User login
Novel SYK inhibitor shows ‘good early evidence’ of activity

LUGANO—The phase 1, first-in-human study of the novel SYK inhibitor TAK-659 is showing “good early evidence” of antitumor activity in patients with lymphoma, according to investigators.
The agent also appears to be fairly well tolerated, with 10 categories of adverse events occurring in 2 or more patients.
Adam M. Petrich, MD, of Northwestern University in Evanston, Illinois, presented results from this ongoing study at the 13th International Congress on Malignant Lymphoma (13-ICML) as abstract 039.*
The study is supported by Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited.
Dr Petrich said the B-cell receptor signaling pathway is “very fertile ground with respect to development for novel targeting, particularly of B-cell malignancies, and SYK—the spleen tyrosine kinase—is an integral component of this.”
Investigators believe SYK has implications beyond B-cell lymphoma, including EBV-related malignancies, solid tumors, and myeloid leukemias.
Preclinical findings
In vitro experiments with TAK-659 showed “profound inhibition” of both SYK and FLT3, as indicated by the low IC50 levels, Dr Petrich said.
He also pointed out that the EC50 levels compare favorably to ibrutinib and idelalisib, with generally lower numbers in a broad panel of diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), and chronic lymphocytic leukemia.
In animal models, TAK-659 exhibited a dose-dependent tumor-inhibitory property.
“And if we look at both germinal center B and non-germinal center B subtypes of large-cell lymphoma, we see activity across both types,” Dr Petrich said.
Phase 1 study
Investigators are currently conducting the phase 1 study, which is a standard 3+3 dose-escalation schema. The data cutoff for the ICML presentation was April 13, although the dose-escalation phase was still underway, and the maximum tolerated dose was not yet reached.
Based on preclinical data, the team projected the efficacious dose for humans to be approximately 600 to 1200 mg per day. Patients were started at 60 mg, and, at the next planned step of 120 mg, 2 patients developed asymptomatic lipase elevations.
“For that reason, we revised the protocol, allowed for those to not be considered dose-limiting toxicities, and explored intermediate doses,” Dr Petrich explained.
So the protocol now includes intermediate doses of 80 and 100 mg. Dr Petrich’s presentation focused on the 4 doses—60, 80, 100, and 120 mg taken orally once daily.
He said the observed human clearance of TAK-659 was approximately 3- to 4-fold lower than predicted based on the mouse pharmacokinetic (PK) data, which led to steady-state area under the curve values 3- to 4-fold higher in humans than predicted.
Patient demographics
The investigators enrolled 21 patients, 12 with solid tumors, 6 with DLBCL, and 3 with FL. The median age was 60 years, 66% were male, and 62% had received 4 or more prior therapies.
The median number of TAK-659 treatment cycles was 2 (range, 1–10), and 5 patients are still on active treatment. Dr Petrich pointed out that 4 of the 5 longest-treated patients have DLBCL, and “the record holder with DLBCL is about to celebrate 1 year on therapy.”
Safety
“The safety profile in humans showed that [TAK-659] was actually quite tolerable,” Dr Petrich said.
There were 10 categories of treatment-related adverse events (AEs) that occurred in 2 or more patients. They were, in descending order, fatigue, anemia, diarrhea, elevated AST, hypophosphatemia, nausea, rash, elevated lipase, elevated ALT, and anorexia.
The majority of AEs were grade 1 or 2. However, there were grade 3/4 cases of anemia, diarrhea, elevated AST, and hypophosphatemia. And elevated lipase—the asymptomatic, dose-limiting toxicity for which the protocol was modified—consisted entirely of grade 3 or 4 events.
Episodes of neutropenia and thrombocytopenia occurred in 1 patient each, and both were grade 1.
“So [TAK-659] seems quite well tolerated in that regard as well,” Dr Petrich observed.
The plasma profile on days 1 and 15 of cycle 1 indicate that PK steady-state conditions are generally achieved by day 8, with moderate accumulation after repeated, once-daily dosing for 15 days.
Antitumor activity
Of the 12 evaluable patients, 5 had tumor shrinkage at the 60, 80, or 100 mg dose levels. Three of the 6 DLBCL patients experienced tumor shrinkage, and there were “2 dramatic responses in patients with follicular lymphoma, including 1 CR [complete response],” Dr Petrich said.
One of these FL patients had an aggressive phenotype and never had a previous response last longer than 20 months.
“[H]e actually achieved a CR within 2 cycles—a dramatic response for his disease—and he remains on treatment, and he’s up to cycle 5 now,” Dr Petrich said.
The team concluded that the PK data support daily dosing, despite lower clearance than originally predicted.
“[There is] good early evidence of antitumor activity and no significant safety signals,” Dr Petrich said. “And the [hematologic] toxicity profile, in particular, seems to suggest this is a well-tolerated drug.”
The investigators are conducting expansion cohorts and are considering future combination studies. They recently activated a study in acute myeloid leukemia because TAK-659 has FLT3 inhibitory properties. ![]()
*Information in the abstract differs from that presented at the meeting.

LUGANO—The phase 1, first-in-human study of the novel SYK inhibitor TAK-659 is showing “good early evidence” of antitumor activity in patients with lymphoma, according to investigators.
The agent also appears to be fairly well tolerated, with 10 categories of adverse events occurring in 2 or more patients.
Adam M. Petrich, MD, of Northwestern University in Evanston, Illinois, presented results from this ongoing study at the 13th International Congress on Malignant Lymphoma (13-ICML) as abstract 039.*
The study is supported by Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited.
Dr Petrich said the B-cell receptor signaling pathway is “very fertile ground with respect to development for novel targeting, particularly of B-cell malignancies, and SYK—the spleen tyrosine kinase—is an integral component of this.”
Investigators believe SYK has implications beyond B-cell lymphoma, including EBV-related malignancies, solid tumors, and myeloid leukemias.
Preclinical findings
In vitro experiments with TAK-659 showed “profound inhibition” of both SYK and FLT3, as indicated by the low IC50 levels, Dr Petrich said.
He also pointed out that the EC50 levels compare favorably to ibrutinib and idelalisib, with generally lower numbers in a broad panel of diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), and chronic lymphocytic leukemia.
In animal models, TAK-659 exhibited a dose-dependent tumor-inhibitory property.
“And if we look at both germinal center B and non-germinal center B subtypes of large-cell lymphoma, we see activity across both types,” Dr Petrich said.
Phase 1 study
Investigators are currently conducting the phase 1 study, which is a standard 3+3 dose-escalation schema. The data cutoff for the ICML presentation was April 13, although the dose-escalation phase was still underway, and the maximum tolerated dose was not yet reached.
Based on preclinical data, the team projected the efficacious dose for humans to be approximately 600 to 1200 mg per day. Patients were started at 60 mg, and, at the next planned step of 120 mg, 2 patients developed asymptomatic lipase elevations.
“For that reason, we revised the protocol, allowed for those to not be considered dose-limiting toxicities, and explored intermediate doses,” Dr Petrich explained.
So the protocol now includes intermediate doses of 80 and 100 mg. Dr Petrich’s presentation focused on the 4 doses—60, 80, 100, and 120 mg taken orally once daily.
He said the observed human clearance of TAK-659 was approximately 3- to 4-fold lower than predicted based on the mouse pharmacokinetic (PK) data, which led to steady-state area under the curve values 3- to 4-fold higher in humans than predicted.
Patient demographics
The investigators enrolled 21 patients, 12 with solid tumors, 6 with DLBCL, and 3 with FL. The median age was 60 years, 66% were male, and 62% had received 4 or more prior therapies.
The median number of TAK-659 treatment cycles was 2 (range, 1–10), and 5 patients are still on active treatment. Dr Petrich pointed out that 4 of the 5 longest-treated patients have DLBCL, and “the record holder with DLBCL is about to celebrate 1 year on therapy.”
Safety
“The safety profile in humans showed that [TAK-659] was actually quite tolerable,” Dr Petrich said.
There were 10 categories of treatment-related adverse events (AEs) that occurred in 2 or more patients. They were, in descending order, fatigue, anemia, diarrhea, elevated AST, hypophosphatemia, nausea, rash, elevated lipase, elevated ALT, and anorexia.
The majority of AEs were grade 1 or 2. However, there were grade 3/4 cases of anemia, diarrhea, elevated AST, and hypophosphatemia. And elevated lipase—the asymptomatic, dose-limiting toxicity for which the protocol was modified—consisted entirely of grade 3 or 4 events.
Episodes of neutropenia and thrombocytopenia occurred in 1 patient each, and both were grade 1.
“So [TAK-659] seems quite well tolerated in that regard as well,” Dr Petrich observed.
The plasma profile on days 1 and 15 of cycle 1 indicate that PK steady-state conditions are generally achieved by day 8, with moderate accumulation after repeated, once-daily dosing for 15 days.
Antitumor activity
Of the 12 evaluable patients, 5 had tumor shrinkage at the 60, 80, or 100 mg dose levels. Three of the 6 DLBCL patients experienced tumor shrinkage, and there were “2 dramatic responses in patients with follicular lymphoma, including 1 CR [complete response],” Dr Petrich said.
One of these FL patients had an aggressive phenotype and never had a previous response last longer than 20 months.
“[H]e actually achieved a CR within 2 cycles—a dramatic response for his disease—and he remains on treatment, and he’s up to cycle 5 now,” Dr Petrich said.
The team concluded that the PK data support daily dosing, despite lower clearance than originally predicted.
“[There is] good early evidence of antitumor activity and no significant safety signals,” Dr Petrich said. “And the [hematologic] toxicity profile, in particular, seems to suggest this is a well-tolerated drug.”
The investigators are conducting expansion cohorts and are considering future combination studies. They recently activated a study in acute myeloid leukemia because TAK-659 has FLT3 inhibitory properties. ![]()
*Information in the abstract differs from that presented at the meeting.

LUGANO—The phase 1, first-in-human study of the novel SYK inhibitor TAK-659 is showing “good early evidence” of antitumor activity in patients with lymphoma, according to investigators.
The agent also appears to be fairly well tolerated, with 10 categories of adverse events occurring in 2 or more patients.
Adam M. Petrich, MD, of Northwestern University in Evanston, Illinois, presented results from this ongoing study at the 13th International Congress on Malignant Lymphoma (13-ICML) as abstract 039.*
The study is supported by Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited.
