User login
Pulmonary Perspectives: Ensuring quality for EBUS bronchoscopy with varying levels of practitioner experience
Dr. Mahajan and colleagues present a compelling case for requiring minimum standards to perform an EBUS-guided bronchoscopy. Their opinion piece epitomizes the classic tension between physicians with advanced training and those who can only have practice-based training. A middle ground may exist, as perhaps competence could be achieved by simulation, clinical cases performed, and observation by a regional expert? Physicians in practice must have a pathway to adopt new technology whether it is thoracic ultrasound or endobronchial ultrasound, but it must be done in a safe manner. As a referring physician, I would only send my patients who required mediastinal staging to a pulmonologist who I knew performed EBUS regularly.
Nitin Puri, MD, FCCP
Endobronchial ultrasound (EBUS) bronchoscopy is a tool that has transformed the diagnosis and staging of lung cancer. Through real-time ultrasound imaging, EBUS provides clear images of lymph nodes and proximal lung masses that can be adequately sampled through transbronchial needle aspiration. EBUS is a minimally invasive, outpatient procedure that can also be used for diagnosing benign disease within the chest. Large studies investigating the use of EBUS for mediastinal staging have shown the procedure to be highly sensitive and specific while harboring an excellent safety profile.1 As a result, EBUS has essentially replaced mediastinoscopy for the staging of lung cancer.
EBUS bronchoscopy was primarily offered at major academic centers when first released and was performed by physicians who were formally trained in the procedure during interventional pulmonology or thoracic surgery fellowships. Over time, the tool has been adopted by established general pulmonologists without formal training in EBUS. Some of these pulmonologists only develop their skills by attending 1- to 2-day courses, which is insufficient supervision to become competent in this important procedure.
An ongoing debate continues as to how many supervised EBUS bronchoscopies should be performed prior to being considered proficient.2 As procedural competence has been associated with the number of EBUS procedures performed, the learning curve required to master EBUS is an important component of proficiency. While most consider learning curves to be variable, evidence produced by Fernandez-Villar and colleagues revealed that EBUS performance continues to improve up to 120 procedures.3 This analysis was performed in unselected consecutive patients based on diagnostic yield, procedure length, number of lymph nodes passes performed in order to obtain adequate samples, and the number of lymph nodes studied per patient. The learning curve was evaluated based on consecutive groups of 20 patients, the number of adequate samples obtained, and the diagnostic accuracy. Their results indicated that the diagnostic effectiveness of EBUS-TBNA improves with increasing number of procedures performed, allowing for access to a greater number of lymph nodes without necessarily increasing the length of the procedure, and by reducing the number of punctures at each nodal station. Based on their results, the first 20 procedures performed yielded a 70% accuracy, 21 to 40 procedures performed resulted in 81.8% accuracy, 41 to 60 procedures performed resulted in 83.3% accuracy, 61 to 80 procedures performed resulted in 89.8% accuracy, 81 to 100 procedures performed resulted in 90.5% accuracy, and 101 to 120 procedures performed resulted in 94.5% accuracy.
While the American Thoracic Society (ATS) and the American College of Chest Physicians (CHEST) both recommend a minimum number of 40 to 50 supervised EBUS bronchoscopies prior to performing the procedure independently, along with 20 procedures per year for maintenance of competency, most institutions do not track the number of EBUS procedures performed and they do not follow the ATS or CHEST recommendations.4,5 As a result, a number of physicians are independently performing EBUS without adequate experience, resulting in possibly poor quality care. Unfortunately, some short courses, intended to generate interest and encourage attendees to pursue further training, are mistakenly assumed to be sufficient by the novice user.
As the number of interventional pulmonary fellowships continues to expand, the growing number of subspecialized pulmonologists with extensive training in EBUS grows. During a dedicated interventional pulmonary fellowship, fellows perform well above the number of EBUS bronchoscopies suggested by the ATS and CHEST in a single year. Recently published accreditation guidelines require a minimum of 100 cases per interventional pulmonary fellow.6 These fellowship-trained interventional pulmonologists are then tested to become board-certified in a wide array of minimally invasive procedures, including EBUS. As a result, a model has developed where both board-certified interventional pulmonologists with extensive training in EBUS and general pulmonologists not meeting ATS or CHEST minimum requirements practice at the same institution. Proponents of a more liberal access to credentialing in EBUS have suggested that adhering to competency requirements constitutes a “barrier to entry” in which incumbent practitioners benefit from limiting competition. However, like any other regulatory metric, the rationale is to prevent asymmetric information. In this example, the physician knows more than the patient. The patient cannot make an informed decision on which provider to choose and what are the minimum requirements that are likely to produce the most useful information (ie, complete staging). For these reasons, it is imperative that regulations protect the patient.
Without question, EBUS bronchoscopy should not be performed only by board-certified interventional pulmonologists. Instead, hospital credentialing committees should adhere to both the ATS and CHEST recommendations for the number of supervised cases necessary prior to performing EBUS independently. As EBUS use continues to grow, fellows in 3- or 4-year pulmonary and critical care fellowships will be likely capable of meeting the minimal number of observed cases, but, if these numbers are not achieved, additional training should be required. Understandably, this could be challenging for physicians who are unable to take time away from their practice to gain this training. However, if these numbers cannot be met, credentialing requirements should be enforced.
Even more challenging than establishing quality measures for EBUS, is to ensure the highest level of care delivery for patients when there exist multiple levels of experience in the same institution. Undoubtedly, patients undergoing EBUS bronchoscopy, or any procedure for that matter, would want the most skilled physician who has attained certification in the procedure. Unfortunately, no formal certification of EBUS exists outside of gaining board certification in interventional pulmonology. To ensure excellence in care, physicians performing EBUS should be involved in quality improvement initiatives and review pathologic yields along with complications on a regular basis in a group setting. Unlike emergency interventions, EBUS bronchoscopy is an entirely elective procedure.
The advent of EBUS bronchoscopy has revolutionized the diagnosis and staging of lung cancer. As use of EBUS continues to become more widespread, the incidence of high volume and low volume proceduralists will become a more commonly encountered scenario. Guidelines have been set by the professional pulmonary societies based on the data and observations available. At the local level, stringent guidelines need to be established by hospitals to ensure a high level of quality with appropriate oversight. Patients undergoing EBUS deserve a physician who is skilled in the procedure and has performed at least the minimum number of procedures to provide the adequate care.
Dr. Mahajan is Medical Director, Interventional Pulmonology, Inova Heart and Vascular Institute - Inova Fairfax Hospital, and Associate Professor, Virginia Commonwealth Medical School; Dr. Khandhar is Medical Director, Thoracic Surgery, Inova Heart and Vascular Institute - Inova Fairfax Hospital, and Assistant Clinical Professor, Virginia Commonwealth Medical School; Falls Church, VA. Dr. Folch is Co-Director, Interventional Pulmonology Chief, Complex Chest Diseases Center, Harvard Medical School, Massachusetts General Hospital, Boston, MA.
References
1. Gomez M, Silvestri GA. Endobronchial ultrasound for the diagnosis and staging of lung cancer. Proc Am Thorac Soc. 2009;6(2):180-186.
2. Folch E, Majid A. Point: Are >50 Supervised Procedures Required to Develop Competency in Performing Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration for Mediastinal Staging? Yes. Chest. 2013;143(4):888-891.
3. Fernandez-Villar A, Leiro-Fernandez V, Botana-Rial M, Represas-Represas C, Nunez-Delgado M. The endobronchial ultrasound-guided transbronchial needle biopsy learning curve for mediastinal and hilar lymph node diagnosis. Chest. 2012; 141(1):278-279.
4. Ernst A, Silvestri GA, Johnstone D. Interventional pulmonary procedures: Guidelines from the American College of Chest Physicians. Chest. 2003;123(5):1693-1717.
5. Bolliger CT, Mathur PN, Beamis JF, et al. ERS/ATS statement on interventional pulmonology. European Respiratory Society/American Thoracic Society. Eur Respir J. 2002;19(2):356-373.
6. Mullon JJ, Burkhart KM, Silvestri G. Interventional Pulmonology Fellowship Accreditation Standards: Executive Summary of the Multi-society Interventional Pulmonology Fellowship Accreditation Committee. Chest. 2017. doi:10.1016/j.chest.2017.01.024.
Dr. Mahajan and colleagues present a compelling case for requiring minimum standards to perform an EBUS-guided bronchoscopy. Their opinion piece epitomizes the classic tension between physicians with advanced training and those who can only have practice-based training. A middle ground may exist, as perhaps competence could be achieved by simulation, clinical cases performed, and observation by a regional expert? Physicians in practice must have a pathway to adopt new technology whether it is thoracic ultrasound or endobronchial ultrasound, but it must be done in a safe manner. As a referring physician, I would only send my patients who required mediastinal staging to a pulmonologist who I knew performed EBUS regularly.
Nitin Puri, MD, FCCP
Endobronchial ultrasound (EBUS) bronchoscopy is a tool that has transformed the diagnosis and staging of lung cancer. Through real-time ultrasound imaging, EBUS provides clear images of lymph nodes and proximal lung masses that can be adequately sampled through transbronchial needle aspiration. EBUS is a minimally invasive, outpatient procedure that can also be used for diagnosing benign disease within the chest. Large studies investigating the use of EBUS for mediastinal staging have shown the procedure to be highly sensitive and specific while harboring an excellent safety profile.1 As a result, EBUS has essentially replaced mediastinoscopy for the staging of lung cancer.
EBUS bronchoscopy was primarily offered at major academic centers when first released and was performed by physicians who were formally trained in the procedure during interventional pulmonology or thoracic surgery fellowships. Over time, the tool has been adopted by established general pulmonologists without formal training in EBUS. Some of these pulmonologists only develop their skills by attending 1- to 2-day courses, which is insufficient supervision to become competent in this important procedure.
An ongoing debate continues as to how many supervised EBUS bronchoscopies should be performed prior to being considered proficient.2 As procedural competence has been associated with the number of EBUS procedures performed, the learning curve required to master EBUS is an important component of proficiency. While most consider learning curves to be variable, evidence produced by Fernandez-Villar and colleagues revealed that EBUS performance continues to improve up to 120 procedures.3 This analysis was performed in unselected consecutive patients based on diagnostic yield, procedure length, number of lymph nodes passes performed in order to obtain adequate samples, and the number of lymph nodes studied per patient. The learning curve was evaluated based on consecutive groups of 20 patients, the number of adequate samples obtained, and the diagnostic accuracy. Their results indicated that the diagnostic effectiveness of EBUS-TBNA improves with increasing number of procedures performed, allowing for access to a greater number of lymph nodes without necessarily increasing the length of the procedure, and by reducing the number of punctures at each nodal station. Based on their results, the first 20 procedures performed yielded a 70% accuracy, 21 to 40 procedures performed resulted in 81.8% accuracy, 41 to 60 procedures performed resulted in 83.3% accuracy, 61 to 80 procedures performed resulted in 89.8% accuracy, 81 to 100 procedures performed resulted in 90.5% accuracy, and 101 to 120 procedures performed resulted in 94.5% accuracy.
