cells to produce high levels of IFN-γ, while levels of IL-4 remained undetectable. The preferential production of IFN-γ supports a TH1-mediated mechanism regulated by NK T cells in the immunopathogenesis of psoriasis.
Dendritic Cells
Dendritic cells are APCs that process antigens in the tissues in which they reside, after which they migrate to local lymph nodes where they present their native antigens to T cells. This process allows the T-cell response to be tailored to the appropriate antigens in the corresponding tissues. Immature DCs that capture antigens mature by migrating to the T-cell center of the lymph node where they present their antigens to either MHC molecules or the CD1 family. This presentation results in T-cell proliferation and differentiation that correlates with the required type of T-cell response. Multiple subsets of APCs, including myeloid and plasmacytoid DCs, are highly represented in the epidermis and dermis of psoriatic plaques as compared with normal skin.31 Dermal DCs are thought to be responsible for activating both the TH1 and TH17 infiltrate by secreting IL-12 and IL-23, respectively. This mixed cellular response secretes cytokines and leads to a cascade of events involving keratinocytes, fibroblasts, endothelial cells, and neutrophils that create the cutaneous lesions seen in psoriasis.3
Although DCs play a pivotal role in eliciting an immune response against a foreign invader, they also contribute to the establishment of tolerance. Throughout their maturation, DCs are continuously sensing their environment, which shapes their production of TH1- versus TH2-type cytokines and subsequently the nature of the T-cell response. When challenged with a virus, bacteria, or unchecked cell growth, DCs mature into APCs. However, in the absence of a strong stimulus, DCs fail to mature into APCs and present self-peptides with MHC molecules, thereby creating regulatory T cells involved in peripheral tolerance.32 If this balance between immunogenic APCs and housekeeping T cells is upset, inflammatory conditions such as psoriasis can result.
Cytokines
Cytokines are low-molecular-weight glycoproteins that function as signals to produce inflammation, defense, tissue repair and remodeling, fibrosis, angiogenesis, and restriction of neoplastic growth.33 Cytokines are produced by immunocytes such as lymphocytes and macrophages as well as nonimmunocytes such as endothelial cells and keratinocytes. Proinflammatory cytokines include IL-1, IL-2, the IL-17 family, IFN-γ, and TNF-α, while anti-inflammatory cytokines include IL-4 and IL-10. A relative preponderance of TH1 proinflammatory cytokines or an insufficiency of TH2 anti-inflammatory cytokines induces local inflammation and recruitment of additional immunocyte populations, which produce added cytokines.34 A vicious cycle of inflammation occurs that results in cutaneous manifestations such as a plaque. Psoriatic lesions are characterized by a relative increase of TH1-type (eg, IL-2, IFN-γ, TNF-α, TNF-β) to TH2-type (eg, IL-4, IL-5, IL-6, IL-9, IL-10, IL-13) cytokines and an increase in TH17-type cytokines. Natural killer T cells stimulated by CD1d-overexpressing keratinocytes increase production of proinflammatory IFN-γ without effect on the anti-inflammatory IL-4. In addition to the cytokines produced by T cells, APCs produce IL-18, IL-23, and TNF-α found in the inflammatory infiltrate of psoriatic plaques. Both IL-18 and IL-23 stimulate TH1 cells to produce IFN-γ, and IL-23 stimulates TH17 cells. Clearly, a TH1- and TH17-type pattern governs the immune effector cells and their respective cytokines present in psoriatic skin.
Tumor Necrosis Factor α
Although a network of cytokines is responsible for the inflammation of psoriasis, TNF-α has been implicated as a master proinflammatory cytokine of the innate immune response due to its widespread targets and sources. Tumor necrosis factor α is produced by activated T cells, keratinocytes, NK cells, macrophages, monocytes, Langerhans APCs, and endothelial cells. Psoriatic lesions demonstrate high concentrations of TNF-α, while the synovial fluid of psoriatic arthritis patients demonstrates elevated concentrations of TNF-α, IL-1, IL-6, and IL-8.34 In psoriasis, TNF-α supports the expression of adhesion molecules (intercellular adhesion molecule 1 and P- and E-selectin), angiogenesis via vascular endothelial growth factor, the synthesis of proinflammatory molecules (IL-1, IL-6, IL-8, and nuclear factor κβ), and keratinocyte hyperproliferation via vasoactive intestinal peptide.35
A role for TNF-α in psoriasis treatment was serendipitously discovered in a trial for Crohn disease in which infliximab, a mouse-human IgG1 anti–TNF-α monoclonal antibody, was observed to clear psoriatic plaques in a patient with both Crohn disease and psoriasis.36 Immunotherapies that target TNF-α, including infliximab, etanercept, and adalimumab, demonstrate notable efficacy in the treatment of psoriasis.37-39 Tumor necrosis factor α is regarded as the driver of the inflammatory cycle of psoriasis due to its numerous modes of production, capability to amplify other proinflammatory signals, and the efficacy and rapidity with which it produces clinical improvements in psoriasis.
IL-23/TH17 Axis
A new distinct population of helper T cells has been shown to play an important role in psoriasis. These cells develop with the help of IL-23 (secreted by dermal DCs) and subsequently secrete cytokines such as IL-17; they are, therefore, named TH17 cells. CD161 is considered a surface marker for these cells.40 Strong evidence for this IL-23/TH17 axis has been shown in mouse and human models as well as in genetic studies.
IL-23 is a cytokine that shares the p40 subunit with IL-12 and has been linked to autoimmune diseases in both mice and humans.3 It is required for optimal development of TH17 cells41 from a committed CD4+ T-cell population after exposure to transforming growth factor β1 in combination with other proinflammatory cytokines.42,43 IL-23 messenger RNA is produced at higher levels in inflammatory psoriatic skin lesions versus uninvolved skin,44 and intradermal IL-23 injections in mice produced lesions resembling psoriasis macroscopically and microscopically.45 Furthermore, several systemic therapies have been shown to modulate IL-23 levels and correlate with clinical benefit.3 Alterations in the gene for the IL-23 receptor have been shown to be protective for psoriasis,46-48 and the gene coding for the p40 subunit is associated with psoriasis.46,47
Type 17 helper T cells produce a number of cytokines, such as IL-22, IL-17A, IL-17F, and IL-26; the latter 3 are considered to be specific to this lineage.42 IL-22 acts on outer body barrier tissues, such as the skin, and has antimicrobial activity. Blocking the activity of IL-22 in mice prevented the development of skin lesions,49 and psoriasis patients have elevated levels of IL-22 in the skin and blood.50,51 The IL-17 cytokines induce the expression of proinflammatory cytokines, colony-stimulating factors, and chemokines, and they recruit, mobilize, and activate neutrophils.52 IL-17 messenger RNA was found in lesional psoriatic skin but not unaffected skin,53 and cells isolated from the dermis of psoriatic skin have been shown to produce IL-17.54 IL-17A is not elevated in the serum of psoriatic patients (unlike other autoimmune diseases),55 and it is, therefore, thought that TH17 cells and IL-17A production are localized to the affected psoriatic skin. Consistent with this concept is the finding that treatments such as cyclosporin A and anti-TNF agents decrease proinflammatory cytokines in lesional skin but not in the periphery.56-58 These cytokines released by TH17 cells in addition to those released by TH1 cells act on keratinocytes and produce epidermal hyperproliferation, acanthosis, and hyperparakeratosis characteristic of psoriasis.3
New therapies have been developed to target the IL-23/TH17 axis. Ustekinumab is approved for moderate to severe plaque psoriasis. This treatment’s effect may be sustained for up to 3 years, it is generally well tolerated, and it may be useful for patients refractory to anti-TNF therapy such as etanercept.59 Briakinumab, another blocker of IL-12 and IL-23, was studied in phase 3 clinical trials, but its development was discontinued due to safety concerns.60 Newer drugs targeting the IL-23/TH17 axis include secukinumab, ixekizumab, brodalumab, guselkumab, and tildrakizumab.
Genetic Basis of Psoriasis
Psoriasis is a disease of overactive immunity in genetically susceptible individuals. Because patients exhibit varying skin phenotypes, extracutaneous manifestations, and disease courses, multiple genes resulting from linkage disequilibrium are believed to be involved in the pathogenesis of psoriasis. A decade of genome-wide linkage scans have established that PSORS1 is the strongest susceptibility locus demonstrable through family linkage studies; PSORS1 is responsible for up to 50% of the genetic component of psoriasis.61 More recently, HLA-Cw6 has received the most attention as a candidate gene of the PSORS1 susceptibility locus on the MHC class I region on chromosome 6p21.3.62 This gene may function in antigen presentation via MHC class I, which aids in the activation of the overactive T cells characteristic of psoriatic inflammation.
Studies involving the IL-23/TH17 axis have shown genetics to play a role. Individuals may be protected from psoriasis with a nonsynonymous nucleotide substitution in the IL23R gene,47-49 and certain haplotypes of the IL23R gene are associated with the disease47,49 in addition to other autoimmune conditions.
Genomic scans have shown additional susceptibility loci for psoriasis on chromosomes 1q21, 3q21, 4q32-35, 16q12, and 17q25. Two regions on chromosome 17q were recently localized via mapping, which demonstrated a 6 megabase pairs separation, thereby indicating independent linkage factors. Genes SLC9A3R1 and NAT9 are present in the first region, while RAPTOR is demonstrated in the second region.63SLC9A3R1 and NAT9 are players that regulate signal transduction, the immunologic synapse, and T-cell growth. RAPTOR is involved in T-cell function and growth pathways. Using these genes as an example, we can predict that the alterations of regulatory genes, even those yet undetermined, can enhance T-cell proliferation and inflammation manifested in psoriasis.
Conclusion
Psoriasis is a complex disease whereby multiple exogenous and endogenous stimuli incite already heightened innate immune responses in genetically predetermined individuals. The disease process is a result of a network of cell types, including T cells, DCs, and keratinocytes that, with the production of cytokines, generate a chronic inflammatory state. Our understanding of these cellular interactions and cytokines originates from developments, some meticulously planned, others serendipitous, in the fields of immunology, cell and molecular biology, and genetics. Such progress has fostered the creation of targeted immune therapy that has demonstrated remarkable efficacy in psoriasis treatment. Further study of the underlying pathophysiology of psoriasis may provide additional targets for therapy.
- Gottlieb A. Psoriasis. Dis Manag Clin Outcome. 1998;1:195-202.
- Gaspari AA. Innate and adaptive immunity and the pathophysiology of psoriasis. J Am Acad Dermatol. 2006;54(3 suppl 2):S67-S80.
- Di Cesare A, Di Meglio P, Nestle F. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009;129:1339-1350.
- Barker J. The pathophysiology of psoriasis. Lancet. 1991;338:227-230.
- Nickoloff BJ, Nestle FO. Recent insights into the immunopathogenesis of psoriasis provide new therapeutic opportunities. J Clin Invest. 2004;113:1664-1675.
- Bos J, Meinardi M, van Joost T, et al. Use of cyclosporine in psoriasis. Lancet. 1989;23:1500-1505.
