User login
Vitreous Hemorrhage in the Setting of a Vascular Loop
Vascular loops are rare congenital optic nerve anomalies that originate from the arterial or venous circulation; 90% arise from the arterial circulation.1 Vascular loops are usually asymptomatic unless an arterial or venous occlusion, hyphema, and vitreous or preretinal hemorrhage should arise.1-8 This article describes a patient who presented with a vitreous hemorrhage secondary to a vascular loop.
Case Presentation
A 67-year-old white male presented to the eye clinic at the Providence VA Medical Center in Rhode Island after experiencing floaters and “snowflakes” in the left eye for 2 days. The patient reported having no photopsias, loss of vision, preceding eye/head trauma, or Valsalva maneuver. His medical history was significant for well-controlled type 2 diabetes mellitus (known duration of 5 years), hypertension, hyperlipidemia, coronary artery disease, and anemia. His medications included aspirin 81 mg, furosemide, clonidine, labetalol, valsartan, glipizide, and lantus injections.
The patient’s ocular history was significant for cataracts in both eyes. On examination, best-corrected visual acuity was 20/20 in each eye with intraocular pressures of 15 mm Hg in the right eye and 14 mm Hg in the left eye. Anterior segment examination was notable for 1+ nuclear sclerotic cataracts in both eyes with red blood cells visible in the anterior chamber in the left eye.
No PVD, retinal break, or detachment was present in the left eye with scleral depression. No background diabetic retinopathy was present in either eye.
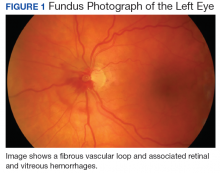
The patient was diagnosed with a vitreous hemorrhage associated with a vascular loop in the left eye.
Discussion
Salient features of this case include the prominent vascular loop at the disc extending anteriorly into the vitreous and an absence of features suggestive of one of the more common etiologies of vitreous hemorrhage, such as PVD, retinal tear/detachment, proliferative diabetic retinopathy (PDR), or retinal vein occlusion.
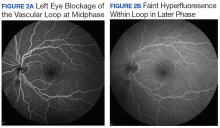
The incidence of venous loops is 1 in 9,000 with no associated systemic conditions.2,3 Typically unilateral, vascular loops arise at the optic disc from the central retinal artery or vein.1-4 An arterial loop is a separate entity from a hyaloid artery.2 The authors were unable to definitively determine whether the loop in this patient was arterial or venous in origin due to blockage from the associated retinal hemorrhage on FA.
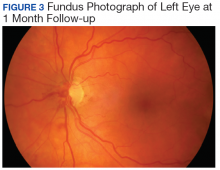
Valsalva maneuver, vitreous traction, trauma, and loop torsion in patients with vascular loops can lead to amaurosis fugax, PVD, and hemorrhagic complications, such as hyphema and vitreous and retinal hemorrhages.1,3,6-8 In addition, retinal ischemia and thrombosis from the vascular loops can lead to retinal artery or vein occlusions.1-8 Vitreous and retinal hemorrhages, such as in this patient, are often observed with complete resolution and visual acuity returning to baseline.4,5 For recurrent or nonresolving vitreous hemorrhages, a vitrectomy can be performed.3,6
Conclusion
Patients with vascular loops should be educated to seek eye care if experiencing new onset floaters or visual loss.
1. Codenotti M, Fogliato G, De Benedetto U, Iuliano L, Bandello F. Simultaneous vitreous hemorrhage and branch retinal artery occlusion after prepapillary arterial loop rupture. J Fr Ophtalmol. 2013;36(4):e63-e65.
2. Brown GC, Magargal L, Augsburger JJ, Shields JA. Preretinal arterial loops and retinal arterial occlusion. Am J Ophthalmol. 1979;87(5):646-651.
3. Degenhart W, Brown GC, Augsburger JJ, Magargal L. Prepapillary vascular loops. Ophthalmology. 1981;88(11):1126-1131.
4. Soltau JB, Olk RJ, Gordon JM. Prepapillary arterial loop associated with vitreous hemorrhage and venous retinal macrovessel. Retina. 1996;16(1):74-75.
5. Fujiwara T, Machida S, Herai T, Tazawa Y. Case of subretinal hemorrhage that developed from a prepapillary vascular loop. Jpn J Ophthalmol. 2004;48(2):175-177.
6. Strassman IB, Desai UR. Prepapillary vascular loop and a recurrent vitreous hemorrhage. Retina. 1997;17(2):166-167.
7. Singh R, Fujinami K, Moore AT. Branch retinal artery occlusion secondary to prepapillary arterial loop. Retin Cases Brief Rep. 2014;8(2):124-126.
8. Takahashi K. Hemodynamics of prepapillary vascular loop in hemi-central retinal vein occlusion [in Japanese]. Nippon Ganka Gakkai Zasshi. 1999;103(5):404-408.
Vascular loops are rare congenital optic nerve anomalies that originate from the arterial or venous circulation; 90% arise from the arterial circulation.1 Vascular loops are usually asymptomatic unless an arterial or venous occlusion, hyphema, and vitreous or preretinal hemorrhage should arise.1-8 This article describes a patient who presented with a vitreous hemorrhage secondary to a vascular loop.
Case Presentation
A 67-year-old white male presented to the eye clinic at the Providence VA Medical Center in Rhode Island after experiencing floaters and “snowflakes” in the left eye for 2 days. The patient reported having no photopsias, loss of vision, preceding eye/head trauma, or Valsalva maneuver. His medical history was significant for well-controlled type 2 diabetes mellitus (known duration of 5 years), hypertension, hyperlipidemia, coronary artery disease, and anemia. His medications included aspirin 81 mg, furosemide, clonidine, labetalol, valsartan, glipizide, and lantus injections.
The patient’s ocular history was significant for cataracts in both eyes. On examination, best-corrected visual acuity was 20/20 in each eye with intraocular pressures of 15 mm Hg in the right eye and 14 mm Hg in the left eye. Anterior segment examination was notable for 1+ nuclear sclerotic cataracts in both eyes with red blood cells visible in the anterior chamber in the left eye.
No PVD, retinal break, or detachment was present in the left eye with scleral depression. No background diabetic retinopathy was present in either eye.
The patient was diagnosed with a vitreous hemorrhage associated with a vascular loop in the left eye.
Discussion
Salient features of this case include the prominent vascular loop at the disc extending anteriorly into the vitreous and an absence of features suggestive of one of the more common etiologies of vitreous hemorrhage, such as PVD, retinal tear/detachment, proliferative diabetic retinopathy (PDR), or retinal vein occlusion.
The incidence of venous loops is 1 in 9,000 with no associated systemic conditions.2,3 Typically unilateral, vascular loops arise at the optic disc from the central retinal artery or vein.1-4 An arterial loop is a separate entity from a hyaloid artery.2 The authors were unable to definitively determine whether the loop in this patient was arterial or venous in origin due to blockage from the associated retinal hemorrhage on FA.
Valsalva maneuver, vitreous traction, trauma, and loop torsion in patients with vascular loops can lead to amaurosis fugax, PVD, and hemorrhagic complications, such as hyphema and vitreous and retinal hemorrhages.1,3,6-8 In addition, retinal ischemia and thrombosis from the vascular loops can lead to retinal artery or vein occlusions.1-8 Vitreous and retinal hemorrhages, such as in this patient, are often observed with complete resolution and visual acuity returning to baseline.4,5 For recurrent or nonresolving vitreous hemorrhages, a vitrectomy can be performed.3,6
Conclusion
Patients with vascular loops should be educated to seek eye care if experiencing new onset floaters or visual loss.
Vascular loops are rare congenital optic nerve anomalies that originate from the arterial or venous circulation; 90% arise from the arterial circulation.1 Vascular loops are usually asymptomatic unless an arterial or venous occlusion, hyphema, and vitreous or preretinal hemorrhage should arise.1-8 This article describes a patient who presented with a vitreous hemorrhage secondary to a vascular loop.
Case Presentation
A 67-year-old white male presented to the eye clinic at the Providence VA Medical Center in Rhode Island after experiencing floaters and “snowflakes” in the left eye for 2 days. The patient reported having no photopsias, loss of vision, preceding eye/head trauma, or Valsalva maneuver. His medical history was significant for well-controlled type 2 diabetes mellitus (known duration of 5 years), hypertension, hyperlipidemia, coronary artery disease, and anemia. His medications included aspirin 81 mg, furosemide, clonidine, labetalol, valsartan, glipizide, and lantus injections.
The patient’s ocular history was significant for cataracts in both eyes. On examination, best-corrected visual acuity was 20/20 in each eye with intraocular pressures of 15 mm Hg in the right eye and 14 mm Hg in the left eye. Anterior segment examination was notable for 1+ nuclear sclerotic cataracts in both eyes with red blood cells visible in the anterior chamber in the left eye.
No PVD, retinal break, or detachment was present in the left eye with scleral depression. No background diabetic retinopathy was present in either eye.
The patient was diagnosed with a vitreous hemorrhage associated with a vascular loop in the left eye.
Discussion
Salient features of this case include the prominent vascular loop at the disc extending anteriorly into the vitreous and an absence of features suggestive of one of the more common etiologies of vitreous hemorrhage, such as PVD, retinal tear/detachment, proliferative diabetic retinopathy (PDR), or retinal vein occlusion.
The incidence of venous loops is 1 in 9,000 with no associated systemic conditions.2,3 Typically unilateral, vascular loops arise at the optic disc from the central retinal artery or vein.1-4 An arterial loop is a separate entity from a hyaloid artery.2 The authors were unable to definitively determine whether the loop in this patient was arterial or venous in origin due to blockage from the associated retinal hemorrhage on FA.
Valsalva maneuver, vitreous traction, trauma, and loop torsion in patients with vascular loops can lead to amaurosis fugax, PVD, and hemorrhagic complications, such as hyphema and vitreous and retinal hemorrhages.1,3,6-8 In addition, retinal ischemia and thrombosis from the vascular loops can lead to retinal artery or vein occlusions.1-8 Vitreous and retinal hemorrhages, such as in this patient, are often observed with complete resolution and visual acuity returning to baseline.4,5 For recurrent or nonresolving vitreous hemorrhages, a vitrectomy can be performed.3,6
Conclusion
Patients with vascular loops should be educated to seek eye care if experiencing new onset floaters or visual loss.
1. Codenotti M, Fogliato G, De Benedetto U, Iuliano L, Bandello F. Simultaneous vitreous hemorrhage and branch retinal artery occlusion after prepapillary arterial loop rupture. J Fr Ophtalmol. 2013;36(4):e63-e65.
2. Brown GC, Magargal L, Augsburger JJ, Shields JA. Preretinal arterial loops and retinal arterial occlusion. Am J Ophthalmol. 1979;87(5):646-651.
3. Degenhart W, Brown GC, Augsburger JJ, Magargal L. Prepapillary vascular loops. Ophthalmology. 1981;88(11):1126-1131.
4. Soltau JB, Olk RJ, Gordon JM. Prepapillary arterial loop associated with vitreous hemorrhage and venous retinal macrovessel. Retina. 1996;16(1):74-75.
5. Fujiwara T, Machida S, Herai T, Tazawa Y. Case of subretinal hemorrhage that developed from a prepapillary vascular loop. Jpn J Ophthalmol. 2004;48(2):175-177.
6. Strassman IB, Desai UR. Prepapillary vascular loop and a recurrent vitreous hemorrhage. Retina. 1997;17(2):166-167.
7. Singh R, Fujinami K, Moore AT. Branch retinal artery occlusion secondary to prepapillary arterial loop. Retin Cases Brief Rep. 2014;8(2):124-126.
