User login
Long-term maintenance deemed feasible in PV
MADRID—Long-term maintenance with ropeginterferon alfa-2b is feasible, effective, and well-tolerated in patients with polycythemia vera (PV), according to researchers.
In the ongoing phase 1/2 PEGINVERA study, patients have received ropeginterferon alfa-2b for a median of 4 years.
After the first 2 years, patients switched from bi-weekly dosing to receiving ropeginterferon alfa-2b once every 4 weeks.
None of the patients discontinued treatment after the switch, and many were able to maintain their best response.
Most adverse events (AEs) were mild, although there were several severe treatment-related AEs.
These results were presented in a poster (abstract P707) at the 22nd Congress of the European Hematology Association (EHA). The research was funded by AOP Orphan Pharmaceuticals AG.
The trial enrolled 51 patients, but the researchers reported results in the 29 patients who had completed 2 years of treatment and switched from bi-weekly dosing to receiving treatment once every 4 weeks.
All 29 patients remained on the 4-week schedule with a median observation period of roughly 2 years. The median monthly dose was 308 μg before the switch and 165 μg after.
At study entry, the patients’ median age was 58 (range, 40-80), and 76% were male. Their median spleen length was 12.8 cm (range, 8.0-22.0), and 34% of patients had prior treatment with hydroxyurea.
Patients’ median hematocrit was 45.40% (range, 36.9-53.8), their median platelet count was 431 G/L (range, 225-1016), their median leukocyte count was 11.1 G/L (range, 4.7-30.9), and their median JAKV617F allelic burden was 78% (range, 2-91.5).
Results
More than 80% of patients achieved a hematologic response, with more than 50% achieving a complete hematologic response. The same percentage of patients maintained their best hematologic response before and 6 months after switching to the 4-week schedule—51.7%.
More than 80% of patients achieved a molecular response, with nearly 20% achieving a complete molecular response. The percentage of patients maintaining their best molecular response was 62.1% before switching to the 4-week schedule and 58.6% 6 months after the switch.
The researchers said changes in hematocrit, platelet count, leukocyte count, and spleen size after the switch were “minimal and without clinical relevance.”
The median hematocrit changed from 42.3% to 42.6%, the median platelet count changed from 201.0 x 109/L to 211.9 x 109/L, the median leukocyte count changed from 5.0 x 109/L to 5.6 x 109/L, and the median spleen size changed from 12.8 cm to 12.4 cm.
The need for phlebotomy did not change, with 24.1% of patients requiring phlebotomy both before and 6 months after the switch.
The researchers also noted that ropeginterferon alfa-2b decreased mutant JAK2 allele burden in all of the patients over time, with the strongest effect observed in the second year of treatment.
After 2 years, most patients had a burden below 10%, and this was not affected by the change in dosing.
There were no cases of progression to myelofibrosis or leukemic transformation.
Seventy-one percent of AEs were mild, and 40.4% were considered likely related to ropeginterferon alfa-2b. The most frequent treatment-related AEs were arthralgia (29.4%) and fatigue (21.6%).
There were 34 severe AEs, 11 of which were related to ropeginterferon alfa-2b. ![]()
MADRID—Long-term maintenance with ropeginterferon alfa-2b is feasible, effective, and well-tolerated in patients with polycythemia vera (PV), according to researchers.
In the ongoing phase 1/2 PEGINVERA study, patients have received ropeginterferon alfa-2b for a median of 4 years.
After the first 2 years, patients switched from bi-weekly dosing to receiving ropeginterferon alfa-2b once every 4 weeks.
None of the patients discontinued treatment after the switch, and many were able to maintain their best response.
Most adverse events (AEs) were mild, although there were several severe treatment-related AEs.
These results were presented in a poster (abstract P707) at the 22nd Congress of the European Hematology Association (EHA). The research was funded by AOP Orphan Pharmaceuticals AG.
The trial enrolled 51 patients, but the researchers reported results in the 29 patients who had completed 2 years of treatment and switched from bi-weekly dosing to receiving treatment once every 4 weeks.
All 29 patients remained on the 4-week schedule with a median observation period of roughly 2 years. The median monthly dose was 308 μg before the switch and 165 μg after.
At study entry, the patients’ median age was 58 (range, 40-80), and 76% were male. Their median spleen length was 12.8 cm (range, 8.0-22.0), and 34% of patients had prior treatment with hydroxyurea.
Patients’ median hematocrit was 45.40% (range, 36.9-53.8), their median platelet count was 431 G/L (range, 225-1016), their median leukocyte count was 11.1 G/L (range, 4.7-30.9), and their median JAKV617F allelic burden was 78% (range, 2-91.5).
Results
More than 80% of patients achieved a hematologic response, with more than 50% achieving a complete hematologic response. The same percentage of patients maintained their best hematologic response before and 6 months after switching to the 4-week schedule—51.7%.
More than 80% of patients achieved a molecular response, with nearly 20% achieving a complete molecular response. The percentage of patients maintaining their best molecular response was 62.1% before switching to the 4-week schedule and 58.6% 6 months after the switch.
The researchers said changes in hematocrit, platelet count, leukocyte count, and spleen size after the switch were “minimal and without clinical relevance.”
The median hematocrit changed from 42.3% to 42.6%, the median platelet count changed from 201.0 x 109/L to 211.9 x 109/L, the median leukocyte count changed from 5.0 x 109/L to 5.6 x 109/L, and the median spleen size changed from 12.8 cm to 12.4 cm.
The need for phlebotomy did not change, with 24.1% of patients requiring phlebotomy both before and 6 months after the switch.
The researchers also noted that ropeginterferon alfa-2b decreased mutant JAK2 allele burden in all of the patients over time, with the strongest effect observed in the second year of treatment.
After 2 years, most patients had a burden below 10%, and this was not affected by the change in dosing.
There were no cases of progression to myelofibrosis or leukemic transformation.
Seventy-one percent of AEs were mild, and 40.4% were considered likely related to ropeginterferon alfa-2b. The most frequent treatment-related AEs were arthralgia (29.4%) and fatigue (21.6%).
There were 34 severe AEs, 11 of which were related to ropeginterferon alfa-2b. ![]()
MADRID—Long-term maintenance with ropeginterferon alfa-2b is feasible, effective, and well-tolerated in patients with polycythemia vera (PV), according to researchers.
In the ongoing phase 1/2 PEGINVERA study, patients have received ropeginterferon alfa-2b for a median of 4 years.
After the first 2 years, patients switched from bi-weekly dosing to receiving ropeginterferon alfa-2b once every 4 weeks.
None of the patients discontinued treatment after the switch, and many were able to maintain their best response.
Most adverse events (AEs) were mild, although there were several severe treatment-related AEs.
These results were presented in a poster (abstract P707) at the 22nd Congress of the European Hematology Association (EHA). The research was funded by AOP Orphan Pharmaceuticals AG.
The trial enrolled 51 patients, but the researchers reported results in the 29 patients who had completed 2 years of treatment and switched from bi-weekly dosing to receiving treatment once every 4 weeks.
All 29 patients remained on the 4-week schedule with a median observation period of roughly 2 years. The median monthly dose was 308 μg before the switch and 165 μg after.
At study entry, the patients’ median age was 58 (range, 40-80), and 76% were male. Their median spleen length was 12.8 cm (range, 8.0-22.0), and 34% of patients had prior treatment with hydroxyurea.
Patients’ median hematocrit was 45.40% (range, 36.9-53.8), their median platelet count was 431 G/L (range, 225-1016), their median leukocyte count was 11.1 G/L (range, 4.7-30.9), and their median JAKV617F allelic burden was 78% (range, 2-91.5).
Results
More than 80% of patients achieved a hematologic response, with more than 50% achieving a complete hematologic response. The same percentage of patients maintained their best hematologic response before and 6 months after switching to the 4-week schedule—51.7%.
More than 80% of patients achieved a molecular response, with nearly 20% achieving a complete molecular response. The percentage of patients maintaining their best molecular response was 62.1% before switching to the 4-week schedule and 58.6% 6 months after the switch.
The researchers said changes in hematocrit, platelet count, leukocyte count, and spleen size after the switch were “minimal and without clinical relevance.”
The median hematocrit changed from 42.3% to 42.6%, the median platelet count changed from 201.0 x 109/L to 211.9 x 109/L, the median leukocyte count changed from 5.0 x 109/L to 5.6 x 109/L, and the median spleen size changed from 12.8 cm to 12.4 cm.
The need for phlebotomy did not change, with 24.1% of patients requiring phlebotomy both before and 6 months after the switch.
The researchers also noted that ropeginterferon alfa-2b decreased mutant JAK2 allele burden in all of the patients over time, with the strongest effect observed in the second year of treatment.
After 2 years, most patients had a burden below 10%, and this was not affected by the change in dosing.
There were no cases of progression to myelofibrosis or leukemic transformation.
Seventy-one percent of AEs were mild, and 40.4% were considered likely related to ropeginterferon alfa-2b. The most frequent treatment-related AEs were arthralgia (29.4%) and fatigue (21.6%).
There were 34 severe AEs, 11 of which were related to ropeginterferon alfa-2b. ![]()
Itchy rash during pregnancy
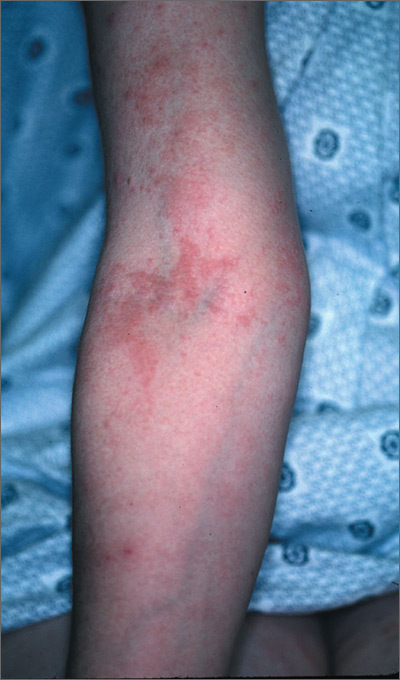
The FP diagnosed pruritic urticarial papules and plaques of pregnancy. PUPPP is usually diagnosed by its characteristic findings in the history and physical examination.
PUPPP typically presents as erythematous papules and plaques within striae (with periumbilical sparing) late in the third trimester. Extreme pruritus is a hallmark of PUPPP and is present in all patients. Abdominal striae are the most common initial symptoms. The lesions usually spread to the extremities and coalesce to form urticarial plaques.
General measures such as cool baths, frequent application of emollients, wet soaks, or cool packs applied to the skin provide some symptomatic relief. First-line pharmacologic therapy consists of topical steroids and oral antihistamines to alleviate symptoms.
In this case, the FP prescribed a mid-potency topical corticosteroid, 0.1% triamcinolone cream twice daily (pregnancy class B). The patient was given the choice of steroid vehicle and chose the cream over the ointment. Both vehicles work to treat PUPPP, but the one that will work best is the one the patient will actually use.
The FP also recommended over-the-counter diphenhydramine 25 mg for additional symptomatic relief of pruritus (pregnancy class B). At her following prenatal visit, the patient’s symptoms were about 80% better and she was tolerating the pruritus. She was relieved to learn that this condition would resolve after pregnancy.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ. Pruritic urticarial papules and plaques of pregnancy. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 467-470.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP diagnosed pruritic urticarial papules and plaques of pregnancy. PUPPP is usually diagnosed by its characteristic findings in the history and physical examination.
PUPPP typically presents as erythematous papules and plaques within striae (with periumbilical sparing) late in the third trimester. Extreme pruritus is a hallmark of PUPPP and is present in all patients. Abdominal striae are the most common initial symptoms. The lesions usually spread to the extremities and coalesce to form urticarial plaques.
General measures such as cool baths, frequent application of emollients, wet soaks, or cool packs applied to the skin provide some symptomatic relief. First-line pharmacologic therapy consists of topical steroids and oral antihistamines to alleviate symptoms.
In this case, the FP prescribed a mid-potency topical corticosteroid, 0.1% triamcinolone cream twice daily (pregnancy class B). The patient was given the choice of steroid vehicle and chose the cream over the ointment. Both vehicles work to treat PUPPP, but the one that will work best is the one the patient will actually use.
The FP also recommended over-the-counter diphenhydramine 25 mg for additional symptomatic relief of pruritus (pregnancy class B). At her following prenatal visit, the patient’s symptoms were about 80% better and she was tolerating the pruritus. She was relieved to learn that this condition would resolve after pregnancy.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ. Pruritic urticarial papules and plaques of pregnancy. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 467-470.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP diagnosed pruritic urticarial papules and plaques of pregnancy. PUPPP is usually diagnosed by its characteristic findings in the history and physical examination.
PUPPP typically presents as erythematous papules and plaques within striae (with periumbilical sparing) late in the third trimester. Extreme pruritus is a hallmark of PUPPP and is present in all patients. Abdominal striae are the most common initial symptoms. The lesions usually spread to the extremities and coalesce to form urticarial plaques.
General measures such as cool baths, frequent application of emollients, wet soaks, or cool packs applied to the skin provide some symptomatic relief. First-line pharmacologic therapy consists of topical steroids and oral antihistamines to alleviate symptoms.
In this case, the FP prescribed a mid-potency topical corticosteroid, 0.1% triamcinolone cream twice daily (pregnancy class B). The patient was given the choice of steroid vehicle and chose the cream over the ointment. Both vehicles work to treat PUPPP, but the one that will work best is the one the patient will actually use.
The FP also recommended over-the-counter diphenhydramine 25 mg for additional symptomatic relief of pruritus (pregnancy class B). At her following prenatal visit, the patient’s symptoms were about 80% better and she was tolerating the pruritus. She was relieved to learn that this condition would resolve after pregnancy.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ. Pruritic urticarial papules and plaques of pregnancy. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 467-470.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Factory contamination seen as likely source of postop endocarditis outbreak
Since 2013, over 100 cases of Mycobacterium chimaera prosthetic valve endocarditis and disseminated disease were detected in Europe and the United States, and these were presumptively linked to contaminated heater-cooler units (HCUs) used during cardiac surgery. A molecular epidemiological analysis of microbial isolate genomes detected a “remarkable clonality of isolates” in almost all of the assessed patients with M. chimaera disease, which “strongly points to a common source of infection,” as reported online in The Lancet Infectious Diseases.
