User login
Ulnar Collateral Ligament Repair: An Old Idea With a New Wrinkle
Repair of the ulnar collateral ligament (UCL) was first reported by Norwood and colleagues1 in a group of athletes who sustained acute UCL ruptures. Of the 4 athletes in their cohort who underwent direct UCL repair, none were noted to have any residual instability 2 years after the surgery. However, none of these 4 were overhead throwing athletes. Jobe and colleagues2 first published Jobe’s technique of UCL reconstruction in 1986, but it was Conway and colleagus’3 1992 publication describing Jobe’s experience with UCL injury and surgical treatment in throwing athletes that set the early standard for management in that population. Since those landmark studies, there has been a tremendous increase in attention to this near-epidemic clinical problem.
Although these studies were the first to describe the surgical procedure that is now often referred to as “Tommy John surgery,” named after Jobe’s initial patient in 1974, Conway and colleagues3 also reported on Jobe’s early experience with UCL repair. In fact, of the 70 patients reported in the Conway and colleagues’3 article, 14 were treated with repair of the ligament. Only 7 of the 14 (50%) of those who underwent UCL repair were able to return to the same level of play, and only 2 of the 7 (29%) of Major League Baseball (MLB) players who underwent UCL repair were able to return to competition at the MLB level. This compared very poorly with the nearly 75% rate of return to competition in patients who underwent UCL reconstructions in the same cohort. In Azar and colleagues’4 2000 report on Dr. James Andrews’ experience with UCL injury and treatment in male college and professional baseball players, UCL repair again did poorly when compared to UCL reconstruction, with only 5 of the 8 (63%) of UCL repair patients returning to the same level of play compared to 41 of the 51 (81%) of UCL reconstructions using a modification of Jobe’s original technique.
Since the mid-1990s, numerous new techniques have been described and shown to have acceptable and largely successful outcomes in treating UCL injuries.5-9 All of them involve placing or anchoring a spanning piece of tendon graft from the native origin on the medial epicondyle of the humerus to the native insertion on the sublime tubercle of the ulna. These palpable and visible anatomic landmarks are important to the UCL surgeon due to the need to place the graft or repair the torn ligament tissue to its normal anatomic origin and/or insertion.10 Regardless of whether the graft is sewn, docked, tunneled, or anchored, these types of procedures have demonstrated rates of return to competition at the same or higher level of play in the 75% to 92% range.3,4,7,11-13 In the largest published series of 1281 UCL reconstructions by Cain and colleagues7 at American Sports Medicine Institute (Birmingham, AL), the rate of return to play at the same or higher level was 84%, with the average time to return to play of 11.4 months. On the basis of these robust clinical studies and numerous basic science studies demonstrating essentially equivalent strength and function among reconstruction techniques, UCL reconstruction now enjoys an acceptance among clinicians, athletes, athletic trainers, coaches, and team management at all levels of overhead sports.
In comparison to UCL reconstruction, relatively little has been published on UCL repair since 2000. Certainly this is in part due to the success of its clinical descendant. UCL repair did not appear on the pages of peer-reviewed literature until 2006, when Argo and colleagues11 published a report on the outcome of 17 UCL repairs in female athletes using a variety of techniques, including plication, anchor-to-bone, and drill holes. Although there was only 1 pitcher in the group, 16 of the 17 (94%) returned to the same or higher level of competition at an average of only 3 months after surgery.11
Savoie and colleagues13 followed this in 2008 with a report on 60 UCL repairs in overhead athletes. Of the 51 patients in this study in which the ligament was repaired to bone using suture anchors, 93% returned to the same or higher level of play at an average of only 6 months after surgery. Including Jobe’s original group, there have been less than 100 patients ever reported to have had a UCL repair performed. In comparison to the thousands of UCL reconstructions that have been reported over the last 20 years, it is not surprising that UCL repair has not gained great popularity among surgeons and patients. It is also important to remember that suture and anchor technology has come a long way since the 1970s, and our overall knowledge of the injury and its treatments and rehabilitation have grown tremendously since that time.
A New Technique for UCL Repair
Since we began data collection in Birmingham, Alabama in the mid 1990s, our practice has successfully treated thousands of overhead athletes of all types with the modified Jobe technique of UCL reconstruction, using either a palmaris longus tendon or a gracilis tendon graft.7 Until August 2013, this technique was exclusively utilized regardless of the amount and location of pathology encountered at the time of surgery. The range of pathology, from partial undersurface tearing to complete disruption of the ligament tissue, was treated by placing a graft at the anatomic insertion points of the native ligament. While the success of this experience cannot be overlooked, we also realized that we were treating a broad spectrum of pathology and injury with the same operation.
Recognizing the valuable contributions of earlier authors who had attempted UCL repair previously, we asked whether we were doing too much of an operation for all of the various pathology we saw at the time of surgery, and whether the availability of modern anchor and suture technology, vast clinical experience with these injuries and their outcomes, and even biologic additives could be applied to some of these patients in order to achieve an equal or superior outcome in less time. In particular, could such a technique be applied to the ever-increasing number of younger athletes with less pathology, who more frequently suffer end-avulsions and partial tears of their UCL?
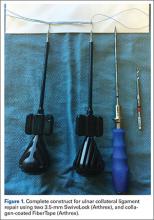
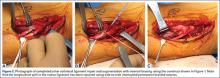
These thoughts, along with Savoie and colleagues’13 experience with UCL repair using suture anchors, led us to create a construct that could be used to not only repair the torn native UCL tissue to bone, but also span the anatomic native ligament from its origin to its insertion. The construct includes an ultra-strong collagen coated tape (FiberTape, Arthrex) attached at the anatomic insertions of the ligament using two 3.5-mm nonabsorbable PEEK corkscrew anchors (SwiveLock, Arthrex), and a suture through the eyelet of one of the anchors (Figure 1). Using this construct, the native ligament disruption can be repaired directly to bone using the suture through the eyelet of the anchor, and the remainder of the native ligament is augmented with the spanning biologic enhanced tape (Figures 2A-2C). The construct is created by placing one end of the tape through the eyelet of the first anchor, and then placing one end of a No. zero braided permanent suture through the same eyelet. Both ends of the tape are then placed through the eyelet of the second anchor. The first anchor is inserted into a hole drilled at the apex of the insertion of the torn end of the native ligament. This anchor is placed first in order to allow for direct repair of the native torn ligament using the free suture through the eyelet of the first anchor. The second hole is then drilled at the insertion of the native ligament on the uninjured end of the native ligament. In order to accommodate the volume of tape in the hole created for the second anchor, a slightly oversized drill and tap were created specifically for this technique (Arthrex).
Before attempting this in vivo, a cadaveric study was carried out in order to ensure that the time-zero function of the construct would be at least as good as the standard UCL reconstruction technique we have used for several decades.14 The time-zero gap formation under valgus load was less for the repair/augmentation than for the standard reconstruction with palmaris longus, and the ultimate failure strength of the repair was the same as in the reconstruction group, with all failures through bone in the cadaveric specimens. No anchors pulled out of bone, and the tape did not tear in any specimen.
This basic science study has given us confidence to proceed with the use of this technique in patients. The first patient was treated with this construct in August 2013. The outcomes of our first series of patients were presented on Saturday, March 5 at American Orthopaedic Society for Sports Medicine Specialty Day during the 2016 American Academy of Orthopaedic Surgeons annual meeting in Orlando, FL.
We do not feel that this technique is adequate for the treatment of the UCL that has sustained attritional injury and contains poor quality native ligament tissue. Before we do these procedures, we always discuss with the patient the possibility that full reconstruction may be required, and that the decision to proceed with UCL repair is contingent upon the quality and quantity of the native UCL tissue present at the time of surgery. If the quality of the native tissue is poor (chronic degenerative changes, etc), full reconstruction with autograft tendon is recommended. It is our hope that this technique will afford the UCL surgeon another option for treating end-avulsions and partial thickness injuries, with a more rapid and successful return to normal function and competition.
1. Norwood LA, Shook JA, Andrews JR. Acute medial elbow ruptures. Am J Sports Med. 1981;9(1):16-19.
2. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
3. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes: treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
4. Azar FM, Andrews JR, Wilk KE, Groh D. Operative treatment of ulnar collateral ligament injuries of the elbow in athletes. Am J Sports Med. 2000;28(1):16-23.
5. Ahmad CS, Lee TQ, ElAttrache NS. Biomechanical evaluation of a new ulnar collateral ligament reconstruction technique with interference screw fixation. Am J Sports Med. 2003;31(3):332-337.
6. Armstrong AD, Dunning CE, Ferreira LM, Faber KJ, Johnson JA, King GJ. A biomechanical comparison of four reconstruction techniques for the medial collateral ligament-deficient elbow. J Shoulder Elbow Surg. 2005;14(2):207-215.
7. Cain EL, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
8. Paletta GA, Klepps SJ, Difelice GS, et al. Biomechanical evaluation of 2 techniques for ulnar collateral ligament reconstruction of the elbow. Am J Sports Med. 2006;34(10):1599-1603.
9. Ruland RT, Hogan CJH, Randall CJ, Richards A, Belkoff SM. Biomechanical comparison of ulnar collateral ligament reconstruction techniques. Am J Sports Med. 2008;36(8):1565-1570.
10. Dugas JR, Ostrander RV, Cain EL, Kingsley D, Andrews JR. Anatomy of the anterior bundle of the ulnar collateral ligament. J Shoulder Elbow Surg. 2007;16(5):657-660.
11. Argo D, Trenhaile SW, Savoie FH, Field LD. Operative treatment of ulnar collateral ligament insufficiency of the elbow in female athletes. Am J Sports Med. 2006;34(3):431-437.
12. Petty DH, Andrews JR, Fleisig GS, Cain EL. Ulnar collateral ligament reconstruction in high school baseball players: clinical results and injury risk factors. Am J Sports Med. 2003;32(5):1158-1164.
13. Savoie FH, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36(6):1066-1072.
14. Dugas JR, Walters BL, Beason DP, Fleisig GS, Chronister JE. Biomechanical comparison of ulnar collateral ligament repair with internal bracing versus modified Jobe reconstruction. Am J Sports Med. 2015. [Epub ahead of print].
Repair of the ulnar collateral ligament (UCL) was first reported by Norwood and colleagues1 in a group of athletes who sustained acute UCL ruptures. Of the 4 athletes in their cohort who underwent direct UCL repair, none were noted to have any residual instability 2 years after the surgery. However, none of these 4 were overhead throwing athletes. Jobe and colleagues2 first published Jobe’s technique of UCL reconstruction in 1986, but it was Conway and colleagus’3 1992 publication describing Jobe’s experience with UCL injury and surgical treatment in throwing athletes that set the early standard for management in that population. Since those landmark studies, there has been a tremendous increase in attention to this near-epidemic clinical problem.
Although these studies were the first to describe the surgical procedure that is now often referred to as “Tommy John surgery,” named after Jobe’s initial patient in 1974, Conway and colleagues3 also reported on Jobe’s early experience with UCL repair. In fact, of the 70 patients reported in the Conway and colleagues’3 article, 14 were treated with repair of the ligament. Only 7 of the 14 (50%) of those who underwent UCL repair were able to return to the same level of play, and only 2 of the 7 (29%) of Major League Baseball (MLB) players who underwent UCL repair were able to return to competition at the MLB level. This compared very poorly with the nearly 75% rate of return to competition in patients who underwent UCL reconstructions in the same cohort. In Azar and colleagues’4 2000 report on Dr. James Andrews’ experience with UCL injury and treatment in male college and professional baseball players, UCL repair again did poorly when compared to UCL reconstruction, with only 5 of the 8 (63%) of UCL repair patients returning to the same level of play compared to 41 of the 51 (81%) of UCL reconstructions using a modification of Jobe’s original technique.
Since the mid-1990s, numerous new techniques have been described and shown to have acceptable and largely successful outcomes in treating UCL injuries.5-9 All of them involve placing or anchoring a spanning piece of tendon graft from the native origin on the medial epicondyle of the humerus to the native insertion on the sublime tubercle of the ulna. These palpable and visible anatomic landmarks are important to the UCL surgeon due to the need to place the graft or repair the torn ligament tissue to its normal anatomic origin and/or insertion.10 Regardless of whether the graft is sewn, docked, tunneled, or anchored, these types of procedures have demonstrated rates of return to competition at the same or higher level of play in the 75% to 92% range.3,4,7,11-13 In the largest published series of 1281 UCL reconstructions by Cain and colleagues7 at American Sports Medicine Institute (Birmingham, AL), the rate of return to play at the same or higher level was 84%, with the average time to return to play of 11.4 months. On the basis of these robust clinical studies and numerous basic science studies demonstrating essentially equivalent strength and function among reconstruction techniques, UCL reconstruction now enjoys an acceptance among clinicians, athletes, athletic trainers, coaches, and team management at all levels of overhead sports.
In comparison to UCL reconstruction, relatively little has been published on UCL repair since 2000. Certainly this is in part due to the success of its clinical descendant. UCL repair did not appear on the pages of peer-reviewed literature until 2006, when Argo and colleagues11 published a report on the outcome of 17 UCL repairs in female athletes using a variety of techniques, including plication, anchor-to-bone, and drill holes. Although there was only 1 pitcher in the group, 16 of the 17 (94%) returned to the same or higher level of competition at an average of only 3 months after surgery.11
Savoie and colleagues13 followed this in 2008 with a report on 60 UCL repairs in overhead athletes. Of the 51 patients in this study in which the ligament was repaired to bone using suture anchors, 93% returned to the same or higher level of play at an average of only 6 months after surgery. Including Jobe’s original group, there have been less than 100 patients ever reported to have had a UCL repair performed. In comparison to the thousands of UCL reconstructions that have been reported over the last 20 years, it is not surprising that UCL repair has not gained great popularity among surgeons and patients. It is also important to remember that suture and anchor technology has come a long way since the 1970s, and our overall knowledge of the injury and its treatments and rehabilitation have grown tremendously since that time.
A New Technique for UCL Repair
Since we began data collection in Birmingham, Alabama in the mid 1990s, our practice has successfully treated thousands of overhead athletes of all types with the modified Jobe technique of UCL reconstruction, using either a palmaris longus tendon or a gracilis tendon graft.7 Until August 2013, this technique was exclusively utilized regardless of the amount and location of pathology encountered at the time of surgery. The range of pathology, from partial undersurface tearing to complete disruption of the ligament tissue, was treated by placing a graft at the anatomic insertion points of the native ligament. While the success of this experience cannot be overlooked, we also realized that we were treating a broad spectrum of pathology and injury with the same operation.
Recognizing the valuable contributions of earlier authors who had attempted UCL repair previously, we asked whether we were doing too much of an operation for all of the various pathology we saw at the time of surgery, and whether the availability of modern anchor and suture technology, vast clinical experience with these injuries and their outcomes, and even biologic additives could be applied to some of these patients in order to achieve an equal or superior outcome in less time. In particular, could such a technique be applied to the ever-increasing number of younger athletes with less pathology, who more frequently suffer end-avulsions and partial tears of their UCL?