While the American Thoracic Society (ATS) and the American College of Chest Physicians (CHEST) both recommend a minimum number of 40 to 50 supervised EBUS bronchoscopies prior to performing the procedure independently, along with 20 procedures per year for maintenance of competency, most institutions do not track the number of EBUS procedures performed and they do not follow the ATS or CHEST recommendations.4,5 As a result, a number of physicians are independently performing EBUS without adequate experience, resulting in possibly poor quality care. Unfortunately, some short courses, intended to generate interest and encourage attendees to pursue further training, are mistakenly assumed to be sufficient by the novice user.
As the number of interventional pulmonary fellowships continues to expand, the growing number of subspecialized pulmonologists with extensive training in EBUS grows. During a dedicated interventional pulmonary fellowship, fellows perform well above the number of EBUS bronchoscopies suggested by the ATS and CHEST in a single year. Recently published accreditation guidelines require a minimum of 100 cases per interventional pulmonary fellow.6 These fellowship-trained interventional pulmonologists are then tested to become board-certified in a wide array of minimally invasive procedures, including EBUS. As a result, a model has developed where both board-certified interventional pulmonologists with extensive training in EBUS and general pulmonologists not meeting ATS or CHEST minimum requirements practice at the same institution. Proponents of a more liberal access to credentialing in EBUS have suggested that adhering to competency requirements constitutes a “barrier to entry” in which incumbent practitioners benefit from limiting competition. However, like any other regulatory metric, the rationale is to prevent asymmetric information. In this example, the physician knows more than the patient. The patient cannot make an informed decision on which provider to choose and what are the minimum requirements that are likely to produce the most useful information (ie, complete staging). For these reasons, it is imperative that regulations protect the patient.
Without question, EBUS bronchoscopy should not be performed only by board-certified interventional pulmonologists. Instead, hospital credentialing committees should adhere to both the ATS and CHEST recommendations for the number of supervised cases necessary prior to performing EBUS independently. As EBUS use continues to grow, fellows in 3- or 4-year pulmonary and critical care fellowships will be likely capable of meeting the minimal number of observed cases, but, if these numbers are not achieved, additional training should be required. Understandably, this could be challenging for physicians who are unable to take time away from their practice to gain this training. However, if these numbers cannot be met, credentialing requirements should be enforced.
Even more challenging than establishing quality measures for EBUS, is to ensure the highest level of care delivery for patients when there exist multiple levels of experience in the same institution. Undoubtedly, patients undergoing EBUS bronchoscopy, or any procedure for that matter, would want the most skilled physician who has attained certification in the procedure. Unfortunately, no formal certification of EBUS exists outside of gaining board certification in interventional pulmonology. To ensure excellence in care, physicians performing EBUS should be involved in quality improvement initiatives and review pathologic yields along with complications on a regular basis in a group setting. Unlike emergency interventions, EBUS bronchoscopy is an entirely elective procedure.
The advent of EBUS bronchoscopy has revolutionized the diagnosis and staging of lung cancer. As use of EBUS continues to become more widespread, the incidence of high volume and low volume proceduralists will become a more commonly encountered scenario. Guidelines have been set by the professional pulmonary societies based on the data and observations available. At the local level, stringent guidelines need to be established by hospitals to ensure a high level of quality with appropriate oversight. Patients undergoing EBUS deserve a physician who is skilled in the procedure and has performed at least the minimum number of procedures to provide the adequate care.
Dr. Mahajan is Medical Director, Interventional Pulmonology, Inova Heart and Vascular Institute - Inova Fairfax Hospital, and Associate Professor, Virginia Commonwealth Medical School; Dr. Khandhar is Medical Director, Thoracic Surgery, Inova Heart and Vascular Institute - Inova Fairfax Hospital, and Assistant Clinical Professor, Virginia Commonwealth Medical School; Falls Church, VA. Dr. Folch is Co-Director, Interventional Pulmonology Chief, Complex Chest Diseases Center, Harvard Medical School, Massachusetts General Hospital, Boston, MA.
References
1. Gomez M, Silvestri GA. Endobronchial ultrasound for the diagnosis and staging of lung cancer. Proc Am Thorac Soc. 2009;6(2):180-186.
2. Folch E, Majid A. Point: Are >50 Supervised Procedures Required to Develop Competency in Performing Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration for Mediastinal Staging? Yes. Chest. 2013;143(4):888-891.
3. Fernandez-Villar A, Leiro-Fernandez V, Botana-Rial M, Represas-Represas C, Nunez-Delgado M. The endobronchial ultrasound-guided transbronchial needle biopsy learning curve for mediastinal and hilar lymph node diagnosis. Chest. 2012; 141(1):278-279.
4. Ernst A, Silvestri GA, Johnstone D. Interventional pulmonary procedures: Guidelines from the American College of Chest Physicians. Chest. 2003;123(5):1693-1717.
5. Bolliger CT, Mathur PN, Beamis JF, et al. ERS/ATS statement on interventional pulmonology. European Respiratory Society/American Thoracic Society. Eur Respir J. 2002;19(2):356-373.
6. Mullon JJ, Burkhart KM, Silvestri G. Interventional Pulmonology Fellowship Accreditation Standards: Executive Summary of the Multi-society Interventional Pulmonology Fellowship Accreditation Committee. Chest. 2017. doi:10.1016/j.chest.2017.01.024.
Dr. Mahajan and colleagues present a compelling case for requiring minimum standards to perform an EBUS-guided bronchoscopy. Their opinion piece epitomizes the classic tension between physicians with advanced training and those who can only have practice-based training. A middle ground may exist, as perhaps competence could be achieved by simulation, clinical cases performed, and observation by a regional expert? Physicians in practice must have a pathway to adopt new technology whether it is thoracic ultrasound or endobronchial ultrasound, but it must be done in a safe manner. As a referring physician, I would only send my patients who required mediastinal staging to a pulmonologist who I knew performed EBUS regularly.
Nitin Puri, MD, FCCP
Endobronchial ultrasound (EBUS) bronchoscopy is a tool that has transformed the diagnosis and staging of lung cancer. Through real-time ultrasound imaging, EBUS provides clear images of lymph nodes and proximal lung masses that can be adequately sampled through transbronchial needle aspiration. EBUS is a minimally invasive, outpatient procedure that can also be used for diagnosing benign disease within the chest. Large studies investigating the use of EBUS for mediastinal staging have shown the procedure to be highly sensitive and specific while harboring an excellent safety profile.1 As a result, EBUS has essentially replaced mediastinoscopy for the staging of lung cancer.
EBUS bronchoscopy was primarily offered at major academic centers when first released and was performed by physicians who were formally trained in the procedure during interventional pulmonology or thoracic surgery fellowships. Over time, the tool has been adopted by established general pulmonologists without formal training in EBUS. Some of these pulmonologists only develop their skills by attending 1- to 2-day courses, which is insufficient supervision to become competent in this important procedure.
An ongoing debate continues as to how many supervised EBUS bronchoscopies should be performed prior to being considered proficient.2 As procedural competence has been associated with the number of EBUS procedures performed, the learning curve required to master EBUS is an important component of proficiency. While most consider learning curves to be variable, evidence produced by Fernandez-Villar and colleagues revealed that EBUS performance continues to improve up to 120 procedures.3 This analysis was performed in unselected consecutive patients based on diagnostic yield, procedure length, number of lymph nodes passes performed in order to obtain adequate samples, and the number of lymph nodes studied per patient. The learning curve was evaluated based on consecutive groups of 20 patients, the number of adequate samples obtained, and the diagnostic accuracy. Their results indicated that the diagnostic effectiveness of EBUS-TBNA improves with increasing number of procedures performed, allowing for access to a greater number of lymph nodes without necessarily increasing the length of the procedure, and by reducing the number of punctures at each nodal station. Based on their results, the first 20 procedures performed yielded a 70% accuracy, 21 to 40 procedures performed resulted in 81.8% accuracy, 41 to 60 procedures performed resulted in 83.3% accuracy, 61 to 80 procedures performed resulted in 89.8% accuracy, 81 to 100 procedures performed resulted in 90.5% accuracy, and 101 to 120 procedures performed resulted in 94.5% accuracy.
While the American Thoracic Society (ATS) and the American College of Chest Physicians (CHEST) both recommend a minimum number of 40 to 50 supervised EBUS bronchoscopies prior to performing the procedure independently, along with 20 procedures per year for maintenance of competency, most institutions do not track the number of EBUS procedures performed and they do not follow the ATS or CHEST recommendations.4,5 As a result, a number of physicians are independently performing EBUS without adequate experience, resulting in possibly poor quality care. Unfortunately, some short courses, intended to generate interest and encourage attendees to pursue further training, are mistakenly assumed to be sufficient by the novice user.
As the number of interventional pulmonary fellowships continues to expand, the growing number of subspecialized pulmonologists with extensive training in EBUS grows. During a dedicated interventional pulmonary fellowship, fellows perform well above the number of EBUS bronchoscopies suggested by the ATS and CHEST in a single year. Recently published accreditation guidelines require a minimum of 100 cases per interventional pulmonary fellow.6 These fellowship-trained interventional pulmonologists are then tested to become board-certified in a wide array of minimally invasive procedures, including EBUS. As a result, a model has developed where both board-certified interventional pulmonologists with extensive training in EBUS and general pulmonologists not meeting ATS or CHEST minimum requirements practice at the same institution. Proponents of a more liberal access to credentialing in EBUS have suggested that adhering to competency requirements constitutes a “barrier to entry” in which incumbent practitioners benefit from limiting competition. However, like any other regulatory metric, the rationale is to prevent asymmetric information. In this example, the physician knows more than the patient. The patient cannot make an informed decision on which provider to choose and what are the minimum requirements that are likely to produce the most useful information (ie, complete staging). For these reasons, it is imperative that regulations protect the patient.
Without question, EBUS bronchoscopy should not be performed only by board-certified interventional pulmonologists. Instead, hospital credentialing committees should adhere to both the ATS and CHEST recommendations for the number of supervised cases necessary prior to performing EBUS independently. As EBUS use continues to grow, fellows in 3- or 4-year pulmonary and critical care fellowships will be likely capable of meeting the minimal number of observed cases, but, if these numbers are not achieved, additional training should be required. Understandably, this could be challenging for physicians who are unable to take time away from their practice to gain this training. However, if these numbers cannot be met, credentialing requirements should be enforced.
Even more challenging than establishing quality measures for EBUS, is to ensure the highest level of care delivery for patients when there exist multiple levels of experience in the same institution. Undoubtedly, patients undergoing EBUS bronchoscopy, or any procedure for that matter, would want the most skilled physician who has attained certification in the procedure. Unfortunately, no formal certification of EBUS exists outside of gaining board certification in interventional pulmonology. To ensure excellence in care, physicians performing EBUS should be involved in quality improvement initiatives and review pathologic yields along with complications on a regular basis in a group setting. Unlike emergency interventions, EBUS bronchoscopy is an entirely elective procedure.
The advent of EBUS bronchoscopy has revolutionized the diagnosis and staging of lung cancer. As use of EBUS continues to become more widespread, the incidence of high volume and low volume proceduralists will become a more commonly encountered scenario. Guidelines have been set by the professional pulmonary societies based on the data and observations available. At the local level, stringent guidelines need to be established by hospitals to ensure a high level of quality with appropriate oversight. Patients undergoing EBUS deserve a physician who is skilled in the procedure and has performed at least the minimum number of procedures to provide the adequate care.
