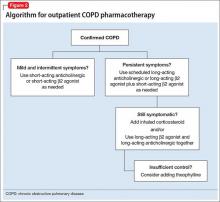
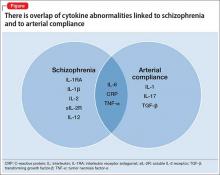
User login
Surveillance finds pancreatic ductal carcinoma in situ at resectable stage
Surveillance of CDNK2A mutation carriers detected most pancreatic ductal carcinoma in situ (PDAC) at a resectable stage, while the surveillance benefit was lower for those with familial prostate cancer.
Among 178 CDKN2A mutation carriers, PDAC was detected in 13 (7.3%), 9 of whom underwent surgery. Compared with previously reported rates of 15%-20% for symptomatic PDAC, this 70% resection rate represents a substantial increase. The 5-year survival rate of 24% for screen-detected PDAC was higher than 4%-7% reported for symptomatic sporadic PDAC. Among individuals with familial prostate cancer (FPC), 13 of 214 individuals (6.1%) underwent surgery, but with a higher proportion of precursor lesions detected, just four high-risk lesions (1.9% of screened FPC patients) were removed.
Whether surveillance improved prognosis for FPC families was difficult to determine, according to the investigators. The yield of PDAC was low at 0.9%, as was the yield of relevant precursor lesions (grade 3 PanIN and high-grade IPMN) at 1.9%.
“However, if surgical removal of multifocal grade 2 PanIN and multifocal BD-IPMNs is regarded as beneficial, the diagnostic yield increases to 3.7% (eight of 214 patients), and surveillance of FPC might also be considered effective,” wrote Dr. Hans Vasen, professor in the department of gastroenterology and hepatology at the Leiden University Medical Center, the Netherlands, and colleagues. “The value of surveillance of FPC is still not clear, and the main effect seems to be prevention of PDAC by removal of” precursor lesions, they added (J Clin Oncol. 2016 Apr 25. doi: 10.1200/JCO.2015.64.0730).
The retrospective evaluation of an ongoing prospective follow-up study included 411 high-risk individuals: 178 with CDKN2A mutations, 214 with familial pancreatic cancer, and 19 with BRCA1/2 or PALB2 mutations. The study was conducted at three expert centers in Marburg, Germany; Leiden, the Netherlands; and Madrid.
In the BRCA1/2 and PALB2 mutation cohort, one individual (3.8%) with a BRCA2 mutation developed PDAC and underwent surgery; 17 months after the surgery this patient died of liver metastasis. Two others underwent surgery for cystic lesions and are in good health at 10 and 21 months after surgery.
In the cohort of CDKN2A mutation carriers, the mean age at the start of surveillance was 56 years (range, 37-75) and the mean follow-up time was 53 months (range, 0-169): in total, 866 MRIs and 106 endoscopic ultrasounds were conducted. In the FPC group, the mean age was 48 years (range, 27-81), and the mean follow up was 2.8 years (range, 0-10.8): 618 MRIs and 402 endoscopic ultrasounds were conducted. Among BRCA1/2 and PALB2 mutation carriers, the mean age was 52.6 years (range, 25-70), and the mean follow up was 32.7 months (range, 1-119).
Given the difficulty of detecting precursor lesions and distinguishing incipient neoplasia from lower grade or nonneoplastic cystic lesions, the authors of the accompanying study achieved impressive results in improving cancer outcomes among high-risk individuals.
Several strategies for earlier cancer detection can be gleaned from the study. Improved outcomes may depend on expert centers running the surveillance. The detection rate of 2%-7%, depending on the cohort studied and the surveillance protocol, may have room for improvement with better risk stratification and refined protocols for cost effectiveness. The age at the start of surveillance may be one place to start: the mean age of pancreatic ductal carcinoma in situ detection was 53-68 years, depending on the center, and it may be possible to shift the starting age upward to improve yield.
The type of mutation conferring susceptibility may aid in risk stratification. For example, CDKN2A mutation carriers had a higher cancer rate (16%) than BRCA/PALB2 mutation carriers (5%). Other factors that could mitigate risk upward include diabetes, family history, and smoking history. A composite risk assessment could aid in identifying the highest-risk patients. Lastly, future studies are needed to determine which surveillance protocols are best. To make valid comparisons, several surveillance protocols must be tested.
These results impact not only high-risk individuals, but the general population as well. The data support that early detection improves outcomes and highlights the need for developing better biomarkers and tests for early detection of PDAC.
Dr. Teresa A. Brentnall is professor in the department of medicine, division of gastroenterology, University of Washington, Seattle. These remarks were part of an accompanying editorial (J Clin Oncol. 2016 Apr 25. doi: 10.1200/JCO.2015.64.0730).
Given the difficulty of detecting precursor lesions and distinguishing incipient neoplasia from lower grade or nonneoplastic cystic lesions, the authors of the accompanying study achieved impressive results in improving cancer outcomes among high-risk individuals.
Several strategies for earlier cancer detection can be gleaned from the study. Improved outcomes may depend on expert centers running the surveillance. The detection rate of 2%-7%, depending on the cohort studied and the surveillance protocol, may have room for improvement with better risk stratification and refined protocols for cost effectiveness. The age at the start of surveillance may be one place to start: the mean age of pancreatic ductal carcinoma in situ detection was 53-68 years, depending on the center, and it may be possible to shift the starting age upward to improve yield.
The type of mutation conferring susceptibility may aid in risk stratification. For example, CDKN2A mutation carriers had a higher cancer rate (16%) than BRCA/PALB2 mutation carriers (5%). Other factors that could mitigate risk upward include diabetes, family history, and smoking history. A composite risk assessment could aid in identifying the highest-risk patients. Lastly, future studies are needed to determine which surveillance protocols are best. To make valid comparisons, several surveillance protocols must be tested.
These results impact not only high-risk individuals, but the general population as well. The data support that early detection improves outcomes and highlights the need for developing better biomarkers and tests for early detection of PDAC.
Dr. Teresa A. Brentnall is professor in the department of medicine, division of gastroenterology, University of Washington, Seattle. These remarks were part of an accompanying editorial (J Clin Oncol. 2016 Apr 25. doi: 10.1200/JCO.2015.64.0730).
Given the difficulty of detecting precursor lesions and distinguishing incipient neoplasia from lower grade or nonneoplastic cystic lesions, the authors of the accompanying study achieved impressive results in improving cancer outcomes among high-risk individuals.
Several strategies for earlier cancer detection can be gleaned from the study. Improved outcomes may depend on expert centers running the surveillance. The detection rate of 2%-7%, depending on the cohort studied and the surveillance protocol, may have room for improvement with better risk stratification and refined protocols for cost effectiveness. The age at the start of surveillance may be one place to start: the mean age of pancreatic ductal carcinoma in situ detection was 53-68 years, depending on the center, and it may be possible to shift the starting age upward to improve yield.
The type of mutation conferring susceptibility may aid in risk stratification. For example, CDKN2A mutation carriers had a higher cancer rate (16%) than BRCA/PALB2 mutation carriers (5%). Other factors that could mitigate risk upward include diabetes, family history, and smoking history. A composite risk assessment could aid in identifying the highest-risk patients. Lastly, future studies are needed to determine which surveillance protocols are best. To make valid comparisons, several surveillance protocols must be tested.
These results impact not only high-risk individuals, but the general population as well. The data support that early detection improves outcomes and highlights the need for developing better biomarkers and tests for early detection of PDAC.
Dr. Teresa A. Brentnall is professor in the department of medicine, division of gastroenterology, University of Washington, Seattle. These remarks were part of an accompanying editorial (J Clin Oncol. 2016 Apr 25. doi: 10.1200/JCO.2015.64.0730).
Surveillance of CDNK2A mutation carriers detected most pancreatic ductal carcinoma in situ (PDAC) at a resectable stage, while the surveillance benefit was lower for those with familial prostate cancer.
Among 178 CDKN2A mutation carriers, PDAC was detected in 13 (7.3%), 9 of whom underwent surgery. Compared with previously reported rates of 15%-20% for symptomatic PDAC, this 70% resection rate represents a substantial increase. The 5-year survival rate of 24% for screen-detected PDAC was higher than 4%-7% reported for symptomatic sporadic PDAC. Among individuals with familial prostate cancer (FPC), 13 of 214 individuals (6.1%) underwent surgery, but with a higher proportion of precursor lesions detected, just four high-risk lesions (1.9% of screened FPC patients) were removed.
Whether surveillance improved prognosis for FPC families was difficult to determine, according to the investigators. The yield of PDAC was low at 0.9%, as was the yield of relevant precursor lesions (grade 3 PanIN and high-grade IPMN) at 1.9%.
“However, if surgical removal of multifocal grade 2 PanIN and multifocal BD-IPMNs is regarded as beneficial, the diagnostic yield increases to 3.7% (eight of 214 patients), and surveillance of FPC might also be considered effective,” wrote Dr. Hans Vasen, professor in the department of gastroenterology and hepatology at the Leiden University Medical Center, the Netherlands, and colleagues. “The value of surveillance of FPC is still not clear, and the main effect seems to be prevention of PDAC by removal of” precursor lesions, they added (J Clin Oncol. 2016 Apr 25. doi: 10.1200/JCO.2015.64.0730).
The retrospective evaluation of an ongoing prospective follow-up study included 411 high-risk individuals: 178 with CDKN2A mutations, 214 with familial pancreatic cancer, and 19 with BRCA1/2 or PALB2 mutations. The study was conducted at three expert centers in Marburg, Germany; Leiden, the Netherlands; and Madrid.
In the BRCA1/2 and PALB2 mutation cohort, one individual (3.8%) with a BRCA2 mutation developed PDAC and underwent surgery; 17 months after the surgery this patient died of liver metastasis. Two others underwent surgery for cystic lesions and are in good health at 10 and 21 months after surgery.
In the cohort of CDKN2A mutation carriers, the mean age at the start of surveillance was 56 years (range, 37-75) and the mean follow-up time was 53 months (range, 0-169): in total, 866 MRIs and 106 endoscopic ultrasounds were conducted. In the FPC group, the mean age was 48 years (range, 27-81), and the mean follow up was 2.8 years (range, 0-10.8): 618 MRIs and 402 endoscopic ultrasounds were conducted. Among BRCA1/2 and PALB2 mutation carriers, the mean age was 52.6 years (range, 25-70), and the mean follow up was 32.7 months (range, 1-119).
Surveillance of CDNK2A mutation carriers detected most pancreatic ductal carcinoma in situ (PDAC) at a resectable stage, while the surveillance benefit was lower for those with familial prostate cancer.
Among 178 CDKN2A mutation carriers, PDAC was detected in 13 (7.3%), 9 of whom underwent surgery. Compared with previously reported rates of 15%-20% for symptomatic PDAC, this 70% resection rate represents a substantial increase. The 5-year survival rate of 24% for screen-detected PDAC was higher than 4%-7% reported for symptomatic sporadic PDAC. Among individuals with familial prostate cancer (FPC), 13 of 214 individuals (6.1%) underwent surgery, but with a higher proportion of precursor lesions detected, just four high-risk lesions (1.9% of screened FPC patients) were removed.
Whether surveillance improved prognosis for FPC families was difficult to determine, according to the investigators. The yield of PDAC was low at 0.9%, as was the yield of relevant precursor lesions (grade 3 PanIN and high-grade IPMN) at 1.9%.
“However, if surgical removal of multifocal grade 2 PanIN and multifocal BD-IPMNs is regarded as beneficial, the diagnostic yield increases to 3.7% (eight of 214 patients), and surveillance of FPC might also be considered effective,” wrote Dr. Hans Vasen, professor in the department of gastroenterology and hepatology at the Leiden University Medical Center, the Netherlands, and colleagues. “The value of surveillance of FPC is still not clear, and the main effect seems to be prevention of PDAC by removal of” precursor lesions, they added (J Clin Oncol. 2016 Apr 25. doi: 10.1200/JCO.2015.64.0730).
The retrospective evaluation of an ongoing prospective follow-up study included 411 high-risk individuals: 178 with CDKN2A mutations, 214 with familial pancreatic cancer, and 19 with BRCA1/2 or PALB2 mutations. The study was conducted at three expert centers in Marburg, Germany; Leiden, the Netherlands; and Madrid.
In the BRCA1/2 and PALB2 mutation cohort, one individual (3.8%) with a BRCA2 mutation developed PDAC and underwent surgery; 17 months after the surgery this patient died of liver metastasis. Two others underwent surgery for cystic lesions and are in good health at 10 and 21 months after surgery.
In the cohort of CDKN2A mutation carriers, the mean age at the start of surveillance was 56 years (range, 37-75) and the mean follow-up time was 53 months (range, 0-169): in total, 866 MRIs and 106 endoscopic ultrasounds were conducted. In the FPC group, the mean age was 48 years (range, 27-81), and the mean follow up was 2.8 years (range, 0-10.8): 618 MRIs and 402 endoscopic ultrasounds were conducted. Among BRCA1/2 and PALB2 mutation carriers, the mean age was 52.6 years (range, 25-70), and the mean follow up was 32.7 months (range, 1-119).
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Surveillance of high-risk individuals was relatively successful in detecting pancreatic ductal carcinoma in situ (PDAC) at a resectable stage.
Major finding: The detection rate in CDKN2A mutation carriers was 7.3% and the resection rate for screen-detected PDAC was 75%, compared with previous reports of 15%-20% for symptomatic PDAC; the PDAC detection rate in individuals with familial prostate cancer was much lower at 0.9%.
Data source: Evaluation of an ongoing prospective follow-up study at three European centers included 411 individuals: 178 with CDKN2A mutations, 214 with familial pancreatic cancer, and 19 with BRCA1/2 or PALB2 mutations.
Disclosures: Dr. Vasen and most coauthors reported having no disclosures. Five coauthors reported financial ties to industry sources.
Offer these interventions to help prevent suicide by firearm
Firearms are the most common means of suicide in the United States, accounting for approximately 20,000 adult deaths annually,1 which is approximately two-thirds of the more than 32,000 gun-related fatalities each year in the United States. Of approximately 3,000 American children who are shot to death annually, one-third are suicides.1-4
Firearms are dangerous; it has been documented that even guns obtained for recreation or protection increase the risk of suicide, homicide, or injury.2,3 This problem has become a public health concern.3-8 Because most suicide attempts with firearms are fatal, psychiatrists have an interest in reducing such outcomes.1-8
Risk factors for suicide by firearm
Easy availability of a gun in the home, with ammunition present—especially a gun that is kept loaded and not locked up—is the one of the biggest risk factors for suicide by firearms.4 Unrestricted, quick access allows people who are impulsive little time to reconsider suicide. The risk presented by easy availability is magnified by dangerous concomitant intoxication (see below), distress, and lack of supervision (of children).
Alcohol consumption is associated with suicide. Approximately one-fourth of the people who commit suicide are intoxicated at the time of death.9 Alcohol use, especially binge drinking, is observed in an even larger percentage of suicide attempts than individuals using guns while sober.
Female sex. In recent years, gun use by women has increased, along with firearm-related suicide. Simply having a gun at home greatly increases the suicide rate for women.2-4
People with a history of high impulsivity, impaired judgment, violence, or psychiatric and neurologic disorders places people at greater risk of shooting themselves, especially those with depression, suicidal ideation, substance abuse, psychosis, or dementia.4
Older age, particularly men who live alone, increases the risk of suicide by firearms, especially in the context of chronic pain or other health problems. Gunfire is the most common means of suicide among geriatric patients of both sexes.8
Lethality. In general, suicide attempts with guns are more likely to be fatal than overdosing, poisoning, or self-mutilation.1,2 Most self-inflicted gunshot wounds result in death, usually on the day of the shooting.1,2
Evidence about these risk factors has led the American Medical Association and other health care groups to encourage physicians—in particular, psychiatric clinicians who focus on suicide prevention—to counsel patients about gun safety.
What can you do to minimize risk?
Gun-related inquiry and counsel by psychiatrists can benefit patients and their family.4 Be aware, however, of restrictions on such discussions by health care providers in some states (Box).10
Ask about the presence of firearms in the home. Our advice and our “doctor’s orders” are a means to promote health; suggestions in the context of a supportive physician-patient relationship could result in compliance.3,4 Firearm-focused discussions might be uncomfortable or unpopular but are critical for preventing suicide. Openly discussing such issues with our patients could avoid tragedies.4 Involving family or significant others in these interventions also might be helpful.
Ask about access to and storage of firearms. Simply talking about gun safety is helpful.4 Seeking information about gun usage is especially called for in psychiatric practices that treat patients with suicidal ideation, depression, substance abuse, and cognitive impairment.8 Discuss firearm availability with patients who have a history of substance use, impulsivity, anger, or violence, or who have a brain disorder or neurologic condition. Talking about firearms with patients and educating them about safety is indicated whenever you observe a risk factor for suicide.
Advise safe storage. Aim to have the entire family agree to a safety policy. Guns should be kept unloaded and not stored with ammunition (eg, keep guns in the attic and ammunition in the basement), which might diminish the risk of (1) an impulsive shooting and (2) a planned attempt by giving people time to consider options other than suicide. Firearm safety includes locking ammunition and weapons in a safe and applying trigger locks. Try to get patients and their family to plan for compliance with such recommendations whenever possible.
Guide dialogue and educate patients about handling guns safely. Be sure that patients know that most firearm deaths that happen inside a home are suicide.2-4 Advise patients, and their family, that firearms should not be handled while intoxicated.4 Encourage families to remove gun access from members who are suicidal, depressed, abusing pharmaceuticals or using illicit drugs, and those in distress or with a significant mental or neurologic illness.
In such circumstances, institute a protective plan to prevent shootings. This can be time-limited, or might include removing guns or ammunition from the home or deactivating firing mechanisms, etc. For safety reasons, some families do not keep ammunition in their home.
Additionally, firearms in the hands of children ought to include close monitoring by a responsible, sober adult. Keeping guns in locked storage is especially important for preventing suicide in children. Despite suicide being less frequent among younger people than in adults, taking steps to avoid 1,000 child suicides each year in the United States is a valuable intervention.
Conclusion
Specific inquiry, overt discussion, and face-to-face counseling about gun safety can be a life-saving aspect of psychiatric intervention. With such recommendations and education, psychiatrists can play a productive role in reducing firearm-related suicide.
1. Center for Disease Control and Prevention. Injury prevention and control: data and statistics. http://www.cdc.gov/injury/wisqars. Updated December 8, 2015. Accessed April 1, 2016.
2. Narang P, Paladugu A, Manda SR, et al. Do guns provide safety? At what cost? South Med J. 2010;103(2):151-153.
3. Cherlopalle S, Kolikonda MK, Enja M, et al. Guns in America: defense or danger? J Trauma Treat. 2014;3(4):207.
4. Lippmann S. Doctors teaching gun safety. Journal of the Kentucky Medical Association. 2015;113(4):112.
5. Cooke BK, Goddard ER, Ginory A, et al. Firearms inquiries in Florida: “medical privacy” or medical neglect? J Am Acad Psychiatry Law. 2012;40(3):399-408.
6. Valeras AB. Patient with gun. Fam Med. 2013;45(8):584-585.
7. Butkus R, Weissman A. Internists’ attitude toward prevention of firearm injury. Ann Intern Med. 2015;160(12):821-827.
8. Kapp MB. Geriatric patients, firearms, and physicians. Ann Intern Med. 2013;159(6):421-422.
9. Kaplan MS, McFarland BH, Huguet N, et al. Acute alcohol intoxication and suicide: a gender-stratified analysis of the National Violent Death Reporting System. Inj Prev. 2013;19(1):38-43.
10. Fla Stat §790.338.
Firearms are the most common means of suicide in the United States, accounting for approximately 20,000 adult deaths annually,1 which is approximately two-thirds of the more than 32,000 gun-related fatalities each year in the United States. Of approximately 3,000 American children who are shot to death annually, one-third are suicides.1-4
Firearms are dangerous; it has been documented that even guns obtained for recreation or protection increase the risk of suicide, homicide, or injury.2,3 This problem has become a public health concern.3-8 Because most suicide attempts with firearms are fatal, psychiatrists have an interest in reducing such outcomes.1-8
Risk factors for suicide by firearm
Easy availability of a gun in the home, with ammunition present—especially a gun that is kept loaded and not locked up—is the one of the biggest risk factors for suicide by firearms.4 Unrestricted, quick access allows people who are impulsive little time to reconsider suicide. The risk presented by easy availability is magnified by dangerous concomitant intoxication (see below), distress, and lack of supervision (of children).
Alcohol consumption is associated with suicide. Approximately one-fourth of the people who commit suicide are intoxicated at the time of death.9 Alcohol use, especially binge drinking, is observed in an even larger percentage of suicide attempts than individuals using guns while sober.
Female sex. In recent years, gun use by women has increased, along with firearm-related suicide. Simply having a gun at home greatly increases the suicide rate for women.2-4
People with a history of high impulsivity, impaired judgment, violence, or psychiatric and neurologic disorders places people at greater risk of shooting themselves, especially those with depression, suicidal ideation, substance abuse, psychosis, or dementia.4
Older age, particularly men who live alone, increases the risk of suicide by firearms, especially in the context of chronic pain or other health problems. Gunfire is the most common means of suicide among geriatric patients of both sexes.8
Lethality. In general, suicide attempts with guns are more likely to be fatal than overdosing, poisoning, or self-mutilation.1,2 Most self-inflicted gunshot wounds result in death, usually on the day of the shooting.1,2
Evidence about these risk factors has led the American Medical Association and other health care groups to encourage physicians—in particular, psychiatric clinicians who focus on suicide prevention—to counsel patients about gun safety.
What can you do to minimize risk?
Gun-related inquiry and counsel by psychiatrists can benefit patients and their family.4 Be aware, however, of restrictions on such discussions by health care providers in some states (Box).10
Ask about the presence of firearms in the home. Our advice and our “doctor’s orders” are a means to promote health; suggestions in the context of a supportive physician-patient relationship could result in compliance.3,4 Firearm-focused discussions might be uncomfortable or unpopular but are critical for preventing suicide. Openly discussing such issues with our patients could avoid tragedies.4 Involving family or significant others in these interventions also might be helpful.
Ask about access to and storage of firearms. Simply talking about gun safety is helpful.4 Seeking information about gun usage is especially called for in psychiatric practices that treat patients with suicidal ideation, depression, substance abuse, and cognitive impairment.8 Discuss firearm availability with patients who have a history of substance use, impulsivity, anger, or violence, or who have a brain disorder or neurologic condition. Talking about firearms with patients and educating them about safety is indicated whenever you observe a risk factor for suicide.
Advise safe storage. Aim to have the entire family agree to a safety policy. Guns should be kept unloaded and not stored with ammunition (eg, keep guns in the attic and ammunition in the basement), which might diminish the risk of (1) an impulsive shooting and (2) a planned attempt by giving people time to consider options other than suicide. Firearm safety includes locking ammunition and weapons in a safe and applying trigger locks. Try to get patients and their family to plan for compliance with such recommendations whenever possible.
Guide dialogue and educate patients about handling guns safely. Be sure that patients know that most firearm deaths that happen inside a home are suicide.2-4 Advise patients, and their family, that firearms should not be handled while intoxicated.4 Encourage families to remove gun access from members who are suicidal, depressed, abusing pharmaceuticals or using illicit drugs, and those in distress or with a significant mental or neurologic illness.
In such circumstances, institute a protective plan to prevent shootings. This can be time-limited, or might include removing guns or ammunition from the home or deactivating firing mechanisms, etc. For safety reasons, some families do not keep ammunition in their home.
Additionally, firearms in the hands of children ought to include close monitoring by a responsible, sober adult. Keeping guns in locked storage is especially important for preventing suicide in children. Despite suicide being less frequent among younger people than in adults, taking steps to avoid 1,000 child suicides each year in the United States is a valuable intervention.
Conclusion
Specific inquiry, overt discussion, and face-to-face counseling about gun safety can be a life-saving aspect of psychiatric intervention. With such recommendations and education, psychiatrists can play a productive role in reducing firearm-related suicide.
Firearms are the most common means of suicide in the United States, accounting for approximately 20,000 adult deaths annually,1 which is approximately two-thirds of the more than 32,000 gun-related fatalities each year in the United States. Of approximately 3,000 American children who are shot to death annually, one-third are suicides.1-4
Firearms are dangerous; it has been documented that even guns obtained for recreation or protection increase the risk of suicide, homicide, or injury.2,3 This problem has become a public health concern.3-8 Because most suicide attempts with firearms are fatal, psychiatrists have an interest in reducing such outcomes.1-8
Risk factors for suicide by firearm
Easy availability of a gun in the home, with ammunition present—especially a gun that is kept loaded and not locked up—is the one of the biggest risk factors for suicide by firearms.4 Unrestricted, quick access allows people who are impulsive little time to reconsider suicide. The risk presented by easy availability is magnified by dangerous concomitant intoxication (see below), distress, and lack of supervision (of children).
Alcohol consumption is associated with suicide. Approximately one-fourth of the people who commit suicide are intoxicated at the time of death.9 Alcohol use, especially binge drinking, is observed in an even larger percentage of suicide attempts than individuals using guns while sober.
Female sex. In recent years, gun use by women has increased, along with firearm-related suicide. Simply having a gun at home greatly increases the suicide rate for women.2-4
People with a history of high impulsivity, impaired judgment, violence, or psychiatric and neurologic disorders places people at greater risk of shooting themselves, especially those with depression, suicidal ideation, substance abuse, psychosis, or dementia.4
Older age, particularly men who live alone, increases the risk of suicide by firearms, especially in the context of chronic pain or other health problems. Gunfire is the most common means of suicide among geriatric patients of both sexes.8
Lethality. In general, suicide attempts with guns are more likely to be fatal than overdosing, poisoning, or self-mutilation.1,2 Most self-inflicted gunshot wounds result in death, usually on the day of the shooting.1,2
Evidence about these risk factors has led the American Medical Association and other health care groups to encourage physicians—in particular, psychiatric clinicians who focus on suicide prevention—to counsel patients about gun safety.
What can you do to minimize risk?
Gun-related inquiry and counsel by psychiatrists can benefit patients and their family.4 Be aware, however, of restrictions on such discussions by health care providers in some states (Box).10
Ask about the presence of firearms in the home. Our advice and our “doctor’s orders” are a means to promote health; suggestions in the context of a supportive physician-patient relationship could result in compliance.3,4 Firearm-focused discussions might be uncomfortable or unpopular but are critical for preventing suicide. Openly discussing such issues with our patients could avoid tragedies.4 Involving family or significant others in these interventions also might be helpful.
Ask about access to and storage of firearms. Simply talking about gun safety is helpful.4 Seeking information about gun usage is especially called for in psychiatric practices that treat patients with suicidal ideation, depression, substance abuse, and cognitive impairment.8 Discuss firearm availability with patients who have a history of substance use, impulsivity, anger, or violence, or who have a brain disorder or neurologic condition. Talking about firearms with patients and educating them about safety is indicated whenever you observe a risk factor for suicide.
Advise safe storage. Aim to have the entire family agree to a safety policy. Guns should be kept unloaded and not stored with ammunition (eg, keep guns in the attic and ammunition in the basement), which might diminish the risk of (1) an impulsive shooting and (2) a planned attempt by giving people time to consider options other than suicide. Firearm safety includes locking ammunition and weapons in a safe and applying trigger locks. Try to get patients and their family to plan for compliance with such recommendations whenever possible.
Guide dialogue and educate patients about handling guns safely. Be sure that patients know that most firearm deaths that happen inside a home are suicide.2-4 Advise patients, and their family, that firearms should not be handled while intoxicated.4 Encourage families to remove gun access from members who are suicidal, depressed, abusing pharmaceuticals or using illicit drugs, and those in distress or with a significant mental or neurologic illness.
In such circumstances, institute a protective plan to prevent shootings. This can be time-limited, or might include removing guns or ammunition from the home or deactivating firing mechanisms, etc. For safety reasons, some families do not keep ammunition in their home.
Additionally, firearms in the hands of children ought to include close monitoring by a responsible, sober adult. Keeping guns in locked storage is especially important for preventing suicide in children. Despite suicide being less frequent among younger people than in adults, taking steps to avoid 1,000 child suicides each year in the United States is a valuable intervention.
Conclusion
Specific inquiry, overt discussion, and face-to-face counseling about gun safety can be a life-saving aspect of psychiatric intervention. With such recommendations and education, psychiatrists can play a productive role in reducing firearm-related suicide.
1. Center for Disease Control and Prevention. Injury prevention and control: data and statistics. http://www.cdc.gov/injury/wisqars. Updated December 8, 2015. Accessed April 1, 2016.
2. Narang P, Paladugu A, Manda SR, et al. Do guns provide safety? At what cost? South Med J. 2010;103(2):151-153.
3. Cherlopalle S, Kolikonda MK, Enja M, et al. Guns in America: defense or danger? J Trauma Treat. 2014;3(4):207.
4. Lippmann S. Doctors teaching gun safety. Journal of the Kentucky Medical Association. 2015;113(4):112.
5. Cooke BK, Goddard ER, Ginory A, et al. Firearms inquiries in Florida: “medical privacy” or medical neglect? J Am Acad Psychiatry Law. 2012;40(3):399-408.
6. Valeras AB. Patient with gun. Fam Med. 2013;45(8):584-585.
7. Butkus R, Weissman A. Internists’ attitude toward prevention of firearm injury. Ann Intern Med. 2015;160(12):821-827.
8. Kapp MB. Geriatric patients, firearms, and physicians. Ann Intern Med. 2013;159(6):421-422.
9. Kaplan MS, McFarland BH, Huguet N, et al. Acute alcohol intoxication and suicide: a gender-stratified analysis of the National Violent Death Reporting System. Inj Prev. 2013;19(1):38-43.
10. Fla Stat §790.338.
1. Center for Disease Control and Prevention. Injury prevention and control: data and statistics. http://www.cdc.gov/injury/wisqars. Updated December 8, 2015. Accessed April 1, 2016.
2. Narang P, Paladugu A, Manda SR, et al. Do guns provide safety? At what cost? South Med J. 2010;103(2):151-153.
3. Cherlopalle S, Kolikonda MK, Enja M, et al. Guns in America: defense or danger? J Trauma Treat. 2014;3(4):207.
4. Lippmann S. Doctors teaching gun safety. Journal of the Kentucky Medical Association. 2015;113(4):112.
5. Cooke BK, Goddard ER, Ginory A, et al. Firearms inquiries in Florida: “medical privacy” or medical neglect? J Am Acad Psychiatry Law. 2012;40(3):399-408.
6. Valeras AB. Patient with gun. Fam Med. 2013;45(8):584-585.
7. Butkus R, Weissman A. Internists’ attitude toward prevention of firearm injury. Ann Intern Med. 2015;160(12):821-827.
8. Kapp MB. Geriatric patients, firearms, and physicians. Ann Intern Med. 2013;159(6):421-422.
9. Kaplan MS, McFarland BH, Huguet N, et al. Acute alcohol intoxication and suicide: a gender-stratified analysis of the National Violent Death Reporting System. Inj Prev. 2013;19(1):38-43.
10. Fla Stat §790.338.
Psoriasis and Erectile Dysfunction

According to a study by Ji et al published online on February 11 in the International Journal of Impotence Research, men with psoriasis may be more prone to erectile dysfunction (ED) than those without this skin disease, and their odds of sexual difficulties are even higher if they are depressed or have other health problems such as diabetes mellitus or high blood pressure.
The investigators evaluated 191 psoriasis patients and 191 healthy men. Of the 191 patients with psoriasis, 52.9% had symptoms of ED compared with 40.3% of the control group, reflecting an age-adjusted odds ratio of 1.965 in favor of the psoriasis group. A univariate analysis of the psoriasis cohort demonstrated that age, hypertension, hyperlipidemia, diabetes mellitus, and depressive symptoms were risk factors for ED. A multivariate logistic regression model indicated that increasing age, hypertension, hyperlipidemia, and depressive symptoms were independent risk factors for ED in those with psoriasis. More severe depressive symptoms increased the risk of ED, especially moderate to severe ED.
Ji et al noted that ED is a predictor of future cardiovascular disease; therefore, it is important to identify ED early in treatment to evaluate cardiovascular issues in psoriasis patients. They noted that screening of ED may become a part of routine care in the management of psoriasis patients.
What’s the issue?
Even though it was a small study from one location, it still sheds light on many important issues. Psoriasis and its comorbidities appear to increase the risk for ED. In addition, ED also may be an indicator of cardiovascular disease.
