User login
Lapatinib plus trastuzumab improves outcomes in HER2+ breast cancer
SAN ANTONIO – Dual HER2 targeting with lapatinib added to 16 weeks of trastuzumab significantly improved event-free survival (EFS), compared with trastuzumab alone, among women with HER2-positive breast cancer.
EFS was significantly longer in the combination arm versus single-blockade therapy (hazard ratio, 0.35; 95% confidence interval, 0.15-0.84; P = .013).
“Overall survival was also increased, but the number of events was very small,” said study author Ian E. Krop, MD, of the Dana-Farber Cancer Institute in Boston, who presented the study results at the San Antonio Breast Cancer Symposium.
As expected, and consistent with other studies, the pathologic complete response (pCR) rate with dual blockade was associated with favorable long-term outcomes, and the effect was most pronounced in HR- and HER2 enriched cancers.
However, Dr. Krop cautioned that these results need to be seen in the context of two larger trials that failed to find a benefit for dual-blockade HER2 therapy in either the adjuvant or neoadjuvant setting.
Previous research has shown that trastuzumab and lapatinib are synergistic, potently inhibiting HER2 signaling in preclinical trials. The combination of both agents is also active in both untreated and heavily pretreated HER2+ advanced breast cancer. The addition of lapatinib to trastuzumab plus chemotherapy has also been associated with a pCR rate that was statistically significant in many studies.
But despite this “wealth of evidence,” the largest of these studies, the phase 3 ALTTO study, failed to show a benefit for dual HER2 blockade. The same was true for the neoadjuvant Neo ALTTO study. Both studies failed to show an improvement in EFS or overall survival.
The current trial, CALGB 40601, a randomized phase 3 trial, assessed the effect of dual HER2 blockade consisting of trastuzumab and lapatinib added to paclitaxel on rates of pCR, and the researchers also looked at tumor and microenvironment molecular features. Dr. Krop noted that they had previously found that rates of pCR were numerically but not significantly increased with combination-blockade therapy; they had also found that the tumor molecular subtype and evidence of immune activation were independent factors affecting rates of pCR.
Dr. Krop presented results from a secondary analysis in which they evaluated the effects of treatment arm and gene expression–defined subgroups on EFS.
A total of 305 patients with stage II and III HER2+ breast cancer were randomized to receive 16 weeks of either paclitaxel plus trastuzumab alone or that combination with lapatinib as well before undergoing surgery. Evaluable information regarding EFS and RNA-sequencing gene expression was available for 265 patients.
“Despite the lack of significant benefits in other studies, we did demonstrate an improvement in EFS,” said Dr. Krop, noting that it was more pronounced in the subset of HR- patients (HR, 0.12; P = .0160).
EFS was also significantly longer among those who achieved pCR than those who didn’t (HR, 0.34; P = .0032).
In an exploratory analysis, Dr. Krop and his colleagues assessed whether there was a difference in rates of pCR by subtype, and they found that luminal A and luminal B did not have much of an effect on rates of pCR. However, patients with HER2-enriched disease who had a pCR had significantly better EFS than those who did not (HR, 0.14; 95% CI, 0.04-0.44; P less than .0001).
Conversely, patients with HER2-enriched disease who did not achieve pCR had a substantial risk of recurrence.
When looking at EFS by intrinsic subtype, the addition of lapatinib had the most effect on luminal A cancers. “This shows that there was some discordance between analyses by pCR and by long-term endpoint,” Dr. Krop said.
Finally, among gene expression signatures, only immune activation measured by an immunoglobulin G signature was associated with an improvement in EFS (HR, 0.70; 95% CI, 0.50-0.98; P = .04).
“These data are hypothesis generating and require validation,” Dr. Krop concluded. He added that a combined analysis of this trial, along with the Neo ALTTO and others, is planned.
SOURCE: Krop IE et al. SABCS 2017 Abstract GS3-02.
SAN ANTONIO – Dual HER2 targeting with lapatinib added to 16 weeks of trastuzumab significantly improved event-free survival (EFS), compared with trastuzumab alone, among women with HER2-positive breast cancer.
EFS was significantly longer in the combination arm versus single-blockade therapy (hazard ratio, 0.35; 95% confidence interval, 0.15-0.84; P = .013).
“Overall survival was also increased, but the number of events was very small,” said study author Ian E. Krop, MD, of the Dana-Farber Cancer Institute in Boston, who presented the study results at the San Antonio Breast Cancer Symposium.
As expected, and consistent with other studies, the pathologic complete response (pCR) rate with dual blockade was associated with favorable long-term outcomes, and the effect was most pronounced in HR- and HER2 enriched cancers.
However, Dr. Krop cautioned that these results need to be seen in the context of two larger trials that failed to find a benefit for dual-blockade HER2 therapy in either the adjuvant or neoadjuvant setting.
Previous research has shown that trastuzumab and lapatinib are synergistic, potently inhibiting HER2 signaling in preclinical trials. The combination of both agents is also active in both untreated and heavily pretreated HER2+ advanced breast cancer. The addition of lapatinib to trastuzumab plus chemotherapy has also been associated with a pCR rate that was statistically significant in many studies.
But despite this “wealth of evidence,” the largest of these studies, the phase 3 ALTTO study, failed to show a benefit for dual HER2 blockade. The same was true for the neoadjuvant Neo ALTTO study. Both studies failed to show an improvement in EFS or overall survival.
The current trial, CALGB 40601, a randomized phase 3 trial, assessed the effect of dual HER2 blockade consisting of trastuzumab and lapatinib added to paclitaxel on rates of pCR, and the researchers also looked at tumor and microenvironment molecular features. Dr. Krop noted that they had previously found that rates of pCR were numerically but not significantly increased with combination-blockade therapy; they had also found that the tumor molecular subtype and evidence of immune activation were independent factors affecting rates of pCR.
Dr. Krop presented results from a secondary analysis in which they evaluated the effects of treatment arm and gene expression–defined subgroups on EFS.
A total of 305 patients with stage II and III HER2+ breast cancer were randomized to receive 16 weeks of either paclitaxel plus trastuzumab alone or that combination with lapatinib as well before undergoing surgery. Evaluable information regarding EFS and RNA-sequencing gene expression was available for 265 patients.
“Despite the lack of significant benefits in other studies, we did demonstrate an improvement in EFS,” said Dr. Krop, noting that it was more pronounced in the subset of HR- patients (HR, 0.12; P = .0160).
EFS was also significantly longer among those who achieved pCR than those who didn’t (HR, 0.34; P = .0032).
In an exploratory analysis, Dr. Krop and his colleagues assessed whether there was a difference in rates of pCR by subtype, and they found that luminal A and luminal B did not have much of an effect on rates of pCR. However, patients with HER2-enriched disease who had a pCR had significantly better EFS than those who did not (HR, 0.14; 95% CI, 0.04-0.44; P less than .0001).
Conversely, patients with HER2-enriched disease who did not achieve pCR had a substantial risk of recurrence.
When looking at EFS by intrinsic subtype, the addition of lapatinib had the most effect on luminal A cancers. “This shows that there was some discordance between analyses by pCR and by long-term endpoint,” Dr. Krop said.
Finally, among gene expression signatures, only immune activation measured by an immunoglobulin G signature was associated with an improvement in EFS (HR, 0.70; 95% CI, 0.50-0.98; P = .04).
“These data are hypothesis generating and require validation,” Dr. Krop concluded. He added that a combined analysis of this trial, along with the Neo ALTTO and others, is planned.
SOURCE: Krop IE et al. SABCS 2017 Abstract GS3-02.
SAN ANTONIO – Dual HER2 targeting with lapatinib added to 16 weeks of trastuzumab significantly improved event-free survival (EFS), compared with trastuzumab alone, among women with HER2-positive breast cancer.
EFS was significantly longer in the combination arm versus single-blockade therapy (hazard ratio, 0.35; 95% confidence interval, 0.15-0.84; P = .013).
“Overall survival was also increased, but the number of events was very small,” said study author Ian E. Krop, MD, of the Dana-Farber Cancer Institute in Boston, who presented the study results at the San Antonio Breast Cancer Symposium.
As expected, and consistent with other studies, the pathologic complete response (pCR) rate with dual blockade was associated with favorable long-term outcomes, and the effect was most pronounced in HR- and HER2 enriched cancers.
However, Dr. Krop cautioned that these results need to be seen in the context of two larger trials that failed to find a benefit for dual-blockade HER2 therapy in either the adjuvant or neoadjuvant setting.
Previous research has shown that trastuzumab and lapatinib are synergistic, potently inhibiting HER2 signaling in preclinical trials. The combination of both agents is also active in both untreated and heavily pretreated HER2+ advanced breast cancer. The addition of lapatinib to trastuzumab plus chemotherapy has also been associated with a pCR rate that was statistically significant in many studies.
But despite this “wealth of evidence,” the largest of these studies, the phase 3 ALTTO study, failed to show a benefit for dual HER2 blockade. The same was true for the neoadjuvant Neo ALTTO study. Both studies failed to show an improvement in EFS or overall survival.
The current trial, CALGB 40601, a randomized phase 3 trial, assessed the effect of dual HER2 blockade consisting of trastuzumab and lapatinib added to paclitaxel on rates of pCR, and the researchers also looked at tumor and microenvironment molecular features. Dr. Krop noted that they had previously found that rates of pCR were numerically but not significantly increased with combination-blockade therapy; they had also found that the tumor molecular subtype and evidence of immune activation were independent factors affecting rates of pCR.
Dr. Krop presented results from a secondary analysis in which they evaluated the effects of treatment arm and gene expression–defined subgroups on EFS.
A total of 305 patients with stage II and III HER2+ breast cancer were randomized to receive 16 weeks of either paclitaxel plus trastuzumab alone or that combination with lapatinib as well before undergoing surgery. Evaluable information regarding EFS and RNA-sequencing gene expression was available for 265 patients.
“Despite the lack of significant benefits in other studies, we did demonstrate an improvement in EFS,” said Dr. Krop, noting that it was more pronounced in the subset of HR- patients (HR, 0.12; P = .0160).
EFS was also significantly longer among those who achieved pCR than those who didn’t (HR, 0.34; P = .0032).
In an exploratory analysis, Dr. Krop and his colleagues assessed whether there was a difference in rates of pCR by subtype, and they found that luminal A and luminal B did not have much of an effect on rates of pCR. However, patients with HER2-enriched disease who had a pCR had significantly better EFS than those who did not (HR, 0.14; 95% CI, 0.04-0.44; P less than .0001).
Conversely, patients with HER2-enriched disease who did not achieve pCR had a substantial risk of recurrence.
When looking at EFS by intrinsic subtype, the addition of lapatinib had the most effect on luminal A cancers. “This shows that there was some discordance between analyses by pCR and by long-term endpoint,” Dr. Krop said.
Finally, among gene expression signatures, only immune activation measured by an immunoglobulin G signature was associated with an improvement in EFS (HR, 0.70; 95% CI, 0.50-0.98; P = .04).
“These data are hypothesis generating and require validation,” Dr. Krop concluded. He added that a combined analysis of this trial, along with the Neo ALTTO and others, is planned.
SOURCE: Krop IE et al. SABCS 2017 Abstract GS3-02.
REPORTING FROM SABCS 2017
Key clinical point: In contrast to previous trials,
Major finding: Event-free survival was significantly longer in the dual HER2 blockade arm versus trastuzumab alone (HR, 0.35; 95% CI, 0.15-0.84; P = .013).
Data source: Phase 3 randomized trial that included 305 women with HER2+ breast cancer.
Disclosures: The study was sponsored by the Alliance for Clinical Trials in Oncology. Dr. Krop reports relationships with Genentech/Roche and MacroGenics.
Source: Krop IE et al. SABCS 2017 Abstract GS3-02.
FDA updates nilotinib product label outlining criteria for discontinuation
for patients who meet certain criteria. Nilotinib, a kinase inhibitor that blocks the BCR-ABL protein that promotes abnormal cell growth, was originally approved in 2007 and was indicated for use in patients with Philadelphia chromosome positive (Ph+) chronic myeloid leukemia (CML). In accordance with the new label update, patients who have early phase CML, have been using nilotinib for 3 years or more, and whose leukemia has responded to treatment according to a test that has received FDA marketing authorization, may be eligible to discontinue use of nilotinib.
The information that led to the FDA approved label update was based on two single-arm trials of patients with chronic phase Ph+ CML. The trial measured the length of time patients were able to discontinue use of nilotinib without leukemia returning, and who had entered treatment-free remission (TFR). In the first trial, among 190 newly diagnosed CML patients who discontinued taking nilotinib after using the drug for 3 or more years, 51.6% were still in TFR after about 1 year (48 weeks) and 48.9% were still in TFR after nearly 2 years (96 weeks). Similar results were seen in the second trial, among 126 patients, with 57.9% in TFR after about a year (48 weeks) and 53.2% in TFR after approximately 2 years (96 weeks).
An important element of these trials was regular monitoring of specific RNA information that specifies the level of BCR-ABL protein in the blood using a diagnostic test that has received FDA marketing authorization. Monitoring with a test that accurately detects the reductions of RNA information in the blood with accuracy and precision is critical in discontinuing the use of nilotinib. This monitoring will allow physicians to detect the first signs of relapse.
“Patients diagnosed with CML generally face a lifetime of treatment to keep their leukemia from growing or recurring,” said Richard Pazdur, MD, acting director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research. “Today’s approval shows that some patients may be able to stop treatment with Tasigna altogether if they are showing a strong response to therapy. While we welcome this progress in patient care, it’s important to note that any discontinuation of treatment still means patients must be regularly monitored for disease recurrence,” Dr. Pazdur said in the FDA statement.
Common side effects after discontinuing use of nilotinib include body aches and pain in the bones and extremities. Severe side effects of taking nilotinib can include myelosuppression, blockages in the heart and arteries, and inflammation of the pancreas. Severe liver damage can also occur.
Severe side effects typically associated with nilotinib administration occurred less frequently in patients who discontinued the drug. However, the long-term outcomes of patients discontinuing versus continuing treatment are unknown at this time, the FDA noted.
for patients who meet certain criteria. Nilotinib, a kinase inhibitor that blocks the BCR-ABL protein that promotes abnormal cell growth, was originally approved in 2007 and was indicated for use in patients with Philadelphia chromosome positive (Ph+) chronic myeloid leukemia (CML). In accordance with the new label update, patients who have early phase CML, have been using nilotinib for 3 years or more, and whose leukemia has responded to treatment according to a test that has received FDA marketing authorization, may be eligible to discontinue use of nilotinib.
The information that led to the FDA approved label update was based on two single-arm trials of patients with chronic phase Ph+ CML. The trial measured the length of time patients were able to discontinue use of nilotinib without leukemia returning, and who had entered treatment-free remission (TFR). In the first trial, among 190 newly diagnosed CML patients who discontinued taking nilotinib after using the drug for 3 or more years, 51.6% were still in TFR after about 1 year (48 weeks) and 48.9% were still in TFR after nearly 2 years (96 weeks). Similar results were seen in the second trial, among 126 patients, with 57.9% in TFR after about a year (48 weeks) and 53.2% in TFR after approximately 2 years (96 weeks).
An important element of these trials was regular monitoring of specific RNA information that specifies the level of BCR-ABL protein in the blood using a diagnostic test that has received FDA marketing authorization. Monitoring with a test that accurately detects the reductions of RNA information in the blood with accuracy and precision is critical in discontinuing the use of nilotinib. This monitoring will allow physicians to detect the first signs of relapse.
“Patients diagnosed with CML generally face a lifetime of treatment to keep their leukemia from growing or recurring,” said Richard Pazdur, MD, acting director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research. “Today’s approval shows that some patients may be able to stop treatment with Tasigna altogether if they are showing a strong response to therapy. While we welcome this progress in patient care, it’s important to note that any discontinuation of treatment still means patients must be regularly monitored for disease recurrence,” Dr. Pazdur said in the FDA statement.
Common side effects after discontinuing use of nilotinib include body aches and pain in the bones and extremities. Severe side effects of taking nilotinib can include myelosuppression, blockages in the heart and arteries, and inflammation of the pancreas. Severe liver damage can also occur.
Severe side effects typically associated with nilotinib administration occurred less frequently in patients who discontinued the drug. However, the long-term outcomes of patients discontinuing versus continuing treatment are unknown at this time, the FDA noted.
for patients who meet certain criteria. Nilotinib, a kinase inhibitor that blocks the BCR-ABL protein that promotes abnormal cell growth, was originally approved in 2007 and was indicated for use in patients with Philadelphia chromosome positive (Ph+) chronic myeloid leukemia (CML). In accordance with the new label update, patients who have early phase CML, have been using nilotinib for 3 years or more, and whose leukemia has responded to treatment according to a test that has received FDA marketing authorization, may be eligible to discontinue use of nilotinib.
The information that led to the FDA approved label update was based on two single-arm trials of patients with chronic phase Ph+ CML. The trial measured the length of time patients were able to discontinue use of nilotinib without leukemia returning, and who had entered treatment-free remission (TFR). In the first trial, among 190 newly diagnosed CML patients who discontinued taking nilotinib after using the drug for 3 or more years, 51.6% were still in TFR after about 1 year (48 weeks) and 48.9% were still in TFR after nearly 2 years (96 weeks). Similar results were seen in the second trial, among 126 patients, with 57.9% in TFR after about a year (48 weeks) and 53.2% in TFR after approximately 2 years (96 weeks).
