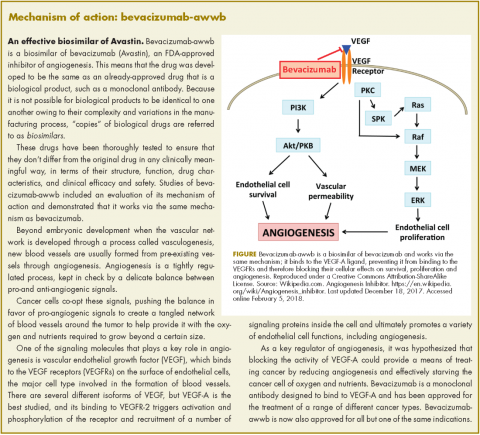
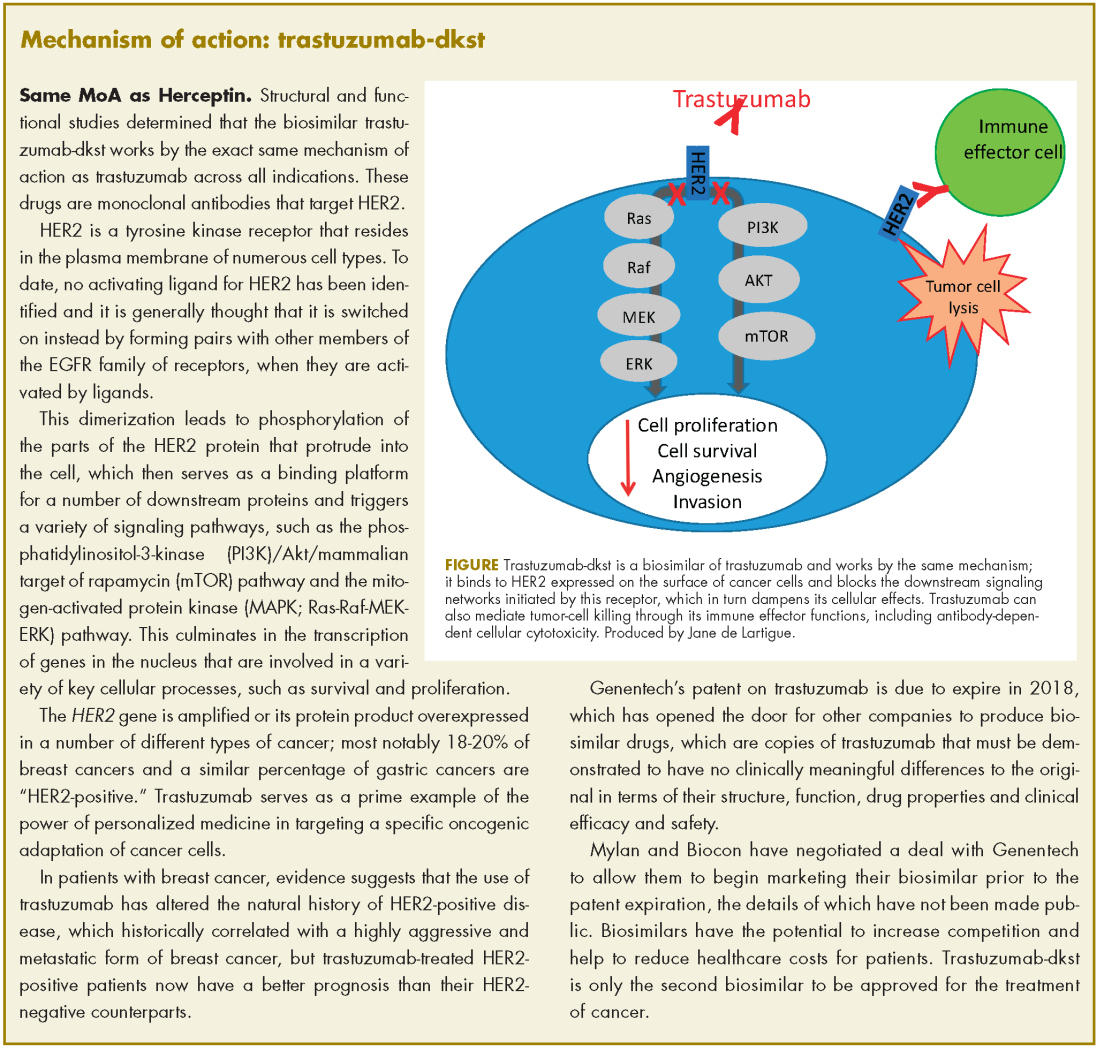
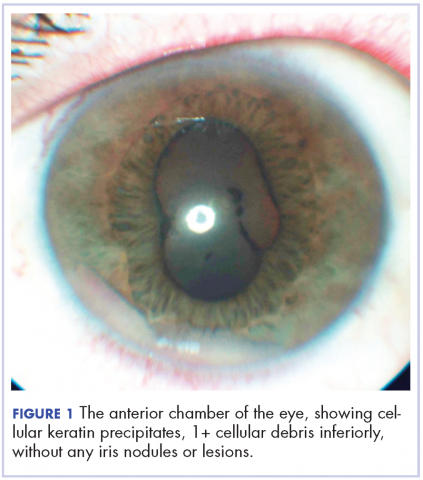
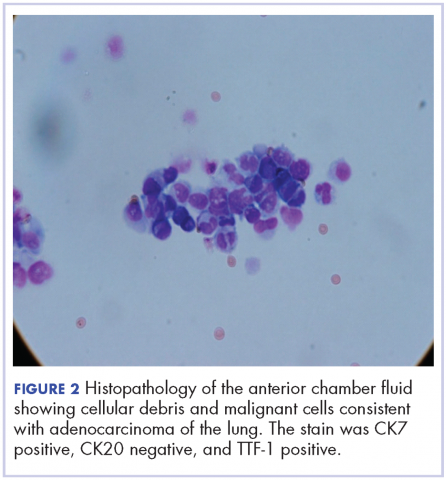
User login
The impact of patient education on consideration of enrollment in clinical trials
The low rate of participation in clinical trials is partly owing to the lack of awareness of these trials not only among potential participants but the US population as a whole.1 This lack of awareness, however, can be reversed. For example, findings from a single-institution observational study showed that systematically sending letters about clinical trial participation to all new lung cancer patients was associated with increased trial participation.2 More recently, a large, multicenter, randomized experiment showed that attitudes toward clinical trials were improved through preparatory education about clinical trials before a patient’s first oncologic visit.3
Such clinical trial education can be used before any medical diagnosis to increase clinical trial awareness in the general population. It may be advantageous to do so because people tend to process information more effectively during less stressful times.4 Clinical trial awareness in the US population has increased slightly over time, but in 2012, one study reported that 26% of its participants lacked general awareness about clinical trials.5
Comprehensive educational material, such as a multimedia psychoeducational intervention,6 a 28-video library,3 or a 160-page book,7 which have been proposed for oncology patients, may be too intensive for someone who is not immediately deciding whether to participate in a clinical trial. However, a simple, concise form of education might be preferable and appropriate to increase basic knowledge and awareness among the general population, especially among those who are less educated.8
Our aim in the present study was to evaluate whether providing brief educational material about clinical trials would increase patient willingness to participate in these trials.
Methods
This is a single-group, cross-sectional design study in which all participants were administered the questions and the 240-word educational statement in the same order.
Sample
An electronic survey was conducted by Marketing and Planning Systems (the analytics practice of Kantar Millwardbrown) on behalf of the Memorial Sloan Kettering Cancer Center (MSK). The survey included a national sample of 1011 participants and a local sample of 500 participants from the MSK catchment area (22 counties across the 5 boroughs of New York City, Long Island, southern New York State, northern New Jersey, and southwestern Connecticut).
Survey participants were aged 18 to 69 years in the national sample and 25 to 69 years in the local sample, representing 87% and 75% of the adult populations of those areas, respectively. Respondents who were or who had a family member currently working in the fields of news, advertising/marketing, or medical care were not surveyed. Participants were sourced from an online incentivized panel with millions of potential respondents representative of the US adult population.
Questionnaire
The questionnaire collected data on participant demographics and main medical history (including previous participation in a clinical trial), and asked questions about clinical trials, focusing on:
Analyses
Descriptive and bivariate statistical analyses of participants’ characteristics were weighted to ensure national representativeness for gender, age, ethnicity, and income. Mean standard deviation (SD) was computed for every quantitative variable. Categorical variables were expressed as proportions.
Student t tests and analyses of variance (ANOVAs) were used to compare continuous variables, while chi-square tests were used to compare categorical data. Repeated measures ANOVAs were then used to determine the sociodemographic and medical characteristics associated with the impression of and willingness to enroll in a clinical trial before and after reading the educational material. The interaction between education level and time (pre- or postreading assessment) was tested to determine if the changes after reading the brief statement were different depending on education level.
All statistical analyses were 2-tailed and considered statistically significant at P < .05. Analyses were performed using SPSS PAWS Statistics 24 (IBM Inc, Armonk, New York). Effect sizes (standardized mean differences) and their 95% confidence intervals (CIs) were computed using the compute.es package for R 3.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Participants
From October 23, 2015, through November 12, 2015, 1511 US participants responded to the survey request, including 1507 respondents (99.7%) who reported their education level and are included in the analyses of this report. The mean age of the respondents was 43.5 years (SD, 4.6). More than half of the respondents (57.8%) reported a current medical condition, mainly cardiovascular (20.0%), arthritis (20.0%), or other type or chronic pain (20.0%), and 9.0% reported a cancer diagnosis (current, 2.9%; previous, 6.1%).
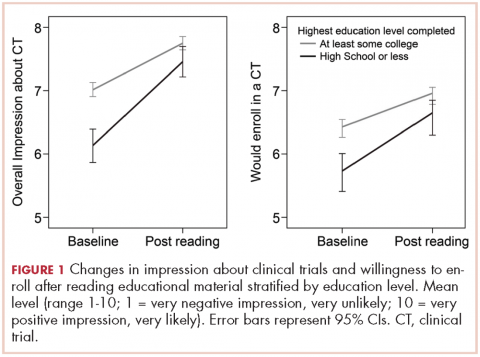
Participants who at most had completed high school (18.9%, including 1.4% who had never even attended high school) were more often white women, lived outside urban areas, had lower household income, and were less likely to have health care insurance (Table 2). They also reported a current or previous cancer diagnosis less often than those of similar age who had attended college.
Previous participation in a clinical trial was reported by 9.6% of participants. Most of the clinical trials (75.0%) were testing a new drug. Previous trial participants were more likely to be older than those who had not participated in trials (46.1 years [SD, 14.8] vs 43.3 [SD, 4.6], repectively; P = .033), have a current health condition (86.2% vs 54.8%; P < .001), and know another trial participant (39.9% vs 9.5%; P < .001).
Education level and baseline impression of and
willingness to enroll in a clinical trial
A lower level of education was associated with a decreased likelihood of previous trial participation or of knowing a trial participant, as well as with less awareness and inaccurate perceptions of clinical trials (Table 2).
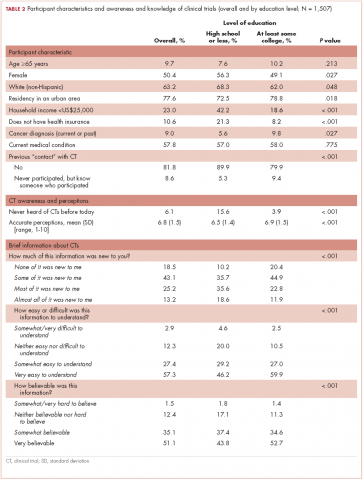
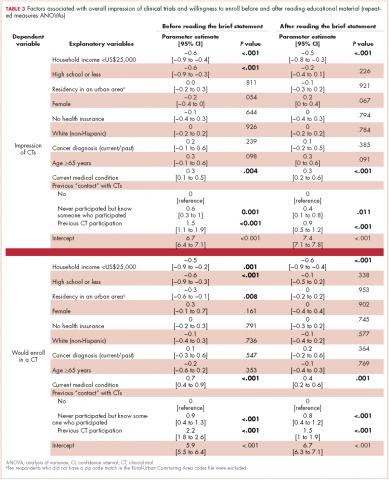
Participants with a high school degree or less were more likely to have a worse impression of and were less likely to enroll in a future hypothetical clinical trial before reading the educational material (Figure 1). Multivariable analyses confirmed that lower education level was associated with lower baseline overall impression, regardless of other personal characteristics (Table 3). Lowest household income was also associated with a more negative impression of trials, whereas participants with a current medical condition and with previous contact with clinical trials had a more positive impression of them. The same effects were observed with likeliness to enroll in a future hypothetical clinical trial (correlated with the overall impression: r, 0.63; P < .001), except that the negative effect of female gender was statistically significant (Table 3).
Posteducation impression of and willingnes to enroll
willingness to enroll in a clinical trial
The brief educational material was mostly considered believable (86.2%), easy to understand (84.8%), and included information that was new to participants (81.5%; Table 2). Participants with a high school diploma or less more often noted that the material provided them with new information, but they also reported more difficulties in fully understanding and believing the information. Overall, however, few participants found the information difficult to understand (4.6%) or hard to believe (1.8%; Table 2).
Most participants had an improved overall impression of clinical trials (standardized mean difference, 0.42; 95% CI, 0.35-0.50; P < .001) after reading the educational material. This increase was higher among participants with a lower completed level of education (Figure 1; standardized mean difference, 0.62; 95% CI, 0.45-0.79; Pinteraction < .001). The same effects were observed for likeliness to enroll in a future hypothetical clinical trial (P < .001; Figure 1).
After reading the informational statement, education level effect was no longer significantly associated with the overall impression of clinical trials (P = .23) and willingness to enroll in a clinical trial (P = .34), whereas the effects of income, current medical condition, and previous engangement with clinical trials remained statistically significant (Table 3).
Remaining challenges
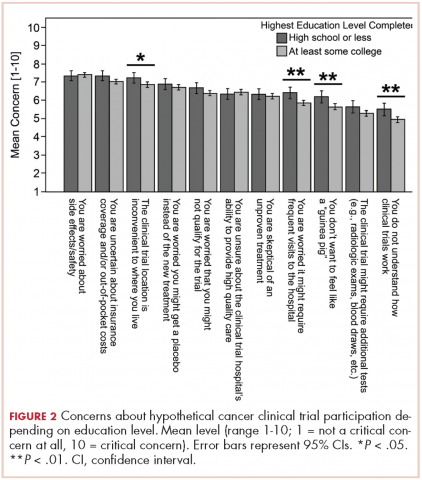
Regarding hypothetical participation in a future clinical trial after a cancer diagnosis, the most critical concerns were related to side effects and the uncertainty of insurance coverage (Figure 2).10 The lack of understanding of clinical trials was the least critical concern; however, it was significantly higher among participants with a lower completed level of education. These participants also expressed more critical concerns about feeling like a “guinea pig,” inconvenient trial location, and the frequent visits needed.
Discussion
Findings from this survey demonstrated that providing brief educational material about cancer clinical trials was associated with a more favorable impression of clinical trials and higher interest in trial participation. Furthermore, as far as we can ascertain, this is the first report showing how a simple intervention such as this may help close the knowledge gap on clinical trials among people of different educational backgrounds. Although most respondents in this interventional survey noted an increased willingness to consider participation in a clinical trial after reading the educational material, those with a lower level of education and knowledge about clinical trials received the most benefit. Previous participation in a clinical trial was also strongly associated with the impression of and willingness to enroll in a trial, both before and after reading the statement.11
Most of the interventions evaluated to date12 have focused on patients who are faced with having to decide whether to participate in a clinical trial2,3 or on very specific populations, such as select ethnic communities.13,14 However, it may be beneficial to provide simple, concise educational information about clinical trials to the general population, especially to those with minimal education. Although level of education concerns the minority of our sample (19%) sourced from an online panel, in the 2015 Current Population Survey, 41% of US participants aged 25 and older had not reached college education.15 Those with a lower level of education have reported a general lack of familiarity with clinical trials and were more likely to have inaccurate perceptions about trials. This is consistent with previous studies that have shown the lack of awareness and knowledge of clinical trials in this population.5,16,17 Our findings suggest that this knowledge gap can be reversed through a simple educational intervention and result in an increased willingness to participate.
The provision of information on clinical trials was positively associated with the 2 outcomes analyzed – improved impression of clinical trials and increased likelihood to enroll in a hypothetical trial. Such improvement might not translate to improved accrual,18 but it is a step toward closing the overall knowledge gap related to clinical trials and increasing the number of people who would consider trial participation. The lack of awareness of clinical trials has been reported as a legitimate explanation for why participation rates are lower in less-educated patient populations.19,20 This brief educational intervention is a simple, technology-sparing way to increase clinical trial awareness in the general population. In a similar survey, most physicians who reviewed an educational statement noted they were likely to use it with patients.21Less-educated patients, those who lived outside of urban areas, and those with lower household incomes were most concerned about trial location and the frequent visits needed when participating in a trial (Figure 2). Living in a nonurban area was not associated with participant impression of clinical trials or willingness to enroll in a trial. However, rural residency may be a barrier to enrollment depending on distance to the hospital22 and out-of-pocket expenses related to travel.23 Some comprehensive cancer centers, such as MSK, have developed alliances with community centers24 as a means of overcoming geographical barriers and increasing clinical trial participation rates.
Another concern shared by most respondents was the uncertainty in insurance coverage and potential out-of-pocket costs related to care. Lower household income, unlike location of residence and lack of insurance, was significantly associated with negative impressions of clinical trials and lower willingness to enroll in a trial, even after adjusting for education level. Cancer patients with higher financial burden have reported more attitudinal barriers, even after accounting for the negative effect of lower education level.25 Recent studies have also discussed the negative impact of lower income on cancer clinical trial participation,19,20,26,27 and new attention has been paid to the negative financial implications or “financial toxicity” of participating in a trial.23,28
White and older survey participants showed similar interest in clinical trial participation after accounting for other characteristics. There is growing evidence that outcome differences attributed to race may in fact be more dependent on socioeconomic status.8 A recent study among breast cancer patients showed that low socioeconomic status, but not race, was associated with decreased participation in clinical trials.29,30 Previous findings have also indicated that interest in clinical trials and barriers to enrollment among older, less-educated patients31 are often related to ineligibility, comorbidity, or communication difficulties.
Among our participants, the fear of side effects also was a common attitudinal barrier to clinical trial participation, as has been reported in previous studies.3,20 However, contrary to one previous study,20 this fear was not significantly increased among our less-educated participants.
Less-educated participants also reported more difficulties in understanding the information they were provided with, and they remained more concerned about being treated like “guinea pigs.” These concerns are consistent with other results showing that decisional conflict about clinical trial participation among patients with a high school diploma or less remained high even after they had received a National Cancer Institute text as pre-education material.3
Limitations
The lack of randomization makes it difficult to attribute with certainty that the change in acceptability of clinical trial participation is owing to the reading of the educational statement. The survey also sampled only English-speaking and well-educated participants from an online panel (81.1% had at least attended college) despite the use of a weighting procedure to ensure representativeness regarding gender, age, ethnicity, and income. Health literacy level or more specific trial literacy level was not evaluated; however, we were able to show less accurate perceptions of clinical trials among participants with a lower level of education by using agreement toward 8 statements about trials. The responses to hypothetical questions from these participants in the general population may also not be generalizable to a restricted population of patients with cancer. In addition, we measured impression of and willingness to enroll in a clinical trial immediately after providing participants with the educational material. We would have to confirm whether the positive effects of the education persist over time and translate to higher clinical trial participation rates.
Conclusions
Participants were receptive of educational material and expressed greater interest in and likelihood of enrolling in a clinical trial after reading it. The information had a greater effect on those with less education, but increased the willingness of all participants to enroll.
1. Ford JG, Howerton MW, Lai GY, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112(2):228-242.
2. Quinn GP, Bell BA, Bell MY, et al. The guinea pig syndrome: improving clinical trial participation among thoracic patients. J Thorac Oncol. 2007;2(3):191-196.
3. Meropol NJ, Wong Y-N, Albrecht T, et al. Randomized trial of a web-based intervention to address barriers to clinical trials. J Clin Oncol. 2016;34(5):469-478.
4. Goldberg RJ. Disclosure of information to adult cancer patients: issues and update. J Clin Oncol. 1984;2(8):948-955.
5. Leiter A, Diefenbach MA, Doucette J, Oh WK, Galsky MD. Clinical trial awareness: changes over time and sociodemographic disparities. Clin Trials. 2015;12(3):215-223.
6. Jacobsen PB, Wells KJ, Meade CD, et al. Effects of a brief multimedia psychoeducational intervention on the attitudes and interest of patients with cancer regarding clinical trial participation: a multicenter randomized controlled trial. J Clin Oncol. 2012;30(20):2516-2521.
7. Carney PA, Tucker EK, Newby TA, Beer TM. Feasibility, acceptability and findings from a pilot randomized controlled intervention study on the impact of a book designed to inform patients about cancer clinical trials. J Cancer Educ. 2014;29(1):181-187.
8. Sharrocks K, Spicer J, Camidge DR, Papa S. The impact of socioeconomic status on access to cancer clinical trials. Br J Cancer. 2014;111(9):1684-1687.
9. Regan J, Hickey C, Targett C, Masuda S, Sabbatini P. Framing clinical research and the importance of trial participation: patient and physician perspective. Poster presented at: 8th Annual AACI Clinical Research Initiative Meeting; July 20-21 2016; Chicago, IL.
10. Mancini J, Genre D, Dalenc F, et al. Patients’ regrets after participating in a randomized controlled trials depended on their involvement in the decision making. J Clin Epidemiol. 2012;65(6):635-642
11. Murphy ST, Frank LB, Chatterjee JS, et al. Comparing the relative efficacy of narrative vs nonnarrative health messages in reducing health disparities using a randomized trial. Am J Public Health. 2015;105(10):2117-2123.
12. Treweek S, Lockhart P, Pitkethly M, et al. Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta-analysis. BMJ Open. 2013;3(2):e002360. doi:10.1136/bmjopen-2012-002360.
13. Ma GX, Tan Y, Blakeney NC, et al. The impact of a community-based clinical trial educational intervention among underrepresented Chinese Americans. Cancer Epidemiol Biomarkers Prev. 2014;23(3):424-432.
14. Cupertino AP, Molina CSP, de los Rios JB, et al. Knowledge, awareness, and interest in cancer clinical trials among rural latinos following brief education by promotores de salud. J Community Med Health Educ. 2015;5:2161. doi:10.4172/2161-0711.1000358
15. Ryan CL, Bauman K. Educational attainment in the United States: 2015. https://www.census.gov/content/dam/Census/library/publications/2016/demo/p20-578.pdf. Published March 2016. Accessed October 18, 2016.
16. Lara PN Jr, Paterniti DA, Chiechi C, et al. Evaluation of factors affecting awareness of and willingness to participate in cancer clinical trials. J Clin Oncol. 2005;23(36):9282-9289.
17. Dhai A, Etheredge H, Cleaton-Jones P. A pilot study evaluating an intervention designed to raise awareness of clinical trials among potential participants in the developing world. J Med Ethics. 2010;36(4):238-242.
18. Denicoff AM, McCaskill-Stevens W, Grubbs SS, et al. The National Cancer Institute–American Society of Clinical Oncology Cancer Trial Accrual Symposium: summary and recommendations. J Oncol Pract. 2013;9(6):267-276.
19. Davison BJ, So A, Goldenberg SL, Berkowitz J, Gleave ME. Measurement of factors influencing the participation of patients with prostate cancer in clinical trials: a Canadian perspective. BJU Int. 2008;101(8):982-987.
20. Unger JM, Hershman DL, Albain KS, et al. Patient income level and cancer clinical trial participation. J Clin Oncol. 2013;31(5):536-542.
21. IRB Advisor. Education may overcome reticence to join trials. Relias website. http://www.ahcmedia.com/articles/138088-education-may-overcome-reticence-to-join-trials. Published July 1, 2016. Accessed July 29, 2016.
22. Vanderpool RC, Kornfeld J, Mills L, Byrne MM. Rural-urban differences in discussions of cancer treatment clinical trials. Patient Educ Couns. 2011;85(2):e69-e74.
23. Meropol NJ. Health policy: overcoming cost barriers to clinical trial participation. Nat Rev Clin Oncol. 2016;13(6):333-334.
24. MSK Cancer Alliance. Memorial Sloan Kettering Cancer Center website. https://www.mskcc.org/about/innovative-collaborations/msk-alliance. Updated March 7, 2018. Accessed August 18, 2016.
25. Manne S, Kashy D, Albrecht T, et al. Attitudinal barriers to participation in oncology clinical trials: factor analysis and correlates of barriers. Eur J Cancer Care (Engl). 2015;24(1):28-38.
26. Unger JM, Gralow JR, Albain KS, Ramsey SD, Hershman DL. Patient income level and cancer clinical trial participation: a prospective survey study. JAMA Oncol. 2016;2(1):137-139.
27. Moy B. Clinical trials, disparities, and financial burden: it’s time to intervene. Oncologist. 2015;20(6):571.
28. Bonevski B, Randell M, Paul C, et al. Reaching the hard-to-reach: a systematic review of strategies for improving health and medical research with socially disadvantaged groups. BMC Med Res Methodol. 2014;14:42.
29. Gross CP, Filardo G, Mayne ST, Krumholz HM. The impact of socioeconomic status and race on trial participation for older women with breast cancer. Cancer. 2005;103(3):483-491.
30. Nickell A, Burke NJ, Cohen E, Caprio M, Joseph G. Educating low-SES and LEP survivors about breast cancer research: pilot test of the Health Research Engagement Intervention. J Cancer Educ. 2014;29(4):746-752.
31. Mancini J, Jansen J, Julian-Reynier C, Bechlian D, Vey N, Chabannon C. Preferences of older adults with cancer for involvement in decision-making about research participation. J Am Geriatr Soc. 2014;62(6):1191-1193.
The low rate of participation in clinical trials is partly owing to the lack of awareness of these trials not only among potential participants but the US population as a whole.1 This lack of awareness, however, can be reversed. For example, findings from a single-institution observational study showed that systematically sending letters about clinical trial participation to all new lung cancer patients was associated with increased trial participation.2 More recently, a large, multicenter, randomized experiment showed that attitudes toward clinical trials were improved through preparatory education about clinical trials before a patient’s first oncologic visit.3
Such clinical trial education can be used before any medical diagnosis to increase clinical trial awareness in the general population. It may be advantageous to do so because people tend to process information more effectively during less stressful times.4 Clinical trial awareness in the US population has increased slightly over time, but in 2012, one study reported that 26% of its participants lacked general awareness about clinical trials.5
Comprehensive educational material, such as a multimedia psychoeducational intervention,6 a 28-video library,3 or a 160-page book,7 which have been proposed for oncology patients, may be too intensive for someone who is not immediately deciding whether to participate in a clinical trial. However, a simple, concise form of education might be preferable and appropriate to increase basic knowledge and awareness among the general population, especially among those who are less educated.8
Our aim in the present study was to evaluate whether providing brief educational material about clinical trials would increase patient willingness to participate in these trials.
Methods
This is a single-group, cross-sectional design study in which all participants were administered the questions and the 240-word educational statement in the same order.
Sample
An electronic survey was conducted by Marketing and Planning Systems (the analytics practice of Kantar Millwardbrown) on behalf of the Memorial Sloan Kettering Cancer Center (MSK). The survey included a national sample of 1011 participants and a local sample of 500 participants from the MSK catchment area (22 counties across the 5 boroughs of New York City, Long Island, southern New York State, northern New Jersey, and southwestern Connecticut).
Survey participants were aged 18 to 69 years in the national sample and 25 to 69 years in the local sample, representing 87% and 75% of the adult populations of those areas, respectively. Respondents who were or who had a family member currently working in the fields of news, advertising/marketing, or medical care were not surveyed. Participants were sourced from an online incentivized panel with millions of potential respondents representative of the US adult population.
Questionnaire
The questionnaire collected data on participant demographics and main medical history (including previous participation in a clinical trial), and asked questions about clinical trials, focusing on:
Analyses
Descriptive and bivariate statistical analyses of participants’ characteristics were weighted to ensure national representativeness for gender, age, ethnicity, and income. Mean standard deviation (SD) was computed for every quantitative variable. Categorical variables were expressed as proportions.
Student t tests and analyses of variance (ANOVAs) were used to compare continuous variables, while chi-square tests were used to compare categorical data. Repeated measures ANOVAs were then used to determine the sociodemographic and medical characteristics associated with the impression of and willingness to enroll in a clinical trial before and after reading the educational material. The interaction between education level and time (pre- or postreading assessment) was tested to determine if the changes after reading the brief statement were different depending on education level.
All statistical analyses were 2-tailed and considered statistically significant at P < .05. Analyses were performed using SPSS PAWS Statistics 24 (IBM Inc, Armonk, New York). Effect sizes (standardized mean differences) and their 95% confidence intervals (CIs) were computed using the compute.es package for R 3.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Participants
From October 23, 2015, through November 12, 2015, 1511 US participants responded to the survey request, including 1507 respondents (99.7%) who reported their education level and are included in the analyses of this report. The mean age of the respondents was 43.5 years (SD, 4.6). More than half of the respondents (57.8%) reported a current medical condition, mainly cardiovascular (20.0%), arthritis (20.0%), or other type or chronic pain (20.0%), and 9.0% reported a cancer diagnosis (current, 2.9%; previous, 6.1%).
Participants who at most had completed high school (18.9%, including 1.4% who had never even attended high school) were more often white women, lived outside urban areas, had lower household income, and were less likely to have health care insurance (Table 2). They also reported a current or previous cancer diagnosis less often than those of similar age who had attended college.
Previous participation in a clinical trial was reported by 9.6% of participants. Most of the clinical trials (75.0%) were testing a new drug. Previous trial participants were more likely to be older than those who had not participated in trials (46.1 years [SD, 14.8] vs 43.3 [SD, 4.6], repectively; P = .033), have a current health condition (86.2% vs 54.8%; P < .001), and know another trial participant (39.9% vs 9.5%; P < .001).
Education level and baseline impression of and
willingness to enroll in a clinical trial
A lower level of education was associated with a decreased likelihood of previous trial participation or of knowing a trial participant, as well as with less awareness and inaccurate perceptions of clinical trials (Table 2).
Participants with a high school degree or less were more likely to have a worse impression of and were less likely to enroll in a future hypothetical clinical trial before reading the educational material (Figure 1). Multivariable analyses confirmed that lower education level was associated with lower baseline overall impression, regardless of other personal characteristics (Table 3). Lowest household income was also associated with a more negative impression of trials, whereas participants with a current medical condition and with previous contact with clinical trials had a more positive impression of them. The same effects were observed with likeliness to enroll in a future hypothetical clinical trial (correlated with the overall impression: r, 0.63; P < .001), except that the negative effect of female gender was statistically significant (Table 3).
Posteducation impression of and willingnes to enroll
willingness to enroll in a clinical trial
The brief educational material was mostly considered believable (86.2%), easy to understand (84.8%), and included information that was new to participants (81.5%; Table 2). Participants with a high school diploma or less more often noted that the material provided them with new information, but they also reported more difficulties in fully understanding and believing the information. Overall, however, few participants found the information difficult to understand (4.6%) or hard to believe (1.8%; Table 2).
Most participants had an improved overall impression of clinical trials (standardized mean difference, 0.42; 95% CI, 0.35-0.50; P < .001) after reading the educational material. This increase was higher among participants with a lower completed level of education (Figure 1; standardized mean difference, 0.62; 95% CI, 0.45-0.79; Pinteraction < .001). The same effects were observed for likeliness to enroll in a future hypothetical clinical trial (P < .001; Figure 1).
After reading the informational statement, education level effect was no longer significantly associated with the overall impression of clinical trials (P = .23) and willingness to enroll in a clinical trial (P = .34), whereas the effects of income, current medical condition, and previous engangement with clinical trials remained statistically significant (Table 3).
Remaining challenges
Regarding hypothetical participation in a future clinical trial after a cancer diagnosis, the most critical concerns were related to side effects and the uncertainty of insurance coverage (Figure 2).10 The lack of understanding of clinical trials was the least critical concern; however, it was significantly higher among participants with a lower completed level of education. These participants also expressed more critical concerns about feeling like a “guinea pig,” inconvenient trial location, and the frequent visits needed.
Discussion
Findings from this survey demonstrated that providing brief educational material about cancer clinical trials was associated with a more favorable impression of clinical trials and higher interest in trial participation. Furthermore, as far as we can ascertain, this is the first report showing how a simple intervention such as this may help close the knowledge gap on clinical trials among people of different educational backgrounds. Although most respondents in this interventional survey noted an increased willingness to consider participation in a clinical trial after reading the educational material, those with a lower level of education and knowledge about clinical trials received the most benefit. Previous participation in a clinical trial was also strongly associated with the impression of and willingness to enroll in a trial, both before and after reading the statement.11
Most of the interventions evaluated to date12 have focused on patients who are faced with having to decide whether to participate in a clinical trial2,3 or on very specific populations, such as select ethnic communities.13,14 However, it may be beneficial to provide simple, concise educational information about clinical trials to the general population, especially to those with minimal education. Although level of education concerns the minority of our sample (19%) sourced from an online panel, in the 2015 Current Population Survey, 41% of US participants aged 25 and older had not reached college education.15 Those with a lower level of education have reported a general lack of familiarity with clinical trials and were more likely to have inaccurate perceptions about trials. This is consistent with previous studies that have shown the lack of awareness and knowledge of clinical trials in this population.5,16,17 Our findings suggest that this knowledge gap can be reversed through a simple educational intervention and result in an increased willingness to participate.
The provision of information on clinical trials was positively associated with the 2 outcomes analyzed – improved impression of clinical trials and increased likelihood to enroll in a hypothetical trial. Such improvement might not translate to improved accrual,18 but it is a step toward closing the overall knowledge gap related to clinical trials and increasing the number of people who would consider trial participation. The lack of awareness of clinical trials has been reported as a legitimate explanation for why participation rates are lower in less-educated patient populations.19,20 This brief educational intervention is a simple, technology-sparing way to increase clinical trial awareness in the general population. In a similar survey, most physicians who reviewed an educational statement noted they were likely to use it with patients.21Less-educated patients, those who lived outside of urban areas, and those with lower household incomes were most concerned about trial location and the frequent visits needed when participating in a trial (Figure 2). Living in a nonurban area was not associated with participant impression of clinical trials or willingness to enroll in a trial. However, rural residency may be a barrier to enrollment depending on distance to the hospital22 and out-of-pocket expenses related to travel.23 Some comprehensive cancer centers, such as MSK, have developed alliances with community centers24 as a means of overcoming geographical barriers and increasing clinical trial participation rates.
Another concern shared by most respondents was the uncertainty in insurance coverage and potential out-of-pocket costs related to care. Lower household income, unlike location of residence and lack of insurance, was significantly associated with negative impressions of clinical trials and lower willingness to enroll in a trial, even after adjusting for education level. Cancer patients with higher financial burden have reported more attitudinal barriers, even after accounting for the negative effect of lower education level.25 Recent studies have also discussed the negative impact of lower income on cancer clinical trial participation,19,20,26,27 and new attention has been paid to the negative financial implications or “financial toxicity” of participating in a trial.23,28
White and older survey participants showed similar interest in clinical trial participation after accounting for other characteristics. There is growing evidence that outcome differences attributed to race may in fact be more dependent on socioeconomic status.8 A recent study among breast cancer patients showed that low socioeconomic status, but not race, was associated with decreased participation in clinical trials.29,30 Previous findings have also indicated that interest in clinical trials and barriers to enrollment among older, less-educated patients31 are often related to ineligibility, comorbidity, or communication difficulties.
Among our participants, the fear of side effects also was a common attitudinal barrier to clinical trial participation, as has been reported in previous studies.3,20 However, contrary to one previous study,20 this fear was not significantly increased among our less-educated participants.