- Khandke L, Krane J, Ashinoff R, et al. Cyclosporine in psoriasis treatment: inhibition of keratinocyte cell-cycle progression in G1 independent effects on transforming growth factor-alpha/epidermal growth factor receptor pathways. Arch Dermatol. 1991;127:1172-1179.
- Gottlieb S, Gilleaudeau P, Johnson R, et al. Response of psoriasis to a lymphocyte-selective toxin (DAB389IL-2) suggests a primary immune, but not keratinocyte, pathogenic basis. Nat Med. 1995;1:442-447.
- Vallat V, Gilleaudeau P, Battat L, et al. PUVA bath therapy strongly suppresses immunological and epidermal activation in psoriasis: a possible cellular basis for remittive therapy. J Exp Med. 1994;180:283-296.
- Gottlieb A, Grossman R, Khandke L, et al. Studies of the effect of cyclosporine in psoriasis in vivo: combined effects on activated T lymphocytes and epidermal regenerative maturation. J Invest Dermatol. 1992;98:302-309.
- Gottlieb S, Hayes E, Gilleaudeau P, et al. Cellular actions of etretinate in psoriasis: enhanced epidermal differentiation and reduced cell-mediated inflammation are unexpected outcomes. J Cutan Pathol. 1996;23:404-418.
- Nickoloff B, Bonish B, Huang B, et al. Characterization of a T cell line bearing natural killer receptors and capable of creating psoriasis in a SCID mouse model system. J Dermatol Sci. 2000;24:212-225.
- Gillet M, Conrad C, Geiges M, et al. Psoriasis triggered by toll-like receptor 7 agonist imiquimod in the presence of dermal plasmacytoid dendritic cell precursors. Arch Dermatol. 2004;140:1490-1495.
- Funk J, Langeland T, Schrumpf E, et al. Psoriasis induced by interferon-alpha. Br J Dermatol. 1991;125:463-465.
- Shiohara T, Kobayahsi M, Abe K, et al. Psoriasis occurring predominantly on warts: possible involvement of interferon alpha. Arch Dermatol. 1988;124:1816-1821.
- Fierlbeck G, Rassner G, Muller C. Psoriasis induced at the injection site of recombinant interferon gamma: results of immunohistologic investigations. Arch Dermatol. 1990;126:351-355.
- Prinz J. The role of T cells in psoriasis. J Eur Acad Dermatol Venereol. 2003;17(suppl):1-5.
- Bos J, de Rie M. The pathogenesis of psoriasis: immunological facts and speculations. Immunol Today. 1999;20:40-46.
- Wucherpfennig KW, Strominger JL. Molecular mimicry in T cell–mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell. 1995;80:695-705.
- Geginat J, Campagnaro S, Sallusto F, et al. TCR-independent proliferation and differentiation of human CD4+ T cell subsets induced by cytokines. Adv Exp Med Biol. 2002;512:107-112.
- Kastelan M, Massari L, Brajac I. Apoptosis mediated by cytolytic molecules might be responsible for maintenance of psoriatic plaques. Med Hypotheses. 2006;67:336-337.
- Austin L, Ozawa M, Kikuchi T, et al. The majority of epidermal T cells in psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J Invest Dermatol. 1999;113:752-759.
- Abrams J, Kelley S, Hayes E, et al. Blockade of T lymphocyte costimulation with cytotoxic T lymphocyte-associated antigen 4-immunoglobulin (CTLA4Ig) reverses the cellular pathology of psoriatic plagues, including the activation of keratinocytes, dendritic cells and endothelial cells. J Exp Med. 2000;192:681-694.
- Lebwohl M, Christophers E, Langley R, et al. An international, randomized, double-blind, placebo-controlled phase 3 trial of intramuscular alefacept in patients with chronic plaque psoriasis. Arch Dermatol. 2003;139:719-727.
- Krueger G, Ellis C. Alefacept therapy produces remission for patients with chronic plaque psoriasis. Br J Dermatol. 2003;148:784-788.
- Gordon K, Leonardi C, Tyring S, et al. Efalizumab (anti-CD11a) is safe and effective in the treatment of psoriasis: pooled results of the 12-week first treatment period from 2 phase III trials. J Invest Dermatol. 2002;119:242.
- Singh A, Wilson M, Hong S, et al. Natural killer T cell activation protects mice against experimental autoimmune encephalomyelitis. J Exp Med. 2001;194:1801-1811.
- Saubermann L, Beck P, De Jong Y, et al. Activation of natural killer T cells by alpha-glactosylceramide in the presence of CD1d provides protection against colitis in mice. Gastroenterology. 2000;119:119-128.
- Campos R, Szczepanik M, Itakura A, et al. Cutaneous immunization rapidly activates liver invariant Valpha 14 NKT cells stimulating B-1 B cells to initiate T cell recruitment for elicitation of contact sensitivity. J Exp Med. 2003;198:1785-1796.
- Bonish B, Jullien D, Dutronc Y, et al. Overexpression of CD1d by keratinocytes in psoriasis and CD1d-dependent IFN-gamma production by NK-T cells. J Immunol. 2000;165:4076-4085.
- Deguchi M, Aiba S, Ohtani H, et al. Comparison of the distribution and numbers of antigen-presenting cells among T-lymphocyte-mediated dermatoses: CD1a+, factor XIIIa+, and CD68+ cells in eczematous dermatitis, psoriasis, lichen planus and graft-versus-host disease. Arch Dermatol Res. 2002;294:297-302.
- Bos J, de Rie M, Teunissen M, et al. Psoriasis: dysregulation of innate immunity. Br J Dermatol. 2005;152:1098-1107.
- Trefzer U, Hofmann M, Sterry W, et al. Cytokine and anticytokine therapy in dermatology. Expert Opin Biol Ther. 2003;3:733-743.
- Nickoloff B. The cytokine network in psoriasis. Arch Dermatol. 1991;127:871-884.
- Victor F, Gottlieb A. TNF-alpha and apoptosis: implications for the pathogenesis and treatment of psoriasis. J Drugs Dermatol. 2002;3:264-275.
- Oh C, Das K, Gottlieb A. Treatment with anti-tumour necrosis factor alpha (TNF-alpha) monoclonal antibody dramatically decreases the clinical activity of psoriasis lesions. J Am Acad Dermatol. 2000;42:829-830.
- Reich K, Nestle FO, Papp K, et al; EXPRESS study investigators. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet. 2005;366:1367-1374.
- Leonardi C, Powers J, Matheson R, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003;349:2014-2022.
- Saini R, Tutrone W, Weinberg J. Advances in therapy for psoriasis: an overview of infliximab, etanercept, efalizumab, alefacept, adalimumab, tazarotene, and pimecrolimus. Curr Pharm Des. 2005;11:273-280.
- Cosmi L, De Palma R, Santarlasci V, et al. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med. 2008;205:1903-1916.
- de Beaucoudrey L, Puel A, Filipe-Santos O, et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med. 2008;205:1543-1550.
- Manel N, Unutmaz D, Littman DR. The differentiation of humanT(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641-649.
- Yang L, Anderson DE, Baecher-Allan C, et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350-352.
- Lee E, Trepicchio WL, Oestreicher JL, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199:125-130.
- Chan JR, Blumenschein W, Murphy E, et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med. 2006;203:2557-2587.
- Capon F, Di Meglio P, Szaub J, et al. Sequence variants in the genes for the interleukin-23 receptor (IL23R) and its ligand (IL12B) confer protection against psoriasis. Hum Genet. 2007;122:201-206.
- Cargill M, Schrodi SJ, Chang M, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet. 2007;80:273-290.
- Nair RP, Ruether A, Stuart PE, et al. Polymorphisms of the IL12B and IL23R genes are associated with psoriasis. J Invest Dermatol. 2008;128:1653-1661.
- Ma HL, Liang S, Li J, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest. 2008;118:597-607.
- Wolk K, Witte E, Wallace E, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36:1309-1323.
- Boniface K, Guignouard E, Pedretti N, et al. A role for T cell-derived interleukin 22 in psoriatic skin inflammation. Clin Exp Immunol. 2007;150:407-415.
- Weaver CT, Hatton RD, Mangan PR, et al. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821-852.
- Teunissen MB, Koomen CW, de Waal Malefyt R, et al. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol. 1998;111:645-649.
- Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207-1211.
- Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005;2005:273-279.
- Zaba LC, Cardinale I, Gilleaudeau P, et al. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J Exp Med. 2007;204:3183-3194.
- Haider AS, Cohen J, Fei J, et al. Insights into gene modulation by therapeutic TNF and IFNgamma antibodies: TNF regulates IFNgamma production by T cells and TNF-regulated genes linked to psoriasis transcriptome. J Invest Dermatol. 2008;128:655-666.
- Haider AS, Lowes MA, Suarez-Farinas M, et al. Identification of cellular pathways of “type 1,” Th17 T cells, and TNF- and inducible nitric oxide synthase-producing dendritic cells in autoimmune inflammation through pharmacogenomic study of cyclosporine A in psoriasis. J Immunol. 2008;180:1913-1920.
- Croxtall JD. Ustekinumab: a review of its use in the management of moderate to severe plaque psoriasis. Drugs. 2011;71:1733-1753.
- Gordon KB, Langely RG, Gottlieb AB, et al. A phase III, randomized, controlled trial of the fully human IL-12/23 mAb briakinumab in moderate-to-severe psoriasis. J Invest Dermatol. 2012;132:304-314.
- Rahman P, Elder JT. Genetic epidemiology of psoriasis and psoriatic arthritis. Ann Rheum Dis. 2005;64(suppl 2):ii37-ii39.
- Elder JT. PSORS1: linking genetics and immunology. J Invest Dermatol. 2006;126:1205-1206.
- Krueger JG, Bowcock A. Psoriasis pathophysiology: current concepts of pathogenesis. Ann Rheum Dis. 2005;64(suppl 2):ii30-ii36.
Increased understanding of the pathophysiology of psoriasis has been one of the driving forces in the development of new therapies. An understanding of the processes involved is important in the optimal management of the disease. The last 30 years of research and clinical practice have revolutionized our understanding of the pathogenesis of psoriasis as the dysregulation of immunity triggered by environmental and genetic stimuli. Psoriasis was originally regarded as a primary disorder of epidermal hyperproliferation. However, experimental models and clinical results from immunomodulating therapies have refined this perspective in conceptualizing psoriasis as a genetically programmed pathologic interaction among resident skin cells; infiltrating immunocytes; and a host of proinflammatory cytokines, chemokines, and growth factors produced by these immunocytes. Two populations of immunocytes and their respective signaling molecules collaborate in the pathogenesis: (1) innate immunocytes, mediated by antigen-presenting cells (APCs)(including natural killer [NK] T lymphocytes, Langerhans cells, and neutrophils), and (2) acquired or adaptive immunocytes, mediated by mature CD4+ and CD8+ T lymphocytes in the skin. Such dysregulation of immunity and subsequent inflammation is responsible for the development and perpetuation of the clinical plaques and histological inflammatory infiltrate characteristic of psoriasis.