An important element of these trials was regular monitoring of specific RNA information that specifies the level of BCR-ABL protein in the blood using a diagnostic test that has received FDA marketing authorization. Monitoring with a test that accurately detects the reductions of RNA information in the blood with accuracy and precision is critical in discontinuing the use of nilotinib. This monitoring will allow physicians to detect the first signs of relapse.
“Patients diagnosed with CML generally face a lifetime of treatment to keep their leukemia from growing or recurring,” said Richard Pazdur, MD, acting director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research. “Today’s approval shows that some patients may be able to stop treatment with Tasigna altogether if they are showing a strong response to therapy. While we welcome this progress in patient care, it’s important to note that any discontinuation of treatment still means patients must be regularly monitored for disease recurrence,” Dr. Pazdur said in the FDA statement.
Common side effects after discontinuing use of nilotinib include body aches and pain in the bones and extremities. Severe side effects of taking nilotinib can include myelosuppression, blockages in the heart and arteries, and inflammation of the pancreas. Severe liver damage can also occur.
Severe side effects typically associated with nilotinib administration occurred less frequently in patients who discontinued the drug. However, the long-term outcomes of patients discontinuing versus continuing treatment are unknown at this time, the FDA noted.
FDA approves raltegravir for newborns at risk for HIV-1 infection
The Food and Drug Administration has approved the use of raltegravir (Isentress), in combination with other antiretrovirals, for HIV-1 exposed newborns who weigh at least 2 kg and are at high risk for acquiring HIV-1 infection from their mothers, according to a press release from Merck, the manufacturer of Isentress.
“With this FDA approval, Isentress becomes the only integrase inhibitor approved in the U.S. for the treatment of HIV-1, in combination with other antiretroviral agents, for neonates weighing at least 2 kg,” Eliav Barr, MD, senior vice president, global clinical development, infectious diseases and vaccines, Merck Research Laboratories, said in a statement.
The study, IMPAACT P1110, was composed of two cohorts receiving different doses of raltegravir at different time intervals. The first cohort, of 16 newborns, received two single doses of raltegravir: the first within 48 hours of birth and the second between 7 and 10 days of age. The second cohort of 26 newborns received daily doses based on weight for 6 weeks. After observation for 24 weeks, all infants were HIV-1 negative.
Raltegravir does not cure HIV-1 infection or AIDS and severe side effects have been reported, including hypersensitivity reaction and toxic epidermal necrolysis, according to the Merck release.
Raltegravir should not be used in newborns and infants weighing under 2 kg. If raltegravir has been administered to the mother 2-24 hours before delivery, the first dose for the newborn should be given between 24 and 48 hours after birth.
The Food and Drug Administration has approved the use of raltegravir (Isentress), in combination with other antiretrovirals, for HIV-1 exposed newborns who weigh at least 2 kg and are at high risk for acquiring HIV-1 infection from their mothers, according to a press release from Merck, the manufacturer of Isentress.
“With this FDA approval, Isentress becomes the only integrase inhibitor approved in the U.S. for the treatment of HIV-1, in combination with other antiretroviral agents, for neonates weighing at least 2 kg,” Eliav Barr, MD, senior vice president, global clinical development, infectious diseases and vaccines, Merck Research Laboratories, said in a statement.
The study, IMPAACT P1110, was composed of two cohorts receiving different doses of raltegravir at different time intervals. The first cohort, of 16 newborns, received two single doses of raltegravir: the first within 48 hours of birth and the second between 7 and 10 days of age. The second cohort of 26 newborns received daily doses based on weight for 6 weeks. After observation for 24 weeks, all infants were HIV-1 negative.
Raltegravir does not cure HIV-1 infection or AIDS and severe side effects have been reported, including hypersensitivity reaction and toxic epidermal necrolysis, according to the Merck release.
Raltegravir should not be used in newborns and infants weighing under 2 kg. If raltegravir has been administered to the mother 2-24 hours before delivery, the first dose for the newborn should be given between 24 and 48 hours after birth.
The Food and Drug Administration has approved the use of raltegravir (Isentress), in combination with other antiretrovirals, for HIV-1 exposed newborns who weigh at least 2 kg and are at high risk for acquiring HIV-1 infection from their mothers, according to a press release from Merck, the manufacturer of Isentress.
“With this FDA approval, Isentress becomes the only integrase inhibitor approved in the U.S. for the treatment of HIV-1, in combination with other antiretroviral agents, for neonates weighing at least 2 kg,” Eliav Barr, MD, senior vice president, global clinical development, infectious diseases and vaccines, Merck Research Laboratories, said in a statement.
The study, IMPAACT P1110, was composed of two cohorts receiving different doses of raltegravir at different time intervals. The first cohort, of 16 newborns, received two single doses of raltegravir: the first within 48 hours of birth and the second between 7 and 10 days of age. The second cohort of 26 newborns received daily doses based on weight for 6 weeks. After observation for 24 weeks, all infants were HIV-1 negative.
Raltegravir does not cure HIV-1 infection or AIDS and severe side effects have been reported, including hypersensitivity reaction and toxic epidermal necrolysis, according to the Merck release.
Raltegravir should not be used in newborns and infants weighing under 2 kg. If raltegravir has been administered to the mother 2-24 hours before delivery, the first dose for the newborn should be given between 24 and 48 hours after birth.
Management of Isolated Greater Tuberosity Fractures: A Systematic Review
Take-Home Points
- Fractures of the greater tuberosity are often mismanaged.
- Comprehension of greater tuberosity fractures involves classification into nonoperative and operative treatment, displacement >5mm or <5 mm, and open vs arthroscopic surgery.
- Nearly a third of patients may suffer concomitant anterior glenohumeral instability.
- Stiffness is the most common postoperative complication.
- Surgery is associated with high patient satisfaction and low rates of complications and reoperations.
Although proximal humerus fractures are common in the elderly, isolated fractures of the greater tuberosity occur less often. Management depends on several factors, including fracture pattern and displacement.1,2 Nondisplaced fractures are often successfully managed with sling immobilization and early range of motion.3,4 Although surgical intervention improves outcomes in displaced greater tuberosity fractures, the ideal surgical treatment is less clear.5
Displaced greater tuberosity fractures may require surgery for prevention of subacromial impingement and range-of-motion deficits.2 Superior fracture displacement results in decreased shoulder abduction, and posterior displacement can limit external rotation.6 Although the greater tuberosity can displace in any direction, posterosuperior displacement has the worst outcomes.1 The exact surgery-warranting displacement amount ranges from 3 mm to 10 mm but is yet to be clearly elucidated.5,6 Less displacement is tolerated by young overhead athletes, and more displacement by older less active patients.5,7,8 Surgical options for isolated greater tuberosity fractures include fragment excision, open reduction and internal fixation (ORIF), closed reduction with percutaneous fixation, and arthroscopically assisted reduction with internal fixation.3,9,10
We conducted a study to determine the management patterns for isolated greater tuberosity fractures. We hypothesized that greater tuberosity fractures displaced <5 mm may be managed nonoperatively and that greater tuberosity fractures displaced >5 mm require surgical fixation.
Methods
Search Strategy
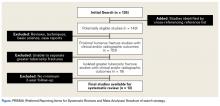
We performed this systematic review according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist11 and registered it (CRD42014010691) with the PROSPERO international prospective register of systematic reviews. Literature searches using the PubMed/Medline database and the Cochrane Central Register of Clinical Trials were completed in August 2014. There were no date or year restrictions. Key words were used to capture all English- language studies with level I to IV evidence (Oxford Centre for Evidence-Based Medicine) and reported clinical or radiographic outcomes. Initial exclusion criteria were cadaveric, biomechanical, histologic, and kinematic results. An electronic search algorithm with key words and a series of NOT phrases was designed to match our exclusion criteria:
((((((((((((((((((((((((((((((((((((((((((((((((((greater[Title/Abstract]) AND tuberosity [Title/Abstract] OR tubercle [Title/Abstract]) AND fracture[Title/Abstract]) AND proximal[Title/Abstract] AND (English[lang]))) NOT intramedullary[Title] AND (English[lang]))) NOT nonunion[Title] AND (English[lang]))) NOT malunion[Title] AND (English[lang]))) NOT biomechanical[Title/Abstract] AND (English[lang]))) NOT cadaveric[Title/Abstract] AND (English[lang]))) NOT cadaver[Title/Abstract] AND (English[lang]))) NOT ((basic[Title/Abstract]) AND science[Title/Abstract] AND (English[lang])) AND (English[lang]))) NOT revision[Title] AND (English[lang]))) NOT pediatric[Title] AND (English[lang]))) NOT physeal[Title] AND (English[lang]))) NOT children[Title] AND (English[lang]))) NOT instability[Title] AND (English[lang]))) NOT imaging[Title])) NOT salter[Title])) NOT physis[Title])) NOT shaft[Title])) NOT distal[Title])) NOT clavicle[Title])) NOT scapula[Title])) NOT ((diaphysis[Title]) AND diaphyseal[Title]))) NOT infection[Title])) NOT laboratory[Title/Abstract])) NOT metastatic[Title/Abstract])) NOT (((((((malignancy[Title/Abstract]) OR malignant[Title/Abstract]) OR tumor[Title/Abstract]) OR oncologic[Title/Abstract]) OR cyst[Title/Abstract]) OR aneurysmal[Title/Abstract]) OR unicameral[Title/Abstract]).
Study Selection
Data Extraction
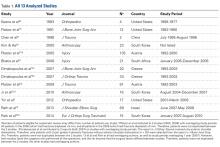
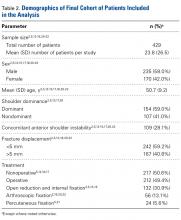
We extracted data from the 13 studies that met the eligibility criteria. Details of study design, sample size, and patient demographics, including age, sex, and hand dominance, were recorded, as were mechanism of injury and concomitant anterior shoulder instability. To capture the most patients, we noted radiographic fracture displacement categorically rather than continuously; patients were divided into 2 displacement groups (<5 mm, >5 mm). Most studies did not define degree of comminution or specific direction of displacement per fracture, so these variables were not included in the data analysis. Nonoperative management and operative management were studied. We abstracted surgical factors, such as approach, method, fixation type (screws or sutures), and technique (suture anchors or transosseous tunnels). Clinical outcomes included physical examination findings, functional assessment results (patient satisfaction; Constant and University of California Los Angeles [UCLA] shoulder scores), and the number of revisions. Radiologic outcomes, retrieved from radiographs or computed tomography scans, focused on loss of reduction (as determined by the respective authors), malunion, nonunion, and heterotopic ossification. Each study’s methodologic quality and bias were evaluated with the 15-item Modified Coleman Methodology Score (MCMS), which was described by Cowan and colleagues.23 The MCMS has been used to assess randomized and nonrandomized patient trials.24,25 Its scaled potential score ranges from 0 to 100 (85-100, excellent; 70-84, good; 55-69, fair; <55, poor).
Statistical Analysis
We report our data as weighted means (SDs). A mean was calculated for each study that reported a respective data point, and each mean was then weighed according to its study sample size. This calculation was performed by multiplying a study’s individual mean by the number of patients enrolled in that study and dividing the sum of these weighted data points by the number of eligible patients in all relevant studies. The result was that the nonweighted means from studies with smaller sample sizes did not carry as much weight as the nonweighted means from larger studies. We compared 3 paired groups: treatment type (nonoperative vs operative), fracture displacement amount (<5 mm vs >5 mm), and surgery type (open vs arthroscopic). Regarding all patient, surgery, and outcomes data, unpaired Student t tests were used for continuous variables and 2-tailed Fisher exact tests for categorical variables with α = 0.05 (SPSS Version 18; IBM).
Results
Postoperative physical examination findings were underreported so that surgical groups could be compared. Of all the surgical studies, 4 reported postoperative forward elevation (mean, 160°; SD, 9.8°) and external rotation (mean, 46.4°; SD 26.3°).14,15,18,22 No malunions and only 1 nonunion were reported in all 13 studies. No deaths or other serious medical complications were reported. Patients with anterior instability more often underwent surgery than were treated nonoperatively (39.2% vs 12.0%; P < .01) and more often had fractures displaced >5 mm than <5 mm (44.3% vs 14.5%; P < .01).
Fisher exact tests were used to perform isolated comparisons of screws and sutures as well as suture anchors and transosseous tunnels. Patients with screw fixation were significantly (P = .051) less likely to require reoperation (0/56; 0%) than patients with suture fixation (8/100; 8.0%). Screw fixation also led to significantly less stiffness (0% vs 12.0%; P < .01) but trended toward a higher rate of superficial infection (3.6% vs 0%; P = .13). There was no statistical difference in nerve injury rates between screws and sutures (1.8% vs 3.0%; P = 1.0). There were no significant differences in reoperations, stiffness, superficial infections, or nerve injuries between suture anchor and transosseous tunnel constructs.
For all 13 studies, mean (SD) MCMS was 41.1 (8.6).
Discussion
Five percent of all fractures involve the proximal humerus, and 20% of proximal humerus fractures are isolated greater tuberosity fractures.26,27 In his classic 1970 article, Neer6 formulated the 4-part proximal humerus fracture classification and defined greater tuberosity fracture “parts” using the same criteria as for other fracture “parts.” Neer6 recommended nonoperative management for isolated greater tuberosity fractures displaced <1 cm but did not present evidence corroborating his recommendation. More recent cutoffs for nonoperative management include 5 mm (general population) and 3 mm (athletes).7,17
In the present systematic review of greater tuberosity fractures, 3 separate comparisons were made: treatment type (nonoperative vs operative), fracture displacement amount (<5 mm vs >5 mm), and surgery type (open vs arthroscopic).
Treatment Type. Only 4 studies reported data on nonoperative treatment outcomes.5,12,16,17 Of these 4 studies, 2 found successful outcomes for fractures displaced <5 mm.12,17 Platzer and colleagues17 found good or excellent results in 97% of 135 shoulders after 4 years. Good results were defined with shoulder scores of ≥80 (Constant), <8 (Vienna), and >28 (UCLA), and excellent results were defined with maximum scores on 2 of the 3 systems. Platzer and colleagues17 also found nonsignificantly worse shoulder scores with superior displacement of 3 mm to 5 mm and recommended surgery for overhead athletes in this group. Rath and colleagues12 described a successful 3-phase rehabilitation protocol of sling immobilization for 3 weeks, pendulum exercises for 3 weeks, and active exercises thereafter. By an average of 31 months, patient satisfaction scores improved to 9.5 from 4.2 (10-point scale), though the authors cautioned that pain and decreased motion lasted 8 months on average. Conservative treatment was far less successful in the 2 studies of fractures displaced >5 mm.5,16 Keene and colleagues16 reported unsatisfactory results in all 4 patients with fractures displaced >1.5 cm. In a study separate from their 2005 analysis,17 Platzer and colleagues5 in 2008 evaluated displaced fractures and found function and patient satisfaction were inferior after nonoperative treatment than after surgery. The studies by Keene and colleagues16 and Platzer and colleagues5 support the finding of an overall lower patient satisfaction rate in nonoperative patients.
Fracture Displacement Amount. Only 2 arthroscopic studies and no open studies addressed surgery for fractures displaced <5 mm. Fewer than 16% of these fractures were managed operatively, and <1% required reoperation. By contrast, almost all fractures displaced >5 mm were managed operatively, and 3.6% required reoperation. Radiographic loss of reduction was more common in fractures displaced <5 mm, primarily because they were managed without fixation. Radiographic loss of reduction was reported in only 9 operatively treated patients, none of whom was symptomatic enough to require another surgery.5 Reoperations were most commonly performed for stiffness, which itself was significantly more common in fractures displaced >5 mm. Bhatia and colleagues14 reported the highest reoperation rate (14.3%; 3/21), but they studied more complex, comminuted fractures of the greater tuberosity. Two of their 3 reoperations were biceps tenodeses for inflamed, stiff tenosynovitis, and the third patient had a foreign body giant cell reaction to suture material. Fewer than 1% of patients with operatively managed displaced fractures required revision ORIF, and <2% developed a superficial infection or postoperative nerve palsy.19,22 For displaced greater tuberosity fractures, surgery is highly successful overall, complication rates are very low, and 90% of patients report being satisfied.
Surgery Type. Patients were divided into 2 groups. In the nonarthroscopic group, open and percutaneous approaches were used. All studies that described a percutaneous approach used screw fixation5,21; in addition, 32 patients were treated with screws through an open approach.2,5 The other open and arthroscopic studies used suture fixation. Interestingly, no studies reported on clinical outcomes of fragment excision. There were no statistically significant differences in rates of reoperation, stiffness, infection, or neurologic injury between the arthroscopic and nonarthroscopic groups. Patient satisfaction scores were slightly higher in the nonarthroscopic group (91.0% vs 87.8%), but the difference was not statistically significant.