How will these data impact your evaluation of psoriasis patients?

According to a study by Ji et al published online on February 11 in the International Journal of Impotence Research, men with psoriasis may be more prone to erectile dysfunction (ED) than those without this skin disease, and their odds of sexual difficulties are even higher if they are depressed or have other health problems such as diabetes mellitus or high blood pressure.
The investigators evaluated 191 psoriasis patients and 191 healthy men. Of the 191 patients with psoriasis, 52.9% had symptoms of ED compared with 40.3% of the control group, reflecting an age-adjusted odds ratio of 1.965 in favor of the psoriasis group. A univariate analysis of the psoriasis cohort demonstrated that age, hypertension, hyperlipidemia, diabetes mellitus, and depressive symptoms were risk factors for ED. A multivariate logistic regression model indicated that increasing age, hypertension, hyperlipidemia, and depressive symptoms were independent risk factors for ED in those with psoriasis. More severe depressive symptoms increased the risk of ED, especially moderate to severe ED.
Ji et al noted that ED is a predictor of future cardiovascular disease; therefore, it is important to identify ED early in treatment to evaluate cardiovascular issues in psoriasis patients. They noted that screening of ED may become a part of routine care in the management of psoriasis patients.
What’s the issue?
Even though it was a small study from one location, it still sheds light on many important issues. Psoriasis and its comorbidities appear to increase the risk for ED. In addition, ED also may be an indicator of cardiovascular disease.
How will these data impact your evaluation of psoriasis patients?

According to a study by Ji et al published online on February 11 in the International Journal of Impotence Research, men with psoriasis may be more prone to erectile dysfunction (ED) than those without this skin disease, and their odds of sexual difficulties are even higher if they are depressed or have other health problems such as diabetes mellitus or high blood pressure.
The investigators evaluated 191 psoriasis patients and 191 healthy men. Of the 191 patients with psoriasis, 52.9% had symptoms of ED compared with 40.3% of the control group, reflecting an age-adjusted odds ratio of 1.965 in favor of the psoriasis group. A univariate analysis of the psoriasis cohort demonstrated that age, hypertension, hyperlipidemia, diabetes mellitus, and depressive symptoms were risk factors for ED. A multivariate logistic regression model indicated that increasing age, hypertension, hyperlipidemia, and depressive symptoms were independent risk factors for ED in those with psoriasis. More severe depressive symptoms increased the risk of ED, especially moderate to severe ED.
Ji et al noted that ED is a predictor of future cardiovascular disease; therefore, it is important to identify ED early in treatment to evaluate cardiovascular issues in psoriasis patients. They noted that screening of ED may become a part of routine care in the management of psoriasis patients.
What’s the issue?
Even though it was a small study from one location, it still sheds light on many important issues. Psoriasis and its comorbidities appear to increase the risk for ED. In addition, ED also may be an indicator of cardiovascular disease.
How will these data impact your evaluation of psoriasis patients?
When ‘eating healthy’ becomes disordered, you can return patients to genuine health
Orthorexia nervosa, from the Greek orthos (straight, proper) and orexia (appetite), is a disorder in which a person demonstrates a pathological obsession not with weight loss but with a “pure” or healthy diet, which can contribute to significant dietary restriction and food-related obsessions. Although the disorder is not a formal diagnosis in DSM 5,1 it is increasingly reported on college campuses and in medical practices, and has been the focus of media attention.
How common is orthorexia?
The precise prevalence of orthorexia nervosa is unknown; some authors have reported estimates as high as 21% of the general population2 and 43.6% of medical students.3 The higher prevalence among medical students might be attributable to the increased focus on factors that can contribute to illnesses (eg, food and diet), and thus underscores the importance of screening for orthorexia symptoms among this population.
How do you identify the disorder?
Orthorexia nervosa was first described by Bratman,4 who observed that a subset of his eating disorder patients were overly obsessed with maintaining an extreme “healthy diet.” Although diagnostic criteria for orthorexia nervosa have not been established, Bratman proposed the following as symptoms indicative of the disorder:
- spending >3 hours a day thinking about a healthy diet
- worrying more about the perceived nutritional quality or “purity” of one’s food than the pleasure of eating it
- feeling guilty about straying from dietary beliefs
- having eating habits that isolate the affected person from others.
Given the focus on this disorder in the media and its presence in medical practice, it is important that you become familiar with the symptoms associated with orthorexia nervosa so you can provide necessary treatment. A patient’s answers to the following questions will aid the savvy clinician in identifying symptoms that suggest orthorexia nervosa5:
- Do you turn to healthy food as a primary source of happiness and meaning, even more so than spirituality?
- Does your diet make you feel superior to other people?
- Does your diet interfere with your personal relationships (family, friends), or with your work?
- Do you use pure foods as a “sword and shield” to ward off anxiety, not just about health problems but about everything that makes you feel insecure?
- Do foods help you feel in control more than really makes sense?
- Do you have to carry your diet to further and further extremes to provide the same “kick”?
- If you stray even minimally from your chosen diet, do you feel a compulsive need to cleanse?
- Has your interest in healthy food expanded past reasonable boundaries to become a kind of brain parasite, so to speak, controlling your life rather than furthering your goals?
No single item is indicative of orthorexia nervosa; however, this list represents a potential clinical picture of how the disorder presents.
Overlap with anorexia nervosa. Although overlap in symptom presentation between these 2 disorders can be significant (eg, diet rigidity can lead to malnutrition, even death), each has important distinguishing features. A low weight status or significant weight loss, or both, is a hallmark characteristic of anorexia nervosa; however, weight loss is not the primary goal in orthorexia nervosa (although extreme dietary restriction in orthorexia could contribute to weight loss). Additionally, a person with anorexia nervosa tends to be preoccupied with weight or shape; a person with orthorexia nervosa is obsessed with food quality and purity. Finally, people with orthorexia have an obsessive preoccupation with health, whereas those with anorexia are more consumed with a fear of fat or weight gain.
Multimodal treatment is indicated
Treating orthorexia typically includes a combination of interventions common to other eating disorders. These include cognitive-behavioral therapy, dietary and nutritional counseling, and medical management of any physical sequelae that result from extreme dietary restriction and malnutrition. Refer patients in whom you suspect orthorexia nervosa to a trained therapist and a dietician who have expertise in managing eating disorders.
It is encouraging to note that, with careful diagnosis and appropriate treatment, recovery from orthorexia is possible,6 and patients can achieve an improved quality of life.
1. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Ramacciotti CE, Perrone P, Coli E, et al. Orthorexia nervosa in the general population: a preliminary screening using a self-administered questionnaire (ORTO-15). Eat Weight Disord. 2011;16(2):e127-e130.
3. Fidan T, Ertekin V, Isikay S, et al. Prevalence of orthorexia among medical students in Erzurum, Turkey. Compr Psychiatry. 2010;51(1):49-54.
4. Bratman S, Knight D. Health food junkies: orthorexia nervosa: overcoming the obsession with healthful eating. New York, NY: Broadway Books; 2000.
5. Bratman S. What is orthorexia? http://www.orthorexia.com. Published January 23, 2014. Accessed March 3, 2016.
6. Fairburn CG, Bohn K. Eating disorder NOS (EDNOS): an example of the troublesome “not otherwise specified” (NOS) category in DSM-IV. Behav Res Ther. 2005;43(6):691-702.
Orthorexia nervosa, from the Greek orthos (straight, proper) and orexia (appetite), is a disorder in which a person demonstrates a pathological obsession not with weight loss but with a “pure” or healthy diet, which can contribute to significant dietary restriction and food-related obsessions. Although the disorder is not a formal diagnosis in DSM 5,1 it is increasingly reported on college campuses and in medical practices, and has been the focus of media attention.
How common is orthorexia?
The precise prevalence of orthorexia nervosa is unknown; some authors have reported estimates as high as 21% of the general population2 and 43.6% of medical students.3 The higher prevalence among medical students might be attributable to the increased focus on factors that can contribute to illnesses (eg, food and diet), and thus underscores the importance of screening for orthorexia symptoms among this population.
How do you identify the disorder?
Orthorexia nervosa was first described by Bratman,4 who observed that a subset of his eating disorder patients were overly obsessed with maintaining an extreme “healthy diet.” Although diagnostic criteria for orthorexia nervosa have not been established, Bratman proposed the following as symptoms indicative of the disorder:
- spending >3 hours a day thinking about a healthy diet
- worrying more about the perceived nutritional quality or “purity” of one’s food than the pleasure of eating it
- feeling guilty about straying from dietary beliefs
- having eating habits that isolate the affected person from others.
Given the focus on this disorder in the media and its presence in medical practice, it is important that you become familiar with the symptoms associated with orthorexia nervosa so you can provide necessary treatment. A patient’s answers to the following questions will aid the savvy clinician in identifying symptoms that suggest orthorexia nervosa5:
- Do you turn to healthy food as a primary source of happiness and meaning, even more so than spirituality?
- Does your diet make you feel superior to other people?
- Does your diet interfere with your personal relationships (family, friends), or with your work?
- Do you use pure foods as a “sword and shield” to ward off anxiety, not just about health problems but about everything that makes you feel insecure?
- Do foods help you feel in control more than really makes sense?
- Do you have to carry your diet to further and further extremes to provide the same “kick”?
- If you stray even minimally from your chosen diet, do you feel a compulsive need to cleanse?
- Has your interest in healthy food expanded past reasonable boundaries to become a kind of brain parasite, so to speak, controlling your life rather than furthering your goals?
No single item is indicative of orthorexia nervosa; however, this list represents a potential clinical picture of how the disorder presents.
Overlap with anorexia nervosa. Although overlap in symptom presentation between these 2 disorders can be significant (eg, diet rigidity can lead to malnutrition, even death), each has important distinguishing features. A low weight status or significant weight loss, or both, is a hallmark characteristic of anorexia nervosa; however, weight loss is not the primary goal in orthorexia nervosa (although extreme dietary restriction in orthorexia could contribute to weight loss). Additionally, a person with anorexia nervosa tends to be preoccupied with weight or shape; a person with orthorexia nervosa is obsessed with food quality and purity. Finally, people with orthorexia have an obsessive preoccupation with health, whereas those with anorexia are more consumed with a fear of fat or weight gain.
Multimodal treatment is indicated
Treating orthorexia typically includes a combination of interventions common to other eating disorders. These include cognitive-behavioral therapy, dietary and nutritional counseling, and medical management of any physical sequelae that result from extreme dietary restriction and malnutrition. Refer patients in whom you suspect orthorexia nervosa to a trained therapist and a dietician who have expertise in managing eating disorders.
It is encouraging to note that, with careful diagnosis and appropriate treatment, recovery from orthorexia is possible,6 and patients can achieve an improved quality of life.
Orthorexia nervosa, from the Greek orthos (straight, proper) and orexia (appetite), is a disorder in which a person demonstrates a pathological obsession not with weight loss but with a “pure” or healthy diet, which can contribute to significant dietary restriction and food-related obsessions. Although the disorder is not a formal diagnosis in DSM 5,1 it is increasingly reported on college campuses and in medical practices, and has been the focus of media attention.
How common is orthorexia?
The precise prevalence of orthorexia nervosa is unknown; some authors have reported estimates as high as 21% of the general population2 and 43.6% of medical students.3 The higher prevalence among medical students might be attributable to the increased focus on factors that can contribute to illnesses (eg, food and diet), and thus underscores the importance of screening for orthorexia symptoms among this population.
How do you identify the disorder?
Orthorexia nervosa was first described by Bratman,4 who observed that a subset of his eating disorder patients were overly obsessed with maintaining an extreme “healthy diet.” Although diagnostic criteria for orthorexia nervosa have not been established, Bratman proposed the following as symptoms indicative of the disorder:
- spending >3 hours a day thinking about a healthy diet
- worrying more about the perceived nutritional quality or “purity” of one’s food than the pleasure of eating it
- feeling guilty about straying from dietary beliefs
- having eating habits that isolate the affected person from others.
Given the focus on this disorder in the media and its presence in medical practice, it is important that you become familiar with the symptoms associated with orthorexia nervosa so you can provide necessary treatment. A patient’s answers to the following questions will aid the savvy clinician in identifying symptoms that suggest orthorexia nervosa5:
- Do you turn to healthy food as a primary source of happiness and meaning, even more so than spirituality?
- Does your diet make you feel superior to other people?
- Does your diet interfere with your personal relationships (family, friends), or with your work?
- Do you use pure foods as a “sword and shield” to ward off anxiety, not just about health problems but about everything that makes you feel insecure?
- Do foods help you feel in control more than really makes sense?
- Do you have to carry your diet to further and further extremes to provide the same “kick”?
- If you stray even minimally from your chosen diet, do you feel a compulsive need to cleanse?
- Has your interest in healthy food expanded past reasonable boundaries to become a kind of brain parasite, so to speak, controlling your life rather than furthering your goals?
No single item is indicative of orthorexia nervosa; however, this list represents a potential clinical picture of how the disorder presents.
Overlap with anorexia nervosa. Although overlap in symptom presentation between these 2 disorders can be significant (eg, diet rigidity can lead to malnutrition, even death), each has important distinguishing features. A low weight status or significant weight loss, or both, is a hallmark characteristic of anorexia nervosa; however, weight loss is not the primary goal in orthorexia nervosa (although extreme dietary restriction in orthorexia could contribute to weight loss). Additionally, a person with anorexia nervosa tends to be preoccupied with weight or shape; a person with orthorexia nervosa is obsessed with food quality and purity. Finally, people with orthorexia have an obsessive preoccupation with health, whereas those with anorexia are more consumed with a fear of fat or weight gain.
Multimodal treatment is indicated
Treating orthorexia typically includes a combination of interventions common to other eating disorders. These include cognitive-behavioral therapy, dietary and nutritional counseling, and medical management of any physical sequelae that result from extreme dietary restriction and malnutrition. Refer patients in whom you suspect orthorexia nervosa to a trained therapist and a dietician who have expertise in managing eating disorders.
It is encouraging to note that, with careful diagnosis and appropriate treatment, recovery from orthorexia is possible,6 and patients can achieve an improved quality of life.
1. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Ramacciotti CE, Perrone P, Coli E, et al. Orthorexia nervosa in the general population: a preliminary screening using a self-administered questionnaire (ORTO-15). Eat Weight Disord. 2011;16(2):e127-e130.
3. Fidan T, Ertekin V, Isikay S, et al. Prevalence of orthorexia among medical students in Erzurum, Turkey. Compr Psychiatry. 2010;51(1):49-54.
4. Bratman S, Knight D. Health food junkies: orthorexia nervosa: overcoming the obsession with healthful eating. New York, NY: Broadway Books; 2000.
5. Bratman S. What is orthorexia? http://www.orthorexia.com. Published January 23, 2014. Accessed March 3, 2016.
6. Fairburn CG, Bohn K. Eating disorder NOS (EDNOS): an example of the troublesome “not otherwise specified” (NOS) category in DSM-IV. Behav Res Ther. 2005;43(6):691-702.
1. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Ramacciotti CE, Perrone P, Coli E, et al. Orthorexia nervosa in the general population: a preliminary screening using a self-administered questionnaire (ORTO-15). Eat Weight Disord. 2011;16(2):e127-e130.
3. Fidan T, Ertekin V, Isikay S, et al. Prevalence of orthorexia among medical students in Erzurum, Turkey. Compr Psychiatry. 2010;51(1):49-54.
4. Bratman S, Knight D. Health food junkies: orthorexia nervosa: overcoming the obsession with healthful eating. New York, NY: Broadway Books; 2000.
5. Bratman S. What is orthorexia? http://www.orthorexia.com. Published January 23, 2014. Accessed March 3, 2016.
6. Fairburn CG, Bohn K. Eating disorder NOS (EDNOS): an example of the troublesome “not otherwise specified” (NOS) category in DSM-IV. Behav Res Ther. 2005;43(6):691-702.
Precipitously and certainly psychotic—but what’s the cause?
CASE Sudden personality change
Ms. L, age 38, is brought to the university hospital’s emergency department (ED) under police escort after she awoke in the middle of the night screaming, “I found it out! I’m a lie! Life is a lie!” and began threatening suicide. This prompted her spouse to call emergency services because of concerns about her safety.
Over the preceding 9 days—and, most precipitously, over the last 24 hours—Ms. L has experienced a dramatic “change in her personality,” according to her spouse. In the ED, she is oriented to person, place, and time. Her vital signs are within normal limits, other than a mild tachycardia. Complete blood count and complete metabolic profile are unremarkable and a urine drug screen is positive only for benzodiazepines (she recently was prescribed alprazolam). Ms. L smiles inappropriately at the ED physicians and confides that she is hearing music by The Lumineers, despite silence in her room.
The psychiatry service is consulted after she is seen making threats of harm to her family members.
EVALUATION Confusion
Over past several weeks, Ms. L has experienced rapid onset of neurovegetative symptoms, with poor oral intake, increased somnolence, neglect of hygiene, excessive time spent in bed, and weight loss of 15 to 20 lb, according to her spouse. She also has been complaining of foggy mentation, weakening handgrip, and tinnitus. She has no previous psychiatric history.
She recently established care with an outpatient neurologist and infectious disease specialist to address these symptoms. Outpatient EEG and sexually transmitted infection (STI) tests were scheduled but not yet obtained. Ms. L’s spouse observes that her drastic “personality change” over the preceding 24 hours coincided with her feeling upset and offended by a physician’s recommendation to obtain STI tests (it is unclear why the physician recommended these tests).
Ms. L had presented to another local ED 4 times over several weeks for various complaints, and had been prescribed alprazolam, 0.5 mg, 3 times a day as needed, and buspirone, 15 mg/d, for anxiety. She also had received a short course of doxycycline, 200 mg/d, which she did not finish, for treatment of presumed Lyme disease. According to her spouse, Ms. L had completed a course of doxycycline for Lyme disease 1 year earlier, but the medical records are not available for review.
During the interview, Ms. L is fairly well groomed but appears confused; she asks her spouse if she is “real” and states that she feels “crazy.” She seems uncomfortable and is guarded, with a minimally reactive, anxious affect. She has general psychomotor slowing and her speech is soft and monotonous, with prominent latency. She reports passive suicidal ideations as well as active auditory hallucinations of a musical quality.
The Mini-Mental State Examination (MMSE) score is 19/30, indicating moderate cognitive impairment, and she is unable to complete attention, executive function, 3-stage command, and delayed word recall tasks. She reports fatigue, diarrhea, and decreased appetite. Her physical examination is notable for an overweight white woman without focal neurologic deficits. Her family psychiatric history reveals bipolar disorder in 2 distant relatives.
In the ED, Ms. L is given 3 provisional diagnoses:
- adjustment disorder, because of her reaction to the proposed STI testing
- psychotic disorder not otherwise specified, because of her obvious psychosis of unknown cause
- rule out delirium due to a general medical condition, because of her sudden onset of attention, perception, and memory difficulties.
As Ms. L sits in her room, her abnormal behaviors become more apparent. She starts to endorse active suicidal ideations and becomes aggressive, trying to choke her spouse, shouting, jumping on her bed, and attempting to strike herself. For her safety, she is physically restrained and given IM haloperidol, 10 mg, and IM lorazepam, 2 mg.
What would you do next to treat Ms. L?
a) Admit her to the psychiatric unit for monitoring and treatment of psychosis and consider additional antipsychotics for agitation
b) Perform a bedside lumbar puncture to assess for findings suggestive of a CNS infection or anomaly
c) Sedate her with IM ketamine, intubate her, and admit her to the intensive care unit (ICU) for further medical workup
d) Begin IV antibiotic therapy with ceftriaxone for early-disseminated Lyme disease with CNS involvement
The authors’ observations
Clearly, Ms. L was psychotic. However, psychosis is a nonspecific term used to describe a heterogeneous group of phenomena in which one experiences an impaired sense of reality. Although commonly caused by psychiatric disorders, psychosis can arise from a variety of causes.1 Ms. L’s initial physical examination and laboratory studies were within the normal range, but her mental status exam and MMSE were abnormal. At this point, selecting the appropriate setting for further observation, workup, and treatment became important.
TREATMENT The right setting
Given the abrupt onset of Ms. L’s symptoms, the treatment team is concerned about active neurologic or infectious disease. However, no acute laboratory or physical examination findings support this hypothesis, and the ED physicians conclude that no further emergent workup is indicated. Because Ms. L is threatening harm to herself and others, she cannot be safely discharged. The treatment team decides the safest option is to admit Ms. L to the inpatient psychiatric unit for observation, further non-emergent workup, and consultation with the neurology service.
At admission. Ms. L is cooperative and calm, lying in bed comfortably. She obeys simple commands; a brief neurologic examination is remarkable for a sedated female without focal motor or sensory deficits. Although her answers to questions are brief, they are appropriate. She sleeps without incident for approximately 10 hours.
The next morning. Ms. L does not awaken to verbal or gentle physical stimuli. Upon sternal rub, she awakens and forcefully squeezes the examiner’s arm, after which she closes her eyes and does not answer further questions (but does resist passive eye opening). After several minutes, she begins exhibiting verbigeration, shouting repeated phrases such as “The birds are in my ears” and “No, I am not okay.”
An emergent EEG is ordered because the team is concerned about nonconvulsive status epilepticus and the neurology service is consulted about the need for an urgent lumbar puncture. Without any obvious abnormal physical examination findings, however, the neurology team’s initial assessment attributes Ms. L’s presentation to a primary psychiatric illness and does not recommend a lumbar puncture or EEG.
That day and night, Ms. L has several episodes of agitation with a disorganized thought process and perseverative speech. She appears distraught and exhibits menacing behaviors. She is poorly redirectable and physically hostile toward staff, requiring several emergent doses of IM haloperidol and IM lorazepam, to which she responds minimally. Ms. L is placed on constant observation, requiring frequent redirection from the rooms of other patients and intermittent seclusion because of her violent, destructive behavior.
The next day. Ms. L remains grossly agitated and psychotic. Although an EEG is ordered, it is not performed because the technicians are concerned about their safety. With her unclear history of Lyme disease and concern for an infectious encephalopathy, Ms. L’s history and symptoms are discussed with the infectious disease service. Given her abrupt onset of symptoms, including auditory hallucinations, they express concern for herpes simplex encephalitis and recommend emergent treatment with IV acyclovir and ceftriaxone.
This recommendation, however, causes a practical conundrum. Because of state laws and differences in staff training, the treatment team believes that the inpatient psychiatric unit is not the appropriate setting to administer these IV treatments. At the same time, hospital security, nursing staff, and the receiving medical team are concerned about transporting Ms. L to the general medical floor.
In the ICU. After discussion, the teams decide that the safest and least traumatic option is to transport Ms. L to the ICU after she is sedated and intubated. In the ICU, she undergoes empirical treatment for herpes simplex encephalitis and further medical workup.
An EEG reveals findings suggestive of severe encephalopathy. A lumbar puncture shows lymphocytic pleocytosis with an opening pressure of 28 cm H2O and normal protein and glucose levels. Her serum C-reactive protein is slightly elevated at 1.4 mg/dL. She also is found to have an elevated herpes simplex virus (HSV)-2 IgG antibody.
Subsequent hospital stay. Ms. L has 2 episodes of seizure-like activity, for which she is treated with levetiracetam, 2,000 mg/d, increased to 3,000 mg/d. She is sedated for several days to allow broad treatment with antiviral and antibiotic medications. Although she experiences intermittent fevers and tachycardia, cultures of blood, urine, and cerebrospinal fluid (CSF) show no growth. Similarly, a test of serum HSV IgM antibodies is negative.
CT of the chest, abdomen, and pelvis reveals no findings suggestive of malignancy but does show a solid-appearing 6-mm nodule in her right lung. Magnetic resonance angiography of the head and neck shows no evidence of abnormalities other than atrophy of the superior cerebellar vermis and a subtle focus of T2/FLAIR signal abnormality in the medial portion of the left occipital lobe.
The following weeks. Ms. L’s cognitive status improves markedly. Extensive studies—including serum ammonia, thyroid-stimulating hormone, Lyme disease antibody, vitamin B12, folate, beta-hCG, HIV, hepatitis B and C, Varicella zoster, syphilis, Lyme disease serology, CSF Eastern equine encephalitis, St. Louis encephalitis virus, West Nile virus, Ehrlichia chaffeensis, Babesia microti, Rocky Mountain spotted fever, John Cunningham virus, typhus fever, cryptococcal antigen, rabies, 2 serum tests for anti-N-methyl-D-aspartate (NMDA) receptor antibodies, and serum ceruloplasmin—are normal.
At discharge, Ms. L’s clinical presentation is thought to be most consistent with viral encephalitis, because of her CSF lymphocytic pleocytosis, fever, and improvement with supportive care. Because she improves, the team does not find it necessary to wait for results of pending studies, including a paraneoplastic autoantibody panel and a CSF anti-NMDA receptor antibody, before discharging her.
Readmission. Although the results of the paraneoplastic autoantibody panel are unremarkable, several weeks after discharge Ms. L’s CSF anti-NMDA receptor antibodies return positive, despite 2 earlier negative serum studies. She is readmitted to the neurology service for treatment with immunomodulators.
A positron-emission tomography scan is negative for malignancy. She is treated on an ongoing basis with immunomodulators; cognition improves such that she is able to start working again with good overall functioning. Despite this improvement, she experiences residual sequelae, including noise sensitivity, amnesia of the events surrounding her hospitalization, mild short-term memory deficits, and persistent affective blunting.
The authors’ observations
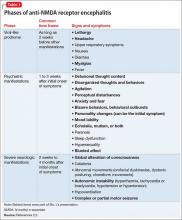
Psychosis is not exclusive to psychiatric syndromes and frequently is a symptom of an underlying neurologic, immunologic, metabolic, infectious, or oncologic abnormality.1 Anti-NMDA receptor encephalitis is an autoimmune disease in which antibodies attack NMDA-type glutamate receptors at central neuronal synapses and can produce psychosis, as seen with Ms. L2 (Table 12,3). The etiology of the disease is not fully understood. Determining the appropriate setting to perform a complete medical workup in a severely agitated patient after an initial negative medical workup can be challenging.
What’s the most appropriate treatment setting?
This case illustrates the importance, with any new-onset psychosis, of weighing heavily a carefully obtained psychiatric history, even in the absence of focal physical examination and initial laboratory abnormalities. It also highlights the challenge of determining the most appropriate initial setting for performing the important task of a complete medical workup for first-episode psychosis.
Ms. L initially was treated in the inpatient psychiatric unit because of safety concerns and practical limitations, but was later found to have a disease that could not be managed in that setting. She proved to be too agitated to obtain a full medical workup on the inpatient psychiatric or general medical floors and required transfer to the ICU. Despite her normal basic laboratory tests, her EEG and CSF studies did demonstrate abnormalities, suggesting these can be useful to the basic workup for psychosis of unknown cause (Table 21,2).
This case also demonstrates that negative serum anti-NMDA receptor antibody tests do not rule out the disease; one study found that only 85% of patients with CSF anti-NMDA receptor antibodies also had detectable antibodies in their serum and that detectability changed during the course of the disease.4 This supports the utility of a lumbar puncture as part of a basic initial workup for some cases of new-onset psychosis. Because clinical outcomes often correlate with early treatment, as with anti-NMDA receptor encephalitis, a timely diagnostic workup of psychosis often can be important.3,5 The ICU can be considered an appropriate setting for working up some patients who develop new, rapid-onset psychosis and severe agitation, even in the absence of initial laboratory or physical examination findings.
Ms. L’s case also illustrates the importance of completing a thorough medical workup for patients with new-onset psychosis before transferring them to an independent psychiatric hospital. Initially, the university’s psychiatric unit was at capacity and a bed was sought at outside psychiatric hospitals while Ms. L waited in the ED. Had Ms. L not been admitted to a large academic medical center, she may not have had access to the multidisciplinary collaboration that proved necessary for the appropriate diagnosis and treatment of her anti-NMDA receptor encephalitis (Table 35,6).
What prodromal symptoms occur as long as 2 weeks as an initial presentation in many patients with anti-NMDA receptor encephalitis?
a) Flu-like symptoms of lethargy, headache, gastrointestinal symptoms, myalgias, fevers, and upper respiratory symptoms
b) Delusions, hallucinations, disorganized behaviors and thoughts, behavioral outbursts, hypersexuality, mood lability, personality change, paranoia, echolalia, mutism, anxiety, agitation, aggression, hyperactivity, sleep dysfunction, and blunted affect
c) Dyskinesias, autonomic instability, central hypoventilation, and seizures
The authors’ observations
Lab results, vital signs, and physical examination should not supplant a careful history when determining an appropriate clinical course of action. As experts in the cognitive sciences, psychiatrists may be the most qualified in determining whether a patient with new-onset psychosis should undergo further medical testing before a condition is deemed to be solely of a psychiatric cause. As a neurologic disease of immunologic origin with psychiatric manifestations, anti-NMDA receptor encephalitis is a complex condition requiring collaboration among several specialists for appropriate management.
1. Freudenreich O. Differential diagnosis of psychotic symptoms: medical “mimics.” Psychiatric Times. http://www.psychiatrictimes.com/forensic-psychiatry/differential-diagnosis-psychotic-symptoms-medical-%E2%80%9Cmimics%E2%80%9D. Published December 3, 2012. Accessed March 31, 2016.
2. Kayser MS, Dalmau J. Anti-NMDA receptor encephalitis in psychiatry. Curr Psychiatry Rev. 2011;7(3):189-193.
3. Dalmau J, Lancaster E, Martinez-Hernandez E, et al. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10(1):63-74.
4. Gresa-Arribas N, Titulaer MJ, Torrents A, et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol. 2014;13(2):167-177.
5. Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7(12):1091-1098.
6. Dalmau J, Tüzün E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61(1):25-36.
CASE Sudden personality change
Ms. L, age 38, is brought to the university hospital’s emergency department (ED) under police escort after she awoke in the middle of the night screaming, “I found it out! I’m a lie! Life is a lie!” and began threatening suicide. This prompted her spouse to call emergency services because of concerns about her safety.
Over the preceding 9 days—and, most precipitously, over the last 24 hours—Ms. L has experienced a dramatic “change in her personality,” according to her spouse. In the ED, she is oriented to person, place, and time. Her vital signs are within normal limits, other than a mild tachycardia. Complete blood count and complete metabolic profile are unremarkable and a urine drug screen is positive only for benzodiazepines (she recently was prescribed alprazolam). Ms. L smiles inappropriately at the ED physicians and confides that she is hearing music by The Lumineers, despite silence in her room.
The psychiatry service is consulted after she is seen making threats of harm to her family members.
EVALUATION Confusion
Over past several weeks, Ms. L has experienced rapid onset of neurovegetative symptoms, with poor oral intake, increased somnolence, neglect of hygiene, excessive time spent in bed, and weight loss of 15 to 20 lb, according to her spouse. She also has been complaining of foggy mentation, weakening handgrip, and tinnitus. She has no previous psychiatric history.
She recently established care with an outpatient neurologist and infectious disease specialist to address these symptoms. Outpatient EEG and sexually transmitted infection (STI) tests were scheduled but not yet obtained. Ms. L’s spouse observes that her drastic “personality change” over the preceding 24 hours coincided with her feeling upset and offended by a physician’s recommendation to obtain STI tests (it is unclear why the physician recommended these tests).
Ms. L had presented to another local ED 4 times over several weeks for various complaints, and had been prescribed alprazolam, 0.5 mg, 3 times a day as needed, and buspirone, 15 mg/d, for anxiety. She also had received a short course of doxycycline, 200 mg/d, which she did not finish, for treatment of presumed Lyme disease. According to her spouse, Ms. L had completed a course of doxycycline for Lyme disease 1 year earlier, but the medical records are not available for review.
During the interview, Ms. L is fairly well groomed but appears confused; she asks her spouse if she is “real” and states that she feels “crazy.” She seems uncomfortable and is guarded, with a minimally reactive, anxious affect. She has general psychomotor slowing and her speech is soft and monotonous, with prominent latency. She reports passive suicidal ideations as well as active auditory hallucinations of a musical quality.
The Mini-Mental State Examination (MMSE) score is 19/30, indicating moderate cognitive impairment, and she is unable to complete attention, executive function, 3-stage command, and delayed word recall tasks. She reports fatigue, diarrhea, and decreased appetite. Her physical examination is notable for an overweight white woman without focal neurologic deficits. Her family psychiatric history reveals bipolar disorder in 2 distant relatives.
