User login
FDA authorizes ClonoSEQ to detect MRD in ALL, myeloma
, the U.S. Food and Drug Administration announced. Marketing authorization of the ClonoSEQ assay was granted to Adaptive Biotechnologies.
The ClonoSEQ assay is an in vitro diagnostic test that uses multiplex polymerase chain reaction and next-generation sequencing to identify and quantify certain gene sequences in DNA extracted from the bone marrow from patients with ALL or multiple myeloma. This is a single-site assay collected by the patient’s provider and sent to Adaptive Biotechnologies for evaluation.
The ClonoSEQ assay is capable of detecting minimal residual disease at levels below 1 in 1 million cells. Currently, providers test for MRD using flow cytometry assays or polymerase chain reaction–based assays. Those methods are usually capable of measuring MRD down to 1 in 10,000 or 1 in 100,000 cells.
“Determining whether a patient has residual cancer cells remaining after treatment provides information on how well a patient has responded to therapy and how long remission may last. Having a highly sensitive test available to measure minimal residual disease in ALL or multiple myeloma patients can help providers manage their patients’ care,” FDA Commissioner Scott Gottlieb, MD, said in a press release.
Along with this authorization, the FDA is establishing criteria, called special controls, which clarify the agency’s expectations in assuring the accuracy, reliability, and effectiveness of tests intended to be used as an aid to measure MRD to assess the change in burden of disease during and after treatment. These special controls, when met along with general controls, provide a reasonable assurance of safety and effectiveness for these tests, the agency said in the release. This action also creates a new regulatory classification, which means that subsequent devices of the same type with the same intended use may go through the FDA’s 510(k) process, whereby devices can obtain marketing authorization by demonstrating substantial equivalence to a previously approved device.
“The FDA is applying novel regulatory approaches to make sure that these rapidly evolving [next-generation sequencing] tests are accurate and reliable. At the same time, we’re seeing more and more laboratory-developed tests seek marketing authorization from the FDA,” he said, adding that the agency has put forward a plan to modernize the regulatory framework for all in vitro clinical tests.
The FDA evaluated data to demonstrate clinical validity from a retrospective analysis of samples obtained from three previously conducted clinical studies including 273 patients with ALL, an ongoing study of 323 patients with multiple myeloma, and a study of 706 patients with multiple myeloma, according to the FDA release.
For patients with ALL, the ClonoSEQ assay was used to assess MRD at various disease burden thresholds to show that the MRD level correlated with event-free survival – the length of time, after treatment, that the patient remains free of certain complications or events. Patients whose ClonoSEQ assay result was MRD negative had longer event-free survival, while patients with higher MRD assay results had lower event-free survival. Similar patterns of results were seen for progression-free and disease-free survival in patients with multiple myeloma.
, the U.S. Food and Drug Administration announced. Marketing authorization of the ClonoSEQ assay was granted to Adaptive Biotechnologies.
The ClonoSEQ assay is an in vitro diagnostic test that uses multiplex polymerase chain reaction and next-generation sequencing to identify and quantify certain gene sequences in DNA extracted from the bone marrow from patients with ALL or multiple myeloma. This is a single-site assay collected by the patient’s provider and sent to Adaptive Biotechnologies for evaluation.
The ClonoSEQ assay is capable of detecting minimal residual disease at levels below 1 in 1 million cells. Currently, providers test for MRD using flow cytometry assays or polymerase chain reaction–based assays. Those methods are usually capable of measuring MRD down to 1 in 10,000 or 1 in 100,000 cells.
“Determining whether a patient has residual cancer cells remaining after treatment provides information on how well a patient has responded to therapy and how long remission may last. Having a highly sensitive test available to measure minimal residual disease in ALL or multiple myeloma patients can help providers manage their patients’ care,” FDA Commissioner Scott Gottlieb, MD, said in a press release.
Along with this authorization, the FDA is establishing criteria, called special controls, which clarify the agency’s expectations in assuring the accuracy, reliability, and effectiveness of tests intended to be used as an aid to measure MRD to assess the change in burden of disease during and after treatment. These special controls, when met along with general controls, provide a reasonable assurance of safety and effectiveness for these tests, the agency said in the release. This action also creates a new regulatory classification, which means that subsequent devices of the same type with the same intended use may go through the FDA’s 510(k) process, whereby devices can obtain marketing authorization by demonstrating substantial equivalence to a previously approved device.
“The FDA is applying novel regulatory approaches to make sure that these rapidly evolving [next-generation sequencing] tests are accurate and reliable. At the same time, we’re seeing more and more laboratory-developed tests seek marketing authorization from the FDA,” he said, adding that the agency has put forward a plan to modernize the regulatory framework for all in vitro clinical tests.
The FDA evaluated data to demonstrate clinical validity from a retrospective analysis of samples obtained from three previously conducted clinical studies including 273 patients with ALL, an ongoing study of 323 patients with multiple myeloma, and a study of 706 patients with multiple myeloma, according to the FDA release.
For patients with ALL, the ClonoSEQ assay was used to assess MRD at various disease burden thresholds to show that the MRD level correlated with event-free survival – the length of time, after treatment, that the patient remains free of certain complications or events. Patients whose ClonoSEQ assay result was MRD negative had longer event-free survival, while patients with higher MRD assay results had lower event-free survival. Similar patterns of results were seen for progression-free and disease-free survival in patients with multiple myeloma.
, the U.S. Food and Drug Administration announced. Marketing authorization of the ClonoSEQ assay was granted to Adaptive Biotechnologies.
The ClonoSEQ assay is an in vitro diagnostic test that uses multiplex polymerase chain reaction and next-generation sequencing to identify and quantify certain gene sequences in DNA extracted from the bone marrow from patients with ALL or multiple myeloma. This is a single-site assay collected by the patient’s provider and sent to Adaptive Biotechnologies for evaluation.
The ClonoSEQ assay is capable of detecting minimal residual disease at levels below 1 in 1 million cells. Currently, providers test for MRD using flow cytometry assays or polymerase chain reaction–based assays. Those methods are usually capable of measuring MRD down to 1 in 10,000 or 1 in 100,000 cells.
“Determining whether a patient has residual cancer cells remaining after treatment provides information on how well a patient has responded to therapy and how long remission may last. Having a highly sensitive test available to measure minimal residual disease in ALL or multiple myeloma patients can help providers manage their patients’ care,” FDA Commissioner Scott Gottlieb, MD, said in a press release.
Along with this authorization, the FDA is establishing criteria, called special controls, which clarify the agency’s expectations in assuring the accuracy, reliability, and effectiveness of tests intended to be used as an aid to measure MRD to assess the change in burden of disease during and after treatment. These special controls, when met along with general controls, provide a reasonable assurance of safety and effectiveness for these tests, the agency said in the release. This action also creates a new regulatory classification, which means that subsequent devices of the same type with the same intended use may go through the FDA’s 510(k) process, whereby devices can obtain marketing authorization by demonstrating substantial equivalence to a previously approved device.
“The FDA is applying novel regulatory approaches to make sure that these rapidly evolving [next-generation sequencing] tests are accurate and reliable. At the same time, we’re seeing more and more laboratory-developed tests seek marketing authorization from the FDA,” he said, adding that the agency has put forward a plan to modernize the regulatory framework for all in vitro clinical tests.
The FDA evaluated data to demonstrate clinical validity from a retrospective analysis of samples obtained from three previously conducted clinical studies including 273 patients with ALL, an ongoing study of 323 patients with multiple myeloma, and a study of 706 patients with multiple myeloma, according to the FDA release.
For patients with ALL, the ClonoSEQ assay was used to assess MRD at various disease burden thresholds to show that the MRD level correlated with event-free survival – the length of time, after treatment, that the patient remains free of certain complications or events. Patients whose ClonoSEQ assay result was MRD negative had longer event-free survival, while patients with higher MRD assay results had lower event-free survival. Similar patterns of results were seen for progression-free and disease-free survival in patients with multiple myeloma.
First NGS assay approved for MRD detection in ALL or MM
The U.S. Food and Drug Administration has authorized the first next-generation sequencing (NGS)-based assay to be marketed for minimal residual disease (MRD) testing in patients with acute lymphoblastic leukemia (ALL) or multiple myeloma (MM).
The assay, called clonoSEQ®, uses both polymerase chain reaction (PCR) and NGS to identify and quantify gene sequences in DNA from patients’ bone marrow.
ClonoSEQ Assay can detect MRD levels below 1 in 1 million cells. By comparison flow cytometry assays or PCR-based assays are capable of measuring MRD down to 1 in 10,000 or 1 in 100,000 cells.
The clonoSEQ Assay is marketed by Adaptive Biotechnologies.
The FDA based its authorization on data from three clinical studies, one with 273 ALL patients, an ongoing study of 323 MM patients, and another MM trial with 706 patients.
Validation in ALL
As described in the clonoSEQ Assay Technical Information, a subset of 273 patients originally enrolled in the Children’s Oncology Group AALL0232 (NCT00075725) and AALL0331 (NCT00103285) studies had left-over bone marrow specimens to evaluate the performance of the clonoSEQ Assay.
MRD as determined by MRD negativity at less than 10-4 predicted improved event-free survival (EFS) irrespective of age. MRD-positive patients had a 2.74 higher event risk compared to MRD-negative patients.
Similar findings between MRD negativity and EFS in pediatric ALL using an earlier version of the assay were published in Blood.
Validation in MM
The ongoing phase 3 DFCI Study 10-106 (NCT01208662) is comparing conventional treatment with lenalidomide, bortezomib and dexamethasone to high-dose treatment with stem cell transplant as initial management of MM patients less than 65 years.
According to clonoSEQ’s technical information, bone marrow samples from 323 of the 720 patients originally enrolled were available and evaluable for MRD assessment.
ClonoSEQ measurements demonstrated that MRD status at a threshold of 10-5 significantly predicts progression-free survival (PFS) in all patients (P=0.027).
And samples from 75 patients who had achieved complete remission (CR) showed a modest association with disease-free survival (DFS) and lower MRD levels (P=0.064).
In the phase 3 ALCYONE trial, investigators randomly assigned 706 treatment-naïve MM patients ineligible for hematopoietic stem cell transplant to bortezomib, melphalan, and prednisone with or without daratumumab.
MRD assessments were made using the clonoSEQ Assay at screening, at confirmation of CR or stringent CR, and at intervals after patients achieved a CR.
Patients who did not achieve CR were considered MRD positive. The threshold for the MRD analysis was 10-5.
Investigators found that patients who were MRD negative by the clonoSEQ Assay had longer PFS compared to MRD-positive patients, regardless of treatment group.
For additional information on the clonoSEQ Assay consult the Technical Information available online.
The U.S. Food and Drug Administration has authorized the first next-generation sequencing (NGS)-based assay to be marketed for minimal residual disease (MRD) testing in patients with acute lymphoblastic leukemia (ALL) or multiple myeloma (MM).
The assay, called clonoSEQ®, uses both polymerase chain reaction (PCR) and NGS to identify and quantify gene sequences in DNA from patients’ bone marrow.
ClonoSEQ Assay can detect MRD levels below 1 in 1 million cells. By comparison flow cytometry assays or PCR-based assays are capable of measuring MRD down to 1 in 10,000 or 1 in 100,000 cells.
The clonoSEQ Assay is marketed by Adaptive Biotechnologies.
The FDA based its authorization on data from three clinical studies, one with 273 ALL patients, an ongoing study of 323 MM patients, and another MM trial with 706 patients.
Validation in ALL
As described in the clonoSEQ Assay Technical Information, a subset of 273 patients originally enrolled in the Children’s Oncology Group AALL0232 (NCT00075725) and AALL0331 (NCT00103285) studies had left-over bone marrow specimens to evaluate the performance of the clonoSEQ Assay.
MRD as determined by MRD negativity at less than 10-4 predicted improved event-free survival (EFS) irrespective of age. MRD-positive patients had a 2.74 higher event risk compared to MRD-negative patients.
Similar findings between MRD negativity and EFS in pediatric ALL using an earlier version of the assay were published in Blood.
Validation in MM
The ongoing phase 3 DFCI Study 10-106 (NCT01208662) is comparing conventional treatment with lenalidomide, bortezomib and dexamethasone to high-dose treatment with stem cell transplant as initial management of MM patients less than 65 years.
According to clonoSEQ’s technical information, bone marrow samples from 323 of the 720 patients originally enrolled were available and evaluable for MRD assessment.
ClonoSEQ measurements demonstrated that MRD status at a threshold of 10-5 significantly predicts progression-free survival (PFS) in all patients (P=0.027).
And samples from 75 patients who had achieved complete remission (CR) showed a modest association with disease-free survival (DFS) and lower MRD levels (P=0.064).
In the phase 3 ALCYONE trial, investigators randomly assigned 706 treatment-naïve MM patients ineligible for hematopoietic stem cell transplant to bortezomib, melphalan, and prednisone with or without daratumumab.
MRD assessments were made using the clonoSEQ Assay at screening, at confirmation of CR or stringent CR, and at intervals after patients achieved a CR.
Patients who did not achieve CR were considered MRD positive. The threshold for the MRD analysis was 10-5.
Investigators found that patients who were MRD negative by the clonoSEQ Assay had longer PFS compared to MRD-positive patients, regardless of treatment group.
For additional information on the clonoSEQ Assay consult the Technical Information available online.
The U.S. Food and Drug Administration has authorized the first next-generation sequencing (NGS)-based assay to be marketed for minimal residual disease (MRD) testing in patients with acute lymphoblastic leukemia (ALL) or multiple myeloma (MM).
The assay, called clonoSEQ®, uses both polymerase chain reaction (PCR) and NGS to identify and quantify gene sequences in DNA from patients’ bone marrow.
ClonoSEQ Assay can detect MRD levels below 1 in 1 million cells. By comparison flow cytometry assays or PCR-based assays are capable of measuring MRD down to 1 in 10,000 or 1 in 100,000 cells.
The clonoSEQ Assay is marketed by Adaptive Biotechnologies.
The FDA based its authorization on data from three clinical studies, one with 273 ALL patients, an ongoing study of 323 MM patients, and another MM trial with 706 patients.
Validation in ALL
As described in the clonoSEQ Assay Technical Information, a subset of 273 patients originally enrolled in the Children’s Oncology Group AALL0232 (NCT00075725) and AALL0331 (NCT00103285) studies had left-over bone marrow specimens to evaluate the performance of the clonoSEQ Assay.
MRD as determined by MRD negativity at less than 10-4 predicted improved event-free survival (EFS) irrespective of age. MRD-positive patients had a 2.74 higher event risk compared to MRD-negative patients.
Similar findings between MRD negativity and EFS in pediatric ALL using an earlier version of the assay were published in Blood.
Validation in MM
The ongoing phase 3 DFCI Study 10-106 (NCT01208662) is comparing conventional treatment with lenalidomide, bortezomib and dexamethasone to high-dose treatment with stem cell transplant as initial management of MM patients less than 65 years.
According to clonoSEQ’s technical information, bone marrow samples from 323 of the 720 patients originally enrolled were available and evaluable for MRD assessment.
ClonoSEQ measurements demonstrated that MRD status at a threshold of 10-5 significantly predicts progression-free survival (PFS) in all patients (P=0.027).
And samples from 75 patients who had achieved complete remission (CR) showed a modest association with disease-free survival (DFS) and lower MRD levels (P=0.064).
In the phase 3 ALCYONE trial, investigators randomly assigned 706 treatment-naïve MM patients ineligible for hematopoietic stem cell transplant to bortezomib, melphalan, and prednisone with or without daratumumab.
MRD assessments were made using the clonoSEQ Assay at screening, at confirmation of CR or stringent CR, and at intervals after patients achieved a CR.
Patients who did not achieve CR were considered MRD positive. The threshold for the MRD analysis was 10-5.
Investigators found that patients who were MRD negative by the clonoSEQ Assay had longer PFS compared to MRD-positive patients, regardless of treatment group.
For additional information on the clonoSEQ Assay consult the Technical Information available online.
Behavioral checklist IDs children at risk of depressive, anxiety disorders
disorders in later adolescent or adult life, according to research published in the Journal of Pediatrics.
“If confirmed, this observation could have very significant clinical and public health implications in the identification of children at the highest risk for the development of major depressive disorder,” Mai Uchida, MD, of the department of psychiatry at Massachusetts General Hospital in Boston, and colleagues wrote. “Early identification is the first step toward effectively allocating limited societal resources to help prevent illness progression and its associated impairments in children most in need.”
Dr. Uchida and her colleagues analyzed a sample of 537 children aged 6-17 years from two longitudinal studies of children and their parents with and without ADHD, excluding the ADHD probands in their analysis. Parents answered the CBCL, which consisted of behavioral questions translated into scores for subscales involving being anxious and/or depressed, withdrawn and/or depressed, social problems, thought problems, aggressive behavior, rule-breaking behavior, attention problems, and somatic complaints.
The sample was then divided into four groups; there were 172 children with parents who had a mood disorder but the children did not have CBCL anxiety/depression subsyndromal elevations (high risk group); 22 children without a parental history of mood disorder but had CBCL anxiety/depression subsyndromal elevations (subsyndromal major depressive disorder); 22 children with a parental history of mood disorder and CBCL anxiety/depression subsyndromal elevations (high-risk and subsyndromal major depressive disorder); and 186 children in a control group with no parental history of mood disorder or CBCL anxiety/depression subsyndromal elevations.
Compared with the control group, children with a history of parental mood disorders and children with baseline CBCL anxiety/depression subsyndromal elevations had intermediate risk of developing major depression and anxiety disorders. However, the highest risk was among children with both a parental history of mood disorder and baseline CBCL anxiety/depression subsyndromal elevations.
Using data from two previously collected longitudinal studies was a potential limitation of the study, Dr. Uchida and her associates said, but they noted the CBCL scale has predictive utility for identifying anxiety and depressive disorders in children later in life.
“Considering its simplicity, strong psychometric properties, and limited cost, the CBCL scale lends itself to be used by health professionals and educators in the community,” they wrote.
This study was partly supported by National Institutes of Health and the Massachusetts General Hospital Pediatric Psychopharmacology Council Fund. Joseph Biederman, MD, received research support from Lundbeck AS, Neurocentria, Pfizer, Shire, Sunovion, and others, and has relationships with multiple other associations and pharmaceutical companies. The other authors have no relevant financial disclosures.
SOURCE: Uchida M et al. J Pediatr. 2018 Oct;201:252-8.
disorders in later adolescent or adult life, according to research published in the Journal of Pediatrics.
“If confirmed, this observation could have very significant clinical and public health implications in the identification of children at the highest risk for the development of major depressive disorder,” Mai Uchida, MD, of the department of psychiatry at Massachusetts General Hospital in Boston, and colleagues wrote. “Early identification is the first step toward effectively allocating limited societal resources to help prevent illness progression and its associated impairments in children most in need.”
Dr. Uchida and her colleagues analyzed a sample of 537 children aged 6-17 years from two longitudinal studies of children and their parents with and without ADHD, excluding the ADHD probands in their analysis. Parents answered the CBCL, which consisted of behavioral questions translated into scores for subscales involving being anxious and/or depressed, withdrawn and/or depressed, social problems, thought problems, aggressive behavior, rule-breaking behavior, attention problems, and somatic complaints.
The sample was then divided into four groups; there were 172 children with parents who had a mood disorder but the children did not have CBCL anxiety/depression subsyndromal elevations (high risk group); 22 children without a parental history of mood disorder but had CBCL anxiety/depression subsyndromal elevations (subsyndromal major depressive disorder); 22 children with a parental history of mood disorder and CBCL anxiety/depression subsyndromal elevations (high-risk and subsyndromal major depressive disorder); and 186 children in a control group with no parental history of mood disorder or CBCL anxiety/depression subsyndromal elevations.
Compared with the control group, children with a history of parental mood disorders and children with baseline CBCL anxiety/depression subsyndromal elevations had intermediate risk of developing major depression and anxiety disorders. However, the highest risk was among children with both a parental history of mood disorder and baseline CBCL anxiety/depression subsyndromal elevations.
Using data from two previously collected longitudinal studies was a potential limitation of the study, Dr. Uchida and her associates said, but they noted the CBCL scale has predictive utility for identifying anxiety and depressive disorders in children later in life.
“Considering its simplicity, strong psychometric properties, and limited cost, the CBCL scale lends itself to be used by health professionals and educators in the community,” they wrote.
This study was partly supported by National Institutes of Health and the Massachusetts General Hospital Pediatric Psychopharmacology Council Fund. Joseph Biederman, MD, received research support from Lundbeck AS, Neurocentria, Pfizer, Shire, Sunovion, and others, and has relationships with multiple other associations and pharmaceutical companies. The other authors have no relevant financial disclosures.
SOURCE: Uchida M et al. J Pediatr. 2018 Oct;201:252-8.
disorders in later adolescent or adult life, according to research published in the Journal of Pediatrics.
