User login
Diabetes Hub contains news and clinical review articles for physicians seeking the most up-to-date information on the rapidly evolving options for treating and preventing Type 2 Diabetes in at-risk patients. The Diabetes Hub is powered by Frontline Medical Communications.
Six PAD diagnostic tests vary widely in patients with diabetes
Six different clinical tests used to identify peripheral arterial disease (PAD) were found to be significantly different in their ability to detect PAD in a population of 50 patients with diabetes, according to a report published online in Primary Care Diabetes.
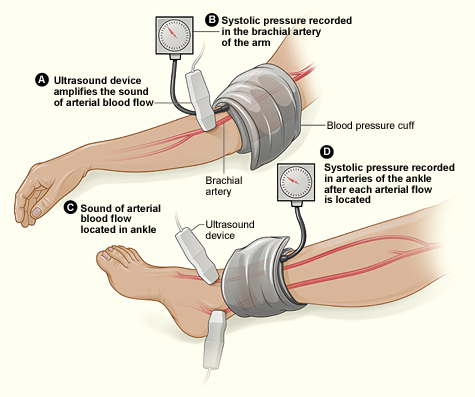
This study assessed the same group of participants with each of the following six tests: Doppler Waveform, toe-brachial pressure index (TBPI), ankle-brachial pressure index (ABPI), posterior tibial artery pulse (ATP), transcutaneous oxygen pressure (TCPO), and pulse palpation. The right and left foot were assessed in each participant, yeilding100 limbs for analysis, according to Yvonne Midolo Azzopardi, MD, of the University of Malta in Msida and her colleagues.
The highest percent of participants who were found to have PAD was 93%, as detected by Doppler Waveform, followed by TBPI (72%), ABPI (57%), ATP (35%), TCPO (30%), and pulse palpation (23%). The difference between these percentages was significant at P less than .0005.
“The reported observations suggest that use of only one screening tool in isolation could yield high false results since it is clear that these tests do not concur with each other to a large extent,” the authors stated.
Dr. Azzopardi and her colleagues pointed out that the use of more specialized tools, such as duplex scanning, could be compared with these six modalities to detect PAD but that such methods were unlikely to be routinely available to primary care physicians who are at the front lines of making the determination of PAD in patients with diabetes.
“The authors advocate for urgent, more robust studies utilizing a gold standard modality for the diagnosis of PAD in order to provide evidence regarding which noninvasive screening modalities would yield the most valid results. This would significantly reduce the proportion of patients with diabetes who would be falsely identified as having no PAD and subsequently denied beneficial and effective secondary risk factor control,” Dr. Azzopardi and her colleagues concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Azzopardi YM et al. 2018. Prim Care Diabetes.. doi: 10.1016/j.pcd.2018.08.005.
Six different clinical tests used to identify peripheral arterial disease (PAD) were found to be significantly different in their ability to detect PAD in a population of 50 patients with diabetes, according to a report published online in Primary Care Diabetes.

This study assessed the same group of participants with each of the following six tests: Doppler Waveform, toe-brachial pressure index (TBPI), ankle-brachial pressure index (ABPI), posterior tibial artery pulse (ATP), transcutaneous oxygen pressure (TCPO), and pulse palpation. The right and left foot were assessed in each participant, yeilding100 limbs for analysis, according to Yvonne Midolo Azzopardi, MD, of the University of Malta in Msida and her colleagues.
The highest percent of participants who were found to have PAD was 93%, as detected by Doppler Waveform, followed by TBPI (72%), ABPI (57%), ATP (35%), TCPO (30%), and pulse palpation (23%). The difference between these percentages was significant at P less than .0005.
“The reported observations suggest that use of only one screening tool in isolation could yield high false results since it is clear that these tests do not concur with each other to a large extent,” the authors stated.
Dr. Azzopardi and her colleagues pointed out that the use of more specialized tools, such as duplex scanning, could be compared with these six modalities to detect PAD but that such methods were unlikely to be routinely available to primary care physicians who are at the front lines of making the determination of PAD in patients with diabetes.
“The authors advocate for urgent, more robust studies utilizing a gold standard modality for the diagnosis of PAD in order to provide evidence regarding which noninvasive screening modalities would yield the most valid results. This would significantly reduce the proportion of patients with diabetes who would be falsely identified as having no PAD and subsequently denied beneficial and effective secondary risk factor control,” Dr. Azzopardi and her colleagues concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Azzopardi YM et al. 2018. Prim Care Diabetes.. doi: 10.1016/j.pcd.2018.08.005.
Six different clinical tests used to identify peripheral arterial disease (PAD) were found to be significantly different in their ability to detect PAD in a population of 50 patients with diabetes, according to a report published online in Primary Care Diabetes.

This study assessed the same group of participants with each of the following six tests: Doppler Waveform, toe-brachial pressure index (TBPI), ankle-brachial pressure index (ABPI), posterior tibial artery pulse (ATP), transcutaneous oxygen pressure (TCPO), and pulse palpation. The right and left foot were assessed in each participant, yeilding100 limbs for analysis, according to Yvonne Midolo Azzopardi, MD, of the University of Malta in Msida and her colleagues.
The highest percent of participants who were found to have PAD was 93%, as detected by Doppler Waveform, followed by TBPI (72%), ABPI (57%), ATP (35%), TCPO (30%), and pulse palpation (23%). The difference between these percentages was significant at P less than .0005.
“The reported observations suggest that use of only one screening tool in isolation could yield high false results since it is clear that these tests do not concur with each other to a large extent,” the authors stated.
Dr. Azzopardi and her colleagues pointed out that the use of more specialized tools, such as duplex scanning, could be compared with these six modalities to detect PAD but that such methods were unlikely to be routinely available to primary care physicians who are at the front lines of making the determination of PAD in patients with diabetes.
“The authors advocate for urgent, more robust studies utilizing a gold standard modality for the diagnosis of PAD in order to provide evidence regarding which noninvasive screening modalities would yield the most valid results. This would significantly reduce the proportion of patients with diabetes who would be falsely identified as having no PAD and subsequently denied beneficial and effective secondary risk factor control,” Dr. Azzopardi and her colleagues concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Azzopardi YM et al. 2018. Prim Care Diabetes.. doi: 10.1016/j.pcd.2018.08.005.
FROM PRIMARY CARE DIABETES
Key clinical point: Six different tests used to identify PAD differed significantly in their ability to detect the disease.
Major finding: Detection ranged from 93% to 23% in the same group of patients.
Study details: Both legs of 50 patients with diabetes were assessed for PAD using six screening modalities.
Disclosures: The authors reported that they had no conflicts of interest.
Source: Azzopardi YM et al. 2018. Prim Care Diabetes. doi: 10.1016/j.pcd.2018.08.005.
Weight-loss drug lorcaserin’s glycemic effects revealed
BERLIN – Lower rates of incident type 2 diabetes mellitus (T2DM) and improved glycemic control were two of the metabolic effects seen with the appetite-suppressant drug lorcaserin versus placebo on top of existing lifestyle management measures in a large-scale trial of more than 12,000 overweight or obese individuals with established cardiovascular disease or T2DM and other cardiovascular risk factors.
In the CAMELLIA-TIMI 61 trial, treatment with a twice-daily, 10-mg dose of lorcaserin for a median of 3.3 years was associated with a significant 19% reduction in the risk of incident T2DM in participants with prediabetes, compared with placebo (8.5% vs. 10.3%; hazard ratio, 0.81; 95% confidence interval, 0.66-0.99; P = .038). The reduction in the risk of incident T2DM was even greater (23%) in people without diabetes at baseline (6.7% lorcaserin vs. 8.4% placebo; HR, 0.77; 95% CI, 0.63-0.94; P = .012).
Furthermore, in patients with T2DM who had a mean baseline glycated hemoglobin (HbA1c) of 7%, an absolute 0.33% reduction was seen at 1 year between the lorcaserin and placebo groups, with more modest but still significant between-group reductions (–0.09% and –0.08%) in individuals with prediabetes or normoglycemia (all P less than .0001). When baseline HbA1c levels were higher in patients with T2DM (8%), greater net reductions (0.52%) versus placebo were seen (P less than .0001).
These were some of the metabolic findings, published online in the Lancet to coincide with their presentation at the annual meeting of the European Association for the Study of Diabetes, that add to those already released from the CAMELLIA-TIMI 61 trial on cardiovascular safety, lead author and TIMI (Thrombolysis in Myocardial Infarction) group investigator Erin A. Bohula May, MD, observed during a press conference.
The cardiovascular safety data were presented at the 2018 annual congress of the European Society for Cardiology in August and published in the New England Journal of Medicine. These showed no increase with lorcaserin versus placebo in the risk of achieving a major cardiovascular endpoint (MACE) of cardiovascular death, MI, or stroke (HR, 0.99; 95% CI, 0.85-1.14; P less than .001 for noninferiority). There was also no difference between groups in the cumulative incidence of MACE+, which included heart failure, hospitalization for unstable angina, and the need for coronary revascularization (HR, 0.97; 95% CI, 0.87-1.07; P = .55 for superiority).
“We know that weight loss can improve cardiovascular and glycemic risk factors, but it’s difficult to achieve and maintain, and weight-loss agents are guideline-recommended adjuncts to lifestyle modification,” said Dr. Bohula May, who is a cardiovascular medicine and critical care specialist at Brigham and Women’s Hospital in Boston.
“However, prior to this study no agent had convincingly demonstrated cardiovascular safety in a rigorous clinical outcomes study,” she said, noting that several agents, such as the now-withdrawn rimonabant (Acomplia/Zimulti) and sibutramine (Meridia), had been shown to precipitate cardiovascular or psychiatric events, which led the Food and Drug Administration to mandate that all weight-loss drugs be assessed for cardiovascular safety. Lorcaserin (Belviq) is a centrally acting 5-HT2C agonist that works by decreasing appetite and was approved by the FDA in 2012 but is not currently available in Europe.
Long-term data on the effects of weight-loss agents on glycemic parameters were limited, hence the remit of the CAMELLIA-TIMI 61 trial was to assess both the cardiovascular and metabolic safety of lorcaserin. The drug was used on a background of lifestyle modification in 6,000 obese or overweight individuals at high risk of cardiovascular events. A further 6,000 individuals received placebo.
“Lorcaserin induced and maintained weight loss across the glycemic categories,” said coauthor and TIMI group investigator Benjamin Scirica, MD, also of Brigham and Women’s Hospital, who presented the metabolic data during a scientific session at the EASD meeting. Specifically, there was a net weight loss beyond that seen with placebo of 2.6 kg, 2.8 kg, and 3.3 kg in individuals with T2DM, prediabetes, and normoglycemia, respectively.
“Roughly 40% of patients with lorcaserin achieved a 5% weight loss, and about 14%-18% achieved a 10% weight loss across the glycemic categories,” Dr. Scirica reported. The corresponding values for the placebo-treated patients were 17%-18% and 4%-7%.
Naveed Sattar, MD, the independent commentator for the trial, noted the weight-loss reduction seen “was modest in the context of this trial, but I think the important point was that it was sustained. Sustained weight loss is difficult, and it was sustained on top of lifestyle and on top of the other drugs, and that is important.”
However, Dr. Sattar, who is professor and honorary consultant in cardiovascular and medical sciences at the University of Glasgow (Scotland), also observed that “as night follows day, glycemic improvements follow weight loss.” So, did the glycemic parameters improve purely because of the weight loss? While there is some preclinical evidence that lorcaserin may have an effect outside of its weight-lowering effects, Dr. Sattar felt this was unlikely to be clinically significant in itself.
“Obesity is probably the biggest challenge we have in the medical profession. We’ve got excellent cholesterol-lowering, blood pressure–lowering, and diabetes drugs. Yet obesity and complications are rising worldwide” and “safe weight-loss drugs remain sparse,” Dr. Sattar said.
He suggested that lorcaserin may well have an adjunctive place in the current treatment paradigm, but that place is probably “down the line” after other measures with greater weight-reducing effects or proven cardiovascular benefits were used. Not only are lifestyle modification approaches improving, Dr. Sattar said, but there are also over-the-counter options such as orlistat (Xenical), metformin, sodium-glucose cotransporter 2 inhibitors, glucagonlike peptide receptor–1 agonists, and bariatric surgery that are likely to be used first.
“This is a fantastically well done trial, we needed it,” Dr. Sattar said. However, because there was modest weight loss and no real cardiovascular benefit (but also no cardiovascular safety concern) he called the results “a bust” saying that “we have to take them at face value for what they are.”
Dr. Sattar noted that his “gut feeling at the moment is that the clinical role for lorcaserin is probably, at best, a down-the-line adjunct in those who are still obese for additional weight reduction on top of other drugs and lifestyle modifications, particularly in those who are ‘super responders.’ ” This is so long as the safety signals remain strong and there are quality of life benefits, he added.
The study was designed by the TIMI Study Group in conjunction with the executive committee and the trial sponsor, Eisai. Dr. Bohula May and Dr. Scirica reported receiving grants from Eisai, during the conduct of the study. Dr. Sattar reported grant support from Boehringer Ingelheim, and being part of an advisory board or speaker’s bureau for Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen Pharmaceuticals, Novo Nordisk, and Sanofi.
SOURCES: Bohula May EA et al. Lancet. 2018 Oct 4. doi: 10.1016/S0140-6736(18)32328-6; Bohula May EA et al. N Engl J Med. 2018; 379:1107-17; Sattar N. EASD 2018, Session S33.
BERLIN – Lower rates of incident type 2 diabetes mellitus (T2DM) and improved glycemic control were two of the metabolic effects seen with the appetite-suppressant drug lorcaserin versus placebo on top of existing lifestyle management measures in a large-scale trial of more than 12,000 overweight or obese individuals with established cardiovascular disease or T2DM and other cardiovascular risk factors.
In the CAMELLIA-TIMI 61 trial, treatment with a twice-daily, 10-mg dose of lorcaserin for a median of 3.3 years was associated with a significant 19% reduction in the risk of incident T2DM in participants with prediabetes, compared with placebo (8.5% vs. 10.3%; hazard ratio, 0.81; 95% confidence interval, 0.66-0.99; P = .038). The reduction in the risk of incident T2DM was even greater (23%) in people without diabetes at baseline (6.7% lorcaserin vs. 8.4% placebo; HR, 0.77; 95% CI, 0.63-0.94; P = .012).
Furthermore, in patients with T2DM who had a mean baseline glycated hemoglobin (HbA1c) of 7%, an absolute 0.33% reduction was seen at 1 year between the lorcaserin and placebo groups, with more modest but still significant between-group reductions (–0.09% and –0.08%) in individuals with prediabetes or normoglycemia (all P less than .0001). When baseline HbA1c levels were higher in patients with T2DM (8%), greater net reductions (0.52%) versus placebo were seen (P less than .0001).
These were some of the metabolic findings, published online in the Lancet to coincide with their presentation at the annual meeting of the European Association for the Study of Diabetes, that add to those already released from the CAMELLIA-TIMI 61 trial on cardiovascular safety, lead author and TIMI (Thrombolysis in Myocardial Infarction) group investigator Erin A. Bohula May, MD, observed during a press conference.
The cardiovascular safety data were presented at the 2018 annual congress of the European Society for Cardiology in August and published in the New England Journal of Medicine. These showed no increase with lorcaserin versus placebo in the risk of achieving a major cardiovascular endpoint (MACE) of cardiovascular death, MI, or stroke (HR, 0.99; 95% CI, 0.85-1.14; P less than .001 for noninferiority). There was also no difference between groups in the cumulative incidence of MACE+, which included heart failure, hospitalization for unstable angina, and the need for coronary revascularization (HR, 0.97; 95% CI, 0.87-1.07; P = .55 for superiority).
“We know that weight loss can improve cardiovascular and glycemic risk factors, but it’s difficult to achieve and maintain, and weight-loss agents are guideline-recommended adjuncts to lifestyle modification,” said Dr. Bohula May, who is a cardiovascular medicine and critical care specialist at Brigham and Women’s Hospital in Boston.
“However, prior to this study no agent had convincingly demonstrated cardiovascular safety in a rigorous clinical outcomes study,” she said, noting that several agents, such as the now-withdrawn rimonabant (Acomplia/Zimulti) and sibutramine (Meridia), had been shown to precipitate cardiovascular or psychiatric events, which led the Food and Drug Administration to mandate that all weight-loss drugs be assessed for cardiovascular safety. Lorcaserin (Belviq) is a centrally acting 5-HT2C agonist that works by decreasing appetite and was approved by the FDA in 2012 but is not currently available in Europe.
Long-term data on the effects of weight-loss agents on glycemic parameters were limited, hence the remit of the CAMELLIA-TIMI 61 trial was to assess both the cardiovascular and metabolic safety of lorcaserin. The drug was used on a background of lifestyle modification in 6,000 obese or overweight individuals at high risk of cardiovascular events. A further 6,000 individuals received placebo.
“Lorcaserin induced and maintained weight loss across the glycemic categories,” said coauthor and TIMI group investigator Benjamin Scirica, MD, also of Brigham and Women’s Hospital, who presented the metabolic data during a scientific session at the EASD meeting. Specifically, there was a net weight loss beyond that seen with placebo of 2.6 kg, 2.8 kg, and 3.3 kg in individuals with T2DM, prediabetes, and normoglycemia, respectively.
“Roughly 40% of patients with lorcaserin achieved a 5% weight loss, and about 14%-18% achieved a 10% weight loss across the glycemic categories,” Dr. Scirica reported. The corresponding values for the placebo-treated patients were 17%-18% and 4%-7%.
Naveed Sattar, MD, the independent commentator for the trial, noted the weight-loss reduction seen “was modest in the context of this trial, but I think the important point was that it was sustained. Sustained weight loss is difficult, and it was sustained on top of lifestyle and on top of the other drugs, and that is important.”
However, Dr. Sattar, who is professor and honorary consultant in cardiovascular and medical sciences at the University of Glasgow (Scotland), also observed that “as night follows day, glycemic improvements follow weight loss.” So, did the glycemic parameters improve purely because of the weight loss? While there is some preclinical evidence that lorcaserin may have an effect outside of its weight-lowering effects, Dr. Sattar felt this was unlikely to be clinically significant in itself.
“Obesity is probably the biggest challenge we have in the medical profession. We’ve got excellent cholesterol-lowering, blood pressure–lowering, and diabetes drugs. Yet obesity and complications are rising worldwide” and “safe weight-loss drugs remain sparse,” Dr. Sattar said.
He suggested that lorcaserin may well have an adjunctive place in the current treatment paradigm, but that place is probably “down the line” after other measures with greater weight-reducing effects or proven cardiovascular benefits were used. Not only are lifestyle modification approaches improving, Dr. Sattar said, but there are also over-the-counter options such as orlistat (Xenical), metformin, sodium-glucose cotransporter 2 inhibitors, glucagonlike peptide receptor–1 agonists, and bariatric surgery that are likely to be used first.
“This is a fantastically well done trial, we needed it,” Dr. Sattar said. However, because there was modest weight loss and no real cardiovascular benefit (but also no cardiovascular safety concern) he called the results “a bust” saying that “we have to take them at face value for what they are.”
Dr. Sattar noted that his “gut feeling at the moment is that the clinical role for lorcaserin is probably, at best, a down-the-line adjunct in those who are still obese for additional weight reduction on top of other drugs and lifestyle modifications, particularly in those who are ‘super responders.’ ” This is so long as the safety signals remain strong and there are quality of life benefits, he added.