These thoughts, along with Savoie and colleagues’13 experience with UCL repair using suture anchors, led us to create a construct that could be used to not only repair the torn native UCL tissue to bone, but also span the anatomic native ligament from its origin to its insertion. The construct includes an ultra-strong collagen coated tape (FiberTape, Arthrex) attached at the anatomic insertions of the ligament using two 3.5-mm nonabsorbable PEEK corkscrew anchors (SwiveLock, Arthrex), and a suture through the eyelet of one of the anchors (Figure 1). Using this construct, the native ligament disruption can be repaired directly to bone using the suture through the eyelet of the anchor, and the remainder of the native ligament is augmented with the spanning biologic enhanced tape (Figures 2A-2C). The construct is created by placing one end of the tape through the eyelet of the first anchor, and then placing one end of a No. zero braided permanent suture through the same eyelet. Both ends of the tape are then placed through the eyelet of the second anchor. The first anchor is inserted into a hole drilled at the apex of the insertion of the torn end of the native ligament. This anchor is placed first in order to allow for direct repair of the native torn ligament using the free suture through the eyelet of the first anchor. The second hole is then drilled at the insertion of the native ligament on the uninjured end of the native ligament. In order to accommodate the volume of tape in the hole created for the second anchor, a slightly oversized drill and tap were created specifically for this technique (Arthrex).
Before attempting this in vivo, a cadaveric study was carried out in order to ensure that the time-zero function of the construct would be at least as good as the standard UCL reconstruction technique we have used for several decades.14 The time-zero gap formation under valgus load was less for the repair/augmentation than for the standard reconstruction with palmaris longus, and the ultimate failure strength of the repair was the same as in the reconstruction group, with all failures through bone in the cadaveric specimens. No anchors pulled out of bone, and the tape did not tear in any specimen.
This basic science study has given us confidence to proceed with the use of this technique in patients. The first patient was treated with this construct in August 2013. The outcomes of our first series of patients were presented on Saturday, March 5 at American Orthopaedic Society for Sports Medicine Specialty Day during the 2016 American Academy of Orthopaedic Surgeons annual meeting in Orlando, FL.
We do not feel that this technique is adequate for the treatment of the UCL that has sustained attritional injury and contains poor quality native ligament tissue. Before we do these procedures, we always discuss with the patient the possibility that full reconstruction may be required, and that the decision to proceed with UCL repair is contingent upon the quality and quantity of the native UCL tissue present at the time of surgery. If the quality of the native tissue is poor (chronic degenerative changes, etc), full reconstruction with autograft tendon is recommended. It is our hope that this technique will afford the UCL surgeon another option for treating end-avulsions and partial thickness injuries, with a more rapid and successful return to normal function and competition.
Repair of the ulnar collateral ligament (UCL) was first reported by Norwood and colleagues1 in a group of athletes who sustained acute UCL ruptures. Of the 4 athletes in their cohort who underwent direct UCL repair, none were noted to have any residual instability 2 years after the surgery. However, none of these 4 were overhead throwing athletes. Jobe and colleagues2 first published Jobe’s technique of UCL reconstruction in 1986, but it was Conway and colleagus’3 1992 publication describing Jobe’s experience with UCL injury and surgical treatment in throwing athletes that set the early standard for management in that population. Since those landmark studies, there has been a tremendous increase in attention to this near-epidemic clinical problem.
Although these studies were the first to describe the surgical procedure that is now often referred to as “Tommy John surgery,” named after Jobe’s initial patient in 1974, Conway and colleagues3 also reported on Jobe’s early experience with UCL repair. In fact, of the 70 patients reported in the Conway and colleagues’3 article, 14 were treated with repair of the ligament. Only 7 of the 14 (50%) of those who underwent UCL repair were able to return to the same level of play, and only 2 of the 7 (29%) of Major League Baseball (MLB) players who underwent UCL repair were able to return to competition at the MLB level. This compared very poorly with the nearly 75% rate of return to competition in patients who underwent UCL reconstructions in the same cohort. In Azar and colleagues’4 2000 report on Dr. James Andrews’ experience with UCL injury and treatment in male college and professional baseball players, UCL repair again did poorly when compared to UCL reconstruction, with only 5 of the 8 (63%) of UCL repair patients returning to the same level of play compared to 41 of the 51 (81%) of UCL reconstructions using a modification of Jobe’s original technique.
Since the mid-1990s, numerous new techniques have been described and shown to have acceptable and largely successful outcomes in treating UCL injuries.5-9 All of them involve placing or anchoring a spanning piece of tendon graft from the native origin on the medial epicondyle of the humerus to the native insertion on the sublime tubercle of the ulna. These palpable and visible anatomic landmarks are important to the UCL surgeon due to the need to place the graft or repair the torn ligament tissue to its normal anatomic origin and/or insertion.10 Regardless of whether the graft is sewn, docked, tunneled, or anchored, these types of procedures have demonstrated rates of return to competition at the same or higher level of play in the 75% to 92% range.3,4,7,11-13 In the largest published series of 1281 UCL reconstructions by Cain and colleagues7 at American Sports Medicine Institute (Birmingham, AL), the rate of return to play at the same or higher level was 84%, with the average time to return to play of 11.4 months. On the basis of these robust clinical studies and numerous basic science studies demonstrating essentially equivalent strength and function among reconstruction techniques, UCL reconstruction now enjoys an acceptance among clinicians, athletes, athletic trainers, coaches, and team management at all levels of overhead sports.
In comparison to UCL reconstruction, relatively little has been published on UCL repair since 2000. Certainly this is in part due to the success of its clinical descendant. UCL repair did not appear on the pages of peer-reviewed literature until 2006, when Argo and colleagues11 published a report on the outcome of 17 UCL repairs in female athletes using a variety of techniques, including plication, anchor-to-bone, and drill holes. Although there was only 1 pitcher in the group, 16 of the 17 (94%) returned to the same or higher level of competition at an average of only 3 months after surgery.11
Savoie and colleagues13 followed this in 2008 with a report on 60 UCL repairs in overhead athletes. Of the 51 patients in this study in which the ligament was repaired to bone using suture anchors, 93% returned to the same or higher level of play at an average of only 6 months after surgery. Including Jobe’s original group, there have been less than 100 patients ever reported to have had a UCL repair performed. In comparison to the thousands of UCL reconstructions that have been reported over the last 20 years, it is not surprising that UCL repair has not gained great popularity among surgeons and patients. It is also important to remember that suture and anchor technology has come a long way since the 1970s, and our overall knowledge of the injury and its treatments and rehabilitation have grown tremendously since that time.
A New Technique for UCL Repair
Since we began data collection in Birmingham, Alabama in the mid 1990s, our practice has successfully treated thousands of overhead athletes of all types with the modified Jobe technique of UCL reconstruction, using either a palmaris longus tendon or a gracilis tendon graft.7 Until August 2013, this technique was exclusively utilized regardless of the amount and location of pathology encountered at the time of surgery. The range of pathology, from partial undersurface tearing to complete disruption of the ligament tissue, was treated by placing a graft at the anatomic insertion points of the native ligament. While the success of this experience cannot be overlooked, we also realized that we were treating a broad spectrum of pathology and injury with the same operation.
Recognizing the valuable contributions of earlier authors who had attempted UCL repair previously, we asked whether we were doing too much of an operation for all of the various pathology we saw at the time of surgery, and whether the availability of modern anchor and suture technology, vast clinical experience with these injuries and their outcomes, and even biologic additives could be applied to some of these patients in order to achieve an equal or superior outcome in less time. In particular, could such a technique be applied to the ever-increasing number of younger athletes with less pathology, who more frequently suffer end-avulsions and partial tears of their UCL?
These thoughts, along with Savoie and colleagues’13 experience with UCL repair using suture anchors, led us to create a construct that could be used to not only repair the torn native UCL tissue to bone, but also span the anatomic native ligament from its origin to its insertion. The construct includes an ultra-strong collagen coated tape (FiberTape, Arthrex) attached at the anatomic insertions of the ligament using two 3.5-mm nonabsorbable PEEK corkscrew anchors (SwiveLock, Arthrex), and a suture through the eyelet of one of the anchors (Figure 1). Using this construct, the native ligament disruption can be repaired directly to bone using the suture through the eyelet of the anchor, and the remainder of the native ligament is augmented with the spanning biologic enhanced tape (Figures 2A-2C). The construct is created by placing one end of the tape through the eyelet of the first anchor, and then placing one end of a No. zero braided permanent suture through the same eyelet. Both ends of the tape are then placed through the eyelet of the second anchor. The first anchor is inserted into a hole drilled at the apex of the insertion of the torn end of the native ligament. This anchor is placed first in order to allow for direct repair of the native torn ligament using the free suture through the eyelet of the first anchor. The second hole is then drilled at the insertion of the native ligament on the uninjured end of the native ligament. In order to accommodate the volume of tape in the hole created for the second anchor, a slightly oversized drill and tap were created specifically for this technique (Arthrex).
Before attempting this in vivo, a cadaveric study was carried out in order to ensure that the time-zero function of the construct would be at least as good as the standard UCL reconstruction technique we have used for several decades.14 The time-zero gap formation under valgus load was less for the repair/augmentation than for the standard reconstruction with palmaris longus, and the ultimate failure strength of the repair was the same as in the reconstruction group, with all failures through bone in the cadaveric specimens. No anchors pulled out of bone, and the tape did not tear in any specimen.
This basic science study has given us confidence to proceed with the use of this technique in patients. The first patient was treated with this construct in August 2013. The outcomes of our first series of patients were presented on Saturday, March 5 at American Orthopaedic Society for Sports Medicine Specialty Day during the 2016 American Academy of Orthopaedic Surgeons annual meeting in Orlando, FL.
We do not feel that this technique is adequate for the treatment of the UCL that has sustained attritional injury and contains poor quality native ligament tissue. Before we do these procedures, we always discuss with the patient the possibility that full reconstruction may be required, and that the decision to proceed with UCL repair is contingent upon the quality and quantity of the native UCL tissue present at the time of surgery. If the quality of the native tissue is poor (chronic degenerative changes, etc), full reconstruction with autograft tendon is recommended. It is our hope that this technique will afford the UCL surgeon another option for treating end-avulsions and partial thickness injuries, with a more rapid and successful return to normal function and competition.
1. Norwood LA, Shook JA, Andrews JR. Acute medial elbow ruptures. Am J Sports Med. 1981;9(1):16-19.
2. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
3. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes: treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
4. Azar FM, Andrews JR, Wilk KE, Groh D. Operative treatment of ulnar collateral ligament injuries of the elbow in athletes. Am J Sports Med. 2000;28(1):16-23.
5. Ahmad CS, Lee TQ, ElAttrache NS. Biomechanical evaluation of a new ulnar collateral ligament reconstruction technique with interference screw fixation. Am J Sports Med. 2003;31(3):332-337.
6. Armstrong AD, Dunning CE, Ferreira LM, Faber KJ, Johnson JA, King GJ. A biomechanical comparison of four reconstruction techniques for the medial collateral ligament-deficient elbow. J Shoulder Elbow Surg. 2005;14(2):207-215.
7. Cain EL, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
8. Paletta GA, Klepps SJ, Difelice GS, et al. Biomechanical evaluation of 2 techniques for ulnar collateral ligament reconstruction of the elbow. Am J Sports Med. 2006;34(10):1599-1603.
9. Ruland RT, Hogan CJH, Randall CJ, Richards A, Belkoff SM. Biomechanical comparison of ulnar collateral ligament reconstruction techniques. Am J Sports Med. 2008;36(8):1565-1570.
10. Dugas JR, Ostrander RV, Cain EL, Kingsley D, Andrews JR. Anatomy of the anterior bundle of the ulnar collateral ligament. J Shoulder Elbow Surg. 2007;16(5):657-660.
11. Argo D, Trenhaile SW, Savoie FH, Field LD. Operative treatment of ulnar collateral ligament insufficiency of the elbow in female athletes. Am J Sports Med. 2006;34(3):431-437.
12. Petty DH, Andrews JR, Fleisig GS, Cain EL. Ulnar collateral ligament reconstruction in high school baseball players: clinical results and injury risk factors. Am J Sports Med. 2003;32(5):1158-1164.
13. Savoie FH, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36(6):1066-1072.
14. Dugas JR, Walters BL, Beason DP, Fleisig GS, Chronister JE. Biomechanical comparison of ulnar collateral ligament repair with internal bracing versus modified Jobe reconstruction. Am J Sports Med. 2015. [Epub ahead of print].
1. Norwood LA, Shook JA, Andrews JR. Acute medial elbow ruptures. Am J Sports Med. 1981;9(1):16-19.
2. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
3. Conway JE, Jobe FW, Glousman RE, Pink M. Medial instability of the elbow in throwing athletes: treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg Am. 1992;74(1):67-83.
4. Azar FM, Andrews JR, Wilk KE, Groh D. Operative treatment of ulnar collateral ligament injuries of the elbow in athletes. Am J Sports Med. 2000;28(1):16-23.
5. Ahmad CS, Lee TQ, ElAttrache NS. Biomechanical evaluation of a new ulnar collateral ligament reconstruction technique with interference screw fixation. Am J Sports Med. 2003;31(3):332-337.
6. Armstrong AD, Dunning CE, Ferreira LM, Faber KJ, Johnson JA, King GJ. A biomechanical comparison of four reconstruction techniques for the medial collateral ligament-deficient elbow. J Shoulder Elbow Surg. 2005;14(2):207-215.
7. Cain EL, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
8. Paletta GA, Klepps SJ, Difelice GS, et al. Biomechanical evaluation of 2 techniques for ulnar collateral ligament reconstruction of the elbow. Am J Sports Med. 2006;34(10):1599-1603.
9. Ruland RT, Hogan CJH, Randall CJ, Richards A, Belkoff SM. Biomechanical comparison of ulnar collateral ligament reconstruction techniques. Am J Sports Med. 2008;36(8):1565-1570.
10. Dugas JR, Ostrander RV, Cain EL, Kingsley D, Andrews JR. Anatomy of the anterior bundle of the ulnar collateral ligament. J Shoulder Elbow Surg. 2007;16(5):657-660.
11. Argo D, Trenhaile SW, Savoie FH, Field LD. Operative treatment of ulnar collateral ligament insufficiency of the elbow in female athletes. Am J Sports Med. 2006;34(3):431-437.
12. Petty DH, Andrews JR, Fleisig GS, Cain EL. Ulnar collateral ligament reconstruction in high school baseball players: clinical results and injury risk factors. Am J Sports Med. 2003;32(5):1158-1164.
13. Savoie FH, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36(6):1066-1072.
14. Dugas JR, Walters BL, Beason DP, Fleisig GS, Chronister JE. Biomechanical comparison of ulnar collateral ligament repair with internal bracing versus modified Jobe reconstruction. Am J Sports Med. 2015. [Epub ahead of print].
A novel approach to overcoming cervical stenosis and false passages