“If confirmed, this observation could have very significant clinical and public health implications in the identification of children at the highest risk for the development of major depressive disorder,” Mai Uchida, MD, of the department of psychiatry at Massachusetts General Hospital in Boston, and colleagues wrote. “Early identification is the first step toward effectively allocating limited societal resources to help prevent illness progression and its associated impairments in children most in need.”
Dr. Uchida and her colleagues analyzed a sample of 537 children aged 6-17 years from two longitudinal studies of children and their parents with and without ADHD, excluding the ADHD probands in their analysis. Parents answered the CBCL, which consisted of behavioral questions translated into scores for subscales involving being anxious and/or depressed, withdrawn and/or depressed, social problems, thought problems, aggressive behavior, rule-breaking behavior, attention problems, and somatic complaints.
The sample was then divided into four groups; there were 172 children with parents who had a mood disorder but the children did not have CBCL anxiety/depression subsyndromal elevations (high risk group); 22 children without a parental history of mood disorder but had CBCL anxiety/depression subsyndromal elevations (subsyndromal major depressive disorder); 22 children with a parental history of mood disorder and CBCL anxiety/depression subsyndromal elevations (high-risk and subsyndromal major depressive disorder); and 186 children in a control group with no parental history of mood disorder or CBCL anxiety/depression subsyndromal elevations.
Compared with the control group, children with a history of parental mood disorders and children with baseline CBCL anxiety/depression subsyndromal elevations had intermediate risk of developing major depression and anxiety disorders. However, the highest risk was among children with both a parental history of mood disorder and baseline CBCL anxiety/depression subsyndromal elevations.
Using data from two previously collected longitudinal studies was a potential limitation of the study, Dr. Uchida and her associates said, but they noted the CBCL scale has predictive utility for identifying anxiety and depressive disorders in children later in life.
“Considering its simplicity, strong psychometric properties, and limited cost, the CBCL scale lends itself to be used by health professionals and educators in the community,” they wrote.
This study was partly supported by National Institutes of Health and the Massachusetts General Hospital Pediatric Psychopharmacology Council Fund. Joseph Biederman, MD, received research support from Lundbeck AS, Neurocentria, Pfizer, Shire, Sunovion, and others, and has relationships with multiple other associations and pharmaceutical companies. The other authors have no relevant financial disclosures.
SOURCE: Uchida M et al. J Pediatr. 2018 Oct;201:252-8.
FROM THE JOURNAL OF PEDIATRICS
Key clinical point: Children with elevated CBCL anxiety/depression scale scores at baseline as well as a parent with a major depressive disorder were at risk of developing a major depressive disorder or anxiety disorder at 10-year follow-up.
Major finding:
Study details: An analysis of 537 children in a sample from two longitudinal case studies of families with and without child and parental history of ADHD.
Disclosures: This study was partly supported by National Institutes of Health and the Massachusetts General Hospital Pediatric Psychopharmacology Council Fund. Joseph Biederman, MD, received research support from Neurocentria, Pfizer, Shire, Sunovion, and others, and has relationships with multiple other associations and pharmaceutical companies. The other authors have no relevant financial disclosures.
Source: Uchida M et al. J Pediatr. 2018 Oct;201:252-8.
Admission eosinopenia predicted severe CDI outcomes
For patients with even in the absence of hypotension and tachycardia, researchers wrote in JAMA Surgery.
“In animal models, peripheral eosinopenia is a biologically plausible predictive factor for adverse outcomes, and human data from this study indicate that this frequent addition to an admission complete blood cell count is an inexpensive, widely available risk index in the treatment of C. difficile infection,” wrote Audrey S. Kulaylat, MD, of Penn State University, Hershey, and her associates.
In their cohort study of 2,065 patients admitted to two tertiary referral centers with C. difficile infection, undetectable eosinophil counts at hospital admission were associated with significantly increased odds of in-hospital mortality in both a training dataset (odds ratio, 2.01; 95% confidence interval, 1.08-3.73; P = .03) and a validation dataset (OR, 2.26; 95% CI, 1.33-3.83; P = .002). Undetectable eosinophil counts also were associated with elevated odds of severe disease requiring intensive care, vasopressor use, and emergency total colectomy. Besides eosinopenia, significant predictors of mortality included having more comorbidities and lower systolic blood pressure at admission. Strikingly, when patients had no initial hypotension or tachycardia, an undetectable eosinophil count was the only identifiable predictor of in-hospital death (OR, 5.76; 95% CI, 1.99-16.64). An elevated white blood cell count was not a significant predictor of mortality in this subgroup.
Dr. Kulaylat and her associates are studying the microbiome in C. difficile infection. Their work has identified a host immune reaction marked by an “exaggerated inflammasome response” and peripheral eosinopenia, they explained. Two recent murine models have produced similar results.
Admission eosinophil counts “allow for an immediate assessment of mortality risk at admission that is inexpensive and part of a differential for a standard complete blood count available at any hospital,” they concluded. They are now prospectively evaluating a prognostic score for C. difficile infection that includes eosinopenia and other easily discernible admission factors. The National Institutes of Health supported the work. The researchers reported having no conflicts of interest.
SOURCE: Kulaylat AS et al. JAMA Surg. 2018 Sep 12. doi: 10.1001/jamasurg.2018.3174.
For patients with even in the absence of hypotension and tachycardia, researchers wrote in JAMA Surgery.
“In animal models, peripheral eosinopenia is a biologically plausible predictive factor for adverse outcomes, and human data from this study indicate that this frequent addition to an admission complete blood cell count is an inexpensive, widely available risk index in the treatment of C. difficile infection,” wrote Audrey S. Kulaylat, MD, of Penn State University, Hershey, and her associates.
In their cohort study of 2,065 patients admitted to two tertiary referral centers with C. difficile infection, undetectable eosinophil counts at hospital admission were associated with significantly increased odds of in-hospital mortality in both a training dataset (odds ratio, 2.01; 95% confidence interval, 1.08-3.73; P = .03) and a validation dataset (OR, 2.26; 95% CI, 1.33-3.83; P = .002). Undetectable eosinophil counts also were associated with elevated odds of severe disease requiring intensive care, vasopressor use, and emergency total colectomy. Besides eosinopenia, significant predictors of mortality included having more comorbidities and lower systolic blood pressure at admission. Strikingly, when patients had no initial hypotension or tachycardia, an undetectable eosinophil count was the only identifiable predictor of in-hospital death (OR, 5.76; 95% CI, 1.99-16.64). An elevated white blood cell count was not a significant predictor of mortality in this subgroup.
Dr. Kulaylat and her associates are studying the microbiome in C. difficile infection. Their work has identified a host immune reaction marked by an “exaggerated inflammasome response” and peripheral eosinopenia, they explained. Two recent murine models have produced similar results.
Admission eosinophil counts “allow for an immediate assessment of mortality risk at admission that is inexpensive and part of a differential for a standard complete blood count available at any hospital,” they concluded. They are now prospectively evaluating a prognostic score for C. difficile infection that includes eosinopenia and other easily discernible admission factors. The National Institutes of Health supported the work. The researchers reported having no conflicts of interest.
SOURCE: Kulaylat AS et al. JAMA Surg. 2018 Sep 12. doi: 10.1001/jamasurg.2018.3174.
For patients with even in the absence of hypotension and tachycardia, researchers wrote in JAMA Surgery.
“In animal models, peripheral eosinopenia is a biologically plausible predictive factor for adverse outcomes, and human data from this study indicate that this frequent addition to an admission complete blood cell count is an inexpensive, widely available risk index in the treatment of C. difficile infection,” wrote Audrey S. Kulaylat, MD, of Penn State University, Hershey, and her associates.
In their cohort study of 2,065 patients admitted to two tertiary referral centers with C. difficile infection, undetectable eosinophil counts at hospital admission were associated with significantly increased odds of in-hospital mortality in both a training dataset (odds ratio, 2.01; 95% confidence interval, 1.08-3.73; P = .03) and a validation dataset (OR, 2.26; 95% CI, 1.33-3.83; P = .002). Undetectable eosinophil counts also were associated with elevated odds of severe disease requiring intensive care, vasopressor use, and emergency total colectomy. Besides eosinopenia, significant predictors of mortality included having more comorbidities and lower systolic blood pressure at admission. Strikingly, when patients had no initial hypotension or tachycardia, an undetectable eosinophil count was the only identifiable predictor of in-hospital death (OR, 5.76; 95% CI, 1.99-16.64). An elevated white blood cell count was not a significant predictor of mortality in this subgroup.
Dr. Kulaylat and her associates are studying the microbiome in C. difficile infection. Their work has identified a host immune reaction marked by an “exaggerated inflammasome response” and peripheral eosinopenia, they explained. Two recent murine models have produced similar results.
Admission eosinophil counts “allow for an immediate assessment of mortality risk at admission that is inexpensive and part of a differential for a standard complete blood count available at any hospital,” they concluded. They are now prospectively evaluating a prognostic score for C. difficile infection that includes eosinopenia and other easily discernible admission factors. The National Institutes of Health supported the work. The researchers reported having no conflicts of interest.
SOURCE: Kulaylat AS et al. JAMA Surg. 2018 Sep 12. doi: 10.1001/jamasurg.2018.3174.
FROM JAMA SURGERY
Key clinical point: Undetectable peripheral eosinophils predicted severe outcomes in patients admitted with Clostridium difficile infection.
Major finding: In the training and validation datasets, odds of in-hospital mortality were 2.01 (95% CI, 1.08-3.73) and 2.26 (95% CI, 1.33-3.83), respectively.
Study details: Two-hospital cohort study of 2,065 patients admitted with C. difficile infection.
Disclosures: The National Institutes of Health supported the work. The researchers reported having no conflicts of interest.
Source: Kulaylat A et al. JAMA Surg. 2018 Sep 12. doi: 10.1001/jamasurg.2018.3174.
Advances in precision medicine help refine – and redefine – cancer care
Among the groundbreaking findings presented at this year’s annual meeting of the American Society of Clinical Oncology were those showing that most women with HR-positive, HER2-negative, early-stage breast cancer who have an intermediate recurrence score can safely skip adjuvant chemotherapy, and that upfront pembrolizumab for patients with NSCLC expressing PD-L1 on at least 1% of tumor cells can not only significantly improve overall survival, but do so with less toxicity than standard chemotherapy.
TAILORx marks major advance for precision medicine in breast cancer
Key clinical point The majority of women with HR-positive, HER2-negative, node-negative early-stage breast cancer who have an intermediate recurrence score can safely skip adjuvant chemotherapy. Major finding In women with an Oncotype DX Recurrence Score in the midrange (11-25), invasive DFS with endocrine therapy alone was not inferior to that with chemotherapy plus endocrine therapy (HR, 1.08; P = .26). Study details A phase 3 trial in 10,273 women with HR-positive, HER2-negative, node-negative, early-stage breast cancer, with a noninferiority randomized component in the 6,711 women with a midrange recurrence score (TAILORx trial). Funding This study received funding primarily from the National Cancer Institute, National Institutes of Health. Additional support was provided by the Breast Cancer Research Foundation, Komen Foundation, and US Postal Service Breast Cancer Stamp. Disclosures Dr Sparano disclosed that he has a consulting or advisory role with Genentech/Roche, Novartis, AstraZeneca, Celgene, Lilly, Celldex, Pfizer, Prescient Therapeutics, Juno Therapeutics, and Merrimack; has stock or other ownership interests with MetaStat; and receives research funding (institutional) from Prescient Therapeutics, Deciphera, Genentech/Roche, Merck, Novartis, and Merrimack. Source Sparano et al. ASCO 2018 Abstract LBA1: https://meetinglibrary.asco.org/record/161490/abstract
Use of the 21-tumor gene expression assay (Oncotype DX Recurrence Score) allows nearly 70% of women with hormone receptor-positive, HER2-negative, node-negative, early-stage breast cancer to safely forgo adjuvant chemotherapy, sparing them adverse effects and preventing overtreatment, TAILORx trial results show.
The findings, which were reported in the plenary session at the meeting and simultaneously published in the New England Journal of Medicine (N Engl J Med. 2018; 379:111-121; [behind paywall]), mark a major advance in precision medicine.
“The rationale for the TAILORx precision medicine trial is that we are really trying to ‘thread the needle,’ “ lead study author Joseph A Sparano, MD, commented in a press briefing. Oncologists typically recommend adjuvant chemotherapy for the half of all breast cancers that are hormone receptor positive, HER2 negative, and node negative, even though its absolute benefit in reducing recurrences in this population is small. “This results in most patients being overtreated because endocrine therapy alone is adequate. But some are undertreated: They do not receive chemotherapy although they could have benefited from it,” he noted.
The recurrence score is known to be prognostic and predictive of benefit from adding chemotherapy to endocrine therapy, Dr Sparano said. “But there was a major gap: There was uncertain benefit for patients who had a midrange score, which is about two-thirds of all patients who are treated,” said Dr Sparano, the associate director for clinical research at Albert Einstein Cancer Center and Montefiore Health System in New York, and vice-chair of the ECOG-ACRIN Cancer Research Group.
The phase 3 TAILORx trial registered 10,273 women with hormone receptor-positive, HER2-negative, node-negative, early-stage breast cancer, making it the largest adjuvant breast cancer trial to date. Analyses focused on the 6,711 evaluable women with a midrange recurrence score (defined as 11 through 25 in the trial), who were randomized to receive endocrine therapy alone or adjuvant chemotherapy plus endocrine therapy, with a noninferiority design. Of note is that contemporary drugs and regimens were used.
Results at a median follow-up of 7.5 years showed that the trial met its primary endpoint: the risk of invasive disease-free survival (DFS) events (invasive disease recurrence, second primary cancer, or death) was not inferior for women given endocrine therapy alone compared with counterparts given chemotherapy plus endocrine therapy (hazard ratio [HR], 1.08; P = .26), Dr Sparano reported.
The groups were also on par, with absolute differences of no more than 1% between rates, with respect to a variety of other efficacy outcomes: freedom from distant recurrence and any recurrence, and overall survival (OS).
Findings were similar across most subgroups. But analyses suggested that women aged 50 years and younger and who had a recurrence score of 16-25 fared better when they received chemotherapy. “Though exploratory from a statistical perspective, this is a highly clinically relevant observation,” Dr Sparano said. “It suggests ... that chemotherapy should be spared with caution in this subgroup, after a careful discussion of potential benefits and risks in a shared decision process.”
In other findings, analyses of the trial’s nonrandomized groups confirmed excellent outcomes in women with a low recurrence score (0-10) who were given endocrine therapy alone, and at the other end of the spectrum, there was need for a more aggressive approach, including chemotherapy, in women with a high recurrence score (26-100).
Ultimately, application of the recurrence score allowed 69% of the trial population to skip chemotherapy: all of the women with a score of 0-10 (16% of the trial population), those older than 50 years with a score of 11-25 (45%), and those aged 50 years or younger with a score of 11-15 (8%).
An ongoing companion phase 3 trial, RxPONDER, is assessing the benefit of applying the recurrence score in women who are similar but ihave node-positive disease.
Study details
All of the women with hormone receptor-positive, HER2-negative, node-negative, early-stage breast cancer enrolled in TAILORx met National Comprehensive Cancer Network guidelines for receiving adjuvant chemotherapy. About 69% had an intermediate recurrence score (11-25) and were randomized. All of the 17% with a low recurrence score (0-10) were given only endocrine therapy, and all of the 14% with a high recurrence score (26-100) were given both adjuvant chemotherapy and endocrine therapy.
Of note, the recurrence scores used to define midrange were adjusted downward from those conventionally used to account for exclusion of patients with higher-risk HER2-positive disease and to minimize potential for undertreatment, Dr Sparano explained.
In the women with midrange scores who were randomized, the hazard ratio of 1.08 for invasive DFS with endocrine therapy alone compared with chemotherapy plus endocrine therapy fell well within the predefined hazard ratio for noninferiority (1.322). The 9-year rate of invasive DFS was 83.3% with endocrine therapy and 84.3% with chemotherapy plus endocrine therapy.
The groups had similar rates of freedom from distant recurrence (94.5% vs 95.0%; HR, 1.10; P = .48) and distant or locoregional recurrence (92.2% vs 92.9%; HR, 1.11; P = .33), and similar OSs (93.9% vs 93.8%; HR for death, 0.99; P = .89).
In exploratory analyses, there was an interaction of age and recurrence score (P = .004) whereby women aged 50 years or younger derived some benefit from chemotherapy if they had a recurrence score of 16-20 (9% fewer invasive DFS events, including 2% fewer distant recurrences) or a recurrence score 21-25 (6% fewer invasive DFS events, mainly distant recurrences). “This is information that could drive some younger women who have a recurrence score in this range to accept chemotherapy,” Dr Sparano said.
The 9-year rate of distant recurrence averaged 5% in women with midrange scores overall. It was 3% in those with a low recurrence score given endocrine therapy alone, but it was still 13% in those with a high recurrence score despite receiving both endocrine therapy and chemotherapy. The latter finding may “indicate the need to explore potentially more effective therapies in this setting,” he proposed.
Tailoring treatment: ‘not too much and not too little’
“These are important data because this is the most common form of breast cancer in the United States and other developed countries, and the most challenging decision we make with these patients is whether or not to recommend adjuvant chemotherapy with all its side effects and potential benefits,” said ASCO expert Harold Burstein, MD, PhD, FASCO. “The data show that the majority of women who have this test performed on their tumor can be told that they don’t need chemotherapy, and that can be said with tremendous confidence and reassurance.”
The recurrence score has been used for a decade, but the trial was necessary because the score was originally developed in patients who were receiving older chemotherapy regimens and older endocrine therapies, and because there have been few data to guide decision making in the large group of patients with midrange scores, he said. “Now we can say with confidence ... that the patients got contemporary chemo regimens and still saw no benefit from chemotherapy.
“This is not so much about de-escalation ... the goal of this study was not to just use less treatment but to tailor treatment. The investigators chose the title very aptly,” said Dr Burstein, a medical oncologist at the Dana-Farber Cancer Institute and associate professor of medicine at the Harvard Medical School, Boston.
“This is extraordinary data for breast cancer doctors and women who have breast cancer. It allows you to individualize treatment based on extraordinary science, which now has tremendous prospective validation,” he said. Overall, “women with breast cancer who are getting modern therapy are doing well, and this test shows us how to tailor that management so that they get exactly the right amount of treatment – not too much and not too little.”
— Susan London
First-line immunotherapy boosts survival in NSCLC patients
Key clinical point Many patients with previously untreated NSCLC could benefit from first-line therapy with the checkpoint inhibitor pembrolizumab. Major finding In all patients with expression of PD-L1 on 1% or more of tumor cells, OS was 16.7 months with pembrolizumab, compared with 12.1 months for chemotherapy. Study details Randomized phase 3 trial of 1,274 patients with advanced or metastatic NSCLC. Funding Merck, the maker of the study drug, funded the study. Disclosures Dr Lopes disclosed institutional research funding from Merck Sharp & Dohme, EMD Serono, and AstraZeneca. Dr Heymach disclosed stock/ownership in Bio-Tree and Cardinal Spine, a consulting or advisory role for Abbvie, ARIAD, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Calithera Biosciences, Genentech, Medivation, Novartis, Oncomed, and Synta, and institutional research funding from AstraZeneca. Dr Gandhi reported having no relevant disclosures. Source Lopes G et al. ASCO 2018, abstract LBA4. https://meetinglibrary.asco.org/record/165950/abstract
Pembrolizumab as first-line treatment of advanced non–small-cell lung cancer (NSCLC) offered longer OS with better tolerability compared with chemotherapy, results of the Keynote-042 phase 3 randomized trial show.
In 1,274 patients with advanced, previously untreated NSCLC with expression of the PD-L1 on 1% or more of tumor cells, median OS after a median follow-up of 12.8 months was 16.7 months for patients treated with pembrolizumab monotherapy, compared with 12.1 months for patients treated with either paclitaxel or pemetrexed plus carboplatin, reported lead author Gilberto Lopes, MD, of the Sylvester Comprehensive Cancer Center at the University of Miami.
The survival benefit for immunotherapy was even greater for patients with higher levels of PD-L1 expression: 20 versus 12.2 months for patients with PD-L1 expression of 50% or greater, and 17.7 versus 13 months for patients with PD-L1 expression of 20% or greater, Dr Lopes noted.
For all 3 PD-L1 expression groups, the median duration of response was 20.2 months, compared with 10.8-8.3 months for patients in the chemotherapy arm.
“These are responses that are unlike anything that we have seen with chemotherapy in the past for non–small-cell lung cancer,” Dr Lopes said at a briefing before his presentation. “In addition to that, and probably more importantly, patients had fewer adverse events [with pembrolizumab]. Overall, about 60% had any treatment-related adverse event with pembrolizumab, versus 90% with chemotherapy,” he added.