Dr Petrich said the B-cell receptor signaling pathway is “very fertile ground with respect to development for novel targeting, particularly of B-cell malignancies, and SYK—the spleen tyrosine kinase—is an integral component of this.”
Investigators believe SYK has implications beyond B-cell lymphoma, including EBV-related malignancies, solid tumors, and myeloid leukemias.
Preclinical findings
In vitro experiments with TAK-659 showed “profound inhibition” of both SYK and FLT3, as indicated by the low IC50 levels, Dr Petrich said.
He also pointed out that the EC50 levels compare favorably to ibrutinib and idelalisib, with generally lower numbers in a broad panel of diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), and chronic lymphocytic leukemia.
In animal models, TAK-659 exhibited a dose-dependent tumor-inhibitory property.
“And if we look at both germinal center B and non-germinal center B subtypes of large-cell lymphoma, we see activity across both types,” Dr Petrich said.
Phase 1 study
Investigators are currently conducting the phase 1 study, which is a standard 3+3 dose-escalation schema. The data cutoff for the ICML presentation was April 13, although the dose-escalation phase was still underway, and the maximum tolerated dose was not yet reached.
Based on preclinical data, the team projected the efficacious dose for humans to be approximately 600 to 1200 mg per day. Patients were started at 60 mg, and, at the next planned step of 120 mg, 2 patients developed asymptomatic lipase elevations.
“For that reason, we revised the protocol, allowed for those to not be considered dose-limiting toxicities, and explored intermediate doses,” Dr Petrich explained.
So the protocol now includes intermediate doses of 80 and 100 mg. Dr Petrich’s presentation focused on the 4 doses—60, 80, 100, and 120 mg taken orally once daily.
He said the observed human clearance of TAK-659 was approximately 3- to 4-fold lower than predicted based on the mouse pharmacokinetic (PK) data, which led to steady-state area under the curve values 3- to 4-fold higher in humans than predicted.
Patient demographics
The investigators enrolled 21 patients, 12 with solid tumors, 6 with DLBCL, and 3 with FL. The median age was 60 years, 66% were male, and 62% had received 4 or more prior therapies.
The median number of TAK-659 treatment cycles was 2 (range, 1–10), and 5 patients are still on active treatment. Dr Petrich pointed out that 4 of the 5 longest-treated patients have DLBCL, and “the record holder with DLBCL is about to celebrate 1 year on therapy.”
Safety
“The safety profile in humans showed that [TAK-659] was actually quite tolerable,” Dr Petrich said.
There were 10 categories of treatment-related adverse events (AEs) that occurred in 2 or more patients. They were, in descending order, fatigue, anemia, diarrhea, elevated AST, hypophosphatemia, nausea, rash, elevated lipase, elevated ALT, and anorexia.
The majority of AEs were grade 1 or 2. However, there were grade 3/4 cases of anemia, diarrhea, elevated AST, and hypophosphatemia. And elevated lipase—the asymptomatic, dose-limiting toxicity for which the protocol was modified—consisted entirely of grade 3 or 4 events.
Episodes of neutropenia and thrombocytopenia occurred in 1 patient each, and both were grade 1.
“So [TAK-659] seems quite well tolerated in that regard as well,” Dr Petrich observed.
The plasma profile on days 1 and 15 of cycle 1 indicate that PK steady-state conditions are generally achieved by day 8, with moderate accumulation after repeated, once-daily dosing for 15 days.
Antitumor activity
Of the 12 evaluable patients, 5 had tumor shrinkage at the 60, 80, or 100 mg dose levels. Three of the 6 DLBCL patients experienced tumor shrinkage, and there were “2 dramatic responses in patients with follicular lymphoma, including 1 CR [complete response],” Dr Petrich said.
One of these FL patients had an aggressive phenotype and never had a previous response last longer than 20 months.
“[H]e actually achieved a CR within 2 cycles—a dramatic response for his disease—and he remains on treatment, and he’s up to cycle 5 now,” Dr Petrich said.
The team concluded that the PK data support daily dosing, despite lower clearance than originally predicted.
“[There is] good early evidence of antitumor activity and no significant safety signals,” Dr Petrich said. “And the [hematologic] toxicity profile, in particular, seems to suggest this is a well-tolerated drug.”
The investigators are conducting expansion cohorts and are considering future combination studies. They recently activated a study in acute myeloid leukemia because TAK-659 has FLT3 inhibitory properties. ![]()
*Information in the abstract differs from that presented at the meeting.
PI3K inhibitors may promote cancer spread

Photo courtesy of
The Wistar Institute
Although PI3K inhibitors have been designed to treat cancer, new research indicates these drugs may actually exacerbate the disease.
Researchers found evidence to suggest that treatment with PI3K inhibitors alone can promote more aggressive tumor cell behavior and increase the likelihood that cancer will spread.
PI3K inhibitors appeared to reprogram the mitochondria of tumor cells and move them to “strategic” positions for invasion.
However, the researchers believe that targeting mitochondrial function along with PI3K could prevent this effect.
Dario C. Altieri, MD, of The Wistar Institute in Philadelphia, Pennsylvania, and his colleagues described these findings in PNAS.
The researchers decided to investigate how mitochondria are reprogrammed when exposed to PI3K inhibition and how mitochondria might prevent targeted agents from being as effective as expected.
“Our prior studies have confirmed that tumor cells rely on energy produced by mitochondria more significantly than previously thought,” Dr Altieri said.
“What we have shown in this study is that, in somewhat of a paradox, treatment with a PI3K inhibitor causes a tumor cell’s mitochondria to produce energy in a localized manner, promoting a far more aggressive and invasive phenotype. The treatment appears to be doing the opposite of its intended effect.”
The study showed that treatment with a PI3K inhibitor causes the mitochondria to migrate to the peripheral cytoskeleton of the tumor cells.
While the mitochondria in untreated cells cluster around the cell’s nucleus, exposure of tumor cells to PI3K therapy causes the mitochondria to move to specialized regions of the cell’s membrane implicated in cell motility and invasion.
In this “strategic” position, tumor mitochondria are ideally positioned to provide a concentrated source of energy to support an increase in cell migration and invasion.
However, the researchers said the dependence of this response on mitochondrial function may offer a new therapeutic angle.
Dr Altieri and his team have shown that targeting mitochondrial functions for tumor therapy is feasible and dramatically enhances the anticancer activity of PI3K inhibitors when used in combination.
“These findings continue to support the idea that the mitochondria of tumor cells are crucial to tumor survival and proliferation,” Dr Altieri said. “It’s certainly counterintuitive that a drug designed to fight cancer may in actuality help it spread, but by identifying why this is happening, we can develop better strategies that allow these drugs to treat tumors the way they should.” ![]()

Photo courtesy of
The Wistar Institute
Although PI3K inhibitors have been designed to treat cancer, new research indicates these drugs may actually exacerbate the disease.
Researchers found evidence to suggest that treatment with PI3K inhibitors alone can promote more aggressive tumor cell behavior and increase the likelihood that cancer will spread.
PI3K inhibitors appeared to reprogram the mitochondria of tumor cells and move them to “strategic” positions for invasion.
However, the researchers believe that targeting mitochondrial function along with PI3K could prevent this effect.
Dario C. Altieri, MD, of The Wistar Institute in Philadelphia, Pennsylvania, and his colleagues described these findings in PNAS.
The researchers decided to investigate how mitochondria are reprogrammed when exposed to PI3K inhibition and how mitochondria might prevent targeted agents from being as effective as expected.
“Our prior studies have confirmed that tumor cells rely on energy produced by mitochondria more significantly than previously thought,” Dr Altieri said.
“What we have shown in this study is that, in somewhat of a paradox, treatment with a PI3K inhibitor causes a tumor cell’s mitochondria to produce energy in a localized manner, promoting a far more aggressive and invasive phenotype. The treatment appears to be doing the opposite of its intended effect.”
The study showed that treatment with a PI3K inhibitor causes the mitochondria to migrate to the peripheral cytoskeleton of the tumor cells.
While the mitochondria in untreated cells cluster around the cell’s nucleus, exposure of tumor cells to PI3K therapy causes the mitochondria to move to specialized regions of the cell’s membrane implicated in cell motility and invasion.
In this “strategic” position, tumor mitochondria are ideally positioned to provide a concentrated source of energy to support an increase in cell migration and invasion.
However, the researchers said the dependence of this response on mitochondrial function may offer a new therapeutic angle.
Dr Altieri and his team have shown that targeting mitochondrial functions for tumor therapy is feasible and dramatically enhances the anticancer activity of PI3K inhibitors when used in combination.
“These findings continue to support the idea that the mitochondria of tumor cells are crucial to tumor survival and proliferation,” Dr Altieri said. “It’s certainly counterintuitive that a drug designed to fight cancer may in actuality help it spread, but by identifying why this is happening, we can develop better strategies that allow these drugs to treat tumors the way they should.” ![]()

Photo courtesy of
The Wistar Institute
Although PI3K inhibitors have been designed to treat cancer, new research indicates these drugs may actually exacerbate the disease.
Researchers found evidence to suggest that treatment with PI3K inhibitors alone can promote more aggressive tumor cell behavior and increase the likelihood that cancer will spread.
PI3K inhibitors appeared to reprogram the mitochondria of tumor cells and move them to “strategic” positions for invasion.
However, the researchers believe that targeting mitochondrial function along with PI3K could prevent this effect.
Dario C. Altieri, MD, of The Wistar Institute in Philadelphia, Pennsylvania, and his colleagues described these findings in PNAS.
The researchers decided to investigate how mitochondria are reprogrammed when exposed to PI3K inhibition and how mitochondria might prevent targeted agents from being as effective as expected.
“Our prior studies have confirmed that tumor cells rely on energy produced by mitochondria more significantly than previously thought,” Dr Altieri said.
“What we have shown in this study is that, in somewhat of a paradox, treatment with a PI3K inhibitor causes a tumor cell’s mitochondria to produce energy in a localized manner, promoting a far more aggressive and invasive phenotype. The treatment appears to be doing the opposite of its intended effect.”
The study showed that treatment with a PI3K inhibitor causes the mitochondria to migrate to the peripheral cytoskeleton of the tumor cells.
