User login
Interim PET scans identify HL patients with better outcomes
CHICAGO—Interim PET scans can identify a subset of Hodgkin lymphoma (HL) patients with a better outcome suitable for de-escalation treatment after upfront BEACOPP without impairing disease control, according to final results of the AHL2011-LYSA study.
BEACOPP, compared to ABVD, improves progression-free survival (PFS) but not overall survival (OS) and is associated with a higher risk of myelodysplasia, acute leukemia, and infertility.
Investigators evaluated whether some patients might be able to reduce treatment intensity without compromising the effectiveness of their therapy.
Olivier Casasnovas, MD, of CHU Le Bocage Service d'Hématologie Clinique, Dijon, France, presented the final analysis at the 2018 ASCO Annual Meeting (abstract 7503).
AHL2011-LYSA study (NCT01358747)
The randomized phase 3 study compared an early PET-driven treatment de-escalation to a non-PET-monitored strategy in patients with advanced-stage HL.
The study included 823 previously untreated patients, median age 30 years (range 16 – 60), with stage III, IV, or high-risk IIB HL.
The PET-driven strategy consisted of 2 BEACOPP* cycles (PET2), followed by 4 cycles of ABVD** for PET2-negative patients, and 4 cycles of BEACOPP for PET2-positive patients.
The experimental PET-driven strategy (410 patients) was randomly compared to a standard treatment delivering 6 cycles of BEACOPP (413 patients). PFS was the primary endpoint with a hypothesis of non-inferiority of the PET-driven arm compared to the standard arm.
Patients characteristics were well balanced between the arms, Dr Casasnovas said. PET2-positivity rate was similar in both arms (experimental 13%, standard 12%).
Based on PET2 results, 346 (84%) patients received 4 cycles of ABVD and 51 (12%) patients received 4 additional cycles of BEACOPP in the experimental arm.
Results
With a median follow-up of 50 months, the 5-year PFS was similar in the standard (86.2%) and the PET-driven arms (85.7%). The 5-year PFS for PET 2-negative/PET 4-negative patients was 90.9%, for PET 2-positive/PET4-negative patients was 75.4%, and for PET 4-positive patients was 46.5%.
The 5-year OS was similar in both arms (96.4% experimental, 95.2% standard).
The treatment toxicity was significantly higher in patients receiving 6 cycles of BEACOPP as compared to those who received 2 cycles of BEACOPP plus 4 cycles of ABVD.
Those who received more cycles of BEACOPP had more frequent grade 3 or higher adverse events than those with fewer cycles, including anemia (11% vs 2%), leukopenia (85% vs 74%), thrombocytopenia (44% vs 15%), and sepsis (7% vs 3%), as well as in serious adverse events (45% vs 28%).
“After 4 cycles of chemotherapy, it [PET positivity] identifies a subset of patients with a particularly poor outcome,” Dr Casasnovas said, “encouraging researchers to develop new treatment options in these patients.”
“PET performed after 2 cycles of BEACOPP escalation can be safely used to guide subsequent treatment,” he concluded.
“This approach allows clinicians to reduce the treatment-related immediate toxicity in most patients,” he added, “and provides similar patient outcomes compared to standard BEACOPP escalation treatment.”
* Bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone
**Adriamycin (doxorubicin), bleomycin, vinblastine, dacarbazine
CHICAGO—Interim PET scans can identify a subset of Hodgkin lymphoma (HL) patients with a better outcome suitable for de-escalation treatment after upfront BEACOPP without impairing disease control, according to final results of the AHL2011-LYSA study.
BEACOPP, compared to ABVD, improves progression-free survival (PFS) but not overall survival (OS) and is associated with a higher risk of myelodysplasia, acute leukemia, and infertility.
Investigators evaluated whether some patients might be able to reduce treatment intensity without compromising the effectiveness of their therapy.
Olivier Casasnovas, MD, of CHU Le Bocage Service d'Hématologie Clinique, Dijon, France, presented the final analysis at the 2018 ASCO Annual Meeting (abstract 7503).
AHL2011-LYSA study (NCT01358747)
The randomized phase 3 study compared an early PET-driven treatment de-escalation to a non-PET-monitored strategy in patients with advanced-stage HL.
The study included 823 previously untreated patients, median age 30 years (range 16 – 60), with stage III, IV, or high-risk IIB HL.
The PET-driven strategy consisted of 2 BEACOPP* cycles (PET2), followed by 4 cycles of ABVD** for PET2-negative patients, and 4 cycles of BEACOPP for PET2-positive patients.
The experimental PET-driven strategy (410 patients) was randomly compared to a standard treatment delivering 6 cycles of BEACOPP (413 patients). PFS was the primary endpoint with a hypothesis of non-inferiority of the PET-driven arm compared to the standard arm.
Patients characteristics were well balanced between the arms, Dr Casasnovas said. PET2-positivity rate was similar in both arms (experimental 13%, standard 12%).
Based on PET2 results, 346 (84%) patients received 4 cycles of ABVD and 51 (12%) patients received 4 additional cycles of BEACOPP in the experimental arm.
Results
With a median follow-up of 50 months, the 5-year PFS was similar in the standard (86.2%) and the PET-driven arms (85.7%). The 5-year PFS for PET 2-negative/PET 4-negative patients was 90.9%, for PET 2-positive/PET4-negative patients was 75.4%, and for PET 4-positive patients was 46.5%.
The 5-year OS was similar in both arms (96.4% experimental, 95.2% standard).
The treatment toxicity was significantly higher in patients receiving 6 cycles of BEACOPP as compared to those who received 2 cycles of BEACOPP plus 4 cycles of ABVD.
Those who received more cycles of BEACOPP had more frequent grade 3 or higher adverse events than those with fewer cycles, including anemia (11% vs 2%), leukopenia (85% vs 74%), thrombocytopenia (44% vs 15%), and sepsis (7% vs 3%), as well as in serious adverse events (45% vs 28%).
“After 4 cycles of chemotherapy, it [PET positivity] identifies a subset of patients with a particularly poor outcome,” Dr Casasnovas said, “encouraging researchers to develop new treatment options in these patients.”
“PET performed after 2 cycles of BEACOPP escalation can be safely used to guide subsequent treatment,” he concluded.
“This approach allows clinicians to reduce the treatment-related immediate toxicity in most patients,” he added, “and provides similar patient outcomes compared to standard BEACOPP escalation treatment.”
* Bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone
**Adriamycin (doxorubicin), bleomycin, vinblastine, dacarbazine
CHICAGO—Interim PET scans can identify a subset of Hodgkin lymphoma (HL) patients with a better outcome suitable for de-escalation treatment after upfront BEACOPP without impairing disease control, according to final results of the AHL2011-LYSA study.
BEACOPP, compared to ABVD, improves progression-free survival (PFS) but not overall survival (OS) and is associated with a higher risk of myelodysplasia, acute leukemia, and infertility.
Investigators evaluated whether some patients might be able to reduce treatment intensity without compromising the effectiveness of their therapy.
Olivier Casasnovas, MD, of CHU Le Bocage Service d'Hématologie Clinique, Dijon, France, presented the final analysis at the 2018 ASCO Annual Meeting (abstract 7503).
AHL2011-LYSA study (NCT01358747)
The randomized phase 3 study compared an early PET-driven treatment de-escalation to a non-PET-monitored strategy in patients with advanced-stage HL.
The study included 823 previously untreated patients, median age 30 years (range 16 – 60), with stage III, IV, or high-risk IIB HL.
The PET-driven strategy consisted of 2 BEACOPP* cycles (PET2), followed by 4 cycles of ABVD** for PET2-negative patients, and 4 cycles of BEACOPP for PET2-positive patients.
The experimental PET-driven strategy (410 patients) was randomly compared to a standard treatment delivering 6 cycles of BEACOPP (413 patients). PFS was the primary endpoint with a hypothesis of non-inferiority of the PET-driven arm compared to the standard arm.
Patients characteristics were well balanced between the arms, Dr Casasnovas said. PET2-positivity rate was similar in both arms (experimental 13%, standard 12%).
Based on PET2 results, 346 (84%) patients received 4 cycles of ABVD and 51 (12%) patients received 4 additional cycles of BEACOPP in the experimental arm.
Results
With a median follow-up of 50 months, the 5-year PFS was similar in the standard (86.2%) and the PET-driven arms (85.7%). The 5-year PFS for PET 2-negative/PET 4-negative patients was 90.9%, for PET 2-positive/PET4-negative patients was 75.4%, and for PET 4-positive patients was 46.5%.
The 5-year OS was similar in both arms (96.4% experimental, 95.2% standard).
The treatment toxicity was significantly higher in patients receiving 6 cycles of BEACOPP as compared to those who received 2 cycles of BEACOPP plus 4 cycles of ABVD.
Those who received more cycles of BEACOPP had more frequent grade 3 or higher adverse events than those with fewer cycles, including anemia (11% vs 2%), leukopenia (85% vs 74%), thrombocytopenia (44% vs 15%), and sepsis (7% vs 3%), as well as in serious adverse events (45% vs 28%).
“After 4 cycles of chemotherapy, it [PET positivity] identifies a subset of patients with a particularly poor outcome,” Dr Casasnovas said, “encouraging researchers to develop new treatment options in these patients.”
“PET performed after 2 cycles of BEACOPP escalation can be safely used to guide subsequent treatment,” he concluded.
“This approach allows clinicians to reduce the treatment-related immediate toxicity in most patients,” he added, “and provides similar patient outcomes compared to standard BEACOPP escalation treatment.”
* Bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone
**Adriamycin (doxorubicin), bleomycin, vinblastine, dacarbazine
Azar blames PBMs for no drop in prescription prices
“The president said that, in reaction to the release of the drug pricing blueprint, drug companies would be ‘announcing voluntary massive drops in prices within 2 weeks,’ ” Sen. Elizabeth Warren (D-Mass.) said during a hearing of the Senate Health, Education, Labor, and Pensions Committee to review the administration’s plan to lower drug costs.
Sen. Warren said she, along with Sen. Tina Smith (D-Minn.), sent letters to the top 10 drug manufacturers to get a pricing update and see what products were going to be the recipient of price cuts in response to the blueprint.
She then asked Alex Azar, Health & Human Services secretary and the hearing’s only witness, which manufacturers the president was referring to when he said drug companies would be reducing prices.
“There are actually several drug companies that are looking at substantial and material decreases of drug prices in competitive classes and actually competing with each other and looking to do that,” Mr. Azar testified. “They are working right now with the pharmacy benefit managers (PBMs) and distributors.”
He went on to blame the PBMs for the inability to lower prices.
“What they are trying to do is work to ensure they are not discriminated against,” he said. “Oddly, the fear is that they would be discriminated against for decreasing their price.”
He noted during the hearing that PBMs get paid based on the rebates they negotiate and could retaliate against manufacturers by placing products on a higher tier or dropping them from formularies in total if manufacturers were to impact the PBM bottom line by dropping prices. He added that one of the options in the blueprint was to ban any financial transactions between the manufacturer and the PBM to ensure there is no conflict of interest and that the PBM is working on behalf of the insurers only to negotiate the best prices for drugs.
Panel Democrats used the hearing to hammer the administration for not following up on President Trump’s campaign promise to allow the government to negotiate Medicare Part D drug pricing. Part of that discussion focused on using government leverage to get “best price” contracts using prices for drugs in other countries, an exercise that Mr. Azar said would theoretically result in manufacturers yanking their drugs out of foreign markets and jacking the prices even more in the United States.
When pressed to try it on a pilot basis with one or two drugs, he pushed back, suggesting that even a pilot trial of it could result in “irreparable harm.”
“The president said that, in reaction to the release of the drug pricing blueprint, drug companies would be ‘announcing voluntary massive drops in prices within 2 weeks,’ ” Sen. Elizabeth Warren (D-Mass.) said during a hearing of the Senate Health, Education, Labor, and Pensions Committee to review the administration’s plan to lower drug costs.
Sen. Warren said she, along with Sen. Tina Smith (D-Minn.), sent letters to the top 10 drug manufacturers to get a pricing update and see what products were going to be the recipient of price cuts in response to the blueprint.
She then asked Alex Azar, Health & Human Services secretary and the hearing’s only witness, which manufacturers the president was referring to when he said drug companies would be reducing prices.
“There are actually several drug companies that are looking at substantial and material decreases of drug prices in competitive classes and actually competing with each other and looking to do that,” Mr. Azar testified. “They are working right now with the pharmacy benefit managers (PBMs) and distributors.”
He went on to blame the PBMs for the inability to lower prices.
“What they are trying to do is work to ensure they are not discriminated against,” he said. “Oddly, the fear is that they would be discriminated against for decreasing their price.”
He noted during the hearing that PBMs get paid based on the rebates they negotiate and could retaliate against manufacturers by placing products on a higher tier or dropping them from formularies in total if manufacturers were to impact the PBM bottom line by dropping prices. He added that one of the options in the blueprint was to ban any financial transactions between the manufacturer and the PBM to ensure there is no conflict of interest and that the PBM is working on behalf of the insurers only to negotiate the best prices for drugs.
Panel Democrats used the hearing to hammer the administration for not following up on President Trump’s campaign promise to allow the government to negotiate Medicare Part D drug pricing. Part of that discussion focused on using government leverage to get “best price” contracts using prices for drugs in other countries, an exercise that Mr. Azar said would theoretically result in manufacturers yanking their drugs out of foreign markets and jacking the prices even more in the United States.
When pressed to try it on a pilot basis with one or two drugs, he pushed back, suggesting that even a pilot trial of it could result in “irreparable harm.”
“The president said that, in reaction to the release of the drug pricing blueprint, drug companies would be ‘announcing voluntary massive drops in prices within 2 weeks,’ ” Sen. Elizabeth Warren (D-Mass.) said during a hearing of the Senate Health, Education, Labor, and Pensions Committee to review the administration’s plan to lower drug costs.
Sen. Warren said she, along with Sen. Tina Smith (D-Minn.), sent letters to the top 10 drug manufacturers to get a pricing update and see what products were going to be the recipient of price cuts in response to the blueprint.
She then asked Alex Azar, Health & Human Services secretary and the hearing’s only witness, which manufacturers the president was referring to when he said drug companies would be reducing prices.
“There are actually several drug companies that are looking at substantial and material decreases of drug prices in competitive classes and actually competing with each other and looking to do that,” Mr. Azar testified. “They are working right now with the pharmacy benefit managers (PBMs) and distributors.”
He went on to blame the PBMs for the inability to lower prices.
“What they are trying to do is work to ensure they are not discriminated against,” he said. “Oddly, the fear is that they would be discriminated against for decreasing their price.”
He noted during the hearing that PBMs get paid based on the rebates they negotiate and could retaliate against manufacturers by placing products on a higher tier or dropping them from formularies in total if manufacturers were to impact the PBM bottom line by dropping prices. He added that one of the options in the blueprint was to ban any financial transactions between the manufacturer and the PBM to ensure there is no conflict of interest and that the PBM is working on behalf of the insurers only to negotiate the best prices for drugs.
Panel Democrats used the hearing to hammer the administration for not following up on President Trump’s campaign promise to allow the government to negotiate Medicare Part D drug pricing. Part of that discussion focused on using government leverage to get “best price” contracts using prices for drugs in other countries, an exercise that Mr. Azar said would theoretically result in manufacturers yanking their drugs out of foreign markets and jacking the prices even more in the United States.
When pressed to try it on a pilot basis with one or two drugs, he pushed back, suggesting that even a pilot trial of it could result in “irreparable harm.”
6 ways to reduce liability by improving doc-nurse teams
Positive relationships between physicians and nurses not only make for a smoother work environment, they also may reduce medical errors and lower the risk of lawsuits.
A recent study of closed claims by national medical malpractice insurer The Doctors Company found that poor physician oversight is a key contributor to lawsuits against nurses. Investigators analyzed 67 nurse practitioner (NP) claims from January 2011 to December 2016 and compared them with 1,358 claims against primary care physicians during the same time period.
Diagnostic and medication errors were the most common allegations against NPs, the study found, a trend that matched the most frequent allegations against primary care (internal medicine and family medicine) doctors. Top administrative factors that prompted lawsuits against nurses included inadequate physician supervision, failure to adhere to scope of practice, and absence of or deviation from written protocols.
The findings illustrate the importance of effective collaboration between physicians and NPs, said Darrell Ranum, vice president for patient safety and risk management for The Doctors Co. Below, legal experts share six ways to strengthen the physician-nurse relationship and at the same time, reduce liability:
1. Foster open dialogue. Cultivating a comfortable environment where nurses and physicians feel at ease sharing concerns and problems is a key step, says Louise B. Andrew, MD, JD, a physician and attorney who specializes in litigation stress management. A common scenario is a nurse who notices an abnormal vital sign but fails to mention it to the supervising physician because they feel they can handle it themselves or because they believe the doctor is too busy or too tired to be bothered, she said. The patient’s condition then worsens, resulting in a poor outcome that could have been avoided with better communication among providers. Delayed/wrong diagnosis accounted for 41% of claims against primary care physicians and 48% of claims against NPs in The Doctors Company study.
Set the tone early by exemplifying positive and clear communication, practicing good listening, and remaining empathetic, yet firm when making your needs known, Dr. Andrew advised.
“In the medical setting, you are always communicating for the benefit of the patient, and it is good to both keep this in mind, and to say it out loud,” she said.
2. Stick to the scope. When hiring an NP, make sure their scope of practice is clearly understood by all parties and respect their limitations, said Melanie L. Balestra, a Newport Beach, Calif., attorney and nurse practitioner who represents health providers.
Nurses practitioners must refrain from overstepping their authority, but physicians also must be careful not to ask too much of their NPs, experts stress. Ms. Balestra notes there is frequent confusion among doctors and NPs over how and whether scope of practice can be expanded as needed.
“This happens all the time,” Ms. Balestra said. “I get at least two questions on this every week [from nurses] asking, ‘Can I do this? Can I do that?’ ”
The answer depends on the circumstances, the nurse’s training, and the type of practice being broadened, Ms. Balestra said. For example, an NP in cardiology care may be allowed to perform more procedures in that field after internal training, but an NP who is trained in the care of adults can see pediatric patients only by going back to school.
“Know who you’re hiring, where their expertise lies, and where they feel comfortable,” she emphasized.
3. Preplan reviews. Early in the doctor-NP relationship, discuss and decide what type of medical cases warrant physician review, Mr. Ranum said. This includes agreeing on the type of patient conditions that will require a physician review and determining the types and percentage of medical records the doctor will evaluate, he said.
“The numbers should be higher at the beginning of the relationship until the physician gains an understanding of the NP’s experience and competence,” Mr. Ranum said. “Setting expectations will open the door to more frequent and more effective communication.”
NPs, meanwhile, should feel confident in requesting the physician’s assistance when a patient’s presentation is complex or a patient has returned with the same complaints, he added.
4. Convene regularly. Schedule regular meetings to catch up and discuss patient cases – not just when something goes awry, said Ms. Balestra. During weekly or monthly meetings, physicians, NPs, and other team members can converse in a more relaxed atmosphere and share any concerns or ideas for improvements.
“Have a meeting, whether by phone or in person, just to see how things are going,” she said. “That way, the NP may be able to take some things off the plate for the physician and the physician can see how [he or she] can assist the NP.”
Short huddles at the start of each day also help clinicians and staff prepare for patients and discuss approaches to managing complex conditions or challenging patient personalities, Mr. Ranum said.
“It is often helpful to debrief on patients who were seen during that day and who represent complex conditions,” he said. “Physicians may see opportunities to improve care following the NP’s assessment and diagnosis.”
5. Consider noncompliant policy. Create a noncompliant patient policy and work together to address uncooperative patients. Noncompliant patients are a top lawsuit risk, Ms. Balestra said. A noncompliant patient for instance, may provide conflicting information to different health professionals or attempt to blame providers for adverse events, she said.
“Your noncompliant patient is your easiest patient for a lawsuit because they’re not following [instructions] and then something happens, and they say, ‘It’s your fault, you didn’t treat me right.’”
Physician and NPs should be on the same page about noncompliant patients, including taking time to discuss when and how to terminate them from the practice if necessary, she said. Consistent documentation about patients by both physician and NPs is also critical, experts emphasize. Insufficient or lack of documentation led to patient injuries in 17% of cases against primary care doctors and in 19% of cases against NPs in The Doctors Company study.
6. Keep patients out of it. When disagreements or grievances occur, discuss the problem in private and ensure all staff members do the same, Dr. Andrew said. Refrain from letting anger or annoyance with another team member carry into patient care or worse, trigger a negative comment about a staff member in front of a patient, she said.
“All it takes is for something to go wrong and a patient or family who has heard such sentiments is tuned into the fact there may be some culpability,” she said. “This is probably a key factor in many a claimant’s decision to seek redress for a bad outcome.”
Instead, address problems or differences as soon as possible and work toward a resolution. It may help to create a conflict resolution policy that outlines behavioral expectations from all team members and suggested solutions when concerns arise.
“We have to put our egos aside,” Ms. Balestra said. “The ultimate goal is the best care of the patient.”
Positive relationships between physicians and nurses not only make for a smoother work environment, they also may reduce medical errors and lower the risk of lawsuits.
A recent study of closed claims by national medical malpractice insurer The Doctors Company found that poor physician oversight is a key contributor to lawsuits against nurses. Investigators analyzed 67 nurse practitioner (NP) claims from January 2011 to December 2016 and compared them with 1,358 claims against primary care physicians during the same time period.
Diagnostic and medication errors were the most common allegations against NPs, the study found, a trend that matched the most frequent allegations against primary care (internal medicine and family medicine) doctors. Top administrative factors that prompted lawsuits against nurses included inadequate physician supervision, failure to adhere to scope of practice, and absence of or deviation from written protocols.
The findings illustrate the importance of effective collaboration between physicians and NPs, said Darrell Ranum, vice president for patient safety and risk management for The Doctors Co. Below, legal experts share six ways to strengthen the physician-nurse relationship and at the same time, reduce liability:
1. Foster open dialogue. Cultivating a comfortable environment where nurses and physicians feel at ease sharing concerns and problems is a key step, says Louise B. Andrew, MD, JD, a physician and attorney who specializes in litigation stress management. A common scenario is a nurse who notices an abnormal vital sign but fails to mention it to the supervising physician because they feel they can handle it themselves or because they believe the doctor is too busy or too tired to be bothered, she said. The patient’s condition then worsens, resulting in a poor outcome that could have been avoided with better communication among providers. Delayed/wrong diagnosis accounted for 41% of claims against primary care physicians and 48% of claims against NPs in The Doctors Company study.
Set the tone early by exemplifying positive and clear communication, practicing good listening, and remaining empathetic, yet firm when making your needs known, Dr. Andrew advised.
“In the medical setting, you are always communicating for the benefit of the patient, and it is good to both keep this in mind, and to say it out loud,” she said.
2. Stick to the scope. When hiring an NP, make sure their scope of practice is clearly understood by all parties and respect their limitations, said Melanie L. Balestra, a Newport Beach, Calif., attorney and nurse practitioner who represents health providers.
Nurses practitioners must refrain from overstepping their authority, but physicians also must be careful not to ask too much of their NPs, experts stress. Ms. Balestra notes there is frequent confusion among doctors and NPs over how and whether scope of practice can be expanded as needed.
“This happens all the time,” Ms. Balestra said. “I get at least two questions on this every week [from nurses] asking, ‘Can I do this? Can I do that?’ ”
The answer depends on the circumstances, the nurse’s training, and the type of practice being broadened, Ms. Balestra said. For example, an NP in cardiology care may be allowed to perform more procedures in that field after internal training, but an NP who is trained in the care of adults can see pediatric patients only by going back to school.
“Know who you’re hiring, where their expertise lies, and where they feel comfortable,” she emphasized.
3. Preplan reviews. Early in the doctor-NP relationship, discuss and decide what type of medical cases warrant physician review, Mr. Ranum said. This includes agreeing on the type of patient conditions that will require a physician review and determining the types and percentage of medical records the doctor will evaluate, he said.
“The numbers should be higher at the beginning of the relationship until the physician gains an understanding of the NP’s experience and competence,” Mr. Ranum said. “Setting expectations will open the door to more frequent and more effective communication.”
NPs, meanwhile, should feel confident in requesting the physician’s assistance when a patient’s presentation is complex or a patient has returned with the same complaints, he added.
4. Convene regularly. Schedule regular meetings to catch up and discuss patient cases – not just when something goes awry, said Ms. Balestra. During weekly or monthly meetings, physicians, NPs, and other team members can converse in a more relaxed atmosphere and share any concerns or ideas for improvements.
“Have a meeting, whether by phone or in person, just to see how things are going,” she said. “That way, the NP may be able to take some things off the plate for the physician and the physician can see how [he or she] can assist the NP.”
Short huddles at the start of each day also help clinicians and staff prepare for patients and discuss approaches to managing complex conditions or challenging patient personalities, Mr. Ranum said.
“It is often helpful to debrief on patients who were seen during that day and who represent complex conditions,” he said. “Physicians may see opportunities to improve care following the NP’s assessment and diagnosis.”
5. Consider noncompliant policy. Create a noncompliant patient policy and work together to address uncooperative patients. Noncompliant patients are a top lawsuit risk, Ms. Balestra said. A noncompliant patient for instance, may provide conflicting information to different health professionals or attempt to blame providers for adverse events, she said.
“Your noncompliant patient is your easiest patient for a lawsuit because they’re not following [instructions] and then something happens, and they say, ‘It’s your fault, you didn’t treat me right.’”
Physician and NPs should be on the same page about noncompliant patients, including taking time to discuss when and how to terminate them from the practice if necessary, she said. Consistent documentation about patients by both physician and NPs is also critical, experts emphasize. Insufficient or lack of documentation led to patient injuries in 17% of cases against primary care doctors and in 19% of cases against NPs in The Doctors Company study.
6. Keep patients out of it. When disagreements or grievances occur, discuss the problem in private and ensure all staff members do the same, Dr. Andrew said. Refrain from letting anger or annoyance with another team member carry into patient care or worse, trigger a negative comment about a staff member in front of a patient, she said.
“All it takes is for something to go wrong and a patient or family who has heard such sentiments is tuned into the fact there may be some culpability,” she said. “This is probably a key factor in many a claimant’s decision to seek redress for a bad outcome.”
Instead, address problems or differences as soon as possible and work toward a resolution. It may help to create a conflict resolution policy that outlines behavioral expectations from all team members and suggested solutions when concerns arise.
“We have to put our egos aside,” Ms. Balestra said. “The ultimate goal is the best care of the patient.”
Positive relationships between physicians and nurses not only make for a smoother work environment, they also may reduce medical errors and lower the risk of lawsuits.
A recent study of closed claims by national medical malpractice insurer The Doctors Company found that poor physician oversight is a key contributor to lawsuits against nurses. Investigators analyzed 67 nurse practitioner (NP) claims from January 2011 to December 2016 and compared them with 1,358 claims against primary care physicians during the same time period.
Diagnostic and medication errors were the most common allegations against NPs, the study found, a trend that matched the most frequent allegations against primary care (internal medicine and family medicine) doctors. Top administrative factors that prompted lawsuits against nurses included inadequate physician supervision, failure to adhere to scope of practice, and absence of or deviation from written protocols.
The findings illustrate the importance of effective collaboration between physicians and NPs, said Darrell Ranum, vice president for patient safety and risk management for The Doctors Co. Below, legal experts share six ways to strengthen the physician-nurse relationship and at the same time, reduce liability:
1. Foster open dialogue. Cultivating a comfortable environment where nurses and physicians feel at ease sharing concerns and problems is a key step, says Louise B. Andrew, MD, JD, a physician and attorney who specializes in litigation stress management. A common scenario is a nurse who notices an abnormal vital sign but fails to mention it to the supervising physician because they feel they can handle it themselves or because they believe the doctor is too busy or too tired to be bothered, she said. The patient’s condition then worsens, resulting in a poor outcome that could have been avoided with better communication among providers. Delayed/wrong diagnosis accounted for 41% of claims against primary care physicians and 48% of claims against NPs in The Doctors Company study.
Set the tone early by exemplifying positive and clear communication, practicing good listening, and remaining empathetic, yet firm when making your needs known, Dr. Andrew advised.
“In the medical setting, you are always communicating for the benefit of the patient, and it is good to both keep this in mind, and to say it out loud,” she said.
2. Stick to the scope. When hiring an NP, make sure their scope of practice is clearly understood by all parties and respect their limitations, said Melanie L. Balestra, a Newport Beach, Calif., attorney and nurse practitioner who represents health providers.
Nurses practitioners must refrain from overstepping their authority, but physicians also must be careful not to ask too much of their NPs, experts stress. Ms. Balestra notes there is frequent confusion among doctors and NPs over how and whether scope of practice can be expanded as needed.
“This happens all the time,” Ms. Balestra said. “I get at least two questions on this every week [from nurses] asking, ‘Can I do this? Can I do that?’ ”
The answer depends on the circumstances, the nurse’s training, and the type of practice being broadened, Ms. Balestra said. For example, an NP in cardiology care may be allowed to perform more procedures in that field after internal training, but an NP who is trained in the care of adults can see pediatric patients only by going back to school.
“Know who you’re hiring, where their expertise lies, and where they feel comfortable,” she emphasized.
3. Preplan reviews. Early in the doctor-NP relationship, discuss and decide what type of medical cases warrant physician review, Mr. Ranum said. This includes agreeing on the type of patient conditions that will require a physician review and determining the types and percentage of medical records the doctor will evaluate, he said.