‘A true milestone’
ASCO expert John Heymach, MD, PhD, of the University of Texas MD Anderson Cancer Center in Houston, said at the briefing that the study was “a true milestone for the field, because now, for the first time, we can say that in non–small-cell lung cancer patients receiving first-line therapy, the vast majority can receive immunotherapy with pembrolizumab instead of chemotherapy.”
He noted that an earlier study, Keynote-024, showed that pembrolizumab significantly improved progression-free survival in patients with tumors expressing PD-L1 on at least 50% of cells compared with standard platinum-based chemotherapy (10.3 vs 6 months).
“This more than doubles that population that can start immunotherapy as a first-line treatment, assuming the [Food and Drug Administration] modifies the label in accordance with this study,” he added.
The Keynote-042 investigators enrolled 1,274 patients with locally advanced or metastatic NSCLC, and randomly assigned them to receive either a maximum of 35 cycles of pembrolizumab 200 mg every 3 weeks, or the investigators’ choice of not more than 6 cycles of either paclitaxel–carboplatin or pemetrexed–carboplatin, with optional pemetrexed maintenance for patients with nonsquamous histologies only.
The randomization was stratified by region (Asia vs non-East Asia), Eastern Cooperative Oncology Group performance status 0 or 1, squamous versus nonsquamous histology, and PD-L1 expression, or TPS (tumor proportion score) greater than 50% versus 1%-49%.
As noted before, the primary endpoint of OS in all patients with a TPS of 1% or greater was met, with respective median OS in the pembrolizumab versus chemotherapy groups of 16.7 and 12.1 months, translating into an HR favoring pembrolizumab of 0.81 (P = .0018). Respective hazard ratios for the TPS 20% or greater and TPS 50% or greater groups were 0.77 (P = .0020) and 0.69 (P = .0003).
At 12.8 months of median follow-up, 13% of patients assigned to pembrolizumab were still on the drug, and 4.3% of patients were receiving maintenance pemetrexed.
Treatment-related adverse events of any grade occurred in 399 of 636 patients assigned to pembrolizumab (62.7%), compared with 553 of 615 patients assigned to chemotherapy (89.9%). Grade 3 or greater events occurred in 17.8% and 41% of patients, respectively. There were 13 deaths related to therapy in the pembrolizumab arm (2.0%), and 14 in the chemotherapy arm (2.3%). Adverse events leading to discontinuation were similar between the groups, at 9% and 9.4%, respectively.
There were more immune-mediated adverse events in the pembrolizumab arm than in the chemotherapy arm (27.8% vs 7.2%, respectively), and of those, grade 3 or higher events occurred in 8% and 1.5% of patients. There was 1 immune-mediated death, from pneumonitis, in the immunotherapy arm; there were no deaths related to immune-mediated side effects in the chemotherapy arm.
“I really view this as a ‘double whammy’ for patients,” Dr Heymach said at the briefing. “Often advances in survival for our lung cancer patients come at the cost of significant toxicities. Here, by contrast, not only are patients living longer and having a much higher likelihood of prolonged survival in years, often instead of months, but they’re also receiving a treatment that has substantially less toxicity across virtually all measures, and this really impacts the day-to-day life of these patients.”
Leena Gandhi, MD, PhD, of the Perlmutter Cancer Center at New York University, the invited discussant at the plenary, agreed that pembrolizumab improves survival, compared with chemotherapy patients with PD-L1 expression levels greater than 1%, but noted that most of the benefit – as also seen in Keynote-024 – was in those patients whose tumors had high levels of PD-L1 expression.
She emphasized that although PD-L1 is an imperfect biomarker, it should still be used to help select patients for therapy and it may be complementary with tumor mutational burden for more precise treatment selection.
“What we know, and what this study adds to, is that PD-L1 really does define a patient population that could receive benefit from pembrolizumab over chemotherapy. Patients with low or no PD-L1 expression likely should get some type of combination therapy,” she said. “This study extends what we’ve seen from other recent studies, which is that chemotherapy alone is no longer a first-line standard of care in non–small-cell lung cancer.”
— Neil Osterweil
Maintenance chemo improves survival in youth with rhabdomyosarcoma
Key clinical point 6 months of maintenance chemotherapy improves survival in youth with high-risk rhabdomyosarcoma. Major finding Patients given maintenance low-dose vinorelbine and cyclophosphamide had better 5-year OS compared with those not receiving any additional treatment (86.5% vs 73.7%; HR, 0.52). Study details A phase 3 randomized controlled trial in 371 patients aged 0-21 years with high-risk rhabdomyosarcoma who had had a complete response to standard intensive therapy. Funding The study received funding from Fondazione Città della Speranza, Italy. Disclosures Dr Bisogno disclosed that he has a consulting or advisory role with Clinigen Group, and receives travel, accommodations, and/or expenses from Jazz Pharmaceuticals. Source Bisogno et al. ASCO 2018, Abstract LBA2. https://meetinglibrary.asco.org/record/161695/abstract
Six months of maintenance chemotherapy prolongs OS in youth with high-risk rhabdomyosarcoma, finds a phase 3 randomized controlled trial of the European Paediatric Soft Tissue Sarcoma Study Group (EpSSG).
Rhabdomyosarcoma is a rare but highly aggressive tumor, lead study author Gianni Bisogno, MD, PhD, a professor at the University Hospital of Padova, Italy, and chair of the EpSSG, noted in a press briefing at the meeting, where the findings were reported. In pediatric patients who achieve complete response to standard therapy, “we know that after 1 or 2 years, one-third of these children relapse, and most of them die,” he said.
The EpSSG trial, which took about 10 years to conduct, enrolled 371 patients aged 0-21 years with high-risk rhabdomyosarcoma who had had a complete response to standard intensive therapy. They were randomized evenly to stop treatment or to receive 6 months of maintenance treatment consisting of low-dose vinorelbine and cyclophosphamide.
Results reported in the meeting’s plenary session showed that giving maintenance chemotherapy improved the 5-year OS rate by an absolute 12.8%, which translated to a near halving of the risk of death. And the maintenance regimen used was generally well tolerated.
“At the end of this long, not-easy study, we concluded that maintenance chemotherapy is an effective and well tolerated treatment for children with high-risk rhabdomyosarcoma,” Dr Bisogno said.
There are three possibilities for its efficacy, he speculated. “It may be the duration, the type of drugs used, or the metronomic approach. Maybe altogether, these three different actions have a benefit to increase survival.
“Our group has decided this is the new standard treatment for patients. At least in Europe, we give standard intensive therapy and then we continue with 6 more months of low-dose chemotherapy,” Dr Bisogno concluded. “We think that this approach – a new way of using old drugs – can be of interest also for other pediatric tumors.”
The trial is noteworthy in that it shows “how to successfully conduct large and important trials in rare diseases,” said ASCO expert Warren Chow, MD.
The standard therapy for rhabdomyosarcomas is somewhat different in the United States, typically a regimen containing vincristine, actinomycin D, cyclophosphamide, and (more recently) irinotecan, he noted. “We have not been traditionally using maintenance chemo for any of the pediatric sarcomas, so this is a paradigm shift. These results will need to be tested with US-based protocols before becoming standard of care in the United States. Also, we will need to determine if these results are applicable to patients older than 21 years of age who are considered high risk based solely on their age.
“Even with these caveats, this is the first significant treatment advance in this rare cancer in more than 30 years,” concluded Dr Chow, a medical oncologist and clinical professor at City of Hope, Duarte, Calif. “No doubt, this trial was a home run.”
Study details
Patients enrolled in the EpSSG trial had had a complete response to the standard intensive therapy used in Europe: high-dose chemotherapy (ifosfamide, vincristine, and actinomycin D, with or without doxorubicin), radiation therapy, and surgery.
The maintenance chemotherapy consisted of a combination of low-dose intravenous vinorelbine given weekly and oral cyclophosphamide given daily. The 6-month duration was somewhat arbitrary, according to Dr Bisogno. “We had to start somewhere. So when we started, we decided to use 6 months because there was some evidence in the past for regimens that long. In our next European trial, we are going to test different kinds and durations of maintenance because this is very important.”
The maintenance regimen was well tolerated compared with the regimen given during standard intensive therapy, with, for example, lower rates of grade 3 and 4 anemia (8.9% vs 48.9%), neutropenia (80.6% vs 91.6%), and thrombocytopenia (0.6% vs 26.0%), which translated to less of a need for transfusions, and a lower rate of grade 3 or 4 infection (29.4% vs 56.4%), Dr Bisogno reported. There were no cases of grade 3 or 4 cardiac, hepatobiliary/pancreatic, or renal toxicity.
Relative to peers who stopped treatment after standard intensive therapy, patients who received maintenance treatment tended to have better DFS (77.6% vs 69.8%; HR, 0.68; P = .0613) and had significantly better OS (86.5% vs 73.7%; HR, 0.52; P = .0111).
— Susan London
Among the groundbreaking findings presented at this year’s annual meeting of the American Society of Clinical Oncology were those showing that most women with HR-positive, HER2-negative, early-stage breast cancer who have an intermediate recurrence score can safely skip adjuvant chemotherapy, and that upfront pembrolizumab for patients with NSCLC expressing PD-L1 on at least 1% of tumor cells can not only significantly improve overall survival, but do so with less toxicity than standard chemotherapy.
TAILORx marks major advance for precision medicine in breast cancer
Key clinical point The majority of women with HR-positive, HER2-negative, node-negative early-stage breast cancer who have an intermediate recurrence score can safely skip adjuvant chemotherapy. Major finding In women with an Oncotype DX Recurrence Score in the midrange (11-25), invasive DFS with endocrine therapy alone was not inferior to that with chemotherapy plus endocrine therapy (HR, 1.08; P = .26). Study details A phase 3 trial in 10,273 women with HR-positive, HER2-negative, node-negative, early-stage breast cancer, with a noninferiority randomized component in the 6,711 women with a midrange recurrence score (TAILORx trial). Funding This study received funding primarily from the National Cancer Institute, National Institutes of Health. Additional support was provided by the Breast Cancer Research Foundation, Komen Foundation, and US Postal Service Breast Cancer Stamp. Disclosures Dr Sparano disclosed that he has a consulting or advisory role with Genentech/Roche, Novartis, AstraZeneca, Celgene, Lilly, Celldex, Pfizer, Prescient Therapeutics, Juno Therapeutics, and Merrimack; has stock or other ownership interests with MetaStat; and receives research funding (institutional) from Prescient Therapeutics, Deciphera, Genentech/Roche, Merck, Novartis, and Merrimack. Source Sparano et al. ASCO 2018 Abstract LBA1: https://meetinglibrary.asco.org/record/161490/abstract
Use of the 21-tumor gene expression assay (Oncotype DX Recurrence Score) allows nearly 70% of women with hormone receptor-positive, HER2-negative, node-negative, early-stage breast cancer to safely forgo adjuvant chemotherapy, sparing them adverse effects and preventing overtreatment, TAILORx trial results show.
The findings, which were reported in the plenary session at the meeting and simultaneously published in the New England Journal of Medicine (N Engl J Med. 2018; 379:111-121; [behind paywall]), mark a major advance in precision medicine.
“The rationale for the TAILORx precision medicine trial is that we are really trying to ‘thread the needle,’ “ lead study author Joseph A Sparano, MD, commented in a press briefing. Oncologists typically recommend adjuvant chemotherapy for the half of all breast cancers that are hormone receptor positive, HER2 negative, and node negative, even though its absolute benefit in reducing recurrences in this population is small. “This results in most patients being overtreated because endocrine therapy alone is adequate. But some are undertreated: They do not receive chemotherapy although they could have benefited from it,” he noted.
The recurrence score is known to be prognostic and predictive of benefit from adding chemotherapy to endocrine therapy, Dr Sparano said. “But there was a major gap: There was uncertain benefit for patients who had a midrange score, which is about two-thirds of all patients who are treated,” said Dr Sparano, the associate director for clinical research at Albert Einstein Cancer Center and Montefiore Health System in New York, and vice-chair of the ECOG-ACRIN Cancer Research Group.
The phase 3 TAILORx trial registered 10,273 women with hormone receptor-positive, HER2-negative, node-negative, early-stage breast cancer, making it the largest adjuvant breast cancer trial to date. Analyses focused on the 6,711 evaluable women with a midrange recurrence score (defined as 11 through 25 in the trial), who were randomized to receive endocrine therapy alone or adjuvant chemotherapy plus endocrine therapy, with a noninferiority design. Of note is that contemporary drugs and regimens were used.
Results at a median follow-up of 7.5 years showed that the trial met its primary endpoint: the risk of invasive disease-free survival (DFS) events (invasive disease recurrence, second primary cancer, or death) was not inferior for women given endocrine therapy alone compared with counterparts given chemotherapy plus endocrine therapy (hazard ratio [HR], 1.08; P = .26), Dr Sparano reported.
The groups were also on par, with absolute differences of no more than 1% between rates, with respect to a variety of other efficacy outcomes: freedom from distant recurrence and any recurrence, and overall survival (OS).
Findings were similar across most subgroups. But analyses suggested that women aged 50 years and younger and who had a recurrence score of 16-25 fared better when they received chemotherapy. “Though exploratory from a statistical perspective, this is a highly clinically relevant observation,” Dr Sparano said. “It suggests ... that chemotherapy should be spared with caution in this subgroup, after a careful discussion of potential benefits and risks in a shared decision process.”
In other findings, analyses of the trial’s nonrandomized groups confirmed excellent outcomes in women with a low recurrence score (0-10) who were given endocrine therapy alone, and at the other end of the spectrum, there was need for a more aggressive approach, including chemotherapy, in women with a high recurrence score (26-100).
Ultimately, application of the recurrence score allowed 69% of the trial population to skip chemotherapy: all of the women with a score of 0-10 (16% of the trial population), those older than 50 years with a score of 11-25 (45%), and those aged 50 years or younger with a score of 11-15 (8%).
An ongoing companion phase 3 trial, RxPONDER, is assessing the benefit of applying the recurrence score in women who are similar but ihave node-positive disease.
Study details
All of the women with hormone receptor-positive, HER2-negative, node-negative, early-stage breast cancer enrolled in TAILORx met National Comprehensive Cancer Network guidelines for receiving adjuvant chemotherapy. About 69% had an intermediate recurrence score (11-25) and were randomized. All of the 17% with a low recurrence score (0-10) were given only endocrine therapy, and all of the 14% with a high recurrence score (26-100) were given both adjuvant chemotherapy and endocrine therapy.
Of note, the recurrence scores used to define midrange were adjusted downward from those conventionally used to account for exclusion of patients with higher-risk HER2-positive disease and to minimize potential for undertreatment, Dr Sparano explained.
In the women with midrange scores who were randomized, the hazard ratio of 1.08 for invasive DFS with endocrine therapy alone compared with chemotherapy plus endocrine therapy fell well within the predefined hazard ratio for noninferiority (1.322). The 9-year rate of invasive DFS was 83.3% with endocrine therapy and 84.3% with chemotherapy plus endocrine therapy.
The groups had similar rates of freedom from distant recurrence (94.5% vs 95.0%; HR, 1.10; P = .48) and distant or locoregional recurrence (92.2% vs 92.9%; HR, 1.11; P = .33), and similar OSs (93.9% vs 93.8%; HR for death, 0.99; P = .89).
In exploratory analyses, there was an interaction of age and recurrence score (P = .004) whereby women aged 50 years or younger derived some benefit from chemotherapy if they had a recurrence score of 16-20 (9% fewer invasive DFS events, including 2% fewer distant recurrences) or a recurrence score 21-25 (6% fewer invasive DFS events, mainly distant recurrences). “This is information that could drive some younger women who have a recurrence score in this range to accept chemotherapy,” Dr Sparano said.
The 9-year rate of distant recurrence averaged 5% in women with midrange scores overall. It was 3% in those with a low recurrence score given endocrine therapy alone, but it was still 13% in those with a high recurrence score despite receiving both endocrine therapy and chemotherapy. The latter finding may “indicate the need to explore potentially more effective therapies in this setting,” he proposed.
Tailoring treatment: ‘not too much and not too little’
“These are important data because this is the most common form of breast cancer in the United States and other developed countries, and the most challenging decision we make with these patients is whether or not to recommend adjuvant chemotherapy with all its side effects and potential benefits,” said ASCO expert Harold Burstein, MD, PhD, FASCO. “The data show that the majority of women who have this test performed on their tumor can be told that they don’t need chemotherapy, and that can be said with tremendous confidence and reassurance.”
The recurrence score has been used for a decade, but the trial was necessary because the score was originally developed in patients who were receiving older chemotherapy regimens and older endocrine therapies, and because there have been few data to guide decision making in the large group of patients with midrange scores, he said. “Now we can say with confidence ... that the patients got contemporary chemo regimens and still saw no benefit from chemotherapy.
“This is not so much about de-escalation ... the goal of this study was not to just use less treatment but to tailor treatment. The investigators chose the title very aptly,” said Dr Burstein, a medical oncologist at the Dana-Farber Cancer Institute and associate professor of medicine at the Harvard Medical School, Boston.
“This is extraordinary data for breast cancer doctors and women who have breast cancer. It allows you to individualize treatment based on extraordinary science, which now has tremendous prospective validation,” he said. Overall, “women with breast cancer who are getting modern therapy are doing well, and this test shows us how to tailor that management so that they get exactly the right amount of treatment – not too much and not too little.”
— Susan London
First-line immunotherapy boosts survival in NSCLC patients
Key clinical point Many patients with previously untreated NSCLC could benefit from first-line therapy with the checkpoint inhibitor pembrolizumab. Major finding In all patients with expression of PD-L1 on 1% or more of tumor cells, OS was 16.7 months with pembrolizumab, compared with 12.1 months for chemotherapy. Study details Randomized phase 3 trial of 1,274 patients with advanced or metastatic NSCLC. Funding Merck, the maker of the study drug, funded the study. Disclosures Dr Lopes disclosed institutional research funding from Merck Sharp & Dohme, EMD Serono, and AstraZeneca. Dr Heymach disclosed stock/ownership in Bio-Tree and Cardinal Spine, a consulting or advisory role for Abbvie, ARIAD, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Calithera Biosciences, Genentech, Medivation, Novartis, Oncomed, and Synta, and institutional research funding from AstraZeneca. Dr Gandhi reported having no relevant disclosures. Source Lopes G et al. ASCO 2018, abstract LBA4. https://meetinglibrary.asco.org/record/165950/abstract
Pembrolizumab as first-line treatment of advanced non–small-cell lung cancer (NSCLC) offered longer OS with better tolerability compared with chemotherapy, results of the Keynote-042 phase 3 randomized trial show.
In 1,274 patients with advanced, previously untreated NSCLC with expression of the PD-L1 on 1% or more of tumor cells, median OS after a median follow-up of 12.8 months was 16.7 months for patients treated with pembrolizumab monotherapy, compared with 12.1 months for patients treated with either paclitaxel or pemetrexed plus carboplatin, reported lead author Gilberto Lopes, MD, of the Sylvester Comprehensive Cancer Center at the University of Miami.
The survival benefit for immunotherapy was even greater for patients with higher levels of PD-L1 expression: 20 versus 12.2 months for patients with PD-L1 expression of 50% or greater, and 17.7 versus 13 months for patients with PD-L1 expression of 20% or greater, Dr Lopes noted.
For all 3 PD-L1 expression groups, the median duration of response was 20.2 months, compared with 10.8-8.3 months for patients in the chemotherapy arm.
“These are responses that are unlike anything that we have seen with chemotherapy in the past for non–small-cell lung cancer,” Dr Lopes said at a briefing before his presentation. “In addition to that, and probably more importantly, patients had fewer adverse events [with pembrolizumab]. Overall, about 60% had any treatment-related adverse event with pembrolizumab, versus 90% with chemotherapy,” he added.
‘A true milestone’
ASCO expert John Heymach, MD, PhD, of the University of Texas MD Anderson Cancer Center in Houston, said at the briefing that the study was “a true milestone for the field, because now, for the first time, we can say that in non–small-cell lung cancer patients receiving first-line therapy, the vast majority can receive immunotherapy with pembrolizumab instead of chemotherapy.”
He noted that an earlier study, Keynote-024, showed that pembrolizumab significantly improved progression-free survival in patients with tumors expressing PD-L1 on at least 50% of cells compared with standard platinum-based chemotherapy (10.3 vs 6 months).
“This more than doubles that population that can start immunotherapy as a first-line treatment, assuming the [Food and Drug Administration] modifies the label in accordance with this study,” he added.
The Keynote-042 investigators enrolled 1,274 patients with locally advanced or metastatic NSCLC, and randomly assigned them to receive either a maximum of 35 cycles of pembrolizumab 200 mg every 3 weeks, or the investigators’ choice of not more than 6 cycles of either paclitaxel–carboplatin or pemetrexed–carboplatin, with optional pemetrexed maintenance for patients with nonsquamous histologies only.
