User login
HELIOS trial: Ibrutinib safely boosts survival in CLL/SLL
CHICAGO – Adding ibrutinib to bendamustine and rituximab improved outcomes without significantly reducing safety in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) in the randomized, placebo-controlled, phase III HELIOS trial.
Efficacy results from the double-blind HELIOS trial, as reported by Dr. Asher Alban Chanan-Khan at the 2015 meeting of the American Society of Clinical Oncology, showed that adding ibrutinib to bendamustine and rituximab (BR) significantly extended progression-free survival, compared with BR plus placebo, in patients with CLL/SLL; the risk of progression and death was reduced by 80%.
The current findings, reported by Dr. Chanan-Khan at the American Society of Hematology Meeting on Hematologic Malignancies, demonstrate that this improvement was achieved without sacrificing safety, and they characterize the management of adverse events.
In 578 patients with active chronic CLL/SLL following at least one prior line of systemic therapy who were randomized to receive 420 mg of ibrutinib plus BR or placebo plus BR for six cycles, exposure was 14.7 months and 12.8 months, respectively. Infection rates were similar in the two groups, but exposure-adjusted analysis showed an overall lower infection rate in the ibrutinib group, compared with the placebo group (10.3/100 vs. 11.2/100 patient months), and the rates of grade 3 or higher infections was similar in the groups, said Dr. Chanan-Khan of the Mayo Clinic, Jacksonville, Fla.
The rates of all-grade and grade 3/4 anemia were 22.3% and 3.5%, respectively, in the ibrutinib group, and 28.9% and 8.0%, respectively, in the BR group. The ibrutinib patients also required fewer transfusions – most often red blood cell transfusions (23% vs. 29% in the BR group).This may have been a reflection of restoration of the hematopoietic system in the ibrutinib group, said Dr. Chanan-Khan.
Grade 3/4 neutropenia was similar in the groups (53.7% and 50.5%), but fewer patients discontinued treatment due to treatment-related neutropenia with ibrutinib (1% vs. 2.8%), he noted.
Thrombocytopenia occurred slightly more often in the ibrutinib group (30.7% vs. 24%), but grade 3/4 events occurred in 15% of patients in each group.
Atrial fibrillation (AF) occurred in a small number of patients, but was observed more often with ibrutinib (7.3% vs. 2.8% overall, and 2.8% vs. 0.7% for grade 3/4 AF). Only seven patients required dose interruption – for a median duration of 7 days – to manage AF.
“No dose reductions were required,” said Dr. Chanan-Khan, adding that four patients, all with grade 3/4 AF and all in the ibrutinib group, discontinued therapy because of AF.
“We then analyzed our data to identify potential risk factors for predisposition to AF ... no one baseline risk factor could be identified as causative. However, most patients who developed AF had a known risk factor,” he said.
He added that among those with a prior history of AF, 28% on the ibrutinib arm, and only 9% on the placebo arm, developed AF.
Baseline cardiac comorbidities also were found to have no effect on progression-free survival in either arm.
“We therefore concluded that the risk of AF is low at around 5%, it does not impact progression-free survival, prior history of AF is not a contraindication in the absence of any great freak event, ibrutinib dose interruption or reduction is not warranted, and you should treat CLL patients first for CLL and manage AF second,” he said.
Another important factor that often impacts clinical decision making is the use of anticoagulants or antiplatelet agents and the bleeding risk with ibrutinib, he said, noting that more than 40% of patients in the ibrutinib arm were using such agents.
“We did not see any impact on the progression-free survival outcomes on either of the arms in patients who were on anticoagulant or antiplatelet therapy,” he said.
Bleeding occurred in 31% and 14.6% of patients in the ibrutinib and placebo groups, respectively, and most cases involved grade 1 bruises and contusions. Only four patients discontinued therapy because of bleeding.
The rates of grade 3/4 major bleeding and major hemorrhage events were low in both groups, at less than 4%, and two patients discontinued therapy because of major bleeding. Two patients in the ibrutinib arm died because of major bleeding, including one who had a large preexisting abdominal aortic aneurysm, and one who experienced a large postsurgical intestinal perforation.
“Overall, these data support the use of ibrutinib in patients on concurrent anticoagulant or antiplatelet therapy, with no significantly increased major risk of bleeding with ibrutinib vs. placebo, and most bleeding events being grade 1 in nature,” said Dr. Chanan-Khan.
The rate of treatment-related lymphocytosis – a known pharmacodynamic effect of ibrutinib – occurred in 7% and 5.9% of the ibrutinib and placebo group patients, and most cases resolved within 2 weeks.
Based on the results of the 2014 phase III RESONATE trial and others looking at ibrutinib as a single-agent treatment for CLL, the agent is considered a new standard of care in patients with previously treated CLL/SLL. HELIOS was the first study to investigate ibrutinib in combination with BR.
“Considering the significant improvement in progression-free survival and overall survival, ibrutinib has a strong overall risk-benefit profile,” Dr. Chanan-Khan concluded.
The HELIOS study was sponsored by Janssen Pharmaceuticals. Dr. Chanan-Khan reported having no disclosures.
CHICAGO – Adding ibrutinib to bendamustine and rituximab improved outcomes without significantly reducing safety in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) in the randomized, placebo-controlled, phase III HELIOS trial.
Efficacy results from the double-blind HELIOS trial, as reported by Dr. Asher Alban Chanan-Khan at the 2015 meeting of the American Society of Clinical Oncology, showed that adding ibrutinib to bendamustine and rituximab (BR) significantly extended progression-free survival, compared with BR plus placebo, in patients with CLL/SLL; the risk of progression and death was reduced by 80%.
The current findings, reported by Dr. Chanan-Khan at the American Society of Hematology Meeting on Hematologic Malignancies, demonstrate that this improvement was achieved without sacrificing safety, and they characterize the management of adverse events.
In 578 patients with active chronic CLL/SLL following at least one prior line of systemic therapy who were randomized to receive 420 mg of ibrutinib plus BR or placebo plus BR for six cycles, exposure was 14.7 months and 12.8 months, respectively. Infection rates were similar in the two groups, but exposure-adjusted analysis showed an overall lower infection rate in the ibrutinib group, compared with the placebo group (10.3/100 vs. 11.2/100 patient months), and the rates of grade 3 or higher infections was similar in the groups, said Dr. Chanan-Khan of the Mayo Clinic, Jacksonville, Fla.
The rates of all-grade and grade 3/4 anemia were 22.3% and 3.5%, respectively, in the ibrutinib group, and 28.9% and 8.0%, respectively, in the BR group. The ibrutinib patients also required fewer transfusions – most often red blood cell transfusions (23% vs. 29% in the BR group).This may have been a reflection of restoration of the hematopoietic system in the ibrutinib group, said Dr. Chanan-Khan.
Grade 3/4 neutropenia was similar in the groups (53.7% and 50.5%), but fewer patients discontinued treatment due to treatment-related neutropenia with ibrutinib (1% vs. 2.8%), he noted.
Thrombocytopenia occurred slightly more often in the ibrutinib group (30.7% vs. 24%), but grade 3/4 events occurred in 15% of patients in each group.
Atrial fibrillation (AF) occurred in a small number of patients, but was observed more often with ibrutinib (7.3% vs. 2.8% overall, and 2.8% vs. 0.7% for grade 3/4 AF). Only seven patients required dose interruption – for a median duration of 7 days – to manage AF.
“No dose reductions were required,” said Dr. Chanan-Khan, adding that four patients, all with grade 3/4 AF and all in the ibrutinib group, discontinued therapy because of AF.
“We then analyzed our data to identify potential risk factors for predisposition to AF ... no one baseline risk factor could be identified as causative. However, most patients who developed AF had a known risk factor,” he said.
He added that among those with a prior history of AF, 28% on the ibrutinib arm, and only 9% on the placebo arm, developed AF.
Baseline cardiac comorbidities also were found to have no effect on progression-free survival in either arm.
“We therefore concluded that the risk of AF is low at around 5%, it does not impact progression-free survival, prior history of AF is not a contraindication in the absence of any great freak event, ibrutinib dose interruption or reduction is not warranted, and you should treat CLL patients first for CLL and manage AF second,” he said.
Another important factor that often impacts clinical decision making is the use of anticoagulants or antiplatelet agents and the bleeding risk with ibrutinib, he said, noting that more than 40% of patients in the ibrutinib arm were using such agents.
“We did not see any impact on the progression-free survival outcomes on either of the arms in patients who were on anticoagulant or antiplatelet therapy,” he said.
Bleeding occurred in 31% and 14.6% of patients in the ibrutinib and placebo groups, respectively, and most cases involved grade 1 bruises and contusions. Only four patients discontinued therapy because of bleeding.