The analysis comprised 250 whole-genome sequencing datasets: 24 isolates from 21 cardiac surgery–related patients in Switzerland, Germany, the Netherlands, and the United Kingdom; 36 from 35 unrelated patients; 126 from LivaNova HCUs in use (85 water cultures, 41 air cultures); 13 from LivaNova HCUs returned to the production site in Germany for disinfection; 4 from the LivaNova production site (3 from newly produced HCUs, 1 from a water source); 2 from Maquet extracorporeal membrane oxygenation (ECMO) devices in use; 14 from Maquet HCUs in use; 15 from new Maquet HCUs sampled at the production site; and 7 from hospital water supplies in Switzerland, Germany, and the Netherlands, plus one M. chimaera DSM 44623–type strain, and eight M. intracellulare strains (from four unrelated patients from Germany and four published genomes).
Isolates were analyzed by next-generation whole-genome sequencing and compared with published M. chimaera genomes, according to Jakko van Ingen, PhD, Radboud University Medical Center, Nijmegen, the Netherlands, and his colleagues. Phylogenetic analysis of these 250 isolates revealed two major M. chimaera groups. They found that all cardiac surgery–related patient isolates could be classified into group 1. They then did a subgroup analysis.
“Three distinct strains of M. chimaera appear to have contaminated the water systems of LivaNova HCUs at the production site, belonging to subgroups 1.1, 1.8, and 2.1,” the authors stated. However, most M. chimaera isolates from air samples taken near operating LivaNova HCUs and those of 23 of the 24 related patients belonged to subgroup 1.1.
“This finding further supports the presumed airborne transmission pathway leading to endocarditis, aortic graft infection, disseminated disease, and surgical site infections in the affected patients,” according to the authors (doi: 10.1016/S1473-3099[17]30324-9).
The results suggest “the possibility that the vast majority of cases of cardiothoracic surgery–related severe M. chimaera infections diagnosed in Switzerland, Germany, the Netherlands, the United Kingdom, the United States, and Australia resulted from a single common source of infection: LivaNova HCUs that were most likely contaminated during production in Germany,” the researchers concluded.
The study was partly funded by the EU Horizon 2020 program, its FP7 program, the German Center for Infection Research (DZIF), the Swiss National Science Foundation, the Swiss Federal Office of Public Health, and National Institute of Health Research Oxford Health Protection Research Units on Healthcare Associated Infection and Antimicrobial Resistance. The authors reported having no relevant conflicts.
Since 2013, over 100 cases of Mycobacterium chimaera prosthetic valve endocarditis and disseminated disease were detected in Europe and the United States, and these were presumptively linked to contaminated heater-cooler units (HCUs) used during cardiac surgery. A molecular epidemiological analysis of microbial isolate genomes detected a “remarkable clonality of isolates” in almost all of the assessed patients with M. chimaera disease, which “strongly points to a common source of infection,” as reported online in The Lancet Infectious Diseases.
The analysis comprised 250 whole-genome sequencing datasets: 24 isolates from 21 cardiac surgery–related patients in Switzerland, Germany, the Netherlands, and the United Kingdom; 36 from 35 unrelated patients; 126 from LivaNova HCUs in use (85 water cultures, 41 air cultures); 13 from LivaNova HCUs returned to the production site in Germany for disinfection; 4 from the LivaNova production site (3 from newly produced HCUs, 1 from a water source); 2 from Maquet extracorporeal membrane oxygenation (ECMO) devices in use; 14 from Maquet HCUs in use; 15 from new Maquet HCUs sampled at the production site; and 7 from hospital water supplies in Switzerland, Germany, and the Netherlands, plus one M. chimaera DSM 44623–type strain, and eight M. intracellulare strains (from four unrelated patients from Germany and four published genomes).
Isolates were analyzed by next-generation whole-genome sequencing and compared with published M. chimaera genomes, according to Jakko van Ingen, PhD, Radboud University Medical Center, Nijmegen, the Netherlands, and his colleagues. Phylogenetic analysis of these 250 isolates revealed two major M. chimaera groups. They found that all cardiac surgery–related patient isolates could be classified into group 1. They then did a subgroup analysis.
“Three distinct strains of M. chimaera appear to have contaminated the water systems of LivaNova HCUs at the production site, belonging to subgroups 1.1, 1.8, and 2.1,” the authors stated. However, most M. chimaera isolates from air samples taken near operating LivaNova HCUs and those of 23 of the 24 related patients belonged to subgroup 1.1.
“This finding further supports the presumed airborne transmission pathway leading to endocarditis, aortic graft infection, disseminated disease, and surgical site infections in the affected patients,” according to the authors (doi: 10.1016/S1473-3099[17]30324-9).
The results suggest “the possibility that the vast majority of cases of cardiothoracic surgery–related severe M. chimaera infections diagnosed in Switzerland, Germany, the Netherlands, the United Kingdom, the United States, and Australia resulted from a single common source of infection: LivaNova HCUs that were most likely contaminated during production in Germany,” the researchers concluded.
The study was partly funded by the EU Horizon 2020 program, its FP7 program, the German Center for Infection Research (DZIF), the Swiss National Science Foundation, the Swiss Federal Office of Public Health, and National Institute of Health Research Oxford Health Protection Research Units on Healthcare Associated Infection and Antimicrobial Resistance. The authors reported having no relevant conflicts.
Since 2013, over 100 cases of Mycobacterium chimaera prosthetic valve endocarditis and disseminated disease were detected in Europe and the United States, and these were presumptively linked to contaminated heater-cooler units (HCUs) used during cardiac surgery. A molecular epidemiological analysis of microbial isolate genomes detected a “remarkable clonality of isolates” in almost all of the assessed patients with M. chimaera disease, which “strongly points to a common source of infection,” as reported online in The Lancet Infectious Diseases.
The analysis comprised 250 whole-genome sequencing datasets: 24 isolates from 21 cardiac surgery–related patients in Switzerland, Germany, the Netherlands, and the United Kingdom; 36 from 35 unrelated patients; 126 from LivaNova HCUs in use (85 water cultures, 41 air cultures); 13 from LivaNova HCUs returned to the production site in Germany for disinfection; 4 from the LivaNova production site (3 from newly produced HCUs, 1 from a water source); 2 from Maquet extracorporeal membrane oxygenation (ECMO) devices in use; 14 from Maquet HCUs in use; 15 from new Maquet HCUs sampled at the production site; and 7 from hospital water supplies in Switzerland, Germany, and the Netherlands, plus one M. chimaera DSM 44623–type strain, and eight M. intracellulare strains (from four unrelated patients from Germany and four published genomes).
Isolates were analyzed by next-generation whole-genome sequencing and compared with published M. chimaera genomes, according to Jakko van Ingen, PhD, Radboud University Medical Center, Nijmegen, the Netherlands, and his colleagues. Phylogenetic analysis of these 250 isolates revealed two major M. chimaera groups. They found that all cardiac surgery–related patient isolates could be classified into group 1. They then did a subgroup analysis.
“Three distinct strains of M. chimaera appear to have contaminated the water systems of LivaNova HCUs at the production site, belonging to subgroups 1.1, 1.8, and 2.1,” the authors stated. However, most M. chimaera isolates from air samples taken near operating LivaNova HCUs and those of 23 of the 24 related patients belonged to subgroup 1.1.
“This finding further supports the presumed airborne transmission pathway leading to endocarditis, aortic graft infection, disseminated disease, and surgical site infections in the affected patients,” according to the authors (doi: 10.1016/S1473-3099[17]30324-9).
The results suggest “the possibility that the vast majority of cases of cardiothoracic surgery–related severe M. chimaera infections diagnosed in Switzerland, Germany, the Netherlands, the United Kingdom, the United States, and Australia resulted from a single common source of infection: LivaNova HCUs that were most likely contaminated during production in Germany,” the researchers concluded.
The study was partly funded by the EU Horizon 2020 program, its FP7 program, the German Center for Infection Research (DZIF), the Swiss National Science Foundation, the Swiss Federal Office of Public Health, and National Institute of Health Research Oxford Health Protection Research Units on Healthcare Associated Infection and Antimicrobial Resistance. The authors reported having no relevant conflicts.
FROM THE LANCET INFECTIOUS DISEASES
Key clinical point:
Major finding: Cardiac surgery–related patient isolates were all classified into the same group, in which all, except one, formed a distinct subgroup of Mycobacterium chimaera, which also comprised most isolates from LivaNova HCUs, and one from the equipment production site.
Data source: Phylogenetic analysis based on whole-genome sequencing of 250 M. chimaera isolates obtained from cardiac surgery patients, hospitals, and other sources.
Disclosures: Partly funded by the EU Horizon 2020 program and several German, Swiss, and U.K. infectious disease–related NGOs. The authors reported having no disclosures.
Depression in adolescence
As many as 20% of children and adolescents experience a psychiatric disorder, with 50% of all lifetime psychiatric illnesses occurring by the age of 14 years. ADHD and depression are among the most common. The National Institutes of Health estimate that, in 2015, 3 million 12- to 17-year-old American children experienced a major depressive episode. Any illness that affects over 10% of adolescents will present regularly in the primary care provider’s office. It is important to know whom to screen and how to start treatment when your patient appears to be suffering from this serious but treatable condition.
While there are many screening instruments, it is important to be ready to ask patients diagnostic questions when your clinical suspicion of depression is high. In addition to asking about mood, sleep, appetite, energy, and the other DSM5 criteria of a major depressive episode, it is important to remember that teens with depression might present with irritability as much as sadness. While they lose interest in school, sports, or hobbies, they still may be distracted or cheered up by friends. And
Explain to your patient (and their parents) that depression is very treatable, but most effective treatments take time. Psychotherapy usually works over several months, and even effective medications can take 6 weeks or more. But, without treatment, their symptoms may persist for over a year and can disrupt their healthy development.
This is also a good time to ask your patient about suicidal thoughts. Have they been imagining how their death would affect others? Wishing they could just sleep? Do they have a plan? Do they have access to a means of killing themselves? Do they feel attached or connected to family, friends, religion, or a goal? Explain to your patient that these thoughts are common symptoms of depression, and work with their parents to ensure that they are connected and safe when starting treatment.
Psychotherapy is considered the first line treatment for mild to moderate episodes of depression and should be used alongside medications in severe episodes. While structured therapies such as cognitive behavioral therapy or interpersonal therapy have a strong evidence base to support their use, the best predictor of an effective therapy appears to be a strong alliance between therapist and patient. So, help your patient to find a therapist, and explain the importance of finding someone with whom they feel comfortable. Suggest to your patients that they have three visits with a new therapist to see if it feels like a “good match,” before considering trying another.
Finally, antidepressant medications are first-line treatment for more severe episodes of depression and episodes in which significant suicidal ideation or functional impairment are present. If the symptoms are more severe, or if therapy alone has not been effective after 4-6 weeks, you might consider starting antidepressant treatment. Psychiatrists usually start with an selective serotonin reuptake inhibitor, typically of a medium half-life, at a low dose to minimize the chances of side effects. While real efficacy takes up to 6 weeks, there should be some improvement in energy within the first 2 weeks on an effective medication. If there is no change, the dose can be raised gradually as tolerated. It is important to tell patients and their families about common side effects (mild GI upset) and the more rare but dangerous ones (such as hypomania or an increase in the frequency or intensity of suicidal thoughts).
Even when you do not refer your patient to someone else for treatment of depression, it is important that you not be alone in their management. Work closely with their therapist or consider having a psychiatric social worker join your team to offer therapy in close connection with your management. You might also periodically consult with a child psychiatrist to address treatment and medication questions and identify needed resources. Staying in touch with parents or connected adults at school (with the appropriate permission) can be very useful with those patients you are more concerned about. The educated and attuned primary care provider can provide thoughtful first-line treatment of depression in young people and can be an important part of managing this public health challenge. It is always rewarding to help an adolescent overcome depression.
Dr. Swick is an attending psychiatrist in the division of child psychiatry at Massachusetts General Hospital, Boston, and director of the Parenting at a Challenging Time (PACT) Program at the Vernon Cancer Center at Newton Wellesley Hospital, also in Boston. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at pdnews@frontlinemedcom.com.
As many as 20% of children and adolescents experience a psychiatric disorder, with 50% of all lifetime psychiatric illnesses occurring by the age of 14 years. ADHD and depression are among the most common. The National Institutes of Health estimate that, in 2015, 3 million 12- to 17-year-old American children experienced a major depressive episode. Any illness that affects over 10% of adolescents will present regularly in the primary care provider’s office. It is important to know whom to screen and how to start treatment when your patient appears to be suffering from this serious but treatable condition.
While there are many screening instruments, it is important to be ready to ask patients diagnostic questions when your clinical suspicion of depression is high. In addition to asking about mood, sleep, appetite, energy, and the other DSM5 criteria of a major depressive episode, it is important to remember that teens with depression might present with irritability as much as sadness. While they lose interest in school, sports, or hobbies, they still may be distracted or cheered up by friends. And
Explain to your patient (and their parents) that depression is very treatable, but most effective treatments take time. Psychotherapy usually works over several months, and even effective medications can take 6 weeks or more. But, without treatment, their symptoms may persist for over a year and can disrupt their healthy development.
This is also a good time to ask your patient about suicidal thoughts. Have they been imagining how their death would affect others? Wishing they could just sleep? Do they have a plan? Do they have access to a means of killing themselves? Do they feel attached or connected to family, friends, religion, or a goal? Explain to your patient that these thoughts are common symptoms of depression, and work with their parents to ensure that they are connected and safe when starting treatment.
