User login
Zika vaccine candidate receives fast track designation
The US Food and Drug Administration (FDA) has granted fast track designation to TAK-426, a candidate vaccine for Zika virus.
TAK-426 is a purified, inactivated, alum-adjuvanted, whole Zika virus vaccine candidate being developed by Takeda Pharmaceutical Company Limited with the aid of federal funding.
TAK-426 is currently being studied in a phase 1 trial, ZIK-101 (NCT03343626).
This randomized, placebo-controlled, double-blind trial was designed to evaluate the safety and immunogenicity of TAK-426 in 240 male and female subjects between the ages of 18 and 49.
The trial is taking place in the continental US and US territories. It is being conducted under a US Investigational New Drug application.
Takeda says that, if initial data from ZIK-101 are supportive, the company will work to progress into phase 2 development as soon as possible.
Takeda’s Zika program is supported by federal funds from the Biomedical Advanced Research and Development Authority (BARDA) within the Office of the Assistant Secretary for Preparedness and Response in the US Department of Health and Human Services (contract No. HHSO100201600015C).
“We recognize the public health threat posed by the Zika virus,” said Laurence De Moerlooze, PhD, lead of the global Zika program at Takeda.
“As soon as Takeda received funding from BARDA, we mobilized a team and prioritized development of this vaccine candidate, initiating a phase 1 trial within 15 months of contract signature. With fast track designation, the ongoing support of BARDA, and the abilities of our organization, we are confident that we will continue to make expedient progress.”
About fast track designation
The FDA’s fast track designation is a process designed to facilitate the development and expedite the review of drugs and vaccines with the potential to treat serious or life-threatening conditions and address unmet medical needs.
The fast track process allows for more frequent interactions with the FDA, rolling reviews of a product application, and eligibility for priority review if relevant criteria are met. The FDA’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months. ![]()
The US Food and Drug Administration (FDA) has granted fast track designation to TAK-426, a candidate vaccine for Zika virus.
TAK-426 is a purified, inactivated, alum-adjuvanted, whole Zika virus vaccine candidate being developed by Takeda Pharmaceutical Company Limited with the aid of federal funding.
TAK-426 is currently being studied in a phase 1 trial, ZIK-101 (NCT03343626).
This randomized, placebo-controlled, double-blind trial was designed to evaluate the safety and immunogenicity of TAK-426 in 240 male and female subjects between the ages of 18 and 49.
The trial is taking place in the continental US and US territories. It is being conducted under a US Investigational New Drug application.
Takeda says that, if initial data from ZIK-101 are supportive, the company will work to progress into phase 2 development as soon as possible.
Takeda’s Zika program is supported by federal funds from the Biomedical Advanced Research and Development Authority (BARDA) within the Office of the Assistant Secretary for Preparedness and Response in the US Department of Health and Human Services (contract No. HHSO100201600015C).
“We recognize the public health threat posed by the Zika virus,” said Laurence De Moerlooze, PhD, lead of the global Zika program at Takeda.
“As soon as Takeda received funding from BARDA, we mobilized a team and prioritized development of this vaccine candidate, initiating a phase 1 trial within 15 months of contract signature. With fast track designation, the ongoing support of BARDA, and the abilities of our organization, we are confident that we will continue to make expedient progress.”
About fast track designation
The FDA’s fast track designation is a process designed to facilitate the development and expedite the review of drugs and vaccines with the potential to treat serious or life-threatening conditions and address unmet medical needs.
The fast track process allows for more frequent interactions with the FDA, rolling reviews of a product application, and eligibility for priority review if relevant criteria are met. The FDA’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months. ![]()
The US Food and Drug Administration (FDA) has granted fast track designation to TAK-426, a candidate vaccine for Zika virus.
TAK-426 is a purified, inactivated, alum-adjuvanted, whole Zika virus vaccine candidate being developed by Takeda Pharmaceutical Company Limited with the aid of federal funding.
TAK-426 is currently being studied in a phase 1 trial, ZIK-101 (NCT03343626).
This randomized, placebo-controlled, double-blind trial was designed to evaluate the safety and immunogenicity of TAK-426 in 240 male and female subjects between the ages of 18 and 49.
The trial is taking place in the continental US and US territories. It is being conducted under a US Investigational New Drug application.
Takeda says that, if initial data from ZIK-101 are supportive, the company will work to progress into phase 2 development as soon as possible.
Takeda’s Zika program is supported by federal funds from the Biomedical Advanced Research and Development Authority (BARDA) within the Office of the Assistant Secretary for Preparedness and Response in the US Department of Health and Human Services (contract No. HHSO100201600015C).
“We recognize the public health threat posed by the Zika virus,” said Laurence De Moerlooze, PhD, lead of the global Zika program at Takeda.
“As soon as Takeda received funding from BARDA, we mobilized a team and prioritized development of this vaccine candidate, initiating a phase 1 trial within 15 months of contract signature. With fast track designation, the ongoing support of BARDA, and the abilities of our organization, we are confident that we will continue to make expedient progress.”
About fast track designation
The FDA’s fast track designation is a process designed to facilitate the development and expedite the review of drugs and vaccines with the potential to treat serious or life-threatening conditions and address unmet medical needs.
The fast track process allows for more frequent interactions with the FDA, rolling reviews of a product application, and eligibility for priority review if relevant criteria are met. The FDA’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months. ![]()
MDedge Daily News: The flu vaccine flop’s Cold War connection
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
APOE4 may determine memory loss in Alzheimer’s, a recent flu vaccine flop may have a Cold War connection, treating women’s urinary incontinence could have a restful side effect, and why the 3 months after a heart attack are particularly perilous.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
APOE4 may determine memory loss in Alzheimer’s, a recent flu vaccine flop may have a Cold War connection, treating women’s urinary incontinence could have a restful side effect, and why the 3 months after a heart attack are particularly perilous.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
APOE4 may determine memory loss in Alzheimer’s, a recent flu vaccine flop may have a Cold War connection, treating women’s urinary incontinence could have a restful side effect, and why the 3 months after a heart attack are particularly perilous.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Letrozole promising as maintenance treatment for high-grade serous ovarian cancer
Maintenance therapy with an aromatase inhibitor may improve progression-free survival in women with estrogen receptor (ER)–positive, high-grade serous ovarian cancers, results of a non-randomized, single-center study suggest.
Patients who received compared with patients who declined the treatment, investigators reported in Gynecologic Oncology.
The benefit of letrozole maintenance treatment was also apparent in women with residual disease who received bevacizumab maintenance treatment, wrote first author Viola Heinzelmann-Schwarz, MD, of the Gynecological Cancer Center, University of Basel, Switzerland, and her coauthors.
The results “strongly suggest that endocrine maintenance therapy has more advantage than disadvantage for use in ovarian cancer patients and seems clearly justified,” when considered alongside other data regarding the potential benefit of letrozole in other treatment settings, Dr. Heinzelmann-Schwarz and her colleagues said.
“In contrast to available expensive maintenance medications like antiangiogenic [drugs] and PARP inhibitors, antihormonal drugs have a favorable safety profile, low cost, easy intake regimen with one tablet daily and an established prognostic target,” they added.
While the role of aromatase inhibitor maintenance therapy in high-grade serous ovarian cancer is unclear, previous studies have shown that patients with low-grade serous ovarian cancer may benefit from endocrine therapy.
Those studies in low-grade serous ovarian cancer include several small case series, and one retrospective analysis showing improved progression-free survival among women treated with endocrine therapy versus women who received observation only (J Clin Oncol. 2017 Apr. doi: 10.1200/JCO.2016.71.0632).
The more recent report by Dr. Heinzelmann-Schwarz and her colleagues included 50 women with ER-positive, FIGO (Fédération Internationale de Gynécologie et d’Obstétrique) stage III/IV high-grade serous ovarian cancer who were offered off-label letrozole treatment after debulking surgery and platinum-based chemotherapy. Twenty-three received letrozole maintenance treatment, and 27 declined.
In the group of women who received letrozole maintenance treatment, 60% were recurrence free at 24 months, compared with 38.5% of patients in the observation group (P = .035).
“This positive effect could particularly be seen when the treatment was initiated within 3 months after the end of adjuvant chemotherapy,” Dr. Heinzelmann-Schwarz and her coauthors wrote.
Among women with residual disease who received maintenance bevacizumab in addition to letrozole, 87.5% were recurrence free at 12 months, compared with 20.8% of patients who received bevacizumab only, the investigators said.
Minor side effects such as hot flushes, fatigue, and bone pain led to discontinuation in two patients (6.4%), and no serious adverse effects were seen during treatment, according to investigators.
Almost half of all high-grade serous ovarian cancers express ER, according to results of separate retrospective analyses also described by Dr. Heinzelmann-Schwarz and her colleagues in Gynecologic Oncology. They found that ER expression was similar regardless of drug resistance status (platinum sensitive versus platinum resistant) or treatment setting (primary or recurrent).
Dr. Heinzelmann-Schwarz and colleagues declared no conflicts of interest.
SOURCE: Heinzelmann-Schwarz V et al. Gyn Oncol. 2018 Jan. doi: 10.1016/j.ygyno.2017.10.036.
Maintenance therapy with an aromatase inhibitor may improve progression-free survival in women with estrogen receptor (ER)–positive, high-grade serous ovarian cancers, results of a non-randomized, single-center study suggest.
Patients who received compared with patients who declined the treatment, investigators reported in Gynecologic Oncology.
The benefit of letrozole maintenance treatment was also apparent in women with residual disease who received bevacizumab maintenance treatment, wrote first author Viola Heinzelmann-Schwarz, MD, of the Gynecological Cancer Center, University of Basel, Switzerland, and her coauthors.
The results “strongly suggest that endocrine maintenance therapy has more advantage than disadvantage for use in ovarian cancer patients and seems clearly justified,” when considered alongside other data regarding the potential benefit of letrozole in other treatment settings, Dr. Heinzelmann-Schwarz and her colleagues said.
“In contrast to available expensive maintenance medications like antiangiogenic [drugs] and PARP inhibitors, antihormonal drugs have a favorable safety profile, low cost, easy intake regimen with one tablet daily and an established prognostic target,” they added.