For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org

For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org

For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org
This video is brought to you by ![]()
STS: Valved conduit shows right ventricular outflow durability
PHOENIX – A prosthetic conduit that contains a porcine valve showed excellent intermediate-term durability for repairing the right ventricular outflow tract in 100 teenagers and young adults at a single U.S. center.
“The Carpentier-Edwards xenograft for right ventricular outflow tract [RVOT] reconstruction provides excellent freedom from reoperation and valve dysfunction, as well as sustained improvement in right-ventricular chamber size at intermediate-term follow-up,” Dr. Heidi B. Schubmehl said at the Society of Thoracic Surgeons annual meeting.
Dr. Schubmehl reported a 92% rate of freedom from valve dysfunction with follow-up out to about 10 years, and significant reductions in right ventricular size at follow-up, compared with baseline, as measured by both echocardiography and by MRI.
The Carpentier-Edwards porcine valve and conduit “seemed to hold up better than a lot of other [prosthetic] valves,” said Dr. George M. Alfieris, director of pediatric cardiac surgery at the University of Rochester (N.Y.), and senior author for the study. In addition to the valve’s durability over approximately the first 10 years following placement, the results also showed the positive impact the valve had on right ventricular size, an important result of the repair’s efficacy, Dr. Alfieris said.
“It’s a mistake to allow the right ventricle to be under high pressure or to reach a large volume. We now focus on preserving the right ventricle,” he said in an interview. “I’ve become very concerned about preventing right ventricular dilation and preserving right ventricular function.”
Dr. Alfieris noted that his prior experience using other types of valves in the pulmonary valve and RVOT position showed those valves “did great for the first 10 years and then failed. What’s different in this series is that after 10 years, we have not seen the same dysfunction as with the prior generation of valves. I will be very interested to see what happens to them” as follow-up continues beyond 10 years. He also expressed dismay that recently the company that had been marketing the valve and conduit used in the current study, the Carpentier-Edwards, stopped selling them. He expects that as his supply of conduits runs out he’ll have to start using a different commercial valve and conduit that he believes will not perform as well or create his own conduits with a porcine valve from a different supplier.
The series of 100 patients comprised individuals aged 17 or older who received a pulmonary artery and had RVOT reconstruction at the University of Rochester during 2000-2010, Dr. Schubmehl reported. The series included 78 patients with a history of tetralogy of Fallot, 8 patients born with transposition of their great arteries, 8 patients with truncus arteriosus, and 6 patients with other congenital heart diseases. Their median age at the time they received the RVOT conduit was 24 years, 59% were men, and 99 had undergone a prior sternotomy. At the time they received the conduit, 55 had pulmonary valve insufficiency, 30 had valve stenosis, and 15 had both. Follow-up occurred an average of 7 years after conduit placement.
Two recipients died: One death occurred perioperatively in a 41-year old who had a massive cerebrovascular event, and the second death was in a 39-year old who died 2.6 years after conduit placement from respiratory failure. Two additional patients required a reintervention during follow-up, said Dr. Schubmehl, a general surgeon at the University of Rochester. One reintervention occurred after 11 years to treat endocarditis, and the second after 11 years to perform balloon valvuloplasty because of valve stenosis.
The results reported by Dr. Schubmehl for echocardiography examinations showed that the patients had a statistically significant reduction in their RVOT pressure gradient from baseline to 1-year follow-up that was sustained through their intermediate-term follow-up. Seventy-seven patients had pulmonary valve insufficiency at baseline that resolved in all patients at 1-year follow-up and remained resolved in all but one patient at extended follow-up. Nineteen patients underwent additional imaging with MRI at an average follow-up of 7 years, and these findings confirmed the echo results.
On Twitter @mitchelzoler
The intermediate-term results reported by Dr. Schubmehl using a Carpentier-Edwards conduit in the right-ventricular outflow tract are clearly better than what we have seen using other types of valves and conduits in this position. If the valve and conduit they used persists with similar performance beyond 10 years, it would be a very good option. However, what typically happens is that replacement valves look good for about 10 years and then start to fail, often with a steep failure curve. I suspect that during the next 10 years of follow-up many more of the valves they placed will start to fail. The 10- to 20-year follow-up period is critical for demonstrating long-term durability of this valve and conduit.
 |
Dr. James Jaggers |
One additional potential advantage of the Carpentier-Edwards prosthesis is that the valve it contains is larger than the usual valve placed in the right ventricular outflow tract (RVOT). Failed valves increasingly are replaced by a transcatheter approach that puts a new valve inside the old, failed valve. As patients who received these replacement valves continue to survive we anticipate their need over time for a series of valve-in-valve procedures. The larger the valve at the outset, the more feasible it will be to have multiple episodes of valve-in-valve replacement.
At one time, we regarded early surgical repair of a tetralogy of Fallot defect as curative. We now know that as children with a repaired tetralogy of Fallot grow into teens and adults they require additional repairs, most often replacement of their RVOTs. This has made pulmonary valve replacement the most common surgery for adult survivors of congenital heart disease. The numbers of teen or adult patients who require a new RVOT will steadily increase as more of these children survive.
Dr. James Jaggers, professor of surgery at the University of Colorado and chief of cardiothoracic surgery at Children’s Hospital Colorado in Denver, made these comments in an interview. He had no disclosures.
The intermediate-term results reported by Dr. Schubmehl using a Carpentier-Edwards conduit in the right-ventricular outflow tract are clearly better than what we have seen using other types of valves and conduits in this position. If the valve and conduit they used persists with similar performance beyond 10 years, it would be a very good option. However, what typically happens is that replacement valves look good for about 10 years and then start to fail, often with a steep failure curve. I suspect that during the next 10 years of follow-up many more of the valves they placed will start to fail. The 10- to 20-year follow-up period is critical for demonstrating long-term durability of this valve and conduit.
 |
Dr. James Jaggers |
One additional potential advantage of the Carpentier-Edwards prosthesis is that the valve it contains is larger than the usual valve placed in the right ventricular outflow tract (RVOT). Failed valves increasingly are replaced by a transcatheter approach that puts a new valve inside the old, failed valve. As patients who received these replacement valves continue to survive we anticipate their need over time for a series of valve-in-valve procedures. The larger the valve at the outset, the more feasible it will be to have multiple episodes of valve-in-valve replacement.
At one time, we regarded early surgical repair of a tetralogy of Fallot defect as curative. We now know that as children with a repaired tetralogy of Fallot grow into teens and adults they require additional repairs, most often replacement of their RVOTs. This has made pulmonary valve replacement the most common surgery for adult survivors of congenital heart disease. The numbers of teen or adult patients who require a new RVOT will steadily increase as more of these children survive.
Dr. James Jaggers, professor of surgery at the University of Colorado and chief of cardiothoracic surgery at Children’s Hospital Colorado in Denver, made these comments in an interview. He had no disclosures.
The intermediate-term results reported by Dr. Schubmehl using a Carpentier-Edwards conduit in the right-ventricular outflow tract are clearly better than what we have seen using other types of valves and conduits in this position. If the valve and conduit they used persists with similar performance beyond 10 years, it would be a very good option. However, what typically happens is that replacement valves look good for about 10 years and then start to fail, often with a steep failure curve. I suspect that during the next 10 years of follow-up many more of the valves they placed will start to fail. The 10- to 20-year follow-up period is critical for demonstrating long-term durability of this valve and conduit.
 |
Dr. James Jaggers |
One additional potential advantage of the Carpentier-Edwards prosthesis is that the valve it contains is larger than the usual valve placed in the right ventricular outflow tract (RVOT). Failed valves increasingly are replaced by a transcatheter approach that puts a new valve inside the old, failed valve. As patients who received these replacement valves continue to survive we anticipate their need over time for a series of valve-in-valve procedures. The larger the valve at the outset, the more feasible it will be to have multiple episodes of valve-in-valve replacement.
At one time, we regarded early surgical repair of a tetralogy of Fallot defect as curative. We now know that as children with a repaired tetralogy of Fallot grow into teens and adults they require additional repairs, most often replacement of their RVOTs. This has made pulmonary valve replacement the most common surgery for adult survivors of congenital heart disease. The numbers of teen or adult patients who require a new RVOT will steadily increase as more of these children survive.
Dr. James Jaggers, professor of surgery at the University of Colorado and chief of cardiothoracic surgery at Children’s Hospital Colorado in Denver, made these comments in an interview. He had no disclosures.
PHOENIX – A prosthetic conduit that contains a porcine valve showed excellent intermediate-term durability for repairing the right ventricular outflow tract in 100 teenagers and young adults at a single U.S. center.
“The Carpentier-Edwards xenograft for right ventricular outflow tract [RVOT] reconstruction provides excellent freedom from reoperation and valve dysfunction, as well as sustained improvement in right-ventricular chamber size at intermediate-term follow-up,” Dr. Heidi B. Schubmehl said at the Society of Thoracic Surgeons annual meeting.
Dr. Schubmehl reported a 92% rate of freedom from valve dysfunction with follow-up out to about 10 years, and significant reductions in right ventricular size at follow-up, compared with baseline, as measured by both echocardiography and by MRI.
The Carpentier-Edwards porcine valve and conduit “seemed to hold up better than a lot of other [prosthetic] valves,” said Dr. George M. Alfieris, director of pediatric cardiac surgery at the University of Rochester (N.Y.), and senior author for the study. In addition to the valve’s durability over approximately the first 10 years following placement, the results also showed the positive impact the valve had on right ventricular size, an important result of the repair’s efficacy, Dr. Alfieris said.
“It’s a mistake to allow the right ventricle to be under high pressure or to reach a large volume. We now focus on preserving the right ventricle,” he said in an interview. “I’ve become very concerned about preventing right ventricular dilation and preserving right ventricular function.”
Dr. Alfieris noted that his prior experience using other types of valves in the pulmonary valve and RVOT position showed those valves “did great for the first 10 years and then failed. What’s different in this series is that after 10 years, we have not seen the same dysfunction as with the prior generation of valves. I will be very interested to see what happens to them” as follow-up continues beyond 10 years. He also expressed dismay that recently the company that had been marketing the valve and conduit used in the current study, the Carpentier-Edwards, stopped selling them. He expects that as his supply of conduits runs out he’ll have to start using a different commercial valve and conduit that he believes will not perform as well or create his own conduits with a porcine valve from a different supplier.
The series of 100 patients comprised individuals aged 17 or older who received a pulmonary artery and had RVOT reconstruction at the University of Rochester during 2000-2010, Dr. Schubmehl reported. The series included 78 patients with a history of tetralogy of Fallot, 8 patients born with transposition of their great arteries, 8 patients with truncus arteriosus, and 6 patients with other congenital heart diseases. Their median age at the time they received the RVOT conduit was 24 years, 59% were men, and 99 had undergone a prior sternotomy. At the time they received the conduit, 55 had pulmonary valve insufficiency, 30 had valve stenosis, and 15 had both. Follow-up occurred an average of 7 years after conduit placement.
Two recipients died: One death occurred perioperatively in a 41-year old who had a massive cerebrovascular event, and the second death was in a 39-year old who died 2.6 years after conduit placement from respiratory failure. Two additional patients required a reintervention during follow-up, said Dr. Schubmehl, a general surgeon at the University of Rochester. One reintervention occurred after 11 years to treat endocarditis, and the second after 11 years to perform balloon valvuloplasty because of valve stenosis.
The results reported by Dr. Schubmehl for echocardiography examinations showed that the patients had a statistically significant reduction in their RVOT pressure gradient from baseline to 1-year follow-up that was sustained through their intermediate-term follow-up. Seventy-seven patients had pulmonary valve insufficiency at baseline that resolved in all patients at 1-year follow-up and remained resolved in all but one patient at extended follow-up. Nineteen patients underwent additional imaging with MRI at an average follow-up of 7 years, and these findings confirmed the echo results.
On Twitter @mitchelzoler
PHOENIX – A prosthetic conduit that contains a porcine valve showed excellent intermediate-term durability for repairing the right ventricular outflow tract in 100 teenagers and young adults at a single U.S. center.
“The Carpentier-Edwards xenograft for right ventricular outflow tract [RVOT] reconstruction provides excellent freedom from reoperation and valve dysfunction, as well as sustained improvement in right-ventricular chamber size at intermediate-term follow-up,” Dr. Heidi B. Schubmehl said at the Society of Thoracic Surgeons annual meeting.
Dr. Schubmehl reported a 92% rate of freedom from valve dysfunction with follow-up out to about 10 years, and significant reductions in right ventricular size at follow-up, compared with baseline, as measured by both echocardiography and by MRI.
The Carpentier-Edwards porcine valve and conduit “seemed to hold up better than a lot of other [prosthetic] valves,” said Dr. George M. Alfieris, director of pediatric cardiac surgery at the University of Rochester (N.Y.), and senior author for the study. In addition to the valve’s durability over approximately the first 10 years following placement, the results also showed the positive impact the valve had on right ventricular size, an important result of the repair’s efficacy, Dr. Alfieris said.
“It’s a mistake to allow the right ventricle to be under high pressure or to reach a large volume. We now focus on preserving the right ventricle,” he said in an interview. “I’ve become very concerned about preventing right ventricular dilation and preserving right ventricular function.”
Dr. Alfieris noted that his prior experience using other types of valves in the pulmonary valve and RVOT position showed those valves “did great for the first 10 years and then failed. What’s different in this series is that after 10 years, we have not seen the same dysfunction as with the prior generation of valves. I will be very interested to see what happens to them” as follow-up continues beyond 10 years. He also expressed dismay that recently the company that had been marketing the valve and conduit used in the current study, the Carpentier-Edwards, stopped selling them. He expects that as his supply of conduits runs out he’ll have to start using a different commercial valve and conduit that he believes will not perform as well or create his own conduits with a porcine valve from a different supplier.
The series of 100 patients comprised individuals aged 17 or older who received a pulmonary artery and had RVOT reconstruction at the University of Rochester during 2000-2010, Dr. Schubmehl reported. The series included 78 patients with a history of tetralogy of Fallot, 8 patients born with transposition of their great arteries, 8 patients with truncus arteriosus, and 6 patients with other congenital heart diseases. Their median age at the time they received the RVOT conduit was 24 years, 59% were men, and 99 had undergone a prior sternotomy. At the time they received the conduit, 55 had pulmonary valve insufficiency, 30 had valve stenosis, and 15 had both. Follow-up occurred an average of 7 years after conduit placement.
Two recipients died: One death occurred perioperatively in a 41-year old who had a massive cerebrovascular event, and the second death was in a 39-year old who died 2.6 years after conduit placement from respiratory failure. Two additional patients required a reintervention during follow-up, said Dr. Schubmehl, a general surgeon at the University of Rochester. One reintervention occurred after 11 years to treat endocarditis, and the second after 11 years to perform balloon valvuloplasty because of valve stenosis.
The results reported by Dr. Schubmehl for echocardiography examinations showed that the patients had a statistically significant reduction in their RVOT pressure gradient from baseline to 1-year follow-up that was sustained through their intermediate-term follow-up. Seventy-seven patients had pulmonary valve insufficiency at baseline that resolved in all patients at 1-year follow-up and remained resolved in all but one patient at extended follow-up. Nineteen patients underwent additional imaging with MRI at an average follow-up of 7 years, and these findings confirmed the echo results.
On Twitter @mitchelzoler