Although psoriasis is considered to be an immune-mediated disease in which intralesional T lymphocytes and their proinflammatory signals trigger primed basal layer keratinocytes to rapidly proliferate, debate and research focus on the stimulus that incites this inflammatory process. Our current understanding considers psoriasis to be triggered by exogenous or endogenous environmental stimuli in genetically susceptible individuals. Such stimuli include group A streptococcal pharyngitis, viremia, allergic drug reactions, antimalarial drugs, lithium, beta-blockers, IFN-α, withdrawal of systemic corticosteroids, local trauma (Köbner phenomenon), and emotional stress. These stimuli correlate with the onset or flares of psoriatic lesions. Psoriasis genetics centers on susceptibility loci and corresponding candidate genes, particularly the psoriasis susceptibility (PSORS) 1 locus on the major histocompatibility complex (MHC) class I region. Current research on the pathogenesis of psoriasis examines the complex interactions among immunologic mechanisms, environmental stimuli, and genetic susceptibility. After discussing the clinical presentation and histopathologic features of psoriasis, we will review the pathophysiology of psoriasis through noteworthy developments, including serendipitous observations, reactions to therapies, clinical trials, and animal model systems that have shaped our view of the disease process. In addition to the classic skin lesions, approximately 23% of psoriasis patients develop psoriatic arthritis, with a 10-year latency after diagnosis of psoriasis.1
Principles of Immunity
The immune system, intended to protect its host from foreign invaders and unregulated cell growth, employs 2 main effector pathways—the innate and the acquired (or adaptive) immune responses—both of which contribute to the pathophysiology of psoriasis.2 Innate immunity responses occur within minutes to hours of antigen exposure but fail to develop memory for when the antigen is encountered again. However, adaptive immunity responses take days to weeks to respond after challenged with an antigen. The adaptive immune cells have the capacity to respond to a greater range of antigens and develop immunologic memory via rearrangement of antigen receptors on B and T cells. These specialized B and T cells can then be promptly mobilized and differentiated into mature effector cells that protect the host from a foreign pathogen.
Innate and adaptive immune responses are highly intertwined; they can initiate, perpetuate, and terminate the immune mechanisms responsible for inflammation. They can modify the nature of the immune response by altering the relative proportions of type 1 (TH1), type 2 (TH2), and the more recently discovered type 17 (TH17) subset of helper T cells and their respective signaling molecules. A TH1 response is essential for a cellular immunologic reaction to intracellular bacteria and viruses or cellular immunity. A TH2 response promotes IgE synthesis, eosinophilia, and mast cell maturation for extracellular parasites and helminthes as well as humoral immunity, while a TH17 response is important for cell-mediated immunity to extracellular bacteria and plays a role in autoimmunity.3 The innate and adaptive immune responses employ common effector molecules such as chemokines and cytokines, which are essential in mediating an immune response.
Implicating Dysregulation of Immunity
Our present appreciation of the pathogenesis of psoriasis is based on the history of trial-and-error therapies; serendipitous discoveries; and the current immune targeting drugs used in a variety of chronic inflammatory conditions, including rheumatoid arthritis, ankylosing spondylitis, and inflammatory bowel disease. Before the mid-1980s, research focused on the hyperproliferative epidermal cells as the primary pathology because a markedly thickened epidermis was indeed demonstrated on histologic specimens. Altered cell-cycle kinetics were thought to be the culprit behind the hyperkeratotic plaques. Thus, initial treatments centered on oncologic and antimitotic therapies used to arrest keratinocyte proliferation with agents such as arsenic, ammoniated mercury, and methotrexate.4
However, a paradigm shift from targeting epidermal keratinocytes to immunocyte populations was recognized when a patient receiving cyclosporine to prevent transplant rejection noted clearing of psoriatic lesions in the 1980s.5 Cyclosporine was observed to inhibit messenger RNA transcription of T-cell cytokines, thereby implicating immunologic dysregulation, specifically T-cell hyperactivity, in the pathogenesis of psoriasis.6 However, the concentrations of oral cyclosporine reached in the epidermis exerted direct effects on keratinocyte proliferation and lymphocyte function in these patients.7 Thus, the question was raised as to whether the keratinocytes or the lymphocytes drove the psoriatic plaques. The use of an IL-2 diphtheria toxin-fusion protein, denileukin diftitox, specific for activated T cells with high-affinity IL-2 receptors and nonreactive with keratinocytes, distinguished which cell type was responsible. This targeted T-cell toxin provided clinical and histological clearing of psoriatic plaques. Thus, T lymphocytes rather than keratinocytes were recognized as the definitive driver behind the psoriatic plaques.8
Additional studies have demonstrated that treatments that induce prolonged clearing of psoriatic lesions without continuous therapy, such as psoralen plus UVA irradiation, decreased the numbers of T cells in plaques by at least 90%.9 However, treatments that require continual therapy for satisfactory clinical results, such as cyclosporine and etretinate, simply suppress T-cell activity and proliferation.10,11 Further evidence has linked cellular immunity with the pathogenesis of psoriasis, defining it as a TH1-type disease. Natural killer T cells were shown to be involved through the use of a severe combined immunodeficient mouse model. They were injected into prepsoriatic skin grafted on immunodeficient mice, creating a psoriatic plaque with an immune response showing cytokines from TH1 cells rather than TH2 cells.12 When psoriatic plaques were treated topically with the toll-like receptor 7 agonist imiquimod, aggravation and spreading of the plaques were noted. The exacerbation of psoriasis was accompanied by an induction of lesional TH1-type interferon produced by plasmacytoid dendritic cell (DC) precursors. Plasmacytoid DCs were observed to compose up to 16% of the total dermal infiltrate in psoriatic skin lesions based on their coexpression of BDCA2 and CD123.13 Additionally, cancer patients being treated with interferon alfa experienced induction of psoriasis.14 Moreover, patients being treated for warts with intralesional interferon alfa developed psoriatic plaques in neighboring prior asymptomatic skin.15 Patients with psoriasis who were treated with interferon gamma, a TH1 cytokine type, also developed new plaques correlating with the sites of injection.16
Intralesional T Lymphocytes
Psoriatic lesions contain a host of innate immunocytes, such as APCs, NK cells, and neutrophils, as well as adaptive T cells and an inflammatory infiltrate. These cells include CD4 and CD8 subtypes in which the CD8+ cells predominate in the epidermis, while CD4+ cells show preference for the dermis.17 There are 2 groups of CD8+ cells: one group migrates to the epidermis, expressing the integrin CD103, while the other group is found in the dermis but may be headed to or from the epidermis. The CD8+ cells residing in the epidermis that express the integrin CD103 are capable of interacting with E-cadherin, which enables these cells to travel to the epidermis and bind resident cells. Immunophenotyping reveals that these mature T cells represent chiefly activated memory cells, including CD2+, CD3+, CD5+, CLA, CD28, and CD45RO+.18 Many of these cells express activation markers such as HLA-DR, CD25, and CD27, in addition to the T-cell receptor (TCR).
T-Lymphocyte Stimulation
Both mature CD4+ and CD8+ T cells can respond to the peptides presented by APCs. Although the specific antigen that these T cells are reacting to has not yet been elucidated, several antigenic stimuli have been proposed, including self-proteins, microbial pathogens, and microbial superantigens. The premise that self-reactive T lymphocytes may contribute to the disease process is derived from the molecular mimicry theory in which an exuberant immune response to a pathogen produces cross-reactivity with self-antigens.19 Considering that infections have been associated with the onset of psoriasis, this theory merits consideration. However, it also has been observed that T cells can be activated without antigens or superantigens but rather with direct contact with accessory cells.20 No single theory has clearly emerged. Researchers continue to search for the inciting stimulus that triggers the T lymphocyte and attempt to determine whether T cells are reacting to a self-derived or non–self-derived antigen.
T-Lymphocyte Signaling
T-cell signaling is a highly coordinated process in which T lymphocytes recognize antigens via presentation by mature APCs in the skin rather than the lymphoid tissues. Such APCs expose antigenic peptides via class I or II MHC molecules for which receptors are present on the T-cell surface. The antigen recognition complex at the T-cell and APC interface, in concert with a host of antigen-independent co-stimulatory signals, regulates T-cell signaling and is referred to as the immunologic synapse. The antigen presentation and network of co-stimulatory and adhesion molecules optimize T-cell activation, and dermal DCs release IL-12 and IL-23 to promote a TH1 and TH17 response, respectively. The growth factors released by these helper T cells sustain neoangiogenesis, stimulate epidermal hyperproliferation, alter epidermal differentiation, and decrease susceptibility to apoptosis that characterizes the erythematous hypertrophic scaling lesions of psoriasis.21 Furthermore, the cytokines produced from the immunologic response, such as tumor necrosis factor (TNF) α, IFN-γ, and IL-2, correspond to cytokines that are upregulated in psoriatic plaques.22
Integral components of the immunologic synapse complex include co-stimulatory signals such as CD28, CD40, CD80, and CD86, as well as adhesion molecules such as cytotoxic T-lymphocyte antigen 4 and lymphocyte function-associated antigen (LFA) 1, which possess corresponding receptors on the T cell. These molecules play a key role in T-cell signaling, as their disruption has been shown to decrease T-cell responsiveness and associated inflammation. The B7 family of molecules routinely interacts with CD28 T cells to co-stimulate T-cell activation. Cytotoxic T-lymphocyte antigen 4 immunoglobulin, an antibody on the T-cell surface, targets B7 and interferes with signaling between B7 and CD28. In psoriatic patients, this blockade was demonstrated to attenuate the T-cell response and correlated with a clinical and histological decrease in psoriasiform hyperplasia.23 Biologic therapies that disrupt the LFA-1 component of the immunologic synapse also have demonstrated efficacy in the treatment of psoriasis. Alefacept is a human LFA-3 fusion protein that binds CD2 on T cells and blocks the interaction between LFA-3 on APCs and CD2 on memory CD45RO+ T cells and induces apoptosis of such T cells. Efalizumab is a human monoclonal antibody to the CD11 chain of LFA-1 that blocks the interaction between LFA-1 on the T cell and intercellular adhesion molecule 1 on an APC or endothelial cell. Both alefacept and efalizumab, 2 formerly marketed biologic therapies, demonstrated remarkable clinical reduction of psoriatic lesions, and alefacept has been shown to produce disease remission for up to 18 months after discontinuation of therapy.24-26
NK T Cells
Natural killer T cells represent a subset of CD3+ T cells present in psoriatic plaques. Although NK T cells possess a TCR, they differ from T cells by displaying NK receptors comprised of lectin and immunoglobulin families. These cells exhibit remarkable specificity and are activated upon recognition of glycolipids presented by CD1d molecules. This process occurs in contrast to CD4+ and CD8+ T cells, which, due to their TCR diversity, respond to peptides processed by APCs and displayed on MHC molecules. Natural killer T cells can be classified into 2 subsets: (1) one group that expresses CD4 and preferentially produces TH1- versus TH2-type cytokines, and (2) another group that lacks CD4 and CD8 that only produces TH1-type cytokines. The innate immune system employs NK T cells early in the immune response because of their direct cytotoxicity and rapid production of cytokines such as IFN-γ, which promotes a TH1 inflammatory response, and IL-4, which promotes the development of TH2 cells. Excessive or dysfunctional NK T cells have been associated with autoimmune diseases such as multiple sclerosis and inflammatory bowel disease as well as allergic contact dermatitis.27-29
In psoriasis, NK T cells are located in the epidermis, closely situated to epidermal keratinocytes, which suggests a role for direct antigen presentation. Furthermore, CD1d is overexpressed throughout the epidermis of psoriatic plaques, whereas normally CD1d expression is confined to terminally differentiated keratinocytes. An in vitro study examining cytokine-based inflammation demonstrative of psoriasis treated cultured CD1d-positive keratinocytes with interferon gamma in the presence of alpha-galactosylceramide of the lectin family.30 Interferon gamma was observed to enhance keratinocyte CD1d expression, and subsequently, CD1d-positive keratinocytes were found to activate NK T cells to produce high levels of IFN-γ, while levels of IL-4 remained undetectable. The preferential production of IFN-γ supports a TH1-mediated mechanism regulated by NK T cells in the immunopathogenesis of psoriasis.
