User login
Ongoing HER2 breast cancer therapy may cost an additional $68,000 per patient
The current funding policy in British Columbia restricts patients to two lines of HER2-directed therapy for metastatic breast cancer, but accessing continued HER2 suppression has become more complex as novel agents have emerged, Emily Jackson, MD, and colleagues explained (in poster PD8-09) at the San Antonio Breast Cancer Symposium.
Continuing HER2 suppression has improved progression free survival (PFS) and overall survival (OS), but the financial implications of adapting funding policies to “reflect increasing lines of proven HER2 treatment” are unclear, they noted.
Drug funding is provided through the provincial government, but it can take months – and sometimes years – from when a drug is approved by Health Canada and when provincial protocols are approved and funding is made available, Dr. Jackson, co-chief resident (PGY5) at BC Cancer, Vancouver, said in an interview.
During that “lag time,” the province is negotiating drug prices with pharmaceutical companies and determining “which patients are eligible and under which circumstances,” she said.
To assess the potential costs, the investigators analyzed data from the BC Cancer outcomes unit, which collects clinical and outcome information on 85% of all patients diagnosed with breast cancer in the province. Information on therapy use was obtained from the BC Cancer pharmacy database.
Of 230 patients who received any HER2 treatment for metastatic breast cancer dispensed by BC Cancer between 2013 and 2018, 112 (49%) were eligible to continue beyond their second line of therapy.
“Of these, 86 patients accessed continued HER2-directed therapy, while 26 were eligible but unable to access continued HER2Rx,” they reported, noting that “the remaining 51% (n = 118) were not eligible for consideration of further HER2Rx due to either stable disease (n = 61) or deterioration precluding treatment (n = 57).”
At median follow-up of 42.2 months, the median number of lines of therapy in the entire study population was three. The median number of cycles in those who received HER2-directed therapy beyond second-line therapy was 33.
The median overall survival was 37.5 months for those who were eligible but did not continue HER2, compared with 57.9 months for those who did continue, they found.
The overall survival difference was not statistically significant (P = .13), but this was likely due to the small number of patients included in the initial analysis, Dr. Jackson said, noting that the finding is “hypothesis generating,” and should be further assessed.
Notably, most patients who continued HER2 therapy did so through pharmaceutical company compassionate access programs or clinical trials, she said.
The “conservative estimated cost per cycle of HER2Rx” was based on currently available trastuzumab biosimilars, and the potential financial implications were calculated based on the current cost of commonly used third-line therapies.
The findings demonstrate that most patients access continued treatment despite prohibitive funding policies, and suggest that significant increases in cost per patient can be expected if funding policies don’t evolve to meet treatment needs, they concluded, noting that “if these trends in survival continue we would expect an additional cost of $68,000 per patient over current costs.
“As the cost of novel therapies are likely to be higher than currently available biosimilars, there will be significant implications for both private payer and public payer healthcare systems,” they added.
A larger, more comprehensive analysis of the data is planned, said Dr. Jackson, who did not disclose any funding or other conflicts of interest associated with this study.
The current funding policy in British Columbia restricts patients to two lines of HER2-directed therapy for metastatic breast cancer, but accessing continued HER2 suppression has become more complex as novel agents have emerged, Emily Jackson, MD, and colleagues explained (in poster PD8-09) at the San Antonio Breast Cancer Symposium.
Continuing HER2 suppression has improved progression free survival (PFS) and overall survival (OS), but the financial implications of adapting funding policies to “reflect increasing lines of proven HER2 treatment” are unclear, they noted.
Drug funding is provided through the provincial government, but it can take months – and sometimes years – from when a drug is approved by Health Canada and when provincial protocols are approved and funding is made available, Dr. Jackson, co-chief resident (PGY5) at BC Cancer, Vancouver, said in an interview.
During that “lag time,” the province is negotiating drug prices with pharmaceutical companies and determining “which patients are eligible and under which circumstances,” she said.
To assess the potential costs, the investigators analyzed data from the BC Cancer outcomes unit, which collects clinical and outcome information on 85% of all patients diagnosed with breast cancer in the province. Information on therapy use was obtained from the BC Cancer pharmacy database.
Of 230 patients who received any HER2 treatment for metastatic breast cancer dispensed by BC Cancer between 2013 and 2018, 112 (49%) were eligible to continue beyond their second line of therapy.
“Of these, 86 patients accessed continued HER2-directed therapy, while 26 were eligible but unable to access continued HER2Rx,” they reported, noting that “the remaining 51% (n = 118) were not eligible for consideration of further HER2Rx due to either stable disease (n = 61) or deterioration precluding treatment (n = 57).”
At median follow-up of 42.2 months, the median number of lines of therapy in the entire study population was three. The median number of cycles in those who received HER2-directed therapy beyond second-line therapy was 33.
The median overall survival was 37.5 months for those who were eligible but did not continue HER2, compared with 57.9 months for those who did continue, they found.
The overall survival difference was not statistically significant (P = .13), but this was likely due to the small number of patients included in the initial analysis, Dr. Jackson said, noting that the finding is “hypothesis generating,” and should be further assessed.
Notably, most patients who continued HER2 therapy did so through pharmaceutical company compassionate access programs or clinical trials, she said.
The “conservative estimated cost per cycle of HER2Rx” was based on currently available trastuzumab biosimilars, and the potential financial implications were calculated based on the current cost of commonly used third-line therapies.
The findings demonstrate that most patients access continued treatment despite prohibitive funding policies, and suggest that significant increases in cost per patient can be expected if funding policies don’t evolve to meet treatment needs, they concluded, noting that “if these trends in survival continue we would expect an additional cost of $68,000 per patient over current costs.
“As the cost of novel therapies are likely to be higher than currently available biosimilars, there will be significant implications for both private payer and public payer healthcare systems,” they added.
A larger, more comprehensive analysis of the data is planned, said Dr. Jackson, who did not disclose any funding or other conflicts of interest associated with this study.
The current funding policy in British Columbia restricts patients to two lines of HER2-directed therapy for metastatic breast cancer, but accessing continued HER2 suppression has become more complex as novel agents have emerged, Emily Jackson, MD, and colleagues explained (in poster PD8-09) at the San Antonio Breast Cancer Symposium.
Continuing HER2 suppression has improved progression free survival (PFS) and overall survival (OS), but the financial implications of adapting funding policies to “reflect increasing lines of proven HER2 treatment” are unclear, they noted.
Drug funding is provided through the provincial government, but it can take months – and sometimes years – from when a drug is approved by Health Canada and when provincial protocols are approved and funding is made available, Dr. Jackson, co-chief resident (PGY5) at BC Cancer, Vancouver, said in an interview.
During that “lag time,” the province is negotiating drug prices with pharmaceutical companies and determining “which patients are eligible and under which circumstances,” she said.
To assess the potential costs, the investigators analyzed data from the BC Cancer outcomes unit, which collects clinical and outcome information on 85% of all patients diagnosed with breast cancer in the province. Information on therapy use was obtained from the BC Cancer pharmacy database.
Of 230 patients who received any HER2 treatment for metastatic breast cancer dispensed by BC Cancer between 2013 and 2018, 112 (49%) were eligible to continue beyond their second line of therapy.
“Of these, 86 patients accessed continued HER2-directed therapy, while 26 were eligible but unable to access continued HER2Rx,” they reported, noting that “the remaining 51% (n = 118) were not eligible for consideration of further HER2Rx due to either stable disease (n = 61) or deterioration precluding treatment (n = 57).”
At median follow-up of 42.2 months, the median number of lines of therapy in the entire study population was three. The median number of cycles in those who received HER2-directed therapy beyond second-line therapy was 33.
The median overall survival was 37.5 months for those who were eligible but did not continue HER2, compared with 57.9 months for those who did continue, they found.
The overall survival difference was not statistically significant (P = .13), but this was likely due to the small number of patients included in the initial analysis, Dr. Jackson said, noting that the finding is “hypothesis generating,” and should be further assessed.
Notably, most patients who continued HER2 therapy did so through pharmaceutical company compassionate access programs or clinical trials, she said.
The “conservative estimated cost per cycle of HER2Rx” was based on currently available trastuzumab biosimilars, and the potential financial implications were calculated based on the current cost of commonly used third-line therapies.
The findings demonstrate that most patients access continued treatment despite prohibitive funding policies, and suggest that significant increases in cost per patient can be expected if funding policies don’t evolve to meet treatment needs, they concluded, noting that “if these trends in survival continue we would expect an additional cost of $68,000 per patient over current costs.
“As the cost of novel therapies are likely to be higher than currently available biosimilars, there will be significant implications for both private payer and public payer healthcare systems,” they added.
A larger, more comprehensive analysis of the data is planned, said Dr. Jackson, who did not disclose any funding or other conflicts of interest associated with this study.
FROM SABCS 2021
Anticoagulant choice in antiphospholipid syndrome–associated thrombosis
Background: DOACs have largely replaced VKAs as first-line therapy for venous thromboembolism in patients with adequate renal function. However, there is concern in APS that DOACs may have higher rates of recurrent thrombosis than VKAs when treating thromboembolism.
Study design: Randomized noninferiority trial.
Setting: Six teaching hospitals in Spain.
Synopsis: Of adults with thrombotic APS, 190 were randomized to receive rivaroxaban or warfarin. Primary outcomes were thrombotic events and major bleeding. Follow-up after 3 years demonstrated new thromboses in 11 patients (11.6%) in the DOAC group and 6 patients (6.3%) in the VKA group (P = .29). Major bleeding occurred in six patients (6.3%) in the DOAC group and seven patients (7.4%) in the VKA group (P = .77). By contrast, stroke occurred in nine patients in the DOAC group while the VKA group had zero events, yielding a significant relative RR of 19.00 (95% CI, 1.12-321.90) for the DOAC group.
The DOAC arm was not proven to be noninferior with respect to the primary outcome of thrombotic events. The higher risk of stroke in this group suggests the need for caution in using DOACs in this population.
Bottom line: DOACs have a higher risk of stroke than VKAs in patients with APS without a significant difference in rate of a major bleed.
Citation: Ordi-Ros J et. al. Rivaroxaban versus vitamin K antagonist in antiphospholipid syndrome. Ann Intern Med. 2019;171(10):685-94. doi: 10.7326/M19-0291.
Dr. Portnoy is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: DOACs have largely replaced VKAs as first-line therapy for venous thromboembolism in patients with adequate renal function. However, there is concern in APS that DOACs may have higher rates of recurrent thrombosis than VKAs when treating thromboembolism.
Study design: Randomized noninferiority trial.
Setting: Six teaching hospitals in Spain.
Synopsis: Of adults with thrombotic APS, 190 were randomized to receive rivaroxaban or warfarin. Primary outcomes were thrombotic events and major bleeding. Follow-up after 3 years demonstrated new thromboses in 11 patients (11.6%) in the DOAC group and 6 patients (6.3%) in the VKA group (P = .29). Major bleeding occurred in six patients (6.3%) in the DOAC group and seven patients (7.4%) in the VKA group (P = .77). By contrast, stroke occurred in nine patients in the DOAC group while the VKA group had zero events, yielding a significant relative RR of 19.00 (95% CI, 1.12-321.90) for the DOAC group.
The DOAC arm was not proven to be noninferior with respect to the primary outcome of thrombotic events. The higher risk of stroke in this group suggests the need for caution in using DOACs in this population.
Bottom line: DOACs have a higher risk of stroke than VKAs in patients with APS without a significant difference in rate of a major bleed.
Citation: Ordi-Ros J et. al. Rivaroxaban versus vitamin K antagonist in antiphospholipid syndrome. Ann Intern Med. 2019;171(10):685-94. doi: 10.7326/M19-0291.
Dr. Portnoy is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: DOACs have largely replaced VKAs as first-line therapy for venous thromboembolism in patients with adequate renal function. However, there is concern in APS that DOACs may have higher rates of recurrent thrombosis than VKAs when treating thromboembolism.
Study design: Randomized noninferiority trial.
Setting: Six teaching hospitals in Spain.
Synopsis: Of adults with thrombotic APS, 190 were randomized to receive rivaroxaban or warfarin. Primary outcomes were thrombotic events and major bleeding. Follow-up after 3 years demonstrated new thromboses in 11 patients (11.6%) in the DOAC group and 6 patients (6.3%) in the VKA group (P = .29). Major bleeding occurred in six patients (6.3%) in the DOAC group and seven patients (7.4%) in the VKA group (P = .77). By contrast, stroke occurred in nine patients in the DOAC group while the VKA group had zero events, yielding a significant relative RR of 19.00 (95% CI, 1.12-321.90) for the DOAC group.
The DOAC arm was not proven to be noninferior with respect to the primary outcome of thrombotic events. The higher risk of stroke in this group suggests the need for caution in using DOACs in this population.
Bottom line: DOACs have a higher risk of stroke than VKAs in patients with APS without a significant difference in rate of a major bleed.
Citation: Ordi-Ros J et. al. Rivaroxaban versus vitamin K antagonist in antiphospholipid syndrome. Ann Intern Med. 2019;171(10):685-94. doi: 10.7326/M19-0291.
Dr. Portnoy is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Vitamin supplementation in healthy patients: What does the evidence support?
Since their discovery in the early 1900s as the treatment for life-threatening deficiency syndromes, vitamins have been touted as panaceas for numerous ailments. While observational data have suggested potential correlations between vitamin status and every imaginable disease, randomized controlled trials (RCTs) have generally failed to find benefits from supplementation. Despite this lack of proven efficacy, more than half of older adults reported taking vitamins regularly.1
While most clinicians consider vitamins to be, at worst, expensive placebos, the potential for harm and dangerous interactions exists. Unlike pharmaceuticals, vitamins are generally unregulated, and the true content of many dietary supplements is often difficult to elucidate. Understanding the physiologic role, foundational evidence, and specific indications for the various vitamins is key to providing the best recommendations to patients.
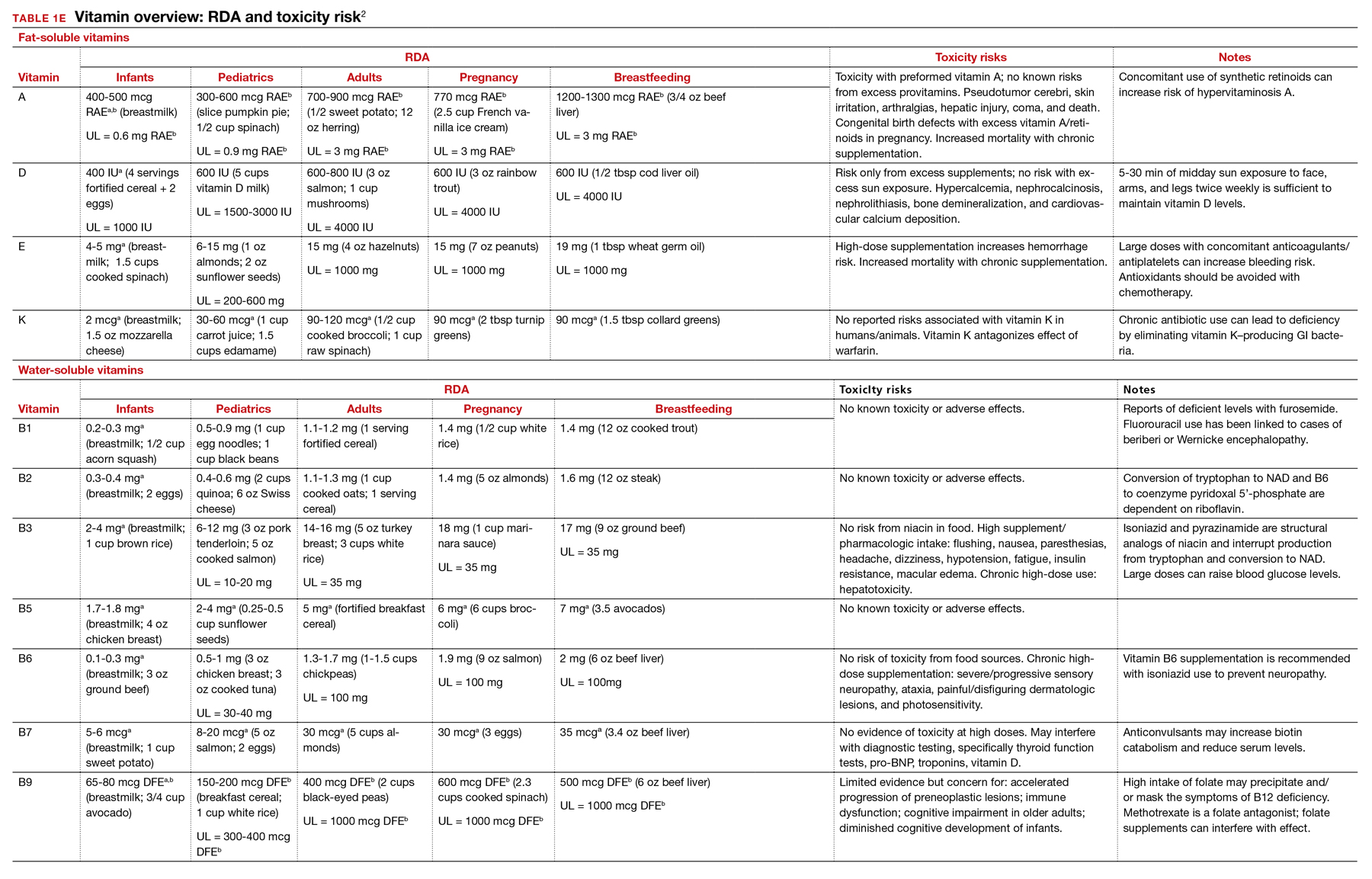
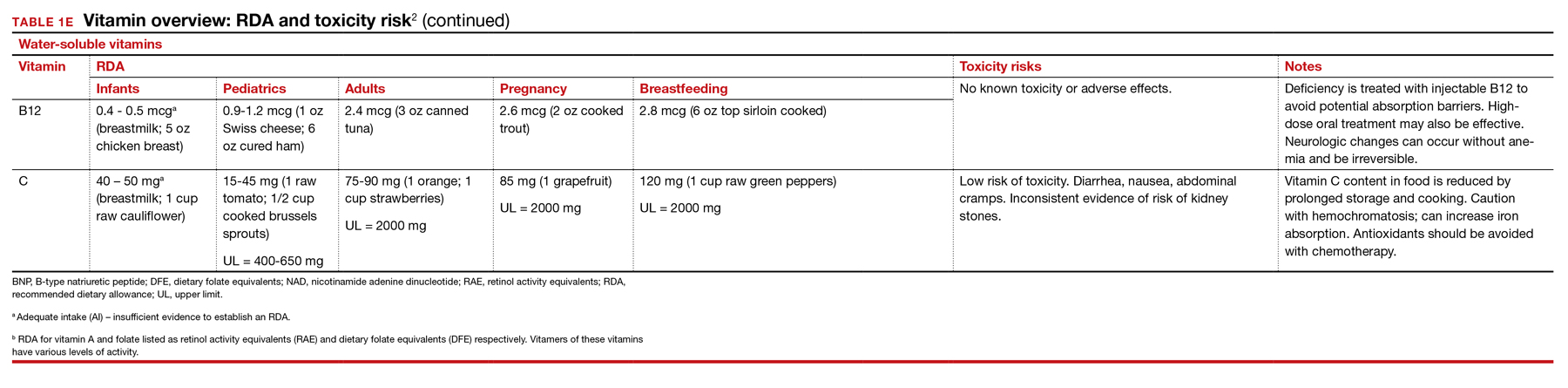
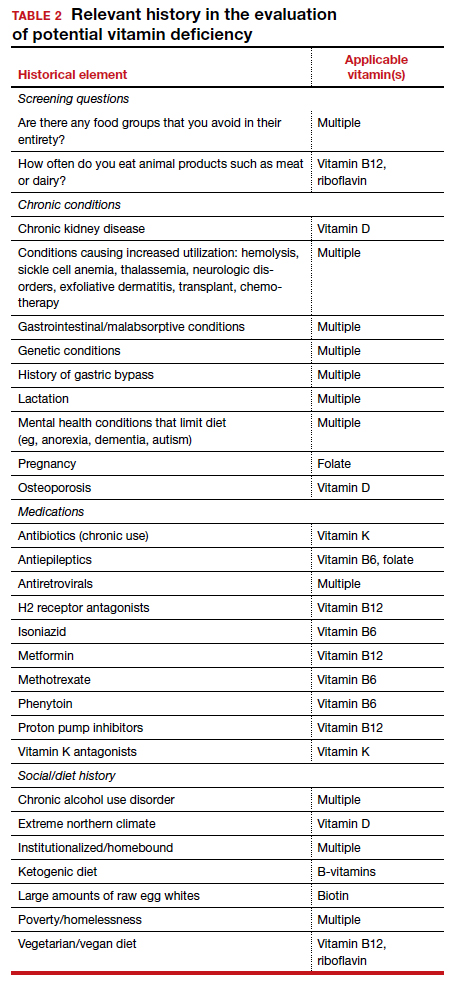
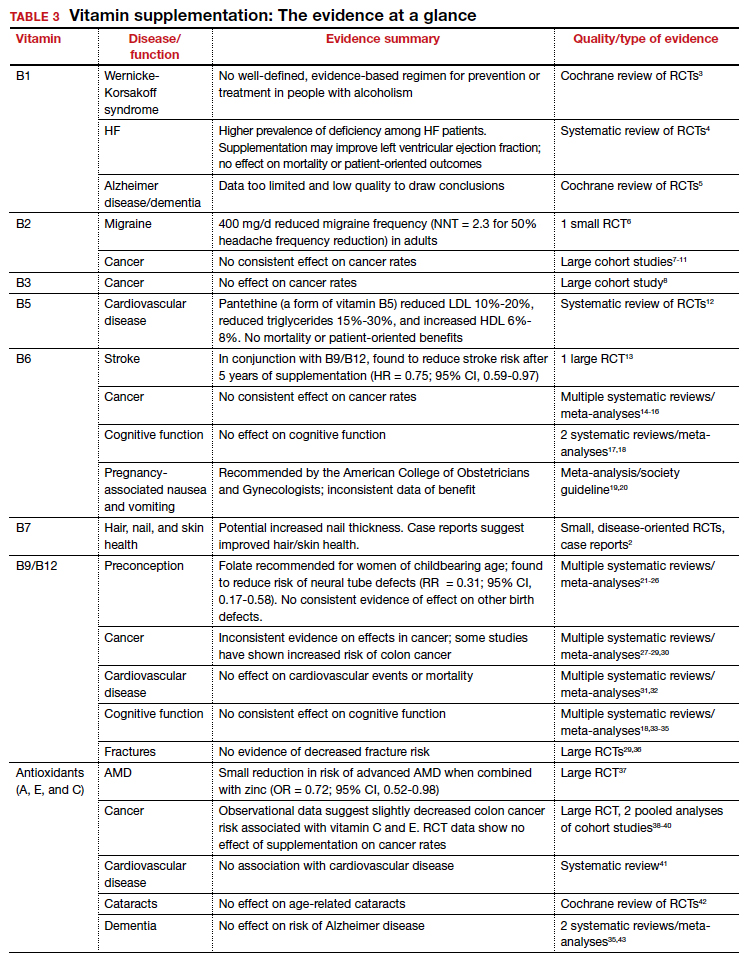
Vitamins are essential organic nutrients, required in small quantities for normal metabolism. Since they are not synthesized endogenously, they must be ingested via food intake. In the developed world, vitamin deficiency syndromes are rare, thanks to sufficiently balanced diets and availability of fortified foods. The focus of this article will be on vitamin supplementation in healthy patients with well-balanced diets. TABLE E12 lists the 13 recognized vitamins, their recommended dietary allowances, and any known toxicity risks. TABLE 22 outlines elements of the history to consider when evaluating for deficiency. A summary of the most clinically significant evidence for vitamin supplementation follows; a more comprehensive review can be found in TABLE 3.3-96
B Complex vitamins
Vitamin B1
Vitamers: Thiamine (thiamin)
Physiologic role: Critical in carbohydrate and amino-acid catabolism and energy metabolism
Dietary sources: Whole grains, meat, fish, fortified cereals, and breads
Thiamine serves as an essential cofactor in energy metabolism.2 Thiamine deficiency is responsible for beriberi syndrome (rare in the developed world) and Wernicke-Korsakoff syndrome, the latter of which is a relatively common complication of chronic alcohol dependence. Although thiamine’s administration in these conditions can be curative, evidence is lacking to support its use preventively in patients with alcoholism.3 Thiamine has additionally been theorized to play a role in cardiac and cognitive function, but RCT data has not shown consistent patient-oriented benefits.4,5
The takeaway: Given the lack of evidence, supplementation in the general population is not recommended.
Continue to: Vitamin B2...
Vitamin B2
Vitamers: Riboflavin
Physiologic role: Essential component of cellular function and growth, energy production, and metabolism of fats and drugs
Dietary sources: Eggs, organ meats, lean meats, milk, green vegetables, fortified cereals and grains Riboflavin is essential to energy production, cellular growth, and metabolism.2
The takeaway: Its use as migraine prophylaxis has limited data,97 but there is otherwise no evidence to support health benefits of riboflavin supplementation.
Vitamin B3
Vitamers: Nicotinic acid (niacin); nicotinamide (niacinamide); nicotinamide riboside
Physiologic role: Converted to nicotinamide adenine dinucleotide (NAD), which is widely required in most cellular metabolic redox processes. Crucial to the synthesis and metabolism of carbohydrates, fatty acids, and proteins
Dietary sources: Poultry, beef, fish, nuts, legumes, grains. (Tryptophan can also be converted to NAD.)
Niacin is readily converted to NAD, an essential coenzyme for multiple catalytic processes in the body. While niacin at doses more than 100 times the recommended dietary allowance (RDA; 1-3 g/d) has been extensively studied for its role in dyslipidemias,2 pharmacologic dosing is beyond the scope of this article.
The takeaway: There is no evidence supporting a clinical benefit from niacin supplementation.
Vitamin B5
Vitamers: Pantothenic acid; pantethine
Physiologic role: Required for synthesis of coenzyme A (CoA) and acyl carrier protein, both essential in fatty acid and other anabolic/catabolic processes
Dietary sources: Almost all plant/animal-based foods. Richest sources include beef, chicken, organ meats, whole grains, and some vegetables
Pantothenic acid is essential to multiple metabolic processes and readily available in sufficient amounts in most foods.2 Although limited RCT data suggest pantethine may improve lipid measures,12,98,99 pantothenic acid itself does not seem to share this effect.
The takeaway: There is no data that supplementation of any form of vitamin B5 has any patient-oriented clinical benefits.
Continue to: Vitamin B6...
Vitamin B6
Vitamers: Pyridoxine; pyridoxamine; pyridoxal
Physiologic role: Widely involved coenzyme for cognitive development, neurotransmitter biosynthesis, homocysteine and glucose metabolism, immune function, and hemoglobin formation
Dietary sources: Fish, organ meats, potatoes/starchy vegetables, fruit (other than citrus), and fortified cereals
Pyridoxine is required for numerous enzymatic processes in the body, including biosynthesis of neurotransmitters and homeostasis of the amino acid homocysteine.2 While overt deficiency is rare, marginal insufficiency may become clinically apparent and has been associated with malabsorption, malignancies, pregnancy, heart disease, alcoholism, and use of drugs such as isoniazid, hydralazine, and levodopa/carbidopa.2 Vitamin B6 supplementation is known to decrease plasma homocysteine levels, a theorized intermediary for cardiovascular disease; however, studies have failed to consistently demonstrate patient-oriented benefits.100-102 While observational data has suggested a correlation between vitamin B6 status and cancer risk, RCTs have not supported benefit from supplementation.14-16 Potential effects of vitamin B6 supplementation on cognitive function have also been studied without observed benefit.17,18
The takeaway: Vitamin B6 is recommended as a potential treatment option for nausea in pregnancy.19 Otherwise, vitamin B6 is readily available in food, deficiency is rare, and no patient-oriented evidence supports supplementation in the general population.
Vitamin B7
Vitamers: Biotin
Physiologic role: Cofactor in the metabolism of fatty acids, glucose, and amino acids. Also plays key role in histone modifications, gene regulation, and cell signaling
Dietary sources: Widely available; most prevalent in organ meats, fish, meat, seeds, nuts, and vegetables (eg, sweet potatoes). Whole cooked eggs are a major source, but raw eggs contain avidin, which blocks absorption
Biotin serves a key role in metabolism, gene regulation, and cell signaling.2 Biotin is known to interfere with laboratory assays— including cardiac enzymes, thyroid studies, and hormone studies—at normal supplementation doses, resulting in both false-positive and false-negative results.103
The takeaway: No evidence supports the health benefits of biotin supplementation.
Vitamin B9
Vitamers: Folates; folic acid
Physiologic role: Functions as a coenzyme in the synthesis of DNA/RNA and metabolism of amino acids
Dietary sources: Highest content in spinach, liver, asparagus, and brussels sprouts. Generally found in green leafy vegetables, fruits, nuts, beans, peas, seafood, eggs, dairy, meat, poultry, grains, and fortified cereals.
Continue to: Vitamin B12...
Vitamin B12
Vitamers: Cyanocobalamin; hydroxocobalamin; methylcobalamin; adenosylcobalamin
Physiologic role: Required for red blood cell formation, neurologic function, and DNA synthesis
Dietary sources: Only in animal products: fish, poultry, meat, eggs, and milk/dairy products. Not present in plant foods. Fortified cereals, nutritional yeast are sources for vegans/vegetarians.
Given their linked physiologic roles, vitamins B9 and B12 are frequently studied together. Folate and cobalamins play key roles in nucleic acid synthesis and amino acid metabolism, with their most clinically significant role in hematopoiesis. Vitamin B12 is also essential to normal neurologic function.2
The US Preventive Services Task Force (USPSTF) recommends preconceptual folate supplementation of 0.4-0.8 mg/d in women of childbearing age to decrease the risk of fetal neural tube defects (grade A).21 This is supported by high-quality RCT evidence demonstrating a protective effect of daily folate supplementation in preventing neural tube defects.22 Folate supplementation’s effect on other fetal birth defects has been investigated, but no benefit has been demonstrated. While observational studies have suggested an inverse relationship with folate status and fetal autism spectrum disorder,23-25 the RCT data is mixed.26
A potential role for folate in cancer prevention has been extensively investigated. An expert panel of the National Toxicology Program (NTP) concluded that folate supplementation does not reduce cancer risk in people with adequate baseline folate status based on high-quality meta-analysis data.27,104 Conversely, long-term follow-up from RCTs demonstrated an increased risk of colorectal adenomas and cancers,28,29 leading the NTP panel to conclude there is sufficient concern for adverse effects of folate on cancer growth to justify further research.104
While observational studies have found a correlation of increased risk for disease with lower antioxidant serum levels, RCTs have not demonstrated a reduction in disease risk with supplementation.
Given folate and vitamin B12’s homocysteine-reducing effects, it has been theorized that supplementation may protect from cardiovascular disease. However, despite extensive research, there remains no consistent patient-oriented outcomes data to support such a benefit.31,32,105
The evidence is mixed but generally has found no benefit of folate or vitamin B12 supplementation on cognitive function.18,33-35 Finally, RCT data has failed to demonstrate a reduction in fracture risk with supplementation.36,106
The takeaway: High-quality RCT evidence demonstrates a protective effect of preconceptual daily folate supplementation in preventing neural tube defects.22 The USPSTF recommends preconceptual folate supplementation of 0.4-0.8 mg/d in women of childbearing age to decrease the risk of fetal neural tube defects.
Antioxidants
In addition to their individual roles, vitamins A, E, and C are antioxidants, functioning to protect cells from oxidative damage by free radical species.2 Due to this shared role, these vitamins are commonly studied together. Antioxidants are hypothesized to protect from various diseases, including cancer, cardiovascular disease, dementia, autoimmune disorders, depression, cataracts, and age-related vision decline.2,37,107-112
Though observational studies have found a correlation of increased risk for disease with lower antioxidant serum levels, RCTs have not demonstrated a reduction in disease risk with supplementation and, in some cases, have found an increased risk of mortality. While several studies have found potential benefit of antioxidant use in reducing colon and breast cancer risk,38,113-115 vitamins A and E have been associated with increased risk of lung and prostate cancer, respectively.47,110 Cardiovascular disease and antioxidant vitamin supplementation has similar inconsistent data, ranging from slight benefit to harm.2,116 After a large Cochrane review in 2012 found a significant increase in all-cause mortality associated with vitamin E and beta-carotene,117 the USPSTF made a specific recommendation against supplementation of these vitamins for the prevention of cardiovascular disease or cancer (grade D).118 Given its limited risk for harm, vitamin C was excluded from this recommendation.
Continue to: Vitamin A...
Vitamin A
Vitamers: Retinol; retinal; retinyl esters; provitamin A carotenoids (beta-carotene, alpha-carotene, beta-cryptoxanthin)
Physiologic role: Essential for vision and corneal development. Also involved in general cell differentiation and immune function
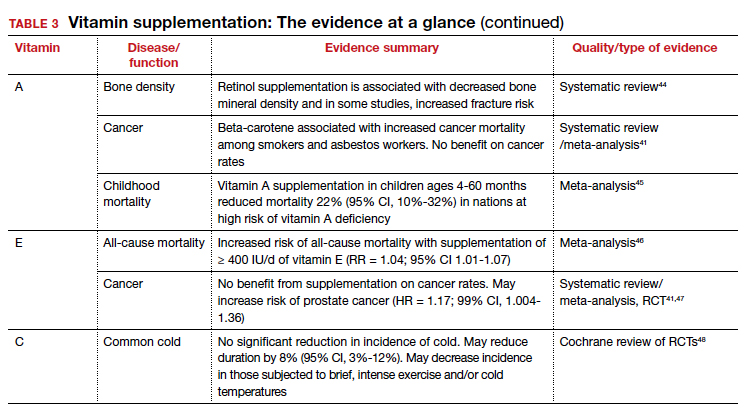
Dietary sources: Liver, fish oil, dairy, and fortified cereals. Provitamin A sources: leafy green vegetables, orange/yellow vegetables, tomato products, fruits, and vegetable oils Retinoids and their precursors, carotenoids, serve a critical function in vision, as well as regulating cell differentiation and proliferation throughout the body.2 While evidence suggests mortality benefit of supplementation in populations at risk of deficiency,45 wide-ranging studies have found either inconsistent benefit or outright harms in the developed world.