In the ED, Ms. L is given 3 provisional diagnoses:
- adjustment disorder, because of her reaction to the proposed STI testing
- psychotic disorder not otherwise specified, because of her obvious psychosis of unknown cause
- rule out delirium due to a general medical condition, because of her sudden onset of attention, perception, and memory difficulties.
As Ms. L sits in her room, her abnormal behaviors become more apparent. She starts to endorse active suicidal ideations and becomes aggressive, trying to choke her spouse, shouting, jumping on her bed, and attempting to strike herself. For her safety, she is physically restrained and given IM haloperidol, 10 mg, and IM lorazepam, 2 mg.
What would you do next to treat Ms. L?
a) Admit her to the psychiatric unit for monitoring and treatment of psychosis and consider additional antipsychotics for agitation
b) Perform a bedside lumbar puncture to assess for findings suggestive of a CNS infection or anomaly
c) Sedate her with IM ketamine, intubate her, and admit her to the intensive care unit (ICU) for further medical workup
d) Begin IV antibiotic therapy with ceftriaxone for early-disseminated Lyme disease with CNS involvement
The authors’ observations
Clearly, Ms. L was psychotic. However, psychosis is a nonspecific term used to describe a heterogeneous group of phenomena in which one experiences an impaired sense of reality. Although commonly caused by psychiatric disorders, psychosis can arise from a variety of causes.1 Ms. L’s initial physical examination and laboratory studies were within the normal range, but her mental status exam and MMSE were abnormal. At this point, selecting the appropriate setting for further observation, workup, and treatment became important.
TREATMENT The right setting
Given the abrupt onset of Ms. L’s symptoms, the treatment team is concerned about active neurologic or infectious disease. However, no acute laboratory or physical examination findings support this hypothesis, and the ED physicians conclude that no further emergent workup is indicated. Because Ms. L is threatening harm to herself and others, she cannot be safely discharged. The treatment team decides the safest option is to admit Ms. L to the inpatient psychiatric unit for observation, further non-emergent workup, and consultation with the neurology service.
At admission. Ms. L is cooperative and calm, lying in bed comfortably. She obeys simple commands; a brief neurologic examination is remarkable for a sedated female without focal motor or sensory deficits. Although her answers to questions are brief, they are appropriate. She sleeps without incident for approximately 10 hours.
The next morning. Ms. L does not awaken to verbal or gentle physical stimuli. Upon sternal rub, she awakens and forcefully squeezes the examiner’s arm, after which she closes her eyes and does not answer further questions (but does resist passive eye opening). After several minutes, she begins exhibiting verbigeration, shouting repeated phrases such as “The birds are in my ears” and “No, I am not okay.”
An emergent EEG is ordered because the team is concerned about nonconvulsive status epilepticus and the neurology service is consulted about the need for an urgent lumbar puncture. Without any obvious abnormal physical examination findings, however, the neurology team’s initial assessment attributes Ms. L’s presentation to a primary psychiatric illness and does not recommend a lumbar puncture or EEG.
That day and night, Ms. L has several episodes of agitation with a disorganized thought process and perseverative speech. She appears distraught and exhibits menacing behaviors. She is poorly redirectable and physically hostile toward staff, requiring several emergent doses of IM haloperidol and IM lorazepam, to which she responds minimally. Ms. L is placed on constant observation, requiring frequent redirection from the rooms of other patients and intermittent seclusion because of her violent, destructive behavior.
The next day. Ms. L remains grossly agitated and psychotic. Although an EEG is ordered, it is not performed because the technicians are concerned about their safety. With her unclear history of Lyme disease and concern for an infectious encephalopathy, Ms. L’s history and symptoms are discussed with the infectious disease service. Given her abrupt onset of symptoms, including auditory hallucinations, they express concern for herpes simplex encephalitis and recommend emergent treatment with IV acyclovir and ceftriaxone.
This recommendation, however, causes a practical conundrum. Because of state laws and differences in staff training, the treatment team believes that the inpatient psychiatric unit is not the appropriate setting to administer these IV treatments. At the same time, hospital security, nursing staff, and the receiving medical team are concerned about transporting Ms. L to the general medical floor.
In the ICU. After discussion, the teams decide that the safest and least traumatic option is to transport Ms. L to the ICU after she is sedated and intubated. In the ICU, she undergoes empirical treatment for herpes simplex encephalitis and further medical workup.
An EEG reveals findings suggestive of severe encephalopathy. A lumbar puncture shows lymphocytic pleocytosis with an opening pressure of 28 cm H2O and normal protein and glucose levels. Her serum C-reactive protein is slightly elevated at 1.4 mg/dL. She also is found to have an elevated herpes simplex virus (HSV)-2 IgG antibody.
Subsequent hospital stay. Ms. L has 2 episodes of seizure-like activity, for which she is treated with levetiracetam, 2,000 mg/d, increased to 3,000 mg/d. She is sedated for several days to allow broad treatment with antiviral and antibiotic medications. Although she experiences intermittent fevers and tachycardia, cultures of blood, urine, and cerebrospinal fluid (CSF) show no growth. Similarly, a test of serum HSV IgM antibodies is negative.
CT of the chest, abdomen, and pelvis reveals no findings suggestive of malignancy but does show a solid-appearing 6-mm nodule in her right lung. Magnetic resonance angiography of the head and neck shows no evidence of abnormalities other than atrophy of the superior cerebellar vermis and a subtle focus of T2/FLAIR signal abnormality in the medial portion of the left occipital lobe.
The following weeks. Ms. L’s cognitive status improves markedly. Extensive studies—including serum ammonia, thyroid-stimulating hormone, Lyme disease antibody, vitamin B12, folate, beta-hCG, HIV, hepatitis B and C, Varicella zoster, syphilis, Lyme disease serology, CSF Eastern equine encephalitis, St. Louis encephalitis virus, West Nile virus, Ehrlichia chaffeensis, Babesia microti, Rocky Mountain spotted fever, John Cunningham virus, typhus fever, cryptococcal antigen, rabies, 2 serum tests for anti-N-methyl-D-aspartate (NMDA) receptor antibodies, and serum ceruloplasmin—are normal.
At discharge, Ms. L’s clinical presentation is thought to be most consistent with viral encephalitis, because of her CSF lymphocytic pleocytosis, fever, and improvement with supportive care. Because she improves, the team does not find it necessary to wait for results of pending studies, including a paraneoplastic autoantibody panel and a CSF anti-NMDA receptor antibody, before discharging her.
Readmission. Although the results of the paraneoplastic autoantibody panel are unremarkable, several weeks after discharge Ms. L’s CSF anti-NMDA receptor antibodies return positive, despite 2 earlier negative serum studies. She is readmitted to the neurology service for treatment with immunomodulators.
A positron-emission tomography scan is negative for malignancy. She is treated on an ongoing basis with immunomodulators; cognition improves such that she is able to start working again with good overall functioning. Despite this improvement, she experiences residual sequelae, including noise sensitivity, amnesia of the events surrounding her hospitalization, mild short-term memory deficits, and persistent affective blunting.
The authors’ observations
Psychosis is not exclusive to psychiatric syndromes and frequently is a symptom of an underlying neurologic, immunologic, metabolic, infectious, or oncologic abnormality.1 Anti-NMDA receptor encephalitis is an autoimmune disease in which antibodies attack NMDA-type glutamate receptors at central neuronal synapses and can produce psychosis, as seen with Ms. L2 (Table 12,3). The etiology of the disease is not fully understood. Determining the appropriate setting to perform a complete medical workup in a severely agitated patient after an initial negative medical workup can be challenging.
What’s the most appropriate treatment setting?
This case illustrates the importance, with any new-onset psychosis, of weighing heavily a carefully obtained psychiatric history, even in the absence of focal physical examination and initial laboratory abnormalities. It also highlights the challenge of determining the most appropriate initial setting for performing the important task of a complete medical workup for first-episode psychosis.
Ms. L initially was treated in the inpatient psychiatric unit because of safety concerns and practical limitations, but was later found to have a disease that could not be managed in that setting. She proved to be too agitated to obtain a full medical workup on the inpatient psychiatric or general medical floors and required transfer to the ICU. Despite her normal basic laboratory tests, her EEG and CSF studies did demonstrate abnormalities, suggesting these can be useful to the basic workup for psychosis of unknown cause (Table 21,2).
This case also demonstrates that negative serum anti-NMDA receptor antibody tests do not rule out the disease; one study found that only 85% of patients with CSF anti-NMDA receptor antibodies also had detectable antibodies in their serum and that detectability changed during the course of the disease.4 This supports the utility of a lumbar puncture as part of a basic initial workup for some cases of new-onset psychosis. Because clinical outcomes often correlate with early treatment, as with anti-NMDA receptor encephalitis, a timely diagnostic workup of psychosis often can be important.3,5 The ICU can be considered an appropriate setting for working up some patients who develop new, rapid-onset psychosis and severe agitation, even in the absence of initial laboratory or physical examination findings.
Ms. L’s case also illustrates the importance of completing a thorough medical workup for patients with new-onset psychosis before transferring them to an independent psychiatric hospital. Initially, the university’s psychiatric unit was at capacity and a bed was sought at outside psychiatric hospitals while Ms. L waited in the ED. Had Ms. L not been admitted to a large academic medical center, she may not have had access to the multidisciplinary collaboration that proved necessary for the appropriate diagnosis and treatment of her anti-NMDA receptor encephalitis (Table 35,6).
What prodromal symptoms occur as long as 2 weeks as an initial presentation in many patients with anti-NMDA receptor encephalitis?
a) Flu-like symptoms of lethargy, headache, gastrointestinal symptoms, myalgias, fevers, and upper respiratory symptoms
b) Delusions, hallucinations, disorganized behaviors and thoughts, behavioral outbursts, hypersexuality, mood lability, personality change, paranoia, echolalia, mutism, anxiety, agitation, aggression, hyperactivity, sleep dysfunction, and blunted affect
c) Dyskinesias, autonomic instability, central hypoventilation, and seizures
The authors’ observations
Lab results, vital signs, and physical examination should not supplant a careful history when determining an appropriate clinical course of action. As experts in the cognitive sciences, psychiatrists may be the most qualified in determining whether a patient with new-onset psychosis should undergo further medical testing before a condition is deemed to be solely of a psychiatric cause. As a neurologic disease of immunologic origin with psychiatric manifestations, anti-NMDA receptor encephalitis is a complex condition requiring collaboration among several specialists for appropriate management.
CASE Sudden personality change
Ms. L, age 38, is brought to the university hospital’s emergency department (ED) under police escort after she awoke in the middle of the night screaming, “I found it out! I’m a lie! Life is a lie!” and began threatening suicide. This prompted her spouse to call emergency services because of concerns about her safety.
Over the preceding 9 days—and, most precipitously, over the last 24 hours—Ms. L has experienced a dramatic “change in her personality,” according to her spouse. In the ED, she is oriented to person, place, and time. Her vital signs are within normal limits, other than a mild tachycardia. Complete blood count and complete metabolic profile are unremarkable and a urine drug screen is positive only for benzodiazepines (she recently was prescribed alprazolam). Ms. L smiles inappropriately at the ED physicians and confides that she is hearing music by The Lumineers, despite silence in her room.
The psychiatry service is consulted after she is seen making threats of harm to her family members.
EVALUATION Confusion
Over past several weeks, Ms. L has experienced rapid onset of neurovegetative symptoms, with poor oral intake, increased somnolence, neglect of hygiene, excessive time spent in bed, and weight loss of 15 to 20 lb, according to her spouse. She also has been complaining of foggy mentation, weakening handgrip, and tinnitus. She has no previous psychiatric history.
She recently established care with an outpatient neurologist and infectious disease specialist to address these symptoms. Outpatient EEG and sexually transmitted infection (STI) tests were scheduled but not yet obtained. Ms. L’s spouse observes that her drastic “personality change” over the preceding 24 hours coincided with her feeling upset and offended by a physician’s recommendation to obtain STI tests (it is unclear why the physician recommended these tests).
Ms. L had presented to another local ED 4 times over several weeks for various complaints, and had been prescribed alprazolam, 0.5 mg, 3 times a day as needed, and buspirone, 15 mg/d, for anxiety. She also had received a short course of doxycycline, 200 mg/d, which she did not finish, for treatment of presumed Lyme disease. According to her spouse, Ms. L had completed a course of doxycycline for Lyme disease 1 year earlier, but the medical records are not available for review.
During the interview, Ms. L is fairly well groomed but appears confused; she asks her spouse if she is “real” and states that she feels “crazy.” She seems uncomfortable and is guarded, with a minimally reactive, anxious affect. She has general psychomotor slowing and her speech is soft and monotonous, with prominent latency. She reports passive suicidal ideations as well as active auditory hallucinations of a musical quality.
The Mini-Mental State Examination (MMSE) score is 19/30, indicating moderate cognitive impairment, and she is unable to complete attention, executive function, 3-stage command, and delayed word recall tasks. She reports fatigue, diarrhea, and decreased appetite. Her physical examination is notable for an overweight white woman without focal neurologic deficits. Her family psychiatric history reveals bipolar disorder in 2 distant relatives.
In the ED, Ms. L is given 3 provisional diagnoses:
- adjustment disorder, because of her reaction to the proposed STI testing
- psychotic disorder not otherwise specified, because of her obvious psychosis of unknown cause
- rule out delirium due to a general medical condition, because of her sudden onset of attention, perception, and memory difficulties.
As Ms. L sits in her room, her abnormal behaviors become more apparent. She starts to endorse active suicidal ideations and becomes aggressive, trying to choke her spouse, shouting, jumping on her bed, and attempting to strike herself. For her safety, she is physically restrained and given IM haloperidol, 10 mg, and IM lorazepam, 2 mg.
What would you do next to treat Ms. L?
a) Admit her to the psychiatric unit for monitoring and treatment of psychosis and consider additional antipsychotics for agitation
b) Perform a bedside lumbar puncture to assess for findings suggestive of a CNS infection or anomaly
c) Sedate her with IM ketamine, intubate her, and admit her to the intensive care unit (ICU) for further medical workup
d) Begin IV antibiotic therapy with ceftriaxone for early-disseminated Lyme disease with CNS involvement
The authors’ observations
Clearly, Ms. L was psychotic. However, psychosis is a nonspecific term used to describe a heterogeneous group of phenomena in which one experiences an impaired sense of reality. Although commonly caused by psychiatric disorders, psychosis can arise from a variety of causes.1 Ms. L’s initial physical examination and laboratory studies were within the normal range, but her mental status exam and MMSE were abnormal. At this point, selecting the appropriate setting for further observation, workup, and treatment became important.
TREATMENT The right setting
Given the abrupt onset of Ms. L’s symptoms, the treatment team is concerned about active neurologic or infectious disease. However, no acute laboratory or physical examination findings support this hypothesis, and the ED physicians conclude that no further emergent workup is indicated. Because Ms. L is threatening harm to herself and others, she cannot be safely discharged. The treatment team decides the safest option is to admit Ms. L to the inpatient psychiatric unit for observation, further non-emergent workup, and consultation with the neurology service.
At admission. Ms. L is cooperative and calm, lying in bed comfortably. She obeys simple commands; a brief neurologic examination is remarkable for a sedated female without focal motor or sensory deficits. Although her answers to questions are brief, they are appropriate. She sleeps without incident for approximately 10 hours.
The next morning. Ms. L does not awaken to verbal or gentle physical stimuli. Upon sternal rub, she awakens and forcefully squeezes the examiner’s arm, after which she closes her eyes and does not answer further questions (but does resist passive eye opening). After several minutes, she begins exhibiting verbigeration, shouting repeated phrases such as “The birds are in my ears” and “No, I am not okay.”
An emergent EEG is ordered because the team is concerned about nonconvulsive status epilepticus and the neurology service is consulted about the need for an urgent lumbar puncture. Without any obvious abnormal physical examination findings, however, the neurology team’s initial assessment attributes Ms. L’s presentation to a primary psychiatric illness and does not recommend a lumbar puncture or EEG.
That day and night, Ms. L has several episodes of agitation with a disorganized thought process and perseverative speech. She appears distraught and exhibits menacing behaviors. She is poorly redirectable and physically hostile toward staff, requiring several emergent doses of IM haloperidol and IM lorazepam, to which she responds minimally. Ms. L is placed on constant observation, requiring frequent redirection from the rooms of other patients and intermittent seclusion because of her violent, destructive behavior.
The next day. Ms. L remains grossly agitated and psychotic. Although an EEG is ordered, it is not performed because the technicians are concerned about their safety. With her unclear history of Lyme disease and concern for an infectious encephalopathy, Ms. L’s history and symptoms are discussed with the infectious disease service. Given her abrupt onset of symptoms, including auditory hallucinations, they express concern for herpes simplex encephalitis and recommend emergent treatment with IV acyclovir and ceftriaxone.
This recommendation, however, causes a practical conundrum. Because of state laws and differences in staff training, the treatment team believes that the inpatient psychiatric unit is not the appropriate setting to administer these IV treatments. At the same time, hospital security, nursing staff, and the receiving medical team are concerned about transporting Ms. L to the general medical floor.
In the ICU. After discussion, the teams decide that the safest and least traumatic option is to transport Ms. L to the ICU after she is sedated and intubated. In the ICU, she undergoes empirical treatment for herpes simplex encephalitis and further medical workup.
An EEG reveals findings suggestive of severe encephalopathy. A lumbar puncture shows lymphocytic pleocytosis with an opening pressure of 28 cm H2O and normal protein and glucose levels. Her serum C-reactive protein is slightly elevated at 1.4 mg/dL. She also is found to have an elevated herpes simplex virus (HSV)-2 IgG antibody.
Subsequent hospital stay. Ms. L has 2 episodes of seizure-like activity, for which she is treated with levetiracetam, 2,000 mg/d, increased to 3,000 mg/d. She is sedated for several days to allow broad treatment with antiviral and antibiotic medications. Although she experiences intermittent fevers and tachycardia, cultures of blood, urine, and cerebrospinal fluid (CSF) show no growth. Similarly, a test of serum HSV IgM antibodies is negative.
CT of the chest, abdomen, and pelvis reveals no findings suggestive of malignancy but does show a solid-appearing 6-mm nodule in her right lung. Magnetic resonance angiography of the head and neck shows no evidence of abnormalities other than atrophy of the superior cerebellar vermis and a subtle focus of T2/FLAIR signal abnormality in the medial portion of the left occipital lobe.
The following weeks. Ms. L’s cognitive status improves markedly. Extensive studies—including serum ammonia, thyroid-stimulating hormone, Lyme disease antibody, vitamin B12, folate, beta-hCG, HIV, hepatitis B and C, Varicella zoster, syphilis, Lyme disease serology, CSF Eastern equine encephalitis, St. Louis encephalitis virus, West Nile virus, Ehrlichia chaffeensis, Babesia microti, Rocky Mountain spotted fever, John Cunningham virus, typhus fever, cryptococcal antigen, rabies, 2 serum tests for anti-N-methyl-D-aspartate (NMDA) receptor antibodies, and serum ceruloplasmin—are normal.
At discharge, Ms. L’s clinical presentation is thought to be most consistent with viral encephalitis, because of her CSF lymphocytic pleocytosis, fever, and improvement with supportive care. Because she improves, the team does not find it necessary to wait for results of pending studies, including a paraneoplastic autoantibody panel and a CSF anti-NMDA receptor antibody, before discharging her.
Readmission. Although the results of the paraneoplastic autoantibody panel are unremarkable, several weeks after discharge Ms. L’s CSF anti-NMDA receptor antibodies return positive, despite 2 earlier negative serum studies. She is readmitted to the neurology service for treatment with immunomodulators.
A positron-emission tomography scan is negative for malignancy. She is treated on an ongoing basis with immunomodulators; cognition improves such that she is able to start working again with good overall functioning. Despite this improvement, she experiences residual sequelae, including noise sensitivity, amnesia of the events surrounding her hospitalization, mild short-term memory deficits, and persistent affective blunting.
The authors’ observations
Psychosis is not exclusive to psychiatric syndromes and frequently is a symptom of an underlying neurologic, immunologic, metabolic, infectious, or oncologic abnormality.1 Anti-NMDA receptor encephalitis is an autoimmune disease in which antibodies attack NMDA-type glutamate receptors at central neuronal synapses and can produce psychosis, as seen with Ms. L2 (Table 12,3). The etiology of the disease is not fully understood. Determining the appropriate setting to perform a complete medical workup in a severely agitated patient after an initial negative medical workup can be challenging.
What’s the most appropriate treatment setting?
This case illustrates the importance, with any new-onset psychosis, of weighing heavily a carefully obtained psychiatric history, even in the absence of focal physical examination and initial laboratory abnormalities. It also highlights the challenge of determining the most appropriate initial setting for performing the important task of a complete medical workup for first-episode psychosis.
Ms. L initially was treated in the inpatient psychiatric unit because of safety concerns and practical limitations, but was later found to have a disease that could not be managed in that setting. She proved to be too agitated to obtain a full medical workup on the inpatient psychiatric or general medical floors and required transfer to the ICU. Despite her normal basic laboratory tests, her EEG and CSF studies did demonstrate abnormalities, suggesting these can be useful to the basic workup for psychosis of unknown cause (Table 21,2).
This case also demonstrates that negative serum anti-NMDA receptor antibody tests do not rule out the disease; one study found that only 85% of patients with CSF anti-NMDA receptor antibodies also had detectable antibodies in their serum and that detectability changed during the course of the disease.4 This supports the utility of a lumbar puncture as part of a basic initial workup for some cases of new-onset psychosis. Because clinical outcomes often correlate with early treatment, as with anti-NMDA receptor encephalitis, a timely diagnostic workup of psychosis often can be important.3,5 The ICU can be considered an appropriate setting for working up some patients who develop new, rapid-onset psychosis and severe agitation, even in the absence of initial laboratory or physical examination findings.
Ms. L’s case also illustrates the importance of completing a thorough medical workup for patients with new-onset psychosis before transferring them to an independent psychiatric hospital. Initially, the university’s psychiatric unit was at capacity and a bed was sought at outside psychiatric hospitals while Ms. L waited in the ED. Had Ms. L not been admitted to a large academic medical center, she may not have had access to the multidisciplinary collaboration that proved necessary for the appropriate diagnosis and treatment of her anti-NMDA receptor encephalitis (Table 35,6).
What prodromal symptoms occur as long as 2 weeks as an initial presentation in many patients with anti-NMDA receptor encephalitis?
a) Flu-like symptoms of lethargy, headache, gastrointestinal symptoms, myalgias, fevers, and upper respiratory symptoms
b) Delusions, hallucinations, disorganized behaviors and thoughts, behavioral outbursts, hypersexuality, mood lability, personality change, paranoia, echolalia, mutism, anxiety, agitation, aggression, hyperactivity, sleep dysfunction, and blunted affect
c) Dyskinesias, autonomic instability, central hypoventilation, and seizures
The authors’ observations
Lab results, vital signs, and physical examination should not supplant a careful history when determining an appropriate clinical course of action. As experts in the cognitive sciences, psychiatrists may be the most qualified in determining whether a patient with new-onset psychosis should undergo further medical testing before a condition is deemed to be solely of a psychiatric cause. As a neurologic disease of immunologic origin with psychiatric manifestations, anti-NMDA receptor encephalitis is a complex condition requiring collaboration among several specialists for appropriate management.
1. Freudenreich O. Differential diagnosis of psychotic symptoms: medical “mimics.” Psychiatric Times. http://www.psychiatrictimes.com/forensic-psychiatry/differential-diagnosis-psychotic-symptoms-medical-%E2%80%9Cmimics%E2%80%9D. Published December 3, 2012. Accessed March 31, 2016.
2. Kayser MS, Dalmau J. Anti-NMDA receptor encephalitis in psychiatry. Curr Psychiatry Rev. 2011;7(3):189-193.
3. Dalmau J, Lancaster E, Martinez-Hernandez E, et al. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10(1):63-74.
4. Gresa-Arribas N, Titulaer MJ, Torrents A, et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol. 2014;13(2):167-177.
5. Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7(12):1091-1098.
6. Dalmau J, Tüzün E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61(1):25-36.
1. Freudenreich O. Differential diagnosis of psychotic symptoms: medical “mimics.” Psychiatric Times. http://www.psychiatrictimes.com/forensic-psychiatry/differential-diagnosis-psychotic-symptoms-medical-%E2%80%9Cmimics%E2%80%9D. Published December 3, 2012. Accessed March 31, 2016.
2. Kayser MS, Dalmau J. Anti-NMDA receptor encephalitis in psychiatry. Curr Psychiatry Rev. 2011;7(3):189-193.
3. Dalmau J, Lancaster E, Martinez-Hernandez E, et al. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10(1):63-74.
4. Gresa-Arribas N, Titulaer MJ, Torrents A, et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol. 2014;13(2):167-177.
5. Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7(12):1091-1098.
6. Dalmau J, Tüzün E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61(1):25-36.
Is the evidence compelling for using ketamine to treat resistant depression?
Ms. B, age 31, experienced her first depressive episode at age 24 during her second year of law school. These episodes are characterized by insomnia, sadness, guilt, suicidal ideation, and impaired concentration that affect her ability to function at work and interfere with her ability to maintain relationships. She has no history of mania, hypomania, or psychosis.
Ms. B has approximately 2 severe episodes a year, lasting 8 to 10 weeks. She has failed adequate (≥6 week) trials of sertraline, 200 mg/d; venlafaxine XR, 300 mg/d; bupropion XL, 450 mg/d; and vortioxetine, 20 mg/d. Adjunctive treatments were not well tolerated; lithium caused severe nausea and aripiprazole lead to intolerable akathisia. Psychotherapy was ineffective. A trial of electroconvulsive therapy relieved her depression but resulted in significant memory impairment.
Is ketamine a treatment option for Ms. B?
Ketamine, an N-methyl-D aspartate antagonist, was approved by the FDA in 1970.
as a dissociative anesthetic. It proved useful in military battlefield situations. The drug then became popular as a “club drug” and is used recreationally as a dissociative agent. It recently has been used clinically for treating post-operative pain and treatment-resistant depression (TRD). It has shown efficacy for several specific symptom clusters in depression, including anhedonia and suicidality.
Several small randomized, double-blind, placebo-controlled trials of ketamine—some of which studied TRD—have reported antidepressant effects after a single IV dose of 0.5 mg/kg in depressed patients.1,2 The response rate, defined as a 50% reduction in symptoms, is reported to be as high as 50% to 71% twenty-four hours after infusion, with significant improvements noted in some patients after just 40 minutes.1 These effects, peaking at 24 hours, last ≥72 hours in approximately 50% of patients, but gradually return to baseline over 1 to 2 weeks (Figure1). The most common post-infusion adverse effects include:
- dissociation
- dizziness
- blurred vision
- poor concentration
- nausea.
Transient sedation and psychotomimetic symptoms, such as hallucinations, abnormal sensations, and confusion, also have been noted, as well as a small but significant increase in blood pressure shortly after infusion.1
Use of repeated doses of ketamine also has been studied, although larger and extended-duration studies are lacking. Two groups3,4 examined thrice weekly infusions (N = 24) and 1 group5 studied twice weekly infusions of 0.5 mg/kg for 2 weeks (6 and 4 doses, respectively) (N = 10). With thrice weekly dosing, 79% to 90% of patients showed symptomatic response overall and 25% to 100% of patients saw improvement after the first dose.3,4 Of the 20 patients who responded, 65% were still reporting improved symptoms 2 weeks after the last infusion and 40% showed response for >28 days.3,4 With twice weekly dosing,5 the response rate was 80% in 10 patients, while 5 patients (50%) achieved remission, lasting at least 28 days in 2 patients.
The authors of a recent Cochrane review6 evaluated ketamine for treating depression and concluded that, although there is evidence for ketamine’s efficacy early in treatment, effects are less certain after 2 weeks post-treatment. The Canadian Agency for Drugs and Technologies in Health also conducted an appraisal7 of ketamine for treating a variety of mental illnesses and similarly noted that, despite evidence in acute studies, (1) the role of the drug in clinical practice is unclear and (2) further comparative studies, as well as longer-term studies, are needed.
Last, the American Psychiatric Association Council of Research Task Force on Novel Biomarkers and Treatments1 conducted a systematic review and meta-analysis, whose authors concluded that ketamine produces a rapid and robust antidepressant effect that appears to be transient. They warn that, although results are promising, “enthusiasm should be tempered” and suggest that “its use in the clinical setting warrants caution.”
Should you consider treating depression with ketamine?
Although evidence for using ketamine as a rapid treatment of TRD is promising and non-IV forms of ketamine are being researched (eg, intranasal esketamine), there are factors that limit clinical application:
- The short duration of effect noted in studies highlights the need for research on maintenance strategies to assess longer-term efficacy as well as safety. For example, long-term ketamine abuse has been associated with cases of ulcerative or hemorrhagic cystitis causing severe and persistent pain, requiring a partial cystectomy.8,9 Further, long-term ketamine use for pain has been associated with a transaminitis. Lastly, ketamine self-treatment for depression with escalating doses has also been associated with severe ketamine addiction and sequelae.10 The incidence and severity of these adverse effects at dosages and administration frequencies that might be required for maintenance treatment of depression is unclear and requires further investigation.
- Psychotomimetic and cardiovascular adverse effects of ketamine warrant monitoring in an acute clinical setting, until longer term safety and monitoring protocols are developed. Of note, the dosing regimen used in most studies requires anesthesia monitoring in many health care systems. Although acute adverse effects in studies to date are infrequent, both cardiovascular and gastrointestinal (vomiting) events requiring IV intervention have been reported,4 underscoring the importance of anesthesiologist involvement.
- Tolerance. It is unknown if patients develop tolerance to ketamine with recurring dosages and may present additional safety concerns with repeated, higher dosages. Lastly, patients on extended ketamine therapy could encounter drug interactions with agents commonly used to treat depression.
Although some authors1,6 advise caution with widespread ketamine use, patients with TRD want effective treatments and may discount these warnings. Even though longer-term studies are needed, ketamine “infusion clinics” are already being established. Before referring patients to such clinics, it is important to understand the current clinical and safety limitations and requirements for ketamine in TRD and to consider and discuss the risks and benefits carefully.
CASE CONTINUED
Because Ms. B has tried several antidepressants and adjunctive therapies without success, and her depression is severe enough to affect her functioning in several domains, it might be reasonable to discuss a trial of ketamine. However, Ms. B also should be presented non-ketamine alternatives, such as other adjunctive strategies (liothyronine, buspirone, cognitive-behavioral therapy) or a trial of nortriptyline or a monoamine oxidase inhibitor.
If ketamine is thought to be the best option for Ms. B, her provider needs to establish a clear expectation that the effects likely will be temporary. Monitoring should include applying a rating scale to assess depressive symptoms, suicidality, and psychotomimetic symptoms. During and shortly after infusion, anesthesia support should be provided and blood pressure and other vital signs should be monitored. Additional monitoring, such as telemetry, might be indicated.
1. Newport DJ, Carpenter LL, McDonald WM, et al; APA Council of Research Task Force on Novel Biomarkers and Treatments. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry. 2015;172(10):950-966.
2. Murrough JW, losifescu DV, Chang LC, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170(10):1134-1142.
3. aan het Rot M, Collins KA, Murrough JW, et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67(2):139-145.
4. Shiroma PR, Johns B, Kuskowski M, et al. Augmentation of response and remission to serial intravenous subanesthetic ketamine in treatment resistant depression. J Affect Disorder. 2014;155:123-129.
5. Ramussen KG, Lineberry TW, Galardy CW, et al. Serial infusions of low-dose ketamine for major depression. J Psychopharmacol. 2013;27(5):444-450.
6. Caddy C, Amit BH, McCloud TL, et al. Ketamine and other glutamate receptor modulators for depression in adults. Cochrane Database Syst Rev. 2015;9:CD011612. doi: 10.1002/14651858.CD011612.pub2.
7. Canadian Agency for Drugs and Technologies in Health. Intravenous ketamine for the treatment of mental health disorders: a review of clinical effectiveness and guidelines. Ottawa, Ontario, Canada: Canadian Agency for Drugs and Technologies in Health; 2014. https://www.cadth.ca/media/pdf/htis/dec-2014/RC0572%20IV%20Ketamine%20Report%20final.pdf. Published August 20, 2014. Accessed April 13, 2016.
8. Jhang JF, Birder LA, Chancellor MB, et al. Patient characteristics for different therapeutic strategies in the management ketamine cystitis [published online March 21, 2016]. Neurourol Urodyn. doi: 10.1002/nau.22996.
9. Busse J, Phillips L, Schechter W. Long-term intravenous ketamine for analgesia in a child with severe chronic intestinal graft versus host disease. Case Rep Anesthesiol. 2015;2015:834168. doi:10.1155/2015/834168.
