User login
Does sharing genetic risk change behavior?
In the era of individualized (or precision) medicine, we are presented with a unique opportunity to peer into the genetic “maps” of our patients. Through this window, we can envision the self-evident present or predict a possible future.
For the front-line provider, knowing that we could someday have a large amount of these data to deal with can be overwhelming. We may be loath to think that, amongst all the other daily battles we wage with current disease states, we may now need to understand and explain risk for future disease states.
But would we be more likely to use these data if we thought that they would change patient behavior? Maybe.
So does it?
Gareth Hollands, Ph.D., of the University of Cambridge, England, and his colleagues conducted a brilliantly timed and welcome systematic review of the literature assessing the impact of communicating DNA-based disease risk estimates on risk-reducing health behaviors and motivation to engage in such behaviors (BMJ. 2016 Mar 15;352:i1102).
Eighteen studies were found reporting on seven behavioral outcomes, including smoking cessation (six studies, n = 2,663), diet (seven studies, n = 1,784), and physical activity (six studies, n = 1,704). The smoking studies related genetic risk for lung or esophageal cancer; the diet studies related risk for diabetes, obesity, cardiovascular disease, hypertension, hyperlipidemia, and Alzheimer’s disease; and the physical activity studies related risks similar to the diet studies.
No evidence was found that communicating DNA-based risk increased smoking cessation or led to positive changes in diet or physical activity. Nor did the investigators find any effects on motivation to change behavior. Although this information is not motivating to patients, no evidence was found suggesting that it is demotivating, either.
If neither behavior nor motivation is modified by DNA-based risk assessment, what is it good for? As the authors pointed out, this information can be used for clinical risk stratification and for refining screening and treatment procedures.
It’s important to note that this puts the responsibility for the required action in response to DNA data in the hands of medical providers – sadly reminding us that the list of ways to motivate patients to change behavior remains frustratingly short.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article.
In the era of individualized (or precision) medicine, we are presented with a unique opportunity to peer into the genetic “maps” of our patients. Through this window, we can envision the self-evident present or predict a possible future.
For the front-line provider, knowing that we could someday have a large amount of these data to deal with can be overwhelming. We may be loath to think that, amongst all the other daily battles we wage with current disease states, we may now need to understand and explain risk for future disease states.
But would we be more likely to use these data if we thought that they would change patient behavior? Maybe.
So does it?
Gareth Hollands, Ph.D., of the University of Cambridge, England, and his colleagues conducted a brilliantly timed and welcome systematic review of the literature assessing the impact of communicating DNA-based disease risk estimates on risk-reducing health behaviors and motivation to engage in such behaviors (BMJ. 2016 Mar 15;352:i1102).
Eighteen studies were found reporting on seven behavioral outcomes, including smoking cessation (six studies, n = 2,663), diet (seven studies, n = 1,784), and physical activity (six studies, n = 1,704). The smoking studies related genetic risk for lung or esophageal cancer; the diet studies related risk for diabetes, obesity, cardiovascular disease, hypertension, hyperlipidemia, and Alzheimer’s disease; and the physical activity studies related risks similar to the diet studies.
No evidence was found that communicating DNA-based risk increased smoking cessation or led to positive changes in diet or physical activity. Nor did the investigators find any effects on motivation to change behavior. Although this information is not motivating to patients, no evidence was found suggesting that it is demotivating, either.
If neither behavior nor motivation is modified by DNA-based risk assessment, what is it good for? As the authors pointed out, this information can be used for clinical risk stratification and for refining screening and treatment procedures.
It’s important to note that this puts the responsibility for the required action in response to DNA data in the hands of medical providers – sadly reminding us that the list of ways to motivate patients to change behavior remains frustratingly short.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article.
In the era of individualized (or precision) medicine, we are presented with a unique opportunity to peer into the genetic “maps” of our patients. Through this window, we can envision the self-evident present or predict a possible future.
For the front-line provider, knowing that we could someday have a large amount of these data to deal with can be overwhelming. We may be loath to think that, amongst all the other daily battles we wage with current disease states, we may now need to understand and explain risk for future disease states.
But would we be more likely to use these data if we thought that they would change patient behavior? Maybe.
So does it?
Gareth Hollands, Ph.D., of the University of Cambridge, England, and his colleagues conducted a brilliantly timed and welcome systematic review of the literature assessing the impact of communicating DNA-based disease risk estimates on risk-reducing health behaviors and motivation to engage in such behaviors (BMJ. 2016 Mar 15;352:i1102).
Eighteen studies were found reporting on seven behavioral outcomes, including smoking cessation (six studies, n = 2,663), diet (seven studies, n = 1,784), and physical activity (six studies, n = 1,704). The smoking studies related genetic risk for lung or esophageal cancer; the diet studies related risk for diabetes, obesity, cardiovascular disease, hypertension, hyperlipidemia, and Alzheimer’s disease; and the physical activity studies related risks similar to the diet studies.
No evidence was found that communicating DNA-based risk increased smoking cessation or led to positive changes in diet or physical activity. Nor did the investigators find any effects on motivation to change behavior. Although this information is not motivating to patients, no evidence was found suggesting that it is demotivating, either.
If neither behavior nor motivation is modified by DNA-based risk assessment, what is it good for? As the authors pointed out, this information can be used for clinical risk stratification and for refining screening and treatment procedures.
It’s important to note that this puts the responsibility for the required action in response to DNA data in the hands of medical providers – sadly reminding us that the list of ways to motivate patients to change behavior remains frustratingly short.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article.
Report from the ABIM GI Specialty Board Meeting
In early March, AGA attended the usually closed-door American Board of Internal Medicine (ABIM) GI Specialty Board meeting. Dr. Suzanne Rose, AGA Institute education and training councillor, along with Lori Marks, Ph.D., AGA vice president for education and training, were there to advocate that ABIM reform maintenance of certification (MOC). Although we are viewing the invitation to attend this meeting as a positive step, we wish we had better news to report. It seems that ABIM has no definitive approach to change the high-stakes examination and that their current efforts are focused on maintaining business as usual.
ABIM acknowledged AGA’s call for ending the every-10-year, closed-book exam. ABIM’s own Assessment 2020 report even suggested consideration of alternative assessment strategies. Despite these appeals, and more from the medical community to end the exam, ABIM pointed to their research proving its validity. AGA leadership is both disappointed and frustrated by ABIM’s intransigence on this point. They are clinging to an exam that flies in the face of adult-learning theory and that is not relevant to practice. Closed-book assessments do not represent the current realities of medicine in the digital age.
Please see AGA’s alternate pathway to recertification, The Gastroenterologist: Accountable Professionalism in Practice or G-APP,which fosters active learning. We support the principles of lifelong learning as evidenced by ongoing CME activities, rather than lifelong testing.
We commit to you that we will keep up the pressure and push on multiple fronts for ABIM to reform MOC, and specifically, to end the MOC exam. We will keep you informed as we move forward.
In early March, AGA attended the usually closed-door American Board of Internal Medicine (ABIM) GI Specialty Board meeting. Dr. Suzanne Rose, AGA Institute education and training councillor, along with Lori Marks, Ph.D., AGA vice president for education and training, were there to advocate that ABIM reform maintenance of certification (MOC). Although we are viewing the invitation to attend this meeting as a positive step, we wish we had better news to report. It seems that ABIM has no definitive approach to change the high-stakes examination and that their current efforts are focused on maintaining business as usual.
ABIM acknowledged AGA’s call for ending the every-10-year, closed-book exam. ABIM’s own Assessment 2020 report even suggested consideration of alternative assessment strategies. Despite these appeals, and more from the medical community to end the exam, ABIM pointed to their research proving its validity. AGA leadership is both disappointed and frustrated by ABIM’s intransigence on this point. They are clinging to an exam that flies in the face of adult-learning theory and that is not relevant to practice. Closed-book assessments do not represent the current realities of medicine in the digital age.
Please see AGA’s alternate pathway to recertification, The Gastroenterologist: Accountable Professionalism in Practice or G-APP,which fosters active learning. We support the principles of lifelong learning as evidenced by ongoing CME activities, rather than lifelong testing.
We commit to you that we will keep up the pressure and push on multiple fronts for ABIM to reform MOC, and specifically, to end the MOC exam. We will keep you informed as we move forward.
In early March, AGA attended the usually closed-door American Board of Internal Medicine (ABIM) GI Specialty Board meeting. Dr. Suzanne Rose, AGA Institute education and training councillor, along with Lori Marks, Ph.D., AGA vice president for education and training, were there to advocate that ABIM reform maintenance of certification (MOC). Although we are viewing the invitation to attend this meeting as a positive step, we wish we had better news to report. It seems that ABIM has no definitive approach to change the high-stakes examination and that their current efforts are focused on maintaining business as usual.
ABIM acknowledged AGA’s call for ending the every-10-year, closed-book exam. ABIM’s own Assessment 2020 report even suggested consideration of alternative assessment strategies. Despite these appeals, and more from the medical community to end the exam, ABIM pointed to their research proving its validity. AGA leadership is both disappointed and frustrated by ABIM’s intransigence on this point. They are clinging to an exam that flies in the face of adult-learning theory and that is not relevant to practice. Closed-book assessments do not represent the current realities of medicine in the digital age.
Please see AGA’s alternate pathway to recertification, The Gastroenterologist: Accountable Professionalism in Practice or G-APP,which fosters active learning. We support the principles of lifelong learning as evidenced by ongoing CME activities, rather than lifelong testing.
We commit to you that we will keep up the pressure and push on multiple fronts for ABIM to reform MOC, and specifically, to end the MOC exam. We will keep you informed as we move forward.
Learn about cancer, colonoscopy, and bundled care in 2016
The 2016 AGA Postgraduate Course: Cognitive and Technical Skills for the Gastroenterologist is set to teach the newest advances in cancer, colonoscopy, and care on May 22, 2016, during Digestive Disease Week® (DDW). John Inadomi, M.D., AGAF, session moderator and AGA clinical research councilor, will address a series of innovations alongside other world-renowned faculty. As gastroenterologists and scientists, you lead the charge in advancing colon care.
Session topics include:
• Advances in Understanding Pathogenesis of Common GI Cancers for the Practicing Gastroenterologist – Implications for Therapy: Richard Boland, M.D., AGAF.
• Colon Cancer Screening: John Inadomi, M.D., AGAF.
• Hereditary and Familial Colorectal Cancer: Lynch, Familial Polyposis and Beyond: Uri Ladabaum, M.D.
• Mastering the Difficult Colonoscopy and Polypectomy – Tricks of the Trade: Douglas Rex, M.D., AGAF, FASGE.
• Quality Using Bundled Care: Rajeev Jain, M.D., AGAF.