The takeaway: Given the USPSTF grade “D” recommendation and concern for potential harms, supplementation is not recommended in healthy patients without risk factors for deficiency.2
Vitamin E
Vitamers: Tocopherols (alpha-, beta-, gamma-, delta-); tocotrienol (alpha-, beta-, gamma-, delta-)
Physiologic role: Antioxidant; protects polyunsaturated fats from free radical oxidative damage. Involved in immune function, cell signaling, and regulation of gene expression
Dietary sources: Nuts, seeds, vegetable oil, green leafy vegetables, and fortified cereals
Vitamin E is the collective name of 8 compounds; alpha-tocopherol is the physiologically active form. Vitamin E is involved with cell proliferation as well as endothelial and platelet function.2
The takeaway: Vitamin E supplementation’s effects on cancer, cardiovascular disease, ophthalmologic disorders, and cognition have been investigated; data is either lacking to support a benefit or demonstrates harms as outlined above. Given this and the USPSTF grade “D” recommendation, supplementation is not recommended in healthy patients.2
Vitamin C
Vitamers: Ascorbic acid
Physiologic role: Required for synthesis of collagen, L-carnitine, and some neurotransmitters. Also involved in protein metabolism
Dietary sources: Primarily in fruits and vegetables: citrus, tomato, potatoes, red/green peppers, kiwi fruit, broccoli, strawberries, brussels sprouts, cantaloupe, and fortified cereals
Vitamin C supplementation at the onset of illness does not seem to have benefit.
Ascorbic acid is a required cofactor for biosynthesis of collagen, neurotransmitters, and protein metabolism.2 In addition to the shared hypothesized benefits of antioxidants, vitamin C supplementation has undergone extensive research into its potential role in augmenting the immune system and preventing the common cold. Systematic reviews have found daily vitamin C supplementation of at least 200 mg did not affect the incidence of the common cold in healthy adults but may shorten duration and could be of benefit in those exposed to extreme physical exercise or cold.48 Vitamin C supplementation at the onset of illness does not seem to have benefit.48 Data is insufficient to draw conclusions about a potential effect on pneumonia incidence or severity.119,120
The takeaway: Overall, data remain inconclusive as to potential benefits of vitamin C supplementation, although risks of potential harms are likely low.
Continue to: Vitamin D...
Vitamin D
Vitamers: Cholecalciferol (D3); ergocalciferol (D2)
Physiologic role: Hydroxylation in liver and kidney required to activate. Promotes dietary calcium absorption, enables normal bone mineralization. Also involved in modulation of cell growth, and neuromuscular and immune function
Dietary sources: Few natural dietary sources, which include fatty fish, fish liver oils; small amount in beef liver, cheese, egg yolks. Primary sources include fortified milk and endogenous synthesis in skin with UV exposure
Calciferol is a fat-soluble vitamin required for calcium and bone homeostasis. It is not naturally available in many foods but is primarily produced endogenously in the skin with ultraviolet light exposure.2
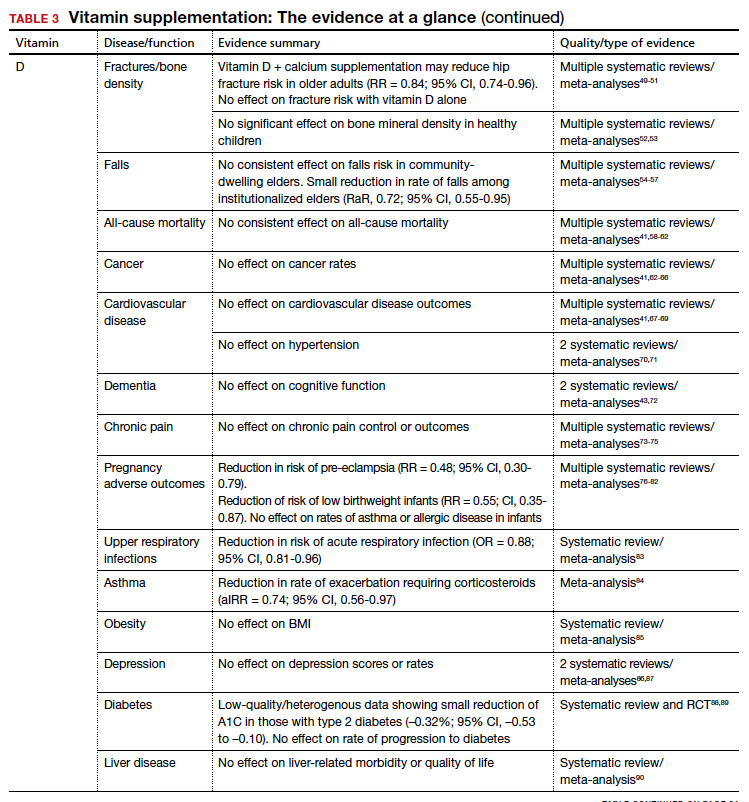
The AAP recommends supplementing exclusively breastfed infants with 400 IU/d of vitamin D to prevent rickets.
Bone density and fracture risk reduction are the most often cited benefits of vitamin D supplementation, but this has not been demonstrated consistently in RCTs. Multiple systematic reviews showing inconsistent benefit of vitamin D (with or without calcium) on fracture risk led the USPSTF to conclude that there is insufficient evidence (grade I) to issue a recommendation on vitamin D and calcium supplementation for primary prevention of fractures in postmenopausal women.49-51 Despite some initial evidence suggesting a benefit of vitamin D supplementation on falls reduction, 3 recent systematic reviews did not demonstrate this in community-dwelling elders,54-56 although a separate Cochrane review did suggest a reduction in rate of falls among institutionalized elders.57
The takeaway: Given these findings, the USPSTF has recommended against (grade D) vitamin D supplementation to prevent falls in community-dwelling elders.55
Beyond falls. While the vitamin D receptor is expressed throughout the body and observational studies have suggested a correlation between vitamin D status and many outcomes, extensive RCT data has generally failed to demonstrate extraskeletal benefits from supplementation. Meta-analysis data have demonstrated potential reductions in acute respiratory infection rates and asthma exacerbations with vitamin D supplementation. There is also limited evidence suggesting a reduction in preeclampsia and low-birthweight infant risk with vitamin D supplementation in pregnancy. However, several large meta-analyses and systematic reviews have investigated vitamin D supplementation’s effect on all-cause mortality and found no consistent data to support an association.41,58-62
Multiple systematic reviews have investigated and found high-quality evidence demonstrating no association between vitamin D supplementation and cancer41,63-66,121 or cardiovascular disease risk.41,70,71 There is high-quality data showing no benefit of vitamin D supplementation for multiple additional diseases, including diabetes, cognitive decline, depression, pain, obesity, and liver disease.43,72-75,85-90,122
The takeaway: Due to poor availability in breastmilk, the American Academy of Pediatrics (AAP) recommends supplementing exclusively breastfed infants with 400 IU/d of vitamin D to prevent rickets.123 RCT data support high-dose supplementation of lactating women (6400 IU/d) as an alternative strategy to supplementation of the infant.124 The AAP recommends that all nonbreastfeeding infants and older children ingesting < 1000 mL/d of vitamin D–fortified formula or milk should also be supplemented with 400 IU/d of vitamin D.123 Despite these universal recommendations for supplementation, evidence is mixed on the effect of vitamin D supplementation on bone health in children.52,53
Although concerns about vitamin D supplementation and increased risk of urolithiasis and hypercalcemia have been raised,51,62,121 systematic reviews have not demonstrated significant, clinically relevant risks, even with high-dose supplementation (> 2800 IU/d).125,126
Vitamin K
Vitamers: Phylloquinone (K1); menaquinones (K2)
Physiologic role: Coenzyme for synthesis of proteins involved in hemostasis and bone metabolism
Dietary sources: Phylloquinone is found in green leafy vegetables, vegetable oils, some fruits, meat, dairy, and eggs. Menaquinone is produced by gut bacteria and present in fermented foods
Vitamin K includes 2 groups of similar compounds: phylloquinone and menaquinones. Unlike other fat-soluble vitamins, vitamin K is rapidly metabolized and has low tissue storage.2
Children taking multivitamins were often found to have excess levels of potentially harmful nutrients, such as retinol, zinc, and folic acid.
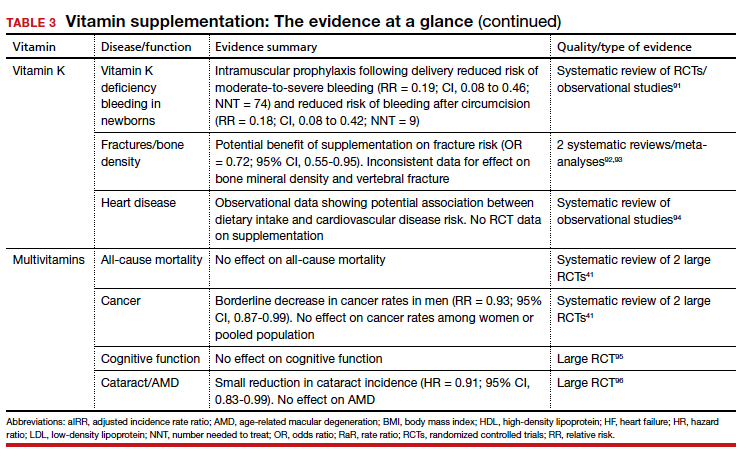
Administration of vitamin K 0.5 to 1 mg intramuscularly (IM) to newborns is standard of care for the prevention of vitamin K deficiency bleeding (VKDB). This is supported by RCT data demonstrating a reduction in classic VKDB (occurring within 7 days)91 and epidemiologic data from various countries showing a reduction in late-onset VKDB with vitamin K prophylaxis programs.127 Oral dosing appears to reduce the risk of VKDB in the setting of parental refusal but is less effective than IM dosing.128,129
Vitamin K’s effects on bone density and fracture risk have also been investigated. Systematic reviews have demonstrated a reduction in fracture risk with vitamin K supplementation,92,93 and European and Asian regulatory bodies have recognized a potential benefit on bone health.2 The FDA considers the evidence insufficient at this time to support such a claim.2 Higher dietary vitamin K consumption has been associated with lower risk of cardiovascular disease in observational studies94 and supplementation was associated with improved disease measures,130 but no patient-oriented outcomes have been demonstrated.131
The takeaway: The administration of vitamin K 0.5 to 1 mg intramuscularly (IM) to newborns is standard of care for the prevention of VKDB. Vitamin K may lead to a reduction in fracture risk, but the FDA considers the evidence insufficient. Vitamin K’s potential link to a lowered risk of cardiovascular disease has not been demonstrated with patient-oriented outcomes. Vitamin K has low potential for toxicity, although its interaction with vitamin K antagonists (ie, warfarin) is clinically relevant.
Multivitamins
Multivitamins are often defined as a supplement containing 3 or more vitamins and minerals but without herbs, hormones, or drugs.132 Many multivitamins do contain additional substances, and some include levels of vitamins that exceed the RDA or even the established tolerable upper intake level.133
Safe medication storage should be practiced, as multivitamins with iron are a leading cause of poisoning in children.
A 2013 systematic review found limited evidence to support any benefit from multivitamin supplementation.41 Two included RCTs demonstrated a narrowly significant decrease in cancer rates among men, but saw no effect in women or the combined population.134,135 This benefit appears to disappear at 5 years of follow-up.136 RCT data have shown no benefit of multivitamin use on cognitive function,95 and high-quality data suggest there is no effect on all-cause mortality.137 Given this lack of supporting evidence, the USPSTF has concluded that there is insufficient evidence (grade I) to recommend vitamin supplementation in general to prevent cardiovascular disease or cancer.41
The use of prenatal multivitamins is generally recommended in the pregnancy and preconception period and has been associated with reduced risk of autism spectrum disorders, pediatric cancer rates, small-for-gestational-age infants, and multiple birth defects in offspring; however, studies have not examined if this benefit exceeds that of folate supplementation alone.138-140 AAP does not recommend multivitamins for children with a well-balanced diet.141 Of concern, children taking multivitamins were often found to have excess levels of potentially harmful nutrients such as retinol, zinc, and folic acid.142
The takeaway: There is limited evidence to support any benefit from multivitamin supplementation. Prenatal multivitamins are generally recommended in the pregnancy and preconception period. Overall, the risks of multivitamins are minimal, although that risk is dependent on the multivitamin’s constituent components.143 Components such as vitamin K may interact with a patient’s medications, and multivitamins have been shown to reduce the circulating levels of antiretrovirals.144 Specifically, multivitamins with iron should be avoided in men and postmenopausal women, and safe medication storage should be practiced as multivitamins with iron are a leading cause of poisoning in children.2
Summary
Vitamin supplementation in the developed world remains common despite a paucity of RCT data supporting it. Supplementation of folate in women planning to conceive, vitamin D in breastfeeding infants, and vitamin K in newborns are well supported by clinical evidence. Otherwise, there is limited evidence supporting clinically significant benefit from supplementation in healthy patients with well-balanced diets—and in the case of vitamins A and E, there may be outright harms.
1. Half of Americans take vitamins regularly. Accessed June 16, 2020. https://news.gallup.com/poll/166541/half-americans-vitamins-regularly.aspx
2. National Institutes of Health. Vitamin and mineral supplement fact sheets. Published 2020. Accessed May 26, 2020. https://ods.od.nih.gov/factsheets/list-VitaminsMinerals/
3. Day E, Bentham PW, Callaghan R, et al. Thiamine for prevention and treatment of Wernicke-Korsakoff syndrome in people who abuse alcohol. Cochrane Database Syst Rev. 2013;(7):CD004033. doi:10.1002/14651858.CD004033.pub3
4. DiNicolantonio JJ, Niazi AK, Lavie CJ, et al. Thiamine supplementation for the treatment of heart failure: a review of the literature. Congest Heart Fail. 2013;19:214-222. doi:10.1111/chf.12037
5. Rodríguez-Martín JL, Qizilbash N, López-Arrieta JM. Thiamine for Alzheimer’s disease. Cochrane Database Syst Rev. 2001;(2):CD001498. doi:10.1002/14651858.CD001498
6. Schoenen J, Jacquy J, Lenaerts M. Effectiveness of high-dose riboflavin in migraine prophylaxis. A randomized controlled trial. Neurology. 1998;50:466-470. doi:10.1212/wnl.50.2.466
7. Johansson M, Relton C, Ueland PM, et al. Serum B vitamin levels and risk of lung cancer. JAMA. 2010;303:2377-2385. doi:10.1001/jama.2010.808
8. Kabat GC, Miller AB, Jain M, et al. Dietary intake of selected B vitamins in relation to risk of major cancers in women. Br J Cancer. 2008;99:816-821. doi:10.1038/sj.bjc.6604540
9. Zschäbitz S, Cheng T-YD, Neuhouser ML, et al. B vitamin intakes and incidence of colorectal cancer: results from the Women’s Health Initiative Observational Study cohort. Am J Clin Nutr. 2013;97:332-343. doi:10.3945/ajcn.112.034736
10. de Vogel S, Dindore V, van Engeland M, et al. Dietary folate, methionine, riboflavin, and vitamin B-6 and risk of sporadic colorectal cancer. J Nutr. 2008;138:2372-2378. doi:10.3945/jn.108.091157
11. Bassett JK, Hodge AM, English DR, et al. Dietary intake of B vitamins and methionine and risk of lung cancer. Eur J Clin Nutr. 2012;66:182-187. doi:10.1038/ejcn.2011.157
12. McRae MP. Treatment of hyperlipoproteinemia with pantethine: a review and analysis of efficacy and tolerability. Nutr Res. 2005; 25:319-333.
13. Saposnik G, Ray JG, Sheridan P, et al; Heart Outcomes Prevention Evaluation 2 Investigators. Homocysteine-lowering therapy and stroke risk, severity, and disability: additional findings from the HOPE 2 trial. Stroke. 2009;40:1365-1372. doi:10.1161/STROKEAHA.108.529503
14. Larsson SC, Orsini N, Wolk A. Vitamin B6 and risk of colorectal cancer: a meta-analysis of prospective studies. JAMA. 2010;303:1077-1083. doi:10.1001/jama.2010.263
15. Mocellin S, Briarava M, Pilati P. Vitamin B6 and cancer risk: a field synopsis and meta-analysis. J Natl Cancer Inst. 2017;109:1-9. doi:10.1093/jnci/djw230
16. Ebbing M, Bønaa KH, Nygård O, et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA. 2009;302:2119-2126. doi:10.1001/jama.2009.1622
17. Malouf R, Grimley Evans J. The effect of vitamin B6 on cognition. Cochrane Database Syst Rev. 2003;(4):CD004393. doi:10.1002/14651858.CD004393
18. Balk EM, Raman G, Tatsioni A, et al. Vitamin B6, B12, and folic acid supplementation and cognitive function: a systematic review of randomized trials. Arch Intern Med. 2007;167:21-30. doi:10.1001/archinte.167.1.21
19. American College of Obstetrics and Gynecology. ACOG Practice Bulletin: nausea and vomiting of pregnancy. Obstet Gynecol. 2004;103:803-814.
20. Matthews A, Dowswell T, Haas DM, et al. Interventions for nausea and vomiting in early pregnancy. Cochrane Database Syst Rev. 2010;(9):CD007575. doi:10.1002/14651858.CD007575.pub2
21. US Preventive Services Task Force. Folic acid for the prevention of neural tube defects: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150:626-631.
22. De-Regil LM, Peña-Rosas JP, Fernández-Gaxiola AC, et al. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst Rev. 2015;(12):CD007950. doi:10.1002/14651858.CD007950.pub3
23. Surén P, Roth C, Bresnahan M, et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA. 2013;309:570-577. doi:10.1001/jama.2012.155925
24. Schmidt RJ, Tancredi DJ, Ozonoff S, et al. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am J Clin Nutr. 2012;96:80-89. doi:10.3945/ajcn.110.004416
25. Levine SZ, Kodesh A, Viktorin A, et al. Association of maternal use of folic acid and multivitamin supplements in the periods before and during pregnancy with the risk of autism spectrum disorder in offspring. JAMA Psychiatry. 2018;75:176-184. doi:10.1001/jamapsychiatry.2017.4050
26. Virk J, Liew Z, Olsen J, et al. Preconceptional and prenatal supplementary folic acid and multivitamin intake and autism spectrum disorders. Autism. 2016;20:710-718. doi:10.1177/1362361315604076
27. Vollset SE, Clarke R, Lewington S, et al. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet. 2013;381:1029-1036. doi:10.1016/S0140-6736(12)62001-7
28. Passarelli MN, Barry EL, Rees JR, et al. Folic acid supplementation and risk of colorectal neoplasia during long-term follow-up of a randomized clinical trial. Am J Clin Nutr. 2019;110:903-911. doi:10.1093/ajcn/nqz160
29. Oliai Araghi S, Kiefte-de Jong JC, van Dijk SC, et al. Folic acid and vitamin B12 supplementation and the risk of cancer: long-term follow-up of the B vitamins for the Prevention of Osteoporotic Fractures (B-PROOF) Trial. Cancer Epidemiol Biomarkers Prev. 2019;28:275-282. doi:10.1158/1055-9965.EPI-17-1198
30. Wan Ismail WR, Abdul Rahman R, et al. The protective effect of maternal folic acid supplementation on childhood cancer: a systematic review and meta-analysis of case-control studies. J Prev Med Public Health. 2019;52:205-213. doi:10.3961/jpmph.19.020
31. Martí-Carvajal AJ, Solà I, Lathyris D, et al. Homocysteine lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2009;(4):CD006612. doi:10.1002/14651858.CD006612.pub2
32. Wang Y, Jin Y, Wang Y, et al. The effect of folic acid in patients with cardiovascular disease: A systematic review and meta-analysis. Medicine. 2019;98:e17095. doi:10.1097/MD.0000000000017095
33. Malouf R, Areosa Sastre A. Vitamin B12 for cognition. Cochrane Database Syst Rev. 2003;(3):CD004326. doi:10.1002/14651858.CD004326
34. Malouf R, Grimley Evans J. Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people. Cochrane Database Syst Rev. 2008;(4):CD004514. doi:10.1002/14651858.CD004514.pub2
35. Suh SW, Kim HS, Han JH, et al. Efficacy of vitamins on cognitive function of non-demented people: a systematic review and meta-analysis. Nutrients. 2020;12(4). doi:10.3390/nu12041168
36. Stone KL, Lui L-Y, Christen WG, et al. Effect of combination folic acid, vitamin B6, and vitamin B12 supplementation on fracture risk in women: a randomized, controlled trial. J Bone Miner Res. 2017;32:2331-2338. doi:10.1002/jbmr.3229
37. Age-related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417-1436. doi:10.1001/archopht.119.10.1417
38. Park Y, Spiegelman D, Hunter DJ, et al. Intakes of vitamins A, C, and E and use of multiple vitamin supplements and risk of colon cancer: a pooled analysis of prospective cohort studies. Cancer Causes Control. 2010;21:1745-1757. doi:10.1007/s10552-010-9549-y
39. Koushik A, Wang M, Anderson KE, et al. Intake of vitamins A, C, and E and folate and the risk of ovarian cancer in a pooled analysis of 10 cohort studies. Cancer Causes Control. 2015;26:1315-1327. doi:10.1007/s10552-015-0626-0
40. Lin J, Cook NR, Albert C, et al. Vitamins C and E and beta carotene supplementation and cancer risk: a randomized controlled trial. J Natl Cancer Inst. 2009;101:14-23. doi:10.1093/jnci/djn438
41. Fortmann SP, Burda BU, Senger CA, et al. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: an updated systematic evidence review for the US Preventive Services Task Force. Ann Intern Med. 2013;159:824-834. doi:10.7326/0003-4819-159-12-201312170-00729
42. Mathew MC, Ervin A-M, Tao J, et al. Antioxidant vitamin supplementation for preventing and slowing the progression of age-related cataract. Cochrane Database Syst Rev. 2012;(6):CD004567. doi:10.1002/14651858.CD004567.pub2
43. Butler M, Nelson VA, Davila H, et al. Over-the-counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med. 2018;168:52-62. doi:10.7326/M17-1530
44. Crandall C. Vitamin A intake and osteoporosis: a clinical review. J Womens Health (Larchmt). 2004;13:939-953. doi:10.1089/jwh.2004.13.939
45. Kranz S, Pimpin L, Fawzi W, et al. Mortality benefits of vitamin A are not affected by varying frequency, total dose, or duration of supplementation. Food Nutr Bull. 2017;38:260-266. doi:10.1177/0379572117696663
46. Miller ER, Pastor-Barriuso R, Dalal D, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37-46. doi:10.7326/0003-4819-142-1-200501040-00110
47. Klein EA, Thompson IM, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2011;306:1549-1556. doi:10.1001/jama.2011.1437
48. Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013;(1):CD000980. doi:10.1002/14651858.CD000980.pub4
49. Avenell A, Mak JCS, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;(4):CD000227. doi:10.1002/14651858.CD000227.pub4
50. Zhao J-G, Zeng X-T, Wang J, et al. Association between calcium or vitamin D supplementation and fracture incidence in community-dwelling older adults: a systematic review and meta-analysis. JAMA. 2017;318:2466-2482. doi:10.1001/jama.2017.19344
51. Kahwati LC, Weber RP, Pan H, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319:1600-1612. doi:10.1001/jama.2017.21640
52. Winzenberg T, Powell S, Shaw KA, et al. Effects of vitamin D supplementation on bone density in healthy children: systematic review and meta-analysis. BMJ. 2011;342:c7254. doi:10.1136/bmj.c7254
53. Winzenberg TM, Powell S, Shaw KA, et al. Vitamin D supplementation for improving bone mineral density in children. Cochrane Database Syst Rev. 2010;(10):CD006944. doi:10.1002/14651858.CD006944.pub2
54. Bolland MJ, Grey A, Gamble GD, et al. Vitamin D supplementation and falls: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014;2:573-580. doi:10.1016/S2213-8587(14)70068-3
55. Guirguis-Blake JM, Michael YL, Perdue LA, et al. Interventions to prevent falls in older adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319:1705-1716. doi:10.1001/jama.2017.21962
56. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;(9):CD007146. doi:10.1002/14651858.CD007146.pub3
57. Cameron ID, Dyer SM, Panagoda CE, et al. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database Syst Rev. 2018;9:CD005465. doi:10.1002/14651858.CD005465.pub4
58. Chung M, Balk EM, Brendel M, et al. Vitamin D and calcium: a systematic review of health outcomes. Evid Rep Technol Assess (Full Rep). 2009;(183):1-420.
59. Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730-1737. doi:10.1001/archinte.167.16.1730
60. Zhang Y, Fang F, Tang J, et al. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ. 2019;366:l4673. doi:10.1136/bmj.l4673
61. Cauley JA, Chlebowski RT, Wactawski-Wende J, et al. Calcium plus vitamin D supplementation and health outcomes five years after active intervention ended: the Women’s Health Initiative. J Womens Health (Larchmt). 2013;22:915-929. doi:10.1089/jwh.2013.4270
62. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2014;(1):CD007470. doi:10.1002/14651858.CD007470.pub3
63. Zhou L, Chen B, Sheng L, et al. The effect of vitamin D supplementation on the risk of breast cancer: a trial sequential meta-analysis. Breast Cancer Res Treat. 2020;182:1-8. doi:10.1007/s10549-020-05669-4
64. Shahvazi S, Soltani S, Ahmadi SM, et al A. The effect of vitamin D supplementation on prostate cancer: a systematic review and meta-analysis of clinical trials. Horm Metab Res. 2019;51:11-21. doi:10.1055/a-0774-8809
65. Buttigliero C, Monagheddu C, Petroni P, et al. Prognostic role of vitamin d status and efficacy of vitamin D supplementation in cancer patients: a systematic review. Oncologist. 2011;16:1215-1227. doi:10.1634/theoncologist.2011-0098
66. Cortés-Jofré M, Rueda J-R, Asenjo-Lobos C, et al. Drugs for preventing lung cancer in healthy people. Cochrane Database Syst Rev. 2020;3:CD002141. doi:10.1002/14651858.CD002141.pub3
67. Elamin MB, Abu Elnour NO, Elamin KB, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:1931-1942. doi:10.1210/jc.2011-0398
68. Pittas AG, Chung M, Trikalinos T, et al. Systematic review: vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152:307-314. doi:10.7326/0003-4819-152-5-201003020-00009
69. Ford JA, MacLennan GS, Avenell A, et al. Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr. 2014;100:746-755. doi:10.3945/ajcn.113.082602
70. Beveridge LA, Struthers AD, Khan F, et al. Effect of vitamin D supplementation on blood pressure: a systematic review and meta-analysis incorporating individual patient data. JAMA Intern Med. 2015;175:745-754. doi:10.1001/jamainternmed.2015.0237
71. Qi D, Nie X, Cai J. The effect of vitamin D supplementation on hypertension in non-CKD populations: a systemic review and meta-analysis. Int J Cardiol. 2017;227:177-186. doi:10.1016/j.ijcard.2016.11.040
72. Rutjes AW, Denton DA, Di Nisio M, et al. Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in mid and late life. Cochrane Database Syst Rev. 2018;12:CD011906. doi:10.1002/14651858.CD011906.pub2
73. Straube S, Derry S, Straube C, et al. Vitamin D for the treatment of chronic painful conditions in adults. Cochrane Database Syst Rev. 2015;(5):CD007771. doi:10.1002/14651858.CD007771.pub3
74. Zadro JR, Shirley D, Ferreira M, et al. Is vitamin D supplementation effective for low back pain? A systematic review and meta-analysis. Pain Physician. 2018;21:121-145.
75. Wu Z, Malihi Z, Stewart AW, et al. Effect of vitamin D supplementation on pain: a systematic review and meta-analysis. Pain Physician. 2016;19:415-427.
76. Palacios C, Kostiuk LK, Peña-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. 2019;7:CD008873. doi:10.1002/14651858.CD008873.pub4
77. Bi WG, Nuyt AM, Weiler H, et al. Association between vitamin D supplementation during pregnancy and offspring growth, morbidity, and mortality: a systematic review and meta-analysis. JAMA Pediatr. 2018;172:635-645. doi:10.1001/jamapediatrics.2018.0302
78. Yepes-Nuñez JJ, Brożek JL, Fiocchi A, et al. Vitamin D supplementation in primary allergy prevention: Systematic review of randomized and non-randomized studies. Allergy. 2018;73:37-49. doi:10.1111/all.13241
79. Purswani JM, Gala P, Dwarkanath P, et al. The role of vitamin D in pre-eclampsia: a systematic review. BMC Pregnancy Childbirth. 2017;17:231. doi:10.1186/s12884-017-1408-3
80. Khaing W, Vallibhakara SA-O, Tantrakul V, et al. Calcium and vitamin D supplementation for prevention of preeclampsia: a systematic review and network meta-analysis. Nutrients. 2017;9:1141. doi:10.3390/nu9101141
81. Palacios C, De-Regil LM, Lombardo LK, et al. Vitamin D supplementation during pregnancy: updated meta-analysis on maternal outcomes. J Steroid Biochem Mol Biol. 2016;164:148-155. doi:10.1016/j.jsbmb.2016.02.008
82. Litonjua AA, Carey VJ, Laranjo N, et al. Six-year follow-up of a trial of antenatal vitamin D for asthma reduction. N Engl J Med. 2020;382:525-533. doi:10.1056/NEJMoa1906137
83. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi:10.1136/bmj.i6583
84. Jolliffe DA, Greenberg L, Hooper RL, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet Respir Med. 2017;5:881-890. doi:10.1016/S2213-2600(17)30306-5
85. Chandler PD, Wang L, Zhang X, et al. Effect of vitamin D supplementation alone or with calcium on adiposity measures: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. 2015;73:577-593. doi:10.1093/nutrit/nuv012
86. Gowda U, Mutowo MP, Smith BJ, et al. Vitamin D supplementation to reduce depression in adults: meta-analysis of randomized controlled trials. Nutrition. 2015;31:421-429. doi:10.1016/j.nut.2014.06.017
87. Li G, Mbuagbaw L, Samaan Z, et al. Efficacy of vitamin D supplementation in depression in adults: a systematic review. J Clin Endocrinol Metab. 2014;99:757-767. doi:10.1210/jc.2013-3450
88. Pittas AG, Dawson-Hughes B, Sheehan P, et al. Vitamin D supplementation and prevention of type 2 diabetes. N Engl J Med. 2019;381:520-530. doi:10.1056/NEJMoa1900906
89. Lee CJ, Iyer G, Liu Y, et al. The effect of vitamin D supplementation on glucose metabolism in type 2 diabetes mellitus: a systematic review and meta-analysis of intervention studies. J Diabetes Complicat. 2017;31:1115-1126. doi:10.1016/j.jdiacomp.2017.04.019
90. Bjelakovic G, Nikolova D, Bjelakovic M, et al. Vitamin D supplementation for chronic liver diseases in adults. Cochrane Database Syst Rev. 2017;11:CD011564. doi:10.1002/14651858.CD011564.pub2
91. Sankar MJ, Chandrasekaran A, Kumar P, et al. Vitamin K prophylaxis for prevention of vitamin K deficiency bleeding: a systematic review. J Perinatol. 2016;36(suppl 1):S29-S35. doi:10.1038/jp.2016.30
92. Mott A, Bradley T, Wright K, et al. Effect of vitamin K on bone mineral density and fractures in adults: an updated systematic review and meta-analysis of randomised controlled trials. Osteoporos Int. 2019;30:1543-1559. doi:10.1007/s00198-019-04949-0
93. Cockayne S, Adamson J, Lanham-New S, et al. Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:1256-1261. doi:10.1001/archinte.166.12.1256
94. Chen H-G, Sheng L-T, Zhang Y-B, et al. Association of vitamin K with cardiovascular events and all-cause mortality: a systematic review and meta-analysis. Eur J Nutr. 2019;58:2191-2205. doi:10.1007/s00394-019-01998-3
95. Grodstein F, O’Brien J, Kang JH, et al. Long-term multivitamin supplementation and cognitive function in men: a randomized trial. Ann Intern Med. 2013;159:806-814. doi:10.7326/0003-4819-159-12-201312170-00006
96. Christen WG, Glynn RJ, Manson JE, et al. Effects of multivitamin supplement on cataract and age-related macular degeneration in a randomized trial of male physicians. Ophthalmology. 2014;121:525-534. doi:10.1016/j.ophtha.2013.09.038
97. Holland S, Silberstein SD, Freitag F, et al. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346-1353. doi:10.1212/WNL.0b013e3182535d0c
98. Rumberger JA, Napolitano J, Azumano I, et al. Pantethine, a derivative of vitamin B(5) used as a nutritional supplement, favorably alters low-density lipoprotein cholesterol metabolism in low- to moderate-cardiovascular risk North American subjects: a triple-blinded placebo and diet-controlled investigation. Nutr Res. 2011;31:608-615. doi:10.1016/j.nutres.2011.08.001
99. Evans M, Rumberger JA, Azumano I, et al. Pantethine, a derivative of vitamin B5, favorably alters total, LDL and non-HDL cholesterol in low to moderate cardiovascular risk subjects eligible for statin therapy: a triple-blinded placebo and diet-controlled investigation. Vasc Health Risk Manag. 2014;10:89-100. doi:10.2147/VHRM.S57116
100. Ebbing M, Bønaa KH, Arnesen E, et al. Combined analyses and extended follow-up of two randomized controlled homocysteine-lowering B-vitamin trials. J Intern Med. 2010;268:367-382. doi:10.1111/j.1365-2796.2010.02259.x
101. Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291:565-575. doi:10.1001/jama.291.5.565
102. Albert CM, Cook NR, Gaziano JM, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008;299:2027-2036. doi:10.1001/jama.299.17.2027
103. FDA. The FDA warns that biotin may interfere with lab tests: FDA Safety Communication. Accessed June 1, 2020. www.fda.gov/medical-devices/safety-communications/update-fda-warns-biotin-may-interfere-lab-tests-fda-safety-communication
104. National Toxicology Program. Identifying research needs for assessing safe use of high intakes of folic acid. Published 2015. Accessed June 7, 2020. https://ntp.niehs.nih.gov/ntp/ohat/folicacid/final_monograph_508.pdf
105. Miller ER, Juraschek S, Pastor-Barriuso R, et al. Meta-analysis of folic acid supplementation trials on risk of cardiovascular disease and risk interaction with baseline homocysteine levels. Am J Cardiol. 2010;106:517-527. doi:10.1016/j.amjcard.2010.03.064
106. van Wijngaarden JP, Swart KMA, Enneman AW, et al. Effect of daily vitamin B-12 and folic acid supplementation on fracture incidence in elderly individuals with an elevated plasma homocysteine concentration: B-PROOF, a randomized controlled trial. Am J Clin Nutr. 2014;100:1578-1586. doi:10.3945/ajcn.114.090043
107. Harirchian MH, Mohammadpour Z, Fatehi F, et al. A systematic review and meta-analysis of randomized controlled trials to evaluating the trend of cytokines to vitamin A supplementation in autoimmune diseases. Clin Nutr. 2019;38:2038-2044. doi:10.1016/j.clnu.2018.10.026
108. Liu T, Zhong S, Liao X, et al. A meta-analysis of oxidative stress markers in depression. PLoS One. 2015;10:e0138904. doi:10.1371/journal.pone.0138904
109. Zeng J, Chen L, Wang Z, et al. Marginal vitamin A deficiency facilitates Alzheimer’s pathogenesis. Acta Neuropathol. 2017;133:967-982. doi:10.1007/s00401-017-1669-y
110. Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150-1155. doi:10.1056/NEJM199605023341802
111. Kanellopoulou A, Riza E, Samoli E, et al. Dietary supplement use after cancer diagnosis in relation to total mortality, cancer mortality and recurrence: a systematic review and meta-analysis. Nutr Cancer. 2021;73:16-30. doi:10.1080/01635581.2020.1734215
112. Sunkara A, Raizner A. Supplemental vitamins and minerals for cardiovascular disease prevention and treatment. Methodist Debakey Cardiovasc J. 2019;15:179-184. doi:10.14797/mdcj-15-3-179
113. Zhang S, Hunter DJ, Forman MR, et al. Dietary carotenoids and vitamins A, C, and E and risk of breast cancer. J Natl Cancer Inst. 1999;91:547-556. doi:10.1093/jnci/91.6.547
114. He J, Gu Y, Zhang S. Vitamin A and breast cancer survival: a systematic review and meta-analysis. Clin Breast Cancer. 2018;18:e1389-e1400. doi:10.1016/j.clbc.2018.07.025
115. Harris HR, Orsini N, Wolk A. Vitamin C and survival among women with breast cancer: a meta-analysis. Eur J Cancer. 2014;50:1223-1231. doi:10.1016/j.ejca.2014.02.013
116. Moser MA, Chun OK. Vitamin C and heart health: a review based on findings from epidemiologic studies. Int J Mol Sci. 2016;17. doi:10.3390/ijms17081328
117. Bjelakovic G, Nikolova D, Gluud LL, et al. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev. 2012;(3):CD007176. doi:10.1002/14651858.CD007176.pub2
118. US Preventive Services Task Force. Vitamin supplementation to prevent cancer and CVD: preventive medication. Accessed May 21, 2020. www.uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-supplementation-to-prevent-cancer-and-cvd-counseling
119. Hemilä H, Louhiala P. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst Rev. 2013;(8):CD005532. doi:10.1002/14651858.CD005532.pub3
120. Padhani ZA, Moazzam Z, Ashraf A, et al. Vitamin C supplementation for prevention and treatment of pneumonia. Cochrane Database Syst Rev. 2020;4:CD013134. doi:10.1002/14651858.CD013134.pub2
121. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database Syst Rev. 2014;(6):CD007469. doi:10.1002/14651858.CD007469.pub2
122. Autier P, Mullie P, Macacu A, et al. Effect of vitamin D supplementation on non-skeletal disorders: a systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol. 2017;5:986-1004. doi:10.1016/S2213-8587(17)30357-1
123. Wagner CL, Greer FR; American Academy of Pediatrics Section on Breastfeeding, American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142-1152. doi:10.1542/peds.2008-1862
124. Hollis BW, Wagner CL, Howard CR, et al. Maternal versus infant vitamin D supplementation during lactation: a randomized controlled trial. Pediatrics. 2015;136:625-634. doi:10.1542/peds.2015-1669
125. Malihi Z, Wu Z, Stewart AW, et al. Hypercalcemia, hypercalciuria, and kidney stones in long-term studies of vitamin D supplementation: a systematic review and meta-analysis. Am J Clin Nutr. 2016;104:1039-1051. doi:10.3945/ajcn.116.134981
126. Vogiatzi MG, Jacobson-Dickman E, DeBoer MD; Drugs, and Therapeutics Committee of The Pediatric Endocrine Society. Vitamin D supplementation and risk of toxicity in pediatrics: a review of current literature. J Clin Endocrinol Metab. 2014;99:1132-1141. doi:10.1210/jc.2013-3655
127. Zurynski Y, Grover CJ, Jalaludin B, et al. Vitamin K deficiency bleeding in Australian infants 1993-2017: an Australian Paediatric Surveillance Unit study. Arch Dis Child. 2020;105:433-438. doi:10.1136/archdischild-2018-316424
128. Ng E, Loewy AD. Guidelines for vitamin K prophylaxis in newborns: a joint statement of the Canadian Paediatric Society and the College of Family Physicians of Canada. Can Fam Physician. 2018;64:736-739.