“The numbers should be higher at the beginning of the relationship until the physician gains an understanding of the NP’s experience and competence,” Mr. Ranum said. “Setting expectations will open the door to more frequent and more effective communication.”
NPs, meanwhile, should feel confident in requesting the physician’s assistance when a patient’s presentation is complex or a patient has returned with the same complaints, he added.
4. Convene regularly. Schedule regular meetings to catch up and discuss patient cases – not just when something goes awry, said Ms. Balestra. During weekly or monthly meetings, physicians, NPs, and other team members can converse in a more relaxed atmosphere and share any concerns or ideas for improvements.
“Have a meeting, whether by phone or in person, just to see how things are going,” she said. “That way, the NP may be able to take some things off the plate for the physician and the physician can see how [he or she] can assist the NP.”
Short huddles at the start of each day also help clinicians and staff prepare for patients and discuss approaches to managing complex conditions or challenging patient personalities, Mr. Ranum said.
“It is often helpful to debrief on patients who were seen during that day and who represent complex conditions,” he said. “Physicians may see opportunities to improve care following the NP’s assessment and diagnosis.”
5. Consider noncompliant policy. Create a noncompliant patient policy and work together to address uncooperative patients. Noncompliant patients are a top lawsuit risk, Ms. Balestra said. A noncompliant patient for instance, may provide conflicting information to different health professionals or attempt to blame providers for adverse events, she said.
“Your noncompliant patient is your easiest patient for a lawsuit because they’re not following [instructions] and then something happens, and they say, ‘It’s your fault, you didn’t treat me right.’”
Physician and NPs should be on the same page about noncompliant patients, including taking time to discuss when and how to terminate them from the practice if necessary, she said. Consistent documentation about patients by both physician and NPs is also critical, experts emphasize. Insufficient or lack of documentation led to patient injuries in 17% of cases against primary care doctors and in 19% of cases against NPs in The Doctors Company study.
6. Keep patients out of it. When disagreements or grievances occur, discuss the problem in private and ensure all staff members do the same, Dr. Andrew said. Refrain from letting anger or annoyance with another team member carry into patient care or worse, trigger a negative comment about a staff member in front of a patient, she said.
“All it takes is for something to go wrong and a patient or family who has heard such sentiments is tuned into the fact there may be some culpability,” she said. “This is probably a key factor in many a claimant’s decision to seek redress for a bad outcome.”
Instead, address problems or differences as soon as possible and work toward a resolution. It may help to create a conflict resolution policy that outlines behavioral expectations from all team members and suggested solutions when concerns arise.
“We have to put our egos aside,” Ms. Balestra said. “The ultimate goal is the best care of the patient.”
The Inpatient Blindside: Comorbid Mental Health Conditions and Readmissions among Hospitalized Children
To ensure hospital quality, the Centers for Medicaid & Medicare Services have tied payments to performance measures, including readmissions.1 One readmission metric, the Potentially Preventable Readmission measure (3M, PPR), was initially developed for Medicare and defined as readmissions related to an index admission, excluding those for treatment of cancer, related to trauma or burns, or following neonatal hospitalization. The PPR includes readmissions for both primary mental health conditions (MHCs) and for other hospitalizations with comorbid MHCs.2 Although controversies surround equating a hospital’s quality with its rate of readmissions, the PPR has been expanded to include numerous states. Since the PPR is also used for the Medicaid population in these states, it also measures pediatric readmissions. Hospitals in states adopting PPR calculations, including children’s hospitals, must either meet these new quality metrics or risk financial penalties. In light of evidence of high readmission rates among adult patients with MHCs, several states have modified the PPR to exclude MHCs and claims for mental health services.3–9
In their study, “Mental Health Conditions and Unplanned Hospital Readmissions in Children,” Doupnik et al. provided compelling evidence that MHCs in children (similar to adults) are closely associated with readmissions.10 MHCs are possibly underappreciated risk factors for readmission penalties and therefore represent a necessary point for increased awareness. Doupnik et al. calculated 30-day unplanned hospital readmissions of children with versus without comorbid MHCs using another standard measure, the Pediatric All-Condition Readmission (PACR) measure. The PACR measure excludes index admissions with a MHC as primary diagnosis but includes children with comorbid MHCs.
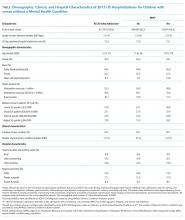
Doupnik et al. used a nationally representative cohort of all index hospitalizations of children aged 3–21 years from the 2013 Nationwide Readmission Database that allowed for estimates of MHC prevalence in the study population.11 A comorbid MHC was identified in almost 1 in 5 medical admissions and 1 in 7 procedural admissions. Comorbid substance abuse was identified in 5.4% of medical admissions and 4.7% of procedure admissions, making this diagnosis the most frequently coded stand-alone MHC. The authors’ findings are particularly noteworthy given that diagnosis of MHCs is highly dependent upon coding and is therefore almost certainly underreported. In pediatric inpatient populations, the true prevalence of comorbid MHCs is probably higher.
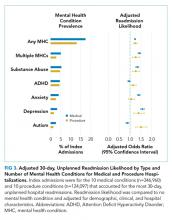
Doupnik et al. observed that comorbid MHCs are a significant risk factor for readmission. After adjustment for demographic, clinical, and hospital characteristics, children with MHCs presented a nearly 25% higher chance of readmission for both medical and procedural hospitalizations. Children admitted with medical conditions and multiple MHCs yielded odds of readmission 50% higher than that of children without MHCs. Overall, the presence of MHCs was associated with more than 2,500 medical and 200 procedure readmissions.
Previous studies in adult populations have also found that comorbid MHCs are an important risk factor for readmissions.12,13 Other research describes that children with MHCs have increased hospital resource use, including longer lengths of stay and higher hospitalization costs.14-17 Further, children with MHCs as a primary diagnosis are more prone to readmission, with readmission rates approaching those observed in children with medical complexity in some cases.18,19 MHCs are common among hospitalized children and have become an increasingly present comorbidity in primary medical or surgical admissions.17
One particular strength of this study lies in its description of the relationship between comorbid (not primary) MHCs and readmission following medical or surgical procedures in hospitalized children. This relationship has been examined in adult inpatient populations but less so in pediatric inpatient populations.12,13 This study provides insights into the relationships between specific MHCs and unplanned readmissions for certain primary medical or surgical diagnoses, including those for attention deficit disorder and autism that are not well-recognized in adult populations.
High-quality inpatient pediatric practice depends not only upon recognition of concurrent MHCs during hospitalizations but also assurance of follow-up outside of such institutions. During the inpatient care of children, pediatric hospitalists often perform myopic inpatient care which fails to routinely address underlying MHCs.20 For example, among children who are admitted with primary medical or procedure diagnoses, it is possible, or perhaps likely, that providers give little attention to an underlying MHC outside of continuation of a current medication. Comorbid MHCs are not accounted for within readmission calculations that directly affect hospital reimbursement. This study suggests that comorbid MHCs in hospitalized children may worsen readmission penalty status. In this manner, comorbid MHCs may represent a hospital’s blindside.
We agree with Doupnik et al. that an integrated approach with medical and mental health professionals may improve the care of children with MHCs in hospitalized settings. This improvement in care may eventually affect hospital-level national quality metrics, such as readmissions. The findings of Doupnik et al. also provide a strong argument that pediatric inpatient providers should consider mental health consultations for patients with frequent admissions associated with chronic conditions, as comorbid MHCs are associated with worsened disease states and account for a disproportionate share of admissions for children with chronic conditions.21,22 Recognition of comorbid MHCs may improve baseline chronic disease states for hospitalized children.
We assert that the current silos in inpatient pediatrics of medical and mental healthcare are outdated. Pediatric hospitalists need to assess for and access effective MHC treatment options in the inpatient setting. In addition to the provision of mental health care within hospital settings, providers should also ensure that appropriate follow-up is arranged at the time of discharge. From a health policy standpoint, providers should clarify how both primary and comorbid MHCs are included within readmission measures while considering the close association of these conditions with readmission. Although the care of children with MHCs requires a long-term and coordinated approach, identification and treatment during hospitalization offer unique opportunities to modify outcomes of MHCs and coexistent medical and surgical diagnoses.
Disclosures
The authors declare no conflict of interest.
1. Centers for Medicare & Medicaid Services. Hospital Readmission Reduction Program. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/HRRP/Hospital-Readmission-Reduction-Program.html. Published September 28, 2015. Accessed February 9, 2018.
2. 3M. Potentially Preventable Readmissions Classification System. http://multimedia.3m.com/mws/media/1042610O/resources-and-references-his-2015.pdf. Accessed February 9, 2018.
3. Illinois Department of Family and Healthcare Services. Hospital Inpatient Potentially Preventable Readmissions Information and Reports. https://www.illinois.gov/hfs/MedicalProviders/hospitals/PPRReports/Pages/default.aspx. Accessed February 9, 2018.
4. New York State Department of Health. Potentially preventable hospital readmissions among medicaid recipients with mental health and/or substance abuse health conditions compared with all others: New York State, 2007. https://www.health.ny.gov/health_care/managed_care/reports/statistics_data/3hospital_readmissions_mentahealth.pdf. Accessed February 9, 2018.
5. Texas Health and Human Services Commission. Potentially preventable readmissions in Texas Medicaid and CHIP Programs, Fiscal Year 2013. https://hhs.texas.gov/reports/2016/08/potentially-preventable-readmissions-texas-medicaid-and-chip-programs-fiscal-year-2013. Accessed February 9, 2018.
6. Oklahoma Healthcare Association. Provider reimbursement notice. https://www.okhca.org/providers.aspx?id=2538. Accessed February 9, 2018.
7. Washington State Hospital Association. Potentially preventable readmission (PPR) adjustments. http://www.wsha.org/articles/hca-implements-potentially-preventable-readmission-ppr-adjustments/. Accessed February 9, 2018.
8. State of Colorado. HQIP 30-day All cause readmission. https://www.colorado.gov/pacific/sites/default/files/2016%20March%20HQIP%2030-day%20all-cause%20readmission%20measure.pdf. Accessed February 9, 2018.
9. Maryland Health Services Cost Review Commission. Readmission reduction incentive program. http://www.hscrc.state.md.us/Pages/init-readm-rip.aspx. Accessed February 9, 2018.
10. Doupnik SK, Lawlor J, Zima BT, et al. Mental health conditions and unplanned hospital readmissions in children. J Hosp Med. 2018(13):445-452. PubMed
11. NRD Overview. https://www.hcup-us.ahrq.gov/nrdoverview.jsp. Accessed February 9, 2018.
12. Singh G, Zhang W, Kuo Y-F, Sharma G. Association of psychological disorders with 30-day readmission rates in patients with COPD. Chest. 2016;149(4):905-915. doi:10.1378/chest.15-0449 PubMed
13. McIntyre LK, Arbabi S, Robinson EF, Maier RV. Analysis of risk factors for patient readmission 30 days following discharge from general surgery. JAMA Surg. 2016;151(9):855-861. doi:10.1001/jamasurg.2016.1258 PubMed
14. Bardach NS, Coker TR, Zima BT, et al. Common and costly hospitalizations for pediatric mental health disorders. Pediatrics. 2014;133(4):602-609. doi:10.1542/peds.2013-3165 PubMed
15. Doupnik SK, Lawlor J, Zima BT, et al. Mental health conditions and medical and surgical hospital utilization. Pediatrics. 2016;138(6): e20162416. doi:10.1542/peds.2016-2416 PubMed
16. Doupnik SK, Mitra N, Feudtner C, Marcus SC. The influence of comorbid mood and anxiety disorders on outcomes of pediatric patients hospitalized for pneumonia. Hosp Pediatr. 2016;6(3):135-142. doi:10.1542/hpeds.2015-0177 PubMed
17. Zima BT, Rodean J, Hall M, Bardach NS, Coker TR, Berry JG. Psychiatric disorders and trends in resource use in pediatric hospitals. Pediatrics. 2016;138(5): e20160909. doi:10.1542/peds.2016-0909 PubMed
18. Feng JY, Toomey SL, Zaslavsky AM, Nakamura MM, Schuster MA. Readmission after pediatric mental health admissions. Pediatrics. 2017;140(6):e20171571. doi:10.1542/peds.2017-1571 PubMed
19. Cohen E, Berry JG, Camacho X, Anderson G, Wodchis W, Guttmann A. Patterns and costs of health care use of children with medical complexity. Pediatrics. 2012;130(6):e1463-e1470. doi:10.1542/peds.2012-0175 PubMed
20. Doupnik SK, Walter JK. Collaboration is key to improving hospital care for patients with medical and psychiatric comorbidity. Hosp Pediatr. 2016;6(12):760-762. doi:10.1542/hpeds.2016-0165 PubMed
21. Richardson LP, Russo JE, Lozano P, McCauley E, Katon W. The effect of comorbid anxiety and depressive disorders on health care utilization and costs among adolescents with asthma. Gen Hosp Psychiatry. 2008;30(5):398-406. doi:10.1016/j.genhosppsych.2008.06.004 PubMed
22. Malik FS, Hall M, Mangione-Smith R, et al. Patient characteristics associated with differences in admission frequency for diabetic ketoacidosis in United States children’s hospitals. J Pediatr. 2016;171:104-110. doi:10.1016/j.jpeds.2015.12.015 PubMed
To ensure hospital quality, the Centers for Medicaid & Medicare Services have tied payments to performance measures, including readmissions.1 One readmission metric, the Potentially Preventable Readmission measure (3M, PPR), was initially developed for Medicare and defined as readmissions related to an index admission, excluding those for treatment of cancer, related to trauma or burns, or following neonatal hospitalization. The PPR includes readmissions for both primary mental health conditions (MHCs) and for other hospitalizations with comorbid MHCs.2 Although controversies surround equating a hospital’s quality with its rate of readmissions, the PPR has been expanded to include numerous states. Since the PPR is also used for the Medicaid population in these states, it also measures pediatric readmissions. Hospitals in states adopting PPR calculations, including children’s hospitals, must either meet these new quality metrics or risk financial penalties. In light of evidence of high readmission rates among adult patients with MHCs, several states have modified the PPR to exclude MHCs and claims for mental health services.3–9
In their study, “Mental Health Conditions and Unplanned Hospital Readmissions in Children,” Doupnik et al. provided compelling evidence that MHCs in children (similar to adults) are closely associated with readmissions.10 MHCs are possibly underappreciated risk factors for readmission penalties and therefore represent a necessary point for increased awareness. Doupnik et al. calculated 30-day unplanned hospital readmissions of children with versus without comorbid MHCs using another standard measure, the Pediatric All-Condition Readmission (PACR) measure. The PACR measure excludes index admissions with a MHC as primary diagnosis but includes children with comorbid MHCs.
Doupnik et al. used a nationally representative cohort of all index hospitalizations of children aged 3–21 years from the 2013 Nationwide Readmission Database that allowed for estimates of MHC prevalence in the study population.11 A comorbid MHC was identified in almost 1 in 5 medical admissions and 1 in 7 procedural admissions. Comorbid substance abuse was identified in 5.4% of medical admissions and 4.7% of procedure admissions, making this diagnosis the most frequently coded stand-alone MHC. The authors’ findings are particularly noteworthy given that diagnosis of MHCs is highly dependent upon coding and is therefore almost certainly underreported. In pediatric inpatient populations, the true prevalence of comorbid MHCs is probably higher.
Doupnik et al. observed that comorbid MHCs are a significant risk factor for readmission. After adjustment for demographic, clinical, and hospital characteristics, children with MHCs presented a nearly 25% higher chance of readmission for both medical and procedural hospitalizations. Children admitted with medical conditions and multiple MHCs yielded odds of readmission 50% higher than that of children without MHCs. Overall, the presence of MHCs was associated with more than 2,500 medical and 200 procedure readmissions.
Previous studies in adult populations have also found that comorbid MHCs are an important risk factor for readmissions.12,13 Other research describes that children with MHCs have increased hospital resource use, including longer lengths of stay and higher hospitalization costs.14-17 Further, children with MHCs as a primary diagnosis are more prone to readmission, with readmission rates approaching those observed in children with medical complexity in some cases.18,19 MHCs are common among hospitalized children and have become an increasingly present comorbidity in primary medical or surgical admissions.17
One particular strength of this study lies in its description of the relationship between comorbid (not primary) MHCs and readmission following medical or surgical procedures in hospitalized children. This relationship has been examined in adult inpatient populations but less so in pediatric inpatient populations.12,13 This study provides insights into the relationships between specific MHCs and unplanned readmissions for certain primary medical or surgical diagnoses, including those for attention deficit disorder and autism that are not well-recognized in adult populations.
High-quality inpatient pediatric practice depends not only upon recognition of concurrent MHCs during hospitalizations but also assurance of follow-up outside of such institutions. During the inpatient care of children, pediatric hospitalists often perform myopic inpatient care which fails to routinely address underlying MHCs.20 For example, among children who are admitted with primary medical or procedure diagnoses, it is possible, or perhaps likely, that providers give little attention to an underlying MHC outside of continuation of a current medication. Comorbid MHCs are not accounted for within readmission calculations that directly affect hospital reimbursement. This study suggests that comorbid MHCs in hospitalized children may worsen readmission penalty status. In this manner, comorbid MHCs may represent a hospital’s blindside.
We agree with Doupnik et al. that an integrated approach with medical and mental health professionals may improve the care of children with MHCs in hospitalized settings. This improvement in care may eventually affect hospital-level national quality metrics, such as readmissions. The findings of Doupnik et al. also provide a strong argument that pediatric inpatient providers should consider mental health consultations for patients with frequent admissions associated with chronic conditions, as comorbid MHCs are associated with worsened disease states and account for a disproportionate share of admissions for children with chronic conditions.21,22 Recognition of comorbid MHCs may improve baseline chronic disease states for hospitalized children.
We assert that the current silos in inpatient pediatrics of medical and mental healthcare are outdated. Pediatric hospitalists need to assess for and access effective MHC treatment options in the inpatient setting. In addition to the provision of mental health care within hospital settings, providers should also ensure that appropriate follow-up is arranged at the time of discharge. From a health policy standpoint, providers should clarify how both primary and comorbid MHCs are included within readmission measures while considering the close association of these conditions with readmission. Although the care of children with MHCs requires a long-term and coordinated approach, identification and treatment during hospitalization offer unique opportunities to modify outcomes of MHCs and coexistent medical and surgical diagnoses.
Disclosures
The authors declare no conflict of interest.
To ensure hospital quality, the Centers for Medicaid & Medicare Services have tied payments to performance measures, including readmissions.1 One readmission metric, the Potentially Preventable Readmission measure (3M, PPR), was initially developed for Medicare and defined as readmissions related to an index admission, excluding those for treatment of cancer, related to trauma or burns, or following neonatal hospitalization. The PPR includes readmissions for both primary mental health conditions (MHCs) and for other hospitalizations with comorbid MHCs.2 Although controversies surround equating a hospital’s quality with its rate of readmissions, the PPR has been expanded to include numerous states. Since the PPR is also used for the Medicaid population in these states, it also measures pediatric readmissions. Hospitals in states adopting PPR calculations, including children’s hospitals, must either meet these new quality metrics or risk financial penalties. In light of evidence of high readmission rates among adult patients with MHCs, several states have modified the PPR to exclude MHCs and claims for mental health services.3–9
In their study, “Mental Health Conditions and Unplanned Hospital Readmissions in Children,” Doupnik et al. provided compelling evidence that MHCs in children (similar to adults) are closely associated with readmissions.10 MHCs are possibly underappreciated risk factors for readmission penalties and therefore represent a necessary point for increased awareness. Doupnik et al. calculated 30-day unplanned hospital readmissions of children with versus without comorbid MHCs using another standard measure, the Pediatric All-Condition Readmission (PACR) measure. The PACR measure excludes index admissions with a MHC as primary diagnosis but includes children with comorbid MHCs.
Doupnik et al. used a nationally representative cohort of all index hospitalizations of children aged 3–21 years from the 2013 Nationwide Readmission Database that allowed for estimates of MHC prevalence in the study population.11 A comorbid MHC was identified in almost 1 in 5 medical admissions and 1 in 7 procedural admissions. Comorbid substance abuse was identified in 5.4% of medical admissions and 4.7% of procedure admissions, making this diagnosis the most frequently coded stand-alone MHC. The authors’ findings are particularly noteworthy given that diagnosis of MHCs is highly dependent upon coding and is therefore almost certainly underreported. In pediatric inpatient populations, the true prevalence of comorbid MHCs is probably higher.
Doupnik et al. observed that comorbid MHCs are a significant risk factor for readmission. After adjustment for demographic, clinical, and hospital characteristics, children with MHCs presented a nearly 25% higher chance of readmission for both medical and procedural hospitalizations. Children admitted with medical conditions and multiple MHCs yielded odds of readmission 50% higher than that of children without MHCs. Overall, the presence of MHCs was associated with more than 2,500 medical and 200 procedure readmissions.
Previous studies in adult populations have also found that comorbid MHCs are an important risk factor for readmissions.12,13 Other research describes that children with MHCs have increased hospital resource use, including longer lengths of stay and higher hospitalization costs.14-17 Further, children with MHCs as a primary diagnosis are more prone to readmission, with readmission rates approaching those observed in children with medical complexity in some cases.18,19 MHCs are common among hospitalized children and have become an increasingly present comorbidity in primary medical or surgical admissions.17
One particular strength of this study lies in its description of the relationship between comorbid (not primary) MHCs and readmission following medical or surgical procedures in hospitalized children. This relationship has been examined in adult inpatient populations but less so in pediatric inpatient populations.12,13 This study provides insights into the relationships between specific MHCs and unplanned readmissions for certain primary medical or surgical diagnoses, including those for attention deficit disorder and autism that are not well-recognized in adult populations.
High-quality inpatient pediatric practice depends not only upon recognition of concurrent MHCs during hospitalizations but also assurance of follow-up outside of such institutions. During the inpatient care of children, pediatric hospitalists often perform myopic inpatient care which fails to routinely address underlying MHCs.20 For example, among children who are admitted with primary medical or procedure diagnoses, it is possible, or perhaps likely, that providers give little attention to an underlying MHC outside of continuation of a current medication. Comorbid MHCs are not accounted for within readmission calculations that directly affect hospital reimbursement. This study suggests that comorbid MHCs in hospitalized children may worsen readmission penalty status. In this manner, comorbid MHCs may represent a hospital’s blindside.
We agree with Doupnik et al. that an integrated approach with medical and mental health professionals may improve the care of children with MHCs in hospitalized settings. This improvement in care may eventually affect hospital-level national quality metrics, such as readmissions. The findings of Doupnik et al. also provide a strong argument that pediatric inpatient providers should consider mental health consultations for patients with frequent admissions associated with chronic conditions, as comorbid MHCs are associated with worsened disease states and account for a disproportionate share of admissions for children with chronic conditions.21,22 Recognition of comorbid MHCs may improve baseline chronic disease states for hospitalized children.
We assert that the current silos in inpatient pediatrics of medical and mental healthcare are outdated. Pediatric hospitalists need to assess for and access effective MHC treatment options in the inpatient setting. In addition to the provision of mental health care within hospital settings, providers should also ensure that appropriate follow-up is arranged at the time of discharge. From a health policy standpoint, providers should clarify how both primary and comorbid MHCs are included within readmission measures while considering the close association of these conditions with readmission. Although the care of children with MHCs requires a long-term and coordinated approach, identification and treatment during hospitalization offer unique opportunities to modify outcomes of MHCs and coexistent medical and surgical diagnoses.
Disclosures
The authors declare no conflict of interest.
1. Centers for Medicare & Medicaid Services. Hospital Readmission Reduction Program. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/HRRP/Hospital-Readmission-Reduction-Program.html. Published September 28, 2015. Accessed February 9, 2018.
2. 3M. Potentially Preventable Readmissions Classification System. http://multimedia.3m.com/mws/media/1042610O/resources-and-references-his-2015.pdf. Accessed February 9, 2018.
3. Illinois Department of Family and Healthcare Services. Hospital Inpatient Potentially Preventable Readmissions Information and Reports. https://www.illinois.gov/hfs/MedicalProviders/hospitals/PPRReports/Pages/default.aspx. Accessed February 9, 2018.
4. New York State Department of Health. Potentially preventable hospital readmissions among medicaid recipients with mental health and/or substance abuse health conditions compared with all others: New York State, 2007. https://www.health.ny.gov/health_care/managed_care/reports/statistics_data/3hospital_readmissions_mentahealth.pdf. Accessed February 9, 2018.
5. Texas Health and Human Services Commission. Potentially preventable readmissions in Texas Medicaid and CHIP Programs, Fiscal Year 2013. https://hhs.texas.gov/reports/2016/08/potentially-preventable-readmissions-texas-medicaid-and-chip-programs-fiscal-year-2013. Accessed February 9, 2018.
6. Oklahoma Healthcare Association. Provider reimbursement notice. https://www.okhca.org/providers.aspx?id=2538. Accessed February 9, 2018.
7. Washington State Hospital Association. Potentially preventable readmission (PPR) adjustments. http://www.wsha.org/articles/hca-implements-potentially-preventable-readmission-ppr-adjustments/. Accessed February 9, 2018.
8. State of Colorado. HQIP 30-day All cause readmission. https://www.colorado.gov/pacific/sites/default/files/2016%20March%20HQIP%2030-day%20all-cause%20readmission%20measure.pdf. Accessed February 9, 2018.
9. Maryland Health Services Cost Review Commission. Readmission reduction incentive program. http://www.hscrc.state.md.us/Pages/init-readm-rip.aspx. Accessed February 9, 2018.
10. Doupnik SK, Lawlor J, Zima BT, et al. Mental health conditions and unplanned hospital readmissions in children. J Hosp Med. 2018(13):445-452. PubMed
11. NRD Overview. https://www.hcup-us.ahrq.gov/nrdoverview.jsp. Accessed February 9, 2018.
12. Singh G, Zhang W, Kuo Y-F, Sharma G. Association of psychological disorders with 30-day readmission rates in patients with COPD. Chest. 2016;149(4):905-915. doi:10.1378/chest.15-0449 PubMed
13. McIntyre LK, Arbabi S, Robinson EF, Maier RV. Analysis of risk factors for patient readmission 30 days following discharge from general surgery. JAMA Surg. 2016;151(9):855-861. doi:10.1001/jamasurg.2016.1258 PubMed
14. Bardach NS, Coker TR, Zima BT, et al. Common and costly hospitalizations for pediatric mental health disorders. Pediatrics. 2014;133(4):602-609. doi:10.1542/peds.2013-3165 PubMed
15. Doupnik SK, Lawlor J, Zima BT, et al. Mental health conditions and medical and surgical hospital utilization. Pediatrics. 2016;138(6): e20162416. doi:10.1542/peds.2016-2416 PubMed
16. Doupnik SK, Mitra N, Feudtner C, Marcus SC. The influence of comorbid mood and anxiety disorders on outcomes of pediatric patients hospitalized for pneumonia. Hosp Pediatr. 2016;6(3):135-142. doi:10.1542/hpeds.2015-0177 PubMed
17. Zima BT, Rodean J, Hall M, Bardach NS, Coker TR, Berry JG. Psychiatric disorders and trends in resource use in pediatric hospitals. Pediatrics. 2016;138(5): e20160909. doi:10.1542/peds.2016-0909 PubMed
18. Feng JY, Toomey SL, Zaslavsky AM, Nakamura MM, Schuster MA. Readmission after pediatric mental health admissions. Pediatrics. 2017;140(6):e20171571. doi:10.1542/peds.2017-1571 PubMed
19. Cohen E, Berry JG, Camacho X, Anderson G, Wodchis W, Guttmann A. Patterns and costs of health care use of children with medical complexity. Pediatrics. 2012;130(6):e1463-e1470. doi:10.1542/peds.2012-0175 PubMed
20. Doupnik SK, Walter JK. Collaboration is key to improving hospital care for patients with medical and psychiatric comorbidity. Hosp Pediatr. 2016;6(12):760-762. doi:10.1542/hpeds.2016-0165 PubMed
21. Richardson LP, Russo JE, Lozano P, McCauley E, Katon W. The effect of comorbid anxiety and depressive disorders on health care utilization and costs among adolescents with asthma. Gen Hosp Psychiatry. 2008;30(5):398-406. doi:10.1016/j.genhosppsych.2008.06.004 PubMed
22. Malik FS, Hall M, Mangione-Smith R, et al. Patient characteristics associated with differences in admission frequency for diabetic ketoacidosis in United States children’s hospitals. J Pediatr. 2016;171:104-110. doi:10.1016/j.jpeds.2015.12.015 PubMed
1. Centers for Medicare & Medicaid Services. Hospital Readmission Reduction Program. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/HRRP/Hospital-Readmission-Reduction-Program.html. Published September 28, 2015. Accessed February 9, 2018.
2. 3M. Potentially Preventable Readmissions Classification System. http://multimedia.3m.com/mws/media/1042610O/resources-and-references-his-2015.pdf. Accessed February 9, 2018.
3. Illinois Department of Family and Healthcare Services. Hospital Inpatient Potentially Preventable Readmissions Information and Reports. https://www.illinois.gov/hfs/MedicalProviders/hospitals/PPRReports/Pages/default.aspx. Accessed February 9, 2018.
4. New York State Department of Health. Potentially preventable hospital readmissions among medicaid recipients with mental health and/or substance abuse health conditions compared with all others: New York State, 2007. https://www.health.ny.gov/health_care/managed_care/reports/statistics_data/3hospital_readmissions_mentahealth.pdf. Accessed February 9, 2018.