The study was designed by the TIMI Study Group in conjunction with the executive committee and the trial sponsor, Eisai. Dr. Bohula May and Dr. Scirica reported receiving grants from Eisai, during the conduct of the study. Dr. Sattar reported grant support from Boehringer Ingelheim, and being part of an advisory board or speaker’s bureau for Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen Pharmaceuticals, Novo Nordisk, and Sanofi.
SOURCES: Bohula May EA et al. Lancet. 2018 Oct 4. doi: 10.1016/S0140-6736(18)32328-6; Bohula May EA et al. N Engl J Med. 2018; 379:1107-17; Sattar N. EASD 2018, Session S33.
BERLIN – Lower rates of incident type 2 diabetes mellitus (T2DM) and improved glycemic control were two of the metabolic effects seen with the appetite-suppressant drug lorcaserin versus placebo on top of existing lifestyle management measures in a large-scale trial of more than 12,000 overweight or obese individuals with established cardiovascular disease or T2DM and other cardiovascular risk factors.
In the CAMELLIA-TIMI 61 trial, treatment with a twice-daily, 10-mg dose of lorcaserin for a median of 3.3 years was associated with a significant 19% reduction in the risk of incident T2DM in participants with prediabetes, compared with placebo (8.5% vs. 10.3%; hazard ratio, 0.81; 95% confidence interval, 0.66-0.99; P = .038). The reduction in the risk of incident T2DM was even greater (23%) in people without diabetes at baseline (6.7% lorcaserin vs. 8.4% placebo; HR, 0.77; 95% CI, 0.63-0.94; P = .012).
Furthermore, in patients with T2DM who had a mean baseline glycated hemoglobin (HbA1c) of 7%, an absolute 0.33% reduction was seen at 1 year between the lorcaserin and placebo groups, with more modest but still significant between-group reductions (–0.09% and –0.08%) in individuals with prediabetes or normoglycemia (all P less than .0001). When baseline HbA1c levels were higher in patients with T2DM (8%), greater net reductions (0.52%) versus placebo were seen (P less than .0001).
These were some of the metabolic findings, published online in the Lancet to coincide with their presentation at the annual meeting of the European Association for the Study of Diabetes, that add to those already released from the CAMELLIA-TIMI 61 trial on cardiovascular safety, lead author and TIMI (Thrombolysis in Myocardial Infarction) group investigator Erin A. Bohula May, MD, observed during a press conference.
The cardiovascular safety data were presented at the 2018 annual congress of the European Society for Cardiology in August and published in the New England Journal of Medicine. These showed no increase with lorcaserin versus placebo in the risk of achieving a major cardiovascular endpoint (MACE) of cardiovascular death, MI, or stroke (HR, 0.99; 95% CI, 0.85-1.14; P less than .001 for noninferiority). There was also no difference between groups in the cumulative incidence of MACE+, which included heart failure, hospitalization for unstable angina, and the need for coronary revascularization (HR, 0.97; 95% CI, 0.87-1.07; P = .55 for superiority).
“We know that weight loss can improve cardiovascular and glycemic risk factors, but it’s difficult to achieve and maintain, and weight-loss agents are guideline-recommended adjuncts to lifestyle modification,” said Dr. Bohula May, who is a cardiovascular medicine and critical care specialist at Brigham and Women’s Hospital in Boston.
“However, prior to this study no agent had convincingly demonstrated cardiovascular safety in a rigorous clinical outcomes study,” she said, noting that several agents, such as the now-withdrawn rimonabant (Acomplia/Zimulti) and sibutramine (Meridia), had been shown to precipitate cardiovascular or psychiatric events, which led the Food and Drug Administration to mandate that all weight-loss drugs be assessed for cardiovascular safety. Lorcaserin (Belviq) is a centrally acting 5-HT2C agonist that works by decreasing appetite and was approved by the FDA in 2012 but is not currently available in Europe.
Long-term data on the effects of weight-loss agents on glycemic parameters were limited, hence the remit of the CAMELLIA-TIMI 61 trial was to assess both the cardiovascular and metabolic safety of lorcaserin. The drug was used on a background of lifestyle modification in 6,000 obese or overweight individuals at high risk of cardiovascular events. A further 6,000 individuals received placebo.
“Lorcaserin induced and maintained weight loss across the glycemic categories,” said coauthor and TIMI group investigator Benjamin Scirica, MD, also of Brigham and Women’s Hospital, who presented the metabolic data during a scientific session at the EASD meeting. Specifically, there was a net weight loss beyond that seen with placebo of 2.6 kg, 2.8 kg, and 3.3 kg in individuals with T2DM, prediabetes, and normoglycemia, respectively.
“Roughly 40% of patients with lorcaserin achieved a 5% weight loss, and about 14%-18% achieved a 10% weight loss across the glycemic categories,” Dr. Scirica reported. The corresponding values for the placebo-treated patients were 17%-18% and 4%-7%.
Naveed Sattar, MD, the independent commentator for the trial, noted the weight-loss reduction seen “was modest in the context of this trial, but I think the important point was that it was sustained. Sustained weight loss is difficult, and it was sustained on top of lifestyle and on top of the other drugs, and that is important.”
However, Dr. Sattar, who is professor and honorary consultant in cardiovascular and medical sciences at the University of Glasgow (Scotland), also observed that “as night follows day, glycemic improvements follow weight loss.” So, did the glycemic parameters improve purely because of the weight loss? While there is some preclinical evidence that lorcaserin may have an effect outside of its weight-lowering effects, Dr. Sattar felt this was unlikely to be clinically significant in itself.
“Obesity is probably the biggest challenge we have in the medical profession. We’ve got excellent cholesterol-lowering, blood pressure–lowering, and diabetes drugs. Yet obesity and complications are rising worldwide” and “safe weight-loss drugs remain sparse,” Dr. Sattar said.
He suggested that lorcaserin may well have an adjunctive place in the current treatment paradigm, but that place is probably “down the line” after other measures with greater weight-reducing effects or proven cardiovascular benefits were used. Not only are lifestyle modification approaches improving, Dr. Sattar said, but there are also over-the-counter options such as orlistat (Xenical), metformin, sodium-glucose cotransporter 2 inhibitors, glucagonlike peptide receptor–1 agonists, and bariatric surgery that are likely to be used first.
“This is a fantastically well done trial, we needed it,” Dr. Sattar said. However, because there was modest weight loss and no real cardiovascular benefit (but also no cardiovascular safety concern) he called the results “a bust” saying that “we have to take them at face value for what they are.”
Dr. Sattar noted that his “gut feeling at the moment is that the clinical role for lorcaserin is probably, at best, a down-the-line adjunct in those who are still obese for additional weight reduction on top of other drugs and lifestyle modifications, particularly in those who are ‘super responders.’ ” This is so long as the safety signals remain strong and there are quality of life benefits, he added.
The study was designed by the TIMI Study Group in conjunction with the executive committee and the trial sponsor, Eisai. Dr. Bohula May and Dr. Scirica reported receiving grants from Eisai, during the conduct of the study. Dr. Sattar reported grant support from Boehringer Ingelheim, and being part of an advisory board or speaker’s bureau for Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen Pharmaceuticals, Novo Nordisk, and Sanofi.
SOURCES: Bohula May EA et al. Lancet. 2018 Oct 4. doi: 10.1016/S0140-6736(18)32328-6; Bohula May EA et al. N Engl J Med. 2018; 379:1107-17; Sattar N. EASD 2018, Session S33.
REPORTING FROM EASD 2018
Key clinical point: Lorcaserin is an adjunctive treatment to lifestyle modification for chronic weight management that may improve metabolic health.
Major finding: A total of 8.5% of lorcaserin-treated individuals with prediabetes versus 10.3% of placebo-treated individuals developed incident type 2 diabetes mellitus at 1 year (hazard ratio, 0.81; 95% confidence interval, 0.66-0.99; P = .038).
Study details: A randomized, double-blind, placebo-controlled trial of 12,000 overweight or obese individuals with established cardiovascular disease, established or no type 2 diabetes mellitus, and other cardiovascular risk factors.
Disclosures: The study was designed by the Thrombolysis in Myocardial Infarction Study Group in conjunction with the executive committee and the trial sponsor, Eisai. Dr. Bohula May and Dr. Scirica reported receiving grants from Eisai, during the conduct of the study. Dr. Sattar reported grant support from Boehringer Ingelheim and being part of an advisory board or speaker’s bureau for Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen Pharmaceuticals, Novo Nordisk, and Sanofi.
Sources: Bohula May EA et al. Lancet. 2018. doi: 10.1016/S0140-6736(18)32328-6; Bohula May EA et al. N Engl J Med. 2018;379:1107-17; Sattar N. EASD 2018, Session S33.
Guidelines outline patient-centered approach to type 2 diabetes
issued jointly by the American Diabetes Association and the European Association for the Study of Diabetes.
The 2018 ADA/EASD Consensus Report also addresses clinical inertia and notes that medication adherence and persistence should be facilitated. All patients should be offered ongoing self-management education and support, Melanie J. Davies, MD, one of the two cochairs of the report-writing committee, said during a press conference at the annual meeting of the European Association for the Study of Diabetes.
The report also addresses preferred choices for glucose-lowering medications, largely based on recent findings of large-scale cardiovascular outcomes trials. There also is specific guidance on how to manage hyperglycemia in patients with atherosclerotic cardiovascular disease, chronic kidney disease, and heart failure.
“The consensus report focuses on not what an individual’s glycemic target should be or how to individualize goals but really addresses how each patient can achieve their individualized glycemic target,” Dr. Davies said.
Dr. Davies, who is professor of diabetes medicine at the University of Leicester (England) and an honorary consultant diabetologist at the University Hospitals of Leicester NHS Trust also said that the report looked at taking patient factors and preferences into account but also considered “the ever-increasing complexity around the availability of glucose-lowering agents.”
Practical guide to managing patients
The consensus report, which was simultaneously published in the official journals of the ADA (Diabetes Care 2018 Sep; dci180033) and the EASD (Diabetologia. 2018 Sep. doi: 10.1007/s00125-018-4729-5) to coincide with its presentation at the EASD meeting, is much more visual and aims to be more of a practical aid than was the previous position statement from 2015 (Diabetologia. 2015 Mar;58:429-42; Diabetes Care 2015 Jan;38[1]:140-9), on which it was based, Dr. Davies said.
The patient has been placed firmly at the center of the decision cycle, she observed, which starts with assessment of patient characteristics and consideration of their lifestyle, comorbidities, and clinical parameters. Specific factors that may affect the choice of treatment, such as the individualized glycosylated hemoglobin (HbA1c) target or side effect profiles of medications, are included, as is working together with the patient to make, continually monitor, and reevaluate a shared decision plan.
In terms of lifestyle, one of the consensus recommendations is that “an individualized program of medical nutritional therapy should be offered to all patients,” with the more specific recommendation that those who are overweight or obese be advised of the health benefits of weight loss and be encouraged to participate in dietary modifications that may include food substitution. Increasing activity is also highly recommended based on long-established evidence that this can help reduce HbA1c level. Recommendations for when to consider bariatric surgery for weight management also are included.
Clarity on treating comorbidities
Previously discussed in June at the ADA’s annual meeting, the consensus report has undergone fine-tuning and multiple revisions. The report was based on a comprehensive and systematic review of the diabetes literature available from 2014 through February 2018. Overall, more than 6,000 randomized trials, reviews, and meta-analyses were considered and distilled down to a list of around 500 papers that were then thoroughly reviewed by an expert panel.
“I guarantee, there’s never been a paper that’s been more peer reviewed,” said John Buse, MD, PhD, the other cochair of the report’s writing committee. A total of 35 named individuals reviewed and provided more than 800 detailed comments among them, which were considered and reflected in the final version.
Dr. Buse is the Verne S. Caviness Distinguished Professor, chief of the division of endocrinology, and director of the diabetes center at the University of North Carolina at Chapel Hill.
“There’s much more clarity now,” added Dr. Davies, referring to the changes made to how patients with comorbidities are managed. If somebody does have atherosclerotic cardiovascular disease or chronic kidney disease, there is now clear direction on which glucose-lowering therapy should be considered first, and what to do if the HbA1c remains above target.
For example, in patients who have established atherosclerotic cardiovascular disease, the recommendation is, after metformin, to choose either a glucagonlike peptide–1 (GLP-1) receptor agonist or a sodium-glucose cotransporter 2 (SGLT2) inhibitor with proven cardiovascular benefit.
If heart failure or chronic kidney disease coexist, then an SGLT2 inhibitor shown to reduce their progression should be favored, or if contraindicated or not preferred, a GLP-1 receptor agonist with proven cardiovascular benefit should be given.
The main action, pros and cons of interventions, and the various medications are shown in tables to clearly guide clinicians in the decision-making process, Dr. Buse said.
First-line management
The first line recommended glucose-lowering therapy for hyperglycemia in type 2 diabetes remains metformin, together with comprehensive lifestyle advice, Dr. Buse observed.
“A huge controversy in the [diabetes] community asks, ‘Is metformin the first-line therapy because it’s cheap and was the first oral agent studied and has a long history?’ or is it something that really is based on medical evidence?” Dr. Buse acknowledged. Although combinations of glucose-lowering drugs have been proposed upfront, “the evidence for that is largely from small studies, in limited numbers of sites, such that, for now, we generally recommend starting on a single-agent medication if lifestyle management is not enough to control glucose.”
If there is a need to intensify treatment as the patient’s HbA1c remains above their individualized target, then other drugs may be added to step up the treatment. The consensus report then looks at which drugs might be best to add, based on the need to avoid hypoglycemia, promote weight loss, and/or if cost or availability is a major issue.
If patients need the greater glucose-lowering effects of an injectable medication, a GLP-1 receptor agonist – not insulin – is recommended, Dr. Buse observed. However, for patients with extreme and symptomatic hyperglycemia, insulin is then recommended.
There also is guidance on when to consider oral therapies in conjunction with injectable therapies, with the consensus recommendation stating: Patients who are unable to maintain glycemic targets on basal insulin in combination with oral medication can have treatment intensified with GLP-1 receptor agonists, SGLT2 inhibitors, or prandial insulin.
The ADA perspective
William T. Cefalu, MD, chief scientific, medical and mission officer of the ADA observed that the “ADA fully endorses the ADA/EASD Consensus Report” and had already added a statement on the recommendations into its Standards of Medical Care in Diabetes – 2018 as part of the organization’s Living Standards Update. This was a change made last year to allow real-time updates of practice recommendations based on new and evolving evidence released in between the annual process of updating the Society’s Standards.
“Much, if not all, of these recommendations from this paper will be incorporated into our Standards,” said Dr. Cefalu. “We applaud the authors of the consensus paper; we think this was an outstanding group, and we really feel that this is a paradigm change in diabetes management,” he added. “Instead of relying on the [HbA1c] number in an algorithm, this puts the patient at the center; patient-related factors, patient preferences, adherence, compliance, and more importantly, the underlying disease state … this really is a comprehensive approach to management.”
The stratification of patients by cardiovascular disease, kidney disease, or heart failure is a particularly noteworthy, as is the advice on which agent to choose if weight management is an issue. Finally, there are the considerations of costs of therapy, and what to do if there is the risk of hypoglycemia. “The consensus recommendations and approach to glycemic management in adults with type 2 diabetes presented within the report reflects the current view of the ADA,” Dr. Cefalu confirmed.
The EASD perspective
“The EASD was again delighted to go into cooperation with our colleagues and friends at the ADA because is it is so important to bring out a consensus on where we need to go in this forest of glucose-lowering therapies,” said Chantal Mathieu, MD, PhD, vice-president of the EASD.
“The fact that this consensus paper puts the patient front and center, and makes that an integral part of glucose-lowering therapy, and also that lifestyle is being accentuated again, together with education in every patient is crucial,” Dr. Mathieu, who is professor of medicine at the Katholieke Universiteit Leuven (Belgium), and a coauthor of the report, added.
“At EASD, we also believe that it is very important to bring this consensus paper to life,” she added, which is part of her role as the chair of postgraduate education at the EASD. Two of the EASD’s main remits is to ensure that the results of research and education are brought to the diabetes community at large, she said.
In every figure in the paper there is a highlight to say, “please avoid clinical inertia, reassess and modify treatment if necessary, at least every 3-6 months,” Dr. Mathieu noted during the EASD congress.
David Matthews, MD, professor of diabetic medicine at the University of Oxford (England) and president-elect of the EASD, commented on the 2018 ADA/EASD Consensus Report after its presentation at the EASD meeting. “The reality is that you’ve got to think extremely hard with your patients about what the balances between risks and benefits are,” Dr. Matthews said. “We encourage you to do this, what you have here is a wonderful handbook to guide you in your decision making.”
Dr. Davies reported receiving personal fees and/or grants from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Gilead, Intarcia Therapeutics/Servier, Janssen, Merck Sharp & Dohme, Mitsubishi Tanabi, Novo Nordisk, Sanofi, and Takeda.
Dr. Buse disclosed acting as a consultant to and/or receiving research support from Adocia, AstraZeneca, Eli Lilly, GI Dynamics, Intarcia Therapeutics, MannKind, NovaTarg, Neurimmune, Novo Nordisk, Senseonics, and vTv Therapeutics with fees paid to the University of North Carolina. He holds stock options in Mellitus Health, PhaseBio and Stability Health.
Dr. Mathieu disclosed relationships with (advisory boards, speakers bureaus, and/or research support) from Abbott, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Hanmi Pharmaceuticals, Intrexon, Janssen Pharmaceuticals, MannKind, Medtronic, MSD, Novartis, Novo Nordisk, Pfizer, Roche Diagnostics, Sanofi, and UCB.
Dr. Cefalu had no disclosures and Dr. Matthews had no relevant conflicts of interest other than becoming the EASD president-elect during the meeting.
issued jointly by the American Diabetes Association and the European Association for the Study of Diabetes.
The 2018 ADA/EASD Consensus Report also addresses clinical inertia and notes that medication adherence and persistence should be facilitated. All patients should be offered ongoing self-management education and support, Melanie J. Davies, MD, one of the two cochairs of the report-writing committee, said during a press conference at the annual meeting of the European Association for the Study of Diabetes.
The report also addresses preferred choices for glucose-lowering medications, largely based on recent findings of large-scale cardiovascular outcomes trials. There also is specific guidance on how to manage hyperglycemia in patients with atherosclerotic cardiovascular disease, chronic kidney disease, and heart failure.
“The consensus report focuses on not what an individual’s glycemic target should be or how to individualize goals but really addresses how each patient can achieve their individualized glycemic target,” Dr. Davies said.
Dr. Davies, who is professor of diabetes medicine at the University of Leicester (England) and an honorary consultant diabetologist at the University Hospitals of Leicester NHS Trust also said that the report looked at taking patient factors and preferences into account but also considered “the ever-increasing complexity around the availability of glucose-lowering agents.”
Practical guide to managing patients
The consensus report, which was simultaneously published in the official journals of the ADA (Diabetes Care 2018 Sep; dci180033) and the EASD (Diabetologia. 2018 Sep. doi: 10.1007/s00125-018-4729-5) to coincide with its presentation at the EASD meeting, is much more visual and aims to be more of a practical aid than was the previous position statement from 2015 (Diabetologia. 2015 Mar;58:429-42; Diabetes Care 2015 Jan;38[1]:140-9), on which it was based, Dr. Davies said.
The patient has been placed firmly at the center of the decision cycle, she observed, which starts with assessment of patient characteristics and consideration of their lifestyle, comorbidities, and clinical parameters. Specific factors that may affect the choice of treatment, such as the individualized glycosylated hemoglobin (HbA1c) target or side effect profiles of medications, are included, as is working together with the patient to make, continually monitor, and reevaluate a shared decision plan.
