User login
... The mother of direction
If you weren’t a young male living in the United States in the 1960s, it may be hard for you to understand my situation. It was a little more than 6 months from my college graduation. Because I couldn’t think of anything else to do, I had applied for and been accepted in a postgraduate fellowship in art history. However, it was clear that this country was becoming entangled in a confusing, unpopular – and from my personal perspective – a dangerous war.
While I was in college I was protected from the draft. But upon graduation, if I were to pursue my studies in something as unrelated to the war effort as art history, I would be ripe for the picking. I’m not sure why, but luckily I had been banking science credits for a rainy day. And in the winter of 1965-1966, it was raining big time.
I was not alone. Even if the term “gap year” had been coined, taking a year off to “find oneself” was not an option for young American males on the verge of high school or college graduation. I share this unflattering anecdote as evidence that there are times when circumstances can provide a floundering young person with a much needed sense of direction.
In May 2017, the Chicago Board of Education approved a plan sponsored by Mayor Rahm Emanuel that will require all high school students planning to graduate to provide evidence that they have secured a job or have been accepted by a college, trade apprenticeship, structured gap year program, or the military. (“Chicago won’t allow high school students to graduate without a plan for the future,” by Emma Brown, the Washington Post, July 3, 2017). Critics of the plan complain, probably with good reason, that the cash-strapped school system with more than 300,000 students doesn’t have the resources to provide its students with the counseling they will need to create the required post-graduation plans.
Even if there are too many devils in too many details in the Chicago plan, the principle underlying it is worth a try and deserves consideration by other school systems. It is not a universal military service requirement. Although, I wonder at times if this country should consider such a thing. It also is not a scheme cooked up by the business community to provide itself with cheap labor, although, it probably will.
In my mind, . Hopefully something that is productive or creative or at least something that improves your chances of living a life that is more likely to provide you with some degree of happiness. It offers a broad enough range of choices so that it is not overly prescriptive. If well administered, the plan would send the message to the graduating student that you must at least have a Plan A.
Regardless of whether a student’s patients come from affluent families with a myriad of post-graduation opportunities or from an economically challenged neighborhood in Chicago, I suspect that many of them would benefit from an artificial dose of necessity in the form of a message that doing nothing is not going to be an option.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@frontlinemedcom.com.
If you weren’t a young male living in the United States in the 1960s, it may be hard for you to understand my situation. It was a little more than 6 months from my college graduation. Because I couldn’t think of anything else to do, I had applied for and been accepted in a postgraduate fellowship in art history. However, it was clear that this country was becoming entangled in a confusing, unpopular – and from my personal perspective – a dangerous war.
While I was in college I was protected from the draft. But upon graduation, if I were to pursue my studies in something as unrelated to the war effort as art history, I would be ripe for the picking. I’m not sure why, but luckily I had been banking science credits for a rainy day. And in the winter of 1965-1966, it was raining big time.
I was not alone. Even if the term “gap year” had been coined, taking a year off to “find oneself” was not an option for young American males on the verge of high school or college graduation. I share this unflattering anecdote as evidence that there are times when circumstances can provide a floundering young person with a much needed sense of direction.
In May 2017, the Chicago Board of Education approved a plan sponsored by Mayor Rahm Emanuel that will require all high school students planning to graduate to provide evidence that they have secured a job or have been accepted by a college, trade apprenticeship, structured gap year program, or the military. (“Chicago won’t allow high school students to graduate without a plan for the future,” by Emma Brown, the Washington Post, July 3, 2017). Critics of the plan complain, probably with good reason, that the cash-strapped school system with more than 300,000 students doesn’t have the resources to provide its students with the counseling they will need to create the required post-graduation plans.
Even if there are too many devils in too many details in the Chicago plan, the principle underlying it is worth a try and deserves consideration by other school systems. It is not a universal military service requirement. Although, I wonder at times if this country should consider such a thing. It also is not a scheme cooked up by the business community to provide itself with cheap labor, although, it probably will.
In my mind, . Hopefully something that is productive or creative or at least something that improves your chances of living a life that is more likely to provide you with some degree of happiness. It offers a broad enough range of choices so that it is not overly prescriptive. If well administered, the plan would send the message to the graduating student that you must at least have a Plan A.
Regardless of whether a student’s patients come from affluent families with a myriad of post-graduation opportunities or from an economically challenged neighborhood in Chicago, I suspect that many of them would benefit from an artificial dose of necessity in the form of a message that doing nothing is not going to be an option.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@frontlinemedcom.com.
If you weren’t a young male living in the United States in the 1960s, it may be hard for you to understand my situation. It was a little more than 6 months from my college graduation. Because I couldn’t think of anything else to do, I had applied for and been accepted in a postgraduate fellowship in art history. However, it was clear that this country was becoming entangled in a confusing, unpopular – and from my personal perspective – a dangerous war.
While I was in college I was protected from the draft. But upon graduation, if I were to pursue my studies in something as unrelated to the war effort as art history, I would be ripe for the picking. I’m not sure why, but luckily I had been banking science credits for a rainy day. And in the winter of 1965-1966, it was raining big time.
I was not alone. Even if the term “gap year” had been coined, taking a year off to “find oneself” was not an option for young American males on the verge of high school or college graduation. I share this unflattering anecdote as evidence that there are times when circumstances can provide a floundering young person with a much needed sense of direction.
In May 2017, the Chicago Board of Education approved a plan sponsored by Mayor Rahm Emanuel that will require all high school students planning to graduate to provide evidence that they have secured a job or have been accepted by a college, trade apprenticeship, structured gap year program, or the military. (“Chicago won’t allow high school students to graduate without a plan for the future,” by Emma Brown, the Washington Post, July 3, 2017). Critics of the plan complain, probably with good reason, that the cash-strapped school system with more than 300,000 students doesn’t have the resources to provide its students with the counseling they will need to create the required post-graduation plans.
Even if there are too many devils in too many details in the Chicago plan, the principle underlying it is worth a try and deserves consideration by other school systems. It is not a universal military service requirement. Although, I wonder at times if this country should consider such a thing. It also is not a scheme cooked up by the business community to provide itself with cheap labor, although, it probably will.
In my mind, . Hopefully something that is productive or creative or at least something that improves your chances of living a life that is more likely to provide you with some degree of happiness. It offers a broad enough range of choices so that it is not overly prescriptive. If well administered, the plan would send the message to the graduating student that you must at least have a Plan A.
Regardless of whether a student’s patients come from affluent families with a myriad of post-graduation opportunities or from an economically challenged neighborhood in Chicago, I suspect that many of them would benefit from an artificial dose of necessity in the form of a message that doing nothing is not going to be an option.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@frontlinemedcom.com.
Implementation of a Patient Medication Disposal Program at a VA Medical Center
Opioid overdoses have quadrupled since 1999, with 78 Americans dying every day of opioid overdoses. More than half of all opioid overdose deaths involve prescription opioids.1 Attacking this problem from both ends—prescribing and disposal—can have a greater impact than focusing on a single strategy.
Background
In 2016, the CDC issued opioid prescription guidelines that included encouraging health care providers to discuss “options for safe disposal of unused opioids.”2 Pharmacies are prohibited from directly taking possession of controlled substances from a user. Historically, the Richard L. Roudebush VAMC (RLRVAMC) in Indianapolis, Indiana, recommended that patients follow FDA guidance for household medication disposal, which includes a list of medications that should be flushed down the toilet.3 As more data became available about the negative downstream environmental effects of pharmaceuticals on the water supply, this method of destruction made many patients feel uncomfortable.4,5
The Secure and Responsible Drug Disposal Act of 2010 presented additional options for hospitals and pharmacies to assist the public with medication disposal.6,7 These options offer convenience and anonymity for the end user, reduce potential for diversion, and enhance patient safety by ridding homes of unwanted and expired medications.
Prior to the 2010 act, the only legal methods of controlled substance disposal were via trash disposal, flushing, or delivery to law enforcement, typically at a community-based drug take-back event. These methods were not always convenient, environmentally friendly, or safe for other family members and pets in the home. The Secure and Responsible Drug Disposal Act of 2010 added 2 additional collection options for pharmacies: collection receptacles and mail-back programs.
The RLRVAMC treats > 62,000 veterans annually. The RLRVAMC Pharmacy Service had been providing pharmaceutical mail-back envelopes to patients since May 2015 with moderate success (271 lb of medications returned and a 22.8% envelope return rate through September 2016). Although the mail-back envelopes offer at-home convenience, there was no on-site disposal option. It is not uncommon for patients to bring medications to their appointment or the emergency department (ED). When medication reconciliation is performed, some medications are discontinued, and the prescriber wants them to be safely out of the patient’s possession to avoid confusion and/or accidental overdose. The purpose of this project was to offer more disposal options to patients through the addition of an on-site medication collection receptacle that would be in compliance with U.S. Drug Enforcement Agency (DEA) regulations.
Methods
A policy was developed between the RLRVAMC pharmacy and police departments for the management of a medication collection receptacle. Police would oversee the disposal program so that the pharmacy would not have to change its DEA registration to collector status. The 2 access keys to the receptacle were maintained by police and secured within a key accountability system in the police station.
Full liners are removed from the receptacle by 2 police officers and sealed securely according to vendor guidelines. In accordance with DEA regulations, a form is completed that documents the dates the inner liner was acquired, installed, removed, and transferred for destruction as well as the unique identification number and size of the liner, the address of the location where it was installed, and the names and signatures of the 2 employees that witnessed the removal.
Arrangements are made to have a mail courier present during the removal of the liner. Once removed, the liner is immediately sealed and released to the mail courier who transports the liner to the DEA authorized reverse distributor. The reverse distributor is a licensed entity that has the authority to take control of the medications, including controlled substances, for disposal. The liner tracking numbers are kept in a police log book so that delivery can be confirmed and destruction certificates obtained from the vendor’s website at a later date. The records are kept for 3 years.
Funding was obtained for a 38-gallon collection receptacle and 12 liners from Pharmacy Benefits Management Services (PBM). Approval was obtained from RLRVAMC leadership to locate the receptacle in a high-traffic area, anchored to the floor, under video surveillance, and away from the ED entrance (a DEA requirement). Public Affairs promoted the receptacle to veterans. E-mails were sent to staff to provide education on regulatory requirements. Weight and frequency of medications returned were obtained from data collected by the reverse distributor. Descriptive statistics are reported.
Results
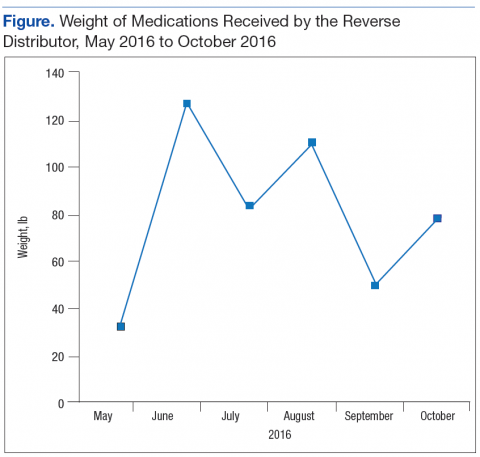
The Federal Supply Schedule cost to procure a DEA-compliant receptacle was $1,450. The additional 12 inner liners cost $2,024.97.8 Staff from Engineering Service were able to install the receptacle at no additional cost to the facility. The collection receptacle was opened to the public in May 2016. From May through October 2016, the facility collected and returned 10 liners to the reverse distributor containing 452 lb of medications. An additional 30 lb of drugs were returned through the mailback envelope program for a total weight of 482 lb over the 6-month period (Figure). The average time between inner liner changes was 2.6 weeks.
Discussion
The most challenging aspect of implementation was identification of a location for the receptacle. The location chosen, an alcove in the hallway between the coffee shop and outpatient pharmacy, was most appropriate. It is a high-traffic area, under video surveillance, and provides easy access for patients. Another challenge was determining the frequency of liner changes. There was no historic data to assist with predicting how quickly the liner would fill up. Initially, Police Service checked the receptacle every week, and it was emptied about every 2 weeks. Aspatients cleaned out their medicine cabinets, the liner needed to be replaced closer to every 4 weeks. An ongoing challenge has been determining how full the liner is without requiring the police to open the receptacle. Consideration is being given to installing a scale in the receptacle under the liner and having the display affixed to the outside of the container.
