User login
Tardive dyskinesia: Screening and management
The DNA of psychiatric practice: A covenant with our patients
As the end of the academic year approaches, I always think of one last message to send to the freshly minted psychiatrists who will complete their 4 years of post-MD training. This year, I thought of emphasizing the principles of psychiatric practice, which the graduates will deliver for the next 4 to 5 decades of their professional lives. Those essential principles are coded in the DNA of psychiatric practice, just as the construction of all organs in the human body is coded within the DNA of the 22,000 genes that comprise our 23 chromosomes.
So here are the principles of psychiatry that I propose govern the relationship of psychiatrists with their patients, encrypted within the DNA of our esteemed medical specialty:
- Provide total dedication to helping psychiatric patients recover from their illness and regain their wellness.
- Maintain total and unimpeachable confidentiality.
- Demonstrate unconditional acceptance and respect to every patient.
- Adopt a nonjudgmental stance toward all patients.
- Establish a strong therapeutic alliance as early as possible. It is the center of the doctor–patient relationship.
- Provide the same standard of care to all patients—the same care you would want your family members to receive.
- Provide evidence-based treatments first, and if no response, use unapproved treatments judiciously, but above all, do no harm.
- Educate patients, and their families, about the illness, and discuss the benefits and risks of various treatments.
- Do not practice “naked psychopharmacology.” Psychotherapy must always be provided side-by-side with medications.
- Support the patient’s family. Their burden often is very heavy.
- Emphasize adherence as a key patient responsibility, and address it at every visit.
- Do not hesitate to consult a seasoned colleague about your complex clinical cases.
- Deal effectively with negative countertransference. Recognize it, and refer the patient to another colleague if you cannot resolve it.
- Always inquire about thoughts of harming self or others and act accordingly.
- Always ask about alcohol and substance use, and about over-the-counter drugs as well. They all can complicate your patient’s treatment course and outcome.
- Never breach boundaries with your patient, and firmly guide the patient about breaching boundaries with you.
- Uphold the medical tenet that all “mental” disorders of thought, mood, affect, behavior, and cognition are generated by disruptions of brain structure and/or function, whether molecular, cellular, or connectomic, caused by various combinations of genetic and/or environmental etiologies.
- Check your patients’ physical health status, including all treatments they received from other specialists, and always rule out iatrogenesis and disruptive pharmacokinetic interactions that may trigger or exacerbate psychiatric symptoms.
- Learn and use clinical rating scales to quantify symptom severity and adverse effects at baseline and at each visit. Measuring the severity of psychosis, depression, or anxiety in psychiatry is like measuring fasting glucose, triglycerides, or blood pressure in internal medicine.
- Use rational adjunctive and augmentation therapies when indicated, but avoid irrational and hazardous polypharmacy.
- Document your clinical findings, diagnosis, and treatment plan conscientiously and accurately. The medical record is a clinical, billing, legal, and research document.
- Advocate tirelessly for psychiatric patients to increase their access to care, and fight the unfair and hurtful stigma vigorously until it is completely erased. A psychiatric disorder should have no more stigma than a broken leg or peptic ulcer, and insurance parity must be identical as well.
- Establish collaborative care for each of your patients and link them to a primary care provider if they do not already have one. Disorders of the body and the brain are bidirectional in their effects and psychiatric patients often suffer from multiple organ diseases.
- Do some pro bono care for indigent or uninsured patients, and actively ask companies to provide free drugs to patients who cannot afford the medication you believe they need.
- Recognize that every treatment you use as the current standard of care was at one time a research project. Know that the research of today is the treatment of tomorrow. So support the creation of new medical knowledge by referring patients to FDA clinical trials or to National Institutes of Health–funded biologic investigations.
- No matter how busy you are, write a case report or a letter to the editor about an unusual response or adverse effect. This generates hypotheses that researchers can pursue and test.
- Volunteer to serve as a clinical supervisor for medical students and residents from your local medical school. Most academic departments of psychiatry appreciate their community-based volunteer faculty.
You, the readers of
As the end of the academic year approaches, I always think of one last message to send to the freshly minted psychiatrists who will complete their 4 years of post-MD training. This year, I thought of emphasizing the principles of psychiatric practice, which the graduates will deliver for the next 4 to 5 decades of their professional lives. Those essential principles are coded in the DNA of psychiatric practice, just as the construction of all organs in the human body is coded within the DNA of the 22,000 genes that comprise our 23 chromosomes.
So here are the principles of psychiatry that I propose govern the relationship of psychiatrists with their patients, encrypted within the DNA of our esteemed medical specialty:
- Provide total dedication to helping psychiatric patients recover from their illness and regain their wellness.
- Maintain total and unimpeachable confidentiality.
- Demonstrate unconditional acceptance and respect to every patient.
- Adopt a nonjudgmental stance toward all patients.
- Establish a strong therapeutic alliance as early as possible. It is the center of the doctor–patient relationship.
- Provide the same standard of care to all patients—the same care you would want your family members to receive.
- Provide evidence-based treatments first, and if no response, use unapproved treatments judiciously, but above all, do no harm.
- Educate patients, and their families, about the illness, and discuss the benefits and risks of various treatments.
- Do not practice “naked psychopharmacology.” Psychotherapy must always be provided side-by-side with medications.
- Support the patient’s family. Their burden often is very heavy.
- Emphasize adherence as a key patient responsibility, and address it at every visit.
- Do not hesitate to consult a seasoned colleague about your complex clinical cases.
- Deal effectively with negative countertransference. Recognize it, and refer the patient to another colleague if you cannot resolve it.
- Always inquire about thoughts of harming self or others and act accordingly.
- Always ask about alcohol and substance use, and about over-the-counter drugs as well. They all can complicate your patient’s treatment course and outcome.
- Never breach boundaries with your patient, and firmly guide the patient about breaching boundaries with you.
- Uphold the medical tenet that all “mental” disorders of thought, mood, affect, behavior, and cognition are generated by disruptions of brain structure and/or function, whether molecular, cellular, or connectomic, caused by various combinations of genetic and/or environmental etiologies.
- Check your patients’ physical health status, including all treatments they received from other specialists, and always rule out iatrogenesis and disruptive pharmacokinetic interactions that may trigger or exacerbate psychiatric symptoms.
- Learn and use clinical rating scales to quantify symptom severity and adverse effects at baseline and at each visit. Measuring the severity of psychosis, depression, or anxiety in psychiatry is like measuring fasting glucose, triglycerides, or blood pressure in internal medicine.
- Use rational adjunctive and augmentation therapies when indicated, but avoid irrational and hazardous polypharmacy.
- Document your clinical findings, diagnosis, and treatment plan conscientiously and accurately. The medical record is a clinical, billing, legal, and research document.
- Advocate tirelessly for psychiatric patients to increase their access to care, and fight the unfair and hurtful stigma vigorously until it is completely erased. A psychiatric disorder should have no more stigma than a broken leg or peptic ulcer, and insurance parity must be identical as well.
- Establish collaborative care for each of your patients and link them to a primary care provider if they do not already have one. Disorders of the body and the brain are bidirectional in their effects and psychiatric patients often suffer from multiple organ diseases.
- Do some pro bono care for indigent or uninsured patients, and actively ask companies to provide free drugs to patients who cannot afford the medication you believe they need.
- Recognize that every treatment you use as the current standard of care was at one time a research project. Know that the research of today is the treatment of tomorrow. So support the creation of new medical knowledge by referring patients to FDA clinical trials or to National Institutes of Health–funded biologic investigations.
- No matter how busy you are, write a case report or a letter to the editor about an unusual response or adverse effect. This generates hypotheses that researchers can pursue and test.
- Volunteer to serve as a clinical supervisor for medical students and residents from your local medical school. Most academic departments of psychiatry appreciate their community-based volunteer faculty.
You, the readers of
As the end of the academic year approaches, I always think of one last message to send to the freshly minted psychiatrists who will complete their 4 years of post-MD training. This year, I thought of emphasizing the principles of psychiatric practice, which the graduates will deliver for the next 4 to 5 decades of their professional lives. Those essential principles are coded in the DNA of psychiatric practice, just as the construction of all organs in the human body is coded within the DNA of the 22,000 genes that comprise our 23 chromosomes.
So here are the principles of psychiatry that I propose govern the relationship of psychiatrists with their patients, encrypted within the DNA of our esteemed medical specialty:
- Provide total dedication to helping psychiatric patients recover from their illness and regain their wellness.
- Maintain total and unimpeachable confidentiality.
- Demonstrate unconditional acceptance and respect to every patient.
- Adopt a nonjudgmental stance toward all patients.
- Establish a strong therapeutic alliance as early as possible. It is the center of the doctor–patient relationship.
- Provide the same standard of care to all patients—the same care you would want your family members to receive.
- Provide evidence-based treatments first, and if no response, use unapproved treatments judiciously, but above all, do no harm.
- Educate patients, and their families, about the illness, and discuss the benefits and risks of various treatments.
- Do not practice “naked psychopharmacology.” Psychotherapy must always be provided side-by-side with medications.
- Support the patient’s family. Their burden often is very heavy.
- Emphasize adherence as a key patient responsibility, and address it at every visit.
- Do not hesitate to consult a seasoned colleague about your complex clinical cases.
- Deal effectively with negative countertransference. Recognize it, and refer the patient to another colleague if you cannot resolve it.
- Always inquire about thoughts of harming self or others and act accordingly.
- Always ask about alcohol and substance use, and about over-the-counter drugs as well. They all can complicate your patient’s treatment course and outcome.
- Never breach boundaries with your patient, and firmly guide the patient about breaching boundaries with you.
- Uphold the medical tenet that all “mental” disorders of thought, mood, affect, behavior, and cognition are generated by disruptions of brain structure and/or function, whether molecular, cellular, or connectomic, caused by various combinations of genetic and/or environmental etiologies.
- Check your patients’ physical health status, including all treatments they received from other specialists, and always rule out iatrogenesis and disruptive pharmacokinetic interactions that may trigger or exacerbate psychiatric symptoms.
- Learn and use clinical rating scales to quantify symptom severity and adverse effects at baseline and at each visit. Measuring the severity of psychosis, depression, or anxiety in psychiatry is like measuring fasting glucose, triglycerides, or blood pressure in internal medicine.
- Use rational adjunctive and augmentation therapies when indicated, but avoid irrational and hazardous polypharmacy.
- Document your clinical findings, diagnosis, and treatment plan conscientiously and accurately. The medical record is a clinical, billing, legal, and research document.
- Advocate tirelessly for psychiatric patients to increase their access to care, and fight the unfair and hurtful stigma vigorously until it is completely erased. A psychiatric disorder should have no more stigma than a broken leg or peptic ulcer, and insurance parity must be identical as well.
- Establish collaborative care for each of your patients and link them to a primary care provider if they do not already have one. Disorders of the body and the brain are bidirectional in their effects and psychiatric patients often suffer from multiple organ diseases.
- Do some pro bono care for indigent or uninsured patients, and actively ask companies to provide free drugs to patients who cannot afford the medication you believe they need.
- Recognize that every treatment you use as the current standard of care was at one time a research project. Know that the research of today is the treatment of tomorrow. So support the creation of new medical knowledge by referring patients to FDA clinical trials or to National Institutes of Health–funded biologic investigations.
- No matter how busy you are, write a case report or a letter to the editor about an unusual response or adverse effect. This generates hypotheses that researchers can pursue and test.
- Volunteer to serve as a clinical supervisor for medical students and residents from your local medical school. Most academic departments of psychiatry appreciate their community-based volunteer faculty.
You, the readers of
The Goldwater Rule and free speech, the current 'political morass', and more
In his editorial, “The toxic zeitgeist of hyper-partisanship: A psychiatric perspective” (From the Editor,
One’s political views do not inform us of his or her mental health status. This appreciation can be obtained only by a thorough psychological assessment. This is the basis of the Goldwater Rule, coupled with the ethical responsibility not to discuss patients’ private communications.
Today, this rule is tested by the behavior and actions of President Donald Trump. Proponents of the Goldwater Rule state that a psychiatrist cannot diagnose someone without performing a face-to-face diagnostic evaluation. This assumes psychiatrists diagnose patients only by interviewing them. However, any psychiatrist who has worked in an emergency room has signed involuntary commitment papers for a patient who refuses to talk to them. This clinical action typically is based on reports of the patient’s potential dangerousness from family, friends, or the police.
The diagnostic criteria for some personality disorders are based only on observed or reported behavior. They do not indicate a need for an interview. The diagnosis of a personality disorder cannot be made solely by interviewing an individual without knowledge of his or her behavior. Interviewing Bernie Madoff would not have revealed his sociopathic behavior.
The critical question may not be whether one could ethically make a psychiatric diagnosis of the President (I believe you can), but rather would it indicate or imply that he is dangerous? History informs us that the existence of a psychiatric disorder does not determine a politician’s fitness for office or if they are dangerous. Behavioral accounts of President Abraham Lincoln and his self-reports seem to confirm that at times he was depressed, but he clearly served our country with distinction.
Finally, it is not clear whether the Goldwater Rule is legal. It arguably interferes with a psychiatrist’s right of free speech without the risk of being accused of unethical behavior. I wonder what would happen if it were tested in court. Does the First Amendment of the U.S. Constitution protect a psychiatrist’s right to speak freely?
Sidney Weissman, MD
Clinical Professor of Psychiatry and Behavioral Science
Feinberg School of Medicine
Northwestern University
Chicago, Illinois
The current ‘political morass’
Thank you, Dr. Nasrallah, for the wonderful synopsis of the current political morass in your editorial (From the Editor,
James Gallagher, MD
Private psychiatric practice
Des Moines, Iowa
Continue to: The biological etiology of compulsive sexual behavior
The biological etiology of compulsive sexual behavior
Dr. Grant’s article, “Compulsive sexual behavior: A nonjudgmental approach” (Evidence-Based Reviews,
Mukesh Sanghadia, MD, MRCPsych (UK), Diplomate ABPN
PsychiatristCommunity Research Foundation
San Diego, California
The author responds
Dr. Sanghadia highlights the lack of possible biological etiology of compulsive sexual behavior (CSB) in my article. This is a fair comment. The lack of agreed-upon diagnostic criteria, however, has resulted in a vast literature discussing sexual behaviors that may or may not be related to each other, and even suggest that what is currently referred to as CSB may in fact be quite heterogeneous. My article mentions the few neuroimaging and neurocognitive studies that address a more rigorously defined CSB. Other possible etiologies have been suggested for a range of out-of-control sexual behaviors, but have not been studied with a focus on this formal diagnostic category. For example, endocrine issues have been explored to some extent in individuals with paraphilic sexual behaviors (behaviors that appear to many to have no relationship to CSB as discussed in my article), and in those cases of paraphilic sexual behavior, a range of endocrine hormones have been examined—gonadotropin-releasing hormone, follicle-stimulating hormone, luteinizing hormone, testosterone/dihydrotestosterone, and estrogen/progesterone. But these studies have yielded no conclusive outcomes in terms of findings or treatments.
In summary, the biology of CSB lags far behind that of other mental health disorders (and even other psychiatric disorders lack conclusive biological etiologies). Establishing this behavior as a legitimate diagnostic entity with agreed-upon criteria may be the first step in furthering our understanding of its possible biology.
Jon E. Grant, JD, MD, MPH
Professor
Department of Psychiatry and Behavioral Neuroscience
University of Chicago, Pritzker School of Medicine
Chicago, Illinois
Continue to: A different view of patients with schizophrenia
A different view of patients with schizophrenia
After treating patients with schizophrenia for more than 30 years, I’ve observed a continuous flood of information about them. This overload has been consistent since my residency back in the 1980s. Theories ranging from the psychoanalytic to the biologic are numerous and valuable additions to our understanding of those who suffer with this malady, yet they provide no summation or overview with which to understand it.
For instance, we know that schizophrenia usually begins in the late teens or early twenties. We know that antidopaminergic medications usually help to varying degrees. Psychosocial interventions may contribute greatly to the ultimate outcome. Substance use invariably makes it worse. Establishing a connection with the patient can often be helpful. Medication compliance is crucial.
It is more or less accepted that there is deterioration of higher brain functions, hypofrontality, as well as so-called dysconnectivity of white matter. There is a genetic vulnerability, and there seems to be an excess of inflammation and changes in mitochondria. Most patients have low functioning, poor compensation, and a lack of social adeptness. However, some patients can recover quite nicely. Although most of us would agree that this is not dementia, we’d also concede that these patients’ cognitive functioning is not what it used to be. Electroconvulsive therapy also can sometimes be helpful.
So, how are we to view our patients with schizophrenia in a way that can be illuminating and give us a deeper sense of understanding this quizzical disorder? It has been helpful to me to regard these individuals as a people whose brain function has been usurped by a more primitive organization that is characterized by:
- a reduction in mental development, where patients function in a more childlike way with magical thinking and impaired reality-testing
- atrophy of higher brain structures, leading to hallucinatory experiences
- a hyper-dominergic state
- a usually gradual onset with some evidence of struggle between the old and new brain organizations
- impaired prepulse inhibition that’s likely secondary to diffuseness of thought
- eventual demise of higher brain structures with an inability to respond to anti-dopaminergics. (Antipsychotics can push the brain organization closer to the adult structure attained before the onset of the disease, at least initially.)
The list goes on. Thinking about patients with schizophrenia in this way allows me to appreciate what I feel is a more encompassing view of who they are and how they got there. I have some theories about where this more primitive organization may have originated, but whatever its origin, in a small percentage of people it is there, ready to assume control of their thinking just as they are reaching reproductive age. Early intervention and medication compliance may minimize damage.
If a theory helps us gain a greater understanding of our patients, then it’s worth considering. This proposition fits much of what we know about schizophrenia. Reading patients’ firsthand accounts of the illness helps confirm, in my opinion, this point of view.
Steven Lesk, MD
Private psychiatric practice
Fridley, Minnesota
Continue to: Cognitive impairment in schizophrenia
Cognitive impairment in schizophrenia
The authors of “Suspicious, sleepless, and smoking” (Cases That Test Your Skills,
During the past 15 years, I have routinely measured cognitive functioning in patients with schizophrenia. Some have no impairment, some have severe impairment, and some fall in between these extremes. Most often, impairment occurs in the area of executive function, which can lead to significant disability. Indeed, positive symptoms can clear up completely with treatment, but the deficits in executive functioning can remain.
I think it is fair to say that cognitive impairment is a common, although not nearly universal, feature of schizophrenia that sometimes improves with antipsychotic medication. I look forward to the advent of more clinicians paying attention to the issue of cognition in schizophrenia and, hopefully, better treatments for it.
John M. Mahoney, PhD
Shasta Psychiatric Hospital
Redding, California
The authors respond
We thank Dr. Mahoney for his thoughtful letter and queries into the case of Mr. F.
First, regarding the prevalence of cognitive impairment in schizophrenia, it is our opinion that cognitive impairment is a distinct, core, and nearly universal feature of schizophrenia. This also is the conclusion of many clinicians and researchers based on their significant work in the field; still, just as in our initial case study, we concede that these symptoms are not part of the DSM-5’s formal diagnostic criteria.
The core question Dr. Mahoney seems to pose is whether we contradicted ourselves. We assert that cognitive impairment in schizophrenia is not effectively treated with existing medications, and yet we described Mr. F’s cognitive improvement after he received risperidone, 2 mg/d, titrated up to 2 mg twice daily. We first pointed out that part of our treatment strategy was to target comorbid depression in this patient; nonetheless, Dr. Mahoney’s question remains valid, and we will attempt to answer.
Dr. Mahoney has observed that his patients with schizophrenia variably experience improved cognition, and notes that executive function is a particularly common lingering impairment. On this we wholly agree; this is a helpful point of clarification, and a useful distinction in light of the above question. Improvement in positive and negative symptoms of schizophrenia, as psychosis resolves, is a well-known and studied effect of antipsychotic therapy. As a result, the sensorium becomes more congruent with external reality, and one would expect the patient to display improved orientation. This then might be reasonably expected to produce mental status improvements; however, while some improvement is frequently observed, this is neither consistent nor complete improvement. In the case of Mr. F, we document improvement, but also significant continued impairment. Thus, we maintain that treating the cognitive symptoms of schizophrenia with antipsychotics has been largely ineffective.