AT THE STS ANNUAL MEETING
Key clinical point: A prosthetic conduit with a porcine valve showed excellent durability for congenital heart defect repairs at intermediate-term follow-up.
Major finding: After an average 7-year follow-up, the replacement valve and conduit had a 92% rate of freedom from valve dysfunction.
Data source: Single-center series of 100 patients.
Disclosures: Dr. Schubmehl and Dr. Alfieris had no disclosures.
Survival is Heightened with the Use of Bisphophonate
NEW YORK (Reuters Health) - Bisphosphonate use is associated with better survival in patients admitted to the intensive care unit (ICU), according to Australian researchers.
As Dr. Paul Lee told Reuters Health by email, "Bone loss in critical illness may have wider effects on the body beyond bone itself, and bisphosphonates, by reducing bone loss, may attenuate these potentially adverse effects on the body."
Increased bone resorption is known to predict mortality in the community setting, Dr. Lee of the Gavan Institute of Medical Research in Sydney and colleagues note in the Journal of Clinical Endocrinology and Metabolism, online January 18. The team theorized that mortality would be lower among patients treated with bisphosphonates prior to their acute illness.
To investigate, they examined data on more than 7,800 patients admitted to the ICU between 2003 and 2014; 245 had received bisphosphonates before admission.
The bisphosphonate users were older and had more co-morbidities, yet their in-hospital mortality rate was significantly lower than that of non-users(mortality rate ratio, 0.41; p<0.01). The difference remained significant after adjusting for factors including age, sex, and principal diagnosis.
Bisphosphonate-associated survival benefit was independent of vitamin D use, but bisphosphonate and vitamin D co-use was associated with a further reduction in mortality (MRR, 0.38).
A substudy involving CT scans of 37 patients with preadmission bisphosphonate use and 74 matched patients without such use found that baseline bone density was significantly lower among bisphosphonate users. However, all users survived admission whereas six of the non-users died.
The researchers speculate that the apparent benefits of bisphosphonate "may be partly related to modulation of systemic inflammation through antibone resorption."
However, Dr. Lee made it clear that "causality is not proven in the study, and prospective intervention trials are required to evaluate effects of bisphosphonates in critical illness."
NEW YORK (Reuters Health) - Bisphosphonate use is associated with better survival in patients admitted to the intensive care unit (ICU), according to Australian researchers.
As Dr. Paul Lee told Reuters Health by email, "Bone loss in critical illness may have wider effects on the body beyond bone itself, and bisphosphonates, by reducing bone loss, may attenuate these potentially adverse effects on the body."
Increased bone resorption is known to predict mortality in the community setting, Dr. Lee of the Gavan Institute of Medical Research in Sydney and colleagues note in the Journal of Clinical Endocrinology and Metabolism, online January 18. The team theorized that mortality would be lower among patients treated with bisphosphonates prior to their acute illness.
To investigate, they examined data on more than 7,800 patients admitted to the ICU between 2003 and 2014; 245 had received bisphosphonates before admission.
The bisphosphonate users were older and had more co-morbidities, yet their in-hospital mortality rate was significantly lower than that of non-users(mortality rate ratio, 0.41; p<0.01). The difference remained significant after adjusting for factors including age, sex, and principal diagnosis.
Bisphosphonate-associated survival benefit was independent of vitamin D use, but bisphosphonate and vitamin D co-use was associated with a further reduction in mortality (MRR, 0.38).
A substudy involving CT scans of 37 patients with preadmission bisphosphonate use and 74 matched patients without such use found that baseline bone density was significantly lower among bisphosphonate users. However, all users survived admission whereas six of the non-users died.
The researchers speculate that the apparent benefits of bisphosphonate "may be partly related to modulation of systemic inflammation through antibone resorption."
However, Dr. Lee made it clear that "causality is not proven in the study, and prospective intervention trials are required to evaluate effects of bisphosphonates in critical illness."
NEW YORK (Reuters Health) - Bisphosphonate use is associated with better survival in patients admitted to the intensive care unit (ICU), according to Australian researchers.
As Dr. Paul Lee told Reuters Health by email, "Bone loss in critical illness may have wider effects on the body beyond bone itself, and bisphosphonates, by reducing bone loss, may attenuate these potentially adverse effects on the body."
Increased bone resorption is known to predict mortality in the community setting, Dr. Lee of the Gavan Institute of Medical Research in Sydney and colleagues note in the Journal of Clinical Endocrinology and Metabolism, online January 18. The team theorized that mortality would be lower among patients treated with bisphosphonates prior to their acute illness.
To investigate, they examined data on more than 7,800 patients admitted to the ICU between 2003 and 2014; 245 had received bisphosphonates before admission.
The bisphosphonate users were older and had more co-morbidities, yet their in-hospital mortality rate was significantly lower than that of non-users(mortality rate ratio, 0.41; p<0.01). The difference remained significant after adjusting for factors including age, sex, and principal diagnosis.
Bisphosphonate-associated survival benefit was independent of vitamin D use, but bisphosphonate and vitamin D co-use was associated with a further reduction in mortality (MRR, 0.38).
A substudy involving CT scans of 37 patients with preadmission bisphosphonate use and 74 matched patients without such use found that baseline bone density was significantly lower among bisphosphonate users. However, all users survived admission whereas six of the non-users died.
The researchers speculate that the apparent benefits of bisphosphonate "may be partly related to modulation of systemic inflammation through antibone resorption."
However, Dr. Lee made it clear that "causality is not proven in the study, and prospective intervention trials are required to evaluate effects of bisphosphonates in critical illness."
VIDEO: New topical acne therapies will target sebum
WAIKOLOA, HAWAII – Three new approaches to topical treatment of acne are on the horizon, and they all share a common foe: sebum.
“One exciting new avenue for topical therapy are drugs that actually target the production of sebum,” explained Dr. Linda F. Stein Gold, director of dermatology research at Henry Ford Health System, Detroit. “For the first time, we have a drug that potentially targets sebum with a topical mechanism. In the past, we’ve only been able to do that with oral therapy.”
In an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Stein Gold discussed three topical, sebum-focused drugs in clinical trials and outlined their differing mechanisms of action.
SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Three new approaches to topical treatment of acne are on the horizon, and they all share a common foe: sebum.
“One exciting new avenue for topical therapy are drugs that actually target the production of sebum,” explained Dr. Linda F. Stein Gold, director of dermatology research at Henry Ford Health System, Detroit. “For the first time, we have a drug that potentially targets sebum with a topical mechanism. In the past, we’ve only been able to do that with oral therapy.”
In an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Stein Gold discussed three topical, sebum-focused drugs in clinical trials and outlined their differing mechanisms of action.
SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Three new approaches to topical treatment of acne are on the horizon, and they all share a common foe: sebum.
“One exciting new avenue for topical therapy are drugs that actually target the production of sebum,” explained Dr. Linda F. Stein Gold, director of dermatology research at Henry Ford Health System, Detroit. “For the first time, we have a drug that potentially targets sebum with a topical mechanism. In the past, we’ve only been able to do that with oral therapy.”
In an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Stein Gold discussed three topical, sebum-focused drugs in clinical trials and outlined their differing mechanisms of action.
SDEF and this news organization are owned by the same parent company.
AT SDEF HAWAII DERMATOLOGY SEMINAR
Dr. Hospitalist: Routine Provider Evaluations Are a Necessary, Valuable Tool
Dear Dr. Hospitalist:
We have several physicians in our large academic group whom I hate to follow when picking up teams. There have only been a few situations when I thought there was a clear knowledge deficit, but the most irritating problem is that they don’t discharge patients. I’ve only been in the group for several years, so I don’t want to come across as a complainer. However, I am concerned about poor patient care and the work left to me to discharge patients. How can I help these physicians improve without damaging my relationship with them?
Dr. Frustrated
Dr. Hospitalist responds:
You bring up a problem that I’m certain many of us in hospital medicine have experienced at some point in our career. Since the “practice” of medicine can often be done with much variability, there are many gray areas that occur during the care of patients. However, we all know it is the transitioning of patients into and out of the hospital that is the most labor-intensive period of their care. If at all possible, the discharge process is best performed by the person with the most longitudinal knowledge of the patient’s hospital course.
Your leadership team has the responsibility to assess the quality and quantity of work of all team members. The periodic assessment of a clinician’s skill and aptitude, as well as the safety of care delivered to patients, can be done in several ways. Typically, the initial assessment is done by focused professional practice evaluations (FPPEs) and later by ongoing professional practice evaluations (OPPEs). The Joint Commission created these tools in 2007 to help determine if the quality of care by clinicians fell below an acceptable level.
FPPEs, as defined by the commission, are “the time limited evaluation of practitioner competence in performing a specific privilege.” They are usually done three to six months after the initial credentialing period, when a new or additional privilege is requested after the initial appointment, or when a condition or issue affecting the delivery of safe and high-quality care is identified.
OPPEs, as the name suggests, are typically done on an ongoing basis (usually annually). These practitioner-specific reports are best utilized as screening tools, and when unusual or aberrant tendencies are observed, a more detailed analysis typically is required.
Although these formal evaluations are carried out by chart review and analysis of data collected by the hospital, they should always be supported by discreet and candid conversations with other frontline team members. It is during these sessions that individuals should take the opportunity to express their opinions regarding the care delivered by their colleagues. From my experience, because of the shared care of patients in hospital medicine, if there is a problem with an individual’s professionalism or clinical abilities, it is usually well-known by others in the group.
If for some reason group leaders are not performing these mandated evaluations (and thus risking regulatory sanctions) or don’t have a formal mechanism in place, I would encourage them to establish one. In the interim, I would discreetly address the individuals and share your concerns. Many times, the problems you mention can be resolved with awareness, mentoring, and/or proctoring, but like any needed corrective actions, they must first be acknowledged.
Good luck! TH
Dear Dr. Hospitalist:
We have several physicians in our large academic group whom I hate to follow when picking up teams. There have only been a few situations when I thought there was a clear knowledge deficit, but the most irritating problem is that they don’t discharge patients. I’ve only been in the group for several years, so I don’t want to come across as a complainer. However, I am concerned about poor patient care and the work left to me to discharge patients. How can I help these physicians improve without damaging my relationship with them?
Dr. Frustrated
Dr. Hospitalist responds:
You bring up a problem that I’m certain many of us in hospital medicine have experienced at some point in our career. Since the “practice” of medicine can often be done with much variability, there are many gray areas that occur during the care of patients. However, we all know it is the transitioning of patients into and out of the hospital that is the most labor-intensive period of their care. If at all possible, the discharge process is best performed by the person with the most longitudinal knowledge of the patient’s hospital course.
Your leadership team has the responsibility to assess the quality and quantity of work of all team members. The periodic assessment of a clinician’s skill and aptitude, as well as the safety of care delivered to patients, can be done in several ways. Typically, the initial assessment is done by focused professional practice evaluations (FPPEs) and later by ongoing professional practice evaluations (OPPEs). The Joint Commission created these tools in 2007 to help determine if the quality of care by clinicians fell below an acceptable level.
FPPEs, as defined by the commission, are “the time limited evaluation of practitioner competence in performing a specific privilege.” They are usually done three to six months after the initial credentialing period, when a new or additional privilege is requested after the initial appointment, or when a condition or issue affecting the delivery of safe and high-quality care is identified.
OPPEs, as the name suggests, are typically done on an ongoing basis (usually annually). These practitioner-specific reports are best utilized as screening tools, and when unusual or aberrant tendencies are observed, a more detailed analysis typically is required.
Although these formal evaluations are carried out by chart review and analysis of data collected by the hospital, they should always be supported by discreet and candid conversations with other frontline team members. It is during these sessions that individuals should take the opportunity to express their opinions regarding the care delivered by their colleagues. From my experience, because of the shared care of patients in hospital medicine, if there is a problem with an individual’s professionalism or clinical abilities, it is usually well-known by others in the group.
If for some reason group leaders are not performing these mandated evaluations (and thus risking regulatory sanctions) or don’t have a formal mechanism in place, I would encourage them to establish one. In the interim, I would discreetly address the individuals and share your concerns. Many times, the problems you mention can be resolved with awareness, mentoring, and/or proctoring, but like any needed corrective actions, they must first be acknowledged.
Good luck! TH
Dear Dr. Hospitalist:
We have several physicians in our large academic group whom I hate to follow when picking up teams. There have only been a few situations when I thought there was a clear knowledge deficit, but the most irritating problem is that they don’t discharge patients. I’ve only been in the group for several years, so I don’t want to come across as a complainer. However, I am concerned about poor patient care and the work left to me to discharge patients. How can I help these physicians improve without damaging my relationship with them?
Dr. Frustrated
Dr. Hospitalist responds:
You bring up a problem that I’m certain many of us in hospital medicine have experienced at some point in our career. Since the “practice” of medicine can often be done with much variability, there are many gray areas that occur during the care of patients. However, we all know it is the transitioning of patients into and out of the hospital that is the most labor-intensive period of their care. If at all possible, the discharge process is best performed by the person with the most longitudinal knowledge of the patient’s hospital course.
Your leadership team has the responsibility to assess the quality and quantity of work of all team members. The periodic assessment of a clinician’s skill and aptitude, as well as the safety of care delivered to patients, can be done in several ways. Typically, the initial assessment is done by focused professional practice evaluations (FPPEs) and later by ongoing professional practice evaluations (OPPEs). The Joint Commission created these tools in 2007 to help determine if the quality of care by clinicians fell below an acceptable level.
FPPEs, as defined by the commission, are “the time limited evaluation of practitioner competence in performing a specific privilege.” They are usually done three to six months after the initial credentialing period, when a new or additional privilege is requested after the initial appointment, or when a condition or issue affecting the delivery of safe and high-quality care is identified.
OPPEs, as the name suggests, are typically done on an ongoing basis (usually annually). These practitioner-specific reports are best utilized as screening tools, and when unusual or aberrant tendencies are observed, a more detailed analysis typically is required.
Although these formal evaluations are carried out by chart review and analysis of data collected by the hospital, they should always be supported by discreet and candid conversations with other frontline team members. It is during these sessions that individuals should take the opportunity to express their opinions regarding the care delivered by their colleagues. From my experience, because of the shared care of patients in hospital medicine, if there is a problem with an individual’s professionalism or clinical abilities, it is usually well-known by others in the group.
If for some reason group leaders are not performing these mandated evaluations (and thus risking regulatory sanctions) or don’t have a formal mechanism in place, I would encourage them to establish one. In the interim, I would discreetly address the individuals and share your concerns. Many times, the problems you mention can be resolved with awareness, mentoring, and/or proctoring, but like any needed corrective actions, they must first be acknowledged.
Good luck! TH
Drug exhibits activity against myeloma, solid tumors