The rates of grade 3/4 major bleeding and major hemorrhage events were low in both groups, at less than 4%, and two patients discontinued therapy because of major bleeding. Two patients in the ibrutinib arm died because of major bleeding, including one who had a large preexisting abdominal aortic aneurysm, and one who experienced a large postsurgical intestinal perforation.
“Overall, these data support the use of ibrutinib in patients on concurrent anticoagulant or antiplatelet therapy, with no significantly increased major risk of bleeding with ibrutinib vs. placebo, and most bleeding events being grade 1 in nature,” said Dr. Chanan-Khan.
The rate of treatment-related lymphocytosis – a known pharmacodynamic effect of ibrutinib – occurred in 7% and 5.9% of the ibrutinib and placebo group patients, and most cases resolved within 2 weeks.
Based on the results of the 2014 phase III RESONATE trial and others looking at ibrutinib as a single-agent treatment for CLL, the agent is considered a new standard of care in patients with previously treated CLL/SLL. HELIOS was the first study to investigate ibrutinib in combination with BR.
“Considering the significant improvement in progression-free survival and overall survival, ibrutinib has a strong overall risk-benefit profile,” Dr. Chanan-Khan concluded.
The HELIOS study was sponsored by Janssen Pharmaceuticals. Dr. Chanan-Khan reported having no disclosures.
CHICAGO – Adding ibrutinib to bendamustine and rituximab improved outcomes without significantly reducing safety in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) in the randomized, placebo-controlled, phase III HELIOS trial.
Efficacy results from the double-blind HELIOS trial, as reported by Dr. Asher Alban Chanan-Khan at the 2015 meeting of the American Society of Clinical Oncology, showed that adding ibrutinib to bendamustine and rituximab (BR) significantly extended progression-free survival, compared with BR plus placebo, in patients with CLL/SLL; the risk of progression and death was reduced by 80%.
The current findings, reported by Dr. Chanan-Khan at the American Society of Hematology Meeting on Hematologic Malignancies, demonstrate that this improvement was achieved without sacrificing safety, and they characterize the management of adverse events.
In 578 patients with active chronic CLL/SLL following at least one prior line of systemic therapy who were randomized to receive 420 mg of ibrutinib plus BR or placebo plus BR for six cycles, exposure was 14.7 months and 12.8 months, respectively. Infection rates were similar in the two groups, but exposure-adjusted analysis showed an overall lower infection rate in the ibrutinib group, compared with the placebo group (10.3/100 vs. 11.2/100 patient months), and the rates of grade 3 or higher infections was similar in the groups, said Dr. Chanan-Khan of the Mayo Clinic, Jacksonville, Fla.
The rates of all-grade and grade 3/4 anemia were 22.3% and 3.5%, respectively, in the ibrutinib group, and 28.9% and 8.0%, respectively, in the BR group. The ibrutinib patients also required fewer transfusions – most often red blood cell transfusions (23% vs. 29% in the BR group).This may have been a reflection of restoration of the hematopoietic system in the ibrutinib group, said Dr. Chanan-Khan.
Grade 3/4 neutropenia was similar in the groups (53.7% and 50.5%), but fewer patients discontinued treatment due to treatment-related neutropenia with ibrutinib (1% vs. 2.8%), he noted.
Thrombocytopenia occurred slightly more often in the ibrutinib group (30.7% vs. 24%), but grade 3/4 events occurred in 15% of patients in each group.
Atrial fibrillation (AF) occurred in a small number of patients, but was observed more often with ibrutinib (7.3% vs. 2.8% overall, and 2.8% vs. 0.7% for grade 3/4 AF). Only seven patients required dose interruption – for a median duration of 7 days – to manage AF.
“No dose reductions were required,” said Dr. Chanan-Khan, adding that four patients, all with grade 3/4 AF and all in the ibrutinib group, discontinued therapy because of AF.
“We then analyzed our data to identify potential risk factors for predisposition to AF ... no one baseline risk factor could be identified as causative. However, most patients who developed AF had a known risk factor,” he said.
He added that among those with a prior history of AF, 28% on the ibrutinib arm, and only 9% on the placebo arm, developed AF.
Baseline cardiac comorbidities also were found to have no effect on progression-free survival in either arm.
“We therefore concluded that the risk of AF is low at around 5%, it does not impact progression-free survival, prior history of AF is not a contraindication in the absence of any great freak event, ibrutinib dose interruption or reduction is not warranted, and you should treat CLL patients first for CLL and manage AF second,” he said.
Another important factor that often impacts clinical decision making is the use of anticoagulants or antiplatelet agents and the bleeding risk with ibrutinib, he said, noting that more than 40% of patients in the ibrutinib arm were using such agents.
“We did not see any impact on the progression-free survival outcomes on either of the arms in patients who were on anticoagulant or antiplatelet therapy,” he said.
Bleeding occurred in 31% and 14.6% of patients in the ibrutinib and placebo groups, respectively, and most cases involved grade 1 bruises and contusions. Only four patients discontinued therapy because of bleeding.
The rates of grade 3/4 major bleeding and major hemorrhage events were low in both groups, at less than 4%, and two patients discontinued therapy because of major bleeding. Two patients in the ibrutinib arm died because of major bleeding, including one who had a large preexisting abdominal aortic aneurysm, and one who experienced a large postsurgical intestinal perforation.
“Overall, these data support the use of ibrutinib in patients on concurrent anticoagulant or antiplatelet therapy, with no significantly increased major risk of bleeding with ibrutinib vs. placebo, and most bleeding events being grade 1 in nature,” said Dr. Chanan-Khan.
The rate of treatment-related lymphocytosis – a known pharmacodynamic effect of ibrutinib – occurred in 7% and 5.9% of the ibrutinib and placebo group patients, and most cases resolved within 2 weeks.
Based on the results of the 2014 phase III RESONATE trial and others looking at ibrutinib as a single-agent treatment for CLL, the agent is considered a new standard of care in patients with previously treated CLL/SLL. HELIOS was the first study to investigate ibrutinib in combination with BR.
“Considering the significant improvement in progression-free survival and overall survival, ibrutinib has a strong overall risk-benefit profile,” Dr. Chanan-Khan concluded.
The HELIOS study was sponsored by Janssen Pharmaceuticals. Dr. Chanan-Khan reported having no disclosures.
AT MHM 2015
Key clinical point: Adding ibrutinib to bendamustine and rituximab improved outcomes without significantly reducing safety in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL).
Major finding: The overall infection rate was lower in the ibrutinib group than in the placebo group (10.3/100 vs. 11.2/100 patient months).
Data source: The phase III HELIOS study involving 578 patients.
Disclosures: Janssen Pharmaceuticals sponsored the study. Dr. Chanan-Khan reported having no disclosures.
Assessment of Body Weight After Completion of Radiotherapy With or Without Chemotherapy and With or Without Prophylactic Feeding Tube Placement in Head and Neck Cancer
Purpose: A majority of the patients with a diagnosis of locally advanced head and neck cancer receiving combined chemoradiotherapy experience mucositis, odynophagia, and dysphagia resulting in reduced oral intake and weight loss during the treatment. A prophylactic feeding tube is generally recommended for these patients to maintain body weight during the treatment. The purpose of this retrospective study is to understand the change in body weight from baseline to last follow-up after completion of radiotherapy or combined chemoradiotherapy and to assess the role of prophylactic feeding tube placement in maintaining body weight.
Methods: Thirty-seven patients with a diagnosis of locally advanced head and neck cancers were treated either with adjuvant or definitive radiotherapy with or without chemotherapy at Kansas City VA Medical Center during 2013. Eleven patients did not receive chemotherapy, and a majority of the patients did not receive prophylactic percutaneous endoscopic gastrostomy tube placement. Twenty-six patient received cisplatin-based chemotherapy during radio-therapy; of these, 9 patients had no feeding tube and 17 patients received a prophylactic feeding tube. The radiation dose ranged from 60 to 70 Gy in 30 to 35 fractions. All these patients were followed on a regular basis, and weights were recorded on each visit. The follow-up period ranged from a minimum of 6 months to a maximum of 18 months. Results: Five patients died either from locoregional recurrence or distant metastases. The average weight loss for patients with combined modality treatments was 9.7% vs 6.3% for patients receiving no chemotherapy. The average weight loss for patients receiving concurrent chemotherapy and with prophylactic feeding tube placement was 7.4% com-pared with average weight loss of 16% for patients receiving chemotherapy and no prophylactic feeding tube placement. The majority of these patients both in the chemotherapy and the no chemotherapy groups never regained their baseline weight.
Conclusions: Patients receiving chemotherapy benefited from prophylactic feeding tube placement in maintaining body weight similar to patients receiving no chemotherapy.