While the role of aromatase inhibitor maintenance therapy in high-grade serous ovarian cancer is unclear, previous studies have shown that patients with low-grade serous ovarian cancer may benefit from endocrine therapy.
Those studies in low-grade serous ovarian cancer include several small case series, and one retrospective analysis showing improved progression-free survival among women treated with endocrine therapy versus women who received observation only (J Clin Oncol. 2017 Apr. doi: 10.1200/JCO.2016.71.0632).
The more recent report by Dr. Heinzelmann-Schwarz and her colleagues included 50 women with ER-positive, FIGO (Fédération Internationale de Gynécologie et d’Obstétrique) stage III/IV high-grade serous ovarian cancer who were offered off-label letrozole treatment after debulking surgery and platinum-based chemotherapy. Twenty-three received letrozole maintenance treatment, and 27 declined.
In the group of women who received letrozole maintenance treatment, 60% were recurrence free at 24 months, compared with 38.5% of patients in the observation group (P = .035).
“This positive effect could particularly be seen when the treatment was initiated within 3 months after the end of adjuvant chemotherapy,” Dr. Heinzelmann-Schwarz and her coauthors wrote.
Among women with residual disease who received maintenance bevacizumab in addition to letrozole, 87.5% were recurrence free at 12 months, compared with 20.8% of patients who received bevacizumab only, the investigators said.
Minor side effects such as hot flushes, fatigue, and bone pain led to discontinuation in two patients (6.4%), and no serious adverse effects were seen during treatment, according to investigators.
Almost half of all high-grade serous ovarian cancers express ER, according to results of separate retrospective analyses also described by Dr. Heinzelmann-Schwarz and her colleagues in Gynecologic Oncology. They found that ER expression was similar regardless of drug resistance status (platinum sensitive versus platinum resistant) or treatment setting (primary or recurrent).
Dr. Heinzelmann-Schwarz and colleagues declared no conflicts of interest.
SOURCE: Heinzelmann-Schwarz V et al. Gyn Oncol. 2018 Jan. doi: 10.1016/j.ygyno.2017.10.036.
Maintenance therapy with an aromatase inhibitor may improve progression-free survival in women with estrogen receptor (ER)–positive, high-grade serous ovarian cancers, results of a non-randomized, single-center study suggest.
Patients who received compared with patients who declined the treatment, investigators reported in Gynecologic Oncology.
The benefit of letrozole maintenance treatment was also apparent in women with residual disease who received bevacizumab maintenance treatment, wrote first author Viola Heinzelmann-Schwarz, MD, of the Gynecological Cancer Center, University of Basel, Switzerland, and her coauthors.
The results “strongly suggest that endocrine maintenance therapy has more advantage than disadvantage for use in ovarian cancer patients and seems clearly justified,” when considered alongside other data regarding the potential benefit of letrozole in other treatment settings, Dr. Heinzelmann-Schwarz and her colleagues said.
“In contrast to available expensive maintenance medications like antiangiogenic [drugs] and PARP inhibitors, antihormonal drugs have a favorable safety profile, low cost, easy intake regimen with one tablet daily and an established prognostic target,” they added.
While the role of aromatase inhibitor maintenance therapy in high-grade serous ovarian cancer is unclear, previous studies have shown that patients with low-grade serous ovarian cancer may benefit from endocrine therapy.
Those studies in low-grade serous ovarian cancer include several small case series, and one retrospective analysis showing improved progression-free survival among women treated with endocrine therapy versus women who received observation only (J Clin Oncol. 2017 Apr. doi: 10.1200/JCO.2016.71.0632).
The more recent report by Dr. Heinzelmann-Schwarz and her colleagues included 50 women with ER-positive, FIGO (Fédération Internationale de Gynécologie et d’Obstétrique) stage III/IV high-grade serous ovarian cancer who were offered off-label letrozole treatment after debulking surgery and platinum-based chemotherapy. Twenty-three received letrozole maintenance treatment, and 27 declined.
In the group of women who received letrozole maintenance treatment, 60% were recurrence free at 24 months, compared with 38.5% of patients in the observation group (P = .035).
“This positive effect could particularly be seen when the treatment was initiated within 3 months after the end of adjuvant chemotherapy,” Dr. Heinzelmann-Schwarz and her coauthors wrote.
Among women with residual disease who received maintenance bevacizumab in addition to letrozole, 87.5% were recurrence free at 12 months, compared with 20.8% of patients who received bevacizumab only, the investigators said.
Minor side effects such as hot flushes, fatigue, and bone pain led to discontinuation in two patients (6.4%), and no serious adverse effects were seen during treatment, according to investigators.
Almost half of all high-grade serous ovarian cancers express ER, according to results of separate retrospective analyses also described by Dr. Heinzelmann-Schwarz and her colleagues in Gynecologic Oncology. They found that ER expression was similar regardless of drug resistance status (platinum sensitive versus platinum resistant) or treatment setting (primary or recurrent).
Dr. Heinzelmann-Schwarz and colleagues declared no conflicts of interest.
SOURCE: Heinzelmann-Schwarz V et al. Gyn Oncol. 2018 Jan. doi: 10.1016/j.ygyno.2017.10.036.
FROM GYNECOLOGIC ONCOLOGY
Key clinical point: Endocrine therapy may have a role in maintenance treatment for patients with estrogen receptor (ER)–positive high-grade serous ovarian cancer (HGSOC) after debulking surgery and platinum-based chemotherapy.
Major finding: Recurrence-free survival at 24 months was 60% for patients receiving the aromatase inhibitor letrozole as maintenance therapy, compared with 38.5% for patients who declined treatment (P = .035).
Data source: A prospective, single-center, nonrandomized study including 50 patients with newly diagnosed FIGO stage III/IV HGSOC and positive estrogen receptor expression.
Disclosures: The authors declared no conflicts of interest.
Source: Heinzelmann-Schwarz V et al. Gyn Oncol. 2018 Jan. doi: 10.1016/j.ygyno.2017.10.036.
Frontline brentuximab vedotin improved Hodgkin lymphoma outcomes
Replacing bleomycin with brentuximab vedotin in the classic ABVD regimen improved a measure of progression-free survival and reduced pulmonary toxicity in patients with previously untreated Hodgkin lymphoma, findings from a randomized, phase 3 trial suggest.
Patients receiving brentuximab plus chemotherapy had a “statistically significant and clinically meaningful improvement” in the rate of modified progression-free survival, according to results published in the New England Journal of Medicine.
Pulmonary toxicity also occurred at a lower rate with the regimen containing brentuximab, an anti-CD30 antibody–drug conjugate, wrote Joseph M. Connors, MD, of the British Columbia Cancer Agency, Vancouver, and his coauthors.
Taken together, these findings suggest brentuximab vedotin and chemotherapy had “substantially less pulmonary toxicity and appears to be more effective for frontline treatment of advanced-stage classic Hodgkin lymphoma,” the researchers wrote.
Bleomycin is often omitted from later cycles of chemotherapy for patients with Hodgkin lymphoma due to pulmonary symptoms, and is sometimes associated with unpredictable or even fatal pulmonary toxicity, the researchers noted.
The brentuximab vedotin arm of the trial did have more neurotoxicity, which was largely reversible, and more myelotoxicity, though that “can be ameliorated with prophylactic granulocyte colony-stimulating factor (G-CSF),” the researchers wrote.
The study by Dr. Connors and colleagues, known as ECHELON-1, was an open-label, multicenter, randomized phase 3 trial including patients with previously untreated stage III or IV classic Hodgkin lymphoma. Among enrolled patients, 664 received brentuximab vedotin plus doxorubicin, vinblastine, and dacarbazine (AVD), and 670 received standard doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD).
The study used a primary endpoint of progression-free survival augmented to include not only time to disease progression or death, but also “modified progression,” which the researchers defined as evidence of noncomplete response at the end of frontline chemotherapy.
It is accepted practice to give more chemotherapy or radiotherapy in Hodgkin lymphoma patients who have a positive PET scan at the end of frontline therapy, since metabolically detectable residual disease reliably predicts imminent progression, Dr. Connors and coauthors wrote.
“In this context, the conventional endpoint of progression-free survival does not accurately assess the curative intent of frontline chemotherapy,” they wrote.
With a median 24.9-month follow-up, modified 2-year progression-free survival in the trial was 82.1% for patients receiving brentuximab plus AVD, versus 77.2% for ABVD (P = .03), a 23% risk reduction.
Pulmonary toxicity of grade 3 or higher occurred in less than 1% of patients in the brentuximab vedotin plus AVD arm of the trial and in 3% of the ABVD arm.
Neutropenia was reported in 58% and 45% in the brentuximab vedotin plus AVD and ABVD arms, respectively, while febrile neutropenia was reported in 19% and 8%, respectively.
In the brentuximab plus AVD group, the rate of febrile neutropenia was 11% for those patients who received primary prophylaxis with G-CSF, and 21% for patients who did not, the researchers noted.
Peripheral neuropathy was seen in 67% and 43% of the brentuximab vedotin plus AVD and ABVD arms, respectively, and about two-thirds of the patients in the brentuximab vedotin plus AVD arm had improvement or resolution at the final follow-up visit.
The study was supported by Millennium Pharmaceuticals and Seattle Genetics. Researchers reported ties to Millennium Pharmaceuticals, Takeda Pharmaceuticals, Seattle Genetics, and other companies.
hematologynews@frontlinemedcom.com
SOURCE: Connors JM et al. N Engl J Med. 2018;378:331-44.
The incorporation of the CD30 immunotoxin brentuximab vedotin into frontline therapy for Hodgkin lymphoma “has been eagerly anticipated, and the wait is over,” Dan L. Longo, MD, and Vincent T. DeVita Jr., MD, wrote in an editorial.
Results of the randomized phase 3 trial by Dr. Connors and colleagues suggest that, with a relatively short follow-up, adding brentuximab vedotin to AVD combination chemotherapy “merits consideration” as a first-line treatment, according to Dr. Longo and Dr. DeVita.
“Although it is too early to rule out unanticipated late side effects … brentuximab vedotin + AVD appears to be more effective than ABVD (and is unlikely to be less effective) and is associated with fewer, more treatable toxicities,” they wrote.
Adverse effects of ABVD are generally modest, but the bleomycin in the regimen can result in serious pulmonary toxicity. While the rate of serious pulmonary toxicity is low, “clinicians have the impression that it is unpredictable,” the authors noted.