Less-educated participants also reported more difficulties in understanding the information they were provided with, and they remained more concerned about being treated like “guinea pigs.” These concerns are consistent with other results showing that decisional conflict about clinical trial participation among patients with a high school diploma or less remained high even after they had received a National Cancer Institute text as pre-education material.3
Limitations
The lack of randomization makes it difficult to attribute with certainty that the change in acceptability of clinical trial participation is owing to the reading of the educational statement. The survey also sampled only English-speaking and well-educated participants from an online panel (81.1% had at least attended college) despite the use of a weighting procedure to ensure representativeness regarding gender, age, ethnicity, and income. Health literacy level or more specific trial literacy level was not evaluated; however, we were able to show less accurate perceptions of clinical trials among participants with a lower level of education by using agreement toward 8 statements about trials. The responses to hypothetical questions from these participants in the general population may also not be generalizable to a restricted population of patients with cancer. In addition, we measured impression of and willingness to enroll in a clinical trial immediately after providing participants with the educational material. We would have to confirm whether the positive effects of the education persist over time and translate to higher clinical trial participation rates.
Conclusions
Participants were receptive of educational material and expressed greater interest in and likelihood of enrolling in a clinical trial after reading it. The information had a greater effect on those with less education, but increased the willingness of all participants to enroll.
The low rate of participation in clinical trials is partly owing to the lack of awareness of these trials not only among potential participants but the US population as a whole.1 This lack of awareness, however, can be reversed. For example, findings from a single-institution observational study showed that systematically sending letters about clinical trial participation to all new lung cancer patients was associated with increased trial participation.2 More recently, a large, multicenter, randomized experiment showed that attitudes toward clinical trials were improved through preparatory education about clinical trials before a patient’s first oncologic visit.3
Such clinical trial education can be used before any medical diagnosis to increase clinical trial awareness in the general population. It may be advantageous to do so because people tend to process information more effectively during less stressful times.4 Clinical trial awareness in the US population has increased slightly over time, but in 2012, one study reported that 26% of its participants lacked general awareness about clinical trials.5
Comprehensive educational material, such as a multimedia psychoeducational intervention,6 a 28-video library,3 or a 160-page book,7 which have been proposed for oncology patients, may be too intensive for someone who is not immediately deciding whether to participate in a clinical trial. However, a simple, concise form of education might be preferable and appropriate to increase basic knowledge and awareness among the general population, especially among those who are less educated.8
Our aim in the present study was to evaluate whether providing brief educational material about clinical trials would increase patient willingness to participate in these trials.
Methods
This is a single-group, cross-sectional design study in which all participants were administered the questions and the 240-word educational statement in the same order.
Sample
An electronic survey was conducted by Marketing and Planning Systems (the analytics practice of Kantar Millwardbrown) on behalf of the Memorial Sloan Kettering Cancer Center (MSK). The survey included a national sample of 1011 participants and a local sample of 500 participants from the MSK catchment area (22 counties across the 5 boroughs of New York City, Long Island, southern New York State, northern New Jersey, and southwestern Connecticut).
Survey participants were aged 18 to 69 years in the national sample and 25 to 69 years in the local sample, representing 87% and 75% of the adult populations of those areas, respectively. Respondents who were or who had a family member currently working in the fields of news, advertising/marketing, or medical care were not surveyed. Participants were sourced from an online incentivized panel with millions of potential respondents representative of the US adult population.
Questionnaire
The questionnaire collected data on participant demographics and main medical history (including previous participation in a clinical trial), and asked questions about clinical trials, focusing on:
Analyses
Descriptive and bivariate statistical analyses of participants’ characteristics were weighted to ensure national representativeness for gender, age, ethnicity, and income. Mean standard deviation (SD) was computed for every quantitative variable. Categorical variables were expressed as proportions.
Student t tests and analyses of variance (ANOVAs) were used to compare continuous variables, while chi-square tests were used to compare categorical data. Repeated measures ANOVAs were then used to determine the sociodemographic and medical characteristics associated with the impression of and willingness to enroll in a clinical trial before and after reading the educational material. The interaction between education level and time (pre- or postreading assessment) was tested to determine if the changes after reading the brief statement were different depending on education level.
All statistical analyses were 2-tailed and considered statistically significant at P < .05. Analyses were performed using SPSS PAWS Statistics 24 (IBM Inc, Armonk, New York). Effect sizes (standardized mean differences) and their 95% confidence intervals (CIs) were computed using the compute.es package for R 3.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Participants
From October 23, 2015, through November 12, 2015, 1511 US participants responded to the survey request, including 1507 respondents (99.7%) who reported their education level and are included in the analyses of this report. The mean age of the respondents was 43.5 years (SD, 4.6). More than half of the respondents (57.8%) reported a current medical condition, mainly cardiovascular (20.0%), arthritis (20.0%), or other type or chronic pain (20.0%), and 9.0% reported a cancer diagnosis (current, 2.9%; previous, 6.1%).
Participants who at most had completed high school (18.9%, including 1.4% who had never even attended high school) were more often white women, lived outside urban areas, had lower household income, and were less likely to have health care insurance (Table 2). They also reported a current or previous cancer diagnosis less often than those of similar age who had attended college.
Previous participation in a clinical trial was reported by 9.6% of participants. Most of the clinical trials (75.0%) were testing a new drug. Previous trial participants were more likely to be older than those who had not participated in trials (46.1 years [SD, 14.8] vs 43.3 [SD, 4.6], repectively; P = .033), have a current health condition (86.2% vs 54.8%; P < .001), and know another trial participant (39.9% vs 9.5%; P < .001).
Education level and baseline impression of and
willingness to enroll in a clinical trial
A lower level of education was associated with a decreased likelihood of previous trial participation or of knowing a trial participant, as well as with less awareness and inaccurate perceptions of clinical trials (Table 2).
Participants with a high school degree or less were more likely to have a worse impression of and were less likely to enroll in a future hypothetical clinical trial before reading the educational material (Figure 1). Multivariable analyses confirmed that lower education level was associated with lower baseline overall impression, regardless of other personal characteristics (Table 3). Lowest household income was also associated with a more negative impression of trials, whereas participants with a current medical condition and with previous contact with clinical trials had a more positive impression of them. The same effects were observed with likeliness to enroll in a future hypothetical clinical trial (correlated with the overall impression: r, 0.63; P < .001), except that the negative effect of female gender was statistically significant (Table 3).
Posteducation impression of and willingnes to enroll
willingness to enroll in a clinical trial
The brief educational material was mostly considered believable (86.2%), easy to understand (84.8%), and included information that was new to participants (81.5%; Table 2). Participants with a high school diploma or less more often noted that the material provided them with new information, but they also reported more difficulties in fully understanding and believing the information. Overall, however, few participants found the information difficult to understand (4.6%) or hard to believe (1.8%; Table 2).
Most participants had an improved overall impression of clinical trials (standardized mean difference, 0.42; 95% CI, 0.35-0.50; P < .001) after reading the educational material. This increase was higher among participants with a lower completed level of education (Figure 1; standardized mean difference, 0.62; 95% CI, 0.45-0.79; Pinteraction < .001). The same effects were observed for likeliness to enroll in a future hypothetical clinical trial (P < .001; Figure 1).
After reading the informational statement, education level effect was no longer significantly associated with the overall impression of clinical trials (P = .23) and willingness to enroll in a clinical trial (P = .34), whereas the effects of income, current medical condition, and previous engangement with clinical trials remained statistically significant (Table 3).
Remaining challenges
Regarding hypothetical participation in a future clinical trial after a cancer diagnosis, the most critical concerns were related to side effects and the uncertainty of insurance coverage (Figure 2).10 The lack of understanding of clinical trials was the least critical concern; however, it was significantly higher among participants with a lower completed level of education. These participants also expressed more critical concerns about feeling like a “guinea pig,” inconvenient trial location, and the frequent visits needed.
Discussion
Findings from this survey demonstrated that providing brief educational material about cancer clinical trials was associated with a more favorable impression of clinical trials and higher interest in trial participation. Furthermore, as far as we can ascertain, this is the first report showing how a simple intervention such as this may help close the knowledge gap on clinical trials among people of different educational backgrounds. Although most respondents in this interventional survey noted an increased willingness to consider participation in a clinical trial after reading the educational material, those with a lower level of education and knowledge about clinical trials received the most benefit. Previous participation in a clinical trial was also strongly associated with the impression of and willingness to enroll in a trial, both before and after reading the statement.11
Most of the interventions evaluated to date12 have focused on patients who are faced with having to decide whether to participate in a clinical trial2,3 or on very specific populations, such as select ethnic communities.13,14 However, it may be beneficial to provide simple, concise educational information about clinical trials to the general population, especially to those with minimal education. Although level of education concerns the minority of our sample (19%) sourced from an online panel, in the 2015 Current Population Survey, 41% of US participants aged 25 and older had not reached college education.15 Those with a lower level of education have reported a general lack of familiarity with clinical trials and were more likely to have inaccurate perceptions about trials. This is consistent with previous studies that have shown the lack of awareness and knowledge of clinical trials in this population.5,16,17 Our findings suggest that this knowledge gap can be reversed through a simple educational intervention and result in an increased willingness to participate.
The provision of information on clinical trials was positively associated with the 2 outcomes analyzed – improved impression of clinical trials and increased likelihood to enroll in a hypothetical trial. Such improvement might not translate to improved accrual,18 but it is a step toward closing the overall knowledge gap related to clinical trials and increasing the number of people who would consider trial participation. The lack of awareness of clinical trials has been reported as a legitimate explanation for why participation rates are lower in less-educated patient populations.19,20 This brief educational intervention is a simple, technology-sparing way to increase clinical trial awareness in the general population. In a similar survey, most physicians who reviewed an educational statement noted they were likely to use it with patients.21Less-educated patients, those who lived outside of urban areas, and those with lower household incomes were most concerned about trial location and the frequent visits needed when participating in a trial (Figure 2). Living in a nonurban area was not associated with participant impression of clinical trials or willingness to enroll in a trial. However, rural residency may be a barrier to enrollment depending on distance to the hospital22 and out-of-pocket expenses related to travel.23 Some comprehensive cancer centers, such as MSK, have developed alliances with community centers24 as a means of overcoming geographical barriers and increasing clinical trial participation rates.
Another concern shared by most respondents was the uncertainty in insurance coverage and potential out-of-pocket costs related to care. Lower household income, unlike location of residence and lack of insurance, was significantly associated with negative impressions of clinical trials and lower willingness to enroll in a trial, even after adjusting for education level. Cancer patients with higher financial burden have reported more attitudinal barriers, even after accounting for the negative effect of lower education level.25 Recent studies have also discussed the negative impact of lower income on cancer clinical trial participation,19,20,26,27 and new attention has been paid to the negative financial implications or “financial toxicity” of participating in a trial.23,28
White and older survey participants showed similar interest in clinical trial participation after accounting for other characteristics. There is growing evidence that outcome differences attributed to race may in fact be more dependent on socioeconomic status.8 A recent study among breast cancer patients showed that low socioeconomic status, but not race, was associated with decreased participation in clinical trials.29,30 Previous findings have also indicated that interest in clinical trials and barriers to enrollment among older, less-educated patients31 are often related to ineligibility, comorbidity, or communication difficulties.
Among our participants, the fear of side effects also was a common attitudinal barrier to clinical trial participation, as has been reported in previous studies.3,20 However, contrary to one previous study,20 this fear was not significantly increased among our less-educated participants.
Less-educated participants also reported more difficulties in understanding the information they were provided with, and they remained more concerned about being treated like “guinea pigs.” These concerns are consistent with other results showing that decisional conflict about clinical trial participation among patients with a high school diploma or less remained high even after they had received a National Cancer Institute text as pre-education material.3
Limitations
The lack of randomization makes it difficult to attribute with certainty that the change in acceptability of clinical trial participation is owing to the reading of the educational statement. The survey also sampled only English-speaking and well-educated participants from an online panel (81.1% had at least attended college) despite the use of a weighting procedure to ensure representativeness regarding gender, age, ethnicity, and income. Health literacy level or more specific trial literacy level was not evaluated; however, we were able to show less accurate perceptions of clinical trials among participants with a lower level of education by using agreement toward 8 statements about trials. The responses to hypothetical questions from these participants in the general population may also not be generalizable to a restricted population of patients with cancer. In addition, we measured impression of and willingness to enroll in a clinical trial immediately after providing participants with the educational material. We would have to confirm whether the positive effects of the education persist over time and translate to higher clinical trial participation rates.
Conclusions
Participants were receptive of educational material and expressed greater interest in and likelihood of enrolling in a clinical trial after reading it. The information had a greater effect on those with less education, but increased the willingness of all participants to enroll.
1. Ford JG, Howerton MW, Lai GY, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112(2):228-242.
2. Quinn GP, Bell BA, Bell MY, et al. The guinea pig syndrome: improving clinical trial participation among thoracic patients. J Thorac Oncol. 2007;2(3):191-196.
3. Meropol NJ, Wong Y-N, Albrecht T, et al. Randomized trial of a web-based intervention to address barriers to clinical trials. J Clin Oncol. 2016;34(5):469-478.
4. Goldberg RJ. Disclosure of information to adult cancer patients: issues and update. J Clin Oncol. 1984;2(8):948-955.
5. Leiter A, Diefenbach MA, Doucette J, Oh WK, Galsky MD. Clinical trial awareness: changes over time and sociodemographic disparities. Clin Trials. 2015;12(3):215-223.
6. Jacobsen PB, Wells KJ, Meade CD, et al. Effects of a brief multimedia psychoeducational intervention on the attitudes and interest of patients with cancer regarding clinical trial participation: a multicenter randomized controlled trial. J Clin Oncol. 2012;30(20):2516-2521.
7. Carney PA, Tucker EK, Newby TA, Beer TM. Feasibility, acceptability and findings from a pilot randomized controlled intervention study on the impact of a book designed to inform patients about cancer clinical trials. J Cancer Educ. 2014;29(1):181-187.
8. Sharrocks K, Spicer J, Camidge DR, Papa S. The impact of socioeconomic status on access to cancer clinical trials. Br J Cancer. 2014;111(9):1684-1687.
9. Regan J, Hickey C, Targett C, Masuda S, Sabbatini P. Framing clinical research and the importance of trial participation: patient and physician perspective. Poster presented at: 8th Annual AACI Clinical Research Initiative Meeting; July 20-21 2016; Chicago, IL.
10. Mancini J, Genre D, Dalenc F, et al. Patients’ regrets after participating in a randomized controlled trials depended on their involvement in the decision making. J Clin Epidemiol. 2012;65(6):635-642
11. Murphy ST, Frank LB, Chatterjee JS, et al. Comparing the relative efficacy of narrative vs nonnarrative health messages in reducing health disparities using a randomized trial. Am J Public Health. 2015;105(10):2117-2123.
12. Treweek S, Lockhart P, Pitkethly M, et al. Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta-analysis. BMJ Open. 2013;3(2):e002360. doi:10.1136/bmjopen-2012-002360.
13. Ma GX, Tan Y, Blakeney NC, et al. The impact of a community-based clinical trial educational intervention among underrepresented Chinese Americans. Cancer Epidemiol Biomarkers Prev. 2014;23(3):424-432.
14. Cupertino AP, Molina CSP, de los Rios JB, et al. Knowledge, awareness, and interest in cancer clinical trials among rural latinos following brief education by promotores de salud. J Community Med Health Educ. 2015;5:2161. doi:10.4172/2161-0711.1000358
15. Ryan CL, Bauman K. Educational attainment in the United States: 2015. https://www.census.gov/content/dam/Census/library/publications/2016/demo/p20-578.pdf. Published March 2016. Accessed October 18, 2016.
16. Lara PN Jr, Paterniti DA, Chiechi C, et al. Evaluation of factors affecting awareness of and willingness to participate in cancer clinical trials. J Clin Oncol. 2005;23(36):9282-9289.
17. Dhai A, Etheredge H, Cleaton-Jones P. A pilot study evaluating an intervention designed to raise awareness of clinical trials among potential participants in the developing world. J Med Ethics. 2010;36(4):238-242.
18. Denicoff AM, McCaskill-Stevens W, Grubbs SS, et al. The National Cancer Institute–American Society of Clinical Oncology Cancer Trial Accrual Symposium: summary and recommendations. J Oncol Pract. 2013;9(6):267-276.
19. Davison BJ, So A, Goldenberg SL, Berkowitz J, Gleave ME. Measurement of factors influencing the participation of patients with prostate cancer in clinical trials: a Canadian perspective. BJU Int. 2008;101(8):982-987.
20. Unger JM, Hershman DL, Albain KS, et al. Patient income level and cancer clinical trial participation. J Clin Oncol. 2013;31(5):536-542.
21. IRB Advisor. Education may overcome reticence to join trials. Relias website. http://www.ahcmedia.com/articles/138088-education-may-overcome-reticence-to-join-trials. Published July 1, 2016. Accessed July 29, 2016.
22. Vanderpool RC, Kornfeld J, Mills L, Byrne MM. Rural-urban differences in discussions of cancer treatment clinical trials. Patient Educ Couns. 2011;85(2):e69-e74.
23. Meropol NJ. Health policy: overcoming cost barriers to clinical trial participation. Nat Rev Clin Oncol. 2016;13(6):333-334.
24. MSK Cancer Alliance. Memorial Sloan Kettering Cancer Center website. https://www.mskcc.org/about/innovative-collaborations/msk-alliance. Updated March 7, 2018. Accessed August 18, 2016.
25. Manne S, Kashy D, Albrecht T, et al. Attitudinal barriers to participation in oncology clinical trials: factor analysis and correlates of barriers. Eur J Cancer Care (Engl). 2015;24(1):28-38.
26. Unger JM, Gralow JR, Albain KS, Ramsey SD, Hershman DL. Patient income level and cancer clinical trial participation: a prospective survey study. JAMA Oncol. 2016;2(1):137-139.
27. Moy B. Clinical trials, disparities, and financial burden: it’s time to intervene. Oncologist. 2015;20(6):571.
28. Bonevski B, Randell M, Paul C, et al. Reaching the hard-to-reach: a systematic review of strategies for improving health and medical research with socially disadvantaged groups. BMC Med Res Methodol. 2014;14:42.
29. Gross CP, Filardo G, Mayne ST, Krumholz HM. The impact of socioeconomic status and race on trial participation for older women with breast cancer. Cancer. 2005;103(3):483-491.
30. Nickell A, Burke NJ, Cohen E, Caprio M, Joseph G. Educating low-SES and LEP survivors about breast cancer research: pilot test of the Health Research Engagement Intervention. J Cancer Educ. 2014;29(4):746-752.
31. Mancini J, Jansen J, Julian-Reynier C, Bechlian D, Vey N, Chabannon C. Preferences of older adults with cancer for involvement in decision-making about research participation. J Am Geriatr Soc. 2014;62(6):1191-1193.
1. Ford JG, Howerton MW, Lai GY, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112(2):228-242.
2. Quinn GP, Bell BA, Bell MY, et al. The guinea pig syndrome: improving clinical trial participation among thoracic patients. J Thorac Oncol. 2007;2(3):191-196.
3. Meropol NJ, Wong Y-N, Albrecht T, et al. Randomized trial of a web-based intervention to address barriers to clinical trials. J Clin Oncol. 2016;34(5):469-478.
4. Goldberg RJ. Disclosure of information to adult cancer patients: issues and update. J Clin Oncol. 1984;2(8):948-955.
5. Leiter A, Diefenbach MA, Doucette J, Oh WK, Galsky MD. Clinical trial awareness: changes over time and sociodemographic disparities. Clin Trials. 2015;12(3):215-223.
6. Jacobsen PB, Wells KJ, Meade CD, et al. Effects of a brief multimedia psychoeducational intervention on the attitudes and interest of patients with cancer regarding clinical trial participation: a multicenter randomized controlled trial. J Clin Oncol. 2012;30(20):2516-2521.
7. Carney PA, Tucker EK, Newby TA, Beer TM. Feasibility, acceptability and findings from a pilot randomized controlled intervention study on the impact of a book designed to inform patients about cancer clinical trials. J Cancer Educ. 2014;29(1):181-187.
8. Sharrocks K, Spicer J, Camidge DR, Papa S. The impact of socioeconomic status on access to cancer clinical trials. Br J Cancer. 2014;111(9):1684-1687.
9. Regan J, Hickey C, Targett C, Masuda S, Sabbatini P. Framing clinical research and the importance of trial participation: patient and physician perspective. Poster presented at: 8th Annual AACI Clinical Research Initiative Meeting; July 20-21 2016; Chicago, IL.
10. Mancini J, Genre D, Dalenc F, et al. Patients’ regrets after participating in a randomized controlled trials depended on their involvement in the decision making. J Clin Epidemiol. 2012;65(6):635-642
11. Murphy ST, Frank LB, Chatterjee JS, et al. Comparing the relative efficacy of narrative vs nonnarrative health messages in reducing health disparities using a randomized trial. Am J Public Health. 2015;105(10):2117-2123.
12. Treweek S, Lockhart P, Pitkethly M, et al. Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta-analysis. BMJ Open. 2013;3(2):e002360. doi:10.1136/bmjopen-2012-002360.
13. Ma GX, Tan Y, Blakeney NC, et al. The impact of a community-based clinical trial educational intervention among underrepresented Chinese Americans. Cancer Epidemiol Biomarkers Prev. 2014;23(3):424-432.
14. Cupertino AP, Molina CSP, de los Rios JB, et al. Knowledge, awareness, and interest in cancer clinical trials among rural latinos following brief education by promotores de salud. J Community Med Health Educ. 2015;5:2161. doi:10.4172/2161-0711.1000358
15. Ryan CL, Bauman K. Educational attainment in the United States: 2015. https://www.census.gov/content/dam/Census/library/publications/2016/demo/p20-578.pdf. Published March 2016. Accessed October 18, 2016.
16. Lara PN Jr, Paterniti DA, Chiechi C, et al. Evaluation of factors affecting awareness of and willingness to participate in cancer clinical trials. J Clin Oncol. 2005;23(36):9282-9289.
17. Dhai A, Etheredge H, Cleaton-Jones P. A pilot study evaluating an intervention designed to raise awareness of clinical trials among potential participants in the developing world. J Med Ethics. 2010;36(4):238-242.
18. Denicoff AM, McCaskill-Stevens W, Grubbs SS, et al. The National Cancer Institute–American Society of Clinical Oncology Cancer Trial Accrual Symposium: summary and recommendations. J Oncol Pract. 2013;9(6):267-276.
19. Davison BJ, So A, Goldenberg SL, Berkowitz J, Gleave ME. Measurement of factors influencing the participation of patients with prostate cancer in clinical trials: a Canadian perspective. BJU Int. 2008;101(8):982-987.
20. Unger JM, Hershman DL, Albain KS, et al. Patient income level and cancer clinical trial participation. J Clin Oncol. 2013;31(5):536-542.
21. IRB Advisor. Education may overcome reticence to join trials. Relias website. http://www.ahcmedia.com/articles/138088-education-may-overcome-reticence-to-join-trials. Published July 1, 2016. Accessed July 29, 2016.
22. Vanderpool RC, Kornfeld J, Mills L, Byrne MM. Rural-urban differences in discussions of cancer treatment clinical trials. Patient Educ Couns. 2011;85(2):e69-e74.
23. Meropol NJ. Health policy: overcoming cost barriers to clinical trial participation. Nat Rev Clin Oncol. 2016;13(6):333-334.
24. MSK Cancer Alliance. Memorial Sloan Kettering Cancer Center website. https://www.mskcc.org/about/innovative-collaborations/msk-alliance. Updated March 7, 2018. Accessed August 18, 2016.
25. Manne S, Kashy D, Albrecht T, et al. Attitudinal barriers to participation in oncology clinical trials: factor analysis and correlates of barriers. Eur J Cancer Care (Engl). 2015;24(1):28-38.
26. Unger JM, Gralow JR, Albain KS, Ramsey SD, Hershman DL. Patient income level and cancer clinical trial participation: a prospective survey study. JAMA Oncol. 2016;2(1):137-139.
27. Moy B. Clinical trials, disparities, and financial burden: it’s time to intervene. Oncologist. 2015;20(6):571.
28. Bonevski B, Randell M, Paul C, et al. Reaching the hard-to-reach: a systematic review of strategies for improving health and medical research with socially disadvantaged groups. BMC Med Res Methodol. 2014;14:42.
29. Gross CP, Filardo G, Mayne ST, Krumholz HM. The impact of socioeconomic status and race on trial participation for older women with breast cancer. Cancer. 2005;103(3):483-491.
30. Nickell A, Burke NJ, Cohen E, Caprio M, Joseph G. Educating low-SES and LEP survivors about breast cancer research: pilot test of the Health Research Engagement Intervention. J Cancer Educ. 2014;29(4):746-752.
31. Mancini J, Jansen J, Julian-Reynier C, Bechlian D, Vey N, Chabannon C. Preferences of older adults with cancer for involvement in decision-making about research participation. J Am Geriatr Soc. 2014;62(6):1191-1193.
Enhancing communication between oncology care providers and patient caregivers during hospice
Improving the delivery of end-of-life care for patients with advanced cancer has become a priority in the United States.1,2 Quality metrics identifying the components of high-quality end-of-life care have focused on improved symptom management, decreased use of chemotherapy at the end of life, fewer hospitalizations, and increased use of hospice care. Patients and caregivers also consider good communication with the medical team to be a critical component of end-of-life care.3-5 Interventions to improve the quality of end-of-life care are needed.
Caregivers of patients with advanced cancer who receive hospice services report better quality of care and death than those receiving end-of-life care in other settings.6-9 However, the transition for patients from active cancer therapy delivered by their oncologists to end-of-life care delivered by a hospice care team can be abrupt. Patients and their caregivers often feel abandoned by oncology clinicians because of the lack of continuity of care and poor communication.10-13 Caregivers who note continued involvement and communication with their oncology clinicians experience a lower caregiving burden, report higher satisfaction with care, and recount a higher quality of death for their loved one.14-16 Therefore, interventions that prevent abrupt transitions in care from oncology to hospice by ensuring continued communication with oncology clinicians are needed to improve the quality of end-of-life care.17 Recent findings have shown that providing concurrent oncology and palliative care is not only feasible but beneficial for patients with advanced cancer and their caregivers.18-24 However, there is no standard of care for the involvement of oncology clinicians in the care of patients receiving hospice services and their families.
Although interventions may be needed, it could be challenging to deliver them given the multiple demands of caregiving during hospice and the lack of regular contact in clinic. We sought to assess the feasibility of an intervention, Ensuring Communication in Hospice by Oncology (ECHO), to facilitate communication between oncology clinicians and caregivers of patients who enroll in hospice. We also explored caregiver-reported outcomes during hospice care, including satisfaction with care, attitudes toward caregiving, stress, decision regret, and perception of the quality of patients’ end-of-life care.
Methods
Study design
During March 2014-June 2015, caregivers of patients with advanced cancer who enrolled in home hospice services were eligible to participate in the study at Massachusetts General Hospital (MGH) in Boston. The Dana Farber/Harvard Cancer Center Institutional Review Board approved all methods and materials. The study opened with an enrollment goal of 30 participating caregivers. However, due to staff transitions, we closed the study early in June 2015 after 25 caregivers enrolled.
Participants
Caregivers of patients receiving care at the cancer center's thoracic, head and neck, sarcoma, melanoma, and gynecological disease centers were eligible within 10 days after a patient’s enrollment in hospice. Five disease sites were selected to participate in the intervention. We defined caregivers as relatives or friends serving as the primary caregiver of the patient at home during hospice care. Other caregiver eligibility criteria included the ability to read and respond to questions in English or with a translator, access to a telephone and/or computer to communicate with oncology clinicians, and willingness to complete questionnaires. Caregivers were ineligible if the patient was participating in an ongoing palliative care trial.
To identify eligible caregivers, case managers from both the inpatient and outpatient settings, as well as the nurses based in participating disease centers, notified the research team of all patients referred to hospice. If the patient had received oncology care in one of our participating disease centers, the research team contacted their oncology clinician/s (physicians, nurse practitioners [NP], registered nurses [RN], and/or physician assistants [PA]) to inquire if the patient had an involved caregiver and to obtain permission to offer study participation. If the oncology clinician/s did not grant permission, we documented the reason. Otherwise, with permission, research staff contacted the caregiver by telephone to offer study participation and obtain verbal consent. We then sent participating caregivers a copy of the informed consent by mail or e-mail.
Intervention
The ECHO intervention consisted of: supportive phone calls from an oncology clinician to the caregiver; an optional clinic visit with the oncology clinician for the patient to address clinical questions or concerns that was offered during the initial telephone consent; a bereavement call to the participating caregiver (Figure 1). Initially, we designed the intervention to have phone calls occurring twice weekly until the patient died. However, 3 months after starting the study, we received feedback from oncology clinicians and caregivers that calls were too frequent, so we amended the protocol to include phone calls twice weekly for the first 2 weeks of the study and then weekly thereafter. Seven months into the study, we again decreased the number of phone calls to weekly for the first 4 weeks, every other week for 4 weeks, and then monthly until patient death. We informed caregivers of changes by e-mail.
Before we started the study, we conducted training sessions with oncology clinicians from the participating disease centers to review study procedures and expectations of the phone calls. Supportive phone calls during hospice were not a part of standard practice prior to the study. The RN, NP or PA, and/or physician who had an established relationship with the patient and caregiver completed the phone calls. They decided based on their respective relationships with the patients and their workloads who would call each week, though the majority of calls were conducted by the RN or NP. All the clinicians had experience comanaging patients with hospice agencies, and our general practice is for the oncology physician to serve as the hospice attending of record. The calls were intended to offer support and reassurance to caregivers. We did not script the calls so that clinicians could tailor their content to the individual needs of the caregiver, as informed by their established relationship. The calls could include the patient if he/she was able to and interested in speaking to the clinician. There was no standardized communication with hospice as part of the intervention. If a caregiver raised concerns about symptom management during a call, the clinician would advise the caregiver to contact the hospice team directly or the clinician would call the hospice to discuss, depending on the clinical scenario and the clinician’s judgment. Research staff reminded oncology clinicians to call caregivers on the scheduled date and to document the discussion in the electronic medical record. The hospice phone number was included in the e-mail. If the call was not documented, research staff sent a reminder e-mail to the oncology clinicians 24 hours after the call was due.
Caregiver-reported measures
Caregivers completed a demographic questionnaire at baseline in which they reported their age, gender, race, ethnicity, religion, employment status, and relationship to the patient. We collected information about patient characteristics from the electronic medical record, including age, gender, and cancer type. In addition, we administered validated, self-report measures (see below). We limited the number of measures to decrease caregiver burden:
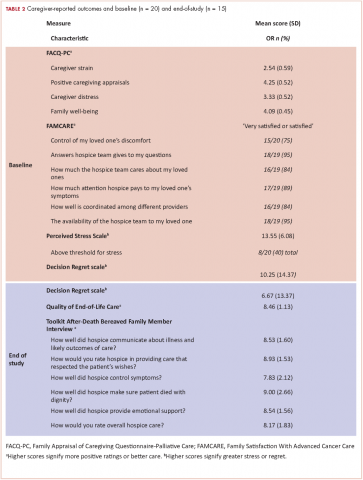
The baseline questionnaire included the FACQ-PC, the FAMCARE scale, the PSS, and the Decision Regret Scale. Initially, the study involved weekly questionnaires after baseline that included the FACQ-PC, the FAMCARE scale, and the PSS. However, after 3 months of study enrollment, we received feedback that the questionnaires were too frequent, so we amended the protocol and changed the frequency to weekly for 2 weeks, then monthly thereafter until the patient died.
Caregiver exit interview
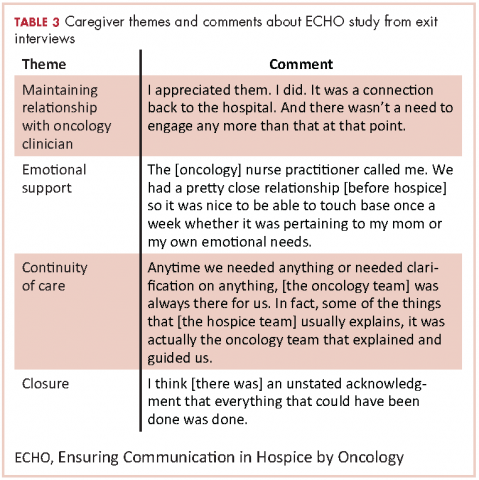
Exit interviews included the toolkit interview, the Quality of End-of-Life Care scale, and the Decision Regret Scale. Caregivers also reported patients’ place and date of death. After the first 6 caregivers enrolled, we amended the exit interview to include open-ended feedback from caregivers. Specifically, we evaluated caregivers’ perceptions of the ECHO intervention by asking them about their perception of and satisfaction with the content and frequency of the oncology clinicians’ phone calls, whether they had an in-person visit with their oncology clinicians after the start of hospice care, whether the clinician/s contacted them after the patient died, and whether there were ways in which the clinician/s could help in the future.
Data collection and storage
Caregivers were given the option of completing study measures by telephone or e-mail so that they could complete them on a computer when it was convenient for them. Caregivers received a link to Research Electronic Data Capture (REDCap), a web-based, HIPAA-compliant application that allows participants to answer questionnaires online. The exit interviews were completed by phone, and research staff entered the data into the REDCap database. In addition, with we obtained caregiver permission to audiorecord the exit interviews, which were then transcribed and de-identified.
Statistical analysis
The primary outcome for the study was feasibility, which we defined as >70% of the caregivers receiving >50% of the phone calls from an oncology clinician, and >70% of the caregivers completing >50% of the questionnaires. All time points for the questionnaires and the exit interview counted toward feasibility. Exploratory endpoints included caregiver-reported satisfaction, stress, quality of end-of-life care, and decision-making regret.
Using STATA (v9.3; StataCorp, College Station, Texas) for all statistical analyses, we summarized participants’ characteristics and outcomes as frequencies and percentages for categorical variables and mean standard deviation for continuous variables. We used the repeated-measures t test to assess changes in caregiver outcomes over time. We used the Fisher exact test to compare clinically meaningful threshold scores of perceived stress between men and women.
We examined caregivers’ open-ended feedback using descriptive analyses to summarize comments about the intervention and to inform possible refinements for a future study.
Results
Baseline characteristics
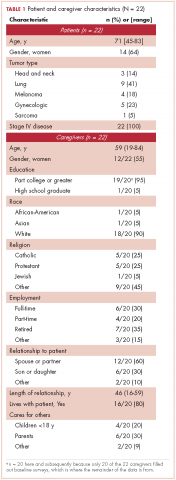
During March 2014-June 2015, we enrolled caregivers of patients with advanced cancer from 5 participating disease centers: thoracic, head and neck, sarcoma, melanoma, and gynecological malignancy. We screened 123 patients to determine the eligibility of their caregivers (Figure 2). Of 38 eligible caregivers, 7 could not be reached, 6 declined participation, and 25 enrolled in the study (81% enrollment rate). Of the 25 caregivers who enrolled, 3 withdrew – 2 because the patients they were caring for died before the intervention began, and 1 who withdrew from the study because the family wanted less contact with the oncology clinician/s. Thus, we had data for 22 caregivers for our feasibility evaluation. One caregiver stopped study assessments after 3 months because the patient dis-enrolled from hospice. Median time from the patients’ hospice enrollment to caregiver study enrollment was 3 days (range, 1-9). Median time from study enrollment to patient death was 36 days (range, 2-135). Patients were receiving care from 10 different hospice agencies.
All of the patients had metastatic cancer, and 64% were women (Table 1).
Most of the caregivers were white (n = 18, 90%) and women (n = 12, 55%). The majority were the patient’s spouse (n = 12, 60%) or child (n = 6, 30%), and they lived with the patient (n = 16, 80%). Many of the caregivers had other responsibilities in addition to caring for the patients, including part- or full-time work (n = 10, 50%) or caring for others in addition to the patient (n = 10, 50%).
Feasibility
Over the study period, oncology clinicians completed 164 of 180 possible phone calls (91%). All 22 caregivers received >50% of the phone calls. Caregivers completed 78 of 99 possible questionnaires (79%), and 16 of 22 completed >50% of the questionnaires (73%). None of the caregivers/patients wanted to schedule the optional visit with an oncology clinician that was offered as part of the intervention; however, 5 patients had a clinic visit after hospice enrollment. In addition, 2 oncologists visited a patient and caregiver at home. All caregivers received bereavement contact from the oncology team.
Caregiver-reported outcomes
In all, 20 of the 22 enrolled caregivers completed baseline measures (Table 2), and they all chose to complete questionnaires by e-mail. Caregivers’ attitudes toward caregiving and satisfaction with hospice services were overall positive. They reported high mean scores on the 2 domains on the FACQ-PC of positive caregiver appraisal (mean, 4.25; SD, 0.52) and family well-being (mean, 4.09; SD, 0.45). The majority of caregivers (75%-95%) reported they were satisfied or very satisfied with various dimensions of hospice care based on the FAMCARE questionnaire.
