User login
HMGB1 might be new biomarker of celiac disease in children
, said Sara Manti, MD, of the unit of pediatric genetics and immunology at the University of Messina, Italy, and her associates.
Serum HMGB1 levels were significantly higher in 49 children with celiac disease, compared with 44 healthy children in the control group (16.4 ng/mL vs. 6.23 ng/mL; P less than .001). Children with typical form celiac disease had significantly higher serum HMGB1 levels (22.03 ng/mL) than both children with atypical form (14.83 ng/mL) and silent form celiac disease (12.3 ng/mL). There was no statistically significant difference in serum HMGB1 levels between children with atypical form and silent form celiac disease.
Higher serum HMGB1 levels were correlated with severity of Marsh-Oberhüber classification.
These data, which need to be confirmed in further studies, suggest that HMGB1 is upregulated and linked to the severity of histologic damage in celiac disease. If other studies confirm these findings, it could be hypothesized that “asymptomatic children only with positive familial history and abnormal serum anti–tTG-IgA levels as well as normal serum HMGB1 levels need not be subjected to endoscopy to rule out the CD diagnosis,” the investigators said.
Perhaps, “neutralizing HMGB1 activity might be identified as a potential therapeutic target,” Dr. Manti and her associates noted.
Read more in the journal Nutrition (2017 May;37:18-21).
, said Sara Manti, MD, of the unit of pediatric genetics and immunology at the University of Messina, Italy, and her associates.
Serum HMGB1 levels were significantly higher in 49 children with celiac disease, compared with 44 healthy children in the control group (16.4 ng/mL vs. 6.23 ng/mL; P less than .001). Children with typical form celiac disease had significantly higher serum HMGB1 levels (22.03 ng/mL) than both children with atypical form (14.83 ng/mL) and silent form celiac disease (12.3 ng/mL). There was no statistically significant difference in serum HMGB1 levels between children with atypical form and silent form celiac disease.
Higher serum HMGB1 levels were correlated with severity of Marsh-Oberhüber classification.
These data, which need to be confirmed in further studies, suggest that HMGB1 is upregulated and linked to the severity of histologic damage in celiac disease. If other studies confirm these findings, it could be hypothesized that “asymptomatic children only with positive familial history and abnormal serum anti–tTG-IgA levels as well as normal serum HMGB1 levels need not be subjected to endoscopy to rule out the CD diagnosis,” the investigators said.
Perhaps, “neutralizing HMGB1 activity might be identified as a potential therapeutic target,” Dr. Manti and her associates noted.
Read more in the journal Nutrition (2017 May;37:18-21).
, said Sara Manti, MD, of the unit of pediatric genetics and immunology at the University of Messina, Italy, and her associates.
Serum HMGB1 levels were significantly higher in 49 children with celiac disease, compared with 44 healthy children in the control group (16.4 ng/mL vs. 6.23 ng/mL; P less than .001). Children with typical form celiac disease had significantly higher serum HMGB1 levels (22.03 ng/mL) than both children with atypical form (14.83 ng/mL) and silent form celiac disease (12.3 ng/mL). There was no statistically significant difference in serum HMGB1 levels between children with atypical form and silent form celiac disease.
Higher serum HMGB1 levels were correlated with severity of Marsh-Oberhüber classification.
These data, which need to be confirmed in further studies, suggest that HMGB1 is upregulated and linked to the severity of histologic damage in celiac disease. If other studies confirm these findings, it could be hypothesized that “asymptomatic children only with positive familial history and abnormal serum anti–tTG-IgA levels as well as normal serum HMGB1 levels need not be subjected to endoscopy to rule out the CD diagnosis,” the investigators said.
Perhaps, “neutralizing HMGB1 activity might be identified as a potential therapeutic target,” Dr. Manti and her associates noted.
Read more in the journal Nutrition (2017 May;37:18-21).
FROM NUTRITION
New evidence bisphosphonates may prevent OA
LAS VEGAS – Bisphosphonates may slow the onset and progression of osteoarthritis (OA), according to data from the National Institutes of Health–sponsored Osteoarthritis Initiative. “Bisphosphonates warrant further study as potential disease-modifying agents in osteoarthritis,” Tuhina Neogi, MD, declared in presenting the study findings at the World Congress on Osteoarthritis.
In addition to the promising signal of a preventive effect for bisphosphonates, her analysis of Osteoarthritis Initiative data yielded two other major findings: Changes over time in the MRI-based three-dimensional bone shape of the knee constitute a novel structural imaging biomarker that appears to be of value in monitoring patients with OA or at high risk for the joint disease, and bisphosphonate-induced suppression of bone turnover had no adverse long-term impact on osteoarthritis risk.
“While bisphosphonates may have beneficial articular cartilage effects, there are potential theoretical concerns regarding long-term effects of bone turnover. This issue hasn’t previously been addressed. Bone turnover suppression may lead to more bone deposition and bone stiffness, with adverse biomechanical consequences. But it did not appear, in this sample at least, that suppression of bone turnover had a negative impact over the long term,” said Dr. Neogi, professor of medicine at Boston University.
The Osteoarthritis Initiative is a multicenter, longitudinal, prospective, observational study of knee osteoarthritis launched by the NIH in 2002. Dr. Neogi’s analysis was limited to the 1,071 female participants free of radiographic knee OA at baseline and who had 3-Tesla MRIs of the right knee at baseline and annually thereafter for 4 years. They were at increased risk for OA on the basis of their age – a mean of 62 years – along with their mean body mass index of 28.3 kg/m2. Just under one-quarter of the women were on bisphosphonate therapy.
Prior studies of the effects of bisphosphonates in patients with knee OA have yielded conflicting results, in part because radiographic findings are a relatively crude indicator of bone and joint changes. 3D bone shape of the knee has been shown to change much more quickly than traditional radiographic measures. These changes predict the incidence of knee OA even years later, the rheumatologist explained at the congress sponsored by the Osteoarthritis Research Society International.
Using bone shape analytic software developed by England-based Imorphics, she and her coinvestigators categorized the women into three distinct groups on the basis of the trajectory of MRI changes toward a more osteoarthritic bone shape over time. Of them, 23% fell into the fast-change group, 49% were in the intermediate group, and 28% were in the slowest-changing group. The rate of MRI bone shape progression toward knee OA was 2.7-times higher in the fastest, compared with the slowest, group.
The incidence of radiographic OA during 4 years of prospective follow-up was 14% in the fastest bone shape-changing group, 8% in the intermediate-speed group, and 4% in the slowest-changing group.
In a multivariate analysis adjusted for age, BMI, education, race, quadriceps strength, and history of knee injury, bisphosphonate users were 41% less likely to be in the fastest bone shape–changing group and 32% less likely to be in the intermediate group, compared with the slowest-changing group.
As a rheumatologist with a PhD in epidemiology, Dr. Neogi was readily prepared to critique her own study. The major limitation in her view was the potential for residual confounding, which is inherent in observational studies. In this instance, the possibility of confounding by indication cannot be excluded. Also, bone mineral density data wasn’t collected in the Osteoarthritis Initiative. Future studies should evaluate the impact over time of new-onset bisphosphonate therapy as a means of altering the slope of the trajectory of MRI-based 3D bone shape of the knee, Dr. Neogi said.
She reported having no financial conflicts regarding the NIH-sponsored study.
LAS VEGAS – Bisphosphonates may slow the onset and progression of osteoarthritis (OA), according to data from the National Institutes of Health–sponsored Osteoarthritis Initiative. “Bisphosphonates warrant further study as potential disease-modifying agents in osteoarthritis,” Tuhina Neogi, MD, declared in presenting the study findings at the World Congress on Osteoarthritis.
In addition to the promising signal of a preventive effect for bisphosphonates, her analysis of Osteoarthritis Initiative data yielded two other major findings: Changes over time in the MRI-based three-dimensional bone shape of the knee constitute a novel structural imaging biomarker that appears to be of value in monitoring patients with OA or at high risk for the joint disease, and bisphosphonate-induced suppression of bone turnover had no adverse long-term impact on osteoarthritis risk.
“While bisphosphonates may have beneficial articular cartilage effects, there are potential theoretical concerns regarding long-term effects of bone turnover. This issue hasn’t previously been addressed. Bone turnover suppression may lead to more bone deposition and bone stiffness, with adverse biomechanical consequences. But it did not appear, in this sample at least, that suppression of bone turnover had a negative impact over the long term,” said Dr. Neogi, professor of medicine at Boston University.
The Osteoarthritis Initiative is a multicenter, longitudinal, prospective, observational study of knee osteoarthritis launched by the NIH in 2002. Dr. Neogi’s analysis was limited to the 1,071 female participants free of radiographic knee OA at baseline and who had 3-Tesla MRIs of the right knee at baseline and annually thereafter for 4 years. They were at increased risk for OA on the basis of their age – a mean of 62 years – along with their mean body mass index of 28.3 kg/m2. Just under one-quarter of the women were on bisphosphonate therapy.
Prior studies of the effects of bisphosphonates in patients with knee OA have yielded conflicting results, in part because radiographic findings are a relatively crude indicator of bone and joint changes. 3D bone shape of the knee has been shown to change much more quickly than traditional radiographic measures. These changes predict the incidence of knee OA even years later, the rheumatologist explained at the congress sponsored by the Osteoarthritis Research Society International.
Using bone shape analytic software developed by England-based Imorphics, she and her coinvestigators categorized the women into three distinct groups on the basis of the trajectory of MRI changes toward a more osteoarthritic bone shape over time. Of them, 23% fell into the fast-change group, 49% were in the intermediate group, and 28% were in the slowest-changing group. The rate of MRI bone shape progression toward knee OA was 2.7-times higher in the fastest, compared with the slowest, group.
The incidence of radiographic OA during 4 years of prospective follow-up was 14% in the fastest bone shape-changing group, 8% in the intermediate-speed group, and 4% in the slowest-changing group.
In a multivariate analysis adjusted for age, BMI, education, race, quadriceps strength, and history of knee injury, bisphosphonate users were 41% less likely to be in the fastest bone shape–changing group and 32% less likely to be in the intermediate group, compared with the slowest-changing group.
As a rheumatologist with a PhD in epidemiology, Dr. Neogi was readily prepared to critique her own study. The major limitation in her view was the potential for residual confounding, which is inherent in observational studies. In this instance, the possibility of confounding by indication cannot be excluded. Also, bone mineral density data wasn’t collected in the Osteoarthritis Initiative. Future studies should evaluate the impact over time of new-onset bisphosphonate therapy as a means of altering the slope of the trajectory of MRI-based 3D bone shape of the knee, Dr. Neogi said.
She reported having no financial conflicts regarding the NIH-sponsored study.
LAS VEGAS – Bisphosphonates may slow the onset and progression of osteoarthritis (OA), according to data from the National Institutes of Health–sponsored Osteoarthritis Initiative. “Bisphosphonates warrant further study as potential disease-modifying agents in osteoarthritis,” Tuhina Neogi, MD, declared in presenting the study findings at the World Congress on Osteoarthritis.
In addition to the promising signal of a preventive effect for bisphosphonates, her analysis of Osteoarthritis Initiative data yielded two other major findings: Changes over time in the MRI-based three-dimensional bone shape of the knee constitute a novel structural imaging biomarker that appears to be of value in monitoring patients with OA or at high risk for the joint disease, and bisphosphonate-induced suppression of bone turnover had no adverse long-term impact on osteoarthritis risk.