Dr. Mahajan is Medical Director, Interventional Pulmonology, Inova Heart and Vascular Institute - Inova Fairfax Hospital, and Associate Professor, Virginia Commonwealth Medical School; Dr. Khandhar is Medical Director, Thoracic Surgery, Inova Heart and Vascular Institute - Inova Fairfax Hospital, and Assistant Clinical Professor, Virginia Commonwealth Medical School; Falls Church, VA. Dr. Folch is Co-Director, Interventional Pulmonology Chief, Complex Chest Diseases Center, Harvard Medical School, Massachusetts General Hospital, Boston, MA.
References
1. Gomez M, Silvestri GA. Endobronchial ultrasound for the diagnosis and staging of lung cancer. Proc Am Thorac Soc. 2009;6(2):180-186.
2. Folch E, Majid A. Point: Are >50 Supervised Procedures Required to Develop Competency in Performing Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration for Mediastinal Staging? Yes. Chest. 2013;143(4):888-891.
3. Fernandez-Villar A, Leiro-Fernandez V, Botana-Rial M, Represas-Represas C, Nunez-Delgado M. The endobronchial ultrasound-guided transbronchial needle biopsy learning curve for mediastinal and hilar lymph node diagnosis. Chest. 2012; 141(1):278-279.
4. Ernst A, Silvestri GA, Johnstone D. Interventional pulmonary procedures: Guidelines from the American College of Chest Physicians. Chest. 2003;123(5):1693-1717.
5. Bolliger CT, Mathur PN, Beamis JF, et al. ERS/ATS statement on interventional pulmonology. European Respiratory Society/American Thoracic Society. Eur Respir J. 2002;19(2):356-373.
6. Mullon JJ, Burkhart KM, Silvestri G. Interventional Pulmonology Fellowship Accreditation Standards: Executive Summary of the Multi-society Interventional Pulmonology Fellowship Accreditation Committee. Chest. 2017. doi:10.1016/j.chest.2017.01.024.
Participate in CHEST Foundation’s NetWorks Challenge






Wait times for dermatologist visits up 3.5 days since 2014
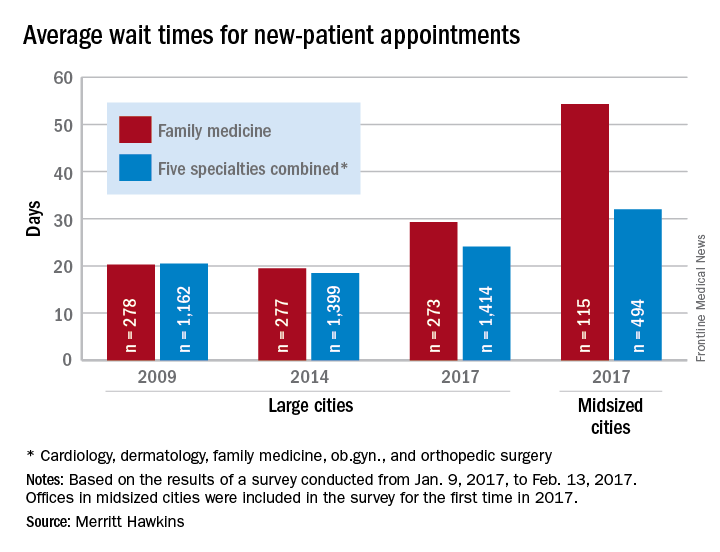
New patients are waiting 3.5 days longer for an appointment with a dermatologist in 2017 than they did in 2014, according to physician recruitment firm Merritt Hawkins.
The average wait time for a new patient to see a dermatologist for a “routine skin exam to detect possible carcinomas/melanomas” was 32.3 days in 2017, a 12.2% increase over the 28.8 days reported in 2014. Investigators called and made appointments with 286 randomly selected dermatologists in 15 large cities in January and February during the fourth such survey the company has conducted since 2004.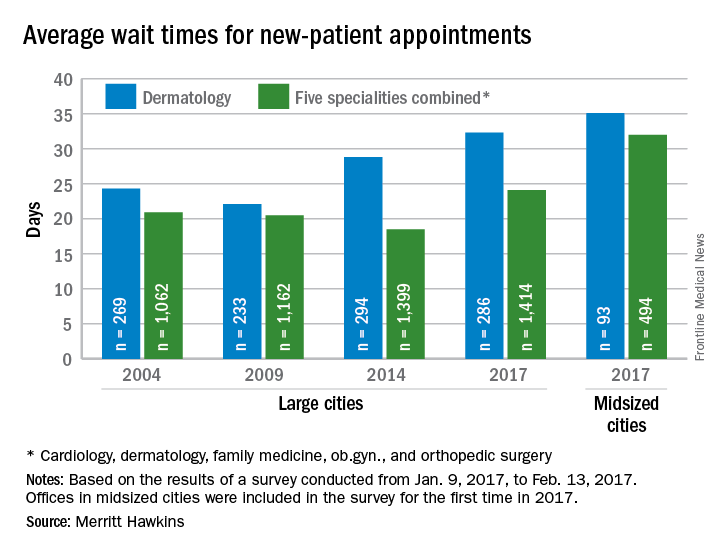
The survey also included four other specialties – cardiology, family medicine, obstetrics and gynecology, and orthopedic surgery – and the average wait time for a new-patient appointment for all 1,414 physicians in all five specialties in the 15 large cities was 24.1 days, an increase of 30% from 2014. The average wait time for all specialties in the midsized cities was 32 days for the 494 offices surveyed, the company said.
“Physician appointment wait times are the longest they have been since we began conducting the survey,” Mark Smith, president of Merritt Hawkins, said in a written statement. “Growing physician appointment wait times are a significant indicator that the nation is experiencing a shortage of physicians.”
New patients are waiting 3.5 days longer for an appointment with a dermatologist in 2017 than they did in 2014, according to physician recruitment firm Merritt Hawkins.
The average wait time for a new patient to see a dermatologist for a “routine skin exam to detect possible carcinomas/melanomas” was 32.3 days in 2017, a 12.2% increase over the 28.8 days reported in 2014. Investigators called and made appointments with 286 randomly selected dermatologists in 15 large cities in January and February during the fourth such survey the company has conducted since 2004.
The survey also included four other specialties – cardiology, family medicine, obstetrics and gynecology, and orthopedic surgery – and the average wait time for a new-patient appointment for all 1,414 physicians in all five specialties in the 15 large cities was 24.1 days, an increase of 30% from 2014. The average wait time for all specialties in the midsized cities was 32 days for the 494 offices surveyed, the company said.
“Physician appointment wait times are the longest they have been since we began conducting the survey,” Mark Smith, president of Merritt Hawkins, said in a written statement. “Growing physician appointment wait times are a significant indicator that the nation is experiencing a shortage of physicians.”
New patients are waiting 3.5 days longer for an appointment with a dermatologist in 2017 than they did in 2014, according to physician recruitment firm Merritt Hawkins.
The average wait time for a new patient to see a dermatologist for a “routine skin exam to detect possible carcinomas/melanomas” was 32.3 days in 2017, a 12.2% increase over the 28.8 days reported in 2014. Investigators called and made appointments with 286 randomly selected dermatologists in 15 large cities in January and February during the fourth such survey the company has conducted since 2004.
The survey also included four other specialties – cardiology, family medicine, obstetrics and gynecology, and orthopedic surgery – and the average wait time for a new-patient appointment for all 1,414 physicians in all five specialties in the 15 large cities was 24.1 days, an increase of 30% from 2014. The average wait time for all specialties in the midsized cities was 32 days for the 494 offices surveyed, the company said.
“Physician appointment wait times are the longest they have been since we began conducting the survey,” Mark Smith, president of Merritt Hawkins, said in a written statement. “Growing physician appointment wait times are a significant indicator that the nation is experiencing a shortage of physicians.”
Debunking Psoriasis Myths: Do Treatments for Psoriasis Cause Suicide?
Myth: Psoriasis Therapies Can Cause Suicidal Ideation in Psoriasis Patients
Psoriasis takes a toll on patients, both physically and emotionally. Depression is one of the comorbidities of psoriasis due to biological changes that cause psoriasis as well as the stigma of visible psoriasis. Severe depression and suicidal ideation have been perceived to be features of life-threatening medical disorders, but dermatologists need to be aware of the relationship between depressive symptoms, suicidal ideation, and psoriasis severity.
A 2010 United Kingdom study of 916,948 patients with mild psoriasis, severe psoriasis, or controls without psoriasis indicated that patients with psoriasis have an increased risk for depression, anxiety, and suicidality. The relative risk of these outcomes is elevated in younger patients with psoriasis, with the greatest relative risk being for depression in patients with severe psoriasis.
Kimball et al conducted a study in the United States of 7404 patients with psoriasis and 37,020 controls without psoriasis (age, <18 years). They reported that pediatric patients with psoriasis were significantly more at risk of developing psychiatric disorders versus controls (P=.0001), especially depression (P=.0036) and anxiety (P=.0048).
In February 2017, the US Food and Drug Administration (FDA) announced approval of brodalumab for use in adults with moderate to severe plaque psoriasis. It is intended for patients who are candidates for systemic therapy or phototherapy but have failed to respond or have stopped responding to other systemic therapies. Lebwohl et al published the results of the phase 3 clinical trials, which showed that brodalumab was highly effective in reducing plaque psoriasis, even compared to ustekinumab. In fact, psoriasis area and severity index scores of 100 were significantly higher in the brodalumab 210-mg group versus ustekinumab group by week 12 (P<.001).
However, the approval is accompanied with a strict warning from the FDA and tightly regulated access to the drug, as suicidal ideation and behavior, including 4 suicides, occurred in patients treated with brodalumab during clinical trials, particularly patients with a history of depression or suicidality. According to the FDA, "[a] causal association between treatment with [brodalumab] and increased risk of suicidal ideation and behavior has not been established." The label includes a black box warning and the drug will only be available through a restricted Risk Evaluation and Mitigation Strategy program, which has the following requirements from the FDA:
- Prescribers must be certified with the program and counsel patients about this risk. Patients with new or worsening symptoms of depression or suicidality should be referred to a mental health professional, as appropriate.
- Patients must sign a Patient-Prescriber Agreement Form and be made aware of the need to seek medical attention should they experience new or worsening suicidal thoughts or behavior, feelings of depression, anxiety, or other mood changes.
- Pharmacies must be certified with the program and must only dispense to patients who are authorized to receive the drug.
A medication guide is available for patients to inform them of the risk for suicidal ideation and behavior. The benefit of treatment must be weighed carefully against the seriousness of the risks associated with use.
Regardless of the therapy prescribed, dermatologists should be aware of the symptoms of depression. The National Psoriasis Foundation suggests you ask patients how they dress: Do they always wear long-sleeved shirts when they leave the house? Do they wear black? These questions can help determine if patients feel socially isolated or stigmatized by the disease. The National Psoriasis Foundation offers a Patient Navigation Center to help patients find a psychologist who specializes in issues related to psoriatic disease. Antidepressants and seeing a mental health professional can help, but ultimately taking control of the disease is the best way to improve depression.
Expert Commentary
According to the prescribing information for brodalumab, "Eight of the 10 subjects who attempted or completed suicide had a history of depression and/or suicidal ideation or behavior." Thus, 80% of these cases were at risk even before receiving 1 injection of brodalumab. Long-term registries will determine if there is truly an increased risk for suicidal ideation or behavior when taking brodalumab.