Dendritic Cells
Dendritic cells are APCs that process antigens in the tissues in which they reside, after which they migrate to local lymph nodes where they present their native antigens to T cells. This process allows the T-cell response to be tailored to the appropriate antigens in the corresponding tissues. Immature DCs that capture antigens mature by migrating to the T-cell center of the lymph node where they present their antigens to either MHC molecules or the CD1 family. This presentation results in T-cell proliferation and differentiation that correlates with the required type of T-cell response. Multiple subsets of APCs, including myeloid and plasmacytoid DCs, are highly represented in the epidermis and dermis of psoriatic plaques as compared with normal skin.31 Dermal DCs are thought to be responsible for activating both the TH1 and TH17 infiltrate by secreting IL-12 and IL-23, respectively. This mixed cellular response secretes cytokines and leads to a cascade of events involving keratinocytes, fibroblasts, endothelial cells, and neutrophils that create the cutaneous lesions seen in psoriasis.3
Although DCs play a pivotal role in eliciting an immune response against a foreign invader, they also contribute to the establishment of tolerance. Throughout their maturation, DCs are continuously sensing their environment, which shapes their production of TH1- versus TH2-type cytokines and subsequently the nature of the T-cell response. When challenged with a virus, bacteria, or unchecked cell growth, DCs mature into APCs. However, in the absence of a strong stimulus, DCs fail to mature into APCs and present self-peptides with MHC molecules, thereby creating regulatory T cells involved in peripheral tolerance.32 If this balance between immunogenic APCs and housekeeping T cells is upset, inflammatory conditions such as psoriasis can result.
Cytokines
Cytokines are low-molecular-weight glycoproteins that function as signals to produce inflammation, defense, tissue repair and remodeling, fibrosis, angiogenesis, and restriction of neoplastic growth.33 Cytokines are produced by immunocytes such as lymphocytes and macrophages as well as nonimmunocytes such as endothelial cells and keratinocytes. Proinflammatory cytokines include IL-1, IL-2, the IL-17 family, IFN-γ, and TNF-α, while anti-inflammatory cytokines include IL-4 and IL-10. A relative preponderance of TH1 proinflammatory cytokines or an insufficiency of TH2 anti-inflammatory cytokines induces local inflammation and recruitment of additional immunocyte populations, which produce added cytokines.34 A vicious cycle of inflammation occurs that results in cutaneous manifestations such as a plaque. Psoriatic lesions are characterized by a relative increase of TH1-type (eg, IL-2, IFN-γ, TNF-α, TNF-β) to TH2-type (eg, IL-4, IL-5, IL-6, IL-9, IL-10, IL-13) cytokines and an increase in TH17-type cytokines. Natural killer T cells stimulated by CD1d-overexpressing keratinocytes increase production of proinflammatory IFN-γ without effect on the anti-inflammatory IL-4. In addition to the cytokines produced by T cells, APCs produce IL-18, IL-23, and TNF-α found in the inflammatory infiltrate of psoriatic plaques. Both IL-18 and IL-23 stimulate TH1 cells to produce IFN-γ, and IL-23 stimulates TH17 cells. Clearly, a TH1- and TH17-type pattern governs the immune effector cells and their respective cytokines present in psoriatic skin.
Tumor Necrosis Factor α
Although a network of cytokines is responsible for the inflammation of psoriasis, TNF-α has been implicated as a master proinflammatory cytokine of the innate immune response due to its widespread targets and sources. Tumor necrosis factor α is produced by activated T cells, keratinocytes, NK cells, macrophages, monocytes, Langerhans APCs, and endothelial cells. Psoriatic lesions demonstrate high concentrations of TNF-α, while the synovial fluid of psoriatic arthritis patients demonstrates elevated concentrations of TNF-α, IL-1, IL-6, and IL-8.34 In psoriasis, TNF-α supports the expression of adhesion molecules (intercellular adhesion molecule 1 and P- and E-selectin), angiogenesis via vascular endothelial growth factor, the synthesis of proinflammatory molecules (IL-1, IL-6, IL-8, and nuclear factor κβ), and keratinocyte hyperproliferation via vasoactive intestinal peptide.35
A role for TNF-α in psoriasis treatment was serendipitously discovered in a trial for Crohn disease in which infliximab, a mouse-human IgG1 anti–TNF-α monoclonal antibody, was observed to clear psoriatic plaques in a patient with both Crohn disease and psoriasis.36 Immunotherapies that target TNF-α, including infliximab, etanercept, and adalimumab, demonstrate notable efficacy in the treatment of psoriasis.37-39 Tumor necrosis factor α is regarded as the driver of the inflammatory cycle of psoriasis due to its numerous modes of production, capability to amplify other proinflammatory signals, and the efficacy and rapidity with which it produces clinical improvements in psoriasis.
IL-23/TH17 Axis
A new distinct population of helper T cells has been shown to play an important role in psoriasis. These cells develop with the help of IL-23 (secreted by dermal DCs) and subsequently secrete cytokines such as IL-17; they are, therefore, named TH17 cells. CD161 is considered a surface marker for these cells.40 Strong evidence for this IL-23/TH17 axis has been shown in mouse and human models as well as in genetic studies.
IL-23 is a cytokine that shares the p40 subunit with IL-12 and has been linked to autoimmune diseases in both mice and humans.3 It is required for optimal development of TH17 cells41 from a committed CD4+ T-cell population after exposure to transforming growth factor β1 in combination with other proinflammatory cytokines.42,43 IL-23 messenger RNA is produced at higher levels in inflammatory psoriatic skin lesions versus uninvolved skin,44 and intradermal IL-23 injections in mice produced lesions resembling psoriasis macroscopically and microscopically.45 Furthermore, several systemic therapies have been shown to modulate IL-23 levels and correlate with clinical benefit.3 Alterations in the gene for the IL-23 receptor have been shown to be protective for psoriasis,46-48 and the gene coding for the p40 subunit is associated with psoriasis.46,47
Type 17 helper T cells produce a number of cytokines, such as IL-22, IL-17A, IL-17F, and IL-26; the latter 3 are considered to be specific to this lineage.42 IL-22 acts on outer body barrier tissues, such as the skin, and has antimicrobial activity. Blocking the activity of IL-22 in mice prevented the development of skin lesions,49 and psoriasis patients have elevated levels of IL-22 in the skin and blood.50,51 The IL-17 cytokines induce the expression of proinflammatory cytokines, colony-stimulating factors, and chemokines, and they recruit, mobilize, and activate neutrophils.52 IL-17 messenger RNA was found in lesional psoriatic skin but not unaffected skin,53 and cells isolated from the dermis of psoriatic skin have been shown to produce IL-17.54 IL-17A is not elevated in the serum of psoriatic patients (unlike other autoimmune diseases),55 and it is, therefore, thought that TH17 cells and IL-17A production are localized to the affected psoriatic skin. Consistent with this concept is the finding that treatments such as cyclosporin A and anti-TNF agents decrease proinflammatory cytokines in lesional skin but not in the periphery.56-58 These cytokines released by TH17 cells in addition to those released by TH1 cells act on keratinocytes and produce epidermal hyperproliferation, acanthosis, and hyperparakeratosis characteristic of psoriasis.3
New therapies have been developed to target the IL-23/TH17 axis. Ustekinumab is approved for moderate to severe plaque psoriasis. This treatment’s effect may be sustained for up to 3 years, it is generally well tolerated, and it may be useful for patients refractory to anti-TNF therapy such as etanercept.59 Briakinumab, another blocker of IL-12 and IL-23, was studied in phase 3 clinical trials, but its development was discontinued due to safety concerns.60 Newer drugs targeting the IL-23/TH17 axis include secukinumab, ixekizumab, brodalumab, guselkumab, and tildrakizumab.
Genetic Basis of Psoriasis
Psoriasis is a disease of overactive immunity in genetically susceptible individuals. Because patients exhibit varying skin phenotypes, extracutaneous manifestations, and disease courses, multiple genes resulting from linkage disequilibrium are believed to be involved in the pathogenesis of psoriasis. A decade of genome-wide linkage scans have established that PSORS1 is the strongest susceptibility locus demonstrable through family linkage studies; PSORS1 is responsible for up to 50% of the genetic component of psoriasis.61 More recently, HLA-Cw6 has received the most attention as a candidate gene of the PSORS1 susceptibility locus on the MHC class I region on chromosome 6p21.3.62 This gene may function in antigen presentation via MHC class I, which aids in the activation of the overactive T cells characteristic of psoriatic inflammation.
Studies involving the IL-23/TH17 axis have shown genetics to play a role. Individuals may be protected from psoriasis with a nonsynonymous nucleotide substitution in the IL23R gene,47-49 and certain haplotypes of the IL23R gene are associated with the disease47,49 in addition to other autoimmune conditions.
Genomic scans have shown additional susceptibility loci for psoriasis on chromosomes 1q21, 3q21, 4q32-35, 16q12, and 17q25. Two regions on chromosome 17q were recently localized via mapping, which demonstrated a 6 megabase pairs separation, thereby indicating independent linkage factors. Genes SLC9A3R1 and NAT9 are present in the first region, while RAPTOR is demonstrated in the second region.63SLC9A3R1 and NAT9 are players that regulate signal transduction, the immunologic synapse, and T-cell growth. RAPTOR is involved in T-cell function and growth pathways. Using these genes as an example, we can predict that the alterations of regulatory genes, even those yet undetermined, can enhance T-cell proliferation and inflammation manifested in psoriasis.
Conclusion
Psoriasis is a complex disease whereby multiple exogenous and endogenous stimuli incite already heightened innate immune responses in genetically predetermined individuals. The disease process is a result of a network of cell types, including T cells, DCs, and keratinocytes that, with the production of cytokines, generate a chronic inflammatory state. Our understanding of these cellular interactions and cytokines originates from developments, some meticulously planned, others serendipitous, in the fields of immunology, cell and molecular biology, and genetics. Such progress has fostered the creation of targeted immune therapy that has demonstrated remarkable efficacy in psoriasis treatment. Further study of the underlying pathophysiology of psoriasis may provide additional targets for therapy.