The receptacle seemed to be the preferred method of disposal, considering that it generated nearly 15 times more waste than did the mail-back envelopes during the same time period. Anecdotal patient feedback has been extremely positive on social media and by word-of-mouth.
Limitations
One limitation of this disposal program is that the specific amount of controlled substance waste vs noncontrolled substance waste cannot be determined since the liner contents are not inventoried. The University of Findlay in Ohio partnered with local law enforcement to host 7 community medication take-back events over a 3-year period, inventoried the drugs, and found that about one-third of the dosing units (eg, tablet or capsule) returned in the analgesic category were controlled substances, suggesting that take-back events may play a role in reducing unauthorized access to prescription painkillers.9 By witnessing the changing of inner liners, it can be anecdotally confirmed that a significant amount of controlled substances were collected and returned at RLRVAMC. These results have been shared with respective VISN leadership, and additional facilities are installing receptacles.
Conclusion
Changes to DEA regulations offer medical centers more options for developing a comprehensive drug disposal program. Implementation of a pharmaceutical take-back program can assist patients with disposal of unwanted and expired medications, promote safety and environmental stewardship, and reduce the risk of diversion.
1. Centers for Disease Control and Prevention. Opioid overdose. http://www.cdc.gov/drugoverdose/index.html. Updated April 16, 2017. Accessed June 5, 2017.
2. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49.
3. U.S. Food and Drug Administration. Disposal of unused medicines: what you should know. http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/EnsuringSafeUseofMedicine/SafeDisposalofMedicines/ucm186187.htm#Flush_List. Updated April 21, 2017. Accessed June 5, 2017.
4. Li WC. Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ Pollut. 2014;187:193-201.
5. Boxall AB. The environmental side effects of medication. EMBO Rep. 2004;5(12):1110-1116.
6. Peterson DM. New DEA rules expand options for controlled substance disposal. J Pain Palliat Care Pharmacother. 2015;29(1):22-26.
7. U.S. Department of Justice, Drug Enforcement Administration. Drug disposal information. https:// www.deadiversion.usdoj.gov/drug_disposal/index.html. Accessed June 5, 2016.
8. GSA Advantage! Online shopping. https://www.gsaadvantage.gov. Accessed June 5, 2017.
9. Perry LA, Shinn BW, Stanovich J. Quantification of an ongoing community-based medication take-back program. J Am Pharm Assoc (2003). 2014;54(3):275-279.
Opioid overdoses have quadrupled since 1999, with 78 Americans dying every day of opioid overdoses. More than half of all opioid overdose deaths involve prescription opioids.1 Attacking this problem from both ends—prescribing and disposal—can have a greater impact than focusing on a single strategy.
Background
In 2016, the CDC issued opioid prescription guidelines that included encouraging health care providers to discuss “options for safe disposal of unused opioids.”2 Pharmacies are prohibited from directly taking possession of controlled substances from a user. Historically, the Richard L. Roudebush VAMC (RLRVAMC) in Indianapolis, Indiana, recommended that patients follow FDA guidance for household medication disposal, which includes a list of medications that should be flushed down the toilet.3 As more data became available about the negative downstream environmental effects of pharmaceuticals on the water supply, this method of destruction made many patients feel uncomfortable.4,5
The Secure and Responsible Drug Disposal Act of 2010 presented additional options for hospitals and pharmacies to assist the public with medication disposal.6,7 These options offer convenience and anonymity for the end user, reduce potential for diversion, and enhance patient safety by ridding homes of unwanted and expired medications.
Prior to the 2010 act, the only legal methods of controlled substance disposal were via trash disposal, flushing, or delivery to law enforcement, typically at a community-based drug take-back event. These methods were not always convenient, environmentally friendly, or safe for other family members and pets in the home. The Secure and Responsible Drug Disposal Act of 2010 added 2 additional collection options for pharmacies: collection receptacles and mail-back programs.
The RLRVAMC treats > 62,000 veterans annually. The RLRVAMC Pharmacy Service had been providing pharmaceutical mail-back envelopes to patients since May 2015 with moderate success (271 lb of medications returned and a 22.8% envelope return rate through September 2016). Although the mail-back envelopes offer at-home convenience, there was no on-site disposal option. It is not uncommon for patients to bring medications to their appointment or the emergency department (ED). When medication reconciliation is performed, some medications are discontinued, and the prescriber wants them to be safely out of the patient’s possession to avoid confusion and/or accidental overdose. The purpose of this project was to offer more disposal options to patients through the addition of an on-site medication collection receptacle that would be in compliance with U.S. Drug Enforcement Agency (DEA) regulations.
Methods
A policy was developed between the RLRVAMC pharmacy and police departments for the management of a medication collection receptacle. Police would oversee the disposal program so that the pharmacy would not have to change its DEA registration to collector status. The 2 access keys to the receptacle were maintained by police and secured within a key accountability system in the police station.
Full liners are removed from the receptacle by 2 police officers and sealed securely according to vendor guidelines. In accordance with DEA regulations, a form is completed that documents the dates the inner liner was acquired, installed, removed, and transferred for destruction as well as the unique identification number and size of the liner, the address of the location where it was installed, and the names and signatures of the 2 employees that witnessed the removal.
Arrangements are made to have a mail courier present during the removal of the liner. Once removed, the liner is immediately sealed and released to the mail courier who transports the liner to the DEA authorized reverse distributor. The reverse distributor is a licensed entity that has the authority to take control of the medications, including controlled substances, for disposal. The liner tracking numbers are kept in a police log book so that delivery can be confirmed and destruction certificates obtained from the vendor’s website at a later date. The records are kept for 3 years.
Funding was obtained for a 38-gallon collection receptacle and 12 liners from Pharmacy Benefits Management Services (PBM). Approval was obtained from RLRVAMC leadership to locate the receptacle in a high-traffic area, anchored to the floor, under video surveillance, and away from the ED entrance (a DEA requirement). Public Affairs promoted the receptacle to veterans. E-mails were sent to staff to provide education on regulatory requirements. Weight and frequency of medications returned were obtained from data collected by the reverse distributor. Descriptive statistics are reported.
Results
The Federal Supply Schedule cost to procure a DEA-compliant receptacle was $1,450. The additional 12 inner liners cost $2,024.97.8 Staff from Engineering Service were able to install the receptacle at no additional cost to the facility. The collection receptacle was opened to the public in May 2016. From May through October 2016, the facility collected and returned 10 liners to the reverse distributor containing 452 lb of medications. An additional 30 lb of drugs were returned through the mailback envelope program for a total weight of 482 lb over the 6-month period (Figure). The average time between inner liner changes was 2.6 weeks.
Discussion
The most challenging aspect of implementation was identification of a location for the receptacle. The location chosen, an alcove in the hallway between the coffee shop and outpatient pharmacy, was most appropriate. It is a high-traffic area, under video surveillance, and provides easy access for patients. Another challenge was determining the frequency of liner changes. There was no historic data to assist with predicting how quickly the liner would fill up. Initially, Police Service checked the receptacle every week, and it was emptied about every 2 weeks. Aspatients cleaned out their medicine cabinets, the liner needed to be replaced closer to every 4 weeks. An ongoing challenge has been determining how full the liner is without requiring the police to open the receptacle. Consideration is being given to installing a scale in the receptacle under the liner and having the display affixed to the outside of the container.
The receptacle seemed to be the preferred method of disposal, considering that it generated nearly 15 times more waste than did the mail-back envelopes during the same time period. Anecdotal patient feedback has been extremely positive on social media and by word-of-mouth.
Limitations
One limitation of this disposal program is that the specific amount of controlled substance waste vs noncontrolled substance waste cannot be determined since the liner contents are not inventoried. The University of Findlay in Ohio partnered with local law enforcement to host 7 community medication take-back events over a 3-year period, inventoried the drugs, and found that about one-third of the dosing units (eg, tablet or capsule) returned in the analgesic category were controlled substances, suggesting that take-back events may play a role in reducing unauthorized access to prescription painkillers.9 By witnessing the changing of inner liners, it can be anecdotally confirmed that a significant amount of controlled substances were collected and returned at RLRVAMC. These results have been shared with respective VISN leadership, and additional facilities are installing receptacles.
Conclusion
Changes to DEA regulations offer medical centers more options for developing a comprehensive drug disposal program. Implementation of a pharmaceutical take-back program can assist patients with disposal of unwanted and expired medications, promote safety and environmental stewardship, and reduce the risk of diversion.
Opioid overdoses have quadrupled since 1999, with 78 Americans dying every day of opioid overdoses. More than half of all opioid overdose deaths involve prescription opioids.1 Attacking this problem from both ends—prescribing and disposal—can have a greater impact than focusing on a single strategy.
Background
In 2016, the CDC issued opioid prescription guidelines that included encouraging health care providers to discuss “options for safe disposal of unused opioids.”2 Pharmacies are prohibited from directly taking possession of controlled substances from a user. Historically, the Richard L. Roudebush VAMC (RLRVAMC) in Indianapolis, Indiana, recommended that patients follow FDA guidance for household medication disposal, which includes a list of medications that should be flushed down the toilet.3 As more data became available about the negative downstream environmental effects of pharmaceuticals on the water supply, this method of destruction made many patients feel uncomfortable.4,5
The Secure and Responsible Drug Disposal Act of 2010 presented additional options for hospitals and pharmacies to assist the public with medication disposal.6,7 These options offer convenience and anonymity for the end user, reduce potential for diversion, and enhance patient safety by ridding homes of unwanted and expired medications.
Prior to the 2010 act, the only legal methods of controlled substance disposal were via trash disposal, flushing, or delivery to law enforcement, typically at a community-based drug take-back event. These methods were not always convenient, environmentally friendly, or safe for other family members and pets in the home. The Secure and Responsible Drug Disposal Act of 2010 added 2 additional collection options for pharmacies: collection receptacles and mail-back programs.
The RLRVAMC treats > 62,000 veterans annually. The RLRVAMC Pharmacy Service had been providing pharmaceutical mail-back envelopes to patients since May 2015 with moderate success (271 lb of medications returned and a 22.8% envelope return rate through September 2016). Although the mail-back envelopes offer at-home convenience, there was no on-site disposal option. It is not uncommon for patients to bring medications to their appointment or the emergency department (ED). When medication reconciliation is performed, some medications are discontinued, and the prescriber wants them to be safely out of the patient’s possession to avoid confusion and/or accidental overdose. The purpose of this project was to offer more disposal options to patients through the addition of an on-site medication collection receptacle that would be in compliance with U.S. Drug Enforcement Agency (DEA) regulations.
Methods
A policy was developed between the RLRVAMC pharmacy and police departments for the management of a medication collection receptacle. Police would oversee the disposal program so that the pharmacy would not have to change its DEA registration to collector status. The 2 access keys to the receptacle were maintained by police and secured within a key accountability system in the police station.
Full liners are removed from the receptacle by 2 police officers and sealed securely according to vendor guidelines. In accordance with DEA regulations, a form is completed that documents the dates the inner liner was acquired, installed, removed, and transferred for destruction as well as the unique identification number and size of the liner, the address of the location where it was installed, and the names and signatures of the 2 employees that witnessed the removal.
Arrangements are made to have a mail courier present during the removal of the liner. Once removed, the liner is immediately sealed and released to the mail courier who transports the liner to the DEA authorized reverse distributor. The reverse distributor is a licensed entity that has the authority to take control of the medications, including controlled substances, for disposal. The liner tracking numbers are kept in a police log book so that delivery can be confirmed and destruction certificates obtained from the vendor’s website at a later date. The records are kept for 3 years.
Funding was obtained for a 38-gallon collection receptacle and 12 liners from Pharmacy Benefits Management Services (PBM). Approval was obtained from RLRVAMC leadership to locate the receptacle in a high-traffic area, anchored to the floor, under video surveillance, and away from the ED entrance (a DEA requirement). Public Affairs promoted the receptacle to veterans. E-mails were sent to staff to provide education on regulatory requirements. Weight and frequency of medications returned were obtained from data collected by the reverse distributor. Descriptive statistics are reported.
Results
The Federal Supply Schedule cost to procure a DEA-compliant receptacle was $1,450. The additional 12 inner liners cost $2,024.97.8 Staff from Engineering Service were able to install the receptacle at no additional cost to the facility. The collection receptacle was opened to the public in May 2016. From May through October 2016, the facility collected and returned 10 liners to the reverse distributor containing 452 lb of medications. An additional 30 lb of drugs were returned through the mailback envelope program for a total weight of 482 lb over the 6-month period (Figure). The average time between inner liner changes was 2.6 weeks.