Overall, caregivers reported moderate levels of stress (mean, 13.55; SD, 6.08) based on the PSS scores. Of the 20 caregivers who completed the baseline measures, 8 had clinically meaningful stress, and stress was numerically higher in female caregivers than in their male counterparts, but it did not reach statistical significance (55% vs 22%, P = .197). Finally, at baseline, caregivers indicated relatively low levels of decisional regret about enrolling in hospice, although there was considerable variation (mean, 10.25; SD, 14.37).
We conducted exit interviews with 15 caregivers because we were not able to reach 6 of the original 21 (Table 2). Caregivers rated hospice services highly for communication, symptom control, emotional support, and overall care. They also rated quality of death highly (mean, 8.46; SD 1.13). Regret was lower at the end of the study, but this did not reach statistical significance (baseline mean 9.29; end of study mean 3.57; P = .161).
In the recorded exit interviews, all of the caregivers responded they were satisfied with the phone calls. Two caregivers commented that they would have preferred the calls were more scheduled or at more suitable times. Overall, they described the phone calls as excellent, supportive, responsive, comfortable, and appreciated. All caregivers reported contact with the oncology team after the patient died, but one caregiver was disappointed she was only contacted by the nurse practitioner and not the oncologist. Participants did not feel as if there were other ways that the oncology team could have been helpful for them while their loved one was in hospice.
Table 3 highlights other representative comments from the exit interviews.
Discussion
As far as we know, this is the first study to assess the feasibility of an intervention to facilitate communication between oncology clinicians and caregivers of patients with advanced cancer who are receiving hospice care. Despite the challenges of oncology clinicians delivering an intervention to caregivers during hospice, we found this intervention was feasible and acceptable. Although the transition to hospice can be stressful, caregivers reported high satisfaction with hospice care and the quality of the patient’s death. In exit interviews, they also reported high satisfaction with the intervention and appreciation for maintaining their relationship with the oncology team. It is worth noting that no caregiver requested the optional clinic visit after hospice enrollment, and most caregivers were not seen again in clinic after hospice enrollment.
These results suggest that a simple, telephone-based intervention of scheduled calls from the oncology team at prompted intervals is not only feasible, but may also help foster continuity between the patient and caregiver and the oncology team. We received feedback from both oncology clinicians and caregivers that the initial call frequency was too often, suggesting that communication may not need to be very frequent to maintain continuity and provide support. This also suggests that if the calls are too frequent, they may be more intrusive than helpful for both oncologists and caregivers. Alternatively, caregiver suggestion for fewer phone calls may indicate that concerns about abandonment are less prevalent than existing literature has suggested.
Limitations
Our study has several important limitations. The sample size was small as this was a feasibility study conducted at a single tertiary care hospital, and the population was 90% white and 95% college educated, which may limit the generalizability of our results. In addition, the median length of stay in hospice for patients on this study was 36 days, which is long compared with national averages,32,33 and thus the outcomes may not represent the experience of a more heterogeneous population. The longer length of stay in hospice may have contributed to caregivers’ high satisfaction with the quality of end-of-life care.
Oncologists did not grant permission for the study team to approach all eligible caregivers, which may have introduced selection bias. We were also not able to reach 6 participants for exit interviews. People less satisfied with the intervention or with hospice may be more likely to have missing data, which could introduce bias into the satisfaction ratings. Furthermore, we did not explore the oncology clinicians’ perspective of the intervention or assess the time commitment of the calls. Oncology clinicians have many competing responsibilities and have variable experience and comfort with hospice care. Therefore, future studies should explore the perspective of oncology clinicians in regard to the intervention.
Finally, we did not require communication with the hospice agency as part of the intervention as there were ten different hospices involved. Thus, we do not know how the intervention impacted the hospice team’s care of the patient. However, based upon the success of this pilot study, future larger studies should explore the impact of the intervention from the perspective of the hospice care team and include oncology clinician communication with the hospice agency.
Conclusion
These findings demonstrate the feasibility and acceptability of an intervention to enhance communication between oncology clinicians and caregivers of patients with advanced cancer receiving hospice care. Importantly, the high caregiver satisfaction with the intervention in this study suggests that maintaining communication with the primary oncology team during hospice care may be an important component of high quality end-of-life care, though the desire for decreased calls suggests that this communication need not be frequent to maintain the continuity. A randomized study with a larger and more diverse patient/caregiver sample would allow us to explore the impact of the intervention on caregiver feelings of abandonment by the oncology team and short- and long-term caregiver outcomes, as well as to understand the perspective of the oncology and hospice clinicians involved.
1. Institute of Medicine. 2015. Dying in america: improving quality and honoring individual preferences near the end of life. Washington, DC: The National Academies Press. https://doi.org/10.17226/18748.
2. Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(1):96-112.
3. Wenrich MD, Curtis JR, Shannon SE, Carline JD, Ambrozy DM, Ramsey PG. Communicating with dying patients within the spectrum of medical care from terminal diagnosis to death. Arch Intern Med. 2001;161(6):868-874.
4. Parker SM, Clayton JM, Hancock K, et al. A systematic review of prognostic/end-of-life communication with adults in the advanced stages of a life-limiting illness: patient/caregiver preferences for the content, style, and timing of information. J Pain Symptom Manage. 2007;34(1):81-93.
5. Steinhauser KE, Clipp EC, McNeilly M, Christakis NA, McIntyre LM, Tulsky JA. In search of a good death: observations of patients, families, and providers. Ann Intern Med. 2000;132(10):825-832.
6. Teno JM, Clarridge BR, Casey V, et al. Family perspectives on end-of-life care at the last place of care. JAMA. 2004;291(1):88-93.
7. Kumar P, Wright AA, Hatfield LA, Temel JS, Keating NL. Family perspectives on hospice care experiences of patients with cancer. J Clin Oncol. 2017;35(4):432-439.
8. Wright AA, Keating NL, Ayanian JZ, et al. Family perspectives on aggressive cancer care near the end of life. JAMA. 2016;315(3):284-292.
9. Duggan KT, Hildebrand Duffus S, D’Agostino RB Jr, Petty WJ, Streer NP, Stephenson RC. The impact of hospice services in the care of patients with advanced stage nonsmall cell lung cancer. J Palliat Med. 2017;20(1):29-34.
10. Curtis JR, Wenrich MD, Carline JD, Shannon SE, Ambrozy DM, Ramsey PG. Understanding physicians’ skills at providing end-of-life care perspectives of patients, families, and health care workers. J Gen Intern Med. 2001;16(1):41-49.
11. Back AL, Young JP, McCown E, et al. Abandonment at the end of life from patient, caregiver, nurse, and physician perspectives: loss of continuity and lack of closure. Arch Intern Med. 2009;169(5):474-479.
12. Vig EK, Starks H, Taylor JS, Hopley EK, Fryer-Edwards K. Why don’t patients enroll in hospice? Can we do anything about it? J Gen Intern Med. 2010;25(10):1009-1019.
13. Waldrop DP, Meeker MA, Kerr C, Skretny J, Tangeman J, Milch R. The nature and timing of family-provider communication in late-stage cancer: a qualitative study of caregivers’ experiences. J Pain Symptom Manage. 2012;43(2):182-194.
14. Emanuel EJ, Fairclough DL, Slutsman J, Emanuel LL. Understanding economic and other burdens of terminal illness: the experience of patients and their caregivers. Ann Intern Med. 2000;132(6):451-459.
15. Curtis JR, Patrick DL, Engelberg RA, Norris K, Asp C, Byock I. A measure of the quality of dying and death. Initial validation using after-death interviews with family members. J Pain Symptom Manage. 2002;24(1):17-31.
16. Fleming DA, Sheppard VB, Mangan PA, et al. Caregiving at the end of life: Perceptions of health care quality and quality of life among patients and caregivers. J Pain Symptom Manage. 2006;31(5):407-420.
17. McMillan SC. Interventions to facilitate family caregiving at the end of life. J Palliat Med. 2005;8(Suppl 1):S132-S139.
18. Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302(7):741-749.
19. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733-742.
20. Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet. 2014;383(9930):1721-1730.
21. Maltoni M, Scarpi E, Dall’Agata M, et al. Systematic versus on-demand early palliative care: a randomised clinical trial assessing quality of care and treatment aggressiveness near the end of life. Eur J Cancer. 2016;69:110-118.
22. El-Jawahri A ea. Early integrated palliative care to improve family caregivers (FC) outcomes for patients with gastrointestinal and lung cancer. J Clin Oncol. 2016;34(suppl; abstr 10131).
23. Temel JS, Greer JA, El-Jawahri A, et al. Effects of early integrated palliative care in patients with lung and gi cancer: a randomized clinical trial. J Clin Oncol. 2017;35(8):834-841.
24. El-Jawahri A, LeBlanc T, VanDusen H, et al. Effect of inpatient palliative care on quality of life 2 weeks after hematopoietic stem cell transplantation: a randomized clinical trial. JAMA. 2016;316(20):2094-2103.
25. Cooper B, Kinsella GJ, Picton C. Development and initial validation of a family appraisal of caregiving questionnaire for palliative care. Psychooncology. 2006;15(7):613-622.
26. Kristjanson LJ. Validity and reliability testing of the FAMCARE Scale: measuring family satisfaction with advanced cancer care. Soc Sci Med. 1993;36(5):693-701.
27. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385-396.
28. Keir ST, Guill AB, Carter KE, Boole LC, Gonzales L, Friedman HS. Differential levels of stress in caregivers of brain tumor patients--observations from a pilot study. Support Care Cancer. 2006;14(12):1258-1261.
29. Brehaut JC, O’Connor AM, Wood TJ, et al. Validation of a decision regret scale. Med Decis Making. 2003;23(4):281-292.
30. Teno JM, Clarridge B, Casey V, Edgman-Levitan S, Fowler J. Validation of toolkit after-death bereaved family member interview. J Pain Symptom Manage. 2001;22(3):752-758.
31. Wright AA, Zhang B, Ray A, Mack JW, Trice E, Balboni T, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665-1673.
32. Earle CC, Neville BA, Landrum MB, Ayanian JZ, Block SD, Weeks JC. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol. 2004;22(2):315-321.
33. Obermeyer Z, Makar M, Abujaber S, Dominici F, Block S, Cutler DM. Association between the Medicare hospice benefit and health care utilization and costs for patients with poor-prognosis cancer. JAMA. 2014;312(18):1888-1896.
Improving the delivery of end-of-life care for patients with advanced cancer has become a priority in the United States.1,2 Quality metrics identifying the components of high-quality end-of-life care have focused on improved symptom management, decreased use of chemotherapy at the end of life, fewer hospitalizations, and increased use of hospice care. Patients and caregivers also consider good communication with the medical team to be a critical component of end-of-life care.3-5 Interventions to improve the quality of end-of-life care are needed.
Caregivers of patients with advanced cancer who receive hospice services report better quality of care and death than those receiving end-of-life care in other settings.6-9 However, the transition for patients from active cancer therapy delivered by their oncologists to end-of-life care delivered by a hospice care team can be abrupt. Patients and their caregivers often feel abandoned by oncology clinicians because of the lack of continuity of care and poor communication.10-13 Caregivers who note continued involvement and communication with their oncology clinicians experience a lower caregiving burden, report higher satisfaction with care, and recount a higher quality of death for their loved one.14-16 Therefore, interventions that prevent abrupt transitions in care from oncology to hospice by ensuring continued communication with oncology clinicians are needed to improve the quality of end-of-life care.17 Recent findings have shown that providing concurrent oncology and palliative care is not only feasible but beneficial for patients with advanced cancer and their caregivers.18-24 However, there is no standard of care for the involvement of oncology clinicians in the care of patients receiving hospice services and their families.
Although interventions may be needed, it could be challenging to deliver them given the multiple demands of caregiving during hospice and the lack of regular contact in clinic. We sought to assess the feasibility of an intervention, Ensuring Communication in Hospice by Oncology (ECHO), to facilitate communication between oncology clinicians and caregivers of patients who enroll in hospice. We also explored caregiver-reported outcomes during hospice care, including satisfaction with care, attitudes toward caregiving, stress, decision regret, and perception of the quality of patients’ end-of-life care.
Methods
Study design
During March 2014-June 2015, caregivers of patients with advanced cancer who enrolled in home hospice services were eligible to participate in the study at Massachusetts General Hospital (MGH) in Boston. The Dana Farber/Harvard Cancer Center Institutional Review Board approved all methods and materials. The study opened with an enrollment goal of 30 participating caregivers. However, due to staff transitions, we closed the study early in June 2015 after 25 caregivers enrolled.
Participants
Caregivers of patients receiving care at the cancer center's thoracic, head and neck, sarcoma, melanoma, and gynecological disease centers were eligible within 10 days after a patient’s enrollment in hospice. Five disease sites were selected to participate in the intervention. We defined caregivers as relatives or friends serving as the primary caregiver of the patient at home during hospice care. Other caregiver eligibility criteria included the ability to read and respond to questions in English or with a translator, access to a telephone and/or computer to communicate with oncology clinicians, and willingness to complete questionnaires. Caregivers were ineligible if the patient was participating in an ongoing palliative care trial.
To identify eligible caregivers, case managers from both the inpatient and outpatient settings, as well as the nurses based in participating disease centers, notified the research team of all patients referred to hospice. If the patient had received oncology care in one of our participating disease centers, the research team contacted their oncology clinician/s (physicians, nurse practitioners [NP], registered nurses [RN], and/or physician assistants [PA]) to inquire if the patient had an involved caregiver and to obtain permission to offer study participation. If the oncology clinician/s did not grant permission, we documented the reason. Otherwise, with permission, research staff contacted the caregiver by telephone to offer study participation and obtain verbal consent. We then sent participating caregivers a copy of the informed consent by mail or e-mail.
Intervention
The ECHO intervention consisted of: supportive phone calls from an oncology clinician to the caregiver; an optional clinic visit with the oncology clinician for the patient to address clinical questions or concerns that was offered during the initial telephone consent; a bereavement call to the participating caregiver (Figure 1). Initially, we designed the intervention to have phone calls occurring twice weekly until the patient died. However, 3 months after starting the study, we received feedback from oncology clinicians and caregivers that calls were too frequent, so we amended the protocol to include phone calls twice weekly for the first 2 weeks of the study and then weekly thereafter. Seven months into the study, we again decreased the number of phone calls to weekly for the first 4 weeks, every other week for 4 weeks, and then monthly until patient death. We informed caregivers of changes by e-mail.
Before we started the study, we conducted training sessions with oncology clinicians from the participating disease centers to review study procedures and expectations of the phone calls. Supportive phone calls during hospice were not a part of standard practice prior to the study. The RN, NP or PA, and/or physician who had an established relationship with the patient and caregiver completed the phone calls. They decided based on their respective relationships with the patients and their workloads who would call each week, though the majority of calls were conducted by the RN or NP. All the clinicians had experience comanaging patients with hospice agencies, and our general practice is for the oncology physician to serve as the hospice attending of record. The calls were intended to offer support and reassurance to caregivers. We did not script the calls so that clinicians could tailor their content to the individual needs of the caregiver, as informed by their established relationship. The calls could include the patient if he/she was able to and interested in speaking to the clinician. There was no standardized communication with hospice as part of the intervention. If a caregiver raised concerns about symptom management during a call, the clinician would advise the caregiver to contact the hospice team directly or the clinician would call the hospice to discuss, depending on the clinical scenario and the clinician’s judgment. Research staff reminded oncology clinicians to call caregivers on the scheduled date and to document the discussion in the electronic medical record. The hospice phone number was included in the e-mail. If the call was not documented, research staff sent a reminder e-mail to the oncology clinicians 24 hours after the call was due.
Caregiver-reported measures
Caregivers completed a demographic questionnaire at baseline in which they reported their age, gender, race, ethnicity, religion, employment status, and relationship to the patient. We collected information about patient characteristics from the electronic medical record, including age, gender, and cancer type. In addition, we administered validated, self-report measures (see below). We limited the number of measures to decrease caregiver burden:
The baseline questionnaire included the FACQ-PC, the FAMCARE scale, the PSS, and the Decision Regret Scale. Initially, the study involved weekly questionnaires after baseline that included the FACQ-PC, the FAMCARE scale, and the PSS. However, after 3 months of study enrollment, we received feedback that the questionnaires were too frequent, so we amended the protocol and changed the frequency to weekly for 2 weeks, then monthly thereafter until the patient died.
Caregiver exit interview
Exit interviews included the toolkit interview, the Quality of End-of-Life Care scale, and the Decision Regret Scale. Caregivers also reported patients’ place and date of death. After the first 6 caregivers enrolled, we amended the exit interview to include open-ended feedback from caregivers. Specifically, we evaluated caregivers’ perceptions of the ECHO intervention by asking them about their perception of and satisfaction with the content and frequency of the oncology clinicians’ phone calls, whether they had an in-person visit with their oncology clinicians after the start of hospice care, whether the clinician/s contacted them after the patient died, and whether there were ways in which the clinician/s could help in the future.
Data collection and storage
Caregivers were given the option of completing study measures by telephone or e-mail so that they could complete them on a computer when it was convenient for them. Caregivers received a link to Research Electronic Data Capture (REDCap), a web-based, HIPAA-compliant application that allows participants to answer questionnaires online. The exit interviews were completed by phone, and research staff entered the data into the REDCap database. In addition, with we obtained caregiver permission to audiorecord the exit interviews, which were then transcribed and de-identified.
Statistical analysis
The primary outcome for the study was feasibility, which we defined as >70% of the caregivers receiving >50% of the phone calls from an oncology clinician, and >70% of the caregivers completing >50% of the questionnaires. All time points for the questionnaires and the exit interview counted toward feasibility. Exploratory endpoints included caregiver-reported satisfaction, stress, quality of end-of-life care, and decision-making regret.
Using STATA (v9.3; StataCorp, College Station, Texas) for all statistical analyses, we summarized participants’ characteristics and outcomes as frequencies and percentages for categorical variables and mean standard deviation for continuous variables. We used the repeated-measures t test to assess changes in caregiver outcomes over time. We used the Fisher exact test to compare clinically meaningful threshold scores of perceived stress between men and women.
We examined caregivers’ open-ended feedback using descriptive analyses to summarize comments about the intervention and to inform possible refinements for a future study.
Results
Baseline characteristics
During March 2014-June 2015, we enrolled caregivers of patients with advanced cancer from 5 participating disease centers: thoracic, head and neck, sarcoma, melanoma, and gynecological malignancy. We screened 123 patients to determine the eligibility of their caregivers (Figure 2). Of 38 eligible caregivers, 7 could not be reached, 6 declined participation, and 25 enrolled in the study (81% enrollment rate). Of the 25 caregivers who enrolled, 3 withdrew – 2 because the patients they were caring for died before the intervention began, and 1 who withdrew from the study because the family wanted less contact with the oncology clinician/s. Thus, we had data for 22 caregivers for our feasibility evaluation. One caregiver stopped study assessments after 3 months because the patient dis-enrolled from hospice. Median time from the patients’ hospice enrollment to caregiver study enrollment was 3 days (range, 1-9). Median time from study enrollment to patient death was 36 days (range, 2-135). Patients were receiving care from 10 different hospice agencies.
All of the patients had metastatic cancer, and 64% were women (Table 1).
Most of the caregivers were white (n = 18, 90%) and women (n = 12, 55%). The majority were the patient’s spouse (n = 12, 60%) or child (n = 6, 30%), and they lived with the patient (n = 16, 80%). Many of the caregivers had other responsibilities in addition to caring for the patients, including part- or full-time work (n = 10, 50%) or caring for others in addition to the patient (n = 10, 50%).
Feasibility
Over the study period, oncology clinicians completed 164 of 180 possible phone calls (91%). All 22 caregivers received >50% of the phone calls. Caregivers completed 78 of 99 possible questionnaires (79%), and 16 of 22 completed >50% of the questionnaires (73%). None of the caregivers/patients wanted to schedule the optional visit with an oncology clinician that was offered as part of the intervention; however, 5 patients had a clinic visit after hospice enrollment. In addition, 2 oncologists visited a patient and caregiver at home. All caregivers received bereavement contact from the oncology team.
Caregiver-reported outcomes
In all, 20 of the 22 enrolled caregivers completed baseline measures (Table 2), and they all chose to complete questionnaires by e-mail. Caregivers’ attitudes toward caregiving and satisfaction with hospice services were overall positive. They reported high mean scores on the 2 domains on the FACQ-PC of positive caregiver appraisal (mean, 4.25; SD, 0.52) and family well-being (mean, 4.09; SD, 0.45). The majority of caregivers (75%-95%) reported they were satisfied or very satisfied with various dimensions of hospice care based on the FAMCARE questionnaire.
Overall, caregivers reported moderate levels of stress (mean, 13.55; SD, 6.08) based on the PSS scores. Of the 20 caregivers who completed the baseline measures, 8 had clinically meaningful stress, and stress was numerically higher in female caregivers than in their male counterparts, but it did not reach statistical significance (55% vs 22%, P = .197). Finally, at baseline, caregivers indicated relatively low levels of decisional regret about enrolling in hospice, although there was considerable variation (mean, 10.25; SD, 14.37).
We conducted exit interviews with 15 caregivers because we were not able to reach 6 of the original 21 (Table 2). Caregivers rated hospice services highly for communication, symptom control, emotional support, and overall care. They also rated quality of death highly (mean, 8.46; SD 1.13). Regret was lower at the end of the study, but this did not reach statistical significance (baseline mean 9.29; end of study mean 3.57; P = .161).
In the recorded exit interviews, all of the caregivers responded they were satisfied with the phone calls. Two caregivers commented that they would have preferred the calls were more scheduled or at more suitable times. Overall, they described the phone calls as excellent, supportive, responsive, comfortable, and appreciated. All caregivers reported contact with the oncology team after the patient died, but one caregiver was disappointed she was only contacted by the nurse practitioner and not the oncologist. Participants did not feel as if there were other ways that the oncology team could have been helpful for them while their loved one was in hospice.
Table 3 highlights other representative comments from the exit interviews.
Discussion
As far as we know, this is the first study to assess the feasibility of an intervention to facilitate communication between oncology clinicians and caregivers of patients with advanced cancer who are receiving hospice care. Despite the challenges of oncology clinicians delivering an intervention to caregivers during hospice, we found this intervention was feasible and acceptable. Although the transition to hospice can be stressful, caregivers reported high satisfaction with hospice care and the quality of the patient’s death. In exit interviews, they also reported high satisfaction with the intervention and appreciation for maintaining their relationship with the oncology team. It is worth noting that no caregiver requested the optional clinic visit after hospice enrollment, and most caregivers were not seen again in clinic after hospice enrollment.
These results suggest that a simple, telephone-based intervention of scheduled calls from the oncology team at prompted intervals is not only feasible, but may also help foster continuity between the patient and caregiver and the oncology team. We received feedback from both oncology clinicians and caregivers that the initial call frequency was too often, suggesting that communication may not need to be very frequent to maintain continuity and provide support. This also suggests that if the calls are too frequent, they may be more intrusive than helpful for both oncologists and caregivers. Alternatively, caregiver suggestion for fewer phone calls may indicate that concerns about abandonment are less prevalent than existing literature has suggested.
Limitations
Our study has several important limitations. The sample size was small as this was a feasibility study conducted at a single tertiary care hospital, and the population was 90% white and 95% college educated, which may limit the generalizability of our results. In addition, the median length of stay in hospice for patients on this study was 36 days, which is long compared with national averages,32,33 and thus the outcomes may not represent the experience of a more heterogeneous population. The longer length of stay in hospice may have contributed to caregivers’ high satisfaction with the quality of end-of-life care.
Oncologists did not grant permission for the study team to approach all eligible caregivers, which may have introduced selection bias. We were also not able to reach 6 participants for exit interviews. People less satisfied with the intervention or with hospice may be more likely to have missing data, which could introduce bias into the satisfaction ratings. Furthermore, we did not explore the oncology clinicians’ perspective of the intervention or assess the time commitment of the calls. Oncology clinicians have many competing responsibilities and have variable experience and comfort with hospice care. Therefore, future studies should explore the perspective of oncology clinicians in regard to the intervention.
Finally, we did not require communication with the hospice agency as part of the intervention as there were ten different hospices involved. Thus, we do not know how the intervention impacted the hospice team’s care of the patient. However, based upon the success of this pilot study, future larger studies should explore the impact of the intervention from the perspective of the hospice care team and include oncology clinician communication with the hospice agency.
Conclusion
These findings demonstrate the feasibility and acceptability of an intervention to enhance communication between oncology clinicians and caregivers of patients with advanced cancer receiving hospice care. Importantly, the high caregiver satisfaction with the intervention in this study suggests that maintaining communication with the primary oncology team during hospice care may be an important component of high quality end-of-life care, though the desire for decreased calls suggests that this communication need not be frequent to maintain the continuity. A randomized study with a larger and more diverse patient/caregiver sample would allow us to explore the impact of the intervention on caregiver feelings of abandonment by the oncology team and short- and long-term caregiver outcomes, as well as to understand the perspective of the oncology and hospice clinicians involved.
Improving the delivery of end-of-life care for patients with advanced cancer has become a priority in the United States.1,2 Quality metrics identifying the components of high-quality end-of-life care have focused on improved symptom management, decreased use of chemotherapy at the end of life, fewer hospitalizations, and increased use of hospice care. Patients and caregivers also consider good communication with the medical team to be a critical component of end-of-life care.3-5 Interventions to improve the quality of end-of-life care are needed.
Caregivers of patients with advanced cancer who receive hospice services report better quality of care and death than those receiving end-of-life care in other settings.6-9 However, the transition for patients from active cancer therapy delivered by their oncologists to end-of-life care delivered by a hospice care team can be abrupt. Patients and their caregivers often feel abandoned by oncology clinicians because of the lack of continuity of care and poor communication.10-13 Caregivers who note continued involvement and communication with their oncology clinicians experience a lower caregiving burden, report higher satisfaction with care, and recount a higher quality of death for their loved one.14-16 Therefore, interventions that prevent abrupt transitions in care from oncology to hospice by ensuring continued communication with oncology clinicians are needed to improve the quality of end-of-life care.17 Recent findings have shown that providing concurrent oncology and palliative care is not only feasible but beneficial for patients with advanced cancer and their caregivers.18-24 However, there is no standard of care for the involvement of oncology clinicians in the care of patients receiving hospice services and their families.
Although interventions may be needed, it could be challenging to deliver them given the multiple demands of caregiving during hospice and the lack of regular contact in clinic. We sought to assess the feasibility of an intervention, Ensuring Communication in Hospice by Oncology (ECHO), to facilitate communication between oncology clinicians and caregivers of patients who enroll in hospice. We also explored caregiver-reported outcomes during hospice care, including satisfaction with care, attitudes toward caregiving, stress, decision regret, and perception of the quality of patients’ end-of-life care.
Methods
Study design
During March 2014-June 2015, caregivers of patients with advanced cancer who enrolled in home hospice services were eligible to participate in the study at Massachusetts General Hospital (MGH) in Boston. The Dana Farber/Harvard Cancer Center Institutional Review Board approved all methods and materials. The study opened with an enrollment goal of 30 participating caregivers. However, due to staff transitions, we closed the study early in June 2015 after 25 caregivers enrolled.
Participants
Caregivers of patients receiving care at the cancer center's thoracic, head and neck, sarcoma, melanoma, and gynecological disease centers were eligible within 10 days after a patient’s enrollment in hospice. Five disease sites were selected to participate in the intervention. We defined caregivers as relatives or friends serving as the primary caregiver of the patient at home during hospice care. Other caregiver eligibility criteria included the ability to read and respond to questions in English or with a translator, access to a telephone and/or computer to communicate with oncology clinicians, and willingness to complete questionnaires. Caregivers were ineligible if the patient was participating in an ongoing palliative care trial.
To identify eligible caregivers, case managers from both the inpatient and outpatient settings, as well as the nurses based in participating disease centers, notified the research team of all patients referred to hospice. If the patient had received oncology care in one of our participating disease centers, the research team contacted their oncology clinician/s (physicians, nurse practitioners [NP], registered nurses [RN], and/or physician assistants [PA]) to inquire if the patient had an involved caregiver and to obtain permission to offer study participation. If the oncology clinician/s did not grant permission, we documented the reason. Otherwise, with permission, research staff contacted the caregiver by telephone to offer study participation and obtain verbal consent. We then sent participating caregivers a copy of the informed consent by mail or e-mail.
Intervention
The ECHO intervention consisted of: supportive phone calls from an oncology clinician to the caregiver; an optional clinic visit with the oncology clinician for the patient to address clinical questions or concerns that was offered during the initial telephone consent; a bereavement call to the participating caregiver (Figure 1). Initially, we designed the intervention to have phone calls occurring twice weekly until the patient died. However, 3 months after starting the study, we received feedback from oncology clinicians and caregivers that calls were too frequent, so we amended the protocol to include phone calls twice weekly for the first 2 weeks of the study and then weekly thereafter. Seven months into the study, we again decreased the number of phone calls to weekly for the first 4 weeks, every other week for 4 weeks, and then monthly until patient death. We informed caregivers of changes by e-mail.
Before we started the study, we conducted training sessions with oncology clinicians from the participating disease centers to review study procedures and expectations of the phone calls. Supportive phone calls during hospice were not a part of standard practice prior to the study. The RN, NP or PA, and/or physician who had an established relationship with the patient and caregiver completed the phone calls. They decided based on their respective relationships with the patients and their workloads who would call each week, though the majority of calls were conducted by the RN or NP. All the clinicians had experience comanaging patients with hospice agencies, and our general practice is for the oncology physician to serve as the hospice attending of record. The calls were intended to offer support and reassurance to caregivers. We did not script the calls so that clinicians could tailor their content to the individual needs of the caregiver, as informed by their established relationship. The calls could include the patient if he/she was able to and interested in speaking to the clinician. There was no standardized communication with hospice as part of the intervention. If a caregiver raised concerns about symptom management during a call, the clinician would advise the caregiver to contact the hospice team directly or the clinician would call the hospice to discuss, depending on the clinical scenario and the clinician’s judgment. Research staff reminded oncology clinicians to call caregivers on the scheduled date and to document the discussion in the electronic medical record. The hospice phone number was included in the e-mail. If the call was not documented, research staff sent a reminder e-mail to the oncology clinicians 24 hours after the call was due.
Caregiver-reported measures
Caregivers completed a demographic questionnaire at baseline in which they reported their age, gender, race, ethnicity, religion, employment status, and relationship to the patient. We collected information about patient characteristics from the electronic medical record, including age, gender, and cancer type. In addition, we administered validated, self-report measures (see below). We limited the number of measures to decrease caregiver burden:
The baseline questionnaire included the FACQ-PC, the FAMCARE scale, the PSS, and the Decision Regret Scale. Initially, the study involved weekly questionnaires after baseline that included the FACQ-PC, the FAMCARE scale, and the PSS. However, after 3 months of study enrollment, we received feedback that the questionnaires were too frequent, so we amended the protocol and changed the frequency to weekly for 2 weeks, then monthly thereafter until the patient died.
Caregiver exit interview
Exit interviews included the toolkit interview, the Quality of End-of-Life Care scale, and the Decision Regret Scale. Caregivers also reported patients’ place and date of death. After the first 6 caregivers enrolled, we amended the exit interview to include open-ended feedback from caregivers. Specifically, we evaluated caregivers’ perceptions of the ECHO intervention by asking them about their perception of and satisfaction with the content and frequency of the oncology clinicians’ phone calls, whether they had an in-person visit with their oncology clinicians after the start of hospice care, whether the clinician/s contacted them after the patient died, and whether there were ways in which the clinician/s could help in the future.
Data collection and storage
Caregivers were given the option of completing study measures by telephone or e-mail so that they could complete them on a computer when it was convenient for them. Caregivers received a link to Research Electronic Data Capture (REDCap), a web-based, HIPAA-compliant application that allows participants to answer questionnaires online. The exit interviews were completed by phone, and research staff entered the data into the REDCap database. In addition, with we obtained caregiver permission to audiorecord the exit interviews, which were then transcribed and de-identified.
Statistical analysis
The primary outcome for the study was feasibility, which we defined as >70% of the caregivers receiving >50% of the phone calls from an oncology clinician, and >70% of the caregivers completing >50% of the questionnaires. All time points for the questionnaires and the exit interview counted toward feasibility. Exploratory endpoints included caregiver-reported satisfaction, stress, quality of end-of-life care, and decision-making regret.
Using STATA (v9.3; StataCorp, College Station, Texas) for all statistical analyses, we summarized participants’ characteristics and outcomes as frequencies and percentages for categorical variables and mean standard deviation for continuous variables. We used the repeated-measures t test to assess changes in caregiver outcomes over time. We used the Fisher exact test to compare clinically meaningful threshold scores of perceived stress between men and women.
We examined caregivers’ open-ended feedback using descriptive analyses to summarize comments about the intervention and to inform possible refinements for a future study.
Results
Baseline characteristics
During March 2014-June 2015, we enrolled caregivers of patients with advanced cancer from 5 participating disease centers: thoracic, head and neck, sarcoma, melanoma, and gynecological malignancy. We screened 123 patients to determine the eligibility of their caregivers (Figure 2). Of 38 eligible caregivers, 7 could not be reached, 6 declined participation, and 25 enrolled in the study (81% enrollment rate). Of the 25 caregivers who enrolled, 3 withdrew – 2 because the patients they were caring for died before the intervention began, and 1 who withdrew from the study because the family wanted less contact with the oncology clinician/s. Thus, we had data for 22 caregivers for our feasibility evaluation. One caregiver stopped study assessments after 3 months because the patient dis-enrolled from hospice. Median time from the patients’ hospice enrollment to caregiver study enrollment was 3 days (range, 1-9). Median time from study enrollment to patient death was 36 days (range, 2-135). Patients were receiving care from 10 different hospice agencies.
All of the patients had metastatic cancer, and 64% were women (Table 1).
Most of the caregivers were white (n = 18, 90%) and women (n = 12, 55%). The majority were the patient’s spouse (n = 12, 60%) or child (n = 6, 30%), and they lived with the patient (n = 16, 80%). Many of the caregivers had other responsibilities in addition to caring for the patients, including part- or full-time work (n = 10, 50%) or caring for others in addition to the patient (n = 10, 50%).
Feasibility
Over the study period, oncology clinicians completed 164 of 180 possible phone calls (91%). All 22 caregivers received >50% of the phone calls. Caregivers completed 78 of 99 possible questionnaires (79%), and 16 of 22 completed >50% of the questionnaires (73%). None of the caregivers/patients wanted to schedule the optional visit with an oncology clinician that was offered as part of the intervention; however, 5 patients had a clinic visit after hospice enrollment. In addition, 2 oncologists visited a patient and caregiver at home. All caregivers received bereavement contact from the oncology team.
Caregiver-reported outcomes
In all, 20 of the 22 enrolled caregivers completed baseline measures (Table 2), and they all chose to complete questionnaires by e-mail. Caregivers’ attitudes toward caregiving and satisfaction with hospice services were overall positive. They reported high mean scores on the 2 domains on the FACQ-PC of positive caregiver appraisal (mean, 4.25; SD, 0.52) and family well-being (mean, 4.09; SD, 0.45). The majority of caregivers (75%-95%) reported they were satisfied or very satisfied with various dimensions of hospice care based on the FAMCARE questionnaire.
Overall, caregivers reported moderate levels of stress (mean, 13.55; SD, 6.08) based on the PSS scores. Of the 20 caregivers who completed the baseline measures, 8 had clinically meaningful stress, and stress was numerically higher in female caregivers than in their male counterparts, but it did not reach statistical significance (55% vs 22%, P = .197). Finally, at baseline, caregivers indicated relatively low levels of decisional regret about enrolling in hospice, although there was considerable variation (mean, 10.25; SD, 14.37).
We conducted exit interviews with 15 caregivers because we were not able to reach 6 of the original 21 (Table 2). Caregivers rated hospice services highly for communication, symptom control, emotional support, and overall care. They also rated quality of death highly (mean, 8.46; SD 1.13). Regret was lower at the end of the study, but this did not reach statistical significance (baseline mean 9.29; end of study mean 3.57; P = .161).