Increased understanding of the pathophysiology of psoriasis has been one of the driving forces in the development of new therapies. An understanding of the processes involved is important in the optimal management of the disease. The last 30 years of research and clinical practice have revolutionized our understanding of the pathogenesis of psoriasis as the dysregulation of immunity triggered by environmental and genetic stimuli. Psoriasis was originally regarded as a primary disorder of epidermal hyperproliferation. However, experimental models and clinical results from immunomodulating therapies have refined this perspective in conceptualizing psoriasis as a genetically programmed pathologic interaction among resident skin cells; infiltrating immunocytes; and a host of proinflammatory cytokines, chemokines, and growth factors produced by these immunocytes. Two populations of immunocytes and their respective signaling molecules collaborate in the pathogenesis: (1) innate immunocytes, mediated by antigen-presenting cells (APCs)(including natural killer [NK] T lymphocytes, Langerhans cells, and neutrophils), and (2) acquired or adaptive immunocytes, mediated by mature CD4+ and CD8+ T lymphocytes in the skin. Such dysregulation of immunity and subsequent inflammation is responsible for the development and perpetuation of the clinical plaques and histological inflammatory infiltrate characteristic of psoriasis.
Although psoriasis is considered to be an immune-mediated disease in which intralesional T lymphocytes and their proinflammatory signals trigger primed basal layer keratinocytes to rapidly proliferate, debate and research focus on the stimulus that incites this inflammatory process. Our current understanding considers psoriasis to be triggered by exogenous or endogenous environmental stimuli in genetically susceptible individuals. Such stimuli include group A streptococcal pharyngitis, viremia, allergic drug reactions, antimalarial drugs, lithium, beta-blockers, IFN-α, withdrawal of systemic corticosteroids, local trauma (Köbner phenomenon), and emotional stress. These stimuli correlate with the onset or flares of psoriatic lesions. Psoriasis genetics centers on susceptibility loci and corresponding candidate genes, particularly the psoriasis susceptibility (PSORS) 1 locus on the major histocompatibility complex (MHC) class I region. Current research on the pathogenesis of psoriasis examines the complex interactions among immunologic mechanisms, environmental stimuli, and genetic susceptibility. After discussing the clinical presentation and histopathologic features of psoriasis, we will review the pathophysiology of psoriasis through noteworthy developments, including serendipitous observations, reactions to therapies, clinical trials, and animal model systems that have shaped our view of the disease process. In addition to the classic skin lesions, approximately 23% of psoriasis patients develop psoriatic arthritis, with a 10-year latency after diagnosis of psoriasis.1
Principles of Immunity
The immune system, intended to protect its host from foreign invaders and unregulated cell growth, employs 2 main effector pathways—the innate and the acquired (or adaptive) immune responses—both of which contribute to the pathophysiology of psoriasis.2 Innate immunity responses occur within minutes to hours of antigen exposure but fail to develop memory for when the antigen is encountered again. However, adaptive immunity responses take days to weeks to respond after challenged with an antigen. The adaptive immune cells have the capacity to respond to a greater range of antigens and develop immunologic memory via rearrangement of antigen receptors on B and T cells. These specialized B and T cells can then be promptly mobilized and differentiated into mature effector cells that protect the host from a foreign pathogen.
Innate and adaptive immune responses are highly intertwined; they can initiate, perpetuate, and terminate the immune mechanisms responsible for inflammation. They can modify the nature of the immune response by altering the relative proportions of type 1 (TH1), type 2 (TH2), and the more recently discovered type 17 (TH17) subset of helper T cells and their respective signaling molecules. A TH1 response is essential for a cellular immunologic reaction to intracellular bacteria and viruses or cellular immunity. A TH2 response promotes IgE synthesis, eosinophilia, and mast cell maturation for extracellular parasites and helminthes as well as humoral immunity, while a TH17 response is important for cell-mediated immunity to extracellular bacteria and plays a role in autoimmunity.3 The innate and adaptive immune responses employ common effector molecules such as chemokines and cytokines, which are essential in mediating an immune response.
Implicating Dysregulation of Immunity
Our present appreciation of the pathogenesis of psoriasis is based on the history of trial-and-error therapies; serendipitous discoveries; and the current immune targeting drugs used in a variety of chronic inflammatory conditions, including rheumatoid arthritis, ankylosing spondylitis, and inflammatory bowel disease. Before the mid-1980s, research focused on the hyperproliferative epidermal cells as the primary pathology because a markedly thickened epidermis was indeed demonstrated on histologic specimens. Altered cell-cycle kinetics were thought to be the culprit behind the hyperkeratotic plaques. Thus, initial treatments centered on oncologic and antimitotic therapies used to arrest keratinocyte proliferation with agents such as arsenic, ammoniated mercury, and methotrexate.4
However, a paradigm shift from targeting epidermal keratinocytes to immunocyte populations was recognized when a patient receiving cyclosporine to prevent transplant rejection noted clearing of psoriatic lesions in the 1980s.5 Cyclosporine was observed to inhibit messenger RNA transcription of T-cell cytokines, thereby implicating immunologic dysregulation, specifically T-cell hyperactivity, in the pathogenesis of psoriasis.6 However, the concentrations of oral cyclosporine reached in the epidermis exerted direct effects on keratinocyte proliferation and lymphocyte function in these patients.7 Thus, the question was raised as to whether the keratinocytes or the lymphocytes drove the psoriatic plaques. The use of an IL-2 diphtheria toxin-fusion protein, denileukin diftitox, specific for activated T cells with high-affinity IL-2 receptors and nonreactive with keratinocytes, distinguished which cell type was responsible. This targeted T-cell toxin provided clinical and histological clearing of psoriatic plaques. Thus, T lymphocytes rather than keratinocytes were recognized as the definitive driver behind the psoriatic plaques.8
Additional studies have demonstrated that treatments that induce prolonged clearing of psoriatic lesions without continuous therapy, such as psoralen plus UVA irradiation, decreased the numbers of T cells in plaques by at least 90%.9 However, treatments that require continual therapy for satisfactory clinical results, such as cyclosporine and etretinate, simply suppress T-cell activity and proliferation.10,11 Further evidence has linked cellular immunity with the pathogenesis of psoriasis, defining it as a TH1-type disease. Natural killer T cells were shown to be involved through the use of a severe combined immunodeficient mouse model. They were injected into prepsoriatic skin grafted on immunodeficient mice, creating a psoriatic plaque with an immune response showing cytokines from TH1 cells rather than TH2 cells.12 When psoriatic plaques were treated topically with the toll-like receptor 7 agonist imiquimod, aggravation and spreading of the plaques were noted. The exacerbation of psoriasis was accompanied by an induction of lesional TH1-type interferon produced by plasmacytoid dendritic cell (DC) precursors. Plasmacytoid DCs were observed to compose up to 16% of the total dermal infiltrate in psoriatic skin lesions based on their coexpression of BDCA2 and CD123.13 Additionally, cancer patients being treated with interferon alfa experienced induction of psoriasis.14 Moreover, patients being treated for warts with intralesional interferon alfa developed psoriatic plaques in neighboring prior asymptomatic skin.15 Patients with psoriasis who were treated with interferon gamma, a TH1 cytokine type, also developed new plaques correlating with the sites of injection.16
Intralesional T Lymphocytes
Psoriatic lesions contain a host of innate immunocytes, such as APCs, NK cells, and neutrophils, as well as adaptive T cells and an inflammatory infiltrate. These cells include CD4 and CD8 subtypes in which the CD8+ cells predominate in the epidermis, while CD4+ cells show preference for the dermis.17 There are 2 groups of CD8+ cells: one group migrates to the epidermis, expressing the integrin CD103, while the other group is found in the dermis but may be headed to or from the epidermis. The CD8+ cells residing in the epidermis that express the integrin CD103 are capable of interacting with E-cadherin, which enables these cells to travel to the epidermis and bind resident cells. Immunophenotyping reveals that these mature T cells represent chiefly activated memory cells, including CD2+, CD3+, CD5+, CLA, CD28, and CD45RO+.18 Many of these cells express activation markers such as HLA-DR, CD25, and CD27, in addition to the T-cell receptor (TCR).
T-Lymphocyte Stimulation
Both mature CD4+ and CD8+ T cells can respond to the peptides presented by APCs. Although the specific antigen that these T cells are reacting to has not yet been elucidated, several antigenic stimuli have been proposed, including self-proteins, microbial pathogens, and microbial superantigens. The premise that self-reactive T lymphocytes may contribute to the disease process is derived from the molecular mimicry theory in which an exuberant immune response to a pathogen produces cross-reactivity with self-antigens.19 Considering that infections have been associated with the onset of psoriasis, this theory merits consideration. However, it also has been observed that T cells can be activated without antigens or superantigens but rather with direct contact with accessory cells.20 No single theory has clearly emerged. Researchers continue to search for the inciting stimulus that triggers the T lymphocyte and attempt to determine whether T cells are reacting to a self-derived or non–self-derived antigen.
T-Lymphocyte Signaling
T-cell signaling is a highly coordinated process in which T lymphocytes recognize antigens via presentation by mature APCs in the skin rather than the lymphoid tissues. Such APCs expose antigenic peptides via class I or II MHC molecules for which receptors are present on the T-cell surface. The antigen recognition complex at the T-cell and APC interface, in concert with a host of antigen-independent co-stimulatory signals, regulates T-cell signaling and is referred to as the immunologic synapse. The antigen presentation and network of co-stimulatory and adhesion molecules optimize T-cell activation, and dermal DCs release IL-12 and IL-23 to promote a TH1 and TH17 response, respectively. The growth factors released by these helper T cells sustain neoangiogenesis, stimulate epidermal hyperproliferation, alter epidermal differentiation, and decrease susceptibility to apoptosis that characterizes the erythematous hypertrophic scaling lesions of psoriasis.21 Furthermore, the cytokines produced from the immunologic response, such as tumor necrosis factor (TNF) α, IFN-γ, and IL-2, correspond to cytokines that are upregulated in psoriatic plaques.22
Integral components of the immunologic synapse complex include co-stimulatory signals such as CD28, CD40, CD80, and CD86, as well as adhesion molecules such as cytotoxic T-lymphocyte antigen 4 and lymphocyte function-associated antigen (LFA) 1, which possess corresponding receptors on the T cell. These molecules play a key role in T-cell signaling, as their disruption has been shown to decrease T-cell responsiveness and associated inflammation. The B7 family of molecules routinely interacts with CD28 T cells to co-stimulate T-cell activation. Cytotoxic T-lymphocyte antigen 4 immunoglobulin, an antibody on the T-cell surface, targets B7 and interferes with signaling between B7 and CD28. In psoriatic patients, this blockade was demonstrated to attenuate the T-cell response and correlated with a clinical and histological decrease in psoriasiform hyperplasia.23 Biologic therapies that disrupt the LFA-1 component of the immunologic synapse also have demonstrated efficacy in the treatment of psoriasis. Alefacept is a human LFA-3 fusion protein that binds CD2 on T cells and blocks the interaction between LFA-3 on APCs and CD2 on memory CD45RO+ T cells and induces apoptosis of such T cells. Efalizumab is a human monoclonal antibody to the CD11 chain of LFA-1 that blocks the interaction between LFA-1 on the T cell and intercellular adhesion molecule 1 on an APC or endothelial cell. Both alefacept and efalizumab, 2 formerly marketed biologic therapies, demonstrated remarkable clinical reduction of psoriatic lesions, and alefacept has been shown to produce disease remission for up to 18 months after discontinuation of therapy.24-26
NK T Cells
Natural killer T cells represent a subset of CD3+ T cells present in psoriatic plaques. Although NK T cells possess a TCR, they differ from T cells by displaying NK receptors comprised of lectin and immunoglobulin families. These cells exhibit remarkable specificity and are activated upon recognition of glycolipids presented by CD1d molecules. This process occurs in contrast to CD4+ and CD8+ T cells, which, due to their TCR diversity, respond to peptides processed by APCs and displayed on MHC molecules. Natural killer T cells can be classified into 2 subsets: (1) one group that expresses CD4 and preferentially produces TH1- versus TH2-type cytokines, and (2) another group that lacks CD4 and CD8 that only produces TH1-type cytokines. The innate immune system employs NK T cells early in the immune response because of their direct cytotoxicity and rapid production of cytokines such as IFN-γ, which promotes a TH1 inflammatory response, and IL-4, which promotes the development of TH2 cells. Excessive or dysfunctional NK T cells have been associated with autoimmune diseases such as multiple sclerosis and inflammatory bowel disease as well as allergic contact dermatitis.27-29
In psoriasis, NK T cells are located in the epidermis, closely situated to epidermal keratinocytes, which suggests a role for direct antigen presentation. Furthermore, CD1d is overexpressed throughout the epidermis of psoriatic plaques, whereas normally CD1d expression is confined to terminally differentiated keratinocytes. An in vitro study examining cytokine-based inflammation demonstrative of psoriasis treated cultured CD1d-positive keratinocytes with interferon gamma in the presence of alpha-galactosylceramide of the lectin family.30 Interferon gamma was observed to enhance keratinocyte CD1d expression, and subsequently, CD1d-positive keratinocytes were found to activate NK T cells to produce high levels of IFN-γ, while levels of IL-4 remained undetectable. The preferential production of IFN-γ supports a TH1-mediated mechanism regulated by NK T cells in the immunopathogenesis of psoriasis.