While the mitochondria in untreated cells cluster around the cell’s nucleus, exposure of tumor cells to PI3K therapy causes the mitochondria to move to specialized regions of the cell’s membrane implicated in cell motility and invasion.
In this “strategic” position, tumor mitochondria are ideally positioned to provide a concentrated source of energy to support an increase in cell migration and invasion.
However, the researchers said the dependence of this response on mitochondrial function may offer a new therapeutic angle.
Dr Altieri and his team have shown that targeting mitochondrial functions for tumor therapy is feasible and dramatically enhances the anticancer activity of PI3K inhibitors when used in combination.
“These findings continue to support the idea that the mitochondria of tumor cells are crucial to tumor survival and proliferation,” Dr Altieri said. “It’s certainly counterintuitive that a drug designed to fight cancer may in actuality help it spread, but by identifying why this is happening, we can develop better strategies that allow these drugs to treat tumors the way they should.” ![]()
Inhibitors increase burden of hemophilia care

TORONTO—When children with hemophilia develop inhibitors, their caregivers shoulder a greater burden, according to a pilot study.
Researchers surveyed 40 subjects on the burden of caring for a child with hemophilia and found that inhibitor development significantly increased the burden of care.
But other factors—such as the number of bleeds a child had experienced in the last 12 months—had no significant impact.
Sylvia von Mackensen, PhD, of the University Medical Centre Hamburg-Eppendorf in Hamburg, Germany, and her colleagues presented these findings at the ISTH 2015 Congress (abstract PO256-WED).
The study was a pilot test of the HEMOCAB questionnaire, which consists of 59 questions mapped to 13 domains. Caregivers were asked to select the response that best qualified their burden and were scored on a scale of 0 to 100, with higher values corresponding with greater burden.
Forty caregivers completed the questionnaire. All of them had children with hemophilia (n=40), with or without inhibitors, who were younger than 22 years of age.
Three-quarters of the caregivers were mothers, 17.5% were fathers, and 7.5% were grandmothers. The mean age of caregivers was 39.32 ± 8.9 years (range, 27-66), and the mean age of the hemophilia patients was 10.98 ± 5.5 years (range, 1-21).
Most of the patients had hemophilia A (95%), and most had severe disease (77.5%). Six children (15%) had inhibitors. Overall, patients had experienced an average of 4.83 ±8.9 bleeds (range, 0-52) in the previous 12 months.
Most of the patients (88.5%) were receiving prophylaxis. Caregivers said they spent 8.69 ± 7.7 hours per month on infusion and 3.84 ± 6.7 hours (range, 0-30) per month traveling to hemophilia treatment facilities.
Size of burden
Caregivers said the most burdensome aspects of care were the caregivers’ perception of the child (38%), emotional stress (36%), financial burden (34%), and the impact of care on the caregiver (31%).
For nearly all of the domains assessed—emotional stress, personal sacrifice, medical management, work situation, etc.—a caregivers’ burden was significantly higher if a child had inhibitors. The only exception was school-related burden.
When the researchers analyzed the impact of other factors on care burden, they found that only inhibitor development had a significant impact.
There was no impact for orthopedic joint score, age of the caregiver, age of the child, time for infusion, time traveling to a hemophilia treatment center, the number of bleeds in the past 12 months, the number of children with hemophilia per household, home treatment, caregiver marital status, location, or caregiver education.
Frequency of burden
The HEMOCAB questionnaire also included scales assessing the frequency of burden. Dr von Mackensen and her colleagues presented data from these scales for the caregivers’ perception of their child, emotional stress, and finances.
Sixty percent of caregivers said they sometimes, often, or always feel their child’s condition is a difficult situation. Seventy-three percent of caregivers expressed feelings of sadness about informing their child of what he can and cannot do due to his illness.
Seventy-four percent of caregivers said they sometimes, often, or always feel afraid their child might get injured when they are not around to help. Forty-six percent of caregivers reported feeling afraid their child’s condition might worsen, and 36% said they feared their child might die from his condition.
Forty-six percent of caregivers said their child’s hemophilia sometimes, often, or always causes financial problems. And 33% of caregivers said that, at least sometimes, their family does not have enough money because of their child’s hemophilia.
HEMOCAB is a trademark of Novo Nordisk Health Care AG. Dr von Mackensen received a consulting fee from Novo Nordisk for developing the HEMOCAB questionnaire. Leonard A. Valentino, MD, of Rush University Medical Center in Chicago, Illinois, was involved in developing the questionnaire as well but did not participate in the pilot study.
The other researchers involved in the study have received funding/consulting fees from—or are employees of—Novo Nordisk, Baxter, Bayer, OctaPharma, CSL Behring, OPKO Health, and Selexys. ![]()

TORONTO—When children with hemophilia develop inhibitors, their caregivers shoulder a greater burden, according to a pilot study.
Researchers surveyed 40 subjects on the burden of caring for a child with hemophilia and found that inhibitor development significantly increased the burden of care.
But other factors—such as the number of bleeds a child had experienced in the last 12 months—had no significant impact.
Sylvia von Mackensen, PhD, of the University Medical Centre Hamburg-Eppendorf in Hamburg, Germany, and her colleagues presented these findings at the ISTH 2015 Congress (abstract PO256-WED).
The study was a pilot test of the HEMOCAB questionnaire, which consists of 59 questions mapped to 13 domains. Caregivers were asked to select the response that best qualified their burden and were scored on a scale of 0 to 100, with higher values corresponding with greater burden.
Forty caregivers completed the questionnaire. All of them had children with hemophilia (n=40), with or without inhibitors, who were younger than 22 years of age.
Three-quarters of the caregivers were mothers, 17.5% were fathers, and 7.5% were grandmothers. The mean age of caregivers was 39.32 ± 8.9 years (range, 27-66), and the mean age of the hemophilia patients was 10.98 ± 5.5 years (range, 1-21).
Most of the patients had hemophilia A (95%), and most had severe disease (77.5%). Six children (15%) had inhibitors. Overall, patients had experienced an average of 4.83 ±8.9 bleeds (range, 0-52) in the previous 12 months.
Most of the patients (88.5%) were receiving prophylaxis. Caregivers said they spent 8.69 ± 7.7 hours per month on infusion and 3.84 ± 6.7 hours (range, 0-30) per month traveling to hemophilia treatment facilities.
Size of burden
Caregivers said the most burdensome aspects of care were the caregivers’ perception of the child (38%), emotional stress (36%), financial burden (34%), and the impact of care on the caregiver (31%).
For nearly all of the domains assessed—emotional stress, personal sacrifice, medical management, work situation, etc.—a caregivers’ burden was significantly higher if a child had inhibitors. The only exception was school-related burden.
When the researchers analyzed the impact of other factors on care burden, they found that only inhibitor development had a significant impact.
There was no impact for orthopedic joint score, age of the caregiver, age of the child, time for infusion, time traveling to a hemophilia treatment center, the number of bleeds in the past 12 months, the number of children with hemophilia per household, home treatment, caregiver marital status, location, or caregiver education.
Frequency of burden
The HEMOCAB questionnaire also included scales assessing the frequency of burden. Dr von Mackensen and her colleagues presented data from these scales for the caregivers’ perception of their child, emotional stress, and finances.
Sixty percent of caregivers said they sometimes, often, or always feel their child’s condition is a difficult situation. Seventy-three percent of caregivers expressed feelings of sadness about informing their child of what he can and cannot do due to his illness.
Seventy-four percent of caregivers said they sometimes, often, or always feel afraid their child might get injured when they are not around to help. Forty-six percent of caregivers reported feeling afraid their child’s condition might worsen, and 36% said they feared their child might die from his condition.
Forty-six percent of caregivers said their child’s hemophilia sometimes, often, or always causes financial problems. And 33% of caregivers said that, at least sometimes, their family does not have enough money because of their child’s hemophilia.
HEMOCAB is a trademark of Novo Nordisk Health Care AG. Dr von Mackensen received a consulting fee from Novo Nordisk for developing the HEMOCAB questionnaire. Leonard A. Valentino, MD, of Rush University Medical Center in Chicago, Illinois, was involved in developing the questionnaire as well but did not participate in the pilot study.
The other researchers involved in the study have received funding/consulting fees from—or are employees of—Novo Nordisk, Baxter, Bayer, OctaPharma, CSL Behring, OPKO Health, and Selexys. ![]()

TORONTO—When children with hemophilia develop inhibitors, their caregivers shoulder a greater burden, according to a pilot study.
Researchers surveyed 40 subjects on the burden of caring for a child with hemophilia and found that inhibitor development significantly increased the burden of care.
But other factors—such as the number of bleeds a child had experienced in the last 12 months—had no significant impact.
Sylvia von Mackensen, PhD, of the University Medical Centre Hamburg-Eppendorf in Hamburg, Germany, and her colleagues presented these findings at the ISTH 2015 Congress (abstract PO256-WED).
The study was a pilot test of the HEMOCAB questionnaire, which consists of 59 questions mapped to 13 domains. Caregivers were asked to select the response that best qualified their burden and were scored on a scale of 0 to 100, with higher values corresponding with greater burden.
Forty caregivers completed the questionnaire. All of them had children with hemophilia (n=40), with or without inhibitors, who were younger than 22 years of age.
Three-quarters of the caregivers were mothers, 17.5% were fathers, and 7.5% were grandmothers. The mean age of caregivers was 39.32 ± 8.9 years (range, 27-66), and the mean age of the hemophilia patients was 10.98 ± 5.5 years (range, 1-21).
Most of the patients had hemophilia A (95%), and most had severe disease (77.5%). Six children (15%) had inhibitors. Overall, patients had experienced an average of 4.83 ±8.9 bleeds (range, 0-52) in the previous 12 months.
Most of the patients (88.5%) were receiving prophylaxis. Caregivers said they spent 8.69 ± 7.7 hours per month on infusion and 3.84 ± 6.7 hours (range, 0-30) per month traveling to hemophilia treatment facilities.
Size of burden
Caregivers said the most burdensome aspects of care were the caregivers’ perception of the child (38%), emotional stress (36%), financial burden (34%), and the impact of care on the caregiver (31%).
