User login
Heavy drinking did not worsen clinical outcomes from drug-induced liver injury
Heavy drinking was not associated with higher proportions of liver-related deaths or liver transplantation among patients with drug-induced liver injury (DILI), according to the results of a prospective multicenter cohort study reported in the May issue of Clinical Gastroenterology and Hepatology.
Anabolic steroids were the most common cause of DILI among heavy drinkers, defined as men who averaged more than three drinks a day or women who averaged more than two drinks daily, said Lara Dakhoul, MD, of Indiana University, Indianapolis, and her associates. There also was no evidence that heavy alcohol consumption increased the risk of liver injury attributable to isoniazid exposure, the researchers wrote in.
Although consuming alcohol significantly increases the risk of acetaminophen-induced liver injury, there is much less clarity about the relationship between drinking and hepatotoxicity from drugs such as duloxetine or antituberculosis medications, the researchers noted. In fact, one recent study found that drinking led to less severe liver injury among individuals with DILI. To better elucidate these links, the investigators studied 1,198 individuals with confirmed or probable DILI who enrolled in the DILI Network study (DILIN) between 2004 and 2016. At enrollment, all participants were asked if they consumed alcohol, and those who reported drinking within the past 12 months were offered a shortened version of the Skinner Alcohol Dependence Scale to collect details on alcohol consumption, including type, amount, and frequency.
In all, 601 persons reported consuming at least one alcoholic drink in the preceding year, of whom 348 completed the Skinner questionnaire. A total of 80 individuals reported heavy alcohol consumption. Heavy drinkers were typically in their early 40s, while nondrinkers tended to be nearly 50 years old (P less than .01). Heavy drinkers were also more often men (63%) while nondrinkers were usually women (65%; P less than .01). Heavy drinkers were significantly more likely to have DILI secondary to anabolic steroid exposure (13%) than were nondrinkers (2%; P less than .001). However, latency, pattern of liver injury, peak enzyme levels, and patterns of recovery from steroid hepatotoxicity were similar regardless of alcohol history.
A total of eight patients with DILI died of liver-related causes or underwent liver transplantation, and proportions of patients with these outcomes were similar regardless of alcohol history. These eight patients had no evidence of hepatitis C virus infection, but three appeared to have underlying alcoholic liver disease with superimposed acute-on-chronic liver failure. Heavy drinkers did not have significantly higher DILI severity scores than nondrinkers, but they did have significantly higher peak serum levels of alanine aminotransferase (1,323 U/L vs. 754, respectively; P = .02) and significantly higher levels of bilirubin (16.1 vs. 12.7 mg/dL; P = .03).
The two fatal cases of DILI among heavy drinkers involved a 44-year-old man with underlying alcoholic cirrhosis and steatohepatitis who developed acute-on-chronic liver failure 11 days after starting niacin, and a 76-year-old man with chronic obstructive pulmonary disease and bronchitis flare who developed severe liver injury and skin rash 6 days after starting azithromycin.
The study was not able to assess whether heavy alcohol consumption contributed to liver injury from specific agents, the researchers said. Additionally, a substantial number of drinkers did not complete the Skinner questionnaire, and those who did might have underestimated or underreported their own alcohol consumption. “Counterbalancing these issues are the [study’s] unique strengths, such as prospective design, larger sample size, well-characterized DILI phenotype, and careful, structured adjudication of causality and severity,” the researchers wrote.
Funders included the National Institute of Diabetes and Digestive and Kidney Diseases and the National Cancer Institute. Dr. Dakhoul had no conflicts of interest. On coinvestigator disclosed ties to numerous pharmaceutical companies.
SOURCE: Dakhoul L et al. Clin Gastro Hepatol. 2018 Jan 3. doi: 10.1016/j.cgh.2017.12.036.
Heavy drinking was not associated with higher proportions of liver-related deaths or liver transplantation among patients with drug-induced liver injury (DILI), according to the results of a prospective multicenter cohort study reported in the May issue of Clinical Gastroenterology and Hepatology.
Anabolic steroids were the most common cause of DILI among heavy drinkers, defined as men who averaged more than three drinks a day or women who averaged more than two drinks daily, said Lara Dakhoul, MD, of Indiana University, Indianapolis, and her associates. There also was no evidence that heavy alcohol consumption increased the risk of liver injury attributable to isoniazid exposure, the researchers wrote in.
Although consuming alcohol significantly increases the risk of acetaminophen-induced liver injury, there is much less clarity about the relationship between drinking and hepatotoxicity from drugs such as duloxetine or antituberculosis medications, the researchers noted. In fact, one recent study found that drinking led to less severe liver injury among individuals with DILI. To better elucidate these links, the investigators studied 1,198 individuals with confirmed or probable DILI who enrolled in the DILI Network study (DILIN) between 2004 and 2016. At enrollment, all participants were asked if they consumed alcohol, and those who reported drinking within the past 12 months were offered a shortened version of the Skinner Alcohol Dependence Scale to collect details on alcohol consumption, including type, amount, and frequency.
In all, 601 persons reported consuming at least one alcoholic drink in the preceding year, of whom 348 completed the Skinner questionnaire. A total of 80 individuals reported heavy alcohol consumption. Heavy drinkers were typically in their early 40s, while nondrinkers tended to be nearly 50 years old (P less than .01). Heavy drinkers were also more often men (63%) while nondrinkers were usually women (65%; P less than .01). Heavy drinkers were significantly more likely to have DILI secondary to anabolic steroid exposure (13%) than were nondrinkers (2%; P less than .001). However, latency, pattern of liver injury, peak enzyme levels, and patterns of recovery from steroid hepatotoxicity were similar regardless of alcohol history.
A total of eight patients with DILI died of liver-related causes or underwent liver transplantation, and proportions of patients with these outcomes were similar regardless of alcohol history. These eight patients had no evidence of hepatitis C virus infection, but three appeared to have underlying alcoholic liver disease with superimposed acute-on-chronic liver failure. Heavy drinkers did not have significantly higher DILI severity scores than nondrinkers, but they did have significantly higher peak serum levels of alanine aminotransferase (1,323 U/L vs. 754, respectively; P = .02) and significantly higher levels of bilirubin (16.1 vs. 12.7 mg/dL; P = .03).
The two fatal cases of DILI among heavy drinkers involved a 44-year-old man with underlying alcoholic cirrhosis and steatohepatitis who developed acute-on-chronic liver failure 11 days after starting niacin, and a 76-year-old man with chronic obstructive pulmonary disease and bronchitis flare who developed severe liver injury and skin rash 6 days after starting azithromycin.
The study was not able to assess whether heavy alcohol consumption contributed to liver injury from specific agents, the researchers said. Additionally, a substantial number of drinkers did not complete the Skinner questionnaire, and those who did might have underestimated or underreported their own alcohol consumption. “Counterbalancing these issues are the [study’s] unique strengths, such as prospective design, larger sample size, well-characterized DILI phenotype, and careful, structured adjudication of causality and severity,” the researchers wrote.
Funders included the National Institute of Diabetes and Digestive and Kidney Diseases and the National Cancer Institute. Dr. Dakhoul had no conflicts of interest. On coinvestigator disclosed ties to numerous pharmaceutical companies.
SOURCE: Dakhoul L et al. Clin Gastro Hepatol. 2018 Jan 3. doi: 10.1016/j.cgh.2017.12.036.
Heavy drinking was not associated with higher proportions of liver-related deaths or liver transplantation among patients with drug-induced liver injury (DILI), according to the results of a prospective multicenter cohort study reported in the May issue of Clinical Gastroenterology and Hepatology.
Anabolic steroids were the most common cause of DILI among heavy drinkers, defined as men who averaged more than three drinks a day or women who averaged more than two drinks daily, said Lara Dakhoul, MD, of Indiana University, Indianapolis, and her associates. There also was no evidence that heavy alcohol consumption increased the risk of liver injury attributable to isoniazid exposure, the researchers wrote in.
Although consuming alcohol significantly increases the risk of acetaminophen-induced liver injury, there is much less clarity about the relationship between drinking and hepatotoxicity from drugs such as duloxetine or antituberculosis medications, the researchers noted. In fact, one recent study found that drinking led to less severe liver injury among individuals with DILI. To better elucidate these links, the investigators studied 1,198 individuals with confirmed or probable DILI who enrolled in the DILI Network study (DILIN) between 2004 and 2016. At enrollment, all participants were asked if they consumed alcohol, and those who reported drinking within the past 12 months were offered a shortened version of the Skinner Alcohol Dependence Scale to collect details on alcohol consumption, including type, amount, and frequency.
In all, 601 persons reported consuming at least one alcoholic drink in the preceding year, of whom 348 completed the Skinner questionnaire. A total of 80 individuals reported heavy alcohol consumption. Heavy drinkers were typically in their early 40s, while nondrinkers tended to be nearly 50 years old (P less than .01). Heavy drinkers were also more often men (63%) while nondrinkers were usually women (65%; P less than .01). Heavy drinkers were significantly more likely to have DILI secondary to anabolic steroid exposure (13%) than were nondrinkers (2%; P less than .001). However, latency, pattern of liver injury, peak enzyme levels, and patterns of recovery from steroid hepatotoxicity were similar regardless of alcohol history.
A total of eight patients with DILI died of liver-related causes or underwent liver transplantation, and proportions of patients with these outcomes were similar regardless of alcohol history. These eight patients had no evidence of hepatitis C virus infection, but three appeared to have underlying alcoholic liver disease with superimposed acute-on-chronic liver failure. Heavy drinkers did not have significantly higher DILI severity scores than nondrinkers, but they did have significantly higher peak serum levels of alanine aminotransferase (1,323 U/L vs. 754, respectively; P = .02) and significantly higher levels of bilirubin (16.1 vs. 12.7 mg/dL; P = .03).
The two fatal cases of DILI among heavy drinkers involved a 44-year-old man with underlying alcoholic cirrhosis and steatohepatitis who developed acute-on-chronic liver failure 11 days after starting niacin, and a 76-year-old man with chronic obstructive pulmonary disease and bronchitis flare who developed severe liver injury and skin rash 6 days after starting azithromycin.
The study was not able to assess whether heavy alcohol consumption contributed to liver injury from specific agents, the researchers said. Additionally, a substantial number of drinkers did not complete the Skinner questionnaire, and those who did might have underestimated or underreported their own alcohol consumption. “Counterbalancing these issues are the [study’s] unique strengths, such as prospective design, larger sample size, well-characterized DILI phenotype, and careful, structured adjudication of causality and severity,” the researchers wrote.
Funders included the National Institute of Diabetes and Digestive and Kidney Diseases and the National Cancer Institute. Dr. Dakhoul had no conflicts of interest. On coinvestigator disclosed ties to numerous pharmaceutical companies.
SOURCE: Dakhoul L et al. Clin Gastro Hepatol. 2018 Jan 3. doi: 10.1016/j.cgh.2017.12.036.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Heavy alcohol consumption was not associated with worse outcomes of drug-induced liver toxicity.
Major finding: Proportions of patients with liver-related deaths and liver transplantation were statistically similar regardless of alcohol consumption history (P = .18).
Study details: Prospective study of 1,198 individuals with probable drug-induced liver injury.
Disclosures: Funders included the National Institute of Diabetes and Digestive and Kidney Diseases and the National Cancer Institute. Dr. Dakhoul had no conflicts. One coinvestigator disclosed ties to numerous pharmaceutical companies.
Source: Dakhoul L et al. Clin Gastro Hepatol. 2018 Jan 3. doi: 10.1016/j.cgh.2017.12.036.
FDA: More COPD patients can use triple therapy
The Food and Drug Administration has approved a new indication for the chronic obstructive pulmonary disease (COPD) therapy fluticasone furoate/umeclidinium/vilanterol (Trelegy Ellipta), which allows physicians to prescribe the drug to a broader class of COPD patients, according to a statement from two pharmaceutical companies.
“Following the initial approval of Trelegy Ellipta in September, we have analysed the data from the IMPACT study and identified additional benefits that this important medicine offers patients with [COPD],” said Hal Barron, MD, chief scientific officer and president of research and development at GlaxoSmithKline, in the statement. “We are pleased that the robust data from the IMPACT study has enabled the expanded indication announced today and the FDA action has been taken so swiftly.”
The results of the IMPACT trial, which was the first study to compare a single-inhaler triple therapy with two dual therapies, were published on April 18 (N Engl J Med 2018. doi: 10.1056/NEJMoa1713901).
This study randomized patients to 52 weeks of either triple inhaled therapy involving a once-daily combination of 100 mcg fluticasone furoate, 62.5 mcg of umeclidinium, and 25 mcg of vilanterol; or dual inhaled therapy involving either 100 mcg fluticasone furoate plus 25 mcg of vilanterol, or 62.5 mcg of umeclidinium plus 25 mcg of vilanterol.
After 1 year, the rate of moderate to severe COPD exacerbations in the triple-therapy group was 0.91 per year, compared with 1.07 in the fluticasone furoate–vilanterol group and 1.21 in the vilanterol-umeclidinium group. This translated to a 15% reduction with triple therapy compared with fluticasone furoate–vilanterol and a 25% reduction, compared with vilanterol-umeclidinium (P less than .001 for both).
The Food and Drug Administration has approved a new indication for the chronic obstructive pulmonary disease (COPD) therapy fluticasone furoate/umeclidinium/vilanterol (Trelegy Ellipta), which allows physicians to prescribe the drug to a broader class of COPD patients, according to a statement from two pharmaceutical companies.
“Following the initial approval of Trelegy Ellipta in September, we have analysed the data from the IMPACT study and identified additional benefits that this important medicine offers patients with [COPD],” said Hal Barron, MD, chief scientific officer and president of research and development at GlaxoSmithKline, in the statement. “We are pleased that the robust data from the IMPACT study has enabled the expanded indication announced today and the FDA action has been taken so swiftly.”
The results of the IMPACT trial, which was the first study to compare a single-inhaler triple therapy with two dual therapies, were published on April 18 (N Engl J Med 2018. doi: 10.1056/NEJMoa1713901).
This study randomized patients to 52 weeks of either triple inhaled therapy involving a once-daily combination of 100 mcg fluticasone furoate, 62.5 mcg of umeclidinium, and 25 mcg of vilanterol; or dual inhaled therapy involving either 100 mcg fluticasone furoate plus 25 mcg of vilanterol, or 62.5 mcg of umeclidinium plus 25 mcg of vilanterol.
After 1 year, the rate of moderate to severe COPD exacerbations in the triple-therapy group was 0.91 per year, compared with 1.07 in the fluticasone furoate–vilanterol group and 1.21 in the vilanterol-umeclidinium group. This translated to a 15% reduction with triple therapy compared with fluticasone furoate–vilanterol and a 25% reduction, compared with vilanterol-umeclidinium (P less than .001 for both).