We do not see this as a slight distinction or an argument of minutiae. That patients frequently experience some degree of lingering impairment is a salient point. Neurocognitive impairment is a strong contributor to and predictor of disability in schizophrenia, and neurocognitive abilities most strongly predict functional outcomes. From a patient’s point of view, these symptoms have real-world consequences. Thus, we believe they should be evaluated and treated as aggressively and consistently as other schizophrenia symptoms.
In our case, we attempted to convey one primary message: Despite the challenges of treatment, there are viable options that should be pursued in the treatment of schizophrenia-related cognitive impairments. Nonpharmacologic modalities have shown encouraging results. Cognitive remediation therapy produces durable cognitive improvement—especially when combined with adjunctive therapies, such as small group therapy and vocational rehabilitation, and when comorbid conditions (major depressive disorder in Mr. F’s case) are treated.
In summary, we reiterate that cognitive impairments in schizophrenia represent a strong predictor of patient-oriented outcomes; we maintain our assertion regarding their inadequate treatment with existing medications; and we suggest that future trials attempt to find effective alternative strategies. We encourage psychiatric clinicians to approach treatment of this facet of pathology with an open mind, and to utilize alternative multi-modal therapies for the benefit of their patients with schizophrenia while waiting for new safe and effective pharmaceutical regimens.
Jarrett Dawson, MD
Family medicine resident
Department of Psychiatry
Saint Louis University
St. Louis, Missouri
Catalina Belean, MD
Assistant Professor
Department of Psychiatry
Saint Louis University
St. Louis, Missouri
In his editorial, “The toxic zeitgeist of hyper-partisanship: A psychiatric perspective” (From the Editor,
One’s political views do not inform us of his or her mental health status. This appreciation can be obtained only by a thorough psychological assessment. This is the basis of the Goldwater Rule, coupled with the ethical responsibility not to discuss patients’ private communications.
Today, this rule is tested by the behavior and actions of President Donald Trump. Proponents of the Goldwater Rule state that a psychiatrist cannot diagnose someone without performing a face-to-face diagnostic evaluation. This assumes psychiatrists diagnose patients only by interviewing them. However, any psychiatrist who has worked in an emergency room has signed involuntary commitment papers for a patient who refuses to talk to them. This clinical action typically is based on reports of the patient’s potential dangerousness from family, friends, or the police.
The diagnostic criteria for some personality disorders are based only on observed or reported behavior. They do not indicate a need for an interview. The diagnosis of a personality disorder cannot be made solely by interviewing an individual without knowledge of his or her behavior. Interviewing Bernie Madoff would not have revealed his sociopathic behavior.
The critical question may not be whether one could ethically make a psychiatric diagnosis of the President (I believe you can), but rather would it indicate or imply that he is dangerous? History informs us that the existence of a psychiatric disorder does not determine a politician’s fitness for office or if they are dangerous. Behavioral accounts of President Abraham Lincoln and his self-reports seem to confirm that at times he was depressed, but he clearly served our country with distinction.
Finally, it is not clear whether the Goldwater Rule is legal. It arguably interferes with a psychiatrist’s right of free speech without the risk of being accused of unethical behavior. I wonder what would happen if it were tested in court. Does the First Amendment of the U.S. Constitution protect a psychiatrist’s right to speak freely?
Sidney Weissman, MD
Clinical Professor of Psychiatry and Behavioral Science
Feinberg School of Medicine
Northwestern University
Chicago, Illinois
The current ‘political morass’
Thank you, Dr. Nasrallah, for the wonderful synopsis of the current political morass in your editorial (From the Editor,
James Gallagher, MD
Private psychiatric practice
Des Moines, Iowa
Continue to: The biological etiology of compulsive sexual behavior
The biological etiology of compulsive sexual behavior
Dr. Grant’s article, “Compulsive sexual behavior: A nonjudgmental approach” (Evidence-Based Reviews,
Mukesh Sanghadia, MD, MRCPsych (UK), Diplomate ABPN
PsychiatristCommunity Research Foundation
San Diego, California
The author responds
Dr. Sanghadia highlights the lack of possible biological etiology of compulsive sexual behavior (CSB) in my article. This is a fair comment. The lack of agreed-upon diagnostic criteria, however, has resulted in a vast literature discussing sexual behaviors that may or may not be related to each other, and even suggest that what is currently referred to as CSB may in fact be quite heterogeneous. My article mentions the few neuroimaging and neurocognitive studies that address a more rigorously defined CSB. Other possible etiologies have been suggested for a range of out-of-control sexual behaviors, but have not been studied with a focus on this formal diagnostic category. For example, endocrine issues have been explored to some extent in individuals with paraphilic sexual behaviors (behaviors that appear to many to have no relationship to CSB as discussed in my article), and in those cases of paraphilic sexual behavior, a range of endocrine hormones have been examined—gonadotropin-releasing hormone, follicle-stimulating hormone, luteinizing hormone, testosterone/dihydrotestosterone, and estrogen/progesterone. But these studies have yielded no conclusive outcomes in terms of findings or treatments.
In summary, the biology of CSB lags far behind that of other mental health disorders (and even other psychiatric disorders lack conclusive biological etiologies). Establishing this behavior as a legitimate diagnostic entity with agreed-upon criteria may be the first step in furthering our understanding of its possible biology.
Jon E. Grant, JD, MD, MPH
Professor
Department of Psychiatry and Behavioral Neuroscience
University of Chicago, Pritzker School of Medicine
Chicago, Illinois
Continue to: A different view of patients with schizophrenia
A different view of patients with schizophrenia
After treating patients with schizophrenia for more than 30 years, I’ve observed a continuous flood of information about them. This overload has been consistent since my residency back in the 1980s. Theories ranging from the psychoanalytic to the biologic are numerous and valuable additions to our understanding of those who suffer with this malady, yet they provide no summation or overview with which to understand it.
For instance, we know that schizophrenia usually begins in the late teens or early twenties. We know that antidopaminergic medications usually help to varying degrees. Psychosocial interventions may contribute greatly to the ultimate outcome. Substance use invariably makes it worse. Establishing a connection with the patient can often be helpful. Medication compliance is crucial.
It is more or less accepted that there is deterioration of higher brain functions, hypofrontality, as well as so-called dysconnectivity of white matter. There is a genetic vulnerability, and there seems to be an excess of inflammation and changes in mitochondria. Most patients have low functioning, poor compensation, and a lack of social adeptness. However, some patients can recover quite nicely. Although most of us would agree that this is not dementia, we’d also concede that these patients’ cognitive functioning is not what it used to be. Electroconvulsive therapy also can sometimes be helpful.
So, how are we to view our patients with schizophrenia in a way that can be illuminating and give us a deeper sense of understanding this quizzical disorder? It has been helpful to me to regard these individuals as a people whose brain function has been usurped by a more primitive organization that is characterized by:
- a reduction in mental development, where patients function in a more childlike way with magical thinking and impaired reality-testing
- atrophy of higher brain structures, leading to hallucinatory experiences
- a hyper-dominergic state
- a usually gradual onset with some evidence of struggle between the old and new brain organizations
- impaired prepulse inhibition that’s likely secondary to diffuseness of thought
- eventual demise of higher brain structures with an inability to respond to anti-dopaminergics. (Antipsychotics can push the brain organization closer to the adult structure attained before the onset of the disease, at least initially.)
The list goes on. Thinking about patients with schizophrenia in this way allows me to appreciate what I feel is a more encompassing view of who they are and how they got there. I have some theories about where this more primitive organization may have originated, but whatever its origin, in a small percentage of people it is there, ready to assume control of their thinking just as they are reaching reproductive age. Early intervention and medication compliance may minimize damage.
If a theory helps us gain a greater understanding of our patients, then it’s worth considering. This proposition fits much of what we know about schizophrenia. Reading patients’ firsthand accounts of the illness helps confirm, in my opinion, this point of view.
Steven Lesk, MD
Private psychiatric practice
Fridley, Minnesota
Continue to: Cognitive impairment in schizophrenia
Cognitive impairment in schizophrenia
The authors of “Suspicious, sleepless, and smoking” (Cases That Test Your Skills,
During the past 15 years, I have routinely measured cognitive functioning in patients with schizophrenia. Some have no impairment, some have severe impairment, and some fall in between these extremes. Most often, impairment occurs in the area of executive function, which can lead to significant disability. Indeed, positive symptoms can clear up completely with treatment, but the deficits in executive functioning can remain.
I think it is fair to say that cognitive impairment is a common, although not nearly universal, feature of schizophrenia that sometimes improves with antipsychotic medication. I look forward to the advent of more clinicians paying attention to the issue of cognition in schizophrenia and, hopefully, better treatments for it.
John M. Mahoney, PhD
Shasta Psychiatric Hospital
Redding, California
The authors respond
We thank Dr. Mahoney for his thoughtful letter and queries into the case of Mr. F.
First, regarding the prevalence of cognitive impairment in schizophrenia, it is our opinion that cognitive impairment is a distinct, core, and nearly universal feature of schizophrenia. This also is the conclusion of many clinicians and researchers based on their significant work in the field; still, just as in our initial case study, we concede that these symptoms are not part of the DSM-5’s formal diagnostic criteria.
The core question Dr. Mahoney seems to pose is whether we contradicted ourselves. We assert that cognitive impairment in schizophrenia is not effectively treated with existing medications, and yet we described Mr. F’s cognitive improvement after he received risperidone, 2 mg/d, titrated up to 2 mg twice daily. We first pointed out that part of our treatment strategy was to target comorbid depression in this patient; nonetheless, Dr. Mahoney’s question remains valid, and we will attempt to answer.
Dr. Mahoney has observed that his patients with schizophrenia variably experience improved cognition, and notes that executive function is a particularly common lingering impairment. On this we wholly agree; this is a helpful point of clarification, and a useful distinction in light of the above question. Improvement in positive and negative symptoms of schizophrenia, as psychosis resolves, is a well-known and studied effect of antipsychotic therapy. As a result, the sensorium becomes more congruent with external reality, and one would expect the patient to display improved orientation. This then might be reasonably expected to produce mental status improvements; however, while some improvement is frequently observed, this is neither consistent nor complete improvement. In the case of Mr. F, we document improvement, but also significant continued impairment. Thus, we maintain that treating the cognitive symptoms of schizophrenia with antipsychotics has been largely ineffective.
We do not see this as a slight distinction or an argument of minutiae. That patients frequently experience some degree of lingering impairment is a salient point. Neurocognitive impairment is a strong contributor to and predictor of disability in schizophrenia, and neurocognitive abilities most strongly predict functional outcomes. From a patient’s point of view, these symptoms have real-world consequences. Thus, we believe they should be evaluated and treated as aggressively and consistently as other schizophrenia symptoms.
In our case, we attempted to convey one primary message: Despite the challenges of treatment, there are viable options that should be pursued in the treatment of schizophrenia-related cognitive impairments. Nonpharmacologic modalities have shown encouraging results. Cognitive remediation therapy produces durable cognitive improvement—especially when combined with adjunctive therapies, such as small group therapy and vocational rehabilitation, and when comorbid conditions (major depressive disorder in Mr. F’s case) are treated.
In summary, we reiterate that cognitive impairments in schizophrenia represent a strong predictor of patient-oriented outcomes; we maintain our assertion regarding their inadequate treatment with existing medications; and we suggest that future trials attempt to find effective alternative strategies. We encourage psychiatric clinicians to approach treatment of this facet of pathology with an open mind, and to utilize alternative multi-modal therapies for the benefit of their patients with schizophrenia while waiting for new safe and effective pharmaceutical regimens.
Jarrett Dawson, MD
Family medicine resident
Department of Psychiatry
Saint Louis University
St. Louis, Missouri
Catalina Belean, MD
Assistant Professor
Department of Psychiatry
Saint Louis University
St. Louis, Missouri
In his editorial, “The toxic zeitgeist of hyper-partisanship: A psychiatric perspective” (From the Editor,
One’s political views do not inform us of his or her mental health status. This appreciation can be obtained only by a thorough psychological assessment. This is the basis of the Goldwater Rule, coupled with the ethical responsibility not to discuss patients’ private communications.
Today, this rule is tested by the behavior and actions of President Donald Trump. Proponents of the Goldwater Rule state that a psychiatrist cannot diagnose someone without performing a face-to-face diagnostic evaluation. This assumes psychiatrists diagnose patients only by interviewing them. However, any psychiatrist who has worked in an emergency room has signed involuntary commitment papers for a patient who refuses to talk to them. This clinical action typically is based on reports of the patient’s potential dangerousness from family, friends, or the police.
The diagnostic criteria for some personality disorders are based only on observed or reported behavior. They do not indicate a need for an interview. The diagnosis of a personality disorder cannot be made solely by interviewing an individual without knowledge of his or her behavior. Interviewing Bernie Madoff would not have revealed his sociopathic behavior.
The critical question may not be whether one could ethically make a psychiatric diagnosis of the President (I believe you can), but rather would it indicate or imply that he is dangerous? History informs us that the existence of a psychiatric disorder does not determine a politician’s fitness for office or if they are dangerous. Behavioral accounts of President Abraham Lincoln and his self-reports seem to confirm that at times he was depressed, but he clearly served our country with distinction.
Finally, it is not clear whether the Goldwater Rule is legal. It arguably interferes with a psychiatrist’s right of free speech without the risk of being accused of unethical behavior. I wonder what would happen if it were tested in court. Does the First Amendment of the U.S. Constitution protect a psychiatrist’s right to speak freely?
Sidney Weissman, MD
Clinical Professor of Psychiatry and Behavioral Science
Feinberg School of Medicine
Northwestern University
Chicago, Illinois
The current ‘political morass’
Thank you, Dr. Nasrallah, for the wonderful synopsis of the current political morass in your editorial (From the Editor,
James Gallagher, MD
Private psychiatric practice
Des Moines, Iowa
Continue to: The biological etiology of compulsive sexual behavior
The biological etiology of compulsive sexual behavior
Dr. Grant’s article, “Compulsive sexual behavior: A nonjudgmental approach” (Evidence-Based Reviews,
Mukesh Sanghadia, MD, MRCPsych (UK), Diplomate ABPN
PsychiatristCommunity Research Foundation
San Diego, California
The author responds
Dr. Sanghadia highlights the lack of possible biological etiology of compulsive sexual behavior (CSB) in my article. This is a fair comment. The lack of agreed-upon diagnostic criteria, however, has resulted in a vast literature discussing sexual behaviors that may or may not be related to each other, and even suggest that what is currently referred to as CSB may in fact be quite heterogeneous. My article mentions the few neuroimaging and neurocognitive studies that address a more rigorously defined CSB. Other possible etiologies have been suggested for a range of out-of-control sexual behaviors, but have not been studied with a focus on this formal diagnostic category. For example, endocrine issues have been explored to some extent in individuals with paraphilic sexual behaviors (behaviors that appear to many to have no relationship to CSB as discussed in my article), and in those cases of paraphilic sexual behavior, a range of endocrine hormones have been examined—gonadotropin-releasing hormone, follicle-stimulating hormone, luteinizing hormone, testosterone/dihydrotestosterone, and estrogen/progesterone. But these studies have yielded no conclusive outcomes in terms of findings or treatments.
In summary, the biology of CSB lags far behind that of other mental health disorders (and even other psychiatric disorders lack conclusive biological etiologies). Establishing this behavior as a legitimate diagnostic entity with agreed-upon criteria may be the first step in furthering our understanding of its possible biology.
Jon E. Grant, JD, MD, MPH
Professor
Department of Psychiatry and Behavioral Neuroscience
University of Chicago, Pritzker School of Medicine
Chicago, Illinois
Continue to: A different view of patients with schizophrenia
A different view of patients with schizophrenia
After treating patients with schizophrenia for more than 30 years, I’ve observed a continuous flood of information about them. This overload has been consistent since my residency back in the 1980s. Theories ranging from the psychoanalytic to the biologic are numerous and valuable additions to our understanding of those who suffer with this malady, yet they provide no summation or overview with which to understand it.
For instance, we know that schizophrenia usually begins in the late teens or early twenties. We know that antidopaminergic medications usually help to varying degrees. Psychosocial interventions may contribute greatly to the ultimate outcome. Substance use invariably makes it worse. Establishing a connection with the patient can often be helpful. Medication compliance is crucial.
It is more or less accepted that there is deterioration of higher brain functions, hypofrontality, as well as so-called dysconnectivity of white matter. There is a genetic vulnerability, and there seems to be an excess of inflammation and changes in mitochondria. Most patients have low functioning, poor compensation, and a lack of social adeptness. However, some patients can recover quite nicely. Although most of us would agree that this is not dementia, we’d also concede that these patients’ cognitive functioning is not what it used to be. Electroconvulsive therapy also can sometimes be helpful.
So, how are we to view our patients with schizophrenia in a way that can be illuminating and give us a deeper sense of understanding this quizzical disorder? It has been helpful to me to regard these individuals as a people whose brain function has been usurped by a more primitive organization that is characterized by:
- a reduction in mental development, where patients function in a more childlike way with magical thinking and impaired reality-testing
- atrophy of higher brain structures, leading to hallucinatory experiences
- a hyper-dominergic state
- a usually gradual onset with some evidence of struggle between the old and new brain organizations
- impaired prepulse inhibition that’s likely secondary to diffuseness of thought
- eventual demise of higher brain structures with an inability to respond to anti-dopaminergics. (Antipsychotics can push the brain organization closer to the adult structure attained before the onset of the disease, at least initially.)
The list goes on. Thinking about patients with schizophrenia in this way allows me to appreciate what I feel is a more encompassing view of who they are and how they got there. I have some theories about where this more primitive organization may have originated, but whatever its origin, in a small percentage of people it is there, ready to assume control of their thinking just as they are reaching reproductive age. Early intervention and medication compliance may minimize damage.
If a theory helps us gain a greater understanding of our patients, then it’s worth considering. This proposition fits much of what we know about schizophrenia. Reading patients’ firsthand accounts of the illness helps confirm, in my opinion, this point of view.
Steven Lesk, MD
Private psychiatric practice
Fridley, Minnesota
Continue to: Cognitive impairment in schizophrenia
Cognitive impairment in schizophrenia
The authors of “Suspicious, sleepless, and smoking” (Cases That Test Your Skills,
During the past 15 years, I have routinely measured cognitive functioning in patients with schizophrenia. Some have no impairment, some have severe impairment, and some fall in between these extremes. Most often, impairment occurs in the area of executive function, which can lead to significant disability. Indeed, positive symptoms can clear up completely with treatment, but the deficits in executive functioning can remain.
I think it is fair to say that cognitive impairment is a common, although not nearly universal, feature of schizophrenia that sometimes improves with antipsychotic medication. I look forward to the advent of more clinicians paying attention to the issue of cognition in schizophrenia and, hopefully, better treatments for it.
John M. Mahoney, PhD
Shasta Psychiatric Hospital
Redding, California
The authors respond
We thank Dr. Mahoney for his thoughtful letter and queries into the case of Mr. F.
First, regarding the prevalence of cognitive impairment in schizophrenia, it is our opinion that cognitive impairment is a distinct, core, and nearly universal feature of schizophrenia. This also is the conclusion of many clinicians and researchers based on their significant work in the field; still, just as in our initial case study, we concede that these symptoms are not part of the DSM-5’s formal diagnostic criteria.
The core question Dr. Mahoney seems to pose is whether we contradicted ourselves. We assert that cognitive impairment in schizophrenia is not effectively treated with existing medications, and yet we described Mr. F’s cognitive improvement after he received risperidone, 2 mg/d, titrated up to 2 mg twice daily. We first pointed out that part of our treatment strategy was to target comorbid depression in this patient; nonetheless, Dr. Mahoney’s question remains valid, and we will attempt to answer.
