User login
Endologix announces FDA approval of AFX2 Bifurcated Endograft
The U.S. Food and Drug Administration has approved the AFX2 Bifurcated Endograft System for the treatment of abdominal aortic aneurysms (AAA), the device’s manufacturer, Endologix, announced in a statement.
Endologix also touts the AFX2 as a way to facilitate percutaneous endovascular aneurysm repair (EVAR) by providing low-profile contralateral access through a 7F introducer. The device incorporates Endologix’s ActiveSeal technology, DuraPly expanded polytetrafluoroethylene graft material, and the Vela proximal endograft.
The AFX2 is expected to hit the market in the United States in the first quarter of 2016.
The U.S. Food and Drug Administration has approved the AFX2 Bifurcated Endograft System for the treatment of abdominal aortic aneurysms (AAA), the device’s manufacturer, Endologix, announced in a statement.
Endologix also touts the AFX2 as a way to facilitate percutaneous endovascular aneurysm repair (EVAR) by providing low-profile contralateral access through a 7F introducer. The device incorporates Endologix’s ActiveSeal technology, DuraPly expanded polytetrafluoroethylene graft material, and the Vela proximal endograft.
The AFX2 is expected to hit the market in the United States in the first quarter of 2016.
The U.S. Food and Drug Administration has approved the AFX2 Bifurcated Endograft System for the treatment of abdominal aortic aneurysms (AAA), the device’s manufacturer, Endologix, announced in a statement.
Endologix also touts the AFX2 as a way to facilitate percutaneous endovascular aneurysm repair (EVAR) by providing low-profile contralateral access through a 7F introducer. The device incorporates Endologix’s ActiveSeal technology, DuraPly expanded polytetrafluoroethylene graft material, and the Vela proximal endograft.
The AFX2 is expected to hit the market in the United States in the first quarter of 2016.
White tea
White tea, like green tea, is derived from the plant Camellia sinensis, a member of the Theaceae family and the source of all the globally popular “true tea” beverages.
Of the four main true teas, green and white are unfermented (white is the least processed), black tea is fermented, and oolong tea is semifermented.1,2,3 White tea actually comes from the tips of the green tea leaves or leaves that have not yet fully opened, with buds covered by fine white hair. As a commodity, white tea is more expensive than green tea because it is more difficult to obtain. EGCG [(-)epigallocatechin-3-O-gallate], the most abundant and biologically active polyphenolic catechin found in green tea, is also the constituent in white tea that accounts for its antioxidant properties.4,5 Indeed, white tea is included in topical products for its antioxidant as well as antiseptic activity, and is considered a more potent antioxidant additive medium than green tea.6,1
As an ingredient in a combination formula
White tea is included in the dietary supplement Imedeen Prime Renewal, along with fish protein polysaccharides, vitamins C and E, zinc, and extracts from soy, grape seed, chamomile, and tomato.
In 2006, Skovgaard et al. conducted a 6-month, double-blind, placebo-controlled randomized study on 80 healthy postmenopausal women (38 in the treatment group, 42 in the placebo group completed the study) to determine antiaging effects on the skin. Subjects took 2 tablets of the supplement or placebo twice daily. Clinical, photo, and ultrasound evaluations showed significantly greater improvements in the treatment group, compared with the placebo group, in the face (forehead, periocular, and perioral wrinkles; mottled pigmentation, laxity, sagging, dark circles under the eyes; and overall appearance), hands, and décolletage.7
Antioxidant and antiaging activity
In 2009, Thring et al. studied the antiaging and antioxidant characteristics of 23 plant extracts (from 21 species) by considering antielastase and anticollagenase activities. White tea was found to exhibit the greatest inhibitory activity against both elastase and collagenase, greater than burdock root and angelica in terms of antielastase activity, and greater than green tea, rose tincture, and lavender in relation to anticollagenase activity. The Trolox equivalent antioxidant capacity assay also showed that white tea displayed the highest antioxidant activity. The investigators noted the very high phenolic content of white tea in characterizing its potent inhibitory activity against enzymes that accelerate cutaneous aging.6
Earlier in 2009, Camouse et al. examined skin samples from volunteers or skin explants treated with topical white or green tea after ultraviolet exposure to ascertain that the antioxidant could prevent simulated solar radiation–induced damage to DNA and Langerhans cells. They noted that each product displayed a sun protection factor of 1, suggesting that the photoprotection conferred was not due to direct UV absorption. Both forms of topically applied tea extracts were equally effective and judged by the researchers to be potential photoprotective agents when used along with other substantiated approaches to skin protection. These findings provided the first reported evidence of topically applied white tea preventing UV-induced immunosuppression. The researchers further suggested that the color of white tea might render it more cosmetically desirable than green tea.8
It should be noted that a systematic review performed by Hunt et al. in 2010 of MEDLINE, Embase, CINAHL (Cumulative Index to Nursing and Allied Health Literature), CENTRAL (Cochrane Central Register of Controlled Trials), and AMED (Allied and Complementary Medicine Database) databases up to 2009 identified 11 randomized clinical or controlled clinical trials evaluating the effectiveness of botanical extracts for diminishing wrinkling and other signs of cutaneous aging. No significant reductions in wrinkling were associated with the use of green tea or Vitaphenol (a combination of green and white teas, mangosteen, and pomegranate extract). The authors noted, however, that all of the trials that they identified were characterized by poor methodologic quality.9
Thring et al. conducted an in vitro study in 2011 to evaluate the antioxidant and anti-inflammatory activity of white tea, rose, and witch hazel extracts in primary human skin fibroblasts. The investigators measured significant anticollagenase, antielastase, and antioxidant activities for the white tea extracts, which also spurred a significant reduction in the interleukin-8 amount synthesized by fibroblasts, compared with controls. They concluded that white tea (as well as the other extracts) yielded a protective effect on fibroblasts against damage induced by hydrogen peroxide exposure.10
In 2014, Azman et al. used the spin trap method and electron paramagnetic resonance (EPR) spectroscopy to show that among white tea constituents, EGCG and epicatechin-3-gallate (ECG) exhibit the greatest antiradical activity against the methoxy radical.1
Conclusion
Tea is one of the most popular beverages in the world and is touted for its antioxidant and anticancer properties. While the ingredients of green tea polyphenols have inspired a spate of recent research, much is yet to be learned about the potential health benefits of white tea, which is even less processed. Some evidence appears to suggest that white tea may be shown to be more effective overall, and in the dermatologic realm, than green tea. I look forward to seeing more research.
References
1. J Agric Food Chem. 2014;62(1):5743-8.
2. Dermatol Surg. 2005;31(7 Pt 2):873-80.
3. Oxid Med Cell Longev. 2012:2012:560682.
4. Mol Cell Biochem. 2000;206(1-2):125-32.
5. Free Radic Biol Med. 1999;26(11-12):1427-35.
6. BMC Complement Altern Med. 2009;9:27.
7. Eur J Clin Nutr. 2006;60(10):1201-6.
8. Exp Dermatol. 2009;18(6):522-6.
9. Drugs Aging. 2010;27(12):973-85.
10. J Inflamm (Lond). 2011;8(1):27).
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.
White tea, like green tea, is derived from the plant Camellia sinensis, a member of the Theaceae family and the source of all the globally popular “true tea” beverages.
Of the four main true teas, green and white are unfermented (white is the least processed), black tea is fermented, and oolong tea is semifermented.1,2,3 White tea actually comes from the tips of the green tea leaves or leaves that have not yet fully opened, with buds covered by fine white hair. As a commodity, white tea is more expensive than green tea because it is more difficult to obtain. EGCG [(-)epigallocatechin-3-O-gallate], the most abundant and biologically active polyphenolic catechin found in green tea, is also the constituent in white tea that accounts for its antioxidant properties.4,5 Indeed, white tea is included in topical products for its antioxidant as well as antiseptic activity, and is considered a more potent antioxidant additive medium than green tea.6,1
As an ingredient in a combination formula
White tea is included in the dietary supplement Imedeen Prime Renewal, along with fish protein polysaccharides, vitamins C and E, zinc, and extracts from soy, grape seed, chamomile, and tomato.
In 2006, Skovgaard et al. conducted a 6-month, double-blind, placebo-controlled randomized study on 80 healthy postmenopausal women (38 in the treatment group, 42 in the placebo group completed the study) to determine antiaging effects on the skin. Subjects took 2 tablets of the supplement or placebo twice daily. Clinical, photo, and ultrasound evaluations showed significantly greater improvements in the treatment group, compared with the placebo group, in the face (forehead, periocular, and perioral wrinkles; mottled pigmentation, laxity, sagging, dark circles under the eyes; and overall appearance), hands, and décolletage.7
Antioxidant and antiaging activity
In 2009, Thring et al. studied the antiaging and antioxidant characteristics of 23 plant extracts (from 21 species) by considering antielastase and anticollagenase activities. White tea was found to exhibit the greatest inhibitory activity against both elastase and collagenase, greater than burdock root and angelica in terms of antielastase activity, and greater than green tea, rose tincture, and lavender in relation to anticollagenase activity. The Trolox equivalent antioxidant capacity assay also showed that white tea displayed the highest antioxidant activity. The investigators noted the very high phenolic content of white tea in characterizing its potent inhibitory activity against enzymes that accelerate cutaneous aging.6
Earlier in 2009, Camouse et al. examined skin samples from volunteers or skin explants treated with topical white or green tea after ultraviolet exposure to ascertain that the antioxidant could prevent simulated solar radiation–induced damage to DNA and Langerhans cells. They noted that each product displayed a sun protection factor of 1, suggesting that the photoprotection conferred was not due to direct UV absorption. Both forms of topically applied tea extracts were equally effective and judged by the researchers to be potential photoprotective agents when used along with other substantiated approaches to skin protection. These findings provided the first reported evidence of topically applied white tea preventing UV-induced immunosuppression. The researchers further suggested that the color of white tea might render it more cosmetically desirable than green tea.8
It should be noted that a systematic review performed by Hunt et al. in 2010 of MEDLINE, Embase, CINAHL (Cumulative Index to Nursing and Allied Health Literature), CENTRAL (Cochrane Central Register of Controlled Trials), and AMED (Allied and Complementary Medicine Database) databases up to 2009 identified 11 randomized clinical or controlled clinical trials evaluating the effectiveness of botanical extracts for diminishing wrinkling and other signs of cutaneous aging. No significant reductions in wrinkling were associated with the use of green tea or Vitaphenol (a combination of green and white teas, mangosteen, and pomegranate extract). The authors noted, however, that all of the trials that they identified were characterized by poor methodologic quality.9
Thring et al. conducted an in vitro study in 2011 to evaluate the antioxidant and anti-inflammatory activity of white tea, rose, and witch hazel extracts in primary human skin fibroblasts. The investigators measured significant anticollagenase, antielastase, and antioxidant activities for the white tea extracts, which also spurred a significant reduction in the interleukin-8 amount synthesized by fibroblasts, compared with controls. They concluded that white tea (as well as the other extracts) yielded a protective effect on fibroblasts against damage induced by hydrogen peroxide exposure.10
In 2014, Azman et al. used the spin trap method and electron paramagnetic resonance (EPR) spectroscopy to show that among white tea constituents, EGCG and epicatechin-3-gallate (ECG) exhibit the greatest antiradical activity against the methoxy radical.1
Conclusion
Tea is one of the most popular beverages in the world and is touted for its antioxidant and anticancer properties. While the ingredients of green tea polyphenols have inspired a spate of recent research, much is yet to be learned about the potential health benefits of white tea, which is even less processed. Some evidence appears to suggest that white tea may be shown to be more effective overall, and in the dermatologic realm, than green tea. I look forward to seeing more research.
References
1. J Agric Food Chem. 2014;62(1):5743-8.
2. Dermatol Surg. 2005;31(7 Pt 2):873-80.
3. Oxid Med Cell Longev. 2012:2012:560682.
4. Mol Cell Biochem. 2000;206(1-2):125-32.
5. Free Radic Biol Med. 1999;26(11-12):1427-35.
6. BMC Complement Altern Med. 2009;9:27.
7. Eur J Clin Nutr. 2006;60(10):1201-6.
8. Exp Dermatol. 2009;18(6):522-6.
9. Drugs Aging. 2010;27(12):973-85.
10. J Inflamm (Lond). 2011;8(1):27).
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.
White tea, like green tea, is derived from the plant Camellia sinensis, a member of the Theaceae family and the source of all the globally popular “true tea” beverages.
Of the four main true teas, green and white are unfermented (white is the least processed), black tea is fermented, and oolong tea is semifermented.1,2,3 White tea actually comes from the tips of the green tea leaves or leaves that have not yet fully opened, with buds covered by fine white hair. As a commodity, white tea is more expensive than green tea because it is more difficult to obtain. EGCG [(-)epigallocatechin-3-O-gallate], the most abundant and biologically active polyphenolic catechin found in green tea, is also the constituent in white tea that accounts for its antioxidant properties.4,5 Indeed, white tea is included in topical products for its antioxidant as well as antiseptic activity, and is considered a more potent antioxidant additive medium than green tea.6,1
As an ingredient in a combination formula
White tea is included in the dietary supplement Imedeen Prime Renewal, along with fish protein polysaccharides, vitamins C and E, zinc, and extracts from soy, grape seed, chamomile, and tomato.
In 2006, Skovgaard et al. conducted a 6-month, double-blind, placebo-controlled randomized study on 80 healthy postmenopausal women (38 in the treatment group, 42 in the placebo group completed the study) to determine antiaging effects on the skin. Subjects took 2 tablets of the supplement or placebo twice daily. Clinical, photo, and ultrasound evaluations showed significantly greater improvements in the treatment group, compared with the placebo group, in the face (forehead, periocular, and perioral wrinkles; mottled pigmentation, laxity, sagging, dark circles under the eyes; and overall appearance), hands, and décolletage.7
Antioxidant and antiaging activity
In 2009, Thring et al. studied the antiaging and antioxidant characteristics of 23 plant extracts (from 21 species) by considering antielastase and anticollagenase activities. White tea was found to exhibit the greatest inhibitory activity against both elastase and collagenase, greater than burdock root and angelica in terms of antielastase activity, and greater than green tea, rose tincture, and lavender in relation to anticollagenase activity. The Trolox equivalent antioxidant capacity assay also showed that white tea displayed the highest antioxidant activity. The investigators noted the very high phenolic content of white tea in characterizing its potent inhibitory activity against enzymes that accelerate cutaneous aging.6
Earlier in 2009, Camouse et al. examined skin samples from volunteers or skin explants treated with topical white or green tea after ultraviolet exposure to ascertain that the antioxidant could prevent simulated solar radiation–induced damage to DNA and Langerhans cells. They noted that each product displayed a sun protection factor of 1, suggesting that the photoprotection conferred was not due to direct UV absorption. Both forms of topically applied tea extracts were equally effective and judged by the researchers to be potential photoprotective agents when used along with other substantiated approaches to skin protection. These findings provided the first reported evidence of topically applied white tea preventing UV-induced immunosuppression. The researchers further suggested that the color of white tea might render it more cosmetically desirable than green tea.8
It should be noted that a systematic review performed by Hunt et al. in 2010 of MEDLINE, Embase, CINAHL (Cumulative Index to Nursing and Allied Health Literature), CENTRAL (Cochrane Central Register of Controlled Trials), and AMED (Allied and Complementary Medicine Database) databases up to 2009 identified 11 randomized clinical or controlled clinical trials evaluating the effectiveness of botanical extracts for diminishing wrinkling and other signs of cutaneous aging. No significant reductions in wrinkling were associated with the use of green tea or Vitaphenol (a combination of green and white teas, mangosteen, and pomegranate extract). The authors noted, however, that all of the trials that they identified were characterized by poor methodologic quality.9
Thring et al. conducted an in vitro study in 2011 to evaluate the antioxidant and anti-inflammatory activity of white tea, rose, and witch hazel extracts in primary human skin fibroblasts. The investigators measured significant anticollagenase, antielastase, and antioxidant activities for the white tea extracts, which also spurred a significant reduction in the interleukin-8 amount synthesized by fibroblasts, compared with controls. They concluded that white tea (as well as the other extracts) yielded a protective effect on fibroblasts against damage induced by hydrogen peroxide exposure.10
In 2014, Azman et al. used the spin trap method and electron paramagnetic resonance (EPR) spectroscopy to show that among white tea constituents, EGCG and epicatechin-3-gallate (ECG) exhibit the greatest antiradical activity against the methoxy radical.1
Conclusion
Tea is one of the most popular beverages in the world and is touted for its antioxidant and anticancer properties. While the ingredients of green tea polyphenols have inspired a spate of recent research, much is yet to be learned about the potential health benefits of white tea, which is even less processed. Some evidence appears to suggest that white tea may be shown to be more effective overall, and in the dermatologic realm, than green tea. I look forward to seeing more research.
References
1. J Agric Food Chem. 2014;62(1):5743-8.
2. Dermatol Surg. 2005;31(7 Pt 2):873-80.
3. Oxid Med Cell Longev. 2012:2012:560682.
4. Mol Cell Biochem. 2000;206(1-2):125-32.
5. Free Radic Biol Med. 1999;26(11-12):1427-35.
6. BMC Complement Altern Med. 2009;9:27.
7. Eur J Clin Nutr. 2006;60(10):1201-6.
8. Exp Dermatol. 2009;18(6):522-6.
9. Drugs Aging. 2010;27(12):973-85.
10. J Inflamm (Lond). 2011;8(1):27).
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.