Brodalumab will be commercially available around the fall 2017. Before prescribing brodalumab, I will counsel patients about this potential increased risk of suicidal ideation or behavior as noted in the prescribing information, but I will tell them that a true risk has not yet been determined in long-term registries. I will mention to patients that if they really do feel depressed or experience suicidal ideation or behavior after starting brodalumab, they should stop taking brodalumab and contact me or a mental health professional.
—Jashin J. Wu, MD (Los Angeles, California)
FDA approves new psoriasis drug [news release]. Silver Spring, MD: US Food and Drug Administration; February 15, 2017. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm541981.htm. Accessed April 5, 2017.
Gupta MA, Schork NJ, Gupta AK, et al. Suicidal ideation in psoriasis. Int J Dermatol. 1993;32:188-190.
Kimball AB, Wu EQ, Guérin A, et al. Risks of developing psychiatric disorders in pediatric patients with psoriasis. J Am Acad Dermatol. 2012;67:651-7.e1-651-7.e2.
Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010;146:891-895.
Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328.
Life with psoriasis: depression. National Psoriasis Foundation website. https://www.psoriasis.org/life-with-psoriasis/depression. Accessed April 5, 2017.
Özkaya Ö. Biologic psoriasis treatment, Siliq, approved by FDA with strong warning of possible suicide risk. https://psoriasisnewstoday.com/2017/02/16/psoriasis-drug-siliq-approved-by-fda-with-warning-of-possible-suicide-risk/. Published February 16, 2017. Accessed April 5, 2017.
Myth: Psoriasis Therapies Can Cause Suicidal Ideation in Psoriasis Patients
Psoriasis takes a toll on patients, both physically and emotionally. Depression is one of the comorbidities of psoriasis due to biological changes that cause psoriasis as well as the stigma of visible psoriasis. Severe depression and suicidal ideation have been perceived to be features of life-threatening medical disorders, but dermatologists need to be aware of the relationship between depressive symptoms, suicidal ideation, and psoriasis severity.
A 2010 United Kingdom study of 916,948 patients with mild psoriasis, severe psoriasis, or controls without psoriasis indicated that patients with psoriasis have an increased risk for depression, anxiety, and suicidality. The relative risk of these outcomes is elevated in younger patients with psoriasis, with the greatest relative risk being for depression in patients with severe psoriasis.
Kimball et al conducted a study in the United States of 7404 patients with psoriasis and 37,020 controls without psoriasis (age, <18 years). They reported that pediatric patients with psoriasis were significantly more at risk of developing psychiatric disorders versus controls (P=.0001), especially depression (P=.0036) and anxiety (P=.0048).
In February 2017, the US Food and Drug Administration (FDA) announced approval of brodalumab for use in adults with moderate to severe plaque psoriasis. It is intended for patients who are candidates for systemic therapy or phototherapy but have failed to respond or have stopped responding to other systemic therapies. Lebwohl et al published the results of the phase 3 clinical trials, which showed that brodalumab was highly effective in reducing plaque psoriasis, even compared to ustekinumab. In fact, psoriasis area and severity index scores of 100 were significantly higher in the brodalumab 210-mg group versus ustekinumab group by week 12 (P<.001).
However, the approval is accompanied with a strict warning from the FDA and tightly regulated access to the drug, as suicidal ideation and behavior, including 4 suicides, occurred in patients treated with brodalumab during clinical trials, particularly patients with a history of depression or suicidality. According to the FDA, "[a] causal association between treatment with [brodalumab] and increased risk of suicidal ideation and behavior has not been established." The label includes a black box warning and the drug will only be available through a restricted Risk Evaluation and Mitigation Strategy program, which has the following requirements from the FDA:
- Prescribers must be certified with the program and counsel patients about this risk. Patients with new or worsening symptoms of depression or suicidality should be referred to a mental health professional, as appropriate.
- Patients must sign a Patient-Prescriber Agreement Form and be made aware of the need to seek medical attention should they experience new or worsening suicidal thoughts or behavior, feelings of depression, anxiety, or other mood changes.
- Pharmacies must be certified with the program and must only dispense to patients who are authorized to receive the drug.
A medication guide is available for patients to inform them of the risk for suicidal ideation and behavior. The benefit of treatment must be weighed carefully against the seriousness of the risks associated with use.
Regardless of the therapy prescribed, dermatologists should be aware of the symptoms of depression. The National Psoriasis Foundation suggests you ask patients how they dress: Do they always wear long-sleeved shirts when they leave the house? Do they wear black? These questions can help determine if patients feel socially isolated or stigmatized by the disease. The National Psoriasis Foundation offers a Patient Navigation Center to help patients find a psychologist who specializes in issues related to psoriatic disease. Antidepressants and seeing a mental health professional can help, but ultimately taking control of the disease is the best way to improve depression.
Expert Commentary
According to the prescribing information for brodalumab, "Eight of the 10 subjects who attempted or completed suicide had a history of depression and/or suicidal ideation or behavior." Thus, 80% of these cases were at risk even before receiving 1 injection of brodalumab. Long-term registries will determine if there is truly an increased risk for suicidal ideation or behavior when taking brodalumab.
Brodalumab will be commercially available around the fall 2017. Before prescribing brodalumab, I will counsel patients about this potential increased risk of suicidal ideation or behavior as noted in the prescribing information, but I will tell them that a true risk has not yet been determined in long-term registries. I will mention to patients that if they really do feel depressed or experience suicidal ideation or behavior after starting brodalumab, they should stop taking brodalumab and contact me or a mental health professional.
—Jashin J. Wu, MD (Los Angeles, California)
Myth: Psoriasis Therapies Can Cause Suicidal Ideation in Psoriasis Patients
Psoriasis takes a toll on patients, both physically and emotionally. Depression is one of the comorbidities of psoriasis due to biological changes that cause psoriasis as well as the stigma of visible psoriasis. Severe depression and suicidal ideation have been perceived to be features of life-threatening medical disorders, but dermatologists need to be aware of the relationship between depressive symptoms, suicidal ideation, and psoriasis severity.
A 2010 United Kingdom study of 916,948 patients with mild psoriasis, severe psoriasis, or controls without psoriasis indicated that patients with psoriasis have an increased risk for depression, anxiety, and suicidality. The relative risk of these outcomes is elevated in younger patients with psoriasis, with the greatest relative risk being for depression in patients with severe psoriasis.
Kimball et al conducted a study in the United States of 7404 patients with psoriasis and 37,020 controls without psoriasis (age, <18 years). They reported that pediatric patients with psoriasis were significantly more at risk of developing psychiatric disorders versus controls (P=.0001), especially depression (P=.0036) and anxiety (P=.0048).
In February 2017, the US Food and Drug Administration (FDA) announced approval of brodalumab for use in adults with moderate to severe plaque psoriasis. It is intended for patients who are candidates for systemic therapy or phototherapy but have failed to respond or have stopped responding to other systemic therapies. Lebwohl et al published the results of the phase 3 clinical trials, which showed that brodalumab was highly effective in reducing plaque psoriasis, even compared to ustekinumab. In fact, psoriasis area and severity index scores of 100 were significantly higher in the brodalumab 210-mg group versus ustekinumab group by week 12 (P<.001).
However, the approval is accompanied with a strict warning from the FDA and tightly regulated access to the drug, as suicidal ideation and behavior, including 4 suicides, occurred in patients treated with brodalumab during clinical trials, particularly patients with a history of depression or suicidality. According to the FDA, "[a] causal association between treatment with [brodalumab] and increased risk of suicidal ideation and behavior has not been established." The label includes a black box warning and the drug will only be available through a restricted Risk Evaluation and Mitigation Strategy program, which has the following requirements from the FDA:
- Prescribers must be certified with the program and counsel patients about this risk. Patients with new or worsening symptoms of depression or suicidality should be referred to a mental health professional, as appropriate.
- Patients must sign a Patient-Prescriber Agreement Form and be made aware of the need to seek medical attention should they experience new or worsening suicidal thoughts or behavior, feelings of depression, anxiety, or other mood changes.
- Pharmacies must be certified with the program and must only dispense to patients who are authorized to receive the drug.
A medication guide is available for patients to inform them of the risk for suicidal ideation and behavior. The benefit of treatment must be weighed carefully against the seriousness of the risks associated with use.
Regardless of the therapy prescribed, dermatologists should be aware of the symptoms of depression. The National Psoriasis Foundation suggests you ask patients how they dress: Do they always wear long-sleeved shirts when they leave the house? Do they wear black? These questions can help determine if patients feel socially isolated or stigmatized by the disease. The National Psoriasis Foundation offers a Patient Navigation Center to help patients find a psychologist who specializes in issues related to psoriatic disease. Antidepressants and seeing a mental health professional can help, but ultimately taking control of the disease is the best way to improve depression.
Expert Commentary
According to the prescribing information for brodalumab, "Eight of the 10 subjects who attempted or completed suicide had a history of depression and/or suicidal ideation or behavior." Thus, 80% of these cases were at risk even before receiving 1 injection of brodalumab. Long-term registries will determine if there is truly an increased risk for suicidal ideation or behavior when taking brodalumab.
Brodalumab will be commercially available around the fall 2017. Before prescribing brodalumab, I will counsel patients about this potential increased risk of suicidal ideation or behavior as noted in the prescribing information, but I will tell them that a true risk has not yet been determined in long-term registries. I will mention to patients that if they really do feel depressed or experience suicidal ideation or behavior after starting brodalumab, they should stop taking brodalumab and contact me or a mental health professional.
—Jashin J. Wu, MD (Los Angeles, California)
FDA approves new psoriasis drug [news release]. Silver Spring, MD: US Food and Drug Administration; February 15, 2017. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm541981.htm. Accessed April 5, 2017.
Gupta MA, Schork NJ, Gupta AK, et al. Suicidal ideation in psoriasis. Int J Dermatol. 1993;32:188-190.
Kimball AB, Wu EQ, Guérin A, et al. Risks of developing psychiatric disorders in pediatric patients with psoriasis. J Am Acad Dermatol. 2012;67:651-7.e1-651-7.e2.
Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010;146:891-895.
Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328.
Life with psoriasis: depression. National Psoriasis Foundation website. https://www.psoriasis.org/life-with-psoriasis/depression. Accessed April 5, 2017.
Özkaya Ö. Biologic psoriasis treatment, Siliq, approved by FDA with strong warning of possible suicide risk. https://psoriasisnewstoday.com/2017/02/16/psoriasis-drug-siliq-approved-by-fda-with-warning-of-possible-suicide-risk/. Published February 16, 2017. Accessed April 5, 2017.
FDA approves new psoriasis drug [news release]. Silver Spring, MD: US Food and Drug Administration; February 15, 2017. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm541981.htm. Accessed April 5, 2017.
Gupta MA, Schork NJ, Gupta AK, et al. Suicidal ideation in psoriasis. Int J Dermatol. 1993;32:188-190.
Kimball AB, Wu EQ, Guérin A, et al. Risks of developing psychiatric disorders in pediatric patients with psoriasis. J Am Acad Dermatol. 2012;67:651-7.e1-651-7.e2.
Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010;146:891-895.
Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328.
Life with psoriasis: depression. National Psoriasis Foundation website. https://www.psoriasis.org/life-with-psoriasis/depression. Accessed April 5, 2017.