“While bisphosphonates may have beneficial articular cartilage effects, there are potential theoretical concerns regarding long-term effects of bone turnover. This issue hasn’t previously been addressed. Bone turnover suppression may lead to more bone deposition and bone stiffness, with adverse biomechanical consequences. But it did not appear, in this sample at least, that suppression of bone turnover had a negative impact over the long term,” said Dr. Neogi, professor of medicine at Boston University.
The Osteoarthritis Initiative is a multicenter, longitudinal, prospective, observational study of knee osteoarthritis launched by the NIH in 2002. Dr. Neogi’s analysis was limited to the 1,071 female participants free of radiographic knee OA at baseline and who had 3-Tesla MRIs of the right knee at baseline and annually thereafter for 4 years. They were at increased risk for OA on the basis of their age – a mean of 62 years – along with their mean body mass index of 28.3 kg/m2. Just under one-quarter of the women were on bisphosphonate therapy.
Prior studies of the effects of bisphosphonates in patients with knee OA have yielded conflicting results, in part because radiographic findings are a relatively crude indicator of bone and joint changes. 3D bone shape of the knee has been shown to change much more quickly than traditional radiographic measures. These changes predict the incidence of knee OA even years later, the rheumatologist explained at the congress sponsored by the Osteoarthritis Research Society International.
Using bone shape analytic software developed by England-based Imorphics, she and her coinvestigators categorized the women into three distinct groups on the basis of the trajectory of MRI changes toward a more osteoarthritic bone shape over time. Of them, 23% fell into the fast-change group, 49% were in the intermediate group, and 28% were in the slowest-changing group. The rate of MRI bone shape progression toward knee OA was 2.7-times higher in the fastest, compared with the slowest, group.
The incidence of radiographic OA during 4 years of prospective follow-up was 14% in the fastest bone shape-changing group, 8% in the intermediate-speed group, and 4% in the slowest-changing group.
In a multivariate analysis adjusted for age, BMI, education, race, quadriceps strength, and history of knee injury, bisphosphonate users were 41% less likely to be in the fastest bone shape–changing group and 32% less likely to be in the intermediate group, compared with the slowest-changing group.
As a rheumatologist with a PhD in epidemiology, Dr. Neogi was readily prepared to critique her own study. The major limitation in her view was the potential for residual confounding, which is inherent in observational studies. In this instance, the possibility of confounding by indication cannot be excluded. Also, bone mineral density data wasn’t collected in the Osteoarthritis Initiative. Future studies should evaluate the impact over time of new-onset bisphosphonate therapy as a means of altering the slope of the trajectory of MRI-based 3D bone shape of the knee, Dr. Neogi said.
She reported having no financial conflicts regarding the NIH-sponsored study.
AT OARSI 2017
Key clinical point:
Major finding: Bisphosphonate users were 41% less likely to be in the group with the fastest trajectory of changes in bone shape known to be highly predictive of knee osteoarthritis.
Data source: This analysis of MRI-based changes in 3D bone shape of the knee over 4 years of follow-up included 1,071 female participants in the multicenter, prospective, observational Osteoarthritis Initiative.
Disclosures: The presenter reported having no financial conflicts regarding the NIH-sponsored study.
Medicaid paperwork adds barrier to postpartum sterilization
SAN DIEGO – Just over half of women who requested immediate postpartum sterilization received it in a prospective study of 334 women, with Medicaid paperwork serving as a barrier for many of the unfulfilled requests.
All of the women in the study delivered a baby and had requested immediate postpartum sterilization at some point before delivery, but just 173 women (52%) received the procedure, Taylor Hahn, MD, reported at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. A total of 161 women (48%) did not receive the procedure.
Dr. Hahn, a fourth-year resident at Indiana University, Indianapolis, and her colleagues followed these women for up to 3 months post partum and found that, within the group that didn’t receive immediate postpartum sterilization, just six women – less than 10% – had received sterilization by the end of the follow-up period. The remaining women had chosen an alternative contraceptive method, were still awaiting interval sterilization, or did not receive postpartum care.
This is concerning, Dr. Hahn said, because Medicaid coverage for sterilization typically expires after 60 days post partum.
The consent form was developed in the 1970s to protect women in vulnerable populations from being coerced into sterilization, but Dr. Hahn said that, today, “it really has created such a barrier to these women getting the care that they want and desire.” She contrasted the Medicaid procedure with what is typical in private insurance, which generally covers immediate postpartum sterilization, and the decision can be made the same day.
The researchers reported having no relevant financial disclosures.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
SAN DIEGO – Just over half of women who requested immediate postpartum sterilization received it in a prospective study of 334 women, with Medicaid paperwork serving as a barrier for many of the unfulfilled requests.
All of the women in the study delivered a baby and had requested immediate postpartum sterilization at some point before delivery, but just 173 women (52%) received the procedure, Taylor Hahn, MD, reported at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. A total of 161 women (48%) did not receive the procedure.
Dr. Hahn, a fourth-year resident at Indiana University, Indianapolis, and her colleagues followed these women for up to 3 months post partum and found that, within the group that didn’t receive immediate postpartum sterilization, just six women – less than 10% – had received sterilization by the end of the follow-up period. The remaining women had chosen an alternative contraceptive method, were still awaiting interval sterilization, or did not receive postpartum care.
This is concerning, Dr. Hahn said, because Medicaid coverage for sterilization typically expires after 60 days post partum.
The consent form was developed in the 1970s to protect women in vulnerable populations from being coerced into sterilization, but Dr. Hahn said that, today, “it really has created such a barrier to these women getting the care that they want and desire.” She contrasted the Medicaid procedure with what is typical in private insurance, which generally covers immediate postpartum sterilization, and the decision can be made the same day.
The researchers reported having no relevant financial disclosures.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
SAN DIEGO – Just over half of women who requested immediate postpartum sterilization received it in a prospective study of 334 women, with Medicaid paperwork serving as a barrier for many of the unfulfilled requests.
All of the women in the study delivered a baby and had requested immediate postpartum sterilization at some point before delivery, but just 173 women (52%) received the procedure, Taylor Hahn, MD, reported at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. A total of 161 women (48%) did not receive the procedure.
Dr. Hahn, a fourth-year resident at Indiana University, Indianapolis, and her colleagues followed these women for up to 3 months post partum and found that, within the group that didn’t receive immediate postpartum sterilization, just six women – less than 10% – had received sterilization by the end of the follow-up period. The remaining women had chosen an alternative contraceptive method, were still awaiting interval sterilization, or did not receive postpartum care.
This is concerning, Dr. Hahn said, because Medicaid coverage for sterilization typically expires after 60 days post partum.
The consent form was developed in the 1970s to protect women in vulnerable populations from being coerced into sterilization, but Dr. Hahn said that, today, “it really has created such a barrier to these women getting the care that they want and desire.” She contrasted the Medicaid procedure with what is typical in private insurance, which generally covers immediate postpartum sterilization, and the decision can be made the same day.
The researchers reported having no relevant financial disclosures.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
Key clinical point:
Major finding: A total of 48% of women who requested immediate postpartum sterilization did not receive it.
Data source: A prospective study of 334 women who delivered and requested immediate postpartum sterilization.
Disclosures: The researchers reported having no relevant financial disclosures.
How bariatric surgery improves knee osteoarthritis
LAS VEGAS – Most of the improvement in knee pain that occurs following bariatric surgery in obese patients with knee osteoarthritis happens in the first month after surgery, well before the bulk of the weight loss takes place, Jonathan Samuels, MD, reported at the World Congress on Osteoarthritis.
This observation suggests that bariatric surgery’s mechanism of benefit in patients with knee osteoarthritis (OA) isn’t simply a matter of reduced mechanical load on the joints caused by a lessened weight burden, Dr. Samuels observed at the World Congress on Osteoarthritis, sponsored by the Osteoarthritis Research Society International.
Indeed, his prospective study of 150 obese patients with comorbid knee OA points to metabolic factors as likely playing a key role.
His study, featuring 2 years of follow-up to date, showed that bariatric surgery improved knee OA proportionate to the percentage of excess weight loss achieved. The greatest reduction in knee pain as well as the most profound weight loss occurred in the 35 patients who underwent gastric bypass and the 97 who opted for sleeve gastrectomy; patients who underwent laparoscopic adjustable gastric banding had more modest outcomes on both scores.
The disparate timing of the reductions in excess weight and knee pain was particularly eye catching. With all three forms of bariatric surgery, weight loss continued steadily for roughly the first 12 months. It then plateaued and was generally maintained at the new body mass index for the second 12 months.
In contrast, improvement in knee pain according to the validated Knee Injury and Osteoarthritis Outcome Score (KOOS) leveled out after just 1 month post surgery and was then sustained through 23 months. Levels of the inflammatory cytokines and adipokines interleukin-6, interleukin-1 receptor antagonist, and lipopolysaccharides were elevated at baseline but dropped steadily in concert with the reduction in excess body weight during the first 12 months after surgery. In contrast, levels of the anti-inflammatory cytokine sRAGE (soluble receptor for advanced glycation end products) were abnormally low prior to surgery but increased sharply for the first 3 months afterward before leveling off. And levels of serum leptin, which were roughly sevenfold greater than in normal controls at baseline, fell precipitously during the first month after bariatric surgery before plateauing, following the same pattern as the improvement in knee pain.
“This suggests that perhaps leptin is the key mediator in this OA population,” said Dr. Samuels.
Obese patients with knee OA are in a catch-22 situation. Obese individuals are at greatly increased lifetime risk of developing knee OA, and patients with chronic knee pain have a tough time losing weight.
“The treatments that might work with either obesity or knee pain alone often fail when both of these are present,” he observed.
That’s why bariatric surgery is becoming an increasingly popular treatment strategy in these patients. Sleeve gastrectomy, gastric bypass, and laparoscopic adjustable gastric banding are all Food and Drug Administration approved treatments for obesity in the presence of at least one qualifying comorbid condition, and knee OA qualifies.
Dr. Samuels reported having no financial conflicts regarding his study.
LAS VEGAS – Most of the improvement in knee pain that occurs following bariatric surgery in obese patients with knee osteoarthritis happens in the first month after surgery, well before the bulk of the weight loss takes place, Jonathan Samuels, MD, reported at the World Congress on Osteoarthritis.
This observation suggests that bariatric surgery’s mechanism of benefit in patients with knee osteoarthritis (OA) isn’t simply a matter of reduced mechanical load on the joints caused by a lessened weight burden, Dr. Samuels observed at the World Congress on Osteoarthritis, sponsored by the Osteoarthritis Research Society International.
Indeed, his prospective study of 150 obese patients with comorbid knee OA points to metabolic factors as likely playing a key role.
His study, featuring 2 years of follow-up to date, showed that bariatric surgery improved knee OA proportionate to the percentage of excess weight loss achieved. The greatest reduction in knee pain as well as the most profound weight loss occurred in the 35 patients who underwent gastric bypass and the 97 who opted for sleeve gastrectomy; patients who underwent laparoscopic adjustable gastric banding had more modest outcomes on both scores.
The disparate timing of the reductions in excess weight and knee pain was particularly eye catching. With all three forms of bariatric surgery, weight loss continued steadily for roughly the first 12 months. It then plateaued and was generally maintained at the new body mass index for the second 12 months.