Dendritic Cells
Dendritic cells are APCs that process antigens in the tissues in which they reside, after which they migrate to local lymph nodes where they present their native antigens to T cells. This process allows the T-cell response to be tailored to the appropriate antigens in the corresponding tissues. Immature DCs that capture antigens mature by migrating to the T-cell center of the lymph node where they present their antigens to either MHC molecules or the CD1 family. This presentation results in T-cell proliferation and differentiation that correlates with the required type of T-cell response. Multiple subsets of APCs, including myeloid and plasmacytoid DCs, are highly represented in the epidermis and dermis of psoriatic plaques as compared with normal skin.31 Dermal DCs are thought to be responsible for activating both the TH1 and TH17 infiltrate by secreting IL-12 and IL-23, respectively. This mixed cellular response secretes cytokines and leads to a cascade of events involving keratinocytes, fibroblasts, endothelial cells, and neutrophils that create the cutaneous lesions seen in psoriasis.3
Although DCs play a pivotal role in eliciting an immune response against a foreign invader, they also contribute to the establishment of tolerance. Throughout their maturation, DCs are continuously sensing their environment, which shapes their production of TH1- versus TH2-type cytokines and subsequently the nature of the T-cell response. When challenged with a virus, bacteria, or unchecked cell growth, DCs mature into APCs. However, in the absence of a strong stimulus, DCs fail to mature into APCs and present self-peptides with MHC molecules, thereby creating regulatory T cells involved in peripheral tolerance.32 If this balance between immunogenic APCs and housekeeping T cells is upset, inflammatory conditions such as psoriasis can result.
Cytokines
Cytokines are low-molecular-weight glycoproteins that function as signals to produce inflammation, defense, tissue repair and remodeling, fibrosis, angiogenesis, and restriction of neoplastic growth.33 Cytokines are produced by immunocytes such as lymphocytes and macrophages as well as nonimmunocytes such as endothelial cells and keratinocytes. Proinflammatory cytokines include IL-1, IL-2, the IL-17 family, IFN-γ, and TNF-α, while anti-inflammatory cytokines include IL-4 and IL-10. A relative preponderance of TH1 proinflammatory cytokines or an insufficiency of TH2 anti-inflammatory cytokines induces local inflammation and recruitment of additional immunocyte populations, which produce added cytokines.34 A vicious cycle of inflammation occurs that results in cutaneous manifestations such as a plaque. Psoriatic lesions are characterized by a relative increase of TH1-type (eg, IL-2, IFN-γ, TNF-α, TNF-β) to TH2-type (eg, IL-4, IL-5, IL-6, IL-9, IL-10, IL-13) cytokines and an increase in TH17-type cytokines. Natural killer T cells stimulated by CD1d-overexpressing keratinocytes increase production of proinflammatory IFN-γ without effect on the anti-inflammatory IL-4. In addition to the cytokines produced by T cells, APCs produce IL-18, IL-23, and TNF-α found in the inflammatory infiltrate of psoriatic plaques. Both IL-18 and IL-23 stimulate TH1 cells to produce IFN-γ, and IL-23 stimulates TH17 cells. Clearly, a TH1- and TH17-type pattern governs the immune effector cells and their respective cytokines present in psoriatic skin.
Tumor Necrosis Factor α
Although a network of cytokines is responsible for the inflammation of psoriasis, TNF-α has been implicated as a master proinflammatory cytokine of the innate immune response due to its widespread targets and sources. Tumor necrosis factor α is produced by activated T cells, keratinocytes, NK cells, macrophages, monocytes, Langerhans APCs, and endothelial cells. Psoriatic lesions demonstrate high concentrations of TNF-α, while the synovial fluid of psoriatic arthritis patients demonstrates elevated concentrations of TNF-α, IL-1, IL-6, and IL-8.34 In psoriasis, TNF-α supports the expression of adhesion molecules (intercellular adhesion molecule 1 and P- and E-selectin), angiogenesis via vascular endothelial growth factor, the synthesis of proinflammatory molecules (IL-1, IL-6, IL-8, and nuclear factor κβ), and keratinocyte hyperproliferation via vasoactive intestinal peptide.35
A role for TNF-α in psoriasis treatment was serendipitously discovered in a trial for Crohn disease in which infliximab, a mouse-human IgG1 anti–TNF-α monoclonal antibody, was observed to clear psoriatic plaques in a patient with both Crohn disease and psoriasis.36 Immunotherapies that target TNF-α, including infliximab, etanercept, and adalimumab, demonstrate notable efficacy in the treatment of psoriasis.37-39 Tumor necrosis factor α is regarded as the driver of the inflammatory cycle of psoriasis due to its numerous modes of production, capability to amplify other proinflammatory signals, and the efficacy and rapidity with which it produces clinical improvements in psoriasis.
IL-23/TH17 Axis
A new distinct population of helper T cells has been shown to play an important role in psoriasis. These cells develop with the help of IL-23 (secreted by dermal DCs) and subsequently secrete cytokines such as IL-17; they are, therefore, named TH17 cells. CD161 is considered a surface marker for these cells.40 Strong evidence for this IL-23/TH17 axis has been shown in mouse and human models as well as in genetic studies.
IL-23 is a cytokine that shares the p40 subunit with IL-12 and has been linked to autoimmune diseases in both mice and humans.3 It is required for optimal development of TH17 cells41 from a committed CD4+ T-cell population after exposure to transforming growth factor β1 in combination with other proinflammatory cytokines.42,43 IL-23 messenger RNA is produced at higher levels in inflammatory psoriatic skin lesions versus uninvolved skin,44 and intradermal IL-23 injections in mice produced lesions resembling psoriasis macroscopically and microscopically.45 Furthermore, several systemic therapies have been shown to modulate IL-23 levels and correlate with clinical benefit.3 Alterations in the gene for the IL-23 receptor have been shown to be protective for psoriasis,46-48 and the gene coding for the p40 subunit is associated with psoriasis.46,47
Type 17 helper T cells produce a number of cytokines, such as IL-22, IL-17A, IL-17F, and IL-26; the latter 3 are considered to be specific to this lineage.42 IL-22 acts on outer body barrier tissues, such as the skin, and has antimicrobial activity. Blocking the activity of IL-22 in mice prevented the development of skin lesions,49 and psoriasis patients have elevated levels of IL-22 in the skin and blood.50,51 The IL-17 cytokines induce the expression of proinflammatory cytokines, colony-stimulating factors, and chemokines, and they recruit, mobilize, and activate neutrophils.52 IL-17 messenger RNA was found in lesional psoriatic skin but not unaffected skin,53 and cells isolated from the dermis of psoriatic skin have been shown to produce IL-17.54 IL-17A is not elevated in the serum of psoriatic patients (unlike other autoimmune diseases),55 and it is, therefore, thought that TH17 cells and IL-17A production are localized to the affected psoriatic skin. Consistent with this concept is the finding that treatments such as cyclosporin A and anti-TNF agents decrease proinflammatory cytokines in lesional skin but not in the periphery.56-58 These cytokines released by TH17 cells in addition to those released by TH1 cells act on keratinocytes and produce epidermal hyperproliferation, acanthosis, and hyperparakeratosis characteristic of psoriasis.3
New therapies have been developed to target the IL-23/TH17 axis. Ustekinumab is approved for moderate to severe plaque psoriasis. This treatment’s effect may be sustained for up to 3 years, it is generally well tolerated, and it may be useful for patients refractory to anti-TNF therapy such as etanercept.59 Briakinumab, another blocker of IL-12 and IL-23, was studied in phase 3 clinical trials, but its development was discontinued due to safety concerns.60 Newer drugs targeting the IL-23/TH17 axis include secukinumab, ixekizumab, brodalumab, guselkumab, and tildrakizumab.
Genetic Basis of Psoriasis
Psoriasis is a disease of overactive immunity in genetically susceptible individuals. Because patients exhibit varying skin phenotypes, extracutaneous manifestations, and disease courses, multiple genes resulting from linkage disequilibrium are believed to be involved in the pathogenesis of psoriasis. A decade of genome-wide linkage scans have established that PSORS1 is the strongest susceptibility locus demonstrable through family linkage studies; PSORS1 is responsible for up to 50% of the genetic component of psoriasis.61 More recently, HLA-Cw6 has received the most attention as a candidate gene of the PSORS1 susceptibility locus on the MHC class I region on chromosome 6p21.3.62 This gene may function in antigen presentation via MHC class I, which aids in the activation of the overactive T cells characteristic of psoriatic inflammation.
Studies involving the IL-23/TH17 axis have shown genetics to play a role. Individuals may be protected from psoriasis with a nonsynonymous nucleotide substitution in the IL23R gene,47-49 and certain haplotypes of the IL23R gene are associated with the disease47,49 in addition to other autoimmune conditions.
Genomic scans have shown additional susceptibility loci for psoriasis on chromosomes 1q21, 3q21, 4q32-35, 16q12, and 17q25. Two regions on chromosome 17q were recently localized via mapping, which demonstrated a 6 megabase pairs separation, thereby indicating independent linkage factors. Genes SLC9A3R1 and NAT9 are present in the first region, while RAPTOR is demonstrated in the second region.63SLC9A3R1 and NAT9 are players that regulate signal transduction, the immunologic synapse, and T-cell growth. RAPTOR is involved in T-cell function and growth pathways. Using these genes as an example, we can predict that the alterations of regulatory genes, even those yet undetermined, can enhance T-cell proliferation and inflammation manifested in psoriasis.