Image courtesy of PNAS
Researchers say they have determined how the investigational drug ONC201 is active against a range of malignancies.
The team found that ONC201 induced apoptosis and cell cycle arrest in multiple myeloma (MM) and solid tumor cell lines.
The drug triggered an increase in the anticancer protein TRAIL and induced cell death through an integrated stress response (ISR) involving the transcription factor ATF4, the transactivator CHOP, and the TRAIL receptor DR5.
The researchers reported these findings in Science Signaling. Some researchers involved in this study are affiliated with Oncoceutics Inc., the company developing ONC201.
“We have revealed, in unprecedented detail, exactly how ONC201 works across a broad range of tumor types, and this has important clinical implications,” said study author Wafik El-Deiry, MD, PhD, of Fox Chase Cancer Center in Philadelphia, Pennsylvania.
“For example, our findings suggest that patients with various solid tumors, as well as multiple myeloma, may be particularly sensitive to the effects of ONC201. We have identified a potential biomarker that could be used to select which patients are most likely to benefit therapeutically from this drug.”
Dr El-Deiry noted that TRAIL has been shown to induce cell death in a range of cancers while sparing normal cells. However, the therapeutic benefit of stimulating TRAIL is limited because of undesirable drug properties, such as a short half-life, difficult and expensive production, the need to give treatment as an intravenous infusion, and poor penetration into certain tissues like the brain.
“This prompted us to look for better options for therapeutics that can kill tumor cells,” Dr El-Deiry said.
He and his colleagues turned to ONC201, which has been shown to stimulate TRAIL. They tested the drug in 23 cancer cell lines representing 9 tumor types—MM, lymphoma, and glioma, as well as lung, colorectal, thyroid, liver, prostate, and breast cancer.
The team found that ONC201 triggers an increase in TRAIL and TRAIL receptor abundance, leading to tumor cell death through the ISR that tumor cells normally use to survive. ONC201 pushes the ISR too far, causing tumor cells to stop dividing and/or die.
ONC201 boosted expression of the gene encoding ATF4, a central component of the ISR, through a translation initiation factor called eIF2α. This process rapidly arrested the cancer’s cell cycle and resulted in cell death.
In essence, ONC201 delivers a double-whammy to tumor cells, Dr El-Deiry said, which may explain why it has such broad-spectrum anticancer activity.
The researchers believe this study has several clinical implications. For one, it suggests that solid tumors or MM cells that normally create large amounts of protein during growth may be particularly sensitive to ONC201. The ISR is often activated in these cells, and ONC201 may push them over the edge.
“Knowing how ONC201 works helps us look for its effects in patient’s tumor cells that have been treated,” Dr El-Deiry said. “Looking in a tumor or liquid biopsy before and after treatment may help predict who is most likely to benefit.”
“We are optimistic that, through basic and clinical research with ONC201, our findings will lead to improved TRAIL-based therapies for individual cancer patients in the future.”
Another study of ONC201, this one focusing only on hematologic malignancies, has been published in Science Signaling.
Early stage clinical trials for ONC201 are currently underway in patients with brain, colorectal, breast, and lung tumors, as well as leukemia and lymphoma. ![]()