In contrast, improvement in knee pain according to the validated Knee Injury and Osteoarthritis Outcome Score (KOOS) leveled out after just 1 month post surgery and was then sustained through 23 months. Levels of the inflammatory cytokines and adipokines interleukin-6, interleukin-1 receptor antagonist, and lipopolysaccharides were elevated at baseline but dropped steadily in concert with the reduction in excess body weight during the first 12 months after surgery. In contrast, levels of the anti-inflammatory cytokine sRAGE (soluble receptor for advanced glycation end products) were abnormally low prior to surgery but increased sharply for the first 3 months afterward before leveling off. And levels of serum leptin, which were roughly sevenfold greater than in normal controls at baseline, fell precipitously during the first month after bariatric surgery before plateauing, following the same pattern as the improvement in knee pain.
“This suggests that perhaps leptin is the key mediator in this OA population,” said Dr. Samuels.
Obese patients with knee OA are in a catch-22 situation. Obese individuals are at greatly increased lifetime risk of developing knee OA, and patients with chronic knee pain have a tough time losing weight.
“The treatments that might work with either obesity or knee pain alone often fail when both of these are present,” he observed.
That’s why bariatric surgery is becoming an increasingly popular treatment strategy in these patients. Sleeve gastrectomy, gastric bypass, and laparoscopic adjustable gastric banding are all Food and Drug Administration approved treatments for obesity in the presence of at least one qualifying comorbid condition, and knee OA qualifies.
Dr. Samuels reported having no financial conflicts regarding his study.
LAS VEGAS – Most of the improvement in knee pain that occurs following bariatric surgery in obese patients with knee osteoarthritis happens in the first month after surgery, well before the bulk of the weight loss takes place, Jonathan Samuels, MD, reported at the World Congress on Osteoarthritis.
This observation suggests that bariatric surgery’s mechanism of benefit in patients with knee osteoarthritis (OA) isn’t simply a matter of reduced mechanical load on the joints caused by a lessened weight burden, Dr. Samuels observed at the World Congress on Osteoarthritis, sponsored by the Osteoarthritis Research Society International.
Indeed, his prospective study of 150 obese patients with comorbid knee OA points to metabolic factors as likely playing a key role.
His study, featuring 2 years of follow-up to date, showed that bariatric surgery improved knee OA proportionate to the percentage of excess weight loss achieved. The greatest reduction in knee pain as well as the most profound weight loss occurred in the 35 patients who underwent gastric bypass and the 97 who opted for sleeve gastrectomy; patients who underwent laparoscopic adjustable gastric banding had more modest outcomes on both scores.
The disparate timing of the reductions in excess weight and knee pain was particularly eye catching. With all three forms of bariatric surgery, weight loss continued steadily for roughly the first 12 months. It then plateaued and was generally maintained at the new body mass index for the second 12 months.
In contrast, improvement in knee pain according to the validated Knee Injury and Osteoarthritis Outcome Score (KOOS) leveled out after just 1 month post surgery and was then sustained through 23 months. Levels of the inflammatory cytokines and adipokines interleukin-6, interleukin-1 receptor antagonist, and lipopolysaccharides were elevated at baseline but dropped steadily in concert with the reduction in excess body weight during the first 12 months after surgery. In contrast, levels of the anti-inflammatory cytokine sRAGE (soluble receptor for advanced glycation end products) were abnormally low prior to surgery but increased sharply for the first 3 months afterward before leveling off. And levels of serum leptin, which were roughly sevenfold greater than in normal controls at baseline, fell precipitously during the first month after bariatric surgery before plateauing, following the same pattern as the improvement in knee pain.
“This suggests that perhaps leptin is the key mediator in this OA population,” said Dr. Samuels.
Obese patients with knee OA are in a catch-22 situation. Obese individuals are at greatly increased lifetime risk of developing knee OA, and patients with chronic knee pain have a tough time losing weight.
“The treatments that might work with either obesity or knee pain alone often fail when both of these are present,” he observed.
That’s why bariatric surgery is becoming an increasingly popular treatment strategy in these patients. Sleeve gastrectomy, gastric bypass, and laparoscopic adjustable gastric banding are all Food and Drug Administration approved treatments for obesity in the presence of at least one qualifying comorbid condition, and knee OA qualifies.
Dr. Samuels reported having no financial conflicts regarding his study.
AT OARSI 2017
Key clinical point:
Major finding: Most of the improvement in knee pain – and most of the accompanying drop in serum leptin – happens in the first month following bariatric surgery, well before most weight loss has occurred.
Data source: A prospective observational study of 150 obese patients with knee osteoarthritis who underwent bariatric surgery.
Disclosures: The study presenter reported having no financial conflicts.
Product News: 05 2017
Dupixent
Sanofi and Regeneron Pharmaceuticals, Inc, announce US Food and Drug Administration approval of Dupixent (dupilumab) injection, a biologic
Renflexis
Samsung Bioepis Co, Ltd, announces US Food and Drug Administration approval of Renflexis (infliximab-abda) injection 100 mg, a biosimilar referencing infliximab. It is indicated in the United States for reducing signs and symptoms in patients with adult and pediatric Crohn disease, adult ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and adult plaque psoriasis. Renflexis will be marketed and distributed in the United States by Merck. For more information, visit www.samsungbioepis.com.
Replenix RetinolForte Treatment Serum
Topix Pharmaceuticals, Inc, introduces Replenix Retinol Forte Treatment Serum containing all- trans -retinol, which helps increase cell turnover to reduce the appearance of fine lines and wrinkles, im- prove skin texture and tone, and promote a collagen-rich appearance. The micropolymer delivery system stabilizes the retinol to protect and shield it from oxidation while on the skin. Its time-released delivery system creates a reservoir that continuously bathes the skin and minimizes irritation. It also contains green tea polyphenols to soothe and calm the skin, caffeine to diminish the appearance of redness, and hyaluronic acid to help skin retain moisture to replenish and repair skin barrier function. For more information, visit www.topixpharm.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at cutis@frontlinemedcom.com.
Dupixent
Sanofi and Regeneron Pharmaceuticals, Inc, announce US Food and Drug Administration approval of Dupixent (dupilumab) injection, a biologic
Renflexis
Samsung Bioepis Co, Ltd, announces US Food and Drug Administration approval of Renflexis (infliximab-abda) injection 100 mg, a biosimilar referencing infliximab. It is indicated in the United States for reducing signs and symptoms in patients with adult and pediatric Crohn disease, adult ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and adult plaque psoriasis. Renflexis will be marketed and distributed in the United States by Merck. For more information, visit www.samsungbioepis.com.
Replenix RetinolForte Treatment Serum
Topix Pharmaceuticals, Inc, introduces Replenix Retinol Forte Treatment Serum containing all- trans -retinol, which helps increase cell turnover to reduce the appearance of fine lines and wrinkles, im- prove skin texture and tone, and promote a collagen-rich appearance. The micropolymer delivery system stabilizes the retinol to protect and shield it from oxidation while on the skin. Its time-released delivery system creates a reservoir that continuously bathes the skin and minimizes irritation. It also contains green tea polyphenols to soothe and calm the skin, caffeine to diminish the appearance of redness, and hyaluronic acid to help skin retain moisture to replenish and repair skin barrier function. For more information, visit www.topixpharm.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at cutis@frontlinemedcom.com.
Dupixent
Sanofi and Regeneron Pharmaceuticals, Inc, announce US Food and Drug Administration approval of Dupixent (dupilumab) injection, a biologic
Renflexis
Samsung Bioepis Co, Ltd, announces US Food and Drug Administration approval of Renflexis (infliximab-abda) injection 100 mg, a biosimilar referencing infliximab. It is indicated in the United States for reducing signs and symptoms in patients with adult and pediatric Crohn disease, adult ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and adult plaque psoriasis. Renflexis will be marketed and distributed in the United States by Merck. For more information, visit www.samsungbioepis.com.
Replenix RetinolForte Treatment Serum
Topix Pharmaceuticals, Inc, introduces Replenix Retinol Forte Treatment Serum containing all- trans -retinol, which helps increase cell turnover to reduce the appearance of fine lines and wrinkles, im- prove skin texture and tone, and promote a collagen-rich appearance. The micropolymer delivery system stabilizes the retinol to protect and shield it from oxidation while on the skin. Its time-released delivery system creates a reservoir that continuously bathes the skin and minimizes irritation. It also contains green tea polyphenols to soothe and calm the skin, caffeine to diminish the appearance of redness, and hyaluronic acid to help skin retain moisture to replenish and repair skin barrier function. For more information, visit www.topixpharm.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at cutis@frontlinemedcom.com.
JAK inhibitors and alopecia: After positive early data, various trials now underway
PORTLAND, ORE. – Janus kinase inhibitors are relatively safe and can produce a full head of hair in patients with moderate to severe alopecia areata (AA), although patients tend to shed hair after stopping treatment, Julian Mackay-Wiggan, MD, said at the annual meeting of the Society for Investigative Dermatology.
“At this point, there are 17 publications in the literature, from clinical trials to case reports, looking at JAK [Janus kinase] inhibitors in patients with alopecia areata,” said Dr. Mackay-Wiggan of the department of dermatology, Columbia University, New York, where she specializes in hair disorders. “Pretty much all report very positive findings. It definitely appears that Janus kinase inhibitors can play a very significant role in treatment.”
In an open label, uncontrolled pilot study at Columbia, 9 of 12 (75%) patients with moderate to severe AA improved by at least 50% on the Severity of Alopecia Tool (SALT) after receiving 20 mg ruxolitinib twice daily for 3 to 6 months (JCI Insight. 2016 Sep 22;1[15]:e89790). Responses started with the first month, and all but one responder achieved at least 50% hair regrowth by week 12, said Dr. Mackay-Wiggan, who is also the director of the Dermatology Clinical Research Unit at Columbia.
By the end of treatment, seven of nine responders achieved more than 95% regrowth, one achieved 85% regrowth, and one achieved 55% regrowth. Importantly, none of these relatively healthy patients experienced serious adverse events on ruxolitinib, and none needed to stop treatment, although one patient experienced declining hemoglobin levels that resolved after dose modification.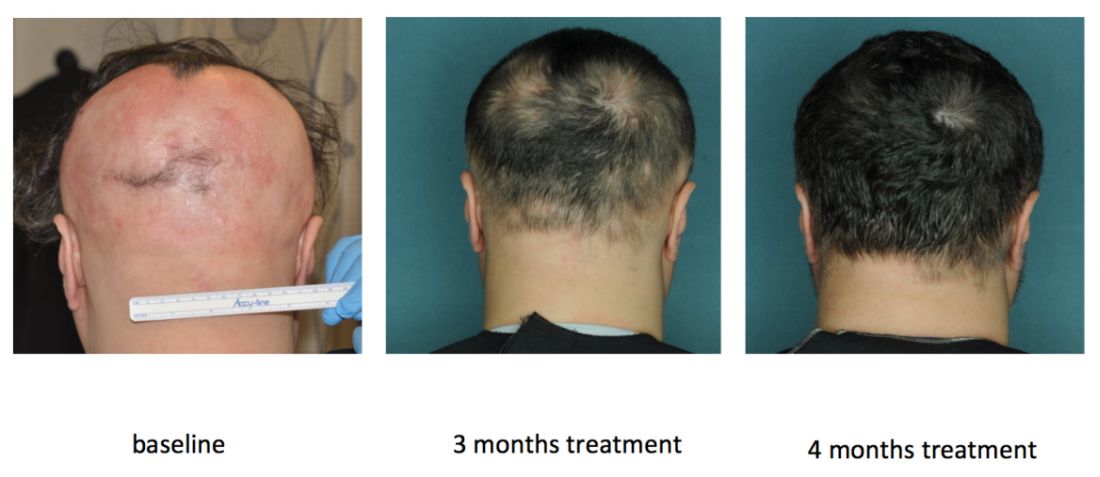
Columbia researchers are also conducting an uncontrolled, open label pilot trial of the JAK inhibitor tofacitinib (Xeljanz) in 12 patients, of whom seven have moderate to severe patchy AA and five have alopecia totalis or universalis. Tofacitinib is approved for treating rheumatoid arthritis at a dose of 5 mg twice daily, but patients have needed up to 10 mg twice daily to achieve hair regrowth, Dr. Mackay-Wiggan said. To date, 11 (92%) have achieved at least some hair regrowth, and 8 (67%) have achieved at least 50% regrowth. So far, there have been no serious adverse events over 6 to 16 months of treatment, although one patient stopped treatment after developing hypertension, a known adverse effect of tofacitinib.