Conclusion
Psoriasis is a complex disease whereby multiple exogenous and endogenous stimuli incite already heightened innate immune responses in genetically predetermined individuals. The disease process is a result of a network of cell types, including T cells, DCs, and keratinocytes that, with the production of cytokines, generate a chronic inflammatory state. Our understanding of these cellular interactions and cytokines originates from developments, some meticulously planned, others serendipitous, in the fields of immunology, cell and molecular biology, and genetics. Such progress has fostered the creation of targeted immune therapy that has demonstrated remarkable efficacy in psoriasis treatment. Further study of the underlying pathophysiology of psoriasis may provide additional targets for therapy.
- Gottlieb A. Psoriasis. Dis Manag Clin Outcome. 1998;1:195-202.
- Gaspari AA. Innate and adaptive immunity and the pathophysiology of psoriasis. J Am Acad Dermatol. 2006;54(3 suppl 2):S67-S80.
- Di Cesare A, Di Meglio P, Nestle F. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009;129:1339-1350.
- Barker J. The pathophysiology of psoriasis. Lancet. 1991;338:227-230.
- Nickoloff BJ, Nestle FO. Recent insights into the immunopathogenesis of psoriasis provide new therapeutic opportunities. J Clin Invest. 2004;113:1664-1675.
- Bos J, Meinardi M, van Joost T, et al. Use of cyclosporine in psoriasis. Lancet. 1989;23:1500-1505.
- Khandke L, Krane J, Ashinoff R, et al. Cyclosporine in psoriasis treatment: inhibition of keratinocyte cell-cycle progression in G1 independent effects on transforming growth factor-alpha/epidermal growth factor receptor pathways. Arch Dermatol. 1991;127:1172-1179.
- Gottlieb S, Gilleaudeau P, Johnson R, et al. Response of psoriasis to a lymphocyte-selective toxin (DAB389IL-2) suggests a primary immune, but not keratinocyte, pathogenic basis. Nat Med. 1995;1:442-447.
- Vallat V, Gilleaudeau P, Battat L, et al. PUVA bath therapy strongly suppresses immunological and epidermal activation in psoriasis: a possible cellular basis for remittive therapy. J Exp Med. 1994;180:283-296.
- Gottlieb A, Grossman R, Khandke L, et al. Studies of the effect of cyclosporine in psoriasis in vivo: combined effects on activated T lymphocytes and epidermal regenerative maturation. J Invest Dermatol. 1992;98:302-309.
- Gottlieb S, Hayes E, Gilleaudeau P, et al. Cellular actions of etretinate in psoriasis: enhanced epidermal differentiation and reduced cell-mediated inflammation are unexpected outcomes. J Cutan Pathol. 1996;23:404-418.
- Nickoloff B, Bonish B, Huang B, et al. Characterization of a T cell line bearing natural killer receptors and capable of creating psoriasis in a SCID mouse model system. J Dermatol Sci. 2000;24:212-225.
- Gillet M, Conrad C, Geiges M, et al. Psoriasis triggered by toll-like receptor 7 agonist imiquimod in the presence of dermal plasmacytoid dendritic cell precursors. Arch Dermatol. 2004;140:1490-1495.
- Funk J, Langeland T, Schrumpf E, et al. Psoriasis induced by interferon-alpha. Br J Dermatol. 1991;125:463-465.
- Shiohara T, Kobayahsi M, Abe K, et al. Psoriasis occurring predominantly on warts: possible involvement of interferon alpha. Arch Dermatol. 1988;124:1816-1821.
- Fierlbeck G, Rassner G, Muller C. Psoriasis induced at the injection site of recombinant interferon gamma: results of immunohistologic investigations. Arch Dermatol. 1990;126:351-355.
- Prinz J. The role of T cells in psoriasis. J Eur Acad Dermatol Venereol. 2003;17(suppl):1-5.
- Bos J, de Rie M. The pathogenesis of psoriasis: immunological facts and speculations. Immunol Today. 1999;20:40-46.
- Wucherpfennig KW, Strominger JL. Molecular mimicry in T cell–mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell. 1995;80:695-705.
- Geginat J, Campagnaro S, Sallusto F, et al. TCR-independent proliferation and differentiation of human CD4+ T cell subsets induced by cytokines. Adv Exp Med Biol. 2002;512:107-112.
- Kastelan M, Massari L, Brajac I. Apoptosis mediated by cytolytic molecules might be responsible for maintenance of psoriatic plaques. Med Hypotheses. 2006;67:336-337.
- Austin L, Ozawa M, Kikuchi T, et al. The majority of epidermal T cells in psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J Invest Dermatol. 1999;113:752-759.
- Abrams J, Kelley S, Hayes E, et al. Blockade of T lymphocyte costimulation with cytotoxic T lymphocyte-associated antigen 4-immunoglobulin (CTLA4Ig) reverses the cellular pathology of psoriatic plagues, including the activation of keratinocytes, dendritic cells and endothelial cells. J Exp Med. 2000;192:681-694.
- Lebwohl M, Christophers E, Langley R, et al. An international, randomized, double-blind, placebo-controlled phase 3 trial of intramuscular alefacept in patients with chronic plaque psoriasis. Arch Dermatol. 2003;139:719-727.
- Krueger G, Ellis C. Alefacept therapy produces remission for patients with chronic plaque psoriasis. Br J Dermatol. 2003;148:784-788.
- Gordon K, Leonardi C, Tyring S, et al. Efalizumab (anti-CD11a) is safe and effective in the treatment of psoriasis: pooled results of the 12-week first treatment period from 2 phase III trials. J Invest Dermatol. 2002;119:242.
- Singh A, Wilson M, Hong S, et al. Natural killer T cell activation protects mice against experimental autoimmune encephalomyelitis. J Exp Med. 2001;194:1801-1811.
- Saubermann L, Beck P, De Jong Y, et al. Activation of natural killer T cells by alpha-glactosylceramide in the presence of CD1d provides protection against colitis in mice. Gastroenterology. 2000;119:119-128.
- Campos R, Szczepanik M, Itakura A, et al. Cutaneous immunization rapidly activates liver invariant Valpha 14 NKT cells stimulating B-1 B cells to initiate T cell recruitment for elicitation of contact sensitivity. J Exp Med. 2003;198:1785-1796.
- Bonish B, Jullien D, Dutronc Y, et al. Overexpression of CD1d by keratinocytes in psoriasis and CD1d-dependent IFN-gamma production by NK-T cells. J Immunol. 2000;165:4076-4085.
- Deguchi M, Aiba S, Ohtani H, et al. Comparison of the distribution and numbers of antigen-presenting cells among T-lymphocyte-mediated dermatoses: CD1a+, factor XIIIa+, and CD68+ cells in eczematous dermatitis, psoriasis, lichen planus and graft-versus-host disease. Arch Dermatol Res. 2002;294:297-302.
- Bos J, de Rie M, Teunissen M, et al. Psoriasis: dysregulation of innate immunity. Br J Dermatol. 2005;152:1098-1107.
- Trefzer U, Hofmann M, Sterry W, et al. Cytokine and anticytokine therapy in dermatology. Expert Opin Biol Ther. 2003;3:733-743.
- Nickoloff B. The cytokine network in psoriasis. Arch Dermatol. 1991;127:871-884.
- Victor F, Gottlieb A. TNF-alpha and apoptosis: implications for the pathogenesis and treatment of psoriasis. J Drugs Dermatol. 2002;3:264-275.
- Oh C, Das K, Gottlieb A. Treatment with anti-tumour necrosis factor alpha (TNF-alpha) monoclonal antibody dramatically decreases the clinical activity of psoriasis lesions. J Am Acad Dermatol. 2000;42:829-830.
- Reich K, Nestle FO, Papp K, et al; EXPRESS study investigators. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet. 2005;366:1367-1374.
- Leonardi C, Powers J, Matheson R, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003;349:2014-2022.
- Saini R, Tutrone W, Weinberg J. Advances in therapy for psoriasis: an overview of infliximab, etanercept, efalizumab, alefacept, adalimumab, tazarotene, and pimecrolimus. Curr Pharm Des. 2005;11:273-280.
- Cosmi L, De Palma R, Santarlasci V, et al. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med. 2008;205:1903-1916.
- de Beaucoudrey L, Puel A, Filipe-Santos O, et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med. 2008;205:1543-1550.
- Manel N, Unutmaz D, Littman DR. The differentiation of humanT(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641-649.
- Yang L, Anderson DE, Baecher-Allan C, et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350-352.
- Lee E, Trepicchio WL, Oestreicher JL, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199:125-130.
- Chan JR, Blumenschein W, Murphy E, et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med. 2006;203:2557-2587.
- Capon F, Di Meglio P, Szaub J, et al. Sequence variants in the genes for the interleukin-23 receptor (IL23R) and its ligand (IL12B) confer protection against psoriasis. Hum Genet. 2007;122:201-206.
- Cargill M, Schrodi SJ, Chang M, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet. 2007;80:273-290.
- Nair RP, Ruether A, Stuart PE, et al. Polymorphisms of the IL12B and IL23R genes are associated with psoriasis. J Invest Dermatol. 2008;128:1653-1661.
- Ma HL, Liang S, Li J, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest. 2008;118:597-607.
- Wolk K, Witte E, Wallace E, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36:1309-1323.
- Boniface K, Guignouard E, Pedretti N, et al. A role for T cell-derived interleukin 22 in psoriatic skin inflammation. Clin Exp Immunol. 2007;150:407-415.
- Weaver CT, Hatton RD, Mangan PR, et al. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821-852.
- Teunissen MB, Koomen CW, de Waal Malefyt R, et al. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol. 1998;111:645-649.
- Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207-1211.
- Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005;2005:273-279.
- Zaba LC, Cardinale I, Gilleaudeau P, et al. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J Exp Med. 2007;204:3183-3194.
- Haider AS, Cohen J, Fei J, et al. Insights into gene modulation by therapeutic TNF and IFNgamma antibodies: TNF regulates IFNgamma production by T cells and TNF-regulated genes linked to psoriasis transcriptome. J Invest Dermatol. 2008;128:655-666.
- Haider AS, Lowes MA, Suarez-Farinas M, et al. Identification of cellular pathways of “type 1,” Th17 T cells, and TNF- and inducible nitric oxide synthase-producing dendritic cells in autoimmune inflammation through pharmacogenomic study of cyclosporine A in psoriasis. J Immunol. 2008;180:1913-1920.
- Croxtall JD. Ustekinumab: a review of its use in the management of moderate to severe plaque psoriasis. Drugs. 2011;71:1733-1753.
- Gordon KB, Langely RG, Gottlieb AB, et al. A phase III, randomized, controlled trial of the fully human IL-12/23 mAb briakinumab in moderate-to-severe psoriasis. J Invest Dermatol. 2012;132:304-314.
- Rahman P, Elder JT. Genetic epidemiology of psoriasis and psoriatic arthritis. Ann Rheum Dis. 2005;64(suppl 2):ii37-ii39.