The randomization was stratified by region (Asia vs non-East Asia), Eastern Cooperative Oncology Group performance status 0 or 1, squamous versus nonsquamous histology, and PD-L1 expression, or TPS (tumor proportion score) greater than 50% versus 1%-49%.
As noted before, the primary endpoint of OS in all patients with a TPS of 1% or greater was met, with respective median OS in the pembrolizumab versus chemotherapy groups of 16.7 and 12.1 months, translating into an HR favoring pembrolizumab of 0.81 (P = .0018). Respective hazard ratios for the TPS 20% or greater and TPS 50% or greater groups were 0.77 (P = .0020) and 0.69 (P = .0003).
At 12.8 months of median follow-up, 13% of patients assigned to pembrolizumab were still on the drug, and 4.3% of patients were receiving maintenance pemetrexed.
Treatment-related adverse events of any grade occurred in 399 of 636 patients assigned to pembrolizumab (62.7%), compared with 553 of 615 patients assigned to chemotherapy (89.9%). Grade 3 or greater events occurred in 17.8% and 41% of patients, respectively. There were 13 deaths related to therapy in the pembrolizumab arm (2.0%), and 14 in the chemotherapy arm (2.3%). Adverse events leading to discontinuation were similar between the groups, at 9% and 9.4%, respectively.
There were more immune-mediated adverse events in the pembrolizumab arm than in the chemotherapy arm (27.8% vs 7.2%, respectively), and of those, grade 3 or higher events occurred in 8% and 1.5% of patients. There was 1 immune-mediated death, from pneumonitis, in the immunotherapy arm; there were no deaths related to immune-mediated side effects in the chemotherapy arm.
“I really view this as a ‘double whammy’ for patients,” Dr Heymach said at the briefing. “Often advances in survival for our lung cancer patients come at the cost of significant toxicities. Here, by contrast, not only are patients living longer and having a much higher likelihood of prolonged survival in years, often instead of months, but they’re also receiving a treatment that has substantially less toxicity across virtually all measures, and this really impacts the day-to-day life of these patients.”
Leena Gandhi, MD, PhD, of the Perlmutter Cancer Center at New York University, the invited discussant at the plenary, agreed that pembrolizumab improves survival, compared with chemotherapy patients with PD-L1 expression levels greater than 1%, but noted that most of the benefit – as also seen in Keynote-024 – was in those patients whose tumors had high levels of PD-L1 expression.
She emphasized that although PD-L1 is an imperfect biomarker, it should still be used to help select patients for therapy and it may be complementary with tumor mutational burden for more precise treatment selection.
“What we know, and what this study adds to, is that PD-L1 really does define a patient population that could receive benefit from pembrolizumab over chemotherapy. Patients with low or no PD-L1 expression likely should get some type of combination therapy,” she said. “This study extends what we’ve seen from other recent studies, which is that chemotherapy alone is no longer a first-line standard of care in non–small-cell lung cancer.”
— Neil Osterweil
Maintenance chemo improves survival in youth with rhabdomyosarcoma
Key clinical point 6 months of maintenance chemotherapy improves survival in youth with high-risk rhabdomyosarcoma. Major finding Patients given maintenance low-dose vinorelbine and cyclophosphamide had better 5-year OS compared with those not receiving any additional treatment (86.5% vs 73.7%; HR, 0.52). Study details A phase 3 randomized controlled trial in 371 patients aged 0-21 years with high-risk rhabdomyosarcoma who had had a complete response to standard intensive therapy. Funding The study received funding from Fondazione Città della Speranza, Italy. Disclosures Dr Bisogno disclosed that he has a consulting or advisory role with Clinigen Group, and receives travel, accommodations, and/or expenses from Jazz Pharmaceuticals. Source Bisogno et al. ASCO 2018, Abstract LBA2. https://meetinglibrary.asco.org/record/161695/abstract
Six months of maintenance chemotherapy prolongs OS in youth with high-risk rhabdomyosarcoma, finds a phase 3 randomized controlled trial of the European Paediatric Soft Tissue Sarcoma Study Group (EpSSG).
Rhabdomyosarcoma is a rare but highly aggressive tumor, lead study author Gianni Bisogno, MD, PhD, a professor at the University Hospital of Padova, Italy, and chair of the EpSSG, noted in a press briefing at the meeting, where the findings were reported. In pediatric patients who achieve complete response to standard therapy, “we know that after 1 or 2 years, one-third of these children relapse, and most of them die,” he said.
The EpSSG trial, which took about 10 years to conduct, enrolled 371 patients aged 0-21 years with high-risk rhabdomyosarcoma who had had a complete response to standard intensive therapy. They were randomized evenly to stop treatment or to receive 6 months of maintenance treatment consisting of low-dose vinorelbine and cyclophosphamide.
Results reported in the meeting’s plenary session showed that giving maintenance chemotherapy improved the 5-year OS rate by an absolute 12.8%, which translated to a near halving of the risk of death. And the maintenance regimen used was generally well tolerated.
“At the end of this long, not-easy study, we concluded that maintenance chemotherapy is an effective and well tolerated treatment for children with high-risk rhabdomyosarcoma,” Dr Bisogno said.
There are three possibilities for its efficacy, he speculated. “It may be the duration, the type of drugs used, or the metronomic approach. Maybe altogether, these three different actions have a benefit to increase survival.
“Our group has decided this is the new standard treatment for patients. At least in Europe, we give standard intensive therapy and then we continue with 6 more months of low-dose chemotherapy,” Dr Bisogno concluded. “We think that this approach – a new way of using old drugs – can be of interest also for other pediatric tumors.”
The trial is noteworthy in that it shows “how to successfully conduct large and important trials in rare diseases,” said ASCO expert Warren Chow, MD.
The standard therapy for rhabdomyosarcomas is somewhat different in the United States, typically a regimen containing vincristine, actinomycin D, cyclophosphamide, and (more recently) irinotecan, he noted. “We have not been traditionally using maintenance chemo for any of the pediatric sarcomas, so this is a paradigm shift. These results will need to be tested with US-based protocols before becoming standard of care in the United States. Also, we will need to determine if these results are applicable to patients older than 21 years of age who are considered high risk based solely on their age.
“Even with these caveats, this is the first significant treatment advance in this rare cancer in more than 30 years,” concluded Dr Chow, a medical oncologist and clinical professor at City of Hope, Duarte, Calif. “No doubt, this trial was a home run.”
Study details
Patients enrolled in the EpSSG trial had had a complete response to the standard intensive therapy used in Europe: high-dose chemotherapy (ifosfamide, vincristine, and actinomycin D, with or without doxorubicin), radiation therapy, and surgery.
The maintenance chemotherapy consisted of a combination of low-dose intravenous vinorelbine given weekly and oral cyclophosphamide given daily. The 6-month duration was somewhat arbitrary, according to Dr Bisogno. “We had to start somewhere. So when we started, we decided to use 6 months because there was some evidence in the past for regimens that long. In our next European trial, we are going to test different kinds and durations of maintenance because this is very important.”
The maintenance regimen was well tolerated compared with the regimen given during standard intensive therapy, with, for example, lower rates of grade 3 and 4 anemia (8.9% vs 48.9%), neutropenia (80.6% vs 91.6%), and thrombocytopenia (0.6% vs 26.0%), which translated to less of a need for transfusions, and a lower rate of grade 3 or 4 infection (29.4% vs 56.4%), Dr Bisogno reported. There were no cases of grade 3 or 4 cardiac, hepatobiliary/pancreatic, or renal toxicity.
Relative to peers who stopped treatment after standard intensive therapy, patients who received maintenance treatment tended to have better DFS (77.6% vs 69.8%; HR, 0.68; P = .0613) and had significantly better OS (86.5% vs 73.7%; HR, 0.52; P = .0111).
— Susan London
Among the groundbreaking findings presented at this year’s annual meeting of the American Society of Clinical Oncology were those showing that most women with HR-positive, HER2-negative, early-stage breast cancer who have an intermediate recurrence score can safely skip adjuvant chemotherapy, and that upfront pembrolizumab for patients with NSCLC expressing PD-L1 on at least 1% of tumor cells can not only significantly improve overall survival, but do so with less toxicity than standard chemotherapy.
TAILORx marks major advance for precision medicine in breast cancer
Key clinical point The majority of women with HR-positive, HER2-negative, node-negative early-stage breast cancer who have an intermediate recurrence score can safely skip adjuvant chemotherapy. Major finding In women with an Oncotype DX Recurrence Score in the midrange (11-25), invasive DFS with endocrine therapy alone was not inferior to that with chemotherapy plus endocrine therapy (HR, 1.08; P = .26). Study details A phase 3 trial in 10,273 women with HR-positive, HER2-negative, node-negative, early-stage breast cancer, with a noninferiority randomized component in the 6,711 women with a midrange recurrence score (TAILORx trial). Funding This study received funding primarily from the National Cancer Institute, National Institutes of Health. Additional support was provided by the Breast Cancer Research Foundation, Komen Foundation, and US Postal Service Breast Cancer Stamp. Disclosures Dr Sparano disclosed that he has a consulting or advisory role with Genentech/Roche, Novartis, AstraZeneca, Celgene, Lilly, Celldex, Pfizer, Prescient Therapeutics, Juno Therapeutics, and Merrimack; has stock or other ownership interests with MetaStat; and receives research funding (institutional) from Prescient Therapeutics, Deciphera, Genentech/Roche, Merck, Novartis, and Merrimack. Source Sparano et al. ASCO 2018 Abstract LBA1: https://meetinglibrary.asco.org/record/161490/abstract
Use of the 21-tumor gene expression assay (Oncotype DX Recurrence Score) allows nearly 70% of women with hormone receptor-positive, HER2-negative, node-negative, early-stage breast cancer to safely forgo adjuvant chemotherapy, sparing them adverse effects and preventing overtreatment, TAILORx trial results show.
The findings, which were reported in the plenary session at the meeting and simultaneously published in the New England Journal of Medicine (N Engl J Med. 2018; 379:111-121; [behind paywall]), mark a major advance in precision medicine.
“The rationale for the TAILORx precision medicine trial is that we are really trying to ‘thread the needle,’ “ lead study author Joseph A Sparano, MD, commented in a press briefing. Oncologists typically recommend adjuvant chemotherapy for the half of all breast cancers that are hormone receptor positive, HER2 negative, and node negative, even though its absolute benefit in reducing recurrences in this population is small. “This results in most patients being overtreated because endocrine therapy alone is adequate. But some are undertreated: They do not receive chemotherapy although they could have benefited from it,” he noted.
The recurrence score is known to be prognostic and predictive of benefit from adding chemotherapy to endocrine therapy, Dr Sparano said. “But there was a major gap: There was uncertain benefit for patients who had a midrange score, which is about two-thirds of all patients who are treated,” said Dr Sparano, the associate director for clinical research at Albert Einstein Cancer Center and Montefiore Health System in New York, and vice-chair of the ECOG-ACRIN Cancer Research Group.
The phase 3 TAILORx trial registered 10,273 women with hormone receptor-positive, HER2-negative, node-negative, early-stage breast cancer, making it the largest adjuvant breast cancer trial to date. Analyses focused on the 6,711 evaluable women with a midrange recurrence score (defined as 11 through 25 in the trial), who were randomized to receive endocrine therapy alone or adjuvant chemotherapy plus endocrine therapy, with a noninferiority design. Of note is that contemporary drugs and regimens were used.
Results at a median follow-up of 7.5 years showed that the trial met its primary endpoint: the risk of invasive disease-free survival (DFS) events (invasive disease recurrence, second primary cancer, or death) was not inferior for women given endocrine therapy alone compared with counterparts given chemotherapy plus endocrine therapy (hazard ratio [HR], 1.08; P = .26), Dr Sparano reported.
The groups were also on par, with absolute differences of no more than 1% between rates, with respect to a variety of other efficacy outcomes: freedom from distant recurrence and any recurrence, and overall survival (OS).
Findings were similar across most subgroups. But analyses suggested that women aged 50 years and younger and who had a recurrence score of 16-25 fared better when they received chemotherapy. “Though exploratory from a statistical perspective, this is a highly clinically relevant observation,” Dr Sparano said. “It suggests ... that chemotherapy should be spared with caution in this subgroup, after a careful discussion of potential benefits and risks in a shared decision process.”
In other findings, analyses of the trial’s nonrandomized groups confirmed excellent outcomes in women with a low recurrence score (0-10) who were given endocrine therapy alone, and at the other end of the spectrum, there was need for a more aggressive approach, including chemotherapy, in women with a high recurrence score (26-100).
Ultimately, application of the recurrence score allowed 69% of the trial population to skip chemotherapy: all of the women with a score of 0-10 (16% of the trial population), those older than 50 years with a score of 11-25 (45%), and those aged 50 years or younger with a score of 11-15 (8%).
An ongoing companion phase 3 trial, RxPONDER, is assessing the benefit of applying the recurrence score in women who are similar but ihave node-positive disease.
Study details
All of the women with hormone receptor-positive, HER2-negative, node-negative, early-stage breast cancer enrolled in TAILORx met National Comprehensive Cancer Network guidelines for receiving adjuvant chemotherapy. About 69% had an intermediate recurrence score (11-25) and were randomized. All of the 17% with a low recurrence score (0-10) were given only endocrine therapy, and all of the 14% with a high recurrence score (26-100) were given both adjuvant chemotherapy and endocrine therapy.
Of note, the recurrence scores used to define midrange were adjusted downward from those conventionally used to account for exclusion of patients with higher-risk HER2-positive disease and to minimize potential for undertreatment, Dr Sparano explained.
In the women with midrange scores who were randomized, the hazard ratio of 1.08 for invasive DFS with endocrine therapy alone compared with chemotherapy plus endocrine therapy fell well within the predefined hazard ratio for noninferiority (1.322). The 9-year rate of invasive DFS was 83.3% with endocrine therapy and 84.3% with chemotherapy plus endocrine therapy.
The groups had similar rates of freedom from distant recurrence (94.5% vs 95.0%; HR, 1.10; P = .48) and distant or locoregional recurrence (92.2% vs 92.9%; HR, 1.11; P = .33), and similar OSs (93.9% vs 93.8%; HR for death, 0.99; P = .89).
In exploratory analyses, there was an interaction of age and recurrence score (P = .004) whereby women aged 50 years or younger derived some benefit from chemotherapy if they had a recurrence score of 16-20 (9% fewer invasive DFS events, including 2% fewer distant recurrences) or a recurrence score 21-25 (6% fewer invasive DFS events, mainly distant recurrences). “This is information that could drive some younger women who have a recurrence score in this range to accept chemotherapy,” Dr Sparano said.
The 9-year rate of distant recurrence averaged 5% in women with midrange scores overall. It was 3% in those with a low recurrence score given endocrine therapy alone, but it was still 13% in those with a high recurrence score despite receiving both endocrine therapy and chemotherapy. The latter finding may “indicate the need to explore potentially more effective therapies in this setting,” he proposed.
Tailoring treatment: ‘not too much and not too little’
“These are important data because this is the most common form of breast cancer in the United States and other developed countries, and the most challenging decision we make with these patients is whether or not to recommend adjuvant chemotherapy with all its side effects and potential benefits,” said ASCO expert Harold Burstein, MD, PhD, FASCO. “The data show that the majority of women who have this test performed on their tumor can be told that they don’t need chemotherapy, and that can be said with tremendous confidence and reassurance.”
The recurrence score has been used for a decade, but the trial was necessary because the score was originally developed in patients who were receiving older chemotherapy regimens and older endocrine therapies, and because there have been few data to guide decision making in the large group of patients with midrange scores, he said. “Now we can say with confidence ... that the patients got contemporary chemo regimens and still saw no benefit from chemotherapy.
“This is not so much about de-escalation ... the goal of this study was not to just use less treatment but to tailor treatment. The investigators chose the title very aptly,” said Dr Burstein, a medical oncologist at the Dana-Farber Cancer Institute and associate professor of medicine at the Harvard Medical School, Boston.
“This is extraordinary data for breast cancer doctors and women who have breast cancer. It allows you to individualize treatment based on extraordinary science, which now has tremendous prospective validation,” he said. Overall, “women with breast cancer who are getting modern therapy are doing well, and this test shows us how to tailor that management so that they get exactly the right amount of treatment – not too much and not too little.”
— Susan London
First-line immunotherapy boosts survival in NSCLC patients
Key clinical point Many patients with previously untreated NSCLC could benefit from first-line therapy with the checkpoint inhibitor pembrolizumab. Major finding In all patients with expression of PD-L1 on 1% or more of tumor cells, OS was 16.7 months with pembrolizumab, compared with 12.1 months for chemotherapy. Study details Randomized phase 3 trial of 1,274 patients with advanced or metastatic NSCLC. Funding Merck, the maker of the study drug, funded the study. Disclosures Dr Lopes disclosed institutional research funding from Merck Sharp & Dohme, EMD Serono, and AstraZeneca. Dr Heymach disclosed stock/ownership in Bio-Tree and Cardinal Spine, a consulting or advisory role for Abbvie, ARIAD, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Calithera Biosciences, Genentech, Medivation, Novartis, Oncomed, and Synta, and institutional research funding from AstraZeneca. Dr Gandhi reported having no relevant disclosures. Source Lopes G et al. ASCO 2018, abstract LBA4. https://meetinglibrary.asco.org/record/165950/abstract
Pembrolizumab as first-line treatment of advanced non–small-cell lung cancer (NSCLC) offered longer OS with better tolerability compared with chemotherapy, results of the Keynote-042 phase 3 randomized trial show.
In 1,274 patients with advanced, previously untreated NSCLC with expression of the PD-L1 on 1% or more of tumor cells, median OS after a median follow-up of 12.8 months was 16.7 months for patients treated with pembrolizumab monotherapy, compared with 12.1 months for patients treated with either paclitaxel or pemetrexed plus carboplatin, reported lead author Gilberto Lopes, MD, of the Sylvester Comprehensive Cancer Center at the University of Miami.
The survival benefit for immunotherapy was even greater for patients with higher levels of PD-L1 expression: 20 versus 12.2 months for patients with PD-L1 expression of 50% or greater, and 17.7 versus 13 months for patients with PD-L1 expression of 20% or greater, Dr Lopes noted.
For all 3 PD-L1 expression groups, the median duration of response was 20.2 months, compared with 10.8-8.3 months for patients in the chemotherapy arm.
“These are responses that are unlike anything that we have seen with chemotherapy in the past for non–small-cell lung cancer,” Dr Lopes said at a briefing before his presentation. “In addition to that, and probably more importantly, patients had fewer adverse events [with pembrolizumab]. Overall, about 60% had any treatment-related adverse event with pembrolizumab, versus 90% with chemotherapy,” he added.
‘A true milestone’
ASCO expert John Heymach, MD, PhD, of the University of Texas MD Anderson Cancer Center in Houston, said at the briefing that the study was “a true milestone for the field, because now, for the first time, we can say that in non–small-cell lung cancer patients receiving first-line therapy, the vast majority can receive immunotherapy with pembrolizumab instead of chemotherapy.”
He noted that an earlier study, Keynote-024, showed that pembrolizumab significantly improved progression-free survival in patients with tumors expressing PD-L1 on at least 50% of cells compared with standard platinum-based chemotherapy (10.3 vs 6 months).
“This more than doubles that population that can start immunotherapy as a first-line treatment, assuming the [Food and Drug Administration] modifies the label in accordance with this study,” he added.
The Keynote-042 investigators enrolled 1,274 patients with locally advanced or metastatic NSCLC, and randomly assigned them to receive either a maximum of 35 cycles of pembrolizumab 200 mg every 3 weeks, or the investigators’ choice of not more than 6 cycles of either paclitaxel–carboplatin or pemetrexed–carboplatin, with optional pemetrexed maintenance for patients with nonsquamous histologies only.
The randomization was stratified by region (Asia vs non-East Asia), Eastern Cooperative Oncology Group performance status 0 or 1, squamous versus nonsquamous histology, and PD-L1 expression, or TPS (tumor proportion score) greater than 50% versus 1%-49%.
As noted before, the primary endpoint of OS in all patients with a TPS of 1% or greater was met, with respective median OS in the pembrolizumab versus chemotherapy groups of 16.7 and 12.1 months, translating into an HR favoring pembrolizumab of 0.81 (P = .0018). Respective hazard ratios for the TPS 20% or greater and TPS 50% or greater groups were 0.77 (P = .0020) and 0.69 (P = .0003).
At 12.8 months of median follow-up, 13% of patients assigned to pembrolizumab were still on the drug, and 4.3% of patients were receiving maintenance pemetrexed.
Treatment-related adverse events of any grade occurred in 399 of 636 patients assigned to pembrolizumab (62.7%), compared with 553 of 615 patients assigned to chemotherapy (89.9%). Grade 3 or greater events occurred in 17.8% and 41% of patients, respectively. There were 13 deaths related to therapy in the pembrolizumab arm (2.0%), and 14 in the chemotherapy arm (2.3%). Adverse events leading to discontinuation were similar between the groups, at 9% and 9.4%, respectively.