8. Takahashi K. Hemodynamics of prepapillary vascular loop in hemi-central retinal vein occlusion [in Japanese]. Nippon Ganka Gakkai Zasshi. 1999;103(5):404-408.
1. Codenotti M, Fogliato G, De Benedetto U, Iuliano L, Bandello F. Simultaneous vitreous hemorrhage and branch retinal artery occlusion after prepapillary arterial loop rupture. J Fr Ophtalmol. 2013;36(4):e63-e65.
2. Brown GC, Magargal L, Augsburger JJ, Shields JA. Preretinal arterial loops and retinal arterial occlusion. Am J Ophthalmol. 1979;87(5):646-651.
3. Degenhart W, Brown GC, Augsburger JJ, Magargal L. Prepapillary vascular loops. Ophthalmology. 1981;88(11):1126-1131.
4. Soltau JB, Olk RJ, Gordon JM. Prepapillary arterial loop associated with vitreous hemorrhage and venous retinal macrovessel. Retina. 1996;16(1):74-75.
5. Fujiwara T, Machida S, Herai T, Tazawa Y. Case of subretinal hemorrhage that developed from a prepapillary vascular loop. Jpn J Ophthalmol. 2004;48(2):175-177.
6. Strassman IB, Desai UR. Prepapillary vascular loop and a recurrent vitreous hemorrhage. Retina. 1997;17(2):166-167.
7. Singh R, Fujinami K, Moore AT. Branch retinal artery occlusion secondary to prepapillary arterial loop. Retin Cases Brief Rep. 2014;8(2):124-126.
8. Takahashi K. Hemodynamics of prepapillary vascular loop in hemi-central retinal vein occlusion [in Japanese]. Nippon Ganka Gakkai Zasshi. 1999;103(5):404-408.
Efficacy of KTE-C19 CAR T cells not compromised by prior blinatumomab
CHICAGO—Prior exposure to blinatumomab does not appear to affect the successful manufacture of KTE-C19 or its efficacy in patients with relapsed/refractory acute lymphoblastic leukemia (ALL), an investigator reported at the 2018 ASCO Annual Meeting.
The clinical benefit of KTE-C19, an anti-CD19 chimeric antigen receptor (CAR) T-cell therapy, was preserved regardless of whether patients were exposed to blinatumomab, said Bijal D. Shah, MD, of Moffitt Cancer Center in Tampa, Florida.
High rates of complete response and undetectable minimal residual disease (MRD) were independent of exposure to blinatumomab, a CD19/CD3 bispecific T-cell engager.
“We feel the results of these data support KTE-C19 as an effective option for adults with refractory leukemia, regardless of prior exposure to CD19-directed therapy,” Dr Shah reported at the meeting (abstract 7006*).
The current standard of care for adults with relapsed/refractory ALL includes blinatumomab, raising the question of whether prior exposure to this CD19-directed treatment could influence the manufacture or efficacy of KTE-C19.
Sara Cooley, MD, of Masonic Medical Center, University of Minnesota in Minneapolis, said results of this analysis suggest prior blinatumomab should not be a contraindication or concern in the context of KTE-C19.
“This remains to be shown with other CAR T-cell therapies,” she said in a presentation at ASCO on cellular therapy in leukemia.
The analysis by Dr Shah and co-investigators was based on ZUMA-3 (NCT02614066), a phase 1 study including adults with relapsed/refractory ALL who received KTE-C19 at doses of 0.5, 1, or 2 x 106 cells/kg.
They excluded patients in the highest dose cohort, who were required to be blinatumomab naïve, per protocol. That left 23 patients who received 0.5 or 1 x 106 cells/kg, of whom 11 had prior blinatumomab exposure and 12 did not.
Best overall response appeared to be independent of prior blinatumomab treatment, with a CR rate of 72% overall, and 63% and 80% for blinatumomab-exposed and blinatumomab-naïve patients, respectively.
The rate of undetectable MRD was likewise high at 88% in the prior blinatumomab group and 100% in the no-blinatumomab group.
Product characteristics did not vary according to blinatumomab exposure, though there was a trend toward a more differentiated phenotype in those patients who had received prior CD19-directed treatment, he said.
There were no significant differences between groups in the rate of grade 3 or greater cytokine release syndrome. Neurologic events were higher in the blinatumomab-naïve patients, though a higher percentage of those patients received the 1 x 106 cells/kg dose, Dr Shah reported.
Investigators also looked at CAR T levels by treatment.
“We cannot appreciate any significant differences between the blinatumomab-naïve and the blinatumomab-exposed groups,” Dr Shah told ASCO attendees.
The ZUMA-3 trial was sponsored by Kite, a Gilead Company.
*Data in the abstract differ from the presentation.
CHICAGO—Prior exposure to blinatumomab does not appear to affect the successful manufacture of KTE-C19 or its efficacy in patients with relapsed/refractory acute lymphoblastic leukemia (ALL), an investigator reported at the 2018 ASCO Annual Meeting.
The clinical benefit of KTE-C19, an anti-CD19 chimeric antigen receptor (CAR) T-cell therapy, was preserved regardless of whether patients were exposed to blinatumomab, said Bijal D. Shah, MD, of Moffitt Cancer Center in Tampa, Florida.
High rates of complete response and undetectable minimal residual disease (MRD) were independent of exposure to blinatumomab, a CD19/CD3 bispecific T-cell engager.
“We feel the results of these data support KTE-C19 as an effective option for adults with refractory leukemia, regardless of prior exposure to CD19-directed therapy,” Dr Shah reported at the meeting (abstract 7006*).
The current standard of care for adults with relapsed/refractory ALL includes blinatumomab, raising the question of whether prior exposure to this CD19-directed treatment could influence the manufacture or efficacy of KTE-C19.
Sara Cooley, MD, of Masonic Medical Center, University of Minnesota in Minneapolis, said results of this analysis suggest prior blinatumomab should not be a contraindication or concern in the context of KTE-C19.
“This remains to be shown with other CAR T-cell therapies,” she said in a presentation at ASCO on cellular therapy in leukemia.
The analysis by Dr Shah and co-investigators was based on ZUMA-3 (NCT02614066), a phase 1 study including adults with relapsed/refractory ALL who received KTE-C19 at doses of 0.5, 1, or 2 x 106 cells/kg.
They excluded patients in the highest dose cohort, who were required to be blinatumomab naïve, per protocol. That left 23 patients who received 0.5 or 1 x 106 cells/kg, of whom 11 had prior blinatumomab exposure and 12 did not.
Best overall response appeared to be independent of prior blinatumomab treatment, with a CR rate of 72% overall, and 63% and 80% for blinatumomab-exposed and blinatumomab-naïve patients, respectively.
The rate of undetectable MRD was likewise high at 88% in the prior blinatumomab group and 100% in the no-blinatumomab group.
Product characteristics did not vary according to blinatumomab exposure, though there was a trend toward a more differentiated phenotype in those patients who had received prior CD19-directed treatment, he said.
There were no significant differences between groups in the rate of grade 3 or greater cytokine release syndrome. Neurologic events were higher in the blinatumomab-naïve patients, though a higher percentage of those patients received the 1 x 106 cells/kg dose, Dr Shah reported.
Investigators also looked at CAR T levels by treatment.
“We cannot appreciate any significant differences between the blinatumomab-naïve and the blinatumomab-exposed groups,” Dr Shah told ASCO attendees.
The ZUMA-3 trial was sponsored by Kite, a Gilead Company.
*Data in the abstract differ from the presentation.
CHICAGO—Prior exposure to blinatumomab does not appear to affect the successful manufacture of KTE-C19 or its efficacy in patients with relapsed/refractory acute lymphoblastic leukemia (ALL), an investigator reported at the 2018 ASCO Annual Meeting.
The clinical benefit of KTE-C19, an anti-CD19 chimeric antigen receptor (CAR) T-cell therapy, was preserved regardless of whether patients were exposed to blinatumomab, said Bijal D. Shah, MD, of Moffitt Cancer Center in Tampa, Florida.
High rates of complete response and undetectable minimal residual disease (MRD) were independent of exposure to blinatumomab, a CD19/CD3 bispecific T-cell engager.
“We feel the results of these data support KTE-C19 as an effective option for adults with refractory leukemia, regardless of prior exposure to CD19-directed therapy,” Dr Shah reported at the meeting (abstract 7006*).
The current standard of care for adults with relapsed/refractory ALL includes blinatumomab, raising the question of whether prior exposure to this CD19-directed treatment could influence the manufacture or efficacy of KTE-C19.
Sara Cooley, MD, of Masonic Medical Center, University of Minnesota in Minneapolis, said results of this analysis suggest prior blinatumomab should not be a contraindication or concern in the context of KTE-C19.
“This remains to be shown with other CAR T-cell therapies,” she said in a presentation at ASCO on cellular therapy in leukemia.
The analysis by Dr Shah and co-investigators was based on ZUMA-3 (NCT02614066), a phase 1 study including adults with relapsed/refractory ALL who received KTE-C19 at doses of 0.5, 1, or 2 x 106 cells/kg.
They excluded patients in the highest dose cohort, who were required to be blinatumomab naïve, per protocol. That left 23 patients who received 0.5 or 1 x 106 cells/kg, of whom 11 had prior blinatumomab exposure and 12 did not.
Best overall response appeared to be independent of prior blinatumomab treatment, with a CR rate of 72% overall, and 63% and 80% for blinatumomab-exposed and blinatumomab-naïve patients, respectively.
The rate of undetectable MRD was likewise high at 88% in the prior blinatumomab group and 100% in the no-blinatumomab group.
Product characteristics did not vary according to blinatumomab exposure, though there was a trend toward a more differentiated phenotype in those patients who had received prior CD19-directed treatment, he said.
There were no significant differences between groups in the rate of grade 3 or greater cytokine release syndrome. Neurologic events were higher in the blinatumomab-naïve patients, though a higher percentage of those patients received the 1 x 106 cells/kg dose, Dr Shah reported.
Investigators also looked at CAR T levels by treatment.
“We cannot appreciate any significant differences between the blinatumomab-naïve and the blinatumomab-exposed groups,” Dr Shah told ASCO attendees.
The ZUMA-3 trial was sponsored by Kite, a Gilead Company.
*Data in the abstract differ from the presentation.
Antipsychotics linked to increased body fat, insulin resistance in children
Twelve weeks of treatment with three different antipsychotics commonly used in children and adolescents with disruptive behavioral disorders was associated with rapid-onset adverse changes in adiposity and insulin sensitivity, results of a randomized clinical trial show.
The adverse effect on adiposity was greatest for children aged 6-18 years who received olanzapine, but was also seen in study participants randomized to receive risperidone or aripiprazole, according to results of the study published in JAMA Psychiatry.
These findings inform the risk-benefit analysis for off-label pediatric use of antipsychotics for disruptive behavior disorders, Ginger E. Nicol, MD, of Washington University, St. Louis, and colleagues wrote.
“The potential psychiatric benefits of antipsychotic use in this population, evident in this trial and others, should be carefully weighed against the potential for childhood onset of abdominal obesity and insulin resistance that – compared with adult onset – further increases long-term risk for [type 2 diabetes], cardiovascular disease, and related conditions,” the researchers wrote.
This randomized, prospective clinical trial included 144 antipsychotic-naive children and adolescents aged 6-18 years who had been diagnosed with one or more psychiatric disorders and clinically significant aggression. They were randomly and evenly assigned to 12 weeks of treatment with oral olanzapine, risperidone, or aripiprazole.