- Elder JT. PSORS1: linking genetics and immunology. J Invest Dermatol. 2006;126:1205-1206.
- Krueger JG, Bowcock A. Psoriasis pathophysiology: current concepts of pathogenesis. Ann Rheum Dis. 2005;64(suppl 2):ii30-ii36.
- Gottlieb A. Psoriasis. Dis Manag Clin Outcome. 1998;1:195-202.
- Gaspari AA. Innate and adaptive immunity and the pathophysiology of psoriasis. J Am Acad Dermatol. 2006;54(3 suppl 2):S67-S80.
- Di Cesare A, Di Meglio P, Nestle F. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009;129:1339-1350.
- Barker J. The pathophysiology of psoriasis. Lancet. 1991;338:227-230.
- Nickoloff BJ, Nestle FO. Recent insights into the immunopathogenesis of psoriasis provide new therapeutic opportunities. J Clin Invest. 2004;113:1664-1675.
- Bos J, Meinardi M, van Joost T, et al. Use of cyclosporine in psoriasis. Lancet. 1989;23:1500-1505.
- Khandke L, Krane J, Ashinoff R, et al. Cyclosporine in psoriasis treatment: inhibition of keratinocyte cell-cycle progression in G1 independent effects on transforming growth factor-alpha/epidermal growth factor receptor pathways. Arch Dermatol. 1991;127:1172-1179.
- Gottlieb S, Gilleaudeau P, Johnson R, et al. Response of psoriasis to a lymphocyte-selective toxin (DAB389IL-2) suggests a primary immune, but not keratinocyte, pathogenic basis. Nat Med. 1995;1:442-447.
- Vallat V, Gilleaudeau P, Battat L, et al. PUVA bath therapy strongly suppresses immunological and epidermal activation in psoriasis: a possible cellular basis for remittive therapy. J Exp Med. 1994;180:283-296.
- Gottlieb A, Grossman R, Khandke L, et al. Studies of the effect of cyclosporine in psoriasis in vivo: combined effects on activated T lymphocytes and epidermal regenerative maturation. J Invest Dermatol. 1992;98:302-309.
- Gottlieb S, Hayes E, Gilleaudeau P, et al. Cellular actions of etretinate in psoriasis: enhanced epidermal differentiation and reduced cell-mediated inflammation are unexpected outcomes. J Cutan Pathol. 1996;23:404-418.
- Nickoloff B, Bonish B, Huang B, et al. Characterization of a T cell line bearing natural killer receptors and capable of creating psoriasis in a SCID mouse model system. J Dermatol Sci. 2000;24:212-225.
- Gillet M, Conrad C, Geiges M, et al. Psoriasis triggered by toll-like receptor 7 agonist imiquimod in the presence of dermal plasmacytoid dendritic cell precursors. Arch Dermatol. 2004;140:1490-1495.
- Funk J, Langeland T, Schrumpf E, et al. Psoriasis induced by interferon-alpha. Br J Dermatol. 1991;125:463-465.
- Shiohara T, Kobayahsi M, Abe K, et al. Psoriasis occurring predominantly on warts: possible involvement of interferon alpha. Arch Dermatol. 1988;124:1816-1821.
- Fierlbeck G, Rassner G, Muller C. Psoriasis induced at the injection site of recombinant interferon gamma: results of immunohistologic investigations. Arch Dermatol. 1990;126:351-355.
- Prinz J. The role of T cells in psoriasis. J Eur Acad Dermatol Venereol. 2003;17(suppl):1-5.
- Bos J, de Rie M. The pathogenesis of psoriasis: immunological facts and speculations. Immunol Today. 1999;20:40-46.
- Wucherpfennig KW, Strominger JL. Molecular mimicry in T cell–mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell. 1995;80:695-705.
- Geginat J, Campagnaro S, Sallusto F, et al. TCR-independent proliferation and differentiation of human CD4+ T cell subsets induced by cytokines. Adv Exp Med Biol. 2002;512:107-112.
- Kastelan M, Massari L, Brajac I. Apoptosis mediated by cytolytic molecules might be responsible for maintenance of psoriatic plaques. Med Hypotheses. 2006;67:336-337.
- Austin L, Ozawa M, Kikuchi T, et al. The majority of epidermal T cells in psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J Invest Dermatol. 1999;113:752-759.
- Abrams J, Kelley S, Hayes E, et al. Blockade of T lymphocyte costimulation with cytotoxic T lymphocyte-associated antigen 4-immunoglobulin (CTLA4Ig) reverses the cellular pathology of psoriatic plagues, including the activation of keratinocytes, dendritic cells and endothelial cells. J Exp Med. 2000;192:681-694.
- Lebwohl M, Christophers E, Langley R, et al. An international, randomized, double-blind, placebo-controlled phase 3 trial of intramuscular alefacept in patients with chronic plaque psoriasis. Arch Dermatol. 2003;139:719-727.
- Krueger G, Ellis C. Alefacept therapy produces remission for patients with chronic plaque psoriasis. Br J Dermatol. 2003;148:784-788.
- Gordon K, Leonardi C, Tyring S, et al. Efalizumab (anti-CD11a) is safe and effective in the treatment of psoriasis: pooled results of the 12-week first treatment period from 2 phase III trials. J Invest Dermatol. 2002;119:242.
- Singh A, Wilson M, Hong S, et al. Natural killer T cell activation protects mice against experimental autoimmune encephalomyelitis. J Exp Med. 2001;194:1801-1811.
- Saubermann L, Beck P, De Jong Y, et al. Activation of natural killer T cells by alpha-glactosylceramide in the presence of CD1d provides protection against colitis in mice. Gastroenterology. 2000;119:119-128.
- Campos R, Szczepanik M, Itakura A, et al. Cutaneous immunization rapidly activates liver invariant Valpha 14 NKT cells stimulating B-1 B cells to initiate T cell recruitment for elicitation of contact sensitivity. J Exp Med. 2003;198:1785-1796.
- Bonish B, Jullien D, Dutronc Y, et al. Overexpression of CD1d by keratinocytes in psoriasis and CD1d-dependent IFN-gamma production by NK-T cells. J Immunol. 2000;165:4076-4085.
- Deguchi M, Aiba S, Ohtani H, et al. Comparison of the distribution and numbers of antigen-presenting cells among T-lymphocyte-mediated dermatoses: CD1a+, factor XIIIa+, and CD68+ cells in eczematous dermatitis, psoriasis, lichen planus and graft-versus-host disease. Arch Dermatol Res. 2002;294:297-302.
- Bos J, de Rie M, Teunissen M, et al. Psoriasis: dysregulation of innate immunity. Br J Dermatol. 2005;152:1098-1107.
- Trefzer U, Hofmann M, Sterry W, et al. Cytokine and anticytokine therapy in dermatology. Expert Opin Biol Ther. 2003;3:733-743.
- Nickoloff B. The cytokine network in psoriasis. Arch Dermatol. 1991;127:871-884.
- Victor F, Gottlieb A. TNF-alpha and apoptosis: implications for the pathogenesis and treatment of psoriasis. J Drugs Dermatol. 2002;3:264-275.
- Oh C, Das K, Gottlieb A. Treatment with anti-tumour necrosis factor alpha (TNF-alpha) monoclonal antibody dramatically decreases the clinical activity of psoriasis lesions. J Am Acad Dermatol. 2000;42:829-830.
- Reich K, Nestle FO, Papp K, et al; EXPRESS study investigators. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet. 2005;366:1367-1374.
- Leonardi C, Powers J, Matheson R, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003;349:2014-2022.
- Saini R, Tutrone W, Weinberg J. Advances in therapy for psoriasis: an overview of infliximab, etanercept, efalizumab, alefacept, adalimumab, tazarotene, and pimecrolimus. Curr Pharm Des. 2005;11:273-280.
- Cosmi L, De Palma R, Santarlasci V, et al. Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor. J Exp Med. 2008;205:1903-1916.
- de Beaucoudrey L, Puel A, Filipe-Santos O, et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med. 2008;205:1543-1550.
- Manel N, Unutmaz D, Littman DR. The differentiation of humanT(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641-649.
- Yang L, Anderson DE, Baecher-Allan C, et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350-352.
- Lee E, Trepicchio WL, Oestreicher JL, et al. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199:125-130.
- Chan JR, Blumenschein W, Murphy E, et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med. 2006;203:2557-2587.
- Capon F, Di Meglio P, Szaub J, et al. Sequence variants in the genes for the interleukin-23 receptor (IL23R) and its ligand (IL12B) confer protection against psoriasis. Hum Genet. 2007;122:201-206.
- Cargill M, Schrodi SJ, Chang M, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet. 2007;80:273-290.
- Nair RP, Ruether A, Stuart PE, et al. Polymorphisms of the IL12B and IL23R genes are associated with psoriasis. J Invest Dermatol. 2008;128:1653-1661.
- Ma HL, Liang S, Li J, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest. 2008;118:597-607.
- Wolk K, Witte E, Wallace E, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36:1309-1323.
- Boniface K, Guignouard E, Pedretti N, et al. A role for T cell-derived interleukin 22 in psoriatic skin inflammation. Clin Exp Immunol. 2007;150:407-415.
- Weaver CT, Hatton RD, Mangan PR, et al. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821-852.
- Teunissen MB, Koomen CW, de Waal Malefyt R, et al. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol. 1998;111:645-649.
- Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207-1211.
- Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005;2005:273-279.
- Zaba LC, Cardinale I, Gilleaudeau P, et al. Amelioration of epidermal hyperplasia by TNF inhibition is associated with reduced Th17 responses. J Exp Med. 2007;204:3183-3194.
- Haider AS, Cohen J, Fei J, et al. Insights into gene modulation by therapeutic TNF and IFNgamma antibodies: TNF regulates IFNgamma production by T cells and TNF-regulated genes linked to psoriasis transcriptome. J Invest Dermatol. 2008;128:655-666.
- Haider AS, Lowes MA, Suarez-Farinas M, et al. Identification of cellular pathways of “type 1,” Th17 T cells, and TNF- and inducible nitric oxide synthase-producing dendritic cells in autoimmune inflammation through pharmacogenomic study of cyclosporine A in psoriasis. J Immunol. 2008;180:1913-1920.
- Croxtall JD. Ustekinumab: a review of its use in the management of moderate to severe plaque psoriasis. Drugs. 2011;71:1733-1753.
- Gordon KB, Langely RG, Gottlieb AB, et al. A phase III, randomized, controlled trial of the fully human IL-12/23 mAb briakinumab in moderate-to-severe psoriasis. J Invest Dermatol. 2012;132:304-314.
- Rahman P, Elder JT. Genetic epidemiology of psoriasis and psoriatic arthritis. Ann Rheum Dis. 2005;64(suppl 2):ii37-ii39.
- Elder JT. PSORS1: linking genetics and immunology. J Invest Dermatol. 2006;126:1205-1206.
- Krueger JG, Bowcock A. Psoriasis pathophysiology: current concepts of pathogenesis. Ann Rheum Dis. 2005;64(suppl 2):ii30-ii36.
Practice Points
- Psoriasis is a systemic inflammatory disease.
- We now have an increased understanding of the specific cytokines involved in the disease.
- Therapies have been developed to target these cytokines.