Dr. Mahoney has observed that his patients with schizophrenia variably experience improved cognition, and notes that executive function is a particularly common lingering impairment. On this we wholly agree; this is a helpful point of clarification, and a useful distinction in light of the above question. Improvement in positive and negative symptoms of schizophrenia, as psychosis resolves, is a well-known and studied effect of antipsychotic therapy. As a result, the sensorium becomes more congruent with external reality, and one would expect the patient to display improved orientation. This then might be reasonably expected to produce mental status improvements; however, while some improvement is frequently observed, this is neither consistent nor complete improvement. In the case of Mr. F, we document improvement, but also significant continued impairment. Thus, we maintain that treating the cognitive symptoms of schizophrenia with antipsychotics has been largely ineffective.
We do not see this as a slight distinction or an argument of minutiae. That patients frequently experience some degree of lingering impairment is a salient point. Neurocognitive impairment is a strong contributor to and predictor of disability in schizophrenia, and neurocognitive abilities most strongly predict functional outcomes. From a patient’s point of view, these symptoms have real-world consequences. Thus, we believe they should be evaluated and treated as aggressively and consistently as other schizophrenia symptoms.
In our case, we attempted to convey one primary message: Despite the challenges of treatment, there are viable options that should be pursued in the treatment of schizophrenia-related cognitive impairments. Nonpharmacologic modalities have shown encouraging results. Cognitive remediation therapy produces durable cognitive improvement—especially when combined with adjunctive therapies, such as small group therapy and vocational rehabilitation, and when comorbid conditions (major depressive disorder in Mr. F’s case) are treated.
In summary, we reiterate that cognitive impairments in schizophrenia represent a strong predictor of patient-oriented outcomes; we maintain our assertion regarding their inadequate treatment with existing medications; and we suggest that future trials attempt to find effective alternative strategies. We encourage psychiatric clinicians to approach treatment of this facet of pathology with an open mind, and to utilize alternative multi-modal therapies for the benefit of their patients with schizophrenia while waiting for new safe and effective pharmaceutical regimens.
Jarrett Dawson, MD
Family medicine resident
Department of Psychiatry
Saint Louis University
St. Louis, Missouri
Catalina Belean, MD
Assistant Professor
Department of Psychiatry
Saint Louis University
St. Louis, Missouri
Aggressive outbursts and emotional lability in a 16-year-old boy
Mr. X, age 16, has cerebral palsy (CP), idiopathic normal pressure hydrocephalus (iNPH), and a history of impulse control disorder and behavioral instability, including episodes of aggression or combativeness. Mr. X’s mother reports that these episodes are almost always preceded by inappropriate laughing or crying. His outbursts and emotional lability have gotten worse during the last 6 months. Due to his disruptive behaviors, Mr. X has been unable to attend school, and his parents are considering group home placement. Although they were previously able to control their son’s aggressive behaviors, they fear for his safety, and after one such episode, they call 911. Mr. X is transported by police in handcuffs to the comprehensive psychiatric emergency room (CPEP) for evaluation.
While in CPEP, Mr. X remains uncooperative and disruptive; subsequently, he is placed in 4-point restraints and given
[polldaddy:9991896]
The authors’ observations
Pseudobulbar affect (PBA) is a disorder characterized by sporadic episodes of inappropriate laughing and/or crying that are incongruent with situational context and are frequently exaggerated in comparison with the actual feelings of the patient. The duration of PBA episodes can last seconds to minutes and arise unpredictably.
PBA typically develops secondary to a neurologic disorder, most commonly Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), Parkinson’s disease (PD), stroke, or traumatic brain injury (TBI).1 PBA symptoms are present in an estimated 29.3% of patients with AD, 44.8% of patients with ALS, 45.8% of patients with MS, 26% of patients with PD, 37.8% of patients with stroke, and 52.4% of patients with TBI.2 Although PBA appears far more frequently in patients with MS or ALS compared with those with PD, PD represents an under-recognized and larger patient population. A small fraction of patients also develops PBA secondary to hyperthyroidism, hypothyroidism, Graves’ disease, Wilson’s disease, brain tumors, and a multitude of encephalopathies.3 These neurologic disorders cause dysregulation of the corticopontine-cerebellar circuitry, resulting in functional impediment to the normal affect modulator action of the cerebellum.4
The neurologic insults that can result in PBA may include CP or iNPH. Cerebellar injury is a frequent pathological finding in CP.5 In patients with iNPH, in addition to altered CSF flow, enlarged ventricles compress the corticospinal tracts in the lateral ventricles,6 which is theorized to induce PBA symptoms.
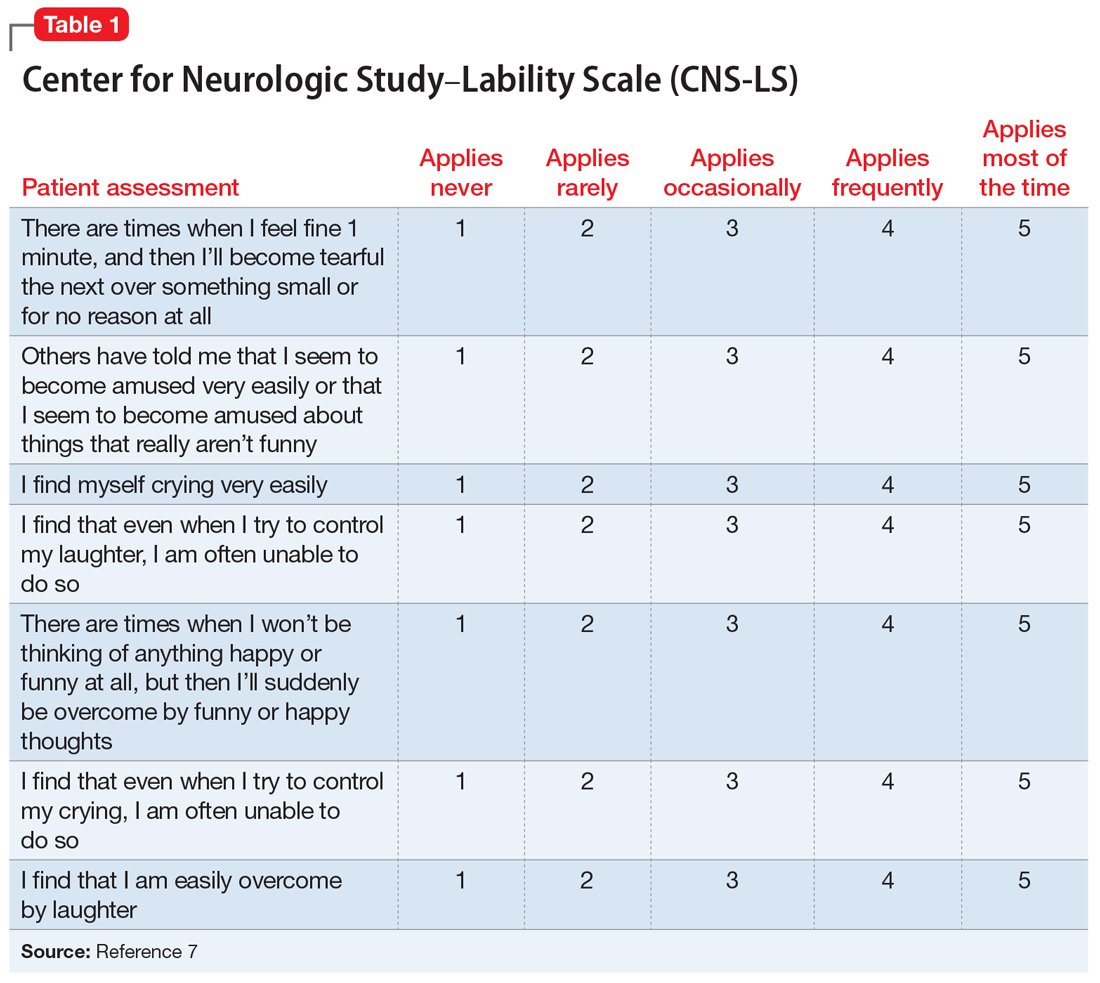
PBA is diagnosed by subjective clinical evaluation and by using the Center for Neurologic Study–Lability Scale (CNS-LS). The CNS-LS is a 7-question survey that addresses the severity of affect lability (Table 17). It may be completed by the patient or caregiver. Each question ranges in score from 1 to 5, with the total score ranging from 7 to 35. The minimum score required for the diagnosis of PBA is 13.7
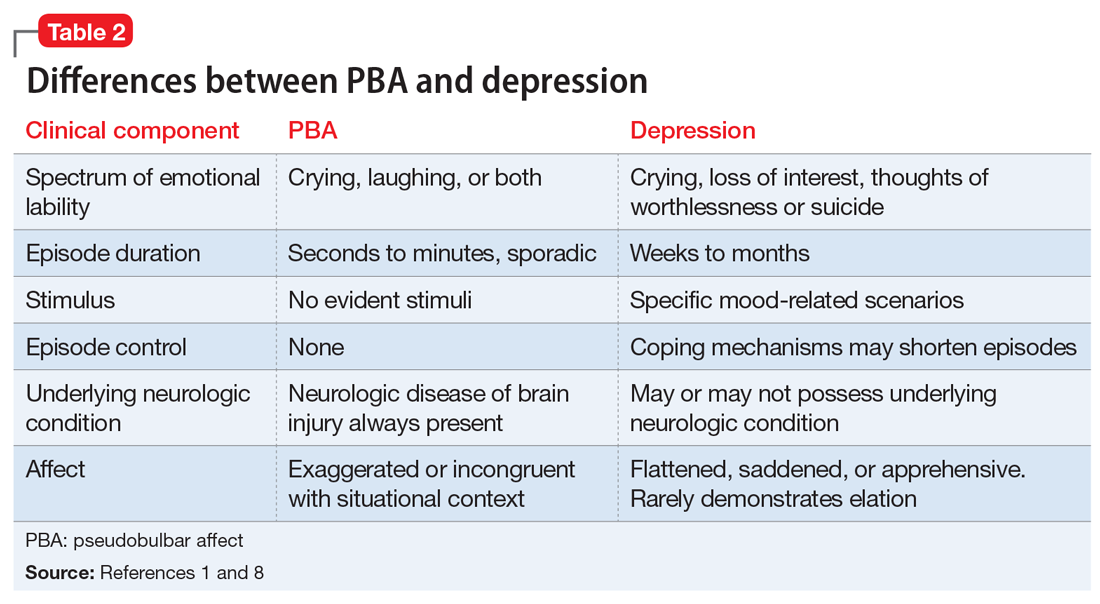
PBA is frequently misdiagnosed as depression, although the 2 disorders can occur simultaneously (Table 21,8). A crucial distinguishing factor between depression and PBA is the extent of symptoms. Depression presents as feelings of sadness associated with crying and disinterest that occur for weeks to months. In contrast, PBA presents as brief, uncontrollable episodes of laughing and/or crying that last seconds to minutes. Unlike depression, the behaviors associated with PBA are exaggerated or do not match the patient’s feelings. Furthermore, a neurologic disease or brain injury is always present in a patient with PBA, but is not imperative for the diagnosis of depression.
Continue to: Compared with individuals without PBA...
Compared with individuals without PBA, patients with PBA also experience more distress, embarrassment, and social disability, and are consequently more likely to suffer from other psychiatric conditions, including depression, anxiety/panic attacks, bipolar disorder, posttraumatic stress disorder, psychotic disorder, and schizophrenia.1 The Patient Health Questionnaire (PHQ-9), a tool for measuring depression severity, can be used in addition to the CNS-LS to determine if the patient has both depression and PBA.
HISTORY Poor response to anxiolytics and antipsychotics
Mr. X previously received a ventriculoperitoneal shunt for treating iNPH. He was not taking any medications for CP. To address his impulse control disorder, he was prescribed olanzapine, 20 mg/d, risperidone, 2 mg/d, and diazepam, 5 mg three times a day. Mr. X is uncontrolled on these medications, experiencing frequent behavioral outbursts at home. His mother completes a CNS-LS for him. He receives a score of 20, which suggests a diagnosis of PBA. His PHQ-9 score is 8, indicating mild depression.
[polldaddy:9991899]
TREATMENT Introducing a new medication
Mr. X is started on
Continue to: The authors' observations
The authors’ observations
Decreasing the severity and frequency of episodes constitutes the mainstay of treating PBA. In the past, off-label treatments, including selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants, were prescribed to reduce PBA symptoms.5 Currently, dextromethorphan/quinidine is the only FDA-approved medication for treating PBA; however, its use in patients younger than age 18 is considered investigational.
Atypical antipsychotics, such as olanzapine and risperidone, have more warnings and precautions than dextromethorphan/quinidine. Risperidone has a “black-box” warning for QT prolongation, in addition to death and stroke in elderly patients.10 Although dextromethorphan/quinidine does not have a black-box warning, it does increase the risk of QT prolongation, and patients with cardiac risk factors should undergo an electrocardiogram before starting this medication. Additionally, risperidone and olanzapine are known to cause significant weight gain, which can increase the risk of developing hyperlipidemia, metabolic syndrome, and type 2 diabetes mellitus.10,11 Neuroleptic malignant syndrome (NMS) is a potentially life-threatening adverse effect of all antipsychotics. NMS is characterized by fever, rigidity, altered consciousness, and increased heart and respiratory rates.12
Quinidine increases the bioavailability of dextromethorphan by inhibiting CYP2D6. When dextromethorphan/quinidine is simultaneously used with an SSRI that also inhibits CYP2D6, such as paroxetine or fluoxetine, the patient may be at increased risk for developing adverse effects such as respiratory depression and serotonin syndrome.13
[polldaddy:9991902]
Continue to: The authors' observations
The authors’ observations
Although the exact pathophysiology of PBA is unknown, multiple theories may explain the principle elements of the condition. In the absence of a neurologic insult, the cerebellum acts as an affect regulator, inhibiting laughter and crying at times in which they are considered inappropriate. Parvizi et al4 have theorized that the lesions involved in PBA disrupt the corticopontine-cerebellar circuitry, which impedes the ability of the cerebellum to function as an affect modulator.3 In addition to the dysregulation of cerebellar circuitry, altered serotonin and glutamate levels are believed to contribute to the deficient affect regulation observed in PBA; therefore, adding dextromethorphan/quinidine potentiates serotonin and glutamate levels in the synaptic cleft, resulting in a reduction in PBA episodes.4
OUTCOME Affect stability
Seven months after beginning dextromethorphan/quinidine, Mr. X has experienced resolution of his PBA episodes. His PHQ-9 score was reduced to 0 (no clinical signs of depression) within 1 month of starting this medication and his PHQ-9 scores remain below 5, representing minimal depressive severity. The CNS-LS scale is not conducted at further visits because the patient’s mother reported no further PBA episodes. Mr. X no longer exhibits episodes of aggression. These episodes seemed to have been a manifestation of his frustration and difficulty in controlling his PBA episodes. Furthermore, his dosage of diazepam was reduced, and he was weaned off risperidone. Mr. X’s parents report that he has a drastically improved affect. He continues to tolerate his medication well and no longer demonstrates any exacerbations of his psychiatric symptoms.
Bottom Line
Pseudobulbar affect (PBA) may occur secondary to various neurologic insults, including cerebral palsy and idiopathic normal pressure hydrocephalus. The condition is diagnosed by a subjective clinical evaluation and use of the Center for Neurologic Study–Lability Scale. Dextromethorphan/quinidine can significantly reduce PBA symptoms.
Acknowledgements
The authors thank Anthony S. Graziano and Rachel M. Watt, both Physician Assistant students, Daemen College, Amherst, New York.
Related Resources
- Frock B, Williams A, Caplan JP. Pseudobulbar affect: when patients laugh or cry, but don’t know why. Current Psychiatry. 2016;15(9):56-60,63.
- Crumpacker DW. Enhancing approaches to the identification and management of pseudobulbar affect. J Clin Psychiatry. 2016;77(9):e1155.
Drug Brand Names
Dextromethorphan/quinidine • Nuedexta
Diazepam • Valium
Fluoxetine • Prozac
Haloperidol • Haldol
Lorazepam • Ativan
Olanzapine • Zyprexa
Paroxetine • Paxil
Risperidone • Risperdal
1. Colamonico J, Formella A, Bradley W. Pseudobulbar affect: burden of illness in the USA. Adv Ther. 2012;29(9):775-798.
2. Brooks BR, Crumpacker D, Fellus J, et al. PRISM: a novel research tool to assess the prevalence of pseudobulbar affect symptoms across neurological conditions. PLoS One. 2013;8(8):e72232. doi: 10.1371/journal.pone.0072232.
3. Schiffer R, Pope LE. Review of pseudobulbar affect including a novel and potential therapy. J Neuropsychiatry Clin Neurosci. 2005;17(4):447-454.
4. Parvizi J, Anderson SW, Martin CO, et al. Pathological laughter and crying: a link to the cerebellum. Brain. 2001;124(pt 9):1708-1719.
5. Johnsen SD, Bodensteiner JB, Lotze TE. Frequency and nature of cerebellar injury in the extremely premature survivor with cerebral palsy. J Child Neurol. 2005;20(1):60-64.
6. Kamiya K, Hori M, Miyajima M, et al. Axon diameter and intra-axonal volume fraction of the corticospinal tract in idiopathic normal pressure hydrocephalus measured by Q-Space imaging. PLoS One. 2014;9(8):e103842. doi: 10.1371/journal.pone.0103842.
7. Moore SR, Gresham LS, Bromberg MB, et al. A self report measuredextromethorphan of affective lability. J Neurol Neurosurg Psychiatry. 1997;63(1):89-93.
8. Ahmed A, Simmons Z. Pseudobulbar affect: prevalence and management. Ther Clinical Risk Manag. 2013;9:483-489.
9. Cruz MP. Nuedexta for the treatment of pseudobulbar affect. A condition of involuntary crying or laughing. P T. 2013;38(6):325-328.
10. Goëb JL, Marco S, Duhamel A, et al. Metabolic side effects of risperidone in children and adolescents with early onset schizophrenia. Prim Care Companion J Clin Psychiatry. 2008;10(6):486-487.
11. Nemeroff CB. Dosing the antipsychotic medication olanzapine. J Clin Psychiatry. 1997;58(suppl 10):45-49.
12. Troller JN, Chen X, Sachdev PS. Neuroleptic malignant syndrome associated with atypical antipsychotic drugs. CNS Drugs. 2009;23(6):477-492.
13. Schoedel KA, Pope LE, Sellers EM. Randomized open-label drug-drug interaction trial of dextromethorphan/quinidine and paroxetine in healthy volunteers. Clin Drug Investig. 2012;32(3):157-169.
Mr. X, age 16, has cerebral palsy (CP), idiopathic normal pressure hydrocephalus (iNPH), and a history of impulse control disorder and behavioral instability, including episodes of aggression or combativeness. Mr. X’s mother reports that these episodes are almost always preceded by inappropriate laughing or crying. His outbursts and emotional lability have gotten worse during the last 6 months. Due to his disruptive behaviors, Mr. X has been unable to attend school, and his parents are considering group home placement. Although they were previously able to control their son’s aggressive behaviors, they fear for his safety, and after one such episode, they call 911. Mr. X is transported by police in handcuffs to the comprehensive psychiatric emergency room (CPEP) for evaluation.
While in CPEP, Mr. X remains uncooperative and disruptive; subsequently, he is placed in 4-point restraints and given
[polldaddy:9991896]
The authors’ observations
Pseudobulbar affect (PBA) is a disorder characterized by sporadic episodes of inappropriate laughing and/or crying that are incongruent with situational context and are frequently exaggerated in comparison with the actual feelings of the patient. The duration of PBA episodes can last seconds to minutes and arise unpredictably.
PBA typically develops secondary to a neurologic disorder, most commonly Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), Parkinson’s disease (PD), stroke, or traumatic brain injury (TBI).1 PBA symptoms are present in an estimated 29.3% of patients with AD, 44.8% of patients with ALS, 45.8% of patients with MS, 26% of patients with PD, 37.8% of patients with stroke, and 52.4% of patients with TBI.2 Although PBA appears far more frequently in patients with MS or ALS compared with those with PD, PD represents an under-recognized and larger patient population. A small fraction of patients also develops PBA secondary to hyperthyroidism, hypothyroidism, Graves’ disease, Wilson’s disease, brain tumors, and a multitude of encephalopathies.3 These neurologic disorders cause dysregulation of the corticopontine-cerebellar circuitry, resulting in functional impediment to the normal affect modulator action of the cerebellum.4
The neurologic insults that can result in PBA may include CP or iNPH. Cerebellar injury is a frequent pathological finding in CP.5 In patients with iNPH, in addition to altered CSF flow, enlarged ventricles compress the corticospinal tracts in the lateral ventricles,6 which is theorized to induce PBA symptoms.