Beyond a significant improvement in modified progression-free survival with a follow-up of 25 months, brentuximab vedotin plus AVD was associated with lower pulmonary toxicity, they noted.
While the brentuximab vedotin had more neutropenia and neuropathy, neutropenia could be addressed with G-CSF between doses, and neuropathy was mainly low grade and completely resolved most of the time, the authors said.
Brentuximab had promising single-agent activity in previous Hodgkin lymphoma studies, so substituting it for bleomycin “had the potential to improve on ABVD. And it did,” Dr. Longo and Dr. DeVita wrote.
Dr. Longo is with Dana-Farber Cancer Institute, Boston, and Dr. DeVita is with the Yale Cancer Center, New Haven, Conn. These comments are based on their editorial appearing in the New England Journal of Medicine (2018 Jan 24. doi: 10.1056/NEJMe1715141). Dr. DeVita reported no disclosures, and Dr. Longo reported employment as Deputy Editor with the New England Journal of Medicine.
The incorporation of the CD30 immunotoxin brentuximab vedotin into frontline therapy for Hodgkin lymphoma “has been eagerly anticipated, and the wait is over,” Dan L. Longo, MD, and Vincent T. DeVita Jr., MD, wrote in an editorial.
Results of the randomized phase 3 trial by Dr. Connors and colleagues suggest that, with a relatively short follow-up, adding brentuximab vedotin to AVD combination chemotherapy “merits consideration” as a first-line treatment, according to Dr. Longo and Dr. DeVita.
“Although it is too early to rule out unanticipated late side effects … brentuximab vedotin + AVD appears to be more effective than ABVD (and is unlikely to be less effective) and is associated with fewer, more treatable toxicities,” they wrote.
Adverse effects of ABVD are generally modest, but the bleomycin in the regimen can result in serious pulmonary toxicity. While the rate of serious pulmonary toxicity is low, “clinicians have the impression that it is unpredictable,” the authors noted.
Beyond a significant improvement in modified progression-free survival with a follow-up of 25 months, brentuximab vedotin plus AVD was associated with lower pulmonary toxicity, they noted.
While the brentuximab vedotin had more neutropenia and neuropathy, neutropenia could be addressed with G-CSF between doses, and neuropathy was mainly low grade and completely resolved most of the time, the authors said.
Brentuximab had promising single-agent activity in previous Hodgkin lymphoma studies, so substituting it for bleomycin “had the potential to improve on ABVD. And it did,” Dr. Longo and Dr. DeVita wrote.
Dr. Longo is with Dana-Farber Cancer Institute, Boston, and Dr. DeVita is with the Yale Cancer Center, New Haven, Conn. These comments are based on their editorial appearing in the New England Journal of Medicine (2018 Jan 24. doi: 10.1056/NEJMe1715141). Dr. DeVita reported no disclosures, and Dr. Longo reported employment as Deputy Editor with the New England Journal of Medicine.
The incorporation of the CD30 immunotoxin brentuximab vedotin into frontline therapy for Hodgkin lymphoma “has been eagerly anticipated, and the wait is over,” Dan L. Longo, MD, and Vincent T. DeVita Jr., MD, wrote in an editorial.
Results of the randomized phase 3 trial by Dr. Connors and colleagues suggest that, with a relatively short follow-up, adding brentuximab vedotin to AVD combination chemotherapy “merits consideration” as a first-line treatment, according to Dr. Longo and Dr. DeVita.
“Although it is too early to rule out unanticipated late side effects … brentuximab vedotin + AVD appears to be more effective than ABVD (and is unlikely to be less effective) and is associated with fewer, more treatable toxicities,” they wrote.
Adverse effects of ABVD are generally modest, but the bleomycin in the regimen can result in serious pulmonary toxicity. While the rate of serious pulmonary toxicity is low, “clinicians have the impression that it is unpredictable,” the authors noted.
Beyond a significant improvement in modified progression-free survival with a follow-up of 25 months, brentuximab vedotin plus AVD was associated with lower pulmonary toxicity, they noted.
While the brentuximab vedotin had more neutropenia and neuropathy, neutropenia could be addressed with G-CSF between doses, and neuropathy was mainly low grade and completely resolved most of the time, the authors said.
Brentuximab had promising single-agent activity in previous Hodgkin lymphoma studies, so substituting it for bleomycin “had the potential to improve on ABVD. And it did,” Dr. Longo and Dr. DeVita wrote.
Dr. Longo is with Dana-Farber Cancer Institute, Boston, and Dr. DeVita is with the Yale Cancer Center, New Haven, Conn. These comments are based on their editorial appearing in the New England Journal of Medicine (2018 Jan 24. doi: 10.1056/NEJMe1715141). Dr. DeVita reported no disclosures, and Dr. Longo reported employment as Deputy Editor with the New England Journal of Medicine.
Replacing bleomycin with brentuximab vedotin in the classic ABVD regimen improved a measure of progression-free survival and reduced pulmonary toxicity in patients with previously untreated Hodgkin lymphoma, findings from a randomized, phase 3 trial suggest.
Patients receiving brentuximab plus chemotherapy had a “statistically significant and clinically meaningful improvement” in the rate of modified progression-free survival, according to results published in the New England Journal of Medicine.
Pulmonary toxicity also occurred at a lower rate with the regimen containing brentuximab, an anti-CD30 antibody–drug conjugate, wrote Joseph M. Connors, MD, of the British Columbia Cancer Agency, Vancouver, and his coauthors.
Taken together, these findings suggest brentuximab vedotin and chemotherapy had “substantially less pulmonary toxicity and appears to be more effective for frontline treatment of advanced-stage classic Hodgkin lymphoma,” the researchers wrote.
Bleomycin is often omitted from later cycles of chemotherapy for patients with Hodgkin lymphoma due to pulmonary symptoms, and is sometimes associated with unpredictable or even fatal pulmonary toxicity, the researchers noted.
The brentuximab vedotin arm of the trial did have more neurotoxicity, which was largely reversible, and more myelotoxicity, though that “can be ameliorated with prophylactic granulocyte colony-stimulating factor (G-CSF),” the researchers wrote.
The study by Dr. Connors and colleagues, known as ECHELON-1, was an open-label, multicenter, randomized phase 3 trial including patients with previously untreated stage III or IV classic Hodgkin lymphoma. Among enrolled patients, 664 received brentuximab vedotin plus doxorubicin, vinblastine, and dacarbazine (AVD), and 670 received standard doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD).
The study used a primary endpoint of progression-free survival augmented to include not only time to disease progression or death, but also “modified progression,” which the researchers defined as evidence of noncomplete response at the end of frontline chemotherapy.
It is accepted practice to give more chemotherapy or radiotherapy in Hodgkin lymphoma patients who have a positive PET scan at the end of frontline therapy, since metabolically detectable residual disease reliably predicts imminent progression, Dr. Connors and coauthors wrote.
“In this context, the conventional endpoint of progression-free survival does not accurately assess the curative intent of frontline chemotherapy,” they wrote.
With a median 24.9-month follow-up, modified 2-year progression-free survival in the trial was 82.1% for patients receiving brentuximab plus AVD, versus 77.2% for ABVD (P = .03), a 23% risk reduction.
Pulmonary toxicity of grade 3 or higher occurred in less than 1% of patients in the brentuximab vedotin plus AVD arm of the trial and in 3% of the ABVD arm.
Neutropenia was reported in 58% and 45% in the brentuximab vedotin plus AVD and ABVD arms, respectively, while febrile neutropenia was reported in 19% and 8%, respectively.
In the brentuximab plus AVD group, the rate of febrile neutropenia was 11% for those patients who received primary prophylaxis with G-CSF, and 21% for patients who did not, the researchers noted.
Peripheral neuropathy was seen in 67% and 43% of the brentuximab vedotin plus AVD and ABVD arms, respectively, and about two-thirds of the patients in the brentuximab vedotin plus AVD arm had improvement or resolution at the final follow-up visit.
The study was supported by Millennium Pharmaceuticals and Seattle Genetics. Researchers reported ties to Millennium Pharmaceuticals, Takeda Pharmaceuticals, Seattle Genetics, and other companies.
hematologynews@frontlinemedcom.com
SOURCE: Connors JM et al. N Engl J Med. 2018;378:331-44.
Replacing bleomycin with brentuximab vedotin in the classic ABVD regimen improved a measure of progression-free survival and reduced pulmonary toxicity in patients with previously untreated Hodgkin lymphoma, findings from a randomized, phase 3 trial suggest.
Patients receiving brentuximab plus chemotherapy had a “statistically significant and clinically meaningful improvement” in the rate of modified progression-free survival, according to results published in the New England Journal of Medicine.
Pulmonary toxicity also occurred at a lower rate with the regimen containing brentuximab, an anti-CD30 antibody–drug conjugate, wrote Joseph M. Connors, MD, of the British Columbia Cancer Agency, Vancouver, and his coauthors.
Taken together, these findings suggest brentuximab vedotin and chemotherapy had “substantially less pulmonary toxicity and appears to be more effective for frontline treatment of advanced-stage classic Hodgkin lymphoma,” the researchers wrote.
Bleomycin is often omitted from later cycles of chemotherapy for patients with Hodgkin lymphoma due to pulmonary symptoms, and is sometimes associated with unpredictable or even fatal pulmonary toxicity, the researchers noted.
The brentuximab vedotin arm of the trial did have more neurotoxicity, which was largely reversible, and more myelotoxicity, though that “can be ameliorated with prophylactic granulocyte colony-stimulating factor (G-CSF),” the researchers wrote.
The study by Dr. Connors and colleagues, known as ECHELON-1, was an open-label, multicenter, randomized phase 3 trial including patients with previously untreated stage III or IV classic Hodgkin lymphoma. Among enrolled patients, 664 received brentuximab vedotin plus doxorubicin, vinblastine, and dacarbazine (AVD), and 670 received standard doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD).
The study used a primary endpoint of progression-free survival augmented to include not only time to disease progression or death, but also “modified progression,” which the researchers defined as evidence of noncomplete response at the end of frontline chemotherapy.
