User login
HSV-2 Has Little to No Effect on HIV Progression
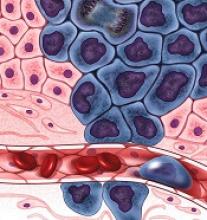
Patients with HIV often also have herpes simplex virus type 2 (HSV-2) infection in part because lesions act as entry portals to susceptible HIV target cells. Some research also has suggested that HSV-2 accelerates HIV progression by upregulating HIV replication and increasing HIV viral load, but data are inconclusive, say researchers from the Iranian Research Center for HIV/AIDS, Pasteur Institute of Iran, Iranian Society for Support of Patients With Infectious Disease, Kermanshah University of Medical Sciences, Tehran University of Medical Sciences, and Zanjan University of Medical Sciences in Iran. They conducted a study to investigate HSV-2 seroprevalence in patients with and without HIV and to find out whether HSV-2 serostatus changed as CD4 counts and HIV viral load changed after 1 year.
The researchers compared 116 HIV patients who were not on HAART with 85 healthy controls. The prevalence and incidence of HSV-2 infection were low in the HIV cases and “negligible” in the control group: 18% of naïve HIV patients had HSV-2 IgG, and none of the control patients did.
Few data exist about HSV-2 seroconversion in HIV patients, the researchers say. In this study, HSV-2 seroconversion was found in 2.43% of HIV patients after 1 year.
Co-infection with HSV-2 had no association with CD4 count and HIV RNA viral load changes in the study participants at baseline or over time, the researchers say. CD4 counts after 1 year were 550 cells/mm3 in the HSV-2 seropositive patients and 563 cells/mm3 in the control group. The viral load in the seropositive group was 3.97 log copies/mL, and 3.49 log copies/mL in the seronegative group.
The researchers conclude that HIV-HSV-2 co-infection does not seem to play a role in HIV infection progression.
Patients with HIV often also have herpes simplex virus type 2 (HSV-2) infection in part because lesions act as entry portals to susceptible HIV target cells. Some research also has suggested that HSV-2 accelerates HIV progression by upregulating HIV replication and increasing HIV viral load, but data are inconclusive, say researchers from the Iranian Research Center for HIV/AIDS, Pasteur Institute of Iran, Iranian Society for Support of Patients With Infectious Disease, Kermanshah University of Medical Sciences, Tehran University of Medical Sciences, and Zanjan University of Medical Sciences in Iran. They conducted a study to investigate HSV-2 seroprevalence in patients with and without HIV and to find out whether HSV-2 serostatus changed as CD4 counts and HIV viral load changed after 1 year.
The researchers compared 116 HIV patients who were not on HAART with 85 healthy controls. The prevalence and incidence of HSV-2 infection were low in the HIV cases and “negligible” in the control group: 18% of naïve HIV patients had HSV-2 IgG, and none of the control patients did.
Few data exist about HSV-2 seroconversion in HIV patients, the researchers say. In this study, HSV-2 seroconversion was found in 2.43% of HIV patients after 1 year.
Co-infection with HSV-2 had no association with CD4 count and HIV RNA viral load changes in the study participants at baseline or over time, the researchers say. CD4 counts after 1 year were 550 cells/mm3 in the HSV-2 seropositive patients and 563 cells/mm3 in the control group. The viral load in the seropositive group was 3.97 log copies/mL, and 3.49 log copies/mL in the seronegative group.
The researchers conclude that HIV-HSV-2 co-infection does not seem to play a role in HIV infection progression.
Patients with HIV often also have herpes simplex virus type 2 (HSV-2) infection in part because lesions act as entry portals to susceptible HIV target cells. Some research also has suggested that HSV-2 accelerates HIV progression by upregulating HIV replication and increasing HIV viral load, but data are inconclusive, say researchers from the Iranian Research Center for HIV/AIDS, Pasteur Institute of Iran, Iranian Society for Support of Patients With Infectious Disease, Kermanshah University of Medical Sciences, Tehran University of Medical Sciences, and Zanjan University of Medical Sciences in Iran. They conducted a study to investigate HSV-2 seroprevalence in patients with and without HIV and to find out whether HSV-2 serostatus changed as CD4 counts and HIV viral load changed after 1 year.
The researchers compared 116 HIV patients who were not on HAART with 85 healthy controls. The prevalence and incidence of HSV-2 infection were low in the HIV cases and “negligible” in the control group: 18% of naïve HIV patients had HSV-2 IgG, and none of the control patients did.
Few data exist about HSV-2 seroconversion in HIV patients, the researchers say. In this study, HSV-2 seroconversion was found in 2.43% of HIV patients after 1 year.
Co-infection with HSV-2 had no association with CD4 count and HIV RNA viral load changes in the study participants at baseline or over time, the researchers say. CD4 counts after 1 year were 550 cells/mm3 in the HSV-2 seropositive patients and 563 cells/mm3 in the control group. The viral load in the seropositive group was 3.97 log copies/mL, and 3.49 log copies/mL in the seronegative group.
The researchers conclude that HIV-HSV-2 co-infection does not seem to play a role in HIV infection progression.
Stopping the Suicide “Contagion” Among Native Americans
American Indians/Alaska Natives (AI/AN) have a disproportionately high rate of suicide—more than 3.5 times those of racial/ethnic groups with the lowest rates, according to a CDC study. And the rate has been steadily rising since 2003.
Those at highest risk are young people aged 10 to 24 years: More than one-third of suicides have occurred in that group compared with 11% of whites in the same age group.
In the CDC study, about 70% of AI/AN decedents lived in nonmetropolitan areas, including rural areas, which underscores the importance of implementing suicide prevention strategies in rural AI/AN communities, the researchers say. Rural areas often have fewer mental health services due to provider shortages and social barriers, among other factors. The researchers point out that in their study AI/AN had lower odds than did white decedents of having received a mental health diagnosis or mental health treatment.
The researchers also found suggestions of “suicide contagion”; AI/AN decedents were more than twice as likely to have a friend’s or family member’s suicide contribute to their death. Community-level programs that focus on “postvention,” such as survivor support groups, should be considered, the researchers say. They also advise that media should focus on “safe reporting of suicides,” for example, by not using sensationalized headlines.
Nearly 28% of the people who died had reported alcohol abuse problems, and 49% had used alcohol in the hours before their death. The researchers caution that differences in the prevalence of alcohol use among AI/AN might be a symptom of “disproportionate exposure to poverty, historical trauma, and other contexts of inequity and should not be viewed as inherent to AI/AN culture.”
American Indians/Alaska Natives (AI/AN) have a disproportionately high rate of suicide—more than 3.5 times those of racial/ethnic groups with the lowest rates, according to a CDC study. And the rate has been steadily rising since 2003.
Those at highest risk are young people aged 10 to 24 years: More than one-third of suicides have occurred in that group compared with 11% of whites in the same age group.
In the CDC study, about 70% of AI/AN decedents lived in nonmetropolitan areas, including rural areas, which underscores the importance of implementing suicide prevention strategies in rural AI/AN communities, the researchers say. Rural areas often have fewer mental health services due to provider shortages and social barriers, among other factors. The researchers point out that in their study AI/AN had lower odds than did white decedents of having received a mental health diagnosis or mental health treatment.
The researchers also found suggestions of “suicide contagion”; AI/AN decedents were more than twice as likely to have a friend’s or family member’s suicide contribute to their death. Community-level programs that focus on “postvention,” such as survivor support groups, should be considered, the researchers say. They also advise that media should focus on “safe reporting of suicides,” for example, by not using sensationalized headlines.
Nearly 28% of the people who died had reported alcohol abuse problems, and 49% had used alcohol in the hours before their death. The researchers caution that differences in the prevalence of alcohol use among AI/AN might be a symptom of “disproportionate exposure to poverty, historical trauma, and other contexts of inequity and should not be viewed as inherent to AI/AN culture.”
American Indians/Alaska Natives (AI/AN) have a disproportionately high rate of suicide—more than 3.5 times those of racial/ethnic groups with the lowest rates, according to a CDC study. And the rate has been steadily rising since 2003.
Those at highest risk are young people aged 10 to 24 years: More than one-third of suicides have occurred in that group compared with 11% of whites in the same age group.
In the CDC study, about 70% of AI/AN decedents lived in nonmetropolitan areas, including rural areas, which underscores the importance of implementing suicide prevention strategies in rural AI/AN communities, the researchers say. Rural areas often have fewer mental health services due to provider shortages and social barriers, among other factors. The researchers point out that in their study AI/AN had lower odds than did white decedents of having received a mental health diagnosis or mental health treatment.
The researchers also found suggestions of “suicide contagion”; AI/AN decedents were more than twice as likely to have a friend’s or family member’s suicide contribute to their death. Community-level programs that focus on “postvention,” such as survivor support groups, should be considered, the researchers say. They also advise that media should focus on “safe reporting of suicides,” for example, by not using sensationalized headlines.
Nearly 28% of the people who died had reported alcohol abuse problems, and 49% had used alcohol in the hours before their death. The researchers caution that differences in the prevalence of alcohol use among AI/AN might be a symptom of “disproportionate exposure to poverty, historical trauma, and other contexts of inequity and should not be viewed as inherent to AI/AN culture.”