129. Araki S, Shirahata A. Vitamin K deficiency bleeding in infancy. Nutrients. 2020;12:780. doi:10.3390/nu12030780
130. Shea MK, Holden RM. Vitamin K status and vascular calcification: evidence from observational and clinical studies. Adv Nutr. 2012;3:158-165. doi:10.3945/an.111.001644
131. Hartley L, Clar C, Ghannam O, et al. Vitamin K for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2015;(9):CD011148. doi:10.1002/14651858.CD011148.pub2
132. Huang H-Y, Caballero B, Chang S, et al. Multivitamin/mineral supplements and prevention of chronic disease. Evid Rep Technol Assess (Full Rep). 2006;(139):1-117.
133. Bailey RL, Gahche JJ, Lentino CV, et al. Dietary supplement use in the United States, 2003-2006. J Nutr. 2011;141:261-266. doi:10.3945/jn.110.133025
134. Gaziano JM, Sesso HD, Christen WG, et al. Multivitamins in the prevention of cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2012;308:1871-1880. doi:10.1001/jama.2012.14641
135. Hercberg S, Galan P, Preziosi P, et al. The SU.VI.MAX Study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med. 2004;164:2335-2342. doi:10.1001/archinte.164.21.2335
136. Hercberg S, Kesse-Guyot E, Druesne-Pecollo N, et al. Incidence of cancers, ischemic cardiovascular diseases and mortality during 5-year follow-up after stopping antioxidant vitamins and minerals supplements: a postintervention follow-up in the SU.VI.MAX Study. Int J Cancer. 2010;127:1875-1881. doi:10.1002/ijc.25201
137. Khan SU, Khan MU, Riaz H, et al. Effects of nutritional supplements and dietary interventions on cardiovascular outcomes: an umbrella review and evidence map. Ann Intern Med. 2019;171:190-198. doi:10.7326/M19-0341
138. Guo B-Q, Li H-B, Zhai D-S, et al. Maternal multivitamin supplementation is associated with a reduced risk of autism spectrum disorder in children: a systematic review and meta-analysis. Nutr Res. 2019;65:4-16. doi:10.1016/j.nutres.2019.02.003
139. Wolf HT, Hegaard HK, Huusom LD, et al. Multivitamin use and adverse birth outcomes in high-income countries: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217:404.e1-404.e30. doi:10.1016/j.ajog.2017.03.029
140. Goh YI, Bollano E, Einarson TR, et al. Prenatal multivitamin supplementation and rates of pediatric cancers: a meta-analysis. Clin Pharmacol Ther. 2007;81:685-691. doi:10.1038/sj.clpt.6100100
141. HealthyChildren.org. Where we stand: vitamins. Accessed June 27, 2020. www.healthychildren.org/English/healthy-living/nutrition/Pages/Where-We-Stand-Vitamins.aspx
142. Bailey RL, Catellier DJ, Jun S, et al. Total usual nutrient intakes of US children (under 48 months): findings from the Feeding Infants and Toddlers Study (FITS) 2016. J Nutr. 2018;148:1557S-1566S. doi:10.1093/jn/nxy042
143. Biesalski HK, Tinz J. Multivitamin/mineral supplements: rationale and safety. Nutrition. 2017;36:60-66. doi:10.1016/j.nut.2016.06.003
144. Jalloh MA, Gregory PJ, Hein D, et al. Dietary supplement interactions with antiretrovirals: a systematic review. Int J STD AIDS. 2017;28:4-15. doi:10.1177/0956462416671087
Since their discovery in the early 1900s as the treatment for life-threatening deficiency syndromes, vitamins have been touted as panaceas for numerous ailments. While observational data have suggested potential correlations between vitamin status and every imaginable disease, randomized controlled trials (RCTs) have generally failed to find benefits from supplementation. Despite this lack of proven efficacy, more than half of older adults reported taking vitamins regularly.1
While most clinicians consider vitamins to be, at worst, expensive placebos, the potential for harm and dangerous interactions exists. Unlike pharmaceuticals, vitamins are generally unregulated, and the true content of many dietary supplements is often difficult to elucidate. Understanding the physiologic role, foundational evidence, and specific indications for the various vitamins is key to providing the best recommendations to patients.
Vitamins are essential organic nutrients, required in small quantities for normal metabolism. Since they are not synthesized endogenously, they must be ingested via food intake. In the developed world, vitamin deficiency syndromes are rare, thanks to sufficiently balanced diets and availability of fortified foods. The focus of this article will be on vitamin supplementation in healthy patients with well-balanced diets. TABLE E12 lists the 13 recognized vitamins, their recommended dietary allowances, and any known toxicity risks. TABLE 22 outlines elements of the history to consider when evaluating for deficiency. A summary of the most clinically significant evidence for vitamin supplementation follows; a more comprehensive review can be found in TABLE 3.3-96







B Complex vitamins
Vitamin B1
Vitamers: Thiamine (thiamin)
Physiologic role: Critical in carbohydrate and amino-acid catabolism and energy metabolism
Dietary sources: Whole grains, meat, fish, fortified cereals, and breads
Thiamine serves as an essential cofactor in energy metabolism.2 Thiamine deficiency is responsible for beriberi syndrome (rare in the developed world) and Wernicke-Korsakoff syndrome, the latter of which is a relatively common complication of chronic alcohol dependence. Although thiamine’s administration in these conditions can be curative, evidence is lacking to support its use preventively in patients with alcoholism.3 Thiamine has additionally been theorized to play a role in cardiac and cognitive function, but RCT data has not shown consistent patient-oriented benefits.4,5
The takeaway: Given the lack of evidence, supplementation in the general population is not recommended.
Continue to: Vitamin B2...
Vitamin B2
Vitamers: Riboflavin
Physiologic role: Essential component of cellular function and growth, energy production, and metabolism of fats and drugs
Dietary sources: Eggs, organ meats, lean meats, milk, green vegetables, fortified cereals and grains Riboflavin is essential to energy production, cellular growth, and metabolism.2
The takeaway: Its use as migraine prophylaxis has limited data,97 but there is otherwise no evidence to support health benefits of riboflavin supplementation.
Vitamin B3
Vitamers: Nicotinic acid (niacin); nicotinamide (niacinamide); nicotinamide riboside
Physiologic role: Converted to nicotinamide adenine dinucleotide (NAD), which is widely required in most cellular metabolic redox processes. Crucial to the synthesis and metabolism of carbohydrates, fatty acids, and proteins
Dietary sources: Poultry, beef, fish, nuts, legumes, grains. (Tryptophan can also be converted to NAD.)
Niacin is readily converted to NAD, an essential coenzyme for multiple catalytic processes in the body. While niacin at doses more than 100 times the recommended dietary allowance (RDA; 1-3 g/d) has been extensively studied for its role in dyslipidemias,2 pharmacologic dosing is beyond the scope of this article.
The takeaway: There is no evidence supporting a clinical benefit from niacin supplementation.
Vitamin B5
Vitamers: Pantothenic acid; pantethine
Physiologic role: Required for synthesis of coenzyme A (CoA) and acyl carrier protein, both essential in fatty acid and other anabolic/catabolic processes
Dietary sources: Almost all plant/animal-based foods. Richest sources include beef, chicken, organ meats, whole grains, and some vegetables
Pantothenic acid is essential to multiple metabolic processes and readily available in sufficient amounts in most foods.2 Although limited RCT data suggest pantethine may improve lipid measures,12,98,99 pantothenic acid itself does not seem to share this effect.
The takeaway: There is no data that supplementation of any form of vitamin B5 has any patient-oriented clinical benefits.
Continue to: Vitamin B6...
Vitamin B6
Vitamers: Pyridoxine; pyridoxamine; pyridoxal
Physiologic role: Widely involved coenzyme for cognitive development, neurotransmitter biosynthesis, homocysteine and glucose metabolism, immune function, and hemoglobin formation
Dietary sources: Fish, organ meats, potatoes/starchy vegetables, fruit (other than citrus), and fortified cereals
Pyridoxine is required for numerous enzymatic processes in the body, including biosynthesis of neurotransmitters and homeostasis of the amino acid homocysteine.2 While overt deficiency is rare, marginal insufficiency may become clinically apparent and has been associated with malabsorption, malignancies, pregnancy, heart disease, alcoholism, and use of drugs such as isoniazid, hydralazine, and levodopa/carbidopa.2 Vitamin B6 supplementation is known to decrease plasma homocysteine levels, a theorized intermediary for cardiovascular disease; however, studies have failed to consistently demonstrate patient-oriented benefits.100-102 While observational data has suggested a correlation between vitamin B6 status and cancer risk, RCTs have not supported benefit from supplementation.14-16 Potential effects of vitamin B6 supplementation on cognitive function have also been studied without observed benefit.17,18
The takeaway: Vitamin B6 is recommended as a potential treatment option for nausea in pregnancy.19 Otherwise, vitamin B6 is readily available in food, deficiency is rare, and no patient-oriented evidence supports supplementation in the general population.
Vitamin B7
Vitamers: Biotin
Physiologic role: Cofactor in the metabolism of fatty acids, glucose, and amino acids. Also plays key role in histone modifications, gene regulation, and cell signaling
Dietary sources: Widely available; most prevalent in organ meats, fish, meat, seeds, nuts, and vegetables (eg, sweet potatoes). Whole cooked eggs are a major source, but raw eggs contain avidin, which blocks absorption
Biotin serves a key role in metabolism, gene regulation, and cell signaling.2 Biotin is known to interfere with laboratory assays— including cardiac enzymes, thyroid studies, and hormone studies—at normal supplementation doses, resulting in both false-positive and false-negative results.103
The takeaway: No evidence supports the health benefits of biotin supplementation.
Vitamin B9
Vitamers: Folates; folic acid
Physiologic role: Functions as a coenzyme in the synthesis of DNA/RNA and metabolism of amino acids
Dietary sources: Highest content in spinach, liver, asparagus, and brussels sprouts. Generally found in green leafy vegetables, fruits, nuts, beans, peas, seafood, eggs, dairy, meat, poultry, grains, and fortified cereals.
Continue to: Vitamin B12...
Vitamin B12
Vitamers: Cyanocobalamin; hydroxocobalamin; methylcobalamin; adenosylcobalamin
Physiologic role: Required for red blood cell formation, neurologic function, and DNA synthesis
Dietary sources: Only in animal products: fish, poultry, meat, eggs, and milk/dairy products. Not present in plant foods. Fortified cereals, nutritional yeast are sources for vegans/vegetarians.
Given their linked physiologic roles, vitamins B9 and B12 are frequently studied together. Folate and cobalamins play key roles in nucleic acid synthesis and amino acid metabolism, with their most clinically significant role in hematopoiesis. Vitamin B12 is also essential to normal neurologic function.2
The US Preventive Services Task Force (USPSTF) recommends preconceptual folate supplementation of 0.4-0.8 mg/d in women of childbearing age to decrease the risk of fetal neural tube defects (grade A).21 This is supported by high-quality RCT evidence demonstrating a protective effect of daily folate supplementation in preventing neural tube defects.22 Folate supplementation’s effect on other fetal birth defects has been investigated, but no benefit has been demonstrated. While observational studies have suggested an inverse relationship with folate status and fetal autism spectrum disorder,23-25 the RCT data is mixed.26
A potential role for folate in cancer prevention has been extensively investigated. An expert panel of the National Toxicology Program (NTP) concluded that folate supplementation does not reduce cancer risk in people with adequate baseline folate status based on high-quality meta-analysis data.27,104 Conversely, long-term follow-up from RCTs demonstrated an increased risk of colorectal adenomas and cancers,28,29 leading the NTP panel to conclude there is sufficient concern for adverse effects of folate on cancer growth to justify further research.104
While observational studies have found a correlation of increased risk for disease with lower antioxidant serum levels, RCTs have not demonstrated a reduction in disease risk with supplementation.
Given folate and vitamin B12’s homocysteine-reducing effects, it has been theorized that supplementation may protect from cardiovascular disease. However, despite extensive research, there remains no consistent patient-oriented outcomes data to support such a benefit.31,32,105
The evidence is mixed but generally has found no benefit of folate or vitamin B12 supplementation on cognitive function.18,33-35 Finally, RCT data has failed to demonstrate a reduction in fracture risk with supplementation.36,106
The takeaway: High-quality RCT evidence demonstrates a protective effect of preconceptual daily folate supplementation in preventing neural tube defects.22 The USPSTF recommends preconceptual folate supplementation of 0.4-0.8 mg/d in women of childbearing age to decrease the risk of fetal neural tube defects.
Antioxidants
In addition to their individual roles, vitamins A, E, and C are antioxidants, functioning to protect cells from oxidative damage by free radical species.2 Due to this shared role, these vitamins are commonly studied together. Antioxidants are hypothesized to protect from various diseases, including cancer, cardiovascular disease, dementia, autoimmune disorders, depression, cataracts, and age-related vision decline.2,37,107-112
Though observational studies have found a correlation of increased risk for disease with lower antioxidant serum levels, RCTs have not demonstrated a reduction in disease risk with supplementation and, in some cases, have found an increased risk of mortality. While several studies have found potential benefit of antioxidant use in reducing colon and breast cancer risk,38,113-115 vitamins A and E have been associated with increased risk of lung and prostate cancer, respectively.47,110 Cardiovascular disease and antioxidant vitamin supplementation has similar inconsistent data, ranging from slight benefit to harm.2,116 After a large Cochrane review in 2012 found a significant increase in all-cause mortality associated with vitamin E and beta-carotene,117 the USPSTF made a specific recommendation against supplementation of these vitamins for the prevention of cardiovascular disease or cancer (grade D).118 Given its limited risk for harm, vitamin C was excluded from this recommendation.
Continue to: Vitamin A...
Vitamin A
Vitamers: Retinol; retinal; retinyl esters; provitamin A carotenoids (beta-carotene, alpha-carotene, beta-cryptoxanthin)
Physiologic role: Essential for vision and corneal development. Also involved in general cell differentiation and immune function
Dietary sources: Liver, fish oil, dairy, and fortified cereals. Provitamin A sources: leafy green vegetables, orange/yellow vegetables, tomato products, fruits, and vegetable oils Retinoids and their precursors, carotenoids, serve a critical function in vision, as well as regulating cell differentiation and proliferation throughout the body.2 While evidence suggests mortality benefit of supplementation in populations at risk of deficiency,45 wide-ranging studies have found either inconsistent benefit or outright harms in the developed world.
The takeaway: Given the USPSTF grade “D” recommendation and concern for potential harms, supplementation is not recommended in healthy patients without risk factors for deficiency.2
Vitamin E
Vitamers: Tocopherols (alpha-, beta-, gamma-, delta-); tocotrienol (alpha-, beta-, gamma-, delta-)
Physiologic role: Antioxidant; protects polyunsaturated fats from free radical oxidative damage. Involved in immune function, cell signaling, and regulation of gene expression
Dietary sources: Nuts, seeds, vegetable oil, green leafy vegetables, and fortified cereals
Vitamin E is the collective name of 8 compounds; alpha-tocopherol is the physiologically active form. Vitamin E is involved with cell proliferation as well as endothelial and platelet function.2
The takeaway: Vitamin E supplementation’s effects on cancer, cardiovascular disease, ophthalmologic disorders, and cognition have been investigated; data is either lacking to support a benefit or demonstrates harms as outlined above. Given this and the USPSTF grade “D” recommendation, supplementation is not recommended in healthy patients.2
Vitamin C
Vitamers: Ascorbic acid
Physiologic role: Required for synthesis of collagen, L-carnitine, and some neurotransmitters. Also involved in protein metabolism
Dietary sources: Primarily in fruits and vegetables: citrus, tomato, potatoes, red/green peppers, kiwi fruit, broccoli, strawberries, brussels sprouts, cantaloupe, and fortified cereals
Vitamin C supplementation at the onset of illness does not seem to have benefit.
Ascorbic acid is a required cofactor for biosynthesis of collagen, neurotransmitters, and protein metabolism.2 In addition to the shared hypothesized benefits of antioxidants, vitamin C supplementation has undergone extensive research into its potential role in augmenting the immune system and preventing the common cold. Systematic reviews have found daily vitamin C supplementation of at least 200 mg did not affect the incidence of the common cold in healthy adults but may shorten duration and could be of benefit in those exposed to extreme physical exercise or cold.48 Vitamin C supplementation at the onset of illness does not seem to have benefit.48 Data is insufficient to draw conclusions about a potential effect on pneumonia incidence or severity.119,120
The takeaway: Overall, data remain inconclusive as to potential benefits of vitamin C supplementation, although risks of potential harms are likely low.
Continue to: Vitamin D...
Vitamin D
Vitamers: Cholecalciferol (D3); ergocalciferol (D2)
Physiologic role: Hydroxylation in liver and kidney required to activate. Promotes dietary calcium absorption, enables normal bone mineralization. Also involved in modulation of cell growth, and neuromuscular and immune function
Dietary sources: Few natural dietary sources, which include fatty fish, fish liver oils; small amount in beef liver, cheese, egg yolks. Primary sources include fortified milk and endogenous synthesis in skin with UV exposure
Calciferol is a fat-soluble vitamin required for calcium and bone homeostasis. It is not naturally available in many foods but is primarily produced endogenously in the skin with ultraviolet light exposure.2
The AAP recommends supplementing exclusively breastfed infants with 400 IU/d of vitamin D to prevent rickets.
Bone density and fracture risk reduction are the most often cited benefits of vitamin D supplementation, but this has not been demonstrated consistently in RCTs. Multiple systematic reviews showing inconsistent benefit of vitamin D (with or without calcium) on fracture risk led the USPSTF to conclude that there is insufficient evidence (grade I) to issue a recommendation on vitamin D and calcium supplementation for primary prevention of fractures in postmenopausal women.49-51 Despite some initial evidence suggesting a benefit of vitamin D supplementation on falls reduction, 3 recent systematic reviews did not demonstrate this in community-dwelling elders,54-56 although a separate Cochrane review did suggest a reduction in rate of falls among institutionalized elders.57
The takeaway: Given these findings, the USPSTF has recommended against (grade D) vitamin D supplementation to prevent falls in community-dwelling elders.55
Beyond falls. While the vitamin D receptor is expressed throughout the body and observational studies have suggested a correlation between vitamin D status and many outcomes, extensive RCT data has generally failed to demonstrate extraskeletal benefits from supplementation. Meta-analysis data have demonstrated potential reductions in acute respiratory infection rates and asthma exacerbations with vitamin D supplementation. There is also limited evidence suggesting a reduction in preeclampsia and low-birthweight infant risk with vitamin D supplementation in pregnancy. However, several large meta-analyses and systematic reviews have investigated vitamin D supplementation’s effect on all-cause mortality and found no consistent data to support an association.41,58-62
Multiple systematic reviews have investigated and found high-quality evidence demonstrating no association between vitamin D supplementation and cancer41,63-66,121 or cardiovascular disease risk.41,70,71 There is high-quality data showing no benefit of vitamin D supplementation for multiple additional diseases, including diabetes, cognitive decline, depression, pain, obesity, and liver disease.43,72-75,85-90,122
The takeaway: Due to poor availability in breastmilk, the American Academy of Pediatrics (AAP) recommends supplementing exclusively breastfed infants with 400 IU/d of vitamin D to prevent rickets.123 RCT data support high-dose supplementation of lactating women (6400 IU/d) as an alternative strategy to supplementation of the infant.124 The AAP recommends that all nonbreastfeeding infants and older children ingesting < 1000 mL/d of vitamin D–fortified formula or milk should also be supplemented with 400 IU/d of vitamin D.123 Despite these universal recommendations for supplementation, evidence is mixed on the effect of vitamin D supplementation on bone health in children.52,53
Although concerns about vitamin D supplementation and increased risk of urolithiasis and hypercalcemia have been raised,51,62,121 systematic reviews have not demonstrated significant, clinically relevant risks, even with high-dose supplementation (> 2800 IU/d).125,126
Vitamin K
Vitamers: Phylloquinone (K1); menaquinones (K2)
Physiologic role: Coenzyme for synthesis of proteins involved in hemostasis and bone metabolism
Dietary sources: Phylloquinone is found in green leafy vegetables, vegetable oils, some fruits, meat, dairy, and eggs. Menaquinone is produced by gut bacteria and present in fermented foods
Vitamin K includes 2 groups of similar compounds: phylloquinone and menaquinones. Unlike other fat-soluble vitamins, vitamin K is rapidly metabolized and has low tissue storage.2
Children taking multivitamins were often found to have excess levels of potentially harmful nutrients, such as retinol, zinc, and folic acid.
Administration of vitamin K 0.5 to 1 mg intramuscularly (IM) to newborns is standard of care for the prevention of vitamin K deficiency bleeding (VKDB). This is supported by RCT data demonstrating a reduction in classic VKDB (occurring within 7 days)91 and epidemiologic data from various countries showing a reduction in late-onset VKDB with vitamin K prophylaxis programs.127 Oral dosing appears to reduce the risk of VKDB in the setting of parental refusal but is less effective than IM dosing.128,129
Vitamin K’s effects on bone density and fracture risk have also been investigated. Systematic reviews have demonstrated a reduction in fracture risk with vitamin K supplementation,92,93 and European and Asian regulatory bodies have recognized a potential benefit on bone health.2 The FDA considers the evidence insufficient at this time to support such a claim.2 Higher dietary vitamin K consumption has been associated with lower risk of cardiovascular disease in observational studies94 and supplementation was associated with improved disease measures,130 but no patient-oriented outcomes have been demonstrated.131
The takeaway: The administration of vitamin K 0.5 to 1 mg intramuscularly (IM) to newborns is standard of care for the prevention of VKDB. Vitamin K may lead to a reduction in fracture risk, but the FDA considers the evidence insufficient. Vitamin K’s potential link to a lowered risk of cardiovascular disease has not been demonstrated with patient-oriented outcomes. Vitamin K has low potential for toxicity, although its interaction with vitamin K antagonists (ie, warfarin) is clinically relevant.
Multivitamins
Multivitamins are often defined as a supplement containing 3 or more vitamins and minerals but without herbs, hormones, or drugs.132 Many multivitamins do contain additional substances, and some include levels of vitamins that exceed the RDA or even the established tolerable upper intake level.133
Safe medication storage should be practiced, as multivitamins with iron are a leading cause of poisoning in children.
A 2013 systematic review found limited evidence to support any benefit from multivitamin supplementation.41 Two included RCTs demonstrated a narrowly significant decrease in cancer rates among men, but saw no effect in women or the combined population.134,135 This benefit appears to disappear at 5 years of follow-up.136 RCT data have shown no benefit of multivitamin use on cognitive function,95 and high-quality data suggest there is no effect on all-cause mortality.137 Given this lack of supporting evidence, the USPSTF has concluded that there is insufficient evidence (grade I) to recommend vitamin supplementation in general to prevent cardiovascular disease or cancer.41
The use of prenatal multivitamins is generally recommended in the pregnancy and preconception period and has been associated with reduced risk of autism spectrum disorders, pediatric cancer rates, small-for-gestational-age infants, and multiple birth defects in offspring; however, studies have not examined if this benefit exceeds that of folate supplementation alone.138-140 AAP does not recommend multivitamins for children with a well-balanced diet.141 Of concern, children taking multivitamins were often found to have excess levels of potentially harmful nutrients such as retinol, zinc, and folic acid.142
The takeaway: There is limited evidence to support any benefit from multivitamin supplementation. Prenatal multivitamins are generally recommended in the pregnancy and preconception period. Overall, the risks of multivitamins are minimal, although that risk is dependent on the multivitamin’s constituent components.143 Components such as vitamin K may interact with a patient’s medications, and multivitamins have been shown to reduce the circulating levels of antiretrovirals.144 Specifically, multivitamins with iron should be avoided in men and postmenopausal women, and safe medication storage should be practiced as multivitamins with iron are a leading cause of poisoning in children.2
Summary
Vitamin supplementation in the developed world remains common despite a paucity of RCT data supporting it. Supplementation of folate in women planning to conceive, vitamin D in breastfeeding infants, and vitamin K in newborns are well supported by clinical evidence. Otherwise, there is limited evidence supporting clinically significant benefit from supplementation in healthy patients with well-balanced diets—and in the case of vitamins A and E, there may be outright harms.
Since their discovery in the early 1900s as the treatment for life-threatening deficiency syndromes, vitamins have been touted as panaceas for numerous ailments. While observational data have suggested potential correlations between vitamin status and every imaginable disease, randomized controlled trials (RCTs) have generally failed to find benefits from supplementation. Despite this lack of proven efficacy, more than half of older adults reported taking vitamins regularly.1
While most clinicians consider vitamins to be, at worst, expensive placebos, the potential for harm and dangerous interactions exists. Unlike pharmaceuticals, vitamins are generally unregulated, and the true content of many dietary supplements is often difficult to elucidate. Understanding the physiologic role, foundational evidence, and specific indications for the various vitamins is key to providing the best recommendations to patients.
Vitamins are essential organic nutrients, required in small quantities for normal metabolism. Since they are not synthesized endogenously, they must be ingested via food intake. In the developed world, vitamin deficiency syndromes are rare, thanks to sufficiently balanced diets and availability of fortified foods. The focus of this article will be on vitamin supplementation in healthy patients with well-balanced diets. TABLE E12 lists the 13 recognized vitamins, their recommended dietary allowances, and any known toxicity risks. TABLE 22 outlines elements of the history to consider when evaluating for deficiency. A summary of the most clinically significant evidence for vitamin supplementation follows; a more comprehensive review can be found in TABLE 3.3-96







B Complex vitamins
Vitamin B1
Vitamers: Thiamine (thiamin)
Physiologic role: Critical in carbohydrate and amino-acid catabolism and energy metabolism
Dietary sources: Whole grains, meat, fish, fortified cereals, and breads
Thiamine serves as an essential cofactor in energy metabolism.2 Thiamine deficiency is responsible for beriberi syndrome (rare in the developed world) and Wernicke-Korsakoff syndrome, the latter of which is a relatively common complication of chronic alcohol dependence. Although thiamine’s administration in these conditions can be curative, evidence is lacking to support its use preventively in patients with alcoholism.3 Thiamine has additionally been theorized to play a role in cardiac and cognitive function, but RCT data has not shown consistent patient-oriented benefits.4,5
The takeaway: Given the lack of evidence, supplementation in the general population is not recommended.
Continue to: Vitamin B2...
Vitamin B2
Vitamers: Riboflavin
Physiologic role: Essential component of cellular function and growth, energy production, and metabolism of fats and drugs
Dietary sources: Eggs, organ meats, lean meats, milk, green vegetables, fortified cereals and grains Riboflavin is essential to energy production, cellular growth, and metabolism.2
The takeaway: Its use as migraine prophylaxis has limited data,97 but there is otherwise no evidence to support health benefits of riboflavin supplementation.
Vitamin B3
Vitamers: Nicotinic acid (niacin); nicotinamide (niacinamide); nicotinamide riboside
Physiologic role: Converted to nicotinamide adenine dinucleotide (NAD), which is widely required in most cellular metabolic redox processes. Crucial to the synthesis and metabolism of carbohydrates, fatty acids, and proteins
Dietary sources: Poultry, beef, fish, nuts, legumes, grains. (Tryptophan can also be converted to NAD.)
Niacin is readily converted to NAD, an essential coenzyme for multiple catalytic processes in the body. While niacin at doses more than 100 times the recommended dietary allowance (RDA; 1-3 g/d) has been extensively studied for its role in dyslipidemias,2 pharmacologic dosing is beyond the scope of this article.
The takeaway: There is no evidence supporting a clinical benefit from niacin supplementation.
Vitamin B5
Vitamers: Pantothenic acid; pantethine
Physiologic role: Required for synthesis of coenzyme A (CoA) and acyl carrier protein, both essential in fatty acid and other anabolic/catabolic processes
Dietary sources: Almost all plant/animal-based foods. Richest sources include beef, chicken, organ meats, whole grains, and some vegetables
Pantothenic acid is essential to multiple metabolic processes and readily available in sufficient amounts in most foods.2 Although limited RCT data suggest pantethine may improve lipid measures,12,98,99 pantothenic acid itself does not seem to share this effect.
The takeaway: There is no data that supplementation of any form of vitamin B5 has any patient-oriented clinical benefits.
Continue to: Vitamin B6...
Vitamin B6
Vitamers: Pyridoxine; pyridoxamine; pyridoxal
Physiologic role: Widely involved coenzyme for cognitive development, neurotransmitter biosynthesis, homocysteine and glucose metabolism, immune function, and hemoglobin formation
Dietary sources: Fish, organ meats, potatoes/starchy vegetables, fruit (other than citrus), and fortified cereals
Pyridoxine is required for numerous enzymatic processes in the body, including biosynthesis of neurotransmitters and homeostasis of the amino acid homocysteine.2 While overt deficiency is rare, marginal insufficiency may become clinically apparent and has been associated with malabsorption, malignancies, pregnancy, heart disease, alcoholism, and use of drugs such as isoniazid, hydralazine, and levodopa/carbidopa.2 Vitamin B6 supplementation is known to decrease plasma homocysteine levels, a theorized intermediary for cardiovascular disease; however, studies have failed to consistently demonstrate patient-oriented benefits.100-102 While observational data has suggested a correlation between vitamin B6 status and cancer risk, RCTs have not supported benefit from supplementation.14-16 Potential effects of vitamin B6 supplementation on cognitive function have also been studied without observed benefit.17,18
The takeaway: Vitamin B6 is recommended as a potential treatment option for nausea in pregnancy.19 Otherwise, vitamin B6 is readily available in food, deficiency is rare, and no patient-oriented evidence supports supplementation in the general population.
Vitamin B7
Vitamers: Biotin
Physiologic role: Cofactor in the metabolism of fatty acids, glucose, and amino acids. Also plays key role in histone modifications, gene regulation, and cell signaling
Dietary sources: Widely available; most prevalent in organ meats, fish, meat, seeds, nuts, and vegetables (eg, sweet potatoes). Whole cooked eggs are a major source, but raw eggs contain avidin, which blocks absorption
Biotin serves a key role in metabolism, gene regulation, and cell signaling.2 Biotin is known to interfere with laboratory assays— including cardiac enzymes, thyroid studies, and hormone studies—at normal supplementation doses, resulting in both false-positive and false-negative results.103
The takeaway: No evidence supports the health benefits of biotin supplementation.
Vitamin B9
Vitamers: Folates; folic acid
Physiologic role: Functions as a coenzyme in the synthesis of DNA/RNA and metabolism of amino acids
Dietary sources: Highest content in spinach, liver, asparagus, and brussels sprouts. Generally found in green leafy vegetables, fruits, nuts, beans, peas, seafood, eggs, dairy, meat, poultry, grains, and fortified cereals.
Continue to: Vitamin B12...