In this study, heatmaps of RNA sequencing of CD8+ T cell populations clearly showed pathogenic signatures for AA and a “robust molecular response to treatment,” Dr. Mackay-Wiggan said. “These two signatures also overlapped statistically, producing 114 genes that may be targetable mediators of disease.” But as with ruxolitinib, regrowth started to decline as patients were taken off treatment.
Research indicates that inhibiting the JAK-STAT signaling pathway induces anagen and subsequent hair growth, but activating STAT 5 in the dermal papilla is also important to induce the growth phase of the hair follicle, according to Dr. Mackay-Wiggan. “Bottom line, it’s complicated,” she added. “The mode of delivery – topical versus systemic – may be important, and the timing of delivery may be crucial.”
Other studies point to a role for JAK inhibition in treating AA. In an uncontrolled, retrospective study of 90 adults with alopecia totalis, alopecia universalis, or moderate to severe AA, 58% had SALT scores of 50% or better after receiving 5 mg tofacitinib twice daily for 4 to 18 months. Patients with AA improved more than those with alopecia totalis or universalis. There were no severe adverse effects, although nearly a third of patients developed upper respiratory tract infections. In another uncontrolled study of 13 patients with AA, totalis, or universalis, 9 (70%) patients achieved full regrowth and there were no serious adverse effects, although patients experienced headaches, upper respiratory infections, and mild increases in liver transaminase levels.
JAK inhibition also has a potential role for treating some scarring alopecias, including lichen planopilaris and frontal fibrosing alopecia. These diseases are histologically “identical” and both exhibit perifollicular erythema, papules, and scale, all of which suggest active inflammation, Dr. Mackay-Wiggan said. Hair follicles from affected patients show immune markers such as interferon-inducible chemokines, cytotoxic T cell responses, and expression of major histocompatibility complexes I and II. “The important message here is that JAK/STAT signaling may play a significant role in other types of hair loss other than alopecia areata,” Dr. Mackay-Wiggan said. “These diseases may also be autoimmune diseases, and may also be treatable with JAK inhibitors.”
Studies continue to evaluate JAK inhibitors for treating alopecia and its variants. Investigators at Yale and Stanford are conducting three uncontrolled trials of oral or topical tofacitinib, while Incyte, the manufacturer of ruxolitinib, is sponsoring a multicenter, randomized, placebo-controlled trial of ruxolitinib phosphate cream for adults with AA, with topline results expected in May 2018. Concert Pharmaceuticals also is recruiting for a trial of a modified, investigational form of ruxolitinib called CTP-543 for treating moderate to severe AA. “Many more trials are in development,” Dr. Mackay-Wiggan noted.
The ruxolitinib pilot study was funded by the Locks of Love Foundation, the Alopecia Areata Initiative, NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases, and by an Irving Institute for Clinical and Translational Research/Columbia University Medical Center Clinical and Translational Science Award. The ongoing tofacitinib pilot study is sponsored by Dr. Mackay-Wiggan, Locks of Love, and Columbia University.
Dr. Mackay-Wiggan also acknowledged support from the Alopecia Areata Initiative – the Gates Foundation, the National Alopecia Areata Registry, and the National Alopecia Areata Foundation. She had no other relevant financial disclosures.
PORTLAND, ORE. – Janus kinase inhibitors are relatively safe and can produce a full head of hair in patients with moderate to severe alopecia areata (AA), although patients tend to shed hair after stopping treatment, Julian Mackay-Wiggan, MD, said at the annual meeting of the Society for Investigative Dermatology.
“At this point, there are 17 publications in the literature, from clinical trials to case reports, looking at JAK [Janus kinase] inhibitors in patients with alopecia areata,” said Dr. Mackay-Wiggan of the department of dermatology, Columbia University, New York, where she specializes in hair disorders. “Pretty much all report very positive findings. It definitely appears that Janus kinase inhibitors can play a very significant role in treatment.”
In an open label, uncontrolled pilot study at Columbia, 9 of 12 (75%) patients with moderate to severe AA improved by at least 50% on the Severity of Alopecia Tool (SALT) after receiving 20 mg ruxolitinib twice daily for 3 to 6 months (JCI Insight. 2016 Sep 22;1[15]:e89790). Responses started with the first month, and all but one responder achieved at least 50% hair regrowth by week 12, said Dr. Mackay-Wiggan, who is also the director of the Dermatology Clinical Research Unit at Columbia.
By the end of treatment, seven of nine responders achieved more than 95% regrowth, one achieved 85% regrowth, and one achieved 55% regrowth. Importantly, none of these relatively healthy patients experienced serious adverse events on ruxolitinib, and none needed to stop treatment, although one patient experienced declining hemoglobin levels that resolved after dose modification.
Columbia researchers are also conducting an uncontrolled, open label pilot trial of the JAK inhibitor tofacitinib (Xeljanz) in 12 patients, of whom seven have moderate to severe patchy AA and five have alopecia totalis or universalis. Tofacitinib is approved for treating rheumatoid arthritis at a dose of 5 mg twice daily, but patients have needed up to 10 mg twice daily to achieve hair regrowth, Dr. Mackay-Wiggan said. To date, 11 (92%) have achieved at least some hair regrowth, and 8 (67%) have achieved at least 50% regrowth. So far, there have been no serious adverse events over 6 to 16 months of treatment, although one patient stopped treatment after developing hypertension, a known adverse effect of tofacitinib.
In this study, heatmaps of RNA sequencing of CD8+ T cell populations clearly showed pathogenic signatures for AA and a “robust molecular response to treatment,” Dr. Mackay-Wiggan said. “These two signatures also overlapped statistically, producing 114 genes that may be targetable mediators of disease.” But as with ruxolitinib, regrowth started to decline as patients were taken off treatment.
Research indicates that inhibiting the JAK-STAT signaling pathway induces anagen and subsequent hair growth, but activating STAT 5 in the dermal papilla is also important to induce the growth phase of the hair follicle, according to Dr. Mackay-Wiggan. “Bottom line, it’s complicated,” she added. “The mode of delivery – topical versus systemic – may be important, and the timing of delivery may be crucial.”
Other studies point to a role for JAK inhibition in treating AA. In an uncontrolled, retrospective study of 90 adults with alopecia totalis, alopecia universalis, or moderate to severe AA, 58% had SALT scores of 50% or better after receiving 5 mg tofacitinib twice daily for 4 to 18 months. Patients with AA improved more than those with alopecia totalis or universalis. There were no severe adverse effects, although nearly a third of patients developed upper respiratory tract infections. In another uncontrolled study of 13 patients with AA, totalis, or universalis, 9 (70%) patients achieved full regrowth and there were no serious adverse effects, although patients experienced headaches, upper respiratory infections, and mild increases in liver transaminase levels.
JAK inhibition also has a potential role for treating some scarring alopecias, including lichen planopilaris and frontal fibrosing alopecia. These diseases are histologically “identical” and both exhibit perifollicular erythema, papules, and scale, all of which suggest active inflammation, Dr. Mackay-Wiggan said. Hair follicles from affected patients show immune markers such as interferon-inducible chemokines, cytotoxic T cell responses, and expression of major histocompatibility complexes I and II. “The important message here is that JAK/STAT signaling may play a significant role in other types of hair loss other than alopecia areata,” Dr. Mackay-Wiggan said. “These diseases may also be autoimmune diseases, and may also be treatable with JAK inhibitors.”
Studies continue to evaluate JAK inhibitors for treating alopecia and its variants. Investigators at Yale and Stanford are conducting three uncontrolled trials of oral or topical tofacitinib, while Incyte, the manufacturer of ruxolitinib, is sponsoring a multicenter, randomized, placebo-controlled trial of ruxolitinib phosphate cream for adults with AA, with topline results expected in May 2018. Concert Pharmaceuticals also is recruiting for a trial of a modified, investigational form of ruxolitinib called CTP-543 for treating moderate to severe AA. “Many more trials are in development,” Dr. Mackay-Wiggan noted.
The ruxolitinib pilot study was funded by the Locks of Love Foundation, the Alopecia Areata Initiative, NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases, and by an Irving Institute for Clinical and Translational Research/Columbia University Medical Center Clinical and Translational Science Award. The ongoing tofacitinib pilot study is sponsored by Dr. Mackay-Wiggan, Locks of Love, and Columbia University.
Dr. Mackay-Wiggan also acknowledged support from the Alopecia Areata Initiative – the Gates Foundation, the National Alopecia Areata Registry, and the National Alopecia Areata Foundation. She had no other relevant financial disclosures.
PORTLAND, ORE. – Janus kinase inhibitors are relatively safe and can produce a full head of hair in patients with moderate to severe alopecia areata (AA), although patients tend to shed hair after stopping treatment, Julian Mackay-Wiggan, MD, said at the annual meeting of the Society for Investigative Dermatology.
“At this point, there are 17 publications in the literature, from clinical trials to case reports, looking at JAK [Janus kinase] inhibitors in patients with alopecia areata,” said Dr. Mackay-Wiggan of the department of dermatology, Columbia University, New York, where she specializes in hair disorders. “Pretty much all report very positive findings. It definitely appears that Janus kinase inhibitors can play a very significant role in treatment.”
In an open label, uncontrolled pilot study at Columbia, 9 of 12 (75%) patients with moderate to severe AA improved by at least 50% on the Severity of Alopecia Tool (SALT) after receiving 20 mg ruxolitinib twice daily for 3 to 6 months (JCI Insight. 2016 Sep 22;1[15]:e89790). Responses started with the first month, and all but one responder achieved at least 50% hair regrowth by week 12, said Dr. Mackay-Wiggan, who is also the director of the Dermatology Clinical Research Unit at Columbia.
By the end of treatment, seven of nine responders achieved more than 95% regrowth, one achieved 85% regrowth, and one achieved 55% regrowth. Importantly, none of these relatively healthy patients experienced serious adverse events on ruxolitinib, and none needed to stop treatment, although one patient experienced declining hemoglobin levels that resolved after dose modification.
Columbia researchers are also conducting an uncontrolled, open label pilot trial of the JAK inhibitor tofacitinib (Xeljanz) in 12 patients, of whom seven have moderate to severe patchy AA and five have alopecia totalis or universalis. Tofacitinib is approved for treating rheumatoid arthritis at a dose of 5 mg twice daily, but patients have needed up to 10 mg twice daily to achieve hair regrowth, Dr. Mackay-Wiggan said. To date, 11 (92%) have achieved at least some hair regrowth, and 8 (67%) have achieved at least 50% regrowth. So far, there have been no serious adverse events over 6 to 16 months of treatment, although one patient stopped treatment after developing hypertension, a known adverse effect of tofacitinib.