BMI negatively associated with acne lesion counts in postadolescent women
Body mass index was negatively associated with the number of acne lesions in a study of Taiwanese women, report Dr. P.H. Lu and coauthors at National Yang-Ming University in Taipei, Taiwan.
A study of 104 Taiwanese women aged 25-45 years with moderate to severe postadolescent acne vulgaris found a negative association between body mass index (BMI) and acne lesion count (P = .001).
In addition to BMI, other recorded measurements included blood pressure, fasting glucose, triglyceride levels, cholesterol levels, waist circumference, and hip circumference. Subjects were classified into four categories: BMI less than 18.5 kg/m2, BMI of 18.5 kg/m2–23.9 kg/m2, BMI of 24 kg/m2–26.9 kg/m2), or BMI greater than or equal to 27.
Acne severity was determined by the Investigator’s Global Assessment (IGA), with inclusion criteria being a score of 3 or 4 on a scale of 0 to 5. Inflammatory lesion counts were recorded by counting papules, pustules, nodules, and cysts. Noninflammatory lesions were recorded by counting comedones.
Women in the lowest BMI category had a mean of 35.5 inflammatory lesions, 7.4 noninflammatory lesions, and 42.9 total lesions, compared with patients in the 18.5-23.9 range (27.9 inflammatory, 6.6 noninflammatory, 34.0 total), and those who were in the two higher categories (18.1 inflammatory, 4.4 noninflammatory, 22.0 total), the authors reported.
“Further investigation is warranted into the association between BMI and acne in this subset of women,” Dr. Lu and associates concluded.
Read the full study in the Journal of the European Academy of Dermatology and Venereology.
Body mass index was negatively associated with the number of acne lesions in a study of Taiwanese women, report Dr. P.H. Lu and coauthors at National Yang-Ming University in Taipei, Taiwan.
A study of 104 Taiwanese women aged 25-45 years with moderate to severe postadolescent acne vulgaris found a negative association between body mass index (BMI) and acne lesion count (P = .001).
In addition to BMI, other recorded measurements included blood pressure, fasting glucose, triglyceride levels, cholesterol levels, waist circumference, and hip circumference. Subjects were classified into four categories: BMI less than 18.5 kg/m2, BMI of 18.5 kg/m2–23.9 kg/m2, BMI of 24 kg/m2–26.9 kg/m2), or BMI greater than or equal to 27.
Acne severity was determined by the Investigator’s Global Assessment (IGA), with inclusion criteria being a score of 3 or 4 on a scale of 0 to 5. Inflammatory lesion counts were recorded by counting papules, pustules, nodules, and cysts. Noninflammatory lesions were recorded by counting comedones.
Women in the lowest BMI category had a mean of 35.5 inflammatory lesions, 7.4 noninflammatory lesions, and 42.9 total lesions, compared with patients in the 18.5-23.9 range (27.9 inflammatory, 6.6 noninflammatory, 34.0 total), and those who were in the two higher categories (18.1 inflammatory, 4.4 noninflammatory, 22.0 total), the authors reported.
“Further investigation is warranted into the association between BMI and acne in this subset of women,” Dr. Lu and associates concluded.
Read the full study in the Journal of the European Academy of Dermatology and Venereology.
Body mass index was negatively associated with the number of acne lesions in a study of Taiwanese women, report Dr. P.H. Lu and coauthors at National Yang-Ming University in Taipei, Taiwan.
A study of 104 Taiwanese women aged 25-45 years with moderate to severe postadolescent acne vulgaris found a negative association between body mass index (BMI) and acne lesion count (P = .001).
In addition to BMI, other recorded measurements included blood pressure, fasting glucose, triglyceride levels, cholesterol levels, waist circumference, and hip circumference. Subjects were classified into four categories: BMI less than 18.5 kg/m2, BMI of 18.5 kg/m2–23.9 kg/m2, BMI of 24 kg/m2–26.9 kg/m2), or BMI greater than or equal to 27.
Acne severity was determined by the Investigator’s Global Assessment (IGA), with inclusion criteria being a score of 3 or 4 on a scale of 0 to 5. Inflammatory lesion counts were recorded by counting papules, pustules, nodules, and cysts. Noninflammatory lesions were recorded by counting comedones.
Women in the lowest BMI category had a mean of 35.5 inflammatory lesions, 7.4 noninflammatory lesions, and 42.9 total lesions, compared with patients in the 18.5-23.9 range (27.9 inflammatory, 6.6 noninflammatory, 34.0 total), and those who were in the two higher categories (18.1 inflammatory, 4.4 noninflammatory, 22.0 total), the authors reported.
“Further investigation is warranted into the association between BMI and acne in this subset of women,” Dr. Lu and associates concluded.
Read the full study in the Journal of the European Academy of Dermatology and Venereology.
Health Canada approves drug for acquired hemophilia A

Photo courtesy of
Baxter International Inc.
Health Canada has approved a recombinant porcine factor VIII (FVIII) product (Obizur) to treat bleeding episodes in patients with acquired hemophilia A caused by autoantibodies to FVIII.
Obizur is the first recombinant porcine treatment to be made available for acquired hemophilia A in Canada.
It is specifically designed so physicians can monitor treatment response by measuring FVIII activity levels in addition to making clinical assessments.
Health Canada’s approval is based on a phase 2/3 trial in which patients with acquired hemophilia A received Obizur as treatment for serious bleeding episodes.
Twenty-nine patients were enrolled in this trial and evaluated for safety. Researchers determined that one of the patients did not actually have acquired hemophilia A, so this patient could not be evaluated for efficacy.
At 24 hours after the initial infusion, all 28 patients in the efficacy analysis had a positive response to Obizur. This meant that bleeding stopped or decreased, the patients experienced clinical stabilization or improvement, and FVIII levels were 20% or higher.
Eighty-six percent of patients (24/28) had successful treatment of their initial bleeding episode. The overall treatment success was determined by the investigator based on the ability to discontinue or reduce the dose and/or dosing frequency of Obizur.
The adverse event most frequently reported in the 29 patients in the safety analysis was the development of inhibitors to porcine FVIII.
Nineteen patients were negative for anti-porcine FVIII antibodies at baseline, and 5 of these patients (26%) developed anti-porcine FVIII antibodies following exposure to Obizur.
Of the 10 patients with detectable anti-porcine FVIII antibodies at baseline, 2 (20%) experienced an increase in titer, and 8 (80%) decreased to a non-detectable titer.
Obizur is under development by Baxalta Incorporated. The drug is currently approved for use in the US and is under regulatory review in the European Union, Switzerland, Australia, and Colombia. ![]()

Photo courtesy of
Baxter International Inc.
Health Canada has approved a recombinant porcine factor VIII (FVIII) product (Obizur) to treat bleeding episodes in patients with acquired hemophilia A caused by autoantibodies to FVIII.
Obizur is the first recombinant porcine treatment to be made available for acquired hemophilia A in Canada.
It is specifically designed so physicians can monitor treatment response by measuring FVIII activity levels in addition to making clinical assessments.
Health Canada’s approval is based on a phase 2/3 trial in which patients with acquired hemophilia A received Obizur as treatment for serious bleeding episodes.
Twenty-nine patients were enrolled in this trial and evaluated for safety. Researchers determined that one of the patients did not actually have acquired hemophilia A, so this patient could not be evaluated for efficacy.
At 24 hours after the initial infusion, all 28 patients in the efficacy analysis had a positive response to Obizur. This meant that bleeding stopped or decreased, the patients experienced clinical stabilization or improvement, and FVIII levels were 20% or higher.
Eighty-six percent of patients (24/28) had successful treatment of their initial bleeding episode. The overall treatment success was determined by the investigator based on the ability to discontinue or reduce the dose and/or dosing frequency of Obizur.
The adverse event most frequently reported in the 29 patients in the safety analysis was the development of inhibitors to porcine FVIII.
Nineteen patients were negative for anti-porcine FVIII antibodies at baseline, and 5 of these patients (26%) developed anti-porcine FVIII antibodies following exposure to Obizur.
Of the 10 patients with detectable anti-porcine FVIII antibodies at baseline, 2 (20%) experienced an increase in titer, and 8 (80%) decreased to a non-detectable titer.
Obizur is under development by Baxalta Incorporated. The drug is currently approved for use in the US and is under regulatory review in the European Union, Switzerland, Australia, and Colombia. ![]()

Photo courtesy of
Baxter International Inc.
Health Canada has approved a recombinant porcine factor VIII (FVIII) product (Obizur) to treat bleeding episodes in patients with acquired hemophilia A caused by autoantibodies to FVIII.
Obizur is the first recombinant porcine treatment to be made available for acquired hemophilia A in Canada.
It is specifically designed so physicians can monitor treatment response by measuring FVIII activity levels in addition to making clinical assessments.
Health Canada’s approval is based on a phase 2/3 trial in which patients with acquired hemophilia A received Obizur as treatment for serious bleeding episodes.
Twenty-nine patients were enrolled in this trial and evaluated for safety. Researchers determined that one of the patients did not actually have acquired hemophilia A, so this patient could not be evaluated for efficacy.
At 24 hours after the initial infusion, all 28 patients in the efficacy analysis had a positive response to Obizur. This meant that bleeding stopped or decreased, the patients experienced clinical stabilization or improvement, and FVIII levels were 20% or higher.
Eighty-six percent of patients (24/28) had successful treatment of their initial bleeding episode. The overall treatment success was determined by the investigator based on the ability to discontinue or reduce the dose and/or dosing frequency of Obizur.
The adverse event most frequently reported in the 29 patients in the safety analysis was the development of inhibitors to porcine FVIII.
Nineteen patients were negative for anti-porcine FVIII antibodies at baseline, and 5 of these patients (26%) developed anti-porcine FVIII antibodies following exposure to Obizur.
Of the 10 patients with detectable anti-porcine FVIII antibodies at baseline, 2 (20%) experienced an increase in titer, and 8 (80%) decreased to a non-detectable titer.
Obizur is under development by Baxalta Incorporated. The drug is currently approved for use in the US and is under regulatory review in the European Union, Switzerland, Australia, and Colombia. ![]()
COMP recommends orphan designations for KTE-C19

The European Medicines Agency’s Committee for Orphan Medicinal Products (COMP) has adopted positive opinions recommending orphan designation for KTE-C19 to treat acute lymphoblastic leukemia, chronic lymphocytic leukemia/small lymphocytic lymphoma, and follicular lymphoma.
KTE-C19 is an investigational chimeric antigen receptor (CAR) T-cell therapy designed to target CD19, a protein expressed on the surface of B cells.
The CAR T-cell therapy already has orphan designation for the treatment of diffuse large B-cell lymphoma in the US and the European Union (EU).
KTE-C19 also has COMP positive opinions for orphan designation in the EU for primary mediastinal B-cell lymphoma and mantle cell lymphoma.
About orphan designation
The COMP adopts an opinion on the granting of orphan designation, and that opinion is submitted to the European Commission for endorsement.
In the EU, orphan designation is granted to therapies intended to treat a life-threatening or chronically debilitating condition that affects no more than 5 in 10,000 persons and where no satisfactory treatment is available.
Companies that obtain orphan designation for a drug benefit from a number of incentives, including protocol assistance, a type of scientific advice specific for designated orphan medicines, and 10 years of market exclusivity once the medicine is approved. Fee reductions are also available, depending on the status of the sponsor and the type of service required.
KTE-C19 research
Last year, researchers reported results with KTE-C19 in the Journal of Clinical Oncology. The study included 15 patients with advanced B-cell malignancies.
The patients received a conditioning regimen of cyclophosphamide and fludarabine, followed 1 day later by a single infusion of the CAR T-cell therapy. The researchers noted that the conditioning regimen is known to be active against B-cell malignancies and could have made a direct contribution to patient responses.
Thirteen patients were evaluable for response. Eight patients achieved a complete response (CR), and 4 had a partial response (PR).
Of the 7 patients with chemotherapy-refractory diffuse large B-cell lymphoma, 4 achieved a CR, 2 achieved a PR, and 1 had stable disease. Of the 4 patients with chronic lymphocytic leukemia, 3 had a CR, and 1 had a PR. Among the 2 patients with indolent lymphomas, 1 achieved a CR, and 1 had a PR.
KTE-C19 was associated with fever, low blood pressure, focal neurological deficits, and delirium. Toxicities largely occurred in the first 2 weeks after infusion.
All but 2 patients experienced grade 3/4 adverse events. Four patients had grade 3/4 hypotension.
All patients had elevations in serum interferon gamma and/or interleukin 6 around the time of peak toxicity, but most did not develop elevations in serum tumor necrosis factor.
Neurologic toxicities included confusion and obtundation, which have been reported in previous studies. However, 3 patients developed unexpected neurologic abnormalities.
KTE-C19 is currently under investigation in a phase 1/2 trial (ZUMA-1) of patients with refractory, aggressive non-Hodgkin lymphomas. Kite Pharma, Inc., the company developing KTE-C19, plans to present top-line phase 1 data at the 2015 ASH Annual Meeting. ![]()

The European Medicines Agency’s Committee for Orphan Medicinal Products (COMP) has adopted positive opinions recommending orphan designation for KTE-C19 to treat acute lymphoblastic leukemia, chronic lymphocytic leukemia/small lymphocytic lymphoma, and follicular lymphoma.
KTE-C19 is an investigational chimeric antigen receptor (CAR) T-cell therapy designed to target CD19, a protein expressed on the surface of B cells.
The CAR T-cell therapy already has orphan designation for the treatment of diffuse large B-cell lymphoma in the US and the European Union (EU).
KTE-C19 also has COMP positive opinions for orphan designation in the EU for primary mediastinal B-cell lymphoma and mantle cell lymphoma.
About orphan designation
The COMP adopts an opinion on the granting of orphan designation, and that opinion is submitted to the European Commission for endorsement.
In the EU, orphan designation is granted to therapies intended to treat a life-threatening or chronically debilitating condition that affects no more than 5 in 10,000 persons and where no satisfactory treatment is available.
Companies that obtain orphan designation for a drug benefit from a number of incentives, including protocol assistance, a type of scientific advice specific for designated orphan medicines, and 10 years of market exclusivity once the medicine is approved. Fee reductions are also available, depending on the status of the sponsor and the type of service required.
KTE-C19 research
Last year, researchers reported results with KTE-C19 in the Journal of Clinical Oncology. The study included 15 patients with advanced B-cell malignancies.
The patients received a conditioning regimen of cyclophosphamide and fludarabine, followed 1 day later by a single infusion of the CAR T-cell therapy. The researchers noted that the conditioning regimen is known to be active against B-cell malignancies and could have made a direct contribution to patient responses.
Thirteen patients were evaluable for response. Eight patients achieved a complete response (CR), and 4 had a partial response (PR).
Of the 7 patients with chemotherapy-refractory diffuse large B-cell lymphoma, 4 achieved a CR, 2 achieved a PR, and 1 had stable disease. Of the 4 patients with chronic lymphocytic leukemia, 3 had a CR, and 1 had a PR. Among the 2 patients with indolent lymphomas, 1 achieved a CR, and 1 had a PR.
KTE-C19 was associated with fever, low blood pressure, focal neurological deficits, and delirium. Toxicities largely occurred in the first 2 weeks after infusion.
All but 2 patients experienced grade 3/4 adverse events. Four patients had grade 3/4 hypotension.
All patients had elevations in serum interferon gamma and/or interleukin 6 around the time of peak toxicity, but most did not develop elevations in serum tumor necrosis factor.
Neurologic toxicities included confusion and obtundation, which have been reported in previous studies. However, 3 patients developed unexpected neurologic abnormalities.
KTE-C19 is currently under investigation in a phase 1/2 trial (ZUMA-1) of patients with refractory, aggressive non-Hodgkin lymphomas. Kite Pharma, Inc., the company developing KTE-C19, plans to present top-line phase 1 data at the 2015 ASH Annual Meeting. ![]()

The European Medicines Agency’s Committee for Orphan Medicinal Products (COMP) has adopted positive opinions recommending orphan designation for KTE-C19 to treat acute lymphoblastic leukemia, chronic lymphocytic leukemia/small lymphocytic lymphoma, and follicular lymphoma.
KTE-C19 is an investigational chimeric antigen receptor (CAR) T-cell therapy designed to target CD19, a protein expressed on the surface of B cells.
The CAR T-cell therapy already has orphan designation for the treatment of diffuse large B-cell lymphoma in the US and the European Union (EU).
KTE-C19 also has COMP positive opinions for orphan designation in the EU for primary mediastinal B-cell lymphoma and mantle cell lymphoma.
About orphan designation
The COMP adopts an opinion on the granting of orphan designation, and that opinion is submitted to the European Commission for endorsement.
In the EU, orphan designation is granted to therapies intended to treat a life-threatening or chronically debilitating condition that affects no more than 5 in 10,000 persons and where no satisfactory treatment is available.
Companies that obtain orphan designation for a drug benefit from a number of incentives, including protocol assistance, a type of scientific advice specific for designated orphan medicines, and 10 years of market exclusivity once the medicine is approved. Fee reductions are also available, depending on the status of the sponsor and the type of service required.