Image courtesy of PNAS
Researchers say they have determined how the investigational drug ONC201 is active against a range of malignancies.
The team found that ONC201 induced apoptosis and cell cycle arrest in multiple myeloma (MM) and solid tumor cell lines.
The drug triggered an increase in the anticancer protein TRAIL and induced cell death through an integrated stress response (ISR) involving the transcription factor ATF4, the transactivator CHOP, and the TRAIL receptor DR5.
The researchers reported these findings in Science Signaling. Some researchers involved in this study are affiliated with Oncoceutics Inc., the company developing ONC201.
“We have revealed, in unprecedented detail, exactly how ONC201 works across a broad range of tumor types, and this has important clinical implications,” said study author Wafik El-Deiry, MD, PhD, of Fox Chase Cancer Center in Philadelphia, Pennsylvania.
“For example, our findings suggest that patients with various solid tumors, as well as multiple myeloma, may be particularly sensitive to the effects of ONC201. We have identified a potential biomarker that could be used to select which patients are most likely to benefit therapeutically from this drug.”
Dr El-Deiry noted that TRAIL has been shown to induce cell death in a range of cancers while sparing normal cells. However, the therapeutic benefit of stimulating TRAIL is limited because of undesirable drug properties, such as a short half-life, difficult and expensive production, the need to give treatment as an intravenous infusion, and poor penetration into certain tissues like the brain.
“This prompted us to look for better options for therapeutics that can kill tumor cells,” Dr El-Deiry said.
He and his colleagues turned to ONC201, which has been shown to stimulate TRAIL. They tested the drug in 23 cancer cell lines representing 9 tumor types—MM, lymphoma, and glioma, as well as lung, colorectal, thyroid, liver, prostate, and breast cancer.
The team found that ONC201 triggers an increase in TRAIL and TRAIL receptor abundance, leading to tumor cell death through the ISR that tumor cells normally use to survive. ONC201 pushes the ISR too far, causing tumor cells to stop dividing and/or die.
ONC201 boosted expression of the gene encoding ATF4, a central component of the ISR, through a translation initiation factor called eIF2α. This process rapidly arrested the cancer’s cell cycle and resulted in cell death.
In essence, ONC201 delivers a double-whammy to tumor cells, Dr El-Deiry said, which may explain why it has such broad-spectrum anticancer activity.
The researchers believe this study has several clinical implications. For one, it suggests that solid tumors or MM cells that normally create large amounts of protein during growth may be particularly sensitive to ONC201. The ISR is often activated in these cells, and ONC201 may push them over the edge.
“Knowing how ONC201 works helps us look for its effects in patient’s tumor cells that have been treated,” Dr El-Deiry said. “Looking in a tumor or liquid biopsy before and after treatment may help predict who is most likely to benefit.”
“We are optimistic that, through basic and clinical research with ONC201, our findings will lead to improved TRAIL-based therapies for individual cancer patients in the future.”
Another study of ONC201, this one focusing only on hematologic malignancies, has been published in Science Signaling.
Early stage clinical trials for ONC201 are currently underway in patients with brain, colorectal, breast, and lung tumors, as well as leukemia and lymphoma. ![]()