There were more immune-mediated adverse events in the pembrolizumab arm than in the chemotherapy arm (27.8% vs 7.2%, respectively), and of those, grade 3 or higher events occurred in 8% and 1.5% of patients. There was 1 immune-mediated death, from pneumonitis, in the immunotherapy arm; there were no deaths related to immune-mediated side effects in the chemotherapy arm.
“I really view this as a ‘double whammy’ for patients,” Dr Heymach said at the briefing. “Often advances in survival for our lung cancer patients come at the cost of significant toxicities. Here, by contrast, not only are patients living longer and having a much higher likelihood of prolonged survival in years, often instead of months, but they’re also receiving a treatment that has substantially less toxicity across virtually all measures, and this really impacts the day-to-day life of these patients.”
Leena Gandhi, MD, PhD, of the Perlmutter Cancer Center at New York University, the invited discussant at the plenary, agreed that pembrolizumab improves survival, compared with chemotherapy patients with PD-L1 expression levels greater than 1%, but noted that most of the benefit – as also seen in Keynote-024 – was in those patients whose tumors had high levels of PD-L1 expression.
She emphasized that although PD-L1 is an imperfect biomarker, it should still be used to help select patients for therapy and it may be complementary with tumor mutational burden for more precise treatment selection.
“What we know, and what this study adds to, is that PD-L1 really does define a patient population that could receive benefit from pembrolizumab over chemotherapy. Patients with low or no PD-L1 expression likely should get some type of combination therapy,” she said. “This study extends what we’ve seen from other recent studies, which is that chemotherapy alone is no longer a first-line standard of care in non–small-cell lung cancer.”
— Neil Osterweil
Maintenance chemo improves survival in youth with rhabdomyosarcoma
Key clinical point 6 months of maintenance chemotherapy improves survival in youth with high-risk rhabdomyosarcoma. Major finding Patients given maintenance low-dose vinorelbine and cyclophosphamide had better 5-year OS compared with those not receiving any additional treatment (86.5% vs 73.7%; HR, 0.52). Study details A phase 3 randomized controlled trial in 371 patients aged 0-21 years with high-risk rhabdomyosarcoma who had had a complete response to standard intensive therapy. Funding The study received funding from Fondazione Città della Speranza, Italy. Disclosures Dr Bisogno disclosed that he has a consulting or advisory role with Clinigen Group, and receives travel, accommodations, and/or expenses from Jazz Pharmaceuticals. Source Bisogno et al. ASCO 2018, Abstract LBA2. https://meetinglibrary.asco.org/record/161695/abstract
Six months of maintenance chemotherapy prolongs OS in youth with high-risk rhabdomyosarcoma, finds a phase 3 randomized controlled trial of the European Paediatric Soft Tissue Sarcoma Study Group (EpSSG).
Rhabdomyosarcoma is a rare but highly aggressive tumor, lead study author Gianni Bisogno, MD, PhD, a professor at the University Hospital of Padova, Italy, and chair of the EpSSG, noted in a press briefing at the meeting, where the findings were reported. In pediatric patients who achieve complete response to standard therapy, “we know that after 1 or 2 years, one-third of these children relapse, and most of them die,” he said.
The EpSSG trial, which took about 10 years to conduct, enrolled 371 patients aged 0-21 years with high-risk rhabdomyosarcoma who had had a complete response to standard intensive therapy. They were randomized evenly to stop treatment or to receive 6 months of maintenance treatment consisting of low-dose vinorelbine and cyclophosphamide.
Results reported in the meeting’s plenary session showed that giving maintenance chemotherapy improved the 5-year OS rate by an absolute 12.8%, which translated to a near halving of the risk of death. And the maintenance regimen used was generally well tolerated.
“At the end of this long, not-easy study, we concluded that maintenance chemotherapy is an effective and well tolerated treatment for children with high-risk rhabdomyosarcoma,” Dr Bisogno said.
There are three possibilities for its efficacy, he speculated. “It may be the duration, the type of drugs used, or the metronomic approach. Maybe altogether, these three different actions have a benefit to increase survival.
“Our group has decided this is the new standard treatment for patients. At least in Europe, we give standard intensive therapy and then we continue with 6 more months of low-dose chemotherapy,” Dr Bisogno concluded. “We think that this approach – a new way of using old drugs – can be of interest also for other pediatric tumors.”
The trial is noteworthy in that it shows “how to successfully conduct large and important trials in rare diseases,” said ASCO expert Warren Chow, MD.
The standard therapy for rhabdomyosarcomas is somewhat different in the United States, typically a regimen containing vincristine, actinomycin D, cyclophosphamide, and (more recently) irinotecan, he noted. “We have not been traditionally using maintenance chemo for any of the pediatric sarcomas, so this is a paradigm shift. These results will need to be tested with US-based protocols before becoming standard of care in the United States. Also, we will need to determine if these results are applicable to patients older than 21 years of age who are considered high risk based solely on their age.
“Even with these caveats, this is the first significant treatment advance in this rare cancer in more than 30 years,” concluded Dr Chow, a medical oncologist and clinical professor at City of Hope, Duarte, Calif. “No doubt, this trial was a home run.”
Study details
Patients enrolled in the EpSSG trial had had a complete response to the standard intensive therapy used in Europe: high-dose chemotherapy (ifosfamide, vincristine, and actinomycin D, with or without doxorubicin), radiation therapy, and surgery.
The maintenance chemotherapy consisted of a combination of low-dose intravenous vinorelbine given weekly and oral cyclophosphamide given daily. The 6-month duration was somewhat arbitrary, according to Dr Bisogno. “We had to start somewhere. So when we started, we decided to use 6 months because there was some evidence in the past for regimens that long. In our next European trial, we are going to test different kinds and durations of maintenance because this is very important.”
The maintenance regimen was well tolerated compared with the regimen given during standard intensive therapy, with, for example, lower rates of grade 3 and 4 anemia (8.9% vs 48.9%), neutropenia (80.6% vs 91.6%), and thrombocytopenia (0.6% vs 26.0%), which translated to less of a need for transfusions, and a lower rate of grade 3 or 4 infection (29.4% vs 56.4%), Dr Bisogno reported. There were no cases of grade 3 or 4 cardiac, hepatobiliary/pancreatic, or renal toxicity.
Relative to peers who stopped treatment after standard intensive therapy, patients who received maintenance treatment tended to have better DFS (77.6% vs 69.8%; HR, 0.68; P = .0613) and had significantly better OS (86.5% vs 73.7%; HR, 0.52; P = .0111).
— Susan London
New guidelines frame treatment options for Castleman disease
The (MCD), although adjuvant chemotherapy can be critical in severe cases, according to the first-ever consensus treatment guidelines based on symptom severity.
Early intervention with combination chemotherapy may prevent a fatal outcome in patients with severe idiopathic MCD, according to the guidelines, which are based on input from 42 experts from 10 countries.
The guidelines should help clinicians select therapy, evaluate response, and thereby improve outcomes for this difficult-to-treat disease, according to guideline co-author David C. Fajgenbaum, MD, who is himself an idiopathic MCD patient. Frits van Rhee, MD, PhD, is the first author of the guidelines.*
“Right now we recommend siltuximab first line for everyone,” Dr. Fajgenbaum said in an interview, “but if we continue to dig deeper, it may be that there are clinical cases within idiopathic MCD that we think are even better candidates than others, and there may be alternative therapies for other patients.”
Experts reviewed published literature and a series of 344 clinical cases to develop the guidelines, which are published in the journal Blood.
Treating idiopathic MCD is challenging because of the rarity and heterogeneity of the disease, among other factors, said Dr. Fajgenbaum, of the division of translational medicine and human genetics at the University of Pennsylvania, Philadelphia.
Some 6,000-7,000 cases of Castleman disease are diagnosed yearly, and of those, only about 1,000 cases are idiopathic MCD, according to Dr. Fajgenbaum.
“Even within idiopathic MCD, there is some heterogeneity,” he said. “Some patients present in the intensive care unit with life-threatening multiple organ failure and will die within weeks of presentation, whereas others will have a slower presentation and certainly not nearly as aggressive presentation.”
Although the exact etiology of idiopathic MCD is unknown, human interleukin (IL)-6 is the most common pathological driver, experts said in the guidelines.
Siltuximab and tocilizumab are two IL-6–directed therapies used to treat multicentric Castleman disease, with siltuximab targeting IL-6 itself and tocilizumab targeting the IL-6 receptor. Siltuximab is recommended as the first choice because of rigorous data supporting its use, including randomized clinical trial data, while tocilizumab is recommended if siltuximab is not available.
However, clinicians need to carefully monitor laboratory results and clinical features for patients on these drugs because about 50% of idiopathic MCD patients don’t have a satisfactory response to first-line anti–IL-6 treatments, Dr. Fajgenbaum said.
“Once you get to second-line therapies, that’s really where the level of evidence is lower,” he said.
Second-line therapy should include rituximab, and immunomodulatory/immunosuppressive agents or steroids may be added, according to the guidelines.
Third line therapy is “less well defined,” according to the guidelines, and experts generally recommended immunomodulatory/immunosuppressive agents such as cyclosporine A, sirolimus, thalidomide, and lenalidomide.
Cytotoxic chemotherapy has a high response rate but also a high rate of relapse and significant toxicities, according to the data analysis conducted as part of the guideline development process. Based on that, the experts said to avoid it unless the patient progresses to severe idiopathic MCD.
“Patients who are literally dying in the intensive care unit, given the right combination chemotherapy, can improve within days to weeks and can even leave the hospital,” Dr. Fajgenbaum said. “It’s not necessarily going to be the answer long term, but it can be life saving in the short term. So we recommended a really quite aggressive approach for these patients.”
To bolster the evidence base, investigators in the Castleman Disease Collaborative Network (CDCN) set up an international registry to collect treatment and outcome data for 500 patients. After the first year and a half, 150 patients were enrolled, and the investigators have identified more than 30 drugs that have been used off label to treat idiopathic MCD, according to Dr. Fajgenbaum.
“Some of the drugs are demonstrating efficacy in small numbers,” he said. “With the goal of 500 patients total, we can certainly hope to see some trends.”
Dr. Fajgenbaum was diagnosed with idiopathic MCD as a medical student.
“That certainly served as a very strong personal motivator for me to get involved in the disease,” he said. “But as I’ve gotten more and more involved, I’ve obviously met a lot of other patients, and that really is a huge motivator for all members of the CDCN. We want more options for more patients more quickly so we can help as many people as possible.”
Dr. Fajgenbaum reported research funding from Janssen. Coauthors reported disclosures related to Janssen, Bristol-Myers Squibb, Genentech, Merck, Celgene, Incyte, Pfizer, Sequenom, and Foundation Medicine, among others.
SOURCE: van Rhee F et al. Blood. 2018 Sep 4. doi: 10.1182/blood-2018-07-862334.
This story was updated 10/01/2018.
The (MCD), although adjuvant chemotherapy can be critical in severe cases, according to the first-ever consensus treatment guidelines based on symptom severity.
Early intervention with combination chemotherapy may prevent a fatal outcome in patients with severe idiopathic MCD, according to the guidelines, which are based on input from 42 experts from 10 countries.
The guidelines should help clinicians select therapy, evaluate response, and thereby improve outcomes for this difficult-to-treat disease, according to guideline co-author David C. Fajgenbaum, MD, who is himself an idiopathic MCD patient. Frits van Rhee, MD, PhD, is the first author of the guidelines.*
“Right now we recommend siltuximab first line for everyone,” Dr. Fajgenbaum said in an interview, “but if we continue to dig deeper, it may be that there are clinical cases within idiopathic MCD that we think are even better candidates than others, and there may be alternative therapies for other patients.”
Experts reviewed published literature and a series of 344 clinical cases to develop the guidelines, which are published in the journal Blood.
Treating idiopathic MCD is challenging because of the rarity and heterogeneity of the disease, among other factors, said Dr. Fajgenbaum, of the division of translational medicine and human genetics at the University of Pennsylvania, Philadelphia.
Some 6,000-7,000 cases of Castleman disease are diagnosed yearly, and of those, only about 1,000 cases are idiopathic MCD, according to Dr. Fajgenbaum.
“Even within idiopathic MCD, there is some heterogeneity,” he said. “Some patients present in the intensive care unit with life-threatening multiple organ failure and will die within weeks of presentation, whereas others will have a slower presentation and certainly not nearly as aggressive presentation.”
Although the exact etiology of idiopathic MCD is unknown, human interleukin (IL)-6 is the most common pathological driver, experts said in the guidelines.
Siltuximab and tocilizumab are two IL-6–directed therapies used to treat multicentric Castleman disease, with siltuximab targeting IL-6 itself and tocilizumab targeting the IL-6 receptor. Siltuximab is recommended as the first choice because of rigorous data supporting its use, including randomized clinical trial data, while tocilizumab is recommended if siltuximab is not available.
However, clinicians need to carefully monitor laboratory results and clinical features for patients on these drugs because about 50% of idiopathic MCD patients don’t have a satisfactory response to first-line anti–IL-6 treatments, Dr. Fajgenbaum said.
“Once you get to second-line therapies, that’s really where the level of evidence is lower,” he said.
Second-line therapy should include rituximab, and immunomodulatory/immunosuppressive agents or steroids may be added, according to the guidelines.
Third line therapy is “less well defined,” according to the guidelines, and experts generally recommended immunomodulatory/immunosuppressive agents such as cyclosporine A, sirolimus, thalidomide, and lenalidomide.
Cytotoxic chemotherapy has a high response rate but also a high rate of relapse and significant toxicities, according to the data analysis conducted as part of the guideline development process. Based on that, the experts said to avoid it unless the patient progresses to severe idiopathic MCD.
“Patients who are literally dying in the intensive care unit, given the right combination chemotherapy, can improve within days to weeks and can even leave the hospital,” Dr. Fajgenbaum said. “It’s not necessarily going to be the answer long term, but it can be life saving in the short term. So we recommended a really quite aggressive approach for these patients.”
To bolster the evidence base, investigators in the Castleman Disease Collaborative Network (CDCN) set up an international registry to collect treatment and outcome data for 500 patients. After the first year and a half, 150 patients were enrolled, and the investigators have identified more than 30 drugs that have been used off label to treat idiopathic MCD, according to Dr. Fajgenbaum.
“Some of the drugs are demonstrating efficacy in small numbers,” he said. “With the goal of 500 patients total, we can certainly hope to see some trends.”
Dr. Fajgenbaum was diagnosed with idiopathic MCD as a medical student.
“That certainly served as a very strong personal motivator for me to get involved in the disease,” he said. “But as I’ve gotten more and more involved, I’ve obviously met a lot of other patients, and that really is a huge motivator for all members of the CDCN. We want more options for more patients more quickly so we can help as many people as possible.”
Dr. Fajgenbaum reported research funding from Janssen. Coauthors reported disclosures related to Janssen, Bristol-Myers Squibb, Genentech, Merck, Celgene, Incyte, Pfizer, Sequenom, and Foundation Medicine, among others.
SOURCE: van Rhee F et al. Blood. 2018 Sep 4. doi: 10.1182/blood-2018-07-862334.
This story was updated 10/01/2018.
The (MCD), although adjuvant chemotherapy can be critical in severe cases, according to the first-ever consensus treatment guidelines based on symptom severity.
Early intervention with combination chemotherapy may prevent a fatal outcome in patients with severe idiopathic MCD, according to the guidelines, which are based on input from 42 experts from 10 countries.
The guidelines should help clinicians select therapy, evaluate response, and thereby improve outcomes for this difficult-to-treat disease, according to guideline co-author David C. Fajgenbaum, MD, who is himself an idiopathic MCD patient. Frits van Rhee, MD, PhD, is the first author of the guidelines.*
“Right now we recommend siltuximab first line for everyone,” Dr. Fajgenbaum said in an interview, “but if we continue to dig deeper, it may be that there are clinical cases within idiopathic MCD that we think are even better candidates than others, and there may be alternative therapies for other patients.”
Experts reviewed published literature and a series of 344 clinical cases to develop the guidelines, which are published in the journal Blood.
Treating idiopathic MCD is challenging because of the rarity and heterogeneity of the disease, among other factors, said Dr. Fajgenbaum, of the division of translational medicine and human genetics at the University of Pennsylvania, Philadelphia.
Some 6,000-7,000 cases of Castleman disease are diagnosed yearly, and of those, only about 1,000 cases are idiopathic MCD, according to Dr. Fajgenbaum.
“Even within idiopathic MCD, there is some heterogeneity,” he said. “Some patients present in the intensive care unit with life-threatening multiple organ failure and will die within weeks of presentation, whereas others will have a slower presentation and certainly not nearly as aggressive presentation.”
Although the exact etiology of idiopathic MCD is unknown, human interleukin (IL)-6 is the most common pathological driver, experts said in the guidelines.
Siltuximab and tocilizumab are two IL-6–directed therapies used to treat multicentric Castleman disease, with siltuximab targeting IL-6 itself and tocilizumab targeting the IL-6 receptor. Siltuximab is recommended as the first choice because of rigorous data supporting its use, including randomized clinical trial data, while tocilizumab is recommended if siltuximab is not available.
However, clinicians need to carefully monitor laboratory results and clinical features for patients on these drugs because about 50% of idiopathic MCD patients don’t have a satisfactory response to first-line anti–IL-6 treatments, Dr. Fajgenbaum said.
“Once you get to second-line therapies, that’s really where the level of evidence is lower,” he said.
Second-line therapy should include rituximab, and immunomodulatory/immunosuppressive agents or steroids may be added, according to the guidelines.
Third line therapy is “less well defined,” according to the guidelines, and experts generally recommended immunomodulatory/immunosuppressive agents such as cyclosporine A, sirolimus, thalidomide, and lenalidomide.
Cytotoxic chemotherapy has a high response rate but also a high rate of relapse and significant toxicities, according to the data analysis conducted as part of the guideline development process. Based on that, the experts said to avoid it unless the patient progresses to severe idiopathic MCD.
“Patients who are literally dying in the intensive care unit, given the right combination chemotherapy, can improve within days to weeks and can even leave the hospital,” Dr. Fajgenbaum said. “It’s not necessarily going to be the answer long term, but it can be life saving in the short term. So we recommended a really quite aggressive approach for these patients.”
To bolster the evidence base, investigators in the Castleman Disease Collaborative Network (CDCN) set up an international registry to collect treatment and outcome data for 500 patients. After the first year and a half, 150 patients were enrolled, and the investigators have identified more than 30 drugs that have been used off label to treat idiopathic MCD, according to Dr. Fajgenbaum.
“Some of the drugs are demonstrating efficacy in small numbers,” he said. “With the goal of 500 patients total, we can certainly hope to see some trends.”
Dr. Fajgenbaum was diagnosed with idiopathic MCD as a medical student.
“That certainly served as a very strong personal motivator for me to get involved in the disease,” he said. “But as I’ve gotten more and more involved, I’ve obviously met a lot of other patients, and that really is a huge motivator for all members of the CDCN. We want more options for more patients more quickly so we can help as many people as possible.”
Dr. Fajgenbaum reported research funding from Janssen. Coauthors reported disclosures related to Janssen, Bristol-Myers Squibb, Genentech, Merck, Celgene, Incyte, Pfizer, Sequenom, and Foundation Medicine, among others.
SOURCE: van Rhee F et al. Blood. 2018 Sep 4. doi: 10.1182/blood-2018-07-862334.
This story was updated 10/01/2018.
FROM BLOOD
Employing irritable bowel syndrome patient-reported outcomes in the clinical trenches
Patients often seek care because they experience symptoms that negatively affect their health-related quality of life (HRQOL). Health care providers must then elicit, measure, and interpret patient symptoms as part of their clinical evaluation. To assist with this goal and to help bridge the gap between patients and providers, investigators have developed and validated a wide range of patient-reported outcomes (PROs) across the breadth and depth of the human health and illness experience. These PROs, which measure any aspect of a patient’s biopsychosocial health and come directly from the patient, may help direct care and improve outcomes. When PROs are collected systematically, efficiently, and in the right place at the right time, they may enhance the patient–provider relationship by improving communication and facilitating shared decision making.1-3
Within gastroenterology and hepatology, PROs have been developed for a number of conditions, including irritable bowel syndrome (IBS), chronic idiopathic constipation, cirrhosis, eosinophilic esophagitis, inflammatory bowel disease, and gastroesophageal reflux disease, among many other chronic diseases. IBS in particular is well suited for PRO measurement because it is symptom based and significantly impacts patients’ HRQOL and emotional health. Moreover, it is the most commonly diagnosed gastrointestinal condition and imparts a significant economic burden. In this article, we review the rationale for measuring IBS PROs in routine clinical practice and detail available measurement instruments.