Discussion
The most challenging aspect of implementation was identification of a location for the receptacle. The location chosen, an alcove in the hallway between the coffee shop and outpatient pharmacy, was most appropriate. It is a high-traffic area, under video surveillance, and provides easy access for patients. Another challenge was determining the frequency of liner changes. There was no historic data to assist with predicting how quickly the liner would fill up. Initially, Police Service checked the receptacle every week, and it was emptied about every 2 weeks. Aspatients cleaned out their medicine cabinets, the liner needed to be replaced closer to every 4 weeks. An ongoing challenge has been determining how full the liner is without requiring the police to open the receptacle. Consideration is being given to installing a scale in the receptacle under the liner and having the display affixed to the outside of the container.
The receptacle seemed to be the preferred method of disposal, considering that it generated nearly 15 times more waste than did the mail-back envelopes during the same time period. Anecdotal patient feedback has been extremely positive on social media and by word-of-mouth.
Limitations
One limitation of this disposal program is that the specific amount of controlled substance waste vs noncontrolled substance waste cannot be determined since the liner contents are not inventoried. The University of Findlay in Ohio partnered with local law enforcement to host 7 community medication take-back events over a 3-year period, inventoried the drugs, and found that about one-third of the dosing units (eg, tablet or capsule) returned in the analgesic category were controlled substances, suggesting that take-back events may play a role in reducing unauthorized access to prescription painkillers.9 By witnessing the changing of inner liners, it can be anecdotally confirmed that a significant amount of controlled substances were collected and returned at RLRVAMC. These results have been shared with respective VISN leadership, and additional facilities are installing receptacles.
Conclusion
Changes to DEA regulations offer medical centers more options for developing a comprehensive drug disposal program. Implementation of a pharmaceutical take-back program can assist patients with disposal of unwanted and expired medications, promote safety and environmental stewardship, and reduce the risk of diversion.
1. Centers for Disease Control and Prevention. Opioid overdose. http://www.cdc.gov/drugoverdose/index.html. Updated April 16, 2017. Accessed June 5, 2017.
2. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49.
3. U.S. Food and Drug Administration. Disposal of unused medicines: what you should know. http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/EnsuringSafeUseofMedicine/SafeDisposalofMedicines/ucm186187.htm#Flush_List. Updated April 21, 2017. Accessed June 5, 2017.
4. Li WC. Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ Pollut. 2014;187:193-201.
5. Boxall AB. The environmental side effects of medication. EMBO Rep. 2004;5(12):1110-1116.
6. Peterson DM. New DEA rules expand options for controlled substance disposal. J Pain Palliat Care Pharmacother. 2015;29(1):22-26.
7. U.S. Department of Justice, Drug Enforcement Administration. Drug disposal information. https:// www.deadiversion.usdoj.gov/drug_disposal/index.html. Accessed June 5, 2016.
8. GSA Advantage! Online shopping. https://www.gsaadvantage.gov. Accessed June 5, 2017.
9. Perry LA, Shinn BW, Stanovich J. Quantification of an ongoing community-based medication take-back program. J Am Pharm Assoc (2003). 2014;54(3):275-279.
1. Centers for Disease Control and Prevention. Opioid overdose. http://www.cdc.gov/drugoverdose/index.html. Updated April 16, 2017. Accessed June 5, 2017.
2. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49.
3. U.S. Food and Drug Administration. Disposal of unused medicines: what you should know. http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/EnsuringSafeUseofMedicine/SafeDisposalofMedicines/ucm186187.htm#Flush_List. Updated April 21, 2017. Accessed June 5, 2017.
4. Li WC. Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ Pollut. 2014;187:193-201.
5. Boxall AB. The environmental side effects of medication. EMBO Rep. 2004;5(12):1110-1116.
6. Peterson DM. New DEA rules expand options for controlled substance disposal. J Pain Palliat Care Pharmacother. 2015;29(1):22-26.
7. U.S. Department of Justice, Drug Enforcement Administration. Drug disposal information. https:// www.deadiversion.usdoj.gov/drug_disposal/index.html. Accessed June 5, 2016.
8. GSA Advantage! Online shopping. https://www.gsaadvantage.gov. Accessed June 5, 2017.
9. Perry LA, Shinn BW, Stanovich J. Quantification of an ongoing community-based medication take-back program. J Am Pharm Assoc (2003). 2014;54(3):275-279.
Modified, CB-derived NK cells target CLL and BL
Natural killer (NK) cells derived from cord blood (CB) can be modified to fight chronic lymphocytic leukemia (CLL) and Burkitt lymphoma (BL), according to research published in Leukemia.
Using a viral vector, researchers transduced CB-derived NK cells with a chimeric antigen receptor (CAR), interleukin-15 (IL-15), and an inducible caspase-9-based suicide gene.
The CAR directed the NK cells to kill CD19-expressing cells, IL-15 prolonged the NK cells’ survival and enhanced their antitumor activity, and the suicide gene allowed researchers to kill off the NK cells in the event of a severe inflammatory response.
“Natural killer cells are the immune system’s most potent killers, but they are short-lived, and cancers manage to evade a patient’s own NK cells to progress,” said study author Katy Rezvani, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston.
“Our cord-blood derived NK cells, genetically equipped with a receptor that focuses them on B-cell malignancies and with interleukin-15 to help them persist longer—potentially for months instead of 2 or 3 weeks—are designed to address these challenges.”
Dr Rezvani and her colleagues tested their CB-derived CAR NK cells in primary CLL cells and a mouse model of BL. Compared to unmodified NK cells, the CAR NK cells killed malignant cells more efficiently and extended the survival of mice.
Another experiment showed the CB-derived CAR NK cells killed CLL cells more efficiently than NK cells that were taken from CLL patients and modified in the same way. The researchers said this highlights the need to transplant CAR-engineered NK cells derived from healthy CB rather than using a patient’s own cells.
Additional experiments in the mouse model of BL showed that a single infusion of low-dose CAR NK cells resulted in prolonged survival.
When CAR NK cells were given at a higher dose, none of the mice died of BL. Half of them survived 100 days and beyond. However, all mice treated with other types of NK cells died by day 41.
Some mice treated with the higher dose of CAR NK cells died of cytokine release syndrome (CRS).
To counteract this toxicity, the researchers activated the suicide gene (iC9) via treatment with a small-molecule dimerizer, AP1903.
The team found that adding as little as 10 nM of AP1903 to cell cultures induced apoptosis/necrosis of the CAR NK cells within 4 hours but had no effect on non-CAR CB-derived NK cells. Similar results were observed in the mouse model.
Next steps
A phase 1/2 trial of these CB-derived CAR NK cells opened at MD Anderson in June for patients with relapsed or refractory CLL, acute lymphocytic leukemia, or non-Hodgkin lymphoma.
Dr Rezvani, the principal investigator of the trial, said the protocol calls for vigilance for signs of CRS, treatment with steroids and tocilizumab for low-grade CRS, and AP1903 added to activate the suicide gene for grade 3 or 4 CRS.
She and her colleagues noted that CB-derived NK cells do not cause graft-vs-host disease. Therefore, they can be an off-the-shelf product, prepared in advance with the necessary receptor and given to patients promptly.
The researchers are developing CB-derived CAR NK cells for other targets in hematologic and solid tumor malignancies.
MD Anderson and the researchers have intellectual property related to the engineered NK cells, which is being managed in accordance with the institution’s conflict-of-interest rules.
Funding for this research was provided by MD Anderson’s Moon Shots Program, the National Cancer Institute of the National Institutes of Health Cancer Center Support Grant (CA016672) to MD Anderson, and grants from the Leukemia and Lymphoma Society and the American Cancer Society. ![]()
Natural killer (NK) cells derived from cord blood (CB) can be modified to fight chronic lymphocytic leukemia (CLL) and Burkitt lymphoma (BL), according to research published in Leukemia.
Using a viral vector, researchers transduced CB-derived NK cells with a chimeric antigen receptor (CAR), interleukin-15 (IL-15), and an inducible caspase-9-based suicide gene.
The CAR directed the NK cells to kill CD19-expressing cells, IL-15 prolonged the NK cells’ survival and enhanced their antitumor activity, and the suicide gene allowed researchers to kill off the NK cells in the event of a severe inflammatory response.
“Natural killer cells are the immune system’s most potent killers, but they are short-lived, and cancers manage to evade a patient’s own NK cells to progress,” said study author Katy Rezvani, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston.
“Our cord-blood derived NK cells, genetically equipped with a receptor that focuses them on B-cell malignancies and with interleukin-15 to help them persist longer—potentially for months instead of 2 or 3 weeks—are designed to address these challenges.”
Dr Rezvani and her colleagues tested their CB-derived CAR NK cells in primary CLL cells and a mouse model of BL. Compared to unmodified NK cells, the CAR NK cells killed malignant cells more efficiently and extended the survival of mice.
Another experiment showed the CB-derived CAR NK cells killed CLL cells more efficiently than NK cells that were taken from CLL patients and modified in the same way. The researchers said this highlights the need to transplant CAR-engineered NK cells derived from healthy CB rather than using a patient’s own cells.
Additional experiments in the mouse model of BL showed that a single infusion of low-dose CAR NK cells resulted in prolonged survival.
When CAR NK cells were given at a higher dose, none of the mice died of BL. Half of them survived 100 days and beyond. However, all mice treated with other types of NK cells died by day 41.
Some mice treated with the higher dose of CAR NK cells died of cytokine release syndrome (CRS).
To counteract this toxicity, the researchers activated the suicide gene (iC9) via treatment with a small-molecule dimerizer, AP1903.
The team found that adding as little as 10 nM of AP1903 to cell cultures induced apoptosis/necrosis of the CAR NK cells within 4 hours but had no effect on non-CAR CB-derived NK cells. Similar results were observed in the mouse model.
Next steps
A phase 1/2 trial of these CB-derived CAR NK cells opened at MD Anderson in June for patients with relapsed or refractory CLL, acute lymphocytic leukemia, or non-Hodgkin lymphoma.
Dr Rezvani, the principal investigator of the trial, said the protocol calls for vigilance for signs of CRS, treatment with steroids and tocilizumab for low-grade CRS, and AP1903 added to activate the suicide gene for grade 3 or 4 CRS.
She and her colleagues noted that CB-derived NK cells do not cause graft-vs-host disease. Therefore, they can be an off-the-shelf product, prepared in advance with the necessary receptor and given to patients promptly.
The researchers are developing CB-derived CAR NK cells for other targets in hematologic and solid tumor malignancies.
MD Anderson and the researchers have intellectual property related to the engineered NK cells, which is being managed in accordance with the institution’s conflict-of-interest rules.
Funding for this research was provided by MD Anderson’s Moon Shots Program, the National Cancer Institute of the National Institutes of Health Cancer Center Support Grant (CA016672) to MD Anderson, and grants from the Leukemia and Lymphoma Society and the American Cancer Society. ![]()
Natural killer (NK) cells derived from cord blood (CB) can be modified to fight chronic lymphocytic leukemia (CLL) and Burkitt lymphoma (BL), according to research published in Leukemia.
Using a viral vector, researchers transduced CB-derived NK cells with a chimeric antigen receptor (CAR), interleukin-15 (IL-15), and an inducible caspase-9-based suicide gene.
The CAR directed the NK cells to kill CD19-expressing cells, IL-15 prolonged the NK cells’ survival and enhanced their antitumor activity, and the suicide gene allowed researchers to kill off the NK cells in the event of a severe inflammatory response.
“Natural killer cells are the immune system’s most potent killers, but they are short-lived, and cancers manage to evade a patient’s own NK cells to progress,” said study author Katy Rezvani, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston.
“Our cord-blood derived NK cells, genetically equipped with a receptor that focuses them on B-cell malignancies and with interleukin-15 to help them persist longer—potentially for months instead of 2 or 3 weeks—are designed to address these challenges.”
Dr Rezvani and her colleagues tested their CB-derived CAR NK cells in primary CLL cells and a mouse model of BL. Compared to unmodified NK cells, the CAR NK cells killed malignant cells more efficiently and extended the survival of mice.
Another experiment showed the CB-derived CAR NK cells killed CLL cells more efficiently than NK cells that were taken from CLL patients and modified in the same way. The researchers said this highlights the need to transplant CAR-engineered NK cells derived from healthy CB rather than using a patient’s own cells.