PBA is diagnosed by subjective clinical evaluation and by using the Center for Neurologic Study–Lability Scale (CNS-LS). The CNS-LS is a 7-question survey that addresses the severity of affect lability (Table 17). It may be completed by the patient or caregiver. Each question ranges in score from 1 to 5, with the total score ranging from 7 to 35. The minimum score required for the diagnosis of PBA is 13.7
PBA is frequently misdiagnosed as depression, although the 2 disorders can occur simultaneously (Table 21,8). A crucial distinguishing factor between depression and PBA is the extent of symptoms. Depression presents as feelings of sadness associated with crying and disinterest that occur for weeks to months. In contrast, PBA presents as brief, uncontrollable episodes of laughing and/or crying that last seconds to minutes. Unlike depression, the behaviors associated with PBA are exaggerated or do not match the patient’s feelings. Furthermore, a neurologic disease or brain injury is always present in a patient with PBA, but is not imperative for the diagnosis of depression.
Continue to: Compared with individuals without PBA...
Compared with individuals without PBA, patients with PBA also experience more distress, embarrassment, and social disability, and are consequently more likely to suffer from other psychiatric conditions, including depression, anxiety/panic attacks, bipolar disorder, posttraumatic stress disorder, psychotic disorder, and schizophrenia.1 The Patient Health Questionnaire (PHQ-9), a tool for measuring depression severity, can be used in addition to the CNS-LS to determine if the patient has both depression and PBA.
HISTORY Poor response to anxiolytics and antipsychotics
Mr. X previously received a ventriculoperitoneal shunt for treating iNPH. He was not taking any medications for CP. To address his impulse control disorder, he was prescribed olanzapine, 20 mg/d, risperidone, 2 mg/d, and diazepam, 5 mg three times a day. Mr. X is uncontrolled on these medications, experiencing frequent behavioral outbursts at home. His mother completes a CNS-LS for him. He receives a score of 20, which suggests a diagnosis of PBA. His PHQ-9 score is 8, indicating mild depression.
[polldaddy:9991899]
TREATMENT Introducing a new medication
Mr. X is started on
Continue to: The authors' observations
The authors’ observations
Decreasing the severity and frequency of episodes constitutes the mainstay of treating PBA. In the past, off-label treatments, including selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants, were prescribed to reduce PBA symptoms.5 Currently, dextromethorphan/quinidine is the only FDA-approved medication for treating PBA; however, its use in patients younger than age 18 is considered investigational.
Atypical antipsychotics, such as olanzapine and risperidone, have more warnings and precautions than dextromethorphan/quinidine. Risperidone has a “black-box” warning for QT prolongation, in addition to death and stroke in elderly patients.10 Although dextromethorphan/quinidine does not have a black-box warning, it does increase the risk of QT prolongation, and patients with cardiac risk factors should undergo an electrocardiogram before starting this medication. Additionally, risperidone and olanzapine are known to cause significant weight gain, which can increase the risk of developing hyperlipidemia, metabolic syndrome, and type 2 diabetes mellitus.10,11 Neuroleptic malignant syndrome (NMS) is a potentially life-threatening adverse effect of all antipsychotics. NMS is characterized by fever, rigidity, altered consciousness, and increased heart and respiratory rates.12
Quinidine increases the bioavailability of dextromethorphan by inhibiting CYP2D6. When dextromethorphan/quinidine is simultaneously used with an SSRI that also inhibits CYP2D6, such as paroxetine or fluoxetine, the patient may be at increased risk for developing adverse effects such as respiratory depression and serotonin syndrome.13
[polldaddy:9991902]
Continue to: The authors' observations
The authors’ observations
Although the exact pathophysiology of PBA is unknown, multiple theories may explain the principle elements of the condition. In the absence of a neurologic insult, the cerebellum acts as an affect regulator, inhibiting laughter and crying at times in which they are considered inappropriate. Parvizi et al4 have theorized that the lesions involved in PBA disrupt the corticopontine-cerebellar circuitry, which impedes the ability of the cerebellum to function as an affect modulator.3 In addition to the dysregulation of cerebellar circuitry, altered serotonin and glutamate levels are believed to contribute to the deficient affect regulation observed in PBA; therefore, adding dextromethorphan/quinidine potentiates serotonin and glutamate levels in the synaptic cleft, resulting in a reduction in PBA episodes.4
OUTCOME Affect stability
Seven months after beginning dextromethorphan/quinidine, Mr. X has experienced resolution of his PBA episodes. His PHQ-9 score was reduced to 0 (no clinical signs of depression) within 1 month of starting this medication and his PHQ-9 scores remain below 5, representing minimal depressive severity. The CNS-LS scale is not conducted at further visits because the patient’s mother reported no further PBA episodes. Mr. X no longer exhibits episodes of aggression. These episodes seemed to have been a manifestation of his frustration and difficulty in controlling his PBA episodes. Furthermore, his dosage of diazepam was reduced, and he was weaned off risperidone. Mr. X’s parents report that he has a drastically improved affect. He continues to tolerate his medication well and no longer demonstrates any exacerbations of his psychiatric symptoms.
Bottom Line
Pseudobulbar affect (PBA) may occur secondary to various neurologic insults, including cerebral palsy and idiopathic normal pressure hydrocephalus. The condition is diagnosed by a subjective clinical evaluation and use of the Center for Neurologic Study–Lability Scale. Dextromethorphan/quinidine can significantly reduce PBA symptoms.
Acknowledgements
The authors thank Anthony S. Graziano and Rachel M. Watt, both Physician Assistant students, Daemen College, Amherst, New York.
Related Resources
- Frock B, Williams A, Caplan JP. Pseudobulbar affect: when patients laugh or cry, but don’t know why. Current Psychiatry. 2016;15(9):56-60,63.
- Crumpacker DW. Enhancing approaches to the identification and management of pseudobulbar affect. J Clin Psychiatry. 2016;77(9):e1155.
Drug Brand Names
Dextromethorphan/quinidine • Nuedexta
Diazepam • Valium
Fluoxetine • Prozac
Haloperidol • Haldol
Lorazepam • Ativan
Olanzapine • Zyprexa
Paroxetine • Paxil
Risperidone • Risperdal
Mr. X, age 16, has cerebral palsy (CP), idiopathic normal pressure hydrocephalus (iNPH), and a history of impulse control disorder and behavioral instability, including episodes of aggression or combativeness. Mr. X’s mother reports that these episodes are almost always preceded by inappropriate laughing or crying. His outbursts and emotional lability have gotten worse during the last 6 months. Due to his disruptive behaviors, Mr. X has been unable to attend school, and his parents are considering group home placement. Although they were previously able to control their son’s aggressive behaviors, they fear for his safety, and after one such episode, they call 911. Mr. X is transported by police in handcuffs to the comprehensive psychiatric emergency room (CPEP) for evaluation.
While in CPEP, Mr. X remains uncooperative and disruptive; subsequently, he is placed in 4-point restraints and given
[polldaddy:9991896]
The authors’ observations
Pseudobulbar affect (PBA) is a disorder characterized by sporadic episodes of inappropriate laughing and/or crying that are incongruent with situational context and are frequently exaggerated in comparison with the actual feelings of the patient. The duration of PBA episodes can last seconds to minutes and arise unpredictably.
PBA typically develops secondary to a neurologic disorder, most commonly Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), Parkinson’s disease (PD), stroke, or traumatic brain injury (TBI).1 PBA symptoms are present in an estimated 29.3% of patients with AD, 44.8% of patients with ALS, 45.8% of patients with MS, 26% of patients with PD, 37.8% of patients with stroke, and 52.4% of patients with TBI.2 Although PBA appears far more frequently in patients with MS or ALS compared with those with PD, PD represents an under-recognized and larger patient population. A small fraction of patients also develops PBA secondary to hyperthyroidism, hypothyroidism, Graves’ disease, Wilson’s disease, brain tumors, and a multitude of encephalopathies.3 These neurologic disorders cause dysregulation of the corticopontine-cerebellar circuitry, resulting in functional impediment to the normal affect modulator action of the cerebellum.4
The neurologic insults that can result in PBA may include CP or iNPH. Cerebellar injury is a frequent pathological finding in CP.5 In patients with iNPH, in addition to altered CSF flow, enlarged ventricles compress the corticospinal tracts in the lateral ventricles,6 which is theorized to induce PBA symptoms.
PBA is diagnosed by subjective clinical evaluation and by using the Center for Neurologic Study–Lability Scale (CNS-LS). The CNS-LS is a 7-question survey that addresses the severity of affect lability (Table 17). It may be completed by the patient or caregiver. Each question ranges in score from 1 to 5, with the total score ranging from 7 to 35. The minimum score required for the diagnosis of PBA is 13.7
PBA is frequently misdiagnosed as depression, although the 2 disorders can occur simultaneously (Table 21,8). A crucial distinguishing factor between depression and PBA is the extent of symptoms. Depression presents as feelings of sadness associated with crying and disinterest that occur for weeks to months. In contrast, PBA presents as brief, uncontrollable episodes of laughing and/or crying that last seconds to minutes. Unlike depression, the behaviors associated with PBA are exaggerated or do not match the patient’s feelings. Furthermore, a neurologic disease or brain injury is always present in a patient with PBA, but is not imperative for the diagnosis of depression.
Continue to: Compared with individuals without PBA...
Compared with individuals without PBA, patients with PBA also experience more distress, embarrassment, and social disability, and are consequently more likely to suffer from other psychiatric conditions, including depression, anxiety/panic attacks, bipolar disorder, posttraumatic stress disorder, psychotic disorder, and schizophrenia.1 The Patient Health Questionnaire (PHQ-9), a tool for measuring depression severity, can be used in addition to the CNS-LS to determine if the patient has both depression and PBA.
HISTORY Poor response to anxiolytics and antipsychotics
Mr. X previously received a ventriculoperitoneal shunt for treating iNPH. He was not taking any medications for CP. To address his impulse control disorder, he was prescribed olanzapine, 20 mg/d, risperidone, 2 mg/d, and diazepam, 5 mg three times a day. Mr. X is uncontrolled on these medications, experiencing frequent behavioral outbursts at home. His mother completes a CNS-LS for him. He receives a score of 20, which suggests a diagnosis of PBA. His PHQ-9 score is 8, indicating mild depression.
[polldaddy:9991899]
TREATMENT Introducing a new medication
Mr. X is started on
Continue to: The authors' observations
The authors’ observations
Decreasing the severity and frequency of episodes constitutes the mainstay of treating PBA. In the past, off-label treatments, including selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants, were prescribed to reduce PBA symptoms.5 Currently, dextromethorphan/quinidine is the only FDA-approved medication for treating PBA; however, its use in patients younger than age 18 is considered investigational.
Atypical antipsychotics, such as olanzapine and risperidone, have more warnings and precautions than dextromethorphan/quinidine. Risperidone has a “black-box” warning for QT prolongation, in addition to death and stroke in elderly patients.10 Although dextromethorphan/quinidine does not have a black-box warning, it does increase the risk of QT prolongation, and patients with cardiac risk factors should undergo an electrocardiogram before starting this medication. Additionally, risperidone and olanzapine are known to cause significant weight gain, which can increase the risk of developing hyperlipidemia, metabolic syndrome, and type 2 diabetes mellitus.10,11 Neuroleptic malignant syndrome (NMS) is a potentially life-threatening adverse effect of all antipsychotics. NMS is characterized by fever, rigidity, altered consciousness, and increased heart and respiratory rates.12
Quinidine increases the bioavailability of dextromethorphan by inhibiting CYP2D6. When dextromethorphan/quinidine is simultaneously used with an SSRI that also inhibits CYP2D6, such as paroxetine or fluoxetine, the patient may be at increased risk for developing adverse effects such as respiratory depression and serotonin syndrome.13
[polldaddy:9991902]
Continue to: The authors' observations
The authors’ observations
Although the exact pathophysiology of PBA is unknown, multiple theories may explain the principle elements of the condition. In the absence of a neurologic insult, the cerebellum acts as an affect regulator, inhibiting laughter and crying at times in which they are considered inappropriate. Parvizi et al4 have theorized that the lesions involved in PBA disrupt the corticopontine-cerebellar circuitry, which impedes the ability of the cerebellum to function as an affect modulator.3 In addition to the dysregulation of cerebellar circuitry, altered serotonin and glutamate levels are believed to contribute to the deficient affect regulation observed in PBA; therefore, adding dextromethorphan/quinidine potentiates serotonin and glutamate levels in the synaptic cleft, resulting in a reduction in PBA episodes.4
OUTCOME Affect stability
Seven months after beginning dextromethorphan/quinidine, Mr. X has experienced resolution of his PBA episodes. His PHQ-9 score was reduced to 0 (no clinical signs of depression) within 1 month of starting this medication and his PHQ-9 scores remain below 5, representing minimal depressive severity. The CNS-LS scale is not conducted at further visits because the patient’s mother reported no further PBA episodes. Mr. X no longer exhibits episodes of aggression. These episodes seemed to have been a manifestation of his frustration and difficulty in controlling his PBA episodes. Furthermore, his dosage of diazepam was reduced, and he was weaned off risperidone. Mr. X’s parents report that he has a drastically improved affect. He continues to tolerate his medication well and no longer demonstrates any exacerbations of his psychiatric symptoms.
Bottom Line
Pseudobulbar affect (PBA) may occur secondary to various neurologic insults, including cerebral palsy and idiopathic normal pressure hydrocephalus. The condition is diagnosed by a subjective clinical evaluation and use of the Center for Neurologic Study–Lability Scale. Dextromethorphan/quinidine can significantly reduce PBA symptoms.
Acknowledgements
The authors thank Anthony S. Graziano and Rachel M. Watt, both Physician Assistant students, Daemen College, Amherst, New York.
Related Resources
- Frock B, Williams A, Caplan JP. Pseudobulbar affect: when patients laugh or cry, but don’t know why. Current Psychiatry. 2016;15(9):56-60,63.
- Crumpacker DW. Enhancing approaches to the identification and management of pseudobulbar affect. J Clin Psychiatry. 2016;77(9):e1155.
Drug Brand Names
Dextromethorphan/quinidine • Nuedexta
Diazepam • Valium
Fluoxetine • Prozac
Haloperidol • Haldol
Lorazepam • Ativan
Olanzapine • Zyprexa
Paroxetine • Paxil
Risperidone • Risperdal
1. Colamonico J, Formella A, Bradley W. Pseudobulbar affect: burden of illness in the USA. Adv Ther. 2012;29(9):775-798.
2. Brooks BR, Crumpacker D, Fellus J, et al. PRISM: a novel research tool to assess the prevalence of pseudobulbar affect symptoms across neurological conditions. PLoS One. 2013;8(8):e72232. doi: 10.1371/journal.pone.0072232.
3. Schiffer R, Pope LE. Review of pseudobulbar affect including a novel and potential therapy. J Neuropsychiatry Clin Neurosci. 2005;17(4):447-454.
4. Parvizi J, Anderson SW, Martin CO, et al. Pathological laughter and crying: a link to the cerebellum. Brain. 2001;124(pt 9):1708-1719.
5. Johnsen SD, Bodensteiner JB, Lotze TE. Frequency and nature of cerebellar injury in the extremely premature survivor with cerebral palsy. J Child Neurol. 2005;20(1):60-64.
6. Kamiya K, Hori M, Miyajima M, et al. Axon diameter and intra-axonal volume fraction of the corticospinal tract in idiopathic normal pressure hydrocephalus measured by Q-Space imaging. PLoS One. 2014;9(8):e103842. doi: 10.1371/journal.pone.0103842.
7. Moore SR, Gresham LS, Bromberg MB, et al. A self report measuredextromethorphan of affective lability. J Neurol Neurosurg Psychiatry. 1997;63(1):89-93.
8. Ahmed A, Simmons Z. Pseudobulbar affect: prevalence and management. Ther Clinical Risk Manag. 2013;9:483-489.
9. Cruz MP. Nuedexta for the treatment of pseudobulbar affect. A condition of involuntary crying or laughing. P T. 2013;38(6):325-328.
10. Goëb JL, Marco S, Duhamel A, et al. Metabolic side effects of risperidone in children and adolescents with early onset schizophrenia. Prim Care Companion J Clin Psychiatry. 2008;10(6):486-487.
11. Nemeroff CB. Dosing the antipsychotic medication olanzapine. J Clin Psychiatry. 1997;58(suppl 10):45-49.
12. Troller JN, Chen X, Sachdev PS. Neuroleptic malignant syndrome associated with atypical antipsychotic drugs. CNS Drugs. 2009;23(6):477-492.
13. Schoedel KA, Pope LE, Sellers EM. Randomized open-label drug-drug interaction trial of dextromethorphan/quinidine and paroxetine in healthy volunteers. Clin Drug Investig. 2012;32(3):157-169.
1. Colamonico J, Formella A, Bradley W. Pseudobulbar affect: burden of illness in the USA. Adv Ther. 2012;29(9):775-798.
2. Brooks BR, Crumpacker D, Fellus J, et al. PRISM: a novel research tool to assess the prevalence of pseudobulbar affect symptoms across neurological conditions. PLoS One. 2013;8(8):e72232. doi: 10.1371/journal.pone.0072232.
3. Schiffer R, Pope LE. Review of pseudobulbar affect including a novel and potential therapy. J Neuropsychiatry Clin Neurosci. 2005;17(4):447-454.
4. Parvizi J, Anderson SW, Martin CO, et al. Pathological laughter and crying: a link to the cerebellum. Brain. 2001;124(pt 9):1708-1719.
5. Johnsen SD, Bodensteiner JB, Lotze TE. Frequency and nature of cerebellar injury in the extremely premature survivor with cerebral palsy. J Child Neurol. 2005;20(1):60-64.
6. Kamiya K, Hori M, Miyajima M, et al. Axon diameter and intra-axonal volume fraction of the corticospinal tract in idiopathic normal pressure hydrocephalus measured by Q-Space imaging. PLoS One. 2014;9(8):e103842. doi: 10.1371/journal.pone.0103842.
7. Moore SR, Gresham LS, Bromberg MB, et al. A self report measuredextromethorphan of affective lability. J Neurol Neurosurg Psychiatry. 1997;63(1):89-93.
8. Ahmed A, Simmons Z. Pseudobulbar affect: prevalence and management. Ther Clinical Risk Manag. 2013;9:483-489.
9. Cruz MP. Nuedexta for the treatment of pseudobulbar affect. A condition of involuntary crying or laughing. P T. 2013;38(6):325-328.
10. Goëb JL, Marco S, Duhamel A, et al. Metabolic side effects of risperidone in children and adolescents with early onset schizophrenia. Prim Care Companion J Clin Psychiatry. 2008;10(6):486-487.
11. Nemeroff CB. Dosing the antipsychotic medication olanzapine. J Clin Psychiatry. 1997;58(suppl 10):45-49.
12. Troller JN, Chen X, Sachdev PS. Neuroleptic malignant syndrome associated with atypical antipsychotic drugs. CNS Drugs. 2009;23(6):477-492.
13. Schoedel KA, Pope LE, Sellers EM. Randomized open-label drug-drug interaction trial of dextromethorphan/quinidine and paroxetine in healthy volunteers. Clin Drug Investig. 2012;32(3):157-169.
Strategies for working with patients with personality disorders
Patients with personality disorders can disrupt the treatment relationship, and may leave us feeling angry, ineffective, inadequate, and defeated. Although their behaviors may appear volitional and purposeful, they often are the result of a dysfunctional personality structure.1 These patients’ unbending patterns of viewing themselves, interacting with others, and navigating the world can be problematic in an inpatient or outpatient setting, causing distress for both the staff and patient. Because no 2 personalities are identical, there is no algorithm for managing patients with personality disorders. However, there are strategies that we can apply to provide effective clinical care.1,2
Discuss the responses the patient evokes. Patients with personality disorders can elicit strong responses from the treatment team. Each clinician can have a different response to the same patient, ranging from feeling the need to protect the patient to strongly disliking him or her. Because cohesion among staff is essential for effective patient care, we need to discuss these responses in an open forum with our team members so we can effectively manage our responses and provide the patient with consistent interactions. Limiting the delivery of inconsistent or conflicting messages will decrease staff splitting and increase team unity.