Project provides ‘unprecedented understanding’ of cancers
Through extensive analyses of data from The Cancer Genome Atlas (TCGA), researchers have produced a new resource known as the Pan-Cancer Atlas.
Multiple research groups analyzed data on more than 10,000 tumors spanning 33 types of cancer, including acute myeloid leukemia and diffuse large B-cell lymphoma.
The work revealed new insights regarding cells of origin, oncogenic processes, and signaling pathways.
These insights make up the Pan-Cancer Atlas and are described in 27 papers published in Cell Press journals. The entire collection of papers is available through a portal on cell.com.
The Pan-Cancer Atlas is the final output of TCGA, a joint effort of the National Cancer Institute (NCI) and the National Human Genome Research Institute (NHGRI) to “collect, select, and analyze human tissues for genomic alterations on a very large scale.”
“This project is the culmination of more than a decade of ground-breaking work,” said Francis S. Collins, MD, PhD, director of the National Institutes of Health.
“This analysis provides cancer researchers with unprecedented understanding of how, where, and why tumors arise in humans, enabling better informed clinical trials and future treatments.”
The project focused on genome sequencing as well as other analyses, such as investigating gene and protein expression profiles and associating them with clinical and imaging data.
“The Pan-Cancer Atlas effort complements the over 30 tumor-specific papers that have been published by TCGA in the last decade and expands upon earlier pan-cancer work that was published in 2013,” said Jean Claude Zenklusen, PhD, director of the TCGA Program Office at NCI.
The Pan-Cancer Atlas is divided into 3 main categories—cell of origin, oncogenic processes, and signaling pathways—each anchored by a summary paper that recaps the core findings for the topic. Companion papers report in-depth explorations of individual topics within these categories.
Cell of origin
In the first Pan-Cancer Atlas summary paper, the authors review the findings from analyses using a technique called molecular clustering, which groups tumors by parameters such as genes being expressed, abnormality of chromosome numbers in tumor cells, and DNA modifications.
The analyses suggest that tumor types cluster by their possible cells of origin, a finding that has implications for the classification and treatment of various cancers.
“Rather than the organ of origin, we can now use molecular features to identify the cancer’s cell of origin,” said Li Ding, PhD, of Washington University School of Medicine in St. Louis, Missouri.
“We are looking at what genes are turned on in the tumor, and that brings us to a particular cell type. For example, squamous cell cancers can arise in the lung, bladder, cervix, and some tumors of the head and neck. We traditionally have treated cancers in these areas as completely different diseases, but, [by] studying their molecular features, we now know such cancers are closely related.”
“This new molecular-based classification system should greatly help in the clinic, where it is already explaining some of the similar clinical behavior of what we thought were different tumor types,” said Charles Perou, PhD, of UNC Lineberger Comprehensive Cancer Center in Chapel Hill, North Carolina.
“These findings also provide many new therapeutic opportunities, which can and will be tested in the next phase of human clinical trials.”
Oncogenic processes
The second Pan-Cancer Atlas summary paper presents a broad view of the TCGA findings on the processes that lead to cancer development and progression.
The research revealed insights into 3 critical oncogenic processes—germline and somatic mutations, the influence of the tumor’s underlying genome and epigenome on gene and protein expression, and the interplay of tumor and immune cells.
“For the 10,000 tumors we analyzed, we now know—in detail—the inherited mutations driving cancer and the genetic errors that accumulate as people age, increasing the risk of cancer,” Dr Ding said. “This is the first definitive summary of the genetics behind 33 major types of cancer.”
“TCGA has created a catalogue of alterations that occur in a variety of cancer types,” said Katherine Hoadley, PhD, of University of North Carolina at Chapel Hill.
“Having this catalogue of alterations is really important for us to look, in future studies, at why these alterations are there and to predict outcomes for patients.”
Signaling pathways
The final Pan-Cancer Atlas summary paper details TCGA research on the genomic alterations in the signaling pathways that control cell-cycle progression, cell death, and cell growth. The work highlights the similarities and differences in these processes across a range of cancers.
The researchers believe these studies have revealed new patterns of potential vulnerabilities that might aid the development of targeted and combination therapies.
Through extensive analyses of data from The Cancer Genome Atlas (TCGA), researchers have produced a new resource known as the Pan-Cancer Atlas.
Multiple research groups analyzed data on more than 10,000 tumors spanning 33 types of cancer, including acute myeloid leukemia and diffuse large B-cell lymphoma.
The work revealed new insights regarding cells of origin, oncogenic processes, and signaling pathways.
These insights make up the Pan-Cancer Atlas and are described in 27 papers published in Cell Press journals. The entire collection of papers is available through a portal on cell.com.
The Pan-Cancer Atlas is the final output of TCGA, a joint effort of the National Cancer Institute (NCI) and the National Human Genome Research Institute (NHGRI) to “collect, select, and analyze human tissues for genomic alterations on a very large scale.”
“This project is the culmination of more than a decade of ground-breaking work,” said Francis S. Collins, MD, PhD, director of the National Institutes of Health.
“This analysis provides cancer researchers with unprecedented understanding of how, where, and why tumors arise in humans, enabling better informed clinical trials and future treatments.”
The project focused on genome sequencing as well as other analyses, such as investigating gene and protein expression profiles and associating them with clinical and imaging data.
“The Pan-Cancer Atlas effort complements the over 30 tumor-specific papers that have been published by TCGA in the last decade and expands upon earlier pan-cancer work that was published in 2013,” said Jean Claude Zenklusen, PhD, director of the TCGA Program Office at NCI.
The Pan-Cancer Atlas is divided into 3 main categories—cell of origin, oncogenic processes, and signaling pathways—each anchored by a summary paper that recaps the core findings for the topic. Companion papers report in-depth explorations of individual topics within these categories.
Cell of origin
In the first Pan-Cancer Atlas summary paper, the authors review the findings from analyses using a technique called molecular clustering, which groups tumors by parameters such as genes being expressed, abnormality of chromosome numbers in tumor cells, and DNA modifications.
The analyses suggest that tumor types cluster by their possible cells of origin, a finding that has implications for the classification and treatment of various cancers.
“Rather than the organ of origin, we can now use molecular features to identify the cancer’s cell of origin,” said Li Ding, PhD, of Washington University School of Medicine in St. Louis, Missouri.
“We are looking at what genes are turned on in the tumor, and that brings us to a particular cell type. For example, squamous cell cancers can arise in the lung, bladder, cervix, and some tumors of the head and neck. We traditionally have treated cancers in these areas as completely different diseases, but, [by] studying their molecular features, we now know such cancers are closely related.”
“This new molecular-based classification system should greatly help in the clinic, where it is already explaining some of the similar clinical behavior of what we thought were different tumor types,” said Charles Perou, PhD, of UNC Lineberger Comprehensive Cancer Center in Chapel Hill, North Carolina.
“These findings also provide many new therapeutic opportunities, which can and will be tested in the next phase of human clinical trials.”
Oncogenic processes
The second Pan-Cancer Atlas summary paper presents a broad view of the TCGA findings on the processes that lead to cancer development and progression.
The research revealed insights into 3 critical oncogenic processes—germline and somatic mutations, the influence of the tumor’s underlying genome and epigenome on gene and protein expression, and the interplay of tumor and immune cells.
“For the 10,000 tumors we analyzed, we now know—in detail—the inherited mutations driving cancer and the genetic errors that accumulate as people age, increasing the risk of cancer,” Dr Ding said. “This is the first definitive summary of the genetics behind 33 major types of cancer.”
“TCGA has created a catalogue of alterations that occur in a variety of cancer types,” said Katherine Hoadley, PhD, of University of North Carolina at Chapel Hill.
“Having this catalogue of alterations is really important for us to look, in future studies, at why these alterations are there and to predict outcomes for patients.”
Signaling pathways
The final Pan-Cancer Atlas summary paper details TCGA research on the genomic alterations in the signaling pathways that control cell-cycle progression, cell death, and cell growth. The work highlights the similarities and differences in these processes across a range of cancers.
The researchers believe these studies have revealed new patterns of potential vulnerabilities that might aid the development of targeted and combination therapies.
Through extensive analyses of data from The Cancer Genome Atlas (TCGA), researchers have produced a new resource known as the Pan-Cancer Atlas.
Multiple research groups analyzed data on more than 10,000 tumors spanning 33 types of cancer, including acute myeloid leukemia and diffuse large B-cell lymphoma.
The work revealed new insights regarding cells of origin, oncogenic processes, and signaling pathways.
These insights make up the Pan-Cancer Atlas and are described in 27 papers published in Cell Press journals. The entire collection of papers is available through a portal on cell.com.
The Pan-Cancer Atlas is the final output of TCGA, a joint effort of the National Cancer Institute (NCI) and the National Human Genome Research Institute (NHGRI) to “collect, select, and analyze human tissues for genomic alterations on a very large scale.”
“This project is the culmination of more than a decade of ground-breaking work,” said Francis S. Collins, MD, PhD, director of the National Institutes of Health.
“This analysis provides cancer researchers with unprecedented understanding of how, where, and why tumors arise in humans, enabling better informed clinical trials and future treatments.”
The project focused on genome sequencing as well as other analyses, such as investigating gene and protein expression profiles and associating them with clinical and imaging data.