KTE-C19 research
Last year, researchers reported results with KTE-C19 in the Journal of Clinical Oncology. The study included 15 patients with advanced B-cell malignancies.
The patients received a conditioning regimen of cyclophosphamide and fludarabine, followed 1 day later by a single infusion of the CAR T-cell therapy. The researchers noted that the conditioning regimen is known to be active against B-cell malignancies and could have made a direct contribution to patient responses.
Thirteen patients were evaluable for response. Eight patients achieved a complete response (CR), and 4 had a partial response (PR).
Of the 7 patients with chemotherapy-refractory diffuse large B-cell lymphoma, 4 achieved a CR, 2 achieved a PR, and 1 had stable disease. Of the 4 patients with chronic lymphocytic leukemia, 3 had a CR, and 1 had a PR. Among the 2 patients with indolent lymphomas, 1 achieved a CR, and 1 had a PR.
KTE-C19 was associated with fever, low blood pressure, focal neurological deficits, and delirium. Toxicities largely occurred in the first 2 weeks after infusion.
All but 2 patients experienced grade 3/4 adverse events. Four patients had grade 3/4 hypotension.
All patients had elevations in serum interferon gamma and/or interleukin 6 around the time of peak toxicity, but most did not develop elevations in serum tumor necrosis factor.
Neurologic toxicities included confusion and obtundation, which have been reported in previous studies. However, 3 patients developed unexpected neurologic abnormalities.
KTE-C19 is currently under investigation in a phase 1/2 trial (ZUMA-1) of patients with refractory, aggressive non-Hodgkin lymphomas. Kite Pharma, Inc., the company developing KTE-C19, plans to present top-line phase 1 data at the 2015 ASH Annual Meeting. ![]()
Watchdog condemns FDA oversight of dabigatran
The US Food and Drug Administration’s (FDA’s) oversight of the oral anticoagulant dabigatran (Pradaxa) raises questions about the agency’s reliability, according to a report by the Project On Government Oversight (POGO).
POGO’s report describes a series of “questionable” calls the FDA has made in its oversight of dabigatran.
The report calls attention to issues with the clinical trial used to support dabigatran’s approval.
It also details concerns regarding FDA advisory committee members, the distribution of potentially misleading information about dabigatran, the possibility that patients receiving dabigatran may actually need to be monitored, and other issues.
“The deeply disturbing part is that the public has to trust the FDA to keep it safe, and the way the agency handled Pradaxa doesn’t inspire confidence,” said Daniel Brian, POGO’s executive director.
This is not the first time the safety of dabigatran and results of dabigatran trials have been called into question.
Following post-marketing reports of adverse events and deaths related to dabigatran, the FDA conducted its own studies investigating the drug’s safety. The agency ultimately concluded that dabigatran’s benefits outweigh any detriments.
Still, a paper published in JAMA in 2012 suggested the FDA may have approved dabigatran too soon.
And several papers published in The BMJ last year indicated that Boehringer Ingelheim, the company developing dabigatran, underreported events associated with the drug and withheld data showing that monitoring and dose adjustment could improve the safety of dabigatran without compromising its efficacy.
The company has denied these allegations, but POGO’s report appears to support these claims, in addition to raising other concerns.
Trial issues
Dabigatran was initially approved by the FDA in 2010 on the basis of the RE-LY trial, in which researchers compared dabigatran and warfarin.
POGO’s report notes that this was an unblinded trial and suggests that investigators treated patients in the dabigatran arm differently than those in the warfarin arm. An FDA review showed that, when study subjects showed “signs of trouble,” those in the dabigatran arm were more likely to stop receiving treatment. This may have prevented adverse events such as hemorrhagic strokes.
FDA inspections also revealed that RE-LY investigators mismanaged parts of the trial. For example, they enrolled patients who were supposed to be excluded and failed to promptly inform Boehringer Ingelheim about possible adverse effects of dabigatran, among other infractions.
POGO’s report also cites FDA documents showing that the agency allowed Boehringer Ingelheim to finalize the scoring system for the RE-LY trial after it was over and the data had been gathered. A subsequent FDA review suggested that this allowance worked in dabigatran’s favor.
Advisory committee concerns
POGO’s report points out that members of the committee that advised the FDA on dabigatran’s approval have financial ties to the drug’s maker.
According to public databases, 2 of the advisory committee members who voted to approve dabigatran went on to receive tens of thousands of dollars from Boehringer Ingelheim.
POGO also found that when Boehringer Ingelheim held a practice session to prepare for questioning by the FDA advisory committee, a former member and a former chairman of that committee were paid to play roles in the rehearsal.
‘Misleading’ information
Another of POGO’s concerns is that the FDA redacted information from a public memo announcing and explaining its decision to approve dabigatran. The deleted section said that patients who were well-treated using warfarin had no reason to switch to dabigatran.
POGO’s report also points out that the FDA initially refused to allow Boehringer Ingelheim to claim that dabigatran was superior to warfarin but later changed its mind without providing much explanation.
In addition, the FDA allowed Boehringer Ingelheim to advertise that, in a clinical trial, dabigatran “reduced stroke risk 35% more than warfarin.” But after dabigatran had been on the market for more than 2.5 years, the FDA decided this claim was misleading.
The agency told Boehringer Ingelheim to add the following clarification: “That means that, in a large clinical study, 3.4% of patients taking warfarin had a stroke, compared to 2.2% of patients taking Pradaxa.” So, in absolute terms, the difference between the drugs was 1.2%.
Potential need for monitoring
According to POGO, Boehringer Ingelheim has been the target of thousands of lawsuits regarding adverse events and deaths thought to be related to dabigatran. In 2013, Boehringer Ingelheim was fined by a federal judge for withholding or failing to preserve records.
Some of these documents suggested that patients taking dabigatran may require monitoring to ensure they remain within a therapeutic range. A scientist at Boehringer Ingelheim, Thorsten Lehr, drafted a paper concluding that there is a therapeutic range for dabigatran.
But company emails indicated that other employees were against publishing this paper. An email from Boehringer Ingelheim’s Jutta Heinrich-Nols said publishing the paper would “make any defense of no monitoring . . . extremely difficult . . . and undermine our efforts to compete” with other new anticoagulants.
For more details, see POGO’s full report. POGO is a nonpartisan, independent watchdog that champions government reform. ![]()
The US Food and Drug Administration’s (FDA’s) oversight of the oral anticoagulant dabigatran (Pradaxa) raises questions about the agency’s reliability, according to a report by the Project On Government Oversight (POGO).
POGO’s report describes a series of “questionable” calls the FDA has made in its oversight of dabigatran.
The report calls attention to issues with the clinical trial used to support dabigatran’s approval.
It also details concerns regarding FDA advisory committee members, the distribution of potentially misleading information about dabigatran, the possibility that patients receiving dabigatran may actually need to be monitored, and other issues.
“The deeply disturbing part is that the public has to trust the FDA to keep it safe, and the way the agency handled Pradaxa doesn’t inspire confidence,” said Daniel Brian, POGO’s executive director.
This is not the first time the safety of dabigatran and results of dabigatran trials have been called into question.
Following post-marketing reports of adverse events and deaths related to dabigatran, the FDA conducted its own studies investigating the drug’s safety. The agency ultimately concluded that dabigatran’s benefits outweigh any detriments.
Still, a paper published in JAMA in 2012 suggested the FDA may have approved dabigatran too soon.
And several papers published in The BMJ last year indicated that Boehringer Ingelheim, the company developing dabigatran, underreported events associated with the drug and withheld data showing that monitoring and dose adjustment could improve the safety of dabigatran without compromising its efficacy.
The company has denied these allegations, but POGO’s report appears to support these claims, in addition to raising other concerns.
Trial issues
Dabigatran was initially approved by the FDA in 2010 on the basis of the RE-LY trial, in which researchers compared dabigatran and warfarin.
POGO’s report notes that this was an unblinded trial and suggests that investigators treated patients in the dabigatran arm differently than those in the warfarin arm. An FDA review showed that, when study subjects showed “signs of trouble,” those in the dabigatran arm were more likely to stop receiving treatment. This may have prevented adverse events such as hemorrhagic strokes.
FDA inspections also revealed that RE-LY investigators mismanaged parts of the trial. For example, they enrolled patients who were supposed to be excluded and failed to promptly inform Boehringer Ingelheim about possible adverse effects of dabigatran, among other infractions.
POGO’s report also cites FDA documents showing that the agency allowed Boehringer Ingelheim to finalize the scoring system for the RE-LY trial after it was over and the data had been gathered. A subsequent FDA review suggested that this allowance worked in dabigatran’s favor.
Advisory committee concerns
POGO’s report points out that members of the committee that advised the FDA on dabigatran’s approval have financial ties to the drug’s maker.
According to public databases, 2 of the advisory committee members who voted to approve dabigatran went on to receive tens of thousands of dollars from Boehringer Ingelheim.
POGO also found that when Boehringer Ingelheim held a practice session to prepare for questioning by the FDA advisory committee, a former member and a former chairman of that committee were paid to play roles in the rehearsal.
‘Misleading’ information
Another of POGO’s concerns is that the FDA redacted information from a public memo announcing and explaining its decision to approve dabigatran. The deleted section said that patients who were well-treated using warfarin had no reason to switch to dabigatran.
POGO’s report also points out that the FDA initially refused to allow Boehringer Ingelheim to claim that dabigatran was superior to warfarin but later changed its mind without providing much explanation.
In addition, the FDA allowed Boehringer Ingelheim to advertise that, in a clinical trial, dabigatran “reduced stroke risk 35% more than warfarin.” But after dabigatran had been on the market for more than 2.5 years, the FDA decided this claim was misleading.
The agency told Boehringer Ingelheim to add the following clarification: “That means that, in a large clinical study, 3.4% of patients taking warfarin had a stroke, compared to 2.2% of patients taking Pradaxa.” So, in absolute terms, the difference between the drugs was 1.2%.
Potential need for monitoring
According to POGO, Boehringer Ingelheim has been the target of thousands of lawsuits regarding adverse events and deaths thought to be related to dabigatran. In 2013, Boehringer Ingelheim was fined by a federal judge for withholding or failing to preserve records.
Some of these documents suggested that patients taking dabigatran may require monitoring to ensure they remain within a therapeutic range. A scientist at Boehringer Ingelheim, Thorsten Lehr, drafted a paper concluding that there is a therapeutic range for dabigatran.
But company emails indicated that other employees were against publishing this paper. An email from Boehringer Ingelheim’s Jutta Heinrich-Nols said publishing the paper would “make any defense of no monitoring . . . extremely difficult . . . and undermine our efforts to compete” with other new anticoagulants.
For more details, see POGO’s full report. POGO is a nonpartisan, independent watchdog that champions government reform. ![]()
The US Food and Drug Administration’s (FDA’s) oversight of the oral anticoagulant dabigatran (Pradaxa) raises questions about the agency’s reliability, according to a report by the Project On Government Oversight (POGO).
POGO’s report describes a series of “questionable” calls the FDA has made in its oversight of dabigatran.
The report calls attention to issues with the clinical trial used to support dabigatran’s approval.
It also details concerns regarding FDA advisory committee members, the distribution of potentially misleading information about dabigatran, the possibility that patients receiving dabigatran may actually need to be monitored, and other issues.
“The deeply disturbing part is that the public has to trust the FDA to keep it safe, and the way the agency handled Pradaxa doesn’t inspire confidence,” said Daniel Brian, POGO’s executive director.
This is not the first time the safety of dabigatran and results of dabigatran trials have been called into question.
Following post-marketing reports of adverse events and deaths related to dabigatran, the FDA conducted its own studies investigating the drug’s safety. The agency ultimately concluded that dabigatran’s benefits outweigh any detriments.
Still, a paper published in JAMA in 2012 suggested the FDA may have approved dabigatran too soon.
And several papers published in The BMJ last year indicated that Boehringer Ingelheim, the company developing dabigatran, underreported events associated with the drug and withheld data showing that monitoring and dose adjustment could improve the safety of dabigatran without compromising its efficacy.
The company has denied these allegations, but POGO’s report appears to support these claims, in addition to raising other concerns.
Trial issues
Dabigatran was initially approved by the FDA in 2010 on the basis of the RE-LY trial, in which researchers compared dabigatran and warfarin.
POGO’s report notes that this was an unblinded trial and suggests that investigators treated patients in the dabigatran arm differently than those in the warfarin arm. An FDA review showed that, when study subjects showed “signs of trouble,” those in the dabigatran arm were more likely to stop receiving treatment. This may have prevented adverse events such as hemorrhagic strokes.
FDA inspections also revealed that RE-LY investigators mismanaged parts of the trial. For example, they enrolled patients who were supposed to be excluded and failed to promptly inform Boehringer Ingelheim about possible adverse effects of dabigatran, among other infractions.
POGO’s report also cites FDA documents showing that the agency allowed Boehringer Ingelheim to finalize the scoring system for the RE-LY trial after it was over and the data had been gathered. A subsequent FDA review suggested that this allowance worked in dabigatran’s favor.
Advisory committee concerns
POGO’s report points out that members of the committee that advised the FDA on dabigatran’s approval have financial ties to the drug’s maker.
According to public databases, 2 of the advisory committee members who voted to approve dabigatran went on to receive tens of thousands of dollars from Boehringer Ingelheim.
POGO also found that when Boehringer Ingelheim held a practice session to prepare for questioning by the FDA advisory committee, a former member and a former chairman of that committee were paid to play roles in the rehearsal.
‘Misleading’ information
Another of POGO’s concerns is that the FDA redacted information from a public memo announcing and explaining its decision to approve dabigatran. The deleted section said that patients who were well-treated using warfarin had no reason to switch to dabigatran.
POGO’s report also points out that the FDA initially refused to allow Boehringer Ingelheim to claim that dabigatran was superior to warfarin but later changed its mind without providing much explanation.
In addition, the FDA allowed Boehringer Ingelheim to advertise that, in a clinical trial, dabigatran “reduced stroke risk 35% more than warfarin.” But after dabigatran had been on the market for more than 2.5 years, the FDA decided this claim was misleading.
The agency told Boehringer Ingelheim to add the following clarification: “That means that, in a large clinical study, 3.4% of patients taking warfarin had a stroke, compared to 2.2% of patients taking Pradaxa.” So, in absolute terms, the difference between the drugs was 1.2%.
Potential need for monitoring
According to POGO, Boehringer Ingelheim has been the target of thousands of lawsuits regarding adverse events and deaths thought to be related to dabigatran. In 2013, Boehringer Ingelheim was fined by a federal judge for withholding or failing to preserve records.
Some of these documents suggested that patients taking dabigatran may require monitoring to ensure they remain within a therapeutic range. A scientist at Boehringer Ingelheim, Thorsten Lehr, drafted a paper concluding that there is a therapeutic range for dabigatran.
But company emails indicated that other employees were against publishing this paper. An email from Boehringer Ingelheim’s Jutta Heinrich-Nols said publishing the paper would “make any defense of no monitoring . . . extremely difficult . . . and undermine our efforts to compete” with other new anticoagulants.
For more details, see POGO’s full report. POGO is a nonpartisan, independent watchdog that champions government reform. ![]()
MMF may increase risk of CNS lymphoma
A new study has linked the immunosuppressive drug mycophenolate mofetil (MMF) to an increased risk of central nervous system (CNS) lymphoma in solid organ transplant recipients.
However, the research also suggests that calcineurin inhibitors (CNIs), when given alone or in combination with MMF, may protect transplant recipients from CNS lymphoma.
Researchers reported these findings in Oncotarget.
“MMF remains one of the best current medications for immunosuppression that we have,” said study author Amy Duffield, MD, PhD, of The Johns Hopkins Medical Institutions in Baltimore, Maryland.
“But a better understanding of its association with CNS lymphoproliferative disease will be crucial to further improving patients’ transplant regimens based on all of the risks these patients face.”
Dr Duffield and her colleagues noted that lymphomas and leukemias are known to be complications of solid organ transplants, but these malignancies rarely start in the CNS.
Still, in recent years, clinicians have begun to notice a rise in primary CNS lymphoproliferative disorders among transplant recipients. The current study is thought to be the first large enough to identify a link between MMF and these tumors.
For this work, Dr Duffield and her colleagues analyzed information on 177 patients with post-transplant lymphoproliferative disorder (PTLD) who were seen at Johns Hopkins Hospital between 1986 and 2014.
In that group, 29 patients—mostly kidney transplant recipients—were diagnosed with primary CNS lymphoproliferative disorders. The researchers said these were predominantly classified as monomorphic PTLD (72%), and most of the classifiable lymphomas were large B-cell lymphomas.
There were no cases of primary CNS PTLD diagnosed between 1986 and 1997, but the diagnosis increased markedly in the next decades.
The proportion of primary CNS PTLD cases compared to other PTLDs was 4.4-fold higher in the period from 2005 to 2014 than in the period from 1995 to 2004 (P<0.0001), even though the total number of PTLD cases remained relatively stable over time.
The researchers had prescription records on 16 of the patients who developed primary CNS lymphoproliferative disease.