For nearly all of the domains assessed—emotional stress, personal sacrifice, medical management, work situation, etc.—a caregivers’ burden was significantly higher if a child had inhibitors. The only exception was school-related burden.
When the researchers analyzed the impact of other factors on care burden, they found that only inhibitor development had a significant impact.
There was no impact for orthopedic joint score, age of the caregiver, age of the child, time for infusion, time traveling to a hemophilia treatment center, the number of bleeds in the past 12 months, the number of children with hemophilia per household, home treatment, caregiver marital status, location, or caregiver education.
Frequency of burden
The HEMOCAB questionnaire also included scales assessing the frequency of burden. Dr von Mackensen and her colleagues presented data from these scales for the caregivers’ perception of their child, emotional stress, and finances.
Sixty percent of caregivers said they sometimes, often, or always feel their child’s condition is a difficult situation. Seventy-three percent of caregivers expressed feelings of sadness about informing their child of what he can and cannot do due to his illness.
Seventy-four percent of caregivers said they sometimes, often, or always feel afraid their child might get injured when they are not around to help. Forty-six percent of caregivers reported feeling afraid their child’s condition might worsen, and 36% said they feared their child might die from his condition.
Forty-six percent of caregivers said their child’s hemophilia sometimes, often, or always causes financial problems. And 33% of caregivers said that, at least sometimes, their family does not have enough money because of their child’s hemophilia.
HEMOCAB is a trademark of Novo Nordisk Health Care AG. Dr von Mackensen received a consulting fee from Novo Nordisk for developing the HEMOCAB questionnaire. Leonard A. Valentino, MD, of Rush University Medical Center in Chicago, Illinois, was involved in developing the questionnaire as well but did not participate in the pilot study.
The other researchers involved in the study have received funding/consulting fees from—or are employees of—Novo Nordisk, Baxter, Bayer, OctaPharma, CSL Behring, OPKO Health, and Selexys. ![]()
DDW: LINX device beneficial, safe for GERD
WASHINGTON – Five-year follow-up data on the magnetic device approved for treating gastroesophageal reflux disease confirm its long-term safety and efficacy, Dr. Robert A. Ganz reported at the annual Digestive Disease Week.
Five years after device implantation, the proportion of patients experiencing moderate to severe regurgitation had dropped to about 1%, from almost 60% at baseline, and two-thirds of patients were not taking any proton pump inhibitors (PPIs), said Dr. Ganz, chief of gastroenterology at Abbott Northwestern Hospital, Minneapolis, and one of the study investigators. These were among the results of the study that evaluated the device, the LINX Reflux Management System. The device was approved by the Food and Drug Administration FDA) in 2012 and is for the treatment of people with GERD as defined by abnormal pH testing, who continue to have chronic GERD symptoms that persist despite maximum medical therapy for the treatment of reflux.
“Magnetic sphincter augmentation should be considered first-line surgical therapy for those with gastroesophageal reflux disease, based on the results of this study,” he said.
The 2-year results of the prospective, multicenter study were the basis of the FDA approval of the device, described by the manufacturer, Torax Medical, as a “small implant [composed] of interlinked titanium beads with magnetic cores,” implanted during standard laparoscopy. The magnetic attraction between the beads augments the existing esophageal sphincter’s barrier function to prevent reflux,” according to the company.
The study enrolled 100 patients with reflux disease with a median age of 53 years, who had experienced typical heartburn for at least 6 months with or without regurgitation and were taking PPIs daily for at least 3 months (median use 5 years). Patients had GERD for a median of 10 years (range: 1-40 years). People who had any type of previous gastric or esophageal surgery, Barrett’s esophagus, a hiatal hernia greater than 3 cm, a body mass index over 35 kg/m2, or grade C or D esophagitis were excluded.
The device was implanted in all patients, who served as their own controls; 85 patients were followed through 5 years (6 were lost to follow-up, the device was explanted in 6 patients, 2 patients did not consent to extended follow-up, and 1 patient died of an unrelated cancer). The median procedure time was 36 minutes with a range of 7-125 minutes); all procedures were successfully completed with no intraoperative complications and all patients were discharged within 24 hours on an unrestricted diet.
The median total Gastroesophageal Reflux Disease–Health-Related Quality of Life (GERD-HRQL) score at baseline was 27 points among those not on PPIs and 11 points on PPIs, dropping to 4 points at 5 years off PPIs. At baseline, 95% of patients expressed dissatisfaction related to reflux, which dropped to 7% at year 5. Moderate to severe heartburn was reported by 89% at baseline, dropping to about 12% at year 5. The proportion of patients experiencing moderate to severe regurgitation dropped from 57% at baseline to about 1% at 5 years, Dr. Ganz said.
At baseline, 100% were taking PPIs every day, compared with 15% at 5 years. (At 5 years, 75% had discontinued PPIs, and about 9% reported PRN use only). Grade A and B esophagitis decreased from 40% at baseline to 16% at 5 years, at which point most cases were grade A, and there were no patients with grade C or D esophagitis, he said. In addition, at 5 years, 100% of patients “reported the ability to belch, and those needing to vomit – about 16% – reported the ability to vomit,” demonstrating that normal physiology was preserved with the device.
At 5 years, there were no device erosions or migrations, or any significant adverse events other than dysphagia, which “was typically mild and not associated with weight loss and tended to resolve over time,” from about 70% in the first few weeks after surgery to 11% at 1 year and 7% at 5 years, Dr. Ganz said.
In seven cases, the device was removed laparoscopically, with no complications and gastric anatomy was preserved for future treatments. All removals were elective. The device was removed in four patients because of dysphagia, which completely resolved in those patients. One patient had the device removed because of vomiting of unknown cause that persisted after removal. Another two patients who “had the device removed for disease management” continued to experience reflux and had “uneventful” Nissen fundoplication,” he said.
“Five years after magnetic augmentation, we have demonstrated objective evidence of reduction in acid exposure and in the majority of patients, normalized pH [and] we demonstrated significant and durable improvement in all group parameters measured, with preservation of fundic anatomy and normal physiology, with the ability to belch and vomit,” Dr. Ganz concluded. The results also show that the “procedure is reproducible, safe and reversible if necessary,” he added, noting that one of the limitations of the study was that subjects served as their own controls. During the discussion period, he was asked about hiatal hernia repairs, an apparent trend to “decay” from years 1 to 5 in some parameters measured, and dysphagia after the procedure.
About 40% of the patients in the study had a hiatal hernia, and about one-third of these patients had a hernia repair. A subgroup analysis of the data is being performed to evaluate the impact of hernia repair, Dr. Ganz said.
PPI use increased from 8% in year 4, to 15% in year 5. The reason for this s difficult to determine but “even though there is a bit of a decay, patients are still quite satisfied at 5 years,” Dr. Ganz remarked, also referring to the marked impact on regurgitation. Many U.S. patients use PPIs for reasons other than reflux, and studies show that many patients are on PPIs after the Nissen procedure in the absence of pathologic pH scores, he pointed out.
Compared with the type of dysphagia patients experience after the Nissen procedure, which is immediate and improves with time, Dr. Ganz said that the dysphagia associated with the device “seemed to peak around 2 weeks and then it slowly improved with time, so this may be more of a scar tissue–associated dysphagia than an edema dysphagia, but … it does improve with time.
Three-year results of the study were published in 2013 (N. Engl. J. Med. 2013;368:719-72), Dr. Ganz was the lead author.
The study was funded by Torax Medical. Dr. Ganz had no disclosures related to the topic of this presentation.
*This story was updated 7/9/2015.
At DDW this year, Dr. Ganz reported on the 5-year follow-up of the original LINX data that was published in the New England Journal of Medicine in 2013 (368:2039-40). The original study enrolled and followed 100 reflux patients for 3 years after implantation of the magnetic sphincter augmentation device, and it appears that the successful outcomes are sustained over the 5-year period. Most notable are the lasting improvement in regurgitation and the dramatic reduction in requirement for maintenance PPI therapy. These findings led the investigators to suggest that this should be considered a first-line surgical therapy for GERD. Overall, this is not an unreasonable statement when one considers the current model wherein antireflux surgery fits in the treatment of GERD. Medical therapy with proton pump inhibitors is extremely safe and effective for a substantial number of patients with GERD and based on this risk/benefit profile should be the first line therapy (Am. J. Gastroenterol. 2013;108:308-28; quiz 329). However, this treatment is not perfect and there are many patients who continue to have persistent symptoms despite PPI therapy (Clin. Gastroenterol. Hepatol. 2012;10:612-9). Although the majority of PPI nonresponders have a functional etiology, there is a distinct population that continue to have refractory reflux-related symptoms, such as regurgitation, that escape the therapeutic target of PPIs. These patients will require an augmentation of the antireflux barrier and the LINX approach appears to be as effective as fundoplication in this regard (J. Am. Coll. Surg. 2015;221:123-8). The question is whether the side effect profile and durability of LINX is better than fundoplication. The answer here is not clear and I would carefully state that LINX and fundoplication can be considered first-line surgical therapies for GERD patients who have documented pathologic acid gastroesophageal reflux and are intolerant to PPIs or not responding to PPIs.
Dr. John E. Pandolfino is professor of medicine and chief of the division of gastroenterology and hepatology at Northwestern University, Chicago. He is a speaker for Astra Zeneca/Takeda and a consultant for EndoGastric Solutions.
At DDW this year, Dr. Ganz reported on the 5-year follow-up of the original LINX data that was published in the New England Journal of Medicine in 2013 (368:2039-40). The original study enrolled and followed 100 reflux patients for 3 years after implantation of the magnetic sphincter augmentation device, and it appears that the successful outcomes are sustained over the 5-year period. Most notable are the lasting improvement in regurgitation and the dramatic reduction in requirement for maintenance PPI therapy. These findings led the investigators to suggest that this should be considered a first-line surgical therapy for GERD. Overall, this is not an unreasonable statement when one considers the current model wherein antireflux surgery fits in the treatment of GERD. Medical therapy with proton pump inhibitors is extremely safe and effective for a substantial number of patients with GERD and based on this risk/benefit profile should be the first line therapy (Am. J. Gastroenterol. 2013;108:308-28; quiz 329). However, this treatment is not perfect and there are many patients who continue to have persistent symptoms despite PPI therapy (Clin. Gastroenterol. Hepatol. 2012;10:612-9). Although the majority of PPI nonresponders have a functional etiology, there is a distinct population that continue to have refractory reflux-related symptoms, such as regurgitation, that escape the therapeutic target of PPIs. These patients will require an augmentation of the antireflux barrier and the LINX approach appears to be as effective as fundoplication in this regard (J. Am. Coll. Surg. 2015;221:123-8). The question is whether the side effect profile and durability of LINX is better than fundoplication. The answer here is not clear and I would carefully state that LINX and fundoplication can be considered first-line surgical therapies for GERD patients who have documented pathologic acid gastroesophageal reflux and are intolerant to PPIs or not responding to PPIs.
