User login
Chemo has greater impact on male fertility

Photo by Nina Matthews
Results of a large study suggest that female survivors of childhood cancer may have more luck than their male peers when it comes to conceiving a child.
Both male and female childhood cancer survivors (CCSs) reported fewer pregnancies and live births than their healthy siblings.
However, chemotherapeutic agents appeared to have a much greater impact on the fertility of male CCSs than female CCSs.
Eric Chow, MD, of the Fred Hutchinson Cancer Research Center in Seattle, Washington, and his colleagues reported these findings in The Lancet Oncology.
Previous research has shown that fertility can be compromised by several types of chemotherapy, mainly alkylating drugs. However, little is known about the dose effects on pregnancy from newer drugs, such as ifosfamide and cisplatin, in CCSs.
With this in mind, Dr Chow and his colleagues analyzed data from the Childhood Cancer Survivor Study, which tracks subjects who were diagnosed with the most common types of childhood cancer before the age of 21 and treated at 27 institutions across the US and Canada between 1970 and 1999.
Patients had been diagnosed with leukemias, lymphomas, and neuroblastoma, as well as kidney, brain, soft tissue, and bone tumors. All had survived at least 5 years after diagnosis.
The researchers examined the impact of various doses of 14 commonly used chemotherapy drugs on pregnancy and live birth in 10,938 male and female CCSs, compared with 3949 siblings.
The team specifically focused on CCSs who were treated with chemotherapy and did not receive any radiotherapy to the pelvis or the brain.
The drugs the researchers evaluated were busulfan, carboplatin, carmustine, chlorambucil, chlormethine, cisplatin, cyclophosphamide, dacarbazine, ifosfamide, lomustine, melphalan, procarbazine, temozolomide, and thiotepa.
Outcomes
Multivariable analysis showed that CCSs were significantly less likely than their siblings to have or sire a pregnancy. For male CCSs, the hazard ratio (HR) was 0.63 (P<0.0001). For female CCSs, the HR was 0.87 (P<0.0001).
CCSs were also significantly less likely to have a live birth. For male CCSs, the HR was 0.63 (P<0.0001). For female CCSs, the HR was 0.82 (P<0.0001).
The researchers noted that, overall, female CCSs were less likely to conceive and have a child when compared to their siblings, but the effect was much smaller than that observed among the men.
In addition, the difference between CCSs and siblings was more pronounced for women who delayed pregnancy until they were 30 or older, possibly because chemotherapy exposure might accelerate the natural depletion of eggs and hasten menopause.
Impact of specific drugs
In male CCSs, the reduced likelihood of siring a pregnancy was associated with upper tertile doses of cyclophosphamide (HR=0.60, P<0.0001), ifosfamide (HR=0.42, P=0.0069), procarbazine (HR=0.30, P<0.0001), and cisplatin (HR=0.56, P=0.0023).
Cyclophosphamide-equivalent dose in male CCSs was significantly associated with a decreased likelihood of siring a pregnancy per 5000 mg/m2 increments (HR=0.82, P<0.0001).
In female CCSs, the reduced likelihood of becoming pregnant was associated with busulfan—both at doses less than 450 mg/m2 (HR=0.22, P=0.020) and at doses of 450 mg/m2 or higher (HR=0.14, P=0.0051)—and with doses of lomustine at 411 mg/m2 or greater (HR=0.41, P=0.046).
Cyclophosphamide-equivalent dose in female CCSs was associated with risk only at the highest doses in analyses categorized by quartile (upper quartile vs no exposure, HR=0.85, P=0.023).
Limitations and implications
The researchers noted that a limitation of this study is that it relied on self-reported pregnancy and live birth, and some pregnancies may go unrecognized.
And although the findings are consistent with others in the field, this study did not account for other factors such as marital or cohabitation status, the intention to conceive, or length of time attempting to conceive.
The researchers also noted that, although the total number of CCSs in this study is large, the number of patients who were exposed to individual drugs varied significantly. So while the overall conclusions of the study are consistent with previous studies, more research is needed to estimate the exact risk of some less commonly used drugs.
“We think these results will be encouraging for most women who were treated with chemotherapy in childhood,” Dr Chow said. “However, I think we, as pediatric oncologists, still need to do a better job discussing fertility and fertility preservation options with patients and families upfront before starting cancer treatment.”
“In particular, all boys diagnosed post-puberty should be encouraged to bank their sperm to maximize their reproductive options in the future. The current options for post-pubertal girls remain more complicated but include oocyte and embryo cryopreservation.” ![]()

Photo by Nina Matthews
Results of a large study suggest that female survivors of childhood cancer may have more luck than their male peers when it comes to conceiving a child.
Both male and female childhood cancer survivors (CCSs) reported fewer pregnancies and live births than their healthy siblings.
However, chemotherapeutic agents appeared to have a much greater impact on the fertility of male CCSs than female CCSs.
Eric Chow, MD, of the Fred Hutchinson Cancer Research Center in Seattle, Washington, and his colleagues reported these findings in The Lancet Oncology.
Previous research has shown that fertility can be compromised by several types of chemotherapy, mainly alkylating drugs. However, little is known about the dose effects on pregnancy from newer drugs, such as ifosfamide and cisplatin, in CCSs.
With this in mind, Dr Chow and his colleagues analyzed data from the Childhood Cancer Survivor Study, which tracks subjects who were diagnosed with the most common types of childhood cancer before the age of 21 and treated at 27 institutions across the US and Canada between 1970 and 1999.
Patients had been diagnosed with leukemias, lymphomas, and neuroblastoma, as well as kidney, brain, soft tissue, and bone tumors. All had survived at least 5 years after diagnosis.
The researchers examined the impact of various doses of 14 commonly used chemotherapy drugs on pregnancy and live birth in 10,938 male and female CCSs, compared with 3949 siblings.
The team specifically focused on CCSs who were treated with chemotherapy and did not receive any radiotherapy to the pelvis or the brain.
The drugs the researchers evaluated were busulfan, carboplatin, carmustine, chlorambucil, chlormethine, cisplatin, cyclophosphamide, dacarbazine, ifosfamide, lomustine, melphalan, procarbazine, temozolomide, and thiotepa.
Outcomes
Multivariable analysis showed that CCSs were significantly less likely than their siblings to have or sire a pregnancy. For male CCSs, the hazard ratio (HR) was 0.63 (P<0.0001). For female CCSs, the HR was 0.87 (P<0.0001).
CCSs were also significantly less likely to have a live birth. For male CCSs, the HR was 0.63 (P<0.0001). For female CCSs, the HR was 0.82 (P<0.0001).
The researchers noted that, overall, female CCSs were less likely to conceive and have a child when compared to their siblings, but the effect was much smaller than that observed among the men.
In addition, the difference between CCSs and siblings was more pronounced for women who delayed pregnancy until they were 30 or older, possibly because chemotherapy exposure might accelerate the natural depletion of eggs and hasten menopause.
Impact of specific drugs
In male CCSs, the reduced likelihood of siring a pregnancy was associated with upper tertile doses of cyclophosphamide (HR=0.60, P<0.0001), ifosfamide (HR=0.42, P=0.0069), procarbazine (HR=0.30, P<0.0001), and cisplatin (HR=0.56, P=0.0023).
Cyclophosphamide-equivalent dose in male CCSs was significantly associated with a decreased likelihood of siring a pregnancy per 5000 mg/m2 increments (HR=0.82, P<0.0001).
In female CCSs, the reduced likelihood of becoming pregnant was associated with busulfan—both at doses less than 450 mg/m2 (HR=0.22, P=0.020) and at doses of 450 mg/m2 or higher (HR=0.14, P=0.0051)—and with doses of lomustine at 411 mg/m2 or greater (HR=0.41, P=0.046).
Cyclophosphamide-equivalent dose in female CCSs was associated with risk only at the highest doses in analyses categorized by quartile (upper quartile vs no exposure, HR=0.85, P=0.023).
Limitations and implications
The researchers noted that a limitation of this study is that it relied on self-reported pregnancy and live birth, and some pregnancies may go unrecognized.
And although the findings are consistent with others in the field, this study did not account for other factors such as marital or cohabitation status, the intention to conceive, or length of time attempting to conceive.
The researchers also noted that, although the total number of CCSs in this study is large, the number of patients who were exposed to individual drugs varied significantly. So while the overall conclusions of the study are consistent with previous studies, more research is needed to estimate the exact risk of some less commonly used drugs.
“We think these results will be encouraging for most women who were treated with chemotherapy in childhood,” Dr Chow said. “However, I think we, as pediatric oncologists, still need to do a better job discussing fertility and fertility preservation options with patients and families upfront before starting cancer treatment.”
“In particular, all boys diagnosed post-puberty should be encouraged to bank their sperm to maximize their reproductive options in the future. The current options for post-pubertal girls remain more complicated but include oocyte and embryo cryopreservation.” ![]()

Photo by Nina Matthews
Results of a large study suggest that female survivors of childhood cancer may have more luck than their male peers when it comes to conceiving a child.
Both male and female childhood cancer survivors (CCSs) reported fewer pregnancies and live births than their healthy siblings.
However, chemotherapeutic agents appeared to have a much greater impact on the fertility of male CCSs than female CCSs.
Eric Chow, MD, of the Fred Hutchinson Cancer Research Center in Seattle, Washington, and his colleagues reported these findings in The Lancet Oncology.
Previous research has shown that fertility can be compromised by several types of chemotherapy, mainly alkylating drugs. However, little is known about the dose effects on pregnancy from newer drugs, such as ifosfamide and cisplatin, in CCSs.
With this in mind, Dr Chow and his colleagues analyzed data from the Childhood Cancer Survivor Study, which tracks subjects who were diagnosed with the most common types of childhood cancer before the age of 21 and treated at 27 institutions across the US and Canada between 1970 and 1999.
Patients had been diagnosed with leukemias, lymphomas, and neuroblastoma, as well as kidney, brain, soft tissue, and bone tumors. All had survived at least 5 years after diagnosis.
The researchers examined the impact of various doses of 14 commonly used chemotherapy drugs on pregnancy and live birth in 10,938 male and female CCSs, compared with 3949 siblings.
The team specifically focused on CCSs who were treated with chemotherapy and did not receive any radiotherapy to the pelvis or the brain.
The drugs the researchers evaluated were busulfan, carboplatin, carmustine, chlorambucil, chlormethine, cisplatin, cyclophosphamide, dacarbazine, ifosfamide, lomustine, melphalan, procarbazine, temozolomide, and thiotepa.
Outcomes
Multivariable analysis showed that CCSs were significantly less likely than their siblings to have or sire a pregnancy. For male CCSs, the hazard ratio (HR) was 0.63 (P<0.0001). For female CCSs, the HR was 0.87 (P<0.0001).
CCSs were also significantly less likely to have a live birth. For male CCSs, the HR was 0.63 (P<0.0001). For female CCSs, the HR was 0.82 (P<0.0001).
The researchers noted that, overall, female CCSs were less likely to conceive and have a child when compared to their siblings, but the effect was much smaller than that observed among the men.
In addition, the difference between CCSs and siblings was more pronounced for women who delayed pregnancy until they were 30 or older, possibly because chemotherapy exposure might accelerate the natural depletion of eggs and hasten menopause.
Impact of specific drugs
In male CCSs, the reduced likelihood of siring a pregnancy was associated with upper tertile doses of cyclophosphamide (HR=0.60, P<0.0001), ifosfamide (HR=0.42, P=0.0069), procarbazine (HR=0.30, P<0.0001), and cisplatin (HR=0.56, P=0.0023).
Cyclophosphamide-equivalent dose in male CCSs was significantly associated with a decreased likelihood of siring a pregnancy per 5000 mg/m2 increments (HR=0.82, P<0.0001).
In female CCSs, the reduced likelihood of becoming pregnant was associated with busulfan—both at doses less than 450 mg/m2 (HR=0.22, P=0.020) and at doses of 450 mg/m2 or higher (HR=0.14, P=0.0051)—and with doses of lomustine at 411 mg/m2 or greater (HR=0.41, P=0.046).
Cyclophosphamide-equivalent dose in female CCSs was associated with risk only at the highest doses in analyses categorized by quartile (upper quartile vs no exposure, HR=0.85, P=0.023).
Limitations and implications
The researchers noted that a limitation of this study is that it relied on self-reported pregnancy and live birth, and some pregnancies may go unrecognized.
And although the findings are consistent with others in the field, this study did not account for other factors such as marital or cohabitation status, the intention to conceive, or length of time attempting to conceive.
The researchers also noted that, although the total number of CCSs in this study is large, the number of patients who were exposed to individual drugs varied significantly. So while the overall conclusions of the study are consistent with previous studies, more research is needed to estimate the exact risk of some less commonly used drugs.
“We think these results will be encouraging for most women who were treated with chemotherapy in childhood,” Dr Chow said. “However, I think we, as pediatric oncologists, still need to do a better job discussing fertility and fertility preservation options with patients and families upfront before starting cancer treatment.”
“In particular, all boys diagnosed post-puberty should be encouraged to bank their sperm to maximize their reproductive options in the future. The current options for post-pubertal girls remain more complicated but include oocyte and embryo cryopreservation.” ![]()
Flooring poses higher cancer risk than previously reported

while woman looks on
US government agencies have released a revised report on the health risks associated with formaldehyde in certain types of laminate flooring.
The new report corrects a previous error and reveals an increase in the estimated lifetime risk of cancers, including leukemias, for individuals who regularly breathe in formaldehyde from the flooring.
The report also suggests that irritation and breathing problems can result in anyone exposed to the flooring.
The report was compiled by the Agency for Toxic Substances and Disease Registry (ATSDR) and the Centers for Disease Control and Prevention’s National Center for Environmental Health (NCEH).
About the report
On March 1, 2015, the CBS news program 60 Minutes reported that an American company, Lumber Liquidators®, was selling laminate wood flooring produced in China that released elevated levels of formaldehyde.
Based on this allegation, the US Consumer Product Safety Commission (CPSC) tested laminate flooring samples manufactured in China from 2012 to 2014 that were sold at Lumber Liquidators® stores. The CPSC then requested that the NCEH and ATSDR evaluate the test results for possible health effects.
The NCEH and ATSDR published a report detailing the possible health effects on February 10, 2016. But the report was pulled on February 19, 2016, after the agencies were informed that the report’s indoor air model incorporated an incorrect value for ceiling height.
As a result, the health risks were calculated using airborne concentration estimates about 3 times lower than they should have been.
Since the discovery of the error, the NCEH and ATSDR revised the value in the model, conducted a review of the revised results, and re-evaluated the possible health implications.
In addition, the revised report has been reviewed by outside experts and experts from the CPSC, the US Environmental Protection Agency, and the US Department of Housing and Urban Development.
Results
The revised report concludes that irritation and breathing problems could occur in everyone exposed to formaldehyde in the tested flooring.
The previous report suggested such problems might only occur in sensitive groups (eg, children) and people with pre-existing health conditions (eg, asthma).
The new report also increased the estimated lifetime cancer risk from breathing the highest levels of formaldehyde from the affected flooring all day, every day for 2 years.
The previous estimate of lifetime cancer risk was 2 to 9 extra cases for every 100,000 people.
The new estimate is 6 to 30 extra cases per 100,000 people.
There is conflicting data regarding the types of cancers associated with formaldehyde exposure, but many studies have suggested that formaldehyde causes cancer of the nasopharynx, sinuses, and nasal cavity, as well as leukemia, particularly myeloid leukemia.
Recommendations
Although the revised report shows an increase in health risks associated with the flooring, the NCEH and ATSDR said their recommendations remain the same.
They recommend that people with the affected flooring:
- Reduce their exposure to formaldehyde in their homes by opening windows, running exhaust fans, avoiding use of other products containing formaldehyde, etc.