It is accepted practice to give more chemotherapy or radiotherapy in Hodgkin lymphoma patients who have a positive PET scan at the end of frontline therapy, since metabolically detectable residual disease reliably predicts imminent progression, Dr. Connors and coauthors wrote.
“In this context, the conventional endpoint of progression-free survival does not accurately assess the curative intent of frontline chemotherapy,” they wrote.
With a median 24.9-month follow-up, modified 2-year progression-free survival in the trial was 82.1% for patients receiving brentuximab plus AVD, versus 77.2% for ABVD (P = .03), a 23% risk reduction.
Pulmonary toxicity of grade 3 or higher occurred in less than 1% of patients in the brentuximab vedotin plus AVD arm of the trial and in 3% of the ABVD arm.
Neutropenia was reported in 58% and 45% in the brentuximab vedotin plus AVD and ABVD arms, respectively, while febrile neutropenia was reported in 19% and 8%, respectively.
In the brentuximab plus AVD group, the rate of febrile neutropenia was 11% for those patients who received primary prophylaxis with G-CSF, and 21% for patients who did not, the researchers noted.
Peripheral neuropathy was seen in 67% and 43% of the brentuximab vedotin plus AVD and ABVD arms, respectively, and about two-thirds of the patients in the brentuximab vedotin plus AVD arm had improvement or resolution at the final follow-up visit.
The study was supported by Millennium Pharmaceuticals and Seattle Genetics. Researchers reported ties to Millennium Pharmaceuticals, Takeda Pharmaceuticals, Seattle Genetics, and other companies.
hematologynews@frontlinemedcom.com
SOURCE: Connors JM et al. N Engl J Med. 2018;378:331-44.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: Modified 2-year progression-free survival was 82.1% with the brentuximab vedotin–containing regimen versus 77.2% for ABVD (P = .03).
Study details: An open-label, multicenter, randomized phase 3 trial including 1,334 patients with previously untreated stage III or IV classic Hodgkin lymphoma.
Disclosures: The study was supported by Millennium Pharmaceuticals and Seattle Genetics. Researchers reported ties to Millennium Pharmaceuticals, Takeda Pharmaceuticals, Seattle Genetics, and other companies.
Source: Connors JM et al. N Engl J Med. 2018;378:331-44.
Integrate brief CBT interventions into medication management visits
Patients who are treated with psychotropics may experience better recovery from their symptoms and improved quality of life when they receive targeted treatment with cognitive-behavioral therapy (CBT). Clinicians can use certain CBT techniques to “jump-start” recovery in patients before prescribed medications produce their intended therapeutic effects. When practitioners are familiar with their use, techniques such as behavioral activation and tools that enhance adherence can be employed during a brief medication management (“med check”) visit.
Take these steps to implement brief CBT interventions into your patient’s routine visits:
- develop a clear, formulation-driven treatment target
- design an intervention that can be explained during a brief visit
- have handouts and worksheets available for patients to use
- provide written explanations and reminders for patients to use in out-of-session practice.
We present a case report that illustrates incorporating brief CBT interventions in a patient with major depressive disorder (MDD).
CASE REPORT
Using CBT to help a patient with MDD
Mr. L, age 52, presents with moderate MDD, and is started on fluoxetine, 20 mg/d. Mr. L has significant anhedonia and poor energy, and has been avoiding going to work and seeing friends. The psychiatrist explains to him how individuals with depression often want to refrain from activity and “shut down,” but that doing so will not improve his quality of life, and his mood will worsen.
The psychiatrist asks Mr. L to identify a pleasurable or important activity to complete before his next appointment. Mr. L decides that he would like to call a friend, because he has been isolated and his friends have been calling him. The psychiatrist encourages him to call one of his golf buddies. She instructs Mr. L to set reminders, such as cell phone alarms and notes on the refrigerator, to prompt him to “Call Phil Saturday at 10
To increase the likelihood that Mr. L will make this call, he and his psychiatrist discuss anticipated obstacles and potential facilitators of this behavior.
The psychiatrist also encourages Mr. L to complete a Behavioral Activation Worksheet (for examples, see http://www.cci.health.wa.gov.au/docs/ACF3B92.pdf or https://www.therapistaid.com/worksheets/behavioral-activation.pdf) to track his depression, pleasure, and sense of achievement before and after completing this activity.
As illustrated by this case, collaborating with the patient is critical to developing a realistic treatment plan that incorporates CBT techniques. With your help and encouragement, patients can use these tools to reach their goals and target the symptoms of their illnesses.
1. Wright JH, McCray LW. Restoring energy and enjoying life. In: Wright JH, McCray LW. Breaking free from depression: pathways to wellness. New York, NY: The Guilford Press; 2012:97-129.
2. Wright JH, Basco MR, Thase ME. Working with automatic thoughts. In: Wright JH, Basco MR, Thase ME. Learning cognitive-behavior therapy: an illustrated guide. Arlington, VA: American Psychiatric Publishing, Inc.; 2005:118-121.
Patients who are treated with psychotropics may experience better recovery from their symptoms and improved quality of life when they receive targeted treatment with cognitive-behavioral therapy (CBT). Clinicians can use certain CBT techniques to “jump-start” recovery in patients before prescribed medications produce their intended therapeutic effects. When practitioners are familiar with their use, techniques such as behavioral activation and tools that enhance adherence can be employed during a brief medication management (“med check”) visit.
Take these steps to implement brief CBT interventions into your patient’s routine visits:
- develop a clear, formulation-driven treatment target
- design an intervention that can be explained during a brief visit
- have handouts and worksheets available for patients to use
- provide written explanations and reminders for patients to use in out-of-session practice.
We present a case report that illustrates incorporating brief CBT interventions in a patient with major depressive disorder (MDD).
CASE REPORT
Using CBT to help a patient with MDD
Mr. L, age 52, presents with moderate MDD, and is started on fluoxetine, 20 mg/d. Mr. L has significant anhedonia and poor energy, and has been avoiding going to work and seeing friends. The psychiatrist explains to him how individuals with depression often want to refrain from activity and “shut down,” but that doing so will not improve his quality of life, and his mood will worsen.
The psychiatrist asks Mr. L to identify a pleasurable or important activity to complete before his next appointment. Mr. L decides that he would like to call a friend, because he has been isolated and his friends have been calling him. The psychiatrist encourages him to call one of his golf buddies. She instructs Mr. L to set reminders, such as cell phone alarms and notes on the refrigerator, to prompt him to “Call Phil Saturday at 10
To increase the likelihood that Mr. L will make this call, he and his psychiatrist discuss anticipated obstacles and potential facilitators of this behavior.
The psychiatrist also encourages Mr. L to complete a Behavioral Activation Worksheet (for examples, see http://www.cci.health.wa.gov.au/docs/ACF3B92.pdf or https://www.therapistaid.com/worksheets/behavioral-activation.pdf) to track his depression, pleasure, and sense of achievement before and after completing this activity.
As illustrated by this case, collaborating with the patient is critical to developing a realistic treatment plan that incorporates CBT techniques. With your help and encouragement, patients can use these tools to reach their goals and target the symptoms of their illnesses.
Patients who are treated with psychotropics may experience better recovery from their symptoms and improved quality of life when they receive targeted treatment with cognitive-behavioral therapy (CBT). Clinicians can use certain CBT techniques to “jump-start” recovery in patients before prescribed medications produce their intended therapeutic effects. When practitioners are familiar with their use, techniques such as behavioral activation and tools that enhance adherence can be employed during a brief medication management (“med check”) visit.
Take these steps to implement brief CBT interventions into your patient’s routine visits:
- develop a clear, formulation-driven treatment target
- design an intervention that can be explained during a brief visit
- have handouts and worksheets available for patients to use
- provide written explanations and reminders for patients to use in out-of-session practice.
We present a case report that illustrates incorporating brief CBT interventions in a patient with major depressive disorder (MDD).
CASE REPORT
Using CBT to help a patient with MDD
Mr. L, age 52, presents with moderate MDD, and is started on fluoxetine, 20 mg/d. Mr. L has significant anhedonia and poor energy, and has been avoiding going to work and seeing friends. The psychiatrist explains to him how individuals with depression often want to refrain from activity and “shut down,” but that doing so will not improve his quality of life, and his mood will worsen.
The psychiatrist asks Mr. L to identify a pleasurable or important activity to complete before his next appointment. Mr. L decides that he would like to call a friend, because he has been isolated and his friends have been calling him. The psychiatrist encourages him to call one of his golf buddies. She instructs Mr. L to set reminders, such as cell phone alarms and notes on the refrigerator, to prompt him to “Call Phil Saturday at 10
To increase the likelihood that Mr. L will make this call, he and his psychiatrist discuss anticipated obstacles and potential facilitators of this behavior.
The psychiatrist also encourages Mr. L to complete a Behavioral Activation Worksheet (for examples, see http://www.cci.health.wa.gov.au/docs/ACF3B92.pdf or https://www.therapistaid.com/worksheets/behavioral-activation.pdf) to track his depression, pleasure, and sense of achievement before and after completing this activity.
As illustrated by this case, collaborating with the patient is critical to developing a realistic treatment plan that incorporates CBT techniques. With your help and encouragement, patients can use these tools to reach their goals and target the symptoms of their illnesses.
1. Wright JH, McCray LW. Restoring energy and enjoying life. In: Wright JH, McCray LW. Breaking free from depression: pathways to wellness. New York, NY: The Guilford Press; 2012:97-129.
2. Wright JH, Basco MR, Thase ME. Working with automatic thoughts. In: Wright JH, Basco MR, Thase ME. Learning cognitive-behavior therapy: an illustrated guide. Arlington, VA: American Psychiatric Publishing, Inc.; 2005:118-121.
1. Wright JH, McCray LW. Restoring energy and enjoying life. In: Wright JH, McCray LW. Breaking free from depression: pathways to wellness. New York, NY: The Guilford Press; 2012:97-129.
2. Wright JH, Basco MR, Thase ME. Working with automatic thoughts. In: Wright JH, Basco MR, Thase ME. Learning cognitive-behavior therapy: an illustrated guide. Arlington, VA: American Psychiatric Publishing, Inc.; 2005:118-121.
Retroperitoneal lymphadenectomy did not impact OS and DFS for high risk, nonmetastatic renal cell carcinoma
according to a secondary analysis of the ASSURE adjuvant trial.