“The Pan-Cancer Atlas effort complements the over 30 tumor-specific papers that have been published by TCGA in the last decade and expands upon earlier pan-cancer work that was published in 2013,” said Jean Claude Zenklusen, PhD, director of the TCGA Program Office at NCI.
The Pan-Cancer Atlas is divided into 3 main categories—cell of origin, oncogenic processes, and signaling pathways—each anchored by a summary paper that recaps the core findings for the topic. Companion papers report in-depth explorations of individual topics within these categories.
Cell of origin
In the first Pan-Cancer Atlas summary paper, the authors review the findings from analyses using a technique called molecular clustering, which groups tumors by parameters such as genes being expressed, abnormality of chromosome numbers in tumor cells, and DNA modifications.
The analyses suggest that tumor types cluster by their possible cells of origin, a finding that has implications for the classification and treatment of various cancers.
“Rather than the organ of origin, we can now use molecular features to identify the cancer’s cell of origin,” said Li Ding, PhD, of Washington University School of Medicine in St. Louis, Missouri.
“We are looking at what genes are turned on in the tumor, and that brings us to a particular cell type. For example, squamous cell cancers can arise in the lung, bladder, cervix, and some tumors of the head and neck. We traditionally have treated cancers in these areas as completely different diseases, but, [by] studying their molecular features, we now know such cancers are closely related.”
“This new molecular-based classification system should greatly help in the clinic, where it is already explaining some of the similar clinical behavior of what we thought were different tumor types,” said Charles Perou, PhD, of UNC Lineberger Comprehensive Cancer Center in Chapel Hill, North Carolina.
“These findings also provide many new therapeutic opportunities, which can and will be tested in the next phase of human clinical trials.”
Oncogenic processes
The second Pan-Cancer Atlas summary paper presents a broad view of the TCGA findings on the processes that lead to cancer development and progression.
The research revealed insights into 3 critical oncogenic processes—germline and somatic mutations, the influence of the tumor’s underlying genome and epigenome on gene and protein expression, and the interplay of tumor and immune cells.
“For the 10,000 tumors we analyzed, we now know—in detail—the inherited mutations driving cancer and the genetic errors that accumulate as people age, increasing the risk of cancer,” Dr Ding said. “This is the first definitive summary of the genetics behind 33 major types of cancer.”
“TCGA has created a catalogue of alterations that occur in a variety of cancer types,” said Katherine Hoadley, PhD, of University of North Carolina at Chapel Hill.
“Having this catalogue of alterations is really important for us to look, in future studies, at why these alterations are there and to predict outcomes for patients.”
Signaling pathways
The final Pan-Cancer Atlas summary paper details TCGA research on the genomic alterations in the signaling pathways that control cell-cycle progression, cell death, and cell growth. The work highlights the similarities and differences in these processes across a range of cancers.
The researchers believe these studies have revealed new patterns of potential vulnerabilities that might aid the development of targeted and combination therapies.
Injectable hydrogels stop bleeding, promote healing
Biomedical engineers have developed hydrogels that can act as an “injectable bandage,” according to preclinical research published in Acta Biomaterialia.
The team engineered injectable hydrogels that were able to promote hemostasis and facilitate wound healing in vitro.
“Injectable hydrogels are promising materials for achieving hemostasis in case of internal injuries and bleeding, as these biomaterials can be introduced into a wound site using minimally invasive approaches,” said study author Akhilesh K. Gaharwar, PhD, of Texas A&M University in College Station, Texas.
“An ideal injectable bandage should solidify after injection in the wound area and promote a natural clotting cascade. In addition, the injectable bandage should initiate wound healing response after achieving hemostasis.”
To create their injectable hydrogels, Dr Gaharwar and his colleagues used a thickening agent known as kappa-carrageenan, which is obtained from seaweed, as well as clay-based nanoparticles known as nanosilicates.
It is the charged characteristics of the nanosilicates that provide the hydrogels with hemostatic ability, according to the researchers.
Specifically, the team said adding nanosilicates to kappa-carrageenan increases protein adsorption on the hydrogels, which enhances cell adhesion and spreading, increases platelet binding, and reduces blood clotting time.
“Interestingly, we also found that these injectable bandages can show a prolonged release of therapeutics that can be used to heal the wound,” said study author Giriraj Lokhande, a graduate student in Dr Gaharwar’s lab.
“The negative surface charge of nanoparticles enabled electrostatic interactions with therapeutics, thus resulting in the slow release of therapeutics.”
The researchers encapsulated vascular endothelial growth factor (VEGF) in the hydrogels and observed sustained release of VEGF in experiments. The hydrogels containing VEGF promoted faster wound healing than control hydrogels.
The researchers said a “range of therapeutic biomacromolecules” could be delivered in the same way as the VEGF.
Biomedical engineers have developed hydrogels that can act as an “injectable bandage,” according to preclinical research published in Acta Biomaterialia.
The team engineered injectable hydrogels that were able to promote hemostasis and facilitate wound healing in vitro.
“Injectable hydrogels are promising materials for achieving hemostasis in case of internal injuries and bleeding, as these biomaterials can be introduced into a wound site using minimally invasive approaches,” said study author Akhilesh K. Gaharwar, PhD, of Texas A&M University in College Station, Texas.
“An ideal injectable bandage should solidify after injection in the wound area and promote a natural clotting cascade. In addition, the injectable bandage should initiate wound healing response after achieving hemostasis.”
To create their injectable hydrogels, Dr Gaharwar and his colleagues used a thickening agent known as kappa-carrageenan, which is obtained from seaweed, as well as clay-based nanoparticles known as nanosilicates.
It is the charged characteristics of the nanosilicates that provide the hydrogels with hemostatic ability, according to the researchers.
Specifically, the team said adding nanosilicates to kappa-carrageenan increases protein adsorption on the hydrogels, which enhances cell adhesion and spreading, increases platelet binding, and reduces blood clotting time.
“Interestingly, we also found that these injectable bandages can show a prolonged release of therapeutics that can be used to heal the wound,” said study author Giriraj Lokhande, a graduate student in Dr Gaharwar’s lab.
“The negative surface charge of nanoparticles enabled electrostatic interactions with therapeutics, thus resulting in the slow release of therapeutics.”
The researchers encapsulated vascular endothelial growth factor (VEGF) in the hydrogels and observed sustained release of VEGF in experiments. The hydrogels containing VEGF promoted faster wound healing than control hydrogels.
The researchers said a “range of therapeutic biomacromolecules” could be delivered in the same way as the VEGF.
Biomedical engineers have developed hydrogels that can act as an “injectable bandage,” according to preclinical research published in Acta Biomaterialia.
The team engineered injectable hydrogels that were able to promote hemostasis and facilitate wound healing in vitro.
“Injectable hydrogels are promising materials for achieving hemostasis in case of internal injuries and bleeding, as these biomaterials can be introduced into a wound site using minimally invasive approaches,” said study author Akhilesh K. Gaharwar, PhD, of Texas A&M University in College Station, Texas.
“An ideal injectable bandage should solidify after injection in the wound area and promote a natural clotting cascade. In addition, the injectable bandage should initiate wound healing response after achieving hemostasis.”
To create their injectable hydrogels, Dr Gaharwar and his colleagues used a thickening agent known as kappa-carrageenan, which is obtained from seaweed, as well as clay-based nanoparticles known as nanosilicates.
It is the charged characteristics of the nanosilicates that provide the hydrogels with hemostatic ability, according to the researchers.
Specifically, the team said adding nanosilicates to kappa-carrageenan increases protein adsorption on the hydrogels, which enhances cell adhesion and spreading, increases platelet binding, and reduces blood clotting time.
“Interestingly, we also found that these injectable bandages can show a prolonged release of therapeutics that can be used to heal the wound,” said study author Giriraj Lokhande, a graduate student in Dr Gaharwar’s lab.
“The negative surface charge of nanoparticles enabled electrostatic interactions with therapeutics, thus resulting in the slow release of therapeutics.”
The researchers encapsulated vascular endothelial growth factor (VEGF) in the hydrogels and observed sustained release of VEGF in experiments. The hydrogels containing VEGF promoted faster wound healing than control hydrogels.
The researchers said a “range of therapeutic biomacromolecules” could be delivered in the same way as the VEGF.
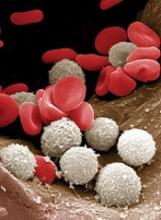
At-home measurement of WBCs
A portable device could be used to monitor patients’ white blood cell (WBC) levels at home, without the need for blood samples, according to researchers.
The team created a prototype that records video of blood cells flowing through capillaries just below the skin surface at the base of the fingernail.
The group is developing a computer algorithm that, in early testing, has been able to analyze the videos and determine if WBC levels are too low.
The researchers believe this type of device could be used to prevent infections among chemotherapy recipients.
“Our vision is that patients will have this portable device that they can take home, and they can monitor daily how they are reacting to the treatment,” said Carlos Castro-Gonzalez, PhD, of the Massachusetts Institute of Technology (MIT) in Cambridge, Massachusetts.
“If they go below the [safe WBC] threshold, then preventive treatment can be deployed.”
Dr Castro-Gonzalez and his colleagues described their prototype, and the testing of it, in Scientific Reports.