Those who received olanzapine had a 4.12% increase in percentage total body fat from baseline to week 12, as measured by dual-energy x-ray absorptiometry (DXA). That increase was significantly greater than the total body fat increases of 1.18% for risperidone and 1.66% for aripiprazole seen over that same time period (P less than .001 for time-by-treatment interaction), the researchers reported.
Insulin sensitivity decreased over time in the pooled study sample, with significant changes reported in insulin-stimulated glucose rate of disappearance, glucose rate of appearance, and glycerol rate of appearance. The changes did not differ significantly across the three treatment groups.
The lack of a placebo group in this study makes it unclear how much of the body fat and insulin sensitivity changes were attributable to antipsychotic medication. Psychiatric symptoms did improve significantly with treatment, the researchers noted.
Dr. Nicol reported research funding from the National Institute of Mental Health, Otsuka America Pharmaceutical, Alkermes PLC, The Sidney R. Baer Jr. Foundation, and the Center for Brain Research in Mood Disorders at Washington University in St Louis. Co-authors reported financial ties to Reviva Pharmaceuticals, Sunovion Pharmaceuticals, Indivior, Amgen, and other companies.
SOURCE: Nicol GE et al. JAMA Psychiatry. 2018 Jun 13. doi: 10.1001/jamapsychiatry.2018.1088.
The findings of this study by Nicol and colleagues corroborate previous reports that used conventional anthropometry to document the metabolic adverse effects of second-generation antipsychotic medications in children and adolescents, according to authors of an accompanying editorial.
The current study used “gold standard” methods of measurement to demonstrate that 12 weeks of treatment with low-dose olanzapine, risperidone, or aripiprazole led to rapid-onset changes in adiposity and insulin sensitivity, with larger increases in those who received olanzapine.
“As such, the study even more emphatically underlines the risks to which these vulnerable populations are exposed,” Marc De Hert, MD, PhD, and Johan Detraux wrote.
It is now is clear that the potential psychiatric benefits of off-label antipsychotics need to be weighed against the potential for childhood-onset obesity and insulin resistance, they added. However, longer-term studies that use hard end points, such as new-onset diabetes or cardiovascular disease are needed.
Marc De Hert, MD, PhD, and Johan Detraux, are with Katholieke Universiteit Leuven, Kortenberg, Belgium. These comments are from an accompanying editorial (JAMA Psychiatry. 2018 Jun 13. doi: 10.1001/jamapsychiatry.2018.1080 ). Dr. De Hert reported no disclosures. Mr. Detraux reported partial support by the Janssen Academy for work on the the Belgian Discussion Board on Antipsychotic Treatment.
The findings of this study by Nicol and colleagues corroborate previous reports that used conventional anthropometry to document the metabolic adverse effects of second-generation antipsychotic medications in children and adolescents, according to authors of an accompanying editorial.
The current study used “gold standard” methods of measurement to demonstrate that 12 weeks of treatment with low-dose olanzapine, risperidone, or aripiprazole led to rapid-onset changes in adiposity and insulin sensitivity, with larger increases in those who received olanzapine.
“As such, the study even more emphatically underlines the risks to which these vulnerable populations are exposed,” Marc De Hert, MD, PhD, and Johan Detraux wrote.
It is now is clear that the potential psychiatric benefits of off-label antipsychotics need to be weighed against the potential for childhood-onset obesity and insulin resistance, they added. However, longer-term studies that use hard end points, such as new-onset diabetes or cardiovascular disease are needed.
Marc De Hert, MD, PhD, and Johan Detraux, are with Katholieke Universiteit Leuven, Kortenberg, Belgium. These comments are from an accompanying editorial (JAMA Psychiatry. 2018 Jun 13. doi: 10.1001/jamapsychiatry.2018.1080 ). Dr. De Hert reported no disclosures. Mr. Detraux reported partial support by the Janssen Academy for work on the the Belgian Discussion Board on Antipsychotic Treatment.
The findings of this study by Nicol and colleagues corroborate previous reports that used conventional anthropometry to document the metabolic adverse effects of second-generation antipsychotic medications in children and adolescents, according to authors of an accompanying editorial.
The current study used “gold standard” methods of measurement to demonstrate that 12 weeks of treatment with low-dose olanzapine, risperidone, or aripiprazole led to rapid-onset changes in adiposity and insulin sensitivity, with larger increases in those who received olanzapine.
“As such, the study even more emphatically underlines the risks to which these vulnerable populations are exposed,” Marc De Hert, MD, PhD, and Johan Detraux wrote.
It is now is clear that the potential psychiatric benefits of off-label antipsychotics need to be weighed against the potential for childhood-onset obesity and insulin resistance, they added. However, longer-term studies that use hard end points, such as new-onset diabetes or cardiovascular disease are needed.
Marc De Hert, MD, PhD, and Johan Detraux, are with Katholieke Universiteit Leuven, Kortenberg, Belgium. These comments are from an accompanying editorial (JAMA Psychiatry. 2018 Jun 13. doi: 10.1001/jamapsychiatry.2018.1080 ). Dr. De Hert reported no disclosures. Mr. Detraux reported partial support by the Janssen Academy for work on the the Belgian Discussion Board on Antipsychotic Treatment.
Twelve weeks of treatment with three different antipsychotics commonly used in children and adolescents with disruptive behavioral disorders was associated with rapid-onset adverse changes in adiposity and insulin sensitivity, results of a randomized clinical trial show.
The adverse effect on adiposity was greatest for children aged 6-18 years who received olanzapine, but was also seen in study participants randomized to receive risperidone or aripiprazole, according to results of the study published in JAMA Psychiatry.
These findings inform the risk-benefit analysis for off-label pediatric use of antipsychotics for disruptive behavior disorders, Ginger E. Nicol, MD, of Washington University, St. Louis, and colleagues wrote.
“The potential psychiatric benefits of antipsychotic use in this population, evident in this trial and others, should be carefully weighed against the potential for childhood onset of abdominal obesity and insulin resistance that – compared with adult onset – further increases long-term risk for [type 2 diabetes], cardiovascular disease, and related conditions,” the researchers wrote.
This randomized, prospective clinical trial included 144 antipsychotic-naive children and adolescents aged 6-18 years who had been diagnosed with one or more psychiatric disorders and clinically significant aggression. They were randomly and evenly assigned to 12 weeks of treatment with oral olanzapine, risperidone, or aripiprazole.
Those who received olanzapine had a 4.12% increase in percentage total body fat from baseline to week 12, as measured by dual-energy x-ray absorptiometry (DXA). That increase was significantly greater than the total body fat increases of 1.18% for risperidone and 1.66% for aripiprazole seen over that same time period (P less than .001 for time-by-treatment interaction), the researchers reported.
Insulin sensitivity decreased over time in the pooled study sample, with significant changes reported in insulin-stimulated glucose rate of disappearance, glucose rate of appearance, and glycerol rate of appearance. The changes did not differ significantly across the three treatment groups.
The lack of a placebo group in this study makes it unclear how much of the body fat and insulin sensitivity changes were attributable to antipsychotic medication. Psychiatric symptoms did improve significantly with treatment, the researchers noted.
Dr. Nicol reported research funding from the National Institute of Mental Health, Otsuka America Pharmaceutical, Alkermes PLC, The Sidney R. Baer Jr. Foundation, and the Center for Brain Research in Mood Disorders at Washington University in St Louis. Co-authors reported financial ties to Reviva Pharmaceuticals, Sunovion Pharmaceuticals, Indivior, Amgen, and other companies.
SOURCE: Nicol GE et al. JAMA Psychiatry. 2018 Jun 13. doi: 10.1001/jamapsychiatry.2018.1088.
Twelve weeks of treatment with three different antipsychotics commonly used in children and adolescents with disruptive behavioral disorders was associated with rapid-onset adverse changes in adiposity and insulin sensitivity, results of a randomized clinical trial show.
The adverse effect on adiposity was greatest for children aged 6-18 years who received olanzapine, but was also seen in study participants randomized to receive risperidone or aripiprazole, according to results of the study published in JAMA Psychiatry.
These findings inform the risk-benefit analysis for off-label pediatric use of antipsychotics for disruptive behavior disorders, Ginger E. Nicol, MD, of Washington University, St. Louis, and colleagues wrote.
“The potential psychiatric benefits of antipsychotic use in this population, evident in this trial and others, should be carefully weighed against the potential for childhood onset of abdominal obesity and insulin resistance that – compared with adult onset – further increases long-term risk for [type 2 diabetes], cardiovascular disease, and related conditions,” the researchers wrote.
This randomized, prospective clinical trial included 144 antipsychotic-naive children and adolescents aged 6-18 years who had been diagnosed with one or more psychiatric disorders and clinically significant aggression. They were randomly and evenly assigned to 12 weeks of treatment with oral olanzapine, risperidone, or aripiprazole.
Those who received olanzapine had a 4.12% increase in percentage total body fat from baseline to week 12, as measured by dual-energy x-ray absorptiometry (DXA). That increase was significantly greater than the total body fat increases of 1.18% for risperidone and 1.66% for aripiprazole seen over that same time period (P less than .001 for time-by-treatment interaction), the researchers reported.
Insulin sensitivity decreased over time in the pooled study sample, with significant changes reported in insulin-stimulated glucose rate of disappearance, glucose rate of appearance, and glycerol rate of appearance. The changes did not differ significantly across the three treatment groups.
The lack of a placebo group in this study makes it unclear how much of the body fat and insulin sensitivity changes were attributable to antipsychotic medication. Psychiatric symptoms did improve significantly with treatment, the researchers noted.
Dr. Nicol reported research funding from the National Institute of Mental Health, Otsuka America Pharmaceutical, Alkermes PLC, The Sidney R. Baer Jr. Foundation, and the Center for Brain Research in Mood Disorders at Washington University in St Louis. Co-authors reported financial ties to Reviva Pharmaceuticals, Sunovion Pharmaceuticals, Indivior, Amgen, and other companies.
SOURCE: Nicol GE et al. JAMA Psychiatry. 2018 Jun 13. doi: 10.1001/jamapsychiatry.2018.1088.
FROM JAMA PSYCHIATRY
Key clinical point:
Major finding: Olanzapine-treated youths had the highest percentage total body fat increase (4.12%), but substantial increases were also noted for risperidone (1.18%) and aripiprazole (1.66%).
Study details: A randomized clinical trial including 144 antipsychotic-naive youths aged 6-18 years with one or more psychiatric disorders and clinically significant aggression.
Disclosures: Study authors reported disclosures related to Otsuka America Pharmaceutical, Alkermes PLC, Reviva Pharmaceuticals, Sunovion Pharmaceuticals, Indivior, Amgen, and other companies.
Source: Nicol GE et al. JAMA Psychiatry. 2018 Jun 13. doi:10.1001/jamapsychiatry.2018.1088.
DEA ruling may have stoked illicit opioid sales
Illicit online sales of opioids increased significantly following a 2014 regulatory decision that restricted the legal supply of opioids in the United States, results of a recent analysis show.
Opioid sales in the so-called cryptomarkets also shifted toward more potent drugs, including fentanyl, after the U.S. Drug Enforcement Administration rescheduled hydrocodone combination products, making them harder to access, authors of the analysis reported.
Those results suggest “the possibility of a causal relation” between the schedule change and sales trends on cryptomarket sites, according to James Martin, PhD, of Swinburne University, Melbourne, Australia, and co-authors.
“Our analysis cannot rule out other possible causal explanatory factors, but our results are consistent with the possibility that the schedule change might have directly contributed to the changes we observed in the supply of illicit opioids,” Dr. Martin and colleagues wrote in the BMJ.