Purpose: A majority of the patients with a diagnosis of locally advanced head and neck cancer receiving combined chemoradiotherapy experience mucositis, odynophagia, and dysphagia resulting in reduced oral intake and weight loss during the treatment. A prophylactic feeding tube is generally recommended for these patients to maintain body weight during the treatment. The purpose of this retrospective study is to understand the change in body weight from baseline to last follow-up after completion of radiotherapy or combined chemoradiotherapy and to assess the role of prophylactic feeding tube placement in maintaining body weight.
Methods: Thirty-seven patients with a diagnosis of locally advanced head and neck cancers were treated either with adjuvant or definitive radiotherapy with or without chemotherapy at Kansas City VA Medical Center during 2013. Eleven patients did not receive chemotherapy, and a majority of the patients did not receive prophylactic percutaneous endoscopic gastrostomy tube placement. Twenty-six patient received cisplatin-based chemotherapy during radio-therapy; of these, 9 patients had no feeding tube and 17 patients received a prophylactic feeding tube. The radiation dose ranged from 60 to 70 Gy in 30 to 35 fractions. All these patients were followed on a regular basis, and weights were recorded on each visit. The follow-up period ranged from a minimum of 6 months to a maximum of 18 months. Results: Five patients died either from locoregional recurrence or distant metastases. The average weight loss for patients with combined modality treatments was 9.7% vs 6.3% for patients receiving no chemotherapy. The average weight loss for patients receiving concurrent chemotherapy and with prophylactic feeding tube placement was 7.4% com-pared with average weight loss of 16% for patients receiving chemotherapy and no prophylactic feeding tube placement. The majority of these patients both in the chemotherapy and the no chemotherapy groups never regained their baseline weight.
Conclusions: Patients receiving chemotherapy benefited from prophylactic feeding tube placement in maintaining body weight similar to patients receiving no chemotherapy.
Purpose: A majority of the patients with a diagnosis of locally advanced head and neck cancer receiving combined chemoradiotherapy experience mucositis, odynophagia, and dysphagia resulting in reduced oral intake and weight loss during the treatment. A prophylactic feeding tube is generally recommended for these patients to maintain body weight during the treatment. The purpose of this retrospective study is to understand the change in body weight from baseline to last follow-up after completion of radiotherapy or combined chemoradiotherapy and to assess the role of prophylactic feeding tube placement in maintaining body weight.
Methods: Thirty-seven patients with a diagnosis of locally advanced head and neck cancers were treated either with adjuvant or definitive radiotherapy with or without chemotherapy at Kansas City VA Medical Center during 2013. Eleven patients did not receive chemotherapy, and a majority of the patients did not receive prophylactic percutaneous endoscopic gastrostomy tube placement. Twenty-six patient received cisplatin-based chemotherapy during radio-therapy; of these, 9 patients had no feeding tube and 17 patients received a prophylactic feeding tube. The radiation dose ranged from 60 to 70 Gy in 30 to 35 fractions. All these patients were followed on a regular basis, and weights were recorded on each visit. The follow-up period ranged from a minimum of 6 months to a maximum of 18 months. Results: Five patients died either from locoregional recurrence or distant metastases. The average weight loss for patients with combined modality treatments was 9.7% vs 6.3% for patients receiving no chemotherapy. The average weight loss for patients receiving concurrent chemotherapy and with prophylactic feeding tube placement was 7.4% com-pared with average weight loss of 16% for patients receiving chemotherapy and no prophylactic feeding tube placement. The majority of these patients both in the chemotherapy and the no chemotherapy groups never regained their baseline weight.
Conclusions: Patients receiving chemotherapy benefited from prophylactic feeding tube placement in maintaining body weight similar to patients receiving no chemotherapy.
Cancer Cachexia and Survival in Patients With Lung Cancer by Histology: Who Is Most at Risk?
Background: Cancer cachexia is a syndrome characterized by wasting of lean body mass and fat, resulting in decreased treatment tolerance and quality of life. Approximately 50% to 60% of patients with lung cancer develop cancer cachexia. Knowledge of cancer cachexia trends and outcomes in different types of lung cancers can help target patients most at risk of decreased survival. In this study, we examined weight loss trends in patients with lung cancer and survival according to cancer histology.
Methods: We conducted a chart review of patients diagnosed with lung cancer from 2007 to 2013 at a medical center in a large metropolitan area. Data collected included demographics, date of diagnosis, treatment received, type of cancer by histology, presence of metastasis, preexisting conditions, and other medications used. Body mass index and weight before, during, and after treatments were recorded, and percentage weight loss and survival days were calculated. Patients were also categorized according to the extent of weight loss (< 5%, 5%-10%, > 10%) through their disease course. Descriptive statistics, ANOVA, t tests, and regression analysis were used for data analysis to assess differences and relationships between percentage of weight loss and survival for all groups.
Results: Data from 197 patients were available for the study. All patients were males with an average age of 68 years; 55% of the patients were white and 35% were African American. Of the total patients, 47.7% had adenocarcinoma, 8.1% had small cell lung cancer (SCLC), and 44.2% had squamous cell cancer (SCC). Percentage weight loss trends for patients with adenocarcinoma showed a 9% loss from baseline till time of diagnosis and a total of 15% loss posttreatments. Patients with SCLC similarly showed a decline from 13% to 22%, and patients with SCC showed a 14% to 17% weight loss. Of the total patients with > 10% weight loss over time, 48% were patients with adenocarcinoma, compared with 10% of patients with SCLC, and 42% of patients with SCC. Average survival for patients with adenocarcinoma was 420 days, for patients with SCLC 321 days, and 492 days for patients with SCC. Weight loss percentage was significantly related to survival in all patients (r = -0.19, P < .01) and patient groups with adenocarcinoma (r = -0.25, P < .01) but not in patients with SCC or SCLC.
Conclusions: Cancer cachexia trends in patients with lung cancer are similar for patients with adenocarcinoma, SCLC, and SCC; however, the percentage of weight loss may adversely impact survival in patients with SCLC.
Background: Cancer cachexia is a syndrome characterized by wasting of lean body mass and fat, resulting in decreased treatment tolerance and quality of life. Approximately 50% to 60% of patients with lung cancer develop cancer cachexia. Knowledge of cancer cachexia trends and outcomes in different types of lung cancers can help target patients most at risk of decreased survival. In this study, we examined weight loss trends in patients with lung cancer and survival according to cancer histology.
Methods: We conducted a chart review of patients diagnosed with lung cancer from 2007 to 2013 at a medical center in a large metropolitan area. Data collected included demographics, date of diagnosis, treatment received, type of cancer by histology, presence of metastasis, preexisting conditions, and other medications used. Body mass index and weight before, during, and after treatments were recorded, and percentage weight loss and survival days were calculated. Patients were also categorized according to the extent of weight loss (< 5%, 5%-10%, > 10%) through their disease course. Descriptive statistics, ANOVA, t tests, and regression analysis were used for data analysis to assess differences and relationships between percentage of weight loss and survival for all groups.
Results: Data from 197 patients were available for the study. All patients were males with an average age of 68 years; 55% of the patients were white and 35% were African American. Of the total patients, 47.7% had adenocarcinoma, 8.1% had small cell lung cancer (SCLC), and 44.2% had squamous cell cancer (SCC). Percentage weight loss trends for patients with adenocarcinoma showed a 9% loss from baseline till time of diagnosis and a total of 15% loss posttreatments. Patients with SCLC similarly showed a decline from 13% to 22%, and patients with SCC showed a 14% to 17% weight loss. Of the total patients with > 10% weight loss over time, 48% were patients with adenocarcinoma, compared with 10% of patients with SCLC, and 42% of patients with SCC. Average survival for patients with adenocarcinoma was 420 days, for patients with SCLC 321 days, and 492 days for patients with SCC. Weight loss percentage was significantly related to survival in all patients (r = -0.19, P < .01) and patient groups with adenocarcinoma (r = -0.25, P < .01) but not in patients with SCC or SCLC.
Conclusions: Cancer cachexia trends in patients with lung cancer are similar for patients with adenocarcinoma, SCLC, and SCC; however, the percentage of weight loss may adversely impact survival in patients with SCLC.
Background: Cancer cachexia is a syndrome characterized by wasting of lean body mass and fat, resulting in decreased treatment tolerance and quality of life. Approximately 50% to 60% of patients with lung cancer develop cancer cachexia. Knowledge of cancer cachexia trends and outcomes in different types of lung cancers can help target patients most at risk of decreased survival. In this study, we examined weight loss trends in patients with lung cancer and survival according to cancer histology.
Methods: We conducted a chart review of patients diagnosed with lung cancer from 2007 to 2013 at a medical center in a large metropolitan area. Data collected included demographics, date of diagnosis, treatment received, type of cancer by histology, presence of metastasis, preexisting conditions, and other medications used. Body mass index and weight before, during, and after treatments were recorded, and percentage weight loss and survival days were calculated. Patients were also categorized according to the extent of weight loss (< 5%, 5%-10%, > 10%) through their disease course. Descriptive statistics, ANOVA, t tests, and regression analysis were used for data analysis to assess differences and relationships between percentage of weight loss and survival for all groups.