Reinforce appropriate behaviors. Patients with personality disorders usually have negative interpersonal interactions, such as acting out, misinterpreting neutral social cues, and seeking constant attention. However, when they are not engaging in detrimental behaviors, we should provide positive reinforcement for appropriate behaviors, such as remaining composed, that help maintain the treatment relationship. When a patient displays disruptive behaviors, take a neutral approach by stating, “You appear upset. I will come back later when you are feeling better.”1
Set limits. These patients are likely to have difficulty conforming to appropriate social boundaries. Our reflex reaction may be to set concrete rules that fit our preferences. This could lead to a power struggle between us and our patients, which is not helpful. Rather than a “one-size-fits-all” approach to rules, it may be prudent to tailor boundaries according to each patient’s unique personality. Also, allowing the patient to help set these limits could increase the chances that he or she will follow your treatment plan and reinforce the more positive aspects of his or her personality structure.
Offer empathy. Empathy can be conceptualized as a step further than sympathy; in addition to expressing concern and compassion, empathy involves recognizing and sharing the patient’s emotions. Seek to comprehend the reasons behind a patient’s negative reactions by
1. Riddle M, Meeks T, Alvarez C, et al. When personality is the problem: managing patients with difficult personalitie s on the acute care unit. J Hosp Med. 2016;11(12):873-878.
2. Strous RD, Ulman AM, Kotler M. The hateful patient revisited: relevance for 21st century medicine. Eur J Intern Med. 2006;17(6):387-393.
Patients with personality disorders can disrupt the treatment relationship, and may leave us feeling angry, ineffective, inadequate, and defeated. Although their behaviors may appear volitional and purposeful, they often are the result of a dysfunctional personality structure.1 These patients’ unbending patterns of viewing themselves, interacting with others, and navigating the world can be problematic in an inpatient or outpatient setting, causing distress for both the staff and patient. Because no 2 personalities are identical, there is no algorithm for managing patients with personality disorders. However, there are strategies that we can apply to provide effective clinical care.1,2
Discuss the responses the patient evokes. Patients with personality disorders can elicit strong responses from the treatment team. Each clinician can have a different response to the same patient, ranging from feeling the need to protect the patient to strongly disliking him or her. Because cohesion among staff is essential for effective patient care, we need to discuss these responses in an open forum with our team members so we can effectively manage our responses and provide the patient with consistent interactions. Limiting the delivery of inconsistent or conflicting messages will decrease staff splitting and increase team unity.
Reinforce appropriate behaviors. Patients with personality disorders usually have negative interpersonal interactions, such as acting out, misinterpreting neutral social cues, and seeking constant attention. However, when they are not engaging in detrimental behaviors, we should provide positive reinforcement for appropriate behaviors, such as remaining composed, that help maintain the treatment relationship. When a patient displays disruptive behaviors, take a neutral approach by stating, “You appear upset. I will come back later when you are feeling better.”1
Set limits. These patients are likely to have difficulty conforming to appropriate social boundaries. Our reflex reaction may be to set concrete rules that fit our preferences. This could lead to a power struggle between us and our patients, which is not helpful. Rather than a “one-size-fits-all” approach to rules, it may be prudent to tailor boundaries according to each patient’s unique personality. Also, allowing the patient to help set these limits could increase the chances that he or she will follow your treatment plan and reinforce the more positive aspects of his or her personality structure.
Offer empathy. Empathy can be conceptualized as a step further than sympathy; in addition to expressing concern and compassion, empathy involves recognizing and sharing the patient’s emotions. Seek to comprehend the reasons behind a patient’s negative reactions by
Patients with personality disorders can disrupt the treatment relationship, and may leave us feeling angry, ineffective, inadequate, and defeated. Although their behaviors may appear volitional and purposeful, they often are the result of a dysfunctional personality structure.1 These patients’ unbending patterns of viewing themselves, interacting with others, and navigating the world can be problematic in an inpatient or outpatient setting, causing distress for both the staff and patient. Because no 2 personalities are identical, there is no algorithm for managing patients with personality disorders. However, there are strategies that we can apply to provide effective clinical care.1,2
Discuss the responses the patient evokes. Patients with personality disorders can elicit strong responses from the treatment team. Each clinician can have a different response to the same patient, ranging from feeling the need to protect the patient to strongly disliking him or her. Because cohesion among staff is essential for effective patient care, we need to discuss these responses in an open forum with our team members so we can effectively manage our responses and provide the patient with consistent interactions. Limiting the delivery of inconsistent or conflicting messages will decrease staff splitting and increase team unity.
Reinforce appropriate behaviors. Patients with personality disorders usually have negative interpersonal interactions, such as acting out, misinterpreting neutral social cues, and seeking constant attention. However, when they are not engaging in detrimental behaviors, we should provide positive reinforcement for appropriate behaviors, such as remaining composed, that help maintain the treatment relationship. When a patient displays disruptive behaviors, take a neutral approach by stating, “You appear upset. I will come back later when you are feeling better.”1
Set limits. These patients are likely to have difficulty conforming to appropriate social boundaries. Our reflex reaction may be to set concrete rules that fit our preferences. This could lead to a power struggle between us and our patients, which is not helpful. Rather than a “one-size-fits-all” approach to rules, it may be prudent to tailor boundaries according to each patient’s unique personality. Also, allowing the patient to help set these limits could increase the chances that he or she will follow your treatment plan and reinforce the more positive aspects of his or her personality structure.
Offer empathy. Empathy can be conceptualized as a step further than sympathy; in addition to expressing concern and compassion, empathy involves recognizing and sharing the patient’s emotions. Seek to comprehend the reasons behind a patient’s negative reactions by
1. Riddle M, Meeks T, Alvarez C, et al. When personality is the problem: managing patients with difficult personalitie s on the acute care unit. J Hosp Med. 2016;11(12):873-878.
2. Strous RD, Ulman AM, Kotler M. The hateful patient revisited: relevance for 21st century medicine. Eur J Intern Med. 2006;17(6):387-393.
1. Riddle M, Meeks T, Alvarez C, et al. When personality is the problem: managing patients with difficult personalitie s on the acute care unit. J Hosp Med. 2016;11(12):873-878.
2. Strous RD, Ulman AM, Kotler M. The hateful patient revisited: relevance for 21st century medicine. Eur J Intern Med. 2006;17(6):387-393.
‘Nocebo’ effects: Address these 4 psychosocial factors
Sorting out the causes of unexplained adverse effects from psychotropic medications can be challenging. Treatment may be further complicated by ‘nocebo’ effects, which are adverse effects based on the patient’s conscious and unconscious expectations of harm. Having strategies for managing nocebo effects can help clinicians better understand and treat patients who have complex medication complaints. When your patient experiences nocebo effects, consider the following 4 psychosocial factors.1
Pills. The impact of a medication is not solely based on its chemical makeup. For example, the appearance of a medication can affect treatment outcomes. Substituting generic medications for branded ones has been shown to negatively impact patient adherence and increase reports of adverse effects that have no physiologic cause.2 Educating patients about medication manufacturing and distribution practices may decrease such consequences.
Patient. A sense of powerlessness is fertile ground for nocebo effects. Patients with an external locus of control may unconsciously employ nocebo effects to express themselves when other outlets are limited. Having a psychosocial formulation of your patient can help you anticipate pitfalls, offer pertinent insights, and mobilize the patient’s adaptive coping mechanisms. Also, clinicians can bolster their patients’ self-agency by encouraging them to participate in healthy activities.
Provider. Irrational factors in the clinician, such as countertransference, may also affect medication outcomes. Unprocessed countertransference can contribute to clinician burnout and impact the therapeutic relationship negatively. Nocebo effects may indicate that the clinician is not “tuned in” to the patient or is acting out harmful unconscious thoughts. Additionally, countertransference can lead to unnecessary prescribing and polypharmacy that confounds nocebo effects. Therefore self-care, consultation, and supervision may be vital in promoting therapeutic outcomes.
Partnership. The doctor–patient relationship can contribute to nocebo effects. A 2016 Gallup Poll found that Americans had low confidence in the honesty and ethics of psychiatrists compared with other healthcare professionals.3 It is important to have conversations with your patients about their reservations and perceived stigma of mental health. Such conversations can bring a patient’s ambivalence into treatment so that it can be further explored and addressed. Psychoeducation about treatment limitations, motivational interviewing techniques, and involving patients in decision-making can be useful tools for fostering a therapeutic alliance and positive outcomes.
Take an active approach
Evidence demonstrates that psychosocial factors significantly impact treatment outcomes.1 Incorporating this evidence into practice and attending to the 4 factors discussed here can enhance a clinician’s ability to flexibly respond to their patients’ complaints, especially in relation to nocebo effects.
1. Mallo CJ, Mintz DL. Teaching all the evidence bases: reintegrating psychodynamic aspects of prescribing into psychopharmacology training. Psychodyn Psychiatry. 2013;41(1):13-37.
2. Weissenfeld J, Stock S, Lüngen M, et al. The nocebo effect: a reason for patients’ non-adherence to generic substitution? Pharmazie. 2010;65(7):451-456.
3. Norman J. Americans rate healthcare providers high on honesty, ethics. Gallup. http://news.gallup.com/poll/200057/americans-rate-healthcare-providers-high-honesty-ethics.aspx. Published December 19, 2016. Accessed October 22, 2017.
Sorting out the causes of unexplained adverse effects from psychotropic medications can be challenging. Treatment may be further complicated by ‘nocebo’ effects, which are adverse effects based on the patient’s conscious and unconscious expectations of harm. Having strategies for managing nocebo effects can help clinicians better understand and treat patients who have complex medication complaints. When your patient experiences nocebo effects, consider the following 4 psychosocial factors.1
Pills. The impact of a medication is not solely based on its chemical makeup. For example, the appearance of a medication can affect treatment outcomes. Substituting generic medications for branded ones has been shown to negatively impact patient adherence and increase reports of adverse effects that have no physiologic cause.2 Educating patients about medication manufacturing and distribution practices may decrease such consequences.
Patient. A sense of powerlessness is fertile ground for nocebo effects. Patients with an external locus of control may unconsciously employ nocebo effects to express themselves when other outlets are limited. Having a psychosocial formulation of your patient can help you anticipate pitfalls, offer pertinent insights, and mobilize the patient’s adaptive coping mechanisms. Also, clinicians can bolster their patients’ self-agency by encouraging them to participate in healthy activities.
Provider. Irrational factors in the clinician, such as countertransference, may also affect medication outcomes. Unprocessed countertransference can contribute to clinician burnout and impact the therapeutic relationship negatively. Nocebo effects may indicate that the clinician is not “tuned in” to the patient or is acting out harmful unconscious thoughts. Additionally, countertransference can lead to unnecessary prescribing and polypharmacy that confounds nocebo effects. Therefore self-care, consultation, and supervision may be vital in promoting therapeutic outcomes.
Partnership. The doctor–patient relationship can contribute to nocebo effects. A 2016 Gallup Poll found that Americans had low confidence in the honesty and ethics of psychiatrists compared with other healthcare professionals.3 It is important to have conversations with your patients about their reservations and perceived stigma of mental health. Such conversations can bring a patient’s ambivalence into treatment so that it can be further explored and addressed. Psychoeducation about treatment limitations, motivational interviewing techniques, and involving patients in decision-making can be useful tools for fostering a therapeutic alliance and positive outcomes.
Take an active approach
Evidence demonstrates that psychosocial factors significantly impact treatment outcomes.1 Incorporating this evidence into practice and attending to the 4 factors discussed here can enhance a clinician’s ability to flexibly respond to their patients’ complaints, especially in relation to nocebo effects.
Sorting out the causes of unexplained adverse effects from psychotropic medications can be challenging. Treatment may be further complicated by ‘nocebo’ effects, which are adverse effects based on the patient’s conscious and unconscious expectations of harm. Having strategies for managing nocebo effects can help clinicians better understand and treat patients who have complex medication complaints. When your patient experiences nocebo effects, consider the following 4 psychosocial factors.1
Pills. The impact of a medication is not solely based on its chemical makeup. For example, the appearance of a medication can affect treatment outcomes. Substituting generic medications for branded ones has been shown to negatively impact patient adherence and increase reports of adverse effects that have no physiologic cause.2 Educating patients about medication manufacturing and distribution practices may decrease such consequences.
Patient. A sense of powerlessness is fertile ground for nocebo effects. Patients with an external locus of control may unconsciously employ nocebo effects to express themselves when other outlets are limited. Having a psychosocial formulation of your patient can help you anticipate pitfalls, offer pertinent insights, and mobilize the patient’s adaptive coping mechanisms. Also, clinicians can bolster their patients’ self-agency by encouraging them to participate in healthy activities.
Provider. Irrational factors in the clinician, such as countertransference, may also affect medication outcomes. Unprocessed countertransference can contribute to clinician burnout and impact the therapeutic relationship negatively. Nocebo effects may indicate that the clinician is not “tuned in” to the patient or is acting out harmful unconscious thoughts. Additionally, countertransference can lead to unnecessary prescribing and polypharmacy that confounds nocebo effects. Therefore self-care, consultation, and supervision may be vital in promoting therapeutic outcomes.
Partnership. The doctor–patient relationship can contribute to nocebo effects. A 2016 Gallup Poll found that Americans had low confidence in the honesty and ethics of psychiatrists compared with other healthcare professionals.3 It is important to have conversations with your patients about their reservations and perceived stigma of mental health. Such conversations can bring a patient’s ambivalence into treatment so that it can be further explored and addressed. Psychoeducation about treatment limitations, motivational interviewing techniques, and involving patients in decision-making can be useful tools for fostering a therapeutic alliance and positive outcomes.
Take an active approach
Evidence demonstrates that psychosocial factors significantly impact treatment outcomes.1 Incorporating this evidence into practice and attending to the 4 factors discussed here can enhance a clinician’s ability to flexibly respond to their patients’ complaints, especially in relation to nocebo effects.
1. Mallo CJ, Mintz DL. Teaching all the evidence bases: reintegrating psychodynamic aspects of prescribing into psychopharmacology training. Psychodyn Psychiatry. 2013;41(1):13-37.
2. Weissenfeld J, Stock S, Lüngen M, et al. The nocebo effect: a reason for patients’ non-adherence to generic substitution? Pharmazie. 2010;65(7):451-456.
3. Norman J. Americans rate healthcare providers high on honesty, ethics. Gallup. http://news.gallup.com/poll/200057/americans-rate-healthcare-providers-high-honesty-ethics.aspx. Published December 19, 2016. Accessed October 22, 2017.
1. Mallo CJ, Mintz DL. Teaching all the evidence bases: reintegrating psychodynamic aspects of prescribing into psychopharmacology training. Psychodyn Psychiatry. 2013;41(1):13-37.
2. Weissenfeld J, Stock S, Lüngen M, et al. The nocebo effect: a reason for patients’ non-adherence to generic substitution? Pharmazie. 2010;65(7):451-456.
3. Norman J. Americans rate healthcare providers high on honesty, ethics. Gallup. http://news.gallup.com/poll/200057/americans-rate-healthcare-providers-high-honesty-ethics.aspx. Published December 19, 2016. Accessed October 22, 2017.
Generalized pustular eruption
A 38-year-old man sought care in the emergency department for an acute, pruritic, generalized cutaneous eruption that manifested in the intertriginous areas of the inner thighs, antecubital fossae, and axilla (FIGURE 1A). He reported associated chills, a 15-pound weight gain, and swelling of his inner thighs. Two weeks before presentation, he had received azithromycin for an upper respiratory tract infection. He was unsure if the rash developed prior to or after taking the medication. He was not taking any other medications and had no history of skin conditions.
On examination, the patient was afebrile and had bilateral thigh edema. Skin examination revealed background erythema with morbilliform papules, plaques, and patches on the bilateral flanks, back, buttocks, arms, legs, and central neck. Pinpoint pustules were present in the intertriginous sites and on the low back and buttocks. The laboratory evaluation revealed leukocytosis (11.0 × 109 cells/L), increased levels of neutrophils and eosinophils, and an elevated C-reactive protein level (12.8 mg/L). The remaining laboratory results were unremarkable. The patient was referred to Dermatology.
An examination by the dermatologist 3 days later revealed small areas of annular desquamation with a few pinpoint pustules, mostly located on the inner thighs and buttocks (FIGURE 1B). Skin biopsies were taken from the anterior hip region. The histopathology revealed subacute dermatitis with mixed dermal inflammatory cells, including neutrophils and eosinophils, and discrete subcorneal spongiform pustules.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Acute generalized exanthematous pustulosis (AGEP)
The acute rash with minute pustules and associated leukocytosis with neutrophilia and eosinophilia led to an early diagnosis of AGEP, which may have been triggered by azithromycin—the patient’s only recent medication. AGEP is a severe cutaneous eruption that may be associated with systemic involvement. Medications are usually implicated, and patients often seek urgent evaluation.
AGEP typically begins as an acute eruption in the intertriginous sites of the axilla, groin, and neck, but often becomes more generalized.1,2 The diagnosis is strongly suggested by the condition’s key features: fever (97% of cases) and leukocytosis (87%) with neutrophilia (91%) and eosinophilia (30%); leukocytosis peaks 4 days after pustulosis occurs and lasts for about 12 days.1 Although common, fever is not always documented in patients with AGEP. 3 (Our patient was a case in point.) While not a key characteristic of AGEP, our patient’s weight gain was likely explained by the severe edema secondary to his inflammatory skin eruption.
Medications are implicated, but pathophysiology is unknown
In approximately 90% of AGEP cases, medications such as antibiotics and calcium channel blockers are implicated; however, the lack of such an association does not preclude the diagnosis.1,4 In cases of drug reactions, the eruption typically develops 1 to 2 days after a medication is begun, and the pustules typically resolve in fewer than 15 days.5 In 17% of patients, systemic involvement can occur and can include the liver, kidneys, bone marrow, and lungs.6 A physical exam, review of systems, and a laboratory evaluation can help rule out systemic involvement and guide additional testing.
AGEP has an incidence of 1 to 5 cases per million people per year, affecting women slightly more frequently than men.7 While the pathophysiology is not well understood, AGEP and its differential diagnoses are categorized as T cell-related inflammatory responses.4,7
Distinguishing AGEP from some look-alikes
There are at least 4 severe cutaneous eruptions that might be confused with AGEP, all of which may be associated with fever. They include: drug reaction with eosinophilia and systemic symptoms (DRESS), also known as drug-induced hypersensitivity syndrome; Stevens-Johnson syndrome (SJS); toxic epidermal necrolysis (TEN); and pustular psoriasis.8-10 The clinical features that may help differentiate these conditions from AGEP include timeline, mucocutaneous features, organ system involvement, and histopathologic findings.4,8
DRESS occurs 2 to 6 weeks after drug exposure, rather than a few days, as is seen with AGEP. It often involves morbilliform erythema and facial edema with substantial eosinophilia and possible nephritis, pneumonitis, myocarditis, and thyroiditis.9 Unlike AGEP, DRESS does not have a predilection for intertriginous anatomic locations.
SJS and TEN occur 1 to 3 weeks after drug exposure. These conditions manifest with the development of bullae, atypical targetoid lesions, painful dusky erythema, epidermal necrosis, and mucosal involvement at multiple sites. Tubular nephritis, tracheobronchial necrosis, and multisystem organ failure can occur, with reported mortality rates of 5% to 35%.8,11
Pustular psoriasis is frequently confused with AGEP. However, AGEP usually develops fewer than 2 days after drug exposure, with pustules that begin in intertriginous sites, and there is associated neutrophilia and possible organ involvement.1,8 Patients who have AGEP typically do not have a history of psoriasis, while patients with pustular psoriasis often do.7 A history of drug reaction is uncommon with pustular psoriasis (although rapid tapering of systemic corticosteroids in patients with psoriasis can trigger the development of pustular psoriasis), whereas a previous history of drug reaction is common in AGEP.3,7
Discontinue medication, treat with corticosteroids
Patients who have AGEP, including those with systemic involvement, generally improve after the offending drug is discontinued and treatment with topical corticosteroids is initiated.6 A brief course of systemic corticosteroids can also be considered for patients with severe skin involvement or systemic involvement.3
Our patient was prescribed topical corticosteroid wet dressing treatments twice daily for 2 weeks. At the 2-week follow-up visit, the rash had completely cleared, and only minimal residual erythema was noted (FIGURE 2). The patient was instructed to avoid azithromycin.