Cryptomarkets outside the United States had no such uptick in opioid traffic over time, reinforcing the possibility that the 2014 DEA decision caused an increase in the illicit opioid supply. The analysis included 31 online illicit markets operating between October 2013 and July 2016.
Investigators collected data using web crawling software that downloaded HTML pages on the cryptomarket sites and extracted relevant information such as listing titles and drug types for later analysis. Generally, each market was fully crawled every 2 weeks. After data was scraped and extracted, they conducted an interrupted time series analysis of drug sales for each day of the data collection period.
Opioids represented 13.7% of all drug sales through U.S. cryptomarkets in July 2016, they found, compared with a modeled estimate of 6.7% of sales that would have occurred in the absence of the new schedule introduction.
“This represents an approximate doubling of the percentage of the total drug sales through cryptomarkets within the United States,” the authors wrote.
Fentanyl was the least purchased product at the beginning of the analysis, but by July 2016 it was the second most frequently purchased. No other drug category had any meaningful changes in the proportion of sales over time.
There was no way for researchers to confirm that U.S.-based cryptomarket sites were selling to customers in the United States or elsewhere. However, they said, cryptomarket buyers often use sites in the same country to avoid shipment losses and increased delivery time.
Part of the study were funded by the Social Sciences and Humanities Research Council of Canada, Macquarie University internal grants, and the Ministry of Justice and Security of the Netherlands. Study authors had no current financial relationships relevant to the study.
SOURCE: Martin J, et al. BMJ. 2018 Jun 13. doi: 10.1136/bmj.k2270.
The overdose crisis in the United States will likely worsen if “supply-side” measures, such as the tighter prescribing regulations evaluated in this study, are not coupled with interventions to reduce harm and decrease demand, according to Scott E. Hadland, MD, and Leo Beletsky.
On a more basic level, the analysis by James Martin and colleagues raises questions about the use of drug scheduling to regulate public health, the authors concluded in an accompanying editorial.
“The U.S. scheduling scheme inexplicably holds such disparate substances as cannabis, heroin, and psilocybin to be equally dangerous,” they wrote. “It is high time to rethink how, why, and when this regulatory framework is deployed to curb drug-related harms.”
The shift from schedule III to schedule II creates barriers to medication access that disproportionately affect individuals in rural areas and those with limited mobility, since refills for schedule II drugs can only be obtained through an in-person visit to a provider and pharmacist, the authors wrote.
Scott E Hadland, MD, is with Grayken Center for Addiction/Department of Pediatrics, Boston Medical Center, and Leo Beletsky is with the School of Law and Bouvé College of Health Sciences, Northeastern University, Boston. Mr. Beletsky reported sitting on the advisory board of a data analytics company that has an interest in the U.S. opioid crisis. These comments are from their accompanying editorial (BMJ. 2018 Jun 13. doi: 10.1136/bmj.k2480 ). Mr. Beletsky reported sitting on the advisory board of a data analytics company that has an interest in the U.S. opioid crisis.
The overdose crisis in the United States will likely worsen if “supply-side” measures, such as the tighter prescribing regulations evaluated in this study, are not coupled with interventions to reduce harm and decrease demand, according to Scott E. Hadland, MD, and Leo Beletsky.
On a more basic level, the analysis by James Martin and colleagues raises questions about the use of drug scheduling to regulate public health, the authors concluded in an accompanying editorial.
“The U.S. scheduling scheme inexplicably holds such disparate substances as cannabis, heroin, and psilocybin to be equally dangerous,” they wrote. “It is high time to rethink how, why, and when this regulatory framework is deployed to curb drug-related harms.”
The shift from schedule III to schedule II creates barriers to medication access that disproportionately affect individuals in rural areas and those with limited mobility, since refills for schedule II drugs can only be obtained through an in-person visit to a provider and pharmacist, the authors wrote.
Scott E Hadland, MD, is with Grayken Center for Addiction/Department of Pediatrics, Boston Medical Center, and Leo Beletsky is with the School of Law and Bouvé College of Health Sciences, Northeastern University, Boston. Mr. Beletsky reported sitting on the advisory board of a data analytics company that has an interest in the U.S. opioid crisis. These comments are from their accompanying editorial (BMJ. 2018 Jun 13. doi: 10.1136/bmj.k2480 ). Mr. Beletsky reported sitting on the advisory board of a data analytics company that has an interest in the U.S. opioid crisis.
The overdose crisis in the United States will likely worsen if “supply-side” measures, such as the tighter prescribing regulations evaluated in this study, are not coupled with interventions to reduce harm and decrease demand, according to Scott E. Hadland, MD, and Leo Beletsky.
On a more basic level, the analysis by James Martin and colleagues raises questions about the use of drug scheduling to regulate public health, the authors concluded in an accompanying editorial.
“The U.S. scheduling scheme inexplicably holds such disparate substances as cannabis, heroin, and psilocybin to be equally dangerous,” they wrote. “It is high time to rethink how, why, and when this regulatory framework is deployed to curb drug-related harms.”
The shift from schedule III to schedule II creates barriers to medication access that disproportionately affect individuals in rural areas and those with limited mobility, since refills for schedule II drugs can only be obtained through an in-person visit to a provider and pharmacist, the authors wrote.
Scott E Hadland, MD, is with Grayken Center for Addiction/Department of Pediatrics, Boston Medical Center, and Leo Beletsky is with the School of Law and Bouvé College of Health Sciences, Northeastern University, Boston. Mr. Beletsky reported sitting on the advisory board of a data analytics company that has an interest in the U.S. opioid crisis. These comments are from their accompanying editorial (BMJ. 2018 Jun 13. doi: 10.1136/bmj.k2480 ). Mr. Beletsky reported sitting on the advisory board of a data analytics company that has an interest in the U.S. opioid crisis.
Illicit online sales of opioids increased significantly following a 2014 regulatory decision that restricted the legal supply of opioids in the United States, results of a recent analysis show.
Opioid sales in the so-called cryptomarkets also shifted toward more potent drugs, including fentanyl, after the U.S. Drug Enforcement Administration rescheduled hydrocodone combination products, making them harder to access, authors of the analysis reported.
Those results suggest “the possibility of a causal relation” between the schedule change and sales trends on cryptomarket sites, according to James Martin, PhD, of Swinburne University, Melbourne, Australia, and co-authors.
“Our analysis cannot rule out other possible causal explanatory factors, but our results are consistent with the possibility that the schedule change might have directly contributed to the changes we observed in the supply of illicit opioids,” Dr. Martin and colleagues wrote in the BMJ.
Cryptomarkets outside the United States had no such uptick in opioid traffic over time, reinforcing the possibility that the 2014 DEA decision caused an increase in the illicit opioid supply. The analysis included 31 online illicit markets operating between October 2013 and July 2016.
Investigators collected data using web crawling software that downloaded HTML pages on the cryptomarket sites and extracted relevant information such as listing titles and drug types for later analysis. Generally, each market was fully crawled every 2 weeks. After data was scraped and extracted, they conducted an interrupted time series analysis of drug sales for each day of the data collection period.
Opioids represented 13.7% of all drug sales through U.S. cryptomarkets in July 2016, they found, compared with a modeled estimate of 6.7% of sales that would have occurred in the absence of the new schedule introduction.
“This represents an approximate doubling of the percentage of the total drug sales through cryptomarkets within the United States,” the authors wrote.
Fentanyl was the least purchased product at the beginning of the analysis, but by July 2016 it was the second most frequently purchased. No other drug category had any meaningful changes in the proportion of sales over time.
There was no way for researchers to confirm that U.S.-based cryptomarket sites were selling to customers in the United States or elsewhere. However, they said, cryptomarket buyers often use sites in the same country to avoid shipment losses and increased delivery time.
Part of the study were funded by the Social Sciences and Humanities Research Council of Canada, Macquarie University internal grants, and the Ministry of Justice and Security of the Netherlands. Study authors had no current financial relationships relevant to the study.
SOURCE: Martin J, et al. BMJ. 2018 Jun 13. doi: 10.1136/bmj.k2270.
Illicit online sales of opioids increased significantly following a 2014 regulatory decision that restricted the legal supply of opioids in the United States, results of a recent analysis show.
Opioid sales in the so-called cryptomarkets also shifted toward more potent drugs, including fentanyl, after the U.S. Drug Enforcement Administration rescheduled hydrocodone combination products, making them harder to access, authors of the analysis reported.
Those results suggest “the possibility of a causal relation” between the schedule change and sales trends on cryptomarket sites, according to James Martin, PhD, of Swinburne University, Melbourne, Australia, and co-authors.
“Our analysis cannot rule out other possible causal explanatory factors, but our results are consistent with the possibility that the schedule change might have directly contributed to the changes we observed in the supply of illicit opioids,” Dr. Martin and colleagues wrote in the BMJ.
Cryptomarkets outside the United States had no such uptick in opioid traffic over time, reinforcing the possibility that the 2014 DEA decision caused an increase in the illicit opioid supply. The analysis included 31 online illicit markets operating between October 2013 and July 2016.
Investigators collected data using web crawling software that downloaded HTML pages on the cryptomarket sites and extracted relevant information such as listing titles and drug types for later analysis. Generally, each market was fully crawled every 2 weeks. After data was scraped and extracted, they conducted an interrupted time series analysis of drug sales for each day of the data collection period.
Opioids represented 13.7% of all drug sales through U.S. cryptomarkets in July 2016, they found, compared with a modeled estimate of 6.7% of sales that would have occurred in the absence of the new schedule introduction.
“This represents an approximate doubling of the percentage of the total drug sales through cryptomarkets within the United States,” the authors wrote.
Fentanyl was the least purchased product at the beginning of the analysis, but by July 2016 it was the second most frequently purchased. No other drug category had any meaningful changes in the proportion of sales over time.
There was no way for researchers to confirm that U.S.-based cryptomarket sites were selling to customers in the United States or elsewhere. However, they said, cryptomarket buyers often use sites in the same country to avoid shipment losses and increased delivery time.
Part of the study were funded by the Social Sciences and Humanities Research Council of Canada, Macquarie University internal grants, and the Ministry of Justice and Security of the Netherlands. Study authors had no current financial relationships relevant to the study.
SOURCE: Martin J, et al. BMJ. 2018 Jun 13. doi: 10.1136/bmj.k2270.
FROM BMJ
Key clinical point: Major finding: Opioids represented 13.7% of all drug sales through U.S. cryptomarkets in July 2016, compared with a modeled estimate of 6.7%.
Study details: An analysis of 31 online cryptomarkets operating between October 2013 and July 2016.
Disclosures: Part of the study were funded by the Social Sciences and Humanities Research Council of Canada, Macquarie University internal grants, and the Ministry of Justice and Security of the Netherlands. Study authors reported having no current financial relationships relevant to the study.
Source: Martin J, et al. BMJ. 2018 Jun 13. doi: 10.1136/bmj.k2270.
SLN mapping is most cost-effective in low-risk endometrial carcinoma
Researchers conducting a cost-utility analysis of sentinel lymph node (SLN) mapping and lymph node dissection (LND), both selective and routine, for low-risk endometrial carcinoma (clinical stage 1 disease with grade 1-2 endometrioid histology on preoperative endometrial biopsy), found that the SLN mapping had the lowest costs and the highest quality-adjusted survival of the three strategies.
Between the two strategies of LND, selective LND based on intraoperative frozen section was more cost-effective than routine LND.