With surgical techniques isolated, there were no significant differences between suture anchors and transosseous tunnel constructs, but screws performed significantly better than suture techniques. Compared with suture fixation, screw fixation led to significantly fewer cases of stiffness and reoperation, which suggests surgeons need to give screws more consideration in the operative management of these fractures. However, the number of patients treated with screws was smaller than the number treated with suture fixation; it is possible the differences between these cohorts would be eliminated if there were more patients in the screw cohort. In addition, screw fixation was universally performed with an open or percutaneous approach and trended toward a higher infection rate. As screw and suture techniques have low rates of complications and reoperations, we recommend leaving fixation choice to the surgeon.
Anterior shoulder instability has been associated with greater tuberosity fractures.1,8,19 The supraspinatus, infraspinatus, and teres minor muscles all insert into the greater tuberosity and resist anterior translation of the proximal humerus. Loss of this dynamic muscle stabilization is amplified by tuberosity fracture displacement: Anterior shoulder instability was significantly more common in fractures displaced >5 mm (44.3%) vs <5 mm (14.5%). In turn, glenohumeral instability was more common in patients treated with surgery, specifically open surgery, because displaced fractures may not be as easily accessed with arthroscopic techniques. No studies reported concomitant labral repair or capsular plication techniques.
This systematic review was limited by the studies analyzed. All but 1 study5 had level IV evidence. Mean (SD) MCMS was 41.8 (8.6). Any MCMS score <54 indicates a poor methodology level, but this scoring system is designed for randomized controlled trials,23 and there were none in this study. Physical examination findings, such as range of motion, were underreported. In addition, radiographic parameters were not consistently described but rather were determined by the respective authors’ subjective interpretations of malunion, nonunion, and loss of reduction. Publication bias is present in that we excluded non- English language studies and medical conference abstracts and may have omitted potentially eligible studies not discoverable with our search methodology. Performance bias is a factor in any systematic review with multiple surgeons and wide variation in surgical technique.
Conclusion
Greater tuberosity fractures displaced <5 mm may be safely managed nonoperatively, as there are no reports of nonoperatively managed fractures that subsequently required surgery. Nonoperative treatment was initially associated with low patient satisfaction, but only because displaced fractures were conservatively managed in early studies.5,16 Fractures displaced >5 mm respond well to operative fixation with screws, suture anchors, or transosseous suture tunnels. Stiffness is the most common postoperative complication (<6%), followed by heterotopic ossification, transient neurapraxias, and superficial infection. There are no discernible differences in outcome between open and arthroscopic techniques, but screw fixation may lead to significantly fewer cases of stiffness and reoperation in comparison with suture constructs.
1. Verdano MA, Aliani D, Pellegrini A, Baudi P, Pedrazzi G, Ceccarelli F. Isolated fractures of the greater tuberosity in proximal humerus: does the direction of displacement influence functional outcome? An analysis of displacement in greater tuberosity fractures. Acta Biomed. 2013;84(3):219-228.
2. Yin B, Moen TC, Thompson SA, Bigliani LU, Ahmad CS, Levine WN. Operative treatment of isolated greater tuberosity fractures: retrospective review of clinical and functional outcomes. Orthopedics. 2012;35(6):e807-e814.
3. Green A, Izzi J. Isolated fractures of the greater tuberosity of the proximal humerus. J Shoulder Elbow Surg. 2003;12(6):641-649.
4. Norouzi M, Naderi MN, Komasi MH, Sharifzadeh SR, Shahrezaei M, Eajazi A. Clinical results of using the proximal humeral internal locking system plate for internal fixation of displaced proximal humeral fractures. Am J Orthop. 2012;41(5):E64-E68.
5. Platzer P, Thalhammer G, Oberleitner G, et al. Displaced fractures of the greater tuberosity: a comparison of operative and nonoperative treatment. J Trauma. 2008;65(4):843-848.
6. Neer CS. Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
7. Park TS, Choi IY, Kim YH, Park MR, Shon JH, Kim SI. A new suggestion for the treatment of minimally displaced fractures of the greater tuberosity of the proximal humerus. Bull Hosp Jt Dis. 1997;56(3):171-176.
8. McLaughlin HL. Dislocation of the shoulder with tuberosity fracture. Surg Clin North Am. 1963;43:1615-1620.
9. DeBottis D, Anavian J, Green A. Surgical management of isolated greater tuberosity fractures of the proximal humerus. Orthop Clin North Am. 2014;45(2):207-218.
10. Monga P, Verma R, Sharma VK. Closed reduction and external fixation for displaced proximal humeral fractures. J Orthop Surg (Hong Kong). 2009;17(2):142-145.
11. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012.
12. Rath E, Alkrinawi N, Levy O, Debbi R, Amar E, Atoun E. Minimally displaced fractures of the greater tuberosity: outcome of non-operative treatment. J Shoulder Elbow Surg. 2013;22(10):e8-e11.
13. Dimakopoulos P, Panagopoulos A, Kasimatis G. Transosseous suture fixation of proximal humeral fractures. J Bone Joint Surg Am. 2007;89(8):1700-1709.
14. Bhatia DN, van Rooyen KS, Toit du DF, de Beer JF. Surgical treatment of comminuted, displaced fractures of the greater tuberosity of the proximal humerus: a new technique of double-row suture-anchor fixation and long-term results. Injury. 2006;37(10):946-952.
15. Flatow EL, Cuomo F, Maday MG, Miller SR, McIlveen SJ, Bigliani LU. Open reduction and internal fixation of two-part displaced fractures of the greater tuberosity of the proximal part of the humerus. J Bone Joint Surg Am. 1991;73(8):1213-1218.
16. Keene JS, Huizenga RE, Engber WD, Rogers SC. Proximal humeral fractures: a correlation of residual deformity with long-term function. Orthopedics. 1983;6(2):173-178.
17. Platzer P, Kutscha-Lissberg F, Lehr S, Vecsei V, Gaebler C. The influence of displacement on shoulder function in patients with minimally displaced fractures of the greater tuberosity. Injury. 2005;36(10):1185-1189.
18. Park SE, Ji JH, Shafi M, Jung JJ, Gil HJ, Lee HH. Arthroscopic management of occult greater tuberosity fracture of the shoulder. Eur J Orthop Surg Traumatol. 2014;24(4):475-482.
19. Dimakopoulos P, Panagopoulos A, Kasimatis G, Syggelos SA, Lambiris E. Anterior traumatic shoulder dislocation associated with displaced greater tuberosity fracture: the necessity of operative treatment. J Orthop Trauma. 2007;21(2):104-112.
20. Kim SH, Ha KI. Arthroscopic treatment of symptomatic shoulders with minimally displaced greater tuberosity fracture. Arthroscopy. 2000;16(7):695-700.
21. Chen CY, Chao EK, Tu YK, Ueng SW, Shih CH. Closed management and percutaneous fixation of unstable proximal humerus fractures. J Trauma. 1998;45(6):1039-1045.
22. Ji JH, Shafi M, Song IS, Kim YY, McFarland EG, Moon CY. Arthroscopic fixation technique for comminuted, displaced greater tuberosity fracture. Arthroscopy. 2010;26(5):600-609.
23. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
24. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
25. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
26. Chun JM, Groh GI, Rockwood CA. Two-part fractures of the proximal humerus. J Shoulder Elbow Surg. 1994;3(5):273-287.
27. Gruson KI, Ruchelsman DE, Tejwani NC. Isolated tuberosity fractures of the proximal humeral: current concepts. Injury. 2008;39(3):284-298.
Take-Home Points
- Fractures of the greater tuberosity are often mismanaged.
- Comprehension of greater tuberosity fractures involves classification into nonoperative and operative treatment, displacement >5mm or <5 mm, and open vs arthroscopic surgery.
- Nearly a third of patients may suffer concomitant anterior glenohumeral instability.
- Stiffness is the most common postoperative complication.
- Surgery is associated with high patient satisfaction and low rates of complications and reoperations.
Although proximal humerus fractures are common in the elderly, isolated fractures of the greater tuberosity occur less often. Management depends on several factors, including fracture pattern and displacement.1,2 Nondisplaced fractures are often successfully managed with sling immobilization and early range of motion.3,4 Although surgical intervention improves outcomes in displaced greater tuberosity fractures, the ideal surgical treatment is less clear.5
Displaced greater tuberosity fractures may require surgery for prevention of subacromial impingement and range-of-motion deficits.2 Superior fracture displacement results in decreased shoulder abduction, and posterior displacement can limit external rotation.6 Although the greater tuberosity can displace in any direction, posterosuperior displacement has the worst outcomes.1 The exact surgery-warranting displacement amount ranges from 3 mm to 10 mm but is yet to be clearly elucidated.5,6 Less displacement is tolerated by young overhead athletes, and more displacement by older less active patients.5,7,8 Surgical options for isolated greater tuberosity fractures include fragment excision, open reduction and internal fixation (ORIF), closed reduction with percutaneous fixation, and arthroscopically assisted reduction with internal fixation.3,9,10
We conducted a study to determine the management patterns for isolated greater tuberosity fractures. We hypothesized that greater tuberosity fractures displaced <5 mm may be managed nonoperatively and that greater tuberosity fractures displaced >5 mm require surgical fixation.
Methods
Search Strategy
We performed this systematic review according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist11 and registered it (CRD42014010691) with the PROSPERO international prospective register of systematic reviews. Literature searches using the PubMed/Medline database and the Cochrane Central Register of Clinical Trials were completed in August 2014. There were no date or year restrictions. Key words were used to capture all English- language studies with level I to IV evidence (Oxford Centre for Evidence-Based Medicine) and reported clinical or radiographic outcomes. Initial exclusion criteria were cadaveric, biomechanical, histologic, and kinematic results. An electronic search algorithm with key words and a series of NOT phrases was designed to match our exclusion criteria:
((((((((((((((((((((((((((((((((((((((((((((((((((greater[Title/Abstract]) AND tuberosity [Title/Abstract] OR tubercle [Title/Abstract]) AND fracture[Title/Abstract]) AND proximal[Title/Abstract] AND (English[lang]))) NOT intramedullary[Title] AND (English[lang]))) NOT nonunion[Title] AND (English[lang]))) NOT malunion[Title] AND (English[lang]))) NOT biomechanical[Title/Abstract] AND (English[lang]))) NOT cadaveric[Title/Abstract] AND (English[lang]))) NOT cadaver[Title/Abstract] AND (English[lang]))) NOT ((basic[Title/Abstract]) AND science[Title/Abstract] AND (English[lang])) AND (English[lang]))) NOT revision[Title] AND (English[lang]))) NOT pediatric[Title] AND (English[lang]))) NOT physeal[Title] AND (English[lang]))) NOT children[Title] AND (English[lang]))) NOT instability[Title] AND (English[lang]))) NOT imaging[Title])) NOT salter[Title])) NOT physis[Title])) NOT shaft[Title])) NOT distal[Title])) NOT clavicle[Title])) NOT scapula[Title])) NOT ((diaphysis[Title]) AND diaphyseal[Title]))) NOT infection[Title])) NOT laboratory[Title/Abstract])) NOT metastatic[Title/Abstract])) NOT (((((((malignancy[Title/Abstract]) OR malignant[Title/Abstract]) OR tumor[Title/Abstract]) OR oncologic[Title/Abstract]) OR cyst[Title/Abstract]) OR aneurysmal[Title/Abstract]) OR unicameral[Title/Abstract]).
Study Selection
Data Extraction
We extracted data from the 13 studies that met the eligibility criteria. Details of study design, sample size, and patient demographics, including age, sex, and hand dominance, were recorded, as were mechanism of injury and concomitant anterior shoulder instability. To capture the most patients, we noted radiographic fracture displacement categorically rather than continuously; patients were divided into 2 displacement groups (<5 mm, >5 mm). Most studies did not define degree of comminution or specific direction of displacement per fracture, so these variables were not included in the data analysis. Nonoperative management and operative management were studied. We abstracted surgical factors, such as approach, method, fixation type (screws or sutures), and technique (suture anchors or transosseous tunnels). Clinical outcomes included physical examination findings, functional assessment results (patient satisfaction; Constant and University of California Los Angeles [UCLA] shoulder scores), and the number of revisions. Radiologic outcomes, retrieved from radiographs or computed tomography scans, focused on loss of reduction (as determined by the respective authors), malunion, nonunion, and heterotopic ossification. Each study’s methodologic quality and bias were evaluated with the 15-item Modified Coleman Methodology Score (MCMS), which was described by Cowan and colleagues.23 The MCMS has been used to assess randomized and nonrandomized patient trials.24,25 Its scaled potential score ranges from 0 to 100 (85-100, excellent; 70-84, good; 55-69, fair; <55, poor).
Statistical Analysis
We report our data as weighted means (SDs). A mean was calculated for each study that reported a respective data point, and each mean was then weighed according to its study sample size. This calculation was performed by multiplying a study’s individual mean by the number of patients enrolled in that study and dividing the sum of these weighted data points by the number of eligible patients in all relevant studies. The result was that the nonweighted means from studies with smaller sample sizes did not carry as much weight as the nonweighted means from larger studies. We compared 3 paired groups: treatment type (nonoperative vs operative), fracture displacement amount (<5 mm vs >5 mm), and surgery type (open vs arthroscopic). Regarding all patient, surgery, and outcomes data, unpaired Student t tests were used for continuous variables and 2-tailed Fisher exact tests for categorical variables with α = 0.05 (SPSS Version 18; IBM).
Results
Postoperative physical examination findings were underreported so that surgical groups could be compared. Of all the surgical studies, 4 reported postoperative forward elevation (mean, 160°; SD, 9.8°) and external rotation (mean, 46.4°; SD 26.3°).14,15,18,22 No malunions and only 1 nonunion were reported in all 13 studies. No deaths or other serious medical complications were reported. Patients with anterior instability more often underwent surgery than were treated nonoperatively (39.2% vs 12.0%; P < .01) and more often had fractures displaced >5 mm than <5 mm (44.3% vs 14.5%; P < .01).
Fisher exact tests were used to perform isolated comparisons of screws and sutures as well as suture anchors and transosseous tunnels. Patients with screw fixation were significantly (P = .051) less likely to require reoperation (0/56; 0%) than patients with suture fixation (8/100; 8.0%). Screw fixation also led to significantly less stiffness (0% vs 12.0%; P < .01) but trended toward a higher rate of superficial infection (3.6% vs 0%; P = .13). There was no statistical difference in nerve injury rates between screws and sutures (1.8% vs 3.0%; P = 1.0). There were no significant differences in reoperations, stiffness, superficial infections, or nerve injuries between suture anchor and transosseous tunnel constructs.
For all 13 studies, mean (SD) MCMS was 41.1 (8.6).
Discussion
Five percent of all fractures involve the proximal humerus, and 20% of proximal humerus fractures are isolated greater tuberosity fractures.26,27 In his classic 1970 article, Neer6 formulated the 4-part proximal humerus fracture classification and defined greater tuberosity fracture “parts” using the same criteria as for other fracture “parts.” Neer6 recommended nonoperative management for isolated greater tuberosity fractures displaced <1 cm but did not present evidence corroborating his recommendation. More recent cutoffs for nonoperative management include 5 mm (general population) and 3 mm (athletes).7,17
In the present systematic review of greater tuberosity fractures, 3 separate comparisons were made: treatment type (nonoperative vs operative), fracture displacement amount (<5 mm vs >5 mm), and surgery type (open vs arthroscopic).
Treatment Type. Only 4 studies reported data on nonoperative treatment outcomes.5,12,16,17 Of these 4 studies, 2 found successful outcomes for fractures displaced <5 mm.12,17 Platzer and colleagues17 found good or excellent results in 97% of 135 shoulders after 4 years. Good results were defined with shoulder scores of ≥80 (Constant), <8 (Vienna), and >28 (UCLA), and excellent results were defined with maximum scores on 2 of the 3 systems. Platzer and colleagues17 also found nonsignificantly worse shoulder scores with superior displacement of 3 mm to 5 mm and recommended surgery for overhead athletes in this group. Rath and colleagues12 described a successful 3-phase rehabilitation protocol of sling immobilization for 3 weeks, pendulum exercises for 3 weeks, and active exercises thereafter. By an average of 31 months, patient satisfaction scores improved to 9.5 from 4.2 (10-point scale), though the authors cautioned that pain and decreased motion lasted 8 months on average. Conservative treatment was far less successful in the 2 studies of fractures displaced >5 mm.5,16 Keene and colleagues16 reported unsatisfactory results in all 4 patients with fractures displaced >1.5 cm. In a study separate from their 2005 analysis,17 Platzer and colleagues5 in 2008 evaluated displaced fractures and found function and patient satisfaction were inferior after nonoperative treatment than after surgery. The studies by Keene and colleagues16 and Platzer and colleagues5 support the finding of an overall lower patient satisfaction rate in nonoperative patients.