In terms of lifestyle, one of the consensus recommendations is that “an individualized program of medical nutritional therapy should be offered to all patients,” with the more specific recommendation that those who are overweight or obese be advised of the health benefits of weight loss and be encouraged to participate in dietary modifications that may include food substitution. Increasing activity is also highly recommended based on long-established evidence that this can help reduce HbA1c level. Recommendations for when to consider bariatric surgery for weight management also are included.
Clarity on treating comorbidities
Previously discussed in June at the ADA’s annual meeting, the consensus report has undergone fine-tuning and multiple revisions. The report was based on a comprehensive and systematic review of the diabetes literature available from 2014 through February 2018. Overall, more than 6,000 randomized trials, reviews, and meta-analyses were considered and distilled down to a list of around 500 papers that were then thoroughly reviewed by an expert panel.
“I guarantee, there’s never been a paper that’s been more peer reviewed,” said John Buse, MD, PhD, the other cochair of the report’s writing committee. A total of 35 named individuals reviewed and provided more than 800 detailed comments among them, which were considered and reflected in the final version.
Dr. Buse is the Verne S. Caviness Distinguished Professor, chief of the division of endocrinology, and director of the diabetes center at the University of North Carolina at Chapel Hill.
“There’s much more clarity now,” added Dr. Davies, referring to the changes made to how patients with comorbidities are managed. If somebody does have atherosclerotic cardiovascular disease or chronic kidney disease, there is now clear direction on which glucose-lowering therapy should be considered first, and what to do if the HbA1c remains above target.
For example, in patients who have established atherosclerotic cardiovascular disease, the recommendation is, after metformin, to choose either a glucagonlike peptide–1 (GLP-1) receptor agonist or a sodium-glucose cotransporter 2 (SGLT2) inhibitor with proven cardiovascular benefit.
If heart failure or chronic kidney disease coexist, then an SGLT2 inhibitor shown to reduce their progression should be favored, or if contraindicated or not preferred, a GLP-1 receptor agonist with proven cardiovascular benefit should be given.
The main action, pros and cons of interventions, and the various medications are shown in tables to clearly guide clinicians in the decision-making process, Dr. Buse said.
First-line management
The first line recommended glucose-lowering therapy for hyperglycemia in type 2 diabetes remains metformin, together with comprehensive lifestyle advice, Dr. Buse observed.
“A huge controversy in the [diabetes] community asks, ‘Is metformin the first-line therapy because it’s cheap and was the first oral agent studied and has a long history?’ or is it something that really is based on medical evidence?” Dr. Buse acknowledged. Although combinations of glucose-lowering drugs have been proposed upfront, “the evidence for that is largely from small studies, in limited numbers of sites, such that, for now, we generally recommend starting on a single-agent medication if lifestyle management is not enough to control glucose.”
If there is a need to intensify treatment as the patient’s HbA1c remains above their individualized target, then other drugs may be added to step up the treatment. The consensus report then looks at which drugs might be best to add, based on the need to avoid hypoglycemia, promote weight loss, and/or if cost or availability is a major issue.
If patients need the greater glucose-lowering effects of an injectable medication, a GLP-1 receptor agonist – not insulin – is recommended, Dr. Buse observed. However, for patients with extreme and symptomatic hyperglycemia, insulin is then recommended.
There also is guidance on when to consider oral therapies in conjunction with injectable therapies, with the consensus recommendation stating: Patients who are unable to maintain glycemic targets on basal insulin in combination with oral medication can have treatment intensified with GLP-1 receptor agonists, SGLT2 inhibitors, or prandial insulin.
The ADA perspective
William T. Cefalu, MD, chief scientific, medical and mission officer of the ADA observed that the “ADA fully endorses the ADA/EASD Consensus Report” and had already added a statement on the recommendations into its Standards of Medical Care in Diabetes – 2018 as part of the organization’s Living Standards Update. This was a change made last year to allow real-time updates of practice recommendations based on new and evolving evidence released in between the annual process of updating the Society’s Standards.
“Much, if not all, of these recommendations from this paper will be incorporated into our Standards,” said Dr. Cefalu. “We applaud the authors of the consensus paper; we think this was an outstanding group, and we really feel that this is a paradigm change in diabetes management,” he added. “Instead of relying on the [HbA1c] number in an algorithm, this puts the patient at the center; patient-related factors, patient preferences, adherence, compliance, and more importantly, the underlying disease state … this really is a comprehensive approach to management.”
The stratification of patients by cardiovascular disease, kidney disease, or heart failure is a particularly noteworthy, as is the advice on which agent to choose if weight management is an issue. Finally, there are the considerations of costs of therapy, and what to do if there is the risk of hypoglycemia. “The consensus recommendations and approach to glycemic management in adults with type 2 diabetes presented within the report reflects the current view of the ADA,” Dr. Cefalu confirmed.
The EASD perspective
“The EASD was again delighted to go into cooperation with our colleagues and friends at the ADA because is it is so important to bring out a consensus on where we need to go in this forest of glucose-lowering therapies,” said Chantal Mathieu, MD, PhD, vice-president of the EASD.
“The fact that this consensus paper puts the patient front and center, and makes that an integral part of glucose-lowering therapy, and also that lifestyle is being accentuated again, together with education in every patient is crucial,” Dr. Mathieu, who is professor of medicine at the Katholieke Universiteit Leuven (Belgium), and a coauthor of the report, added.
“At EASD, we also believe that it is very important to bring this consensus paper to life,” she added, which is part of her role as the chair of postgraduate education at the EASD. Two of the EASD’s main remits is to ensure that the results of research and education are brought to the diabetes community at large, she said.
In every figure in the paper there is a highlight to say, “please avoid clinical inertia, reassess and modify treatment if necessary, at least every 3-6 months,” Dr. Mathieu noted during the EASD congress.
David Matthews, MD, professor of diabetic medicine at the University of Oxford (England) and president-elect of the EASD, commented on the 2018 ADA/EASD Consensus Report after its presentation at the EASD meeting. “The reality is that you’ve got to think extremely hard with your patients about what the balances between risks and benefits are,” Dr. Matthews said. “We encourage you to do this, what you have here is a wonderful handbook to guide you in your decision making.”
Dr. Davies reported receiving personal fees and/or grants from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Gilead, Intarcia Therapeutics/Servier, Janssen, Merck Sharp & Dohme, Mitsubishi Tanabi, Novo Nordisk, Sanofi, and Takeda.
Dr. Buse disclosed acting as a consultant to and/or receiving research support from Adocia, AstraZeneca, Eli Lilly, GI Dynamics, Intarcia Therapeutics, MannKind, NovaTarg, Neurimmune, Novo Nordisk, Senseonics, and vTv Therapeutics with fees paid to the University of North Carolina. He holds stock options in Mellitus Health, PhaseBio and Stability Health.
Dr. Mathieu disclosed relationships with (advisory boards, speakers bureaus, and/or research support) from Abbott, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Hanmi Pharmaceuticals, Intrexon, Janssen Pharmaceuticals, MannKind, Medtronic, MSD, Novartis, Novo Nordisk, Pfizer, Roche Diagnostics, Sanofi, and UCB.
Dr. Cefalu had no disclosures and Dr. Matthews had no relevant conflicts of interest other than becoming the EASD president-elect during the meeting.
issued jointly by the American Diabetes Association and the European Association for the Study of Diabetes.
The 2018 ADA/EASD Consensus Report also addresses clinical inertia and notes that medication adherence and persistence should be facilitated. All patients should be offered ongoing self-management education and support, Melanie J. Davies, MD, one of the two cochairs of the report-writing committee, said during a press conference at the annual meeting of the European Association for the Study of Diabetes.
The report also addresses preferred choices for glucose-lowering medications, largely based on recent findings of large-scale cardiovascular outcomes trials. There also is specific guidance on how to manage hyperglycemia in patients with atherosclerotic cardiovascular disease, chronic kidney disease, and heart failure.
“The consensus report focuses on not what an individual’s glycemic target should be or how to individualize goals but really addresses how each patient can achieve their individualized glycemic target,” Dr. Davies said.
Dr. Davies, who is professor of diabetes medicine at the University of Leicester (England) and an honorary consultant diabetologist at the University Hospitals of Leicester NHS Trust also said that the report looked at taking patient factors and preferences into account but also considered “the ever-increasing complexity around the availability of glucose-lowering agents.”
Practical guide to managing patients
The consensus report, which was simultaneously published in the official journals of the ADA (Diabetes Care 2018 Sep; dci180033) and the EASD (Diabetologia. 2018 Sep. doi: 10.1007/s00125-018-4729-5) to coincide with its presentation at the EASD meeting, is much more visual and aims to be more of a practical aid than was the previous position statement from 2015 (Diabetologia. 2015 Mar;58:429-42; Diabetes Care 2015 Jan;38[1]:140-9), on which it was based, Dr. Davies said.
The patient has been placed firmly at the center of the decision cycle, she observed, which starts with assessment of patient characteristics and consideration of their lifestyle, comorbidities, and clinical parameters. Specific factors that may affect the choice of treatment, such as the individualized glycosylated hemoglobin (HbA1c) target or side effect profiles of medications, are included, as is working together with the patient to make, continually monitor, and reevaluate a shared decision plan.
In terms of lifestyle, one of the consensus recommendations is that “an individualized program of medical nutritional therapy should be offered to all patients,” with the more specific recommendation that those who are overweight or obese be advised of the health benefits of weight loss and be encouraged to participate in dietary modifications that may include food substitution. Increasing activity is also highly recommended based on long-established evidence that this can help reduce HbA1c level. Recommendations for when to consider bariatric surgery for weight management also are included.
Clarity on treating comorbidities
Previously discussed in June at the ADA’s annual meeting, the consensus report has undergone fine-tuning and multiple revisions. The report was based on a comprehensive and systematic review of the diabetes literature available from 2014 through February 2018. Overall, more than 6,000 randomized trials, reviews, and meta-analyses were considered and distilled down to a list of around 500 papers that were then thoroughly reviewed by an expert panel.
“I guarantee, there’s never been a paper that’s been more peer reviewed,” said John Buse, MD, PhD, the other cochair of the report’s writing committee. A total of 35 named individuals reviewed and provided more than 800 detailed comments among them, which were considered and reflected in the final version.
Dr. Buse is the Verne S. Caviness Distinguished Professor, chief of the division of endocrinology, and director of the diabetes center at the University of North Carolina at Chapel Hill.
“There’s much more clarity now,” added Dr. Davies, referring to the changes made to how patients with comorbidities are managed. If somebody does have atherosclerotic cardiovascular disease or chronic kidney disease, there is now clear direction on which glucose-lowering therapy should be considered first, and what to do if the HbA1c remains above target.
For example, in patients who have established atherosclerotic cardiovascular disease, the recommendation is, after metformin, to choose either a glucagonlike peptide–1 (GLP-1) receptor agonist or a sodium-glucose cotransporter 2 (SGLT2) inhibitor with proven cardiovascular benefit.
If heart failure or chronic kidney disease coexist, then an SGLT2 inhibitor shown to reduce their progression should be favored, or if contraindicated or not preferred, a GLP-1 receptor agonist with proven cardiovascular benefit should be given.
The main action, pros and cons of interventions, and the various medications are shown in tables to clearly guide clinicians in the decision-making process, Dr. Buse said.
First-line management
The first line recommended glucose-lowering therapy for hyperglycemia in type 2 diabetes remains metformin, together with comprehensive lifestyle advice, Dr. Buse observed.
“A huge controversy in the [diabetes] community asks, ‘Is metformin the first-line therapy because it’s cheap and was the first oral agent studied and has a long history?’ or is it something that really is based on medical evidence?” Dr. Buse acknowledged. Although combinations of glucose-lowering drugs have been proposed upfront, “the evidence for that is largely from small studies, in limited numbers of sites, such that, for now, we generally recommend starting on a single-agent medication if lifestyle management is not enough to control glucose.”
If there is a need to intensify treatment as the patient’s HbA1c remains above their individualized target, then other drugs may be added to step up the treatment. The consensus report then looks at which drugs might be best to add, based on the need to avoid hypoglycemia, promote weight loss, and/or if cost or availability is a major issue.
If patients need the greater glucose-lowering effects of an injectable medication, a GLP-1 receptor agonist – not insulin – is recommended, Dr. Buse observed. However, for patients with extreme and symptomatic hyperglycemia, insulin is then recommended.
There also is guidance on when to consider oral therapies in conjunction with injectable therapies, with the consensus recommendation stating: Patients who are unable to maintain glycemic targets on basal insulin in combination with oral medication can have treatment intensified with GLP-1 receptor agonists, SGLT2 inhibitors, or prandial insulin.
The ADA perspective
William T. Cefalu, MD, chief scientific, medical and mission officer of the ADA observed that the “ADA fully endorses the ADA/EASD Consensus Report” and had already added a statement on the recommendations into its Standards of Medical Care in Diabetes – 2018 as part of the organization’s Living Standards Update. This was a change made last year to allow real-time updates of practice recommendations based on new and evolving evidence released in between the annual process of updating the Society’s Standards.
“Much, if not all, of these recommendations from this paper will be incorporated into our Standards,” said Dr. Cefalu. “We applaud the authors of the consensus paper; we think this was an outstanding group, and we really feel that this is a paradigm change in diabetes management,” he added. “Instead of relying on the [HbA1c] number in an algorithm, this puts the patient at the center; patient-related factors, patient preferences, adherence, compliance, and more importantly, the underlying disease state … this really is a comprehensive approach to management.”
The stratification of patients by cardiovascular disease, kidney disease, or heart failure is a particularly noteworthy, as is the advice on which agent to choose if weight management is an issue. Finally, there are the considerations of costs of therapy, and what to do if there is the risk of hypoglycemia. “The consensus recommendations and approach to glycemic management in adults with type 2 diabetes presented within the report reflects the current view of the ADA,” Dr. Cefalu confirmed.
The EASD perspective
“The EASD was again delighted to go into cooperation with our colleagues and friends at the ADA because is it is so important to bring out a consensus on where we need to go in this forest of glucose-lowering therapies,” said Chantal Mathieu, MD, PhD, vice-president of the EASD.
“The fact that this consensus paper puts the patient front and center, and makes that an integral part of glucose-lowering therapy, and also that lifestyle is being accentuated again, together with education in every patient is crucial,” Dr. Mathieu, who is professor of medicine at the Katholieke Universiteit Leuven (Belgium), and a coauthor of the report, added.
“At EASD, we also believe that it is very important to bring this consensus paper to life,” she added, which is part of her role as the chair of postgraduate education at the EASD. Two of the EASD’s main remits is to ensure that the results of research and education are brought to the diabetes community at large, she said.
In every figure in the paper there is a highlight to say, “please avoid clinical inertia, reassess and modify treatment if necessary, at least every 3-6 months,” Dr. Mathieu noted during the EASD congress.
David Matthews, MD, professor of diabetic medicine at the University of Oxford (England) and president-elect of the EASD, commented on the 2018 ADA/EASD Consensus Report after its presentation at the EASD meeting. “The reality is that you’ve got to think extremely hard with your patients about what the balances between risks and benefits are,” Dr. Matthews said. “We encourage you to do this, what you have here is a wonderful handbook to guide you in your decision making.”
Dr. Davies reported receiving personal fees and/or grants from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Gilead, Intarcia Therapeutics/Servier, Janssen, Merck Sharp & Dohme, Mitsubishi Tanabi, Novo Nordisk, Sanofi, and Takeda.
Dr. Buse disclosed acting as a consultant to and/or receiving research support from Adocia, AstraZeneca, Eli Lilly, GI Dynamics, Intarcia Therapeutics, MannKind, NovaTarg, Neurimmune, Novo Nordisk, Senseonics, and vTv Therapeutics with fees paid to the University of North Carolina. He holds stock options in Mellitus Health, PhaseBio and Stability Health.
Dr. Mathieu disclosed relationships with (advisory boards, speakers bureaus, and/or research support) from Abbott, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Hanmi Pharmaceuticals, Intrexon, Janssen Pharmaceuticals, MannKind, Medtronic, MSD, Novartis, Novo Nordisk, Pfizer, Roche Diagnostics, Sanofi, and UCB.
Dr. Cefalu had no disclosures and Dr. Matthews had no relevant conflicts of interest other than becoming the EASD president-elect during the meeting.
REPORTING FROM EASD 2018
‘Twincreatin’ produces ‘impressive’ HbA1c, weight control in T2DM
BERLIN – Patients with type 2 diabetes mellitus (T2DM) achieved “impressive” drops in glycated hemoglobin, or HbA1c, of up to 2.4% and in body weight of up to 11.3 kg with the investigational drug LY3298176 in a phase 2b trial.
The primary endpoint was the change in HbA1c at 26 weeks, which showed significant reductions from baseline of 1.6%, 2%, and 2.4% for the 5-mg, 10-mg, and 15-mg doses, respectively, of the drug tested. By comparison, a drop of just 1.1% was seen for dulaglutide, and 0.1% for placebo.
The higher doses of LY3298176, which is a dual agonist of glucose-dependent insulinotropic polypeptide (GIP) and the glucagonlike peptide–1 (GLP-1) receptor, used in the trial helped up to 90% of patients achieve an HbA1c of 7% or less, 82% of patients to achieve an HbA1c of 6.5% or less, and up to 30% of patients to achieve an HbA1c of less than 5.7%, a range which is considered normal for people without diabetes.
As for weight loss, the 5-mg, 10-mg, and 15-mg doses of LY3298176 helped patients achieve significant –4.8-kg, –8.7-kg, and –11.3-kg changes from baseline, respectively. These were greater than those seen with dulaglutide (–2.7 kg) and placebo (–0.4 kg).
More than one third of patients treated with the novel drug achieved at least a 10% or more change in their starting body weight, with a quarter of patients on the 15-mg dose achieving a 15% or more change in bodyweight.
“These are very, very impressive data both from an A1c-lowering perspective and also from a weight reduction perspective,” said the study’s principal investigator Juan Frias, MD, at a press briefing held at the annual meeting of the European Association for the Study of Diabetes (EASD).
GLP-1 receptor agonist therapy is a recommended treatment for T2DM because it addresses many of the important pathophysiologic problems, from blood glucose control and weight loss to reducing cardiovascular risk, Dr. Frias observed.
He added that the first GLP-1 receptor agonists became available in the United States in 2005. Their use in T2DM has recently been further endorsed in a consensus report (Diabetes Care. 2018 Sep. doi: rg/10.2337/dci18-0033; Diabetologia. 2018. doi: 10.1007/s00125-018-4729-5) issued jointly by the American Diabetes Association and the EASD.
“Despite the potency of these agents, many of our patients are not achieving adequate glycemic or weight control, so we could always use new agents that are more potent as long as they’re safe for the patient,” said Dr. Frias, who is a clinical endocrinologist and the chief executive officer at the National Research Institute in Los Angeles
LY3298176 is a novel unimolecular, multifunctional peptide that is being developed by Eli Lilly as a dual GIP/GLP-1 receptor agonist for the treatment of T2DM. It consists of 39 amino acids linked to a C20 fatty–diacid moiety and has a mean half-life of around 5 days, which means it can be dosed weekly, Dr. Frias observed.
The effect of targeting both GIP and the GLP-1 receptor simultaneously is possibly both additive and complementary, Dr. Frias suggested. Additive effects may include a decrease in food intake and an increase in insulin secretion, with opposing effects on glucagon secretion, with the additional effects of increased glucose uptake with GIP and delayed gastric emptying with GLP-1 receptor agonism.
“When you think back on what the history, biology, and discovery of these compounds was, it isn’t at all obvious you’d pair these two [to make] a so-called ‘twincreatin’ – there is so much overlap,” said Matthias Tschöp, MD, who was invited to discuss the findings after the presentation at the EASD meeting.