Vitamin B12
Vitamers: Cyanocobalamin; hydroxocobalamin; methylcobalamin; adenosylcobalamin
Physiologic role: Required for red blood cell formation, neurologic function, and DNA synthesis
Dietary sources: Only in animal products: fish, poultry, meat, eggs, and milk/dairy products. Not present in plant foods. Fortified cereals, nutritional yeast are sources for vegans/vegetarians.
Given their linked physiologic roles, vitamins B9 and B12 are frequently studied together. Folate and cobalamins play key roles in nucleic acid synthesis and amino acid metabolism, with their most clinically significant role in hematopoiesis. Vitamin B12 is also essential to normal neurologic function.2
The US Preventive Services Task Force (USPSTF) recommends preconceptual folate supplementation of 0.4-0.8 mg/d in women of childbearing age to decrease the risk of fetal neural tube defects (grade A).21 This is supported by high-quality RCT evidence demonstrating a protective effect of daily folate supplementation in preventing neural tube defects.22 Folate supplementation’s effect on other fetal birth defects has been investigated, but no benefit has been demonstrated. While observational studies have suggested an inverse relationship with folate status and fetal autism spectrum disorder,23-25 the RCT data is mixed.26
A potential role for folate in cancer prevention has been extensively investigated. An expert panel of the National Toxicology Program (NTP) concluded that folate supplementation does not reduce cancer risk in people with adequate baseline folate status based on high-quality meta-analysis data.27,104 Conversely, long-term follow-up from RCTs demonstrated an increased risk of colorectal adenomas and cancers,28,29 leading the NTP panel to conclude there is sufficient concern for adverse effects of folate on cancer growth to justify further research.104
While observational studies have found a correlation of increased risk for disease with lower antioxidant serum levels, RCTs have not demonstrated a reduction in disease risk with supplementation.
Given folate and vitamin B12’s homocysteine-reducing effects, it has been theorized that supplementation may protect from cardiovascular disease. However, despite extensive research, there remains no consistent patient-oriented outcomes data to support such a benefit.31,32,105
The evidence is mixed but generally has found no benefit of folate or vitamin B12 supplementation on cognitive function.18,33-35 Finally, RCT data has failed to demonstrate a reduction in fracture risk with supplementation.36,106
The takeaway: High-quality RCT evidence demonstrates a protective effect of preconceptual daily folate supplementation in preventing neural tube defects.22 The USPSTF recommends preconceptual folate supplementation of 0.4-0.8 mg/d in women of childbearing age to decrease the risk of fetal neural tube defects.
Antioxidants
In addition to their individual roles, vitamins A, E, and C are antioxidants, functioning to protect cells from oxidative damage by free radical species.2 Due to this shared role, these vitamins are commonly studied together. Antioxidants are hypothesized to protect from various diseases, including cancer, cardiovascular disease, dementia, autoimmune disorders, depression, cataracts, and age-related vision decline.2,37,107-112
Though observational studies have found a correlation of increased risk for disease with lower antioxidant serum levels, RCTs have not demonstrated a reduction in disease risk with supplementation and, in some cases, have found an increased risk of mortality. While several studies have found potential benefit of antioxidant use in reducing colon and breast cancer risk,38,113-115 vitamins A and E have been associated with increased risk of lung and prostate cancer, respectively.47,110 Cardiovascular disease and antioxidant vitamin supplementation has similar inconsistent data, ranging from slight benefit to harm.2,116 After a large Cochrane review in 2012 found a significant increase in all-cause mortality associated with vitamin E and beta-carotene,117 the USPSTF made a specific recommendation against supplementation of these vitamins for the prevention of cardiovascular disease or cancer (grade D).118 Given its limited risk for harm, vitamin C was excluded from this recommendation.
Continue to: Vitamin A...
Vitamin A
Vitamers: Retinol; retinal; retinyl esters; provitamin A carotenoids (beta-carotene, alpha-carotene, beta-cryptoxanthin)
Physiologic role: Essential for vision and corneal development. Also involved in general cell differentiation and immune function
Dietary sources: Liver, fish oil, dairy, and fortified cereals. Provitamin A sources: leafy green vegetables, orange/yellow vegetables, tomato products, fruits, and vegetable oils Retinoids and their precursors, carotenoids, serve a critical function in vision, as well as regulating cell differentiation and proliferation throughout the body.2 While evidence suggests mortality benefit of supplementation in populations at risk of deficiency,45 wide-ranging studies have found either inconsistent benefit or outright harms in the developed world.
The takeaway: Given the USPSTF grade “D” recommendation and concern for potential harms, supplementation is not recommended in healthy patients without risk factors for deficiency.2
Vitamin E
Vitamers: Tocopherols (alpha-, beta-, gamma-, delta-); tocotrienol (alpha-, beta-, gamma-, delta-)
Physiologic role: Antioxidant; protects polyunsaturated fats from free radical oxidative damage. Involved in immune function, cell signaling, and regulation of gene expression
Dietary sources: Nuts, seeds, vegetable oil, green leafy vegetables, and fortified cereals
Vitamin E is the collective name of 8 compounds; alpha-tocopherol is the physiologically active form. Vitamin E is involved with cell proliferation as well as endothelial and platelet function.2
The takeaway: Vitamin E supplementation’s effects on cancer, cardiovascular disease, ophthalmologic disorders, and cognition have been investigated; data is either lacking to support a benefit or demonstrates harms as outlined above. Given this and the USPSTF grade “D” recommendation, supplementation is not recommended in healthy patients.2
Vitamin C
Vitamers: Ascorbic acid
Physiologic role: Required for synthesis of collagen, L-carnitine, and some neurotransmitters. Also involved in protein metabolism
Dietary sources: Primarily in fruits and vegetables: citrus, tomato, potatoes, red/green peppers, kiwi fruit, broccoli, strawberries, brussels sprouts, cantaloupe, and fortified cereals
Vitamin C supplementation at the onset of illness does not seem to have benefit.
Ascorbic acid is a required cofactor for biosynthesis of collagen, neurotransmitters, and protein metabolism.2 In addition to the shared hypothesized benefits of antioxidants, vitamin C supplementation has undergone extensive research into its potential role in augmenting the immune system and preventing the common cold. Systematic reviews have found daily vitamin C supplementation of at least 200 mg did not affect the incidence of the common cold in healthy adults but may shorten duration and could be of benefit in those exposed to extreme physical exercise or cold.48 Vitamin C supplementation at the onset of illness does not seem to have benefit.48 Data is insufficient to draw conclusions about a potential effect on pneumonia incidence or severity.119,120
The takeaway: Overall, data remain inconclusive as to potential benefits of vitamin C supplementation, although risks of potential harms are likely low.
Continue to: Vitamin D...
Vitamin D
Vitamers: Cholecalciferol (D3); ergocalciferol (D2)
Physiologic role: Hydroxylation in liver and kidney required to activate. Promotes dietary calcium absorption, enables normal bone mineralization. Also involved in modulation of cell growth, and neuromuscular and immune function
Dietary sources: Few natural dietary sources, which include fatty fish, fish liver oils; small amount in beef liver, cheese, egg yolks. Primary sources include fortified milk and endogenous synthesis in skin with UV exposure
Calciferol is a fat-soluble vitamin required for calcium and bone homeostasis. It is not naturally available in many foods but is primarily produced endogenously in the skin with ultraviolet light exposure.2
The AAP recommends supplementing exclusively breastfed infants with 400 IU/d of vitamin D to prevent rickets.
Bone density and fracture risk reduction are the most often cited benefits of vitamin D supplementation, but this has not been demonstrated consistently in RCTs. Multiple systematic reviews showing inconsistent benefit of vitamin D (with or without calcium) on fracture risk led the USPSTF to conclude that there is insufficient evidence (grade I) to issue a recommendation on vitamin D and calcium supplementation for primary prevention of fractures in postmenopausal women.49-51 Despite some initial evidence suggesting a benefit of vitamin D supplementation on falls reduction, 3 recent systematic reviews did not demonstrate this in community-dwelling elders,54-56 although a separate Cochrane review did suggest a reduction in rate of falls among institutionalized elders.57
The takeaway: Given these findings, the USPSTF has recommended against (grade D) vitamin D supplementation to prevent falls in community-dwelling elders.55
Beyond falls. While the vitamin D receptor is expressed throughout the body and observational studies have suggested a correlation between vitamin D status and many outcomes, extensive RCT data has generally failed to demonstrate extraskeletal benefits from supplementation. Meta-analysis data have demonstrated potential reductions in acute respiratory infection rates and asthma exacerbations with vitamin D supplementation. There is also limited evidence suggesting a reduction in preeclampsia and low-birthweight infant risk with vitamin D supplementation in pregnancy. However, several large meta-analyses and systematic reviews have investigated vitamin D supplementation’s effect on all-cause mortality and found no consistent data to support an association.41,58-62
Multiple systematic reviews have investigated and found high-quality evidence demonstrating no association between vitamin D supplementation and cancer41,63-66,121 or cardiovascular disease risk.41,70,71 There is high-quality data showing no benefit of vitamin D supplementation for multiple additional diseases, including diabetes, cognitive decline, depression, pain, obesity, and liver disease.43,72-75,85-90,122
The takeaway: Due to poor availability in breastmilk, the American Academy of Pediatrics (AAP) recommends supplementing exclusively breastfed infants with 400 IU/d of vitamin D to prevent rickets.123 RCT data support high-dose supplementation of lactating women (6400 IU/d) as an alternative strategy to supplementation of the infant.124 The AAP recommends that all nonbreastfeeding infants and older children ingesting < 1000 mL/d of vitamin D–fortified formula or milk should also be supplemented with 400 IU/d of vitamin D.123 Despite these universal recommendations for supplementation, evidence is mixed on the effect of vitamin D supplementation on bone health in children.52,53
Although concerns about vitamin D supplementation and increased risk of urolithiasis and hypercalcemia have been raised,51,62,121 systematic reviews have not demonstrated significant, clinically relevant risks, even with high-dose supplementation (> 2800 IU/d).125,126
Vitamin K
Vitamers: Phylloquinone (K1); menaquinones (K2)
Physiologic role: Coenzyme for synthesis of proteins involved in hemostasis and bone metabolism
Dietary sources: Phylloquinone is found in green leafy vegetables, vegetable oils, some fruits, meat, dairy, and eggs. Menaquinone is produced by gut bacteria and present in fermented foods
Vitamin K includes 2 groups of similar compounds: phylloquinone and menaquinones. Unlike other fat-soluble vitamins, vitamin K is rapidly metabolized and has low tissue storage.2
Children taking multivitamins were often found to have excess levels of potentially harmful nutrients, such as retinol, zinc, and folic acid.
Administration of vitamin K 0.5 to 1 mg intramuscularly (IM) to newborns is standard of care for the prevention of vitamin K deficiency bleeding (VKDB). This is supported by RCT data demonstrating a reduction in classic VKDB (occurring within 7 days)91 and epidemiologic data from various countries showing a reduction in late-onset VKDB with vitamin K prophylaxis programs.127 Oral dosing appears to reduce the risk of VKDB in the setting of parental refusal but is less effective than IM dosing.128,129
Vitamin K’s effects on bone density and fracture risk have also been investigated. Systematic reviews have demonstrated a reduction in fracture risk with vitamin K supplementation,92,93 and European and Asian regulatory bodies have recognized a potential benefit on bone health.2 The FDA considers the evidence insufficient at this time to support such a claim.2 Higher dietary vitamin K consumption has been associated with lower risk of cardiovascular disease in observational studies94 and supplementation was associated with improved disease measures,130 but no patient-oriented outcomes have been demonstrated.131
The takeaway: The administration of vitamin K 0.5 to 1 mg intramuscularly (IM) to newborns is standard of care for the prevention of VKDB. Vitamin K may lead to a reduction in fracture risk, but the FDA considers the evidence insufficient. Vitamin K’s potential link to a lowered risk of cardiovascular disease has not been demonstrated with patient-oriented outcomes. Vitamin K has low potential for toxicity, although its interaction with vitamin K antagonists (ie, warfarin) is clinically relevant.
Multivitamins
Multivitamins are often defined as a supplement containing 3 or more vitamins and minerals but without herbs, hormones, or drugs.132 Many multivitamins do contain additional substances, and some include levels of vitamins that exceed the RDA or even the established tolerable upper intake level.133
Safe medication storage should be practiced, as multivitamins with iron are a leading cause of poisoning in children.
A 2013 systematic review found limited evidence to support any benefit from multivitamin supplementation.41 Two included RCTs demonstrated a narrowly significant decrease in cancer rates among men, but saw no effect in women or the combined population.134,135 This benefit appears to disappear at 5 years of follow-up.136 RCT data have shown no benefit of multivitamin use on cognitive function,95 and high-quality data suggest there is no effect on all-cause mortality.137 Given this lack of supporting evidence, the USPSTF has concluded that there is insufficient evidence (grade I) to recommend vitamin supplementation in general to prevent cardiovascular disease or cancer.41
The use of prenatal multivitamins is generally recommended in the pregnancy and preconception period and has been associated with reduced risk of autism spectrum disorders, pediatric cancer rates, small-for-gestational-age infants, and multiple birth defects in offspring; however, studies have not examined if this benefit exceeds that of folate supplementation alone.138-140 AAP does not recommend multivitamins for children with a well-balanced diet.141 Of concern, children taking multivitamins were often found to have excess levels of potentially harmful nutrients such as retinol, zinc, and folic acid.142
The takeaway: There is limited evidence to support any benefit from multivitamin supplementation. Prenatal multivitamins are generally recommended in the pregnancy and preconception period. Overall, the risks of multivitamins are minimal, although that risk is dependent on the multivitamin’s constituent components.143 Components such as vitamin K may interact with a patient’s medications, and multivitamins have been shown to reduce the circulating levels of antiretrovirals.144 Specifically, multivitamins with iron should be avoided in men and postmenopausal women, and safe medication storage should be practiced as multivitamins with iron are a leading cause of poisoning in children.2
Summary
Vitamin supplementation in the developed world remains common despite a paucity of RCT data supporting it. Supplementation of folate in women planning to conceive, vitamin D in breastfeeding infants, and vitamin K in newborns are well supported by clinical evidence. Otherwise, there is limited evidence supporting clinically significant benefit from supplementation in healthy patients with well-balanced diets—and in the case of vitamins A and E, there may be outright harms.
1. Half of Americans take vitamins regularly. Accessed June 16, 2020. https://news.gallup.com/poll/166541/half-americans-vitamins-regularly.aspx
2. National Institutes of Health. Vitamin and mineral supplement fact sheets. Published 2020. Accessed May 26, 2020. https://ods.od.nih.gov/factsheets/list-VitaminsMinerals/
3. Day E, Bentham PW, Callaghan R, et al. Thiamine for prevention and treatment of Wernicke-Korsakoff syndrome in people who abuse alcohol. Cochrane Database Syst Rev. 2013;(7):CD004033. doi:10.1002/14651858.CD004033.pub3
4. DiNicolantonio JJ, Niazi AK, Lavie CJ, et al. Thiamine supplementation for the treatment of heart failure: a review of the literature. Congest Heart Fail. 2013;19:214-222. doi:10.1111/chf.12037
5. Rodríguez-Martín JL, Qizilbash N, López-Arrieta JM. Thiamine for Alzheimer’s disease. Cochrane Database Syst Rev. 2001;(2):CD001498. doi:10.1002/14651858.CD001498
6. Schoenen J, Jacquy J, Lenaerts M. Effectiveness of high-dose riboflavin in migraine prophylaxis. A randomized controlled trial. Neurology. 1998;50:466-470. doi:10.1212/wnl.50.2.466
7. Johansson M, Relton C, Ueland PM, et al. Serum B vitamin levels and risk of lung cancer. JAMA. 2010;303:2377-2385. doi:10.1001/jama.2010.808
8. Kabat GC, Miller AB, Jain M, et al. Dietary intake of selected B vitamins in relation to risk of major cancers in women. Br J Cancer. 2008;99:816-821. doi:10.1038/sj.bjc.6604540
9. Zschäbitz S, Cheng T-YD, Neuhouser ML, et al. B vitamin intakes and incidence of colorectal cancer: results from the Women’s Health Initiative Observational Study cohort. Am J Clin Nutr. 2013;97:332-343. doi:10.3945/ajcn.112.034736
10. de Vogel S, Dindore V, van Engeland M, et al. Dietary folate, methionine, riboflavin, and vitamin B-6 and risk of sporadic colorectal cancer. J Nutr. 2008;138:2372-2378. doi:10.3945/jn.108.091157
11. Bassett JK, Hodge AM, English DR, et al. Dietary intake of B vitamins and methionine and risk of lung cancer. Eur J Clin Nutr. 2012;66:182-187. doi:10.1038/ejcn.2011.157
12. McRae MP. Treatment of hyperlipoproteinemia with pantethine: a review and analysis of efficacy and tolerability. Nutr Res. 2005; 25:319-333.
13. Saposnik G, Ray JG, Sheridan P, et al; Heart Outcomes Prevention Evaluation 2 Investigators. Homocysteine-lowering therapy and stroke risk, severity, and disability: additional findings from the HOPE 2 trial. Stroke. 2009;40:1365-1372. doi:10.1161/STROKEAHA.108.529503
14. Larsson SC, Orsini N, Wolk A. Vitamin B6 and risk of colorectal cancer: a meta-analysis of prospective studies. JAMA. 2010;303:1077-1083. doi:10.1001/jama.2010.263
15. Mocellin S, Briarava M, Pilati P. Vitamin B6 and cancer risk: a field synopsis and meta-analysis. J Natl Cancer Inst. 2017;109:1-9. doi:10.1093/jnci/djw230
16. Ebbing M, Bønaa KH, Nygård O, et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA. 2009;302:2119-2126. doi:10.1001/jama.2009.1622
17. Malouf R, Grimley Evans J. The effect of vitamin B6 on cognition. Cochrane Database Syst Rev. 2003;(4):CD004393. doi:10.1002/14651858.CD004393
18. Balk EM, Raman G, Tatsioni A, et al. Vitamin B6, B12, and folic acid supplementation and cognitive function: a systematic review of randomized trials. Arch Intern Med. 2007;167:21-30. doi:10.1001/archinte.167.1.21
19. American College of Obstetrics and Gynecology. ACOG Practice Bulletin: nausea and vomiting of pregnancy. Obstet Gynecol. 2004;103:803-814.
20. Matthews A, Dowswell T, Haas DM, et al. Interventions for nausea and vomiting in early pregnancy. Cochrane Database Syst Rev. 2010;(9):CD007575. doi:10.1002/14651858.CD007575.pub2
21. US Preventive Services Task Force. Folic acid for the prevention of neural tube defects: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150:626-631.
22. De-Regil LM, Peña-Rosas JP, Fernández-Gaxiola AC, et al. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst Rev. 2015;(12):CD007950. doi:10.1002/14651858.CD007950.pub3
23. Surén P, Roth C, Bresnahan M, et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA. 2013;309:570-577. doi:10.1001/jama.2012.155925
24. Schmidt RJ, Tancredi DJ, Ozonoff S, et al. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am J Clin Nutr. 2012;96:80-89. doi:10.3945/ajcn.110.004416
25. Levine SZ, Kodesh A, Viktorin A, et al. Association of maternal use of folic acid and multivitamin supplements in the periods before and during pregnancy with the risk of autism spectrum disorder in offspring. JAMA Psychiatry. 2018;75:176-184. doi:10.1001/jamapsychiatry.2017.4050
26. Virk J, Liew Z, Olsen J, et al. Preconceptional and prenatal supplementary folic acid and multivitamin intake and autism spectrum disorders. Autism. 2016;20:710-718. doi:10.1177/1362361315604076
27. Vollset SE, Clarke R, Lewington S, et al. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet. 2013;381:1029-1036. doi:10.1016/S0140-6736(12)62001-7
28. Passarelli MN, Barry EL, Rees JR, et al. Folic acid supplementation and risk of colorectal neoplasia during long-term follow-up of a randomized clinical trial. Am J Clin Nutr. 2019;110:903-911. doi:10.1093/ajcn/nqz160
29. Oliai Araghi S, Kiefte-de Jong JC, van Dijk SC, et al. Folic acid and vitamin B12 supplementation and the risk of cancer: long-term follow-up of the B vitamins for the Prevention of Osteoporotic Fractures (B-PROOF) Trial. Cancer Epidemiol Biomarkers Prev. 2019;28:275-282. doi:10.1158/1055-9965.EPI-17-1198
30. Wan Ismail WR, Abdul Rahman R, et al. The protective effect of maternal folic acid supplementation on childhood cancer: a systematic review and meta-analysis of case-control studies. J Prev Med Public Health. 2019;52:205-213. doi:10.3961/jpmph.19.020
31. Martí-Carvajal AJ, Solà I, Lathyris D, et al. Homocysteine lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2009;(4):CD006612. doi:10.1002/14651858.CD006612.pub2
32. Wang Y, Jin Y, Wang Y, et al. The effect of folic acid in patients with cardiovascular disease: A systematic review and meta-analysis. Medicine. 2019;98:e17095. doi:10.1097/MD.0000000000017095
33. Malouf R, Areosa Sastre A. Vitamin B12 for cognition. Cochrane Database Syst Rev. 2003;(3):CD004326. doi:10.1002/14651858.CD004326
34. Malouf R, Grimley Evans J. Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people. Cochrane Database Syst Rev. 2008;(4):CD004514. doi:10.1002/14651858.CD004514.pub2
35. Suh SW, Kim HS, Han JH, et al. Efficacy of vitamins on cognitive function of non-demented people: a systematic review and meta-analysis. Nutrients. 2020;12(4). doi:10.3390/nu12041168
36. Stone KL, Lui L-Y, Christen WG, et al. Effect of combination folic acid, vitamin B6, and vitamin B12 supplementation on fracture risk in women: a randomized, controlled trial. J Bone Miner Res. 2017;32:2331-2338. doi:10.1002/jbmr.3229
37. Age-related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417-1436. doi:10.1001/archopht.119.10.1417
38. Park Y, Spiegelman D, Hunter DJ, et al. Intakes of vitamins A, C, and E and use of multiple vitamin supplements and risk of colon cancer: a pooled analysis of prospective cohort studies. Cancer Causes Control. 2010;21:1745-1757. doi:10.1007/s10552-010-9549-y
39. Koushik A, Wang M, Anderson KE, et al. Intake of vitamins A, C, and E and folate and the risk of ovarian cancer in a pooled analysis of 10 cohort studies. Cancer Causes Control. 2015;26:1315-1327. doi:10.1007/s10552-015-0626-0
40. Lin J, Cook NR, Albert C, et al. Vitamins C and E and beta carotene supplementation and cancer risk: a randomized controlled trial. J Natl Cancer Inst. 2009;101:14-23. doi:10.1093/jnci/djn438
41. Fortmann SP, Burda BU, Senger CA, et al. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: an updated systematic evidence review for the US Preventive Services Task Force. Ann Intern Med. 2013;159:824-834. doi:10.7326/0003-4819-159-12-201312170-00729
42. Mathew MC, Ervin A-M, Tao J, et al. Antioxidant vitamin supplementation for preventing and slowing the progression of age-related cataract. Cochrane Database Syst Rev. 2012;(6):CD004567. doi:10.1002/14651858.CD004567.pub2
43. Butler M, Nelson VA, Davila H, et al. Over-the-counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med. 2018;168:52-62. doi:10.7326/M17-1530
44. Crandall C. Vitamin A intake and osteoporosis: a clinical review. J Womens Health (Larchmt). 2004;13:939-953. doi:10.1089/jwh.2004.13.939
45. Kranz S, Pimpin L, Fawzi W, et al. Mortality benefits of vitamin A are not affected by varying frequency, total dose, or duration of supplementation. Food Nutr Bull. 2017;38:260-266. doi:10.1177/0379572117696663
46. Miller ER, Pastor-Barriuso R, Dalal D, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37-46. doi:10.7326/0003-4819-142-1-200501040-00110
47. Klein EA, Thompson IM, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2011;306:1549-1556. doi:10.1001/jama.2011.1437
48. Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013;(1):CD000980. doi:10.1002/14651858.CD000980.pub4
49. Avenell A, Mak JCS, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;(4):CD000227. doi:10.1002/14651858.CD000227.pub4
50. Zhao J-G, Zeng X-T, Wang J, et al. Association between calcium or vitamin D supplementation and fracture incidence in community-dwelling older adults: a systematic review and meta-analysis. JAMA. 2017;318:2466-2482. doi:10.1001/jama.2017.19344
51. Kahwati LC, Weber RP, Pan H, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319:1600-1612. doi:10.1001/jama.2017.21640
52. Winzenberg T, Powell S, Shaw KA, et al. Effects of vitamin D supplementation on bone density in healthy children: systematic review and meta-analysis. BMJ. 2011;342:c7254. doi:10.1136/bmj.c7254
53. Winzenberg TM, Powell S, Shaw KA, et al. Vitamin D supplementation for improving bone mineral density in children. Cochrane Database Syst Rev. 2010;(10):CD006944. doi:10.1002/14651858.CD006944.pub2
54. Bolland MJ, Grey A, Gamble GD, et al. Vitamin D supplementation and falls: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014;2:573-580. doi:10.1016/S2213-8587(14)70068-3
55. Guirguis-Blake JM, Michael YL, Perdue LA, et al. Interventions to prevent falls in older adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319:1705-1716. doi:10.1001/jama.2017.21962
56. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;(9):CD007146. doi:10.1002/14651858.CD007146.pub3
57. Cameron ID, Dyer SM, Panagoda CE, et al. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database Syst Rev. 2018;9:CD005465. doi:10.1002/14651858.CD005465.pub4
58. Chung M, Balk EM, Brendel M, et al. Vitamin D and calcium: a systematic review of health outcomes. Evid Rep Technol Assess (Full Rep). 2009;(183):1-420.
59. Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730-1737. doi:10.1001/archinte.167.16.1730
60. Zhang Y, Fang F, Tang J, et al. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ. 2019;366:l4673. doi:10.1136/bmj.l4673
61. Cauley JA, Chlebowski RT, Wactawski-Wende J, et al. Calcium plus vitamin D supplementation and health outcomes five years after active intervention ended: the Women’s Health Initiative. J Womens Health (Larchmt). 2013;22:915-929. doi:10.1089/jwh.2013.4270
62. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2014;(1):CD007470. doi:10.1002/14651858.CD007470.pub3
63. Zhou L, Chen B, Sheng L, et al. The effect of vitamin D supplementation on the risk of breast cancer: a trial sequential meta-analysis. Breast Cancer Res Treat. 2020;182:1-8. doi:10.1007/s10549-020-05669-4
64. Shahvazi S, Soltani S, Ahmadi SM, et al A. The effect of vitamin D supplementation on prostate cancer: a systematic review and meta-analysis of clinical trials. Horm Metab Res. 2019;51:11-21. doi:10.1055/a-0774-8809
65. Buttigliero C, Monagheddu C, Petroni P, et al. Prognostic role of vitamin d status and efficacy of vitamin D supplementation in cancer patients: a systematic review. Oncologist. 2011;16:1215-1227. doi:10.1634/theoncologist.2011-0098
66. Cortés-Jofré M, Rueda J-R, Asenjo-Lobos C, et al. Drugs for preventing lung cancer in healthy people. Cochrane Database Syst Rev. 2020;3:CD002141. doi:10.1002/14651858.CD002141.pub3
67. Elamin MB, Abu Elnour NO, Elamin KB, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:1931-1942. doi:10.1210/jc.2011-0398
68. Pittas AG, Chung M, Trikalinos T, et al. Systematic review: vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152:307-314. doi:10.7326/0003-4819-152-5-201003020-00009
69. Ford JA, MacLennan GS, Avenell A, et al. Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr. 2014;100:746-755. doi:10.3945/ajcn.113.082602
70. Beveridge LA, Struthers AD, Khan F, et al. Effect of vitamin D supplementation on blood pressure: a systematic review and meta-analysis incorporating individual patient data. JAMA Intern Med. 2015;175:745-754. doi:10.1001/jamainternmed.2015.0237
71. Qi D, Nie X, Cai J. The effect of vitamin D supplementation on hypertension in non-CKD populations: a systemic review and meta-analysis. Int J Cardiol. 2017;227:177-186. doi:10.1016/j.ijcard.2016.11.040
72. Rutjes AW, Denton DA, Di Nisio M, et al. Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in mid and late life. Cochrane Database Syst Rev. 2018;12:CD011906. doi:10.1002/14651858.CD011906.pub2
73. Straube S, Derry S, Straube C, et al. Vitamin D for the treatment of chronic painful conditions in adults. Cochrane Database Syst Rev. 2015;(5):CD007771. doi:10.1002/14651858.CD007771.pub3
74. Zadro JR, Shirley D, Ferreira M, et al. Is vitamin D supplementation effective for low back pain? A systematic review and meta-analysis. Pain Physician. 2018;21:121-145.
75. Wu Z, Malihi Z, Stewart AW, et al. Effect of vitamin D supplementation on pain: a systematic review and meta-analysis. Pain Physician. 2016;19:415-427.