Psychotherapy is considered the first line treatment for mild to moderate episodes of depression and should be used alongside medications in severe episodes. While structured therapies such as cognitive behavioral therapy or interpersonal therapy have a strong evidence base to support their use, the best predictor of an effective therapy appears to be a strong alliance between therapist and patient. So, help your patient to find a therapist, and explain the importance of finding someone with whom they feel comfortable. Suggest to your patients that they have three visits with a new therapist to see if it feels like a “good match,” before considering trying another.
Finally, antidepressant medications are first-line treatment for more severe episodes of depression and episodes in which significant suicidal ideation or functional impairment are present. If the symptoms are more severe, or if therapy alone has not been effective after 4-6 weeks, you might consider starting antidepressant treatment. Psychiatrists usually start with an selective serotonin reuptake inhibitor, typically of a medium half-life, at a low dose to minimize the chances of side effects. While real efficacy takes up to 6 weeks, there should be some improvement in energy within the first 2 weeks on an effective medication. If there is no change, the dose can be raised gradually as tolerated. It is important to tell patients and their families about common side effects (mild GI upset) and the more rare but dangerous ones (such as hypomania or an increase in the frequency or intensity of suicidal thoughts).
Even when you do not refer your patient to someone else for treatment of depression, it is important that you not be alone in their management. Work closely with their therapist or consider having a psychiatric social worker join your team to offer therapy in close connection with your management. You might also periodically consult with a child psychiatrist to address treatment and medication questions and identify needed resources. Staying in touch with parents or connected adults at school (with the appropriate permission) can be very useful with those patients you are more concerned about. The educated and attuned primary care provider can provide thoughtful first-line treatment of depression in young people and can be an important part of managing this public health challenge. It is always rewarding to help an adolescent overcome depression.
Dr. Swick is an attending psychiatrist in the division of child psychiatry at Massachusetts General Hospital, Boston, and director of the Parenting at a Challenging Time (PACT) Program at the Vernon Cancer Center at Newton Wellesley Hospital, also in Boston. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at pdnews@frontlinemedcom.com.
As many as 20% of children and adolescents experience a psychiatric disorder, with 50% of all lifetime psychiatric illnesses occurring by the age of 14 years. ADHD and depression are among the most common. The National Institutes of Health estimate that, in 2015, 3 million 12- to 17-year-old American children experienced a major depressive episode. Any illness that affects over 10% of adolescents will present regularly in the primary care provider’s office. It is important to know whom to screen and how to start treatment when your patient appears to be suffering from this serious but treatable condition.
While there are many screening instruments, it is important to be ready to ask patients diagnostic questions when your clinical suspicion of depression is high. In addition to asking about mood, sleep, appetite, energy, and the other DSM5 criteria of a major depressive episode, it is important to remember that teens with depression might present with irritability as much as sadness. While they lose interest in school, sports, or hobbies, they still may be distracted or cheered up by friends. And
Explain to your patient (and their parents) that depression is very treatable, but most effective treatments take time. Psychotherapy usually works over several months, and even effective medications can take 6 weeks or more. But, without treatment, their symptoms may persist for over a year and can disrupt their healthy development.
This is also a good time to ask your patient about suicidal thoughts. Have they been imagining how their death would affect others? Wishing they could just sleep? Do they have a plan? Do they have access to a means of killing themselves? Do they feel attached or connected to family, friends, religion, or a goal? Explain to your patient that these thoughts are common symptoms of depression, and work with their parents to ensure that they are connected and safe when starting treatment.
Psychotherapy is considered the first line treatment for mild to moderate episodes of depression and should be used alongside medications in severe episodes. While structured therapies such as cognitive behavioral therapy or interpersonal therapy have a strong evidence base to support their use, the best predictor of an effective therapy appears to be a strong alliance between therapist and patient. So, help your patient to find a therapist, and explain the importance of finding someone with whom they feel comfortable. Suggest to your patients that they have three visits with a new therapist to see if it feels like a “good match,” before considering trying another.
Finally, antidepressant medications are first-line treatment for more severe episodes of depression and episodes in which significant suicidal ideation or functional impairment are present. If the symptoms are more severe, or if therapy alone has not been effective after 4-6 weeks, you might consider starting antidepressant treatment. Psychiatrists usually start with an selective serotonin reuptake inhibitor, typically of a medium half-life, at a low dose to minimize the chances of side effects. While real efficacy takes up to 6 weeks, there should be some improvement in energy within the first 2 weeks on an effective medication. If there is no change, the dose can be raised gradually as tolerated. It is important to tell patients and their families about common side effects (mild GI upset) and the more rare but dangerous ones (such as hypomania or an increase in the frequency or intensity of suicidal thoughts).
Even when you do not refer your patient to someone else for treatment of depression, it is important that you not be alone in their management. Work closely with their therapist or consider having a psychiatric social worker join your team to offer therapy in close connection with your management. You might also periodically consult with a child psychiatrist to address treatment and medication questions and identify needed resources. Staying in touch with parents or connected adults at school (with the appropriate permission) can be very useful with those patients you are more concerned about. The educated and attuned primary care provider can provide thoughtful first-line treatment of depression in young people and can be an important part of managing this public health challenge. It is always rewarding to help an adolescent overcome depression.
Dr. Swick is an attending psychiatrist in the division of child psychiatry at Massachusetts General Hospital, Boston, and director of the Parenting at a Challenging Time (PACT) Program at the Vernon Cancer Center at Newton Wellesley Hospital, also in Boston. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at pdnews@frontlinemedcom.com.
Expanded urine culture identified more pathogens
NEW ORLEANS – With the trade-off of an extra 24 hours for results, an enhanced protocol to culture clinically relevant urinary pathogens detected significantly more unique pathogens associated with urinary tract infection, compared with standard cultures, in a study of 150 women.
“What we were able to see is that for about 90% of the samples that were called negative by standard [approach], we were able to detect bacteria through our protocol,” said Travis K. Price, a PhD candidate in the department of microbiology and immunology at Loyola University, Chicago.
Typically, when a urine sample is cultured for a UTI at Loyola University Medical Center, the standard protocol is for the lab to test 1 mcL of urine using agar plates incubated aerobically for 24 hours, Mr. Price said. “When we’re testing the urinary microbiome, we expand on that protocol. We use 100 times more urine, different plates, different environmental conditions, and we hold them for 48 hours instead of 24.”
The investigators prospectively recruited 150 women coming in to the urogynecology clinic – half who felt they had a UTI that day, half who did not. “We wanted to understand if using our enhanced protocol was beneficial and essentially leading to better patient outcomes,” Mr. Price said at the annual meeting of the American Society for Microbiology.
“Among the women who felt they had a UTI, standard culture only picked up 50% of the pathogens we were picking up with our protocol,” Mr. Price said. “And when we looked closer, we realized most of that was Escherichia coli.” Excluding samples positive for E. coli, standard culture detected only 12% of UTI pathogens, he added, compared with 77% detected with the expanded quantitative protocol.
The expanded protocol detected significantly more unique pathogen species, 95, compared with 11 with standard cultures. In addition, of all the uropathogens detected by the new protocol, the standard protocol missed 67%, or 122 of the total 182.
In terms of clinical practicality, Mr. Price and his colleagues looked at “different conditions, multiple volumes of urine, different plates, 24 versus 48 hours – and at the end tried to figure out what is the least amount of work you can do to get the most information.” They then developed a streamlined protocol that involves 100 mcL of urine, a CNA agar plate that selects for gram-positive organisms, a MacConkey agar using 5% CO2, and 48 hours of incubation. “It’s easy to implement,” he added. “The only issue is the longer incubation time could lead to delayed treatment, potentially.”
The streamlined protocol detected more uropathogens – 152 of the 182, for an 84% detection rate – compared with standard cultures, which detected 60 of the 182, or 33%.
The streamlined protocol markedly improved uropathogen detection, the authors wrote. “These findings support the necessity for an immediate change in urine culture procedures.”
Another aim of the study was to evaluate the optimal threshold for UTI colony counts. Traditionally, the cutoff is set at 105 colonies or greater for diagnosis of a UTI, Mr. Price said. “We found there were always higher pathogen colony counts in people who thought they had a UTI. But there wasn’t one threshold that would have caught all of these.”
Next, the investigators looked for a correlation between the colony count cutoff and clinical outcomes. “For people who had a colony count greater than 105 – typically, it was a gram-negative organism – most people were treated with an antibiotic, and a week later most people, 62%, reported feeling better,” Mr. Price said. “But people who didn’t have a pathogen greater than 105, some were not treated, and when we called them a week later, most reported they were not feeling better. ... This suggests this threshold is not actually appropriate.”
Going forward, the investigators just started a clinical trial using the enhanced culture to confirm whether or not their protocol leads to better outcomes for women with UTIs.
Mr. Price did not have any relevant disclosures.
NEW ORLEANS – With the trade-off of an extra 24 hours for results, an enhanced protocol to culture clinically relevant urinary pathogens detected significantly more unique pathogens associated with urinary tract infection, compared with standard cultures, in a study of 150 women.
“What we were able to see is that for about 90% of the samples that were called negative by standard [approach], we were able to detect bacteria through our protocol,” said Travis K. Price, a PhD candidate in the department of microbiology and immunology at Loyola University, Chicago.
Typically, when a urine sample is cultured for a UTI at Loyola University Medical Center, the standard protocol is for the lab to test 1 mcL of urine using agar plates incubated aerobically for 24 hours, Mr. Price said. “When we’re testing the urinary microbiome, we expand on that protocol. We use 100 times more urine, different plates, different environmental conditions, and we hold them for 48 hours instead of 24.”
The investigators prospectively recruited 150 women coming in to the urogynecology clinic – half who felt they had a UTI that day, half who did not. “We wanted to understand if using our enhanced protocol was beneficial and essentially leading to better patient outcomes,” Mr. Price said at the annual meeting of the American Society for Microbiology.
“Among the women who felt they had a UTI, standard culture only picked up 50% of the pathogens we were picking up with our protocol,” Mr. Price said. “And when we looked closer, we realized most of that was Escherichia coli.” Excluding samples positive for E. coli, standard culture detected only 12% of UTI pathogens, he added, compared with 77% detected with the expanded quantitative protocol.
The expanded protocol detected significantly more unique pathogen species, 95, compared with 11 with standard cultures. In addition, of all the uropathogens detected by the new protocol, the standard protocol missed 67%, or 122 of the total 182.
In terms of clinical practicality, Mr. Price and his colleagues looked at “different conditions, multiple volumes of urine, different plates, 24 versus 48 hours – and at the end tried to figure out what is the least amount of work you can do to get the most information.” They then developed a streamlined protocol that involves 100 mcL of urine, a CNA agar plate that selects for gram-positive organisms, a MacConkey agar using 5% CO2, and 48 hours of incubation. “It’s easy to implement,” he added. “The only issue is the longer incubation time could lead to delayed treatment, potentially.”
The streamlined protocol detected more uropathogens – 152 of the 182, for an 84% detection rate – compared with standard cultures, which detected 60 of the 182, or 33%.
The streamlined protocol markedly improved uropathogen detection, the authors wrote. “These findings support the necessity for an immediate change in urine culture procedures.”
Another aim of the study was to evaluate the optimal threshold for UTI colony counts. Traditionally, the cutoff is set at 105 colonies or greater for diagnosis of a UTI, Mr. Price said. “We found there were always higher pathogen colony counts in people who thought they had a UTI. But there wasn’t one threshold that would have caught all of these.”
Next, the investigators looked for a correlation between the colony count cutoff and clinical outcomes. “For people who had a colony count greater than 105 – typically, it was a gram-negative organism – most people were treated with an antibiotic, and a week later most people, 62%, reported feeling better,” Mr. Price said. “But people who didn’t have a pathogen greater than 105, some were not treated, and when we called them a week later, most reported they were not feeling better. ... This suggests this threshold is not actually appropriate.”
Going forward, the investigators just started a clinical trial using the enhanced culture to confirm whether or not their protocol leads to better outcomes for women with UTIs.
Mr. Price did not have any relevant disclosures.
NEW ORLEANS – With the trade-off of an extra 24 hours for results, an enhanced protocol to culture clinically relevant urinary pathogens detected significantly more unique pathogens associated with urinary tract infection, compared with standard cultures, in a study of 150 women.
“What we were able to see is that for about 90% of the samples that were called negative by standard [approach], we were able to detect bacteria through our protocol,” said Travis K. Price, a PhD candidate in the department of microbiology and immunology at Loyola University, Chicago.
Typically, when a urine sample is cultured for a UTI at Loyola University Medical Center, the standard protocol is for the lab to test 1 mcL of urine using agar plates incubated aerobically for 24 hours, Mr. Price said. “When we’re testing the urinary microbiome, we expand on that protocol. We use 100 times more urine, different plates, different environmental conditions, and we hold them for 48 hours instead of 24.”
The investigators prospectively recruited 150 women coming in to the urogynecology clinic – half who felt they had a UTI that day, half who did not. “We wanted to understand if using our enhanced protocol was beneficial and essentially leading to better patient outcomes,” Mr. Price said at the annual meeting of the American Society for Microbiology.
“Among the women who felt they had a UTI, standard culture only picked up 50% of the pathogens we were picking up with our protocol,” Mr. Price said. “And when we looked closer, we realized most of that was Escherichia coli.” Excluding samples positive for E. coli, standard culture detected only 12% of UTI pathogens, he added, compared with 77% detected with the expanded quantitative protocol.
The expanded protocol detected significantly more unique pathogen species, 95, compared with 11 with standard cultures. In addition, of all the uropathogens detected by the new protocol, the standard protocol missed 67%, or 122 of the total 182.
In terms of clinical practicality, Mr. Price and his colleagues looked at “different conditions, multiple volumes of urine, different plates, 24 versus 48 hours – and at the end tried to figure out what is the least amount of work you can do to get the most information.” They then developed a streamlined protocol that involves 100 mcL of urine, a CNA agar plate that selects for gram-positive organisms, a MacConkey agar using 5% CO2, and 48 hours of incubation. “It’s easy to implement,” he added. “The only issue is the longer incubation time could lead to delayed treatment, potentially.”