Dr. Tschöp, who is the head of the division of neuroendocrinology at the Institute for Diabetes and Obesity at Helmholtz Zentrum München in Neuherberg , Germany, noted that it’s an interesting combination, but it’s not the only approach being tested – there are other twincretin combinations. GIP on its own doesn’t seem to do much, but it must be the combination that is important, it was suggested during discussion.
A total of 318 patients with T2DM and a starting HbA1c of 7%-10.5% were studied and randomized equally to one of six weekly treatment groups: LY3298176 at doses of either 1 mg, 5 mg, 10 mg, or 15 mg, given subcutaneously; 1.5 mg of dulaglutide; or a placebo injection.
The two highest doses of LY3298176 were titrated rather quickly, Dr. Frias said, and this is important when it comes to the side effect profile. In a subsequent study, the dose titration schedule was amended to prolong the titration period to try to avoid some of the side effects seen, according to Dr. Frias.
Adverse events predominantly affected the gastrointestinal system, with any grade nausea, vomiting, and diarrhea seen in a respective 20%-40%, 7%-26%, and 24%-32% of patients taking the 5-mg, 10-mg, and 15-mg doses. Corresponding rates in dulaglutide-treated patients were, approximately, 30%, 9%, and 17%.
“The safety profile of the dual agonist is really similar to that which you see with selective GLP-1 receptor agonists,” Dr. Frias observed. “The most common adverse events were seen at the higher doses and pertained to GI tolerability, but the majority of these events were mild to moderate and dissipated with time and [could] be reduced significantly by appropriate titration.”
Dr. Frias said: “This novel dual GIP/GLP-1 receptor agonist certainly has the potential to be become a very promising treatment option for patients with type 2 diabetes.”
He added: “In my experience as an investigator, as a clinician, I’ve never really seen this magnitude of A1c reduction in this percentage of patients and also with this level of weight loss as well, certainly greater than with a selective GLP-1 receptor agonist.”
Further long-term studies are needed, and the phase 3 program in T2DM will be called SURPASS, according to a press statement issued by Eli Lilly. The phase 3 studies are expected to begin “no later than early 2019,” the statement said, and be completed in late 2021. The drug company also noted that it is “evaluating next steps in the study of GIP/GLP-1 RA for obesity and other conditions.”
SOURCE: Frias JP et al. EASD 2018, Session S31; Frias JP et al. Lancet. 2018 Oct 4. doi: 10.1016/S0140-6736(18)32260-8.
BERLIN – Patients with type 2 diabetes mellitus (T2DM) achieved “impressive” drops in glycated hemoglobin, or HbA1c, of up to 2.4% and in body weight of up to 11.3 kg with the investigational drug LY3298176 in a phase 2b trial.
The primary endpoint was the change in HbA1c at 26 weeks, which showed significant reductions from baseline of 1.6%, 2%, and 2.4% for the 5-mg, 10-mg, and 15-mg doses, respectively, of the drug tested. By comparison, a drop of just 1.1% was seen for dulaglutide, and 0.1% for placebo.
The higher doses of LY3298176, which is a dual agonist of glucose-dependent insulinotropic polypeptide (GIP) and the glucagonlike peptide–1 (GLP-1) receptor, used in the trial helped up to 90% of patients achieve an HbA1c of 7% or less, 82% of patients to achieve an HbA1c of 6.5% or less, and up to 30% of patients to achieve an HbA1c of less than 5.7%, a range which is considered normal for people without diabetes.
As for weight loss, the 5-mg, 10-mg, and 15-mg doses of LY3298176 helped patients achieve significant –4.8-kg, –8.7-kg, and –11.3-kg changes from baseline, respectively. These were greater than those seen with dulaglutide (–2.7 kg) and placebo (–0.4 kg).
More than one third of patients treated with the novel drug achieved at least a 10% or more change in their starting body weight, with a quarter of patients on the 15-mg dose achieving a 15% or more change in bodyweight.
“These are very, very impressive data both from an A1c-lowering perspective and also from a weight reduction perspective,” said the study’s principal investigator Juan Frias, MD, at a press briefing held at the annual meeting of the European Association for the Study of Diabetes (EASD).
GLP-1 receptor agonist therapy is a recommended treatment for T2DM because it addresses many of the important pathophysiologic problems, from blood glucose control and weight loss to reducing cardiovascular risk, Dr. Frias observed.
He added that the first GLP-1 receptor agonists became available in the United States in 2005. Their use in T2DM has recently been further endorsed in a consensus report (Diabetes Care. 2018 Sep. doi: rg/10.2337/dci18-0033; Diabetologia. 2018. doi: 10.1007/s00125-018-4729-5) issued jointly by the American Diabetes Association and the EASD.
“Despite the potency of these agents, many of our patients are not achieving adequate glycemic or weight control, so we could always use new agents that are more potent as long as they’re safe for the patient,” said Dr. Frias, who is a clinical endocrinologist and the chief executive officer at the National Research Institute in Los Angeles
LY3298176 is a novel unimolecular, multifunctional peptide that is being developed by Eli Lilly as a dual GIP/GLP-1 receptor agonist for the treatment of T2DM. It consists of 39 amino acids linked to a C20 fatty–diacid moiety and has a mean half-life of around 5 days, which means it can be dosed weekly, Dr. Frias observed.
The effect of targeting both GIP and the GLP-1 receptor simultaneously is possibly both additive and complementary, Dr. Frias suggested. Additive effects may include a decrease in food intake and an increase in insulin secretion, with opposing effects on glucagon secretion, with the additional effects of increased glucose uptake with GIP and delayed gastric emptying with GLP-1 receptor agonism.
“When you think back on what the history, biology, and discovery of these compounds was, it isn’t at all obvious you’d pair these two [to make] a so-called ‘twincreatin’ – there is so much overlap,” said Matthias Tschöp, MD, who was invited to discuss the findings after the presentation at the EASD meeting.
Dr. Tschöp, who is the head of the division of neuroendocrinology at the Institute for Diabetes and Obesity at Helmholtz Zentrum München in Neuherberg , Germany, noted that it’s an interesting combination, but it’s not the only approach being tested – there are other twincretin combinations. GIP on its own doesn’t seem to do much, but it must be the combination that is important, it was suggested during discussion.
A total of 318 patients with T2DM and a starting HbA1c of 7%-10.5% were studied and randomized equally to one of six weekly treatment groups: LY3298176 at doses of either 1 mg, 5 mg, 10 mg, or 15 mg, given subcutaneously; 1.5 mg of dulaglutide; or a placebo injection.
The two highest doses of LY3298176 were titrated rather quickly, Dr. Frias said, and this is important when it comes to the side effect profile. In a subsequent study, the dose titration schedule was amended to prolong the titration period to try to avoid some of the side effects seen, according to Dr. Frias.
Adverse events predominantly affected the gastrointestinal system, with any grade nausea, vomiting, and diarrhea seen in a respective 20%-40%, 7%-26%, and 24%-32% of patients taking the 5-mg, 10-mg, and 15-mg doses. Corresponding rates in dulaglutide-treated patients were, approximately, 30%, 9%, and 17%.
“The safety profile of the dual agonist is really similar to that which you see with selective GLP-1 receptor agonists,” Dr. Frias observed. “The most common adverse events were seen at the higher doses and pertained to GI tolerability, but the majority of these events were mild to moderate and dissipated with time and [could] be reduced significantly by appropriate titration.”
Dr. Frias said: “This novel dual GIP/GLP-1 receptor agonist certainly has the potential to be become a very promising treatment option for patients with type 2 diabetes.”
He added: “In my experience as an investigator, as a clinician, I’ve never really seen this magnitude of A1c reduction in this percentage of patients and also with this level of weight loss as well, certainly greater than with a selective GLP-1 receptor agonist.”
Further long-term studies are needed, and the phase 3 program in T2DM will be called SURPASS, according to a press statement issued by Eli Lilly. The phase 3 studies are expected to begin “no later than early 2019,” the statement said, and be completed in late 2021. The drug company also noted that it is “evaluating next steps in the study of GIP/GLP-1 RA for obesity and other conditions.”
SOURCE: Frias JP et al. EASD 2018, Session S31; Frias JP et al. Lancet. 2018 Oct 4. doi: 10.1016/S0140-6736(18)32260-8.
BERLIN – Patients with type 2 diabetes mellitus (T2DM) achieved “impressive” drops in glycated hemoglobin, or HbA1c, of up to 2.4% and in body weight of up to 11.3 kg with the investigational drug LY3298176 in a phase 2b trial.
The primary endpoint was the change in HbA1c at 26 weeks, which showed significant reductions from baseline of 1.6%, 2%, and 2.4% for the 5-mg, 10-mg, and 15-mg doses, respectively, of the drug tested. By comparison, a drop of just 1.1% was seen for dulaglutide, and 0.1% for placebo.
The higher doses of LY3298176, which is a dual agonist of glucose-dependent insulinotropic polypeptide (GIP) and the glucagonlike peptide–1 (GLP-1) receptor, used in the trial helped up to 90% of patients achieve an HbA1c of 7% or less, 82% of patients to achieve an HbA1c of 6.5% or less, and up to 30% of patients to achieve an HbA1c of less than 5.7%, a range which is considered normal for people without diabetes.
As for weight loss, the 5-mg, 10-mg, and 15-mg doses of LY3298176 helped patients achieve significant –4.8-kg, –8.7-kg, and –11.3-kg changes from baseline, respectively. These were greater than those seen with dulaglutide (–2.7 kg) and placebo (–0.4 kg).
More than one third of patients treated with the novel drug achieved at least a 10% or more change in their starting body weight, with a quarter of patients on the 15-mg dose achieving a 15% or more change in bodyweight.
“These are very, very impressive data both from an A1c-lowering perspective and also from a weight reduction perspective,” said the study’s principal investigator Juan Frias, MD, at a press briefing held at the annual meeting of the European Association for the Study of Diabetes (EASD).
GLP-1 receptor agonist therapy is a recommended treatment for T2DM because it addresses many of the important pathophysiologic problems, from blood glucose control and weight loss to reducing cardiovascular risk, Dr. Frias observed.
He added that the first GLP-1 receptor agonists became available in the United States in 2005. Their use in T2DM has recently been further endorsed in a consensus report (Diabetes Care. 2018 Sep. doi: rg/10.2337/dci18-0033; Diabetologia. 2018. doi: 10.1007/s00125-018-4729-5) issued jointly by the American Diabetes Association and the EASD.
“Despite the potency of these agents, many of our patients are not achieving adequate glycemic or weight control, so we could always use new agents that are more potent as long as they’re safe for the patient,” said Dr. Frias, who is a clinical endocrinologist and the chief executive officer at the National Research Institute in Los Angeles
LY3298176 is a novel unimolecular, multifunctional peptide that is being developed by Eli Lilly as a dual GIP/GLP-1 receptor agonist for the treatment of T2DM. It consists of 39 amino acids linked to a C20 fatty–diacid moiety and has a mean half-life of around 5 days, which means it can be dosed weekly, Dr. Frias observed.
The effect of targeting both GIP and the GLP-1 receptor simultaneously is possibly both additive and complementary, Dr. Frias suggested. Additive effects may include a decrease in food intake and an increase in insulin secretion, with opposing effects on glucagon secretion, with the additional effects of increased glucose uptake with GIP and delayed gastric emptying with GLP-1 receptor agonism.
“When you think back on what the history, biology, and discovery of these compounds was, it isn’t at all obvious you’d pair these two [to make] a so-called ‘twincreatin’ – there is so much overlap,” said Matthias Tschöp, MD, who was invited to discuss the findings after the presentation at the EASD meeting.
Dr. Tschöp, who is the head of the division of neuroendocrinology at the Institute for Diabetes and Obesity at Helmholtz Zentrum München in Neuherberg , Germany, noted that it’s an interesting combination, but it’s not the only approach being tested – there are other twincretin combinations. GIP on its own doesn’t seem to do much, but it must be the combination that is important, it was suggested during discussion.
A total of 318 patients with T2DM and a starting HbA1c of 7%-10.5% were studied and randomized equally to one of six weekly treatment groups: LY3298176 at doses of either 1 mg, 5 mg, 10 mg, or 15 mg, given subcutaneously; 1.5 mg of dulaglutide; or a placebo injection.
The two highest doses of LY3298176 were titrated rather quickly, Dr. Frias said, and this is important when it comes to the side effect profile. In a subsequent study, the dose titration schedule was amended to prolong the titration period to try to avoid some of the side effects seen, according to Dr. Frias.
Adverse events predominantly affected the gastrointestinal system, with any grade nausea, vomiting, and diarrhea seen in a respective 20%-40%, 7%-26%, and 24%-32% of patients taking the 5-mg, 10-mg, and 15-mg doses. Corresponding rates in dulaglutide-treated patients were, approximately, 30%, 9%, and 17%.
“The safety profile of the dual agonist is really similar to that which you see with selective GLP-1 receptor agonists,” Dr. Frias observed. “The most common adverse events were seen at the higher doses and pertained to GI tolerability, but the majority of these events were mild to moderate and dissipated with time and [could] be reduced significantly by appropriate titration.”
Dr. Frias said: “This novel dual GIP/GLP-1 receptor agonist certainly has the potential to be become a very promising treatment option for patients with type 2 diabetes.”
He added: “In my experience as an investigator, as a clinician, I’ve never really seen this magnitude of A1c reduction in this percentage of patients and also with this level of weight loss as well, certainly greater than with a selective GLP-1 receptor agonist.”
Further long-term studies are needed, and the phase 3 program in T2DM will be called SURPASS, according to a press statement issued by Eli Lilly. The phase 3 studies are expected to begin “no later than early 2019,” the statement said, and be completed in late 2021. The drug company also noted that it is “evaluating next steps in the study of GIP/GLP-1 RA for obesity and other conditions.”
SOURCE: Frias JP et al. EASD 2018, Session S31; Frias JP et al. Lancet. 2018 Oct 4. doi: 10.1016/S0140-6736(18)32260-8.
REPORTING FROM EASD 2018
Key clinical point: The investigational dual GIP/GLP-1 receptor agonist LY3298176 could prove to be a promising treatment for T2DM.
Major finding: HbA1c fell by up to 2.4%, and body weight reduced by up to 11.3 kg depending on the dose tested.
Study details: A 26-week, phase 2b, double-blind, placebo-controlled study of 318 subjects with T2DM and a starting HbA1c of 7%-10.5%.
Disclosures: The study was funded by Eli Lilly. The presenting author Dr. Frias declared receiving research support and consulting honorarium from the company, as well as from multiple other pharmaceutical companies. The commentator Dr. Tschöp disclosed being a scientific advisory board member of ERX Pharmaceuticals.
Sources: Frias JP et al. EASD 2018, Session S31; Frias JP et al. Lancet. 2018 Oct 4. doi: 10.1016/S0140-6736(18)32260-8.
Gastric banding, metformin “equal” for slowing early T2DM progression
BERLIN – Gastric banding surgery and metformin produce similar improvements in insulin sensitivity and parameters indicative of preserved beta-cell function in patients with impaired glucose tolerance (IGT) or newly diagnosed type 2 diabetes mellitus (T2DM), according to the results of a study conducted by the Restoring Insulin Secretion (RISE) Consortium.
“Both interventions resulted in about 50% improvements in insulin sensitivity at 1 year, which was attenuated at 2 years,” reported study investigator Thomas Buchanan, MD, of the University of Southern California, Los Angeles, at the annual meeting of the European Association for the Study of Diabetes.
“The beta-cell responses fell in a pattern that maintained relatively, but not perfectly, stable compensation for insulin resistance,” he added.
Although glucose levels improved “only slightly,” he said, “acute compensation to glucose improved significantly with gastric banding and beta-cell compensation at maximal stimulation fell significantly with metformin.”
Results of the BetaFat (Beta Cell Restoration through Fat Mitigation) study, which are now published online in Diabetes Care, also showed that greater weight loss could be achieved with surgery versus metformin, with a 8.9 kg difference between the groups at 2 years (10.6 vs. 1.7 kg, respectively, P less than .01).
HDL cholesterol levels also rose with both interventions, and gastric banding resulted in a greater effect on very low–density lipoprotein cholesterol and triglycerides, as well as serum ALT, Dr. Buchanan said.
The BetaFat study is one of three “proof-of principle” studies currently being conducted by the RISE Consortium in patients with IGT, sometimes called prediabetes, and T2DM, explained Steven E. Kahn, MB, ChB, the chair for the RISE studies.
The other two multicenter, randomized trials being conducted by the RISE Consortium are looking at the effects of medications on preserving beta-cell function in pediatric/adolescent (10-19 years) and adult (21-65 years) populations with IGT or mild, recently diagnosed T2DM. The design, and some results, of these trials can be viewed on a dedicated section of the Diabetes Care website.
Beta-cell function is being assessed using “state-of-the-art” methods; the coprimary endpoint of the surgery versus metformin study was the steady state C-peptide level and acute C-peptide response at maximal glycemia measured using a hyperglycemic “clamp.”
The goal of the RISE studies is to test different approaches to preserve beta-cell function. It is designed to answer the question of which is more effective in this setting: sustained weight loss through gastric banding such as in the BetaFat study or medication.
Patients were eligible for inclusion in the study if they were aged 21-65 years, had a body mass index of 30-40 kg/m2, and had IGT or a diagnosis of T2DM within the past year for which they had received no diabetes medication at recruitment.
A total of 88 individuals were randomized with exactly half undergoing gastric banding. This consisted of a gastric band placed laparoscopically and adjusted every 2 months for the first year, and then every 3 months for the following year depending on symptoms and weight change.
Normoglycemia was observed in none of the study subjects at baseline but in 22% and 15% of those who had gastric banding or metformin, respectively, at 2 years (P = .66).
As for tolerability, five patients who underwent gastric banding experienced serious adverse events, of which two were caused by band slippage and three were caused other reasons. In the metformin arm, there were two serious adverse events, both unrelated to the medication.
“Gastric banding and metformin offered approximately equal approaches for improving insulin sensitivity in adults with mild to moderate obesity and impaired glucose tolerance or early, mild type 2 diabetes,” Dr. Buchanan concluded. “The predominant beta-cell response was a reduction in secretion to maintain a relatively constant compensation for insulin resistance, with only a small improvement in glucose. Whether these interventions will have different effects on beta-cell function over the long-term remains to be determined.”
Commenting on the study, Roy Taylor, MD, professor of medicine and metabolism at Newcastle University (England), noted that the changes in the lipid and liver parameters were important. Fasting plasma triglyceride levels fell from 1.3 mmol/L at baseline to 1.1 mmol/L at 2 years with surgery but stayed more or less the same with metformin (1.23 mmol/L and 1.28 mmol/L; P less than .009 comparing surgery and metformin groups at 2 years). Change in ALT levels were also significant comparing baseline values with results at 2 years, decreasing in the surgical group to a greater extent than in the metformin groups.
“There’s a really important message here, the predictors of a better response to the weight loss [i.e. changes in triglycerides and liver enzymes] are all there,” Dr. Taylor observed. “RISE has looked at 2 years of this effect, but the conversion to type 2 diabetes is probably going to happen over a longer time course.”
He added that “although the primary outcome measure of change in insulin secretion was not achieved, the writing is on the wall. These people, provided they maintain their weight loss, are likely to succeed. We see all the hallmarks of a successful outcome for the weight loss group – remove the primary driver for type 2 diabetes, and that group is on track.”