76. Palacios C, Kostiuk LK, Peña-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. 2019;7:CD008873. doi:10.1002/14651858.CD008873.pub4
77. Bi WG, Nuyt AM, Weiler H, et al. Association between vitamin D supplementation during pregnancy and offspring growth, morbidity, and mortality: a systematic review and meta-analysis. JAMA Pediatr. 2018;172:635-645. doi:10.1001/jamapediatrics.2018.0302
78. Yepes-Nuñez JJ, Brożek JL, Fiocchi A, et al. Vitamin D supplementation in primary allergy prevention: Systematic review of randomized and non-randomized studies. Allergy. 2018;73:37-49. doi:10.1111/all.13241
79. Purswani JM, Gala P, Dwarkanath P, et al. The role of vitamin D in pre-eclampsia: a systematic review. BMC Pregnancy Childbirth. 2017;17:231. doi:10.1186/s12884-017-1408-3
80. Khaing W, Vallibhakara SA-O, Tantrakul V, et al. Calcium and vitamin D supplementation for prevention of preeclampsia: a systematic review and network meta-analysis. Nutrients. 2017;9:1141. doi:10.3390/nu9101141
81. Palacios C, De-Regil LM, Lombardo LK, et al. Vitamin D supplementation during pregnancy: updated meta-analysis on maternal outcomes. J Steroid Biochem Mol Biol. 2016;164:148-155. doi:10.1016/j.jsbmb.2016.02.008
82. Litonjua AA, Carey VJ, Laranjo N, et al. Six-year follow-up of a trial of antenatal vitamin D for asthma reduction. N Engl J Med. 2020;382:525-533. doi:10.1056/NEJMoa1906137
83. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi:10.1136/bmj.i6583
84. Jolliffe DA, Greenberg L, Hooper RL, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet Respir Med. 2017;5:881-890. doi:10.1016/S2213-2600(17)30306-5
85. Chandler PD, Wang L, Zhang X, et al. Effect of vitamin D supplementation alone or with calcium on adiposity measures: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. 2015;73:577-593. doi:10.1093/nutrit/nuv012
86. Gowda U, Mutowo MP, Smith BJ, et al. Vitamin D supplementation to reduce depression in adults: meta-analysis of randomized controlled trials. Nutrition. 2015;31:421-429. doi:10.1016/j.nut.2014.06.017
87. Li G, Mbuagbaw L, Samaan Z, et al. Efficacy of vitamin D supplementation in depression in adults: a systematic review. J Clin Endocrinol Metab. 2014;99:757-767. doi:10.1210/jc.2013-3450
88. Pittas AG, Dawson-Hughes B, Sheehan P, et al. Vitamin D supplementation and prevention of type 2 diabetes. N Engl J Med. 2019;381:520-530. doi:10.1056/NEJMoa1900906
89. Lee CJ, Iyer G, Liu Y, et al. The effect of vitamin D supplementation on glucose metabolism in type 2 diabetes mellitus: a systematic review and meta-analysis of intervention studies. J Diabetes Complicat. 2017;31:1115-1126. doi:10.1016/j.jdiacomp.2017.04.019
90. Bjelakovic G, Nikolova D, Bjelakovic M, et al. Vitamin D supplementation for chronic liver diseases in adults. Cochrane Database Syst Rev. 2017;11:CD011564. doi:10.1002/14651858.CD011564.pub2
91. Sankar MJ, Chandrasekaran A, Kumar P, et al. Vitamin K prophylaxis for prevention of vitamin K deficiency bleeding: a systematic review. J Perinatol. 2016;36(suppl 1):S29-S35. doi:10.1038/jp.2016.30
92. Mott A, Bradley T, Wright K, et al. Effect of vitamin K on bone mineral density and fractures in adults: an updated systematic review and meta-analysis of randomised controlled trials. Osteoporos Int. 2019;30:1543-1559. doi:10.1007/s00198-019-04949-0
93. Cockayne S, Adamson J, Lanham-New S, et al. Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:1256-1261. doi:10.1001/archinte.166.12.1256
94. Chen H-G, Sheng L-T, Zhang Y-B, et al. Association of vitamin K with cardiovascular events and all-cause mortality: a systematic review and meta-analysis. Eur J Nutr. 2019;58:2191-2205. doi:10.1007/s00394-019-01998-3
95. Grodstein F, O’Brien J, Kang JH, et al. Long-term multivitamin supplementation and cognitive function in men: a randomized trial. Ann Intern Med. 2013;159:806-814. doi:10.7326/0003-4819-159-12-201312170-00006
96. Christen WG, Glynn RJ, Manson JE, et al. Effects of multivitamin supplement on cataract and age-related macular degeneration in a randomized trial of male physicians. Ophthalmology. 2014;121:525-534. doi:10.1016/j.ophtha.2013.09.038
97. Holland S, Silberstein SD, Freitag F, et al. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346-1353. doi:10.1212/WNL.0b013e3182535d0c
98. Rumberger JA, Napolitano J, Azumano I, et al. Pantethine, a derivative of vitamin B(5) used as a nutritional supplement, favorably alters low-density lipoprotein cholesterol metabolism in low- to moderate-cardiovascular risk North American subjects: a triple-blinded placebo and diet-controlled investigation. Nutr Res. 2011;31:608-615. doi:10.1016/j.nutres.2011.08.001
99. Evans M, Rumberger JA, Azumano I, et al. Pantethine, a derivative of vitamin B5, favorably alters total, LDL and non-HDL cholesterol in low to moderate cardiovascular risk subjects eligible for statin therapy: a triple-blinded placebo and diet-controlled investigation. Vasc Health Risk Manag. 2014;10:89-100. doi:10.2147/VHRM.S57116
100. Ebbing M, Bønaa KH, Arnesen E, et al. Combined analyses and extended follow-up of two randomized controlled homocysteine-lowering B-vitamin trials. J Intern Med. 2010;268:367-382. doi:10.1111/j.1365-2796.2010.02259.x
101. Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291:565-575. doi:10.1001/jama.291.5.565
102. Albert CM, Cook NR, Gaziano JM, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008;299:2027-2036. doi:10.1001/jama.299.17.2027
103. FDA. The FDA warns that biotin may interfere with lab tests: FDA Safety Communication. Accessed June 1, 2020. www.fda.gov/medical-devices/safety-communications/update-fda-warns-biotin-may-interfere-lab-tests-fda-safety-communication
104. National Toxicology Program. Identifying research needs for assessing safe use of high intakes of folic acid. Published 2015. Accessed June 7, 2020. https://ntp.niehs.nih.gov/ntp/ohat/folicacid/final_monograph_508.pdf
105. Miller ER, Juraschek S, Pastor-Barriuso R, et al. Meta-analysis of folic acid supplementation trials on risk of cardiovascular disease and risk interaction with baseline homocysteine levels. Am J Cardiol. 2010;106:517-527. doi:10.1016/j.amjcard.2010.03.064
106. van Wijngaarden JP, Swart KMA, Enneman AW, et al. Effect of daily vitamin B-12 and folic acid supplementation on fracture incidence in elderly individuals with an elevated plasma homocysteine concentration: B-PROOF, a randomized controlled trial. Am J Clin Nutr. 2014;100:1578-1586. doi:10.3945/ajcn.114.090043
107. Harirchian MH, Mohammadpour Z, Fatehi F, et al. A systematic review and meta-analysis of randomized controlled trials to evaluating the trend of cytokines to vitamin A supplementation in autoimmune diseases. Clin Nutr. 2019;38:2038-2044. doi:10.1016/j.clnu.2018.10.026
108. Liu T, Zhong S, Liao X, et al. A meta-analysis of oxidative stress markers in depression. PLoS One. 2015;10:e0138904. doi:10.1371/journal.pone.0138904
109. Zeng J, Chen L, Wang Z, et al. Marginal vitamin A deficiency facilitates Alzheimer’s pathogenesis. Acta Neuropathol. 2017;133:967-982. doi:10.1007/s00401-017-1669-y
110. Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150-1155. doi:10.1056/NEJM199605023341802
111. Kanellopoulou A, Riza E, Samoli E, et al. Dietary supplement use after cancer diagnosis in relation to total mortality, cancer mortality and recurrence: a systematic review and meta-analysis. Nutr Cancer. 2021;73:16-30. doi:10.1080/01635581.2020.1734215
112. Sunkara A, Raizner A. Supplemental vitamins and minerals for cardiovascular disease prevention and treatment. Methodist Debakey Cardiovasc J. 2019;15:179-184. doi:10.14797/mdcj-15-3-179
113. Zhang S, Hunter DJ, Forman MR, et al. Dietary carotenoids and vitamins A, C, and E and risk of breast cancer. J Natl Cancer Inst. 1999;91:547-556. doi:10.1093/jnci/91.6.547
114. He J, Gu Y, Zhang S. Vitamin A and breast cancer survival: a systematic review and meta-analysis. Clin Breast Cancer. 2018;18:e1389-e1400. doi:10.1016/j.clbc.2018.07.025
115. Harris HR, Orsini N, Wolk A. Vitamin C and survival among women with breast cancer: a meta-analysis. Eur J Cancer. 2014;50:1223-1231. doi:10.1016/j.ejca.2014.02.013
116. Moser MA, Chun OK. Vitamin C and heart health: a review based on findings from epidemiologic studies. Int J Mol Sci. 2016;17. doi:10.3390/ijms17081328
117. Bjelakovic G, Nikolova D, Gluud LL, et al. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev. 2012;(3):CD007176. doi:10.1002/14651858.CD007176.pub2
118. US Preventive Services Task Force. Vitamin supplementation to prevent cancer and CVD: preventive medication. Accessed May 21, 2020. www.uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-supplementation-to-prevent-cancer-and-cvd-counseling
119. Hemilä H, Louhiala P. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst Rev. 2013;(8):CD005532. doi:10.1002/14651858.CD005532.pub3
120. Padhani ZA, Moazzam Z, Ashraf A, et al. Vitamin C supplementation for prevention and treatment of pneumonia. Cochrane Database Syst Rev. 2020;4:CD013134. doi:10.1002/14651858.CD013134.pub2
121. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database Syst Rev. 2014;(6):CD007469. doi:10.1002/14651858.CD007469.pub2
122. Autier P, Mullie P, Macacu A, et al. Effect of vitamin D supplementation on non-skeletal disorders: a systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol. 2017;5:986-1004. doi:10.1016/S2213-8587(17)30357-1
123. Wagner CL, Greer FR; American Academy of Pediatrics Section on Breastfeeding, American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142-1152. doi:10.1542/peds.2008-1862
124. Hollis BW, Wagner CL, Howard CR, et al. Maternal versus infant vitamin D supplementation during lactation: a randomized controlled trial. Pediatrics. 2015;136:625-634. doi:10.1542/peds.2015-1669
125. Malihi Z, Wu Z, Stewart AW, et al. Hypercalcemia, hypercalciuria, and kidney stones in long-term studies of vitamin D supplementation: a systematic review and meta-analysis. Am J Clin Nutr. 2016;104:1039-1051. doi:10.3945/ajcn.116.134981
126. Vogiatzi MG, Jacobson-Dickman E, DeBoer MD; Drugs, and Therapeutics Committee of The Pediatric Endocrine Society. Vitamin D supplementation and risk of toxicity in pediatrics: a review of current literature. J Clin Endocrinol Metab. 2014;99:1132-1141. doi:10.1210/jc.2013-3655
127. Zurynski Y, Grover CJ, Jalaludin B, et al. Vitamin K deficiency bleeding in Australian infants 1993-2017: an Australian Paediatric Surveillance Unit study. Arch Dis Child. 2020;105:433-438. doi:10.1136/archdischild-2018-316424
128. Ng E, Loewy AD. Guidelines for vitamin K prophylaxis in newborns: a joint statement of the Canadian Paediatric Society and the College of Family Physicians of Canada. Can Fam Physician. 2018;64:736-739.
129. Araki S, Shirahata A. Vitamin K deficiency bleeding in infancy. Nutrients. 2020;12:780. doi:10.3390/nu12030780
130. Shea MK, Holden RM. Vitamin K status and vascular calcification: evidence from observational and clinical studies. Adv Nutr. 2012;3:158-165. doi:10.3945/an.111.001644
131. Hartley L, Clar C, Ghannam O, et al. Vitamin K for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2015;(9):CD011148. doi:10.1002/14651858.CD011148.pub2
132. Huang H-Y, Caballero B, Chang S, et al. Multivitamin/mineral supplements and prevention of chronic disease. Evid Rep Technol Assess (Full Rep). 2006;(139):1-117.
133. Bailey RL, Gahche JJ, Lentino CV, et al. Dietary supplement use in the United States, 2003-2006. J Nutr. 2011;141:261-266. doi:10.3945/jn.110.133025
134. Gaziano JM, Sesso HD, Christen WG, et al. Multivitamins in the prevention of cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2012;308:1871-1880. doi:10.1001/jama.2012.14641
135. Hercberg S, Galan P, Preziosi P, et al. The SU.VI.MAX Study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med. 2004;164:2335-2342. doi:10.1001/archinte.164.21.2335
136. Hercberg S, Kesse-Guyot E, Druesne-Pecollo N, et al. Incidence of cancers, ischemic cardiovascular diseases and mortality during 5-year follow-up after stopping antioxidant vitamins and minerals supplements: a postintervention follow-up in the SU.VI.MAX Study. Int J Cancer. 2010;127:1875-1881. doi:10.1002/ijc.25201
137. Khan SU, Khan MU, Riaz H, et al. Effects of nutritional supplements and dietary interventions on cardiovascular outcomes: an umbrella review and evidence map. Ann Intern Med. 2019;171:190-198. doi:10.7326/M19-0341
138. Guo B-Q, Li H-B, Zhai D-S, et al. Maternal multivitamin supplementation is associated with a reduced risk of autism spectrum disorder in children: a systematic review and meta-analysis. Nutr Res. 2019;65:4-16. doi:10.1016/j.nutres.2019.02.003
139. Wolf HT, Hegaard HK, Huusom LD, et al. Multivitamin use and adverse birth outcomes in high-income countries: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217:404.e1-404.e30. doi:10.1016/j.ajog.2017.03.029
140. Goh YI, Bollano E, Einarson TR, et al. Prenatal multivitamin supplementation and rates of pediatric cancers: a meta-analysis. Clin Pharmacol Ther. 2007;81:685-691. doi:10.1038/sj.clpt.6100100
141. HealthyChildren.org. Where we stand: vitamins. Accessed June 27, 2020. www.healthychildren.org/English/healthy-living/nutrition/Pages/Where-We-Stand-Vitamins.aspx
142. Bailey RL, Catellier DJ, Jun S, et al. Total usual nutrient intakes of US children (under 48 months): findings from the Feeding Infants and Toddlers Study (FITS) 2016. J Nutr. 2018;148:1557S-1566S. doi:10.1093/jn/nxy042
143. Biesalski HK, Tinz J. Multivitamin/mineral supplements: rationale and safety. Nutrition. 2017;36:60-66. doi:10.1016/j.nut.2016.06.003
144. Jalloh MA, Gregory PJ, Hein D, et al. Dietary supplement interactions with antiretrovirals: a systematic review. Int J STD AIDS. 2017;28:4-15. doi:10.1177/0956462416671087
1. Half of Americans take vitamins regularly. Accessed June 16, 2020. https://news.gallup.com/poll/166541/half-americans-vitamins-regularly.aspx
2. National Institutes of Health. Vitamin and mineral supplement fact sheets. Published 2020. Accessed May 26, 2020. https://ods.od.nih.gov/factsheets/list-VitaminsMinerals/
3. Day E, Bentham PW, Callaghan R, et al. Thiamine for prevention and treatment of Wernicke-Korsakoff syndrome in people who abuse alcohol. Cochrane Database Syst Rev. 2013;(7):CD004033. doi:10.1002/14651858.CD004033.pub3
4. DiNicolantonio JJ, Niazi AK, Lavie CJ, et al. Thiamine supplementation for the treatment of heart failure: a review of the literature. Congest Heart Fail. 2013;19:214-222. doi:10.1111/chf.12037
5. Rodríguez-Martín JL, Qizilbash N, López-Arrieta JM. Thiamine for Alzheimer’s disease. Cochrane Database Syst Rev. 2001;(2):CD001498. doi:10.1002/14651858.CD001498
6. Schoenen J, Jacquy J, Lenaerts M. Effectiveness of high-dose riboflavin in migraine prophylaxis. A randomized controlled trial. Neurology. 1998;50:466-470. doi:10.1212/wnl.50.2.466
7. Johansson M, Relton C, Ueland PM, et al. Serum B vitamin levels and risk of lung cancer. JAMA. 2010;303:2377-2385. doi:10.1001/jama.2010.808
8. Kabat GC, Miller AB, Jain M, et al. Dietary intake of selected B vitamins in relation to risk of major cancers in women. Br J Cancer. 2008;99:816-821. doi:10.1038/sj.bjc.6604540
9. Zschäbitz S, Cheng T-YD, Neuhouser ML, et al. B vitamin intakes and incidence of colorectal cancer: results from the Women’s Health Initiative Observational Study cohort. Am J Clin Nutr. 2013;97:332-343. doi:10.3945/ajcn.112.034736
10. de Vogel S, Dindore V, van Engeland M, et al. Dietary folate, methionine, riboflavin, and vitamin B-6 and risk of sporadic colorectal cancer. J Nutr. 2008;138:2372-2378. doi:10.3945/jn.108.091157
11. Bassett JK, Hodge AM, English DR, et al. Dietary intake of B vitamins and methionine and risk of lung cancer. Eur J Clin Nutr. 2012;66:182-187. doi:10.1038/ejcn.2011.157
12. McRae MP. Treatment of hyperlipoproteinemia with pantethine: a review and analysis of efficacy and tolerability. Nutr Res. 2005; 25:319-333.
13. Saposnik G, Ray JG, Sheridan P, et al; Heart Outcomes Prevention Evaluation 2 Investigators. Homocysteine-lowering therapy and stroke risk, severity, and disability: additional findings from the HOPE 2 trial. Stroke. 2009;40:1365-1372. doi:10.1161/STROKEAHA.108.529503
14. Larsson SC, Orsini N, Wolk A. Vitamin B6 and risk of colorectal cancer: a meta-analysis of prospective studies. JAMA. 2010;303:1077-1083. doi:10.1001/jama.2010.263
15. Mocellin S, Briarava M, Pilati P. Vitamin B6 and cancer risk: a field synopsis and meta-analysis. J Natl Cancer Inst. 2017;109:1-9. doi:10.1093/jnci/djw230
16. Ebbing M, Bønaa KH, Nygård O, et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA. 2009;302:2119-2126. doi:10.1001/jama.2009.1622
17. Malouf R, Grimley Evans J. The effect of vitamin B6 on cognition. Cochrane Database Syst Rev. 2003;(4):CD004393. doi:10.1002/14651858.CD004393
18. Balk EM, Raman G, Tatsioni A, et al. Vitamin B6, B12, and folic acid supplementation and cognitive function: a systematic review of randomized trials. Arch Intern Med. 2007;167:21-30. doi:10.1001/archinte.167.1.21
19. American College of Obstetrics and Gynecology. ACOG Practice Bulletin: nausea and vomiting of pregnancy. Obstet Gynecol. 2004;103:803-814.
20. Matthews A, Dowswell T, Haas DM, et al. Interventions for nausea and vomiting in early pregnancy. Cochrane Database Syst Rev. 2010;(9):CD007575. doi:10.1002/14651858.CD007575.pub2
21. US Preventive Services Task Force. Folic acid for the prevention of neural tube defects: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150:626-631.
22. De-Regil LM, Peña-Rosas JP, Fernández-Gaxiola AC, et al. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst Rev. 2015;(12):CD007950. doi:10.1002/14651858.CD007950.pub3
23. Surén P, Roth C, Bresnahan M, et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA. 2013;309:570-577. doi:10.1001/jama.2012.155925
24. Schmidt RJ, Tancredi DJ, Ozonoff S, et al. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am J Clin Nutr. 2012;96:80-89. doi:10.3945/ajcn.110.004416
25. Levine SZ, Kodesh A, Viktorin A, et al. Association of maternal use of folic acid and multivitamin supplements in the periods before and during pregnancy with the risk of autism spectrum disorder in offspring. JAMA Psychiatry. 2018;75:176-184. doi:10.1001/jamapsychiatry.2017.4050
26. Virk J, Liew Z, Olsen J, et al. Preconceptional and prenatal supplementary folic acid and multivitamin intake and autism spectrum disorders. Autism. 2016;20:710-718. doi:10.1177/1362361315604076
27. Vollset SE, Clarke R, Lewington S, et al. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet. 2013;381:1029-1036. doi:10.1016/S0140-6736(12)62001-7
28. Passarelli MN, Barry EL, Rees JR, et al. Folic acid supplementation and risk of colorectal neoplasia during long-term follow-up of a randomized clinical trial. Am J Clin Nutr. 2019;110:903-911. doi:10.1093/ajcn/nqz160
29. Oliai Araghi S, Kiefte-de Jong JC, van Dijk SC, et al. Folic acid and vitamin B12 supplementation and the risk of cancer: long-term follow-up of the B vitamins for the Prevention of Osteoporotic Fractures (B-PROOF) Trial. Cancer Epidemiol Biomarkers Prev. 2019;28:275-282. doi:10.1158/1055-9965.EPI-17-1198
30. Wan Ismail WR, Abdul Rahman R, et al. The protective effect of maternal folic acid supplementation on childhood cancer: a systematic review and meta-analysis of case-control studies. J Prev Med Public Health. 2019;52:205-213. doi:10.3961/jpmph.19.020
31. Martí-Carvajal AJ, Solà I, Lathyris D, et al. Homocysteine lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2009;(4):CD006612. doi:10.1002/14651858.CD006612.pub2
32. Wang Y, Jin Y, Wang Y, et al. The effect of folic acid in patients with cardiovascular disease: A systematic review and meta-analysis. Medicine. 2019;98:e17095. doi:10.1097/MD.0000000000017095
33. Malouf R, Areosa Sastre A. Vitamin B12 for cognition. Cochrane Database Syst Rev. 2003;(3):CD004326. doi:10.1002/14651858.CD004326
34. Malouf R, Grimley Evans J. Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people. Cochrane Database Syst Rev. 2008;(4):CD004514. doi:10.1002/14651858.CD004514.pub2
35. Suh SW, Kim HS, Han JH, et al. Efficacy of vitamins on cognitive function of non-demented people: a systematic review and meta-analysis. Nutrients. 2020;12(4). doi:10.3390/nu12041168
36. Stone KL, Lui L-Y, Christen WG, et al. Effect of combination folic acid, vitamin B6, and vitamin B12 supplementation on fracture risk in women: a randomized, controlled trial. J Bone Miner Res. 2017;32:2331-2338. doi:10.1002/jbmr.3229
37. Age-related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417-1436. doi:10.1001/archopht.119.10.1417
38. Park Y, Spiegelman D, Hunter DJ, et al. Intakes of vitamins A, C, and E and use of multiple vitamin supplements and risk of colon cancer: a pooled analysis of prospective cohort studies. Cancer Causes Control. 2010;21:1745-1757. doi:10.1007/s10552-010-9549-y
39. Koushik A, Wang M, Anderson KE, et al. Intake of vitamins A, C, and E and folate and the risk of ovarian cancer in a pooled analysis of 10 cohort studies. Cancer Causes Control. 2015;26:1315-1327. doi:10.1007/s10552-015-0626-0
40. Lin J, Cook NR, Albert C, et al. Vitamins C and E and beta carotene supplementation and cancer risk: a randomized controlled trial. J Natl Cancer Inst. 2009;101:14-23. doi:10.1093/jnci/djn438
41. Fortmann SP, Burda BU, Senger CA, et al. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: an updated systematic evidence review for the US Preventive Services Task Force. Ann Intern Med. 2013;159:824-834. doi:10.7326/0003-4819-159-12-201312170-00729
42. Mathew MC, Ervin A-M, Tao J, et al. Antioxidant vitamin supplementation for preventing and slowing the progression of age-related cataract. Cochrane Database Syst Rev. 2012;(6):CD004567. doi:10.1002/14651858.CD004567.pub2
43. Butler M, Nelson VA, Davila H, et al. Over-the-counter supplement interventions to prevent cognitive decline, mild cognitive impairment, and clinical Alzheimer-type dementia: a systematic review. Ann Intern Med. 2018;168:52-62. doi:10.7326/M17-1530
44. Crandall C. Vitamin A intake and osteoporosis: a clinical review. J Womens Health (Larchmt). 2004;13:939-953. doi:10.1089/jwh.2004.13.939
45. Kranz S, Pimpin L, Fawzi W, et al. Mortality benefits of vitamin A are not affected by varying frequency, total dose, or duration of supplementation. Food Nutr Bull. 2017;38:260-266. doi:10.1177/0379572117696663
46. Miller ER, Pastor-Barriuso R, Dalal D, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37-46. doi:10.7326/0003-4819-142-1-200501040-00110
47. Klein EA, Thompson IM, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2011;306:1549-1556. doi:10.1001/jama.2011.1437
48. Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013;(1):CD000980. doi:10.1002/14651858.CD000980.pub4
49. Avenell A, Mak JCS, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;(4):CD000227. doi:10.1002/14651858.CD000227.pub4
50. Zhao J-G, Zeng X-T, Wang J, et al. Association between calcium or vitamin D supplementation and fracture incidence in community-dwelling older adults: a systematic review and meta-analysis. JAMA. 2017;318:2466-2482. doi:10.1001/jama.2017.19344
51. Kahwati LC, Weber RP, Pan H, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319:1600-1612. doi:10.1001/jama.2017.21640
52. Winzenberg T, Powell S, Shaw KA, et al. Effects of vitamin D supplementation on bone density in healthy children: systematic review and meta-analysis. BMJ. 2011;342:c7254. doi:10.1136/bmj.c7254
53. Winzenberg TM, Powell S, Shaw KA, et al. Vitamin D supplementation for improving bone mineral density in children. Cochrane Database Syst Rev. 2010;(10):CD006944. doi:10.1002/14651858.CD006944.pub2
54. Bolland MJ, Grey A, Gamble GD, et al. Vitamin D supplementation and falls: a trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2014;2:573-580. doi:10.1016/S2213-8587(14)70068-3
55. Guirguis-Blake JM, Michael YL, Perdue LA, et al. Interventions to prevent falls in older adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319:1705-1716. doi:10.1001/jama.2017.21962
56. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;(9):CD007146. doi:10.1002/14651858.CD007146.pub3
57. Cameron ID, Dyer SM, Panagoda CE, et al. Interventions for preventing falls in older people in care facilities and hospitals. Cochrane Database Syst Rev. 2018;9:CD005465. doi:10.1002/14651858.CD005465.pub4
58. Chung M, Balk EM, Brendel M, et al. Vitamin D and calcium: a systematic review of health outcomes. Evid Rep Technol Assess (Full Rep). 2009;(183):1-420.
59. Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730-1737. doi:10.1001/archinte.167.16.1730
60. Zhang Y, Fang F, Tang J, et al. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ. 2019;366:l4673. doi:10.1136/bmj.l4673
61. Cauley JA, Chlebowski RT, Wactawski-Wende J, et al. Calcium plus vitamin D supplementation and health outcomes five years after active intervention ended: the Women’s Health Initiative. J Womens Health (Larchmt). 2013;22:915-929. doi:10.1089/jwh.2013.4270
62. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2014;(1):CD007470. doi:10.1002/14651858.CD007470.pub3
63. Zhou L, Chen B, Sheng L, et al. The effect of vitamin D supplementation on the risk of breast cancer: a trial sequential meta-analysis. Breast Cancer Res Treat. 2020;182:1-8. doi:10.1007/s10549-020-05669-4
64. Shahvazi S, Soltani S, Ahmadi SM, et al A. The effect of vitamin D supplementation on prostate cancer: a systematic review and meta-analysis of clinical trials. Horm Metab Res. 2019;51:11-21. doi:10.1055/a-0774-8809
65. Buttigliero C, Monagheddu C, Petroni P, et al. Prognostic role of vitamin d status and efficacy of vitamin D supplementation in cancer patients: a systematic review. Oncologist. 2011;16:1215-1227. doi:10.1634/theoncologist.2011-0098
66. Cortés-Jofré M, Rueda J-R, Asenjo-Lobos C, et al. Drugs for preventing lung cancer in healthy people. Cochrane Database Syst Rev. 2020;3:CD002141. doi:10.1002/14651858.CD002141.pub3
67. Elamin MB, Abu Elnour NO, Elamin KB, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:1931-1942. doi:10.1210/jc.2011-0398
68. Pittas AG, Chung M, Trikalinos T, et al. Systematic review: vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152:307-314. doi:10.7326/0003-4819-152-5-201003020-00009
69. Ford JA, MacLennan GS, Avenell A, et al. Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr. 2014;100:746-755. doi:10.3945/ajcn.113.082602
70. Beveridge LA, Struthers AD, Khan F, et al. Effect of vitamin D supplementation on blood pressure: a systematic review and meta-analysis incorporating individual patient data. JAMA Intern Med. 2015;175:745-754. doi:10.1001/jamainternmed.2015.0237
71. Qi D, Nie X, Cai J. The effect of vitamin D supplementation on hypertension in non-CKD populations: a systemic review and meta-analysis. Int J Cardiol. 2017;227:177-186. doi:10.1016/j.ijcard.2016.11.040
72. Rutjes AW, Denton DA, Di Nisio M, et al. Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in mid and late life. Cochrane Database Syst Rev. 2018;12:CD011906. doi:10.1002/14651858.CD011906.pub2
73. Straube S, Derry S, Straube C, et al. Vitamin D for the treatment of chronic painful conditions in adults. Cochrane Database Syst Rev. 2015;(5):CD007771. doi:10.1002/14651858.CD007771.pub3
74. Zadro JR, Shirley D, Ferreira M, et al. Is vitamin D supplementation effective for low back pain? A systematic review and meta-analysis. Pain Physician. 2018;21:121-145.
75. Wu Z, Malihi Z, Stewart AW, et al. Effect of vitamin D supplementation on pain: a systematic review and meta-analysis. Pain Physician. 2016;19:415-427.
76. Palacios C, Kostiuk LK, Peña-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. 2019;7:CD008873. doi:10.1002/14651858.CD008873.pub4
77. Bi WG, Nuyt AM, Weiler H, et al. Association between vitamin D supplementation during pregnancy and offspring growth, morbidity, and mortality: a systematic review and meta-analysis. JAMA Pediatr. 2018;172:635-645. doi:10.1001/jamapediatrics.2018.0302
78. Yepes-Nuñez JJ, Brożek JL, Fiocchi A, et al. Vitamin D supplementation in primary allergy prevention: Systematic review of randomized and non-randomized studies. Allergy. 2018;73:37-49. doi:10.1111/all.13241
79. Purswani JM, Gala P, Dwarkanath P, et al. The role of vitamin D in pre-eclampsia: a systematic review. BMC Pregnancy Childbirth. 2017;17:231. doi:10.1186/s12884-017-1408-3
80. Khaing W, Vallibhakara SA-O, Tantrakul V, et al. Calcium and vitamin D supplementation for prevention of preeclampsia: a systematic review and network meta-analysis. Nutrients. 2017;9:1141. doi:10.3390/nu9101141
81. Palacios C, De-Regil LM, Lombardo LK, et al. Vitamin D supplementation during pregnancy: updated meta-analysis on maternal outcomes. J Steroid Biochem Mol Biol. 2016;164:148-155. doi:10.1016/j.jsbmb.2016.02.008
82. Litonjua AA, Carey VJ, Laranjo N, et al. Six-year follow-up of a trial of antenatal vitamin D for asthma reduction. N Engl J Med. 2020;382:525-533. doi:10.1056/NEJMoa1906137
83. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi:10.1136/bmj.i6583
84. Jolliffe DA, Greenberg L, Hooper RL, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet Respir Med. 2017;5:881-890. doi:10.1016/S2213-2600(17)30306-5
85. Chandler PD, Wang L, Zhang X, et al. Effect of vitamin D supplementation alone or with calcium on adiposity measures: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. 2015;73:577-593. doi:10.1093/nutrit/nuv012
86. Gowda U, Mutowo MP, Smith BJ, et al. Vitamin D supplementation to reduce depression in adults: meta-analysis of randomized controlled trials. Nutrition. 2015;31:421-429. doi:10.1016/j.nut.2014.06.017
87. Li G, Mbuagbaw L, Samaan Z, et al. Efficacy of vitamin D supplementation in depression in adults: a systematic review. J Clin Endocrinol Metab. 2014;99:757-767. doi:10.1210/jc.2013-3450
88. Pittas AG, Dawson-Hughes B, Sheehan P, et al. Vitamin D supplementation and prevention of type 2 diabetes. N Engl J Med. 2019;381:520-530. doi:10.1056/NEJMoa1900906
89. Lee CJ, Iyer G, Liu Y, et al. The effect of vitamin D supplementation on glucose metabolism in type 2 diabetes mellitus: a systematic review and meta-analysis of intervention studies. J Diabetes Complicat. 2017;31:1115-1126. doi:10.1016/j.jdiacomp.2017.04.019
90. Bjelakovic G, Nikolova D, Bjelakovic M, et al. Vitamin D supplementation for chronic liver diseases in adults. Cochrane Database Syst Rev. 2017;11:CD011564. doi:10.1002/14651858.CD011564.pub2
91. Sankar MJ, Chandrasekaran A, Kumar P, et al. Vitamin K prophylaxis for prevention of vitamin K deficiency bleeding: a systematic review. J Perinatol. 2016;36(suppl 1):S29-S35. doi:10.1038/jp.2016.30
92. Mott A, Bradley T, Wright K, et al. Effect of vitamin K on bone mineral density and fractures in adults: an updated systematic review and meta-analysis of randomised controlled trials. Osteoporos Int. 2019;30:1543-1559. doi:10.1007/s00198-019-04949-0
93. Cockayne S, Adamson J, Lanham-New S, et al. Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:1256-1261. doi:10.1001/archinte.166.12.1256
94. Chen H-G, Sheng L-T, Zhang Y-B, et al. Association of vitamin K with cardiovascular events and all-cause mortality: a systematic review and meta-analysis. Eur J Nutr. 2019;58:2191-2205. doi:10.1007/s00394-019-01998-3
95. Grodstein F, O’Brien J, Kang JH, et al. Long-term multivitamin supplementation and cognitive function in men: a randomized trial. Ann Intern Med. 2013;159:806-814. doi:10.7326/0003-4819-159-12-201312170-00006
96. Christen WG, Glynn RJ, Manson JE, et al. Effects of multivitamin supplement on cataract and age-related macular degeneration in a randomized trial of male physicians. Ophthalmology. 2014;121:525-534. doi:10.1016/j.ophtha.2013.09.038
97. Holland S, Silberstein SD, Freitag F, et al. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346-1353. doi:10.1212/WNL.0b013e3182535d0c
98. Rumberger JA, Napolitano J, Azumano I, et al. Pantethine, a derivative of vitamin B(5) used as a nutritional supplement, favorably alters low-density lipoprotein cholesterol metabolism in low- to moderate-cardiovascular risk North American subjects: a triple-blinded placebo and diet-controlled investigation. Nutr Res. 2011;31:608-615. doi:10.1016/j.nutres.2011.08.001
99. Evans M, Rumberger JA, Azumano I, et al. Pantethine, a derivative of vitamin B5, favorably alters total, LDL and non-HDL cholesterol in low to moderate cardiovascular risk subjects eligible for statin therapy: a triple-blinded placebo and diet-controlled investigation. Vasc Health Risk Manag. 2014;10:89-100. doi:10.2147/VHRM.S57116
100. Ebbing M, Bønaa KH, Arnesen E, et al. Combined analyses and extended follow-up of two randomized controlled homocysteine-lowering B-vitamin trials. J Intern Med. 2010;268:367-382. doi:10.1111/j.1365-2796.2010.02259.x
101. Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291:565-575. doi:10.1001/jama.291.5.565
102. Albert CM, Cook NR, Gaziano JM, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008;299:2027-2036. doi:10.1001/jama.299.17.2027
103. FDA. The FDA warns that biotin may interfere with lab tests: FDA Safety Communication. Accessed June 1, 2020. www.fda.gov/medical-devices/safety-communications/update-fda-warns-biotin-may-interfere-lab-tests-fda-safety-communication
104. National Toxicology Program. Identifying research needs for assessing safe use of high intakes of folic acid. Published 2015. Accessed June 7, 2020. https://ntp.niehs.nih.gov/ntp/ohat/folicacid/final_monograph_508.pdf
105. Miller ER, Juraschek S, Pastor-Barriuso R, et al. Meta-analysis of folic acid supplementation trials on risk of cardiovascular disease and risk interaction with baseline homocysteine levels. Am J Cardiol. 2010;106:517-527. doi:10.1016/j.amjcard.2010.03.064
106. van Wijngaarden JP, Swart KMA, Enneman AW, et al. Effect of daily vitamin B-12 and folic acid supplementation on fracture incidence in elderly individuals with an elevated plasma homocysteine concentration: B-PROOF, a randomized controlled trial. Am J Clin Nutr. 2014;100:1578-1586. doi:10.3945/ajcn.114.090043
107. Harirchian MH, Mohammadpour Z, Fatehi F, et al. A systematic review and meta-analysis of randomized controlled trials to evaluating the trend of cytokines to vitamin A supplementation in autoimmune diseases. Clin Nutr. 2019;38:2038-2044. doi:10.1016/j.clnu.2018.10.026
108. Liu T, Zhong S, Liao X, et al. A meta-analysis of oxidative stress markers in depression. PLoS One. 2015;10:e0138904. doi:10.1371/journal.pone.0138904
109. Zeng J, Chen L, Wang Z, et al. Marginal vitamin A deficiency facilitates Alzheimer’s pathogenesis. Acta Neuropathol. 2017;133:967-982. doi:10.1007/s00401-017-1669-y
110. Omenn GS, Goodman GE, Thornquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150-1155. doi:10.1056/NEJM199605023341802
111. Kanellopoulou A, Riza E, Samoli E, et al. Dietary supplement use after cancer diagnosis in relation to total mortality, cancer mortality and recurrence: a systematic review and meta-analysis. Nutr Cancer. 2021;73:16-30. doi:10.1080/01635581.2020.1734215
112. Sunkara A, Raizner A. Supplemental vitamins and minerals for cardiovascular disease prevention and treatment. Methodist Debakey Cardiovasc J. 2019;15:179-184. doi:10.14797/mdcj-15-3-179
113. Zhang S, Hunter DJ, Forman MR, et al. Dietary carotenoids and vitamins A, C, and E and risk of breast cancer. J Natl Cancer Inst. 1999;91:547-556. doi:10.1093/jnci/91.6.547
114. He J, Gu Y, Zhang S. Vitamin A and breast cancer survival: a systematic review and meta-analysis. Clin Breast Cancer. 2018;18:e1389-e1400. doi:10.1016/j.clbc.2018.07.025
115. Harris HR, Orsini N, Wolk A. Vitamin C and survival among women with breast cancer: a meta-analysis. Eur J Cancer. 2014;50:1223-1231. doi:10.1016/j.ejca.2014.02.013
116. Moser MA, Chun OK. Vitamin C and heart health: a review based on findings from epidemiologic studies. Int J Mol Sci. 2016;17. doi:10.3390/ijms17081328
117. Bjelakovic G, Nikolova D, Gluud LL, et al. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev. 2012;(3):CD007176. doi:10.1002/14651858.CD007176.pub2
118. US Preventive Services Task Force. Vitamin supplementation to prevent cancer and CVD: preventive medication. Accessed May 21, 2020. www.uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-supplementation-to-prevent-cancer-and-cvd-counseling
119. Hemilä H, Louhiala P. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst Rev. 2013;(8):CD005532. doi:10.1002/14651858.CD005532.pub3
120. Padhani ZA, Moazzam Z, Ashraf A, et al. Vitamin C supplementation for prevention and treatment of pneumonia. Cochrane Database Syst Rev. 2020;4:CD013134. doi:10.1002/14651858.CD013134.pub2
121. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database Syst Rev. 2014;(6):CD007469. doi:10.1002/14651858.CD007469.pub2
122. Autier P, Mullie P, Macacu A, et al. Effect of vitamin D supplementation on non-skeletal disorders: a systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol. 2017;5:986-1004. doi:10.1016/S2213-8587(17)30357-1
123. Wagner CL, Greer FR; American Academy of Pediatrics Section on Breastfeeding, American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142-1152. doi:10.1542/peds.2008-1862
124. Hollis BW, Wagner CL, Howard CR, et al. Maternal versus infant vitamin D supplementation during lactation: a randomized controlled trial. Pediatrics. 2015;136:625-634. doi:10.1542/peds.2015-1669
125. Malihi Z, Wu Z, Stewart AW, et al. Hypercalcemia, hypercalciuria, and kidney stones in long-term studies of vitamin D supplementation: a systematic review and meta-analysis. Am J Clin Nutr. 2016;104:1039-1051. doi:10.3945/ajcn.116.134981
126. Vogiatzi MG, Jacobson-Dickman E, DeBoer MD; Drugs, and Therapeutics Committee of The Pediatric Endocrine Society. Vitamin D supplementation and risk of toxicity in pediatrics: a review of current literature. J Clin Endocrinol Metab. 2014;99:1132-1141. doi:10.1210/jc.2013-3655
127. Zurynski Y, Grover CJ, Jalaludin B, et al. Vitamin K deficiency bleeding in Australian infants 1993-2017: an Australian Paediatric Surveillance Unit study. Arch Dis Child. 2020;105:433-438. doi:10.1136/archdischild-2018-316424
128. Ng E, Loewy AD. Guidelines for vitamin K prophylaxis in newborns: a joint statement of the Canadian Paediatric Society and the College of Family Physicians of Canada. Can Fam Physician. 2018;64:736-739.