The Food and Drug Administration has approved a new indication for the chronic obstructive pulmonary disease (COPD) therapy fluticasone furoate/umeclidinium/vilanterol (Trelegy Ellipta), which allows physicians to prescribe the drug to a broader class of COPD patients, according to a statement from two pharmaceutical companies.
“Following the initial approval of Trelegy Ellipta in September, we have analysed the data from the IMPACT study and identified additional benefits that this important medicine offers patients with [COPD],” said Hal Barron, MD, chief scientific officer and president of research and development at GlaxoSmithKline, in the statement. “We are pleased that the robust data from the IMPACT study has enabled the expanded indication announced today and the FDA action has been taken so swiftly.”
The results of the IMPACT trial, which was the first study to compare a single-inhaler triple therapy with two dual therapies, were published on April 18 (N Engl J Med 2018. doi: 10.1056/NEJMoa1713901).
This study randomized patients to 52 weeks of either triple inhaled therapy involving a once-daily combination of 100 mcg fluticasone furoate, 62.5 mcg of umeclidinium, and 25 mcg of vilanterol; or dual inhaled therapy involving either 100 mcg fluticasone furoate plus 25 mcg of vilanterol, or 62.5 mcg of umeclidinium plus 25 mcg of vilanterol.
After 1 year, the rate of moderate to severe COPD exacerbations in the triple-therapy group was 0.91 per year, compared with 1.07 in the fluticasone furoate–vilanterol group and 1.21 in the vilanterol-umeclidinium group. This translated to a 15% reduction with triple therapy compared with fluticasone furoate–vilanterol and a 25% reduction, compared with vilanterol-umeclidinium (P less than .001 for both).
Is medical aid in dying suicide?
WASHINGTON – Medical aid in dying is not the same as suicide; it is a way to give people with terminal illness and facing imminent death a way to die better and avoid the terrible, drawn-out deaths from chronic disease that often now occur, Margaret P. Battin, PhD, said at the annual conference of the American Association of Suicidology.
and is allowed now by law in Colorado, Hawaii, Montana, Oregon, Vermont, Washington, the District of Columbia, and by court decision in California. It’s also a legal option in Belgium, Canada, Luxembourg, the Netherlands, Switzerland, and parts of Germany, she said. Dr. Battin attributed the spread of legalized MAiD to the frequent terrible and protracted deaths people in industrialized countries face from chronic diseases such as cancer, heart disease, organ failure, dementia, stroke, and other neurologic disorders, and the comforting option of choice that MAiD offers these terminally ill patients. All the laws that have legalized MAiD draw distinctions between it and suicide, she noted.
“Even it the patient doesn’t act on this, someone facing death can find comfort knowing that if things get too bad, there is a way to avoid it, that they can have some control over their death. People want to remain in control, and this gives people a sense of control” that is not pathologic; it’s reassuring. “It can also make bereavement easier for others,” she said. Medical aid in dying “ helps people with terminal illness reduce their confusion, despair, and sense of impotence. When we can’t prevent death, this can make it better.” Dr. Battin also cited the restrictions these laws make to rule out people with mental illness or psychiatric problems that influence their choice of MAiD. That plus cultural and societal attitudes toward self-directed death mean that very few people actually wind up taking MAiD to completion.
But Thomas Joiner, PhD, dissented that the MAiD process successfully winnows out people who are acting on a desire for suicide in the traditional sense.
Another major problem with MAiD is that it focuses too much on self-determination and autonomy and downplays the importance of the social reverberations that this form of death has on others, he explained. “The social dimension is an afterthought [of MAiD], and that’s a problem.”
The American Association of Suicidality statement “has evolved, and Professor Battin had a lot to do with that, and I respect” the writing process. It shows better balance between autonomy and social connections, but I believe that [MAiD] is often an expression of [traditional] suicide,” Dr. Joiner said. MAiD has become more acceptable, “but that doesn’t make it right. It is trying to dodge the stigma of suicide.”
Dr. Battin and Dr. Joiner had no disclosures.
WASHINGTON – Medical aid in dying is not the same as suicide; it is a way to give people with terminal illness and facing imminent death a way to die better and avoid the terrible, drawn-out deaths from chronic disease that often now occur, Margaret P. Battin, PhD, said at the annual conference of the American Association of Suicidology.
and is allowed now by law in Colorado, Hawaii, Montana, Oregon, Vermont, Washington, the District of Columbia, and by court decision in California. It’s also a legal option in Belgium, Canada, Luxembourg, the Netherlands, Switzerland, and parts of Germany, she said. Dr. Battin attributed the spread of legalized MAiD to the frequent terrible and protracted deaths people in industrialized countries face from chronic diseases such as cancer, heart disease, organ failure, dementia, stroke, and other neurologic disorders, and the comforting option of choice that MAiD offers these terminally ill patients. All the laws that have legalized MAiD draw distinctions between it and suicide, she noted.
“Even it the patient doesn’t act on this, someone facing death can find comfort knowing that if things get too bad, there is a way to avoid it, that they can have some control over their death. People want to remain in control, and this gives people a sense of control” that is not pathologic; it’s reassuring. “It can also make bereavement easier for others,” she said. Medical aid in dying “ helps people with terminal illness reduce their confusion, despair, and sense of impotence. When we can’t prevent death, this can make it better.” Dr. Battin also cited the restrictions these laws make to rule out people with mental illness or psychiatric problems that influence their choice of MAiD. That plus cultural and societal attitudes toward self-directed death mean that very few people actually wind up taking MAiD to completion.
But Thomas Joiner, PhD, dissented that the MAiD process successfully winnows out people who are acting on a desire for suicide in the traditional sense.
Another major problem with MAiD is that it focuses too much on self-determination and autonomy and downplays the importance of the social reverberations that this form of death has on others, he explained. “The social dimension is an afterthought [of MAiD], and that’s a problem.”
The American Association of Suicidality statement “has evolved, and Professor Battin had a lot to do with that, and I respect” the writing process. It shows better balance between autonomy and social connections, but I believe that [MAiD] is often an expression of [traditional] suicide,” Dr. Joiner said. MAiD has become more acceptable, “but that doesn’t make it right. It is trying to dodge the stigma of suicide.”
Dr. Battin and Dr. Joiner had no disclosures.
WASHINGTON – Medical aid in dying is not the same as suicide; it is a way to give people with terminal illness and facing imminent death a way to die better and avoid the terrible, drawn-out deaths from chronic disease that often now occur, Margaret P. Battin, PhD, said at the annual conference of the American Association of Suicidology.
and is allowed now by law in Colorado, Hawaii, Montana, Oregon, Vermont, Washington, the District of Columbia, and by court decision in California. It’s also a legal option in Belgium, Canada, Luxembourg, the Netherlands, Switzerland, and parts of Germany, she said. Dr. Battin attributed the spread of legalized MAiD to the frequent terrible and protracted deaths people in industrialized countries face from chronic diseases such as cancer, heart disease, organ failure, dementia, stroke, and other neurologic disorders, and the comforting option of choice that MAiD offers these terminally ill patients. All the laws that have legalized MAiD draw distinctions between it and suicide, she noted.
“Even it the patient doesn’t act on this, someone facing death can find comfort knowing that if things get too bad, there is a way to avoid it, that they can have some control over their death. People want to remain in control, and this gives people a sense of control” that is not pathologic; it’s reassuring. “It can also make bereavement easier for others,” she said. Medical aid in dying “ helps people with terminal illness reduce their confusion, despair, and sense of impotence. When we can’t prevent death, this can make it better.” Dr. Battin also cited the restrictions these laws make to rule out people with mental illness or psychiatric problems that influence their choice of MAiD. That plus cultural and societal attitudes toward self-directed death mean that very few people actually wind up taking MAiD to completion.
But Thomas Joiner, PhD, dissented that the MAiD process successfully winnows out people who are acting on a desire for suicide in the traditional sense.
Another major problem with MAiD is that it focuses too much on self-determination and autonomy and downplays the importance of the social reverberations that this form of death has on others, he explained. “The social dimension is an afterthought [of MAiD], and that’s a problem.”
The American Association of Suicidality statement “has evolved, and Professor Battin had a lot to do with that, and I respect” the writing process. It shows better balance between autonomy and social connections, but I believe that [MAiD] is often an expression of [traditional] suicide,” Dr. Joiner said. MAiD has become more acceptable, “but that doesn’t make it right. It is trying to dodge the stigma of suicide.”
Dr. Battin and Dr. Joiner had no disclosures.
REPORTING FROM THE AAS ANNUAL CONFERENCE
Penile Squamous Cell Carcinoma With Urethral Extension Treated With Mohs Micrographic Surgery
Penile squamous cell carcinoma (SCC) with considerable urethral extension is uncommon and difficult to manage. It often is resistant to less invasive and nonsurgical treatments and frequently results in partial or total penectomy, which can lead to cosmetic disfigurement, functional issues, and psychological distress. We report a case of penile SCC in situ with considerable urethral extension with a focus of cells suspicious for moderately well-differentiated and invasive SCC that was treated with
Mohs micrographic surgery with distal urethrectomy and reconstruction is a valuable treatment technique for cases of SCC involving the glans penis and distal urethra. It offers equivalent or better overall cure rates compared to more radical interventions. Additionally, preservation of the penis with MMS spares patients from considerable physical and psychosocial morbidity. Our case, along with growing body of literature,1-4 calls on dermatologists and urologists to consider MMS as a treatment for penile SCC with or without urethral involvement.
Case Report
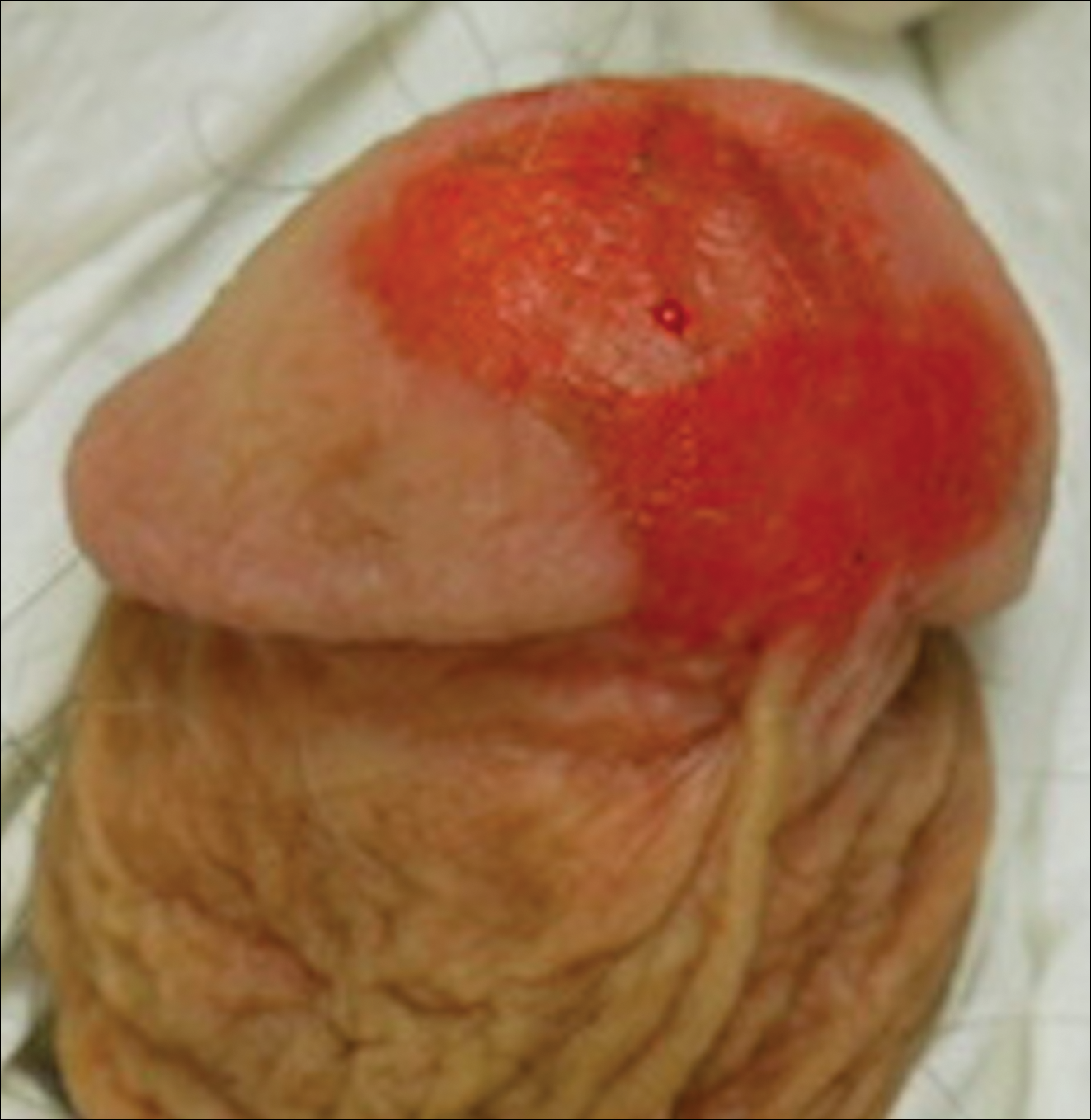
A 61-year-old man presented to the dermatology department with a pruritic lesion on the penis that had been present for 6 years. Shave biopsy demonstrated SCC in situ with a focus of cells suspicious for moderately well-differentiated and invasive SCC. Physical examination revealed an ill-defined, 2.2×1.9-cm, pink, eroded plaque involving the tip of the penis and surrounding the external urinary meatus (Figure 1). There was no palpable inguinal lymphadenopathy.
