User login
Boosting bedside skills in hands-on session
A low faculty-to-learner ratio helped HM18 attendees get the most from their learning experience in the Sunday pre-conference course “Bedside Procedures for the Hospitalist.”
The pre-course blended live didactic teaching and hands-on training with simulators so participants could not only learn but also review and demonstrate techniques for many common invasive procedures hospitalists encounter in practice.
“Our goal is to make the entire bedside procedures pre-course a unique experience,” course codirector Alyssa Burkhart, MD, of the Billings (Mont.) Clinic, said in an interview before the session.
“We carefully select the curriculum to create a program most relevant to the participants and their day-to-day work in patient care,” said Dr. Burkhart.
“The low faculty-to-learner ratio coupled with ample time to practice under expert guidance separates us from others. ... It’s a privilege to share our love of procedures with this year’s SHM participants,” said Dr. Burkhart, who comoderated the session with Joshua Lenchus, DO, SFHM, of the University of Miami.
An interactive focus on bedside procedures benefits novices and experienced clinicians, said Dr. Lenchus.
The simulation experience involved practice with ultrasound as well as anatomically representative training equipment.
“Our hope is that many hospitalists may once again find that spark of interest in performing more of their own procedures. The interactive sessions embedded within the pre-course are vital to the success of our program. Many other training sessions are didactics based. We strive to keep lecture time to a minimum so that small groups can learn from the expert facilitators,” Dr. Burkhart added.
“Ample hands-on practice time, interactive experience, and direct supervision separate our pre-course from other commercially available offerings,” Dr. Lenchus said.
The agenda kicked off with vascular and intraosseous access in the morning, followed by paracentesis, thoracentesis, lumbar puncture, and basic airway management, including the use of supraglottic devices.
Dr. Burkhart noted that the course included two separate practice sessions for vascular access because of the number of technical steps and potential complications. “Attendees typically wish to spend a considerable amount of time on vascular access,” she said. “The intraosseous access station and its exceptional trainers always receive very positive feedback.”
Dr. Burkhart and Dr. Lenchus had no financial conflicts to disclose.
A low faculty-to-learner ratio helped HM18 attendees get the most from their learning experience in the Sunday pre-conference course “Bedside Procedures for the Hospitalist.”
The pre-course blended live didactic teaching and hands-on training with simulators so participants could not only learn but also review and demonstrate techniques for many common invasive procedures hospitalists encounter in practice.
“Our goal is to make the entire bedside procedures pre-course a unique experience,” course codirector Alyssa Burkhart, MD, of the Billings (Mont.) Clinic, said in an interview before the session.
“We carefully select the curriculum to create a program most relevant to the participants and their day-to-day work in patient care,” said Dr. Burkhart.
“The low faculty-to-learner ratio coupled with ample time to practice under expert guidance separates us from others. ... It’s a privilege to share our love of procedures with this year’s SHM participants,” said Dr. Burkhart, who comoderated the session with Joshua Lenchus, DO, SFHM, of the University of Miami.
An interactive focus on bedside procedures benefits novices and experienced clinicians, said Dr. Lenchus.
The simulation experience involved practice with ultrasound as well as anatomically representative training equipment.
“Our hope is that many hospitalists may once again find that spark of interest in performing more of their own procedures. The interactive sessions embedded within the pre-course are vital to the success of our program. Many other training sessions are didactics based. We strive to keep lecture time to a minimum so that small groups can learn from the expert facilitators,” Dr. Burkhart added.
“Ample hands-on practice time, interactive experience, and direct supervision separate our pre-course from other commercially available offerings,” Dr. Lenchus said.
The agenda kicked off with vascular and intraosseous access in the morning, followed by paracentesis, thoracentesis, lumbar puncture, and basic airway management, including the use of supraglottic devices.
Dr. Burkhart noted that the course included two separate practice sessions for vascular access because of the number of technical steps and potential complications. “Attendees typically wish to spend a considerable amount of time on vascular access,” she said. “The intraosseous access station and its exceptional trainers always receive very positive feedback.”
Dr. Burkhart and Dr. Lenchus had no financial conflicts to disclose.
A low faculty-to-learner ratio helped HM18 attendees get the most from their learning experience in the Sunday pre-conference course “Bedside Procedures for the Hospitalist.”
The pre-course blended live didactic teaching and hands-on training with simulators so participants could not only learn but also review and demonstrate techniques for many common invasive procedures hospitalists encounter in practice.
“Our goal is to make the entire bedside procedures pre-course a unique experience,” course codirector Alyssa Burkhart, MD, of the Billings (Mont.) Clinic, said in an interview before the session.
“We carefully select the curriculum to create a program most relevant to the participants and their day-to-day work in patient care,” said Dr. Burkhart.
“The low faculty-to-learner ratio coupled with ample time to practice under expert guidance separates us from others. ... It’s a privilege to share our love of procedures with this year’s SHM participants,” said Dr. Burkhart, who comoderated the session with Joshua Lenchus, DO, SFHM, of the University of Miami.
An interactive focus on bedside procedures benefits novices and experienced clinicians, said Dr. Lenchus.
The simulation experience involved practice with ultrasound as well as anatomically representative training equipment.
“Our hope is that many hospitalists may once again find that spark of interest in performing more of their own procedures. The interactive sessions embedded within the pre-course are vital to the success of our program. Many other training sessions are didactics based. We strive to keep lecture time to a minimum so that small groups can learn from the expert facilitators,” Dr. Burkhart added.
“Ample hands-on practice time, interactive experience, and direct supervision separate our pre-course from other commercially available offerings,” Dr. Lenchus said.
The agenda kicked off with vascular and intraosseous access in the morning, followed by paracentesis, thoracentesis, lumbar puncture, and basic airway management, including the use of supraglottic devices.
Dr. Burkhart noted that the course included two separate practice sessions for vascular access because of the number of technical steps and potential complications. “Attendees typically wish to spend a considerable amount of time on vascular access,” she said. “The intraosseous access station and its exceptional trainers always receive very positive feedback.”
Dr. Burkhart and Dr. Lenchus had no financial conflicts to disclose.
Practical changes for improving practice management
An all-day HM18 pre-course – “Hospitalist Practice Management: How to Thrive in a Time of Intense Change” – for hospitalist leaders and practice administrators was all about practicality.
One of the goals of the session was to provide “quick, actionable interventions that attendees can implement right away, as well as alternatives for attendees to consider, which will require some work to employ,” said John Nelson, MD, MHM, a partner at Nelson Flores Hospital Medicine Consultants, La Quinta, Calif., the medical director of the Overlake Medical Center, Bellevue, Wash., and a course codirector and a faculty presenter.
Dr. Nelson pointed out that the hospitalist practice is a unique practice model. “We can’t effectively use the same approaches that other medical specialties use to ensure we have successful practices,” he said.
The pre-course, held Sunday before the official start of HM18, included more commentary and specifics than in past years about how to prosper in the rapidly changing health care landscape and how to reduce the chance of burnout.
Topics addressed included how to find, measure, and demonstrate value; how to incorporate different types of providers and clinical support staffing into a practice to support hospitalists; and how to recruit the right people and build a desirable culture.
The pre-course also covered effective roles for a variety of providers in a hospitalist group, including nurses, scribes, and coordinators, and delineated the benefits of providing telemedicine.
For group leaders and administrators in attendance, the session also shed light on how to interact with individual providers in their group and how to collaborate to build a healthy culture and practice, Ms. Flores said.
The day began with presentations that laid out valuable information and frameworks, including “A Tour of Survey Data: What It Does and Doesn’t Tell You” and “Defining and Measuring Value.” Sessions included six didactic lectures with a question-and-answer period, as well as what Dr. Nelson has dubbed “point/counterpoint” sessions in which faculty members debated particular issues, such as work scheduling models. During the last session, “Learning From Each Other,” participants shared with other attendees their own best practices in the areas covered.
Although there is no single best way to organize a hospitalist’s practice, the course provided lots of information and perspective to help listeners decide what is best for their practice.
“Even though we work in a stressful environment of constant change, hospitalists do have some control over their destiny, and there are things they can do to make hospitalist groups thrive in this challenging environment,” Ms. Flores concluded.
An all-day HM18 pre-course – “Hospitalist Practice Management: How to Thrive in a Time of Intense Change” – for hospitalist leaders and practice administrators was all about practicality.
One of the goals of the session was to provide “quick, actionable interventions that attendees can implement right away, as well as alternatives for attendees to consider, which will require some work to employ,” said John Nelson, MD, MHM, a partner at Nelson Flores Hospital Medicine Consultants, La Quinta, Calif., the medical director of the Overlake Medical Center, Bellevue, Wash., and a course codirector and a faculty presenter.
Dr. Nelson pointed out that the hospitalist practice is a unique practice model. “We can’t effectively use the same approaches that other medical specialties use to ensure we have successful practices,” he said.
The pre-course, held Sunday before the official start of HM18, included more commentary and specifics than in past years about how to prosper in the rapidly changing health care landscape and how to reduce the chance of burnout.
Topics addressed included how to find, measure, and demonstrate value; how to incorporate different types of providers and clinical support staffing into a practice to support hospitalists; and how to recruit the right people and build a desirable culture.
The pre-course also covered effective roles for a variety of providers in a hospitalist group, including nurses, scribes, and coordinators, and delineated the benefits of providing telemedicine.
For group leaders and administrators in attendance, the session also shed light on how to interact with individual providers in their group and how to collaborate to build a healthy culture and practice, Ms. Flores said.
The day began with presentations that laid out valuable information and frameworks, including “A Tour of Survey Data: What It Does and Doesn’t Tell You” and “Defining and Measuring Value.” Sessions included six didactic lectures with a question-and-answer period, as well as what Dr. Nelson has dubbed “point/counterpoint” sessions in which faculty members debated particular issues, such as work scheduling models. During the last session, “Learning From Each Other,” participants shared with other attendees their own best practices in the areas covered.
Although there is no single best way to organize a hospitalist’s practice, the course provided lots of information and perspective to help listeners decide what is best for their practice.
“Even though we work in a stressful environment of constant change, hospitalists do have some control over their destiny, and there are things they can do to make hospitalist groups thrive in this challenging environment,” Ms. Flores concluded.
An all-day HM18 pre-course – “Hospitalist Practice Management: How to Thrive in a Time of Intense Change” – for hospitalist leaders and practice administrators was all about practicality.
One of the goals of the session was to provide “quick, actionable interventions that attendees can implement right away, as well as alternatives for attendees to consider, which will require some work to employ,” said John Nelson, MD, MHM, a partner at Nelson Flores Hospital Medicine Consultants, La Quinta, Calif., the medical director of the Overlake Medical Center, Bellevue, Wash., and a course codirector and a faculty presenter.
Dr. Nelson pointed out that the hospitalist practice is a unique practice model. “We can’t effectively use the same approaches that other medical specialties use to ensure we have successful practices,” he said.
The pre-course, held Sunday before the official start of HM18, included more commentary and specifics than in past years about how to prosper in the rapidly changing health care landscape and how to reduce the chance of burnout.
Topics addressed included how to find, measure, and demonstrate value; how to incorporate different types of providers and clinical support staffing into a practice to support hospitalists; and how to recruit the right people and build a desirable culture.
The pre-course also covered effective roles for a variety of providers in a hospitalist group, including nurses, scribes, and coordinators, and delineated the benefits of providing telemedicine.
For group leaders and administrators in attendance, the session also shed light on how to interact with individual providers in their group and how to collaborate to build a healthy culture and practice, Ms. Flores said.
The day began with presentations that laid out valuable information and frameworks, including “A Tour of Survey Data: What It Does and Doesn’t Tell You” and “Defining and Measuring Value.” Sessions included six didactic lectures with a question-and-answer period, as well as what Dr. Nelson has dubbed “point/counterpoint” sessions in which faculty members debated particular issues, such as work scheduling models. During the last session, “Learning From Each Other,” participants shared with other attendees their own best practices in the areas covered.
Although there is no single best way to organize a hospitalist’s practice, the course provided lots of information and perspective to help listeners decide what is best for their practice.
“Even though we work in a stressful environment of constant change, hospitalists do have some control over their destiny, and there are things they can do to make hospitalist groups thrive in this challenging environment,” Ms. Flores concluded.
Gastrointestinal cancers: new standards of care from landmark trials
In this interview, Dr David Henry, the Editor-in-Chief of JCSO, and Dr Daniel Haller, of the Abramson Cancer Center, University of Pennsylvania Perelman School of Medicine in Philadelphia, discuss the findings from recent groundbreaking studies in gastrointestinal cancers.
Listen to the podcast below.
In this interview, Dr David Henry, the Editor-in-Chief of JCSO, and Dr Daniel Haller, of the Abramson Cancer Center, University of Pennsylvania Perelman School of Medicine in Philadelphia, discuss the findings from recent groundbreaking studies in gastrointestinal cancers.
Listen to the podcast below.
In this interview, Dr David Henry, the Editor-in-Chief of JCSO, and Dr Daniel Haller, of the Abramson Cancer Center, University of Pennsylvania Perelman School of Medicine in Philadelphia, discuss the findings from recent groundbreaking studies in gastrointestinal cancers.
Listen to the podcast below.
Journal of Hospital Medicine releases consensus statement on inpatient opioid prescribing
The Journal of Hospital Medicine, the official peer-reviewed journal of the Society of Hospital Medicine, has released a statement titled “Improving the Safety of Opioid Use for Acute Noncancer Pain in Hospitalized Adults: A Consensus Statement from the Society of Hospital Medicine” in response to the growing issue of managing opioid prescribing in the inpatient setting.
The statement offers 16 recommendations covering whether to use opioids in the hospital and how to improve the safety of opioid prescribing both during hospitalization and at discharge. The statement is available in the April 2018 issue of the journal.
SHM convened a working group to develop the consensus statement, intended for clinicians practicing in the inpatient setting. The development of the statement began with the working group conducting a systemic review of relevant guidelines and composing a draft based on extracted recommendations. The working group then obtained feedback from external experts in hospital-based opioid prescribing, SHM members, the SHM Patient-Family Advisory Council, other professional societies and peer reviewers.
The statement reads, “Despite a paucity of data on the comparative effectiveness of different management strategies for acute pain, several areas of expert consensus emerged across existing guidelines, which were felt to be relevant and applicable to the hospital setting. The objective of these recommendations is to provide information that can be used to inform and support opioid-related management decisions for acute pain by clinicians practicing medicine in the inpatient setting.”