Fracture Displacement Amount. Only 2 arthroscopic studies and no open studies addressed surgery for fractures displaced <5 mm. Fewer than 16% of these fractures were managed operatively, and <1% required reoperation. By contrast, almost all fractures displaced >5 mm were managed operatively, and 3.6% required reoperation. Radiographic loss of reduction was more common in fractures displaced <5 mm, primarily because they were managed without fixation. Radiographic loss of reduction was reported in only 9 operatively treated patients, none of whom was symptomatic enough to require another surgery.5 Reoperations were most commonly performed for stiffness, which itself was significantly more common in fractures displaced >5 mm. Bhatia and colleagues14 reported the highest reoperation rate (14.3%; 3/21), but they studied more complex, comminuted fractures of the greater tuberosity. Two of their 3 reoperations were biceps tenodeses for inflamed, stiff tenosynovitis, and the third patient had a foreign body giant cell reaction to suture material. Fewer than 1% of patients with operatively managed displaced fractures required revision ORIF, and <2% developed a superficial infection or postoperative nerve palsy.19,22 For displaced greater tuberosity fractures, surgery is highly successful overall, complication rates are very low, and 90% of patients report being satisfied.
Surgery Type. Patients were divided into 2 groups. In the nonarthroscopic group, open and percutaneous approaches were used. All studies that described a percutaneous approach used screw fixation5,21; in addition, 32 patients were treated with screws through an open approach.2,5 The other open and arthroscopic studies used suture fixation. Interestingly, no studies reported on clinical outcomes of fragment excision. There were no statistically significant differences in rates of reoperation, stiffness, infection, or neurologic injury between the arthroscopic and nonarthroscopic groups. Patient satisfaction scores were slightly higher in the nonarthroscopic group (91.0% vs 87.8%), but the difference was not statistically significant.
With surgical techniques isolated, there were no significant differences between suture anchors and transosseous tunnel constructs, but screws performed significantly better than suture techniques. Compared with suture fixation, screw fixation led to significantly fewer cases of stiffness and reoperation, which suggests surgeons need to give screws more consideration in the operative management of these fractures. However, the number of patients treated with screws was smaller than the number treated with suture fixation; it is possible the differences between these cohorts would be eliminated if there were more patients in the screw cohort. In addition, screw fixation was universally performed with an open or percutaneous approach and trended toward a higher infection rate. As screw and suture techniques have low rates of complications and reoperations, we recommend leaving fixation choice to the surgeon.
Anterior shoulder instability has been associated with greater tuberosity fractures.1,8,19 The supraspinatus, infraspinatus, and teres minor muscles all insert into the greater tuberosity and resist anterior translation of the proximal humerus. Loss of this dynamic muscle stabilization is amplified by tuberosity fracture displacement: Anterior shoulder instability was significantly more common in fractures displaced >5 mm (44.3%) vs <5 mm (14.5%). In turn, glenohumeral instability was more common in patients treated with surgery, specifically open surgery, because displaced fractures may not be as easily accessed with arthroscopic techniques. No studies reported concomitant labral repair or capsular plication techniques.
This systematic review was limited by the studies analyzed. All but 1 study5 had level IV evidence. Mean (SD) MCMS was 41.8 (8.6). Any MCMS score <54 indicates a poor methodology level, but this scoring system is designed for randomized controlled trials,23 and there were none in this study. Physical examination findings, such as range of motion, were underreported. In addition, radiographic parameters were not consistently described but rather were determined by the respective authors’ subjective interpretations of malunion, nonunion, and loss of reduction. Publication bias is present in that we excluded non- English language studies and medical conference abstracts and may have omitted potentially eligible studies not discoverable with our search methodology. Performance bias is a factor in any systematic review with multiple surgeons and wide variation in surgical technique.
Conclusion
Greater tuberosity fractures displaced <5 mm may be safely managed nonoperatively, as there are no reports of nonoperatively managed fractures that subsequently required surgery. Nonoperative treatment was initially associated with low patient satisfaction, but only because displaced fractures were conservatively managed in early studies.5,16 Fractures displaced >5 mm respond well to operative fixation with screws, suture anchors, or transosseous suture tunnels. Stiffness is the most common postoperative complication (<6%), followed by heterotopic ossification, transient neurapraxias, and superficial infection. There are no discernible differences in outcome between open and arthroscopic techniques, but screw fixation may lead to significantly fewer cases of stiffness and reoperation in comparison with suture constructs.
Take-Home Points
- Fractures of the greater tuberosity are often mismanaged.
- Comprehension of greater tuberosity fractures involves classification into nonoperative and operative treatment, displacement >5mm or <5 mm, and open vs arthroscopic surgery.
- Nearly a third of patients may suffer concomitant anterior glenohumeral instability.
- Stiffness is the most common postoperative complication.
- Surgery is associated with high patient satisfaction and low rates of complications and reoperations.
Although proximal humerus fractures are common in the elderly, isolated fractures of the greater tuberosity occur less often. Management depends on several factors, including fracture pattern and displacement.1,2 Nondisplaced fractures are often successfully managed with sling immobilization and early range of motion.3,4 Although surgical intervention improves outcomes in displaced greater tuberosity fractures, the ideal surgical treatment is less clear.5
Displaced greater tuberosity fractures may require surgery for prevention of subacromial impingement and range-of-motion deficits.2 Superior fracture displacement results in decreased shoulder abduction, and posterior displacement can limit external rotation.6 Although the greater tuberosity can displace in any direction, posterosuperior displacement has the worst outcomes.1 The exact surgery-warranting displacement amount ranges from 3 mm to 10 mm but is yet to be clearly elucidated.5,6 Less displacement is tolerated by young overhead athletes, and more displacement by older less active patients.5,7,8 Surgical options for isolated greater tuberosity fractures include fragment excision, open reduction and internal fixation (ORIF), closed reduction with percutaneous fixation, and arthroscopically assisted reduction with internal fixation.3,9,10
We conducted a study to determine the management patterns for isolated greater tuberosity fractures. We hypothesized that greater tuberosity fractures displaced <5 mm may be managed nonoperatively and that greater tuberosity fractures displaced >5 mm require surgical fixation.
Methods
Search Strategy
We performed this systematic review according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist11 and registered it (CRD42014010691) with the PROSPERO international prospective register of systematic reviews. Literature searches using the PubMed/Medline database and the Cochrane Central Register of Clinical Trials were completed in August 2014. There were no date or year restrictions. Key words were used to capture all English- language studies with level I to IV evidence (Oxford Centre for Evidence-Based Medicine) and reported clinical or radiographic outcomes. Initial exclusion criteria were cadaveric, biomechanical, histologic, and kinematic results. An electronic search algorithm with key words and a series of NOT phrases was designed to match our exclusion criteria:
((((((((((((((((((((((((((((((((((((((((((((((((((greater[Title/Abstract]) AND tuberosity [Title/Abstract] OR tubercle [Title/Abstract]) AND fracture[Title/Abstract]) AND proximal[Title/Abstract] AND (English[lang]))) NOT intramedullary[Title] AND (English[lang]))) NOT nonunion[Title] AND (English[lang]))) NOT malunion[Title] AND (English[lang]))) NOT biomechanical[Title/Abstract] AND (English[lang]))) NOT cadaveric[Title/Abstract] AND (English[lang]))) NOT cadaver[Title/Abstract] AND (English[lang]))) NOT ((basic[Title/Abstract]) AND science[Title/Abstract] AND (English[lang])) AND (English[lang]))) NOT revision[Title] AND (English[lang]))) NOT pediatric[Title] AND (English[lang]))) NOT physeal[Title] AND (English[lang]))) NOT children[Title] AND (English[lang]))) NOT instability[Title] AND (English[lang]))) NOT imaging[Title])) NOT salter[Title])) NOT physis[Title])) NOT shaft[Title])) NOT distal[Title])) NOT clavicle[Title])) NOT scapula[Title])) NOT ((diaphysis[Title]) AND diaphyseal[Title]))) NOT infection[Title])) NOT laboratory[Title/Abstract])) NOT metastatic[Title/Abstract])) NOT (((((((malignancy[Title/Abstract]) OR malignant[Title/Abstract]) OR tumor[Title/Abstract]) OR oncologic[Title/Abstract]) OR cyst[Title/Abstract]) OR aneurysmal[Title/Abstract]) OR unicameral[Title/Abstract]).
Study Selection
Data Extraction
We extracted data from the 13 studies that met the eligibility criteria. Details of study design, sample size, and patient demographics, including age, sex, and hand dominance, were recorded, as were mechanism of injury and concomitant anterior shoulder instability. To capture the most patients, we noted radiographic fracture displacement categorically rather than continuously; patients were divided into 2 displacement groups (<5 mm, >5 mm). Most studies did not define degree of comminution or specific direction of displacement per fracture, so these variables were not included in the data analysis. Nonoperative management and operative management were studied. We abstracted surgical factors, such as approach, method, fixation type (screws or sutures), and technique (suture anchors or transosseous tunnels). Clinical outcomes included physical examination findings, functional assessment results (patient satisfaction; Constant and University of California Los Angeles [UCLA] shoulder scores), and the number of revisions. Radiologic outcomes, retrieved from radiographs or computed tomography scans, focused on loss of reduction (as determined by the respective authors), malunion, nonunion, and heterotopic ossification. Each study’s methodologic quality and bias were evaluated with the 15-item Modified Coleman Methodology Score (MCMS), which was described by Cowan and colleagues.23 The MCMS has been used to assess randomized and nonrandomized patient trials.24,25 Its scaled potential score ranges from 0 to 100 (85-100, excellent; 70-84, good; 55-69, fair; <55, poor).
Statistical Analysis
We report our data as weighted means (SDs). A mean was calculated for each study that reported a respective data point, and each mean was then weighed according to its study sample size. This calculation was performed by multiplying a study’s individual mean by the number of patients enrolled in that study and dividing the sum of these weighted data points by the number of eligible patients in all relevant studies. The result was that the nonweighted means from studies with smaller sample sizes did not carry as much weight as the nonweighted means from larger studies. We compared 3 paired groups: treatment type (nonoperative vs operative), fracture displacement amount (<5 mm vs >5 mm), and surgery type (open vs arthroscopic). Regarding all patient, surgery, and outcomes data, unpaired Student t tests were used for continuous variables and 2-tailed Fisher exact tests for categorical variables with α = 0.05 (SPSS Version 18; IBM).
Results
Postoperative physical examination findings were underreported so that surgical groups could be compared. Of all the surgical studies, 4 reported postoperative forward elevation (mean, 160°; SD, 9.8°) and external rotation (mean, 46.4°; SD 26.3°).14,15,18,22 No malunions and only 1 nonunion were reported in all 13 studies. No deaths or other serious medical complications were reported. Patients with anterior instability more often underwent surgery than were treated nonoperatively (39.2% vs 12.0%; P < .01) and more often had fractures displaced >5 mm than <5 mm (44.3% vs 14.5%; P < .01).
Fisher exact tests were used to perform isolated comparisons of screws and sutures as well as suture anchors and transosseous tunnels. Patients with screw fixation were significantly (P = .051) less likely to require reoperation (0/56; 0%) than patients with suture fixation (8/100; 8.0%). Screw fixation also led to significantly less stiffness (0% vs 12.0%; P < .01) but trended toward a higher rate of superficial infection (3.6% vs 0%; P = .13). There was no statistical difference in nerve injury rates between screws and sutures (1.8% vs 3.0%; P = 1.0). There were no significant differences in reoperations, stiffness, superficial infections, or nerve injuries between suture anchor and transosseous tunnel constructs.
For all 13 studies, mean (SD) MCMS was 41.1 (8.6).
Discussion
Five percent of all fractures involve the proximal humerus, and 20% of proximal humerus fractures are isolated greater tuberosity fractures.26,27 In his classic 1970 article, Neer6 formulated the 4-part proximal humerus fracture classification and defined greater tuberosity fracture “parts” using the same criteria as for other fracture “parts.” Neer6 recommended nonoperative management for isolated greater tuberosity fractures displaced <1 cm but did not present evidence corroborating his recommendation. More recent cutoffs for nonoperative management include 5 mm (general population) and 3 mm (athletes).7,17
In the present systematic review of greater tuberosity fractures, 3 separate comparisons were made: treatment type (nonoperative vs operative), fracture displacement amount (<5 mm vs >5 mm), and surgery type (open vs arthroscopic).
Treatment Type. Only 4 studies reported data on nonoperative treatment outcomes.5,12,16,17 Of these 4 studies, 2 found successful outcomes for fractures displaced <5 mm.12,17 Platzer and colleagues17 found good or excellent results in 97% of 135 shoulders after 4 years. Good results were defined with shoulder scores of ≥80 (Constant), <8 (Vienna), and >28 (UCLA), and excellent results were defined with maximum scores on 2 of the 3 systems. Platzer and colleagues17 also found nonsignificantly worse shoulder scores with superior displacement of 3 mm to 5 mm and recommended surgery for overhead athletes in this group. Rath and colleagues12 described a successful 3-phase rehabilitation protocol of sling immobilization for 3 weeks, pendulum exercises for 3 weeks, and active exercises thereafter. By an average of 31 months, patient satisfaction scores improved to 9.5 from 4.2 (10-point scale), though the authors cautioned that pain and decreased motion lasted 8 months on average. Conservative treatment was far less successful in the 2 studies of fractures displaced >5 mm.5,16 Keene and colleagues16 reported unsatisfactory results in all 4 patients with fractures displaced >1.5 cm. In a study separate from their 2005 analysis,17 Platzer and colleagues5 in 2008 evaluated displaced fractures and found function and patient satisfaction were inferior after nonoperative treatment than after surgery. The studies by Keene and colleagues16 and Platzer and colleagues5 support the finding of an overall lower patient satisfaction rate in nonoperative patients.
Fracture Displacement Amount. Only 2 arthroscopic studies and no open studies addressed surgery for fractures displaced <5 mm. Fewer than 16% of these fractures were managed operatively, and <1% required reoperation. By contrast, almost all fractures displaced >5 mm were managed operatively, and 3.6% required reoperation. Radiographic loss of reduction was more common in fractures displaced <5 mm, primarily because they were managed without fixation. Radiographic loss of reduction was reported in only 9 operatively treated patients, none of whom was symptomatic enough to require another surgery.5 Reoperations were most commonly performed for stiffness, which itself was significantly more common in fractures displaced >5 mm. Bhatia and colleagues14 reported the highest reoperation rate (14.3%; 3/21), but they studied more complex, comminuted fractures of the greater tuberosity. Two of their 3 reoperations were biceps tenodeses for inflamed, stiff tenosynovitis, and the third patient had a foreign body giant cell reaction to suture material. Fewer than 1% of patients with operatively managed displaced fractures required revision ORIF, and <2% developed a superficial infection or postoperative nerve palsy.19,22 For displaced greater tuberosity fractures, surgery is highly successful overall, complication rates are very low, and 90% of patients report being satisfied.
Surgery Type. Patients were divided into 2 groups. In the nonarthroscopic group, open and percutaneous approaches were used. All studies that described a percutaneous approach used screw fixation5,21; in addition, 32 patients were treated with screws through an open approach.2,5 The other open and arthroscopic studies used suture fixation. Interestingly, no studies reported on clinical outcomes of fragment excision. There were no statistically significant differences in rates of reoperation, stiffness, infection, or neurologic injury between the arthroscopic and nonarthroscopic groups. Patient satisfaction scores were slightly higher in the nonarthroscopic group (91.0% vs 87.8%), but the difference was not statistically significant.
With surgical techniques isolated, there were no significant differences between suture anchors and transosseous tunnel constructs, but screws performed significantly better than suture techniques. Compared with suture fixation, screw fixation led to significantly fewer cases of stiffness and reoperation, which suggests surgeons need to give screws more consideration in the operative management of these fractures. However, the number of patients treated with screws was smaller than the number treated with suture fixation; it is possible the differences between these cohorts would be eliminated if there were more patients in the screw cohort. In addition, screw fixation was universally performed with an open or percutaneous approach and trended toward a higher infection rate. As screw and suture techniques have low rates of complications and reoperations, we recommend leaving fixation choice to the surgeon.
Anterior shoulder instability has been associated with greater tuberosity fractures.1,8,19 The supraspinatus, infraspinatus, and teres minor muscles all insert into the greater tuberosity and resist anterior translation of the proximal humerus. Loss of this dynamic muscle stabilization is amplified by tuberosity fracture displacement: Anterior shoulder instability was significantly more common in fractures displaced >5 mm (44.3%) vs <5 mm (14.5%). In turn, glenohumeral instability was more common in patients treated with surgery, specifically open surgery, because displaced fractures may not be as easily accessed with arthroscopic techniques. No studies reported concomitant labral repair or capsular plication techniques.
This systematic review was limited by the studies analyzed. All but 1 study5 had level IV evidence. Mean (SD) MCMS was 41.8 (8.6). Any MCMS score <54 indicates a poor methodology level, but this scoring system is designed for randomized controlled trials,23 and there were none in this study. Physical examination findings, such as range of motion, were underreported. In addition, radiographic parameters were not consistently described but rather were determined by the respective authors’ subjective interpretations of malunion, nonunion, and loss of reduction. Publication bias is present in that we excluded non- English language studies and medical conference abstracts and may have omitted potentially eligible studies not discoverable with our search methodology. Performance bias is a factor in any systematic review with multiple surgeons and wide variation in surgical technique.
Conclusion
Greater tuberosity fractures displaced <5 mm may be safely managed nonoperatively, as there are no reports of nonoperatively managed fractures that subsequently required surgery. Nonoperative treatment was initially associated with low patient satisfaction, but only because displaced fractures were conservatively managed in early studies.5,16 Fractures displaced >5 mm respond well to operative fixation with screws, suture anchors, or transosseous suture tunnels. Stiffness is the most common postoperative complication (<6%), followed by heterotopic ossification, transient neurapraxias, and superficial infection. There are no discernible differences in outcome between open and arthroscopic techniques, but screw fixation may lead to significantly fewer cases of stiffness and reoperation in comparison with suture constructs.
