User login
Cathepsin K inhibitor exhibits bone protecting effects in osteoarthritis
LIVERPOOL, ENGLAND – The investigational cathepsin K inhibitor MIV-711 had positive effects on both bone and cartilage at 6 months in patients with knee osteoarthritis (OA) in a phase 2a study.
The MRI measures of the “area of bone in the medial femur region” and “average cartilage thickness” showed reduced progression with MIV-711 versus placebo.
MIV-711 also produced rapid and sustained reductions in the bone biomarkers CTX-I and CTX-II, study investigator Philip Conaghan, MBBS, PhD, reported at the World Congress on Osteoarthritis.
The primary endpoint of the trial – the change in average knee pain over 26 weeks assessed using an 11-point numerical rating scale (NRS) – was not met, however, with no differences between MIV-711 treatment and placebo.
While there were also no statistical differences in Western Ontario and McMaster Universities Osteoarthritis Index pain scores, one of several secondary endpoints assessed, there was a trend for less pain with MIV-711 than with placebo.
“The lack of symptom benefits may reflect that the study duration was not sufficient to see symptom reduction following structure modification,” Dr. Conaghan and his coauthors stated in their abstract.
“That’s something we’re seeing with all the drugs that are trying to get DMOAD [disease modifying osteoarthritis drug] licenses at present,“ Dr. Conaghan said at the Congress, sponsored by the Osteoarthritis Research Society International.
Dr. Conaghan, who is professor of musculoskeletal medicine and director of the Leeds (England) Institute of Rheumatic and Musculoskeletal Medicine, noted: “Cathepsin K is a cysteine protease that degrades the collagen matrix and stops bone resorption, so it’s got a number of potential actions,” in the development of OA.
MIV-711 is a potent, selective, and reversible inhibitor of cathepsin K, he added, which has previously been shown to have bone structure–modifying properties in preclinical models. It also has been shown to reduce CTX-I and CTX-II in healthy volunteers.
The current randomized, double-blind, placebo-controlled phase 2a study included two active treatment (100 mg and 200 mg, once daily) arms and one placebo arm. For inclusion, patients had to have knee pain rated as 4 or higher on an 11-point NRS and Kellgren-Lawrence grade 2 or 3 knee OA.
Patients also were allowed to remain on any analgesic medication they were currently taking, provided this was stable. “That enables recruitment as a trialist, and it’s more like my usual practice as it’s unlikely I’d stop an analgesic before starting a new one,” Dr. Conaghan said.
In all, 240 patients were enrolled at six European sites. They had a mean age of 62 years, a body mass index of 32 kg/m2, and 77% were women. MRIs were assessed at baseline and at week 26, with additional clinic visits scheduled at 2, 4, 8, 14, and 20 weeks.
“Overall, there are not any great safety signals,” Dr. Conaghan said. The placebo, 100-mg, and 200-mg MIV-117 arms had similar rates of any adverse event (55%, 54.9%, and 52.4%) or treatment-related adverse events (21.2%, 20.7%, and 24.4%). There were more serious adverse events in the 100-mg and 200-mg MIV-711 treatment arms (3.7% and 2.4%) than in the placebo arm (1.3%), and more discontinuations (7.3%, 4.9%, and 3.8%).
“The primary endpoint of knee pain was not met, but there was a consistent trend, and it looks like there’s a benefit, but the statistically endpoint was not achieved,” Dr. Conaghan summarized.
“MRI measures do show structural modification in terms of bone shape and probably an effect on cartilage,” although the study was not powered to show an effect on the latter, Dr. Conaghan noted.
“The overall data, and from what we’ve seen regarding structural effects, do warrant this drug moving forward into further trials,” he added.
The study was sponsored by Medivir. Dr. Conaghan disclosed being a member of the speaker’s bureau for Kolon TissueGene and Samumed and on advisory boards for AbbVie, Centrexion, Flexion Therapeutics, Medivir, Novartis, and ONO Pharmaceutical.
SOURCE: Conaghan P et al. Osteoarthritis Cartilage. 2018 Apr 16:26(1):S25-26. Abstract 29.
LIVERPOOL, ENGLAND – The investigational cathepsin K inhibitor MIV-711 had positive effects on both bone and cartilage at 6 months in patients with knee osteoarthritis (OA) in a phase 2a study.
The MRI measures of the “area of bone in the medial femur region” and “average cartilage thickness” showed reduced progression with MIV-711 versus placebo.
MIV-711 also produced rapid and sustained reductions in the bone biomarkers CTX-I and CTX-II, study investigator Philip Conaghan, MBBS, PhD, reported at the World Congress on Osteoarthritis.
The primary endpoint of the trial – the change in average knee pain over 26 weeks assessed using an 11-point numerical rating scale (NRS) – was not met, however, with no differences between MIV-711 treatment and placebo.
While there were also no statistical differences in Western Ontario and McMaster Universities Osteoarthritis Index pain scores, one of several secondary endpoints assessed, there was a trend for less pain with MIV-711 than with placebo.
“The lack of symptom benefits may reflect that the study duration was not sufficient to see symptom reduction following structure modification,” Dr. Conaghan and his coauthors stated in their abstract.
“That’s something we’re seeing with all the drugs that are trying to get DMOAD [disease modifying osteoarthritis drug] licenses at present,“ Dr. Conaghan said at the Congress, sponsored by the Osteoarthritis Research Society International.
Dr. Conaghan, who is professor of musculoskeletal medicine and director of the Leeds (England) Institute of Rheumatic and Musculoskeletal Medicine, noted: “Cathepsin K is a cysteine protease that degrades the collagen matrix and stops bone resorption, so it’s got a number of potential actions,” in the development of OA.
MIV-711 is a potent, selective, and reversible inhibitor of cathepsin K, he added, which has previously been shown to have bone structure–modifying properties in preclinical models. It also has been shown to reduce CTX-I and CTX-II in healthy volunteers.
The current randomized, double-blind, placebo-controlled phase 2a study included two active treatment (100 mg and 200 mg, once daily) arms and one placebo arm. For inclusion, patients had to have knee pain rated as 4 or higher on an 11-point NRS and Kellgren-Lawrence grade 2 or 3 knee OA.
Patients also were allowed to remain on any analgesic medication they were currently taking, provided this was stable. “That enables recruitment as a trialist, and it’s more like my usual practice as it’s unlikely I’d stop an analgesic before starting a new one,” Dr. Conaghan said.
In all, 240 patients were enrolled at six European sites. They had a mean age of 62 years, a body mass index of 32 kg/m2, and 77% were women. MRIs were assessed at baseline and at week 26, with additional clinic visits scheduled at 2, 4, 8, 14, and 20 weeks.
“Overall, there are not any great safety signals,” Dr. Conaghan said. The placebo, 100-mg, and 200-mg MIV-117 arms had similar rates of any adverse event (55%, 54.9%, and 52.4%) or treatment-related adverse events (21.2%, 20.7%, and 24.4%). There were more serious adverse events in the 100-mg and 200-mg MIV-711 treatment arms (3.7% and 2.4%) than in the placebo arm (1.3%), and more discontinuations (7.3%, 4.9%, and 3.8%).
“The primary endpoint of knee pain was not met, but there was a consistent trend, and it looks like there’s a benefit, but the statistically endpoint was not achieved,” Dr. Conaghan summarized.
“MRI measures do show structural modification in terms of bone shape and probably an effect on cartilage,” although the study was not powered to show an effect on the latter, Dr. Conaghan noted.
“The overall data, and from what we’ve seen regarding structural effects, do warrant this drug moving forward into further trials,” he added.
The study was sponsored by Medivir. Dr. Conaghan disclosed being a member of the speaker’s bureau for Kolon TissueGene and Samumed and on advisory boards for AbbVie, Centrexion, Flexion Therapeutics, Medivir, Novartis, and ONO Pharmaceutical.
SOURCE: Conaghan P et al. Osteoarthritis Cartilage. 2018 Apr 16:26(1):S25-26. Abstract 29.
LIVERPOOL, ENGLAND – The investigational cathepsin K inhibitor MIV-711 had positive effects on both bone and cartilage at 6 months in patients with knee osteoarthritis (OA) in a phase 2a study.
The MRI measures of the “area of bone in the medial femur region” and “average cartilage thickness” showed reduced progression with MIV-711 versus placebo.
MIV-711 also produced rapid and sustained reductions in the bone biomarkers CTX-I and CTX-II, study investigator Philip Conaghan, MBBS, PhD, reported at the World Congress on Osteoarthritis.
The primary endpoint of the trial – the change in average knee pain over 26 weeks assessed using an 11-point numerical rating scale (NRS) – was not met, however, with no differences between MIV-711 treatment and placebo.
While there were also no statistical differences in Western Ontario and McMaster Universities Osteoarthritis Index pain scores, one of several secondary endpoints assessed, there was a trend for less pain with MIV-711 than with placebo.
“The lack of symptom benefits may reflect that the study duration was not sufficient to see symptom reduction following structure modification,” Dr. Conaghan and his coauthors stated in their abstract.
“That’s something we’re seeing with all the drugs that are trying to get DMOAD [disease modifying osteoarthritis drug] licenses at present,“ Dr. Conaghan said at the Congress, sponsored by the Osteoarthritis Research Society International.
Dr. Conaghan, who is professor of musculoskeletal medicine and director of the Leeds (England) Institute of Rheumatic and Musculoskeletal Medicine, noted: “Cathepsin K is a cysteine protease that degrades the collagen matrix and stops bone resorption, so it’s got a number of potential actions,” in the development of OA.
MIV-711 is a potent, selective, and reversible inhibitor of cathepsin K, he added, which has previously been shown to have bone structure–modifying properties in preclinical models. It also has been shown to reduce CTX-I and CTX-II in healthy volunteers.
The current randomized, double-blind, placebo-controlled phase 2a study included two active treatment (100 mg and 200 mg, once daily) arms and one placebo arm. For inclusion, patients had to have knee pain rated as 4 or higher on an 11-point NRS and Kellgren-Lawrence grade 2 or 3 knee OA.
Patients also were allowed to remain on any analgesic medication they were currently taking, provided this was stable. “That enables recruitment as a trialist, and it’s more like my usual practice as it’s unlikely I’d stop an analgesic before starting a new one,” Dr. Conaghan said.
In all, 240 patients were enrolled at six European sites. They had a mean age of 62 years, a body mass index of 32 kg/m2, and 77% were women. MRIs were assessed at baseline and at week 26, with additional clinic visits scheduled at 2, 4, 8, 14, and 20 weeks.
“Overall, there are not any great safety signals,” Dr. Conaghan said. The placebo, 100-mg, and 200-mg MIV-117 arms had similar rates of any adverse event (55%, 54.9%, and 52.4%) or treatment-related adverse events (21.2%, 20.7%, and 24.4%). There were more serious adverse events in the 100-mg and 200-mg MIV-711 treatment arms (3.7% and 2.4%) than in the placebo arm (1.3%), and more discontinuations (7.3%, 4.9%, and 3.8%).
“The primary endpoint of knee pain was not met, but there was a consistent trend, and it looks like there’s a benefit, but the statistically endpoint was not achieved,” Dr. Conaghan summarized.
“MRI measures do show structural modification in terms of bone shape and probably an effect on cartilage,” although the study was not powered to show an effect on the latter, Dr. Conaghan noted.
“The overall data, and from what we’ve seen regarding structural effects, do warrant this drug moving forward into further trials,” he added.
The study was sponsored by Medivir. Dr. Conaghan disclosed being a member of the speaker’s bureau for Kolon TissueGene and Samumed and on advisory boards for AbbVie, Centrexion, Flexion Therapeutics, Medivir, Novartis, and ONO Pharmaceutical.
SOURCE: Conaghan P et al. Osteoarthritis Cartilage. 2018 Apr 16:26(1):S25-26. Abstract 29.
REPORTING FROM OARSI 2018
Key clinical point:
Major finding: A 63%-66% reduction in the medial femur bone area was observed with MIV-711, compared with placebo.
Study details: Randomized, double-blind, placebo-controlled phase 2a study of 240 patients with osteoarthritis knee pain.
Disclosures: The study was sponsored by Medivir. Dr. Conaghan disclosed being a member of the speakers bureau for Kolon TissueGene and Samumed and on advisory boards for AbbVie, Centrexion, Flexion Therapeutics, Medivir, Novartis, and ONO Pharmaceutical.
Source: Conaghan P et al. Osteoarthritis Cartilage. 2018 Apr 16:26(1):S25-26. Abstract 29.
VIDEO: Postpartum care gets a new look
AUSTIN, TEX. – While women may have a plethora of options for care during pregnancy, attention given to women after birth is seriously lacking, with detrimental effect.
Currently, postpartum care is limited to a follow-up appointment 6 weeks after pregnancy, but according to Alison Stuebe, MD, medical director of lactation services at the University of North Carolina, Chapel Hill, there is too much going on in those 6 weeks to continue this model.
To address preferred changes to this system of care, the .
“What we’d like to do with the new committee opinion is move from this one-off visit at 6 weeks where we tell people ‘you’re good to go, you can have sex, get out of my office,’ to a much more comprehensive approach that reaches out to moms in the first couple of weeks,” explained Dr. Stuebe. “Whether that’s by phone, by asynchronous communication, by in-person visit, [the physician] finds out what’s going on, and then makes appropriate recommendations to help her rather than waiting to see what’s left after 6 weeks,” she said at ACOG’s annual clinical and scientific meeting.
Paying for these services is a big barrier right now, said Dr. Stuebe, but some solutions have already shown signs of being cost effective.
One example, in Dr. Stuebe’s hometown of Durham County, N.C., is a program called Durham Connect, which puts nurses in contact with women at 3 weeks postpartum to make assessments of what care the mother needs, and then offers service referrals to help with those needs.
According to Dr. Stuebe, studies have found every dollar invested in the program would save $3 in emergency department visits for children.
As postpartum care evolves, the most important thing is to remember that when it comes to pregnancy and birth, just because the baby is out doesn’t mean the mother can be ignored, she said.
“When we think about the way postpartum care exists today, you think about the mom being the candy wrapper and the baby being the candy; when the candy’s out of the wrapper, we toss the wrapper,” said Dr. Stuebe. “What these new guidelines are saying is this wrapper is actually pretty important.”
The revised committee opinion states: “The comprehensive postpartum visit should include a full assessment of physical, social, and psychological well-being, including the following domains: mood and emotional well-being; infant care and feeding; sexuality, contraception, and birth spacing; sleep and fatigue; physical recovery from birth; chronic disease management; and health maintenance.”
Dr. Stuebe receives support from Janssen.
ezimmerman@mdedge.com
AUSTIN, TEX. – While women may have a plethora of options for care during pregnancy, attention given to women after birth is seriously lacking, with detrimental effect.
Currently, postpartum care is limited to a follow-up appointment 6 weeks after pregnancy, but according to Alison Stuebe, MD, medical director of lactation services at the University of North Carolina, Chapel Hill, there is too much going on in those 6 weeks to continue this model.
To address preferred changes to this system of care, the .
“What we’d like to do with the new committee opinion is move from this one-off visit at 6 weeks where we tell people ‘you’re good to go, you can have sex, get out of my office,’ to a much more comprehensive approach that reaches out to moms in the first couple of weeks,” explained Dr. Stuebe. “Whether that’s by phone, by asynchronous communication, by in-person visit, [the physician] finds out what’s going on, and then makes appropriate recommendations to help her rather than waiting to see what’s left after 6 weeks,” she said at ACOG’s annual clinical and scientific meeting.
Paying for these services is a big barrier right now, said Dr. Stuebe, but some solutions have already shown signs of being cost effective.
One example, in Dr. Stuebe’s hometown of Durham County, N.C., is a program called Durham Connect, which puts nurses in contact with women at 3 weeks postpartum to make assessments of what care the mother needs, and then offers service referrals to help with those needs.
According to Dr. Stuebe, studies have found every dollar invested in the program would save $3 in emergency department visits for children.
As postpartum care evolves, the most important thing is to remember that when it comes to pregnancy and birth, just because the baby is out doesn’t mean the mother can be ignored, she said.
“When we think about the way postpartum care exists today, you think about the mom being the candy wrapper and the baby being the candy; when the candy’s out of the wrapper, we toss the wrapper,” said Dr. Stuebe. “What these new guidelines are saying is this wrapper is actually pretty important.”
The revised committee opinion states: “The comprehensive postpartum visit should include a full assessment of physical, social, and psychological well-being, including the following domains: mood and emotional well-being; infant care and feeding; sexuality, contraception, and birth spacing; sleep and fatigue; physical recovery from birth; chronic disease management; and health maintenance.”
Dr. Stuebe receives support from Janssen.
ezimmerman@mdedge.com
AUSTIN, TEX. – While women may have a plethora of options for care during pregnancy, attention given to women after birth is seriously lacking, with detrimental effect.
Currently, postpartum care is limited to a follow-up appointment 6 weeks after pregnancy, but according to Alison Stuebe, MD, medical director of lactation services at the University of North Carolina, Chapel Hill, there is too much going on in those 6 weeks to continue this model.