“The journal is always pleased to be able to publish results of rigorous and innovative work, and the consensus document authored by Dr. Herzig and her team represents an outstanding example,” said Andrew Auerbach, MD, MPH, MHM, Professor of Medicine in Residence at the University of California, San Francisco, and editor in chief for the Journal of Hospital Medicine. “As we enter the ‘post-opioid’ era of pain management, papers like these will provide critical guidance for how to improve pain control among hospitalized patients; they are important first steps in the transition to new pain care strategies that are safer, more effective and patient-centered.”
Click here to access the consensus statement.
The Journal of Hospital Medicine, the official peer-reviewed journal of the Society of Hospital Medicine, has released a statement titled “Improving the Safety of Opioid Use for Acute Noncancer Pain in Hospitalized Adults: A Consensus Statement from the Society of Hospital Medicine” in response to the growing issue of managing opioid prescribing in the inpatient setting.
The statement offers 16 recommendations covering whether to use opioids in the hospital and how to improve the safety of opioid prescribing both during hospitalization and at discharge. The statement is available in the April 2018 issue of the journal.
SHM convened a working group to develop the consensus statement, intended for clinicians practicing in the inpatient setting. The development of the statement began with the working group conducting a systemic review of relevant guidelines and composing a draft based on extracted recommendations. The working group then obtained feedback from external experts in hospital-based opioid prescribing, SHM members, the SHM Patient-Family Advisory Council, other professional societies and peer reviewers.
The statement reads, “Despite a paucity of data on the comparative effectiveness of different management strategies for acute pain, several areas of expert consensus emerged across existing guidelines, which were felt to be relevant and applicable to the hospital setting. The objective of these recommendations is to provide information that can be used to inform and support opioid-related management decisions for acute pain by clinicians practicing medicine in the inpatient setting.”
“The journal is always pleased to be able to publish results of rigorous and innovative work, and the consensus document authored by Dr. Herzig and her team represents an outstanding example,” said Andrew Auerbach, MD, MPH, MHM, Professor of Medicine in Residence at the University of California, San Francisco, and editor in chief for the Journal of Hospital Medicine. “As we enter the ‘post-opioid’ era of pain management, papers like these will provide critical guidance for how to improve pain control among hospitalized patients; they are important first steps in the transition to new pain care strategies that are safer, more effective and patient-centered.”
Click here to access the consensus statement.
The Journal of Hospital Medicine, the official peer-reviewed journal of the Society of Hospital Medicine, has released a statement titled “Improving the Safety of Opioid Use for Acute Noncancer Pain in Hospitalized Adults: A Consensus Statement from the Society of Hospital Medicine” in response to the growing issue of managing opioid prescribing in the inpatient setting.
The statement offers 16 recommendations covering whether to use opioids in the hospital and how to improve the safety of opioid prescribing both during hospitalization and at discharge. The statement is available in the April 2018 issue of the journal.
SHM convened a working group to develop the consensus statement, intended for clinicians practicing in the inpatient setting. The development of the statement began with the working group conducting a systemic review of relevant guidelines and composing a draft based on extracted recommendations. The working group then obtained feedback from external experts in hospital-based opioid prescribing, SHM members, the SHM Patient-Family Advisory Council, other professional societies and peer reviewers.
The statement reads, “Despite a paucity of data on the comparative effectiveness of different management strategies for acute pain, several areas of expert consensus emerged across existing guidelines, which were felt to be relevant and applicable to the hospital setting. The objective of these recommendations is to provide information that can be used to inform and support opioid-related management decisions for acute pain by clinicians practicing medicine in the inpatient setting.”
“The journal is always pleased to be able to publish results of rigorous and innovative work, and the consensus document authored by Dr. Herzig and her team represents an outstanding example,” said Andrew Auerbach, MD, MPH, MHM, Professor of Medicine in Residence at the University of California, San Francisco, and editor in chief for the Journal of Hospital Medicine. “As we enter the ‘post-opioid’ era of pain management, papers like these will provide critical guidance for how to improve pain control among hospitalized patients; they are important first steps in the transition to new pain care strategies that are safer, more effective and patient-centered.”
Click here to access the consensus statement.
Study: Type 2 narcolepsy is significantly different from type 1
Patients with type 1 narcolepsy have more clinical impairments and distinct functional abnormalities than do patients with type 2 narcolepsy, according to investigators.
Patients with type 2 “do not present with such severe handicaps and are clinically closer to hypersomniac patients than the patients with type 1 narcolepsy,” reported Yu-Shu Huang, MD, of Chang Gung Memorial Hospital and Chang Gung University, Taoyuan City, Taiwan, and associates. The study was published in Neurology.
The researchers used brain scans, neuropsychological tests, and other screening tests to analyze three groups of subjects – 104 patients with Na-1, 29 with Na-2, and a control group of 26 subjects. Depending on the group, 62%-66% of the subjects were men, and the mean age ranged from 19 to 20.
The mean age of onset for the narcolepsy groups was 12-13. Those with Na-1 had higher mean body mass indexes – 27 kg/m2 vs. 24 (Na-2) vs. 20 (control), (P = .001).
The patients in both narcolepsy groups showed similar levels of sleepiness, but those with Na-2 had significantly fewer abnormal findings and disturbances.
Patients with Na-2 had significantly fewer sleep-onset REM periods, longer mean sleep latencies, and lower apnea-hypopnea indexes. The human leukocyte antigen DQ-Beta1*0602 was also found less frequently in Na-2 compared to Na-1 (52% vs. 97%, respectively, P less than .001).
PET findings also revealed less impairment in Na-2 compared to Na-1. The researchers noted increased metabolic rate in several brain areas in Na-1, although hypometabolism is more common in some areas in Na-2.
Based on their findings, the authors challenge a previous study of insurance data that suggests patients with both types have similarly poor outcomes over the long term. (PLoS One 2012;7:e33525.)
“In our study,” the authors wrote, “compared to patients with type 2 narcolepsy, patients with type 1 narcolepsy present with much more severe handicaps as early as adolescence. Further studies are needed to clarify the issue.”
The Taiwan Ministry of Science and Technology funded the study. The authors report no relevant disclosures.
SOURCE: Huang Y, et al. March 30, 2018, Neurology. 2018 Mar 30. doi: 10.1212/WNL.0000000000005346.
Patients with type 1 narcolepsy have more clinical impairments and distinct functional abnormalities than do patients with type 2 narcolepsy, according to investigators.
Patients with type 2 “do not present with such severe handicaps and are clinically closer to hypersomniac patients than the patients with type 1 narcolepsy,” reported Yu-Shu Huang, MD, of Chang Gung Memorial Hospital and Chang Gung University, Taoyuan City, Taiwan, and associates. The study was published in Neurology.
The researchers used brain scans, neuropsychological tests, and other screening tests to analyze three groups of subjects – 104 patients with Na-1, 29 with Na-2, and a control group of 26 subjects. Depending on the group, 62%-66% of the subjects were men, and the mean age ranged from 19 to 20.
The mean age of onset for the narcolepsy groups was 12-13. Those with Na-1 had higher mean body mass indexes – 27 kg/m2 vs. 24 (Na-2) vs. 20 (control), (P = .001).
The patients in both narcolepsy groups showed similar levels of sleepiness, but those with Na-2 had significantly fewer abnormal findings and disturbances.
Patients with Na-2 had significantly fewer sleep-onset REM periods, longer mean sleep latencies, and lower apnea-hypopnea indexes. The human leukocyte antigen DQ-Beta1*0602 was also found less frequently in Na-2 compared to Na-1 (52% vs. 97%, respectively, P less than .001).
PET findings also revealed less impairment in Na-2 compared to Na-1. The researchers noted increased metabolic rate in several brain areas in Na-1, although hypometabolism is more common in some areas in Na-2.
Based on their findings, the authors challenge a previous study of insurance data that suggests patients with both types have similarly poor outcomes over the long term. (PLoS One 2012;7:e33525.)
“In our study,” the authors wrote, “compared to patients with type 2 narcolepsy, patients with type 1 narcolepsy present with much more severe handicaps as early as adolescence. Further studies are needed to clarify the issue.”
The Taiwan Ministry of Science and Technology funded the study. The authors report no relevant disclosures.
SOURCE: Huang Y, et al. March 30, 2018, Neurology. 2018 Mar 30. doi: 10.1212/WNL.0000000000005346.
Patients with type 1 narcolepsy have more clinical impairments and distinct functional abnormalities than do patients with type 2 narcolepsy, according to investigators.
Patients with type 2 “do not present with such severe handicaps and are clinically closer to hypersomniac patients than the patients with type 1 narcolepsy,” reported Yu-Shu Huang, MD, of Chang Gung Memorial Hospital and Chang Gung University, Taoyuan City, Taiwan, and associates. The study was published in Neurology.
The researchers used brain scans, neuropsychological tests, and other screening tests to analyze three groups of subjects – 104 patients with Na-1, 29 with Na-2, and a control group of 26 subjects. Depending on the group, 62%-66% of the subjects were men, and the mean age ranged from 19 to 20.
The mean age of onset for the narcolepsy groups was 12-13. Those with Na-1 had higher mean body mass indexes – 27 kg/m2 vs. 24 (Na-2) vs. 20 (control), (P = .001).
The patients in both narcolepsy groups showed similar levels of sleepiness, but those with Na-2 had significantly fewer abnormal findings and disturbances.
Patients with Na-2 had significantly fewer sleep-onset REM periods, longer mean sleep latencies, and lower apnea-hypopnea indexes. The human leukocyte antigen DQ-Beta1*0602 was also found less frequently in Na-2 compared to Na-1 (52% vs. 97%, respectively, P less than .001).
PET findings also revealed less impairment in Na-2 compared to Na-1. The researchers noted increased metabolic rate in several brain areas in Na-1, although hypometabolism is more common in some areas in Na-2.
Based on their findings, the authors challenge a previous study of insurance data that suggests patients with both types have similarly poor outcomes over the long term. (PLoS One 2012;7:e33525.)
“In our study,” the authors wrote, “compared to patients with type 2 narcolepsy, patients with type 1 narcolepsy present with much more severe handicaps as early as adolescence. Further studies are needed to clarify the issue.”
The Taiwan Ministry of Science and Technology funded the study. The authors report no relevant disclosures.
SOURCE: Huang Y, et al. March 30, 2018, Neurology. 2018 Mar 30. doi: 10.1212/WNL.0000000000005346.
FROM NEUROLOGY
Four-gene signature predicted TB progression
A whole blood, four-gene polymerase chain reaction (PCR) signature predicted progression to tuberculosis disease up to 2 years after exposure, investigators reported.
The four-gene signature dubbed RISK4 performed similarly well in four diverse cohorts of HIV-negative household contacts of TB patients in sub-Saharan Africa, reported Sara Suliman, PhD, of the University of Cape Town, South Africa, and her associates. Testing for such a signature could be a cost-effective, point-of-care method to prioritize recipients of prophylactic treatment, the researchers said.
Worldwide, about 1.7 people are infected with M. tuberculosis, but only 5%-20% of these individuals develop TB. Finding a reliable biomarker for increased risk of progression would be “an important step forward towards better TB control,” especially in resource-strapped areas, the investigators said. Unfortunately, the predictive value of a positive tuberculin skin test or a positive interferon gamma release assay is too low to be useful for this purpose, they wrote in the American Journal of Respiratory and Critical Care Medicine.
Accordingly, the investigators searched for gene transcripts whose upregulation or downregulation reliably predicted progression to TB disease. To do so, they compared whole blood PCR test results from 79 cases (who developed TB after exposure to a household index case) and 328 controls (household contacts who did not progress to TB disease). Progressors developed TB disease within 3 to 24 months of exposure. Nonprogressors were matched by site, sex, age, and year of recruitment.
The RISK4 signature comprised four unique genes: GAS6 and SEPT4, which were upregulated in progressors compared with matched controls, and CD1C and BLK, which were downregulated, the researchers reported. For the overall data set, RISK4 predicted TB progression with an area under the curve (AUC) of 0.67 (95% confidence interval, 0.57 to 0.77; P = .0002). The AUC for individual sites ranged from 0.66 to 0.72 (P less than .03) and was 0.69 (P = .0004) among household contacts who were tested within 2 months of index case diagnosis. Furthermore, RISK4 performed comparably in an external cohort South African adolescents who tested positive on Interferon Gamma Release Assay (IGRA) or Tuberculin skin tests (AUC, 0.69; 95% CI, 0.62 to 0.76; P = .0003).
The groups in this study represented diverse genetic backgrounds, TB epidemiology, and circulating strains of M.tb, suggesting that RISK4 reliably predicts TB progression among household contacts across sub-Saharan Africa, the researchers said. Previously published TB signatures (which include DIAG3, DIAG4, and ACS COR) performed as well as RISK4 on the overall test cohort, but not at individual sites, they added.
In unblinded post hoc analyses, two of the four transcripts (SEPT4 and BLK) performed as well as the four-gene RISK4 signature, according to the investigators. Upregulation of the complement C1q C-chain (C1QC) with downregulation of T-cell receptor alpha variable gene 27 (TRAV27) predicted progression even more reliably, with AUCs exceeding 0.76 at all study sites. However, this transcript pair did not perform as well in the separate adolescent cohort (AUC = 0.57).
“Importantly, samples from household contact progressors were collected mostly at enrollment, immediately following exposure to the respective TB index cases, thus possibly representing a signature of recent M.tb exposure,” the researchers noted. “The next steps include assessment of the performance of RISK4 and the 2-transcript C1QC/TRAV27 signature in other settings, including non-African populations, and [determining] the feasibility of developing a near-patient test for targeted intervention.”
Funding sources included the Bill and Melinda Gates Foundation, the National Institutes of Health, the South African Medical Research Council, the Carnegie Corporation of New York, the South African National Research Foundation, and the Claude Leon Foundation. The researchers had no disclosures.
SOURCE: Am J Respir Crit Care Med. 2018 Apr 6. doi: 10.1164/rccm.201711-2340OC.
Eric Gartman, MD, FCCP, comments: Given the poor performance of our current latent TB testing to predict progression to active TB, this is a very welcome development. Refinement of these personalized approaches not only allows resource-limited areas to target their efforts, but holds the potential to minimize therapeutic harm in those not at high risk for developing active disease. It should be noted that this modality was tested in a particular area and in non-HIV infected people - and adapting its use to other populations may be inappropriate (especially the immunocompromised).