The researchers created a model using data from past studies and clinical estimates. “Our biggest assumption was that sentinel lymph node mapping is associated with a decreased risk of lymphedema compared with lymph node dissection ... [from] several studies showing that having less than five lymph nodes excised was associated with a much smaller risk of developing lymphedema,” wrote Rudy S. Suidan, MD, of the MD Anderson Cancer Center, Houston, and his coauthors. Lymphedema was the main factor affecting quality of life in the analysis.
The analysis included estimates of rates of lymphadenectomy, bilateral mapping, and unilateral mapping, 3-year disease-specific survival, and overall survival, all of which were compared with third-party reimbursement costs at 2016 Medicare rates.
SLN mapping cost $16,401 per patient, while selective LND cost $17,036 per patient and routine LND cost $18,041 per patient. These strategies had quality-adjusted life years of 2.87, 2.81, and 2.79, respectively.
The superior cost-effectiveness of SLN mapping held, even when the researchers altered several of the variables in the model, including assuming open surgery instead of minimally invasive, and altering the assumed risk of lymphedema.
In addition to the possible limitation of making assumptions about SLN mapping and lymphedema, the researchers also pointed to the 3-year survival rates as shorter-term than preferable, driven by the available literature. The quality-adjusted life years did not differ much between the strategies because “most patients with low-risk cancer tend to have good clinical outcomes,” they wrote.
“This adds to the body of literature evaluating the clinical benefits of this strategy and may help health care providers in the decision-making process as they consider which approach to use,” the researchers wrote.
Dr. Suidan is supported by an NIH grant. Three coauthors reported other grants and fellowships. Five coauthors reported research support from AstraZeneca, Bayer, Clovis Oncology, and several other companies.
SOURCE: Suidan RS et al. Obstet Gynecol. 2018 Jun 11;132:52-8.
Researchers conducting a cost-utility analysis of sentinel lymph node (SLN) mapping and lymph node dissection (LND), both selective and routine, for low-risk endometrial carcinoma (clinical stage 1 disease with grade 1-2 endometrioid histology on preoperative endometrial biopsy), found that the SLN mapping had the lowest costs and the highest quality-adjusted survival of the three strategies.
Between the two strategies of LND, selective LND based on intraoperative frozen section was more cost-effective than routine LND.
The researchers created a model using data from past studies and clinical estimates. “Our biggest assumption was that sentinel lymph node mapping is associated with a decreased risk of lymphedema compared with lymph node dissection ... [from] several studies showing that having less than five lymph nodes excised was associated with a much smaller risk of developing lymphedema,” wrote Rudy S. Suidan, MD, of the MD Anderson Cancer Center, Houston, and his coauthors. Lymphedema was the main factor affecting quality of life in the analysis.
The analysis included estimates of rates of lymphadenectomy, bilateral mapping, and unilateral mapping, 3-year disease-specific survival, and overall survival, all of which were compared with third-party reimbursement costs at 2016 Medicare rates.
SLN mapping cost $16,401 per patient, while selective LND cost $17,036 per patient and routine LND cost $18,041 per patient. These strategies had quality-adjusted life years of 2.87, 2.81, and 2.79, respectively.
The superior cost-effectiveness of SLN mapping held, even when the researchers altered several of the variables in the model, including assuming open surgery instead of minimally invasive, and altering the assumed risk of lymphedema.
In addition to the possible limitation of making assumptions about SLN mapping and lymphedema, the researchers also pointed to the 3-year survival rates as shorter-term than preferable, driven by the available literature. The quality-adjusted life years did not differ much between the strategies because “most patients with low-risk cancer tend to have good clinical outcomes,” they wrote.
“This adds to the body of literature evaluating the clinical benefits of this strategy and may help health care providers in the decision-making process as they consider which approach to use,” the researchers wrote.
Dr. Suidan is supported by an NIH grant. Three coauthors reported other grants and fellowships. Five coauthors reported research support from AstraZeneca, Bayer, Clovis Oncology, and several other companies.
SOURCE: Suidan RS et al. Obstet Gynecol. 2018 Jun 11;132:52-8.
Researchers conducting a cost-utility analysis of sentinel lymph node (SLN) mapping and lymph node dissection (LND), both selective and routine, for low-risk endometrial carcinoma (clinical stage 1 disease with grade 1-2 endometrioid histology on preoperative endometrial biopsy), found that the SLN mapping had the lowest costs and the highest quality-adjusted survival of the three strategies.
Between the two strategies of LND, selective LND based on intraoperative frozen section was more cost-effective than routine LND.
The researchers created a model using data from past studies and clinical estimates. “Our biggest assumption was that sentinel lymph node mapping is associated with a decreased risk of lymphedema compared with lymph node dissection ... [from] several studies showing that having less than five lymph nodes excised was associated with a much smaller risk of developing lymphedema,” wrote Rudy S. Suidan, MD, of the MD Anderson Cancer Center, Houston, and his coauthors. Lymphedema was the main factor affecting quality of life in the analysis.
The analysis included estimates of rates of lymphadenectomy, bilateral mapping, and unilateral mapping, 3-year disease-specific survival, and overall survival, all of which were compared with third-party reimbursement costs at 2016 Medicare rates.
SLN mapping cost $16,401 per patient, while selective LND cost $17,036 per patient and routine LND cost $18,041 per patient. These strategies had quality-adjusted life years of 2.87, 2.81, and 2.79, respectively.
The superior cost-effectiveness of SLN mapping held, even when the researchers altered several of the variables in the model, including assuming open surgery instead of minimally invasive, and altering the assumed risk of lymphedema.
In addition to the possible limitation of making assumptions about SLN mapping and lymphedema, the researchers also pointed to the 3-year survival rates as shorter-term than preferable, driven by the available literature. The quality-adjusted life years did not differ much between the strategies because “most patients with low-risk cancer tend to have good clinical outcomes,” they wrote.
“This adds to the body of literature evaluating the clinical benefits of this strategy and may help health care providers in the decision-making process as they consider which approach to use,” the researchers wrote.
Dr. Suidan is supported by an NIH grant. Three coauthors reported other grants and fellowships. Five coauthors reported research support from AstraZeneca, Bayer, Clovis Oncology, and several other companies.
SOURCE: Suidan RS et al. Obstet Gynecol. 2018 Jun 11;132:52-8.
FROM OBSTETRICS & GYNECOLOGY
Ankylosing spondylitis diagnosis linked to self-harm attempts
AMSTERDAM – There is an increased relative risk of deliberate self-harm that results in emergency treatment among individuals newly diagnosed with ankylosing spondylitis, according to the results of a large, Canadian population-based study.
A diagnosis of ankylosing spondylitis was associated with a 59% increased risk of deliberate self-harm, compared with no diagnosis (HR = 1.59, 95% CI, 1.16-2.21). While the risk of deliberate self-harm in patients diagnosed with rheumatoid arthritis (RA) was initially elevated, the association was not significant after adjustment for confounding factors (HR = 1.08, 95% CI, 0.87-1.34).
These findings call for heightened awareness among clinicians, study investigator Nigil Haroon, MD, PhD, said in an interview at the European Congress of Rheumatology. “Depression is generally well known to be increased in patients with chronic diseases, especially so with chronic inflammatory rheumatic diseases like ankylosing spondylitis and rheumatoid arthritis,” he said. This may in turn be linked to increased cases of deliberate self-harm, but there have been few studies to determine if this is the case, he said, which may be because it is a relatively rare event in routine clinical practice.
Dr. Haroon, who runs a specialist clinic in ankylosing spondylitis in Toronto, has seen the long-term effects of chronic pain, lack of social support, and inability to sleep on patients’ mood first hand. This is what drove him and other colleagues at the University of Toronto and University Health Network to look at the possibility that this could be linked to an increased risk for depression and perhaps deliberate self-harm among newly diagnosed patients.
To try to estimate the risk, they obtained administrative data on more than 100,000 individuals diagnosed with ankylosing spondylitis or RA in the province of Ontario, Canada, between 2002 and 2014. Excluding those with a history of mental illness or a prior self-harm attempt resulted in the creation of two cohorts of patients – 13,964 with ankylosing spondylitis and 53,240 with RA. Indviduals in these two cohorts were then matched, 4:1, to similar controls in the general population.
The average age of those diagnosed with ankylosing spondylitis was 46 years and of those with RA was 57 years, with more males than females in the ankylosing spondylitis group (57% vs. 43%) and more females than males in the RA group (67% vs. 33%).
The main outcome assessed was the first episode of intentional self-injury or self-poisoning that required emergency treatment that occurred after the diagnosis of ankylosing spondylitis or RA.
Overall, there were 69 deliberate self-harm attempts recorded in the ankylosing spondylitis patient group, compared with 131 attempts in the non-ankylosing spondylitis group. In the RA patient group, there were 129 attempts, and 372 attempts in the non-RA group.
Poisoning was “by far the most common modality” used to intentionally self-harm, used by 67% of patients with ankylosing spondylitis and by 81% of those with RA, Dr. Haroon reported. Contact with a sharp object was the second most common method used to deliberately self-harm by 30% of ankylosing spondylitis patients and 16% of RA patients.
Most (70%) patients were discharged following emergency treatment for a deliberate self-harm attempt, with around 15% of ankylosing spondylitis and 22% of RA patients requiring hospital admission.
“For any chronic disease there is a potential for depression to settle, and we should identify [patients] early, even at the primary care levels itself and try to address it,” Dr. Haroon advised. It’s important to spend time and to develop a good rapport with your patients, he added, which can help them open up and talk about their mood.
The work was funded by the Division of Rheumatology Pfizer Research Chair, University of Toronto. Dr. Haroon reported having no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SOURCE: Kuriya B et al. Ann Rheum Dis. 2018;77(Suppl 2):195. Abstract OP0296.
AMSTERDAM – There is an increased relative risk of deliberate self-harm that results in emergency treatment among individuals newly diagnosed with ankylosing spondylitis, according to the results of a large, Canadian population-based study.
A diagnosis of ankylosing spondylitis was associated with a 59% increased risk of deliberate self-harm, compared with no diagnosis (HR = 1.59, 95% CI, 1.16-2.21). While the risk of deliberate self-harm in patients diagnosed with rheumatoid arthritis (RA) was initially elevated, the association was not significant after adjustment for confounding factors (HR = 1.08, 95% CI, 0.87-1.34).
These findings call for heightened awareness among clinicians, study investigator Nigil Haroon, MD, PhD, said in an interview at the European Congress of Rheumatology. “Depression is generally well known to be increased in patients with chronic diseases, especially so with chronic inflammatory rheumatic diseases like ankylosing spondylitis and rheumatoid arthritis,” he said. This may in turn be linked to increased cases of deliberate self-harm, but there have been few studies to determine if this is the case, he said, which may be because it is a relatively rare event in routine clinical practice.
Dr. Haroon, who runs a specialist clinic in ankylosing spondylitis in Toronto, has seen the long-term effects of chronic pain, lack of social support, and inability to sleep on patients’ mood first hand. This is what drove him and other colleagues at the University of Toronto and University Health Network to look at the possibility that this could be linked to an increased risk for depression and perhaps deliberate self-harm among newly diagnosed patients.
To try to estimate the risk, they obtained administrative data on more than 100,000 individuals diagnosed with ankylosing spondylitis or RA in the province of Ontario, Canada, between 2002 and 2014. Excluding those with a history of mental illness or a prior self-harm attempt resulted in the creation of two cohorts of patients – 13,964 with ankylosing spondylitis and 53,240 with RA. Indviduals in these two cohorts were then matched, 4:1, to similar controls in the general population.
The average age of those diagnosed with ankylosing spondylitis was 46 years and of those with RA was 57 years, with more males than females in the ankylosing spondylitis group (57% vs. 43%) and more females than males in the RA group (67% vs. 33%).