Results: Data from 197 patients were available for the study. All patients were males with an average age of 68 years; 55% of the patients were white and 35% were African American. Of the total patients, 47.7% had adenocarcinoma, 8.1% had small cell lung cancer (SCLC), and 44.2% had squamous cell cancer (SCC). Percentage weight loss trends for patients with adenocarcinoma showed a 9% loss from baseline till time of diagnosis and a total of 15% loss posttreatments. Patients with SCLC similarly showed a decline from 13% to 22%, and patients with SCC showed a 14% to 17% weight loss. Of the total patients with > 10% weight loss over time, 48% were patients with adenocarcinoma, compared with 10% of patients with SCLC, and 42% of patients with SCC. Average survival for patients with adenocarcinoma was 420 days, for patients with SCLC 321 days, and 492 days for patients with SCC. Weight loss percentage was significantly related to survival in all patients (r = -0.19, P < .01) and patient groups with adenocarcinoma (r = -0.25, P < .01) but not in patients with SCC or SCLC.
Conclusions: Cancer cachexia trends in patients with lung cancer are similar for patients with adenocarcinoma, SCLC, and SCC; however, the percentage of weight loss may adversely impact survival in patients with SCLC.
Frequency of EGFR Mutations and ALK Rearrangements in Pulmonary Carcinomas at the Minneapolis VA Medical Center
Purpose: To evaluate the frequency of molecular testing in pulmonary carcinomas.
Background: Pulmonary carcinoma represents the second most common type of new malignancies in males and females. Although its incidence is plateauing, it represents the most common cause of cancer-related death in both genders. The 5-year survival rate is only 18%. The main risk factor in both genders is smoking. Carcinogenesis and tumor progression in some tumors involve mutations in relevant genes that further cell survival and proliferation and provide advantage over neighboring cells. Although therapy has traditionally been guided by tumor histology, drugs have been developed that specifically target driver mutation metabolic pathways. These drugs promise higher efficacy and lower toxicity; however, their use must be guided by the genetic makeup of individual tumors. One such mutation occurs in the EGFR gene and is more common in women with no smoking history. Another, mutually exclusive mutation consists of rearrangements in the ALK gene. These alterations render the cells sensitive to targeted therapy with tyrosine kinase inhibitors.
Data Analysis/Results: At the Minneapolis VA Medical Center, these tests are performed when ordered by the oncologists. We analyzed the frequency of mutations in exons 18-21 of the EGFR gene by PCR-based essay and ALK rearrangements by FISH technique in lung carcinomas. One hundred eighty-three biopsy and cytology specimens collected between January 2009 and June 2015 were evaluated. Of these, 11 (6%) were positive for EGFR mutations; none was positive for ALK rearrangements. All positive cases were morphologically adenocarcinomas; pulmonary origin was supported by clinical, radiologic and immunohistochemical criteria (positivity for cytokeratin 7 and for TTF-1). Specimens consisted of cell blocks (pleural effusion, fine needle aspirates) and needle biopsy cores of primary tumors or metastases or lobectomy specimen.
Implications: Compared with the literature, our frequency of mutant cases for the 2 targets analyzed is lower. This may be due to the fact that our patient population consisted predominantly of men with a history of smoking. It is important to analyze local statistics and compare them with the VA systemwide data for establishing guidelines and cost analysis.
Purpose: To evaluate the frequency of molecular testing in pulmonary carcinomas.
Background: Pulmonary carcinoma represents the second most common type of new malignancies in males and females. Although its incidence is plateauing, it represents the most common cause of cancer-related death in both genders. The 5-year survival rate is only 18%. The main risk factor in both genders is smoking. Carcinogenesis and tumor progression in some tumors involve mutations in relevant genes that further cell survival and proliferation and provide advantage over neighboring cells. Although therapy has traditionally been guided by tumor histology, drugs have been developed that specifically target driver mutation metabolic pathways. These drugs promise higher efficacy and lower toxicity; however, their use must be guided by the genetic makeup of individual tumors. One such mutation occurs in the EGFR gene and is more common in women with no smoking history. Another, mutually exclusive mutation consists of rearrangements in the ALK gene. These alterations render the cells sensitive to targeted therapy with tyrosine kinase inhibitors.
Data Analysis/Results: At the Minneapolis VA Medical Center, these tests are performed when ordered by the oncologists. We analyzed the frequency of mutations in exons 18-21 of the EGFR gene by PCR-based essay and ALK rearrangements by FISH technique in lung carcinomas. One hundred eighty-three biopsy and cytology specimens collected between January 2009 and June 2015 were evaluated. Of these, 11 (6%) were positive for EGFR mutations; none was positive for ALK rearrangements. All positive cases were morphologically adenocarcinomas; pulmonary origin was supported by clinical, radiologic and immunohistochemical criteria (positivity for cytokeratin 7 and for TTF-1). Specimens consisted of cell blocks (pleural effusion, fine needle aspirates) and needle biopsy cores of primary tumors or metastases or lobectomy specimen.
Implications: Compared with the literature, our frequency of mutant cases for the 2 targets analyzed is lower. This may be due to the fact that our patient population consisted predominantly of men with a history of smoking. It is important to analyze local statistics and compare them with the VA systemwide data for establishing guidelines and cost analysis.
Purpose: To evaluate the frequency of molecular testing in pulmonary carcinomas.
Background: Pulmonary carcinoma represents the second most common type of new malignancies in males and females. Although its incidence is plateauing, it represents the most common cause of cancer-related death in both genders. The 5-year survival rate is only 18%. The main risk factor in both genders is smoking. Carcinogenesis and tumor progression in some tumors involve mutations in relevant genes that further cell survival and proliferation and provide advantage over neighboring cells. Although therapy has traditionally been guided by tumor histology, drugs have been developed that specifically target driver mutation metabolic pathways. These drugs promise higher efficacy and lower toxicity; however, their use must be guided by the genetic makeup of individual tumors. One such mutation occurs in the EGFR gene and is more common in women with no smoking history. Another, mutually exclusive mutation consists of rearrangements in the ALK gene. These alterations render the cells sensitive to targeted therapy with tyrosine kinase inhibitors.
Data Analysis/Results: At the Minneapolis VA Medical Center, these tests are performed when ordered by the oncologists. We analyzed the frequency of mutations in exons 18-21 of the EGFR gene by PCR-based essay and ALK rearrangements by FISH technique in lung carcinomas. One hundred eighty-three biopsy and cytology specimens collected between January 2009 and June 2015 were evaluated. Of these, 11 (6%) were positive for EGFR mutations; none was positive for ALK rearrangements. All positive cases were morphologically adenocarcinomas; pulmonary origin was supported by clinical, radiologic and immunohistochemical criteria (positivity for cytokeratin 7 and for TTF-1). Specimens consisted of cell blocks (pleural effusion, fine needle aspirates) and needle biopsy cores of primary tumors or metastases or lobectomy specimen.
Implications: Compared with the literature, our frequency of mutant cases for the 2 targets analyzed is lower. This may be due to the fact that our patient population consisted predominantly of men with a history of smoking. It is important to analyze local statistics and compare them with the VA systemwide data for establishing guidelines and cost analysis.
The Importance of Lymph Node Retrieval and Lymph Node Ratio in Male Patients With Colorectal Cancer: A 5-Year Retrospective Single Institution Study
Background: The National Comprehensive Cancer Network and the American Joint Committee on Cancer recommend retrieving > 12 lymph nodes for adequate colorectal cancer (CRC) staging. Nodal status and presence of metastasis is an important prognostic factor and may guide decision making for adjuvant chemotherapy. Recent data have shown variable results for survival based on the number of lymph nodes sampled and the ratio of positive lymph nodes of the total sampled. In our study, we aimed to assess the influence of lymph node retrieval and positive lymph node status on overall survival of male veteran patients with CRC.