Eric Gartman, MD, FCCP, comments: Given the poor performance of our current latent TB testing to predict progression to active TB, this is a very welcome development. Refinement of these personalized approaches not only allows resource-limited areas to target their efforts, but holds the potential to minimize therapeutic harm in those not at high risk for developing active disease. It should be noted that this modality was tested in a particular area and in non-HIV infected people - and adapting its use to other populations may be inappropriate (especially the immunocompromised).
Eric Gartman, MD, FCCP, comments: Given the poor performance of our current latent TB testing to predict progression to active TB, this is a very welcome development. Refinement of these personalized approaches not only allows resource-limited areas to target their efforts, but holds the potential to minimize therapeutic harm in those not at high risk for developing active disease. It should be noted that this modality was tested in a particular area and in non-HIV infected people - and adapting its use to other populations may be inappropriate (especially the immunocompromised).
A whole blood, four-gene polymerase chain reaction (PCR) signature predicted progression to tuberculosis disease up to 2 years after exposure, investigators reported.
The four-gene signature dubbed RISK4 performed similarly well in four diverse cohorts of HIV-negative household contacts of TB patients in sub-Saharan Africa, reported Sara Suliman, PhD, of the University of Cape Town, South Africa, and her associates. Testing for such a signature could be a cost-effective, point-of-care method to prioritize recipients of prophylactic treatment, the researchers said.
Worldwide, about 1.7 people are infected with M. tuberculosis, but only 5%-20% of these individuals develop TB. Finding a reliable biomarker for increased risk of progression would be “an important step forward towards better TB control,” especially in resource-strapped areas, the investigators said. Unfortunately, the predictive value of a positive tuberculin skin test or a positive interferon gamma release assay is too low to be useful for this purpose, they wrote in the American Journal of Respiratory and Critical Care Medicine.
Accordingly, the investigators searched for gene transcripts whose upregulation or downregulation reliably predicted progression to TB disease. To do so, they compared whole blood PCR test results from 79 cases (who developed TB after exposure to a household index case) and 328 controls (household contacts who did not progress to TB disease). Progressors developed TB disease within 3 to 24 months of exposure. Nonprogressors were matched by site, sex, age, and year of recruitment.
The RISK4 signature comprised four unique genes: GAS6 and SEPT4, which were upregulated in progressors compared with matched controls, and CD1C and BLK, which were downregulated, the researchers reported. For the overall data set, RISK4 predicted TB progression with an area under the curve (AUC) of 0.67 (95% confidence interval, 0.57 to 0.77; P = .0002). The AUC for individual sites ranged from 0.66 to 0.72 (P less than .03) and was 0.69 (P = .0004) among household contacts who were tested within 2 months of index case diagnosis. Furthermore, RISK4 performed comparably in an external cohort South African adolescents who tested positive on Interferon Gamma Release Assay (IGRA) or Tuberculin skin tests (AUC, 0.69; 95% CI, 0.62 to 0.76; P = .0003).
The groups in this study represented diverse genetic backgrounds, TB epidemiology, and circulating strains of M.tb, suggesting that RISK4 reliably predicts TB progression among household contacts across sub-Saharan Africa, the researchers said. Previously published TB signatures (which include DIAG3, DIAG4, and ACS COR) performed as well as RISK4 on the overall test cohort, but not at individual sites, they added.
In unblinded post hoc analyses, two of the four transcripts (SEPT4 and BLK) performed as well as the four-gene RISK4 signature, according to the investigators. Upregulation of the complement C1q C-chain (C1QC) with downregulation of T-cell receptor alpha variable gene 27 (TRAV27) predicted progression even more reliably, with AUCs exceeding 0.76 at all study sites. However, this transcript pair did not perform as well in the separate adolescent cohort (AUC = 0.57).
“Importantly, samples from household contact progressors were collected mostly at enrollment, immediately following exposure to the respective TB index cases, thus possibly representing a signature of recent M.tb exposure,” the researchers noted. “The next steps include assessment of the performance of RISK4 and the 2-transcript C1QC/TRAV27 signature in other settings, including non-African populations, and [determining] the feasibility of developing a near-patient test for targeted intervention.”
Funding sources included the Bill and Melinda Gates Foundation, the National Institutes of Health, the South African Medical Research Council, the Carnegie Corporation of New York, the South African National Research Foundation, and the Claude Leon Foundation. The researchers had no disclosures.
SOURCE: Am J Respir Crit Care Med. 2018 Apr 6. doi: 10.1164/rccm.201711-2340OC.
A whole blood, four-gene polymerase chain reaction (PCR) signature predicted progression to tuberculosis disease up to 2 years after exposure, investigators reported.
The four-gene signature dubbed RISK4 performed similarly well in four diverse cohorts of HIV-negative household contacts of TB patients in sub-Saharan Africa, reported Sara Suliman, PhD, of the University of Cape Town, South Africa, and her associates. Testing for such a signature could be a cost-effective, point-of-care method to prioritize recipients of prophylactic treatment, the researchers said.
Worldwide, about 1.7 people are infected with M. tuberculosis, but only 5%-20% of these individuals develop TB. Finding a reliable biomarker for increased risk of progression would be “an important step forward towards better TB control,” especially in resource-strapped areas, the investigators said. Unfortunately, the predictive value of a positive tuberculin skin test or a positive interferon gamma release assay is too low to be useful for this purpose, they wrote in the American Journal of Respiratory and Critical Care Medicine.
Accordingly, the investigators searched for gene transcripts whose upregulation or downregulation reliably predicted progression to TB disease. To do so, they compared whole blood PCR test results from 79 cases (who developed TB after exposure to a household index case) and 328 controls (household contacts who did not progress to TB disease). Progressors developed TB disease within 3 to 24 months of exposure. Nonprogressors were matched by site, sex, age, and year of recruitment.
The RISK4 signature comprised four unique genes: GAS6 and SEPT4, which were upregulated in progressors compared with matched controls, and CD1C and BLK, which were downregulated, the researchers reported. For the overall data set, RISK4 predicted TB progression with an area under the curve (AUC) of 0.67 (95% confidence interval, 0.57 to 0.77; P = .0002). The AUC for individual sites ranged from 0.66 to 0.72 (P less than .03) and was 0.69 (P = .0004) among household contacts who were tested within 2 months of index case diagnosis. Furthermore, RISK4 performed comparably in an external cohort South African adolescents who tested positive on Interferon Gamma Release Assay (IGRA) or Tuberculin skin tests (AUC, 0.69; 95% CI, 0.62 to 0.76; P = .0003).
The groups in this study represented diverse genetic backgrounds, TB epidemiology, and circulating strains of M.tb, suggesting that RISK4 reliably predicts TB progression among household contacts across sub-Saharan Africa, the researchers said. Previously published TB signatures (which include DIAG3, DIAG4, and ACS COR) performed as well as RISK4 on the overall test cohort, but not at individual sites, they added.
In unblinded post hoc analyses, two of the four transcripts (SEPT4 and BLK) performed as well as the four-gene RISK4 signature, according to the investigators. Upregulation of the complement C1q C-chain (C1QC) with downregulation of T-cell receptor alpha variable gene 27 (TRAV27) predicted progression even more reliably, with AUCs exceeding 0.76 at all study sites. However, this transcript pair did not perform as well in the separate adolescent cohort (AUC = 0.57).
“Importantly, samples from household contact progressors were collected mostly at enrollment, immediately following exposure to the respective TB index cases, thus possibly representing a signature of recent M.tb exposure,” the researchers noted. “The next steps include assessment of the performance of RISK4 and the 2-transcript C1QC/TRAV27 signature in other settings, including non-African populations, and [determining] the feasibility of developing a near-patient test for targeted intervention.”
Funding sources included the Bill and Melinda Gates Foundation, the National Institutes of Health, the South African Medical Research Council, the Carnegie Corporation of New York, the South African National Research Foundation, and the Claude Leon Foundation. The researchers had no disclosures.
SOURCE: Am J Respir Crit Care Med. 2018 Apr 6. doi: 10.1164/rccm.201711-2340OC.
FROM AMERICAN JOURNAL OF RESPIRATORY AND CRITICAL CARE MEDICINE
Key clinical point: A novel four-gene signature predicted progression to tuberculosis disease among high-risk individuals.
Major finding: The RISK4 signature predicted TB progression with an area under the curve (AUC) of 0.67 (95% confidence interval, 0.57 to 0.77; P = .0002).
Study details: Comparison of 79 cases (household contacts who progressed to TB disease) with 328 controls (nonprogressors).
Disclosures: Funding sources included the Bill and Melinda Gates Foundation, the National Institutes of Health, the South African Medical Research Council, the Carnegie Corporation of New York, the South African National Research Foundation, and the Claude Leon Foundation. The researchers had no disclosures.
Source: Am J Respir Crit Care Med. 2018 Apr 6. doi: 10.1164/rccm.201711-2340OC.
Diabetes from checkpoint inhibitors probably means lifelong insulin
CHICAGO – , according to Priyanka Iyer, MD, an endocrinology fellow at MD Anderson Cancer Center, Houston.
“As long as we get glycemic control, they can continue,” she said at the annual meeting of the Endocrine Society.
Diabetes is a known side effect of immune checkpoint inhibitors (ICIs) but it’s rare, occurring in maybe 0.17% of patients, and its natural history and risk factors are unknown.
ICIs are fairly new agents, and as their use expands beyond clinical trials, “we anticipate seeing larger numbers of cases. Patients should be educated about the symptoms of uncontrolled blood sugars while on ICIs,” and endocrinologists “have to get involved and recognize this entity sooner,” Dr. Iyer said.
In short, her team found that ICI-mediated diabetes can occur in patients with or without preexisting diabetes, and that most patients have evidence of beta-cell failure, likely T-cell mediated destruction due to immune activation. In all but one case, patients remained on insulin at a median follow-up of 44 weeks, even after stopping ICIs. For most, ICI-mediated diabetes likely means lifelong insulin.
They were all on the programmed cell death protein (PD-1) inhibitors nivolumab (Opdivo) or pembrolizumab (Keytruda), or the PD-1 ligand (PD-L) inhibitor durvalumab (Imfinzi). The agents are used for a range of cancers, including renal cell, melanoma, and Hodgkin lymphoma. There were no diabetes cases in patients on single-agent ipilimumab (Yervoy) or tremelimumab, which target cytotoxic T-lymphocyte associated antigen-4 and are used for melanoma and mesothelioma.
Median time to diabetes presentation after the start of ICI treatment was 12.3 weeks but ranged from 1 to 67.2 weeks. Half of the cases presented in diabetic ketoacidosis (DKA). Patients had upward trending hyperglycemia and most had diabetes symptoms for a while before diagnosis. They presented with a blood glucose above 250 mg/dL, and more than half above 500 mg/dL. Median hemoglobin A1c at presentation was 8%, but ranged up to 12.5%.
Every patient required insulin, including the six that discontinued ICIs after developing diabetes. Diabetes resolved in just one patient at 10.2 months; she presented with DKA.
There were no obvious predisposing factors. None of the patients had histories of type 1 diabetes or other autoimmune disease. Five patients had well-controlled type 2 diabetes prior to ICI initiation; four had prediabetes. Some had family members with type 2 diabetes, but not type 1. Four had prior ICI exposure. Just three patients were on concomitant steroids.
A few patients also developed thyroid or pituitary dysfunction, which are more common side effects of ICIs.
The median age at diabetes presentation was 61 years and ranged from 32 to 82 years. The majority of patients were men, which reflects MD Anderson demographics, not a predisposing risk factor, Dr. Iyer said.
Melanoma was the most common cancer, followed by renal cell and prostate; patients had stage 2-4 disease. About half the subjects were on single agent anti-PD-1 treatment, about a third on anti-PD-1 combination treatment, and the rest on anti-PD-L1 combination therapy. C-peptide levels were below 0.9 ng/mL at diabetes diagnosis in most of the patients. Eleven of the 20 tested (55%) were positive for the pancreatic islet cell antibody GAD65.
The investigators had no disclosures. A funding source was not reported.
SOURCE: Iyer PC et al. Abstract OR05-5.
CHICAGO – , according to Priyanka Iyer, MD, an endocrinology fellow at MD Anderson Cancer Center, Houston.
“As long as we get glycemic control, they can continue,” she said at the annual meeting of the Endocrine Society.
Diabetes is a known side effect of immune checkpoint inhibitors (ICIs) but it’s rare, occurring in maybe 0.17% of patients, and its natural history and risk factors are unknown.
ICIs are fairly new agents, and as their use expands beyond clinical trials, “we anticipate seeing larger numbers of cases. Patients should be educated about the symptoms of uncontrolled blood sugars while on ICIs,” and endocrinologists “have to get involved and recognize this entity sooner,” Dr. Iyer said.
In short, her team found that ICI-mediated diabetes can occur in patients with or without preexisting diabetes, and that most patients have evidence of beta-cell failure, likely T-cell mediated destruction due to immune activation. In all but one case, patients remained on insulin at a median follow-up of 44 weeks, even after stopping ICIs. For most, ICI-mediated diabetes likely means lifelong insulin.
They were all on the programmed cell death protein (PD-1) inhibitors nivolumab (Opdivo) or pembrolizumab (Keytruda), or the PD-1 ligand (PD-L) inhibitor durvalumab (Imfinzi). The agents are used for a range of cancers, including renal cell, melanoma, and Hodgkin lymphoma. There were no diabetes cases in patients on single-agent ipilimumab (Yervoy) or tremelimumab, which target cytotoxic T-lymphocyte associated antigen-4 and are used for melanoma and mesothelioma.
Median time to diabetes presentation after the start of ICI treatment was 12.3 weeks but ranged from 1 to 67.2 weeks. Half of the cases presented in diabetic ketoacidosis (DKA). Patients had upward trending hyperglycemia and most had diabetes symptoms for a while before diagnosis. They presented with a blood glucose above 250 mg/dL, and more than half above 500 mg/dL. Median hemoglobin A1c at presentation was 8%, but ranged up to 12.5%.
Every patient required insulin, including the six that discontinued ICIs after developing diabetes. Diabetes resolved in just one patient at 10.2 months; she presented with DKA.
There were no obvious predisposing factors. None of the patients had histories of type 1 diabetes or other autoimmune disease. Five patients had well-controlled type 2 diabetes prior to ICI initiation; four had prediabetes. Some had family members with type 2 diabetes, but not type 1. Four had prior ICI exposure. Just three patients were on concomitant steroids.
A few patients also developed thyroid or pituitary dysfunction, which are more common side effects of ICIs.