- See a doctor for ongoing health symptoms such as breathing problems or irritation of the eyes, nose, or throat
- Weigh the pros and cons of professional air testing
- Consult a professional before removing the flooring, as removing it may release more formaldehyde into the home.


while woman looks on
US government agencies have released a revised report on the health risks associated with formaldehyde in certain types of laminate flooring.
The new report corrects a previous error and reveals an increase in the estimated lifetime risk of cancers, including leukemias, for individuals who regularly breathe in formaldehyde from the flooring.
The report also suggests that irritation and breathing problems can result in anyone exposed to the flooring.
The report was compiled by the Agency for Toxic Substances and Disease Registry (ATSDR) and the Centers for Disease Control and Prevention’s National Center for Environmental Health (NCEH).
About the report
On March 1, 2015, the CBS news program 60 Minutes reported that an American company, Lumber Liquidators®, was selling laminate wood flooring produced in China that released elevated levels of formaldehyde.
Based on this allegation, the US Consumer Product Safety Commission (CPSC) tested laminate flooring samples manufactured in China from 2012 to 2014 that were sold at Lumber Liquidators® stores. The CPSC then requested that the NCEH and ATSDR evaluate the test results for possible health effects.
The NCEH and ATSDR published a report detailing the possible health effects on February 10, 2016. But the report was pulled on February 19, 2016, after the agencies were informed that the report’s indoor air model incorporated an incorrect value for ceiling height.
As a result, the health risks were calculated using airborne concentration estimates about 3 times lower than they should have been.
Since the discovery of the error, the NCEH and ATSDR revised the value in the model, conducted a review of the revised results, and re-evaluated the possible health implications.
In addition, the revised report has been reviewed by outside experts and experts from the CPSC, the US Environmental Protection Agency, and the US Department of Housing and Urban Development.
Results
The revised report concludes that irritation and breathing problems could occur in everyone exposed to formaldehyde in the tested flooring.
The previous report suggested such problems might only occur in sensitive groups (eg, children) and people with pre-existing health conditions (eg, asthma).
The new report also increased the estimated lifetime cancer risk from breathing the highest levels of formaldehyde from the affected flooring all day, every day for 2 years.
The previous estimate of lifetime cancer risk was 2 to 9 extra cases for every 100,000 people.
The new estimate is 6 to 30 extra cases per 100,000 people.
There is conflicting data regarding the types of cancers associated with formaldehyde exposure, but many studies have suggested that formaldehyde causes cancer of the nasopharynx, sinuses, and nasal cavity, as well as leukemia, particularly myeloid leukemia.
Recommendations
Although the revised report shows an increase in health risks associated with the flooring, the NCEH and ATSDR said their recommendations remain the same.
They recommend that people with the affected flooring:
- Reduce their exposure to formaldehyde in their homes by opening windows, running exhaust fans, avoiding use of other products containing formaldehyde, etc.
- See a doctor for ongoing health symptoms such as breathing problems or irritation of the eyes, nose, or throat
- Weigh the pros and cons of professional air testing
- Consult a professional before removing the flooring, as removing it may release more formaldehyde into the home.


while woman looks on
US government agencies have released a revised report on the health risks associated with formaldehyde in certain types of laminate flooring.
The new report corrects a previous error and reveals an increase in the estimated lifetime risk of cancers, including leukemias, for individuals who regularly breathe in formaldehyde from the flooring.
The report also suggests that irritation and breathing problems can result in anyone exposed to the flooring.
The report was compiled by the Agency for Toxic Substances and Disease Registry (ATSDR) and the Centers for Disease Control and Prevention’s National Center for Environmental Health (NCEH).
About the report
On March 1, 2015, the CBS news program 60 Minutes reported that an American company, Lumber Liquidators®, was selling laminate wood flooring produced in China that released elevated levels of formaldehyde.
Based on this allegation, the US Consumer Product Safety Commission (CPSC) tested laminate flooring samples manufactured in China from 2012 to 2014 that were sold at Lumber Liquidators® stores. The CPSC then requested that the NCEH and ATSDR evaluate the test results for possible health effects.
The NCEH and ATSDR published a report detailing the possible health effects on February 10, 2016. But the report was pulled on February 19, 2016, after the agencies were informed that the report’s indoor air model incorporated an incorrect value for ceiling height.
As a result, the health risks were calculated using airborne concentration estimates about 3 times lower than they should have been.
Since the discovery of the error, the NCEH and ATSDR revised the value in the model, conducted a review of the revised results, and re-evaluated the possible health implications.
In addition, the revised report has been reviewed by outside experts and experts from the CPSC, the US Environmental Protection Agency, and the US Department of Housing and Urban Development.
Results
The revised report concludes that irritation and breathing problems could occur in everyone exposed to formaldehyde in the tested flooring.
The previous report suggested such problems might only occur in sensitive groups (eg, children) and people with pre-existing health conditions (eg, asthma).
The new report also increased the estimated lifetime cancer risk from breathing the highest levels of formaldehyde from the affected flooring all day, every day for 2 years.
The previous estimate of lifetime cancer risk was 2 to 9 extra cases for every 100,000 people.
The new estimate is 6 to 30 extra cases per 100,000 people.
There is conflicting data regarding the types of cancers associated with formaldehyde exposure, but many studies have suggested that formaldehyde causes cancer of the nasopharynx, sinuses, and nasal cavity, as well as leukemia, particularly myeloid leukemia.
Recommendations
Although the revised report shows an increase in health risks associated with the flooring, the NCEH and ATSDR said their recommendations remain the same.
They recommend that people with the affected flooring:
- Reduce their exposure to formaldehyde in their homes by opening windows, running exhaust fans, avoiding use of other products containing formaldehyde, etc.
- See a doctor for ongoing health symptoms such as breathing problems or irritation of the eyes, nose, or throat
- Weigh the pros and cons of professional air testing
- Consult a professional before removing the flooring, as removing it may release more formaldehyde into the home.

Parasite competition influences drug resistance

infecting a red blood cell
Image courtesy of St. Jude
Children’s Research Hospital
Researchers say they have documented how competition among different malaria parasite strains in human hosts could influence the spread of drug resistance.
“We found that when hosts are co-infected with drug-resistant and drug-sensitive strains, both strains are competitively suppressed,” said Mary Bushman, of Emory University in Atlanta, Georgia.
“Antimalarial therapy, by clearing drug-sensitive parasites from mixed infections, may result in competitive release of resistant strains.”
Bushman and her colleagues described these findings in Proceedings of the Royal Society B.
The researchers focused on the parasite Plasmodium falciparum, which has developed resistance to former first-line therapies chloroquine and sulfadoxine-pyrimethamine.
“We’re now down to our last treatment, artemisinin combination therapy, or ACT, and resistance to that recently emerged in Southeast Asia,” Bushman said. “If ACT resistance continues to follow the same pattern, the world may soon be without reliable antimalarial drugs.”
In addition, people infected with P falciparum often have multiple strains of the parasite, especially in high-transmission areas such as sub-Saharan Africa, where infectious mosquito bites occur frequently. Many people have developed partial immunity, making asymptomatic infections common and further complicating control efforts.
With previous work in lab mice, the researchers found that competition between mixed strains of malaria parasites were a crucial determinant to the spread of resistance.
“In the mouse studies, we found that drug-sensitive parasites suppress resistant parasites,” said Jaap de Roode, PhD, of Emory University.
“We also found that by clearing these sensitive parasites with drugs, the resistant parasites had a big advantage, growing up to high numbers and transmitting to mosquitoes at high rates. Ever since doing that work, I have wanted to see if the same could apply to humans.”
To find out, Dr de Roode and his colleagues analyzed 1341 blood samples from untreated children with malaria living in Angola, Ghana, and Tanzania.
The researchers extracted the DNA of malaria parasites from the blood samples and used polymerase chain reaction technology to determine the densities of drug-resistant strains and drug-sensitive ones. About 15% of the samples had mixtures of both types.
Analyses showed that, in mixed-strain infections, densities of chloroquine-sensitive and chloroquine-resistant strains were reduced in the presence of competitors.
The results also showed that, in the absence of chloroquine, the resistant strains had lower densities than sensitive strains.
“The results were really clear cut, which rarely happens in human studies,” Bushman said. “We found almost complete consistency between the 3 data sets [divided by country].”
Bushman added that the tendency is to use a “one-size-fits-all” strategy for controlling malaria, but this research suggests that more tailored approaches are needed.
For example, a strategy of mass drug administration might be effective in a place with a low prevalence of malaria and less likelihood of mixed-strain infections. However, that same strategy might actually boost drug resistance without reducing the burden of disease in areas where most of the population is infected with multiple strains of malaria parasites.
“The epidemiology of malaria infection is different for different places and different conditions,” Bushman said. “We hope that our work will spur development of new strategies to minimize resistance while maximizing the benefits of control measures.”
However, more questions must be answered to guide the development of these new strategies.
“As a first step, we need to determine if the observed suppression of resistance in humans also results in reduced transmission to mosquitoes,” Dr de Roode said.
Another avenue to explore is resistance among patients who have received antimalarial treatment.
“We need to find out if drug treatment of people infected with malaria removes competition and gives resistance a boost, as we have found in mice before,” Dr de Roode noted. ![]()

infecting a red blood cell
Image courtesy of St. Jude
Children’s Research Hospital
Researchers say they have documented how competition among different malaria parasite strains in human hosts could influence the spread of drug resistance.
“We found that when hosts are co-infected with drug-resistant and drug-sensitive strains, both strains are competitively suppressed,” said Mary Bushman, of Emory University in Atlanta, Georgia.
“Antimalarial therapy, by clearing drug-sensitive parasites from mixed infections, may result in competitive release of resistant strains.”
Bushman and her colleagues described these findings in Proceedings of the Royal Society B.
The researchers focused on the parasite Plasmodium falciparum, which has developed resistance to former first-line therapies chloroquine and sulfadoxine-pyrimethamine.
“We’re now down to our last treatment, artemisinin combination therapy, or ACT, and resistance to that recently emerged in Southeast Asia,” Bushman said. “If ACT resistance continues to follow the same pattern, the world may soon be without reliable antimalarial drugs.”
In addition, people infected with P falciparum often have multiple strains of the parasite, especially in high-transmission areas such as sub-Saharan Africa, where infectious mosquito bites occur frequently. Many people have developed partial immunity, making asymptomatic infections common and further complicating control efforts.
With previous work in lab mice, the researchers found that competition between mixed strains of malaria parasites were a crucial determinant to the spread of resistance.
“In the mouse studies, we found that drug-sensitive parasites suppress resistant parasites,” said Jaap de Roode, PhD, of Emory University.
“We also found that by clearing these sensitive parasites with drugs, the resistant parasites had a big advantage, growing up to high numbers and transmitting to mosquitoes at high rates. Ever since doing that work, I have wanted to see if the same could apply to humans.”
To find out, Dr de Roode and his colleagues analyzed 1341 blood samples from untreated children with malaria living in Angola, Ghana, and Tanzania.
The researchers extracted the DNA of malaria parasites from the blood samples and used polymerase chain reaction technology to determine the densities of drug-resistant strains and drug-sensitive ones. About 15% of the samples had mixtures of both types.
Analyses showed that, in mixed-strain infections, densities of chloroquine-sensitive and chloroquine-resistant strains were reduced in the presence of competitors.
The results also showed that, in the absence of chloroquine, the resistant strains had lower densities than sensitive strains.
“The results were really clear cut, which rarely happens in human studies,” Bushman said. “We found almost complete consistency between the 3 data sets [divided by country].”
Bushman added that the tendency is to use a “one-size-fits-all” strategy for controlling malaria, but this research suggests that more tailored approaches are needed.
For example, a strategy of mass drug administration might be effective in a place with a low prevalence of malaria and less likelihood of mixed-strain infections. However, that same strategy might actually boost drug resistance without reducing the burden of disease in areas where most of the population is infected with multiple strains of malaria parasites.
“The epidemiology of malaria infection is different for different places and different conditions,” Bushman said. “We hope that our work will spur development of new strategies to minimize resistance while maximizing the benefits of control measures.”
However, more questions must be answered to guide the development of these new strategies.
“As a first step, we need to determine if the observed suppression of resistance in humans also results in reduced transmission to mosquitoes,” Dr de Roode said.
Another avenue to explore is resistance among patients who have received antimalarial treatment.
“We need to find out if drug treatment of people infected with malaria removes competition and gives resistance a boost, as we have found in mice before,” Dr de Roode noted. ![]()

infecting a red blood cell
Image courtesy of St. Jude
Children’s Research Hospital
Researchers say they have documented how competition among different malaria parasite strains in human hosts could influence the spread of drug resistance.
“We found that when hosts are co-infected with drug-resistant and drug-sensitive strains, both strains are competitively suppressed,” said Mary Bushman, of Emory University in Atlanta, Georgia.
“Antimalarial therapy, by clearing drug-sensitive parasites from mixed infections, may result in competitive release of resistant strains.”
Bushman and her colleagues described these findings in Proceedings of the Royal Society B.
The researchers focused on the parasite Plasmodium falciparum, which has developed resistance to former first-line therapies chloroquine and sulfadoxine-pyrimethamine.
“We’re now down to our last treatment, artemisinin combination therapy, or ACT, and resistance to that recently emerged in Southeast Asia,” Bushman said. “If ACT resistance continues to follow the same pattern, the world may soon be without reliable antimalarial drugs.”
In addition, people infected with P falciparum often have multiple strains of the parasite, especially in high-transmission areas such as sub-Saharan Africa, where infectious mosquito bites occur frequently. Many people have developed partial immunity, making asymptomatic infections common and further complicating control efforts.
With previous work in lab mice, the researchers found that competition between mixed strains of malaria parasites were a crucial determinant to the spread of resistance.
“In the mouse studies, we found that drug-sensitive parasites suppress resistant parasites,” said Jaap de Roode, PhD, of Emory University.
“We also found that by clearing these sensitive parasites with drugs, the resistant parasites had a big advantage, growing up to high numbers and transmitting to mosquitoes at high rates. Ever since doing that work, I have wanted to see if the same could apply to humans.”
To find out, Dr de Roode and his colleagues analyzed 1341 blood samples from untreated children with malaria living in Angola, Ghana, and Tanzania.
The researchers extracted the DNA of malaria parasites from the blood samples and used polymerase chain reaction technology to determine the densities of drug-resistant strains and drug-sensitive ones. About 15% of the samples had mixtures of both types.
Analyses showed that, in mixed-strain infections, densities of chloroquine-sensitive and chloroquine-resistant strains were reduced in the presence of competitors.
The results also showed that, in the absence of chloroquine, the resistant strains had lower densities than sensitive strains.
“The results were really clear cut, which rarely happens in human studies,” Bushman said. “We found almost complete consistency between the 3 data sets [divided by country].”
Bushman added that the tendency is to use a “one-size-fits-all” strategy for controlling malaria, but this research suggests that more tailored approaches are needed.
For example, a strategy of mass drug administration might be effective in a place with a low prevalence of malaria and less likelihood of mixed-strain infections. However, that same strategy might actually boost drug resistance without reducing the burden of disease in areas where most of the population is infected with multiple strains of malaria parasites.
“The epidemiology of malaria infection is different for different places and different conditions,” Bushman said. “We hope that our work will spur development of new strategies to minimize resistance while maximizing the benefits of control measures.”
However, more questions must be answered to guide the development of these new strategies.
“As a first step, we need to determine if the observed suppression of resistance in humans also results in reduced transmission to mosquitoes,” Dr de Roode said.
Another avenue to explore is resistance among patients who have received antimalarial treatment.