In the recorded exit interviews, all of the caregivers responded they were satisfied with the phone calls. Two caregivers commented that they would have preferred the calls were more scheduled or at more suitable times. Overall, they described the phone calls as excellent, supportive, responsive, comfortable, and appreciated. All caregivers reported contact with the oncology team after the patient died, but one caregiver was disappointed she was only contacted by the nurse practitioner and not the oncologist. Participants did not feel as if there were other ways that the oncology team could have been helpful for them while their loved one was in hospice.
Table 3 highlights other representative comments from the exit interviews.
Discussion
As far as we know, this is the first study to assess the feasibility of an intervention to facilitate communication between oncology clinicians and caregivers of patients with advanced cancer who are receiving hospice care. Despite the challenges of oncology clinicians delivering an intervention to caregivers during hospice, we found this intervention was feasible and acceptable. Although the transition to hospice can be stressful, caregivers reported high satisfaction with hospice care and the quality of the patient’s death. In exit interviews, they also reported high satisfaction with the intervention and appreciation for maintaining their relationship with the oncology team. It is worth noting that no caregiver requested the optional clinic visit after hospice enrollment, and most caregivers were not seen again in clinic after hospice enrollment.
These results suggest that a simple, telephone-based intervention of scheduled calls from the oncology team at prompted intervals is not only feasible, but may also help foster continuity between the patient and caregiver and the oncology team. We received feedback from both oncology clinicians and caregivers that the initial call frequency was too often, suggesting that communication may not need to be very frequent to maintain continuity and provide support. This also suggests that if the calls are too frequent, they may be more intrusive than helpful for both oncologists and caregivers. Alternatively, caregiver suggestion for fewer phone calls may indicate that concerns about abandonment are less prevalent than existing literature has suggested.
Limitations
Our study has several important limitations. The sample size was small as this was a feasibility study conducted at a single tertiary care hospital, and the population was 90% white and 95% college educated, which may limit the generalizability of our results. In addition, the median length of stay in hospice for patients on this study was 36 days, which is long compared with national averages,32,33 and thus the outcomes may not represent the experience of a more heterogeneous population. The longer length of stay in hospice may have contributed to caregivers’ high satisfaction with the quality of end-of-life care.
Oncologists did not grant permission for the study team to approach all eligible caregivers, which may have introduced selection bias. We were also not able to reach 6 participants for exit interviews. People less satisfied with the intervention or with hospice may be more likely to have missing data, which could introduce bias into the satisfaction ratings. Furthermore, we did not explore the oncology clinicians’ perspective of the intervention or assess the time commitment of the calls. Oncology clinicians have many competing responsibilities and have variable experience and comfort with hospice care. Therefore, future studies should explore the perspective of oncology clinicians in regard to the intervention.
Finally, we did not require communication with the hospice agency as part of the intervention as there were ten different hospices involved. Thus, we do not know how the intervention impacted the hospice team’s care of the patient. However, based upon the success of this pilot study, future larger studies should explore the impact of the intervention from the perspective of the hospice care team and include oncology clinician communication with the hospice agency.
Conclusion
These findings demonstrate the feasibility and acceptability of an intervention to enhance communication between oncology clinicians and caregivers of patients with advanced cancer receiving hospice care. Importantly, the high caregiver satisfaction with the intervention in this study suggests that maintaining communication with the primary oncology team during hospice care may be an important component of high quality end-of-life care, though the desire for decreased calls suggests that this communication need not be frequent to maintain the continuity. A randomized study with a larger and more diverse patient/caregiver sample would allow us to explore the impact of the intervention on caregiver feelings of abandonment by the oncology team and short- and long-term caregiver outcomes, as well as to understand the perspective of the oncology and hospice clinicians involved.
1. Institute of Medicine. 2015. Dying in america: improving quality and honoring individual preferences near the end of life. Washington, DC: The National Academies Press. https://doi.org/10.17226/18748.
2. Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(1):96-112.
3. Wenrich MD, Curtis JR, Shannon SE, Carline JD, Ambrozy DM, Ramsey PG. Communicating with dying patients within the spectrum of medical care from terminal diagnosis to death. Arch Intern Med. 2001;161(6):868-874.
4. Parker SM, Clayton JM, Hancock K, et al. A systematic review of prognostic/end-of-life communication with adults in the advanced stages of a life-limiting illness: patient/caregiver preferences for the content, style, and timing of information. J Pain Symptom Manage. 2007;34(1):81-93.
5. Steinhauser KE, Clipp EC, McNeilly M, Christakis NA, McIntyre LM, Tulsky JA. In search of a good death: observations of patients, families, and providers. Ann Intern Med. 2000;132(10):825-832.
6. Teno JM, Clarridge BR, Casey V, et al. Family perspectives on end-of-life care at the last place of care. JAMA. 2004;291(1):88-93.
7. Kumar P, Wright AA, Hatfield LA, Temel JS, Keating NL. Family perspectives on hospice care experiences of patients with cancer. J Clin Oncol. 2017;35(4):432-439.
8. Wright AA, Keating NL, Ayanian JZ, et al. Family perspectives on aggressive cancer care near the end of life. JAMA. 2016;315(3):284-292.
9. Duggan KT, Hildebrand Duffus S, D’Agostino RB Jr, Petty WJ, Streer NP, Stephenson RC. The impact of hospice services in the care of patients with advanced stage nonsmall cell lung cancer. J Palliat Med. 2017;20(1):29-34.
10. Curtis JR, Wenrich MD, Carline JD, Shannon SE, Ambrozy DM, Ramsey PG. Understanding physicians’ skills at providing end-of-life care perspectives of patients, families, and health care workers. J Gen Intern Med. 2001;16(1):41-49.
11. Back AL, Young JP, McCown E, et al. Abandonment at the end of life from patient, caregiver, nurse, and physician perspectives: loss of continuity and lack of closure. Arch Intern Med. 2009;169(5):474-479.
12. Vig EK, Starks H, Taylor JS, Hopley EK, Fryer-Edwards K. Why don’t patients enroll in hospice? Can we do anything about it? J Gen Intern Med. 2010;25(10):1009-1019.
13. Waldrop DP, Meeker MA, Kerr C, Skretny J, Tangeman J, Milch R. The nature and timing of family-provider communication in late-stage cancer: a qualitative study of caregivers’ experiences. J Pain Symptom Manage. 2012;43(2):182-194.
14. Emanuel EJ, Fairclough DL, Slutsman J, Emanuel LL. Understanding economic and other burdens of terminal illness: the experience of patients and their caregivers. Ann Intern Med. 2000;132(6):451-459.
15. Curtis JR, Patrick DL, Engelberg RA, Norris K, Asp C, Byock I. A measure of the quality of dying and death. Initial validation using after-death interviews with family members. J Pain Symptom Manage. 2002;24(1):17-31.
16. Fleming DA, Sheppard VB, Mangan PA, et al. Caregiving at the end of life: Perceptions of health care quality and quality of life among patients and caregivers. J Pain Symptom Manage. 2006;31(5):407-420.
17. McMillan SC. Interventions to facilitate family caregiving at the end of life. J Palliat Med. 2005;8(Suppl 1):S132-S139.
18. Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302(7):741-749.
19. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733-742.
20. Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet. 2014;383(9930):1721-1730.
21. Maltoni M, Scarpi E, Dall’Agata M, et al. Systematic versus on-demand early palliative care: a randomised clinical trial assessing quality of care and treatment aggressiveness near the end of life. Eur J Cancer. 2016;69:110-118.
22. El-Jawahri A ea. Early integrated palliative care to improve family caregivers (FC) outcomes for patients with gastrointestinal and lung cancer. J Clin Oncol. 2016;34(suppl; abstr 10131).
23. Temel JS, Greer JA, El-Jawahri A, et al. Effects of early integrated palliative care in patients with lung and gi cancer: a randomized clinical trial. J Clin Oncol. 2017;35(8):834-841.
24. El-Jawahri A, LeBlanc T, VanDusen H, et al. Effect of inpatient palliative care on quality of life 2 weeks after hematopoietic stem cell transplantation: a randomized clinical trial. JAMA. 2016;316(20):2094-2103.
25. Cooper B, Kinsella GJ, Picton C. Development and initial validation of a family appraisal of caregiving questionnaire for palliative care. Psychooncology. 2006;15(7):613-622.
26. Kristjanson LJ. Validity and reliability testing of the FAMCARE Scale: measuring family satisfaction with advanced cancer care. Soc Sci Med. 1993;36(5):693-701.
27. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385-396.
28. Keir ST, Guill AB, Carter KE, Boole LC, Gonzales L, Friedman HS. Differential levels of stress in caregivers of brain tumor patients--observations from a pilot study. Support Care Cancer. 2006;14(12):1258-1261.
29. Brehaut JC, O’Connor AM, Wood TJ, et al. Validation of a decision regret scale. Med Decis Making. 2003;23(4):281-292.
30. Teno JM, Clarridge B, Casey V, Edgman-Levitan S, Fowler J. Validation of toolkit after-death bereaved family member interview. J Pain Symptom Manage. 2001;22(3):752-758.
31. Wright AA, Zhang B, Ray A, Mack JW, Trice E, Balboni T, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665-1673.
32. Earle CC, Neville BA, Landrum MB, Ayanian JZ, Block SD, Weeks JC. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol. 2004;22(2):315-321.
33. Obermeyer Z, Makar M, Abujaber S, Dominici F, Block S, Cutler DM. Association between the Medicare hospice benefit and health care utilization and costs for patients with poor-prognosis cancer. JAMA. 2014;312(18):1888-1896.
1. Institute of Medicine. 2015. Dying in america: improving quality and honoring individual preferences near the end of life. Washington, DC: The National Academies Press. https://doi.org/10.17226/18748.
2. Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(1):96-112.
3. Wenrich MD, Curtis JR, Shannon SE, Carline JD, Ambrozy DM, Ramsey PG. Communicating with dying patients within the spectrum of medical care from terminal diagnosis to death. Arch Intern Med. 2001;161(6):868-874.
4. Parker SM, Clayton JM, Hancock K, et al. A systematic review of prognostic/end-of-life communication with adults in the advanced stages of a life-limiting illness: patient/caregiver preferences for the content, style, and timing of information. J Pain Symptom Manage. 2007;34(1):81-93.
5. Steinhauser KE, Clipp EC, McNeilly M, Christakis NA, McIntyre LM, Tulsky JA. In search of a good death: observations of patients, families, and providers. Ann Intern Med. 2000;132(10):825-832.
6. Teno JM, Clarridge BR, Casey V, et al. Family perspectives on end-of-life care at the last place of care. JAMA. 2004;291(1):88-93.
7. Kumar P, Wright AA, Hatfield LA, Temel JS, Keating NL. Family perspectives on hospice care experiences of patients with cancer. J Clin Oncol. 2017;35(4):432-439.
8. Wright AA, Keating NL, Ayanian JZ, et al. Family perspectives on aggressive cancer care near the end of life. JAMA. 2016;315(3):284-292.
9. Duggan KT, Hildebrand Duffus S, D’Agostino RB Jr, Petty WJ, Streer NP, Stephenson RC. The impact of hospice services in the care of patients with advanced stage nonsmall cell lung cancer. J Palliat Med. 2017;20(1):29-34.
10. Curtis JR, Wenrich MD, Carline JD, Shannon SE, Ambrozy DM, Ramsey PG. Understanding physicians’ skills at providing end-of-life care perspectives of patients, families, and health care workers. J Gen Intern Med. 2001;16(1):41-49.
11. Back AL, Young JP, McCown E, et al. Abandonment at the end of life from patient, caregiver, nurse, and physician perspectives: loss of continuity and lack of closure. Arch Intern Med. 2009;169(5):474-479.
12. Vig EK, Starks H, Taylor JS, Hopley EK, Fryer-Edwards K. Why don’t patients enroll in hospice? Can we do anything about it? J Gen Intern Med. 2010;25(10):1009-1019.
13. Waldrop DP, Meeker MA, Kerr C, Skretny J, Tangeman J, Milch R. The nature and timing of family-provider communication in late-stage cancer: a qualitative study of caregivers’ experiences. J Pain Symptom Manage. 2012;43(2):182-194.
14. Emanuel EJ, Fairclough DL, Slutsman J, Emanuel LL. Understanding economic and other burdens of terminal illness: the experience of patients and their caregivers. Ann Intern Med. 2000;132(6):451-459.
15. Curtis JR, Patrick DL, Engelberg RA, Norris K, Asp C, Byock I. A measure of the quality of dying and death. Initial validation using after-death interviews with family members. J Pain Symptom Manage. 2002;24(1):17-31.
16. Fleming DA, Sheppard VB, Mangan PA, et al. Caregiving at the end of life: Perceptions of health care quality and quality of life among patients and caregivers. J Pain Symptom Manage. 2006;31(5):407-420.
17. McMillan SC. Interventions to facilitate family caregiving at the end of life. J Palliat Med. 2005;8(Suppl 1):S132-S139.
18. Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302(7):741-749.
19. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733-742.
20. Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet. 2014;383(9930):1721-1730.
21. Maltoni M, Scarpi E, Dall’Agata M, et al. Systematic versus on-demand early palliative care: a randomised clinical trial assessing quality of care and treatment aggressiveness near the end of life. Eur J Cancer. 2016;69:110-118.
22. El-Jawahri A ea. Early integrated palliative care to improve family caregivers (FC) outcomes for patients with gastrointestinal and lung cancer. J Clin Oncol. 2016;34(suppl; abstr 10131).
23. Temel JS, Greer JA, El-Jawahri A, et al. Effects of early integrated palliative care in patients with lung and gi cancer: a randomized clinical trial. J Clin Oncol. 2017;35(8):834-841.
24. El-Jawahri A, LeBlanc T, VanDusen H, et al. Effect of inpatient palliative care on quality of life 2 weeks after hematopoietic stem cell transplantation: a randomized clinical trial. JAMA. 2016;316(20):2094-2103.
25. Cooper B, Kinsella GJ, Picton C. Development and initial validation of a family appraisal of caregiving questionnaire for palliative care. Psychooncology. 2006;15(7):613-622.
26. Kristjanson LJ. Validity and reliability testing of the FAMCARE Scale: measuring family satisfaction with advanced cancer care. Soc Sci Med. 1993;36(5):693-701.
27. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385-396.
28. Keir ST, Guill AB, Carter KE, Boole LC, Gonzales L, Friedman HS. Differential levels of stress in caregivers of brain tumor patients--observations from a pilot study. Support Care Cancer. 2006;14(12):1258-1261.
29. Brehaut JC, O’Connor AM, Wood TJ, et al. Validation of a decision regret scale. Med Decis Making. 2003;23(4):281-292.
30. Teno JM, Clarridge B, Casey V, Edgman-Levitan S, Fowler J. Validation of toolkit after-death bereaved family member interview. J Pain Symptom Manage. 2001;22(3):752-758.
31. Wright AA, Zhang B, Ray A, Mack JW, Trice E, Balboni T, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665-1673.
32. Earle CC, Neville BA, Landrum MB, Ayanian JZ, Block SD, Weeks JC. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol. 2004;22(2):315-321.
33. Obermeyer Z, Makar M, Abujaber S, Dominici F, Block S, Cutler DM. Association between the Medicare hospice benefit and health care utilization and costs for patients with poor-prognosis cancer. JAMA. 2014;312(18):1888-1896.
Integrating survivorship care planning in radiation oncology workflow
In January 2016 there were an estimated 15.5 million people in the United States who were living with a cancer diagnosis, representing 4.8% of the population. That number is expected to increase to 20.3 million by 2026.1 The 5-year relative survival rate for all cancers diagnosed during 2005 to 2011 was 69%.2 As more individuals with a cancer diagnosis now live longer, cancer survivorship is receiving increased attention. A report from the Institute of Medicine3 identified the essential components of survivorship care, including the provision of a survivorship care plan (SCP) containing specific diagnosis, treatment, and follow-up information (Table 1). To maintain accreditation in their respective organizations, the American College of Surgeons’ Commission on Cancer and the National Accreditation Program for Breast Centers (NAPBC) have included standards on providing treatment summaries and SCPs in person to those patients who have completed cancer treatments given with curative intent.4,5
SCPs are personalized documents presented to cancer patients at the end of treatment that summarize key aspects of cancer treatment and recommend appropriate ongoing medical care and self-management. The purpose of the SCP is both to educate cancer survivors and to create a portable document that can be shared with primary care providers to facilitate coordinated care.6 There are multiple barriers to SCP implementation, which may include the time required to create an SCP, inadequate reimbursement for the time spent creating and delivering the plan, a lack of risk-stratified guidelines for coordinated care, and the incomplete automation of diagnosis and treatment summarization by the electronic health record (EHR).7
Survivorship care in radiation oncology
The American College of Radiology includes the recommendation for regular, ongoing follow-up in the standards for accreditation for radiation oncology practice.8 Radiation oncology practices often provide the initial follow-up appointment about a month after the prescribed radiation treatment has been completed. The twofold purpose of this appointment is to assess the response to treatment and to evaluate acute treatment-related effects.9 The appointment may include a skin evaluation, assessment for any acute treatment effects, informal counseling on maintaining a healthy lifestyle, and recommendations for posttreatment care and follow-up. The appointment may also be an opportune time for delivering the SCP because radiation therapy is often the final treatment modality in active therapy for breast cancer patients.
A review of the literature yields scant data on the incorporation of SCPs into a radiation oncology practice. A 2014 survey of members of the American Society of Radiation Oncology10for a response percentage of 14.7%. Almost all providers follow their patients after treatment (97% (n = 574 respondents/3987 total membership, 14.4% response rate) showed that although most radiation oncologists provide long-term follow-up care to their patients after treatment completion (97%), fewer than half of those surveyed indicated that they delivered SCPs for curative-intent patients (40%), and even fewer delivered for palliative-intent patients (19%). Standards for the American Society for Radiation Oncology’s Accreditation Program for Excellence11 outline content for end-of-treatment documentation. Typically, the documentation includes a detailed treatment summary prepared by the treating radiation oncologist. This treatment summary includes the patient’s diagnosis, the area treated, radiation doses received, number of fractions delivered, therapy start date, therapy completion date, and overall tolerance of treatment in a clinical summary. The treatment summary is communicated to other providers involved in the patient’s care to promote care coordination, but it is not typically provided to patients.
Development of University of Wisconsin survivorship care planning
As an important component of maintaining NAPBC accreditation, the University of Wisconsin (UW) Health Breast Center began the process of formalizing and optimizing SCPs for breast cancer survivors who are followed at the center. Multidisciplinary input from surgical, medical, and radiation oncology was obtained. Representatives from those disciplines met regularly to reach consensus on the treatment summary and SCP content. The following 3 documents were created for use during a transition visit at the end of treatment: the written individualized SCP to be provided to the survivor and his/her primary care providers, a general survivorship patient education booklet, and a patient questionnaire to identify survivors’ concerns and additional resources that may be beneficial.
Treatment summary
Working in collaboration with IT specialists, we enabled out-of-the-box functionality within our EHR. This cancer-specific functionality provides a central and standard location within each survivor’s problem list to systematically document information regarding cancer diagnosis, stage, and treatment associated with a specific cancer diagnosis. Each treating provider (surgeon, medical oncologist, radiation oncologist, genetic counselor, etc) is responsible for entering and updating the relevant components within the treatment summary (ie, the surgeon enters and maintains the surgical details, the medical oncologist does likewise for chemotherapy and other medical therapies, etc). Information is updated and current, creating a dynamic documentation of diagnosis and treatment that can be used in clinic notes, patient after-visit summaries, and SCPs.
Survivorship care plan
This same EHR functionality is leveraged to generate, populate, and maintain the individualized SCP for each breast cancer survivor. The Treatment Summary section of the SCP can be quickly prepared within the EHR by autopopulating data previously entered by treating providers. Content and language for SCP templates in breast, colorectal, prostate, and gynecologic cancers are in use at the time of publication. The templates are developed as a collaborative effort between oncology subspecialists, with input from the UW Health survivor and family advocacy councils.
Each template contains a Treatment Summary section and an SCP section. The Treatment Summary section includes survivor general information, diagnosis and treatment information, and the clinical and supportive/survivor care team names and contact information. The SCP section includes follow-up recommendations, signs of recurrence and/or symptoms to report, healthy lifestyle and maintenance, chronic or late effects of specific treatment if applicable (eg, surgery, chemotherapy by drug, radiation therapy, and endocrine therapy), and general resources for common psychosocial concerns (Table 1).12,13
Each SCP is visible to the entire health care team, including other specialists and primary care, as long as they have access to UW Health’s EHR.14 The result is a readily accessible, comprehensive document that is individualized for each survivor, residing in a standard location with standardized format and content to facilitate review and use.15
General survivorship patient education booklet
Many cancer survivors request additional information about their posttreatment concerns. The “UW Health Facts for You: Cancer Survivorship, Carbone Cancer Center” booklet was developed by a multidisciplinary team including oncologists, advanced practice providers (APPs), navigators, social workers, program leadership, cancer survivors, and caregivers. The guide includes detailed information for the cancer survivor on topics including nutrition, exercise, sleep, tobacco cessation, sexual health, and spirituality. Common concerns and symptom management are addressed as well as a comprehensive list of community resources. The booklet can be found at http://www.uwhealth.org/healthfacts/cancer/7834.
Survivorship questionnaire
Breast cancer survivors often have multiple concerns as they transition from active treatment to the survivorship phase of their cancer journey. Specific concerns may vary slightly form one survivor to another. Guided by recommendations for the American Society of Clinical Oncology and the National Comprehensive Cancer Network, we developed a 10-question, 2-page questionnaire to identify those concerns with input from members of the Breast Cancer Steering Committee. Members of the committee include surgical, medical, and radiation oncologists, AAPs, radiologists, pathologists, program leadership, and nurses, along with breast cancer survivors. Elements in the questionnaire include nutrition, activity, mood, sleep, sexual health, employment/insurance, pain/swelling, desires regarding pregnancy or prevention, memory/concentration, smoking, alcohol, genetic testing/counseling, and assistance with establishing care with a primary care provider. By completing the questionnaire, breast cancer survivors identify specific concerns within each category and are able to request additional information about those concerns and/or a referral to appropriate resources. They may also select the I need nothing further option if the concern is present but already being addressed.
SCP delivery and the transition visit
The next task in implementation of the care process for survivors encompassed the development of clinical workflows and processes to provide the document to the breast cancer survivor at the completion of treatment. In a study of breast cancer survivors, it was found that the preferred format for survivorship care planning is generally an in-person consultation at completion of treatment with an oncology professional.16 The best time for distribution of the written SCP is, however, unclear. Intuitively, it seems optimal to distribute SCPs around the time of completion of active treatment. However, for SCP delivery to be feasible and sustainable, delivery must be integrated into existing clinical care-delivery processes, and content must be streamlined and focused to meet the needs of their intended recipients without becoming overly burdensome to prepare and deliver.17
Ultimately, and after significant multidisciplinary discussion, it was determined that Stage 0-III breast cancer patients would have a visit focusing on symptoms and transitioning to surveillance follow-up (Transition Visit) as they completed active curative-intent cancer treatment. During this Transition Visit, the SCP document would be provided and reviewed with survivors. The Transition Visit for breast cancer survivors would be conducted by an APP following the completion of their final stage of active, primary treatment (surgery, chemotherapy, and/or radiation therapy). Additional long-term adjuvant therapy for breast cancer survivors (ie, trastuzumab, endocrine therapy) would continue as indicated during and after delivery of the SCP.
The radiation oncology clinic was chosen as a venue for these Transition Visits for breast cancer survivors whose treatment included radiotherapy. Despite little historical experience with delivery of SCPs in radiation oncology clinics, this was a logical choice given that radiotherapy is usually the final phase of active treatment for these breast cancer survivors, and a follow-up visit about a month after completing radiotherapy is already part of standard practice. Collaborating with the multidisciplinary UW Health Breast Center, we therefore integrated the formal breast survivorship care planning process and provision of the SCP into the current radiation oncology workflow. About 40% of the roughly 600 breast cancer patients treated by surgical and/or medical oncology at our institution annually also receive radiation therapy at our site. For the remaining 60% of breast cancer survivors who do not receive radiation therapy or who completed radiotherapy at an outside facility, the SCP is provided by an APP within the UW Health Breast Center.
UW radiation oncology survivorship transition visit
The overall workflow of our Transition Visit is depicted in the Figure. Toward the end of the breast cancer survivor’s radiation treatments, the radiation oncologist instructs the schedulers to arrange the 1-month, post-radiation Transition Visit with the APP and informs the survivor about the nature of the appointment. The Transition Visit is scheduled as a 60-minute appointment. Before the survivor’s arrival, an APP generates the written SCP. The activity includes completing the Treatment Summary, or verifying the accuracy of a prepopulated Treatment Summary, and individualizing the SCP section for the patient based on treatment received and follow-up recommendations using drop-down functionality. As the SCP is printed for review with the survivor, it is simultaneously sent to the survivor’s primary care provider. This is accomplished by using EHR functionality to route the document internally to UW primary care providers or automatically faxing the document to external primary care providers. Each SCP is also marked as complete within the EHR for the purposes of documenting compliance with this activity for later data analysis.
On arrival for the appointment, each breast cancer survivor completes the survivorship questionnaire. During the Transition Visit, the questionnaire is reviewed with the survivor and additional information is provided. Referral options are discussed if indicated with desired referrals made by the APP. The survivor is interviewed and examined for any persistent side effects of treatment. Next, the Treatment Summary and SCP are reviewed with the survivor, emphasizing the follow-up plan, signs or symptoms of breast cancer recurrence, and chronic or late treatment-related toxicities. Ample opportunity is provided for the survivor to ask questions and voice concerns.
Follow-up appointments with members of the patient’s care team (ie, medical, surgical, or radiation oncology) as well as necessary breast imaging (ie, mammogram, MRI) are coordinated and scheduled before the survivor leaves the department. A survey of oncologists (medical, surgical, radiation) identified specific cancer-related components of survivorship care that oncologists felt most responsible for as well as opportunities to improve the quality and efficiency of care provided by oncologists.18 At our institution, the breast surgical, medical, and radiation oncologists all generally participate in follow-up care through at least 1 year following completion of active, primary treatment.
Outcomes, quality improvement opportunities, and continued challenges with the process
There is presently a lack of long-term outcome data about the impact of SCPs. As mandates for the provision of SCPs are made, research focusing on whether SCPs result in improved health behaviors and outcomes, reduced burden in care transitions from the oncology setting, and increased cost-effectiveness will be needed.19 The long-term effects of SCPs on psychological, oncologic, and resource outcomes should be evaluated,20 as well as the impact on health behaviors, such as smoking cessation or participation in rehabilitation programs.21
Following the implementation of our Transition Visits in 2015, we conducted a quality improvement review. This review included summation of 69 recent breast cancer questionnaires from Transition Visits with our APPs (Table 2 and Table 3). The most common concerns raised by our breast cancer survivors include desire for weight loss, improving diet, and increasing physical activity. Of note, concerns did not often translate into a desire for more information or referrals.22 Survivors were generally satisfied with the timing of the Transition Visits and generally indicated that the visits were helpful, with self-reported improvements in their understanding of planned follow-up. A Canadian group evaluating breast and head and neck cancer survivors has suggested that SCPs could produce long-term improvements in healthy lifestyle behaviors; however, further research is needed to determine the extent to which SCPs might improve follow-up care over the long term.23
Finally, although efforts to date have been focused on the breast cancer survivor at the completion of treatment, long-term survivors may also benefit from receiving the SCP. A study by the American Cancer Society found that long-term cancer survivors had unmet informational needs, particularly with regard to screening, long-term cancer and treatment effects, and healthy lifestyle behaviors.24 Identifying and subsequently delivering an SCP to eligible long-term survivors is a challenging prospect, which depends on further refinement of EHR-based tracking of the date of diagnosis, cancer stage, and end-of-treatment date.
Summary and recommendations
Survivorship care has been efficiently integrated into our 1-month post-radiation follow-up appointment for breast cancer survivors. By using current resources in the radiation oncology department, the process has provided an effective way to deliver the SCP to breast cancer survivors. Future plans include implementing the process for all patients receiving curative-intent radiation for additional solid tumor survivors. Quality improvement projects will be developed to assess survivor satisfaction and the impact on health behaviors.
Acknowledgments
The authors thank Amy Heath, MS, RTT, for editorial and manuscript preparation assistance.
1. Statistics. National Cancer Institute, Division of Cancer Control & Population Sciences website. http:///cancercontrol.cancer.gov/ocs/statistics/statistics.html. Updated October 17, 2016. Accessed March 6, 2018.
2. Cancer facts & figures 2016. American Cancer Society website. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2016.html. Published 2016. Accessed February 27, 2018.
3. Hewitt M, Greenfield S, Stovall E, eds. From cancer patient to cancer survivor: lost in transition. Washington, DC: National Academies Press; 2006.
4. Knutson A, McNamara E. Cancer program standards: ensuring patient-centered care. American College of Surgeons website. https://www.facs.org/quality-programs/cancer/coc/standards. Published August 2016. Accessed March 6, 2018.
5. National Accreditation Program for Breast Centers. NAPBC standards manual. American College of Surgeons website. https://www.facs.org/~/media/files/quality%20programs/napbc/2014%20napbc%20standards%20manual.ashx. Published 2014. Accessed March 6, 2018.
6. Salz T, McCabe MS, Onstad EE, et al. Survivorship care plans: is there buy-in from community oncology providers? Cancer. 2014;120(5):722-730.
7. Mayer DK, Nekhlyudov L, Snyder CF, Merrill JK, Wollins DS, Shulman LN. American Society of Clinical Oncology clinical expert statement on cancer survivorship care planning. J Oncol Pract. 2014;10(6):345-351.
8. Dobelbower RR, Cotter G, Schilling PJ, Parsai EI, Carroll JM. Radiation oncology practice accreditation. Rays. 2001;26(3):191-198.
9. Hartford AC, Conway PD, Desai NB, et al. ACR-ASTRO practice parameter for communication: radiation oncology. The American College of Radiology website. http://www.acr.org/-/media/ACR/Files/Practice-Parameters/RadOnc.pdf. Updated 2014. Accessed March 6, 2018.
10. Koontz BF, Benda R, De Los Santos J, et al. US radiation oncology practice patterns for posttreatment survivor care. Pract Radiat Oncol. 2016;6(1):50-56.
11. American Society of Therapeutic Radiation Oncologists. APEx program standards. ASTRO website. http://www.astro.org/uploadedFiles/_MAIN_SITE/Daily_Practice/Accreditation/Content_Pieces/ProgramStandards.pdf. Published February 1, 2016. Accessed March 6, 2018.
12. Clinical practice survivorship guidelines and adaptations. American Society of Clinical Oncology website. http://www.asco.org/practice-guidelines/cancer-care-initiatives/prevention-survivorship. Published 2013. Accessed March 6, 2018.
13. National Comprehensive Cancer Network. Supportive care guidelines. NCNN website. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#supportive. Updated February 16, 2018. Accessed March 6, 2018.
14. Donohue S, Sesto ME, Hahn DL, et al. Evaluating primary care providers’ views on survivorship care plans generated by an electronic health record system. J Oncol Pract. 2015;11(3):e329-e335.
15. Tevaarwerk AJ, Wisinski KB, Buhr KA, et al. Leveraging electronic health record systems to create and provide electronic cancer survivorship care plans: a pilot study. J Oncol Pract. 2014;10(3):e150-e159.
16. Smith SL, Singh-Carlson S, Downie L, Payeur N, Wai ES. Survivors of breast cancer: patient perspectives on survivorship care planning. J Cancer Surviv. 2011;5(4):337-344.
17. Stricker CT, O’Brien M. Implementing the commission on cancer standards for survivorship care plans. Clin J Oncol Nurs. 2014;18(suppl 1):15-22.
18. Neuman HB, Steffens NM, Jacobson N, et al. Oncologists’ perspectives of their roles and responsibilities during multi-disciplinary breast cancer follow-up. Ann Surg Oncol. 2016;23(3):708-714.
19. Palmer SC, Stricker CT, Panzer SL, et al. Outcomes and satisfaction after delivery of a breast cancer survivorship care plan: Results of a multicenter trial. J Oncol Pract. 2015;11(2):e222-e229.
20. Brennan ME, Gormally JF, Butow P, Boyle FM, Spillane AJ. Survivorship care plans in cancer: a systematic review of care plan outcomes. Br J Cancer. 2014;111(10):1899-1908.
21. Chen RC, Hoffman KE, Sher DJ, et al. Development of a standard survivorship care plan template for radiation oncologists. Pract Radiat Oncol. 2016;6(1):57-65.
22. Seaborne LA, Huenerberg KJ, Bohler A, et al. Developing electronic health record based program to deliver survivorship care plans and visits at the UW breast center. Poster presented at American Society of Clinical Oncology Survivorship Symposium; January 15-16, 2016; San Francisco CA.
23. Collie K, McCormick J, Waller A, et al. Qualitative evaluation of care plans for Canadian breast and head-and-neck cancer survivors. Curr Oncol. 2014;21(1):18-28.
24. Playdon M, Ferrucci LM, McCorkle R, et al. Health information needs and preferences in relation to survivorship care plans of long-term cancer survivors in the American Cancer Society’s study of cancer survivors-I. J Cancer Surviv. 2016;10(4):674-685.
In January 2016 there were an estimated 15.5 million people in the United States who were living with a cancer diagnosis, representing 4.8% of the population. That number is expected to increase to 20.3 million by 2026.1 The 5-year relative survival rate for all cancers diagnosed during 2005 to 2011 was 69%.2 As more individuals with a cancer diagnosis now live longer, cancer survivorship is receiving increased attention. A report from the Institute of Medicine3 identified the essential components of survivorship care, including the provision of a survivorship care plan (SCP) containing specific diagnosis, treatment, and follow-up information (Table 1). To maintain accreditation in their respective organizations, the American College of Surgeons’ Commission on Cancer and the National Accreditation Program for Breast Centers (NAPBC) have included standards on providing treatment summaries and SCPs in person to those patients who have completed cancer treatments given with curative intent.4,5
SCPs are personalized documents presented to cancer patients at the end of treatment that summarize key aspects of cancer treatment and recommend appropriate ongoing medical care and self-management. The purpose of the SCP is both to educate cancer survivors and to create a portable document that can be shared with primary care providers to facilitate coordinated care.6 There are multiple barriers to SCP implementation, which may include the time required to create an SCP, inadequate reimbursement for the time spent creating and delivering the plan, a lack of risk-stratified guidelines for coordinated care, and the incomplete automation of diagnosis and treatment summarization by the electronic health record (EHR).7
Survivorship care in radiation oncology
The American College of Radiology includes the recommendation for regular, ongoing follow-up in the standards for accreditation for radiation oncology practice.8 Radiation oncology practices often provide the initial follow-up appointment about a month after the prescribed radiation treatment has been completed. The twofold purpose of this appointment is to assess the response to treatment and to evaluate acute treatment-related effects.9 The appointment may include a skin evaluation, assessment for any acute treatment effects, informal counseling on maintaining a healthy lifestyle, and recommendations for posttreatment care and follow-up. The appointment may also be an opportune time for delivering the SCP because radiation therapy is often the final treatment modality in active therapy for breast cancer patients.
A review of the literature yields scant data on the incorporation of SCPs into a radiation oncology practice. A 2014 survey of members of the American Society of Radiation Oncology10for a response percentage of 14.7%. Almost all providers follow their patients after treatment (97% (n = 574 respondents/3987 total membership, 14.4% response rate) showed that although most radiation oncologists provide long-term follow-up care to their patients after treatment completion (97%), fewer than half of those surveyed indicated that they delivered SCPs for curative-intent patients (40%), and even fewer delivered for palliative-intent patients (19%). Standards for the American Society for Radiation Oncology’s Accreditation Program for Excellence11 outline content for end-of-treatment documentation. Typically, the documentation includes a detailed treatment summary prepared by the treating radiation oncologist. This treatment summary includes the patient’s diagnosis, the area treated, radiation doses received, number of fractions delivered, therapy start date, therapy completion date, and overall tolerance of treatment in a clinical summary. The treatment summary is communicated to other providers involved in the patient’s care to promote care coordination, but it is not typically provided to patients.