The median age at diabetes presentation was 61 years and ranged from 32 to 82 years. The majority of patients were men, which reflects MD Anderson demographics, not a predisposing risk factor, Dr. Iyer said.
Melanoma was the most common cancer, followed by renal cell and prostate; patients had stage 2-4 disease. About half the subjects were on single agent anti-PD-1 treatment, about a third on anti-PD-1 combination treatment, and the rest on anti-PD-L1 combination therapy. C-peptide levels were below 0.9 ng/mL at diabetes diagnosis in most of the patients. Eleven of the 20 tested (55%) were positive for the pancreatic islet cell antibody GAD65.
The investigators had no disclosures. A funding source was not reported.
SOURCE: Iyer PC et al. Abstract OR05-5.
CHICAGO – , according to Priyanka Iyer, MD, an endocrinology fellow at MD Anderson Cancer Center, Houston.
“As long as we get glycemic control, they can continue,” she said at the annual meeting of the Endocrine Society.
Diabetes is a known side effect of immune checkpoint inhibitors (ICIs) but it’s rare, occurring in maybe 0.17% of patients, and its natural history and risk factors are unknown.
ICIs are fairly new agents, and as their use expands beyond clinical trials, “we anticipate seeing larger numbers of cases. Patients should be educated about the symptoms of uncontrolled blood sugars while on ICIs,” and endocrinologists “have to get involved and recognize this entity sooner,” Dr. Iyer said.
In short, her team found that ICI-mediated diabetes can occur in patients with or without preexisting diabetes, and that most patients have evidence of beta-cell failure, likely T-cell mediated destruction due to immune activation. In all but one case, patients remained on insulin at a median follow-up of 44 weeks, even after stopping ICIs. For most, ICI-mediated diabetes likely means lifelong insulin.
They were all on the programmed cell death protein (PD-1) inhibitors nivolumab (Opdivo) or pembrolizumab (Keytruda), or the PD-1 ligand (PD-L) inhibitor durvalumab (Imfinzi). The agents are used for a range of cancers, including renal cell, melanoma, and Hodgkin lymphoma. There were no diabetes cases in patients on single-agent ipilimumab (Yervoy) or tremelimumab, which target cytotoxic T-lymphocyte associated antigen-4 and are used for melanoma and mesothelioma.
Median time to diabetes presentation after the start of ICI treatment was 12.3 weeks but ranged from 1 to 67.2 weeks. Half of the cases presented in diabetic ketoacidosis (DKA). Patients had upward trending hyperglycemia and most had diabetes symptoms for a while before diagnosis. They presented with a blood glucose above 250 mg/dL, and more than half above 500 mg/dL. Median hemoglobin A1c at presentation was 8%, but ranged up to 12.5%.
Every patient required insulin, including the six that discontinued ICIs after developing diabetes. Diabetes resolved in just one patient at 10.2 months; she presented with DKA.
There were no obvious predisposing factors. None of the patients had histories of type 1 diabetes or other autoimmune disease. Five patients had well-controlled type 2 diabetes prior to ICI initiation; four had prediabetes. Some had family members with type 2 diabetes, but not type 1. Four had prior ICI exposure. Just three patients were on concomitant steroids.
A few patients also developed thyroid or pituitary dysfunction, which are more common side effects of ICIs.
The median age at diabetes presentation was 61 years and ranged from 32 to 82 years. The majority of patients were men, which reflects MD Anderson demographics, not a predisposing risk factor, Dr. Iyer said.
Melanoma was the most common cancer, followed by renal cell and prostate; patients had stage 2-4 disease. About half the subjects were on single agent anti-PD-1 treatment, about a third on anti-PD-1 combination treatment, and the rest on anti-PD-L1 combination therapy. C-peptide levels were below 0.9 ng/mL at diabetes diagnosis in most of the patients. Eleven of the 20 tested (55%) were positive for the pancreatic islet cell antibody GAD65.
The investigators had no disclosures. A funding source was not reported.
SOURCE: Iyer PC et al. Abstract OR05-5.
REPORTING FROM ENDO 2018
Key clinical point: Be on the lookout for new-onset diabetes when patients start immune checkpoint inhibitors.
Major finding: In all but one case, patients remained on insulin at a median follow-up of 44 weeks, even after stopping ICIs.
Study details: Review of 24 cases.
Disclosures: The investigators had no disclosures. A funding source was not reported.
Source: Iyer PC et al. Abstract OR05-5.
Analgesic management in radiation oncology for painful bone metastases
Bone metastases are a common cause of pain in patients with advanced cancer, with about three-quarters of patients with bone metastases experiencing pain as the dominant symptom.1 Inadequately treated cancer pain impairs patient quality of life, and is associated with higher rates of depression, anxiety, and fatigue. Palliative radiotherapy (RT) is effective in alleviating pain from bone metastases.4 Local field external beam radiotherapy can provide some pain relief at the site of treated metastasis in 80%-90% of cases, with complete pain relief in 50%-60% of cases.5,6 However, maximal pain relief from RT is delayed, in some cases taking days to up to multiple weeks to attain.7,8 Therefore, optimal management of bone metastases pain may require the use of analgesics until RT takes adequate effect.
National Comprehensive Cancer Network (NCCN) Guidelines for Adult Cancer Pain (v. 2.2015) recommend that pain intensity rating (PIR; range, 0-10, where 0 denotes no pain and 10, worst pain imaginable) be used to quantify pain for all symptomatic patients. These guidelines also recommend the pain medication regimen be assessed for all symptomatic patients. For patients with moderate or severe pain (PIR of ≥4), NCCN guidelines recommend that analgesic regimen be intervened upon by alteration of the analgesic regimen (initiating, rotating, or titrating analgesic) or consideration of referral to pain/symptom management specialty.
Previous findings have demonstrated inadequate analgesic management for cancer pain,2,9 including within the radiation oncology (RO) clinic, suggesting that patients seen in consultation for palliative RT may experience uncontrolled pain for days to weeks before the onset of relief from RT. Possible reasons for inadequate acute pain intervention in the RO clinic may be provider discomfort with analgesic management and infrequent formal integration of palliative care within RO.10
Limited single-institution data from the few institutions with dedicated palliative RO services have suggested that these services improve the quality of palliative care delivery, as demonstrated by providers perceptions’ of the clinical impact of a dedicated service11 and the implementation of expedited palliative RT delivery for acute cancer pain.12,13 To our knowledge, the impact of a dedicated palliative RO service on analgesic management for cancer pain has not been assessed.
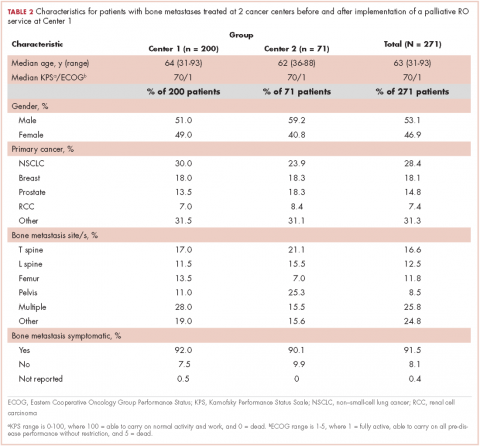
Here, we report how often patients with symptomatic bone metastases had assessments of existing analgesic regimens and interventions at RO consultation at 2 cancer centers. Center 1 had implemented a dedicated palliative RO service in 2011, consisting of rotating attending physicians and residents as well as dedicated palliative care trained nurse practitioners and a fellow, with the service structured around daily rounds,11 whereas Center 2 had not yet implemented a dedicated service. Using data from both centers, we assessed the impact of a palliative RO service on analgesic assessment and management in patients with bone metastases.
Methods
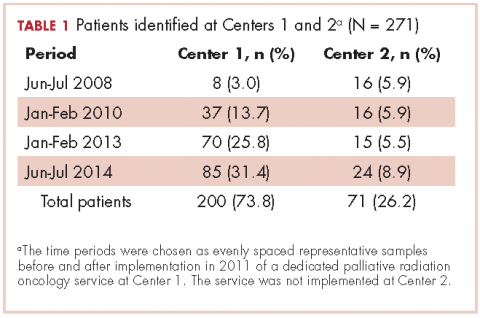
We searched our institutional databases for patients seen in RO consultation for bone metastases using ICD-9 code 198.5, and retrospectively reviewed consultation notes for those patients during June-July 2008, January-February 2010, January-February 2013, and June-July 2014. Those time periods were chosen as evenly spaced representative samples before and after implementation of a dedicated palliative RO service in 2011 at Center 1. Center 2 did not implement a dedicated palliative RO service in these time periods.
Within consultation notes, we recorded the following data from the History of the Present Illness section: symptoms from bone metastases (symptomatic was defined as any pain present); PIR (range, 0-10); and whether or not the preconsultation analgesic regimen was reported for symptomatic patients (including analgesic type, dosing, effectiveness, and adherence).
Documentation of the analgesic regimen in the history section of the notes was considered the proxy for analgesic regimen assessment at time of RO consultation. Analgesics within the Medications list, which were autopopulated in the consultation note by the electronic medical record, were recorded.
Whether or not pain was addressed with initiation or titration of analgesics for patients with a PIR of ≥4 was recorded from the Assessment and Plan portion of the notes, and that metric was considered the proxy for pain intervention. In addition, the case was coded as having had pain intervention if there was documentation of the patient declining recommended analgesic intervention, or the patient had been referred to a symptom management service for intervention (eg, referral to a specialty palliative care clinic), or there was recommendation for the patient to discuss uncontrolled pain with the original prescriber. A PIR of 4 was chosen as the threshold for analgesic intervention because at that level, NCCN guidelines for cancer pain state that the analgesic regimen should be titrated, whereas for a PIR of 3 or less, the guidelines recommend only consideration of titrating the analgesic. Only patients with a documented PIR were included in the pain intervention analysis.
Frequencies of analgesic assessment and analgesic intervention were compared using t tests (Wizard Pro, v1.8.5; Evan Miller, Chicago IL).
Results
A total of 271 patients with RO consultation notes were identified at the 2 centers within the 4 time periods (Table 1).
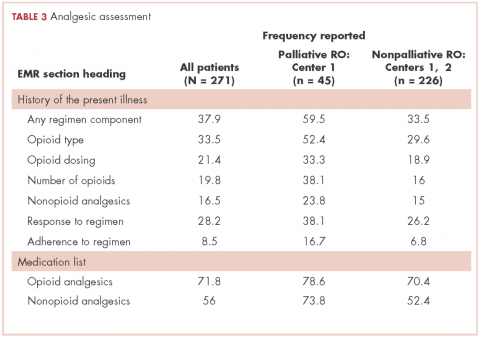
Among symptomatic patients, any component of the preconsultation analgesic regimen (including analgesic type, dosing, pain response, and adherence) was documented for 37.9% of the entire cohort at RO consultation (Table 3). At Centers 1 and 2, the frequencies of analgesic regimen assessment were documented for 41.3% and 28.1%, respectively (P = .06). Among symptomatic patients, 81.5% had an opioid or nonopioid analgesic listed in the Medications section in the electronic medical record at time of consultation.
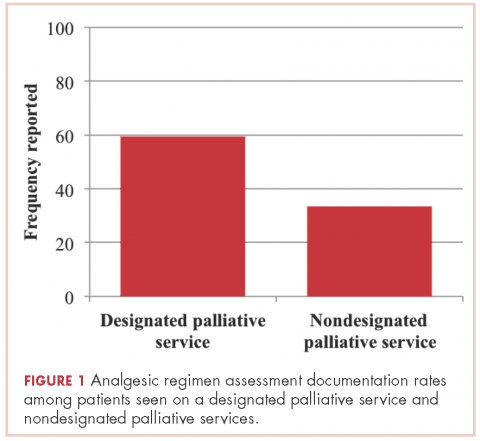
Patients seen on the dedicated palliative RO service at Center 1 had an analgesic assessment documentation rate of 59.5%, whereas the patients not seen on a palliative RO service (ie, patients seen on a nonpalliative RO service at Center 1 plus all patients at Center 2) had an assessment documentation rate of 33.5% (P = .002; Figure 1). There was no significant difference between rates of analgesic regimen assessment between patients seen at Center 2 and patients seen within nondedicated palliative RO services at Center 1 (28.1% vs 35.9%, respectively; P = .27).
In patients seen at Center 1 only, those seen on the palliative RO service had a higher documentation rate of analgesic assessment compared with those seen by other services after implementation of the dedicated service (59.5% vs 38%, respectively; P = .018). Time period (after versus before 2011) was not significantly associated with the rate of documentation of analgesic assessment at either Center 1 (after vs before 2011: 44.4% vs 31%, P = .23) or Center 2 (31.4% vs 24.1%, P = .60).
Among patients with a PIR of ≥4, analgesic intervention was reported for 17.2% of patients within the entire cohort (20.8% at Center 1 and 0% at Center 2, P = .05). Among those with a PIR of ≥4, documentation of analgesic assessment noted in the History of the Present Illness section was associated with increased documentation of an analgesic intervention in the Assessment and Plan section (25% vs 7.3%; odds ratio [OR], 4.22; 95% confidence interval [CI], 1.1-16.0; P = .03).
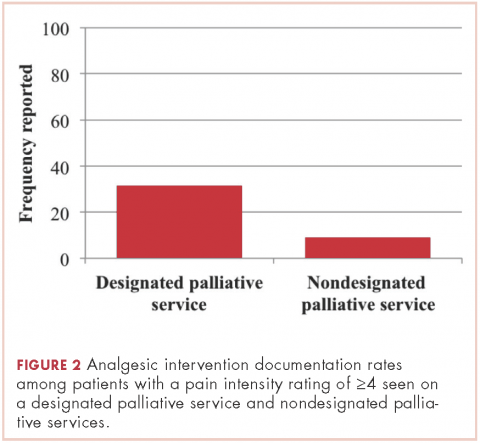
Patients seen on the dedicated palliative RO service at Center 1 had a documented analgesic intervention rate of 31.6%, whereas the patients not seen on a palliative RO service (ie, those seen on a nonpalliative RO service at Center 1 plus all patients at Center 2) had a documented analgesic intervention rate of 9.2% (P = .01; Figure 2). There was no statistically significant difference between rates of documentation of an analgesic regimen intervention between patients seen at Center 2 and patients seen within nondedicated palliative RO services at Center 1 (0% vs 17.2%, respectively; P = .07).
Looking at only patients seen at Center 1, patients with a PIR of ≥4 seen on the dedicated palliative RO service had a nearly significant higher rate of documented analgesic interventions in the time period after implementation of the dedicate service (31.6% if seen on the dedicated service vs 12% if seen on a nondedicated service, P = .06).