Visit pgcourse.gastro.org/pgcourse/home to learn more and register.
The 2016 AGA Postgraduate Course: Cognitive and Technical Skills for the Gastroenterologist is set to teach the newest advances in cancer, colonoscopy, and care on May 22, 2016, during Digestive Disease Week® (DDW). John Inadomi, M.D., AGAF, session moderator and AGA clinical research councilor, will address a series of innovations alongside other world-renowned faculty. As gastroenterologists and scientists, you lead the charge in advancing colon care.
Session topics include:
• Advances in Understanding Pathogenesis of Common GI Cancers for the Practicing Gastroenterologist – Implications for Therapy: Richard Boland, M.D., AGAF.
• Colon Cancer Screening: John Inadomi, M.D., AGAF.
• Hereditary and Familial Colorectal Cancer: Lynch, Familial Polyposis and Beyond: Uri Ladabaum, M.D.
• Mastering the Difficult Colonoscopy and Polypectomy – Tricks of the Trade: Douglas Rex, M.D., AGAF, FASGE.
• Quality Using Bundled Care: Rajeev Jain, M.D., AGAF.
Visit pgcourse.gastro.org/pgcourse/home to learn more and register.
The 2016 AGA Postgraduate Course: Cognitive and Technical Skills for the Gastroenterologist is set to teach the newest advances in cancer, colonoscopy, and care on May 22, 2016, during Digestive Disease Week® (DDW). John Inadomi, M.D., AGAF, session moderator and AGA clinical research councilor, will address a series of innovations alongside other world-renowned faculty. As gastroenterologists and scientists, you lead the charge in advancing colon care.
Session topics include:
• Advances in Understanding Pathogenesis of Common GI Cancers for the Practicing Gastroenterologist – Implications for Therapy: Richard Boland, M.D., AGAF.
• Colon Cancer Screening: John Inadomi, M.D., AGAF.
• Hereditary and Familial Colorectal Cancer: Lynch, Familial Polyposis and Beyond: Uri Ladabaum, M.D.
• Mastering the Difficult Colonoscopy and Polypectomy – Tricks of the Trade: Douglas Rex, M.D., AGAF, FASGE.
• Quality Using Bundled Care: Rajeev Jain, M.D., AGAF.
Visit pgcourse.gastro.org/pgcourse/home to learn more and register.
VIDEO: Medication reconciliation can improve patient outcomes
SAN DIEGO – Prescription medications are a major contributor to unnecessary health care spending.
According to data from the Centers for Medicare & Medicaid Services, retail spending on prescription drugs grew 12.2% to $297.7 billion in 2014, compared with the 2.4% growth in 2013. That’s one key reason why medication reconciliation should be performed at every inpatient and outpatient visit and prior to every hospital discharge, Dr. Aparna Kamath said in a video interview at the annual meeting of the Society of Hospital Medicine. “The focus should be on clear indications for each medication prescribed, substitution of generics when possible, and consideration of an individual patient’s insurance formulary and ability to meet out-of-pocket costs.”
A recent article in JAMA Internal Medicine discussed the practice of “deprescribing” in an effort to reduce the number of prescribed drugs (2015;175[5]:827-34). According to Dr. Kamath of the department of medicine at Duke University Health System, Durham, N.C., who was not involved with the article, deprescribing “means safely narrowing, discontinuing, or withdrawing medications for our patients. It has been shown that deprescribing might actually improve outpatient outcomes by making the medication list safer for our patients and hopefully also improve medication adherence by making them more affordable for our patients.”
The study authors proposed a five-step protocol for deprescribing:
• Ascertain all drugs the patient is currently taking and the reasons for each one.
• Consider overall risk of drug-induced harm in individual patients in determining the required intensity of deprescribing intervention.
• Assess each drug in regard to its current or future benefit potential, compared with current or future harm or burden potential.
• Prioritize drugs for discontinuation that have the lowest benefit-harm ratio and lowest likelihood of adverse withdrawal reactions or disease rebound syndromes.
• Implement a discontinuation regimen and monitor patients closely for improvement in outcomes or onset of adverse effects.
According to Dr. Kamath, other medication reconciliation strategies include referring patients to a social worker to inquire about drug assistance programs; following up with the patient’s primary care or prescribing physician; partnering with pharmacists; and educating patients about variance in prescription drug prices. “I think it’s important to inform the patients that these drugs are priced differently in different pharmacies,” she said. “According to Consumer Reports, we should ask the patient to shop around, maybe call the medication pharmacies in their local area to find out where they can find the drugs at a most affordable price. We can also advise our patients to ask for discounts or coupons, and check for monthly price changes,” Dr. Kamath said. She recommended the following websites, which allow patients to compare costs and/or inquire about discounts:
• https://www.rxpricequotes.com.
• https://www.blinkhealth.com.
Dr. Kamath reported having no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – Prescription medications are a major contributor to unnecessary health care spending.
According to data from the Centers for Medicare & Medicaid Services, retail spending on prescription drugs grew 12.2% to $297.7 billion in 2014, compared with the 2.4% growth in 2013. That’s one key reason why medication reconciliation should be performed at every inpatient and outpatient visit and prior to every hospital discharge, Dr. Aparna Kamath said in a video interview at the annual meeting of the Society of Hospital Medicine. “The focus should be on clear indications for each medication prescribed, substitution of generics when possible, and consideration of an individual patient’s insurance formulary and ability to meet out-of-pocket costs.”
A recent article in JAMA Internal Medicine discussed the practice of “deprescribing” in an effort to reduce the number of prescribed drugs (2015;175[5]:827-34). According to Dr. Kamath of the department of medicine at Duke University Health System, Durham, N.C., who was not involved with the article, deprescribing “means safely narrowing, discontinuing, or withdrawing medications for our patients. It has been shown that deprescribing might actually improve outpatient outcomes by making the medication list safer for our patients and hopefully also improve medication adherence by making them more affordable for our patients.”
The study authors proposed a five-step protocol for deprescribing:
• Ascertain all drugs the patient is currently taking and the reasons for each one.
• Consider overall risk of drug-induced harm in individual patients in determining the required intensity of deprescribing intervention.
• Assess each drug in regard to its current or future benefit potential, compared with current or future harm or burden potential.
• Prioritize drugs for discontinuation that have the lowest benefit-harm ratio and lowest likelihood of adverse withdrawal reactions or disease rebound syndromes.
• Implement a discontinuation regimen and monitor patients closely for improvement in outcomes or onset of adverse effects.
According to Dr. Kamath, other medication reconciliation strategies include referring patients to a social worker to inquire about drug assistance programs; following up with the patient’s primary care or prescribing physician; partnering with pharmacists; and educating patients about variance in prescription drug prices. “I think it’s important to inform the patients that these drugs are priced differently in different pharmacies,” she said. “According to Consumer Reports, we should ask the patient to shop around, maybe call the medication pharmacies in their local area to find out where they can find the drugs at a most affordable price. We can also advise our patients to ask for discounts or coupons, and check for monthly price changes,” Dr. Kamath said. She recommended the following websites, which allow patients to compare costs and/or inquire about discounts:
• https://www.rxpricequotes.com.
• https://www.blinkhealth.com.
Dr. Kamath reported having no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – Prescription medications are a major contributor to unnecessary health care spending.
According to data from the Centers for Medicare & Medicaid Services, retail spending on prescription drugs grew 12.2% to $297.7 billion in 2014, compared with the 2.4% growth in 2013. That’s one key reason why medication reconciliation should be performed at every inpatient and outpatient visit and prior to every hospital discharge, Dr. Aparna Kamath said in a video interview at the annual meeting of the Society of Hospital Medicine. “The focus should be on clear indications for each medication prescribed, substitution of generics when possible, and consideration of an individual patient’s insurance formulary and ability to meet out-of-pocket costs.”
A recent article in JAMA Internal Medicine discussed the practice of “deprescribing” in an effort to reduce the number of prescribed drugs (2015;175[5]:827-34). According to Dr. Kamath of the department of medicine at Duke University Health System, Durham, N.C., who was not involved with the article, deprescribing “means safely narrowing, discontinuing, or withdrawing medications for our patients. It has been shown that deprescribing might actually improve outpatient outcomes by making the medication list safer for our patients and hopefully also improve medication adherence by making them more affordable for our patients.”
The study authors proposed a five-step protocol for deprescribing:
• Ascertain all drugs the patient is currently taking and the reasons for each one.
• Consider overall risk of drug-induced harm in individual patients in determining the required intensity of deprescribing intervention.
• Assess each drug in regard to its current or future benefit potential, compared with current or future harm or burden potential.
• Prioritize drugs for discontinuation that have the lowest benefit-harm ratio and lowest likelihood of adverse withdrawal reactions or disease rebound syndromes.
• Implement a discontinuation regimen and monitor patients closely for improvement in outcomes or onset of adverse effects.
According to Dr. Kamath, other medication reconciliation strategies include referring patients to a social worker to inquire about drug assistance programs; following up with the patient’s primary care or prescribing physician; partnering with pharmacists; and educating patients about variance in prescription drug prices. “I think it’s important to inform the patients that these drugs are priced differently in different pharmacies,” she said. “According to Consumer Reports, we should ask the patient to shop around, maybe call the medication pharmacies in their local area to find out where they can find the drugs at a most affordable price. We can also advise our patients to ask for discounts or coupons, and check for monthly price changes,” Dr. Kamath said. She recommended the following websites, which allow patients to compare costs and/or inquire about discounts:
• https://www.rxpricequotes.com.
• https://www.blinkhealth.com.
Dr. Kamath reported having no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS AT HOSPITAL MEDICINE 16
Registration now open for Freston 2016
Connect and engage with your fellow researchers and clinicians at the 2016 James W. Freston Conference: Intestinal Metaplasia in the Esophagus and Stomach – Origins, Differences, Similarities and Significance. The conference will take place Aug. 19-21 in Chicago, Ill.
By attending, you’ll be able to explore novel ideas that may lead to enhanced therapies and management strategies for the prevention of intestinal metaplasia. Faculty will guide you through an interactive program that provides greater insight on the clinical and histological issues of intestinal metaplasia, and the role of inflammation in the development of intestinal metaplasia.
A consensus will take place at the end of the conference, which will attempt to develop standard terminology, identify potential preventive strategies, and suggest future directions for research.
Don’t miss this unique and intimate forum, bringing together more than 100 attendees from around the world. For program and registration details, visit the conference Web page.
Connect and engage with your fellow researchers and clinicians at the 2016 James W. Freston Conference: Intestinal Metaplasia in the Esophagus and Stomach – Origins, Differences, Similarities and Significance. The conference will take place Aug. 19-21 in Chicago, Ill.