The main outcome assessed was the first episode of intentional self-injury or self-poisoning that required emergency treatment that occurred after the diagnosis of ankylosing spondylitis or RA.
Overall, there were 69 deliberate self-harm attempts recorded in the ankylosing spondylitis patient group, compared with 131 attempts in the non-ankylosing spondylitis group. In the RA patient group, there were 129 attempts, and 372 attempts in the non-RA group.
Poisoning was “by far the most common modality” used to intentionally self-harm, used by 67% of patients with ankylosing spondylitis and by 81% of those with RA, Dr. Haroon reported. Contact with a sharp object was the second most common method used to deliberately self-harm by 30% of ankylosing spondylitis patients and 16% of RA patients.
Most (70%) patients were discharged following emergency treatment for a deliberate self-harm attempt, with around 15% of ankylosing spondylitis and 22% of RA patients requiring hospital admission.
“For any chronic disease there is a potential for depression to settle, and we should identify [patients] early, even at the primary care levels itself and try to address it,” Dr. Haroon advised. It’s important to spend time and to develop a good rapport with your patients, he added, which can help them open up and talk about their mood.
The work was funded by the Division of Rheumatology Pfizer Research Chair, University of Toronto. Dr. Haroon reported having no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SOURCE: Kuriya B et al. Ann Rheum Dis. 2018;77(Suppl 2):195. Abstract OP0296.
AMSTERDAM – There is an increased relative risk of deliberate self-harm that results in emergency treatment among individuals newly diagnosed with ankylosing spondylitis, according to the results of a large, Canadian population-based study.
A diagnosis of ankylosing spondylitis was associated with a 59% increased risk of deliberate self-harm, compared with no diagnosis (HR = 1.59, 95% CI, 1.16-2.21). While the risk of deliberate self-harm in patients diagnosed with rheumatoid arthritis (RA) was initially elevated, the association was not significant after adjustment for confounding factors (HR = 1.08, 95% CI, 0.87-1.34).
These findings call for heightened awareness among clinicians, study investigator Nigil Haroon, MD, PhD, said in an interview at the European Congress of Rheumatology. “Depression is generally well known to be increased in patients with chronic diseases, especially so with chronic inflammatory rheumatic diseases like ankylosing spondylitis and rheumatoid arthritis,” he said. This may in turn be linked to increased cases of deliberate self-harm, but there have been few studies to determine if this is the case, he said, which may be because it is a relatively rare event in routine clinical practice.
Dr. Haroon, who runs a specialist clinic in ankylosing spondylitis in Toronto, has seen the long-term effects of chronic pain, lack of social support, and inability to sleep on patients’ mood first hand. This is what drove him and other colleagues at the University of Toronto and University Health Network to look at the possibility that this could be linked to an increased risk for depression and perhaps deliberate self-harm among newly diagnosed patients.
To try to estimate the risk, they obtained administrative data on more than 100,000 individuals diagnosed with ankylosing spondylitis or RA in the province of Ontario, Canada, between 2002 and 2014. Excluding those with a history of mental illness or a prior self-harm attempt resulted in the creation of two cohorts of patients – 13,964 with ankylosing spondylitis and 53,240 with RA. Indviduals in these two cohorts were then matched, 4:1, to similar controls in the general population.
The average age of those diagnosed with ankylosing spondylitis was 46 years and of those with RA was 57 years, with more males than females in the ankylosing spondylitis group (57% vs. 43%) and more females than males in the RA group (67% vs. 33%).
The main outcome assessed was the first episode of intentional self-injury or self-poisoning that required emergency treatment that occurred after the diagnosis of ankylosing spondylitis or RA.
Overall, there were 69 deliberate self-harm attempts recorded in the ankylosing spondylitis patient group, compared with 131 attempts in the non-ankylosing spondylitis group. In the RA patient group, there were 129 attempts, and 372 attempts in the non-RA group.
Poisoning was “by far the most common modality” used to intentionally self-harm, used by 67% of patients with ankylosing spondylitis and by 81% of those with RA, Dr. Haroon reported. Contact with a sharp object was the second most common method used to deliberately self-harm by 30% of ankylosing spondylitis patients and 16% of RA patients.
Most (70%) patients were discharged following emergency treatment for a deliberate self-harm attempt, with around 15% of ankylosing spondylitis and 22% of RA patients requiring hospital admission.
“For any chronic disease there is a potential for depression to settle, and we should identify [patients] early, even at the primary care levels itself and try to address it,” Dr. Haroon advised. It’s important to spend time and to develop a good rapport with your patients, he added, which can help them open up and talk about their mood.
The work was funded by the Division of Rheumatology Pfizer Research Chair, University of Toronto. Dr. Haroon reported having no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SOURCE: Kuriya B et al. Ann Rheum Dis. 2018;77(Suppl 2):195. Abstract OP0296.
REPORTING FROM THE EULAR 2018 CONGRESS
Key clinical point: Major finding: Newly-diagnosed individuals with ankylosing spondylitis are more likely to attempt self harm than those without the diagnosis (HR = 1.59, 95% CI, 1.16-2.21).
Study details: Population-based study of 13,964 individuals with ankylosing spondylitis, 53,240 individuals with RA, and matched controls from the general population.
Disclosures: The work was funded by the Division of Rheumatology Pfizer Research Chair, University of Toronto. Dr. Haroon reported having no relevant financial disclosures.
Source: Kuriya B et al. Ann Rheum Dis. 2018;77(Suppl 2):195. Abstract OP0296.
Axial SpA diagnostic strategies need not be sex-specific
AMSTERDAM – Imaging and a positive HLA-B27 test are effective tools for early diagnosis of axial spondyloarthritis in both men and women, according to data from a study of 719 patients with chronic back pain reported at the European Congress of Rheumatology.
Data from previous studies have shown greater severity of axial spondyloarthritis (axSpA) in men, but gender differences at first presentation of the disease have not been well studied, noted Dr. Augusta Ortolan of the University of Padova (Italy) and her colleagues.
The researchers analyzed baseline data from 444 women and 275 men in the Spondyloarthritis Caught Early (SPACE) cohort, which included patients with chronic back pain that had lasted 3 months to 2 years. The patients were younger than 45 years at the onset of symptoms.
Overall, 53% of men and 35% of women were diagnosed with axSpA. The duration of symptoms was similar between genders, but the average age at diagnosis was significantly younger for men, compared with women (27 years vs. 30 years; P = .021). A positive HLA-B27 test was more common among men with axSpA than among women with axSpA (80% vs. 60%; P less than .001).
Similarly, the presence of axial spondyloarthritis features on imaging was greater among men with axSpA than women with axSpA (78% vs. 64%; P = .007).
Dr. Ortolan said in an interview that she was somewhat surprised by the findings. “I probably expected to see more differences between male and female patients,” she said. “Although it has been demonstrated that the proportion of men to women is more balanced in axial spondyloarthritis in general [nearly 1:1] as compared to the more advanced stages of the disease [known as ankylosing spondylitis or radiographic axial spondyloarthritis, where the male-to-female ratio is around 3:1], there is a tendency to believe that spondyloarthritis in females is rarer or at least more difficult to detect,” she said. “In fact, the study does not completely contradict this belief as we found that from our chronic back pain population, males are twice as likely to be diagnosed. However, all in all, they do not present so much differently than women,” she noted.
The take home message is for clinicians to examine all features that may lead to a diagnosis of axSpA in patients regardless of gender, Dr. Ortolan said. The study results showing that HLA-B27 and imaging are strongly associated with axSpA diagnosis in both genders in a multivariate analysis, which suggests that clinicians do not need to adopt different diagnostic strategies, she said.
The study findings were the result of a cross-sectional approach that was based on an examination of baseline data, Dr. Ortolan noted. “However, it would be really interesting to see what happens in the long term: Do these gender differences tend to increase? Does the disease have a different long-term impact in men and women? Should we treat them differently? These are open questions that need to be addressed in the future,” she said.
Dr. Ortolan and her colleagues had no relevant disclosures.
Mitchel L. Zoler contributed to this story.
SOURCE: Ortolan A et al. Ann Rheum Dis. 2018;77(Suppl 2):207-8. Abstract OP0323.
AMSTERDAM – Imaging and a positive HLA-B27 test are effective tools for early diagnosis of axial spondyloarthritis in both men and women, according to data from a study of 719 patients with chronic back pain reported at the European Congress of Rheumatology.
Data from previous studies have shown greater severity of axial spondyloarthritis (axSpA) in men, but gender differences at first presentation of the disease have not been well studied, noted Dr. Augusta Ortolan of the University of Padova (Italy) and her colleagues.
The researchers analyzed baseline data from 444 women and 275 men in the Spondyloarthritis Caught Early (SPACE) cohort, which included patients with chronic back pain that had lasted 3 months to 2 years. The patients were younger than 45 years at the onset of symptoms.
Overall, 53% of men and 35% of women were diagnosed with axSpA. The duration of symptoms was similar between genders, but the average age at diagnosis was significantly younger for men, compared with women (27 years vs. 30 years; P = .021). A positive HLA-B27 test was more common among men with axSpA than among women with axSpA (80% vs. 60%; P less than .001).
Similarly, the presence of axial spondyloarthritis features on imaging was greater among men with axSpA than women with axSpA (78% vs. 64%; P = .007).
Dr. Ortolan said in an interview that she was somewhat surprised by the findings. “I probably expected to see more differences between male and female patients,” she said. “Although it has been demonstrated that the proportion of men to women is more balanced in axial spondyloarthritis in general [nearly 1:1] as compared to the more advanced stages of the disease [known as ankylosing spondylitis or radiographic axial spondyloarthritis, where the male-to-female ratio is around 3:1], there is a tendency to believe that spondyloarthritis in females is rarer or at least more difficult to detect,” she said. “In fact, the study does not completely contradict this belief as we found that from our chronic back pain population, males are twice as likely to be diagnosed. However, all in all, they do not present so much differently than women,” she noted.
The take home message is for clinicians to examine all features that may lead to a diagnosis of axSpA in patients regardless of gender, Dr. Ortolan said. The study results showing that HLA-B27 and imaging are strongly associated with axSpA diagnosis in both genders in a multivariate analysis, which suggests that clinicians do not need to adopt different diagnostic strategies, she said.
The study findings were the result of a cross-sectional approach that was based on an examination of baseline data, Dr. Ortolan noted. “However, it would be really interesting to see what happens in the long term: Do these gender differences tend to increase? Does the disease have a different long-term impact in men and women? Should we treat them differently? These are open questions that need to be addressed in the future,” she said.
Dr. Ortolan and her colleagues had no relevant disclosures.
Mitchel L. Zoler contributed to this story.
SOURCE: Ortolan A et al. Ann Rheum Dis. 2018;77(Suppl 2):207-8. Abstract OP0323.
AMSTERDAM – Imaging and a positive HLA-B27 test are effective tools for early diagnosis of axial spondyloarthritis in both men and women, according to data from a study of 719 patients with chronic back pain reported at the European Congress of Rheumatology.
Data from previous studies have shown greater severity of axial spondyloarthritis (axSpA) in men, but gender differences at first presentation of the disease have not been well studied, noted Dr. Augusta Ortolan of the University of Padova (Italy) and her colleagues.
The researchers analyzed baseline data from 444 women and 275 men in the Spondyloarthritis Caught Early (SPACE) cohort, which included patients with chronic back pain that had lasted 3 months to 2 years. The patients were younger than 45 years at the onset of symptoms.