Discussion
Multiple studies demonstrate the undertreatment of cancer pain in the outpatient setting.2,9,14,15 At 2 cancer centers, we found that about half of patients who present for consideration of palliative RT for bone metastases had a PIR of ≥4, yet only 17% of them had documentation of analgesic intervention as recommended by NCCN guidelines for cancer pain. Underlying this low rate of appropriate intervention may be the assumption of rapid pain relief by RT. However, RT often does not begin at time of consultation,16 and maximal pain relief may take days to weeks after commencement of RT.17 It is estimated that a quarter of all patients with cancer develop bone metastases during the course of their disease,12 and most of those patients suffer from pain. Thus, inherent delay in pain relief before, during, and after RT results in significant morbidity for the cancer patient population if adequate analgesic management is not provided.
The low rate of appropriate analgesic intervention at the time of RO consultation may also be related to the low incidence of proper analgesic assessment. In our cohort, 80% of symptomatic patients had an opioid or nonopioid analgesic listed in their medications within the electronic medical record at time of consultation, but only 38% had the analgesic regimen and/or its effectiveness described in the History of the Present Illness section of the record. Inattentiveness to analgesic type, dosing, and effectiveness during consultation may result in any inadequacies of the analgesic regimen going unnoticed. Consistent with this notion, we found that the rate of appropriate intervention for patients with a PIR of ≥4 was higher among patients who had analgesic regimen reported in the consultation note. Thus, interventions to implement routine review and documentation of the analgesic regimen, for example within the electronic medical record, may be one way to improve pain management.
Another possible reason for low rates of acute pain management within the RO clinic is low provider confidence in regard to analgesic management. In a recent national survey, 96% of radiation oncologists stated they were at least moderately confident with assessment of pain, yet only 77% were at least moderately confident with titrating opioids, and just 56% were at least moderately confident with rotating opioids.10 Educational interventions that improve providers’ facility with analgesic management may increase the frequency of pain management in the RO clinic.
Patients seen on the dedicated palliative RO service had significantly higher rates of documented analgesic regimen assessment and appropriate intervention during RO consultation, compared with patients seen at Center 2 and those not seen on the dedicated palliative RO service at Center 1. The improvements we observed in analgesic assessment and intervention at Center 1 for patients seen on the palliative RO service are likely owing to involvement of palliative RO and not to secular trends, because there were not similar improvements for patients at Center 1 who were not seen by the palliative RO service and those at Center 2, where there was no service.
At Center 1, the dedicated palliative RO service was created to provide specialized care to patients with metastatic disease undergoing palliative radiation. Within its structure, topics within palliative RO, such as technical aspects of palliative RT, symptom management, and communication are taught and reinforced in a case-based approach. Such palliative care awareness, integration, and education within RO achieved by the palliative RO service likely contribute to the improved rates of analgesic management we found in our study. We do note that rate of analgesic intervention in the palliative RO cohort, though higher than in the nonpalliative RO group, was still low, with only a third of patients receiving proper analgesic management. These findings highlight the importance of continued effort in increasing providers’ awareness of the need to assess pain and raise comfort with analgesic initiation and titration and of having dedicated palliative care clinicians embedded within the RO setting.
Since the data for this study was acquired, Center 2 has implemented a short palliative RO didactic course for residents, which improved their comfort levels in assessing analgesic effectiveness and intervening for uncontrolled pain.18 The impact of this intervention on clinical care will need to be evaluated, but the improved provider comfort levels may translate into better-quality care.
Limitations
An important limitation of this retrospective study is the reliance on the documentation provided in the consultation note for determining frequencies of analgesic regimen assessment and intervention. The actual rates of analgesic management that occurred in clinic may have been higher than reported in the documentation. However, such discrepancy in documentation of analgesic management would also be an area for quality improvement. Inadequate documentation limits the ability for proper follow-up of cancer pain as recommended by a joint guidance statement from the American Society of Clinical Oncology and the American Academy of Hospice and Palliative Medicine.19,20 The results of our study may also partly reflect a positive impact in documentation of analgesic management by a dedicated palliative RO service.
Given the multi-institutional nature of this study, it may be that general practice differences confound the impact of the dedicated palliative RO service at Center 1. However, with excluding Center 2, the dedicated service was still strongly associated with a higher rate of analgesic assessment within Center 1 and was almost significantly associated with appropriate analgesic intervention within Center 1.
We used a PIR of ≥4 as a threshold for appropriate analgesic regimen intervention because it is what is recommended by the NCCN guidelines. However, close attention should be paid to the impact that any amount of pain has on an individual patient. The functional, spiritual, and existential impact of pain is unique to each patient’s experience, and optimal symptom management should take those elements into account.
Conclusion
In conclusion, this study indicates that advanced cancer patient pain assessment and intervention according to NCCN cancer pain management guidelines is not common in the RO setting, and it is an area that should be targeted for quality improvement because of the positive implications for patient well-being. Pain assessment and intervention were greater in the setting of a dedicated structure for palliative care within RO, suggesting that the integration of palliative care within RO is a promising means of improving quality of pain management.
This work was presented at the 2016 ASCO Palliative Care in Oncology Symposium (September 9-10, 2016), where this work received a Conquer Cancer Foundation Merit Award.
1. Amichetti M, Orrù P, Madeddu A, et al. Comparative evaluation of two hypofractionated radiotherapy regimens for painful bone metastases. Tumori. 2004;90(1):91-95.
2. Vuong S, Pulenzas N, DeAngelis C, et al. Inadequate pain management in cancer patients attending an outpatient palliative radiotherapy clinic. Support Care Cancer. 2016;24(2):887-892.
3. Portenoy RK, Payne D, Jacobsen P. Breakthrough pain: characteristics and impact in patients with cancer pain. Pain. 1999;81(1-2):129-134.
4. Sze WM, Shelley M, Held I, Mason M. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy - a systematic review of the randomised trials. Sze WM, ed. Cochrane Database Syst Rev. 2004;(2):CD004721-CD004721.
5. Ratanatharathorn V, Powers WE, Moss WT, Perez CA. Bone metastasis: review and critical analysis of random allocation trials of local field treatment. Int J Radiat Oncol Biol Phys. 1999;44(1):1-18.
6. Kirou-Mauro A, Hird A, Wong J, et al. Is response to radiotherapy in patients related to the severity of pretreatment pain? Int J Radiat Oncol Biol Phys. 2008;71(4):1208-1212.
7. Frassica DA. General principles of external beam radiation therapy for skeletal metastases. Clin Orthop Relat Res. 2003;(415 Suppl):S158-S164.
8. McDonald R, Ding K, Brundage M, et al. Effect of radiotherapy on painful bone metastases: a secondary analysis of the NCIC Clinical Trials Group Symptom Control Trial SC.23. JAMA Oncol. 2017 Jul 1;3(7):953-959.
9. Greco MT, Roberto A, Corli O, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. 2014;32(36):4149-4154.
10. Wei RL, Mattes MD, Yu J, et al. Attitudes of radiation oncologists toward palliative and supportive care in the united states: report on national membership survey by the American Society for Radiation Oncology (ASTRO). Pract Radiat Oncol. 2017;7(2):113-119.
11. Tseng YD, Krishnan MS, Jones JA, et al. Supportive and palliative radiation oncology service: impact of a dedicated service on palliative cancer care. Pract Radiat Oncol. 2014;4(4):247-253.
12. Fairchild A, Pituskin E, Rose B, et al. The rapid access palliative radiotherapy program: blueprint for initiation of a one-stop multidisciplinary bone metastases clinic. Support Care Cancer. 2009;17(2):163-170.
13. de Sa E, Sinclair E, Mitera G, et al. Continued success of the rapid response radiotherapy program: a review of 2004-2008. Support Care Cancer. 2009;17(7):757-762.
14. Deandrea S, Montanari M, Moja L, Apolone G. Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol. 2008;19(12):1985-1991.
15. Mitera G, Zeiadin N, Kirou-Mauro A, et al. Retrospective assessment of cancer pain management in an outpatient palliative radiotherapy clinic using the Pain Management Index. J Pain Symptom Manage. 2010;39(2):259-267.
16. Danjoux C, Chow E, Drossos A, et al. An innovative rapid response radiotherapy program to reduce waiting time for palliative radiotherapy. Support Care Cancer. 2006;14(1):38-43.
17. Feyer PC, Steingraeber M. Radiotherapy of bone metastasis in breast cancer patients – current approaches. Breast Care (Basel). 2012;7(2):108-112.
18. Garcia MA, Braunstein SE, Anderson WG. Palliative Care Didactic Course for Radiation Oncology Residents. Int J Radiat Oncol Biol Phys. 2017;97(5):884-885.
19. Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(1):96-112.
20. Bickel KE, McNiff K, Buss MK, et al. Defining high-quality palliative care in oncology practice: an American Society of Clinical Oncology/American Academy of Hospice and Palliative Medicine guidance statement. J Oncol Pract. 2016;12(9):e828-e838.
Bone metastases are a common cause of pain in patients with advanced cancer, with about three-quarters of patients with bone metastases experiencing pain as the dominant symptom.1 Inadequately treated cancer pain impairs patient quality of life, and is associated with higher rates of depression, anxiety, and fatigue. Palliative radiotherapy (RT) is effective in alleviating pain from bone metastases.4 Local field external beam radiotherapy can provide some pain relief at the site of treated metastasis in 80%-90% of cases, with complete pain relief in 50%-60% of cases.5,6 However, maximal pain relief from RT is delayed, in some cases taking days to up to multiple weeks to attain.7,8 Therefore, optimal management of bone metastases pain may require the use of analgesics until RT takes adequate effect.
National Comprehensive Cancer Network (NCCN) Guidelines for Adult Cancer Pain (v. 2.2015) recommend that pain intensity rating (PIR; range, 0-10, where 0 denotes no pain and 10, worst pain imaginable) be used to quantify pain for all symptomatic patients. These guidelines also recommend the pain medication regimen be assessed for all symptomatic patients. For patients with moderate or severe pain (PIR of ≥4), NCCN guidelines recommend that analgesic regimen be intervened upon by alteration of the analgesic regimen (initiating, rotating, or titrating analgesic) or consideration of referral to pain/symptom management specialty.
Previous findings have demonstrated inadequate analgesic management for cancer pain,2,9 including within the radiation oncology (RO) clinic, suggesting that patients seen in consultation for palliative RT may experience uncontrolled pain for days to weeks before the onset of relief from RT. Possible reasons for inadequate acute pain intervention in the RO clinic may be provider discomfort with analgesic management and infrequent formal integration of palliative care within RO.10
Limited single-institution data from the few institutions with dedicated palliative RO services have suggested that these services improve the quality of palliative care delivery, as demonstrated by providers perceptions’ of the clinical impact of a dedicated service11 and the implementation of expedited palliative RT delivery for acute cancer pain.12,13 To our knowledge, the impact of a dedicated palliative RO service on analgesic management for cancer pain has not been assessed.
Here, we report how often patients with symptomatic bone metastases had assessments of existing analgesic regimens and interventions at RO consultation at 2 cancer centers. Center 1 had implemented a dedicated palliative RO service in 2011, consisting of rotating attending physicians and residents as well as dedicated palliative care trained nurse practitioners and a fellow, with the service structured around daily rounds,11 whereas Center 2 had not yet implemented a dedicated service. Using data from both centers, we assessed the impact of a palliative RO service on analgesic assessment and management in patients with bone metastases.
Methods
We searched our institutional databases for patients seen in RO consultation for bone metastases using ICD-9 code 198.5, and retrospectively reviewed consultation notes for those patients during June-July 2008, January-February 2010, January-February 2013, and June-July 2014. Those time periods were chosen as evenly spaced representative samples before and after implementation of a dedicated palliative RO service in 2011 at Center 1. Center 2 did not implement a dedicated palliative RO service in these time periods.
Within consultation notes, we recorded the following data from the History of the Present Illness section: symptoms from bone metastases (symptomatic was defined as any pain present); PIR (range, 0-10); and whether or not the preconsultation analgesic regimen was reported for symptomatic patients (including analgesic type, dosing, effectiveness, and adherence).
Documentation of the analgesic regimen in the history section of the notes was considered the proxy for analgesic regimen assessment at time of RO consultation. Analgesics within the Medications list, which were autopopulated in the consultation note by the electronic medical record, were recorded.
Whether or not pain was addressed with initiation or titration of analgesics for patients with a PIR of ≥4 was recorded from the Assessment and Plan portion of the notes, and that metric was considered the proxy for pain intervention. In addition, the case was coded as having had pain intervention if there was documentation of the patient declining recommended analgesic intervention, or the patient had been referred to a symptom management service for intervention (eg, referral to a specialty palliative care clinic), or there was recommendation for the patient to discuss uncontrolled pain with the original prescriber. A PIR of 4 was chosen as the threshold for analgesic intervention because at that level, NCCN guidelines for cancer pain state that the analgesic regimen should be titrated, whereas for a PIR of 3 or less, the guidelines recommend only consideration of titrating the analgesic. Only patients with a documented PIR were included in the pain intervention analysis.
Frequencies of analgesic assessment and analgesic intervention were compared using t tests (Wizard Pro, v1.8.5; Evan Miller, Chicago IL).
Results
A total of 271 patients with RO consultation notes were identified at the 2 centers within the 4 time periods (Table 1).
Among symptomatic patients, any component of the preconsultation analgesic regimen (including analgesic type, dosing, pain response, and adherence) was documented for 37.9% of the entire cohort at RO consultation (Table 3). At Centers 1 and 2, the frequencies of analgesic regimen assessment were documented for 41.3% and 28.1%, respectively (P = .06). Among symptomatic patients, 81.5% had an opioid or nonopioid analgesic listed in the Medications section in the electronic medical record at time of consultation.
Patients seen on the dedicated palliative RO service at Center 1 had an analgesic assessment documentation rate of 59.5%, whereas the patients not seen on a palliative RO service (ie, patients seen on a nonpalliative RO service at Center 1 plus all patients at Center 2) had an assessment documentation rate of 33.5% (P = .002; Figure 1). There was no significant difference between rates of analgesic regimen assessment between patients seen at Center 2 and patients seen within nondedicated palliative RO services at Center 1 (28.1% vs 35.9%, respectively; P = .27).
