User login
Pseudobulbar affect: More common than you’d think
ORLANDO – The prevalence of pseudobulbar affect symptoms – that is, uncontrollable, disruptive outbursts of crying and/or laughing – is considerably greater across a range of neurologic disorders than previously appreciated, according to the largest-ever study to screen for this condition.
Pseudobulbar affect (PBA) symptoms were found in the study to be more common among neurology patients under age 65; however, the adverse impact of PBA symptoms upon quality of life was greater in the elderly, Dr. David W. Crumpacker reported at the annual meeting of the American Association for Geriatric Psychiatry.
He presented the results of the PRISM (PBA Registry Series) study, which enrolled 5,290 patients on the basis of having any of six neurologic disorders: Alzheimer’s disease, amyotrophic lateral sclerosis (ALS), multiple sclerosis, Parkinson’s disease, stroke, or traumatic brain injury. They were screened for the presence of PBA symptoms using the validated Center for Neurologic Study–Lability Scale (CNS-LS). A score of 13 or more was deemed positive, based upon its demonstrated good predictive value for physician diagnosis of PBA in patients with ALS.
The CNS-LS is a simple test that can be completed quickly by either the patient or caregiver. The test is well-suited for routine use in clinical practice, noted Dr. W. Crumpacker, a psychiatrist at Baylor University Medical Center, Dallas.
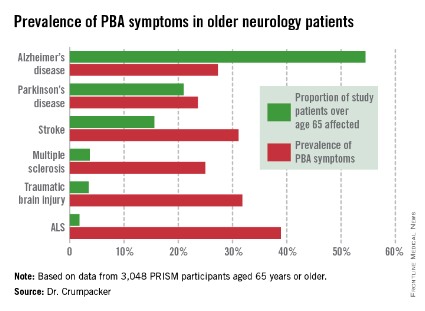
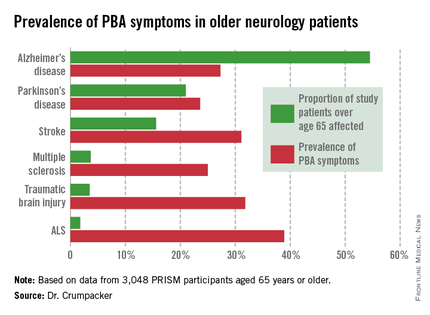
The overall prevalence of PBA symptoms among the 3,048 PRISM participants aged 65 years or older was 27.4%, with the highest rate seen in patients having ALS (see chart). In contrast, the prevalence of PBA symptoms among patients under age 65 years was 49.5%, with the highest rate – 56.9% – being seen in traumatic brain injury patients.
Patients or caregivers were asked to rate on a 0-10 scale the impact their primary neurologic disease has had on their quality of life. Patients 65 years and older with PBA symptoms reported a significantly greater negative impact than did those without PBA symptoms, with mean scores on the quality of life impact scale of 6.3 vs. 4.6. The quality of life difference between those with PBA symptoms and those without was significant for patients with each of the neurologic diseases except for ALS.
As another measure of the adverse impact of having PBA symptoms, 56% of affected older patients were on at least one antipsychotic or antidepressant, compared with 35% of older patients without PBA symptoms.
PBA is thought to result from injury to neurologic pathways that regulate emotional expression as a secondary consequence of a variety of neurologic disorders.
In an interview, Dr. Crumpacker said PBA is greatly underdiagnosed and often gets misdiagnosed as depression.
"The symptoms are extremely disturbing to others, and patients are acutely aware of that. I tell my friends in neurology, it’s the psychiatric pathology that causes people problems in their lives. No one gets divorced over neurologic pathology, they get divorced over psychiatric pathology. It’s not, ‘I got a divorce because he had a stroke.’ " "It’s "We got divorced because he had a stroke and it changed his personality; he was a different person and I couldn’t be around him anymore,’ " the psychiatrist said.
PBA became a diagnosable disorder with its own ICD-9 code, albeit a diagnosis that can’t be made in the absence of neurologic pathology, at the behest of the Food and Drug Administration, Dr. Crumpacker explained. The impetus was the discovery of an effective treatment, dextromethorphan HBr and quinidine sulfate (Nuedexta), which received FDA approval for PBA 3 years ago.
Nuedexta’s development as the sole medication indicated for PBA was serendipitous, according to Dr. Crumpacker.
"The drug was being tested in Alzheimer’s disease. The jury is still out on whether it helps. But families of study participants came back saying, ‘You know that stuff dad used to do – the crying, the inappropriate laughter, the anger? He doesn’t do those kinds of things anymore,’ " Dr. Crumpacker recalled.