The device consists of a wide-field microscope that emits blue light, which penetrates about 50 to 150 microns below the skin and is reflected back to a video camera.
The researchers decided to image the skin at the base of the nail because the capillaries there are very close to the skin surface. These capillaries are so narrow that WBCs must squeeze through one at a time, making them easier to see.
The researchers tested the device in 11 patients at various points during their chemotherapy treatment.
The device does not provide precise WBC counts but allowed the team to differentiate cases of severe neutropenia (<500 neutrophils per μL) from non-neutropenic cases (>1500 neutrophils per μL).
To obtain enough data to make these classifications, the researchers recorded 1 minute of video per patient. Three blinded human assistants then watched the videos and noted whenever a WBC passed by.
However, since submitting their paper, the researchers have been developing a computer algorithm to perform the same task automatically.
“Based on the feature-set that our human raters identified, we are now developing an AI and machine-vision algorithm, with preliminary results that indicate the same accuracy as the raters,” said study author Aurélien Bourquard, PhD, of MIT.
The researchers have applied for patents on the technology and launched a company called Leuko, which is working on commercializing the technology with help from MIT.
To move the technology further toward commercialization, the researchers are building a new automated prototype.
“Automating the measurement process is key to making a viable home-use device,” said study author Ian Butterworth, of MIT. “The imaging needs to take place in the right spot on the patient’s finger, and the operation of the device must be straightforward.”
Using this new prototype, the researchers plan to test the device with additional cancer patients. And the team is investigating whether they can get accurate results with shorter lengths of video.
They also plan to adapt the technology so it can generate more precise WBC counts, which would make it useful for monitoring bone marrow transplant recipients or people with certain infectious diseases, Dr Castro-Gonzalez said.
This could also make it possible to determine whether chemotherapy patients can receive their next dose sooner than usual.
“There is a balancing act that oncologists must do,” said study author Alvaro Sanchez-Ferro, MD, of Centro Integral en Neurociencias A.C. HM CINAC in Madrid, Spain.
“Normally, doctors want to make chemotherapy as intensive as possible but without getting people too immunosuppressed. Current 21-day cycles are based on statistics of what most patients can take, but if you are ready early, then they can potentially bring you back early, and that can translate into better survival.”
A portable device could be used to monitor patients’ white blood cell (WBC) levels at home, without the need for blood samples, according to researchers.
The team created a prototype that records video of blood cells flowing through capillaries just below the skin surface at the base of the fingernail.
The group is developing a computer algorithm that, in early testing, has been able to analyze the videos and determine if WBC levels are too low.
The researchers believe this type of device could be used to prevent infections among chemotherapy recipients.
“Our vision is that patients will have this portable device that they can take home, and they can monitor daily how they are reacting to the treatment,” said Carlos Castro-Gonzalez, PhD, of the Massachusetts Institute of Technology (MIT) in Cambridge, Massachusetts.
“If they go below the [safe WBC] threshold, then preventive treatment can be deployed.”
Dr Castro-Gonzalez and his colleagues described their prototype, and the testing of it, in Scientific Reports.
The device consists of a wide-field microscope that emits blue light, which penetrates about 50 to 150 microns below the skin and is reflected back to a video camera.
The researchers decided to image the skin at the base of the nail because the capillaries there are very close to the skin surface. These capillaries are so narrow that WBCs must squeeze through one at a time, making them easier to see.
The researchers tested the device in 11 patients at various points during their chemotherapy treatment.
The device does not provide precise WBC counts but allowed the team to differentiate cases of severe neutropenia (<500 neutrophils per μL) from non-neutropenic cases (>1500 neutrophils per μL).
To obtain enough data to make these classifications, the researchers recorded 1 minute of video per patient. Three blinded human assistants then watched the videos and noted whenever a WBC passed by.
However, since submitting their paper, the researchers have been developing a computer algorithm to perform the same task automatically.
“Based on the feature-set that our human raters identified, we are now developing an AI and machine-vision algorithm, with preliminary results that indicate the same accuracy as the raters,” said study author Aurélien Bourquard, PhD, of MIT.
The researchers have applied for patents on the technology and launched a company called Leuko, which is working on commercializing the technology with help from MIT.
To move the technology further toward commercialization, the researchers are building a new automated prototype.
“Automating the measurement process is key to making a viable home-use device,” said study author Ian Butterworth, of MIT. “The imaging needs to take place in the right spot on the patient’s finger, and the operation of the device must be straightforward.”
Using this new prototype, the researchers plan to test the device with additional cancer patients. And the team is investigating whether they can get accurate results with shorter lengths of video.
They also plan to adapt the technology so it can generate more precise WBC counts, which would make it useful for monitoring bone marrow transplant recipients or people with certain infectious diseases, Dr Castro-Gonzalez said.
This could also make it possible to determine whether chemotherapy patients can receive their next dose sooner than usual.
“There is a balancing act that oncologists must do,” said study author Alvaro Sanchez-Ferro, MD, of Centro Integral en Neurociencias A.C. HM CINAC in Madrid, Spain.
“Normally, doctors want to make chemotherapy as intensive as possible but without getting people too immunosuppressed. Current 21-day cycles are based on statistics of what most patients can take, but if you are ready early, then they can potentially bring you back early, and that can translate into better survival.”
A portable device could be used to monitor patients’ white blood cell (WBC) levels at home, without the need for blood samples, according to researchers.
The team created a prototype that records video of blood cells flowing through capillaries just below the skin surface at the base of the fingernail.
The group is developing a computer algorithm that, in early testing, has been able to analyze the videos and determine if WBC levels are too low.
The researchers believe this type of device could be used to prevent infections among chemotherapy recipients.
“Our vision is that patients will have this portable device that they can take home, and they can monitor daily how they are reacting to the treatment,” said Carlos Castro-Gonzalez, PhD, of the Massachusetts Institute of Technology (MIT) in Cambridge, Massachusetts.
“If they go below the [safe WBC] threshold, then preventive treatment can be deployed.”
Dr Castro-Gonzalez and his colleagues described their prototype, and the testing of it, in Scientific Reports.
The device consists of a wide-field microscope that emits blue light, which penetrates about 50 to 150 microns below the skin and is reflected back to a video camera.
The researchers decided to image the skin at the base of the nail because the capillaries there are very close to the skin surface. These capillaries are so narrow that WBCs must squeeze through one at a time, making them easier to see.
The researchers tested the device in 11 patients at various points during their chemotherapy treatment.
The device does not provide precise WBC counts but allowed the team to differentiate cases of severe neutropenia (<500 neutrophils per μL) from non-neutropenic cases (>1500 neutrophils per μL).
To obtain enough data to make these classifications, the researchers recorded 1 minute of video per patient. Three blinded human assistants then watched the videos and noted whenever a WBC passed by.
However, since submitting their paper, the researchers have been developing a computer algorithm to perform the same task automatically.
“Based on the feature-set that our human raters identified, we are now developing an AI and machine-vision algorithm, with preliminary results that indicate the same accuracy as the raters,” said study author Aurélien Bourquard, PhD, of MIT.
The researchers have applied for patents on the technology and launched a company called Leuko, which is working on commercializing the technology with help from MIT.
To move the technology further toward commercialization, the researchers are building a new automated prototype.
“Automating the measurement process is key to making a viable home-use device,” said study author Ian Butterworth, of MIT. “The imaging needs to take place in the right spot on the patient’s finger, and the operation of the device must be straightforward.”
Using this new prototype, the researchers plan to test the device with additional cancer patients. And the team is investigating whether they can get accurate results with shorter lengths of video.
They also plan to adapt the technology so it can generate more precise WBC counts, which would make it useful for monitoring bone marrow transplant recipients or people with certain infectious diseases, Dr Castro-Gonzalez said.
This could also make it possible to determine whether chemotherapy patients can receive their next dose sooner than usual.
“There is a balancing act that oncologists must do,” said study author Alvaro Sanchez-Ferro, MD, of Centro Integral en Neurociencias A.C. HM CINAC in Madrid, Spain.
“Normally, doctors want to make chemotherapy as intensive as possible but without getting people too immunosuppressed. Current 21-day cycles are based on statistics of what most patients can take, but if you are ready early, then they can potentially bring you back early, and that can translate into better survival.”
Abstract: Collaborative Care for Opioid and Alcohol Use Disorders in Primary Care: The SUMMIT Randomized Clinical Trial
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Watkins, K.E., et al, JAMA Intern Med 177(10):1480, October 1, 2017
BACKGROUND: Collaborative care has been reported to be an effective strategy for the delivery of evidence-based treatments and improving patient outcomes, but its utility for substance abuse treatment in primary care has not been evaluated.
METHODS: The Substance Use Motivation and Medication Integrated Treatment (SUMMIT) study, coordinated at RAND Corporation, included 377 adults (mean age 42 years; 80% male) attending two community health clinics for opioid and alcohol use disorders. The study excluded patients with bipolar disorder, schizophrenia, or current substance abuse treatment. Study participants were randomized to collaborative care (n=187) or usual care (n=190). Patients were assessed for their use of evidence-based treatments (brief six-session psychotherapy treatment and treatment with buprenorphine/naloxone or naltrexone) and self-reported abstinence from opioids and alcohol at six months.