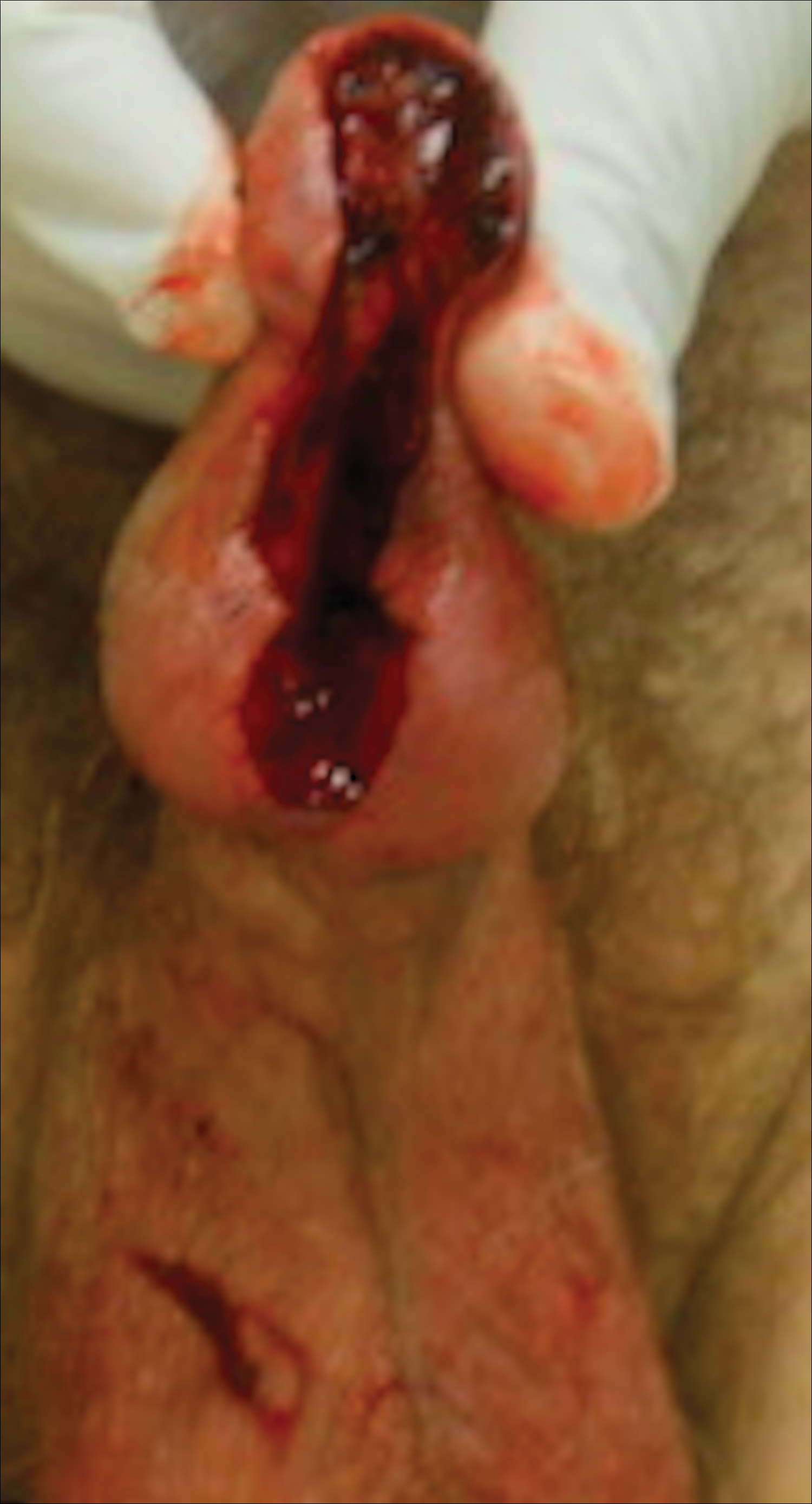
Distal penectomy and lymph node biopsy was recommended following evaluation by the urologic oncology department, but the patient declined these interventions and presented to our dermatology department (A.H.) for a second opinion. The tumor, including the invasive perineural portion, was removed using MMS several weeks after initially presenting to urologic oncology. Ventral meatotomy allowed access to the SCC in situ portion extending proximally up the pendulous urethra (Figure 2). Clear margins were obtained after the eighth stage of MMS, which required removal of 4 to 5 cm of the distal urethra (Figure 3). Reconstruction of the wound required urethral advancement, urethrostomy, and meatoplasty. A positive outcome was achieved with preservation of the length and shape of the penis as well as the cosmetic appearance of the glans penis (Figure 4). The patient was satisfied with the outcome. At 49 months’ follow-up, no evidence of local recurrence or disease progression was noted, and the distal urethrostomy remained intact and functional.


Comment
Penile SCC is a rare malignancy that represents between 0.4% and 0.6% of all malignant tumors in the United States and occurs most commonly in men aged 50 to 70 years.4 The incidence is higher in developing countries, approaching 10% of malignancies in men. It occurs most commonly on the glans penis, prepuce, and coronal sulcus, and has multiple possible appearances, including erythematous and indurated, warty and exophytic, or flat and ulcerated lesions.5 Some reports indicate that more than 40% of penile SCCs are attributable to human papilloma virus,6 while lack of circumcision, chronic inflammation, poor hygiene, balanitis xerotica obliterans, penile trauma, human immunodeficiency virus, UVA treatment of penile psoriasis, and tobacco use are known risk factors.5
Invasive penile SCC generally is treated with penectomy (partial or total), radiation therapy, or MMS; SCC in situ can be treated with topical chemotherapy, laser therapy, and wide local excision (2-cm margins) including circumcision, complete glansectomy, or MMS.5 Squamous cell carcinoma in situ with urethral involvement treated with nonsurgical therapies is associated with higher recurrence rates, ultimately necessitating more aggressive treatments, most commonly partial penectomy.7 The high local recurrence rate of SCC in situ with urethral involvement treated with nonsurgical therapies reflects the fact that determining the presence of urethral extension is difficult and, if present, is inherently inaccessible to these local therapies because the urethra is not an outward-facing tissue surface; MMS represents one possible solution to these issues.
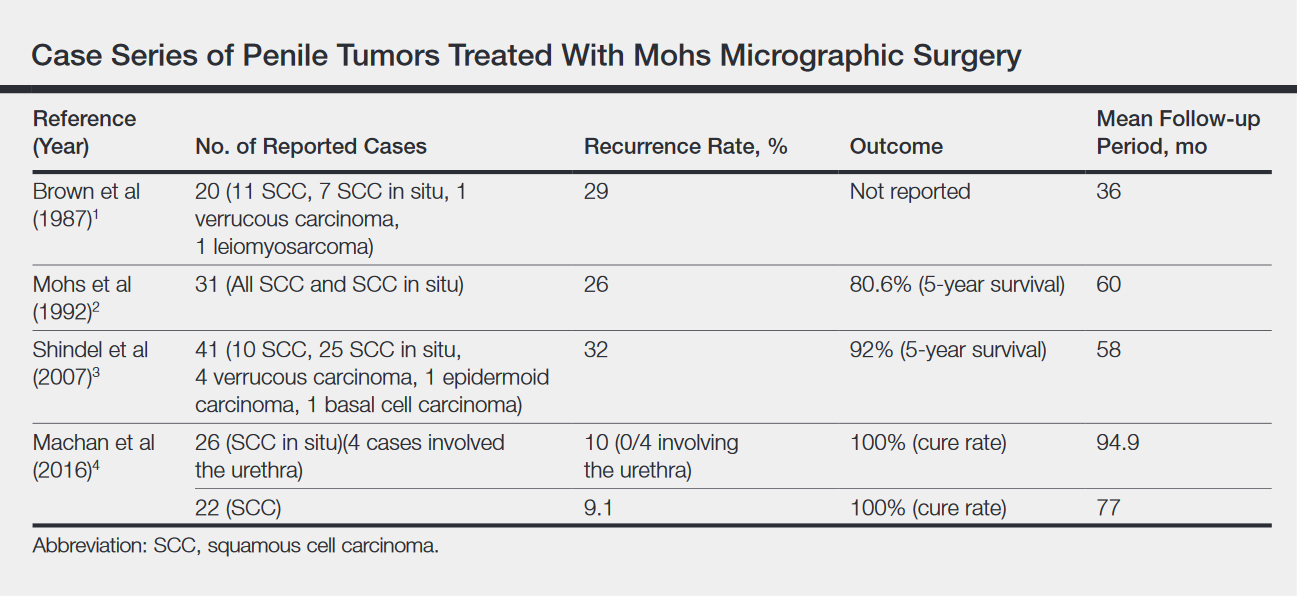
Across all treatment modalities, the most prognostic factor of cancer-specific survival in patients with penile SCC is pelvic lymph node involvement. Some reports cite 5-year survival rates as low as 0% in the setting of pelvic lymph node involvement,5 whereas others had cited rates of 29% to 40%4; 5-year survival rates of higher than 85% have been reported in node-negative patients.4 Recurrence rates vary widely by treatment modality, ranging from less than 10% with partial penectomy and long-term follow-up8 and up to 50% within 2 years with penile-preserving approaches (eg, topical chemotherapy, laser therapy, radiotherapy).5 Multiple case series of penile cancer (the most common of which was SCC/SCC in situ) treated with MMS report comparable and at times superior survival and recurrence data (Table).1-4 Slightly higher recurrences of penile SCC treated with MMS compared to penectomy have been reported, along with considerably higher recurrence rates compared to nonpenile cutaneous SCC treated with MMS (reported to be less than 3%).4 The elastic and expansile nature of penile tissue may lead to distortion from swelling/local anesthesia when taking individual Mohs layers. Additionally, as a large percentage of penile SCCs are attributable to human papillomavirus, difficulty in detecting human papilloma virus–infected cells (which may have oncogenic potential) with the naked eye or histologically with typical staining techniques may help explain the higher recurrence rate of penile SCC treated with MMS compared to penectomy. Despite the higher recurrence rates, survival is comparable or higher in cases treated with MMS (Table).
Partial penectomy also has a negative impact on health-related quality of life. Kieffer et al9 compared the impact of penile-sparing surgery (PSS)(including MMS) versus partial or total penectomy on sexual function and health-related quality of life in 90 patients with penile cancer. Although the association between the extent of surgery (partial penectomy/total penectomy/PSS) surgery type and extent and most outcome measures was not statistically significant, partial penectomy was associated with significantly more problems with orgasm (P=.031), concerns about appearance (P=.008), interference in daily life (P=.032), and urinary function (P<.0001) when compared to patients treated with PSS.9 Although this study included only laser/local excision with or without circumcision or glans penis amputation with or without reconstruction as PSSs and did not explicitly include MMS, MMS is clearly a tissue-sparing technique and the study results are generaliz
Conclusion
Penile SCC with considerable urethral extension is uncommon, difficult to manage, and often is resistant to less invasive and nonsurgical treatments. As a result, partial or total penectomy is sometimes necessary. Such cases benefit from MMS with distal urethrectomy and reconstruction because MMS provides equivalent or better overall cure rates compared to more radical interventions.1-4 Importantly, preservation of the penis with MMS can spare patients considerable physical and psychosocial morbidity. Partial penectomy is associated with more health-related quality-of-life problems with orgasm, concerns about appearance, interference in daily life, and urinary function compared to PSSs such as MMS.9 This case, and a growing body of literature, are a call to dermatologists and urologists to consider MMS as a treatment for penile SCC, even with involvement of the urethra.
- Brown MD, Zachary CB, Grekin RC, et al. Penile tumors: their management by Mohs micrographic surgery. J Dermatol Surg Oncol. 1987;13:1163-1167.
- Mohs FE, Snow SN, Larson PO. Mohs micrographic surgery for penile tumors. Urol Clin North Am. 1992;19:291-304.
- Shindel AW, Mann MW, Lev RY, et al. Mohs micrographic surgery for penile cancer: management and long-term followup. J Urol. 2007;178:1980-1985.
- Machan M, Brodland D, Zitelli J. Penile squamous cell carcinoma: penis-preserving treatment with Mohs micrographic surgery. Dermatol Surg. 2016;42:936-944.
- Spiess PE, Horenblas S, Pagliaro LC, et al. Current concepts in penile cancer. J Natl Compr Canc Netw. 2013;11:617-624.
- Hernandez BY, Barnholtz-Sloan J, German RR, et al. Burden of invasive squamous cell carcinoma of the penis in the United States, 1998-2003. Cancer. 2008;113(10 suppl):2883-2891.
- Nash PA, Bihrle R, Gleason PE, et al. Mohs micrographic surgery and distal urethrectomy with immediate urethral reconstruction for glanular carcinoma in situ with significant urethral extension. Urology. 1996;47:108-110.
- Djordjevic ML, Palminteri E, Martins F. Male genital reconstruction for the penile cancer survivor. Curr Opin Urol. 2014;24:427-433.
- Kieffer JM, Djajadiningrat RS, van Muilekom EA, et al. Quality of life for patients treated for penile cancer. J Urol. 2014;192:1105-1110.
Penile squamous cell carcinoma (SCC) with considerable urethral extension is uncommon and difficult to manage. It often is resistant to less invasive and nonsurgical treatments and frequently results in partial or total penectomy, which can lead to cosmetic disfigurement, functional issues, and psychological distress. We report a case of penile SCC in situ with considerable urethral extension with a focus of cells suspicious for moderately well-differentiated and invasive SCC that was treated with
Mohs micrographic surgery with distal urethrectomy and reconstruction is a valuable treatment technique for cases of SCC involving the glans penis and distal urethra. It offers equivalent or better overall cure rates compared to more radical interventions. Additionally, preservation of the penis with MMS spares patients from considerable physical and psychosocial morbidity. Our case, along with growing body of literature,1-4 calls on dermatologists and urologists to consider MMS as a treatment for penile SCC with or without urethral involvement.
Case Report
A 61-year-old man presented to the dermatology department with a pruritic lesion on the penis that had been present for 6 years. Shave biopsy demonstrated SCC in situ with a focus of cells suspicious for moderately well-differentiated and invasive SCC. Physical examination revealed an ill-defined, 2.2×1.9-cm, pink, eroded plaque involving the tip of the penis and surrounding the external urinary meatus (Figure 1). There was no palpable inguinal lymphadenopathy.

Distal penectomy and lymph node biopsy was recommended following evaluation by the urologic oncology department, but the patient declined these interventions and presented to our dermatology department (A.H.) for a second opinion. The tumor, including the invasive perineural portion, was removed using MMS several weeks after initially presenting to urologic oncology. Ventral meatotomy allowed access to the SCC in situ portion extending proximally up the pendulous urethra (Figure 2). Clear margins were obtained after the eighth stage of MMS, which required removal of 4 to 5 cm of the distal urethra (Figure 3). Reconstruction of the wound required urethral advancement, urethrostomy, and meatoplasty. A positive outcome was achieved with preservation of the length and shape of the penis as well as the cosmetic appearance of the glans penis (Figure 4). The patient was satisfied with the outcome. At 49 months’ follow-up, no evidence of local recurrence or disease progression was noted, and the distal urethrostomy remained intact and functional.



Comment
Penile SCC is a rare malignancy that represents between 0.4% and 0.6% of all malignant tumors in the United States and occurs most commonly in men aged 50 to 70 years.4 The incidence is higher in developing countries, approaching 10% of malignancies in men. It occurs most commonly on the glans penis, prepuce, and coronal sulcus, and has multiple possible appearances, including erythematous and indurated, warty and exophytic, or flat and ulcerated lesions.5 Some reports indicate that more than 40% of penile SCCs are attributable to human papilloma virus,6 while lack of circumcision, chronic inflammation, poor hygiene, balanitis xerotica obliterans, penile trauma, human immunodeficiency virus, UVA treatment of penile psoriasis, and tobacco use are known risk factors.5
Invasive penile SCC generally is treated with penectomy (partial or total), radiation therapy, or MMS; SCC in situ can be treated with topical chemotherapy, laser therapy, and wide local excision (2-cm margins) including circumcision, complete glansectomy, or MMS.5 Squamous cell carcinoma in situ with urethral involvement treated with nonsurgical therapies is associated with higher recurrence rates, ultimately necessitating more aggressive treatments, most commonly partial penectomy.7 The high local recurrence rate of SCC in situ with urethral involvement treated with nonsurgical therapies reflects the fact that determining the presence of urethral extension is difficult and, if present, is inherently inaccessible to these local therapies because the urethra is not an outward-facing tissue surface; MMS represents one possible solution to these issues.