129. Araki S, Shirahata A. Vitamin K deficiency bleeding in infancy. Nutrients. 2020;12:780. doi:10.3390/nu12030780
130. Shea MK, Holden RM. Vitamin K status and vascular calcification: evidence from observational and clinical studies. Adv Nutr. 2012;3:158-165. doi:10.3945/an.111.001644
131. Hartley L, Clar C, Ghannam O, et al. Vitamin K for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2015;(9):CD011148. doi:10.1002/14651858.CD011148.pub2
132. Huang H-Y, Caballero B, Chang S, et al. Multivitamin/mineral supplements and prevention of chronic disease. Evid Rep Technol Assess (Full Rep). 2006;(139):1-117.
133. Bailey RL, Gahche JJ, Lentino CV, et al. Dietary supplement use in the United States, 2003-2006. J Nutr. 2011;141:261-266. doi:10.3945/jn.110.133025
134. Gaziano JM, Sesso HD, Christen WG, et al. Multivitamins in the prevention of cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2012;308:1871-1880. doi:10.1001/jama.2012.14641
135. Hercberg S, Galan P, Preziosi P, et al. The SU.VI.MAX Study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med. 2004;164:2335-2342. doi:10.1001/archinte.164.21.2335
136. Hercberg S, Kesse-Guyot E, Druesne-Pecollo N, et al. Incidence of cancers, ischemic cardiovascular diseases and mortality during 5-year follow-up after stopping antioxidant vitamins and minerals supplements: a postintervention follow-up in the SU.VI.MAX Study. Int J Cancer. 2010;127:1875-1881. doi:10.1002/ijc.25201
137. Khan SU, Khan MU, Riaz H, et al. Effects of nutritional supplements and dietary interventions on cardiovascular outcomes: an umbrella review and evidence map. Ann Intern Med. 2019;171:190-198. doi:10.7326/M19-0341
138. Guo B-Q, Li H-B, Zhai D-S, et al. Maternal multivitamin supplementation is associated with a reduced risk of autism spectrum disorder in children: a systematic review and meta-analysis. Nutr Res. 2019;65:4-16. doi:10.1016/j.nutres.2019.02.003
139. Wolf HT, Hegaard HK, Huusom LD, et al. Multivitamin use and adverse birth outcomes in high-income countries: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217:404.e1-404.e30. doi:10.1016/j.ajog.2017.03.029
140. Goh YI, Bollano E, Einarson TR, et al. Prenatal multivitamin supplementation and rates of pediatric cancers: a meta-analysis. Clin Pharmacol Ther. 2007;81:685-691. doi:10.1038/sj.clpt.6100100
141. HealthyChildren.org. Where we stand: vitamins. Accessed June 27, 2020. www.healthychildren.org/English/healthy-living/nutrition/Pages/Where-We-Stand-Vitamins.aspx
142. Bailey RL, Catellier DJ, Jun S, et al. Total usual nutrient intakes of US children (under 48 months): findings from the Feeding Infants and Toddlers Study (FITS) 2016. J Nutr. 2018;148:1557S-1566S. doi:10.1093/jn/nxy042
143. Biesalski HK, Tinz J. Multivitamin/mineral supplements: rationale and safety. Nutrition. 2017;36:60-66. doi:10.1016/j.nut.2016.06.003
144. Jalloh MA, Gregory PJ, Hein D, et al. Dietary supplement interactions with antiretrovirals: a systematic review. Int J STD AIDS. 2017;28:4-15. doi:10.1177/0956462416671087
1 in 7 breast cancers are overdiagnosed
A new model based on data from the Breast Cancer Surveillance Consortium (BCSC) suggests that overdiagnosis of screen-detected breast cancer is less frequent than estimates from excess-incidence studies, but the model also takes into account indolent tumors and produced a higher estimate than previous models that didn’t consider this factor.
“There is a pronounced lack of consensus of the true rate of overdiagnosis in the contemporary U.S. mammography practice. This uncertainty about the extent of overdiagnosis is a problem for the development of guidelines and policies. By overcoming shortcomings of previous studies, we produced a defensible estimate of overdiagnosis in contemporary U.S. mammography practice. About one in seven screen-detected cancers in women (between 50 and 74 years) undergoing biennial screening will be overdiagnosed, and about one in three overdiagnosed cancers are attributed to the detection of nonprogressive cancers,” said Marc D. Ryser, PhD, in an interview. Dr. Ryser is an expert in mathematical and statistical modeling in population health science at Duke University, Durham, N.C. He presented the results of the model at the 2021 San Antonio Breast Cancer Symposium.
Previous models have come up with estimates ranging from 0% to 54%, but the heterogeneity makes them difficult to compare. “They differ in study populations, estimation methods and their definitions of overdiagnosis,” Dr. Ryser said.
There are two general ways to estimate overdiagnosis. One is a model-based approach that works out the tumor latency using models of disease natural history and clinical data, and then uses that to predict overdiagnosis. But these models may not account or indolent tumors, or tumors that would not likely cause death during the patient’s lifetime, and the assumptions behind the models can be opaque. On the other hand, the excess-incidence strategy compares incidence in screened versus unscreened populations and assumes that excess cancers in the screened group is caused by overdiagnosis, but this can be affected by bias.
To get around these limitations, Dr. Ryser’s group used a model-based approach, but also allowed for indolent tumors. They ensured transparency of the underlying assumptions of the model, and took advantage of a contemporary, high-quality data source in the BCSC.
They used individual mammography screening and breast cancer diagnosis records from 35,986 women aged 50-74 years, who were first screened between 2000 and 2018. To estimate overdiagnosis caused by indolent tumors, they used the risk of non–breast cancer mortality from age cohort–adjusted annual mortality risks. There were a total 82,677 screens and 718 cases of breast cancer diagnosed. 3.6% of detected tumor were indolent (95% credible interval, 0.2%-13.8%). The predicted overdiagnosis rate for a biennial screening program was 15.3% (95% prediction interval, 9.7%-25.2%). 6.0% of overdiagnosis was projected to be caused by indolent tumors (95% PI, 0.2%-19.0%) that don’t progress at all, and 9.3% to tumors that would progress, but not fast enough to cause mortality during the individual’s lifetime. An annual screening program had a predicted overdiagnosis rate of 14.6% (95% PI, 9.4%-23.9%).
Dr. Ryser identified some specific studies that used the same definition of overdiagnosis as his group used, and compared them with the 15.3% incidence that his group determined. Excess-incidence studies produced higher estimates, while modeling studies produced lower estimates.
The model did not distinguish between ductal carcinoma in situ and invasive cancers, and it did not account for patient race and breast density.
The study was funded by the National Institutes of Health. Dr. Ryser has no relevant financial disclosures.
A new model based on data from the Breast Cancer Surveillance Consortium (BCSC) suggests that overdiagnosis of screen-detected breast cancer is less frequent than estimates from excess-incidence studies, but the model also takes into account indolent tumors and produced a higher estimate than previous models that didn’t consider this factor.
“There is a pronounced lack of consensus of the true rate of overdiagnosis in the contemporary U.S. mammography practice. This uncertainty about the extent of overdiagnosis is a problem for the development of guidelines and policies. By overcoming shortcomings of previous studies, we produced a defensible estimate of overdiagnosis in contemporary U.S. mammography practice. About one in seven screen-detected cancers in women (between 50 and 74 years) undergoing biennial screening will be overdiagnosed, and about one in three overdiagnosed cancers are attributed to the detection of nonprogressive cancers,” said Marc D. Ryser, PhD, in an interview. Dr. Ryser is an expert in mathematical and statistical modeling in population health science at Duke University, Durham, N.C. He presented the results of the model at the 2021 San Antonio Breast Cancer Symposium.
Previous models have come up with estimates ranging from 0% to 54%, but the heterogeneity makes them difficult to compare. “They differ in study populations, estimation methods and their definitions of overdiagnosis,” Dr. Ryser said.
There are two general ways to estimate overdiagnosis. One is a model-based approach that works out the tumor latency using models of disease natural history and clinical data, and then uses that to predict overdiagnosis. But these models may not account or indolent tumors, or tumors that would not likely cause death during the patient’s lifetime, and the assumptions behind the models can be opaque. On the other hand, the excess-incidence strategy compares incidence in screened versus unscreened populations and assumes that excess cancers in the screened group is caused by overdiagnosis, but this can be affected by bias.
To get around these limitations, Dr. Ryser’s group used a model-based approach, but also allowed for indolent tumors. They ensured transparency of the underlying assumptions of the model, and took advantage of a contemporary, high-quality data source in the BCSC.
They used individual mammography screening and breast cancer diagnosis records from 35,986 women aged 50-74 years, who were first screened between 2000 and 2018. To estimate overdiagnosis caused by indolent tumors, they used the risk of non–breast cancer mortality from age cohort–adjusted annual mortality risks. There were a total 82,677 screens and 718 cases of breast cancer diagnosed. 3.6% of detected tumor were indolent (95% credible interval, 0.2%-13.8%). The predicted overdiagnosis rate for a biennial screening program was 15.3% (95% prediction interval, 9.7%-25.2%). 6.0% of overdiagnosis was projected to be caused by indolent tumors (95% PI, 0.2%-19.0%) that don’t progress at all, and 9.3% to tumors that would progress, but not fast enough to cause mortality during the individual’s lifetime. An annual screening program had a predicted overdiagnosis rate of 14.6% (95% PI, 9.4%-23.9%).
Dr. Ryser identified some specific studies that used the same definition of overdiagnosis as his group used, and compared them with the 15.3% incidence that his group determined. Excess-incidence studies produced higher estimates, while modeling studies produced lower estimates.
The model did not distinguish between ductal carcinoma in situ and invasive cancers, and it did not account for patient race and breast density.
The study was funded by the National Institutes of Health. Dr. Ryser has no relevant financial disclosures.
A new model based on data from the Breast Cancer Surveillance Consortium (BCSC) suggests that overdiagnosis of screen-detected breast cancer is less frequent than estimates from excess-incidence studies, but the model also takes into account indolent tumors and produced a higher estimate than previous models that didn’t consider this factor.
“There is a pronounced lack of consensus of the true rate of overdiagnosis in the contemporary U.S. mammography practice. This uncertainty about the extent of overdiagnosis is a problem for the development of guidelines and policies. By overcoming shortcomings of previous studies, we produced a defensible estimate of overdiagnosis in contemporary U.S. mammography practice. About one in seven screen-detected cancers in women (between 50 and 74 years) undergoing biennial screening will be overdiagnosed, and about one in three overdiagnosed cancers are attributed to the detection of nonprogressive cancers,” said Marc D. Ryser, PhD, in an interview. Dr. Ryser is an expert in mathematical and statistical modeling in population health science at Duke University, Durham, N.C. He presented the results of the model at the 2021 San Antonio Breast Cancer Symposium.
Previous models have come up with estimates ranging from 0% to 54%, but the heterogeneity makes them difficult to compare. “They differ in study populations, estimation methods and their definitions of overdiagnosis,” Dr. Ryser said.
There are two general ways to estimate overdiagnosis. One is a model-based approach that works out the tumor latency using models of disease natural history and clinical data, and then uses that to predict overdiagnosis. But these models may not account or indolent tumors, or tumors that would not likely cause death during the patient’s lifetime, and the assumptions behind the models can be opaque. On the other hand, the excess-incidence strategy compares incidence in screened versus unscreened populations and assumes that excess cancers in the screened group is caused by overdiagnosis, but this can be affected by bias.
To get around these limitations, Dr. Ryser’s group used a model-based approach, but also allowed for indolent tumors. They ensured transparency of the underlying assumptions of the model, and took advantage of a contemporary, high-quality data source in the BCSC.
They used individual mammography screening and breast cancer diagnosis records from 35,986 women aged 50-74 years, who were first screened between 2000 and 2018. To estimate overdiagnosis caused by indolent tumors, they used the risk of non–breast cancer mortality from age cohort–adjusted annual mortality risks. There were a total 82,677 screens and 718 cases of breast cancer diagnosed. 3.6% of detected tumor were indolent (95% credible interval, 0.2%-13.8%). The predicted overdiagnosis rate for a biennial screening program was 15.3% (95% prediction interval, 9.7%-25.2%). 6.0% of overdiagnosis was projected to be caused by indolent tumors (95% PI, 0.2%-19.0%) that don’t progress at all, and 9.3% to tumors that would progress, but not fast enough to cause mortality during the individual’s lifetime. An annual screening program had a predicted overdiagnosis rate of 14.6% (95% PI, 9.4%-23.9%).
Dr. Ryser identified some specific studies that used the same definition of overdiagnosis as his group used, and compared them with the 15.3% incidence that his group determined. Excess-incidence studies produced higher estimates, while modeling studies produced lower estimates.
The model did not distinguish between ductal carcinoma in situ and invasive cancers, and it did not account for patient race and breast density.
The study was funded by the National Institutes of Health. Dr. Ryser has no relevant financial disclosures.
FROM SABCS 2021
Vitamin D counters bone density loss with aromatase inhibitors
Among women with breast cancer being treated with aromatase inhibitors (AI), supplementation with vitamin D and calcium protected against bone loss after 5 years, according to results from a prospective cohort study in Brazil. The study found no difference in bone mineral density outcomes at 5 years between women with hormone receptor–positive cancers treated with aromatase inhibitors (AIG) and triple negative or HER-2 positive patients who were treated with another therapy (CG).
About two-thirds of women with breast cancer have tumors that are positive for hormone receptors, and so are often treated with endocrine therapy such as selective estrogen receptor modulators or AI. However, there are concerns that AI treatment may lead to a loss of bone mineral density and impacts on quality of life. This loss is influenced by a range of factors, including body weight, physical activity, smoking, alcohol consumption, corticosteroid use, calcium in the diet, and circulating levels of vitamin D.
Vitamin D helps to regulate absorption of calcium and phosphorus, ensuring that their plasma concentrations are high enough for adequate bone health. But vitamin D deficiency is a common problem, even in tropical areas such as Brazil. “It is high in the general population and especially in postmenopausal breast cancer patients. Thus, vitamin D and calcium supplementation has an impact on these women’s lives,” said lead author Marcelo Antonini, MD, who presented the study (abstract P1-13-04) at the San Antonio Breast Cancer Symposium. He is a researcher at Hospital Servidor Publico Estadual in São Paulo, Brazil.
Although the findings are encouraging, more work needs to be done before it leads to a change in practice. “Larger studies must be carried out to prove this theory; however, in noncancer patients we have already well established the benefits of vitamin D and calcium supplementation,” Dr. Antonini said in an interview
The researchers examined women before the start of treatment, at 6 months, and at 5 years. Those with vitamin D levels below 30 ng/mL received 7,000 IU/day for 8 weeks, followed by a 1,000 IU/day maintenance dose. Subjects with osteopenia received a calcium supplement (500 mg calcium carbonate), and those with osteoporosis received 4 mg zoledronic acid (Zometa, Novartis).
There were 140 patients in both the AIG and CG groups. The average age was 65 years. Sixty-four percent of the AIG group and 71% of the CG group were vitamin D deficient at baseline. At 5 years, the frequencies were 17% and 16%, respectively. Both groups showed significant declines in bone mineral density in the femoral neck and femur at both 6 months and 5 years, but there was no significant difference between them. There was no significant difference between the two groups with respect to bone density loss in the spine.
The study is limited by the fact that it was conducted at a single center and had a small population size.
Another prospective observational study, published earlier this year, looked at vitamin D supplementation in 741 patients (mean age 61.9 years) being treated with aromatase inhibitors, whose baseline vitamin D levels were less 30 ng/mL. They received 16,000 IU dose of oral calcifediol every 2 weeks. At 3 months, individuals who achieved vitamin D levels of 40 ng/mL or higher were less likely to have joint pain (P < .05). At 12 months, data from 473 patients showed that for every 10-ng/mL increase in serum vitamin D at 3 months, there was a reduction in loss of bone marrow density in the lumbar spine (adjusted beta = +0.177%, P < .05), though there were no associations between vitamin D levels and BMD of the femur or total hip.
“Our results suggest that optimal levels of vitamin D are associated with a reduced risk of joint pain related to AI treatment. A target threshold (of vitamin D) levels was set at 40 ng/mL to significantly reduce the increase in joint pain. The authors noted that this threshold is well above the goal of 20 ng/mL recommended by the 2010 Institute of Medicine report.
The study did not receive external funding. Dr. Antonini has no relevant financial disclosures.
Among women with breast cancer being treated with aromatase inhibitors (AI), supplementation with vitamin D and calcium protected against bone loss after 5 years, according to results from a prospective cohort study in Brazil. The study found no difference in bone mineral density outcomes at 5 years between women with hormone receptor–positive cancers treated with aromatase inhibitors (AIG) and triple negative or HER-2 positive patients who were treated with another therapy (CG).
About two-thirds of women with breast cancer have tumors that are positive for hormone receptors, and so are often treated with endocrine therapy such as selective estrogen receptor modulators or AI. However, there are concerns that AI treatment may lead to a loss of bone mineral density and impacts on quality of life. This loss is influenced by a range of factors, including body weight, physical activity, smoking, alcohol consumption, corticosteroid use, calcium in the diet, and circulating levels of vitamin D.
Vitamin D helps to regulate absorption of calcium and phosphorus, ensuring that their plasma concentrations are high enough for adequate bone health. But vitamin D deficiency is a common problem, even in tropical areas such as Brazil. “It is high in the general population and especially in postmenopausal breast cancer patients. Thus, vitamin D and calcium supplementation has an impact on these women’s lives,” said lead author Marcelo Antonini, MD, who presented the study (abstract P1-13-04) at the San Antonio Breast Cancer Symposium. He is a researcher at Hospital Servidor Publico Estadual in São Paulo, Brazil.
Although the findings are encouraging, more work needs to be done before it leads to a change in practice. “Larger studies must be carried out to prove this theory; however, in noncancer patients we have already well established the benefits of vitamin D and calcium supplementation,” Dr. Antonini said in an interview
The researchers examined women before the start of treatment, at 6 months, and at 5 years. Those with vitamin D levels below 30 ng/mL received 7,000 IU/day for 8 weeks, followed by a 1,000 IU/day maintenance dose. Subjects with osteopenia received a calcium supplement (500 mg calcium carbonate), and those with osteoporosis received 4 mg zoledronic acid (Zometa, Novartis).
There were 140 patients in both the AIG and CG groups. The average age was 65 years. Sixty-four percent of the AIG group and 71% of the CG group were vitamin D deficient at baseline. At 5 years, the frequencies were 17% and 16%, respectively. Both groups showed significant declines in bone mineral density in the femoral neck and femur at both 6 months and 5 years, but there was no significant difference between them. There was no significant difference between the two groups with respect to bone density loss in the spine.
The study is limited by the fact that it was conducted at a single center and had a small population size.
Another prospective observational study, published earlier this year, looked at vitamin D supplementation in 741 patients (mean age 61.9 years) being treated with aromatase inhibitors, whose baseline vitamin D levels were less 30 ng/mL. They received 16,000 IU dose of oral calcifediol every 2 weeks. At 3 months, individuals who achieved vitamin D levels of 40 ng/mL or higher were less likely to have joint pain (P < .05). At 12 months, data from 473 patients showed that for every 10-ng/mL increase in serum vitamin D at 3 months, there was a reduction in loss of bone marrow density in the lumbar spine (adjusted beta = +0.177%, P < .05), though there were no associations between vitamin D levels and BMD of the femur or total hip.
“Our results suggest that optimal levels of vitamin D are associated with a reduced risk of joint pain related to AI treatment. A target threshold (of vitamin D) levels was set at 40 ng/mL to significantly reduce the increase in joint pain. The authors noted that this threshold is well above the goal of 20 ng/mL recommended by the 2010 Institute of Medicine report.
The study did not receive external funding. Dr. Antonini has no relevant financial disclosures.
Among women with breast cancer being treated with aromatase inhibitors (AI), supplementation with vitamin D and calcium protected against bone loss after 5 years, according to results from a prospective cohort study in Brazil. The study found no difference in bone mineral density outcomes at 5 years between women with hormone receptor–positive cancers treated with aromatase inhibitors (AIG) and triple negative or HER-2 positive patients who were treated with another therapy (CG).
About two-thirds of women with breast cancer have tumors that are positive for hormone receptors, and so are often treated with endocrine therapy such as selective estrogen receptor modulators or AI. However, there are concerns that AI treatment may lead to a loss of bone mineral density and impacts on quality of life. This loss is influenced by a range of factors, including body weight, physical activity, smoking, alcohol consumption, corticosteroid use, calcium in the diet, and circulating levels of vitamin D.
Vitamin D helps to regulate absorption of calcium and phosphorus, ensuring that their plasma concentrations are high enough for adequate bone health. But vitamin D deficiency is a common problem, even in tropical areas such as Brazil. “It is high in the general population and especially in postmenopausal breast cancer patients. Thus, vitamin D and calcium supplementation has an impact on these women’s lives,” said lead author Marcelo Antonini, MD, who presented the study (abstract P1-13-04) at the San Antonio Breast Cancer Symposium. He is a researcher at Hospital Servidor Publico Estadual in São Paulo, Brazil.
Although the findings are encouraging, more work needs to be done before it leads to a change in practice. “Larger studies must be carried out to prove this theory; however, in noncancer patients we have already well established the benefits of vitamin D and calcium supplementation,” Dr. Antonini said in an interview
The researchers examined women before the start of treatment, at 6 months, and at 5 years. Those with vitamin D levels below 30 ng/mL received 7,000 IU/day for 8 weeks, followed by a 1,000 IU/day maintenance dose. Subjects with osteopenia received a calcium supplement (500 mg calcium carbonate), and those with osteoporosis received 4 mg zoledronic acid (Zometa, Novartis).
There were 140 patients in both the AIG and CG groups. The average age was 65 years. Sixty-four percent of the AIG group and 71% of the CG group were vitamin D deficient at baseline. At 5 years, the frequencies were 17% and 16%, respectively. Both groups showed significant declines in bone mineral density in the femoral neck and femur at both 6 months and 5 years, but there was no significant difference between them. There was no significant difference between the two groups with respect to bone density loss in the spine.
The study is limited by the fact that it was conducted at a single center and had a small population size.
Another prospective observational study, published earlier this year, looked at vitamin D supplementation in 741 patients (mean age 61.9 years) being treated with aromatase inhibitors, whose baseline vitamin D levels were less 30 ng/mL. They received 16,000 IU dose of oral calcifediol every 2 weeks. At 3 months, individuals who achieved vitamin D levels of 40 ng/mL or higher were less likely to have joint pain (P < .05). At 12 months, data from 473 patients showed that for every 10-ng/mL increase in serum vitamin D at 3 months, there was a reduction in loss of bone marrow density in the lumbar spine (adjusted beta = +0.177%, P < .05), though there were no associations between vitamin D levels and BMD of the femur or total hip.
“Our results suggest that optimal levels of vitamin D are associated with a reduced risk of joint pain related to AI treatment. A target threshold (of vitamin D) levels was set at 40 ng/mL to significantly reduce the increase in joint pain. The authors noted that this threshold is well above the goal of 20 ng/mL recommended by the 2010 Institute of Medicine report.
The study did not receive external funding. Dr. Antonini has no relevant financial disclosures.
FROM SABCS 2021
IDF Atlas: 1 in 10 adults worldwide now has diabetes
One in 10 adults worldwide currently has diabetes, accounting for an estimated global health expenditure of $966 billion in U.S. dollars in 2021, according to the new International Diabetes Federation Diabetes Atlas.
The IDF Atlas, 10th edition, was published online Dec. 6, 2021.
Highlights from it were presented during two sessions at the IDF Virtual Congress 2021, covering global diabetes incidence and prevalence, mortality, and costs, as well as new sections in this edition devoted to adult-onset type 1 diabetes, childhood-onset type 2 diabetes, and the interactions between diabetes and COVID-19.
More detailed data from some of the Atlas chapters were also published Dec. 6, 2021, in separate papers in the IDF journal Diabetes Research and Clinical Practice, with more publications planned.
Information for the Atlas comes from peer-reviewed literature, unpublished reports, and national registries. This latest edition includes 219 data sources from 144 countries, with figures for other countries extrapolated.
Atlas cochair Dianna Magliano, PhD, reviewed some of the highlights. Half of those currently with diabetes, or about 240 million adults, are undiagnosed, and another 319 million have impaired fasting glucose. Over three-quarters of all adults with diabetes now live in low- and middle-income countries. And about 6.7 million deaths in 2021 can be attributed to diabetes.
The Atlas also predicts increases in these numbers over the coming decades if current trends continue.
“Our data and projections tell a sobering story. Diabetes prevalence is expected to increase globally. The number of adults with diabetes will rise from 537 million in 2021 to 786 million ... by the year 2045, an increase of 46%. Rises are expected in every region of the world, with the largest increases expected to occur in the regions of Africa, the Middle East, and Southeast Asia,” said Dr. Magliano, head of diabetes and population health at the Baker Heart and Diabetes Institute, Melbourne.
Since 2019, when the last Atlas was published, the 2021 numbers represent increases of 73.6 million more adults with diabetes including 7.8 million more undiagnosed, 2.5 million more deaths attributed to diabetes, and an additional global expenditure of $206 billion.
Increases have also occurred in the number of people with prediabetes, children with type 1 diabetes, and pregnancies affected by diabetes, Dr. Magliano reported.
“There is a strong need for effective intervention strategies and policies to stall the increase in the number of people developing diabetes across the world,” she added.
Projected rise in expenditures for diabetes will be ‘unsustainable’
The current $966 billion global health expenditure caused by diabetes represents a 316% increase from the $232 billion reported in 2006, according to William H. Herman, MD, professor of internal medicine and epidemiology at the University of Michigan, Ann Arbor.
By region, 43% of current diabetes-related global expenditures are in North America, 25% in the Western Pacific, and 20% in Europe, while 12% are from the regions of South and Central America, North Africa, Africa, and Southeast Asia combined, Herman said.
The direct costs of diabetes are projected to grow to $1054 billion in 2045, an increase of just 9% over 25 years. The reason for the far lower increase going forward, compared with the tripling in the 15 years prior, is because of the anticipated diabetes rise in regions of the world where per-person spending on diabetes is low, a situation Dr. Herman called “unsustainable.”
“The keys to controlling the global costs of diabetes care are diabetes prevention and providing effective care to the largest number of people at the lowest possible cost,” he said.
Diabetes-related mortality: Some shifts since 2019
One third of the current 6.7 million diabetes-related deaths in 2021 were in people younger than 60 years, said Elbert S. Huang, MD, professor of medicine and public health sciences at the University of Chicago.
Overall, diabetes accounted for 11.8% of total global deaths in people younger than 60 years, but that varied widely, from 24.5% in the Middle East/North Africa to just 6.9% in Southeast Asia.
The regions with the highest number of diabetes-related deaths in people younger than 60 years in 2021 were the Western Pacific and the Middle East/North Africa, a major change from just 2 years ago, when Southeast Asia and Africa saw the greatest numbers of diabetes-related deaths in working-age adults.
“These findings mirror recent reports on inadequate uptake of diabetes prevention programs as well as stagnant quality of care trends for the past decade and reemphasize the need to address noncommunicable diseases across the globe,” Dr. Huang said.
Diabetes and COVID-19: Other factors partly explain the increased risk
Gillian Booth, MD, summarized the current literature on COVID-19 and diabetes including a meta-analysis her group conducted of 300 studies from around the world, with 58% from high-income countries.
The risk for increased COVID-19 severity in people with diabetes could be at least partly explained by factors such as age, sex, and comorbidities, said Dr. Booth, professor in the department of medicine and the Institute of Health Policy, Management, and Evaluation at the University of Toronto.
For example, the unadjusted pooled odds of hospitalization with COVID-19 in patients with diabetes, compared with those without diabetes, was 3.69, but dropped to 1.73 after adjustment for age, sex, and having one or more comorbidities. For COVID-19–related death, those odds ratios were 2.32 unadjusted versus 1.59 adjusted. In both cases, the values were still significant after adjustment, she emphasized.
Overall, hyperglycemia and hemoglobin A1c at admission emerged as significant independent predictors of severe outcomes.
“Further research is needed to understand the interplay between COVID-19 and diabetes and how best to address the disproportionate burden of COVID-19 among people living with diabetes,” she stressed.
Adult-onset type 1 diabetes: Growing recognition of the burden
Ascertainment of data for both adult-onset type 1 and type 2 diabetes in youth was subject to significant limitations.
For adult-onset type 1 diabetes, Jessica Harding, PhD, pointed to the fact that the epidemiology of adult-onset type 1 diabetes hasn’t been well characterized because of the historical focus on children, the difficulty of distinguishing it from type 2 diabetes in adults, and that many registries simply don’t include incident data across the lifespan for type 1 diabetes.
Nonetheless, she said, “there is growing recognition of the burden of adult-onset type 1,” noting that the American Diabetes Association and European Association for the Study of Diabetes just published a consensus statement addressing the topic.
A systematic review of 46 studies representing 32 countries or regions revealed that countries with the highest incidence of type 1 diabetes onset per population of 100,000 ages 20 or above were Eritrea, at 46.2, followed by Sweden and Ireland, both at 30.6, and Finland, at 0. The lowest rates were in Asian countries.
While the Nordic countries (Finland, Sweden, and Norway) are among the top for incidence of both childhood-onset (0-14 years) and adult-onset type 1 diabetes, Eritrea isn’t even among the top 10 for childhood onset.
The unusual situation in Eritrea is the subject of current study but the reasons aren’t yet clear, noted Dr. Magliano, of Emory University, Atlanta, during the question-and-answer period.
And only seven studies, 15%, used biomarkers to determine type 1 diabetes status, suggesting “there is a pressing need to improve the quality and quantity of information on adult-onset type 1 diabetes, particularly in those low- and middle-income countries,” Dr. Harding said.
Type 2 diabetes in youth: A call for better data
When presenting the data for childhood-onset type 2 diabetes, Andrea Luk, MD, noted: “The onset of advanced complications during the most productive time of life has significant impact on individuals, communities, and health economies.”
In 19 studies, the highest reported prevalence of type 2 diabetes in youth was in Brazil, Mexico, indigenous populations of the United States and Canada, and the Black population in the United States, with rates ranging from 160 per 100,000 to 3300 per 100,000. The lowest prevalence rates of 0.6 per 100,000 to 2.7 per 100,000 were reported in Europe. Incidence data were similar, with the highest rates from 31 per 100,000 to 94 per 100,000 and the lowest 0.1 per 100,000 to 0.8 per 100,000 per year.
Of note, Dr. Luk pointed out that childhood obesity is an important factor but not the only one.
“Some populations that have a low prevalence of obesity, such as East Asians, reported higher incidence rates of youth-onset type 2 diabetes than populations with a greater burden of childhood obesity.”
There was variability in incidence rates for youth of similar ethnic background but from different countries. “Apart from genetic predisposition and background obesogenic environment, disparity in socioeconomic status, access to health care, and cultural practices are other contributors to differences in risk of type 2 diabetes in youth,” noted Dr. Luk, associate professor in the division of endocrinology, Department of Medicine and Therapeutics, Chinese University of Hong Kong.
She also noted that the incidence of type 2 diabetes was extremely low in prepubertal children and rises gradually during puberty, and that the incidence is higher in girls than boys but that reverses in adulthood.
Compared with adults with type 2 diabetes, youth with type 2 diabetes had a more adverse glycemic trajectory and higher rates of metformin failure.
And compared with youth with type 1 diabetes, those with type 2 diabetes had more adverse metabolic profiles and higher rates of vascular complications.
“A strong call must be made for the collection of trend data to assess global burden of type 2 diabetes in youth,” she concluded.
Dr. Luk reported serving as an advisory panel member for and/or receiving research support from Amgen, AstraZeneca, Boehringer Ingelheim, Sanofi, the Asia Diabetes Foundation, Bayer, Lee’s Pharmaceutical, MSD, Novo Nordisk, Roche, Sugardown, and Takeda. The other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
One in 10 adults worldwide currently has diabetes, accounting for an estimated global health expenditure of $966 billion in U.S. dollars in 2021, according to the new International Diabetes Federation Diabetes Atlas.
The IDF Atlas, 10th edition, was published online Dec. 6, 2021.
Highlights from it were presented during two sessions at the IDF Virtual Congress 2021, covering global diabetes incidence and prevalence, mortality, and costs, as well as new sections in this edition devoted to adult-onset type 1 diabetes, childhood-onset type 2 diabetes, and the interactions between diabetes and COVID-19.
More detailed data from some of the Atlas chapters were also published Dec. 6, 2021, in separate papers in the IDF journal Diabetes Research and Clinical Practice, with more publications planned.
Information for the Atlas comes from peer-reviewed literature, unpublished reports, and national registries. This latest edition includes 219 data sources from 144 countries, with figures for other countries extrapolated.
Atlas cochair Dianna Magliano, PhD, reviewed some of the highlights. Half of those currently with diabetes, or about 240 million adults, are undiagnosed, and another 319 million have impaired fasting glucose. Over three-quarters of all adults with diabetes now live in low- and middle-income countries. And about 6.7 million deaths in 2021 can be attributed to diabetes.
The Atlas also predicts increases in these numbers over the coming decades if current trends continue.
“Our data and projections tell a sobering story. Diabetes prevalence is expected to increase globally. The number of adults with diabetes will rise from 537 million in 2021 to 786 million ... by the year 2045, an increase of 46%. Rises are expected in every region of the world, with the largest increases expected to occur in the regions of Africa, the Middle East, and Southeast Asia,” said Dr. Magliano, head of diabetes and population health at the Baker Heart and Diabetes Institute, Melbourne.