By attending, you’ll be able to explore novel ideas that may lead to enhanced therapies and management strategies for the prevention of intestinal metaplasia. Faculty will guide you through an interactive program that provides greater insight on the clinical and histological issues of intestinal metaplasia, and the role of inflammation in the development of intestinal metaplasia.
A consensus will take place at the end of the conference, which will attempt to develop standard terminology, identify potential preventive strategies, and suggest future directions for research.
Don’t miss this unique and intimate forum, bringing together more than 100 attendees from around the world. For program and registration details, visit the conference Web page.
Connect and engage with your fellow researchers and clinicians at the 2016 James W. Freston Conference: Intestinal Metaplasia in the Esophagus and Stomach – Origins, Differences, Similarities and Significance. The conference will take place Aug. 19-21 in Chicago, Ill.
By attending, you’ll be able to explore novel ideas that may lead to enhanced therapies and management strategies for the prevention of intestinal metaplasia. Faculty will guide you through an interactive program that provides greater insight on the clinical and histological issues of intestinal metaplasia, and the role of inflammation in the development of intestinal metaplasia.
A consensus will take place at the end of the conference, which will attempt to develop standard terminology, identify potential preventive strategies, and suggest future directions for research.
Don’t miss this unique and intimate forum, bringing together more than 100 attendees from around the world. For program and registration details, visit the conference Web page.
Idarucizumab may reverse dabigatran anticoagulation in intracranial hemorrhage
LOS ANGELES – It only took a few minutes for idarucizumab to normalize blood-clotting parameters in 18 patients with dabigatran-associated intracranial hemorrhages, according to interim results from an ongoing phase III trial presented at the International Stroke Conference.
“When I put patients on [dabigatran], they always ask me what happens if they bleed or need surgery. … Until now, I haven’t been able to tell them with any confidence that I have a way of reversing it. Now I think I can. ... It makes a big difference” in their comfort, said lead investigator Dr. Richard Bernstein, a Northwestern University neurology professor and director of the stroke program at Northwestern Memorial Hospital, in Chicago.
“We would love to know if hematoma expansion was limited [and outcomes improved] by giving this reversal agent,” but the study so far is too small. “We hope to have a larger cohort of brain hemorrhage patients to answer these questions,” he said.
Approved in October 2015, idarucizumab (Praxbind) was fast tracked by the Food and Drug Administration to reverse the blockbuster atrial fibrillation anticoagulant dabigatran (Pradaxa); the labeling for idarucizumab doesn’t mention intracranial hemorrhage patients specifically. Boehringer Ingelheim makes both drugs, and funded Dr. Bernstein’s work.
Eleven of the 18 patients were men, and the average age in the study was about 80 years. The patients had either subdural hematomas or bleeding into the brain itself. They were culled from the 90 subjects analyzed so far in the idarucizumab trial, dubbed RE-VERSE AD (Reversal Effects of Idarucizumab in Patients on Active Dabigatran).
The team followed label dosing: 5 g total given as two separate 2.5-g infusions. Blood samples were taken in between to check how well idarucizumab worked. The whole process took no more than 15 minutes.
The first 2.5 g completely reversed dabigatran in all 18 patients, based on their dilute thrombin or ecarin clotting times. Patients “remained reversed out to 12 hours, and all but one out to 24 hours,” Dr. Bernstein said at the conference, sponsored by the American Heart Association.
Idarucizumab is a monoclonal antibody fragment that binds dabigatran more powerfully than dabigatran binds thrombin. In vitro studies have found no prothrombotic effects. “It has no endogenous target, so the drug has no effect on any other clotting factors that we can tell. We did have, I think, five thrombotic events in our cohort, most of them many days after the dabigatran was reversed. It may have just been a reversion to [patient] clotting risks,” he said.
When – or if – to restart dabigatran “is a clinical question.” If bleeding is controlled or patients are stable after surgery, you can go back on the next day,” he said.
Idarucizumab’s labeling notes that 5% or more of patients developed hypokalemia, delirium, constipation, pyrexia, and pneumonia. It wasn’t clear these events were drug related. Patients had dabigatran reversed either for serious bleeding or emergency surgery.
Dr. Bernstein is a speaker and adviser for Boehringer Ingelheim, and reported honoraria from the company.
LOS ANGELES – It only took a few minutes for idarucizumab to normalize blood-clotting parameters in 18 patients with dabigatran-associated intracranial hemorrhages, according to interim results from an ongoing phase III trial presented at the International Stroke Conference.
“When I put patients on [dabigatran], they always ask me what happens if they bleed or need surgery. … Until now, I haven’t been able to tell them with any confidence that I have a way of reversing it. Now I think I can. ... It makes a big difference” in their comfort, said lead investigator Dr. Richard Bernstein, a Northwestern University neurology professor and director of the stroke program at Northwestern Memorial Hospital, in Chicago.
“We would love to know if hematoma expansion was limited [and outcomes improved] by giving this reversal agent,” but the study so far is too small. “We hope to have a larger cohort of brain hemorrhage patients to answer these questions,” he said.
Approved in October 2015, idarucizumab (Praxbind) was fast tracked by the Food and Drug Administration to reverse the blockbuster atrial fibrillation anticoagulant dabigatran (Pradaxa); the labeling for idarucizumab doesn’t mention intracranial hemorrhage patients specifically. Boehringer Ingelheim makes both drugs, and funded Dr. Bernstein’s work.
Eleven of the 18 patients were men, and the average age in the study was about 80 years. The patients had either subdural hematomas or bleeding into the brain itself. They were culled from the 90 subjects analyzed so far in the idarucizumab trial, dubbed RE-VERSE AD (Reversal Effects of Idarucizumab in Patients on Active Dabigatran).
The team followed label dosing: 5 g total given as two separate 2.5-g infusions. Blood samples were taken in between to check how well idarucizumab worked. The whole process took no more than 15 minutes.
The first 2.5 g completely reversed dabigatran in all 18 patients, based on their dilute thrombin or ecarin clotting times. Patients “remained reversed out to 12 hours, and all but one out to 24 hours,” Dr. Bernstein said at the conference, sponsored by the American Heart Association.
Idarucizumab is a monoclonal antibody fragment that binds dabigatran more powerfully than dabigatran binds thrombin. In vitro studies have found no prothrombotic effects. “It has no endogenous target, so the drug has no effect on any other clotting factors that we can tell. We did have, I think, five thrombotic events in our cohort, most of them many days after the dabigatran was reversed. It may have just been a reversion to [patient] clotting risks,” he said.
When – or if – to restart dabigatran “is a clinical question.” If bleeding is controlled or patients are stable after surgery, you can go back on the next day,” he said.
Idarucizumab’s labeling notes that 5% or more of patients developed hypokalemia, delirium, constipation, pyrexia, and pneumonia. It wasn’t clear these events were drug related. Patients had dabigatran reversed either for serious bleeding or emergency surgery.
Dr. Bernstein is a speaker and adviser for Boehringer Ingelheim, and reported honoraria from the company.
LOS ANGELES – It only took a few minutes for idarucizumab to normalize blood-clotting parameters in 18 patients with dabigatran-associated intracranial hemorrhages, according to interim results from an ongoing phase III trial presented at the International Stroke Conference.
“When I put patients on [dabigatran], they always ask me what happens if they bleed or need surgery. … Until now, I haven’t been able to tell them with any confidence that I have a way of reversing it. Now I think I can. ... It makes a big difference” in their comfort, said lead investigator Dr. Richard Bernstein, a Northwestern University neurology professor and director of the stroke program at Northwestern Memorial Hospital, in Chicago.
“We would love to know if hematoma expansion was limited [and outcomes improved] by giving this reversal agent,” but the study so far is too small. “We hope to have a larger cohort of brain hemorrhage patients to answer these questions,” he said.
Approved in October 2015, idarucizumab (Praxbind) was fast tracked by the Food and Drug Administration to reverse the blockbuster atrial fibrillation anticoagulant dabigatran (Pradaxa); the labeling for idarucizumab doesn’t mention intracranial hemorrhage patients specifically. Boehringer Ingelheim makes both drugs, and funded Dr. Bernstein’s work.
Eleven of the 18 patients were men, and the average age in the study was about 80 years. The patients had either subdural hematomas or bleeding into the brain itself. They were culled from the 90 subjects analyzed so far in the idarucizumab trial, dubbed RE-VERSE AD (Reversal Effects of Idarucizumab in Patients on Active Dabigatran).
The team followed label dosing: 5 g total given as two separate 2.5-g infusions. Blood samples were taken in between to check how well idarucizumab worked. The whole process took no more than 15 minutes.
The first 2.5 g completely reversed dabigatran in all 18 patients, based on their dilute thrombin or ecarin clotting times. Patients “remained reversed out to 12 hours, and all but one out to 24 hours,” Dr. Bernstein said at the conference, sponsored by the American Heart Association.
Idarucizumab is a monoclonal antibody fragment that binds dabigatran more powerfully than dabigatran binds thrombin. In vitro studies have found no prothrombotic effects. “It has no endogenous target, so the drug has no effect on any other clotting factors that we can tell. We did have, I think, five thrombotic events in our cohort, most of them many days after the dabigatran was reversed. It may have just been a reversion to [patient] clotting risks,” he said.
When – or if – to restart dabigatran “is a clinical question.” If bleeding is controlled or patients are stable after surgery, you can go back on the next day,” he said.
Idarucizumab’s labeling notes that 5% or more of patients developed hypokalemia, delirium, constipation, pyrexia, and pneumonia. It wasn’t clear these events were drug related. Patients had dabigatran reversed either for serious bleeding or emergency surgery.
Dr. Bernstein is a speaker and adviser for Boehringer Ingelheim, and reported honoraria from the company.
AT THE INTERNATIONAL STROKE CONFERENCE
Key clinical point: An ongoing investigation suggests that idarucizumab can reliably stop intracranial hemorrhage associated with dabigatran anticoagulation.
Major finding: It took only a few minutes for idarucizumab to normalize blood-clotting parameters in 18 patients with dabigatran intracranial hemorrhage.
Source: Interim results from an ongoing phase III trial.
Disclosures: The work was funded by Boehringer Ingelheim, maker of both dabigatran and idarucizumab. The lead investigator is a speaker and adviser for Boehringer, and reported honoraria from the company.
A gift in your will: Getting started
A simple, flexible, and versatile way to ensure The AGA Research Foundation can continue our work for years to come is a gift in your will or living trust, known as a charitable bequest. To make a charitable bequest, you need a current will or living trust.
Your gift can be made as a percentage of your estate. Or you can make a specific bequest by contributing a certain amount of cash, securities, or property. After your lifetime, the AGA Research Foundation receives your gift.