Image courtesy of PNAS
Researchers say they have determined how the investigational drug ONC201 is active against a range of malignancies.
The team found that ONC201 induced apoptosis and cell cycle arrest in multiple myeloma (MM) and solid tumor cell lines.
The drug triggered an increase in the anticancer protein TRAIL and induced cell death through an integrated stress response (ISR) involving the transcription factor ATF4, the transactivator CHOP, and the TRAIL receptor DR5.
The researchers reported these findings in Science Signaling. Some researchers involved in this study are affiliated with Oncoceutics Inc., the company developing ONC201.
“We have revealed, in unprecedented detail, exactly how ONC201 works across a broad range of tumor types, and this has important clinical implications,” said study author Wafik El-Deiry, MD, PhD, of Fox Chase Cancer Center in Philadelphia, Pennsylvania.
“For example, our findings suggest that patients with various solid tumors, as well as multiple myeloma, may be particularly sensitive to the effects of ONC201. We have identified a potential biomarker that could be used to select which patients are most likely to benefit therapeutically from this drug.”
Dr El-Deiry noted that TRAIL has been shown to induce cell death in a range of cancers while sparing normal cells. However, the therapeutic benefit of stimulating TRAIL is limited because of undesirable drug properties, such as a short half-life, difficult and expensive production, the need to give treatment as an intravenous infusion, and poor penetration into certain tissues like the brain.
“This prompted us to look for better options for therapeutics that can kill tumor cells,” Dr El-Deiry said.
He and his colleagues turned to ONC201, which has been shown to stimulate TRAIL. They tested the drug in 23 cancer cell lines representing 9 tumor types—MM, lymphoma, and glioma, as well as lung, colorectal, thyroid, liver, prostate, and breast cancer.
The team found that ONC201 triggers an increase in TRAIL and TRAIL receptor abundance, leading to tumor cell death through the ISR that tumor cells normally use to survive. ONC201 pushes the ISR too far, causing tumor cells to stop dividing and/or die.
ONC201 boosted expression of the gene encoding ATF4, a central component of the ISR, through a translation initiation factor called eIF2α. This process rapidly arrested the cancer’s cell cycle and resulted in cell death.
In essence, ONC201 delivers a double-whammy to tumor cells, Dr El-Deiry said, which may explain why it has such broad-spectrum anticancer activity.
The researchers believe this study has several clinical implications. For one, it suggests that solid tumors or MM cells that normally create large amounts of protein during growth may be particularly sensitive to ONC201. The ISR is often activated in these cells, and ONC201 may push them over the edge.
“Knowing how ONC201 works helps us look for its effects in patient’s tumor cells that have been treated,” Dr El-Deiry said. “Looking in a tumor or liquid biopsy before and after treatment may help predict who is most likely to benefit.”
“We are optimistic that, through basic and clinical research with ONC201, our findings will lead to improved TRAIL-based therapies for individual cancer patients in the future.”
Another study of ONC201, this one focusing only on hematologic malignancies, has been published in Science Signaling.
Early stage clinical trials for ONC201 are currently underway in patients with brain, colorectal, breast, and lung tumors, as well as leukemia and lymphoma. ![]()
Drug shows promise for treating resistant AML, MCL

Preclinical research suggests the investigational anticancer drug ONC201 can be effective against mantle cell lymphoma (MCL) and acute myeloid leukemia (AML).
ONC201 induced p53-independent apoptosis in AML and MCL cell lines and in samples from patients with either disease.
Investigators noted that p53 dysfunction occurs in more than half of malignancies and can promote resistance to standard chemotherapy.
“The clinical challenge posed by p53 abnormalities in blood malignancies is that therapeutic strategies other than standard chemotherapies are required,” said Michael Andreeff, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston.
“We found that ONC201 caused p53-independent cell death and cell cycle arrest in cell lines and in lymphoma and acute leukemia patient samples.”
Dr Andreeff and his colleagues reported these findings in Science Signaling. Some of the investigators involved in this research are affiliated with Oncoceutics Inc., the company developing ONC201.
Dr Andreeff and his colleagues assessed the effects of ONC201 against AML and MCL, in both cultured cell lines and primary cells bearing either wild-type or mutant p53.
The patient samples included those that demonstrated genetic abnormalities linked to poor prognosis (FLT3 mutations, TP53 mutations) or resistance to ibrutinib. The team also tested ONC201 in a bortezomib-resistant myeloma cell line.
The experiments showed that ONC201 exerted anticancer activity regardless of p53 status, FLT3 mutations, or drug resistance. ONC201 proved active in the bortezomib-resistant myeloma cell line and in ibrutinib-resistant samples from MCL patients.
Experiments in mice showed that ONC201 caused cell death in AML and leukemia stem cells while sparing normal bone marrow cells.
And the investigators found that combining ONC201 with the BCL-2 antagonist venetoclax (ABT-199) synergistically increased apoptosis.
Further investigation revealed that ONC201 increased translation of the stress-induced protein ATF4 through stress signals similar to those caused by unfolded protein response (UPR) and integrated stress response (ISR).
“This increase in ATF4 in ONC201-treated hematopoietic cells promoted cell death,” Dr Andreeff explained. “However, unlike with UPR and ISR, the increase in ATF4 in ONC201-treated cells was not regulated by standard molecular signaling, indicating a novel mechanism of stressing cancer cells to death regardless of p53 status.”
The investigators noted that the mechanisms of ONC201 identified in solid tumors—namely, induction of TRAIL and DR5—were not operational in leukemia and lymphoma.
A study of ONC201 in solid tumors and multiple myeloma was published alongside this study in Science Signaling.
“There is clear evidence that ONC201 has clinical potential in hematological malignancies,” Dr Andreeff noted. “Clinical trials in leukemia and lymphoma patients have recently been initiated at MD Anderson.” ![]()

Preclinical research suggests the investigational anticancer drug ONC201 can be effective against mantle cell lymphoma (MCL) and acute myeloid leukemia (AML).
ONC201 induced p53-independent apoptosis in AML and MCL cell lines and in samples from patients with either disease.
Investigators noted that p53 dysfunction occurs in more than half of malignancies and can promote resistance to standard chemotherapy.
“The clinical challenge posed by p53 abnormalities in blood malignancies is that therapeutic strategies other than standard chemotherapies are required,” said Michael Andreeff, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston.
“We found that ONC201 caused p53-independent cell death and cell cycle arrest in cell lines and in lymphoma and acute leukemia patient samples.”
Dr Andreeff and his colleagues reported these findings in Science Signaling. Some of the investigators involved in this research are affiliated with Oncoceutics Inc., the company developing ONC201.
Dr Andreeff and his colleagues assessed the effects of ONC201 against AML and MCL, in both cultured cell lines and primary cells bearing either wild-type or mutant p53.
The patient samples included those that demonstrated genetic abnormalities linked to poor prognosis (FLT3 mutations, TP53 mutations) or resistance to ibrutinib. The team also tested ONC201 in a bortezomib-resistant myeloma cell line.
The experiments showed that ONC201 exerted anticancer activity regardless of p53 status, FLT3 mutations, or drug resistance. ONC201 proved active in the bortezomib-resistant myeloma cell line and in ibrutinib-resistant samples from MCL patients.
Experiments in mice showed that ONC201 caused cell death in AML and leukemia stem cells while sparing normal bone marrow cells.
And the investigators found that combining ONC201 with the BCL-2 antagonist venetoclax (ABT-199) synergistically increased apoptosis.
Further investigation revealed that ONC201 increased translation of the stress-induced protein ATF4 through stress signals similar to those caused by unfolded protein response (UPR) and integrated stress response (ISR).
“This increase in ATF4 in ONC201-treated hematopoietic cells promoted cell death,” Dr Andreeff explained. “However, unlike with UPR and ISR, the increase in ATF4 in ONC201-treated cells was not regulated by standard molecular signaling, indicating a novel mechanism of stressing cancer cells to death regardless of p53 status.”
The investigators noted that the mechanisms of ONC201 identified in solid tumors—namely, induction of TRAIL and DR5—were not operational in leukemia and lymphoma.
A study of ONC201 in solid tumors and multiple myeloma was published alongside this study in Science Signaling.
“There is clear evidence that ONC201 has clinical potential in hematological malignancies,” Dr Andreeff noted. “Clinical trials in leukemia and lymphoma patients have recently been initiated at MD Anderson.” ![]()

Preclinical research suggests the investigational anticancer drug ONC201 can be effective against mantle cell lymphoma (MCL) and acute myeloid leukemia (AML).
ONC201 induced p53-independent apoptosis in AML and MCL cell lines and in samples from patients with either disease.
Investigators noted that p53 dysfunction occurs in more than half of malignancies and can promote resistance to standard chemotherapy.
“The clinical challenge posed by p53 abnormalities in blood malignancies is that therapeutic strategies other than standard chemotherapies are required,” said Michael Andreeff, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston.
“We found that ONC201 caused p53-independent cell death and cell cycle arrest in cell lines and in lymphoma and acute leukemia patient samples.”
Dr Andreeff and his colleagues reported these findings in Science Signaling. Some of the investigators involved in this research are affiliated with Oncoceutics Inc., the company developing ONC201.
Dr Andreeff and his colleagues assessed the effects of ONC201 against AML and MCL, in both cultured cell lines and primary cells bearing either wild-type or mutant p53.
The patient samples included those that demonstrated genetic abnormalities linked to poor prognosis (FLT3 mutations, TP53 mutations) or resistance to ibrutinib. The team also tested ONC201 in a bortezomib-resistant myeloma cell line.
The experiments showed that ONC201 exerted anticancer activity regardless of p53 status, FLT3 mutations, or drug resistance. ONC201 proved active in the bortezomib-resistant myeloma cell line and in ibrutinib-resistant samples from MCL patients.
Experiments in mice showed that ONC201 caused cell death in AML and leukemia stem cells while sparing normal bone marrow cells.
And the investigators found that combining ONC201 with the BCL-2 antagonist venetoclax (ABT-199) synergistically increased apoptosis.
Further investigation revealed that ONC201 increased translation of the stress-induced protein ATF4 through stress signals similar to those caused by unfolded protein response (UPR) and integrated stress response (ISR).
“This increase in ATF4 in ONC201-treated hematopoietic cells promoted cell death,” Dr Andreeff explained. “However, unlike with UPR and ISR, the increase in ATF4 in ONC201-treated cells was not regulated by standard molecular signaling, indicating a novel mechanism of stressing cancer cells to death regardless of p53 status.”
The investigators noted that the mechanisms of ONC201 identified in solid tumors—namely, induction of TRAIL and DR5—were not operational in leukemia and lymphoma.
A study of ONC201 in solid tumors and multiple myeloma was published alongside this study in Science Signaling.
“There is clear evidence that ONC201 has clinical potential in hematological malignancies,” Dr Andreeff noted. “Clinical trials in leukemia and lymphoma patients have recently been initiated at MD Anderson.” ![]()
NIH’s peer-review process is flawed, team says

Photo by Rhoda Baer
The peer-review process the National Institutes of Health (NIH) use to allocate government research funds to US scientists may work no better than distributing those dollars at random, according to a group of researchers.
The group said their findings, published in eLife, suggest that peer review is not necessarily funding the best science, and awarding grants by lottery might actually produce equally good, if not better, results.
“The NIH claims that they are funding the best grants by the best scientists,” said study author Arturo Casadevall, MD, PhD, of the Johns Hopkins Bloomberg School of Public Health in Baltimore, Maryland.
“While [our] data would argue that the NIH is funding a lot of very good science, they are also leaving a lot of very good science on the table. The government can’t afford to fund every good grant proposal, but the problems with the current system make it worse than awarding grants through a lottery.”
The researchers noted that the NIH rejects the majority of research grant proposals it receives. To decide which proposals to fund, the organization relies on expert panels whose members score each application. Funding decisions are made on the basis of these scores and the amount of available funds.
In recent years, the NIH has only funded those proposals ranked around the top 10%. The 2015 annual research budget for the NIH was $30.1 billion.
For their study, Dr Casadevall and his colleagues reanalyzed data on the 102,740 research project grants funded by the NIH from 1980 through 2008.
Another group of researchers previously collected the data. Their research, published in Science in 2015, suggested that peer review works, as the highest ranked research projects funded by the NIH earned the most citations.
For the current study, Dr Casadevall and his colleagues decided to look only at the top 20% of grants awarded. They found very little difference between the top-ranked projects and those projects ranked in the 20th percentile when it came to citations.
What the peer-review process can do, they determined, is discriminate between very good science and very bad science—that is, those in the top 20% versus those below the 50th percentile.
“We are not criticizing the peer reviewers,” said study author Ferric Fang, MD, of the University of Washington in Seattle.
“We are simply showing that there are limits to the ability of peer review to predict future productivity based on grant applications. This suggests that some of the resources and effort spent on ranking applications might be better spent elsewhere. While the average productivity of grants with better scores was somewhat higher, the differences were extremely small, raising questions as to whether the effort is worthwhile.”
The researchers noted that peer review isn’t cheap. The annual budget of the NIH Center for Scientific Review is $110 million. Individual NIH institutes and centers also spend money on peer review. The team said that money could be used toward more grants.
They also noted that peer review allows for substantial subjectivity. The objection of a single member of the committee can effectively kill a grant proposal, whether that objection is legitimate or not.
“When people’s opinions count a lot, we may be doing worse than choosing at random,” Dr Casadevall said. “A negative word at the table can often swing the debate. And this is how we allocate research funding in this country.”
However, Dr Casadevall and his colleagues do not recommend abandoning the peer-review process completely. They believe a way to improve the system might be to continue using peer review to identify the top proposals but then place those proposals into a lottery, with grants awarded at random. ![]()