Methods: A retrospective chart review study at a VA medical center in a large metropolitan area was conducted. Charts of patients diagnosed with colon cancer from January 1, 2008, to January 1, 2012, were reviewed, and data on age, diagnosis of cancer, symptoms, histologic type of tumor, stage, number of lymph nodes harvested, number of lymph nodes positive for cancer, tumor invasion, date of diagnosis, and date of death were recorded. Descriptive statistics, including average/median, range, and standard deviation were calculated. Lymph node ratio (LNR) was calculated from the number of lymph nodes positive for cancer of the total number of lymph nodes harvested. Survival was calculated from date of diagnosis to date of death. Differences in survival were assessed through t tests for different groups. Pearson’s correlations and regression analysis were carried out for survival for the 4 groups of interest (< 12 nodes harvested, ≥ 12 nodes harvested. Lymph node ratio < 0.2, and LNR > 0.2)
Results: Data from 84 patients were obtained with a median survival of 299 days. On diagnosis, 26 (31%) were stage I, 21 (25%) were stage II, 16 (18%) were stage III, and 21 (25%) were stage IV. Twenty-three (27.3%) patients had local invasion at time of diagnosis. An average of 14.5 lymph nodes (range 4-29) were sampled per patient. Twenty-two (26%) patients had < 12 nodes sampled, and 42 (50%) had ≥ 12 nodes sampled. The average LNR for the whole group was 0.07 (SD ± 0.15). There was no significant difference in sur-vival between the patient groups who had < 12 LNs sampled vs those who had 12 or more LNs sampled (mean 316.6 days vs 543.6 days, t = 0.82, df 11, P = .42). There was no significant difference in survival between the patient groups who had LNR < 0.2 vs those who had LNR > 0.2 (mean 450.7 days vs 580.0 days, t = 0.50, df 9, P = .62).There were no significant differences in survival based on mode of diagnosis (screening colonoscopy vs presence of symptoms) or presence of local invasion at diagnosis.
Conclusion: This study did not find that number of harvested LNs as well as LNR had an impact on survival.
Background: The National Comprehensive Cancer Network and the American Joint Committee on Cancer recommend retrieving > 12 lymph nodes for adequate colorectal cancer (CRC) staging. Nodal status and presence of metastasis is an important prognostic factor and may guide decision making for adjuvant chemotherapy. Recent data have shown variable results for survival based on the number of lymph nodes sampled and the ratio of positive lymph nodes of the total sampled. In our study, we aimed to assess the influence of lymph node retrieval and positive lymph node status on overall survival of male veteran patients with CRC.
Methods: A retrospective chart review study at a VA medical center in a large metropolitan area was conducted. Charts of patients diagnosed with colon cancer from January 1, 2008, to January 1, 2012, were reviewed, and data on age, diagnosis of cancer, symptoms, histologic type of tumor, stage, number of lymph nodes harvested, number of lymph nodes positive for cancer, tumor invasion, date of diagnosis, and date of death were recorded. Descriptive statistics, including average/median, range, and standard deviation were calculated. Lymph node ratio (LNR) was calculated from the number of lymph nodes positive for cancer of the total number of lymph nodes harvested. Survival was calculated from date of diagnosis to date of death. Differences in survival were assessed through t tests for different groups. Pearson’s correlations and regression analysis were carried out for survival for the 4 groups of interest (< 12 nodes harvested, ≥ 12 nodes harvested. Lymph node ratio < 0.2, and LNR > 0.2)
Results: Data from 84 patients were obtained with a median survival of 299 days. On diagnosis, 26 (31%) were stage I, 21 (25%) were stage II, 16 (18%) were stage III, and 21 (25%) were stage IV. Twenty-three (27.3%) patients had local invasion at time of diagnosis. An average of 14.5 lymph nodes (range 4-29) were sampled per patient. Twenty-two (26%) patients had < 12 nodes sampled, and 42 (50%) had ≥ 12 nodes sampled. The average LNR for the whole group was 0.07 (SD ± 0.15). There was no significant difference in sur-vival between the patient groups who had < 12 LNs sampled vs those who had 12 or more LNs sampled (mean 316.6 days vs 543.6 days, t = 0.82, df 11, P = .42). There was no significant difference in survival between the patient groups who had LNR < 0.2 vs those who had LNR > 0.2 (mean 450.7 days vs 580.0 days, t = 0.50, df 9, P = .62).There were no significant differences in survival based on mode of diagnosis (screening colonoscopy vs presence of symptoms) or presence of local invasion at diagnosis.
Conclusion: This study did not find that number of harvested LNs as well as LNR had an impact on survival.
Background: The National Comprehensive Cancer Network and the American Joint Committee on Cancer recommend retrieving > 12 lymph nodes for adequate colorectal cancer (CRC) staging. Nodal status and presence of metastasis is an important prognostic factor and may guide decision making for adjuvant chemotherapy. Recent data have shown variable results for survival based on the number of lymph nodes sampled and the ratio of positive lymph nodes of the total sampled. In our study, we aimed to assess the influence of lymph node retrieval and positive lymph node status on overall survival of male veteran patients with CRC.
Methods: A retrospective chart review study at a VA medical center in a large metropolitan area was conducted. Charts of patients diagnosed with colon cancer from January 1, 2008, to January 1, 2012, were reviewed, and data on age, diagnosis of cancer, symptoms, histologic type of tumor, stage, number of lymph nodes harvested, number of lymph nodes positive for cancer, tumor invasion, date of diagnosis, and date of death were recorded. Descriptive statistics, including average/median, range, and standard deviation were calculated. Lymph node ratio (LNR) was calculated from the number of lymph nodes positive for cancer of the total number of lymph nodes harvested. Survival was calculated from date of diagnosis to date of death. Differences in survival were assessed through t tests for different groups. Pearson’s correlations and regression analysis were carried out for survival for the 4 groups of interest (< 12 nodes harvested, ≥ 12 nodes harvested. Lymph node ratio < 0.2, and LNR > 0.2)
Results: Data from 84 patients were obtained with a median survival of 299 days. On diagnosis, 26 (31%) were stage I, 21 (25%) were stage II, 16 (18%) were stage III, and 21 (25%) were stage IV. Twenty-three (27.3%) patients had local invasion at time of diagnosis. An average of 14.5 lymph nodes (range 4-29) were sampled per patient. Twenty-two (26%) patients had < 12 nodes sampled, and 42 (50%) had ≥ 12 nodes sampled. The average LNR for the whole group was 0.07 (SD ± 0.15). There was no significant difference in sur-vival between the patient groups who had < 12 LNs sampled vs those who had 12 or more LNs sampled (mean 316.6 days vs 543.6 days, t = 0.82, df 11, P = .42). There was no significant difference in survival between the patient groups who had LNR < 0.2 vs those who had LNR > 0.2 (mean 450.7 days vs 580.0 days, t = 0.50, df 9, P = .62).There were no significant differences in survival based on mode of diagnosis (screening colonoscopy vs presence of symptoms) or presence of local invasion at diagnosis.
Conclusion: This study did not find that number of harvested LNs as well as LNR had an impact on survival.
LISTEN NOW: Pediatric Hospital Medicine and the “Right Care” Movement
Three pediatric hospitalists – Dr. Ricardo Quiñonez of San Antonio Children’s Hospital, Dr. Shawn Ralston of Dartmouth-Hitchcock, and Dr. Alan Schroeder of Santa Clara Valley Medical Center – talk about the concept of “right care” in hospital medicine, and their participation in the Lown Institute’s Right Care movement.
Three pediatric hospitalists – Dr. Ricardo Quiñonez of San Antonio Children’s Hospital, Dr. Shawn Ralston of Dartmouth-Hitchcock, and Dr. Alan Schroeder of Santa Clara Valley Medical Center – talk about the concept of “right care” in hospital medicine, and their participation in the Lown Institute’s Right Care movement.
Three pediatric hospitalists – Dr. Ricardo Quiñonez of San Antonio Children’s Hospital, Dr. Shawn Ralston of Dartmouth-Hitchcock, and Dr. Alan Schroeder of Santa Clara Valley Medical Center – talk about the concept of “right care” in hospital medicine, and their participation in the Lown Institute’s Right Care movement.
No evidence for CLL transmission via blood transfusion
Analysis of data from blood transfusions that took place in Sweden and Denmark over a 30-year period showed no indication that chronic lymphocytic leukemia (CLL) risk is higher among recipients of blood from donors who subsequently developed CLL, according to researchers.
The study compared 7,413 recipients of blood from 796 donors who subsequently developed CLL (exposed group), with 80,431 recipients from 7,477 donors free of CLL (unexposed group). In total, 12 recipients in the exposed group and 107 in the unexposed group were later diagnosed with CLL, for an incidence rate ratio of 0.94 (95% confidence interval, 0.52-1.71). When defining “exposed” as receiving blood less than 10 years before donor CLL diagnosis, the incidence rate ratio was 0.46 (95% CI, 0.12-1.85).
“The analyses provided little evidence that donor MBL [monoclonal B-cell lymphocytosis]/CLL transmission in blood products influences recipient CLL risk,” wrote Dr. Henrik Hjalgrim of the department of epidemiology research at Statens Serum Institut, Copenhagen, and his colleagues (Blood 2015 doi: 10.1182/blood-2015-03-632844).
MBL is fairly common in healthy individuals (estimated at 7.1% in a study of American blood donors aged 45-91 years) and may progress to CLL at various rates depending on the MBL cell count. Results from previous studies investigating the association between transfusion and risk of CLL or small lymphocytic lymphoma have been mixed, they noted.
Using a retrospective approach, Dr. Hjalgrim and his associates first identified donors subsequently diagnosed with CLL, then identified control donors free from CLL who were matched for age, sex, county, number of donations, and blood type.