Importance of IBS PROs in clinical practice
IBS is a functional GI disorder that is characterized by recurrent abdominal pain and altered bowel habits (i.e., diarrhea, constipation, or a mix of both). It has an estimated worldwide prevalence of 11%, and total costs are estimated at $30 billion annually in the United States alone.4 Because of the chronic relapsing nature of IBS, along with its impact on physical, mental, and social distress, it becomes important to accurately capture a patient’s illness experience with PROs. This is especially relevant to patients with IBS because we currently lack objective measurable biomarkers to assess their GI symptom burden. Instead, clinicians often are relegated to informal assessments of the severity of a patient’s symptoms, which ultimately guide their treatment recommendations: How many bowel movements have you had in the past week? Were your bowel movements hard or soft? How bad is your abdominal pain on a scale of 1 to 10? However, these traditional outcomes measured by health care providers often fail to capture other aspects of their health. For example, simply asking patients about the frequency and character of their stools will not provide any insight into how their symptoms impact their HRQOL and psychosocial health. An individual may report only two loose stools per day, but this may lead to substantial anxiety and negatively affect his or her performance at work. Similarly, the significance of the symptoms will vary from person to person; a patient with IBS who has five loose daily bowel movements may not be bothered by it, whereas another individual with three loose stools per day may feel that it severely hampers his or her daily activities. This is where PROs provide value because they provide a key component to understanding the true burden of IBS, and accounts for the HRQOL and psychosocial decrement engendered by the disease.
Although past literature has reported inconsistent benefits of using PROs in clinical practice,5 including that from our own work,6 there is a growing number of studies that have noted improved clinical outcomes through employing PROs.7-9 A compelling example is provided by Basch et al,7 who randomized patients receiving chemotherapy for metastatic cancer to either weekly symptom reporting using electronically delivered PRO instruments vs. usual care, which consisted of symptom reporting at the discretion of clinicians. Here, they found that the intervention group had higher HRQOL, were less likely to visit the emergency department, and remained on chemotherapy for a longer period of time. Interestingly, the benefits from PROs were greater among participants who lacked prior computer experience vs. those who were computer savvy. Basch et al9 also noted that the use of PROs extended survival by 5 months when compared with the control group (31.2 vs. 26.0 mo; P = .03). Longitudinal symptom reporting among IBS patients using PROs, when implemented well, may similarly lead to improved patient satisfaction, HRQOL, and clinical outcomes.
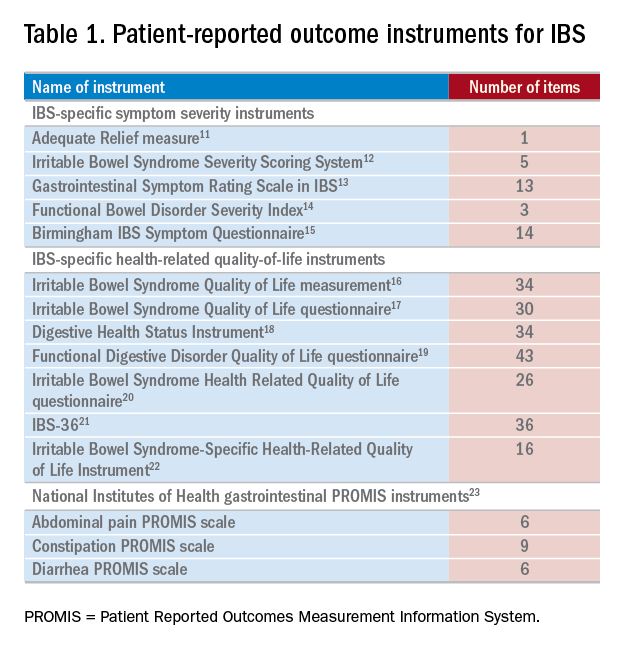
Measuring PROs in the clinical trenches
PROs generally are measured with patient questionnaires that collect data across several areas, including physical, social, and psychological functioning. Although PROs may enhance the patient–provider relationship and improve communication and shared decision making, we acknowledge that there are important barriers to its use in routine clinical practice.10 First, many providers (and their patients) may find use of PRO instruments burdensome, and it can be time consuming to collect PROs from patients and securely transmit the data into the electronic health record (EHR). This can make it untenable for use in busy practices. Second, many gastroenterologists have not received formal training in performing complete biopsychosocial evaluations with PROs, and it can be difficult to understand and act upon PRO scores. Third, there are many PROs to choose from and there is a lack of measurement standards across questionnaires. These challenges limit widespread use of PROs in clinical practice, and it is understandable why most providers instead opt for informal measurement of symptoms and function. Later, we detail strategies for overcoming the earlier-described challenges in employing PROs in everyday practice along with relevant IBS PRO instruments.
IBS–specific PRO instruments
There have been several IBS-specific PRO instruments described in the literature, all of which vary in length, content, and amount of data supporting their validity (Table 1). Examples of IBS symptoms scales include the Adequate Relief measure, Irritable Bowel Syndrome Severity Scoring System, Gastrointestinal Symptom Rating Scale in IBS, Functional Bowel Disorder Severity Index, IBS Symptom Questionnaire, and Birmingham IBS Symptom Questionnaire.15,24 There are also IBS-specific HRQOL instruments, such as the Irritable Bowel Syndrome Quality of Life measurement, Digestive Health Status Instrument, Functional Digestive Disorder Quality of Life questionnaire, Irritable Bowel Syndrome Health Related Quality of Life questionnaire, and IBS-36, among others.21,24
Bijkerk et al24 evaluated and compared the validity and appropriateness of both the symptom and QOL scales. Among the examined IBS symptom instruments, they found that the Adequate Relief question (Did you have adequate relief of IBS-related abdominal pain or discomfort?) is the best choice for assessing global symptomatology, and the Irritable Bowel Syndrome Severity Scoring System is optimal for obtaining information on more specific symptoms.24 As for the QOL scales, the Irritable Bowel Syndrome Quality of Life measurement, which comprises 34 items, is the preferred instrument for assessing changes in HRQOL because it is the most extensively validated.24 Bijkerk et al24 also concluded that although the studied instruments showed reasonable psychometric and methodologic qualities, the use of these instruments in daily clinical practice is debatable because the measures (save for the Adequate Relief question) are lengthy and/or cumbersome to use. Because these instruments may not be practical for use during everyday care, this leads to a discussion of the National Institutes of Health (NIH) Patient Reported Outcomes Measurement Information System (PROMIS), a novel approach to measuring PROs in the clinical trenches.
NIH PROMIS
Although there have been many efforts to implement PROs in routine clinical care, a recent confluence of scientific, regulatory, and political factors, coupled with technological advancements in PRO measurement techniques, have justified re-evaluation of the use of PROs in everyday practice. In response to the practical and technical challenges to employing PROs in the clinical trenches as described earlier, the NIH PROMIS (www.healthmeasures.net) was created in 2004 with the goal of developing and validating a toolbox of PROs that cover the breadth and depth of the human health and illness experience. The PROMIS initiative also was borne from the realization that patients are the ultimate consumers of health care and are the final judge on whether their health care needs are being addressed adequately.
By using modern psychometric techniques, such as item response theory and computerized adaptive testing, PROMIS offers state-of-the-art psychometrics, establishes common-language benchmarks for symptoms across conditions, and identifies clinical thresholds for action and meaningful clinical improvement or decline. PROMIS questionnaires, in light of accelerated EHR adoption in recent years, also are designed to be administered electronically and efficiently, allowing implementation in busy clinical settings. As of December 2017, these instruments can be administered and scored through EHRs such as Epic (Epic Systems, Verona, WI) and Cerner (Cerner Corporation, North Kansas City, MO), the PROMIS iPad (Apple Inc, Cupertino, CA) App, and online data collection tools such as the Assessment Center (www.assessmentcenter.net) and REDCap (Research Electronic Data Capture; Vanderbilt University, Nashville, TN).25 An increasing number of health systems are making PROMIS measures available through their EHRs. For example, the University of Rochester Medical Center collects PROMIS scores for physical function, pain interference, and depression from more than 80% of their patients with in-clinic testing, and individual departments are able to further tailor their administered questionnaires.26
Gastrointestinal PROs measurement information system scales
Because of the extraordinary burden of illness from digestive diseases, the PROMIS consortium added a GI item bank, which our research group developed.23 By using the NIH PROMIS framework, we constructed and validated eight GI PROMIS symptom scales: abdominal pain, bloating/gas, constipation, diarrhea, bowel incontinence, dysphagia, heartburn/reflux, and nausea/vomiting.23 GI PROMIS was designed from the outset to not be a disease-targeted item bank (e.g., IBS-, cirrhosis-, or inflammatory bowel disease specific), but rather symptom targeted, measuring the physical symptoms of the GI tract, because it is more useful across the population as a whole. In Supplementary Figure 1 (at https://doi.org/10.1016/j.cgh.2017.12.026), we include the abdominal pain, constipation, and diarrhea PROMIS scales because they form the cardinal symptoms of IBS.
GI PROMIS scales are readily accessible via the Assessment Center,25 and we also have made them freely available via MyGiHealth — an iOS (Apple Inc) and online app (go.mygihealth.io) endorsed by the American Gastroenterological Association. The patient’s responses to the questionnaires are converted to percentile scores and compared with the general U.S. population, and then displayed in a symptom heat map. The app also allows users to track GI PROMIS scores longitudinally, empowering IBS patients (and any patient with GI symptoms for that matter) and their providers to see if they are objectively responding to prescribed therapies and potentially improving satisfaction and patient–provider communication.
Conclusions
IBS is a common, chronic, relapsing disease that often leads to physical, mental, and social distress. Without objective measurable biomarkers to assess IBS patients’ GI symptom burden, along with health care’s increased emphasis on patient-centered care, it becomes important to accurately capture a patient’s illness experience with PROs. A number of IBS symptom and QOL PRO instruments have been described in the literature, but most are beset by lengthy completion times and are impractical for use in everyday care. GI PROMIS, on the other hand, is a versatile and efficient instrument for collecting PRO data from not only IBS patients, but also all those who seek care in our GI clinics. Improvements in PRO and implementation science combined with technological advances have lessened the barriers to employing PROs in routine clinical care, and an increasing number of institutions are beginning to take up this challenge. In doing so and by seamlessly incorporating PROs in clinical practice, it facilitates placement of our patients’ voices at the forefront of their health care, changes how we monitor and manage patients, and, ultimately, may improve patient satisfaction and clinical outcomes.
Content from this column was originally published in the “Practice Management: The Road Ahead” section of Clinical Gastroenterology and Hepatology (2018;16:462-6).
References
1. Detmar S.B., Muller M.J., Schornagel J.H., et al. Role of health-related quality of life in palliative chemotherapy treatment decisions. J Clin Oncol. 2002;20:1056-62.
2. Detmar S.B., Muller M.J., Schornagel J.H., et al. Health-related quality-of-life assessments and patient-physician communication: a randomized controlled trial. JAMA. 2002;288:3027-34.
3. Neumann M., Edelhauser F., Kreps G.L., et al. Can patient-provider interaction increase the effectiveness of medical treatment or even substitute it? An exploration on why and how to study the specific effect of the provider. Patient Educ Couns. 2010;80:307-14.
4. Lembo A.J. The clinical and economic burden of irritable bowel syndrome. Pract Gastroenterol. 2007;31:3-9.
5. Valderas J.M., Kotzeva A., Espallargues M., et al. The impact of measuring patient-reported outcomes in clinical practice: a systematic review of the literature. Qual Life Res. 2008;17:179-93.
6. Almario C.V., Chey W.D., Khanna D., et al. Impact of National Institutes of Health gastrointestinal PROMIS measures in clinical practice: results of a multicenter controlled trial. Am J Gastroenterol. 2016;111:1546-56.
7. Basch E., Deal A.M., Kris M.G., et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34:557-65.
8. Kotronoulas G., Kearney N., Maguire R., et al. What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. J Clin Oncol. 2014;32:1480-501.
9. Basch E., Deal A.M., Dueck A.C., et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318:197-8.
10. Spiegel B.M. Patient-reported outcomes in gastroenterology: clinical and research applications. J Neurogastroenterol Motil. 2013;19:137-48.
11. Mangel A.W., Hahn B.A., Heath A.T., et al. Adequate relief as an endpoint in clinical trials in irritable bowel syndrome. J Int Med Res. 1998;26:76-81.
12. Francis C.Y., Morris J., Whorwell P.J. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395-402.
13. Wiklund I.K., Fullerton S., Hawkey C.J., et al. An irritable bowel syndrome-specific symptom questionnaire: development and validation. Scand J Gastroenterol. 2003;38:947-54.
14. Drossman D.A., Li Z., Toner B.B., et al. Functional bowel disorders. A multicenter comparison of health status and development of illness severity index. Dig Dis Sci. 1995;40:986-95.
15. Roalfe A.K., Roberts L.M., Wilson S. Evaluation of the Birmingham IBS symptom questionnaire. BMC Gastroenterol. 2008;8:30.
16. Patrick D.L., Drossman D.A., Frederick I.O., et al. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43:400-11.
17. Hahn B.A., Kirchdoerfer L.J., Fullerton S., et al. Evaluation of a new quality of life questionnaire for patients with irritable bowel syndrome. Aliment Pharmacol Ther. 1997;11:547-52.
18. Shaw M., Talley N.J., Adlis S., et al. Development of a digestive health status instrument: tests of scaling assumptions, structure and reliability in a primary care population. Aliment Pharmacol Ther. 1998;12:1067-78.
19. Chassany O., Marquis P., Scherrer B., et al. Validation of a specific quality of life questionnaire for functional digestive disorders. Gut. 1999;44:527-33.
20. Wong E., Guyatt G.H., Cook D.J., et al. Development of a questionnaire to measure quality of life in patients with irritable bowel syndrome. Eur J Surg Suppl. 1998;583:50-6.
21. Groll D., Vanner S.J., Depew W.T., et al. The IBS-36: a new quality of life measure for irritable bowel syndrome. Am J Gastroenterol. 2002;97:962-71.
22. Lee E.H., Kwon O., Hahm K.B., et al. Irritable bowel syndrome-specific health-related quality of life instrument: development and psychometric evaluation. Health Qual Life Outcomes. 2016;14:22.
23. Spiegel B.M., Hays R.D., Bolus R., et al. Development of the NIH Patient-Reported Outcomes Measurement Information System (PROMIS) gastrointestinal symptom scales. Am J Gastroenterol. 2014;109:1804-14.
24. Bijkerk C.J., de Wit N.J., Muris J.W., et al. Outcome measures in irritable bowel syndrome: comparison of psychometric and methodological characteristics. Am J Gastroenterol. 2003;98:122-7.
25. HealthMeasures. Data collection tools. 2017. Available from http://www.healthmeasures.net/resource-center/data-collection-tools. Accessed August 20, 2017.
26. Baumhauer J.F. Patient-reported outcomes – are they living up to their potential?. N Engl J Med. 2017;377:6-9.
Dr. Almario is assistant professor-in-residence of medicine and public health, division of digestive and liver diseases, Dr. Spiegel professor-in-residence of medicine and public health, division of digestive and liver diseases, Cedars-Sinai Medical Center; Cedars-Sinai Center for Outcomes Research and Education, Los Angeles. Dr. Spiegel is a principal at My Total Health, which operates and maintains MyGiHealth; Dr. Almario has a stock option grant in My Total Health.
Patients often seek care because they experience symptoms that negatively affect their health-related quality of life (HRQOL). Health care providers must then elicit, measure, and interpret patient symptoms as part of their clinical evaluation. To assist with this goal and to help bridge the gap between patients and providers, investigators have developed and validated a wide range of patient-reported outcomes (PROs) across the breadth and depth of the human health and illness experience. These PROs, which measure any aspect of a patient’s biopsychosocial health and come directly from the patient, may help direct care and improve outcomes. When PROs are collected systematically, efficiently, and in the right place at the right time, they may enhance the patient–provider relationship by improving communication and facilitating shared decision making.1-3
Within gastroenterology and hepatology, PROs have been developed for a number of conditions, including irritable bowel syndrome (IBS), chronic idiopathic constipation, cirrhosis, eosinophilic esophagitis, inflammatory bowel disease, and gastroesophageal reflux disease, among many other chronic diseases. IBS in particular is well suited for PRO measurement because it is symptom based and significantly impacts patients’ HRQOL and emotional health. Moreover, it is the most commonly diagnosed gastrointestinal condition and imparts a significant economic burden. In this article, we review the rationale for measuring IBS PROs in routine clinical practice and detail available measurement instruments.
Importance of IBS PROs in clinical practice
IBS is a functional GI disorder that is characterized by recurrent abdominal pain and altered bowel habits (i.e., diarrhea, constipation, or a mix of both). It has an estimated worldwide prevalence of 11%, and total costs are estimated at $30 billion annually in the United States alone.4 Because of the chronic relapsing nature of IBS, along with its impact on physical, mental, and social distress, it becomes important to accurately capture a patient’s illness experience with PROs. This is especially relevant to patients with IBS because we currently lack objective measurable biomarkers to assess their GI symptom burden. Instead, clinicians often are relegated to informal assessments of the severity of a patient’s symptoms, which ultimately guide their treatment recommendations: How many bowel movements have you had in the past week? Were your bowel movements hard or soft? How bad is your abdominal pain on a scale of 1 to 10? However, these traditional outcomes measured by health care providers often fail to capture other aspects of their health. For example, simply asking patients about the frequency and character of their stools will not provide any insight into how their symptoms impact their HRQOL and psychosocial health. An individual may report only two loose stools per day, but this may lead to substantial anxiety and negatively affect his or her performance at work. Similarly, the significance of the symptoms will vary from person to person; a patient with IBS who has five loose daily bowel movements may not be bothered by it, whereas another individual with three loose stools per day may feel that it severely hampers his or her daily activities. This is where PROs provide value because they provide a key component to understanding the true burden of IBS, and accounts for the HRQOL and psychosocial decrement engendered by the disease.
Although past literature has reported inconsistent benefits of using PROs in clinical practice,5 including that from our own work,6 there is a growing number of studies that have noted improved clinical outcomes through employing PROs.7-9 A compelling example is provided by Basch et al,7 who randomized patients receiving chemotherapy for metastatic cancer to either weekly symptom reporting using electronically delivered PRO instruments vs. usual care, which consisted of symptom reporting at the discretion of clinicians. Here, they found that the intervention group had higher HRQOL, were less likely to visit the emergency department, and remained on chemotherapy for a longer period of time. Interestingly, the benefits from PROs were greater among participants who lacked prior computer experience vs. those who were computer savvy. Basch et al9 also noted that the use of PROs extended survival by 5 months when compared with the control group (31.2 vs. 26.0 mo; P = .03). Longitudinal symptom reporting among IBS patients using PROs, when implemented well, may similarly lead to improved patient satisfaction, HRQOL, and clinical outcomes.

Measuring PROs in the clinical trenches
PROs generally are measured with patient questionnaires that collect data across several areas, including physical, social, and psychological functioning. Although PROs may enhance the patient–provider relationship and improve communication and shared decision making, we acknowledge that there are important barriers to its use in routine clinical practice.10 First, many providers (and their patients) may find use of PRO instruments burdensome, and it can be time consuming to collect PROs from patients and securely transmit the data into the electronic health record (EHR). This can make it untenable for use in busy practices. Second, many gastroenterologists have not received formal training in performing complete biopsychosocial evaluations with PROs, and it can be difficult to understand and act upon PRO scores. Third, there are many PROs to choose from and there is a lack of measurement standards across questionnaires. These challenges limit widespread use of PROs in clinical practice, and it is understandable why most providers instead opt for informal measurement of symptoms and function. Later, we detail strategies for overcoming the earlier-described challenges in employing PROs in everyday practice along with relevant IBS PRO instruments.
IBS–specific PRO instruments
There have been several IBS-specific PRO instruments described in the literature, all of which vary in length, content, and amount of data supporting their validity (Table 1). Examples of IBS symptoms scales include the Adequate Relief measure, Irritable Bowel Syndrome Severity Scoring System, Gastrointestinal Symptom Rating Scale in IBS, Functional Bowel Disorder Severity Index, IBS Symptom Questionnaire, and Birmingham IBS Symptom Questionnaire.15,24 There are also IBS-specific HRQOL instruments, such as the Irritable Bowel Syndrome Quality of Life measurement, Digestive Health Status Instrument, Functional Digestive Disorder Quality of Life questionnaire, Irritable Bowel Syndrome Health Related Quality of Life questionnaire, and IBS-36, among others.21,24
Bijkerk et al24 evaluated and compared the validity and appropriateness of both the symptom and QOL scales. Among the examined IBS symptom instruments, they found that the Adequate Relief question (Did you have adequate relief of IBS-related abdominal pain or discomfort?) is the best choice for assessing global symptomatology, and the Irritable Bowel Syndrome Severity Scoring System is optimal for obtaining information on more specific symptoms.24 As for the QOL scales, the Irritable Bowel Syndrome Quality of Life measurement, which comprises 34 items, is the preferred instrument for assessing changes in HRQOL because it is the most extensively validated.24 Bijkerk et al24 also concluded that although the studied instruments showed reasonable psychometric and methodologic qualities, the use of these instruments in daily clinical practice is debatable because the measures (save for the Adequate Relief question) are lengthy and/or cumbersome to use. Because these instruments may not be practical for use during everyday care, this leads to a discussion of the National Institutes of Health (NIH) Patient Reported Outcomes Measurement Information System (PROMIS), a novel approach to measuring PROs in the clinical trenches.