5. Texas Health and Human Services Commission. Potentially preventable readmissions in Texas Medicaid and CHIP Programs, Fiscal Year 2013. https://hhs.texas.gov/reports/2016/08/potentially-preventable-readmissions-texas-medicaid-and-chip-programs-fiscal-year-2013. Accessed February 9, 2018.
6. Oklahoma Healthcare Association. Provider reimbursement notice. https://www.okhca.org/providers.aspx?id=2538. Accessed February 9, 2018.
7. Washington State Hospital Association. Potentially preventable readmission (PPR) adjustments. http://www.wsha.org/articles/hca-implements-potentially-preventable-readmission-ppr-adjustments/. Accessed February 9, 2018.
8. State of Colorado. HQIP 30-day All cause readmission. https://www.colorado.gov/pacific/sites/default/files/2016%20March%20HQIP%2030-day%20all-cause%20readmission%20measure.pdf. Accessed February 9, 2018.
9. Maryland Health Services Cost Review Commission. Readmission reduction incentive program. http://www.hscrc.state.md.us/Pages/init-readm-rip.aspx. Accessed February 9, 2018.
10. Doupnik SK, Lawlor J, Zima BT, et al. Mental health conditions and unplanned hospital readmissions in children. J Hosp Med. 2018(13):445-452. PubMed
11. NRD Overview. https://www.hcup-us.ahrq.gov/nrdoverview.jsp. Accessed February 9, 2018.
12. Singh G, Zhang W, Kuo Y-F, Sharma G. Association of psychological disorders with 30-day readmission rates in patients with COPD. Chest. 2016;149(4):905-915. doi:10.1378/chest.15-0449 PubMed
13. McIntyre LK, Arbabi S, Robinson EF, Maier RV. Analysis of risk factors for patient readmission 30 days following discharge from general surgery. JAMA Surg. 2016;151(9):855-861. doi:10.1001/jamasurg.2016.1258 PubMed
14. Bardach NS, Coker TR, Zima BT, et al. Common and costly hospitalizations for pediatric mental health disorders. Pediatrics. 2014;133(4):602-609. doi:10.1542/peds.2013-3165 PubMed
15. Doupnik SK, Lawlor J, Zima BT, et al. Mental health conditions and medical and surgical hospital utilization. Pediatrics. 2016;138(6): e20162416. doi:10.1542/peds.2016-2416 PubMed
16. Doupnik SK, Mitra N, Feudtner C, Marcus SC. The influence of comorbid mood and anxiety disorders on outcomes of pediatric patients hospitalized for pneumonia. Hosp Pediatr. 2016;6(3):135-142. doi:10.1542/hpeds.2015-0177 PubMed
17. Zima BT, Rodean J, Hall M, Bardach NS, Coker TR, Berry JG. Psychiatric disorders and trends in resource use in pediatric hospitals. Pediatrics. 2016;138(5): e20160909. doi:10.1542/peds.2016-0909 PubMed
18. Feng JY, Toomey SL, Zaslavsky AM, Nakamura MM, Schuster MA. Readmission after pediatric mental health admissions. Pediatrics. 2017;140(6):e20171571. doi:10.1542/peds.2017-1571 PubMed
19. Cohen E, Berry JG, Camacho X, Anderson G, Wodchis W, Guttmann A. Patterns and costs of health care use of children with medical complexity. Pediatrics. 2012;130(6):e1463-e1470. doi:10.1542/peds.2012-0175 PubMed
20. Doupnik SK, Walter JK. Collaboration is key to improving hospital care for patients with medical and psychiatric comorbidity. Hosp Pediatr. 2016;6(12):760-762. doi:10.1542/hpeds.2016-0165 PubMed
21. Richardson LP, Russo JE, Lozano P, McCauley E, Katon W. The effect of comorbid anxiety and depressive disorders on health care utilization and costs among adolescents with asthma. Gen Hosp Psychiatry. 2008;30(5):398-406. doi:10.1016/j.genhosppsych.2008.06.004 PubMed
22. Malik FS, Hall M, Mangione-Smith R, et al. Patient characteristics associated with differences in admission frequency for diabetic ketoacidosis in United States children’s hospitals. J Pediatr. 2016;171:104-110. doi:10.1016/j.jpeds.2015.12.015 PubMed
© 2018 Society of Hospital Medicine
Things We Do For No Reason: Blood Cultures for Uncomplicated Skin and Soft Tissue Infections in Children
The “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CLINICAL SCENARIO
An 8-year-old previously healthy girl presented to the emergency department (ED) with 2 days of warmth, swelling, and pain over her right upper thigh. Three days prior before presentation, a “pimple” appeared on her leg and drained a small amount of pus. Over the next 24 hours, the lesion became swollen, red, and painful. Her pediatrician prescribed trimethoprim-sulfamethoxazole. The patient took 3 doses of this medication but still experienced worsening pain and swelling.
In the ED, she had normal vital signs for her age except for temperature of 100.8 °F. A 2 cm × 3 cm area of fluctuance, erythema, and warmth was noted, and bedside ultrasound demonstrated a simple fluid collection. Incision and drainage was performed with expression of several milliliters of pus. The patient was referred for admission due to worsening symptoms despite outpatient antibiotic therapy. The ED providers ordered a blood culture at the time of admission.
BACKGROUND
Skin and soft tissue infections (SSTIs) are common pediatric diagnoses, which account for an estimated 390,000 ED visits annually1 and represent the 7th most common reason for pediatric hospital admission in the United States.2 The rates of SSTIs have increased over the past several decades partly due to the rise of methicillin-resistant Staphylococcus aureus (MRSA).3
Why You Might Think Blood Cultures are Helpful In Children with SSTIs?
Prior to the introduction of the Haemophilus influenzae vaccine, the rates of SSTI-associated bacteremia ranged from 8% to 20%.4,5 Although the rate of bacteremia has declined significantly, blood cultures are still commonly performed as part of the evaluation of uncomplicated SSTIs in children; studies have shown that blood culture rates are 46% in the combined outpatient/inpatient setting,6 34% in the ED setting,7 and 47%-94% in the inpatient setting.7-11 Clinicians still feel that bacteremia detection is important to guide the selection of antibiotics and treatment duration. Providers may also underestimate the risk of obtaining a contaminant result and associated charges. Lastly, clinicians may perform blood cultures due to cultural norms at their institution.
Why Blood Cultures are Unnecessary in Children with Uncomplicated SSTIs
Several decades into the post vaccine era, the current guidelines from the Infectious Diseases Society of America (IDSA) do not recommend blood cultures as part of the routine evaluation of uncomplicated SSTIs.12 Multiple single-center studies have failed to demonstrate the benefits of obtaining blood cultures in pediatric patients with uncomplicated SSTIs in the post-H. influenzae vaccine era.6–11
Sadow et al11 performed a retrospective case series of 381 children hospitalized with cellulitis to determine the rate and yield of blood cultures. Of the 266 (70%) patients who had a blood culture performed, 5 (1.9%) were true positives and 13 (5.4%) were contaminants. Notably, the true positive results included 3 children with active varicella infection and 2 children with septic joints; the latter would qualify as a complicated SSTI or as a separate infectious process altogether. No significant change in management resulted the positive blood cultures.
Wathen et al7 conducted a similar retrospective case series of 385 children with cellulitis who presented to the ED of a single tertiary-care children’s hospital to determine the rate and yield of blood cultures. Of the 129 (33.5%) blood cultures performed, there were no true positives and 4 (3.1%) contaminants. Obtaining a blood culture was also associated with high rates of ordering complete blood count and hospitalization.
Malone et al8 performed a retrospective case series of 580 children hospitalized with an SSTI at a single children’s hospital to determine the yield of blood cultures for uncomplicated versus complicated SSTIs. Of the 482 patients with uncomplicated SSTIs, 455 (94.4%) had a blood culture, with no true positive cultures and 3 (0.7%) contaminants. Obtaining a blood culture in this study was associated with an almost 1 day increase in length of stay (LOS; mean LOS 3.24 vs 2.33 days, P = .04).
Parikh et al6 conducted a retrospective cohort study of 304 children with SSTIs in both inpatient and outpatient settings to determine the yield and rate of blood cultures. Of this group, 140 (46.1%) patients had a blood culture performed, of which there were 3 (2.9%) true positives and 1 (0.7%) contaminant. True-positive bacteria included MRSA and Streptococcus pyogenes, neither of which was associated with a change in antibiotic regimen or increase in hospital LOS. The total charges associated with the original 140 blood cultures were estimated to be $42,450 annually in the authors’ institution.
Lastly, Trenchs et al9 performed a retrospective case series of 445 children hospitalized with SSTI in a Spanish children’s hospital and found 353 (79.3%) blood cultures with 2 (0.6%) true positives and 10 (2.8%) contaminants. Methicillin-sensitive Staphylococcus aureus (MSSA) and S. pyogenes were the sole true-positive bacteria, and no change in management was reported. Obtaining blood cultures was associated with an increased hospital LOS (median LOS 4 vs. 3 days, P
Across these studies, the reported rates of true-positive blood cultures ranged from 0%-2.9%. Of the 1997 patients included in the studies, only 10 (0.5%) had true-positive blood cultures. This rate decreased to 0.4% if the 2 patients with septic arthritis from the study of Sadow et al were excluded. Isolated organisms included MRSA, MSSA, S. pyogenes, and Streptococcus pneumoniae. No unusual organisms were isolated in uncomplicated SSTIs, and the true-positive results were not associated with any reported change in antibiotic management.6–9,11 False-positive blood culture results were found in 0%-5.4% of patients,6–9,11 accounting for 30 patients or 1.5% of the total patients.
Harms Associated With Unnecessary Blood Cultures in SSTIs
Blood cultures necessitate venipunctures, which are painful for children and families. The inevitable false-positive contaminants also lead to repeat venipunctures and, potentially, unnecessary antibiotic exposure. From a high-value care perspective, Parikh et al reported hospital charges of $300 per blood culture and $250 for identification and sensitivity of positives.6 Assuming that these single-center charges are representative of national charges and using 0.5% true positivity and 1.5% false positivity rates, subjecting all children with uncomplicated SSTIs to blood culture would result in $60,250 charges to find one true positive blood culture, with no resultant changes in management. Additionally, among the 200 children cultured to find one true positive, there would be 3 false positives, necessitating another $1650 in charges for identification, sensitivity analysis, and repeat culture. These amounts do not factor in the significant expenditures associated with increased LOS. The potential savings associated with forgoing blood cultures in children with SSTIs should be an incentive for institutional change.
When Blood Cultures May Be Reasonable
The current IDSA guidelines recommend blood cultures for SSTIs in patients with immunodeficiency, animal bites, and immersion injuries (soft tissue injuries occurring in fresh or saltwater).12 Previous studies also delineated criteria for “complicated” SSTIs, typically defined as surgical or traumatic wounds, infections requiring surgical intervention (not including simple incision and drainage), or infected ulcers or burns.8,9 In the study of Malone et al, 10 (12.5%) positives were found among 80 patients with complicated SSTIs who had blood cultures performed.8 Although this work had a single-center study design with a relatively small sample size, no unusual organisms were found; the grown cultures included MRSA, MSSA, and S. pneumoniae. In addition to patients with complicated SSTIs, immunocompromised children, such as those receiving chemotherapy or other immunosuppressive agents, were excluded from the studies of blood culture yield in SSTIs and may warrant blood cultures given the risk of overwhelming infection and susceptibility to rare or invasive organisms.12 In a study of 57 pediatric patients with leukemia and no central catheters who experienced skin or soft tissue complications, Demircioglu et al13 reported 6 positive blood cultures, including Klebsiella oxytoca, Pseudomonas aeruginosa, and Escherichia coli. These organisms would not be covered by typical SSTI antibiotic regimens, illustrating the value of blood cultures in this selected group of patients. Lastly, although the above studies included some infants, the data on utility of blood cultures in neonates are limited. Blood cultures may be reasonable in this group given the relative immunocompromised state of neonates compared with older children. Additionally, any infants aged
What You Should Do Instead Of Blood Cultures for Uncomplicated SSTIs
Gram stain and wound culture of any purulent material may assist with choice of empiric antibiotic therapy and appropriate narrowing of regimen for antibiotic stewardship. Wound cultures of purulent material can identify the causative organism in 58%-66% of the cases.9,14 The rate of wound culture varies widely from 29% to 81% in studies across different healthcare systems.9,10,15 The use of visually appealing posters advising clinicians to “culture pus, not blood” has been shown to significantly decreased the number of blood cultures performed at a single pediatric hospital.10
RECOMMENDATIONS
- Do not obtain blood cultures in pediatric patients with uncomplicated SSTIs.
- If purulent material is available spontaneously or after incision and drainage, then send it for Gram stain and bacterial culture.
- Blood cultures are reasonable in patients with complicated SSTIs and in immunocompromised patients with SSTIs.
- Despite limited data, blood cultures may be reasonable in neonates with SSTIs. Febrile infants with SSTIs aged less than 90 days should be managed under existing febrile infant guidelines.
CONCLUSIONS
Blood cultures in pediatric patients with uncomplicated SSTIs have no proven benefit and are associated with increased LOS, non-negligible false-positive rate, and associated increase in financial charges to the patient and healthcare system. The patient described in the clinical scenario would have an extremely low likelihood of having any meaningful clinical information provided by blood culture as part of her evaluation.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
DISCLOSURES
The authors have no conflicts of interest relevant to this article to disclose.
1. Mistry R, Shapiro D, Goyal M, et al. Clinical management of skin and soft tissue infections in the U.S. Emergency Departments. West J Emerg Med. 2014;15(4):491-498. doi:10.5811/westjem.2014.4.20583. PubMed
2. Witt WP, Weiss AJ, Elixhauser A. Overview of hospital stays for children in the United States, 2012; Statistical Brief #187. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb187-Hospital-Stays-Children-2012.pdf.
3. Dukic VM, Lauderdale DS, Wilder J, Daum RS, David MZ. Epidemics of community-associated methicillin-resistant Staphylococcus aureus in the United States: a meta-analysis. Otto M, ed. PLoS One. 2013;8(1):e52722. doi: 10.1371/journal.pone.0052722. PubMed
4. Fleisher G, Ludwig S, Henretig F, Ruddy R, Henry W. Cellulitis: initial management. Ann Emerg Med. 1981;10(7):356-359. PubMed
5. Fleisher G, Ludwig S, Campos J. Cellulitis: bacterial etiology, clinical features, and laboratory findings. J Pediatr. 1980;97(4):591-593. doi: 10.1016/S0022-3476(80)80014-X http://www.ncbi.nlm.nih.gov/pubmed/6775063. Accessed July 26, 2017.
6. Parikh K, Davis AB, Pavuluri P. Do we need this blood culture? Hosp Pediatr. 2014;4(2):78-84. doi:10.1542/hpeds.2013-0053. PubMed
7. Wathen D, Halloran DR. Blood culture associations in children with a diagnosis of cellulitis in the era of methicillin-resistant Staphylococcus aureus. Hosp Pediatr. 2013;3(2):103-107. http://www.ncbi.nlm.nih.gov/pubmed/24340410. Accessed July 26, 2017.
8. Malone JR, Durica SR, Thompson DM, Bogie A, Naifeh M. Blood cultures in the evaluation of uncomplicated skin and soft tissue infections. Pediatrics. 2013;132(3):454-459. doi:10.1542/peds.2013-1384. PubMed
9. Trenchs V, Hernandez-Bou S, Bianchi C, Arnan M, Gene A, Luaces C. Blood cultures are not useful in the evaluation of children with uncomplicated superficial skin and soft tissue infections. Pediatr Infect Dis J. 2015;34(9):924-927. doi:10.1097/INF.0000000000000768. PubMed
10. Sloane AJ, Pressel DM. Culture pus, not blood: decreasing routine laboratory testing in patients with uncomplicated skin and soft tissue infections. Hosp Pediatr. 2016;6(7):394-398. doi:10.1542/hpeds.2015-0186. PubMed
11. Sadow KB, Chamberlain JM. Blood cultures in the evaluation of children with cellulitis. Pediatrics. 1998;101(3):E4. doi: 10.1542/peds.101.3.e4 http://www.ncbi.nlm.nih.gov/pubmed/9481023. Accessed July 26, 2017.
12. Stevens DL, Bisno AL, Chambers HF, et al. Executive Summary: practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of America. Clin Infect Dis. 2014;59(2):147-159. doi:10.1093/cid/ciu444.
13. Demircioğlu F, Ylmaz S, Oren H, Ozgüven AA, Irken G. Skin and soft tissue complications in pediatric leukemia patients with and without central venous catheters. J Pediatr Hematol Oncol. 2008;30(1):32-35. doi:10.1097/MPH.0b013e31815cc429. PubMed
14. Ray GT, Suaya JA, Baxter R. Microbiology of skin and soft tissue infections in the age of community-acquired methicillin-resistant Staphylococcus aureus. Diagn Microbiol Infect Dis. 2013;76(1):24-30. doi:10.1016/j.diagmicrobio.2013.02.020. PubMed
15. Baumann BM, Russo CJ, Pavlik D, et al. Management of pediatric skin abscesses in pediatric, general academic and community emergency departments. West J Emerg Med. 2011;12(2):159-167. http://www.ncbi.nlm.nih.gov/pubmed/21691519. Accessed July 26, 2017.
The “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CLINICAL SCENARIO
An 8-year-old previously healthy girl presented to the emergency department (ED) with 2 days of warmth, swelling, and pain over her right upper thigh. Three days prior before presentation, a “pimple” appeared on her leg and drained a small amount of pus. Over the next 24 hours, the lesion became swollen, red, and painful. Her pediatrician prescribed trimethoprim-sulfamethoxazole. The patient took 3 doses of this medication but still experienced worsening pain and swelling.
In the ED, she had normal vital signs for her age except for temperature of 100.8 °F. A 2 cm × 3 cm area of fluctuance, erythema, and warmth was noted, and bedside ultrasound demonstrated a simple fluid collection. Incision and drainage was performed with expression of several milliliters of pus. The patient was referred for admission due to worsening symptoms despite outpatient antibiotic therapy. The ED providers ordered a blood culture at the time of admission.
BACKGROUND
Skin and soft tissue infections (SSTIs) are common pediatric diagnoses, which account for an estimated 390,000 ED visits annually1 and represent the 7th most common reason for pediatric hospital admission in the United States.2 The rates of SSTIs have increased over the past several decades partly due to the rise of methicillin-resistant Staphylococcus aureus (MRSA).3
Why You Might Think Blood Cultures are Helpful In Children with SSTIs?
Prior to the introduction of the Haemophilus influenzae vaccine, the rates of SSTI-associated bacteremia ranged from 8% to 20%.4,5 Although the rate of bacteremia has declined significantly, blood cultures are still commonly performed as part of the evaluation of uncomplicated SSTIs in children; studies have shown that blood culture rates are 46% in the combined outpatient/inpatient setting,6 34% in the ED setting,7 and 47%-94% in the inpatient setting.7-11 Clinicians still feel that bacteremia detection is important to guide the selection of antibiotics and treatment duration. Providers may also underestimate the risk of obtaining a contaminant result and associated charges. Lastly, clinicians may perform blood cultures due to cultural norms at their institution.
Why Blood Cultures are Unnecessary in Children with Uncomplicated SSTIs
Several decades into the post vaccine era, the current guidelines from the Infectious Diseases Society of America (IDSA) do not recommend blood cultures as part of the routine evaluation of uncomplicated SSTIs.12 Multiple single-center studies have failed to demonstrate the benefits of obtaining blood cultures in pediatric patients with uncomplicated SSTIs in the post-H. influenzae vaccine era.6–11
Sadow et al11 performed a retrospective case series of 381 children hospitalized with cellulitis to determine the rate and yield of blood cultures. Of the 266 (70%) patients who had a blood culture performed, 5 (1.9%) were true positives and 13 (5.4%) were contaminants. Notably, the true positive results included 3 children with active varicella infection and 2 children with septic joints; the latter would qualify as a complicated SSTI or as a separate infectious process altogether. No significant change in management resulted the positive blood cultures.
Wathen et al7 conducted a similar retrospective case series of 385 children with cellulitis who presented to the ED of a single tertiary-care children’s hospital to determine the rate and yield of blood cultures. Of the 129 (33.5%) blood cultures performed, there were no true positives and 4 (3.1%) contaminants. Obtaining a blood culture was also associated with high rates of ordering complete blood count and hospitalization.
Malone et al8 performed a retrospective case series of 580 children hospitalized with an SSTI at a single children’s hospital to determine the yield of blood cultures for uncomplicated versus complicated SSTIs. Of the 482 patients with uncomplicated SSTIs, 455 (94.4%) had a blood culture, with no true positive cultures and 3 (0.7%) contaminants. Obtaining a blood culture in this study was associated with an almost 1 day increase in length of stay (LOS; mean LOS 3.24 vs 2.33 days, P = .04).
Parikh et al6 conducted a retrospective cohort study of 304 children with SSTIs in both inpatient and outpatient settings to determine the yield and rate of blood cultures. Of this group, 140 (46.1%) patients had a blood culture performed, of which there were 3 (2.9%) true positives and 1 (0.7%) contaminant. True-positive bacteria included MRSA and Streptococcus pyogenes, neither of which was associated with a change in antibiotic regimen or increase in hospital LOS. The total charges associated with the original 140 blood cultures were estimated to be $42,450 annually in the authors’ institution.
Lastly, Trenchs et al9 performed a retrospective case series of 445 children hospitalized with SSTI in a Spanish children’s hospital and found 353 (79.3%) blood cultures with 2 (0.6%) true positives and 10 (2.8%) contaminants. Methicillin-sensitive Staphylococcus aureus (MSSA) and S. pyogenes were the sole true-positive bacteria, and no change in management was reported. Obtaining blood cultures was associated with an increased hospital LOS (median LOS 4 vs. 3 days, P
Across these studies, the reported rates of true-positive blood cultures ranged from 0%-2.9%. Of the 1997 patients included in the studies, only 10 (0.5%) had true-positive blood cultures. This rate decreased to 0.4% if the 2 patients with septic arthritis from the study of Sadow et al were excluded. Isolated organisms included MRSA, MSSA, S. pyogenes, and Streptococcus pneumoniae. No unusual organisms were isolated in uncomplicated SSTIs, and the true-positive results were not associated with any reported change in antibiotic management.6–9,11 False-positive blood culture results were found in 0%-5.4% of patients,6–9,11 accounting for 30 patients or 1.5% of the total patients.
Harms Associated With Unnecessary Blood Cultures in SSTIs
Blood cultures necessitate venipunctures, which are painful for children and families. The inevitable false-positive contaminants also lead to repeat venipunctures and, potentially, unnecessary antibiotic exposure. From a high-value care perspective, Parikh et al reported hospital charges of $300 per blood culture and $250 for identification and sensitivity of positives.6 Assuming that these single-center charges are representative of national charges and using 0.5% true positivity and 1.5% false positivity rates, subjecting all children with uncomplicated SSTIs to blood culture would result in $60,250 charges to find one true positive blood culture, with no resultant changes in management. Additionally, among the 200 children cultured to find one true positive, there would be 3 false positives, necessitating another $1650 in charges for identification, sensitivity analysis, and repeat culture. These amounts do not factor in the significant expenditures associated with increased LOS. The potential savings associated with forgoing blood cultures in children with SSTIs should be an incentive for institutional change.
When Blood Cultures May Be Reasonable
The current IDSA guidelines recommend blood cultures for SSTIs in patients with immunodeficiency, animal bites, and immersion injuries (soft tissue injuries occurring in fresh or saltwater).12 Previous studies also delineated criteria for “complicated” SSTIs, typically defined as surgical or traumatic wounds, infections requiring surgical intervention (not including simple incision and drainage), or infected ulcers or burns.8,9 In the study of Malone et al, 10 (12.5%) positives were found among 80 patients with complicated SSTIs who had blood cultures performed.8 Although this work had a single-center study design with a relatively small sample size, no unusual organisms were found; the grown cultures included MRSA, MSSA, and S. pneumoniae. In addition to patients with complicated SSTIs, immunocompromised children, such as those receiving chemotherapy or other immunosuppressive agents, were excluded from the studies of blood culture yield in SSTIs and may warrant blood cultures given the risk of overwhelming infection and susceptibility to rare or invasive organisms.12 In a study of 57 pediatric patients with leukemia and no central catheters who experienced skin or soft tissue complications, Demircioglu et al13 reported 6 positive blood cultures, including Klebsiella oxytoca, Pseudomonas aeruginosa, and Escherichia coli. These organisms would not be covered by typical SSTI antibiotic regimens, illustrating the value of blood cultures in this selected group of patients. Lastly, although the above studies included some infants, the data on utility of blood cultures in neonates are limited. Blood cultures may be reasonable in this group given the relative immunocompromised state of neonates compared with older children. Additionally, any infants aged
What You Should Do Instead Of Blood Cultures for Uncomplicated SSTIs
Gram stain and wound culture of any purulent material may assist with choice of empiric antibiotic therapy and appropriate narrowing of regimen for antibiotic stewardship. Wound cultures of purulent material can identify the causative organism in 58%-66% of the cases.9,14 The rate of wound culture varies widely from 29% to 81% in studies across different healthcare systems.9,10,15 The use of visually appealing posters advising clinicians to “culture pus, not blood” has been shown to significantly decreased the number of blood cultures performed at a single pediatric hospital.10
RECOMMENDATIONS
- Do not obtain blood cultures in pediatric patients with uncomplicated SSTIs.
- If purulent material is available spontaneously or after incision and drainage, then send it for Gram stain and bacterial culture.
- Blood cultures are reasonable in patients with complicated SSTIs and in immunocompromised patients with SSTIs.
- Despite limited data, blood cultures may be reasonable in neonates with SSTIs. Febrile infants with SSTIs aged less than 90 days should be managed under existing febrile infant guidelines.
CONCLUSIONS
Blood cultures in pediatric patients with uncomplicated SSTIs have no proven benefit and are associated with increased LOS, non-negligible false-positive rate, and associated increase in financial charges to the patient and healthcare system. The patient described in the clinical scenario would have an extremely low likelihood of having any meaningful clinical information provided by blood culture as part of her evaluation.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
DISCLOSURES
The authors have no conflicts of interest relevant to this article to disclose.
The “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CLINICAL SCENARIO
An 8-year-old previously healthy girl presented to the emergency department (ED) with 2 days of warmth, swelling, and pain over her right upper thigh. Three days prior before presentation, a “pimple” appeared on her leg and drained a small amount of pus. Over the next 24 hours, the lesion became swollen, red, and painful. Her pediatrician prescribed trimethoprim-sulfamethoxazole. The patient took 3 doses of this medication but still experienced worsening pain and swelling.
In the ED, she had normal vital signs for her age except for temperature of 100.8 °F. A 2 cm × 3 cm area of fluctuance, erythema, and warmth was noted, and bedside ultrasound demonstrated a simple fluid collection. Incision and drainage was performed with expression of several milliliters of pus. The patient was referred for admission due to worsening symptoms despite outpatient antibiotic therapy. The ED providers ordered a blood culture at the time of admission.
BACKGROUND
Skin and soft tissue infections (SSTIs) are common pediatric diagnoses, which account for an estimated 390,000 ED visits annually1 and represent the 7th most common reason for pediatric hospital admission in the United States.2 The rates of SSTIs have increased over the past several decades partly due to the rise of methicillin-resistant Staphylococcus aureus (MRSA).3
Why You Might Think Blood Cultures are Helpful In Children with SSTIs?
Prior to the introduction of the Haemophilus influenzae vaccine, the rates of SSTI-associated bacteremia ranged from 8% to 20%.4,5 Although the rate of bacteremia has declined significantly, blood cultures are still commonly performed as part of the evaluation of uncomplicated SSTIs in children; studies have shown that blood culture rates are 46% in the combined outpatient/inpatient setting,6 34% in the ED setting,7 and 47%-94% in the inpatient setting.7-11 Clinicians still feel that bacteremia detection is important to guide the selection of antibiotics and treatment duration. Providers may also underestimate the risk of obtaining a contaminant result and associated charges. Lastly, clinicians may perform blood cultures due to cultural norms at their institution.
Why Blood Cultures are Unnecessary in Children with Uncomplicated SSTIs
Several decades into the post vaccine era, the current guidelines from the Infectious Diseases Society of America (IDSA) do not recommend blood cultures as part of the routine evaluation of uncomplicated SSTIs.12 Multiple single-center studies have failed to demonstrate the benefits of obtaining blood cultures in pediatric patients with uncomplicated SSTIs in the post-H. influenzae vaccine era.6–11
Sadow et al11 performed a retrospective case series of 381 children hospitalized with cellulitis to determine the rate and yield of blood cultures. Of the 266 (70%) patients who had a blood culture performed, 5 (1.9%) were true positives and 13 (5.4%) were contaminants. Notably, the true positive results included 3 children with active varicella infection and 2 children with septic joints; the latter would qualify as a complicated SSTI or as a separate infectious process altogether. No significant change in management resulted the positive blood cultures.
Wathen et al7 conducted a similar retrospective case series of 385 children with cellulitis who presented to the ED of a single tertiary-care children’s hospital to determine the rate and yield of blood cultures. Of the 129 (33.5%) blood cultures performed, there were no true positives and 4 (3.1%) contaminants. Obtaining a blood culture was also associated with high rates of ordering complete blood count and hospitalization.