The streamlined protocol detected more uropathogens – 152 of the 182, for an 84% detection rate – compared with standard cultures, which detected 60 of the 182, or 33%.
The streamlined protocol markedly improved uropathogen detection, the authors wrote. “These findings support the necessity for an immediate change in urine culture procedures.”
Another aim of the study was to evaluate the optimal threshold for UTI colony counts. Traditionally, the cutoff is set at 105 colonies or greater for diagnosis of a UTI, Mr. Price said. “We found there were always higher pathogen colony counts in people who thought they had a UTI. But there wasn’t one threshold that would have caught all of these.”
Next, the investigators looked for a correlation between the colony count cutoff and clinical outcomes. “For people who had a colony count greater than 105 – typically, it was a gram-negative organism – most people were treated with an antibiotic, and a week later most people, 62%, reported feeling better,” Mr. Price said. “But people who didn’t have a pathogen greater than 105, some were not treated, and when we called them a week later, most reported they were not feeling better. ... This suggests this threshold is not actually appropriate.”
Going forward, the investigators just started a clinical trial using the enhanced culture to confirm whether or not their protocol leads to better outcomes for women with UTIs.
Mr. Price did not have any relevant disclosures.
AT ASM MICROBE 2017
Key clinical point:
Major finding: Standard cultures missed 67% (122 of 182) of the uropathogens identified with the expanded culture protocol.
Data source: A prospective study of 150 women comparing UTI pathogen detection between standard and expanded culture analysis.
Disclosures: Mr. Price did not have any relevant disclosures.
Add-on azithromycin cuts asthma exacerbations
Adults with persistent symptomatic asthma who took azithromycin as an add-on therapy experienced fewer exacerbations and had improved quality of life, compared with their peers who took a placebo, a multicenter, randomized trial demonstrated.
“Macrolide antibiotics have antibacterial, antiviral, and anti-inflammatory effects, and are reported to be beneficial in both eosinophilic and noneosinophilic subtypes,” a group of Australian researchers wrote online July 4 in The Lancet (doi: org/10.1016/S0140-6736[17]31281-3). “Systematic reviews of randomized, controlled trials report benefits of macrolides on asthma symptoms but [we] are unable to draw conclusions about the effects on other endpoints, including exacerbations, due to lack of data, heterogeneity of results, and inadequate study design and sample size.”
The researchers observed a significant reduction in the incidence of total asthma exacerbations in the azithromycin-treated group: 1.07/patient-year, compared with 1.86/patient year in the placebo group, which translated into an incidence rate ratio of 0.59 (P less than .0001). Specifically, 127 patients in the placebo group (61%) experienced at least one asthma exacerbation compared with 94 patients in the azithromycin group (44%; P less than .0001). A significant improvement in asthma-related quality of life was also seen among patients in the azithromycin group (adjusted mean difference of 0.36; P = .001).
Though the mechanism of the antiviral effect of macrolides is not yet determined, Dr. Gibson and his associates noted that respiratory viral infection is associated with severe exacerbations in eosinophilic asthma and causes most respiratory infections. “There is a known interaction between eosinophilic airway inflammation, exacerbation rate, and impaired innate antiviral immunity,” they wrote. “Since we observed a benefit of azithromycin on both asthma exacerbations and respiratory infections, we speculate that azithromycin might be acting to prevent viral-induced episodes in asthma.”
“Given the major impact of asthma exacerbations on patients and the community and the ongoing risk posed by these events in patients who remain symptomatic on maintenance therapy, we consider that azithromycin is a valuable addition to existing regimens for treating asthma,” the researchers concluded. “The long-term effects of this therapy on community microbial resistance require further evaluation.”
The overall rates and types of serious adverse events seen in both groups were not significantly different from each other, with serious adverse events having occurred in 16 (8%) patients treated with azithromycin and 26 (13%) patients given the placebo.
The study was funded by the National Health and Medical Research Council of Australia and the John Hunter Hospital Charitable Trust. The authors reported having no financial conflicts directly related to the study.
Since microbial resistance is a well known side effect of antibiotic use, add-on therapy with azithromycin in asthma needs to be restricted to those patients with the highest unmet medical need (for example, frequent exacerbators) and to time periods with the greatest risk of exacerbations (such as winter). Biomarkers that predict the therapeutic response to macrolides might facilitate optimal patient selection. Further research is needed to elucidate the most important mechanism of action of these pleiotropic drugs. Macrolides have anti-inflammatory, antibacterial, and antiviral effects. However, the authors did not observe a reduction in inflammatory cell counts in sputum to support a definite anti-inflammatory effect. Azithromycin also was effective in patients with and without potentially pathogenic microorganisms in sputum cultures at baseline. Since azithromycin reduced both asthma exacerbations and respiratory infections, the benefits of azithromycin might be caused by preventing viral-induced attacks in asthma. Azithromycin stimulates phagocytosis of microbes and dead cells by macrophages (i.e., efferocytosis), an effect that is likely to be independent of the nature of the accompanying neutrophilic or eosinophilic airway inflammation.
Gibson and colleagues have clearly shown that add-on therapy with azithromycin is effective and safe in adult patients with uncontrolled asthma despite treatment with inhaled corticosteroids and long-acting beta-agonists. Azithromycin benefited patients with both eosinophilic and noneosinophilic asthma. However, the effects of long-term therapy with macrolides on community microbial resistance remain a public health concern. Future studies with potentially safer nonantibiotic macrolides in uncontrolled severe asthma are warranted. Since the antimicrobial effects probably contribute to the overall efficacy of macrolides, the beneficial effects of nonantibiotic macrolides might be intermediate between macrolide antibiotics and placebo.
This text is excerpted from a commentary published online July 4 in The Lancet (doi. org/10.1016/S0140-6736[17]31547-7). Guy Brusselle, MD, is with the department of respiratory medicine at Ghent (Belgium) University Hospital and Ian Pavord, MD, is with the University of Oxford’s Nuffield Department of Medicine, England. Both authors disclosed having received honoraria and other financial support from numerous pharmaceutical companies.
Since microbial resistance is a well known side effect of antibiotic use, add-on therapy with azithromycin in asthma needs to be restricted to those patients with the highest unmet medical need (for example, frequent exacerbators) and to time periods with the greatest risk of exacerbations (such as winter). Biomarkers that predict the therapeutic response to macrolides might facilitate optimal patient selection. Further research is needed to elucidate the most important mechanism of action of these pleiotropic drugs. Macrolides have anti-inflammatory, antibacterial, and antiviral effects. However, the authors did not observe a reduction in inflammatory cell counts in sputum to support a definite anti-inflammatory effect. Azithromycin also was effective in patients with and without potentially pathogenic microorganisms in sputum cultures at baseline. Since azithromycin reduced both asthma exacerbations and respiratory infections, the benefits of azithromycin might be caused by preventing viral-induced attacks in asthma. Azithromycin stimulates phagocytosis of microbes and dead cells by macrophages (i.e., efferocytosis), an effect that is likely to be independent of the nature of the accompanying neutrophilic or eosinophilic airway inflammation.
Gibson and colleagues have clearly shown that add-on therapy with azithromycin is effective and safe in adult patients with uncontrolled asthma despite treatment with inhaled corticosteroids and long-acting beta-agonists. Azithromycin benefited patients with both eosinophilic and noneosinophilic asthma. However, the effects of long-term therapy with macrolides on community microbial resistance remain a public health concern. Future studies with potentially safer nonantibiotic macrolides in uncontrolled severe asthma are warranted. Since the antimicrobial effects probably contribute to the overall efficacy of macrolides, the beneficial effects of nonantibiotic macrolides might be intermediate between macrolide antibiotics and placebo.
This text is excerpted from a commentary published online July 4 in The Lancet (doi. org/10.1016/S0140-6736[17]31547-7). Guy Brusselle, MD, is with the department of respiratory medicine at Ghent (Belgium) University Hospital and Ian Pavord, MD, is with the University of Oxford’s Nuffield Department of Medicine, England. Both authors disclosed having received honoraria and other financial support from numerous pharmaceutical companies.
Since microbial resistance is a well known side effect of antibiotic use, add-on therapy with azithromycin in asthma needs to be restricted to those patients with the highest unmet medical need (for example, frequent exacerbators) and to time periods with the greatest risk of exacerbations (such as winter). Biomarkers that predict the therapeutic response to macrolides might facilitate optimal patient selection. Further research is needed to elucidate the most important mechanism of action of these pleiotropic drugs. Macrolides have anti-inflammatory, antibacterial, and antiviral effects. However, the authors did not observe a reduction in inflammatory cell counts in sputum to support a definite anti-inflammatory effect. Azithromycin also was effective in patients with and without potentially pathogenic microorganisms in sputum cultures at baseline. Since azithromycin reduced both asthma exacerbations and respiratory infections, the benefits of azithromycin might be caused by preventing viral-induced attacks in asthma. Azithromycin stimulates phagocytosis of microbes and dead cells by macrophages (i.e., efferocytosis), an effect that is likely to be independent of the nature of the accompanying neutrophilic or eosinophilic airway inflammation.
Gibson and colleagues have clearly shown that add-on therapy with azithromycin is effective and safe in adult patients with uncontrolled asthma despite treatment with inhaled corticosteroids and long-acting beta-agonists. Azithromycin benefited patients with both eosinophilic and noneosinophilic asthma. However, the effects of long-term therapy with macrolides on community microbial resistance remain a public health concern. Future studies with potentially safer nonantibiotic macrolides in uncontrolled severe asthma are warranted. Since the antimicrobial effects probably contribute to the overall efficacy of macrolides, the beneficial effects of nonantibiotic macrolides might be intermediate between macrolide antibiotics and placebo.
This text is excerpted from a commentary published online July 4 in The Lancet (doi. org/10.1016/S0140-6736[17]31547-7). Guy Brusselle, MD, is with the department of respiratory medicine at Ghent (Belgium) University Hospital and Ian Pavord, MD, is with the University of Oxford’s Nuffield Department of Medicine, England. Both authors disclosed having received honoraria and other financial support from numerous pharmaceutical companies.
Adults with persistent symptomatic asthma who took azithromycin as an add-on therapy experienced fewer exacerbations and had improved quality of life, compared with their peers who took a placebo, a multicenter, randomized trial demonstrated.
“Macrolide antibiotics have antibacterial, antiviral, and anti-inflammatory effects, and are reported to be beneficial in both eosinophilic and noneosinophilic subtypes,” a group of Australian researchers wrote online July 4 in The Lancet (doi: org/10.1016/S0140-6736[17]31281-3). “Systematic reviews of randomized, controlled trials report benefits of macrolides on asthma symptoms but [we] are unable to draw conclusions about the effects on other endpoints, including exacerbations, due to lack of data, heterogeneity of results, and inadequate study design and sample size.”
The researchers observed a significant reduction in the incidence of total asthma exacerbations in the azithromycin-treated group: 1.07/patient-year, compared with 1.86/patient year in the placebo group, which translated into an incidence rate ratio of 0.59 (P less than .0001). Specifically, 127 patients in the placebo group (61%) experienced at least one asthma exacerbation compared with 94 patients in the azithromycin group (44%; P less than .0001). A significant improvement in asthma-related quality of life was also seen among patients in the azithromycin group (adjusted mean difference of 0.36; P = .001).
Though the mechanism of the antiviral effect of macrolides is not yet determined, Dr. Gibson and his associates noted that respiratory viral infection is associated with severe exacerbations in eosinophilic asthma and causes most respiratory infections. “There is a known interaction between eosinophilic airway inflammation, exacerbation rate, and impaired innate antiviral immunity,” they wrote. “Since we observed a benefit of azithromycin on both asthma exacerbations and respiratory infections, we speculate that azithromycin might be acting to prevent viral-induced episodes in asthma.”
“Given the major impact of asthma exacerbations on patients and the community and the ongoing risk posed by these events in patients who remain symptomatic on maintenance therapy, we consider that azithromycin is a valuable addition to existing regimens for treating asthma,” the researchers concluded. “The long-term effects of this therapy on community microbial resistance require further evaluation.”
The overall rates and types of serious adverse events seen in both groups were not significantly different from each other, with serious adverse events having occurred in 16 (8%) patients treated with azithromycin and 26 (13%) patients given the placebo.
The study was funded by the National Health and Medical Research Council of Australia and the John Hunter Hospital Charitable Trust. The authors reported having no financial conflicts directly related to the study.
Adults with persistent symptomatic asthma who took azithromycin as an add-on therapy experienced fewer exacerbations and had improved quality of life, compared with their peers who took a placebo, a multicenter, randomized trial demonstrated.
“Macrolide antibiotics have antibacterial, antiviral, and anti-inflammatory effects, and are reported to be beneficial in both eosinophilic and noneosinophilic subtypes,” a group of Australian researchers wrote online July 4 in The Lancet (doi: org/10.1016/S0140-6736[17]31281-3). “Systematic reviews of randomized, controlled trials report benefits of macrolides on asthma symptoms but [we] are unable to draw conclusions about the effects on other endpoints, including exacerbations, due to lack of data, heterogeneity of results, and inadequate study design and sample size.”
The researchers observed a significant reduction in the incidence of total asthma exacerbations in the azithromycin-treated group: 1.07/patient-year, compared with 1.86/patient year in the placebo group, which translated into an incidence rate ratio of 0.59 (P less than .0001). Specifically, 127 patients in the placebo group (61%) experienced at least one asthma exacerbation compared with 94 patients in the azithromycin group (44%; P less than .0001). A significant improvement in asthma-related quality of life was also seen among patients in the azithromycin group (adjusted mean difference of 0.36; P = .001).