Dr. John E. Pandolfino is professor of medicine and chief of the division of gastroenterology and hepatology at Northwestern University, Chicago. He is a speaker for Astra Zeneca/Takeda and a consultant for EndoGastric Solutions.
At DDW this year, Dr. Ganz reported on the 5-year follow-up of the original LINX data that was published in the New England Journal of Medicine in 2013 (368:2039-40). The original study enrolled and followed 100 reflux patients for 3 years after implantation of the magnetic sphincter augmentation device, and it appears that the successful outcomes are sustained over the 5-year period. Most notable are the lasting improvement in regurgitation and the dramatic reduction in requirement for maintenance PPI therapy. These findings led the investigators to suggest that this should be considered a first-line surgical therapy for GERD. Overall, this is not an unreasonable statement when one considers the current model wherein antireflux surgery fits in the treatment of GERD. Medical therapy with proton pump inhibitors is extremely safe and effective for a substantial number of patients with GERD and based on this risk/benefit profile should be the first line therapy (Am. J. Gastroenterol. 2013;108:308-28; quiz 329). However, this treatment is not perfect and there are many patients who continue to have persistent symptoms despite PPI therapy (Clin. Gastroenterol. Hepatol. 2012;10:612-9). Although the majority of PPI nonresponders have a functional etiology, there is a distinct population that continue to have refractory reflux-related symptoms, such as regurgitation, that escape the therapeutic target of PPIs. These patients will require an augmentation of the antireflux barrier and the LINX approach appears to be as effective as fundoplication in this regard (J. Am. Coll. Surg. 2015;221:123-8). The question is whether the side effect profile and durability of LINX is better than fundoplication. The answer here is not clear and I would carefully state that LINX and fundoplication can be considered first-line surgical therapies for GERD patients who have documented pathologic acid gastroesophageal reflux and are intolerant to PPIs or not responding to PPIs.
Dr. John E. Pandolfino is professor of medicine and chief of the division of gastroenterology and hepatology at Northwestern University, Chicago. He is a speaker for Astra Zeneca/Takeda and a consultant for EndoGastric Solutions.
WASHINGTON – Five-year follow-up data on the magnetic device approved for treating gastroesophageal reflux disease confirm its long-term safety and efficacy, Dr. Robert A. Ganz reported at the annual Digestive Disease Week.
Five years after device implantation, the proportion of patients experiencing moderate to severe regurgitation had dropped to about 1%, from almost 60% at baseline, and two-thirds of patients were not taking any proton pump inhibitors (PPIs), said Dr. Ganz, chief of gastroenterology at Abbott Northwestern Hospital, Minneapolis, and one of the study investigators. These were among the results of the study that evaluated the device, the LINX Reflux Management System. The device was approved by the Food and Drug Administration FDA) in 2012 and is for the treatment of people with GERD as defined by abnormal pH testing, who continue to have chronic GERD symptoms that persist despite maximum medical therapy for the treatment of reflux.
“Magnetic sphincter augmentation should be considered first-line surgical therapy for those with gastroesophageal reflux disease, based on the results of this study,” he said.
The 2-year results of the prospective, multicenter study were the basis of the FDA approval of the device, described by the manufacturer, Torax Medical, as a “small implant [composed] of interlinked titanium beads with magnetic cores,” implanted during standard laparoscopy. The magnetic attraction between the beads augments the existing esophageal sphincter’s barrier function to prevent reflux,” according to the company.
The study enrolled 100 patients with reflux disease with a median age of 53 years, who had experienced typical heartburn for at least 6 months with or without regurgitation and were taking PPIs daily for at least 3 months (median use 5 years). Patients had GERD for a median of 10 years (range: 1-40 years). People who had any type of previous gastric or esophageal surgery, Barrett’s esophagus, a hiatal hernia greater than 3 cm, a body mass index over 35 kg/m2, or grade C or D esophagitis were excluded.
The device was implanted in all patients, who served as their own controls; 85 patients were followed through 5 years (6 were lost to follow-up, the device was explanted in 6 patients, 2 patients did not consent to extended follow-up, and 1 patient died of an unrelated cancer). The median procedure time was 36 minutes with a range of 7-125 minutes); all procedures were successfully completed with no intraoperative complications and all patients were discharged within 24 hours on an unrestricted diet.
The median total Gastroesophageal Reflux Disease–Health-Related Quality of Life (GERD-HRQL) score at baseline was 27 points among those not on PPIs and 11 points on PPIs, dropping to 4 points at 5 years off PPIs. At baseline, 95% of patients expressed dissatisfaction related to reflux, which dropped to 7% at year 5. Moderate to severe heartburn was reported by 89% at baseline, dropping to about 12% at year 5. The proportion of patients experiencing moderate to severe regurgitation dropped from 57% at baseline to about 1% at 5 years, Dr. Ganz said.
At baseline, 100% were taking PPIs every day, compared with 15% at 5 years. (At 5 years, 75% had discontinued PPIs, and about 9% reported PRN use only). Grade A and B esophagitis decreased from 40% at baseline to 16% at 5 years, at which point most cases were grade A, and there were no patients with grade C or D esophagitis, he said. In addition, at 5 years, 100% of patients “reported the ability to belch, and those needing to vomit – about 16% – reported the ability to vomit,” demonstrating that normal physiology was preserved with the device.
At 5 years, there were no device erosions or migrations, or any significant adverse events other than dysphagia, which “was typically mild and not associated with weight loss and tended to resolve over time,” from about 70% in the first few weeks after surgery to 11% at 1 year and 7% at 5 years, Dr. Ganz said.
In seven cases, the device was removed laparoscopically, with no complications and gastric anatomy was preserved for future treatments. All removals were elective. The device was removed in four patients because of dysphagia, which completely resolved in those patients. One patient had the device removed because of vomiting of unknown cause that persisted after removal. Another two patients who “had the device removed for disease management” continued to experience reflux and had “uneventful” Nissen fundoplication,” he said.
“Five years after magnetic augmentation, we have demonstrated objective evidence of reduction in acid exposure and in the majority of patients, normalized pH [and] we demonstrated significant and durable improvement in all group parameters measured, with preservation of fundic anatomy and normal physiology, with the ability to belch and vomit,” Dr. Ganz concluded. The results also show that the “procedure is reproducible, safe and reversible if necessary,” he added, noting that one of the limitations of the study was that subjects served as their own controls. During the discussion period, he was asked about hiatal hernia repairs, an apparent trend to “decay” from years 1 to 5 in some parameters measured, and dysphagia after the procedure.
About 40% of the patients in the study had a hiatal hernia, and about one-third of these patients had a hernia repair. A subgroup analysis of the data is being performed to evaluate the impact of hernia repair, Dr. Ganz said.
PPI use increased from 8% in year 4, to 15% in year 5. The reason for this s difficult to determine but “even though there is a bit of a decay, patients are still quite satisfied at 5 years,” Dr. Ganz remarked, also referring to the marked impact on regurgitation. Many U.S. patients use PPIs for reasons other than reflux, and studies show that many patients are on PPIs after the Nissen procedure in the absence of pathologic pH scores, he pointed out.
Compared with the type of dysphagia patients experience after the Nissen procedure, which is immediate and improves with time, Dr. Ganz said that the dysphagia associated with the device “seemed to peak around 2 weeks and then it slowly improved with time, so this may be more of a scar tissue–associated dysphagia than an edema dysphagia, but … it does improve with time.
Three-year results of the study were published in 2013 (N. Engl. J. Med. 2013;368:719-72), Dr. Ganz was the lead author.
The study was funded by Torax Medical. Dr. Ganz had no disclosures related to the topic of this presentation.
*This story was updated 7/9/2015.
WASHINGTON – Five-year follow-up data on the magnetic device approved for treating gastroesophageal reflux disease confirm its long-term safety and efficacy, Dr. Robert A. Ganz reported at the annual Digestive Disease Week.
Five years after device implantation, the proportion of patients experiencing moderate to severe regurgitation had dropped to about 1%, from almost 60% at baseline, and two-thirds of patients were not taking any proton pump inhibitors (PPIs), said Dr. Ganz, chief of gastroenterology at Abbott Northwestern Hospital, Minneapolis, and one of the study investigators. These were among the results of the study that evaluated the device, the LINX Reflux Management System. The device was approved by the Food and Drug Administration FDA) in 2012 and is for the treatment of people with GERD as defined by abnormal pH testing, who continue to have chronic GERD symptoms that persist despite maximum medical therapy for the treatment of reflux.
“Magnetic sphincter augmentation should be considered first-line surgical therapy for those with gastroesophageal reflux disease, based on the results of this study,” he said.
The 2-year results of the prospective, multicenter study were the basis of the FDA approval of the device, described by the manufacturer, Torax Medical, as a “small implant [composed] of interlinked titanium beads with magnetic cores,” implanted during standard laparoscopy. The magnetic attraction between the beads augments the existing esophageal sphincter’s barrier function to prevent reflux,” according to the company.
The study enrolled 100 patients with reflux disease with a median age of 53 years, who had experienced typical heartburn for at least 6 months with or without regurgitation and were taking PPIs daily for at least 3 months (median use 5 years). Patients had GERD for a median of 10 years (range: 1-40 years). People who had any type of previous gastric or esophageal surgery, Barrett’s esophagus, a hiatal hernia greater than 3 cm, a body mass index over 35 kg/m2, or grade C or D esophagitis were excluded.
The device was implanted in all patients, who served as their own controls; 85 patients were followed through 5 years (6 were lost to follow-up, the device was explanted in 6 patients, 2 patients did not consent to extended follow-up, and 1 patient died of an unrelated cancer). The median procedure time was 36 minutes with a range of 7-125 minutes); all procedures were successfully completed with no intraoperative complications and all patients were discharged within 24 hours on an unrestricted diet.