Malone et al8 performed a retrospective case series of 580 children hospitalized with an SSTI at a single children’s hospital to determine the yield of blood cultures for uncomplicated versus complicated SSTIs. Of the 482 patients with uncomplicated SSTIs, 455 (94.4%) had a blood culture, with no true positive cultures and 3 (0.7%) contaminants. Obtaining a blood culture in this study was associated with an almost 1 day increase in length of stay (LOS; mean LOS 3.24 vs 2.33 days, P = .04).
Parikh et al6 conducted a retrospective cohort study of 304 children with SSTIs in both inpatient and outpatient settings to determine the yield and rate of blood cultures. Of this group, 140 (46.1%) patients had a blood culture performed, of which there were 3 (2.9%) true positives and 1 (0.7%) contaminant. True-positive bacteria included MRSA and Streptococcus pyogenes, neither of which was associated with a change in antibiotic regimen or increase in hospital LOS. The total charges associated with the original 140 blood cultures were estimated to be $42,450 annually in the authors’ institution.
Lastly, Trenchs et al9 performed a retrospective case series of 445 children hospitalized with SSTI in a Spanish children’s hospital and found 353 (79.3%) blood cultures with 2 (0.6%) true positives and 10 (2.8%) contaminants. Methicillin-sensitive Staphylococcus aureus (MSSA) and S. pyogenes were the sole true-positive bacteria, and no change in management was reported. Obtaining blood cultures was associated with an increased hospital LOS (median LOS 4 vs. 3 days, P
Across these studies, the reported rates of true-positive blood cultures ranged from 0%-2.9%. Of the 1997 patients included in the studies, only 10 (0.5%) had true-positive blood cultures. This rate decreased to 0.4% if the 2 patients with septic arthritis from the study of Sadow et al were excluded. Isolated organisms included MRSA, MSSA, S. pyogenes, and Streptococcus pneumoniae. No unusual organisms were isolated in uncomplicated SSTIs, and the true-positive results were not associated with any reported change in antibiotic management.6–9,11 False-positive blood culture results were found in 0%-5.4% of patients,6–9,11 accounting for 30 patients or 1.5% of the total patients.
Harms Associated With Unnecessary Blood Cultures in SSTIs
Blood cultures necessitate venipunctures, which are painful for children and families. The inevitable false-positive contaminants also lead to repeat venipunctures and, potentially, unnecessary antibiotic exposure. From a high-value care perspective, Parikh et al reported hospital charges of $300 per blood culture and $250 for identification and sensitivity of positives.6 Assuming that these single-center charges are representative of national charges and using 0.5% true positivity and 1.5% false positivity rates, subjecting all children with uncomplicated SSTIs to blood culture would result in $60,250 charges to find one true positive blood culture, with no resultant changes in management. Additionally, among the 200 children cultured to find one true positive, there would be 3 false positives, necessitating another $1650 in charges for identification, sensitivity analysis, and repeat culture. These amounts do not factor in the significant expenditures associated with increased LOS. The potential savings associated with forgoing blood cultures in children with SSTIs should be an incentive for institutional change.
When Blood Cultures May Be Reasonable
The current IDSA guidelines recommend blood cultures for SSTIs in patients with immunodeficiency, animal bites, and immersion injuries (soft tissue injuries occurring in fresh or saltwater).12 Previous studies also delineated criteria for “complicated” SSTIs, typically defined as surgical or traumatic wounds, infections requiring surgical intervention (not including simple incision and drainage), or infected ulcers or burns.8,9 In the study of Malone et al, 10 (12.5%) positives were found among 80 patients with complicated SSTIs who had blood cultures performed.8 Although this work had a single-center study design with a relatively small sample size, no unusual organisms were found; the grown cultures included MRSA, MSSA, and S. pneumoniae. In addition to patients with complicated SSTIs, immunocompromised children, such as those receiving chemotherapy or other immunosuppressive agents, were excluded from the studies of blood culture yield in SSTIs and may warrant blood cultures given the risk of overwhelming infection and susceptibility to rare or invasive organisms.12 In a study of 57 pediatric patients with leukemia and no central catheters who experienced skin or soft tissue complications, Demircioglu et al13 reported 6 positive blood cultures, including Klebsiella oxytoca, Pseudomonas aeruginosa, and Escherichia coli. These organisms would not be covered by typical SSTI antibiotic regimens, illustrating the value of blood cultures in this selected group of patients. Lastly, although the above studies included some infants, the data on utility of blood cultures in neonates are limited. Blood cultures may be reasonable in this group given the relative immunocompromised state of neonates compared with older children. Additionally, any infants aged
What You Should Do Instead Of Blood Cultures for Uncomplicated SSTIs
Gram stain and wound culture of any purulent material may assist with choice of empiric antibiotic therapy and appropriate narrowing of regimen for antibiotic stewardship. Wound cultures of purulent material can identify the causative organism in 58%-66% of the cases.9,14 The rate of wound culture varies widely from 29% to 81% in studies across different healthcare systems.9,10,15 The use of visually appealing posters advising clinicians to “culture pus, not blood” has been shown to significantly decreased the number of blood cultures performed at a single pediatric hospital.10
RECOMMENDATIONS
- Do not obtain blood cultures in pediatric patients with uncomplicated SSTIs.
- If purulent material is available spontaneously or after incision and drainage, then send it for Gram stain and bacterial culture.
- Blood cultures are reasonable in patients with complicated SSTIs and in immunocompromised patients with SSTIs.
- Despite limited data, blood cultures may be reasonable in neonates with SSTIs. Febrile infants with SSTIs aged less than 90 days should be managed under existing febrile infant guidelines.
CONCLUSIONS
Blood cultures in pediatric patients with uncomplicated SSTIs have no proven benefit and are associated with increased LOS, non-negligible false-positive rate, and associated increase in financial charges to the patient and healthcare system. The patient described in the clinical scenario would have an extremely low likelihood of having any meaningful clinical information provided by blood culture as part of her evaluation.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
DISCLOSURES
The authors have no conflicts of interest relevant to this article to disclose.
1. Mistry R, Shapiro D, Goyal M, et al. Clinical management of skin and soft tissue infections in the U.S. Emergency Departments. West J Emerg Med. 2014;15(4):491-498. doi:10.5811/westjem.2014.4.20583. PubMed
2. Witt WP, Weiss AJ, Elixhauser A. Overview of hospital stays for children in the United States, 2012; Statistical Brief #187. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb187-Hospital-Stays-Children-2012.pdf.
3. Dukic VM, Lauderdale DS, Wilder J, Daum RS, David MZ. Epidemics of community-associated methicillin-resistant Staphylococcus aureus in the United States: a meta-analysis. Otto M, ed. PLoS One. 2013;8(1):e52722. doi: 10.1371/journal.pone.0052722. PubMed
4. Fleisher G, Ludwig S, Henretig F, Ruddy R, Henry W. Cellulitis: initial management. Ann Emerg Med. 1981;10(7):356-359. PubMed
5. Fleisher G, Ludwig S, Campos J. Cellulitis: bacterial etiology, clinical features, and laboratory findings. J Pediatr. 1980;97(4):591-593. doi: 10.1016/S0022-3476(80)80014-X http://www.ncbi.nlm.nih.gov/pubmed/6775063. Accessed July 26, 2017.
6. Parikh K, Davis AB, Pavuluri P. Do we need this blood culture? Hosp Pediatr. 2014;4(2):78-84. doi:10.1542/hpeds.2013-0053. PubMed
7. Wathen D, Halloran DR. Blood culture associations in children with a diagnosis of cellulitis in the era of methicillin-resistant Staphylococcus aureus. Hosp Pediatr. 2013;3(2):103-107. http://www.ncbi.nlm.nih.gov/pubmed/24340410. Accessed July 26, 2017.
8. Malone JR, Durica SR, Thompson DM, Bogie A, Naifeh M. Blood cultures in the evaluation of uncomplicated skin and soft tissue infections. Pediatrics. 2013;132(3):454-459. doi:10.1542/peds.2013-1384. PubMed
9. Trenchs V, Hernandez-Bou S, Bianchi C, Arnan M, Gene A, Luaces C. Blood cultures are not useful in the evaluation of children with uncomplicated superficial skin and soft tissue infections. Pediatr Infect Dis J. 2015;34(9):924-927. doi:10.1097/INF.0000000000000768. PubMed
10. Sloane AJ, Pressel DM. Culture pus, not blood: decreasing routine laboratory testing in patients with uncomplicated skin and soft tissue infections. Hosp Pediatr. 2016;6(7):394-398. doi:10.1542/hpeds.2015-0186. PubMed
11. Sadow KB, Chamberlain JM. Blood cultures in the evaluation of children with cellulitis. Pediatrics. 1998;101(3):E4. doi: 10.1542/peds.101.3.e4 http://www.ncbi.nlm.nih.gov/pubmed/9481023. Accessed July 26, 2017.
12. Stevens DL, Bisno AL, Chambers HF, et al. Executive Summary: practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of America. Clin Infect Dis. 2014;59(2):147-159. doi:10.1093/cid/ciu444.
13. Demircioğlu F, Ylmaz S, Oren H, Ozgüven AA, Irken G. Skin and soft tissue complications in pediatric leukemia patients with and without central venous catheters. J Pediatr Hematol Oncol. 2008;30(1):32-35. doi:10.1097/MPH.0b013e31815cc429. PubMed
14. Ray GT, Suaya JA, Baxter R. Microbiology of skin and soft tissue infections in the age of community-acquired methicillin-resistant Staphylococcus aureus. Diagn Microbiol Infect Dis. 2013;76(1):24-30. doi:10.1016/j.diagmicrobio.2013.02.020. PubMed
15. Baumann BM, Russo CJ, Pavlik D, et al. Management of pediatric skin abscesses in pediatric, general academic and community emergency departments. West J Emerg Med. 2011;12(2):159-167. http://www.ncbi.nlm.nih.gov/pubmed/21691519. Accessed July 26, 2017.
1. Mistry R, Shapiro D, Goyal M, et al. Clinical management of skin and soft tissue infections in the U.S. Emergency Departments. West J Emerg Med. 2014;15(4):491-498. doi:10.5811/westjem.2014.4.20583. PubMed
2. Witt WP, Weiss AJ, Elixhauser A. Overview of hospital stays for children in the United States, 2012; Statistical Brief #187. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb187-Hospital-Stays-Children-2012.pdf.
3. Dukic VM, Lauderdale DS, Wilder J, Daum RS, David MZ. Epidemics of community-associated methicillin-resistant Staphylococcus aureus in the United States: a meta-analysis. Otto M, ed. PLoS One. 2013;8(1):e52722. doi: 10.1371/journal.pone.0052722. PubMed
4. Fleisher G, Ludwig S, Henretig F, Ruddy R, Henry W. Cellulitis: initial management. Ann Emerg Med. 1981;10(7):356-359. PubMed
5. Fleisher G, Ludwig S, Campos J. Cellulitis: bacterial etiology, clinical features, and laboratory findings. J Pediatr. 1980;97(4):591-593. doi: 10.1016/S0022-3476(80)80014-X http://www.ncbi.nlm.nih.gov/pubmed/6775063. Accessed July 26, 2017.
6. Parikh K, Davis AB, Pavuluri P. Do we need this blood culture? Hosp Pediatr. 2014;4(2):78-84. doi:10.1542/hpeds.2013-0053. PubMed
7. Wathen D, Halloran DR. Blood culture associations in children with a diagnosis of cellulitis in the era of methicillin-resistant Staphylococcus aureus. Hosp Pediatr. 2013;3(2):103-107. http://www.ncbi.nlm.nih.gov/pubmed/24340410. Accessed July 26, 2017.
8. Malone JR, Durica SR, Thompson DM, Bogie A, Naifeh M. Blood cultures in the evaluation of uncomplicated skin and soft tissue infections. Pediatrics. 2013;132(3):454-459. doi:10.1542/peds.2013-1384. PubMed
9. Trenchs V, Hernandez-Bou S, Bianchi C, Arnan M, Gene A, Luaces C. Blood cultures are not useful in the evaluation of children with uncomplicated superficial skin and soft tissue infections. Pediatr Infect Dis J. 2015;34(9):924-927. doi:10.1097/INF.0000000000000768. PubMed
10. Sloane AJ, Pressel DM. Culture pus, not blood: decreasing routine laboratory testing in patients with uncomplicated skin and soft tissue infections. Hosp Pediatr. 2016;6(7):394-398. doi:10.1542/hpeds.2015-0186. PubMed
11. Sadow KB, Chamberlain JM. Blood cultures in the evaluation of children with cellulitis. Pediatrics. 1998;101(3):E4. doi: 10.1542/peds.101.3.e4 http://www.ncbi.nlm.nih.gov/pubmed/9481023. Accessed July 26, 2017.
12. Stevens DL, Bisno AL, Chambers HF, et al. Executive Summary: practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of America. Clin Infect Dis. 2014;59(2):147-159. doi:10.1093/cid/ciu444.
13. Demircioğlu F, Ylmaz S, Oren H, Ozgüven AA, Irken G. Skin and soft tissue complications in pediatric leukemia patients with and without central venous catheters. J Pediatr Hematol Oncol. 2008;30(1):32-35. doi:10.1097/MPH.0b013e31815cc429. PubMed
14. Ray GT, Suaya JA, Baxter R. Microbiology of skin and soft tissue infections in the age of community-acquired methicillin-resistant Staphylococcus aureus. Diagn Microbiol Infect Dis. 2013;76(1):24-30. doi:10.1016/j.diagmicrobio.2013.02.020. PubMed
15. Baumann BM, Russo CJ, Pavlik D, et al. Management of pediatric skin abscesses in pediatric, general academic and community emergency departments. West J Emerg Med. 2011;12(2):159-167. http://www.ncbi.nlm.nih.gov/pubmed/21691519. Accessed July 26, 2017.
© 2018 Society of Hospital Medicine
Mental Health Conditions and Unplanned Hospital Readmissions in Children
Readmission prevention is a focus of national efforts to improve the quality of hospital care for children.1-5 Several factors contribute to the risk of readmission for hospitalized children, including age, race or ethnicity, payer, and the type and number of comorbid health conditions.6-9 Mental health conditions (MHCs) are a prevalent comorbidity in children hospitalized for physical health reasons that could influence their postdischarge health and safety.
MHCs are increasingly common in children hospitalized for physical health indications; a comorbid MHC is currently present in 10% to 25% of hospitalized children ages 3 years and older.10,11 Hospital length of stay (LOS) and cost are higher in children with an MHC.12,13 Increased resource use may occur because MHCs can impede hospital treatment effectiveness and the child’s recovery from physical illness. MHCs are associated with a lower adherence with medications14-16 and a lower ability to cope with health events and problems.17-19 In adults, MHCs are a well-established risk factor for hospital readmission for a variety of physical health conditions.20-24 Although the influence of MHCs on readmissions in children has not been extensively investigated, higher readmission rates have been reported in adolescents hospitalized for diabetes with an MHC compared with those with no MHC.25,26
To our knowledge, no large studies have examined the relationship between the presence of a comorbid MHC and hospital readmissions in children or adolescents hospitalized for a broad array of medical or procedure conditions. Therefore, we conducted this study to (1) assess the likelihood of 30-day hospital readmission in children with versus without MHC who were hospitalized for one of 10 medical or 10 procedure conditions, and (2) to assess which MHCs are associated with the highest likelihood of hospital readmission.
METHODS
Study Design and Setting
We conducted a national, retrospective cohort study of index hospitalizations for children ages 3 to 21 years who were discharged from January 1, 2013, to November 30, 2013, in the Agency for Healthcare Research and Quality’s (AHRQ) Nationwide Readmissions Database (NRD). Admissions occurring in December 2013 were excluded because they did not have a 30-day timeframe available for readmission measurement. The 2013 NRD includes administrative data for a nationally representative sample of 14 million hospitalizations in 21 states, accounting for 49% of all US hospitalizations and weighted to represent 35.6 million hospitalizations. The database includes deidentified, verified patient linkage numbers so that patients can be tracked across multiple hospitalizations at the same institution or different institutions within a state. The NRD includes hospital information, patient demographic information, and the International Classification of Diseases, 9th Revision-Clinical Modification (ICD-9-CM) discharge diagnoses and procedures, with 1 primary diagnosis and up to 24 additional fields for comorbid diagnoses. This study was approved for exemption by the Children’s Hospital of Philadelphia Institutional Review Board.
Index Admissions
We used the methods described below to create a study cohort of the 10 medical and 10 procedure index admissions associated with the highest volume (ie, the greatest absolute number) of 30-day hospital readmissions. Conditions with a high volume of readmissions were chosen in an effort to identify conditions in which readmission-prevention interventions had the greatest potential to reduce the absolute number of readmissions. We first categorized index hospitalizations for medical and procedure conditions by using the All Patient Refined Diagnosis Related Groups (APR-DRGs; 3M Health Information Systems, Wallingford, CT).27 APR-DRGs use all diagnosis and/or procedure ICD-9-CM codes registered for a hospital discharge to assign 1 reason that best explains the need for hospitalization. We then excluded obstetric hospitalizations, psychiatric hospitalizations, and hospitalizations resulting in death or transfer from being considered as index admissions. Afterwards, we ranked each APR-DRG index hospitalization by the total number of 30-day hospital readmissions that occurred afterward and selected the 10 medical and 10 procedure index admissions with the highest number of readmissions. The APR-DRG index admissions are listed in Figures 1 and 2. For the APR-DRG “digestive system diagnoses,” the most common diagnosis was constipation, and we refer to that category as “constipation.” The most common diagnosis for the APR-DRG called “other operating room procedure for neoplasm” was tumor biopsy, and we refer to that category as “tumor biopsy.”
Main Outcome Measure
The primary study outcome was unplanned, all-cause readmission to any hospital within 30 days of index hospitalization. All-cause readmissions include any hospitalization for the same or different condition as the index admission, including conditions not eligible to be considered as index admissions (obstetric, psychiatric, and hospitalizations resulting in death or transfer). Planned readmissions, identified by using pediatric-specific measure specifications endorsed by AHRQ and the National Quality Forum,28 were excluded from measurement. For index admissions with multiple 30-day readmissions, only the first readmission was counted. Each readmission was treated as an index admission.
Main Independent Variable
The main independent variable was the presence of an MHC documented during the index hospitalization. MHCs were identified and classified into diagnosis categories derived from the AHRQ Chronic Condition Indicator system by using ICD-9-CM codes.29 MHC categories included anxiety disorders, attention-deficit/hyperactivity disorder (ADHD), autism, depression, and substance abuse. Less common MHCs included bipolar disorder, schizophrenia, disruptive behavior disorders, somatoform disorders, and eating disorders. These conditions are included in the group with any MHC, but we did not calculate the adjusted odds ratios (AORs) of readmission for these conditions. Children were identified as having multiple MHCs if they had more than 1 MHC.
Other Characteristics of Index Hospitalizations
A priori, we selected for analysis the known demographic, clinical, and hospital factors associated with the risk of readmission.20-24 The demographic characteristics included patient age, gender, payer category, urban or rural residence, and the median income quartile for a patient’s ZIP code. The hospital characteristics included location, ownership, and teaching hospital designation. The clinical characteristics included the number of chronic conditions30 and indicators for the presence of a complex chronic condition in each of 12 organ systems.31
Statistical Analysis
We calculated descriptive summary statistics for the characteristics of index hospitalizations. We compared characteristics in index admissions of children with versus without MHC by using Wilcoxon Rank-Sum tests for continuous variables and Wald χ2 tests for categorical variables. In the multivariable analysis, we derived logistic regression models to assess the relationship of 30-day hospital readmission with each type of MHC, adjusting for index admission demographic, hospital, and clinical characteristics. MHCs were modeled as binary indicator variables with the presence of any MHC, more than 1 MHC, or each of 5 MHC categories (anxiety disorders, ADHD, autism, depression, substance abuse) compared with no MHC. Four types of logistic regression models were derived (1) for the combined sample of all 10 index medical admissions with each MHC category versus no MHC as a primary predictor, (2) for each medical index admission with any MHC versus no MHC as the primary predictor, (3) for the combined sample of all 10 index procedure admissions with each MHC category versus no MHC as a primary predictor, and (4) for each procedure index admission with any MHC versus no MHC as the primary predictor. All analyses were weighted to achieve national estimates and clustered by hospital by using AHRQ-recommended survey procedures. SAS version 9.4 (SAS Institute, Cary, NC) was used for all analyses. All tests were two-sided, and a P < .05 was
considered statistically significant.
RESULTS
Study Population
Across all index admissions, 16.3% were for children with an MHC. Overall, children with MHCs were older and more likely to have a chronic30 or complex chronic31 physical health condition than children with no MHCs (Table).
Index Medical Admissions, Mental Health Conditions, and Hospital Readmission
The 10 index medical hospitalizations with the most readmissions for children ages 3 to 20 years were asthma, chemotherapy, constipation, diabetes, gastroenteritis, inflammatory bowel disease, neutropenia, pneumonia, seizure, and sickle cell crisis. Across all index medical hospitalizations, 17.5% were for patients with an MHC (Figure 1). Of index medical admissions with any MHC, 26.3% had ADHD, 22.9% had an anxiety disorder, 14.9% had autism, 18.3% had depression, and 30.9% had substance abuse. Among all admissions with MHCs, 28.9% had 2 or more MHCs.
Index Medical Admissions Combined
For all index medical hospitalizations combined, 17.0% (n = 59,138) had an unplanned, 30-day hospital readmission. The rate of 30-day hospital readmissions was higher with versus without an MHC (17.5 vs 16.8%; P < .001). In a multivariable analysis, presence of an MHC was associated with a higher likelihood of hospital readmission following an index medical admission (AOR, 1.23; 95% confidence interval [CI], 1.19-1.26); Figure 1). All MHCs except autism and ADHD had a higher likelihood of readmission (Figure 3).
Specific Index Medical Admissions
Index Procedure Admissions, Mental Health Conditions, and Hospital Readmission
Index Procedure Admissions Combined
Specific Index Procedure Admissions
For specific index procedure admissions, the rate of 30-day hospital readmission ranged from 2.2% for knee procedures to 33.6% for tumor biopsy. For 3 (ie, urinary tract, ventricular shunt, and bowel procedures) of the 10 specific index procedure hospitalizations, having an MHC was associated with higher adjusted odds of 30-day readmission (AOR range, 1.38-2.27; Figure 2).
In total, adjusting for sociodemographic, clinical, and hospital characteristics, MHCs were associated with an additional 2501 medical readmissions and 217 procedure readmissions beyond what would have been expected if MHCs were not associated with readmissions.
DISCUSSION
MHCs are common among pediatric hospitalizations with the highest volume of readmissions; MHCs were present in approximately 1 in 5 medical and 1 in 7 procedure index hospitalizations. Across medical and procedure admissions, the adjusted likelihood of unplanned, all-cause 30-day readmission was 25% higher for children with versus without an MHC. The readmission likelihood varied by the type of medical or procedure admission and by the type of MHC. MHCs had the strongest associations with readmissions following hospitalization for diabetes and urinary tract procedures. The MHC categories associated with the highest readmission likelihood were depression, substance abuse, and multiple MHCs.
The current study complements existing literature by helping establish MHCs as a prevalent and important risk factor for hospital readmission in children. Estimates of the prevalence of MHCs in hospitalized children are between 10% and 25%,10,11,32 and prevalence has increased by as much as 160% over the last 10 years.29 Prior investigations have found that children with an MHC tend to stay longer in the hospital compared with children with no MHC.32 Results from the present study suggest that children with MHCs also experience more inpatient days because of rehospitalizations. Subsequent investigations should strive to understand the mechanisms in the hospital, community, and family environment that are responsible for the increased inpatient utilization in children with MHCs. Understanding how the receipt of mental health services before, during, and after hospitalization influences readmissions could help identify opportunities for practice improvement. Families report the need for better coordination of their child’s medical and mental health care,33 and opportunities exist to improve attendance at mental health visits after acute care encounters.34 Among adults, interventions that address posthospital access to mental healthcare have prevented readmissions.35
Depression was associated with an increased risk of readmission in medical and procedure hospitalizations. As a well-known risk factor for readmission in adult patients,21 depression can adversely affect and exacerbate the physical health recovery of patients experiencing acute and chronic illnesses.14,36,37 Depression is considered a modifiable contributor that, when controlled, may help lower readmission risk. Optimal adherence with behavior and medication treatment for depression is associated with a lower risk of unplanned 30-day readmissions.14-16,19 Emerging evidence demonstrates how multifaceted, psychosocial approaches can improve patients’ adherence with depression treatment plans.38 Increased attention to depression in hospitalized children may uncover new ways to manage symptoms as children transition from hospital to home.
Other MHCs were associated with a different risk of readmission among medical and procedure hospitalizations. For example, ADHD or autism documented during index hospitalization was associated with an increased risk of readmission following procedure hospitalizations and a decreased risk following medical hospitalizations. Perhaps children with ADHD or autism who exhibit hyperactive, impulsive, or repetitive behaviors39,40 are at risk for disrupting their postprocedure wound healing, nutrition recovery, or pain tolerance, which might contribute to increased readmission risk.
MHCs were associated with different readmission risks across specific types of medical or procedure hospitalizations. For example, among medical conditions, the association of readmissions with MHCs was highest for diabetes, which is consistent with prior research.26 Factors that might mediate this relationship include changes in diet and appetite, difficulty with diabetes care plan adherence, and intentional nonadherence as a form of self-harm. Similarly, a higher risk of readmission in chronic medical conditions like asthma, constipation, and sickle cell disease might be mediated by difficulty adhering to medical plans or managing exacerbations at home. In contrast, MHCs had no association with readmission following chemotherapy. In our clinical experience, readmissions following chemotherapy are driven by physiologic problems, such as thrombocytopenia, fever, and/or neutropenia. MHCs might have limited influence over those health issues. For procedure hospitalizations, MHCs had 1 of the strongest associations with ventricular shunt procedures. We hypothesize that MHCs might lead some children to experience general health symptoms that might be associated with shunt malfunction (eg, fatigue, headache, behavior change), which could lead to an increased risk of readmission to evaluate for shunt malfunction. Conversely, we found no relationship between MHCs and readmissions following appendectomy. For appendectomy, MHCs might have limited influence over the development of postsurgical complications (eg, wound infection or ileus). Future research to better elucidate mediators of increased risk of readmission associated with MHCs in certain medical and procedure conditions could help explain these relationships and identify possible future intervention targets to prevent readmissions.
This study has several limitations. The administrative data are not positioned to discover the mechanisms by which MHCs are associated with a higher likelihood of readmission. We used hospital ICD-9-CM codes to identify patients with MHCs. Other methods using more clinically rich data (eg, chart review, prescription medications, etc.) may be preferable to identify patients with MHCs. Although the use of ICD-9-CM codes may have sufficient specificity, some hospitalized children may have an MHC that is not coded. Patients identified by using diagnosis codes could represent patients with a higher severity of illness, patients using medications, or patients whose outpatient records are accessible to make the hospital team aware of the MHC. If documentation of MHCs during hospitalization represents a higher severity of illness, findings may not extrapolate to lower-severity MHCs. As hospitals transition from ICD-9 -CM to ICD-10 coding, and health systems develop more integrated inpatient and outpatient EHRs, diagnostic specificity may improve. We could not analyze the relationships with several potential confounders and explanatory variables that may be related both to the likelihood of having an MHC and the risk of readmission, including medication administration, psychiatric consultation, and parent mental health. Postdischarge health services, including access to a medical home or a usual source of mental healthcare and measures of medication adherence, were not available in the NRD.
Despite these limitations, the current study underscores the importance of MHCs in hospitalized children upon discharge. As subsequent investigations uncover the key drivers explaining the influence of MHCs on hospital readmission risk, hospitals and their local outpatient and community practices may find it useful to consider MHCs when (1) developing contingency plans and establishing follow-up care at discharge,41 (2) exploring opportunities of care integration between mental and physical health care professionals, and (3) devising strategies to reduce hospital readmissions among populations of children.
CONCLUSIONS
MHCs are prevalent in hospitalized children and are associated with an increased risk of 30-day, unplanned hospital readmission. Future readmission prevention efforts may uncover new ways to improve children’s transitions from hospital to home by investigating strategies to address their MHCs.
Acknowledgments
The authors thank Donjo Lau and Troy Richardson for their assistance with the analysis.
Disclosures
Dr. Doupnik was supported by a Ruth L. Kirschstein National Research Service Award institutional training grant (T32-HP010026), funded by the National Institutes of Health. Dr. Zima was supported by the Behavioral Health Centers of Excellence for California (SB852). Dr. Bardach was supported by the National Institute of Child Health and Human Development (K23-HD065836). Dr. Berry was supported by the Agency for Healthcare Research and Quality (R21 HS023092-01). The authors have no financial relationships relevant to this article to disclose. The authors have no potential conflicts of interest to disclose. Dr. Doupnik led the study design and analysis and drafted the initial manuscript. Mr. Lawlor performed the data analysis. Dr. Hall provided statistical consultation. All authors participated in the design of the study, interpretation of the data, revised the manuscript for key intellectual content, and all authors read and approved the final manuscript.