In this study, heatmaps of RNA sequencing of CD8+ T cell populations clearly showed pathogenic signatures for AA and a “robust molecular response to treatment,” Dr. Mackay-Wiggan said. “These two signatures also overlapped statistically, producing 114 genes that may be targetable mediators of disease.” But as with ruxolitinib, regrowth started to decline as patients were taken off treatment.
Research indicates that inhibiting the JAK-STAT signaling pathway induces anagen and subsequent hair growth, but activating STAT 5 in the dermal papilla is also important to induce the growth phase of the hair follicle, according to Dr. Mackay-Wiggan. “Bottom line, it’s complicated,” she added. “The mode of delivery – topical versus systemic – may be important, and the timing of delivery may be crucial.”
Other studies point to a role for JAK inhibition in treating AA. In an uncontrolled, retrospective study of 90 adults with alopecia totalis, alopecia universalis, or moderate to severe AA, 58% had SALT scores of 50% or better after receiving 5 mg tofacitinib twice daily for 4 to 18 months. Patients with AA improved more than those with alopecia totalis or universalis. There were no severe adverse effects, although nearly a third of patients developed upper respiratory tract infections. In another uncontrolled study of 13 patients with AA, totalis, or universalis, 9 (70%) patients achieved full regrowth and there were no serious adverse effects, although patients experienced headaches, upper respiratory infections, and mild increases in liver transaminase levels.
JAK inhibition also has a potential role for treating some scarring alopecias, including lichen planopilaris and frontal fibrosing alopecia. These diseases are histologically “identical” and both exhibit perifollicular erythema, papules, and scale, all of which suggest active inflammation, Dr. Mackay-Wiggan said. Hair follicles from affected patients show immune markers such as interferon-inducible chemokines, cytotoxic T cell responses, and expression of major histocompatibility complexes I and II. “The important message here is that JAK/STAT signaling may play a significant role in other types of hair loss other than alopecia areata,” Dr. Mackay-Wiggan said. “These diseases may also be autoimmune diseases, and may also be treatable with JAK inhibitors.”
Studies continue to evaluate JAK inhibitors for treating alopecia and its variants. Investigators at Yale and Stanford are conducting three uncontrolled trials of oral or topical tofacitinib, while Incyte, the manufacturer of ruxolitinib, is sponsoring a multicenter, randomized, placebo-controlled trial of ruxolitinib phosphate cream for adults with AA, with topline results expected in May 2018. Concert Pharmaceuticals also is recruiting for a trial of a modified, investigational form of ruxolitinib called CTP-543 for treating moderate to severe AA. “Many more trials are in development,” Dr. Mackay-Wiggan noted.
The ruxolitinib pilot study was funded by the Locks of Love Foundation, the Alopecia Areata Initiative, NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases, and by an Irving Institute for Clinical and Translational Research/Columbia University Medical Center Clinical and Translational Science Award. The ongoing tofacitinib pilot study is sponsored by Dr. Mackay-Wiggan, Locks of Love, and Columbia University.
Dr. Mackay-Wiggan also acknowledged support from the Alopecia Areata Initiative – the Gates Foundation, the National Alopecia Areata Registry, and the National Alopecia Areata Foundation. She had no other relevant financial disclosures.
AT SID 2017
Transverse Melanonychia and Palmar Hyperpigmentation Secondary to Hydroxyurea Therapy
To the Editor:
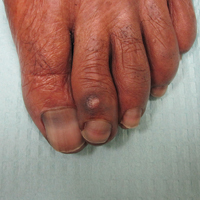
An 85-year-old woman with a history of hypertension, hyperlipidemia, stroke, hypothyroidism, chronic obstructive pulmonary disease, and chronic myeloproliferative disorder presented to our clinic for evaluation of brown lesions on the hands and discoloration of the fingernails and toenails of 4 months’ duration. Six months prior to visiting our clinic she was admitted to the hospital for a pulmonary embolism. On admission she was noted to have a platelet count of more than 2 million/μL (reference range, 150,000–350,000/μL). She received urgent plasmapheresis and started hydroxyurea 500 mg twice daily, which she continued as an outpatient.
On physical examination at our clinic she had diffusely scattered red and brown macules on the bilateral palms and transverse hyperpigmented bands of various intensities on all fingernails and toenails (Figure). Her platelet count was 372,000/μL, white blood cell count was 5200/μL (reference range, 4500–11,000/μL), hemoglobin was 12.6 g/dL (reference range, 14.0–17.5 g/dL), hematocrit was 39.0% (reference range, 41%–50%), and mean corpuscular volume was 87.5 fL per red cell (reference range, 80–96 fL per red cell).
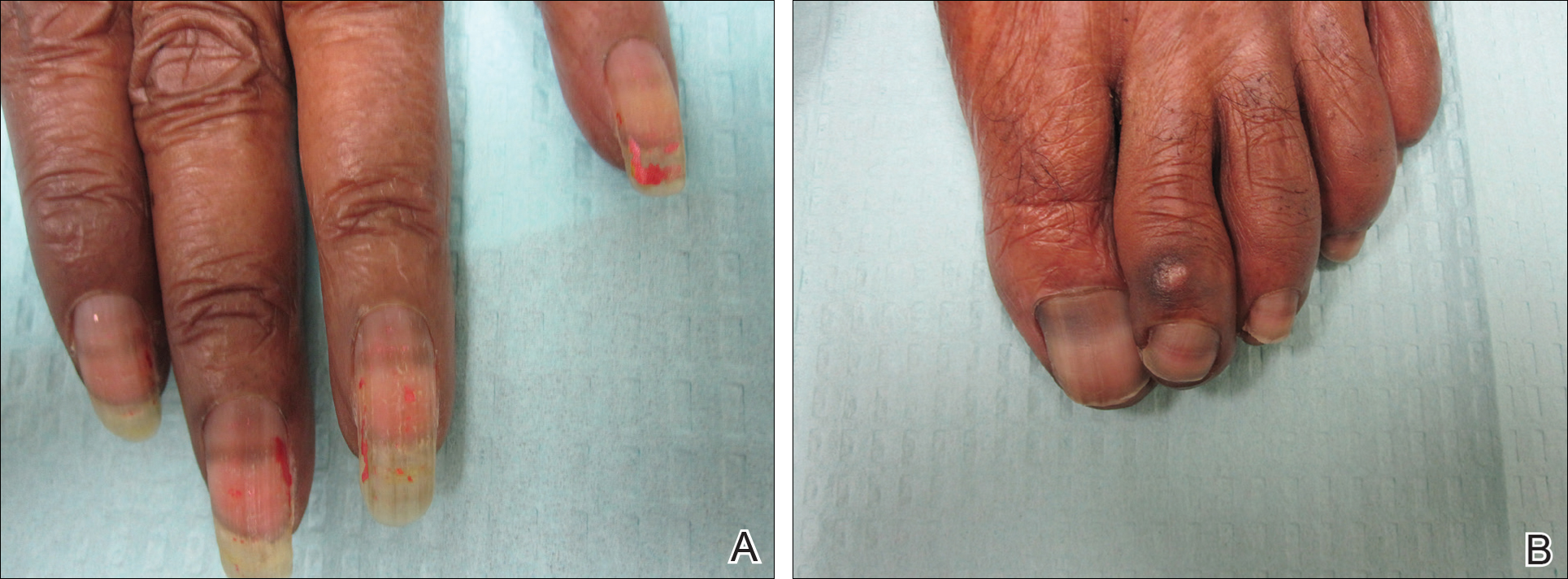
The patient was diagnosed with hydroxyurea-induced nail hyperpigmentation and was counseled on the benign nature of the condition. Three months later her platelet count decreased to below 100,000/μL, and hydroxyurea was discontinued. She noticed considerable improvement in the lesions on the hands and nails with the cessation of hydroxyurea.
Hydroxyurea is a cytostatic agent that has been used for more than 40 years in the treatment of myeloproliferative disorders including chronic myelogenous leukemia, polycythemia vera, essential thrombocythemia, and sickle cell anemia.1 It inhibits ribonucleoside diphosphate reductase and promotes cell death in the S phase of the cell cycle.1-3
Several adverse cutaneous reactions have been associated with hydroxyurea including increased pigmentation, hyperkeratosis, skin atrophy, xerosis, lichenoid eruptions, palmoplantar keratoderma, cutaneous vasculitis, alopecia, chronic leg ulcers, cutaneous carcinomas, and melanonychia.3,4
Hydroxyurea-induced melanonychia most often occurs after several months of therapy but has been reported to occur as early as 4 months and as late as 5 years after initiating the drug.1,4-6 The prevalence of melanonychia in the general population has been estimated at 1% and is thought to increase to approximately 4% in patients treated with hydroxyurea.1,2,6,7 The prevalence of affected individuals increases with age; it is more common in females as well as black and Hispanic patients.2
Multiple patterns of hydroxyurea-induced melanonychia have been described, including longitudinal bands, transverse bands, and diffuse hyperpigmentation.1-3,6 By far the most common pattern described in the literature is longitudinal banding1-3,8; transverse bands are more rare. Although there are sporadic case reports linking the transverse bands with hydroxyurea, these bands occur more frequently with systemic chemotherapy such as doxorubicin and cyclosphosphamide.1,6
The exact pathogenesis of hydroxyurea-induced melanonychia remains unclear, though it is thought to result from focal melanogenesis in the nail bed or matrix followed by deposition of melanin granules on the nail plate.5,8 When these melanocytes are activated, melanosomes filled with melanin are transferred to differentiating matrix cells, which migrate distally as they become nail plate oncocytes, resulting in a visible band of pigmentation in the nail plate.2 There also may be a genetic and photosensitivity component.1,2
Prior case series have described spontaneous remission of nail hyperpigmentation following discontinuation of hydroxyurea therapy.1 In many patients, however, the chronic nature of the myeloproliferative disorder and lack of alternative treatments make a therapeutic change difficult. Although the melanonychia itself is benign, it may precede the appearance of more serious mucocutaneous side effects, such as skin ulceration or development of cutaneous carcinomas, so careful monitoring should be performed.2
Our patient presented with melanonychia that was transverse, polydactylic, monochromic, stable in size and shape, and associated with palmar hyperpigmentation. Of note, the pigmentation remitted over time along with discontinuation of the drug. Although this presentation did not warrant a nail matrix biopsy, it should be noted that patients with single nail melanonychia suspicious for melanoma should have a biopsy, even with concomitant use of hydroxyurea.2 Although transverse melanonychia most commonly is associated with other systemic chemotherapeutics, in the absence of such medications hydroxyurea was the likely culprit in our patient. The palmar hyperpigmentation, which has previously been reported with hydroxyurea use, further solidifies the diagnosis.
- Aste N, Futmo G, Contu F, et al. Nail pigmentation caused by hydroxyurea: report of 9 cases. J Am Acad Dermatol. 2002;47:146-147.
- Murray N, Tapia P, Porcell J, et al. Acquired melanonychia in Chilean patients with essential thrombocythemia treated with hydroxyurea: a report of 7 clinical cases and review of the literature [published online February 7, 2013]. ISRN Dermatol. 2013;2013:325246.
- Utas S. A case of hydroxyurea-induced longitudinal melanonychia. Int J Dermatol. 2010;49:469-470.
- Saraceno R, Teoli M, Chimenti S. Hydroxyurea associated with concomitant occurrence of diffuse longitudinal melanonychia and multiple squamous cell carcinomas in an elderly subject. Clin Ther. 2008;30:1324-1329.