We hope you’ll consider including a gift to the AGA Research Foundation in your will or living trust. It’s simple – just a few sentences in your will or trust are all that is needed. The official bequest language for the AGA Research Foundation is: “I, [name], of [city, state, ZIP], give, devise, and bequeath to the AGA Research Foundation [written amount or percentage of the estate or description of property] for its unrestricted use and purpose.”
When planning a future gift, it’s sometimes difficult to determine what size donation will make sense. Emergencies happen, and you need to make sure your family is financially taken care of first. Including a bequest of a percentage of your estate ensures that your gift will remain proportionate, no matter how your estate’s value fluctuates over the years.
Whether you would like to put your donation to work today or benefit us after your lifetime, you can find a charitable plan that lets you provide for your family and support the AGA Research Foundation.
Please contact us for more information at foundation@gastro.org or visit http://gastro.planmylegacy.org.
A simple, flexible, and versatile way to ensure The AGA Research Foundation can continue our work for years to come is a gift in your will or living trust, known as a charitable bequest. To make a charitable bequest, you need a current will or living trust.
Your gift can be made as a percentage of your estate. Or you can make a specific bequest by contributing a certain amount of cash, securities, or property. After your lifetime, the AGA Research Foundation receives your gift.
We hope you’ll consider including a gift to the AGA Research Foundation in your will or living trust. It’s simple – just a few sentences in your will or trust are all that is needed. The official bequest language for the AGA Research Foundation is: “I, [name], of [city, state, ZIP], give, devise, and bequeath to the AGA Research Foundation [written amount or percentage of the estate or description of property] for its unrestricted use and purpose.”
When planning a future gift, it’s sometimes difficult to determine what size donation will make sense. Emergencies happen, and you need to make sure your family is financially taken care of first. Including a bequest of a percentage of your estate ensures that your gift will remain proportionate, no matter how your estate’s value fluctuates over the years.
Whether you would like to put your donation to work today or benefit us after your lifetime, you can find a charitable plan that lets you provide for your family and support the AGA Research Foundation.
Please contact us for more information at foundation@gastro.org or visit http://gastro.planmylegacy.org.
A simple, flexible, and versatile way to ensure The AGA Research Foundation can continue our work for years to come is a gift in your will or living trust, known as a charitable bequest. To make a charitable bequest, you need a current will or living trust.
Your gift can be made as a percentage of your estate. Or you can make a specific bequest by contributing a certain amount of cash, securities, or property. After your lifetime, the AGA Research Foundation receives your gift.
We hope you’ll consider including a gift to the AGA Research Foundation in your will or living trust. It’s simple – just a few sentences in your will or trust are all that is needed. The official bequest language for the AGA Research Foundation is: “I, [name], of [city, state, ZIP], give, devise, and bequeath to the AGA Research Foundation [written amount or percentage of the estate or description of property] for its unrestricted use and purpose.”
When planning a future gift, it’s sometimes difficult to determine what size donation will make sense. Emergencies happen, and you need to make sure your family is financially taken care of first. Including a bequest of a percentage of your estate ensures that your gift will remain proportionate, no matter how your estate’s value fluctuates over the years.
Whether you would like to put your donation to work today or benefit us after your lifetime, you can find a charitable plan that lets you provide for your family and support the AGA Research Foundation.
Please contact us for more information at foundation@gastro.org or visit http://gastro.planmylegacy.org.
Perinatal depression screening: New recommendations and challenges
It was almost a year ago that the American College of Obstetricians and Gynecologists came out unequivocally in favor of universal screening for perinatal depression.
In the revised policy statement from ACOG’s Committee on Obstetric Practice, the college recommended that physicians screen women for depression and anxiety symptoms at least once during the perinatal period using a standard, validated tool. ACOG also noted that screening must be coupled with appropriate follow-up and that clinical staff must be prepared to start therapy or refer patients to treatment (Obstet. Gynecol. 2015;125:1268-71).
This move toward routine screening was intuitive given the prevalence of perinatal mood and anxiety disorders.
Fast forward to January 2016 and the U.S. Preventive Services Task Force final recommendation calling for screening all adults for depression, including the at-risk populations of pregnant and postpartum women. Much like the ACOG guidelines, the USPSTF recommendations call for adequate systems to ensure treatment and follow-up (JAMA. 2016 Jan. 26;315[4]:380-7).
These recommendations, although timely, derive from relatively sparse data on the actual effectiveness of perinatal screening. Although the move toward screening is welcome and simply commonsense, it is concerning that there has been very little systematic study of the effectiveness of screening for such a prevalent and impactful illness. At the end of the day, the question remains: Will screening for perinatal depression in obstetric and possibly pediatric settings lead to improved outcomes for patients and families?
We’re screening, but will it make a difference?
As more U.S. states, along with other countries around the world, have begun routine screening of women in the perinatal period, it’s become clear that screening itself is easy to do. What has yet to be adequately demonstrated is how screening moves us toward getting women into treatment and ultimately toward getting women well.
New Jersey and Illinois are good examples of states that should be applauded for recognizing early on how important it is to identify women with perinatal depression. But even in these early-adopter states, the actual implementation of referral systems has been lacking.
Here in Massachusetts, we have a state-funded program designed to teach local women’s health providers – including ob.gyns. – about diagnosing perinatal depression. The MCPAP (Massachusetts Child Psychiatry Access Project) for Moms program also offers resources for consultation and referral. The program is fairly new, so it’s still unclear whether ob.gyns. and primary care physicians will accept the role of de facto mental health treaters, as well as whether the women who are identified through screening will go on to recover acutely and, more importantly, over the long term.
These experiences among the states highlight how great a challenge it is to go from screening to positive health outcomes for women.
Downstream difficulties
A lack of evidence isn’t the only problem. A recent editorial in the Lancet raised the concern that the currently available screening tests are not suitable for clinical practice. The suggestion read to some like heresy.
The Edinburgh Postnatal Depression Scale, which is the most commonly used screening instrument, has a positive predictive value of detecting major depressive disorder of 47%-64%, according to the editorial, making it prone to delivering false positives (doi: 10.1016/S0140-6736[16]00265-8).
“This situation is potentially dangerous,” the Lancet editorial noted, since results of qualitative studies “suggest that women are extremely concerned about depression screening, about the stigma associated with a diagnosis of depression, and that a positive result might lead to an automatic social service referral, and potentially removal of their baby.”
A recent article, published in the New York Times, raises an additional concern about what a depression diagnosis could mean for insurability. The article highlights the experience of a woman whose diagnosis of postpartum depression is creating difficulties for her in getting life insurance. The point is underscored that it is perfectly legal for life and disability insurers to charge more to patients with a diagnosis of mental illness or to deny coverage outright.
No going back
The whole issue of perinatal depression screening has opened a Pandora’s box, and that is a good thing. The conversation is long overdue in America. It is time for greater national awareness and focus on a disease that is as prevalent as perinatal depression and as disabling for women and their families.
The focus up to this point has been on perinatal depression screening, but we’re about to see a shift toward building the community infrastructure that will be critical for managing patients, including those women who have previously been marginalized and have had very poor access to care.
Widespread screening and treatment will also require a level of cooperation between advocacy groups and providers who are multidisciplinary in their approach, taking advantage of both pharmacologic and nonpharmacologic approaches. A model of crossdisciplinary collaboration will include, for example, providers from psychiatrists to therapists to doulas to social workers to clinicians who focus on mother-infant interaction. It is a long list and models for such collaboration are somewhat lacking.
One good example of a pilot effort for such a collaboration is the Massachusetts Postpartum Depression Commission, which includes a full spectrum of participants from doulas, social workers, and perinatal psychiatrists to lay people. The partnerships and the networking that’s going on across disciplines is absolutely new and is going to be essential if we’re going to manage an issue as large as the treatment of perinatal depression.
The enhanced awareness of the need to screen for, identify, and treat postpartum depression will also lead to better tools with greater specificity, perhaps using new technologies for better identification and treatment, everything from telemedicine to smartphone applications.
There will certainly be growing pains as we gather evidence, refine our screening instruments, and build referral systems, but I don’t see this as a reason not to identify illness in this vulnerable population. Rather, it is a charge to the field that there is work to be done.
Dr. Cohen is the director of the Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications.
It was almost a year ago that the American College of Obstetricians and Gynecologists came out unequivocally in favor of universal screening for perinatal depression.
In the revised policy statement from ACOG’s Committee on Obstetric Practice, the college recommended that physicians screen women for depression and anxiety symptoms at least once during the perinatal period using a standard, validated tool. ACOG also noted that screening must be coupled with appropriate follow-up and that clinical staff must be prepared to start therapy or refer patients to treatment (Obstet. Gynecol. 2015;125:1268-71).
This move toward routine screening was intuitive given the prevalence of perinatal mood and anxiety disorders.
Fast forward to January 2016 and the U.S. Preventive Services Task Force final recommendation calling for screening all adults for depression, including the at-risk populations of pregnant and postpartum women. Much like the ACOG guidelines, the USPSTF recommendations call for adequate systems to ensure treatment and follow-up (JAMA. 2016 Jan. 26;315[4]:380-7).
These recommendations, although timely, derive from relatively sparse data on the actual effectiveness of perinatal screening. Although the move toward screening is welcome and simply commonsense, it is concerning that there has been very little systematic study of the effectiveness of screening for such a prevalent and impactful illness. At the end of the day, the question remains: Will screening for perinatal depression in obstetric and possibly pediatric settings lead to improved outcomes for patients and families?
We’re screening, but will it make a difference?
As more U.S. states, along with other countries around the world, have begun routine screening of women in the perinatal period, it’s become clear that screening itself is easy to do. What has yet to be adequately demonstrated is how screening moves us toward getting women into treatment and ultimately toward getting women well.
New Jersey and Illinois are good examples of states that should be applauded for recognizing early on how important it is to identify women with perinatal depression. But even in these early-adopter states, the actual implementation of referral systems has been lacking.
Here in Massachusetts, we have a state-funded program designed to teach local women’s health providers – including ob.gyns. – about diagnosing perinatal depression. The MCPAP (Massachusetts Child Psychiatry Access Project) for Moms program also offers resources for consultation and referral. The program is fairly new, so it’s still unclear whether ob.gyns. and primary care physicians will accept the role of de facto mental health treaters, as well as whether the women who are identified through screening will go on to recover acutely and, more importantly, over the long term.
These experiences among the states highlight how great a challenge it is to go from screening to positive health outcomes for women.
Downstream difficulties
A lack of evidence isn’t the only problem. A recent editorial in the Lancet raised the concern that the currently available screening tests are not suitable for clinical practice. The suggestion read to some like heresy.