Across all treatment modalities, the most prognostic factor of cancer-specific survival in patients with penile SCC is pelvic lymph node involvement. Some reports cite 5-year survival rates as low as 0% in the setting of pelvic lymph node involvement,5 whereas others had cited rates of 29% to 40%4; 5-year survival rates of higher than 85% have been reported in node-negative patients.4 Recurrence rates vary widely by treatment modality, ranging from less than 10% with partial penectomy and long-term follow-up8 and up to 50% within 2 years with penile-preserving approaches (eg, topical chemotherapy, laser therapy, radiotherapy).5 Multiple case series of penile cancer (the most common of which was SCC/SCC in situ) treated with MMS report comparable and at times superior survival and recurrence data (Table).1-4 Slightly higher recurrences of penile SCC treated with MMS compared to penectomy have been reported, along with considerably higher recurrence rates compared to nonpenile cutaneous SCC treated with MMS (reported to be less than 3%).4 The elastic and expansile nature of penile tissue may lead to distortion from swelling/local anesthesia when taking individual Mohs layers. Additionally, as a large percentage of penile SCCs are attributable to human papillomavirus, difficulty in detecting human papilloma virus–infected cells (which may have oncogenic potential) with the naked eye or histologically with typical staining techniques may help explain the higher recurrence rate of penile SCC treated with MMS compared to penectomy. Despite the higher recurrence rates, survival is comparable or higher in cases treated with MMS (Table).

Partial penectomy also has a negative impact on health-related quality of life. Kieffer et al9 compared the impact of penile-sparing surgery (PSS)(including MMS) versus partial or total penectomy on sexual function and health-related quality of life in 90 patients with penile cancer. Although the association between the extent of surgery (partial penectomy/total penectomy/PSS) surgery type and extent and most outcome measures was not statistically significant, partial penectomy was associated with significantly more problems with orgasm (P=.031), concerns about appearance (P=.008), interference in daily life (P=.032), and urinary function (P<.0001) when compared to patients treated with PSS.9 Although this study included only laser/local excision with or without circumcision or glans penis amputation with or without reconstruction as PSSs and did not explicitly include MMS, MMS is clearly a tissue-sparing technique and the study results are generaliz
Conclusion
Penile SCC with considerable urethral extension is uncommon, difficult to manage, and often is resistant to less invasive and nonsurgical treatments. As a result, partial or total penectomy is sometimes necessary. Such cases benefit from MMS with distal urethrectomy and reconstruction because MMS provides equivalent or better overall cure rates compared to more radical interventions.1-4 Importantly, preservation of the penis with MMS can spare patients considerable physical and psychosocial morbidity. Partial penectomy is associated with more health-related quality-of-life problems with orgasm, concerns about appearance, interference in daily life, and urinary function compared to PSSs such as MMS.9 This case, and a growing body of literature, are a call to dermatologists and urologists to consider MMS as a treatment for penile SCC, even with involvement of the urethra.
Penile squamous cell carcinoma (SCC) with considerable urethral extension is uncommon and difficult to manage. It often is resistant to less invasive and nonsurgical treatments and frequently results in partial or total penectomy, which can lead to cosmetic disfigurement, functional issues, and psychological distress. We report a case of penile SCC in situ with considerable urethral extension with a focus of cells suspicious for moderately well-differentiated and invasive SCC that was treated with
Mohs micrographic surgery with distal urethrectomy and reconstruction is a valuable treatment technique for cases of SCC involving the glans penis and distal urethra. It offers equivalent or better overall cure rates compared to more radical interventions. Additionally, preservation of the penis with MMS spares patients from considerable physical and psychosocial morbidity. Our case, along with growing body of literature,1-4 calls on dermatologists and urologists to consider MMS as a treatment for penile SCC with or without urethral involvement.
Case Report
A 61-year-old man presented to the dermatology department with a pruritic lesion on the penis that had been present for 6 years. Shave biopsy demonstrated SCC in situ with a focus of cells suspicious for moderately well-differentiated and invasive SCC. Physical examination revealed an ill-defined, 2.2×1.9-cm, pink, eroded plaque involving the tip of the penis and surrounding the external urinary meatus (Figure 1). There was no palpable inguinal lymphadenopathy.

Distal penectomy and lymph node biopsy was recommended following evaluation by the urologic oncology department, but the patient declined these interventions and presented to our dermatology department (A.H.) for a second opinion. The tumor, including the invasive perineural portion, was removed using MMS several weeks after initially presenting to urologic oncology. Ventral meatotomy allowed access to the SCC in situ portion extending proximally up the pendulous urethra (Figure 2). Clear margins were obtained after the eighth stage of MMS, which required removal of 4 to 5 cm of the distal urethra (Figure 3). Reconstruction of the wound required urethral advancement, urethrostomy, and meatoplasty. A positive outcome was achieved with preservation of the length and shape of the penis as well as the cosmetic appearance of the glans penis (Figure 4). The patient was satisfied with the outcome. At 49 months’ follow-up, no evidence of local recurrence or disease progression was noted, and the distal urethrostomy remained intact and functional.



Comment
Penile SCC is a rare malignancy that represents between 0.4% and 0.6% of all malignant tumors in the United States and occurs most commonly in men aged 50 to 70 years.4 The incidence is higher in developing countries, approaching 10% of malignancies in men. It occurs most commonly on the glans penis, prepuce, and coronal sulcus, and has multiple possible appearances, including erythematous and indurated, warty and exophytic, or flat and ulcerated lesions.5 Some reports indicate that more than 40% of penile SCCs are attributable to human papilloma virus,6 while lack of circumcision, chronic inflammation, poor hygiene, balanitis xerotica obliterans, penile trauma, human immunodeficiency virus, UVA treatment of penile psoriasis, and tobacco use are known risk factors.5
Invasive penile SCC generally is treated with penectomy (partial or total), radiation therapy, or MMS; SCC in situ can be treated with topical chemotherapy, laser therapy, and wide local excision (2-cm margins) including circumcision, complete glansectomy, or MMS.5 Squamous cell carcinoma in situ with urethral involvement treated with nonsurgical therapies is associated with higher recurrence rates, ultimately necessitating more aggressive treatments, most commonly partial penectomy.7 The high local recurrence rate of SCC in situ with urethral involvement treated with nonsurgical therapies reflects the fact that determining the presence of urethral extension is difficult and, if present, is inherently inaccessible to these local therapies because the urethra is not an outward-facing tissue surface; MMS represents one possible solution to these issues.
Across all treatment modalities, the most prognostic factor of cancer-specific survival in patients with penile SCC is pelvic lymph node involvement. Some reports cite 5-year survival rates as low as 0% in the setting of pelvic lymph node involvement,5 whereas others had cited rates of 29% to 40%4; 5-year survival rates of higher than 85% have been reported in node-negative patients.4 Recurrence rates vary widely by treatment modality, ranging from less than 10% with partial penectomy and long-term follow-up8 and up to 50% within 2 years with penile-preserving approaches (eg, topical chemotherapy, laser therapy, radiotherapy).5 Multiple case series of penile cancer (the most common of which was SCC/SCC in situ) treated with MMS report comparable and at times superior survival and recurrence data (Table).1-4 Slightly higher recurrences of penile SCC treated with MMS compared to penectomy have been reported, along with considerably higher recurrence rates compared to nonpenile cutaneous SCC treated with MMS (reported to be less than 3%).4 The elastic and expansile nature of penile tissue may lead to distortion from swelling/local anesthesia when taking individual Mohs layers. Additionally, as a large percentage of penile SCCs are attributable to human papillomavirus, difficulty in detecting human papilloma virus–infected cells (which may have oncogenic potential) with the naked eye or histologically with typical staining techniques may help explain the higher recurrence rate of penile SCC treated with MMS compared to penectomy. Despite the higher recurrence rates, survival is comparable or higher in cases treated with MMS (Table).

Partial penectomy also has a negative impact on health-related quality of life. Kieffer et al9 compared the impact of penile-sparing surgery (PSS)(including MMS) versus partial or total penectomy on sexual function and health-related quality of life in 90 patients with penile cancer. Although the association between the extent of surgery (partial penectomy/total penectomy/PSS) surgery type and extent and most outcome measures was not statistically significant, partial penectomy was associated with significantly more problems with orgasm (P=.031), concerns about appearance (P=.008), interference in daily life (P=.032), and urinary function (P<.0001) when compared to patients treated with PSS.9 Although this study included only laser/local excision with or without circumcision or glans penis amputation with or without reconstruction as PSSs and did not explicitly include MMS, MMS is clearly a tissue-sparing technique and the study results are generaliz
Conclusion
Penile SCC with considerable urethral extension is uncommon, difficult to manage, and often is resistant to less invasive and nonsurgical treatments. As a result, partial or total penectomy is sometimes necessary. Such cases benefit from MMS with distal urethrectomy and reconstruction because MMS provides equivalent or better overall cure rates compared to more radical interventions.1-4 Importantly, preservation of the penis with MMS can spare patients considerable physical and psychosocial morbidity. Partial penectomy is associated with more health-related quality-of-life problems with orgasm, concerns about appearance, interference in daily life, and urinary function compared to PSSs such as MMS.9 This case, and a growing body of literature, are a call to dermatologists and urologists to consider MMS as a treatment for penile SCC, even with involvement of the urethra.
- Brown MD, Zachary CB, Grekin RC, et al. Penile tumors: their management by Mohs micrographic surgery. J Dermatol Surg Oncol. 1987;13:1163-1167.
- Mohs FE, Snow SN, Larson PO. Mohs micrographic surgery for penile tumors. Urol Clin North Am. 1992;19:291-304.
- Shindel AW, Mann MW, Lev RY, et al. Mohs micrographic surgery for penile cancer: management and long-term followup. J Urol. 2007;178:1980-1985.
- Machan M, Brodland D, Zitelli J. Penile squamous cell carcinoma: penis-preserving treatment with Mohs micrographic surgery. Dermatol Surg. 2016;42:936-944.
- Spiess PE, Horenblas S, Pagliaro LC, et al. Current concepts in penile cancer. J Natl Compr Canc Netw. 2013;11:617-624.
- Hernandez BY, Barnholtz-Sloan J, German RR, et al. Burden of invasive squamous cell carcinoma of the penis in the United States, 1998-2003. Cancer. 2008;113(10 suppl):2883-2891.
- Nash PA, Bihrle R, Gleason PE, et al. Mohs micrographic surgery and distal urethrectomy with immediate urethral reconstruction for glanular carcinoma in situ with significant urethral extension. Urology. 1996;47:108-110.
- Djordjevic ML, Palminteri E, Martins F. Male genital reconstruction for the penile cancer survivor. Curr Opin Urol. 2014;24:427-433.
- Kieffer JM, Djajadiningrat RS, van Muilekom EA, et al. Quality of life for patients treated for penile cancer. J Urol. 2014;192:1105-1110.
- Brown MD, Zachary CB, Grekin RC, et al. Penile tumors: their management by Mohs micrographic surgery. J Dermatol Surg Oncol. 1987;13:1163-1167.
- Mohs FE, Snow SN, Larson PO. Mohs micrographic surgery for penile tumors. Urol Clin North Am. 1992;19:291-304.
- Shindel AW, Mann MW, Lev RY, et al. Mohs micrographic surgery for penile cancer: management and long-term followup. J Urol. 2007;178:1980-1985.
- Machan M, Brodland D, Zitelli J. Penile squamous cell carcinoma: penis-preserving treatment with Mohs micrographic surgery. Dermatol Surg. 2016;42:936-944.
- Spiess PE, Horenblas S, Pagliaro LC, et al. Current concepts in penile cancer. J Natl Compr Canc Netw. 2013;11:617-624.
- Hernandez BY, Barnholtz-Sloan J, German RR, et al. Burden of invasive squamous cell carcinoma of the penis in the United States, 1998-2003. Cancer. 2008;113(10 suppl):2883-2891.
- Nash PA, Bihrle R, Gleason PE, et al. Mohs micrographic surgery and distal urethrectomy with immediate urethral reconstruction for glanular carcinoma in situ with significant urethral extension. Urology. 1996;47:108-110.
- Djordjevic ML, Palminteri E, Martins F. Male genital reconstruction for the penile cancer survivor. Curr Opin Urol. 2014;24:427-433.
- Kieffer JM, Djajadiningrat RS, van Muilekom EA, et al. Quality of life for patients treated for penile cancer. J Urol. 2014;192:1105-1110.
Resident Pearl
- Penile squamous cell carcinoma (SCC) often is treated with partial or total penectomy, especially when there is urethral extension. Mohs micrographic surgery for penile SCC results in equivalent or better overall cure rates and decreases morbidity.
One-fifth of Medicaid kids receive mental health diagnoses
Twenty percent of children insured by Medicaid received a psychiatric diagnosis before 8 years of age, according to data from more than 35,000 Medicaid-insured children in a mid-Atlantic state.
Previous cross-sectional studies have addressed trends in psychiatric treatment of children. “However, little is known about the longitudinal patterns of pediatric use of psychiatric services,” wrote Dinci Pennap, MPH, of the University of Maryland, Baltimore, and her colleagues.
By the age of 8 years, 20% of the children had received a psychiatric diagnosis; 58% of these diagnoses were behavioral. The most common psychiatric diagnoses were ADHD (44%) and learning disorder (32%).
In addition, 10% (2,196) of children had received psychotropic medications. Of those receiving psychotropic medications, 81% received a single medication, 16% received two medications, and 4% received three medications for 60 days or more, the researchers said. Girls were significantly more likely than boys to be diagnosed with adjustment disorder (22% vs. 15%, respectively) or anxiety disorder (7% vs. 4%, respectively). Boys were significantly more likely than girls to be diagnosed with ADHD (30% vs. 22%).
the researchers said.
The study findings were limited by several factors, including the use of clinician-reported diagnoses rather than research-identified diagnoses and the possible lack of generalizability to Medicaid populations in other regions or to privately insured children, the researchers noted. However, the results captured long-term psychotropic use and “highlight the need for safety and outcomes research, particularly for health outcomes such as metabolic imbalance, weight gain, and sleep disturbances after initiation of psychotropic medication for very young children.”
Dr. Pennap had no financial conflicts to disclose. One of the study coauthors disclosed research grants from the National Institutes of Health.
SOURCE: Pennap D et al. JAMA Pediatr. 2018. doi: 10.1001/jamapediatrics.2018.0240.
Twenty percent of children insured by Medicaid received a psychiatric diagnosis before 8 years of age, according to data from more than 35,000 Medicaid-insured children in a mid-Atlantic state.