Development of University of Wisconsin survivorship care planning
As an important component of maintaining NAPBC accreditation, the University of Wisconsin (UW) Health Breast Center began the process of formalizing and optimizing SCPs for breast cancer survivors who are followed at the center. Multidisciplinary input from surgical, medical, and radiation oncology was obtained. Representatives from those disciplines met regularly to reach consensus on the treatment summary and SCP content. The following 3 documents were created for use during a transition visit at the end of treatment: the written individualized SCP to be provided to the survivor and his/her primary care providers, a general survivorship patient education booklet, and a patient questionnaire to identify survivors’ concerns and additional resources that may be beneficial.
Treatment summary
Working in collaboration with IT specialists, we enabled out-of-the-box functionality within our EHR. This cancer-specific functionality provides a central and standard location within each survivor’s problem list to systematically document information regarding cancer diagnosis, stage, and treatment associated with a specific cancer diagnosis. Each treating provider (surgeon, medical oncologist, radiation oncologist, genetic counselor, etc) is responsible for entering and updating the relevant components within the treatment summary (ie, the surgeon enters and maintains the surgical details, the medical oncologist does likewise for chemotherapy and other medical therapies, etc). Information is updated and current, creating a dynamic documentation of diagnosis and treatment that can be used in clinic notes, patient after-visit summaries, and SCPs.
Survivorship care plan
This same EHR functionality is leveraged to generate, populate, and maintain the individualized SCP for each breast cancer survivor. The Treatment Summary section of the SCP can be quickly prepared within the EHR by autopopulating data previously entered by treating providers. Content and language for SCP templates in breast, colorectal, prostate, and gynecologic cancers are in use at the time of publication. The templates are developed as a collaborative effort between oncology subspecialists, with input from the UW Health survivor and family advocacy councils.
Each template contains a Treatment Summary section and an SCP section. The Treatment Summary section includes survivor general information, diagnosis and treatment information, and the clinical and supportive/survivor care team names and contact information. The SCP section includes follow-up recommendations, signs of recurrence and/or symptoms to report, healthy lifestyle and maintenance, chronic or late effects of specific treatment if applicable (eg, surgery, chemotherapy by drug, radiation therapy, and endocrine therapy), and general resources for common psychosocial concerns (Table 1).12,13
Each SCP is visible to the entire health care team, including other specialists and primary care, as long as they have access to UW Health’s EHR.14 The result is a readily accessible, comprehensive document that is individualized for each survivor, residing in a standard location with standardized format and content to facilitate review and use.15
General survivorship patient education booklet
Many cancer survivors request additional information about their posttreatment concerns. The “UW Health Facts for You: Cancer Survivorship, Carbone Cancer Center” booklet was developed by a multidisciplinary team including oncologists, advanced practice providers (APPs), navigators, social workers, program leadership, cancer survivors, and caregivers. The guide includes detailed information for the cancer survivor on topics including nutrition, exercise, sleep, tobacco cessation, sexual health, and spirituality. Common concerns and symptom management are addressed as well as a comprehensive list of community resources. The booklet can be found at http://www.uwhealth.org/healthfacts/cancer/7834.
Survivorship questionnaire
Breast cancer survivors often have multiple concerns as they transition from active treatment to the survivorship phase of their cancer journey. Specific concerns may vary slightly form one survivor to another. Guided by recommendations for the American Society of Clinical Oncology and the National Comprehensive Cancer Network, we developed a 10-question, 2-page questionnaire to identify those concerns with input from members of the Breast Cancer Steering Committee. Members of the committee include surgical, medical, and radiation oncologists, AAPs, radiologists, pathologists, program leadership, and nurses, along with breast cancer survivors. Elements in the questionnaire include nutrition, activity, mood, sleep, sexual health, employment/insurance, pain/swelling, desires regarding pregnancy or prevention, memory/concentration, smoking, alcohol, genetic testing/counseling, and assistance with establishing care with a primary care provider. By completing the questionnaire, breast cancer survivors identify specific concerns within each category and are able to request additional information about those concerns and/or a referral to appropriate resources. They may also select the I need nothing further option if the concern is present but already being addressed.
SCP delivery and the transition visit
The next task in implementation of the care process for survivors encompassed the development of clinical workflows and processes to provide the document to the breast cancer survivor at the completion of treatment. In a study of breast cancer survivors, it was found that the preferred format for survivorship care planning is generally an in-person consultation at completion of treatment with an oncology professional.16 The best time for distribution of the written SCP is, however, unclear. Intuitively, it seems optimal to distribute SCPs around the time of completion of active treatment. However, for SCP delivery to be feasible and sustainable, delivery must be integrated into existing clinical care-delivery processes, and content must be streamlined and focused to meet the needs of their intended recipients without becoming overly burdensome to prepare and deliver.17
Ultimately, and after significant multidisciplinary discussion, it was determined that Stage 0-III breast cancer patients would have a visit focusing on symptoms and transitioning to surveillance follow-up (Transition Visit) as they completed active curative-intent cancer treatment. During this Transition Visit, the SCP document would be provided and reviewed with survivors. The Transition Visit for breast cancer survivors would be conducted by an APP following the completion of their final stage of active, primary treatment (surgery, chemotherapy, and/or radiation therapy). Additional long-term adjuvant therapy for breast cancer survivors (ie, trastuzumab, endocrine therapy) would continue as indicated during and after delivery of the SCP.
The radiation oncology clinic was chosen as a venue for these Transition Visits for breast cancer survivors whose treatment included radiotherapy. Despite little historical experience with delivery of SCPs in radiation oncology clinics, this was a logical choice given that radiotherapy is usually the final phase of active treatment for these breast cancer survivors, and a follow-up visit about a month after completing radiotherapy is already part of standard practice. Collaborating with the multidisciplinary UW Health Breast Center, we therefore integrated the formal breast survivorship care planning process and provision of the SCP into the current radiation oncology workflow. About 40% of the roughly 600 breast cancer patients treated by surgical and/or medical oncology at our institution annually also receive radiation therapy at our site. For the remaining 60% of breast cancer survivors who do not receive radiation therapy or who completed radiotherapy at an outside facility, the SCP is provided by an APP within the UW Health Breast Center.
UW radiation oncology survivorship transition visit
The overall workflow of our Transition Visit is depicted in the Figure. Toward the end of the breast cancer survivor’s radiation treatments, the radiation oncologist instructs the schedulers to arrange the 1-month, post-radiation Transition Visit with the APP and informs the survivor about the nature of the appointment. The Transition Visit is scheduled as a 60-minute appointment. Before the survivor’s arrival, an APP generates the written SCP. The activity includes completing the Treatment Summary, or verifying the accuracy of a prepopulated Treatment Summary, and individualizing the SCP section for the patient based on treatment received and follow-up recommendations using drop-down functionality. As the SCP is printed for review with the survivor, it is simultaneously sent to the survivor’s primary care provider. This is accomplished by using EHR functionality to route the document internally to UW primary care providers or automatically faxing the document to external primary care providers. Each SCP is also marked as complete within the EHR for the purposes of documenting compliance with this activity for later data analysis.
On arrival for the appointment, each breast cancer survivor completes the survivorship questionnaire. During the Transition Visit, the questionnaire is reviewed with the survivor and additional information is provided. Referral options are discussed if indicated with desired referrals made by the APP. The survivor is interviewed and examined for any persistent side effects of treatment. Next, the Treatment Summary and SCP are reviewed with the survivor, emphasizing the follow-up plan, signs or symptoms of breast cancer recurrence, and chronic or late treatment-related toxicities. Ample opportunity is provided for the survivor to ask questions and voice concerns.
Follow-up appointments with members of the patient’s care team (ie, medical, surgical, or radiation oncology) as well as necessary breast imaging (ie, mammogram, MRI) are coordinated and scheduled before the survivor leaves the department. A survey of oncologists (medical, surgical, radiation) identified specific cancer-related components of survivorship care that oncologists felt most responsible for as well as opportunities to improve the quality and efficiency of care provided by oncologists.18 At our institution, the breast surgical, medical, and radiation oncologists all generally participate in follow-up care through at least 1 year following completion of active, primary treatment.
Outcomes, quality improvement opportunities, and continued challenges with the process
There is presently a lack of long-term outcome data about the impact of SCPs. As mandates for the provision of SCPs are made, research focusing on whether SCPs result in improved health behaviors and outcomes, reduced burden in care transitions from the oncology setting, and increased cost-effectiveness will be needed.19 The long-term effects of SCPs on psychological, oncologic, and resource outcomes should be evaluated,20 as well as the impact on health behaviors, such as smoking cessation or participation in rehabilitation programs.21
Following the implementation of our Transition Visits in 2015, we conducted a quality improvement review. This review included summation of 69 recent breast cancer questionnaires from Transition Visits with our APPs (Table 2 and Table 3). The most common concerns raised by our breast cancer survivors include desire for weight loss, improving diet, and increasing physical activity. Of note, concerns did not often translate into a desire for more information or referrals.22 Survivors were generally satisfied with the timing of the Transition Visits and generally indicated that the visits were helpful, with self-reported improvements in their understanding of planned follow-up. A Canadian group evaluating breast and head and neck cancer survivors has suggested that SCPs could produce long-term improvements in healthy lifestyle behaviors; however, further research is needed to determine the extent to which SCPs might improve follow-up care over the long term.23
Finally, although efforts to date have been focused on the breast cancer survivor at the completion of treatment, long-term survivors may also benefit from receiving the SCP. A study by the American Cancer Society found that long-term cancer survivors had unmet informational needs, particularly with regard to screening, long-term cancer and treatment effects, and healthy lifestyle behaviors.24 Identifying and subsequently delivering an SCP to eligible long-term survivors is a challenging prospect, which depends on further refinement of EHR-based tracking of the date of diagnosis, cancer stage, and end-of-treatment date.
Summary and recommendations
Survivorship care has been efficiently integrated into our 1-month post-radiation follow-up appointment for breast cancer survivors. By using current resources in the radiation oncology department, the process has provided an effective way to deliver the SCP to breast cancer survivors. Future plans include implementing the process for all patients receiving curative-intent radiation for additional solid tumor survivors. Quality improvement projects will be developed to assess survivor satisfaction and the impact on health behaviors.
Acknowledgments
The authors thank Amy Heath, MS, RTT, for editorial and manuscript preparation assistance.
In January 2016 there were an estimated 15.5 million people in the United States who were living with a cancer diagnosis, representing 4.8% of the population. That number is expected to increase to 20.3 million by 2026.1 The 5-year relative survival rate for all cancers diagnosed during 2005 to 2011 was 69%.2 As more individuals with a cancer diagnosis now live longer, cancer survivorship is receiving increased attention. A report from the Institute of Medicine3 identified the essential components of survivorship care, including the provision of a survivorship care plan (SCP) containing specific diagnosis, treatment, and follow-up information (Table 1). To maintain accreditation in their respective organizations, the American College of Surgeons’ Commission on Cancer and the National Accreditation Program for Breast Centers (NAPBC) have included standards on providing treatment summaries and SCPs in person to those patients who have completed cancer treatments given with curative intent.4,5
SCPs are personalized documents presented to cancer patients at the end of treatment that summarize key aspects of cancer treatment and recommend appropriate ongoing medical care and self-management. The purpose of the SCP is both to educate cancer survivors and to create a portable document that can be shared with primary care providers to facilitate coordinated care.6 There are multiple barriers to SCP implementation, which may include the time required to create an SCP, inadequate reimbursement for the time spent creating and delivering the plan, a lack of risk-stratified guidelines for coordinated care, and the incomplete automation of diagnosis and treatment summarization by the electronic health record (EHR).7
Survivorship care in radiation oncology
The American College of Radiology includes the recommendation for regular, ongoing follow-up in the standards for accreditation for radiation oncology practice.8 Radiation oncology practices often provide the initial follow-up appointment about a month after the prescribed radiation treatment has been completed. The twofold purpose of this appointment is to assess the response to treatment and to evaluate acute treatment-related effects.9 The appointment may include a skin evaluation, assessment for any acute treatment effects, informal counseling on maintaining a healthy lifestyle, and recommendations for posttreatment care and follow-up. The appointment may also be an opportune time for delivering the SCP because radiation therapy is often the final treatment modality in active therapy for breast cancer patients.
A review of the literature yields scant data on the incorporation of SCPs into a radiation oncology practice. A 2014 survey of members of the American Society of Radiation Oncology10for a response percentage of 14.7%. Almost all providers follow their patients after treatment (97% (n = 574 respondents/3987 total membership, 14.4% response rate) showed that although most radiation oncologists provide long-term follow-up care to their patients after treatment completion (97%), fewer than half of those surveyed indicated that they delivered SCPs for curative-intent patients (40%), and even fewer delivered for palliative-intent patients (19%). Standards for the American Society for Radiation Oncology’s Accreditation Program for Excellence11 outline content for end-of-treatment documentation. Typically, the documentation includes a detailed treatment summary prepared by the treating radiation oncologist. This treatment summary includes the patient’s diagnosis, the area treated, radiation doses received, number of fractions delivered, therapy start date, therapy completion date, and overall tolerance of treatment in a clinical summary. The treatment summary is communicated to other providers involved in the patient’s care to promote care coordination, but it is not typically provided to patients.
Development of University of Wisconsin survivorship care planning
As an important component of maintaining NAPBC accreditation, the University of Wisconsin (UW) Health Breast Center began the process of formalizing and optimizing SCPs for breast cancer survivors who are followed at the center. Multidisciplinary input from surgical, medical, and radiation oncology was obtained. Representatives from those disciplines met regularly to reach consensus on the treatment summary and SCP content. The following 3 documents were created for use during a transition visit at the end of treatment: the written individualized SCP to be provided to the survivor and his/her primary care providers, a general survivorship patient education booklet, and a patient questionnaire to identify survivors’ concerns and additional resources that may be beneficial.
Treatment summary
Working in collaboration with IT specialists, we enabled out-of-the-box functionality within our EHR. This cancer-specific functionality provides a central and standard location within each survivor’s problem list to systematically document information regarding cancer diagnosis, stage, and treatment associated with a specific cancer diagnosis. Each treating provider (surgeon, medical oncologist, radiation oncologist, genetic counselor, etc) is responsible for entering and updating the relevant components within the treatment summary (ie, the surgeon enters and maintains the surgical details, the medical oncologist does likewise for chemotherapy and other medical therapies, etc). Information is updated and current, creating a dynamic documentation of diagnosis and treatment that can be used in clinic notes, patient after-visit summaries, and SCPs.
Survivorship care plan
This same EHR functionality is leveraged to generate, populate, and maintain the individualized SCP for each breast cancer survivor. The Treatment Summary section of the SCP can be quickly prepared within the EHR by autopopulating data previously entered by treating providers. Content and language for SCP templates in breast, colorectal, prostate, and gynecologic cancers are in use at the time of publication. The templates are developed as a collaborative effort between oncology subspecialists, with input from the UW Health survivor and family advocacy councils.
Each template contains a Treatment Summary section and an SCP section. The Treatment Summary section includes survivor general information, diagnosis and treatment information, and the clinical and supportive/survivor care team names and contact information. The SCP section includes follow-up recommendations, signs of recurrence and/or symptoms to report, healthy lifestyle and maintenance, chronic or late effects of specific treatment if applicable (eg, surgery, chemotherapy by drug, radiation therapy, and endocrine therapy), and general resources for common psychosocial concerns (Table 1).12,13
Each SCP is visible to the entire health care team, including other specialists and primary care, as long as they have access to UW Health’s EHR.14 The result is a readily accessible, comprehensive document that is individualized for each survivor, residing in a standard location with standardized format and content to facilitate review and use.15
General survivorship patient education booklet
Many cancer survivors request additional information about their posttreatment concerns. The “UW Health Facts for You: Cancer Survivorship, Carbone Cancer Center” booklet was developed by a multidisciplinary team including oncologists, advanced practice providers (APPs), navigators, social workers, program leadership, cancer survivors, and caregivers. The guide includes detailed information for the cancer survivor on topics including nutrition, exercise, sleep, tobacco cessation, sexual health, and spirituality. Common concerns and symptom management are addressed as well as a comprehensive list of community resources. The booklet can be found at http://www.uwhealth.org/healthfacts/cancer/7834.
Survivorship questionnaire
Breast cancer survivors often have multiple concerns as they transition from active treatment to the survivorship phase of their cancer journey. Specific concerns may vary slightly form one survivor to another. Guided by recommendations for the American Society of Clinical Oncology and the National Comprehensive Cancer Network, we developed a 10-question, 2-page questionnaire to identify those concerns with input from members of the Breast Cancer Steering Committee. Members of the committee include surgical, medical, and radiation oncologists, AAPs, radiologists, pathologists, program leadership, and nurses, along with breast cancer survivors. Elements in the questionnaire include nutrition, activity, mood, sleep, sexual health, employment/insurance, pain/swelling, desires regarding pregnancy or prevention, memory/concentration, smoking, alcohol, genetic testing/counseling, and assistance with establishing care with a primary care provider. By completing the questionnaire, breast cancer survivors identify specific concerns within each category and are able to request additional information about those concerns and/or a referral to appropriate resources. They may also select the I need nothing further option if the concern is present but already being addressed.
SCP delivery and the transition visit
The next task in implementation of the care process for survivors encompassed the development of clinical workflows and processes to provide the document to the breast cancer survivor at the completion of treatment. In a study of breast cancer survivors, it was found that the preferred format for survivorship care planning is generally an in-person consultation at completion of treatment with an oncology professional.16 The best time for distribution of the written SCP is, however, unclear. Intuitively, it seems optimal to distribute SCPs around the time of completion of active treatment. However, for SCP delivery to be feasible and sustainable, delivery must be integrated into existing clinical care-delivery processes, and content must be streamlined and focused to meet the needs of their intended recipients without becoming overly burdensome to prepare and deliver.17
Ultimately, and after significant multidisciplinary discussion, it was determined that Stage 0-III breast cancer patients would have a visit focusing on symptoms and transitioning to surveillance follow-up (Transition Visit) as they completed active curative-intent cancer treatment. During this Transition Visit, the SCP document would be provided and reviewed with survivors. The Transition Visit for breast cancer survivors would be conducted by an APP following the completion of their final stage of active, primary treatment (surgery, chemotherapy, and/or radiation therapy). Additional long-term adjuvant therapy for breast cancer survivors (ie, trastuzumab, endocrine therapy) would continue as indicated during and after delivery of the SCP.
The radiation oncology clinic was chosen as a venue for these Transition Visits for breast cancer survivors whose treatment included radiotherapy. Despite little historical experience with delivery of SCPs in radiation oncology clinics, this was a logical choice given that radiotherapy is usually the final phase of active treatment for these breast cancer survivors, and a follow-up visit about a month after completing radiotherapy is already part of standard practice. Collaborating with the multidisciplinary UW Health Breast Center, we therefore integrated the formal breast survivorship care planning process and provision of the SCP into the current radiation oncology workflow. About 40% of the roughly 600 breast cancer patients treated by surgical and/or medical oncology at our institution annually also receive radiation therapy at our site. For the remaining 60% of breast cancer survivors who do not receive radiation therapy or who completed radiotherapy at an outside facility, the SCP is provided by an APP within the UW Health Breast Center.
UW radiation oncology survivorship transition visit
The overall workflow of our Transition Visit is depicted in the Figure. Toward the end of the breast cancer survivor’s radiation treatments, the radiation oncologist instructs the schedulers to arrange the 1-month, post-radiation Transition Visit with the APP and informs the survivor about the nature of the appointment. The Transition Visit is scheduled as a 60-minute appointment. Before the survivor’s arrival, an APP generates the written SCP. The activity includes completing the Treatment Summary, or verifying the accuracy of a prepopulated Treatment Summary, and individualizing the SCP section for the patient based on treatment received and follow-up recommendations using drop-down functionality. As the SCP is printed for review with the survivor, it is simultaneously sent to the survivor’s primary care provider. This is accomplished by using EHR functionality to route the document internally to UW primary care providers or automatically faxing the document to external primary care providers. Each SCP is also marked as complete within the EHR for the purposes of documenting compliance with this activity for later data analysis.
On arrival for the appointment, each breast cancer survivor completes the survivorship questionnaire. During the Transition Visit, the questionnaire is reviewed with the survivor and additional information is provided. Referral options are discussed if indicated with desired referrals made by the APP. The survivor is interviewed and examined for any persistent side effects of treatment. Next, the Treatment Summary and SCP are reviewed with the survivor, emphasizing the follow-up plan, signs or symptoms of breast cancer recurrence, and chronic or late treatment-related toxicities. Ample opportunity is provided for the survivor to ask questions and voice concerns.
Follow-up appointments with members of the patient’s care team (ie, medical, surgical, or radiation oncology) as well as necessary breast imaging (ie, mammogram, MRI) are coordinated and scheduled before the survivor leaves the department. A survey of oncologists (medical, surgical, radiation) identified specific cancer-related components of survivorship care that oncologists felt most responsible for as well as opportunities to improve the quality and efficiency of care provided by oncologists.18 At our institution, the breast surgical, medical, and radiation oncologists all generally participate in follow-up care through at least 1 year following completion of active, primary treatment.
Outcomes, quality improvement opportunities, and continued challenges with the process
There is presently a lack of long-term outcome data about the impact of SCPs. As mandates for the provision of SCPs are made, research focusing on whether SCPs result in improved health behaviors and outcomes, reduced burden in care transitions from the oncology setting, and increased cost-effectiveness will be needed.19 The long-term effects of SCPs on psychological, oncologic, and resource outcomes should be evaluated,20 as well as the impact on health behaviors, such as smoking cessation or participation in rehabilitation programs.21
Following the implementation of our Transition Visits in 2015, we conducted a quality improvement review. This review included summation of 69 recent breast cancer questionnaires from Transition Visits with our APPs (Table 2 and Table 3). The most common concerns raised by our breast cancer survivors include desire for weight loss, improving diet, and increasing physical activity. Of note, concerns did not often translate into a desire for more information or referrals.22 Survivors were generally satisfied with the timing of the Transition Visits and generally indicated that the visits were helpful, with self-reported improvements in their understanding of planned follow-up. A Canadian group evaluating breast and head and neck cancer survivors has suggested that SCPs could produce long-term improvements in healthy lifestyle behaviors; however, further research is needed to determine the extent to which SCPs might improve follow-up care over the long term.23
Finally, although efforts to date have been focused on the breast cancer survivor at the completion of treatment, long-term survivors may also benefit from receiving the SCP. A study by the American Cancer Society found that long-term cancer survivors had unmet informational needs, particularly with regard to screening, long-term cancer and treatment effects, and healthy lifestyle behaviors.24 Identifying and subsequently delivering an SCP to eligible long-term survivors is a challenging prospect, which depends on further refinement of EHR-based tracking of the date of diagnosis, cancer stage, and end-of-treatment date.
Summary and recommendations
Survivorship care has been efficiently integrated into our 1-month post-radiation follow-up appointment for breast cancer survivors. By using current resources in the radiation oncology department, the process has provided an effective way to deliver the SCP to breast cancer survivors. Future plans include implementing the process for all patients receiving curative-intent radiation for additional solid tumor survivors. Quality improvement projects will be developed to assess survivor satisfaction and the impact on health behaviors.
Acknowledgments
The authors thank Amy Heath, MS, RTT, for editorial and manuscript preparation assistance.
1. Statistics. National Cancer Institute, Division of Cancer Control & Population Sciences website. http:///cancercontrol.cancer.gov/ocs/statistics/statistics.html. Updated October 17, 2016. Accessed March 6, 2018.
2. Cancer facts & figures 2016. American Cancer Society website. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2016.html. Published 2016. Accessed February 27, 2018.
3. Hewitt M, Greenfield S, Stovall E, eds. From cancer patient to cancer survivor: lost in transition. Washington, DC: National Academies Press; 2006.
4. Knutson A, McNamara E. Cancer program standards: ensuring patient-centered care. American College of Surgeons website. https://www.facs.org/quality-programs/cancer/coc/standards. Published August 2016. Accessed March 6, 2018.
5. National Accreditation Program for Breast Centers. NAPBC standards manual. American College of Surgeons website. https://www.facs.org/~/media/files/quality%20programs/napbc/2014%20napbc%20standards%20manual.ashx. Published 2014. Accessed March 6, 2018.
6. Salz T, McCabe MS, Onstad EE, et al. Survivorship care plans: is there buy-in from community oncology providers? Cancer. 2014;120(5):722-730.
7. Mayer DK, Nekhlyudov L, Snyder CF, Merrill JK, Wollins DS, Shulman LN. American Society of Clinical Oncology clinical expert statement on cancer survivorship care planning. J Oncol Pract. 2014;10(6):345-351.
8. Dobelbower RR, Cotter G, Schilling PJ, Parsai EI, Carroll JM. Radiation oncology practice accreditation. Rays. 2001;26(3):191-198.
9. Hartford AC, Conway PD, Desai NB, et al. ACR-ASTRO practice parameter for communication: radiation oncology. The American College of Radiology website. http://www.acr.org/-/media/ACR/Files/Practice-Parameters/RadOnc.pdf. Updated 2014. Accessed March 6, 2018.
10. Koontz BF, Benda R, De Los Santos J, et al. US radiation oncology practice patterns for posttreatment survivor care. Pract Radiat Oncol. 2016;6(1):50-56.
11. American Society of Therapeutic Radiation Oncologists. APEx program standards. ASTRO website. http://www.astro.org/uploadedFiles/_MAIN_SITE/Daily_Practice/Accreditation/Content_Pieces/ProgramStandards.pdf. Published February 1, 2016. Accessed March 6, 2018.
12. Clinical practice survivorship guidelines and adaptations. American Society of Clinical Oncology website. http://www.asco.org/practice-guidelines/cancer-care-initiatives/prevention-survivorship. Published 2013. Accessed March 6, 2018.
13. National Comprehensive Cancer Network. Supportive care guidelines. NCNN website. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#supportive. Updated February 16, 2018. Accessed March 6, 2018.
14. Donohue S, Sesto ME, Hahn DL, et al. Evaluating primary care providers’ views on survivorship care plans generated by an electronic health record system. J Oncol Pract. 2015;11(3):e329-e335.
15. Tevaarwerk AJ, Wisinski KB, Buhr KA, et al. Leveraging electronic health record systems to create and provide electronic cancer survivorship care plans: a pilot study. J Oncol Pract. 2014;10(3):e150-e159.
16. Smith SL, Singh-Carlson S, Downie L, Payeur N, Wai ES. Survivors of breast cancer: patient perspectives on survivorship care planning. J Cancer Surviv. 2011;5(4):337-344.
17. Stricker CT, O’Brien M. Implementing the commission on cancer standards for survivorship care plans. Clin J Oncol Nurs. 2014;18(suppl 1):15-22.
18. Neuman HB, Steffens NM, Jacobson N, et al. Oncologists’ perspectives of their roles and responsibilities during multi-disciplinary breast cancer follow-up. Ann Surg Oncol. 2016;23(3):708-714.
19. Palmer SC, Stricker CT, Panzer SL, et al. Outcomes and satisfaction after delivery of a breast cancer survivorship care plan: Results of a multicenter trial. J Oncol Pract. 2015;11(2):e222-e229.
20. Brennan ME, Gormally JF, Butow P, Boyle FM, Spillane AJ. Survivorship care plans in cancer: a systematic review of care plan outcomes. Br J Cancer. 2014;111(10):1899-1908.
21. Chen RC, Hoffman KE, Sher DJ, et al. Development of a standard survivorship care plan template for radiation oncologists. Pract Radiat Oncol. 2016;6(1):57-65.
22. Seaborne LA, Huenerberg KJ, Bohler A, et al. Developing electronic health record based program to deliver survivorship care plans and visits at the UW breast center. Poster presented at American Society of Clinical Oncology Survivorship Symposium; January 15-16, 2016; San Francisco CA.
23. Collie K, McCormick J, Waller A, et al. Qualitative evaluation of care plans for Canadian breast and head-and-neck cancer survivors. Curr Oncol. 2014;21(1):18-28.
24. Playdon M, Ferrucci LM, McCorkle R, et al. Health information needs and preferences in relation to survivorship care plans of long-term cancer survivors in the American Cancer Society’s study of cancer survivors-I. J Cancer Surviv. 2016;10(4):674-685.
1. Statistics. National Cancer Institute, Division of Cancer Control & Population Sciences website. http:///cancercontrol.cancer.gov/ocs/statistics/statistics.html. Updated October 17, 2016. Accessed March 6, 2018.
2. Cancer facts & figures 2016. American Cancer Society website. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2016.html. Published 2016. Accessed February 27, 2018.
3. Hewitt M, Greenfield S, Stovall E, eds. From cancer patient to cancer survivor: lost in transition. Washington, DC: National Academies Press; 2006.
4. Knutson A, McNamara E. Cancer program standards: ensuring patient-centered care. American College of Surgeons website. https://www.facs.org/quality-programs/cancer/coc/standards. Published August 2016. Accessed March 6, 2018.
5. National Accreditation Program for Breast Centers. NAPBC standards manual. American College of Surgeons website. https://www.facs.org/~/media/files/quality%20programs/napbc/2014%20napbc%20standards%20manual.ashx. Published 2014. Accessed March 6, 2018.
6. Salz T, McCabe MS, Onstad EE, et al. Survivorship care plans: is there buy-in from community oncology providers? Cancer. 2014;120(5):722-730.
7. Mayer DK, Nekhlyudov L, Snyder CF, Merrill JK, Wollins DS, Shulman LN. American Society of Clinical Oncology clinical expert statement on cancer survivorship care planning. J Oncol Pract. 2014;10(6):345-351.
8. Dobelbower RR, Cotter G, Schilling PJ, Parsai EI, Carroll JM. Radiation oncology practice accreditation. Rays. 2001;26(3):191-198.
9. Hartford AC, Conway PD, Desai NB, et al. ACR-ASTRO practice parameter for communication: radiation oncology. The American College of Radiology website. http://www.acr.org/-/media/ACR/Files/Practice-Parameters/RadOnc.pdf. Updated 2014. Accessed March 6, 2018.
10. Koontz BF, Benda R, De Los Santos J, et al. US radiation oncology practice patterns for posttreatment survivor care. Pract Radiat Oncol. 2016;6(1):50-56.
11. American Society of Therapeutic Radiation Oncologists. APEx program standards. ASTRO website. http://www.astro.org/uploadedFiles/_MAIN_SITE/Daily_Practice/Accreditation/Content_Pieces/ProgramStandards.pdf. Published February 1, 2016. Accessed March 6, 2018.
12. Clinical practice survivorship guidelines and adaptations. American Society of Clinical Oncology website. http://www.asco.org/practice-guidelines/cancer-care-initiatives/prevention-survivorship. Published 2013. Accessed March 6, 2018.
13. National Comprehensive Cancer Network. Supportive care guidelines. NCNN website. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#supportive. Updated February 16, 2018. Accessed March 6, 2018.
14. Donohue S, Sesto ME, Hahn DL, et al. Evaluating primary care providers’ views on survivorship care plans generated by an electronic health record system. J Oncol Pract. 2015;11(3):e329-e335.
15. Tevaarwerk AJ, Wisinski KB, Buhr KA, et al. Leveraging electronic health record systems to create and provide electronic cancer survivorship care plans: a pilot study. J Oncol Pract. 2014;10(3):e150-e159.
16. Smith SL, Singh-Carlson S, Downie L, Payeur N, Wai ES. Survivors of breast cancer: patient perspectives on survivorship care planning. J Cancer Surviv. 2011;5(4):337-344.
17. Stricker CT, O’Brien M. Implementing the commission on cancer standards for survivorship care plans. Clin J Oncol Nurs. 2014;18(suppl 1):15-22.
18. Neuman HB, Steffens NM, Jacobson N, et al. Oncologists’ perspectives of their roles and responsibilities during multi-disciplinary breast cancer follow-up. Ann Surg Oncol. 2016;23(3):708-714.
19. Palmer SC, Stricker CT, Panzer SL, et al. Outcomes and satisfaction after delivery of a breast cancer survivorship care plan: Results of a multicenter trial. J Oncol Pract. 2015;11(2):e222-e229.
20. Brennan ME, Gormally JF, Butow P, Boyle FM, Spillane AJ. Survivorship care plans in cancer: a systematic review of care plan outcomes. Br J Cancer. 2014;111(10):1899-1908.
21. Chen RC, Hoffman KE, Sher DJ, et al. Development of a standard survivorship care plan template for radiation oncologists. Pract Radiat Oncol. 2016;6(1):57-65.
22. Seaborne LA, Huenerberg KJ, Bohler A, et al. Developing electronic health record based program to deliver survivorship care plans and visits at the UW breast center. Poster presented at American Society of Clinical Oncology Survivorship Symposium; January 15-16, 2016; San Francisco CA.
23. Collie K, McCormick J, Waller A, et al. Qualitative evaluation of care plans for Canadian breast and head-and-neck cancer survivors. Curr Oncol. 2014;21(1):18-28.
24. Playdon M, Ferrucci LM, McCorkle R, et al. Health information needs and preferences in relation to survivorship care plans of long-term cancer survivors in the American Cancer Society’s study of cancer survivors-I. J Cancer Surviv. 2016;10(4):674-685.
Bevacizumab-awwb becomes first biosimilar approved for cancer treatment
Targeted therapies have revolutionized the treatment of numerous different cancer types and ushered in an era of personalized medicine, yet they can be prohibitively costly. As patent protection expires on many of the first FDA-approved monoclonal antibodies developed for oncologic indications, the doors are opened for other companies to develop their own version of these drugs, known as biosimilars. The price of biosimilars is expected to be considerably lower than the original drugs upon which they are based.
Bevacizumab-awwb, marketed as Mvasi by Amgen and Allergen, became the first such drug to receive approval by the US Food and Drug Administration for the treatment of cancer in fall last year.1 It is a biosimilar of Genentech’s anti-angiogenesis drug, bevacizumab (Avastin), a monoclonal antibody that targets vascular endothelial growth factor-A (VEGF-A).
The approval of biosimilars is based on rigorous demonstration of a high level of similarity between the biosimilar and the already-approved reference drug, in terms of structure, function, pharmacokinetics, pharmacodynamics, and clinical efficacy and safety.
Bevacizumab-awwb was approved for the first- or second-line treatment of metastatic colorectal cancer (mCRC) in combination with 5-fluorouracil-based chemotherapy; the second-line treatment of mCRC in combination with fluoropyrimidine-oxaliplatin chemotherapy in patients who progressed on first-line bevacizumab; the first-line treatment of unresectable, locally advanced, recurrent or metastatic nonsquamous non-small cell lung cancer (NSCLC) in combination with carboplatin and paclitaxel; the second-line treatment of glioblastoma (GBM) as monotherapy; and in patients with persistent, recurrent, or metastatic cervical cancer in combination with paclitaxel and cisplatin or paclitaxel and topotecan. It was not approved for the treatment of ovarian cancer, for which bevacizumab is indicated.
The majority of the data used to support approval came from 2 studies – a 3-arm, single-dose pharmacokinetics study, and a comparative clinical study in patients with advanced/metastatic NSCLC. In the pharmacokinetics study, 202 healthy men received an infusion of 3 mg/kg of bevacizumab-awwb, US-approved bevacizumab, or EU-approved bevacizumab. Bevacizumab-awwb was shown to have pharmacokinetic similarity to both approved forms of bevacizumab, and safety and tolerability were comparable, with none of the participants developing binding or neutralizing antidrug antibodies.2
In the clinical study, 648 patients received an infusion of bevacizumab-awwb or EU-approved bevacizumab at a dose of 15 mg/kg every 3 weeks in combination with 6 AUC carboplatin and 200 mg/m2 paclitaxel for 6 cycles. The overall response rate was 39% for bevacizumab-awwb, compared with 41.7% for EU-bevacizumab, and there were 2 complete responses in each group. The median duration of response for bevacizumab-awwb compared with EU-bevacizumab was 5.8 months versus 5.6 months, respectively, and median progression-free survival was 6.6 months versus 7.9 months.3
In terms of safety, the rates of grade 3/4 adverse events (AEs) were 42.9% in the biosimilar arm, compared with 44.3% for the reference drug. Overall, there were no clinically meaningful differences in AEs, serious AEs, deaths, or treatment discontinuations.
The recommended dose for bevacizumab-awwb in patients with mCRC is a 5 mg/kg intravenous dose administered every 2 weeks with bolus-IFL, a 10 mg/kg IV dose administered every 2 weeks with FOLFOX4, or a 5 mg/kg IV dose administered every 2 weeks or 7.5 mg/kg IV dose administered every 3 weeks with fluoropyrimidine-irinotecan or fluoropyrimidine-oxaliplatin-based chemotherapy.