Overall, 53% of men and 35% of women were diagnosed with axSpA. The duration of symptoms was similar between genders, but the average age at diagnosis was significantly younger for men, compared with women (27 years vs. 30 years; P = .021). A positive HLA-B27 test was more common among men with axSpA than among women with axSpA (80% vs. 60%; P less than .001).
Similarly, the presence of axial spondyloarthritis features on imaging was greater among men with axSpA than women with axSpA (78% vs. 64%; P = .007).
Dr. Ortolan said in an interview that she was somewhat surprised by the findings. “I probably expected to see more differences between male and female patients,” she said. “Although it has been demonstrated that the proportion of men to women is more balanced in axial spondyloarthritis in general [nearly 1:1] as compared to the more advanced stages of the disease [known as ankylosing spondylitis or radiographic axial spondyloarthritis, where the male-to-female ratio is around 3:1], there is a tendency to believe that spondyloarthritis in females is rarer or at least more difficult to detect,” she said. “In fact, the study does not completely contradict this belief as we found that from our chronic back pain population, males are twice as likely to be diagnosed. However, all in all, they do not present so much differently than women,” she noted.
The take home message is for clinicians to examine all features that may lead to a diagnosis of axSpA in patients regardless of gender, Dr. Ortolan said. The study results showing that HLA-B27 and imaging are strongly associated with axSpA diagnosis in both genders in a multivariate analysis, which suggests that clinicians do not need to adopt different diagnostic strategies, she said.
The study findings were the result of a cross-sectional approach that was based on an examination of baseline data, Dr. Ortolan noted. “However, it would be really interesting to see what happens in the long term: Do these gender differences tend to increase? Does the disease have a different long-term impact in men and women? Should we treat them differently? These are open questions that need to be addressed in the future,” she said.
Dr. Ortolan and her colleagues had no relevant disclosures.
Mitchel L. Zoler contributed to this story.
SOURCE: Ortolan A et al. Ann Rheum Dis. 2018;77(Suppl 2):207-8. Abstract OP0323.
REPORTING FROM THE EULAR 2018 CONGRESS
Key clinical point: Although there are clear sex differences in early axSpA, HLA-B27 and imaging are key elements for a diagnosis of axSpA in both sexes.
Major finding: The presence of axSpA features on imaging was greater among men with axSpA than women with axSpA (78% vs. 64%; P = .007).
Study details: A cross-sectional study of baseline data from 444 women and 275 men in the Spondyloarthritis Caught Early (SPACE) cohort.
Disclosures: Dr. Ortolan and her colleagues had no relevant disclosures.
Source: Ortolan A et al. Ann Rheum Dis. 2018;77(Suppl 2):207-8. Abstract OP0323.
Maternal use of pot and tobacco may boost birth defect risk
SAN DIEGO – New research suggests that pregnant users of both cannabis and tobacco may put their unborn children at higher risk of birth defects and small head circumference than if they used either alone.
Researchers also found that 13% of pregnant Medicaid recipients surveyed reported using both cannabis and tobacco within the past month.
, especially if they smoke tobacco, said study lead author Victoria H. Coleman-Cowger, PhD, of the research organization Battelle, in an interview.
In some cases, in fact, pregnant women might think that marijuana is healthier than regular cigarettes, said Dr. Coleman-Cowger. She observed this phenomenon while conducting a smoking intervention study at a prenatal clinic that largely served poor, African American women.
“I learned that many participants were also smoking cannabis and felt that there was lower risk associated with cannabis use than with tobacco use,” she said. “Some women were decreasing their use of tobacco during pregnancy but increasing their use of cannabis.”
Dr. Coleman-Cowger’s observations at the clinic inspired the new study, which reports the findings of a convenience survey of 500 pregnant women.
The mean age in the group was 28, and 71% were black. Two-thirds were employed, and 29% were college graduates.
By comparison, the 45 women in the co-user group – who reported both cannabis and tobacco use in the past month – were 93% black, 42% employed, and 7% college graduates. (An additional 39 women reported tobacco use only, and 60 reported cannabis use only.)
Co-use also was correlated with “never married, being in the first trimester of pregnancy, not wanting to be pregnant when they were, past-month other substance use, and more frequent use of both cannabis and tobacco than either exclusive group,” Dr. Coleman-Cowger said.
In adjusted models, co-users were more likely (odds ratio, 5.7; P = .05) to give birth to babies with small head circumference than nonusers. The risks of giving birth to babies with small head circumference also were more likely among the tobacco-only users (OR, 4.8; P = .05) and cannabis-only users (OR, 2.0; P = .05), compared with nonusers. Birth defects also were more likely in the co-user group.
The study did not allow researchers to speculate on whether co-use may multiply risk vs. cannabis or tobacco use alone.
Dr. Coleman-Cowger said in light of the small sample size, the results should be interpreted with caution. One possible confounder is quantity of use, she said. “We did not assess quantity of use, but given our finding that frequency of use is higher among the co-use group, it could be that the co-use group is simply using more of each substance and thus impacting the health consequences.”
Current clinical practice guidelines do not suggest screening for cannabis use in pregnant women. But Dr. Coleman-Cowger said it’s “particularly important when tobacco use has been identified, though in states where substance use is considered child abuse, professional judgment should be utilized in terms of the legal implications of asking about use.”
More research is planned to better understand issues like quantity of use, and reasons why pregnant women co-use cannabis and tobacco, Dr. Coleman-Cowger said.
The National Institute on Drug Abuse funded the study, which Dr. Coleman-Cowger said is part of a larger project “to compare and validate screeners that assess prescription drug misuse and illicit drug use during pregnancy.” The study authors report no relevant disclosures.
SAN DIEGO – New research suggests that pregnant users of both cannabis and tobacco may put their unborn children at higher risk of birth defects and small head circumference than if they used either alone.
Researchers also found that 13% of pregnant Medicaid recipients surveyed reported using both cannabis and tobacco within the past month.
, especially if they smoke tobacco, said study lead author Victoria H. Coleman-Cowger, PhD, of the research organization Battelle, in an interview.
In some cases, in fact, pregnant women might think that marijuana is healthier than regular cigarettes, said Dr. Coleman-Cowger. She observed this phenomenon while conducting a smoking intervention study at a prenatal clinic that largely served poor, African American women.
“I learned that many participants were also smoking cannabis and felt that there was lower risk associated with cannabis use than with tobacco use,” she said. “Some women were decreasing their use of tobacco during pregnancy but increasing their use of cannabis.”
Dr. Coleman-Cowger’s observations at the clinic inspired the new study, which reports the findings of a convenience survey of 500 pregnant women.
The mean age in the group was 28, and 71% were black. Two-thirds were employed, and 29% were college graduates.
By comparison, the 45 women in the co-user group – who reported both cannabis and tobacco use in the past month – were 93% black, 42% employed, and 7% college graduates. (An additional 39 women reported tobacco use only, and 60 reported cannabis use only.)
Co-use also was correlated with “never married, being in the first trimester of pregnancy, not wanting to be pregnant when they were, past-month other substance use, and more frequent use of both cannabis and tobacco than either exclusive group,” Dr. Coleman-Cowger said.
In adjusted models, co-users were more likely (odds ratio, 5.7; P = .05) to give birth to babies with small head circumference than nonusers. The risks of giving birth to babies with small head circumference also were more likely among the tobacco-only users (OR, 4.8; P = .05) and cannabis-only users (OR, 2.0; P = .05), compared with nonusers. Birth defects also were more likely in the co-user group.
The study did not allow researchers to speculate on whether co-use may multiply risk vs. cannabis or tobacco use alone.
Dr. Coleman-Cowger said in light of the small sample size, the results should be interpreted with caution. One possible confounder is quantity of use, she said. “We did not assess quantity of use, but given our finding that frequency of use is higher among the co-use group, it could be that the co-use group is simply using more of each substance and thus impacting the health consequences.”
Current clinical practice guidelines do not suggest screening for cannabis use in pregnant women. But Dr. Coleman-Cowger said it’s “particularly important when tobacco use has been identified, though in states where substance use is considered child abuse, professional judgment should be utilized in terms of the legal implications of asking about use.”
More research is planned to better understand issues like quantity of use, and reasons why pregnant women co-use cannabis and tobacco, Dr. Coleman-Cowger said.
The National Institute on Drug Abuse funded the study, which Dr. Coleman-Cowger said is part of a larger project “to compare and validate screeners that assess prescription drug misuse and illicit drug use during pregnancy.” The study authors report no relevant disclosures.
SAN DIEGO – New research suggests that pregnant users of both cannabis and tobacco may put their unborn children at higher risk of birth defects and small head circumference than if they used either alone.
Researchers also found that 13% of pregnant Medicaid recipients surveyed reported using both cannabis and tobacco within the past month.
, especially if they smoke tobacco, said study lead author Victoria H. Coleman-Cowger, PhD, of the research organization Battelle, in an interview.
In some cases, in fact, pregnant women might think that marijuana is healthier than regular cigarettes, said Dr. Coleman-Cowger. She observed this phenomenon while conducting a smoking intervention study at a prenatal clinic that largely served poor, African American women.
“I learned that many participants were also smoking cannabis and felt that there was lower risk associated with cannabis use than with tobacco use,” she said. “Some women were decreasing their use of tobacco during pregnancy but increasing their use of cannabis.”
Dr. Coleman-Cowger’s observations at the clinic inspired the new study, which reports the findings of a convenience survey of 500 pregnant women.
The mean age in the group was 28, and 71% were black. Two-thirds were employed, and 29% were college graduates.
By comparison, the 45 women in the co-user group – who reported both cannabis and tobacco use in the past month – were 93% black, 42% employed, and 7% college graduates. (An additional 39 women reported tobacco use only, and 60 reported cannabis use only.)
Co-use also was correlated with “never married, being in the first trimester of pregnancy, not wanting to be pregnant when they were, past-month other substance use, and more frequent use of both cannabis and tobacco than either exclusive group,” Dr. Coleman-Cowger said.
In adjusted models, co-users were more likely (odds ratio, 5.7; P = .05) to give birth to babies with small head circumference than nonusers. The risks of giving birth to babies with small head circumference also were more likely among the tobacco-only users (OR, 4.8; P = .05) and cannabis-only users (OR, 2.0; P = .05), compared with nonusers. Birth defects also were more likely in the co-user group.
The study did not allow researchers to speculate on whether co-use may multiply risk vs. cannabis or tobacco use alone.
Dr. Coleman-Cowger said in light of the small sample size, the results should be interpreted with caution. One possible confounder is quantity of use, she said. “We did not assess quantity of use, but given our finding that frequency of use is higher among the co-use group, it could be that the co-use group is simply using more of each substance and thus impacting the health consequences.”
Current clinical practice guidelines do not suggest screening for cannabis use in pregnant women. But Dr. Coleman-Cowger said it’s “particularly important when tobacco use has been identified, though in states where substance use is considered child abuse, professional judgment should be utilized in terms of the legal implications of asking about use.”
More research is planned to better understand issues like quantity of use, and reasons why pregnant women co-use cannabis and tobacco, Dr. Coleman-Cowger said.
The National Institute on Drug Abuse funded the study, which Dr. Coleman-Cowger said is part of a larger project “to compare and validate screeners that assess prescription drug misuse and illicit drug use during pregnancy.” The study authors report no relevant disclosures.
REPORTING FROM CPDD 2018
Key clinical point: Couse of cannabis and tobacco by pregnant mothers may pose more risk to unborn children than use of either alone.