The Edinburgh Postnatal Depression Scale, which is the most commonly used screening instrument, has a positive predictive value of detecting major depressive disorder of 47%-64%, according to the editorial, making it prone to delivering false positives (doi: 10.1016/S0140-6736[16]00265-8).
“This situation is potentially dangerous,” the Lancet editorial noted, since results of qualitative studies “suggest that women are extremely concerned about depression screening, about the stigma associated with a diagnosis of depression, and that a positive result might lead to an automatic social service referral, and potentially removal of their baby.”
A recent article, published in the New York Times, raises an additional concern about what a depression diagnosis could mean for insurability. The article highlights the experience of a woman whose diagnosis of postpartum depression is creating difficulties for her in getting life insurance. The point is underscored that it is perfectly legal for life and disability insurers to charge more to patients with a diagnosis of mental illness or to deny coverage outright.
No going back
The whole issue of perinatal depression screening has opened a Pandora’s box, and that is a good thing. The conversation is long overdue in America. It is time for greater national awareness and focus on a disease that is as prevalent as perinatal depression and as disabling for women and their families.
The focus up to this point has been on perinatal depression screening, but we’re about to see a shift toward building the community infrastructure that will be critical for managing patients, including those women who have previously been marginalized and have had very poor access to care.
Widespread screening and treatment will also require a level of cooperation between advocacy groups and providers who are multidisciplinary in their approach, taking advantage of both pharmacologic and nonpharmacologic approaches. A model of crossdisciplinary collaboration will include, for example, providers from psychiatrists to therapists to doulas to social workers to clinicians who focus on mother-infant interaction. It is a long list and models for such collaboration are somewhat lacking.
One good example of a pilot effort for such a collaboration is the Massachusetts Postpartum Depression Commission, which includes a full spectrum of participants from doulas, social workers, and perinatal psychiatrists to lay people. The partnerships and the networking that’s going on across disciplines is absolutely new and is going to be essential if we’re going to manage an issue as large as the treatment of perinatal depression.
The enhanced awareness of the need to screen for, identify, and treat postpartum depression will also lead to better tools with greater specificity, perhaps using new technologies for better identification and treatment, everything from telemedicine to smartphone applications.
There will certainly be growing pains as we gather evidence, refine our screening instruments, and build referral systems, but I don’t see this as a reason not to identify illness in this vulnerable population. Rather, it is a charge to the field that there is work to be done.
Dr. Cohen is the director of the Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications.
It was almost a year ago that the American College of Obstetricians and Gynecologists came out unequivocally in favor of universal screening for perinatal depression.
In the revised policy statement from ACOG’s Committee on Obstetric Practice, the college recommended that physicians screen women for depression and anxiety symptoms at least once during the perinatal period using a standard, validated tool. ACOG also noted that screening must be coupled with appropriate follow-up and that clinical staff must be prepared to start therapy or refer patients to treatment (Obstet. Gynecol. 2015;125:1268-71).
This move toward routine screening was intuitive given the prevalence of perinatal mood and anxiety disorders.
Fast forward to January 2016 and the U.S. Preventive Services Task Force final recommendation calling for screening all adults for depression, including the at-risk populations of pregnant and postpartum women. Much like the ACOG guidelines, the USPSTF recommendations call for adequate systems to ensure treatment and follow-up (JAMA. 2016 Jan. 26;315[4]:380-7).
These recommendations, although timely, derive from relatively sparse data on the actual effectiveness of perinatal screening. Although the move toward screening is welcome and simply commonsense, it is concerning that there has been very little systematic study of the effectiveness of screening for such a prevalent and impactful illness. At the end of the day, the question remains: Will screening for perinatal depression in obstetric and possibly pediatric settings lead to improved outcomes for patients and families?
We’re screening, but will it make a difference?
As more U.S. states, along with other countries around the world, have begun routine screening of women in the perinatal period, it’s become clear that screening itself is easy to do. What has yet to be adequately demonstrated is how screening moves us toward getting women into treatment and ultimately toward getting women well.
New Jersey and Illinois are good examples of states that should be applauded for recognizing early on how important it is to identify women with perinatal depression. But even in these early-adopter states, the actual implementation of referral systems has been lacking.
Here in Massachusetts, we have a state-funded program designed to teach local women’s health providers – including ob.gyns. – about diagnosing perinatal depression. The MCPAP (Massachusetts Child Psychiatry Access Project) for Moms program also offers resources for consultation and referral. The program is fairly new, so it’s still unclear whether ob.gyns. and primary care physicians will accept the role of de facto mental health treaters, as well as whether the women who are identified through screening will go on to recover acutely and, more importantly, over the long term.
These experiences among the states highlight how great a challenge it is to go from screening to positive health outcomes for women.
Downstream difficulties
A lack of evidence isn’t the only problem. A recent editorial in the Lancet raised the concern that the currently available screening tests are not suitable for clinical practice. The suggestion read to some like heresy.
The Edinburgh Postnatal Depression Scale, which is the most commonly used screening instrument, has a positive predictive value of detecting major depressive disorder of 47%-64%, according to the editorial, making it prone to delivering false positives (doi: 10.1016/S0140-6736[16]00265-8).
“This situation is potentially dangerous,” the Lancet editorial noted, since results of qualitative studies “suggest that women are extremely concerned about depression screening, about the stigma associated with a diagnosis of depression, and that a positive result might lead to an automatic social service referral, and potentially removal of their baby.”
A recent article, published in the New York Times, raises an additional concern about what a depression diagnosis could mean for insurability. The article highlights the experience of a woman whose diagnosis of postpartum depression is creating difficulties for her in getting life insurance. The point is underscored that it is perfectly legal for life and disability insurers to charge more to patients with a diagnosis of mental illness or to deny coverage outright.
No going back
The whole issue of perinatal depression screening has opened a Pandora’s box, and that is a good thing. The conversation is long overdue in America. It is time for greater national awareness and focus on a disease that is as prevalent as perinatal depression and as disabling for women and their families.
The focus up to this point has been on perinatal depression screening, but we’re about to see a shift toward building the community infrastructure that will be critical for managing patients, including those women who have previously been marginalized and have had very poor access to care.
Widespread screening and treatment will also require a level of cooperation between advocacy groups and providers who are multidisciplinary in their approach, taking advantage of both pharmacologic and nonpharmacologic approaches. A model of crossdisciplinary collaboration will include, for example, providers from psychiatrists to therapists to doulas to social workers to clinicians who focus on mother-infant interaction. It is a long list and models for such collaboration are somewhat lacking.
One good example of a pilot effort for such a collaboration is the Massachusetts Postpartum Depression Commission, which includes a full spectrum of participants from doulas, social workers, and perinatal psychiatrists to lay people. The partnerships and the networking that’s going on across disciplines is absolutely new and is going to be essential if we’re going to manage an issue as large as the treatment of perinatal depression.
The enhanced awareness of the need to screen for, identify, and treat postpartum depression will also lead to better tools with greater specificity, perhaps using new technologies for better identification and treatment, everything from telemedicine to smartphone applications.
There will certainly be growing pains as we gather evidence, refine our screening instruments, and build referral systems, but I don’t see this as a reason not to identify illness in this vulnerable population. Rather, it is a charge to the field that there is work to be done.
Dr. Cohen is the director of the Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications.
The Pimple That Wasn’t
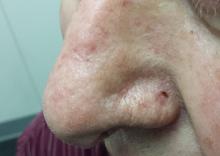
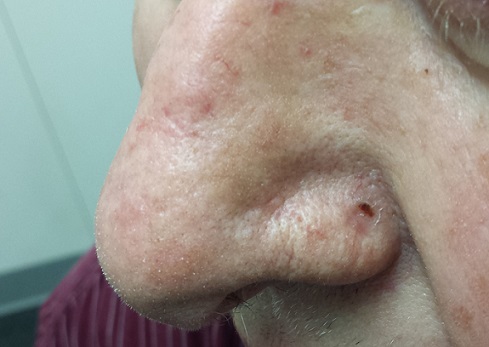
At first, this 70-year-old man thought the lesion on his nose was a pimple, although it seemed oddly resilient. When he attempted to pop it, nothing came out.
When it not only failed to heal but also grew over a six-month period, he finally decided to consult his primary care provider (PCP). The PCP told him not to worry about it but offered to refer him to dermatology—mostly because the patient’s wife was concerned.
The patient gives a history of extensive sun exposure in childhood and young adulthood. In the 1960s, he was drafted into the military and served two tours of duty in the jungles of Vietnam, where he acquired an almost permanent sunburn.
EXAMINATION
The patient is quite fair, with modest facial sun damage manifesting as telangiectasias and solar elastosis. The lesion in question is a glassy, scarlike, concave papule with a scab at one end. It is located in the left alar groove and measures about 5 mm in diameter.
Under local anesthesia, the lesion is biopsied and sent to pathology.
What is the diagnosis?
DISCUSSION
The pathology report indicated basal cell carcinoma (BCC). Given the location, the patient was referred to a Mohs surgeon, who needed two passes to clear the site of cancer. Closure of the resulting 2.5-cm defect required a skin graft, using the postauricular neck as the donor site. Afterward, the PCP, the patient, and his wife all expressed astonishment that such a tiny lesion had required so complex a procedure.
BCC is, by far, the most common of the sun-caused skin cancers, with more than a million new cases diagnosed in the US each year. While rarely dangerous, BCCs can be highly destructive of local structures (eyelids, noses, ears, faces) if left untreated for extended periods. Virtually all BCCs grow relentlessly, but some are more aggressive than others—a trait confirmed not only by the behavior of the cancer, but also, in some cases, by characteristic histologic findings.
Location has a major impact on the behavior of a given BCC. The posterior portion of the nose (alar groove) is particularly prone to harbor more extensive and aggressive BCCs.
Given the additional cosmetic implications of removing cancers from this area, these often require Mohs surgery to ensure complete, mircroscopically controlled removal of the cancer, as well as closure of the wound in a cosmetically acceptable manner.
Some aggressive types of BCC will require radiation therapy even after Mohs or conventional surgical removal. Fortunately, deaths from BCCs usually result from local invasion and not from metastasis, which is extremely rare.
For providers, the key is a low threshold for the necessity of biopsy. Simple shave biopsy is usually adequate. When PCPs are uncomfortable performing such biopsies, expedited referral to dermatology is called for.
TAKE-HOME LEARNING POINTS
• BCC is the most common of the sun-caused skin cancers, with more than a million new cases diagnosed every year in the US.
• While they rarely metastasize, BCCs can result in extensive invasion of local structures (eg, bone, cartilage, and even brain).
• Some BCCs are more aggressive than others in terms of demonstrated biologic activity.
• Some anatomical locations are themselves predictors of increased biologic activity; the nose is a prime example of this phenomenon.