Additional experiments in the mouse model of BL showed that a single infusion of low-dose CAR NK cells resulted in prolonged survival.
When CAR NK cells were given at a higher dose, none of the mice died of BL. Half of them survived 100 days and beyond. However, all mice treated with other types of NK cells died by day 41.
Some mice treated with the higher dose of CAR NK cells died of cytokine release syndrome (CRS).
To counteract this toxicity, the researchers activated the suicide gene (iC9) via treatment with a small-molecule dimerizer, AP1903.
The team found that adding as little as 10 nM of AP1903 to cell cultures induced apoptosis/necrosis of the CAR NK cells within 4 hours but had no effect on non-CAR CB-derived NK cells. Similar results were observed in the mouse model.
Next steps
A phase 1/2 trial of these CB-derived CAR NK cells opened at MD Anderson in June for patients with relapsed or refractory CLL, acute lymphocytic leukemia, or non-Hodgkin lymphoma.
Dr Rezvani, the principal investigator of the trial, said the protocol calls for vigilance for signs of CRS, treatment with steroids and tocilizumab for low-grade CRS, and AP1903 added to activate the suicide gene for grade 3 or 4 CRS.
She and her colleagues noted that CB-derived NK cells do not cause graft-vs-host disease. Therefore, they can be an off-the-shelf product, prepared in advance with the necessary receptor and given to patients promptly.
The researchers are developing CB-derived CAR NK cells for other targets in hematologic and solid tumor malignancies.
MD Anderson and the researchers have intellectual property related to the engineered NK cells, which is being managed in accordance with the institution’s conflict-of-interest rules.
Funding for this research was provided by MD Anderson’s Moon Shots Program, the National Cancer Institute of the National Institutes of Health Cancer Center Support Grant (CA016672) to MD Anderson, and grants from the Leukemia and Lymphoma Society and the American Cancer Society. ![]()
AYA cancer survivors struggle with social functioning
New research suggests young cancer survivors struggle to get their social lives “back to normal” within the first 2 years of their diagnosis.
The study showed that adolescents and young adults (AYAs) with cancer had significantly worse social functioning than the general population around the time of cancer diagnosis as well as 1 year and 2 years later.
These findings were published in Cancer.
“The research is important to help these young survivors better reintegrate into society,” said study author Brad Zebrack, PhD, of the University of Michigan in Ann Arbor.
He and his colleagues collected data from 141 AYA cancer patients (ages 14 to 39) who visited 1 of 5 US medical facilities between March 2008 and April 2010.
The patients completed a self-report measure of social functioning within the first 4 months of diagnosis, then again at 12 months and 24 months.
Compared to the general population, the AYA cancer patients had significantly worse social functioning scores at all time points:
- Around the time of diagnosis—52.0 vs 85.1 (P<0.001)
- At 12 months—73.1 vs 85.1 (P<0.001)
- At 24 months—69.2 vs 85.1 (P<0.001).
Overall, the patients did experience improvements in social functioning from baseline to the 12-month time point, but their scores remained stable after that.
The researchers noted that 9% of patients had consistently high/normal social functioning, 47% had improvements in social functioning over time, 13% had worsening social functioning over time, and 32% had consistently low social functioning.
“This finding highlights the need to screen, identify, and respond to the needs of high-risk young adult-adolescent patients at the time of diagnosis and then monitor them over time,” Dr Zebrack said.
“They are likely the ones most in need of help in managing work, school, and potentially problematic relationships with family members and friends.”![]()
New research suggests young cancer survivors struggle to get their social lives “back to normal” within the first 2 years of their diagnosis.
The study showed that adolescents and young adults (AYAs) with cancer had significantly worse social functioning than the general population around the time of cancer diagnosis as well as 1 year and 2 years later.
These findings were published in Cancer.
“The research is important to help these young survivors better reintegrate into society,” said study author Brad Zebrack, PhD, of the University of Michigan in Ann Arbor.
He and his colleagues collected data from 141 AYA cancer patients (ages 14 to 39) who visited 1 of 5 US medical facilities between March 2008 and April 2010.
The patients completed a self-report measure of social functioning within the first 4 months of diagnosis, then again at 12 months and 24 months.
Compared to the general population, the AYA cancer patients had significantly worse social functioning scores at all time points:
- Around the time of diagnosis—52.0 vs 85.1 (P<0.001)
- At 12 months—73.1 vs 85.1 (P<0.001)
- At 24 months—69.2 vs 85.1 (P<0.001).
Overall, the patients did experience improvements in social functioning from baseline to the 12-month time point, but their scores remained stable after that.
The researchers noted that 9% of patients had consistently high/normal social functioning, 47% had improvements in social functioning over time, 13% had worsening social functioning over time, and 32% had consistently low social functioning.
“This finding highlights the need to screen, identify, and respond to the needs of high-risk young adult-adolescent patients at the time of diagnosis and then monitor them over time,” Dr Zebrack said.
“They are likely the ones most in need of help in managing work, school, and potentially problematic relationships with family members and friends.”![]()
New research suggests young cancer survivors struggle to get their social lives “back to normal” within the first 2 years of their diagnosis.
The study showed that adolescents and young adults (AYAs) with cancer had significantly worse social functioning than the general population around the time of cancer diagnosis as well as 1 year and 2 years later.
These findings were published in Cancer.
“The research is important to help these young survivors better reintegrate into society,” said study author Brad Zebrack, PhD, of the University of Michigan in Ann Arbor.
He and his colleagues collected data from 141 AYA cancer patients (ages 14 to 39) who visited 1 of 5 US medical facilities between March 2008 and April 2010.
The patients completed a self-report measure of social functioning within the first 4 months of diagnosis, then again at 12 months and 24 months.
Compared to the general population, the AYA cancer patients had significantly worse social functioning scores at all time points:
- Around the time of diagnosis—52.0 vs 85.1 (P<0.001)
- At 12 months—73.1 vs 85.1 (P<0.001)
- At 24 months—69.2 vs 85.1 (P<0.001).
Overall, the patients did experience improvements in social functioning from baseline to the 12-month time point, but their scores remained stable after that.
The researchers noted that 9% of patients had consistently high/normal social functioning, 47% had improvements in social functioning over time, 13% had worsening social functioning over time, and 32% had consistently low social functioning.
“This finding highlights the need to screen, identify, and respond to the needs of high-risk young adult-adolescent patients at the time of diagnosis and then monitor them over time,” Dr Zebrack said.
“They are likely the ones most in need of help in managing work, school, and potentially problematic relationships with family members and friends.”![]()
FDA grants orphan designation to gilteritinib in AML
The US Food and Drug Administration (FDA) has granted orphan drug designation to gilteritinib for the treatment of patients with acute myeloid leukemia (AML).
Gilteritinib, an inhibitor of FLT3 and AXL, has demonstrated activity against FLT3 internal tandem duplication (ITD) as well as tyrosine kinase domain, 2 mutations that are seen in up to a third of patients with AML.
Astellas Pharma Inc. is currently investigating gilteritinib in phase 3 trials of AML patients.
Results from a phase 1/2 study of gilteritinib in AML were presented at the 2017 ASCO Annual Meeting.
The goal of the study was to determine the tolerability and antileukemic activity of once-daily gilteritinib in a FLT3-ITD-enriched, relapsed/refractory AML population.
The drug exhibited “potent” FLT3 inhibition at doses greater than 80 mg/day. In patients who received such doses, the greatest overall response rate was 52%, and the longest median overall survival was 31 weeks.
The maximum tolerated dose of gilteritinib was 300 mg/day. Dose-limiting toxicities included diarrhea and liver function abnormalities.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation to gilteritinib for the treatment of patients with acute myeloid leukemia (AML).
Gilteritinib, an inhibitor of FLT3 and AXL, has demonstrated activity against FLT3 internal tandem duplication (ITD) as well as tyrosine kinase domain, 2 mutations that are seen in up to a third of patients with AML.
Astellas Pharma Inc. is currently investigating gilteritinib in phase 3 trials of AML patients.
Results from a phase 1/2 study of gilteritinib in AML were presented at the 2017 ASCO Annual Meeting.
The goal of the study was to determine the tolerability and antileukemic activity of once-daily gilteritinib in a FLT3-ITD-enriched, relapsed/refractory AML population.
The drug exhibited “potent” FLT3 inhibition at doses greater than 80 mg/day. In patients who received such doses, the greatest overall response rate was 52%, and the longest median overall survival was 31 weeks.
The maximum tolerated dose of gilteritinib was 300 mg/day. Dose-limiting toxicities included diarrhea and liver function abnormalities.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation to gilteritinib for the treatment of patients with acute myeloid leukemia (AML).
Gilteritinib, an inhibitor of FLT3 and AXL, has demonstrated activity against FLT3 internal tandem duplication (ITD) as well as tyrosine kinase domain, 2 mutations that are seen in up to a third of patients with AML.
Astellas Pharma Inc. is currently investigating gilteritinib in phase 3 trials of AML patients.
Results from a phase 1/2 study of gilteritinib in AML were presented at the 2017 ASCO Annual Meeting.
The goal of the study was to determine the tolerability and antileukemic activity of once-daily gilteritinib in a FLT3-ITD-enriched, relapsed/refractory AML population.
The drug exhibited “potent” FLT3 inhibition at doses greater than 80 mg/day. In patients who received such doses, the greatest overall response rate was 52%, and the longest median overall survival was 31 weeks.
The maximum tolerated dose of gilteritinib was 300 mg/day. Dose-limiting toxicities included diarrhea and liver function abnormalities.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
FDA approves generic tranexamic acid
Zydus Cadila has received approval from the US Food and Drug Administration (FDA) to market a tranexamic acid product for use in patients with hemophilia.
The company’s product—Tranexamic Acid Injection, 1000 mg/10 mL (100 mg/mL) Single Dose Vial—can be used to prevent or reduce bleeding in hemophilia patients undergoing tooth extraction.
Zydus Cadila’s tranexamic acid will be produced at a manufacturing facility in Moraiya, Gujarat, India. ![]()
Zydus Cadila has received approval from the US Food and Drug Administration (FDA) to market a tranexamic acid product for use in patients with hemophilia.
The company’s product—Tranexamic Acid Injection, 1000 mg/10 mL (100 mg/mL) Single Dose Vial—can be used to prevent or reduce bleeding in hemophilia patients undergoing tooth extraction.
Zydus Cadila’s tranexamic acid will be produced at a manufacturing facility in Moraiya, Gujarat, India. ![]()
Zydus Cadila has received approval from the US Food and Drug Administration (FDA) to market a tranexamic acid product for use in patients with hemophilia.
The company’s product—Tranexamic Acid Injection, 1000 mg/10 mL (100 mg/mL) Single Dose Vial—can be used to prevent or reduce bleeding in hemophilia patients undergoing tooth extraction.
Zydus Cadila’s tranexamic acid will be produced at a manufacturing facility in Moraiya, Gujarat, India. ![]()
Statewide QI learning collaboratives improve asthma care
Care of children with asthma can be improved using statewide quality improvement (QI) learning collaboratives, said Judith C. Dolins, of the American Academy of Pediatrics (AAP), Elk Grove Village, Ill., and her associates.
AAP chapters demonstrated this through their implementation of the Chapter Quality Network (CQN) project, which was supported by the national organization. This project sought to encourage practice changes in asthma care in a number of states using statewide QI learning collaboratives and lead practices to implement the National Heart, Lung, and Blood Institute’s (NHLBI) 2007 asthma guidelines over the course of four time periods, which the researchers referred to as waves. In nine states, 19 AAP chapters engaged 180 practices to participate, and involved 749 pediatricians treating 45,431 patients (Pediatrics. 2017. doi: 10.1542/peds.2016-1612).
Across all waves, practices had reasonably high baseline use of the stepwise approach (81%-89%) and a controller medication for patients with persistent asthma (74%-88%). The patients with a current written asthma action plan increased from 49%-57% to 75%-91%. Physicians’ and parents’ ratings of asthma in children as “well controlled” rose modestly from 58%-63% to 71%-74% and from 67%-73% to 78%-87%, respectively. Patients receiving self-management materials increased from 57%-62% to 62%-88%.
Here’s how the project worked. The CQN had three linked frameworks.: One provided a methodology for spreading practice changes, another outlined the elements of care, and a third provided a framework so the practices could test, implement, and adapt changes.