10. Bonnet U. Long-term ketamine self-injections in major depressive disorder: focus on tolerance in ketamine’s antidepressant response and the development of ketamine addiction. J Psychoactive Drugs. 2015;47(4):276-285.
Ms. B, age 31, experienced her first depressive episode at age 24 during her second year of law school. These episodes are characterized by insomnia, sadness, guilt, suicidal ideation, and impaired concentration that affect her ability to function at work and interfere with her ability to maintain relationships. She has no history of mania, hypomania, or psychosis.
Ms. B has approximately 2 severe episodes a year, lasting 8 to 10 weeks. She has failed adequate (≥6 week) trials of sertraline, 200 mg/d; venlafaxine XR, 300 mg/d; bupropion XL, 450 mg/d; and vortioxetine, 20 mg/d. Adjunctive treatments were not well tolerated; lithium caused severe nausea and aripiprazole lead to intolerable akathisia. Psychotherapy was ineffective. A trial of electroconvulsive therapy relieved her depression but resulted in significant memory impairment.
Is ketamine a treatment option for Ms. B?
Ketamine, an N-methyl-D aspartate antagonist, was approved by the FDA in 1970.
as a dissociative anesthetic. It proved useful in military battlefield situations. The drug then became popular as a “club drug” and is used recreationally as a dissociative agent. It recently has been used clinically for treating post-operative pain and treatment-resistant depression (TRD). It has shown efficacy for several specific symptom clusters in depression, including anhedonia and suicidality.
Several small randomized, double-blind, placebo-controlled trials of ketamine—some of which studied TRD—have reported antidepressant effects after a single IV dose of 0.5 mg/kg in depressed patients.1,2 The response rate, defined as a 50% reduction in symptoms, is reported to be as high as 50% to 71% twenty-four hours after infusion, with significant improvements noted in some patients after just 40 minutes.1 These effects, peaking at 24 hours, last ≥72 hours in approximately 50% of patients, but gradually return to baseline over 1 to 2 weeks (Figure1). The most common post-infusion adverse effects include:
- dissociation
- dizziness
- blurred vision
- poor concentration
- nausea.
Transient sedation and psychotomimetic symptoms, such as hallucinations, abnormal sensations, and confusion, also have been noted, as well as a small but significant increase in blood pressure shortly after infusion.1
Use of repeated doses of ketamine also has been studied, although larger and extended-duration studies are lacking. Two groups3,4 examined thrice weekly infusions (N = 24) and 1 group5 studied twice weekly infusions of 0.5 mg/kg for 2 weeks (6 and 4 doses, respectively) (N = 10). With thrice weekly dosing, 79% to 90% of patients showed symptomatic response overall and 25% to 100% of patients saw improvement after the first dose.3,4 Of the 20 patients who responded, 65% were still reporting improved symptoms 2 weeks after the last infusion and 40% showed response for >28 days.3,4 With twice weekly dosing,5 the response rate was 80% in 10 patients, while 5 patients (50%) achieved remission, lasting at least 28 days in 2 patients.
The authors of a recent Cochrane review6 evaluated ketamine for treating depression and concluded that, although there is evidence for ketamine’s efficacy early in treatment, effects are less certain after 2 weeks post-treatment. The Canadian Agency for Drugs and Technologies in Health also conducted an appraisal7 of ketamine for treating a variety of mental illnesses and similarly noted that, despite evidence in acute studies, (1) the role of the drug in clinical practice is unclear and (2) further comparative studies, as well as longer-term studies, are needed.
Last, the American Psychiatric Association Council of Research Task Force on Novel Biomarkers and Treatments1 conducted a systematic review and meta-analysis, whose authors concluded that ketamine produces a rapid and robust antidepressant effect that appears to be transient. They warn that, although results are promising, “enthusiasm should be tempered” and suggest that “its use in the clinical setting warrants caution.”
Should you consider treating depression with ketamine?
Although evidence for using ketamine as a rapid treatment of TRD is promising and non-IV forms of ketamine are being researched (eg, intranasal esketamine), there are factors that limit clinical application:
- The short duration of effect noted in studies highlights the need for research on maintenance strategies to assess longer-term efficacy as well as safety. For example, long-term ketamine abuse has been associated with cases of ulcerative or hemorrhagic cystitis causing severe and persistent pain, requiring a partial cystectomy.8,9 Further, long-term ketamine use for pain has been associated with a transaminitis. Lastly, ketamine self-treatment for depression with escalating doses has also been associated with severe ketamine addiction and sequelae.10 The incidence and severity of these adverse effects at dosages and administration frequencies that might be required for maintenance treatment of depression is unclear and requires further investigation.
- Psychotomimetic and cardiovascular adverse effects of ketamine warrant monitoring in an acute clinical setting, until longer term safety and monitoring protocols are developed. Of note, the dosing regimen used in most studies requires anesthesia monitoring in many health care systems. Although acute adverse effects in studies to date are infrequent, both cardiovascular and gastrointestinal (vomiting) events requiring IV intervention have been reported,4 underscoring the importance of anesthesiologist involvement.
- Tolerance. It is unknown if patients develop tolerance to ketamine with recurring dosages and may present additional safety concerns with repeated, higher dosages. Lastly, patients on extended ketamine therapy could encounter drug interactions with agents commonly used to treat depression.
Although some authors1,6 advise caution with widespread ketamine use, patients with TRD want effective treatments and may discount these warnings. Even though longer-term studies are needed, ketamine “infusion clinics” are already being established. Before referring patients to such clinics, it is important to understand the current clinical and safety limitations and requirements for ketamine in TRD and to consider and discuss the risks and benefits carefully.
CASE CONTINUED
Because Ms. B has tried several antidepressants and adjunctive therapies without success, and her depression is severe enough to affect her functioning in several domains, it might be reasonable to discuss a trial of ketamine. However, Ms. B also should be presented non-ketamine alternatives, such as other adjunctive strategies (liothyronine, buspirone, cognitive-behavioral therapy) or a trial of nortriptyline or a monoamine oxidase inhibitor.
If ketamine is thought to be the best option for Ms. B, her provider needs to establish a clear expectation that the effects likely will be temporary. Monitoring should include applying a rating scale to assess depressive symptoms, suicidality, and psychotomimetic symptoms. During and shortly after infusion, anesthesia support should be provided and blood pressure and other vital signs should be monitored. Additional monitoring, such as telemetry, might be indicated.
Ms. B, age 31, experienced her first depressive episode at age 24 during her second year of law school. These episodes are characterized by insomnia, sadness, guilt, suicidal ideation, and impaired concentration that affect her ability to function at work and interfere with her ability to maintain relationships. She has no history of mania, hypomania, or psychosis.
Ms. B has approximately 2 severe episodes a year, lasting 8 to 10 weeks. She has failed adequate (≥6 week) trials of sertraline, 200 mg/d; venlafaxine XR, 300 mg/d; bupropion XL, 450 mg/d; and vortioxetine, 20 mg/d. Adjunctive treatments were not well tolerated; lithium caused severe nausea and aripiprazole lead to intolerable akathisia. Psychotherapy was ineffective. A trial of electroconvulsive therapy relieved her depression but resulted in significant memory impairment.
Is ketamine a treatment option for Ms. B?
Ketamine, an N-methyl-D aspartate antagonist, was approved by the FDA in 1970.
as a dissociative anesthetic. It proved useful in military battlefield situations. The drug then became popular as a “club drug” and is used recreationally as a dissociative agent. It recently has been used clinically for treating post-operative pain and treatment-resistant depression (TRD). It has shown efficacy for several specific symptom clusters in depression, including anhedonia and suicidality.
Several small randomized, double-blind, placebo-controlled trials of ketamine—some of which studied TRD—have reported antidepressant effects after a single IV dose of 0.5 mg/kg in depressed patients.1,2 The response rate, defined as a 50% reduction in symptoms, is reported to be as high as 50% to 71% twenty-four hours after infusion, with significant improvements noted in some patients after just 40 minutes.1 These effects, peaking at 24 hours, last ≥72 hours in approximately 50% of patients, but gradually return to baseline over 1 to 2 weeks (Figure1). The most common post-infusion adverse effects include:
- dissociation
- dizziness
- blurred vision
- poor concentration
- nausea.
Transient sedation and psychotomimetic symptoms, such as hallucinations, abnormal sensations, and confusion, also have been noted, as well as a small but significant increase in blood pressure shortly after infusion.1
Use of repeated doses of ketamine also has been studied, although larger and extended-duration studies are lacking. Two groups3,4 examined thrice weekly infusions (N = 24) and 1 group5 studied twice weekly infusions of 0.5 mg/kg for 2 weeks (6 and 4 doses, respectively) (N = 10). With thrice weekly dosing, 79% to 90% of patients showed symptomatic response overall and 25% to 100% of patients saw improvement after the first dose.3,4 Of the 20 patients who responded, 65% were still reporting improved symptoms 2 weeks after the last infusion and 40% showed response for >28 days.3,4 With twice weekly dosing,5 the response rate was 80% in 10 patients, while 5 patients (50%) achieved remission, lasting at least 28 days in 2 patients.
The authors of a recent Cochrane review6 evaluated ketamine for treating depression and concluded that, although there is evidence for ketamine’s efficacy early in treatment, effects are less certain after 2 weeks post-treatment. The Canadian Agency for Drugs and Technologies in Health also conducted an appraisal7 of ketamine for treating a variety of mental illnesses and similarly noted that, despite evidence in acute studies, (1) the role of the drug in clinical practice is unclear and (2) further comparative studies, as well as longer-term studies, are needed.
Last, the American Psychiatric Association Council of Research Task Force on Novel Biomarkers and Treatments1 conducted a systematic review and meta-analysis, whose authors concluded that ketamine produces a rapid and robust antidepressant effect that appears to be transient. They warn that, although results are promising, “enthusiasm should be tempered” and suggest that “its use in the clinical setting warrants caution.”
Should you consider treating depression with ketamine?
Although evidence for using ketamine as a rapid treatment of TRD is promising and non-IV forms of ketamine are being researched (eg, intranasal esketamine), there are factors that limit clinical application:
- The short duration of effect noted in studies highlights the need for research on maintenance strategies to assess longer-term efficacy as well as safety. For example, long-term ketamine abuse has been associated with cases of ulcerative or hemorrhagic cystitis causing severe and persistent pain, requiring a partial cystectomy.8,9 Further, long-term ketamine use for pain has been associated with a transaminitis. Lastly, ketamine self-treatment for depression with escalating doses has also been associated with severe ketamine addiction and sequelae.10 The incidence and severity of these adverse effects at dosages and administration frequencies that might be required for maintenance treatment of depression is unclear and requires further investigation.
- Psychotomimetic and cardiovascular adverse effects of ketamine warrant monitoring in an acute clinical setting, until longer term safety and monitoring protocols are developed. Of note, the dosing regimen used in most studies requires anesthesia monitoring in many health care systems. Although acute adverse effects in studies to date are infrequent, both cardiovascular and gastrointestinal (vomiting) events requiring IV intervention have been reported,4 underscoring the importance of anesthesiologist involvement.
- Tolerance. It is unknown if patients develop tolerance to ketamine with recurring dosages and may present additional safety concerns with repeated, higher dosages. Lastly, patients on extended ketamine therapy could encounter drug interactions with agents commonly used to treat depression.
Although some authors1,6 advise caution with widespread ketamine use, patients with TRD want effective treatments and may discount these warnings. Even though longer-term studies are needed, ketamine “infusion clinics” are already being established. Before referring patients to such clinics, it is important to understand the current clinical and safety limitations and requirements for ketamine in TRD and to consider and discuss the risks and benefits carefully.
CASE CONTINUED
Because Ms. B has tried several antidepressants and adjunctive therapies without success, and her depression is severe enough to affect her functioning in several domains, it might be reasonable to discuss a trial of ketamine. However, Ms. B also should be presented non-ketamine alternatives, such as other adjunctive strategies (liothyronine, buspirone, cognitive-behavioral therapy) or a trial of nortriptyline or a monoamine oxidase inhibitor.
If ketamine is thought to be the best option for Ms. B, her provider needs to establish a clear expectation that the effects likely will be temporary. Monitoring should include applying a rating scale to assess depressive symptoms, suicidality, and psychotomimetic symptoms. During and shortly after infusion, anesthesia support should be provided and blood pressure and other vital signs should be monitored. Additional monitoring, such as telemetry, might be indicated.
1. Newport DJ, Carpenter LL, McDonald WM, et al; APA Council of Research Task Force on Novel Biomarkers and Treatments. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry. 2015;172(10):950-966.
2. Murrough JW, losifescu DV, Chang LC, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170(10):1134-1142.
3. aan het Rot M, Collins KA, Murrough JW, et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67(2):139-145.
4. Shiroma PR, Johns B, Kuskowski M, et al. Augmentation of response and remission to serial intravenous subanesthetic ketamine in treatment resistant depression. J Affect Disorder. 2014;155:123-129.
5. Ramussen KG, Lineberry TW, Galardy CW, et al. Serial infusions of low-dose ketamine for major depression. J Psychopharmacol. 2013;27(5):444-450.
6. Caddy C, Amit BH, McCloud TL, et al. Ketamine and other glutamate receptor modulators for depression in adults. Cochrane Database Syst Rev. 2015;9:CD011612. doi: 10.1002/14651858.CD011612.pub2.
7. Canadian Agency for Drugs and Technologies in Health. Intravenous ketamine for the treatment of mental health disorders: a review of clinical effectiveness and guidelines. Ottawa, Ontario, Canada: Canadian Agency for Drugs and Technologies in Health; 2014. https://www.cadth.ca/media/pdf/htis/dec-2014/RC0572%20IV%20Ketamine%20Report%20final.pdf. Published August 20, 2014. Accessed April 13, 2016.
8. Jhang JF, Birder LA, Chancellor MB, et al. Patient characteristics for different therapeutic strategies in the management ketamine cystitis [published online March 21, 2016]. Neurourol Urodyn. doi: 10.1002/nau.22996.
9. Busse J, Phillips L, Schechter W. Long-term intravenous ketamine for analgesia in a child with severe chronic intestinal graft versus host disease. Case Rep Anesthesiol. 2015;2015:834168. doi:10.1155/2015/834168.
10. Bonnet U. Long-term ketamine self-injections in major depressive disorder: focus on tolerance in ketamine’s antidepressant response and the development of ketamine addiction. J Psychoactive Drugs. 2015;47(4):276-285.
1. Newport DJ, Carpenter LL, McDonald WM, et al; APA Council of Research Task Force on Novel Biomarkers and Treatments. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry. 2015;172(10):950-966.
2. Murrough JW, losifescu DV, Chang LC, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170(10):1134-1142.
3. aan het Rot M, Collins KA, Murrough JW, et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67(2):139-145.
4. Shiroma PR, Johns B, Kuskowski M, et al. Augmentation of response and remission to serial intravenous subanesthetic ketamine in treatment resistant depression. J Affect Disorder. 2014;155:123-129.
5. Ramussen KG, Lineberry TW, Galardy CW, et al. Serial infusions of low-dose ketamine for major depression. J Psychopharmacol. 2013;27(5):444-450.
6. Caddy C, Amit BH, McCloud TL, et al. Ketamine and other glutamate receptor modulators for depression in adults. Cochrane Database Syst Rev. 2015;9:CD011612. doi: 10.1002/14651858.CD011612.pub2.
7. Canadian Agency for Drugs and Technologies in Health. Intravenous ketamine for the treatment of mental health disorders: a review of clinical effectiveness and guidelines. Ottawa, Ontario, Canada: Canadian Agency for Drugs and Technologies in Health; 2014. https://www.cadth.ca/media/pdf/htis/dec-2014/RC0572%20IV%20Ketamine%20Report%20final.pdf. Published August 20, 2014. Accessed April 13, 2016.
8. Jhang JF, Birder LA, Chancellor MB, et al. Patient characteristics for different therapeutic strategies in the management ketamine cystitis [published online March 21, 2016]. Neurourol Urodyn. doi: 10.1002/nau.22996.
9. Busse J, Phillips L, Schechter W. Long-term intravenous ketamine for analgesia in a child with severe chronic intestinal graft versus host disease. Case Rep Anesthesiol. 2015;2015:834168. doi:10.1155/2015/834168.
10. Bonnet U. Long-term ketamine self-injections in major depressive disorder: focus on tolerance in ketamine’s antidepressant response and the development of ketamine addiction. J Psychoactive Drugs. 2015;47(4):276-285.
The doctor is sick
The doctor is sick.
Her feet are swollen, as if her heart is failing. But it is strong and beating faster than usual, as she runs her fingers through her hair. Her vision gets blurry sometimes, and she has to hold onto something when she stands up or she will fall down. The doctor is sick because she is taking medicine to make her well. Lithium, Seroquel, Depakote. Mood stabilizer, antipsychotic, mood stabilizer. Two pills, 4 pills, 3 pills. Plus a multivitamin because Depakote can cause her hair to fall out. Skinny and fat bottles, next to her bed so she won’t forget. As if she would forget.
The doctor is sick because 3 weeks ago tomorrow she made a concrete plan to take her life. She wrote 5 letters, the longest to her sweet and supportive husband. She put some pills and some alcohol and the letters in her backpack and made it to the front stoop before she sat down, her heart breaking, and called her best friend.
The doctor is sick. Sicker now that she is home. As a patient in the psychiatric hospital, she was the doctor, and she held “office hours” (24 hours a day) to answer questions about GERD, schizophrenia, and stomachaches. This made her feel a lot less sick. Everyone said she was clever. She is only a medical student and offers a disclaimer with her advice (along with the suggestion to see a “real doctor”). But to most of the patients she is a doctor. And she feels the calling to be a doctor. Deep down inside herself she feels she was born to be a doctor.
In the psychiatric hospital, she felt alive when she gave advice. It made her feel she was helping people. She lives for people. And it distinguished her from the rest of them. They were her friends and she shared illness with them, but somehow she was different. Sometimes, this gave her comfort. Other times she held onto them as anchors in the madness they were swimming through together. Those times she found comfort in being the same as them. Exactly the same.
The doctor is sick. On the discharge sheet, 18 days after admission, her acceptance of this fact (“judgment and insight”) was judged “fair.” This is because the doctor does not believe in her diagnosis. She does not want to be sick. She wants to be the person she was before—minus those highs and lows. She would trade in the agitated anxious state that caused her to drop out of her pediatric rotation. She would trade in the days spent in bed in college, while everyone seemed to be having fun. She would trade in the Google searches of “suicide methods.” She would trade in the panic that caused her to stop her rotation, and therefore caused her to stop playing with the pediatric patients in the playroom at night, after her clinical duties were finished. In the playroom she was not a doctor or a medical student. She was just someone’s playmate. She held the little hand of her little patient and she felt like she was exactly where she was supposed to be. She knew she belonged when she helped her patients forget they were sick.
The doctor is sick. She is drinking hard lemonade, even though it makes the dizziness worse. The doctor has a swollen face and swollen fingers and her wedding ring does not fit anymore. She has gained 10, no, 15 lb because of the medicine, and she knows her husband has noticed. The doctor knows that she has enough pills beside her bed to end her life, but she also knows she is calm now. She is calm in a way she has not felt for as long as she can remember. She thinks back on college, on that 2-month period when she slept until 2 PM, and got out of bed only to smoke marijuana and change the CD on her stereo. She thinks back on the way she pulled the blanket over her head and she remembers that she wanted to be dead.
The doctor remembers writing her treatises later that year on the meaning of life, of the world, of peace and joy and love, on her desktop computer. She wrote and wrote and wrote, as she often did, and sleep did not seem very necessary or desired. She felt she was more connected to the world than anyone else on the planet. She felt more intensely than anyone else on the planet. She ran 5 miles a day, 15 miles on Saturdays. Tears streamed down her face as she bicycled back from her volunteer work with the elderly. She felt so much love and she felt so different from everyone else. Tears came again when she read literature; she felt alive with politics and meaning and urgency. Her heart pounded and she felt words run through her mind that told her to keep working, harder, and that she was capable of more efficient, more emotive, more effective, more productive work than anyone else on the planet. She had more insight than anyone else on the planet. But all of this was directed to help other people, and so she was connected to the whole world.
The doctor feels sick now as she recalls those events. Now she fears her past is part of this illness. Those thoughts and actions that were her proudest moments, the organizations that offered her awards and accolades, the papers that got high marks—those were just the consequence of neurotransmitters being sucked in and spat out in the wrong ratios. It is that simple. Something we can fix with around 10 pills a day, pills that will make the doctor better but will make the doctor sick. What about the doctor’s sense of humor? What about her energy? What about her wild dancing and her disinhibition? What about her ability to be the life of a party even when she is stone cold sober and everyone else has had 4 or 5 drinks? What about her accomplishments and her intensity? What about her ever-present belief that the world’s people are all connected, and she is to play a small but important, even vital, role in bringing them together? Sick, sick, all sick.
As a patient in the psychiatric hospital, the doctor would dance sometimes. She would be dancing, twirling, laughing, making others laugh. Then, on the same day, she would burst into tears and sit in her room, blanket over head, turning over the possibilities for a painless but sure death. She didn’t have much time to think on it because soon it would be time to line up for medicine.
Next … it’s time for her office hours, to reassure other patients, to pretend she is a doctor. But soon enough … the shrink beckons her for the daily session, and she is reminded of his infinite wisdom and her relative ignorance. She nods and agrees with some of what he says, believes it even when she is talking to him in his little room, but not later, when she is back in bed, nighttime, waiting for nightmares. The psychiatrist says that her frantic sleepless days before Christmas, the ones where her friends couldn’t understand why she couldn’t even sit down for lunch (“You’re going to have a stroke!” a less tactful one declared), the ones where she feigned listening to someone talking while in her head she entertained a disorganized, discordant symphony of thoughts trying to hammer out a requiem, a death march … those days were a “mixed state.” Mumbo jumbo. Nonsense. She tells herself she is just moody. At least now there are moments of heady delicious delight, no matter if they are brief.
So the doctor is improving! And she has reason to doubt their boxes and their labels, as she reads on this illness in rare moments when her mind is still, and she finds there is controversy surrounding every diagnosis and delineation. And so that is fuel for her disapproving, disbelieving fire. All of this is just an expression of eccentricity, she tells herself, and these lousy doctors don’t appreciate someone “as brilliant and beautiful as me.” But later, watching the nurses as they fill out their assessments of the day, she wonders how sick they think she really is, and she hates herself and this lunatic frenzy and she is embarrassed and ashamed.
If she does not believe she is sick, then she has to blame herself, and that hurts. But she can handle that pain and that bleeding, for she alone can suffer pain and bleeding like no others, and that is part of what makes her beautiful. But she is not sick …. According to them, the doctor is sick every day and so she stays in the hospital. She retorts, “Of course that is what they say. Isn’t that their job, to call me crazy?” And then, “If I was not crazy, wouldn’t that put them out of business?”
Nightmares have tormented the doctor since she was a child. But lately they are more twisted. They pull her out of the safety of the day such that she plunges, screaming, down, head-first, into black night, afraid. Some of her medicines exacerbate nightmares. In one awful dream, she is a patient, then a doctor, then a patient again, and she keeps waking up inside the dream to be transformed into doctor or patient, one then the other; it never stops. So first she is giving medicines, then she is being intubated, then she is finishing rounds, then she is enduring electroconvulsive therapy. Finally she drops out of the dream as if from the sky, and she is shivering and afraid.
Now, as she dozes, she awakens to every noise, and the shadows look like people, and she knows the nightmares wait for her again, so she fights sleep. Paxil made her hear voices (the psychiatrists proclaimed this “classic” for her illness; she shrugged her shoulders in response) and so now she listens carefully, holding her breath, in bed, trying to hear voices. She thought she heard something. Of course, now she is hypersensitive. Now it is hard not to describe everything as “classic” for the illness. If she thinks about it, from junior high school onwards she was “classic.” What nonsense! Her vision is 20/20 in hindsight. Who wouldn’t find madness in their past if they looked hard enough?
The doctor is sick. The night lasts forever. Somebody opens the door because, at this hospital, they check every 15 minutes, 24 hours per day, to make sure the doctor has not slit her wrists or hanged herself or found another way to end her sick life. For this reason, they have confiscated her shoelaces, and she has to floss at the nurses’ station. She startles as they open the door, and screams out loud. They reassure her they are just checking on her, and now they are gone, and the doctor is left to imagine them walking down the corridor to the nurses’ station, shaking their heads, saying, “That doctor sure is sick!”.
Yes, the doctor is sick. Who is she anyway? She is not even a doctor! She is just a third-year medical student who is fighting, fists up, her descent into madness. Has she won? She is trying to find a way to accept what she is. Does that mean she is a winner or a loser? It is chronic, but manageable, bipolar madness. There are lots of other people like her. But it is still overwhelming, and she is not even sure she wants to join anybody else. She never defined herself as part of any group before. Now she tries to get back to normal—but what can that mean now, after all this time, after release from a mental hospital, after taking a leave from school, after swallowing 10 pills a day (every day, for the rest of her life?), after finding out she is so sick? It is time for her meds. She hasn’t told most of her classmates or her parents because she doesn’t want them to find out that the doctor is sick. She doesn’t want to know if the doctor is sick. She doesn’t want to ponder if the doctor can ever get well.
The doctor misses seeing her patients. Tears are in her eyes and she swallows her sadness as best as she can. She aims to keep a brave face, but she is sad for what she has lost. She tries to shake that off, but it keeps returning. Like a virus, it sneaks up on her and she is shivering, feverish with grief. She hears stories from her friends as they continue to round, heal, cut and sew, take histories, stay up all night, get yelled at by senior physicians, and she wants so much to be there. She is in nowhere land. To some, a few, those who know the twisted tale of the last few months, she is sick. To those who know nothing of it, she is her usual slightly eccentric self, but well. But really she knows she is a liar and a fraud. Because somewhere in the middle she exists alone.
Well, no, she is not alone. Her husband stands by her and he is wonderful. He holds her and laughs with her and tells her she is beautiful and amazing. But she misses seeing lots of people; she thrives on people. Doesn’t anyone understand that she needs people to be alive? Is that so wrong? Is that so sick? She feels sorry for herself, sorry for what she must give up, sorry that she must graduate a year later, sorry that she cannot be with people, sorry that she feels so sorry so much of the time. And then she gets angry with herself for feeling so sorry and being so sick.
Every now and then, the doctor sees another side of things. She surfs the Internet and reads books and she finds out about lots of smart and creative and beautiful people who were sick like she is. These people have twisted, wonderful abilities but so many of them plunged to their death or swallowed pills or died so young. They wrote books or composed symphonies or made people laugh or created works of art but then they died. And so she is left feeling strong and beautiful sometimes, but also lonely and sad that all those souls have deserted her. She is sad that they have left her in this madness, and she must fight to stay alive all by herself. Then, every now and then, there are flickers of wisdom and insight and she knows she will stay alive and she will create beautiful things too, one day.
The doctor is sick. Getting sick stinks. She admits, “Nobody, especially not me, counted on the doctor getting sick. Please! Enough!”
She concedes: “I won’t forget this. I promise. OK, I have learned my lesson now. Please, enough of this!”
She pleads: “I need the doctor to get well. I am afraid of being the doctor that is sick, so afraid.”
She ponders: “Does this mean I will never be free? Does this mean I will never be well? Does this mean I will never be a mother? Oh, God, does this mean I will never be a doctor?”
Then there is silence because she asks these questions of herself, and she has no answers, and no office hours today.
Somebody better call a doctor.
Acknowledgment
The author is grateful for the support of her medical school and residency program during training.
The doctor is sick.
Her feet are swollen, as if her heart is failing. But it is strong and beating faster than usual, as she runs her fingers through her hair. Her vision gets blurry sometimes, and she has to hold onto something when she stands up or she will fall down. The doctor is sick because she is taking medicine to make her well. Lithium, Seroquel, Depakote. Mood stabilizer, antipsychotic, mood stabilizer. Two pills, 4 pills, 3 pills. Plus a multivitamin because Depakote can cause her hair to fall out. Skinny and fat bottles, next to her bed so she won’t forget. As if she would forget.
The doctor is sick because 3 weeks ago tomorrow she made a concrete plan to take her life. She wrote 5 letters, the longest to her sweet and supportive husband. She put some pills and some alcohol and the letters in her backpack and made it to the front stoop before she sat down, her heart breaking, and called her best friend.
The doctor is sick. Sicker now that she is home. As a patient in the psychiatric hospital, she was the doctor, and she held “office hours” (24 hours a day) to answer questions about GERD, schizophrenia, and stomachaches. This made her feel a lot less sick. Everyone said she was clever. She is only a medical student and offers a disclaimer with her advice (along with the suggestion to see a “real doctor”). But to most of the patients she is a doctor. And she feels the calling to be a doctor. Deep down inside herself she feels she was born to be a doctor.
In the psychiatric hospital, she felt alive when she gave advice. It made her feel she was helping people. She lives for people. And it distinguished her from the rest of them. They were her friends and she shared illness with them, but somehow she was different. Sometimes, this gave her comfort. Other times she held onto them as anchors in the madness they were swimming through together. Those times she found comfort in being the same as them. Exactly the same.
The doctor is sick. On the discharge sheet, 18 days after admission, her acceptance of this fact (“judgment and insight”) was judged “fair.” This is because the doctor does not believe in her diagnosis. She does not want to be sick. She wants to be the person she was before—minus those highs and lows. She would trade in the agitated anxious state that caused her to drop out of her pediatric rotation. She would trade in the days spent in bed in college, while everyone seemed to be having fun. She would trade in the Google searches of “suicide methods.” She would trade in the panic that caused her to stop her rotation, and therefore caused her to stop playing with the pediatric patients in the playroom at night, after her clinical duties were finished. In the playroom she was not a doctor or a medical student. She was just someone’s playmate. She held the little hand of her little patient and she felt like she was exactly where she was supposed to be. She knew she belonged when she helped her patients forget they were sick.
The doctor is sick. She is drinking hard lemonade, even though it makes the dizziness worse. The doctor has a swollen face and swollen fingers and her wedding ring does not fit anymore. She has gained 10, no, 15 lb because of the medicine, and she knows her husband has noticed. The doctor knows that she has enough pills beside her bed to end her life, but she also knows she is calm now. She is calm in a way she has not felt for as long as she can remember. She thinks back on college, on that 2-month period when she slept until 2 PM, and got out of bed only to smoke marijuana and change the CD on her stereo. She thinks back on the way she pulled the blanket over her head and she remembers that she wanted to be dead.
The doctor remembers writing her treatises later that year on the meaning of life, of the world, of peace and joy and love, on her desktop computer. She wrote and wrote and wrote, as she often did, and sleep did not seem very necessary or desired. She felt she was more connected to the world than anyone else on the planet. She felt more intensely than anyone else on the planet. She ran 5 miles a day, 15 miles on Saturdays. Tears streamed down her face as she bicycled back from her volunteer work with the elderly. She felt so much love and she felt so different from everyone else. Tears came again when she read literature; she felt alive with politics and meaning and urgency. Her heart pounded and she felt words run through her mind that told her to keep working, harder, and that she was capable of more efficient, more emotive, more effective, more productive work than anyone else on the planet. She had more insight than anyone else on the planet. But all of this was directed to help other people, and so she was connected to the whole world.
The doctor feels sick now as she recalls those events. Now she fears her past is part of this illness. Those thoughts and actions that were her proudest moments, the organizations that offered her awards and accolades, the papers that got high marks—those were just the consequence of neurotransmitters being sucked in and spat out in the wrong ratios. It is that simple. Something we can fix with around 10 pills a day, pills that will make the doctor better but will make the doctor sick. What about the doctor’s sense of humor? What about her energy? What about her wild dancing and her disinhibition? What about her ability to be the life of a party even when she is stone cold sober and everyone else has had 4 or 5 drinks? What about her accomplishments and her intensity? What about her ever-present belief that the world’s people are all connected, and she is to play a small but important, even vital, role in bringing them together? Sick, sick, all sick.
As a patient in the psychiatric hospital, the doctor would dance sometimes. She would be dancing, twirling, laughing, making others laugh. Then, on the same day, she would burst into tears and sit in her room, blanket over head, turning over the possibilities for a painless but sure death. She didn’t have much time to think on it because soon it would be time to line up for medicine.