“We need to find out if drug treatment of people infected with malaria removes competition and gives resistance a boost, as we have found in mice before,” Dr de Roode noted. ![]()
A Click Is Not a Clunk: Developmental Dysplasia of the Hip in a Newborn
IN THIS ARTICLE
- Diagnosis
- Management
- Newborn hip evaluation algorithm
Developmental dysplasia of the hip (DDH), previously known as congenital dislocation of the hip, follows a spectrum of irregular anatomic hip development spanning from acetabular dysplasia to irreducible dislocation at birth. Early detection is critical to improve the overall prognosis. Prompt diagnosis requires understanding of potential risk factors, proficiency in physical examination techniques, and implementation of appropriate screening tools when indicated. Although current guidelines direct timing for physical exam screenings, imaging, and treatment, it is ultimately up to the provider to determine the best course of action on a case-by-case basis. This article provides a review of these topics and more.
CURRENT GUIDELINES
In 2000, the American Academy of Pediatrics (AAP) developed guidelines for detection of hip dysplasia, including recommendation of relevant physical exam screenings for all newborns.1 In 2007, the Pediatric Orthopaedic Society of North America (POSNA) encouraged providers to follow the AAP guidelines with a continued recommendation to perform newborn screening for hip instability and routine follow-up evaluations until the child achieves walking.2 The American Academy of Orthopaedic Surgeons (AAOS) also established clinical guidelines in 2014 that are endorsed by both AAP and POSNA.3 These guidelines support routine clinical screening; research evaluated infants up to 6 months old, however, limiting the recommendations to that age-group.
Failure to treat DDH early has been associated with serious negative sequelae that include chronic pain, degenerative arthritis, postural scoliosis, and early gait disturbances.4 Primary care providers are expected to perform thorough newborn hip exams with associated specialized tests (ie, Ortolani and Barlow, which are discussed in “Physical exam”) at each routine follow-up. Heightened clinical suspicion and risk factor awareness are key for primary care providers to promptly identify patients requiring orthopedic referral. With early diagnosis, a removable soft abduction brace can be applied as the initial treatment. When treatment is delayed, however, closed reduction under anesthesia or complex surgical intervention may be required.
EPIDEMIOLOGY
The etiology for DDH remains unknown. Hip dysplasia typically presents unilaterally but can also occur bilaterally. DDH is more likely to affect the left hip than the right.5
Reported incidence varies, ranging from 0.06 to 76.1 per 1,000 live births, and is largely affected by race and geographic location.5 Incidence is higher in countries where routine screening is required, by either physical examination or ultrasound (1.6 to 28.5 and 34.0 to 60.3 per 1,000, respectively), compared with countries not requiring routine screening (1.3 per 1,000). This may suggest that the majority of hip dysplasia cases are transient and resolve spontaneously without treatment.6,7
RISK FACTORS AND PATIENT HISTORY
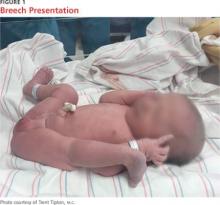
Known risk factors for DDH include breech presentation (see Figure 1), positive family history, and female gender.5,8-10 Female infants are eight times more likely than males to develop DDH.10 Firstborn status is also recognized as an associated risk factor, which may be attributable to space constraints in utero. This hypothesis is further supported by the relative DDH-protective effect of prematurity and low birth weight. Other potential risk factors include advanced maternal age, birth weight that is high for gestational age, decreased hip abduction, and joint laxity. However, the majority of patients with hip dysplasia have no identifiable risk factors.3,5,9,11,12
Swaddling, which often maintains the hips in an adducted and/or extended position, has also been strongly associated with hip dysplasia.5,13 Multiple organizations, including the AAOS,AAP, POSNA, and the International Hip Dysplasia Institute, have developed or endorsed hip-healthy swaddling recommendations to minimize the risk for DDH in swaddled infants.13-15 Such practices allow the infant’s legs to bend up and out at the hips, promoting free hip movement, flexion, and abduction.13,15 Swaddling has demonstrated multiple benefits (including improved sleep and relief of excessive crying13) and continues to be recommended by many US providers; however, those caring for infants at risk for DDH should avoid traditional swaddling and/or practice hip-healthy swaddling techniques.10,13,14 Early diagnosis starts with the clinician’s knowledge of DDH risk factors and the recommended screening protocols. The presence of multiple risk factors will increase the likelihood of this condition and should lower the clinician’s threshold for ordering additional screening, regardless of hip exam findings.
PHYSICAL EXAM
Both AAP and AAOS guidelines recommend clinical screening for DDH with physical exam in all newborns.1,3 A head-to-toe musculoskeletal exam is warranted during the initial evaluation of every newborn in order to assess for any known DDH-associated conditions, which may include neuromuscular disorders, torticollis, and metatarsus adductus.5
Initial evaluation of an infant with DDH may reveal nonspecific findings, including asymmetric skin folds and limb-length inequality. The Galeazzi sign should be sought by aligning flexed knees with the child in the supine position and assessing for uneven knee heights (see Figure 2). Unilateral posterior hip dislocation or femoral shortening represents a positive Galeazzi sign.16 Joint laxity and limited hip abduction have also been associated with DDH.1,10
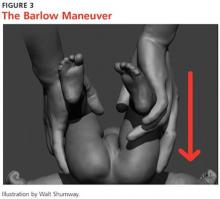
Barlow and Ortolani exams are more specific to DDH and should be completed at newborn screening and each subsequent well-baby exam.1 The Barlow maneuver is a provocative test with flexion, adduction, and posterior pressure through the infant’s hip (Figure 3). A palpable clunk during the Barlow maneuver indicates positive instability with posterior displacement. The Ortolani test is a reductive maneuver requiring abduction with posterior pressure to lift the greater trochanter (Figure 4). A clunk sensation with this test is positive for reduction of the hip.
The infant’s diaper should be removed during the hip evaluation. These exams are more reliable when each hip is evaluated separately with the pelvis stabilized.10 All physical exam findings must be carefully documented at each encounter.1,17
It is critical for the examiner to understand the appropriate technique and potential results when conducting each of these specialized hip exams. A true positive finding is the clunking sensation that occurs with the dislocation or relocation of the affected hip; this finding is better felt than heard. In contrast, a benign hip click with these maneuvers is a more subtle sensation—typically, a soft-tissue snapping or catching—and is not diagnostic of DDH. A click is not a clunk and is not indicative of DDH.1,3
DDH may present later in infancy or early childhood; therefore, DDH should remain within the differential diagnosis for gait asymmetry, unequal hip motion, or limb-length discrepancy. It may be beneficial to continue to evaluate for these developments during routine exams as part of a thorough pediatric musculoskeletal assessment, particularly in patients with documented risk factors for DDH.1,3,4 Delay in diagnosis of DDH, it should be noted, is a relatively common complaint in pediatric medical malpractice lawsuits; until the early 2000s, this condition represented about 75% of claims in one medical malpractice database.The decrease in claims has been attributed to better awareness and earlier diagnosis of DDH. 17
Continue for the diagnosis >>
DIAGNOSIS
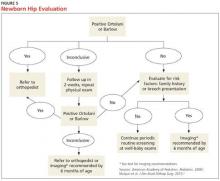
A positive Ortolani or Barlow sign is diagnostic and warrants prompt orthopedic referral (Figure 5). If physical examination results are equivocal or inconclusive, follow-up at two weeks is recommended, with continued routine follow-up until walking is achieved. Patients with persistent equivocal findings at the two-week follow-up warrant ultrasound at age 3 to 4 weeks or orthopedic referral. Infants with significant risk factors, particularly breech presentation at birth, should also undergo imaging.18 AAP recommends ultrasound at age 6 weeks or radiograph after 4 months of age.1,18 AAOS recommends performing an imaging study before age 6 months when at least one of the following risk factors is present: breech presentation, positive family history of DDH, or previous clinical instability (moderate level of evidence).3
IMAGING
Ultrasound is the diagnostic test of choice for infants because radiographs have limited value until the femoral heads begin to ossify at age 4 to 6 months.18 Ultrasonography allows for visualization of the cartilaginous portion of the acetabulum and femoral head.1 Dynamic stressing is performed during ultrasound to assess the level of hip stability. A provider trained in ultrasound will measure the depth of the acetabulum and identify any potential laxity or instability of the hip joint. Accuracy of these findings is largely dependent on the experience and skill of the examiner.
Ultrasound evaluation is not recommended until after age 3 to 4 weeks. Earlier findings may include mild laxity and immature morphology of the acetabulum, which often resolve spontaneously.1,18 Use of ultrasound is currently recommended only to confirm diagnostic suspicion, based on clinical findings, or for infants with significant risk factors.18 Universal ultrasound screening in newborns is not recommended and would incur unnecessary costs.1,3,9 Plain radiographs are used after age 4 months to confirm a diagnosis of DDH or to assess for residual dysplasia.3,18
Continue for management >>
MANAGEMENT
Once hip dysplasia is suggested by physical exam or imaging study, the child’s subsequent care should be provided by an orthopedic specialist with experience in treating this condition. Treatment is preferably initiated before age 6 weeks.12 The specifics of treatment are largely based on age at diagnosis and the severity of dysplasia.
The goal of treatment is to maintain the hips in a stable position with the femoral head well covered by the acetabulum. This will improve anatomic development and function. Early clinical diagnosis is often sufficient to justify initiating conservative treatment; additionally, early detection of DDH can considerably reduce the need for surgical intervention.12 Although the potential for spontaneous resolution is high, the consequences associated with delay in care can be significant.
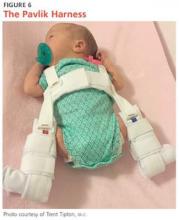
Preferred initial management, which can be initiated before confirmation of DDH by ultrasound, involves implementation of soft abduction support.19 The Pavlik harness is the support design of choice (Figure 6).12 This harness maintains hip flexion and abduction, creating concentric reduction of the femoral head. The brace is highly successful when its use is initiated early. Treatment in a Pavlik harness requires nearly full-time wear and close monitoring by a clinician. Unlikely potential risks associated with this treatment include avascular necrosis and femoral nerve palsy.4
Ultrasonography is used to further monitor treatment and to determine length of wear. Long-term results suggest a success rate exceeding 90%.20,21 However, this rate may be falsely elevated due to the number of hips that likely would have improved spontaneously without treatment.6,19
The Pavlik harness becomes less effective with increasing age, and a more rigid abduction brace may be considered in infants older than 6 months.20 Overall outcomes improve once the femoral head is consistently maintained in the acetabulum. Delay in treatment is associated with an increase in the long-term complications associated with residual hip dysplasia.22
Once an infant is undergoing treatment for DDH in a Pavlik harness, there is no need for primary care providers to continue to perform provocative testing, such as the Ortolani or Barlow test, at routine well-baby checks. Unnecessary stress to the hips is not beneficial, and any new results will not change the treatment being provided by the orthopedic specialist. Adjustments to the fit of the harness should be made only by the orthopedist, unless femoral nerve palsy is noted on exam. This development warrants immediate discontinuation of harness use until symptoms resolve.21
Abduction bracing may not be suitable for all cases of hip dysplasia. Newborns with irreducible hips, more advanced dysplasia, or associated neuromuscular or syndromic disorder may require closed versus open reduction and casting. More invasive surgical options may also be considered in advanced dysplasia in order to reshape the joint and improve function.20,22
Continue for patient education >>
PATIENT EDUCATION
Parents should be fully educated on the options for managing hip dysplasia. Once DDH is diagnosed, prompt referral to an orthopedic specialist is critical in order to weigh the treatment options and to develop the appropriate individualized plan for each child. Once treatment is initiated, parental compliance is essential; frequent meetings between parents and the specialist are important.
Parents of infants with known risk factors for and/or suspicion of hip dysplasia should also be educated on hip-healthy swaddling to allow for free motion of the hips and knees.10,13 Advise them that some commercial baby carriers and slings may maintain the hips in an undesirable extended position. In both swaddling and with baby carriers, care should be taken to allow for hip abduction and flexion. Caution should also be taken during diaper changes to avoid lifting the legs and thereby causing unnecessary stress to the hips.
CONCLUSION
Developmental dysplasia of the hip can be a disabling pediatric condition. Early diagnosis improves the likelihood of successful treatment during infancy and can prevent serious complications. If untreated, DDH can lead to joint degeneration and premature arthritis. Recognition and treatment within the first six weeks of life is crucial to the overall outcome.
The role of a primary care provider is to identify hip dysplasia risk factors and recognize associated physical exam findings in order to refer to an orthopedic specialist in a timely manner. Guidelines from the AAP, POSNA, and AAOS help direct this process in order to effectively identify infants at risk and in need of treatment.
REFERENCES
1. American Academy of Pediatrics. Committee on Quality Improvement, Subcommittee on Developmental Dysplasia of the Hip. Clinical practice guideline: early detection of developmental dysplasia of the hip. Pediatrics. 2000;105(4 pt 1):896-905.
2. Schwend RM, Schoenecker P, Richards BS, et al. Screening the newborn for developmental dysplasia of the hip: now what do we do? J Pediatr Orthop. 2007;27(6):607-610.
3. Mulpuri K, Song KM, Goldberg MJ, Sevarino K. Detection and nonoperative management of pediatric developmental dysplasia of the hip in infants up to six months of age. J Am Acad Orthop Surg. 2015;23(3):202-205.
4. Thomas SRYW. A review of long-term outcomes for late presenting developmental hip dysplasia. Bone Joint J. 2015;97-B(6):729-733.
5. Loder RT, Skopelja EN. The epidemiology and demographics of hip dysplasia. ISRN Orthop. 2011;2011:238607.
6. US Preventive Services Task Force. Screening for developmental dysplasia of the hip: recommendation statement. Pediatrics. 2006;117(3):898-902.
7. Shorter D, Hong T, Osborn DA. Screening programmes for developmental dysplasia of the hip in newborn infants. Cochrane Database Syst Rev. 2011;(9):CD004595.
8. Loder RT, Shafer C. The demographics of developmental hip dysplasia in the Midwestern United States (Indiana). J Child Orthop. 2015;9(1):93-98.
9. Paton RW, Hinduja K, Thomas CD. The significance of at-risk factors in ultrasound surveillance of developmental dysplasia of the hip: a ten-year prospective study. J Bone Joint Surg Br. 2005;87(9):1264-1266.
10. Alsaleem M, Set KK, Saadeh L. Developmental dysplasia of hip: a review. Clin Pediatr (Phila). 2015;54(10):921-928.
11. Chan A, McCaul KA, Cundy PJ, et al. Perinatal risk factors for developmental dysplasia of the hip. Arch Dis Child. 1997;76(2):F94-F100.
12. Godley DR. Assessment, diagnosis, and treatment of developmental dysplasia of the hip. JAAPA. 2013;26(3):54-58.
13. Van Sleuwen BE, Engelberts AC, Boere-Boonekamp MM, et al. Swaddling: a systematic review. Pediatrics. 2007;120(4):e1097-e1106.
14. American Academy of Orthopaedic Surgeons, American Association of Orthopaedic Surgeons. Position statement: swaddling and developmental hip dysplasia. www.aaos.org/uploadedFiles/PreProduction/About/Opinion_Statements/position/1186%20Swaddling%20and%20Developmental%20Hip%20Dysplasia.pdf. Accessed January 22, 2016.
15. Clarke NM. Swaddling and hip dysplasia: an orthopaedic perspective. Arch Dis Child. 2014;99(1):5-6.
16. Storer SK, Skaggs DL. Developmental dysplasia of the hip. Am Fam Physician. 2006;74(8):1310-1316.
17. McAbee GN, Donn SM, Mendelson RA, et al. Medical diagnoses commonly associated with pediatric malpractice lawsuits in the United States. Pediatrics. 2008;122(6):e1282-e1286.
18. Imrie M, Scott V, Stearns P, et al. Is ultrasound screening for DDH in babies born breech sufficient? J Child Orthop. 2010;4(1):3-8.