1. Dougherty D, Schiff J, Mangione-Smith R. The Children’s Health Insurance Program Reauthorization Act quality measures initiatives: moving forward to improve measurement, care, and child and adolescent outcomes. Acad Pediatr. 2011;11(3):S1-S10. PubMed
2. Bardach NS, Vittinghoff E, Asteria-Penaloza R, et al. Measuring Hospital Quality Using Pediatric Readmission and Revisit Rates. Pediatrics. 2013;132(3):429-436. doi:10.1542/peds.2012-3527. PubMed
3. Khan A, Nakamura MM, Zaslavsky AM, et al. Same-Hospital Readmission Rates as a Measure of Pediatric Quality of Care. JAMA Pediatr. 2015;169(10):905-912. doi:10.1001/jamapediatrics.2015.1129. PubMed
4. Fassl BA, Nkoy FL, Stone BL, et al. The Joint Commission Children’s Asthma Care quality measures and asthma readmissions. Pediatrics. 2012;130(3):482-491. doi:10.1542/peds.2011-3318. PubMed
5. Hain PD, Gay JC, Berutti TW, Whitney GM, Wang W, Saville BR. Preventability of Early Readmissions at a Children’s Hospital. Pediatrics. 2013;131(1):e171-e181. doi:10.1542/peds.2012-0820. PubMed
6. Nagasako E, Reidhead B, Waterman B, et al. Adding Socioeconomic Data to Hospital Readmissions Calculations May Produce More Useful Results. Health Aff. 2014;33(5):786-791. PubMed
7. Hu J, Gonsahn MD, Nerenz DR. Socioeconomic Status and Readmissions: Evidence from an Urban Teaching Hospital. Health Aff. 2014;33(5):778-785. doi:10.1377/hlthaff.2013.0816. PubMed
8. Sills MR, Hall M, Colvin JD, et al. Association of Social Determinants with Children’s Hospitals’ Preventable Readmissions Performance. JAMA Pediatr. 2016;170(4):350-358. doi:10.1001/jamapediatrics.2015.4440. PubMed
9. Eselius LL, Cleary PD, Zaslavsky AM, Huskamp HA, Busch SH. Case-Mix Adjustment of Consumer Reports about Managed Behavioral Health Care and Health Plans. Health Serv Res. 2008;43(6):2014-2032. doi:10.1111/j.1475-6773.2008.00894.x. PubMed
10. Doupnik SK, Henry MK, Bae H, et al. Mental Health Conditions and Symptoms in Pediatric Hospitalizations: A Single-Center Point Prevalence Study. Acad Pediatr. 2017;17(2):184-190. PubMed
11. Bardach NS, Coker TR, Zima BT, et al. Common and Costly Hospitalizations for Pediatric Mental Health Disorders. Pediatrics. 2014;133(4):602-609. doi:10.1542/peds.2013-3165. PubMed
12. Doupnik SK, Mitra N, Feudtner C, Marcus SC. The Influence of Comorbid Mood and Anxiety Disorders on Outcomes of Pediatric Patients Hospitalized for Pneumonia. Hosp Pediatr. 2016;6(3):135-142. doi:10.1542/hpeds.2015-0177. PubMed
13. Snell C, Fernandes S, Bujoreanu IS, Garcia G. Depression, illness severity, and healthcare utilization in cystic fibrosis. Pediatr Pulmonol. 2014;49(12):1177-1181. doi:10.1002/ppul.22990. PubMed
14. DiMatteo MR, Lepper HS, Croghan TW. Depression Is a Risk Factor for Noncompliance with Medical Treatment: Meta-analysis of the Effects of Anxiety and Depression on Patient Adherence. Arch Intern Med . 2000;160(14):2101-2107. doi:10.1001/archinte.160.14.2101. PubMed
15. Gray WN, Denson LA, Baldassano RN, Hommel KA. Treatment Adherence in Adolescents with Inflammatory Bowel Disease: The Collective Impact of Barriers to Adherence and Anxiety/Depressive Symptoms. J Pediatr Psychol. 2012;37(3):282-291. doi:10.1093/jpepsy/jsr092. PubMed
16. Mosnaim G, Li H, Martin M, et al. Factors associated with levels of adherence to inhaled corticosteroids in minority adolescents with asthma. Ann Allergy Asthma Immunol. 2014;112(2):116-120. doi:10.1016/j.anai.2013.11.021. PubMed
17. Compas BE, Jaser SS, Dunn MJ, Rodriguez EM. Coping with Chronic Illness in Childhood and Adolescence. Ann Rev Clin Psychol. 2012;8(1):455-480. doi:10.1146/annurev-clinpsy-032511-143108. PubMed
18. Graue M, Wentzel-Larsen T, Bru E, Hanestad BR, Søvik O. The coping styles of adolescents with type 1 diabetes are associated with degree of metabolic control. Diabetes Care. 2004;27(6):1313-1317. PubMed
19. Jaser SS, White LE. Coping and resilience in adolescents with type 1 diabetes. Child Care Health Dev. 2011;37(3):335-342. doi:10.1111/j.1365-2214.2010.01184.x. PubMed
20. Cancino RS, Culpepper L, Sadikova E, Martin J, Jack BW, Mitchell SE. Dose-response relationship between depressive symptoms and hospital readmission. J Hosp Med. 2014;9(6):358-364. doi:10.1002/jhm.2180. PubMed
21. Pederson JL, Warkentin LM, Majumdar SR, McAlister FA. Depressive symptoms are associated with higher rates of readmission or mortality after medical hospitalization: A systematic review and meta-analysis. J Hosp Med. 2016;11(5):373-380. doi:10.1002/jhm.2547. PubMed
22. Chwastiak LA, Davydow DS, McKibbin CL, et al. The Effect of Serious Mental Illness on the Risk of Rehospitalization Among Patients with Diabetes. Psychosomatics. 2014;55(2):134-143. PubMed
23. Daratha KB, Barbosa-Leiker C, H Burley M, et al. Co-occurring mood disorders among hospitalized patients and risk for subsequent medical hospitalization. Gen Hosp Psychiatry. 2012;34(5):500-505. doi:10.1016/j.genhosppsych.2012.05.001. PubMed
24. Kartha A, Anthony D, Manasseh CS, et al. Depression is a risk factor for rehospitalization in medical inpatients. Prim Care Companion J Clin Psychiatry. 2007;9(4):256-262. PubMed
25. Myrvik MP, Burks LM, Hoffman RG, Dasgupta M, Panepinto JA. Mental health disorders influence admission rates for pain in children with sickle cell disease. Pediatr Blood Cancer. 2013;60(7):1211-1214. doi:10.1002/pbc.24394. PubMed
26. Garrison MM, Katon WJ, Richardson LP. The impact of psychiatric comorbidities on readmissions for diabetes in youth. Diabetes Care. 2005;28(9):2150-2154. PubMed
27. Averill R, Goldfield N, Hughes JS, et al. All Patient Refined Diagnosis Related Groups (APR-DRGs) Version 20.0: Methodology Overview. https://www.hcup-us.ahrq.gov/db/nation/nis/APR-DRGsV20MethodologyOverviewandBibliography.pdf. Accessed on November 2, 2016.
28. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. PubMed
29. Zima BT, Rodean J, Hall M, Bardach NS, Coker TR, Berry JG. Psychiatric Disorders and Trends in Resource Use in Pediatric Hospitals. Pediatrics. 2016;138(5):e20160909-e20160909. doi:10.1542/peds.2016-0909. PubMed
30. Chronic Condition Indicator (CCI) for ICD-9-CM. Healthcare Cost and Utilization Project (HCUP) Tools & Software Page. http://www.hcup-us.ahrq.gov/toolssoftware/chronic/chronic.jsp. Accessed on October 30, 2015.
31. Feudtner C, Feinstein J, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14(1):199-205. PubMed
32. Doupnik S, Lawlor J, Zima BT, et al. Mental Health Conditions and Medical and Surgical Hospital Utilization. Pediatrics. 2016;138(6):e20162416. doi:10.1542/peds.2016-2416. PubMed
33. Brown NM, Green JC, Desai MM, Weitzman CC, Rosenthal MS. Need and Unmet Need for Care Coordination Among Children with Mental Health Conditions. Pediatrics. 2014;133(3):e530-e537. doi:10.1542/peds.2013-2590. PubMed
34. Sobolewski B, Richey L, Kowatch RA, Grupp-Phelan J. Mental health follow-up among adolescents with suicidal behaviors after emergency department discharge. Arch Suicide Res. 2013;17(4):323-334. doi:10.1080/13811118.2013.801807. PubMed
35. Hansen LO, Greenwald JL, Budnitz T, et al. Project BOOST: Effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8(8):421-427. doi:10.1002/jhm.2054. PubMed
36. Di Marco F, Verga M, Santus P, et al. Close correlation between anxiety, depression, and asthma control. Respir Med. 2010;104(1):22-28. doi:10.1016/j.rmed.2009.08.005. PubMed
37. Ghose SS, Williams LS, Swindle RW. Depression and other mental health diagnoses after stroke increase inpatient and outpatient medical utilization three years poststroke. Med Care. 2005;43(12):1259-1264. PubMed
38. Szigethy E, Bujoreanu SI, Youk AO, et al. Randomized efficacy trial of two psychotherapies for depression in youth with inflammatory bowel disease. J Am Acad Child Adolesc Psychiatry. 2014;53(7):726-735. PubMed
39. Swensen A, Birnbaum HG, Ben Hamadi R, Greenberg P, Cremieux PY, Secnik K. Incidence and costs of accidents among attention-deficit/hyperactivity disorder patients. J Adolesc Health. 2004;35(4):346.e1-346.e9. doi:10.1016/j.jadohealth.2003.12.003. PubMed
40. Chan E, Zhan C, Homer CJ. Health Care Use and Costs for Children with Attention-Deficit/Hyperactivity Disorder: National Estimates from the Medical Expenditure Panel Survey. Arch Pediatr Adolesc Med. 2002;156(5):504-511. doi:10.1001/archpedi.156.5.504. PubMed
41. Berry JG, Blaine K, Rogers J, et al. A Framework of Pediatric Hospital Discharge Care Informed by Legislation, Research, and Practice. JAMA Pediatr. 2014;168(10):955-962. doi:10.1001/jamapediatrics.2014.891. PubMed
Readmission prevention is a focus of national efforts to improve the quality of hospital care for children.1-5 Several factors contribute to the risk of readmission for hospitalized children, including age, race or ethnicity, payer, and the type and number of comorbid health conditions.6-9 Mental health conditions (MHCs) are a prevalent comorbidity in children hospitalized for physical health reasons that could influence their postdischarge health and safety.
MHCs are increasingly common in children hospitalized for physical health indications; a comorbid MHC is currently present in 10% to 25% of hospitalized children ages 3 years and older.10,11 Hospital length of stay (LOS) and cost are higher in children with an MHC.12,13 Increased resource use may occur because MHCs can impede hospital treatment effectiveness and the child’s recovery from physical illness. MHCs are associated with a lower adherence with medications14-16 and a lower ability to cope with health events and problems.17-19 In adults, MHCs are a well-established risk factor for hospital readmission for a variety of physical health conditions.20-24 Although the influence of MHCs on readmissions in children has not been extensively investigated, higher readmission rates have been reported in adolescents hospitalized for diabetes with an MHC compared with those with no MHC.25,26
To our knowledge, no large studies have examined the relationship between the presence of a comorbid MHC and hospital readmissions in children or adolescents hospitalized for a broad array of medical or procedure conditions. Therefore, we conducted this study to (1) assess the likelihood of 30-day hospital readmission in children with versus without MHC who were hospitalized for one of 10 medical or 10 procedure conditions, and (2) to assess which MHCs are associated with the highest likelihood of hospital readmission.
METHODS
Study Design and Setting
We conducted a national, retrospective cohort study of index hospitalizations for children ages 3 to 21 years who were discharged from January 1, 2013, to November 30, 2013, in the Agency for Healthcare Research and Quality’s (AHRQ) Nationwide Readmissions Database (NRD). Admissions occurring in December 2013 were excluded because they did not have a 30-day timeframe available for readmission measurement. The 2013 NRD includes administrative data for a nationally representative sample of 14 million hospitalizations in 21 states, accounting for 49% of all US hospitalizations and weighted to represent 35.6 million hospitalizations. The database includes deidentified, verified patient linkage numbers so that patients can be tracked across multiple hospitalizations at the same institution or different institutions within a state. The NRD includes hospital information, patient demographic information, and the International Classification of Diseases, 9th Revision-Clinical Modification (ICD-9-CM) discharge diagnoses and procedures, with 1 primary diagnosis and up to 24 additional fields for comorbid diagnoses. This study was approved for exemption by the Children’s Hospital of Philadelphia Institutional Review Board.
Index Admissions
We used the methods described below to create a study cohort of the 10 medical and 10 procedure index admissions associated with the highest volume (ie, the greatest absolute number) of 30-day hospital readmissions. Conditions with a high volume of readmissions were chosen in an effort to identify conditions in which readmission-prevention interventions had the greatest potential to reduce the absolute number of readmissions. We first categorized index hospitalizations for medical and procedure conditions by using the All Patient Refined Diagnosis Related Groups (APR-DRGs; 3M Health Information Systems, Wallingford, CT).27 APR-DRGs use all diagnosis and/or procedure ICD-9-CM codes registered for a hospital discharge to assign 1 reason that best explains the need for hospitalization. We then excluded obstetric hospitalizations, psychiatric hospitalizations, and hospitalizations resulting in death or transfer from being considered as index admissions. Afterwards, we ranked each APR-DRG index hospitalization by the total number of 30-day hospital readmissions that occurred afterward and selected the 10 medical and 10 procedure index admissions with the highest number of readmissions. The APR-DRG index admissions are listed in Figures 1 and 2. For the APR-DRG “digestive system diagnoses,” the most common diagnosis was constipation, and we refer to that category as “constipation.” The most common diagnosis for the APR-DRG called “other operating room procedure for neoplasm” was tumor biopsy, and we refer to that category as “tumor biopsy.”
Main Outcome Measure
The primary study outcome was unplanned, all-cause readmission to any hospital within 30 days of index hospitalization. All-cause readmissions include any hospitalization for the same or different condition as the index admission, including conditions not eligible to be considered as index admissions (obstetric, psychiatric, and hospitalizations resulting in death or transfer). Planned readmissions, identified by using pediatric-specific measure specifications endorsed by AHRQ and the National Quality Forum,28 were excluded from measurement. For index admissions with multiple 30-day readmissions, only the first readmission was counted. Each readmission was treated as an index admission.
Main Independent Variable
The main independent variable was the presence of an MHC documented during the index hospitalization. MHCs were identified and classified into diagnosis categories derived from the AHRQ Chronic Condition Indicator system by using ICD-9-CM codes.29 MHC categories included anxiety disorders, attention-deficit/hyperactivity disorder (ADHD), autism, depression, and substance abuse. Less common MHCs included bipolar disorder, schizophrenia, disruptive behavior disorders, somatoform disorders, and eating disorders. These conditions are included in the group with any MHC, but we did not calculate the adjusted odds ratios (AORs) of readmission for these conditions. Children were identified as having multiple MHCs if they had more than 1 MHC.
Other Characteristics of Index Hospitalizations
A priori, we selected for analysis the known demographic, clinical, and hospital factors associated with the risk of readmission.20-24 The demographic characteristics included patient age, gender, payer category, urban or rural residence, and the median income quartile for a patient’s ZIP code. The hospital characteristics included location, ownership, and teaching hospital designation. The clinical characteristics included the number of chronic conditions30 and indicators for the presence of a complex chronic condition in each of 12 organ systems.31
Statistical Analysis
We calculated descriptive summary statistics for the characteristics of index hospitalizations. We compared characteristics in index admissions of children with versus without MHC by using Wilcoxon Rank-Sum tests for continuous variables and Wald χ2 tests for categorical variables. In the multivariable analysis, we derived logistic regression models to assess the relationship of 30-day hospital readmission with each type of MHC, adjusting for index admission demographic, hospital, and clinical characteristics. MHCs were modeled as binary indicator variables with the presence of any MHC, more than 1 MHC, or each of 5 MHC categories (anxiety disorders, ADHD, autism, depression, substance abuse) compared with no MHC. Four types of logistic regression models were derived (1) for the combined sample of all 10 index medical admissions with each MHC category versus no MHC as a primary predictor, (2) for each medical index admission with any MHC versus no MHC as the primary predictor, (3) for the combined sample of all 10 index procedure admissions with each MHC category versus no MHC as a primary predictor, and (4) for each procedure index admission with any MHC versus no MHC as the primary predictor. All analyses were weighted to achieve national estimates and clustered by hospital by using AHRQ-recommended survey procedures. SAS version 9.4 (SAS Institute, Cary, NC) was used for all analyses. All tests were two-sided, and a P < .05 was
considered statistically significant.
RESULTS
Study Population
Across all index admissions, 16.3% were for children with an MHC. Overall, children with MHCs were older and more likely to have a chronic30 or complex chronic31 physical health condition than children with no MHCs (Table).
Index Medical Admissions, Mental Health Conditions, and Hospital Readmission
The 10 index medical hospitalizations with the most readmissions for children ages 3 to 20 years were asthma, chemotherapy, constipation, diabetes, gastroenteritis, inflammatory bowel disease, neutropenia, pneumonia, seizure, and sickle cell crisis. Across all index medical hospitalizations, 17.5% were for patients with an MHC (Figure 1). Of index medical admissions with any MHC, 26.3% had ADHD, 22.9% had an anxiety disorder, 14.9% had autism, 18.3% had depression, and 30.9% had substance abuse. Among all admissions with MHCs, 28.9% had 2 or more MHCs.
Index Medical Admissions Combined
For all index medical hospitalizations combined, 17.0% (n = 59,138) had an unplanned, 30-day hospital readmission. The rate of 30-day hospital readmissions was higher with versus without an MHC (17.5 vs 16.8%; P < .001). In a multivariable analysis, presence of an MHC was associated with a higher likelihood of hospital readmission following an index medical admission (AOR, 1.23; 95% confidence interval [CI], 1.19-1.26); Figure 1). All MHCs except autism and ADHD had a higher likelihood of readmission (Figure 3).
Specific Index Medical Admissions
Index Procedure Admissions, Mental Health Conditions, and Hospital Readmission
Index Procedure Admissions Combined
Specific Index Procedure Admissions
For specific index procedure admissions, the rate of 30-day hospital readmission ranged from 2.2% for knee procedures to 33.6% for tumor biopsy. For 3 (ie, urinary tract, ventricular shunt, and bowel procedures) of the 10 specific index procedure hospitalizations, having an MHC was associated with higher adjusted odds of 30-day readmission (AOR range, 1.38-2.27; Figure 2).
In total, adjusting for sociodemographic, clinical, and hospital characteristics, MHCs were associated with an additional 2501 medical readmissions and 217 procedure readmissions beyond what would have been expected if MHCs were not associated with readmissions.
DISCUSSION
MHCs are common among pediatric hospitalizations with the highest volume of readmissions; MHCs were present in approximately 1 in 5 medical and 1 in 7 procedure index hospitalizations. Across medical and procedure admissions, the adjusted likelihood of unplanned, all-cause 30-day readmission was 25% higher for children with versus without an MHC. The readmission likelihood varied by the type of medical or procedure admission and by the type of MHC. MHCs had the strongest associations with readmissions following hospitalization for diabetes and urinary tract procedures. The MHC categories associated with the highest readmission likelihood were depression, substance abuse, and multiple MHCs.
The current study complements existing literature by helping establish MHCs as a prevalent and important risk factor for hospital readmission in children. Estimates of the prevalence of MHCs in hospitalized children are between 10% and 25%,10,11,32 and prevalence has increased by as much as 160% over the last 10 years.29 Prior investigations have found that children with an MHC tend to stay longer in the hospital compared with children with no MHC.32 Results from the present study suggest that children with MHCs also experience more inpatient days because of rehospitalizations. Subsequent investigations should strive to understand the mechanisms in the hospital, community, and family environment that are responsible for the increased inpatient utilization in children with MHCs. Understanding how the receipt of mental health services before, during, and after hospitalization influences readmissions could help identify opportunities for practice improvement. Families report the need for better coordination of their child’s medical and mental health care,33 and opportunities exist to improve attendance at mental health visits after acute care encounters.34 Among adults, interventions that address posthospital access to mental healthcare have prevented readmissions.35
Depression was associated with an increased risk of readmission in medical and procedure hospitalizations. As a well-known risk factor for readmission in adult patients,21 depression can adversely affect and exacerbate the physical health recovery of patients experiencing acute and chronic illnesses.14,36,37 Depression is considered a modifiable contributor that, when controlled, may help lower readmission risk. Optimal adherence with behavior and medication treatment for depression is associated with a lower risk of unplanned 30-day readmissions.14-16,19 Emerging evidence demonstrates how multifaceted, psychosocial approaches can improve patients’ adherence with depression treatment plans.38 Increased attention to depression in hospitalized children may uncover new ways to manage symptoms as children transition from hospital to home.
Other MHCs were associated with a different risk of readmission among medical and procedure hospitalizations. For example, ADHD or autism documented during index hospitalization was associated with an increased risk of readmission following procedure hospitalizations and a decreased risk following medical hospitalizations. Perhaps children with ADHD or autism who exhibit hyperactive, impulsive, or repetitive behaviors39,40 are at risk for disrupting their postprocedure wound healing, nutrition recovery, or pain tolerance, which might contribute to increased readmission risk.
MHCs were associated with different readmission risks across specific types of medical or procedure hospitalizations. For example, among medical conditions, the association of readmissions with MHCs was highest for diabetes, which is consistent with prior research.26 Factors that might mediate this relationship include changes in diet and appetite, difficulty with diabetes care plan adherence, and intentional nonadherence as a form of self-harm. Similarly, a higher risk of readmission in chronic medical conditions like asthma, constipation, and sickle cell disease might be mediated by difficulty adhering to medical plans or managing exacerbations at home. In contrast, MHCs had no association with readmission following chemotherapy. In our clinical experience, readmissions following chemotherapy are driven by physiologic problems, such as thrombocytopenia, fever, and/or neutropenia. MHCs might have limited influence over those health issues. For procedure hospitalizations, MHCs had 1 of the strongest associations with ventricular shunt procedures. We hypothesize that MHCs might lead some children to experience general health symptoms that might be associated with shunt malfunction (eg, fatigue, headache, behavior change), which could lead to an increased risk of readmission to evaluate for shunt malfunction. Conversely, we found no relationship between MHCs and readmissions following appendectomy. For appendectomy, MHCs might have limited influence over the development of postsurgical complications (eg, wound infection or ileus). Future research to better elucidate mediators of increased risk of readmission associated with MHCs in certain medical and procedure conditions could help explain these relationships and identify possible future intervention targets to prevent readmissions.
This study has several limitations. The administrative data are not positioned to discover the mechanisms by which MHCs are associated with a higher likelihood of readmission. We used hospital ICD-9-CM codes to identify patients with MHCs. Other methods using more clinically rich data (eg, chart review, prescription medications, etc.) may be preferable to identify patients with MHCs. Although the use of ICD-9-CM codes may have sufficient specificity, some hospitalized children may have an MHC that is not coded. Patients identified by using diagnosis codes could represent patients with a higher severity of illness, patients using medications, or patients whose outpatient records are accessible to make the hospital team aware of the MHC. If documentation of MHCs during hospitalization represents a higher severity of illness, findings may not extrapolate to lower-severity MHCs. As hospitals transition from ICD-9 -CM to ICD-10 coding, and health systems develop more integrated inpatient and outpatient EHRs, diagnostic specificity may improve. We could not analyze the relationships with several potential confounders and explanatory variables that may be related both to the likelihood of having an MHC and the risk of readmission, including medication administration, psychiatric consultation, and parent mental health. Postdischarge health services, including access to a medical home or a usual source of mental healthcare and measures of medication adherence, were not available in the NRD.
Despite these limitations, the current study underscores the importance of MHCs in hospitalized children upon discharge. As subsequent investigations uncover the key drivers explaining the influence of MHCs on hospital readmission risk, hospitals and their local outpatient and community practices may find it useful to consider MHCs when (1) developing contingency plans and establishing follow-up care at discharge,41 (2) exploring opportunities of care integration between mental and physical health care professionals, and (3) devising strategies to reduce hospital readmissions among populations of children.
CONCLUSIONS
MHCs are prevalent in hospitalized children and are associated with an increased risk of 30-day, unplanned hospital readmission. Future readmission prevention efforts may uncover new ways to improve children’s transitions from hospital to home by investigating strategies to address their MHCs.
Acknowledgments
The authors thank Donjo Lau and Troy Richardson for their assistance with the analysis.
Disclosures
Dr. Doupnik was supported by a Ruth L. Kirschstein National Research Service Award institutional training grant (T32-HP010026), funded by the National Institutes of Health. Dr. Zima was supported by the Behavioral Health Centers of Excellence for California (SB852). Dr. Bardach was supported by the National Institute of Child Health and Human Development (K23-HD065836). Dr. Berry was supported by the Agency for Healthcare Research and Quality (R21 HS023092-01). The authors have no financial relationships relevant to this article to disclose. The authors have no potential conflicts of interest to disclose. Dr. Doupnik led the study design and analysis and drafted the initial manuscript. Mr. Lawlor performed the data analysis. Dr. Hall provided statistical consultation. All authors participated in the design of the study, interpretation of the data, revised the manuscript for key intellectual content, and all authors read and approved the final manuscript.
Readmission prevention is a focus of national efforts to improve the quality of hospital care for children.1-5 Several factors contribute to the risk of readmission for hospitalized children, including age, race or ethnicity, payer, and the type and number of comorbid health conditions.6-9 Mental health conditions (MHCs) are a prevalent comorbidity in children hospitalized for physical health reasons that could influence their postdischarge health and safety.
MHCs are increasingly common in children hospitalized for physical health indications; a comorbid MHC is currently present in 10% to 25% of hospitalized children ages 3 years and older.10,11 Hospital length of stay (LOS) and cost are higher in children with an MHC.12,13 Increased resource use may occur because MHCs can impede hospital treatment effectiveness and the child’s recovery from physical illness. MHCs are associated with a lower adherence with medications14-16 and a lower ability to cope with health events and problems.17-19 In adults, MHCs are a well-established risk factor for hospital readmission for a variety of physical health conditions.20-24 Although the influence of MHCs on readmissions in children has not been extensively investigated, higher readmission rates have been reported in adolescents hospitalized for diabetes with an MHC compared with those with no MHC.25,26
To our knowledge, no large studies have examined the relationship between the presence of a comorbid MHC and hospital readmissions in children or adolescents hospitalized for a broad array of medical or procedure conditions. Therefore, we conducted this study to (1) assess the likelihood of 30-day hospital readmission in children with versus without MHC who were hospitalized for one of 10 medical or 10 procedure conditions, and (2) to assess which MHCs are associated with the highest likelihood of hospital readmission.
METHODS
Study Design and Setting
We conducted a national, retrospective cohort study of index hospitalizations for children ages 3 to 21 years who were discharged from January 1, 2013, to November 30, 2013, in the Agency for Healthcare Research and Quality’s (AHRQ) Nationwide Readmissions Database (NRD). Admissions occurring in December 2013 were excluded because they did not have a 30-day timeframe available for readmission measurement. The 2013 NRD includes administrative data for a nationally representative sample of 14 million hospitalizations in 21 states, accounting for 49% of all US hospitalizations and weighted to represent 35.6 million hospitalizations. The database includes deidentified, verified patient linkage numbers so that patients can be tracked across multiple hospitalizations at the same institution or different institutions within a state. The NRD includes hospital information, patient demographic information, and the International Classification of Diseases, 9th Revision-Clinical Modification (ICD-9-CM) discharge diagnoses and procedures, with 1 primary diagnosis and up to 24 additional fields for comorbid diagnoses. This study was approved for exemption by the Children’s Hospital of Philadelphia Institutional Review Board.
Index Admissions
We used the methods described below to create a study cohort of the 10 medical and 10 procedure index admissions associated with the highest volume (ie, the greatest absolute number) of 30-day hospital readmissions. Conditions with a high volume of readmissions were chosen in an effort to identify conditions in which readmission-prevention interventions had the greatest potential to reduce the absolute number of readmissions. We first categorized index hospitalizations for medical and procedure conditions by using the All Patient Refined Diagnosis Related Groups (APR-DRGs; 3M Health Information Systems, Wallingford, CT).27 APR-DRGs use all diagnosis and/or procedure ICD-9-CM codes registered for a hospital discharge to assign 1 reason that best explains the need for hospitalization. We then excluded obstetric hospitalizations, psychiatric hospitalizations, and hospitalizations resulting in death or transfer from being considered as index admissions. Afterwards, we ranked each APR-DRG index hospitalization by the total number of 30-day hospital readmissions that occurred afterward and selected the 10 medical and 10 procedure index admissions with the highest number of readmissions. The APR-DRG index admissions are listed in Figures 1 and 2. For the APR-DRG “digestive system diagnoses,” the most common diagnosis was constipation, and we refer to that category as “constipation.” The most common diagnosis for the APR-DRG called “other operating room procedure for neoplasm” was tumor biopsy, and we refer to that category as “tumor biopsy.”
Main Outcome Measure
The primary study outcome was unplanned, all-cause readmission to any hospital within 30 days of index hospitalization. All-cause readmissions include any hospitalization for the same or different condition as the index admission, including conditions not eligible to be considered as index admissions (obstetric, psychiatric, and hospitalizations resulting in death or transfer). Planned readmissions, identified by using pediatric-specific measure specifications endorsed by AHRQ and the National Quality Forum,28 were excluded from measurement. For index admissions with multiple 30-day readmissions, only the first readmission was counted. Each readmission was treated as an index admission.