The RISE Consortium conducted the BetaFat study. The RISE Consortium is supported by grants from the National Institutes for Health. Further support came from the Department of Veterans Affairs, Kaiser Permanente Southern California, the American Diabetes Association, and Allergan. Additional donations of supplies were provided by Allergan, Apollo Endosurgery, Abbott, and Novo Nordisk. Dr. Buchanan reported receiving research funding from Allergan and Apollo Endosurgery. Dr. Taylor had no conflicts of interest.
SOURCES: Buchanan T et al. EASD 2018, Session S09; Xiang AH et al. Diabetes Care. 2018 Oct; dc181662.
BERLIN – Gastric banding surgery and metformin produce similar improvements in insulin sensitivity and parameters indicative of preserved beta-cell function in patients with impaired glucose tolerance (IGT) or newly diagnosed type 2 diabetes mellitus (T2DM), according to the results of a study conducted by the Restoring Insulin Secretion (RISE) Consortium.
“Both interventions resulted in about 50% improvements in insulin sensitivity at 1 year, which was attenuated at 2 years,” reported study investigator Thomas Buchanan, MD, of the University of Southern California, Los Angeles, at the annual meeting of the European Association for the Study of Diabetes.
“The beta-cell responses fell in a pattern that maintained relatively, but not perfectly, stable compensation for insulin resistance,” he added.
Although glucose levels improved “only slightly,” he said, “acute compensation to glucose improved significantly with gastric banding and beta-cell compensation at maximal stimulation fell significantly with metformin.”
Results of the BetaFat (Beta Cell Restoration through Fat Mitigation) study, which are now published online in Diabetes Care, also showed that greater weight loss could be achieved with surgery versus metformin, with a 8.9 kg difference between the groups at 2 years (10.6 vs. 1.7 kg, respectively, P less than .01).
HDL cholesterol levels also rose with both interventions, and gastric banding resulted in a greater effect on very low–density lipoprotein cholesterol and triglycerides, as well as serum ALT, Dr. Buchanan said.
The BetaFat study is one of three “proof-of principle” studies currently being conducted by the RISE Consortium in patients with IGT, sometimes called prediabetes, and T2DM, explained Steven E. Kahn, MB, ChB, the chair for the RISE studies.
The other two multicenter, randomized trials being conducted by the RISE Consortium are looking at the effects of medications on preserving beta-cell function in pediatric/adolescent (10-19 years) and adult (21-65 years) populations with IGT or mild, recently diagnosed T2DM. The design, and some results, of these trials can be viewed on a dedicated section of the Diabetes Care website.
Beta-cell function is being assessed using “state-of-the-art” methods; the coprimary endpoint of the surgery versus metformin study was the steady state C-peptide level and acute C-peptide response at maximal glycemia measured using a hyperglycemic “clamp.”
The goal of the RISE studies is to test different approaches to preserve beta-cell function. It is designed to answer the question of which is more effective in this setting: sustained weight loss through gastric banding such as in the BetaFat study or medication.
Patients were eligible for inclusion in the study if they were aged 21-65 years, had a body mass index of 30-40 kg/m2, and had IGT or a diagnosis of T2DM within the past year for which they had received no diabetes medication at recruitment.
A total of 88 individuals were randomized with exactly half undergoing gastric banding. This consisted of a gastric band placed laparoscopically and adjusted every 2 months for the first year, and then every 3 months for the following year depending on symptoms and weight change.
Normoglycemia was observed in none of the study subjects at baseline but in 22% and 15% of those who had gastric banding or metformin, respectively, at 2 years (P = .66).
As for tolerability, five patients who underwent gastric banding experienced serious adverse events, of which two were caused by band slippage and three were caused other reasons. In the metformin arm, there were two serious adverse events, both unrelated to the medication.
“Gastric banding and metformin offered approximately equal approaches for improving insulin sensitivity in adults with mild to moderate obesity and impaired glucose tolerance or early, mild type 2 diabetes,” Dr. Buchanan concluded. “The predominant beta-cell response was a reduction in secretion to maintain a relatively constant compensation for insulin resistance, with only a small improvement in glucose. Whether these interventions will have different effects on beta-cell function over the long-term remains to be determined.”
Commenting on the study, Roy Taylor, MD, professor of medicine and metabolism at Newcastle University (England), noted that the changes in the lipid and liver parameters were important. Fasting plasma triglyceride levels fell from 1.3 mmol/L at baseline to 1.1 mmol/L at 2 years with surgery but stayed more or less the same with metformin (1.23 mmol/L and 1.28 mmol/L; P less than .009 comparing surgery and metformin groups at 2 years). Change in ALT levels were also significant comparing baseline values with results at 2 years, decreasing in the surgical group to a greater extent than in the metformin groups.
“There’s a really important message here, the predictors of a better response to the weight loss [i.e. changes in triglycerides and liver enzymes] are all there,” Dr. Taylor observed. “RISE has looked at 2 years of this effect, but the conversion to type 2 diabetes is probably going to happen over a longer time course.”
He added that “although the primary outcome measure of change in insulin secretion was not achieved, the writing is on the wall. These people, provided they maintain their weight loss, are likely to succeed. We see all the hallmarks of a successful outcome for the weight loss group – remove the primary driver for type 2 diabetes, and that group is on track.”
The RISE Consortium conducted the BetaFat study. The RISE Consortium is supported by grants from the National Institutes for Health. Further support came from the Department of Veterans Affairs, Kaiser Permanente Southern California, the American Diabetes Association, and Allergan. Additional donations of supplies were provided by Allergan, Apollo Endosurgery, Abbott, and Novo Nordisk. Dr. Buchanan reported receiving research funding from Allergan and Apollo Endosurgery. Dr. Taylor had no conflicts of interest.
SOURCES: Buchanan T et al. EASD 2018, Session S09; Xiang AH et al. Diabetes Care. 2018 Oct; dc181662.
BERLIN – Gastric banding surgery and metformin produce similar improvements in insulin sensitivity and parameters indicative of preserved beta-cell function in patients with impaired glucose tolerance (IGT) or newly diagnosed type 2 diabetes mellitus (T2DM), according to the results of a study conducted by the Restoring Insulin Secretion (RISE) Consortium.
“Both interventions resulted in about 50% improvements in insulin sensitivity at 1 year, which was attenuated at 2 years,” reported study investigator Thomas Buchanan, MD, of the University of Southern California, Los Angeles, at the annual meeting of the European Association for the Study of Diabetes.
“The beta-cell responses fell in a pattern that maintained relatively, but not perfectly, stable compensation for insulin resistance,” he added.
Although glucose levels improved “only slightly,” he said, “acute compensation to glucose improved significantly with gastric banding and beta-cell compensation at maximal stimulation fell significantly with metformin.”
Results of the BetaFat (Beta Cell Restoration through Fat Mitigation) study, which are now published online in Diabetes Care, also showed that greater weight loss could be achieved with surgery versus metformin, with a 8.9 kg difference between the groups at 2 years (10.6 vs. 1.7 kg, respectively, P less than .01).
HDL cholesterol levels also rose with both interventions, and gastric banding resulted in a greater effect on very low–density lipoprotein cholesterol and triglycerides, as well as serum ALT, Dr. Buchanan said.
The BetaFat study is one of three “proof-of principle” studies currently being conducted by the RISE Consortium in patients with IGT, sometimes called prediabetes, and T2DM, explained Steven E. Kahn, MB, ChB, the chair for the RISE studies.
The other two multicenter, randomized trials being conducted by the RISE Consortium are looking at the effects of medications on preserving beta-cell function in pediatric/adolescent (10-19 years) and adult (21-65 years) populations with IGT or mild, recently diagnosed T2DM. The design, and some results, of these trials can be viewed on a dedicated section of the Diabetes Care website.
Beta-cell function is being assessed using “state-of-the-art” methods; the coprimary endpoint of the surgery versus metformin study was the steady state C-peptide level and acute C-peptide response at maximal glycemia measured using a hyperglycemic “clamp.”
The goal of the RISE studies is to test different approaches to preserve beta-cell function. It is designed to answer the question of which is more effective in this setting: sustained weight loss through gastric banding such as in the BetaFat study or medication.
Patients were eligible for inclusion in the study if they were aged 21-65 years, had a body mass index of 30-40 kg/m2, and had IGT or a diagnosis of T2DM within the past year for which they had received no diabetes medication at recruitment.
A total of 88 individuals were randomized with exactly half undergoing gastric banding. This consisted of a gastric band placed laparoscopically and adjusted every 2 months for the first year, and then every 3 months for the following year depending on symptoms and weight change.
Normoglycemia was observed in none of the study subjects at baseline but in 22% and 15% of those who had gastric banding or metformin, respectively, at 2 years (P = .66).
As for tolerability, five patients who underwent gastric banding experienced serious adverse events, of which two were caused by band slippage and three were caused other reasons. In the metformin arm, there were two serious adverse events, both unrelated to the medication.
“Gastric banding and metformin offered approximately equal approaches for improving insulin sensitivity in adults with mild to moderate obesity and impaired glucose tolerance or early, mild type 2 diabetes,” Dr. Buchanan concluded. “The predominant beta-cell response was a reduction in secretion to maintain a relatively constant compensation for insulin resistance, with only a small improvement in glucose. Whether these interventions will have different effects on beta-cell function over the long-term remains to be determined.”
Commenting on the study, Roy Taylor, MD, professor of medicine and metabolism at Newcastle University (England), noted that the changes in the lipid and liver parameters were important. Fasting plasma triglyceride levels fell from 1.3 mmol/L at baseline to 1.1 mmol/L at 2 years with surgery but stayed more or less the same with metformin (1.23 mmol/L and 1.28 mmol/L; P less than .009 comparing surgery and metformin groups at 2 years). Change in ALT levels were also significant comparing baseline values with results at 2 years, decreasing in the surgical group to a greater extent than in the metformin groups.
“There’s a really important message here, the predictors of a better response to the weight loss [i.e. changes in triglycerides and liver enzymes] are all there,” Dr. Taylor observed. “RISE has looked at 2 years of this effect, but the conversion to type 2 diabetes is probably going to happen over a longer time course.”
He added that “although the primary outcome measure of change in insulin secretion was not achieved, the writing is on the wall. These people, provided they maintain their weight loss, are likely to succeed. We see all the hallmarks of a successful outcome for the weight loss group – remove the primary driver for type 2 diabetes, and that group is on track.”
The RISE Consortium conducted the BetaFat study. The RISE Consortium is supported by grants from the National Institutes for Health. Further support came from the Department of Veterans Affairs, Kaiser Permanente Southern California, the American Diabetes Association, and Allergan. Additional donations of supplies were provided by Allergan, Apollo Endosurgery, Abbott, and Novo Nordisk. Dr. Buchanan reported receiving research funding from Allergan and Apollo Endosurgery. Dr. Taylor had no conflicts of interest.
SOURCES: Buchanan T et al. EASD 2018, Session S09; Xiang AH et al. Diabetes Care. 2018 Oct; dc181662.
REPORTING FROM EASD 2018
Key clinical point: Over 2 years, gastric banding surgery and metformin produced similar improvements in insulin sensitivity and parameters suggestive of preserved beta-cell function in patients with prediabetes or early type 2 diabetes.
Major finding: Around a 50% improvement in insulin sensitivity was seen in both study groups at 1 year with attenuation of the effect at 2 years.
Study details: The BetaFat study included 88 obese adults with impaired glucose tolerance or newly diagnosed early type 2 diabetes.
Disclosures: The study was part of the RISE studies, which are supported by grants from the National Institutes for Health. Further support comes from the Department of Veterans Affairs, Kaiser Permanente Southern California, the American Diabetes Association, and Allergan. Additional donations of supplies are provided by Allergan, Apollo Endosurgery, Abbott Laboratories, and Novo Nordisk. Dr. Buchanan reported research funding from Allergan and Apollo Endosurgery. Dr. Taylor had no conflicts of interest.
Sources: Buchanan T et al. EASD 2018, Session S09; Xiang AH et al. Diabetes Care. 2018 Oct; dc181662.
Breast cancer risk in type 2 diabetes related to adiposity
ORLANDO – findings from meta-analyses suggest.
In one meta-analysis of data from 21 prospective studies with a total of nearly 15.2 million women, 325,117 breast cancer cases, and a mean follow-up time of 8 years (nearly 33 million person-years), the risk of breast cancer was significantly greater among patients with diabetes than it was among patients without diabetes (summary relative risk, 1.11), Maria Bota reported at the annual scientific sessions of the American Diabetes Association.
However, there was substantial unexplained heterogeneity of results across the individual studies (I2 = 82%), said Ms. Bota, a faculty member at the International Prevention Research Institute, Lyon, France.
“When the analysis was restricted to the 12 studies that adjusted for [body mass index], the summary relative risk decreased to 1.09 and the heterogeneity also decreased to a moderate value of 32%; when the analysis was restricted to the 9 studies that did not adjust for BMI, the summary relative risk increased to 1.14 again, and the heterogeneity increased even more to 91%,” she said.
In an analysis that combined the results of the nine studies that did not adjust for BMI along with crude relative risks from studies that reported both crude and BMI-adjusted relative risks (17 studies in all), the SRR was 1.12, and heterogeneity among the studies was high at 84%.
Additionally, an analysis by menopausal status based on four studies that reported breast cancer in both pre- and postmenopausal women showed SRRs for breast cancer of 0.97 (a 3% decrease in risk) and 1.14 among diabetic vs. nondiabetic premenopausal women and postmenopausal women, respectively, she said, noting that heterogeneity was low (I2 = 0%) among the premenopausal breast cancer study groups and high (I2 = 70%) among the postmenopausal study groups.
The findings provide evidence for a moderately increased risk of breast cancer in women with T2DM, Ms. Bota said.
“However, the effect of the adjustment or lack of adjustment on the heterogeneity suggests that the higher risk of breast cancer in women with diabetes may not be due to diabetes itself, but to adiposity,” she said, adding that “this hypothesis is equally supported by our subgroup analysis according to menopausal status because we saw that the risk of breast cancer was only associated with diabetes in postmenopausal women and this pattern resembles the pattern of the risk of breast cancer associated with adiposity, which is also only increased in postmenopausal women.”
This study was limited by insufficient data for investigating the sources of heterogeneity, she said.
“Therefore we propose ... future pooled analyses based on individual data from good quality prospective studies in order to increase the study power and to do some detailed analysis of the links between adiposity, diabetes, and breast cancer,” she concluded, adding that new studies to examine those relationships only in premenopausal women are also needed
In a separate meta-analysis, she and her colleagues, including Peter Boyle, PhD, president of the International Prevention Research Institute, assessed the association between insulin treatment and breast cancer risk in patients with diabetes.
“The long-acting insulin analogues glargine and detemir have been shown in some studies to be associated with increased risk of breast cancer, and other studies have shown no association between the use of these two compounds and the risk of breast cancer,” Dr. Boyle said in a separate presentation at the ADA meeting. “It was important to sort out a little bit what was going on in the literature.”
Overall, the meta-analysis of data from 12 longitudinal cohort studies – including more than 6,000 cases of breast cancer – showed a slight increase in breast cancer risk in patients taking long-acting insulin (SRR, 1.13) with “a relatively reasonable” level of heterogeneity (I2 = 23%).
“But we see that the story is not that simple,” he said.
For example, some studies included only patients who were prescribed insulin for the first time after the study began (new users), some included only patients who were prescribed insulin before the study began (prevalent users), and some included both (ever users), which may have introduced bias in the results, he explained.
Studies of glargine included 4,168 breast cancer cases over a total of 1,418,743 person-years of observation, and studies of detemir included fewer than 2,047 breast cancer cases (not all studies reported case numbers). Among both new users of glargine and detemir, the SRR was 1.12, suggesting no real association between either glargine or detemir use and breast cancer, he said.
“One important take-home message is that you have to be careful that these pharmaco-epidemiological studies, even when working with the same database, may have conflicting results ... so we still need more robust standards in methods for [such] studies,” he concluded.
Ms. Bota reported having no disclosures. The study presented by Dr. Boyle was funded by Sanofi. Dr. Boyle is president of a charity that has received donations from Pfizer, Roche, Novartis, and Lilly.
SOURCE: Bota M. ADA 2018, Abstract 180-OR; Boyle P. ADA 2018, Abstract 133-OR.
ORLANDO – findings from meta-analyses suggest.
In one meta-analysis of data from 21 prospective studies with a total of nearly 15.2 million women, 325,117 breast cancer cases, and a mean follow-up time of 8 years (nearly 33 million person-years), the risk of breast cancer was significantly greater among patients with diabetes than it was among patients without diabetes (summary relative risk, 1.11), Maria Bota reported at the annual scientific sessions of the American Diabetes Association.
However, there was substantial unexplained heterogeneity of results across the individual studies (I2 = 82%), said Ms. Bota, a faculty member at the International Prevention Research Institute, Lyon, France.
“When the analysis was restricted to the 12 studies that adjusted for [body mass index], the summary relative risk decreased to 1.09 and the heterogeneity also decreased to a moderate value of 32%; when the analysis was restricted to the 9 studies that did not adjust for BMI, the summary relative risk increased to 1.14 again, and the heterogeneity increased even more to 91%,” she said.
In an analysis that combined the results of the nine studies that did not adjust for BMI along with crude relative risks from studies that reported both crude and BMI-adjusted relative risks (17 studies in all), the SRR was 1.12, and heterogeneity among the studies was high at 84%.
Additionally, an analysis by menopausal status based on four studies that reported breast cancer in both pre- and postmenopausal women showed SRRs for breast cancer of 0.97 (a 3% decrease in risk) and 1.14 among diabetic vs. nondiabetic premenopausal women and postmenopausal women, respectively, she said, noting that heterogeneity was low (I2 = 0%) among the premenopausal breast cancer study groups and high (I2 = 70%) among the postmenopausal study groups.
The findings provide evidence for a moderately increased risk of breast cancer in women with T2DM, Ms. Bota said.
“However, the effect of the adjustment or lack of adjustment on the heterogeneity suggests that the higher risk of breast cancer in women with diabetes may not be due to diabetes itself, but to adiposity,” she said, adding that “this hypothesis is equally supported by our subgroup analysis according to menopausal status because we saw that the risk of breast cancer was only associated with diabetes in postmenopausal women and this pattern resembles the pattern of the risk of breast cancer associated with adiposity, which is also only increased in postmenopausal women.”
This study was limited by insufficient data for investigating the sources of heterogeneity, she said.
“Therefore we propose ... future pooled analyses based on individual data from good quality prospective studies in order to increase the study power and to do some detailed analysis of the links between adiposity, diabetes, and breast cancer,” she concluded, adding that new studies to examine those relationships only in premenopausal women are also needed
In a separate meta-analysis, she and her colleagues, including Peter Boyle, PhD, president of the International Prevention Research Institute, assessed the association between insulin treatment and breast cancer risk in patients with diabetes.
“The long-acting insulin analogues glargine and detemir have been shown in some studies to be associated with increased risk of breast cancer, and other studies have shown no association between the use of these two compounds and the risk of breast cancer,” Dr. Boyle said in a separate presentation at the ADA meeting. “It was important to sort out a little bit what was going on in the literature.”
Overall, the meta-analysis of data from 12 longitudinal cohort studies – including more than 6,000 cases of breast cancer – showed a slight increase in breast cancer risk in patients taking long-acting insulin (SRR, 1.13) with “a relatively reasonable” level of heterogeneity (I2 = 23%).
“But we see that the story is not that simple,” he said.
For example, some studies included only patients who were prescribed insulin for the first time after the study began (new users), some included only patients who were prescribed insulin before the study began (prevalent users), and some included both (ever users), which may have introduced bias in the results, he explained.