Previous cross-sectional studies have addressed trends in psychiatric treatment of children. “However, little is known about the longitudinal patterns of pediatric use of psychiatric services,” wrote Dinci Pennap, MPH, of the University of Maryland, Baltimore, and her colleagues.
By the age of 8 years, 20% of the children had received a psychiatric diagnosis; 58% of these diagnoses were behavioral. The most common psychiatric diagnoses were ADHD (44%) and learning disorder (32%).
In addition, 10% (2,196) of children had received psychotropic medications. Of those receiving psychotropic medications, 81% received a single medication, 16% received two medications, and 4% received three medications for 60 days or more, the researchers said. Girls were significantly more likely than boys to be diagnosed with adjustment disorder (22% vs. 15%, respectively) or anxiety disorder (7% vs. 4%, respectively). Boys were significantly more likely than girls to be diagnosed with ADHD (30% vs. 22%).
the researchers said.
The study findings were limited by several factors, including the use of clinician-reported diagnoses rather than research-identified diagnoses and the possible lack of generalizability to Medicaid populations in other regions or to privately insured children, the researchers noted. However, the results captured long-term psychotropic use and “highlight the need for safety and outcomes research, particularly for health outcomes such as metabolic imbalance, weight gain, and sleep disturbances after initiation of psychotropic medication for very young children.”
Dr. Pennap had no financial conflicts to disclose. One of the study coauthors disclosed research grants from the National Institutes of Health.
SOURCE: Pennap D et al. JAMA Pediatr. 2018. doi: 10.1001/jamapediatrics.2018.0240.
Twenty percent of children insured by Medicaid received a psychiatric diagnosis before 8 years of age, according to data from more than 35,000 Medicaid-insured children in a mid-Atlantic state.
Previous cross-sectional studies have addressed trends in psychiatric treatment of children. “However, little is known about the longitudinal patterns of pediatric use of psychiatric services,” wrote Dinci Pennap, MPH, of the University of Maryland, Baltimore, and her colleagues.
By the age of 8 years, 20% of the children had received a psychiatric diagnosis; 58% of these diagnoses were behavioral. The most common psychiatric diagnoses were ADHD (44%) and learning disorder (32%).
In addition, 10% (2,196) of children had received psychotropic medications. Of those receiving psychotropic medications, 81% received a single medication, 16% received two medications, and 4% received three medications for 60 days or more, the researchers said. Girls were significantly more likely than boys to be diagnosed with adjustment disorder (22% vs. 15%, respectively) or anxiety disorder (7% vs. 4%, respectively). Boys were significantly more likely than girls to be diagnosed with ADHD (30% vs. 22%).
the researchers said.
The study findings were limited by several factors, including the use of clinician-reported diagnoses rather than research-identified diagnoses and the possible lack of generalizability to Medicaid populations in other regions or to privately insured children, the researchers noted. However, the results captured long-term psychotropic use and “highlight the need for safety and outcomes research, particularly for health outcomes such as metabolic imbalance, weight gain, and sleep disturbances after initiation of psychotropic medication for very young children.”
Dr. Pennap had no financial conflicts to disclose. One of the study coauthors disclosed research grants from the National Institutes of Health.
SOURCE: Pennap D et al. JAMA Pediatr. 2018. doi: 10.1001/jamapediatrics.2018.0240.
FROM JAMA PEDIATRICS
Key clinical point: More safety and outcome data are needed for young children receiving psychiatric medications.
Major finding: Twenty percent of Medicaid-insured children in a mid-Atlantic state were diagnosed with a psychiatric disorder before 8 years of age.
Study details: The data come from a longitudinal study of 35,244 children insured with Medicaid born in 2007 in a mid-Atlantic state.
Disclosures: Dr. Pennap had no financial conflicts to disclose. One of the study coauthors disclosed research grants from the National Institutes of Health.
Source: Pennap D et al. JAMA Pediatr. 2018. doi: 10.1001/jamapediatrics.2018.0240.
Use these two questions to simplify H. pylori treatment choice
Recent clinical guidelines have expanded not only the pool of patients who should be tested for Helicobacter pylori infection, but also the number of first-line treatment strategies clinicians should consider.
The American College of Gastroenterology guidelines from 2007 recommended just two treatments: clarithromycin-based triple therapy or bismuth-based quadruple therapy.
The 2017 update to ACG guidelines adds five additional recommended treatment possibilities, not all of which have been well studied in U.S. clinical practice, Colin W. Howden, MD, AGAF, said in a presentation at the inaugural Perspectives in Digestive Diseases meeting held by Global Academy for Medical Education.
“There are a variety of options, and unfortunately for us as practitioners, antibiotic sensitivity testing is not routinely or easily available in contemporary U.S. practice,” said Dr. Howden, professor of medicine–gastroenterology at the University of Tennessee Health Sciences Center, Memphis.
Dr. Howden, a coauthor of the latest ACG guidelines, said asking two pointed questions outlined in the document can help simplify the treatment decision:
- Is there a penicillin allergy?
- Has there been previous macrolide exposure?
“The ideal situation is that the patient is not penicillin allergic, and they’ve never had a macrolide before,” Dr. Howden said. In that case, bismuth-based quadruple therapy would be an appropriate choice.
“Bismuth quadruple therapy is never the wrong answer,” he added.
Clarithromycin-based triple therapy might be considered, according to Dr. Howden, if the local rate of resistance to H. pylori is known to be low.
Bismuth-based quadruple therapy consists of a proton pump inhibitor (PPI) or H2 blocker, bismuth, tetracycline, and metronidazole for 10-14 days, while clarithromycin triple therapy consists of a PPI, clarithromycin, and amoxicillin or metronidazole for 10-14 days.
Several other options recently added to the guidelines have been tried in this scenario, he noted, including concomitant therapy, which consists of a PPI, clarithromycin, amoxicillin, and metronidazole for 10-14 days.
If there has been previous macrolide use but the patient is not penicillin allergic, bismuth quadruple therapy is again recommended, Dr. Howden said, and an additional approach might be the introduction of a levofloxacin-based regimen, as outlined in the guidelines.
Conversely, if there has been no previous macrolide use but the patient is confirmed to be penicillin allergic, the current guideline-recommended options are limited to bismuth quadruple therapy, or clarithromycin triple therapy with metronidazole instead of amoxicillin, Dr. Howden said at the meeting.
Finally, for penicillin-allergic patients with previous macrolide use, recommended options are whittled down to just bismuth-based quadruple therapy. “Again, it’s never the wrong answer,” Dr. Howden said.
Global Academy and this news organization are owned by the same parent company.
Dr. Howden reported disclosures related to Horizon, Otsuka, Allergan, Aralaez, EndoStim, Ironwood, Pfizer, and SynteractHCR.
Recent clinical guidelines have expanded not only the pool of patients who should be tested for Helicobacter pylori infection, but also the number of first-line treatment strategies clinicians should consider.
The American College of Gastroenterology guidelines from 2007 recommended just two treatments: clarithromycin-based triple therapy or bismuth-based quadruple therapy.
The 2017 update to ACG guidelines adds five additional recommended treatment possibilities, not all of which have been well studied in U.S. clinical practice, Colin W. Howden, MD, AGAF, said in a presentation at the inaugural Perspectives in Digestive Diseases meeting held by Global Academy for Medical Education.
“There are a variety of options, and unfortunately for us as practitioners, antibiotic sensitivity testing is not routinely or easily available in contemporary U.S. practice,” said Dr. Howden, professor of medicine–gastroenterology at the University of Tennessee Health Sciences Center, Memphis.
Dr. Howden, a coauthor of the latest ACG guidelines, said asking two pointed questions outlined in the document can help simplify the treatment decision:
- Is there a penicillin allergy?
- Has there been previous macrolide exposure?
“The ideal situation is that the patient is not penicillin allergic, and they’ve never had a macrolide before,” Dr. Howden said. In that case, bismuth-based quadruple therapy would be an appropriate choice.
“Bismuth quadruple therapy is never the wrong answer,” he added.
Clarithromycin-based triple therapy might be considered, according to Dr. Howden, if the local rate of resistance to H. pylori is known to be low.
Bismuth-based quadruple therapy consists of a proton pump inhibitor (PPI) or H2 blocker, bismuth, tetracycline, and metronidazole for 10-14 days, while clarithromycin triple therapy consists of a PPI, clarithromycin, and amoxicillin or metronidazole for 10-14 days.
Several other options recently added to the guidelines have been tried in this scenario, he noted, including concomitant therapy, which consists of a PPI, clarithromycin, amoxicillin, and metronidazole for 10-14 days.
If there has been previous macrolide use but the patient is not penicillin allergic, bismuth quadruple therapy is again recommended, Dr. Howden said, and an additional approach might be the introduction of a levofloxacin-based regimen, as outlined in the guidelines.
Conversely, if there has been no previous macrolide use but the patient is confirmed to be penicillin allergic, the current guideline-recommended options are limited to bismuth quadruple therapy, or clarithromycin triple therapy with metronidazole instead of amoxicillin, Dr. Howden said at the meeting.
Finally, for penicillin-allergic patients with previous macrolide use, recommended options are whittled down to just bismuth-based quadruple therapy. “Again, it’s never the wrong answer,” Dr. Howden said.
Global Academy and this news organization are owned by the same parent company.
Dr. Howden reported disclosures related to Horizon, Otsuka, Allergan, Aralaez, EndoStim, Ironwood, Pfizer, and SynteractHCR.
Recent clinical guidelines have expanded not only the pool of patients who should be tested for Helicobacter pylori infection, but also the number of first-line treatment strategies clinicians should consider.
The American College of Gastroenterology guidelines from 2007 recommended just two treatments: clarithromycin-based triple therapy or bismuth-based quadruple therapy.
The 2017 update to ACG guidelines adds five additional recommended treatment possibilities, not all of which have been well studied in U.S. clinical practice, Colin W. Howden, MD, AGAF, said in a presentation at the inaugural Perspectives in Digestive Diseases meeting held by Global Academy for Medical Education.
“There are a variety of options, and unfortunately for us as practitioners, antibiotic sensitivity testing is not routinely or easily available in contemporary U.S. practice,” said Dr. Howden, professor of medicine–gastroenterology at the University of Tennessee Health Sciences Center, Memphis.
Dr. Howden, a coauthor of the latest ACG guidelines, said asking two pointed questions outlined in the document can help simplify the treatment decision:
- Is there a penicillin allergy?
- Has there been previous macrolide exposure?
“The ideal situation is that the patient is not penicillin allergic, and they’ve never had a macrolide before,” Dr. Howden said. In that case, bismuth-based quadruple therapy would be an appropriate choice.
“Bismuth quadruple therapy is never the wrong answer,” he added.
Clarithromycin-based triple therapy might be considered, according to Dr. Howden, if the local rate of resistance to H. pylori is known to be low.
Bismuth-based quadruple therapy consists of a proton pump inhibitor (PPI) or H2 blocker, bismuth, tetracycline, and metronidazole for 10-14 days, while clarithromycin triple therapy consists of a PPI, clarithromycin, and amoxicillin or metronidazole for 10-14 days.
Several other options recently added to the guidelines have been tried in this scenario, he noted, including concomitant therapy, which consists of a PPI, clarithromycin, amoxicillin, and metronidazole for 10-14 days.
If there has been previous macrolide use but the patient is not penicillin allergic, bismuth quadruple therapy is again recommended, Dr. Howden said, and an additional approach might be the introduction of a levofloxacin-based regimen, as outlined in the guidelines.
Conversely, if there has been no previous macrolide use but the patient is confirmed to be penicillin allergic, the current guideline-recommended options are limited to bismuth quadruple therapy, or clarithromycin triple therapy with metronidazole instead of amoxicillin, Dr. Howden said at the meeting.
Finally, for penicillin-allergic patients with previous macrolide use, recommended options are whittled down to just bismuth-based quadruple therapy. “Again, it’s never the wrong answer,” Dr. Howden said.
Global Academy and this news organization are owned by the same parent company.
Dr. Howden reported disclosures related to Horizon, Otsuka, Allergan, Aralaez, EndoStim, Ironwood, Pfizer, and SynteractHCR.
EXPERT ANALYSIS FROM PERSPECTIVES IN DIGESTIVE DISEASES
Reduced intensity conditioning doesn’t protect fertility
SALT LAKE CITY – Both male and female recipients of childhood hematopoietic stem cell transplantation (HSCT) were very likely to have severely decreased fertility potential, even in the setting of preserved puberty, according to a recent study of adolescent and young adult HSCT recipients.
A reduced intensity conditioning regimen did not protect this cohort from decreased fertility, a finding that surprised the study’s lead author.
“We had hypothesized that, as compared to myeloablative conditioning, reduced intensity conditioning in children who received HSCT would lower the risk of infertility and lessen gonadal failure,” said Helen Oquendo del Toro, MD. In fact, Dr. Oquendo del Toro and her collaborators found that more than 90% of semen samples available for analysis had results that indicated infertility or severely impaired fertility, regardless of the type of pretransplant conditioning the patient had received.
The study highlights the need for fertility preservation when possible before HSCT, and makes clear that “normal puberty does not equate to normal fertility,” said Dr. Oquendo del Toro, of Cincinnati Children’s Hospital Medical Center.
Dr. Oquendo del Toro presented results of an observational cohort study of late effects of HSCT that included individuals aged 1-40 years old who received a single HSCT at, or after, 1 year of age.
Twenty-one males in the study had semen available for analysis. Of the 10 males who received myeloablative conditioning (MAC), 8 had azoospermia, and 2 more had oligoteratospermia (low sperm count with abnormal morphology). For the 11 males who received reduced intensity conditioning (RIC), eight had azoospermia, two had semen samples that showed oligoteratospermia, and one had a normal semen analysis.
The median age at transplant for these males was 14.5 years, and patients were a median of 19 years old at follow-up, Dr. Oquendo del Toro said at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
For females in the study, low levels of anti-Müllerian hormone (AMH) – generally considered the best surrogate lab value for ovarian reserve – were nearly as common. Of 14 females receiving MAC, 13 (93%) had low AMH, as did 6 of 8 (75%) female patients who received RIC.
Individuals with more than one HSCT were excluded, as were those with Fanconi anemia, which itself carries a risk of gonadal failure. The study’s two aims were to investigate gonadal function as well as fertility potential after receipt of either RIC or MAC for HSCT.
Patients were seen by an endocrinologist who assessed testicular volume and assigned a Tanner stage. At age 11 and older, patients’ gonadal function was assessed on an annual basis by obtaining levels of luteinizing hormone and follicle stimulating hormone for all patients; female estradiol levels were tracked, as were male testosterone levels.
Assessment of fertility potential required additional laboratory testing: For females, the investigators obtained AMH levels, while for males, semen analysis was coupled with serum levels of inhibin B, an indicator of Sertoli cell function.