To address preferred changes to this system of care, the .
“What we’d like to do with the new committee opinion is move from this one-off visit at 6 weeks where we tell people ‘you’re good to go, you can have sex, get out of my office,’ to a much more comprehensive approach that reaches out to moms in the first couple of weeks,” explained Dr. Stuebe. “Whether that’s by phone, by asynchronous communication, by in-person visit, [the physician] finds out what’s going on, and then makes appropriate recommendations to help her rather than waiting to see what’s left after 6 weeks,” she said at ACOG’s annual clinical and scientific meeting.
Paying for these services is a big barrier right now, said Dr. Stuebe, but some solutions have already shown signs of being cost effective.
One example, in Dr. Stuebe’s hometown of Durham County, N.C., is a program called Durham Connect, which puts nurses in contact with women at 3 weeks postpartum to make assessments of what care the mother needs, and then offers service referrals to help with those needs.
According to Dr. Stuebe, studies have found every dollar invested in the program would save $3 in emergency department visits for children.
As postpartum care evolves, the most important thing is to remember that when it comes to pregnancy and birth, just because the baby is out doesn’t mean the mother can be ignored, she said.
“When we think about the way postpartum care exists today, you think about the mom being the candy wrapper and the baby being the candy; when the candy’s out of the wrapper, we toss the wrapper,” said Dr. Stuebe. “What these new guidelines are saying is this wrapper is actually pretty important.”
The revised committee opinion states: “The comprehensive postpartum visit should include a full assessment of physical, social, and psychological well-being, including the following domains: mood and emotional well-being; infant care and feeding; sexuality, contraception, and birth spacing; sleep and fatigue; physical recovery from birth; chronic disease management; and health maintenance.”
Dr. Stuebe receives support from Janssen.
ezimmerman@mdedge.com
REPORTING FROM ACOG 2018
Prompt palliative care cut hospital costs in pooled study
For adults with serious illness, consulting with a palliative care team within 3 days of hospital admission significantly reduced hospital costs, according to findings from a systematic review and meta-analysis.
In a pooled analysis of six cohort studies, average cost savings per admission were $3,237 (95% confidence interval, –$3,581 to −$2,893) overall, $4,251 for patients with cancer, and $2,105 for patients with other serious illnesses (all P values less than .001), reported Peter May, PhD, of Trinity College Dublin, and his associates.
In this latter group, prompt palliative care consultations saved more when patients had at least four comorbidities rather than two or fewer comorbidities, the reviewers wrote. The report was published in JAMA Internal Medicine.
About one in four Medicare beneficiaries dies in acute care hospitals, often after weeks of intensive, costly care that may not reflect personal wishes, according to an earlier study (JAMA. 2013;309:470-7). Economic studies have tried to pinpoint the cost savings of palliative care. These studies have found it important to consider both the clinical characteristics of patients and the amount of time between admission and palliative consultations, the reviewers noted. However, heterogeneity among older studies had precluded pooled analyses.
The six studies in this meta-analysis were identified by a search of Embase, PsycINFO, CENTRAL, PubMed, CINAHL, and EconLit databases for economic studies of hospital-based palliative care consultations. The studies were published between 2008 and 2017 and included 133,118 adults with cancer, chronic obstructive pulmonary disease, major organ failure, AIDS/HIV, or serious neurodegenerative disease. Patients tended to be in their 60s and were usually Medicare beneficiaries, although one study focused only on Medicaid enrollees. Forty-one percent of patients had a primary diagnosis of cancer, and 93% were discharged alive. Most also had at least two comorbidities. Only 3.6% received a palliative care consultation (range, 2.2% to 22.3%).
The link that they found between more comorbidities and greater cost savings “is the reverse of prior research that assumed that long-stay, high-cost hospitalized patients could not have their care trajectories affected by palliative care,” the researchers wrote. “Current palliative care provision in the United States is characterized by widespread understaffing. Our results suggest that acute care hospitals may be able to reduce costs for this population by increasing palliative care capacity to meet national guidelines.”
Dr. May received grant support from The Atlantic Philanthropies. The reviewers reported having no conflicts of interest.
SOURCE: May P et al. JAMA Intern Med. 2018 Apr 30. doi: 10.1001/jamainternmed.2018.0750.
For adults with serious illness, consulting with a palliative care team within 3 days of hospital admission significantly reduced hospital costs, according to findings from a systematic review and meta-analysis.
In a pooled analysis of six cohort studies, average cost savings per admission were $3,237 (95% confidence interval, –$3,581 to −$2,893) overall, $4,251 for patients with cancer, and $2,105 for patients with other serious illnesses (all P values less than .001), reported Peter May, PhD, of Trinity College Dublin, and his associates.
In this latter group, prompt palliative care consultations saved more when patients had at least four comorbidities rather than two or fewer comorbidities, the reviewers wrote. The report was published in JAMA Internal Medicine.
About one in four Medicare beneficiaries dies in acute care hospitals, often after weeks of intensive, costly care that may not reflect personal wishes, according to an earlier study (JAMA. 2013;309:470-7). Economic studies have tried to pinpoint the cost savings of palliative care. These studies have found it important to consider both the clinical characteristics of patients and the amount of time between admission and palliative consultations, the reviewers noted. However, heterogeneity among older studies had precluded pooled analyses.
The six studies in this meta-analysis were identified by a search of Embase, PsycINFO, CENTRAL, PubMed, CINAHL, and EconLit databases for economic studies of hospital-based palliative care consultations. The studies were published between 2008 and 2017 and included 133,118 adults with cancer, chronic obstructive pulmonary disease, major organ failure, AIDS/HIV, or serious neurodegenerative disease. Patients tended to be in their 60s and were usually Medicare beneficiaries, although one study focused only on Medicaid enrollees. Forty-one percent of patients had a primary diagnosis of cancer, and 93% were discharged alive. Most also had at least two comorbidities. Only 3.6% received a palliative care consultation (range, 2.2% to 22.3%).
The link that they found between more comorbidities and greater cost savings “is the reverse of prior research that assumed that long-stay, high-cost hospitalized patients could not have their care trajectories affected by palliative care,” the researchers wrote. “Current palliative care provision in the United States is characterized by widespread understaffing. Our results suggest that acute care hospitals may be able to reduce costs for this population by increasing palliative care capacity to meet national guidelines.”
Dr. May received grant support from The Atlantic Philanthropies. The reviewers reported having no conflicts of interest.
SOURCE: May P et al. JAMA Intern Med. 2018 Apr 30. doi: 10.1001/jamainternmed.2018.0750.
For adults with serious illness, consulting with a palliative care team within 3 days of hospital admission significantly reduced hospital costs, according to findings from a systematic review and meta-analysis.
In a pooled analysis of six cohort studies, average cost savings per admission were $3,237 (95% confidence interval, –$3,581 to −$2,893) overall, $4,251 for patients with cancer, and $2,105 for patients with other serious illnesses (all P values less than .001), reported Peter May, PhD, of Trinity College Dublin, and his associates.
In this latter group, prompt palliative care consultations saved more when patients had at least four comorbidities rather than two or fewer comorbidities, the reviewers wrote. The report was published in JAMA Internal Medicine.
About one in four Medicare beneficiaries dies in acute care hospitals, often after weeks of intensive, costly care that may not reflect personal wishes, according to an earlier study (JAMA. 2013;309:470-7). Economic studies have tried to pinpoint the cost savings of palliative care. These studies have found it important to consider both the clinical characteristics of patients and the amount of time between admission and palliative consultations, the reviewers noted. However, heterogeneity among older studies had precluded pooled analyses.
The six studies in this meta-analysis were identified by a search of Embase, PsycINFO, CENTRAL, PubMed, CINAHL, and EconLit databases for economic studies of hospital-based palliative care consultations. The studies were published between 2008 and 2017 and included 133,118 adults with cancer, chronic obstructive pulmonary disease, major organ failure, AIDS/HIV, or serious neurodegenerative disease. Patients tended to be in their 60s and were usually Medicare beneficiaries, although one study focused only on Medicaid enrollees. Forty-one percent of patients had a primary diagnosis of cancer, and 93% were discharged alive. Most also had at least two comorbidities. Only 3.6% received a palliative care consultation (range, 2.2% to 22.3%).
The link that they found between more comorbidities and greater cost savings “is the reverse of prior research that assumed that long-stay, high-cost hospitalized patients could not have their care trajectories affected by palliative care,” the researchers wrote. “Current palliative care provision in the United States is characterized by widespread understaffing. Our results suggest that acute care hospitals may be able to reduce costs for this population by increasing palliative care capacity to meet national guidelines.”
Dr. May received grant support from The Atlantic Philanthropies. The reviewers reported having no conflicts of interest.
SOURCE: May P et al. JAMA Intern Med. 2018 Apr 30. doi: 10.1001/jamainternmed.2018.0750.
FROM JAMA INTERNAL MEDICINE
Key clinical point:
Major finding: Average cost savings per admission were $3,237 overall, $4,251 for patients with cancer, and $2,105 for patients with other serious illnesses (all P-values less than .001).
Study details: Systematic review and meta-analysis of six cohort studies of 133,118 adults with cancer, chronic obstructive pulmonary disease, major organ failure, AIDS/HIV, or serious neurodegenerative disease.
Disclosures: Dr. May received grant support from The Atlantic Philanthropies. The reviewers reported having no conflicts of interest.
Source: May P et al. JAMA Intern Med. 2018 Apr 30. doi: 10.1001/jamainternmed.2018.0750.
VRIC is May 9; Register On-Site
Learn about emerging vascular science, engage with researchers, network with colleagues, and support your peers at VRIC, our ‘Annual Meeting’ for basic and translational vascular researchers. Online registration has ended by attendees can register in person the day of the conference. VRIC 2018 will be held at the Hilton San Francisco Union Square, on May 9. Learn more and find the program schedule here.
Learn about emerging vascular science, engage with researchers, network with colleagues, and support your peers at VRIC, our ‘Annual Meeting’ for basic and translational vascular researchers. Online registration has ended by attendees can register in person the day of the conference. VRIC 2018 will be held at the Hilton San Francisco Union Square, on May 9. Learn more and find the program schedule here.
Learn about emerging vascular science, engage with researchers, network with colleagues, and support your peers at VRIC, our ‘Annual Meeting’ for basic and translational vascular researchers. Online registration has ended by attendees can register in person the day of the conference. VRIC 2018 will be held at the Hilton San Francisco Union Square, on May 9. Learn more and find the program schedule here.
Circulating biomarkers predicted melanoma survival
Among patients with metastatic melanoma, detectable circulating levels of BRAFV600 mutated circulating tumor DNA and elevated levels of circulating hepatocyte growth factor separately predicted significantly worse survival, regardless of treatment.
After adjusting for lactate dehydrogenase, ECOG (Eastern Cooperative Oncology Group) status, disease stage, and treatment, hazard ratios for overall survival were 1.75 (95% confidence interval, 1.35-2.28) for detectable versus undetectable circulating tumor DNA (ctDNA) and 1.24 (95% CI, 1.00-1.53) for high versus low circulating hepatocyte growth factor (cHGF), reported William Lu, PhD, of Genentech in South San Francisco, with his associates. Their retrospective, exploratory analysis of the phase 3 BRIM-3 trial was published in JCO Precision Oncology.
BRIM-3 was a multicenter, open-label trial of 675 patients with previously untreated unresectable stage IIIC or stage IV melanoma that tested positive for the BRAFV600 mutation. Patients received either vemurafenib or dacarbazine therapy.
Baseline ctDNA, baseline cHGF, and ECOG performance status most strongly predicted overall survival, said the investigators. Patients whose mutant ctDNA fraction exceeded 0.039 had an 11.5-month shorter median overall survival on vemurafenib (P less than .001) and a 13.9-month shorter median overall survival on dacarbazine (P less than .001) than patients whose mutant ctDNA fraction was less than 0.039, they explained. Similarly, median overall survival times were 5.4 months shorter for vemurafenib (P = .002) and 12.3 months shorter for dacarbazine (P less than .001) when patients had high (more than 438 pg/mL) versus low circulating cHGF.
Median overall survival for vemurafenib was shortest (7.3 months) in the subgroup of 64 patients with detectable BRAFV600 ctDNA and elevated cHGF, according to the researchers. In contrast, median overall survival for vemurafenib was longest (22.0 months) when patients had undetectable ctDNA and an ECOG status of 0.
“Although similar studies have been performed, to our knowledge, our study is unique in its size and in its design as a randomized trial,” Dr. Lu and associates concluded. “The biomarkers in this study can be readily acquired and measured, and require only small volumes of plasma.” They suggested validating the findings in a separate dataset.
F. Hoffman-La Roche provided funding. Roche Molecular Systems makes the Cobas 4800 BRAFV600 Mutation Test used in the study. Dr. Lu disclosed employment and research funding from Genentech/Roche.
SOURCE: Lu W et al. JCO Precis Oncol. 2018 Apr 25. doi: 10.1200/PO.17.00168.
Among patients with metastatic melanoma, detectable circulating levels of BRAFV600 mutated circulating tumor DNA and elevated levels of circulating hepatocyte growth factor separately predicted significantly worse survival, regardless of treatment.
After adjusting for lactate dehydrogenase, ECOG (Eastern Cooperative Oncology Group) status, disease stage, and treatment, hazard ratios for overall survival were 1.75 (95% confidence interval, 1.35-2.28) for detectable versus undetectable circulating tumor DNA (ctDNA) and 1.24 (95% CI, 1.00-1.53) for high versus low circulating hepatocyte growth factor (cHGF), reported William Lu, PhD, of Genentech in South San Francisco, with his associates. Their retrospective, exploratory analysis of the phase 3 BRIM-3 trial was published in JCO Precision Oncology.
BRIM-3 was a multicenter, open-label trial of 675 patients with previously untreated unresectable stage IIIC or stage IV melanoma that tested positive for the BRAFV600 mutation. Patients received either vemurafenib or dacarbazine therapy.
Baseline ctDNA, baseline cHGF, and ECOG performance status most strongly predicted overall survival, said the investigators. Patients whose mutant ctDNA fraction exceeded 0.039 had an 11.5-month shorter median overall survival on vemurafenib (P less than .001) and a 13.9-month shorter median overall survival on dacarbazine (P less than .001) than patients whose mutant ctDNA fraction was less than 0.039, they explained. Similarly, median overall survival times were 5.4 months shorter for vemurafenib (P = .002) and 12.3 months shorter for dacarbazine (P less than .001) when patients had high (more than 438 pg/mL) versus low circulating cHGF.
Median overall survival for vemurafenib was shortest (7.3 months) in the subgroup of 64 patients with detectable BRAFV600 ctDNA and elevated cHGF, according to the researchers. In contrast, median overall survival for vemurafenib was longest (22.0 months) when patients had undetectable ctDNA and an ECOG status of 0.
“Although similar studies have been performed, to our knowledge, our study is unique in its size and in its design as a randomized trial,” Dr. Lu and associates concluded. “The biomarkers in this study can be readily acquired and measured, and require only small volumes of plasma.” They suggested validating the findings in a separate dataset.
F. Hoffman-La Roche provided funding. Roche Molecular Systems makes the Cobas 4800 BRAFV600 Mutation Test used in the study. Dr. Lu disclosed employment and research funding from Genentech/Roche.
SOURCE: Lu W et al. JCO Precis Oncol. 2018 Apr 25. doi: 10.1200/PO.17.00168.
Among patients with metastatic melanoma, detectable circulating levels of BRAFV600 mutated circulating tumor DNA and elevated levels of circulating hepatocyte growth factor separately predicted significantly worse survival, regardless of treatment.
After adjusting for lactate dehydrogenase, ECOG (Eastern Cooperative Oncology Group) status, disease stage, and treatment, hazard ratios for overall survival were 1.75 (95% confidence interval, 1.35-2.28) for detectable versus undetectable circulating tumor DNA (ctDNA) and 1.24 (95% CI, 1.00-1.53) for high versus low circulating hepatocyte growth factor (cHGF), reported William Lu, PhD, of Genentech in South San Francisco, with his associates. Their retrospective, exploratory analysis of the phase 3 BRIM-3 trial was published in JCO Precision Oncology.
BRIM-3 was a multicenter, open-label trial of 675 patients with previously untreated unresectable stage IIIC or stage IV melanoma that tested positive for the BRAFV600 mutation. Patients received either vemurafenib or dacarbazine therapy.
Baseline ctDNA, baseline cHGF, and ECOG performance status most strongly predicted overall survival, said the investigators. Patients whose mutant ctDNA fraction exceeded 0.039 had an 11.5-month shorter median overall survival on vemurafenib (P less than .001) and a 13.9-month shorter median overall survival on dacarbazine (P less than .001) than patients whose mutant ctDNA fraction was less than 0.039, they explained. Similarly, median overall survival times were 5.4 months shorter for vemurafenib (P = .002) and 12.3 months shorter for dacarbazine (P less than .001) when patients had high (more than 438 pg/mL) versus low circulating cHGF.