• Mohs surgery—named after Frederic Mohs, MD, who pioneered the concept—ensures complete removal of the cancer and provides cosmetically acceptable closure as well.
At first, this 70-year-old man thought the lesion on his nose was a pimple, although it seemed oddly resilient. When he attempted to pop it, nothing came out.
When it not only failed to heal but also grew over a six-month period, he finally decided to consult his primary care provider (PCP). The PCP told him not to worry about it but offered to refer him to dermatology—mostly because the patient’s wife was concerned.
The patient gives a history of extensive sun exposure in childhood and young adulthood. In the 1960s, he was drafted into the military and served two tours of duty in the jungles of Vietnam, where he acquired an almost permanent sunburn.
EXAMINATION
The patient is quite fair, with modest facial sun damage manifesting as telangiectasias and solar elastosis. The lesion in question is a glassy, scarlike, concave papule with a scab at one end. It is located in the left alar groove and measures about 5 mm in diameter.
Under local anesthesia, the lesion is biopsied and sent to pathology.
What is the diagnosis?
DISCUSSION
The pathology report indicated basal cell carcinoma (BCC). Given the location, the patient was referred to a Mohs surgeon, who needed two passes to clear the site of cancer. Closure of the resulting 2.5-cm defect required a skin graft, using the postauricular neck as the donor site. Afterward, the PCP, the patient, and his wife all expressed astonishment that such a tiny lesion had required so complex a procedure.
BCC is, by far, the most common of the sun-caused skin cancers, with more than a million new cases diagnosed in the US each year. While rarely dangerous, BCCs can be highly destructive of local structures (eyelids, noses, ears, faces) if left untreated for extended periods. Virtually all BCCs grow relentlessly, but some are more aggressive than others—a trait confirmed not only by the behavior of the cancer, but also, in some cases, by characteristic histologic findings.
Location has a major impact on the behavior of a given BCC. The posterior portion of the nose (alar groove) is particularly prone to harbor more extensive and aggressive BCCs.
Given the additional cosmetic implications of removing cancers from this area, these often require Mohs surgery to ensure complete, mircroscopically controlled removal of the cancer, as well as closure of the wound in a cosmetically acceptable manner.
Some aggressive types of BCC will require radiation therapy even after Mohs or conventional surgical removal. Fortunately, deaths from BCCs usually result from local invasion and not from metastasis, which is extremely rare.
For providers, the key is a low threshold for the necessity of biopsy. Simple shave biopsy is usually adequate. When PCPs are uncomfortable performing such biopsies, expedited referral to dermatology is called for.
TAKE-HOME LEARNING POINTS
• BCC is the most common of the sun-caused skin cancers, with more than a million new cases diagnosed every year in the US.
• While they rarely metastasize, BCCs can result in extensive invasion of local structures (eg, bone, cartilage, and even brain).
• Some BCCs are more aggressive than others in terms of demonstrated biologic activity.
• Some anatomical locations are themselves predictors of increased biologic activity; the nose is a prime example of this phenomenon.
• Mohs surgery—named after Frederic Mohs, MD, who pioneered the concept—ensures complete removal of the cancer and provides cosmetically acceptable closure as well.
At first, this 70-year-old man thought the lesion on his nose was a pimple, although it seemed oddly resilient. When he attempted to pop it, nothing came out.
When it not only failed to heal but also grew over a six-month period, he finally decided to consult his primary care provider (PCP). The PCP told him not to worry about it but offered to refer him to dermatology—mostly because the patient’s wife was concerned.
The patient gives a history of extensive sun exposure in childhood and young adulthood. In the 1960s, he was drafted into the military and served two tours of duty in the jungles of Vietnam, where he acquired an almost permanent sunburn.
EXAMINATION
The patient is quite fair, with modest facial sun damage manifesting as telangiectasias and solar elastosis. The lesion in question is a glassy, scarlike, concave papule with a scab at one end. It is located in the left alar groove and measures about 5 mm in diameter.
Under local anesthesia, the lesion is biopsied and sent to pathology.
What is the diagnosis?
DISCUSSION
The pathology report indicated basal cell carcinoma (BCC). Given the location, the patient was referred to a Mohs surgeon, who needed two passes to clear the site of cancer. Closure of the resulting 2.5-cm defect required a skin graft, using the postauricular neck as the donor site. Afterward, the PCP, the patient, and his wife all expressed astonishment that such a tiny lesion had required so complex a procedure.
BCC is, by far, the most common of the sun-caused skin cancers, with more than a million new cases diagnosed in the US each year. While rarely dangerous, BCCs can be highly destructive of local structures (eyelids, noses, ears, faces) if left untreated for extended periods. Virtually all BCCs grow relentlessly, but some are more aggressive than others—a trait confirmed not only by the behavior of the cancer, but also, in some cases, by characteristic histologic findings.
Location has a major impact on the behavior of a given BCC. The posterior portion of the nose (alar groove) is particularly prone to harbor more extensive and aggressive BCCs.
Given the additional cosmetic implications of removing cancers from this area, these often require Mohs surgery to ensure complete, mircroscopically controlled removal of the cancer, as well as closure of the wound in a cosmetically acceptable manner.
Some aggressive types of BCC will require radiation therapy even after Mohs or conventional surgical removal. Fortunately, deaths from BCCs usually result from local invasion and not from metastasis, which is extremely rare.
For providers, the key is a low threshold for the necessity of biopsy. Simple shave biopsy is usually adequate. When PCPs are uncomfortable performing such biopsies, expedited referral to dermatology is called for.
TAKE-HOME LEARNING POINTS
• BCC is the most common of the sun-caused skin cancers, with more than a million new cases diagnosed every year in the US.
• While they rarely metastasize, BCCs can result in extensive invasion of local structures (eg, bone, cartilage, and even brain).
• Some BCCs are more aggressive than others in terms of demonstrated biologic activity.
• Some anatomical locations are themselves predictors of increased biologic activity; the nose is a prime example of this phenomenon.
• Mohs surgery—named after Frederic Mohs, MD, who pioneered the concept—ensures complete removal of the cancer and provides cosmetically acceptable closure as well.
Giant-Cell Arteritis May Underlie New Headache
OJAI, CA—Headache specialists may play an important role in diagnosing giant-cell arteritis, a vasculitis of large and medium vessels, because new headache is the initial symptom in about a third of cases, according to a lecture provided at the Ninth Annual Headache Cooperative of the Pacific Winter Conference. Early treatment with corticosteroids can help prevent permanent visual loss in this disorder. “If you strongly suspect the diagnosis, start prednisone and then proceed, in terms of trying to confirm the diagnosis,” said Jerry W. Swanson, MD, Professor of Neurology at the Mayo Clinic College of Medicine in Rochester, Minnesota. “If there have been any ischemic complications, which most commonly would affect the eye, therapy should be started immediately.”
Diagnosing giant-cell arteritis without sufficient evidence, however, can subject patients to the risks of long-term steroid use unnecessarily, he noted.
A Feared Complication
In the United States, an estimated 1% of women and 0.5% of men develop giant-cell arteritis. Prevalence is estimated to be one in 500 individuals over age 50, and peak incidence is in the 70s. “You virtually never see this before the age of 50,” said Dr. Swanson. The disorder has a genetic component and is most common in individuals of Scandinavian descent.
Research indicates that giant-cell arteritis “is an immunologic, not an infectious, problem,” said Dr. Swanson. The pathophysiology of the disease appears to involve dendritic cells in the vessel wall, which initiate a cascade of events that involves two major immune-response networks and leads to lumen-obstructive intimal hyperplasia and remodeling of the arterial wall.
Permanent visual loss is the most frequent and feared complication. It occurs in about 20% of patients. Transient monocular, and rarely binocular, vision loss can be an early manifestation of the disorder. This transient visual loss may soon be followed by permanent visual loss. The usual mechanism of visual loss is anterior ischemic optic neuropathy.
Headache Features Have Limited Use
The diagnosis of giant-cell arteritis should be considered in a patient age 50 or older with new headaches, abrupt onset of visual disturbances, symptoms of polymyalgia rheumatica, or jaw claudication. Systemic symptoms such as low-grade fever and anemia may arise, as well as high erythrocyte sedimentation rate or high serum C-reactive protein.
Headache is the most common symptom, occurring in almost three-quarters of patients. The headaches typically are acute or subacute and are associated with scalp tenderness near the temporal or occipital arteries. The headache features otherwise are not particularly helpful. “The headaches have been described as persistent or remittent, and they can resemble primary headache disorders just as cardiac cephalalgia can,” said Dr. Swanson. Features similar to migraine, cluster headache, or idiopathic stabbing headache have been described.
Jaw claudication is highly specific for giant-cell arteritis. Persistent dry cough and manifestations such as sore throat, tongue pain, trismus, and choking also have been described. Audiovestibular manifestations are fairly common, and polymyalgia rheumatica is present in 40% to 50% of patients with giant-cell arteritis.
Ophthalmologic exam can be normal, although some patients have cotton wool spots in the retina, suggesting retinal ischemia. Physicians should carefully examine the superficial temporal arteries for tenderness, absent pulsations, or marked prominence of the arteries.
Screening Test of Choice
Elevated erythrocyte sedimentation rate is one of the major hallmarks of giant-cell arteritis and “the screening test of choice,” said Dr. Swanson. In a series of more than 940 biopsy-proven cases, only 4% of patients had a normal sedimentation rate. “If the [sedimentation] rate is normal, still consider the diagnosis if clinical features are present, but it’s much less likely,” he said.
Although imperfect, temporal artery biopsy is the gold standard for diagnosing giant-cell arteritis. “Unfortunately, attempts to develop an algorithm that would say, ‘If they have a certain score, for instance, then do a biopsy or not,’ have failed,” he said. “You have to take the entire picture and then develop some sort of index of suspicion.”
Of all patients suspected of giant-cell arteritis, about a third of biopsies are positive. Positive yield increases if biopsies are 2 cm or longer. There is no clear consensus on whether to biopsy the temporal artery on one or both sides. At the Mayo Clinic, surgical pathologists often biopsy the side that is more symptomatic. Surgeons have the patient remain and may biopsy the other side if the first biopsy is negative and there is a high index of suspicion. “I realize that may not be possible at most institutions, but it seems to be a prudent approach,” Dr. Swanson said.
Younge et al found that certain factors and combinations of factors could be highly predictive of temporal artery biopsy results, including jaw claudication, new headache, scalp tenderness, and decreased vision.
In one series, approximately 9% of patients with negative biopsy subsequently were diagnosed with giant-cell arteritis, “so that unfortunately can occur.” One variant of giant-cell arteritis tends to affect the large vessels leaving the aorta and to spare the temporal arteries, which could lead to a negative biopsy. The most common cause of a negative biopsy, however, is that “it is a different diagnosis.”