A total of 72 males were more than 1 year post-HSCT in the cohort, and of these, 41 were at least 11 years old and had achieved pubertal status according to laboratory evaluation. In all, 22 of the male patients received RIC, and 19 received MAC.
Males receiving MAC were a median 20 years old at their follow-up evaluation, and a median 6 years post-HSCT, while the RIC group were a median of 18.5 years old and 5.5 years out from their transplant.
Of the 50 females who were more than 1 year post-HSCT, 25 were pubertal and 11 years old or older. Nine of the female patients received RIC, and 16 received MAC.
Females who received MAC were a median 12.1 years old and 4.1 years post-HSCT at their follow-up evaluation. Females receiving RIC were a median 16 years old, and 6.5 years post-HSCT at the time of evaluation.
Patients received their transplants for a variety of malignant and nonmalignant conditions.
“We saw relatively normal gonadotropins after both reduced intensity and myeloablative conditioning in males,” Dr. Oquendo del Toro said. Of the MAC group, 4 of 15 (27%) had elevated follicle stimulating hormone levels, as did 2 of 17 (12%) of the RIC group. Elevated luteinizing hormone levels were seen in 2 of 15 (13%) of the MAC group and 1 of 17 (6%) of the RIC group. Four patients in each group had abnormally low testosterone levels.
However, when the investigators looked at inhibin B levels in males, they found abnormally low levels in 9 of 15 (60%) of those who received MAC, and in 6 of 15 (40%) of those who received RIC. These results meshed with the severely abnormal semen analyses investigators found from those participants for whom a sample was available, Dr. Oquendo del Toro said.
For females, estradiol levels were significantly lower for those who had received MAC, with 7 of 11 (64%) of that group having abnormally low estradiol levels. The levels approached 0 pg/mL for many, said Dr. Oquendo del Toro. None of the eight patients who had received RIC had abnormally low estradiol levels (P = .0008).
“Male puberty is relatively well preserved after both myeloablative and reduced intensity conditioning, but there is a greater than 90% risk of male infertility associated with both reduced intensity and myeloablative conditioning for HSCT,” Dr. Oquendo del Toro said.
For females, the study paints a different picture. “We saw decreased premature ovarian failure after reduced intensity conditioning … but the fertility potential as assessed by anti-Müllerian hormone was decreased” after both conditioning regimens, she said.
Dr. Oquendo del Toro reported having no conflicts of interest.
SOURCE: Oquendo del Toro H et al. The 2018 BMT Tandem Meetings, Abstract 88.
SALT LAKE CITY – Both male and female recipients of childhood hematopoietic stem cell transplantation (HSCT) were very likely to have severely decreased fertility potential, even in the setting of preserved puberty, according to a recent study of adolescent and young adult HSCT recipients.
A reduced intensity conditioning regimen did not protect this cohort from decreased fertility, a finding that surprised the study’s lead author.
“We had hypothesized that, as compared to myeloablative conditioning, reduced intensity conditioning in children who received HSCT would lower the risk of infertility and lessen gonadal failure,” said Helen Oquendo del Toro, MD. In fact, Dr. Oquendo del Toro and her collaborators found that more than 90% of semen samples available for analysis had results that indicated infertility or severely impaired fertility, regardless of the type of pretransplant conditioning the patient had received.
The study highlights the need for fertility preservation when possible before HSCT, and makes clear that “normal puberty does not equate to normal fertility,” said Dr. Oquendo del Toro, of Cincinnati Children’s Hospital Medical Center.
Dr. Oquendo del Toro presented results of an observational cohort study of late effects of HSCT that included individuals aged 1-40 years old who received a single HSCT at, or after, 1 year of age.
Twenty-one males in the study had semen available for analysis. Of the 10 males who received myeloablative conditioning (MAC), 8 had azoospermia, and 2 more had oligoteratospermia (low sperm count with abnormal morphology). For the 11 males who received reduced intensity conditioning (RIC), eight had azoospermia, two had semen samples that showed oligoteratospermia, and one had a normal semen analysis.
The median age at transplant for these males was 14.5 years, and patients were a median of 19 years old at follow-up, Dr. Oquendo del Toro said at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
For females in the study, low levels of anti-Müllerian hormone (AMH) – generally considered the best surrogate lab value for ovarian reserve – were nearly as common. Of 14 females receiving MAC, 13 (93%) had low AMH, as did 6 of 8 (75%) female patients who received RIC.
Individuals with more than one HSCT were excluded, as were those with Fanconi anemia, which itself carries a risk of gonadal failure. The study’s two aims were to investigate gonadal function as well as fertility potential after receipt of either RIC or MAC for HSCT.
Patients were seen by an endocrinologist who assessed testicular volume and assigned a Tanner stage. At age 11 and older, patients’ gonadal function was assessed on an annual basis by obtaining levels of luteinizing hormone and follicle stimulating hormone for all patients; female estradiol levels were tracked, as were male testosterone levels.
Assessment of fertility potential required additional laboratory testing: For females, the investigators obtained AMH levels, while for males, semen analysis was coupled with serum levels of inhibin B, an indicator of Sertoli cell function.
A total of 72 males were more than 1 year post-HSCT in the cohort, and of these, 41 were at least 11 years old and had achieved pubertal status according to laboratory evaluation. In all, 22 of the male patients received RIC, and 19 received MAC.
Males receiving MAC were a median 20 years old at their follow-up evaluation, and a median 6 years post-HSCT, while the RIC group were a median of 18.5 years old and 5.5 years out from their transplant.
Of the 50 females who were more than 1 year post-HSCT, 25 were pubertal and 11 years old or older. Nine of the female patients received RIC, and 16 received MAC.
Females who received MAC were a median 12.1 years old and 4.1 years post-HSCT at their follow-up evaluation. Females receiving RIC were a median 16 years old, and 6.5 years post-HSCT at the time of evaluation.
Patients received their transplants for a variety of malignant and nonmalignant conditions.
“We saw relatively normal gonadotropins after both reduced intensity and myeloablative conditioning in males,” Dr. Oquendo del Toro said. Of the MAC group, 4 of 15 (27%) had elevated follicle stimulating hormone levels, as did 2 of 17 (12%) of the RIC group. Elevated luteinizing hormone levels were seen in 2 of 15 (13%) of the MAC group and 1 of 17 (6%) of the RIC group. Four patients in each group had abnormally low testosterone levels.
However, when the investigators looked at inhibin B levels in males, they found abnormally low levels in 9 of 15 (60%) of those who received MAC, and in 6 of 15 (40%) of those who received RIC. These results meshed with the severely abnormal semen analyses investigators found from those participants for whom a sample was available, Dr. Oquendo del Toro said.
For females, estradiol levels were significantly lower for those who had received MAC, with 7 of 11 (64%) of that group having abnormally low estradiol levels. The levels approached 0 pg/mL for many, said Dr. Oquendo del Toro. None of the eight patients who had received RIC had abnormally low estradiol levels (P = .0008).
“Male puberty is relatively well preserved after both myeloablative and reduced intensity conditioning, but there is a greater than 90% risk of male infertility associated with both reduced intensity and myeloablative conditioning for HSCT,” Dr. Oquendo del Toro said.
For females, the study paints a different picture. “We saw decreased premature ovarian failure after reduced intensity conditioning … but the fertility potential as assessed by anti-Müllerian hormone was decreased” after both conditioning regimens, she said.
Dr. Oquendo del Toro reported having no conflicts of interest.
SOURCE: Oquendo del Toro H et al. The 2018 BMT Tandem Meetings, Abstract 88.
SALT LAKE CITY – Both male and female recipients of childhood hematopoietic stem cell transplantation (HSCT) were very likely to have severely decreased fertility potential, even in the setting of preserved puberty, according to a recent study of adolescent and young adult HSCT recipients.
A reduced intensity conditioning regimen did not protect this cohort from decreased fertility, a finding that surprised the study’s lead author.
“We had hypothesized that, as compared to myeloablative conditioning, reduced intensity conditioning in children who received HSCT would lower the risk of infertility and lessen gonadal failure,” said Helen Oquendo del Toro, MD. In fact, Dr. Oquendo del Toro and her collaborators found that more than 90% of semen samples available for analysis had results that indicated infertility or severely impaired fertility, regardless of the type of pretransplant conditioning the patient had received.
The study highlights the need for fertility preservation when possible before HSCT, and makes clear that “normal puberty does not equate to normal fertility,” said Dr. Oquendo del Toro, of Cincinnati Children’s Hospital Medical Center.
Dr. Oquendo del Toro presented results of an observational cohort study of late effects of HSCT that included individuals aged 1-40 years old who received a single HSCT at, or after, 1 year of age.
Twenty-one males in the study had semen available for analysis. Of the 10 males who received myeloablative conditioning (MAC), 8 had azoospermia, and 2 more had oligoteratospermia (low sperm count with abnormal morphology). For the 11 males who received reduced intensity conditioning (RIC), eight had azoospermia, two had semen samples that showed oligoteratospermia, and one had a normal semen analysis.
The median age at transplant for these males was 14.5 years, and patients were a median of 19 years old at follow-up, Dr. Oquendo del Toro said at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
For females in the study, low levels of anti-Müllerian hormone (AMH) – generally considered the best surrogate lab value for ovarian reserve – were nearly as common. Of 14 females receiving MAC, 13 (93%) had low AMH, as did 6 of 8 (75%) female patients who received RIC.
Individuals with more than one HSCT were excluded, as were those with Fanconi anemia, which itself carries a risk of gonadal failure. The study’s two aims were to investigate gonadal function as well as fertility potential after receipt of either RIC or MAC for HSCT.
Patients were seen by an endocrinologist who assessed testicular volume and assigned a Tanner stage. At age 11 and older, patients’ gonadal function was assessed on an annual basis by obtaining levels of luteinizing hormone and follicle stimulating hormone for all patients; female estradiol levels were tracked, as were male testosterone levels.
Assessment of fertility potential required additional laboratory testing: For females, the investigators obtained AMH levels, while for males, semen analysis was coupled with serum levels of inhibin B, an indicator of Sertoli cell function.
A total of 72 males were more than 1 year post-HSCT in the cohort, and of these, 41 were at least 11 years old and had achieved pubertal status according to laboratory evaluation. In all, 22 of the male patients received RIC, and 19 received MAC.
Males receiving MAC were a median 20 years old at their follow-up evaluation, and a median 6 years post-HSCT, while the RIC group were a median of 18.5 years old and 5.5 years out from their transplant.
Of the 50 females who were more than 1 year post-HSCT, 25 were pubertal and 11 years old or older. Nine of the female patients received RIC, and 16 received MAC.
Females who received MAC were a median 12.1 years old and 4.1 years post-HSCT at their follow-up evaluation. Females receiving RIC were a median 16 years old, and 6.5 years post-HSCT at the time of evaluation.
Patients received their transplants for a variety of malignant and nonmalignant conditions.
“We saw relatively normal gonadotropins after both reduced intensity and myeloablative conditioning in males,” Dr. Oquendo del Toro said. Of the MAC group, 4 of 15 (27%) had elevated follicle stimulating hormone levels, as did 2 of 17 (12%) of the RIC group. Elevated luteinizing hormone levels were seen in 2 of 15 (13%) of the MAC group and 1 of 17 (6%) of the RIC group. Four patients in each group had abnormally low testosterone levels.
However, when the investigators looked at inhibin B levels in males, they found abnormally low levels in 9 of 15 (60%) of those who received MAC, and in 6 of 15 (40%) of those who received RIC. These results meshed with the severely abnormal semen analyses investigators found from those participants for whom a sample was available, Dr. Oquendo del Toro said.
For females, estradiol levels were significantly lower for those who had received MAC, with 7 of 11 (64%) of that group having abnormally low estradiol levels. The levels approached 0 pg/mL for many, said Dr. Oquendo del Toro. None of the eight patients who had received RIC had abnormally low estradiol levels (P = .0008).
“Male puberty is relatively well preserved after both myeloablative and reduced intensity conditioning, but there is a greater than 90% risk of male infertility associated with both reduced intensity and myeloablative conditioning for HSCT,” Dr. Oquendo del Toro said.
For females, the study paints a different picture. “We saw decreased premature ovarian failure after reduced intensity conditioning … but the fertility potential as assessed by anti-Müllerian hormone was decreased” after both conditioning regimens, she said.
Dr. Oquendo del Toro reported having no conflicts of interest.
SOURCE: Oquendo del Toro H et al. The 2018 BMT Tandem Meetings, Abstract 88.
REPORTING FROM THE 2018 BMT TANDEM MEETINGS
Key clinical point:
Major finding: Of 21 males receiving reduced intensity conditioning or myeloablative conditioning, all but one had azoospermia or oligoteratospermia.
Study details: Observational cohort study of 41 males and 25 females receiving pediatric HSCT.
Disclosures: Dr. Oquendo del Toro reported having no conflicts of interest.
Source: Oquendo del Toro H et al. The 2018 BMT Tandem Meetings, Abstract 88.
Women’s representation in CV drug trials still lagging
Although women have been well represented recently in certain types of cardiovascular drug trials, they remain underrepresented in studies of heart failure, coronary artery disease, and acute coronary syndrome, a recent study authored by Food and Drug Administration researchers has revealed.
However, representation of women fell below an acceptable participation-to-prevalence ratio in those other trial types that were evaluated, according to researchers, including lead author Pamela E. Scott, PhD, of the FDA’s Office of Women’s Health.
“As we move into the era of precision medicine – that is, assessing the impact of a wide range of patient and disease characteristics on drug effects – it is imperative that clinical trial participants represent the full spectrum of patients for whom the drug will be prescribed,” Dr. Scott and her colleagues said in the report.
To quantify the participation of women in clinical trials, Dr. Scott and coinvestigators reviewed publicly available FDA reviews from 2005 to 2015 supporting the approval of 36 drugs.
They used a metric called the participation-to-prevalence ratio (PPR) to compare representation of women in a study relative to the representation of women in the disease population being studied. The range of PPR of 0.8-1.2 represented similar representation of women in the study and disease population.
Participation of women in drug trials varied widely – from a low of 22% to a high of 81% – with a mean of 46%, they found.
The PPR was within the desirable range for hypertension, at 0.9, and atrial fibrillation trials, at 0.8-1.1, while participation for pulmonary arterial hypertension trials was above the desirable range, at 1.4, according to the report.
However, a PPR of less than 0.8 was found for coronary artery disease trials, at 0.6; acute coronary syndrome/myocardial infarction trials, also at 0.6; and heart failure trials, at 0.5 to 0.6.
Few clinically meaningful gender differences were found in efficacy or safety, they added, noting that such differences were discussed in the prescribing information for four different drugs.
To ensure that representative patient populations are enrolled in drug trials, clinical researchers, patient advocacy groups, federal agencies, and the industry as a whole should work together, Dr. Scott and her colleagues said.
“These steps will move us closer toward the goal of providing the best information possible about the use of drugs for every patient,” they wrote.