Patients were randomized to adjuvant sorafenib, sunitinib, or placebo in the ASSURE (Adjuvant Sorafenib and Sunitinib for Unfavorable Renal Carcinoma) trial, and those at high risk – which was defined by cN+ disease or determined at their surgeon’s discretion – underwent LND. The primary objective was to assess the effect of LND on overall survival; secondary objectives included the effect of LND on disease-free survival and the benefit of adjuvant therapy vs. placebo in patients who underwent LND.
Overall, 1,943 patients were enrolled in the ASSURE trial, of which 36.1% (701 patients) underwent LND. A median of three lymph nodes (interquartile range, one to eight) was examined, and disease was pN+ in 23.4% patients. A majority of the patients were male (67.4%), with a median age of 56 years. Most (94.5%) patients underwent radical nephrectomy, and 57.2% patients had open surgery rather than laparoscopic. Tumors were clear cell in 81.7% of cases and Fuhrman grade 3-4 in 66.1%, investigators reported in the Journal Of Urology.
“There was no improvement in overall survival for lymphadenectomy relative to no lymphadenectomy (HR, 1.14; 95% CI, 0.93-1.39; P = .20). For patients who underwent lymphadenectomy with pN+ disease, no improvement in overall or disease-free survival was observed for adjuvant therapy relative to placebo. Lymphadenectomy was overall safe, and did not increase the risk of surgical complications (14.2% vs. 13.4%; P = .63),” wrote Benjamin Ristau, MD, of Fox Chase Cancer Center in Philadelphia and his colleagues. LND was independently associated with other markers of aggressive surgical resection, such as open surgery, radical nephrectomy, and adrenalectomy.
The role of lymphadenectomy in patients undergoing surgery for high-risk renal cell carcinoma remains elusive, the authors wrote. Future strategies include a prospective trial in which patients with high-risk renal cell carcinoma are randomized to specific lymphadenectomy templates.
This study was supported by the National Cancer Institute of National Institutes of Health and the Canadian Cancer Research Institute. Christopher G. Wood reported conflicts of interest with Pfizer, Novartis and Argos. Other authors reported no conflicts of interest.
SOURCE: Ristau BT et al. J Urol. 2018 Jan. doi: 10.1016/j.juro.2017.07.042.
according to a secondary analysis of the ASSURE adjuvant trial.
Patients were randomized to adjuvant sorafenib, sunitinib, or placebo in the ASSURE (Adjuvant Sorafenib and Sunitinib for Unfavorable Renal Carcinoma) trial, and those at high risk – which was defined by cN+ disease or determined at their surgeon’s discretion – underwent LND. The primary objective was to assess the effect of LND on overall survival; secondary objectives included the effect of LND on disease-free survival and the benefit of adjuvant therapy vs. placebo in patients who underwent LND.
Overall, 1,943 patients were enrolled in the ASSURE trial, of which 36.1% (701 patients) underwent LND. A median of three lymph nodes (interquartile range, one to eight) was examined, and disease was pN+ in 23.4% patients. A majority of the patients were male (67.4%), with a median age of 56 years. Most (94.5%) patients underwent radical nephrectomy, and 57.2% patients had open surgery rather than laparoscopic. Tumors were clear cell in 81.7% of cases and Fuhrman grade 3-4 in 66.1%, investigators reported in the Journal Of Urology.
“There was no improvement in overall survival for lymphadenectomy relative to no lymphadenectomy (HR, 1.14; 95% CI, 0.93-1.39; P = .20). For patients who underwent lymphadenectomy with pN+ disease, no improvement in overall or disease-free survival was observed for adjuvant therapy relative to placebo. Lymphadenectomy was overall safe, and did not increase the risk of surgical complications (14.2% vs. 13.4%; P = .63),” wrote Benjamin Ristau, MD, of Fox Chase Cancer Center in Philadelphia and his colleagues. LND was independently associated with other markers of aggressive surgical resection, such as open surgery, radical nephrectomy, and adrenalectomy.
The role of lymphadenectomy in patients undergoing surgery for high-risk renal cell carcinoma remains elusive, the authors wrote. Future strategies include a prospective trial in which patients with high-risk renal cell carcinoma are randomized to specific lymphadenectomy templates.
This study was supported by the National Cancer Institute of National Institutes of Health and the Canadian Cancer Research Institute. Christopher G. Wood reported conflicts of interest with Pfizer, Novartis and Argos. Other authors reported no conflicts of interest.
SOURCE: Ristau BT et al. J Urol. 2018 Jan. doi: 10.1016/j.juro.2017.07.042.
according to a secondary analysis of the ASSURE adjuvant trial.
Patients were randomized to adjuvant sorafenib, sunitinib, or placebo in the ASSURE (Adjuvant Sorafenib and Sunitinib for Unfavorable Renal Carcinoma) trial, and those at high risk – which was defined by cN+ disease or determined at their surgeon’s discretion – underwent LND. The primary objective was to assess the effect of LND on overall survival; secondary objectives included the effect of LND on disease-free survival and the benefit of adjuvant therapy vs. placebo in patients who underwent LND.
Overall, 1,943 patients were enrolled in the ASSURE trial, of which 36.1% (701 patients) underwent LND. A median of three lymph nodes (interquartile range, one to eight) was examined, and disease was pN+ in 23.4% patients. A majority of the patients were male (67.4%), with a median age of 56 years. Most (94.5%) patients underwent radical nephrectomy, and 57.2% patients had open surgery rather than laparoscopic. Tumors were clear cell in 81.7% of cases and Fuhrman grade 3-4 in 66.1%, investigators reported in the Journal Of Urology.
“There was no improvement in overall survival for lymphadenectomy relative to no lymphadenectomy (HR, 1.14; 95% CI, 0.93-1.39; P = .20). For patients who underwent lymphadenectomy with pN+ disease, no improvement in overall or disease-free survival was observed for adjuvant therapy relative to placebo. Lymphadenectomy was overall safe, and did not increase the risk of surgical complications (14.2% vs. 13.4%; P = .63),” wrote Benjamin Ristau, MD, of Fox Chase Cancer Center in Philadelphia and his colleagues. LND was independently associated with other markers of aggressive surgical resection, such as open surgery, radical nephrectomy, and adrenalectomy.
The role of lymphadenectomy in patients undergoing surgery for high-risk renal cell carcinoma remains elusive, the authors wrote. Future strategies include a prospective trial in which patients with high-risk renal cell carcinoma are randomized to specific lymphadenectomy templates.
This study was supported by the National Cancer Institute of National Institutes of Health and the Canadian Cancer Research Institute. Christopher G. Wood reported conflicts of interest with Pfizer, Novartis and Argos. Other authors reported no conflicts of interest.
SOURCE: Ristau BT et al. J Urol. 2018 Jan. doi: 10.1016/j.juro.2017.07.042.
FROM THE JOURNAL OF UROLOGY
Key clinical point: Lymphadenectomy did not improve overall survival or disease-free survival in patients with high-risk, nonmetastatic renal cell carcinoma who received either adjuvant therapy or placebo.
Major finding: There was no overall survival benefit for lymphadenectomy relative to no lymphadenectomy (HR, 1.14; 95% CI, 0.93-1.39; P = .20).
Study details: Patients enrolled prospectively in the ASSURE trial.
Disclosures: The study was funded by the National Cancer Institute of National Institutes of Health and the Canadian Cancer Research Institute. Although one author did report conflicts of interest with Pfizer, Novartis, and Argos, the rest reported no conflicts of interest.
Source: Ristau BT et al. J Urol. Jan 2018. doi: 10.1016/j.juro.2017.07.042.
‘Smoker’s paradox’ found in study of IBD patients
Las Vegas – Smoking is more prevalent in Crohn’s disease (CD) patients than in patients with ulcerative colitis (UC), results from a retrospective analysis of national data showed. In addition, , a so-called “smoker’s paradox.”
“This paradox seems to be real, because we know that it has been shown in some heart diseases, that the patients who were smokers had better outcomes,” Zubair Khan, MD, said in an interview at the Crohn’s & Colitis Congress, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association. In fact, a recent analysis of a nationwide cohort of patients who underwent primary percutaneous coronary intervention for ST-segment elevation myocardial infarction found that smokers had significantly lower risk‐adjusted in‐hospital mortality, compared with nonsmokers (J Am Heart Assoc. 2016 Apr 22;5:e003370. doi: 10.1161/JAHA.116.003370).
Between 2002 and 2014, a higher proportion of CD patients than UC patients were smokers (25.1% vs. 17.2%; P less than .001), while CD patients who smoked were more likely to be younger than age 50 years, compared with UC patients who smoked (53.9% vs. 36.9%; P less than .001). The researchers also found that African Americans with CD were more likely than were those with UC to smoke (10% vs. 7.8%, respectively; P less than .001). On the other hand, both Hispanics and Asians with UC were more likely to be smokers than were their counterparts with CD (5% vs. 2.9% and 3.4% vs. 2.5%, respectively). From a geographical standpoint, UC patients in the Northeast and Western United States were more likely to be smokers, compared with CD patients in those regions (20.7% vs. 18.3% and 21.4% vs. 15%, respectively). Meanwhile, CD patients in the Midwest and South were more likely to be smokers, compared with UC patients in those regions (29.3% vs 26% and 37.2% vs. 31.9%, respectively).
Dr. Khan and his associates also found that a higher proportion of female CD patients were smokers, compared with female UC patients (57% vs. 47.3%; P less than .001), and that mortality among UC and CD patients with no smoking history was higher than that of their counterparts who had a smoking history (2.5% vs. 1.2% and 1.2% vs. 0.7%, respectively; P less than .001 for both associations).
“I would certainly not encourage IBD patients to smoke, but maybe we need to so some more prospective studies to better understand this smoker’s paradox,” Dr. Khan said. He reported having no financial disclosures.
*This story was updated on 3/26.
SOURCE: Khan et al. Crohn’s & Colitis Congress, Poster 213.
Las Vegas – Smoking is more prevalent in Crohn’s disease (CD) patients than in patients with ulcerative colitis (UC), results from a retrospective analysis of national data showed. In addition, , a so-called “smoker’s paradox.”