1. Verdano MA, Aliani D, Pellegrini A, Baudi P, Pedrazzi G, Ceccarelli F. Isolated fractures of the greater tuberosity in proximal humerus: does the direction of displacement influence functional outcome? An analysis of displacement in greater tuberosity fractures. Acta Biomed. 2013;84(3):219-228.
2. Yin B, Moen TC, Thompson SA, Bigliani LU, Ahmad CS, Levine WN. Operative treatment of isolated greater tuberosity fractures: retrospective review of clinical and functional outcomes. Orthopedics. 2012;35(6):e807-e814.
3. Green A, Izzi J. Isolated fractures of the greater tuberosity of the proximal humerus. J Shoulder Elbow Surg. 2003;12(6):641-649.
4. Norouzi M, Naderi MN, Komasi MH, Sharifzadeh SR, Shahrezaei M, Eajazi A. Clinical results of using the proximal humeral internal locking system plate for internal fixation of displaced proximal humeral fractures. Am J Orthop. 2012;41(5):E64-E68.
5. Platzer P, Thalhammer G, Oberleitner G, et al. Displaced fractures of the greater tuberosity: a comparison of operative and nonoperative treatment. J Trauma. 2008;65(4):843-848.
6. Neer CS. Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
7. Park TS, Choi IY, Kim YH, Park MR, Shon JH, Kim SI. A new suggestion for the treatment of minimally displaced fractures of the greater tuberosity of the proximal humerus. Bull Hosp Jt Dis. 1997;56(3):171-176.
8. McLaughlin HL. Dislocation of the shoulder with tuberosity fracture. Surg Clin North Am. 1963;43:1615-1620.
9. DeBottis D, Anavian J, Green A. Surgical management of isolated greater tuberosity fractures of the proximal humerus. Orthop Clin North Am. 2014;45(2):207-218.
10. Monga P, Verma R, Sharma VK. Closed reduction and external fixation for displaced proximal humeral fractures. J Orthop Surg (Hong Kong). 2009;17(2):142-145.
11. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012.
12. Rath E, Alkrinawi N, Levy O, Debbi R, Amar E, Atoun E. Minimally displaced fractures of the greater tuberosity: outcome of non-operative treatment. J Shoulder Elbow Surg. 2013;22(10):e8-e11.
13. Dimakopoulos P, Panagopoulos A, Kasimatis G. Transosseous suture fixation of proximal humeral fractures. J Bone Joint Surg Am. 2007;89(8):1700-1709.
14. Bhatia DN, van Rooyen KS, Toit du DF, de Beer JF. Surgical treatment of comminuted, displaced fractures of the greater tuberosity of the proximal humerus: a new technique of double-row suture-anchor fixation and long-term results. Injury. 2006;37(10):946-952.
15. Flatow EL, Cuomo F, Maday MG, Miller SR, McIlveen SJ, Bigliani LU. Open reduction and internal fixation of two-part displaced fractures of the greater tuberosity of the proximal part of the humerus. J Bone Joint Surg Am. 1991;73(8):1213-1218.
16. Keene JS, Huizenga RE, Engber WD, Rogers SC. Proximal humeral fractures: a correlation of residual deformity with long-term function. Orthopedics. 1983;6(2):173-178.
17. Platzer P, Kutscha-Lissberg F, Lehr S, Vecsei V, Gaebler C. The influence of displacement on shoulder function in patients with minimally displaced fractures of the greater tuberosity. Injury. 2005;36(10):1185-1189.
18. Park SE, Ji JH, Shafi M, Jung JJ, Gil HJ, Lee HH. Arthroscopic management of occult greater tuberosity fracture of the shoulder. Eur J Orthop Surg Traumatol. 2014;24(4):475-482.
19. Dimakopoulos P, Panagopoulos A, Kasimatis G, Syggelos SA, Lambiris E. Anterior traumatic shoulder dislocation associated with displaced greater tuberosity fracture: the necessity of operative treatment. J Orthop Trauma. 2007;21(2):104-112.
20. Kim SH, Ha KI. Arthroscopic treatment of symptomatic shoulders with minimally displaced greater tuberosity fracture. Arthroscopy. 2000;16(7):695-700.
21. Chen CY, Chao EK, Tu YK, Ueng SW, Shih CH. Closed management and percutaneous fixation of unstable proximal humerus fractures. J Trauma. 1998;45(6):1039-1045.
22. Ji JH, Shafi M, Song IS, Kim YY, McFarland EG, Moon CY. Arthroscopic fixation technique for comminuted, displaced greater tuberosity fracture. Arthroscopy. 2010;26(5):600-609.
23. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
24. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
25. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
26. Chun JM, Groh GI, Rockwood CA. Two-part fractures of the proximal humerus. J Shoulder Elbow Surg. 1994;3(5):273-287.
27. Gruson KI, Ruchelsman DE, Tejwani NC. Isolated tuberosity fractures of the proximal humeral: current concepts. Injury. 2008;39(3):284-298.
1. Verdano MA, Aliani D, Pellegrini A, Baudi P, Pedrazzi G, Ceccarelli F. Isolated fractures of the greater tuberosity in proximal humerus: does the direction of displacement influence functional outcome? An analysis of displacement in greater tuberosity fractures. Acta Biomed. 2013;84(3):219-228.
2. Yin B, Moen TC, Thompson SA, Bigliani LU, Ahmad CS, Levine WN. Operative treatment of isolated greater tuberosity fractures: retrospective review of clinical and functional outcomes. Orthopedics. 2012;35(6):e807-e814.
3. Green A, Izzi J. Isolated fractures of the greater tuberosity of the proximal humerus. J Shoulder Elbow Surg. 2003;12(6):641-649.
4. Norouzi M, Naderi MN, Komasi MH, Sharifzadeh SR, Shahrezaei M, Eajazi A. Clinical results of using the proximal humeral internal locking system plate for internal fixation of displaced proximal humeral fractures. Am J Orthop. 2012;41(5):E64-E68.
5. Platzer P, Thalhammer G, Oberleitner G, et al. Displaced fractures of the greater tuberosity: a comparison of operative and nonoperative treatment. J Trauma. 2008;65(4):843-848.
6. Neer CS. Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
7. Park TS, Choi IY, Kim YH, Park MR, Shon JH, Kim SI. A new suggestion for the treatment of minimally displaced fractures of the greater tuberosity of the proximal humerus. Bull Hosp Jt Dis. 1997;56(3):171-176.
8. McLaughlin HL. Dislocation of the shoulder with tuberosity fracture. Surg Clin North Am. 1963;43:1615-1620.
9. DeBottis D, Anavian J, Green A. Surgical management of isolated greater tuberosity fractures of the proximal humerus. Orthop Clin North Am. 2014;45(2):207-218.
10. Monga P, Verma R, Sharma VK. Closed reduction and external fixation for displaced proximal humeral fractures. J Orthop Surg (Hong Kong). 2009;17(2):142-145.
11. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012.
12. Rath E, Alkrinawi N, Levy O, Debbi R, Amar E, Atoun E. Minimally displaced fractures of the greater tuberosity: outcome of non-operative treatment. J Shoulder Elbow Surg. 2013;22(10):e8-e11.
13. Dimakopoulos P, Panagopoulos A, Kasimatis G. Transosseous suture fixation of proximal humeral fractures. J Bone Joint Surg Am. 2007;89(8):1700-1709.
14. Bhatia DN, van Rooyen KS, Toit du DF, de Beer JF. Surgical treatment of comminuted, displaced fractures of the greater tuberosity of the proximal humerus: a new technique of double-row suture-anchor fixation and long-term results. Injury. 2006;37(10):946-952.
15. Flatow EL, Cuomo F, Maday MG, Miller SR, McIlveen SJ, Bigliani LU. Open reduction and internal fixation of two-part displaced fractures of the greater tuberosity of the proximal part of the humerus. J Bone Joint Surg Am. 1991;73(8):1213-1218.
16. Keene JS, Huizenga RE, Engber WD, Rogers SC. Proximal humeral fractures: a correlation of residual deformity with long-term function. Orthopedics. 1983;6(2):173-178.
17. Platzer P, Kutscha-Lissberg F, Lehr S, Vecsei V, Gaebler C. The influence of displacement on shoulder function in patients with minimally displaced fractures of the greater tuberosity. Injury. 2005;36(10):1185-1189.
18. Park SE, Ji JH, Shafi M, Jung JJ, Gil HJ, Lee HH. Arthroscopic management of occult greater tuberosity fracture of the shoulder. Eur J Orthop Surg Traumatol. 2014;24(4):475-482.
19. Dimakopoulos P, Panagopoulos A, Kasimatis G, Syggelos SA, Lambiris E. Anterior traumatic shoulder dislocation associated with displaced greater tuberosity fracture: the necessity of operative treatment. J Orthop Trauma. 2007;21(2):104-112.
20. Kim SH, Ha KI. Arthroscopic treatment of symptomatic shoulders with minimally displaced greater tuberosity fracture. Arthroscopy. 2000;16(7):695-700.
21. Chen CY, Chao EK, Tu YK, Ueng SW, Shih CH. Closed management and percutaneous fixation of unstable proximal humerus fractures. J Trauma. 1998;45(6):1039-1045.
22. Ji JH, Shafi M, Song IS, Kim YY, McFarland EG, Moon CY. Arthroscopic fixation technique for comminuted, displaced greater tuberosity fracture. Arthroscopy. 2010;26(5):600-609.
23. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
24. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
25. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
26. Chun JM, Groh GI, Rockwood CA. Two-part fractures of the proximal humerus. J Shoulder Elbow Surg. 1994;3(5):273-287.
27. Gruson KI, Ruchelsman DE, Tejwani NC. Isolated tuberosity fractures of the proximal humeral: current concepts. Injury. 2008;39(3):284-298.
How Long Can Corneas Be Saved Before Transplantation?
The belief that corneas that have been preserved for > 7 days are not viable for transplantation is not based on evidence, says Jonathan Lass, MD. In fact, he led a study that found corneas can be preserved safely for 11 days without negative impact on the success of transplantation. In the Cornea Preservation Time Study, funded by the National Eye Institute, Lass and other researchers looked at 3-year graft success rates among 1,090 participants (1,330 eyes) who underwent transplantation via Descemet’s stripping automated endothelial keratoplasty by 70 surgeons at 40 surgical sites. Most of the patients had Fuchs’ endothelial corneal dystrophy, a progressive disease.
The researchers were “unable to conclude” that the success rates were the same for corneas preserved for 8 to 14 days, versus up to 7 days (92% vs 95%). However, they found that much of the difference between the groups was accounted for by patients receiving corneas preserved for 12 to 14 days.
In a separate analysis, the researchers looked to see if differences in corneal preservation time affected endothelial cell loss after 3 years. They found that corneas preserved for up to 7 days had a 37% loss of cells versus 40% in those preserved for 8 to 14 days. A closer look at the data showed that the effect of corneal preservation time on the loss of endothelial cells was comparable from 4 to 13 days.
Dr. Lass emphasizes that while patients who received the older corneas had lower success rates, even those success rates were “impressively high” at 89%.
Donor corneas are not in short supply in the U.S. Outside the U.S., however, corneal disease is the third leading cause of blindness and corneal donor tissue is scarce.
The belief that corneas that have been preserved for > 7 days are not viable for transplantation is not based on evidence, says Jonathan Lass, MD. In fact, he led a study that found corneas can be preserved safely for 11 days without negative impact on the success of transplantation. In the Cornea Preservation Time Study, funded by the National Eye Institute, Lass and other researchers looked at 3-year graft success rates among 1,090 participants (1,330 eyes) who underwent transplantation via Descemet’s stripping automated endothelial keratoplasty by 70 surgeons at 40 surgical sites. Most of the patients had Fuchs’ endothelial corneal dystrophy, a progressive disease.
The researchers were “unable to conclude” that the success rates were the same for corneas preserved for 8 to 14 days, versus up to 7 days (92% vs 95%). However, they found that much of the difference between the groups was accounted for by patients receiving corneas preserved for 12 to 14 days.
In a separate analysis, the researchers looked to see if differences in corneal preservation time affected endothelial cell loss after 3 years. They found that corneas preserved for up to 7 days had a 37% loss of cells versus 40% in those preserved for 8 to 14 days. A closer look at the data showed that the effect of corneal preservation time on the loss of endothelial cells was comparable from 4 to 13 days.
Dr. Lass emphasizes that while patients who received the older corneas had lower success rates, even those success rates were “impressively high” at 89%.
Donor corneas are not in short supply in the U.S. Outside the U.S., however, corneal disease is the third leading cause of blindness and corneal donor tissue is scarce.
The belief that corneas that have been preserved for > 7 days are not viable for transplantation is not based on evidence, says Jonathan Lass, MD. In fact, he led a study that found corneas can be preserved safely for 11 days without negative impact on the success of transplantation. In the Cornea Preservation Time Study, funded by the National Eye Institute, Lass and other researchers looked at 3-year graft success rates among 1,090 participants (1,330 eyes) who underwent transplantation via Descemet’s stripping automated endothelial keratoplasty by 70 surgeons at 40 surgical sites. Most of the patients had Fuchs’ endothelial corneal dystrophy, a progressive disease.
The researchers were “unable to conclude” that the success rates were the same for corneas preserved for 8 to 14 days, versus up to 7 days (92% vs 95%). However, they found that much of the difference between the groups was accounted for by patients receiving corneas preserved for 12 to 14 days.
In a separate analysis, the researchers looked to see if differences in corneal preservation time affected endothelial cell loss after 3 years. They found that corneas preserved for up to 7 days had a 37% loss of cells versus 40% in those preserved for 8 to 14 days. A closer look at the data showed that the effect of corneal preservation time on the loss of endothelial cells was comparable from 4 to 13 days.
Dr. Lass emphasizes that while patients who received the older corneas had lower success rates, even those success rates were “impressively high” at 89%.
Donor corneas are not in short supply in the U.S. Outside the U.S., however, corneal disease is the third leading cause of blindness and corneal donor tissue is scarce.
Gorham Disease
Take-Home Points
- Gorham disease is a rare condition that manifests as an acute, spontaneous osteolysis.
- There is no clear hereditary pattern of transmission. Bones of any type or location can be affected.
- Imaging studies are nonspecific, but show permeative osteolysis involving the subcortical and intramedullary regions and typically affect regional, contiguous bones, without adjacent sclerosis, somewhat resembling osteoporosis.
- Tissue biopsy is indicated to rule out other potential etiologies of osteolysis, and the histologic findings help confirm a diagnosis of Gorham disease.
- There is no single or combined treatment modality that is considered as the gold standard. Surgical treatment includes resection of the lesion and reconstruction. Also, antiosteoclastic medication can be used.
Gorham disease, a rare condition of unknown etiology, manifests as acute, spontaneous osteolysis associated with benign hemangiomatosis or lymphangiomatosis, which presents as skeletal lucency on radiographs, prompting the classic eponym of vanishing bone disease.1-6 There is no evidence supporting the idea that osteoclasts are present in any meaningful amount in the resorption areas or that local reparative osteogenesis occurs.4,6
Jackson and colleagues first described idiopathic osteolysis in 1838,1,2 and Gorham and Stout3 introduced the syndrome to the orthopedic community in 1955. Since then, few strides have been made in identifying the disease origin.1,2,4 Diagnosis is possible only after meticulous work-up has excluded neoplastic and infectious etiologies.7,8
Clinical Presentation
Gorham disease affects patients ranging widely in age, from 2 months to 78 years, but typically presents in those under 40 years. There is a questionable predilection for males but no correlation with ethnicity or geographic region. There is no clear hereditary pattern of transmission.7 Although the bones of the head, neck, and upper extremities are involved in most cases, bone of any type or location can be affected.6 Pelvic bones seem to be involved least often.6,7
Initial clinical presentation varies considerably but typically involves prolonged soreness in the affected region and, rarely, acute pathologic fracture.1,2,4 The nonspecific nature of complaints, lack of markers of systemic illness, and rarity of the disease contribute to delayed diagnosis.1,2
Imaging
Computed tomography (CT) better defines the severity and extent of these changes.
Magnetic resonance imaging shows an infiltrative and irregular T2 hyperintense signal throughout regions of bone affected by osteolysis, but this finding is not characteristic. There is heterogeneous enhancement on postcontrast sequences, and, though masslike enhancement is absent, signal abnormalities may extend into adjacent soft tissues.
Bone scintigraphy using technetium-99m is similarly nonspecific, typically revealing radiotracer uptake that is consistent with bony reaction to an underlying osteolytic process (Figure 4) but turning negative with ongoing resorption.