RESULTS: Only 13% of the patients had received any substance abuse treatment in the previous year. After six months, more patients in the collaborative care group than controls had received psychotherapy or medications (39.0% versus 16.8%; adjusted odds ratio [OR] 3.97, 95% CI 2.32-6.79; p<0.001); the difference was explained by a higher rate of psychotherapy (35.8% versus 10.5%; OR 6.22, 95% CI 3.4-11.5; p<0.001), while rates of medication use in the two groups were similar (13.4% versus 12.6%). Self-reported abstinence at six months was also more frequent with collaborative care (32.8% versus 22.3%; adjusted effect estimate, beta = 0.12; 95% CI 0.01-0.23; p=0.03). Healthcare Effectiveness Data and Information Set (HEDIS) measures of initiation and engagement increased significantly with collaborative care (both, p<0.001).
CONCLUSIONS: A collaborative care intervention increased treatment uptake and six-month abstinence in these primary care patients with opioid and alcohol abuse disorders. 69 references (kwatkins@rand.org – no reprints)
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Watkins, K.E., et al, JAMA Intern Med 177(10):1480, October 1, 2017
BACKGROUND: Collaborative care has been reported to be an effective strategy for the delivery of evidence-based treatments and improving patient outcomes, but its utility for substance abuse treatment in primary care has not been evaluated.
METHODS: The Substance Use Motivation and Medication Integrated Treatment (SUMMIT) study, coordinated at RAND Corporation, included 377 adults (mean age 42 years; 80% male) attending two community health clinics for opioid and alcohol use disorders. The study excluded patients with bipolar disorder, schizophrenia, or current substance abuse treatment. Study participants were randomized to collaborative care (n=187) or usual care (n=190). Patients were assessed for their use of evidence-based treatments (brief six-session psychotherapy treatment and treatment with buprenorphine/naloxone or naltrexone) and self-reported abstinence from opioids and alcohol at six months.
RESULTS: Only 13% of the patients had received any substance abuse treatment in the previous year. After six months, more patients in the collaborative care group than controls had received psychotherapy or medications (39.0% versus 16.8%; adjusted odds ratio [OR] 3.97, 95% CI 2.32-6.79; p<0.001); the difference was explained by a higher rate of psychotherapy (35.8% versus 10.5%; OR 6.22, 95% CI 3.4-11.5; p<0.001), while rates of medication use in the two groups were similar (13.4% versus 12.6%). Self-reported abstinence at six months was also more frequent with collaborative care (32.8% versus 22.3%; adjusted effect estimate, beta = 0.12; 95% CI 0.01-0.23; p=0.03). Healthcare Effectiveness Data and Information Set (HEDIS) measures of initiation and engagement increased significantly with collaborative care (both, p<0.001).
CONCLUSIONS: A collaborative care intervention increased treatment uptake and six-month abstinence in these primary care patients with opioid and alcohol abuse disorders. 69 references (kwatkins@rand.org – no reprints)
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Watkins, K.E., et al, JAMA Intern Med 177(10):1480, October 1, 2017
BACKGROUND: Collaborative care has been reported to be an effective strategy for the delivery of evidence-based treatments and improving patient outcomes, but its utility for substance abuse treatment in primary care has not been evaluated.
METHODS: The Substance Use Motivation and Medication Integrated Treatment (SUMMIT) study, coordinated at RAND Corporation, included 377 adults (mean age 42 years; 80% male) attending two community health clinics for opioid and alcohol use disorders. The study excluded patients with bipolar disorder, schizophrenia, or current substance abuse treatment. Study participants were randomized to collaborative care (n=187) or usual care (n=190). Patients were assessed for their use of evidence-based treatments (brief six-session psychotherapy treatment and treatment with buprenorphine/naloxone or naltrexone) and self-reported abstinence from opioids and alcohol at six months.
RESULTS: Only 13% of the patients had received any substance abuse treatment in the previous year. After six months, more patients in the collaborative care group than controls had received psychotherapy or medications (39.0% versus 16.8%; adjusted odds ratio [OR] 3.97, 95% CI 2.32-6.79; p<0.001); the difference was explained by a higher rate of psychotherapy (35.8% versus 10.5%; OR 6.22, 95% CI 3.4-11.5; p<0.001), while rates of medication use in the two groups were similar (13.4% versus 12.6%). Self-reported abstinence at six months was also more frequent with collaborative care (32.8% versus 22.3%; adjusted effect estimate, beta = 0.12; 95% CI 0.01-0.23; p=0.03). Healthcare Effectiveness Data and Information Set (HEDIS) measures of initiation and engagement increased significantly with collaborative care (both, p<0.001).
CONCLUSIONS: A collaborative care intervention increased treatment uptake and six-month abstinence in these primary care patients with opioid and alcohol abuse disorders. 69 references (kwatkins@rand.org – no reprints)
Learn more about the Primary Care Medical Abstracts and podcasts, for which you can earn up to 9 CME credits per month.
Copyright © The Center for Medical Education
Sound and light levels are similarly disruptive in ICU and non-ICU wards
Clinical question: While it is generally thought that ICU wards are not conducive for sleep because of light and noise disruptions, are general wards any better?
Background: Hospitalized patients frequently report poor sleep, partly because of the inpatient environment. Sound level changes (SLCs), rather than the total volumes, are important in disrupting sleep. The World Health Organization recommends that nighttime baseline noise levels do not exceed 30 decibels (dB) and that nighttime noise peaks (i.e., loud noises) do not exceed 40 dB. The circadian rhythm system depends on ambient light to regulate the internal clock. Insufficient and inappropriately timed light exposure can desynchronize the biological clock, thereby negatively affecting sleep quality.
Setting: Tertiary care hospital in La Jolla, Calif.
Synopsis: For approximately 24-72 hours, recordings of sound and light were performed. ICU rooms were louder (hourly averages ranged from 56.1 dB to 60.3 dB) than were non-ICU wards (44.6-55.1 dB). However, SLCs of 17.5 dB or greater were not statistically different (ICU, 203.9 ± 28.8 times; non-ICU, 270.9 ± 39.5; P = .11). In both ICU and non-ICU wards, average daytime light levels were less than 250 lux and generally were brightest in the afternoon. This corresponds to low, office-level lighting, which may not be conducive for maintaining circadian rhythm.
Bottom line: While ICU wards are generally louder than non-ICU wards, sound level changes are equivalent and probably more important concerning sleep disruption. While no significant differences were seen in light levels, the amount and timing of lighting may not be optimal for keeping circadian rhythm.
Citation: Jaiswal SJ et al. Sound and light levels are similarly disruptive in ICU and non-ICU wards. J Hosp Med. 2017 Oct;12(10):798-804.
Dr. Kim is assistant professor of medicine in the division of hospital medicine, Emory University, Atlanta.
Clinical question: While it is generally thought that ICU wards are not conducive for sleep because of light and noise disruptions, are general wards any better?
Background: Hospitalized patients frequently report poor sleep, partly because of the inpatient environment. Sound level changes (SLCs), rather than the total volumes, are important in disrupting sleep. The World Health Organization recommends that nighttime baseline noise levels do not exceed 30 decibels (dB) and that nighttime noise peaks (i.e., loud noises) do not exceed 40 dB. The circadian rhythm system depends on ambient light to regulate the internal clock. Insufficient and inappropriately timed light exposure can desynchronize the biological clock, thereby negatively affecting sleep quality.
Setting: Tertiary care hospital in La Jolla, Calif.
Synopsis: For approximately 24-72 hours, recordings of sound and light were performed. ICU rooms were louder (hourly averages ranged from 56.1 dB to 60.3 dB) than were non-ICU wards (44.6-55.1 dB). However, SLCs of 17.5 dB or greater were not statistically different (ICU, 203.9 ± 28.8 times; non-ICU, 270.9 ± 39.5; P = .11). In both ICU and non-ICU wards, average daytime light levels were less than 250 lux and generally were brightest in the afternoon. This corresponds to low, office-level lighting, which may not be conducive for maintaining circadian rhythm.
Bottom line: While ICU wards are generally louder than non-ICU wards, sound level changes are equivalent and probably more important concerning sleep disruption. While no significant differences were seen in light levels, the amount and timing of lighting may not be optimal for keeping circadian rhythm.
Citation: Jaiswal SJ et al. Sound and light levels are similarly disruptive in ICU and non-ICU wards. J Hosp Med. 2017 Oct;12(10):798-804.
Dr. Kim is assistant professor of medicine in the division of hospital medicine, Emory University, Atlanta.
Clinical question: While it is generally thought that ICU wards are not conducive for sleep because of light and noise disruptions, are general wards any better?
Background: Hospitalized patients frequently report poor sleep, partly because of the inpatient environment. Sound level changes (SLCs), rather than the total volumes, are important in disrupting sleep. The World Health Organization recommends that nighttime baseline noise levels do not exceed 30 decibels (dB) and that nighttime noise peaks (i.e., loud noises) do not exceed 40 dB. The circadian rhythm system depends on ambient light to regulate the internal clock. Insufficient and inappropriately timed light exposure can desynchronize the biological clock, thereby negatively affecting sleep quality.
Setting: Tertiary care hospital in La Jolla, Calif.