Though the mechanism of the antiviral effect of macrolides is not yet determined, Dr. Gibson and his associates noted that respiratory viral infection is associated with severe exacerbations in eosinophilic asthma and causes most respiratory infections. “There is a known interaction between eosinophilic airway inflammation, exacerbation rate, and impaired innate antiviral immunity,” they wrote. “Since we observed a benefit of azithromycin on both asthma exacerbations and respiratory infections, we speculate that azithromycin might be acting to prevent viral-induced episodes in asthma.”
“Given the major impact of asthma exacerbations on patients and the community and the ongoing risk posed by these events in patients who remain symptomatic on maintenance therapy, we consider that azithromycin is a valuable addition to existing regimens for treating asthma,” the researchers concluded. “The long-term effects of this therapy on community microbial resistance require further evaluation.”
The overall rates and types of serious adverse events seen in both groups were not significantly different from each other, with serious adverse events having occurred in 16 (8%) patients treated with azithromycin and 26 (13%) patients given the placebo.
The study was funded by the National Health and Medical Research Council of Australia and the John Hunter Hospital Charitable Trust. The authors reported having no financial conflicts directly related to the study.
FROM THE LANCET
Key clinical point:
Major finding: Azithromycin reduced the incidence of total asthma exacerbations, compared with placebo (1.07/patient-year vs. 1.86/patient-year, respectively, for an incidence rate ratio of 0.59; P less than .0001).
Data source: A randomized, placebo-controlled, multicenter trial of 420 adults with persistent, uncontrolled asthma.
Disclosures: The study was funded by the National Health and Medical Research Council of Australia and the John Hunter Hospital Charitable Trust. The authors reported having no financial conflicts directly related to the study.
Recovery: Where TAVR gains advantage over SAVR
A post hoc analysis of the first randomized clinical to show the superiority of an interventional technique for aortic valve repair over surgery in terms of postoperative death has found the period of 30 days to 4 months after the procedure to be the most perilous for surgery patients, when their risk of death was almost twice that of interventional patients, likely because surgery patients were more vulnerable to complications and were less likely to go home after the procedure.
“This mortality difference was largely driven by higher rates of technical failure, surgical complications, and lack of recovery following surgery,” said Vincent A. Gaudiani, MD, of El Camino Hospital, Mountain View, Calif., and his coauthors (J Thorac Cardiovasc Surg. 2017;153:1293-99). The analysis investigated causes and timing of death in the CoreValve US Pivotal High-Risk Trial, a randomized, high-risk trial of the CoreValve self-expanding bioprosthesis (Medtronic). The trial favored transcatheter aortic valve replacement (TAVR) over surgical aortic valve replacement (SAVR).
The post hoc analysis evaluated all-cause mortality through the first year based on three time periods: early, up to 30 days; recovery, 31-120 days; and late, 121-365 days. Death rates for the two procedures were similar in the early and late postoperative periods, but deviated significantly in the recovery period: 4% for TAVR vs. 7.9% for SAVR (P = .25). SAVR patients were more likely affected by the overall influence of physical stress associated with surgery, the study found, whereas rates of technical failure and complications were similar between the two groups. “This suggests that early TAVR results can improve with technical refinements and that high-risk surgical patients will benefit from reducing complications,” wrote Dr. Gaudiani and his coauthors.
They noted the CoreValve trial findings, in terms of the survival differences between TAVR and SAVR, are significant because previous trials that compared TAVR and SAVR, including the Placement of Aortic Transcatheter Valves A trial (Lancet. 2015;385:2477-84), showed equivalent survival between the two procedures at up to 5 years. “This unique finding is provocative and the reason for this survival difference is important to understanding TAVR and SAVR and improving both therapies,” said Dr. Gaudiani and his coauthors.
While SAVR patients had a higher overall death rate in the recovery period, TAVR patients had a larger proportion of cardiovascular deaths – 12 of 15 (80%) vs. 16 of 27 (59.3%) for SAVR. The leading noncardiovascular cause of death in the SAVR group was sepsis (six), followed by malignancy (one), chronic obstructive pulmonary disease (one) and other (three). “Although these deaths were adjudicated as noncardiovascular by the CEC [clinical events committee], our review showed that some of these patients had never really recovered from the initial procedure,” the researchers wrote.
In the early period, death rates were 3.3% for TAVR and 4.5% for SAVR, a nonsignificant difference. TAVR patients who died had higher rates of peripheral vascular disease and recent falls; SAVR patients who died were more likely to have had a pacemaker. In the late period, the death rates were 7.5% for TAVR and 7.7% for SAVR, and the researchers also found no significant difference in the number of cardiovascular deaths (4.4% and 4.2%, respectively). “Hierarchical causes of death were primarily due to other reasons deemed unrelated to the initial aortic valve replacement,” noted Dr. Gaudiani and his coauthors.
However, the study also found that TAVR patients were significantly more likely to go home after hospital discharge rather than to a rehabilitation facility or another hospital – 66.9% vs. 39.7% (P less than .001).
In the SAVR group, five cardiovascular deaths in the recovery period occurred because the operation failed to correct aortic stenosis – all related to placement of a valve too small for the patient. “Placing a valve appropriately sized to the patient should be a priority for surgeons if we are to improve our outcomes,” the researchers noted. “Most other deaths were the result of patients’ inability to cope with the physical trauma of surgery.”
Dr. Gaudiani disclosed that he is a consultant and paid instructor for Medtronic, St. Jude Medical, and Edwards Lifesciences. Coauthors disclosed relationships with Edwards Lifesciences, Terumo, Gore Medical, Medtronic, Boston Scientific, and other device companies.
In his invited discussion, Craig R. Smith, MD, of New York, noted that comparisons “are odious” and that comparing clinical trials requires caution. (J Thorac Cardiovasc Surg. 2017;153:1300-1) He also acknowledged that surgeons would hope for evidence that the findings of the CoreValve US Pivotal High-Risk Trial were somehow wrong.
Dr. Smith raised a question about the CoreValve trial, which was designed to enroll high-risk patients, “but actually enrolled at the upper end of the intermediate risk range with a Society of Thoracic Surgeons (STS) score of 7.4 versus 11.3 in the high-risk PARTNER 1.” However, he noted that it would not be fair to consider the self-expanding TAVR trial intermediate risk, because the intermediate risk PARTNER 2 trial had an STS score of 5.8. And while outcomes for SAVR in the CoreValve trial were within the expected variable of less than 1 using the STS Predicted Risk for Mortality, the “bulge” of deaths in the recovery phase raises “a whiff of concern.”
Dr. Smith said that the early technical mortalities with TAVR in the trial are already disappearing with experience. He also noted that Dr. Gaudiani and his coauthors pointed out the frequency of failure to repair and failure to recover. “Whether competing against TAVR in a randomized trial or operating on TAVR in eligible patients in the future, as the authors have emphasized, it behooves us to correct the problem as completely as possible and take the best possible care of our patients afterward,” Dr. Smith said. He also noted the difference in discharge rates home “illustrates a very significant advantage of TAVR.”
Dr. Smith disclosed he has received reimbursement for expenses in his leadership role in the Placement of Aortic Transcatheter Valves (PARTNER) trials.
In his invited discussion, Craig R. Smith, MD, of New York, noted that comparisons “are odious” and that comparing clinical trials requires caution. (J Thorac Cardiovasc Surg. 2017;153:1300-1) He also acknowledged that surgeons would hope for evidence that the findings of the CoreValve US Pivotal High-Risk Trial were somehow wrong.
Dr. Smith raised a question about the CoreValve trial, which was designed to enroll high-risk patients, “but actually enrolled at the upper end of the intermediate risk range with a Society of Thoracic Surgeons (STS) score of 7.4 versus 11.3 in the high-risk PARTNER 1.” However, he noted that it would not be fair to consider the self-expanding TAVR trial intermediate risk, because the intermediate risk PARTNER 2 trial had an STS score of 5.8. And while outcomes for SAVR in the CoreValve trial were within the expected variable of less than 1 using the STS Predicted Risk for Mortality, the “bulge” of deaths in the recovery phase raises “a whiff of concern.”
Dr. Smith said that the early technical mortalities with TAVR in the trial are already disappearing with experience. He also noted that Dr. Gaudiani and his coauthors pointed out the frequency of failure to repair and failure to recover. “Whether competing against TAVR in a randomized trial or operating on TAVR in eligible patients in the future, as the authors have emphasized, it behooves us to correct the problem as completely as possible and take the best possible care of our patients afterward,” Dr. Smith said. He also noted the difference in discharge rates home “illustrates a very significant advantage of TAVR.”
Dr. Smith disclosed he has received reimbursement for expenses in his leadership role in the Placement of Aortic Transcatheter Valves (PARTNER) trials.
In his invited discussion, Craig R. Smith, MD, of New York, noted that comparisons “are odious” and that comparing clinical trials requires caution. (J Thorac Cardiovasc Surg. 2017;153:1300-1) He also acknowledged that surgeons would hope for evidence that the findings of the CoreValve US Pivotal High-Risk Trial were somehow wrong.
Dr. Smith raised a question about the CoreValve trial, which was designed to enroll high-risk patients, “but actually enrolled at the upper end of the intermediate risk range with a Society of Thoracic Surgeons (STS) score of 7.4 versus 11.3 in the high-risk PARTNER 1.” However, he noted that it would not be fair to consider the self-expanding TAVR trial intermediate risk, because the intermediate risk PARTNER 2 trial had an STS score of 5.8. And while outcomes for SAVR in the CoreValve trial were within the expected variable of less than 1 using the STS Predicted Risk for Mortality, the “bulge” of deaths in the recovery phase raises “a whiff of concern.”
Dr. Smith said that the early technical mortalities with TAVR in the trial are already disappearing with experience. He also noted that Dr. Gaudiani and his coauthors pointed out the frequency of failure to repair and failure to recover. “Whether competing against TAVR in a randomized trial or operating on TAVR in eligible patients in the future, as the authors have emphasized, it behooves us to correct the problem as completely as possible and take the best possible care of our patients afterward,” Dr. Smith said. He also noted the difference in discharge rates home “illustrates a very significant advantage of TAVR.”
Dr. Smith disclosed he has received reimbursement for expenses in his leadership role in the Placement of Aortic Transcatheter Valves (PARTNER) trials.
A post hoc analysis of the first randomized clinical to show the superiority of an interventional technique for aortic valve repair over surgery in terms of postoperative death has found the period of 30 days to 4 months after the procedure to be the most perilous for surgery patients, when their risk of death was almost twice that of interventional patients, likely because surgery patients were more vulnerable to complications and were less likely to go home after the procedure.
“This mortality difference was largely driven by higher rates of technical failure, surgical complications, and lack of recovery following surgery,” said Vincent A. Gaudiani, MD, of El Camino Hospital, Mountain View, Calif., and his coauthors (J Thorac Cardiovasc Surg. 2017;153:1293-99). The analysis investigated causes and timing of death in the CoreValve US Pivotal High-Risk Trial, a randomized, high-risk trial of the CoreValve self-expanding bioprosthesis (Medtronic). The trial favored transcatheter aortic valve replacement (TAVR) over surgical aortic valve replacement (SAVR).
The post hoc analysis evaluated all-cause mortality through the first year based on three time periods: early, up to 30 days; recovery, 31-120 days; and late, 121-365 days. Death rates for the two procedures were similar in the early and late postoperative periods, but deviated significantly in the recovery period: 4% for TAVR vs. 7.9% for SAVR (P = .25). SAVR patients were more likely affected by the overall influence of physical stress associated with surgery, the study found, whereas rates of technical failure and complications were similar between the two groups. “This suggests that early TAVR results can improve with technical refinements and that high-risk surgical patients will benefit from reducing complications,” wrote Dr. Gaudiani and his coauthors.
They noted the CoreValve trial findings, in terms of the survival differences between TAVR and SAVR, are significant because previous trials that compared TAVR and SAVR, including the Placement of Aortic Transcatheter Valves A trial (Lancet. 2015;385:2477-84), showed equivalent survival between the two procedures at up to 5 years. “This unique finding is provocative and the reason for this survival difference is important to understanding TAVR and SAVR and improving both therapies,” said Dr. Gaudiani and his coauthors.
While SAVR patients had a higher overall death rate in the recovery period, TAVR patients had a larger proportion of cardiovascular deaths – 12 of 15 (80%) vs. 16 of 27 (59.3%) for SAVR. The leading noncardiovascular cause of death in the SAVR group was sepsis (six), followed by malignancy (one), chronic obstructive pulmonary disease (one) and other (three). “Although these deaths were adjudicated as noncardiovascular by the CEC [clinical events committee], our review showed that some of these patients had never really recovered from the initial procedure,” the researchers wrote.
In the early period, death rates were 3.3% for TAVR and 4.5% for SAVR, a nonsignificant difference. TAVR patients who died had higher rates of peripheral vascular disease and recent falls; SAVR patients who died were more likely to have had a pacemaker. In the late period, the death rates were 7.5% for TAVR and 7.7% for SAVR, and the researchers also found no significant difference in the number of cardiovascular deaths (4.4% and 4.2%, respectively). “Hierarchical causes of death were primarily due to other reasons deemed unrelated to the initial aortic valve replacement,” noted Dr. Gaudiani and his coauthors.
However, the study also found that TAVR patients were significantly more likely to go home after hospital discharge rather than to a rehabilitation facility or another hospital – 66.9% vs. 39.7% (P less than .001).
In the SAVR group, five cardiovascular deaths in the recovery period occurred because the operation failed to correct aortic stenosis – all related to placement of a valve too small for the patient. “Placing a valve appropriately sized to the patient should be a priority for surgeons if we are to improve our outcomes,” the researchers noted. “Most other deaths were the result of patients’ inability to cope with the physical trauma of surgery.”
Dr. Gaudiani disclosed that he is a consultant and paid instructor for Medtronic, St. Jude Medical, and Edwards Lifesciences. Coauthors disclosed relationships with Edwards Lifesciences, Terumo, Gore Medical, Medtronic, Boston Scientific, and other device companies.