In patients seen at Center 1 only, those seen on the palliative RO service had a higher documentation rate of analgesic assessment compared with those seen by other services after implementation of the dedicated service (59.5% vs 38%, respectively; P = .018). Time period (after versus before 2011) was not significantly associated with the rate of documentation of analgesic assessment at either Center 1 (after vs before 2011: 44.4% vs 31%, P = .23) or Center 2 (31.4% vs 24.1%, P = .60).
Among patients with a PIR of ≥4, analgesic intervention was reported for 17.2% of patients within the entire cohort (20.8% at Center 1 and 0% at Center 2, P = .05). Among those with a PIR of ≥4, documentation of analgesic assessment noted in the History of the Present Illness section was associated with increased documentation of an analgesic intervention in the Assessment and Plan section (25% vs 7.3%; odds ratio [OR], 4.22; 95% confidence interval [CI], 1.1-16.0; P = .03).
Patients seen on the dedicated palliative RO service at Center 1 had a documented analgesic intervention rate of 31.6%, whereas the patients not seen on a palliative RO service (ie, those seen on a nonpalliative RO service at Center 1 plus all patients at Center 2) had a documented analgesic intervention rate of 9.2% (P = .01; Figure 2). There was no statistically significant difference between rates of documentation of an analgesic regimen intervention between patients seen at Center 2 and patients seen within nondedicated palliative RO services at Center 1 (0% vs 17.2%, respectively; P = .07).
Looking at only patients seen at Center 1, patients with a PIR of ≥4 seen on the dedicated palliative RO service had a nearly significant higher rate of documented analgesic interventions in the time period after implementation of the dedicate service (31.6% if seen on the dedicated service vs 12% if seen on a nondedicated service, P = .06).
Discussion
Multiple studies demonstrate the undertreatment of cancer pain in the outpatient setting.2,9,14,15 At 2 cancer centers, we found that about half of patients who present for consideration of palliative RT for bone metastases had a PIR of ≥4, yet only 17% of them had documentation of analgesic intervention as recommended by NCCN guidelines for cancer pain. Underlying this low rate of appropriate intervention may be the assumption of rapid pain relief by RT. However, RT often does not begin at time of consultation,16 and maximal pain relief may take days to weeks after commencement of RT.17 It is estimated that a quarter of all patients with cancer develop bone metastases during the course of their disease,12 and most of those patients suffer from pain. Thus, inherent delay in pain relief before, during, and after RT results in significant morbidity for the cancer patient population if adequate analgesic management is not provided.
The low rate of appropriate analgesic intervention at the time of RO consultation may also be related to the low incidence of proper analgesic assessment. In our cohort, 80% of symptomatic patients had an opioid or nonopioid analgesic listed in their medications within the electronic medical record at time of consultation, but only 38% had the analgesic regimen and/or its effectiveness described in the History of the Present Illness section of the record. Inattentiveness to analgesic type, dosing, and effectiveness during consultation may result in any inadequacies of the analgesic regimen going unnoticed. Consistent with this notion, we found that the rate of appropriate intervention for patients with a PIR of ≥4 was higher among patients who had analgesic regimen reported in the consultation note. Thus, interventions to implement routine review and documentation of the analgesic regimen, for example within the electronic medical record, may be one way to improve pain management.
Another possible reason for low rates of acute pain management within the RO clinic is low provider confidence in regard to analgesic management. In a recent national survey, 96% of radiation oncologists stated they were at least moderately confident with assessment of pain, yet only 77% were at least moderately confident with titrating opioids, and just 56% were at least moderately confident with rotating opioids.10 Educational interventions that improve providers’ facility with analgesic management may increase the frequency of pain management in the RO clinic.
Patients seen on the dedicated palliative RO service had significantly higher rates of documented analgesic regimen assessment and appropriate intervention during RO consultation, compared with patients seen at Center 2 and those not seen on the dedicated palliative RO service at Center 1. The improvements we observed in analgesic assessment and intervention at Center 1 for patients seen on the palliative RO service are likely owing to involvement of palliative RO and not to secular trends, because there were not similar improvements for patients at Center 1 who were not seen by the palliative RO service and those at Center 2, where there was no service.
At Center 1, the dedicated palliative RO service was created to provide specialized care to patients with metastatic disease undergoing palliative radiation. Within its structure, topics within palliative RO, such as technical aspects of palliative RT, symptom management, and communication are taught and reinforced in a case-based approach. Such palliative care awareness, integration, and education within RO achieved by the palliative RO service likely contribute to the improved rates of analgesic management we found in our study. We do note that rate of analgesic intervention in the palliative RO cohort, though higher than in the nonpalliative RO group, was still low, with only a third of patients receiving proper analgesic management. These findings highlight the importance of continued effort in increasing providers’ awareness of the need to assess pain and raise comfort with analgesic initiation and titration and of having dedicated palliative care clinicians embedded within the RO setting.
Since the data for this study was acquired, Center 2 has implemented a short palliative RO didactic course for residents, which improved their comfort levels in assessing analgesic effectiveness and intervening for uncontrolled pain.18 The impact of this intervention on clinical care will need to be evaluated, but the improved provider comfort levels may translate into better-quality care.
Limitations
An important limitation of this retrospective study is the reliance on the documentation provided in the consultation note for determining frequencies of analgesic regimen assessment and intervention. The actual rates of analgesic management that occurred in clinic may have been higher than reported in the documentation. However, such discrepancy in documentation of analgesic management would also be an area for quality improvement. Inadequate documentation limits the ability for proper follow-up of cancer pain as recommended by a joint guidance statement from the American Society of Clinical Oncology and the American Academy of Hospice and Palliative Medicine.19,20 The results of our study may also partly reflect a positive impact in documentation of analgesic management by a dedicated palliative RO service.
Given the multi-institutional nature of this study, it may be that general practice differences confound the impact of the dedicated palliative RO service at Center 1. However, with excluding Center 2, the dedicated service was still strongly associated with a higher rate of analgesic assessment within Center 1 and was almost significantly associated with appropriate analgesic intervention within Center 1.
We used a PIR of ≥4 as a threshold for appropriate analgesic regimen intervention because it is what is recommended by the NCCN guidelines. However, close attention should be paid to the impact that any amount of pain has on an individual patient. The functional, spiritual, and existential impact of pain is unique to each patient’s experience, and optimal symptom management should take those elements into account.
Conclusion
In conclusion, this study indicates that advanced cancer patient pain assessment and intervention according to NCCN cancer pain management guidelines is not common in the RO setting, and it is an area that should be targeted for quality improvement because of the positive implications for patient well-being. Pain assessment and intervention were greater in the setting of a dedicated structure for palliative care within RO, suggesting that the integration of palliative care within RO is a promising means of improving quality of pain management.
This work was presented at the 2016 ASCO Palliative Care in Oncology Symposium (September 9-10, 2016), where this work received a Conquer Cancer Foundation Merit Award.
Bone metastases are a common cause of pain in patients with advanced cancer, with about three-quarters of patients with bone metastases experiencing pain as the dominant symptom.1 Inadequately treated cancer pain impairs patient quality of life, and is associated with higher rates of depression, anxiety, and fatigue. Palliative radiotherapy (RT) is effective in alleviating pain from bone metastases.4 Local field external beam radiotherapy can provide some pain relief at the site of treated metastasis in 80%-90% of cases, with complete pain relief in 50%-60% of cases.5,6 However, maximal pain relief from RT is delayed, in some cases taking days to up to multiple weeks to attain.7,8 Therefore, optimal management of bone metastases pain may require the use of analgesics until RT takes adequate effect.
National Comprehensive Cancer Network (NCCN) Guidelines for Adult Cancer Pain (v. 2.2015) recommend that pain intensity rating (PIR; range, 0-10, where 0 denotes no pain and 10, worst pain imaginable) be used to quantify pain for all symptomatic patients. These guidelines also recommend the pain medication regimen be assessed for all symptomatic patients. For patients with moderate or severe pain (PIR of ≥4), NCCN guidelines recommend that analgesic regimen be intervened upon by alteration of the analgesic regimen (initiating, rotating, or titrating analgesic) or consideration of referral to pain/symptom management specialty.
Previous findings have demonstrated inadequate analgesic management for cancer pain,2,9 including within the radiation oncology (RO) clinic, suggesting that patients seen in consultation for palliative RT may experience uncontrolled pain for days to weeks before the onset of relief from RT. Possible reasons for inadequate acute pain intervention in the RO clinic may be provider discomfort with analgesic management and infrequent formal integration of palliative care within RO.10
Limited single-institution data from the few institutions with dedicated palliative RO services have suggested that these services improve the quality of palliative care delivery, as demonstrated by providers perceptions’ of the clinical impact of a dedicated service11 and the implementation of expedited palliative RT delivery for acute cancer pain.12,13 To our knowledge, the impact of a dedicated palliative RO service on analgesic management for cancer pain has not been assessed.
Here, we report how often patients with symptomatic bone metastases had assessments of existing analgesic regimens and interventions at RO consultation at 2 cancer centers. Center 1 had implemented a dedicated palliative RO service in 2011, consisting of rotating attending physicians and residents as well as dedicated palliative care trained nurse practitioners and a fellow, with the service structured around daily rounds,11 whereas Center 2 had not yet implemented a dedicated service. Using data from both centers, we assessed the impact of a palliative RO service on analgesic assessment and management in patients with bone metastases.
Methods
We searched our institutional databases for patients seen in RO consultation for bone metastases using ICD-9 code 198.5, and retrospectively reviewed consultation notes for those patients during June-July 2008, January-February 2010, January-February 2013, and June-July 2014. Those time periods were chosen as evenly spaced representative samples before and after implementation of a dedicated palliative RO service in 2011 at Center 1. Center 2 did not implement a dedicated palliative RO service in these time periods.
Within consultation notes, we recorded the following data from the History of the Present Illness section: symptoms from bone metastases (symptomatic was defined as any pain present); PIR (range, 0-10); and whether or not the preconsultation analgesic regimen was reported for symptomatic patients (including analgesic type, dosing, effectiveness, and adherence).
Documentation of the analgesic regimen in the history section of the notes was considered the proxy for analgesic regimen assessment at time of RO consultation. Analgesics within the Medications list, which were autopopulated in the consultation note by the electronic medical record, were recorded.
Whether or not pain was addressed with initiation or titration of analgesics for patients with a PIR of ≥4 was recorded from the Assessment and Plan portion of the notes, and that metric was considered the proxy for pain intervention. In addition, the case was coded as having had pain intervention if there was documentation of the patient declining recommended analgesic intervention, or the patient had been referred to a symptom management service for intervention (eg, referral to a specialty palliative care clinic), or there was recommendation for the patient to discuss uncontrolled pain with the original prescriber. A PIR of 4 was chosen as the threshold for analgesic intervention because at that level, NCCN guidelines for cancer pain state that the analgesic regimen should be titrated, whereas for a PIR of 3 or less, the guidelines recommend only consideration of titrating the analgesic. Only patients with a documented PIR were included in the pain intervention analysis.
Frequencies of analgesic assessment and analgesic intervention were compared using t tests (Wizard Pro, v1.8.5; Evan Miller, Chicago IL).
Results
A total of 271 patients with RO consultation notes were identified at the 2 centers within the 4 time periods (Table 1).
Among symptomatic patients, any component of the preconsultation analgesic regimen (including analgesic type, dosing, pain response, and adherence) was documented for 37.9% of the entire cohort at RO consultation (Table 3). At Centers 1 and 2, the frequencies of analgesic regimen assessment were documented for 41.3% and 28.1%, respectively (P = .06). Among symptomatic patients, 81.5% had an opioid or nonopioid analgesic listed in the Medications section in the electronic medical record at time of consultation.
Patients seen on the dedicated palliative RO service at Center 1 had an analgesic assessment documentation rate of 59.5%, whereas the patients not seen on a palliative RO service (ie, patients seen on a nonpalliative RO service at Center 1 plus all patients at Center 2) had an assessment documentation rate of 33.5% (P = .002; Figure 1). There was no significant difference between rates of analgesic regimen assessment between patients seen at Center 2 and patients seen within nondedicated palliative RO services at Center 1 (28.1% vs 35.9%, respectively; P = .27).
In patients seen at Center 1 only, those seen on the palliative RO service had a higher documentation rate of analgesic assessment compared with those seen by other services after implementation of the dedicated service (59.5% vs 38%, respectively; P = .018). Time period (after versus before 2011) was not significantly associated with the rate of documentation of analgesic assessment at either Center 1 (after vs before 2011: 44.4% vs 31%, P = .23) or Center 2 (31.4% vs 24.1%, P = .60).
Among patients with a PIR of ≥4, analgesic intervention was reported for 17.2% of patients within the entire cohort (20.8% at Center 1 and 0% at Center 2, P = .05). Among those with a PIR of ≥4, documentation of analgesic assessment noted in the History of the Present Illness section was associated with increased documentation of an analgesic intervention in the Assessment and Plan section (25% vs 7.3%; odds ratio [OR], 4.22; 95% confidence interval [CI], 1.1-16.0; P = .03).
Patients seen on the dedicated palliative RO service at Center 1 had a documented analgesic intervention rate of 31.6%, whereas the patients not seen on a palliative RO service (ie, those seen on a nonpalliative RO service at Center 1 plus all patients at Center 2) had a documented analgesic intervention rate of 9.2% (P = .01; Figure 2). There was no statistically significant difference between rates of documentation of an analgesic regimen intervention between patients seen at Center 2 and patients seen within nondedicated palliative RO services at Center 1 (0% vs 17.2%, respectively; P = .07).
Looking at only patients seen at Center 1, patients with a PIR of ≥4 seen on the dedicated palliative RO service had a nearly significant higher rate of documented analgesic interventions in the time period after implementation of the dedicate service (31.6% if seen on the dedicated service vs 12% if seen on a nondedicated service, P = .06).
Discussion
Multiple studies demonstrate the undertreatment of cancer pain in the outpatient setting.2,9,14,15 At 2 cancer centers, we found that about half of patients who present for consideration of palliative RT for bone metastases had a PIR of ≥4, yet only 17% of them had documentation of analgesic intervention as recommended by NCCN guidelines for cancer pain. Underlying this low rate of appropriate intervention may be the assumption of rapid pain relief by RT. However, RT often does not begin at time of consultation,16 and maximal pain relief may take days to weeks after commencement of RT.17 It is estimated that a quarter of all patients with cancer develop bone metastases during the course of their disease,12 and most of those patients suffer from pain. Thus, inherent delay in pain relief before, during, and after RT results in significant morbidity for the cancer patient population if adequate analgesic management is not provided.