“If the biopsy is negative, but you suspect this [disorder] very, very strongly, then continue the treatment because then there should be a marked improvement in symptoms within a week.” As this is an autoimmune disorder, consultation with a rheumatologist is usually helpful.
Imaging studies are not part of routine testing, but FDG PET may be useful in making the diagnosis.
Old and New Therapies
The FDA has not approved medications specifically for giant-cell arteritis. The mainstay of treatment is corticosteroids. Rheumatologists principally treat giant-cell arteritis, and neurologists should involve them early in the process, Dr. Swanson said.
Low-dose aspirin is frequently added for its antiplatelet effect, and this practice is supported by observational studies. Follow-up includes monitoring and treating the effects of glucocorticoids. Once glucocorticoid therapy is initiated, the risk of blindness is estimated to be about 1%.
To avoid corticosteroid or glucocorticoid adverse effects, such as osteopenia and bone fracture, methotrexate has been used. Three studies using methotrexate have shown reductions in the cumulative dose of glucocorticoid over 48 weeks and a reduced risk of relapse after prednisone is stopped. It takes 24 to 36 weeks for methotrexate to have its full anti-immune effect, Dr. Swanson noted.
Several case series have reported that tocilizumab, a humanized monoclonal antibody that targets interleukin 6, may be effective in patients in whom it has been difficult to taper glucocorticoids to an acceptable level or who have had refractory or relapsing disease. Controlled trials of this drug are under way, and “it will be important to have those results to know if this [drug] is in fact worthwhile,” Dr. Swanson said.
Results from a randomized, double-blind, placebo-controlled trial of tocilizumab in giant-cell arteritis were published online ahead of print March 4 in Lancet. In the phase II trial, 20 patients received IV tocilizumab and 10 patients received placebo infusions at four-week intervals for a year. Both groups received oral prednisolone. At 12 weeks, 85% of patients in the tocilizumab group achieved complete remission of disease, compared with 40% of patients in the placebo group. By 52 weeks, 85% of patients in the tocilizumab group achieved relapse-free survival, compared with 20% in the placebo group. The mean cumulative prednisolone dose was 43 mg/kg in the tocilizumab group versus 110 mg/kg in the placebo group. Rates of serious adverse events were higher in the placebo group than in the active treatment group. These preliminary results suggest that tocilizumab may become an important new management tool, Dr. Swanson said.
Cyclophosphamide has been used in patients at high risk of glucocorticoid-related adverse effects who have not responded adequately to other agents. In a meta-analysis of 88 cases, 84% of patients responded to cyclophosphamide, but 19% relapsed and 2.5% discontinued the therapy because of adverse effects.
Several other potential treatments may be on the horizon, including abatacept, a novel fusion protein that consists of cytotoxic T lymphocyte antigen (CTLA) linked to a modified heavy-chain constant region of human immunoglobulin G1; anakinra, which blocks the biologic activity of interleukin-1 (IL-1) by competitively inhibiting IL-1 binding to the IL-1 type I receptor; and gevokizumab, a humanized anti-IL-1β monoclonal antibody. Randomized trials have found that tumor necrosis factor inhibition therapies, including infliximab, etanercept, and adalimumab, are not effective in giant-cell arteritis.
—Jake Remaly
Suggested Reading
Quartuccio L, Maset M, De Maglio G, et al. Role of oral cyclophosphamide in the treatment of giant cell arteritis. Rheumatology (Oxford). 2012;51(9):1677-1686.
Smith JH, Swanson JW. Giant cell arteritis. Headache. 2014;54(8):1273-1289.
Steel L, Khan A, Dasgupta B. Giant cell arteritis: beyond corticosteroids. Drugs Aging. 2015;32(8):591-599.
Villiger PM, Adler S, Kuchen S, et al. Tocilizumab for induction and maintenance of remission in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2016 Mar 4 [Epub ahead of print].
Younge BR, Cook BE Jr, Bartley GB, et al. Initiation of glucocorticoid therapy: before or after temporal artery biopsy? Mayo Clin Proc. 2004;79(4):483-491.
OJAI, CA—Headache specialists may play an important role in diagnosing giant-cell arteritis, a vasculitis of large and medium vessels, because new headache is the initial symptom in about a third of cases, according to a lecture provided at the Ninth Annual Headache Cooperative of the Pacific Winter Conference. Early treatment with corticosteroids can help prevent permanent visual loss in this disorder. “If you strongly suspect the diagnosis, start prednisone and then proceed, in terms of trying to confirm the diagnosis,” said Jerry W. Swanson, MD, Professor of Neurology at the Mayo Clinic College of Medicine in Rochester, Minnesota. “If there have been any ischemic complications, which most commonly would affect the eye, therapy should be started immediately.”
Diagnosing giant-cell arteritis without sufficient evidence, however, can subject patients to the risks of long-term steroid use unnecessarily, he noted.
A Feared Complication
In the United States, an estimated 1% of women and 0.5% of men develop giant-cell arteritis. Prevalence is estimated to be one in 500 individuals over age 50, and peak incidence is in the 70s. “You virtually never see this before the age of 50,” said Dr. Swanson. The disorder has a genetic component and is most common in individuals of Scandinavian descent.
Research indicates that giant-cell arteritis “is an immunologic, not an infectious, problem,” said Dr. Swanson. The pathophysiology of the disease appears to involve dendritic cells in the vessel wall, which initiate a cascade of events that involves two major immune-response networks and leads to lumen-obstructive intimal hyperplasia and remodeling of the arterial wall.
Permanent visual loss is the most frequent and feared complication. It occurs in about 20% of patients. Transient monocular, and rarely binocular, vision loss can be an early manifestation of the disorder. This transient visual loss may soon be followed by permanent visual loss. The usual mechanism of visual loss is anterior ischemic optic neuropathy.
Headache Features Have Limited Use
The diagnosis of giant-cell arteritis should be considered in a patient age 50 or older with new headaches, abrupt onset of visual disturbances, symptoms of polymyalgia rheumatica, or jaw claudication. Systemic symptoms such as low-grade fever and anemia may arise, as well as high erythrocyte sedimentation rate or high serum C-reactive protein.
Headache is the most common symptom, occurring in almost three-quarters of patients. The headaches typically are acute or subacute and are associated with scalp tenderness near the temporal or occipital arteries. The headache features otherwise are not particularly helpful. “The headaches have been described as persistent or remittent, and they can resemble primary headache disorders just as cardiac cephalalgia can,” said Dr. Swanson. Features similar to migraine, cluster headache, or idiopathic stabbing headache have been described.
Jaw claudication is highly specific for giant-cell arteritis. Persistent dry cough and manifestations such as sore throat, tongue pain, trismus, and choking also have been described. Audiovestibular manifestations are fairly common, and polymyalgia rheumatica is present in 40% to 50% of patients with giant-cell arteritis.
Ophthalmologic exam can be normal, although some patients have cotton wool spots in the retina, suggesting retinal ischemia. Physicians should carefully examine the superficial temporal arteries for tenderness, absent pulsations, or marked prominence of the arteries.
Screening Test of Choice
Elevated erythrocyte sedimentation rate is one of the major hallmarks of giant-cell arteritis and “the screening test of choice,” said Dr. Swanson. In a series of more than 940 biopsy-proven cases, only 4% of patients had a normal sedimentation rate. “If the [sedimentation] rate is normal, still consider the diagnosis if clinical features are present, but it’s much less likely,” he said.
Although imperfect, temporal artery biopsy is the gold standard for diagnosing giant-cell arteritis. “Unfortunately, attempts to develop an algorithm that would say, ‘If they have a certain score, for instance, then do a biopsy or not,’ have failed,” he said. “You have to take the entire picture and then develop some sort of index of suspicion.”
Of all patients suspected of giant-cell arteritis, about a third of biopsies are positive. Positive yield increases if biopsies are 2 cm or longer. There is no clear consensus on whether to biopsy the temporal artery on one or both sides. At the Mayo Clinic, surgical pathologists often biopsy the side that is more symptomatic. Surgeons have the patient remain and may biopsy the other side if the first biopsy is negative and there is a high index of suspicion. “I realize that may not be possible at most institutions, but it seems to be a prudent approach,” Dr. Swanson said.
Younge et al found that certain factors and combinations of factors could be highly predictive of temporal artery biopsy results, including jaw claudication, new headache, scalp tenderness, and decreased vision.
In one series, approximately 9% of patients with negative biopsy subsequently were diagnosed with giant-cell arteritis, “so that unfortunately can occur.” One variant of giant-cell arteritis tends to affect the large vessels leaving the aorta and to spare the temporal arteries, which could lead to a negative biopsy. The most common cause of a negative biopsy, however, is that “it is a different diagnosis.”
“If the biopsy is negative, but you suspect this [disorder] very, very strongly, then continue the treatment because then there should be a marked improvement in symptoms within a week.” As this is an autoimmune disorder, consultation with a rheumatologist is usually helpful.
Imaging studies are not part of routine testing, but FDG PET may be useful in making the diagnosis.
Old and New Therapies
The FDA has not approved medications specifically for giant-cell arteritis. The mainstay of treatment is corticosteroids. Rheumatologists principally treat giant-cell arteritis, and neurologists should involve them early in the process, Dr. Swanson said.
Low-dose aspirin is frequently added for its antiplatelet effect, and this practice is supported by observational studies. Follow-up includes monitoring and treating the effects of glucocorticoids. Once glucocorticoid therapy is initiated, the risk of blindness is estimated to be about 1%.
To avoid corticosteroid or glucocorticoid adverse effects, such as osteopenia and bone fracture, methotrexate has been used. Three studies using methotrexate have shown reductions in the cumulative dose of glucocorticoid over 48 weeks and a reduced risk of relapse after prednisone is stopped. It takes 24 to 36 weeks for methotrexate to have its full anti-immune effect, Dr. Swanson noted.
Several case series have reported that tocilizumab, a humanized monoclonal antibody that targets interleukin 6, may be effective in patients in whom it has been difficult to taper glucocorticoids to an acceptable level or who have had refractory or relapsing disease. Controlled trials of this drug are under way, and “it will be important to have those results to know if this [drug] is in fact worthwhile,” Dr. Swanson said.
Results from a randomized, double-blind, placebo-controlled trial of tocilizumab in giant-cell arteritis were published online ahead of print March 4 in Lancet. In the phase II trial, 20 patients received IV tocilizumab and 10 patients received placebo infusions at four-week intervals for a year. Both groups received oral prednisolone. At 12 weeks, 85% of patients in the tocilizumab group achieved complete remission of disease, compared with 40% of patients in the placebo group. By 52 weeks, 85% of patients in the tocilizumab group achieved relapse-free survival, compared with 20% in the placebo group. The mean cumulative prednisolone dose was 43 mg/kg in the tocilizumab group versus 110 mg/kg in the placebo group. Rates of serious adverse events were higher in the placebo group than in the active treatment group. These preliminary results suggest that tocilizumab may become an important new management tool, Dr. Swanson said.