A national AAP leadership team of experts in asthma care, QI, and primary care practice systems developed a set of key drivers and interventions, and an implementation guide that included resources, tools and methods, and a set of measures for the chapters. They also designed reporting tools and a curriculum fostering QI learning by chapter leaders. Then, in a state chapter leadership learning network and a practice collaborative model in each state, there were “multiple in-person learning sessions and defined action periods, best practices, and tests of change that were spread and incorporated” for the chapters to use, according to the study report.
Chapters in each state had monthly webinars and two in-person learning sessions for participating practices. They also reviewed data reports at the state and practice level and provided coaching for practices.
Practices were asked to “incorporate optimal NHLBI asthma care practices, including assessment of control, a stepwise approach to treatment, appropriate use of controller medication, and an updated asthma action plan for self-management.” Other interventions included use of an asthma encounter form (which collected 16 measures, including outcome and process measures that were entered into a national database at least monthly); introduction of population registries; workflow assessment; motivational interviews; plan-do-study-act cycles for QI; and review of data for QI.
All the physicians were eligible to earn MOC part 4 credit and continuing medical education credit, and 80% of the physicians did get MOC credit across the 4 waves.
“Our findings are notable for achieving improvement in asthma care through training multiple state coordinating entities instead of directly leading the learning collaborative itself. Few other projects have achieved this level of results in pediatric practice improvement in asthma care on such a widespread scale across multiple states. This project may serve as a model for other statewide and national organizations attempting to achieve improvements in population health through support and training for statewide organizations that, in turn, support individual health care providers and practices,” Ms. Dolins and her associates said. “Although none of the waves achieved the preset goal of 90%, all showed substantial improvement and remarkable consistency in the rate of improvement. We, therefore, regarded the project as an overall success.”
The study was funded by the Merck Childhood Asthma Network, American Board of Pediatrics Foundation, American Academy of Pediatrics Friends of Children Fund, the JPB Foundation, and GlaxoSmithKline. Ms. Dolins and Mr. Wise received salary support. Ms. Powell and Dr. Stemmler received consulting fees.
We can do a better job of taking care of our patients. And in no other area is there more room for quality improvement (QI) than in ambulatory care. In the article by Dolins et al., substantial improvements were reported in outpatient management meeting the standard of care for asthma. In my experience, doing so not only gives children a better functional outcome but also saves costs by avoiding unnecessary ED visits and hospitalization and generates new dollars for pediatric offices through the additional services they provide. We have seen similar improvements in our own work in South Carolina and Tennessee. The practices that have wanted to become involved in our asthma QI are those that are already doing a better than average job. Yet, they still generate substantial additional improvements.
The AAP study relied heavily on national experts and standards. Although their input is valued, the true advantage of QI is the opportunity for pediatricians and their staff at the service delivery level to generate their own changes within the context of a learning collaborative. True improvement and innovation is facilitated by allowing flexibility at the local level and sharing the ideas that result. Those that design the QI efforts of the future need to make sure they allow for local expertise rather than leaning too heavily on centrally predetermined change concepts.
Part IV Maintenance of Certification requirements from the American Board of Pediatrics help provide momentum for successful pediatric QI. The National Improvement Partnership Network has formed pediatric outpatient learning collaboratives to share ideas. As we identify more successes with ambulatory care QI, as more and more organizations like AAP state chapters become involved, as all practices – rather than just the best – recognize the obligation to promote quality, we have a tremendous opportunity to improve the developmental and health outcomes of the children. QI can promote work and cost efficiencies that facilitate our work as pediatricians, making our lives more productive and rewarding.
Francis E. Rushton Jr, MD, is a pediatrician and medical director of South Carolina’s QTIP (Quality Through Innovation in Pediatrics) network and the Quality Director for PHIIT (Pediatric Health Improvement In Tennessee). He reported not having any relevant financial disclosures.
We can do a better job of taking care of our patients. And in no other area is there more room for quality improvement (QI) than in ambulatory care. In the article by Dolins et al., substantial improvements were reported in outpatient management meeting the standard of care for asthma. In my experience, doing so not only gives children a better functional outcome but also saves costs by avoiding unnecessary ED visits and hospitalization and generates new dollars for pediatric offices through the additional services they provide. We have seen similar improvements in our own work in South Carolina and Tennessee. The practices that have wanted to become involved in our asthma QI are those that are already doing a better than average job. Yet, they still generate substantial additional improvements.
The AAP study relied heavily on national experts and standards. Although their input is valued, the true advantage of QI is the opportunity for pediatricians and their staff at the service delivery level to generate their own changes within the context of a learning collaborative. True improvement and innovation is facilitated by allowing flexibility at the local level and sharing the ideas that result. Those that design the QI efforts of the future need to make sure they allow for local expertise rather than leaning too heavily on centrally predetermined change concepts.
Part IV Maintenance of Certification requirements from the American Board of Pediatrics help provide momentum for successful pediatric QI. The National Improvement Partnership Network has formed pediatric outpatient learning collaboratives to share ideas. As we identify more successes with ambulatory care QI, as more and more organizations like AAP state chapters become involved, as all practices – rather than just the best – recognize the obligation to promote quality, we have a tremendous opportunity to improve the developmental and health outcomes of the children. QI can promote work and cost efficiencies that facilitate our work as pediatricians, making our lives more productive and rewarding.
Francis E. Rushton Jr, MD, is a pediatrician and medical director of South Carolina’s QTIP (Quality Through Innovation in Pediatrics) network and the Quality Director for PHIIT (Pediatric Health Improvement In Tennessee). He reported not having any relevant financial disclosures.
We can do a better job of taking care of our patients. And in no other area is there more room for quality improvement (QI) than in ambulatory care. In the article by Dolins et al., substantial improvements were reported in outpatient management meeting the standard of care for asthma. In my experience, doing so not only gives children a better functional outcome but also saves costs by avoiding unnecessary ED visits and hospitalization and generates new dollars for pediatric offices through the additional services they provide. We have seen similar improvements in our own work in South Carolina and Tennessee. The practices that have wanted to become involved in our asthma QI are those that are already doing a better than average job. Yet, they still generate substantial additional improvements.
The AAP study relied heavily on national experts and standards. Although their input is valued, the true advantage of QI is the opportunity for pediatricians and their staff at the service delivery level to generate their own changes within the context of a learning collaborative. True improvement and innovation is facilitated by allowing flexibility at the local level and sharing the ideas that result. Those that design the QI efforts of the future need to make sure they allow for local expertise rather than leaning too heavily on centrally predetermined change concepts.
Part IV Maintenance of Certification requirements from the American Board of Pediatrics help provide momentum for successful pediatric QI. The National Improvement Partnership Network has formed pediatric outpatient learning collaboratives to share ideas. As we identify more successes with ambulatory care QI, as more and more organizations like AAP state chapters become involved, as all practices – rather than just the best – recognize the obligation to promote quality, we have a tremendous opportunity to improve the developmental and health outcomes of the children. QI can promote work and cost efficiencies that facilitate our work as pediatricians, making our lives more productive and rewarding.
Francis E. Rushton Jr, MD, is a pediatrician and medical director of South Carolina’s QTIP (Quality Through Innovation in Pediatrics) network and the Quality Director for PHIIT (Pediatric Health Improvement In Tennessee). He reported not having any relevant financial disclosures.
Care of children with asthma can be improved using statewide quality improvement (QI) learning collaboratives, said Judith C. Dolins, of the American Academy of Pediatrics (AAP), Elk Grove Village, Ill., and her associates.
AAP chapters demonstrated this through their implementation of the Chapter Quality Network (CQN) project, which was supported by the national organization. This project sought to encourage practice changes in asthma care in a number of states using statewide QI learning collaboratives and lead practices to implement the National Heart, Lung, and Blood Institute’s (NHLBI) 2007 asthma guidelines over the course of four time periods, which the researchers referred to as waves. In nine states, 19 AAP chapters engaged 180 practices to participate, and involved 749 pediatricians treating 45,431 patients (Pediatrics. 2017. doi: 10.1542/peds.2016-1612).
Across all waves, practices had reasonably high baseline use of the stepwise approach (81%-89%) and a controller medication for patients with persistent asthma (74%-88%). The patients with a current written asthma action plan increased from 49%-57% to 75%-91%. Physicians’ and parents’ ratings of asthma in children as “well controlled” rose modestly from 58%-63% to 71%-74% and from 67%-73% to 78%-87%, respectively. Patients receiving self-management materials increased from 57%-62% to 62%-88%.
Here’s how the project worked. The CQN had three linked frameworks.: One provided a methodology for spreading practice changes, another outlined the elements of care, and a third provided a framework so the practices could test, implement, and adapt changes.
A national AAP leadership team of experts in asthma care, QI, and primary care practice systems developed a set of key drivers and interventions, and an implementation guide that included resources, tools and methods, and a set of measures for the chapters. They also designed reporting tools and a curriculum fostering QI learning by chapter leaders. Then, in a state chapter leadership learning network and a practice collaborative model in each state, there were “multiple in-person learning sessions and defined action periods, best practices, and tests of change that were spread and incorporated” for the chapters to use, according to the study report.
Chapters in each state had monthly webinars and two in-person learning sessions for participating practices. They also reviewed data reports at the state and practice level and provided coaching for practices.
Practices were asked to “incorporate optimal NHLBI asthma care practices, including assessment of control, a stepwise approach to treatment, appropriate use of controller medication, and an updated asthma action plan for self-management.” Other interventions included use of an asthma encounter form (which collected 16 measures, including outcome and process measures that were entered into a national database at least monthly); introduction of population registries; workflow assessment; motivational interviews; plan-do-study-act cycles for QI; and review of data for QI.
All the physicians were eligible to earn MOC part 4 credit and continuing medical education credit, and 80% of the physicians did get MOC credit across the 4 waves.
“Our findings are notable for achieving improvement in asthma care through training multiple state coordinating entities instead of directly leading the learning collaborative itself. Few other projects have achieved this level of results in pediatric practice improvement in asthma care on such a widespread scale across multiple states. This project may serve as a model for other statewide and national organizations attempting to achieve improvements in population health through support and training for statewide organizations that, in turn, support individual health care providers and practices,” Ms. Dolins and her associates said. “Although none of the waves achieved the preset goal of 90%, all showed substantial improvement and remarkable consistency in the rate of improvement. We, therefore, regarded the project as an overall success.”
The study was funded by the Merck Childhood Asthma Network, American Board of Pediatrics Foundation, American Academy of Pediatrics Friends of Children Fund, the JPB Foundation, and GlaxoSmithKline. Ms. Dolins and Mr. Wise received salary support. Ms. Powell and Dr. Stemmler received consulting fees.
Care of children with asthma can be improved using statewide quality improvement (QI) learning collaboratives, said Judith C. Dolins, of the American Academy of Pediatrics (AAP), Elk Grove Village, Ill., and her associates.
AAP chapters demonstrated this through their implementation of the Chapter Quality Network (CQN) project, which was supported by the national organization. This project sought to encourage practice changes in asthma care in a number of states using statewide QI learning collaboratives and lead practices to implement the National Heart, Lung, and Blood Institute’s (NHLBI) 2007 asthma guidelines over the course of four time periods, which the researchers referred to as waves. In nine states, 19 AAP chapters engaged 180 practices to participate, and involved 749 pediatricians treating 45,431 patients (Pediatrics. 2017. doi: 10.1542/peds.2016-1612).
Across all waves, practices had reasonably high baseline use of the stepwise approach (81%-89%) and a controller medication for patients with persistent asthma (74%-88%). The patients with a current written asthma action plan increased from 49%-57% to 75%-91%. Physicians’ and parents’ ratings of asthma in children as “well controlled” rose modestly from 58%-63% to 71%-74% and from 67%-73% to 78%-87%, respectively. Patients receiving self-management materials increased from 57%-62% to 62%-88%.
Here’s how the project worked. The CQN had three linked frameworks.: One provided a methodology for spreading practice changes, another outlined the elements of care, and a third provided a framework so the practices could test, implement, and adapt changes.
A national AAP leadership team of experts in asthma care, QI, and primary care practice systems developed a set of key drivers and interventions, and an implementation guide that included resources, tools and methods, and a set of measures for the chapters. They also designed reporting tools and a curriculum fostering QI learning by chapter leaders. Then, in a state chapter leadership learning network and a practice collaborative model in each state, there were “multiple in-person learning sessions and defined action periods, best practices, and tests of change that were spread and incorporated” for the chapters to use, according to the study report.