In case MBL may have progressed in the recipient but not the donor, investigators also examined whether CLL clustered among recipients from an individual donor, regardless of donor CLL status, but found no such clusters.
Limiting the analysis was the lack of donor MBL status, for which postdonation CLL diagnosis substituted. Some recipients in the exposed group may have received blood drawn before the donor developed MBL.
Dr. Hjalgrim and his coauthors reported having no disclosures.
Analysis of data from blood transfusions that took place in Sweden and Denmark over a 30-year period showed no indication that chronic lymphocytic leukemia (CLL) risk is higher among recipients of blood from donors who subsequently developed CLL, according to researchers.
The study compared 7,413 recipients of blood from 796 donors who subsequently developed CLL (exposed group), with 80,431 recipients from 7,477 donors free of CLL (unexposed group). In total, 12 recipients in the exposed group and 107 in the unexposed group were later diagnosed with CLL, for an incidence rate ratio of 0.94 (95% confidence interval, 0.52-1.71). When defining “exposed” as receiving blood less than 10 years before donor CLL diagnosis, the incidence rate ratio was 0.46 (95% CI, 0.12-1.85).
“The analyses provided little evidence that donor MBL [monoclonal B-cell lymphocytosis]/CLL transmission in blood products influences recipient CLL risk,” wrote Dr. Henrik Hjalgrim of the department of epidemiology research at Statens Serum Institut, Copenhagen, and his colleagues (Blood 2015 doi: 10.1182/blood-2015-03-632844).
MBL is fairly common in healthy individuals (estimated at 7.1% in a study of American blood donors aged 45-91 years) and may progress to CLL at various rates depending on the MBL cell count. Results from previous studies investigating the association between transfusion and risk of CLL or small lymphocytic lymphoma have been mixed, they noted.
Using a retrospective approach, Dr. Hjalgrim and his associates first identified donors subsequently diagnosed with CLL, then identified control donors free from CLL who were matched for age, sex, county, number of donations, and blood type.
In case MBL may have progressed in the recipient but not the donor, investigators also examined whether CLL clustered among recipients from an individual donor, regardless of donor CLL status, but found no such clusters.
Limiting the analysis was the lack of donor MBL status, for which postdonation CLL diagnosis substituted. Some recipients in the exposed group may have received blood drawn before the donor developed MBL.
Dr. Hjalgrim and his coauthors reported having no disclosures.
Analysis of data from blood transfusions that took place in Sweden and Denmark over a 30-year period showed no indication that chronic lymphocytic leukemia (CLL) risk is higher among recipients of blood from donors who subsequently developed CLL, according to researchers.
The study compared 7,413 recipients of blood from 796 donors who subsequently developed CLL (exposed group), with 80,431 recipients from 7,477 donors free of CLL (unexposed group). In total, 12 recipients in the exposed group and 107 in the unexposed group were later diagnosed with CLL, for an incidence rate ratio of 0.94 (95% confidence interval, 0.52-1.71). When defining “exposed” as receiving blood less than 10 years before donor CLL diagnosis, the incidence rate ratio was 0.46 (95% CI, 0.12-1.85).
“The analyses provided little evidence that donor MBL [monoclonal B-cell lymphocytosis]/CLL transmission in blood products influences recipient CLL risk,” wrote Dr. Henrik Hjalgrim of the department of epidemiology research at Statens Serum Institut, Copenhagen, and his colleagues (Blood 2015 doi: 10.1182/blood-2015-03-632844).
MBL is fairly common in healthy individuals (estimated at 7.1% in a study of American blood donors aged 45-91 years) and may progress to CLL at various rates depending on the MBL cell count. Results from previous studies investigating the association between transfusion and risk of CLL or small lymphocytic lymphoma have been mixed, they noted.
Using a retrospective approach, Dr. Hjalgrim and his associates first identified donors subsequently diagnosed with CLL, then identified control donors free from CLL who were matched for age, sex, county, number of donations, and blood type.
In case MBL may have progressed in the recipient but not the donor, investigators also examined whether CLL clustered among recipients from an individual donor, regardless of donor CLL status, but found no such clusters.
Limiting the analysis was the lack of donor MBL status, for which postdonation CLL diagnosis substituted. Some recipients in the exposed group may have received blood drawn before the donor developed MBL.
Dr. Hjalgrim and his coauthors reported having no disclosures.
FROM BLOOD
Key clinical point: There is no evidence for higher risk of chronic lymphocytic leukemia (CLL) among recipients of blood products from donors who subsequently were diagnosed with CLL.
Major finding: Among exposed recipients (7,413 who received blood from 796 donors who subsequently developed CLL), 12 were diagnosed with CLL. Among unexposed recipients (80,431 who received blood from 7,477 donors free of CLL), 107 were diagnosed with CLL, for an incidence rate ratio of 0.94 (95% CI, 0.52-1.71).
Data source: The Scandinavian Donations and Transfusions (SCANDAT2) database comprises information, including donor and recipient health outcomes, for more than 20 million blood products handled by blood banks from 1968 to 2010.
Disclosures: Dr. Hjalgrim and his coauthors reported having no disclosures.
Diabetic foot ulcer: Early closure post debridement best
SAN DIEGO – Early wound closure prior to hospital discharge after surgical debridement of infected diabetic foot ulcers yields higher ulcer healing rates and a shorter time to healing, compared with various nonclosure wound management methods, according to a propensity-matched study.
How best to manage the open wound following nonamputative surgery of infected diabetic foot ulcers has been controversial. But early wound closure during the index hospitalization was the clear winner in this comparative study, Dr. Shey-Ying Chen reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.
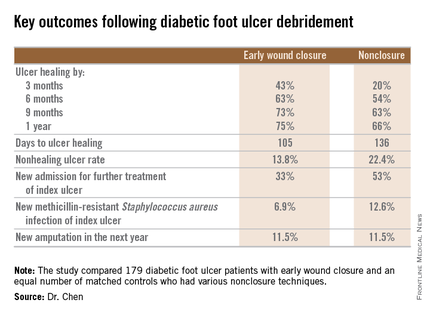
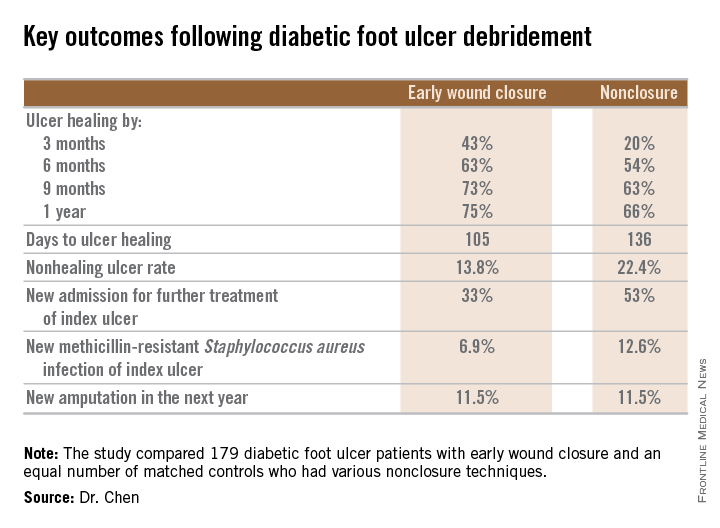
He presented a retrospective comparison between 179 diabetic foot ulcer (DFU) patients with early wound closure after surgical debridement and an equal number of matched controls treated with various nonclosure techniques, including negative pressure wound therapy and the repeated application of moist dressings. The two study groups were matched first on the basis of DFU location – toe, forefoot, midfoot, or rear foot – and then further propensity matched based on demographics, comorbid conditions, the presence of neuropathy, ulcer status by Wagner classification, infection severity, revascularization procedures, and other variables.
During 1 year of follow-up post discharge, ulcer healing occurred in 75% of the early wound closure group, compared with 66% of the nonclosure patients. Readmission for further treatment of the index ulcer occurred in 33% of the early closure group and 52% of the nonclosure group. Other outcomes were also superior in the early wound closure group, noted Dr. Chen of Beth Israel Deaconess Medical Center, Boston.
Two independent predictors of DFU healing during the follow-up period emerged from a Cox regression analysis: early wound closure, with an adjusted odds ratio of 1.63, and acute as opposed to chronic DFU, with an OR of 1.35.
Ulcer healing was significantly less likely in patients with peripheral vascular disease, with an OR of 0.62; neuropathy, with an OR of 0.53; and methicillin-resistant Staphylococcus aureus wound infection, with an OR of 0.59, he continued.
Underscoring the longer-term difficulties faced by patients with DFUs, it’s noteworthy that 11.5% of patients in both study arms underwent new amputations during the year of follow-up. Moreover, a new diagnosis of osteomyelitis was made in 20% of the early wound closure group and 26% of the nonclosure group, a nonsignificant difference.