Main Independent Variable
The main independent variable was the presence of an MHC documented during the index hospitalization. MHCs were identified and classified into diagnosis categories derived from the AHRQ Chronic Condition Indicator system by using ICD-9-CM codes.29 MHC categories included anxiety disorders, attention-deficit/hyperactivity disorder (ADHD), autism, depression, and substance abuse. Less common MHCs included bipolar disorder, schizophrenia, disruptive behavior disorders, somatoform disorders, and eating disorders. These conditions are included in the group with any MHC, but we did not calculate the adjusted odds ratios (AORs) of readmission for these conditions. Children were identified as having multiple MHCs if they had more than 1 MHC.
Other Characteristics of Index Hospitalizations
A priori, we selected for analysis the known demographic, clinical, and hospital factors associated with the risk of readmission.20-24 The demographic characteristics included patient age, gender, payer category, urban or rural residence, and the median income quartile for a patient’s ZIP code. The hospital characteristics included location, ownership, and teaching hospital designation. The clinical characteristics included the number of chronic conditions30 and indicators for the presence of a complex chronic condition in each of 12 organ systems.31
Statistical Analysis
We calculated descriptive summary statistics for the characteristics of index hospitalizations. We compared characteristics in index admissions of children with versus without MHC by using Wilcoxon Rank-Sum tests for continuous variables and Wald χ2 tests for categorical variables. In the multivariable analysis, we derived logistic regression models to assess the relationship of 30-day hospital readmission with each type of MHC, adjusting for index admission demographic, hospital, and clinical characteristics. MHCs were modeled as binary indicator variables with the presence of any MHC, more than 1 MHC, or each of 5 MHC categories (anxiety disorders, ADHD, autism, depression, substance abuse) compared with no MHC. Four types of logistic regression models were derived (1) for the combined sample of all 10 index medical admissions with each MHC category versus no MHC as a primary predictor, (2) for each medical index admission with any MHC versus no MHC as the primary predictor, (3) for the combined sample of all 10 index procedure admissions with each MHC category versus no MHC as a primary predictor, and (4) for each procedure index admission with any MHC versus no MHC as the primary predictor. All analyses were weighted to achieve national estimates and clustered by hospital by using AHRQ-recommended survey procedures. SAS version 9.4 (SAS Institute, Cary, NC) was used for all analyses. All tests were two-sided, and a P < .05 was
considered statistically significant.
RESULTS
Study Population
Across all index admissions, 16.3% were for children with an MHC. Overall, children with MHCs were older and more likely to have a chronic30 or complex chronic31 physical health condition than children with no MHCs (Table).
Index Medical Admissions, Mental Health Conditions, and Hospital Readmission
The 10 index medical hospitalizations with the most readmissions for children ages 3 to 20 years were asthma, chemotherapy, constipation, diabetes, gastroenteritis, inflammatory bowel disease, neutropenia, pneumonia, seizure, and sickle cell crisis. Across all index medical hospitalizations, 17.5% were for patients with an MHC (Figure 1). Of index medical admissions with any MHC, 26.3% had ADHD, 22.9% had an anxiety disorder, 14.9% had autism, 18.3% had depression, and 30.9% had substance abuse. Among all admissions with MHCs, 28.9% had 2 or more MHCs.
Index Medical Admissions Combined
For all index medical hospitalizations combined, 17.0% (n = 59,138) had an unplanned, 30-day hospital readmission. The rate of 30-day hospital readmissions was higher with versus without an MHC (17.5 vs 16.8%; P < .001). In a multivariable analysis, presence of an MHC was associated with a higher likelihood of hospital readmission following an index medical admission (AOR, 1.23; 95% confidence interval [CI], 1.19-1.26); Figure 1). All MHCs except autism and ADHD had a higher likelihood of readmission (Figure 3).
Specific Index Medical Admissions
Index Procedure Admissions, Mental Health Conditions, and Hospital Readmission
Index Procedure Admissions Combined
Specific Index Procedure Admissions
For specific index procedure admissions, the rate of 30-day hospital readmission ranged from 2.2% for knee procedures to 33.6% for tumor biopsy. For 3 (ie, urinary tract, ventricular shunt, and bowel procedures) of the 10 specific index procedure hospitalizations, having an MHC was associated with higher adjusted odds of 30-day readmission (AOR range, 1.38-2.27; Figure 2).
In total, adjusting for sociodemographic, clinical, and hospital characteristics, MHCs were associated with an additional 2501 medical readmissions and 217 procedure readmissions beyond what would have been expected if MHCs were not associated with readmissions.
DISCUSSION
MHCs are common among pediatric hospitalizations with the highest volume of readmissions; MHCs were present in approximately 1 in 5 medical and 1 in 7 procedure index hospitalizations. Across medical and procedure admissions, the adjusted likelihood of unplanned, all-cause 30-day readmission was 25% higher for children with versus without an MHC. The readmission likelihood varied by the type of medical or procedure admission and by the type of MHC. MHCs had the strongest associations with readmissions following hospitalization for diabetes and urinary tract procedures. The MHC categories associated with the highest readmission likelihood were depression, substance abuse, and multiple MHCs.
The current study complements existing literature by helping establish MHCs as a prevalent and important risk factor for hospital readmission in children. Estimates of the prevalence of MHCs in hospitalized children are between 10% and 25%,10,11,32 and prevalence has increased by as much as 160% over the last 10 years.29 Prior investigations have found that children with an MHC tend to stay longer in the hospital compared with children with no MHC.32 Results from the present study suggest that children with MHCs also experience more inpatient days because of rehospitalizations. Subsequent investigations should strive to understand the mechanisms in the hospital, community, and family environment that are responsible for the increased inpatient utilization in children with MHCs. Understanding how the receipt of mental health services before, during, and after hospitalization influences readmissions could help identify opportunities for practice improvement. Families report the need for better coordination of their child’s medical and mental health care,33 and opportunities exist to improve attendance at mental health visits after acute care encounters.34 Among adults, interventions that address posthospital access to mental healthcare have prevented readmissions.35
Depression was associated with an increased risk of readmission in medical and procedure hospitalizations. As a well-known risk factor for readmission in adult patients,21 depression can adversely affect and exacerbate the physical health recovery of patients experiencing acute and chronic illnesses.14,36,37 Depression is considered a modifiable contributor that, when controlled, may help lower readmission risk. Optimal adherence with behavior and medication treatment for depression is associated with a lower risk of unplanned 30-day readmissions.14-16,19 Emerging evidence demonstrates how multifaceted, psychosocial approaches can improve patients’ adherence with depression treatment plans.38 Increased attention to depression in hospitalized children may uncover new ways to manage symptoms as children transition from hospital to home.
Other MHCs were associated with a different risk of readmission among medical and procedure hospitalizations. For example, ADHD or autism documented during index hospitalization was associated with an increased risk of readmission following procedure hospitalizations and a decreased risk following medical hospitalizations. Perhaps children with ADHD or autism who exhibit hyperactive, impulsive, or repetitive behaviors39,40 are at risk for disrupting their postprocedure wound healing, nutrition recovery, or pain tolerance, which might contribute to increased readmission risk.
MHCs were associated with different readmission risks across specific types of medical or procedure hospitalizations. For example, among medical conditions, the association of readmissions with MHCs was highest for diabetes, which is consistent with prior research.26 Factors that might mediate this relationship include changes in diet and appetite, difficulty with diabetes care plan adherence, and intentional nonadherence as a form of self-harm. Similarly, a higher risk of readmission in chronic medical conditions like asthma, constipation, and sickle cell disease might be mediated by difficulty adhering to medical plans or managing exacerbations at home. In contrast, MHCs had no association with readmission following chemotherapy. In our clinical experience, readmissions following chemotherapy are driven by physiologic problems, such as thrombocytopenia, fever, and/or neutropenia. MHCs might have limited influence over those health issues. For procedure hospitalizations, MHCs had 1 of the strongest associations with ventricular shunt procedures. We hypothesize that MHCs might lead some children to experience general health symptoms that might be associated with shunt malfunction (eg, fatigue, headache, behavior change), which could lead to an increased risk of readmission to evaluate for shunt malfunction. Conversely, we found no relationship between MHCs and readmissions following appendectomy. For appendectomy, MHCs might have limited influence over the development of postsurgical complications (eg, wound infection or ileus). Future research to better elucidate mediators of increased risk of readmission associated with MHCs in certain medical and procedure conditions could help explain these relationships and identify possible future intervention targets to prevent readmissions.
This study has several limitations. The administrative data are not positioned to discover the mechanisms by which MHCs are associated with a higher likelihood of readmission. We used hospital ICD-9-CM codes to identify patients with MHCs. Other methods using more clinically rich data (eg, chart review, prescription medications, etc.) may be preferable to identify patients with MHCs. Although the use of ICD-9-CM codes may have sufficient specificity, some hospitalized children may have an MHC that is not coded. Patients identified by using diagnosis codes could represent patients with a higher severity of illness, patients using medications, or patients whose outpatient records are accessible to make the hospital team aware of the MHC. If documentation of MHCs during hospitalization represents a higher severity of illness, findings may not extrapolate to lower-severity MHCs. As hospitals transition from ICD-9 -CM to ICD-10 coding, and health systems develop more integrated inpatient and outpatient EHRs, diagnostic specificity may improve. We could not analyze the relationships with several potential confounders and explanatory variables that may be related both to the likelihood of having an MHC and the risk of readmission, including medication administration, psychiatric consultation, and parent mental health. Postdischarge health services, including access to a medical home or a usual source of mental healthcare and measures of medication adherence, were not available in the NRD.
Despite these limitations, the current study underscores the importance of MHCs in hospitalized children upon discharge. As subsequent investigations uncover the key drivers explaining the influence of MHCs on hospital readmission risk, hospitals and their local outpatient and community practices may find it useful to consider MHCs when (1) developing contingency plans and establishing follow-up care at discharge,41 (2) exploring opportunities of care integration between mental and physical health care professionals, and (3) devising strategies to reduce hospital readmissions among populations of children.
CONCLUSIONS
MHCs are prevalent in hospitalized children and are associated with an increased risk of 30-day, unplanned hospital readmission. Future readmission prevention efforts may uncover new ways to improve children’s transitions from hospital to home by investigating strategies to address their MHCs.
Acknowledgments
The authors thank Donjo Lau and Troy Richardson for their assistance with the analysis.
Disclosures
Dr. Doupnik was supported by a Ruth L. Kirschstein National Research Service Award institutional training grant (T32-HP010026), funded by the National Institutes of Health. Dr. Zima was supported by the Behavioral Health Centers of Excellence for California (SB852). Dr. Bardach was supported by the National Institute of Child Health and Human Development (K23-HD065836). Dr. Berry was supported by the Agency for Healthcare Research and Quality (R21 HS023092-01). The authors have no financial relationships relevant to this article to disclose. The authors have no potential conflicts of interest to disclose. Dr. Doupnik led the study design and analysis and drafted the initial manuscript. Mr. Lawlor performed the data analysis. Dr. Hall provided statistical consultation. All authors participated in the design of the study, interpretation of the data, revised the manuscript for key intellectual content, and all authors read and approved the final manuscript.
1. Dougherty D, Schiff J, Mangione-Smith R. The Children’s Health Insurance Program Reauthorization Act quality measures initiatives: moving forward to improve measurement, care, and child and adolescent outcomes. Acad Pediatr. 2011;11(3):S1-S10. PubMed
2. Bardach NS, Vittinghoff E, Asteria-Penaloza R, et al. Measuring Hospital Quality Using Pediatric Readmission and Revisit Rates. Pediatrics. 2013;132(3):429-436. doi:10.1542/peds.2012-3527. PubMed
3. Khan A, Nakamura MM, Zaslavsky AM, et al. Same-Hospital Readmission Rates as a Measure of Pediatric Quality of Care. JAMA Pediatr. 2015;169(10):905-912. doi:10.1001/jamapediatrics.2015.1129. PubMed
4. Fassl BA, Nkoy FL, Stone BL, et al. The Joint Commission Children’s Asthma Care quality measures and asthma readmissions. Pediatrics. 2012;130(3):482-491. doi:10.1542/peds.2011-3318. PubMed
5. Hain PD, Gay JC, Berutti TW, Whitney GM, Wang W, Saville BR. Preventability of Early Readmissions at a Children’s Hospital. Pediatrics. 2013;131(1):e171-e181. doi:10.1542/peds.2012-0820. PubMed
6. Nagasako E, Reidhead B, Waterman B, et al. Adding Socioeconomic Data to Hospital Readmissions Calculations May Produce More Useful Results. Health Aff. 2014;33(5):786-791. PubMed
7. Hu J, Gonsahn MD, Nerenz DR. Socioeconomic Status and Readmissions: Evidence from an Urban Teaching Hospital. Health Aff. 2014;33(5):778-785. doi:10.1377/hlthaff.2013.0816. PubMed
8. Sills MR, Hall M, Colvin JD, et al. Association of Social Determinants with Children’s Hospitals’ Preventable Readmissions Performance. JAMA Pediatr. 2016;170(4):350-358. doi:10.1001/jamapediatrics.2015.4440. PubMed
9. Eselius LL, Cleary PD, Zaslavsky AM, Huskamp HA, Busch SH. Case-Mix Adjustment of Consumer Reports about Managed Behavioral Health Care and Health Plans. Health Serv Res. 2008;43(6):2014-2032. doi:10.1111/j.1475-6773.2008.00894.x. PubMed
10. Doupnik SK, Henry MK, Bae H, et al. Mental Health Conditions and Symptoms in Pediatric Hospitalizations: A Single-Center Point Prevalence Study. Acad Pediatr. 2017;17(2):184-190. PubMed
11. Bardach NS, Coker TR, Zima BT, et al. Common and Costly Hospitalizations for Pediatric Mental Health Disorders. Pediatrics. 2014;133(4):602-609. doi:10.1542/peds.2013-3165. PubMed
12. Doupnik SK, Mitra N, Feudtner C, Marcus SC. The Influence of Comorbid Mood and Anxiety Disorders on Outcomes of Pediatric Patients Hospitalized for Pneumonia. Hosp Pediatr. 2016;6(3):135-142. doi:10.1542/hpeds.2015-0177. PubMed
13. Snell C, Fernandes S, Bujoreanu IS, Garcia G. Depression, illness severity, and healthcare utilization in cystic fibrosis. Pediatr Pulmonol. 2014;49(12):1177-1181. doi:10.1002/ppul.22990. PubMed
14. DiMatteo MR, Lepper HS, Croghan TW. Depression Is a Risk Factor for Noncompliance with Medical Treatment: Meta-analysis of the Effects of Anxiety and Depression on Patient Adherence. Arch Intern Med . 2000;160(14):2101-2107. doi:10.1001/archinte.160.14.2101. PubMed
15. Gray WN, Denson LA, Baldassano RN, Hommel KA. Treatment Adherence in Adolescents with Inflammatory Bowel Disease: The Collective Impact of Barriers to Adherence and Anxiety/Depressive Symptoms. J Pediatr Psychol. 2012;37(3):282-291. doi:10.1093/jpepsy/jsr092. PubMed
16. Mosnaim G, Li H, Martin M, et al. Factors associated with levels of adherence to inhaled corticosteroids in minority adolescents with asthma. Ann Allergy Asthma Immunol. 2014;112(2):116-120. doi:10.1016/j.anai.2013.11.021. PubMed
17. Compas BE, Jaser SS, Dunn MJ, Rodriguez EM. Coping with Chronic Illness in Childhood and Adolescence. Ann Rev Clin Psychol. 2012;8(1):455-480. doi:10.1146/annurev-clinpsy-032511-143108. PubMed
18. Graue M, Wentzel-Larsen T, Bru E, Hanestad BR, Søvik O. The coping styles of adolescents with type 1 diabetes are associated with degree of metabolic control. Diabetes Care. 2004;27(6):1313-1317. PubMed
19. Jaser SS, White LE. Coping and resilience in adolescents with type 1 diabetes. Child Care Health Dev. 2011;37(3):335-342. doi:10.1111/j.1365-2214.2010.01184.x. PubMed
20. Cancino RS, Culpepper L, Sadikova E, Martin J, Jack BW, Mitchell SE. Dose-response relationship between depressive symptoms and hospital readmission. J Hosp Med. 2014;9(6):358-364. doi:10.1002/jhm.2180. PubMed
21. Pederson JL, Warkentin LM, Majumdar SR, McAlister FA. Depressive symptoms are associated with higher rates of readmission or mortality after medical hospitalization: A systematic review and meta-analysis. J Hosp Med. 2016;11(5):373-380. doi:10.1002/jhm.2547. PubMed
22. Chwastiak LA, Davydow DS, McKibbin CL, et al. The Effect of Serious Mental Illness on the Risk of Rehospitalization Among Patients with Diabetes. Psychosomatics. 2014;55(2):134-143. PubMed
23. Daratha KB, Barbosa-Leiker C, H Burley M, et al. Co-occurring mood disorders among hospitalized patients and risk for subsequent medical hospitalization. Gen Hosp Psychiatry. 2012;34(5):500-505. doi:10.1016/j.genhosppsych.2012.05.001. PubMed
24. Kartha A, Anthony D, Manasseh CS, et al. Depression is a risk factor for rehospitalization in medical inpatients. Prim Care Companion J Clin Psychiatry. 2007;9(4):256-262. PubMed
25. Myrvik MP, Burks LM, Hoffman RG, Dasgupta M, Panepinto JA. Mental health disorders influence admission rates for pain in children with sickle cell disease. Pediatr Blood Cancer. 2013;60(7):1211-1214. doi:10.1002/pbc.24394. PubMed
26. Garrison MM, Katon WJ, Richardson LP. The impact of psychiatric comorbidities on readmissions for diabetes in youth. Diabetes Care. 2005;28(9):2150-2154. PubMed
27. Averill R, Goldfield N, Hughes JS, et al. All Patient Refined Diagnosis Related Groups (APR-DRGs) Version 20.0: Methodology Overview. https://www.hcup-us.ahrq.gov/db/nation/nis/APR-DRGsV20MethodologyOverviewandBibliography.pdf. Accessed on November 2, 2016.
28. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. PubMed
29. Zima BT, Rodean J, Hall M, Bardach NS, Coker TR, Berry JG. Psychiatric Disorders and Trends in Resource Use in Pediatric Hospitals. Pediatrics. 2016;138(5):e20160909-e20160909. doi:10.1542/peds.2016-0909. PubMed
30. Chronic Condition Indicator (CCI) for ICD-9-CM. Healthcare Cost and Utilization Project (HCUP) Tools & Software Page. http://www.hcup-us.ahrq.gov/toolssoftware/chronic/chronic.jsp. Accessed on October 30, 2015.
31. Feudtner C, Feinstein J, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14(1):199-205. PubMed
32. Doupnik S, Lawlor J, Zima BT, et al. Mental Health Conditions and Medical and Surgical Hospital Utilization. Pediatrics. 2016;138(6):e20162416. doi:10.1542/peds.2016-2416. PubMed
33. Brown NM, Green JC, Desai MM, Weitzman CC, Rosenthal MS. Need and Unmet Need for Care Coordination Among Children with Mental Health Conditions. Pediatrics. 2014;133(3):e530-e537. doi:10.1542/peds.2013-2590. PubMed
34. Sobolewski B, Richey L, Kowatch RA, Grupp-Phelan J. Mental health follow-up among adolescents with suicidal behaviors after emergency department discharge. Arch Suicide Res. 2013;17(4):323-334. doi:10.1080/13811118.2013.801807. PubMed
35. Hansen LO, Greenwald JL, Budnitz T, et al. Project BOOST: Effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8(8):421-427. doi:10.1002/jhm.2054. PubMed
36. Di Marco F, Verga M, Santus P, et al. Close correlation between anxiety, depression, and asthma control. Respir Med. 2010;104(1):22-28. doi:10.1016/j.rmed.2009.08.005. PubMed
37. Ghose SS, Williams LS, Swindle RW. Depression and other mental health diagnoses after stroke increase inpatient and outpatient medical utilization three years poststroke. Med Care. 2005;43(12):1259-1264. PubMed
38. Szigethy E, Bujoreanu SI, Youk AO, et al. Randomized efficacy trial of two psychotherapies for depression in youth with inflammatory bowel disease. J Am Acad Child Adolesc Psychiatry. 2014;53(7):726-735. PubMed
39. Swensen A, Birnbaum HG, Ben Hamadi R, Greenberg P, Cremieux PY, Secnik K. Incidence and costs of accidents among attention-deficit/hyperactivity disorder patients. J Adolesc Health. 2004;35(4):346.e1-346.e9. doi:10.1016/j.jadohealth.2003.12.003. PubMed
40. Chan E, Zhan C, Homer CJ. Health Care Use and Costs for Children with Attention-Deficit/Hyperactivity Disorder: National Estimates from the Medical Expenditure Panel Survey. Arch Pediatr Adolesc Med. 2002;156(5):504-511. doi:10.1001/archpedi.156.5.504. PubMed
41. Berry JG, Blaine K, Rogers J, et al. A Framework of Pediatric Hospital Discharge Care Informed by Legislation, Research, and Practice. JAMA Pediatr. 2014;168(10):955-962. doi:10.1001/jamapediatrics.2014.891. PubMed
1. Dougherty D, Schiff J, Mangione-Smith R. The Children’s Health Insurance Program Reauthorization Act quality measures initiatives: moving forward to improve measurement, care, and child and adolescent outcomes. Acad Pediatr. 2011;11(3):S1-S10. PubMed
2. Bardach NS, Vittinghoff E, Asteria-Penaloza R, et al. Measuring Hospital Quality Using Pediatric Readmission and Revisit Rates. Pediatrics. 2013;132(3):429-436. doi:10.1542/peds.2012-3527. PubMed
3. Khan A, Nakamura MM, Zaslavsky AM, et al. Same-Hospital Readmission Rates as a Measure of Pediatric Quality of Care. JAMA Pediatr. 2015;169(10):905-912. doi:10.1001/jamapediatrics.2015.1129. PubMed
4. Fassl BA, Nkoy FL, Stone BL, et al. The Joint Commission Children’s Asthma Care quality measures and asthma readmissions. Pediatrics. 2012;130(3):482-491. doi:10.1542/peds.2011-3318. PubMed
5. Hain PD, Gay JC, Berutti TW, Whitney GM, Wang W, Saville BR. Preventability of Early Readmissions at a Children’s Hospital. Pediatrics. 2013;131(1):e171-e181. doi:10.1542/peds.2012-0820. PubMed
6. Nagasako E, Reidhead B, Waterman B, et al. Adding Socioeconomic Data to Hospital Readmissions Calculations May Produce More Useful Results. Health Aff. 2014;33(5):786-791. PubMed
7. Hu J, Gonsahn MD, Nerenz DR. Socioeconomic Status and Readmissions: Evidence from an Urban Teaching Hospital. Health Aff. 2014;33(5):778-785. doi:10.1377/hlthaff.2013.0816. PubMed
8. Sills MR, Hall M, Colvin JD, et al. Association of Social Determinants with Children’s Hospitals’ Preventable Readmissions Performance. JAMA Pediatr. 2016;170(4):350-358. doi:10.1001/jamapediatrics.2015.4440. PubMed
9. Eselius LL, Cleary PD, Zaslavsky AM, Huskamp HA, Busch SH. Case-Mix Adjustment of Consumer Reports about Managed Behavioral Health Care and Health Plans. Health Serv Res. 2008;43(6):2014-2032. doi:10.1111/j.1475-6773.2008.00894.x. PubMed
10. Doupnik SK, Henry MK, Bae H, et al. Mental Health Conditions and Symptoms in Pediatric Hospitalizations: A Single-Center Point Prevalence Study. Acad Pediatr. 2017;17(2):184-190. PubMed
11. Bardach NS, Coker TR, Zima BT, et al. Common and Costly Hospitalizations for Pediatric Mental Health Disorders. Pediatrics. 2014;133(4):602-609. doi:10.1542/peds.2013-3165. PubMed
12. Doupnik SK, Mitra N, Feudtner C, Marcus SC. The Influence of Comorbid Mood and Anxiety Disorders on Outcomes of Pediatric Patients Hospitalized for Pneumonia. Hosp Pediatr. 2016;6(3):135-142. doi:10.1542/hpeds.2015-0177. PubMed
13. Snell C, Fernandes S, Bujoreanu IS, Garcia G. Depression, illness severity, and healthcare utilization in cystic fibrosis. Pediatr Pulmonol. 2014;49(12):1177-1181. doi:10.1002/ppul.22990. PubMed
14. DiMatteo MR, Lepper HS, Croghan TW. Depression Is a Risk Factor for Noncompliance with Medical Treatment: Meta-analysis of the Effects of Anxiety and Depression on Patient Adherence. Arch Intern Med . 2000;160(14):2101-2107. doi:10.1001/archinte.160.14.2101. PubMed
15. Gray WN, Denson LA, Baldassano RN, Hommel KA. Treatment Adherence in Adolescents with Inflammatory Bowel Disease: The Collective Impact of Barriers to Adherence and Anxiety/Depressive Symptoms. J Pediatr Psychol. 2012;37(3):282-291. doi:10.1093/jpepsy/jsr092. PubMed
16. Mosnaim G, Li H, Martin M, et al. Factors associated with levels of adherence to inhaled corticosteroids in minority adolescents with asthma. Ann Allergy Asthma Immunol. 2014;112(2):116-120. doi:10.1016/j.anai.2013.11.021. PubMed
17. Compas BE, Jaser SS, Dunn MJ, Rodriguez EM. Coping with Chronic Illness in Childhood and Adolescence. Ann Rev Clin Psychol. 2012;8(1):455-480. doi:10.1146/annurev-clinpsy-032511-143108. PubMed
18. Graue M, Wentzel-Larsen T, Bru E, Hanestad BR, Søvik O. The coping styles of adolescents with type 1 diabetes are associated with degree of metabolic control. Diabetes Care. 2004;27(6):1313-1317. PubMed
19. Jaser SS, White LE. Coping and resilience in adolescents with type 1 diabetes. Child Care Health Dev. 2011;37(3):335-342. doi:10.1111/j.1365-2214.2010.01184.x. PubMed
20. Cancino RS, Culpepper L, Sadikova E, Martin J, Jack BW, Mitchell SE. Dose-response relationship between depressive symptoms and hospital readmission. J Hosp Med. 2014;9(6):358-364. doi:10.1002/jhm.2180. PubMed
21. Pederson JL, Warkentin LM, Majumdar SR, McAlister FA. Depressive symptoms are associated with higher rates of readmission or mortality after medical hospitalization: A systematic review and meta-analysis. J Hosp Med. 2016;11(5):373-380. doi:10.1002/jhm.2547. PubMed
22. Chwastiak LA, Davydow DS, McKibbin CL, et al. The Effect of Serious Mental Illness on the Risk of Rehospitalization Among Patients with Diabetes. Psychosomatics. 2014;55(2):134-143. PubMed
23. Daratha KB, Barbosa-Leiker C, H Burley M, et al. Co-occurring mood disorders among hospitalized patients and risk for subsequent medical hospitalization. Gen Hosp Psychiatry. 2012;34(5):500-505. doi:10.1016/j.genhosppsych.2012.05.001. PubMed
24. Kartha A, Anthony D, Manasseh CS, et al. Depression is a risk factor for rehospitalization in medical inpatients. Prim Care Companion J Clin Psychiatry. 2007;9(4):256-262. PubMed
25. Myrvik MP, Burks LM, Hoffman RG, Dasgupta M, Panepinto JA. Mental health disorders influence admission rates for pain in children with sickle cell disease. Pediatr Blood Cancer. 2013;60(7):1211-1214. doi:10.1002/pbc.24394. PubMed
26. Garrison MM, Katon WJ, Richardson LP. The impact of psychiatric comorbidities on readmissions for diabetes in youth. Diabetes Care. 2005;28(9):2150-2154. PubMed
27. Averill R, Goldfield N, Hughes JS, et al. All Patient Refined Diagnosis Related Groups (APR-DRGs) Version 20.0: Methodology Overview. https://www.hcup-us.ahrq.gov/db/nation/nis/APR-DRGsV20MethodologyOverviewandBibliography.pdf. Accessed on November 2, 2016.
28. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. PubMed
29. Zima BT, Rodean J, Hall M, Bardach NS, Coker TR, Berry JG. Psychiatric Disorders and Trends in Resource Use in Pediatric Hospitals. Pediatrics. 2016;138(5):e20160909-e20160909. doi:10.1542/peds.2016-0909. PubMed
30. Chronic Condition Indicator (CCI) for ICD-9-CM. Healthcare Cost and Utilization Project (HCUP) Tools & Software Page. http://www.hcup-us.ahrq.gov/toolssoftware/chronic/chronic.jsp. Accessed on October 30, 2015.