Median overall survival for vemurafenib was shortest (7.3 months) in the subgroup of 64 patients with detectable BRAFV600 ctDNA and elevated cHGF, according to the researchers. In contrast, median overall survival for vemurafenib was longest (22.0 months) when patients had undetectable ctDNA and an ECOG status of 0.
“Although similar studies have been performed, to our knowledge, our study is unique in its size and in its design as a randomized trial,” Dr. Lu and associates concluded. “The biomarkers in this study can be readily acquired and measured, and require only small volumes of plasma.” They suggested validating the findings in a separate dataset.
F. Hoffman-La Roche provided funding. Roche Molecular Systems makes the Cobas 4800 BRAFV600 Mutation Test used in the study. Dr. Lu disclosed employment and research funding from Genentech/Roche.
SOURCE: Lu W et al. JCO Precis Oncol. 2018 Apr 25. doi: 10.1200/PO.17.00168.
FROM JCO PRECISION ONCOLOGY
Key clinical point: Among patients with metastatic melanoma, high circulating levels of BRAFV600 mutated circulating tumor DNA and circulating hepatocyte growth factor predicted significantly worse overall survival, irrespective of treatment.
Major finding: Adjusted hazard ratios for overall survival were 1.75 for high versus undetectable circulating tumor DNA and 1.24 for high versus low circulating hepatocyte growth factor.
Study details: A retrospective, exploratory analysis of 675 patients with metastatic BRAFV600 mutated advanced melanoma.
Disclosures: F. Hoffman-La Roche provided funding. Roche Molecular Systems makes the Cobas 4800 BRAFV600 Mutation Test used in the study. Dr. Lu disclosed employment and research funding from Genentech/Roche.
Source: Lu W et al. JCO Precis Oncol. 2018 Apr 25. doi: 10.1200/PO.17.00168.
MDedge Daily News: Female physicans face enduring wage gap
A metabolic syndrome scoring system predicts cardiovascular disease risk in type 2 diabetes. A national suicide hotline could become a reality. And anticholinergics’ link to dementia calls for vigilance in elderly patients.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
A metabolic syndrome scoring system predicts cardiovascular disease risk in type 2 diabetes. A national suicide hotline could become a reality. And anticholinergics’ link to dementia calls for vigilance in elderly patients.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
A metabolic syndrome scoring system predicts cardiovascular disease risk in type 2 diabetes. A national suicide hotline could become a reality. And anticholinergics’ link to dementia calls for vigilance in elderly patients.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Getting At-Risk Kids to Change Sexual Behavior
In 2015, young people aged 13- 24 years accounted for > 1 in 5 new HIV diagnoses in the US. Young people aged 15-24 years also account for half of all new sexually transmitted disease infections. Many of those high-risk youth are caught in the juvenile justice system, where they experience more mental illness, substance abuse, and STDs than their peers in the general population.
It is a challenge to identify and implement effective prevention strategies for these young people, but a study at the University of Illinois shows potential. PHAT Life: Preventing HIV/AIDS Among Teens uses role-playing, videos, games, and skill-building exercises to promote knowledge about HIV/AIDS, positive coping, and problem-solving skills.
The study was conducted with 310 urban teens, mostly African American boys, on probation in Chicago’s Cook County, the second-largest county justice system in the US. The participants were assigned to PHAT Life or an equally intensive health information program. Both 2-week programs consisted of 8 90- to 120-minute sessions, delivered at detention-alternative after-school programs.
The study measured the degree of condom use and number of sexual partners in the 6 months before and after the intervention. Among participants who reported the highest-risk sexual behavior at baseline, those assigned to PHAT Life were over 4 times more likely than those in the control group to report a reduction in number of sexual partners and more consistent condom use. Of those who reported having had sex before age 12, the PHAT Life participants reported significantly fewer sexual partners after the intervention.
“Uniquely tailored interventions like PHAT Life … are essential to mitigate young offenders’ poor long-term trajectories,” says Geri Donenberg, PhD, study leader.
The next step, the researchers say, is to identify how best to disseminate PHAT Life resources to ensure it’s self-sustaining in the juvenile justice system.
In 2015, young people aged 13- 24 years accounted for > 1 in 5 new HIV diagnoses in the US. Young people aged 15-24 years also account for half of all new sexually transmitted disease infections. Many of those high-risk youth are caught in the juvenile justice system, where they experience more mental illness, substance abuse, and STDs than their peers in the general population.
It is a challenge to identify and implement effective prevention strategies for these young people, but a study at the University of Illinois shows potential. PHAT Life: Preventing HIV/AIDS Among Teens uses role-playing, videos, games, and skill-building exercises to promote knowledge about HIV/AIDS, positive coping, and problem-solving skills.
The study was conducted with 310 urban teens, mostly African American boys, on probation in Chicago’s Cook County, the second-largest county justice system in the US. The participants were assigned to PHAT Life or an equally intensive health information program. Both 2-week programs consisted of 8 90- to 120-minute sessions, delivered at detention-alternative after-school programs.
The study measured the degree of condom use and number of sexual partners in the 6 months before and after the intervention. Among participants who reported the highest-risk sexual behavior at baseline, those assigned to PHAT Life were over 4 times more likely than those in the control group to report a reduction in number of sexual partners and more consistent condom use. Of those who reported having had sex before age 12, the PHAT Life participants reported significantly fewer sexual partners after the intervention.
“Uniquely tailored interventions like PHAT Life … are essential to mitigate young offenders’ poor long-term trajectories,” says Geri Donenberg, PhD, study leader.
The next step, the researchers say, is to identify how best to disseminate PHAT Life resources to ensure it’s self-sustaining in the juvenile justice system.
In 2015, young people aged 13- 24 years accounted for > 1 in 5 new HIV diagnoses in the US. Young people aged 15-24 years also account for half of all new sexually transmitted disease infections. Many of those high-risk youth are caught in the juvenile justice system, where they experience more mental illness, substance abuse, and STDs than their peers in the general population.
It is a challenge to identify and implement effective prevention strategies for these young people, but a study at the University of Illinois shows potential. PHAT Life: Preventing HIV/AIDS Among Teens uses role-playing, videos, games, and skill-building exercises to promote knowledge about HIV/AIDS, positive coping, and problem-solving skills.
The study was conducted with 310 urban teens, mostly African American boys, on probation in Chicago’s Cook County, the second-largest county justice system in the US. The participants were assigned to PHAT Life or an equally intensive health information program. Both 2-week programs consisted of 8 90- to 120-minute sessions, delivered at detention-alternative after-school programs.
The study measured the degree of condom use and number of sexual partners in the 6 months before and after the intervention. Among participants who reported the highest-risk sexual behavior at baseline, those assigned to PHAT Life were over 4 times more likely than those in the control group to report a reduction in number of sexual partners and more consistent condom use. Of those who reported having had sex before age 12, the PHAT Life participants reported significantly fewer sexual partners after the intervention.
“Uniquely tailored interventions like PHAT Life … are essential to mitigate young offenders’ poor long-term trajectories,” says Geri Donenberg, PhD, study leader.
The next step, the researchers say, is to identify how best to disseminate PHAT Life resources to ensure it’s self-sustaining in the juvenile justice system.
CHMP backs approval of dasatinib for kids
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended changes to the marketing authorization for dasatinib (Sprycel).
The CHMP is recommending approval for dasatinib as a treatment for pediatric patients with newly diagnosed, Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia (CML) in chronic phase (CP) or Ph+ CML-CP that is resistant or intolerant to prior therapy, including imatinib.
The CHMP has also recommended approval of a new formulation of dasatinib—a powder for oral suspension (PFOS)—for use in pediatric patients.
Dasatinib is already approved in the European Union to treat adults with:
- Newly diagnosed Ph+ CML-CP
- Chronic, accelerated, or blast phase CML with resistance or intolerance to prior therapy, including imatinib
- Ph+ acute lymphoblastic leukemia and lymphoid blast CML with resistance or intolerance to prior therapy.
The CHMP’s opinion on dasatinib for pediatric patients will be reviewed by the European Commission (EC).
If the EC agrees with the CHMP, the commission will grant a centralized marketing authorization that will be valid in the European Union. Norway, Iceland, and Liechtenstein will make corresponding decisions on the basis of the EC’s decision.
The EC typically makes a decision within 67 days of the CHMP’s recommendation.
The CHMP’s opinion on dasatinib for pediatric patients is supported by 2 studies. Results from the phase 1 study (NCT00306202) were published in the Journal of Clinical Oncology in 2013. Phase 2 (NCT00777036) results were published in the same journal this year.
Phase 1
The phase 1 trial included 17 patients with CML-CP, all of whom had received prior imatinib.
Eleven patients received dasatinib at a starting dose of 60 mg/m2 once daily, and 6 received the drug at a starting dose of 80 mg/m2 once daily. Dose escalation was allowed based on tolerance and response. The median duration of treatment was 24.1 months (range, 2.3 to 50.6 months).
The 60 mg/m2 starting dose appeared more tolerable than 80 mg/m2 dose.
Drug-related adverse events (AEs) occurring in at least 20% of patients included neutropenia (82.4%), anemia (70.6%), thrombocytopenia (64.7%), nausea (29.4%), headache (35.3%), diarrhea (23.5%), and pain in extremity (23.5%). Grade 3-4 AEs included neutropenia (23.5%), thrombocytopenia (11.8%), and headache (5.9%). There were no drug-related deaths.
Ninety-four percent of patients achieved a complete hematologic response (CHR), 88% had a major cytogenetic response (MCyR), 82% had a complete cytogenetic response (CCyR), 47% had a major molecular response (MMR), and 24% had a complete molecular response (CMR).
Patients who received the lower starting dose of dasatinib had lower rates of cumulative CCyR (72.7% vs 100%) and CHR (90.9% vs 100%) but higher rates of cumulative MMR (54.5% vs 33.3%) and CMR (27.3% vs 16.7).
The median progression-free survival (PFS) and overall survival (OS) had not been reached at last follow-up. At 24 months, the estimated PFS was 61%, and the estimated OS was 88%.
Phase 2
The phase 2 trial included 29 patients with imatinib-resistant/intolerant CML-CP and 84 with newly diagnosed CML-CP.
The previously treated patients received dasatinib tablets. Newly diagnosed patients were treated with dasatinib tablets (n=51) or PFOS (n=33). Patients who started on PFOS could switch to tablets after receiving PFOS for at least 1 year. Sixty-seven percent of patients on PFOS switched to tablets due to patient preference.
The average daily dose of dasatinib was 58.18 mg/m2 in the previously treated patients and 59.84 mg/m2 in the newly diagnosed patients (for both tablets and PFOS). The median duration of treatment was 49.91 months (range, 1.9 to 90.2) and 42.30 months (range, 0.1 to 75.2), respectively.
Rates of confirmed CHR (at any time) were 93% in the previously treated patients and 96% in the newly diagnosed patients.
At 12 months, previously treated patients had an MMR rate of 41% and a CMR rate of 7%. In newly diagnosed patients, MMR was 52%, and CMR was 8%.
At 24 months, previously treated patients had an MMR rate of 55% and a CMR rate of 17%. In the newly diagnosed patients, MMR was 70%, and CMR was 21%.
The rate of MCyR at any time was 89.7% in all previously treated patients and 90% when the researchers excluded patients with MCyR or unknown cytogenetic status at baseline.
The rate of CCyR at any time was 94% in all newly diagnosed patients and 93.9% when the researchers excluded patients with CCyR or unknown cytogenetic status at baseline.
The median PFS and OS had not been reached at last follow-up. The estimated 48-month PFS was 78% in the previously treated patients and 93% in the newly diagnosed patients. The estimated 48-month OS was 96% and 100%, respectively.
Dasatinib-related AEs occurring in at least 10% of the previously treated patients included nausea/vomiting (31%), myalgia/arthralgia (17%), fatigue (14%), rash (14%), diarrhea (14%), hemorrhage (10%), bone growth and development events (10%), and shortness of breath (10%).
Dasatinib-related AEs occurring in at least 10% of the newly diagnosed patients included nausea/vomiting (20%), myalgia/arthralgia (10%), fatigue (11%), rash (19%), diarrhea (18%), and hemorrhage (10%).
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended changes to the marketing authorization for dasatinib (Sprycel).
The CHMP is recommending approval for dasatinib as a treatment for pediatric patients with newly diagnosed, Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia (CML) in chronic phase (CP) or Ph+ CML-CP that is resistant or intolerant to prior therapy, including imatinib.
The CHMP has also recommended approval of a new formulation of dasatinib—a powder for oral suspension (PFOS)—for use in pediatric patients.
Dasatinib is already approved in the European Union to treat adults with:
- Newly diagnosed Ph+ CML-CP
- Chronic, accelerated, or blast phase CML with resistance or intolerance to prior therapy, including imatinib
- Ph+ acute lymphoblastic leukemia and lymphoid blast CML with resistance or intolerance to prior therapy.
The CHMP’s opinion on dasatinib for pediatric patients will be reviewed by the European Commission (EC).
If the EC agrees with the CHMP, the commission will grant a centralized marketing authorization that will be valid in the European Union. Norway, Iceland, and Liechtenstein will make corresponding decisions on the basis of the EC’s decision.
The EC typically makes a decision within 67 days of the CHMP’s recommendation.
The CHMP’s opinion on dasatinib for pediatric patients is supported by 2 studies. Results from the phase 1 study (NCT00306202) were published in the Journal of Clinical Oncology in 2013. Phase 2 (NCT00777036) results were published in the same journal this year.
Phase 1
The phase 1 trial included 17 patients with CML-CP, all of whom had received prior imatinib.
Eleven patients received dasatinib at a starting dose of 60 mg/m2 once daily, and 6 received the drug at a starting dose of 80 mg/m2 once daily. Dose escalation was allowed based on tolerance and response. The median duration of treatment was 24.1 months (range, 2.3 to 50.6 months).
The 60 mg/m2 starting dose appeared more tolerable than 80 mg/m2 dose.
Drug-related adverse events (AEs) occurring in at least 20% of patients included neutropenia (82.4%), anemia (70.6%), thrombocytopenia (64.7%), nausea (29.4%), headache (35.3%), diarrhea (23.5%), and pain in extremity (23.5%). Grade 3-4 AEs included neutropenia (23.5%), thrombocytopenia (11.8%), and headache (5.9%). There were no drug-related deaths.
Ninety-four percent of patients achieved a complete hematologic response (CHR), 88% had a major cytogenetic response (MCyR), 82% had a complete cytogenetic response (CCyR), 47% had a major molecular response (MMR), and 24% had a complete molecular response (CMR).
Patients who received the lower starting dose of dasatinib had lower rates of cumulative CCyR (72.7% vs 100%) and CHR (90.9% vs 100%) but higher rates of cumulative MMR (54.5% vs 33.3%) and CMR (27.3% vs 16.7).
The median progression-free survival (PFS) and overall survival (OS) had not been reached at last follow-up. At 24 months, the estimated PFS was 61%, and the estimated OS was 88%.
Phase 2
The phase 2 trial included 29 patients with imatinib-resistant/intolerant CML-CP and 84 with newly diagnosed CML-CP.
The previously treated patients received dasatinib tablets. Newly diagnosed patients were treated with dasatinib tablets (n=51) or PFOS (n=33). Patients who started on PFOS could switch to tablets after receiving PFOS for at least 1 year. Sixty-seven percent of patients on PFOS switched to tablets due to patient preference.
The average daily dose of dasatinib was 58.18 mg/m2 in the previously treated patients and 59.84 mg/m2 in the newly diagnosed patients (for both tablets and PFOS). The median duration of treatment was 49.91 months (range, 1.9 to 90.2) and 42.30 months (range, 0.1 to 75.2), respectively.
Rates of confirmed CHR (at any time) were 93% in the previously treated patients and 96% in the newly diagnosed patients.
At 12 months, previously treated patients had an MMR rate of 41% and a CMR rate of 7%. In newly diagnosed patients, MMR was 52%, and CMR was 8%.
At 24 months, previously treated patients had an MMR rate of 55% and a CMR rate of 17%. In the newly diagnosed patients, MMR was 70%, and CMR was 21%.
The rate of MCyR at any time was 89.7% in all previously treated patients and 90% when the researchers excluded patients with MCyR or unknown cytogenetic status at baseline.
The rate of CCyR at any time was 94% in all newly diagnosed patients and 93.9% when the researchers excluded patients with CCyR or unknown cytogenetic status at baseline.
The median PFS and OS had not been reached at last follow-up. The estimated 48-month PFS was 78% in the previously treated patients and 93% in the newly diagnosed patients. The estimated 48-month OS was 96% and 100%, respectively.
Dasatinib-related AEs occurring in at least 10% of the previously treated patients included nausea/vomiting (31%), myalgia/arthralgia (17%), fatigue (14%), rash (14%), diarrhea (14%), hemorrhage (10%), bone growth and development events (10%), and shortness of breath (10%).
Dasatinib-related AEs occurring in at least 10% of the newly diagnosed patients included nausea/vomiting (20%), myalgia/arthralgia (10%), fatigue (11%), rash (19%), diarrhea (18%), and hemorrhage (10%).
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended changes to the marketing authorization for dasatinib (Sprycel).