For patients with NSCLC, bevacizumab-awwb should be administered at a 15 mg/kg IV dose every 3 weeks with the carboplatin–paclitaxel combination; for GBM patients, a 10 mg/kg IV dose should be administered every 3 weeks; and for patients with cervical cancer, an IV dose of 15 mg/kg every 3 weeks in combination with paclitaxel–cisplatin or paclitaxel–topotecan is recommended.
The prescribing information outlines warnings and precautions to advise clinicians administering the new biosimilar of the risks of gastrointestinal (GI) perforations, surgery and wound healing complications, and severe and potentially fatal pulmonary, GI, central nervous system, and vaginal bleeding.4
Treatment should be discontinued if GI perforation occurs. Patients should not take bevacizumab-awwb in the 28 days before elective surgery and after surgery until the wound is healed, and treatment should be discontinued if the surgical wound breaks open. Bevacizumab-awwb should not be administered to patients with severe hemorrhage or those with hemoptysis.
Blood pressure should be monitored every 2-3 weeks during treatment and hypertension treated with antihypertensive therapy. Treatment should be temporarily suspended in patients with severe hypertension that is not controlled with antihypertensive therapy and discontinued in patients who experience hypertensive crisis or hypertensive encephalopathy.
Proteinuria should be monitored by dipstick urine analysis during treatment, and patients with a 2+ or greater reading (concentration, 100 mg/dL) should undergo further assessment with 24-hour urine collection. Treatment should be suspended if proteinuria levels are ≥2 g/24h and can be resumed when they fall below that level, but should be discontinued in patients with nephrotic syndrome. Treatment should also be discontinued in patients who develop posterior reversible encephalopathy syndrome, and patients should be advised of the potential for fetal harm
1. FDA approves first biosimilar for the treatment of cancer. FDA News Release. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm576112.htm. September 14, 2017. Accessed January 31, 2018.
2. Markus R, Chow V, Pan X, and Hanes V. A phase I, randomized, single-dose study evaluating the pharmacokinetic equivalence of biosimilar ABP 215 and bevacizumab in healthy adult men. Cancer Chemother. Pharmacol. 2017;80:755-763.
3. Thatcher N, Thomas M, Ostoros G, et al. Randomized, double-blind, phase 3 study comparing biosimilar candidate ABP-215 with bevacizumab in patients with non-squamous NSCLC. J Thorac Oncol. 2017;12(1):S902-S903.
4. Mvasi (bevacizumab-awwb) solution, for intravenous infusion. Prescribing information. Amgen Inc, https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761028s000lbl.pdf. September 2017. Accessed January 31, 2018.
Targeted therapies have revolutionized the treatment of numerous different cancer types and ushered in an era of personalized medicine, yet they can be prohibitively costly. As patent protection expires on many of the first FDA-approved monoclonal antibodies developed for oncologic indications, the doors are opened for other companies to develop their own version of these drugs, known as biosimilars. The price of biosimilars is expected to be considerably lower than the original drugs upon which they are based.
Bevacizumab-awwb, marketed as Mvasi by Amgen and Allergen, became the first such drug to receive approval by the US Food and Drug Administration for the treatment of cancer in fall last year.1 It is a biosimilar of Genentech’s anti-angiogenesis drug, bevacizumab (Avastin), a monoclonal antibody that targets vascular endothelial growth factor-A (VEGF-A).
The approval of biosimilars is based on rigorous demonstration of a high level of similarity between the biosimilar and the already-approved reference drug, in terms of structure, function, pharmacokinetics, pharmacodynamics, and clinical efficacy and safety.
Bevacizumab-awwb was approved for the first- or second-line treatment of metastatic colorectal cancer (mCRC) in combination with 5-fluorouracil-based chemotherapy; the second-line treatment of mCRC in combination with fluoropyrimidine-oxaliplatin chemotherapy in patients who progressed on first-line bevacizumab; the first-line treatment of unresectable, locally advanced, recurrent or metastatic nonsquamous non-small cell lung cancer (NSCLC) in combination with carboplatin and paclitaxel; the second-line treatment of glioblastoma (GBM) as monotherapy; and in patients with persistent, recurrent, or metastatic cervical cancer in combination with paclitaxel and cisplatin or paclitaxel and topotecan. It was not approved for the treatment of ovarian cancer, for which bevacizumab is indicated.
The majority of the data used to support approval came from 2 studies – a 3-arm, single-dose pharmacokinetics study, and a comparative clinical study in patients with advanced/metastatic NSCLC. In the pharmacokinetics study, 202 healthy men received an infusion of 3 mg/kg of bevacizumab-awwb, US-approved bevacizumab, or EU-approved bevacizumab. Bevacizumab-awwb was shown to have pharmacokinetic similarity to both approved forms of bevacizumab, and safety and tolerability were comparable, with none of the participants developing binding or neutralizing antidrug antibodies.2
In the clinical study, 648 patients received an infusion of bevacizumab-awwb or EU-approved bevacizumab at a dose of 15 mg/kg every 3 weeks in combination with 6 AUC carboplatin and 200 mg/m2 paclitaxel for 6 cycles. The overall response rate was 39% for bevacizumab-awwb, compared with 41.7% for EU-bevacizumab, and there were 2 complete responses in each group. The median duration of response for bevacizumab-awwb compared with EU-bevacizumab was 5.8 months versus 5.6 months, respectively, and median progression-free survival was 6.6 months versus 7.9 months.3
In terms of safety, the rates of grade 3/4 adverse events (AEs) were 42.9% in the biosimilar arm, compared with 44.3% for the reference drug. Overall, there were no clinically meaningful differences in AEs, serious AEs, deaths, or treatment discontinuations.
The recommended dose for bevacizumab-awwb in patients with mCRC is a 5 mg/kg intravenous dose administered every 2 weeks with bolus-IFL, a 10 mg/kg IV dose administered every 2 weeks with FOLFOX4, or a 5 mg/kg IV dose administered every 2 weeks or 7.5 mg/kg IV dose administered every 3 weeks with fluoropyrimidine-irinotecan or fluoropyrimidine-oxaliplatin-based chemotherapy.
For patients with NSCLC, bevacizumab-awwb should be administered at a 15 mg/kg IV dose every 3 weeks with the carboplatin–paclitaxel combination; for GBM patients, a 10 mg/kg IV dose should be administered every 3 weeks; and for patients with cervical cancer, an IV dose of 15 mg/kg every 3 weeks in combination with paclitaxel–cisplatin or paclitaxel–topotecan is recommended.
The prescribing information outlines warnings and precautions to advise clinicians administering the new biosimilar of the risks of gastrointestinal (GI) perforations, surgery and wound healing complications, and severe and potentially fatal pulmonary, GI, central nervous system, and vaginal bleeding.4
Treatment should be discontinued if GI perforation occurs. Patients should not take bevacizumab-awwb in the 28 days before elective surgery and after surgery until the wound is healed, and treatment should be discontinued if the surgical wound breaks open. Bevacizumab-awwb should not be administered to patients with severe hemorrhage or those with hemoptysis.
Blood pressure should be monitored every 2-3 weeks during treatment and hypertension treated with antihypertensive therapy. Treatment should be temporarily suspended in patients with severe hypertension that is not controlled with antihypertensive therapy and discontinued in patients who experience hypertensive crisis or hypertensive encephalopathy.
Proteinuria should be monitored by dipstick urine analysis during treatment, and patients with a 2+ or greater reading (concentration, 100 mg/dL) should undergo further assessment with 24-hour urine collection. Treatment should be suspended if proteinuria levels are ≥2 g/24h and can be resumed when they fall below that level, but should be discontinued in patients with nephrotic syndrome. Treatment should also be discontinued in patients who develop posterior reversible encephalopathy syndrome, and patients should be advised of the potential for fetal harm
Targeted therapies have revolutionized the treatment of numerous different cancer types and ushered in an era of personalized medicine, yet they can be prohibitively costly. As patent protection expires on many of the first FDA-approved monoclonal antibodies developed for oncologic indications, the doors are opened for other companies to develop their own version of these drugs, known as biosimilars. The price of biosimilars is expected to be considerably lower than the original drugs upon which they are based.
Bevacizumab-awwb, marketed as Mvasi by Amgen and Allergen, became the first such drug to receive approval by the US Food and Drug Administration for the treatment of cancer in fall last year.1 It is a biosimilar of Genentech’s anti-angiogenesis drug, bevacizumab (Avastin), a monoclonal antibody that targets vascular endothelial growth factor-A (VEGF-A).
The approval of biosimilars is based on rigorous demonstration of a high level of similarity between the biosimilar and the already-approved reference drug, in terms of structure, function, pharmacokinetics, pharmacodynamics, and clinical efficacy and safety.
Bevacizumab-awwb was approved for the first- or second-line treatment of metastatic colorectal cancer (mCRC) in combination with 5-fluorouracil-based chemotherapy; the second-line treatment of mCRC in combination with fluoropyrimidine-oxaliplatin chemotherapy in patients who progressed on first-line bevacizumab; the first-line treatment of unresectable, locally advanced, recurrent or metastatic nonsquamous non-small cell lung cancer (NSCLC) in combination with carboplatin and paclitaxel; the second-line treatment of glioblastoma (GBM) as monotherapy; and in patients with persistent, recurrent, or metastatic cervical cancer in combination with paclitaxel and cisplatin or paclitaxel and topotecan. It was not approved for the treatment of ovarian cancer, for which bevacizumab is indicated.
The majority of the data used to support approval came from 2 studies – a 3-arm, single-dose pharmacokinetics study, and a comparative clinical study in patients with advanced/metastatic NSCLC. In the pharmacokinetics study, 202 healthy men received an infusion of 3 mg/kg of bevacizumab-awwb, US-approved bevacizumab, or EU-approved bevacizumab. Bevacizumab-awwb was shown to have pharmacokinetic similarity to both approved forms of bevacizumab, and safety and tolerability were comparable, with none of the participants developing binding or neutralizing antidrug antibodies.2
In the clinical study, 648 patients received an infusion of bevacizumab-awwb or EU-approved bevacizumab at a dose of 15 mg/kg every 3 weeks in combination with 6 AUC carboplatin and 200 mg/m2 paclitaxel for 6 cycles. The overall response rate was 39% for bevacizumab-awwb, compared with 41.7% for EU-bevacizumab, and there were 2 complete responses in each group. The median duration of response for bevacizumab-awwb compared with EU-bevacizumab was 5.8 months versus 5.6 months, respectively, and median progression-free survival was 6.6 months versus 7.9 months.3
In terms of safety, the rates of grade 3/4 adverse events (AEs) were 42.9% in the biosimilar arm, compared with 44.3% for the reference drug. Overall, there were no clinically meaningful differences in AEs, serious AEs, deaths, or treatment discontinuations.
The recommended dose for bevacizumab-awwb in patients with mCRC is a 5 mg/kg intravenous dose administered every 2 weeks with bolus-IFL, a 10 mg/kg IV dose administered every 2 weeks with FOLFOX4, or a 5 mg/kg IV dose administered every 2 weeks or 7.5 mg/kg IV dose administered every 3 weeks with fluoropyrimidine-irinotecan or fluoropyrimidine-oxaliplatin-based chemotherapy.
For patients with NSCLC, bevacizumab-awwb should be administered at a 15 mg/kg IV dose every 3 weeks with the carboplatin–paclitaxel combination; for GBM patients, a 10 mg/kg IV dose should be administered every 3 weeks; and for patients with cervical cancer, an IV dose of 15 mg/kg every 3 weeks in combination with paclitaxel–cisplatin or paclitaxel–topotecan is recommended.
The prescribing information outlines warnings and precautions to advise clinicians administering the new biosimilar of the risks of gastrointestinal (GI) perforations, surgery and wound healing complications, and severe and potentially fatal pulmonary, GI, central nervous system, and vaginal bleeding.4
Treatment should be discontinued if GI perforation occurs. Patients should not take bevacizumab-awwb in the 28 days before elective surgery and after surgery until the wound is healed, and treatment should be discontinued if the surgical wound breaks open. Bevacizumab-awwb should not be administered to patients with severe hemorrhage or those with hemoptysis.
Blood pressure should be monitored every 2-3 weeks during treatment and hypertension treated with antihypertensive therapy. Treatment should be temporarily suspended in patients with severe hypertension that is not controlled with antihypertensive therapy and discontinued in patients who experience hypertensive crisis or hypertensive encephalopathy.
Proteinuria should be monitored by dipstick urine analysis during treatment, and patients with a 2+ or greater reading (concentration, 100 mg/dL) should undergo further assessment with 24-hour urine collection. Treatment should be suspended if proteinuria levels are ≥2 g/24h and can be resumed when they fall below that level, but should be discontinued in patients with nephrotic syndrome. Treatment should also be discontinued in patients who develop posterior reversible encephalopathy syndrome, and patients should be advised of the potential for fetal harm
1. FDA approves first biosimilar for the treatment of cancer. FDA News Release. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm576112.htm. September 14, 2017. Accessed January 31, 2018.
2. Markus R, Chow V, Pan X, and Hanes V. A phase I, randomized, single-dose study evaluating the pharmacokinetic equivalence of biosimilar ABP 215 and bevacizumab in healthy adult men. Cancer Chemother. Pharmacol. 2017;80:755-763.
3. Thatcher N, Thomas M, Ostoros G, et al. Randomized, double-blind, phase 3 study comparing biosimilar candidate ABP-215 with bevacizumab in patients with non-squamous NSCLC. J Thorac Oncol. 2017;12(1):S902-S903.
4. Mvasi (bevacizumab-awwb) solution, for intravenous infusion. Prescribing information. Amgen Inc, https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761028s000lbl.pdf. September 2017. Accessed January 31, 2018.
1. FDA approves first biosimilar for the treatment of cancer. FDA News Release. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm576112.htm. September 14, 2017. Accessed January 31, 2018.
2. Markus R, Chow V, Pan X, and Hanes V. A phase I, randomized, single-dose study evaluating the pharmacokinetic equivalence of biosimilar ABP 215 and bevacizumab in healthy adult men. Cancer Chemother. Pharmacol. 2017;80:755-763.
3. Thatcher N, Thomas M, Ostoros G, et al. Randomized, double-blind, phase 3 study comparing biosimilar candidate ABP-215 with bevacizumab in patients with non-squamous NSCLC. J Thorac Oncol. 2017;12(1):S902-S903.
4. Mvasi (bevacizumab-awwb) solution, for intravenous infusion. Prescribing information. Amgen Inc, https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761028s000lbl.pdf. September 2017. Accessed January 31, 2018.
Trastuzumab-dkst approval adds to the biosimilar cancer drug market
The human epidermal growth factor receptor-2 (HER2)-targeting monoclonal antibody trastuzumab-dkst, was approved by the US Food and Drug Administration in 2017 for the treatment of patients with HER2-positive breast or metastatic gastric or gastroesophageal junction adenocarcinoma.1 Trastuzumab-dkst, marketed as Ogviri by Mylan NV and Biocon Ltd, is a copy, known as a biosimilar, of Genentech’s trastuzumab (Herceptin), which has been approved in the US since 1998. Genentech’s patent on trastuzumab expires in 2018
Approval was based on a comparison of the 2 drugs, which demonstrated that there were no clinically meaningful differences between the biosimilar and the reference product (trastuzumab) in terms of structure and function, pharmacokinetics (PKs), pharmacodynamics, and clinical efficacy and safety.
In structural and functional studies, trastuzumab-dkst was shown to have an identical amino acid sequence and a highly similar 3-dimensional structure, as well as equivalency in an inhibition of proliferation assay, a HER2-binding assay, and an antibody-dependent cellular cytotoxicity assay, compared with trastuzumab.
Two nonclinical animal studies were performed in cynomolgus monkeys; a single-dose comparative PK study and a 4-week, repeat-dose toxicity study. That was further supported by data from a single-dose, randomized, double-blind, comparative 3-way PK study (MYL-HER-1002) in which 120 healthy men were given an 8 mg/kg infusion of trastuzumab-dkst, US-approved trastuzumab, or European Union (EU)-approved trastuzumab.
The key clinical study was the phase 3 HERiTAge trial, a 2-part, multicenter, double-blind, randomized, parallel group trial that was performed in patients with HER2-positive metastatic breast cancer who had not been previously treated with either chemotherapy or trastuzumab in the metastatic setting.2
Eligible patients included males or females with measurably HER2-positive disease (as defined by HER2 overexpression determined by immunohistochemistry performed by a central laboratory), no exposure to chemotherapy or trastuzumab in the metastatic setting, an Eastern Cooperative Oncology Group Performance Status of 0 or 2, left ventricular ejection fraction (LVEF) within institutional range of normal, and who had completed adjuvant trastuzumab therapy at least 1 year before.
Patients with central nervous system metastases had to have stable disease after treatment, and hormonal agents were required to be discontinued before the start of the study. Patients with a history of unstable angina, heart failure, myocardial infarction less than 1 year from randomization, other clinically significant cardiac disease, grade 2 or higher peripheral neuropathy, a history of any other cancer within 4 years before screening, or any significant medical illness that increased treatment risk or impeded evaluation, were excluded from the study.
Patients were randomly assigned 1:1 to receive trastuzumab-dkst or trastuzumab, both in combination with paclitaxel or docetaxel, at a loading dose of 8 mg/kg, followed by a maintenance dose of 6 mg/kg, every 3 weeks for a minimum of 7 cycles in part 1 of the study. Patients who had stable disease or better were enrolled in part 2 and continued treatment until disease progression or unacceptable toxicity.
The primary endpoint was overall response rate (ORR) and, after 24 weeks, the ORR was 69.6% in the trastuzumab-dkst arm, compared with 64% in the trastuzumab arm, with a ratio of ORR of 1.09. Progression-free survival was also nearly identical in the 2 groups and median overall survival had not been reached in either arm.
The safety of the biosimilar and reference product were also highly similar. Serious adverse events occurred in 39.3%, compared with 37% of patients, respectively, with neutropenia the most frequently reported in both arms. Overall, treatment-emergent AEs occurred in 96.8%, compared with 94.7% of patients, respectively, with the majority of events mild or moderate in severity in both groups. This study also confirmed the low immunogenicity of the 2 drug products.
The prescribing information details the recommended doses of trastuzumab-dkst for each approved indication and warnings and precautions for cardiomyopathy, infusion reactions, pulmonary toxicity, exacerbation of chemotherapy-induced neutropenia and embryofetal toxicity.3
Patients should undergo thorough cardiac assessments, including baseline LVEF measurement immediately before starting therapy, every 3 months during therapy, and upon completion of therapy. Patients who complete adjuvant therapy should have cardiac assessments every 6 months for at least 2 years. Treatment should be withheld for ≥16% absolute decrease in LVEF from pre-treatment values or an LVEF value below institutional limits of normal and ≥10% absolute decrease in LVEF from pre-treatment values. When treatment is withheld for significant LVEF cardiac dysfunction, patients should undergo cardiac assessment at 4-week intervals.
To combat infusion reactions, infusion should be interrupted in all patients experiencing dyspnea or clinically significant hypotension and medical therapy administered. Patients should be evaluated and monitored carefully until signs and symptoms resolve and permanent discontinuation considered in patients with severe reactions. Patients should be warned of the potential for fetal harm with trastuzumab-dkst and of the need for effective contraceptive use during and for 6 months after treatment
1. FDA approves first biosimilar for the treatment of certain breast and stomach cancers. FDA News Release. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm587378.htm. December 1, 2017. Accessed January 31, 2018.
2. Rugo HS, Barve A, Waller CF, et al. Effect of a proposed trastuzumab biosimilar compared with trastuzumab on overall response rate in patients with ERBB2 (HER2)-positive metastatic breast cancer: a randomized clinical trial. JAMA. 2017;317(1):37-47.
3. Ogviri (trastuzumab-dkst) injection, for intravenous use. Prescribing information. Mylan, GMBH. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761074s000lbl.pdf. December, 2017. Accessed July 31, 2015.
The human epidermal growth factor receptor-2 (HER2)-targeting monoclonal antibody trastuzumab-dkst, was approved by the US Food and Drug Administration in 2017 for the treatment of patients with HER2-positive breast or metastatic gastric or gastroesophageal junction adenocarcinoma.1 Trastuzumab-dkst, marketed as Ogviri by Mylan NV and Biocon Ltd, is a copy, known as a biosimilar, of Genentech’s trastuzumab (Herceptin), which has been approved in the US since 1998. Genentech’s patent on trastuzumab expires in 2018
Approval was based on a comparison of the 2 drugs, which demonstrated that there were no clinically meaningful differences between the biosimilar and the reference product (trastuzumab) in terms of structure and function, pharmacokinetics (PKs), pharmacodynamics, and clinical efficacy and safety.
In structural and functional studies, trastuzumab-dkst was shown to have an identical amino acid sequence and a highly similar 3-dimensional structure, as well as equivalency in an inhibition of proliferation assay, a HER2-binding assay, and an antibody-dependent cellular cytotoxicity assay, compared with trastuzumab.
Two nonclinical animal studies were performed in cynomolgus monkeys; a single-dose comparative PK study and a 4-week, repeat-dose toxicity study. That was further supported by data from a single-dose, randomized, double-blind, comparative 3-way PK study (MYL-HER-1002) in which 120 healthy men were given an 8 mg/kg infusion of trastuzumab-dkst, US-approved trastuzumab, or European Union (EU)-approved trastuzumab.
The key clinical study was the phase 3 HERiTAge trial, a 2-part, multicenter, double-blind, randomized, parallel group trial that was performed in patients with HER2-positive metastatic breast cancer who had not been previously treated with either chemotherapy or trastuzumab in the metastatic setting.2
Eligible patients included males or females with measurably HER2-positive disease (as defined by HER2 overexpression determined by immunohistochemistry performed by a central laboratory), no exposure to chemotherapy or trastuzumab in the metastatic setting, an Eastern Cooperative Oncology Group Performance Status of 0 or 2, left ventricular ejection fraction (LVEF) within institutional range of normal, and who had completed adjuvant trastuzumab therapy at least 1 year before.
Patients with central nervous system metastases had to have stable disease after treatment, and hormonal agents were required to be discontinued before the start of the study. Patients with a history of unstable angina, heart failure, myocardial infarction less than 1 year from randomization, other clinically significant cardiac disease, grade 2 or higher peripheral neuropathy, a history of any other cancer within 4 years before screening, or any significant medical illness that increased treatment risk or impeded evaluation, were excluded from the study.
Patients were randomly assigned 1:1 to receive trastuzumab-dkst or trastuzumab, both in combination with paclitaxel or docetaxel, at a loading dose of 8 mg/kg, followed by a maintenance dose of 6 mg/kg, every 3 weeks for a minimum of 7 cycles in part 1 of the study. Patients who had stable disease or better were enrolled in part 2 and continued treatment until disease progression or unacceptable toxicity.
The primary endpoint was overall response rate (ORR) and, after 24 weeks, the ORR was 69.6% in the trastuzumab-dkst arm, compared with 64% in the trastuzumab arm, with a ratio of ORR of 1.09. Progression-free survival was also nearly identical in the 2 groups and median overall survival had not been reached in either arm.
The safety of the biosimilar and reference product were also highly similar. Serious adverse events occurred in 39.3%, compared with 37% of patients, respectively, with neutropenia the most frequently reported in both arms. Overall, treatment-emergent AEs occurred in 96.8%, compared with 94.7% of patients, respectively, with the majority of events mild or moderate in severity in both groups. This study also confirmed the low immunogenicity of the 2 drug products.
The prescribing information details the recommended doses of trastuzumab-dkst for each approved indication and warnings and precautions for cardiomyopathy, infusion reactions, pulmonary toxicity, exacerbation of chemotherapy-induced neutropenia and embryofetal toxicity.3
Patients should undergo thorough cardiac assessments, including baseline LVEF measurement immediately before starting therapy, every 3 months during therapy, and upon completion of therapy. Patients who complete adjuvant therapy should have cardiac assessments every 6 months for at least 2 years. Treatment should be withheld for ≥16% absolute decrease in LVEF from pre-treatment values or an LVEF value below institutional limits of normal and ≥10% absolute decrease in LVEF from pre-treatment values. When treatment is withheld for significant LVEF cardiac dysfunction, patients should undergo cardiac assessment at 4-week intervals.
To combat infusion reactions, infusion should be interrupted in all patients experiencing dyspnea or clinically significant hypotension and medical therapy administered. Patients should be evaluated and monitored carefully until signs and symptoms resolve and permanent discontinuation considered in patients with severe reactions. Patients should be warned of the potential for fetal harm with trastuzumab-dkst and of the need for effective contraceptive use during and for 6 months after treatment
The human epidermal growth factor receptor-2 (HER2)-targeting monoclonal antibody trastuzumab-dkst, was approved by the US Food and Drug Administration in 2017 for the treatment of patients with HER2-positive breast or metastatic gastric or gastroesophageal junction adenocarcinoma.1 Trastuzumab-dkst, marketed as Ogviri by Mylan NV and Biocon Ltd, is a copy, known as a biosimilar, of Genentech’s trastuzumab (Herceptin), which has been approved in the US since 1998. Genentech’s patent on trastuzumab expires in 2018
Approval was based on a comparison of the 2 drugs, which demonstrated that there were no clinically meaningful differences between the biosimilar and the reference product (trastuzumab) in terms of structure and function, pharmacokinetics (PKs), pharmacodynamics, and clinical efficacy and safety.
In structural and functional studies, trastuzumab-dkst was shown to have an identical amino acid sequence and a highly similar 3-dimensional structure, as well as equivalency in an inhibition of proliferation assay, a HER2-binding assay, and an antibody-dependent cellular cytotoxicity assay, compared with trastuzumab.
Two nonclinical animal studies were performed in cynomolgus monkeys; a single-dose comparative PK study and a 4-week, repeat-dose toxicity study. That was further supported by data from a single-dose, randomized, double-blind, comparative 3-way PK study (MYL-HER-1002) in which 120 healthy men were given an 8 mg/kg infusion of trastuzumab-dkst, US-approved trastuzumab, or European Union (EU)-approved trastuzumab.
The key clinical study was the phase 3 HERiTAge trial, a 2-part, multicenter, double-blind, randomized, parallel group trial that was performed in patients with HER2-positive metastatic breast cancer who had not been previously treated with either chemotherapy or trastuzumab in the metastatic setting.2
Eligible patients included males or females with measurably HER2-positive disease (as defined by HER2 overexpression determined by immunohistochemistry performed by a central laboratory), no exposure to chemotherapy or trastuzumab in the metastatic setting, an Eastern Cooperative Oncology Group Performance Status of 0 or 2, left ventricular ejection fraction (LVEF) within institutional range of normal, and who had completed adjuvant trastuzumab therapy at least 1 year before.
Patients with central nervous system metastases had to have stable disease after treatment, and hormonal agents were required to be discontinued before the start of the study. Patients with a history of unstable angina, heart failure, myocardial infarction less than 1 year from randomization, other clinically significant cardiac disease, grade 2 or higher peripheral neuropathy, a history of any other cancer within 4 years before screening, or any significant medical illness that increased treatment risk or impeded evaluation, were excluded from the study.
Patients were randomly assigned 1:1 to receive trastuzumab-dkst or trastuzumab, both in combination with paclitaxel or docetaxel, at a loading dose of 8 mg/kg, followed by a maintenance dose of 6 mg/kg, every 3 weeks for a minimum of 7 cycles in part 1 of the study. Patients who had stable disease or better were enrolled in part 2 and continued treatment until disease progression or unacceptable toxicity.
The primary endpoint was overall response rate (ORR) and, after 24 weeks, the ORR was 69.6% in the trastuzumab-dkst arm, compared with 64% in the trastuzumab arm, with a ratio of ORR of 1.09. Progression-free survival was also nearly identical in the 2 groups and median overall survival had not been reached in either arm.
The safety of the biosimilar and reference product were also highly similar. Serious adverse events occurred in 39.3%, compared with 37% of patients, respectively, with neutropenia the most frequently reported in both arms. Overall, treatment-emergent AEs occurred in 96.8%, compared with 94.7% of patients, respectively, with the majority of events mild or moderate in severity in both groups. This study also confirmed the low immunogenicity of the 2 drug products.
The prescribing information details the recommended doses of trastuzumab-dkst for each approved indication and warnings and precautions for cardiomyopathy, infusion reactions, pulmonary toxicity, exacerbation of chemotherapy-induced neutropenia and embryofetal toxicity.3
Patients should undergo thorough cardiac assessments, including baseline LVEF measurement immediately before starting therapy, every 3 months during therapy, and upon completion of therapy. Patients who complete adjuvant therapy should have cardiac assessments every 6 months for at least 2 years. Treatment should be withheld for ≥16% absolute decrease in LVEF from pre-treatment values or an LVEF value below institutional limits of normal and ≥10% absolute decrease in LVEF from pre-treatment values. When treatment is withheld for significant LVEF cardiac dysfunction, patients should undergo cardiac assessment at 4-week intervals.
To combat infusion reactions, infusion should be interrupted in all patients experiencing dyspnea or clinically significant hypotension and medical therapy administered. Patients should be evaluated and monitored carefully until signs and symptoms resolve and permanent discontinuation considered in patients with severe reactions. Patients should be warned of the potential for fetal harm with trastuzumab-dkst and of the need for effective contraceptive use during and for 6 months after treatment
1. FDA approves first biosimilar for the treatment of certain breast and stomach cancers. FDA News Release. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm587378.htm. December 1, 2017. Accessed January 31, 2018.
2. Rugo HS, Barve A, Waller CF, et al. Effect of a proposed trastuzumab biosimilar compared with trastuzumab on overall response rate in patients with ERBB2 (HER2)-positive metastatic breast cancer: a randomized clinical trial. JAMA. 2017;317(1):37-47.
3. Ogviri (trastuzumab-dkst) injection, for intravenous use. Prescribing information. Mylan, GMBH. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761074s000lbl.pdf. December, 2017. Accessed July 31, 2015.
1. FDA approves first biosimilar for the treatment of certain breast and stomach cancers. FDA News Release. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm587378.htm. December 1, 2017. Accessed January 31, 2018.
2. Rugo HS, Barve A, Waller CF, et al. Effect of a proposed trastuzumab biosimilar compared with trastuzumab on overall response rate in patients with ERBB2 (HER2)-positive metastatic breast cancer: a randomized clinical trial. JAMA. 2017;317(1):37-47.
3. Ogviri (trastuzumab-dkst) injection, for intravenous use. Prescribing information. Mylan, GMBH. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761074s000lbl.pdf. December, 2017. Accessed July 31, 2015.
Biosimilars: same ol’ – but with a suffix, and cheaper
Biosimilars have arrived, and chances are that you’re already prescribing them. Last September, the US Food and Drug Administration (FDA) approved the first cancer-specific biosimilar, bevacizumab-awwb, for multiple cancer types (p. e60);1 and in November, it approved trastuzumab-dkst for HER2-positive breast and gastrointestinal cancers (p. e63).1 Briefly, biosimilars are biologic products that show comparable quality, efficacy, and safety to an existing, approved biologic known as the reference product.
Small-molecule drugs such as aspirin are easy to replicate identically, whereas biosimilars are large, complex proteins that are manufactured in nature’s factory, a micro-organism or biologic cell.2 The manufacturing process must be nearly identical to that for the reference product, so that only insignificant/nonclinically significant impurities occur in the final product. The protein-amino acid sequence is key and must therefore be identical. The 2010 Biologics Price Competition and Innovation Act established an abbreviated pathway for the FDA to consider and approve biosimilars, and 5 years later, the bone marrow stimulant filgrastim-sndz became the first biosimilar approved for use in the United States.3 The development of biosimilars is not inexpensive. The law and the FDA approval system require preclinical and phase 1 testing, and a robust phase 3 trial against the reference product to demonstrate that safety and efficacy are statistically not different and that any chemical differences between the biosimilar and reference product are clinically and safety or immunogenically insignificant. When those criteria have been met, and the biosimilar approved, the clinical and cost benefits to patients could be significant. In general, the cost of a biosimilar is about 20% to 30% lower than that of the reference product.
Biosimilarity does not yet allow interchangeability. Small-molecule generics under FDA regulations are interchangeable in the drug store and the hospital without the prescriber or patient being aware. That is not yet the case with biosimilars, but their lower prices could have a notable impact on overall cost of care. In 2013, 7 of the top 8 best-selling drugs in the global market were biologics.4 Three of the top 8 – rituximab, trastuzumab, and bevacizumab – were used to treat cancer, and 1 (pegfilgrastim) was for therapy-related neutropenia. Their total cost was US$27 billion. Biosimilars of those therapies could significantly lower that amount.
Nabhan and colleagues interviewed 510 US-based community oncologists about their understanding of biosimilars. They found that only 29% of respondents said they prescribed filgrastim-sndz for supportive care by personal choice, but upward of 73% said they would prescribe biosimilars for the active anticancer therapies, trastuzumab and bevacizumab. There’s no question that biosimilars are here to stay. The requirements to make them have been well worked out. Their safety and efficacy therefore can be assured, and their lower prices promise cost savings for patients and society as a whole.
1. Bosserman L. Cancer care in 2017: the promise of more cures with the challenges of an unstable health care system. https://www. mdedge.com/jcso/article/154559/cancer-care-2017-promise-morecures- challenges-unstable-health-care-system. December 15, 2017. Accessed April 23, 2018.
2. Biosimilar and interchangeable products. FDA website. https://www. fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsare DevelopedandApproved/ApprovalApplications/Therapeutic BiologicApplications/Biosimilars/ucm580419.htm#biological. Last updated October 23, 2017. Accessed April 25, 2018.
3. de Lartigue J. Filgrastim-sndz debuts as the first biosimilar approved in United States. https://www.mdedge.com/jcso/article/105177/patientsurvivor- care/filgrastim-sndz-debuts-first-biosimilar-approved-united. Published December 2015. Accessed April 23, 2018.
4 . The Dish. Biologics still on top in best selling drugs of 2013. http:// cellculturedish.com/2014/03/top-ten-biologics-2013-us-pharmaceutical- sales-2/. March 13, 2014. Accessed April 26, 2018.
5. Nabhan C, Jeune-Smith Y, Valley A, Feinberg BA. Community Oncologists’ Perception and Acceptance of Biosimilars in Oncology. https://www.journalofclinicalpathways.com/article/communityoncologists- perception-and-acceptance-biosimilars-oncology. Published March 2018. Accessed April 24, 2018.
Biosimilars have arrived, and chances are that you’re already prescribing them. Last September, the US Food and Drug Administration (FDA) approved the first cancer-specific biosimilar, bevacizumab-awwb, for multiple cancer types (p. e60);1 and in November, it approved trastuzumab-dkst for HER2-positive breast and gastrointestinal cancers (p. e63).1 Briefly, biosimilars are biologic products that show comparable quality, efficacy, and safety to an existing, approved biologic known as the reference product.
Small-molecule drugs such as aspirin are easy to replicate identically, whereas biosimilars are large, complex proteins that are manufactured in nature’s factory, a micro-organism or biologic cell.2 The manufacturing process must be nearly identical to that for the reference product, so that only insignificant/nonclinically significant impurities occur in the final product. The protein-amino acid sequence is key and must therefore be identical. The 2010 Biologics Price Competition and Innovation Act established an abbreviated pathway for the FDA to consider and approve biosimilars, and 5 years later, the bone marrow stimulant filgrastim-sndz became the first biosimilar approved for use in the United States.3 The development of biosimilars is not inexpensive. The law and the FDA approval system require preclinical and phase 1 testing, and a robust phase 3 trial against the reference product to demonstrate that safety and efficacy are statistically not different and that any chemical differences between the biosimilar and reference product are clinically and safety or immunogenically insignificant. When those criteria have been met, and the biosimilar approved, the clinical and cost benefits to patients could be significant. In general, the cost of a biosimilar is about 20% to 30% lower than that of the reference product.
Biosimilarity does not yet allow interchangeability. Small-molecule generics under FDA regulations are interchangeable in the drug store and the hospital without the prescriber or patient being aware. That is not yet the case with biosimilars, but their lower prices could have a notable impact on overall cost of care. In 2013, 7 of the top 8 best-selling drugs in the global market were biologics.4 Three of the top 8 – rituximab, trastuzumab, and bevacizumab – were used to treat cancer, and 1 (pegfilgrastim) was for therapy-related neutropenia. Their total cost was US$27 billion. Biosimilars of those therapies could significantly lower that amount.