Photo by Rhoda Baer
The peer-review process the National Institutes of Health (NIH) use to allocate government research funds to US scientists may work no better than distributing those dollars at random, according to a group of researchers.
The group said their findings, published in eLife, suggest that peer review is not necessarily funding the best science, and awarding grants by lottery might actually produce equally good, if not better, results.
“The NIH claims that they are funding the best grants by the best scientists,” said study author Arturo Casadevall, MD, PhD, of the Johns Hopkins Bloomberg School of Public Health in Baltimore, Maryland.
“While [our] data would argue that the NIH is funding a lot of very good science, they are also leaving a lot of very good science on the table. The government can’t afford to fund every good grant proposal, but the problems with the current system make it worse than awarding grants through a lottery.”
The researchers noted that the NIH rejects the majority of research grant proposals it receives. To decide which proposals to fund, the organization relies on expert panels whose members score each application. Funding decisions are made on the basis of these scores and the amount of available funds.
In recent years, the NIH has only funded those proposals ranked around the top 10%. The 2015 annual research budget for the NIH was $30.1 billion.
For their study, Dr Casadevall and his colleagues reanalyzed data on the 102,740 research project grants funded by the NIH from 1980 through 2008.
Another group of researchers previously collected the data. Their research, published in Science in 2015, suggested that peer review works, as the highest ranked research projects funded by the NIH earned the most citations.
For the current study, Dr Casadevall and his colleagues decided to look only at the top 20% of grants awarded. They found very little difference between the top-ranked projects and those projects ranked in the 20th percentile when it came to citations.
What the peer-review process can do, they determined, is discriminate between very good science and very bad science—that is, those in the top 20% versus those below the 50th percentile.
“We are not criticizing the peer reviewers,” said study author Ferric Fang, MD, of the University of Washington in Seattle.
“We are simply showing that there are limits to the ability of peer review to predict future productivity based on grant applications. This suggests that some of the resources and effort spent on ranking applications might be better spent elsewhere. While the average productivity of grants with better scores was somewhat higher, the differences were extremely small, raising questions as to whether the effort is worthwhile.”
The researchers noted that peer review isn’t cheap. The annual budget of the NIH Center for Scientific Review is $110 million. Individual NIH institutes and centers also spend money on peer review. The team said that money could be used toward more grants.
They also noted that peer review allows for substantial subjectivity. The objection of a single member of the committee can effectively kill a grant proposal, whether that objection is legitimate or not.
“When people’s opinions count a lot, we may be doing worse than choosing at random,” Dr Casadevall said. “A negative word at the table can often swing the debate. And this is how we allocate research funding in this country.”
However, Dr Casadevall and his colleagues do not recommend abandoning the peer-review process completely. They believe a way to improve the system might be to continue using peer review to identify the top proposals but then place those proposals into a lottery, with grants awarded at random. ![]()

Photo by Rhoda Baer
The peer-review process the National Institutes of Health (NIH) use to allocate government research funds to US scientists may work no better than distributing those dollars at random, according to a group of researchers.
The group said their findings, published in eLife, suggest that peer review is not necessarily funding the best science, and awarding grants by lottery might actually produce equally good, if not better, results.
“The NIH claims that they are funding the best grants by the best scientists,” said study author Arturo Casadevall, MD, PhD, of the Johns Hopkins Bloomberg School of Public Health in Baltimore, Maryland.
“While [our] data would argue that the NIH is funding a lot of very good science, they are also leaving a lot of very good science on the table. The government can’t afford to fund every good grant proposal, but the problems with the current system make it worse than awarding grants through a lottery.”
The researchers noted that the NIH rejects the majority of research grant proposals it receives. To decide which proposals to fund, the organization relies on expert panels whose members score each application. Funding decisions are made on the basis of these scores and the amount of available funds.
In recent years, the NIH has only funded those proposals ranked around the top 10%. The 2015 annual research budget for the NIH was $30.1 billion.
For their study, Dr Casadevall and his colleagues reanalyzed data on the 102,740 research project grants funded by the NIH from 1980 through 2008.
Another group of researchers previously collected the data. Their research, published in Science in 2015, suggested that peer review works, as the highest ranked research projects funded by the NIH earned the most citations.
For the current study, Dr Casadevall and his colleagues decided to look only at the top 20% of grants awarded. They found very little difference between the top-ranked projects and those projects ranked in the 20th percentile when it came to citations.
What the peer-review process can do, they determined, is discriminate between very good science and very bad science—that is, those in the top 20% versus those below the 50th percentile.
“We are not criticizing the peer reviewers,” said study author Ferric Fang, MD, of the University of Washington in Seattle.
“We are simply showing that there are limits to the ability of peer review to predict future productivity based on grant applications. This suggests that some of the resources and effort spent on ranking applications might be better spent elsewhere. While the average productivity of grants with better scores was somewhat higher, the differences were extremely small, raising questions as to whether the effort is worthwhile.”
The researchers noted that peer review isn’t cheap. The annual budget of the NIH Center for Scientific Review is $110 million. Individual NIH institutes and centers also spend money on peer review. The team said that money could be used toward more grants.
They also noted that peer review allows for substantial subjectivity. The objection of a single member of the committee can effectively kill a grant proposal, whether that objection is legitimate or not.
“When people’s opinions count a lot, we may be doing worse than choosing at random,” Dr Casadevall said. “A negative word at the table can often swing the debate. And this is how we allocate research funding in this country.”
However, Dr Casadevall and his colleagues do not recommend abandoning the peer-review process completely. They believe a way to improve the system might be to continue using peer review to identify the top proposals but then place those proposals into a lottery, with grants awarded at random. ![]()
Group identifies genes that may impact HSCT

Photo by Aaron Logan
A new screening method has revealed genes that regulate how hematopoietic stem and progenitor cells (HSPCs) grow and thrive in mice.
Researchers used this method to uncover 17 genes that are regulators of hematopoietic stem cell transplant (HSCT).
Thirteen of these genes had never before been linked to HSPC engraftment.
The researchers reported their findings in the Journal of Experimental Medicine.
“We recognized that one barrier to improving [HSCT] is a lack of understanding of how [HSPCs] successfully grow in the challenged environment of transplant, so we set out to identify the genes that control this process,” said Shannon McKinney-Freeman, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
Dr McKinney-Freeman and her colleagues transplanted more than 1300 mice with shRNA-transduced HSPCs and searched for genes that regulate HSPC repopulation.
The team identified 17 such genes—Arhgef5, Armcx1, Cadps2, Crispld1, Emcn, Foxa3, Fstl1, Glis2, Gprasp2, Gpr56, Myct1, Nbea, P2ry14, Smarca2, Sox4, Stat4, and Zfp251.
For most of these genes, knockdown yielded a loss of function. The exceptions were Armcx1 and Gprasp2, whose loss enhanced HSPC repopulation.
“Our functional screen in mice is a first step to enhancing [HSCT],” Dr McKinney-Freeman said. “If we are to improve transplant outcomes in patients, we next need to study these identified genes and the molecules they specify in much more detail.”
“The more we understand the full scope of the molecular mechanisms that regulate stable engraftment of [HSPCs], the better equipped we will be to develop and clinically test novel therapies to improve health outcomes.” ![]()

Photo by Aaron Logan
A new screening method has revealed genes that regulate how hematopoietic stem and progenitor cells (HSPCs) grow and thrive in mice.
Researchers used this method to uncover 17 genes that are regulators of hematopoietic stem cell transplant (HSCT).
Thirteen of these genes had never before been linked to HSPC engraftment.
The researchers reported their findings in the Journal of Experimental Medicine.
“We recognized that one barrier to improving [HSCT] is a lack of understanding of how [HSPCs] successfully grow in the challenged environment of transplant, so we set out to identify the genes that control this process,” said Shannon McKinney-Freeman, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
Dr McKinney-Freeman and her colleagues transplanted more than 1300 mice with shRNA-transduced HSPCs and searched for genes that regulate HSPC repopulation.
The team identified 17 such genes—Arhgef5, Armcx1, Cadps2, Crispld1, Emcn, Foxa3, Fstl1, Glis2, Gprasp2, Gpr56, Myct1, Nbea, P2ry14, Smarca2, Sox4, Stat4, and Zfp251.
For most of these genes, knockdown yielded a loss of function. The exceptions were Armcx1 and Gprasp2, whose loss enhanced HSPC repopulation.
“Our functional screen in mice is a first step to enhancing [HSCT],” Dr McKinney-Freeman said. “If we are to improve transplant outcomes in patients, we next need to study these identified genes and the molecules they specify in much more detail.”
“The more we understand the full scope of the molecular mechanisms that regulate stable engraftment of [HSPCs], the better equipped we will be to develop and clinically test novel therapies to improve health outcomes.” ![]()

Photo by Aaron Logan
A new screening method has revealed genes that regulate how hematopoietic stem and progenitor cells (HSPCs) grow and thrive in mice.
Researchers used this method to uncover 17 genes that are regulators of hematopoietic stem cell transplant (HSCT).
Thirteen of these genes had never before been linked to HSPC engraftment.
The researchers reported their findings in the Journal of Experimental Medicine.
“We recognized that one barrier to improving [HSCT] is a lack of understanding of how [HSPCs] successfully grow in the challenged environment of transplant, so we set out to identify the genes that control this process,” said Shannon McKinney-Freeman, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
Dr McKinney-Freeman and her colleagues transplanted more than 1300 mice with shRNA-transduced HSPCs and searched for genes that regulate HSPC repopulation.
The team identified 17 such genes—Arhgef5, Armcx1, Cadps2, Crispld1, Emcn, Foxa3, Fstl1, Glis2, Gprasp2, Gpr56, Myct1, Nbea, P2ry14, Smarca2, Sox4, Stat4, and Zfp251.
For most of these genes, knockdown yielded a loss of function. The exceptions were Armcx1 and Gprasp2, whose loss enhanced HSPC repopulation.
“Our functional screen in mice is a first step to enhancing [HSCT],” Dr McKinney-Freeman said. “If we are to improve transplant outcomes in patients, we next need to study these identified genes and the molecules they specify in much more detail.”
“The more we understand the full scope of the molecular mechanisms that regulate stable engraftment of [HSPCs], the better equipped we will be to develop and clinically test novel therapies to improve health outcomes.” ![]()