Dr. Adolf W. Karchmer, Dr. Chen’s senior coinvestigator, said the outcome data are too new to be able to gauge how vascular, orthopedic, and podiatric surgeons will react.
The investigators reported having no financial conflicts with regard to this study, conducted without commercial sponsorship.
SAN DIEGO – Early wound closure prior to hospital discharge after surgical debridement of infected diabetic foot ulcers yields higher ulcer healing rates and a shorter time to healing, compared with various nonclosure wound management methods, according to a propensity-matched study.
How best to manage the open wound following nonamputative surgery of infected diabetic foot ulcers has been controversial. But early wound closure during the index hospitalization was the clear winner in this comparative study, Dr. Shey-Ying Chen reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.

He presented a retrospective comparison between 179 diabetic foot ulcer (DFU) patients with early wound closure after surgical debridement and an equal number of matched controls treated with various nonclosure techniques, including negative pressure wound therapy and the repeated application of moist dressings. The two study groups were matched first on the basis of DFU location – toe, forefoot, midfoot, or rear foot – and then further propensity matched based on demographics, comorbid conditions, the presence of neuropathy, ulcer status by Wagner classification, infection severity, revascularization procedures, and other variables.
During 1 year of follow-up post discharge, ulcer healing occurred in 75% of the early wound closure group, compared with 66% of the nonclosure patients. Readmission for further treatment of the index ulcer occurred in 33% of the early closure group and 52% of the nonclosure group. Other outcomes were also superior in the early wound closure group, noted Dr. Chen of Beth Israel Deaconess Medical Center, Boston.
Two independent predictors of DFU healing during the follow-up period emerged from a Cox regression analysis: early wound closure, with an adjusted odds ratio of 1.63, and acute as opposed to chronic DFU, with an OR of 1.35.
Ulcer healing was significantly less likely in patients with peripheral vascular disease, with an OR of 0.62; neuropathy, with an OR of 0.53; and methicillin-resistant Staphylococcus aureus wound infection, with an OR of 0.59, he continued.
Underscoring the longer-term difficulties faced by patients with DFUs, it’s noteworthy that 11.5% of patients in both study arms underwent new amputations during the year of follow-up. Moreover, a new diagnosis of osteomyelitis was made in 20% of the early wound closure group and 26% of the nonclosure group, a nonsignificant difference.
Dr. Adolf W. Karchmer, Dr. Chen’s senior coinvestigator, said the outcome data are too new to be able to gauge how vascular, orthopedic, and podiatric surgeons will react.
The investigators reported having no financial conflicts with regard to this study, conducted without commercial sponsorship.
SAN DIEGO – Early wound closure prior to hospital discharge after surgical debridement of infected diabetic foot ulcers yields higher ulcer healing rates and a shorter time to healing, compared with various nonclosure wound management methods, according to a propensity-matched study.
How best to manage the open wound following nonamputative surgery of infected diabetic foot ulcers has been controversial. But early wound closure during the index hospitalization was the clear winner in this comparative study, Dr. Shey-Ying Chen reported at the annual Interscience Conference on Antimicrobial Agents and Chemotherapy.

He presented a retrospective comparison between 179 diabetic foot ulcer (DFU) patients with early wound closure after surgical debridement and an equal number of matched controls treated with various nonclosure techniques, including negative pressure wound therapy and the repeated application of moist dressings. The two study groups were matched first on the basis of DFU location – toe, forefoot, midfoot, or rear foot – and then further propensity matched based on demographics, comorbid conditions, the presence of neuropathy, ulcer status by Wagner classification, infection severity, revascularization procedures, and other variables.
During 1 year of follow-up post discharge, ulcer healing occurred in 75% of the early wound closure group, compared with 66% of the nonclosure patients. Readmission for further treatment of the index ulcer occurred in 33% of the early closure group and 52% of the nonclosure group. Other outcomes were also superior in the early wound closure group, noted Dr. Chen of Beth Israel Deaconess Medical Center, Boston.
Two independent predictors of DFU healing during the follow-up period emerged from a Cox regression analysis: early wound closure, with an adjusted odds ratio of 1.63, and acute as opposed to chronic DFU, with an OR of 1.35.
Ulcer healing was significantly less likely in patients with peripheral vascular disease, with an OR of 0.62; neuropathy, with an OR of 0.53; and methicillin-resistant Staphylococcus aureus wound infection, with an OR of 0.59, he continued.
Underscoring the longer-term difficulties faced by patients with DFUs, it’s noteworthy that 11.5% of patients in both study arms underwent new amputations during the year of follow-up. Moreover, a new diagnosis of osteomyelitis was made in 20% of the early wound closure group and 26% of the nonclosure group, a nonsignificant difference.
Dr. Adolf W. Karchmer, Dr. Chen’s senior coinvestigator, said the outcome data are too new to be able to gauge how vascular, orthopedic, and podiatric surgeons will react.
The investigators reported having no financial conflicts with regard to this study, conducted without commercial sponsorship.
AT ICAAC 2015
Key clinical point: Diabetic foot ulcers are more likely to heal with early wound closure following surgical debridement than with nonclosure techniques.
Major finding: Healing of diabetic foot ulcers after surgical debridement took an average of 105 days in patients who underwent early wound closure prior to hospital discharge, compared with 136 days in those whose wounds were managed with nonclosure techniques.
Data source: A retrospective, nonrandomized study featuring two propensity score–matched groups, with 179 patients in each, who were followed for 1 year post discharge for surgical debridement of a diabetic foot ulcer.
Disclosures: The presenter reported having no financial conflicts regarding this study, conducted free of commercial support.
Travel Burden and Distress in Veterans With Head and Neck Cancer
Purpose: To investigate whether traveling long distances to a cancer treatment facility increases self-reported distress among veterans with head and neck cancer.
Background: Veterans within VISN 20 receive radiation therapy for head and neck cancer in Portland, Oregon, or Seattle, Washington. Given the geography, many travel and stay in lodging for the duration of treatment. As they cannot access usual sources of support within their communities, these veterans may be at risk for greater distress while undergoing cancer treatment.
Methods: The National Comprehensive Cancer Network Distress Thermometer (DT) is a validated tool for self-reported distress by cancer patients. Respondents report distress on a 0 to 10 scale and answer 28 questions regarding physical, emotional, and practical problems. In Seattle, the DT is completed shortly before starting treatment. Patient demographics, treatment plan (chemoradiation vs radiation alone), and DT data for veterans with head and neck cancer were abstracted from the Computerized Patient Record System. A DT score of 7 or higher was considered significant distress. Distance to the VA was calculated by zip code from the veteran’s address. Data were analyzed with logistic regression to control for possible effects of cancer stage, age category, or treatment plan.
Results: Sixty veterans with head and neck cancer completed the DT between April 2014 and April 2015. The average age was 65.4 years (range 39-91), all were male, 77% were white, 77% had stage III or IV cancer at diagnosis, and 47% traveled > 50 miles. The average DT score was 5.4. Veterans traveling > 50 miles were more likely to report significant distress compared with those who traveled < 50 miles (odds ratio (OR) = 1.6, P = .02). Sleep was the only problem significantly more likely for veterans traveling > 50 miles (OR = 1.71, P = .01).
Implications: Veterans with head and neck cancer traveling > 50 miles for cancer care are more likely to report significant distress or distress related to sleep. This small study suggests travel burden may be an underappreciated source of distress for veterans with cancer. Further research is warranted to better understand how travel burden affects distress and identify opportunities for intervention
Purpose: To investigate whether traveling long distances to a cancer treatment facility increases self-reported distress among veterans with head and neck cancer.
Background: Veterans within VISN 20 receive radiation therapy for head and neck cancer in Portland, Oregon, or Seattle, Washington. Given the geography, many travel and stay in lodging for the duration of treatment. As they cannot access usual sources of support within their communities, these veterans may be at risk for greater distress while undergoing cancer treatment.
Methods: The National Comprehensive Cancer Network Distress Thermometer (DT) is a validated tool for self-reported distress by cancer patients. Respondents report distress on a 0 to 10 scale and answer 28 questions regarding physical, emotional, and practical problems. In Seattle, the DT is completed shortly before starting treatment. Patient demographics, treatment plan (chemoradiation vs radiation alone), and DT data for veterans with head and neck cancer were abstracted from the Computerized Patient Record System. A DT score of 7 or higher was considered significant distress. Distance to the VA was calculated by zip code from the veteran’s address. Data were analyzed with logistic regression to control for possible effects of cancer stage, age category, or treatment plan.