Next … it’s time for her office hours, to reassure other patients, to pretend she is a doctor. But soon enough … the shrink beckons her for the daily session, and she is reminded of his infinite wisdom and her relative ignorance. She nods and agrees with some of what he says, believes it even when she is talking to him in his little room, but not later, when she is back in bed, nighttime, waiting for nightmares. The psychiatrist says that her frantic sleepless days before Christmas, the ones where her friends couldn’t understand why she couldn’t even sit down for lunch (“You’re going to have a stroke!” a less tactful one declared), the ones where she feigned listening to someone talking while in her head she entertained a disorganized, discordant symphony of thoughts trying to hammer out a requiem, a death march … those days were a “mixed state.” Mumbo jumbo. Nonsense. She tells herself she is just moody. At least now there are moments of heady delicious delight, no matter if they are brief.
So the doctor is improving! And she has reason to doubt their boxes and their labels, as she reads on this illness in rare moments when her mind is still, and she finds there is controversy surrounding every diagnosis and delineation. And so that is fuel for her disapproving, disbelieving fire. All of this is just an expression of eccentricity, she tells herself, and these lousy doctors don’t appreciate someone “as brilliant and beautiful as me.” But later, watching the nurses as they fill out their assessments of the day, she wonders how sick they think she really is, and she hates herself and this lunatic frenzy and she is embarrassed and ashamed.
If she does not believe she is sick, then she has to blame herself, and that hurts. But she can handle that pain and that bleeding, for she alone can suffer pain and bleeding like no others, and that is part of what makes her beautiful. But she is not sick …. According to them, the doctor is sick every day and so she stays in the hospital. She retorts, “Of course that is what they say. Isn’t that their job, to call me crazy?” And then, “If I was not crazy, wouldn’t that put them out of business?”
Nightmares have tormented the doctor since she was a child. But lately they are more twisted. They pull her out of the safety of the day such that she plunges, screaming, down, head-first, into black night, afraid. Some of her medicines exacerbate nightmares. In one awful dream, she is a patient, then a doctor, then a patient again, and she keeps waking up inside the dream to be transformed into doctor or patient, one then the other; it never stops. So first she is giving medicines, then she is being intubated, then she is finishing rounds, then she is enduring electroconvulsive therapy. Finally she drops out of the dream as if from the sky, and she is shivering and afraid.
Now, as she dozes, she awakens to every noise, and the shadows look like people, and she knows the nightmares wait for her again, so she fights sleep. Paxil made her hear voices (the psychiatrists proclaimed this “classic” for her illness; she shrugged her shoulders in response) and so now she listens carefully, holding her breath, in bed, trying to hear voices. She thought she heard something. Of course, now she is hypersensitive. Now it is hard not to describe everything as “classic” for the illness. If she thinks about it, from junior high school onwards she was “classic.” What nonsense! Her vision is 20/20 in hindsight. Who wouldn’t find madness in their past if they looked hard enough?
The doctor is sick. The night lasts forever. Somebody opens the door because, at this hospital, they check every 15 minutes, 24 hours per day, to make sure the doctor has not slit her wrists or hanged herself or found another way to end her sick life. For this reason, they have confiscated her shoelaces, and she has to floss at the nurses’ station. She startles as they open the door, and screams out loud. They reassure her they are just checking on her, and now they are gone, and the doctor is left to imagine them walking down the corridor to the nurses’ station, shaking their heads, saying, “That doctor sure is sick!”.
Yes, the doctor is sick. Who is she anyway? She is not even a doctor! She is just a third-year medical student who is fighting, fists up, her descent into madness. Has she won? She is trying to find a way to accept what she is. Does that mean she is a winner or a loser? It is chronic, but manageable, bipolar madness. There are lots of other people like her. But it is still overwhelming, and she is not even sure she wants to join anybody else. She never defined herself as part of any group before. Now she tries to get back to normal—but what can that mean now, after all this time, after release from a mental hospital, after taking a leave from school, after swallowing 10 pills a day (every day, for the rest of her life?), after finding out she is so sick? It is time for her meds. She hasn’t told most of her classmates or her parents because she doesn’t want them to find out that the doctor is sick. She doesn’t want to know if the doctor is sick. She doesn’t want to ponder if the doctor can ever get well.
The doctor misses seeing her patients. Tears are in her eyes and she swallows her sadness as best as she can. She aims to keep a brave face, but she is sad for what she has lost. She tries to shake that off, but it keeps returning. Like a virus, it sneaks up on her and she is shivering, feverish with grief. She hears stories from her friends as they continue to round, heal, cut and sew, take histories, stay up all night, get yelled at by senior physicians, and she wants so much to be there. She is in nowhere land. To some, a few, those who know the twisted tale of the last few months, she is sick. To those who know nothing of it, she is her usual slightly eccentric self, but well. But really she knows she is a liar and a fraud. Because somewhere in the middle she exists alone.
Well, no, she is not alone. Her husband stands by her and he is wonderful. He holds her and laughs with her and tells her she is beautiful and amazing. But she misses seeing lots of people; she thrives on people. Doesn’t anyone understand that she needs people to be alive? Is that so wrong? Is that so sick? She feels sorry for herself, sorry for what she must give up, sorry that she must graduate a year later, sorry that she cannot be with people, sorry that she feels so sorry so much of the time. And then she gets angry with herself for feeling so sorry and being so sick.
Every now and then, the doctor sees another side of things. She surfs the Internet and reads books and she finds out about lots of smart and creative and beautiful people who were sick like she is. These people have twisted, wonderful abilities but so many of them plunged to their death or swallowed pills or died so young. They wrote books or composed symphonies or made people laugh or created works of art but then they died. And so she is left feeling strong and beautiful sometimes, but also lonely and sad that all those souls have deserted her. She is sad that they have left her in this madness, and she must fight to stay alive all by herself. Then, every now and then, there are flickers of wisdom and insight and she knows she will stay alive and she will create beautiful things too, one day.
The doctor is sick. Getting sick stinks. She admits, “Nobody, especially not me, counted on the doctor getting sick. Please! Enough!”
She concedes: “I won’t forget this. I promise. OK, I have learned my lesson now. Please, enough of this!”
She pleads: “I need the doctor to get well. I am afraid of being the doctor that is sick, so afraid.”
She ponders: “Does this mean I will never be free? Does this mean I will never be well? Does this mean I will never be a mother? Oh, God, does this mean I will never be a doctor?”
Then there is silence because she asks these questions of herself, and she has no answers, and no office hours today.
Somebody better call a doctor.
Acknowledgment
The author is grateful for the support of her medical school and residency program during training.
The doctor is sick.
Her feet are swollen, as if her heart is failing. But it is strong and beating faster than usual, as she runs her fingers through her hair. Her vision gets blurry sometimes, and she has to hold onto something when she stands up or she will fall down. The doctor is sick because she is taking medicine to make her well. Lithium, Seroquel, Depakote. Mood stabilizer, antipsychotic, mood stabilizer. Two pills, 4 pills, 3 pills. Plus a multivitamin because Depakote can cause her hair to fall out. Skinny and fat bottles, next to her bed so she won’t forget. As if she would forget.
The doctor is sick because 3 weeks ago tomorrow she made a concrete plan to take her life. She wrote 5 letters, the longest to her sweet and supportive husband. She put some pills and some alcohol and the letters in her backpack and made it to the front stoop before she sat down, her heart breaking, and called her best friend.
The doctor is sick. Sicker now that she is home. As a patient in the psychiatric hospital, she was the doctor, and she held “office hours” (24 hours a day) to answer questions about GERD, schizophrenia, and stomachaches. This made her feel a lot less sick. Everyone said she was clever. She is only a medical student and offers a disclaimer with her advice (along with the suggestion to see a “real doctor”). But to most of the patients she is a doctor. And she feels the calling to be a doctor. Deep down inside herself she feels she was born to be a doctor.
In the psychiatric hospital, she felt alive when she gave advice. It made her feel she was helping people. She lives for people. And it distinguished her from the rest of them. They were her friends and she shared illness with them, but somehow she was different. Sometimes, this gave her comfort. Other times she held onto them as anchors in the madness they were swimming through together. Those times she found comfort in being the same as them. Exactly the same.
The doctor is sick. On the discharge sheet, 18 days after admission, her acceptance of this fact (“judgment and insight”) was judged “fair.” This is because the doctor does not believe in her diagnosis. She does not want to be sick. She wants to be the person she was before—minus those highs and lows. She would trade in the agitated anxious state that caused her to drop out of her pediatric rotation. She would trade in the days spent in bed in college, while everyone seemed to be having fun. She would trade in the Google searches of “suicide methods.” She would trade in the panic that caused her to stop her rotation, and therefore caused her to stop playing with the pediatric patients in the playroom at night, after her clinical duties were finished. In the playroom she was not a doctor or a medical student. She was just someone’s playmate. She held the little hand of her little patient and she felt like she was exactly where she was supposed to be. She knew she belonged when she helped her patients forget they were sick.
The doctor is sick. She is drinking hard lemonade, even though it makes the dizziness worse. The doctor has a swollen face and swollen fingers and her wedding ring does not fit anymore. She has gained 10, no, 15 lb because of the medicine, and she knows her husband has noticed. The doctor knows that she has enough pills beside her bed to end her life, but she also knows she is calm now. She is calm in a way she has not felt for as long as she can remember. She thinks back on college, on that 2-month period when she slept until 2 PM, and got out of bed only to smoke marijuana and change the CD on her stereo. She thinks back on the way she pulled the blanket over her head and she remembers that she wanted to be dead.
The doctor remembers writing her treatises later that year on the meaning of life, of the world, of peace and joy and love, on her desktop computer. She wrote and wrote and wrote, as she often did, and sleep did not seem very necessary or desired. She felt she was more connected to the world than anyone else on the planet. She felt more intensely than anyone else on the planet. She ran 5 miles a day, 15 miles on Saturdays. Tears streamed down her face as she bicycled back from her volunteer work with the elderly. She felt so much love and she felt so different from everyone else. Tears came again when she read literature; she felt alive with politics and meaning and urgency. Her heart pounded and she felt words run through her mind that told her to keep working, harder, and that she was capable of more efficient, more emotive, more effective, more productive work than anyone else on the planet. She had more insight than anyone else on the planet. But all of this was directed to help other people, and so she was connected to the whole world.
The doctor feels sick now as she recalls those events. Now she fears her past is part of this illness. Those thoughts and actions that were her proudest moments, the organizations that offered her awards and accolades, the papers that got high marks—those were just the consequence of neurotransmitters being sucked in and spat out in the wrong ratios. It is that simple. Something we can fix with around 10 pills a day, pills that will make the doctor better but will make the doctor sick. What about the doctor’s sense of humor? What about her energy? What about her wild dancing and her disinhibition? What about her ability to be the life of a party even when she is stone cold sober and everyone else has had 4 or 5 drinks? What about her accomplishments and her intensity? What about her ever-present belief that the world’s people are all connected, and she is to play a small but important, even vital, role in bringing them together? Sick, sick, all sick.
As a patient in the psychiatric hospital, the doctor would dance sometimes. She would be dancing, twirling, laughing, making others laugh. Then, on the same day, she would burst into tears and sit in her room, blanket over head, turning over the possibilities for a painless but sure death. She didn’t have much time to think on it because soon it would be time to line up for medicine.
Next … it’s time for her office hours, to reassure other patients, to pretend she is a doctor. But soon enough … the shrink beckons her for the daily session, and she is reminded of his infinite wisdom and her relative ignorance. She nods and agrees with some of what he says, believes it even when she is talking to him in his little room, but not later, when she is back in bed, nighttime, waiting for nightmares. The psychiatrist says that her frantic sleepless days before Christmas, the ones where her friends couldn’t understand why she couldn’t even sit down for lunch (“You’re going to have a stroke!” a less tactful one declared), the ones where she feigned listening to someone talking while in her head she entertained a disorganized, discordant symphony of thoughts trying to hammer out a requiem, a death march … those days were a “mixed state.” Mumbo jumbo. Nonsense. She tells herself she is just moody. At least now there are moments of heady delicious delight, no matter if they are brief.
So the doctor is improving! And she has reason to doubt their boxes and their labels, as she reads on this illness in rare moments when her mind is still, and she finds there is controversy surrounding every diagnosis and delineation. And so that is fuel for her disapproving, disbelieving fire. All of this is just an expression of eccentricity, she tells herself, and these lousy doctors don’t appreciate someone “as brilliant and beautiful as me.” But later, watching the nurses as they fill out their assessments of the day, she wonders how sick they think she really is, and she hates herself and this lunatic frenzy and she is embarrassed and ashamed.
If she does not believe she is sick, then she has to blame herself, and that hurts. But she can handle that pain and that bleeding, for she alone can suffer pain and bleeding like no others, and that is part of what makes her beautiful. But she is not sick …. According to them, the doctor is sick every day and so she stays in the hospital. She retorts, “Of course that is what they say. Isn’t that their job, to call me crazy?” And then, “If I was not crazy, wouldn’t that put them out of business?”
Nightmares have tormented the doctor since she was a child. But lately they are more twisted. They pull her out of the safety of the day such that she plunges, screaming, down, head-first, into black night, afraid. Some of her medicines exacerbate nightmares. In one awful dream, she is a patient, then a doctor, then a patient again, and she keeps waking up inside the dream to be transformed into doctor or patient, one then the other; it never stops. So first she is giving medicines, then she is being intubated, then she is finishing rounds, then she is enduring electroconvulsive therapy. Finally she drops out of the dream as if from the sky, and she is shivering and afraid.
Now, as she dozes, she awakens to every noise, and the shadows look like people, and she knows the nightmares wait for her again, so she fights sleep. Paxil made her hear voices (the psychiatrists proclaimed this “classic” for her illness; she shrugged her shoulders in response) and so now she listens carefully, holding her breath, in bed, trying to hear voices. She thought she heard something. Of course, now she is hypersensitive. Now it is hard not to describe everything as “classic” for the illness. If she thinks about it, from junior high school onwards she was “classic.” What nonsense! Her vision is 20/20 in hindsight. Who wouldn’t find madness in their past if they looked hard enough?
The doctor is sick. The night lasts forever. Somebody opens the door because, at this hospital, they check every 15 minutes, 24 hours per day, to make sure the doctor has not slit her wrists or hanged herself or found another way to end her sick life. For this reason, they have confiscated her shoelaces, and she has to floss at the nurses’ station. She startles as they open the door, and screams out loud. They reassure her they are just checking on her, and now they are gone, and the doctor is left to imagine them walking down the corridor to the nurses’ station, shaking their heads, saying, “That doctor sure is sick!”.
Yes, the doctor is sick. Who is she anyway? She is not even a doctor! She is just a third-year medical student who is fighting, fists up, her descent into madness. Has she won? She is trying to find a way to accept what she is. Does that mean she is a winner or a loser? It is chronic, but manageable, bipolar madness. There are lots of other people like her. But it is still overwhelming, and she is not even sure she wants to join anybody else. She never defined herself as part of any group before. Now she tries to get back to normal—but what can that mean now, after all this time, after release from a mental hospital, after taking a leave from school, after swallowing 10 pills a day (every day, for the rest of her life?), after finding out she is so sick? It is time for her meds. She hasn’t told most of her classmates or her parents because she doesn’t want them to find out that the doctor is sick. She doesn’t want to know if the doctor is sick. She doesn’t want to ponder if the doctor can ever get well.
The doctor misses seeing her patients. Tears are in her eyes and she swallows her sadness as best as she can. She aims to keep a brave face, but she is sad for what she has lost. She tries to shake that off, but it keeps returning. Like a virus, it sneaks up on her and she is shivering, feverish with grief. She hears stories from her friends as they continue to round, heal, cut and sew, take histories, stay up all night, get yelled at by senior physicians, and she wants so much to be there. She is in nowhere land. To some, a few, those who know the twisted tale of the last few months, she is sick. To those who know nothing of it, she is her usual slightly eccentric self, but well. But really she knows she is a liar and a fraud. Because somewhere in the middle she exists alone.
Well, no, she is not alone. Her husband stands by her and he is wonderful. He holds her and laughs with her and tells her she is beautiful and amazing. But she misses seeing lots of people; she thrives on people. Doesn’t anyone understand that she needs people to be alive? Is that so wrong? Is that so sick? She feels sorry for herself, sorry for what she must give up, sorry that she must graduate a year later, sorry that she cannot be with people, sorry that she feels so sorry so much of the time. And then she gets angry with herself for feeling so sorry and being so sick.
Every now and then, the doctor sees another side of things. She surfs the Internet and reads books and she finds out about lots of smart and creative and beautiful people who were sick like she is. These people have twisted, wonderful abilities but so many of them plunged to their death or swallowed pills or died so young. They wrote books or composed symphonies or made people laugh or created works of art but then they died. And so she is left feeling strong and beautiful sometimes, but also lonely and sad that all those souls have deserted her. She is sad that they have left her in this madness, and she must fight to stay alive all by herself. Then, every now and then, there are flickers of wisdom and insight and she knows she will stay alive and she will create beautiful things too, one day.
The doctor is sick. Getting sick stinks. She admits, “Nobody, especially not me, counted on the doctor getting sick. Please! Enough!”
She concedes: “I won’t forget this. I promise. OK, I have learned my lesson now. Please, enough of this!”
She pleads: “I need the doctor to get well. I am afraid of being the doctor that is sick, so afraid.”
She ponders: “Does this mean I will never be free? Does this mean I will never be well? Does this mean I will never be a mother? Oh, God, does this mean I will never be a doctor?”
Then there is silence because she asks these questions of herself, and she has no answers, and no office hours today.
Somebody better call a doctor.
Acknowledgment
The author is grateful for the support of her medical school and residency program during training.
‘Druggable’ genes, promiscuous drugs, repurposed medications
Unprecedented collaboration among 900 genetics investigators across 40 countries led to creation of the highly productive Psychiatric Genomics Consortium (PGC), which is analyzing 400,000 individual DNA samples.1 The Consortium has an open-source approach, with data freely available to all who are interested.a
The PGC recently published the results of a large Genome Wide Association Study (GWAS) of 36,989 people with schizophrenia and 113,075 controls. Investigators discovered 108 genetic loci (each containing as many as 26 genes), adding up to 341 protein-coding risk genes for schizophrenia, distributed across all 23 chromosomes.2 One of these risk genes, on chromosome 6, is in the major histocompatibility complex and has the strongest association with schizophrenia (P = 10–31). This finding provides insight that schizophrenia might be related to immune dysfunction, supported by evidence for neuro-inflammation and elevated pro-inflammatory biomarkers in this syndrome.3
In addition to heritable risk genes, the PGC has found many copy number variants (CNVs) and rare de novo mutations that are found significantly more often (10-fold or greater) in schizophrenia. But, as reflected by the 50% concordance rate for schizophrenia in monozygotic twins, non-genetic pathways to schizophrenia obviously exist; this is especially so through adverse events during pregnancy, which can disrupt brain development in a manner similar to disruption caused by risk genes, CNVs, and mutations.
The most exciting consequence of these breakthroughs?These genetic discoveries have great implications for novel drug development for the hundreds of biological subtypes of schizophrenia. At latest count, 23,345 genes that code for proteins, the building blocks of the body, are found in the human set of 23 chromosomes.2 Approximately 7,000 of those genes are druggable and can open the way to developing new agents. In fact, identifying potential targets for pharmacotherapeutic intervention is the major goal of conducting a GWAS.4
What it means to be ‘druggable.’ Two conditions must be met for a gene to be druggable: First, it must code for a protein with folds that can interact with chemical compounds; second, that protein must be associated with a human disease.5 A drug that interacts with several target proteins (eg, kinases, proteases, transporters, enzymes) is considered promiscuous. After such a drug is found to have efficacy in 1 disease, it can be repurposed for treating other diseases. Such repositioning of an already approved drug for other conditions could save the pharmaceutical industry an enormous amount of time and billions of research and development dollars in developing new drugs for psychiatric illnesses that might have been used to treat various other medical conditions.
To exploit the principle of re-purposing, Lencz and Malhotra2 examined the 341 coding genes associated with schizophrenia, to determine whether available drugs interact with the proteins produced by some of those genes. They identified 40 druggable genes (11.7% of the 341) and reported that:
- 27 coding genes (7.92% of the 341) are drug targets6
- 20 of the 40 druggable genes are already approved by the FDA to treat a range of medical disorders, including glaucoma, epilepsy, hypertension, angina, irritable bowel syndrome, incontinence, smoking cessation, nausea, hypertension, prostate cancer, type 2 diabetes mellitus, pulmonary fibrosis, and acute promyelocytic leukemia; in addition, some genes act as a diuretic or an nonsteroidal anti-inflammatory drug
- another 20 druggable genes are not approved for use but are in clinical trials for disorders such as Alzheimer’s disease, heart failure, neuropathic pain, depression, cancer, immune-supported acne psoriasis, and myeloma.
The opportunity to repurpose some of those promiscuous drugs for various medical indications for the treatment of schizophrenia is exciting, and presents Pandora’s box of new mechanisms of action.7 It is intriguing how therapeutic mechanisms for a wide range of unrelated medical conditions may have commonality with the neurobiological underpinnings of a serious brain disorder such as schizophrenia.
Journey from genome to clinicPsychiatrists should be heartened by this translational research into the pharmacotherapeutic promise of emerging genetic advances. The parched terrain of psychopharmacology—the result of a drought of truly innovative medications for serious psychiatric brain disorders—soon may be drenched by a shower of translational discoveries from druggable genes.8 An auspicious scientific journey, from the genome to the clinic, has begun in earnest.
That is great news for our patients, and uplifting to us as well. Breakthroughs to cure intractable and persistent psychiatric brain disorders will not only vanquish disability and restore functioning, but also will be a powerful, long-awaited antidote to the virulent stigma of mental illness.
aAvailable at http://pgc.unc.edu/downloads.
1. Corvin A, Sullivan PF. What next in schizophrenia genetics for the Psychiatric Genomics Consortium [published online March 18, 2016]. Schizophr Bull. pii: sbw014.
2. Lencz T, Malhotra AK. Targeting the schizophrenia genome: a fast-track strategy from GWAS to clinic. Mol Psychiatry. 2015;20(7):820-826.
3. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421-427.
4. Russ AP, Lampels S. The druggable genome: an update. Drug Discov Today. 2005;10(23-24):1607-1610.
5. Sakharkar MK, Sakharkar KR. Targetability of human disease genes. Curr Drug Discov Technol. 2007;4(1):48-58.
6. Rask-Anderson M, Masuram S, Schiöth HB. The druggable genome: evaluation of drug targets in clinical trials supports major shifts in molecular class and indication. Annu Rev Pharmacol Toxicol. 2014;54:9-26.
7. Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1(9):727-730.
8. Lipinski CA, Lombardo F, Dominy BW, et al. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46(1-3):3-26.
Unprecedented collaboration among 900 genetics investigators across 40 countries led to creation of the highly productive Psychiatric Genomics Consortium (PGC), which is analyzing 400,000 individual DNA samples.1 The Consortium has an open-source approach, with data freely available to all who are interested.a
The PGC recently published the results of a large Genome Wide Association Study (GWAS) of 36,989 people with schizophrenia and 113,075 controls. Investigators discovered 108 genetic loci (each containing as many as 26 genes), adding up to 341 protein-coding risk genes for schizophrenia, distributed across all 23 chromosomes.2 One of these risk genes, on chromosome 6, is in the major histocompatibility complex and has the strongest association with schizophrenia (P = 10–31). This finding provides insight that schizophrenia might be related to immune dysfunction, supported by evidence for neuro-inflammation and elevated pro-inflammatory biomarkers in this syndrome.3
In addition to heritable risk genes, the PGC has found many copy number variants (CNVs) and rare de novo mutations that are found significantly more often (10-fold or greater) in schizophrenia. But, as reflected by the 50% concordance rate for schizophrenia in monozygotic twins, non-genetic pathways to schizophrenia obviously exist; this is especially so through adverse events during pregnancy, which can disrupt brain development in a manner similar to disruption caused by risk genes, CNVs, and mutations.
The most exciting consequence of these breakthroughs?These genetic discoveries have great implications for novel drug development for the hundreds of biological subtypes of schizophrenia. At latest count, 23,345 genes that code for proteins, the building blocks of the body, are found in the human set of 23 chromosomes.2 Approximately 7,000 of those genes are druggable and can open the way to developing new agents. In fact, identifying potential targets for pharmacotherapeutic intervention is the major goal of conducting a GWAS.4
What it means to be ‘druggable.’ Two conditions must be met for a gene to be druggable: First, it must code for a protein with folds that can interact with chemical compounds; second, that protein must be associated with a human disease.5 A drug that interacts with several target proteins (eg, kinases, proteases, transporters, enzymes) is considered promiscuous. After such a drug is found to have efficacy in 1 disease, it can be repurposed for treating other diseases. Such repositioning of an already approved drug for other conditions could save the pharmaceutical industry an enormous amount of time and billions of research and development dollars in developing new drugs for psychiatric illnesses that might have been used to treat various other medical conditions.
To exploit the principle of re-purposing, Lencz and Malhotra2 examined the 341 coding genes associated with schizophrenia, to determine whether available drugs interact with the proteins produced by some of those genes. They identified 40 druggable genes (11.7% of the 341) and reported that:
- 27 coding genes (7.92% of the 341) are drug targets6
- 20 of the 40 druggable genes are already approved by the FDA to treat a range of medical disorders, including glaucoma, epilepsy, hypertension, angina, irritable bowel syndrome, incontinence, smoking cessation, nausea, hypertension, prostate cancer, type 2 diabetes mellitus, pulmonary fibrosis, and acute promyelocytic leukemia; in addition, some genes act as a diuretic or an nonsteroidal anti-inflammatory drug
- another 20 druggable genes are not approved for use but are in clinical trials for disorders such as Alzheimer’s disease, heart failure, neuropathic pain, depression, cancer, immune-supported acne psoriasis, and myeloma.
The opportunity to repurpose some of those promiscuous drugs for various medical indications for the treatment of schizophrenia is exciting, and presents Pandora’s box of new mechanisms of action.7 It is intriguing how therapeutic mechanisms for a wide range of unrelated medical conditions may have commonality with the neurobiological underpinnings of a serious brain disorder such as schizophrenia.
Journey from genome to clinicPsychiatrists should be heartened by this translational research into the pharmacotherapeutic promise of emerging genetic advances. The parched terrain of psychopharmacology—the result of a drought of truly innovative medications for serious psychiatric brain disorders—soon may be drenched by a shower of translational discoveries from druggable genes.8 An auspicious scientific journey, from the genome to the clinic, has begun in earnest.
That is great news for our patients, and uplifting to us as well. Breakthroughs to cure intractable and persistent psychiatric brain disorders will not only vanquish disability and restore functioning, but also will be a powerful, long-awaited antidote to the virulent stigma of mental illness.
aAvailable at http://pgc.unc.edu/downloads.
Unprecedented collaboration among 900 genetics investigators across 40 countries led to creation of the highly productive Psychiatric Genomics Consortium (PGC), which is analyzing 400,000 individual DNA samples.1 The Consortium has an open-source approach, with data freely available to all who are interested.a
The PGC recently published the results of a large Genome Wide Association Study (GWAS) of 36,989 people with schizophrenia and 113,075 controls. Investigators discovered 108 genetic loci (each containing as many as 26 genes), adding up to 341 protein-coding risk genes for schizophrenia, distributed across all 23 chromosomes.2 One of these risk genes, on chromosome 6, is in the major histocompatibility complex and has the strongest association with schizophrenia (P = 10–31). This finding provides insight that schizophrenia might be related to immune dysfunction, supported by evidence for neuro-inflammation and elevated pro-inflammatory biomarkers in this syndrome.3
In addition to heritable risk genes, the PGC has found many copy number variants (CNVs) and rare de novo mutations that are found significantly more often (10-fold or greater) in schizophrenia. But, as reflected by the 50% concordance rate for schizophrenia in monozygotic twins, non-genetic pathways to schizophrenia obviously exist; this is especially so through adverse events during pregnancy, which can disrupt brain development in a manner similar to disruption caused by risk genes, CNVs, and mutations.
The most exciting consequence of these breakthroughs?These genetic discoveries have great implications for novel drug development for the hundreds of biological subtypes of schizophrenia. At latest count, 23,345 genes that code for proteins, the building blocks of the body, are found in the human set of 23 chromosomes.2 Approximately 7,000 of those genes are druggable and can open the way to developing new agents. In fact, identifying potential targets for pharmacotherapeutic intervention is the major goal of conducting a GWAS.4
What it means to be ‘druggable.’ Two conditions must be met for a gene to be druggable: First, it must code for a protein with folds that can interact with chemical compounds; second, that protein must be associated with a human disease.5 A drug that interacts with several target proteins (eg, kinases, proteases, transporters, enzymes) is considered promiscuous. After such a drug is found to have efficacy in 1 disease, it can be repurposed for treating other diseases. Such repositioning of an already approved drug for other conditions could save the pharmaceutical industry an enormous amount of time and billions of research and development dollars in developing new drugs for psychiatric illnesses that might have been used to treat various other medical conditions.
To exploit the principle of re-purposing, Lencz and Malhotra2 examined the 341 coding genes associated with schizophrenia, to determine whether available drugs interact with the proteins produced by some of those genes. They identified 40 druggable genes (11.7% of the 341) and reported that:
- 27 coding genes (7.92% of the 341) are drug targets6
- 20 of the 40 druggable genes are already approved by the FDA to treat a range of medical disorders, including glaucoma, epilepsy, hypertension, angina, irritable bowel syndrome, incontinence, smoking cessation, nausea, hypertension, prostate cancer, type 2 diabetes mellitus, pulmonary fibrosis, and acute promyelocytic leukemia; in addition, some genes act as a diuretic or an nonsteroidal anti-inflammatory drug
- another 20 druggable genes are not approved for use but are in clinical trials for disorders such as Alzheimer’s disease, heart failure, neuropathic pain, depression, cancer, immune-supported acne psoriasis, and myeloma.
The opportunity to repurpose some of those promiscuous drugs for various medical indications for the treatment of schizophrenia is exciting, and presents Pandora’s box of new mechanisms of action.7 It is intriguing how therapeutic mechanisms for a wide range of unrelated medical conditions may have commonality with the neurobiological underpinnings of a serious brain disorder such as schizophrenia.
Journey from genome to clinicPsychiatrists should be heartened by this translational research into the pharmacotherapeutic promise of emerging genetic advances. The parched terrain of psychopharmacology—the result of a drought of truly innovative medications for serious psychiatric brain disorders—soon may be drenched by a shower of translational discoveries from druggable genes.8 An auspicious scientific journey, from the genome to the clinic, has begun in earnest.
That is great news for our patients, and uplifting to us as well. Breakthroughs to cure intractable and persistent psychiatric brain disorders will not only vanquish disability and restore functioning, but also will be a powerful, long-awaited antidote to the virulent stigma of mental illness.
aAvailable at http://pgc.unc.edu/downloads.
1. Corvin A, Sullivan PF. What next in schizophrenia genetics for the Psychiatric Genomics Consortium [published online March 18, 2016]. Schizophr Bull. pii: sbw014.
2. Lencz T, Malhotra AK. Targeting the schizophrenia genome: a fast-track strategy from GWAS to clinic. Mol Psychiatry. 2015;20(7):820-826.
3. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421-427.
4. Russ AP, Lampels S. The druggable genome: an update. Drug Discov Today. 2005;10(23-24):1607-1610.
5. Sakharkar MK, Sakharkar KR. Targetability of human disease genes. Curr Drug Discov Technol. 2007;4(1):48-58.
6. Rask-Anderson M, Masuram S, Schiöth HB. The druggable genome: evaluation of drug targets in clinical trials supports major shifts in molecular class and indication. Annu Rev Pharmacol Toxicol. 2014;54:9-26.
7. Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1(9):727-730.
8. Lipinski CA, Lombardo F, Dominy BW, et al. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46(1-3):3-26.
1. Corvin A, Sullivan PF. What next in schizophrenia genetics for the Psychiatric Genomics Consortium [published online March 18, 2016]. Schizophr Bull. pii: sbw014.
2. Lencz T, Malhotra AK. Targeting the schizophrenia genome: a fast-track strategy from GWAS to clinic. Mol Psychiatry. 2015;20(7):820-826.
3. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421-427.
4. Russ AP, Lampels S. The druggable genome: an update. Drug Discov Today. 2005;10(23-24):1607-1610.
5. Sakharkar MK, Sakharkar KR. Targetability of human disease genes. Curr Drug Discov Technol. 2007;4(1):48-58.
6. Rask-Anderson M, Masuram S, Schiöth HB. The druggable genome: evaluation of drug targets in clinical trials supports major shifts in molecular class and indication. Annu Rev Pharmacol Toxicol. 2014;54:9-26.
7. Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1(9):727-730.
8. Lipinski CA, Lombardo F, Dominy BW, et al. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46(1-3):3-26.
COPD comorbid with mental illness: What psychiatrists can do
Chronic obstructive pulmonary disease (COPD) usually is not diagnosed until clinically apparent and moderately advanced. Patients might not notice chronic dyspnea and smoker’s cough, or might consider their symptoms “normal” and not seek medical care. Delayed diagnosis is particularly prevalent in the psychiatric population, in which co-existing medical problems tend to remain unrecognized and untreated.1
Life expectancy of people with serious mental illness (SMI) is 13 to 30 years less than that of the general population—a gap that has widened over time.2 Pulmonary disease is a leading cause of elevated mortality risk in SMI, along with cardiovascular and infectious disease, diabetes, and barriers to care. Having a comorbid mental illness triples the mortality risk of chronic lower respiratory disease (Table 1).3
This article describes how you can intervene and improve quality of life for your patients with COPD by:
- asking all patients, especially smokers, if they are experiencing classic symptoms of COPD
- advocating for and supporting smoking cessation efforts
- avoiding drug interactions and off-target dosing related to COPD and nicotine replacement therapy
- considering, if feasible, a switch from typical to atypical antipsychotic therapy, which could reduce smoking behavior.
What is COPD?
COPD is preventable and treatable. It is characterized by “persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response in the airways and the lungs to inhaled noxious particles or gases.”4
Smoking tobacco is the greatest risk factor for developing COPD.5 An estimated 50% to 80% of people with schizophrenia are smokers, as are 55% of people with bipolar disorder.6 COPD is a leading cause of morbidity and mortality worldwide,7,8 and its prevalence is projected to increase as the global population and smoking rates grow.9
A simplified schema of the pathophysiology of COPD implicates 4 lung areas: parenchyma, pulmonary vasculature, central airways, and peripheral airways.10 Variation in the areas affected and severity of change contributes to the disease’s heterogeneous presentation, which can include pulmonary hypertension, hypersecretion of mucus, ciliary dysfunction, airway hyperinflation, and impaired gas exchange.11,12 Many of these features lead to systemic effects as well, particularly on cardiac function.
When to test a patient for COPD
Early diagnosis and treatment can substantially improve quality-of-life outcomes for patients with COPD. The clinical approach (Figure 1) begins with recognizing classic symptoms. Consider COPD in any patient with:
- dyspnea (particularly if becoming worse, persistent, or associated with exercise)
- chronic cough
- chronic sputum production
- history of risk-factor exposure (particularly tobacco smoke)
- family history of COPD.4
If the history and physical exam suggest COPD (Table 2), spirometry is the most reliable test to quantify and characterize lung dysfunction. It is not indicated as a screening tool for healthy adults or appropriate when a patient is acutely ill. Forced expiratory volume in the first second of expiration divided by the measured forced vital capacity (FEV1/FVC) < 0.7 defines clinical COPD and determines the need for pharmacologic intervention. Laboratory studies could be useful in certain clinical scenarios, such as serum testing for alpha1-antitrypsin deficiency in patients age <45 with emphysema. Plain film imaging might be useful to support a COPD diagnosis or rule out alternate diagnoses.
Psychopharmacology issues with comorbid COPD
Pharmacotherapy for psychiatric disorders can exacerbate comorbid COPD. For example, long-term use of phenothiazine-related typical antipsychotics for schizophrenia has been linked to an increased incidence of COPD.13 Antipsychotic side effects such as acute laryngeal dystonia and tardive dyskinesia, most commonly seen with first-generation antipsychotic use, can aggravate dyspnea caused by COPD. Opioids and most hypnotics, sedatives, and anxiolytics suppress the respiratory drive, and therefore should be used with caution in patients with COPD.
Carefully monitor serum levels of medications before and during attempts at smoking cessation. Nicotine’s induction of the cytochrome P450 1A2 system increases the metabolism of antipsychotics such as clozapine, fluvoxamine, olanzapine, and haloperidol. As a result, potentially toxic drug levels can occur when a smoker tries to quit.14
Screen patients with COPD for comorbid psychiatric conditions. New psychiatric symptoms can emerge after COPD has been diagnosed, even in patients without pre-existing psychopathology.
Anxiety is a particularly common COPD comorbidity that can be difficult to manage. Selective serotonin reuptake inhibitors, buspirone, cognitive-behavioral therapy, and pulmonary rehabilitation can be helpful, although the effect of antidepressants on respiration is controversial. Nortriptyline has been shown to be effective in treating both anxiety and depressive symptoms in patients with COPD.15 Avoid using hypnotics to manage sleep problems related to COPD; instead, focus on minimizing sleep disturbance by limiting cough and dyspnea.
Antipsychotics and nicotine metabolism
Multiple studies have focused on the interplay among nicotine, dopamine, and antipsychotic agents. Nicotine receptors are present in the ventral tegmental dopaminergic cell bodies, which induce the release of dopamine and other neurotransmitters when stimulated. Smoking has been noted to increase in patients administered haloperidol (a dopamine antagonist) and to decrease with administration of bromocriptine (a dopamine agonist).16 This suggests that psychiatric patients might smoke to overcome the dopamine blockade caused by most typical antipsychotics, therefore alleviating their negative and extrapyramidal side effects.17
Alternatively, some studies suggest that a difference in dopamine receptor occupancy between typical and atypical antipsychotics leads to different effects on smoking behavior.18 When used long term, typical antipsychotics might increase dopamine receptors or dopamine sensitivity, and thus reinforce the positive effect of nicotine by increasing the number of receptors that can be stimulated, whereas atypical antipsychotics help stimulate the release of dopamine directly through partial agonist of serotonin 5-HT1A receptors.19,20 Atypical antipsychotics also appear to decrease cue-elicited cravings in people who are not mentally ill, whereas haloperidol does not.21
Based on these findings, switching patients with COPD from a typical to an atypical antipsychotic, if feasible, might make smoking cessation more manageable.22 Multiple studies have shown that clozapine is the preferred atypical antipsychotic because it is associated with the most significant decrease in smoking behaviors.23
First-line therapy: Nicotine replacement
Smoking cessation slows the progression of COPD and leads to marked improvements in cough, expectoration, breathlessness, and wheezing.24,25 Nicotine replacement therapy (NRT)—gum, inhaler, lozenges, nasal spray, and skin patch—is considered first-line pharmacotherapy. These nicotine substitutes can decrease withdrawal symptoms, although they do not appear to be as effective for light smokers (eg, <10 cigarettes/d), compared with heavy smokers (eg, ≥20 cigarettes/d).26
Long-term smoking abstinence can be improved with combination therapies. A nicotine patch, kept in place for as long as 24 hours, often is used with a nicotine gum or nasal spray. Another option combines the patch with a first-line, non-NRT intervention, such as sustained-release bupropion. Use bupropion with caution in psychiatric patients, however. Do not combine it with a monoamine oxidase inhibitor, and do not prescribe it to patients with an eating disorder or history of seizures.26 Bupropion could induce mania in patients with bipolar disorder.
Varenicline, a nicotinic receptor partial agonist indicated to aid in smoking cessation, has been shown to reduce pleasure gained from tobacco as well as cravings. It can increase the likelihood of abstinence from smoking for as long as 1 year, but it also can provoke behavioral changes, depressed mood, and suicidal ideation. These risks—described in an FDA black-box warning of serious neuropsychiatric events—warrant due caution when prescribing varenicline to patients with depression. The FDA also has warned that varenicline could lead to decreased alcohol tolerance and atypically aggressive behavior during intoxication, which is of particular concern because of the high rate of alcohol use among people with SMI.
Motivating and supporting change
When counseling patients with mental illness about smoking cessation, consider unique motivations that, if disregarded, could undermine your efforts. As described above, smoking can ameliorate negative and extrapyramidal symptoms associated with typical antipsychotics. This could explain the significantly higher rates of smoking associated with typical antipsychotics, compared with atypical antipsychotics.27 Patients also could use smoking as self-medication for depression and anxiety. Therefore, take care to offer alternate methods for coping, along with smoking cessation recommendations.22
Screen all adult patients for tobacco use, and offer prompt cessation counseling and pharmacologic interventions.28As a motivational intervention, the “5 As” framework—ask, advise, assess, assist, arrange—can help gauge patients’ smoking status and willingness to quit, as well as emphasize the importance of establishing a concrete, manageable plan.29
Keep in mind the barriers all patients face in their fight to quit smoking, such as nicotine withdrawal, weight gain, and loss of a coping mechanism for stress.29 Patients with schizophrenia can be motivated to quit smoking and participate in treatment for nicotine dependence.30
Besides encouraging smoking cessation, you can educate patients in behaviors that will improve COPD symptoms and management. These include:
- reducing the risk of lung infections through vaccinations (influenza yearly, pneumonia once in adulthood) and avoiding crowds during peak cold and influenza season
- participating in physical activity, which could slow lung function decline
- adhering to prescribed medication
- eating a balanced diet
- seeking medical care early during an exacerbation.
Coaching patients in symptom control
Smoking cessation may have the greatest long-term benefit for patients with COPD, but symptom management is important as well (Figure 2). Pharmacotherapy for COPD usually is advanced in steps, but a more aggressive approach may be necessary for patients presenting with severe symptoms.
Mainstays of COPD therapy are inhaled bronchodilators, consisting of β2 agonists and anticholinergics, alone or in combination. Short-acting formulations are used for mild and intermittent symptoms; long-acting bronchodilators are added if symptoms persist.4 When dyspnea, wheezing, and activity intolerance are not well-controlled with bronchodilators, an inhaled corticosteroid can be tried, either alone or in combination with a long-acting bronchodilator.4
Adherence to medical recommendations is critical for successful COPD management, but inhaled therapy can be difficult for psychiatric patients—especially patients with cognitive or functional impairment. Asking them to demonstrate their inhaler technique can help assess treatment effectiveness.31
Referral to a pulmonologist is strongly advised in cases of:
- advanced, end-stage COPD (FEV1 <50% predicted value despite adherence to recommended treatment, or rapid decline of FEV1)
- COPD in patients age <50
- frequent exacerbations
- possible complications related to chronic heart failure
- indications for oxygen treatment (eg, resting or ambulatory oxygen saturation ≤88% or PaO2 ≤55 mm Hg).32
1. Miller BJ, Paschall CB 3rd, Svendsen DP. Mortality and medical comorbidity among patients with serious mental illness. Psychiatr Serv. 2006;57(10):1482-1487.
2. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123-1131.
3. Freeman E, Yoe JT. The poor health status of consumers of mental healthcare: behavioral disorders and chronic disease. Paper presented at: the National Association of State Mental Health Program Directors Medical Directors Workgroup; May 2006; Alexandria, VA.
4. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf. Published February 20, 2013. Accessed March 2, 2016.
5. AntÒ JM, Vermeire P, Vestbo J, et al. Epidemiology of chronic obstructive pulmonary disease. Eur Respir J. 2001;17(5):982-994.
6. Newcomer JW. Antipsychotic medications: metabolic and cardiovascular risk. J Clin Psychiatry. 2007;68(suppl 4):8-13.
7. Calverley PM, Walker P. Chronic obstructive pulmonary disease. Lancet. 2003;362(9389):1053-1061.
8. Centers for Disease Control and Prevention. Chronic obstructive pulmonary disease among adults—United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:938-943.
9. Feenstra TL, van Genugten ML, Hoogenveen RT, et al. The impact of aging and smoking on the future burden of chronic obstructive pulmonary disease: a model analysis in the Netherlands. Am J Respir Crit Care Med. 2001;164(4):590-596.
10. Celli BR, MacNee W; ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper [Erratum in: Eur Respir J. 2006;27(1):242]. Eur Respir J. 2004;23(6):932-946.
11. Matsuba K, Wright JL, Wiggs BR, et al. The changes in airways structure associated with reduced forced expiratory volume in one second. Eur Respir J. 1989;2(9):834-839.
12. O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(5):770-777.
13. Volkov VP. Respiratory diseases as a cause of death in schizophrenia [article in Russian]. Probl Tuberk Bolezn Legk. 2009;(6):24-27.
14. Kroon LA. Drug interactions and smoking: raising awareness for acute and critical care providers. Crit Care Nurs Clin North Am. 2006;18(1):53-62, xii.
15. Borson S, McDonald GJ, Gayle T, et al. Improvement in mood, physical symptoms, and function with nortriptyline for depression in patients with chronic obstructive pulmonary disease. Psychosomatics. 1992;33(2):190-201.
16. Caskey NH, Jarvik ME, Wirshing WC. The effects of dopaminergic D2 stimulation and blockade on smoking behavior. Exp Clin Psychopharmacol. 1999;7(1):72-78.
17. Dawe S, Gerada C, Russell MA, et al. Nicotine intake in smokers increases following a single dose of haloperidol. Psychopharmacol (Berl). 1995;117(1):110-115.
18. de Haan L, Booji J, Lavalaye J, et al. Occupancy of dopamine D2 receptors by antipsychotic drugs is related to nicotine addiction in young patients with schizophrenia. Psychopharmacology (Berl). 2006;183(4):500-505.
19. Hertel P, Nomikos GG, Iurlo M, et al. Risperidone: regional effects in vivo on release and metabolism of dopamine and serotonin in the rat brain. Psychopharmacology (Berl). 1996;124(1-2):74-86.
20. Silvestri S, Seeman MV, Negrete JC, et al. Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl). 2000;152(2):174-180.
21. Hutchison KE, Rutter MC, Niaura R, et al. Olanzapine attenuates cue-elicited craving for tobacco. Psychopharmacology (Berl). 2004;175(4):407-413.
22. Kumari V, Postma P. Nicotine use in schizophrenia: the self medication hypotheses. Neurosci Biobehav Rev. 2005;29(6):1021-1034.
23. Procyshyn RM, Tse G, Sin O, et al. Concomitant clozapine reduces smoking in patients treated with risperidone. Eur Neuropsychopharmacol. 2002;12(1):77-80.
24. Anthonisen NR, Connett JE, Kiley JP, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA. 1994;272(19):1497-1505.
25. Pisinger C, Godtfredsen NS. Is there a health benefit of reduced tobacco consumption? A systematic review. Nicotine Tob Res. 2007;9(6):631-646.
26. Fiore MC, Jaén CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Clinical Practice Guideline. Rockville, MD: Public Health Service, US Department of Health and Human Services; 2008.
27. Barnes M, Lawford BR, Burton SC, et al. Smoking and schizophrenia: is symptom profile related to smoking and which antipsychotic medication is of benefit in reducing cigarette use? Aust N Z J Psychiatry. 2006;40(6-7):575-580.
28. Screening for chronic obstructive pulmonary disease using spirometry: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;148(7):529-534.
29. Agency for Healthcare Research and Quality. Five major steps to intervention (The “5 A’s”). http://www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/5steps.html. Published 2012. Accessed March 2, 2016.
30. Addington J, el-Guebaly N, Campbell W, et al. Smoking cessation treatment for patients with schizophrenia. Am J Psychiatry. 1998;155(7):974-976.
31. Zarowitz BJ, O’Shea T. Chronic obstructive pulmonary disease: prevalence, characteristics, and pharmacologic treatment in nursing home residents with cognitive impairment. J Manag Care Pharm. 2012;18(8):598-606.
32. Schermer T, Smeenk F, van Weel C. Referral and consultation in asthma and COPD: an exploration of pulmonologists’ views. Neth J Med. 2003;61(3):71-81.
Chronic obstructive pulmonary disease (COPD) usually is not diagnosed until clinically apparent and moderately advanced. Patients might not notice chronic dyspnea and smoker’s cough, or might consider their symptoms “normal” and not seek medical care. Delayed diagnosis is particularly prevalent in the psychiatric population, in which co-existing medical problems tend to remain unrecognized and untreated.1
Life expectancy of people with serious mental illness (SMI) is 13 to 30 years less than that of the general population—a gap that has widened over time.2 Pulmonary disease is a leading cause of elevated mortality risk in SMI, along with cardiovascular and infectious disease, diabetes, and barriers to care. Having a comorbid mental illness triples the mortality risk of chronic lower respiratory disease (Table 1).3
This article describes how you can intervene and improve quality of life for your patients with COPD by:
- asking all patients, especially smokers, if they are experiencing classic symptoms of COPD
- advocating for and supporting smoking cessation efforts
- avoiding drug interactions and off-target dosing related to COPD and nicotine replacement therapy
- considering, if feasible, a switch from typical to atypical antipsychotic therapy, which could reduce smoking behavior.
What is COPD?
COPD is preventable and treatable. It is characterized by “persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response in the airways and the lungs to inhaled noxious particles or gases.”4
Smoking tobacco is the greatest risk factor for developing COPD.5 An estimated 50% to 80% of people with schizophrenia are smokers, as are 55% of people with bipolar disorder.6 COPD is a leading cause of morbidity and mortality worldwide,7,8 and its prevalence is projected to increase as the global population and smoking rates grow.9
A simplified schema of the pathophysiology of COPD implicates 4 lung areas: parenchyma, pulmonary vasculature, central airways, and peripheral airways.10 Variation in the areas affected and severity of change contributes to the disease’s heterogeneous presentation, which can include pulmonary hypertension, hypersecretion of mucus, ciliary dysfunction, airway hyperinflation, and impaired gas exchange.11,12 Many of these features lead to systemic effects as well, particularly on cardiac function.
When to test a patient for COPD
Early diagnosis and treatment can substantially improve quality-of-life outcomes for patients with COPD. The clinical approach (Figure 1) begins with recognizing classic symptoms. Consider COPD in any patient with:
- dyspnea (particularly if becoming worse, persistent, or associated with exercise)
- chronic cough
- chronic sputum production
- history of risk-factor exposure (particularly tobacco smoke)
- family history of COPD.4
If the history and physical exam suggest COPD (Table 2), spirometry is the most reliable test to quantify and characterize lung dysfunction. It is not indicated as a screening tool for healthy adults or appropriate when a patient is acutely ill. Forced expiratory volume in the first second of expiration divided by the measured forced vital capacity (FEV1/FVC) < 0.7 defines clinical COPD and determines the need for pharmacologic intervention. Laboratory studies could be useful in certain clinical scenarios, such as serum testing for alpha1-antitrypsin deficiency in patients age <45 with emphysema. Plain film imaging might be useful to support a COPD diagnosis or rule out alternate diagnoses.
Psychopharmacology issues with comorbid COPD
Pharmacotherapy for psychiatric disorders can exacerbate comorbid COPD. For example, long-term use of phenothiazine-related typical antipsychotics for schizophrenia has been linked to an increased incidence of COPD.13 Antipsychotic side effects such as acute laryngeal dystonia and tardive dyskinesia, most commonly seen with first-generation antipsychotic use, can aggravate dyspnea caused by COPD. Opioids and most hypnotics, sedatives, and anxiolytics suppress the respiratory drive, and therefore should be used with caution in patients with COPD.
Carefully monitor serum levels of medications before and during attempts at smoking cessation. Nicotine’s induction of the cytochrome P450 1A2 system increases the metabolism of antipsychotics such as clozapine, fluvoxamine, olanzapine, and haloperidol. As a result, potentially toxic drug levels can occur when a smoker tries to quit.14
Screen patients with COPD for comorbid psychiatric conditions. New psychiatric symptoms can emerge after COPD has been diagnosed, even in patients without pre-existing psychopathology.
Anxiety is a particularly common COPD comorbidity that can be difficult to manage. Selective serotonin reuptake inhibitors, buspirone, cognitive-behavioral therapy, and pulmonary rehabilitation can be helpful, although the effect of antidepressants on respiration is controversial. Nortriptyline has been shown to be effective in treating both anxiety and depressive symptoms in patients with COPD.15 Avoid using hypnotics to manage sleep problems related to COPD; instead, focus on minimizing sleep disturbance by limiting cough and dyspnea.
Antipsychotics and nicotine metabolism
Multiple studies have focused on the interplay among nicotine, dopamine, and antipsychotic agents. Nicotine receptors are present in the ventral tegmental dopaminergic cell bodies, which induce the release of dopamine and other neurotransmitters when stimulated. Smoking has been noted to increase in patients administered haloperidol (a dopamine antagonist) and to decrease with administration of bromocriptine (a dopamine agonist).16 This suggests that psychiatric patients might smoke to overcome the dopamine blockade caused by most typical antipsychotics, therefore alleviating their negative and extrapyramidal side effects.17
Alternatively, some studies suggest that a difference in dopamine receptor occupancy between typical and atypical antipsychotics leads to different effects on smoking behavior.18 When used long term, typical antipsychotics might increase dopamine receptors or dopamine sensitivity, and thus reinforce the positive effect of nicotine by increasing the number of receptors that can be stimulated, whereas atypical antipsychotics help stimulate the release of dopamine directly through partial agonist of serotonin 5-HT1A receptors.19,20 Atypical antipsychotics also appear to decrease cue-elicited cravings in people who are not mentally ill, whereas haloperidol does not.21
Based on these findings, switching patients with COPD from a typical to an atypical antipsychotic, if feasible, might make smoking cessation more manageable.22 Multiple studies have shown that clozapine is the preferred atypical antipsychotic because it is associated with the most significant decrease in smoking behaviors.23
First-line therapy: Nicotine replacement
Smoking cessation slows the progression of COPD and leads to marked improvements in cough, expectoration, breathlessness, and wheezing.24,25 Nicotine replacement therapy (NRT)—gum, inhaler, lozenges, nasal spray, and skin patch—is considered first-line pharmacotherapy. These nicotine substitutes can decrease withdrawal symptoms, although they do not appear to be as effective for light smokers (eg, <10 cigarettes/d), compared with heavy smokers (eg, ≥20 cigarettes/d).26
Long-term smoking abstinence can be improved with combination therapies. A nicotine patch, kept in place for as long as 24 hours, often is used with a nicotine gum or nasal spray. Another option combines the patch with a first-line, non-NRT intervention, such as sustained-release bupropion. Use bupropion with caution in psychiatric patients, however. Do not combine it with a monoamine oxidase inhibitor, and do not prescribe it to patients with an eating disorder or history of seizures.26 Bupropion could induce mania in patients with bipolar disorder.
Varenicline, a nicotinic receptor partial agonist indicated to aid in smoking cessation, has been shown to reduce pleasure gained from tobacco as well as cravings. It can increase the likelihood of abstinence from smoking for as long as 1 year, but it also can provoke behavioral changes, depressed mood, and suicidal ideation. These risks—described in an FDA black-box warning of serious neuropsychiatric events—warrant due caution when prescribing varenicline to patients with depression. The FDA also has warned that varenicline could lead to decreased alcohol tolerance and atypically aggressive behavior during intoxication, which is of particular concern because of the high rate of alcohol use among people with SMI.
Motivating and supporting change
When counseling patients with mental illness about smoking cessation, consider unique motivations that, if disregarded, could undermine your efforts. As described above, smoking can ameliorate negative and extrapyramidal symptoms associated with typical antipsychotics. This could explain the significantly higher rates of smoking associated with typical antipsychotics, compared with atypical antipsychotics.27 Patients also could use smoking as self-medication for depression and anxiety. Therefore, take care to offer alternate methods for coping, along with smoking cessation recommendations.22
Screen all adult patients for tobacco use, and offer prompt cessation counseling and pharmacologic interventions.28As a motivational intervention, the “5 As” framework—ask, advise, assess, assist, arrange—can help gauge patients’ smoking status and willingness to quit, as well as emphasize the importance of establishing a concrete, manageable plan.29
Keep in mind the barriers all patients face in their fight to quit smoking, such as nicotine withdrawal, weight gain, and loss of a coping mechanism for stress.29 Patients with schizophrenia can be motivated to quit smoking and participate in treatment for nicotine dependence.30
Besides encouraging smoking cessation, you can educate patients in behaviors that will improve COPD symptoms and management. These include:
- reducing the risk of lung infections through vaccinations (influenza yearly, pneumonia once in adulthood) and avoiding crowds during peak cold and influenza season
- participating in physical activity, which could slow lung function decline
- adhering to prescribed medication
- eating a balanced diet
- seeking medical care early during an exacerbation.
Coaching patients in symptom control
Smoking cessation may have the greatest long-term benefit for patients with COPD, but symptom management is important as well (Figure 2). Pharmacotherapy for COPD usually is advanced in steps, but a more aggressive approach may be necessary for patients presenting with severe symptoms.
Mainstays of COPD therapy are inhaled bronchodilators, consisting of β2 agonists and anticholinergics, alone or in combination. Short-acting formulations are used for mild and intermittent symptoms; long-acting bronchodilators are added if symptoms persist.4 When dyspnea, wheezing, and activity intolerance are not well-controlled with bronchodilators, an inhaled corticosteroid can be tried, either alone or in combination with a long-acting bronchodilator.4
Adherence to medical recommendations is critical for successful COPD management, but inhaled therapy can be difficult for psychiatric patients—especially patients with cognitive or functional impairment. Asking them to demonstrate their inhaler technique can help assess treatment effectiveness.31
Referral to a pulmonologist is strongly advised in cases of:
- advanced, end-stage COPD (FEV1 <50% predicted value despite adherence to recommended treatment, or rapid decline of FEV1)
- COPD in patients age <50
- frequent exacerbations
- possible complications related to chronic heart failure
- indications for oxygen treatment (eg, resting or ambulatory oxygen saturation ≤88% or PaO2 ≤55 mm Hg).32
Chronic obstructive pulmonary disease (COPD) usually is not diagnosed until clinically apparent and moderately advanced. Patients might not notice chronic dyspnea and smoker’s cough, or might consider their symptoms “normal” and not seek medical care. Delayed diagnosis is particularly prevalent in the psychiatric population, in which co-existing medical problems tend to remain unrecognized and untreated.1
Life expectancy of people with serious mental illness (SMI) is 13 to 30 years less than that of the general population—a gap that has widened over time.2 Pulmonary disease is a leading cause of elevated mortality risk in SMI, along with cardiovascular and infectious disease, diabetes, and barriers to care. Having a comorbid mental illness triples the mortality risk of chronic lower respiratory disease (Table 1).3
This article describes how you can intervene and improve quality of life for your patients with COPD by:
- asking all patients, especially smokers, if they are experiencing classic symptoms of COPD
- advocating for and supporting smoking cessation efforts
- avoiding drug interactions and off-target dosing related to COPD and nicotine replacement therapy
- considering, if feasible, a switch from typical to atypical antipsychotic therapy, which could reduce smoking behavior.
What is COPD?
COPD is preventable and treatable. It is characterized by “persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response in the airways and the lungs to inhaled noxious particles or gases.”4
Smoking tobacco is the greatest risk factor for developing COPD.5 An estimated 50% to 80% of people with schizophrenia are smokers, as are 55% of people with bipolar disorder.6 COPD is a leading cause of morbidity and mortality worldwide,7,8 and its prevalence is projected to increase as the global population and smoking rates grow.9
A simplified schema of the pathophysiology of COPD implicates 4 lung areas: parenchyma, pulmonary vasculature, central airways, and peripheral airways.10 Variation in the areas affected and severity of change contributes to the disease’s heterogeneous presentation, which can include pulmonary hypertension, hypersecretion of mucus, ciliary dysfunction, airway hyperinflation, and impaired gas exchange.11,12 Many of these features lead to systemic effects as well, particularly on cardiac function.
When to test a patient for COPD
Early diagnosis and treatment can substantially improve quality-of-life outcomes for patients with COPD. The clinical approach (Figure 1) begins with recognizing classic symptoms. Consider COPD in any patient with:
- dyspnea (particularly if becoming worse, persistent, or associated with exercise)
- chronic cough
- chronic sputum production
- history of risk-factor exposure (particularly tobacco smoke)
- family history of COPD.4
If the history and physical exam suggest COPD (Table 2), spirometry is the most reliable test to quantify and characterize lung dysfunction. It is not indicated as a screening tool for healthy adults or appropriate when a patient is acutely ill. Forced expiratory volume in the first second of expiration divided by the measured forced vital capacity (FEV1/FVC) < 0.7 defines clinical COPD and determines the need for pharmacologic intervention. Laboratory studies could be useful in certain clinical scenarios, such as serum testing for alpha1-antitrypsin deficiency in patients age <45 with emphysema. Plain film imaging might be useful to support a COPD diagnosis or rule out alternate diagnoses.
Psychopharmacology issues with comorbid COPD
Pharmacotherapy for psychiatric disorders can exacerbate comorbid COPD. For example, long-term use of phenothiazine-related typical antipsychotics for schizophrenia has been linked to an increased incidence of COPD.13 Antipsychotic side effects such as acute laryngeal dystonia and tardive dyskinesia, most commonly seen with first-generation antipsychotic use, can aggravate dyspnea caused by COPD. Opioids and most hypnotics, sedatives, and anxiolytics suppress the respiratory drive, and therefore should be used with caution in patients with COPD.
Carefully monitor serum levels of medications before and during attempts at smoking cessation. Nicotine’s induction of the cytochrome P450 1A2 system increases the metabolism of antipsychotics such as clozapine, fluvoxamine, olanzapine, and haloperidol. As a result, potentially toxic drug levels can occur when a smoker tries to quit.14
Screen patients with COPD for comorbid psychiatric conditions. New psychiatric symptoms can emerge after COPD has been diagnosed, even in patients without pre-existing psychopathology.
Anxiety is a particularly common COPD comorbidity that can be difficult to manage. Selective serotonin reuptake inhibitors, buspirone, cognitive-behavioral therapy, and pulmonary rehabilitation can be helpful, although the effect of antidepressants on respiration is controversial. Nortriptyline has been shown to be effective in treating both anxiety and depressive symptoms in patients with COPD.15 Avoid using hypnotics to manage sleep problems related to COPD; instead, focus on minimizing sleep disturbance by limiting cough and dyspnea.
Antipsychotics and nicotine metabolism
Multiple studies have focused on the interplay among nicotine, dopamine, and antipsychotic agents. Nicotine receptors are present in the ventral tegmental dopaminergic cell bodies, which induce the release of dopamine and other neurotransmitters when stimulated. Smoking has been noted to increase in patients administered haloperidol (a dopamine antagonist) and to decrease with administration of bromocriptine (a dopamine agonist).16 This suggests that psychiatric patients might smoke to overcome the dopamine blockade caused by most typical antipsychotics, therefore alleviating their negative and extrapyramidal side effects.17
Alternatively, some studies suggest that a difference in dopamine receptor occupancy between typical and atypical antipsychotics leads to different effects on smoking behavior.18 When used long term, typical antipsychotics might increase dopamine receptors or dopamine sensitivity, and thus reinforce the positive effect of nicotine by increasing the number of receptors that can be stimulated, whereas atypical antipsychotics help stimulate the release of dopamine directly through partial agonist of serotonin 5-HT1A receptors.19,20 Atypical antipsychotics also appear to decrease cue-elicited cravings in people who are not mentally ill, whereas haloperidol does not.21
Based on these findings, switching patients with COPD from a typical to an atypical antipsychotic, if feasible, might make smoking cessation more manageable.22 Multiple studies have shown that clozapine is the preferred atypical antipsychotic because it is associated with the most significant decrease in smoking behaviors.23
First-line therapy: Nicotine replacement
Smoking cessation slows the progression of COPD and leads to marked improvements in cough, expectoration, breathlessness, and wheezing.24,25 Nicotine replacement therapy (NRT)—gum, inhaler, lozenges, nasal spray, and skin patch—is considered first-line pharmacotherapy. These nicotine substitutes can decrease withdrawal symptoms, although they do not appear to be as effective for light smokers (eg, <10 cigarettes/d), compared with heavy smokers (eg, ≥20 cigarettes/d).26
Long-term smoking abstinence can be improved with combination therapies. A nicotine patch, kept in place for as long as 24 hours, often is used with a nicotine gum or nasal spray. Another option combines the patch with a first-line, non-NRT intervention, such as sustained-release bupropion. Use bupropion with caution in psychiatric patients, however. Do not combine it with a monoamine oxidase inhibitor, and do not prescribe it to patients with an eating disorder or history of seizures.26 Bupropion could induce mania in patients with bipolar disorder.
Varenicline, a nicotinic receptor partial agonist indicated to aid in smoking cessation, has been shown to reduce pleasure gained from tobacco as well as cravings. It can increase the likelihood of abstinence from smoking for as long as 1 year, but it also can provoke behavioral changes, depressed mood, and suicidal ideation. These risks—described in an FDA black-box warning of serious neuropsychiatric events—warrant due caution when prescribing varenicline to patients with depression. The FDA also has warned that varenicline could lead to decreased alcohol tolerance and atypically aggressive behavior during intoxication, which is of particular concern because of the high rate of alcohol use among people with SMI.