CORRESPONDENCE
David A. Wetter, MD, Department of Dermatology, Mayo Clinic, 200 First Street SW, Rochester, MN 55905; wetter.david@mayo.edu.
1. Roujeau JC, Bioulac-Sage P, Bourseau C, et al. Acute generalized exanthematous pustulosis. Analysis of 63 cases. Arch Dermatol. 1991;127:1333-1338.
2. Lee HY, Chou D, Pang SM, et al. Acute generalized exanthematous pustulosis: analysis of cases managed in a tertiary hospital in Singapore. Int J Dermatol. 2010;49:507-512.
3. Alniemi DT, Wetter DA, Bridges AG, et al. Acute generalized exanthematous pustulosis: clinical characteristics, etiologic associations, treatments, and outcomes in a series of 28 patients at Mayo Clinic, 1996-2013. Int J Dermatol. 2017;56:405-414.
4. Bouvresse S, Valeyrie-Allanore L, Ortonne N, et al. Toxic epidermal necrolysis, DRESS, AGEP: do overlap cases exist? Orphanet J Rare Dis. 2012;7:72.
5. Sidoroff A, Halevy S, Bavinck JN, et al. Acute generalized exanthematous pustulosis (AGEP)—a clinical reaction pattern. J Cutan Pathol. 2001;28:113-119.
6. Hotz C, Valeyrie-Allanore L, Haddad C, et al. Systemic involvement of acute generalized exanthematous pustulosis: a retrospective study on 58 patients. Br J Dermatol. 2013;169:1223-1232.
7. Feldmeyer L, Heidemeyer K, Yawalkar N. Acute generalized exanthematous pustulosis: pathogenesis, genetic background, clinical variants and therapy. Int J Mol Sci. 2016;17:E1214.
8. Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: Part II. Management and therapeutics. J Am Acad Dermatol. 2013;68:709.e1-e9.
9. Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: Part I. Clinical perspectives. J Am Acad Dermatol. 2013;68:693.e1-e14.
10. Bastuji-Garin S, Rzany B, Stern RS, et al. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129:92-96.
11. Roujeau JC. The spectrum of Stevens-Johnson syndrome and toxic epidermal necrolysis: a clinical classification. J Invest Dermatol. 1994;102:28S-30S.
A 38-year-old man sought care in the emergency department for an acute, pruritic, generalized cutaneous eruption that manifested in the intertriginous areas of the inner thighs, antecubital fossae, and axilla (FIGURE 1A). He reported associated chills, a 15-pound weight gain, and swelling of his inner thighs. Two weeks before presentation, he had received azithromycin for an upper respiratory tract infection. He was unsure if the rash developed prior to or after taking the medication. He was not taking any other medications and had no history of skin conditions.
On examination, the patient was afebrile and had bilateral thigh edema. Skin examination revealed background erythema with morbilliform papules, plaques, and patches on the bilateral flanks, back, buttocks, arms, legs, and central neck. Pinpoint pustules were present in the intertriginous sites and on the low back and buttocks. The laboratory evaluation revealed leukocytosis (11.0 × 109 cells/L), increased levels of neutrophils and eosinophils, and an elevated C-reactive protein level (12.8 mg/L). The remaining laboratory results were unremarkable. The patient was referred to Dermatology.
An examination by the dermatologist 3 days later revealed small areas of annular desquamation with a few pinpoint pustules, mostly located on the inner thighs and buttocks (FIGURE 1B). Skin biopsies were taken from the anterior hip region. The histopathology revealed subacute dermatitis with mixed dermal inflammatory cells, including neutrophils and eosinophils, and discrete subcorneal spongiform pustules.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Acute generalized exanthematous pustulosis (AGEP)
The acute rash with minute pustules and associated leukocytosis with neutrophilia and eosinophilia led to an early diagnosis of AGEP, which may have been triggered by azithromycin—the patient’s only recent medication. AGEP is a severe cutaneous eruption that may be associated with systemic involvement. Medications are usually implicated, and patients often seek urgent evaluation.
AGEP typically begins as an acute eruption in the intertriginous sites of the axilla, groin, and neck, but often becomes more generalized.1,2 The diagnosis is strongly suggested by the condition’s key features: fever (97% of cases) and leukocytosis (87%) with neutrophilia (91%) and eosinophilia (30%); leukocytosis peaks 4 days after pustulosis occurs and lasts for about 12 days.1 Although common, fever is not always documented in patients with AGEP. 3 (Our patient was a case in point.) While not a key characteristic of AGEP, our patient’s weight gain was likely explained by the severe edema secondary to his inflammatory skin eruption.
Medications are implicated, but pathophysiology is unknown
In approximately 90% of AGEP cases, medications such as antibiotics and calcium channel blockers are implicated; however, the lack of such an association does not preclude the diagnosis.1,4 In cases of drug reactions, the eruption typically develops 1 to 2 days after a medication is begun, and the pustules typically resolve in fewer than 15 days.5 In 17% of patients, systemic involvement can occur and can include the liver, kidneys, bone marrow, and lungs.6 A physical exam, review of systems, and a laboratory evaluation can help rule out systemic involvement and guide additional testing.
AGEP has an incidence of 1 to 5 cases per million people per year, affecting women slightly more frequently than men.7 While the pathophysiology is not well understood, AGEP and its differential diagnoses are categorized as T cell-related inflammatory responses.4,7
Distinguishing AGEP from some look-alikes
There are at least 4 severe cutaneous eruptions that might be confused with AGEP, all of which may be associated with fever. They include: drug reaction with eosinophilia and systemic symptoms (DRESS), also known as drug-induced hypersensitivity syndrome; Stevens-Johnson syndrome (SJS); toxic epidermal necrolysis (TEN); and pustular psoriasis.8-10 The clinical features that may help differentiate these conditions from AGEP include timeline, mucocutaneous features, organ system involvement, and histopathologic findings.4,8
DRESS occurs 2 to 6 weeks after drug exposure, rather than a few days, as is seen with AGEP. It often involves morbilliform erythema and facial edema with substantial eosinophilia and possible nephritis, pneumonitis, myocarditis, and thyroiditis.9 Unlike AGEP, DRESS does not have a predilection for intertriginous anatomic locations.
SJS and TEN occur 1 to 3 weeks after drug exposure. These conditions manifest with the development of bullae, atypical targetoid lesions, painful dusky erythema, epidermal necrosis, and mucosal involvement at multiple sites. Tubular nephritis, tracheobronchial necrosis, and multisystem organ failure can occur, with reported mortality rates of 5% to 35%.8,11
Pustular psoriasis is frequently confused with AGEP. However, AGEP usually develops fewer than 2 days after drug exposure, with pustules that begin in intertriginous sites, and there is associated neutrophilia and possible organ involvement.1,8 Patients who have AGEP typically do not have a history of psoriasis, while patients with pustular psoriasis often do.7 A history of drug reaction is uncommon with pustular psoriasis (although rapid tapering of systemic corticosteroids in patients with psoriasis can trigger the development of pustular psoriasis), whereas a previous history of drug reaction is common in AGEP.3,7
Discontinue medication, treat with corticosteroids
Patients who have AGEP, including those with systemic involvement, generally improve after the offending drug is discontinued and treatment with topical corticosteroids is initiated.6 A brief course of systemic corticosteroids can also be considered for patients with severe skin involvement or systemic involvement.3
Our patient was prescribed topical corticosteroid wet dressing treatments twice daily for 2 weeks. At the 2-week follow-up visit, the rash had completely cleared, and only minimal residual erythema was noted (FIGURE 2). The patient was instructed to avoid azithromycin.
CORRESPONDENCE
David A. Wetter, MD, Department of Dermatology, Mayo Clinic, 200 First Street SW, Rochester, MN 55905; wetter.david@mayo.edu.
A 38-year-old man sought care in the emergency department for an acute, pruritic, generalized cutaneous eruption that manifested in the intertriginous areas of the inner thighs, antecubital fossae, and axilla (FIGURE 1A). He reported associated chills, a 15-pound weight gain, and swelling of his inner thighs. Two weeks before presentation, he had received azithromycin for an upper respiratory tract infection. He was unsure if the rash developed prior to or after taking the medication. He was not taking any other medications and had no history of skin conditions.
On examination, the patient was afebrile and had bilateral thigh edema. Skin examination revealed background erythema with morbilliform papules, plaques, and patches on the bilateral flanks, back, buttocks, arms, legs, and central neck. Pinpoint pustules were present in the intertriginous sites and on the low back and buttocks. The laboratory evaluation revealed leukocytosis (11.0 × 109 cells/L), increased levels of neutrophils and eosinophils, and an elevated C-reactive protein level (12.8 mg/L). The remaining laboratory results were unremarkable. The patient was referred to Dermatology.
An examination by the dermatologist 3 days later revealed small areas of annular desquamation with a few pinpoint pustules, mostly located on the inner thighs and buttocks (FIGURE 1B). Skin biopsies were taken from the anterior hip region. The histopathology revealed subacute dermatitis with mixed dermal inflammatory cells, including neutrophils and eosinophils, and discrete subcorneal spongiform pustules.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Acute generalized exanthematous pustulosis (AGEP)
The acute rash with minute pustules and associated leukocytosis with neutrophilia and eosinophilia led to an early diagnosis of AGEP, which may have been triggered by azithromycin—the patient’s only recent medication. AGEP is a severe cutaneous eruption that may be associated with systemic involvement. Medications are usually implicated, and patients often seek urgent evaluation.
AGEP typically begins as an acute eruption in the intertriginous sites of the axilla, groin, and neck, but often becomes more generalized.1,2 The diagnosis is strongly suggested by the condition’s key features: fever (97% of cases) and leukocytosis (87%) with neutrophilia (91%) and eosinophilia (30%); leukocytosis peaks 4 days after pustulosis occurs and lasts for about 12 days.1 Although common, fever is not always documented in patients with AGEP. 3 (Our patient was a case in point.) While not a key characteristic of AGEP, our patient’s weight gain was likely explained by the severe edema secondary to his inflammatory skin eruption.
Medications are implicated, but pathophysiology is unknown
In approximately 90% of AGEP cases, medications such as antibiotics and calcium channel blockers are implicated; however, the lack of such an association does not preclude the diagnosis.1,4 In cases of drug reactions, the eruption typically develops 1 to 2 days after a medication is begun, and the pustules typically resolve in fewer than 15 days.5 In 17% of patients, systemic involvement can occur and can include the liver, kidneys, bone marrow, and lungs.6 A physical exam, review of systems, and a laboratory evaluation can help rule out systemic involvement and guide additional testing.
AGEP has an incidence of 1 to 5 cases per million people per year, affecting women slightly more frequently than men.7 While the pathophysiology is not well understood, AGEP and its differential diagnoses are categorized as T cell-related inflammatory responses.4,7
Distinguishing AGEP from some look-alikes
There are at least 4 severe cutaneous eruptions that might be confused with AGEP, all of which may be associated with fever. They include: drug reaction with eosinophilia and systemic symptoms (DRESS), also known as drug-induced hypersensitivity syndrome; Stevens-Johnson syndrome (SJS); toxic epidermal necrolysis (TEN); and pustular psoriasis.8-10 The clinical features that may help differentiate these conditions from AGEP include timeline, mucocutaneous features, organ system involvement, and histopathologic findings.4,8
DRESS occurs 2 to 6 weeks after drug exposure, rather than a few days, as is seen with AGEP. It often involves morbilliform erythema and facial edema with substantial eosinophilia and possible nephritis, pneumonitis, myocarditis, and thyroiditis.9 Unlike AGEP, DRESS does not have a predilection for intertriginous anatomic locations.
SJS and TEN occur 1 to 3 weeks after drug exposure. These conditions manifest with the development of bullae, atypical targetoid lesions, painful dusky erythema, epidermal necrosis, and mucosal involvement at multiple sites. Tubular nephritis, tracheobronchial necrosis, and multisystem organ failure can occur, with reported mortality rates of 5% to 35%.8,11
Pustular psoriasis is frequently confused with AGEP. However, AGEP usually develops fewer than 2 days after drug exposure, with pustules that begin in intertriginous sites, and there is associated neutrophilia and possible organ involvement.1,8 Patients who have AGEP typically do not have a history of psoriasis, while patients with pustular psoriasis often do.7 A history of drug reaction is uncommon with pustular psoriasis (although rapid tapering of systemic corticosteroids in patients with psoriasis can trigger the development of pustular psoriasis), whereas a previous history of drug reaction is common in AGEP.3,7
Discontinue medication, treat with corticosteroids
Patients who have AGEP, including those with systemic involvement, generally improve after the offending drug is discontinued and treatment with topical corticosteroids is initiated.6 A brief course of systemic corticosteroids can also be considered for patients with severe skin involvement or systemic involvement.3
Our patient was prescribed topical corticosteroid wet dressing treatments twice daily for 2 weeks. At the 2-week follow-up visit, the rash had completely cleared, and only minimal residual erythema was noted (FIGURE 2). The patient was instructed to avoid azithromycin.
CORRESPONDENCE
David A. Wetter, MD, Department of Dermatology, Mayo Clinic, 200 First Street SW, Rochester, MN 55905; wetter.david@mayo.edu.
1. Roujeau JC, Bioulac-Sage P, Bourseau C, et al. Acute generalized exanthematous pustulosis. Analysis of 63 cases. Arch Dermatol. 1991;127:1333-1338.
2. Lee HY, Chou D, Pang SM, et al. Acute generalized exanthematous pustulosis: analysis of cases managed in a tertiary hospital in Singapore. Int J Dermatol. 2010;49:507-512.
3. Alniemi DT, Wetter DA, Bridges AG, et al. Acute generalized exanthematous pustulosis: clinical characteristics, etiologic associations, treatments, and outcomes in a series of 28 patients at Mayo Clinic, 1996-2013. Int J Dermatol. 2017;56:405-414.
4. Bouvresse S, Valeyrie-Allanore L, Ortonne N, et al. Toxic epidermal necrolysis, DRESS, AGEP: do overlap cases exist? Orphanet J Rare Dis. 2012;7:72.
5. Sidoroff A, Halevy S, Bavinck JN, et al. Acute generalized exanthematous pustulosis (AGEP)—a clinical reaction pattern. J Cutan Pathol. 2001;28:113-119.
6. Hotz C, Valeyrie-Allanore L, Haddad C, et al. Systemic involvement of acute generalized exanthematous pustulosis: a retrospective study on 58 patients. Br J Dermatol. 2013;169:1223-1232.
7. Feldmeyer L, Heidemeyer K, Yawalkar N. Acute generalized exanthematous pustulosis: pathogenesis, genetic background, clinical variants and therapy. Int J Mol Sci. 2016;17:E1214.
8. Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: Part II. Management and therapeutics. J Am Acad Dermatol. 2013;68:709.e1-e9.
9. Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: Part I. Clinical perspectives. J Am Acad Dermatol. 2013;68:693.e1-e14.
10. Bastuji-Garin S, Rzany B, Stern RS, et al. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129:92-96.
11. Roujeau JC. The spectrum of Stevens-Johnson syndrome and toxic epidermal necrolysis: a clinical classification. J Invest Dermatol. 1994;102:28S-30S.
1. Roujeau JC, Bioulac-Sage P, Bourseau C, et al. Acute generalized exanthematous pustulosis. Analysis of 63 cases. Arch Dermatol. 1991;127:1333-1338.
2. Lee HY, Chou D, Pang SM, et al. Acute generalized exanthematous pustulosis: analysis of cases managed in a tertiary hospital in Singapore. Int J Dermatol. 2010;49:507-512.
3. Alniemi DT, Wetter DA, Bridges AG, et al. Acute generalized exanthematous pustulosis: clinical characteristics, etiologic associations, treatments, and outcomes in a series of 28 patients at Mayo Clinic, 1996-2013. Int J Dermatol. 2017;56:405-414.
4. Bouvresse S, Valeyrie-Allanore L, Ortonne N, et al. Toxic epidermal necrolysis, DRESS, AGEP: do overlap cases exist? Orphanet J Rare Dis. 2012;7:72.
5. Sidoroff A, Halevy S, Bavinck JN, et al. Acute generalized exanthematous pustulosis (AGEP)—a clinical reaction pattern. J Cutan Pathol. 2001;28:113-119.
6. Hotz C, Valeyrie-Allanore L, Haddad C, et al. Systemic involvement of acute generalized exanthematous pustulosis: a retrospective study on 58 patients. Br J Dermatol. 2013;169:1223-1232.
7. Feldmeyer L, Heidemeyer K, Yawalkar N. Acute generalized exanthematous pustulosis: pathogenesis, genetic background, clinical variants and therapy. Int J Mol Sci. 2016;17:E1214.
8. Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: Part II. Management and therapeutics. J Am Acad Dermatol. 2013;68:709.e1-e9.
9. Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: Part I. Clinical perspectives. J Am Acad Dermatol. 2013;68:693.e1-e14.
10. Bastuji-Garin S, Rzany B, Stern RS, et al. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129:92-96.
11. Roujeau JC. The spectrum of Stevens-Johnson syndrome and toxic epidermal necrolysis: a clinical classification. J Invest Dermatol. 1994;102:28S-30S.
A new protocol for RhD-negative pregnant women?
ILLUSTRATIVE CASE
A 30-year-old G1P0 woman presents to your office for routine obstetric care at 18 weeks’ gestation. Her pregnancy has been uncomplicated, but her prenatal lab evaluation is notable for blood type A-negative. She wants to know if she really needs the anti-D immune globulin injection.
Rhesus (Rh)D-negative women carrying an RhD-positive fetus are at risk of developing anti-D antibodies, placing the fetus at risk for HDFN (hemolytic disease of the fetus and newborn). If undiagnosed and/or untreated, HDFN carries significant risk of perinatal morbidity and mortality.2
With routine postnatal anti-D immunoglobulin prophylaxis of RhD-negative women who delivered an RhD-positive child (which began around 1970), the risk of maternal alloimmunization was reduced from 16% to 1.12%-1.3%.3-5 The risk was further reduced to approximately 0.28% with the addition of consistent prophylaxis at 28 weeks’ gestation.4 As a result, the current standard of care is to administer anti-D immunoglobulin at 28 weeks’ gestation, within 72 hours of delivery of an RhD-positive fetus, and after events with risk of fetal-to-maternal transfusion (eg, spontaneous, threatened, or induced abortion; invasive prenatal diagnostic procedures such as amniocentesis; blunt abdominal trauma; external cephalic version; second or third trimester antepartum bleeding).6
The problem of unnecessary Tx. However, under this current practice, many RhD-negative women are receiving anti-D immunoglobulin unnecessarily. This is because the fetus’s RhD status is not routinely known during the prenatal period.
Enter cell-free DNA testing. Cell-free DNA testing analyzes fragments of fetal DNA found in maternal blood. The use of cell-free DNA testing at 10 to 13 weeks’ gestation to screen for fetal chromosomal abnormalities is reliable (91%-99% sensitivity for trisomies 21, 18, and 137) and becoming increasingly more common.
A notable meta-analysis. A 2017 meta-analysis of 30 studies of cell-free DNA testing of RhD status in the first and second trimester calculated a sensitivity of 99.3% (95% confidence interval [CI], 98.2-99.7) and a specificity of 98.4% (95% CI, 96.4-99.3).7
This study evaluated the accuracy of using cell-free DNA testing at 27 weeks’ gestation to determine fetal RhD status compared with serologic typing of cord blood at delivery.