Özkaya Ö. Biologic psoriasis treatment, Siliq, approved by FDA with strong warning of possible suicide risk. https://psoriasisnewstoday.com/2017/02/16/psoriasis-drug-siliq-approved-by-fda-with-warning-of-possible-suicide-risk/. Published February 16, 2017. Accessed April 5, 2017.
Wait times for ob.gyn. visits up by 9 days since 2014
New patients are waiting 9 days longer for an appointment with an ob.gyn. in 2017 than they did in 2014, according to physician recruitment firm Merritt Hawkins.
The average wait time for a new patient to see an ob.gyn. for a routine gynecologic exam was 26.4 days in 2017, a nearly 53% increase from the 17.3 days reported in 2014. Investigators called and made appointments with 286 randomly-selected ob.gyns. in 15 large cities in January and February during the fourth such survey the company has conducted since 2004.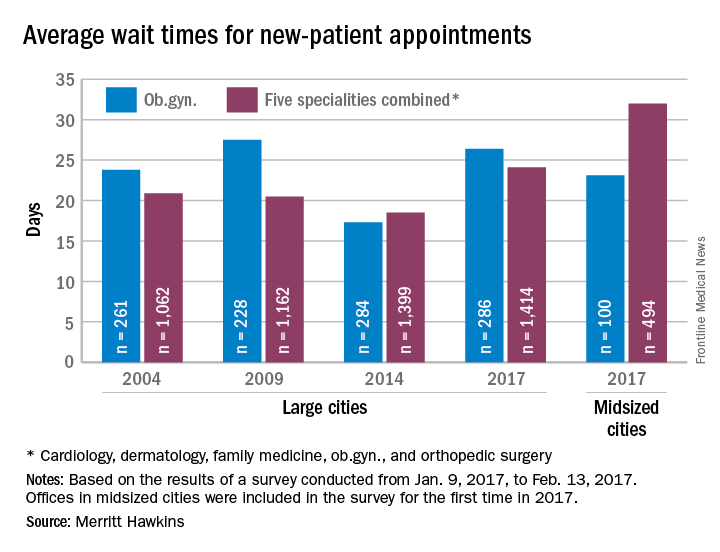
The survey also included four other specialties – cardiology, dermatology, family medicine, and orthopedic surgery – and the average wait time for a new-patient appointment for all 1,414 physicians in all five specialties in the 15 large cities was 24.1 days, an increase of 30% over 2014. The average wait time for all specialties in the mid-sized cities was 32 days for the 494 offices surveyed, the company said.
“Physician appointment wait times are the longest they have been since we began conducting the survey,” Mark Smith, president of Merritt Hawkins, said in a statement. “Growing physician appointment wait times are a significant indicator that the nation is experiencing a shortage of physicians.”
New patients are waiting 9 days longer for an appointment with an ob.gyn. in 2017 than they did in 2014, according to physician recruitment firm Merritt Hawkins.
The average wait time for a new patient to see an ob.gyn. for a routine gynecologic exam was 26.4 days in 2017, a nearly 53% increase from the 17.3 days reported in 2014. Investigators called and made appointments with 286 randomly-selected ob.gyns. in 15 large cities in January and February during the fourth such survey the company has conducted since 2004.
The survey also included four other specialties – cardiology, dermatology, family medicine, and orthopedic surgery – and the average wait time for a new-patient appointment for all 1,414 physicians in all five specialties in the 15 large cities was 24.1 days, an increase of 30% over 2014. The average wait time for all specialties in the mid-sized cities was 32 days for the 494 offices surveyed, the company said.
“Physician appointment wait times are the longest they have been since we began conducting the survey,” Mark Smith, president of Merritt Hawkins, said in a statement. “Growing physician appointment wait times are a significant indicator that the nation is experiencing a shortage of physicians.”
New patients are waiting 9 days longer for an appointment with an ob.gyn. in 2017 than they did in 2014, according to physician recruitment firm Merritt Hawkins.
The average wait time for a new patient to see an ob.gyn. for a routine gynecologic exam was 26.4 days in 2017, a nearly 53% increase from the 17.3 days reported in 2014. Investigators called and made appointments with 286 randomly-selected ob.gyns. in 15 large cities in January and February during the fourth such survey the company has conducted since 2004.
The survey also included four other specialties – cardiology, dermatology, family medicine, and orthopedic surgery – and the average wait time for a new-patient appointment for all 1,414 physicians in all five specialties in the 15 large cities was 24.1 days, an increase of 30% over 2014. The average wait time for all specialties in the mid-sized cities was 32 days for the 494 offices surveyed, the company said.
“Physician appointment wait times are the longest they have been since we began conducting the survey,” Mark Smith, president of Merritt Hawkins, said in a statement. “Growing physician appointment wait times are a significant indicator that the nation is experiencing a shortage of physicians.”
This month in CHEST: Editor’s picks
Original Research
Clinical Predictors of Hospital Mortality Differ Between Direct and Indirect ARDS. By Dr. L. Luo, et al.
Giants in Chest Medicine
Professor James C. Hogg. By Dr. Manuel G. Cosio.
Commentary
Pulmonary Hypertension Care Center Network: Improving Care and Outcomes in Pulmonary Hypertension. By Dr. S. Sahay, et al.
Evidence-Based Medicine
Use of Management Pathways or Algorithms in Children With Chronic Cough: CHEST Guideline and Expert Panel Report. By Dr. A. B. Chang, et al; on behalf of the CHEST Expert Cough Panel.
Symptomatic Treatment of Cough Among Adult Patients With Lung Cancer: CHEST Guideline and Expert Panel Report. By Dr. A. Molassiotis, et al; on behalf of the CHEST Expert Cough Panel.
Management of Children With Chronic Wet Cough and Protracted Bacterial Bronchitis: CHEST Guideline and Expert Panel Report. By Dr. A. B. Chang, et al; on behalf of the CHEST Expert Cough Panel.
Original Research
Clinical Predictors of Hospital Mortality Differ Between Direct and Indirect ARDS. By Dr. L. Luo, et al.
Giants in Chest Medicine
Professor James C. Hogg. By Dr. Manuel G. Cosio.
Commentary
Pulmonary Hypertension Care Center Network: Improving Care and Outcomes in Pulmonary Hypertension. By Dr. S. Sahay, et al.
Evidence-Based Medicine
Use of Management Pathways or Algorithms in Children With Chronic Cough: CHEST Guideline and Expert Panel Report. By Dr. A. B. Chang, et al; on behalf of the CHEST Expert Cough Panel.
Symptomatic Treatment of Cough Among Adult Patients With Lung Cancer: CHEST Guideline and Expert Panel Report. By Dr. A. Molassiotis, et al; on behalf of the CHEST Expert Cough Panel.
Management of Children With Chronic Wet Cough and Protracted Bacterial Bronchitis: CHEST Guideline and Expert Panel Report. By Dr. A. B. Chang, et al; on behalf of the CHEST Expert Cough Panel.
Original Research
Clinical Predictors of Hospital Mortality Differ Between Direct and Indirect ARDS. By Dr. L. Luo, et al.
Giants in Chest Medicine
Professor James C. Hogg. By Dr. Manuel G. Cosio.
Commentary
Pulmonary Hypertension Care Center Network: Improving Care and Outcomes in Pulmonary Hypertension. By Dr. S. Sahay, et al.
Evidence-Based Medicine
Use of Management Pathways or Algorithms in Children With Chronic Cough: CHEST Guideline and Expert Panel Report. By Dr. A. B. Chang, et al; on behalf of the CHEST Expert Cough Panel.
Symptomatic Treatment of Cough Among Adult Patients With Lung Cancer: CHEST Guideline and Expert Panel Report. By Dr. A. Molassiotis, et al; on behalf of the CHEST Expert Cough Panel.
Management of Children With Chronic Wet Cough and Protracted Bacterial Bronchitis: CHEST Guideline and Expert Panel Report. By Dr. A. B. Chang, et al; on behalf of the CHEST Expert Cough Panel.
NetWorks: Uranium mining, hyperoxia, palliative care education, OSA impact
Health effects of uranium mining
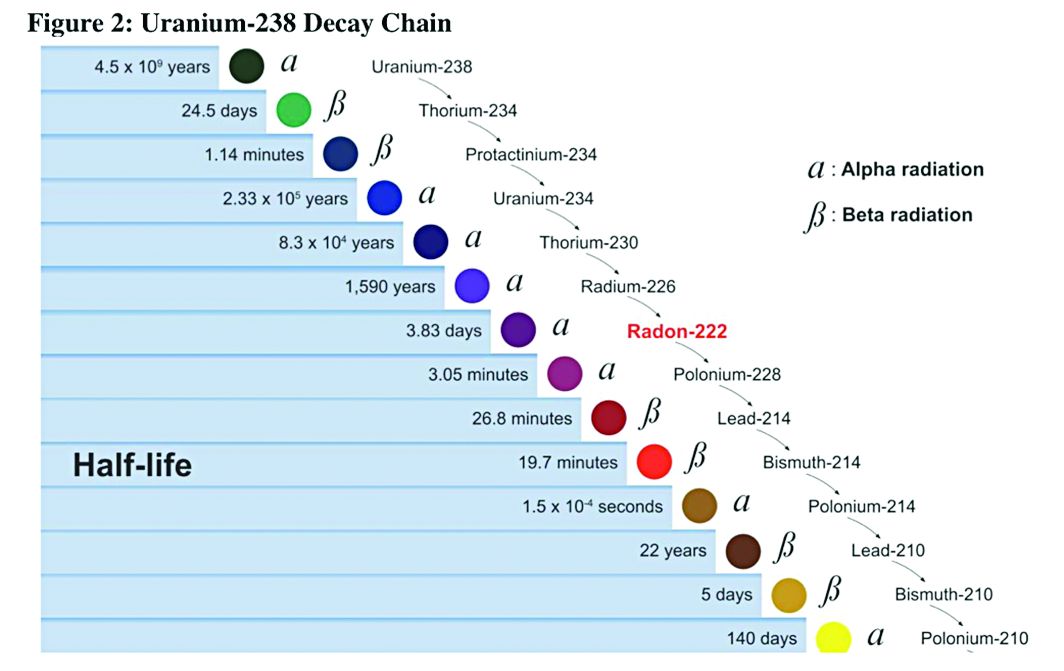
Decay series of U 238
Prior to 1900, uranium was used only for coloring glass. After discovery of radium by Madame Curie in 1898, uranium was widely mined to obtain radium (a decay product of uranium).
While uranium was not directly mined until 1900, uranium contaminates were in the ore in silver and cobalt mines in Czechoslovakia, which were heavily mined in the 18th and 19th centuries.
There were no reports (written in English) of lung cancer associated with radiation until 1942; but in 1944, these results were called into question in a monograph from the National Cancer Institute. The carcinogenicity of radon was confirmed in 1951; however, this remained an internal government document until 1980. By 1967, the increased prevalence of lung cancer in uranium miners was widely known. By 1970, new ventilation standards for uranium mines were established.