19. Chen HW, Chang CH, Tsai ST, et al. Natural progression of hip dysplasia in newborns: a reflection of hip ultrasonographic screenings in newborn nurseries. J Pediatr Orthop B. 2010;19(5):418-423.
20. Gans I, Flynn JM, Sankar WN. Abduction bracing for residual acetabular dysplasia in infantile DDH. J Pediatr Orthop. 2013;33(7):714-718.
21. Murnaghan ML, Browne RH, Sucato DJ, Birch J. Femoral nerve palsy in Pavlik harness treatment for developmental dysplasia of the hip. J Bone Joint Surg Am. 2011;93(5):493-499.
22. Dezateux C, Rosendahl K. Developmental dysplasia of the hip. Lancet. 2007;369(9572):1541-1552.
IN THIS ARTICLE
- Diagnosis
- Management
- Newborn hip evaluation algorithm
Developmental dysplasia of the hip (DDH), previously known as congenital dislocation of the hip, follows a spectrum of irregular anatomic hip development spanning from acetabular dysplasia to irreducible dislocation at birth. Early detection is critical to improve the overall prognosis. Prompt diagnosis requires understanding of potential risk factors, proficiency in physical examination techniques, and implementation of appropriate screening tools when indicated. Although current guidelines direct timing for physical exam screenings, imaging, and treatment, it is ultimately up to the provider to determine the best course of action on a case-by-case basis. This article provides a review of these topics and more.
CURRENT GUIDELINES
In 2000, the American Academy of Pediatrics (AAP) developed guidelines for detection of hip dysplasia, including recommendation of relevant physical exam screenings for all newborns.1 In 2007, the Pediatric Orthopaedic Society of North America (POSNA) encouraged providers to follow the AAP guidelines with a continued recommendation to perform newborn screening for hip instability and routine follow-up evaluations until the child achieves walking.2 The American Academy of Orthopaedic Surgeons (AAOS) also established clinical guidelines in 2014 that are endorsed by both AAP and POSNA.3 These guidelines support routine clinical screening; research evaluated infants up to 6 months old, however, limiting the recommendations to that age-group.
Failure to treat DDH early has been associated with serious negative sequelae that include chronic pain, degenerative arthritis, postural scoliosis, and early gait disturbances.4 Primary care providers are expected to perform thorough newborn hip exams with associated specialized tests (ie, Ortolani and Barlow, which are discussed in “Physical exam”) at each routine follow-up. Heightened clinical suspicion and risk factor awareness are key for primary care providers to promptly identify patients requiring orthopedic referral. With early diagnosis, a removable soft abduction brace can be applied as the initial treatment. When treatment is delayed, however, closed reduction under anesthesia or complex surgical intervention may be required.
EPIDEMIOLOGY
The etiology for DDH remains unknown. Hip dysplasia typically presents unilaterally but can also occur bilaterally. DDH is more likely to affect the left hip than the right.5
Reported incidence varies, ranging from 0.06 to 76.1 per 1,000 live births, and is largely affected by race and geographic location.5 Incidence is higher in countries where routine screening is required, by either physical examination or ultrasound (1.6 to 28.5 and 34.0 to 60.3 per 1,000, respectively), compared with countries not requiring routine screening (1.3 per 1,000). This may suggest that the majority of hip dysplasia cases are transient and resolve spontaneously without treatment.6,7
RISK FACTORS AND PATIENT HISTORY
Known risk factors for DDH include breech presentation (see Figure 1), positive family history, and female gender.5,8-10 Female infants are eight times more likely than males to develop DDH.10 Firstborn status is also recognized as an associated risk factor, which may be attributable to space constraints in utero. This hypothesis is further supported by the relative DDH-protective effect of prematurity and low birth weight. Other potential risk factors include advanced maternal age, birth weight that is high for gestational age, decreased hip abduction, and joint laxity. However, the majority of patients with hip dysplasia have no identifiable risk factors.3,5,9,11,12
Swaddling, which often maintains the hips in an adducted and/or extended position, has also been strongly associated with hip dysplasia.5,13 Multiple organizations, including the AAOS,AAP, POSNA, and the International Hip Dysplasia Institute, have developed or endorsed hip-healthy swaddling recommendations to minimize the risk for DDH in swaddled infants.13-15 Such practices allow the infant’s legs to bend up and out at the hips, promoting free hip movement, flexion, and abduction.13,15 Swaddling has demonstrated multiple benefits (including improved sleep and relief of excessive crying13) and continues to be recommended by many US providers; however, those caring for infants at risk for DDH should avoid traditional swaddling and/or practice hip-healthy swaddling techniques.10,13,14 Early diagnosis starts with the clinician’s knowledge of DDH risk factors and the recommended screening protocols. The presence of multiple risk factors will increase the likelihood of this condition and should lower the clinician’s threshold for ordering additional screening, regardless of hip exam findings.
PHYSICAL EXAM
Both AAP and AAOS guidelines recommend clinical screening for DDH with physical exam in all newborns.1,3 A head-to-toe musculoskeletal exam is warranted during the initial evaluation of every newborn in order to assess for any known DDH-associated conditions, which may include neuromuscular disorders, torticollis, and metatarsus adductus.5
Initial evaluation of an infant with DDH may reveal nonspecific findings, including asymmetric skin folds and limb-length inequality. The Galeazzi sign should be sought by aligning flexed knees with the child in the supine position and assessing for uneven knee heights (see Figure 2). Unilateral posterior hip dislocation or femoral shortening represents a positive Galeazzi sign.16 Joint laxity and limited hip abduction have also been associated with DDH.1,10
Barlow and Ortolani exams are more specific to DDH and should be completed at newborn screening and each subsequent well-baby exam.1 The Barlow maneuver is a provocative test with flexion, adduction, and posterior pressure through the infant’s hip (Figure 3). A palpable clunk during the Barlow maneuver indicates positive instability with posterior displacement. The Ortolani test is a reductive maneuver requiring abduction with posterior pressure to lift the greater trochanter (Figure 4). A clunk sensation with this test is positive for reduction of the hip.
The infant’s diaper should be removed during the hip evaluation. These exams are more reliable when each hip is evaluated separately with the pelvis stabilized.10 All physical exam findings must be carefully documented at each encounter.1,17
It is critical for the examiner to understand the appropriate technique and potential results when conducting each of these specialized hip exams. A true positive finding is the clunking sensation that occurs with the dislocation or relocation of the affected hip; this finding is better felt than heard. In contrast, a benign hip click with these maneuvers is a more subtle sensation—typically, a soft-tissue snapping or catching—and is not diagnostic of DDH. A click is not a clunk and is not indicative of DDH.1,3
DDH may present later in infancy or early childhood; therefore, DDH should remain within the differential diagnosis for gait asymmetry, unequal hip motion, or limb-length discrepancy. It may be beneficial to continue to evaluate for these developments during routine exams as part of a thorough pediatric musculoskeletal assessment, particularly in patients with documented risk factors for DDH.1,3,4 Delay in diagnosis of DDH, it should be noted, is a relatively common complaint in pediatric medical malpractice lawsuits; until the early 2000s, this condition represented about 75% of claims in one medical malpractice database.The decrease in claims has been attributed to better awareness and earlier diagnosis of DDH. 17
Continue for the diagnosis >>
DIAGNOSIS
A positive Ortolani or Barlow sign is diagnostic and warrants prompt orthopedic referral (Figure 5). If physical examination results are equivocal or inconclusive, follow-up at two weeks is recommended, with continued routine follow-up until walking is achieved. Patients with persistent equivocal findings at the two-week follow-up warrant ultrasound at age 3 to 4 weeks or orthopedic referral. Infants with significant risk factors, particularly breech presentation at birth, should also undergo imaging.18 AAP recommends ultrasound at age 6 weeks or radiograph after 4 months of age.1,18 AAOS recommends performing an imaging study before age 6 months when at least one of the following risk factors is present: breech presentation, positive family history of DDH, or previous clinical instability (moderate level of evidence).3
IMAGING
Ultrasound is the diagnostic test of choice for infants because radiographs have limited value until the femoral heads begin to ossify at age 4 to 6 months.18 Ultrasonography allows for visualization of the cartilaginous portion of the acetabulum and femoral head.1 Dynamic stressing is performed during ultrasound to assess the level of hip stability. A provider trained in ultrasound will measure the depth of the acetabulum and identify any potential laxity or instability of the hip joint. Accuracy of these findings is largely dependent on the experience and skill of the examiner.
Ultrasound evaluation is not recommended until after age 3 to 4 weeks. Earlier findings may include mild laxity and immature morphology of the acetabulum, which often resolve spontaneously.1,18 Use of ultrasound is currently recommended only to confirm diagnostic suspicion, based on clinical findings, or for infants with significant risk factors.18 Universal ultrasound screening in newborns is not recommended and would incur unnecessary costs.1,3,9 Plain radiographs are used after age 4 months to confirm a diagnosis of DDH or to assess for residual dysplasia.3,18
Continue for management >>
MANAGEMENT
Once hip dysplasia is suggested by physical exam or imaging study, the child’s subsequent care should be provided by an orthopedic specialist with experience in treating this condition. Treatment is preferably initiated before age 6 weeks.12 The specifics of treatment are largely based on age at diagnosis and the severity of dysplasia.
The goal of treatment is to maintain the hips in a stable position with the femoral head well covered by the acetabulum. This will improve anatomic development and function. Early clinical diagnosis is often sufficient to justify initiating conservative treatment; additionally, early detection of DDH can considerably reduce the need for surgical intervention.12 Although the potential for spontaneous resolution is high, the consequences associated with delay in care can be significant.
Preferred initial management, which can be initiated before confirmation of DDH by ultrasound, involves implementation of soft abduction support.19 The Pavlik harness is the support design of choice (Figure 6).12 This harness maintains hip flexion and abduction, creating concentric reduction of the femoral head. The brace is highly successful when its use is initiated early. Treatment in a Pavlik harness requires nearly full-time wear and close monitoring by a clinician. Unlikely potential risks associated with this treatment include avascular necrosis and femoral nerve palsy.4
Ultrasonography is used to further monitor treatment and to determine length of wear. Long-term results suggest a success rate exceeding 90%.20,21 However, this rate may be falsely elevated due to the number of hips that likely would have improved spontaneously without treatment.6,19
The Pavlik harness becomes less effective with increasing age, and a more rigid abduction brace may be considered in infants older than 6 months.20 Overall outcomes improve once the femoral head is consistently maintained in the acetabulum. Delay in treatment is associated with an increase in the long-term complications associated with residual hip dysplasia.22
Once an infant is undergoing treatment for DDH in a Pavlik harness, there is no need for primary care providers to continue to perform provocative testing, such as the Ortolani or Barlow test, at routine well-baby checks. Unnecessary stress to the hips is not beneficial, and any new results will not change the treatment being provided by the orthopedic specialist. Adjustments to the fit of the harness should be made only by the orthopedist, unless femoral nerve palsy is noted on exam. This development warrants immediate discontinuation of harness use until symptoms resolve.21
Abduction bracing may not be suitable for all cases of hip dysplasia. Newborns with irreducible hips, more advanced dysplasia, or associated neuromuscular or syndromic disorder may require closed versus open reduction and casting. More invasive surgical options may also be considered in advanced dysplasia in order to reshape the joint and improve function.20,22
Continue for patient education >>
PATIENT EDUCATION
Parents should be fully educated on the options for managing hip dysplasia. Once DDH is diagnosed, prompt referral to an orthopedic specialist is critical in order to weigh the treatment options and to develop the appropriate individualized plan for each child. Once treatment is initiated, parental compliance is essential; frequent meetings between parents and the specialist are important.
Parents of infants with known risk factors for and/or suspicion of hip dysplasia should also be educated on hip-healthy swaddling to allow for free motion of the hips and knees.10,13 Advise them that some commercial baby carriers and slings may maintain the hips in an undesirable extended position. In both swaddling and with baby carriers, care should be taken to allow for hip abduction and flexion. Caution should also be taken during diaper changes to avoid lifting the legs and thereby causing unnecessary stress to the hips.
CONCLUSION
Developmental dysplasia of the hip can be a disabling pediatric condition. Early diagnosis improves the likelihood of successful treatment during infancy and can prevent serious complications. If untreated, DDH can lead to joint degeneration and premature arthritis. Recognition and treatment within the first six weeks of life is crucial to the overall outcome.
The role of a primary care provider is to identify hip dysplasia risk factors and recognize associated physical exam findings in order to refer to an orthopedic specialist in a timely manner. Guidelines from the AAP, POSNA, and AAOS help direct this process in order to effectively identify infants at risk and in need of treatment.
REFERENCES
1. American Academy of Pediatrics. Committee on Quality Improvement, Subcommittee on Developmental Dysplasia of the Hip. Clinical practice guideline: early detection of developmental dysplasia of the hip. Pediatrics. 2000;105(4 pt 1):896-905.
2. Schwend RM, Schoenecker P, Richards BS, et al. Screening the newborn for developmental dysplasia of the hip: now what do we do? J Pediatr Orthop. 2007;27(6):607-610.
3. Mulpuri K, Song KM, Goldberg MJ, Sevarino K. Detection and nonoperative management of pediatric developmental dysplasia of the hip in infants up to six months of age. J Am Acad Orthop Surg. 2015;23(3):202-205.
4. Thomas SRYW. A review of long-term outcomes for late presenting developmental hip dysplasia. Bone Joint J. 2015;97-B(6):729-733.
5. Loder RT, Skopelja EN. The epidemiology and demographics of hip dysplasia. ISRN Orthop. 2011;2011:238607.
6. US Preventive Services Task Force. Screening for developmental dysplasia of the hip: recommendation statement. Pediatrics. 2006;117(3):898-902.
7. Shorter D, Hong T, Osborn DA. Screening programmes for developmental dysplasia of the hip in newborn infants. Cochrane Database Syst Rev. 2011;(9):CD004595.
8. Loder RT, Shafer C. The demographics of developmental hip dysplasia in the Midwestern United States (Indiana). J Child Orthop. 2015;9(1):93-98.
9. Paton RW, Hinduja K, Thomas CD. The significance of at-risk factors in ultrasound surveillance of developmental dysplasia of the hip: a ten-year prospective study. J Bone Joint Surg Br. 2005;87(9):1264-1266.
10. Alsaleem M, Set KK, Saadeh L. Developmental dysplasia of hip: a review. Clin Pediatr (Phila). 2015;54(10):921-928.
11. Chan A, McCaul KA, Cundy PJ, et al. Perinatal risk factors for developmental dysplasia of the hip. Arch Dis Child. 1997;76(2):F94-F100.
12. Godley DR. Assessment, diagnosis, and treatment of developmental dysplasia of the hip. JAAPA. 2013;26(3):54-58.
13. Van Sleuwen BE, Engelberts AC, Boere-Boonekamp MM, et al. Swaddling: a systematic review. Pediatrics. 2007;120(4):e1097-e1106.
14. American Academy of Orthopaedic Surgeons, American Association of Orthopaedic Surgeons. Position statement: swaddling and developmental hip dysplasia. www.aaos.org/uploadedFiles/PreProduction/About/Opinion_Statements/position/1186%20Swaddling%20and%20Developmental%20Hip%20Dysplasia.pdf. Accessed January 22, 2016.
15. Clarke NM. Swaddling and hip dysplasia: an orthopaedic perspective. Arch Dis Child. 2014;99(1):5-6.
16. Storer SK, Skaggs DL. Developmental dysplasia of the hip. Am Fam Physician. 2006;74(8):1310-1316.
17. McAbee GN, Donn SM, Mendelson RA, et al. Medical diagnoses commonly associated with pediatric malpractice lawsuits in the United States. Pediatrics. 2008;122(6):e1282-e1286.
18. Imrie M, Scott V, Stearns P, et al. Is ultrasound screening for DDH in babies born breech sufficient? J Child Orthop. 2010;4(1):3-8.