Fifteen of the 16 patients had been taking MMF in the year prior to, or at the time of, their PTLD diagnosis. On the other hand, 37 of the 102 patients with PTLD outside the CNS had taken MMF (P<0.001).
The researchers also found that patients who took CNIs, either alone or in combination with MMF, seemed to be protected from developing primary CNS disease.
Primary CNS lymphoproliferative disease accounted for 66.7% of PTLDs among patients who took MMF but not a CNI (n=6), 23.9% of PTLDs among patients who took both an MMF and a CNI (n=46), and 1.7% of PTLDs among patients who took just a CNI (n=60).
The researchers found similar trends in a set of 6966 patients with PTLD. Those patients’ records were gleaned from an organ transplant database managed by the Organ Procurement and Transplantation Network and the United Network for Organ Sharing.
“More research needs to be done to confirm our results,” said Genevieve Crane, MD, PhD, of The Johns Hopkins Medical Institutions.
“But our work suggests that, at least in some patients, the combination of MMF and CNIs may be protective against CNS lymphoproliferative disease in a way that had not previously been appreciated.” ![]()

A new study has linked the immunosuppressive drug mycophenolate mofetil (MMF) to an increased risk of central nervous system (CNS) lymphoma in solid organ transplant recipients.
However, the research also suggests that calcineurin inhibitors (CNIs), when given alone or in combination with MMF, may protect transplant recipients from CNS lymphoma.
Researchers reported these findings in Oncotarget.
“MMF remains one of the best current medications for immunosuppression that we have,” said study author Amy Duffield, MD, PhD, of The Johns Hopkins Medical Institutions in Baltimore, Maryland.
“But a better understanding of its association with CNS lymphoproliferative disease will be crucial to further improving patients’ transplant regimens based on all of the risks these patients face.”
Dr Duffield and her colleagues noted that lymphomas and leukemias are known to be complications of solid organ transplants, but these malignancies rarely start in the CNS.
Still, in recent years, clinicians have begun to notice a rise in primary CNS lymphoproliferative disorders among transplant recipients. The current study is thought to be the first large enough to identify a link between MMF and these tumors.
For this work, Dr Duffield and her colleagues analyzed information on 177 patients with post-transplant lymphoproliferative disorder (PTLD) who were seen at Johns Hopkins Hospital between 1986 and 2014.
In that group, 29 patients—mostly kidney transplant recipients—were diagnosed with primary CNS lymphoproliferative disorders. The researchers said these were predominantly classified as monomorphic PTLD (72%), and most of the classifiable lymphomas were large B-cell lymphomas.
There were no cases of primary CNS PTLD diagnosed between 1986 and 1997, but the diagnosis increased markedly in the next decades.
The proportion of primary CNS PTLD cases compared to other PTLDs was 4.4-fold higher in the period from 2005 to 2014 than in the period from 1995 to 2004 (P<0.0001), even though the total number of PTLD cases remained relatively stable over time.
The researchers had prescription records on 16 of the patients who developed primary CNS lymphoproliferative disease.
Fifteen of the 16 patients had been taking MMF in the year prior to, or at the time of, their PTLD diagnosis. On the other hand, 37 of the 102 patients with PTLD outside the CNS had taken MMF (P<0.001).
The researchers also found that patients who took CNIs, either alone or in combination with MMF, seemed to be protected from developing primary CNS disease.
Primary CNS lymphoproliferative disease accounted for 66.7% of PTLDs among patients who took MMF but not a CNI (n=6), 23.9% of PTLDs among patients who took both an MMF and a CNI (n=46), and 1.7% of PTLDs among patients who took just a CNI (n=60).
The researchers found similar trends in a set of 6966 patients with PTLD. Those patients’ records were gleaned from an organ transplant database managed by the Organ Procurement and Transplantation Network and the United Network for Organ Sharing.
“More research needs to be done to confirm our results,” said Genevieve Crane, MD, PhD, of The Johns Hopkins Medical Institutions.
“But our work suggests that, at least in some patients, the combination of MMF and CNIs may be protective against CNS lymphoproliferative disease in a way that had not previously been appreciated.” ![]()

A new study has linked the immunosuppressive drug mycophenolate mofetil (MMF) to an increased risk of central nervous system (CNS) lymphoma in solid organ transplant recipients.
However, the research also suggests that calcineurin inhibitors (CNIs), when given alone or in combination with MMF, may protect transplant recipients from CNS lymphoma.
Researchers reported these findings in Oncotarget.
“MMF remains one of the best current medications for immunosuppression that we have,” said study author Amy Duffield, MD, PhD, of The Johns Hopkins Medical Institutions in Baltimore, Maryland.
“But a better understanding of its association with CNS lymphoproliferative disease will be crucial to further improving patients’ transplant regimens based on all of the risks these patients face.”
Dr Duffield and her colleagues noted that lymphomas and leukemias are known to be complications of solid organ transplants, but these malignancies rarely start in the CNS.
Still, in recent years, clinicians have begun to notice a rise in primary CNS lymphoproliferative disorders among transplant recipients. The current study is thought to be the first large enough to identify a link between MMF and these tumors.
For this work, Dr Duffield and her colleagues analyzed information on 177 patients with post-transplant lymphoproliferative disorder (PTLD) who were seen at Johns Hopkins Hospital between 1986 and 2014.
In that group, 29 patients—mostly kidney transplant recipients—were diagnosed with primary CNS lymphoproliferative disorders. The researchers said these were predominantly classified as monomorphic PTLD (72%), and most of the classifiable lymphomas were large B-cell lymphomas.
There were no cases of primary CNS PTLD diagnosed between 1986 and 1997, but the diagnosis increased markedly in the next decades.
The proportion of primary CNS PTLD cases compared to other PTLDs was 4.4-fold higher in the period from 2005 to 2014 than in the period from 1995 to 2004 (P<0.0001), even though the total number of PTLD cases remained relatively stable over time.
The researchers had prescription records on 16 of the patients who developed primary CNS lymphoproliferative disease.
Fifteen of the 16 patients had been taking MMF in the year prior to, or at the time of, their PTLD diagnosis. On the other hand, 37 of the 102 patients with PTLD outside the CNS had taken MMF (P<0.001).
The researchers also found that patients who took CNIs, either alone or in combination with MMF, seemed to be protected from developing primary CNS disease.
Primary CNS lymphoproliferative disease accounted for 66.7% of PTLDs among patients who took MMF but not a CNI (n=6), 23.9% of PTLDs among patients who took both an MMF and a CNI (n=46), and 1.7% of PTLDs among patients who took just a CNI (n=60).
The researchers found similar trends in a set of 6966 patients with PTLD. Those patients’ records were gleaned from an organ transplant database managed by the Organ Procurement and Transplantation Network and the United Network for Organ Sharing.
“More research needs to be done to confirm our results,” said Genevieve Crane, MD, PhD, of The Johns Hopkins Medical Institutions.
“But our work suggests that, at least in some patients, the combination of MMF and CNIs may be protective against CNS lymphoproliferative disease in a way that had not previously been appreciated.” ![]()
Code Status Discussions
Informed consent is one of the ethical, legal, and moral foundations of modern medicine.[1] Key elements of informed consent include: details of the procedure, benefits of the procedure, significant risks involved, likelihood of the outcome if predictable, and alternative therapeutic options.[2] Although rarely identified as such, conversations eliciting patient preferences about cardiopulmonary resuscitation (CPR) are among the most common examples of obtaining informed consent. Nevertheless, discussing CPR preference, often called code status discussions, differs from other examples of obtaining informed consent in 2 important ways. First, they occur well in advance of the potential need for CPR, so that the patient is well enough to participate meaningfully in the discussion. Second, because the default assumption is for patients to undergo the intervention (i.e. CPR), the focus of code status discussions is often on informed refusal, namely a decision about a do not resuscitate(DNR) order.
Since the institution of the Patient Self‐Determination Act in 1990, hospitals are obliged to educate patients about choices regarding end‐of‐life care at the time of hospital admission.[3] In many teaching hospitals, this responsibility falls to the admitting physician, often a trainee, who determines the patient's preferences regarding CPR and documents whether the patient is full code or DNR.
Prior studies have raised concerns about the quality of these conversations, highlighting their superficial nature and revealing trainee dissatisfaction with the results.[4, 5] Importantly, studies have shown that patients are capable of assimilating information about CPR when presented accurately and completely, and that such information can dramatically alter their choices.[6, 7, 8] These findings suggest that patients who are adequately educated will make more informed decisions regarding CPR, and that well‐informed choices about CPR may differ from poorly informed ones.
Although several studies have questioned the quality of code status discussions, none of these studies frames these interactions as examples of informed consent. Therefore, the purpose of the study was to examine the content of code status discussions as reported by internal medicine residents to determine whether they meet the basic tenets of informed consent, thereby facilitating informed decision making.
METHODS
In an iterative, collaborative process, authors A.F.B. and M.K.B. (an internal medicine resident at the time of the study and a board‐certified palliative care specialist/oncologist with experience in survey development, respectively) developed a survey adapted from previously published surveys.[9, 10, 11] The survey solicited respondent demographics, frequency of code status conversations, content of these discussions, and barriers to discussions. The survey instrument can be viewed in the Supporting Information, Appendix A, in the online version of this article. We used a 5‐point frequency scale (almost nevernearly always) for questions regarding: specific aspects of the informed consent related to code status discussions, resident confidence in conducting code status discussions, and barriers to code status discussions. We used a checklist for questions regarding content of code status discussions and patient characteristics influencing code status discussions. Residents provided a numeric percentage answer to 2 knowledge‐based questions of postarrest outcomes: (1) likelihood a patient would survive a witnessed pulseless ventricular tachycardia event and (2) likelihood of survival of a pulseless electrical activity event. The survey was revised by a hospitalist with experience in survey design (G.C.H.). We piloted the survey with 15 residents not part of the subject population and made revisions based on their input.
We sent a link to the online survey over secure email to all 159 internal medicine residents at our urban‐based academic medical center in January 2012. The email described the purpose of the study and stated that participation in the study (or lack thereof) was voluntary, anonymous, and would not have ramifications within the residency program. As part of the recruitment email, we explicitly included the elements of informed consent for the study participants. Not all the questions were mandatory to complete the survey. We sent a reminder e‐mail on a weekly basis for a total of 3 times and closed the survey after 1 month. Our goal was a 60% (N = 95) response rate.
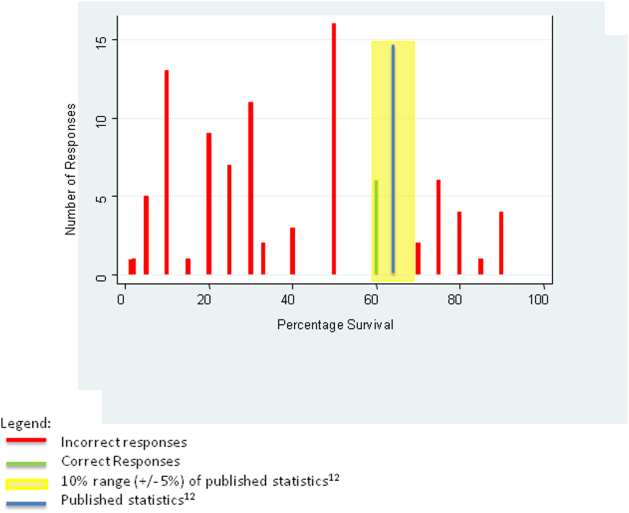
We tabulated the results by question. For analytic purposes, we aligned the content questions with key elements of informed consent as follows: step‐by‐step description of the events (details), patient‐specific likelihood of discharge if resuscitated (benefits), complications of resuscitation (risks), population‐based likelihood of discharge if resuscitated (likelihood), and opportunity for changing code status (alternatives). For the knowledge‐based questions, we deemed the answer correct if it was within 10% (5%) of published statistics from the 2010 national registry of cardiopulmonary resuscitation.[12] We stratified the key elements of informed consent and level of confidence by postgraduate year (PGY), comparing PGY1 residents versus PGY2 and PGY3 residents using 2 tests (or Fisher exact test for observations 5). We performed a univariate logistic regression analysis examining the relationship between confidence and reported use of informed consent elements in code discussions. The dependent variable of confidence in sufficient information having been provided for fully informed decision making was dichotomized as most of the time or nearly always versus other responses, whereas the independent variable was dichotomized as residents who reported using all 5 informed consent elements versus those who did not. We analyzed data using Stata 12 (StataCorp, College Station, TX).
The institutional review board of the Beth Israel Deaconess reviewed the study protocol and determined that it was exempt from institutional review board review.
RESULTS
One hundred of 159 (62.3%) internal medicine residents responded to the survey. Of the respondents 93% (N = 93) completed the survey. The 7% (N = 7) who did not complete the survey omitted the knowledge‐based questions and demographics. Approximately half of participants (54%, N = 50) were male. The majority of residents (85%, N = 79) had either occasional or frequent exposure to palliative care, with 10% (N = 9) having completed a palliative care rotation (Table 1).
| Characteristic | N (%) |
|---|---|
| |
| Sex, male | 50 (54) |
| PGY level | |
| PGY1 | 35 (38) |
| PGY2 | 33 (35) |
| PGY3 | 25 (27) |
| Exposure to palliative care | |
| Very little | 5 (5) |
| Occasional | 55 (59) |
| Frequent | 24 (26) |
| Completed palliative care elective | 9 (10) |
| What type of teaching did you have with code status discussions (check all that apply)? | |
| No teaching | 6 (6) |
| Lectures | 35 (38) |
| Small group teaching sessions | 57 (61) |
| Direct observation and feedback | 50 (54) |
| Exposure to palliative care consultation while rotating on the wards | 54 (58) |
| Other | 4 (4) |
| How much has your previous teaching about resuscitative measures influenced your behavior? | |
| Not at all | 1 (1) |
| Not very much | 15 (16) |
| A little bit | 39 (42) |
| A lot | 38 (41) |
The vast majority of residents (96%, N = 95) discussed code status with more than 40% of patients they admitted to the hospital (Table 2). Two‐thirds (66%, N = 65) of all residents had the conversation with at least 4 out of 5 (81%99% and 100%) patients they admitted to the hospital. Only 1% (N = 1) of residents who responded to the survey reported conducting code status discussions with 20% or fewer of the patients they admitted to the hospital.
| N (%) | |
|---|---|
| Percentage of inpatients with which you discuss code status, n = 99 | |
| 100% | 12 (12) |
| 8199% | 53 (54) |
| 6180% | 19 (19) |
| 4160% | 11 (11) |
| 2140% | 3 (3) |
| 120% | 1 (1) |
| Aspects of resuscitative measures routinely discussed, n = 100 | |
| Intubation/ventilation | 100 (100) |
| Chest compressions | 99 (99) |
| Defibrillation | 86 (86) |
| Surrogate decision maker | 61 (61) |
| Likelihood of success | 35 (35) |
| Quality of life | 32 (32) |
| Vasopressors | 13 (13) |
| Likelihood of discharge | 10 (10) |
| Possible role of depression | 10 (10) |
| Physical states worse than death | 7 (7) |
| Religious beliefs as a factor | 6 (6) |
| Makes recommendations for code status, n = 93 | |
| Never | 19 (20) |
| Rarely | 33 (35) |
| Sometimes | 33 (35) |
| Often | 7 (8) |
| Nearly always | 1 (1) |
Most residents (66%, N = 66) identified the healthcare proxy or surrogate decision maker most of the time or nearly always. In addition, most residents (62%, N = 62) reminded patients that they could reverse their code status at any time. Almost half included a description of step‐by‐step events during resuscitation (45%, N = 45) or factored in patient's comorbidities (43%, N = 43) when discussing resuscitation at least most of the time. Few residents described complications (31%, N = 31) or outcomes (17%, N = 17) of cardiopulmonary arrests to patients most of the time or nearly always. Most residents did not explore factors such as quality of life, role of depression or physical states worse than death, factors that could potentially affect patient decision making (Table 2). Few (9%, N = 8) internal medicine residents (often or nearly always) offered their opinion regarding a patient's code status.
Many factors influenced residents' decisions to have a code status conversation. At least 85% (N = 86) of residents reported that older age, particular admitting diagnoses, and multiple comorbidities made them more likely to have a code status discussion (see Supporting Table 1 in the online version of this article). Patient race/ethnicity did not influence this decision, with only 1 respondent reporting this factor as relevant.
Residents identified lack of time (49%, N = 49 responding often or nearly always) as the most frequent barrier to having a code status discussion, followed by lack of rapport (29%, N = 29). Lack of experience (6%, N = 6), lack of information about the patient's clinical status (11%, N = 11), and lack of knowledge about outcomes (13%, N = 13) did not represent frequent barriers for residents.