The median total Gastroesophageal Reflux Disease–Health-Related Quality of Life (GERD-HRQL) score at baseline was 27 points among those not on PPIs and 11 points on PPIs, dropping to 4 points at 5 years off PPIs. At baseline, 95% of patients expressed dissatisfaction related to reflux, which dropped to 7% at year 5. Moderate to severe heartburn was reported by 89% at baseline, dropping to about 12% at year 5. The proportion of patients experiencing moderate to severe regurgitation dropped from 57% at baseline to about 1% at 5 years, Dr. Ganz said.
At baseline, 100% were taking PPIs every day, compared with 15% at 5 years. (At 5 years, 75% had discontinued PPIs, and about 9% reported PRN use only). Grade A and B esophagitis decreased from 40% at baseline to 16% at 5 years, at which point most cases were grade A, and there were no patients with grade C or D esophagitis, he said. In addition, at 5 years, 100% of patients “reported the ability to belch, and those needing to vomit – about 16% – reported the ability to vomit,” demonstrating that normal physiology was preserved with the device.
At 5 years, there were no device erosions or migrations, or any significant adverse events other than dysphagia, which “was typically mild and not associated with weight loss and tended to resolve over time,” from about 70% in the first few weeks after surgery to 11% at 1 year and 7% at 5 years, Dr. Ganz said.
In seven cases, the device was removed laparoscopically, with no complications and gastric anatomy was preserved for future treatments. All removals were elective. The device was removed in four patients because of dysphagia, which completely resolved in those patients. One patient had the device removed because of vomiting of unknown cause that persisted after removal. Another two patients who “had the device removed for disease management” continued to experience reflux and had “uneventful” Nissen fundoplication,” he said.
“Five years after magnetic augmentation, we have demonstrated objective evidence of reduction in acid exposure and in the majority of patients, normalized pH [and] we demonstrated significant and durable improvement in all group parameters measured, with preservation of fundic anatomy and normal physiology, with the ability to belch and vomit,” Dr. Ganz concluded. The results also show that the “procedure is reproducible, safe and reversible if necessary,” he added, noting that one of the limitations of the study was that subjects served as their own controls. During the discussion period, he was asked about hiatal hernia repairs, an apparent trend to “decay” from years 1 to 5 in some parameters measured, and dysphagia after the procedure.
About 40% of the patients in the study had a hiatal hernia, and about one-third of these patients had a hernia repair. A subgroup analysis of the data is being performed to evaluate the impact of hernia repair, Dr. Ganz said.
PPI use increased from 8% in year 4, to 15% in year 5. The reason for this s difficult to determine but “even though there is a bit of a decay, patients are still quite satisfied at 5 years,” Dr. Ganz remarked, also referring to the marked impact on regurgitation. Many U.S. patients use PPIs for reasons other than reflux, and studies show that many patients are on PPIs after the Nissen procedure in the absence of pathologic pH scores, he pointed out.
Compared with the type of dysphagia patients experience after the Nissen procedure, which is immediate and improves with time, Dr. Ganz said that the dysphagia associated with the device “seemed to peak around 2 weeks and then it slowly improved with time, so this may be more of a scar tissue–associated dysphagia than an edema dysphagia, but … it does improve with time.
Three-year results of the study were published in 2013 (N. Engl. J. Med. 2013;368:719-72), Dr. Ganz was the lead author.
The study was funded by Torax Medical. Dr. Ganz had no disclosures related to the topic of this presentation.
*This story was updated 7/9/2015.
AT DDW 2015
Key clinical point: A magnetic device designed to augment the lower esophageal sphincter is a surgical option that can be expected to provide long-term control of reflux symptoms in patients with GERD.
Major finding: Improvements 5 years after treatment with the Linx Reflux Management System include a drop from 60% experiencing regurgitation to 1%, and two-third of patients no longer taking PPIs.
Data source: The multicenter, prospective study evaluated the long-term safety and efficacy of the device over 5 years in 100 patients with GERD, who served as their own controls; 85 were included in the 5-year follow-up.
Disclosures: The study was funded by the device manufacturer, Torax Medical. Dr. Ganz had no disclosures related to the topic of this presentation.
He Tried So Hard to Avoid It …
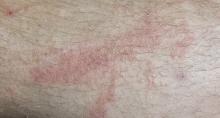
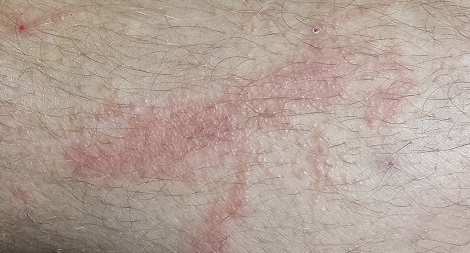
A 38-year-old man presents to dermatology with what he assumes is poison ivy: an itchy, blistery rash that usually appears in the summer, more years than not. Each year, it’s a bit worse in terms of extent and symptomatology, despite his efforts to avoid the problem altogether.
The rash pretty consistently manifests on his leg, although there are other areas of involvement. Equally predictable, at this point, is his wife’s reaction: She banishes him to the couch for fear of “catching” whatever he has.
After so many years’ experience with the condition, the patient is understandably alert to coming in contact with the offending plant. He has even taken photos of it, to demonstrate how abundantly it grows in his yard.
EXAMINATION
The patient’s lesions are classic collections of vesicles crisscrossing his legs in linear configurations. There is faint underlying erythema. Smaller but similar lesions are scattered over his arms and trunk; the patient is sure he spread the rash with his scratching.
The plant in the patient’s photos has five dart-shaped leaves with uniformly serrated margins extending from a single stem. It grows as a vine on fences and masonry. He has scrupulously avoided contact with it and therefore cannot understand how he keeps developing the rash.
What is the diagnosis?
DISCUSSION
It has been said that humor is tragedy plus time; certain skin diseases, such as shingles or poison ivy, certainly provide fodder. In the midst of an attack, however, these conditions can produce not only miserable symptoms of itching, pain, and sleeplessness, but also considerable mental anguish regarding contagion.
Repeated polls of the general public reveal an almost universal belief that poison ivy is contagious and that scratching spreads it around the body. Research, and a thorough knowledge of the pathophysiology involved, have long since disproven both concepts. So while the inclination to distance oneself from an affected person is understandable, there is actually no need to do so. For the patient, facing six weeks or more of isolation is no laughing matter (at least, until long after the fact).
This particular case highlights one other pertinent issue with poison ivy: As the photos established, the patient had carefully avoided the wrong plant. The five dart-shaped serrated leaves suggest Virginia creeper—certainly not poison ivy. This may sound like a minor issue, but it is quite possible that while the patient was steering clear of a harmless plant, he was in fact coming in contact with the one he should have been concerned about.
True poison ivy, Toxicodendron radicans, can develop as a bush, a vine that stays low to the ground, a small tree, or even a climbing vine that can reach 30 feet or higher into mature trees, especially along waterways or in low, boggy land. The vines can attain a thickness of more than 3 in and have a surface covered by tiny “rootlets,” giving them a shaggy look.
Regardless of the plant’s form, its leaves are always found in threes, protruding from the same stem. The leaves are diamond shaped, can reach a length of 10 in, and often have a single notch on the margin that is said to resemble the outline of a thumb. The plant produces white berries in late summer or early fall.
It has been estimated that the number of poison ivy plants has doubled since 1960, for at least two reasons. First, an increase in population has led to more land being cleared for housing; many properties now border woodlands, which are an ideal environment for this plant.
Second, and more surprising, poison ivy thrives in a CO2-rich environment. Carbon dioxide in the atmosphere has increased significantly in the past 50 years and will continue to do so. Experts predict that the density of poison ivy will double again in the next 20 years as a result. The potency of the urushiol, the offending substance in the stems and leaves, is expected to increase as well.
The patient (height, 6’3” and weight, > 300 lb) was treated with a 60-mg IM injection of triamcinolone, a two-week, 40-mg taper of prednisone, and twice-daily application of betamethasone cream. This, of course, followed a discussion of the risks versus benefits of such a course of action.
TAKE-HOME LEARNING POINTS
• Climbing vines with five serrated leaves coming off the same stem are probably Virginia creeper and not poison ivy.
• Poison ivy is not contagious, cannot be spread by scratching, and is not poisonous in any way.
• The rash produced by poison ivy exposure can be severe and can last six weeks or more without treatment.
• The number of poison ivy plants has doubled in the past 50 years and is expected to double again within 20 years. The potency of the plant’s allergen is also expected to increase.
A 38-year-old man presents to dermatology with what he assumes is poison ivy: an itchy, blistery rash that usually appears in the summer, more years than not. Each year, it’s a bit worse in terms of extent and symptomatology, despite his efforts to avoid the problem altogether.
The rash pretty consistently manifests on his leg, although there are other areas of involvement. Equally predictable, at this point, is his wife’s reaction: She banishes him to the couch for fear of “catching” whatever he has.
After so many years’ experience with the condition, the patient is understandably alert to coming in contact with the offending plant. He has even taken photos of it, to demonstrate how abundantly it grows in his yard.
EXAMINATION
The patient’s lesions are classic collections of vesicles crisscrossing his legs in linear configurations. There is faint underlying erythema. Smaller but similar lesions are scattered over his arms and trunk; the patient is sure he spread the rash with his scratching.
The plant in the patient’s photos has five dart-shaped leaves with uniformly serrated margins extending from a single stem. It grows as a vine on fences and masonry. He has scrupulously avoided contact with it and therefore cannot understand how he keeps developing the rash.
What is the diagnosis?
DISCUSSION
It has been said that humor is tragedy plus time; certain skin diseases, such as shingles or poison ivy, certainly provide fodder. In the midst of an attack, however, these conditions can produce not only miserable symptoms of itching, pain, and sleeplessness, but also considerable mental anguish regarding contagion.
Repeated polls of the general public reveal an almost universal belief that poison ivy is contagious and that scratching spreads it around the body. Research, and a thorough knowledge of the pathophysiology involved, have long since disproven both concepts. So while the inclination to distance oneself from an affected person is understandable, there is actually no need to do so. For the patient, facing six weeks or more of isolation is no laughing matter (at least, until long after the fact).