19. Chen HW, Chang CH, Tsai ST, et al. Natural progression of hip dysplasia in newborns: a reflection of hip ultrasonographic screenings in newborn nurseries. J Pediatr Orthop B. 2010;19(5):418-423.
20. Gans I, Flynn JM, Sankar WN. Abduction bracing for residual acetabular dysplasia in infantile DDH. J Pediatr Orthop. 2013;33(7):714-718.
21. Murnaghan ML, Browne RH, Sucato DJ, Birch J. Femoral nerve palsy in Pavlik harness treatment for developmental dysplasia of the hip. J Bone Joint Surg Am. 2011;93(5):493-499.
22. Dezateux C, Rosendahl K. Developmental dysplasia of the hip. Lancet. 2007;369(9572):1541-1552.
IN THIS ARTICLE
- Diagnosis
- Management
- Newborn hip evaluation algorithm
Developmental dysplasia of the hip (DDH), previously known as congenital dislocation of the hip, follows a spectrum of irregular anatomic hip development spanning from acetabular dysplasia to irreducible dislocation at birth. Early detection is critical to improve the overall prognosis. Prompt diagnosis requires understanding of potential risk factors, proficiency in physical examination techniques, and implementation of appropriate screening tools when indicated. Although current guidelines direct timing for physical exam screenings, imaging, and treatment, it is ultimately up to the provider to determine the best course of action on a case-by-case basis. This article provides a review of these topics and more.
CURRENT GUIDELINES
In 2000, the American Academy of Pediatrics (AAP) developed guidelines for detection of hip dysplasia, including recommendation of relevant physical exam screenings for all newborns.1 In 2007, the Pediatric Orthopaedic Society of North America (POSNA) encouraged providers to follow the AAP guidelines with a continued recommendation to perform newborn screening for hip instability and routine follow-up evaluations until the child achieves walking.2 The American Academy of Orthopaedic Surgeons (AAOS) also established clinical guidelines in 2014 that are endorsed by both AAP and POSNA.3 These guidelines support routine clinical screening; research evaluated infants up to 6 months old, however, limiting the recommendations to that age-group.
Failure to treat DDH early has been associated with serious negative sequelae that include chronic pain, degenerative arthritis, postural scoliosis, and early gait disturbances.4 Primary care providers are expected to perform thorough newborn hip exams with associated specialized tests (ie, Ortolani and Barlow, which are discussed in “Physical exam”) at each routine follow-up. Heightened clinical suspicion and risk factor awareness are key for primary care providers to promptly identify patients requiring orthopedic referral. With early diagnosis, a removable soft abduction brace can be applied as the initial treatment. When treatment is delayed, however, closed reduction under anesthesia or complex surgical intervention may be required.
EPIDEMIOLOGY
The etiology for DDH remains unknown. Hip dysplasia typically presents unilaterally but can also occur bilaterally. DDH is more likely to affect the left hip than the right.5
Reported incidence varies, ranging from 0.06 to 76.1 per 1,000 live births, and is largely affected by race and geographic location.5 Incidence is higher in countries where routine screening is required, by either physical examination or ultrasound (1.6 to 28.5 and 34.0 to 60.3 per 1,000, respectively), compared with countries not requiring routine screening (1.3 per 1,000). This may suggest that the majority of hip dysplasia cases are transient and resolve spontaneously without treatment.6,7
RISK FACTORS AND PATIENT HISTORY
Known risk factors for DDH include breech presentation (see Figure 1), positive family history, and female gender.5,8-10 Female infants are eight times more likely than males to develop DDH.10 Firstborn status is also recognized as an associated risk factor, which may be attributable to space constraints in utero. This hypothesis is further supported by the relative DDH-protective effect of prematurity and low birth weight. Other potential risk factors include advanced maternal age, birth weight that is high for gestational age, decreased hip abduction, and joint laxity. However, the majority of patients with hip dysplasia have no identifiable risk factors.3,5,9,11,12
Swaddling, which often maintains the hips in an adducted and/or extended position, has also been strongly associated with hip dysplasia.5,13 Multiple organizations, including the AAOS,AAP, POSNA, and the International Hip Dysplasia Institute, have developed or endorsed hip-healthy swaddling recommendations to minimize the risk for DDH in swaddled infants.13-15 Such practices allow the infant’s legs to bend up and out at the hips, promoting free hip movement, flexion, and abduction.13,15 Swaddling has demonstrated multiple benefits (including improved sleep and relief of excessive crying13) and continues to be recommended by many US providers; however, those caring for infants at risk for DDH should avoid traditional swaddling and/or practice hip-healthy swaddling techniques.10,13,14 Early diagnosis starts with the clinician’s knowledge of DDH risk factors and the recommended screening protocols. The presence of multiple risk factors will increase the likelihood of this condition and should lower the clinician’s threshold for ordering additional screening, regardless of hip exam findings.
PHYSICAL EXAM
Both AAP and AAOS guidelines recommend clinical screening for DDH with physical exam in all newborns.1,3 A head-to-toe musculoskeletal exam is warranted during the initial evaluation of every newborn in order to assess for any known DDH-associated conditions, which may include neuromuscular disorders, torticollis, and metatarsus adductus.5
Initial evaluation of an infant with DDH may reveal nonspecific findings, including asymmetric skin folds and limb-length inequality. The Galeazzi sign should be sought by aligning flexed knees with the child in the supine position and assessing for uneven knee heights (see Figure 2). Unilateral posterior hip dislocation or femoral shortening represents a positive Galeazzi sign.16 Joint laxity and limited hip abduction have also been associated with DDH.1,10
Barlow and Ortolani exams are more specific to DDH and should be completed at newborn screening and each subsequent well-baby exam.1 The Barlow maneuver is a provocative test with flexion, adduction, and posterior pressure through the infant’s hip (Figure 3). A palpable clunk during the Barlow maneuver indicates positive instability with posterior displacement. The Ortolani test is a reductive maneuver requiring abduction with posterior pressure to lift the greater trochanter (Figure 4). A clunk sensation with this test is positive for reduction of the hip.
The infant’s diaper should be removed during the hip evaluation. These exams are more reliable when each hip is evaluated separately with the pelvis stabilized.10 All physical exam findings must be carefully documented at each encounter.1,17
It is critical for the examiner to understand the appropriate technique and potential results when conducting each of these specialized hip exams. A true positive finding is the clunking sensation that occurs with the dislocation or relocation of the affected hip; this finding is better felt than heard. In contrast, a benign hip click with these maneuvers is a more subtle sensation—typically, a soft-tissue snapping or catching—and is not diagnostic of DDH. A click is not a clunk and is not indicative of DDH.1,3
DDH may present later in infancy or early childhood; therefore, DDH should remain within the differential diagnosis for gait asymmetry, unequal hip motion, or limb-length discrepancy. It may be beneficial to continue to evaluate for these developments during routine exams as part of a thorough pediatric musculoskeletal assessment, particularly in patients with documented risk factors for DDH.1,3,4 Delay in diagnosis of DDH, it should be noted, is a relatively common complaint in pediatric medical malpractice lawsuits; until the early 2000s, this condition represented about 75% of claims in one medical malpractice database.The decrease in claims has been attributed to better awareness and earlier diagnosis of DDH. 17
Continue for the diagnosis >>
DIAGNOSIS
A positive Ortolani or Barlow sign is diagnostic and warrants prompt orthopedic referral (Figure 5). If physical examination results are equivocal or inconclusive, follow-up at two weeks is recommended, with continued routine follow-up until walking is achieved. Patients with persistent equivocal findings at the two-week follow-up warrant ultrasound at age 3 to 4 weeks or orthopedic referral. Infants with significant risk factors, particularly breech presentation at birth, should also undergo imaging.18 AAP recommends ultrasound at age 6 weeks or radiograph after 4 months of age.1,18 AAOS recommends performing an imaging study before age 6 months when at least one of the following risk factors is present: breech presentation, positive family history of DDH, or previous clinical instability (moderate level of evidence).3
IMAGING
Ultrasound is the diagnostic test of choice for infants because radiographs have limited value until the femoral heads begin to ossify at age 4 to 6 months.18 Ultrasonography allows for visualization of the cartilaginous portion of the acetabulum and femoral head.1 Dynamic stressing is performed during ultrasound to assess the level of hip stability. A provider trained in ultrasound will measure the depth of the acetabulum and identify any potential laxity or instability of the hip joint. Accuracy of these findings is largely dependent on the experience and skill of the examiner.
Ultrasound evaluation is not recommended until after age 3 to 4 weeks. Earlier findings may include mild laxity and immature morphology of the acetabulum, which often resolve spontaneously.1,18 Use of ultrasound is currently recommended only to confirm diagnostic suspicion, based on clinical findings, or for infants with significant risk factors.18 Universal ultrasound screening in newborns is not recommended and would incur unnecessary costs.1,3,9 Plain radiographs are used after age 4 months to confirm a diagnosis of DDH or to assess for residual dysplasia.3,18
Continue for management >>
MANAGEMENT
Once hip dysplasia is suggested by physical exam or imaging study, the child’s subsequent care should be provided by an orthopedic specialist with experience in treating this condition. Treatment is preferably initiated before age 6 weeks.12 The specifics of treatment are largely based on age at diagnosis and the severity of dysplasia.
The goal of treatment is to maintain the hips in a stable position with the femoral head well covered by the acetabulum. This will improve anatomic development and function. Early clinical diagnosis is often sufficient to justify initiating conservative treatment; additionally, early detection of DDH can considerably reduce the need for surgical intervention.12 Although the potential for spontaneous resolution is high, the consequences associated with delay in care can be significant.
Preferred initial management, which can be initiated before confirmation of DDH by ultrasound, involves implementation of soft abduction support.19 The Pavlik harness is the support design of choice (Figure 6).12 This harness maintains hip flexion and abduction, creating concentric reduction of the femoral head. The brace is highly successful when its use is initiated early. Treatment in a Pavlik harness requires nearly full-time wear and close monitoring by a clinician. Unlikely potential risks associated with this treatment include avascular necrosis and femoral nerve palsy.4
Ultrasonography is used to further monitor treatment and to determine length of wear. Long-term results suggest a success rate exceeding 90%.20,21 However, this rate may be falsely elevated due to the number of hips that likely would have improved spontaneously without treatment.6,19
The Pavlik harness becomes less effective with increasing age, and a more rigid abduction brace may be considered in infants older than 6 months.20 Overall outcomes improve once the femoral head is consistently maintained in the acetabulum. Delay in treatment is associated with an increase in the long-term complications associated with residual hip dysplasia.22
Once an infant is undergoing treatment for DDH in a Pavlik harness, there is no need for primary care providers to continue to perform provocative testing, such as the Ortolani or Barlow test, at routine well-baby checks. Unnecessary stress to the hips is not beneficial, and any new results will not change the treatment being provided by the orthopedic specialist. Adjustments to the fit of the harness should be made only by the orthopedist, unless femoral nerve palsy is noted on exam. This development warrants immediate discontinuation of harness use until symptoms resolve.21
Abduction bracing may not be suitable for all cases of hip dysplasia. Newborns with irreducible hips, more advanced dysplasia, or associated neuromuscular or syndromic disorder may require closed versus open reduction and casting. More invasive surgical options may also be considered in advanced dysplasia in order to reshape the joint and improve function.20,22
Continue for patient education >>
PATIENT EDUCATION
Parents should be fully educated on the options for managing hip dysplasia. Once DDH is diagnosed, prompt referral to an orthopedic specialist is critical in order to weigh the treatment options and to develop the appropriate individualized plan for each child. Once treatment is initiated, parental compliance is essential; frequent meetings between parents and the specialist are important.
Parents of infants with known risk factors for and/or suspicion of hip dysplasia should also be educated on hip-healthy swaddling to allow for free motion of the hips and knees.10,13 Advise them that some commercial baby carriers and slings may maintain the hips in an undesirable extended position. In both swaddling and with baby carriers, care should be taken to allow for hip abduction and flexion. Caution should also be taken during diaper changes to avoid lifting the legs and thereby causing unnecessary stress to the hips.
CONCLUSION
Developmental dysplasia of the hip can be a disabling pediatric condition. Early diagnosis improves the likelihood of successful treatment during infancy and can prevent serious complications. If untreated, DDH can lead to joint degeneration and premature arthritis. Recognition and treatment within the first six weeks of life is crucial to the overall outcome.
The role of a primary care provider is to identify hip dysplasia risk factors and recognize associated physical exam findings in order to refer to an orthopedic specialist in a timely manner. Guidelines from the AAP, POSNA, and AAOS help direct this process in order to effectively identify infants at risk and in need of treatment.
REFERENCES
1. American Academy of Pediatrics. Committee on Quality Improvement, Subcommittee on Developmental Dysplasia of the Hip. Clinical practice guideline: early detection of developmental dysplasia of the hip. Pediatrics. 2000;105(4 pt 1):896-905.
2. Schwend RM, Schoenecker P, Richards BS, et al. Screening the newborn for developmental dysplasia of the hip: now what do we do? J Pediatr Orthop. 2007;27(6):607-610.
3. Mulpuri K, Song KM, Goldberg MJ, Sevarino K. Detection and nonoperative management of pediatric developmental dysplasia of the hip in infants up to six months of age. J Am Acad Orthop Surg. 2015;23(3):202-205.
4. Thomas SRYW. A review of long-term outcomes for late presenting developmental hip dysplasia. Bone Joint J. 2015;97-B(6):729-733.
5. Loder RT, Skopelja EN. The epidemiology and demographics of hip dysplasia. ISRN Orthop. 2011;2011:238607.
6. US Preventive Services Task Force. Screening for developmental dysplasia of the hip: recommendation statement. Pediatrics. 2006;117(3):898-902.
7. Shorter D, Hong T, Osborn DA. Screening programmes for developmental dysplasia of the hip in newborn infants. Cochrane Database Syst Rev. 2011;(9):CD004595.
8. Loder RT, Shafer C. The demographics of developmental hip dysplasia in the Midwestern United States (Indiana). J Child Orthop. 2015;9(1):93-98.
9. Paton RW, Hinduja K, Thomas CD. The significance of at-risk factors in ultrasound surveillance of developmental dysplasia of the hip: a ten-year prospective study. J Bone Joint Surg Br. 2005;87(9):1264-1266.
10. Alsaleem M, Set KK, Saadeh L. Developmental dysplasia of hip: a review. Clin Pediatr (Phila). 2015;54(10):921-928.
11. Chan A, McCaul KA, Cundy PJ, et al. Perinatal risk factors for developmental dysplasia of the hip. Arch Dis Child. 1997;76(2):F94-F100.
12. Godley DR. Assessment, diagnosis, and treatment of developmental dysplasia of the hip. JAAPA. 2013;26(3):54-58.
13. Van Sleuwen BE, Engelberts AC, Boere-Boonekamp MM, et al. Swaddling: a systematic review. Pediatrics. 2007;120(4):e1097-e1106.
14. American Academy of Orthopaedic Surgeons, American Association of Orthopaedic Surgeons. Position statement: swaddling and developmental hip dysplasia. www.aaos.org/uploadedFiles/PreProduction/About/Opinion_Statements/position/1186%20Swaddling%20and%20Developmental%20Hip%20Dysplasia.pdf. Accessed January 22, 2016.
15. Clarke NM. Swaddling and hip dysplasia: an orthopaedic perspective. Arch Dis Child. 2014;99(1):5-6.
16. Storer SK, Skaggs DL. Developmental dysplasia of the hip. Am Fam Physician. 2006;74(8):1310-1316.
17. McAbee GN, Donn SM, Mendelson RA, et al. Medical diagnoses commonly associated with pediatric malpractice lawsuits in the United States. Pediatrics. 2008;122(6):e1282-e1286.
18. Imrie M, Scott V, Stearns P, et al. Is ultrasound screening for DDH in babies born breech sufficient? J Child Orthop. 2010;4(1):3-8.