Fifty‐five percent (N = 53) of residents often or nearly always felt confident that they provided enough information for patients to make fully informed decisions about code status, and this did not differ by PGY status (PGY1 vs PGY2/3, P = 0.80, 2 test). However, only 8% (N = 8) of residents most of the time or nearly always addressed all 5 key elements of informed consent in reporting the content of their code status discussions. When stratified by training year, PGY2/3 residents were significantly more likely than PGY1 residents to factor in a patient's comorbidities when discussing resuscitation and were also significantly more likely to relay the likelihood of hospital discharge. They were not significantly more likely to discuss other key elements of informed consent (Table 3).
| Elements of Code Status Discussion (Most of the Time or Nearly Always), n = 100 | Elements | Total, N (%) | PGY1, N (%) | PGY2/3, N (%) | P Value |
|---|---|---|---|---|---|
| |||||
| Identify the patient's HCP or surrogate | 66 (66) | N/A | N/A | N/A | |
| Describe the step‐by‐step events that occur during resuscitative measures | Details | 45 (45) | 14 (40) | 28 (33) | 0.437 |
| Describe the complications associated with resuscitative measures | Risks | 31 (31) | 8 (23) | 19 (33) | 0.308 |
| Describe the likelihood the patient will be discharged from the hospital if resuscitated | Likelihood | 17 (17) | 2 (6) | 14 (24) | 0.025 |
| Factor in the patient's comorbidities when discussing the likelihood of discharge from the hospital if resuscitated | Benefits | 43 (43) | 8 (23) | 33 (57) | 0.002 |
| Tell the patient that decisions regarding code status can be changed at any time | Alternatives | 62 (62) | 18 (51) | 38 (66) | 0.179 |
Our subanalysis showed that residents reporting all 5 key elements of informed consent were associated with higher levels of confidence that they had provided enough information to patients for them to make an informed decision (odds ratio of 1.7, 95% confidence interval 1.2‐2.3).
For the first knowledge‐based question about witnessed pulseless ventricular tachycardia, according to the 2010 registry,[12] 64% survived the event (range of responses 1%90%). Six out of 92 (7%) respondents were within 5% of the correct answer. For the second question about survival after unwitnessed pulseless electrical activity, 41.5% survived the event according to the registry (range of responses 1%50%). Three out of 92 (3%) respondents gave estimates within 5% of the correct answer. Figures 1 and 2 display the ranges of responses from residents.
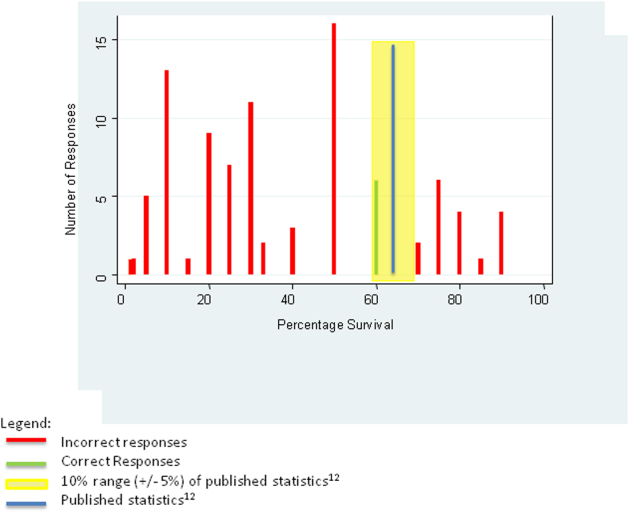
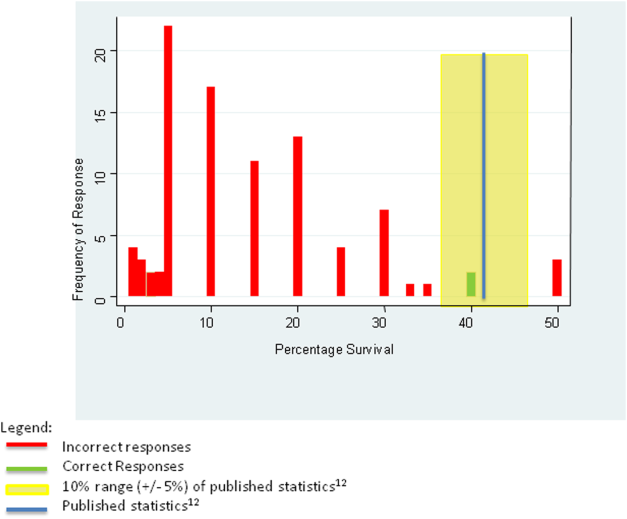
DISCUSSION
We found that although our internal medicine residents frequently have code status discussions with their patients, very few routinely report addressing all 5 key elements of informed consent. Furthermore, residents lack accurate knowledge about the outcomes of CPR, with most tending to underestimate the benefit expected of resuscitation. These deficiencies raise serious concerns about whether patients are receiving all the information essential to making fully informed decisions about their preferences for resuscitation.
The data demonstrate that the residents are routinely discussing code status and regularly discussing some aspects of the procedure itself, such as chest compressions, intubation, or defibrillation; the actual step‐by‐step events of CPR are being described less than half the time. It seems that residents mentally list the possible procedures that may occur in a code without a context for how one intervention would lead to another. Placing CPR into context is important, because studies have shown that more comprehensive discussions or the use of visual aids/videos that depict CPR in more detail improves patients' understanding of CPR and changes their decision about CPR, making them more likely to forego the procedure.[7, 8]
Residents report that they are more likely to have a code status discussion with patient's with multiple comorbidities, suggesting that they take into account information about the patient's clinical condition when deciding with which patients to address code status. They also recognize which patients are at increased risk for an in hospital cardiopulmonary arrest. Additionally, nearly half of residents factor in patient's comorbidities when discussing likelihood of discharge from the hospital, suggesting that they recognize that comorbidities can alter the outcome of CPR. Importantly, however, very few residents describe the likelihood the patient will be discharged from the hospital if resuscitated. Thus, residents in our sample have some insight into the impact of comorbidities on outcomes of CPR, but fail to provide their patients with any information about the outcome of CPR.
One reason residents may not discuss outcomes of CPR is because they do not know the data regarding outcomes. Although few residents reported that lack of knowledge of the risks and outcomes of CPR was a barrier, very few respondents answered the knowledge questions appropriately. Given how few residents gave an accurate estimate of CPR outcomes and simultaneously reported confidence in their code status discussions suggests that many residents fail to recognize their knowledge deficits. This finding corroborates other studies showing that residents don't know what they don't know[10] and may reflect their lack of education on CPR outcomes. Alternatively, some residents who underestimated the outcomes in the examples provided may have done so because, in their experience caring for patients with multiple comorbidites, the outcomes of CPR are in fact poorer than those in the cases described. Outcomes of CPR at our institution might differ from those quoted in the registry. However, given the prevalence of inaccuracy, both for under‐ and overestimation, it seems likely that a true knowledge deficit on the part of the residents still accounts for much of the error and should be a target for education. Understanding CPR outcomes is vital for informed decision making, and studies have shown that when patients have more information, it can substantially affect a patient's decision regarding resuscitation.[7, 13]
Residents are infrequently exploring key determinants that affect a patient's decision‐making process. Only one‐third of residents report discussing quality‐of‐life issues with patients during code status discussions. Understanding an individual patient's values and goals and how he or she describes a good quality of life can help guide the discussion and potential recommendations. For example, some patients may feel it is important to be alive regardless of the physical state, whereas others may feel that if there is not a chance to be independent in their activities of daily living, then they would not want to be resuscitated. By exploring patient's perceptions of what quality of life and physical states worse than death means, residents can better understand and assist in the decision‐making process of their patients.
Our data show that few residents offer a recommendation regarding code status. Thus, residents expect patients to make their own decision with the data provided. At the same time, many residents focus on the details of the procedural components of CPR with little mention of anticipated outcomes or inquiries into key determinants discussed above. Additionally, based on their response to the knowledge‐based questions, residents' estimates of survival, if offered, would be inaccurate. Thus, code status conversations by residents leave patients to make uninformed choices to consent to or refuse resuscitative measures.
When stratified by training year, PGY2/3 residents were significantly more likely than PGY1 residents to discuss likelihood of discharge from the hospital as well as factor in patients' comorbidities when discussing outcomes. Although there is a statistically significant improvement between PGY2/3 residents as compared to PGY1 residents, the numbers still show that most PGY2/3 residents and almost all PGY1 residents do not discuss the likelihood of discharge if resuscitated during code status discussions. In addition, there is no difference reported in other key areas of informed consent. Thus, though there is some improvement as housestaff advance in their training, PGY2 and PGY3 residents still do not discuss all 5 key elements of informed consent significantly more than PGY1 residents.
Our findings suggest an opportunity for additional education regarding how to address code status for internal medicine housestaff. Over half of the respondents reported small group teaching sessions, direct observation and feedback, and exposure to palliative care consultation during their clinical rotations; yet, very few of them included all the key elements of informed consent in their discussions. To address this, our institution is developing additional educational initiatives, including a faculty development program for teaching communication skills, using direct observation and feedback. The orientation didactic lecture series for housestaff now includes a lecture on CPR that highlights the data on outcomes and the importance of putting the step‐by‐step procedures of CPR into the context of potential benefits, such as survival to hospital discharge. The curriculum also includes a module on advance care planning for junior and senior residents during their ambulatory block, using simulation and feedback as part of the teaching methods.
There are limitations to this study. Studies based on surveys are subject to recall and selection bias, and we lack objective assessment of actual code status discussions. Furthermore, the nature of the study may lead to an overestimation of the quality of the code status discussions due to social acceptability bias; yet, our data clearly show that the key elements of informed consent are not included during these conversations. Another limitation is that our subjects were residents at a single institution, and our clinical practice may differ from other academic settings in the teaching environment and culture; yet, our findings mirror similar work done in other locations.[10, 14]
In conclusion, our results demonstrate that residents fail to meet standards of informed consent when discussing code status in that they do not provide sufficient information for patients to make an informed decision regarding resuscitation. Residents would benefit from education aimed at improving their knowledge of CPR outcomes as well as training on how to conduct these conversations effectively. Framing code status discussions as an example of an informed consent may help residents recognize the need for the key elements to be included in these conversations. In addition, training should focus on how to conduct these conversations in an efficient yet effective manner. This will require clear simple language, good communication skills, and training with observation and feedback by specialists trained in this field.
Disclosures
This work was presented at the Society of General Internal Medicine New England Regional Meeting, March 8, 2013, Yale Medical Center, New Haven, Connecticut. The authors report no conflicts of interest.
- , , , , Medical informed consent: general considerations for physicians. Mayo Clin Proc. 2008;83(3):313–319.
- Beth Israel Deaconess Medical Center. Policy #PR‐02 45 CFR 46.11679(4):240–243.
- , , , . Medical residents' perspectives on discussions of advanced directives: can prior experience affect how they approach patients? J Palliat Med. 2007;10(3):712–720.
- , , , , . Code status discussions between attending hospitalist physicians and medical patients at hospital admission. J Gen Intern Med. 2010;26(4):359–366.
- , , . The influence of the probability of survival on patient's preferences regarding cardiopulmonary resuscitation. N Engl J Med. 1994;330:545–549.
- , , , , . Using video images to improve the accuracy of surrogate decision‐making: a randomized controlled trial. J Am Med Dir Assoc. 2009;10(8):575–580.
- , , , et al. Use of video to facilitate end‐of‐life discussions with patients with cancer: a randomized controlled trial. J Clin Oncol. 2010;28(2):305–310.
- , , , , , . Resident Approaches to Advance Care Planning on the Day of Hospital Admission. Arch Intern Med. 2006;166:1597–1602.
- , , , . Assessing competence of residents to discuss end‐of‐life issues. J Palliat Med. 2005;8(2):363–371.
- , , , et al. Code status discussions and goals of care among hospitalised adults. J Med Ethics. 2009;35:338–342.
- , , , . Pre‐resuscitation factors associated with mortality in 49,130 cases of in‐hospital cardiac arrest: a report from the National Registry for Cardiopulmonary Resuscitation. Resuscitation. 2010;81:302–311.
- , , , . Resuscitation decision making in the elderly: the value of outcome data. J Gen Intern Med. 1993;8:295–300.
- , , . How do medical residents discuss resuscitation with patients? J Gen Intern Med. 1995;10:436–442.
Informed consent is one of the ethical, legal, and moral foundations of modern medicine.[1] Key elements of informed consent include: details of the procedure, benefits of the procedure, significant risks involved, likelihood of the outcome if predictable, and alternative therapeutic options.[2] Although rarely identified as such, conversations eliciting patient preferences about cardiopulmonary resuscitation (CPR) are among the most common examples of obtaining informed consent. Nevertheless, discussing CPR preference, often called code status discussions, differs from other examples of obtaining informed consent in 2 important ways. First, they occur well in advance of the potential need for CPR, so that the patient is well enough to participate meaningfully in the discussion. Second, because the default assumption is for patients to undergo the intervention (i.e. CPR), the focus of code status discussions is often on informed refusal, namely a decision about a do not resuscitate(DNR) order.
Since the institution of the Patient Self‐Determination Act in 1990, hospitals are obliged to educate patients about choices regarding end‐of‐life care at the time of hospital admission.[3] In many teaching hospitals, this responsibility falls to the admitting physician, often a trainee, who determines the patient's preferences regarding CPR and documents whether the patient is full code or DNR.
Prior studies have raised concerns about the quality of these conversations, highlighting their superficial nature and revealing trainee dissatisfaction with the results.[4, 5] Importantly, studies have shown that patients are capable of assimilating information about CPR when presented accurately and completely, and that such information can dramatically alter their choices.[6, 7, 8] These findings suggest that patients who are adequately educated will make more informed decisions regarding CPR, and that well‐informed choices about CPR may differ from poorly informed ones.
Although several studies have questioned the quality of code status discussions, none of these studies frames these interactions as examples of informed consent. Therefore, the purpose of the study was to examine the content of code status discussions as reported by internal medicine residents to determine whether they meet the basic tenets of informed consent, thereby facilitating informed decision making.
METHODS
In an iterative, collaborative process, authors A.F.B. and M.K.B. (an internal medicine resident at the time of the study and a board‐certified palliative care specialist/oncologist with experience in survey development, respectively) developed a survey adapted from previously published surveys.[9, 10, 11] The survey solicited respondent demographics, frequency of code status conversations, content of these discussions, and barriers to discussions. The survey instrument can be viewed in the Supporting Information, Appendix A, in the online version of this article. We used a 5‐point frequency scale (almost nevernearly always) for questions regarding: specific aspects of the informed consent related to code status discussions, resident confidence in conducting code status discussions, and barriers to code status discussions. We used a checklist for questions regarding content of code status discussions and patient characteristics influencing code status discussions. Residents provided a numeric percentage answer to 2 knowledge‐based questions of postarrest outcomes: (1) likelihood a patient would survive a witnessed pulseless ventricular tachycardia event and (2) likelihood of survival of a pulseless electrical activity event. The survey was revised by a hospitalist with experience in survey design (G.C.H.). We piloted the survey with 15 residents not part of the subject population and made revisions based on their input.
We sent a link to the online survey over secure email to all 159 internal medicine residents at our urban‐based academic medical center in January 2012. The email described the purpose of the study and stated that participation in the study (or lack thereof) was voluntary, anonymous, and would not have ramifications within the residency program. As part of the recruitment email, we explicitly included the elements of informed consent for the study participants. Not all the questions were mandatory to complete the survey. We sent a reminder e‐mail on a weekly basis for a total of 3 times and closed the survey after 1 month. Our goal was a 60% (N = 95) response rate.
We tabulated the results by question. For analytic purposes, we aligned the content questions with key elements of informed consent as follows: step‐by‐step description of the events (details), patient‐specific likelihood of discharge if resuscitated (benefits), complications of resuscitation (risks), population‐based likelihood of discharge if resuscitated (likelihood), and opportunity for changing code status (alternatives). For the knowledge‐based questions, we deemed the answer correct if it was within 10% (5%) of published statistics from the 2010 national registry of cardiopulmonary resuscitation.[12] We stratified the key elements of informed consent and level of confidence by postgraduate year (PGY), comparing PGY1 residents versus PGY2 and PGY3 residents using 2 tests (or Fisher exact test for observations 5). We performed a univariate logistic regression analysis examining the relationship between confidence and reported use of informed consent elements in code discussions. The dependent variable of confidence in sufficient information having been provided for fully informed decision making was dichotomized as most of the time or nearly always versus other responses, whereas the independent variable was dichotomized as residents who reported using all 5 informed consent elements versus those who did not. We analyzed data using Stata 12 (StataCorp, College Station, TX).
The institutional review board of the Beth Israel Deaconess reviewed the study protocol and determined that it was exempt from institutional review board review.