He reported serving on a scientific advisory board for Avanir Pharmaceuticals, which markets Nuedexta.
ORLANDO – The prevalence of pseudobulbar affect symptoms – that is, uncontrollable, disruptive outbursts of crying and/or laughing – is considerably greater across a range of neurologic disorders than previously appreciated, according to the largest-ever study to screen for this condition.
Pseudobulbar affect (PBA) symptoms were found in the study to be more common among neurology patients under age 65; however, the adverse impact of PBA symptoms upon quality of life was greater in the elderly, Dr. David W. Crumpacker reported at the annual meeting of the American Association for Geriatric Psychiatry.

He presented the results of the PRISM (PBA Registry Series) study, which enrolled 5,290 patients on the basis of having any of six neurologic disorders: Alzheimer’s disease, amyotrophic lateral sclerosis (ALS), multiple sclerosis, Parkinson’s disease, stroke, or traumatic brain injury. They were screened for the presence of PBA symptoms using the validated Center for Neurologic Study–Lability Scale (CNS-LS). A score of 13 or more was deemed positive, based upon its demonstrated good predictive value for physician diagnosis of PBA in patients with ALS.
The CNS-LS is a simple test that can be completed quickly by either the patient or caregiver. The test is well-suited for routine use in clinical practice, noted Dr. W. Crumpacker, a psychiatrist at Baylor University Medical Center, Dallas.
The overall prevalence of PBA symptoms among the 3,048 PRISM participants aged 65 years or older was 27.4%, with the highest rate seen in patients having ALS (see chart). In contrast, the prevalence of PBA symptoms among patients under age 65 years was 49.5%, with the highest rate – 56.9% – being seen in traumatic brain injury patients.
Patients or caregivers were asked to rate on a 0-10 scale the impact their primary neurologic disease has had on their quality of life. Patients 65 years and older with PBA symptoms reported a significantly greater negative impact than did those without PBA symptoms, with mean scores on the quality of life impact scale of 6.3 vs. 4.6. The quality of life difference between those with PBA symptoms and those without was significant for patients with each of the neurologic diseases except for ALS.
As another measure of the adverse impact of having PBA symptoms, 56% of affected older patients were on at least one antipsychotic or antidepressant, compared with 35% of older patients without PBA symptoms.
PBA is thought to result from injury to neurologic pathways that regulate emotional expression as a secondary consequence of a variety of neurologic disorders.
In an interview, Dr. Crumpacker said PBA is greatly underdiagnosed and often gets misdiagnosed as depression.
"The symptoms are extremely disturbing to others, and patients are acutely aware of that. I tell my friends in neurology, it’s the psychiatric pathology that causes people problems in their lives. No one gets divorced over neurologic pathology, they get divorced over psychiatric pathology. It’s not, ‘I got a divorce because he had a stroke.’ " "It’s "We got divorced because he had a stroke and it changed his personality; he was a different person and I couldn’t be around him anymore,’ " the psychiatrist said.
PBA became a diagnosable disorder with its own ICD-9 code, albeit a diagnosis that can’t be made in the absence of neurologic pathology, at the behest of the Food and Drug Administration, Dr. Crumpacker explained. The impetus was the discovery of an effective treatment, dextromethorphan HBr and quinidine sulfate (Nuedexta), which received FDA approval for PBA 3 years ago.
Nuedexta’s development as the sole medication indicated for PBA was serendipitous, according to Dr. Crumpacker.
"The drug was being tested in Alzheimer’s disease. The jury is still out on whether it helps. But families of study participants came back saying, ‘You know that stuff dad used to do – the crying, the inappropriate laughter, the anger? He doesn’t do those kinds of things anymore,’ " Dr. Crumpacker recalled.
He reported serving on a scientific advisory board for Avanir Pharmaceuticals, which markets Nuedexta.
ORLANDO – The prevalence of pseudobulbar affect symptoms – that is, uncontrollable, disruptive outbursts of crying and/or laughing – is considerably greater across a range of neurologic disorders than previously appreciated, according to the largest-ever study to screen for this condition.
Pseudobulbar affect (PBA) symptoms were found in the study to be more common among neurology patients under age 65; however, the adverse impact of PBA symptoms upon quality of life was greater in the elderly, Dr. David W. Crumpacker reported at the annual meeting of the American Association for Geriatric Psychiatry.

He presented the results of the PRISM (PBA Registry Series) study, which enrolled 5,290 patients on the basis of having any of six neurologic disorders: Alzheimer’s disease, amyotrophic lateral sclerosis (ALS), multiple sclerosis, Parkinson’s disease, stroke, or traumatic brain injury. They were screened for the presence of PBA symptoms using the validated Center for Neurologic Study–Lability Scale (CNS-LS). A score of 13 or more was deemed positive, based upon its demonstrated good predictive value for physician diagnosis of PBA in patients with ALS.