STUDY SUMMARY
Cell-free DNA test gets high marks in Netherlands trial
This large observational cohort trial from the Netherlands examined the accuracy of identifying RhD-positive fetuses using cell-free DNA isolates in maternal plasma. Over the 15-month study period, fetal RhD testing was conducted during Week 27 of gestation, and results were compared with those obtained using neonatal cord blood at birth. If the fetal RhD test was positive, providers administered 200 mcg anti-D immunoglobulin during the 30th week of gestation and within 48 hours of birth. If fetal RhD was negative, providers were told immunoglobulin was unnecessary.
More than 32,000 RhD-negative women were screened. The cell-free DNA test showed fetal RhD-positive results 62% of the time and RhD-negative results in the remainder. Cord blood samples were available for 25,789 pregnancies (80%).
Sensitivity, specificity. The sensitivity for identifying fetal RhD was 99% and the specificity was 98%. Both negative and positive predictive values were 99%. Overall, there were 225 false-positive results and 9 false-negative results. In the 9 false negatives, 6 were due to a lack of fetal DNA in the sample and 3 were due to technical error (defined as an operator ignoring a failure of the robot pipetting the plasma or other technical failures).
The false-negative rate (0.03%) was lower than the predetermined estimated false-negative rate of cord blood serology (0.25%). In 22 of the supposed false positives, follow-up serology or molecular testing found an RhD gene was actually present, meaning the results of the neonatal cord blood serology in these cases were falsely negative. If you recalculate with these data in mind, the false-negative rate for fetal DNA testing was actually less than half that of typical serologic determination.
WHAT’S NEW
An accurate test with the potential to reduce unnecessary Tx
Fetal RhD testing at 27 weeks’ gestation appears to be highly accurate and could reduce the unnecessary use of anti-D immunoglobulin when the fetal RhD is negative.
CAVEATS
Different results with different ethnicities?
Dutch participants are not necessarily reflective of the US population. Known variation in the rate of fetal RhD positivity among RhD-negative pregnant women by race and ethnicity could mean that the number of women able to forego anti-D-immunoglobulin prophylaxis would be different in the United States from that in other countries.
Also, in this study, polymerase chain reaction (PCR) for 2 RhD sequences was run in triplicate, and a computer-based algorithm was used to automatically score samples to provide results. For safe implementation, the cell-free fetal RhD DNA testing process would need to follow similar methods.
CHALLENGES TO IMPLEMENTATION
Test cost and availability are big unknowns
Cost and availability of the test may be barriers, but there is currently too little information on either subject in the United States to make a determination. A 2013 study indicated that the use of cell-free DNA testing to determine fetal RhD status was then approximately $682.10
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. de Haas M, Thurik FF, van der Ploeg CP, et al. Sensitivity of fetal RHD screening for safe guidance of targeted anti-D immunoglobulin prophylaxis: prospective cohort study of a nationwide programme in the Netherlands. BMJ. 2016;355:i5789.
2. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 75: Management of alloimmunization during pregnancy. Obstet Gynecol. 2006;108:457-464.
3. Urbaniak S, Greiss MA. RhD haemolytic disease of the fetus and the newborn. Blood Rev. 2000;14:44-61.
4. Mayne S, Parker JH, Harden TA, et al. Rate of RhD sensitisation before and after implementation of a community based antenatal prophylaxis programme. BMJ. 1997;315:1588-1588.
5. MacKenzie IZ, Bowell P, Gregory H, et al. Routine antenatal Rhesus D immunoglobulin prophylaxis: the results of a prospective 10 year study. Br J Obstet Gynecol: 1999;106:492-497.
6. Zolotor AJ, Carlough MC. Update on prenatal care. Am Fam Physician. 2014;89:199-208.
7. Mackie FL, Hemming K, Allen S, et al. The accuracy of cell-free fetal DNA-based non-invasive prenatal testing in singleton pregnancies: a systematic review and bivariate meta-analysis. BJOG. 2017;124:32-46.
8. Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 181: Prevention of Rh D Alloimmunization. Obstet Gynecol. 2017;130:e57-e70.
9. National Institute for Health and Care Excellence. High-throughput non-invasive prenatal testing for fetal RHD genotype 1: Recommendations. Available at: https://www.nice.org.uk/guidance/dg25/chapter/1-Recommendations. Accessed August 9, 2017.
10. Hawk AF, Chang EY, Shields SM, et al. Costs and clinical outcomes of noninvasive fetal RhD typing for targeted prophylaxis. Obstet Gynecol. 2013;122:579-585.
ILLUSTRATIVE CASE
A 30-year-old G1P0 woman presents to your office for routine obstetric care at 18 weeks’ gestation. Her pregnancy has been uncomplicated, but her prenatal lab evaluation is notable for blood type A-negative. She wants to know if she really needs the anti-D immune globulin injection.
Rhesus (Rh)D-negative women carrying an RhD-positive fetus are at risk of developing anti-D antibodies, placing the fetus at risk for HDFN (hemolytic disease of the fetus and newborn). If undiagnosed and/or untreated, HDFN carries significant risk of perinatal morbidity and mortality.2
With routine postnatal anti-D immunoglobulin prophylaxis of RhD-negative women who delivered an RhD-positive child (which began around 1970), the risk of maternal alloimmunization was reduced from 16% to 1.12%-1.3%.3-5 The risk was further reduced to approximately 0.28% with the addition of consistent prophylaxis at 28 weeks’ gestation.4 As a result, the current standard of care is to administer anti-D immunoglobulin at 28 weeks’ gestation, within 72 hours of delivery of an RhD-positive fetus, and after events with risk of fetal-to-maternal transfusion (eg, spontaneous, threatened, or induced abortion; invasive prenatal diagnostic procedures such as amniocentesis; blunt abdominal trauma; external cephalic version; second or third trimester antepartum bleeding).6
The problem of unnecessary Tx. However, under this current practice, many RhD-negative women are receiving anti-D immunoglobulin unnecessarily. This is because the fetus’s RhD status is not routinely known during the prenatal period.
Enter cell-free DNA testing. Cell-free DNA testing analyzes fragments of fetal DNA found in maternal blood. The use of cell-free DNA testing at 10 to 13 weeks’ gestation to screen for fetal chromosomal abnormalities is reliable (91%-99% sensitivity for trisomies 21, 18, and 137) and becoming increasingly more common.
A notable meta-analysis. A 2017 meta-analysis of 30 studies of cell-free DNA testing of RhD status in the first and second trimester calculated a sensitivity of 99.3% (95% confidence interval [CI], 98.2-99.7) and a specificity of 98.4% (95% CI, 96.4-99.3).7
This study evaluated the accuracy of using cell-free DNA testing at 27 weeks’ gestation to determine fetal RhD status compared with serologic typing of cord blood at delivery.
STUDY SUMMARY
Cell-free DNA test gets high marks in Netherlands trial
This large observational cohort trial from the Netherlands examined the accuracy of identifying RhD-positive fetuses using cell-free DNA isolates in maternal plasma. Over the 15-month study period, fetal RhD testing was conducted during Week 27 of gestation, and results were compared with those obtained using neonatal cord blood at birth. If the fetal RhD test was positive, providers administered 200 mcg anti-D immunoglobulin during the 30th week of gestation and within 48 hours of birth. If fetal RhD was negative, providers were told immunoglobulin was unnecessary.
More than 32,000 RhD-negative women were screened. The cell-free DNA test showed fetal RhD-positive results 62% of the time and RhD-negative results in the remainder. Cord blood samples were available for 25,789 pregnancies (80%).
Sensitivity, specificity. The sensitivity for identifying fetal RhD was 99% and the specificity was 98%. Both negative and positive predictive values were 99%. Overall, there were 225 false-positive results and 9 false-negative results. In the 9 false negatives, 6 were due to a lack of fetal DNA in the sample and 3 were due to technical error (defined as an operator ignoring a failure of the robot pipetting the plasma or other technical failures).
The false-negative rate (0.03%) was lower than the predetermined estimated false-negative rate of cord blood serology (0.25%). In 22 of the supposed false positives, follow-up serology or molecular testing found an RhD gene was actually present, meaning the results of the neonatal cord blood serology in these cases were falsely negative. If you recalculate with these data in mind, the false-negative rate for fetal DNA testing was actually less than half that of typical serologic determination.
WHAT’S NEW
An accurate test with the potential to reduce unnecessary Tx
Fetal RhD testing at 27 weeks’ gestation appears to be highly accurate and could reduce the unnecessary use of anti-D immunoglobulin when the fetal RhD is negative.
CAVEATS
Different results with different ethnicities?
Dutch participants are not necessarily reflective of the US population. Known variation in the rate of fetal RhD positivity among RhD-negative pregnant women by race and ethnicity could mean that the number of women able to forego anti-D-immunoglobulin prophylaxis would be different in the United States from that in other countries.
Also, in this study, polymerase chain reaction (PCR) for 2 RhD sequences was run in triplicate, and a computer-based algorithm was used to automatically score samples to provide results. For safe implementation, the cell-free fetal RhD DNA testing process would need to follow similar methods.
CHALLENGES TO IMPLEMENTATION
Test cost and availability are big unknowns
Cost and availability of the test may be barriers, but there is currently too little information on either subject in the United States to make a determination. A 2013 study indicated that the use of cell-free DNA testing to determine fetal RhD status was then approximately $682.10
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 30-year-old G1P0 woman presents to your office for routine obstetric care at 18 weeks’ gestation. Her pregnancy has been uncomplicated, but her prenatal lab evaluation is notable for blood type A-negative. She wants to know if she really needs the anti-D immune globulin injection.
Rhesus (Rh)D-negative women carrying an RhD-positive fetus are at risk of developing anti-D antibodies, placing the fetus at risk for HDFN (hemolytic disease of the fetus and newborn). If undiagnosed and/or untreated, HDFN carries significant risk of perinatal morbidity and mortality.2
With routine postnatal anti-D immunoglobulin prophylaxis of RhD-negative women who delivered an RhD-positive child (which began around 1970), the risk of maternal alloimmunization was reduced from 16% to 1.12%-1.3%.3-5 The risk was further reduced to approximately 0.28% with the addition of consistent prophylaxis at 28 weeks’ gestation.4 As a result, the current standard of care is to administer anti-D immunoglobulin at 28 weeks’ gestation, within 72 hours of delivery of an RhD-positive fetus, and after events with risk of fetal-to-maternal transfusion (eg, spontaneous, threatened, or induced abortion; invasive prenatal diagnostic procedures such as amniocentesis; blunt abdominal trauma; external cephalic version; second or third trimester antepartum bleeding).6
The problem of unnecessary Tx. However, under this current practice, many RhD-negative women are receiving anti-D immunoglobulin unnecessarily. This is because the fetus’s RhD status is not routinely known during the prenatal period.
Enter cell-free DNA testing. Cell-free DNA testing analyzes fragments of fetal DNA found in maternal blood. The use of cell-free DNA testing at 10 to 13 weeks’ gestation to screen for fetal chromosomal abnormalities is reliable (91%-99% sensitivity for trisomies 21, 18, and 137) and becoming increasingly more common.
A notable meta-analysis. A 2017 meta-analysis of 30 studies of cell-free DNA testing of RhD status in the first and second trimester calculated a sensitivity of 99.3% (95% confidence interval [CI], 98.2-99.7) and a specificity of 98.4% (95% CI, 96.4-99.3).7
This study evaluated the accuracy of using cell-free DNA testing at 27 weeks’ gestation to determine fetal RhD status compared with serologic typing of cord blood at delivery.
STUDY SUMMARY
Cell-free DNA test gets high marks in Netherlands trial
This large observational cohort trial from the Netherlands examined the accuracy of identifying RhD-positive fetuses using cell-free DNA isolates in maternal plasma. Over the 15-month study period, fetal RhD testing was conducted during Week 27 of gestation, and results were compared with those obtained using neonatal cord blood at birth. If the fetal RhD test was positive, providers administered 200 mcg anti-D immunoglobulin during the 30th week of gestation and within 48 hours of birth. If fetal RhD was negative, providers were told immunoglobulin was unnecessary.
More than 32,000 RhD-negative women were screened. The cell-free DNA test showed fetal RhD-positive results 62% of the time and RhD-negative results in the remainder. Cord blood samples were available for 25,789 pregnancies (80%).
Sensitivity, specificity. The sensitivity for identifying fetal RhD was 99% and the specificity was 98%. Both negative and positive predictive values were 99%. Overall, there were 225 false-positive results and 9 false-negative results. In the 9 false negatives, 6 were due to a lack of fetal DNA in the sample and 3 were due to technical error (defined as an operator ignoring a failure of the robot pipetting the plasma or other technical failures).
The false-negative rate (0.03%) was lower than the predetermined estimated false-negative rate of cord blood serology (0.25%). In 22 of the supposed false positives, follow-up serology or molecular testing found an RhD gene was actually present, meaning the results of the neonatal cord blood serology in these cases were falsely negative. If you recalculate with these data in mind, the false-negative rate for fetal DNA testing was actually less than half that of typical serologic determination.
WHAT’S NEW
An accurate test with the potential to reduce unnecessary Tx
Fetal RhD testing at 27 weeks’ gestation appears to be highly accurate and could reduce the unnecessary use of anti-D immunoglobulin when the fetal RhD is negative.
CAVEATS
Different results with different ethnicities?
Dutch participants are not necessarily reflective of the US population. Known variation in the rate of fetal RhD positivity among RhD-negative pregnant women by race and ethnicity could mean that the number of women able to forego anti-D-immunoglobulin prophylaxis would be different in the United States from that in other countries.
Also, in this study, polymerase chain reaction (PCR) for 2 RhD sequences was run in triplicate, and a computer-based algorithm was used to automatically score samples to provide results. For safe implementation, the cell-free fetal RhD DNA testing process would need to follow similar methods.
CHALLENGES TO IMPLEMENTATION
Test cost and availability are big unknowns
Cost and availability of the test may be barriers, but there is currently too little information on either subject in the United States to make a determination. A 2013 study indicated that the use of cell-free DNA testing to determine fetal RhD status was then approximately $682.10
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. de Haas M, Thurik FF, van der Ploeg CP, et al. Sensitivity of fetal RHD screening for safe guidance of targeted anti-D immunoglobulin prophylaxis: prospective cohort study of a nationwide programme in the Netherlands. BMJ. 2016;355:i5789.
2. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 75: Management of alloimmunization during pregnancy. Obstet Gynecol. 2006;108:457-464.
3. Urbaniak S, Greiss MA. RhD haemolytic disease of the fetus and the newborn. Blood Rev. 2000;14:44-61.
4. Mayne S, Parker JH, Harden TA, et al. Rate of RhD sensitisation before and after implementation of a community based antenatal prophylaxis programme. BMJ. 1997;315:1588-1588.
5. MacKenzie IZ, Bowell P, Gregory H, et al. Routine antenatal Rhesus D immunoglobulin prophylaxis: the results of a prospective 10 year study. Br J Obstet Gynecol: 1999;106:492-497.
6. Zolotor AJ, Carlough MC. Update on prenatal care. Am Fam Physician. 2014;89:199-208.
7. Mackie FL, Hemming K, Allen S, et al. The accuracy of cell-free fetal DNA-based non-invasive prenatal testing in singleton pregnancies: a systematic review and bivariate meta-analysis. BJOG. 2017;124:32-46.
8. Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 181: Prevention of Rh D Alloimmunization. Obstet Gynecol. 2017;130:e57-e70.
9. National Institute for Health and Care Excellence. High-throughput non-invasive prenatal testing for fetal RHD genotype 1: Recommendations. Available at: https://www.nice.org.uk/guidance/dg25/chapter/1-Recommendations. Accessed August 9, 2017.
10. Hawk AF, Chang EY, Shields SM, et al. Costs and clinical outcomes of noninvasive fetal RhD typing for targeted prophylaxis. Obstet Gynecol. 2013;122:579-585.
1. de Haas M, Thurik FF, van der Ploeg CP, et al. Sensitivity of fetal RHD screening for safe guidance of targeted anti-D immunoglobulin prophylaxis: prospective cohort study of a nationwide programme in the Netherlands. BMJ. 2016;355:i5789.
2. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 75: Management of alloimmunization during pregnancy. Obstet Gynecol. 2006;108:457-464.
3. Urbaniak S, Greiss MA. RhD haemolytic disease of the fetus and the newborn. Blood Rev. 2000;14:44-61.
4. Mayne S, Parker JH, Harden TA, et al. Rate of RhD sensitisation before and after implementation of a community based antenatal prophylaxis programme. BMJ. 1997;315:1588-1588.
5. MacKenzie IZ, Bowell P, Gregory H, et al. Routine antenatal Rhesus D immunoglobulin prophylaxis: the results of a prospective 10 year study. Br J Obstet Gynecol: 1999;106:492-497.
6. Zolotor AJ, Carlough MC. Update on prenatal care. Am Fam Physician. 2014;89:199-208.
7. Mackie FL, Hemming K, Allen S, et al. The accuracy of cell-free fetal DNA-based non-invasive prenatal testing in singleton pregnancies: a systematic review and bivariate meta-analysis. BJOG. 2017;124:32-46.
8. Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 181: Prevention of Rh D Alloimmunization. Obstet Gynecol. 2017;130:e57-e70.
9. National Institute for Health and Care Excellence. High-throughput non-invasive prenatal testing for fetal RHD genotype 1: Recommendations. Available at: https://www.nice.org.uk/guidance/dg25/chapter/1-Recommendations. Accessed August 9, 2017.
10. Hawk AF, Chang EY, Shields SM, et al. Costs and clinical outcomes of noninvasive fetal RhD typing for targeted prophylaxis. Obstet Gynecol. 2013;122:579-585.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
Employ cell-free DNA testing at 27 weeks’ gestation in your RhD-negative obstetric patients to reduce unnecessary use of anti-D immunoglobulin.1
STRENGTH OF RECOMMENDATION
B: Based on a single, prospective, cohort study.
de Haas M, Thurik FF, van der Ploeg CP, et al. Sensitivity of fetal RHD screening for safe guidance of targeted anti-D immunoglobulin prophylaxis: prospective cohort study of a nationwide programme in the Netherlands. BMJ. 2016;355:i5789.
Critical anemia • light-headedness • bilateral leg swelling • Dx?
THE CASE
A 40-year-old man was referred to the emergency department (ED) with critical anemia after routine blood work at an outside clinic showed a hemoglobin level of 3.5 g/dL. On presentation, he reported symptoms of fatigue, shortness of breath, bilateral leg swelling, dizziness (characterized as light-headedness), and frequent heartburn. He said that the symptoms began 5 weeks earlier, after he was exposed to a relative with hand, foot, and mouth disease.
Additionally, the patient reported an intentional 14-lb weight loss over the 6 months prior to presentation. He denied fever, rash, chest pain, loss of consciousness, headache, abdominal pain, hematemesis, melena, and hematochezia. His medical history was significant for peptic ulcer disease (diagnosed and treated at age 8). He did not recall the specifics, and he denied any related chronic symptoms or complications. His family history (paternal) was significant for colon cancer.
The physical exam revealed conjunctival pallor, skin pallor, jaundice, +1 bilateral lower extremity edema, tachycardia, and tachypnea. Stool Hemoccult was negative. On repeat complete blood count (performed in the ED), hemoglobin was found to be 3.1 g/dL with a mean corpuscular volume of 47 fL.
THE DIAGNOSIS
The patient was admitted to the family medicine service and received 4 units of packed red blood cells, which increased his hemoglobin to the target goal of >7 g/dL. A colonoscopy and an esophagogastroduodenoscopy (EGD) were performed (FIGURE 1A-1C), with results suggestive of diverticulosis, probable Barrett’s mucosa, esophageal ulcer, huge hiatal hernia (at least one-half of the stomach was in the chest), and Cameron ulcers. Esophageal biopsies showed cardiac mucosa with chronic inflammation. Esophageal ulcer biopsies revealed Barrett’s esophagus without dysplasia. Duodenal biopsies displayed normal mucosa.
DISCUSSION
Although rare, Cameron ulcers must be considered in the differential diagnosis of patients with chronic anemia of unknown origin. The potential for these lesions to result in chronic blood loss, which could over time manifest as severe anemia or hypovolemic shock, makes proper diagnosis and prompt treatment especially important.4,5
In our patient’s case, his severe anemia was likely the result of a combination of the esophageal and Cameron ulcers evidenced on EGD, rather than any single ulcer. In our review of the literature, we found no reports of any patients with anemia and Cameron ulcers who presented with hemoglobin levels as low as our patient had.