Motivating and supporting change
When counseling patients with mental illness about smoking cessation, consider unique motivations that, if disregarded, could undermine your efforts. As described above, smoking can ameliorate negative and extrapyramidal symptoms associated with typical antipsychotics. This could explain the significantly higher rates of smoking associated with typical antipsychotics, compared with atypical antipsychotics.27 Patients also could use smoking as self-medication for depression and anxiety. Therefore, take care to offer alternate methods for coping, along with smoking cessation recommendations.22
Screen all adult patients for tobacco use, and offer prompt cessation counseling and pharmacologic interventions.28As a motivational intervention, the “5 As” framework—ask, advise, assess, assist, arrange—can help gauge patients’ smoking status and willingness to quit, as well as emphasize the importance of establishing a concrete, manageable plan.29
Keep in mind the barriers all patients face in their fight to quit smoking, such as nicotine withdrawal, weight gain, and loss of a coping mechanism for stress.29 Patients with schizophrenia can be motivated to quit smoking and participate in treatment for nicotine dependence.30
Besides encouraging smoking cessation, you can educate patients in behaviors that will improve COPD symptoms and management. These include:
- reducing the risk of lung infections through vaccinations (influenza yearly, pneumonia once in adulthood) and avoiding crowds during peak cold and influenza season
- participating in physical activity, which could slow lung function decline
- adhering to prescribed medication
- eating a balanced diet
- seeking medical care early during an exacerbation.
Coaching patients in symptom control
Smoking cessation may have the greatest long-term benefit for patients with COPD, but symptom management is important as well (Figure 2). Pharmacotherapy for COPD usually is advanced in steps, but a more aggressive approach may be necessary for patients presenting with severe symptoms.
Mainstays of COPD therapy are inhaled bronchodilators, consisting of β2 agonists and anticholinergics, alone or in combination. Short-acting formulations are used for mild and intermittent symptoms; long-acting bronchodilators are added if symptoms persist.4 When dyspnea, wheezing, and activity intolerance are not well-controlled with bronchodilators, an inhaled corticosteroid can be tried, either alone or in combination with a long-acting bronchodilator.4
Adherence to medical recommendations is critical for successful COPD management, but inhaled therapy can be difficult for psychiatric patients—especially patients with cognitive or functional impairment. Asking them to demonstrate their inhaler technique can help assess treatment effectiveness.31
Referral to a pulmonologist is strongly advised in cases of:
- advanced, end-stage COPD (FEV1 <50% predicted value despite adherence to recommended treatment, or rapid decline of FEV1)
- COPD in patients age <50
- frequent exacerbations
- possible complications related to chronic heart failure
- indications for oxygen treatment (eg, resting or ambulatory oxygen saturation ≤88% or PaO2 ≤55 mm Hg).32
1. Miller BJ, Paschall CB 3rd, Svendsen DP. Mortality and medical comorbidity among patients with serious mental illness. Psychiatr Serv. 2006;57(10):1482-1487.
2. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123-1131.
3. Freeman E, Yoe JT. The poor health status of consumers of mental healthcare: behavioral disorders and chronic disease. Paper presented at: the National Association of State Mental Health Program Directors Medical Directors Workgroup; May 2006; Alexandria, VA.
4. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf. Published February 20, 2013. Accessed March 2, 2016.
5. AntÒ JM, Vermeire P, Vestbo J, et al. Epidemiology of chronic obstructive pulmonary disease. Eur Respir J. 2001;17(5):982-994.
6. Newcomer JW. Antipsychotic medications: metabolic and cardiovascular risk. J Clin Psychiatry. 2007;68(suppl 4):8-13.
7. Calverley PM, Walker P. Chronic obstructive pulmonary disease. Lancet. 2003;362(9389):1053-1061.
8. Centers for Disease Control and Prevention. Chronic obstructive pulmonary disease among adults—United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:938-943.
9. Feenstra TL, van Genugten ML, Hoogenveen RT, et al. The impact of aging and smoking on the future burden of chronic obstructive pulmonary disease: a model analysis in the Netherlands. Am J Respir Crit Care Med. 2001;164(4):590-596.
10. Celli BR, MacNee W; ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper [Erratum in: Eur Respir J. 2006;27(1):242]. Eur Respir J. 2004;23(6):932-946.
11. Matsuba K, Wright JL, Wiggs BR, et al. The changes in airways structure associated with reduced forced expiratory volume in one second. Eur Respir J. 1989;2(9):834-839.
12. O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(5):770-777.
13. Volkov VP. Respiratory diseases as a cause of death in schizophrenia [article in Russian]. Probl Tuberk Bolezn Legk. 2009;(6):24-27.
14. Kroon LA. Drug interactions and smoking: raising awareness for acute and critical care providers. Crit Care Nurs Clin North Am. 2006;18(1):53-62, xii.
15. Borson S, McDonald GJ, Gayle T, et al. Improvement in mood, physical symptoms, and function with nortriptyline for depression in patients with chronic obstructive pulmonary disease. Psychosomatics. 1992;33(2):190-201.
16. Caskey NH, Jarvik ME, Wirshing WC. The effects of dopaminergic D2 stimulation and blockade on smoking behavior. Exp Clin Psychopharmacol. 1999;7(1):72-78.
17. Dawe S, Gerada C, Russell MA, et al. Nicotine intake in smokers increases following a single dose of haloperidol. Psychopharmacol (Berl). 1995;117(1):110-115.
18. de Haan L, Booji J, Lavalaye J, et al. Occupancy of dopamine D2 receptors by antipsychotic drugs is related to nicotine addiction in young patients with schizophrenia. Psychopharmacology (Berl). 2006;183(4):500-505.
19. Hertel P, Nomikos GG, Iurlo M, et al. Risperidone: regional effects in vivo on release and metabolism of dopamine and serotonin in the rat brain. Psychopharmacology (Berl). 1996;124(1-2):74-86.
20. Silvestri S, Seeman MV, Negrete JC, et al. Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl). 2000;152(2):174-180.
21. Hutchison KE, Rutter MC, Niaura R, et al. Olanzapine attenuates cue-elicited craving for tobacco. Psychopharmacology (Berl). 2004;175(4):407-413.
22. Kumari V, Postma P. Nicotine use in schizophrenia: the self medication hypotheses. Neurosci Biobehav Rev. 2005;29(6):1021-1034.
23. Procyshyn RM, Tse G, Sin O, et al. Concomitant clozapine reduces smoking in patients treated with risperidone. Eur Neuropsychopharmacol. 2002;12(1):77-80.
24. Anthonisen NR, Connett JE, Kiley JP, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA. 1994;272(19):1497-1505.
25. Pisinger C, Godtfredsen NS. Is there a health benefit of reduced tobacco consumption? A systematic review. Nicotine Tob Res. 2007;9(6):631-646.
26. Fiore MC, Jaén CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Clinical Practice Guideline. Rockville, MD: Public Health Service, US Department of Health and Human Services; 2008.
27. Barnes M, Lawford BR, Burton SC, et al. Smoking and schizophrenia: is symptom profile related to smoking and which antipsychotic medication is of benefit in reducing cigarette use? Aust N Z J Psychiatry. 2006;40(6-7):575-580.
28. Screening for chronic obstructive pulmonary disease using spirometry: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;148(7):529-534.
29. Agency for Healthcare Research and Quality. Five major steps to intervention (The “5 A’s”). http://www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/5steps.html. Published 2012. Accessed March 2, 2016.
30. Addington J, el-Guebaly N, Campbell W, et al. Smoking cessation treatment for patients with schizophrenia. Am J Psychiatry. 1998;155(7):974-976.
31. Zarowitz BJ, O’Shea T. Chronic obstructive pulmonary disease: prevalence, characteristics, and pharmacologic treatment in nursing home residents with cognitive impairment. J Manag Care Pharm. 2012;18(8):598-606.
32. Schermer T, Smeenk F, van Weel C. Referral and consultation in asthma and COPD: an exploration of pulmonologists’ views. Neth J Med. 2003;61(3):71-81.
1. Miller BJ, Paschall CB 3rd, Svendsen DP. Mortality and medical comorbidity among patients with serious mental illness. Psychiatr Serv. 2006;57(10):1482-1487.
2. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123-1131.
3. Freeman E, Yoe JT. The poor health status of consumers of mental healthcare: behavioral disorders and chronic disease. Paper presented at: the National Association of State Mental Health Program Directors Medical Directors Workgroup; May 2006; Alexandria, VA.
4. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf. Published February 20, 2013. Accessed March 2, 2016.
5. AntÒ JM, Vermeire P, Vestbo J, et al. Epidemiology of chronic obstructive pulmonary disease. Eur Respir J. 2001;17(5):982-994.
6. Newcomer JW. Antipsychotic medications: metabolic and cardiovascular risk. J Clin Psychiatry. 2007;68(suppl 4):8-13.
7. Calverley PM, Walker P. Chronic obstructive pulmonary disease. Lancet. 2003;362(9389):1053-1061.
8. Centers for Disease Control and Prevention. Chronic obstructive pulmonary disease among adults—United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:938-943.
9. Feenstra TL, van Genugten ML, Hoogenveen RT, et al. The impact of aging and smoking on the future burden of chronic obstructive pulmonary disease: a model analysis in the Netherlands. Am J Respir Crit Care Med. 2001;164(4):590-596.
10. Celli BR, MacNee W; ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper [Erratum in: Eur Respir J. 2006;27(1):242]. Eur Respir J. 2004;23(6):932-946.
11. Matsuba K, Wright JL, Wiggs BR, et al. The changes in airways structure associated with reduced forced expiratory volume in one second. Eur Respir J. 1989;2(9):834-839.
12. O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(5):770-777.
13. Volkov VP. Respiratory diseases as a cause of death in schizophrenia [article in Russian]. Probl Tuberk Bolezn Legk. 2009;(6):24-27.
14. Kroon LA. Drug interactions and smoking: raising awareness for acute and critical care providers. Crit Care Nurs Clin North Am. 2006;18(1):53-62, xii.
15. Borson S, McDonald GJ, Gayle T, et al. Improvement in mood, physical symptoms, and function with nortriptyline for depression in patients with chronic obstructive pulmonary disease. Psychosomatics. 1992;33(2):190-201.
16. Caskey NH, Jarvik ME, Wirshing WC. The effects of dopaminergic D2 stimulation and blockade on smoking behavior. Exp Clin Psychopharmacol. 1999;7(1):72-78.
17. Dawe S, Gerada C, Russell MA, et al. Nicotine intake in smokers increases following a single dose of haloperidol. Psychopharmacol (Berl). 1995;117(1):110-115.
18. de Haan L, Booji J, Lavalaye J, et al. Occupancy of dopamine D2 receptors by antipsychotic drugs is related to nicotine addiction in young patients with schizophrenia. Psychopharmacology (Berl). 2006;183(4):500-505.
19. Hertel P, Nomikos GG, Iurlo M, et al. Risperidone: regional effects in vivo on release and metabolism of dopamine and serotonin in the rat brain. Psychopharmacology (Berl). 1996;124(1-2):74-86.
20. Silvestri S, Seeman MV, Negrete JC, et al. Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl). 2000;152(2):174-180.
21. Hutchison KE, Rutter MC, Niaura R, et al. Olanzapine attenuates cue-elicited craving for tobacco. Psychopharmacology (Berl). 2004;175(4):407-413.
22. Kumari V, Postma P. Nicotine use in schizophrenia: the self medication hypotheses. Neurosci Biobehav Rev. 2005;29(6):1021-1034.
23. Procyshyn RM, Tse G, Sin O, et al. Concomitant clozapine reduces smoking in patients treated with risperidone. Eur Neuropsychopharmacol. 2002;12(1):77-80.
24. Anthonisen NR, Connett JE, Kiley JP, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA. 1994;272(19):1497-1505.
25. Pisinger C, Godtfredsen NS. Is there a health benefit of reduced tobacco consumption? A systematic review. Nicotine Tob Res. 2007;9(6):631-646.
26. Fiore MC, Jaén CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Clinical Practice Guideline. Rockville, MD: Public Health Service, US Department of Health and Human Services; 2008.
27. Barnes M, Lawford BR, Burton SC, et al. Smoking and schizophrenia: is symptom profile related to smoking and which antipsychotic medication is of benefit in reducing cigarette use? Aust N Z J Psychiatry. 2006;40(6-7):575-580.
28. Screening for chronic obstructive pulmonary disease using spirometry: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;148(7):529-534.
29. Agency for Healthcare Research and Quality. Five major steps to intervention (The “5 A’s”). http://www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/5steps.html. Published 2012. Accessed March 2, 2016.
30. Addington J, el-Guebaly N, Campbell W, et al. Smoking cessation treatment for patients with schizophrenia. Am J Psychiatry. 1998;155(7):974-976.
31. Zarowitz BJ, O’Shea T. Chronic obstructive pulmonary disease: prevalence, characteristics, and pharmacologic treatment in nursing home residents with cognitive impairment. J Manag Care Pharm. 2012;18(8):598-606.
32. Schermer T, Smeenk F, van Weel C. Referral and consultation in asthma and COPD: an exploration of pulmonologists’ views. Neth J Med. 2003;61(3):71-81.
Can anti-inflammatory medications improve symptoms and reduce mortality in schizophrenia?
Consider 3 observations:
- Evidence is mounting that cytokine abnormalities are present in schizophrenia (Box1-8).
- Reduced arterial compliance (change in volume divided by change in pressure [ΔV/ΔP] in an artery during the cardiac cycle) is an early marker of cardiovascular disease (CVD) and a robust predictor of mortality, and is associated with cytokine abnormalities.
- People with schizophrenia experience increased mortality from CVD.
Taken together, the 3 statements hint at a hypothesis: a common inflammatory process involving cytokine imbalance is associated with symptoms of schizophrenia, reduced arterial compliance, and CVD.
Anti-inflammatory therapeutics that target specific cytokines might both decrease psychiatric symptoms and reduce cardiac mortality in people with schizophrenia. In this article, we (1) highlight the potential role of anti-inflammatory medications in reducing both psychiatric symptoms and cardiac mortality in people with schizophrenia and (2) review the pathophysiological basis of this inflammatory commonality and the evidence for its presence in schizophrenia.
The ‘membrane hypothesis’ of schizophrenia
In this hypothesis, a disturbance in the synthesis and structure of membrane phospholipids results in a subsequent disturbance in the function of neuronal membrane proteins, which might be associated with symptoms and mortality in schizophrenia.9-12 The synaptic vesicle protein synaptophysin, a marker for synaptic density, was found to be decreased in postmortem tissue from the gyrus cinguli in 11 patients with schizophrenia, compared with 13 controls.10 Intracellular phospholipases A2 (inPLA2) act as key enzymes in cell membrane repair and remodeling and in neuroplasticity, neurodevelopment, apoptosis, synaptic pruning, neurodegenerative processes, and neuroinflammation.
In a study, people with first-episode schizophrenia (n = 24) who were drug-naïve or off antipsychotic medication were compared with 25 healthy controls using voxel-based morphometry analysis of T1 high-resolution MRI. inPLA2 activity was increased in the patient group compared with controls; the analysis revealed abnormalities of the frontal and medial temporal cortices, hippocampus, and left-middle and superior temporal gyri in first-episode patients.11 In another study, inPLA2 activity was increased in 35 people with first-episode schizophrenia, compared with 22 controls, and was associated with symptom severity and outcome after 12 weeks of antipsychotic treatment.12
Early CVD mortality in schizophrenia
People with schizophrenia have an elevated rate of CVD compared with the general population; in part, this elevation is linked to magnified risk factors for CVD, including obesity, metabolic syndrome, cigarette smoking, and diabetes13-17; furthermore, most antipsychotics can cause or worsen metabolic syndrome.17
CVD is one of the most common causes of death among people with schizophrenia.17,18 Their life expectancy is reported to be 51 to 61 years—20 to 25 years less than what is seen in the general population.19-21
Arterial compliance in schizophrenia
Reduced arterial compliance has been found to be a robust predictor of atherosclerosis, stroke, and myocardial infarction22-29:
- In 376 subjects who had routine diagnostic coronary angiography associated with coronary stenosis, arterial compliance was reduced significantly—even after controlling for age, sex, smoking, diabetes, hypertension, hyperlipidemia, and obesity.24
In a cross-sectional study, 63 male U.S. veterans age 18 to 70 who had a psychiatric diagnosis (16 taking quetiapine, 19 taking risperidone, and 28 treated in the past but off antipsychotics for 2 months) had significantly reduced compliance in thigh- and calf-level arteries than male controls (n = 111), adjusting for body mass index and Framingham Risk Score (FRS). Of the 63 patients, 23 had a diagnosis of schizophrenia or schizoaffective disorder.30 (The FRS is an estimate of a person’s 10-year cardiovascular risk, calculated using age, sex, total cholesterol, high-density lipoprotein, smoker or not, systolic blood pressure, and whether taking an antihypertensive or not. Compliance was measured using computerized plethysmography). Although not statistically significant, secondary analyses from this data set (n = 77, including men for whom factors for metabolic syndrome were available) showed that calf-level compliance (1.82 vs 2.06 mL) and thigh-level compliance (3.6 vs 4.26 mL; P = .06) were reduced in subjects with schizophrenia, compared with those who had another psychiatric diagnosis.31
- In another study, arterial compliance was significantly reduced in 10 subjects with schizophrenia, compared with 10 healthy controls.32
- Last, reduced total arterial compliance has been shown to be a robust predictor of mortality in older people, compared with reduced local or regional arterial compliance.33
Cytokine abnormalities in arterial compliance
The mechanism by which reduced arterial compliance is associated with cardiovascular pathology is not entirely clear. Arterial compliance is a predictor of cardiovascular disorders independent of hypertension.34 Two studies show that vascular inflammation is associated with reduced arterial compliance.35,36 Reduced arterial compliance is associated with increased angiotensin II activity; increased nicotinamide adenine dinucleotide phosphate oxidase activity; reduced nitric oxide activity; and increased reactive oxygen species.37-39 Angiotensin-II signaling activates transforming growth factor-β, tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-17, IL-6, and C-reactive protein (CRP)—all of which are associated with reduced arterial compliance.39-46 In addition, high-sensitivity CRP is significantly associated with reduced arterial compliance.47-49
The overlap of cytokine abnormalities linked to schizophrenia and to arterial compliance is depicted in the Figure.
Anti-inflammatory medications and arterial compliance
Evidence suggests that anti-inflammatory medications increase arterial compliance:
- In 10 patients who had coronary artery disease or diabetes, or both, simvastatin (40 mg/d) was administered for 4 months. Arterial compliance improved in all 10 after 2 months of treatment and increased by 34% after 4 months.27
- Evidence also suggests that the use of omega-3 fatty acids was associated with increased arterial compliance in people with dyslipidemia.50
- Last, in people with rheumatoid arthritis, infliximab, a monoclonal antibody against TNF-Symbol Stdα, reduced aortic inflammation; this effect correlated with an increase in aortic compliance.51
Anti-inflammatory medications in schizophrenia
Two studies have yielded notable findings:
- A meta-analysis of 5 randomized controlled trials (RCTs) involving 264 subjects, comprising 4 studies of celecoxib and 1 of acetylsalicylic acid, had an effect size of 0.43 on total symptom severity. Investigators argued that acetylsalicylic acid might have the additional benefit of decreasing the risk of cardiac death in schizophrenia.52
- A review of 26 RCTs examined the efficacy of anti-inflammatory medications on symptom severity in schizophrenia. Acetylsalicylic acid, N-acetylcysteine, and estrogens had an effect size of 0.3, 0.45, and 0.51, respectively.53
Significance of these findings
A revelation that cytokine abnormalities are associated with schizophrenia symptoms and co-occurring somatic illness might offer an important new avenue of therapeutic discovery. On average, people with schizophrenia die 20 to 25 years earlier than the general population; CVD is the major cause of their death. Measuring arterial compliance, a novel noninvasive technology in psychiatry, as well as metabolic parameters, could serve as an early biomarker for assessing risk of CVD.
Implications for psychiatric practice. If inflammation plays a role in CVD in schizophrenia—either independently of factors such as metabolic syndrome, obesity, and smoking, or on the causal pathway linking these factors to reduced arterial compliance and to CVD—treatment with anti-inflammatory medications might reduce the alarming disparity of mortality that accompanies schizophrenia. In short, anti-inflammatory medications may offer a double benefit in this setting. Furthermore, success in this approach could spur clarification of the role of abnormal cytokines in other psychiatric disorders.
At this time, for your patients, consider that anti-inflammatory medications routinely used in medical practice, such as nonsteroidal anti-inflammatory drugs, omega-3 fatty acids, and statins, might alleviate psychiatric symptoms and might reduce cardiovascular mortality in schizophrenia.
Future directions
Perhaps only a limited number of cytokines are common to schizophrenia and reduced arterial compliance. Targeting those specific cytokines might, however, provide the dual benefit in schizophrenia of:
- alleviating symptoms
- reducing the rate of CVD-related mortality.
Studies are warranted to determine the value of (1) anti-inflammatory medications, such as N-acetylcysteine and infliximab and (2) anti-inflammatory combination therapy for this dual purpose. In fact, recruitment of subjects is underway for a study, Anti-Inflammatory Combination Therapy for the Treatment of Schizophrenia, at the University of Maryland (ClinicalTrials.gov Identifier: NCT01514682).
1. Potvin S, Stip E, Sepehry AA, et al. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63(8):801-808.
2. Miller BJ, Buckley P, Seabolt W, et al. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663-671.
3. Frydecka D, Misiak B, Pawlak-Adamska E, et al. Interleukin-6: the missing element of the neurocognitive deterioration in schizophrenia? The focus on genetic underpinnings, cognitive impairment and clinical manifestation. Eur Arch Psychiatry Clin Neurosci. 2015;265(6):449-459.
4. Dickerson F, Stallings C, Origoni A, et al. Additive effects of elevated C-reactive protein and exposure to herpes simplex virus type 1 on cognitive impairment in individuals with schizophrenia. Schizophr Res. 2012;134(1):83-88.
5. Dickerson F, Stallings C, Origoni A, et al. C-reactive protein is associated with the severity of cognitive impairment but not of psychiatric symptoms in individuals with schizophrenia. Schizophr Res. 2007;93(1-3):261-265.
6. Asevedo E, Rizzo LB, Gadelha A, et al. Peripheral interleukin-2 level is associated with negative symptoms and cognitive performance in schizophrenia. Physiol Behav. 2014;129:194-198.
7. Miller BJ, Culpepper N, Rapaport MH. C-reactive protein levels in schizophrenia: a review and meta-analysis. Clin Schizophr Relat Psychoses. 2014;7(4):223-230.
8. Micoulaud-Franchi JA, Faugere M, Boyer L, et al. Elevated C-reactive protein is associated with sensory gating deficit in schizophrenia. Schizophr Res. 2015;165(1):94-96.
9. Horrobin DF. The membrane phospholipid hypothesis as a biochemical basis for the neurodevelopmental concept of schizophrenia. Schizophr Res. 1998;30(3):193-208.
10. Landén M, Davidsson P, Gottfries CG, et al. Reduction of the synaptophysin level but normal levels of glycerophospholipids in the gyrus cinguli in schizophrenia. Schizophr Res. 2002;55(1-2):83-98.
11. Smesny S, Milleit B, Nenadic I, et al. Phospholipase A2 activity is associated with structural brain changes in schizophrenia. Neuroimage. 2010;52(4):1314-1327.
12. Smesny S, Kunstmann C, Kunstmann S, et al. Phospholipase A2 activity in first episode schizophrenia: associations with symptom severity and outcome at week 12. World J Biol Psychiatry. 2011;12(8):598-607.
13. Fontaine KR, Heo M, Harrigan EP, et al. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res. 2001;101(3):277-288.
14. Homel P, Casey D, Allison DB. Changes in body mass index for individuals with and without schizophrenia, 1987-1996. Schizophr Res. 2002;55(3):277-284.
15. Hedley AA, Ogden CL, Johnson CL, et al. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. JAMA. 2004;291(23):2847-2850.
16. Dickerson FB, Brown CH, Kreyenbuhl JA, et al. Obesity among individuals with serious mental illness. Acta Psychiatr Scand. 2006;113(4):306-313.
17. Newcomer JW. Metabolic syndrome and mental illness. Am J Manag Care. 2007;13(suppl 7):S170-S177.
18. Healy D, Le Noury J, Harris M, et al. Mortality in schizophrenia and related psychoses: data from two cohorts, 1875-1924 and 1994-2010. BMJ Open. 2012;2(5). doi: 10.1136/bmjopen-2012-001810.
19. Newman SC, Bland RC. Mortality in a cohort of patients with schizophrenia: a record linkage study. Can J Psychiatry. 1991;36(4):239-245.
20. Harris EC, Barraclough B. Excess mortality of mental disorder. Br J Psychiatry. 1998;173:11-53.
21. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
22. Farrar DJ, Bond MG, Riley WA, et al. Anatomic correlates of aortic pulse wave velocity and carotid artery elasticity during atherosclerosis progression and regression in monkeys. Circulation. 1991;83(5):1754-1763.
23. Wada T, Kodaira K, Fujishiro K, et al. Correlation of ultrasound-measured common carotid artery stiffness with pathological findings. Arterioscler Thromb. 1994;14(3):479-482.
24. Herrington DM, Kesler K, Reiber JH, et al. Arterial compliance adds to conventional risk factors for prediction of angiographic coronary artery disease. Am Heart J. 2013;146(4):662-667.
25. Willens HJ, Davis W, Herrington DM, et al. Relationship of peripheral arterial compliance and standard cardiovascular risk factors. Vasc Endovascular Surg. 2003;37(3):197-206.
26. Herrington DM, Brown WV, Mosca L, et al. Relationship between arterial stiffness and subclinical aortic atherosclerosis. Circulation. 2004;110(4):432-437.
27. Saliashvili G, Davis WW, Harris MT, et al. Simvastatin improved arterial compliance in high-risk patients. Vasc Endovascular Surg. 2004;38(6):519-523.
28. Le NA, Brown WV, Davis WW, et al. Comparison of the relation of triglyceride-rich lipoproteins and muscular artery compliance in healthy women versus healthy men. Am J Cardiol. 2005;95(9):1049-1054.
29. Willens HJ, Chirinos JA, Brown WV, et al. Usefulness of arterial compliance in the thigh in predicting exercise capacity in individuals without coronary heart disease. Am J Cardiol. 2005;96(2):306-310.
30. Koola MM, Brown WV, Qualls C, et al. Reduced arterial compliance in patients with psychiatric diagnoses. Schizophr Res. 2012;137(1-3):251-253.
31. Koola MM, Sorkin JD, Fargotstein M, et al. Predictors of calf arterial compliance in male veterans with psychiatric diagnoses. The Primary Care Companion for CNS Disorders. In press.
32. Phillips AA, Warburton DE, Flynn SW, et al. Assessment of arterial stiffness among schizophrenia-spectrum disorders using aortic pulse wave velocity and arterial compliance: a pilot study. Psychiatry Res. 2014;215(1):14-19.
33. Papaioannou TG, Protogerou AD, Stergiopulos N, et al. Total arterial compliance estimated by a novel method and all-cause mortality in the elderly: the PROTEGER study. Age (Dordr). 2014;36(3):9661.
34. Park S, Lakatta EG. Role of inflammation in the pathogenesis of arterial stiffness. Yonsei Med J. 2012;53(2):258-261.
35. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107(2):346-354.
36. Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25(5):932-943.
37. van der Loo B, Labugger R, Skepper JN, et al. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192(12):1731-1744.
38. Csiszar A, Ungvari Z, Edwards JG, et al. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90(11):1159-1166.
39. Wang MC, Tsai WC, Chen JY, et al. Arterial stiffness correlated with cardiac remodelling in patients with chronic kidney disease. Nephrology (Carlton). 2007;12(6):591-597.
40. Belmin J, Bernard C, Corman B, et al. Increased production of tumor necrosis factor and interleukin-6 by arterial wall of aged rats. Am J Physiol. 1995;268(6 pt 2):H2288-2293.
41. Gerli R, Monti D, Bistoni O, et al. Chemokines, sTNF-Rs and sCD30 serum levels in healthy aged people and centenarians. Mech Ageing Dev. 2000;121(1-3):37-46.
42. Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102(18):2165-2168.
43. Torzewski M, Rist C, Mortensen RF, et al. C-reactive protein in the arterial intima: role of C-reactive protein receptor-dependent monocyte recruitment in atherogenesis. Arterioscler Thromb Vasc Biol. 2000;20(9):2094-2099.
44. Venugopal SK, Devaraj S, Yuhanna I, et al. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation. 2002;106(12):1439-1441.
45. Csiszar A, Ungvari Z, Koller A, et al. Aging-induced proinflammatory shift in cytokine expression profile in coronary arteries. FASEB J. 2003;17(9):1183-1185.
46. Spinetti G, Wang M, Monticone R, et al. Rat aortic MCP-1 and its receptor CCR2 increase with age and alter vascular smooth muscle cell function. Arterioscler Thromb Vasc Biol. 2004;24(8):1397-1402.
47. Mattace-Raso FU, van der Cammen TJ, van der Meer IM, et al. C-reactive protein and arterial stiffness in older adults: the Rotterdam Study. Atherosclerosis. 2004;176(1):111-116.
48. Mahmud A, Feely J. Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension. 2005;46(5):1118-1122.
49. Nagano M, Nakamura M, Sato K, et al. Association between serum C-reactive protein levels and pulse wave velocity: a population-based cross-sectional study in a general population. Atherosclerosis. 2005;180(1):189-195.
50. Nestel P, Shige H, Pomeroy S, et al. The n-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid increase systemic arterial compliance in humans. Am J Clin Nutr. 2002;76(2):326-330.
51. Mäki-Petäjä KM, Elkhawad M, Cheriyan J, et al. Anti-tumor necrosis factor-α therapy reduces aortic inflammation and stiffness in patients with rheumatoid arthritis. Circulation. 2012;126(21):2473-2480.
52. Sommer IE, de Witte L, Begemann M, et al. Nonsteroidal anti-inflammatory drugs in schizophrenia: ready for practice or a good start? A meta-analysis. J Clin Psychiatry. 2012;73(4):414-419.
53. Sommer IE, van Westrhenen R, Begemann MJ, et al. Efficacy of anti-inflammatory agents to improve symptoms in patients with schizophrenia: an update. Schizophr Bull. 2014;40(1):181-191.
Consider 3 observations:
- Evidence is mounting that cytokine abnormalities are present in schizophrenia (Box1-8).
- Reduced arterial compliance (change in volume divided by change in pressure [ΔV/ΔP] in an artery during the cardiac cycle) is an early marker of cardiovascular disease (CVD) and a robust predictor of mortality, and is associated with cytokine abnormalities.
- People with schizophrenia experience increased mortality from CVD.
Taken together, the 3 statements hint at a hypothesis: a common inflammatory process involving cytokine imbalance is associated with symptoms of schizophrenia, reduced arterial compliance, and CVD.
Anti-inflammatory therapeutics that target specific cytokines might both decrease psychiatric symptoms and reduce cardiac mortality in people with schizophrenia. In this article, we (1) highlight the potential role of anti-inflammatory medications in reducing both psychiatric symptoms and cardiac mortality in people with schizophrenia and (2) review the pathophysiological basis of this inflammatory commonality and the evidence for its presence in schizophrenia.
The ‘membrane hypothesis’ of schizophrenia
In this hypothesis, a disturbance in the synthesis and structure of membrane phospholipids results in a subsequent disturbance in the function of neuronal membrane proteins, which might be associated with symptoms and mortality in schizophrenia.9-12 The synaptic vesicle protein synaptophysin, a marker for synaptic density, was found to be decreased in postmortem tissue from the gyrus cinguli in 11 patients with schizophrenia, compared with 13 controls.10 Intracellular phospholipases A2 (inPLA2) act as key enzymes in cell membrane repair and remodeling and in neuroplasticity, neurodevelopment, apoptosis, synaptic pruning, neurodegenerative processes, and neuroinflammation.