The CHMP is recommending approval for dasatinib as a treatment for pediatric patients with newly diagnosed, Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia (CML) in chronic phase (CP) or Ph+ CML-CP that is resistant or intolerant to prior therapy, including imatinib.
The CHMP has also recommended approval of a new formulation of dasatinib—a powder for oral suspension (PFOS)—for use in pediatric patients.
Dasatinib is already approved in the European Union to treat adults with:
- Newly diagnosed Ph+ CML-CP
- Chronic, accelerated, or blast phase CML with resistance or intolerance to prior therapy, including imatinib
- Ph+ acute lymphoblastic leukemia and lymphoid blast CML with resistance or intolerance to prior therapy.
The CHMP’s opinion on dasatinib for pediatric patients will be reviewed by the European Commission (EC).
If the EC agrees with the CHMP, the commission will grant a centralized marketing authorization that will be valid in the European Union. Norway, Iceland, and Liechtenstein will make corresponding decisions on the basis of the EC’s decision.
The EC typically makes a decision within 67 days of the CHMP’s recommendation.
The CHMP’s opinion on dasatinib for pediatric patients is supported by 2 studies. Results from the phase 1 study (NCT00306202) were published in the Journal of Clinical Oncology in 2013. Phase 2 (NCT00777036) results were published in the same journal this year.
Phase 1
The phase 1 trial included 17 patients with CML-CP, all of whom had received prior imatinib.
Eleven patients received dasatinib at a starting dose of 60 mg/m2 once daily, and 6 received the drug at a starting dose of 80 mg/m2 once daily. Dose escalation was allowed based on tolerance and response. The median duration of treatment was 24.1 months (range, 2.3 to 50.6 months).
The 60 mg/m2 starting dose appeared more tolerable than 80 mg/m2 dose.
Drug-related adverse events (AEs) occurring in at least 20% of patients included neutropenia (82.4%), anemia (70.6%), thrombocytopenia (64.7%), nausea (29.4%), headache (35.3%), diarrhea (23.5%), and pain in extremity (23.5%). Grade 3-4 AEs included neutropenia (23.5%), thrombocytopenia (11.8%), and headache (5.9%). There were no drug-related deaths.
Ninety-four percent of patients achieved a complete hematologic response (CHR), 88% had a major cytogenetic response (MCyR), 82% had a complete cytogenetic response (CCyR), 47% had a major molecular response (MMR), and 24% had a complete molecular response (CMR).
Patients who received the lower starting dose of dasatinib had lower rates of cumulative CCyR (72.7% vs 100%) and CHR (90.9% vs 100%) but higher rates of cumulative MMR (54.5% vs 33.3%) and CMR (27.3% vs 16.7).
The median progression-free survival (PFS) and overall survival (OS) had not been reached at last follow-up. At 24 months, the estimated PFS was 61%, and the estimated OS was 88%.
Phase 2
The phase 2 trial included 29 patients with imatinib-resistant/intolerant CML-CP and 84 with newly diagnosed CML-CP.
The previously treated patients received dasatinib tablets. Newly diagnosed patients were treated with dasatinib tablets (n=51) or PFOS (n=33). Patients who started on PFOS could switch to tablets after receiving PFOS for at least 1 year. Sixty-seven percent of patients on PFOS switched to tablets due to patient preference.
The average daily dose of dasatinib was 58.18 mg/m2 in the previously treated patients and 59.84 mg/m2 in the newly diagnosed patients (for both tablets and PFOS). The median duration of treatment was 49.91 months (range, 1.9 to 90.2) and 42.30 months (range, 0.1 to 75.2), respectively.
Rates of confirmed CHR (at any time) were 93% in the previously treated patients and 96% in the newly diagnosed patients.
At 12 months, previously treated patients had an MMR rate of 41% and a CMR rate of 7%. In newly diagnosed patients, MMR was 52%, and CMR was 8%.
At 24 months, previously treated patients had an MMR rate of 55% and a CMR rate of 17%. In the newly diagnosed patients, MMR was 70%, and CMR was 21%.
The rate of MCyR at any time was 89.7% in all previously treated patients and 90% when the researchers excluded patients with MCyR or unknown cytogenetic status at baseline.
The rate of CCyR at any time was 94% in all newly diagnosed patients and 93.9% when the researchers excluded patients with CCyR or unknown cytogenetic status at baseline.
The median PFS and OS had not been reached at last follow-up. The estimated 48-month PFS was 78% in the previously treated patients and 93% in the newly diagnosed patients. The estimated 48-month OS was 96% and 100%, respectively.
Dasatinib-related AEs occurring in at least 10% of the previously treated patients included nausea/vomiting (31%), myalgia/arthralgia (17%), fatigue (14%), rash (14%), diarrhea (14%), hemorrhage (10%), bone growth and development events (10%), and shortness of breath (10%).
Dasatinib-related AEs occurring in at least 10% of the newly diagnosed patients included nausea/vomiting (20%), myalgia/arthralgia (10%), fatigue (11%), rash (19%), diarrhea (18%), and hemorrhage (10%).
Readmitted patients less likely to be “very satisfied” with index admission
Clinical question: Are patient perceptions of care during index hospitalization associated with likelihood of 30-day readmission?
Background: Hospital readmissions are costly (more than $40 billion annually) and common. Nearly one readmission in five is thought to be preventable. While many risk-prediction models exist, few incorporate patient perceptions during the index hospitalization or factors associated with patient experience.
Setting: Single-center academic medical center.
Synopsis: A total of 846 patients – admitted to one of two inpatient general medicine wards at Massachusetts General Hospital in Boston, and were English speaking, aged 18 years or older, and possessed the ability to complete a 20-item study questionnaire – were screened from January 2012 to January 2016. An interviewer-assisted questionnaire was coupled with structured medical records review. Among items assessed were demographic information, patient perceptions of health, satisfaction with inpatient care, confidence in ability to perform self-care and understanding of the care plan, presence of a caregiver, and patient-predicted likelihood of readmission. Of 846 enrolled patients, 201 were readmitted within 30 days. Readmitted patients were less likely to report being “very satisfied” with their overall care during the index admission, and less likely to report that physicians “always listened” to them during the index stay.
Bottom line: This is the first study to relate patients’ perceptions of their care during index hospitalization to the likelihood of readmission. Further investigation will be necessary to determine whether timely assessment of these perceptions can prompt effective intervention that improves likelihood of an enduringly successful transition home.
Citation: Carter J et al. The association between patient experience factors and likelihood of 30-day readmission: A prospective cohort study. BMJ Qual Saf. 2017 Nov 16. doi: 10.1136/bmjqs-2017-007184.
Dr. Helgerson is associate professor of medicine and section head, division of hospital medicine, University of Virginia.
Clinical question: Are patient perceptions of care during index hospitalization associated with likelihood of 30-day readmission?
Background: Hospital readmissions are costly (more than $40 billion annually) and common. Nearly one readmission in five is thought to be preventable. While many risk-prediction models exist, few incorporate patient perceptions during the index hospitalization or factors associated with patient experience.
Setting: Single-center academic medical center.
Synopsis: A total of 846 patients – admitted to one of two inpatient general medicine wards at Massachusetts General Hospital in Boston, and were English speaking, aged 18 years or older, and possessed the ability to complete a 20-item study questionnaire – were screened from January 2012 to January 2016. An interviewer-assisted questionnaire was coupled with structured medical records review. Among items assessed were demographic information, patient perceptions of health, satisfaction with inpatient care, confidence in ability to perform self-care and understanding of the care plan, presence of a caregiver, and patient-predicted likelihood of readmission. Of 846 enrolled patients, 201 were readmitted within 30 days. Readmitted patients were less likely to report being “very satisfied” with their overall care during the index admission, and less likely to report that physicians “always listened” to them during the index stay.
Bottom line: This is the first study to relate patients’ perceptions of their care during index hospitalization to the likelihood of readmission. Further investigation will be necessary to determine whether timely assessment of these perceptions can prompt effective intervention that improves likelihood of an enduringly successful transition home.
Citation: Carter J et al. The association between patient experience factors and likelihood of 30-day readmission: A prospective cohort study. BMJ Qual Saf. 2017 Nov 16. doi: 10.1136/bmjqs-2017-007184.
Dr. Helgerson is associate professor of medicine and section head, division of hospital medicine, University of Virginia.
Clinical question: Are patient perceptions of care during index hospitalization associated with likelihood of 30-day readmission?
Background: Hospital readmissions are costly (more than $40 billion annually) and common. Nearly one readmission in five is thought to be preventable. While many risk-prediction models exist, few incorporate patient perceptions during the index hospitalization or factors associated with patient experience.
Setting: Single-center academic medical center.
Synopsis: A total of 846 patients – admitted to one of two inpatient general medicine wards at Massachusetts General Hospital in Boston, and were English speaking, aged 18 years or older, and possessed the ability to complete a 20-item study questionnaire – were screened from January 2012 to January 2016. An interviewer-assisted questionnaire was coupled with structured medical records review. Among items assessed were demographic information, patient perceptions of health, satisfaction with inpatient care, confidence in ability to perform self-care and understanding of the care plan, presence of a caregiver, and patient-predicted likelihood of readmission. Of 846 enrolled patients, 201 were readmitted within 30 days. Readmitted patients were less likely to report being “very satisfied” with their overall care during the index admission, and less likely to report that physicians “always listened” to them during the index stay.
Bottom line: This is the first study to relate patients’ perceptions of their care during index hospitalization to the likelihood of readmission. Further investigation will be necessary to determine whether timely assessment of these perceptions can prompt effective intervention that improves likelihood of an enduringly successful transition home.
Citation: Carter J et al. The association between patient experience factors and likelihood of 30-day readmission: A prospective cohort study. BMJ Qual Saf. 2017 Nov 16. doi: 10.1136/bmjqs-2017-007184.
Dr. Helgerson is associate professor of medicine and section head, division of hospital medicine, University of Virginia.
Qualitative assessment of organizational barriers to optimal lung cancer care in a community hospital setting in the United States
Lung cancer is a major public health challenge in the United States. It is the leading cause of cancer death in the United States, accounting for 27% of all cancer deaths, and it has an aggregate 5-year survival rate of 18%.1 Advances in diagnostic and treatment options are rapidly increasing the complexity of lung cancer care delivery, which involves multiple specialty providers and often cuts across health care institutions.2-4 Navigating the process of care while coping with the complexities of the illness can be overwhelming for both the patient and the caregiver.5 With increasing regulations and cost-cutting measures, the health care system in the United States can pose many challenges, especially for those dealing with catastrophic and life-threatening illnesses. Any barrier to accessing care often increases anxiety in patients, who are already trying to cope with the management of their disease.6-8
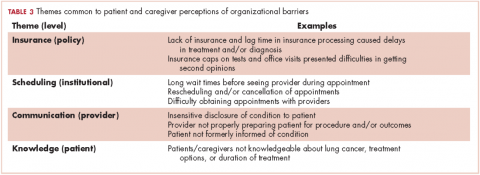
The concept of barriers to quality care (such as the receipt of timely and appropriate diagnostic and staging work-up and treatment selection according to evidence-based guidelines) is generally used in the context of improving health care management or prevention programs.9-13 Barriers might include high costs, transportation, distance, underinsurance, limited hours for access to care, patient sharing by physicians, and a lack of access to information about physicians’ recommendations.10,14-16 Such barriers have been categorized as organizational (leadership and workforce), structural (process of care), clinical (provider-patient encounter), and macro (policy and population).17,18 Organizational barriers are defined as impediments encountered within the medical system and health care organizations when accessing, receiving, and delivering care.12 Several organizational barriers have been identified in the literature based on characteristics of the targeted population (eg, race, ethnicity, type of illness), key stakeholder views, and aspects of care (eg, screening, preventive practice, care, and treatment).
In a systematic review, Betancourt and colleagues reported provider-patient interactions, processes of care, and language as some of the barriers to receiving quality care.17 Although cancer screening has been shown to reduce mortality in the adult population for several types of cancer,19-21 barriers that impede access to services have been identified as emanating not only from the macro level (eg, age of screening, reimbursement problems, screening guidelines) or inter- and intra-individual levels (eg, awareness of screening, various perspectives on life and cancer, comorbidities, social support), but also from the organization (organizational infrastructure that inhibits screening because of limited participation in research trials) and provider levels (impaired communication regarding screening between patient and physician, low commitment to shared decision-making, provider’s awareness of screening and screening guidelines).18 Other organizational barriers, such as difficulty navigating the health care system, poor interaction between patients and medical staff, and language barriers, have been identified in a systematic review of breast cancer screening in immigrant and minority women.22
Other barriers to quality cancer care reported by patients include knowledge about the disease and treatment, poor communication with providers, lack of coordination and timeliness of care, and lack of attention to care. Providers have identified other barriers to quality care, which include a lack of access to care, reimbursement problems, poor psychosocial support services, accountability of care, provider workload, and inadequate patient education.23 Few qualitative studies have been conducted to understand the organizational barriers that lung cancer patients and their caregivers face within the health care system.
Through the use of focus groups, we sought the perspectives of lung cancer patients and their caregivers on the organizational barriers that they experience while navigating the health care system. Identifying and understanding these barriers can help health care professionals work with patients and their caregivers to alleviate these stressors in an already difficult time.3,24 In addition, a more thorough understanding of patients’ and caregivers’ perspectives on organizational barriers may help improve health care delivery and, thus, patient satisfaction.
Methods
With the approval of the Institutional Review Boards of the University of Memphis and the Baptist Memorial Health Care Corporation, we conducted focus groups with lung cancer patients and their informal caregivers to understand the challenges they encounter while navigating the health care system during their illness. The Baptist Memorial Health Care system is centered in the Mid-South region of the United States, which has some of the highest US lung cancer incidence rates.25
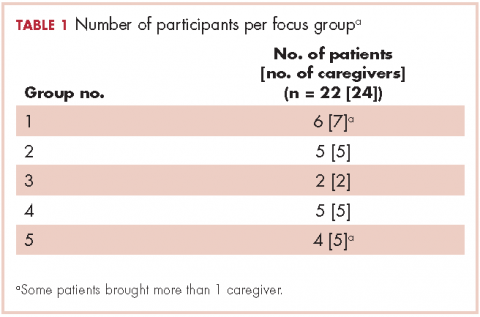
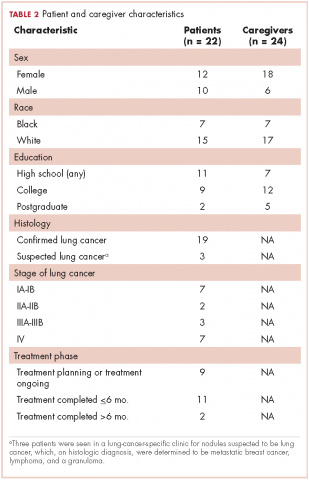
Research staff identified potential participants from a roster of patients provided by treatment clinics within the system. Patients eligible for this study had received care for suspected lung cancer within a community-based health care system within 6 months preceding the date of the focus group. Eligible patients were approached by the research staff by cold calling or in-person contact during clinic visits for their consent to participate in the study. From a compiled list of 219 patients, 89 received initial contact to gauge interest. Of those, 42 patients were formally approached and asked to participate; 22 agreed to participate, and 20 did not participate for reasons including illness, previous participation in other forms of patient feedback, lack of interest, failure to show up to focus group sessions, change of mind, lack of transportation, or other commitments. Patients identified their informal caregivers to form patient-caregiver dyads. All patients and caregivers provided written informed consent before participating in the focus groups.
We conducted 10 focus groups during March 2013 through January 2014 – 5 with 22 patients and 5 with 24 caregivers (Table 1). Eight of the focus groups were conducted in Memphis, Tennessee, and to obtain the perspectives of patients from a rural setting, we conducted 2 focus groups in Grenada, Mississippi. All of the focus groups were facilitated by a medical anthropologist (SK) and a clinical psychologist (KDW), neither of whom was affiliated with the health care system. Each facilitator was accompanied by a note-taker. Patient-caregiver dyads came to the designated location together. Two focus groups (one for patients, the other for caregivers) were then conducted simultaneously in 2 separate rooms. The facilitators used a pilot-tested focus group interview guide during each session. The items in the focus group guide revolved around experience with the health care system in diagnosis and treatment; timeliness with appointments and procedures for diagnosis and subsequent care; physician communication in being informed about the disease, treatment, and getting questions answered; coordination of care; other challenges in receiving quality care; and suggestions for improving the patient and caregiver experience with the health care system.