- Cohen AD, Hallel-Halevy D, Hatskelzon L, et al. Longitudinal melanonychia associated with hydroxyurea therapy in a patient with essential thrombocytosis. J Eur Acad Dermatol. 1999;13:137-139.
- Hernández-Martín A, Ros-Forteza S, de Unamuno P. Longitudinal, transverse, and diffuse nail hyperpigmentation induced by hydroxyurea. J Am Acad Dermatol. 1999;41(2, pt 2):333-334.
- Kwong Y. Hydroxyurea-induced nail pigmentation. J Am Acad Dermatol. 1996;35:275-276.
- O’Branski E, Ware R, Prose N, et al. Skin and nail changes in children with sickle cell anemia receiving hydroxyurea therapy. J Am Acad Dermatol. 2001;44:859-861.
To the Editor:
An 85-year-old woman with a history of hypertension, hyperlipidemia, stroke, hypothyroidism, chronic obstructive pulmonary disease, and chronic myeloproliferative disorder presented to our clinic for evaluation of brown lesions on the hands and discoloration of the fingernails and toenails of 4 months’ duration. Six months prior to visiting our clinic she was admitted to the hospital for a pulmonary embolism. On admission she was noted to have a platelet count of more than 2 million/μL (reference range, 150,000–350,000/μL). She received urgent plasmapheresis and started hydroxyurea 500 mg twice daily, which she continued as an outpatient.
On physical examination at our clinic she had diffusely scattered red and brown macules on the bilateral palms and transverse hyperpigmented bands of various intensities on all fingernails and toenails (Figure). Her platelet count was 372,000/μL, white blood cell count was 5200/μL (reference range, 4500–11,000/μL), hemoglobin was 12.6 g/dL (reference range, 14.0–17.5 g/dL), hematocrit was 39.0% (reference range, 41%–50%), and mean corpuscular volume was 87.5 fL per red cell (reference range, 80–96 fL per red cell).

The patient was diagnosed with hydroxyurea-induced nail hyperpigmentation and was counseled on the benign nature of the condition. Three months later her platelet count decreased to below 100,000/μL, and hydroxyurea was discontinued. She noticed considerable improvement in the lesions on the hands and nails with the cessation of hydroxyurea.
Hydroxyurea is a cytostatic agent that has been used for more than 40 years in the treatment of myeloproliferative disorders including chronic myelogenous leukemia, polycythemia vera, essential thrombocythemia, and sickle cell anemia.1 It inhibits ribonucleoside diphosphate reductase and promotes cell death in the S phase of the cell cycle.1-3
Several adverse cutaneous reactions have been associated with hydroxyurea including increased pigmentation, hyperkeratosis, skin atrophy, xerosis, lichenoid eruptions, palmoplantar keratoderma, cutaneous vasculitis, alopecia, chronic leg ulcers, cutaneous carcinomas, and melanonychia.3,4
Hydroxyurea-induced melanonychia most often occurs after several months of therapy but has been reported to occur as early as 4 months and as late as 5 years after initiating the drug.1,4-6 The prevalence of melanonychia in the general population has been estimated at 1% and is thought to increase to approximately 4% in patients treated with hydroxyurea.1,2,6,7 The prevalence of affected individuals increases with age; it is more common in females as well as black and Hispanic patients.2
Multiple patterns of hydroxyurea-induced melanonychia have been described, including longitudinal bands, transverse bands, and diffuse hyperpigmentation.1-3,6 By far the most common pattern described in the literature is longitudinal banding1-3,8; transverse bands are more rare. Although there are sporadic case reports linking the transverse bands with hydroxyurea, these bands occur more frequently with systemic chemotherapy such as doxorubicin and cyclosphosphamide.1,6
The exact pathogenesis of hydroxyurea-induced melanonychia remains unclear, though it is thought to result from focal melanogenesis in the nail bed or matrix followed by deposition of melanin granules on the nail plate.5,8 When these melanocytes are activated, melanosomes filled with melanin are transferred to differentiating matrix cells, which migrate distally as they become nail plate oncocytes, resulting in a visible band of pigmentation in the nail plate.2 There also may be a genetic and photosensitivity component.1,2
Prior case series have described spontaneous remission of nail hyperpigmentation following discontinuation of hydroxyurea therapy.1 In many patients, however, the chronic nature of the myeloproliferative disorder and lack of alternative treatments make a therapeutic change difficult. Although the melanonychia itself is benign, it may precede the appearance of more serious mucocutaneous side effects, such as skin ulceration or development of cutaneous carcinomas, so careful monitoring should be performed.2
Our patient presented with melanonychia that was transverse, polydactylic, monochromic, stable in size and shape, and associated with palmar hyperpigmentation. Of note, the pigmentation remitted over time along with discontinuation of the drug. Although this presentation did not warrant a nail matrix biopsy, it should be noted that patients with single nail melanonychia suspicious for melanoma should have a biopsy, even with concomitant use of hydroxyurea.2 Although transverse melanonychia most commonly is associated with other systemic chemotherapeutics, in the absence of such medications hydroxyurea was the likely culprit in our patient. The palmar hyperpigmentation, which has previously been reported with hydroxyurea use, further solidifies the diagnosis.
To the Editor:
An 85-year-old woman with a history of hypertension, hyperlipidemia, stroke, hypothyroidism, chronic obstructive pulmonary disease, and chronic myeloproliferative disorder presented to our clinic for evaluation of brown lesions on the hands and discoloration of the fingernails and toenails of 4 months’ duration. Six months prior to visiting our clinic she was admitted to the hospital for a pulmonary embolism. On admission she was noted to have a platelet count of more than 2 million/μL (reference range, 150,000–350,000/μL). She received urgent plasmapheresis and started hydroxyurea 500 mg twice daily, which she continued as an outpatient.
On physical examination at our clinic she had diffusely scattered red and brown macules on the bilateral palms and transverse hyperpigmented bands of various intensities on all fingernails and toenails (Figure). Her platelet count was 372,000/μL, white blood cell count was 5200/μL (reference range, 4500–11,000/μL), hemoglobin was 12.6 g/dL (reference range, 14.0–17.5 g/dL), hematocrit was 39.0% (reference range, 41%–50%), and mean corpuscular volume was 87.5 fL per red cell (reference range, 80–96 fL per red cell).

The patient was diagnosed with hydroxyurea-induced nail hyperpigmentation and was counseled on the benign nature of the condition. Three months later her platelet count decreased to below 100,000/μL, and hydroxyurea was discontinued. She noticed considerable improvement in the lesions on the hands and nails with the cessation of hydroxyurea.
Hydroxyurea is a cytostatic agent that has been used for more than 40 years in the treatment of myeloproliferative disorders including chronic myelogenous leukemia, polycythemia vera, essential thrombocythemia, and sickle cell anemia.1 It inhibits ribonucleoside diphosphate reductase and promotes cell death in the S phase of the cell cycle.1-3
Several adverse cutaneous reactions have been associated with hydroxyurea including increased pigmentation, hyperkeratosis, skin atrophy, xerosis, lichenoid eruptions, palmoplantar keratoderma, cutaneous vasculitis, alopecia, chronic leg ulcers, cutaneous carcinomas, and melanonychia.3,4
Hydroxyurea-induced melanonychia most often occurs after several months of therapy but has been reported to occur as early as 4 months and as late as 5 years after initiating the drug.1,4-6 The prevalence of melanonychia in the general population has been estimated at 1% and is thought to increase to approximately 4% in patients treated with hydroxyurea.1,2,6,7 The prevalence of affected individuals increases with age; it is more common in females as well as black and Hispanic patients.2
Multiple patterns of hydroxyurea-induced melanonychia have been described, including longitudinal bands, transverse bands, and diffuse hyperpigmentation.1-3,6 By far the most common pattern described in the literature is longitudinal banding1-3,8; transverse bands are more rare. Although there are sporadic case reports linking the transverse bands with hydroxyurea, these bands occur more frequently with systemic chemotherapy such as doxorubicin and cyclosphosphamide.1,6
The exact pathogenesis of hydroxyurea-induced melanonychia remains unclear, though it is thought to result from focal melanogenesis in the nail bed or matrix followed by deposition of melanin granules on the nail plate.5,8 When these melanocytes are activated, melanosomes filled with melanin are transferred to differentiating matrix cells, which migrate distally as they become nail plate oncocytes, resulting in a visible band of pigmentation in the nail plate.2 There also may be a genetic and photosensitivity component.1,2
Prior case series have described spontaneous remission of nail hyperpigmentation following discontinuation of hydroxyurea therapy.1 In many patients, however, the chronic nature of the myeloproliferative disorder and lack of alternative treatments make a therapeutic change difficult. Although the melanonychia itself is benign, it may precede the appearance of more serious mucocutaneous side effects, such as skin ulceration or development of cutaneous carcinomas, so careful monitoring should be performed.2
Our patient presented with melanonychia that was transverse, polydactylic, monochromic, stable in size and shape, and associated with palmar hyperpigmentation. Of note, the pigmentation remitted over time along with discontinuation of the drug. Although this presentation did not warrant a nail matrix biopsy, it should be noted that patients with single nail melanonychia suspicious for melanoma should have a biopsy, even with concomitant use of hydroxyurea.2 Although transverse melanonychia most commonly is associated with other systemic chemotherapeutics, in the absence of such medications hydroxyurea was the likely culprit in our patient. The palmar hyperpigmentation, which has previously been reported with hydroxyurea use, further solidifies the diagnosis.
- Aste N, Futmo G, Contu F, et al. Nail pigmentation caused by hydroxyurea: report of 9 cases. J Am Acad Dermatol. 2002;47:146-147.
- Murray N, Tapia P, Porcell J, et al. Acquired melanonychia in Chilean patients with essential thrombocythemia treated with hydroxyurea: a report of 7 clinical cases and review of the literature [published online February 7, 2013]. ISRN Dermatol. 2013;2013:325246.
- Utas S. A case of hydroxyurea-induced longitudinal melanonychia. Int J Dermatol. 2010;49:469-470.
- Saraceno R, Teoli M, Chimenti S. Hydroxyurea associated with concomitant occurrence of diffuse longitudinal melanonychia and multiple squamous cell carcinomas in an elderly subject. Clin Ther. 2008;30:1324-1329.
- Cohen AD, Hallel-Halevy D, Hatskelzon L, et al. Longitudinal melanonychia associated with hydroxyurea therapy in a patient with essential thrombocytosis. J Eur Acad Dermatol. 1999;13:137-139.
- Hernández-Martín A, Ros-Forteza S, de Unamuno P. Longitudinal, transverse, and diffuse nail hyperpigmentation induced by hydroxyurea. J Am Acad Dermatol. 1999;41(2, pt 2):333-334.
- Kwong Y. Hydroxyurea-induced nail pigmentation. J Am Acad Dermatol. 1996;35:275-276.
- O’Branski E, Ware R, Prose N, et al. Skin and nail changes in children with sickle cell anemia receiving hydroxyurea therapy. J Am Acad Dermatol. 2001;44:859-861.
- Aste N, Futmo G, Contu F, et al. Nail pigmentation caused by hydroxyurea: report of 9 cases. J Am Acad Dermatol. 2002;47:146-147.
- Murray N, Tapia P, Porcell J, et al. Acquired melanonychia in Chilean patients with essential thrombocythemia treated with hydroxyurea: a report of 7 clinical cases and review of the literature [published online February 7, 2013]. ISRN Dermatol. 2013;2013:325246.
- Utas S. A case of hydroxyurea-induced longitudinal melanonychia. Int J Dermatol. 2010;49:469-470.