Synopsis: For approximately 24-72 hours, recordings of sound and light were performed. ICU rooms were louder (hourly averages ranged from 56.1 dB to 60.3 dB) than were non-ICU wards (44.6-55.1 dB). However, SLCs of 17.5 dB or greater were not statistically different (ICU, 203.9 ± 28.8 times; non-ICU, 270.9 ± 39.5; P = .11). In both ICU and non-ICU wards, average daytime light levels were less than 250 lux and generally were brightest in the afternoon. This corresponds to low, office-level lighting, which may not be conducive for maintaining circadian rhythm.
Bottom line: While ICU wards are generally louder than non-ICU wards, sound level changes are equivalent and probably more important concerning sleep disruption. While no significant differences were seen in light levels, the amount and timing of lighting may not be optimal for keeping circadian rhythm.
Citation: Jaiswal SJ et al. Sound and light levels are similarly disruptive in ICU and non-ICU wards. J Hosp Med. 2017 Oct;12(10):798-804.
Dr. Kim is assistant professor of medicine in the division of hospital medicine, Emory University, Atlanta.
CMS finalizes measures to help combat opioid crisis
Federal agencies and leaders are taking a multipronged approach to the opioid crisis.
Efforts by the Centers for Medicare & Medicaid Services focus on changes to the Medicare Part D program and Medicare Advantage, via two policy changes issued April 2: The Part D/Medicare Advantage annual update and final calendar year 2019 guidance to insurers who provide coverage under those two programs.
The annual Part D/Medicare Advantage update implements provisions of the Comprehensive Addiction and Recovery Act of 2016 (S. 524), requiring “CMS to establish through regulation a framework that allows Part D sponsors to implement drug management programs,” according to a CMS fact sheet. “Under such programs, a sponsor can limit at-risk beneficiaries’ access to coverage for frequently abused drugs beginning with the 2019 plan year.” The update designates opioids and benzodiazepines as frequently abused drugs.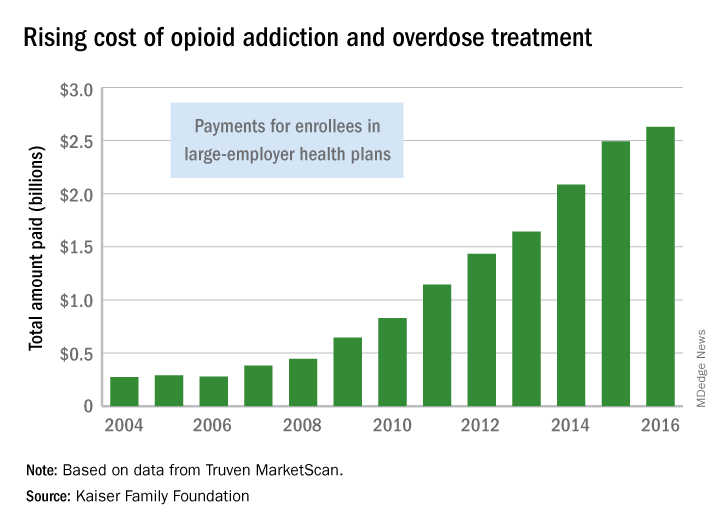
The agency is taking the further step of limiting special enrollment periods for dual eligibles – those beneficiaries eligible for both Medicare and Medicaid – if they are identified as at-risk or potentially at-risk for prescription drug abuse under the programs. Beneficiaries maintain the right to challenge the determination.
Patients who are being treated for cancer-related pain and those receiving hospice/palliative/end-of-life care are exempted from the drug management programs.
The policies also update opioid prescription limits. CMS is calling on “all Part D sponsors to implement a hard safety edit to limit initial opioid prescription fills for the treatment of acute pain to no more than a 7-day supply,” according to the fact sheet.
For chronic opioid users, CMS expects “all sponsors to implement an opioid care coordination edit at 90 morphine milligram equivalent (MME) per day. This formulary-level safety edit should trigger when a beneficiary’s cumulative MME per day across their opioid prescriptions reaches or exceeds 90 MME.”
There is flexibility: Pharmacists may contact prescribers and confirm that more pain medication is needed and then override the 90 MME/day threshold, if appropriate.
Finally, CMS expects “sponsors to implement additional soft safety edits to alert the pharmacist about duplicative opioid therapy and concurrent use of opioids and benzodiazepines,” according to the guidance.
The executive and legislative branches also are taking action.
President Trump in March detailed initiatives to address the opioid crisis across three domains: reducing demand through education, awareness, and prevention of overprescribing; reducing illicit drug importation and distribution; and expanding opportunities for proven treatment options.
For prescribers, the president’s plan calls for a nationwide reduction in opioid prescriptions filled by one-third within 3 years. It also looks aims to ensure that 75% of opioid prescriptions paid for by the government are “issued using best practices within 3 years, and 95% within 5 years,” a White House fact sheet notes.
When it comes to federal health care providers, those standards are to be met by half within 2 years and all within 5.
The White House also is calling on states to transition to a nationally interoperable Prescription Drug Monitoring Program (PDMP) network.
The president’s plan also calls for ensuring first-responder access to naloxone, improving overdose tracking systems, and expanding access to medication-assisted treatment. It also aims to change the Medicaid law that prohibits reimbursement of residential treatment at certain facilities with more than 16 beds.
On the legislative side, the House Energy and Commerce Committee is in the process of hosting a series of hearings and is expected to introduce a comprehensive package of bills aimed at various aspects of the opioid crisis. Across the four hearings, more than 30 pieces of individual legislation have been examined.
In the Senate, the Health, Education, Labor and Pensions Committee on April 4 released a discussion draft of the Opioid Crisis Response Act of 2018 and has scheduled a hearing for April 11 to discuss it.
The bill would spur development of nonaddictive pain killers, clarify FDA authority on small-quantity blister packs for opioids, provide states with better PDMP support; increase access to mental health services in schools, and improve substance use disorder treatment in underserved areas.
Also on April 4, the National Institutes of Health launched the HEAL Initiative (Helping to End Addiction Long-Term) to increase research to help prevent addiction through advanced pain management, and improve treatments for opioid misuse disorder and addiction. NIH is nearly doubling funding into opioid misuse/addiction research from $600 million in fiscal 2016 to $1.1 billion in fiscal 2018.
Federal agencies and leaders are taking a multipronged approach to the opioid crisis.
Efforts by the Centers for Medicare & Medicaid Services focus on changes to the Medicare Part D program and Medicare Advantage, via two policy changes issued April 2: The Part D/Medicare Advantage annual update and final calendar year 2019 guidance to insurers who provide coverage under those two programs.
The annual Part D/Medicare Advantage update implements provisions of the Comprehensive Addiction and Recovery Act of 2016 (S. 524), requiring “CMS to establish through regulation a framework that allows Part D sponsors to implement drug management programs,” according to a CMS fact sheet. “Under such programs, a sponsor can limit at-risk beneficiaries’ access to coverage for frequently abused drugs beginning with the 2019 plan year.” The update designates opioids and benzodiazepines as frequently abused drugs.
The agency is taking the further step of limiting special enrollment periods for dual eligibles – those beneficiaries eligible for both Medicare and Medicaid – if they are identified as at-risk or potentially at-risk for prescription drug abuse under the programs. Beneficiaries maintain the right to challenge the determination.
Patients who are being treated for cancer-related pain and those receiving hospice/palliative/end-of-life care are exempted from the drug management programs.
The policies also update opioid prescription limits. CMS is calling on “all Part D sponsors to implement a hard safety edit to limit initial opioid prescription fills for the treatment of acute pain to no more than a 7-day supply,” according to the fact sheet.
For chronic opioid users, CMS expects “all sponsors to implement an opioid care coordination edit at 90 morphine milligram equivalent (MME) per day. This formulary-level safety edit should trigger when a beneficiary’s cumulative MME per day across their opioid prescriptions reaches or exceeds 90 MME.”
There is flexibility: Pharmacists may contact prescribers and confirm that more pain medication is needed and then override the 90 MME/day threshold, if appropriate.
Finally, CMS expects “sponsors to implement additional soft safety edits to alert the pharmacist about duplicative opioid therapy and concurrent use of opioids and benzodiazepines,” according to the guidance.
The executive and legislative branches also are taking action.
President Trump in March detailed initiatives to address the opioid crisis across three domains: reducing demand through education, awareness, and prevention of overprescribing; reducing illicit drug importation and distribution; and expanding opportunities for proven treatment options.
For prescribers, the president’s plan calls for a nationwide reduction in opioid prescriptions filled by one-third within 3 years. It also looks aims to ensure that 75% of opioid prescriptions paid for by the government are “issued using best practices within 3 years, and 95% within 5 years,” a White House fact sheet notes.
When it comes to federal health care providers, those standards are to be met by half within 2 years and all within 5.
The White House also is calling on states to transition to a nationally interoperable Prescription Drug Monitoring Program (PDMP) network.
The president’s plan also calls for ensuring first-responder access to naloxone, improving overdose tracking systems, and expanding access to medication-assisted treatment. It also aims to change the Medicaid law that prohibits reimbursement of residential treatment at certain facilities with more than 16 beds.