The focus group sessions lasted 1 to 2 hours and were audiorecorded and transcribed verbatim. The data were analyzed by using Dedoose software version 5.0.11 (Sociocultural Research Consultants, Los Angeles, California). Data collection and analysis were conducted concurrently to achieve theoretical saturation. Creswell’s 7-step analysis framework was used as a guide to code and interpret the data.26 The process involved collecting raw data, preparing and organizing transcripts, reading the transcripts, coding the data with the help of qualitative software, analyzing the data for themes and subthemes, interpreting the themes, and devising the meaning of the themes.26 Initial codes were categorized and compared to determine recurrent themes. Three members of the research team independently reviewed the transcripts, extensively discussed the content, and developed consensus around the identified themes. Critical and rigorous steps were taken throughout data collection and analysis to ensure the credibility, transferability, dependability, and confirmability of the qualitative data.27-29 In addition, elements of the Consolidated Criteria for Reporting Qualitative Research checklist were used to strengthen the data collection, analysis, and reporting process.30
Results
The 10 focus groups included 46 participants: 22 patients and 24 caregivers (some patients brought multiple caregivers). Of the 22 patients, 12 were women and 7 were black. An equal proportion of patients had at least a high school education as had a college or postgraduate degree. Although all of the patients had had a lung lesion suspicious for lung cancer, 19 eventually had a histologic diagnosis of lung cancer. The remaining 3 patients were all evaluated in a thoracic oncology clinic but were eventually found to have metastatic breast cancer, lymphoma, and a granuloma. Nine patients were either currently in the treatment decision-making process or actively receiving treatment, 11 had completed treatment within the preceding 6 months, and 2 had completed treatment more than 6 months previously. Treatment covered the spectrum from curative intent to palliative care. Of the 24 caregivers, 18 were women and 7 were black; 12 caregivers had at least a college education, of whom 2 had postgraduate degrees (Table 2).
Based on participants’ feedback, we identified 4 main levels within the system where barriers to optimal care occurred: policy, institutional, provider, and patient. From our qualitative analyses, we identified a central theme associated with each level, around which the barriers coalesced. The themes were insurance, scheduling, provider communication, and patient knowledge. At the policy level, medical insurance was perceived to affect the timeliness of care and to be a deterrent to timely diagnosis and quality treatment. Lack of insurance was a daunting obstacle for indigent patients. However, even those who were insured felt that dealing with insurance companies was a significant barrier to care. At the institutional level, appointment scheduling caused problems for both patients and their caregivers. At the health care provider level, communication was perceived as a major problem. And finally, at the patient level, both patient and caregiver lack of knowledge of lung cancer and the processes inherent in lung cancer treatment were barriers for optimal diagnosis and treatment (Table 3).
Insurance barriers
At the policy level, health insurance was reported as a significant barrier to accessing health care. Patients and caregivers reported delays in diagnosis and/or treatment because of either lack of insurance or lag time in insurance processing of clinician requests. Insurance restrictions on tests, procedures, and office visits presented difficulties in getting additional opinions from providers regarding diagnosis, prognosis, or treatment plans. Some patients were no longer able to see providers after they had met the test or office visit limit allotted by insurance providers. One patient shared the following experience with office visits leading up to their lung cancer diagnosis:
Patient … with my insurance I just had only 12 office visits … and I had already maxed those out.
In other instances, insurers would not cover hospital or clinic visits if certain logistic protocols were not met. This would sometimes leave patients stranded for a period of time without receiving any care.
Caretaker … went home and went to one of those minor emergency clinics and they sent her to [xx hospital in another city]. There they did nothing. That was then over the weekend, and my son wanted to get her out of there to get her into practice because they weren’t doing anything. They said, ‘No. Your insurance won’t pay for it unless you stay here till we sign the papers to be transferred.’ … It was a bandage. Period.
Some insurers would not cover certain health services outside of routine testing protocols for the patients’ conditions. This lack of coverage caused patients to pay out of pocket for needed care.
Patient Nothing in the lymph nodes, but if it hadn’t been for me going ahead with this [coronary] calcium score, the insurance wasn’t gonna pay anything. If it wouldn’t been for the 79 bucks or the family situation, I wouldn’t be sitting [here] today.
Individuals who had not yet met the age requirement for Medicare reported being without insurance for a period of time, which contributed to delays in accessing care.
Caregiver [xx patient] probably … could’ve been diagnosed maybe even months ago, but she is in that in-between where she gets Social Security but she’s not 65 until November, so she has no insurance.
Scheduling barriers
At the institutional level, patients and caregivers reported problems with appointment scheduling. Logistic problems with adjusting work schedules and arranging for transportation as well as long wait times before evaluation by a provider were recurrent themes expressed by both patients and caregivers. Many had become resigned to the expectation of long wait times during appointments.
Patient I have to call the month before to make the appointment because they don’t take appointments so far—‘Oh, we’re not working on that yet.’ I find that very annoying ....
Caregiver The last time I was there I waited four hours.
Caregiver … your appointment at 9:00 and you get called back at 9:30 or 10:00 and you get to see the doctor by 11:00, but that’s not any different than anywhere, unfortunately ….
Rescheduled appointments also posed a problem for participants. Constant rescheduling was an inconvenience for both patients and caregivers. Many were unhappy with rescheduling because both patients and caregivers had prepared mentally and physically for an appointment, only to be told that they would have to reschedule, which caused delays in the care process.
Patient I think every single visit I had with him gets rescheduled at least twice ....
Caregiver Three times this week we’ve been geared up, ready to have chemo and they keep changing it.
Patient Everything was fine with me, but they keep cancelling my appointments ....
Some participants perceived that the popularity of physicians might explain the difficulty with scheduling. Patients suggested that it is challenging to get appointments with better-known physicians, so they are more accepting of appointments at any time, even if the time is inconvenient for them.
Caregiver Of course, … if you have a popular doctor, sometimes you don’t always get the appointment you want …
Participants also expressed frustration with the way appointments were rescheduled. They felt as though the physicians were not concerned about their lives outside of office visits.
Patient … patients actually have lives. Many of them have jobs or families or responsibilities.
Communication barriers
At the provider level, poor communication between health care providers and patients was perceived as a major impediment to the quality of care patients received. Both patients and caregivers emphasized the importance of open patient-provider interactions and that there was a lack of such open communications in many instances. There was concern regarding the way diagnoses or prognoses were relayed to patients. Many times, physicians were insensitive and disregarded the sentiments of the patients and caregivers when delivering news about the patients’ condition, as one caregiver shared,
Caregiver … the pulmonary man came … in the room and said, ‘Oh, don’t worry about your lungs. Something else will get you first,’ which was a very, very bad thing to say.
Participants also expressed concerns that they were not properly prepared for treatments by their physicians because vital information was not discussed. They felt as if physicians were not realistic about potential outcomes. This resulted in patients and caregivers being too optimistic and later disappointed when the outcome was not what they had originally expected.
Patient Until I got to this office, I was totally oversold on everything. I was told surgery … robotic, not invasive. Day one, surgery. Day two, tubes out. Day three, go home. I expected to be home on Sunday night, stir-frying vegetables, and making dinner, feeding my cat. I was in ICU four days … I went home with oxygen. I mean I thought I was just gonna walk outta there…. You take a little thing out and you put a Band-Aid on, and you go home.
Data also revealed that patients were unsure of their condition, even following treatment. Information was not communicated to patients about the specifics of their disease, either because of miscommunication or minimal patient-provider time spent during office visits. This lack of communication between patients and providers often left patients and caregivers uncertain about exactly what condition they had or what they were being treated for.
Patient I just can’t have the time with Dr. xx, cuz he’s so busy...
Patient … I didn’t understand. Which exactly what type of cancer did I have, cuz I’m—really to tell you the truth—I’m still wondering.
Knowledge barriers
Patients and caregivers also identified a lack of education and knowledge about lung cancer diagnosis and treatment as a barrier to their care. Patients and caregivers were not always fully knowledgeable about lung cancer, treatment options, or the duration of treatments. They relied on the provider to disclose such information or direct them to credible sources. In many instances, patients were misinformed about the causes of lung cancer. There were misconceptions that lung cancer was only caused by a history of smoking or genetic predisposition. Patients who did not smoke or did not have a family history of lung cancer were often confused and dismayed by the diagnosis.
Patient I was trying to figure out, why do I have lung cancer. Never smoked a day in my life.
Patients were often unaware of treatment options or side effects of various treatments. They relied on physicians to relay information and make decisions for them about treatment plans.
Patient I was told chemo would probably be the best thing for me, and I just had faith that Dr. xx knows more about it than me.
Patient I’m doing chemo but it’s — what I’m doing is different. Of course, I don’t know anything about it actually either. It’s what I hear from other people.
Other patients relied on their own sources for information about their condition, either through the Internet, from family members and/or friends, or from preconceived notions.
Patient Well — of course in the meantime, I read — because they said it was a small cell, very aggressive, so I felt like everything I read — that’s the deal. I think we’ve become where we can get on the internet and look up so much, that to me, I was gonna be gone.
Patient When he said, ‘Cancer,’ I said, ‘Well, I thought cancer was a heredity thing? That you have to have somebody in your family that has it…’
Discussion
Organizational barriers are an important consideration in the delivery and receipt of high-quality, patient-centered lung cancer care. This qualitative study of patients being treated for lung cancer and their informal caregivers revealed several common perceived organizational barriers to receiving care, including health insurance coverage restrictions, appointment scheduling difficulties, quality of communication with physicians, and failure to properly educate the patient and family about the disease and what to expect of the treatment process.
The provider communication and patient knowledge barriers seem to reinforce each other and could be improved through focused efforts on the quality of communication between patients and their caregivers and clinical care providers. Patients expect, but are often deprived of, open and active dialogue with their providers. Improved communication can be helpful in educating patients and their caregivers about their disease, prognosis, and treatment goals. Although communication ranks highly as a patient and caregiver priority, there is often a disconnect between patients and caregivers and their physicians.31 Patients and caregivers often want to be more involved in the decision-making process, and effective communication between physicians and patients has been linked to the patient’s ability to understand, and also receive high-quality care.32,33 Failure to communicate effectively and educate patients on key aspects of their condition strips them of their autonomy in decision-making.
The involvement of a navigator for patients being treated for lung cancer could be pivotal in relieving the communication and scheduling barriers. The nurse navigator assists with coordinating effective communication and providing needed information between providers and patients and their caregivers. A navigator also serves as a single point of contact for patients and caregivers to communicate questions outside of physician visits or concerns that may not be urgent enough to warrant immediate physician response.34 The navigator coordinates patients’ appointment schedules and physician referrals and communicates the details of the next steps in the care-delivery process. This helps remove the barriers to care and improve patient outcomes and the quality of health care delivery, especially for patients and caregivers dealing with a life-threatening illness within a complex referral process.35
Multidisciplinary care, a much-recommended alternative care-delivery model, should, in theory, promote connectivity of providers and collaboration between providers, patients, and family members. This model could help reduce barriers for patients and caregivers.3,24,36 A network of connected providers can better coordinate treatment plans, easily share test results, and provide built-in second opinions. Given the increasingly multimodal approach to the diagnosis, staging, and treatment of lung cancer, the multidisciplinary model could allow physicians to consider multiple perspectives and care-delivery options and, ideally, develop consensus around the optimal approach for each individual patient in one setting. This can shorten the length of time before treatment and establish a plan that is tailored to the patient’s needs.24
The National Academy of Medicine (formerly, Institute of Medicine) proposes that modern health care systems have 6 aims for quality improvement: safety, effectiveness, patient-centeredness, timeliness, efficiency, and equity.37 It would take changes in the design and implementation of organizational support systems at the policy, institutional, and provider level for those aims to be achieved. Further investigation of the problems identified by patients and caregivers could lead to innovative solutions to improve lung cancer care. Future work should evaluate the most effective communication styles in patient-provider interactions, particularly in regard to to lung cancer diagnosis and treatment, and investigate how multidisciplinary models influence patient-provider communication and patient care.
Limitations
This study has several limitations. Less than half of those approached for the study participated in the study for various reasons, which may have introduced selection bias in terms of not having the perspectives of patients not willing or able to participate in the study. Though focus groups are known to generate rich in-depth views of certain issues, they have been criticized as potentially lacking rigor and generalizability. To address this concern, we used a standardized script for each focus group and involved multiple members of the research team in data analysis and interpretation. Also, this study enrolled participants from a single health care institution and did not use a comparison group. There might be institutional and geographic differences in the experience of lung cancer care, which might further limit the generalizability of the results of this study.
Conclusions
Despite those limitations, this study offers valuable insight into the barriers that lung cancer patients and caregivers encounter while navigating a community-level health care system. Eliminating or minimizing these barriers will require strategic plans that help mitigate insurance-related, scheduling, provider-patient communication, and patient/caregiver knowledge acquisition problems and translate them into tactical actions for quality improvement. This is one of the first qualitative studies conducted to understand the organizational barriers that lung cancer patients and their caregivers face within a health care system. Additional research is needed to explore these barriers and develop viable solutions.
Disclaimer
All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute or its Board of Governors or Methodology Committee.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30.
2. Riedel RF, Wang X, McCormack M, et al. Impact of a multidisciplinary thoracic oncology clinic on the timeliness of care. J Thorac Oncol. 2006;1(7):692-696.
3. Seek A, Hogle WP. Modeling a better way: navigating the health care system for patients with lung cancer. Clin J Oncol Nurs. 2007;11(1):81-85.
4. Sorensen R, Iedema R. Redefining accountability in health care: managing the plurality of medical interests. Health. 2008;12(1):87-106.
5. Mosher CE, Jaynes HA, Hanna N, Ostroff JS. Distressed family caregivers of lung cancer patients: an examination of psychosocial and practical challenges. Support Care Cancer. 2013;21(2):431-437.
6. Cantril C, Haylock PJ. Patient navigation in the oncology care setting. Semin Oncol Nurs. 2013;29(2):76-90.
7. Freeman HP. Patient navigation: a community based strategy to reduce cancer disparities. J Urban Health. 2006;83(2):139-141.
8. Kwon DH, Tisnado DM, Keating NL, et al. Physician reported barriers to referring cancer patients to specialists: prevalence, factors, and association with career satisfaction. Cancer. 2015;121(1):113-122.
9. Andersen SE. Implementation of a new prescription system. A qualitative survey of organizational barriers. Ugeskr Laeger. 2002;164(38):4449-4453.
10. Rousseau L, Guay M, Archambault D, El m’ala Z, Abdelaziz N. Do organizational barriers to pneumococcal and influenza vaccine access exist? Can J Public Health. 2007;98(2):105-110.
11. Storey J, Buchanan D. Health care governance and organizational barriers to learning from mistakes. J Health Organ Manag. 2008;22(6):642-651.
12. Ziegenfuss JT Jr. Organizational barriers to quality improvement in medical and health care organizations. Qual Assur Util Rev. 1991;6(4):115-122.
13. Rihari-Thomas J, DiGiacomo M, Phillips J, Newton P, Davidson PM. Clinician perspectives of barriers to effective implementation of a rapid response system in an academic health centre: a focus group study. Int J Health Policy Manag. 2017;6(8):447-456.
14. Dutton D. Financial, organizational and professional factors affecting health care utilization. Soc Sci Med. 1986;23(7):721-735.
15. King CJ, Chen J, Dagher RK, Holt CL, Thomas SB. Decomposing differences in medical care access among cancer survivors by race and ethnicity. Am J Med Qual. 2015;30(5):459-469.
16. Renzaho AM, Romios P, Crock C, Sønderlund AL. The effectiveness of cultural competence programs in ethnic minority patient-centered health care—a systematic review of the literature. Int J Qual Health Care. 2013;25(3):261-269.
17. Betancourt JR, Green AR, Carrillo JE, Ananeh-Firempong O. Defining cultural competence: a practical framework for addressing racial/ethnic disparities in health and health care. Public Health Rep. 2003;118(4):293-302.
18. Scheinfeld Gorin S, Gauthier J, Hay J, Miles A, Wardle J. Cancer screening and aging: research barriers and opportunities. Cancer. 2008;113(suppl 12):3493-3504.
19. Humphrey L, Deffebach M, Pappas M, et al. Screening for lung cancer: systematic review to update the US preventive services task force recommendation. Evidence syntheses No. 105. Rockville, MD: Agency for Health Care Research and Quality; 2013.
20. Nelson HD, Fu R, Cantor A, Pappas M, Daeges M, Humphrey L. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 U.S. Preventive Services Task Force Recommendation. Ann Intern Med. 2016;164(4):244-255.
21. Zauber AG. The impact of screening on colorectal cancer mortality and incidence: has it really made a difference? Dig Dis Sci. 2015;60(3):681-691.
22. Remennick L. The challenge of early breast cancer detection among immigrant and minority women in multicultural societies. Breast J. 2006;12(suppl 1):103-110.
23. Hess LM, Pohl G. Perspectives of quality care in cancer treatment: a review of the literature. Am Health Drug Benefits. 2013;6(6):321-329.
24. Kedia SK, Ward KD, Digney SA, et al. ‘One-stop shop’: lung cancer patients’ and caregivers’ perceptions of multidisciplinary care in a community health care setting. Transl Lung Cancer Res. 2015;4(4):456-464.
25. US Cancer Statistics Working Group. United States cancer statistics: 1999-2014 incidence and mortality web-based report. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute. http://www.cdc.gov/uscs. Published 2017. Accessed November 21, 2017.
26. Creswell JW. Research design: qualitative, quantitative, and mixed methods approaches. 4th ed. Thousand Oaks, CA: SAGE Publications; 2014.