A post hoc analysis of the first randomized clinical to show the superiority of an interventional technique for aortic valve repair over surgery in terms of postoperative death has found the period of 30 days to 4 months after the procedure to be the most perilous for surgery patients, when their risk of death was almost twice that of interventional patients, likely because surgery patients were more vulnerable to complications and were less likely to go home after the procedure.
“This mortality difference was largely driven by higher rates of technical failure, surgical complications, and lack of recovery following surgery,” said Vincent A. Gaudiani, MD, of El Camino Hospital, Mountain View, Calif., and his coauthors (J Thorac Cardiovasc Surg. 2017;153:1293-99). The analysis investigated causes and timing of death in the CoreValve US Pivotal High-Risk Trial, a randomized, high-risk trial of the CoreValve self-expanding bioprosthesis (Medtronic). The trial favored transcatheter aortic valve replacement (TAVR) over surgical aortic valve replacement (SAVR).
The post hoc analysis evaluated all-cause mortality through the first year based on three time periods: early, up to 30 days; recovery, 31-120 days; and late, 121-365 days. Death rates for the two procedures were similar in the early and late postoperative periods, but deviated significantly in the recovery period: 4% for TAVR vs. 7.9% for SAVR (P = .25). SAVR patients were more likely affected by the overall influence of physical stress associated with surgery, the study found, whereas rates of technical failure and complications were similar between the two groups. “This suggests that early TAVR results can improve with technical refinements and that high-risk surgical patients will benefit from reducing complications,” wrote Dr. Gaudiani and his coauthors.
They noted the CoreValve trial findings, in terms of the survival differences between TAVR and SAVR, are significant because previous trials that compared TAVR and SAVR, including the Placement of Aortic Transcatheter Valves A trial (Lancet. 2015;385:2477-84), showed equivalent survival between the two procedures at up to 5 years. “This unique finding is provocative and the reason for this survival difference is important to understanding TAVR and SAVR and improving both therapies,” said Dr. Gaudiani and his coauthors.
While SAVR patients had a higher overall death rate in the recovery period, TAVR patients had a larger proportion of cardiovascular deaths – 12 of 15 (80%) vs. 16 of 27 (59.3%) for SAVR. The leading noncardiovascular cause of death in the SAVR group was sepsis (six), followed by malignancy (one), chronic obstructive pulmonary disease (one) and other (three). “Although these deaths were adjudicated as noncardiovascular by the CEC [clinical events committee], our review showed that some of these patients had never really recovered from the initial procedure,” the researchers wrote.
In the early period, death rates were 3.3% for TAVR and 4.5% for SAVR, a nonsignificant difference. TAVR patients who died had higher rates of peripheral vascular disease and recent falls; SAVR patients who died were more likely to have had a pacemaker. In the late period, the death rates were 7.5% for TAVR and 7.7% for SAVR, and the researchers also found no significant difference in the number of cardiovascular deaths (4.4% and 4.2%, respectively). “Hierarchical causes of death were primarily due to other reasons deemed unrelated to the initial aortic valve replacement,” noted Dr. Gaudiani and his coauthors.
However, the study also found that TAVR patients were significantly more likely to go home after hospital discharge rather than to a rehabilitation facility or another hospital – 66.9% vs. 39.7% (P less than .001).
In the SAVR group, five cardiovascular deaths in the recovery period occurred because the operation failed to correct aortic stenosis – all related to placement of a valve too small for the patient. “Placing a valve appropriately sized to the patient should be a priority for surgeons if we are to improve our outcomes,” the researchers noted. “Most other deaths were the result of patients’ inability to cope with the physical trauma of surgery.”
Dr. Gaudiani disclosed that he is a consultant and paid instructor for Medtronic, St. Jude Medical, and Edwards Lifesciences. Coauthors disclosed relationships with Edwards Lifesciences, Terumo, Gore Medical, Medtronic, Boston Scientific, and other device companies.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Differences in survival between transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR) in high-risk patients appear to be driven primarily by issues with disease repair and patient recovery from SAVR.
Major finding: During the recovery period (31-120 days) the death rate was 4% for TAVR vs. 7.9% for SAVR (P = .025).
Data source: Post hoc analysis of CoreValve US Pivotal High-Risk Trial involving 750 patients and 45 sites with outcomes reported through 3 years.
Disclosures: Dr. Gaudiani disclosed that he is a consultant and paid instructor for Medtronic, St. Jude Medical, and Edwards Lifesciences. Coauthors disclosed relationships with Edwards Lifesciences, Terumo, Gore Medical, Medtronic, Boston Scientific, and other device companies.
Supportive care alone linked to worse outcomes in mucocutaneous reactions
CHICAGO – , compared with other treatment modalities, a systematic review suggested.
Although rare in the pediatric population – with an incidence of approximately 1 case in 2 million – Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are both life-threatening mucocutaneous reactions.
Because reliable and validated data in the management of these conditions are lacking, he and his associates performed a systematic review to evaluate the effectiveness of reported treatment modalities using specific outcome measures.
“This study is unique in that it aims to not only capture more commonly employed interventions such as supportive care, surgical debridement, intravenous immunoglobulin [IVIG], and corticosteroids but also newer modalities that coincide with our improved understanding of the pathogenesis of the disease, such as the use of cyclosporine and biologics,” said Mr. Mansour, a third-year medical student at the University of Toronto.
The researchers systematically reviewed English and non-English articles using EMBASE, MEDLINE, the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, the Database of Abstracts of Reviews of Effects, the NHS Economic Evaluation Database, and the Health Technology Assessment Database. They excluded nonpediatric cases and those in which specific treatment modalities and outcome measures of interest were missing. In all, 302 articles were included in the study – 197 that examined mortality and 105 that examined time for arrest of progression of blistering, re-epithelialization, and length of hospital stay.
The main treatment modalities included supportive care alone, IVIG, corticosteroids, cyclosporine, surgical debridement, and biologics. The average time for arrest of progression of blistering was 8.4 days for supportive care, 4 days for IVIG, 6 days for corticosteroids, 2.5 days for cyclosporine, and 1 for infliximab. No data were available for time to arrest of progression for surgical debridement alone.
The average time for re-epithelialization was 24 days for supportive care, 18 days for surgical debridement, 8.7 days for IVIG, 12.2 days for corticosteroids, 10.6 days for cyclosporine, and 13 days for infliximab. Average length of hospital stay varied from 15.6 days for supportive care, 24.3 days for surgical debridement, 17.4 days for IVIG, 11.5 for corticosteroids, 15.5 for cyclosporine, and 26.3 for biologics.
The overall mortality was 4.6% among those who received supportive care alone, 8.3% among those who received IVIG, 4.5% among those who received corticosteroids, and 0.9% among those who received IVIG plus corticosteroids. Specifically, the rates of mortality for SJS and TEN cases treated with supportive care alone were 2% for SJS cases and 15.3% for TEN cases, respectively. The rates for other treatment modalities were 3.1% and 11% for IVIG, 1.7% and 9.3% for corticosteroids, and 0% and 2.1% for IVIG plus corticosteroids.
“While it was not surprising that mortality rates in pediatric SJS and TEN are low, the rates were slightly higher than anticipated,” Mr. Mansour said. “In addition, our data show that, although mortality is not influenced by certain therapeutic interventions in SJS patients, it may play a role in the management of the more severe disease, TEN.”
He acknowledged certain limitations of the study, including the fact that many of the data were derived from small-cohort retrospective studies and isolated case reports, giving rise to data heterogeneity and publication bias. “In addition, the mortality data we gathered may not necessarily represent patient survival, as many studies had variable follow-up duration,” he said. “Lastly, the additive effect of combination treatment and variable dosing was not captured in the present analysis, and further statistical analysis is required for re-epithelialization–based outcome measures and length of stay to ascertain significance.”
Mr. Mansour reported having no financial disclosures.
CHICAGO – , compared with other treatment modalities, a systematic review suggested.
Although rare in the pediatric population – with an incidence of approximately 1 case in 2 million – Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are both life-threatening mucocutaneous reactions.
Because reliable and validated data in the management of these conditions are lacking, he and his associates performed a systematic review to evaluate the effectiveness of reported treatment modalities using specific outcome measures.
“This study is unique in that it aims to not only capture more commonly employed interventions such as supportive care, surgical debridement, intravenous immunoglobulin [IVIG], and corticosteroids but also newer modalities that coincide with our improved understanding of the pathogenesis of the disease, such as the use of cyclosporine and biologics,” said Mr. Mansour, a third-year medical student at the University of Toronto.
The researchers systematically reviewed English and non-English articles using EMBASE, MEDLINE, the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, the Database of Abstracts of Reviews of Effects, the NHS Economic Evaluation Database, and the Health Technology Assessment Database. They excluded nonpediatric cases and those in which specific treatment modalities and outcome measures of interest were missing. In all, 302 articles were included in the study – 197 that examined mortality and 105 that examined time for arrest of progression of blistering, re-epithelialization, and length of hospital stay.
The main treatment modalities included supportive care alone, IVIG, corticosteroids, cyclosporine, surgical debridement, and biologics. The average time for arrest of progression of blistering was 8.4 days for supportive care, 4 days for IVIG, 6 days for corticosteroids, 2.5 days for cyclosporine, and 1 for infliximab. No data were available for time to arrest of progression for surgical debridement alone.
The average time for re-epithelialization was 24 days for supportive care, 18 days for surgical debridement, 8.7 days for IVIG, 12.2 days for corticosteroids, 10.6 days for cyclosporine, and 13 days for infliximab. Average length of hospital stay varied from 15.6 days for supportive care, 24.3 days for surgical debridement, 17.4 days for IVIG, 11.5 for corticosteroids, 15.5 for cyclosporine, and 26.3 for biologics.
The overall mortality was 4.6% among those who received supportive care alone, 8.3% among those who received IVIG, 4.5% among those who received corticosteroids, and 0.9% among those who received IVIG plus corticosteroids. Specifically, the rates of mortality for SJS and TEN cases treated with supportive care alone were 2% for SJS cases and 15.3% for TEN cases, respectively. The rates for other treatment modalities were 3.1% and 11% for IVIG, 1.7% and 9.3% for corticosteroids, and 0% and 2.1% for IVIG plus corticosteroids.
“While it was not surprising that mortality rates in pediatric SJS and TEN are low, the rates were slightly higher than anticipated,” Mr. Mansour said. “In addition, our data show that, although mortality is not influenced by certain therapeutic interventions in SJS patients, it may play a role in the management of the more severe disease, TEN.”
He acknowledged certain limitations of the study, including the fact that many of the data were derived from small-cohort retrospective studies and isolated case reports, giving rise to data heterogeneity and publication bias. “In addition, the mortality data we gathered may not necessarily represent patient survival, as many studies had variable follow-up duration,” he said. “Lastly, the additive effect of combination treatment and variable dosing was not captured in the present analysis, and further statistical analysis is required for re-epithelialization–based outcome measures and length of stay to ascertain significance.”
Mr. Mansour reported having no financial disclosures.
CHICAGO – , compared with other treatment modalities, a systematic review suggested.
Although rare in the pediatric population – with an incidence of approximately 1 case in 2 million – Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are both life-threatening mucocutaneous reactions.
Because reliable and validated data in the management of these conditions are lacking, he and his associates performed a systematic review to evaluate the effectiveness of reported treatment modalities using specific outcome measures.
“This study is unique in that it aims to not only capture more commonly employed interventions such as supportive care, surgical debridement, intravenous immunoglobulin [IVIG], and corticosteroids but also newer modalities that coincide with our improved understanding of the pathogenesis of the disease, such as the use of cyclosporine and biologics,” said Mr. Mansour, a third-year medical student at the University of Toronto.
The researchers systematically reviewed English and non-English articles using EMBASE, MEDLINE, the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, the Database of Abstracts of Reviews of Effects, the NHS Economic Evaluation Database, and the Health Technology Assessment Database. They excluded nonpediatric cases and those in which specific treatment modalities and outcome measures of interest were missing. In all, 302 articles were included in the study – 197 that examined mortality and 105 that examined time for arrest of progression of blistering, re-epithelialization, and length of hospital stay.
The main treatment modalities included supportive care alone, IVIG, corticosteroids, cyclosporine, surgical debridement, and biologics. The average time for arrest of progression of blistering was 8.4 days for supportive care, 4 days for IVIG, 6 days for corticosteroids, 2.5 days for cyclosporine, and 1 for infliximab. No data were available for time to arrest of progression for surgical debridement alone.
The average time for re-epithelialization was 24 days for supportive care, 18 days for surgical debridement, 8.7 days for IVIG, 12.2 days for corticosteroids, 10.6 days for cyclosporine, and 13 days for infliximab. Average length of hospital stay varied from 15.6 days for supportive care, 24.3 days for surgical debridement, 17.4 days for IVIG, 11.5 for corticosteroids, 15.5 for cyclosporine, and 26.3 for biologics.
The overall mortality was 4.6% among those who received supportive care alone, 8.3% among those who received IVIG, 4.5% among those who received corticosteroids, and 0.9% among those who received IVIG plus corticosteroids. Specifically, the rates of mortality for SJS and TEN cases treated with supportive care alone were 2% for SJS cases and 15.3% for TEN cases, respectively. The rates for other treatment modalities were 3.1% and 11% for IVIG, 1.7% and 9.3% for corticosteroids, and 0% and 2.1% for IVIG plus corticosteroids.
“While it was not surprising that mortality rates in pediatric SJS and TEN are low, the rates were slightly higher than anticipated,” Mr. Mansour said. “In addition, our data show that, although mortality is not influenced by certain therapeutic interventions in SJS patients, it may play a role in the management of the more severe disease, TEN.”
He acknowledged certain limitations of the study, including the fact that many of the data were derived from small-cohort retrospective studies and isolated case reports, giving rise to data heterogeneity and publication bias. “In addition, the mortality data we gathered may not necessarily represent patient survival, as many studies had variable follow-up duration,” he said. “Lastly, the additive effect of combination treatment and variable dosing was not captured in the present analysis, and further statistical analysis is required for re-epithelialization–based outcome measures and length of stay to ascertain significance.”