This particular case highlights one other pertinent issue with poison ivy: As the photos established, the patient had carefully avoided the wrong plant. The five dart-shaped serrated leaves suggest Virginia creeper—certainly not poison ivy. This may sound like a minor issue, but it is quite possible that while the patient was steering clear of a harmless plant, he was in fact coming in contact with the one he should have been concerned about.
True poison ivy, Toxicodendron radicans, can develop as a bush, a vine that stays low to the ground, a small tree, or even a climbing vine that can reach 30 feet or higher into mature trees, especially along waterways or in low, boggy land. The vines can attain a thickness of more than 3 in and have a surface covered by tiny “rootlets,” giving them a shaggy look.
Regardless of the plant’s form, its leaves are always found in threes, protruding from the same stem. The leaves are diamond shaped, can reach a length of 10 in, and often have a single notch on the margin that is said to resemble the outline of a thumb. The plant produces white berries in late summer or early fall.
It has been estimated that the number of poison ivy plants has doubled since 1960, for at least two reasons. First, an increase in population has led to more land being cleared for housing; many properties now border woodlands, which are an ideal environment for this plant.
Second, and more surprising, poison ivy thrives in a CO2-rich environment. Carbon dioxide in the atmosphere has increased significantly in the past 50 years and will continue to do so. Experts predict that the density of poison ivy will double again in the next 20 years as a result. The potency of the urushiol, the offending substance in the stems and leaves, is expected to increase as well.
The patient (height, 6’3” and weight, > 300 lb) was treated with a 60-mg IM injection of triamcinolone, a two-week, 40-mg taper of prednisone, and twice-daily application of betamethasone cream. This, of course, followed a discussion of the risks versus benefits of such a course of action.
TAKE-HOME LEARNING POINTS
• Climbing vines with five serrated leaves coming off the same stem are probably Virginia creeper and not poison ivy.
• Poison ivy is not contagious, cannot be spread by scratching, and is not poisonous in any way.
• The rash produced by poison ivy exposure can be severe and can last six weeks or more without treatment.
• The number of poison ivy plants has doubled in the past 50 years and is expected to double again within 20 years. The potency of the plant’s allergen is also expected to increase.
A 38-year-old man presents to dermatology with what he assumes is poison ivy: an itchy, blistery rash that usually appears in the summer, more years than not. Each year, it’s a bit worse in terms of extent and symptomatology, despite his efforts to avoid the problem altogether.
The rash pretty consistently manifests on his leg, although there are other areas of involvement. Equally predictable, at this point, is his wife’s reaction: She banishes him to the couch for fear of “catching” whatever he has.
After so many years’ experience with the condition, the patient is understandably alert to coming in contact with the offending plant. He has even taken photos of it, to demonstrate how abundantly it grows in his yard.
EXAMINATION
The patient’s lesions are classic collections of vesicles crisscrossing his legs in linear configurations. There is faint underlying erythema. Smaller but similar lesions are scattered over his arms and trunk; the patient is sure he spread the rash with his scratching.
The plant in the patient’s photos has five dart-shaped leaves with uniformly serrated margins extending from a single stem. It grows as a vine on fences and masonry. He has scrupulously avoided contact with it and therefore cannot understand how he keeps developing the rash.
What is the diagnosis?
DISCUSSION
It has been said that humor is tragedy plus time; certain skin diseases, such as shingles or poison ivy, certainly provide fodder. In the midst of an attack, however, these conditions can produce not only miserable symptoms of itching, pain, and sleeplessness, but also considerable mental anguish regarding contagion.
Repeated polls of the general public reveal an almost universal belief that poison ivy is contagious and that scratching spreads it around the body. Research, and a thorough knowledge of the pathophysiology involved, have long since disproven both concepts. So while the inclination to distance oneself from an affected person is understandable, there is actually no need to do so. For the patient, facing six weeks or more of isolation is no laughing matter (at least, until long after the fact).
This particular case highlights one other pertinent issue with poison ivy: As the photos established, the patient had carefully avoided the wrong plant. The five dart-shaped serrated leaves suggest Virginia creeper—certainly not poison ivy. This may sound like a minor issue, but it is quite possible that while the patient was steering clear of a harmless plant, he was in fact coming in contact with the one he should have been concerned about.
True poison ivy, Toxicodendron radicans, can develop as a bush, a vine that stays low to the ground, a small tree, or even a climbing vine that can reach 30 feet or higher into mature trees, especially along waterways or in low, boggy land. The vines can attain a thickness of more than 3 in and have a surface covered by tiny “rootlets,” giving them a shaggy look.
Regardless of the plant’s form, its leaves are always found in threes, protruding from the same stem. The leaves are diamond shaped, can reach a length of 10 in, and often have a single notch on the margin that is said to resemble the outline of a thumb. The plant produces white berries in late summer or early fall.
It has been estimated that the number of poison ivy plants has doubled since 1960, for at least two reasons. First, an increase in population has led to more land being cleared for housing; many properties now border woodlands, which are an ideal environment for this plant.
Second, and more surprising, poison ivy thrives in a CO2-rich environment. Carbon dioxide in the atmosphere has increased significantly in the past 50 years and will continue to do so. Experts predict that the density of poison ivy will double again in the next 20 years as a result. The potency of the urushiol, the offending substance in the stems and leaves, is expected to increase as well.
The patient (height, 6’3” and weight, > 300 lb) was treated with a 60-mg IM injection of triamcinolone, a two-week, 40-mg taper of prednisone, and twice-daily application of betamethasone cream. This, of course, followed a discussion of the risks versus benefits of such a course of action.
TAKE-HOME LEARNING POINTS
• Climbing vines with five serrated leaves coming off the same stem are probably Virginia creeper and not poison ivy.
• Poison ivy is not contagious, cannot be spread by scratching, and is not poisonous in any way.
• The rash produced by poison ivy exposure can be severe and can last six weeks or more without treatment.
• The number of poison ivy plants has doubled in the past 50 years and is expected to double again within 20 years. The potency of the plant’s allergen is also expected to increase.
LISTEN NOW: Hospitalist Manya Gupta on Choosing Wisely
Excerpt of our interviews with Choosing Wisely, hospitalist Manya Gupta, MD, an assistant professor in the department of internal medicine of Rush University Medical Center in Chicago, discusses an example of a Choosing Wisely program.
Excerpt of our interviews with Choosing Wisely, hospitalist Manya Gupta, MD, an assistant professor in the department of internal medicine of Rush University Medical Center in Chicago, discusses an example of a Choosing Wisely program.
Excerpt of our interviews with Choosing Wisely, hospitalist Manya Gupta, MD, an assistant professor in the department of internal medicine of Rush University Medical Center in Chicago, discusses an example of a Choosing Wisely program.
LISTEN NOW: Cheryl DeVita on Evaluating Contracts, Hospitalist Groups
Cheryl DeVita, senior search consultant at Cejka Search, Inc., in St. Louis, Mo., offers advice on evaluating contracts and hospitalist groups.
Cheryl DeVita, senior search consultant at Cejka Search, Inc., in St. Louis, Mo., offers advice on evaluating contracts and hospitalist groups.
Cheryl DeVita, senior search consultant at Cejka Search, Inc., in St. Louis, Mo., offers advice on evaluating contracts and hospitalist groups.
LISTEN NOW: Gastroenterologist, Robert Coben, MD, on GI Bleeds, Colon Cancer
ROBERT COBEN, MD, Program director of the gastroenterology fellowship program at Thomas Jefferson University Hospital in Philadelphia, discusses GI bleeds and colon cancer.
ROBERT COBEN, MD, Program director of the gastroenterology fellowship program at Thomas Jefferson University Hospital in Philadelphia, discusses GI bleeds and colon cancer.
ROBERT COBEN, MD, Program director of the gastroenterology fellowship program at Thomas Jefferson University Hospital in Philadelphia, discusses GI bleeds and colon cancer.
Early relapse signals high mortality in follicular lymphoma
Patients with follicular lymphoma who relapse within 2 years of receiving R-CHOP chemoimmunotherapy are at high risk of death, unlike those who do not relapse early, according to a report published online in Journal of Clinical Oncology.
Survival in follicular lymphoma, the second most common non-Hodgkin lymphoma in the United States, has dramatically improved over time, and the median survival after first-line chemoimmunotherapy now exceeds 18 years. But researchers have noted a remarkably consistent 20% rate of early relapse across numerous forms of treatment and varied study populations. Until now, the clinical significance of early relapse and its impact on overall survival has not been explored, said Dr. Carla Casulo of the University of Rochester, New York, and her associates.
They examined this issue using data from a national cohort of patients with newly diagnosed follicular lymphoma, focusing on 588 patients with stage II, III, or IV disease who were treated using first-line rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). A total of 19% of these patients relapsed within 24 months of diagnosis. Median follow-up was 7 years. With early disease progression, overall survival was only 68% at 2 years and only 50% at 5 years, compared with 97% and 90%, respectively, among patients who didn’t have early disease progression. Early progression was associated with markedly reduced survival, with a hazard ratio of 7.17.
To verify their findings in a separate cohort, Dr. Casulo and her associates assessed survival in 147 similar patients participating in a different study who were followed for a mean of 5.5 years. A total of 26% of this cohort had early relapse after receiving a variety of first-line chemoimmunotherapy regimens. With early disease progression, overall survival was only 64% at 2 years and only 34% at 5 years, compared with 98% and 94%, respectively, among patients who didn’t have early progression. Again, early progression was associated with markedly reduced survival, with an HR of 20.0 (J. Clin. Oncol. 2015 June 29 [doi: 10.1200/JCO.2014.59.7534]).
These two studies confirm that patients with follicular lymphoma who relapse within 2 years constitute a distinct subgroup at very high risk of death. “Given their poor prognosis, consideration of aggressive second-line treatments, including possibly autologous stem-cell transplantation, seem reasonable,” the investigators said.
Patients with follicular lymphoma who relapse within 2 years of receiving R-CHOP chemoimmunotherapy are at high risk of death, unlike those who do not relapse early, according to a report published online in Journal of Clinical Oncology.