31. Feudtner C, Feinstein J, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14(1):199-205. PubMed
32. Doupnik S, Lawlor J, Zima BT, et al. Mental Health Conditions and Medical and Surgical Hospital Utilization. Pediatrics. 2016;138(6):e20162416. doi:10.1542/peds.2016-2416. PubMed
33. Brown NM, Green JC, Desai MM, Weitzman CC, Rosenthal MS. Need and Unmet Need for Care Coordination Among Children with Mental Health Conditions. Pediatrics. 2014;133(3):e530-e537. doi:10.1542/peds.2013-2590. PubMed
34. Sobolewski B, Richey L, Kowatch RA, Grupp-Phelan J. Mental health follow-up among adolescents with suicidal behaviors after emergency department discharge. Arch Suicide Res. 2013;17(4):323-334. doi:10.1080/13811118.2013.801807. PubMed
35. Hansen LO, Greenwald JL, Budnitz T, et al. Project BOOST: Effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8(8):421-427. doi:10.1002/jhm.2054. PubMed
36. Di Marco F, Verga M, Santus P, et al. Close correlation between anxiety, depression, and asthma control. Respir Med. 2010;104(1):22-28. doi:10.1016/j.rmed.2009.08.005. PubMed
37. Ghose SS, Williams LS, Swindle RW. Depression and other mental health diagnoses after stroke increase inpatient and outpatient medical utilization three years poststroke. Med Care. 2005;43(12):1259-1264. PubMed
38. Szigethy E, Bujoreanu SI, Youk AO, et al. Randomized efficacy trial of two psychotherapies for depression in youth with inflammatory bowel disease. J Am Acad Child Adolesc Psychiatry. 2014;53(7):726-735. PubMed
39. Swensen A, Birnbaum HG, Ben Hamadi R, Greenberg P, Cremieux PY, Secnik K. Incidence and costs of accidents among attention-deficit/hyperactivity disorder patients. J Adolesc Health. 2004;35(4):346.e1-346.e9. doi:10.1016/j.jadohealth.2003.12.003. PubMed
40. Chan E, Zhan C, Homer CJ. Health Care Use and Costs for Children with Attention-Deficit/Hyperactivity Disorder: National Estimates from the Medical Expenditure Panel Survey. Arch Pediatr Adolesc Med. 2002;156(5):504-511. doi:10.1001/archpedi.156.5.504. PubMed
41. Berry JG, Blaine K, Rogers J, et al. A Framework of Pediatric Hospital Discharge Care Informed by Legislation, Research, and Practice. JAMA Pediatr. 2014;168(10):955-962. doi:10.1001/jamapediatrics.2014.891. PubMed
© 2018 Society of Hospital Medicine
Make adult immunization a profit center
NEW ORLEANS – It’s a widespread misconception among internists: Implementing an office-based adult immunization program is a potential financial sinkhole and just isn’t worth the hassle.
That’s utterly wrong, Jason M. Goldman, MD, declared at the annual meeting of the American College of Physicians.
“But it is virtually impossible to lose money giving vaccines,” he countered. “You may not be able to retire on it, but you’re certainly not going to break the bank – and you’re not going to lose money. And more importantly, you’re doing what’s best for the patient. This is one of the few times where the payers and the government recognize that doing what’s best for the patient can actually be profitable in running a practice.”
At the annual meeting of the American College of Physicians, he detailed how to create a successful immunization program, offering money-saving tips on vaccine purchasing and proper storage, as well as wading into the complexities of coding and billing – which, by the way, he insisted actually is not daunting.
“The vaccine schedule is not nearly as complicated as it appears,” according to Dr. Goldman. “Read through it. Look at it. As automatically as you say, ‘You’re over 50, get a colonoscopy,’ you can very quickly learn to look at a patient and say, ‘These are your diseases, this is your age, these are the vaccines you need.’
“This is not difficult. If I can do it, anyone can do it,” Dr. Goldman noted. “Start simple with one or two vaccines until you hit your comfort level; then you can get more advanced. I do the travel vaccines – yellow fever, typhus, the whole gamut. And it’s just as easy vaccinating for that as for any of the others.”
Why implement adult immunization?
Many internists send patients off to a pharmacy for their vaccinations. That’s simply not good medical care, Dr. Goldman said.
“We are the primary care doctors,” he said. “We are the ones who should be vaccinating our patients, for several reasons: It’s the standard of care. It’s good medical practice.”
And Dr. Goldman frequently doesn’t receive any reports from the pharmacies. That means patients come to his office and have no idea what vaccines they received.
“That’s not good documentation,” he cautioned. “And when patients go into the hospital, they all get Pneumovax every single week because the hospital isn’t keeping documentation.”
The bottom line with vaccinating: “Whether you’re in a small group, a solo practitioner, or in a large health system, the vaccine programs work. They prevent disease and save lives. It’s easy to incorporate into your practice. And it is profitable.”
How profitable?
Dr. Goldman has the answer. For a great many different vaccines, he has calculated his average cost for the needle, syringe, medical assistant, time in the room, and other factors involved in running his practice. He also knows from experience the average purchase price paid for a given vaccine, the typical reimbursement for that vaccine, plus the reimbursement for its administration, which is a separate yet necessary coding/billing item.
The typical net profit ranges from $21.50 for high-dose influenza vaccine to, at the top end, $47.41 for meningococcal group B vaccine (Bexsero) and $49.58 for recombinant human papillomavirus 9-valent vaccine (Gardasil-9).
Purchasing and storage considerations
Always buy vaccines directly from the manufacturer; it’s a better deal than going through a middleman, who’ll invariably take a cut out of what should be the physician’s profit.
Each of the major vaccine makers has a dedicated vaccine purchase website where a physician can sign up for an account and order the company’s vaccines. These include Merck (www.merckvaccines.com), Aventis (www.vaccineshoppe.com), Pfizer (www.pfizerprime.com), and GlaxoSmithKline (www.gskdirect.com).
You’ll get a discount by buying multiple different vaccines on the same order.
“You can defer payment of your invoice for several months,” Dr. Goldman explained. “You purchase the vaccines now, but you don’t have to pay for them until 3-4 months later. By then, hopefully, you’ll have received reimbursement. So, your cost is covered, and you have profit on the side.”
For paying promptly on the due date, the manufacturer will provide an additional discount. The easiest way to do that is to have the money automatically charged to a credit card on that date.
Also, the vaccine manufacturers’ staff are happy to provide reliably expert reimbursement guidance.
With a little experience, it’s easy to predict how many vaccines will be used per month, Dr. Goldman said. Order what’s needed, so there aren’t a bunch of vaccines expiring in the office.
“However, even if that does happen, all is not lost,” he noted. “You can call up the manufacturer, and many of them will take back unused or even expired vaccines for full credit to the account. So, again, you really can’t lose money.”
With regard to vaccine storage, don’t skimp on the refrigerator and/or freezer. Get a professional model. And follow the best practices as described in the Centers for Disease Control and Prevention toolkit.
“It’s really common sense: Don’t use a dorm-type refrigerator; don’t put food or beverages in there; make sure the vaccines are appropriately stored; check the temperature every day; make sure if you lose power, your building has a backup generator,” he explained. “If you train your staff the right way, they’ll be able to handle it so you don’t have to worry about it. You just have to look at the logs and make sure they’re doing it.”
Use standing orders
Studies show that standing orders result in higher vaccination rates.
“You’re empowering the nurses or other staff members to act within the full extent of their license,” Dr. Goldman said. “It takes the burden off the physician to have to do anything that can be delegated to other individuals to make sure patients get vaccinated.”
Coding and billing for commercially insured patients
All vaccines have the same ICD-10 diagnostic code: Z23. And each vaccine has its own CPT code. For example, 90750 for Shingrix, the new herpes zoster vaccine; 90715 for Tdap; and 90686 for quadrivalent influenza.
But there are two components to the CPT code for a vaccination: the individual vaccine code and the administration code.
If you give one vaccination to a non-Medicare patient, the administration code is 90471. If you give a second vaccination during the same visit, its administration code is 90472. If you give a patient, say, four vaccines during one visit, you would bill the first using the administration code 90471, and the others as 90472 times three units.
If the vaccines are being given during a legitimate office visit, the physician can bill for both by employing modifiers 25 and 59. Modifier 25 goes with the appropriate E/M code for the office visit; it serves to tell the coding system that other things are going on in addition to the billable office visit. Modifier 59 needs to be attached to both the specific vaccine code and the vaccine administration code for reimbursement to occur.
Billing for vaccines for all commercially insured patients go through the office’s normal claims process.
Immunizing Medicare patients
For patients under Medicare Part B, vaccines for influenza, pneumonia, and hepatitis B have their own individual G codes: G0008 for influenza, G0009 for a pneumonia vaccine, and G0010 for hepatitis B. If a Medicare patient also gets an additional vaccine other than one of those three during the visit, administration code 90472 is applied to it. Those G-code bills are also submitted through the office’s normal claims process.
Under Medicare, vaccines for herpes zoster, hepatitis A, and Tdap are a special case. They are considered drugs and are covered under Medicare Part D.
“To bill that, you have to tell Medicare that you’re acting as a pharmacy,” Dr. Goldman explained. “You go to www.mytransactRX.com. You request there to be seen as a pharmacy billing for a drug. You will then be able to receive direct payment into your bank account from your Medicare payer. It will also allow you to check out patient coverage, print out proof of coverage, and submit the claim through the portal.”
If the Medicare patient doesn’t have a drug plan for those vaccines, or if the information in the system isn’t up to date, it’s a good idea to download the Advanced Beneficiary Notice of Noncoverage from the Medicare website and have the patient sign it. It spells out what the patient’s financial responsibility could be.
“The ABN also protects you as a provider, because it shows you’re not trying to balance-bill the patient,” he noted.
Dr. Goldman implored his internist colleagues to stand up and become the stewards of adult immunization.
“Remember: Keep calm and vaccinate,” he urged.
He reported having no relevant financial conflicts.
NEW ORLEANS – It’s a widespread misconception among internists: Implementing an office-based adult immunization program is a potential financial sinkhole and just isn’t worth the hassle.
That’s utterly wrong, Jason M. Goldman, MD, declared at the annual meeting of the American College of Physicians.
“But it is virtually impossible to lose money giving vaccines,” he countered. “You may not be able to retire on it, but you’re certainly not going to break the bank – and you’re not going to lose money. And more importantly, you’re doing what’s best for the patient. This is one of the few times where the payers and the government recognize that doing what’s best for the patient can actually be profitable in running a practice.”
At the annual meeting of the American College of Physicians, he detailed how to create a successful immunization program, offering money-saving tips on vaccine purchasing and proper storage, as well as wading into the complexities of coding and billing – which, by the way, he insisted actually is not daunting.
“The vaccine schedule is not nearly as complicated as it appears,” according to Dr. Goldman. “Read through it. Look at it. As automatically as you say, ‘You’re over 50, get a colonoscopy,’ you can very quickly learn to look at a patient and say, ‘These are your diseases, this is your age, these are the vaccines you need.’
“This is not difficult. If I can do it, anyone can do it,” Dr. Goldman noted. “Start simple with one or two vaccines until you hit your comfort level; then you can get more advanced. I do the travel vaccines – yellow fever, typhus, the whole gamut. And it’s just as easy vaccinating for that as for any of the others.”
Why implement adult immunization?
Many internists send patients off to a pharmacy for their vaccinations. That’s simply not good medical care, Dr. Goldman said.
“We are the primary care doctors,” he said. “We are the ones who should be vaccinating our patients, for several reasons: It’s the standard of care. It’s good medical practice.”
And Dr. Goldman frequently doesn’t receive any reports from the pharmacies. That means patients come to his office and have no idea what vaccines they received.
“That’s not good documentation,” he cautioned. “And when patients go into the hospital, they all get Pneumovax every single week because the hospital isn’t keeping documentation.”
The bottom line with vaccinating: “Whether you’re in a small group, a solo practitioner, or in a large health system, the vaccine programs work. They prevent disease and save lives. It’s easy to incorporate into your practice. And it is profitable.”
How profitable?
Dr. Goldman has the answer. For a great many different vaccines, he has calculated his average cost for the needle, syringe, medical assistant, time in the room, and other factors involved in running his practice. He also knows from experience the average purchase price paid for a given vaccine, the typical reimbursement for that vaccine, plus the reimbursement for its administration, which is a separate yet necessary coding/billing item.
The typical net profit ranges from $21.50 for high-dose influenza vaccine to, at the top end, $47.41 for meningococcal group B vaccine (Bexsero) and $49.58 for recombinant human papillomavirus 9-valent vaccine (Gardasil-9).
Purchasing and storage considerations
Always buy vaccines directly from the manufacturer; it’s a better deal than going through a middleman, who’ll invariably take a cut out of what should be the physician’s profit.
Each of the major vaccine makers has a dedicated vaccine purchase website where a physician can sign up for an account and order the company’s vaccines. These include Merck (www.merckvaccines.com), Aventis (www.vaccineshoppe.com), Pfizer (www.pfizerprime.com), and GlaxoSmithKline (www.gskdirect.com).
You’ll get a discount by buying multiple different vaccines on the same order.
“You can defer payment of your invoice for several months,” Dr. Goldman explained. “You purchase the vaccines now, but you don’t have to pay for them until 3-4 months later. By then, hopefully, you’ll have received reimbursement. So, your cost is covered, and you have profit on the side.”
For paying promptly on the due date, the manufacturer will provide an additional discount. The easiest way to do that is to have the money automatically charged to a credit card on that date.
Also, the vaccine manufacturers’ staff are happy to provide reliably expert reimbursement guidance.
With a little experience, it’s easy to predict how many vaccines will be used per month, Dr. Goldman said. Order what’s needed, so there aren’t a bunch of vaccines expiring in the office.
“However, even if that does happen, all is not lost,” he noted. “You can call up the manufacturer, and many of them will take back unused or even expired vaccines for full credit to the account. So, again, you really can’t lose money.”
With regard to vaccine storage, don’t skimp on the refrigerator and/or freezer. Get a professional model. And follow the best practices as described in the Centers for Disease Control and Prevention toolkit.
“It’s really common sense: Don’t use a dorm-type refrigerator; don’t put food or beverages in there; make sure the vaccines are appropriately stored; check the temperature every day; make sure if you lose power, your building has a backup generator,” he explained. “If you train your staff the right way, they’ll be able to handle it so you don’t have to worry about it. You just have to look at the logs and make sure they’re doing it.”
Use standing orders
Studies show that standing orders result in higher vaccination rates.
“You’re empowering the nurses or other staff members to act within the full extent of their license,” Dr. Goldman said. “It takes the burden off the physician to have to do anything that can be delegated to other individuals to make sure patients get vaccinated.”
Coding and billing for commercially insured patients
All vaccines have the same ICD-10 diagnostic code: Z23. And each vaccine has its own CPT code. For example, 90750 for Shingrix, the new herpes zoster vaccine; 90715 for Tdap; and 90686 for quadrivalent influenza.
But there are two components to the CPT code for a vaccination: the individual vaccine code and the administration code.
If you give one vaccination to a non-Medicare patient, the administration code is 90471. If you give a second vaccination during the same visit, its administration code is 90472. If you give a patient, say, four vaccines during one visit, you would bill the first using the administration code 90471, and the others as 90472 times three units.
If the vaccines are being given during a legitimate office visit, the physician can bill for both by employing modifiers 25 and 59. Modifier 25 goes with the appropriate E/M code for the office visit; it serves to tell the coding system that other things are going on in addition to the billable office visit. Modifier 59 needs to be attached to both the specific vaccine code and the vaccine administration code for reimbursement to occur.
Billing for vaccines for all commercially insured patients go through the office’s normal claims process.
Immunizing Medicare patients
For patients under Medicare Part B, vaccines for influenza, pneumonia, and hepatitis B have their own individual G codes: G0008 for influenza, G0009 for a pneumonia vaccine, and G0010 for hepatitis B. If a Medicare patient also gets an additional vaccine other than one of those three during the visit, administration code 90472 is applied to it. Those G-code bills are also submitted through the office’s normal claims process.
Under Medicare, vaccines for herpes zoster, hepatitis A, and Tdap are a special case. They are considered drugs and are covered under Medicare Part D.
“To bill that, you have to tell Medicare that you’re acting as a pharmacy,” Dr. Goldman explained. “You go to www.mytransactRX.com. You request there to be seen as a pharmacy billing for a drug. You will then be able to receive direct payment into your bank account from your Medicare payer. It will also allow you to check out patient coverage, print out proof of coverage, and submit the claim through the portal.”
If the Medicare patient doesn’t have a drug plan for those vaccines, or if the information in the system isn’t up to date, it’s a good idea to download the Advanced Beneficiary Notice of Noncoverage from the Medicare website and have the patient sign it. It spells out what the patient’s financial responsibility could be.
“The ABN also protects you as a provider, because it shows you’re not trying to balance-bill the patient,” he noted.
Dr. Goldman implored his internist colleagues to stand up and become the stewards of adult immunization.
“Remember: Keep calm and vaccinate,” he urged.
He reported having no relevant financial conflicts.
NEW ORLEANS – It’s a widespread misconception among internists: Implementing an office-based adult immunization program is a potential financial sinkhole and just isn’t worth the hassle.
That’s utterly wrong, Jason M. Goldman, MD, declared at the annual meeting of the American College of Physicians.
“But it is virtually impossible to lose money giving vaccines,” he countered. “You may not be able to retire on it, but you’re certainly not going to break the bank – and you’re not going to lose money. And more importantly, you’re doing what’s best for the patient. This is one of the few times where the payers and the government recognize that doing what’s best for the patient can actually be profitable in running a practice.”
At the annual meeting of the American College of Physicians, he detailed how to create a successful immunization program, offering money-saving tips on vaccine purchasing and proper storage, as well as wading into the complexities of coding and billing – which, by the way, he insisted actually is not daunting.
“The vaccine schedule is not nearly as complicated as it appears,” according to Dr. Goldman. “Read through it. Look at it. As automatically as you say, ‘You’re over 50, get a colonoscopy,’ you can very quickly learn to look at a patient and say, ‘These are your diseases, this is your age, these are the vaccines you need.’
“This is not difficult. If I can do it, anyone can do it,” Dr. Goldman noted. “Start simple with one or two vaccines until you hit your comfort level; then you can get more advanced. I do the travel vaccines – yellow fever, typhus, the whole gamut. And it’s just as easy vaccinating for that as for any of the others.”
Why implement adult immunization?
Many internists send patients off to a pharmacy for their vaccinations. That’s simply not good medical care, Dr. Goldman said.
“We are the primary care doctors,” he said. “We are the ones who should be vaccinating our patients, for several reasons: It’s the standard of care. It’s good medical practice.”
And Dr. Goldman frequently doesn’t receive any reports from the pharmacies. That means patients come to his office and have no idea what vaccines they received.
“That’s not good documentation,” he cautioned. “And when patients go into the hospital, they all get Pneumovax every single week because the hospital isn’t keeping documentation.”
The bottom line with vaccinating: “Whether you’re in a small group, a solo practitioner, or in a large health system, the vaccine programs work. They prevent disease and save lives. It’s easy to incorporate into your practice. And it is profitable.”
How profitable?
Dr. Goldman has the answer. For a great many different vaccines, he has calculated his average cost for the needle, syringe, medical assistant, time in the room, and other factors involved in running his practice. He also knows from experience the average purchase price paid for a given vaccine, the typical reimbursement for that vaccine, plus the reimbursement for its administration, which is a separate yet necessary coding/billing item.
The typical net profit ranges from $21.50 for high-dose influenza vaccine to, at the top end, $47.41 for meningococcal group B vaccine (Bexsero) and $49.58 for recombinant human papillomavirus 9-valent vaccine (Gardasil-9).
Purchasing and storage considerations
Always buy vaccines directly from the manufacturer; it’s a better deal than going through a middleman, who’ll invariably take a cut out of what should be the physician’s profit.
Each of the major vaccine makers has a dedicated vaccine purchase website where a physician can sign up for an account and order the company’s vaccines. These include Merck (www.merckvaccines.com), Aventis (www.vaccineshoppe.com), Pfizer (www.pfizerprime.com), and GlaxoSmithKline (www.gskdirect.com).
You’ll get a discount by buying multiple different vaccines on the same order.
“You can defer payment of your invoice for several months,” Dr. Goldman explained. “You purchase the vaccines now, but you don’t have to pay for them until 3-4 months later. By then, hopefully, you’ll have received reimbursement. So, your cost is covered, and you have profit on the side.”
For paying promptly on the due date, the manufacturer will provide an additional discount. The easiest way to do that is to have the money automatically charged to a credit card on that date.
Also, the vaccine manufacturers’ staff are happy to provide reliably expert reimbursement guidance.
With a little experience, it’s easy to predict how many vaccines will be used per month, Dr. Goldman said. Order what’s needed, so there aren’t a bunch of vaccines expiring in the office.
“However, even if that does happen, all is not lost,” he noted. “You can call up the manufacturer, and many of them will take back unused or even expired vaccines for full credit to the account. So, again, you really can’t lose money.”
With regard to vaccine storage, don’t skimp on the refrigerator and/or freezer. Get a professional model. And follow the best practices as described in the Centers for Disease Control and Prevention toolkit.
“It’s really common sense: Don’t use a dorm-type refrigerator; don’t put food or beverages in there; make sure the vaccines are appropriately stored; check the temperature every day; make sure if you lose power, your building has a backup generator,” he explained. “If you train your staff the right way, they’ll be able to handle it so you don’t have to worry about it. You just have to look at the logs and make sure they’re doing it.”
Use standing orders
Studies show that standing orders result in higher vaccination rates.
“You’re empowering the nurses or other staff members to act within the full extent of their license,” Dr. Goldman said. “It takes the burden off the physician to have to do anything that can be delegated to other individuals to make sure patients get vaccinated.”
Coding and billing for commercially insured patients
All vaccines have the same ICD-10 diagnostic code: Z23. And each vaccine has its own CPT code. For example, 90750 for Shingrix, the new herpes zoster vaccine; 90715 for Tdap; and 90686 for quadrivalent influenza.
But there are two components to the CPT code for a vaccination: the individual vaccine code and the administration code.
If you give one vaccination to a non-Medicare patient, the administration code is 90471. If you give a second vaccination during the same visit, its administration code is 90472. If you give a patient, say, four vaccines during one visit, you would bill the first using the administration code 90471, and the others as 90472 times three units.
If the vaccines are being given during a legitimate office visit, the physician can bill for both by employing modifiers 25 and 59. Modifier 25 goes with the appropriate E/M code for the office visit; it serves to tell the coding system that other things are going on in addition to the billable office visit. Modifier 59 needs to be attached to both the specific vaccine code and the vaccine administration code for reimbursement to occur.
Billing for vaccines for all commercially insured patients go through the office’s normal claims process.
Immunizing Medicare patients
For patients under Medicare Part B, vaccines for influenza, pneumonia, and hepatitis B have their own individual G codes: G0008 for influenza, G0009 for a pneumonia vaccine, and G0010 for hepatitis B. If a Medicare patient also gets an additional vaccine other than one of those three during the visit, administration code 90472 is applied to it. Those G-code bills are also submitted through the office’s normal claims process.
Under Medicare, vaccines for herpes zoster, hepatitis A, and Tdap are a special case. They are considered drugs and are covered under Medicare Part D.
“To bill that, you have to tell Medicare that you’re acting as a pharmacy,” Dr. Goldman explained. “You go to www.mytransactRX.com. You request there to be seen as a pharmacy billing for a drug. You will then be able to receive direct payment into your bank account from your Medicare payer. It will also allow you to check out patient coverage, print out proof of coverage, and submit the claim through the portal.”
If the Medicare patient doesn’t have a drug plan for those vaccines, or if the information in the system isn’t up to date, it’s a good idea to download the Advanced Beneficiary Notice of Noncoverage from the Medicare website and have the patient sign it. It spells out what the patient’s financial responsibility could be.
“The ABN also protects you as a provider, because it shows you’re not trying to balance-bill the patient,” he noted.
Dr. Goldman implored his internist colleagues to stand up and become the stewards of adult immunization.
“Remember: Keep calm and vaccinate,” he urged.
He reported having no relevant financial conflicts.
REPORTING FROM ACP INTERNAL MEDICINE
NIH cans study that relied on millions in funding from alcohol companies
NIH Francis Collins, MD, said the ethical violations resulted in a fundamentally flawed study that could not proceed.
“NIH has strong policies that detail the standards of conduct for NIH employees, including prohibiting the solicitation of gifts and promoting fairness in grant competitions. We take very seriously any violations of these standards,” Dr. Collins said in a statement, which added that the agency will take appropriate personnel actions.
While testifying before the Senate Appropriations Committee in mid-May on NIH’s budget request for 2019, Dr. Collins vowed not only to appropriately close the Moderate Alcohol and Cardiovascular Health (MACH) study, but to investigate whether other potential conflicts exist in other NIH-funded studies.
The story broke in mid-March, when The New York Times reported that scientists and officials from the National Institute on Alcohol Abuse and Alcoholism who were working on the MACH trial met at informational sessions with five liquor and beer companies in 2013 and 2014. The officials suggested that “the research might reflect favorably on moderate drinking, while institute officials pressed the groups for support,” according to documents obtained by the Times.
In all, the Times reported, the alcohol companies agreed to foot $67 million of the trial’s total $100 million bill. Such action violates NIH policy. An NIH report named those companies as Anheuser-Busch InBev, Carlsberg Breweries A/S, Diageo plc, Heineken, and Pernod Ricard USA LLC.
The MACH study was a multicenter, randomized clinical trial to determine the effects of one serving of alcohol (approximately 15 grams) daily, compared to no alcohol intake, on the rate of new cases of cardiovascular disease and the rate of new cases of diabetes among participants free of diabetes at baseline.
“The study was launched because some epidemiological studies have shown that moderate alcohol consumption has health benefits by reducing risk for coronary artery disease, type 2 diabetes, and rheumatoid arthritis,” according to the NIH statement. “The study aimed to enroll 7,800 participants. After a planning phase, it began enrollment on Feb. 5, 2018, and was suspended on May 10, 2018, at which time there were 105 participants enrolled.”
The trial was being led by researchers at Beth Israel Deaconess Medical Center, Boston.
In response to the public disclosure of the study’s funding, NIH convened a working group to ascertain:
- the circumstances that led to securing private funding for MACH trial
- the scientific premise of and planning for the MACH trial
- the process used to decide to support the MACH trial
- program development and oversight once funding was secured by the secured by the Foundation for NIH (FNIH)
- a review of the NIAAA portfolio prior to and during the leadership of the current NIAAA Director to assess what programmatic shifts, if any, could be discerned.
While noting that public-private partnerships are key to advancing science, the committee found that soliciting funds from alcoholic beverage companies for a study that could prove such beverages are beneficial, crossed the “firewall” between public funds and private resources. The committee recommended terminating the study.
The committee also recommended an expanded investigation into measures that would prevent NIH staff from soliciting external funds to support research programs.
The committee uncovered an email trail strongly suggesting that the solicitation of funds was planned and intended to be secretive.
According to the working group report, there was “frequent email correspondence among members of NIAAA senior staff, select extramural investigators (including the eventual PI of the MACH trial), and industry representatives occurred prior to involvement of the FNIH and the development of the NIH funding opportunity announcement for a multi-site clinical trial on moderate drinking and cardiovascular health. These communications appear to be an attempt to persuade industry to provide funding for the MACH trial. Moreover, these senior members of NIAAA staff appear to have purposefully kept other key members of NIAAA staff and the FNIH ignorant of these efforts. For example, correspondence between NIAAA staff draws attention to a February 2014 wine industry blog that reports that FNIH is initiating a search for industry funding to support a major clinical study on the health effects of moderate alcohol consumption. One senior staff member at NIAAA is unaware of any such potential planning, asking another senior staff member about the article. ‘... Anything seem broken here?’ even though such a trial to test moderate drinking effects on cardiovascular health should very likely involve the programmatic division to which this senior staff member belongs. In response to receiving the forwarded discussion, NIAAA senior leadership communicates among one other, ‘Best not to respond right now but we can’t keep him totally in the dark.’ "
The trial was also funded in part by NIAAA, which expected to commit $20 million to the overall project over 10 years, of which $4 million has been spent.
“The integrity of the NIH grants administrative process, peer review, and the quality of NIH-supported research must always be above reproach,” Dr. Collins said in the statement. “When any problems are uncovered, however, efforts to correct them must be swift and comprehensive.”
NIH Francis Collins, MD, said the ethical violations resulted in a fundamentally flawed study that could not proceed.
“NIH has strong policies that detail the standards of conduct for NIH employees, including prohibiting the solicitation of gifts and promoting fairness in grant competitions. We take very seriously any violations of these standards,” Dr. Collins said in a statement, which added that the agency will take appropriate personnel actions.
While testifying before the Senate Appropriations Committee in mid-May on NIH’s budget request for 2019, Dr. Collins vowed not only to appropriately close the Moderate Alcohol and Cardiovascular Health (MACH) study, but to investigate whether other potential conflicts exist in other NIH-funded studies.
The story broke in mid-March, when The New York Times reported that scientists and officials from the National Institute on Alcohol Abuse and Alcoholism who were working on the MACH trial met at informational sessions with five liquor and beer companies in 2013 and 2014. The officials suggested that “the research might reflect favorably on moderate drinking, while institute officials pressed the groups for support,” according to documents obtained by the Times.
In all, the Times reported, the alcohol companies agreed to foot $67 million of the trial’s total $100 million bill. Such action violates NIH policy. An NIH report named those companies as Anheuser-Busch InBev, Carlsberg Breweries A/S, Diageo plc, Heineken, and Pernod Ricard USA LLC.
The MACH study was a multicenter, randomized clinical trial to determine the effects of one serving of alcohol (approximately 15 grams) daily, compared to no alcohol intake, on the rate of new cases of cardiovascular disease and the rate of new cases of diabetes among participants free of diabetes at baseline.
“The study was launched because some epidemiological studies have shown that moderate alcohol consumption has health benefits by reducing risk for coronary artery disease, type 2 diabetes, and rheumatoid arthritis,” according to the NIH statement. “The study aimed to enroll 7,800 participants. After a planning phase, it began enrollment on Feb. 5, 2018, and was suspended on May 10, 2018, at which time there were 105 participants enrolled.”