RESULTS
One hundred of 159 (62.3%) internal medicine residents responded to the survey. Of the respondents 93% (N = 93) completed the survey. The 7% (N = 7) who did not complete the survey omitted the knowledge‐based questions and demographics. Approximately half of participants (54%, N = 50) were male. The majority of residents (85%, N = 79) had either occasional or frequent exposure to palliative care, with 10% (N = 9) having completed a palliative care rotation (Table 1).
| Characteristic | N (%) |
|---|---|
| |
| Sex, male | 50 (54) |
| PGY level | |
| PGY1 | 35 (38) |
| PGY2 | 33 (35) |
| PGY3 | 25 (27) |
| Exposure to palliative care | |
| Very little | 5 (5) |
| Occasional | 55 (59) |
| Frequent | 24 (26) |
| Completed palliative care elective | 9 (10) |
| What type of teaching did you have with code status discussions (check all that apply)? | |
| No teaching | 6 (6) |
| Lectures | 35 (38) |
| Small group teaching sessions | 57 (61) |
| Direct observation and feedback | 50 (54) |
| Exposure to palliative care consultation while rotating on the wards | 54 (58) |
| Other | 4 (4) |
| How much has your previous teaching about resuscitative measures influenced your behavior? | |
| Not at all | 1 (1) |
| Not very much | 15 (16) |
| A little bit | 39 (42) |
| A lot | 38 (41) |
The vast majority of residents (96%, N = 95) discussed code status with more than 40% of patients they admitted to the hospital (Table 2). Two‐thirds (66%, N = 65) of all residents had the conversation with at least 4 out of 5 (81%99% and 100%) patients they admitted to the hospital. Only 1% (N = 1) of residents who responded to the survey reported conducting code status discussions with 20% or fewer of the patients they admitted to the hospital.
| N (%) | |
|---|---|
| Percentage of inpatients with which you discuss code status, n = 99 | |
| 100% | 12 (12) |
| 8199% | 53 (54) |
| 6180% | 19 (19) |
| 4160% | 11 (11) |
| 2140% | 3 (3) |
| 120% | 1 (1) |
| Aspects of resuscitative measures routinely discussed, n = 100 | |
| Intubation/ventilation | 100 (100) |
| Chest compressions | 99 (99) |
| Defibrillation | 86 (86) |
| Surrogate decision maker | 61 (61) |
| Likelihood of success | 35 (35) |
| Quality of life | 32 (32) |
| Vasopressors | 13 (13) |
| Likelihood of discharge | 10 (10) |
| Possible role of depression | 10 (10) |
| Physical states worse than death | 7 (7) |
| Religious beliefs as a factor | 6 (6) |
| Makes recommendations for code status, n = 93 | |
| Never | 19 (20) |
| Rarely | 33 (35) |
| Sometimes | 33 (35) |
| Often | 7 (8) |
| Nearly always | 1 (1) |
Most residents (66%, N = 66) identified the healthcare proxy or surrogate decision maker most of the time or nearly always. In addition, most residents (62%, N = 62) reminded patients that they could reverse their code status at any time. Almost half included a description of step‐by‐step events during resuscitation (45%, N = 45) or factored in patient's comorbidities (43%, N = 43) when discussing resuscitation at least most of the time. Few residents described complications (31%, N = 31) or outcomes (17%, N = 17) of cardiopulmonary arrests to patients most of the time or nearly always. Most residents did not explore factors such as quality of life, role of depression or physical states worse than death, factors that could potentially affect patient decision making (Table 2). Few (9%, N = 8) internal medicine residents (often or nearly always) offered their opinion regarding a patient's code status.
Many factors influenced residents' decisions to have a code status conversation. At least 85% (N = 86) of residents reported that older age, particular admitting diagnoses, and multiple comorbidities made them more likely to have a code status discussion (see Supporting Table 1 in the online version of this article). Patient race/ethnicity did not influence this decision, with only 1 respondent reporting this factor as relevant.
Residents identified lack of time (49%, N = 49 responding often or nearly always) as the most frequent barrier to having a code status discussion, followed by lack of rapport (29%, N = 29). Lack of experience (6%, N = 6), lack of information about the patient's clinical status (11%, N = 11), and lack of knowledge about outcomes (13%, N = 13) did not represent frequent barriers for residents.
Fifty‐five percent (N = 53) of residents often or nearly always felt confident that they provided enough information for patients to make fully informed decisions about code status, and this did not differ by PGY status (PGY1 vs PGY2/3, P = 0.80, 2 test). However, only 8% (N = 8) of residents most of the time or nearly always addressed all 5 key elements of informed consent in reporting the content of their code status discussions. When stratified by training year, PGY2/3 residents were significantly more likely than PGY1 residents to factor in a patient's comorbidities when discussing resuscitation and were also significantly more likely to relay the likelihood of hospital discharge. They were not significantly more likely to discuss other key elements of informed consent (Table 3).
| Elements of Code Status Discussion (Most of the Time or Nearly Always), n = 100 | Elements | Total, N (%) | PGY1, N (%) | PGY2/3, N (%) | P Value |
|---|---|---|---|---|---|
| |||||
| Identify the patient's HCP or surrogate | 66 (66) | N/A | N/A | N/A | |
| Describe the step‐by‐step events that occur during resuscitative measures | Details | 45 (45) | 14 (40) | 28 (33) | 0.437 |
| Describe the complications associated with resuscitative measures | Risks | 31 (31) | 8 (23) | 19 (33) | 0.308 |
| Describe the likelihood the patient will be discharged from the hospital if resuscitated | Likelihood | 17 (17) | 2 (6) | 14 (24) | 0.025 |
| Factor in the patient's comorbidities when discussing the likelihood of discharge from the hospital if resuscitated | Benefits | 43 (43) | 8 (23) | 33 (57) | 0.002 |
| Tell the patient that decisions regarding code status can be changed at any time | Alternatives | 62 (62) | 18 (51) | 38 (66) | 0.179 |
Our subanalysis showed that residents reporting all 5 key elements of informed consent were associated with higher levels of confidence that they had provided enough information to patients for them to make an informed decision (odds ratio of 1.7, 95% confidence interval 1.2‐2.3).
For the first knowledge‐based question about witnessed pulseless ventricular tachycardia, according to the 2010 registry,[12] 64% survived the event (range of responses 1%90%). Six out of 92 (7%) respondents were within 5% of the correct answer. For the second question about survival after unwitnessed pulseless electrical activity, 41.5% survived the event according to the registry (range of responses 1%50%). Three out of 92 (3%) respondents gave estimates within 5% of the correct answer. Figures 1 and 2 display the ranges of responses from residents.


DISCUSSION
We found that although our internal medicine residents frequently have code status discussions with their patients, very few routinely report addressing all 5 key elements of informed consent. Furthermore, residents lack accurate knowledge about the outcomes of CPR, with most tending to underestimate the benefit expected of resuscitation. These deficiencies raise serious concerns about whether patients are receiving all the information essential to making fully informed decisions about their preferences for resuscitation.
The data demonstrate that the residents are routinely discussing code status and regularly discussing some aspects of the procedure itself, such as chest compressions, intubation, or defibrillation; the actual step‐by‐step events of CPR are being described less than half the time. It seems that residents mentally list the possible procedures that may occur in a code without a context for how one intervention would lead to another. Placing CPR into context is important, because studies have shown that more comprehensive discussions or the use of visual aids/videos that depict CPR in more detail improves patients' understanding of CPR and changes their decision about CPR, making them more likely to forego the procedure.[7, 8]
Residents report that they are more likely to have a code status discussion with patient's with multiple comorbidities, suggesting that they take into account information about the patient's clinical condition when deciding with which patients to address code status. They also recognize which patients are at increased risk for an in hospital cardiopulmonary arrest. Additionally, nearly half of residents factor in patient's comorbidities when discussing likelihood of discharge from the hospital, suggesting that they recognize that comorbidities can alter the outcome of CPR. Importantly, however, very few residents describe the likelihood the patient will be discharged from the hospital if resuscitated. Thus, residents in our sample have some insight into the impact of comorbidities on outcomes of CPR, but fail to provide their patients with any information about the outcome of CPR.
One reason residents may not discuss outcomes of CPR is because they do not know the data regarding outcomes. Although few residents reported that lack of knowledge of the risks and outcomes of CPR was a barrier, very few respondents answered the knowledge questions appropriately. Given how few residents gave an accurate estimate of CPR outcomes and simultaneously reported confidence in their code status discussions suggests that many residents fail to recognize their knowledge deficits. This finding corroborates other studies showing that residents don't know what they don't know[10] and may reflect their lack of education on CPR outcomes. Alternatively, some residents who underestimated the outcomes in the examples provided may have done so because, in their experience caring for patients with multiple comorbidites, the outcomes of CPR are in fact poorer than those in the cases described. Outcomes of CPR at our institution might differ from those quoted in the registry. However, given the prevalence of inaccuracy, both for under‐ and overestimation, it seems likely that a true knowledge deficit on the part of the residents still accounts for much of the error and should be a target for education. Understanding CPR outcomes is vital for informed decision making, and studies have shown that when patients have more information, it can substantially affect a patient's decision regarding resuscitation.[7, 13]
Residents are infrequently exploring key determinants that affect a patient's decision‐making process. Only one‐third of residents report discussing quality‐of‐life issues with patients during code status discussions. Understanding an individual patient's values and goals and how he or she describes a good quality of life can help guide the discussion and potential recommendations. For example, some patients may feel it is important to be alive regardless of the physical state, whereas others may feel that if there is not a chance to be independent in their activities of daily living, then they would not want to be resuscitated. By exploring patient's perceptions of what quality of life and physical states worse than death means, residents can better understand and assist in the decision‐making process of their patients.
Our data show that few residents offer a recommendation regarding code status. Thus, residents expect patients to make their own decision with the data provided. At the same time, many residents focus on the details of the procedural components of CPR with little mention of anticipated outcomes or inquiries into key determinants discussed above. Additionally, based on their response to the knowledge‐based questions, residents' estimates of survival, if offered, would be inaccurate. Thus, code status conversations by residents leave patients to make uninformed choices to consent to or refuse resuscitative measures.
When stratified by training year, PGY2/3 residents were significantly more likely than PGY1 residents to discuss likelihood of discharge from the hospital as well as factor in patients' comorbidities when discussing outcomes. Although there is a statistically significant improvement between PGY2/3 residents as compared to PGY1 residents, the numbers still show that most PGY2/3 residents and almost all PGY1 residents do not discuss the likelihood of discharge if resuscitated during code status discussions. In addition, there is no difference reported in other key areas of informed consent. Thus, though there is some improvement as housestaff advance in their training, PGY2 and PGY3 residents still do not discuss all 5 key elements of informed consent significantly more than PGY1 residents.
Our findings suggest an opportunity for additional education regarding how to address code status for internal medicine housestaff. Over half of the respondents reported small group teaching sessions, direct observation and feedback, and exposure to palliative care consultation during their clinical rotations; yet, very few of them included all the key elements of informed consent in their discussions. To address this, our institution is developing additional educational initiatives, including a faculty development program for teaching communication skills, using direct observation and feedback. The orientation didactic lecture series for housestaff now includes a lecture on CPR that highlights the data on outcomes and the importance of putting the step‐by‐step procedures of CPR into the context of potential benefits, such as survival to hospital discharge. The curriculum also includes a module on advance care planning for junior and senior residents during their ambulatory block, using simulation and feedback as part of the teaching methods.
There are limitations to this study. Studies based on surveys are subject to recall and selection bias, and we lack objective assessment of actual code status discussions. Furthermore, the nature of the study may lead to an overestimation of the quality of the code status discussions due to social acceptability bias; yet, our data clearly show that the key elements of informed consent are not included during these conversations. Another limitation is that our subjects were residents at a single institution, and our clinical practice may differ from other academic settings in the teaching environment and culture; yet, our findings mirror similar work done in other locations.[10, 14]
In conclusion, our results demonstrate that residents fail to meet standards of informed consent when discussing code status in that they do not provide sufficient information for patients to make an informed decision regarding resuscitation. Residents would benefit from education aimed at improving their knowledge of CPR outcomes as well as training on how to conduct these conversations effectively. Framing code status discussions as an example of an informed consent may help residents recognize the need for the key elements to be included in these conversations. In addition, training should focus on how to conduct these conversations in an efficient yet effective manner. This will require clear simple language, good communication skills, and training with observation and feedback by specialists trained in this field.
Disclosures
This work was presented at the Society of General Internal Medicine New England Regional Meeting, March 8, 2013, Yale Medical Center, New Haven, Connecticut. The authors report no conflicts of interest.
Informed consent is one of the ethical, legal, and moral foundations of modern medicine.[1] Key elements of informed consent include: details of the procedure, benefits of the procedure, significant risks involved, likelihood of the outcome if predictable, and alternative therapeutic options.[2] Although rarely identified as such, conversations eliciting patient preferences about cardiopulmonary resuscitation (CPR) are among the most common examples of obtaining informed consent. Nevertheless, discussing CPR preference, often called code status discussions, differs from other examples of obtaining informed consent in 2 important ways. First, they occur well in advance of the potential need for CPR, so that the patient is well enough to participate meaningfully in the discussion. Second, because the default assumption is for patients to undergo the intervention (i.e. CPR), the focus of code status discussions is often on informed refusal, namely a decision about a do not resuscitate(DNR) order.
Since the institution of the Patient Self‐Determination Act in 1990, hospitals are obliged to educate patients about choices regarding end‐of‐life care at the time of hospital admission.[3] In many teaching hospitals, this responsibility falls to the admitting physician, often a trainee, who determines the patient's preferences regarding CPR and documents whether the patient is full code or DNR.
Prior studies have raised concerns about the quality of these conversations, highlighting their superficial nature and revealing trainee dissatisfaction with the results.[4, 5] Importantly, studies have shown that patients are capable of assimilating information about CPR when presented accurately and completely, and that such information can dramatically alter their choices.[6, 7, 8] These findings suggest that patients who are adequately educated will make more informed decisions regarding CPR, and that well‐informed choices about CPR may differ from poorly informed ones.
Although several studies have questioned the quality of code status discussions, none of these studies frames these interactions as examples of informed consent. Therefore, the purpose of the study was to examine the content of code status discussions as reported by internal medicine residents to determine whether they meet the basic tenets of informed consent, thereby facilitating informed decision making.
METHODS
In an iterative, collaborative process, authors A.F.B. and M.K.B. (an internal medicine resident at the time of the study and a board‐certified palliative care specialist/oncologist with experience in survey development, respectively) developed a survey adapted from previously published surveys.[9, 10, 11] The survey solicited respondent demographics, frequency of code status conversations, content of these discussions, and barriers to discussions. The survey instrument can be viewed in the Supporting Information, Appendix A, in the online version of this article. We used a 5‐point frequency scale (almost nevernearly always) for questions regarding: specific aspects of the informed consent related to code status discussions, resident confidence in conducting code status discussions, and barriers to code status discussions. We used a checklist for questions regarding content of code status discussions and patient characteristics influencing code status discussions. Residents provided a numeric percentage answer to 2 knowledge‐based questions of postarrest outcomes: (1) likelihood a patient would survive a witnessed pulseless ventricular tachycardia event and (2) likelihood of survival of a pulseless electrical activity event. The survey was revised by a hospitalist with experience in survey design (G.C.H.). We piloted the survey with 15 residents not part of the subject population and made revisions based on their input.
We sent a link to the online survey over secure email to all 159 internal medicine residents at our urban‐based academic medical center in January 2012. The email described the purpose of the study and stated that participation in the study (or lack thereof) was voluntary, anonymous, and would not have ramifications within the residency program. As part of the recruitment email, we explicitly included the elements of informed consent for the study participants. Not all the questions were mandatory to complete the survey. We sent a reminder e‐mail on a weekly basis for a total of 3 times and closed the survey after 1 month. Our goal was a 60% (N = 95) response rate.
We tabulated the results by question. For analytic purposes, we aligned the content questions with key elements of informed consent as follows: step‐by‐step description of the events (details), patient‐specific likelihood of discharge if resuscitated (benefits), complications of resuscitation (risks), population‐based likelihood of discharge if resuscitated (likelihood), and opportunity for changing code status (alternatives). For the knowledge‐based questions, we deemed the answer correct if it was within 10% (5%) of published statistics from the 2010 national registry of cardiopulmonary resuscitation.[12] We stratified the key elements of informed consent and level of confidence by postgraduate year (PGY), comparing PGY1 residents versus PGY2 and PGY3 residents using 2 tests (or Fisher exact test for observations 5). We performed a univariate logistic regression analysis examining the relationship between confidence and reported use of informed consent elements in code discussions. The dependent variable of confidence in sufficient information having been provided for fully informed decision making was dichotomized as most of the time or nearly always versus other responses, whereas the independent variable was dichotomized as residents who reported using all 5 informed consent elements versus those who did not. We analyzed data using Stata 12 (StataCorp, College Station, TX).
The institutional review board of the Beth Israel Deaconess reviewed the study protocol and determined that it was exempt from institutional review board review.