Since 2019, when the last Atlas was published, the 2021 numbers represent increases of 73.6 million more adults with diabetes including 7.8 million more undiagnosed, 2.5 million more deaths attributed to diabetes, and an additional global expenditure of $206 billion.
Increases have also occurred in the number of people with prediabetes, children with type 1 diabetes, and pregnancies affected by diabetes, Dr. Magliano reported.
“There is a strong need for effective intervention strategies and policies to stall the increase in the number of people developing diabetes across the world,” she added.
Projected rise in expenditures for diabetes will be ‘unsustainable’
The current $966 billion global health expenditure caused by diabetes represents a 316% increase from the $232 billion reported in 2006, according to William H. Herman, MD, professor of internal medicine and epidemiology at the University of Michigan, Ann Arbor.
By region, 43% of current diabetes-related global expenditures are in North America, 25% in the Western Pacific, and 20% in Europe, while 12% are from the regions of South and Central America, North Africa, Africa, and Southeast Asia combined, Herman said.
The direct costs of diabetes are projected to grow to $1054 billion in 2045, an increase of just 9% over 25 years. The reason for the far lower increase going forward, compared with the tripling in the 15 years prior, is because of the anticipated diabetes rise in regions of the world where per-person spending on diabetes is low, a situation Dr. Herman called “unsustainable.”
“The keys to controlling the global costs of diabetes care are diabetes prevention and providing effective care to the largest number of people at the lowest possible cost,” he said.
Diabetes-related mortality: Some shifts since 2019
One third of the current 6.7 million diabetes-related deaths in 2021 were in people younger than 60 years, said Elbert S. Huang, MD, professor of medicine and public health sciences at the University of Chicago.
Overall, diabetes accounted for 11.8% of total global deaths in people younger than 60 years, but that varied widely, from 24.5% in the Middle East/North Africa to just 6.9% in Southeast Asia.
The regions with the highest number of diabetes-related deaths in people younger than 60 years in 2021 were the Western Pacific and the Middle East/North Africa, a major change from just 2 years ago, when Southeast Asia and Africa saw the greatest numbers of diabetes-related deaths in working-age adults.
“These findings mirror recent reports on inadequate uptake of diabetes prevention programs as well as stagnant quality of care trends for the past decade and reemphasize the need to address noncommunicable diseases across the globe,” Dr. Huang said.
Diabetes and COVID-19: Other factors partly explain the increased risk
Gillian Booth, MD, summarized the current literature on COVID-19 and diabetes including a meta-analysis her group conducted of 300 studies from around the world, with 58% from high-income countries.
The risk for increased COVID-19 severity in people with diabetes could be at least partly explained by factors such as age, sex, and comorbidities, said Dr. Booth, professor in the department of medicine and the Institute of Health Policy, Management, and Evaluation at the University of Toronto.
For example, the unadjusted pooled odds of hospitalization with COVID-19 in patients with diabetes, compared with those without diabetes, was 3.69, but dropped to 1.73 after adjustment for age, sex, and having one or more comorbidities. For COVID-19–related death, those odds ratios were 2.32 unadjusted versus 1.59 adjusted. In both cases, the values were still significant after adjustment, she emphasized.
Overall, hyperglycemia and hemoglobin A1c at admission emerged as significant independent predictors of severe outcomes.
“Further research is needed to understand the interplay between COVID-19 and diabetes and how best to address the disproportionate burden of COVID-19 among people living with diabetes,” she stressed.
Adult-onset type 1 diabetes: Growing recognition of the burden
Ascertainment of data for both adult-onset type 1 and type 2 diabetes in youth was subject to significant limitations.
For adult-onset type 1 diabetes, Jessica Harding, PhD, pointed to the fact that the epidemiology of adult-onset type 1 diabetes hasn’t been well characterized because of the historical focus on children, the difficulty of distinguishing it from type 2 diabetes in adults, and that many registries simply don’t include incident data across the lifespan for type 1 diabetes.
Nonetheless, she said, “there is growing recognition of the burden of adult-onset type 1,” noting that the American Diabetes Association and European Association for the Study of Diabetes just published a consensus statement addressing the topic.
A systematic review of 46 studies representing 32 countries or regions revealed that countries with the highest incidence of type 1 diabetes onset per population of 100,000 ages 20 or above were Eritrea, at 46.2, followed by Sweden and Ireland, both at 30.6, and Finland, at 0. The lowest rates were in Asian countries.
While the Nordic countries (Finland, Sweden, and Norway) are among the top for incidence of both childhood-onset (0-14 years) and adult-onset type 1 diabetes, Eritrea isn’t even among the top 10 for childhood onset.
The unusual situation in Eritrea is the subject of current study but the reasons aren’t yet clear, noted Dr. Magliano, of Emory University, Atlanta, during the question-and-answer period.
And only seven studies, 15%, used biomarkers to determine type 1 diabetes status, suggesting “there is a pressing need to improve the quality and quantity of information on adult-onset type 1 diabetes, particularly in those low- and middle-income countries,” Dr. Harding said.
Type 2 diabetes in youth: A call for better data
When presenting the data for childhood-onset type 2 diabetes, Andrea Luk, MD, noted: “The onset of advanced complications during the most productive time of life has significant impact on individuals, communities, and health economies.”
In 19 studies, the highest reported prevalence of type 2 diabetes in youth was in Brazil, Mexico, indigenous populations of the United States and Canada, and the Black population in the United States, with rates ranging from 160 per 100,000 to 3300 per 100,000. The lowest prevalence rates of 0.6 per 100,000 to 2.7 per 100,000 were reported in Europe. Incidence data were similar, with the highest rates from 31 per 100,000 to 94 per 100,000 and the lowest 0.1 per 100,000 to 0.8 per 100,000 per year.
Of note, Dr. Luk pointed out that childhood obesity is an important factor but not the only one.
“Some populations that have a low prevalence of obesity, such as East Asians, reported higher incidence rates of youth-onset type 2 diabetes than populations with a greater burden of childhood obesity.”
There was variability in incidence rates for youth of similar ethnic background but from different countries. “Apart from genetic predisposition and background obesogenic environment, disparity in socioeconomic status, access to health care, and cultural practices are other contributors to differences in risk of type 2 diabetes in youth,” noted Dr. Luk, associate professor in the division of endocrinology, Department of Medicine and Therapeutics, Chinese University of Hong Kong.
She also noted that the incidence of type 2 diabetes was extremely low in prepubertal children and rises gradually during puberty, and that the incidence is higher in girls than boys but that reverses in adulthood.
Compared with adults with type 2 diabetes, youth with type 2 diabetes had a more adverse glycemic trajectory and higher rates of metformin failure.
And compared with youth with type 1 diabetes, those with type 2 diabetes had more adverse metabolic profiles and higher rates of vascular complications.
“A strong call must be made for the collection of trend data to assess global burden of type 2 diabetes in youth,” she concluded.
Dr. Luk reported serving as an advisory panel member for and/or receiving research support from Amgen, AstraZeneca, Boehringer Ingelheim, Sanofi, the Asia Diabetes Foundation, Bayer, Lee’s Pharmaceutical, MSD, Novo Nordisk, Roche, Sugardown, and Takeda. The other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
One in 10 adults worldwide currently has diabetes, accounting for an estimated global health expenditure of $966 billion in U.S. dollars in 2021, according to the new International Diabetes Federation Diabetes Atlas.
The IDF Atlas, 10th edition, was published online Dec. 6, 2021.
Highlights from it were presented during two sessions at the IDF Virtual Congress 2021, covering global diabetes incidence and prevalence, mortality, and costs, as well as new sections in this edition devoted to adult-onset type 1 diabetes, childhood-onset type 2 diabetes, and the interactions between diabetes and COVID-19.
More detailed data from some of the Atlas chapters were also published Dec. 6, 2021, in separate papers in the IDF journal Diabetes Research and Clinical Practice, with more publications planned.
Information for the Atlas comes from peer-reviewed literature, unpublished reports, and national registries. This latest edition includes 219 data sources from 144 countries, with figures for other countries extrapolated.
Atlas cochair Dianna Magliano, PhD, reviewed some of the highlights. Half of those currently with diabetes, or about 240 million adults, are undiagnosed, and another 319 million have impaired fasting glucose. Over three-quarters of all adults with diabetes now live in low- and middle-income countries. And about 6.7 million deaths in 2021 can be attributed to diabetes.
The Atlas also predicts increases in these numbers over the coming decades if current trends continue.
“Our data and projections tell a sobering story. Diabetes prevalence is expected to increase globally. The number of adults with diabetes will rise from 537 million in 2021 to 786 million ... by the year 2045, an increase of 46%. Rises are expected in every region of the world, with the largest increases expected to occur in the regions of Africa, the Middle East, and Southeast Asia,” said Dr. Magliano, head of diabetes and population health at the Baker Heart and Diabetes Institute, Melbourne.
Since 2019, when the last Atlas was published, the 2021 numbers represent increases of 73.6 million more adults with diabetes including 7.8 million more undiagnosed, 2.5 million more deaths attributed to diabetes, and an additional global expenditure of $206 billion.
Increases have also occurred in the number of people with prediabetes, children with type 1 diabetes, and pregnancies affected by diabetes, Dr. Magliano reported.
“There is a strong need for effective intervention strategies and policies to stall the increase in the number of people developing diabetes across the world,” she added.
Projected rise in expenditures for diabetes will be ‘unsustainable’
The current $966 billion global health expenditure caused by diabetes represents a 316% increase from the $232 billion reported in 2006, according to William H. Herman, MD, professor of internal medicine and epidemiology at the University of Michigan, Ann Arbor.
By region, 43% of current diabetes-related global expenditures are in North America, 25% in the Western Pacific, and 20% in Europe, while 12% are from the regions of South and Central America, North Africa, Africa, and Southeast Asia combined, Herman said.
The direct costs of diabetes are projected to grow to $1054 billion in 2045, an increase of just 9% over 25 years. The reason for the far lower increase going forward, compared with the tripling in the 15 years prior, is because of the anticipated diabetes rise in regions of the world where per-person spending on diabetes is low, a situation Dr. Herman called “unsustainable.”
“The keys to controlling the global costs of diabetes care are diabetes prevention and providing effective care to the largest number of people at the lowest possible cost,” he said.
Diabetes-related mortality: Some shifts since 2019
One third of the current 6.7 million diabetes-related deaths in 2021 were in people younger than 60 years, said Elbert S. Huang, MD, professor of medicine and public health sciences at the University of Chicago.
Overall, diabetes accounted for 11.8% of total global deaths in people younger than 60 years, but that varied widely, from 24.5% in the Middle East/North Africa to just 6.9% in Southeast Asia.
The regions with the highest number of diabetes-related deaths in people younger than 60 years in 2021 were the Western Pacific and the Middle East/North Africa, a major change from just 2 years ago, when Southeast Asia and Africa saw the greatest numbers of diabetes-related deaths in working-age adults.
“These findings mirror recent reports on inadequate uptake of diabetes prevention programs as well as stagnant quality of care trends for the past decade and reemphasize the need to address noncommunicable diseases across the globe,” Dr. Huang said.
Diabetes and COVID-19: Other factors partly explain the increased risk
Gillian Booth, MD, summarized the current literature on COVID-19 and diabetes including a meta-analysis her group conducted of 300 studies from around the world, with 58% from high-income countries.
The risk for increased COVID-19 severity in people with diabetes could be at least partly explained by factors such as age, sex, and comorbidities, said Dr. Booth, professor in the department of medicine and the Institute of Health Policy, Management, and Evaluation at the University of Toronto.
For example, the unadjusted pooled odds of hospitalization with COVID-19 in patients with diabetes, compared with those without diabetes, was 3.69, but dropped to 1.73 after adjustment for age, sex, and having one or more comorbidities. For COVID-19–related death, those odds ratios were 2.32 unadjusted versus 1.59 adjusted. In both cases, the values were still significant after adjustment, she emphasized.
Overall, hyperglycemia and hemoglobin A1c at admission emerged as significant independent predictors of severe outcomes.
“Further research is needed to understand the interplay between COVID-19 and diabetes and how best to address the disproportionate burden of COVID-19 among people living with diabetes,” she stressed.
Adult-onset type 1 diabetes: Growing recognition of the burden
Ascertainment of data for both adult-onset type 1 and type 2 diabetes in youth was subject to significant limitations.
For adult-onset type 1 diabetes, Jessica Harding, PhD, pointed to the fact that the epidemiology of adult-onset type 1 diabetes hasn’t been well characterized because of the historical focus on children, the difficulty of distinguishing it from type 2 diabetes in adults, and that many registries simply don’t include incident data across the lifespan for type 1 diabetes.
Nonetheless, she said, “there is growing recognition of the burden of adult-onset type 1,” noting that the American Diabetes Association and European Association for the Study of Diabetes just published a consensus statement addressing the topic.
A systematic review of 46 studies representing 32 countries or regions revealed that countries with the highest incidence of type 1 diabetes onset per population of 100,000 ages 20 or above were Eritrea, at 46.2, followed by Sweden and Ireland, both at 30.6, and Finland, at 0. The lowest rates were in Asian countries.
While the Nordic countries (Finland, Sweden, and Norway) are among the top for incidence of both childhood-onset (0-14 years) and adult-onset type 1 diabetes, Eritrea isn’t even among the top 10 for childhood onset.
The unusual situation in Eritrea is the subject of current study but the reasons aren’t yet clear, noted Dr. Magliano, of Emory University, Atlanta, during the question-and-answer period.
And only seven studies, 15%, used biomarkers to determine type 1 diabetes status, suggesting “there is a pressing need to improve the quality and quantity of information on adult-onset type 1 diabetes, particularly in those low- and middle-income countries,” Dr. Harding said.
Type 2 diabetes in youth: A call for better data
When presenting the data for childhood-onset type 2 diabetes, Andrea Luk, MD, noted: “The onset of advanced complications during the most productive time of life has significant impact on individuals, communities, and health economies.”
In 19 studies, the highest reported prevalence of type 2 diabetes in youth was in Brazil, Mexico, indigenous populations of the United States and Canada, and the Black population in the United States, with rates ranging from 160 per 100,000 to 3300 per 100,000. The lowest prevalence rates of 0.6 per 100,000 to 2.7 per 100,000 were reported in Europe. Incidence data were similar, with the highest rates from 31 per 100,000 to 94 per 100,000 and the lowest 0.1 per 100,000 to 0.8 per 100,000 per year.
Of note, Dr. Luk pointed out that childhood obesity is an important factor but not the only one.
“Some populations that have a low prevalence of obesity, such as East Asians, reported higher incidence rates of youth-onset type 2 diabetes than populations with a greater burden of childhood obesity.”
There was variability in incidence rates for youth of similar ethnic background but from different countries. “Apart from genetic predisposition and background obesogenic environment, disparity in socioeconomic status, access to health care, and cultural practices are other contributors to differences in risk of type 2 diabetes in youth,” noted Dr. Luk, associate professor in the division of endocrinology, Department of Medicine and Therapeutics, Chinese University of Hong Kong.
She also noted that the incidence of type 2 diabetes was extremely low in prepubertal children and rises gradually during puberty, and that the incidence is higher in girls than boys but that reverses in adulthood.
Compared with adults with type 2 diabetes, youth with type 2 diabetes had a more adverse glycemic trajectory and higher rates of metformin failure.
And compared with youth with type 1 diabetes, those with type 2 diabetes had more adverse metabolic profiles and higher rates of vascular complications.
“A strong call must be made for the collection of trend data to assess global burden of type 2 diabetes in youth,” she concluded.
Dr. Luk reported serving as an advisory panel member for and/or receiving research support from Amgen, AstraZeneca, Boehringer Ingelheim, Sanofi, the Asia Diabetes Foundation, Bayer, Lee’s Pharmaceutical, MSD, Novo Nordisk, Roche, Sugardown, and Takeda. The other authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
More brain aging observed in older patients with child-onset epilepsy
While the meaning of the findings aren’t entirely clear, new research offers insight into the aging brains of people who developed child-onset epilepsy: A cohort with an average age of 63 appears to be more likely than controls to show signs of brain deterioration, according to a study presented at the annual meeting of the American Epilepsy Society.
“,” lead author Matti Sillanpää, MD, PhD, a researcher and former child neurologist based at the University of Turko in Finland, said in an interview.
The study began 60 years ago when Finnish researchers started to track 99 subjects who were under 16 and had developed uncomplicated epilepsy. In 2012, 51 participants returned for assessments (9 of the original cohort had died, 2 didn’t speak Finnish as a mother tongue, and 15 had left the country or couldn’t be found).
In 2017, 41 participants agreed to take part in follow-up assessments (1 of the 2012 cohort could not be traced, and 9 declined to participate.)
Researchers launched the follow-up assessments to provide more insight into aging and epilepsy, Dr. Sillanpää said. “While we are in the early stages of understanding the brain and cognitive aging processes of people with epilepsy, there are enough worrisome signs from neuroimaging and cognitive studies to suggest that much more clinical and research attention is warranted. Especially important are population-based investigations that include persons with both remitted as well as active epilepsy in order to obtain a clearer understanding of the overall aging risks involved.”
The average age of the 41 subjects in the second assessment was 63.2 (4.1), and 58% were female. Just over half (52%) had focal epilepsy, and 48% had generalized epilepsy. In 74%, epilepsy had remitted, and it remained active in the rest (26%).
For the study, researchers compared the subjects with a control group of 46 subjects, 50% of whom were female, with an average age of 63.0 (4.13). The original control group had 99 participants, and 52 took part in 2012. Of those, 6 declined to participate in the 2017 assessments.
The researchers report these findings:
- Patients with active epilepsy were more likely to have neurologic signs than were those with remitted epilepsy (P = .015), especially the most common signs – cerebellar signs (P < .001). There was a trend toward cerebellar atrophy but it wasn’t statistically significant (P = .06).
- Patients with focal epilepsies were more likely to have neurologic signs (P = .008) and, specifically, cerebellar signs (P = .018) than were those with generalized epilepsies.
- The study authors calculated the lifetime usage of four drugs: carbamazepine, diphenylhydantoin, phenobarbital, and valproate. They found that patients with higher usage had more peripheral neuropathy, especially those with high levels of diphenylhydantoin, and phenobarbital usage.
- Overall, patients with epilepsy versus controls and those with active epilepsy versus remitting epilepsy were more likely to show adjusted declines in “cognitive trajectories” (both P < .05)
The researchers also estimated beta-amyloid levels via Pittsburgh Compound B positron emission tomography (PIB-PET); some specialists consider PIB-positive levels to be a sign of more beta amyloid.
From 2012 to 2017, the percentage of patients with epilepsy who were PIB positive grew from 22% to 33% (P = .03), while the percentage grew from 7% to 11% in the controls (P = .04). “The presence of amyloid and increasing positivity is cause for concern, and further research into the course of the participants is critical,” Dr. Sillanpää said.
It’s not clear if higher levels of brain aging are affecting the lives of participants, he said. “No one in the cohort has a diagnosed dementia at present, but going forward it will be important to pay close attention to the day-to-day functional status of participants.”
The mechanisms that may cause more brain aging in epilepsy aren’t known. However, “the CDC has shown through population-based investigations that people with epilepsy as a group may be more socially isolated, more physically inactive, and may harbor other lifestyle issues that we now know to be counterproductive to successful cognitive and brain aging in the general population,” Dr. Sillanpää said. “These factors need to be examined in depth in aging persons with epilepsy to gain a sound understanding of the risk and resilience factors that are most important so that people with epilepsy can act accordingly.”
The researchers also report that in patients with epilepsy, there’s evidence of a link between hypertension and hippocampal atrophy. They reported trends toward links between obesity and ischemic disease and between type 2 diabetes and hippocampal atrophy.
Going forward, “the findings may be helpful in the treatment and counseling of patients with epilepsy and especially advocating for those health and lifestyle practices that may be beneficial to long-term courses,” Dr. Sillanpää said. As for the study cohort, he said, researchers plan to continue monitoring them to track their long-term outcomes and any development of neurological disorders such as Alzheimer’s disease.
This work was funded by CURE Epilepsy, the National Governmental Research Grant, and the Pro Humanitate Foundation Grant. The study authors report no disclosures.
While the meaning of the findings aren’t entirely clear, new research offers insight into the aging brains of people who developed child-onset epilepsy: A cohort with an average age of 63 appears to be more likely than controls to show signs of brain deterioration, according to a study presented at the annual meeting of the American Epilepsy Society.
“,” lead author Matti Sillanpää, MD, PhD, a researcher and former child neurologist based at the University of Turko in Finland, said in an interview.
The study began 60 years ago when Finnish researchers started to track 99 subjects who were under 16 and had developed uncomplicated epilepsy. In 2012, 51 participants returned for assessments (9 of the original cohort had died, 2 didn’t speak Finnish as a mother tongue, and 15 had left the country or couldn’t be found).
In 2017, 41 participants agreed to take part in follow-up assessments (1 of the 2012 cohort could not be traced, and 9 declined to participate.)
Researchers launched the follow-up assessments to provide more insight into aging and epilepsy, Dr. Sillanpää said. “While we are in the early stages of understanding the brain and cognitive aging processes of people with epilepsy, there are enough worrisome signs from neuroimaging and cognitive studies to suggest that much more clinical and research attention is warranted. Especially important are population-based investigations that include persons with both remitted as well as active epilepsy in order to obtain a clearer understanding of the overall aging risks involved.”
The average age of the 41 subjects in the second assessment was 63.2 (4.1), and 58% were female. Just over half (52%) had focal epilepsy, and 48% had generalized epilepsy. In 74%, epilepsy had remitted, and it remained active in the rest (26%).
For the study, researchers compared the subjects with a control group of 46 subjects, 50% of whom were female, with an average age of 63.0 (4.13). The original control group had 99 participants, and 52 took part in 2012. Of those, 6 declined to participate in the 2017 assessments.
The researchers report these findings:
- Patients with active epilepsy were more likely to have neurologic signs than were those with remitted epilepsy (P = .015), especially the most common signs – cerebellar signs (P < .001). There was a trend toward cerebellar atrophy but it wasn’t statistically significant (P = .06).
- Patients with focal epilepsies were more likely to have neurologic signs (P = .008) and, specifically, cerebellar signs (P = .018) than were those with generalized epilepsies.
- The study authors calculated the lifetime usage of four drugs: carbamazepine, diphenylhydantoin, phenobarbital, and valproate. They found that patients with higher usage had more peripheral neuropathy, especially those with high levels of diphenylhydantoin, and phenobarbital usage.
- Overall, patients with epilepsy versus controls and those with active epilepsy versus remitting epilepsy were more likely to show adjusted declines in “cognitive trajectories” (both P < .05)
The researchers also estimated beta-amyloid levels via Pittsburgh Compound B positron emission tomography (PIB-PET); some specialists consider PIB-positive levels to be a sign of more beta amyloid.
From 2012 to 2017, the percentage of patients with epilepsy who were PIB positive grew from 22% to 33% (P = .03), while the percentage grew from 7% to 11% in the controls (P = .04). “The presence of amyloid and increasing positivity is cause for concern, and further research into the course of the participants is critical,” Dr. Sillanpää said.
It’s not clear if higher levels of brain aging are affecting the lives of participants, he said. “No one in the cohort has a diagnosed dementia at present, but going forward it will be important to pay close attention to the day-to-day functional status of participants.”
The mechanisms that may cause more brain aging in epilepsy aren’t known. However, “the CDC has shown through population-based investigations that people with epilepsy as a group may be more socially isolated, more physically inactive, and may harbor other lifestyle issues that we now know to be counterproductive to successful cognitive and brain aging in the general population,” Dr. Sillanpää said. “These factors need to be examined in depth in aging persons with epilepsy to gain a sound understanding of the risk and resilience factors that are most important so that people with epilepsy can act accordingly.”
The researchers also report that in patients with epilepsy, there’s evidence of a link between hypertension and hippocampal atrophy. They reported trends toward links between obesity and ischemic disease and between type 2 diabetes and hippocampal atrophy.
Going forward, “the findings may be helpful in the treatment and counseling of patients with epilepsy and especially advocating for those health and lifestyle practices that may be beneficial to long-term courses,” Dr. Sillanpää said. As for the study cohort, he said, researchers plan to continue monitoring them to track their long-term outcomes and any development of neurological disorders such as Alzheimer’s disease.
This work was funded by CURE Epilepsy, the National Governmental Research Grant, and the Pro Humanitate Foundation Grant. The study authors report no disclosures.
While the meaning of the findings aren’t entirely clear, new research offers insight into the aging brains of people who developed child-onset epilepsy: A cohort with an average age of 63 appears to be more likely than controls to show signs of brain deterioration, according to a study presented at the annual meeting of the American Epilepsy Society.
“,” lead author Matti Sillanpää, MD, PhD, a researcher and former child neurologist based at the University of Turko in Finland, said in an interview.
The study began 60 years ago when Finnish researchers started to track 99 subjects who were under 16 and had developed uncomplicated epilepsy. In 2012, 51 participants returned for assessments (9 of the original cohort had died, 2 didn’t speak Finnish as a mother tongue, and 15 had left the country or couldn’t be found).
In 2017, 41 participants agreed to take part in follow-up assessments (1 of the 2012 cohort could not be traced, and 9 declined to participate.)
Researchers launched the follow-up assessments to provide more insight into aging and epilepsy, Dr. Sillanpää said. “While we are in the early stages of understanding the brain and cognitive aging processes of people with epilepsy, there are enough worrisome signs from neuroimaging and cognitive studies to suggest that much more clinical and research attention is warranted. Especially important are population-based investigations that include persons with both remitted as well as active epilepsy in order to obtain a clearer understanding of the overall aging risks involved.”
The average age of the 41 subjects in the second assessment was 63.2 (4.1), and 58% were female. Just over half (52%) had focal epilepsy, and 48% had generalized epilepsy. In 74%, epilepsy had remitted, and it remained active in the rest (26%).
For the study, researchers compared the subjects with a control group of 46 subjects, 50% of whom were female, with an average age of 63.0 (4.13). The original control group had 99 participants, and 52 took part in 2012. Of those, 6 declined to participate in the 2017 assessments.
The researchers report these findings:
- Patients with active epilepsy were more likely to have neurologic signs than were those with remitted epilepsy (P = .015), especially the most common signs – cerebellar signs (P < .001). There was a trend toward cerebellar atrophy but it wasn’t statistically significant (P = .06).
- Patients with focal epilepsies were more likely to have neurologic signs (P = .008) and, specifically, cerebellar signs (P = .018) than were those with generalized epilepsies.
- The study authors calculated the lifetime usage of four drugs: carbamazepine, diphenylhydantoin, phenobarbital, and valproate. They found that patients with higher usage had more peripheral neuropathy, especially those with high levels of diphenylhydantoin, and phenobarbital usage.
- Overall, patients with epilepsy versus controls and those with active epilepsy versus remitting epilepsy were more likely to show adjusted declines in “cognitive trajectories” (both P < .05)
The researchers also estimated beta-amyloid levels via Pittsburgh Compound B positron emission tomography (PIB-PET); some specialists consider PIB-positive levels to be a sign of more beta amyloid.
From 2012 to 2017, the percentage of patients with epilepsy who were PIB positive grew from 22% to 33% (P = .03), while the percentage grew from 7% to 11% in the controls (P = .04). “The presence of amyloid and increasing positivity is cause for concern, and further research into the course of the participants is critical,” Dr. Sillanpää said.
It’s not clear if higher levels of brain aging are affecting the lives of participants, he said. “No one in the cohort has a diagnosed dementia at present, but going forward it will be important to pay close attention to the day-to-day functional status of participants.”
The mechanisms that may cause more brain aging in epilepsy aren’t known. However, “the CDC has shown through population-based investigations that people with epilepsy as a group may be more socially isolated, more physically inactive, and may harbor other lifestyle issues that we now know to be counterproductive to successful cognitive and brain aging in the general population,” Dr. Sillanpää said. “These factors need to be examined in depth in aging persons with epilepsy to gain a sound understanding of the risk and resilience factors that are most important so that people with epilepsy can act accordingly.”
The researchers also report that in patients with epilepsy, there’s evidence of a link between hypertension and hippocampal atrophy. They reported trends toward links between obesity and ischemic disease and between type 2 diabetes and hippocampal atrophy.
Going forward, “the findings may be helpful in the treatment and counseling of patients with epilepsy and especially advocating for those health and lifestyle practices that may be beneficial to long-term courses,” Dr. Sillanpää said. As for the study cohort, he said, researchers plan to continue monitoring them to track their long-term outcomes and any development of neurological disorders such as Alzheimer’s disease.
This work was funded by CURE Epilepsy, the National Governmental Research Grant, and the Pro Humanitate Foundation Grant. The study authors report no disclosures.
FROM AES 2021
FIB-4 could ID liver risk in primary care
Fibrosis-4 index (FIB-4) scores are strongly associated with severe liver disease outcomes in a primary care population, both in patients with known chronic liver disease and those without known CLD. The result could help identify patients with CLD before their condition becomes severe.
FIB-4 has previously shown utility in predicting the risk of advanced fibrosis in patients with viral hepatitis B and C, nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH), and alcohol-related liver disease.
“This is really important in primary care because FIB-4 is easy to calculate. Its inputs are accessible, and it is inexpensive, often taking advantage of labs that we’ve ordered anyway. And if we can use it to find advanced fibrosis, it will be critically important because we know that advanced fibrosis is associated with severe liver outcomes – these are going to be patients that we need to make sure are in touch with our hepatology colleagues,” said Andrew Schreiner, MD, during a presentation of the results at the annual meeting of the American Association for the Study of Liver Diseases. Dr. Schreiner is general internist at the Medical University of South Carolina, Charleston.
He also noted that FIB-4 is playing an important role in the assessment of NAFLD and NASH. Many newer algorithms to manage NAFLD in the primary care setting rely on FIB-4, but that application is limited because NAFLD is underdiagnosed according to administrative database studies, which found rates of about 2%-5% despite the fact that estimates put it at having a prevalence of 25%-30% in the U.S. population.
To determine if FIB-4 scores could assist in identifying primary care patients at risk of severe outcomes, including cirrhosis, hepatocellular carcinoma, and liver transplant, the researchers conducted a retrospective analysis of primary care electronic health care data from 20,556 patients between 2007 and 2018 who were seen at their institution. Participants had ALT and AST values less than 500 IU/L, as well as a platelet count within two months preceding or on the day of the liver enzyme tests. They excluded individuals with known chronic or severe liver disease.
65% of patients were female, 45% were Black, and the mean BMI was 29.8 kg/m2. 64% of participants were ranked as low risk (FIB-4 ≤1.3), 29% with undetermined risk (1.3-2.67), and 7% with high risk (>2.67).
The population had more liver risk than expected. “[It is] a distribution that certainly may have more high risk and indeterminant risks than we would have anticipated, but we have seen this in external studies,” said Dr. Schreiner.
Over a mean follow-up period of 8.2 years, 11% were diagnosed with CLD: 2.3% developed NAFLD, 8.2% another CLD, and 0.5% had NAFLD and another CLD. About 4% developed a severe CLD. A severe liver outcome occurred in 2.2% of those who had been classified as FIB-4 low risk, 4.2% classified as indeterminate risk, and 20.8% of those classified as high risk.
“Troublingly,” said Dr. Schreiner, 49% of those who went on to develop a severe liver outcome had no CLD diagnosis before it occurred. “This is a tremendous opportunity to improve diagnosis in this setting.”
After adjustment for race, gender, marital status, smoking history, BMI, and various comorbidities, the researchers found a higher risk of severe liver disease associated with indeterminate FIB-4 risk score (hazard ratio, 1.62; 95% confidence interval, 1.36-1.92) and a high FIB-4 risk score (HR, 6.64; 95% CI, 5.58-7.90), compared with those with a low FIB-4 risk score. The same was true for individual liver diseases, including NAFLD (indeterminate HR, 1.88; 95% CI, 0.99-3.60; high HR, 7.32; 95% CI, 3.44-15.58), other liver diagnosis (indeterminate HR, 2.65; 95% CI, 1.93-3.63; high HR, 11.39; 95% CI, 8.53-15.20), and NAFLD plus another liver disease (intermediate HR, 2.53; 95% CI, 0.79-8.12; high HR, 6.89; 95% CI, 1.82-26.14).
Dr. Schreiner conceded that the study may not be generalizable, since FIB-4 was not designed for use in general populations, and it was conducted at a single center.
During the question-and-answer session after the talk, Dr. Schreiner was asked if the majority of the 49% who had a severe liver outcome without previous liver disease had NAFLD. He said that was the team’s hypothesis, and they are in the process of examining that data, but a significant number appear to be alcohol related. “For us in the primary care setting, it’s just another opportunity to emphasize that we have to do a better job getting exposure histories, and alcohol histories in particular, and finding ways to document those in ways that we can make diagnoses for patients and for our hepatology colleagues,” said Dr. Schreiner.
Comoderator Kathleen Corey, MD, asked Dr. Schreiner if he had any concerns about false positives from FIB-4 screening, and whether that could lead to overtreatment. “We’ve seen other screening tests leading to patient distress and overutilization of resources. How do you think we might be able to mitigate that?” asked Dr. Corey, who is an assistant professor of medicine at Harvard Medical School and director of the Fatty Liver Clinic at Massachusetts General Hospital, both in Boston.
Dr. Schreiner underscored the need for more physician education about FIB-4, both its potential and its pitfalls, since many primary care providers don’t use it or even know about it. “FIB-4 is very popular in the hepatology literature, but in primary care, we don’t talk about it as often. So I think educational efforts about its possible utility, about some of the drawbacks, or some of the things that might lead to inappropriately positive results – like advanced age, for those of us who see patients 60 and older. Those are really important considerations both for the patient and the provider for management of expectations and concerns. I’m worried too about application in our younger cohorts. The explosion of NAFLD in adolescence, and the likelihood that we might get a false negative in maybe a 28-year-old who might have problematic disease, is a concern as well,” said Dr. Schreiner.
Dr. Schreiner has no relevant financial disclosures. Dr. Corey has been on an advisory committee or review panel for Bristol-Myers Squibb, Novo Nordisk, and Gilead. She has consulted for Novo Nordisk and received research support from BMS, Boehringer Ingelheim, Novartis, and Boehringer Ingelheim.
Fibrosis-4 index (FIB-4) scores are strongly associated with severe liver disease outcomes in a primary care population, both in patients with known chronic liver disease and those without known CLD. The result could help identify patients with CLD before their condition becomes severe.
FIB-4 has previously shown utility in predicting the risk of advanced fibrosis in patients with viral hepatitis B and C, nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH), and alcohol-related liver disease.
“This is really important in primary care because FIB-4 is easy to calculate. Its inputs are accessible, and it is inexpensive, often taking advantage of labs that we’ve ordered anyway. And if we can use it to find advanced fibrosis, it will be critically important because we know that advanced fibrosis is associated with severe liver outcomes – these are going to be patients that we need to make sure are in touch with our hepatology colleagues,” said Andrew Schreiner, MD, during a presentation of the results at the annual meeting of the American Association for the Study of Liver Diseases. Dr. Schreiner is general internist at the Medical University of South Carolina, Charleston.