NIH PROMIS
Although there have been many efforts to implement PROs in routine clinical care, a recent confluence of scientific, regulatory, and political factors, coupled with technological advancements in PRO measurement techniques, have justified re-evaluation of the use of PROs in everyday practice. In response to the practical and technical challenges to employing PROs in the clinical trenches as described earlier, the NIH PROMIS (www.healthmeasures.net) was created in 2004 with the goal of developing and validating a toolbox of PROs that cover the breadth and depth of the human health and illness experience. The PROMIS initiative also was borne from the realization that patients are the ultimate consumers of health care and are the final judge on whether their health care needs are being addressed adequately.
By using modern psychometric techniques, such as item response theory and computerized adaptive testing, PROMIS offers state-of-the-art psychometrics, establishes common-language benchmarks for symptoms across conditions, and identifies clinical thresholds for action and meaningful clinical improvement or decline. PROMIS questionnaires, in light of accelerated EHR adoption in recent years, also are designed to be administered electronically and efficiently, allowing implementation in busy clinical settings. As of December 2017, these instruments can be administered and scored through EHRs such as Epic (Epic Systems, Verona, WI) and Cerner (Cerner Corporation, North Kansas City, MO), the PROMIS iPad (Apple Inc, Cupertino, CA) App, and online data collection tools such as the Assessment Center (www.assessmentcenter.net) and REDCap (Research Electronic Data Capture; Vanderbilt University, Nashville, TN).25 An increasing number of health systems are making PROMIS measures available through their EHRs. For example, the University of Rochester Medical Center collects PROMIS scores for physical function, pain interference, and depression from more than 80% of their patients with in-clinic testing, and individual departments are able to further tailor their administered questionnaires.26
Gastrointestinal PROs measurement information system scales
Because of the extraordinary burden of illness from digestive diseases, the PROMIS consortium added a GI item bank, which our research group developed.23 By using the NIH PROMIS framework, we constructed and validated eight GI PROMIS symptom scales: abdominal pain, bloating/gas, constipation, diarrhea, bowel incontinence, dysphagia, heartburn/reflux, and nausea/vomiting.23 GI PROMIS was designed from the outset to not be a disease-targeted item bank (e.g., IBS-, cirrhosis-, or inflammatory bowel disease specific), but rather symptom targeted, measuring the physical symptoms of the GI tract, because it is more useful across the population as a whole. In Supplementary Figure 1 (at https://doi.org/10.1016/j.cgh.2017.12.026), we include the abdominal pain, constipation, and diarrhea PROMIS scales because they form the cardinal symptoms of IBS.
GI PROMIS scales are readily accessible via the Assessment Center,25 and we also have made them freely available via MyGiHealth — an iOS (Apple Inc) and online app (go.mygihealth.io) endorsed by the American Gastroenterological Association. The patient’s responses to the questionnaires are converted to percentile scores and compared with the general U.S. population, and then displayed in a symptom heat map. The app also allows users to track GI PROMIS scores longitudinally, empowering IBS patients (and any patient with GI symptoms for that matter) and their providers to see if they are objectively responding to prescribed therapies and potentially improving satisfaction and patient–provider communication.
Conclusions
IBS is a common, chronic, relapsing disease that often leads to physical, mental, and social distress. Without objective measurable biomarkers to assess IBS patients’ GI symptom burden, along with health care’s increased emphasis on patient-centered care, it becomes important to accurately capture a patient’s illness experience with PROs. A number of IBS symptom and QOL PRO instruments have been described in the literature, but most are beset by lengthy completion times and are impractical for use in everyday care. GI PROMIS, on the other hand, is a versatile and efficient instrument for collecting PRO data from not only IBS patients, but also all those who seek care in our GI clinics. Improvements in PRO and implementation science combined with technological advances have lessened the barriers to employing PROs in routine clinical care, and an increasing number of institutions are beginning to take up this challenge. In doing so and by seamlessly incorporating PROs in clinical practice, it facilitates placement of our patients’ voices at the forefront of their health care, changes how we monitor and manage patients, and, ultimately, may improve patient satisfaction and clinical outcomes.
Content from this column was originally published in the “Practice Management: The Road Ahead” section of Clinical Gastroenterology and Hepatology (2018;16:462-6).
References
1. Detmar S.B., Muller M.J., Schornagel J.H., et al. Role of health-related quality of life in palliative chemotherapy treatment decisions. J Clin Oncol. 2002;20:1056-62.
2. Detmar S.B., Muller M.J., Schornagel J.H., et al. Health-related quality-of-life assessments and patient-physician communication: a randomized controlled trial. JAMA. 2002;288:3027-34.
3. Neumann M., Edelhauser F., Kreps G.L., et al. Can patient-provider interaction increase the effectiveness of medical treatment or even substitute it? An exploration on why and how to study the specific effect of the provider. Patient Educ Couns. 2010;80:307-14.
4. Lembo A.J. The clinical and economic burden of irritable bowel syndrome. Pract Gastroenterol. 2007;31:3-9.
5. Valderas J.M., Kotzeva A., Espallargues M., et al. The impact of measuring patient-reported outcomes in clinical practice: a systematic review of the literature. Qual Life Res. 2008;17:179-93.
6. Almario C.V., Chey W.D., Khanna D., et al. Impact of National Institutes of Health gastrointestinal PROMIS measures in clinical practice: results of a multicenter controlled trial. Am J Gastroenterol. 2016;111:1546-56.
7. Basch E., Deal A.M., Kris M.G., et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34:557-65.
8. Kotronoulas G., Kearney N., Maguire R., et al. What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. J Clin Oncol. 2014;32:1480-501.
9. Basch E., Deal A.M., Dueck A.C., et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318:197-8.
10. Spiegel B.M. Patient-reported outcomes in gastroenterology: clinical and research applications. J Neurogastroenterol Motil. 2013;19:137-48.
11. Mangel A.W., Hahn B.A., Heath A.T., et al. Adequate relief as an endpoint in clinical trials in irritable bowel syndrome. J Int Med Res. 1998;26:76-81.
12. Francis C.Y., Morris J., Whorwell P.J. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395-402.
13. Wiklund I.K., Fullerton S., Hawkey C.J., et al. An irritable bowel syndrome-specific symptom questionnaire: development and validation. Scand J Gastroenterol. 2003;38:947-54.
14. Drossman D.A., Li Z., Toner B.B., et al. Functional bowel disorders. A multicenter comparison of health status and development of illness severity index. Dig Dis Sci. 1995;40:986-95.
15. Roalfe A.K., Roberts L.M., Wilson S. Evaluation of the Birmingham IBS symptom questionnaire. BMC Gastroenterol. 2008;8:30.
16. Patrick D.L., Drossman D.A., Frederick I.O., et al. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43:400-11.
17. Hahn B.A., Kirchdoerfer L.J., Fullerton S., et al. Evaluation of a new quality of life questionnaire for patients with irritable bowel syndrome. Aliment Pharmacol Ther. 1997;11:547-52.
18. Shaw M., Talley N.J., Adlis S., et al. Development of a digestive health status instrument: tests of scaling assumptions, structure and reliability in a primary care population. Aliment Pharmacol Ther. 1998;12:1067-78.
19. Chassany O., Marquis P., Scherrer B., et al. Validation of a specific quality of life questionnaire for functional digestive disorders. Gut. 1999;44:527-33.
20. Wong E., Guyatt G.H., Cook D.J., et al. Development of a questionnaire to measure quality of life in patients with irritable bowel syndrome. Eur J Surg Suppl. 1998;583:50-6.
21. Groll D., Vanner S.J., Depew W.T., et al. The IBS-36: a new quality of life measure for irritable bowel syndrome. Am J Gastroenterol. 2002;97:962-71.
22. Lee E.H., Kwon O., Hahm K.B., et al. Irritable bowel syndrome-specific health-related quality of life instrument: development and psychometric evaluation. Health Qual Life Outcomes. 2016;14:22.
23. Spiegel B.M., Hays R.D., Bolus R., et al. Development of the NIH Patient-Reported Outcomes Measurement Information System (PROMIS) gastrointestinal symptom scales. Am J Gastroenterol. 2014;109:1804-14.
24. Bijkerk C.J., de Wit N.J., Muris J.W., et al. Outcome measures in irritable bowel syndrome: comparison of psychometric and methodological characteristics. Am J Gastroenterol. 2003;98:122-7.
25. HealthMeasures. Data collection tools. 2017. Available from http://www.healthmeasures.net/resource-center/data-collection-tools. Accessed August 20, 2017.
26. Baumhauer J.F. Patient-reported outcomes – are they living up to their potential?. N Engl J Med. 2017;377:6-9.
Dr. Almario is assistant professor-in-residence of medicine and public health, division of digestive and liver diseases, Dr. Spiegel professor-in-residence of medicine and public health, division of digestive and liver diseases, Cedars-Sinai Medical Center; Cedars-Sinai Center for Outcomes Research and Education, Los Angeles. Dr. Spiegel is a principal at My Total Health, which operates and maintains MyGiHealth; Dr. Almario has a stock option grant in My Total Health.
Patients often seek care because they experience symptoms that negatively affect their health-related quality of life (HRQOL). Health care providers must then elicit, measure, and interpret patient symptoms as part of their clinical evaluation. To assist with this goal and to help bridge the gap between patients and providers, investigators have developed and validated a wide range of patient-reported outcomes (PROs) across the breadth and depth of the human health and illness experience. These PROs, which measure any aspect of a patient’s biopsychosocial health and come directly from the patient, may help direct care and improve outcomes. When PROs are collected systematically, efficiently, and in the right place at the right time, they may enhance the patient–provider relationship by improving communication and facilitating shared decision making.1-3
Within gastroenterology and hepatology, PROs have been developed for a number of conditions, including irritable bowel syndrome (IBS), chronic idiopathic constipation, cirrhosis, eosinophilic esophagitis, inflammatory bowel disease, and gastroesophageal reflux disease, among many other chronic diseases. IBS in particular is well suited for PRO measurement because it is symptom based and significantly impacts patients’ HRQOL and emotional health. Moreover, it is the most commonly diagnosed gastrointestinal condition and imparts a significant economic burden. In this article, we review the rationale for measuring IBS PROs in routine clinical practice and detail available measurement instruments.
Importance of IBS PROs in clinical practice
IBS is a functional GI disorder that is characterized by recurrent abdominal pain and altered bowel habits (i.e., diarrhea, constipation, or a mix of both). It has an estimated worldwide prevalence of 11%, and total costs are estimated at $30 billion annually in the United States alone.4 Because of the chronic relapsing nature of IBS, along with its impact on physical, mental, and social distress, it becomes important to accurately capture a patient’s illness experience with PROs. This is especially relevant to patients with IBS because we currently lack objective measurable biomarkers to assess their GI symptom burden. Instead, clinicians often are relegated to informal assessments of the severity of a patient’s symptoms, which ultimately guide their treatment recommendations: How many bowel movements have you had in the past week? Were your bowel movements hard or soft? How bad is your abdominal pain on a scale of 1 to 10? However, these traditional outcomes measured by health care providers often fail to capture other aspects of their health. For example, simply asking patients about the frequency and character of their stools will not provide any insight into how their symptoms impact their HRQOL and psychosocial health. An individual may report only two loose stools per day, but this may lead to substantial anxiety and negatively affect his or her performance at work. Similarly, the significance of the symptoms will vary from person to person; a patient with IBS who has five loose daily bowel movements may not be bothered by it, whereas another individual with three loose stools per day may feel that it severely hampers his or her daily activities. This is where PROs provide value because they provide a key component to understanding the true burden of IBS, and accounts for the HRQOL and psychosocial decrement engendered by the disease.
Although past literature has reported inconsistent benefits of using PROs in clinical practice,5 including that from our own work,6 there is a growing number of studies that have noted improved clinical outcomes through employing PROs.7-9 A compelling example is provided by Basch et al,7 who randomized patients receiving chemotherapy for metastatic cancer to either weekly symptom reporting using electronically delivered PRO instruments vs. usual care, which consisted of symptom reporting at the discretion of clinicians. Here, they found that the intervention group had higher HRQOL, were less likely to visit the emergency department, and remained on chemotherapy for a longer period of time. Interestingly, the benefits from PROs were greater among participants who lacked prior computer experience vs. those who were computer savvy. Basch et al9 also noted that the use of PROs extended survival by 5 months when compared with the control group (31.2 vs. 26.0 mo; P = .03). Longitudinal symptom reporting among IBS patients using PROs, when implemented well, may similarly lead to improved patient satisfaction, HRQOL, and clinical outcomes.

Measuring PROs in the clinical trenches
PROs generally are measured with patient questionnaires that collect data across several areas, including physical, social, and psychological functioning. Although PROs may enhance the patient–provider relationship and improve communication and shared decision making, we acknowledge that there are important barriers to its use in routine clinical practice.10 First, many providers (and their patients) may find use of PRO instruments burdensome, and it can be time consuming to collect PROs from patients and securely transmit the data into the electronic health record (EHR). This can make it untenable for use in busy practices. Second, many gastroenterologists have not received formal training in performing complete biopsychosocial evaluations with PROs, and it can be difficult to understand and act upon PRO scores. Third, there are many PROs to choose from and there is a lack of measurement standards across questionnaires. These challenges limit widespread use of PROs in clinical practice, and it is understandable why most providers instead opt for informal measurement of symptoms and function. Later, we detail strategies for overcoming the earlier-described challenges in employing PROs in everyday practice along with relevant IBS PRO instruments.
IBS–specific PRO instruments
There have been several IBS-specific PRO instruments described in the literature, all of which vary in length, content, and amount of data supporting their validity (Table 1). Examples of IBS symptoms scales include the Adequate Relief measure, Irritable Bowel Syndrome Severity Scoring System, Gastrointestinal Symptom Rating Scale in IBS, Functional Bowel Disorder Severity Index, IBS Symptom Questionnaire, and Birmingham IBS Symptom Questionnaire.15,24 There are also IBS-specific HRQOL instruments, such as the Irritable Bowel Syndrome Quality of Life measurement, Digestive Health Status Instrument, Functional Digestive Disorder Quality of Life questionnaire, Irritable Bowel Syndrome Health Related Quality of Life questionnaire, and IBS-36, among others.21,24
Bijkerk et al24 evaluated and compared the validity and appropriateness of both the symptom and QOL scales. Among the examined IBS symptom instruments, they found that the Adequate Relief question (Did you have adequate relief of IBS-related abdominal pain or discomfort?) is the best choice for assessing global symptomatology, and the Irritable Bowel Syndrome Severity Scoring System is optimal for obtaining information on more specific symptoms.24 As for the QOL scales, the Irritable Bowel Syndrome Quality of Life measurement, which comprises 34 items, is the preferred instrument for assessing changes in HRQOL because it is the most extensively validated.24 Bijkerk et al24 also concluded that although the studied instruments showed reasonable psychometric and methodologic qualities, the use of these instruments in daily clinical practice is debatable because the measures (save for the Adequate Relief question) are lengthy and/or cumbersome to use. Because these instruments may not be practical for use during everyday care, this leads to a discussion of the National Institutes of Health (NIH) Patient Reported Outcomes Measurement Information System (PROMIS), a novel approach to measuring PROs in the clinical trenches.
NIH PROMIS
Although there have been many efforts to implement PROs in routine clinical care, a recent confluence of scientific, regulatory, and political factors, coupled with technological advancements in PRO measurement techniques, have justified re-evaluation of the use of PROs in everyday practice. In response to the practical and technical challenges to employing PROs in the clinical trenches as described earlier, the NIH PROMIS (www.healthmeasures.net) was created in 2004 with the goal of developing and validating a toolbox of PROs that cover the breadth and depth of the human health and illness experience. The PROMIS initiative also was borne from the realization that patients are the ultimate consumers of health care and are the final judge on whether their health care needs are being addressed adequately.
By using modern psychometric techniques, such as item response theory and computerized adaptive testing, PROMIS offers state-of-the-art psychometrics, establishes common-language benchmarks for symptoms across conditions, and identifies clinical thresholds for action and meaningful clinical improvement or decline. PROMIS questionnaires, in light of accelerated EHR adoption in recent years, also are designed to be administered electronically and efficiently, allowing implementation in busy clinical settings. As of December 2017, these instruments can be administered and scored through EHRs such as Epic (Epic Systems, Verona, WI) and Cerner (Cerner Corporation, North Kansas City, MO), the PROMIS iPad (Apple Inc, Cupertino, CA) App, and online data collection tools such as the Assessment Center (www.assessmentcenter.net) and REDCap (Research Electronic Data Capture; Vanderbilt University, Nashville, TN).25 An increasing number of health systems are making PROMIS measures available through their EHRs. For example, the University of Rochester Medical Center collects PROMIS scores for physical function, pain interference, and depression from more than 80% of their patients with in-clinic testing, and individual departments are able to further tailor their administered questionnaires.26
Gastrointestinal PROs measurement information system scales
Because of the extraordinary burden of illness from digestive diseases, the PROMIS consortium added a GI item bank, which our research group developed.23 By using the NIH PROMIS framework, we constructed and validated eight GI PROMIS symptom scales: abdominal pain, bloating/gas, constipation, diarrhea, bowel incontinence, dysphagia, heartburn/reflux, and nausea/vomiting.23 GI PROMIS was designed from the outset to not be a disease-targeted item bank (e.g., IBS-, cirrhosis-, or inflammatory bowel disease specific), but rather symptom targeted, measuring the physical symptoms of the GI tract, because it is more useful across the population as a whole. In Supplementary Figure 1 (at https://doi.org/10.1016/j.cgh.2017.12.026), we include the abdominal pain, constipation, and diarrhea PROMIS scales because they form the cardinal symptoms of IBS.
GI PROMIS scales are readily accessible via the Assessment Center,25 and we also have made them freely available via MyGiHealth — an iOS (Apple Inc) and online app (go.mygihealth.io) endorsed by the American Gastroenterological Association. The patient’s responses to the questionnaires are converted to percentile scores and compared with the general U.S. population, and then displayed in a symptom heat map. The app also allows users to track GI PROMIS scores longitudinally, empowering IBS patients (and any patient with GI symptoms for that matter) and their providers to see if they are objectively responding to prescribed therapies and potentially improving satisfaction and patient–provider communication.
Conclusions
IBS is a common, chronic, relapsing disease that often leads to physical, mental, and social distress. Without objective measurable biomarkers to assess IBS patients’ GI symptom burden, along with health care’s increased emphasis on patient-centered care, it becomes important to accurately capture a patient’s illness experience with PROs. A number of IBS symptom and QOL PRO instruments have been described in the literature, but most are beset by lengthy completion times and are impractical for use in everyday care. GI PROMIS, on the other hand, is a versatile and efficient instrument for collecting PRO data from not only IBS patients, but also all those who seek care in our GI clinics. Improvements in PRO and implementation science combined with technological advances have lessened the barriers to employing PROs in routine clinical care, and an increasing number of institutions are beginning to take up this challenge. In doing so and by seamlessly incorporating PROs in clinical practice, it facilitates placement of our patients’ voices at the forefront of their health care, changes how we monitor and manage patients, and, ultimately, may improve patient satisfaction and clinical outcomes.
Content from this column was originally published in the “Practice Management: The Road Ahead” section of Clinical Gastroenterology and Hepatology (2018;16:462-6).
References
1. Detmar S.B., Muller M.J., Schornagel J.H., et al. Role of health-related quality of life in palliative chemotherapy treatment decisions. J Clin Oncol. 2002;20:1056-62.
2. Detmar S.B., Muller M.J., Schornagel J.H., et al. Health-related quality-of-life assessments and patient-physician communication: a randomized controlled trial. JAMA. 2002;288:3027-34.
3. Neumann M., Edelhauser F., Kreps G.L., et al. Can patient-provider interaction increase the effectiveness of medical treatment or even substitute it? An exploration on why and how to study the specific effect of the provider. Patient Educ Couns. 2010;80:307-14.
4. Lembo A.J. The clinical and economic burden of irritable bowel syndrome. Pract Gastroenterol. 2007;31:3-9.
5. Valderas J.M., Kotzeva A., Espallargues M., et al. The impact of measuring patient-reported outcomes in clinical practice: a systematic review of the literature. Qual Life Res. 2008;17:179-93.
6. Almario C.V., Chey W.D., Khanna D., et al. Impact of National Institutes of Health gastrointestinal PROMIS measures in clinical practice: results of a multicenter controlled trial. Am J Gastroenterol. 2016;111:1546-56.