The trial was being led by researchers at Beth Israel Deaconess Medical Center, Boston.
In response to the public disclosure of the study’s funding, NIH convened a working group to ascertain:
- the circumstances that led to securing private funding for MACH trial
- the scientific premise of and planning for the MACH trial
- the process used to decide to support the MACH trial
- program development and oversight once funding was secured by the secured by the Foundation for NIH (FNIH)
- a review of the NIAAA portfolio prior to and during the leadership of the current NIAAA Director to assess what programmatic shifts, if any, could be discerned.
While noting that public-private partnerships are key to advancing science, the committee found that soliciting funds from alcoholic beverage companies for a study that could prove such beverages are beneficial, crossed the “firewall” between public funds and private resources. The committee recommended terminating the study.
The committee also recommended an expanded investigation into measures that would prevent NIH staff from soliciting external funds to support research programs.
The committee uncovered an email trail strongly suggesting that the solicitation of funds was planned and intended to be secretive.
According to the working group report, there was “frequent email correspondence among members of NIAAA senior staff, select extramural investigators (including the eventual PI of the MACH trial), and industry representatives occurred prior to involvement of the FNIH and the development of the NIH funding opportunity announcement for a multi-site clinical trial on moderate drinking and cardiovascular health. These communications appear to be an attempt to persuade industry to provide funding for the MACH trial. Moreover, these senior members of NIAAA staff appear to have purposefully kept other key members of NIAAA staff and the FNIH ignorant of these efforts. For example, correspondence between NIAAA staff draws attention to a February 2014 wine industry blog that reports that FNIH is initiating a search for industry funding to support a major clinical study on the health effects of moderate alcohol consumption. One senior staff member at NIAAA is unaware of any such potential planning, asking another senior staff member about the article. ‘... Anything seem broken here?’ even though such a trial to test moderate drinking effects on cardiovascular health should very likely involve the programmatic division to which this senior staff member belongs. In response to receiving the forwarded discussion, NIAAA senior leadership communicates among one other, ‘Best not to respond right now but we can’t keep him totally in the dark.’ "
The trial was also funded in part by NIAAA, which expected to commit $20 million to the overall project over 10 years, of which $4 million has been spent.
“The integrity of the NIH grants administrative process, peer review, and the quality of NIH-supported research must always be above reproach,” Dr. Collins said in the statement. “When any problems are uncovered, however, efforts to correct them must be swift and comprehensive.”
NIH Francis Collins, MD, said the ethical violations resulted in a fundamentally flawed study that could not proceed.
“NIH has strong policies that detail the standards of conduct for NIH employees, including prohibiting the solicitation of gifts and promoting fairness in grant competitions. We take very seriously any violations of these standards,” Dr. Collins said in a statement, which added that the agency will take appropriate personnel actions.
While testifying before the Senate Appropriations Committee in mid-May on NIH’s budget request for 2019, Dr. Collins vowed not only to appropriately close the Moderate Alcohol and Cardiovascular Health (MACH) study, but to investigate whether other potential conflicts exist in other NIH-funded studies.
The story broke in mid-March, when The New York Times reported that scientists and officials from the National Institute on Alcohol Abuse and Alcoholism who were working on the MACH trial met at informational sessions with five liquor and beer companies in 2013 and 2014. The officials suggested that “the research might reflect favorably on moderate drinking, while institute officials pressed the groups for support,” according to documents obtained by the Times.
In all, the Times reported, the alcohol companies agreed to foot $67 million of the trial’s total $100 million bill. Such action violates NIH policy. An NIH report named those companies as Anheuser-Busch InBev, Carlsberg Breweries A/S, Diageo plc, Heineken, and Pernod Ricard USA LLC.
The MACH study was a multicenter, randomized clinical trial to determine the effects of one serving of alcohol (approximately 15 grams) daily, compared to no alcohol intake, on the rate of new cases of cardiovascular disease and the rate of new cases of diabetes among participants free of diabetes at baseline.
“The study was launched because some epidemiological studies have shown that moderate alcohol consumption has health benefits by reducing risk for coronary artery disease, type 2 diabetes, and rheumatoid arthritis,” according to the NIH statement. “The study aimed to enroll 7,800 participants. After a planning phase, it began enrollment on Feb. 5, 2018, and was suspended on May 10, 2018, at which time there were 105 participants enrolled.”
The trial was being led by researchers at Beth Israel Deaconess Medical Center, Boston.
In response to the public disclosure of the study’s funding, NIH convened a working group to ascertain:
- the circumstances that led to securing private funding for MACH trial
- the scientific premise of and planning for the MACH trial
- the process used to decide to support the MACH trial
- program development and oversight once funding was secured by the secured by the Foundation for NIH (FNIH)
- a review of the NIAAA portfolio prior to and during the leadership of the current NIAAA Director to assess what programmatic shifts, if any, could be discerned.
While noting that public-private partnerships are key to advancing science, the committee found that soliciting funds from alcoholic beverage companies for a study that could prove such beverages are beneficial, crossed the “firewall” between public funds and private resources. The committee recommended terminating the study.
The committee also recommended an expanded investigation into measures that would prevent NIH staff from soliciting external funds to support research programs.
The committee uncovered an email trail strongly suggesting that the solicitation of funds was planned and intended to be secretive.
According to the working group report, there was “frequent email correspondence among members of NIAAA senior staff, select extramural investigators (including the eventual PI of the MACH trial), and industry representatives occurred prior to involvement of the FNIH and the development of the NIH funding opportunity announcement for a multi-site clinical trial on moderate drinking and cardiovascular health. These communications appear to be an attempt to persuade industry to provide funding for the MACH trial. Moreover, these senior members of NIAAA staff appear to have purposefully kept other key members of NIAAA staff and the FNIH ignorant of these efforts. For example, correspondence between NIAAA staff draws attention to a February 2014 wine industry blog that reports that FNIH is initiating a search for industry funding to support a major clinical study on the health effects of moderate alcohol consumption. One senior staff member at NIAAA is unaware of any such potential planning, asking another senior staff member about the article. ‘... Anything seem broken here?’ even though such a trial to test moderate drinking effects on cardiovascular health should very likely involve the programmatic division to which this senior staff member belongs. In response to receiving the forwarded discussion, NIAAA senior leadership communicates among one other, ‘Best not to respond right now but we can’t keep him totally in the dark.’ "
The trial was also funded in part by NIAAA, which expected to commit $20 million to the overall project over 10 years, of which $4 million has been spent.
“The integrity of the NIH grants administrative process, peer review, and the quality of NIH-supported research must always be above reproach,” Dr. Collins said in the statement. “When any problems are uncovered, however, efforts to correct them must be swift and comprehensive.”
Fundoplication works best for true PPI-refractory heartburn
WASHINGTON – Less than a quarter of patients with heartburn that appears refractory to proton pump inhibitor treatment truly have reflux-related, drug-refractory heartburn with a high symptom–related probability, but patients who fall into this select subgroup often have significant symptom relief from surgical fundoplication, based on results from a randomized, multicenter, Department of Veterans Affairs study with 78 patients.
Although laparoscopic Nissen fundoplication relieved the heartburn symptoms of just two-thirds of patients who met the study’s definition of having true proton pump inhibitor (PPI)–refractory heartburn, this level of efficacy far exceeded the impact of drug therapy with baclofen or desipramine, which was little better than placebo, Stuart J. Spechler, MD, said at the annual Digestive Disease Week®.
“Fundoplication fell out of favor because of the success of PPI treatment, and because of complications from the surgery, but what our results show is that there is a subgroup of patients who can benefit from fundoplication. The challenge is identifying them,” said Dr. Spechler, a gastroenterologist and professor of medicine at the University of Texas, Dallas. “If you go through a careful work-up you will find the patients who have true PPI-refractory acid reflux and heartburn, and in the end we don’t have good medical treatments for these patients,” leaving fundoplication as their best hope for symptom relief.
The study he ran included 366 patients seen at about 30 VA Medical Centers across the United States who had been referred to his center because of presumed PPI-refractory heartburn. The careful work-up that Dr. Spechler and his associates ran included a closely supervised, 2-week trial of a standardized PPI regimen with omeprazole, careful symptom scoring on this treatment with a reflux-specific, health-related quality of life questionnaire, endoscopic esophageal manometry, and esophageal pH monitoring while on omeprazole.
This process placed patients into several distinct subgroups: About 19% dropped out of the study during this assessment, and another 15% left the study because of their intolerance of various stages of the work-up. Nearly 12% of patients wound up being responsive to the PPI regimen, about 6% had organic disorders not related to gastroesophageal reflux disease, and 27% had functional heartburn with a normal level of acid reflux, which left 78 patients (21%) who demonstrated true reflux-related, PPI-refractory heartburn symptoms.
The researchers then randomized this 78-patient subgroup into three treatment arms, with one group of 27 underwent fundoplication surgery. A group of 25 underwent active medical therapy with 20 mg omeprazole b.i.d. plus baclofen, which was started at 5 mg t.i.d. and increased to 20 mg t.i.d. In baclofen-intolerant or nonresponding patients, this treatment was followed up with desipramine, increasing from a starting dosage of 25 mg/day to 100 mg/day. A third group of 26 control patients received active omeprazole at the same dosage but placebo in place of the baclofen and desipramine. These three subgroups showed no statistically significant differences at baseline for all demographic and clinical parameters recorded.
The study’s primary endpoint was the percentage of patients in each treatment arm who had a “successful” outcome, defined as at least a 50% improvement in their gastroesophageal reflux health-related quality of life score (J Gastrointest Surg. 1998 Mar-Apr;2[2]:141-5) after 1 year on treatment, which occurred in 67% of the fundoplication patients, 28% in the active medical arm, and 12% in the control arm. The fundoplication-treated patients had a significantly higher rate of a successful outcome, compared with patients in each of the other two treatment groups, while the success rates among patients in the active medical group and the control group did not differ significantly, Dr. Spechler said.
Dr. Spechler had no disclosures to report.
WASHINGTON – Less than a quarter of patients with heartburn that appears refractory to proton pump inhibitor treatment truly have reflux-related, drug-refractory heartburn with a high symptom–related probability, but patients who fall into this select subgroup often have significant symptom relief from surgical fundoplication, based on results from a randomized, multicenter, Department of Veterans Affairs study with 78 patients.
Although laparoscopic Nissen fundoplication relieved the heartburn symptoms of just two-thirds of patients who met the study’s definition of having true proton pump inhibitor (PPI)–refractory heartburn, this level of efficacy far exceeded the impact of drug therapy with baclofen or desipramine, which was little better than placebo, Stuart J. Spechler, MD, said at the annual Digestive Disease Week®.
“Fundoplication fell out of favor because of the success of PPI treatment, and because of complications from the surgery, but what our results show is that there is a subgroup of patients who can benefit from fundoplication. The challenge is identifying them,” said Dr. Spechler, a gastroenterologist and professor of medicine at the University of Texas, Dallas. “If you go through a careful work-up you will find the patients who have true PPI-refractory acid reflux and heartburn, and in the end we don’t have good medical treatments for these patients,” leaving fundoplication as their best hope for symptom relief.
The study he ran included 366 patients seen at about 30 VA Medical Centers across the United States who had been referred to his center because of presumed PPI-refractory heartburn. The careful work-up that Dr. Spechler and his associates ran included a closely supervised, 2-week trial of a standardized PPI regimen with omeprazole, careful symptom scoring on this treatment with a reflux-specific, health-related quality of life questionnaire, endoscopic esophageal manometry, and esophageal pH monitoring while on omeprazole.
This process placed patients into several distinct subgroups: About 19% dropped out of the study during this assessment, and another 15% left the study because of their intolerance of various stages of the work-up. Nearly 12% of patients wound up being responsive to the PPI regimen, about 6% had organic disorders not related to gastroesophageal reflux disease, and 27% had functional heartburn with a normal level of acid reflux, which left 78 patients (21%) who demonstrated true reflux-related, PPI-refractory heartburn symptoms.
The researchers then randomized this 78-patient subgroup into three treatment arms, with one group of 27 underwent fundoplication surgery. A group of 25 underwent active medical therapy with 20 mg omeprazole b.i.d. plus baclofen, which was started at 5 mg t.i.d. and increased to 20 mg t.i.d. In baclofen-intolerant or nonresponding patients, this treatment was followed up with desipramine, increasing from a starting dosage of 25 mg/day to 100 mg/day. A third group of 26 control patients received active omeprazole at the same dosage but placebo in place of the baclofen and desipramine. These three subgroups showed no statistically significant differences at baseline for all demographic and clinical parameters recorded.
The study’s primary endpoint was the percentage of patients in each treatment arm who had a “successful” outcome, defined as at least a 50% improvement in their gastroesophageal reflux health-related quality of life score (J Gastrointest Surg. 1998 Mar-Apr;2[2]:141-5) after 1 year on treatment, which occurred in 67% of the fundoplication patients, 28% in the active medical arm, and 12% in the control arm. The fundoplication-treated patients had a significantly higher rate of a successful outcome, compared with patients in each of the other two treatment groups, while the success rates among patients in the active medical group and the control group did not differ significantly, Dr. Spechler said.
Dr. Spechler had no disclosures to report.
WASHINGTON – Less than a quarter of patients with heartburn that appears refractory to proton pump inhibitor treatment truly have reflux-related, drug-refractory heartburn with a high symptom–related probability, but patients who fall into this select subgroup often have significant symptom relief from surgical fundoplication, based on results from a randomized, multicenter, Department of Veterans Affairs study with 78 patients.
Although laparoscopic Nissen fundoplication relieved the heartburn symptoms of just two-thirds of patients who met the study’s definition of having true proton pump inhibitor (PPI)–refractory heartburn, this level of efficacy far exceeded the impact of drug therapy with baclofen or desipramine, which was little better than placebo, Stuart J. Spechler, MD, said at the annual Digestive Disease Week®.
“Fundoplication fell out of favor because of the success of PPI treatment, and because of complications from the surgery, but what our results show is that there is a subgroup of patients who can benefit from fundoplication. The challenge is identifying them,” said Dr. Spechler, a gastroenterologist and professor of medicine at the University of Texas, Dallas. “If you go through a careful work-up you will find the patients who have true PPI-refractory acid reflux and heartburn, and in the end we don’t have good medical treatments for these patients,” leaving fundoplication as their best hope for symptom relief.
The study he ran included 366 patients seen at about 30 VA Medical Centers across the United States who had been referred to his center because of presumed PPI-refractory heartburn. The careful work-up that Dr. Spechler and his associates ran included a closely supervised, 2-week trial of a standardized PPI regimen with omeprazole, careful symptom scoring on this treatment with a reflux-specific, health-related quality of life questionnaire, endoscopic esophageal manometry, and esophageal pH monitoring while on omeprazole.
This process placed patients into several distinct subgroups: About 19% dropped out of the study during this assessment, and another 15% left the study because of their intolerance of various stages of the work-up. Nearly 12% of patients wound up being responsive to the PPI regimen, about 6% had organic disorders not related to gastroesophageal reflux disease, and 27% had functional heartburn with a normal level of acid reflux, which left 78 patients (21%) who demonstrated true reflux-related, PPI-refractory heartburn symptoms.
The researchers then randomized this 78-patient subgroup into three treatment arms, with one group of 27 underwent fundoplication surgery. A group of 25 underwent active medical therapy with 20 mg omeprazole b.i.d. plus baclofen, which was started at 5 mg t.i.d. and increased to 20 mg t.i.d. In baclofen-intolerant or nonresponding patients, this treatment was followed up with desipramine, increasing from a starting dosage of 25 mg/day to 100 mg/day. A third group of 26 control patients received active omeprazole at the same dosage but placebo in place of the baclofen and desipramine. These three subgroups showed no statistically significant differences at baseline for all demographic and clinical parameters recorded.
The study’s primary endpoint was the percentage of patients in each treatment arm who had a “successful” outcome, defined as at least a 50% improvement in their gastroesophageal reflux health-related quality of life score (J Gastrointest Surg. 1998 Mar-Apr;2[2]:141-5) after 1 year on treatment, which occurred in 67% of the fundoplication patients, 28% in the active medical arm, and 12% in the control arm. The fundoplication-treated patients had a significantly higher rate of a successful outcome, compared with patients in each of the other two treatment groups, while the success rates among patients in the active medical group and the control group did not differ significantly, Dr. Spechler said.
Dr. Spechler had no disclosures to report.
REPORTING FROM DDW 2018
Key clinical point: Fundoplication produces the best outcomes in patients with true proton pump inhibitor–refractory heartburn.
Major finding: Two-thirds of patients treated with fundoplication had successful outcomes, compared with 28% in medical controls and 12% in placebo controls.
Study details: A multicenter, randomized study with 78 patients.
Disclosures: Dr. Spechler had no disclosures to report.
Summer colds
Enteroviruses cause most summer colds. The enteroviruses include echoviruses, coxsackieviruses, numbered enteroviruses, and the polioviruses. Most summer colds seen in private practice are self limited, presenting with fever alone or clinically distinctive pictures such as hand-foot-and-mouth disease (HFMD), herpangina, or pleurodynia. However, enteroviruses also cause serious illnesses such as meningitis, myocarditis, encephalitis, and neonatal sepsis. Enterovirus infections often are confused with bacterial infections and treated unnecessarily with antibiotics.
Enteroviral infections spread predominantly by the fecal-oral route. Contaminated swimming pools also may serve as a source of transmission. Enteroviruses colonize the respiratory and the gastrointestinal tract. The infection spreads to the lymph nodes, where the virus replicates and an initial viremia occurs on approximately the third postexposure day. The viremia results in subsequent spread to the throat (herpangina), and/or hands and feet (HFMD), lungs (pleurodynia), heart (myocarditis) or meninges (viral meningitis). Infection at the secondary sites corresponds to the onset of clinical symptoms 4-6 days after exposure. The clinical manifestations of enteroviral infections result from the damage caused by the virus at the secondary sites of infection.
Enterovirus pharyngitis starts abruptly and often is accompanied by fever. Younger children may present with increased drooling, hands in the mouth, and refusal to eat. Older children complain of sore throat as well as headache, myalgias, and malaise. Mild vomiting and diarrhea commonly accompany the respiratory symptoms. Herpangina is a specific syndrome of enterovirus pharyngitis; children with this syndrome have fever and characteristic papulovesicular lesions on the anterior tonsillar pillars, soft palate, uvula, tonsils, and pharyngeal wall. The lesions are discrete and average five per patient. They do not appear in the anterior part of the mouth.
Hand-foot-and-mouth disease is well recognized by clinicians who care for young children. The child presents with fever and papulovesicular lesions within the mouth that quickly become ulcerated and papulovesicular lesions on the palms and soles. The palms and soles often are puffy and red, and the child may act as though her hands and feet hurt, refusing to use her hands or walk. The fever accompanying herpangina and HFMD usually lasts 3 or 4 days, but fever that persists for a week is not uncommon. The pharyngitis follows a pattern similar to the fever.
Pleurodynia has a sudden onset of pain in the chest or upper abdomen. The pain appears to be muscular in origin; its intensity varies. It can be excruciatingly severe and accompanied by sweating and pallor. Older children describe the pain as sharp and stabbing. It occurs in spasms that can last for a few minutes to a few hours. During spasms, the patient has rapid, shallow respirations that suggest pneumonia. The symptoms usually last 1 or 2 days, but the illness can be biphasic, with symptoms resolving only to reappear a few days later.
Gastrointestinal manifestations are almost universal in enterovirus infections. The most common symptoms are anorexia, nausea, vomiting, and diarrhea. They usually are not severe and often occur in combination with other symptoms, such as fever and sore throat. Abdominal pain may be the only manifestation of infection; when severe, it can mimic appendicitis.
Enterovirus infections once were thought to be mild diseases that lasted 2-3 days. But a study of 380 children aged 4-18 years during July to October from private pediatric practices found that illness is prolonged in many patients (Pediatrics. 1998 Nov;102[5]:1126-34). The mean duration of illness was found to be 10 days for myalgia-malaise syndrome, 7 days for herpangina, and 7 days for HFMD.
Spread of enteroviral infections within a household was common. More than 50% of children studied had a family member with enterovirus illness. Half of siblings and 25% of adults within the household of the index case contracted an enteroviral infection. Some had the same presentation as the index patient, but it was not uncommon for other household members to have quite different presentations. For example, the first child seen might present with hand-foot-and-mouth disease, and a few days later a sibling might be brought for care with myalgia-malaise, and the parent might appear ill and complain of pleurodynia.
Summer colds can be costly to families. The duration of the illness and the multitude of nonspecific symptoms sometimes leads to concern about a possible bacterial cause, which prompts a diagnostic workup, including laboratory tests and empiric treatment with antibiotics. The direct costs vary with the syndrome; stomatitis and HFMD are the least expensive to treat because the clinical picture is diagnostic with a single office visit, but a severe manifestation such as aseptic meningitis are expensive to treat with associated emergency department visits, spinal tap, and sometimes hospitalization.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He reported having no conflicts of interest. Email him at pdnews@mdedge.com.
Enteroviruses cause most summer colds. The enteroviruses include echoviruses, coxsackieviruses, numbered enteroviruses, and the polioviruses. Most summer colds seen in private practice are self limited, presenting with fever alone or clinically distinctive pictures such as hand-foot-and-mouth disease (HFMD), herpangina, or pleurodynia. However, enteroviruses also cause serious illnesses such as meningitis, myocarditis, encephalitis, and neonatal sepsis. Enterovirus infections often are confused with bacterial infections and treated unnecessarily with antibiotics.
Enteroviral infections spread predominantly by the fecal-oral route. Contaminated swimming pools also may serve as a source of transmission. Enteroviruses colonize the respiratory and the gastrointestinal tract. The infection spreads to the lymph nodes, where the virus replicates and an initial viremia occurs on approximately the third postexposure day. The viremia results in subsequent spread to the throat (herpangina), and/or hands and feet (HFMD), lungs (pleurodynia), heart (myocarditis) or meninges (viral meningitis). Infection at the secondary sites corresponds to the onset of clinical symptoms 4-6 days after exposure. The clinical manifestations of enteroviral infections result from the damage caused by the virus at the secondary sites of infection.
Enterovirus pharyngitis starts abruptly and often is accompanied by fever. Younger children may present with increased drooling, hands in the mouth, and refusal to eat. Older children complain of sore throat as well as headache, myalgias, and malaise. Mild vomiting and diarrhea commonly accompany the respiratory symptoms. Herpangina is a specific syndrome of enterovirus pharyngitis; children with this syndrome have fever and characteristic papulovesicular lesions on the anterior tonsillar pillars, soft palate, uvula, tonsils, and pharyngeal wall. The lesions are discrete and average five per patient. They do not appear in the anterior part of the mouth.
Hand-foot-and-mouth disease is well recognized by clinicians who care for young children. The child presents with fever and papulovesicular lesions within the mouth that quickly become ulcerated and papulovesicular lesions on the palms and soles. The palms and soles often are puffy and red, and the child may act as though her hands and feet hurt, refusing to use her hands or walk. The fever accompanying herpangina and HFMD usually lasts 3 or 4 days, but fever that persists for a week is not uncommon. The pharyngitis follows a pattern similar to the fever.
Pleurodynia has a sudden onset of pain in the chest or upper abdomen. The pain appears to be muscular in origin; its intensity varies. It can be excruciatingly severe and accompanied by sweating and pallor. Older children describe the pain as sharp and stabbing. It occurs in spasms that can last for a few minutes to a few hours. During spasms, the patient has rapid, shallow respirations that suggest pneumonia. The symptoms usually last 1 or 2 days, but the illness can be biphasic, with symptoms resolving only to reappear a few days later.
Gastrointestinal manifestations are almost universal in enterovirus infections. The most common symptoms are anorexia, nausea, vomiting, and diarrhea. They usually are not severe and often occur in combination with other symptoms, such as fever and sore throat. Abdominal pain may be the only manifestation of infection; when severe, it can mimic appendicitis.
Enterovirus infections once were thought to be mild diseases that lasted 2-3 days. But a study of 380 children aged 4-18 years during July to October from private pediatric practices found that illness is prolonged in many patients (Pediatrics. 1998 Nov;102[5]:1126-34). The mean duration of illness was found to be 10 days for myalgia-malaise syndrome, 7 days for herpangina, and 7 days for HFMD.
Spread of enteroviral infections within a household was common. More than 50% of children studied had a family member with enterovirus illness. Half of siblings and 25% of adults within the household of the index case contracted an enteroviral infection. Some had the same presentation as the index patient, but it was not uncommon for other household members to have quite different presentations. For example, the first child seen might present with hand-foot-and-mouth disease, and a few days later a sibling might be brought for care with myalgia-malaise, and the parent might appear ill and complain of pleurodynia.
Summer colds can be costly to families. The duration of the illness and the multitude of nonspecific symptoms sometimes leads to concern about a possible bacterial cause, which prompts a diagnostic workup, including laboratory tests and empiric treatment with antibiotics. The direct costs vary with the syndrome; stomatitis and HFMD are the least expensive to treat because the clinical picture is diagnostic with a single office visit, but a severe manifestation such as aseptic meningitis are expensive to treat with associated emergency department visits, spinal tap, and sometimes hospitalization.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He reported having no conflicts of interest. Email him at pdnews@mdedge.com.
Enteroviruses cause most summer colds. The enteroviruses include echoviruses, coxsackieviruses, numbered enteroviruses, and the polioviruses. Most summer colds seen in private practice are self limited, presenting with fever alone or clinically distinctive pictures such as hand-foot-and-mouth disease (HFMD), herpangina, or pleurodynia. However, enteroviruses also cause serious illnesses such as meningitis, myocarditis, encephalitis, and neonatal sepsis. Enterovirus infections often are confused with bacterial infections and treated unnecessarily with antibiotics.
Enteroviral infections spread predominantly by the fecal-oral route. Contaminated swimming pools also may serve as a source of transmission. Enteroviruses colonize the respiratory and the gastrointestinal tract. The infection spreads to the lymph nodes, where the virus replicates and an initial viremia occurs on approximately the third postexposure day. The viremia results in subsequent spread to the throat (herpangina), and/or hands and feet (HFMD), lungs (pleurodynia), heart (myocarditis) or meninges (viral meningitis). Infection at the secondary sites corresponds to the onset of clinical symptoms 4-6 days after exposure. The clinical manifestations of enteroviral infections result from the damage caused by the virus at the secondary sites of infection.
Enterovirus pharyngitis starts abruptly and often is accompanied by fever. Younger children may present with increased drooling, hands in the mouth, and refusal to eat. Older children complain of sore throat as well as headache, myalgias, and malaise. Mild vomiting and diarrhea commonly accompany the respiratory symptoms. Herpangina is a specific syndrome of enterovirus pharyngitis; children with this syndrome have fever and characteristic papulovesicular lesions on the anterior tonsillar pillars, soft palate, uvula, tonsils, and pharyngeal wall. The lesions are discrete and average five per patient. They do not appear in the anterior part of the mouth.
Hand-foot-and-mouth disease is well recognized by clinicians who care for young children. The child presents with fever and papulovesicular lesions within the mouth that quickly become ulcerated and papulovesicular lesions on the palms and soles. The palms and soles often are puffy and red, and the child may act as though her hands and feet hurt, refusing to use her hands or walk. The fever accompanying herpangina and HFMD usually lasts 3 or 4 days, but fever that persists for a week is not uncommon. The pharyngitis follows a pattern similar to the fever.
Pleurodynia has a sudden onset of pain in the chest or upper abdomen. The pain appears to be muscular in origin; its intensity varies. It can be excruciatingly severe and accompanied by sweating and pallor. Older children describe the pain as sharp and stabbing. It occurs in spasms that can last for a few minutes to a few hours. During spasms, the patient has rapid, shallow respirations that suggest pneumonia. The symptoms usually last 1 or 2 days, but the illness can be biphasic, with symptoms resolving only to reappear a few days later.
Gastrointestinal manifestations are almost universal in enterovirus infections. The most common symptoms are anorexia, nausea, vomiting, and diarrhea. They usually are not severe and often occur in combination with other symptoms, such as fever and sore throat. Abdominal pain may be the only manifestation of infection; when severe, it can mimic appendicitis.
Enterovirus infections once were thought to be mild diseases that lasted 2-3 days. But a study of 380 children aged 4-18 years during July to October from private pediatric practices found that illness is prolonged in many patients (Pediatrics. 1998 Nov;102[5]:1126-34). The mean duration of illness was found to be 10 days for myalgia-malaise syndrome, 7 days for herpangina, and 7 days for HFMD.
Spread of enteroviral infections within a household was common. More than 50% of children studied had a family member with enterovirus illness. Half of siblings and 25% of adults within the household of the index case contracted an enteroviral infection. Some had the same presentation as the index patient, but it was not uncommon for other household members to have quite different presentations. For example, the first child seen might present with hand-foot-and-mouth disease, and a few days later a sibling might be brought for care with myalgia-malaise, and the parent might appear ill and complain of pleurodynia.
Summer colds can be costly to families. The duration of the illness and the multitude of nonspecific symptoms sometimes leads to concern about a possible bacterial cause, which prompts a diagnostic workup, including laboratory tests and empiric treatment with antibiotics. The direct costs vary with the syndrome; stomatitis and HFMD are the least expensive to treat because the clinical picture is diagnostic with a single office visit, but a severe manifestation such as aseptic meningitis are expensive to treat with associated emergency department visits, spinal tap, and sometimes hospitalization.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He reported having no conflicts of interest. Email him at pdnews@mdedge.com.