Nabhan and colleagues interviewed 510 US-based community oncologists about their understanding of biosimilars. They found that only 29% of respondents said they prescribed filgrastim-sndz for supportive care by personal choice, but upward of 73% said they would prescribe biosimilars for the active anticancer therapies, trastuzumab and bevacizumab. There’s no question that biosimilars are here to stay. The requirements to make them have been well worked out. Their safety and efficacy therefore can be assured, and their lower prices promise cost savings for patients and society as a whole.
Biosimilars have arrived, and chances are that you’re already prescribing them. Last September, the US Food and Drug Administration (FDA) approved the first cancer-specific biosimilar, bevacizumab-awwb, for multiple cancer types (p. e60);1 and in November, it approved trastuzumab-dkst for HER2-positive breast and gastrointestinal cancers (p. e63).1 Briefly, biosimilars are biologic products that show comparable quality, efficacy, and safety to an existing, approved biologic known as the reference product.
Small-molecule drugs such as aspirin are easy to replicate identically, whereas biosimilars are large, complex proteins that are manufactured in nature’s factory, a micro-organism or biologic cell.2 The manufacturing process must be nearly identical to that for the reference product, so that only insignificant/nonclinically significant impurities occur in the final product. The protein-amino acid sequence is key and must therefore be identical. The 2010 Biologics Price Competition and Innovation Act established an abbreviated pathway for the FDA to consider and approve biosimilars, and 5 years later, the bone marrow stimulant filgrastim-sndz became the first biosimilar approved for use in the United States.3 The development of biosimilars is not inexpensive. The law and the FDA approval system require preclinical and phase 1 testing, and a robust phase 3 trial against the reference product to demonstrate that safety and efficacy are statistically not different and that any chemical differences between the biosimilar and reference product are clinically and safety or immunogenically insignificant. When those criteria have been met, and the biosimilar approved, the clinical and cost benefits to patients could be significant. In general, the cost of a biosimilar is about 20% to 30% lower than that of the reference product.
Biosimilarity does not yet allow interchangeability. Small-molecule generics under FDA regulations are interchangeable in the drug store and the hospital without the prescriber or patient being aware. That is not yet the case with biosimilars, but their lower prices could have a notable impact on overall cost of care. In 2013, 7 of the top 8 best-selling drugs in the global market were biologics.4 Three of the top 8 – rituximab, trastuzumab, and bevacizumab – were used to treat cancer, and 1 (pegfilgrastim) was for therapy-related neutropenia. Their total cost was US$27 billion. Biosimilars of those therapies could significantly lower that amount.
Nabhan and colleagues interviewed 510 US-based community oncologists about their understanding of biosimilars. They found that only 29% of respondents said they prescribed filgrastim-sndz for supportive care by personal choice, but upward of 73% said they would prescribe biosimilars for the active anticancer therapies, trastuzumab and bevacizumab. There’s no question that biosimilars are here to stay. The requirements to make them have been well worked out. Their safety and efficacy therefore can be assured, and their lower prices promise cost savings for patients and society as a whole.
1. Bosserman L. Cancer care in 2017: the promise of more cures with the challenges of an unstable health care system. https://www. mdedge.com/jcso/article/154559/cancer-care-2017-promise-morecures- challenges-unstable-health-care-system. December 15, 2017. Accessed April 23, 2018.
2. Biosimilar and interchangeable products. FDA website. https://www. fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsare DevelopedandApproved/ApprovalApplications/Therapeutic BiologicApplications/Biosimilars/ucm580419.htm#biological. Last updated October 23, 2017. Accessed April 25, 2018.
3. de Lartigue J. Filgrastim-sndz debuts as the first biosimilar approved in United States. https://www.mdedge.com/jcso/article/105177/patientsurvivor- care/filgrastim-sndz-debuts-first-biosimilar-approved-united. Published December 2015. Accessed April 23, 2018.
4 . The Dish. Biologics still on top in best selling drugs of 2013. http:// cellculturedish.com/2014/03/top-ten-biologics-2013-us-pharmaceutical- sales-2/. March 13, 2014. Accessed April 26, 2018.
5. Nabhan C, Jeune-Smith Y, Valley A, Feinberg BA. Community Oncologists’ Perception and Acceptance of Biosimilars in Oncology. https://www.journalofclinicalpathways.com/article/communityoncologists- perception-and-acceptance-biosimilars-oncology. Published March 2018. Accessed April 24, 2018.
1. Bosserman L. Cancer care in 2017: the promise of more cures with the challenges of an unstable health care system. https://www. mdedge.com/jcso/article/154559/cancer-care-2017-promise-morecures- challenges-unstable-health-care-system. December 15, 2017. Accessed April 23, 2018.
2. Biosimilar and interchangeable products. FDA website. https://www. fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsare DevelopedandApproved/ApprovalApplications/Therapeutic BiologicApplications/Biosimilars/ucm580419.htm#biological. Last updated October 23, 2017. Accessed April 25, 2018.
3. de Lartigue J. Filgrastim-sndz debuts as the first biosimilar approved in United States. https://www.mdedge.com/jcso/article/105177/patientsurvivor- care/filgrastim-sndz-debuts-first-biosimilar-approved-united. Published December 2015. Accessed April 23, 2018.
4 . The Dish. Biologics still on top in best selling drugs of 2013. http:// cellculturedish.com/2014/03/top-ten-biologics-2013-us-pharmaceutical- sales-2/. March 13, 2014. Accessed April 26, 2018.
5. Nabhan C, Jeune-Smith Y, Valley A, Feinberg BA. Community Oncologists’ Perception and Acceptance of Biosimilars in Oncology. https://www.journalofclinicalpathways.com/article/communityoncologists- perception-and-acceptance-biosimilars-oncology. Published March 2018. Accessed April 24, 2018.
Isolated ocular metastases from lung cancer
Non–small cell lung cancer constitutes 80%-85% of lung cancers, and 40% of NSCLC are adenocarcinoma. It is rare to find intraocular metastasis from lung cancer. In this article, we present the case of a patient who presented with complaints of diminished vision redness of the eye and was found to have intra-ocular metastases from lung cancer.
Case presentation and summary
A 60-year-old man with a 40-pack per year history of smoking presented to multiple ophthalmologists with complaints of decreased vision and redness of the left eye. He was eventually evaluated by an ophthalmologist who performed a biopsy of the anterior chamber of the eye. Histologic findings were consistent with adenocarcinoma of lung primary (Figures 1 and 2).
After the diagnosis, a chest X-ray showed that the patient had a left lower lung mass. The results of his physical exam were all within normal limits, with the exception of decreased visual acuity in the left eye. The results of his laboratory studies, including complete blood count and serum chemistries, were also within normal limits. Imaging studies – including a computed-tomography (CT) scan of the chest, abdomen, and pelvis and a full-body positron-emission tomography–CT scan – showed a hypermetabolic left lower lobe mass 4.5 cm and right lower paratracheal lymph node metastasis 2 cm with a small focus of increased uptake alone the medial aspect of the left globe (Figures 3 and 4).
An MRI orbit was performed in an attempt to better characterize the left eye mass, but no optic lesion was identified. A biopsy of the left lower lung mass was consistent with non–small-cell lung cancer (NSCLC). Aside from the isolated left eye metastases, the patient did not have evidence of other distant metastatic involvement.
He was started on palliative chemotherapy on a clinical trial and received intravenous carboplatin AUC 6, pemetrexed 500 mg/m2, and bevacizumab 15 mg/kg every 3 weeks. He received 1 dose intraocular bevacizumab injection before initiation of systemic chemotherapy as he was symptomatic from the intraocular metastases. Within 2 weeks after intravitreal bevacizumab was administered, the patient had subjective improvement in vision. Mutational analysis to identify if the patient would benefit from targeted therapy showed no presence of EGFR mutation and ALK gene rearrangement, and that the patient was K-RAS mutant.
After treatment initiation, interval imaging studies (a computed-tomography scan of the chest, abdomen, pelvis; and magnetic-resonance imaging of the brain) after 3 cycles showed no evidence of disease progression, and after 4 cycles of chemotherapy with these drugs, the patient was started on maintenance chemotherapy with bevacizumab 15 mg/kg and pemetrexed 500 mg/m2.
Discussion
Choroidal metastasis is the most common site of intraocular tumor. In an autopsy study of 230 patients with carcinoma, 12% of cases demonstrated histologic evidence of ocular metastasis.1 A retrospective series of patients with malignant involvement of the eye, 66% of patients had a known history of primary cancer and in 34% of patients the ocular tumor was the first sign of cancer.2 The most common cancers that were found to have ocular metastasis were lung and breast cancer.2 Adenocarcinoma was the most common histologic type of lung cancer to result in ocular metastases and was seen in 41% of patients.3
Decreased or blurred vision with redness as the primary complaint of NSCLC is rare. Only a few case reports are available. Abundo and colleagues reported that 0.7%-12% of patients with lung cancer develop ocular metastases.4 Therefore, routine ophthalmologic screening for ocular metastases in patients with cancer has not been pursued in asymptomatic patients.5 Ophthalmological evaluation is recommended in symptomatic patients.
Metastatic involvement of two or more other organs was found to be a risk factor for development of choroidal metastasis in patients with lung cancer though in our patient no evidence of other organ involvement was found.5 The most common site of metastases in patients with NSCLC with ocular metastases was found to be the liver. Choroidal metastases was reported to be the sixth common site of metastases in patients with lung cancer.5
Treatment of ocular manifestations has been generally confined to surgical resection or radiation therapy, but advances in chemotherapy and development of novel targeted agents have shown promising results.7 Median life expectancy after a diagnosis of uveal metastases was reported to be 12 months in a retrospective study, which is similar to the reported median survival in metastatic NSCLC.8
Our patient was enrolled in a clinical trial and was treated with a regimen of carboplatin, paclitaxel, and bevacizumab. On presentation, he had significant impairment of vision with pain. He was treated with intravitreal bevacizumab yielding improvement in his visual symptoms. Bevacizumab is a vascular endothelial growth factor receptor monoclonal antibody approved for use in patients with metastatic lung cancer. Other pathways that have been reported in development of lung cancer involve the ALK gene translocation, and EGFR and K-RAS mutations, and targeted therapy has shown good results in cancer patients with these molecular defects. Randomized clinical trials in patients with advanced NSCLC and an EGFR mutation have shown significant improvement in overall survival with the use of erlotinib, a tyrosine kinase inhibitor targeting the epidermal growth factor receptor.9 Similarly, crizotinib has shown promising results in patients with metastatic NSCLC who have ELM-ALK rearrangement.10 As our patient’s tumor did not have either of these mutations, he was initiated on chemotherapy with bevacizumab. The presence of a K-RAS mutation in this patient further supported the use of front-line chemotherapy given that it may confer resistance against agents that target the EGFR pathway.
In our review of the literature, we found cases of patients with ocular metastases who responded well to therapy with targeted agents (Table).
Singh and colleagues did a systematic review of 55 cases of patients with lung cancer and choroidal metastases and found that the type of therapy depended on when the diagnosis had been made in relation to the advent of targeted therapy: cases diagnosed before targeted therapy had received radiation therapy or enucleation.6 As far as we could ascertain, there have been no randomized studies evaluating the impact of various targeted therapies or systemic chemotherapy on ocular metastases, although case reports have documented improvement in vision and regression of metastases with such therapy.
Conclusion
The goal of therapy in metastatic lung cancer is palliation of symptoms and improvement in patient quality of life with prolongation in overall survival. The newer targeted chemotherapeutic agents assist in achieving these goals and may decrease the morbidity associated from radiation or surgery with improvement in vision and regression of ocular metastatic lesions. Targeted therapies should be considered in the treatment of patients with ocular metastases from NSCLC.
1. Bloch RS, Gartner S. The incidence of ocular metastatic carcinoma. Arch Ophthalmol-Chic. 1971;85(6):673-675.
2. Shields CL, Shields JA, Gross NE, Schwartz GP, Lally SE. Survey of 520 eyes with uveal metastases. Ophthalmology. 1997;104(8):1265-1276.
3. Kreusel KM, Bechrakis NE, Wiegel T, Krause L, Foerster MH. Incidence and clinical characteristics of symptomatic choroidal metastasis from lung cancer. Acta Ophthalmol. 2008;86(5):515-519.
4. Abundo RE, Orenic CJ, Anderson SF, Townsend JC. Choroidal metastases resulting from carcinoma of the lung. J Am Optom Assoc. 1997;68(2):95-108.
5. Kreusel KM, Wiegel T, Stange M, Bornfeld N, Hinkelbein W, Foerster MH. Choroidal metastasis in disseminated lung cancer: frequency and risk factors. Am J Ophthalmol. 2002;134(3):445-447.
6. Singh N, Kulkarni P, Aggarwal AN, et al. Choroidal metastasis as a presenting manifestation of lung cancer: a report of 3 cases and systematic review of the literature. Medicine (Baltimore). 2012;91(4):179-194.
7. Chen CJ, McCoy AN, Brahmer J, Handa JT. Emerging treatments for choroidal metastases. Surv Ophthalmol. 2011;56(6):511-521.
8. Shah SU, Mashayekhi A, Shields CL, et al. Uveal metastasis from lung cancer: clinical features, treatment, and outcome in 194 patients. Ophthalmology. 2014;121(1):352-357.
9. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353(2):123-132.
10. Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385-2394.
11. Kim SW, Kim MJ, Huh K, Oh J. Complete regression of choroidal metastasis secondary to non-small-cell lung cancer with intravitreal bevacizumab and oral erlotinib combination therapy. Ophthalmologica. 2009;223(6):411-413.
12. George B, Wirostko WJ, Connor TB, Choong NW. Complete and durable response of choroid metastasis from non-small cell lung cancer with systemic bevacizumab and chemotherapy. J Thorac Oncol. 2009;4(5):661-662.
13. Inoue M, Watanabe Y, Yamane S, et al. Choroidal metastasis with adenocarcinoma of the lung treated with gefitinib. Eur J Ophthalmol. 2010;20(5):963-965.
14. Shimomura I, Tada Y, Miura G, et al. Choroidal metastasis of non-small cell lung cancer that responded to gefitinib. https://www.hindawi.com/journals/criopm/2013/213124/. Published 2013. Accessed May 4, 2017.
15. Feng Y, Singh AD, Lanigan C, Tubbs RR, Ma PC. Choroidal metastases responsive to crizotinib therapy in a lung adenocarcinoma patient with ALK 2p23 fusion identified by ALK immunohistochemistry. J Thorac Oncol. 2013;8(12):e109-111.
Non–small cell lung cancer constitutes 80%-85% of lung cancers, and 40% of NSCLC are adenocarcinoma. It is rare to find intraocular metastasis from lung cancer. In this article, we present the case of a patient who presented with complaints of diminished vision redness of the eye and was found to have intra-ocular metastases from lung cancer.
Case presentation and summary
A 60-year-old man with a 40-pack per year history of smoking presented to multiple ophthalmologists with complaints of decreased vision and redness of the left eye. He was eventually evaluated by an ophthalmologist who performed a biopsy of the anterior chamber of the eye. Histologic findings were consistent with adenocarcinoma of lung primary (Figures 1 and 2).
After the diagnosis, a chest X-ray showed that the patient had a left lower lung mass. The results of his physical exam were all within normal limits, with the exception of decreased visual acuity in the left eye. The results of his laboratory studies, including complete blood count and serum chemistries, were also within normal limits. Imaging studies – including a computed-tomography (CT) scan of the chest, abdomen, and pelvis and a full-body positron-emission tomography–CT scan – showed a hypermetabolic left lower lobe mass 4.5 cm and right lower paratracheal lymph node metastasis 2 cm with a small focus of increased uptake alone the medial aspect of the left globe (Figures 3 and 4).
An MRI orbit was performed in an attempt to better characterize the left eye mass, but no optic lesion was identified. A biopsy of the left lower lung mass was consistent with non–small-cell lung cancer (NSCLC). Aside from the isolated left eye metastases, the patient did not have evidence of other distant metastatic involvement.
He was started on palliative chemotherapy on a clinical trial and received intravenous carboplatin AUC 6, pemetrexed 500 mg/m2, and bevacizumab 15 mg/kg every 3 weeks. He received 1 dose intraocular bevacizumab injection before initiation of systemic chemotherapy as he was symptomatic from the intraocular metastases. Within 2 weeks after intravitreal bevacizumab was administered, the patient had subjective improvement in vision. Mutational analysis to identify if the patient would benefit from targeted therapy showed no presence of EGFR mutation and ALK gene rearrangement, and that the patient was K-RAS mutant.
After treatment initiation, interval imaging studies (a computed-tomography scan of the chest, abdomen, pelvis; and magnetic-resonance imaging of the brain) after 3 cycles showed no evidence of disease progression, and after 4 cycles of chemotherapy with these drugs, the patient was started on maintenance chemotherapy with bevacizumab 15 mg/kg and pemetrexed 500 mg/m2.
Discussion
Choroidal metastasis is the most common site of intraocular tumor. In an autopsy study of 230 patients with carcinoma, 12% of cases demonstrated histologic evidence of ocular metastasis.1 A retrospective series of patients with malignant involvement of the eye, 66% of patients had a known history of primary cancer and in 34% of patients the ocular tumor was the first sign of cancer.2 The most common cancers that were found to have ocular metastasis were lung and breast cancer.2 Adenocarcinoma was the most common histologic type of lung cancer to result in ocular metastases and was seen in 41% of patients.3
Decreased or blurred vision with redness as the primary complaint of NSCLC is rare. Only a few case reports are available. Abundo and colleagues reported that 0.7%-12% of patients with lung cancer develop ocular metastases.4 Therefore, routine ophthalmologic screening for ocular metastases in patients with cancer has not been pursued in asymptomatic patients.5 Ophthalmological evaluation is recommended in symptomatic patients.
Metastatic involvement of two or more other organs was found to be a risk factor for development of choroidal metastasis in patients with lung cancer though in our patient no evidence of other organ involvement was found.5 The most common site of metastases in patients with NSCLC with ocular metastases was found to be the liver. Choroidal metastases was reported to be the sixth common site of metastases in patients with lung cancer.5
Treatment of ocular manifestations has been generally confined to surgical resection or radiation therapy, but advances in chemotherapy and development of novel targeted agents have shown promising results.7 Median life expectancy after a diagnosis of uveal metastases was reported to be 12 months in a retrospective study, which is similar to the reported median survival in metastatic NSCLC.8
Our patient was enrolled in a clinical trial and was treated with a regimen of carboplatin, paclitaxel, and bevacizumab. On presentation, he had significant impairment of vision with pain. He was treated with intravitreal bevacizumab yielding improvement in his visual symptoms. Bevacizumab is a vascular endothelial growth factor receptor monoclonal antibody approved for use in patients with metastatic lung cancer. Other pathways that have been reported in development of lung cancer involve the ALK gene translocation, and EGFR and K-RAS mutations, and targeted therapy has shown good results in cancer patients with these molecular defects. Randomized clinical trials in patients with advanced NSCLC and an EGFR mutation have shown significant improvement in overall survival with the use of erlotinib, a tyrosine kinase inhibitor targeting the epidermal growth factor receptor.9 Similarly, crizotinib has shown promising results in patients with metastatic NSCLC who have ELM-ALK rearrangement.10 As our patient’s tumor did not have either of these mutations, he was initiated on chemotherapy with bevacizumab. The presence of a K-RAS mutation in this patient further supported the use of front-line chemotherapy given that it may confer resistance against agents that target the EGFR pathway.
In our review of the literature, we found cases of patients with ocular metastases who responded well to therapy with targeted agents (Table).
Singh and colleagues did a systematic review of 55 cases of patients with lung cancer and choroidal metastases and found that the type of therapy depended on when the diagnosis had been made in relation to the advent of targeted therapy: cases diagnosed before targeted therapy had received radiation therapy or enucleation.6 As far as we could ascertain, there have been no randomized studies evaluating the impact of various targeted therapies or systemic chemotherapy on ocular metastases, although case reports have documented improvement in vision and regression of metastases with such therapy.
Conclusion
The goal of therapy in metastatic lung cancer is palliation of symptoms and improvement in patient quality of life with prolongation in overall survival. The newer targeted chemotherapeutic agents assist in achieving these goals and may decrease the morbidity associated from radiation or surgery with improvement in vision and regression of ocular metastatic lesions. Targeted therapies should be considered in the treatment of patients with ocular metastases from NSCLC.
Non–small cell lung cancer constitutes 80%-85% of lung cancers, and 40% of NSCLC are adenocarcinoma. It is rare to find intraocular metastasis from lung cancer. In this article, we present the case of a patient who presented with complaints of diminished vision redness of the eye and was found to have intra-ocular metastases from lung cancer.
Case presentation and summary
A 60-year-old man with a 40-pack per year history of smoking presented to multiple ophthalmologists with complaints of decreased vision and redness of the left eye. He was eventually evaluated by an ophthalmologist who performed a biopsy of the anterior chamber of the eye. Histologic findings were consistent with adenocarcinoma of lung primary (Figures 1 and 2).
After the diagnosis, a chest X-ray showed that the patient had a left lower lung mass. The results of his physical exam were all within normal limits, with the exception of decreased visual acuity in the left eye. The results of his laboratory studies, including complete blood count and serum chemistries, were also within normal limits. Imaging studies – including a computed-tomography (CT) scan of the chest, abdomen, and pelvis and a full-body positron-emission tomography–CT scan – showed a hypermetabolic left lower lobe mass 4.5 cm and right lower paratracheal lymph node metastasis 2 cm with a small focus of increased uptake alone the medial aspect of the left globe (Figures 3 and 4).
An MRI orbit was performed in an attempt to better characterize the left eye mass, but no optic lesion was identified. A biopsy of the left lower lung mass was consistent with non–small-cell lung cancer (NSCLC). Aside from the isolated left eye metastases, the patient did not have evidence of other distant metastatic involvement.
He was started on palliative chemotherapy on a clinical trial and received intravenous carboplatin AUC 6, pemetrexed 500 mg/m2, and bevacizumab 15 mg/kg every 3 weeks. He received 1 dose intraocular bevacizumab injection before initiation of systemic chemotherapy as he was symptomatic from the intraocular metastases. Within 2 weeks after intravitreal bevacizumab was administered, the patient had subjective improvement in vision. Mutational analysis to identify if the patient would benefit from targeted therapy showed no presence of EGFR mutation and ALK gene rearrangement, and that the patient was K-RAS mutant.
After treatment initiation, interval imaging studies (a computed-tomography scan of the chest, abdomen, pelvis; and magnetic-resonance imaging of the brain) after 3 cycles showed no evidence of disease progression, and after 4 cycles of chemotherapy with these drugs, the patient was started on maintenance chemotherapy with bevacizumab 15 mg/kg and pemetrexed 500 mg/m2.
Discussion
Choroidal metastasis is the most common site of intraocular tumor. In an autopsy study of 230 patients with carcinoma, 12% of cases demonstrated histologic evidence of ocular metastasis.1 A retrospective series of patients with malignant involvement of the eye, 66% of patients had a known history of primary cancer and in 34% of patients the ocular tumor was the first sign of cancer.2 The most common cancers that were found to have ocular metastasis were lung and breast cancer.2 Adenocarcinoma was the most common histologic type of lung cancer to result in ocular metastases and was seen in 41% of patients.3
Decreased or blurred vision with redness as the primary complaint of NSCLC is rare. Only a few case reports are available. Abundo and colleagues reported that 0.7%-12% of patients with lung cancer develop ocular metastases.4 Therefore, routine ophthalmologic screening for ocular metastases in patients with cancer has not been pursued in asymptomatic patients.5 Ophthalmological evaluation is recommended in symptomatic patients.
Metastatic involvement of two or more other organs was found to be a risk factor for development of choroidal metastasis in patients with lung cancer though in our patient no evidence of other organ involvement was found.5 The most common site of metastases in patients with NSCLC with ocular metastases was found to be the liver. Choroidal metastases was reported to be the sixth common site of metastases in patients with lung cancer.5
Treatment of ocular manifestations has been generally confined to surgical resection or radiation therapy, but advances in chemotherapy and development of novel targeted agents have shown promising results.7 Median life expectancy after a diagnosis of uveal metastases was reported to be 12 months in a retrospective study, which is similar to the reported median survival in metastatic NSCLC.8
Our patient was enrolled in a clinical trial and was treated with a regimen of carboplatin, paclitaxel, and bevacizumab. On presentation, he had significant impairment of vision with pain. He was treated with intravitreal bevacizumab yielding improvement in his visual symptoms. Bevacizumab is a vascular endothelial growth factor receptor monoclonal antibody approved for use in patients with metastatic lung cancer. Other pathways that have been reported in development of lung cancer involve the ALK gene translocation, and EGFR and K-RAS mutations, and targeted therapy has shown good results in cancer patients with these molecular defects. Randomized clinical trials in patients with advanced NSCLC and an EGFR mutation have shown significant improvement in overall survival with the use of erlotinib, a tyrosine kinase inhibitor targeting the epidermal growth factor receptor.9 Similarly, crizotinib has shown promising results in patients with metastatic NSCLC who have ELM-ALK rearrangement.10 As our patient’s tumor did not have either of these mutations, he was initiated on chemotherapy with bevacizumab. The presence of a K-RAS mutation in this patient further supported the use of front-line chemotherapy given that it may confer resistance against agents that target the EGFR pathway.
In our review of the literature, we found cases of patients with ocular metastases who responded well to therapy with targeted agents (Table).
Singh and colleagues did a systematic review of 55 cases of patients with lung cancer and choroidal metastases and found that the type of therapy depended on when the diagnosis had been made in relation to the advent of targeted therapy: cases diagnosed before targeted therapy had received radiation therapy or enucleation.6 As far as we could ascertain, there have been no randomized studies evaluating the impact of various targeted therapies or systemic chemotherapy on ocular metastases, although case reports have documented improvement in vision and regression of metastases with such therapy.
Conclusion
The goal of therapy in metastatic lung cancer is palliation of symptoms and improvement in patient quality of life with prolongation in overall survival. The newer targeted chemotherapeutic agents assist in achieving these goals and may decrease the morbidity associated from radiation or surgery with improvement in vision and regression of ocular metastatic lesions. Targeted therapies should be considered in the treatment of patients with ocular metastases from NSCLC.
1. Bloch RS, Gartner S. The incidence of ocular metastatic carcinoma. Arch Ophthalmol-Chic. 1971;85(6):673-675.
2. Shields CL, Shields JA, Gross NE, Schwartz GP, Lally SE. Survey of 520 eyes with uveal metastases. Ophthalmology. 1997;104(8):1265-1276.
3. Kreusel KM, Bechrakis NE, Wiegel T, Krause L, Foerster MH. Incidence and clinical characteristics of symptomatic choroidal metastasis from lung cancer. Acta Ophthalmol. 2008;86(5):515-519.
4. Abundo RE, Orenic CJ, Anderson SF, Townsend JC. Choroidal metastases resulting from carcinoma of the lung. J Am Optom Assoc. 1997;68(2):95-108.
5. Kreusel KM, Wiegel T, Stange M, Bornfeld N, Hinkelbein W, Foerster MH. Choroidal metastasis in disseminated lung cancer: frequency and risk factors. Am J Ophthalmol. 2002;134(3):445-447.
6. Singh N, Kulkarni P, Aggarwal AN, et al. Choroidal metastasis as a presenting manifestation of lung cancer: a report of 3 cases and systematic review of the literature. Medicine (Baltimore). 2012;91(4):179-194.
7. Chen CJ, McCoy AN, Brahmer J, Handa JT. Emerging treatments for choroidal metastases. Surv Ophthalmol. 2011;56(6):511-521.
8. Shah SU, Mashayekhi A, Shields CL, et al. Uveal metastasis from lung cancer: clinical features, treatment, and outcome in 194 patients. Ophthalmology. 2014;121(1):352-357.
9. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353(2):123-132.
10. Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385-2394.
11. Kim SW, Kim MJ, Huh K, Oh J. Complete regression of choroidal metastasis secondary to non-small-cell lung cancer with intravitreal bevacizumab and oral erlotinib combination therapy. Ophthalmologica. 2009;223(6):411-413.
12. George B, Wirostko WJ, Connor TB, Choong NW. Complete and durable response of choroid metastasis from non-small cell lung cancer with systemic bevacizumab and chemotherapy. J Thorac Oncol. 2009;4(5):661-662.
13. Inoue M, Watanabe Y, Yamane S, et al. Choroidal metastasis with adenocarcinoma of the lung treated with gefitinib. Eur J Ophthalmol. 2010;20(5):963-965.
14. Shimomura I, Tada Y, Miura G, et al. Choroidal metastasis of non-small cell lung cancer that responded to gefitinib. https://www.hindawi.com/journals/criopm/2013/213124/. Published 2013. Accessed May 4, 2017.
15. Feng Y, Singh AD, Lanigan C, Tubbs RR, Ma PC. Choroidal metastases responsive to crizotinib therapy in a lung adenocarcinoma patient with ALK 2p23 fusion identified by ALK immunohistochemistry. J Thorac Oncol. 2013;8(12):e109-111.
1. Bloch RS, Gartner S. The incidence of ocular metastatic carcinoma. Arch Ophthalmol-Chic. 1971;85(6):673-675.
2. Shields CL, Shields JA, Gross NE, Schwartz GP, Lally SE. Survey of 520 eyes with uveal metastases. Ophthalmology. 1997;104(8):1265-1276.
3. Kreusel KM, Bechrakis NE, Wiegel T, Krause L, Foerster MH. Incidence and clinical characteristics of symptomatic choroidal metastasis from lung cancer. Acta Ophthalmol. 2008;86(5):515-519.
4. Abundo RE, Orenic CJ, Anderson SF, Townsend JC. Choroidal metastases resulting from carcinoma of the lung. J Am Optom Assoc. 1997;68(2):95-108.
5. Kreusel KM, Wiegel T, Stange M, Bornfeld N, Hinkelbein W, Foerster MH. Choroidal metastasis in disseminated lung cancer: frequency and risk factors. Am J Ophthalmol. 2002;134(3):445-447.
6. Singh N, Kulkarni P, Aggarwal AN, et al. Choroidal metastasis as a presenting manifestation of lung cancer: a report of 3 cases and systematic review of the literature. Medicine (Baltimore). 2012;91(4):179-194.
7. Chen CJ, McCoy AN, Brahmer J, Handa JT. Emerging treatments for choroidal metastases. Surv Ophthalmol. 2011;56(6):511-521.
8. Shah SU, Mashayekhi A, Shields CL, et al. Uveal metastasis from lung cancer: clinical features, treatment, and outcome in 194 patients. Ophthalmology. 2014;121(1):352-357.
9. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353(2):123-132.
10. Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385-2394.
11. Kim SW, Kim MJ, Huh K, Oh J. Complete regression of choroidal metastasis secondary to non-small-cell lung cancer with intravitreal bevacizumab and oral erlotinib combination therapy. Ophthalmologica. 2009;223(6):411-413.
12. George B, Wirostko WJ, Connor TB, Choong NW. Complete and durable response of choroid metastasis from non-small cell lung cancer with systemic bevacizumab and chemotherapy. J Thorac Oncol. 2009;4(5):661-662.
13. Inoue M, Watanabe Y, Yamane S, et al. Choroidal metastasis with adenocarcinoma of the lung treated with gefitinib. Eur J Ophthalmol. 2010;20(5):963-965.
14. Shimomura I, Tada Y, Miura G, et al. Choroidal metastasis of non-small cell lung cancer that responded to gefitinib. https://www.hindawi.com/journals/criopm/2013/213124/. Published 2013. Accessed May 4, 2017.
15. Feng Y, Singh AD, Lanigan C, Tubbs RR, Ma PC. Choroidal metastases responsive to crizotinib therapy in a lung adenocarcinoma patient with ALK 2p23 fusion identified by ALK immunohistochemistry. J Thorac Oncol. 2013;8(12):e109-111.
Resolution of refractory pruritus with aprepitant in a patient with microcystic adnexal carcinoma
Substance P is an important neurotransmitter implicated in itch pathways.1 After binding to its receptor, neurokinin-1 (NK-1), substance P induces release of factors including histamine, which may cause pruritus.2 Recent literature has reported successful use of aprepitant, an NK-1 antagonist that has been approved by the US Food and Drug Administration for the treatment of chemotherapy-induced nausea and vomiting, for treatment of pruritus. We report here the case of a patient with microcystic adnexal carcinoma (MAC) who presented with refractory pruritus and who had rapid and complete resolution of itch after administration of aprepitant.
Case presentation and summary
A 73-year-old man presented with a 12-year history of a small nodule on his philtrum, which had been increasing in size. He subsequently developed upper-lip numbness and nasal induration. He complained of 2.5 months of severe, debilitating, full-body pruritus. His symptoms were refractory to treatment with prednisone, gabapentin, doxycycline, doxepin, antihistamines, and topical steroids. At the time of consultation, he was being treated with hydroxyzine and topical pramocaine lotion with minimal relief.
At initial dermatologic evaluation, his tumor involved the lower two-thirds of the nose and entire upper cutaneous lip. There was a 4-mm rolled ulcer on the nasal tip and a 1-cm exophytic, smooth nodule on the left upper lip with palpable 4-cm submandibular adenopathy (Figure). Skin examination otherwise revealed linear excoriations on the upper back with no additional primary lesions. The nodule was biopsied, and the patient was diagnosed with MAC with gross nodal involvement. Laboratory findings including serum chemistries, blood urea nitrogen, complete blood cell count, thyroid, and liver function were normal. Positron emission tomography-computed tomography (PET-CT) imaging was negative for distant metastases.
Treatment was initiated with oral aprepitant – 125 mg on day 1, 80 mg on day 2, and 80 mg on day 3 –with concomitant weekly carboplatin (AUC 1.5) and paclitaxel (30 mg/m2) as well as radiation. Within hours after the first dose of aprepitant, the patient reported a notable cessation in his pruritus. He reported that after 5 hours, his skin “finally turned off” and over the hour that followed, he had complete resolution of symptoms. He completed chemoradiation with a significant disease response. Despite persistent MAC confined to the philtrum, he has been followed for over 2 years without recurrence of itch.
Discussion
MAC is an uncommon cutaneous malignancy of sweat and eccrine gland differentiation. In all, 700 cases of MAC have been described in the literature; a 2008 review estimated the incidence of metastasis at around 2.1%.3 Though metastasis is exceedingly rare, the tumor is locally aggressive and there are reports of invasion into the muscle, perichondrium, periosteum, bone marrow, as well as perineural spaces and vascular adventitia.4
The clinical presentation of MAC includes smooth, flesh-colored or yellow papules, nodules, or plaques.3 Patients often present with numbness, paresthesia, and burning in the area of involvement because of neural infiltration with tumor. Despite the rarity of MAC, pruritus has been reported as a presenting symptom in 1 other case in the literature.4 Our case represents the first report of MAC presenting with a grossly enlarging centrofacial mass, lymph node involvement, and severe full-body pruritus. Our patient responded completely, and within hours, to treatment with aprepitant after experiencing months of failure with conventional antipruritus treatments and without recurrence in symptoms in more than 2 years of follow-up.
Aprepitant blocks the binding of substance P to its receptor NK-1 and has been approved as an anti-emetic for chemotherapy patients. Substance P has been shown to be important in both nausea and itch pathways. The largest prospective study to date on aprepitant for the indication of pruritus in 45 patients with metastatic solid tumors demonstrated a 91% response rate, defined by >50% reduction in pruritus intensity, and 13% recurrence rate that occurred at a median of 7 weeks after initial treatment.5 Aprepitant treatment has been used with success for pruritus associated with both malignant and nonmalignant conditions in at least 74 patients,6 among whom the malignant conditions included cutaneous T-cell lymphoma, Hodgkin lymphoma, and metastatic solid tumors.5-7 Aprepitant has also been used for erlotinib- and nivolumab-induced pruritus in non–small cell lung cancer, which suggests a possible future role for aprepitant in the treatment of pruritus secondary to novel cancer therapies, perhaps including immune checkpoint inhibitors.8-10
However, despite those reports, and likely owing to the multifactorial nature of pruritus, aprepitant is not unviversally effective. Mechanisms of malignancy-associated itch are yet to be elucidated, and optimal patient selection for aprepitant use needs to be determined. However, our patient’s notable response supports the increasing evidence that substance P is a key mediator of pruritus and that disruption of binding to its receptor may result in significant improvement in symptoms in certain patients. It remains to be seen whether the cell type or the tendency toward neural invasion plays a role. Large, randomized studies are needed to guide patient selection and confirm the findings reported here and in the literature, with careful documentation of and close attention paid to timing of pruritus relief and improvement in patient quality of life. Aprepitant might be an important therapeutic tool for refractory, malignancy-associated pruritus, in which patient quality of life is especially critical.