The CNS-LS is a simple test that can be completed quickly by either the patient or caregiver. The test is well-suited for routine use in clinical practice, noted Dr. W. Crumpacker, a psychiatrist at Baylor University Medical Center, Dallas.
The overall prevalence of PBA symptoms among the 3,048 PRISM participants aged 65 years or older was 27.4%, with the highest rate seen in patients having ALS (see chart). In contrast, the prevalence of PBA symptoms among patients under age 65 years was 49.5%, with the highest rate – 56.9% – being seen in traumatic brain injury patients.
Patients or caregivers were asked to rate on a 0-10 scale the impact their primary neurologic disease has had on their quality of life. Patients 65 years and older with PBA symptoms reported a significantly greater negative impact than did those without PBA symptoms, with mean scores on the quality of life impact scale of 6.3 vs. 4.6. The quality of life difference between those with PBA symptoms and those without was significant for patients with each of the neurologic diseases except for ALS.
As another measure of the adverse impact of having PBA symptoms, 56% of affected older patients were on at least one antipsychotic or antidepressant, compared with 35% of older patients without PBA symptoms.
PBA is thought to result from injury to neurologic pathways that regulate emotional expression as a secondary consequence of a variety of neurologic disorders.
In an interview, Dr. Crumpacker said PBA is greatly underdiagnosed and often gets misdiagnosed as depression.
"The symptoms are extremely disturbing to others, and patients are acutely aware of that. I tell my friends in neurology, it’s the psychiatric pathology that causes people problems in their lives. No one gets divorced over neurologic pathology, they get divorced over psychiatric pathology. It’s not, ‘I got a divorce because he had a stroke.’ " "It’s "We got divorced because he had a stroke and it changed his personality; he was a different person and I couldn’t be around him anymore,’ " the psychiatrist said.
PBA became a diagnosable disorder with its own ICD-9 code, albeit a diagnosis that can’t be made in the absence of neurologic pathology, at the behest of the Food and Drug Administration, Dr. Crumpacker explained. The impetus was the discovery of an effective treatment, dextromethorphan HBr and quinidine sulfate (Nuedexta), which received FDA approval for PBA 3 years ago.
Nuedexta’s development as the sole medication indicated for PBA was serendipitous, according to Dr. Crumpacker.
"The drug was being tested in Alzheimer’s disease. The jury is still out on whether it helps. But families of study participants came back saying, ‘You know that stuff dad used to do – the crying, the inappropriate laughter, the anger? He doesn’t do those kinds of things anymore,’ " Dr. Crumpacker recalled.
He reported serving on a scientific advisory board for Avanir Pharmaceuticals, which markets Nuedexta.
AT THE AAGP ANNUAL MEETING
Major finding: The prevalence of PBA symptoms among patients over age 65 years with any of six underlying neurologic disorders was 27.4%. That was significantly less than in younger patients with the same disorders, but the adverse effect of having PBA symptoms upon quality of life was markedly greater in the older group.
Data source: The PRISM study included 5,290 patients with Alzheimer’s disease or any of five other less common neurologic disorders, all of whom were screened for the presence of PBA symptoms using a brief validated measure.
Disclosures: The presenter serves on a scientific advisory board for Avanir Pharmaceuticals, which funded the PRISM study.
Why the ACA makes me appreciate hospital medicine more each day
When I moved to Maryland over a decade ago, my first job was at Kaiser Permanente, where I had a panel of office patients and occasionally rounded at the hospital. Ultimately, management gave us the option of being solely office-based and giving up hospital rounds or continuing to do both. Most of my colleagues jumped at the chance to give up the grueling 24-hour shifts – a full day in the office followed by in-house night call at our hospital. Ouch!
But a little voice inside my head told me not to give up my hospital skills, and I’m so glad I listened. Little did I know that I would soon be offered a full-time hospitalist position. What a lifestyle change! I went from working Monday through Friday with occasional weekend and night shifts, counting the months until my next vacation, to working block shifts and having "vacation" time every month. What’s more, unlike my days in private practice, when I often struggled to make ends meet, I could count on a steady paycheck.
And while many of our office-based colleagues currently thrive in primary care, the Affordable Care Act has made many rethink their future. The ACA has ushered in new payment rates and regulations that make it more challenging for some small practices to stay afloat, and impossible for others.