19. Chen HW, Chang CH, Tsai ST, et al. Natural progression of hip dysplasia in newborns: a reflection of hip ultrasonographic screenings in newborn nurseries. J Pediatr Orthop B. 2010;19(5):418-423.
20. Gans I, Flynn JM, Sankar WN. Abduction bracing for residual acetabular dysplasia in infantile DDH. J Pediatr Orthop. 2013;33(7):714-718.
21. Murnaghan ML, Browne RH, Sucato DJ, Birch J. Femoral nerve palsy in Pavlik harness treatment for developmental dysplasia of the hip. J Bone Joint Surg Am. 2011;93(5):493-499.
22. Dezateux C, Rosendahl K. Developmental dysplasia of the hip. Lancet. 2007;369(9572):1541-1552.
Man Is in Thick of Skin Problem
ANSWER
The correct answer is palmoplantar hyperkeratosis (PPK; choice “c”). As a category, it includes many conditions that are characterized by a heterogeneous thickening of the palms and soles, although each has distinct features.
Psoriasis (choice “a”) can present in somewhat similar fashion. However, it rarely appears so early in life and almost never involves such uniform hyperkeratosis.
Xerosis (choice “b”) merely means dry skin—a common enough problem, but one that does not involve such uniform and deep hyperkeratosis. It is almost never congenital.
Occult cancers (eg, GI, breast) can occasionally trigger a hyperkeratotic “perineoplastic” reaction (choice “d”) in the palms, soles, and other areas. However, it is acquired relatively late in life.
DISCUSSION
PPK is a relatively common problem, especially in those of northern European ancestry. The incidence in Northern Ireland, for example, is 4.4/100,000. In its more severe forms, such as this case, it can be debilitating.
Categorization of this extraordinarily diverse group of disorders is a challenge, to say the least. For purposes of this discussion, let’s start with this patient’s condition and then clarify its place in the panoply of hyperkeratotic disorders.
Our patient has one of the more common forms of diffuse PPK, generically called nonepidermolytic PPK, which presents with a waxy, uniform yellowed hyperkeratosis confined to the palms and soles. The old eponymic term for it was Unna-Thost disorder, named for the Viennese and Norwegian dermatologists who first described it late in the 19th century. A more modern term is tylosis, but many variations exist. This particular form is inherited in autosomal dominant fashion, but other forms of PPK can be transmitted by autosomal recessive or even X-linked genes.
Other types include focal (thick calluses over points of friction, especially on the feet) versions that can present in linear configuration and punctate forms with deep calluses on palms and soles that can be discrete or widespread, with lesions that range in size from pinpoint (“speculated”) to pea-sized.
New-onset PPK should prompt a search for an occult malignancy. In terms of establishing a firm diagnosis, punch biopsy can be performed for clarification when necessary.
As might be expected, treatment is difficult at best. Urea or salicylic acid–based topical preparations can help to thin it out. This patient was started on acitretin, an oral retinoid (artificial form of vitamin A) that may help; it was one of the few treatments he hadn’t already tried. A one-month course of terbinafine had already been tried, without success, just in case there was any secondary fungal involvement—a fairly common complication of PPK.
ANSWER
The correct answer is palmoplantar hyperkeratosis (PPK; choice “c”). As a category, it includes many conditions that are characterized by a heterogeneous thickening of the palms and soles, although each has distinct features.
Psoriasis (choice “a”) can present in somewhat similar fashion. However, it rarely appears so early in life and almost never involves such uniform hyperkeratosis.
Xerosis (choice “b”) merely means dry skin—a common enough problem, but one that does not involve such uniform and deep hyperkeratosis. It is almost never congenital.
Occult cancers (eg, GI, breast) can occasionally trigger a hyperkeratotic “perineoplastic” reaction (choice “d”) in the palms, soles, and other areas. However, it is acquired relatively late in life.
DISCUSSION
PPK is a relatively common problem, especially in those of northern European ancestry. The incidence in Northern Ireland, for example, is 4.4/100,000. In its more severe forms, such as this case, it can be debilitating.
Categorization of this extraordinarily diverse group of disorders is a challenge, to say the least. For purposes of this discussion, let’s start with this patient’s condition and then clarify its place in the panoply of hyperkeratotic disorders.
Our patient has one of the more common forms of diffuse PPK, generically called nonepidermolytic PPK, which presents with a waxy, uniform yellowed hyperkeratosis confined to the palms and soles. The old eponymic term for it was Unna-Thost disorder, named for the Viennese and Norwegian dermatologists who first described it late in the 19th century. A more modern term is tylosis, but many variations exist. This particular form is inherited in autosomal dominant fashion, but other forms of PPK can be transmitted by autosomal recessive or even X-linked genes.
Other types include focal (thick calluses over points of friction, especially on the feet) versions that can present in linear configuration and punctate forms with deep calluses on palms and soles that can be discrete or widespread, with lesions that range in size from pinpoint (“speculated”) to pea-sized.
New-onset PPK should prompt a search for an occult malignancy. In terms of establishing a firm diagnosis, punch biopsy can be performed for clarification when necessary.
As might be expected, treatment is difficult at best. Urea or salicylic acid–based topical preparations can help to thin it out. This patient was started on acitretin, an oral retinoid (artificial form of vitamin A) that may help; it was one of the few treatments he hadn’t already tried. A one-month course of terbinafine had already been tried, without success, just in case there was any secondary fungal involvement—a fairly common complication of PPK.
ANSWER
The correct answer is palmoplantar hyperkeratosis (PPK; choice “c”). As a category, it includes many conditions that are characterized by a heterogeneous thickening of the palms and soles, although each has distinct features.
Psoriasis (choice “a”) can present in somewhat similar fashion. However, it rarely appears so early in life and almost never involves such uniform hyperkeratosis.
Xerosis (choice “b”) merely means dry skin—a common enough problem, but one that does not involve such uniform and deep hyperkeratosis. It is almost never congenital.
Occult cancers (eg, GI, breast) can occasionally trigger a hyperkeratotic “perineoplastic” reaction (choice “d”) in the palms, soles, and other areas. However, it is acquired relatively late in life.
DISCUSSION
PPK is a relatively common problem, especially in those of northern European ancestry. The incidence in Northern Ireland, for example, is 4.4/100,000. In its more severe forms, such as this case, it can be debilitating.
Categorization of this extraordinarily diverse group of disorders is a challenge, to say the least. For purposes of this discussion, let’s start with this patient’s condition and then clarify its place in the panoply of hyperkeratotic disorders.
Our patient has one of the more common forms of diffuse PPK, generically called nonepidermolytic PPK, which presents with a waxy, uniform yellowed hyperkeratosis confined to the palms and soles. The old eponymic term for it was Unna-Thost disorder, named for the Viennese and Norwegian dermatologists who first described it late in the 19th century. A more modern term is tylosis, but many variations exist. This particular form is inherited in autosomal dominant fashion, but other forms of PPK can be transmitted by autosomal recessive or even X-linked genes.
Other types include focal (thick calluses over points of friction, especially on the feet) versions that can present in linear configuration and punctate forms with deep calluses on palms and soles that can be discrete or widespread, with lesions that range in size from pinpoint (“speculated”) to pea-sized.
New-onset PPK should prompt a search for an occult malignancy. In terms of establishing a firm diagnosis, punch biopsy can be performed for clarification when necessary.
As might be expected, treatment is difficult at best. Urea or salicylic acid–based topical preparations can help to thin it out. This patient was started on acitretin, an oral retinoid (artificial form of vitamin A) that may help; it was one of the few treatments he hadn’t already tried. A one-month course of terbinafine had already been tried, without success, just in case there was any secondary fungal involvement—a fairly common complication of PPK.

Since birth, a now 60-year-old man has been affected by the same dermatologic condition as several members of his mother’s family. As he has aged, the problem has become more intrusive: His hands and feet are rough and painful, and he experiences loss of sensation, particularly on his soles. Over the years, he has been evaluated in multiple venues, both private practices and medical schools, by a variety of providers, including pediatricians and dermatologists. Many treatments have been tried; few have produced any good effect. Still seeking an explanation for his condition, he self-refers to dermatology. Evaluation reveals that the surfaces of both feet and hands are uniformly covered by rough and, in some focal areas, waxy hyperkeratotic skin. The nails, hair, teeth, and dorsal surfaces of the extremities are unaffected. The patient’s affect and level of intelligence appear well within normal limits.
Higher levels of function before hip fracture tied to greater fears of falling at 1 year
WASHINGTON – Fear of falling at 12 weeks was associated with poorer functional recovery up to 1 year after hip fracture, particularly if the person had a high level of function before the fracture, a study has shown.
Inherent in this fear is a tendency of the patient to limit his activities, which in turn affects his sense of balance, visual attention, and gait. This leads to an increased risk of further falls, according to Emily Bower, a doctoral candidate who presented the findings at the annual meeting of the American Association for Geriatric Psychiatry.
The study included 241 hip fracture patients, three-quarters of whom were female, with an average age of 77 years. All of the patients lived in the community, nearly all were able to participate in basic activities of daily living, and three-quarters could walk without assistance.
Patients were assessed for their level of functionality at week 4, week 12, week 26, and week 52, as well as their fear of falling using the Falls Efficacy Scale International (FES-I).
The investigators found that by week 52, 48% of all patients had reached full recovery of functional status. At week 12, 53% of all patients had FES-I scores indicating a lower fear of falling.
“That means that almost half of our participants were reporting high fear of falling 3 months after hip fracture, which is a substantial number when you consider that in the general population, fear of falling is reported by about one in five older adults,” Ms. Bower, who is enrolled at the San Diego State University/University of California, San Diego, joint doctoral program in clinical psychology, said in an interview.
At 1-year after fracture, for each 1 point increase in FES-I scores, the odds of reaching full recovery were lowered by 12% (P = .003). Meanwhile, in patients with low baseline levels of functioning, there was “no effect of fear of falling,” according to Ms. Bower.
The upshot is that for patients with high levels of functional independence prior to injury, more intervention may be required to bring them back to their levels of activity before the fracture, according to Ms. Bower, who suggested multicomponent activities that combine psychological, physical, and environmental factors, such as tai chi.
On Twitter @whitneymcknight
WASHINGTON – Fear of falling at 12 weeks was associated with poorer functional recovery up to 1 year after hip fracture, particularly if the person had a high level of function before the fracture, a study has shown.
Inherent in this fear is a tendency of the patient to limit his activities, which in turn affects his sense of balance, visual attention, and gait. This leads to an increased risk of further falls, according to Emily Bower, a doctoral candidate who presented the findings at the annual meeting of the American Association for Geriatric Psychiatry.
The study included 241 hip fracture patients, three-quarters of whom were female, with an average age of 77 years. All of the patients lived in the community, nearly all were able to participate in basic activities of daily living, and three-quarters could walk without assistance.
Patients were assessed for their level of functionality at week 4, week 12, week 26, and week 52, as well as their fear of falling using the Falls Efficacy Scale International (FES-I).
The investigators found that by week 52, 48% of all patients had reached full recovery of functional status. At week 12, 53% of all patients had FES-I scores indicating a lower fear of falling.
“That means that almost half of our participants were reporting high fear of falling 3 months after hip fracture, which is a substantial number when you consider that in the general population, fear of falling is reported by about one in five older adults,” Ms. Bower, who is enrolled at the San Diego State University/University of California, San Diego, joint doctoral program in clinical psychology, said in an interview.
At 1-year after fracture, for each 1 point increase in FES-I scores, the odds of reaching full recovery were lowered by 12% (P = .003). Meanwhile, in patients with low baseline levels of functioning, there was “no effect of fear of falling,” according to Ms. Bower.
The upshot is that for patients with high levels of functional independence prior to injury, more intervention may be required to bring them back to their levels of activity before the fracture, according to Ms. Bower, who suggested multicomponent activities that combine psychological, physical, and environmental factors, such as tai chi.
On Twitter @whitneymcknight
WASHINGTON – Fear of falling at 12 weeks was associated with poorer functional recovery up to 1 year after hip fracture, particularly if the person had a high level of function before the fracture, a study has shown.
Inherent in this fear is a tendency of the patient to limit his activities, which in turn affects his sense of balance, visual attention, and gait. This leads to an increased risk of further falls, according to Emily Bower, a doctoral candidate who presented the findings at the annual meeting of the American Association for Geriatric Psychiatry.
The study included 241 hip fracture patients, three-quarters of whom were female, with an average age of 77 years. All of the patients lived in the community, nearly all were able to participate in basic activities of daily living, and three-quarters could walk without assistance.
Patients were assessed for their level of functionality at week 4, week 12, week 26, and week 52, as well as their fear of falling using the Falls Efficacy Scale International (FES-I).
The investigators found that by week 52, 48% of all patients had reached full recovery of functional status. At week 12, 53% of all patients had FES-I scores indicating a lower fear of falling.
“That means that almost half of our participants were reporting high fear of falling 3 months after hip fracture, which is a substantial number when you consider that in the general population, fear of falling is reported by about one in five older adults,” Ms. Bower, who is enrolled at the San Diego State University/University of California, San Diego, joint doctoral program in clinical psychology, said in an interview.
At 1-year after fracture, for each 1 point increase in FES-I scores, the odds of reaching full recovery were lowered by 12% (P = .003). Meanwhile, in patients with low baseline levels of functioning, there was “no effect of fear of falling,” according to Ms. Bower.
The upshot is that for patients with high levels of functional independence prior to injury, more intervention may be required to bring them back to their levels of activity before the fracture, according to Ms. Bower, who suggested multicomponent activities that combine psychological, physical, and environmental factors, such as tai chi.
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM AAGP 2016
Incretin-based diabetes drugs don’t raise heart failure risk
Incretin-based antidiabetic drugs didn’t raise the risk of hospitalization for heart failure in an international observational study involving 1.5 million patients reported online March 24 in the New England Journal of Medicine.
The safety of dipeptidyl peptidase 4 (DPP-4) inhibitors such as sitagliptin, saxagliptin, and linagliptin, and of glucagon-like peptide–1 (GLP-1) analogues such as exenatide and liraglutide is controversial. Some clinical trials have reported these agents raise the risk of heart failure (HF) while others have found no increase in risk, but all of the studies are underpowered to settle the question, said Kristian B. Filion, Ph.D., of McGill University and the Center for Clinical Epidemiology, Lady Davis Research Institute, Jewish General Hospital, and his associates.
They examined this issue by analyzing data from several large cohorts of diabetes patients treated in routine clinical practice in the United States, Canada, and England. Their study population comprised 1,499,650 adults who began taking noninsulin antidiabetic drugs at or after the date that incretin-based agents entered the market. “With 3.2 million person-years of observations, we had the statistical power to robustly assess this important drug safety issue,” the investigators said.
Patients taking DPP-4 inhibitors and GLP-1 analogues were compared with those taking non–incretin-based drugs such as biguanides, sulfonylureas, thiazolidinediones, alpha-glucosidase inhibitors, meglitinides, and sodium-glucose cotransporter-2 inhibitors. A total of 29,741 patients were hospitalized for HF, for an overall rate of 9.2 events per 1,000 person-years.
Incretin-based drugs were not associated with an increased rate of hospitalization for HF when compared with other antidiabetic drugs (hazard ratio, 0.82) among the roughly 1.4 million patients who had no history of HF at baseline. Individually, neither DPP-4 inhibitors (HR, 0.84) nor GLP-1 analogues (HR, 0.95) were associated with an increased risk of hospitalization for HF. These findings remained consistent through several subgroup and sensitivity analyses that categorized the data according to duration of exposure, presence or absence of a history of MI, and duration of diabetes, Dr. Filion and his associates said (N Engl J Med. 2016 Mar 24. doi: 10.1056/NEJMoa1506115).