- Saraceno R, Teoli M, Chimenti S. Hydroxyurea associated with concomitant occurrence of diffuse longitudinal melanonychia and multiple squamous cell carcinomas in an elderly subject. Clin Ther. 2008;30:1324-1329.
- Cohen AD, Hallel-Halevy D, Hatskelzon L, et al. Longitudinal melanonychia associated with hydroxyurea therapy in a patient with essential thrombocytosis. J Eur Acad Dermatol. 1999;13:137-139.
- Hernández-Martín A, Ros-Forteza S, de Unamuno P. Longitudinal, transverse, and diffuse nail hyperpigmentation induced by hydroxyurea. J Am Acad Dermatol. 1999;41(2, pt 2):333-334.
- Kwong Y. Hydroxyurea-induced nail pigmentation. J Am Acad Dermatol. 1996;35:275-276.
- O’Branski E, Ware R, Prose N, et al. Skin and nail changes in children with sickle cell anemia receiving hydroxyurea therapy. J Am Acad Dermatol. 2001;44:859-861.
Practice Points
- Transverse melanonychia may result as a side effect of hydroxyurea.
- Discontinuation of hydroxyurea typically results in a resolution of symptoms. If the medication cannot be stopped, however, pigmentary changes may precede the development of severe mucocutaneous side effects and close monitoring is warranted.
- Patients with single nail melanonychia suspicious for melanoma should have a biopsy, even with concomitant use of hydroxyurea.
Big data study looks at safety of concurrent surgical procedures
PHILADELPHIA – Overlapping and concurrent surgical procedures operations are not uncommon, but data on their safety and quality are scarce.
An analysis of the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) data suggests that concurrent operations do not pose any greater risk to patients than do nonconcurrent operations.
“We found that concurrent operations were more often elective inpatient operations at large academic hospitals,” Jason Liu, MD, a clinical scholar-in-residence with the American College of Surgeons, Chicago, said at the annual meeting of the American Surgical Association.
The study evaluated 12,010 concurrent and 521,656 nonconcurrent operations in the ASC NSQIP registry between 2014 and 2015, and propensity-score matched 11,044 operations of each type to evaluate safety. “We then analyzed the three primary outcomes – death or serious morbidity, unplanned reoperation, and unplanned readmission,” Dr. Liu said. “After propensity matching and risk adjustment, we detected no association of concurrent operations with these adverse outcomes.”
Death or serious morbidity had an odds ratio of 1.08, while the odds ratio for reoperation and readmission were 1.16 and 1.14, respectively.
Of the five surgery subspecialty groups presented, ear, nose and throat surgeons performed the highest proportion of concurrent operations, comprising 11.2% of all operations within the subspecialty, followed by neurosurgeons (8.4%) and urological surgeons (5.2%). General surgeons performed more total concurrent cases than did any of the other surgical subspecialties, but these comprised only 1.5% of all general surgery cases.
Among the individual operations presented, the highest percentage of concurrent cases was for spinal operations, comprising 7.1% of all spinal surgeries, followed by total knee and hip replacements, at 2.1% each. Among general surgery procedures, concurrent operations comprised 1.9% of colon surgeries, 1.5% of ventral hernia repairs, and 0.9% of cholecystectomies.
“We found that patients who had concurrent operations tended to have fewer comorbidities,” Dr. Liu said.
Despite the findings suggesting concurrent operations are safe, Dr. Liu added that “failure to detect an effect does not prove its absence. Additional studies and continued vigilance are certainly needed moving forward.”
In her discussion, Valerie W. Rusch, MD, FACS, of Memorial Sloan-Kettering Cancer Center, New York, pointed out that the American College of Surgeons has not revised its statement that considers concurrent surgery “not appropriate,” and that the study itself “reflects both the strengths and weaknesses of ‘big data.’ ” While ACS NSQIP provides a large number of patients, the study authors were still constrained in using time as a proxy for concurrence, she said. “Also, as noted, this analysis reports only a sample of operations rather than all the procedures performed, and the authors were constrained to using simulated statistical methods to avoid misclassification of cases,” Dr. Rusch said.
The death or serious morbidity composite measure the study used may not identify procedure-specific or specialty-specific adverse events that could reflect differences in outcomes between concurrent and nonconcurrent operations, she said. “Nonetheless, this study represents an important step toward bringing science to an extremely important and rightfully contentious aspect of surgical practice,” Dr. Rusch said.
Senior coauthor David Hoyt, MD, FACS, of the ACS, acknowledged the many study limitations Dr. Rusch pointed out. “What this analysis shows is that probably there’s not a huge problem with morbidity and mortality,” Dr. Hoyt said. “It doesn’t really say that we should pursue this without some care of how we do this.”
A key element in planning concurrent surgery is disclosing that to the patient. “Much of what came out of the discussion that led to this was because patients felt that they were not informed,” Dr. Hoyt said. “It really has to do with an ethical issue of patient autonomy.”
Dr. Liu, Dr. Hoyt, and Dr. Rusch had no financial relationships to disclose.
The complete manuscript of this study and its presentation at the American Surgical Association’s 137th Annual Meeting, April 2017, in Philadelphia, Pennsylvania, is anticipated to be published in the Annals of Surgery pending editorial review.
PHILADELPHIA – Overlapping and concurrent surgical procedures operations are not uncommon, but data on their safety and quality are scarce.
An analysis of the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) data suggests that concurrent operations do not pose any greater risk to patients than do nonconcurrent operations.
“We found that concurrent operations were more often elective inpatient operations at large academic hospitals,” Jason Liu, MD, a clinical scholar-in-residence with the American College of Surgeons, Chicago, said at the annual meeting of the American Surgical Association.
The study evaluated 12,010 concurrent and 521,656 nonconcurrent operations in the ASC NSQIP registry between 2014 and 2015, and propensity-score matched 11,044 operations of each type to evaluate safety. “We then analyzed the three primary outcomes – death or serious morbidity, unplanned reoperation, and unplanned readmission,” Dr. Liu said. “After propensity matching and risk adjustment, we detected no association of concurrent operations with these adverse outcomes.”
Death or serious morbidity had an odds ratio of 1.08, while the odds ratio for reoperation and readmission were 1.16 and 1.14, respectively.
Of the five surgery subspecialty groups presented, ear, nose and throat surgeons performed the highest proportion of concurrent operations, comprising 11.2% of all operations within the subspecialty, followed by neurosurgeons (8.4%) and urological surgeons (5.2%). General surgeons performed more total concurrent cases than did any of the other surgical subspecialties, but these comprised only 1.5% of all general surgery cases.
Among the individual operations presented, the highest percentage of concurrent cases was for spinal operations, comprising 7.1% of all spinal surgeries, followed by total knee and hip replacements, at 2.1% each. Among general surgery procedures, concurrent operations comprised 1.9% of colon surgeries, 1.5% of ventral hernia repairs, and 0.9% of cholecystectomies.
“We found that patients who had concurrent operations tended to have fewer comorbidities,” Dr. Liu said.
Despite the findings suggesting concurrent operations are safe, Dr. Liu added that “failure to detect an effect does not prove its absence. Additional studies and continued vigilance are certainly needed moving forward.”
In her discussion, Valerie W. Rusch, MD, FACS, of Memorial Sloan-Kettering Cancer Center, New York, pointed out that the American College of Surgeons has not revised its statement that considers concurrent surgery “not appropriate,” and that the study itself “reflects both the strengths and weaknesses of ‘big data.’ ” While ACS NSQIP provides a large number of patients, the study authors were still constrained in using time as a proxy for concurrence, she said. “Also, as noted, this analysis reports only a sample of operations rather than all the procedures performed, and the authors were constrained to using simulated statistical methods to avoid misclassification of cases,” Dr. Rusch said.
The death or serious morbidity composite measure the study used may not identify procedure-specific or specialty-specific adverse events that could reflect differences in outcomes between concurrent and nonconcurrent operations, she said. “Nonetheless, this study represents an important step toward bringing science to an extremely important and rightfully contentious aspect of surgical practice,” Dr. Rusch said.
Senior coauthor David Hoyt, MD, FACS, of the ACS, acknowledged the many study limitations Dr. Rusch pointed out. “What this analysis shows is that probably there’s not a huge problem with morbidity and mortality,” Dr. Hoyt said. “It doesn’t really say that we should pursue this without some care of how we do this.”
A key element in planning concurrent surgery is disclosing that to the patient. “Much of what came out of the discussion that led to this was because patients felt that they were not informed,” Dr. Hoyt said. “It really has to do with an ethical issue of patient autonomy.”
Dr. Liu, Dr. Hoyt, and Dr. Rusch had no financial relationships to disclose.
The complete manuscript of this study and its presentation at the American Surgical Association’s 137th Annual Meeting, April 2017, in Philadelphia, Pennsylvania, is anticipated to be published in the Annals of Surgery pending editorial review.
PHILADELPHIA – Overlapping and concurrent surgical procedures operations are not uncommon, but data on their safety and quality are scarce.
An analysis of the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) data suggests that concurrent operations do not pose any greater risk to patients than do nonconcurrent operations.
“We found that concurrent operations were more often elective inpatient operations at large academic hospitals,” Jason Liu, MD, a clinical scholar-in-residence with the American College of Surgeons, Chicago, said at the annual meeting of the American Surgical Association.
The study evaluated 12,010 concurrent and 521,656 nonconcurrent operations in the ASC NSQIP registry between 2014 and 2015, and propensity-score matched 11,044 operations of each type to evaluate safety. “We then analyzed the three primary outcomes – death or serious morbidity, unplanned reoperation, and unplanned readmission,” Dr. Liu said. “After propensity matching and risk adjustment, we detected no association of concurrent operations with these adverse outcomes.”
Death or serious morbidity had an odds ratio of 1.08, while the odds ratio for reoperation and readmission were 1.16 and 1.14, respectively.
Of the five surgery subspecialty groups presented, ear, nose and throat surgeons performed the highest proportion of concurrent operations, comprising 11.2% of all operations within the subspecialty, followed by neurosurgeons (8.4%) and urological surgeons (5.2%). General surgeons performed more total concurrent cases than did any of the other surgical subspecialties, but these comprised only 1.5% of all general surgery cases.
Among the individual operations presented, the highest percentage of concurrent cases was for spinal operations, comprising 7.1% of all spinal surgeries, followed by total knee and hip replacements, at 2.1% each. Among general surgery procedures, concurrent operations comprised 1.9% of colon surgeries, 1.5% of ventral hernia repairs, and 0.9% of cholecystectomies.
“We found that patients who had concurrent operations tended to have fewer comorbidities,” Dr. Liu said.
Despite the findings suggesting concurrent operations are safe, Dr. Liu added that “failure to detect an effect does not prove its absence. Additional studies and continued vigilance are certainly needed moving forward.”
In her discussion, Valerie W. Rusch, MD, FACS, of Memorial Sloan-Kettering Cancer Center, New York, pointed out that the American College of Surgeons has not revised its statement that considers concurrent surgery “not appropriate,” and that the study itself “reflects both the strengths and weaknesses of ‘big data.’ ” While ACS NSQIP provides a large number of patients, the study authors were still constrained in using time as a proxy for concurrence, she said. “Also, as noted, this analysis reports only a sample of operations rather than all the procedures performed, and the authors were constrained to using simulated statistical methods to avoid misclassification of cases,” Dr. Rusch said.