Chapters in each state had monthly webinars and two in-person learning sessions for participating practices. They also reviewed data reports at the state and practice level and provided coaching for practices.
Practices were asked to “incorporate optimal NHLBI asthma care practices, including assessment of control, a stepwise approach to treatment, appropriate use of controller medication, and an updated asthma action plan for self-management.” Other interventions included use of an asthma encounter form (which collected 16 measures, including outcome and process measures that were entered into a national database at least monthly); introduction of population registries; workflow assessment; motivational interviews; plan-do-study-act cycles for QI; and review of data for QI.
All the physicians were eligible to earn MOC part 4 credit and continuing medical education credit, and 80% of the physicians did get MOC credit across the 4 waves.
“Our findings are notable for achieving improvement in asthma care through training multiple state coordinating entities instead of directly leading the learning collaborative itself. Few other projects have achieved this level of results in pediatric practice improvement in asthma care on such a widespread scale across multiple states. This project may serve as a model for other statewide and national organizations attempting to achieve improvements in population health through support and training for statewide organizations that, in turn, support individual health care providers and practices,” Ms. Dolins and her associates said. “Although none of the waves achieved the preset goal of 90%, all showed substantial improvement and remarkable consistency in the rate of improvement. We, therefore, regarded the project as an overall success.”
The study was funded by the Merck Childhood Asthma Network, American Board of Pediatrics Foundation, American Academy of Pediatrics Friends of Children Fund, the JPB Foundation, and GlaxoSmithKline. Ms. Dolins and Mr. Wise received salary support. Ms. Powell and Dr. Stemmler received consulting fees.
FROM PEDIATRICS
Key clinical point:
Major finding: In wave 4, during which data were collected for 9 months, the rate of optimal asthma care at each encounter improved from 38% to 72%.
Data source: In nine states, 19 AAP chapters engaged 180 practices to participate in quality improvement learning collaboratives, which involved 749 pediatricians treating 45,431 patients.
Disclosures: The study was funded by the Merck Childhood Asthma Network, American Board of Pediatrics Foundation, American Academy of Pediatrics Friends of Children Fund, the JPB Foundation, and GlaxoSmithKline. Ms. Dolins and Mr. Wise received salary support. Ms. Powell and Dr. Stemmler received consulting fees.
Rivaroxaban or aspirin for extended treatment of VTE
TITLE: Low-dose or full-dose rivaroxaban is superior to aspirin for long-term anticoagulation
CLINICAL QUESTION: Is full or lower intensity rivaroxaban better for extended treatment of venous thromboembolism (VTE) as compared to aspirin?
STUDY DESIGN: Multicenter randomized double-blinded phase III trial.
SETTING: 230 Medical centers worldwide in 20 countries.
SYNOPSIS: 3,365 patients with a history of VTE who had undergone 6-12 months of initial anticoagulation therapy and in whom continuation of therapy was thought to be beneficial were enrolled. Patients were randomly assigned to daily high-dose rivaroxaban (20 mg) or daily low-dose rivaroxaban (10 mg) or aspirin (100 mg). After a median of 351 days, symptomatic recurrent VTE or unexplained death occurred in 17 of the 1,107 patients (1.5%) who were assigned to the high-dose group, in 13 of 1,127 patients (1.2%) who were assigned to the low-dose group, and in 50 of 1,131 patients (4.4%) who were assigned to aspirin. Bleeding rates were not significantly different between the three groups (2%-3%). The major limitation of this study is the short duration of follow-up and the lack of power to demonstrate noninferiority of the low-dose as compared to the high-dose regimen for rivaroxaban.
BOTTOM LINE: In patients with a history of VTE, in whom prolonged anticoagulation could be beneficial, low or high-dose rivaroxaban is superior to aspirin in preventing recurrent VTE without increasing bleeding risk.
CITATION: Weitz JI, Lensing MH, Prins R, et al. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med. 2017; 376:1211-22.
Dr. Mayasy is assistant professor in the department of hospital medicine at the University of New Mexico.
TITLE: Low-dose or full-dose rivaroxaban is superior to aspirin for long-term anticoagulation
CLINICAL QUESTION: Is full or lower intensity rivaroxaban better for extended treatment of venous thromboembolism (VTE) as compared to aspirin?
STUDY DESIGN: Multicenter randomized double-blinded phase III trial.
SETTING: 230 Medical centers worldwide in 20 countries.
SYNOPSIS: 3,365 patients with a history of VTE who had undergone 6-12 months of initial anticoagulation therapy and in whom continuation of therapy was thought to be beneficial were enrolled. Patients were randomly assigned to daily high-dose rivaroxaban (20 mg) or daily low-dose rivaroxaban (10 mg) or aspirin (100 mg). After a median of 351 days, symptomatic recurrent VTE or unexplained death occurred in 17 of the 1,107 patients (1.5%) who were assigned to the high-dose group, in 13 of 1,127 patients (1.2%) who were assigned to the low-dose group, and in 50 of 1,131 patients (4.4%) who were assigned to aspirin. Bleeding rates were not significantly different between the three groups (2%-3%). The major limitation of this study is the short duration of follow-up and the lack of power to demonstrate noninferiority of the low-dose as compared to the high-dose regimen for rivaroxaban.
BOTTOM LINE: In patients with a history of VTE, in whom prolonged anticoagulation could be beneficial, low or high-dose rivaroxaban is superior to aspirin in preventing recurrent VTE without increasing bleeding risk.
CITATION: Weitz JI, Lensing MH, Prins R, et al. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med. 2017; 376:1211-22.
Dr. Mayasy is assistant professor in the department of hospital medicine at the University of New Mexico.
TITLE: Low-dose or full-dose rivaroxaban is superior to aspirin for long-term anticoagulation
CLINICAL QUESTION: Is full or lower intensity rivaroxaban better for extended treatment of venous thromboembolism (VTE) as compared to aspirin?
STUDY DESIGN: Multicenter randomized double-blinded phase III trial.
SETTING: 230 Medical centers worldwide in 20 countries.
SYNOPSIS: 3,365 patients with a history of VTE who had undergone 6-12 months of initial anticoagulation therapy and in whom continuation of therapy was thought to be beneficial were enrolled. Patients were randomly assigned to daily high-dose rivaroxaban (20 mg) or daily low-dose rivaroxaban (10 mg) or aspirin (100 mg). After a median of 351 days, symptomatic recurrent VTE or unexplained death occurred in 17 of the 1,107 patients (1.5%) who were assigned to the high-dose group, in 13 of 1,127 patients (1.2%) who were assigned to the low-dose group, and in 50 of 1,131 patients (4.4%) who were assigned to aspirin. Bleeding rates were not significantly different between the three groups (2%-3%). The major limitation of this study is the short duration of follow-up and the lack of power to demonstrate noninferiority of the low-dose as compared to the high-dose regimen for rivaroxaban.
BOTTOM LINE: In patients with a history of VTE, in whom prolonged anticoagulation could be beneficial, low or high-dose rivaroxaban is superior to aspirin in preventing recurrent VTE without increasing bleeding risk.
CITATION: Weitz JI, Lensing MH, Prins R, et al. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med. 2017; 376:1211-22.
Dr. Mayasy is assistant professor in the department of hospital medicine at the University of New Mexico.
Life-long risk reduction could cut late-life dementia by up to 35%
LONDON – As many as a third of dementia cases could be prevented worldwide if society could adopt a life course–focused approach of supporting brain health with mostly common-sense measures.
Improving childhood education, controlling blood pressure and cholesterol, keeping socially and intellectually active, exercising, and ceasing tobacco use are among the recommendations to reduce the incidence of dementia made by a worldwide panel of expert clinicians and researchers.
The findings are part of an exhaustive report commission by The Lancet and released at the Alzheimer’s Association International Conference. The report has concluded that nine lifestyle factors, most of which are modifiable from childhood though middle age, account for 35% of dementia that strikes elderly persons, Gillian Livingston, MD, said at the conference. The report was simultaneously published.
Being homozygous for the ApoE4 allele confers about an immutable 7% increased chance of developing Alzheimer’s disease. But two of the other factors identified in the Lancet report, low education in childhood and hearing loss at middle age, confer even higher individual risks of 8% and 9%. And when combined with other mid-life risks of hypertension and obesity, and late-life risks imposed by smoking, depression, inactivity, social isolation, and diabetes, these factors not only dwarf the potential impact of ApoE4, but offer a lifelong chance to forestall or even prevent dementia.
The findings, all gathered from an exhaustive literature review, bolster the notion that public health interventions could block the tsunami of dementia cases that threaten to overwhelm the world’s health care resources by 2050, Dr. Livingston said.
“While public health interventions won’t prevent or cure all potentially modifiable dementia, intervention for cardiovascular risk factors, mental health, and hearing may push back the onset in many people for years. Even if only some of this promise is realized, it could make a huge difference. We have, in fact, already seen that in some populations dementia is being delayed for years. If we could achieve an overall delay of onset by 5 years, we could cut the global prevalence by half.”
The Lancet commissioned the panel of global dementia experts to review the extant literature and construct a lifespan-focused risk model. In addition to examining risk and making recommendations to ameliorate it, the panel issued recommendations about treating cognition and psychiatric and behavioral problems; protecting dementia patients in both home and long-term care settings; supporting the family members who provide most of the care for dementia patients; and helping patients and families navigate end-of-life situations.
The literature review identified nine modifiable risk factors that account for 35% of dementia risk worldwide:
• Education in youth. Less education in childhood, which the commission identified as a lack of secondary schooling, increased the risk of dementia by 8%. Improving education at this age would remove this portion of the population attributable risk factor (PAF), Dr. Livingston said.
This finding represents an enormous opportunity for improvement: The decline in dementia incidence seen in some populations occurs mostly among the better-educated. “The mechanism of prevention here appears to be increasing brain resilience,” said Lon Schneider, MD, a member of the Lancet panel.
Tackling poor childhood education is a daunting task and requires commitment from both public and private sectors, the report noted, but its importance cannot be overstated.
• Hearing loss at mid-life. This emerged as the most powerful risk factor in the analysis, conferring an independent 9% increased risk of dementia, “a relatively new idea that has not been included in previous calculations of population attributable factors,” the commission wrote. The mechanism of increased risk isn’t clear, but may be a combination of neurodegeneration and social isolation imposed by being shut out of easy communication. It’s unclear whether hearing aids can mitigate the effects of hearing loss on dementia risk, the report noted.
• Hypertension, obesity, and diabetes. Respectively, these accounted for 2%, 1%, and 1% of the PAF. Obesity is linked to prediabetes and diabetes, which are in turn linked to insulin resistance, decreased brain amyloid clearance, high blood glucose, and inflammation – all risks for Alzheimer’s disease.
The report recommends that anyone aged 45-65 years who has hypertension should be actively treated for the disorder.
• Smoking. At 5%, smoking posted the third-highest PAF. The risk is probably mediated through smoking’s detrimental effects on cardiovascular health. But the report noted that tobacco smoke contains known neurotoxins as well.
Preventing the smoking/dementia connection is simple, Dr. Livingston said. “Simply stop smoking. If you’re smoking, just stop. Please.”
• Depression. Depression in late life imposed a 4% PAF. The evidence reviewed suggested that depression is not, in fact, linked to dementia when experienced at mid-life. But late-life depression may be a prodromal symptom of dementia and biologically linked to increased stress hormones, decreased neuronal growth factors, and decreased hippocampal volume. The commission noted animal models that suggest some antidepressants, including citalopram, decrease amyloid progression.
• Social isolation. Associated with a 2% PAF, social isolation may, like depression, be a prodromal symptom. But, the report said, there is growing evidence that it actually is an independent risk factor as well. It has been shown to also increase the risk of hypertension, cardiovascular disease, and depression, all dementia risk factors in their own right.
Longitudinal studies suggest that social activities and personal connections may prevent or delay dementia, although top-grade evidence is lacking. Still, maintaining a rich social network not only reduces the chance of isolation, but helps prevent depression as well.
• Physical inactivity. Sedentary lifestyle carried a 3% PAF for dementia. Older adults who maintain physical activity are more likely to remain cognitively intact. Physical exercise improves mood, reduces the risk of falls, and maintains normal physical function. The report cited a meta-analysis of 16 studies and almost 164,000 participants without dementia; it concluded that those in the highest level of activity had a 25% decreased risk of all-cause dementia and a 45% decreased risk of Alzheimer’s disease.