“This paradox seems to be real, because we know that it has been shown in some heart diseases, that the patients who were smokers had better outcomes,” Zubair Khan, MD, said in an interview at the Crohn’s & Colitis Congress, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association. In fact, a recent analysis of a nationwide cohort of patients who underwent primary percutaneous coronary intervention for ST-segment elevation myocardial infarction found that smokers had significantly lower risk‐adjusted in‐hospital mortality, compared with nonsmokers (J Am Heart Assoc. 2016 Apr 22;5:e003370. doi: 10.1161/JAHA.116.003370).
Between 2002 and 2014, a higher proportion of CD patients than UC patients were smokers (25.1% vs. 17.2%; P less than .001), while CD patients who smoked were more likely to be younger than age 50 years, compared with UC patients who smoked (53.9% vs. 36.9%; P less than .001). The researchers also found that African Americans with CD were more likely than were those with UC to smoke (10% vs. 7.8%, respectively; P less than .001). On the other hand, both Hispanics and Asians with UC were more likely to be smokers than were their counterparts with CD (5% vs. 2.9% and 3.4% vs. 2.5%, respectively). From a geographical standpoint, UC patients in the Northeast and Western United States were more likely to be smokers, compared with CD patients in those regions (20.7% vs. 18.3% and 21.4% vs. 15%, respectively). Meanwhile, CD patients in the Midwest and South were more likely to be smokers, compared with UC patients in those regions (29.3% vs 26% and 37.2% vs. 31.9%, respectively).
Dr. Khan and his associates also found that a higher proportion of female CD patients were smokers, compared with female UC patients (57% vs. 47.3%; P less than .001), and that mortality among UC and CD patients with no smoking history was higher than that of their counterparts who had a smoking history (2.5% vs. 1.2% and 1.2% vs. 0.7%, respectively; P less than .001 for both associations).
“I would certainly not encourage IBD patients to smoke, but maybe we need to so some more prospective studies to better understand this smoker’s paradox,” Dr. Khan said. He reported having no financial disclosures.
*This story was updated on 3/26.
SOURCE: Khan et al. Crohn’s & Colitis Congress, Poster 213.
Las Vegas – Smoking is more prevalent in Crohn’s disease (CD) patients than in patients with ulcerative colitis (UC), results from a retrospective analysis of national data showed. In addition, , a so-called “smoker’s paradox.”
“This paradox seems to be real, because we know that it has been shown in some heart diseases, that the patients who were smokers had better outcomes,” Zubair Khan, MD, said in an interview at the Crohn’s & Colitis Congress, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association. In fact, a recent analysis of a nationwide cohort of patients who underwent primary percutaneous coronary intervention for ST-segment elevation myocardial infarction found that smokers had significantly lower risk‐adjusted in‐hospital mortality, compared with nonsmokers (J Am Heart Assoc. 2016 Apr 22;5:e003370. doi: 10.1161/JAHA.116.003370).
Between 2002 and 2014, a higher proportion of CD patients than UC patients were smokers (25.1% vs. 17.2%; P less than .001), while CD patients who smoked were more likely to be younger than age 50 years, compared with UC patients who smoked (53.9% vs. 36.9%; P less than .001). The researchers also found that African Americans with CD were more likely than were those with UC to smoke (10% vs. 7.8%, respectively; P less than .001). On the other hand, both Hispanics and Asians with UC were more likely to be smokers than were their counterparts with CD (5% vs. 2.9% and 3.4% vs. 2.5%, respectively). From a geographical standpoint, UC patients in the Northeast and Western United States were more likely to be smokers, compared with CD patients in those regions (20.7% vs. 18.3% and 21.4% vs. 15%, respectively). Meanwhile, CD patients in the Midwest and South were more likely to be smokers, compared with UC patients in those regions (29.3% vs 26% and 37.2% vs. 31.9%, respectively).
Dr. Khan and his associates also found that a higher proportion of female CD patients were smokers, compared with female UC patients (57% vs. 47.3%; P less than .001), and that mortality among UC and CD patients with no smoking history was higher than that of their counterparts who had a smoking history (2.5% vs. 1.2% and 1.2% vs. 0.7%, respectively; P less than .001 for both associations).
“I would certainly not encourage IBD patients to smoke, but maybe we need to so some more prospective studies to better understand this smoker’s paradox,” Dr. Khan said. He reported having no financial disclosures.
*This story was updated on 3/26.
SOURCE: Khan et al. Crohn’s & Colitis Congress, Poster 213.
REPORTING FROM THE CROHN’S & COLITIS CONGRESS
Key clinical point: In IBD patients, smoker status was paradoxically associated with mortality and other outcomes.
Major finding: Mortality among UC and CD patients with no smoking history was higher than that of their counterparts who had a smoking history (2.5% vs. 1.2% and 1.2% vs. 0.7%, respectively; P less than .001 for both associations).
Study details: An analysis of 22,620 patients with a primary or secondary discharge diagnosis of IBD during 2002-2014.
Disclosures: Dr. Khan reported having no financial disclosures.
Source: Khan et al. Crohn’s & Colitis Congress, Poster 213.
States judged on smoking cessation services
Minnesota and South Carolina are at the top of the class for access to shows that the treatment coverage in most states earned barely passable or failing grades.
In fact, 31 states received either a D (11 states) or an F (20 states) on the grading system. There were also 11 C’s and 7 B’s to go along with the two A’s, the ALA said in “State of Tobacco Control 2018.”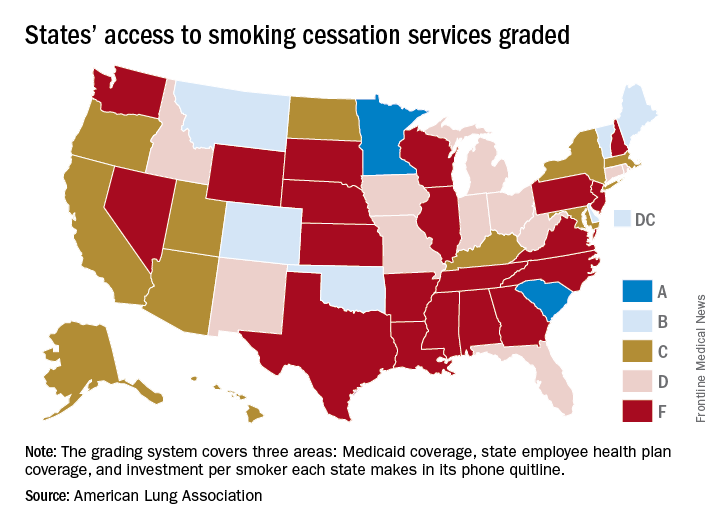
Minnesota received 66 points and South Carolina earned 63 after a 5-point deduction for not expanding Medicaid up to Affordable Care Act standards. The highest-finishing states with B’s were Vermont with 62 points and Maine with 61, and the lowest total score was the 23 points earned by Virginia and Washington, although Washington’s grade did not include the state employee category since the state did not provide data on its plan, the ALA noted.
The Department of Health & Human Services recommends that tobacco cessation coverage include the use of five nicotine-replacement therapies (gum, patch, lozenge, nasal spray, inhaler), bupropion and varenicline (nonnicotine medications), and three types of counseling (individual, group, and phone), the report said.
“It’s imperative that all state Medicaid programs cover a comprehensive tobacco cessation benefit, with no barriers, to help smokers quit, including all seven [Food and Drug Administration]–approved medications and three forms of counseling for Medicaid enrollees. In 2017, only Kentucky, Missouri, and South Carolina provided this coverage,” wrote Harold P. Wimmer, national president and CEO of the ALA.
Minnesota and South Carolina are at the top of the class for access to shows that the treatment coverage in most states earned barely passable or failing grades.
In fact, 31 states received either a D (11 states) or an F (20 states) on the grading system. There were also 11 C’s and 7 B’s to go along with the two A’s, the ALA said in “State of Tobacco Control 2018.”
Minnesota received 66 points and South Carolina earned 63 after a 5-point deduction for not expanding Medicaid up to Affordable Care Act standards. The highest-finishing states with B’s were Vermont with 62 points and Maine with 61, and the lowest total score was the 23 points earned by Virginia and Washington, although Washington’s grade did not include the state employee category since the state did not provide data on its plan, the ALA noted.
The Department of Health & Human Services recommends that tobacco cessation coverage include the use of five nicotine-replacement therapies (gum, patch, lozenge, nasal spray, inhaler), bupropion and varenicline (nonnicotine medications), and three types of counseling (individual, group, and phone), the report said.
“It’s imperative that all state Medicaid programs cover a comprehensive tobacco cessation benefit, with no barriers, to help smokers quit, including all seven [Food and Drug Administration]–approved medications and three forms of counseling for Medicaid enrollees. In 2017, only Kentucky, Missouri, and South Carolina provided this coverage,” wrote Harold P. Wimmer, national president and CEO of the ALA.
Minnesota and South Carolina are at the top of the class for access to shows that the treatment coverage in most states earned barely passable or failing grades.
In fact, 31 states received either a D (11 states) or an F (20 states) on the grading system. There were also 11 C’s and 7 B’s to go along with the two A’s, the ALA said in “State of Tobacco Control 2018.”
Minnesota received 66 points and South Carolina earned 63 after a 5-point deduction for not expanding Medicaid up to Affordable Care Act standards. The highest-finishing states with B’s were Vermont with 62 points and Maine with 61, and the lowest total score was the 23 points earned by Virginia and Washington, although Washington’s grade did not include the state employee category since the state did not provide data on its plan, the ALA noted.
The Department of Health & Human Services recommends that tobacco cessation coverage include the use of five nicotine-replacement therapies (gum, patch, lozenge, nasal spray, inhaler), bupropion and varenicline (nonnicotine medications), and three types of counseling (individual, group, and phone), the report said.
“It’s imperative that all state Medicaid programs cover a comprehensive tobacco cessation benefit, with no barriers, to help smokers quit, including all seven [Food and Drug Administration]–approved medications and three forms of counseling for Medicaid enrollees. In 2017, only Kentucky, Missouri, and South Carolina provided this coverage,” wrote Harold P. Wimmer, national president and CEO of the ALA.