Results: Sixty veterans with head and neck cancer completed the DT between April 2014 and April 2015. The average age was 65.4 years (range 39-91), all were male, 77% were white, 77% had stage III or IV cancer at diagnosis, and 47% traveled > 50 miles. The average DT score was 5.4. Veterans traveling > 50 miles were more likely to report significant distress compared with those who traveled < 50 miles (odds ratio (OR) = 1.6, P = .02). Sleep was the only problem significantly more likely for veterans traveling > 50 miles (OR = 1.71, P = .01).
Implications: Veterans with head and neck cancer traveling > 50 miles for cancer care are more likely to report significant distress or distress related to sleep. This small study suggests travel burden may be an underappreciated source of distress for veterans with cancer. Further research is warranted to better understand how travel burden affects distress and identify opportunities for intervention
Purpose: To investigate whether traveling long distances to a cancer treatment facility increases self-reported distress among veterans with head and neck cancer.
Background: Veterans within VISN 20 receive radiation therapy for head and neck cancer in Portland, Oregon, or Seattle, Washington. Given the geography, many travel and stay in lodging for the duration of treatment. As they cannot access usual sources of support within their communities, these veterans may be at risk for greater distress while undergoing cancer treatment.
Methods: The National Comprehensive Cancer Network Distress Thermometer (DT) is a validated tool for self-reported distress by cancer patients. Respondents report distress on a 0 to 10 scale and answer 28 questions regarding physical, emotional, and practical problems. In Seattle, the DT is completed shortly before starting treatment. Patient demographics, treatment plan (chemoradiation vs radiation alone), and DT data for veterans with head and neck cancer were abstracted from the Computerized Patient Record System. A DT score of 7 or higher was considered significant distress. Distance to the VA was calculated by zip code from the veteran’s address. Data were analyzed with logistic regression to control for possible effects of cancer stage, age category, or treatment plan.
Results: Sixty veterans with head and neck cancer completed the DT between April 2014 and April 2015. The average age was 65.4 years (range 39-91), all were male, 77% were white, 77% had stage III or IV cancer at diagnosis, and 47% traveled > 50 miles. The average DT score was 5.4. Veterans traveling > 50 miles were more likely to report significant distress compared with those who traveled < 50 miles (odds ratio (OR) = 1.6, P = .02). Sleep was the only problem significantly more likely for veterans traveling > 50 miles (OR = 1.71, P = .01).
Implications: Veterans with head and neck cancer traveling > 50 miles for cancer care are more likely to report significant distress or distress related to sleep. This small study suggests travel burden may be an underappreciated source of distress for veterans with cancer. Further research is warranted to better understand how travel burden affects distress and identify opportunities for intervention
Patterns of Initial Treatment in Veteran Patients With Chronic Lymphocytic Leukemia: A National VA Tumor Registry Study
Background: Chronic lymphocytic leukemia (CLL) is the most common leukemia in adults, including elderly veterans, with many new treatment options now available. Data on patterns of treatment in elderly veteran patients with CLL is limited. We sought to assess initial treatment patterns over a 13-year period among veteran patients in the Minneapolis VA Health Care System.
Methods: We identified 6,756 CLL cases diagnosed from 2000 to 2013 and are presenting interim data on 2015. We reviewed clinical data from 2,015 patients with CLL diagnosed from 2000 to 2013 and identified through the National VA Tumor Registry. Baseline demographics and treatment information were collected. The objective of this study was to assess initial treatment patterns, time to initial treatment, and variation of these parameters by age.
Results: At diagnosis, median age was 69 years (range, 37-96 years); 98% were male (1,979); Rai stage was 0 (n = 1,331, 66%), 1 (n = 317, 16%), 2 (n = 156, 8%), 3 (n = 91, 5%), 4 (n = 113, 6%). The majority of patients were white (n = 1,752, 87%); followed by African American (n = 203, 10%); and Hispanic (n = 33, 2%). Of the 2,015 patients, 751 (37%) received therapy over this period of follow-up. Median time from diagnosis to initial treatment was 1.3 years (range, 0-13 years). The most common initial therapies utilized were chlorambucil (39.4%); fludarabine/cyclophosphamide/ritux-imab (FCR) (12.4%); and single-agent fludarabine (10.5%). When examining these parameters by age in decades, we found that there were no differences in Rai stage at diagnosis by age-decade. There was a progressive increase in initial chlorambucil usage by advancing age. Likewise, the majority of FCR usage was in patients aged < 70 years.
Conclusions: In this veteran population, including many elderly patients, the majority of patients requiring therapy initiated it within 2 years of diagnosis. These patients were most commonly treated with chlorambucil. These patterns of care will be changing with the introduction of newer oral agents, such as ibrutinib and idelalisib, but at a significantly higher cost. The National VA Tumor Registry data will allow future opportunity to examine evolving treatment patterns in both an elderly as well as a veteran population. Updated data will be presented at the AVAHO annual meeting.
Background: Chronic lymphocytic leukemia (CLL) is the most common leukemia in adults, including elderly veterans, with many new treatment options now available. Data on patterns of treatment in elderly veteran patients with CLL is limited. We sought to assess initial treatment patterns over a 13-year period among veteran patients in the Minneapolis VA Health Care System.
Methods: We identified 6,756 CLL cases diagnosed from 2000 to 2013 and are presenting interim data on 2015. We reviewed clinical data from 2,015 patients with CLL diagnosed from 2000 to 2013 and identified through the National VA Tumor Registry. Baseline demographics and treatment information were collected. The objective of this study was to assess initial treatment patterns, time to initial treatment, and variation of these parameters by age.
Results: At diagnosis, median age was 69 years (range, 37-96 years); 98% were male (1,979); Rai stage was 0 (n = 1,331, 66%), 1 (n = 317, 16%), 2 (n = 156, 8%), 3 (n = 91, 5%), 4 (n = 113, 6%). The majority of patients were white (n = 1,752, 87%); followed by African American (n = 203, 10%); and Hispanic (n = 33, 2%). Of the 2,015 patients, 751 (37%) received therapy over this period of follow-up. Median time from diagnosis to initial treatment was 1.3 years (range, 0-13 years). The most common initial therapies utilized were chlorambucil (39.4%); fludarabine/cyclophosphamide/ritux-imab (FCR) (12.4%); and single-agent fludarabine (10.5%). When examining these parameters by age in decades, we found that there were no differences in Rai stage at diagnosis by age-decade. There was a progressive increase in initial chlorambucil usage by advancing age. Likewise, the majority of FCR usage was in patients aged < 70 years.
Conclusions: In this veteran population, including many elderly patients, the majority of patients requiring therapy initiated it within 2 years of diagnosis. These patients were most commonly treated with chlorambucil. These patterns of care will be changing with the introduction of newer oral agents, such as ibrutinib and idelalisib, but at a significantly higher cost. The National VA Tumor Registry data will allow future opportunity to examine evolving treatment patterns in both an elderly as well as a veteran population. Updated data will be presented at the AVAHO annual meeting.
Background: Chronic lymphocytic leukemia (CLL) is the most common leukemia in adults, including elderly veterans, with many new treatment options now available. Data on patterns of treatment in elderly veteran patients with CLL is limited. We sought to assess initial treatment patterns over a 13-year period among veteran patients in the Minneapolis VA Health Care System.
Methods: We identified 6,756 CLL cases diagnosed from 2000 to 2013 and are presenting interim data on 2015. We reviewed clinical data from 2,015 patients with CLL diagnosed from 2000 to 2013 and identified through the National VA Tumor Registry. Baseline demographics and treatment information were collected. The objective of this study was to assess initial treatment patterns, time to initial treatment, and variation of these parameters by age.
Results: At diagnosis, median age was 69 years (range, 37-96 years); 98% were male (1,979); Rai stage was 0 (n = 1,331, 66%), 1 (n = 317, 16%), 2 (n = 156, 8%), 3 (n = 91, 5%), 4 (n = 113, 6%). The majority of patients were white (n = 1,752, 87%); followed by African American (n = 203, 10%); and Hispanic (n = 33, 2%). Of the 2,015 patients, 751 (37%) received therapy over this period of follow-up. Median time from diagnosis to initial treatment was 1.3 years (range, 0-13 years). The most common initial therapies utilized were chlorambucil (39.4%); fludarabine/cyclophosphamide/ritux-imab (FCR) (12.4%); and single-agent fludarabine (10.5%). When examining these parameters by age in decades, we found that there were no differences in Rai stage at diagnosis by age-decade. There was a progressive increase in initial chlorambucil usage by advancing age. Likewise, the majority of FCR usage was in patients aged < 70 years.
Conclusions: In this veteran population, including many elderly patients, the majority of patients requiring therapy initiated it within 2 years of diagnosis. These patients were most commonly treated with chlorambucil. These patterns of care will be changing with the introduction of newer oral agents, such as ibrutinib and idelalisib, but at a significantly higher cost. The National VA Tumor Registry data will allow future opportunity to examine evolving treatment patterns in both an elderly as well as a veteran population. Updated data will be presented at the AVAHO annual meeting.