Major finding: Cousers had a higher risk of giving birth to children with birth detects and small head circumference than tobacco-only or cannabis-only users. In adjusted models, cousers were more likely (odds ratio, 5.7; P = .05) to give birth to babies with small head circumference than nonusers. The risks of giving birth to babies with small head circumference also were more likely among the tobacco-only users (OR, 4.8; P = .05) and cannabis-only users (OR, 2.0; P = .05), compared with nonusers.
Study details: Survey of 500 pregnant Medicaid recipients.
Disclosures: The National Institute on Drug Abuse funded the study. The study authors report no relevant disclosures.
Epstein-Barr ‘Switches On” Autoimmune Genes
The Epstein-Barr virus (EBV) protein can “switch on” risk genes for autoimmune diseases, according to researchers from the National Institute of Allergy and Infectious Diseases. They found that when EBV infects human immune cells, the virus produces a protein (EBNA2) that “recruits” human proteins (transcription factors) to bind to regions of both the EBV genome and the cell’s own genome. Together the viral protein and the human transcription factors change the expression of neighboring viral genes.
In the study, EBNA2 and its related transcription factors activated some of the genes associated with the risk for lupus, multiple sclerosis, rheumatoid arthritis, inflammatory bowel disease, type 1 diabetes, juvenile idiopathic arthritis, and celiac disease.
Previous research had also suggested that EBV infection plays a role in autoimmune diseases, particularly lupus. The researchers in the current study speculated that genetic analysis could further explain the relationship. They used a new computational and biochemical technique to sift through genetic and protein data, looking for potential regulatory regions in genes associated with the risk of developing lupus that also bound to EBNA2 and its transcription factors.
“We were surprised to see that nearly half of the locations on the human genome known to contribute to lupus risk were also binding sites for EBNA2,” said John Harley, MD, PhD, lead investigator.
The researchers caution, however, that many of the regulatory genes that contribute to lupus and other autoimmune disorders did not interact with EBNA2, and some individuals with activated regulatory genes do not develop disease.
The Epstein-Barr virus (EBV) protein can “switch on” risk genes for autoimmune diseases, according to researchers from the National Institute of Allergy and Infectious Diseases. They found that when EBV infects human immune cells, the virus produces a protein (EBNA2) that “recruits” human proteins (transcription factors) to bind to regions of both the EBV genome and the cell’s own genome. Together the viral protein and the human transcription factors change the expression of neighboring viral genes.
In the study, EBNA2 and its related transcription factors activated some of the genes associated with the risk for lupus, multiple sclerosis, rheumatoid arthritis, inflammatory bowel disease, type 1 diabetes, juvenile idiopathic arthritis, and celiac disease.
Previous research had also suggested that EBV infection plays a role in autoimmune diseases, particularly lupus. The researchers in the current study speculated that genetic analysis could further explain the relationship. They used a new computational and biochemical technique to sift through genetic and protein data, looking for potential regulatory regions in genes associated with the risk of developing lupus that also bound to EBNA2 and its transcription factors.
“We were surprised to see that nearly half of the locations on the human genome known to contribute to lupus risk were also binding sites for EBNA2,” said John Harley, MD, PhD, lead investigator.
The researchers caution, however, that many of the regulatory genes that contribute to lupus and other autoimmune disorders did not interact with EBNA2, and some individuals with activated regulatory genes do not develop disease.
The Epstein-Barr virus (EBV) protein can “switch on” risk genes for autoimmune diseases, according to researchers from the National Institute of Allergy and Infectious Diseases. They found that when EBV infects human immune cells, the virus produces a protein (EBNA2) that “recruits” human proteins (transcription factors) to bind to regions of both the EBV genome and the cell’s own genome. Together the viral protein and the human transcription factors change the expression of neighboring viral genes.
In the study, EBNA2 and its related transcription factors activated some of the genes associated with the risk for lupus, multiple sclerosis, rheumatoid arthritis, inflammatory bowel disease, type 1 diabetes, juvenile idiopathic arthritis, and celiac disease.
Previous research had also suggested that EBV infection plays a role in autoimmune diseases, particularly lupus. The researchers in the current study speculated that genetic analysis could further explain the relationship. They used a new computational and biochemical technique to sift through genetic and protein data, looking for potential regulatory regions in genes associated with the risk of developing lupus that also bound to EBNA2 and its transcription factors.
“We were surprised to see that nearly half of the locations on the human genome known to contribute to lupus risk were also binding sites for EBNA2,” said John Harley, MD, PhD, lead investigator.
The researchers caution, however, that many of the regulatory genes that contribute to lupus and other autoimmune disorders did not interact with EBNA2, and some individuals with activated regulatory genes do not develop disease.
‘Excellent’ survival with HCT despite early treatment failure in FL
Autologous and allogeneic hematopoietic stem cell transplantation (HCT) both offer excellent long-term survival in follicular lymphoma (FL) patients who experience early treatment failure, an analysis of a large transplant registry suggests.
Five-year survival rates exceeded 70% for patients who received autologous or matched sibling donor (MSD) transplants, according to the analysis of the Center for International Blood and Marrow Transplant Research (CIBMTR) database. The database included 440 patients who underwent a procedure between 2002 and 2014.
“Until better risk-stratification tools are available for FL, auto-HCT and MSD allo-HCT should be considered as effective treatment options with excellent long-term survival for high-risk patients as defined by early treatment failure,” Sonali M. Smith, MD, of the University of Chicago, and co-investigators wrote in the journal Cancer.
Early treatment failure in FL is associated with worse overall survival. In the National LymphoCare Study (NLCS), patients who received upfront R-CHOP therapy and progressed within 24 months had a 5-year overall survival of 50%, versus 90% for patients without early progression.
By contrast, survival figures in the present study are “provocatively higher” than those in the NLCS, in which only 8 out of 110 patients underwent HCT, Dr Smith and co-authors said.
Dr Smith’s study showed that with a median follow-up of 69 to 73 months, adjusted probability of 5-year overall survival was 70% for autologous and 73% for MSD HCT, versus 49% for matched unrelated donor HCT (P=0.0008).
Ryan C. Lynch, MD, and Ajay K. Gopal, MD, of the Fred Hutchinson Cancer Research Center in Seattle, Washington, said that the finding “convincingly demonstrates” the benefit of transplant in the setting of early treatment failure.
“Select patients (particularly younger patients) with chemoresponsive disease who understand the risk-benefit ratio in comparison with currently approved and experimental therapies still remain good candidates for autologous HCT,” Drs Lynch and Gopal said in an editorial.
“For older patients or patients with comorbidities, we would continue to recommend clinical trials or treatment with an approved PI3K inhibitor,” they added.
The study by Dr Smith and colleagues is not the first to show a benefit of HCT in this clinical scenario. In a recent NLCS/CIBMTR analysis of FL patients, 5-year overall survival was 73% for those undergoing autologous HCT done within a year of early treatment failure, versus 60% for those who did not (P=0.05).
The two studies “collectively suggest that transplantation should be considered in this high-risk group of patients with early relapse,” Dr Smith and co-authors wrote.
Autologous and allogeneic hematopoietic stem cell transplantation (HCT) both offer excellent long-term survival in follicular lymphoma (FL) patients who experience early treatment failure, an analysis of a large transplant registry suggests.
Five-year survival rates exceeded 70% for patients who received autologous or matched sibling donor (MSD) transplants, according to the analysis of the Center for International Blood and Marrow Transplant Research (CIBMTR) database. The database included 440 patients who underwent a procedure between 2002 and 2014.
“Until better risk-stratification tools are available for FL, auto-HCT and MSD allo-HCT should be considered as effective treatment options with excellent long-term survival for high-risk patients as defined by early treatment failure,” Sonali M. Smith, MD, of the University of Chicago, and co-investigators wrote in the journal Cancer.
Early treatment failure in FL is associated with worse overall survival. In the National LymphoCare Study (NLCS), patients who received upfront R-CHOP therapy and progressed within 24 months had a 5-year overall survival of 50%, versus 90% for patients without early progression.
By contrast, survival figures in the present study are “provocatively higher” than those in the NLCS, in which only 8 out of 110 patients underwent HCT, Dr Smith and co-authors said.
Dr Smith’s study showed that with a median follow-up of 69 to 73 months, adjusted probability of 5-year overall survival was 70% for autologous and 73% for MSD HCT, versus 49% for matched unrelated donor HCT (P=0.0008).
Ryan C. Lynch, MD, and Ajay K. Gopal, MD, of the Fred Hutchinson Cancer Research Center in Seattle, Washington, said that the finding “convincingly demonstrates” the benefit of transplant in the setting of early treatment failure.
“Select patients (particularly younger patients) with chemoresponsive disease who understand the risk-benefit ratio in comparison with currently approved and experimental therapies still remain good candidates for autologous HCT,” Drs Lynch and Gopal said in an editorial.
“For older patients or patients with comorbidities, we would continue to recommend clinical trials or treatment with an approved PI3K inhibitor,” they added.
The study by Dr Smith and colleagues is not the first to show a benefit of HCT in this clinical scenario. In a recent NLCS/CIBMTR analysis of FL patients, 5-year overall survival was 73% for those undergoing autologous HCT done within a year of early treatment failure, versus 60% for those who did not (P=0.05).
The two studies “collectively suggest that transplantation should be considered in this high-risk group of patients with early relapse,” Dr Smith and co-authors wrote.
Autologous and allogeneic hematopoietic stem cell transplantation (HCT) both offer excellent long-term survival in follicular lymphoma (FL) patients who experience early treatment failure, an analysis of a large transplant registry suggests.
Five-year survival rates exceeded 70% for patients who received autologous or matched sibling donor (MSD) transplants, according to the analysis of the Center for International Blood and Marrow Transplant Research (CIBMTR) database. The database included 440 patients who underwent a procedure between 2002 and 2014.
“Until better risk-stratification tools are available for FL, auto-HCT and MSD allo-HCT should be considered as effective treatment options with excellent long-term survival for high-risk patients as defined by early treatment failure,” Sonali M. Smith, MD, of the University of Chicago, and co-investigators wrote in the journal Cancer.
Early treatment failure in FL is associated with worse overall survival. In the National LymphoCare Study (NLCS), patients who received upfront R-CHOP therapy and progressed within 24 months had a 5-year overall survival of 50%, versus 90% for patients without early progression.
By contrast, survival figures in the present study are “provocatively higher” than those in the NLCS, in which only 8 out of 110 patients underwent HCT, Dr Smith and co-authors said.
Dr Smith’s study showed that with a median follow-up of 69 to 73 months, adjusted probability of 5-year overall survival was 70% for autologous and 73% for MSD HCT, versus 49% for matched unrelated donor HCT (P=0.0008).
Ryan C. Lynch, MD, and Ajay K. Gopal, MD, of the Fred Hutchinson Cancer Research Center in Seattle, Washington, said that the finding “convincingly demonstrates” the benefit of transplant in the setting of early treatment failure.
“Select patients (particularly younger patients) with chemoresponsive disease who understand the risk-benefit ratio in comparison with currently approved and experimental therapies still remain good candidates for autologous HCT,” Drs Lynch and Gopal said in an editorial.
“For older patients or patients with comorbidities, we would continue to recommend clinical trials or treatment with an approved PI3K inhibitor,” they added.
The study by Dr Smith and colleagues is not the first to show a benefit of HCT in this clinical scenario. In a recent NLCS/CIBMTR analysis of FL patients, 5-year overall survival was 73% for those undergoing autologous HCT done within a year of early treatment failure, versus 60% for those who did not (P=0.05).
The two studies “collectively suggest that transplantation should be considered in this high-risk group of patients with early relapse,” Dr Smith and co-authors wrote.