Treat with a PPI and iron supplementation
Multiple EGDs may be needed to properly diagnose Cameron ulcers, as they can be difficult to identify. Once a patient receives the diagnosis, he or she will typically be put on a daily proton pump inhibitor (PPI) regimen, such as omeprazole 20 mg bid. However, since many patients with Cameron ulcers also have acid-related problems (as was true in this case), a multifactorial acid suppression approach may be warranted.1 This may include recommending lifestyle modifications (eg, eating small meals, avoiding foods that provoke symptoms, or losing weight) and prescribing medications in addition to a PPI, such as an H2 blocker (eg, 300 mg qid, before meals and at bedtime).
In addition, iron sulfate (325 mg/d, in this case) and blood transfusions may be required to treat the anemia. In refractory cases, endoscopic or surgical interventions, such as hemoclipping, Nissen fundoplication, or laparoscopic gastropexy, may need to be performed.2
Our patient was given a prescription for ferrous sulfate 325 mg/d and omeprazole 20 mg bid. His symptoms improved with treatment, and he was discharged on Day 5; his hemoglobin remained >7 g/dL.
THE TAKEAWAY
The association between chronic iron deficiency anemia and Cameron ulcers has been established but is commonly overlooked in patients presenting with unexplained anemia or an undiagnosed hiatal hernia. This is likely due to their rarity as a cause of anemia, in general.
Furthermore, the lesions can be missed on EGD; multiple EGDs may be needed to make the diagnosis. Once diagnosed, Cameron ulcers typically respond well to twice daily PPI treatment. Patients with refractory, recurrent, or severe lesions, or large, symptomatic hiatal hernias should be referred for surgical assessment.
CORRESPONDENCE
Megan Yee, 801 Broadward Avenue NW, Grand Rapids, MI 49504; Megan.Yee@mercyhealth.com.
1. Maganty K, Smith RL. Cameron lesions: unusual cause of gastrointestinal bleeding and anemia. Digestion. 2008;77:214-217.
2. Camus M, Jensen DM, Ohning GV, et al. Severe upper gastrointestinal hemorrhage from linear gastric ulcers in large hiatal hernias: a large prospective case series of Cameron ulcers. Endoscopy. 2013;45:397-400.
3. Kimer N, Schmidt PN, Krag, A. Cameron lesions: an often overlooked cause of iron deficiency anaemia in patients with large hiatal hernias. BMJ Case Rep. 2010;2010.
4. Kapadia S, Jagroop S, Kumar, A. Cameron ulcers: an atypical source for a massive upper gastrointestinal bleed. World J Gastroenterol. 2012;18:4959-4961.
5. Gupta P, Suryadevara M, Das A, et al. Cameron ulcer causing severe anemia in a patient with diaphragmatic hernia. Am J Case Rep. 2015;16:733-736.
THE CASE
A 40-year-old man was referred to the emergency department (ED) with critical anemia after routine blood work at an outside clinic showed a hemoglobin level of 3.5 g/dL. On presentation, he reported symptoms of fatigue, shortness of breath, bilateral leg swelling, dizziness (characterized as light-headedness), and frequent heartburn. He said that the symptoms began 5 weeks earlier, after he was exposed to a relative with hand, foot, and mouth disease.
Additionally, the patient reported an intentional 14-lb weight loss over the 6 months prior to presentation. He denied fever, rash, chest pain, loss of consciousness, headache, abdominal pain, hematemesis, melena, and hematochezia. His medical history was significant for peptic ulcer disease (diagnosed and treated at age 8). He did not recall the specifics, and he denied any related chronic symptoms or complications. His family history (paternal) was significant for colon cancer.
The physical exam revealed conjunctival pallor, skin pallor, jaundice, +1 bilateral lower extremity edema, tachycardia, and tachypnea. Stool Hemoccult was negative. On repeat complete blood count (performed in the ED), hemoglobin was found to be 3.1 g/dL with a mean corpuscular volume of 47 fL.
THE DIAGNOSIS
The patient was admitted to the family medicine service and received 4 units of packed red blood cells, which increased his hemoglobin to the target goal of >7 g/dL. A colonoscopy and an esophagogastroduodenoscopy (EGD) were performed (FIGURE 1A-1C), with results suggestive of diverticulosis, probable Barrett’s mucosa, esophageal ulcer, huge hiatal hernia (at least one-half of the stomach was in the chest), and Cameron ulcers. Esophageal biopsies showed cardiac mucosa with chronic inflammation. Esophageal ulcer biopsies revealed Barrett’s esophagus without dysplasia. Duodenal biopsies displayed normal mucosa.
DISCUSSION
Although rare, Cameron ulcers must be considered in the differential diagnosis of patients with chronic anemia of unknown origin. The potential for these lesions to result in chronic blood loss, which could over time manifest as severe anemia or hypovolemic shock, makes proper diagnosis and prompt treatment especially important.4,5
In our patient’s case, his severe anemia was likely the result of a combination of the esophageal and Cameron ulcers evidenced on EGD, rather than any single ulcer. In our review of the literature, we found no reports of any patients with anemia and Cameron ulcers who presented with hemoglobin levels as low as our patient had.
Treat with a PPI and iron supplementation
Multiple EGDs may be needed to properly diagnose Cameron ulcers, as they can be difficult to identify. Once a patient receives the diagnosis, he or she will typically be put on a daily proton pump inhibitor (PPI) regimen, such as omeprazole 20 mg bid. However, since many patients with Cameron ulcers also have acid-related problems (as was true in this case), a multifactorial acid suppression approach may be warranted.1 This may include recommending lifestyle modifications (eg, eating small meals, avoiding foods that provoke symptoms, or losing weight) and prescribing medications in addition to a PPI, such as an H2 blocker (eg, 300 mg qid, before meals and at bedtime).
In addition, iron sulfate (325 mg/d, in this case) and blood transfusions may be required to treat the anemia. In refractory cases, endoscopic or surgical interventions, such as hemoclipping, Nissen fundoplication, or laparoscopic gastropexy, may need to be performed.2
Our patient was given a prescription for ferrous sulfate 325 mg/d and omeprazole 20 mg bid. His symptoms improved with treatment, and he was discharged on Day 5; his hemoglobin remained >7 g/dL.
THE TAKEAWAY
The association between chronic iron deficiency anemia and Cameron ulcers has been established but is commonly overlooked in patients presenting with unexplained anemia or an undiagnosed hiatal hernia. This is likely due to their rarity as a cause of anemia, in general.
Furthermore, the lesions can be missed on EGD; multiple EGDs may be needed to make the diagnosis. Once diagnosed, Cameron ulcers typically respond well to twice daily PPI treatment. Patients with refractory, recurrent, or severe lesions, or large, symptomatic hiatal hernias should be referred for surgical assessment.
CORRESPONDENCE
Megan Yee, 801 Broadward Avenue NW, Grand Rapids, MI 49504; Megan.Yee@mercyhealth.com.
THE CASE
A 40-year-old man was referred to the emergency department (ED) with critical anemia after routine blood work at an outside clinic showed a hemoglobin level of 3.5 g/dL. On presentation, he reported symptoms of fatigue, shortness of breath, bilateral leg swelling, dizziness (characterized as light-headedness), and frequent heartburn. He said that the symptoms began 5 weeks earlier, after he was exposed to a relative with hand, foot, and mouth disease.
Additionally, the patient reported an intentional 14-lb weight loss over the 6 months prior to presentation. He denied fever, rash, chest pain, loss of consciousness, headache, abdominal pain, hematemesis, melena, and hematochezia. His medical history was significant for peptic ulcer disease (diagnosed and treated at age 8). He did not recall the specifics, and he denied any related chronic symptoms or complications. His family history (paternal) was significant for colon cancer.
The physical exam revealed conjunctival pallor, skin pallor, jaundice, +1 bilateral lower extremity edema, tachycardia, and tachypnea. Stool Hemoccult was negative. On repeat complete blood count (performed in the ED), hemoglobin was found to be 3.1 g/dL with a mean corpuscular volume of 47 fL.
THE DIAGNOSIS
The patient was admitted to the family medicine service and received 4 units of packed red blood cells, which increased his hemoglobin to the target goal of >7 g/dL. A colonoscopy and an esophagogastroduodenoscopy (EGD) were performed (FIGURE 1A-1C), with results suggestive of diverticulosis, probable Barrett’s mucosa, esophageal ulcer, huge hiatal hernia (at least one-half of the stomach was in the chest), and Cameron ulcers. Esophageal biopsies showed cardiac mucosa with chronic inflammation. Esophageal ulcer biopsies revealed Barrett’s esophagus without dysplasia. Duodenal biopsies displayed normal mucosa.
DISCUSSION
Although rare, Cameron ulcers must be considered in the differential diagnosis of patients with chronic anemia of unknown origin. The potential for these lesions to result in chronic blood loss, which could over time manifest as severe anemia or hypovolemic shock, makes proper diagnosis and prompt treatment especially important.4,5
In our patient’s case, his severe anemia was likely the result of a combination of the esophageal and Cameron ulcers evidenced on EGD, rather than any single ulcer. In our review of the literature, we found no reports of any patients with anemia and Cameron ulcers who presented with hemoglobin levels as low as our patient had.
Treat with a PPI and iron supplementation
Multiple EGDs may be needed to properly diagnose Cameron ulcers, as they can be difficult to identify. Once a patient receives the diagnosis, he or she will typically be put on a daily proton pump inhibitor (PPI) regimen, such as omeprazole 20 mg bid. However, since many patients with Cameron ulcers also have acid-related problems (as was true in this case), a multifactorial acid suppression approach may be warranted.1 This may include recommending lifestyle modifications (eg, eating small meals, avoiding foods that provoke symptoms, or losing weight) and prescribing medications in addition to a PPI, such as an H2 blocker (eg, 300 mg qid, before meals and at bedtime).
In addition, iron sulfate (325 mg/d, in this case) and blood transfusions may be required to treat the anemia. In refractory cases, endoscopic or surgical interventions, such as hemoclipping, Nissen fundoplication, or laparoscopic gastropexy, may need to be performed.2
Our patient was given a prescription for ferrous sulfate 325 mg/d and omeprazole 20 mg bid. His symptoms improved with treatment, and he was discharged on Day 5; his hemoglobin remained >7 g/dL.
THE TAKEAWAY
The association between chronic iron deficiency anemia and Cameron ulcers has been established but is commonly overlooked in patients presenting with unexplained anemia or an undiagnosed hiatal hernia. This is likely due to their rarity as a cause of anemia, in general.
Furthermore, the lesions can be missed on EGD; multiple EGDs may be needed to make the diagnosis. Once diagnosed, Cameron ulcers typically respond well to twice daily PPI treatment. Patients with refractory, recurrent, or severe lesions, or large, symptomatic hiatal hernias should be referred for surgical assessment.
CORRESPONDENCE
Megan Yee, 801 Broadward Avenue NW, Grand Rapids, MI 49504; Megan.Yee@mercyhealth.com.
1. Maganty K, Smith RL. Cameron lesions: unusual cause of gastrointestinal bleeding and anemia. Digestion. 2008;77:214-217.
2. Camus M, Jensen DM, Ohning GV, et al. Severe upper gastrointestinal hemorrhage from linear gastric ulcers in large hiatal hernias: a large prospective case series of Cameron ulcers. Endoscopy. 2013;45:397-400.
3. Kimer N, Schmidt PN, Krag, A. Cameron lesions: an often overlooked cause of iron deficiency anaemia in patients with large hiatal hernias. BMJ Case Rep. 2010;2010.
4. Kapadia S, Jagroop S, Kumar, A. Cameron ulcers: an atypical source for a massive upper gastrointestinal bleed. World J Gastroenterol. 2012;18:4959-4961.
5. Gupta P, Suryadevara M, Das A, et al. Cameron ulcer causing severe anemia in a patient with diaphragmatic hernia. Am J Case Rep. 2015;16:733-736.
1. Maganty K, Smith RL. Cameron lesions: unusual cause of gastrointestinal bleeding and anemia. Digestion. 2008;77:214-217.
2. Camus M, Jensen DM, Ohning GV, et al. Severe upper gastrointestinal hemorrhage from linear gastric ulcers in large hiatal hernias: a large prospective case series of Cameron ulcers. Endoscopy. 2013;45:397-400.
3. Kimer N, Schmidt PN, Krag, A. Cameron lesions: an often overlooked cause of iron deficiency anaemia in patients with large hiatal hernias. BMJ Case Rep. 2010;2010.
4. Kapadia S, Jagroop S, Kumar, A. Cameron ulcers: an atypical source for a massive upper gastrointestinal bleed. World J Gastroenterol. 2012;18:4959-4961.
5. Gupta P, Suryadevara M, Das A, et al. Cameron ulcer causing severe anemia in a patient with diaphragmatic hernia. Am J Case Rep. 2015;16:733-736.
When the correct Dx is elusive
In this issue of JFP, Dr. Mendoza reminds us that “Parkinson’s disease can be a tough diagnosis to navigate.”1 Classically, Parkinson’s disease (PD) is associated with a resting tremor, but bradykinesia is actually the hallmark of the disease. PD can also present with subtle movement disorders, as well as depression and early dementia. It is, indeed, a difficult clinical diagnosis, and consultation with an expert to confirm or deny its presence can be quite helpful.
Other conundrums. PD, however, is not the only illness whose signs and symptoms can present a challenge. Chronic and intermittent shortness of breath, for example, can be very difficult to sort out. Is the shortness of breath due to congestive heart failure, chronic obstructive pulmonary disease, asthma, or a neurologic condition such as myasthenia gravis? Or is it the result of several causes?
When asthma isn’t asthma. Because it is a common illness, physicians often diagnose asthma in patients with shortness of breath or wheezing. But a recent study suggests that as many as 30% of primary care patients with a current diagnosis of asthma do not have asthma at all.2
In the study, Canadian researchers recruited 701 adults with physician-diagnosed asthma, all of whom were taking asthma medications regularly. The researchers did baseline pulmonary function testing (including methacholine challenge testing, if needed) and monitored symptoms frequently. Then they gradually withdrew asthma medications from those who did not appear to have a definitive diagnosis of asthma. They followed these patients for one year. One-third (203 of 613) of the patients with complete follow-up data were no longer taking asthma medications one year later and had no symptoms of asthma. Twelve patients had serious alternative diagnoses such as coronary artery disease and bronchiectasis.
Closer to home. In my practice, I found 2 patients with long-standing diagnoses of asthma who didn’t, in fact, have the condition at all. In both cases, my suspicion was raised by lung examination. In one case, fine bibasilar rales suggested pulmonary fibrosis, which was the correct diagnosis, and the patient is now on the lung transplant list. In the other case, a loud venous hum suggested an arteriovenous malformation. Surgery corrected the patient’s “asthma.”
I urge you to reevaluate your asthma patients to be sure they have the correct diagnosis and to keep PD in your differential for patients who present with atypical symptoms. Primary care clinicians must be expert diagnosticians, willing to question prior diagnoses.
1. Young J, Mendoza M. Parkinson’s disease: a treatment guide. J Fam Pract. 2018;67:276-286.
2. Aaron SD, Vandemheen KL, FitzGerald JM, et al for the Canadian Respiratory Research Network. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017:317:269-279.
In this issue of JFP, Dr. Mendoza reminds us that “Parkinson’s disease can be a tough diagnosis to navigate.”1 Classically, Parkinson’s disease (PD) is associated with a resting tremor, but bradykinesia is actually the hallmark of the disease. PD can also present with subtle movement disorders, as well as depression and early dementia. It is, indeed, a difficult clinical diagnosis, and consultation with an expert to confirm or deny its presence can be quite helpful.
Other conundrums. PD, however, is not the only illness whose signs and symptoms can present a challenge. Chronic and intermittent shortness of breath, for example, can be very difficult to sort out. Is the shortness of breath due to congestive heart failure, chronic obstructive pulmonary disease, asthma, or a neurologic condition such as myasthenia gravis? Or is it the result of several causes?
When asthma isn’t asthma. Because it is a common illness, physicians often diagnose asthma in patients with shortness of breath or wheezing. But a recent study suggests that as many as 30% of primary care patients with a current diagnosis of asthma do not have asthma at all.2
In the study, Canadian researchers recruited 701 adults with physician-diagnosed asthma, all of whom were taking asthma medications regularly. The researchers did baseline pulmonary function testing (including methacholine challenge testing, if needed) and monitored symptoms frequently. Then they gradually withdrew asthma medications from those who did not appear to have a definitive diagnosis of asthma. They followed these patients for one year. One-third (203 of 613) of the patients with complete follow-up data were no longer taking asthma medications one year later and had no symptoms of asthma. Twelve patients had serious alternative diagnoses such as coronary artery disease and bronchiectasis.
Closer to home. In my practice, I found 2 patients with long-standing diagnoses of asthma who didn’t, in fact, have the condition at all. In both cases, my suspicion was raised by lung examination. In one case, fine bibasilar rales suggested pulmonary fibrosis, which was the correct diagnosis, and the patient is now on the lung transplant list. In the other case, a loud venous hum suggested an arteriovenous malformation. Surgery corrected the patient’s “asthma.”
I urge you to reevaluate your asthma patients to be sure they have the correct diagnosis and to keep PD in your differential for patients who present with atypical symptoms. Primary care clinicians must be expert diagnosticians, willing to question prior diagnoses.
In this issue of JFP, Dr. Mendoza reminds us that “Parkinson’s disease can be a tough diagnosis to navigate.”1 Classically, Parkinson’s disease (PD) is associated with a resting tremor, but bradykinesia is actually the hallmark of the disease. PD can also present with subtle movement disorders, as well as depression and early dementia. It is, indeed, a difficult clinical diagnosis, and consultation with an expert to confirm or deny its presence can be quite helpful.
Other conundrums. PD, however, is not the only illness whose signs and symptoms can present a challenge. Chronic and intermittent shortness of breath, for example, can be very difficult to sort out. Is the shortness of breath due to congestive heart failure, chronic obstructive pulmonary disease, asthma, or a neurologic condition such as myasthenia gravis? Or is it the result of several causes?
When asthma isn’t asthma. Because it is a common illness, physicians often diagnose asthma in patients with shortness of breath or wheezing. But a recent study suggests that as many as 30% of primary care patients with a current diagnosis of asthma do not have asthma at all.2
In the study, Canadian researchers recruited 701 adults with physician-diagnosed asthma, all of whom were taking asthma medications regularly. The researchers did baseline pulmonary function testing (including methacholine challenge testing, if needed) and monitored symptoms frequently. Then they gradually withdrew asthma medications from those who did not appear to have a definitive diagnosis of asthma. They followed these patients for one year. One-third (203 of 613) of the patients with complete follow-up data were no longer taking asthma medications one year later and had no symptoms of asthma. Twelve patients had serious alternative diagnoses such as coronary artery disease and bronchiectasis.
Closer to home. In my practice, I found 2 patients with long-standing diagnoses of asthma who didn’t, in fact, have the condition at all. In both cases, my suspicion was raised by lung examination. In one case, fine bibasilar rales suggested pulmonary fibrosis, which was the correct diagnosis, and the patient is now on the lung transplant list. In the other case, a loud venous hum suggested an arteriovenous malformation. Surgery corrected the patient’s “asthma.”
I urge you to reevaluate your asthma patients to be sure they have the correct diagnosis and to keep PD in your differential for patients who present with atypical symptoms. Primary care clinicians must be expert diagnosticians, willing to question prior diagnoses.
1. Young J, Mendoza M. Parkinson’s disease: a treatment guide. J Fam Pract. 2018;67:276-286.
2. Aaron SD, Vandemheen KL, FitzGerald JM, et al for the Canadian Respiratory Research Network. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017:317:269-279.
1. Young J, Mendoza M. Parkinson’s disease: a treatment guide. J Fam Pract. 2018;67:276-286.
2. Aaron SD, Vandemheen KL, FitzGerald JM, et al for the Canadian Respiratory Research Network. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017:317:269-279.