Postcolonoscopy cancer rates persist despite quality protocols
The number of colorectal cancers diagnosed after a colonoscopy remained consistent at approximately 8% over a 15-year period despite the introduction of quality improvement measures, according to data from a population-based cohort study of more than 1 million individuals in Canada.
“It is believed that the majority of PCCRCs [postcolonoscopy colorectal cancers] arise due to cancers or near cancers that were either missed or incompletely treated during colonoscopy,” wrote Sanjay K. Murthy, MD, of the University of Ottawa, and colleagues.
Established quality improvement measures included adenoma detection rate, cecal intubation rate, colonoscopy withdrawal time, and endoscopy training standards, but how well the measures have been implemented remains uncertain, the researchers said. In a study published in Gastrointestinal Endoscopy (2018 Jan 6. doi: 10.1016/j.gie.2017.12.027), the researchers assessed data from 1,093,658 eligible adults aged 50-74 years over a 15-year period. The time period was divided into three sections: July 1, 1996, to June 30, 2001; July 1, 2001, to June 30, 2006; and July 1, 2006, to Dec. 31, 2010.
Overall, the number of colonoscopy procedures increased during the study period, from 305 per 10,000 people in 1996-1997 to 870 per 10,000 people in 2010-2011, and the percentage of individuals who underwent complete colonoscopies increased from 67% in the 1996-2001 period to 88% in the 2006-2010 period. “There was a considerable increase in the proportion of colonoscopies performed in younger age groups and community clinics in successive study periods,” the researchers noted.
Comparing the 2006-2010 and 1996-2001 time periods yielded adjusted odds of PCCRC, distal PCCRC, and proximal PCCRC of 1.14, 1.11, and 1.14, respectively; the trends were not affected by endoscopist specialty or institutional setting.
“Our findings are concerning for lack of improvement in colonoscopy practice quality in Ontario, particularly in the wake of greater emphasis having been placed on colonoscopy quality metrics during the study period,” the researchers said. The findings contrast with the decline in PCCRC rates in the United Kingdom reported in a previous study of a similar time period, they noted.
The study findings were limited by several factors, including possible patient and outcome misclassification, an unvalidated definition for PCCRC, and unmeasured confounders such as changes in practice or changes in the definition of PCCRC. Although more research is needed in other jurisdictions to confirm, the results “call for increased population-based practice audit as well as endoscopy educational programs and certification requirements.”
The study was supported by a research grant to Dr. Murthy from the University of Ottawa. The researchers had no financial conflicts to disclose.
SOURCE: Murthy S et al. Gastrointest Endosc. 2018 Jan 6. doi: 10.1016/j.gie.2017.12.027
Postcolonoscopy colorectal cancers (PCCRCs) are those cancers that occur between 6 and 36 months after a complete colonoscopy. For cancers diagnosed less than 6 months from exam, it is presumed that the exam itself was diagnostic. Most of these cancers grow from cancers or near cancers missed or incompletely resected during the baseline colonoscopy. Clinical researchers have published extensively about reasons for missed lesions and we know that age, female sex, and proximal location of cancers increase rates of PCCRC. GI societies worldwide have developed training initiatives, performance metrics (adenoma detection rate or ADR, withdrawal time, and prep quality documentation), and postcolonoscopy guidelines, all intended to mitigate risk of PCCRCs. It would be nice to know whether such efforts have made a difference.
Murthy and colleagues studied PCCRC rates in Ottawa, Canada during three different time periods to determine whether quality and educational efforts impacted PCCRC rates. More than 99% of this population has health care covered under a single public payer system where all encounters are carefully tracked. Using population-level health data derived from over 1 million people (screen eligible people, 50-74 years of age with low to moderate CRC risk) they identified cancers diagnosed within 36 months of a colonoscopy and compared three 5-year periods (1996-2001, 2001-2006, and 2006-2010).
Their method of calculating PCCRC rates essentially says, “If I am destined to develop CRC in the next 3 years, what is my chance of a false-negative colonoscopy?” There are five published methods of calculating PCCRC rates (summarized in Gut 2015;64:1248-56) and each method uses different inclusion criteria and denominators. The question posed above yields “rates” that would terrify patients (4%-10%) without a detailed explanation (it took me about an hour of focused attention to finally understand this methodology). In essence, if we could, a priori, identify and examine only patients who have a prevalent cancer or near cancer, how close can we come to 100% accuracy with a colonoscopy? Turns out, that rate is somewhere between 90% and 96% and really hasn’t changed over time. Thus, these studies speak to the impact of our efforts around colonoscopy quality.
John I. Allen MD, MBA, AGAF, professor of medicine, department of gastroenterology and hepatology, University of Michigan, Ann Arbor, and Editor in Chief of GI & Hepatology News.
Postcolonoscopy colorectal cancers (PCCRCs) are those cancers that occur between 6 and 36 months after a complete colonoscopy. For cancers diagnosed less than 6 months from exam, it is presumed that the exam itself was diagnostic. Most of these cancers grow from cancers or near cancers missed or incompletely resected during the baseline colonoscopy. Clinical researchers have published extensively about reasons for missed lesions and we know that age, female sex, and proximal location of cancers increase rates of PCCRC. GI societies worldwide have developed training initiatives, performance metrics (adenoma detection rate or ADR, withdrawal time, and prep quality documentation), and postcolonoscopy guidelines, all intended to mitigate risk of PCCRCs. It would be nice to know whether such efforts have made a difference.
Murthy and colleagues studied PCCRC rates in Ottawa, Canada during three different time periods to determine whether quality and educational efforts impacted PCCRC rates. More than 99% of this population has health care covered under a single public payer system where all encounters are carefully tracked. Using population-level health data derived from over 1 million people (screen eligible people, 50-74 years of age with low to moderate CRC risk) they identified cancers diagnosed within 36 months of a colonoscopy and compared three 5-year periods (1996-2001, 2001-2006, and 2006-2010).
Their method of calculating PCCRC rates essentially says, “If I am destined to develop CRC in the next 3 years, what is my chance of a false-negative colonoscopy?” There are five published methods of calculating PCCRC rates (summarized in Gut 2015;64:1248-56) and each method uses different inclusion criteria and denominators. The question posed above yields “rates” that would terrify patients (4%-10%) without a detailed explanation (it took me about an hour of focused attention to finally understand this methodology). In essence, if we could, a priori, identify and examine only patients who have a prevalent cancer or near cancer, how close can we come to 100% accuracy with a colonoscopy? Turns out, that rate is somewhere between 90% and 96% and really hasn’t changed over time. Thus, these studies speak to the impact of our efforts around colonoscopy quality.
John I. Allen MD, MBA, AGAF, professor of medicine, department of gastroenterology and hepatology, University of Michigan, Ann Arbor, and Editor in Chief of GI & Hepatology News.
Postcolonoscopy colorectal cancers (PCCRCs) are those cancers that occur between 6 and 36 months after a complete colonoscopy. For cancers diagnosed less than 6 months from exam, it is presumed that the exam itself was diagnostic. Most of these cancers grow from cancers or near cancers missed or incompletely resected during the baseline colonoscopy. Clinical researchers have published extensively about reasons for missed lesions and we know that age, female sex, and proximal location of cancers increase rates of PCCRC. GI societies worldwide have developed training initiatives, performance metrics (adenoma detection rate or ADR, withdrawal time, and prep quality documentation), and postcolonoscopy guidelines, all intended to mitigate risk of PCCRCs. It would be nice to know whether such efforts have made a difference.
Murthy and colleagues studied PCCRC rates in Ottawa, Canada during three different time periods to determine whether quality and educational efforts impacted PCCRC rates. More than 99% of this population has health care covered under a single public payer system where all encounters are carefully tracked. Using population-level health data derived from over 1 million people (screen eligible people, 50-74 years of age with low to moderate CRC risk) they identified cancers diagnosed within 36 months of a colonoscopy and compared three 5-year periods (1996-2001, 2001-2006, and 2006-2010).
Their method of calculating PCCRC rates essentially says, “If I am destined to develop CRC in the next 3 years, what is my chance of a false-negative colonoscopy?” There are five published methods of calculating PCCRC rates (summarized in Gut 2015;64:1248-56) and each method uses different inclusion criteria and denominators. The question posed above yields “rates” that would terrify patients (4%-10%) without a detailed explanation (it took me about an hour of focused attention to finally understand this methodology). In essence, if we could, a priori, identify and examine only patients who have a prevalent cancer or near cancer, how close can we come to 100% accuracy with a colonoscopy? Turns out, that rate is somewhere between 90% and 96% and really hasn’t changed over time. Thus, these studies speak to the impact of our efforts around colonoscopy quality.
John I. Allen MD, MBA, AGAF, professor of medicine, department of gastroenterology and hepatology, University of Michigan, Ann Arbor, and Editor in Chief of GI & Hepatology News.
The number of colorectal cancers diagnosed after a colonoscopy remained consistent at approximately 8% over a 15-year period despite the introduction of quality improvement measures, according to data from a population-based cohort study of more than 1 million individuals in Canada.
“It is believed that the majority of PCCRCs [postcolonoscopy colorectal cancers] arise due to cancers or near cancers that were either missed or incompletely treated during colonoscopy,” wrote Sanjay K. Murthy, MD, of the University of Ottawa, and colleagues.
Established quality improvement measures included adenoma detection rate, cecal intubation rate, colonoscopy withdrawal time, and endoscopy training standards, but how well the measures have been implemented remains uncertain, the researchers said. In a study published in Gastrointestinal Endoscopy (2018 Jan 6. doi: 10.1016/j.gie.2017.12.027), the researchers assessed data from 1,093,658 eligible adults aged 50-74 years over a 15-year period. The time period was divided into three sections: July 1, 1996, to June 30, 2001; July 1, 2001, to June 30, 2006; and July 1, 2006, to Dec. 31, 2010.
Overall, the number of colonoscopy procedures increased during the study period, from 305 per 10,000 people in 1996-1997 to 870 per 10,000 people in 2010-2011, and the percentage of individuals who underwent complete colonoscopies increased from 67% in the 1996-2001 period to 88% in the 2006-2010 period. “There was a considerable increase in the proportion of colonoscopies performed in younger age groups and community clinics in successive study periods,” the researchers noted.