In a study, people with first-episode schizophrenia (n = 24) who were drug-naïve or off antipsychotic medication were compared with 25 healthy controls using voxel-based morphometry analysis of T1 high-resolution MRI. inPLA2 activity was increased in the patient group compared with controls; the analysis revealed abnormalities of the frontal and medial temporal cortices, hippocampus, and left-middle and superior temporal gyri in first-episode patients.11 In another study, inPLA2 activity was increased in 35 people with first-episode schizophrenia, compared with 22 controls, and was associated with symptom severity and outcome after 12 weeks of antipsychotic treatment.12
Early CVD mortality in schizophrenia
People with schizophrenia have an elevated rate of CVD compared with the general population; in part, this elevation is linked to magnified risk factors for CVD, including obesity, metabolic syndrome, cigarette smoking, and diabetes13-17; furthermore, most antipsychotics can cause or worsen metabolic syndrome.17
CVD is one of the most common causes of death among people with schizophrenia.17,18 Their life expectancy is reported to be 51 to 61 years—20 to 25 years less than what is seen in the general population.19-21
Arterial compliance in schizophrenia
Reduced arterial compliance has been found to be a robust predictor of atherosclerosis, stroke, and myocardial infarction22-29:
- In 376 subjects who had routine diagnostic coronary angiography associated with coronary stenosis, arterial compliance was reduced significantly—even after controlling for age, sex, smoking, diabetes, hypertension, hyperlipidemia, and obesity.24
In a cross-sectional study, 63 male U.S. veterans age 18 to 70 who had a psychiatric diagnosis (16 taking quetiapine, 19 taking risperidone, and 28 treated in the past but off antipsychotics for 2 months) had significantly reduced compliance in thigh- and calf-level arteries than male controls (n = 111), adjusting for body mass index and Framingham Risk Score (FRS). Of the 63 patients, 23 had a diagnosis of schizophrenia or schizoaffective disorder.30 (The FRS is an estimate of a person’s 10-year cardiovascular risk, calculated using age, sex, total cholesterol, high-density lipoprotein, smoker or not, systolic blood pressure, and whether taking an antihypertensive or not. Compliance was measured using computerized plethysmography). Although not statistically significant, secondary analyses from this data set (n = 77, including men for whom factors for metabolic syndrome were available) showed that calf-level compliance (1.82 vs 2.06 mL) and thigh-level compliance (3.6 vs 4.26 mL; P = .06) were reduced in subjects with schizophrenia, compared with those who had another psychiatric diagnosis.31
- In another study, arterial compliance was significantly reduced in 10 subjects with schizophrenia, compared with 10 healthy controls.32
- Last, reduced total arterial compliance has been shown to be a robust predictor of mortality in older people, compared with reduced local or regional arterial compliance.33
Cytokine abnormalities in arterial compliance
The mechanism by which reduced arterial compliance is associated with cardiovascular pathology is not entirely clear. Arterial compliance is a predictor of cardiovascular disorders independent of hypertension.34 Two studies show that vascular inflammation is associated with reduced arterial compliance.35,36 Reduced arterial compliance is associated with increased angiotensin II activity; increased nicotinamide adenine dinucleotide phosphate oxidase activity; reduced nitric oxide activity; and increased reactive oxygen species.37-39 Angiotensin-II signaling activates transforming growth factor-β, tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-17, IL-6, and C-reactive protein (CRP)—all of which are associated with reduced arterial compliance.39-46 In addition, high-sensitivity CRP is significantly associated with reduced arterial compliance.47-49
The overlap of cytokine abnormalities linked to schizophrenia and to arterial compliance is depicted in the Figure.
Anti-inflammatory medications and arterial compliance
Evidence suggests that anti-inflammatory medications increase arterial compliance:
- In 10 patients who had coronary artery disease or diabetes, or both, simvastatin (40 mg/d) was administered for 4 months. Arterial compliance improved in all 10 after 2 months of treatment and increased by 34% after 4 months.27
- Evidence also suggests that the use of omega-3 fatty acids was associated with increased arterial compliance in people with dyslipidemia.50
- Last, in people with rheumatoid arthritis, infliximab, a monoclonal antibody against TNF-Symbol Stdα, reduced aortic inflammation; this effect correlated with an increase in aortic compliance.51
Anti-inflammatory medications in schizophrenia
Two studies have yielded notable findings:
- A meta-analysis of 5 randomized controlled trials (RCTs) involving 264 subjects, comprising 4 studies of celecoxib and 1 of acetylsalicylic acid, had an effect size of 0.43 on total symptom severity. Investigators argued that acetylsalicylic acid might have the additional benefit of decreasing the risk of cardiac death in schizophrenia.52
- A review of 26 RCTs examined the efficacy of anti-inflammatory medications on symptom severity in schizophrenia. Acetylsalicylic acid, N-acetylcysteine, and estrogens had an effect size of 0.3, 0.45, and 0.51, respectively.53
Significance of these findings
A revelation that cytokine abnormalities are associated with schizophrenia symptoms and co-occurring somatic illness might offer an important new avenue of therapeutic discovery. On average, people with schizophrenia die 20 to 25 years earlier than the general population; CVD is the major cause of their death. Measuring arterial compliance, a novel noninvasive technology in psychiatry, as well as metabolic parameters, could serve as an early biomarker for assessing risk of CVD.
Implications for psychiatric practice. If inflammation plays a role in CVD in schizophrenia—either independently of factors such as metabolic syndrome, obesity, and smoking, or on the causal pathway linking these factors to reduced arterial compliance and to CVD—treatment with anti-inflammatory medications might reduce the alarming disparity of mortality that accompanies schizophrenia. In short, anti-inflammatory medications may offer a double benefit in this setting. Furthermore, success in this approach could spur clarification of the role of abnormal cytokines in other psychiatric disorders.
At this time, for your patients, consider that anti-inflammatory medications routinely used in medical practice, such as nonsteroidal anti-inflammatory drugs, omega-3 fatty acids, and statins, might alleviate psychiatric symptoms and might reduce cardiovascular mortality in schizophrenia.
Future directions
Perhaps only a limited number of cytokines are common to schizophrenia and reduced arterial compliance. Targeting those specific cytokines might, however, provide the dual benefit in schizophrenia of:
- alleviating symptoms
- reducing the rate of CVD-related mortality.
Studies are warranted to determine the value of (1) anti-inflammatory medications, such as N-acetylcysteine and infliximab and (2) anti-inflammatory combination therapy for this dual purpose. In fact, recruitment of subjects is underway for a study, Anti-Inflammatory Combination Therapy for the Treatment of Schizophrenia, at the University of Maryland (ClinicalTrials.gov Identifier: NCT01514682).
Consider 3 observations:
- Evidence is mounting that cytokine abnormalities are present in schizophrenia (Box1-8).
- Reduced arterial compliance (change in volume divided by change in pressure [ΔV/ΔP] in an artery during the cardiac cycle) is an early marker of cardiovascular disease (CVD) and a robust predictor of mortality, and is associated with cytokine abnormalities.
- People with schizophrenia experience increased mortality from CVD.
Taken together, the 3 statements hint at a hypothesis: a common inflammatory process involving cytokine imbalance is associated with symptoms of schizophrenia, reduced arterial compliance, and CVD.
Anti-inflammatory therapeutics that target specific cytokines might both decrease psychiatric symptoms and reduce cardiac mortality in people with schizophrenia. In this article, we (1) highlight the potential role of anti-inflammatory medications in reducing both psychiatric symptoms and cardiac mortality in people with schizophrenia and (2) review the pathophysiological basis of this inflammatory commonality and the evidence for its presence in schizophrenia.
The ‘membrane hypothesis’ of schizophrenia
In this hypothesis, a disturbance in the synthesis and structure of membrane phospholipids results in a subsequent disturbance in the function of neuronal membrane proteins, which might be associated with symptoms and mortality in schizophrenia.9-12 The synaptic vesicle protein synaptophysin, a marker for synaptic density, was found to be decreased in postmortem tissue from the gyrus cinguli in 11 patients with schizophrenia, compared with 13 controls.10 Intracellular phospholipases A2 (inPLA2) act as key enzymes in cell membrane repair and remodeling and in neuroplasticity, neurodevelopment, apoptosis, synaptic pruning, neurodegenerative processes, and neuroinflammation.
In a study, people with first-episode schizophrenia (n = 24) who were drug-naïve or off antipsychotic medication were compared with 25 healthy controls using voxel-based morphometry analysis of T1 high-resolution MRI. inPLA2 activity was increased in the patient group compared with controls; the analysis revealed abnormalities of the frontal and medial temporal cortices, hippocampus, and left-middle and superior temporal gyri in first-episode patients.11 In another study, inPLA2 activity was increased in 35 people with first-episode schizophrenia, compared with 22 controls, and was associated with symptom severity and outcome after 12 weeks of antipsychotic treatment.12
Early CVD mortality in schizophrenia
People with schizophrenia have an elevated rate of CVD compared with the general population; in part, this elevation is linked to magnified risk factors for CVD, including obesity, metabolic syndrome, cigarette smoking, and diabetes13-17; furthermore, most antipsychotics can cause or worsen metabolic syndrome.17
CVD is one of the most common causes of death among people with schizophrenia.17,18 Their life expectancy is reported to be 51 to 61 years—20 to 25 years less than what is seen in the general population.19-21
Arterial compliance in schizophrenia
Reduced arterial compliance has been found to be a robust predictor of atherosclerosis, stroke, and myocardial infarction22-29:
- In 376 subjects who had routine diagnostic coronary angiography associated with coronary stenosis, arterial compliance was reduced significantly—even after controlling for age, sex, smoking, diabetes, hypertension, hyperlipidemia, and obesity.24
In a cross-sectional study, 63 male U.S. veterans age 18 to 70 who had a psychiatric diagnosis (16 taking quetiapine, 19 taking risperidone, and 28 treated in the past but off antipsychotics for 2 months) had significantly reduced compliance in thigh- and calf-level arteries than male controls (n = 111), adjusting for body mass index and Framingham Risk Score (FRS). Of the 63 patients, 23 had a diagnosis of schizophrenia or schizoaffective disorder.30 (The FRS is an estimate of a person’s 10-year cardiovascular risk, calculated using age, sex, total cholesterol, high-density lipoprotein, smoker or not, systolic blood pressure, and whether taking an antihypertensive or not. Compliance was measured using computerized plethysmography). Although not statistically significant, secondary analyses from this data set (n = 77, including men for whom factors for metabolic syndrome were available) showed that calf-level compliance (1.82 vs 2.06 mL) and thigh-level compliance (3.6 vs 4.26 mL; P = .06) were reduced in subjects with schizophrenia, compared with those who had another psychiatric diagnosis.31
- In another study, arterial compliance was significantly reduced in 10 subjects with schizophrenia, compared with 10 healthy controls.32
- Last, reduced total arterial compliance has been shown to be a robust predictor of mortality in older people, compared with reduced local or regional arterial compliance.33
Cytokine abnormalities in arterial compliance
The mechanism by which reduced arterial compliance is associated with cardiovascular pathology is not entirely clear. Arterial compliance is a predictor of cardiovascular disorders independent of hypertension.34 Two studies show that vascular inflammation is associated with reduced arterial compliance.35,36 Reduced arterial compliance is associated with increased angiotensin II activity; increased nicotinamide adenine dinucleotide phosphate oxidase activity; reduced nitric oxide activity; and increased reactive oxygen species.37-39 Angiotensin-II signaling activates transforming growth factor-β, tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-17, IL-6, and C-reactive protein (CRP)—all of which are associated with reduced arterial compliance.39-46 In addition, high-sensitivity CRP is significantly associated with reduced arterial compliance.47-49
The overlap of cytokine abnormalities linked to schizophrenia and to arterial compliance is depicted in the Figure.
Anti-inflammatory medications and arterial compliance
Evidence suggests that anti-inflammatory medications increase arterial compliance:
- In 10 patients who had coronary artery disease or diabetes, or both, simvastatin (40 mg/d) was administered for 4 months. Arterial compliance improved in all 10 after 2 months of treatment and increased by 34% after 4 months.27
- Evidence also suggests that the use of omega-3 fatty acids was associated with increased arterial compliance in people with dyslipidemia.50
- Last, in people with rheumatoid arthritis, infliximab, a monoclonal antibody against TNF-Symbol Stdα, reduced aortic inflammation; this effect correlated with an increase in aortic compliance.51
Anti-inflammatory medications in schizophrenia
Two studies have yielded notable findings:
- A meta-analysis of 5 randomized controlled trials (RCTs) involving 264 subjects, comprising 4 studies of celecoxib and 1 of acetylsalicylic acid, had an effect size of 0.43 on total symptom severity. Investigators argued that acetylsalicylic acid might have the additional benefit of decreasing the risk of cardiac death in schizophrenia.52
- A review of 26 RCTs examined the efficacy of anti-inflammatory medications on symptom severity in schizophrenia. Acetylsalicylic acid, N-acetylcysteine, and estrogens had an effect size of 0.3, 0.45, and 0.51, respectively.53
Significance of these findings
A revelation that cytokine abnormalities are associated with schizophrenia symptoms and co-occurring somatic illness might offer an important new avenue of therapeutic discovery. On average, people with schizophrenia die 20 to 25 years earlier than the general population; CVD is the major cause of their death. Measuring arterial compliance, a novel noninvasive technology in psychiatry, as well as metabolic parameters, could serve as an early biomarker for assessing risk of CVD.
Implications for psychiatric practice. If inflammation plays a role in CVD in schizophrenia—either independently of factors such as metabolic syndrome, obesity, and smoking, or on the causal pathway linking these factors to reduced arterial compliance and to CVD—treatment with anti-inflammatory medications might reduce the alarming disparity of mortality that accompanies schizophrenia. In short, anti-inflammatory medications may offer a double benefit in this setting. Furthermore, success in this approach could spur clarification of the role of abnormal cytokines in other psychiatric disorders.
At this time, for your patients, consider that anti-inflammatory medications routinely used in medical practice, such as nonsteroidal anti-inflammatory drugs, omega-3 fatty acids, and statins, might alleviate psychiatric symptoms and might reduce cardiovascular mortality in schizophrenia.
Future directions
Perhaps only a limited number of cytokines are common to schizophrenia and reduced arterial compliance. Targeting those specific cytokines might, however, provide the dual benefit in schizophrenia of:
- alleviating symptoms
- reducing the rate of CVD-related mortality.
Studies are warranted to determine the value of (1) anti-inflammatory medications, such as N-acetylcysteine and infliximab and (2) anti-inflammatory combination therapy for this dual purpose. In fact, recruitment of subjects is underway for a study, Anti-Inflammatory Combination Therapy for the Treatment of Schizophrenia, at the University of Maryland (ClinicalTrials.gov Identifier: NCT01514682).
1. Potvin S, Stip E, Sepehry AA, et al. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63(8):801-808.
2. Miller BJ, Buckley P, Seabolt W, et al. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663-671.
3. Frydecka D, Misiak B, Pawlak-Adamska E, et al. Interleukin-6: the missing element of the neurocognitive deterioration in schizophrenia? The focus on genetic underpinnings, cognitive impairment and clinical manifestation. Eur Arch Psychiatry Clin Neurosci. 2015;265(6):449-459.
4. Dickerson F, Stallings C, Origoni A, et al. Additive effects of elevated C-reactive protein and exposure to herpes simplex virus type 1 on cognitive impairment in individuals with schizophrenia. Schizophr Res. 2012;134(1):83-88.
5. Dickerson F, Stallings C, Origoni A, et al. C-reactive protein is associated with the severity of cognitive impairment but not of psychiatric symptoms in individuals with schizophrenia. Schizophr Res. 2007;93(1-3):261-265.
6. Asevedo E, Rizzo LB, Gadelha A, et al. Peripheral interleukin-2 level is associated with negative symptoms and cognitive performance in schizophrenia. Physiol Behav. 2014;129:194-198.
7. Miller BJ, Culpepper N, Rapaport MH. C-reactive protein levels in schizophrenia: a review and meta-analysis. Clin Schizophr Relat Psychoses. 2014;7(4):223-230.
8. Micoulaud-Franchi JA, Faugere M, Boyer L, et al. Elevated C-reactive protein is associated with sensory gating deficit in schizophrenia. Schizophr Res. 2015;165(1):94-96.
9. Horrobin DF. The membrane phospholipid hypothesis as a biochemical basis for the neurodevelopmental concept of schizophrenia. Schizophr Res. 1998;30(3):193-208.
10. Landén M, Davidsson P, Gottfries CG, et al. Reduction of the synaptophysin level but normal levels of glycerophospholipids in the gyrus cinguli in schizophrenia. Schizophr Res. 2002;55(1-2):83-98.
11. Smesny S, Milleit B, Nenadic I, et al. Phospholipase A2 activity is associated with structural brain changes in schizophrenia. Neuroimage. 2010;52(4):1314-1327.
12. Smesny S, Kunstmann C, Kunstmann S, et al. Phospholipase A2 activity in first episode schizophrenia: associations with symptom severity and outcome at week 12. World J Biol Psychiatry. 2011;12(8):598-607.
13. Fontaine KR, Heo M, Harrigan EP, et al. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res. 2001;101(3):277-288.
14. Homel P, Casey D, Allison DB. Changes in body mass index for individuals with and without schizophrenia, 1987-1996. Schizophr Res. 2002;55(3):277-284.
15. Hedley AA, Ogden CL, Johnson CL, et al. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. JAMA. 2004;291(23):2847-2850.
16. Dickerson FB, Brown CH, Kreyenbuhl JA, et al. Obesity among individuals with serious mental illness. Acta Psychiatr Scand. 2006;113(4):306-313.
17. Newcomer JW. Metabolic syndrome and mental illness. Am J Manag Care. 2007;13(suppl 7):S170-S177.
18. Healy D, Le Noury J, Harris M, et al. Mortality in schizophrenia and related psychoses: data from two cohorts, 1875-1924 and 1994-2010. BMJ Open. 2012;2(5). doi: 10.1136/bmjopen-2012-001810.
19. Newman SC, Bland RC. Mortality in a cohort of patients with schizophrenia: a record linkage study. Can J Psychiatry. 1991;36(4):239-245.
20. Harris EC, Barraclough B. Excess mortality of mental disorder. Br J Psychiatry. 1998;173:11-53.
21. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
22. Farrar DJ, Bond MG, Riley WA, et al. Anatomic correlates of aortic pulse wave velocity and carotid artery elasticity during atherosclerosis progression and regression in monkeys. Circulation. 1991;83(5):1754-1763.
23. Wada T, Kodaira K, Fujishiro K, et al. Correlation of ultrasound-measured common carotid artery stiffness with pathological findings. Arterioscler Thromb. 1994;14(3):479-482.
24. Herrington DM, Kesler K, Reiber JH, et al. Arterial compliance adds to conventional risk factors for prediction of angiographic coronary artery disease. Am Heart J. 2013;146(4):662-667.
25. Willens HJ, Davis W, Herrington DM, et al. Relationship of peripheral arterial compliance and standard cardiovascular risk factors. Vasc Endovascular Surg. 2003;37(3):197-206.
26. Herrington DM, Brown WV, Mosca L, et al. Relationship between arterial stiffness and subclinical aortic atherosclerosis. Circulation. 2004;110(4):432-437.
27. Saliashvili G, Davis WW, Harris MT, et al. Simvastatin improved arterial compliance in high-risk patients. Vasc Endovascular Surg. 2004;38(6):519-523.
28. Le NA, Brown WV, Davis WW, et al. Comparison of the relation of triglyceride-rich lipoproteins and muscular artery compliance in healthy women versus healthy men. Am J Cardiol. 2005;95(9):1049-1054.
29. Willens HJ, Chirinos JA, Brown WV, et al. Usefulness of arterial compliance in the thigh in predicting exercise capacity in individuals without coronary heart disease. Am J Cardiol. 2005;96(2):306-310.
30. Koola MM, Brown WV, Qualls C, et al. Reduced arterial compliance in patients with psychiatric diagnoses. Schizophr Res. 2012;137(1-3):251-253.
31. Koola MM, Sorkin JD, Fargotstein M, et al. Predictors of calf arterial compliance in male veterans with psychiatric diagnoses. The Primary Care Companion for CNS Disorders. In press.
32. Phillips AA, Warburton DE, Flynn SW, et al. Assessment of arterial stiffness among schizophrenia-spectrum disorders using aortic pulse wave velocity and arterial compliance: a pilot study. Psychiatry Res. 2014;215(1):14-19.
33. Papaioannou TG, Protogerou AD, Stergiopulos N, et al. Total arterial compliance estimated by a novel method and all-cause mortality in the elderly: the PROTEGER study. Age (Dordr). 2014;36(3):9661.
34. Park S, Lakatta EG. Role of inflammation in the pathogenesis of arterial stiffness. Yonsei Med J. 2012;53(2):258-261.
35. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107(2):346-354.
36. Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25(5):932-943.
37. van der Loo B, Labugger R, Skepper JN, et al. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192(12):1731-1744.
38. Csiszar A, Ungvari Z, Edwards JG, et al. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90(11):1159-1166.
39. Wang MC, Tsai WC, Chen JY, et al. Arterial stiffness correlated with cardiac remodelling in patients with chronic kidney disease. Nephrology (Carlton). 2007;12(6):591-597.
40. Belmin J, Bernard C, Corman B, et al. Increased production of tumor necrosis factor and interleukin-6 by arterial wall of aged rats. Am J Physiol. 1995;268(6 pt 2):H2288-2293.
41. Gerli R, Monti D, Bistoni O, et al. Chemokines, sTNF-Rs and sCD30 serum levels in healthy aged people and centenarians. Mech Ageing Dev. 2000;121(1-3):37-46.
42. Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102(18):2165-2168.
43. Torzewski M, Rist C, Mortensen RF, et al. C-reactive protein in the arterial intima: role of C-reactive protein receptor-dependent monocyte recruitment in atherogenesis. Arterioscler Thromb Vasc Biol. 2000;20(9):2094-2099.
44. Venugopal SK, Devaraj S, Yuhanna I, et al. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation. 2002;106(12):1439-1441.
45. Csiszar A, Ungvari Z, Koller A, et al. Aging-induced proinflammatory shift in cytokine expression profile in coronary arteries. FASEB J. 2003;17(9):1183-1185.
46. Spinetti G, Wang M, Monticone R, et al. Rat aortic MCP-1 and its receptor CCR2 increase with age and alter vascular smooth muscle cell function. Arterioscler Thromb Vasc Biol. 2004;24(8):1397-1402.
47. Mattace-Raso FU, van der Cammen TJ, van der Meer IM, et al. C-reactive protein and arterial stiffness in older adults: the Rotterdam Study. Atherosclerosis. 2004;176(1):111-116.
48. Mahmud A, Feely J. Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension. 2005;46(5):1118-1122.
49. Nagano M, Nakamura M, Sato K, et al. Association between serum C-reactive protein levels and pulse wave velocity: a population-based cross-sectional study in a general population. Atherosclerosis. 2005;180(1):189-195.
50. Nestel P, Shige H, Pomeroy S, et al. The n-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid increase systemic arterial compliance in humans. Am J Clin Nutr. 2002;76(2):326-330.
51. Mäki-Petäjä KM, Elkhawad M, Cheriyan J, et al. Anti-tumor necrosis factor-α therapy reduces aortic inflammation and stiffness in patients with rheumatoid arthritis. Circulation. 2012;126(21):2473-2480.
52. Sommer IE, de Witte L, Begemann M, et al. Nonsteroidal anti-inflammatory drugs in schizophrenia: ready for practice or a good start? A meta-analysis. J Clin Psychiatry. 2012;73(4):414-419.
53. Sommer IE, van Westrhenen R, Begemann MJ, et al. Efficacy of anti-inflammatory agents to improve symptoms in patients with schizophrenia: an update. Schizophr Bull. 2014;40(1):181-191.
1. Potvin S, Stip E, Sepehry AA, et al. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63(8):801-808.
2. Miller BJ, Buckley P, Seabolt W, et al. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663-671.
3. Frydecka D, Misiak B, Pawlak-Adamska E, et al. Interleukin-6: the missing element of the neurocognitive deterioration in schizophrenia? The focus on genetic underpinnings, cognitive impairment and clinical manifestation. Eur Arch Psychiatry Clin Neurosci. 2015;265(6):449-459.
4. Dickerson F, Stallings C, Origoni A, et al. Additive effects of elevated C-reactive protein and exposure to herpes simplex virus type 1 on cognitive impairment in individuals with schizophrenia. Schizophr Res. 2012;134(1):83-88.
5. Dickerson F, Stallings C, Origoni A, et al. C-reactive protein is associated with the severity of cognitive impairment but not of psychiatric symptoms in individuals with schizophrenia. Schizophr Res. 2007;93(1-3):261-265.
6. Asevedo E, Rizzo LB, Gadelha A, et al. Peripheral interleukin-2 level is associated with negative symptoms and cognitive performance in schizophrenia. Physiol Behav. 2014;129:194-198.
7. Miller BJ, Culpepper N, Rapaport MH. C-reactive protein levels in schizophrenia: a review and meta-analysis. Clin Schizophr Relat Psychoses. 2014;7(4):223-230.
8. Micoulaud-Franchi JA, Faugere M, Boyer L, et al. Elevated C-reactive protein is associated with sensory gating deficit in schizophrenia. Schizophr Res. 2015;165(1):94-96.
9. Horrobin DF. The membrane phospholipid hypothesis as a biochemical basis for the neurodevelopmental concept of schizophrenia. Schizophr Res. 1998;30(3):193-208.
10. Landén M, Davidsson P, Gottfries CG, et al. Reduction of the synaptophysin level but normal levels of glycerophospholipids in the gyrus cinguli in schizophrenia. Schizophr Res. 2002;55(1-2):83-98.
11. Smesny S, Milleit B, Nenadic I, et al. Phospholipase A2 activity is associated with structural brain changes in schizophrenia. Neuroimage. 2010;52(4):1314-1327.
12. Smesny S, Kunstmann C, Kunstmann S, et al. Phospholipase A2 activity in first episode schizophrenia: associations with symptom severity and outcome at week 12. World J Biol Psychiatry. 2011;12(8):598-607.
13. Fontaine KR, Heo M, Harrigan EP, et al. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res. 2001;101(3):277-288.
14. Homel P, Casey D, Allison DB. Changes in body mass index for individuals with and without schizophrenia, 1987-1996. Schizophr Res. 2002;55(3):277-284.
15. Hedley AA, Ogden CL, Johnson CL, et al. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. JAMA. 2004;291(23):2847-2850.
16. Dickerson FB, Brown CH, Kreyenbuhl JA, et al. Obesity among individuals with serious mental illness. Acta Psychiatr Scand. 2006;113(4):306-313.
17. Newcomer JW. Metabolic syndrome and mental illness. Am J Manag Care. 2007;13(suppl 7):S170-S177.
18. Healy D, Le Noury J, Harris M, et al. Mortality in schizophrenia and related psychoses: data from two cohorts, 1875-1924 and 1994-2010. BMJ Open. 2012;2(5). doi: 10.1136/bmjopen-2012-001810.
19. Newman SC, Bland RC. Mortality in a cohort of patients with schizophrenia: a record linkage study. Can J Psychiatry. 1991;36(4):239-245.
20. Harris EC, Barraclough B. Excess mortality of mental disorder. Br J Psychiatry. 1998;173:11-53.
21. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
22. Farrar DJ, Bond MG, Riley WA, et al. Anatomic correlates of aortic pulse wave velocity and carotid artery elasticity during atherosclerosis progression and regression in monkeys. Circulation. 1991;83(5):1754-1763.
23. Wada T, Kodaira K, Fujishiro K, et al. Correlation of ultrasound-measured common carotid artery stiffness with pathological findings. Arterioscler Thromb. 1994;14(3):479-482.
24. Herrington DM, Kesler K, Reiber JH, et al. Arterial compliance adds to conventional risk factors for prediction of angiographic coronary artery disease. Am Heart J. 2013;146(4):662-667.
25. Willens HJ, Davis W, Herrington DM, et al. Relationship of peripheral arterial compliance and standard cardiovascular risk factors. Vasc Endovascular Surg. 2003;37(3):197-206.
26. Herrington DM, Brown WV, Mosca L, et al. Relationship between arterial stiffness and subclinical aortic atherosclerosis. Circulation. 2004;110(4):432-437.
27. Saliashvili G, Davis WW, Harris MT, et al. Simvastatin improved arterial compliance in high-risk patients. Vasc Endovascular Surg. 2004;38(6):519-523.
28. Le NA, Brown WV, Davis WW, et al. Comparison of the relation of triglyceride-rich lipoproteins and muscular artery compliance in healthy women versus healthy men. Am J Cardiol. 2005;95(9):1049-1054.
29. Willens HJ, Chirinos JA, Brown WV, et al. Usefulness of arterial compliance in the thigh in predicting exercise capacity in individuals without coronary heart disease. Am J Cardiol. 2005;96(2):306-310.
30. Koola MM, Brown WV, Qualls C, et al. Reduced arterial compliance in patients with psychiatric diagnoses. Schizophr Res. 2012;137(1-3):251-253.
31. Koola MM, Sorkin JD, Fargotstein M, et al. Predictors of calf arterial compliance in male veterans with psychiatric diagnoses. The Primary Care Companion for CNS Disorders. In press.
32. Phillips AA, Warburton DE, Flynn SW, et al. Assessment of arterial stiffness among schizophrenia-spectrum disorders using aortic pulse wave velocity and arterial compliance: a pilot study. Psychiatry Res. 2014;215(1):14-19.
33. Papaioannou TG, Protogerou AD, Stergiopulos N, et al. Total arterial compliance estimated by a novel method and all-cause mortality in the elderly: the PROTEGER study. Age (Dordr). 2014;36(3):9661.
34. Park S, Lakatta EG. Role of inflammation in the pathogenesis of arterial stiffness. Yonsei Med J. 2012;53(2):258-261.
35. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107(2):346-354.
36. Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25(5):932-943.
37. van der Loo B, Labugger R, Skepper JN, et al. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192(12):1731-1744.
38. Csiszar A, Ungvari Z, Edwards JG, et al. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90(11):1159-1166.
39. Wang MC, Tsai WC, Chen JY, et al. Arterial stiffness correlated with cardiac remodelling in patients with chronic kidney disease. Nephrology (Carlton). 2007;12(6):591-597.
40. Belmin J, Bernard C, Corman B, et al. Increased production of tumor necrosis factor and interleukin-6 by arterial wall of aged rats. Am J Physiol. 1995;268(6 pt 2):H2288-2293.
41. Gerli R, Monti D, Bistoni O, et al. Chemokines, sTNF-Rs and sCD30 serum levels in healthy aged people and centenarians. Mech Ageing Dev. 2000;121(1-3):37-46.
42. Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102(18):2165-2168.
43. Torzewski M, Rist C, Mortensen RF, et al. C-reactive protein in the arterial intima: role of C-reactive protein receptor-dependent monocyte recruitment in atherogenesis. Arterioscler Thromb Vasc Biol. 2000;20(9):2094-2099.
44. Venugopal SK, Devaraj S, Yuhanna I, et al. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation. 2002;106(12):1439-1441.
45. Csiszar A, Ungvari Z, Koller A, et al. Aging-induced proinflammatory shift in cytokine expression profile in coronary arteries. FASEB J. 2003;17(9):1183-1185.
46. Spinetti G, Wang M, Monticone R, et al. Rat aortic MCP-1 and its receptor CCR2 increase with age and alter vascular smooth muscle cell function. Arterioscler Thromb Vasc Biol. 2004;24(8):1397-1402.
47. Mattace-Raso FU, van der Cammen TJ, van der Meer IM, et al. C-reactive protein and arterial stiffness in older adults: the Rotterdam Study. Atherosclerosis. 2004;176(1):111-116.
48. Mahmud A, Feely J. Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension. 2005;46(5):1118-1122.
49. Nagano M, Nakamura M, Sato K, et al. Association between serum C-reactive protein levels and pulse wave velocity: a population-based cross-sectional study in a general population. Atherosclerosis. 2005;180(1):189-195.
50. Nestel P, Shige H, Pomeroy S, et al. The n-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid increase systemic arterial compliance in humans. Am J Clin Nutr. 2002;76(2):326-330.
51. Mäki-Petäjä KM, Elkhawad M, Cheriyan J, et al. Anti-tumor necrosis factor-α therapy reduces aortic inflammation and stiffness in patients with rheumatoid arthritis. Circulation. 2012;126(21):2473-2480.
52. Sommer IE, de Witte L, Begemann M, et al. Nonsteroidal anti-inflammatory drugs in schizophrenia: ready for practice or a good start? A meta-analysis. J Clin Psychiatry. 2012;73(4):414-419.
53. Sommer IE, van Westrhenen R, Begemann MJ, et al. Efficacy of anti-inflammatory agents to improve symptoms in patients with schizophrenia: an update. Schizophr Bull. 2014;40(1):181-191.