Similarly, incretin-based drugs were not associated with an increased rate of hospitalization for HF when compared with other antidiabetic drugs among the approximately 80,000 patients who had a history of HF at baseline (HR, 0.86).
This study was supported by the Canadian Institutes of Health Research and the Quebec Foundation for Health Research. Dr. Filion reported having no relevant financial disclosures; some of his associates reported ties to numerous industry sources.
Incretin-based antidiabetic drugs didn’t raise the risk of hospitalization for heart failure in an international observational study involving 1.5 million patients reported online March 24 in the New England Journal of Medicine.
The safety of dipeptidyl peptidase 4 (DPP-4) inhibitors such as sitagliptin, saxagliptin, and linagliptin, and of glucagon-like peptide–1 (GLP-1) analogues such as exenatide and liraglutide is controversial. Some clinical trials have reported these agents raise the risk of heart failure (HF) while others have found no increase in risk, but all of the studies are underpowered to settle the question, said Kristian B. Filion, Ph.D., of McGill University and the Center for Clinical Epidemiology, Lady Davis Research Institute, Jewish General Hospital, and his associates.
They examined this issue by analyzing data from several large cohorts of diabetes patients treated in routine clinical practice in the United States, Canada, and England. Their study population comprised 1,499,650 adults who began taking noninsulin antidiabetic drugs at or after the date that incretin-based agents entered the market. “With 3.2 million person-years of observations, we had the statistical power to robustly assess this important drug safety issue,” the investigators said.
Patients taking DPP-4 inhibitors and GLP-1 analogues were compared with those taking non–incretin-based drugs such as biguanides, sulfonylureas, thiazolidinediones, alpha-glucosidase inhibitors, meglitinides, and sodium-glucose cotransporter-2 inhibitors. A total of 29,741 patients were hospitalized for HF, for an overall rate of 9.2 events per 1,000 person-years.
Incretin-based drugs were not associated with an increased rate of hospitalization for HF when compared with other antidiabetic drugs (hazard ratio, 0.82) among the roughly 1.4 million patients who had no history of HF at baseline. Individually, neither DPP-4 inhibitors (HR, 0.84) nor GLP-1 analogues (HR, 0.95) were associated with an increased risk of hospitalization for HF. These findings remained consistent through several subgroup and sensitivity analyses that categorized the data according to duration of exposure, presence or absence of a history of MI, and duration of diabetes, Dr. Filion and his associates said (N Engl J Med. 2016 Mar 24. doi: 10.1056/NEJMoa1506115).
Similarly, incretin-based drugs were not associated with an increased rate of hospitalization for HF when compared with other antidiabetic drugs among the approximately 80,000 patients who had a history of HF at baseline (HR, 0.86).
This study was supported by the Canadian Institutes of Health Research and the Quebec Foundation for Health Research. Dr. Filion reported having no relevant financial disclosures; some of his associates reported ties to numerous industry sources.
Incretin-based antidiabetic drugs didn’t raise the risk of hospitalization for heart failure in an international observational study involving 1.5 million patients reported online March 24 in the New England Journal of Medicine.
The safety of dipeptidyl peptidase 4 (DPP-4) inhibitors such as sitagliptin, saxagliptin, and linagliptin, and of glucagon-like peptide–1 (GLP-1) analogues such as exenatide and liraglutide is controversial. Some clinical trials have reported these agents raise the risk of heart failure (HF) while others have found no increase in risk, but all of the studies are underpowered to settle the question, said Kristian B. Filion, Ph.D., of McGill University and the Center for Clinical Epidemiology, Lady Davis Research Institute, Jewish General Hospital, and his associates.
They examined this issue by analyzing data from several large cohorts of diabetes patients treated in routine clinical practice in the United States, Canada, and England. Their study population comprised 1,499,650 adults who began taking noninsulin antidiabetic drugs at or after the date that incretin-based agents entered the market. “With 3.2 million person-years of observations, we had the statistical power to robustly assess this important drug safety issue,” the investigators said.
Patients taking DPP-4 inhibitors and GLP-1 analogues were compared with those taking non–incretin-based drugs such as biguanides, sulfonylureas, thiazolidinediones, alpha-glucosidase inhibitors, meglitinides, and sodium-glucose cotransporter-2 inhibitors. A total of 29,741 patients were hospitalized for HF, for an overall rate of 9.2 events per 1,000 person-years.
Incretin-based drugs were not associated with an increased rate of hospitalization for HF when compared with other antidiabetic drugs (hazard ratio, 0.82) among the roughly 1.4 million patients who had no history of HF at baseline. Individually, neither DPP-4 inhibitors (HR, 0.84) nor GLP-1 analogues (HR, 0.95) were associated with an increased risk of hospitalization for HF. These findings remained consistent through several subgroup and sensitivity analyses that categorized the data according to duration of exposure, presence or absence of a history of MI, and duration of diabetes, Dr. Filion and his associates said (N Engl J Med. 2016 Mar 24. doi: 10.1056/NEJMoa1506115).
Similarly, incretin-based drugs were not associated with an increased rate of hospitalization for HF when compared with other antidiabetic drugs among the approximately 80,000 patients who had a history of HF at baseline (HR, 0.86).
This study was supported by the Canadian Institutes of Health Research and the Quebec Foundation for Health Research. Dr. Filion reported having no relevant financial disclosures; some of his associates reported ties to numerous industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Incretin-based antidiabetic drugs did not raise the risk of hospitalization for heart failure in a large international observational study.
Major finding: Neither DPP-4 inhibitors (HR, 0.84) nor GLP-1 analogues (HR, 0.95) were associated with an increased risk of hospitalization for HF, compared with non–incretin-based antidiabetic drugs.
Data source: A retrospective international observational cohort study involving roughly 1.5 million diabetes patients, of whom 29,741 were hospitalized for HF.
Disclosures: This study was supported by the Canadian Institutes of Health Research and the Quebec Foundation for Health Research. Dr. Filion reported having no relevant financial disclosures; some of his associates reported ties to numerous industry sources.
Tips for Living With Essential Tremor
Considering learning disorders
Introduction
Often, it can be difficult for providers to fully understand why children may be presenting with behavioral problems that appear to be occurring principally in specific environments. Parents may bring their children for an evaluation based upon reports they are receiving from teachers and other school personnel who may be observing more prominent oppositionality, social struggles, troubles following instructions, and frustration intolerance than the parents are experiencing in the home. Additionally, youth may begin to display school refusal and voice strong negative feelings about school. Although at face value, these problems may indicate potential diagnoses like oppositional defiant disorder and attention-deficit/hyperactivity disorder, one also should consider other underlying “non–mental health” issues that could greatly influence school success and present with a range of emotional and behavioral struggles.
Case Summary
Brynn is an animated, social, and strong-willed 4 year-old girl who experienced delays in her receptive and expressive language that prompted her engagement in early intervention around 18 months of age. She and her family continued to receive developmental services over the next few years and, at the age of 3 years, because of ongoing speech and language challenges, she was enrolled in a preschool individualized education program. In her program, Brynn participates in an array of specialized instruction, but her educators comment that she is not making expected academic progress and has troubles “holding onto information,” focusing, and participating meaningfully with peers in a consistent manner.
At times, Brynn can be impulsive, aggressive, and volatile – behavioral traits that mom denies are occurring in the home. A diagnosis of an autism spectrum disorder has been ruled out, and Brynn’s hearing and vision are tested to be normal. Brynn’s mother feels confused by the reports she’s getting from school; “I want Brynn to be happy,” she shares. “I needed special help to read in high school, and I worry about her future.”
Discussion
Learning disorders (or learning disabilities, using educational terminology) are defined as neurologically rooted problems that affect academic achievement via the receiving, processing, or communication of information. They occur in 1 in 10 children (Pediatrics. 2007 Feb;119 Suppl 1:S77-83) and can present with specific problems in reading, writing, and mathematics while having considerable influence on related aptitudes in language, social ability, self-help, and motor functioning. Dyslexia – a developmental reading disorder – is the most common type of learning disorder (LD). Although it’s not clear what causes learning disorders, there are several factors that are thought to play a role in their development, including hereditary factors and problems with pregnancy and birth. Having developmental delays also can place children at risk for having later learning problems that may not be identifiable until a child enters a more structured learning environment. At clinical visits during the preschool years, particularly with a child who may have had earlier developmental concerns, the pediatrician should inquire about a range of “warning signs” that may indicate a need for additional screening and evaluation for specific learning issues.
The National Center for Learning Disabilities offers a range of practical tips for pediatricians who may want to further explore parental and teacher concerns by asking questions related to literacy, writing, language, and social-emotional skills, attention, and gross and fine motor movements in a developmentally informed manner. Further exploring Brynn’s mother’s comments in the context of her daughter not progressing in school revealed a history of difficulty retaining new words, troubles learning colors and shapes, challenges remembering rules, and particular difficulties engaging in group play with other 4-year-olds – all potential signals for a learning disorder. This alerted educators that she may indeed be struggling with issues beyond that of an enduring speech-language delay.
With the suspicion that Brynn was presenting with signs and symptoms suggestive of a learning disorder, her family was educated about the Individuals with Disabilities Education Act, and it was recommended she receive a comprehensive psychoeducational evaluation to further assess her intellectual profile, academic achievement, social functioning, and performance in the classroom using standardized tools. These tools, among other objectives, can help the child’s team offer a more definitive LD diagnosis while informing the potential development of special education supports and assessing for an intellectual disability. The DSM 5 indicates that LD diagnostic criteria are to be met based upon a clinical synthesis of history, school reports, and psychoeducational assessment.
It’s been long established that children with learning problems frequently have co-occurring emotional and behavioral troubles (Arch Dis Child. 1974 Apr; 49[4]:249-56), many of which also should be considered as differential diagnoses in a child with school problems – as such, a complicated interplay of learning disorders and internalizing and externalizing conditions is often appreciated in school-age children with academic difficulties. At times, learning disorders can lead to emotional distress and could also be misdiagnosed as primary emotional and behavioral challenges. Specifically, children with learning problems are at risk for struggling with low self-esteem, loneliness, and anxiety, which also can be associated with mood disorders, school dropout, victimization, and engaging in substance use.
In Brynn’s case, the results of the Teacher Report Forms and Child Behavior Checklists were reviewed, revealing some evidence that she was experiencing clinical-level problems with her attention, but a discrepancy was noted between the teacher and parent report (the teachers endorsing more clinically significant symptoms). Although co-occurring attention-deficit/hyperactivity disorder (ADHD) is not uncommon in children with a learning disorder (Pediatrics. 2011 Mar;127[3]:462-70), we did not feel Brynn met criteria for this. We elected not to provide an ADHD diagnosis but are mindful that her attentional concerns should be closely monitored over time; a diagnosis may be more relevant in the future, perhaps influencing Brynn’s eligibility for services and treatment planning. Furthermore, comorbidity with ADHD is predictive of worse mental health outcomes, compared with learning disabilities presenting without ADHD.
Clinical Pearl
Pediatricians should consider the possibility of a child having a learning disorder in youth who display risk factors (family history of learning concerns, atypical development, prematurity, etc.) and have problems at school. Such problems may be presenting with emotional and behavioral symptoms that could mask a child’s primary learning impairments. Learning disorders also frequently co-occur with psychiatric conditions, but engaging children in effective interventions (school-based supports) could potentially attenuate the risk for the development of mental health problems. Also, promoting emotional wellness and fostering self-worth may improve the academic performance of children with learning disorders.
Dr. Dickerson, a child and adolescent psychiatrist, is an assistant professor of psychiatry at the University of Vermont, Burlington. He is the director of the university’s autism diagnostic clinic. Dr. Dickerson said he had no relevant financial disclosures. Contact Dr. Dickerson at pdnews@frontlinemedcom.com.
Introduction
Often, it can be difficult for providers to fully understand why children may be presenting with behavioral problems that appear to be occurring principally in specific environments. Parents may bring their children for an evaluation based upon reports they are receiving from teachers and other school personnel who may be observing more prominent oppositionality, social struggles, troubles following instructions, and frustration intolerance than the parents are experiencing in the home. Additionally, youth may begin to display school refusal and voice strong negative feelings about school. Although at face value, these problems may indicate potential diagnoses like oppositional defiant disorder and attention-deficit/hyperactivity disorder, one also should consider other underlying “non–mental health” issues that could greatly influence school success and present with a range of emotional and behavioral struggles.
Case Summary
Brynn is an animated, social, and strong-willed 4 year-old girl who experienced delays in her receptive and expressive language that prompted her engagement in early intervention around 18 months of age. She and her family continued to receive developmental services over the next few years and, at the age of 3 years, because of ongoing speech and language challenges, she was enrolled in a preschool individualized education program. In her program, Brynn participates in an array of specialized instruction, but her educators comment that she is not making expected academic progress and has troubles “holding onto information,” focusing, and participating meaningfully with peers in a consistent manner.
At times, Brynn can be impulsive, aggressive, and volatile – behavioral traits that mom denies are occurring in the home. A diagnosis of an autism spectrum disorder has been ruled out, and Brynn’s hearing and vision are tested to be normal. Brynn’s mother feels confused by the reports she’s getting from school; “I want Brynn to be happy,” she shares. “I needed special help to read in high school, and I worry about her future.”
Discussion
Learning disorders (or learning disabilities, using educational terminology) are defined as neurologically rooted problems that affect academic achievement via the receiving, processing, or communication of information. They occur in 1 in 10 children (Pediatrics. 2007 Feb;119 Suppl 1:S77-83) and can present with specific problems in reading, writing, and mathematics while having considerable influence on related aptitudes in language, social ability, self-help, and motor functioning. Dyslexia – a developmental reading disorder – is the most common type of learning disorder (LD). Although it’s not clear what causes learning disorders, there are several factors that are thought to play a role in their development, including hereditary factors and problems with pregnancy and birth. Having developmental delays also can place children at risk for having later learning problems that may not be identifiable until a child enters a more structured learning environment. At clinical visits during the preschool years, particularly with a child who may have had earlier developmental concerns, the pediatrician should inquire about a range of “warning signs” that may indicate a need for additional screening and evaluation for specific learning issues.
The National Center for Learning Disabilities offers a range of practical tips for pediatricians who may want to further explore parental and teacher concerns by asking questions related to literacy, writing, language, and social-emotional skills, attention, and gross and fine motor movements in a developmentally informed manner. Further exploring Brynn’s mother’s comments in the context of her daughter not progressing in school revealed a history of difficulty retaining new words, troubles learning colors and shapes, challenges remembering rules, and particular difficulties engaging in group play with other 4-year-olds – all potential signals for a learning disorder. This alerted educators that she may indeed be struggling with issues beyond that of an enduring speech-language delay.
With the suspicion that Brynn was presenting with signs and symptoms suggestive of a learning disorder, her family was educated about the Individuals with Disabilities Education Act, and it was recommended she receive a comprehensive psychoeducational evaluation to further assess her intellectual profile, academic achievement, social functioning, and performance in the classroom using standardized tools. These tools, among other objectives, can help the child’s team offer a more definitive LD diagnosis while informing the potential development of special education supports and assessing for an intellectual disability. The DSM 5 indicates that LD diagnostic criteria are to be met based upon a clinical synthesis of history, school reports, and psychoeducational assessment.
It’s been long established that children with learning problems frequently have co-occurring emotional and behavioral troubles (Arch Dis Child. 1974 Apr; 49[4]:249-56), many of which also should be considered as differential diagnoses in a child with school problems – as such, a complicated interplay of learning disorders and internalizing and externalizing conditions is often appreciated in school-age children with academic difficulties. At times, learning disorders can lead to emotional distress and could also be misdiagnosed as primary emotional and behavioral challenges. Specifically, children with learning problems are at risk for struggling with low self-esteem, loneliness, and anxiety, which also can be associated with mood disorders, school dropout, victimization, and engaging in substance use.