RESULTS
One hundred of 159 (62.3%) internal medicine residents responded to the survey. Of the respondents 93% (N = 93) completed the survey. The 7% (N = 7) who did not complete the survey omitted the knowledge‐based questions and demographics. Approximately half of participants (54%, N = 50) were male. The majority of residents (85%, N = 79) had either occasional or frequent exposure to palliative care, with 10% (N = 9) having completed a palliative care rotation (Table 1).
| Characteristic | N (%) |
|---|---|
| |
| Sex, male | 50 (54) |
| PGY level | |
| PGY1 | 35 (38) |
| PGY2 | 33 (35) |
| PGY3 | 25 (27) |
| Exposure to palliative care | |
| Very little | 5 (5) |
| Occasional | 55 (59) |
| Frequent | 24 (26) |
| Completed palliative care elective | 9 (10) |
| What type of teaching did you have with code status discussions (check all that apply)? | |
| No teaching | 6 (6) |
| Lectures | 35 (38) |
| Small group teaching sessions | 57 (61) |
| Direct observation and feedback | 50 (54) |
| Exposure to palliative care consultation while rotating on the wards | 54 (58) |
| Other | 4 (4) |
| How much has your previous teaching about resuscitative measures influenced your behavior? | |
| Not at all | 1 (1) |
| Not very much | 15 (16) |
| A little bit | 39 (42) |
| A lot | 38 (41) |
The vast majority of residents (96%, N = 95) discussed code status with more than 40% of patients they admitted to the hospital (Table 2). Two‐thirds (66%, N = 65) of all residents had the conversation with at least 4 out of 5 (81%99% and 100%) patients they admitted to the hospital. Only 1% (N = 1) of residents who responded to the survey reported conducting code status discussions with 20% or fewer of the patients they admitted to the hospital.
| N (%) | |
|---|---|
| Percentage of inpatients with which you discuss code status, n = 99 | |
| 100% | 12 (12) |
| 8199% | 53 (54) |
| 6180% | 19 (19) |
| 4160% | 11 (11) |
| 2140% | 3 (3) |
| 120% | 1 (1) |
| Aspects of resuscitative measures routinely discussed, n = 100 | |
| Intubation/ventilation | 100 (100) |
| Chest compressions | 99 (99) |
| Defibrillation | 86 (86) |
| Surrogate decision maker | 61 (61) |
| Likelihood of success | 35 (35) |
| Quality of life | 32 (32) |
| Vasopressors | 13 (13) |
| Likelihood of discharge | 10 (10) |
| Possible role of depression | 10 (10) |
| Physical states worse than death | 7 (7) |
| Religious beliefs as a factor | 6 (6) |
| Makes recommendations for code status, n = 93 | |
| Never | 19 (20) |
| Rarely | 33 (35) |
| Sometimes | 33 (35) |
| Often | 7 (8) |
| Nearly always | 1 (1) |
Most residents (66%, N = 66) identified the healthcare proxy or surrogate decision maker most of the time or nearly always. In addition, most residents (62%, N = 62) reminded patients that they could reverse their code status at any time. Almost half included a description of step‐by‐step events during resuscitation (45%, N = 45) or factored in patient's comorbidities (43%, N = 43) when discussing resuscitation at least most of the time. Few residents described complications (31%, N = 31) or outcomes (17%, N = 17) of cardiopulmonary arrests to patients most of the time or nearly always. Most residents did not explore factors such as quality of life, role of depression or physical states worse than death, factors that could potentially affect patient decision making (Table 2). Few (9%, N = 8) internal medicine residents (often or nearly always) offered their opinion regarding a patient's code status.
Many factors influenced residents' decisions to have a code status conversation. At least 85% (N = 86) of residents reported that older age, particular admitting diagnoses, and multiple comorbidities made them more likely to have a code status discussion (see Supporting Table 1 in the online version of this article). Patient race/ethnicity did not influence this decision, with only 1 respondent reporting this factor as relevant.
Residents identified lack of time (49%, N = 49 responding often or nearly always) as the most frequent barrier to having a code status discussion, followed by lack of rapport (29%, N = 29). Lack of experience (6%, N = 6), lack of information about the patient's clinical status (11%, N = 11), and lack of knowledge about outcomes (13%, N = 13) did not represent frequent barriers for residents.
Fifty‐five percent (N = 53) of residents often or nearly always felt confident that they provided enough information for patients to make fully informed decisions about code status, and this did not differ by PGY status (PGY1 vs PGY2/3, P = 0.80, 2 test). However, only 8% (N = 8) of residents most of the time or nearly always addressed all 5 key elements of informed consent in reporting the content of their code status discussions. When stratified by training year, PGY2/3 residents were significantly more likely than PGY1 residents to factor in a patient's comorbidities when discussing resuscitation and were also significantly more likely to relay the likelihood of hospital discharge. They were not significantly more likely to discuss other key elements of informed consent (Table 3).
| Elements of Code Status Discussion (Most of the Time or Nearly Always), n = 100 | Elements | Total, N (%) | PGY1, N (%) | PGY2/3, N (%) | P Value |
|---|---|---|---|---|---|
| |||||
| Identify the patient's HCP or surrogate | 66 (66) | N/A | N/A | N/A | |
| Describe the step‐by‐step events that occur during resuscitative measures | Details | 45 (45) | 14 (40) | 28 (33) | 0.437 |
| Describe the complications associated with resuscitative measures | Risks | 31 (31) | 8 (23) | 19 (33) | 0.308 |
| Describe the likelihood the patient will be discharged from the hospital if resuscitated | Likelihood | 17 (17) | 2 (6) | 14 (24) | 0.025 |
| Factor in the patient's comorbidities when discussing the likelihood of discharge from the hospital if resuscitated | Benefits | 43 (43) | 8 (23) | 33 (57) | 0.002 |
| Tell the patient that decisions regarding code status can be changed at any time | Alternatives | 62 (62) | 18 (51) | 38 (66) | 0.179 |
Our subanalysis showed that residents reporting all 5 key elements of informed consent were associated with higher levels of confidence that they had provided enough information to patients for them to make an informed decision (odds ratio of 1.7, 95% confidence interval 1.2‐2.3).
For the first knowledge‐based question about witnessed pulseless ventricular tachycardia, according to the 2010 registry,[12] 64% survived the event (range of responses 1%90%). Six out of 92 (7%) respondents were within 5% of the correct answer. For the second question about survival after unwitnessed pulseless electrical activity, 41.5% survived the event according to the registry (range of responses 1%50%). Three out of 92 (3%) respondents gave estimates within 5% of the correct answer. Figures 1 and 2 display the ranges of responses from residents.


DISCUSSION
We found that although our internal medicine residents frequently have code status discussions with their patients, very few routinely report addressing all 5 key elements of informed consent. Furthermore, residents lack accurate knowledge about the outcomes of CPR, with most tending to underestimate the benefit expected of resuscitation. These deficiencies raise serious concerns about whether patients are receiving all the information essential to making fully informed decisions about their preferences for resuscitation.
The data demonstrate that the residents are routinely discussing code status and regularly discussing some aspects of the procedure itself, such as chest compressions, intubation, or defibrillation; the actual step‐by‐step events of CPR are being described less than half the time. It seems that residents mentally list the possible procedures that may occur in a code without a context for how one intervention would lead to another. Placing CPR into context is important, because studies have shown that more comprehensive discussions or the use of visual aids/videos that depict CPR in more detail improves patients' understanding of CPR and changes their decision about CPR, making them more likely to forego the procedure.[7, 8]
Residents report that they are more likely to have a code status discussion with patient's with multiple comorbidities, suggesting that they take into account information about the patient's clinical condition when deciding with which patients to address code status. They also recognize which patients are at increased risk for an in hospital cardiopulmonary arrest. Additionally, nearly half of residents factor in patient's comorbidities when discussing likelihood of discharge from the hospital, suggesting that they recognize that comorbidities can alter the outcome of CPR. Importantly, however, very few residents describe the likelihood the patient will be discharged from the hospital if resuscitated. Thus, residents in our sample have some insight into the impact of comorbidities on outcomes of CPR, but fail to provide their patients with any information about the outcome of CPR.
One reason residents may not discuss outcomes of CPR is because they do not know the data regarding outcomes. Although few residents reported that lack of knowledge of the risks and outcomes of CPR was a barrier, very few respondents answered the knowledge questions appropriately. Given how few residents gave an accurate estimate of CPR outcomes and simultaneously reported confidence in their code status discussions suggests that many residents fail to recognize their knowledge deficits. This finding corroborates other studies showing that residents don't know what they don't know[10] and may reflect their lack of education on CPR outcomes. Alternatively, some residents who underestimated the outcomes in the examples provided may have done so because, in their experience caring for patients with multiple comorbidites, the outcomes of CPR are in fact poorer than those in the cases described. Outcomes of CPR at our institution might differ from those quoted in the registry. However, given the prevalence of inaccuracy, both for under‐ and overestimation, it seems likely that a true knowledge deficit on the part of the residents still accounts for much of the error and should be a target for education. Understanding CPR outcomes is vital for informed decision making, and studies have shown that when patients have more information, it can substantially affect a patient's decision regarding resuscitation.[7, 13]
Residents are infrequently exploring key determinants that affect a patient's decision‐making process. Only one‐third of residents report discussing quality‐of‐life issues with patients during code status discussions. Understanding an individual patient's values and goals and how he or she describes a good quality of life can help guide the discussion and potential recommendations. For example, some patients may feel it is important to be alive regardless of the physical state, whereas others may feel that if there is not a chance to be independent in their activities of daily living, then they would not want to be resuscitated. By exploring patient's perceptions of what quality of life and physical states worse than death means, residents can better understand and assist in the decision‐making process of their patients.
Our data show that few residents offer a recommendation regarding code status. Thus, residents expect patients to make their own decision with the data provided. At the same time, many residents focus on the details of the procedural components of CPR with little mention of anticipated outcomes or inquiries into key determinants discussed above. Additionally, based on their response to the knowledge‐based questions, residents' estimates of survival, if offered, would be inaccurate. Thus, code status conversations by residents leave patients to make uninformed choices to consent to or refuse resuscitative measures.
When stratified by training year, PGY2/3 residents were significantly more likely than PGY1 residents to discuss likelihood of discharge from the hospital as well as factor in patients' comorbidities when discussing outcomes. Although there is a statistically significant improvement between PGY2/3 residents as compared to PGY1 residents, the numbers still show that most PGY2/3 residents and almost all PGY1 residents do not discuss the likelihood of discharge if resuscitated during code status discussions. In addition, there is no difference reported in other key areas of informed consent. Thus, though there is some improvement as housestaff advance in their training, PGY2 and PGY3 residents still do not discuss all 5 key elements of informed consent significantly more than PGY1 residents.
Our findings suggest an opportunity for additional education regarding how to address code status for internal medicine housestaff. Over half of the respondents reported small group teaching sessions, direct observation and feedback, and exposure to palliative care consultation during their clinical rotations; yet, very few of them included all the key elements of informed consent in their discussions. To address this, our institution is developing additional educational initiatives, including a faculty development program for teaching communication skills, using direct observation and feedback. The orientation didactic lecture series for housestaff now includes a lecture on CPR that highlights the data on outcomes and the importance of putting the step‐by‐step procedures of CPR into the context of potential benefits, such as survival to hospital discharge. The curriculum also includes a module on advance care planning for junior and senior residents during their ambulatory block, using simulation and feedback as part of the teaching methods.
There are limitations to this study. Studies based on surveys are subject to recall and selection bias, and we lack objective assessment of actual code status discussions. Furthermore, the nature of the study may lead to an overestimation of the quality of the code status discussions due to social acceptability bias; yet, our data clearly show that the key elements of informed consent are not included during these conversations. Another limitation is that our subjects were residents at a single institution, and our clinical practice may differ from other academic settings in the teaching environment and culture; yet, our findings mirror similar work done in other locations.[10, 14]
In conclusion, our results demonstrate that residents fail to meet standards of informed consent when discussing code status in that they do not provide sufficient information for patients to make an informed decision regarding resuscitation. Residents would benefit from education aimed at improving their knowledge of CPR outcomes as well as training on how to conduct these conversations effectively. Framing code status discussions as an example of an informed consent may help residents recognize the need for the key elements to be included in these conversations. In addition, training should focus on how to conduct these conversations in an efficient yet effective manner. This will require clear simple language, good communication skills, and training with observation and feedback by specialists trained in this field.
Disclosures
This work was presented at the Society of General Internal Medicine New England Regional Meeting, March 8, 2013, Yale Medical Center, New Haven, Connecticut. The authors report no conflicts of interest.
- , , , , Medical informed consent: general considerations for physicians. Mayo Clin Proc. 2008;83(3):313–319.
- Beth Israel Deaconess Medical Center. Policy #PR‐02 45 CFR 46.11679(4):240–243.
- , , , . Medical residents' perspectives on discussions of advanced directives: can prior experience affect how they approach patients? J Palliat Med. 2007;10(3):712–720.
- , , , , . Code status discussions between attending hospitalist physicians and medical patients at hospital admission. J Gen Intern Med. 2010;26(4):359–366.
- , , . The influence of the probability of survival on patient's preferences regarding cardiopulmonary resuscitation. N Engl J Med. 1994;330:545–549.
- , , , , . Using video images to improve the accuracy of surrogate decision‐making: a randomized controlled trial. J Am Med Dir Assoc. 2009;10(8):575–580.
- , , , et al. Use of video to facilitate end‐of‐life discussions with patients with cancer: a randomized controlled trial. J Clin Oncol. 2010;28(2):305–310.
- , , , , , . Resident Approaches to Advance Care Planning on the Day of Hospital Admission. Arch Intern Med. 2006;166:1597–1602.
- , , , . Assessing competence of residents to discuss end‐of‐life issues. J Palliat Med. 2005;8(2):363–371.
- , , , et al. Code status discussions and goals of care among hospitalised adults. J Med Ethics. 2009;35:338–342.
- , , , . Pre‐resuscitation factors associated with mortality in 49,130 cases of in‐hospital cardiac arrest: a report from the National Registry for Cardiopulmonary Resuscitation. Resuscitation. 2010;81:302–311.
- , , , . Resuscitation decision making in the elderly: the value of outcome data. J Gen Intern Med. 1993;8:295–300.
- , , . How do medical residents discuss resuscitation with patients? J Gen Intern Med. 1995;10:436–442.
- , , , , Medical informed consent: general considerations for physicians. Mayo Clin Proc. 2008;83(3):313–319.
- Beth Israel Deaconess Medical Center. Policy #PR‐02 45 CFR 46.11679(4):240–243.
- , , , . Medical residents' perspectives on discussions of advanced directives: can prior experience affect how they approach patients? J Palliat Med. 2007;10(3):712–720.
- , , , , . Code status discussions between attending hospitalist physicians and medical patients at hospital admission. J Gen Intern Med. 2010;26(4):359–366.
- , , . The influence of the probability of survival on patient's preferences regarding cardiopulmonary resuscitation. N Engl J Med. 1994;330:545–549.
- , , , , . Using video images to improve the accuracy of surrogate decision‐making: a randomized controlled trial. J Am Med Dir Assoc. 2009;10(8):575–580.
- , , , et al. Use of video to facilitate end‐of‐life discussions with patients with cancer: a randomized controlled trial. J Clin Oncol. 2010;28(2):305–310.
- , , , , , . Resident Approaches to Advance Care Planning on the Day of Hospital Admission. Arch Intern Med. 2006;166:1597–1602.
- , , , . Assessing competence of residents to discuss end‐of‐life issues. J Palliat Med. 2005;8(2):363–371.
- , , , et al. Code status discussions and goals of care among hospitalised adults. J Med Ethics. 2009;35:338–342.
- , , , . Pre‐resuscitation factors associated with mortality in 49,130 cases of in‐hospital cardiac arrest: a report from the National Registry for Cardiopulmonary Resuscitation. Resuscitation. 2010;81:302–311.
- , , , . Resuscitation decision making in the elderly: the value of outcome data. J Gen Intern Med. 1993;8:295–300.
- , , . How do medical residents discuss resuscitation with patients? J Gen Intern Med. 1995;10:436–442.
© 2015 Society of Hospital Medicine
Small nucleolar RNA signals colorectal cancer outcomes
Expression of a set of small nucleolar RNAs (snoRNAs) was found to be significantly elevated in cancer tissue, compared with normal tissue, and expression of a specific snoRNA, SNORA42, was associated with poor overall and disease-free survival in patients with colorectal cancer (CRC).
Elevated expression of the specific snoRNA SNORA42 was an independent prognostic indicator for overall survival (hazard ratio, 2.11; P = .021), disease-free survival (HR, 3.17; P = .011), and distant metastasis (HR, 2.66; P .023). In a subset of patients with stage II CRC, the significant association between high expression of SNORA42 and poor prognosis remained.
The majority of patients with stage II CRC are cured by surgery alone, but a significant proportion experience disease progression. According to Dr. Yoshinaga Okugawa of the Center for Gastrointestinal Cancer Research at the Baylor University Medical Center, Dallas, and colleagues, adjuvant chemotherapy treatment for all patients with stage II cancer is controversial.
“Therefore, identification of such high-risk patients with CRC using molecular biomarkers such as SNORA42 expression will allow use of adjuvant chemotherapy after surgery only in a select subgroup of high-risk patients to improve their prognosis,” they wrote (Gut 2015 Oct 15. doi: 10.1136/gutjnl-2015.309359).
The research examined expression levels of RNA extracted from formalin-fixed paraffin-embedded (FFPE) tissue samples from 250 patients with colorectal cancer and 24 matched controls. The initial focus was on four snoRNAs that were reported to be dysregulated in other cancers. The study found that all four of the snoRNAs were significantly upregulated in CRC tissue, compared with normal tissue. Receiver operating characteristic (ROC) curves showed that expression levels of all four snoRNAs distinguished between cancer and noncancer tissue; area under the ROC curves values ranged from 0.75 to 0.88.
SnoRNAs are single-stranded, noncoding RNAs that have recently emerged as important components in the control of cell fate and oncogenesis in various cancers. In addition, studies have suggested the potential of snoRNAs as prognostic indicators.
While the expression levels of all four snoRNAs distinguished cancer tissue from normal tissue, only one of the snoRNAs, SNORA42, had expression levels that correlated with disease outcomes (overall survival, disease-free survival, and distant metastasis).