Lung cancer risk associated with uranium mining is the result of exposure to radon gas and specifically radon progeny of Polonium 218 and 210. These radon progeny remain suspended in air, attached to ambient particles (diesel exhaust, silica) and are then inhaled into the lung, where they tend to precipitate on the major airways. Polonium 218 and 210 are alpha emitters, which have a 20-fold increase in energy compared with gamma rays (the primary radiation source in radiation therapy). Given the mass of alpha particles (two protons and two neutrons), they interact with superficial tissues; thus, once deposited in the large airways, a large radiation dose is directed to the respiratory epithelium of these airways.
Occupational control of exposure to radon and radon progeny is accomplished primarily by ventilation. In high-grade deposits of uranium, such as the 20% ore grades in the Athabasca Basin of Saskatchewan, remote control mining is performed.
Smoking, in combination with occupational exposure to radon progeny, carries a greater than additive but less than multiplicative risk of lung cancer.
In addition to the lung cancer risk associated with radon progeny exposure, uranium miners share the occupational risks of other miners: exposure to silica and diesel exhaust. Miners are also at risk for traumatic injuries, including electrocution.
Health effects associated with uranium milling, enrichment, and tailings will be discussed in a subsequent CHEST Physician article.
Richard B. Evans, MD, MPH, FCCP
Steering Committee Chair
Hyperoxia in critically ill patients: What’s the verdict?
Oxygen saturation is considered to be the “fifth vital sign,” and current guidelines recommend target oxygen saturation (SpO2) between 94% and 98%, with lower targets for patients at risk for hypercapnic respiratory failure (O’Driscoll BR et al. Thorax. 2008;63(suppl):vi1). Oxygen toxicity is well-demonstrated in experimental animal studies. While its incidence and impact on outcomes is difficult to determine in the clinical setting, increases in-hospital mortality have been associated with hyperoxia in patients with cardiac arrest, acute myocardial infarction, and stroke (Kligannon et al. JAMA. 2010;303[21]:2165; Stub et al. Circulation. 2015;131[24]:2143; Rincon et al. Crit Care Med. 2014;42[2]:387).
Complementing the findings of Girardis and colleagues, a recent analysis of more than 14,000 critically ill patients, found that time spent at PaO2 > 200 mm Hg was associated with excess mortality and fewer ventilator-free days (Helmerhorst et al. Crit Care Med. 2017;45[2]:187).
While other trials demonstrated safety and feasibility of conservative oxygen therapy in critically ill patients (Panwar et al. Am J Respir Crit Care Med. 2016;193[1]:43; Helmerhorst et al. Crit Care Med. 2016; 44[3]:554; Suzuki et al. Crit Care Med. 2014;42[6]:1414), they did not find significant differences between conservative and liberal oxygen therapy with regards to new organ dysfunction or mortality. However, the degree of hyperoxia was usually more modest than in either the Girardis trial or the Helmerhorst (2017) analysis.
Amanpreet Kaur, MD
Steering Committee Fellow-in-Training
David L. Bowton, MD, FCCP
Steering Committee Chair
Education in palliative medicine
Prompted by concerns that the Affordable Care Act would be instituting “death panels” as part of cost-containment measures, “Dying in America” (a 2015 report of the Institute of Medicine [IOM]) identified compassionate, affordable, and effective care for patients at the end of their lives as a “national priority” in American health care. The IOM identified the education of all primary care providers in the delivery of basic palliative care, specifically commenting that all clinicians who manage patients with serious, life-threatening illnesses should be “competent in basic palliative care” (IOM, The National Academies Press 2015).
Check out our NetWork Storify page later this year for links to the ongoing discussion surrounding palliative care in medicine and for useful tools in the effort to provide palliative care to all our patients.
Laura Johnson, MD, FCCP
Steering Committee Vice Chair
The impact of sleep apnea: Why should we care?
With recent large trials such as the SAVE and the SERVE-HF studies challenging the cardiovascular benefits of treating sleep-disordered breathing in specific patient subsets, many physicians may start to question, “Why all the fuss?” The Sleep NetWork is bringing the leaders in the field to CHEST 2017 to discuss their take on where we stand with the connection between sleep-disordered breathing and cardiovascular disease, so stay tuned!
Our relationships, general health, and work productivity can be affected by untreated OSA. The effect on daily life may not be initially obvious. Patients often present only at the insistence of their partner or physician, only to be surprised at how much better they feel once treated. Symptoms of OSA are associated with a higher rate of impaired work performance, sick leave, and divorce (Grunstein et al. Sleep. 1995;18[8]:635). A recent survey estimates an $86.9 billion loss of workplace productivity due to sleep apnea in 2015 (Frost & Sullivan. Hidden health crisis costing America billions. AASM; 2016. http://www.aasmnet.org/Resources/pdf/sleep-apnea-economic-crisis.pdf. Accessed March 21, 2017.). The same survey found that among those who are employed, treating OSA was associated with a decline in absences by 1.8 days per year and an increase in productivity 17.3% on average. Considering that the majority of OSA remains undiagnosed, this could have tremendous economic impact.
OSA is an important public health burden. The Sleep NetWork is committed to increasing awareness among individuals (patients and clinicians) and institutions (transportation agencies, government) of the impact of sleep-disordered breathing on society.
Aneesa Das, MD, FCCP
Steering Committee Chair
Health effects of uranium mining
Decay series of U 238
Prior to 1900, uranium was used only for coloring glass. After discovery of radium by Madame Curie in 1898, uranium was widely mined to obtain radium (a decay product of uranium).
While uranium was not directly mined until 1900, uranium contaminates were in the ore in silver and cobalt mines in Czechoslovakia, which were heavily mined in the 18th and 19th centuries.
There were no reports (written in English) of lung cancer associated with radiation until 1942; but in 1944, these results were called into question in a monograph from the National Cancer Institute. The carcinogenicity of radon was confirmed in 1951; however, this remained an internal government document until 1980. By 1967, the increased prevalence of lung cancer in uranium miners was widely known. By 1970, new ventilation standards for uranium mines were established.
Lung cancer risk associated with uranium mining is the result of exposure to radon gas and specifically radon progeny of Polonium 218 and 210. These radon progeny remain suspended in air, attached to ambient particles (diesel exhaust, silica) and are then inhaled into the lung, where they tend to precipitate on the major airways. Polonium 218 and 210 are alpha emitters, which have a 20-fold increase in energy compared with gamma rays (the primary radiation source in radiation therapy). Given the mass of alpha particles (two protons and two neutrons), they interact with superficial tissues; thus, once deposited in the large airways, a large radiation dose is directed to the respiratory epithelium of these airways.
Occupational control of exposure to radon and radon progeny is accomplished primarily by ventilation. In high-grade deposits of uranium, such as the 20% ore grades in the Athabasca Basin of Saskatchewan, remote control mining is performed.
Smoking, in combination with occupational exposure to radon progeny, carries a greater than additive but less than multiplicative risk of lung cancer.
In addition to the lung cancer risk associated with radon progeny exposure, uranium miners share the occupational risks of other miners: exposure to silica and diesel exhaust. Miners are also at risk for traumatic injuries, including electrocution.
Health effects associated with uranium milling, enrichment, and tailings will be discussed in a subsequent CHEST Physician article.
Richard B. Evans, MD, MPH, FCCP
Steering Committee Chair
Hyperoxia in critically ill patients: What’s the verdict?
Oxygen saturation is considered to be the “fifth vital sign,” and current guidelines recommend target oxygen saturation (SpO2) between 94% and 98%, with lower targets for patients at risk for hypercapnic respiratory failure (O’Driscoll BR et al. Thorax. 2008;63(suppl):vi1). Oxygen toxicity is well-demonstrated in experimental animal studies. While its incidence and impact on outcomes is difficult to determine in the clinical setting, increases in-hospital mortality have been associated with hyperoxia in patients with cardiac arrest, acute myocardial infarction, and stroke (Kligannon et al. JAMA. 2010;303[21]:2165; Stub et al. Circulation. 2015;131[24]:2143; Rincon et al. Crit Care Med. 2014;42[2]:387).
Complementing the findings of Girardis and colleagues, a recent analysis of more than 14,000 critically ill patients, found that time spent at PaO2 > 200 mm Hg was associated with excess mortality and fewer ventilator-free days (Helmerhorst et al. Crit Care Med. 2017;45[2]:187).
While other trials demonstrated safety and feasibility of conservative oxygen therapy in critically ill patients (Panwar et al. Am J Respir Crit Care Med. 2016;193[1]:43; Helmerhorst et al. Crit Care Med. 2016; 44[3]:554; Suzuki et al. Crit Care Med. 2014;42[6]:1414), they did not find significant differences between conservative and liberal oxygen therapy with regards to new organ dysfunction or mortality. However, the degree of hyperoxia was usually more modest than in either the Girardis trial or the Helmerhorst (2017) analysis.
Amanpreet Kaur, MD
Steering Committee Fellow-in-Training
David L. Bowton, MD, FCCP
Steering Committee Chair
Education in palliative medicine
Prompted by concerns that the Affordable Care Act would be instituting “death panels” as part of cost-containment measures, “Dying in America” (a 2015 report of the Institute of Medicine [IOM]) identified compassionate, affordable, and effective care for patients at the end of their lives as a “national priority” in American health care. The IOM identified the education of all primary care providers in the delivery of basic palliative care, specifically commenting that all clinicians who manage patients with serious, life-threatening illnesses should be “competent in basic palliative care” (IOM, The National Academies Press 2015).
Check out our NetWork Storify page later this year for links to the ongoing discussion surrounding palliative care in medicine and for useful tools in the effort to provide palliative care to all our patients.
Laura Johnson, MD, FCCP
Steering Committee Vice Chair
The impact of sleep apnea: Why should we care?
With recent large trials such as the SAVE and the SERVE-HF studies challenging the cardiovascular benefits of treating sleep-disordered breathing in specific patient subsets, many physicians may start to question, “Why all the fuss?” The Sleep NetWork is bringing the leaders in the field to CHEST 2017 to discuss their take on where we stand with the connection between sleep-disordered breathing and cardiovascular disease, so stay tuned!
Our relationships, general health, and work productivity can be affected by untreated OSA. The effect on daily life may not be initially obvious. Patients often present only at the insistence of their partner or physician, only to be surprised at how much better they feel once treated. Symptoms of OSA are associated with a higher rate of impaired work performance, sick leave, and divorce (Grunstein et al. Sleep. 1995;18[8]:635). A recent survey estimates an $86.9 billion loss of workplace productivity due to sleep apnea in 2015 (Frost & Sullivan. Hidden health crisis costing America billions. AASM; 2016. http://www.aasmnet.org/Resources/pdf/sleep-apnea-economic-crisis.pdf. Accessed March 21, 2017.). The same survey found that among those who are employed, treating OSA was associated with a decline in absences by 1.8 days per year and an increase in productivity 17.3% on average. Considering that the majority of OSA remains undiagnosed, this could have tremendous economic impact.
OSA is an important public health burden. The Sleep NetWork is committed to increasing awareness among individuals (patients and clinicians) and institutions (transportation agencies, government) of the impact of sleep-disordered breathing on society.
Aneesa Das, MD, FCCP
Steering Committee Chair
Health effects of uranium mining
Decay series of U 238
Prior to 1900, uranium was used only for coloring glass. After discovery of radium by Madame Curie in 1898, uranium was widely mined to obtain radium (a decay product of uranium).
While uranium was not directly mined until 1900, uranium contaminates were in the ore in silver and cobalt mines in Czechoslovakia, which were heavily mined in the 18th and 19th centuries.