7. Basch E., Deal A.M., Kris M.G., et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34:557-65.
8. Kotronoulas G., Kearney N., Maguire R., et al. What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. J Clin Oncol. 2014;32:1480-501.
9. Basch E., Deal A.M., Dueck A.C., et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318:197-8.
10. Spiegel B.M. Patient-reported outcomes in gastroenterology: clinical and research applications. J Neurogastroenterol Motil. 2013;19:137-48.
11. Mangel A.W., Hahn B.A., Heath A.T., et al. Adequate relief as an endpoint in clinical trials in irritable bowel syndrome. J Int Med Res. 1998;26:76-81.
12. Francis C.Y., Morris J., Whorwell P.J. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395-402.
13. Wiklund I.K., Fullerton S., Hawkey C.J., et al. An irritable bowel syndrome-specific symptom questionnaire: development and validation. Scand J Gastroenterol. 2003;38:947-54.
14. Drossman D.A., Li Z., Toner B.B., et al. Functional bowel disorders. A multicenter comparison of health status and development of illness severity index. Dig Dis Sci. 1995;40:986-95.
15. Roalfe A.K., Roberts L.M., Wilson S. Evaluation of the Birmingham IBS symptom questionnaire. BMC Gastroenterol. 2008;8:30.
16. Patrick D.L., Drossman D.A., Frederick I.O., et al. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43:400-11.
17. Hahn B.A., Kirchdoerfer L.J., Fullerton S., et al. Evaluation of a new quality of life questionnaire for patients with irritable bowel syndrome. Aliment Pharmacol Ther. 1997;11:547-52.
18. Shaw M., Talley N.J., Adlis S., et al. Development of a digestive health status instrument: tests of scaling assumptions, structure and reliability in a primary care population. Aliment Pharmacol Ther. 1998;12:1067-78.
19. Chassany O., Marquis P., Scherrer B., et al. Validation of a specific quality of life questionnaire for functional digestive disorders. Gut. 1999;44:527-33.
20. Wong E., Guyatt G.H., Cook D.J., et al. Development of a questionnaire to measure quality of life in patients with irritable bowel syndrome. Eur J Surg Suppl. 1998;583:50-6.
21. Groll D., Vanner S.J., Depew W.T., et al. The IBS-36: a new quality of life measure for irritable bowel syndrome. Am J Gastroenterol. 2002;97:962-71.
22. Lee E.H., Kwon O., Hahm K.B., et al. Irritable bowel syndrome-specific health-related quality of life instrument: development and psychometric evaluation. Health Qual Life Outcomes. 2016;14:22.
23. Spiegel B.M., Hays R.D., Bolus R., et al. Development of the NIH Patient-Reported Outcomes Measurement Information System (PROMIS) gastrointestinal symptom scales. Am J Gastroenterol. 2014;109:1804-14.
24. Bijkerk C.J., de Wit N.J., Muris J.W., et al. Outcome measures in irritable bowel syndrome: comparison of psychometric and methodological characteristics. Am J Gastroenterol. 2003;98:122-7.
25. HealthMeasures. Data collection tools. 2017. Available from http://www.healthmeasures.net/resource-center/data-collection-tools. Accessed August 20, 2017.
26. Baumhauer J.F. Patient-reported outcomes – are they living up to their potential?. N Engl J Med. 2017;377:6-9.
Dr. Almario is assistant professor-in-residence of medicine and public health, division of digestive and liver diseases, Dr. Spiegel professor-in-residence of medicine and public health, division of digestive and liver diseases, Cedars-Sinai Medical Center; Cedars-Sinai Center for Outcomes Research and Education, Los Angeles. Dr. Spiegel is a principal at My Total Health, which operates and maintains MyGiHealth; Dr. Almario has a stock option grant in My Total Health.
Mayo Clinic announces new president and CEO: Gianrico Farrugia, MD
The Mayo Clinic Board of Trustees has announced that Gianrico Farrugia, MD, vice president and CEO of Mayo Clinic Florida, will take over as president and CEO of Mayo Clinic at the end of the year. AGA congratulates Dr. Farrugia on this accomplishment.
Here’s three reasons why AGA is excited by this news:
1. Dr. Farrugia is an accomplished GI investigator. Dr. Farrugia runs an NIH-funded translational laboratory focused on disorders of GI motility. The aim of Dr. Farrugia’s work is to understand at a cellular, subcellular and molecular level how the normal functions of the GI tract determine the defects that result in diseases such as diabetic gastroparesis, slow transit constipation, and irritable bowel syndrome (IBS), which will ultimately lead to new strategies to treat these diseases by developing targeted disease-modifying agents.
2. Dr. Farrugia is an alumnus of the AGA Research Foundation Research Scholar Award program. Dr. Farrugia received his Research Scholar Award in 1994 for his project titled “Jejunal Smooth Muscle Ion Channel Regulation in Health and Disease.”
3. Dr. Farrugia has given back to AGA both with his time – serving on the AGA Nominating Committee, AGA Institute Council, and Cellular and Molecular Gastroenterology and Hepatology editorial board – and by contributing, with his wife Geraldine Farrugia, to the AGA Research Foundation at the highest level as an AGA Legacy Society member.
Join AGA members in congratulating Dr. Farrugia in the AGA Community, community.gastro.org.
The Mayo Clinic Board of Trustees has announced that Gianrico Farrugia, MD, vice president and CEO of Mayo Clinic Florida, will take over as president and CEO of Mayo Clinic at the end of the year. AGA congratulates Dr. Farrugia on this accomplishment.
Here’s three reasons why AGA is excited by this news:
1. Dr. Farrugia is an accomplished GI investigator. Dr. Farrugia runs an NIH-funded translational laboratory focused on disorders of GI motility. The aim of Dr. Farrugia’s work is to understand at a cellular, subcellular and molecular level how the normal functions of the GI tract determine the defects that result in diseases such as diabetic gastroparesis, slow transit constipation, and irritable bowel syndrome (IBS), which will ultimately lead to new strategies to treat these diseases by developing targeted disease-modifying agents.
2. Dr. Farrugia is an alumnus of the AGA Research Foundation Research Scholar Award program. Dr. Farrugia received his Research Scholar Award in 1994 for his project titled “Jejunal Smooth Muscle Ion Channel Regulation in Health and Disease.”
3. Dr. Farrugia has given back to AGA both with his time – serving on the AGA Nominating Committee, AGA Institute Council, and Cellular and Molecular Gastroenterology and Hepatology editorial board – and by contributing, with his wife Geraldine Farrugia, to the AGA Research Foundation at the highest level as an AGA Legacy Society member.
Join AGA members in congratulating Dr. Farrugia in the AGA Community, community.gastro.org.
The Mayo Clinic Board of Trustees has announced that Gianrico Farrugia, MD, vice president and CEO of Mayo Clinic Florida, will take over as president and CEO of Mayo Clinic at the end of the year. AGA congratulates Dr. Farrugia on this accomplishment.
Here’s three reasons why AGA is excited by this news:
1. Dr. Farrugia is an accomplished GI investigator. Dr. Farrugia runs an NIH-funded translational laboratory focused on disorders of GI motility. The aim of Dr. Farrugia’s work is to understand at a cellular, subcellular and molecular level how the normal functions of the GI tract determine the defects that result in diseases such as diabetic gastroparesis, slow transit constipation, and irritable bowel syndrome (IBS), which will ultimately lead to new strategies to treat these diseases by developing targeted disease-modifying agents.
2. Dr. Farrugia is an alumnus of the AGA Research Foundation Research Scholar Award program. Dr. Farrugia received his Research Scholar Award in 1994 for his project titled “Jejunal Smooth Muscle Ion Channel Regulation in Health and Disease.”
3. Dr. Farrugia has given back to AGA both with his time – serving on the AGA Nominating Committee, AGA Institute Council, and Cellular and Molecular Gastroenterology and Hepatology editorial board – and by contributing, with his wife Geraldine Farrugia, to the AGA Research Foundation at the highest level as an AGA Legacy Society member.
Join AGA members in congratulating Dr. Farrugia in the AGA Community, community.gastro.org.
Rising microbiome investigator: Ting-Chin David Shen, MD, PhD
We spoke with Dr. Shen, instructor of medicine at the University of Pennsylvania and the recipient of the AGA Research Foundation’s 2016 Microbiome Junior Investigator Award, to learn about his passion for gut microbiome research.
How would you sum up your research in one sentence?
My research examines the metabolic interactions between the gut microbiota and the mammalian host, with a particular emphasis on amino acid metabolism and nitrogen flux via the bacterial enzyme urease.
What impact do you hope your research will have on patients?
My hope is that by better understanding the biological mechanisms by which the gut microbiota impacts host metabolism, we can modulate its effects to treat a variety of conditions and diseases including hepatic encephalopathy, inborn errors of metabolism, obesity, malnutrition, etc.
What inspired you to focus your research career on the gut microbiome?
My clinical experience as a gastroenterologist inspired my interest in metabolic and nutritional research. When I learned of the impact that the gut microbiota has on host metabolism, it created an entirely different perspective for me in terms of thinking about how to treat metabolic and nutritional disorders. There are tremendous opportunities in modifying our gut microbiota in concert with dietary interventions in order to modulate our metabolism.
What recent publication from your lab best represents your work, if anyone wants to learn more?
The following work examined how the use of a defined bacterial consortium without urease activity can reduce colonic ammonia level upon inoculation into the gut and ameliorate morbidity and mortality in a murine model of liver disease: Shen T.D., Albenberg L.A., Bittinger K., et al, Engineering the gut microbiota to treat hyperammonemia. J Clin Invest. 2015 Jul 1;125(7):2841-50. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4563680/.
We spoke with Dr. Shen, instructor of medicine at the University of Pennsylvania and the recipient of the AGA Research Foundation’s 2016 Microbiome Junior Investigator Award, to learn about his passion for gut microbiome research.
How would you sum up your research in one sentence?
My research examines the metabolic interactions between the gut microbiota and the mammalian host, with a particular emphasis on amino acid metabolism and nitrogen flux via the bacterial enzyme urease.
What impact do you hope your research will have on patients?
My hope is that by better understanding the biological mechanisms by which the gut microbiota impacts host metabolism, we can modulate its effects to treat a variety of conditions and diseases including hepatic encephalopathy, inborn errors of metabolism, obesity, malnutrition, etc.
What inspired you to focus your research career on the gut microbiome?
My clinical experience as a gastroenterologist inspired my interest in metabolic and nutritional research. When I learned of the impact that the gut microbiota has on host metabolism, it created an entirely different perspective for me in terms of thinking about how to treat metabolic and nutritional disorders. There are tremendous opportunities in modifying our gut microbiota in concert with dietary interventions in order to modulate our metabolism.
What recent publication from your lab best represents your work, if anyone wants to learn more?
The following work examined how the use of a defined bacterial consortium without urease activity can reduce colonic ammonia level upon inoculation into the gut and ameliorate morbidity and mortality in a murine model of liver disease: Shen T.D., Albenberg L.A., Bittinger K., et al, Engineering the gut microbiota to treat hyperammonemia. J Clin Invest. 2015 Jul 1;125(7):2841-50. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4563680/.
We spoke with Dr. Shen, instructor of medicine at the University of Pennsylvania and the recipient of the AGA Research Foundation’s 2016 Microbiome Junior Investigator Award, to learn about his passion for gut microbiome research.
How would you sum up your research in one sentence?
My research examines the metabolic interactions between the gut microbiota and the mammalian host, with a particular emphasis on amino acid metabolism and nitrogen flux via the bacterial enzyme urease.
What impact do you hope your research will have on patients?
My hope is that by better understanding the biological mechanisms by which the gut microbiota impacts host metabolism, we can modulate its effects to treat a variety of conditions and diseases including hepatic encephalopathy, inborn errors of metabolism, obesity, malnutrition, etc.
What inspired you to focus your research career on the gut microbiome?
My clinical experience as a gastroenterologist inspired my interest in metabolic and nutritional research. When I learned of the impact that the gut microbiota has on host metabolism, it created an entirely different perspective for me in terms of thinking about how to treat metabolic and nutritional disorders. There are tremendous opportunities in modifying our gut microbiota in concert with dietary interventions in order to modulate our metabolism.
What recent publication from your lab best represents your work, if anyone wants to learn more?
The following work examined how the use of a defined bacterial consortium without urease activity can reduce colonic ammonia level upon inoculation into the gut and ameliorate morbidity and mortality in a murine model of liver disease: Shen T.D., Albenberg L.A., Bittinger K., et al, Engineering the gut microbiota to treat hyperammonemia. J Clin Invest. 2015 Jul 1;125(7):2841-50. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4563680/.
Red flag raised on CMS indication-based formulary policy
Physician groups are expressing concerns regarding a new policy that will allow indication-based formulary design in the Medicare Part D prescription drug benefit.
The Centers for Medicare & Medicaid Services announced the new policy in an Aug. 29 memo to Part D plan sponsors.
According to a fact sheet issued by CMS on the same day, indication-based formulary design “is a formulary management tool that allows health plans to tailor on-formulary coverage of drugs predicated on specific indications.”
Current Part D policy requires plan sponsors to cover all Food and Drug Administration–approved indications for each drug that is on a plan formulary. Sponsors can begin to implement the new indication-based formulary design policy for plans issued in 2020.
The memo notes that, if a Part D plan sponsor chooses to opt into this policy, “it must ensure that there is another therapeutically similar drug on formulary for the nonformulary indication. For example, if a tumor necrosis factor (TNF) blocker is FDA-approved for both Crohn’s disease and plaque psoriasis, but the Part D plan will include it on formulary for plaque psoriasis only, the plan must ensure that there is another TNF blocker on formulary that will be covered for Crohn’s disease.”
Beneficiaries can use the exceptions process to get coverage for a drug that has an indication not on the formulary.
“By allowing Medicare’s prescription drug plans to cover the best drug for each patient condition, plans will have more negotiating power with drug companies, which will result in lower prices for Medicare beneficiaries,” CMS Administrator Seema Verma said in a statement.
However, physician groups should be concerned about the definition of “best drug.” Is this definition based upon efficacy, results of clinical trials, clinical effectiveness research, or just cost? Will there be transparenecy surrounding rebates?
The “proposed changes will exacerbate many of the access issues patients currently face with plan usage of existing utilization management practices, such as step therapy,” the American College of Rheumatology said in a statement. “Unlike step therapy, which often delays effective treatments, this proposal would go even further and allow plans to remove therapies from the formulary altogether, leaving patients completely unable to access treatments that doctors and patients choose together. ... We also have concerns on what this would mean for work being done on compendia inclusion to secure off-label drug coverage if plans don’t have to cover all FDA-approved indications.”
A similar situation exists in patients with inflammatory bowel disease in which step therapy has largely been replaced by risk assessments. The AGA Crohn’s and UC Care Pathways are based on this principle.
Under the plan, Medicare patients will face increased challenges as they navigate health plans to make sure that their needed drug is on their selected formulary, which can change based on what health conditions they have,” AMA President Barbara McAneny, MD, said in a statement. Dr. McAneny added that it will be even more difficult for physicians who are working with patients to get them on the best medicines covered by the patient’s formulary.
“Physicians already lack ready access to accurate formulary information – preferred/tier status, on/off formulary, PA [prior authorization] and step therapy requirements – at the point of care in their EHRs,” she said. “These transparency problems will expand by an order of magnitude by the complications this change introduces.”
Physician groups are expressing concerns regarding a new policy that will allow indication-based formulary design in the Medicare Part D prescription drug benefit.
The Centers for Medicare & Medicaid Services announced the new policy in an Aug. 29 memo to Part D plan sponsors.
According to a fact sheet issued by CMS on the same day, indication-based formulary design “is a formulary management tool that allows health plans to tailor on-formulary coverage of drugs predicated on specific indications.”
Current Part D policy requires plan sponsors to cover all Food and Drug Administration–approved indications for each drug that is on a plan formulary. Sponsors can begin to implement the new indication-based formulary design policy for plans issued in 2020.
The memo notes that, if a Part D plan sponsor chooses to opt into this policy, “it must ensure that there is another therapeutically similar drug on formulary for the nonformulary indication. For example, if a tumor necrosis factor (TNF) blocker is FDA-approved for both Crohn’s disease and plaque psoriasis, but the Part D plan will include it on formulary for plaque psoriasis only, the plan must ensure that there is another TNF blocker on formulary that will be covered for Crohn’s disease.”
Beneficiaries can use the exceptions process to get coverage for a drug that has an indication not on the formulary.
“By allowing Medicare’s prescription drug plans to cover the best drug for each patient condition, plans will have more negotiating power with drug companies, which will result in lower prices for Medicare beneficiaries,” CMS Administrator Seema Verma said in a statement.
However, physician groups should be concerned about the definition of “best drug.” Is this definition based upon efficacy, results of clinical trials, clinical effectiveness research, or just cost? Will there be transparenecy surrounding rebates?
The “proposed changes will exacerbate many of the access issues patients currently face with plan usage of existing utilization management practices, such as step therapy,” the American College of Rheumatology said in a statement. “Unlike step therapy, which often delays effective treatments, this proposal would go even further and allow plans to remove therapies from the formulary altogether, leaving patients completely unable to access treatments that doctors and patients choose together. ... We also have concerns on what this would mean for work being done on compendia inclusion to secure off-label drug coverage if plans don’t have to cover all FDA-approved indications.”
A similar situation exists in patients with inflammatory bowel disease in which step therapy has largely been replaced by risk assessments. The AGA Crohn’s and UC Care Pathways are based on this principle.
Under the plan, Medicare patients will face increased challenges as they navigate health plans to make sure that their needed drug is on their selected formulary, which can change based on what health conditions they have,” AMA President Barbara McAneny, MD, said in a statement. Dr. McAneny added that it will be even more difficult for physicians who are working with patients to get them on the best medicines covered by the patient’s formulary.
“Physicians already lack ready access to accurate formulary information – preferred/tier status, on/off formulary, PA [prior authorization] and step therapy requirements – at the point of care in their EHRs,” she said. “These transparency problems will expand by an order of magnitude by the complications this change introduces.”
Physician groups are expressing concerns regarding a new policy that will allow indication-based formulary design in the Medicare Part D prescription drug benefit.
The Centers for Medicare & Medicaid Services announced the new policy in an Aug. 29 memo to Part D plan sponsors.
According to a fact sheet issued by CMS on the same day, indication-based formulary design “is a formulary management tool that allows health plans to tailor on-formulary coverage of drugs predicated on specific indications.”
Current Part D policy requires plan sponsors to cover all Food and Drug Administration–approved indications for each drug that is on a plan formulary. Sponsors can begin to implement the new indication-based formulary design policy for plans issued in 2020.
The memo notes that, if a Part D plan sponsor chooses to opt into this policy, “it must ensure that there is another therapeutically similar drug on formulary for the nonformulary indication. For example, if a tumor necrosis factor (TNF) blocker is FDA-approved for both Crohn’s disease and plaque psoriasis, but the Part D plan will include it on formulary for plaque psoriasis only, the plan must ensure that there is another TNF blocker on formulary that will be covered for Crohn’s disease.”
Beneficiaries can use the exceptions process to get coverage for a drug that has an indication not on the formulary.
“By allowing Medicare’s prescription drug plans to cover the best drug for each patient condition, plans will have more negotiating power with drug companies, which will result in lower prices for Medicare beneficiaries,” CMS Administrator Seema Verma said in a statement.
However, physician groups should be concerned about the definition of “best drug.” Is this definition based upon efficacy, results of clinical trials, clinical effectiveness research, or just cost? Will there be transparenecy surrounding rebates?
The “proposed changes will exacerbate many of the access issues patients currently face with plan usage of existing utilization management practices, such as step therapy,” the American College of Rheumatology said in a statement. “Unlike step therapy, which often delays effective treatments, this proposal would go even further and allow plans to remove therapies from the formulary altogether, leaving patients completely unable to access treatments that doctors and patients choose together. ... We also have concerns on what this would mean for work being done on compendia inclusion to secure off-label drug coverage if plans don’t have to cover all FDA-approved indications.”
A similar situation exists in patients with inflammatory bowel disease in which step therapy has largely been replaced by risk assessments. The AGA Crohn’s and UC Care Pathways are based on this principle.
Under the plan, Medicare patients will face increased challenges as they navigate health plans to make sure that their needed drug is on their selected formulary, which can change based on what health conditions they have,” AMA President Barbara McAneny, MD, said in a statement. Dr. McAneny added that it will be even more difficult for physicians who are working with patients to get them on the best medicines covered by the patient’s formulary.
“Physicians already lack ready access to accurate formulary information – preferred/tier status, on/off formulary, PA [prior authorization] and step therapy requirements – at the point of care in their EHRs,” she said. “These transparency problems will expand by an order of magnitude by the complications this change introduces.”

