27. Ballinger C. Demonstrating rigour and quality? In Finlay L, Ballinger C, eds. Qualitative research for allied health professionals: challenging choices. Chichester, England: John Wiley & Sons Ltd; 2006:235-246.
28. Finlay L. ‘Rigour’, ‘ethical integrity’ or ‘artistry’? Reflexively reviewing criteria for evaluating qualitative research. Br J Occup Ther. 2006;69(7):319-326.
29. Lincoln YS, Guba EG. Naturalistic inquiry. Newbury Park, CA: SAGE Publications; 1985.
30. Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus group. Int J Qual Health Care. 2007;19(6):349-357.
31. Neeman N, Quinn K, Shoeb M, Mourad M, Sehgal NL, Sliwka D. Postdischarge focus groups to improve the hospital experience. Am J Med Qual. 2013;28(6):536-538.
32. O’Day BL, Killeen M, Iezzoni LI. Improving health care experiences of persons who are blind or have low vision: suggestions from focus groups. Am J Med Qual. 2004;19(5):193-200.
33. Smith B, Lynch WD, Markow C, Lifsey S, Slover M. Consumers’ understanding of and interest in provider- versus practice-level quality characteristics: findings from a focus group study. Am J Med Qual. 2015;30(4):367-373.
34. Islam KM, Opoku ST, Apenteng BA, et al. Coping with an advanced stage lung cancer diagnosis: patient, caregiver, and provider perspectives on the role of the health care system. J Cancer Educ. 2016;31(3):554-558.
35. Pedersen A, Hack TF. Pilots of oncology health care: a concept analysis of the patient navigator role. Oncol Nurs Forum. 2010;37(1):55-60.
36. Gopal R. How to maintain multidisciplinary treatment schedules. J Support Oncol. 2005;3(3):248-256.
37. Committee on Quality of Health Care in America. Improving the 21st century health care system. In: Institute of Medicine, ed. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academies Press; 2001:39-60.
Lung cancer is a major public health challenge in the United States. It is the leading cause of cancer death in the United States, accounting for 27% of all cancer deaths, and it has an aggregate 5-year survival rate of 18%.1 Advances in diagnostic and treatment options are rapidly increasing the complexity of lung cancer care delivery, which involves multiple specialty providers and often cuts across health care institutions.2-4 Navigating the process of care while coping with the complexities of the illness can be overwhelming for both the patient and the caregiver.5 With increasing regulations and cost-cutting measures, the health care system in the United States can pose many challenges, especially for those dealing with catastrophic and life-threatening illnesses. Any barrier to accessing care often increases anxiety in patients, who are already trying to cope with the management of their disease.6-8
The concept of barriers to quality care (such as the receipt of timely and appropriate diagnostic and staging work-up and treatment selection according to evidence-based guidelines) is generally used in the context of improving health care management or prevention programs.9-13 Barriers might include high costs, transportation, distance, underinsurance, limited hours for access to care, patient sharing by physicians, and a lack of access to information about physicians’ recommendations.10,14-16 Such barriers have been categorized as organizational (leadership and workforce), structural (process of care), clinical (provider-patient encounter), and macro (policy and population).17,18 Organizational barriers are defined as impediments encountered within the medical system and health care organizations when accessing, receiving, and delivering care.12 Several organizational barriers have been identified in the literature based on characteristics of the targeted population (eg, race, ethnicity, type of illness), key stakeholder views, and aspects of care (eg, screening, preventive practice, care, and treatment).
In a systematic review, Betancourt and colleagues reported provider-patient interactions, processes of care, and language as some of the barriers to receiving quality care.17 Although cancer screening has been shown to reduce mortality in the adult population for several types of cancer,19-21 barriers that impede access to services have been identified as emanating not only from the macro level (eg, age of screening, reimbursement problems, screening guidelines) or inter- and intra-individual levels (eg, awareness of screening, various perspectives on life and cancer, comorbidities, social support), but also from the organization (organizational infrastructure that inhibits screening because of limited participation in research trials) and provider levels (impaired communication regarding screening between patient and physician, low commitment to shared decision-making, provider’s awareness of screening and screening guidelines).18 Other organizational barriers, such as difficulty navigating the health care system, poor interaction between patients and medical staff, and language barriers, have been identified in a systematic review of breast cancer screening in immigrant and minority women.22
Other barriers to quality cancer care reported by patients include knowledge about the disease and treatment, poor communication with providers, lack of coordination and timeliness of care, and lack of attention to care. Providers have identified other barriers to quality care, which include a lack of access to care, reimbursement problems, poor psychosocial support services, accountability of care, provider workload, and inadequate patient education.23 Few qualitative studies have been conducted to understand the organizational barriers that lung cancer patients and their caregivers face within the health care system.
Through the use of focus groups, we sought the perspectives of lung cancer patients and their caregivers on the organizational barriers that they experience while navigating the health care system. Identifying and understanding these barriers can help health care professionals work with patients and their caregivers to alleviate these stressors in an already difficult time.3,24 In addition, a more thorough understanding of patients’ and caregivers’ perspectives on organizational barriers may help improve health care delivery and, thus, patient satisfaction.
Methods
With the approval of the Institutional Review Boards of the University of Memphis and the Baptist Memorial Health Care Corporation, we conducted focus groups with lung cancer patients and their informal caregivers to understand the challenges they encounter while navigating the health care system during their illness. The Baptist Memorial Health Care system is centered in the Mid-South region of the United States, which has some of the highest US lung cancer incidence rates.25
Research staff identified potential participants from a roster of patients provided by treatment clinics within the system. Patients eligible for this study had received care for suspected lung cancer within a community-based health care system within 6 months preceding the date of the focus group. Eligible patients were approached by the research staff by cold calling or in-person contact during clinic visits for their consent to participate in the study. From a compiled list of 219 patients, 89 received initial contact to gauge interest. Of those, 42 patients were formally approached and asked to participate; 22 agreed to participate, and 20 did not participate for reasons including illness, previous participation in other forms of patient feedback, lack of interest, failure to show up to focus group sessions, change of mind, lack of transportation, or other commitments. Patients identified their informal caregivers to form patient-caregiver dyads. All patients and caregivers provided written informed consent before participating in the focus groups.
We conducted 10 focus groups during March 2013 through January 2014 – 5 with 22 patients and 5 with 24 caregivers (Table 1). Eight of the focus groups were conducted in Memphis, Tennessee, and to obtain the perspectives of patients from a rural setting, we conducted 2 focus groups in Grenada, Mississippi. All of the focus groups were facilitated by a medical anthropologist (SK) and a clinical psychologist (KDW), neither of whom was affiliated with the health care system. Each facilitator was accompanied by a note-taker. Patient-caregiver dyads came to the designated location together. Two focus groups (one for patients, the other for caregivers) were then conducted simultaneously in 2 separate rooms. The facilitators used a pilot-tested focus group interview guide during each session. The items in the focus group guide revolved around experience with the health care system in diagnosis and treatment; timeliness with appointments and procedures for diagnosis and subsequent care; physician communication in being informed about the disease, treatment, and getting questions answered; coordination of care; other challenges in receiving quality care; and suggestions for improving the patient and caregiver experience with the health care system.
The focus group sessions lasted 1 to 2 hours and were audiorecorded and transcribed verbatim. The data were analyzed by using Dedoose software version 5.0.11 (Sociocultural Research Consultants, Los Angeles, California). Data collection and analysis were conducted concurrently to achieve theoretical saturation. Creswell’s 7-step analysis framework was used as a guide to code and interpret the data.26 The process involved collecting raw data, preparing and organizing transcripts, reading the transcripts, coding the data with the help of qualitative software, analyzing the data for themes and subthemes, interpreting the themes, and devising the meaning of the themes.26 Initial codes were categorized and compared to determine recurrent themes. Three members of the research team independently reviewed the transcripts, extensively discussed the content, and developed consensus around the identified themes. Critical and rigorous steps were taken throughout data collection and analysis to ensure the credibility, transferability, dependability, and confirmability of the qualitative data.27-29 In addition, elements of the Consolidated Criteria for Reporting Qualitative Research checklist were used to strengthen the data collection, analysis, and reporting process.30
Results
The 10 focus groups included 46 participants: 22 patients and 24 caregivers (some patients brought multiple caregivers). Of the 22 patients, 12 were women and 7 were black. An equal proportion of patients had at least a high school education as had a college or postgraduate degree. Although all of the patients had had a lung lesion suspicious for lung cancer, 19 eventually had a histologic diagnosis of lung cancer. The remaining 3 patients were all evaluated in a thoracic oncology clinic but were eventually found to have metastatic breast cancer, lymphoma, and a granuloma. Nine patients were either currently in the treatment decision-making process or actively receiving treatment, 11 had completed treatment within the preceding 6 months, and 2 had completed treatment more than 6 months previously. Treatment covered the spectrum from curative intent to palliative care. Of the 24 caregivers, 18 were women and 7 were black; 12 caregivers had at least a college education, of whom 2 had postgraduate degrees (Table 2).
Based on participants’ feedback, we identified 4 main levels within the system where barriers to optimal care occurred: policy, institutional, provider, and patient. From our qualitative analyses, we identified a central theme associated with each level, around which the barriers coalesced. The themes were insurance, scheduling, provider communication, and patient knowledge. At the policy level, medical insurance was perceived to affect the timeliness of care and to be a deterrent to timely diagnosis and quality treatment. Lack of insurance was a daunting obstacle for indigent patients. However, even those who were insured felt that dealing with insurance companies was a significant barrier to care. At the institutional level, appointment scheduling caused problems for both patients and their caregivers. At the health care provider level, communication was perceived as a major problem. And finally, at the patient level, both patient and caregiver lack of knowledge of lung cancer and the processes inherent in lung cancer treatment were barriers for optimal diagnosis and treatment (Table 3).
Insurance barriers
At the policy level, health insurance was reported as a significant barrier to accessing health care. Patients and caregivers reported delays in diagnosis and/or treatment because of either lack of insurance or lag time in insurance processing of clinician requests. Insurance restrictions on tests, procedures, and office visits presented difficulties in getting additional opinions from providers regarding diagnosis, prognosis, or treatment plans. Some patients were no longer able to see providers after they had met the test or office visit limit allotted by insurance providers. One patient shared the following experience with office visits leading up to their lung cancer diagnosis:
Patient … with my insurance I just had only 12 office visits … and I had already maxed those out.
In other instances, insurers would not cover hospital or clinic visits if certain logistic protocols were not met. This would sometimes leave patients stranded for a period of time without receiving any care.
Caretaker … went home and went to one of those minor emergency clinics and they sent her to [xx hospital in another city]. There they did nothing. That was then over the weekend, and my son wanted to get her out of there to get her into practice because they weren’t doing anything. They said, ‘No. Your insurance won’t pay for it unless you stay here till we sign the papers to be transferred.’ … It was a bandage. Period.
Some insurers would not cover certain health services outside of routine testing protocols for the patients’ conditions. This lack of coverage caused patients to pay out of pocket for needed care.
Patient Nothing in the lymph nodes, but if it hadn’t been for me going ahead with this [coronary] calcium score, the insurance wasn’t gonna pay anything. If it wouldn’t been for the 79 bucks or the family situation, I wouldn’t be sitting [here] today.
Individuals who had not yet met the age requirement for Medicare reported being without insurance for a period of time, which contributed to delays in accessing care.
Caregiver [xx patient] probably … could’ve been diagnosed maybe even months ago, but she is in that in-between where she gets Social Security but she’s not 65 until November, so she has no insurance.
Scheduling barriers
At the institutional level, patients and caregivers reported problems with appointment scheduling. Logistic problems with adjusting work schedules and arranging for transportation as well as long wait times before evaluation by a provider were recurrent themes expressed by both patients and caregivers. Many had become resigned to the expectation of long wait times during appointments.
Patient I have to call the month before to make the appointment because they don’t take appointments so far—‘Oh, we’re not working on that yet.’ I find that very annoying ....
Caregiver The last time I was there I waited four hours.
Caregiver … your appointment at 9:00 and you get called back at 9:30 or 10:00 and you get to see the doctor by 11:00, but that’s not any different than anywhere, unfortunately ….
Rescheduled appointments also posed a problem for participants. Constant rescheduling was an inconvenience for both patients and caregivers. Many were unhappy with rescheduling because both patients and caregivers had prepared mentally and physically for an appointment, only to be told that they would have to reschedule, which caused delays in the care process.
Patient I think every single visit I had with him gets rescheduled at least twice ....
Caregiver Three times this week we’ve been geared up, ready to have chemo and they keep changing it.
Patient Everything was fine with me, but they keep cancelling my appointments ....
Some participants perceived that the popularity of physicians might explain the difficulty with scheduling. Patients suggested that it is challenging to get appointments with better-known physicians, so they are more accepting of appointments at any time, even if the time is inconvenient for them.
Caregiver Of course, … if you have a popular doctor, sometimes you don’t always get the appointment you want …
Participants also expressed frustration with the way appointments were rescheduled. They felt as though the physicians were not concerned about their lives outside of office visits.
Patient … patients actually have lives. Many of them have jobs or families or responsibilities.
Communication barriers
At the provider level, poor communication between health care providers and patients was perceived as a major impediment to the quality of care patients received. Both patients and caregivers emphasized the importance of open patient-provider interactions and that there was a lack of such open communications in many instances. There was concern regarding the way diagnoses or prognoses were relayed to patients. Many times, physicians were insensitive and disregarded the sentiments of the patients and caregivers when delivering news about the patients’ condition, as one caregiver shared,
Caregiver … the pulmonary man came … in the room and said, ‘Oh, don’t worry about your lungs. Something else will get you first,’ which was a very, very bad thing to say.
Participants also expressed concerns that they were not properly prepared for treatments by their physicians because vital information was not discussed. They felt as if physicians were not realistic about potential outcomes. This resulted in patients and caregivers being too optimistic and later disappointed when the outcome was not what they had originally expected.
Patient Until I got to this office, I was totally oversold on everything. I was told surgery … robotic, not invasive. Day one, surgery. Day two, tubes out. Day three, go home. I expected to be home on Sunday night, stir-frying vegetables, and making dinner, feeding my cat. I was in ICU four days … I went home with oxygen. I mean I thought I was just gonna walk outta there…. You take a little thing out and you put a Band-Aid on, and you go home.
Data also revealed that patients were unsure of their condition, even following treatment. Information was not communicated to patients about the specifics of their disease, either because of miscommunication or minimal patient-provider time spent during office visits. This lack of communication between patients and providers often left patients and caregivers uncertain about exactly what condition they had or what they were being treated for.
Patient I just can’t have the time with Dr. xx, cuz he’s so busy...
Patient … I didn’t understand. Which exactly what type of cancer did I have, cuz I’m—really to tell you the truth—I’m still wondering.
Knowledge barriers
Patients and caregivers also identified a lack of education and knowledge about lung cancer diagnosis and treatment as a barrier to their care. Patients and caregivers were not always fully knowledgeable about lung cancer, treatment options, or the duration of treatments. They relied on the provider to disclose such information or direct them to credible sources. In many instances, patients were misinformed about the causes of lung cancer. There were misconceptions that lung cancer was only caused by a history of smoking or genetic predisposition. Patients who did not smoke or did not have a family history of lung cancer were often confused and dismayed by the diagnosis.
Patient I was trying to figure out, why do I have lung cancer. Never smoked a day in my life.
Patients were often unaware of treatment options or side effects of various treatments. They relied on physicians to relay information and make decisions for them about treatment plans.
Patient I was told chemo would probably be the best thing for me, and I just had faith that Dr. xx knows more about it than me.
Patient I’m doing chemo but it’s — what I’m doing is different. Of course, I don’t know anything about it actually either. It’s what I hear from other people.
Other patients relied on their own sources for information about their condition, either through the Internet, from family members and/or friends, or from preconceived notions.
Patient Well — of course in the meantime, I read — because they said it was a small cell, very aggressive, so I felt like everything I read — that’s the deal. I think we’ve become where we can get on the internet and look up so much, that to me, I was gonna be gone.
Patient When he said, ‘Cancer,’ I said, ‘Well, I thought cancer was a heredity thing? That you have to have somebody in your family that has it…’
Discussion
Organizational barriers are an important consideration in the delivery and receipt of high-quality, patient-centered lung cancer care. This qualitative study of patients being treated for lung cancer and their informal caregivers revealed several common perceived organizational barriers to receiving care, including health insurance coverage restrictions, appointment scheduling difficulties, quality of communication with physicians, and failure to properly educate the patient and family about the disease and what to expect of the treatment process.
The provider communication and patient knowledge barriers seem to reinforce each other and could be improved through focused efforts on the quality of communication between patients and their caregivers and clinical care providers. Patients expect, but are often deprived of, open and active dialogue with their providers. Improved communication can be helpful in educating patients and their caregivers about their disease, prognosis, and treatment goals. Although communication ranks highly as a patient and caregiver priority, there is often a disconnect between patients and caregivers and their physicians.31 Patients and caregivers often want to be more involved in the decision-making process, and effective communication between physicians and patients has been linked to the patient’s ability to understand, and also receive high-quality care.32,33 Failure to communicate effectively and educate patients on key aspects of their condition strips them of their autonomy in decision-making.