There were no reports (written in English) of lung cancer associated with radiation until 1942; but in 1944, these results were called into question in a monograph from the National Cancer Institute. The carcinogenicity of radon was confirmed in 1951; however, this remained an internal government document until 1980. By 1967, the increased prevalence of lung cancer in uranium miners was widely known. By 1970, new ventilation standards for uranium mines were established.
Lung cancer risk associated with uranium mining is the result of exposure to radon gas and specifically radon progeny of Polonium 218 and 210. These radon progeny remain suspended in air, attached to ambient particles (diesel exhaust, silica) and are then inhaled into the lung, where they tend to precipitate on the major airways. Polonium 218 and 210 are alpha emitters, which have a 20-fold increase in energy compared with gamma rays (the primary radiation source in radiation therapy). Given the mass of alpha particles (two protons and two neutrons), they interact with superficial tissues; thus, once deposited in the large airways, a large radiation dose is directed to the respiratory epithelium of these airways.
Occupational control of exposure to radon and radon progeny is accomplished primarily by ventilation. In high-grade deposits of uranium, such as the 20% ore grades in the Athabasca Basin of Saskatchewan, remote control mining is performed.
Smoking, in combination with occupational exposure to radon progeny, carries a greater than additive but less than multiplicative risk of lung cancer.
In addition to the lung cancer risk associated with radon progeny exposure, uranium miners share the occupational risks of other miners: exposure to silica and diesel exhaust. Miners are also at risk for traumatic injuries, including electrocution.
Health effects associated with uranium milling, enrichment, and tailings will be discussed in a subsequent CHEST Physician article.
Richard B. Evans, MD, MPH, FCCP
Steering Committee Chair
Hyperoxia in critically ill patients: What’s the verdict?
Oxygen saturation is considered to be the “fifth vital sign,” and current guidelines recommend target oxygen saturation (SpO2) between 94% and 98%, with lower targets for patients at risk for hypercapnic respiratory failure (O’Driscoll BR et al. Thorax. 2008;63(suppl):vi1). Oxygen toxicity is well-demonstrated in experimental animal studies. While its incidence and impact on outcomes is difficult to determine in the clinical setting, increases in-hospital mortality have been associated with hyperoxia in patients with cardiac arrest, acute myocardial infarction, and stroke (Kligannon et al. JAMA. 2010;303[21]:2165; Stub et al. Circulation. 2015;131[24]:2143; Rincon et al. Crit Care Med. 2014;42[2]:387).
Complementing the findings of Girardis and colleagues, a recent analysis of more than 14,000 critically ill patients, found that time spent at PaO2 > 200 mm Hg was associated with excess mortality and fewer ventilator-free days (Helmerhorst et al. Crit Care Med. 2017;45[2]:187).
While other trials demonstrated safety and feasibility of conservative oxygen therapy in critically ill patients (Panwar et al. Am J Respir Crit Care Med. 2016;193[1]:43; Helmerhorst et al. Crit Care Med. 2016; 44[3]:554; Suzuki et al. Crit Care Med. 2014;42[6]:1414), they did not find significant differences between conservative and liberal oxygen therapy with regards to new organ dysfunction or mortality. However, the degree of hyperoxia was usually more modest than in either the Girardis trial or the Helmerhorst (2017) analysis.
Amanpreet Kaur, MD
Steering Committee Fellow-in-Training
David L. Bowton, MD, FCCP
Steering Committee Chair
Education in palliative medicine
Prompted by concerns that the Affordable Care Act would be instituting “death panels” as part of cost-containment measures, “Dying in America” (a 2015 report of the Institute of Medicine [IOM]) identified compassionate, affordable, and effective care for patients at the end of their lives as a “national priority” in American health care. The IOM identified the education of all primary care providers in the delivery of basic palliative care, specifically commenting that all clinicians who manage patients with serious, life-threatening illnesses should be “competent in basic palliative care” (IOM, The National Academies Press 2015).
Check out our NetWork Storify page later this year for links to the ongoing discussion surrounding palliative care in medicine and for useful tools in the effort to provide palliative care to all our patients.
Laura Johnson, MD, FCCP
Steering Committee Vice Chair
The impact of sleep apnea: Why should we care?
With recent large trials such as the SAVE and the SERVE-HF studies challenging the cardiovascular benefits of treating sleep-disordered breathing in specific patient subsets, many physicians may start to question, “Why all the fuss?” The Sleep NetWork is bringing the leaders in the field to CHEST 2017 to discuss their take on where we stand with the connection between sleep-disordered breathing and cardiovascular disease, so stay tuned!
Our relationships, general health, and work productivity can be affected by untreated OSA. The effect on daily life may not be initially obvious. Patients often present only at the insistence of their partner or physician, only to be surprised at how much better they feel once treated. Symptoms of OSA are associated with a higher rate of impaired work performance, sick leave, and divorce (Grunstein et al. Sleep. 1995;18[8]:635). A recent survey estimates an $86.9 billion loss of workplace productivity due to sleep apnea in 2015 (Frost & Sullivan. Hidden health crisis costing America billions. AASM; 2016. http://www.aasmnet.org/Resources/pdf/sleep-apnea-economic-crisis.pdf. Accessed March 21, 2017.). The same survey found that among those who are employed, treating OSA was associated with a decline in absences by 1.8 days per year and an increase in productivity 17.3% on average. Considering that the majority of OSA remains undiagnosed, this could have tremendous economic impact.
OSA is an important public health burden. The Sleep NetWork is committed to increasing awareness among individuals (patients and clinicians) and institutions (transportation agencies, government) of the impact of sleep-disordered breathing on society.
Aneesa Das, MD, FCCP
Steering Committee Chair
Wait times for family physician visits up almost 10 days since 2014
New patients are waiting almost 10 days longer for an appointment with a family physician in 2017 than they did in 2014, according to physician recruitment firm Merritt Hawkins.
The average wait time for a new patient to see a family physician for a routine physical was 29.3 days in 2017, a 50% increase from the 19.5 days reported in 2014. Investigators called and made appointments with 273 randomly selected family physicians in 15 large cities in January and February. This was the fourth such survey the company has conducted since 2004, although family physicians were not included until the second survey in 2009.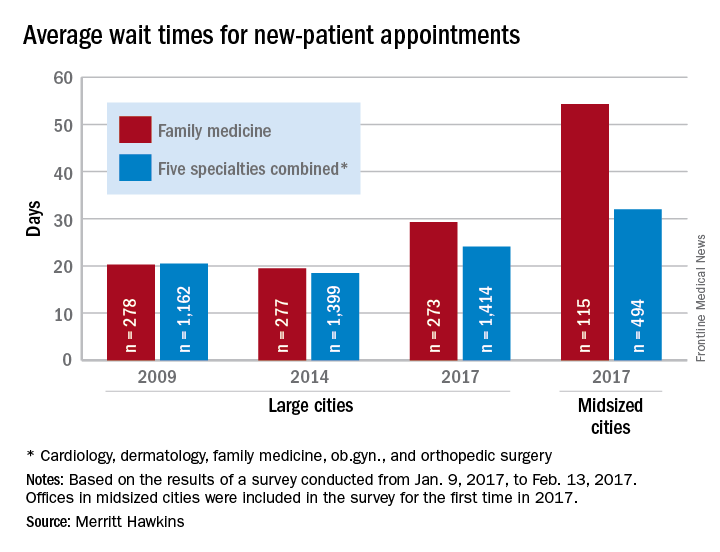
The survey also included four other specialties – cardiology, dermatology, obstetrics and gynecology, and orthopedic surgery – and the average wait time for a new-patient appointment for all 1,414 physicians in all five specialties in the 15 large cities was 24.1 days, an increase of 30% from 2014. The average wait time for all specialties in the midsized cities was 32 days for the 494 offices surveyed, the company said.
“Physician appointment wait times are the longest they have been since we began conducting the survey,” Mark Smith, president of Merritt Hawkins, said in a written statement. “Growing physician appointment wait times are a significant indicator that the nation is experiencing a shortage of physicians.”
New patients are waiting almost 10 days longer for an appointment with a family physician in 2017 than they did in 2014, according to physician recruitment firm Merritt Hawkins.
The average wait time for a new patient to see a family physician for a routine physical was 29.3 days in 2017, a 50% increase from the 19.5 days reported in 2014. Investigators called and made appointments with 273 randomly selected family physicians in 15 large cities in January and February. This was the fourth such survey the company has conducted since 2004, although family physicians were not included until the second survey in 2009.
The survey also included four other specialties – cardiology, dermatology, obstetrics and gynecology, and orthopedic surgery – and the average wait time for a new-patient appointment for all 1,414 physicians in all five specialties in the 15 large cities was 24.1 days, an increase of 30% from 2014. The average wait time for all specialties in the midsized cities was 32 days for the 494 offices surveyed, the company said.
“Physician appointment wait times are the longest they have been since we began conducting the survey,” Mark Smith, president of Merritt Hawkins, said in a written statement. “Growing physician appointment wait times are a significant indicator that the nation is experiencing a shortage of physicians.”
New patients are waiting almost 10 days longer for an appointment with a family physician in 2017 than they did in 2014, according to physician recruitment firm Merritt Hawkins.
The average wait time for a new patient to see a family physician for a routine physical was 29.3 days in 2017, a 50% increase from the 19.5 days reported in 2014. Investigators called and made appointments with 273 randomly selected family physicians in 15 large cities in January and February. This was the fourth such survey the company has conducted since 2004, although family physicians were not included until the second survey in 2009.
The survey also included four other specialties – cardiology, dermatology, obstetrics and gynecology, and orthopedic surgery – and the average wait time for a new-patient appointment for all 1,414 physicians in all five specialties in the 15 large cities was 24.1 days, an increase of 30% from 2014. The average wait time for all specialties in the midsized cities was 32 days for the 494 offices surveyed, the company said.
“Physician appointment wait times are the longest they have been since we began conducting the survey,” Mark Smith, president of Merritt Hawkins, said in a written statement. “Growing physician appointment wait times are a significant indicator that the nation is experiencing a shortage of physicians.”
In Memoriam
Sandra K. Willsie, DO, FCCP, died on March 26, 2017, after a courageous battle with brain cancer. As an osteopathic physician with board certification in internal medicine, pulmonary diseases, and critical care medicine, Sandra worked diligently for over 30 years to further scientific discovery and health-care education.
An NIH-funded career academic awardee, a Macy Institute scholar, and an invited faculty member on health-care leadership at Harvard University, Sandra was very involved in academic medicine. She served as professor of medicine, interim chair of medicine and docent at the University of Missouri–Kansas City School of Medicine; and as provost, dean, vice-dean, and department chair at Kansas City University of Osteopathic Medicine. Sandra earned a master’s degree in bioethics and health policy focusing on research ethics from Loyola University of Chicago Stritch School of Medicine. She made countless scholarly presentations and published regularly.
Sandra made eight pro bono trips to provide physicians in Honduras, Panama, Costa Rica, and the Dominican Republic the latest research updates on asthma and COPD research. She was honored to serve as president of Women Executives in Science and Healthcare and as board president of the American Heart Association’s Midwest Affiliate. She had been volunteering for over 30 years at the KC CARE Clinic in downtown Kansas City, Missouri, and was a committee member of the FDA advisory panel on respiratory and anesthesiology devices.