On the legislative side, the House Energy and Commerce Committee is in the process of hosting a series of hearings and is expected to introduce a comprehensive package of bills aimed at various aspects of the opioid crisis. Across the four hearings, more than 30 pieces of individual legislation have been examined.
In the Senate, the Health, Education, Labor and Pensions Committee on April 4 released a discussion draft of the Opioid Crisis Response Act of 2018 and has scheduled a hearing for April 11 to discuss it.
The bill would spur development of nonaddictive pain killers, clarify FDA authority on small-quantity blister packs for opioids, provide states with better PDMP support; increase access to mental health services in schools, and improve substance use disorder treatment in underserved areas.
Also on April 4, the National Institutes of Health launched the HEAL Initiative (Helping to End Addiction Long-Term) to increase research to help prevent addiction through advanced pain management, and improve treatments for opioid misuse disorder and addiction. NIH is nearly doubling funding into opioid misuse/addiction research from $600 million in fiscal 2016 to $1.1 billion in fiscal 2018.
Federal agencies and leaders are taking a multipronged approach to the opioid crisis.
Efforts by the Centers for Medicare & Medicaid Services focus on changes to the Medicare Part D program and Medicare Advantage, via two policy changes issued April 2: The Part D/Medicare Advantage annual update and final calendar year 2019 guidance to insurers who provide coverage under those two programs.
The annual Part D/Medicare Advantage update implements provisions of the Comprehensive Addiction and Recovery Act of 2016 (S. 524), requiring “CMS to establish through regulation a framework that allows Part D sponsors to implement drug management programs,” according to a CMS fact sheet. “Under such programs, a sponsor can limit at-risk beneficiaries’ access to coverage for frequently abused drugs beginning with the 2019 plan year.” The update designates opioids and benzodiazepines as frequently abused drugs.
The agency is taking the further step of limiting special enrollment periods for dual eligibles – those beneficiaries eligible for both Medicare and Medicaid – if they are identified as at-risk or potentially at-risk for prescription drug abuse under the programs. Beneficiaries maintain the right to challenge the determination.
Patients who are being treated for cancer-related pain and those receiving hospice/palliative/end-of-life care are exempted from the drug management programs.
The policies also update opioid prescription limits. CMS is calling on “all Part D sponsors to implement a hard safety edit to limit initial opioid prescription fills for the treatment of acute pain to no more than a 7-day supply,” according to the fact sheet.
For chronic opioid users, CMS expects “all sponsors to implement an opioid care coordination edit at 90 morphine milligram equivalent (MME) per day. This formulary-level safety edit should trigger when a beneficiary’s cumulative MME per day across their opioid prescriptions reaches or exceeds 90 MME.”
There is flexibility: Pharmacists may contact prescribers and confirm that more pain medication is needed and then override the 90 MME/day threshold, if appropriate.
Finally, CMS expects “sponsors to implement additional soft safety edits to alert the pharmacist about duplicative opioid therapy and concurrent use of opioids and benzodiazepines,” according to the guidance.
The executive and legislative branches also are taking action.
President Trump in March detailed initiatives to address the opioid crisis across three domains: reducing demand through education, awareness, and prevention of overprescribing; reducing illicit drug importation and distribution; and expanding opportunities for proven treatment options.
For prescribers, the president’s plan calls for a nationwide reduction in opioid prescriptions filled by one-third within 3 years. It also looks aims to ensure that 75% of opioid prescriptions paid for by the government are “issued using best practices within 3 years, and 95% within 5 years,” a White House fact sheet notes.
When it comes to federal health care providers, those standards are to be met by half within 2 years and all within 5.
The White House also is calling on states to transition to a nationally interoperable Prescription Drug Monitoring Program (PDMP) network.
The president’s plan also calls for ensuring first-responder access to naloxone, improving overdose tracking systems, and expanding access to medication-assisted treatment. It also aims to change the Medicaid law that prohibits reimbursement of residential treatment at certain facilities with more than 16 beds.
On the legislative side, the House Energy and Commerce Committee is in the process of hosting a series of hearings and is expected to introduce a comprehensive package of bills aimed at various aspects of the opioid crisis. Across the four hearings, more than 30 pieces of individual legislation have been examined.
In the Senate, the Health, Education, Labor and Pensions Committee on April 4 released a discussion draft of the Opioid Crisis Response Act of 2018 and has scheduled a hearing for April 11 to discuss it.
The bill would spur development of nonaddictive pain killers, clarify FDA authority on small-quantity blister packs for opioids, provide states with better PDMP support; increase access to mental health services in schools, and improve substance use disorder treatment in underserved areas.
Also on April 4, the National Institutes of Health launched the HEAL Initiative (Helping to End Addiction Long-Term) to increase research to help prevent addiction through advanced pain management, and improve treatments for opioid misuse disorder and addiction. NIH is nearly doubling funding into opioid misuse/addiction research from $600 million in fiscal 2016 to $1.1 billion in fiscal 2018.
From weekend warriors to pros, athletes are plagued by skin disorders
SAN DIEGO – Think beyond the foot: Fungal infections are just the beginning when it comes to skin disorders in athletes, which include ringworm in wrestlers, “jogger’s nipple” in runners, and more serious conditions – like skin cancer.
“from weekend warriors to professional athletes,” said Brian B. Adams, MD, MPH, professor and chair of the department of dermatology at the University of Cincinnati.
In an interview, he discussed specific risks for athletes, the scarcity of data on skin cancer in athletes, and the hazards posed by cotton clothing.
Skin cancer: Risk seems clear, but data are sparse
“Athletes in general appear to be at increased risk in the long term for skin cancer” since they often play and practice during the hours between 10 a.m. and 4 p.m., when the danger of sun exposure is at its highest, he said. In addition, “sweating removes the sunscreen that athletes put on, and there is evidence that sweating actually increases the chance of burning,” he said.
Skiers and snowboarders face unique sun exposure risks, he added. “Snow reflects up to 100% of UV, so even though they may be in shade, they experience UV. And mountain athletes experience greater amounts of UV as the altitude of the mountain increases: At higher altitudes, the atmosphere has less chance of filtering out the damaging rays.”
While it’s obvious that many athletes face extra sun exposure, Dr. Adams pointed out, “very little is definitively known about the actual degree of risk of athletic activities.”
Still, the research that does exist provides plenty of hints about risk. “Large epidemiological studies showed that recreational activities such as sun exposure on the beach or during water sports were associated with an increased risk of basal cell carcinoma, whereas skiing has been shown to be at increased risk for squamous cell carcinoma,” according to a 2008 report on outdoor sports and skin cancer (Clin Dermatol. 2008 Jan-Feb;26[1]:12-5).
“Risk factors of cutaneous melanoma, such as the number of melanocytic nevi and solar lentigines, have been found to be more frequent in subjects practicing endurance outdoor sports. An increased risk for cutaneous melanoma may be assumed for these athletes,” Dr. Adams commented.
Another study, this one published in 2006, found more atypical melanocytic nevi, solar lentigines, and lesions suggestive of nonmelanoma skin cancer in marathon runners, compared with a control group, and the risk was associated with the level of training intensity. The control subjects were more sensitive to the sun and had more common melanocytic nevi (Arch Dermatol. 2006 Nov;142[11]:1471-4).
Counseling about sunscreen may actually work
One strategy to reduce sun exposure is to advise athletes to avoid peak sun hours. However, “the key to caring for the athletes is not only recognizing that their sport may play a role in their disease but also realizing that your therapeutic approach must be tailored to minimize disruption to their practices and competitions,” Dr. Adams said.
However, there’s good news for dermatologists who are willing to push: The study also reported that athletes who were encouraged to use sunscreen were significantly more likely to use sunscreen (P less than .0001).
Watch for other conditions, from jogger’s nipple to ringworm
Dr. Adams offered advice about detection, treatment, and prevention of other skin disorders that affect athletes:
- “Jogger’s nipples” and other kinds of chafing. He has learned to recognize the “red eleven” – two vertical streaks of blood on a runner’s shirt – that represent a case of “jogger’s nipples” caused by chafing. Antibacterial ointment or petroleum jelly are useful treatments, he said, and an application of plenty of petroleum jelly on the nipples prior to a run can be helpful. Cotton shirts should be avoided, he said, in favor of synthetic, moisture-wicking shirts and bras. Chafing can also occur in the underarms and inner thighs, he said, and the same treatments and preventive techniques are useful.
- Callused and bleeding “jogger’s toes.” This can strike runners, especially on the second toe, which is often the longest and most likely to strike the toe box of a shoe. Specialty shoes can help prevent this condition, he said.
- Tinea corporis (ringworm) and herpes gladiatorum. In wrestlers, ringworm is known as tinea corporis gladiatorum because the intensity of skin-to-skin contact in wrestling makes the condition especially common in these athletes. Lesions don’t develop as rings at first; instead, they first appear as relatively nonspecific red round lesions and are most likely to be found in the head, neck, and upper extremities, Dr. Adams noted. Herpes gladiatorum is caused by herpes simplex virus 1; it is also seen in wrestlers and caused by skin-to-skin contact. Topical and oral antifungals clear ringworm, while oral antiviral agents are appropriate for herpes gladiatorum, Dr. Adams said. While herpes gladiatorum clears up and is no longer contagious after 4-5 days, he said, it’s not clear how long wrestlers with ringworm should be disqualified from playing.