Studies of glargine included 4,168 breast cancer cases over a total of 1,418,743 person-years of observation, and studies of detemir included fewer than 2,047 breast cancer cases (not all studies reported case numbers). Among both new users of glargine and detemir, the SRR was 1.12, suggesting no real association between either glargine or detemir use and breast cancer, he said.
“One important take-home message is that you have to be careful that these pharmaco-epidemiological studies, even when working with the same database, may have conflicting results ... so we still need more robust standards in methods for [such] studies,” he concluded.
Ms. Bota reported having no disclosures. The study presented by Dr. Boyle was funded by Sanofi. Dr. Boyle is president of a charity that has received donations from Pfizer, Roche, Novartis, and Lilly.
SOURCE: Bota M. ADA 2018, Abstract 180-OR; Boyle P. ADA 2018, Abstract 133-OR.
ORLANDO – findings from meta-analyses suggest.
In one meta-analysis of data from 21 prospective studies with a total of nearly 15.2 million women, 325,117 breast cancer cases, and a mean follow-up time of 8 years (nearly 33 million person-years), the risk of breast cancer was significantly greater among patients with diabetes than it was among patients without diabetes (summary relative risk, 1.11), Maria Bota reported at the annual scientific sessions of the American Diabetes Association.
However, there was substantial unexplained heterogeneity of results across the individual studies (I2 = 82%), said Ms. Bota, a faculty member at the International Prevention Research Institute, Lyon, France.
“When the analysis was restricted to the 12 studies that adjusted for [body mass index], the summary relative risk decreased to 1.09 and the heterogeneity also decreased to a moderate value of 32%; when the analysis was restricted to the 9 studies that did not adjust for BMI, the summary relative risk increased to 1.14 again, and the heterogeneity increased even more to 91%,” she said.
In an analysis that combined the results of the nine studies that did not adjust for BMI along with crude relative risks from studies that reported both crude and BMI-adjusted relative risks (17 studies in all), the SRR was 1.12, and heterogeneity among the studies was high at 84%.
Additionally, an analysis by menopausal status based on four studies that reported breast cancer in both pre- and postmenopausal women showed SRRs for breast cancer of 0.97 (a 3% decrease in risk) and 1.14 among diabetic vs. nondiabetic premenopausal women and postmenopausal women, respectively, she said, noting that heterogeneity was low (I2 = 0%) among the premenopausal breast cancer study groups and high (I2 = 70%) among the postmenopausal study groups.
The findings provide evidence for a moderately increased risk of breast cancer in women with T2DM, Ms. Bota said.
“However, the effect of the adjustment or lack of adjustment on the heterogeneity suggests that the higher risk of breast cancer in women with diabetes may not be due to diabetes itself, but to adiposity,” she said, adding that “this hypothesis is equally supported by our subgroup analysis according to menopausal status because we saw that the risk of breast cancer was only associated with diabetes in postmenopausal women and this pattern resembles the pattern of the risk of breast cancer associated with adiposity, which is also only increased in postmenopausal women.”
This study was limited by insufficient data for investigating the sources of heterogeneity, she said.
“Therefore we propose ... future pooled analyses based on individual data from good quality prospective studies in order to increase the study power and to do some detailed analysis of the links between adiposity, diabetes, and breast cancer,” she concluded, adding that new studies to examine those relationships only in premenopausal women are also needed
In a separate meta-analysis, she and her colleagues, including Peter Boyle, PhD, president of the International Prevention Research Institute, assessed the association between insulin treatment and breast cancer risk in patients with diabetes.
“The long-acting insulin analogues glargine and detemir have been shown in some studies to be associated with increased risk of breast cancer, and other studies have shown no association between the use of these two compounds and the risk of breast cancer,” Dr. Boyle said in a separate presentation at the ADA meeting. “It was important to sort out a little bit what was going on in the literature.”
Overall, the meta-analysis of data from 12 longitudinal cohort studies – including more than 6,000 cases of breast cancer – showed a slight increase in breast cancer risk in patients taking long-acting insulin (SRR, 1.13) with “a relatively reasonable” level of heterogeneity (I2 = 23%).
“But we see that the story is not that simple,” he said.
For example, some studies included only patients who were prescribed insulin for the first time after the study began (new users), some included only patients who were prescribed insulin before the study began (prevalent users), and some included both (ever users), which may have introduced bias in the results, he explained.
Studies of glargine included 4,168 breast cancer cases over a total of 1,418,743 person-years of observation, and studies of detemir included fewer than 2,047 breast cancer cases (not all studies reported case numbers). Among both new users of glargine and detemir, the SRR was 1.12, suggesting no real association between either glargine or detemir use and breast cancer, he said.
“One important take-home message is that you have to be careful that these pharmaco-epidemiological studies, even when working with the same database, may have conflicting results ... so we still need more robust standards in methods for [such] studies,” he concluded.
Ms. Bota reported having no disclosures. The study presented by Dr. Boyle was funded by Sanofi. Dr. Boyle is president of a charity that has received donations from Pfizer, Roche, Novartis, and Lilly.
SOURCE: Bota M. ADA 2018, Abstract 180-OR; Boyle P. ADA 2018, Abstract 133-OR.
REPORTING FROM ADA 2018
Key clinical point: Adiposity accounts for the increased risk of breast cancer among women with diabetes.
Major finding: An analysis of 12 studies that adjusted for BMI showed a summary relative risk for breast cancer of 1.09 in diabetic versus nondiabetic women, with moderate study heterogeneity.
Study details: Meta-analyses including 21 and 12 studies, respectively.
Disclosures: Ms. Bota reported having no disclosures. The study presented by Dr. Boyle was funded by Sanofi. Dr. Boyle is president of a charity that has received donations from Pfizer, Roche, Novartis, and Lilly.
Source: Bota M. ADA 2018, Abstract 180-OR; Boyle P. ADA 2018, Abstract 133-OR.
GBS in T2DM patients: Study highlights pros and cons, need for better patient selection
ORLANDO – , according to a nationwide, matched, observational cohort study in Sweden.
After 9 years of follow-up, all-cause mortality was 49% lower among 5,321 patients with T2DM compared with 5,321 matched control (183 vs. 351 deaths; hazard ratio, 0.51), as has been reported in prior studies, Vasileios Liakopoulos, MD, of the University of Gothenburg (Sweden) reported at the annual scientific sessions of the American Diabetes Association.
Cardiovascular disease (CVD) risk was 34% lower (108 vs. 150 patients; HR, 0.66), fatal CVD risk was 66% lower (21 vs. 64 patients; HR, 0.34), acute myocardial infarction risk was 45% lower (51 vs. 85 events; HR, 0.55) congestive heart failure risk was 51% lower (109 vs. 225 events; HR, 0.49), and cancer risk was 22% lower (153 vs. 188 cases; HR, 0.78) in cases vs. controls, respectively.
“[As for] the diagnoses that related to diabetes, hyperglycemia was lower by 66%, admission to the hospital due to amputation was 49% lower, and we also found something relatively new – that renal disease was lower by 42%,” Dr. Liakopoulos said.
Renal disease occurred in 105 cases vs. 187 controls (HR, 0.58), with the difference between the groups intensifying after the third year of follow-up, he noted.
However, numerous adverse events occurred more often in case patients, he said.
For example, hospitalizations for psychiatric disorders were increased by 33% (317 vs. 268; HR, 1.33), alcohol-related diagnoses were nearly threefold higher (180 vs. 65; HR, 2.90), malnutrition occurred nearly three times more often (128 vs. 46 patients; HR, 2.81), and anemia occurred nearly twice as often (84 vs. 46 cases; HR, 1.92) in cases vs. controls.
Of course, all the surgery-related adverse events occurred more often in the case patients, but interestingly, those events – which included things like gastrointestinal surgery other than gastric bypass, abdominal pain, gallstones/pancreatitis, gastrointestinal ulcers and reflux, and bowel obstruction – did not occur more often in case patients than in gastric bypass patients without diabetes in other studies, he noted.
The findings were based on merged data from the Scandinavian Obesity Surgery Registry, the Swedish National Diabetes Register, and other national databases, and persons with T2DM who had undergone gastric bypass surgery between 2007 and 2013 were matched by propensity score (based on sex, age, body mass index, and calendar time from the beginning of the study) with obese individuals who were not surgically treated for obesity. The risks of postoperative outcomes were assessed using a Cox regression model adjusted for sex, age, body mass index, and socioeconomic status, Dr. Liakopoulos said.
This study, though limited by its observational nature, minor differences in patient characteristics between the cases and controls, and potential residual confounding, confirms the benefits of gastric bypass surgery in obese patients with T2DM but also characterizes an array of both short- and long-term adverse events after bariatric surgery in these patients, he said.
“The beneficial effects of gastric bypass have been presented in terms of weight reduction, improvements in risk factors and cardiovascular disease, and mortality reduction in people with or without diabetes,” he said, noting, however, that only a few reports have addressed long-term incidence of complications after gastric bypass – and type 2 diabetes has only been addressed in small randomized studies or in low proportions in large prospective studies.
“[Based on the findings] we suggest better selection of patients for bariatric surgery, and we think improved long-term postoperative monitoring might further improve the results of such treatment,” he concluded.
Dr. Liakopoulos reported having no disclosures.
SOURCE: Liakopoulos V et al. ADA 2018, Abstract 131-OR.
ORLANDO – , according to a nationwide, matched, observational cohort study in Sweden.
After 9 years of follow-up, all-cause mortality was 49% lower among 5,321 patients with T2DM compared with 5,321 matched control (183 vs. 351 deaths; hazard ratio, 0.51), as has been reported in prior studies, Vasileios Liakopoulos, MD, of the University of Gothenburg (Sweden) reported at the annual scientific sessions of the American Diabetes Association.
Cardiovascular disease (CVD) risk was 34% lower (108 vs. 150 patients; HR, 0.66), fatal CVD risk was 66% lower (21 vs. 64 patients; HR, 0.34), acute myocardial infarction risk was 45% lower (51 vs. 85 events; HR, 0.55) congestive heart failure risk was 51% lower (109 vs. 225 events; HR, 0.49), and cancer risk was 22% lower (153 vs. 188 cases; HR, 0.78) in cases vs. controls, respectively.
“[As for] the diagnoses that related to diabetes, hyperglycemia was lower by 66%, admission to the hospital due to amputation was 49% lower, and we also found something relatively new – that renal disease was lower by 42%,” Dr. Liakopoulos said.
Renal disease occurred in 105 cases vs. 187 controls (HR, 0.58), with the difference between the groups intensifying after the third year of follow-up, he noted.
However, numerous adverse events occurred more often in case patients, he said.
For example, hospitalizations for psychiatric disorders were increased by 33% (317 vs. 268; HR, 1.33), alcohol-related diagnoses were nearly threefold higher (180 vs. 65; HR, 2.90), malnutrition occurred nearly three times more often (128 vs. 46 patients; HR, 2.81), and anemia occurred nearly twice as often (84 vs. 46 cases; HR, 1.92) in cases vs. controls.
Of course, all the surgery-related adverse events occurred more often in the case patients, but interestingly, those events – which included things like gastrointestinal surgery other than gastric bypass, abdominal pain, gallstones/pancreatitis, gastrointestinal ulcers and reflux, and bowel obstruction – did not occur more often in case patients than in gastric bypass patients without diabetes in other studies, he noted.
The findings were based on merged data from the Scandinavian Obesity Surgery Registry, the Swedish National Diabetes Register, and other national databases, and persons with T2DM who had undergone gastric bypass surgery between 2007 and 2013 were matched by propensity score (based on sex, age, body mass index, and calendar time from the beginning of the study) with obese individuals who were not surgically treated for obesity. The risks of postoperative outcomes were assessed using a Cox regression model adjusted for sex, age, body mass index, and socioeconomic status, Dr. Liakopoulos said.
This study, though limited by its observational nature, minor differences in patient characteristics between the cases and controls, and potential residual confounding, confirms the benefits of gastric bypass surgery in obese patients with T2DM but also characterizes an array of both short- and long-term adverse events after bariatric surgery in these patients, he said.
“The beneficial effects of gastric bypass have been presented in terms of weight reduction, improvements in risk factors and cardiovascular disease, and mortality reduction in people with or without diabetes,” he said, noting, however, that only a few reports have addressed long-term incidence of complications after gastric bypass – and type 2 diabetes has only been addressed in small randomized studies or in low proportions in large prospective studies.
“[Based on the findings] we suggest better selection of patients for bariatric surgery, and we think improved long-term postoperative monitoring might further improve the results of such treatment,” he concluded.
Dr. Liakopoulos reported having no disclosures.
SOURCE: Liakopoulos V et al. ADA 2018, Abstract 131-OR.
ORLANDO – , according to a nationwide, matched, observational cohort study in Sweden.
After 9 years of follow-up, all-cause mortality was 49% lower among 5,321 patients with T2DM compared with 5,321 matched control (183 vs. 351 deaths; hazard ratio, 0.51), as has been reported in prior studies, Vasileios Liakopoulos, MD, of the University of Gothenburg (Sweden) reported at the annual scientific sessions of the American Diabetes Association.
Cardiovascular disease (CVD) risk was 34% lower (108 vs. 150 patients; HR, 0.66), fatal CVD risk was 66% lower (21 vs. 64 patients; HR, 0.34), acute myocardial infarction risk was 45% lower (51 vs. 85 events; HR, 0.55) congestive heart failure risk was 51% lower (109 vs. 225 events; HR, 0.49), and cancer risk was 22% lower (153 vs. 188 cases; HR, 0.78) in cases vs. controls, respectively.
“[As for] the diagnoses that related to diabetes, hyperglycemia was lower by 66%, admission to the hospital due to amputation was 49% lower, and we also found something relatively new – that renal disease was lower by 42%,” Dr. Liakopoulos said.
Renal disease occurred in 105 cases vs. 187 controls (HR, 0.58), with the difference between the groups intensifying after the third year of follow-up, he noted.
However, numerous adverse events occurred more often in case patients, he said.
For example, hospitalizations for psychiatric disorders were increased by 33% (317 vs. 268; HR, 1.33), alcohol-related diagnoses were nearly threefold higher (180 vs. 65; HR, 2.90), malnutrition occurred nearly three times more often (128 vs. 46 patients; HR, 2.81), and anemia occurred nearly twice as often (84 vs. 46 cases; HR, 1.92) in cases vs. controls.
Of course, all the surgery-related adverse events occurred more often in the case patients, but interestingly, those events – which included things like gastrointestinal surgery other than gastric bypass, abdominal pain, gallstones/pancreatitis, gastrointestinal ulcers and reflux, and bowel obstruction – did not occur more often in case patients than in gastric bypass patients without diabetes in other studies, he noted.
The findings were based on merged data from the Scandinavian Obesity Surgery Registry, the Swedish National Diabetes Register, and other national databases, and persons with T2DM who had undergone gastric bypass surgery between 2007 and 2013 were matched by propensity score (based on sex, age, body mass index, and calendar time from the beginning of the study) with obese individuals who were not surgically treated for obesity. The risks of postoperative outcomes were assessed using a Cox regression model adjusted for sex, age, body mass index, and socioeconomic status, Dr. Liakopoulos said.
This study, though limited by its observational nature, minor differences in patient characteristics between the cases and controls, and potential residual confounding, confirms the benefits of gastric bypass surgery in obese patients with T2DM but also characterizes an array of both short- and long-term adverse events after bariatric surgery in these patients, he said.
“The beneficial effects of gastric bypass have been presented in terms of weight reduction, improvements in risk factors and cardiovascular disease, and mortality reduction in people with or without diabetes,” he said, noting, however, that only a few reports have addressed long-term incidence of complications after gastric bypass – and type 2 diabetes has only been addressed in small randomized studies or in low proportions in large prospective studies.
“[Based on the findings] we suggest better selection of patients for bariatric surgery, and we think improved long-term postoperative monitoring might further improve the results of such treatment,” he concluded.
Dr. Liakopoulos reported having no disclosures.
SOURCE: Liakopoulos V et al. ADA 2018, Abstract 131-OR.
REPORTING FROM ADA 2018
Key clinical point: Bariatric surgery lowers mortality, CVD, and renal and other risks in obese T2DM patients but also has high complication rates.
Major finding: All-cause mortality, CVD, and renal disease risks were 49%, 34%, and 42% lower, respectively, in cases vs. controls.
Study details: A matched observational cohort study of 5,321 cases and 5,321 controls.
Disclosures: Dr. Liakopoulos reported having no disclosures.
Source: Liakopoulos V et al. ADA 2018, Abstract 131-OR.
Task force advises behavioral intervention for obese adults
The U.S. Preventive Services Task Force advises clinicians to refer or offer intensive behavioral weight-loss interventions to obese adults, according to an updated recommendation statement published in JAMA.
Obesity affects more than one-third of U.S. adults, according to federal statistics. It carries increased risk for comorbidities including heart disease, diabetes, and various cancers, as well as increased risk of death among adults younger than 65 years, noted lead author Susan J. Curry, PhD, of the University of Iowa, Iowa City, and members of the Task Force.
The B recommendation applies to obese adults; obesity was defined as a body mass index of 30 kg/m2 or higher. The evidence review focused on interventions for weight loss and weight maintenance that could be provided in primary care or referred from primary care, such as nutrition counseling, exercise strategies, and goal setting.
The Task Force found adequate evidence that behavior-based weight-loss interventions improved weight, reduced incidence of type 2 diabetes, and helped maintain weight loss after interventions ended.
The Task Force found small to no evidence of harm associated with any of the behavioral weight-loss interventions, which included group sessions, personal sessions, print-based interventions, and technology-based interventions (such as text messages). Although interventions that combined drug therapy with behavioral intervention resulted in greater weight loss over 12-18 months, compared with behavioral interventions alone, the attrition rates were high and data on weight-loss maintenance after discontinuation of the drugs were limited, the Task Force noted.
“As a result, the USPSTF encourages clinicians to promote behavioral interventions as the primary focus of effective interventions for weight loss in adults,” they wrote.
The Task Force acknowledged the need for future research in subgroups and to explore whether factors such as genetics and untreated conditions are barriers to behavior-based weight loss interventions.
In the evidence review published in JAMA, Erin S. LeBlanc, MD, of Kaiser Permanente in Portland, Ore., and her colleagues reviewed data from 122 randomized, controlled trials including more than 62,000 persons and 2 observational studies including more than 209,000 persons.
The researchers found behavioral interventions were associated with greater weight loss and less risk of developing diabetes, compared with control interventions.
Intensive behavioral interventions included counseling patients about healthy eating, encouraging physical activity, setting weight and health goals, and assisting with weight monitoring. The interventions ranged from text messaging to in-person sessions for individuals or groups. The average absolute weight loss in the trials included in the review ranged from –0.5 kg to –9.3 kg (–1.1 lb to –20.5 lb) for intervention patients and from +1.4 kg to –5.6 kg (+3.1 lb to –12.3 lb) in controls.
Limitations of the review included a lack of data on population subgroups and a lack of long-term data on weight and health outcomes, the researchers noted. However, the results support the value of behavior-based therapy for obesity treatment.
The final recommendation is consistent with the 2018 draft recommendation and updates the 2012 final recommendation on obesity management.
The researchers and Task Force members had no relevant financial conflicts to disclose.
SOURCE: U.S. Preventive Services Task Force. JAMA. 2018;320(11):1163-71. doi: 10.1001/jama.2018.13022.
For most primary care clinicians, referring obese patients for more advanced behavioral therapy will be the most practical integration of the recommendation, Susan Z. Yanovski, MD, wrote in an accompanying editorial. Clinicians with training in motivational interviewing or counseling may help assess a patient’s readiness for treatment, but even being familiar with weight-management resources in the community can help patients find the right fit.