Cyclophosphamide has been used in patients at high risk of glucocorticoid-related adverse effects who have not responded adequately to other agents. In a meta-analysis of 88 cases, 84% of patients responded to cyclophosphamide, but 19% relapsed and 2.5% discontinued the therapy because of adverse effects.
Several other potential treatments may be on the horizon, including abatacept, a novel fusion protein that consists of cytotoxic T lymphocyte antigen (CTLA) linked to a modified heavy-chain constant region of human immunoglobulin G1; anakinra, which blocks the biologic activity of interleukin-1 (IL-1) by competitively inhibiting IL-1 binding to the IL-1 type I receptor; and gevokizumab, a humanized anti-IL-1β monoclonal antibody. Randomized trials have found that tumor necrosis factor inhibition therapies, including infliximab, etanercept, and adalimumab, are not effective in giant-cell arteritis.
—Jake Remaly
OJAI, CA—Headache specialists may play an important role in diagnosing giant-cell arteritis, a vasculitis of large and medium vessels, because new headache is the initial symptom in about a third of cases, according to a lecture provided at the Ninth Annual Headache Cooperative of the Pacific Winter Conference. Early treatment with corticosteroids can help prevent permanent visual loss in this disorder. “If you strongly suspect the diagnosis, start prednisone and then proceed, in terms of trying to confirm the diagnosis,” said Jerry W. Swanson, MD, Professor of Neurology at the Mayo Clinic College of Medicine in Rochester, Minnesota. “If there have been any ischemic complications, which most commonly would affect the eye, therapy should be started immediately.”
Diagnosing giant-cell arteritis without sufficient evidence, however, can subject patients to the risks of long-term steroid use unnecessarily, he noted.
A Feared Complication
In the United States, an estimated 1% of women and 0.5% of men develop giant-cell arteritis. Prevalence is estimated to be one in 500 individuals over age 50, and peak incidence is in the 70s. “You virtually never see this before the age of 50,” said Dr. Swanson. The disorder has a genetic component and is most common in individuals of Scandinavian descent.
Research indicates that giant-cell arteritis “is an immunologic, not an infectious, problem,” said Dr. Swanson. The pathophysiology of the disease appears to involve dendritic cells in the vessel wall, which initiate a cascade of events that involves two major immune-response networks and leads to lumen-obstructive intimal hyperplasia and remodeling of the arterial wall.
Permanent visual loss is the most frequent and feared complication. It occurs in about 20% of patients. Transient monocular, and rarely binocular, vision loss can be an early manifestation of the disorder. This transient visual loss may soon be followed by permanent visual loss. The usual mechanism of visual loss is anterior ischemic optic neuropathy.
Headache Features Have Limited Use
The diagnosis of giant-cell arteritis should be considered in a patient age 50 or older with new headaches, abrupt onset of visual disturbances, symptoms of polymyalgia rheumatica, or jaw claudication. Systemic symptoms such as low-grade fever and anemia may arise, as well as high erythrocyte sedimentation rate or high serum C-reactive protein.
Headache is the most common symptom, occurring in almost three-quarters of patients. The headaches typically are acute or subacute and are associated with scalp tenderness near the temporal or occipital arteries. The headache features otherwise are not particularly helpful. “The headaches have been described as persistent or remittent, and they can resemble primary headache disorders just as cardiac cephalalgia can,” said Dr. Swanson. Features similar to migraine, cluster headache, or idiopathic stabbing headache have been described.
Jaw claudication is highly specific for giant-cell arteritis. Persistent dry cough and manifestations such as sore throat, tongue pain, trismus, and choking also have been described. Audiovestibular manifestations are fairly common, and polymyalgia rheumatica is present in 40% to 50% of patients with giant-cell arteritis.
Ophthalmologic exam can be normal, although some patients have cotton wool spots in the retina, suggesting retinal ischemia. Physicians should carefully examine the superficial temporal arteries for tenderness, absent pulsations, or marked prominence of the arteries.
Screening Test of Choice
Elevated erythrocyte sedimentation rate is one of the major hallmarks of giant-cell arteritis and “the screening test of choice,” said Dr. Swanson. In a series of more than 940 biopsy-proven cases, only 4% of patients had a normal sedimentation rate. “If the [sedimentation] rate is normal, still consider the diagnosis if clinical features are present, but it’s much less likely,” he said.
Although imperfect, temporal artery biopsy is the gold standard for diagnosing giant-cell arteritis. “Unfortunately, attempts to develop an algorithm that would say, ‘If they have a certain score, for instance, then do a biopsy or not,’ have failed,” he said. “You have to take the entire picture and then develop some sort of index of suspicion.”
Of all patients suspected of giant-cell arteritis, about a third of biopsies are positive. Positive yield increases if biopsies are 2 cm or longer. There is no clear consensus on whether to biopsy the temporal artery on one or both sides. At the Mayo Clinic, surgical pathologists often biopsy the side that is more symptomatic. Surgeons have the patient remain and may biopsy the other side if the first biopsy is negative and there is a high index of suspicion. “I realize that may not be possible at most institutions, but it seems to be a prudent approach,” Dr. Swanson said.
Younge et al found that certain factors and combinations of factors could be highly predictive of temporal artery biopsy results, including jaw claudication, new headache, scalp tenderness, and decreased vision.
In one series, approximately 9% of patients with negative biopsy subsequently were diagnosed with giant-cell arteritis, “so that unfortunately can occur.” One variant of giant-cell arteritis tends to affect the large vessels leaving the aorta and to spare the temporal arteries, which could lead to a negative biopsy. The most common cause of a negative biopsy, however, is that “it is a different diagnosis.”
“If the biopsy is negative, but you suspect this [disorder] very, very strongly, then continue the treatment because then there should be a marked improvement in symptoms within a week.” As this is an autoimmune disorder, consultation with a rheumatologist is usually helpful.
Imaging studies are not part of routine testing, but FDG PET may be useful in making the diagnosis.
Old and New Therapies
The FDA has not approved medications specifically for giant-cell arteritis. The mainstay of treatment is corticosteroids. Rheumatologists principally treat giant-cell arteritis, and neurologists should involve them early in the process, Dr. Swanson said.
Low-dose aspirin is frequently added for its antiplatelet effect, and this practice is supported by observational studies. Follow-up includes monitoring and treating the effects of glucocorticoids. Once glucocorticoid therapy is initiated, the risk of blindness is estimated to be about 1%.
To avoid corticosteroid or glucocorticoid adverse effects, such as osteopenia and bone fracture, methotrexate has been used. Three studies using methotrexate have shown reductions in the cumulative dose of glucocorticoid over 48 weeks and a reduced risk of relapse after prednisone is stopped. It takes 24 to 36 weeks for methotrexate to have its full anti-immune effect, Dr. Swanson noted.
Several case series have reported that tocilizumab, a humanized monoclonal antibody that targets interleukin 6, may be effective in patients in whom it has been difficult to taper glucocorticoids to an acceptable level or who have had refractory or relapsing disease. Controlled trials of this drug are under way, and “it will be important to have those results to know if this [drug] is in fact worthwhile,” Dr. Swanson said.
Results from a randomized, double-blind, placebo-controlled trial of tocilizumab in giant-cell arteritis were published online ahead of print March 4 in Lancet. In the phase II trial, 20 patients received IV tocilizumab and 10 patients received placebo infusions at four-week intervals for a year. Both groups received oral prednisolone. At 12 weeks, 85% of patients in the tocilizumab group achieved complete remission of disease, compared with 40% of patients in the placebo group. By 52 weeks, 85% of patients in the tocilizumab group achieved relapse-free survival, compared with 20% in the placebo group. The mean cumulative prednisolone dose was 43 mg/kg in the tocilizumab group versus 110 mg/kg in the placebo group. Rates of serious adverse events were higher in the placebo group than in the active treatment group. These preliminary results suggest that tocilizumab may become an important new management tool, Dr. Swanson said.
Cyclophosphamide has been used in patients at high risk of glucocorticoid-related adverse effects who have not responded adequately to other agents. In a meta-analysis of 88 cases, 84% of patients responded to cyclophosphamide, but 19% relapsed and 2.5% discontinued the therapy because of adverse effects.
Several other potential treatments may be on the horizon, including abatacept, a novel fusion protein that consists of cytotoxic T lymphocyte antigen (CTLA) linked to a modified heavy-chain constant region of human immunoglobulin G1; anakinra, which blocks the biologic activity of interleukin-1 (IL-1) by competitively inhibiting IL-1 binding to the IL-1 type I receptor; and gevokizumab, a humanized anti-IL-1β monoclonal antibody. Randomized trials have found that tumor necrosis factor inhibition therapies, including infliximab, etanercept, and adalimumab, are not effective in giant-cell arteritis.
—Jake Remaly
Suggested Reading
Quartuccio L, Maset M, De Maglio G, et al. Role of oral cyclophosphamide in the treatment of giant cell arteritis. Rheumatology (Oxford). 2012;51(9):1677-1686.
Smith JH, Swanson JW. Giant cell arteritis. Headache. 2014;54(8):1273-1289.
Steel L, Khan A, Dasgupta B. Giant cell arteritis: beyond corticosteroids. Drugs Aging. 2015;32(8):591-599.
Villiger PM, Adler S, Kuchen S, et al. Tocilizumab for induction and maintenance of remission in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2016 Mar 4 [Epub ahead of print].
Younge BR, Cook BE Jr, Bartley GB, et al. Initiation of glucocorticoid therapy: before or after temporal artery biopsy? Mayo Clin Proc. 2004;79(4):483-491.
Suggested Reading
Quartuccio L, Maset M, De Maglio G, et al. Role of oral cyclophosphamide in the treatment of giant cell arteritis. Rheumatology (Oxford). 2012;51(9):1677-1686.
Smith JH, Swanson JW. Giant cell arteritis. Headache. 2014;54(8):1273-1289.
Steel L, Khan A, Dasgupta B. Giant cell arteritis: beyond corticosteroids. Drugs Aging. 2015;32(8):591-599.
Villiger PM, Adler S, Kuchen S, et al. Tocilizumab for induction and maintenance of remission in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2016 Mar 4 [Epub ahead of print].
Younge BR, Cook BE Jr, Bartley GB, et al. Initiation of glucocorticoid therapy: before or after temporal artery biopsy? Mayo Clin Proc. 2004;79(4):483-491.