The involvement of a navigator for patients being treated for lung cancer could be pivotal in relieving the communication and scheduling barriers. The nurse navigator assists with coordinating effective communication and providing needed information between providers and patients and their caregivers. A navigator also serves as a single point of contact for patients and caregivers to communicate questions outside of physician visits or concerns that may not be urgent enough to warrant immediate physician response.34 The navigator coordinates patients’ appointment schedules and physician referrals and communicates the details of the next steps in the care-delivery process. This helps remove the barriers to care and improve patient outcomes and the quality of health care delivery, especially for patients and caregivers dealing with a life-threatening illness within a complex referral process.35
Multidisciplinary care, a much-recommended alternative care-delivery model, should, in theory, promote connectivity of providers and collaboration between providers, patients, and family members. This model could help reduce barriers for patients and caregivers.3,24,36 A network of connected providers can better coordinate treatment plans, easily share test results, and provide built-in second opinions. Given the increasingly multimodal approach to the diagnosis, staging, and treatment of lung cancer, the multidisciplinary model could allow physicians to consider multiple perspectives and care-delivery options and, ideally, develop consensus around the optimal approach for each individual patient in one setting. This can shorten the length of time before treatment and establish a plan that is tailored to the patient’s needs.24
The National Academy of Medicine (formerly, Institute of Medicine) proposes that modern health care systems have 6 aims for quality improvement: safety, effectiveness, patient-centeredness, timeliness, efficiency, and equity.37 It would take changes in the design and implementation of organizational support systems at the policy, institutional, and provider level for those aims to be achieved. Further investigation of the problems identified by patients and caregivers could lead to innovative solutions to improve lung cancer care. Future work should evaluate the most effective communication styles in patient-provider interactions, particularly in regard to to lung cancer diagnosis and treatment, and investigate how multidisciplinary models influence patient-provider communication and patient care.
Limitations
This study has several limitations. Less than half of those approached for the study participated in the study for various reasons, which may have introduced selection bias in terms of not having the perspectives of patients not willing or able to participate in the study. Though focus groups are known to generate rich in-depth views of certain issues, they have been criticized as potentially lacking rigor and generalizability. To address this concern, we used a standardized script for each focus group and involved multiple members of the research team in data analysis and interpretation. Also, this study enrolled participants from a single health care institution and did not use a comparison group. There might be institutional and geographic differences in the experience of lung cancer care, which might further limit the generalizability of the results of this study.
Conclusions
Despite those limitations, this study offers valuable insight into the barriers that lung cancer patients and caregivers encounter while navigating a community-level health care system. Eliminating or minimizing these barriers will require strategic plans that help mitigate insurance-related, scheduling, provider-patient communication, and patient/caregiver knowledge acquisition problems and translate them into tactical actions for quality improvement. This is one of the first qualitative studies conducted to understand the organizational barriers that lung cancer patients and their caregivers face within a health care system. Additional research is needed to explore these barriers and develop viable solutions.
Disclaimer
All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute or its Board of Governors or Methodology Committee.
Lung cancer is a major public health challenge in the United States. It is the leading cause of cancer death in the United States, accounting for 27% of all cancer deaths, and it has an aggregate 5-year survival rate of 18%.1 Advances in diagnostic and treatment options are rapidly increasing the complexity of lung cancer care delivery, which involves multiple specialty providers and often cuts across health care institutions.2-4 Navigating the process of care while coping with the complexities of the illness can be overwhelming for both the patient and the caregiver.5 With increasing regulations and cost-cutting measures, the health care system in the United States can pose many challenges, especially for those dealing with catastrophic and life-threatening illnesses. Any barrier to accessing care often increases anxiety in patients, who are already trying to cope with the management of their disease.6-8
The concept of barriers to quality care (such as the receipt of timely and appropriate diagnostic and staging work-up and treatment selection according to evidence-based guidelines) is generally used in the context of improving health care management or prevention programs.9-13 Barriers might include high costs, transportation, distance, underinsurance, limited hours for access to care, patient sharing by physicians, and a lack of access to information about physicians’ recommendations.10,14-16 Such barriers have been categorized as organizational (leadership and workforce), structural (process of care), clinical (provider-patient encounter), and macro (policy and population).17,18 Organizational barriers are defined as impediments encountered within the medical system and health care organizations when accessing, receiving, and delivering care.12 Several organizational barriers have been identified in the literature based on characteristics of the targeted population (eg, race, ethnicity, type of illness), key stakeholder views, and aspects of care (eg, screening, preventive practice, care, and treatment).
In a systematic review, Betancourt and colleagues reported provider-patient interactions, processes of care, and language as some of the barriers to receiving quality care.17 Although cancer screening has been shown to reduce mortality in the adult population for several types of cancer,19-21 barriers that impede access to services have been identified as emanating not only from the macro level (eg, age of screening, reimbursement problems, screening guidelines) or inter- and intra-individual levels (eg, awareness of screening, various perspectives on life and cancer, comorbidities, social support), but also from the organization (organizational infrastructure that inhibits screening because of limited participation in research trials) and provider levels (impaired communication regarding screening between patient and physician, low commitment to shared decision-making, provider’s awareness of screening and screening guidelines).18 Other organizational barriers, such as difficulty navigating the health care system, poor interaction between patients and medical staff, and language barriers, have been identified in a systematic review of breast cancer screening in immigrant and minority women.22
Other barriers to quality cancer care reported by patients include knowledge about the disease and treatment, poor communication with providers, lack of coordination and timeliness of care, and lack of attention to care. Providers have identified other barriers to quality care, which include a lack of access to care, reimbursement problems, poor psychosocial support services, accountability of care, provider workload, and inadequate patient education.23 Few qualitative studies have been conducted to understand the organizational barriers that lung cancer patients and their caregivers face within the health care system.
Through the use of focus groups, we sought the perspectives of lung cancer patients and their caregivers on the organizational barriers that they experience while navigating the health care system. Identifying and understanding these barriers can help health care professionals work with patients and their caregivers to alleviate these stressors in an already difficult time.3,24 In addition, a more thorough understanding of patients’ and caregivers’ perspectives on organizational barriers may help improve health care delivery and, thus, patient satisfaction.
Methods
With the approval of the Institutional Review Boards of the University of Memphis and the Baptist Memorial Health Care Corporation, we conducted focus groups with lung cancer patients and their informal caregivers to understand the challenges they encounter while navigating the health care system during their illness. The Baptist Memorial Health Care system is centered in the Mid-South region of the United States, which has some of the highest US lung cancer incidence rates.25
Research staff identified potential participants from a roster of patients provided by treatment clinics within the system. Patients eligible for this study had received care for suspected lung cancer within a community-based health care system within 6 months preceding the date of the focus group. Eligible patients were approached by the research staff by cold calling or in-person contact during clinic visits for their consent to participate in the study. From a compiled list of 219 patients, 89 received initial contact to gauge interest. Of those, 42 patients were formally approached and asked to participate; 22 agreed to participate, and 20 did not participate for reasons including illness, previous participation in other forms of patient feedback, lack of interest, failure to show up to focus group sessions, change of mind, lack of transportation, or other commitments. Patients identified their informal caregivers to form patient-caregiver dyads. All patients and caregivers provided written informed consent before participating in the focus groups.
We conducted 10 focus groups during March 2013 through January 2014 – 5 with 22 patients and 5 with 24 caregivers (Table 1). Eight of the focus groups were conducted in Memphis, Tennessee, and to obtain the perspectives of patients from a rural setting, we conducted 2 focus groups in Grenada, Mississippi. All of the focus groups were facilitated by a medical anthropologist (SK) and a clinical psychologist (KDW), neither of whom was affiliated with the health care system. Each facilitator was accompanied by a note-taker. Patient-caregiver dyads came to the designated location together. Two focus groups (one for patients, the other for caregivers) were then conducted simultaneously in 2 separate rooms. The facilitators used a pilot-tested focus group interview guide during each session. The items in the focus group guide revolved around experience with the health care system in diagnosis and treatment; timeliness with appointments and procedures for diagnosis and subsequent care; physician communication in being informed about the disease, treatment, and getting questions answered; coordination of care; other challenges in receiving quality care; and suggestions for improving the patient and caregiver experience with the health care system.
The focus group sessions lasted 1 to 2 hours and were audiorecorded and transcribed verbatim. The data were analyzed by using Dedoose software version 5.0.11 (Sociocultural Research Consultants, Los Angeles, California). Data collection and analysis were conducted concurrently to achieve theoretical saturation. Creswell’s 7-step analysis framework was used as a guide to code and interpret the data.26 The process involved collecting raw data, preparing and organizing transcripts, reading the transcripts, coding the data with the help of qualitative software, analyzing the data for themes and subthemes, interpreting the themes, and devising the meaning of the themes.26 Initial codes were categorized and compared to determine recurrent themes. Three members of the research team independently reviewed the transcripts, extensively discussed the content, and developed consensus around the identified themes. Critical and rigorous steps were taken throughout data collection and analysis to ensure the credibility, transferability, dependability, and confirmability of the qualitative data.27-29 In addition, elements of the Consolidated Criteria for Reporting Qualitative Research checklist were used to strengthen the data collection, analysis, and reporting process.30
Results
The 10 focus groups included 46 participants: 22 patients and 24 caregivers (some patients brought multiple caregivers). Of the 22 patients, 12 were women and 7 were black. An equal proportion of patients had at least a high school education as had a college or postgraduate degree. Although all of the patients had had a lung lesion suspicious for lung cancer, 19 eventually had a histologic diagnosis of lung cancer. The remaining 3 patients were all evaluated in a thoracic oncology clinic but were eventually found to have metastatic breast cancer, lymphoma, and a granuloma. Nine patients were either currently in the treatment decision-making process or actively receiving treatment, 11 had completed treatment within the preceding 6 months, and 2 had completed treatment more than 6 months previously. Treatment covered the spectrum from curative intent to palliative care. Of the 24 caregivers, 18 were women and 7 were black; 12 caregivers had at least a college education, of whom 2 had postgraduate degrees (Table 2).
Based on participants’ feedback, we identified 4 main levels within the system where barriers to optimal care occurred: policy, institutional, provider, and patient. From our qualitative analyses, we identified a central theme associated with each level, around which the barriers coalesced. The themes were insurance, scheduling, provider communication, and patient knowledge. At the policy level, medical insurance was perceived to affect the timeliness of care and to be a deterrent to timely diagnosis and quality treatment. Lack of insurance was a daunting obstacle for indigent patients. However, even those who were insured felt that dealing with insurance companies was a significant barrier to care. At the institutional level, appointment scheduling caused problems for both patients and their caregivers. At the health care provider level, communication was perceived as a major problem. And finally, at the patient level, both patient and caregiver lack of knowledge of lung cancer and the processes inherent in lung cancer treatment were barriers for optimal diagnosis and treatment (Table 3).
Insurance barriers
At the policy level, health insurance was reported as a significant barrier to accessing health care. Patients and caregivers reported delays in diagnosis and/or treatment because of either lack of insurance or lag time in insurance processing of clinician requests. Insurance restrictions on tests, procedures, and office visits presented difficulties in getting additional opinions from providers regarding diagnosis, prognosis, or treatment plans. Some patients were no longer able to see providers after they had met the test or office visit limit allotted by insurance providers. One patient shared the following experience with office visits leading up to their lung cancer diagnosis:
Patient … with my insurance I just had only 12 office visits … and I had already maxed those out.
In other instances, insurers would not cover hospital or clinic visits if certain logistic protocols were not met. This would sometimes leave patients stranded for a period of time without receiving any care.
Caretaker … went home and went to one of those minor emergency clinics and they sent her to [xx hospital in another city]. There they did nothing. That was then over the weekend, and my son wanted to get her out of there to get her into practice because they weren’t doing anything. They said, ‘No. Your insurance won’t pay for it unless you stay here till we sign the papers to be transferred.’ … It was a bandage. Period.
Some insurers would not cover certain health services outside of routine testing protocols for the patients’ conditions. This lack of coverage caused patients to pay out of pocket for needed care.
Patient Nothing in the lymph nodes, but if it hadn’t been for me going ahead with this [coronary] calcium score, the insurance wasn’t gonna pay anything. If it wouldn’t been for the 79 bucks or the family situation, I wouldn’t be sitting [here] today.
Individuals who had not yet met the age requirement for Medicare reported being without insurance for a period of time, which contributed to delays in accessing care.
Caregiver [xx patient] probably … could’ve been diagnosed maybe even months ago, but she is in that in-between where she gets Social Security but she’s not 65 until November, so she has no insurance.
Scheduling barriers
At the institutional level, patients and caregivers reported problems with appointment scheduling. Logistic problems with adjusting work schedules and arranging for transportation as well as long wait times before evaluation by a provider were recurrent themes expressed by both patients and caregivers. Many had become resigned to the expectation of long wait times during appointments.
Patient I have to call the month before to make the appointment because they don’t take appointments so far—‘Oh, we’re not working on that yet.’ I find that very annoying ....
Caregiver The last time I was there I waited four hours.
Caregiver … your appointment at 9:00 and you get called back at 9:30 or 10:00 and you get to see the doctor by 11:00, but that’s not any different than anywhere, unfortunately ….
Rescheduled appointments also posed a problem for participants. Constant rescheduling was an inconvenience for both patients and caregivers. Many were unhappy with rescheduling because both patients and caregivers had prepared mentally and physically for an appointment, only to be told that they would have to reschedule, which caused delays in the care process.
Patient I think every single visit I had with him gets rescheduled at least twice ....
Caregiver Three times this week we’ve been geared up, ready to have chemo and they keep changing it.
Patient Everything was fine with me, but they keep cancelling my appointments ....
Some participants perceived that the popularity of physicians might explain the difficulty with scheduling. Patients suggested that it is challenging to get appointments with better-known physicians, so they are more accepting of appointments at any time, even if the time is inconvenient for them.
Caregiver Of course, … if you have a popular doctor, sometimes you don’t always get the appointment you want …
Participants also expressed frustration with the way appointments were rescheduled. They felt as though the physicians were not concerned about their lives outside of office visits.
Patient … patients actually have lives. Many of them have jobs or families or responsibilities.
Communication barriers
At the provider level, poor communication between health care providers and patients was perceived as a major impediment to the quality of care patients received. Both patients and caregivers emphasized the importance of open patient-provider interactions and that there was a lack of such open communications in many instances. There was concern regarding the way diagnoses or prognoses were relayed to patients. Many times, physicians were insensitive and disregarded the sentiments of the patients and caregivers when delivering news about the patients’ condition, as one caregiver shared,
Caregiver … the pulmonary man came … in the room and said, ‘Oh, don’t worry about your lungs. Something else will get you first,’ which was a very, very bad thing to say.
Participants also expressed concerns that they were not properly prepared for treatments by their physicians because vital information was not discussed. They felt as if physicians were not realistic about potential outcomes. This resulted in patients and caregivers being too optimistic and later disappointed when the outcome was not what they had originally expected.
Patient Until I got to this office, I was totally oversold on everything. I was told surgery … robotic, not invasive. Day one, surgery. Day two, tubes out. Day three, go home. I expected to be home on Sunday night, stir-frying vegetables, and making dinner, feeding my cat. I was in ICU four days … I went home with oxygen. I mean I thought I was just gonna walk outta there…. You take a little thing out and you put a Band-Aid on, and you go home.
Data also revealed that patients were unsure of their condition, even following treatment. Information was not communicated to patients about the specifics of their disease, either because of miscommunication or minimal patient-provider time spent during office visits. This lack of communication between patients and providers often left patients and caregivers uncertain about exactly what condition they had or what they were being treated for.
Patient I just can’t have the time with Dr. xx, cuz he’s so busy...
Patient … I didn’t understand. Which exactly what type of cancer did I have, cuz I’m—really to tell you the truth—I’m still wondering.
Knowledge barriers
Patients and caregivers also identified a lack of education and knowledge about lung cancer diagnosis and treatment as a barrier to their care. Patients and caregivers were not always fully knowledgeable about lung cancer, treatment options, or the duration of treatments. They relied on the provider to disclose such information or direct them to credible sources. In many instances, patients were misinformed about the causes of lung cancer. There were misconceptions that lung cancer was only caused by a history of smoking or genetic predisposition. Patients who did not smoke or did not have a family history of lung cancer were often confused and dismayed by the diagnosis.
Patient I was trying to figure out, why do I have lung cancer. Never smoked a day in my life.
Patients were often unaware of treatment options or side effects of various treatments. They relied on physicians to relay information and make decisions for them about treatment plans.
Patient I was told chemo would probably be the best thing for me, and I just had faith that Dr. xx knows more about it than me.
Patient I’m doing chemo but it’s — what I’m doing is different. Of course, I don’t know anything about it actually either. It’s what I hear from other people.
Other patients relied on their own sources for information about their condition, either through the Internet, from family members and/or friends, or from preconceived notions.
Patient Well — of course in the meantime, I read — because they said it was a small cell, very aggressive, so I felt like everything I read — that’s the deal. I think we’ve become where we can get on the internet and look up so much, that to me, I was gonna be gone.