In Brynn’s case, the results of the Teacher Report Forms and Child Behavior Checklists were reviewed, revealing some evidence that she was experiencing clinical-level problems with her attention, but a discrepancy was noted between the teacher and parent report (the teachers endorsing more clinically significant symptoms). Although co-occurring attention-deficit/hyperactivity disorder (ADHD) is not uncommon in children with a learning disorder (Pediatrics. 2011 Mar;127[3]:462-70), we did not feel Brynn met criteria for this. We elected not to provide an ADHD diagnosis but are mindful that her attentional concerns should be closely monitored over time; a diagnosis may be more relevant in the future, perhaps influencing Brynn’s eligibility for services and treatment planning. Furthermore, comorbidity with ADHD is predictive of worse mental health outcomes, compared with learning disabilities presenting without ADHD.
Clinical Pearl
Pediatricians should consider the possibility of a child having a learning disorder in youth who display risk factors (family history of learning concerns, atypical development, prematurity, etc.) and have problems at school. Such problems may be presenting with emotional and behavioral symptoms that could mask a child’s primary learning impairments. Learning disorders also frequently co-occur with psychiatric conditions, but engaging children in effective interventions (school-based supports) could potentially attenuate the risk for the development of mental health problems. Also, promoting emotional wellness and fostering self-worth may improve the academic performance of children with learning disorders.
Dr. Dickerson, a child and adolescent psychiatrist, is an assistant professor of psychiatry at the University of Vermont, Burlington. He is the director of the university’s autism diagnostic clinic. Dr. Dickerson said he had no relevant financial disclosures. Contact Dr. Dickerson at pdnews@frontlinemedcom.com.
Introduction
Often, it can be difficult for providers to fully understand why children may be presenting with behavioral problems that appear to be occurring principally in specific environments. Parents may bring their children for an evaluation based upon reports they are receiving from teachers and other school personnel who may be observing more prominent oppositionality, social struggles, troubles following instructions, and frustration intolerance than the parents are experiencing in the home. Additionally, youth may begin to display school refusal and voice strong negative feelings about school. Although at face value, these problems may indicate potential diagnoses like oppositional defiant disorder and attention-deficit/hyperactivity disorder, one also should consider other underlying “non–mental health” issues that could greatly influence school success and present with a range of emotional and behavioral struggles.
Case Summary
Brynn is an animated, social, and strong-willed 4 year-old girl who experienced delays in her receptive and expressive language that prompted her engagement in early intervention around 18 months of age. She and her family continued to receive developmental services over the next few years and, at the age of 3 years, because of ongoing speech and language challenges, she was enrolled in a preschool individualized education program. In her program, Brynn participates in an array of specialized instruction, but her educators comment that she is not making expected academic progress and has troubles “holding onto information,” focusing, and participating meaningfully with peers in a consistent manner.
At times, Brynn can be impulsive, aggressive, and volatile – behavioral traits that mom denies are occurring in the home. A diagnosis of an autism spectrum disorder has been ruled out, and Brynn’s hearing and vision are tested to be normal. Brynn’s mother feels confused by the reports she’s getting from school; “I want Brynn to be happy,” she shares. “I needed special help to read in high school, and I worry about her future.”
Discussion
Learning disorders (or learning disabilities, using educational terminology) are defined as neurologically rooted problems that affect academic achievement via the receiving, processing, or communication of information. They occur in 1 in 10 children (Pediatrics. 2007 Feb;119 Suppl 1:S77-83) and can present with specific problems in reading, writing, and mathematics while having considerable influence on related aptitudes in language, social ability, self-help, and motor functioning. Dyslexia – a developmental reading disorder – is the most common type of learning disorder (LD). Although it’s not clear what causes learning disorders, there are several factors that are thought to play a role in their development, including hereditary factors and problems with pregnancy and birth. Having developmental delays also can place children at risk for having later learning problems that may not be identifiable until a child enters a more structured learning environment. At clinical visits during the preschool years, particularly with a child who may have had earlier developmental concerns, the pediatrician should inquire about a range of “warning signs” that may indicate a need for additional screening and evaluation for specific learning issues.
The National Center for Learning Disabilities offers a range of practical tips for pediatricians who may want to further explore parental and teacher concerns by asking questions related to literacy, writing, language, and social-emotional skills, attention, and gross and fine motor movements in a developmentally informed manner. Further exploring Brynn’s mother’s comments in the context of her daughter not progressing in school revealed a history of difficulty retaining new words, troubles learning colors and shapes, challenges remembering rules, and particular difficulties engaging in group play with other 4-year-olds – all potential signals for a learning disorder. This alerted educators that she may indeed be struggling with issues beyond that of an enduring speech-language delay.
With the suspicion that Brynn was presenting with signs and symptoms suggestive of a learning disorder, her family was educated about the Individuals with Disabilities Education Act, and it was recommended she receive a comprehensive psychoeducational evaluation to further assess her intellectual profile, academic achievement, social functioning, and performance in the classroom using standardized tools. These tools, among other objectives, can help the child’s team offer a more definitive LD diagnosis while informing the potential development of special education supports and assessing for an intellectual disability. The DSM 5 indicates that LD diagnostic criteria are to be met based upon a clinical synthesis of history, school reports, and psychoeducational assessment.
It’s been long established that children with learning problems frequently have co-occurring emotional and behavioral troubles (Arch Dis Child. 1974 Apr; 49[4]:249-56), many of which also should be considered as differential diagnoses in a child with school problems – as such, a complicated interplay of learning disorders and internalizing and externalizing conditions is often appreciated in school-age children with academic difficulties. At times, learning disorders can lead to emotional distress and could also be misdiagnosed as primary emotional and behavioral challenges. Specifically, children with learning problems are at risk for struggling with low self-esteem, loneliness, and anxiety, which also can be associated with mood disorders, school dropout, victimization, and engaging in substance use.
In Brynn’s case, the results of the Teacher Report Forms and Child Behavior Checklists were reviewed, revealing some evidence that she was experiencing clinical-level problems with her attention, but a discrepancy was noted between the teacher and parent report (the teachers endorsing more clinically significant symptoms). Although co-occurring attention-deficit/hyperactivity disorder (ADHD) is not uncommon in children with a learning disorder (Pediatrics. 2011 Mar;127[3]:462-70), we did not feel Brynn met criteria for this. We elected not to provide an ADHD diagnosis but are mindful that her attentional concerns should be closely monitored over time; a diagnosis may be more relevant in the future, perhaps influencing Brynn’s eligibility for services and treatment planning. Furthermore, comorbidity with ADHD is predictive of worse mental health outcomes, compared with learning disabilities presenting without ADHD.
Clinical Pearl
Pediatricians should consider the possibility of a child having a learning disorder in youth who display risk factors (family history of learning concerns, atypical development, prematurity, etc.) and have problems at school. Such problems may be presenting with emotional and behavioral symptoms that could mask a child’s primary learning impairments. Learning disorders also frequently co-occur with psychiatric conditions, but engaging children in effective interventions (school-based supports) could potentially attenuate the risk for the development of mental health problems. Also, promoting emotional wellness and fostering self-worth may improve the academic performance of children with learning disorders.
Dr. Dickerson, a child and adolescent psychiatrist, is an assistant professor of psychiatry at the University of Vermont, Burlington. He is the director of the university’s autism diagnostic clinic. Dr. Dickerson said he had no relevant financial disclosures. Contact Dr. Dickerson at pdnews@frontlinemedcom.com.
Better sarcoma outcomes at high-volume centers
BOSTON – In sarcoma as in other cancers, experience counts.
That’s the conclusion of investigators who found that patients with extra-abdominal sarcomas who were treated in high-volume hospitals had half the 30-day mortality rate, higher likelihood of negative surgical margins, and better overall survival, compared with patients treated in low-volume hospitals.
“It appears there is a direct association between hospital volume and short-term as well as long-term outcomes for soft-tissue sarcomas outside the abdomen,” said Dr. Sanjay P. Bagaria of the Mayo Clinic in Jacksonville, Fla.
The findings support centralization of services at the national level in centers specializing in the management of sarcomas, he said at the annual Society of Surgical Oncology Cancer Symposium.
Previous studies have shown that patients treated in high-volume centers for cancers of the esophagus, pancreas, and lung have better outcomes than patients treated in low-volume centers, he noted.
Given the rarity of sarcomas, with an incidence of approximately 12,000 in the U.S. annually, their complexity, with more than 60 histologic subtypes, and the multimodality approach required for them, it seemed likely that a positive association between volume and outcomes could be found, Dr. Bagaria said.
He and colleagues queried the U.S. National Cancer Database, a hospital registry of data from more than 1,500 facilities accredited by the Commission on Cancer.
They drew records on all patients diagnosed with extra-abdominal sarcomas from 2003 through 2007 who underwent surgery at the reporting hospitals, and divided the cases into terciles as either low volume (3 or fewer surgical cases per year), medium volume (3.2-11.6 per year), or high volume (12 or more cases per year).
One third (33%) of all cases were concentrated in just 44 high-volume hospitals, which comprised just 4% of the total hospital sample of 1,163. An additional third (34%) of cases were managed among 196 medium-volume hospitals (17% of the hospital sample), and the remaining third (33%) were spread among 923 low-volume hospitals (79%).
The 30-day mortality rates for low-, medium-, and high-volume hospitals, respectively, were 1.7%, 1.1%, and 0.6% (P less than .0001).
Similarly, the rates of negative margins (R0 resections) were 73.%, 78.2%, and 84.2% (P less than .0001).
Five-year overall survival was identical for low- and medium-volume centers (65% each), but was significantly better for patients treated at high-volume centers (69%, P less than .001)
Compared with low-volume centers, patients treated at high-volume centers had an adjusted odds ratio (OR) for 30-day mortality of 0.46 (P = .01), an adjusted OR for R0 margins of 1.87 (P less than .001), and OR for overall mortality of 0.92 (P = .04).
Dr. Bagaria noted that the study was limited by missing data about disease-specific survival and by possible selection bias associated with the choice of Commission on Cancer-accredited institutions, which account for 70% of cancer cases nationwide but comprise one-third of all hospitals.
BOSTON – In sarcoma as in other cancers, experience counts.
That’s the conclusion of investigators who found that patients with extra-abdominal sarcomas who were treated in high-volume hospitals had half the 30-day mortality rate, higher likelihood of negative surgical margins, and better overall survival, compared with patients treated in low-volume hospitals.
“It appears there is a direct association between hospital volume and short-term as well as long-term outcomes for soft-tissue sarcomas outside the abdomen,” said Dr. Sanjay P. Bagaria of the Mayo Clinic in Jacksonville, Fla.
The findings support centralization of services at the national level in centers specializing in the management of sarcomas, he said at the annual Society of Surgical Oncology Cancer Symposium.
Previous studies have shown that patients treated in high-volume centers for cancers of the esophagus, pancreas, and lung have better outcomes than patients treated in low-volume centers, he noted.
Given the rarity of sarcomas, with an incidence of approximately 12,000 in the U.S. annually, their complexity, with more than 60 histologic subtypes, and the multimodality approach required for them, it seemed likely that a positive association between volume and outcomes could be found, Dr. Bagaria said.
He and colleagues queried the U.S. National Cancer Database, a hospital registry of data from more than 1,500 facilities accredited by the Commission on Cancer.
They drew records on all patients diagnosed with extra-abdominal sarcomas from 2003 through 2007 who underwent surgery at the reporting hospitals, and divided the cases into terciles as either low volume (3 or fewer surgical cases per year), medium volume (3.2-11.6 per year), or high volume (12 or more cases per year).
One third (33%) of all cases were concentrated in just 44 high-volume hospitals, which comprised just 4% of the total hospital sample of 1,163. An additional third (34%) of cases were managed among 196 medium-volume hospitals (17% of the hospital sample), and the remaining third (33%) were spread among 923 low-volume hospitals (79%).
The 30-day mortality rates for low-, medium-, and high-volume hospitals, respectively, were 1.7%, 1.1%, and 0.6% (P less than .0001).
Similarly, the rates of negative margins (R0 resections) were 73.%, 78.2%, and 84.2% (P less than .0001).
Five-year overall survival was identical for low- and medium-volume centers (65% each), but was significantly better for patients treated at high-volume centers (69%, P less than .001)
Compared with low-volume centers, patients treated at high-volume centers had an adjusted odds ratio (OR) for 30-day mortality of 0.46 (P = .01), an adjusted OR for R0 margins of 1.87 (P less than .001), and OR for overall mortality of 0.92 (P = .04).
Dr. Bagaria noted that the study was limited by missing data about disease-specific survival and by possible selection bias associated with the choice of Commission on Cancer-accredited institutions, which account for 70% of cancer cases nationwide but comprise one-third of all hospitals.
BOSTON – In sarcoma as in other cancers, experience counts.
That’s the conclusion of investigators who found that patients with extra-abdominal sarcomas who were treated in high-volume hospitals had half the 30-day mortality rate, higher likelihood of negative surgical margins, and better overall survival, compared with patients treated in low-volume hospitals.
“It appears there is a direct association between hospital volume and short-term as well as long-term outcomes for soft-tissue sarcomas outside the abdomen,” said Dr. Sanjay P. Bagaria of the Mayo Clinic in Jacksonville, Fla.
The findings support centralization of services at the national level in centers specializing in the management of sarcomas, he said at the annual Society of Surgical Oncology Cancer Symposium.
Previous studies have shown that patients treated in high-volume centers for cancers of the esophagus, pancreas, and lung have better outcomes than patients treated in low-volume centers, he noted.
Given the rarity of sarcomas, with an incidence of approximately 12,000 in the U.S. annually, their complexity, with more than 60 histologic subtypes, and the multimodality approach required for them, it seemed likely that a positive association between volume and outcomes could be found, Dr. Bagaria said.
He and colleagues queried the U.S. National Cancer Database, a hospital registry of data from more than 1,500 facilities accredited by the Commission on Cancer.
They drew records on all patients diagnosed with extra-abdominal sarcomas from 2003 through 2007 who underwent surgery at the reporting hospitals, and divided the cases into terciles as either low volume (3 or fewer surgical cases per year), medium volume (3.2-11.6 per year), or high volume (12 or more cases per year).
One third (33%) of all cases were concentrated in just 44 high-volume hospitals, which comprised just 4% of the total hospital sample of 1,163. An additional third (34%) of cases were managed among 196 medium-volume hospitals (17% of the hospital sample), and the remaining third (33%) were spread among 923 low-volume hospitals (79%).
The 30-day mortality rates for low-, medium-, and high-volume hospitals, respectively, were 1.7%, 1.1%, and 0.6% (P less than .0001).
Similarly, the rates of negative margins (R0 resections) were 73.%, 78.2%, and 84.2% (P less than .0001).
Five-year overall survival was identical for low- and medium-volume centers (65% each), but was significantly better for patients treated at high-volume centers (69%, P less than .001)
Compared with low-volume centers, patients treated at high-volume centers had an adjusted odds ratio (OR) for 30-day mortality of 0.46 (P = .01), an adjusted OR for R0 margins of 1.87 (P less than .001), and OR for overall mortality of 0.92 (P = .04).
Dr. Bagaria noted that the study was limited by missing data about disease-specific survival and by possible selection bias associated with the choice of Commission on Cancer-accredited institutions, which account for 70% of cancer cases nationwide but comprise one-third of all hospitals.
Key clinical point: Surgical volume, a surrogate for experience, has been shown to have a direct correlation with patient outcomes for cancers of the esophagus, lung, and pancreas, and this appears to be true for sarcomas as well.
Major finding: Patients treated for sarcoma at high-volume centers had lower 30-day and overall mortality and a higher probability of negative margins than those treated at low-volume centers.
Data source: Retrospective review of data on 14,634 patients treated at 1,163 U.S. hospitals.
Disclosures: The authors reported no relevant disclosures.