Positron emission tomography/CT typically shows foci of increased metabolic activity in the areas of osteolysis.10
Diagnosis
There have been 8 histologic and clinical criteria described to diagnose Gorham disease: (1) biopsy positive for presence of angiomatous tissue, (2) complete absence of any cellular atypia, (3) lack of osteoclastic response and lack of dystrophic calcifications, (4) evidence of progressive resorption of native bone, (5) no evidence of expansive or ulcerative lesion, (6) lack of visceral involvement, (7) osteolytic radiographic pattern, and (8) no concrete diagnosis after hereditary, metabolic, neoplastic, immunologic, and infectious work-up.4-6 These criteria confirm that the diagnosis can be rendered only after exclusion of neoplastic and infectious etiologies through clinical and laboratory work-up, imaging studies, and tissue sampling.
Tissue biopsy is indicated to rule out other potential etiologies of osteolysis, and the histologic findings help confirm a diagnosis of Gorham disease.
The differential diagnosis includes infection (osteomyelitis, Brodie abscess), benign tumors (eosinophilic granuloma/Langerhans cell histiocytosis), malignant tumors (Ewing sarcoma and angiosarcoma), inflammatory conditions (eg, apatite- associated destructive arthritis), endocrine disorders (eg, osteolytic hyperparathyroidism), benign non-neoplastic conditions (venous or venolymphatic malformation), and other syndromes that present with osteolysis.1,2 Nevertheless, progressive and unusually substantial bone destruction without evidence of repair is almost pathognomonic for Gorham disease.9
Treatment
Surgical treatment usually includes lesion resection and subsequent reconstruction using combinations of bone grafts (allogenic) and prostheses. Bone graft alone is quickly resorbed and has not been found to be beneficial.1,2,4,20
1. Saify FY, Gosavi SR. Gorham’s disease: a diagnostic challenge. J Oral Maxillofac Pathol. 2014;18(3):411-414.
2. Patel DV. Gorham’s disease or massive osteolysis. Clin Med Res. 2005;3(2):65-74.
3. Gorham LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone); its relation to hemangiomatosis. J Bone Joint Surg Am. 1955;37(5):985-1004.
4. Heffez L, Doku HC, Carter BL, Feeney JE. Perspectives on massive osteolysis. Report of a case and review of the literature. Oral Surg Oral Med Oral Pathol. 1983;55(4):331-343.
5. Gulati U, Mohanty S, Dabas J, Chandra N. “Vanishing bone disease” in maxillofacial region: a review and our experience. J Maxillofac Oral Surg. 2015;14(3):548-557.
6. Nikolaou VS, Chytas D, Korres D, Efstathopoulos N. Vanishing bone disease (Gorham-Stout syndrome): a review of a rare entity. World J Orthop. 2014;5(5):694-698.
7. Möller G, Priemel M, Amling M, Werner M, Kuhlmey AS, Delling G. The Gorham-Stout syndrome (Gorham’s massive osteolysis). A report of six cases with histopathological findings. J Bone Joint Surg Br. 1999;81(3):501-506.
8. Dominguez R, Washowich TL. Gorham’s disease or vanishing bone disease: plain film, CT, and MRI findings of two cases. Pediatr Radiol. 1994;24(5):316-318.
9. Kotecha R, Mascarenhas L, Jackson HA, Venkatramani R. Radiological features of Gorham’s disease. Clin Radiol. 2012;67(8):782-788.
10. Dong A, Bai Y, Wang Y, Zuo C. Bone scan, MRI, and FDG PET/CT findings in composite hemangioendothelioma of the manubrium sterni. Clin Nucl Med. 2014;39(2):e180-e183.
11. Baulieu F, De Pinieux G, Maruani A, Vaillant L, Lorette G. Serial lymphoscintigraphic findings in a patient with Gorham’s disease with lymphedema. Lymphology. 2014;47(3):118-122.
12. Manisali M, Ozaksoy D. Gorham disease: correlation of MR findings with histopathologic changes. Eur Radiol. 1998;8(9):1647-1650.
13. Brodszki N, Länsberg JK, Dictor M, et al. A novel treatment approach for paediatric Gorham-Stout syndrome with chylothorax. Acta Paediatr. 2011;100(11):1448-1453.
14. Nir V, Guralnik L, Livnat G, et al. Propranolol as a treatment option in Gorham-Stout syndrome: a case report. Pediatr Pulmonol. 2014;49(4):417-419.
15. Fontanesi J. Radiation therapy in the treatment of Gorham disease. J Pediatr Hematol. 2003;25(10):816-817.
16. Pfleger A, Schwinger W, Maier A, Tauss J, Popper HH, Zach MS. Gorham-Stout syndrome in a male adolescent—case report and review of the literature. J Pediatr Hematol Oncol. 2006;28(4):231-233.
17. Patrick JH. Massive osteolysis complicated by chylothorax successfully treated by pleurodesis. J Bone Joint Surg Br. 1976;58(3):347-349.
18. Hagberg H, Lamberg K, Åström G. α-2b interferon and oral clodronate for Gorham’s disease. Lancet. 1997;350(9094):1822-1823.
19. Takahashi A, Ogawa C, Kanazawa T, et al. Remission induced by interferon alfa in a patient with massive osteolysis and extension of lymph-hemangiomatosis: a severe case of Gorham-Stout syndrome. J Pediatr Surg. 2005;40(3):E47-E50.
20. Paley MD, Lloyd CJ, Penfold CN. Total mandibular reconstruction for massive osteolysis of the mandible (Gorham-Stout syndrome). Br J Oral Maxillofac Surg. 2005;43(2):166-168.
21. Avelar RL, Martins VB, Antunes AA, de Oliveira Neto PJ, de Souza Andrade ES. Use of zoledronic acid in the treatment of Gorham’s disease. Int J Pediatr Otorhinolaryngol. 2010;74(3):319-322.
22. Holroyd I, Dillon M, Roberts GJ. Gorham’s disease: a case (including dental presentation) of vanishing bone disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89(1):125-129.
23. Lee S, Finn L, Sze RW, Perkins JA, Sie KC. Gorham Stout syndrome (disappearing bone disease): two additional case reports and a review of the literature. Arch Otolaryngol Head Neck Surg. 2003;129(12):1340-1343.
Take-Home Points
- Gorham disease is a rare condition that manifests as an acute, spontaneous osteolysis.
- There is no clear hereditary pattern of transmission. Bones of any type or location can be affected.
- Imaging studies are nonspecific, but show permeative osteolysis involving the subcortical and intramedullary regions and typically affect regional, contiguous bones, without adjacent sclerosis, somewhat resembling osteoporosis.
- Tissue biopsy is indicated to rule out other potential etiologies of osteolysis, and the histologic findings help confirm a diagnosis of Gorham disease.
- There is no single or combined treatment modality that is considered as the gold standard. Surgical treatment includes resection of the lesion and reconstruction. Also, antiosteoclastic medication can be used.
Gorham disease, a rare condition of unknown etiology, manifests as acute, spontaneous osteolysis associated with benign hemangiomatosis or lymphangiomatosis, which presents as skeletal lucency on radiographs, prompting the classic eponym of vanishing bone disease.1-6 There is no evidence supporting the idea that osteoclasts are present in any meaningful amount in the resorption areas or that local reparative osteogenesis occurs.4,6
Jackson and colleagues first described idiopathic osteolysis in 1838,1,2 and Gorham and Stout3 introduced the syndrome to the orthopedic community in 1955. Since then, few strides have been made in identifying the disease origin.1,2,4 Diagnosis is possible only after meticulous work-up has excluded neoplastic and infectious etiologies.7,8
Clinical Presentation
Gorham disease affects patients ranging widely in age, from 2 months to 78 years, but typically presents in those under 40 years. There is a questionable predilection for males but no correlation with ethnicity or geographic region. There is no clear hereditary pattern of transmission.7 Although the bones of the head, neck, and upper extremities are involved in most cases, bone of any type or location can be affected.6 Pelvic bones seem to be involved least often.6,7
Initial clinical presentation varies considerably but typically involves prolonged soreness in the affected region and, rarely, acute pathologic fracture.1,2,4 The nonspecific nature of complaints, lack of markers of systemic illness, and rarity of the disease contribute to delayed diagnosis.1,2
Imaging
Computed tomography (CT) better defines the severity and extent of these changes.
Magnetic resonance imaging shows an infiltrative and irregular T2 hyperintense signal throughout regions of bone affected by osteolysis, but this finding is not characteristic. There is heterogeneous enhancement on postcontrast sequences, and, though masslike enhancement is absent, signal abnormalities may extend into adjacent soft tissues.
Bone scintigraphy using technetium-99m is similarly nonspecific, typically revealing radiotracer uptake that is consistent with bony reaction to an underlying osteolytic process (Figure 4) but turning negative with ongoing resorption.
Positron emission tomography/CT typically shows foci of increased metabolic activity in the areas of osteolysis.10
Diagnosis
There have been 8 histologic and clinical criteria described to diagnose Gorham disease: (1) biopsy positive for presence of angiomatous tissue, (2) complete absence of any cellular atypia, (3) lack of osteoclastic response and lack of dystrophic calcifications, (4) evidence of progressive resorption of native bone, (5) no evidence of expansive or ulcerative lesion, (6) lack of visceral involvement, (7) osteolytic radiographic pattern, and (8) no concrete diagnosis after hereditary, metabolic, neoplastic, immunologic, and infectious work-up.4-6 These criteria confirm that the diagnosis can be rendered only after exclusion of neoplastic and infectious etiologies through clinical and laboratory work-up, imaging studies, and tissue sampling.
Tissue biopsy is indicated to rule out other potential etiologies of osteolysis, and the histologic findings help confirm a diagnosis of Gorham disease.
The differential diagnosis includes infection (osteomyelitis, Brodie abscess), benign tumors (eosinophilic granuloma/Langerhans cell histiocytosis), malignant tumors (Ewing sarcoma and angiosarcoma), inflammatory conditions (eg, apatite- associated destructive arthritis), endocrine disorders (eg, osteolytic hyperparathyroidism), benign non-neoplastic conditions (venous or venolymphatic malformation), and other syndromes that present with osteolysis.1,2 Nevertheless, progressive and unusually substantial bone destruction without evidence of repair is almost pathognomonic for Gorham disease.9
Treatment
Surgical treatment usually includes lesion resection and subsequent reconstruction using combinations of bone grafts (allogenic) and prostheses. Bone graft alone is quickly resorbed and has not been found to be beneficial.1,2,4,20
Take-Home Points
- Gorham disease is a rare condition that manifests as an acute, spontaneous osteolysis.
- There is no clear hereditary pattern of transmission. Bones of any type or location can be affected.
- Imaging studies are nonspecific, but show permeative osteolysis involving the subcortical and intramedullary regions and typically affect regional, contiguous bones, without adjacent sclerosis, somewhat resembling osteoporosis.
- Tissue biopsy is indicated to rule out other potential etiologies of osteolysis, and the histologic findings help confirm a diagnosis of Gorham disease.
- There is no single or combined treatment modality that is considered as the gold standard. Surgical treatment includes resection of the lesion and reconstruction. Also, antiosteoclastic medication can be used.
Gorham disease, a rare condition of unknown etiology, manifests as acute, spontaneous osteolysis associated with benign hemangiomatosis or lymphangiomatosis, which presents as skeletal lucency on radiographs, prompting the classic eponym of vanishing bone disease.1-6 There is no evidence supporting the idea that osteoclasts are present in any meaningful amount in the resorption areas or that local reparative osteogenesis occurs.4,6
Jackson and colleagues first described idiopathic osteolysis in 1838,1,2 and Gorham and Stout3 introduced the syndrome to the orthopedic community in 1955. Since then, few strides have been made in identifying the disease origin.1,2,4 Diagnosis is possible only after meticulous work-up has excluded neoplastic and infectious etiologies.7,8
Clinical Presentation
Gorham disease affects patients ranging widely in age, from 2 months to 78 years, but typically presents in those under 40 years. There is a questionable predilection for males but no correlation with ethnicity or geographic region. There is no clear hereditary pattern of transmission.7 Although the bones of the head, neck, and upper extremities are involved in most cases, bone of any type or location can be affected.6 Pelvic bones seem to be involved least often.6,7
Initial clinical presentation varies considerably but typically involves prolonged soreness in the affected region and, rarely, acute pathologic fracture.1,2,4 The nonspecific nature of complaints, lack of markers of systemic illness, and rarity of the disease contribute to delayed diagnosis.1,2
Imaging
Computed tomography (CT) better defines the severity and extent of these changes.
Magnetic resonance imaging shows an infiltrative and irregular T2 hyperintense signal throughout regions of bone affected by osteolysis, but this finding is not characteristic. There is heterogeneous enhancement on postcontrast sequences, and, though masslike enhancement is absent, signal abnormalities may extend into adjacent soft tissues.
Bone scintigraphy using technetium-99m is similarly nonspecific, typically revealing radiotracer uptake that is consistent with bony reaction to an underlying osteolytic process (Figure 4) but turning negative with ongoing resorption.
Positron emission tomography/CT typically shows foci of increased metabolic activity in the areas of osteolysis.10
Diagnosis
There have been 8 histologic and clinical criteria described to diagnose Gorham disease: (1) biopsy positive for presence of angiomatous tissue, (2) complete absence of any cellular atypia, (3) lack of osteoclastic response and lack of dystrophic calcifications, (4) evidence of progressive resorption of native bone, (5) no evidence of expansive or ulcerative lesion, (6) lack of visceral involvement, (7) osteolytic radiographic pattern, and (8) no concrete diagnosis after hereditary, metabolic, neoplastic, immunologic, and infectious work-up.4-6 These criteria confirm that the diagnosis can be rendered only after exclusion of neoplastic and infectious etiologies through clinical and laboratory work-up, imaging studies, and tissue sampling.
Tissue biopsy is indicated to rule out other potential etiologies of osteolysis, and the histologic findings help confirm a diagnosis of Gorham disease.
The differential diagnosis includes infection (osteomyelitis, Brodie abscess), benign tumors (eosinophilic granuloma/Langerhans cell histiocytosis), malignant tumors (Ewing sarcoma and angiosarcoma), inflammatory conditions (eg, apatite- associated destructive arthritis), endocrine disorders (eg, osteolytic hyperparathyroidism), benign non-neoplastic conditions (venous or venolymphatic malformation), and other syndromes that present with osteolysis.1,2 Nevertheless, progressive and unusually substantial bone destruction without evidence of repair is almost pathognomonic for Gorham disease.9
Treatment
Surgical treatment usually includes lesion resection and subsequent reconstruction using combinations of bone grafts (allogenic) and prostheses. Bone graft alone is quickly resorbed and has not been found to be beneficial.1,2,4,20
1. Saify FY, Gosavi SR. Gorham’s disease: a diagnostic challenge. J Oral Maxillofac Pathol. 2014;18(3):411-414.
2. Patel DV. Gorham’s disease or massive osteolysis. Clin Med Res. 2005;3(2):65-74.
3. Gorham LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone); its relation to hemangiomatosis. J Bone Joint Surg Am. 1955;37(5):985-1004.
4. Heffez L, Doku HC, Carter BL, Feeney JE. Perspectives on massive osteolysis. Report of a case and review of the literature. Oral Surg Oral Med Oral Pathol. 1983;55(4):331-343.
5. Gulati U, Mohanty S, Dabas J, Chandra N. “Vanishing bone disease” in maxillofacial region: a review and our experience. J Maxillofac Oral Surg. 2015;14(3):548-557.
6. Nikolaou VS, Chytas D, Korres D, Efstathopoulos N. Vanishing bone disease (Gorham-Stout syndrome): a review of a rare entity. World J Orthop. 2014;5(5):694-698.
7. Möller G, Priemel M, Amling M, Werner M, Kuhlmey AS, Delling G. The Gorham-Stout syndrome (Gorham’s massive osteolysis). A report of six cases with histopathological findings. J Bone Joint Surg Br. 1999;81(3):501-506.
8. Dominguez R, Washowich TL. Gorham’s disease or vanishing bone disease: plain film, CT, and MRI findings of two cases. Pediatr Radiol. 1994;24(5):316-318.
9. Kotecha R, Mascarenhas L, Jackson HA, Venkatramani R. Radiological features of Gorham’s disease. Clin Radiol. 2012;67(8):782-788.
10. Dong A, Bai Y, Wang Y, Zuo C. Bone scan, MRI, and FDG PET/CT findings in composite hemangioendothelioma of the manubrium sterni. Clin Nucl Med. 2014;39(2):e180-e183.
11. Baulieu F, De Pinieux G, Maruani A, Vaillant L, Lorette G. Serial lymphoscintigraphic findings in a patient with Gorham’s disease with lymphedema. Lymphology. 2014;47(3):118-122.
12. Manisali M, Ozaksoy D. Gorham disease: correlation of MR findings with histopathologic changes. Eur Radiol. 1998;8(9):1647-1650.
13. Brodszki N, Länsberg JK, Dictor M, et al. A novel treatment approach for paediatric Gorham-Stout syndrome with chylothorax. Acta Paediatr. 2011;100(11):1448-1453.
14. Nir V, Guralnik L, Livnat G, et al. Propranolol as a treatment option in Gorham-Stout syndrome: a case report. Pediatr Pulmonol. 2014;49(4):417-419.
15. Fontanesi J. Radiation therapy in the treatment of Gorham disease. J Pediatr Hematol. 2003;25(10):816-817.
16. Pfleger A, Schwinger W, Maier A, Tauss J, Popper HH, Zach MS. Gorham-Stout syndrome in a male adolescent—case report and review of the literature. J Pediatr Hematol Oncol. 2006;28(4):231-233.