“Clinicians can do their patients a great service by showing respect for their patients’ struggles with weight management, screening for obesity-related comorbidities, and providing treatment for identified conditions regardless of the patient’s motivation for, or success with, weight-loss treatment,” she said.
Dr. Yanovski noted that pharmacotherapy options have increased since the 2012 recommendations, when orlistat was the only approved drug for long-term treatment of obesity. Five medications are currently available for this indication.
The USPSTF review was limited in scope for both drug and behavior therapy, noted Dr. Yanovski. “Because the recommendations are meant to apply to adults without diseases for which weight loss is part of disease management, some large and long-term clinical trials conducted among patients with type 2 diabetes or cardiovascular disease were not included.”
Another limitation was the exclusion of surgical treatments as being outside the primary care setting, but bariatric surgery remains a viable option for many patients, especially for prevention or resolution of type 2 diabetes. Primary care clinicians are in a position to identify patients who might benefit and to provide referrals to surgeons if appropriate, she wrote.
Dr. Yanovski agreed with the recommendations but concluded that early strategies to prevent obesity should not be neglected. “Research to develop effective prevention strategies throughout the life course, including infancy and early childhood, could ultimately decrease the number of adults who must confront the difficult challenge of losing excess weight.”
Dr. Yanovski is affiliated with the National Institute of Diabetes and Digestive and Kidney Diseases. She disclosed that her spouse has received research funding from Zafgen and Rhythm Pharmaceuticals for studies of investigational products to treat obesity. Her comments are summarized from an editorial accompanying the articles by Curry SJ et al. and LeBlanc ES et al. (JAMA. 2018;320[11]:1111-3).
For most primary care clinicians, referring obese patients for more advanced behavioral therapy will be the most practical integration of the recommendation, Susan Z. Yanovski, MD, wrote in an accompanying editorial. Clinicians with training in motivational interviewing or counseling may help assess a patient’s readiness for treatment, but even being familiar with weight-management resources in the community can help patients find the right fit.
“Clinicians can do their patients a great service by showing respect for their patients’ struggles with weight management, screening for obesity-related comorbidities, and providing treatment for identified conditions regardless of the patient’s motivation for, or success with, weight-loss treatment,” she said.
Dr. Yanovski noted that pharmacotherapy options have increased since the 2012 recommendations, when orlistat was the only approved drug for long-term treatment of obesity. Five medications are currently available for this indication.
The USPSTF review was limited in scope for both drug and behavior therapy, noted Dr. Yanovski. “Because the recommendations are meant to apply to adults without diseases for which weight loss is part of disease management, some large and long-term clinical trials conducted among patients with type 2 diabetes or cardiovascular disease were not included.”
Another limitation was the exclusion of surgical treatments as being outside the primary care setting, but bariatric surgery remains a viable option for many patients, especially for prevention or resolution of type 2 diabetes. Primary care clinicians are in a position to identify patients who might benefit and to provide referrals to surgeons if appropriate, she wrote.
Dr. Yanovski agreed with the recommendations but concluded that early strategies to prevent obesity should not be neglected. “Research to develop effective prevention strategies throughout the life course, including infancy and early childhood, could ultimately decrease the number of adults who must confront the difficult challenge of losing excess weight.”
Dr. Yanovski is affiliated with the National Institute of Diabetes and Digestive and Kidney Diseases. She disclosed that her spouse has received research funding from Zafgen and Rhythm Pharmaceuticals for studies of investigational products to treat obesity. Her comments are summarized from an editorial accompanying the articles by Curry SJ et al. and LeBlanc ES et al. (JAMA. 2018;320[11]:1111-3).
For most primary care clinicians, referring obese patients for more advanced behavioral therapy will be the most practical integration of the recommendation, Susan Z. Yanovski, MD, wrote in an accompanying editorial. Clinicians with training in motivational interviewing or counseling may help assess a patient’s readiness for treatment, but even being familiar with weight-management resources in the community can help patients find the right fit.
“Clinicians can do their patients a great service by showing respect for their patients’ struggles with weight management, screening for obesity-related comorbidities, and providing treatment for identified conditions regardless of the patient’s motivation for, or success with, weight-loss treatment,” she said.
Dr. Yanovski noted that pharmacotherapy options have increased since the 2012 recommendations, when orlistat was the only approved drug for long-term treatment of obesity. Five medications are currently available for this indication.
The USPSTF review was limited in scope for both drug and behavior therapy, noted Dr. Yanovski. “Because the recommendations are meant to apply to adults without diseases for which weight loss is part of disease management, some large and long-term clinical trials conducted among patients with type 2 diabetes or cardiovascular disease were not included.”
Another limitation was the exclusion of surgical treatments as being outside the primary care setting, but bariatric surgery remains a viable option for many patients, especially for prevention or resolution of type 2 diabetes. Primary care clinicians are in a position to identify patients who might benefit and to provide referrals to surgeons if appropriate, she wrote.
Dr. Yanovski agreed with the recommendations but concluded that early strategies to prevent obesity should not be neglected. “Research to develop effective prevention strategies throughout the life course, including infancy and early childhood, could ultimately decrease the number of adults who must confront the difficult challenge of losing excess weight.”
Dr. Yanovski is affiliated with the National Institute of Diabetes and Digestive and Kidney Diseases. She disclosed that her spouse has received research funding from Zafgen and Rhythm Pharmaceuticals for studies of investigational products to treat obesity. Her comments are summarized from an editorial accompanying the articles by Curry SJ et al. and LeBlanc ES et al. (JAMA. 2018;320[11]:1111-3).
The U.S. Preventive Services Task Force advises clinicians to refer or offer intensive behavioral weight-loss interventions to obese adults, according to an updated recommendation statement published in JAMA.
Obesity affects more than one-third of U.S. adults, according to federal statistics. It carries increased risk for comorbidities including heart disease, diabetes, and various cancers, as well as increased risk of death among adults younger than 65 years, noted lead author Susan J. Curry, PhD, of the University of Iowa, Iowa City, and members of the Task Force.
The B recommendation applies to obese adults; obesity was defined as a body mass index of 30 kg/m2 or higher. The evidence review focused on interventions for weight loss and weight maintenance that could be provided in primary care or referred from primary care, such as nutrition counseling, exercise strategies, and goal setting.
The Task Force found adequate evidence that behavior-based weight-loss interventions improved weight, reduced incidence of type 2 diabetes, and helped maintain weight loss after interventions ended.
The Task Force found small to no evidence of harm associated with any of the behavioral weight-loss interventions, which included group sessions, personal sessions, print-based interventions, and technology-based interventions (such as text messages). Although interventions that combined drug therapy with behavioral intervention resulted in greater weight loss over 12-18 months, compared with behavioral interventions alone, the attrition rates were high and data on weight-loss maintenance after discontinuation of the drugs were limited, the Task Force noted.
“As a result, the USPSTF encourages clinicians to promote behavioral interventions as the primary focus of effective interventions for weight loss in adults,” they wrote.
The Task Force acknowledged the need for future research in subgroups and to explore whether factors such as genetics and untreated conditions are barriers to behavior-based weight loss interventions.
In the evidence review published in JAMA, Erin S. LeBlanc, MD, of Kaiser Permanente in Portland, Ore., and her colleagues reviewed data from 122 randomized, controlled trials including more than 62,000 persons and 2 observational studies including more than 209,000 persons.
The researchers found behavioral interventions were associated with greater weight loss and less risk of developing diabetes, compared with control interventions.
Intensive behavioral interventions included counseling patients about healthy eating, encouraging physical activity, setting weight and health goals, and assisting with weight monitoring. The interventions ranged from text messaging to in-person sessions for individuals or groups. The average absolute weight loss in the trials included in the review ranged from –0.5 kg to –9.3 kg (–1.1 lb to –20.5 lb) for intervention patients and from +1.4 kg to –5.6 kg (+3.1 lb to –12.3 lb) in controls.
Limitations of the review included a lack of data on population subgroups and a lack of long-term data on weight and health outcomes, the researchers noted. However, the results support the value of behavior-based therapy for obesity treatment.
The final recommendation is consistent with the 2018 draft recommendation and updates the 2012 final recommendation on obesity management.
The researchers and Task Force members had no relevant financial conflicts to disclose.
SOURCE: U.S. Preventive Services Task Force. JAMA. 2018;320(11):1163-71. doi: 10.1001/jama.2018.13022.
The U.S. Preventive Services Task Force advises clinicians to refer or offer intensive behavioral weight-loss interventions to obese adults, according to an updated recommendation statement published in JAMA.
Obesity affects more than one-third of U.S. adults, according to federal statistics. It carries increased risk for comorbidities including heart disease, diabetes, and various cancers, as well as increased risk of death among adults younger than 65 years, noted lead author Susan J. Curry, PhD, of the University of Iowa, Iowa City, and members of the Task Force.
The B recommendation applies to obese adults; obesity was defined as a body mass index of 30 kg/m2 or higher. The evidence review focused on interventions for weight loss and weight maintenance that could be provided in primary care or referred from primary care, such as nutrition counseling, exercise strategies, and goal setting.
The Task Force found adequate evidence that behavior-based weight-loss interventions improved weight, reduced incidence of type 2 diabetes, and helped maintain weight loss after interventions ended.
The Task Force found small to no evidence of harm associated with any of the behavioral weight-loss interventions, which included group sessions, personal sessions, print-based interventions, and technology-based interventions (such as text messages). Although interventions that combined drug therapy with behavioral intervention resulted in greater weight loss over 12-18 months, compared with behavioral interventions alone, the attrition rates were high and data on weight-loss maintenance after discontinuation of the drugs were limited, the Task Force noted.
“As a result, the USPSTF encourages clinicians to promote behavioral interventions as the primary focus of effective interventions for weight loss in adults,” they wrote.
The Task Force acknowledged the need for future research in subgroups and to explore whether factors such as genetics and untreated conditions are barriers to behavior-based weight loss interventions.
In the evidence review published in JAMA, Erin S. LeBlanc, MD, of Kaiser Permanente in Portland, Ore., and her colleagues reviewed data from 122 randomized, controlled trials including more than 62,000 persons and 2 observational studies including more than 209,000 persons.
The researchers found behavioral interventions were associated with greater weight loss and less risk of developing diabetes, compared with control interventions.
Intensive behavioral interventions included counseling patients about healthy eating, encouraging physical activity, setting weight and health goals, and assisting with weight monitoring. The interventions ranged from text messaging to in-person sessions for individuals or groups. The average absolute weight loss in the trials included in the review ranged from –0.5 kg to –9.3 kg (–1.1 lb to –20.5 lb) for intervention patients and from +1.4 kg to –5.6 kg (+3.1 lb to –12.3 lb) in controls.
Limitations of the review included a lack of data on population subgroups and a lack of long-term data on weight and health outcomes, the researchers noted. However, the results support the value of behavior-based therapy for obesity treatment.
The final recommendation is consistent with the 2018 draft recommendation and updates the 2012 final recommendation on obesity management.
The researchers and Task Force members had no relevant financial conflicts to disclose.
SOURCE: U.S. Preventive Services Task Force. JAMA. 2018;320(11):1163-71. doi: 10.1001/jama.2018.13022.
FROM JAMA
One-step gestational diabetes screening doesn’t improve outcomes
according to data from a before-and-after cohort study of women in the state of Washington.
The one-step test, a 75-g 2-hour oral glucose tolerance test (OGTT), was recommended for all pregnant women in 2010, although the traditional two-step test – a 50-g screening glucose challenge test followed by a 100-g 3-hour OGTT – remains widely used, wrote Gaia Pocobelli, PhD, of Kaiser Permanente Washington Health Research Institute, Seattle, and her colleagues. “No randomized trial has been published comparing outcomes of the two approaches.”
In a study published in Obstetrics & Gynecology, the researchers compared data from 23,257 women who received prenatal care in Washington State between January 2009 and December 2014, including 8,363 women who received care before the guideline change, 4,103 who received care during a transition period, and 10,791 after the guideline change. Approximately 60% of the women received care from clinicians internal to Kaiser Permanente; 40% received care from external providers. Most (87%) of the internal clinicians switched to the one-step approach, the researchers said. Only 5% of external providers did so.
Overall, adopting the one-step approach was associated with a 41% increase in the diagnosis of GDM without improved maternal or neonatal outcomes, the researchers noted.
The incidence of GDM increased from 7% before the guideline change to 11% afterward for women seen by internal providers. For women seen by external providers, gestational diabetes incidence increased from 10% to 11%.
For women seen by internal providers, the use of insulin increased from 1% before the guideline change to 4% afterward; for women seen by external providers, use of insulin increased from 1.3% to 1.4% (change between the groups P less than .001).
In addition, women seen by internal providers were more likely to undergo induction of labor after the guideline change (25% to 29%), while labor induction decreased for women seen by external providers (31% to 29%) for a relative risk of 1.2.
Neonatal hypoglycemia increased from 1% to 2% among women seen by internal providers, but decreased slightly from 2.4% to 2.1% for women seen by external providers, for a relative risk of 1.77.
There were no significant differences between the women seen by internal and external providers in risk of primary cesarean section, large for gestational age, small for gestational age, or neonatal ICU admission.
The main limitation of the study was the potential confounding variables including maternal diet and exercise, and possible underreporting of risk factors such as smoking, the researchers noted. However, the results were strengthened by the large study population, and the results “do not suggest a benefit of adopting the one-step over the two-step approach.
“Kaiser Permanente Washington has revised [its] guidelines to return to a two-step process. We recommend that any health care system considering switching to the one-step approach incorporate a rigorous evaluation of changes in maternal and neonatal outcomes,” Dr. Pocobelli and her associates added.
Dr. Pocobelli disclosed funding from Jazz Pharmaceuticals for work unrelated to this study. The study was supported in part by a grant from the Group Health Foundation Momentum Fund.
Diabetes is a significant global public health concern, but is especially problematic for women of reproductive age because diabetes in pregnancy can cause significant health complications for the mother and baby. Gestational diabetes mellitus (GDM) affects up to 10% of pregnancies in the United States annually, and is associated with perinatal loss, operative delivery, macrosomia, hypoglycemia, respiratory distress syndrome, and metabolic derangements for the offspring. For the mother, GDM is associated with hypertensive disorders, infections, hydramnios, and increased risk for developing type 2 diabetes later in life. As the incidence of GDM continues to rise, studies examining how to reduce, manage or prevent this condition become increasingly important.
The authors’ conclusions, that adopting the one-step approach increased the number of women with diagnosed GDM but did not significantly improve maternal or neonatal outcomes, are not surprising. Since the initial publication of the Hyperglycemia and Adverse Pregnancy Outcome Study, upon which the International Association of the Diabetes in Pregnancy Study Groups based its recommendations to go to a one-step approach, much debate has ensued about the best method to diagnose GDM. Indeed, the National Institutes of Health convened a consensus panel to review the literature and determine whether the one-step approach should be universally adopted (the panel concluded that more information was needed, and that the current two-step approach should continue to be used).
As the authors concede, studies have shown conflicting results, and no large-scale randomized controlled trial has been conducted to date. However, the literature does not bear out the idea that the one-step approach is truly better. The current study, although including a significant number of women and a reasonable control group, only serves as yet another study to reinforce what has previously been published.
I would agree with the researchers’ conclusions that the one-step approach is not necessarily beneficial. Although the one-step approach may identify a subset of patients who might not otherwise be diagnosed with GDM, it still remains unclear whether the outcomes for these patients will be improved. Furthermore, additional testing, need for insulin or other oral antidiabetic medications, etc., would result in additional stress to the patient and the health care system. Based on the authors’ findings, and results of other studies, it remains to be determined if the effort (diagnosing additional patients with GDM) is justified medically, economically, or otherwise.
As ob.gyns., we must continually ask ourselves: “By not doing something, are we causing harm to our patients?” If we change the diagnostic criteria for GDM, thereby increasing the number of women with the condition who would then require additional care, medications, and, potentially, more complex decisions around timing and mode of delivery, we need to be certain that we are not doing harm. This, and other studies examining the use of the one- versus two-step approach have yet to demonstrate, unequivocally, that changing the criteria reduces harm, and, perhaps, might – unintentionally – cause more.
As the study authors and the NIH consensus panel concluded, more rigorous evaluation is needed; that is, a large, multicenter randomized controlled trial that examines not only the benefits during pregnancy but also the long-term benefits to women and their children.
E. Albert Reece, MD, PhD, MBA, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He provided commentary on the study by Pocobelli et al. Dr. Reece said he had no relevant financial disclosures.
Diabetes is a significant global public health concern, but is especially problematic for women of reproductive age because diabetes in pregnancy can cause significant health complications for the mother and baby. Gestational diabetes mellitus (GDM) affects up to 10% of pregnancies in the United States annually, and is associated with perinatal loss, operative delivery, macrosomia, hypoglycemia, respiratory distress syndrome, and metabolic derangements for the offspring. For the mother, GDM is associated with hypertensive disorders, infections, hydramnios, and increased risk for developing type 2 diabetes later in life. As the incidence of GDM continues to rise, studies examining how to reduce, manage or prevent this condition become increasingly important.
The authors’ conclusions, that adopting the one-step approach increased the number of women with diagnosed GDM but did not significantly improve maternal or neonatal outcomes, are not surprising. Since the initial publication of the Hyperglycemia and Adverse Pregnancy Outcome Study, upon which the International Association of the Diabetes in Pregnancy Study Groups based its recommendations to go to a one-step approach, much debate has ensued about the best method to diagnose GDM. Indeed, the National Institutes of Health convened a consensus panel to review the literature and determine whether the one-step approach should be universally adopted (the panel concluded that more information was needed, and that the current two-step approach should continue to be used).
As the authors concede, studies have shown conflicting results, and no large-scale randomized controlled trial has been conducted to date. However, the literature does not bear out the idea that the one-step approach is truly better. The current study, although including a significant number of women and a reasonable control group, only serves as yet another study to reinforce what has previously been published.
I would agree with the researchers’ conclusions that the one-step approach is not necessarily beneficial. Although the one-step approach may identify a subset of patients who might not otherwise be diagnosed with GDM, it still remains unclear whether the outcomes for these patients will be improved. Furthermore, additional testing, need for insulin or other oral antidiabetic medications, etc., would result in additional stress to the patient and the health care system. Based on the authors’ findings, and results of other studies, it remains to be determined if the effort (diagnosing additional patients with GDM) is justified medically, economically, or otherwise.
As ob.gyns., we must continually ask ourselves: “By not doing something, are we causing harm to our patients?” If we change the diagnostic criteria for GDM, thereby increasing the number of women with the condition who would then require additional care, medications, and, potentially, more complex decisions around timing and mode of delivery, we need to be certain that we are not doing harm. This, and other studies examining the use of the one- versus two-step approach have yet to demonstrate, unequivocally, that changing the criteria reduces harm, and, perhaps, might – unintentionally – cause more.
As the study authors and the NIH consensus panel concluded, more rigorous evaluation is needed; that is, a large, multicenter randomized controlled trial that examines not only the benefits during pregnancy but also the long-term benefits to women and their children.
E. Albert Reece, MD, PhD, MBA, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He provided commentary on the study by Pocobelli et al. Dr. Reece said he had no relevant financial disclosures.
Diabetes is a significant global public health concern, but is especially problematic for women of reproductive age because diabetes in pregnancy can cause significant health complications for the mother and baby. Gestational diabetes mellitus (GDM) affects up to 10% of pregnancies in the United States annually, and is associated with perinatal loss, operative delivery, macrosomia, hypoglycemia, respiratory distress syndrome, and metabolic derangements for the offspring. For the mother, GDM is associated with hypertensive disorders, infections, hydramnios, and increased risk for developing type 2 diabetes later in life. As the incidence of GDM continues to rise, studies examining how to reduce, manage or prevent this condition become increasingly important.