Dr. Adams disclosed advising Mission, a company that focuses on sunscreen designed by and for athletes.
SAN DIEGO – Think beyond the foot: Fungal infections are just the beginning when it comes to skin disorders in athletes, which include ringworm in wrestlers, “jogger’s nipple” in runners, and more serious conditions – like skin cancer.
“from weekend warriors to professional athletes,” said Brian B. Adams, MD, MPH, professor and chair of the department of dermatology at the University of Cincinnati.
In an interview, he discussed specific risks for athletes, the scarcity of data on skin cancer in athletes, and the hazards posed by cotton clothing.
Skin cancer: Risk seems clear, but data are sparse
“Athletes in general appear to be at increased risk in the long term for skin cancer” since they often play and practice during the hours between 10 a.m. and 4 p.m., when the danger of sun exposure is at its highest, he said. In addition, “sweating removes the sunscreen that athletes put on, and there is evidence that sweating actually increases the chance of burning,” he said.
Skiers and snowboarders face unique sun exposure risks, he added. “Snow reflects up to 100% of UV, so even though they may be in shade, they experience UV. And mountain athletes experience greater amounts of UV as the altitude of the mountain increases: At higher altitudes, the atmosphere has less chance of filtering out the damaging rays.”
While it’s obvious that many athletes face extra sun exposure, Dr. Adams pointed out, “very little is definitively known about the actual degree of risk of athletic activities.”
Still, the research that does exist provides plenty of hints about risk. “Large epidemiological studies showed that recreational activities such as sun exposure on the beach or during water sports were associated with an increased risk of basal cell carcinoma, whereas skiing has been shown to be at increased risk for squamous cell carcinoma,” according to a 2008 report on outdoor sports and skin cancer (Clin Dermatol. 2008 Jan-Feb;26[1]:12-5).
“Risk factors of cutaneous melanoma, such as the number of melanocytic nevi and solar lentigines, have been found to be more frequent in subjects practicing endurance outdoor sports. An increased risk for cutaneous melanoma may be assumed for these athletes,” Dr. Adams commented.
Another study, this one published in 2006, found more atypical melanocytic nevi, solar lentigines, and lesions suggestive of nonmelanoma skin cancer in marathon runners, compared with a control group, and the risk was associated with the level of training intensity. The control subjects were more sensitive to the sun and had more common melanocytic nevi (Arch Dermatol. 2006 Nov;142[11]:1471-4).
Counseling about sunscreen may actually work
One strategy to reduce sun exposure is to advise athletes to avoid peak sun hours. However, “the key to caring for the athletes is not only recognizing that their sport may play a role in their disease but also realizing that your therapeutic approach must be tailored to minimize disruption to their practices and competitions,” Dr. Adams said.
However, there’s good news for dermatologists who are willing to push: The study also reported that athletes who were encouraged to use sunscreen were significantly more likely to use sunscreen (P less than .0001).
Watch for other conditions, from jogger’s nipple to ringworm
Dr. Adams offered advice about detection, treatment, and prevention of other skin disorders that affect athletes:
- “Jogger’s nipples” and other kinds of chafing. He has learned to recognize the “red eleven” – two vertical streaks of blood on a runner’s shirt – that represent a case of “jogger’s nipples” caused by chafing. Antibacterial ointment or petroleum jelly are useful treatments, he said, and an application of plenty of petroleum jelly on the nipples prior to a run can be helpful. Cotton shirts should be avoided, he said, in favor of synthetic, moisture-wicking shirts and bras. Chafing can also occur in the underarms and inner thighs, he said, and the same treatments and preventive techniques are useful.
- Callused and bleeding “jogger’s toes.” This can strike runners, especially on the second toe, which is often the longest and most likely to strike the toe box of a shoe. Specialty shoes can help prevent this condition, he said.
- Tinea corporis (ringworm) and herpes gladiatorum. In wrestlers, ringworm is known as tinea corporis gladiatorum because the intensity of skin-to-skin contact in wrestling makes the condition especially common in these athletes. Lesions don’t develop as rings at first; instead, they first appear as relatively nonspecific red round lesions and are most likely to be found in the head, neck, and upper extremities, Dr. Adams noted. Herpes gladiatorum is caused by herpes simplex virus 1; it is also seen in wrestlers and caused by skin-to-skin contact. Topical and oral antifungals clear ringworm, while oral antiviral agents are appropriate for herpes gladiatorum, Dr. Adams said. While herpes gladiatorum clears up and is no longer contagious after 4-5 days, he said, it’s not clear how long wrestlers with ringworm should be disqualified from playing.
Dr. Adams disclosed advising Mission, a company that focuses on sunscreen designed by and for athletes.
SAN DIEGO – Think beyond the foot: Fungal infections are just the beginning when it comes to skin disorders in athletes, which include ringworm in wrestlers, “jogger’s nipple” in runners, and more serious conditions – like skin cancer.
“from weekend warriors to professional athletes,” said Brian B. Adams, MD, MPH, professor and chair of the department of dermatology at the University of Cincinnati.
In an interview, he discussed specific risks for athletes, the scarcity of data on skin cancer in athletes, and the hazards posed by cotton clothing.
Skin cancer: Risk seems clear, but data are sparse
“Athletes in general appear to be at increased risk in the long term for skin cancer” since they often play and practice during the hours between 10 a.m. and 4 p.m., when the danger of sun exposure is at its highest, he said. In addition, “sweating removes the sunscreen that athletes put on, and there is evidence that sweating actually increases the chance of burning,” he said.
Skiers and snowboarders face unique sun exposure risks, he added. “Snow reflects up to 100% of UV, so even though they may be in shade, they experience UV. And mountain athletes experience greater amounts of UV as the altitude of the mountain increases: At higher altitudes, the atmosphere has less chance of filtering out the damaging rays.”
While it’s obvious that many athletes face extra sun exposure, Dr. Adams pointed out, “very little is definitively known about the actual degree of risk of athletic activities.”
Still, the research that does exist provides plenty of hints about risk. “Large epidemiological studies showed that recreational activities such as sun exposure on the beach or during water sports were associated with an increased risk of basal cell carcinoma, whereas skiing has been shown to be at increased risk for squamous cell carcinoma,” according to a 2008 report on outdoor sports and skin cancer (Clin Dermatol. 2008 Jan-Feb;26[1]:12-5).
“Risk factors of cutaneous melanoma, such as the number of melanocytic nevi and solar lentigines, have been found to be more frequent in subjects practicing endurance outdoor sports. An increased risk for cutaneous melanoma may be assumed for these athletes,” Dr. Adams commented.
Another study, this one published in 2006, found more atypical melanocytic nevi, solar lentigines, and lesions suggestive of nonmelanoma skin cancer in marathon runners, compared with a control group, and the risk was associated with the level of training intensity. The control subjects were more sensitive to the sun and had more common melanocytic nevi (Arch Dermatol. 2006 Nov;142[11]:1471-4).
Counseling about sunscreen may actually work
One strategy to reduce sun exposure is to advise athletes to avoid peak sun hours. However, “the key to caring for the athletes is not only recognizing that their sport may play a role in their disease but also realizing that your therapeutic approach must be tailored to minimize disruption to their practices and competitions,” Dr. Adams said.
However, there’s good news for dermatologists who are willing to push: The study also reported that athletes who were encouraged to use sunscreen were significantly more likely to use sunscreen (P less than .0001).
Watch for other conditions, from jogger’s nipple to ringworm
Dr. Adams offered advice about detection, treatment, and prevention of other skin disorders that affect athletes:
- “Jogger’s nipples” and other kinds of chafing. He has learned to recognize the “red eleven” – two vertical streaks of blood on a runner’s shirt – that represent a case of “jogger’s nipples” caused by chafing. Antibacterial ointment or petroleum jelly are useful treatments, he said, and an application of plenty of petroleum jelly on the nipples prior to a run can be helpful. Cotton shirts should be avoided, he said, in favor of synthetic, moisture-wicking shirts and bras. Chafing can also occur in the underarms and inner thighs, he said, and the same treatments and preventive techniques are useful.
- Callused and bleeding “jogger’s toes.” This can strike runners, especially on the second toe, which is often the longest and most likely to strike the toe box of a shoe. Specialty shoes can help prevent this condition, he said.
- Tinea corporis (ringworm) and herpes gladiatorum. In wrestlers, ringworm is known as tinea corporis gladiatorum because the intensity of skin-to-skin contact in wrestling makes the condition especially common in these athletes. Lesions don’t develop as rings at first; instead, they first appear as relatively nonspecific red round lesions and are most likely to be found in the head, neck, and upper extremities, Dr. Adams noted. Herpes gladiatorum is caused by herpes simplex virus 1; it is also seen in wrestlers and caused by skin-to-skin contact. Topical and oral antifungals clear ringworm, while oral antiviral agents are appropriate for herpes gladiatorum, Dr. Adams said. While herpes gladiatorum clears up and is no longer contagious after 4-5 days, he said, it’s not clear how long wrestlers with ringworm should be disqualified from playing.
Dr. Adams disclosed advising Mission, a company that focuses on sunscreen designed by and for athletes.
EXPERT ANALYSIS FROM AAD 18