The death or serious morbidity composite measure the study used may not identify procedure-specific or specialty-specific adverse events that could reflect differences in outcomes between concurrent and nonconcurrent operations, she said. “Nonetheless, this study represents an important step toward bringing science to an extremely important and rightfully contentious aspect of surgical practice,” Dr. Rusch said.
Senior coauthor David Hoyt, MD, FACS, of the ACS, acknowledged the many study limitations Dr. Rusch pointed out. “What this analysis shows is that probably there’s not a huge problem with morbidity and mortality,” Dr. Hoyt said. “It doesn’t really say that we should pursue this without some care of how we do this.”
A key element in planning concurrent surgery is disclosing that to the patient. “Much of what came out of the discussion that led to this was because patients felt that they were not informed,” Dr. Hoyt said. “It really has to do with an ethical issue of patient autonomy.”
Dr. Liu, Dr. Hoyt, and Dr. Rusch had no financial relationships to disclose.
The complete manuscript of this study and its presentation at the American Surgical Association’s 137th Annual Meeting, April 2017, in Philadelphia, Pennsylvania, is anticipated to be published in the Annals of Surgery pending editorial review.
AT THE ASA ANNUAL MEETING
Key clinical point: Concurrent operations were not associated with an increased risk for worse outcomes, compared with nonconcurrent operations.
Major finding: After propensity-score matching and risk adjustment, the adjusted odds ratio for death or serious mortality was 1.08, and for reoperation, 1.16, for concurrent operations vs. nonconcurrent operations.
Data source: Propensity-score-matched concurrent and nonconcurrent operations (n = 11,044 for each) done in 2014 and 2015 in the American College of Surgeons National Surgical Quality Improvement Program registry.
Disclosures: Dr. Liu and coauthors had no financial relationships to disclose.
Breast milk bacteria seed infant gut microbiome
Beneficial bacteria in the mother’s breast milk and on the breast skin seed the infant gut microbiome and maintain it even after solid foods are introduced, according to a report published in JAMA Pediatrics.
Noting that little is known about the vertical transfer of breast milk microbes from mother to infant, researchers examined bacteria in samples from breast milk, areolar skin swabs, and infant stools in 107 healthy mother-infant pairs. The samples were obtained during a 5-year period from mothers and infants living in the community in Los Angeles and St. Petersburg, Fla., said Pia S. Pannaraj, MD, of the division of infectious diseases, Children’s Hospital Los Angeles, and her associates.
The transferred bacteria are known to “have prominent carbohydrate, amino acid, and energy metabolism functions.” In addition, “The amount of daily breastfeeding as a proportion of total milk intake continued to influence the infant stool microbiome diversity and membership even after solid foods were introduced,” Dr. Pannaraj and her associates said.
These findings highlight “the importance of breastfeeding in the assembly of the infant gut microbiome.” They also support current World Health Organization and American Academy of Pediatrics recommendations “for exclusive breastfeeding during the first 6 months, with continued breastfeeding until at least 12 months,” the investigators added.
Beneficial bacteria in the mother’s breast milk and on the breast skin seed the infant gut microbiome and maintain it even after solid foods are introduced, according to a report published in JAMA Pediatrics.
Noting that little is known about the vertical transfer of breast milk microbes from mother to infant, researchers examined bacteria in samples from breast milk, areolar skin swabs, and infant stools in 107 healthy mother-infant pairs. The samples were obtained during a 5-year period from mothers and infants living in the community in Los Angeles and St. Petersburg, Fla., said Pia S. Pannaraj, MD, of the division of infectious diseases, Children’s Hospital Los Angeles, and her associates.
The transferred bacteria are known to “have prominent carbohydrate, amino acid, and energy metabolism functions.” In addition, “The amount of daily breastfeeding as a proportion of total milk intake continued to influence the infant stool microbiome diversity and membership even after solid foods were introduced,” Dr. Pannaraj and her associates said.
These findings highlight “the importance of breastfeeding in the assembly of the infant gut microbiome.” They also support current World Health Organization and American Academy of Pediatrics recommendations “for exclusive breastfeeding during the first 6 months, with continued breastfeeding until at least 12 months,” the investigators added.
Beneficial bacteria in the mother’s breast milk and on the breast skin seed the infant gut microbiome and maintain it even after solid foods are introduced, according to a report published in JAMA Pediatrics.
Noting that little is known about the vertical transfer of breast milk microbes from mother to infant, researchers examined bacteria in samples from breast milk, areolar skin swabs, and infant stools in 107 healthy mother-infant pairs. The samples were obtained during a 5-year period from mothers and infants living in the community in Los Angeles and St. Petersburg, Fla., said Pia S. Pannaraj, MD, of the division of infectious diseases, Children’s Hospital Los Angeles, and her associates.
The transferred bacteria are known to “have prominent carbohydrate, amino acid, and energy metabolism functions.” In addition, “The amount of daily breastfeeding as a proportion of total milk intake continued to influence the infant stool microbiome diversity and membership even after solid foods were introduced,” Dr. Pannaraj and her associates said.
These findings highlight “the importance of breastfeeding in the assembly of the infant gut microbiome.” They also support current World Health Organization and American Academy of Pediatrics recommendations “for exclusive breastfeeding during the first 6 months, with continued breastfeeding until at least 12 months,” the investigators added.
FROM JAMA PEDIATRICS
Key clinical point: Beneficial bacteria in the mother’s breast milk and on the areola seed and maintain the infant gut microbiome.
Major finding: During their first 30 days of life, breastfed infants obtained a mean of 28% of their gut bacteria from breast milk and 10% from the mothers’ areolar skin.
Data source: A prospective longitudinal study involving 107 healthy mother-infant pairs.
Disclosures: This study was supported by the National Institutes of Health and the University of Pennsylvania Center for AIDS Research. Dr. Pannaraj reported ties to AstraZeneca and Pfizer.
Novel robotic camera photographs colon
A novel robotically driven capsule successfully navigated a colon in a pig model in 100% of 30 retroflexion maneuvers, setting the stage for further study of robotics in colonoscopy. The data were presented at the annual Digestive Disease Week.
Although capsule technology has expanded exploration of the GI tract, current capsules are limited by passive movement and lack therapeutic capability, said Keith L. Obstein, MD, of Vanderbilt University in Nashville, Tenn.
The researchers developed a capsule with an 18-mm head and interior permanent magnets designed to be automatically controlled by an external robotic arm in difficult areas.
“Retroflexion is a common but mechanically complex maneuver during colonoscopy, and therefore serves as an excellent subject for autonomous control,” the researchers noted.
Logistically, the capsule is soft-tethered after it is introduced through the rectum, which allows the potential for therapeutic procedures such as biopsies and polyp removal. In addition, the use of an external magnet to pull the robot with the tether may reduce the pressure on the colon that typically created when a physician pushes the colonoscope from behind. Therefore, use of a robotic capsule may help reduce patients’ discomfort and make them more amenable to the procedure, Dr. Obstein said.
In the study, an endoscopist performed the first retroflexion with a standard colonoscope at 15 cm from the anal sphincter, and these parameters were used to set the robotic system for autonomous retroflexion at 15 cm from the anal sphincter.
The success rate was 100% for both the standard endoscope and the automatically controlled robot for each of 30 maneuvers. The average time for a robotic maneuver was 12 seconds, and no leaks or histologic abnormalities were noted.
Studies to evaluate additional control algorithms are in progress, and the robot capsule may be ready for human trials in 2018, Dr. Obstein said.
This study was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under award number R01EB018992. Dr. Obstein had no relevant financial conflicts to disclose.
Digestive Disease Week® is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE) and the Society for Surgery of the Alimentary Tract (SSAT).
A novel robotically driven capsule successfully navigated a colon in a pig model in 100% of 30 retroflexion maneuvers, setting the stage for further study of robotics in colonoscopy. The data were presented at the annual Digestive Disease Week.
Although capsule technology has expanded exploration of the GI tract, current capsules are limited by passive movement and lack therapeutic capability, said Keith L. Obstein, MD, of Vanderbilt University in Nashville, Tenn.
The researchers developed a capsule with an 18-mm head and interior permanent magnets designed to be automatically controlled by an external robotic arm in difficult areas.
“Retroflexion is a common but mechanically complex maneuver during colonoscopy, and therefore serves as an excellent subject for autonomous control,” the researchers noted.
Logistically, the capsule is soft-tethered after it is introduced through the rectum, which allows the potential for therapeutic procedures such as biopsies and polyp removal. In addition, the use of an external magnet to pull the robot with the tether may reduce the pressure on the colon that typically created when a physician pushes the colonoscope from behind. Therefore, use of a robotic capsule may help reduce patients’ discomfort and make them more amenable to the procedure, Dr. Obstein said.
In the study, an endoscopist performed the first retroflexion with a standard colonoscope at 15 cm from the anal sphincter, and these parameters were used to set the robotic system for autonomous retroflexion at 15 cm from the anal sphincter.
The success rate was 100% for both the standard endoscope and the automatically controlled robot for each of 30 maneuvers. The average time for a robotic maneuver was 12 seconds, and no leaks or histologic abnormalities were noted.
Studies to evaluate additional control algorithms are in progress, and the robot capsule may be ready for human trials in 2018, Dr. Obstein said.
This study was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under award number R01EB018992. Dr. Obstein had no relevant financial conflicts to disclose.
Digestive Disease Week® is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE) and the Society for Surgery of the Alimentary Tract (SSAT).
A novel robotically driven capsule successfully navigated a colon in a pig model in 100% of 30 retroflexion maneuvers, setting the stage for further study of robotics in colonoscopy. The data were presented at the annual Digestive Disease Week.
Although capsule technology has expanded exploration of the GI tract, current capsules are limited by passive movement and lack therapeutic capability, said Keith L. Obstein, MD, of Vanderbilt University in Nashville, Tenn.
The researchers developed a capsule with an 18-mm head and interior permanent magnets designed to be automatically controlled by an external robotic arm in difficult areas.
“Retroflexion is a common but mechanically complex maneuver during colonoscopy, and therefore serves as an excellent subject for autonomous control,” the researchers noted.
Logistically, the capsule is soft-tethered after it is introduced through the rectum, which allows the potential for therapeutic procedures such as biopsies and polyp removal. In addition, the use of an external magnet to pull the robot with the tether may reduce the pressure on the colon that typically created when a physician pushes the colonoscope from behind. Therefore, use of a robotic capsule may help reduce patients’ discomfort and make them more amenable to the procedure, Dr. Obstein said.
In the study, an endoscopist performed the first retroflexion with a standard colonoscope at 15 cm from the anal sphincter, and these parameters were used to set the robotic system for autonomous retroflexion at 15 cm from the anal sphincter.
The success rate was 100% for both the standard endoscope and the automatically controlled robot for each of 30 maneuvers. The average time for a robotic maneuver was 12 seconds, and no leaks or histologic abnormalities were noted.
Studies to evaluate additional control algorithms are in progress, and the robot capsule may be ready for human trials in 2018, Dr. Obstein said.
This study was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under award number R01EB018992. Dr. Obstein had no relevant financial conflicts to disclose.
Digestive Disease Week® is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE) and the Society for Surgery of the Alimentary Tract (SSAT).
FROM DDW
Key clinical point: An 18-mm magnetized capsule colonoscope successfully navigated a pig colon, and researchers are planning human trials for 2018.
Major finding: An automated robot capsule successfully completed 30 retroflexion maneuvers in a pig colon with an average time of 12 seconds.
Data source: The data come from a test of 30 maneuvers.
Disclosures: This study was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under award number R01EB018992. Dr. Obstein had no relevant financial conflicts to disclose.