The strongest evidence for exercise’s benefit on cognition may be from the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER). Patients with a high risk of dementia who completed the lifestyle modification program of healthy diet cognitive training, vascular risk factor management, and aerobic exercise showed a slowing of cognitive decline and improvements in executive function and processing speed.
Becoming aware of the risk factors is one thing, the report said. Doing something about them is another. In general, the first step is to “be ambitious” about prevention.
“Prevention is always better than treatment,” Dr. Livingston said in an interview. “We need to start thinking about dementia not as something that simply happens outside our control, but as something that we can have an effect on.”
The Lancet commissioned the report. Dr. Livingston did not have any financial declarations but many of the other authors reported multiple relationships with pharmaceutical companies.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
LONDON – As many as a third of dementia cases could be prevented worldwide if society could adopt a life course–focused approach of supporting brain health with mostly common-sense measures.
Improving childhood education, controlling blood pressure and cholesterol, keeping socially and intellectually active, exercising, and ceasing tobacco use are among the recommendations to reduce the incidence of dementia made by a worldwide panel of expert clinicians and researchers.
The findings are part of an exhaustive report commission by The Lancet and released at the Alzheimer’s Association International Conference. The report has concluded that nine lifestyle factors, most of which are modifiable from childhood though middle age, account for 35% of dementia that strikes elderly persons, Gillian Livingston, MD, said at the conference. The report was simultaneously published.
Being homozygous for the ApoE4 allele confers about an immutable 7% increased chance of developing Alzheimer’s disease. But two of the other factors identified in the Lancet report, low education in childhood and hearing loss at middle age, confer even higher individual risks of 8% and 9%. And when combined with other mid-life risks of hypertension and obesity, and late-life risks imposed by smoking, depression, inactivity, social isolation, and diabetes, these factors not only dwarf the potential impact of ApoE4, but offer a lifelong chance to forestall or even prevent dementia.
The findings, all gathered from an exhaustive literature review, bolster the notion that public health interventions could block the tsunami of dementia cases that threaten to overwhelm the world’s health care resources by 2050, Dr. Livingston said.
“While public health interventions won’t prevent or cure all potentially modifiable dementia, intervention for cardiovascular risk factors, mental health, and hearing may push back the onset in many people for years. Even if only some of this promise is realized, it could make a huge difference. We have, in fact, already seen that in some populations dementia is being delayed for years. If we could achieve an overall delay of onset by 5 years, we could cut the global prevalence by half.”
The Lancet commissioned the panel of global dementia experts to review the extant literature and construct a lifespan-focused risk model. In addition to examining risk and making recommendations to ameliorate it, the panel issued recommendations about treating cognition and psychiatric and behavioral problems; protecting dementia patients in both home and long-term care settings; supporting the family members who provide most of the care for dementia patients; and helping patients and families navigate end-of-life situations.
The literature review identified nine modifiable risk factors that account for 35% of dementia risk worldwide:
• Education in youth. Less education in childhood, which the commission identified as a lack of secondary schooling, increased the risk of dementia by 8%. Improving education at this age would remove this portion of the population attributable risk factor (PAF), Dr. Livingston said.
This finding represents an enormous opportunity for improvement: The decline in dementia incidence seen in some populations occurs mostly among the better-educated. “The mechanism of prevention here appears to be increasing brain resilience,” said Lon Schneider, MD, a member of the Lancet panel.
Tackling poor childhood education is a daunting task and requires commitment from both public and private sectors, the report noted, but its importance cannot be overstated.
• Hearing loss at mid-life. This emerged as the most powerful risk factor in the analysis, conferring an independent 9% increased risk of dementia, “a relatively new idea that has not been included in previous calculations of population attributable factors,” the commission wrote. The mechanism of increased risk isn’t clear, but may be a combination of neurodegeneration and social isolation imposed by being shut out of easy communication. It’s unclear whether hearing aids can mitigate the effects of hearing loss on dementia risk, the report noted.
• Hypertension, obesity, and diabetes. Respectively, these accounted for 2%, 1%, and 1% of the PAF. Obesity is linked to prediabetes and diabetes, which are in turn linked to insulin resistance, decreased brain amyloid clearance, high blood glucose, and inflammation – all risks for Alzheimer’s disease.
The report recommends that anyone aged 45-65 years who has hypertension should be actively treated for the disorder.
• Smoking. At 5%, smoking posted the third-highest PAF. The risk is probably mediated through smoking’s detrimental effects on cardiovascular health. But the report noted that tobacco smoke contains known neurotoxins as well.
Preventing the smoking/dementia connection is simple, Dr. Livingston said. “Simply stop smoking. If you’re smoking, just stop. Please.”
• Depression. Depression in late life imposed a 4% PAF. The evidence reviewed suggested that depression is not, in fact, linked to dementia when experienced at mid-life. But late-life depression may be a prodromal symptom of dementia and biologically linked to increased stress hormones, decreased neuronal growth factors, and decreased hippocampal volume. The commission noted animal models that suggest some antidepressants, including citalopram, decrease amyloid progression.
• Social isolation. Associated with a 2% PAF, social isolation may, like depression, be a prodromal symptom. But, the report said, there is growing evidence that it actually is an independent risk factor as well. It has been shown to also increase the risk of hypertension, cardiovascular disease, and depression, all dementia risk factors in their own right.
Longitudinal studies suggest that social activities and personal connections may prevent or delay dementia, although top-grade evidence is lacking. Still, maintaining a rich social network not only reduces the chance of isolation, but helps prevent depression as well.
• Physical inactivity. Sedentary lifestyle carried a 3% PAF for dementia. Older adults who maintain physical activity are more likely to remain cognitively intact. Physical exercise improves mood, reduces the risk of falls, and maintains normal physical function. The report cited a meta-analysis of 16 studies and almost 164,000 participants without dementia; it concluded that those in the highest level of activity had a 25% decreased risk of all-cause dementia and a 45% decreased risk of Alzheimer’s disease.
The strongest evidence for exercise’s benefit on cognition may be from the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER). Patients with a high risk of dementia who completed the lifestyle modification program of healthy diet cognitive training, vascular risk factor management, and aerobic exercise showed a slowing of cognitive decline and improvements in executive function and processing speed.
Becoming aware of the risk factors is one thing, the report said. Doing something about them is another. In general, the first step is to “be ambitious” about prevention.
“Prevention is always better than treatment,” Dr. Livingston said in an interview. “We need to start thinking about dementia not as something that simply happens outside our control, but as something that we can have an effect on.”
The Lancet commissioned the report. Dr. Livingston did not have any financial declarations but many of the other authors reported multiple relationships with pharmaceutical companies.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
LONDON – As many as a third of dementia cases could be prevented worldwide if society could adopt a life course–focused approach of supporting brain health with mostly common-sense measures.
Improving childhood education, controlling blood pressure and cholesterol, keeping socially and intellectually active, exercising, and ceasing tobacco use are among the recommendations to reduce the incidence of dementia made by a worldwide panel of expert clinicians and researchers.
The findings are part of an exhaustive report commission by The Lancet and released at the Alzheimer’s Association International Conference. The report has concluded that nine lifestyle factors, most of which are modifiable from childhood though middle age, account for 35% of dementia that strikes elderly persons, Gillian Livingston, MD, said at the conference. The report was simultaneously published.
Being homozygous for the ApoE4 allele confers about an immutable 7% increased chance of developing Alzheimer’s disease. But two of the other factors identified in the Lancet report, low education in childhood and hearing loss at middle age, confer even higher individual risks of 8% and 9%. And when combined with other mid-life risks of hypertension and obesity, and late-life risks imposed by smoking, depression, inactivity, social isolation, and diabetes, these factors not only dwarf the potential impact of ApoE4, but offer a lifelong chance to forestall or even prevent dementia.
The findings, all gathered from an exhaustive literature review, bolster the notion that public health interventions could block the tsunami of dementia cases that threaten to overwhelm the world’s health care resources by 2050, Dr. Livingston said.
“While public health interventions won’t prevent or cure all potentially modifiable dementia, intervention for cardiovascular risk factors, mental health, and hearing may push back the onset in many people for years. Even if only some of this promise is realized, it could make a huge difference. We have, in fact, already seen that in some populations dementia is being delayed for years. If we could achieve an overall delay of onset by 5 years, we could cut the global prevalence by half.”
The Lancet commissioned the panel of global dementia experts to review the extant literature and construct a lifespan-focused risk model. In addition to examining risk and making recommendations to ameliorate it, the panel issued recommendations about treating cognition and psychiatric and behavioral problems; protecting dementia patients in both home and long-term care settings; supporting the family members who provide most of the care for dementia patients; and helping patients and families navigate end-of-life situations.
The literature review identified nine modifiable risk factors that account for 35% of dementia risk worldwide:
• Education in youth. Less education in childhood, which the commission identified as a lack of secondary schooling, increased the risk of dementia by 8%. Improving education at this age would remove this portion of the population attributable risk factor (PAF), Dr. Livingston said.
This finding represents an enormous opportunity for improvement: The decline in dementia incidence seen in some populations occurs mostly among the better-educated. “The mechanism of prevention here appears to be increasing brain resilience,” said Lon Schneider, MD, a member of the Lancet panel.
Tackling poor childhood education is a daunting task and requires commitment from both public and private sectors, the report noted, but its importance cannot be overstated.
• Hearing loss at mid-life. This emerged as the most powerful risk factor in the analysis, conferring an independent 9% increased risk of dementia, “a relatively new idea that has not been included in previous calculations of population attributable factors,” the commission wrote. The mechanism of increased risk isn’t clear, but may be a combination of neurodegeneration and social isolation imposed by being shut out of easy communication. It’s unclear whether hearing aids can mitigate the effects of hearing loss on dementia risk, the report noted.
• Hypertension, obesity, and diabetes. Respectively, these accounted for 2%, 1%, and 1% of the PAF. Obesity is linked to prediabetes and diabetes, which are in turn linked to insulin resistance, decreased brain amyloid clearance, high blood glucose, and inflammation – all risks for Alzheimer’s disease.
The report recommends that anyone aged 45-65 years who has hypertension should be actively treated for the disorder.
• Smoking. At 5%, smoking posted the third-highest PAF. The risk is probably mediated through smoking’s detrimental effects on cardiovascular health. But the report noted that tobacco smoke contains known neurotoxins as well.
Preventing the smoking/dementia connection is simple, Dr. Livingston said. “Simply stop smoking. If you’re smoking, just stop. Please.”
• Depression. Depression in late life imposed a 4% PAF. The evidence reviewed suggested that depression is not, in fact, linked to dementia when experienced at mid-life. But late-life depression may be a prodromal symptom of dementia and biologically linked to increased stress hormones, decreased neuronal growth factors, and decreased hippocampal volume. The commission noted animal models that suggest some antidepressants, including citalopram, decrease amyloid progression.
• Social isolation. Associated with a 2% PAF, social isolation may, like depression, be a prodromal symptom. But, the report said, there is growing evidence that it actually is an independent risk factor as well. It has been shown to also increase the risk of hypertension, cardiovascular disease, and depression, all dementia risk factors in their own right.
Longitudinal studies suggest that social activities and personal connections may prevent or delay dementia, although top-grade evidence is lacking. Still, maintaining a rich social network not only reduces the chance of isolation, but helps prevent depression as well.
• Physical inactivity. Sedentary lifestyle carried a 3% PAF for dementia. Older adults who maintain physical activity are more likely to remain cognitively intact. Physical exercise improves mood, reduces the risk of falls, and maintains normal physical function. The report cited a meta-analysis of 16 studies and almost 164,000 participants without dementia; it concluded that those in the highest level of activity had a 25% decreased risk of all-cause dementia and a 45% decreased risk of Alzheimer’s disease.
The strongest evidence for exercise’s benefit on cognition may be from the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER). Patients with a high risk of dementia who completed the lifestyle modification program of healthy diet cognitive training, vascular risk factor management, and aerobic exercise showed a slowing of cognitive decline and improvements in executive function and processing speed.
Becoming aware of the risk factors is one thing, the report said. Doing something about them is another. In general, the first step is to “be ambitious” about prevention.