Patient When he said, ‘Cancer,’ I said, ‘Well, I thought cancer was a heredity thing? That you have to have somebody in your family that has it…’
Discussion
Organizational barriers are an important consideration in the delivery and receipt of high-quality, patient-centered lung cancer care. This qualitative study of patients being treated for lung cancer and their informal caregivers revealed several common perceived organizational barriers to receiving care, including health insurance coverage restrictions, appointment scheduling difficulties, quality of communication with physicians, and failure to properly educate the patient and family about the disease and what to expect of the treatment process.
The provider communication and patient knowledge barriers seem to reinforce each other and could be improved through focused efforts on the quality of communication between patients and their caregivers and clinical care providers. Patients expect, but are often deprived of, open and active dialogue with their providers. Improved communication can be helpful in educating patients and their caregivers about their disease, prognosis, and treatment goals. Although communication ranks highly as a patient and caregiver priority, there is often a disconnect between patients and caregivers and their physicians.31 Patients and caregivers often want to be more involved in the decision-making process, and effective communication between physicians and patients has been linked to the patient’s ability to understand, and also receive high-quality care.32,33 Failure to communicate effectively and educate patients on key aspects of their condition strips them of their autonomy in decision-making.
The involvement of a navigator for patients being treated for lung cancer could be pivotal in relieving the communication and scheduling barriers. The nurse navigator assists with coordinating effective communication and providing needed information between providers and patients and their caregivers. A navigator also serves as a single point of contact for patients and caregivers to communicate questions outside of physician visits or concerns that may not be urgent enough to warrant immediate physician response.34 The navigator coordinates patients’ appointment schedules and physician referrals and communicates the details of the next steps in the care-delivery process. This helps remove the barriers to care and improve patient outcomes and the quality of health care delivery, especially for patients and caregivers dealing with a life-threatening illness within a complex referral process.35
Multidisciplinary care, a much-recommended alternative care-delivery model, should, in theory, promote connectivity of providers and collaboration between providers, patients, and family members. This model could help reduce barriers for patients and caregivers.3,24,36 A network of connected providers can better coordinate treatment plans, easily share test results, and provide built-in second opinions. Given the increasingly multimodal approach to the diagnosis, staging, and treatment of lung cancer, the multidisciplinary model could allow physicians to consider multiple perspectives and care-delivery options and, ideally, develop consensus around the optimal approach for each individual patient in one setting. This can shorten the length of time before treatment and establish a plan that is tailored to the patient’s needs.24
The National Academy of Medicine (formerly, Institute of Medicine) proposes that modern health care systems have 6 aims for quality improvement: safety, effectiveness, patient-centeredness, timeliness, efficiency, and equity.37 It would take changes in the design and implementation of organizational support systems at the policy, institutional, and provider level for those aims to be achieved. Further investigation of the problems identified by patients and caregivers could lead to innovative solutions to improve lung cancer care. Future work should evaluate the most effective communication styles in patient-provider interactions, particularly in regard to to lung cancer diagnosis and treatment, and investigate how multidisciplinary models influence patient-provider communication and patient care.
Limitations
This study has several limitations. Less than half of those approached for the study participated in the study for various reasons, which may have introduced selection bias in terms of not having the perspectives of patients not willing or able to participate in the study. Though focus groups are known to generate rich in-depth views of certain issues, they have been criticized as potentially lacking rigor and generalizability. To address this concern, we used a standardized script for each focus group and involved multiple members of the research team in data analysis and interpretation. Also, this study enrolled participants from a single health care institution and did not use a comparison group. There might be institutional and geographic differences in the experience of lung cancer care, which might further limit the generalizability of the results of this study.
Conclusions
Despite those limitations, this study offers valuable insight into the barriers that lung cancer patients and caregivers encounter while navigating a community-level health care system. Eliminating or minimizing these barriers will require strategic plans that help mitigate insurance-related, scheduling, provider-patient communication, and patient/caregiver knowledge acquisition problems and translate them into tactical actions for quality improvement. This is one of the first qualitative studies conducted to understand the organizational barriers that lung cancer patients and their caregivers face within a health care system. Additional research is needed to explore these barriers and develop viable solutions.
Disclaimer
All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute or its Board of Governors or Methodology Committee.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30.
2. Riedel RF, Wang X, McCormack M, et al. Impact of a multidisciplinary thoracic oncology clinic on the timeliness of care. J Thorac Oncol. 2006;1(7):692-696.
3. Seek A, Hogle WP. Modeling a better way: navigating the health care system for patients with lung cancer. Clin J Oncol Nurs. 2007;11(1):81-85.
4. Sorensen R, Iedema R. Redefining accountability in health care: managing the plurality of medical interests. Health. 2008;12(1):87-106.
5. Mosher CE, Jaynes HA, Hanna N, Ostroff JS. Distressed family caregivers of lung cancer patients: an examination of psychosocial and practical challenges. Support Care Cancer. 2013;21(2):431-437.
6. Cantril C, Haylock PJ. Patient navigation in the oncology care setting. Semin Oncol Nurs. 2013;29(2):76-90.
7. Freeman HP. Patient navigation: a community based strategy to reduce cancer disparities. J Urban Health. 2006;83(2):139-141.
8. Kwon DH, Tisnado DM, Keating NL, et al. Physician reported barriers to referring cancer patients to specialists: prevalence, factors, and association with career satisfaction. Cancer. 2015;121(1):113-122.
9. Andersen SE. Implementation of a new prescription system. A qualitative survey of organizational barriers. Ugeskr Laeger. 2002;164(38):4449-4453.
10. Rousseau L, Guay M, Archambault D, El m’ala Z, Abdelaziz N. Do organizational barriers to pneumococcal and influenza vaccine access exist? Can J Public Health. 2007;98(2):105-110.
11. Storey J, Buchanan D. Health care governance and organizational barriers to learning from mistakes. J Health Organ Manag. 2008;22(6):642-651.
12. Ziegenfuss JT Jr. Organizational barriers to quality improvement in medical and health care organizations. Qual Assur Util Rev. 1991;6(4):115-122.
13. Rihari-Thomas J, DiGiacomo M, Phillips J, Newton P, Davidson PM. Clinician perspectives of barriers to effective implementation of a rapid response system in an academic health centre: a focus group study. Int J Health Policy Manag. 2017;6(8):447-456.
14. Dutton D. Financial, organizational and professional factors affecting health care utilization. Soc Sci Med. 1986;23(7):721-735.
15. King CJ, Chen J, Dagher RK, Holt CL, Thomas SB. Decomposing differences in medical care access among cancer survivors by race and ethnicity. Am J Med Qual. 2015;30(5):459-469.
16. Renzaho AM, Romios P, Crock C, Sønderlund AL. The effectiveness of cultural competence programs in ethnic minority patient-centered health care—a systematic review of the literature. Int J Qual Health Care. 2013;25(3):261-269.
17. Betancourt JR, Green AR, Carrillo JE, Ananeh-Firempong O. Defining cultural competence: a practical framework for addressing racial/ethnic disparities in health and health care. Public Health Rep. 2003;118(4):293-302.
18. Scheinfeld Gorin S, Gauthier J, Hay J, Miles A, Wardle J. Cancer screening and aging: research barriers and opportunities. Cancer. 2008;113(suppl 12):3493-3504.
19. Humphrey L, Deffebach M, Pappas M, et al. Screening for lung cancer: systematic review to update the US preventive services task force recommendation. Evidence syntheses No. 105. Rockville, MD: Agency for Health Care Research and Quality; 2013.
20. Nelson HD, Fu R, Cantor A, Pappas M, Daeges M, Humphrey L. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 U.S. Preventive Services Task Force Recommendation. Ann Intern Med. 2016;164(4):244-255.
21. Zauber AG. The impact of screening on colorectal cancer mortality and incidence: has it really made a difference? Dig Dis Sci. 2015;60(3):681-691.
22. Remennick L. The challenge of early breast cancer detection among immigrant and minority women in multicultural societies. Breast J. 2006;12(suppl 1):103-110.
23. Hess LM, Pohl G. Perspectives of quality care in cancer treatment: a review of the literature. Am Health Drug Benefits. 2013;6(6):321-329.
24. Kedia SK, Ward KD, Digney SA, et al. ‘One-stop shop’: lung cancer patients’ and caregivers’ perceptions of multidisciplinary care in a community health care setting. Transl Lung Cancer Res. 2015;4(4):456-464.
25. US Cancer Statistics Working Group. United States cancer statistics: 1999-2014 incidence and mortality web-based report. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute. http://www.cdc.gov/uscs. Published 2017. Accessed November 21, 2017.
26. Creswell JW. Research design: qualitative, quantitative, and mixed methods approaches. 4th ed. Thousand Oaks, CA: SAGE Publications; 2014.
27. Ballinger C. Demonstrating rigour and quality? In Finlay L, Ballinger C, eds. Qualitative research for allied health professionals: challenging choices. Chichester, England: John Wiley & Sons Ltd; 2006:235-246.
28. Finlay L. ‘Rigour’, ‘ethical integrity’ or ‘artistry’? Reflexively reviewing criteria for evaluating qualitative research. Br J Occup Ther. 2006;69(7):319-326.
29. Lincoln YS, Guba EG. Naturalistic inquiry. Newbury Park, CA: SAGE Publications; 1985.
30. Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus group. Int J Qual Health Care. 2007;19(6):349-357.
31. Neeman N, Quinn K, Shoeb M, Mourad M, Sehgal NL, Sliwka D. Postdischarge focus groups to improve the hospital experience. Am J Med Qual. 2013;28(6):536-538.
32. O’Day BL, Killeen M, Iezzoni LI. Improving health care experiences of persons who are blind or have low vision: suggestions from focus groups. Am J Med Qual. 2004;19(5):193-200.
33. Smith B, Lynch WD, Markow C, Lifsey S, Slover M. Consumers’ understanding of and interest in provider- versus practice-level quality characteristics: findings from a focus group study. Am J Med Qual. 2015;30(4):367-373.
34. Islam KM, Opoku ST, Apenteng BA, et al. Coping with an advanced stage lung cancer diagnosis: patient, caregiver, and provider perspectives on the role of the health care system. J Cancer Educ. 2016;31(3):554-558.
35. Pedersen A, Hack TF. Pilots of oncology health care: a concept analysis of the patient navigator role. Oncol Nurs Forum. 2010;37(1):55-60.
36. Gopal R. How to maintain multidisciplinary treatment schedules. J Support Oncol. 2005;3(3):248-256.
37. Committee on Quality of Health Care in America. Improving the 21st century health care system. In: Institute of Medicine, ed. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academies Press; 2001:39-60.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30.
2. Riedel RF, Wang X, McCormack M, et al. Impact of a multidisciplinary thoracic oncology clinic on the timeliness of care. J Thorac Oncol. 2006;1(7):692-696.
3. Seek A, Hogle WP. Modeling a better way: navigating the health care system for patients with lung cancer. Clin J Oncol Nurs. 2007;11(1):81-85.
4. Sorensen R, Iedema R. Redefining accountability in health care: managing the plurality of medical interests. Health. 2008;12(1):87-106.
5. Mosher CE, Jaynes HA, Hanna N, Ostroff JS. Distressed family caregivers of lung cancer patients: an examination of psychosocial and practical challenges. Support Care Cancer. 2013;21(2):431-437.
6. Cantril C, Haylock PJ. Patient navigation in the oncology care setting. Semin Oncol Nurs. 2013;29(2):76-90.
7. Freeman HP. Patient navigation: a community based strategy to reduce cancer disparities. J Urban Health. 2006;83(2):139-141.
8. Kwon DH, Tisnado DM, Keating NL, et al. Physician reported barriers to referring cancer patients to specialists: prevalence, factors, and association with career satisfaction. Cancer. 2015;121(1):113-122.
9. Andersen SE. Implementation of a new prescription system. A qualitative survey of organizational barriers. Ugeskr Laeger. 2002;164(38):4449-4453.
10. Rousseau L, Guay M, Archambault D, El m’ala Z, Abdelaziz N. Do organizational barriers to pneumococcal and influenza vaccine access exist? Can J Public Health. 2007;98(2):105-110.
11. Storey J, Buchanan D. Health care governance and organizational barriers to learning from mistakes. J Health Organ Manag. 2008;22(6):642-651.
12. Ziegenfuss JT Jr. Organizational barriers to quality improvement in medical and health care organizations. Qual Assur Util Rev. 1991;6(4):115-122.
13. Rihari-Thomas J, DiGiacomo M, Phillips J, Newton P, Davidson PM. Clinician perspectives of barriers to effective implementation of a rapid response system in an academic health centre: a focus group study. Int J Health Policy Manag. 2017;6(8):447-456.
14. Dutton D. Financial, organizational and professional factors affecting health care utilization. Soc Sci Med. 1986;23(7):721-735.
15. King CJ, Chen J, Dagher RK, Holt CL, Thomas SB. Decomposing differences in medical care access among cancer survivors by race and ethnicity. Am J Med Qual. 2015;30(5):459-469.
16. Renzaho AM, Romios P, Crock C, Sønderlund AL. The effectiveness of cultural competence programs in ethnic minority patient-centered health care—a systematic review of the literature. Int J Qual Health Care. 2013;25(3):261-269.
17. Betancourt JR, Green AR, Carrillo JE, Ananeh-Firempong O. Defining cultural competence: a practical framework for addressing racial/ethnic disparities in health and health care. Public Health Rep. 2003;118(4):293-302.
18. Scheinfeld Gorin S, Gauthier J, Hay J, Miles A, Wardle J. Cancer screening and aging: research barriers and opportunities. Cancer. 2008;113(suppl 12):3493-3504.
19. Humphrey L, Deffebach M, Pappas M, et al. Screening for lung cancer: systematic review to update the US preventive services task force recommendation. Evidence syntheses No. 105. Rockville, MD: Agency for Health Care Research and Quality; 2013.
20. Nelson HD, Fu R, Cantor A, Pappas M, Daeges M, Humphrey L. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 U.S. Preventive Services Task Force Recommendation. Ann Intern Med. 2016;164(4):244-255.
21. Zauber AG. The impact of screening on colorectal cancer mortality and incidence: has it really made a difference? Dig Dis Sci. 2015;60(3):681-691.
22. Remennick L. The challenge of early breast cancer detection among immigrant and minority women in multicultural societies. Breast J. 2006;12(suppl 1):103-110.
23. Hess LM, Pohl G. Perspectives of quality care in cancer treatment: a review of the literature. Am Health Drug Benefits. 2013;6(6):321-329.
24. Kedia SK, Ward KD, Digney SA, et al. ‘One-stop shop’: lung cancer patients’ and caregivers’ perceptions of multidisciplinary care in a community health care setting. Transl Lung Cancer Res. 2015;4(4):456-464.
25. US Cancer Statistics Working Group. United States cancer statistics: 1999-2014 incidence and mortality web-based report. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute. http://www.cdc.gov/uscs. Published 2017. Accessed November 21, 2017.
26. Creswell JW. Research design: qualitative, quantitative, and mixed methods approaches. 4th ed. Thousand Oaks, CA: SAGE Publications; 2014.
27. Ballinger C. Demonstrating rigour and quality? In Finlay L, Ballinger C, eds. Qualitative research for allied health professionals: challenging choices. Chichester, England: John Wiley & Sons Ltd; 2006:235-246.
28. Finlay L. ‘Rigour’, ‘ethical integrity’ or ‘artistry’? Reflexively reviewing criteria for evaluating qualitative research. Br J Occup Ther. 2006;69(7):319-326.
29. Lincoln YS, Guba EG. Naturalistic inquiry. Newbury Park, CA: SAGE Publications; 1985.
30. Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus group. Int J Qual Health Care. 2007;19(6):349-357.
31. Neeman N, Quinn K, Shoeb M, Mourad M, Sehgal NL, Sliwka D. Postdischarge focus groups to improve the hospital experience. Am J Med Qual. 2013;28(6):536-538.
32. O’Day BL, Killeen M, Iezzoni LI. Improving health care experiences of persons who are blind or have low vision: suggestions from focus groups. Am J Med Qual. 2004;19(5):193-200.
33. Smith B, Lynch WD, Markow C, Lifsey S, Slover M. Consumers’ understanding of and interest in provider- versus practice-level quality characteristics: findings from a focus group study. Am J Med Qual. 2015;30(4):367-373.
34. Islam KM, Opoku ST, Apenteng BA, et al. Coping with an advanced stage lung cancer diagnosis: patient, caregiver, and provider perspectives on the role of the health care system. J Cancer Educ. 2016;31(3):554-558.
35. Pedersen A, Hack TF. Pilots of oncology health care: a concept analysis of the patient navigator role. Oncol Nurs Forum. 2010;37(1):55-60.
36. Gopal R. How to maintain multidisciplinary treatment schedules. J Support Oncol. 2005;3(3):248-256.
37. Committee on Quality of Health Care in America. Improving the 21st century health care system. In: Institute of Medicine, ed. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academies Press; 2001:39-60.