Sandra devoted many years of active participation to the American College of Chest Physicians and will be missed by so many colleagues and friends. She served on the Board of Regents and on the US and Canadian Council of Governors, was a member of numerous committees, including Education, Ethics, Marketing, Nominating, and Chair of the Scientific Presentations and Awards Committee. A staunch supporter of the CHEST Foundation, she was instrumental in its creation and served as a board and committee member. We extend our heartfelt condolences to her husband, Tom, and her family and many friends.
Sandra K. Willsie, DO, FCCP, died on March 26, 2017, after a courageous battle with brain cancer. As an osteopathic physician with board certification in internal medicine, pulmonary diseases, and critical care medicine, Sandra worked diligently for over 30 years to further scientific discovery and health-care education.
An NIH-funded career academic awardee, a Macy Institute scholar, and an invited faculty member on health-care leadership at Harvard University, Sandra was very involved in academic medicine. She served as professor of medicine, interim chair of medicine and docent at the University of Missouri–Kansas City School of Medicine; and as provost, dean, vice-dean, and department chair at Kansas City University of Osteopathic Medicine. Sandra earned a master’s degree in bioethics and health policy focusing on research ethics from Loyola University of Chicago Stritch School of Medicine. She made countless scholarly presentations and published regularly.
Sandra made eight pro bono trips to provide physicians in Honduras, Panama, Costa Rica, and the Dominican Republic the latest research updates on asthma and COPD research. She was honored to serve as president of Women Executives in Science and Healthcare and as board president of the American Heart Association’s Midwest Affiliate. She had been volunteering for over 30 years at the KC CARE Clinic in downtown Kansas City, Missouri, and was a committee member of the FDA advisory panel on respiratory and anesthesiology devices.
Sandra devoted many years of active participation to the American College of Chest Physicians and will be missed by so many colleagues and friends. She served on the Board of Regents and on the US and Canadian Council of Governors, was a member of numerous committees, including Education, Ethics, Marketing, Nominating, and Chair of the Scientific Presentations and Awards Committee. A staunch supporter of the CHEST Foundation, she was instrumental in its creation and served as a board and committee member. We extend our heartfelt condolences to her husband, Tom, and her family and many friends.
Sandra K. Willsie, DO, FCCP, died on March 26, 2017, after a courageous battle with brain cancer. As an osteopathic physician with board certification in internal medicine, pulmonary diseases, and critical care medicine, Sandra worked diligently for over 30 years to further scientific discovery and health-care education.
An NIH-funded career academic awardee, a Macy Institute scholar, and an invited faculty member on health-care leadership at Harvard University, Sandra was very involved in academic medicine. She served as professor of medicine, interim chair of medicine and docent at the University of Missouri–Kansas City School of Medicine; and as provost, dean, vice-dean, and department chair at Kansas City University of Osteopathic Medicine. Sandra earned a master’s degree in bioethics and health policy focusing on research ethics from Loyola University of Chicago Stritch School of Medicine. She made countless scholarly presentations and published regularly.
Sandra made eight pro bono trips to provide physicians in Honduras, Panama, Costa Rica, and the Dominican Republic the latest research updates on asthma and COPD research. She was honored to serve as president of Women Executives in Science and Healthcare and as board president of the American Heart Association’s Midwest Affiliate. She had been volunteering for over 30 years at the KC CARE Clinic in downtown Kansas City, Missouri, and was a committee member of the FDA advisory panel on respiratory and anesthesiology devices.
Sandra devoted many years of active participation to the American College of Chest Physicians and will be missed by so many colleagues and friends. She served on the Board of Regents and on the US and Canadian Council of Governors, was a member of numerous committees, including Education, Ethics, Marketing, Nominating, and Chair of the Scientific Presentations and Awards Committee. A staunch supporter of the CHEST Foundation, she was instrumental in its creation and served as a board and committee member. We extend our heartfelt condolences to her husband, Tom, and her family and many friends.
Being a Teenager Is Rough Enough
Since she was about 2 years old, this now 14-year-old girl has had asymptomatic bumps on her arms and face. They are most noticeable in the wintertime and a bit less conspicuous during humid months. Besides the appearance of the lesions, the patient is annoyed by the rough feel of them, which is unaffected by her use of OTC moisturizers and lotions.
Her mother recalls having the same problem as a child but says it improved with time. The patient’s immediate family members are all atopic, with seasonal allergies and eczema.
EXAMINATION
The patient is Hispanic with type IV skin. Dense patches of brown, tiny, rough, papulofollicular lesions cover the surface of both posterior triceps. They are also visible on the patient’s anterior thighs and the posterior two-thirds of her face.

What is the diagnosis?
DISCUSSION
Keratosis pilaris (KP) is an inherited condition that affects 30% to 50% of all children without respect to race or gender. It can be a problem unto itself, or it can be part of the atopic diathesis in patients with seasonal allergies, dry skin, and eczema.
KP is caused by an excessive production of keratin that plugs the follicles, often trapping tiny fine hairs inside and resulting in a firm follicular papule. The distribution exhibited in this case is quite typical, as is the brown color on the patient’s arms (common in those with darker skin). KP can also affect the skin on other convex areas (eg, buttocks, deltoids, and thighs). It spares glabrous skin completely. A common variant is rubra facei, characterized by redness and bumps on the posterior two-thirds of the face.
Though it cannot be cured, the condition can be controlled with keratolytics containing salicylic acid, urea, or glycolic acid, or with pure emollients, which trap moisture in the skin. For the occasional itch, topical steroid creams or ointments can be helpful.
Patient education is a key aspect of treatment. Patients can be reassured of the condition’s benignancy, and they may be relieved to know that most KP patients see major improvement as they reach their third decade of life (and beyond).
TAKE-HOME LEARNING POINTS
- Keratosis pilaris (KP) is an extremely common condition inherited by autosomal dominant mode that affects more than 30% of children.
- Excessive keratin production is the cause of KP; it plugs follicular orifices, trapping fine hairs inside and causing crops of firm scaly papules to develop on triceps, deltoids, anterior thighs, and other areas (eg, the face).
- KP is considered part of the minor diagnostic criteria for atopy, but it can also be a standalone condition.
- Treatment of KP is far from perfect, but improvement is seen with the use of either keratolytics or emollients. Anything that dries it out more (eg, long, hot showers) or irritates individual lesions (eg, picking) is to be avoided.
- Most KP patients report improvement after the third decade of life.
Since she was about 2 years old, this now 14-year-old girl has had asymptomatic bumps on her arms and face. They are most noticeable in the wintertime and a bit less conspicuous during humid months. Besides the appearance of the lesions, the patient is annoyed by the rough feel of them, which is unaffected by her use of OTC moisturizers and lotions.
Her mother recalls having the same problem as a child but says it improved with time. The patient’s immediate family members are all atopic, with seasonal allergies and eczema.
EXAMINATION
The patient is Hispanic with type IV skin. Dense patches of brown, tiny, rough, papulofollicular lesions cover the surface of both posterior triceps. They are also visible on the patient’s anterior thighs and the posterior two-thirds of her face.

What is the diagnosis?
DISCUSSION
Keratosis pilaris (KP) is an inherited condition that affects 30% to 50% of all children without respect to race or gender. It can be a problem unto itself, or it can be part of the atopic diathesis in patients with seasonal allergies, dry skin, and eczema.
KP is caused by an excessive production of keratin that plugs the follicles, often trapping tiny fine hairs inside and resulting in a firm follicular papule. The distribution exhibited in this case is quite typical, as is the brown color on the patient’s arms (common in those with darker skin). KP can also affect the skin on other convex areas (eg, buttocks, deltoids, and thighs). It spares glabrous skin completely. A common variant is rubra facei, characterized by redness and bumps on the posterior two-thirds of the face.
Though it cannot be cured, the condition can be controlled with keratolytics containing salicylic acid, urea, or glycolic acid, or with pure emollients, which trap moisture in the skin. For the occasional itch, topical steroid creams or ointments can be helpful.
Patient education is a key aspect of treatment. Patients can be reassured of the condition’s benignancy, and they may be relieved to know that most KP patients see major improvement as they reach their third decade of life (and beyond).
TAKE-HOME LEARNING POINTS
- Keratosis pilaris (KP) is an extremely common condition inherited by autosomal dominant mode that affects more than 30% of children.
- Excessive keratin production is the cause of KP; it plugs follicular orifices, trapping fine hairs inside and causing crops of firm scaly papules to develop on triceps, deltoids, anterior thighs, and other areas (eg, the face).
- KP is considered part of the minor diagnostic criteria for atopy, but it can also be a standalone condition.
- Treatment of KP is far from perfect, but improvement is seen with the use of either keratolytics or emollients. Anything that dries it out more (eg, long, hot showers) or irritates individual lesions (eg, picking) is to be avoided.
- Most KP patients report improvement after the third decade of life.
Since she was about 2 years old, this now 14-year-old girl has had asymptomatic bumps on her arms and face. They are most noticeable in the wintertime and a bit less conspicuous during humid months. Besides the appearance of the lesions, the patient is annoyed by the rough feel of them, which is unaffected by her use of OTC moisturizers and lotions.
Her mother recalls having the same problem as a child but says it improved with time. The patient’s immediate family members are all atopic, with seasonal allergies and eczema.
EXAMINATION
The patient is Hispanic with type IV skin. Dense patches of brown, tiny, rough, papulofollicular lesions cover the surface of both posterior triceps. They are also visible on the patient’s anterior thighs and the posterior two-thirds of her face.

What is the diagnosis?
DISCUSSION
Keratosis pilaris (KP) is an inherited condition that affects 30% to 50% of all children without respect to race or gender. It can be a problem unto itself, or it can be part of the atopic diathesis in patients with seasonal allergies, dry skin, and eczema.
KP is caused by an excessive production of keratin that plugs the follicles, often trapping tiny fine hairs inside and resulting in a firm follicular papule. The distribution exhibited in this case is quite typical, as is the brown color on the patient’s arms (common in those with darker skin). KP can also affect the skin on other convex areas (eg, buttocks, deltoids, and thighs). It spares glabrous skin completely. A common variant is rubra facei, characterized by redness and bumps on the posterior two-thirds of the face.
Though it cannot be cured, the condition can be controlled with keratolytics containing salicylic acid, urea, or glycolic acid, or with pure emollients, which trap moisture in the skin. For the occasional itch, topical steroid creams or ointments can be helpful.
Patient education is a key aspect of treatment. Patients can be reassured of the condition’s benignancy, and they may be relieved to know that most KP patients see major improvement as they reach their third decade of life (and beyond).
TAKE-HOME LEARNING POINTS
- Keratosis pilaris (KP) is an extremely common condition inherited by autosomal dominant mode that affects more than 30% of children.
- Excessive keratin production is the cause of KP; it plugs follicular orifices, trapping fine hairs inside and causing crops of firm scaly papules to develop on triceps, deltoids, anterior thighs, and other areas (eg, the face).
- KP is considered part of the minor diagnostic criteria for atopy, but it can also be a standalone condition.
- Treatment of KP is far from perfect, but improvement is seen with the use of either keratolytics or emollients. Anything that dries it out more (eg, long, hot showers) or irritates individual lesions (eg, picking) is to be avoided.
- Most KP patients report improvement after the third decade of life.