He also noted that FIB-4 is playing an important role in the assessment of NAFLD and NASH. Many newer algorithms to manage NAFLD in the primary care setting rely on FIB-4, but that application is limited because NAFLD is underdiagnosed according to administrative database studies, which found rates of about 2%-5% despite the fact that estimates put it at having a prevalence of 25%-30% in the U.S. population.
To determine if FIB-4 scores could assist in identifying primary care patients at risk of severe outcomes, including cirrhosis, hepatocellular carcinoma, and liver transplant, the researchers conducted a retrospective analysis of primary care electronic health care data from 20,556 patients between 2007 and 2018 who were seen at their institution. Participants had ALT and AST values less than 500 IU/L, as well as a platelet count within two months preceding or on the day of the liver enzyme tests. They excluded individuals with known chronic or severe liver disease.
65% of patients were female, 45% were Black, and the mean BMI was 29.8 kg/m2. 64% of participants were ranked as low risk (FIB-4 ≤1.3), 29% with undetermined risk (1.3-2.67), and 7% with high risk (>2.67).
The population had more liver risk than expected. “[It is] a distribution that certainly may have more high risk and indeterminant risks than we would have anticipated, but we have seen this in external studies,” said Dr. Schreiner.
Over a mean follow-up period of 8.2 years, 11% were diagnosed with CLD: 2.3% developed NAFLD, 8.2% another CLD, and 0.5% had NAFLD and another CLD. About 4% developed a severe CLD. A severe liver outcome occurred in 2.2% of those who had been classified as FIB-4 low risk, 4.2% classified as indeterminate risk, and 20.8% of those classified as high risk.
“Troublingly,” said Dr. Schreiner, 49% of those who went on to develop a severe liver outcome had no CLD diagnosis before it occurred. “This is a tremendous opportunity to improve diagnosis in this setting.”
After adjustment for race, gender, marital status, smoking history, BMI, and various comorbidities, the researchers found a higher risk of severe liver disease associated with indeterminate FIB-4 risk score (hazard ratio, 1.62; 95% confidence interval, 1.36-1.92) and a high FIB-4 risk score (HR, 6.64; 95% CI, 5.58-7.90), compared with those with a low FIB-4 risk score. The same was true for individual liver diseases, including NAFLD (indeterminate HR, 1.88; 95% CI, 0.99-3.60; high HR, 7.32; 95% CI, 3.44-15.58), other liver diagnosis (indeterminate HR, 2.65; 95% CI, 1.93-3.63; high HR, 11.39; 95% CI, 8.53-15.20), and NAFLD plus another liver disease (intermediate HR, 2.53; 95% CI, 0.79-8.12; high HR, 6.89; 95% CI, 1.82-26.14).
Dr. Schreiner conceded that the study may not be generalizable, since FIB-4 was not designed for use in general populations, and it was conducted at a single center.
During the question-and-answer session after the talk, Dr. Schreiner was asked if the majority of the 49% who had a severe liver outcome without previous liver disease had NAFLD. He said that was the team’s hypothesis, and they are in the process of examining that data, but a significant number appear to be alcohol related. “For us in the primary care setting, it’s just another opportunity to emphasize that we have to do a better job getting exposure histories, and alcohol histories in particular, and finding ways to document those in ways that we can make diagnoses for patients and for our hepatology colleagues,” said Dr. Schreiner.
Comoderator Kathleen Corey, MD, asked Dr. Schreiner if he had any concerns about false positives from FIB-4 screening, and whether that could lead to overtreatment. “We’ve seen other screening tests leading to patient distress and overutilization of resources. How do you think we might be able to mitigate that?” asked Dr. Corey, who is an assistant professor of medicine at Harvard Medical School and director of the Fatty Liver Clinic at Massachusetts General Hospital, both in Boston.
Dr. Schreiner underscored the need for more physician education about FIB-4, both its potential and its pitfalls, since many primary care providers don’t use it or even know about it. “FIB-4 is very popular in the hepatology literature, but in primary care, we don’t talk about it as often. So I think educational efforts about its possible utility, about some of the drawbacks, or some of the things that might lead to inappropriately positive results – like advanced age, for those of us who see patients 60 and older. Those are really important considerations both for the patient and the provider for management of expectations and concerns. I’m worried too about application in our younger cohorts. The explosion of NAFLD in adolescence, and the likelihood that we might get a false negative in maybe a 28-year-old who might have problematic disease, is a concern as well,” said Dr. Schreiner.
Dr. Schreiner has no relevant financial disclosures. Dr. Corey has been on an advisory committee or review panel for Bristol-Myers Squibb, Novo Nordisk, and Gilead. She has consulted for Novo Nordisk and received research support from BMS, Boehringer Ingelheim, Novartis, and Boehringer Ingelheim.
Fibrosis-4 index (FIB-4) scores are strongly associated with severe liver disease outcomes in a primary care population, both in patients with known chronic liver disease and those without known CLD. The result could help identify patients with CLD before their condition becomes severe.
FIB-4 has previously shown utility in predicting the risk of advanced fibrosis in patients with viral hepatitis B and C, nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH), and alcohol-related liver disease.
“This is really important in primary care because FIB-4 is easy to calculate. Its inputs are accessible, and it is inexpensive, often taking advantage of labs that we’ve ordered anyway. And if we can use it to find advanced fibrosis, it will be critically important because we know that advanced fibrosis is associated with severe liver outcomes – these are going to be patients that we need to make sure are in touch with our hepatology colleagues,” said Andrew Schreiner, MD, during a presentation of the results at the annual meeting of the American Association for the Study of Liver Diseases. Dr. Schreiner is general internist at the Medical University of South Carolina, Charleston.
He also noted that FIB-4 is playing an important role in the assessment of NAFLD and NASH. Many newer algorithms to manage NAFLD in the primary care setting rely on FIB-4, but that application is limited because NAFLD is underdiagnosed according to administrative database studies, which found rates of about 2%-5% despite the fact that estimates put it at having a prevalence of 25%-30% in the U.S. population.
To determine if FIB-4 scores could assist in identifying primary care patients at risk of severe outcomes, including cirrhosis, hepatocellular carcinoma, and liver transplant, the researchers conducted a retrospective analysis of primary care electronic health care data from 20,556 patients between 2007 and 2018 who were seen at their institution. Participants had ALT and AST values less than 500 IU/L, as well as a platelet count within two months preceding or on the day of the liver enzyme tests. They excluded individuals with known chronic or severe liver disease.
65% of patients were female, 45% were Black, and the mean BMI was 29.8 kg/m2. 64% of participants were ranked as low risk (FIB-4 ≤1.3), 29% with undetermined risk (1.3-2.67), and 7% with high risk (>2.67).
The population had more liver risk than expected. “[It is] a distribution that certainly may have more high risk and indeterminant risks than we would have anticipated, but we have seen this in external studies,” said Dr. Schreiner.
Over a mean follow-up period of 8.2 years, 11% were diagnosed with CLD: 2.3% developed NAFLD, 8.2% another CLD, and 0.5% had NAFLD and another CLD. About 4% developed a severe CLD. A severe liver outcome occurred in 2.2% of those who had been classified as FIB-4 low risk, 4.2% classified as indeterminate risk, and 20.8% of those classified as high risk.
“Troublingly,” said Dr. Schreiner, 49% of those who went on to develop a severe liver outcome had no CLD diagnosis before it occurred. “This is a tremendous opportunity to improve diagnosis in this setting.”
After adjustment for race, gender, marital status, smoking history, BMI, and various comorbidities, the researchers found a higher risk of severe liver disease associated with indeterminate FIB-4 risk score (hazard ratio, 1.62; 95% confidence interval, 1.36-1.92) and a high FIB-4 risk score (HR, 6.64; 95% CI, 5.58-7.90), compared with those with a low FIB-4 risk score. The same was true for individual liver diseases, including NAFLD (indeterminate HR, 1.88; 95% CI, 0.99-3.60; high HR, 7.32; 95% CI, 3.44-15.58), other liver diagnosis (indeterminate HR, 2.65; 95% CI, 1.93-3.63; high HR, 11.39; 95% CI, 8.53-15.20), and NAFLD plus another liver disease (intermediate HR, 2.53; 95% CI, 0.79-8.12; high HR, 6.89; 95% CI, 1.82-26.14).
Dr. Schreiner conceded that the study may not be generalizable, since FIB-4 was not designed for use in general populations, and it was conducted at a single center.
During the question-and-answer session after the talk, Dr. Schreiner was asked if the majority of the 49% who had a severe liver outcome without previous liver disease had NAFLD. He said that was the team’s hypothesis, and they are in the process of examining that data, but a significant number appear to be alcohol related. “For us in the primary care setting, it’s just another opportunity to emphasize that we have to do a better job getting exposure histories, and alcohol histories in particular, and finding ways to document those in ways that we can make diagnoses for patients and for our hepatology colleagues,” said Dr. Schreiner.
Comoderator Kathleen Corey, MD, asked Dr. Schreiner if he had any concerns about false positives from FIB-4 screening, and whether that could lead to overtreatment. “We’ve seen other screening tests leading to patient distress and overutilization of resources. How do you think we might be able to mitigate that?” asked Dr. Corey, who is an assistant professor of medicine at Harvard Medical School and director of the Fatty Liver Clinic at Massachusetts General Hospital, both in Boston.
Dr. Schreiner underscored the need for more physician education about FIB-4, both its potential and its pitfalls, since many primary care providers don’t use it or even know about it. “FIB-4 is very popular in the hepatology literature, but in primary care, we don’t talk about it as often. So I think educational efforts about its possible utility, about some of the drawbacks, or some of the things that might lead to inappropriately positive results – like advanced age, for those of us who see patients 60 and older. Those are really important considerations both for the patient and the provider for management of expectations and concerns. I’m worried too about application in our younger cohorts. The explosion of NAFLD in adolescence, and the likelihood that we might get a false negative in maybe a 28-year-old who might have problematic disease, is a concern as well,” said Dr. Schreiner.
Dr. Schreiner has no relevant financial disclosures. Dr. Corey has been on an advisory committee or review panel for Bristol-Myers Squibb, Novo Nordisk, and Gilead. She has consulted for Novo Nordisk and received research support from BMS, Boehringer Ingelheim, Novartis, and Boehringer Ingelheim.
FROM THE LIVER MEETING
Metformin does not improve outcomes in early breast cancer
In the primary analysis, the addition of metformin to standard therapy in moderate/high-risk hormone receptor positive or negative breast cancer did not improve invasive disease–free survival (IDFS), overall survival, or other breast outcomes, explained lead author Pamela J. Goodwin, MD, FRCPC, professor of medicine at the University of Toronto. “Metformin should not be used as breast cancer treatment in this population.”
However, an exploratory analysis suggested that metformin may have a beneficial effect in women with HER2-positive breast cancer, Dr. Goodwin noted.
In this subset, IDFS was improved in patients who received metformin (hazard ratio, 0.64; P = .03), as was overall survival (HR, 0.53; P = .04).
The findings were presented at the San Antonio Breast Cancer Symposium.
“This trial arose from the observation that obesity is associated with poor breast cancer outcomes, and insulin levels are higher in obesity and may be more strongly associated with breast cancer outcomes than obesity,” said Dr. Goodwin.
Metformin was used because of its ability to promote modest weight loss and lower insulin by about 15%-20% in nondiabetic breast cancer survivors. It has also shown anticancer effects in preclinical studies. “In some window of opportunity neoadjuvant studies, it has been shown to reduce Ki67 in breast cancer cells,” she said. “And in preclinical in vitro and in vivo research, it slows growth of breast cancer.”
In addition, emerging evidence from observational studies suggests that the use of metformin to treat diabetes in breast cancer patients may be associated with better outcomes, strengthening the rationale for the study.
The negative results in breast cancer follow recent reports of negative findings in lung cancer, when metformin was found to be ineffective when used alongside chemotherapy in locally advanced lung cancer, as reported by this news organization.
No benefit seen
Metformin was compared to placebo in the phase 3 CCTG MA.32 trial, conducted in 3,649 patients aged 18-74 years with T1-3 N0-3 M0 breast cancer. All patients were treated with standard therapy and were randomized to receive metformin 850 mg twice daily for 5 years or placebo.
In 2016, “futility was declared in ER/PR-negative patients” after a second interim analysis conducted at 29.6 months’ median follow-up, Dr. Goodwin noted. The intervention was stopped in that group, although blinding and follow-up continued.
After that, the study’s primary analysis focused on the 2,533 ER/PR-positive patients (mean age, 52.7 years; mean body mass index, 28.8; approx. 60% postmenopausal).
Just over half of these patients had T2 tumor stage, and most disease was grade 2 or 3.
In addition, 16.5% (of metformin) and 17.4% (of placebo) patients had HER2-positive disease, with the majority (97%) of all HER2 patients receiving trastuzumab.
There was no difference between the two groups in IDFS events, occurring in 18.5% of patients receiving metformin and 18.3% who received placebo, with most (75.6%) events due to breast cancer (HR, 1.01; P = .92).
There were 131 deaths in the metformin arm and 119 in the placebo arm, with most (75.8%) of the deaths related to breast cancer (HR, 1.10; P = .47).
Other breast cancer outcomes had similar results, including distant disease-free survival (HR, 0.99; P = .94) and breast cancer–free interval (HR, 0.98; P = .87), both of which showed no advantage for metformin.
Possible HER2 advantage
However, the exploratory analysis suggested there may be an advantage for patients with HER2-positive disease, but primarily those who had at least one C allele of a prespecified ATM associated rs11212617 SNP. These patients achieved a higher pathologic complete response rate with metformin than that of those without the allele.
There were 620 patients with HER2-positive disease analyzed, with 99.4% receiving chemotherapy and 96.5% receiving trastuzumab. There were 99 IDFS events, and 47 OS events.
In the entire HER2-positive cohort, patients who received metformin had fewer IDFS events (HR, 0.64; P = .026) compared with the placebo arm. Mortality was lower with metformin (HR for overall survival, 0.53; P = .038).
“Subjects with HER2-positive breast cancer, notably those with at least one C allele of the ATM-associated rs11212617 SNP, experienced improved IDFS and overall survival with metformin,” Dr. Goodwin concluded. “However, no P-value ‘spend’ was allocated to this comparison. As a result, it requires replication in a prospective trial focusing on the HER2-positive population.”
More research?
Stephanie Bernik, MD, chief of breast surgery, Mount Sinai West, and associate professor of breast surgery, Icahn School of Medicine at Mount Sinai, New York, was approached by this news organization for an independent comment.
“It has long been known that obesity, which often correlates with diabetes, increases a woman’s risk of breast cancer,” she said.
“This study tried to show that using a medication that helps control insulin levels, even in those without diabetes, might decrease one’s risk of breast cancer,” she said. “Unfortunately, using metformin had no effect on outcomes in this study, even though it has shown promise in other studies. Perhaps more research needs to be carried out to try to pinpoint which mechanisms of action, if any, might be helpful to combat cancer in those with and without diabetes.”
The study was funded by the Canadian Cancer Trials Group, Cancer Therapy Evaluation Program, Breast Cancer Researcher Foundation, Susan G. Komen for the Cure, Canadian Cancer Society, Apotex, Swiss Cancer Research, and the Canadian Breast Cancer Foundation. Dr. Goodwin has no disclosures.
A version of this article first appeared on Medscape.com.
In the primary analysis, the addition of metformin to standard therapy in moderate/high-risk hormone receptor positive or negative breast cancer did not improve invasive disease–free survival (IDFS), overall survival, or other breast outcomes, explained lead author Pamela J. Goodwin, MD, FRCPC, professor of medicine at the University of Toronto. “Metformin should not be used as breast cancer treatment in this population.”
However, an exploratory analysis suggested that metformin may have a beneficial effect in women with HER2-positive breast cancer, Dr. Goodwin noted.
In this subset, IDFS was improved in patients who received metformin (hazard ratio, 0.64; P = .03), as was overall survival (HR, 0.53; P = .04).
The findings were presented at the San Antonio Breast Cancer Symposium.
“This trial arose from the observation that obesity is associated with poor breast cancer outcomes, and insulin levels are higher in obesity and may be more strongly associated with breast cancer outcomes than obesity,” said Dr. Goodwin.
Metformin was used because of its ability to promote modest weight loss and lower insulin by about 15%-20% in nondiabetic breast cancer survivors. It has also shown anticancer effects in preclinical studies. “In some window of opportunity neoadjuvant studies, it has been shown to reduce Ki67 in breast cancer cells,” she said. “And in preclinical in vitro and in vivo research, it slows growth of breast cancer.”
In addition, emerging evidence from observational studies suggests that the use of metformin to treat diabetes in breast cancer patients may be associated with better outcomes, strengthening the rationale for the study.
The negative results in breast cancer follow recent reports of negative findings in lung cancer, when metformin was found to be ineffective when used alongside chemotherapy in locally advanced lung cancer, as reported by this news organization.
No benefit seen
Metformin was compared to placebo in the phase 3 CCTG MA.32 trial, conducted in 3,649 patients aged 18-74 years with T1-3 N0-3 M0 breast cancer. All patients were treated with standard therapy and were randomized to receive metformin 850 mg twice daily for 5 years or placebo.
In 2016, “futility was declared in ER/PR-negative patients” after a second interim analysis conducted at 29.6 months’ median follow-up, Dr. Goodwin noted. The intervention was stopped in that group, although blinding and follow-up continued.
After that, the study’s primary analysis focused on the 2,533 ER/PR-positive patients (mean age, 52.7 years; mean body mass index, 28.8; approx. 60% postmenopausal).
Just over half of these patients had T2 tumor stage, and most disease was grade 2 or 3.
In addition, 16.5% (of metformin) and 17.4% (of placebo) patients had HER2-positive disease, with the majority (97%) of all HER2 patients receiving trastuzumab.
There was no difference between the two groups in IDFS events, occurring in 18.5% of patients receiving metformin and 18.3% who received placebo, with most (75.6%) events due to breast cancer (HR, 1.01; P = .92).
There were 131 deaths in the metformin arm and 119 in the placebo arm, with most (75.8%) of the deaths related to breast cancer (HR, 1.10; P = .47).
Other breast cancer outcomes had similar results, including distant disease-free survival (HR, 0.99; P = .94) and breast cancer–free interval (HR, 0.98; P = .87), both of which showed no advantage for metformin.
Possible HER2 advantage
However, the exploratory analysis suggested there may be an advantage for patients with HER2-positive disease, but primarily those who had at least one C allele of a prespecified ATM associated rs11212617 SNP. These patients achieved a higher pathologic complete response rate with metformin than that of those without the allele.
There were 620 patients with HER2-positive disease analyzed, with 99.4% receiving chemotherapy and 96.5% receiving trastuzumab. There were 99 IDFS events, and 47 OS events.
In the entire HER2-positive cohort, patients who received metformin had fewer IDFS events (HR, 0.64; P = .026) compared with the placebo arm. Mortality was lower with metformin (HR for overall survival, 0.53; P = .038).
“Subjects with HER2-positive breast cancer, notably those with at least one C allele of the ATM-associated rs11212617 SNP, experienced improved IDFS and overall survival with metformin,” Dr. Goodwin concluded. “However, no P-value ‘spend’ was allocated to this comparison. As a result, it requires replication in a prospective trial focusing on the HER2-positive population.”
More research?
Stephanie Bernik, MD, chief of breast surgery, Mount Sinai West, and associate professor of breast surgery, Icahn School of Medicine at Mount Sinai, New York, was approached by this news organization for an independent comment.
“It has long been known that obesity, which often correlates with diabetes, increases a woman’s risk of breast cancer,” she said.
“This study tried to show that using a medication that helps control insulin levels, even in those without diabetes, might decrease one’s risk of breast cancer,” she said. “Unfortunately, using metformin had no effect on outcomes in this study, even though it has shown promise in other studies. Perhaps more research needs to be carried out to try to pinpoint which mechanisms of action, if any, might be helpful to combat cancer in those with and without diabetes.”
The study was funded by the Canadian Cancer Trials Group, Cancer Therapy Evaluation Program, Breast Cancer Researcher Foundation, Susan G. Komen for the Cure, Canadian Cancer Society, Apotex, Swiss Cancer Research, and the Canadian Breast Cancer Foundation. Dr. Goodwin has no disclosures.
A version of this article first appeared on Medscape.com.
In the primary analysis, the addition of metformin to standard therapy in moderate/high-risk hormone receptor positive or negative breast cancer did not improve invasive disease–free survival (IDFS), overall survival, or other breast outcomes, explained lead author Pamela J. Goodwin, MD, FRCPC, professor of medicine at the University of Toronto. “Metformin should not be used as breast cancer treatment in this population.”
However, an exploratory analysis suggested that metformin may have a beneficial effect in women with HER2-positive breast cancer, Dr. Goodwin noted.
In this subset, IDFS was improved in patients who received metformin (hazard ratio, 0.64; P = .03), as was overall survival (HR, 0.53; P = .04).
The findings were presented at the San Antonio Breast Cancer Symposium.
“This trial arose from the observation that obesity is associated with poor breast cancer outcomes, and insulin levels are higher in obesity and may be more strongly associated with breast cancer outcomes than obesity,” said Dr. Goodwin.
Metformin was used because of its ability to promote modest weight loss and lower insulin by about 15%-20% in nondiabetic breast cancer survivors. It has also shown anticancer effects in preclinical studies. “In some window of opportunity neoadjuvant studies, it has been shown to reduce Ki67 in breast cancer cells,” she said. “And in preclinical in vitro and in vivo research, it slows growth of breast cancer.”
In addition, emerging evidence from observational studies suggests that the use of metformin to treat diabetes in breast cancer patients may be associated with better outcomes, strengthening the rationale for the study.
The negative results in breast cancer follow recent reports of negative findings in lung cancer, when metformin was found to be ineffective when used alongside chemotherapy in locally advanced lung cancer, as reported by this news organization.
No benefit seen
Metformin was compared to placebo in the phase 3 CCTG MA.32 trial, conducted in 3,649 patients aged 18-74 years with T1-3 N0-3 M0 breast cancer. All patients were treated with standard therapy and were randomized to receive metformin 850 mg twice daily for 5 years or placebo.
In 2016, “futility was declared in ER/PR-negative patients” after a second interim analysis conducted at 29.6 months’ median follow-up, Dr. Goodwin noted. The intervention was stopped in that group, although blinding and follow-up continued.
After that, the study’s primary analysis focused on the 2,533 ER/PR-positive patients (mean age, 52.7 years; mean body mass index, 28.8; approx. 60% postmenopausal).
Just over half of these patients had T2 tumor stage, and most disease was grade 2 or 3.
In addition, 16.5% (of metformin) and 17.4% (of placebo) patients had HER2-positive disease, with the majority (97%) of all HER2 patients receiving trastuzumab.
There was no difference between the two groups in IDFS events, occurring in 18.5% of patients receiving metformin and 18.3% who received placebo, with most (75.6%) events due to breast cancer (HR, 1.01; P = .92).
There were 131 deaths in the metformin arm and 119 in the placebo arm, with most (75.8%) of the deaths related to breast cancer (HR, 1.10; P = .47).
Other breast cancer outcomes had similar results, including distant disease-free survival (HR, 0.99; P = .94) and breast cancer–free interval (HR, 0.98; P = .87), both of which showed no advantage for metformin.
Possible HER2 advantage
However, the exploratory analysis suggested there may be an advantage for patients with HER2-positive disease, but primarily those who had at least one C allele of a prespecified ATM associated rs11212617 SNP. These patients achieved a higher pathologic complete response rate with metformin than that of those without the allele.
There were 620 patients with HER2-positive disease analyzed, with 99.4% receiving chemotherapy and 96.5% receiving trastuzumab. There were 99 IDFS events, and 47 OS events.
In the entire HER2-positive cohort, patients who received metformin had fewer IDFS events (HR, 0.64; P = .026) compared with the placebo arm. Mortality was lower with metformin (HR for overall survival, 0.53; P = .038).
“Subjects with HER2-positive breast cancer, notably those with at least one C allele of the ATM-associated rs11212617 SNP, experienced improved IDFS and overall survival with metformin,” Dr. Goodwin concluded. “However, no P-value ‘spend’ was allocated to this comparison. As a result, it requires replication in a prospective trial focusing on the HER2-positive population.”
More research?
Stephanie Bernik, MD, chief of breast surgery, Mount Sinai West, and associate professor of breast surgery, Icahn School of Medicine at Mount Sinai, New York, was approached by this news organization for an independent comment.
“It has long been known that obesity, which often correlates with diabetes, increases a woman’s risk of breast cancer,” she said.
“This study tried to show that using a medication that helps control insulin levels, even in those without diabetes, might decrease one’s risk of breast cancer,” she said. “Unfortunately, using metformin had no effect on outcomes in this study, even though it has shown promise in other studies. Perhaps more research needs to be carried out to try to pinpoint which mechanisms of action, if any, might be helpful to combat cancer in those with and without diabetes.”
The study was funded by the Canadian Cancer Trials Group, Cancer Therapy Evaluation Program, Breast Cancer Researcher Foundation, Susan G. Komen for the Cure, Canadian Cancer Society, Apotex, Swiss Cancer Research, and the Canadian Breast Cancer Foundation. Dr. Goodwin has no disclosures.
A version of this article first appeared on Medscape.com.
FROM SABCS 2021
Filler complications involving vascular necrosis, vision changes on the rise
analysis showed.
“The ASDS estimates that 1.6 million soft tissue filler procedures were performed in 2019, a 78% increase from 2012,” presenting author Michelle Xiong, a 4th-year student at Brown University, Providence, R.I., said during a virtual abstract session at the annual meeting of the American Society for Dermatologic Surgery. “The popularity of dermal fillers continues to increase. With that, there is increasing concern of possible associated adverse events. Most concerning are those related to vascular occlusion.”
Under the supervision of senior author Kachiu C. Lee, MD, MPH, of Main Line Center for Laser Surgery in Ardmore, Pa., Ms. Xiong and colleagues analyzed the Food and Drug Administration’s Manufacturer and User Facility Device Experience (MAUDE) database of medical device–related adverse event reports, to better understand and characterize dermal filler-related complications. They limited the analysis to adverse events involving injectable fillers from January 2014 to December 2020 and determined the number of complications by type per year and reviewed reports to identify injection site locations. Next, they used the binomial test to compare the proportion of complication categories from 2014 through 2016 and from 2017 through 2020.
In all, 5,994 reports were identified during the 7-year study period. To evaluate trends over time, the researchers estimated the rate of complications per 100 reports each year. While the absolute number of reports increased over time, the rate of adverse events per 100 reports decreased, suggesting an overall improvement in safety.
When the researchers focused on complications involving vascular occlusion, they found that vascular necrosis accounted for 3.5% of all complications, compared with vision changes (1.5% of all complications), and stroke (0.3% of all complications). When comparing the years 2014-2016 with 2017-2020, there was a significant increase in adverse events involving vascular necrosis (0.9%; P = .018) and vision changes (0.94%; P = .001), but no significant difference in the number of reports of stroke (-0.1%; P = .409). “This highlights that serious complications like necrosis and vision changes have increased over time,” Ms. Xiong said.
Overall, the three most common injection sites involving necrosis and vision changes were the cheek, the nose, and the nasolabial fold. The cheek was the most common site associated with stroke. “These findings are similar to those of previous studies, further emphasizing that the nose, nasolabial fold, and cheek are possibly challenging injection sites,” she said.
“In general, as the face is a highly vascular area with many anastomoses, it’s especially important to be aware of facial anatomy when injecting. In addition to awareness of anatomy, injection techniques can influence vascular complications. Unfortunately, the event narratives in the MAUDE database did not go into detail about the procedural technique.”
Ms. Xiong said that as the popularity of dermal fillers continues to grow, “it’s important for providers to understand the possible adverse events, both to better counsel patients and to improve safety management. The proportion of serious complications such as vascular necrosis and vision changes have increased from 2014 to 2020. This highlights an increased need for training to better understand facial anatomy and to emphasize practice techniques to minimize risk.”
Dr. Lee acknowledged certain limitations of the study, including that “submission of adverse events to the MAUDE database are not verified or standardized,” she told this news organization.
“With the ever-increasing popularity of fillers, it is not surprising that the absolute number of complications is rising, but it is also reassuring to see that the overall ratio of complications per hundred reports is down,” said Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study. “I would be curious to know what proportion of filler complications are due to non–core practitioners compared to dermatologists and plastic surgeons.”
The researchers reported having no financial disclosures.
Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
analysis showed.
“The ASDS estimates that 1.6 million soft tissue filler procedures were performed in 2019, a 78% increase from 2012,” presenting author Michelle Xiong, a 4th-year student at Brown University, Providence, R.I., said during a virtual abstract session at the annual meeting of the American Society for Dermatologic Surgery. “The popularity of dermal fillers continues to increase. With that, there is increasing concern of possible associated adverse events. Most concerning are those related to vascular occlusion.”
Under the supervision of senior author Kachiu C. Lee, MD, MPH, of Main Line Center for Laser Surgery in Ardmore, Pa., Ms. Xiong and colleagues analyzed the Food and Drug Administration’s Manufacturer and User Facility Device Experience (MAUDE) database of medical device–related adverse event reports, to better understand and characterize dermal filler-related complications. They limited the analysis to adverse events involving injectable fillers from January 2014 to December 2020 and determined the number of complications by type per year and reviewed reports to identify injection site locations. Next, they used the binomial test to compare the proportion of complication categories from 2014 through 2016 and from 2017 through 2020.
In all, 5,994 reports were identified during the 7-year study period. To evaluate trends over time, the researchers estimated the rate of complications per 100 reports each year. While the absolute number of reports increased over time, the rate of adverse events per 100 reports decreased, suggesting an overall improvement in safety.
When the researchers focused on complications involving vascular occlusion, they found that vascular necrosis accounted for 3.5% of all complications, compared with vision changes (1.5% of all complications), and stroke (0.3% of all complications). When comparing the years 2014-2016 with 2017-2020, there was a significant increase in adverse events involving vascular necrosis (0.9%; P = .018) and vision changes (0.94%; P = .001), but no significant difference in the number of reports of stroke (-0.1%; P = .409). “This highlights that serious complications like necrosis and vision changes have increased over time,” Ms. Xiong said.
Overall, the three most common injection sites involving necrosis and vision changes were the cheek, the nose, and the nasolabial fold. The cheek was the most common site associated with stroke. “These findings are similar to those of previous studies, further emphasizing that the nose, nasolabial fold, and cheek are possibly challenging injection sites,” she said.
“In general, as the face is a highly vascular area with many anastomoses, it’s especially important to be aware of facial anatomy when injecting. In addition to awareness of anatomy, injection techniques can influence vascular complications. Unfortunately, the event narratives in the MAUDE database did not go into detail about the procedural technique.”
Ms. Xiong said that as the popularity of dermal fillers continues to grow, “it’s important for providers to understand the possible adverse events, both to better counsel patients and to improve safety management. The proportion of serious complications such as vascular necrosis and vision changes have increased from 2014 to 2020. This highlights an increased need for training to better understand facial anatomy and to emphasize practice techniques to minimize risk.”
Dr. Lee acknowledged certain limitations of the study, including that “submission of adverse events to the MAUDE database are not verified or standardized,” she told this news organization.
“With the ever-increasing popularity of fillers, it is not surprising that the absolute number of complications is rising, but it is also reassuring to see that the overall ratio of complications per hundred reports is down,” said Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study. “I would be curious to know what proportion of filler complications are due to non–core practitioners compared to dermatologists and plastic surgeons.”
The researchers reported having no financial disclosures.
Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
analysis showed.
“The ASDS estimates that 1.6 million soft tissue filler procedures were performed in 2019, a 78% increase from 2012,” presenting author Michelle Xiong, a 4th-year student at Brown University, Providence, R.I., said during a virtual abstract session at the annual meeting of the American Society for Dermatologic Surgery. “The popularity of dermal fillers continues to increase. With that, there is increasing concern of possible associated adverse events. Most concerning are those related to vascular occlusion.”
Under the supervision of senior author Kachiu C. Lee, MD, MPH, of Main Line Center for Laser Surgery in Ardmore, Pa., Ms. Xiong and colleagues analyzed the Food and Drug Administration’s Manufacturer and User Facility Device Experience (MAUDE) database of medical device–related adverse event reports, to better understand and characterize dermal filler-related complications. They limited the analysis to adverse events involving injectable fillers from January 2014 to December 2020 and determined the number of complications by type per year and reviewed reports to identify injection site locations. Next, they used the binomial test to compare the proportion of complication categories from 2014 through 2016 and from 2017 through 2020.
In all, 5,994 reports were identified during the 7-year study period. To evaluate trends over time, the researchers estimated the rate of complications per 100 reports each year. While the absolute number of reports increased over time, the rate of adverse events per 100 reports decreased, suggesting an overall improvement in safety.
When the researchers focused on complications involving vascular occlusion, they found that vascular necrosis accounted for 3.5% of all complications, compared with vision changes (1.5% of all complications), and stroke (0.3% of all complications). When comparing the years 2014-2016 with 2017-2020, there was a significant increase in adverse events involving vascular necrosis (0.9%; P = .018) and vision changes (0.94%; P = .001), but no significant difference in the number of reports of stroke (-0.1%; P = .409). “This highlights that serious complications like necrosis and vision changes have increased over time,” Ms. Xiong said.
Overall, the three most common injection sites involving necrosis and vision changes were the cheek, the nose, and the nasolabial fold. The cheek was the most common site associated with stroke. “These findings are similar to those of previous studies, further emphasizing that the nose, nasolabial fold, and cheek are possibly challenging injection sites,” she said.
“In general, as the face is a highly vascular area with many anastomoses, it’s especially important to be aware of facial anatomy when injecting. In addition to awareness of anatomy, injection techniques can influence vascular complications. Unfortunately, the event narratives in the MAUDE database did not go into detail about the procedural technique.”
Ms. Xiong said that as the popularity of dermal fillers continues to grow, “it’s important for providers to understand the possible adverse events, both to better counsel patients and to improve safety management. The proportion of serious complications such as vascular necrosis and vision changes have increased from 2014 to 2020. This highlights an increased need for training to better understand facial anatomy and to emphasize practice techniques to minimize risk.”
Dr. Lee acknowledged certain limitations of the study, including that “submission of adverse events to the MAUDE database are not verified or standardized,” she told this news organization.
“With the ever-increasing popularity of fillers, it is not surprising that the absolute number of complications is rising, but it is also reassuring to see that the overall ratio of complications per hundred reports is down,” said Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study. “I would be curious to know what proportion of filler complications are due to non–core practitioners compared to dermatologists and plastic surgeons.”
The researchers reported having no financial disclosures.
Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
FROM ASDS 2021