Dr. Scott and coauthors reported that they had no relationships relevant to their study.
SOURCE: Scott PE et al. J Am Coll Cardiol. 2018 May 18;71:1960-9.
Any optimism for results of this study by FDA authors are dampened significantly by the continued under-representation of women in heart failure and ischemic heart disease.
Even though progress has been made toward a higher participation of women in pivotal clinical trials, it still is not time to rest on our laurels.
One key contributor to the persistently low representation of women is the eligibility criteria of clinical trials. Those criteria often describe a male pattern of disease, particularly in drug trials for ischemic heart disease and heart failure.
As an example, heart failure trials have included eligibility criteria that consistently exclude women, such as a glomerular filtration rate less than 30 mL/min per 1.73 m2, and an ejection fraction of 40% or less.
The inclusion criteria in randomized, controlled trials impose homogeneous clinical characteristics for men and women and may be responsible for the lack of sex differences in efficacy outcomes.
Thus, the current analysis exposes not only successes but also failings of clinical trial design for FDA approval. We are still far from providing equitable cardiovascular health care for women.
Louise Pilote, MD, MPH, PhD, and Valeria Raparelli, MD, PhD, are both affiliated with McGill University Health Centre, Montreal. These comments are derived from their editorial in the Journal of the American College of Cardiology . Both reported that they had no relationships relevant to the contents of their editorial.
Any optimism for results of this study by FDA authors are dampened significantly by the continued under-representation of women in heart failure and ischemic heart disease.
Even though progress has been made toward a higher participation of women in pivotal clinical trials, it still is not time to rest on our laurels.
One key contributor to the persistently low representation of women is the eligibility criteria of clinical trials. Those criteria often describe a male pattern of disease, particularly in drug trials for ischemic heart disease and heart failure.
As an example, heart failure trials have included eligibility criteria that consistently exclude women, such as a glomerular filtration rate less than 30 mL/min per 1.73 m2, and an ejection fraction of 40% or less.
The inclusion criteria in randomized, controlled trials impose homogeneous clinical characteristics for men and women and may be responsible for the lack of sex differences in efficacy outcomes.
Thus, the current analysis exposes not only successes but also failings of clinical trial design for FDA approval. We are still far from providing equitable cardiovascular health care for women.
Louise Pilote, MD, MPH, PhD, and Valeria Raparelli, MD, PhD, are both affiliated with McGill University Health Centre, Montreal. These comments are derived from their editorial in the Journal of the American College of Cardiology . Both reported that they had no relationships relevant to the contents of their editorial.
Any optimism for results of this study by FDA authors are dampened significantly by the continued under-representation of women in heart failure and ischemic heart disease.
Even though progress has been made toward a higher participation of women in pivotal clinical trials, it still is not time to rest on our laurels.
One key contributor to the persistently low representation of women is the eligibility criteria of clinical trials. Those criteria often describe a male pattern of disease, particularly in drug trials for ischemic heart disease and heart failure.
As an example, heart failure trials have included eligibility criteria that consistently exclude women, such as a glomerular filtration rate less than 30 mL/min per 1.73 m2, and an ejection fraction of 40% or less.
The inclusion criteria in randomized, controlled trials impose homogeneous clinical characteristics for men and women and may be responsible for the lack of sex differences in efficacy outcomes.
Thus, the current analysis exposes not only successes but also failings of clinical trial design for FDA approval. We are still far from providing equitable cardiovascular health care for women.
Louise Pilote, MD, MPH, PhD, and Valeria Raparelli, MD, PhD, are both affiliated with McGill University Health Centre, Montreal. These comments are derived from their editorial in the Journal of the American College of Cardiology . Both reported that they had no relationships relevant to the contents of their editorial.
Although women have been well represented recently in certain types of cardiovascular drug trials, they remain underrepresented in studies of heart failure, coronary artery disease, and acute coronary syndrome, a recent study authored by Food and Drug Administration researchers has revealed.
However, representation of women fell below an acceptable participation-to-prevalence ratio in those other trial types that were evaluated, according to researchers, including lead author Pamela E. Scott, PhD, of the FDA’s Office of Women’s Health.
“As we move into the era of precision medicine – that is, assessing the impact of a wide range of patient and disease characteristics on drug effects – it is imperative that clinical trial participants represent the full spectrum of patients for whom the drug will be prescribed,” Dr. Scott and her colleagues said in the report.
To quantify the participation of women in clinical trials, Dr. Scott and coinvestigators reviewed publicly available FDA reviews from 2005 to 2015 supporting the approval of 36 drugs.
They used a metric called the participation-to-prevalence ratio (PPR) to compare representation of women in a study relative to the representation of women in the disease population being studied. The range of PPR of 0.8-1.2 represented similar representation of women in the study and disease population.
Participation of women in drug trials varied widely – from a low of 22% to a high of 81% – with a mean of 46%, they found.
The PPR was within the desirable range for hypertension, at 0.9, and atrial fibrillation trials, at 0.8-1.1, while participation for pulmonary arterial hypertension trials was above the desirable range, at 1.4, according to the report.
However, a PPR of less than 0.8 was found for coronary artery disease trials, at 0.6; acute coronary syndrome/myocardial infarction trials, also at 0.6; and heart failure trials, at 0.5 to 0.6.
Few clinically meaningful gender differences were found in efficacy or safety, they added, noting that such differences were discussed in the prescribing information for four different drugs.
To ensure that representative patient populations are enrolled in drug trials, clinical researchers, patient advocacy groups, federal agencies, and the industry as a whole should work together, Dr. Scott and her colleagues said.
“These steps will move us closer toward the goal of providing the best information possible about the use of drugs for every patient,” they wrote.
Dr. Scott and coauthors reported that they had no relationships relevant to their study.
SOURCE: Scott PE et al. J Am Coll Cardiol. 2018 May 18;71:1960-9.
Although women have been well represented recently in certain types of cardiovascular drug trials, they remain underrepresented in studies of heart failure, coronary artery disease, and acute coronary syndrome, a recent study authored by Food and Drug Administration researchers has revealed.
However, representation of women fell below an acceptable participation-to-prevalence ratio in those other trial types that were evaluated, according to researchers, including lead author Pamela E. Scott, PhD, of the FDA’s Office of Women’s Health.
“As we move into the era of precision medicine – that is, assessing the impact of a wide range of patient and disease characteristics on drug effects – it is imperative that clinical trial participants represent the full spectrum of patients for whom the drug will be prescribed,” Dr. Scott and her colleagues said in the report.
To quantify the participation of women in clinical trials, Dr. Scott and coinvestigators reviewed publicly available FDA reviews from 2005 to 2015 supporting the approval of 36 drugs.
They used a metric called the participation-to-prevalence ratio (PPR) to compare representation of women in a study relative to the representation of women in the disease population being studied. The range of PPR of 0.8-1.2 represented similar representation of women in the study and disease population.
Participation of women in drug trials varied widely – from a low of 22% to a high of 81% – with a mean of 46%, they found.
The PPR was within the desirable range for hypertension, at 0.9, and atrial fibrillation trials, at 0.8-1.1, while participation for pulmonary arterial hypertension trials was above the desirable range, at 1.4, according to the report.
However, a PPR of less than 0.8 was found for coronary artery disease trials, at 0.6; acute coronary syndrome/myocardial infarction trials, also at 0.6; and heart failure trials, at 0.5 to 0.6.
Few clinically meaningful gender differences were found in efficacy or safety, they added, noting that such differences were discussed in the prescribing information for four different drugs.
To ensure that representative patient populations are enrolled in drug trials, clinical researchers, patient advocacy groups, federal agencies, and the industry as a whole should work together, Dr. Scott and her colleagues said.
“These steps will move us closer toward the goal of providing the best information possible about the use of drugs for every patient,” they wrote.
Dr. Scott and coauthors reported that they had no relationships relevant to their study.
SOURCE: Scott PE et al. J Am Coll Cardiol. 2018 May 18;71:1960-9.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Key clinical point: Although women are well represented in clinical trials for hypertension, atrial fibrillation, and pulmonary arterial hypertension, they are not well represented in other trial types.
Major finding: The participation-to-prevalence ratio fell below 0.8 (that is, the bottom end of the range reflecting a similar representation of women) for coronary artery disease, acute coronary syndrome, and heart failure.
Study details: An assessment of publicly available FDA data from 2005 to 2015 on enrollment of women in clinical trials representing 36 drug approvals.
Disclosures: Authors reported that they had no relationships relevant to their report.
Source: Scott PE et al. J Am Coll Cardiol. 2018 May 18;71:1960-9.
Wearing lateral wedge insoles reduces osteoarthritis knee pain
LIVERPOOL, ENGLAND – Lateral wedge insoles reduced osteoarthritis knee pain to a greater extent than did wearing neutral insoles in a randomized, controlled, crossover trial.
An overall significant treatment difference of 0.7 points on a 0-10 numerical rating scale global pain score was observed when patients with painful medial osteoarthritis (OA) wore lateral wedge insoles compared with when they wore neutral insoles in their own shoes (95% confidence interval [CI], 0.1-1.2 points; P = .02).
The study’s findings are in contrast to previous research that has found no benefit of lateral wedge insoles in patients with medial compartment knee OA (JAMA. 2013;310[7]:722-30) because patients were specifically prescreened to find those who would be biomechanical responders, David T. Felson, MD, said at the World Congress on Osteoarthritis.
“There is increased medial load in medial knee OA, and that is thought to cause pain and medial joint damage,” Dr. Felson, of Boston University, said at the congress, sponsored by the Osteoarthritis Research Society International.
There is evidence, however, that by moving the center of pressure laterally during walking with the use of lateral wedge insoles, the external knee adduction movement (KAM) can be reduced by around 5%-6%, which in turn can reduce the load across the medial component.
“We asked the following question: ‘If patients were screened to remove those with painful patellofemoral OA and those who did not show a biomechanical response to lateral wedge insoles, would lateral wedge insoles actually reduce pain?’ ” Dr. Felson noted.
For inclusion in the trial, patients needed to be aged between 40 and 80 years and have x-rays showing Kellgren-Lawrence grade 2-4 OA with definite medial and no lateral joint space narrowing in the last 2 years. They then also needed to have experienced knee pain that was rated at least 4 or more on a 0-10 numerical rating scale in the last week. Patients then had to be examined by an experienced physiotherapist or have x-rays to exclude patellofemoral OA and inflammatory arthritis.
All patients then underwent biomechanical assessment which consisted of motion analysis screening. Patients who did not show at least a 2% decrease in KAM after wearing the lateral wedge insoles versus the neutral insoles were excluded.
Of 112 subjects who were screened for the study, there remained 62 with a mean age of 64 years and body mass index of 28 kg/m2 who could be randomized. The subjects, 73% of whom were male, were randomized to first wear either neutral insoles or insoles with a lateral five-degree wedge for 8 weeks and then, after an 8-week washout period, to wear the other type of insole for a further 8 weeks. Participants wore the insoles for at least 4 hours a day and for a median of 7 hours per day.
Results showed that the lateral wedge insoles reduced KAM by 7.5% versus their own shoes and by 6.6% versus a neutral insole, and of the 62 patients randomized, 56 completed the trial. Two patients in each group stopped participating in the trial because of adverse events, which with lateral wedge insoles were cramps in the calf and foot and increased pain in the knee, and with the neutral insoles included a blister and increased pain in knee and foot.
At baseline, the mean global knee pain score in the last week was 5.46. After use of the lateral wedge or neutral insoles, this fell to a respective 4.2 and 4.9.
Patients were asked to report on the pain experience during a nominated activity. The baseline value for pain was 6.18, which decreased to 4.9 and 5.8 in the two groups, respectively, with an overall treatment difference of 0.9 (P = .001) favoring the use of the lateral wedge insoles.
There was improvement in knee pain assessed by the Knee injury and Osteoarthritis Outcomes Score from a baseline of 55 points to a score of 60.6 for lateral wedge and 58.9 for the neutral insoles, a between group difference of –1.7 (P = .45).
“Lateral wedge insoles reduce knee pain in selected subjects with painful medial OA, those without patellofemoral OA, and those who are biomechanical responders to these insoles,” Dr. Felson said.
“I think the importance of this study is that it opens the door to a treatment that we were frustrated wasn’t working,” Dr. Felson said. Insoles are inexpensive, safe, and potentially widely useful, and the research paves the way for further study of these insoles and for perhaps for shoes that could modify knee loads.
The findings open the door to “stratified approaches to knee OA that may also offer us an avenue toward success in developing treatments,” he added.
The National Institute for Health Research, Arthritis Research U.K., and the National Institutes of Health sponsored the study. Dr. Felson had no disclosures.
SOURCE: Felson D et al. Osteoarthritis Cartilage. 2018:26(1):S17. Abstract 14.
LIVERPOOL, ENGLAND – Lateral wedge insoles reduced osteoarthritis knee pain to a greater extent than did wearing neutral insoles in a randomized, controlled, crossover trial.
An overall significant treatment difference of 0.7 points on a 0-10 numerical rating scale global pain score was observed when patients with painful medial osteoarthritis (OA) wore lateral wedge insoles compared with when they wore neutral insoles in their own shoes (95% confidence interval [CI], 0.1-1.2 points; P = .02).
The study’s findings are in contrast to previous research that has found no benefit of lateral wedge insoles in patients with medial compartment knee OA (JAMA. 2013;310[7]:722-30) because patients were specifically prescreened to find those who would be biomechanical responders, David T. Felson, MD, said at the World Congress on Osteoarthritis.
“There is increased medial load in medial knee OA, and that is thought to cause pain and medial joint damage,” Dr. Felson, of Boston University, said at the congress, sponsored by the Osteoarthritis Research Society International.
There is evidence, however, that by moving the center of pressure laterally during walking with the use of lateral wedge insoles, the external knee adduction movement (KAM) can be reduced by around 5%-6%, which in turn can reduce the load across the medial component.
“We asked the following question: ‘If patients were screened to remove those with painful patellofemoral OA and those who did not show a biomechanical response to lateral wedge insoles, would lateral wedge insoles actually reduce pain?’ ” Dr. Felson noted.
For inclusion in the trial, patients needed to be aged between 40 and 80 years and have x-rays showing Kellgren-Lawrence grade 2-4 OA with definite medial and no lateral joint space narrowing in the last 2 years. They then also needed to have experienced knee pain that was rated at least 4 or more on a 0-10 numerical rating scale in the last week. Patients then had to be examined by an experienced physiotherapist or have x-rays to exclude patellofemoral OA and inflammatory arthritis.
All patients then underwent biomechanical assessment which consisted of motion analysis screening. Patients who did not show at least a 2% decrease in KAM after wearing the lateral wedge insoles versus the neutral insoles were excluded.
Of 112 subjects who were screened for the study, there remained 62 with a mean age of 64 years and body mass index of 28 kg/m2 who could be randomized. The subjects, 73% of whom were male, were randomized to first wear either neutral insoles or insoles with a lateral five-degree wedge for 8 weeks and then, after an 8-week washout period, to wear the other type of insole for a further 8 weeks. Participants wore the insoles for at least 4 hours a day and for a median of 7 hours per day.