To characterize the biologic role of SNORN42 in CRC progression, the research team investigated its expression and impact on cancer cell lines and animal models. Overexpressed SNORN42 enhanced cell proliferation and tumorigenicity in cultured cells and in an animal model of CRC.
Taken together, the results suggest potential clinical usefulness of SNORA42 as a diagnostic and predictive biomarker in patients with CRC.
Dr. Okugawa and coauthors reported having no disclosures.
Expression of a set of small nucleolar RNAs (snoRNAs) was found to be significantly elevated in cancer tissue, compared with normal tissue, and expression of a specific snoRNA, SNORA42, was associated with poor overall and disease-free survival in patients with colorectal cancer (CRC).
Elevated expression of the specific snoRNA SNORA42 was an independent prognostic indicator for overall survival (hazard ratio, 2.11; P = .021), disease-free survival (HR, 3.17; P = .011), and distant metastasis (HR, 2.66; P .023). In a subset of patients with stage II CRC, the significant association between high expression of SNORA42 and poor prognosis remained.
The majority of patients with stage II CRC are cured by surgery alone, but a significant proportion experience disease progression. According to Dr. Yoshinaga Okugawa of the Center for Gastrointestinal Cancer Research at the Baylor University Medical Center, Dallas, and colleagues, adjuvant chemotherapy treatment for all patients with stage II cancer is controversial.
“Therefore, identification of such high-risk patients with CRC using molecular biomarkers such as SNORA42 expression will allow use of adjuvant chemotherapy after surgery only in a select subgroup of high-risk patients to improve their prognosis,” they wrote (Gut 2015 Oct 15. doi: 10.1136/gutjnl-2015.309359).
The research examined expression levels of RNA extracted from formalin-fixed paraffin-embedded (FFPE) tissue samples from 250 patients with colorectal cancer and 24 matched controls. The initial focus was on four snoRNAs that were reported to be dysregulated in other cancers. The study found that all four of the snoRNAs were significantly upregulated in CRC tissue, compared with normal tissue. Receiver operating characteristic (ROC) curves showed that expression levels of all four snoRNAs distinguished between cancer and noncancer tissue; area under the ROC curves values ranged from 0.75 to 0.88.
SnoRNAs are single-stranded, noncoding RNAs that have recently emerged as important components in the control of cell fate and oncogenesis in various cancers. In addition, studies have suggested the potential of snoRNAs as prognostic indicators.
While the expression levels of all four snoRNAs distinguished cancer tissue from normal tissue, only one of the snoRNAs, SNORA42, had expression levels that correlated with disease outcomes (overall survival, disease-free survival, and distant metastasis).
To characterize the biologic role of SNORN42 in CRC progression, the research team investigated its expression and impact on cancer cell lines and animal models. Overexpressed SNORN42 enhanced cell proliferation and tumorigenicity in cultured cells and in an animal model of CRC.
Taken together, the results suggest potential clinical usefulness of SNORA42 as a diagnostic and predictive biomarker in patients with CRC.
Dr. Okugawa and coauthors reported having no disclosures.
Expression of a set of small nucleolar RNAs (snoRNAs) was found to be significantly elevated in cancer tissue, compared with normal tissue, and expression of a specific snoRNA, SNORA42, was associated with poor overall and disease-free survival in patients with colorectal cancer (CRC).
Elevated expression of the specific snoRNA SNORA42 was an independent prognostic indicator for overall survival (hazard ratio, 2.11; P = .021), disease-free survival (HR, 3.17; P = .011), and distant metastasis (HR, 2.66; P .023). In a subset of patients with stage II CRC, the significant association between high expression of SNORA42 and poor prognosis remained.
The majority of patients with stage II CRC are cured by surgery alone, but a significant proportion experience disease progression. According to Dr. Yoshinaga Okugawa of the Center for Gastrointestinal Cancer Research at the Baylor University Medical Center, Dallas, and colleagues, adjuvant chemotherapy treatment for all patients with stage II cancer is controversial.
“Therefore, identification of such high-risk patients with CRC using molecular biomarkers such as SNORA42 expression will allow use of adjuvant chemotherapy after surgery only in a select subgroup of high-risk patients to improve their prognosis,” they wrote (Gut 2015 Oct 15. doi: 10.1136/gutjnl-2015.309359).
The research examined expression levels of RNA extracted from formalin-fixed paraffin-embedded (FFPE) tissue samples from 250 patients with colorectal cancer and 24 matched controls. The initial focus was on four snoRNAs that were reported to be dysregulated in other cancers. The study found that all four of the snoRNAs were significantly upregulated in CRC tissue, compared with normal tissue. Receiver operating characteristic (ROC) curves showed that expression levels of all four snoRNAs distinguished between cancer and noncancer tissue; area under the ROC curves values ranged from 0.75 to 0.88.
SnoRNAs are single-stranded, noncoding RNAs that have recently emerged as important components in the control of cell fate and oncogenesis in various cancers. In addition, studies have suggested the potential of snoRNAs as prognostic indicators.
While the expression levels of all four snoRNAs distinguished cancer tissue from normal tissue, only one of the snoRNAs, SNORA42, had expression levels that correlated with disease outcomes (overall survival, disease-free survival, and distant metastasis).
To characterize the biologic role of SNORN42 in CRC progression, the research team investigated its expression and impact on cancer cell lines and animal models. Overexpressed SNORN42 enhanced cell proliferation and tumorigenicity in cultured cells and in an animal model of CRC.
Taken together, the results suggest potential clinical usefulness of SNORA42 as a diagnostic and predictive biomarker in patients with CRC.
Dr. Okugawa and coauthors reported having no disclosures.
FROM GUT
Key clinical point: Elevated expression of a small nucleolar RNA was associated with decreased overall and disease-free survival in patients with colorectal cancer.
Major finding: Elevated expression of the specific snoRNA SNORA42 was an independent prognostic indicator for overall survival (HR, 2.11; P = .021), disease-free survival (HR, 3.17; P = .011), and distant metastasis (HR, 2.66; P = .023).
Data source: RNA was extracted from FFPE tissue samples from 250 patients with colorectal cancer and 24 samples from matched controls.
Disclosures: Dr. Okugawa and coauthors reported having no disclosures.
ESC: Air quality level linked to STEMI risk in men
LONDON – The risk for ST-elevation myocardial infarction (STEMI) increased in men when air quality dipped below acceptable levels as designated by the World Health Organization (WHO), based on the results of an observational study in Belgian.
The relative risk for a STEMI increased by 2.8% (1.026; 95% confidence interval, 1.005-1.048) for every 10 mcg/cm3 rise in fine particulate air pollution and by 5.1% (RR, 1.051; 95% CI, 1.018-1.084) for a comparable rise in nitric oxide air pollution. Ozone level was not associated with the risk for STEMI. Air pollution levels did not affect in-hospital mortality due to STEMI and had no clear effect on patients with coronary artery disease, diabetes or hypertension.
“Of course, it is very interesting that the results are only observed in men. This may be due to a statistical issue, because in our study population, the female group represents less than 25% of the population,” Dr. Jean-François Argacha, a study investigator, said during a press briefing at the annual congress of the European Society of Cardiology.
Subgroup analysis suggested that patients who were aged 75 years and older were more likely to develop STEMI in relation to exposure to particulate matter (RR, 1.046; 95% CI, 1.002-1.092, P = .041). Those aged 54 years and younger seemed more susceptible to nitric oxide levels (RR, 1.071; 95% CI, 1.010-1.136; P = .021).“The detrimental impact of nitric oxide exceeded that of fine particles and may be of particular concern in the younger population,” said Dr. Argacha, a cardiologist at University Hospital Brussels.
Previous research had shown that particulate matter is associated with an increased risk for acute myocardial infarction, but there had been no specific assessments on air pollution’s potential effects on STEMI, according to Dr. Argacha.
The study considered information on 11,428 patients in the Belgian Interdisciplinary Working Group on Acute Cardiology STEMI Registry between 2009 and 2013. Data on national air pollution parameters – particulate matter with an aerodynamic diameter of less than 10 (PM10) or 2.5 (PM2.5) mcm, nitric oxide and ozone – and air temperature were obtained from the Belgian Environment Agency database and adjusted for population density.
A case crossover analysis of STEMI risk was then performed, with risk being adjusted for ambient temperature, day of the week, and the season. The study’s case crossover design ensured that the effects were limited to air pollution and excluded other confounders including respiratory disease and air temperature.
Worldwide, WHO has estimated that poor urban air quality is responsible for around 1.3 million deaths per year, Dr. Argacha observed, noting that air pollution consists of fine (PM2.5) and larger (PM10) particles, nitric oxide, and ozone as well as sulfur dioxide and carbon monoxide.
During the observation period, the mean air pollution levels over the course of 1 year in Belgium were 23.9 mcg/cm3 for PM10, 16.1 mcg/cm3 for PM2.5, and 23.7 mcg/cm3 for nitric oxide. WHO guidelines set a daily limit of 25 mcg/cm3 for PM2.5. Dr. Argacha noted that this limit was exceeded in Belgium on 17.5% of days in the study.
Dr. Oscar Franco, professor of preventative medicine at the Erasmus University Medical Center in Rotterdam, the Netherlands, who cochaired the press conference noted that the European Society of Cardiology recently published a position paper on air pollution on cardiovascular disease (Eur Heart J. 2015;36:83-93) and has launched a major campaign to raise awareness of the detrimental effects that the environment can have on the heart.
The campaign notes that air and noise pollution are modifiable risk factors for the prevention and control of cardiovascular diseases, and advocated for acceptable limits that reflect WHO levels. Individuals “need to take action,” Dr. Franco urged. The decisions that people make every day – how they get to work for example – could have a potentially huge impact on the environment and health.
Dr. Argacha and Dr. Franco had no disclosures to report.
LONDON – The risk for ST-elevation myocardial infarction (STEMI) increased in men when air quality dipped below acceptable levels as designated by the World Health Organization (WHO), based on the results of an observational study in Belgian.
The relative risk for a STEMI increased by 2.8% (1.026; 95% confidence interval, 1.005-1.048) for every 10 mcg/cm3 rise in fine particulate air pollution and by 5.1% (RR, 1.051; 95% CI, 1.018-1.084) for a comparable rise in nitric oxide air pollution. Ozone level was not associated with the risk for STEMI. Air pollution levels did not affect in-hospital mortality due to STEMI and had no clear effect on patients with coronary artery disease, diabetes or hypertension.
“Of course, it is very interesting that the results are only observed in men. This may be due to a statistical issue, because in our study population, the female group represents less than 25% of the population,” Dr. Jean-François Argacha, a study investigator, said during a press briefing at the annual congress of the European Society of Cardiology.
Subgroup analysis suggested that patients who were aged 75 years and older were more likely to develop STEMI in relation to exposure to particulate matter (RR, 1.046; 95% CI, 1.002-1.092, P = .041). Those aged 54 years and younger seemed more susceptible to nitric oxide levels (RR, 1.071; 95% CI, 1.010-1.136; P = .021).“The detrimental impact of nitric oxide exceeded that of fine particles and may be of particular concern in the younger population,” said Dr. Argacha, a cardiologist at University Hospital Brussels.
Previous research had shown that particulate matter is associated with an increased risk for acute myocardial infarction, but there had been no specific assessments on air pollution’s potential effects on STEMI, according to Dr. Argacha.
The study considered information on 11,428 patients in the Belgian Interdisciplinary Working Group on Acute Cardiology STEMI Registry between 2009 and 2013. Data on national air pollution parameters – particulate matter with an aerodynamic diameter of less than 10 (PM10) or 2.5 (PM2.5) mcm, nitric oxide and ozone – and air temperature were obtained from the Belgian Environment Agency database and adjusted for population density.
A case crossover analysis of STEMI risk was then performed, with risk being adjusted for ambient temperature, day of the week, and the season. The study’s case crossover design ensured that the effects were limited to air pollution and excluded other confounders including respiratory disease and air temperature.
Worldwide, WHO has estimated that poor urban air quality is responsible for around 1.3 million deaths per year, Dr. Argacha observed, noting that air pollution consists of fine (PM2.5) and larger (PM10) particles, nitric oxide, and ozone as well as sulfur dioxide and carbon monoxide.
During the observation period, the mean air pollution levels over the course of 1 year in Belgium were 23.9 mcg/cm3 for PM10, 16.1 mcg/cm3 for PM2.5, and 23.7 mcg/cm3 for nitric oxide. WHO guidelines set a daily limit of 25 mcg/cm3 for PM2.5. Dr. Argacha noted that this limit was exceeded in Belgium on 17.5% of days in the study.
Dr. Oscar Franco, professor of preventative medicine at the Erasmus University Medical Center in Rotterdam, the Netherlands, who cochaired the press conference noted that the European Society of Cardiology recently published a position paper on air pollution on cardiovascular disease (Eur Heart J. 2015;36:83-93) and has launched a major campaign to raise awareness of the detrimental effects that the environment can have on the heart.
The campaign notes that air and noise pollution are modifiable risk factors for the prevention and control of cardiovascular diseases, and advocated for acceptable limits that reflect WHO levels. Individuals “need to take action,” Dr. Franco urged. The decisions that people make every day – how they get to work for example – could have a potentially huge impact on the environment and health.
Dr. Argacha and Dr. Franco had no disclosures to report.
LONDON – The risk for ST-elevation myocardial infarction (STEMI) increased in men when air quality dipped below acceptable levels as designated by the World Health Organization (WHO), based on the results of an observational study in Belgian.
The relative risk for a STEMI increased by 2.8% (1.026; 95% confidence interval, 1.005-1.048) for every 10 mcg/cm3 rise in fine particulate air pollution and by 5.1% (RR, 1.051; 95% CI, 1.018-1.084) for a comparable rise in nitric oxide air pollution. Ozone level was not associated with the risk for STEMI. Air pollution levels did not affect in-hospital mortality due to STEMI and had no clear effect on patients with coronary artery disease, diabetes or hypertension.
“Of course, it is very interesting that the results are only observed in men. This may be due to a statistical issue, because in our study population, the female group represents less than 25% of the population,” Dr. Jean-François Argacha, a study investigator, said during a press briefing at the annual congress of the European Society of Cardiology.
Subgroup analysis suggested that patients who were aged 75 years and older were more likely to develop STEMI in relation to exposure to particulate matter (RR, 1.046; 95% CI, 1.002-1.092, P = .041). Those aged 54 years and younger seemed more susceptible to nitric oxide levels (RR, 1.071; 95% CI, 1.010-1.136; P = .021).“The detrimental impact of nitric oxide exceeded that of fine particles and may be of particular concern in the younger population,” said Dr. Argacha, a cardiologist at University Hospital Brussels.
Previous research had shown that particulate matter is associated with an increased risk for acute myocardial infarction, but there had been no specific assessments on air pollution’s potential effects on STEMI, according to Dr. Argacha.
The study considered information on 11,428 patients in the Belgian Interdisciplinary Working Group on Acute Cardiology STEMI Registry between 2009 and 2013. Data on national air pollution parameters – particulate matter with an aerodynamic diameter of less than 10 (PM10) or 2.5 (PM2.5) mcm, nitric oxide and ozone – and air temperature were obtained from the Belgian Environment Agency database and adjusted for population density.
A case crossover analysis of STEMI risk was then performed, with risk being adjusted for ambient temperature, day of the week, and the season. The study’s case crossover design ensured that the effects were limited to air pollution and excluded other confounders including respiratory disease and air temperature.
Worldwide, WHO has estimated that poor urban air quality is responsible for around 1.3 million deaths per year, Dr. Argacha observed, noting that air pollution consists of fine (PM2.5) and larger (PM10) particles, nitric oxide, and ozone as well as sulfur dioxide and carbon monoxide.
During the observation period, the mean air pollution levels over the course of 1 year in Belgium were 23.9 mcg/cm3 for PM10, 16.1 mcg/cm3 for PM2.5, and 23.7 mcg/cm3 for nitric oxide. WHO guidelines set a daily limit of 25 mcg/cm3 for PM2.5. Dr. Argacha noted that this limit was exceeded in Belgium on 17.5% of days in the study.
Dr. Oscar Franco, professor of preventative medicine at the Erasmus University Medical Center in Rotterdam, the Netherlands, who cochaired the press conference noted that the European Society of Cardiology recently published a position paper on air pollution on cardiovascular disease (Eur Heart J. 2015;36:83-93) and has launched a major campaign to raise awareness of the detrimental effects that the environment can have on the heart.
The campaign notes that air and noise pollution are modifiable risk factors for the prevention and control of cardiovascular diseases, and advocated for acceptable limits that reflect WHO levels. Individuals “need to take action,” Dr. Franco urged. The decisions that people make every day – how they get to work for example – could have a potentially huge impact on the environment and health.
Dr. Argacha and Dr. Franco had no disclosures to report.
AT THE ESC CONGRESS 2015
Key clinical point: Air pollution may be a potentially modifiable risk factor for STEMI in men.
Major finding: The relative risk for a STEMI increased by 2.8% for a 10 mcg/cm3 rise in fine particulate air pollution and by 5.1% for a comparable rise in nitric oxide air pollution.
Data source: 11,428 patients logged in the Belgian Interdisciplinary Working Group on Acute Cardiology STEMI Registry between 2009 and 2013.
Disclosures: Dr. Argacha and Dr. Franco had no disclosures to report.