The low rate of appropriate analgesic intervention at the time of RO consultation may also be related to the low incidence of proper analgesic assessment. In our cohort, 80% of symptomatic patients had an opioid or nonopioid analgesic listed in their medications within the electronic medical record at time of consultation, but only 38% had the analgesic regimen and/or its effectiveness described in the History of the Present Illness section of the record. Inattentiveness to analgesic type, dosing, and effectiveness during consultation may result in any inadequacies of the analgesic regimen going unnoticed. Consistent with this notion, we found that the rate of appropriate intervention for patients with a PIR of ≥4 was higher among patients who had analgesic regimen reported in the consultation note. Thus, interventions to implement routine review and documentation of the analgesic regimen, for example within the electronic medical record, may be one way to improve pain management.
Another possible reason for low rates of acute pain management within the RO clinic is low provider confidence in regard to analgesic management. In a recent national survey, 96% of radiation oncologists stated they were at least moderately confident with assessment of pain, yet only 77% were at least moderately confident with titrating opioids, and just 56% were at least moderately confident with rotating opioids.10 Educational interventions that improve providers’ facility with analgesic management may increase the frequency of pain management in the RO clinic.
Patients seen on the dedicated palliative RO service had significantly higher rates of documented analgesic regimen assessment and appropriate intervention during RO consultation, compared with patients seen at Center 2 and those not seen on the dedicated palliative RO service at Center 1. The improvements we observed in analgesic assessment and intervention at Center 1 for patients seen on the palliative RO service are likely owing to involvement of palliative RO and not to secular trends, because there were not similar improvements for patients at Center 1 who were not seen by the palliative RO service and those at Center 2, where there was no service.
At Center 1, the dedicated palliative RO service was created to provide specialized care to patients with metastatic disease undergoing palliative radiation. Within its structure, topics within palliative RO, such as technical aspects of palliative RT, symptom management, and communication are taught and reinforced in a case-based approach. Such palliative care awareness, integration, and education within RO achieved by the palliative RO service likely contribute to the improved rates of analgesic management we found in our study. We do note that rate of analgesic intervention in the palliative RO cohort, though higher than in the nonpalliative RO group, was still low, with only a third of patients receiving proper analgesic management. These findings highlight the importance of continued effort in increasing providers’ awareness of the need to assess pain and raise comfort with analgesic initiation and titration and of having dedicated palliative care clinicians embedded within the RO setting.
Since the data for this study was acquired, Center 2 has implemented a short palliative RO didactic course for residents, which improved their comfort levels in assessing analgesic effectiveness and intervening for uncontrolled pain.18 The impact of this intervention on clinical care will need to be evaluated, but the improved provider comfort levels may translate into better-quality care.
Limitations
An important limitation of this retrospective study is the reliance on the documentation provided in the consultation note for determining frequencies of analgesic regimen assessment and intervention. The actual rates of analgesic management that occurred in clinic may have been higher than reported in the documentation. However, such discrepancy in documentation of analgesic management would also be an area for quality improvement. Inadequate documentation limits the ability for proper follow-up of cancer pain as recommended by a joint guidance statement from the American Society of Clinical Oncology and the American Academy of Hospice and Palliative Medicine.19,20 The results of our study may also partly reflect a positive impact in documentation of analgesic management by a dedicated palliative RO service.
Given the multi-institutional nature of this study, it may be that general practice differences confound the impact of the dedicated palliative RO service at Center 1. However, with excluding Center 2, the dedicated service was still strongly associated with a higher rate of analgesic assessment within Center 1 and was almost significantly associated with appropriate analgesic intervention within Center 1.
We used a PIR of ≥4 as a threshold for appropriate analgesic regimen intervention because it is what is recommended by the NCCN guidelines. However, close attention should be paid to the impact that any amount of pain has on an individual patient. The functional, spiritual, and existential impact of pain is unique to each patient’s experience, and optimal symptom management should take those elements into account.
Conclusion
In conclusion, this study indicates that advanced cancer patient pain assessment and intervention according to NCCN cancer pain management guidelines is not common in the RO setting, and it is an area that should be targeted for quality improvement because of the positive implications for patient well-being. Pain assessment and intervention were greater in the setting of a dedicated structure for palliative care within RO, suggesting that the integration of palliative care within RO is a promising means of improving quality of pain management.
This work was presented at the 2016 ASCO Palliative Care in Oncology Symposium (September 9-10, 2016), where this work received a Conquer Cancer Foundation Merit Award.
1. Amichetti M, Orrù P, Madeddu A, et al. Comparative evaluation of two hypofractionated radiotherapy regimens for painful bone metastases. Tumori. 2004;90(1):91-95.
2. Vuong S, Pulenzas N, DeAngelis C, et al. Inadequate pain management in cancer patients attending an outpatient palliative radiotherapy clinic. Support Care Cancer. 2016;24(2):887-892.
3. Portenoy RK, Payne D, Jacobsen P. Breakthrough pain: characteristics and impact in patients with cancer pain. Pain. 1999;81(1-2):129-134.
4. Sze WM, Shelley M, Held I, Mason M. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy - a systematic review of the randomised trials. Sze WM, ed. Cochrane Database Syst Rev. 2004;(2):CD004721-CD004721.
5. Ratanatharathorn V, Powers WE, Moss WT, Perez CA. Bone metastasis: review and critical analysis of random allocation trials of local field treatment. Int J Radiat Oncol Biol Phys. 1999;44(1):1-18.
6. Kirou-Mauro A, Hird A, Wong J, et al. Is response to radiotherapy in patients related to the severity of pretreatment pain? Int J Radiat Oncol Biol Phys. 2008;71(4):1208-1212.
7. Frassica DA. General principles of external beam radiation therapy for skeletal metastases. Clin Orthop Relat Res. 2003;(415 Suppl):S158-S164.
8. McDonald R, Ding K, Brundage M, et al. Effect of radiotherapy on painful bone metastases: a secondary analysis of the NCIC Clinical Trials Group Symptom Control Trial SC.23. JAMA Oncol. 2017 Jul 1;3(7):953-959.
9. Greco MT, Roberto A, Corli O, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. 2014;32(36):4149-4154.
10. Wei RL, Mattes MD, Yu J, et al. Attitudes of radiation oncologists toward palliative and supportive care in the united states: report on national membership survey by the American Society for Radiation Oncology (ASTRO). Pract Radiat Oncol. 2017;7(2):113-119.
11. Tseng YD, Krishnan MS, Jones JA, et al. Supportive and palliative radiation oncology service: impact of a dedicated service on palliative cancer care. Pract Radiat Oncol. 2014;4(4):247-253.
12. Fairchild A, Pituskin E, Rose B, et al. The rapid access palliative radiotherapy program: blueprint for initiation of a one-stop multidisciplinary bone metastases clinic. Support Care Cancer. 2009;17(2):163-170.
13. de Sa E, Sinclair E, Mitera G, et al. Continued success of the rapid response radiotherapy program: a review of 2004-2008. Support Care Cancer. 2009;17(7):757-762.
14. Deandrea S, Montanari M, Moja L, Apolone G. Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol. 2008;19(12):1985-1991.
15. Mitera G, Zeiadin N, Kirou-Mauro A, et al. Retrospective assessment of cancer pain management in an outpatient palliative radiotherapy clinic using the Pain Management Index. J Pain Symptom Manage. 2010;39(2):259-267.
16. Danjoux C, Chow E, Drossos A, et al. An innovative rapid response radiotherapy program to reduce waiting time for palliative radiotherapy. Support Care Cancer. 2006;14(1):38-43.
17. Feyer PC, Steingraeber M. Radiotherapy of bone metastasis in breast cancer patients – current approaches. Breast Care (Basel). 2012;7(2):108-112.
18. Garcia MA, Braunstein SE, Anderson WG. Palliative Care Didactic Course for Radiation Oncology Residents. Int J Radiat Oncol Biol Phys. 2017;97(5):884-885.
19. Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(1):96-112.
20. Bickel KE, McNiff K, Buss MK, et al. Defining high-quality palliative care in oncology practice: an American Society of Clinical Oncology/American Academy of Hospice and Palliative Medicine guidance statement. J Oncol Pract. 2016;12(9):e828-e838.
1. Amichetti M, Orrù P, Madeddu A, et al. Comparative evaluation of two hypofractionated radiotherapy regimens for painful bone metastases. Tumori. 2004;90(1):91-95.
2. Vuong S, Pulenzas N, DeAngelis C, et al. Inadequate pain management in cancer patients attending an outpatient palliative radiotherapy clinic. Support Care Cancer. 2016;24(2):887-892.
3. Portenoy RK, Payne D, Jacobsen P. Breakthrough pain: characteristics and impact in patients with cancer pain. Pain. 1999;81(1-2):129-134.
4. Sze WM, Shelley M, Held I, Mason M. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy - a systematic review of the randomised trials. Sze WM, ed. Cochrane Database Syst Rev. 2004;(2):CD004721-CD004721.
5. Ratanatharathorn V, Powers WE, Moss WT, Perez CA. Bone metastasis: review and critical analysis of random allocation trials of local field treatment. Int J Radiat Oncol Biol Phys. 1999;44(1):1-18.
6. Kirou-Mauro A, Hird A, Wong J, et al. Is response to radiotherapy in patients related to the severity of pretreatment pain? Int J Radiat Oncol Biol Phys. 2008;71(4):1208-1212.
7. Frassica DA. General principles of external beam radiation therapy for skeletal metastases. Clin Orthop Relat Res. 2003;(415 Suppl):S158-S164.
8. McDonald R, Ding K, Brundage M, et al. Effect of radiotherapy on painful bone metastases: a secondary analysis of the NCIC Clinical Trials Group Symptom Control Trial SC.23. JAMA Oncol. 2017 Jul 1;3(7):953-959.
9. Greco MT, Roberto A, Corli O, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. 2014;32(36):4149-4154.
10. Wei RL, Mattes MD, Yu J, et al. Attitudes of radiation oncologists toward palliative and supportive care in the united states: report on national membership survey by the American Society for Radiation Oncology (ASTRO). Pract Radiat Oncol. 2017;7(2):113-119.
11. Tseng YD, Krishnan MS, Jones JA, et al. Supportive and palliative radiation oncology service: impact of a dedicated service on palliative cancer care. Pract Radiat Oncol. 2014;4(4):247-253.
12. Fairchild A, Pituskin E, Rose B, et al. The rapid access palliative radiotherapy program: blueprint for initiation of a one-stop multidisciplinary bone metastases clinic. Support Care Cancer. 2009;17(2):163-170.
13. de Sa E, Sinclair E, Mitera G, et al. Continued success of the rapid response radiotherapy program: a review of 2004-2008. Support Care Cancer. 2009;17(7):757-762.
14. Deandrea S, Montanari M, Moja L, Apolone G. Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol. 2008;19(12):1985-1991.
15. Mitera G, Zeiadin N, Kirou-Mauro A, et al. Retrospective assessment of cancer pain management in an outpatient palliative radiotherapy clinic using the Pain Management Index. J Pain Symptom Manage. 2010;39(2):259-267.
16. Danjoux C, Chow E, Drossos A, et al. An innovative rapid response radiotherapy program to reduce waiting time for palliative radiotherapy. Support Care Cancer. 2006;14(1):38-43.
17. Feyer PC, Steingraeber M. Radiotherapy of bone metastasis in breast cancer patients – current approaches. Breast Care (Basel). 2012;7(2):108-112.
18. Garcia MA, Braunstein SE, Anderson WG. Palliative Care Didactic Course for Radiation Oncology Residents. Int J Radiat Oncol Biol Phys. 2017;97(5):884-885.
19. Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(1):96-112.
20. Bickel KE, McNiff K, Buss MK, et al. Defining high-quality palliative care in oncology practice: an American Society of Clinical Oncology/American Academy of Hospice and Palliative Medicine guidance statement. J Oncol Pract. 2016;12(9):e828-e838.
Why iron can worsen malaria infection
Researchers believe they may have discovered why iron can sometimes worsen malaria infection.
By studying mice and samples from malaria patients, the researchers found that extra iron interferes with ferroportin, a protein that prevents a toxic buildup of iron in red blood cells and helps protect these cells against malaria infection.
The team also found a mutant form of ferroportin that occurs in African populations appears to protect against malaria.
These findings, published in Science, may help researchers and healthcare officials develop strategies to prevent and treat malaria.
“Our study helps solve a long-standing mystery,” said study author Tracey Rouault, MD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development in Bethesda, Maryland.
“Iron supplements can sometimes worsen malaria infection and, conversely, iron deficiency can be protective in some cases. Our findings reveal that ferroportin—its function, as well as its regulation by iron levels—helps to explain these observations.”
The team found that red blood cells use ferroportin to remove excess iron, which malaria parasites consume as a food source.
In studies of mice, the researchers found the absence of ferroportin in erythroid cells caused iron to accumulate to toxic levels inside red blood cells. This, in turn, stressed the cells and shortened their life span.
In addition, the team found that mice lacking ferroportin had more parasites and worse outcomes when infected with malaria, compared to malaria-infected mice with intact ferroportin.
When they fed mice a high-iron diet, the researchers found that hepcidin regulated ferroportin in erythroid cells. The hormone, which is more abundant in high-iron environments, lowered ferroportin levels on erythroblasts and, subsequently, in red blood cells.
Additionally, hepcidin physically bound to ferroportin, preventing iron removal from the cells.
Next, the researchers sought to determine whether the ferroportin mutation Q248H, which is found in African populations, protects against malaria. This mutation shields ferroportin from hepcidin’s effects.
The team analyzed patient samples from 2 existing malaria studies. In one study, which enrolled children hospitalized for malaria in Zambia, 19.7% of the 66 patients had the Q248H mutation.
Children with the mutation tended to have fewer malarial parasites in their blood and tolerated their fevers for a longer period before coming to the hospital. While the trends were not statistically significant, they raise the possibility that Q248H reduces the iron available in the blood, therefore reducing the malaria parasite’s food source.
In the other study, which enrolled 290 pregnant women in Ghana, 8.6% had the Q248H mutation. Women with the mutation were significantly less likely to have pregnancy-associated malaria, in which parasites accumulate in the placenta and can cause adverse pregnancy and birth outcomes.
“Our findings suggest that Q248H does protect against malaria, possibly explaining why it occurs in people who live in malaria-endemic regions,” said study author De-Liang Zhang, PhD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
“Given the importance of iron metabolism overall, we will continue studying the ferroportin mutation and explore its other potential health effects.”
Researchers believe they may have discovered why iron can sometimes worsen malaria infection.