Mr. Mansour reported having no financial disclosures.
AT WCPD 2017
Key clinical point: Reliable and validated data informing the best treatment intervention for Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) are lacking.
Major finding: The overall mortality was 4.6% among those who received supportive care alone, 8.3% among those who received IVIG, 4.5% among those who received corticosteroids, and 0.9% among those who received IVIG plus corticosteroids.
Data source: A systematic review of 302 studies that evaluated the effectiveness of reported treatment modalities for SJS/TEN using specific outcome measures.
Disclosures: Mr. Mansour reported having no financial disclosures.
Pediatric hospital medicine marches toward subspecialty recognition
Pediatric hospital medicine is moving quickly toward recognition as a board-certified, fellowship-trained medical subspecialty, joining 14 other pediatric subspecialties now certified by the American Board of Pediatrics (ABP).
It was approved as a subspecialty by the American Board of Medical Specialties (ABMS) at its October 2016 board meeting in Chicago in response to a petition from the ABP. Following years of discussion within the field,1 it will take 2 more years to describe pediatric hospital medicine’s specialized knowledge base and write test questions for biannual board exams that are projected to commence in the fall of 2019.
Eventually, starting in 2025, pediatric hospitalists will need to complete a fellowship of 2 years or more if they wish to sit for the exam and become board-certified in the field. But for the next 7 years, hospitalists in current practice will be able to qualify based on their work experience. Maintenance of certification requirements likely will be similar to those in other subspecialties, and doctors certified in pediatric hospital medicine won’t be required to maintain general pediatric certification, Dr. Rauch said.
Formal eligibility criteria have not been set, but likely will include working half-time overall in pediatric-related activities, and quarter-time in clinical practice in pediatric hospital medicine for 4 years prior to qualifying for the exam. How the hours might break down between clinical and other hospital responsibilities, and between pediatric or adult patients, still needs to be determined, Dr. Rauch said. He added that the experiential pathway is likely to be defined broadly, with latitude for determinations based on percentages of time worked, rather than absolute number of hours worked. Local pediatric institutions will be granted latitude to determine how that “plays out” in real world situations, he said, “and an ABP credentialing committee will be available to hear appeals for people who have complicated life circumstances.”
“I was part of a committee that explored these issues. We were an independent group of hospitalists who decided that board certification was a good way to advance the field,” Dr. Quinonez said. “We’ve seen what subspecialty certification has done, for example, for pediatric emergency medicine and pediatric critical care medicine – advancing them tremendously from a research standpoint and helping to develop a distinct body of knowledge reflecting increased severity of illness in hospitalized children.”
Discrepancy between practicing hospitalists, fellowships
An estimated 4,000 pediatric hospitalists now practice in the United States, and 2,100 of those belong to the American Academy of Pediatrics’ Section on Hospital Medicine. There are 40 pediatric hospital medicine fellowship programs listed on the website of AAP’s Section on Hospital Medicine (http://phmfellows.org/phm-programs/), although formal training assessment criteria will be needed for the American College of Graduate Medical Education to recognize programs that qualify their fellows to sit for the PHM exam. A wide gap is anticipated between the demand for pediatric hospitalists and currently available fellowship training slots to generate new candidates for board certification, although Dr. Rauch projects that fellowship slots will double in coming years.
“My message to the field is that historically, board certification has been the launching point for further development of the field,” Dr. Rauch said. “It leads to standardization of who is a subspecialist. Right now, who is a pediatric hospitalist is subject to wide variation. We need to standardize training and to create for this field the same distinction and stature as other medical subspecialties,” he said, noting that subspecialty status also has ramifications for academic settings, and for career advancement and career satisfaction for the individuals who choose to pursue it.
“I know there has been some hue and cry about this in the field, but in most cases certification will not change a pediatric hospitalist’s ability to obtain a job,” he said. “Already, you can’t become a division leader at a children’s hospital without additional training. This isn’t going to change that reality. But for people who don’t want to follow an academic career path, there will never be enough board-certified or fellowship-trained pediatric hospitalists to fill all of the pediatric positions in all the hospitals in the country. Community hospitals aren’t going to say: We won’t hire you unless you are board certified.”
Is the fellowship good for the field?
The subspecialty development process clearly is moving forward. Those in favor believe it will increase scholarship, research, and recognition for the subspecialty by the public for its specialized body of knowledge. But not everyone in the field agrees. Last fall The Hospitalist published3 an opinion piece questioning the need for fellowship-based board certification in pediatric hospital medicine. The author recommended instead retaining the current voluntary approach to fellowships and establishing a pediatric “focused practice” incorporated into residency training, much as the American Board of Internal Medicine and the American Board of Family Medicine have done for hospitalists in adult medicine.
“Will it lead to uncertainty among those currently in residency programs? If you are a pediatric resident and you want to become a board-certified pediatric hospitalist, you’ll need at least 2 years more of training. Is that going to deter qualified individuals?” Dr. Chang said. “The people this decision will impact the most are med-peds doctors – who complete a combined internal medicine and pediatrics residency – and part-timers. They may find themselves in a difficult position if the number of hours don’t add up for them to sit for the boards. For the most part, we’ll have to wait and see for answers to these questions.”
Brian Alverson, MD, FAAP, current chair of the AAP’s Section on Hospital Medicine and associate professor of pediatrics at Brown University, Providence, R.I., says he can see both sides of the debate.
But at the same time, there is a significant opportunity cost for doing 2 more years of fellowship training, Dr. Alverson said.
“We don’t know how much the board certification test will improve actual care,” he noted. “Does it truly identify higher quality doctors, or just doctors who are good at taking multiple choice exams? There are a number of people in pediatrics who do a lot of different things in their jobs, and it’s important that they not lose their ability to practice in the field. Two-thirds of our work force is in community hospitals, not academic medical centers. They work hard to provide the backbone of hospital care for young patients, and many of them are unlikely to ever do a fellowship.”
Nonetheless, Dr. Alverson believes pediatric hospitalists needn’t worry. “You still have plenty of time to figure out what’s going to happen in your hospital,” he said.
References
1. Section on Hospital Medicine. Guiding principles for pediatric hospital medicine program. Pediatrics; 2013; 132:782-786.
2. Stucky E. The Pediatric Hospital Medicine Core Competencies. Wiley-Blackwell; 2010.
3. Feldman LS, Monash B, Eniasivam A. Why required pediatric hospital medicine fellowships are unnecessary. The Hospitalist Magazine, October 8, 2016.
Pediatric hospital medicine is moving quickly toward recognition as a board-certified, fellowship-trained medical subspecialty, joining 14 other pediatric subspecialties now certified by the American Board of Pediatrics (ABP).
It was approved as a subspecialty by the American Board of Medical Specialties (ABMS) at its October 2016 board meeting in Chicago in response to a petition from the ABP. Following years of discussion within the field,1 it will take 2 more years to describe pediatric hospital medicine’s specialized knowledge base and write test questions for biannual board exams that are projected to commence in the fall of 2019.
Eventually, starting in 2025, pediatric hospitalists will need to complete a fellowship of 2 years or more if they wish to sit for the exam and become board-certified in the field. But for the next 7 years, hospitalists in current practice will be able to qualify based on their work experience. Maintenance of certification requirements likely will be similar to those in other subspecialties, and doctors certified in pediatric hospital medicine won’t be required to maintain general pediatric certification, Dr. Rauch said.
Formal eligibility criteria have not been set, but likely will include working half-time overall in pediatric-related activities, and quarter-time in clinical practice in pediatric hospital medicine for 4 years prior to qualifying for the exam. How the hours might break down between clinical and other hospital responsibilities, and between pediatric or adult patients, still needs to be determined, Dr. Rauch said. He added that the experiential pathway is likely to be defined broadly, with latitude for determinations based on percentages of time worked, rather than absolute number of hours worked. Local pediatric institutions will be granted latitude to determine how that “plays out” in real world situations, he said, “and an ABP credentialing committee will be available to hear appeals for people who have complicated life circumstances.”
“I was part of a committee that explored these issues. We were an independent group of hospitalists who decided that board certification was a good way to advance the field,” Dr. Quinonez said. “We’ve seen what subspecialty certification has done, for example, for pediatric emergency medicine and pediatric critical care medicine – advancing them tremendously from a research standpoint and helping to develop a distinct body of knowledge reflecting increased severity of illness in hospitalized children.”
Discrepancy between practicing hospitalists, fellowships
An estimated 4,000 pediatric hospitalists now practice in the United States, and 2,100 of those belong to the American Academy of Pediatrics’ Section on Hospital Medicine. There are 40 pediatric hospital medicine fellowship programs listed on the website of AAP’s Section on Hospital Medicine (http://phmfellows.org/phm-programs/), although formal training assessment criteria will be needed for the American College of Graduate Medical Education to recognize programs that qualify their fellows to sit for the PHM exam. A wide gap is anticipated between the demand for pediatric hospitalists and currently available fellowship training slots to generate new candidates for board certification, although Dr. Rauch projects that fellowship slots will double in coming years.
“My message to the field is that historically, board certification has been the launching point for further development of the field,” Dr. Rauch said. “It leads to standardization of who is a subspecialist. Right now, who is a pediatric hospitalist is subject to wide variation. We need to standardize training and to create for this field the same distinction and stature as other medical subspecialties,” he said, noting that subspecialty status also has ramifications for academic settings, and for career advancement and career satisfaction for the individuals who choose to pursue it.
“I know there has been some hue and cry about this in the field, but in most cases certification will not change a pediatric hospitalist’s ability to obtain a job,” he said. “Already, you can’t become a division leader at a children’s hospital without additional training. This isn’t going to change that reality. But for people who don’t want to follow an academic career path, there will never be enough board-certified or fellowship-trained pediatric hospitalists to fill all of the pediatric positions in all the hospitals in the country. Community hospitals aren’t going to say: We won’t hire you unless you are board certified.”
Is the fellowship good for the field?
The subspecialty development process clearly is moving forward. Those in favor believe it will increase scholarship, research, and recognition for the subspecialty by the public for its specialized body of knowledge. But not everyone in the field agrees. Last fall The Hospitalist published3 an opinion piece questioning the need for fellowship-based board certification in pediatric hospital medicine. The author recommended instead retaining the current voluntary approach to fellowships and establishing a pediatric “focused practice” incorporated into residency training, much as the American Board of Internal Medicine and the American Board of Family Medicine have done for hospitalists in adult medicine.
“Will it lead to uncertainty among those currently in residency programs? If you are a pediatric resident and you want to become a board-certified pediatric hospitalist, you’ll need at least 2 years more of training. Is that going to deter qualified individuals?” Dr. Chang said. “The people this decision will impact the most are med-peds doctors – who complete a combined internal medicine and pediatrics residency – and part-timers. They may find themselves in a difficult position if the number of hours don’t add up for them to sit for the boards. For the most part, we’ll have to wait and see for answers to these questions.”
Brian Alverson, MD, FAAP, current chair of the AAP’s Section on Hospital Medicine and associate professor of pediatrics at Brown University, Providence, R.I., says he can see both sides of the debate.
But at the same time, there is a significant opportunity cost for doing 2 more years of fellowship training, Dr. Alverson said.
“We don’t know how much the board certification test will improve actual care,” he noted. “Does it truly identify higher quality doctors, or just doctors who are good at taking multiple choice exams? There are a number of people in pediatrics who do a lot of different things in their jobs, and it’s important that they not lose their ability to practice in the field. Two-thirds of our work force is in community hospitals, not academic medical centers. They work hard to provide the backbone of hospital care for young patients, and many of them are unlikely to ever do a fellowship.”
Nonetheless, Dr. Alverson believes pediatric hospitalists needn’t worry. “You still have plenty of time to figure out what’s going to happen in your hospital,” he said.
References
1. Section on Hospital Medicine. Guiding principles for pediatric hospital medicine program. Pediatrics; 2013; 132:782-786.
2. Stucky E. The Pediatric Hospital Medicine Core Competencies. Wiley-Blackwell; 2010.
3. Feldman LS, Monash B, Eniasivam A. Why required pediatric hospital medicine fellowships are unnecessary. The Hospitalist Magazine, October 8, 2016.
Pediatric hospital medicine is moving quickly toward recognition as a board-certified, fellowship-trained medical subspecialty, joining 14 other pediatric subspecialties now certified by the American Board of Pediatrics (ABP).
It was approved as a subspecialty by the American Board of Medical Specialties (ABMS) at its October 2016 board meeting in Chicago in response to a petition from the ABP. Following years of discussion within the field,1 it will take 2 more years to describe pediatric hospital medicine’s specialized knowledge base and write test questions for biannual board exams that are projected to commence in the fall of 2019.
Eventually, starting in 2025, pediatric hospitalists will need to complete a fellowship of 2 years or more if they wish to sit for the exam and become board-certified in the field. But for the next 7 years, hospitalists in current practice will be able to qualify based on their work experience. Maintenance of certification requirements likely will be similar to those in other subspecialties, and doctors certified in pediatric hospital medicine won’t be required to maintain general pediatric certification, Dr. Rauch said.
Formal eligibility criteria have not been set, but likely will include working half-time overall in pediatric-related activities, and quarter-time in clinical practice in pediatric hospital medicine for 4 years prior to qualifying for the exam. How the hours might break down between clinical and other hospital responsibilities, and between pediatric or adult patients, still needs to be determined, Dr. Rauch said. He added that the experiential pathway is likely to be defined broadly, with latitude for determinations based on percentages of time worked, rather than absolute number of hours worked. Local pediatric institutions will be granted latitude to determine how that “plays out” in real world situations, he said, “and an ABP credentialing committee will be available to hear appeals for people who have complicated life circumstances.”
“I was part of a committee that explored these issues. We were an independent group of hospitalists who decided that board certification was a good way to advance the field,” Dr. Quinonez said. “We’ve seen what subspecialty certification has done, for example, for pediatric emergency medicine and pediatric critical care medicine – advancing them tremendously from a research standpoint and helping to develop a distinct body of knowledge reflecting increased severity of illness in hospitalized children.”