The authors’ conclusions, that adopting the one-step approach increased the number of women with diagnosed GDM but did not significantly improve maternal or neonatal outcomes, are not surprising. Since the initial publication of the Hyperglycemia and Adverse Pregnancy Outcome Study, upon which the International Association of the Diabetes in Pregnancy Study Groups based its recommendations to go to a one-step approach, much debate has ensued about the best method to diagnose GDM. Indeed, the National Institutes of Health convened a consensus panel to review the literature and determine whether the one-step approach should be universally adopted (the panel concluded that more information was needed, and that the current two-step approach should continue to be used).
As the authors concede, studies have shown conflicting results, and no large-scale randomized controlled trial has been conducted to date. However, the literature does not bear out the idea that the one-step approach is truly better. The current study, although including a significant number of women and a reasonable control group, only serves as yet another study to reinforce what has previously been published.
I would agree with the researchers’ conclusions that the one-step approach is not necessarily beneficial. Although the one-step approach may identify a subset of patients who might not otherwise be diagnosed with GDM, it still remains unclear whether the outcomes for these patients will be improved. Furthermore, additional testing, need for insulin or other oral antidiabetic medications, etc., would result in additional stress to the patient and the health care system. Based on the authors’ findings, and results of other studies, it remains to be determined if the effort (diagnosing additional patients with GDM) is justified medically, economically, or otherwise.
As ob.gyns., we must continually ask ourselves: “By not doing something, are we causing harm to our patients?” If we change the diagnostic criteria for GDM, thereby increasing the number of women with the condition who would then require additional care, medications, and, potentially, more complex decisions around timing and mode of delivery, we need to be certain that we are not doing harm. This, and other studies examining the use of the one- versus two-step approach have yet to demonstrate, unequivocally, that changing the criteria reduces harm, and, perhaps, might – unintentionally – cause more.
As the study authors and the NIH consensus panel concluded, more rigorous evaluation is needed; that is, a large, multicenter randomized controlled trial that examines not only the benefits during pregnancy but also the long-term benefits to women and their children.
E. Albert Reece, MD, PhD, MBA, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He provided commentary on the study by Pocobelli et al. Dr. Reece said he had no relevant financial disclosures.
according to data from a before-and-after cohort study of women in the state of Washington.
The one-step test, a 75-g 2-hour oral glucose tolerance test (OGTT), was recommended for all pregnant women in 2010, although the traditional two-step test – a 50-g screening glucose challenge test followed by a 100-g 3-hour OGTT – remains widely used, wrote Gaia Pocobelli, PhD, of Kaiser Permanente Washington Health Research Institute, Seattle, and her colleagues. “No randomized trial has been published comparing outcomes of the two approaches.”
In a study published in Obstetrics & Gynecology, the researchers compared data from 23,257 women who received prenatal care in Washington State between January 2009 and December 2014, including 8,363 women who received care before the guideline change, 4,103 who received care during a transition period, and 10,791 after the guideline change. Approximately 60% of the women received care from clinicians internal to Kaiser Permanente; 40% received care from external providers. Most (87%) of the internal clinicians switched to the one-step approach, the researchers said. Only 5% of external providers did so.
Overall, adopting the one-step approach was associated with a 41% increase in the diagnosis of GDM without improved maternal or neonatal outcomes, the researchers noted.
The incidence of GDM increased from 7% before the guideline change to 11% afterward for women seen by internal providers. For women seen by external providers, gestational diabetes incidence increased from 10% to 11%.
For women seen by internal providers, the use of insulin increased from 1% before the guideline change to 4% afterward; for women seen by external providers, use of insulin increased from 1.3% to 1.4% (change between the groups P less than .001).
In addition, women seen by internal providers were more likely to undergo induction of labor after the guideline change (25% to 29%), while labor induction decreased for women seen by external providers (31% to 29%) for a relative risk of 1.2.
Neonatal hypoglycemia increased from 1% to 2% among women seen by internal providers, but decreased slightly from 2.4% to 2.1% for women seen by external providers, for a relative risk of 1.77.
There were no significant differences between the women seen by internal and external providers in risk of primary cesarean section, large for gestational age, small for gestational age, or neonatal ICU admission.
The main limitation of the study was the potential confounding variables including maternal diet and exercise, and possible underreporting of risk factors such as smoking, the researchers noted. However, the results were strengthened by the large study population, and the results “do not suggest a benefit of adopting the one-step over the two-step approach.
“Kaiser Permanente Washington has revised [its] guidelines to return to a two-step process. We recommend that any health care system considering switching to the one-step approach incorporate a rigorous evaluation of changes in maternal and neonatal outcomes,” Dr. Pocobelli and her associates added.
Dr. Pocobelli disclosed funding from Jazz Pharmaceuticals for work unrelated to this study. The study was supported in part by a grant from the Group Health Foundation Momentum Fund.
according to data from a before-and-after cohort study of women in the state of Washington.
The one-step test, a 75-g 2-hour oral glucose tolerance test (OGTT), was recommended for all pregnant women in 2010, although the traditional two-step test – a 50-g screening glucose challenge test followed by a 100-g 3-hour OGTT – remains widely used, wrote Gaia Pocobelli, PhD, of Kaiser Permanente Washington Health Research Institute, Seattle, and her colleagues. “No randomized trial has been published comparing outcomes of the two approaches.”
In a study published in Obstetrics & Gynecology, the researchers compared data from 23,257 women who received prenatal care in Washington State between January 2009 and December 2014, including 8,363 women who received care before the guideline change, 4,103 who received care during a transition period, and 10,791 after the guideline change. Approximately 60% of the women received care from clinicians internal to Kaiser Permanente; 40% received care from external providers. Most (87%) of the internal clinicians switched to the one-step approach, the researchers said. Only 5% of external providers did so.
Overall, adopting the one-step approach was associated with a 41% increase in the diagnosis of GDM without improved maternal or neonatal outcomes, the researchers noted.
The incidence of GDM increased from 7% before the guideline change to 11% afterward for women seen by internal providers. For women seen by external providers, gestational diabetes incidence increased from 10% to 11%.
For women seen by internal providers, the use of insulin increased from 1% before the guideline change to 4% afterward; for women seen by external providers, use of insulin increased from 1.3% to 1.4% (change between the groups P less than .001).
In addition, women seen by internal providers were more likely to undergo induction of labor after the guideline change (25% to 29%), while labor induction decreased for women seen by external providers (31% to 29%) for a relative risk of 1.2.
Neonatal hypoglycemia increased from 1% to 2% among women seen by internal providers, but decreased slightly from 2.4% to 2.1% for women seen by external providers, for a relative risk of 1.77.
There were no significant differences between the women seen by internal and external providers in risk of primary cesarean section, large for gestational age, small for gestational age, or neonatal ICU admission.
The main limitation of the study was the potential confounding variables including maternal diet and exercise, and possible underreporting of risk factors such as smoking, the researchers noted. However, the results were strengthened by the large study population, and the results “do not suggest a benefit of adopting the one-step over the two-step approach.
“Kaiser Permanente Washington has revised [its] guidelines to return to a two-step process. We recommend that any health care system considering switching to the one-step approach incorporate a rigorous evaluation of changes in maternal and neonatal outcomes,” Dr. Pocobelli and her associates added.
Dr. Pocobelli disclosed funding from Jazz Pharmaceuticals for work unrelated to this study. The study was supported in part by a grant from the Group Health Foundation Momentum Fund.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: Increased diagnoses of gestational diabetes did not significantly improve maternal or fetal outcomes.
Major finding: Adoption of a one-step screening process for gestational diabetes increased diagnoses by 41%.
Study details: The data come from a before-and-after cohort study with a population of 23,257 women.
Disclosures: Dr. Pocobelli disclosed funding from Jazz Pharmaceuticals for work unrelated to this study. The study was supported in part by a grant from the Group Health Foundation Momentum Fund.
Source: Pocobelli G et al. Obstet Gynecol. 2018;132:859-67.
Study suggests “alarming” diabetes med discontinuation
ORLANDO – Most patients with type 2 diabetes mellitus stop taking their medication within a year, and nearly one-third stop within the first 3 months, a retrospective analysis of claims data for more than 324,000 patients suggests.
The findings in this population of commercially insured adults are startling and highlight a need for interventions to improve treatment persistence, according to Lisa Latts, MD, deputy chief health officer for IBM Watson Health in Cambridge, Mass.
Dr. Latts and her colleagues reviewed medication claims data for 324,136 patients with at least one diagnosis for type 2 diabetes mellitus and one outpatient pharmacy claim for a type 2 diabetes medication after at least 12 prior months without such a claim.
Of those patients, 58% discontinued treatment within 12 months, 31% discontinued within the first 3 months, and 44% discontinued within 6 months, Dr. Latts reported at the annual scientific sessions of the American Diabetes Association.
“Less than half of those patients who discontinued had a restart within the following year. So what we’re seeing here is a huge percentage of individuals who had a diagnosis of type 2 diabetes, were prescribed a medication, and then did not continue the medication,” she said.
Of those who discontinued within the 12-month follow-up, 27% restarted therapy within 60 days and 39% restarted therapy anytime during the 12-month follow-up (mean treatment gap, 107 days). Of those who discontinued by 3 months, 45% restarted within a year (mean treatment gap, 112 days), and of those who discontinued by 6 months, 44% restarted within a year (mean treatment gap, 119 days).
Patients included in the review, which was a collaborative effort of IBM Watson Health and the ADA, had at least 12 months of continuous enrollment in the Truven Health MarketScan Commercial and Medicare Supplemental Databases between 2013 and 2017, before and after the therapy initiation date.
They had a mean age of 55 years, with 28% aged 45-54 years and 35% aged 55-64 years. About 46% were women, and all had at least one diagnosis for type 2 diabetes mellitus during the study period and at least one outpatient pharmacy claim for a type 2 diabetes medication that was prescribed to be taken for at least 30 days.
“As you would expect, far and away the majority [68%] were given metformin,” Dr. Latts said.
Other prescribed treatments after the initial diagnosis included sulfonylureas (7%), insulin (6%), dipeptidyl peptidase–4 inhibitors (6%), sodium-glucose cotransporter 2 inhibitors (1.5%), and a variety of combination treatments – typically metformin plus sulfonylureas (5%).
This study provides real-world evidence that a majority of patients with type 2 diabetes discontinue their treatment within 1 year – an important finding given that medication persistence is imperative for successful treatment, Dr. Latts said. She noted that prior research has shown treatment discontinuation of prescribed medication within the first year is common for a number of chronic disease treatments and is associated with poor clinical outcomes.
“If you treat diabetes, this is alarming,” she said. “These are people who should be on a diabetes med, their doctor probably thinks they’re on a diabetes med, and they’re not taking it.”
The findings are limited by factors associated with the use of administrative claims data, such as possible coding inaccuracies and missed cases in which patients paid out of pocket for medications through low-cost pharmacy offers, as well as by the 12-month window used for the study. She added that the findings may not be generalizable to uninsured or Medicare patients.
Nevertheless, the findings are concerning and may reflect misunderstandings among patients about the need to refill prescriptions after the initial supply runs out, or may relate to side effects that patients don’t report to their physicians, Sherita Golden, MD, of Johns Hopkins University, Baltimore, said during a question-and-answer period following Dr. Latts’ presentation.
“I do treat patients with diabetes so I am very alarmed,” she said, adding that there is a need to improve communication between patients and physicians about treatment and side effects.
Dr. Latts reported relationships with Medtronic, Novo Nordisk, and Sanofi. Her coauthors from IBM Watson and the ADA all reported having no financial disclosures.
SOURCE: Latts L et al. ADA 2018, Abstract 135-OR.
ORLANDO – Most patients with type 2 diabetes mellitus stop taking their medication within a year, and nearly one-third stop within the first 3 months, a retrospective analysis of claims data for more than 324,000 patients suggests.
The findings in this population of commercially insured adults are startling and highlight a need for interventions to improve treatment persistence, according to Lisa Latts, MD, deputy chief health officer for IBM Watson Health in Cambridge, Mass.
Dr. Latts and her colleagues reviewed medication claims data for 324,136 patients with at least one diagnosis for type 2 diabetes mellitus and one outpatient pharmacy claim for a type 2 diabetes medication after at least 12 prior months without such a claim.
Of those patients, 58% discontinued treatment within 12 months, 31% discontinued within the first 3 months, and 44% discontinued within 6 months, Dr. Latts reported at the annual scientific sessions of the American Diabetes Association.
“Less than half of those patients who discontinued had a restart within the following year. So what we’re seeing here is a huge percentage of individuals who had a diagnosis of type 2 diabetes, were prescribed a medication, and then did not continue the medication,” she said.
Of those who discontinued within the 12-month follow-up, 27% restarted therapy within 60 days and 39% restarted therapy anytime during the 12-month follow-up (mean treatment gap, 107 days). Of those who discontinued by 3 months, 45% restarted within a year (mean treatment gap, 112 days), and of those who discontinued by 6 months, 44% restarted within a year (mean treatment gap, 119 days).
Patients included in the review, which was a collaborative effort of IBM Watson Health and the ADA, had at least 12 months of continuous enrollment in the Truven Health MarketScan Commercial and Medicare Supplemental Databases between 2013 and 2017, before and after the therapy initiation date.
They had a mean age of 55 years, with 28% aged 45-54 years and 35% aged 55-64 years. About 46% were women, and all had at least one diagnosis for type 2 diabetes mellitus during the study period and at least one outpatient pharmacy claim for a type 2 diabetes medication that was prescribed to be taken for at least 30 days.
“As you would expect, far and away the majority [68%] were given metformin,” Dr. Latts said.
Other prescribed treatments after the initial diagnosis included sulfonylureas (7%), insulin (6%), dipeptidyl peptidase–4 inhibitors (6%), sodium-glucose cotransporter 2 inhibitors (1.5%), and a variety of combination treatments – typically metformin plus sulfonylureas (5%).
This study provides real-world evidence that a majority of patients with type 2 diabetes discontinue their treatment within 1 year – an important finding given that medication persistence is imperative for successful treatment, Dr. Latts said. She noted that prior research has shown treatment discontinuation of prescribed medication within the first year is common for a number of chronic disease treatments and is associated with poor clinical outcomes.
“If you treat diabetes, this is alarming,” she said. “These are people who should be on a diabetes med, their doctor probably thinks they’re on a diabetes med, and they’re not taking it.”
The findings are limited by factors associated with the use of administrative claims data, such as possible coding inaccuracies and missed cases in which patients paid out of pocket for medications through low-cost pharmacy offers, as well as by the 12-month window used for the study. She added that the findings may not be generalizable to uninsured or Medicare patients.
Nevertheless, the findings are concerning and may reflect misunderstandings among patients about the need to refill prescriptions after the initial supply runs out, or may relate to side effects that patients don’t report to their physicians, Sherita Golden, MD, of Johns Hopkins University, Baltimore, said during a question-and-answer period following Dr. Latts’ presentation.
“I do treat patients with diabetes so I am very alarmed,” she said, adding that there is a need to improve communication between patients and physicians about treatment and side effects.
Dr. Latts reported relationships with Medtronic, Novo Nordisk, and Sanofi. Her coauthors from IBM Watson and the ADA all reported having no financial disclosures.
SOURCE: Latts L et al. ADA 2018, Abstract 135-OR.
ORLANDO – Most patients with type 2 diabetes mellitus stop taking their medication within a year, and nearly one-third stop within the first 3 months, a retrospective analysis of claims data for more than 324,000 patients suggests.
The findings in this population of commercially insured adults are startling and highlight a need for interventions to improve treatment persistence, according to Lisa Latts, MD, deputy chief health officer for IBM Watson Health in Cambridge, Mass.
Dr. Latts and her colleagues reviewed medication claims data for 324,136 patients with at least one diagnosis for type 2 diabetes mellitus and one outpatient pharmacy claim for a type 2 diabetes medication after at least 12 prior months without such a claim.
Of those patients, 58% discontinued treatment within 12 months, 31% discontinued within the first 3 months, and 44% discontinued within 6 months, Dr. Latts reported at the annual scientific sessions of the American Diabetes Association.
“Less than half of those patients who discontinued had a restart within the following year. So what we’re seeing here is a huge percentage of individuals who had a diagnosis of type 2 diabetes, were prescribed a medication, and then did not continue the medication,” she said.
Of those who discontinued within the 12-month follow-up, 27% restarted therapy within 60 days and 39% restarted therapy anytime during the 12-month follow-up (mean treatment gap, 107 days). Of those who discontinued by 3 months, 45% restarted within a year (mean treatment gap, 112 days), and of those who discontinued by 6 months, 44% restarted within a year (mean treatment gap, 119 days).
Patients included in the review, which was a collaborative effort of IBM Watson Health and the ADA, had at least 12 months of continuous enrollment in the Truven Health MarketScan Commercial and Medicare Supplemental Databases between 2013 and 2017, before and after the therapy initiation date.
They had a mean age of 55 years, with 28% aged 45-54 years and 35% aged 55-64 years. About 46% were women, and all had at least one diagnosis for type 2 diabetes mellitus during the study period and at least one outpatient pharmacy claim for a type 2 diabetes medication that was prescribed to be taken for at least 30 days.
“As you would expect, far and away the majority [68%] were given metformin,” Dr. Latts said.
Other prescribed treatments after the initial diagnosis included sulfonylureas (7%), insulin (6%), dipeptidyl peptidase–4 inhibitors (6%), sodium-glucose cotransporter 2 inhibitors (1.5%), and a variety of combination treatments – typically metformin plus sulfonylureas (5%).
This study provides real-world evidence that a majority of patients with type 2 diabetes discontinue their treatment within 1 year – an important finding given that medication persistence is imperative for successful treatment, Dr. Latts said. She noted that prior research has shown treatment discontinuation of prescribed medication within the first year is common for a number of chronic disease treatments and is associated with poor clinical outcomes.
“If you treat diabetes, this is alarming,” she said. “These are people who should be on a diabetes med, their doctor probably thinks they’re on a diabetes med, and they’re not taking it.”
The findings are limited by factors associated with the use of administrative claims data, such as possible coding inaccuracies and missed cases in which patients paid out of pocket for medications through low-cost pharmacy offers, as well as by the 12-month window used for the study. She added that the findings may not be generalizable to uninsured or Medicare patients.
Nevertheless, the findings are concerning and may reflect misunderstandings among patients about the need to refill prescriptions after the initial supply runs out, or may relate to side effects that patients don’t report to their physicians, Sherita Golden, MD, of Johns Hopkins University, Baltimore, said during a question-and-answer period following Dr. Latts’ presentation.
“I do treat patients with diabetes so I am very alarmed,” she said, adding that there is a need to improve communication between patients and physicians about treatment and side effects.
Dr. Latts reported relationships with Medtronic, Novo Nordisk, and Sanofi. Her coauthors from IBM Watson and the ADA all reported having no financial disclosures.
SOURCE: Latts L et al. ADA 2018, Abstract 135-OR.
REPORTING FROM ADA 2018
Key clinical point:
Major finding: In total, 58% of patients discontinued treatment within 12 months, and just 39% of those patients restarted within 1 year.
Study details: An analysis of claims data for more than 324,000 patients.
Disclosures: Dr. Latts reported relationships with Medtronic, Novo Nordisk, and Sanofi. Her coauthors from IBM Watson and the American Diabetes Association all reported having no financial disclosures.
Source: Latts L et al. ADA 2018, Abstract 135-OR.
