“Prevention is always better than treatment,” Dr. Livingston said in an interview. “We need to start thinking about dementia not as something that simply happens outside our control, but as something that we can have an effect on.”
The Lancet commissioned the report. Dr. Livingston did not have any financial declarations but many of the other authors reported multiple relationships with pharmaceutical companies.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
AT AAIC 2017
Amyloid PET imaging impacts diagnoses, management for those with MCI and dementia
LONDON – A study to prove the practical clinical utility of amyloid PET scanning appears to be doing just that: The knowledge of patients’ brain amyloid status changed clinical management for 68% of those who had the imaging done.
Interim results of the Imaging Dementia–Evidence for Amyloid Scanning (IDEAS) study far exceeded the 30% change rate that investigators hoped to see, Gil Rabinovici, MD, said at the Alzheimer’s Association International Conference. Not only did many patients receive a more suitable care plan, the scans actually changed diagnoses in three-quarters of those who tested positive, said Dr. Rabinovici of the University of California, San Francisco.
The U.S.-wide, open-label study is being conducted in Medicare beneficiaries who have been diagnosed with mild cognitive impairment (MCI) or dementia of uncertain cause. Its two goals are to show that amyloid PET imaging affects a patient’s care plan within 3 months of the scan and to show an impact on major medical outcomes 12 months later. In diagnostically uncertain cases, investigators theorized, amyloid PET imaging would lead to significant changes in patient management, which would then translate into improved medical outcomes.
At the meeting, Dr. Rabinovici discussed the preplanned interim analysis of 3-month care plan changes. After the scan was completed, physicians related the results to patients and recommended any appropriate management changes. At the 90-day post-scan visit, physicians documented how any changes had been implemented. Data on the 12-month outcomes have not been announced on any portion of the cohort.
The patients were a mean of 76 years old; 64% had a diagnosis of MCI and 36% a diagnosis of dementia. Alzheimer’s disease was the suspected etiology in 74% of the MCI group and in 80% of the dementia group. About one-third of MCI patients and two-thirds of dementia patients were taking Alzheimer’s medications at enrollment.
The scans were positive in 54% of the MCI patients and in 70% of dementia patients. In amyloid-positive patients, the rate of Alzheimer’s diagnosis increased from 78% to 85%. In amyloid-negative patients, the rate of Alzheimer’s diagnosis decreased from 73% to 14%.
More than two-thirds of patients in both groups experienced a change in management after the scans were read. Changes in Alzheimer’s drugs occurred in 48% of both groups, while changes in non-Alzheimer’s drugs occurred in 36% of the MCI group and 32% of the dementia group. In amyloid-positive patients, use of Alzheimer’s drugs increased from 51% to 84%. In amyloid-negative patients, the use of Alzheimer’s drugs decreased from 39% to 31%.
Patient counseling also changed in 24% of the MCI group and 16% of the dementia group.
Dr. Rabinovici was cautiously optimistic about the interim results. “The study has two aims, and Medicare has made it clear that we need to complete both before they will consider covering the scans. I hope they understand that these changes in management are incredibly important, but they also want to see that they result in improved outcomes. That will take a year from when we enroll our last patient.”
Although the study is designed to show change in clinical outcomes, it’s also changing something less tangible, but no less important: patients’ understanding of their situation.
“We may be looking at objective changes in management, but we are also seeing that patients really want to know what’s going on in their brain, what the cause of their cognitive impairment is. They don’t want to be told that it’s normal aging, because they know it isn’t. There is a real value to getting that diagnosis, even if it’s the bad news that there are amyloid plaques in the brain. It leads to a clear plan in terms of what drugs to use, the possibility of enrolling in clinical trials, and getting some clarity about what’s going on. A lot of times, the certainty is better than the uncertainty.”
As of June, IDEAS had recruited 12,484 patients and completed 11,712 amyloid PET scans. Dr. Rabinovici hopes the recruitment will be completed by late this year, or early 2018.
IDEAS is being funded by the Centers for Medicare & Medicaid Services, Avid Radiopharmaceuticals/Eli Lilly, GE Healthcare, Piramal Imaging, the Alzheimer’s Association, and the American College of Radiology. Dr. Rabinovici has received honoraria or consulting fees from Eisai, Genentech, Lundbeck, Merck, Putnam, and Roche.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
LONDON – A study to prove the practical clinical utility of amyloid PET scanning appears to be doing just that: The knowledge of patients’ brain amyloid status changed clinical management for 68% of those who had the imaging done.
Interim results of the Imaging Dementia–Evidence for Amyloid Scanning (IDEAS) study far exceeded the 30% change rate that investigators hoped to see, Gil Rabinovici, MD, said at the Alzheimer’s Association International Conference. Not only did many patients receive a more suitable care plan, the scans actually changed diagnoses in three-quarters of those who tested positive, said Dr. Rabinovici of the University of California, San Francisco.
The U.S.-wide, open-label study is being conducted in Medicare beneficiaries who have been diagnosed with mild cognitive impairment (MCI) or dementia of uncertain cause. Its two goals are to show that amyloid PET imaging affects a patient’s care plan within 3 months of the scan and to show an impact on major medical outcomes 12 months later. In diagnostically uncertain cases, investigators theorized, amyloid PET imaging would lead to significant changes in patient management, which would then translate into improved medical outcomes.
At the meeting, Dr. Rabinovici discussed the preplanned interim analysis of 3-month care plan changes. After the scan was completed, physicians related the results to patients and recommended any appropriate management changes. At the 90-day post-scan visit, physicians documented how any changes had been implemented. Data on the 12-month outcomes have not been announced on any portion of the cohort.
The patients were a mean of 76 years old; 64% had a diagnosis of MCI and 36% a diagnosis of dementia. Alzheimer’s disease was the suspected etiology in 74% of the MCI group and in 80% of the dementia group. About one-third of MCI patients and two-thirds of dementia patients were taking Alzheimer’s medications at enrollment.
The scans were positive in 54% of the MCI patients and in 70% of dementia patients. In amyloid-positive patients, the rate of Alzheimer’s diagnosis increased from 78% to 85%. In amyloid-negative patients, the rate of Alzheimer’s diagnosis decreased from 73% to 14%.
More than two-thirds of patients in both groups experienced a change in management after the scans were read. Changes in Alzheimer’s drugs occurred in 48% of both groups, while changes in non-Alzheimer’s drugs occurred in 36% of the MCI group and 32% of the dementia group. In amyloid-positive patients, use of Alzheimer’s drugs increased from 51% to 84%. In amyloid-negative patients, the use of Alzheimer’s drugs decreased from 39% to 31%.
Patient counseling also changed in 24% of the MCI group and 16% of the dementia group.
Dr. Rabinovici was cautiously optimistic about the interim results. “The study has two aims, and Medicare has made it clear that we need to complete both before they will consider covering the scans. I hope they understand that these changes in management are incredibly important, but they also want to see that they result in improved outcomes. That will take a year from when we enroll our last patient.”
Although the study is designed to show change in clinical outcomes, it’s also changing something less tangible, but no less important: patients’ understanding of their situation.
“We may be looking at objective changes in management, but we are also seeing that patients really want to know what’s going on in their brain, what the cause of their cognitive impairment is. They don’t want to be told that it’s normal aging, because they know it isn’t. There is a real value to getting that diagnosis, even if it’s the bad news that there are amyloid plaques in the brain. It leads to a clear plan in terms of what drugs to use, the possibility of enrolling in clinical trials, and getting some clarity about what’s going on. A lot of times, the certainty is better than the uncertainty.”
As of June, IDEAS had recruited 12,484 patients and completed 11,712 amyloid PET scans. Dr. Rabinovici hopes the recruitment will be completed by late this year, or early 2018.
IDEAS is being funded by the Centers for Medicare & Medicaid Services, Avid Radiopharmaceuticals/Eli Lilly, GE Healthcare, Piramal Imaging, the Alzheimer’s Association, and the American College of Radiology. Dr. Rabinovici has received honoraria or consulting fees from Eisai, Genentech, Lundbeck, Merck, Putnam, and Roche.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
LONDON – A study to prove the practical clinical utility of amyloid PET scanning appears to be doing just that: The knowledge of patients’ brain amyloid status changed clinical management for 68% of those who had the imaging done.
Interim results of the Imaging Dementia–Evidence for Amyloid Scanning (IDEAS) study far exceeded the 30% change rate that investigators hoped to see, Gil Rabinovici, MD, said at the Alzheimer’s Association International Conference. Not only did many patients receive a more suitable care plan, the scans actually changed diagnoses in three-quarters of those who tested positive, said Dr. Rabinovici of the University of California, San Francisco.
The U.S.-wide, open-label study is being conducted in Medicare beneficiaries who have been diagnosed with mild cognitive impairment (MCI) or dementia of uncertain cause. Its two goals are to show that amyloid PET imaging affects a patient’s care plan within 3 months of the scan and to show an impact on major medical outcomes 12 months later. In diagnostically uncertain cases, investigators theorized, amyloid PET imaging would lead to significant changes in patient management, which would then translate into improved medical outcomes.
At the meeting, Dr. Rabinovici discussed the preplanned interim analysis of 3-month care plan changes. After the scan was completed, physicians related the results to patients and recommended any appropriate management changes. At the 90-day post-scan visit, physicians documented how any changes had been implemented. Data on the 12-month outcomes have not been announced on any portion of the cohort.
The patients were a mean of 76 years old; 64% had a diagnosis of MCI and 36% a diagnosis of dementia. Alzheimer’s disease was the suspected etiology in 74% of the MCI group and in 80% of the dementia group. About one-third of MCI patients and two-thirds of dementia patients were taking Alzheimer’s medications at enrollment.
The scans were positive in 54% of the MCI patients and in 70% of dementia patients. In amyloid-positive patients, the rate of Alzheimer’s diagnosis increased from 78% to 85%. In amyloid-negative patients, the rate of Alzheimer’s diagnosis decreased from 73% to 14%.
More than two-thirds of patients in both groups experienced a change in management after the scans were read. Changes in Alzheimer’s drugs occurred in 48% of both groups, while changes in non-Alzheimer’s drugs occurred in 36% of the MCI group and 32% of the dementia group. In amyloid-positive patients, use of Alzheimer’s drugs increased from 51% to 84%. In amyloid-negative patients, the use of Alzheimer’s drugs decreased from 39% to 31%.
Patient counseling also changed in 24% of the MCI group and 16% of the dementia group.
Dr. Rabinovici was cautiously optimistic about the interim results. “The study has two aims, and Medicare has made it clear that we need to complete both before they will consider covering the scans. I hope they understand that these changes in management are incredibly important, but they also want to see that they result in improved outcomes. That will take a year from when we enroll our last patient.”
Although the study is designed to show change in clinical outcomes, it’s also changing something less tangible, but no less important: patients’ understanding of their situation.
“We may be looking at objective changes in management, but we are also seeing that patients really want to know what’s going on in their brain, what the cause of their cognitive impairment is. They don’t want to be told that it’s normal aging, because they know it isn’t. There is a real value to getting that diagnosis, even if it’s the bad news that there are amyloid plaques in the brain. It leads to a clear plan in terms of what drugs to use, the possibility of enrolling in clinical trials, and getting some clarity about what’s going on. A lot of times, the certainty is better than the uncertainty.”
As of June, IDEAS had recruited 12,484 patients and completed 11,712 amyloid PET scans. Dr. Rabinovici hopes the recruitment will be completed by late this year, or early 2018.
IDEAS is being funded by the Centers for Medicare & Medicaid Services, Avid Radiopharmaceuticals/Eli Lilly, GE Healthcare, Piramal Imaging, the Alzheimer’s Association, and the American College of Radiology. Dr. Rabinovici has received honoraria or consulting fees from Eisai, Genentech, Lundbeck, Merck, Putnam, and Roche.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
AT AAIC 2017
Key clinical point:
Major finding: Amyloid status changed clinical management in 68% of patients diagnosed with mild cognitive impairment or dementia.
Data source: An interim analysis of 4,000 out of 18,500 planned for enrollment in the IDEAS study.
Disclosures: IDEAS is being funded by the Centers for Medicare & Medicaid Services, Avid Radiopharmaceuticals/Eli Lilly, GE Healthcare, Piramal Imaging, the Alzheimer’s Association, and the American College of Radiology. Dr. Rabinovici has received honoraria or consulting fees from Eisai, Genentech, Lundbeck, Merck, Putnam, and Roche.