Results showed that the lateral wedge insoles reduced KAM by 7.5% versus their own shoes and by 6.6% versus a neutral insole, and of the 62 patients randomized, 56 completed the trial. Two patients in each group stopped participating in the trial because of adverse events, which with lateral wedge insoles were cramps in the calf and foot and increased pain in the knee, and with the neutral insoles included a blister and increased pain in knee and foot.
At baseline, the mean global knee pain score in the last week was 5.46. After use of the lateral wedge or neutral insoles, this fell to a respective 4.2 and 4.9.
Patients were asked to report on the pain experience during a nominated activity. The baseline value for pain was 6.18, which decreased to 4.9 and 5.8 in the two groups, respectively, with an overall treatment difference of 0.9 (P = .001) favoring the use of the lateral wedge insoles.
There was improvement in knee pain assessed by the Knee injury and Osteoarthritis Outcomes Score from a baseline of 55 points to a score of 60.6 for lateral wedge and 58.9 for the neutral insoles, a between group difference of –1.7 (P = .45).
“Lateral wedge insoles reduce knee pain in selected subjects with painful medial OA, those without patellofemoral OA, and those who are biomechanical responders to these insoles,” Dr. Felson said.
“I think the importance of this study is that it opens the door to a treatment that we were frustrated wasn’t working,” Dr. Felson said. Insoles are inexpensive, safe, and potentially widely useful, and the research paves the way for further study of these insoles and for perhaps for shoes that could modify knee loads.
The findings open the door to “stratified approaches to knee OA that may also offer us an avenue toward success in developing treatments,” he added.
The National Institute for Health Research, Arthritis Research U.K., and the National Institutes of Health sponsored the study. Dr. Felson had no disclosures.
SOURCE: Felson D et al. Osteoarthritis Cartilage. 2018:26(1):S17. Abstract 14.
LIVERPOOL, ENGLAND – Lateral wedge insoles reduced osteoarthritis knee pain to a greater extent than did wearing neutral insoles in a randomized, controlled, crossover trial.
An overall significant treatment difference of 0.7 points on a 0-10 numerical rating scale global pain score was observed when patients with painful medial osteoarthritis (OA) wore lateral wedge insoles compared with when they wore neutral insoles in their own shoes (95% confidence interval [CI], 0.1-1.2 points; P = .02).
The study’s findings are in contrast to previous research that has found no benefit of lateral wedge insoles in patients with medial compartment knee OA (JAMA. 2013;310[7]:722-30) because patients were specifically prescreened to find those who would be biomechanical responders, David T. Felson, MD, said at the World Congress on Osteoarthritis.
“There is increased medial load in medial knee OA, and that is thought to cause pain and medial joint damage,” Dr. Felson, of Boston University, said at the congress, sponsored by the Osteoarthritis Research Society International.
There is evidence, however, that by moving the center of pressure laterally during walking with the use of lateral wedge insoles, the external knee adduction movement (KAM) can be reduced by around 5%-6%, which in turn can reduce the load across the medial component.
“We asked the following question: ‘If patients were screened to remove those with painful patellofemoral OA and those who did not show a biomechanical response to lateral wedge insoles, would lateral wedge insoles actually reduce pain?’ ” Dr. Felson noted.
For inclusion in the trial, patients needed to be aged between 40 and 80 years and have x-rays showing Kellgren-Lawrence grade 2-4 OA with definite medial and no lateral joint space narrowing in the last 2 years. They then also needed to have experienced knee pain that was rated at least 4 or more on a 0-10 numerical rating scale in the last week. Patients then had to be examined by an experienced physiotherapist or have x-rays to exclude patellofemoral OA and inflammatory arthritis.
All patients then underwent biomechanical assessment which consisted of motion analysis screening. Patients who did not show at least a 2% decrease in KAM after wearing the lateral wedge insoles versus the neutral insoles were excluded.
Of 112 subjects who were screened for the study, there remained 62 with a mean age of 64 years and body mass index of 28 kg/m2 who could be randomized. The subjects, 73% of whom were male, were randomized to first wear either neutral insoles or insoles with a lateral five-degree wedge for 8 weeks and then, after an 8-week washout period, to wear the other type of insole for a further 8 weeks. Participants wore the insoles for at least 4 hours a day and for a median of 7 hours per day.
Results showed that the lateral wedge insoles reduced KAM by 7.5% versus their own shoes and by 6.6% versus a neutral insole, and of the 62 patients randomized, 56 completed the trial. Two patients in each group stopped participating in the trial because of adverse events, which with lateral wedge insoles were cramps in the calf and foot and increased pain in the knee, and with the neutral insoles included a blister and increased pain in knee and foot.
At baseline, the mean global knee pain score in the last week was 5.46. After use of the lateral wedge or neutral insoles, this fell to a respective 4.2 and 4.9.
Patients were asked to report on the pain experience during a nominated activity. The baseline value for pain was 6.18, which decreased to 4.9 and 5.8 in the two groups, respectively, with an overall treatment difference of 0.9 (P = .001) favoring the use of the lateral wedge insoles.
There was improvement in knee pain assessed by the Knee injury and Osteoarthritis Outcomes Score from a baseline of 55 points to a score of 60.6 for lateral wedge and 58.9 for the neutral insoles, a between group difference of –1.7 (P = .45).
“Lateral wedge insoles reduce knee pain in selected subjects with painful medial OA, those without patellofemoral OA, and those who are biomechanical responders to these insoles,” Dr. Felson said.
“I think the importance of this study is that it opens the door to a treatment that we were frustrated wasn’t working,” Dr. Felson said. Insoles are inexpensive, safe, and potentially widely useful, and the research paves the way for further study of these insoles and for perhaps for shoes that could modify knee loads.
The findings open the door to “stratified approaches to knee OA that may also offer us an avenue toward success in developing treatments,” he added.
The National Institute for Health Research, Arthritis Research U.K., and the National Institutes of Health sponsored the study. Dr. Felson had no disclosures.
SOURCE: Felson D et al. Osteoarthritis Cartilage. 2018:26(1):S17. Abstract 14.
REPORTING FROM OARSI 2018
Key clinical point:
Major finding: There was a mean treatment difference of 0.7 points in a global pain score favoring lateral wedge over neutral insoles (P = .02).
Study details: Randomized, controlled, crossover study of 62 patients with painful medial knee OA.
Disclosures: The National Institute for Health Research, Arthritis Research U.K., and the National Institutes of Health sponsored the study. Dr. Felson had no disclosures.
Source: Felson D et al. Osteoarthritis Cartilage. 2018:26(1):S17. Abstract 14.
Hep B therapy: Indefinite or FINITE for e-negative patients?
LAS VEGAS – Current guidelines recommend indefinite continuation of antiviral therapy in chronic hepatitis B patients who are hepatitis B e-antigen (HBeAg)-negative. But emerging data suggest that this may not always be the case.
“It’s very provocative data, though not at the guideline level,” W. Ray Kim, MD, said in a presentation at the inaugural Perspectives in Digestive Diseases meeting held by Global Academy for Medical Education.
“There are patients who really have begged to go off treatment because they are sick of taking the medication for year after year after year,” said Dr. Kim, professor of medicine, gastroenterology and hepatology, Stanford (Calif.) University.
In light of new data, taking them off medication might be “something to consider” in noncirrhotic patients if they are completely suppressed, have normal ALT, and have a low level of quantitative hepatitis B surface antigen (HBsAg), Dr. Kim told attendees.
The most current Association for the Advancement of the Study of Liver Diseases guidelines state that unless there is a competing rationale, antiviral therapy should be continued indefinitely for noncirrhotic adults with HBeAg‐negative immune‐active chronic hepatitis B.
They do also say that treatment discontinuation “may be considered” for individuals with proven loss of HBsAg. “However, there is currently insufficient evidence to definitively guide treatment decisions for such persons,” the guidelines say.
Evidence has emerged since those guideline statements were written. Most recently, German investigators published results of the FINITE study showing some long-term responses after stopping tenofovir disoproxil fumarate (TDF) in noncirrhotic, HBeAg-negative patients.
In that prospective, controlled study, 62% of patients who stopped TDF therapy (n = 13) stayed off therapy to week 144 of treatment follow-up. Four of the patients achieved HBsAg loss, and median HBsAg change was –0.59log10IU/mL vs. 0.21log10IU/mL in patients who stayed on TDF therapy.
Investigators said that result demonstrated the potential of stopping long-term TDF treatment and seeing either HBsAg loss or sustained virologic response.
Before that, a retrospective study from investigators in Taiwan showed that age plus level of HBsAg were associated with HBV relapse after entecavir treatment in HBeAg-negative patients. According to investigators, those results suggested HBsAg levels could be used to guide timing of entecavir cessation.
If antiviral therapy is stopped in an HBeAg-negative patient, that patient should be monitored every 3 month for a year for recurrent viremia, ALT flares, and hepatic decompensation, Dr. Kim said at the meeting.
Even before stopping, “there are a number of factors to consider, including biological relapse, flare, hepatic decompensation,” he said.
On the other hand, there is the burden of continued therapy, financial concerns of continuing treatment due not only to medication costs but also long-term monitoring, as well as patient and provider preference, he noted in his presentation.
Global Academy and this news organization are owned by the same parent company.
Dr. Kim reported serving as a consultant to Gilead.
LAS VEGAS – Current guidelines recommend indefinite continuation of antiviral therapy in chronic hepatitis B patients who are hepatitis B e-antigen (HBeAg)-negative. But emerging data suggest that this may not always be the case.
“It’s very provocative data, though not at the guideline level,” W. Ray Kim, MD, said in a presentation at the inaugural Perspectives in Digestive Diseases meeting held by Global Academy for Medical Education.
“There are patients who really have begged to go off treatment because they are sick of taking the medication for year after year after year,” said Dr. Kim, professor of medicine, gastroenterology and hepatology, Stanford (Calif.) University.
In light of new data, taking them off medication might be “something to consider” in noncirrhotic patients if they are completely suppressed, have normal ALT, and have a low level of quantitative hepatitis B surface antigen (HBsAg), Dr. Kim told attendees.
The most current Association for the Advancement of the Study of Liver Diseases guidelines state that unless there is a competing rationale, antiviral therapy should be continued indefinitely for noncirrhotic adults with HBeAg‐negative immune‐active chronic hepatitis B.
They do also say that treatment discontinuation “may be considered” for individuals with proven loss of HBsAg. “However, there is currently insufficient evidence to definitively guide treatment decisions for such persons,” the guidelines say.
Evidence has emerged since those guideline statements were written. Most recently, German investigators published results of the FINITE study showing some long-term responses after stopping tenofovir disoproxil fumarate (TDF) in noncirrhotic, HBeAg-negative patients.
In that prospective, controlled study, 62% of patients who stopped TDF therapy (n = 13) stayed off therapy to week 144 of treatment follow-up. Four of the patients achieved HBsAg loss, and median HBsAg change was –0.59log10IU/mL vs. 0.21log10IU/mL in patients who stayed on TDF therapy.
Investigators said that result demonstrated the potential of stopping long-term TDF treatment and seeing either HBsAg loss or sustained virologic response.
Before that, a retrospective study from investigators in Taiwan showed that age plus level of HBsAg were associated with HBV relapse after entecavir treatment in HBeAg-negative patients. According to investigators, those results suggested HBsAg levels could be used to guide timing of entecavir cessation.
If antiviral therapy is stopped in an HBeAg-negative patient, that patient should be monitored every 3 month for a year for recurrent viremia, ALT flares, and hepatic decompensation, Dr. Kim said at the meeting.
Even before stopping, “there are a number of factors to consider, including biological relapse, flare, hepatic decompensation,” he said.
On the other hand, there is the burden of continued therapy, financial concerns of continuing treatment due not only to medication costs but also long-term monitoring, as well as patient and provider preference, he noted in his presentation.
Global Academy and this news organization are owned by the same parent company.
Dr. Kim reported serving as a consultant to Gilead.
LAS VEGAS – Current guidelines recommend indefinite continuation of antiviral therapy in chronic hepatitis B patients who are hepatitis B e-antigen (HBeAg)-negative. But emerging data suggest that this may not always be the case.
“It’s very provocative data, though not at the guideline level,” W. Ray Kim, MD, said in a presentation at the inaugural Perspectives in Digestive Diseases meeting held by Global Academy for Medical Education.
“There are patients who really have begged to go off treatment because they are sick of taking the medication for year after year after year,” said Dr. Kim, professor of medicine, gastroenterology and hepatology, Stanford (Calif.) University.
In light of new data, taking them off medication might be “something to consider” in noncirrhotic patients if they are completely suppressed, have normal ALT, and have a low level of quantitative hepatitis B surface antigen (HBsAg), Dr. Kim told attendees.
The most current Association for the Advancement of the Study of Liver Diseases guidelines state that unless there is a competing rationale, antiviral therapy should be continued indefinitely for noncirrhotic adults with HBeAg‐negative immune‐active chronic hepatitis B.
They do also say that treatment discontinuation “may be considered” for individuals with proven loss of HBsAg. “However, there is currently insufficient evidence to definitively guide treatment decisions for such persons,” the guidelines say.
Evidence has emerged since those guideline statements were written. Most recently, German investigators published results of the FINITE study showing some long-term responses after stopping tenofovir disoproxil fumarate (TDF) in noncirrhotic, HBeAg-negative patients.
In that prospective, controlled study, 62% of patients who stopped TDF therapy (n = 13) stayed off therapy to week 144 of treatment follow-up. Four of the patients achieved HBsAg loss, and median HBsAg change was –0.59log10IU/mL vs. 0.21log10IU/mL in patients who stayed on TDF therapy.
Investigators said that result demonstrated the potential of stopping long-term TDF treatment and seeing either HBsAg loss or sustained virologic response.
Before that, a retrospective study from investigators in Taiwan showed that age plus level of HBsAg were associated with HBV relapse after entecavir treatment in HBeAg-negative patients. According to investigators, those results suggested HBsAg levels could be used to guide timing of entecavir cessation.
If antiviral therapy is stopped in an HBeAg-negative patient, that patient should be monitored every 3 month for a year for recurrent viremia, ALT flares, and hepatic decompensation, Dr. Kim said at the meeting.
Even before stopping, “there are a number of factors to consider, including biological relapse, flare, hepatic decompensation,” he said.
On the other hand, there is the burden of continued therapy, financial concerns of continuing treatment due not only to medication costs but also long-term monitoring, as well as patient and provider preference, he noted in his presentation.
Global Academy and this news organization are owned by the same parent company.
Dr. Kim reported serving as a consultant to Gilead.
EXPERT ANALYSIS FROM PERSPECTIVES IN DIGESTIVE DISEASES