17. Patrick JH. Massive osteolysis complicated by chylothorax successfully treated by pleurodesis. J Bone Joint Surg Br. 1976;58(3):347-349.
18. Hagberg H, Lamberg K, Åström G. α-2b interferon and oral clodronate for Gorham’s disease. Lancet. 1997;350(9094):1822-1823.
19. Takahashi A, Ogawa C, Kanazawa T, et al. Remission induced by interferon alfa in a patient with massive osteolysis and extension of lymph-hemangiomatosis: a severe case of Gorham-Stout syndrome. J Pediatr Surg. 2005;40(3):E47-E50.
20. Paley MD, Lloyd CJ, Penfold CN. Total mandibular reconstruction for massive osteolysis of the mandible (Gorham-Stout syndrome). Br J Oral Maxillofac Surg. 2005;43(2):166-168.
21. Avelar RL, Martins VB, Antunes AA, de Oliveira Neto PJ, de Souza Andrade ES. Use of zoledronic acid in the treatment of Gorham’s disease. Int J Pediatr Otorhinolaryngol. 2010;74(3):319-322.
22. Holroyd I, Dillon M, Roberts GJ. Gorham’s disease: a case (including dental presentation) of vanishing bone disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89(1):125-129.
23. Lee S, Finn L, Sze RW, Perkins JA, Sie KC. Gorham Stout syndrome (disappearing bone disease): two additional case reports and a review of the literature. Arch Otolaryngol Head Neck Surg. 2003;129(12):1340-1343.
1. Saify FY, Gosavi SR. Gorham’s disease: a diagnostic challenge. J Oral Maxillofac Pathol. 2014;18(3):411-414.
2. Patel DV. Gorham’s disease or massive osteolysis. Clin Med Res. 2005;3(2):65-74.
3. Gorham LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone); its relation to hemangiomatosis. J Bone Joint Surg Am. 1955;37(5):985-1004.
4. Heffez L, Doku HC, Carter BL, Feeney JE. Perspectives on massive osteolysis. Report of a case and review of the literature. Oral Surg Oral Med Oral Pathol. 1983;55(4):331-343.
5. Gulati U, Mohanty S, Dabas J, Chandra N. “Vanishing bone disease” in maxillofacial region: a review and our experience. J Maxillofac Oral Surg. 2015;14(3):548-557.
6. Nikolaou VS, Chytas D, Korres D, Efstathopoulos N. Vanishing bone disease (Gorham-Stout syndrome): a review of a rare entity. World J Orthop. 2014;5(5):694-698.
7. Möller G, Priemel M, Amling M, Werner M, Kuhlmey AS, Delling G. The Gorham-Stout syndrome (Gorham’s massive osteolysis). A report of six cases with histopathological findings. J Bone Joint Surg Br. 1999;81(3):501-506.
8. Dominguez R, Washowich TL. Gorham’s disease or vanishing bone disease: plain film, CT, and MRI findings of two cases. Pediatr Radiol. 1994;24(5):316-318.
9. Kotecha R, Mascarenhas L, Jackson HA, Venkatramani R. Radiological features of Gorham’s disease. Clin Radiol. 2012;67(8):782-788.
10. Dong A, Bai Y, Wang Y, Zuo C. Bone scan, MRI, and FDG PET/CT findings in composite hemangioendothelioma of the manubrium sterni. Clin Nucl Med. 2014;39(2):e180-e183.
11. Baulieu F, De Pinieux G, Maruani A, Vaillant L, Lorette G. Serial lymphoscintigraphic findings in a patient with Gorham’s disease with lymphedema. Lymphology. 2014;47(3):118-122.
12. Manisali M, Ozaksoy D. Gorham disease: correlation of MR findings with histopathologic changes. Eur Radiol. 1998;8(9):1647-1650.
13. Brodszki N, Länsberg JK, Dictor M, et al. A novel treatment approach for paediatric Gorham-Stout syndrome with chylothorax. Acta Paediatr. 2011;100(11):1448-1453.
14. Nir V, Guralnik L, Livnat G, et al. Propranolol as a treatment option in Gorham-Stout syndrome: a case report. Pediatr Pulmonol. 2014;49(4):417-419.
15. Fontanesi J. Radiation therapy in the treatment of Gorham disease. J Pediatr Hematol. 2003;25(10):816-817.
16. Pfleger A, Schwinger W, Maier A, Tauss J, Popper HH, Zach MS. Gorham-Stout syndrome in a male adolescent—case report and review of the literature. J Pediatr Hematol Oncol. 2006;28(4):231-233.
17. Patrick JH. Massive osteolysis complicated by chylothorax successfully treated by pleurodesis. J Bone Joint Surg Br. 1976;58(3):347-349.
18. Hagberg H, Lamberg K, Åström G. α-2b interferon and oral clodronate for Gorham’s disease. Lancet. 1997;350(9094):1822-1823.
19. Takahashi A, Ogawa C, Kanazawa T, et al. Remission induced by interferon alfa in a patient with massive osteolysis and extension of lymph-hemangiomatosis: a severe case of Gorham-Stout syndrome. J Pediatr Surg. 2005;40(3):E47-E50.
20. Paley MD, Lloyd CJ, Penfold CN. Total mandibular reconstruction for massive osteolysis of the mandible (Gorham-Stout syndrome). Br J Oral Maxillofac Surg. 2005;43(2):166-168.
21. Avelar RL, Martins VB, Antunes AA, de Oliveira Neto PJ, de Souza Andrade ES. Use of zoledronic acid in the treatment of Gorham’s disease. Int J Pediatr Otorhinolaryngol. 2010;74(3):319-322.
22. Holroyd I, Dillon M, Roberts GJ. Gorham’s disease: a case (including dental presentation) of vanishing bone disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89(1):125-129.
23. Lee S, Finn L, Sze RW, Perkins JA, Sie KC. Gorham Stout syndrome (disappearing bone disease): two additional case reports and a review of the literature. Arch Otolaryngol Head Neck Surg. 2003;129(12):1340-1343.
Team develops new scoring systems for PMF
ATLANTA—Two novel prognostic scoring systems can help clinicians decide how to treat certain patients with primary myelofibrosis (PMF), according to a new study.
The scoring systems, which build upon the International Prognostic Scoring System (IPSS), were developed for use in PMF patients age 70 and younger who are potential candidates for hematopoietic stem cell transplant (HSCT).
One of the scoring systems—MIPSS70—integrates clinical, histologic, and molecular information. The other—MIPSS70-plus—also includes cytogenetic information.
Alessandro M. Vannucchi, MD, of the University of Florence in Italy, presented details on these prognostic scoring systems at the 2017 ASH Annual Meeting (abstract 200*).
The information was published simultaneously in the Journal of Clinical Oncology.
Dr Vannucchi noted that, in PMF, survival is currently predicted by the IPSS, the dynamic IPSS, and the dynamic IPSS-plus.
“The IPSS score is used at the time of diagnosis, while the dynamic IPSS or dynamic IPSS-plus are used to provide survival estimates at the time of patient referral,” he explained. “In clinical practice, these prognostic risk scores are mainly used for [HSCT] decision-making in younger patients.”
Driver mutations and other myeloid neoplasm-associated mutations provide prognostic information that is independent of the IPSS/dynamic IPSS/dynamic IPSS-plus scoring systems.
The degree of bone marrow fibrosis and cytogenetic abnormalities configuring an unfavorable category also contribute prognostic information that is independent of these scoring systems.
With this in mind, Dr Vannucchi and his colleagues set out to develop an updated prognostic score that included molecular information (MIPSS70) and, if possible, cytogenetic information (MIPSS70-plus) for PMF patients age 70 and younger who are potential candidates for HSCT.
The researchers developed 2 prognostic models using a training/validation cohort approach.
For MIPSS70, the training cohort included 490 patients from 6 Italian institutions associated with the Associazione Italiana per la Ricerca sul Cancro Gruppo Italiano Malattie Mieloproliferative project (AGIMM group), and the validation cohort included 211 patients from the Mayo Clinic in Rochester, Minnesota.
For MIPSS70-plus, the training cohort included 315 patients from the Mayo Clinic, and the validation cohort included 261 patients from the AGIMM group.
Using the MIPSS70 risk score in the validation cohort, the 5-year overall survival rate was 96% in low-risk, 67% in intermediate-risk, and 34% in high-risk patients.
“MIPSS70 performed better than IPSS in predicting survival,” Dr Vannucchi said. “About 30% of patients who were high-risk with MIPPS70 were missed by IPSS.”
Using the MIPSS70-plus risk score in the validation cohort, the 5-year overall survival rate was 100% in low-risk, 90% in intermediate-risk, 76% in high-risk, and 46.5% in very high-risk patients.
The MIPSS70-plus risk score also identified patients at very high risk for leukemic transformation, Dr Vannucchi said.
Furthermore, both MIPSS70 and MIPSS70-plus remained predictive of survival when the researchers evaluated patients older than 70 years of age.
“The new MIPSS70 and MIPSS70-plus scores include modern disease-associated risk variables pertinent to both pre-PMF and overt-PMF according to the 2016 WHO classification,” Dr Vannucchi said. “They integrate prognostically relevant clinical, cytogenetic, and mutation data and provide complementary systems of improved risk stratification for transplantation-age patients with PMF.”
Dr Vannucchi disclosed membership in speaker’s bureaus with Gilead, Shire, and Novartis, and research funding and membership on Board of Directors or advisory committees with Novartis. ![]()
*Data in the presentation differ from the abstract.
ATLANTA—Two novel prognostic scoring systems can help clinicians decide how to treat certain patients with primary myelofibrosis (PMF), according to a new study.
The scoring systems, which build upon the International Prognostic Scoring System (IPSS), were developed for use in PMF patients age 70 and younger who are potential candidates for hematopoietic stem cell transplant (HSCT).
One of the scoring systems—MIPSS70—integrates clinical, histologic, and molecular information. The other—MIPSS70-plus—also includes cytogenetic information.
Alessandro M. Vannucchi, MD, of the University of Florence in Italy, presented details on these prognostic scoring systems at the 2017 ASH Annual Meeting (abstract 200*).
The information was published simultaneously in the Journal of Clinical Oncology.
Dr Vannucchi noted that, in PMF, survival is currently predicted by the IPSS, the dynamic IPSS, and the dynamic IPSS-plus.
“The IPSS score is used at the time of diagnosis, while the dynamic IPSS or dynamic IPSS-plus are used to provide survival estimates at the time of patient referral,” he explained. “In clinical practice, these prognostic risk scores are mainly used for [HSCT] decision-making in younger patients.”
Driver mutations and other myeloid neoplasm-associated mutations provide prognostic information that is independent of the IPSS/dynamic IPSS/dynamic IPSS-plus scoring systems.
The degree of bone marrow fibrosis and cytogenetic abnormalities configuring an unfavorable category also contribute prognostic information that is independent of these scoring systems.
With this in mind, Dr Vannucchi and his colleagues set out to develop an updated prognostic score that included molecular information (MIPSS70) and, if possible, cytogenetic information (MIPSS70-plus) for PMF patients age 70 and younger who are potential candidates for HSCT.
The researchers developed 2 prognostic models using a training/validation cohort approach.
For MIPSS70, the training cohort included 490 patients from 6 Italian institutions associated with the Associazione Italiana per la Ricerca sul Cancro Gruppo Italiano Malattie Mieloproliferative project (AGIMM group), and the validation cohort included 211 patients from the Mayo Clinic in Rochester, Minnesota.
For MIPSS70-plus, the training cohort included 315 patients from the Mayo Clinic, and the validation cohort included 261 patients from the AGIMM group.
Using the MIPSS70 risk score in the validation cohort, the 5-year overall survival rate was 96% in low-risk, 67% in intermediate-risk, and 34% in high-risk patients.
“MIPSS70 performed better than IPSS in predicting survival,” Dr Vannucchi said. “About 30% of patients who were high-risk with MIPPS70 were missed by IPSS.”
Using the MIPSS70-plus risk score in the validation cohort, the 5-year overall survival rate was 100% in low-risk, 90% in intermediate-risk, 76% in high-risk, and 46.5% in very high-risk patients.
The MIPSS70-plus risk score also identified patients at very high risk for leukemic transformation, Dr Vannucchi said.
Furthermore, both MIPSS70 and MIPSS70-plus remained predictive of survival when the researchers evaluated patients older than 70 years of age.
“The new MIPSS70 and MIPSS70-plus scores include modern disease-associated risk variables pertinent to both pre-PMF and overt-PMF according to the 2016 WHO classification,” Dr Vannucchi said. “They integrate prognostically relevant clinical, cytogenetic, and mutation data and provide complementary systems of improved risk stratification for transplantation-age patients with PMF.”
Dr Vannucchi disclosed membership in speaker’s bureaus with Gilead, Shire, and Novartis, and research funding and membership on Board of Directors or advisory committees with Novartis. ![]()
*Data in the presentation differ from the abstract.
ATLANTA—Two novel prognostic scoring systems can help clinicians decide how to treat certain patients with primary myelofibrosis (PMF), according to a new study.
The scoring systems, which build upon the International Prognostic Scoring System (IPSS), were developed for use in PMF patients age 70 and younger who are potential candidates for hematopoietic stem cell transplant (HSCT).
One of the scoring systems—MIPSS70—integrates clinical, histologic, and molecular information. The other—MIPSS70-plus—also includes cytogenetic information.
Alessandro M. Vannucchi, MD, of the University of Florence in Italy, presented details on these prognostic scoring systems at the 2017 ASH Annual Meeting (abstract 200*).
The information was published simultaneously in the Journal of Clinical Oncology.
Dr Vannucchi noted that, in PMF, survival is currently predicted by the IPSS, the dynamic IPSS, and the dynamic IPSS-plus.
“The IPSS score is used at the time of diagnosis, while the dynamic IPSS or dynamic IPSS-plus are used to provide survival estimates at the time of patient referral,” he explained. “In clinical practice, these prognostic risk scores are mainly used for [HSCT] decision-making in younger patients.”
Driver mutations and other myeloid neoplasm-associated mutations provide prognostic information that is independent of the IPSS/dynamic IPSS/dynamic IPSS-plus scoring systems.
The degree of bone marrow fibrosis and cytogenetic abnormalities configuring an unfavorable category also contribute prognostic information that is independent of these scoring systems.
With this in mind, Dr Vannucchi and his colleagues set out to develop an updated prognostic score that included molecular information (MIPSS70) and, if possible, cytogenetic information (MIPSS70-plus) for PMF patients age 70 and younger who are potential candidates for HSCT.
The researchers developed 2 prognostic models using a training/validation cohort approach.
For MIPSS70, the training cohort included 490 patients from 6 Italian institutions associated with the Associazione Italiana per la Ricerca sul Cancro Gruppo Italiano Malattie Mieloproliferative project (AGIMM group), and the validation cohort included 211 patients from the Mayo Clinic in Rochester, Minnesota.
For MIPSS70-plus, the training cohort included 315 patients from the Mayo Clinic, and the validation cohort included 261 patients from the AGIMM group.
Using the MIPSS70 risk score in the validation cohort, the 5-year overall survival rate was 96% in low-risk, 67% in intermediate-risk, and 34% in high-risk patients.
“MIPSS70 performed better than IPSS in predicting survival,” Dr Vannucchi said. “About 30% of patients who were high-risk with MIPPS70 were missed by IPSS.”
Using the MIPSS70-plus risk score in the validation cohort, the 5-year overall survival rate was 100% in low-risk, 90% in intermediate-risk, 76% in high-risk, and 46.5% in very high-risk patients.
The MIPSS70-plus risk score also identified patients at very high risk for leukemic transformation, Dr Vannucchi said.
Furthermore, both MIPSS70 and MIPSS70-plus remained predictive of survival when the researchers evaluated patients older than 70 years of age.
“The new MIPSS70 and MIPSS70-plus scores include modern disease-associated risk variables pertinent to both pre-PMF and overt-PMF according to the 2016 WHO classification,” Dr Vannucchi said. “They integrate prognostically relevant clinical, cytogenetic, and mutation data and provide complementary systems of improved risk stratification for transplantation-age patients with PMF.”
Dr Vannucchi disclosed membership in speaker’s bureaus with Gilead, Shire, and Novartis, and research funding and membership on Board of Directors or advisory committees with Novartis. ![]()
*Data in the presentation differ from the abstract.
Drug receives fast track, orphan designations for PTCL
The US Food and Drug Administration (FDA) has granted orphan drug and fast track designations to tenalisib (RP6530) for the treatment of peripheral T-cell lymphoma (PTCL).
Tenalisib is a dual PI3K delta/gamma inhibitor being developed by Rhizen Pharmaceuticals.
Research has shown that tenalisib inhibits the growth of immortalized cancerous cell lines and primary leukemia/lymphoma cells.
In preclinical studies, tenalisib reprogrammed macrophages from an immunosuppressive M2-like phenotype (pro-tumor) to an inflammatory M1-like state (anti-tumor).
Researchers are currently conducting a phase 1 study of tenalisib in patients with relapsed/refractory PTCL. Results from this study were presented at the 2017 ASH Annual Meeting (abstract 2791*).