Survival in follicular lymphoma, the second most common non-Hodgkin lymphoma in the United States, has dramatically improved over time, and the median survival after first-line chemoimmunotherapy now exceeds 18 years. But researchers have noted a remarkably consistent 20% rate of early relapse across numerous forms of treatment and varied study populations. Until now, the clinical significance of early relapse and its impact on overall survival has not been explored, said Dr. Carla Casulo of the University of Rochester, New York, and her associates.
They examined this issue using data from a national cohort of patients with newly diagnosed follicular lymphoma, focusing on 588 patients with stage II, III, or IV disease who were treated using first-line rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). A total of 19% of these patients relapsed within 24 months of diagnosis. Median follow-up was 7 years. With early disease progression, overall survival was only 68% at 2 years and only 50% at 5 years, compared with 97% and 90%, respectively, among patients who didn’t have early disease progression. Early progression was associated with markedly reduced survival, with a hazard ratio of 7.17.
To verify their findings in a separate cohort, Dr. Casulo and her associates assessed survival in 147 similar patients participating in a different study who were followed for a mean of 5.5 years. A total of 26% of this cohort had early relapse after receiving a variety of first-line chemoimmunotherapy regimens. With early disease progression, overall survival was only 64% at 2 years and only 34% at 5 years, compared with 98% and 94%, respectively, among patients who didn’t have early progression. Again, early progression was associated with markedly reduced survival, with an HR of 20.0 (J. Clin. Oncol. 2015 June 29 [doi: 10.1200/JCO.2014.59.7534]).
These two studies confirm that patients with follicular lymphoma who relapse within 2 years constitute a distinct subgroup at very high risk of death. “Given their poor prognosis, consideration of aggressive second-line treatments, including possibly autologous stem-cell transplantation, seem reasonable,” the investigators said.
Patients with follicular lymphoma who relapse within 2 years of receiving R-CHOP chemoimmunotherapy are at high risk of death, unlike those who do not relapse early, according to a report published online in Journal of Clinical Oncology.
Survival in follicular lymphoma, the second most common non-Hodgkin lymphoma in the United States, has dramatically improved over time, and the median survival after first-line chemoimmunotherapy now exceeds 18 years. But researchers have noted a remarkably consistent 20% rate of early relapse across numerous forms of treatment and varied study populations. Until now, the clinical significance of early relapse and its impact on overall survival has not been explored, said Dr. Carla Casulo of the University of Rochester, New York, and her associates.
They examined this issue using data from a national cohort of patients with newly diagnosed follicular lymphoma, focusing on 588 patients with stage II, III, or IV disease who were treated using first-line rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). A total of 19% of these patients relapsed within 24 months of diagnosis. Median follow-up was 7 years. With early disease progression, overall survival was only 68% at 2 years and only 50% at 5 years, compared with 97% and 90%, respectively, among patients who didn’t have early disease progression. Early progression was associated with markedly reduced survival, with a hazard ratio of 7.17.
To verify their findings in a separate cohort, Dr. Casulo and her associates assessed survival in 147 similar patients participating in a different study who were followed for a mean of 5.5 years. A total of 26% of this cohort had early relapse after receiving a variety of first-line chemoimmunotherapy regimens. With early disease progression, overall survival was only 64% at 2 years and only 34% at 5 years, compared with 98% and 94%, respectively, among patients who didn’t have early progression. Again, early progression was associated with markedly reduced survival, with an HR of 20.0 (J. Clin. Oncol. 2015 June 29 [doi: 10.1200/JCO.2014.59.7534]).
These two studies confirm that patients with follicular lymphoma who relapse within 2 years constitute a distinct subgroup at very high risk of death. “Given their poor prognosis, consideration of aggressive second-line treatments, including possibly autologous stem-cell transplantation, seem reasonable,” the investigators said.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Patients with follicular lymphoma who relapse within 2 years of receiving R-CHOP are at high risk of death, unlike those who don’t relapse early.
Major finding: In a validation cohort, overall survival was only 64% at 2 years and only 34% at 5 years among patients who relapsed early, compared with 98% and 94% among patients who didn’t relapse early (HR, 20.0).
Data source: : A secondary analysis of a study involving 588 patients with newly diagnosed follicular lymphoma, and a validation study in an independent cohort of 147 similar patients.
Disclosures: This study was supported by Genentech and F. Hoffmann-La Roche. Dr. Casulo reported having no financial disclosures; her associates reported ties to numerous industry sources.
Naloxone lotion improves disabling itch in CTCL
VANCOUVER, B.C. – Naloxone lotion appears to be a safe and effective treatment for the severe chronic itching that occurs in most patients with cutaneous T-cell lymphoma, Dr. Madeleine Duvic reported at the World Congress of Dermatology.
A major unmet need exists for better treatments for pruritis in CTCL. Antihistamines are generally ineffective. Chemotherapeutic agents provide little relief. Moreover, it has been estimated that up to half of all patients with CTCL die as a result of systemic infections arising secondary to pruritic skin excoriations, according to Dr. Duvic, professor of medicine and dermatology at the University of Texas and MD Anderson Cancer Center, Houston.
Naloxone is a pure opiate antagonist with no agonist effects. Naloxone lotion is an investigational agent that has received orphan drug status for treatment of pruritis in CTCL and a fast-track evaluation designation from the Food and Drug Administration.
Dr. Duvic presented a double-blind, vehicle-controlled, multicenter, crossover study involving 15 CTCL patients with severe itching. They were assigned to apply naloxone lotion 0.5% or its vehicle four times daily for 8 days and then cross over to the other regimen for another 8 days following a washout period.
After 8 days of naloxone lotion, patients reported a mean 66% reduction in itch severity from baseline on a visual analog scale, a significantly better result than the 45% reduction on vehicle.
The study suffered from small numbers, as four patients withdrew during part 1 while on vehicle, two dropped out while on naloxone lotion, and one was excluded for a concomitant medication violation. Of the nine patients available for Physician Global Assessment, seven were rated better or much better. Seven of the nine patients also rated themselves as globally better or much better while on naloxone lotion. These ratings were numerically better than while patients were on vehicle.
Adverse events were limited to two cases of mild or moderate application-site erythema.
The study was funded by Elorac. Dr. Duvic reported having no financial conflicts. She noted that the University of Texas received a research grant to conduct the study.
VANCOUVER, B.C. – Naloxone lotion appears to be a safe and effective treatment for the severe chronic itching that occurs in most patients with cutaneous T-cell lymphoma, Dr. Madeleine Duvic reported at the World Congress of Dermatology.
A major unmet need exists for better treatments for pruritis in CTCL. Antihistamines are generally ineffective. Chemotherapeutic agents provide little relief. Moreover, it has been estimated that up to half of all patients with CTCL die as a result of systemic infections arising secondary to pruritic skin excoriations, according to Dr. Duvic, professor of medicine and dermatology at the University of Texas and MD Anderson Cancer Center, Houston.
Naloxone is a pure opiate antagonist with no agonist effects. Naloxone lotion is an investigational agent that has received orphan drug status for treatment of pruritis in CTCL and a fast-track evaluation designation from the Food and Drug Administration.
Dr. Duvic presented a double-blind, vehicle-controlled, multicenter, crossover study involving 15 CTCL patients with severe itching. They were assigned to apply naloxone lotion 0.5% or its vehicle four times daily for 8 days and then cross over to the other regimen for another 8 days following a washout period.
After 8 days of naloxone lotion, patients reported a mean 66% reduction in itch severity from baseline on a visual analog scale, a significantly better result than the 45% reduction on vehicle.
The study suffered from small numbers, as four patients withdrew during part 1 while on vehicle, two dropped out while on naloxone lotion, and one was excluded for a concomitant medication violation. Of the nine patients available for Physician Global Assessment, seven were rated better or much better. Seven of the nine patients also rated themselves as globally better or much better while on naloxone lotion. These ratings were numerically better than while patients were on vehicle.
Adverse events were limited to two cases of mild or moderate application-site erythema.
The study was funded by Elorac. Dr. Duvic reported having no financial conflicts. She noted that the University of Texas received a research grant to conduct the study.
VANCOUVER, B.C. – Naloxone lotion appears to be a safe and effective treatment for the severe chronic itching that occurs in most patients with cutaneous T-cell lymphoma, Dr. Madeleine Duvic reported at the World Congress of Dermatology.
A major unmet need exists for better treatments for pruritis in CTCL. Antihistamines are generally ineffective. Chemotherapeutic agents provide little relief. Moreover, it has been estimated that up to half of all patients with CTCL die as a result of systemic infections arising secondary to pruritic skin excoriations, according to Dr. Duvic, professor of medicine and dermatology at the University of Texas and MD Anderson Cancer Center, Houston.
Naloxone is a pure opiate antagonist with no agonist effects. Naloxone lotion is an investigational agent that has received orphan drug status for treatment of pruritis in CTCL and a fast-track evaluation designation from the Food and Drug Administration.
Dr. Duvic presented a double-blind, vehicle-controlled, multicenter, crossover study involving 15 CTCL patients with severe itching. They were assigned to apply naloxone lotion 0.5% or its vehicle four times daily for 8 days and then cross over to the other regimen for another 8 days following a washout period.
After 8 days of naloxone lotion, patients reported a mean 66% reduction in itch severity from baseline on a visual analog scale, a significantly better result than the 45% reduction on vehicle.
The study suffered from small numbers, as four patients withdrew during part 1 while on vehicle, two dropped out while on naloxone lotion, and one was excluded for a concomitant medication violation. Of the nine patients available for Physician Global Assessment, seven were rated better or much better. Seven of the nine patients also rated themselves as globally better or much better while on naloxone lotion. These ratings were numerically better than while patients were on vehicle.
Adverse events were limited to two cases of mild or moderate application-site erythema.
The study was funded by Elorac. Dr. Duvic reported having no financial conflicts. She noted that the University of Texas received a research grant to conduct the study.
AT WCD 2015
Key clinical point: Naloxone lotion shows promise for the severe pruritis that accompanies cutaneous T-cell lymphoma.
Major finding: Patients with cutaneous T-cell lymphoma reported an absolute 21% greater reduction in pruritis with naloxone lotion than with its vehicle.
Data source: This was a 15-patient, multicenter, double-blind, crossover study.
Disclosures: The study was funded by Elorac. The presenter reported having no financial conflicts.