Acknowledgments
This work was presented at the Multinational Association of Supportive Care and Cancer Meeting, in Miami Florida, June 26-28, 2014. The authors are indebted to Saajar Jadeja for his assistance preparing the manuscript.
1. Wallengren J. Neuroanatomy and neurophysiology of itch. Dermatol Ther. 2005;18(4):292-303.
2. Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology. 2008;123(3):398-410.
3. Wetter R, Goldstein GD. Microcystic adnexal carcinoma: a diagnostic and therapeutic challenge. Dermatol Ther. 2008;21(6):452-458.
4. Adamson T. Microcystic adnexal carcinoma. Dermatol Nurs. 2004;16(4):365.
5. Santini D, Vincenzi B, Guida FM, et al. Aprepitant for management of severe pruritus related to biological cancer treatments: a pilot study. Lancet Oncol. 2012;13(10):1020-1024.
6. Song JS, Tawa M, Chau NG, Kupper TS, LeBoeuf NR. Aprepitant for refractory cutaneous T-cell lymphoma-associated pruritus: 4 cases and a review of the literature. BMC Cancer. 2017;17.
7. Villafranca JJA, Siles MG, Casanova M, Goitia BT, Domínguez AR. Paraneoplastic pruritus presenting with Hodgkin’s lymphoma: a case report. J Med Case Reports. 2014;8:300.
8. Ito J, Fujimoto D, Nakamura A, et al. Aprepitant for refractory nivolumab-induced pruritus. Lung Cancer Amst Neth. 2017;109:58-61.
9. Levêque D. Aprepitant for erlotinib-induced pruritus. N Engl J Med. 2010;363(17):1680-1681; author reply 1681.
10. Gerber PA, Buhren BA, Homey B. More on aprepitant for erlotinib-induced pruritus. N Engl J Med. 2011;364(5):486-487.
Substance P is an important neurotransmitter implicated in itch pathways.1 After binding to its receptor, neurokinin-1 (NK-1), substance P induces release of factors including histamine, which may cause pruritus.2 Recent literature has reported successful use of aprepitant, an NK-1 antagonist that has been approved by the US Food and Drug Administration for the treatment of chemotherapy-induced nausea and vomiting, for treatment of pruritus. We report here the case of a patient with microcystic adnexal carcinoma (MAC) who presented with refractory pruritus and who had rapid and complete resolution of itch after administration of aprepitant.
Case presentation and summary
A 73-year-old man presented with a 12-year history of a small nodule on his philtrum, which had been increasing in size. He subsequently developed upper-lip numbness and nasal induration. He complained of 2.5 months of severe, debilitating, full-body pruritus. His symptoms were refractory to treatment with prednisone, gabapentin, doxycycline, doxepin, antihistamines, and topical steroids. At the time of consultation, he was being treated with hydroxyzine and topical pramocaine lotion with minimal relief.
At initial dermatologic evaluation, his tumor involved the lower two-thirds of the nose and entire upper cutaneous lip. There was a 4-mm rolled ulcer on the nasal tip and a 1-cm exophytic, smooth nodule on the left upper lip with palpable 4-cm submandibular adenopathy (Figure). Skin examination otherwise revealed linear excoriations on the upper back with no additional primary lesions. The nodule was biopsied, and the patient was diagnosed with MAC with gross nodal involvement. Laboratory findings including serum chemistries, blood urea nitrogen, complete blood cell count, thyroid, and liver function were normal. Positron emission tomography-computed tomography (PET-CT) imaging was negative for distant metastases.
Treatment was initiated with oral aprepitant – 125 mg on day 1, 80 mg on day 2, and 80 mg on day 3 –with concomitant weekly carboplatin (AUC 1.5) and paclitaxel (30 mg/m2) as well as radiation. Within hours after the first dose of aprepitant, the patient reported a notable cessation in his pruritus. He reported that after 5 hours, his skin “finally turned off” and over the hour that followed, he had complete resolution of symptoms. He completed chemoradiation with a significant disease response. Despite persistent MAC confined to the philtrum, he has been followed for over 2 years without recurrence of itch.
Discussion
MAC is an uncommon cutaneous malignancy of sweat and eccrine gland differentiation. In all, 700 cases of MAC have been described in the literature; a 2008 review estimated the incidence of metastasis at around 2.1%.3 Though metastasis is exceedingly rare, the tumor is locally aggressive and there are reports of invasion into the muscle, perichondrium, periosteum, bone marrow, as well as perineural spaces and vascular adventitia.4
The clinical presentation of MAC includes smooth, flesh-colored or yellow papules, nodules, or plaques.3 Patients often present with numbness, paresthesia, and burning in the area of involvement because of neural infiltration with tumor. Despite the rarity of MAC, pruritus has been reported as a presenting symptom in 1 other case in the literature.4 Our case represents the first report of MAC presenting with a grossly enlarging centrofacial mass, lymph node involvement, and severe full-body pruritus. Our patient responded completely, and within hours, to treatment with aprepitant after experiencing months of failure with conventional antipruritus treatments and without recurrence in symptoms in more than 2 years of follow-up.
Aprepitant blocks the binding of substance P to its receptor NK-1 and has been approved as an anti-emetic for chemotherapy patients. Substance P has been shown to be important in both nausea and itch pathways. The largest prospective study to date on aprepitant for the indication of pruritus in 45 patients with metastatic solid tumors demonstrated a 91% response rate, defined by >50% reduction in pruritus intensity, and 13% recurrence rate that occurred at a median of 7 weeks after initial treatment.5 Aprepitant treatment has been used with success for pruritus associated with both malignant and nonmalignant conditions in at least 74 patients,6 among whom the malignant conditions included cutaneous T-cell lymphoma, Hodgkin lymphoma, and metastatic solid tumors.5-7 Aprepitant has also been used for erlotinib- and nivolumab-induced pruritus in non–small cell lung cancer, which suggests a possible future role for aprepitant in the treatment of pruritus secondary to novel cancer therapies, perhaps including immune checkpoint inhibitors.8-10
However, despite those reports, and likely owing to the multifactorial nature of pruritus, aprepitant is not unviversally effective. Mechanisms of malignancy-associated itch are yet to be elucidated, and optimal patient selection for aprepitant use needs to be determined. However, our patient’s notable response supports the increasing evidence that substance P is a key mediator of pruritus and that disruption of binding to its receptor may result in significant improvement in symptoms in certain patients. It remains to be seen whether the cell type or the tendency toward neural invasion plays a role. Large, randomized studies are needed to guide patient selection and confirm the findings reported here and in the literature, with careful documentation of and close attention paid to timing of pruritus relief and improvement in patient quality of life. Aprepitant might be an important therapeutic tool for refractory, malignancy-associated pruritus, in which patient quality of life is especially critical.
Acknowledgments
This work was presented at the Multinational Association of Supportive Care and Cancer Meeting, in Miami Florida, June 26-28, 2014. The authors are indebted to Saajar Jadeja for his assistance preparing the manuscript.
Substance P is an important neurotransmitter implicated in itch pathways.1 After binding to its receptor, neurokinin-1 (NK-1), substance P induces release of factors including histamine, which may cause pruritus.2 Recent literature has reported successful use of aprepitant, an NK-1 antagonist that has been approved by the US Food and Drug Administration for the treatment of chemotherapy-induced nausea and vomiting, for treatment of pruritus. We report here the case of a patient with microcystic adnexal carcinoma (MAC) who presented with refractory pruritus and who had rapid and complete resolution of itch after administration of aprepitant.
Case presentation and summary
A 73-year-old man presented with a 12-year history of a small nodule on his philtrum, which had been increasing in size. He subsequently developed upper-lip numbness and nasal induration. He complained of 2.5 months of severe, debilitating, full-body pruritus. His symptoms were refractory to treatment with prednisone, gabapentin, doxycycline, doxepin, antihistamines, and topical steroids. At the time of consultation, he was being treated with hydroxyzine and topical pramocaine lotion with minimal relief.
At initial dermatologic evaluation, his tumor involved the lower two-thirds of the nose and entire upper cutaneous lip. There was a 4-mm rolled ulcer on the nasal tip and a 1-cm exophytic, smooth nodule on the left upper lip with palpable 4-cm submandibular adenopathy (Figure). Skin examination otherwise revealed linear excoriations on the upper back with no additional primary lesions. The nodule was biopsied, and the patient was diagnosed with MAC with gross nodal involvement. Laboratory findings including serum chemistries, blood urea nitrogen, complete blood cell count, thyroid, and liver function were normal. Positron emission tomography-computed tomography (PET-CT) imaging was negative for distant metastases.
Treatment was initiated with oral aprepitant – 125 mg on day 1, 80 mg on day 2, and 80 mg on day 3 –with concomitant weekly carboplatin (AUC 1.5) and paclitaxel (30 mg/m2) as well as radiation. Within hours after the first dose of aprepitant, the patient reported a notable cessation in his pruritus. He reported that after 5 hours, his skin “finally turned off” and over the hour that followed, he had complete resolution of symptoms. He completed chemoradiation with a significant disease response. Despite persistent MAC confined to the philtrum, he has been followed for over 2 years without recurrence of itch.
Discussion
MAC is an uncommon cutaneous malignancy of sweat and eccrine gland differentiation. In all, 700 cases of MAC have been described in the literature; a 2008 review estimated the incidence of metastasis at around 2.1%.3 Though metastasis is exceedingly rare, the tumor is locally aggressive and there are reports of invasion into the muscle, perichondrium, periosteum, bone marrow, as well as perineural spaces and vascular adventitia.4
The clinical presentation of MAC includes smooth, flesh-colored or yellow papules, nodules, or plaques.3 Patients often present with numbness, paresthesia, and burning in the area of involvement because of neural infiltration with tumor. Despite the rarity of MAC, pruritus has been reported as a presenting symptom in 1 other case in the literature.4 Our case represents the first report of MAC presenting with a grossly enlarging centrofacial mass, lymph node involvement, and severe full-body pruritus. Our patient responded completely, and within hours, to treatment with aprepitant after experiencing months of failure with conventional antipruritus treatments and without recurrence in symptoms in more than 2 years of follow-up.
Aprepitant blocks the binding of substance P to its receptor NK-1 and has been approved as an anti-emetic for chemotherapy patients. Substance P has been shown to be important in both nausea and itch pathways. The largest prospective study to date on aprepitant for the indication of pruritus in 45 patients with metastatic solid tumors demonstrated a 91% response rate, defined by >50% reduction in pruritus intensity, and 13% recurrence rate that occurred at a median of 7 weeks after initial treatment.5 Aprepitant treatment has been used with success for pruritus associated with both malignant and nonmalignant conditions in at least 74 patients,6 among whom the malignant conditions included cutaneous T-cell lymphoma, Hodgkin lymphoma, and metastatic solid tumors.5-7 Aprepitant has also been used for erlotinib- and nivolumab-induced pruritus in non–small cell lung cancer, which suggests a possible future role for aprepitant in the treatment of pruritus secondary to novel cancer therapies, perhaps including immune checkpoint inhibitors.8-10
However, despite those reports, and likely owing to the multifactorial nature of pruritus, aprepitant is not unviversally effective. Mechanisms of malignancy-associated itch are yet to be elucidated, and optimal patient selection for aprepitant use needs to be determined. However, our patient’s notable response supports the increasing evidence that substance P is a key mediator of pruritus and that disruption of binding to its receptor may result in significant improvement in symptoms in certain patients. It remains to be seen whether the cell type or the tendency toward neural invasion plays a role. Large, randomized studies are needed to guide patient selection and confirm the findings reported here and in the literature, with careful documentation of and close attention paid to timing of pruritus relief and improvement in patient quality of life. Aprepitant might be an important therapeutic tool for refractory, malignancy-associated pruritus, in which patient quality of life is especially critical.
Acknowledgments
This work was presented at the Multinational Association of Supportive Care and Cancer Meeting, in Miami Florida, June 26-28, 2014. The authors are indebted to Saajar Jadeja for his assistance preparing the manuscript.
1. Wallengren J. Neuroanatomy and neurophysiology of itch. Dermatol Ther. 2005;18(4):292-303.
2. Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology. 2008;123(3):398-410.
3. Wetter R, Goldstein GD. Microcystic adnexal carcinoma: a diagnostic and therapeutic challenge. Dermatol Ther. 2008;21(6):452-458.
4. Adamson T. Microcystic adnexal carcinoma. Dermatol Nurs. 2004;16(4):365.
5. Santini D, Vincenzi B, Guida FM, et al. Aprepitant for management of severe pruritus related to biological cancer treatments: a pilot study. Lancet Oncol. 2012;13(10):1020-1024.
6. Song JS, Tawa M, Chau NG, Kupper TS, LeBoeuf NR. Aprepitant for refractory cutaneous T-cell lymphoma-associated pruritus: 4 cases and a review of the literature. BMC Cancer. 2017;17.
7. Villafranca JJA, Siles MG, Casanova M, Goitia BT, Domínguez AR. Paraneoplastic pruritus presenting with Hodgkin’s lymphoma: a case report. J Med Case Reports. 2014;8:300.
8. Ito J, Fujimoto D, Nakamura A, et al. Aprepitant for refractory nivolumab-induced pruritus. Lung Cancer Amst Neth. 2017;109:58-61.
9. Levêque D. Aprepitant for erlotinib-induced pruritus. N Engl J Med. 2010;363(17):1680-1681; author reply 1681.
10. Gerber PA, Buhren BA, Homey B. More on aprepitant for erlotinib-induced pruritus. N Engl J Med. 2011;364(5):486-487.
1. Wallengren J. Neuroanatomy and neurophysiology of itch. Dermatol Ther. 2005;18(4):292-303.
2. Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology. 2008;123(3):398-410.
3. Wetter R, Goldstein GD. Microcystic adnexal carcinoma: a diagnostic and therapeutic challenge. Dermatol Ther. 2008;21(6):452-458.
4. Adamson T. Microcystic adnexal carcinoma. Dermatol Nurs. 2004;16(4):365.
5. Santini D, Vincenzi B, Guida FM, et al. Aprepitant for management of severe pruritus related to biological cancer treatments: a pilot study. Lancet Oncol. 2012;13(10):1020-1024.
6. Song JS, Tawa M, Chau NG, Kupper TS, LeBoeuf NR. Aprepitant for refractory cutaneous T-cell lymphoma-associated pruritus: 4 cases and a review of the literature. BMC Cancer. 2017;17.
7. Villafranca JJA, Siles MG, Casanova M, Goitia BT, Domínguez AR. Paraneoplastic pruritus presenting with Hodgkin’s lymphoma: a case report. J Med Case Reports. 2014;8:300.
8. Ito J, Fujimoto D, Nakamura A, et al. Aprepitant for refractory nivolumab-induced pruritus. Lung Cancer Amst Neth. 2017;109:58-61.
9. Levêque D. Aprepitant for erlotinib-induced pruritus. N Engl J Med. 2010;363(17):1680-1681; author reply 1681.
10. Gerber PA, Buhren BA, Homey B. More on aprepitant for erlotinib-induced pruritus. N Engl J Med. 2011;364(5):486-487.
VIDEO: To boost newborn breastfeeding rates, hide the EHR formula order
AUSTIN, TEXAS – When the check box for ordering formula for newborns was removed as a standard newborn order option in the electronic health record (EHR), rates of exclusive breastfeeding climbed significantly in Los Angeles County hospitals, according to a recent study.
“The saying, ‘out of sight, out of mind’ cannot be overstated when it comes to physician order entry,” wrote Ramy Eskander, MD, and his colleagues in the poster accompanying the presentation at the annual clinical and scientific sessions of the American College of Obstetricians and Gynecologists.
In a video interview, Dr. Eskander said that he and his colleagues at the University of California, Los Angeles, were looking for an intervention that would use the EHR as a quality improvement tool.
What they decided to do was to see “how could we possibly ‘get in the way’ and have an intervention between the provider and the patient that didn’t necessarily involve much work on the provider’s end, that had a significant impact on the back end,” he said. What they ended up doing was remove the order to request formula for mothers from the physician order set in the EHR.
Study data were collected in three stages for the academic tertiary care hospital within the Los Angeles County Department of Health Services system.
First, Dr. Eskander and his colleagues collected baseline data from January to July of 2016. Then, data were collected from July to the end of 2016, while a campaign was underway to bring staff and patients up to speed on the benefits of exclusive breastfeeding. There were no statistically significant differences in the rates of exclusive breastfeeding on discharge between these two time periods, when rates hovered between 30% and 40%.
The final data collection period began in January 2017. At that time, the option to order formula for a newborn was removed as an option for the EHR newborn order set.
“When we did that, providers weren’t looking at the possibility of having that easy check box right there to fill in … and we know that when people have to go through more steps, they invariably don’t do it,” Dr. Eskander said.
He and his colleagues saw an almost immediate . Once the formula order was removed, breastfeeding rates rose from 40.57% to 53.90% (P less than .001). Rates have been sustained since the removal of the EHR option for formula.
There was no difference in how infants fared after the intervention, said Dr. Eskander. “The outcomes for those infants was identical. There were no increased NICU admissions, there were no increased poor outcomes.”
Length of stay remained the same as well. “The babies were being discharged in the same state of health, just more of them were getting breast milk only, and we know the benefits that tends to portend,” he added.
There was some initial grumbling when the formula order was pulled from the newborn order set, he conceded. “The providers were not very happy about having to look for the newborn order for formula.” However, it took just about a month for the new workflow to seem normal, he said.
Dr. Eskander envisions a future where the EHR is “smart” enough to prompt appropriate orders and interventions for serious conditions such as preeclampsia. The electronic record, he said, could recognize the maternal diagnosis “and immediately create a system and structure around that mother to be able to help protect her and her baby. ... then having those diagnoses be able to drive outcomes can be very significant.”
Dr. Eskander reported no relevant financial disclosures.
SOURCE: Eskander, R et al. ACOG 2018, Abstract 31I.
AUSTIN, TEXAS – When the check box for ordering formula for newborns was removed as a standard newborn order option in the electronic health record (EHR), rates of exclusive breastfeeding climbed significantly in Los Angeles County hospitals, according to a recent study.
“The saying, ‘out of sight, out of mind’ cannot be overstated when it comes to physician order entry,” wrote Ramy Eskander, MD, and his colleagues in the poster accompanying the presentation at the annual clinical and scientific sessions of the American College of Obstetricians and Gynecologists.
In a video interview, Dr. Eskander said that he and his colleagues at the University of California, Los Angeles, were looking for an intervention that would use the EHR as a quality improvement tool.
What they decided to do was to see “how could we possibly ‘get in the way’ and have an intervention between the provider and the patient that didn’t necessarily involve much work on the provider’s end, that had a significant impact on the back end,” he said. What they ended up doing was remove the order to request formula for mothers from the physician order set in the EHR.
Study data were collected in three stages for the academic tertiary care hospital within the Los Angeles County Department of Health Services system.
First, Dr. Eskander and his colleagues collected baseline data from January to July of 2016. Then, data were collected from July to the end of 2016, while a campaign was underway to bring staff and patients up to speed on the benefits of exclusive breastfeeding. There were no statistically significant differences in the rates of exclusive breastfeeding on discharge between these two time periods, when rates hovered between 30% and 40%.
The final data collection period began in January 2017. At that time, the option to order formula for a newborn was removed as an option for the EHR newborn order set.
“When we did that, providers weren’t looking at the possibility of having that easy check box right there to fill in … and we know that when people have to go through more steps, they invariably don’t do it,” Dr. Eskander said.
He and his colleagues saw an almost immediate . Once the formula order was removed, breastfeeding rates rose from 40.57% to 53.90% (P less than .001). Rates have been sustained since the removal of the EHR option for formula.
There was no difference in how infants fared after the intervention, said Dr. Eskander. “The outcomes for those infants was identical. There were no increased NICU admissions, there were no increased poor outcomes.”
Length of stay remained the same as well. “The babies were being discharged in the same state of health, just more of them were getting breast milk only, and we know the benefits that tends to portend,” he added.
There was some initial grumbling when the formula order was pulled from the newborn order set, he conceded. “The providers were not very happy about having to look for the newborn order for formula.” However, it took just about a month for the new workflow to seem normal, he said.
Dr. Eskander envisions a future where the EHR is “smart” enough to prompt appropriate orders and interventions for serious conditions such as preeclampsia. The electronic record, he said, could recognize the maternal diagnosis “and immediately create a system and structure around that mother to be able to help protect her and her baby. ... then having those diagnoses be able to drive outcomes can be very significant.”
Dr. Eskander reported no relevant financial disclosures.
SOURCE: Eskander, R et al. ACOG 2018, Abstract 31I.
AUSTIN, TEXAS – When the check box for ordering formula for newborns was removed as a standard newborn order option in the electronic health record (EHR), rates of exclusive breastfeeding climbed significantly in Los Angeles County hospitals, according to a recent study.
“The saying, ‘out of sight, out of mind’ cannot be overstated when it comes to physician order entry,” wrote Ramy Eskander, MD, and his colleagues in the poster accompanying the presentation at the annual clinical and scientific sessions of the American College of Obstetricians and Gynecologists.
In a video interview, Dr. Eskander said that he and his colleagues at the University of California, Los Angeles, were looking for an intervention that would use the EHR as a quality improvement tool.
What they decided to do was to see “how could we possibly ‘get in the way’ and have an intervention between the provider and the patient that didn’t necessarily involve much work on the provider’s end, that had a significant impact on the back end,” he said. What they ended up doing was remove the order to request formula for mothers from the physician order set in the EHR.
Study data were collected in three stages for the academic tertiary care hospital within the Los Angeles County Department of Health Services system.
First, Dr. Eskander and his colleagues collected baseline data from January to July of 2016. Then, data were collected from July to the end of 2016, while a campaign was underway to bring staff and patients up to speed on the benefits of exclusive breastfeeding. There were no statistically significant differences in the rates of exclusive breastfeeding on discharge between these two time periods, when rates hovered between 30% and 40%.
The final data collection period began in January 2017. At that time, the option to order formula for a newborn was removed as an option for the EHR newborn order set.
“When we did that, providers weren’t looking at the possibility of having that easy check box right there to fill in … and we know that when people have to go through more steps, they invariably don’t do it,” Dr. Eskander said.
He and his colleagues saw an almost immediate . Once the formula order was removed, breastfeeding rates rose from 40.57% to 53.90% (P less than .001). Rates have been sustained since the removal of the EHR option for formula.
There was no difference in how infants fared after the intervention, said Dr. Eskander. “The outcomes for those infants was identical. There were no increased NICU admissions, there were no increased poor outcomes.”
Length of stay remained the same as well. “The babies were being discharged in the same state of health, just more of them were getting breast milk only, and we know the benefits that tends to portend,” he added.
There was some initial grumbling when the formula order was pulled from the newborn order set, he conceded. “The providers were not very happy about having to look for the newborn order for formula.” However, it took just about a month for the new workflow to seem normal, he said.
Dr. Eskander envisions a future where the EHR is “smart” enough to prompt appropriate orders and interventions for serious conditions such as preeclampsia. The electronic record, he said, could recognize the maternal diagnosis “and immediately create a system and structure around that mother to be able to help protect her and her baby. ... then having those diagnoses be able to drive outcomes can be very significant.”
Dr. Eskander reported no relevant financial disclosures.
SOURCE: Eskander, R et al. ACOG 2018, Abstract 31I.
REPORTING FROM ACOG 2018
Patient-reported outcomes show impairment decades after acute knee injury
LIVERPOOL, ENGLAND – Decades after they were sustained, acute knee injuries caused clinically significant impairments in patient-reported outcomes, as well as upped the risk for knee osteoarthritis (OA) in an observational study.
Results of the study, which followed up individuals 32-37 years after they were treated for a ruptured anterior cruciate ligament (ACL) injury between 1980 and 1985, showed that, compared with the general population, they experienced greater levels of knee pain, participated less in physical activities, and had a reduced quality of life.
The link between OA and ACL injury is not new, with prior estimates suggesting that up to half of all patients with ACL injury develop OA within 10 years of the injury, said Stephanie Filbay, PhD, who presented the results of the study at the World Congress on Osteoarthritis. There have also been reports of knee pain and other symptoms, and poor quality of life more than 5 years later. What’s not been known until now, however, is what happens with even longer term follow-up, said Dr. Filbay, a postdoctoral research fellow in sport, exercise, and osteoarthritis at the University of Oxford, England.
The aims of the study were to compare patient-reported outcomes at 32-37-years’ follow-up against the general population, then to see if the baseline injury or treatment approach, or knee function 3-7 years after the initial injury had any influence on outcomes.
The study included 223 patients who were between aged 15 and 40 years at the time of the acute ACL injury between 1980 and 1985 and who had been seen within 2 weeks of ACL rupture at Linköping University Hospital in Linköping, Sweden. Patients had been allocated to early surgical or non-surgical treatment based on having an odd or even birth year. They had then been assessed 3-7 years later using a variety of tests to determine the strength of their quadriceps and hamstrings and the ability to hop on one leg.
All patients were then invited 32-37 years later after the initial injury to complete questionnaires and undergo clinical examination and X-rays. Only four people declined and 38 did not answer, leaving 181 (81%) people who agreed to participate and complete the Knee injury and Osteoarthritis Outcome Score (KOOS) and the ACL quality of life questionnaire (ACL-QOL).
The average age of participants at follow-up was 59 years (range, 47-74 years); 30% were female. 58% of all patients had been treated non-surgically initially, and 38% remained non-surgically treated at the longterm follow-up. At baseline, 58% had a meniscus injury.
Compared with an age- and sex-matched Swedish population, patients with ACL injuries had a lower KOOS for pain, sport/recreational activities, and quality of life. For example, KOOS for knee pain was around 65-70 for those with prior ACL injuries, compared with 80-90 for those without ACL injuries, where 100 indicates the best outcome or least pain and zero the worst.
KOOS was not affected by whether or not patients had initial ACL surgery or surgery at any point in their follow up. It also did not appear to matter if patients had a meniscal injury at baseline or not.
Quadriceps and hamstring strength at the 3-7 year postinjury assessment did not affect the longterm KOOS, but the ability to hop on one leg did: Those who were not able to hop on one leg for more than 90% of the time on the unaffected limb at the 3-7 years follow-up had worse pain, symptoms, function, and quality of life at the longterm follow-up point.
With regards to OA, “overall, more than one in two individuals had Kellgren-Lawrence grade 4 that could be considered severe radiographic changes in at least one compartment,” Dr. Filbay said at the meeting, which is sponsored by the Osteoarthritis Research Society International.
Severe radiographic changes were most common in the tibiofemoral joint, with around 47% having Kellgren-Lawrence (KL) grade 4. About 35% of tibiofemoral joints and about 60% of patellofemoral joints were KL grade 1.
Interestingly, different factors were found to be associated with OA in the tibiofemoral and patellofemoral joints, according to Dr. Filbay. Patients who had been treated non-surgically, whether initially or at any time during the 32-37 year follow-up, were more likely to have tibiofemoral OA, whereas those who had been treated surgically tended to have patellofemoral OA.
“Perhaps not surprisingly, meniscal injury at baseline was related to a higher percentage of tibiofemoral OA at long-term follow-up,” Dr. Filbay said.
Another finding was that patients with weaker hamstrings 3-7 years after the injury were more likely to develop patellofemoral joint OA.
Dr. Filbay had no disclosures.
SOURCE: Filbay S, et al. Osteoarthritis Cartilage 2018:26(1):S52-3. Abstract 80.
LIVERPOOL, ENGLAND – Decades after they were sustained, acute knee injuries caused clinically significant impairments in patient-reported outcomes, as well as upped the risk for knee osteoarthritis (OA) in an observational study.
Results of the study, which followed up individuals 32-37 years after they were treated for a ruptured anterior cruciate ligament (ACL) injury between 1980 and 1985, showed that, compared with the general population, they experienced greater levels of knee pain, participated less in physical activities, and had a reduced quality of life.
The link between OA and ACL injury is not new, with prior estimates suggesting that up to half of all patients with ACL injury develop OA within 10 years of the injury, said Stephanie Filbay, PhD, who presented the results of the study at the World Congress on Osteoarthritis. There have also been reports of knee pain and other symptoms, and poor quality of life more than 5 years later. What’s not been known until now, however, is what happens with even longer term follow-up, said Dr. Filbay, a postdoctoral research fellow in sport, exercise, and osteoarthritis at the University of Oxford, England.
The aims of the study were to compare patient-reported outcomes at 32-37-years’ follow-up against the general population, then to see if the baseline injury or treatment approach, or knee function 3-7 years after the initial injury had any influence on outcomes.
The study included 223 patients who were between aged 15 and 40 years at the time of the acute ACL injury between 1980 and 1985 and who had been seen within 2 weeks of ACL rupture at Linköping University Hospital in Linköping, Sweden. Patients had been allocated to early surgical or non-surgical treatment based on having an odd or even birth year. They had then been assessed 3-7 years later using a variety of tests to determine the strength of their quadriceps and hamstrings and the ability to hop on one leg.
All patients were then invited 32-37 years later after the initial injury to complete questionnaires and undergo clinical examination and X-rays. Only four people declined and 38 did not answer, leaving 181 (81%) people who agreed to participate and complete the Knee injury and Osteoarthritis Outcome Score (KOOS) and the ACL quality of life questionnaire (ACL-QOL).
The average age of participants at follow-up was 59 years (range, 47-74 years); 30% were female. 58% of all patients had been treated non-surgically initially, and 38% remained non-surgically treated at the longterm follow-up. At baseline, 58% had a meniscus injury.
Compared with an age- and sex-matched Swedish population, patients with ACL injuries had a lower KOOS for pain, sport/recreational activities, and quality of life. For example, KOOS for knee pain was around 65-70 for those with prior ACL injuries, compared with 80-90 for those without ACL injuries, where 100 indicates the best outcome or least pain and zero the worst.
KOOS was not affected by whether or not patients had initial ACL surgery or surgery at any point in their follow up. It also did not appear to matter if patients had a meniscal injury at baseline or not.
Quadriceps and hamstring strength at the 3-7 year postinjury assessment did not affect the longterm KOOS, but the ability to hop on one leg did: Those who were not able to hop on one leg for more than 90% of the time on the unaffected limb at the 3-7 years follow-up had worse pain, symptoms, function, and quality of life at the longterm follow-up point.
With regards to OA, “overall, more than one in two individuals had Kellgren-Lawrence grade 4 that could be considered severe radiographic changes in at least one compartment,” Dr. Filbay said at the meeting, which is sponsored by the Osteoarthritis Research Society International.
Severe radiographic changes were most common in the tibiofemoral joint, with around 47% having Kellgren-Lawrence (KL) grade 4. About 35% of tibiofemoral joints and about 60% of patellofemoral joints were KL grade 1.
Interestingly, different factors were found to be associated with OA in the tibiofemoral and patellofemoral joints, according to Dr. Filbay. Patients who had been treated non-surgically, whether initially or at any time during the 32-37 year follow-up, were more likely to have tibiofemoral OA, whereas those who had been treated surgically tended to have patellofemoral OA.
“Perhaps not surprisingly, meniscal injury at baseline was related to a higher percentage of tibiofemoral OA at long-term follow-up,” Dr. Filbay said.
Another finding was that patients with weaker hamstrings 3-7 years after the injury were more likely to develop patellofemoral joint OA.
Dr. Filbay had no disclosures.
SOURCE: Filbay S, et al. Osteoarthritis Cartilage 2018:26(1):S52-3. Abstract 80.
LIVERPOOL, ENGLAND – Decades after they were sustained, acute knee injuries caused clinically significant impairments in patient-reported outcomes, as well as upped the risk for knee osteoarthritis (OA) in an observational study.
Results of the study, which followed up individuals 32-37 years after they were treated for a ruptured anterior cruciate ligament (ACL) injury between 1980 and 1985, showed that, compared with the general population, they experienced greater levels of knee pain, participated less in physical activities, and had a reduced quality of life.
The link between OA and ACL injury is not new, with prior estimates suggesting that up to half of all patients with ACL injury develop OA within 10 years of the injury, said Stephanie Filbay, PhD, who presented the results of the study at the World Congress on Osteoarthritis. There have also been reports of knee pain and other symptoms, and poor quality of life more than 5 years later. What’s not been known until now, however, is what happens with even longer term follow-up, said Dr. Filbay, a postdoctoral research fellow in sport, exercise, and osteoarthritis at the University of Oxford, England.
The aims of the study were to compare patient-reported outcomes at 32-37-years’ follow-up against the general population, then to see if the baseline injury or treatment approach, or knee function 3-7 years after the initial injury had any influence on outcomes.
The study included 223 patients who were between aged 15 and 40 years at the time of the acute ACL injury between 1980 and 1985 and who had been seen within 2 weeks of ACL rupture at Linköping University Hospital in Linköping, Sweden. Patients had been allocated to early surgical or non-surgical treatment based on having an odd or even birth year. They had then been assessed 3-7 years later using a variety of tests to determine the strength of their quadriceps and hamstrings and the ability to hop on one leg.
All patients were then invited 32-37 years later after the initial injury to complete questionnaires and undergo clinical examination and X-rays. Only four people declined and 38 did not answer, leaving 181 (81%) people who agreed to participate and complete the Knee injury and Osteoarthritis Outcome Score (KOOS) and the ACL quality of life questionnaire (ACL-QOL).
The average age of participants at follow-up was 59 years (range, 47-74 years); 30% were female. 58% of all patients had been treated non-surgically initially, and 38% remained non-surgically treated at the longterm follow-up. At baseline, 58% had a meniscus injury.
Compared with an age- and sex-matched Swedish population, patients with ACL injuries had a lower KOOS for pain, sport/recreational activities, and quality of life. For example, KOOS for knee pain was around 65-70 for those with prior ACL injuries, compared with 80-90 for those without ACL injuries, where 100 indicates the best outcome or least pain and zero the worst.
KOOS was not affected by whether or not patients had initial ACL surgery or surgery at any point in their follow up. It also did not appear to matter if patients had a meniscal injury at baseline or not.
Quadriceps and hamstring strength at the 3-7 year postinjury assessment did not affect the longterm KOOS, but the ability to hop on one leg did: Those who were not able to hop on one leg for more than 90% of the time on the unaffected limb at the 3-7 years follow-up had worse pain, symptoms, function, and quality of life at the longterm follow-up point.
With regards to OA, “overall, more than one in two individuals had Kellgren-Lawrence grade 4 that could be considered severe radiographic changes in at least one compartment,” Dr. Filbay said at the meeting, which is sponsored by the Osteoarthritis Research Society International.
Severe radiographic changes were most common in the tibiofemoral joint, with around 47% having Kellgren-Lawrence (KL) grade 4. About 35% of tibiofemoral joints and about 60% of patellofemoral joints were KL grade 1.
Interestingly, different factors were found to be associated with OA in the tibiofemoral and patellofemoral joints, according to Dr. Filbay. Patients who had been treated non-surgically, whether initially or at any time during the 32-37 year follow-up, were more likely to have tibiofemoral OA, whereas those who had been treated surgically tended to have patellofemoral OA.
“Perhaps not surprisingly, meniscal injury at baseline was related to a higher percentage of tibiofemoral OA at long-term follow-up,” Dr. Filbay said.
Another finding was that patients with weaker hamstrings 3-7 years after the injury were more likely to develop patellofemoral joint OA.
Dr. Filbay had no disclosures.
SOURCE: Filbay S, et al. Osteoarthritis Cartilage 2018:26(1):S52-3. Abstract 80.
REPORTING FROM OARSI 2018
Key clinical point: Decades after rupturing the anterior cruciate ligament (ACL), patients can experience significant impairments.
Major finding: 70% of 136 of the patients in the study developed knee osteoarthritis 32-37 years after an ACL injury.Study details: A population-based, observational follow-up study of 181 individuals who had an acute ACL injury in 1980-1985. Disclosures: Stephanie Filbay, PhD., had no disclosures. Source: Filbay S, et al. Osteoarthritis Cartilage 2018:26(1):S52-53. Abstract 80.