The presentation included data on 50 patients—24 with PTCL and 26 with cutaneous T-cell lymphoma (CTCL).
For the PTCL patients, the median age was 63 (range, 40-89), and 67% were male. The median number of prior therapies was 3 (range, 1-7). All patients had an ECOG status of 0 (n=14) or 1 (n=10). More patients had relapsed disease (n=17, 58%) than refractory disease (n=10, 42%).
For the CTCL patients, the median age was 67 (range, 37-84), and 46% were male. The median number of prior therapies was 5.5 (range, 2-15). All patients had an ECOG status of 0 (n=23) or 1 (n=3). More patients had refractory disease (n=15, 58%) than relapsed disease (n=11, 42%).
In the dose-escalation portion of the study, patients received tenalisib at 200 mg twice daily (BID), 400 mg BID, 800 mg BID fasting, or 800 mg BID fed. The maximum tolerated dose was 800 mg BID fasting, so this dose is being used in the expansion cohort.
Twelve PTCL patients were evaluable for efficacy. The overall response rate in these patients was 58% (7/12), with a 25% complete response rate (3/12).
Sixteen CTCL patients were evaluable for efficacy. The overall response rate was 56% (9/16). All responders had partial responses.
In both PTCL and CTCL patients, treatment-related grade 3 or higher adverse events (AEs) included transaminitis (22%), rash (6%), neutropenia (6%), hypophosphatemia (2%), increased international normalized ratio (2%), diplopia secondary to neuropathy (2%), and sepsis (2%).
Treatment-related serious AEs included sepsis, increased international normalized ratio, diplopia secondary to neuropathy, and pyrexia. Five patients discontinued treatment due to AEs.
About orphan and fast track designations
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The FDA’s fast track drug development program is designed to expedite clinical development and submission of new drug applications for medicines with the potential to treat serious or life-threatening conditions and address unmet medical needs.
Fast track designation facilitates frequent interactions with the FDA review team, including meetings to discuss all aspects of development to support a drug’s approval, and also provides the opportunity to submit sections of a new drug application on a rolling basis as data become available. ![]()
*Data in the abstract differ from the presentation.
The US Food and Drug Administration (FDA) has granted orphan drug and fast track designations to tenalisib (RP6530) for the treatment of peripheral T-cell lymphoma (PTCL).
Tenalisib is a dual PI3K delta/gamma inhibitor being developed by Rhizen Pharmaceuticals.
Research has shown that tenalisib inhibits the growth of immortalized cancerous cell lines and primary leukemia/lymphoma cells.
In preclinical studies, tenalisib reprogrammed macrophages from an immunosuppressive M2-like phenotype (pro-tumor) to an inflammatory M1-like state (anti-tumor).
Researchers are currently conducting a phase 1 study of tenalisib in patients with relapsed/refractory PTCL. Results from this study were presented at the 2017 ASH Annual Meeting (abstract 2791*).
The presentation included data on 50 patients—24 with PTCL and 26 with cutaneous T-cell lymphoma (CTCL).
For the PTCL patients, the median age was 63 (range, 40-89), and 67% were male. The median number of prior therapies was 3 (range, 1-7). All patients had an ECOG status of 0 (n=14) or 1 (n=10). More patients had relapsed disease (n=17, 58%) than refractory disease (n=10, 42%).
For the CTCL patients, the median age was 67 (range, 37-84), and 46% were male. The median number of prior therapies was 5.5 (range, 2-15). All patients had an ECOG status of 0 (n=23) or 1 (n=3). More patients had refractory disease (n=15, 58%) than relapsed disease (n=11, 42%).
In the dose-escalation portion of the study, patients received tenalisib at 200 mg twice daily (BID), 400 mg BID, 800 mg BID fasting, or 800 mg BID fed. The maximum tolerated dose was 800 mg BID fasting, so this dose is being used in the expansion cohort.
Twelve PTCL patients were evaluable for efficacy. The overall response rate in these patients was 58% (7/12), with a 25% complete response rate (3/12).
Sixteen CTCL patients were evaluable for efficacy. The overall response rate was 56% (9/16). All responders had partial responses.
In both PTCL and CTCL patients, treatment-related grade 3 or higher adverse events (AEs) included transaminitis (22%), rash (6%), neutropenia (6%), hypophosphatemia (2%), increased international normalized ratio (2%), diplopia secondary to neuropathy (2%), and sepsis (2%).
Treatment-related serious AEs included sepsis, increased international normalized ratio, diplopia secondary to neuropathy, and pyrexia. Five patients discontinued treatment due to AEs.
About orphan and fast track designations
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The FDA’s fast track drug development program is designed to expedite clinical development and submission of new drug applications for medicines with the potential to treat serious or life-threatening conditions and address unmet medical needs.
Fast track designation facilitates frequent interactions with the FDA review team, including meetings to discuss all aspects of development to support a drug’s approval, and also provides the opportunity to submit sections of a new drug application on a rolling basis as data become available. ![]()
*Data in the abstract differ from the presentation.
The US Food and Drug Administration (FDA) has granted orphan drug and fast track designations to tenalisib (RP6530) for the treatment of peripheral T-cell lymphoma (PTCL).
Tenalisib is a dual PI3K delta/gamma inhibitor being developed by Rhizen Pharmaceuticals.
Research has shown that tenalisib inhibits the growth of immortalized cancerous cell lines and primary leukemia/lymphoma cells.
In preclinical studies, tenalisib reprogrammed macrophages from an immunosuppressive M2-like phenotype (pro-tumor) to an inflammatory M1-like state (anti-tumor).
Researchers are currently conducting a phase 1 study of tenalisib in patients with relapsed/refractory PTCL. Results from this study were presented at the 2017 ASH Annual Meeting (abstract 2791*).
The presentation included data on 50 patients—24 with PTCL and 26 with cutaneous T-cell lymphoma (CTCL).
For the PTCL patients, the median age was 63 (range, 40-89), and 67% were male. The median number of prior therapies was 3 (range, 1-7). All patients had an ECOG status of 0 (n=14) or 1 (n=10). More patients had relapsed disease (n=17, 58%) than refractory disease (n=10, 42%).
For the CTCL patients, the median age was 67 (range, 37-84), and 46% were male. The median number of prior therapies was 5.5 (range, 2-15). All patients had an ECOG status of 0 (n=23) or 1 (n=3). More patients had refractory disease (n=15, 58%) than relapsed disease (n=11, 42%).
In the dose-escalation portion of the study, patients received tenalisib at 200 mg twice daily (BID), 400 mg BID, 800 mg BID fasting, or 800 mg BID fed. The maximum tolerated dose was 800 mg BID fasting, so this dose is being used in the expansion cohort.
Twelve PTCL patients were evaluable for efficacy. The overall response rate in these patients was 58% (7/12), with a 25% complete response rate (3/12).
Sixteen CTCL patients were evaluable for efficacy. The overall response rate was 56% (9/16). All responders had partial responses.
In both PTCL and CTCL patients, treatment-related grade 3 or higher adverse events (AEs) included transaminitis (22%), rash (6%), neutropenia (6%), hypophosphatemia (2%), increased international normalized ratio (2%), diplopia secondary to neuropathy (2%), and sepsis (2%).
Treatment-related serious AEs included sepsis, increased international normalized ratio, diplopia secondary to neuropathy, and pyrexia. Five patients discontinued treatment due to AEs.
About orphan and fast track designations
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The FDA’s fast track drug development program is designed to expedite clinical development and submission of new drug applications for medicines with the potential to treat serious or life-threatening conditions and address unmet medical needs.
Fast track designation facilitates frequent interactions with the FDA review team, including meetings to discuss all aspects of development to support a drug’s approval, and also provides the opportunity to submit sections of a new drug application on a rolling basis as data become available. ![]()
*Data in the abstract differ from the presentation.
FDA grants orphan designation to drug for AML
The US Food and Drug Administration (FDA) has granted orphan drug designation to CG’806 for the treatment of patients with acute myeloid leukemia (AML).
CG’806 is an oral, first-in-class pan-FLT3/pan-BTK inhibitor being developed by Aptose Biosciences Inc.
In preclinical studies, CG’806 inhibited all wild-type and mutant forms of FLT3 tested, suppressed multiple oncogenic pathways operative in AML, and eliminated AML tumors (without toxicity) in murine xenograft models.
In addition, CG’806 demonstrated non-covalent inhibition of the wild-type and Cys481Ser mutant forms of the BTK enzyme, as well as other oncogenic kinases operative in B-cell malignancies.
Preclinical results with CG’806 were presented as posters at the AACR conference “Hematologic Malignancies: Translating Discoveries to Novel Therapies,” which took place last May.
“Results from non-clinical studies that we and our research collaborators have generated are promising and give reason for our eagerness to begin clinical trials in both AML and B-cell malignancies in 2018,” said William G. Rice, PhD, chairman, president, and chief executive officer at Aptose.
“We are pleased that the FDA has recognized the unique potential of CG’806 to address AML and has assigned CG’806 the status of orphan drug designation.”
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation to CG’806 for the treatment of patients with acute myeloid leukemia (AML).
CG’806 is an oral, first-in-class pan-FLT3/pan-BTK inhibitor being developed by Aptose Biosciences Inc.
In preclinical studies, CG’806 inhibited all wild-type and mutant forms of FLT3 tested, suppressed multiple oncogenic pathways operative in AML, and eliminated AML tumors (without toxicity) in murine xenograft models.
In addition, CG’806 demonstrated non-covalent inhibition of the wild-type and Cys481Ser mutant forms of the BTK enzyme, as well as other oncogenic kinases operative in B-cell malignancies.
Preclinical results with CG’806 were presented as posters at the AACR conference “Hematologic Malignancies: Translating Discoveries to Novel Therapies,” which took place last May.
“Results from non-clinical studies that we and our research collaborators have generated are promising and give reason for our eagerness to begin clinical trials in both AML and B-cell malignancies in 2018,” said William G. Rice, PhD, chairman, president, and chief executive officer at Aptose.
“We are pleased that the FDA has recognized the unique potential of CG’806 to address AML and has assigned CG’806 the status of orphan drug designation.”
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation to CG’806 for the treatment of patients with acute myeloid leukemia (AML).
CG’806 is an oral, first-in-class pan-FLT3/pan-BTK inhibitor being developed by Aptose Biosciences Inc.
In preclinical studies, CG’806 inhibited all wild-type and mutant forms of FLT3 tested, suppressed multiple oncogenic pathways operative in AML, and eliminated AML tumors (without toxicity) in murine xenograft models.
In addition, CG’806 demonstrated non-covalent inhibition of the wild-type and Cys481Ser mutant forms of the BTK enzyme, as well as other oncogenic kinases operative in B-cell malignancies.
Preclinical results with CG’806 were presented as posters at the AACR conference “Hematologic Malignancies: Translating Discoveries to Novel Therapies,” which took place last May.
“Results from non-clinical studies that we and our research collaborators have generated are promising and give reason for our eagerness to begin clinical trials in both AML and B-cell malignancies in 2018,” said William G. Rice, PhD, chairman, president, and chief executive officer at Aptose.
“We are pleased that the FDA has recognized the unique potential of CG’806 to address AML and has assigned CG’806 the status of orphan drug designation.”
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
Addition of azithromycin to maintenance therapy is beneficial in adults with uncontrolled asthma
Clinical question: Does azithromycin decrease the frequency of asthma exacerbations in adults with persistent asthma symptoms despite use of inhaled corticosteroid (ICS) and a long-acting beta-agonist (LABA)?
Background: Asthma is a chronic inflammatory airway disease that is highly prevalent worldwide within a subset of people with asthma who have symptoms that are poorly controlled despite ICS and LABA maintenance therapy. Currently, add-on therapy options include monoclonal antibodies, which are cost prohibitive. The need for additional therapeutic options exists. At the same time, macrolide antibiotics are known to have anti-inflammatory, antiviral, and antibacterial effects and have proven to have beneficial effects on asthma symptoms.
Setting: Multiple sites throughout Australia.
Synopsis: The AMAZES trial enrolled 420 adult patients with symptomatic asthma despite current use of ICS and LABA. Patients were randomly assigned to receive azithromycin 500 mg or placebo three times a week for 48 weeks. Patients who had hearing impairment, prolonged QTc interval, or emphysema were excluded.
Azithromycin reduced the frequency of asthma exacerbations, compared with placebo (1.07 vs. 1.86 exacerbations/patient-year; incidence rate ratio 0.59; 95% confidence interval, 0.47-0.74; P less than .0001). It also significantly improved asthma-related quality of life according to the Asthma Quality of Life Questionnaire (adjusted mean difference, 0.36; 95% CI, 0.21-0.52; P = .001). Diarrhea occurred more commonly in the azithromycin group but did not result in a higher withdrawal rate.
A significant limitation of this study was generalizability, as the median patient age was 60 years and race was not reported. More research is needed to determine the effect of long-term azithromycin use on microbial resistance.
Bottom line: Adding azithromycin to maintenance ICS and LABA therapy in patients with symptomatic asthma decreased the frequency of asthma exacerbations and improved quality of life.
Citation: Gibson PG et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): A randomised, double-blind, placebo-controlled trial. Lancet. 2017 Aug 12;390(10095):659-68.
Dr. Farber is a medical instructor, Duke University Health System.
Clinical question: Does azithromycin decrease the frequency of asthma exacerbations in adults with persistent asthma symptoms despite use of inhaled corticosteroid (ICS) and a long-acting beta-agonist (LABA)?
Background: Asthma is a chronic inflammatory airway disease that is highly prevalent worldwide within a subset of people with asthma who have symptoms that are poorly controlled despite ICS and LABA maintenance therapy. Currently, add-on therapy options include monoclonal antibodies, which are cost prohibitive. The need for additional therapeutic options exists. At the same time, macrolide antibiotics are known to have anti-inflammatory, antiviral, and antibacterial effects and have proven to have beneficial effects on asthma symptoms.
Setting: Multiple sites throughout Australia.
Synopsis: The AMAZES trial enrolled 420 adult patients with symptomatic asthma despite current use of ICS and LABA. Patients were randomly assigned to receive azithromycin 500 mg or placebo three times a week for 48 weeks. Patients who had hearing impairment, prolonged QTc interval, or emphysema were excluded.
Azithromycin reduced the frequency of asthma exacerbations, compared with placebo (1.07 vs. 1.86 exacerbations/patient-year; incidence rate ratio 0.59; 95% confidence interval, 0.47-0.74; P less than .0001). It also significantly improved asthma-related quality of life according to the Asthma Quality of Life Questionnaire (adjusted mean difference, 0.36; 95% CI, 0.21-0.52; P = .001). Diarrhea occurred more commonly in the azithromycin group but did not result in a higher withdrawal rate.
A significant limitation of this study was generalizability, as the median patient age was 60 years and race was not reported. More research is needed to determine the effect of long-term azithromycin use on microbial resistance.
Bottom line: Adding azithromycin to maintenance ICS and LABA therapy in patients with symptomatic asthma decreased the frequency of asthma exacerbations and improved quality of life.
Citation: Gibson PG et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): A randomised, double-blind, placebo-controlled trial. Lancet. 2017 Aug 12;390(10095):659-68.
Dr. Farber is a medical instructor, Duke University Health System.
Clinical question: Does azithromycin decrease the frequency of asthma exacerbations in adults with persistent asthma symptoms despite use of inhaled corticosteroid (ICS) and a long-acting beta-agonist (LABA)?
Background: Asthma is a chronic inflammatory airway disease that is highly prevalent worldwide within a subset of people with asthma who have symptoms that are poorly controlled despite ICS and LABA maintenance therapy. Currently, add-on therapy options include monoclonal antibodies, which are cost prohibitive. The need for additional therapeutic options exists. At the same time, macrolide antibiotics are known to have anti-inflammatory, antiviral, and antibacterial effects and have proven to have beneficial effects on asthma symptoms.
Setting: Multiple sites throughout Australia.
Synopsis: The AMAZES trial enrolled 420 adult patients with symptomatic asthma despite current use of ICS and LABA. Patients were randomly assigned to receive azithromycin 500 mg or placebo three times a week for 48 weeks. Patients who had hearing impairment, prolonged QTc interval, or emphysema were excluded.
Azithromycin reduced the frequency of asthma exacerbations, compared with placebo (1.07 vs. 1.86 exacerbations/patient-year; incidence rate ratio 0.59; 95% confidence interval, 0.47-0.74; P less than .0001). It also significantly improved asthma-related quality of life according to the Asthma Quality of Life Questionnaire (adjusted mean difference, 0.36; 95% CI, 0.21-0.52; P = .001). Diarrhea occurred more commonly in the azithromycin group but did not result in a higher withdrawal rate.
A significant limitation of this study was generalizability, as the median patient age was 60 years and race was not reported. More research is needed to determine the effect of long-term azithromycin use on microbial resistance.
Bottom line: Adding azithromycin to maintenance ICS and LABA therapy in patients with symptomatic asthma decreased the frequency of asthma exacerbations and improved quality of life.
Citation: Gibson PG et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): A randomised, double-blind, placebo-controlled trial. Lancet. 2017 Aug 12;390(10095):659-68.
Dr. Farber is a medical instructor, Duke University Health System.