Comparing the 2006-2010 and 1996-2001 time periods yielded adjusted odds of PCCRC, distal PCCRC, and proximal PCCRC of 1.14, 1.11, and 1.14, respectively; the trends were not affected by endoscopist specialty or institutional setting.
“Our findings are concerning for lack of improvement in colonoscopy practice quality in Ontario, particularly in the wake of greater emphasis having been placed on colonoscopy quality metrics during the study period,” the researchers said. The findings contrast with the decline in PCCRC rates in the United Kingdom reported in a previous study of a similar time period, they noted.
The study findings were limited by several factors, including possible patient and outcome misclassification, an unvalidated definition for PCCRC, and unmeasured confounders such as changes in practice or changes in the definition of PCCRC. Although more research is needed in other jurisdictions to confirm, the results “call for increased population-based practice audit as well as endoscopy educational programs and certification requirements.”
The study was supported by a research grant to Dr. Murthy from the University of Ottawa. The researchers had no financial conflicts to disclose.
SOURCE: Murthy S et al. Gastrointest Endosc. 2018 Jan 6. doi: 10.1016/j.gie.2017.12.027
The number of colorectal cancers diagnosed after a colonoscopy remained consistent at approximately 8% over a 15-year period despite the introduction of quality improvement measures, according to data from a population-based cohort study of more than 1 million individuals in Canada.
“It is believed that the majority of PCCRCs [postcolonoscopy colorectal cancers] arise due to cancers or near cancers that were either missed or incompletely treated during colonoscopy,” wrote Sanjay K. Murthy, MD, of the University of Ottawa, and colleagues.
Established quality improvement measures included adenoma detection rate, cecal intubation rate, colonoscopy withdrawal time, and endoscopy training standards, but how well the measures have been implemented remains uncertain, the researchers said. In a study published in Gastrointestinal Endoscopy (2018 Jan 6. doi: 10.1016/j.gie.2017.12.027), the researchers assessed data from 1,093,658 eligible adults aged 50-74 years over a 15-year period. The time period was divided into three sections: July 1, 1996, to June 30, 2001; July 1, 2001, to June 30, 2006; and July 1, 2006, to Dec. 31, 2010.
Overall, the number of colonoscopy procedures increased during the study period, from 305 per 10,000 people in 1996-1997 to 870 per 10,000 people in 2010-2011, and the percentage of individuals who underwent complete colonoscopies increased from 67% in the 1996-2001 period to 88% in the 2006-2010 period. “There was a considerable increase in the proportion of colonoscopies performed in younger age groups and community clinics in successive study periods,” the researchers noted.
Comparing the 2006-2010 and 1996-2001 time periods yielded adjusted odds of PCCRC, distal PCCRC, and proximal PCCRC of 1.14, 1.11, and 1.14, respectively; the trends were not affected by endoscopist specialty or institutional setting.
“Our findings are concerning for lack of improvement in colonoscopy practice quality in Ontario, particularly in the wake of greater emphasis having been placed on colonoscopy quality metrics during the study period,” the researchers said. The findings contrast with the decline in PCCRC rates in the United Kingdom reported in a previous study of a similar time period, they noted.
The study findings were limited by several factors, including possible patient and outcome misclassification, an unvalidated definition for PCCRC, and unmeasured confounders such as changes in practice or changes in the definition of PCCRC. Although more research is needed in other jurisdictions to confirm, the results “call for increased population-based practice audit as well as endoscopy educational programs and certification requirements.”
The study was supported by a research grant to Dr. Murthy from the University of Ottawa. The researchers had no financial conflicts to disclose.
SOURCE: Murthy S et al. Gastrointest Endosc. 2018 Jan 6. doi: 10.1016/j.gie.2017.12.027
FROM GASTROINTESTINAL ENDOSCOPY
Key clinical point: Rates of postcolonoscopy colorectal cancer have not declined despite the introduction of quality improvement measures.
Major finding: The rate of colorectal cancers diagnosed after a colonoscopy has remained at approximately 8% over the past 15 years.
Study details: A population-based retrospective cohort study of Canadian adults aged 50-74 years without risk factors for CRC.
Disclosures: The researchers had no financial conflicts to disclose.
Source: Murthy S et al. Gastrointest Endosc. 2018 Jan 6. doi: 10.1016/j.gie.2017.12.027.
Beta-blocker therapy post AMI
reported that the treatment of AMI in patients with propranolol, the beta-blocker of the day, reduced mortality by over 25% over 3 years (JAMA. 1981 Nov 6;246[18]:2073-4), and a European trial of timolol confirmed those findings.
New medications have been developed, including ACE inhibitors, statins, and a variety of drugs that modify the thrombotic process that occurs with the event. In addition, endovascular procedures have modified the obstructive coronary vascular anatomy that precipitated the event. At the same time, the definition of an AMI has changed dramatically, now depending in many instances on transitory elevation of the highly sensitive troponins. The BHAT definition depended largely on electrocardiographic changes associated with the event, which were ST-segment elevations in 79% of the occurrences, or significant ST-segment changes associated often with elevation of the insensitive enzyme, serum glutamic transaminase, and significant symptoms. The characteristics of the BHAT patient only faintly resemble the patients who we now classify with AMIs, and its definition expanded well beyond the BHAT patients. And yet beta-blocker therapy is still part of the class I or II recommendations for the treatment of an AMI.
Reasonable questions have been raised as to the wisdom of throwing the full bag of therapy at this population rather than a more selective choice of drugs. In order to gain further insight into the benefits of beta-blocker therapy in the total spectrum of post MI therapy, large populations studies using a variety of statistical gymnastics have tried to identify the unique benefit that can be attributed to beta-blocker therapy relative to the other drugs and surgical interventions used in the post-AMI populations. The result has been a mixed message without any clear guidance. One can make a good case for carrying out clinical trials in specific subsets of the currently defined post-AMI population. Such an endeavor is unlikely. Pharma is clearly not interested in old drug research and the National Heart, Lung, and Blood Institute doesn’t have the funds. So it comes down to clinician’s decisions.
As an avowed beta-blocker enthusiast, I use it in most of my post-AMI patients, particularly if they have left ventricular dysfunction, and in patients with concomitant hypertension. I do not use it in patents with transitory troponin elevations without convincing evidence of clinical symptoms. I will continue beta-blockade as long as I am able to write the prescription or until someone has answered the question.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
reported that the treatment of AMI in patients with propranolol, the beta-blocker of the day, reduced mortality by over 25% over 3 years (JAMA. 1981 Nov 6;246[18]:2073-4), and a European trial of timolol confirmed those findings.
New medications have been developed, including ACE inhibitors, statins, and a variety of drugs that modify the thrombotic process that occurs with the event. In addition, endovascular procedures have modified the obstructive coronary vascular anatomy that precipitated the event. At the same time, the definition of an AMI has changed dramatically, now depending in many instances on transitory elevation of the highly sensitive troponins. The BHAT definition depended largely on electrocardiographic changes associated with the event, which were ST-segment elevations in 79% of the occurrences, or significant ST-segment changes associated often with elevation of the insensitive enzyme, serum glutamic transaminase, and significant symptoms. The characteristics of the BHAT patient only faintly resemble the patients who we now classify with AMIs, and its definition expanded well beyond the BHAT patients. And yet beta-blocker therapy is still part of the class I or II recommendations for the treatment of an AMI.
Reasonable questions have been raised as to the wisdom of throwing the full bag of therapy at this population rather than a more selective choice of drugs. In order to gain further insight into the benefits of beta-blocker therapy in the total spectrum of post MI therapy, large populations studies using a variety of statistical gymnastics have tried to identify the unique benefit that can be attributed to beta-blocker therapy relative to the other drugs and surgical interventions used in the post-AMI populations. The result has been a mixed message without any clear guidance. One can make a good case for carrying out clinical trials in specific subsets of the currently defined post-AMI population. Such an endeavor is unlikely. Pharma is clearly not interested in old drug research and the National Heart, Lung, and Blood Institute doesn’t have the funds. So it comes down to clinician’s decisions.
As an avowed beta-blocker enthusiast, I use it in most of my post-AMI patients, particularly if they have left ventricular dysfunction, and in patients with concomitant hypertension. I do not use it in patents with transitory troponin elevations without convincing evidence of clinical symptoms. I will continue beta-blockade as long as I am able to write the prescription or until someone has answered the question.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
reported that the treatment of AMI in patients with propranolol, the beta-blocker of the day, reduced mortality by over 25% over 3 years (JAMA. 1981 Nov 6;246[18]:2073-4), and a European trial of timolol confirmed those findings.
New medications have been developed, including ACE inhibitors, statins, and a variety of drugs that modify the thrombotic process that occurs with the event. In addition, endovascular procedures have modified the obstructive coronary vascular anatomy that precipitated the event. At the same time, the definition of an AMI has changed dramatically, now depending in many instances on transitory elevation of the highly sensitive troponins. The BHAT definition depended largely on electrocardiographic changes associated with the event, which were ST-segment elevations in 79% of the occurrences, or significant ST-segment changes associated often with elevation of the insensitive enzyme, serum glutamic transaminase, and significant symptoms. The characteristics of the BHAT patient only faintly resemble the patients who we now classify with AMIs, and its definition expanded well beyond the BHAT patients. And yet beta-blocker therapy is still part of the class I or II recommendations for the treatment of an AMI.
Reasonable questions have been raised as to the wisdom of throwing the full bag of therapy at this population rather than a more selective choice of drugs. In order to gain further insight into the benefits of beta-blocker therapy in the total spectrum of post MI therapy, large populations studies using a variety of statistical gymnastics have tried to identify the unique benefit that can be attributed to beta-blocker therapy relative to the other drugs and surgical interventions used in the post-AMI populations. The result has been a mixed message without any clear guidance. One can make a good case for carrying out clinical trials in specific subsets of the currently defined post-AMI population. Such an endeavor is unlikely. Pharma is clearly not interested in old drug research and the National Heart, Lung, and Blood Institute doesn’t have the funds. So it comes down to clinician’s decisions.
As an avowed beta-blocker enthusiast, I use it in most of my post-AMI patients, particularly if they have left ventricular dysfunction, and in patients with concomitant hypertension. I do not use it in patents with transitory troponin elevations without convincing evidence of clinical symptoms. I will continue beta-blockade as long as I am able to write the prescription or until someone has answered the question.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.