Discrepancy between practicing hospitalists, fellowships
An estimated 4,000 pediatric hospitalists now practice in the United States, and 2,100 of those belong to the American Academy of Pediatrics’ Section on Hospital Medicine. There are 40 pediatric hospital medicine fellowship programs listed on the website of AAP’s Section on Hospital Medicine (http://phmfellows.org/phm-programs/), although formal training assessment criteria will be needed for the American College of Graduate Medical Education to recognize programs that qualify their fellows to sit for the PHM exam. A wide gap is anticipated between the demand for pediatric hospitalists and currently available fellowship training slots to generate new candidates for board certification, although Dr. Rauch projects that fellowship slots will double in coming years.
“My message to the field is that historically, board certification has been the launching point for further development of the field,” Dr. Rauch said. “It leads to standardization of who is a subspecialist. Right now, who is a pediatric hospitalist is subject to wide variation. We need to standardize training and to create for this field the same distinction and stature as other medical subspecialties,” he said, noting that subspecialty status also has ramifications for academic settings, and for career advancement and career satisfaction for the individuals who choose to pursue it.
“I know there has been some hue and cry about this in the field, but in most cases certification will not change a pediatric hospitalist’s ability to obtain a job,” he said. “Already, you can’t become a division leader at a children’s hospital without additional training. This isn’t going to change that reality. But for people who don’t want to follow an academic career path, there will never be enough board-certified or fellowship-trained pediatric hospitalists to fill all of the pediatric positions in all the hospitals in the country. Community hospitals aren’t going to say: We won’t hire you unless you are board certified.”
Is the fellowship good for the field?
The subspecialty development process clearly is moving forward. Those in favor believe it will increase scholarship, research, and recognition for the subspecialty by the public for its specialized body of knowledge. But not everyone in the field agrees. Last fall The Hospitalist published3 an opinion piece questioning the need for fellowship-based board certification in pediatric hospital medicine. The author recommended instead retaining the current voluntary approach to fellowships and establishing a pediatric “focused practice” incorporated into residency training, much as the American Board of Internal Medicine and the American Board of Family Medicine have done for hospitalists in adult medicine.
“Will it lead to uncertainty among those currently in residency programs? If you are a pediatric resident and you want to become a board-certified pediatric hospitalist, you’ll need at least 2 years more of training. Is that going to deter qualified individuals?” Dr. Chang said. “The people this decision will impact the most are med-peds doctors – who complete a combined internal medicine and pediatrics residency – and part-timers. They may find themselves in a difficult position if the number of hours don’t add up for them to sit for the boards. For the most part, we’ll have to wait and see for answers to these questions.”
Brian Alverson, MD, FAAP, current chair of the AAP’s Section on Hospital Medicine and associate professor of pediatrics at Brown University, Providence, R.I., says he can see both sides of the debate.
But at the same time, there is a significant opportunity cost for doing 2 more years of fellowship training, Dr. Alverson said.
“We don’t know how much the board certification test will improve actual care,” he noted. “Does it truly identify higher quality doctors, or just doctors who are good at taking multiple choice exams? There are a number of people in pediatrics who do a lot of different things in their jobs, and it’s important that they not lose their ability to practice in the field. Two-thirds of our work force is in community hospitals, not academic medical centers. They work hard to provide the backbone of hospital care for young patients, and many of them are unlikely to ever do a fellowship.”
Nonetheless, Dr. Alverson believes pediatric hospitalists needn’t worry. “You still have plenty of time to figure out what’s going to happen in your hospital,” he said.
References
1. Section on Hospital Medicine. Guiding principles for pediatric hospital medicine program. Pediatrics; 2013; 132:782-786.
2. Stucky E. The Pediatric Hospital Medicine Core Competencies. Wiley-Blackwell; 2010.
3. Feldman LS, Monash B, Eniasivam A. Why required pediatric hospital medicine fellowships are unnecessary. The Hospitalist Magazine, October 8, 2016.
IMiD/Anti-CD20 combo induces complete responses in r/r NHL
Lugano, Switzerland – A combination of obinutuzumab (Gazyva) and the experimental immunomodulatory agent CC-122 showed “clinically meaningful” activity against relapsed/refractory diffuse large B cell lymphoma (DLBCL) and indolent non-Hodgkin lymphoma (NHL) in a phase 1b study.
Among 38 patients with heavily pretreated, relapsed/refractory DLBCL, follicular lymphoma (FL), or marginal zone lymphoma (MZL), the overall response rate was 66%, including 12 patients (32%) with a complete response (CR), reported Jean-Marie Michot, MD, from the Goustave-Roussy Cancer Center in Villejuif, France.
CC-122 is a thalidomide analog that shares a molecular target with its cousin lenalidomide (Revlimid). Both molecules bind to the protein cereblon to cause degradation of the lymphoid transcription factors Aiolos and Ikaros.
As a single agent, CC-122 has been shown to have immunomodulatory effects on T-cell and natural killer (NK)–cell functions and has shown clinical activity in heavily pretreated patients with relapsed refractory NHL, including various cell-of-origin–based DLBCL subtypes, Dr, Michot said.
In preclinical studies, the combination of CC-122 and obinutuzumab, an anti-CD20 monoclonal antibody, has shown synergistic effects against FL and greater antilymphoma effects against DLBCL than either agent alone, he added.
In a multicenter, open-label, phase 1b dose-escalation and expansion study, investigators enrolled 19 patients with FL or MZL for whom at least one prior regimen had failed and 19 patients with relapsed/refractory DLBCL following at least two prior regimens and failed autologous stem cell transplant.
The patients received oral CC-122 at different dose levels for 5 of 7 days in each 28 day treatment cycle, plus intravenous obinutuzumab 1000 mg on days 2, 8, and 15 of cycle 1 and day 1 of cycles 2 through 8.
Responses were assessed according to International Working Group 2007 revised response criteria for malignant lymphoma.
Among all 38 patients, 25 (66%) had a response. Responses consisted of 12 CR (3 in patients with DLBCL, and 9 in patients with FL/MZL) and 13 partial responses (six and seven patients, respectively),
The median time to best response was 57 days. Responses were seen in 23 of the 30 patients who received CC-122 at dose level of 3 mg or higher.
“To date, patients receiving CC-122 at a dose of 3 mg and higher have the best and more durable responses to CC-122 plus obinutuzumab,” Dr. Michot said.
Patients generally tolerated the combination well. The most common grade 3 or 4 adverse events were hematologic and included grade 4 febrile neutropenia in two patients. Two patients discontinued treatment because of adverse events.
There was a dose-limiting toxicity, grade 4 neutropenia in one patient who received CC-122 at the 3 mg dose level, and one death from a tumor flare reaction in a patient treated at the 4 mg dose level.
The dose-escalation arm of the study has completed, and investigators are enrolling patients in a dose expansion phase at the 3 mg level.
The study was sponsored by Celgene. Hoffman La-Roche contributed obinutuzumab for the study. Dr. Michot reported serving as an advisor to Bristol-Myers Squibb and receiving travel grants from BMS, Pfizer, and Roche. Seven coauthors are Celgene employees and stockholders.
Lugano, Switzerland – A combination of obinutuzumab (Gazyva) and the experimental immunomodulatory agent CC-122 showed “clinically meaningful” activity against relapsed/refractory diffuse large B cell lymphoma (DLBCL) and indolent non-Hodgkin lymphoma (NHL) in a phase 1b study.
Among 38 patients with heavily pretreated, relapsed/refractory DLBCL, follicular lymphoma (FL), or marginal zone lymphoma (MZL), the overall response rate was 66%, including 12 patients (32%) with a complete response (CR), reported Jean-Marie Michot, MD, from the Goustave-Roussy Cancer Center in Villejuif, France.
CC-122 is a thalidomide analog that shares a molecular target with its cousin lenalidomide (Revlimid). Both molecules bind to the protein cereblon to cause degradation of the lymphoid transcription factors Aiolos and Ikaros.
As a single agent, CC-122 has been shown to have immunomodulatory effects on T-cell and natural killer (NK)–cell functions and has shown clinical activity in heavily pretreated patients with relapsed refractory NHL, including various cell-of-origin–based DLBCL subtypes, Dr, Michot said.
In preclinical studies, the combination of CC-122 and obinutuzumab, an anti-CD20 monoclonal antibody, has shown synergistic effects against FL and greater antilymphoma effects against DLBCL than either agent alone, he added.
In a multicenter, open-label, phase 1b dose-escalation and expansion study, investigators enrolled 19 patients with FL or MZL for whom at least one prior regimen had failed and 19 patients with relapsed/refractory DLBCL following at least two prior regimens and failed autologous stem cell transplant.
The patients received oral CC-122 at different dose levels for 5 of 7 days in each 28 day treatment cycle, plus intravenous obinutuzumab 1000 mg on days 2, 8, and 15 of cycle 1 and day 1 of cycles 2 through 8.
Responses were assessed according to International Working Group 2007 revised response criteria for malignant lymphoma.
Among all 38 patients, 25 (66%) had a response. Responses consisted of 12 CR (3 in patients with DLBCL, and 9 in patients with FL/MZL) and 13 partial responses (six and seven patients, respectively),
The median time to best response was 57 days. Responses were seen in 23 of the 30 patients who received CC-122 at dose level of 3 mg or higher.
“To date, patients receiving CC-122 at a dose of 3 mg and higher have the best and more durable responses to CC-122 plus obinutuzumab,” Dr. Michot said.
Patients generally tolerated the combination well. The most common grade 3 or 4 adverse events were hematologic and included grade 4 febrile neutropenia in two patients. Two patients discontinued treatment because of adverse events.
There was a dose-limiting toxicity, grade 4 neutropenia in one patient who received CC-122 at the 3 mg dose level, and one death from a tumor flare reaction in a patient treated at the 4 mg dose level.
The dose-escalation arm of the study has completed, and investigators are enrolling patients in a dose expansion phase at the 3 mg level.
The study was sponsored by Celgene. Hoffman La-Roche contributed obinutuzumab for the study. Dr. Michot reported serving as an advisor to Bristol-Myers Squibb and receiving travel grants from BMS, Pfizer, and Roche. Seven coauthors are Celgene employees and stockholders.
Lugano, Switzerland – A combination of obinutuzumab (Gazyva) and the experimental immunomodulatory agent CC-122 showed “clinically meaningful” activity against relapsed/refractory diffuse large B cell lymphoma (DLBCL) and indolent non-Hodgkin lymphoma (NHL) in a phase 1b study.
Among 38 patients with heavily pretreated, relapsed/refractory DLBCL, follicular lymphoma (FL), or marginal zone lymphoma (MZL), the overall response rate was 66%, including 12 patients (32%) with a complete response (CR), reported Jean-Marie Michot, MD, from the Goustave-Roussy Cancer Center in Villejuif, France.
CC-122 is a thalidomide analog that shares a molecular target with its cousin lenalidomide (Revlimid). Both molecules bind to the protein cereblon to cause degradation of the lymphoid transcription factors Aiolos and Ikaros.
As a single agent, CC-122 has been shown to have immunomodulatory effects on T-cell and natural killer (NK)–cell functions and has shown clinical activity in heavily pretreated patients with relapsed refractory NHL, including various cell-of-origin–based DLBCL subtypes, Dr, Michot said.
In preclinical studies, the combination of CC-122 and obinutuzumab, an anti-CD20 monoclonal antibody, has shown synergistic effects against FL and greater antilymphoma effects against DLBCL than either agent alone, he added.
In a multicenter, open-label, phase 1b dose-escalation and expansion study, investigators enrolled 19 patients with FL or MZL for whom at least one prior regimen had failed and 19 patients with relapsed/refractory DLBCL following at least two prior regimens and failed autologous stem cell transplant.
The patients received oral CC-122 at different dose levels for 5 of 7 days in each 28 day treatment cycle, plus intravenous obinutuzumab 1000 mg on days 2, 8, and 15 of cycle 1 and day 1 of cycles 2 through 8.
Responses were assessed according to International Working Group 2007 revised response criteria for malignant lymphoma.
Among all 38 patients, 25 (66%) had a response. Responses consisted of 12 CR (3 in patients with DLBCL, and 9 in patients with FL/MZL) and 13 partial responses (six and seven patients, respectively),
The median time to best response was 57 days. Responses were seen in 23 of the 30 patients who received CC-122 at dose level of 3 mg or higher.
“To date, patients receiving CC-122 at a dose of 3 mg and higher have the best and more durable responses to CC-122 plus obinutuzumab,” Dr. Michot said.
Patients generally tolerated the combination well. The most common grade 3 or 4 adverse events were hematologic and included grade 4 febrile neutropenia in two patients. Two patients discontinued treatment because of adverse events.
There was a dose-limiting toxicity, grade 4 neutropenia in one patient who received CC-122 at the 3 mg dose level, and one death from a tumor flare reaction in a patient treated at the 4 mg dose level.
The dose-escalation arm of the study has completed, and investigators are enrolling patients in a dose expansion phase at the 3 mg level.
The study was sponsored by Celgene. Hoffman La-Roche contributed obinutuzumab for the study. Dr. Michot reported serving as an advisor to Bristol-Myers Squibb and receiving travel grants from BMS, Pfizer, and Roche. Seven coauthors are Celgene employees and stockholders.
AT 14-ICML
Key clinical point: A combination of the experimental immunomodulator CC-122 and obinutuzumab showed significant activity against relapsed/refractory non-Hodgkin lymphoma.
Major finding: The overall response rate was 66%, including 32% complete responses.
Data source: A multicenter open-label phase 1b dose-escalation study in 19 patients with DLBCL and 19 with follicular lymphoma or marginal zone lymphoma.
Disclosures: The study was sponsored by Celgene. Hoffman La-Roche contributed obinutuzumab for the study. Dr. Michot reported serving as an advisor to Bristol-Myers Squibb and receiving travel grants from BMS, Pfizer, and Roche. Seven coauthors are Celgene employees and stockholders.