By studying mice and samples from malaria patients, the researchers found that extra iron interferes with ferroportin, a protein that prevents a toxic buildup of iron in red blood cells and helps protect these cells against malaria infection.
The team also found a mutant form of ferroportin that occurs in African populations appears to protect against malaria.
These findings, published in Science, may help researchers and healthcare officials develop strategies to prevent and treat malaria.
“Our study helps solve a long-standing mystery,” said study author Tracey Rouault, MD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development in Bethesda, Maryland.
“Iron supplements can sometimes worsen malaria infection and, conversely, iron deficiency can be protective in some cases. Our findings reveal that ferroportin—its function, as well as its regulation by iron levels—helps to explain these observations.”
The team found that red blood cells use ferroportin to remove excess iron, which malaria parasites consume as a food source.
In studies of mice, the researchers found the absence of ferroportin in erythroid cells caused iron to accumulate to toxic levels inside red blood cells. This, in turn, stressed the cells and shortened their life span.
In addition, the team found that mice lacking ferroportin had more parasites and worse outcomes when infected with malaria, compared to malaria-infected mice with intact ferroportin.
When they fed mice a high-iron diet, the researchers found that hepcidin regulated ferroportin in erythroid cells. The hormone, which is more abundant in high-iron environments, lowered ferroportin levels on erythroblasts and, subsequently, in red blood cells.
Additionally, hepcidin physically bound to ferroportin, preventing iron removal from the cells.
Next, the researchers sought to determine whether the ferroportin mutation Q248H, which is found in African populations, protects against malaria. This mutation shields ferroportin from hepcidin’s effects.
The team analyzed patient samples from 2 existing malaria studies. In one study, which enrolled children hospitalized for malaria in Zambia, 19.7% of the 66 patients had the Q248H mutation.
Children with the mutation tended to have fewer malarial parasites in their blood and tolerated their fevers for a longer period before coming to the hospital. While the trends were not statistically significant, they raise the possibility that Q248H reduces the iron available in the blood, therefore reducing the malaria parasite’s food source.
In the other study, which enrolled 290 pregnant women in Ghana, 8.6% had the Q248H mutation. Women with the mutation were significantly less likely to have pregnancy-associated malaria, in which parasites accumulate in the placenta and can cause adverse pregnancy and birth outcomes.
“Our findings suggest that Q248H does protect against malaria, possibly explaining why it occurs in people who live in malaria-endemic regions,” said study author De-Liang Zhang, PhD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
“Given the importance of iron metabolism overall, we will continue studying the ferroportin mutation and explore its other potential health effects.”
Researchers believe they may have discovered why iron can sometimes worsen malaria infection.
By studying mice and samples from malaria patients, the researchers found that extra iron interferes with ferroportin, a protein that prevents a toxic buildup of iron in red blood cells and helps protect these cells against malaria infection.
The team also found a mutant form of ferroportin that occurs in African populations appears to protect against malaria.
These findings, published in Science, may help researchers and healthcare officials develop strategies to prevent and treat malaria.
“Our study helps solve a long-standing mystery,” said study author Tracey Rouault, MD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development in Bethesda, Maryland.
“Iron supplements can sometimes worsen malaria infection and, conversely, iron deficiency can be protective in some cases. Our findings reveal that ferroportin—its function, as well as its regulation by iron levels—helps to explain these observations.”
The team found that red blood cells use ferroportin to remove excess iron, which malaria parasites consume as a food source.
In studies of mice, the researchers found the absence of ferroportin in erythroid cells caused iron to accumulate to toxic levels inside red blood cells. This, in turn, stressed the cells and shortened their life span.
In addition, the team found that mice lacking ferroportin had more parasites and worse outcomes when infected with malaria, compared to malaria-infected mice with intact ferroportin.
When they fed mice a high-iron diet, the researchers found that hepcidin regulated ferroportin in erythroid cells. The hormone, which is more abundant in high-iron environments, lowered ferroportin levels on erythroblasts and, subsequently, in red blood cells.
Additionally, hepcidin physically bound to ferroportin, preventing iron removal from the cells.
Next, the researchers sought to determine whether the ferroportin mutation Q248H, which is found in African populations, protects against malaria. This mutation shields ferroportin from hepcidin’s effects.
The team analyzed patient samples from 2 existing malaria studies. In one study, which enrolled children hospitalized for malaria in Zambia, 19.7% of the 66 patients had the Q248H mutation.
Children with the mutation tended to have fewer malarial parasites in their blood and tolerated their fevers for a longer period before coming to the hospital. While the trends were not statistically significant, they raise the possibility that Q248H reduces the iron available in the blood, therefore reducing the malaria parasite’s food source.
In the other study, which enrolled 290 pregnant women in Ghana, 8.6% had the Q248H mutation. Women with the mutation were significantly less likely to have pregnancy-associated malaria, in which parasites accumulate in the placenta and can cause adverse pregnancy and birth outcomes.
“Our findings suggest that Q248H does protect against malaria, possibly explaining why it occurs in people who live in malaria-endemic regions,” said study author De-Liang Zhang, PhD, of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
“Given the importance of iron metabolism overall, we will continue studying the ferroportin mutation and explore its other potential health effects.”
Ponatinib bests older TKIs against Ph+ALL
Every generation aspires to be better than its predecessors, and for the third-generation tyrosine kinase inhibitor ponatinib (Iclusig), that might just be true, investigators claim.
A retrospective analysis comparing clinical trial outcomes for patients with newly diagnosed acute lymphoblastic leukemia positive for the Philadelphia chromosome (Ph+ALL) suggests that first-line ponatinib offers modestly better complete molecular response (CMR) rates and 3-year overall survival (OS) than either first-generation TKIs such as imatinib (Gleevec) or second-generation agents such as dasatinib (Sprycel) and nilotinib (Tasigna).
“Although only 1 relevant study of ponatinib combined with chemotherapy in Ph+ALL has been reported and our ability to adjust for baseline patient characteristics was limited, the results suggest that ponatinib combined with chemotherapy might represent a more effective front-line treatment option than chemotherapy combined with an earlier generation TKI for patients with newly diagnosed Ph+ALL, including those who cannot or choose not to undergo [stem cell transplant],” wrote Elias Jabbour, MD, of the University of Texas MD Anderson Cancer Center in Houston, and his colleagues.
They based their conclusions on a meta-regression analysis of 25 studies looking at first- or second-generation TKIs and one study of ponatinib as frontline therapy for patients with Ph+ALL. They described their results in Clinical Lymphoma, Myeloma & Leukemia.
The investigators created pooled estimates of outcomes from studies of earlier-generation TKIs plus combination chemotherapy using a random-effects meta-analysis method. For the sole ponatinib study – a single-arm trial of combination chemotherapy plus ponatinib – they used a binomial distribution method to calculate 95% confidence intervals (CI). The method essentially estimates the probability of success or failure of a repeated experiment.
They found that 79% of patients in the ponatinib trial achieved a CMR, compared with 34% of patients treated with earlier generation TKIs plus chemotherapy. This translates into an odds ratio (OR) for CMR with ponatinib of 6.09 (P = .034).
Two-year OS rates were 83% with ponatinib versus 58% for all patients treated with other TKIs. Although the OR (3.70) seemed to run in favor of ponatinib, the difference was not statistically significant (P = .062).
For 3-year OS, however, ponatinib was superior to the pooled data for the other TKIs, at 79% versus 50%, translating into an OR of 4.49 (P = .050).
The authors acknowledged that the study was limited by differences in treatment regimens and centers, and a limited ability to adjust for patient characteristics, due to the study’s reliance on only those covariates published across different studies. Head-to-head comparison trials are needed to confirm their results, they noted.
Nonetheless, “[w]e believe that the improved efficacy of ponatinib combined with chemotherapy for newly diagnosed Ph+ALL might prevent the need for allogeneic SCT,” they wrote.
Ariad Pharmaceuticals funded the study. Dr. Jabbour and three coauthors reported research funding from the company; other study authors reported employment or other financial relationships with Ariad or its parent company Takeda.
SOURCE: Jabbour E et al. Clin Lymphoma Myeloma Leuk. 2018 18(4):257-65.
Every generation aspires to be better than its predecessors, and for the third-generation tyrosine kinase inhibitor ponatinib (Iclusig), that might just be true, investigators claim.
A retrospective analysis comparing clinical trial outcomes for patients with newly diagnosed acute lymphoblastic leukemia positive for the Philadelphia chromosome (Ph+ALL) suggests that first-line ponatinib offers modestly better complete molecular response (CMR) rates and 3-year overall survival (OS) than either first-generation TKIs such as imatinib (Gleevec) or second-generation agents such as dasatinib (Sprycel) and nilotinib (Tasigna).
“Although only 1 relevant study of ponatinib combined with chemotherapy in Ph+ALL has been reported and our ability to adjust for baseline patient characteristics was limited, the results suggest that ponatinib combined with chemotherapy might represent a more effective front-line treatment option than chemotherapy combined with an earlier generation TKI for patients with newly diagnosed Ph+ALL, including those who cannot or choose not to undergo [stem cell transplant],” wrote Elias Jabbour, MD, of the University of Texas MD Anderson Cancer Center in Houston, and his colleagues.
They based their conclusions on a meta-regression analysis of 25 studies looking at first- or second-generation TKIs and one study of ponatinib as frontline therapy for patients with Ph+ALL. They described their results in Clinical Lymphoma, Myeloma & Leukemia.
The investigators created pooled estimates of outcomes from studies of earlier-generation TKIs plus combination chemotherapy using a random-effects meta-analysis method. For the sole ponatinib study – a single-arm trial of combination chemotherapy plus ponatinib – they used a binomial distribution method to calculate 95% confidence intervals (CI). The method essentially estimates the probability of success or failure of a repeated experiment.
They found that 79% of patients in the ponatinib trial achieved a CMR, compared with 34% of patients treated with earlier generation TKIs plus chemotherapy. This translates into an odds ratio (OR) for CMR with ponatinib of 6.09 (P = .034).
Two-year OS rates were 83% with ponatinib versus 58% for all patients treated with other TKIs. Although the OR (3.70) seemed to run in favor of ponatinib, the difference was not statistically significant (P = .062).
For 3-year OS, however, ponatinib was superior to the pooled data for the other TKIs, at 79% versus 50%, translating into an OR of 4.49 (P = .050).
The authors acknowledged that the study was limited by differences in treatment regimens and centers, and a limited ability to adjust for patient characteristics, due to the study’s reliance on only those covariates published across different studies. Head-to-head comparison trials are needed to confirm their results, they noted.
Nonetheless, “[w]e believe that the improved efficacy of ponatinib combined with chemotherapy for newly diagnosed Ph+ALL might prevent the need for allogeneic SCT,” they wrote.
Ariad Pharmaceuticals funded the study. Dr. Jabbour and three coauthors reported research funding from the company; other study authors reported employment or other financial relationships with Ariad or its parent company Takeda.
SOURCE: Jabbour E et al. Clin Lymphoma Myeloma Leuk. 2018 18(4):257-65.
Every generation aspires to be better than its predecessors, and for the third-generation tyrosine kinase inhibitor ponatinib (Iclusig), that might just be true, investigators claim.
A retrospective analysis comparing clinical trial outcomes for patients with newly diagnosed acute lymphoblastic leukemia positive for the Philadelphia chromosome (Ph+ALL) suggests that first-line ponatinib offers modestly better complete molecular response (CMR) rates and 3-year overall survival (OS) than either first-generation TKIs such as imatinib (Gleevec) or second-generation agents such as dasatinib (Sprycel) and nilotinib (Tasigna).
“Although only 1 relevant study of ponatinib combined with chemotherapy in Ph+ALL has been reported and our ability to adjust for baseline patient characteristics was limited, the results suggest that ponatinib combined with chemotherapy might represent a more effective front-line treatment option than chemotherapy combined with an earlier generation TKI for patients with newly diagnosed Ph+ALL, including those who cannot or choose not to undergo [stem cell transplant],” wrote Elias Jabbour, MD, of the University of Texas MD Anderson Cancer Center in Houston, and his colleagues.
They based their conclusions on a meta-regression analysis of 25 studies looking at first- or second-generation TKIs and one study of ponatinib as frontline therapy for patients with Ph+ALL. They described their results in Clinical Lymphoma, Myeloma & Leukemia.
The investigators created pooled estimates of outcomes from studies of earlier-generation TKIs plus combination chemotherapy using a random-effects meta-analysis method. For the sole ponatinib study – a single-arm trial of combination chemotherapy plus ponatinib – they used a binomial distribution method to calculate 95% confidence intervals (CI). The method essentially estimates the probability of success or failure of a repeated experiment.
They found that 79% of patients in the ponatinib trial achieved a CMR, compared with 34% of patients treated with earlier generation TKIs plus chemotherapy. This translates into an odds ratio (OR) for CMR with ponatinib of 6.09 (P = .034).
Two-year OS rates were 83% with ponatinib versus 58% for all patients treated with other TKIs. Although the OR (3.70) seemed to run in favor of ponatinib, the difference was not statistically significant (P = .062).
For 3-year OS, however, ponatinib was superior to the pooled data for the other TKIs, at 79% versus 50%, translating into an OR of 4.49 (P = .050).
The authors acknowledged that the study was limited by differences in treatment regimens and centers, and a limited ability to adjust for patient characteristics, due to the study’s reliance on only those covariates published across different studies. Head-to-head comparison trials are needed to confirm their results, they noted.
Nonetheless, “[w]e believe that the improved efficacy of ponatinib combined with chemotherapy for newly diagnosed Ph+ALL might prevent the need for allogeneic SCT,” they wrote.
Ariad Pharmaceuticals funded the study. Dr. Jabbour and three coauthors reported research funding from the company; other study authors reported employment or other financial relationships with Ariad or its parent company Takeda.
SOURCE: Jabbour E et al. Clin Lymphoma Myeloma Leuk. 2018 18(4):257-65.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Key clinical point:
Major finding: Complete molecular response rates and 3-year overall survival were better with ponatinib in combination with chemotherapy.
Study details: Meta-regression analysis of 26 studies of TKIs in combination with chemotherapy as first-line therapy for patients with Ph+ALL.
Disclosures: Ariad Pharmaceuticals funded the study. Dr. Jabbour and three coauthors reported research funding from the company; other study authors reported employment or other financial relationships with Ariad or its parent company, Takeda.
Source: Jabbour E et al. Clin Lymphoma Myeloma Leuk. 2018 18(4):257-65.