Since the ACA was passed in 2010, many hospitals have aggressively pursued and acquired physician practices, which allows them to reap the benefits of some incentives available under the Affordable Care Act, potentially a win-win for hospitals and struggling physicians alike. In addition, many primary care physicians have joined independent accountable care organizations to mitigate the challenges and reap the potential rewards of the ACA.
But this is only the tip of the iceberg. For instance, in its recently released 2015 budget request, the administration proposed cutting an additional $2 billion from health care through decreased payments to rural hospitals, reductions to postacute care, and reimbursements for care given to those Medicare beneficiaries whose bills go unpaid. Meanwhile, the Federation of American Hospitals, an organization representing over 1,000 providers of health care, is working on a study it hopes will help persuade lawmakers to forgo the proposed cuts.
In this seemingly never-ending flux of our new health care system, it appears that we hospitalists, for the moment, are faring quite well. And, relatively unburdened by these forces of flux, we are free to focus our energies on top-notch patient care.
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center who has a passion for empowering patients to partner in their health care. She is the creator of the Patient Whiz, a patient-engagement app for iOS.
When I moved to Maryland over a decade ago, my first job was at Kaiser Permanente, where I had a panel of office patients and occasionally rounded at the hospital. Ultimately, management gave us the option of being solely office-based and giving up hospital rounds or continuing to do both. Most of my colleagues jumped at the chance to give up the grueling 24-hour shifts – a full day in the office followed by in-house night call at our hospital. Ouch!
But a little voice inside my head told me not to give up my hospital skills, and I’m so glad I listened. Little did I know that I would soon be offered a full-time hospitalist position. What a lifestyle change! I went from working Monday through Friday with occasional weekend and night shifts, counting the months until my next vacation, to working block shifts and having "vacation" time every month. What’s more, unlike my days in private practice, when I often struggled to make ends meet, I could count on a steady paycheck.
And while many of our office-based colleagues currently thrive in primary care, the Affordable Care Act has made many rethink their future. The ACA has ushered in new payment rates and regulations that make it more challenging for some small practices to stay afloat, and impossible for others.
Since the ACA was passed in 2010, many hospitals have aggressively pursued and acquired physician practices, which allows them to reap the benefits of some incentives available under the Affordable Care Act, potentially a win-win for hospitals and struggling physicians alike. In addition, many primary care physicians have joined independent accountable care organizations to mitigate the challenges and reap the potential rewards of the ACA.
But this is only the tip of the iceberg. For instance, in its recently released 2015 budget request, the administration proposed cutting an additional $2 billion from health care through decreased payments to rural hospitals, reductions to postacute care, and reimbursements for care given to those Medicare beneficiaries whose bills go unpaid. Meanwhile, the Federation of American Hospitals, an organization representing over 1,000 providers of health care, is working on a study it hopes will help persuade lawmakers to forgo the proposed cuts.
In this seemingly never-ending flux of our new health care system, it appears that we hospitalists, for the moment, are faring quite well. And, relatively unburdened by these forces of flux, we are free to focus our energies on top-notch patient care.
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center who has a passion for empowering patients to partner in their health care. She is the creator of the Patient Whiz, a patient-engagement app for iOS.
When I moved to Maryland over a decade ago, my first job was at Kaiser Permanente, where I had a panel of office patients and occasionally rounded at the hospital. Ultimately, management gave us the option of being solely office-based and giving up hospital rounds or continuing to do both. Most of my colleagues jumped at the chance to give up the grueling 24-hour shifts – a full day in the office followed by in-house night call at our hospital. Ouch!
But a little voice inside my head told me not to give up my hospital skills, and I’m so glad I listened. Little did I know that I would soon be offered a full-time hospitalist position. What a lifestyle change! I went from working Monday through Friday with occasional weekend and night shifts, counting the months until my next vacation, to working block shifts and having "vacation" time every month. What’s more, unlike my days in private practice, when I often struggled to make ends meet, I could count on a steady paycheck.
And while many of our office-based colleagues currently thrive in primary care, the Affordable Care Act has made many rethink their future. The ACA has ushered in new payment rates and regulations that make it more challenging for some small practices to stay afloat, and impossible for others.
Since the ACA was passed in 2010, many hospitals have aggressively pursued and acquired physician practices, which allows them to reap the benefits of some incentives available under the Affordable Care Act, potentially a win-win for hospitals and struggling physicians alike. In addition, many primary care physicians have joined independent accountable care organizations to mitigate the challenges and reap the potential rewards of the ACA.
But this is only the tip of the iceberg. For instance, in its recently released 2015 budget request, the administration proposed cutting an additional $2 billion from health care through decreased payments to rural hospitals, reductions to postacute care, and reimbursements for care given to those Medicare beneficiaries whose bills go unpaid. Meanwhile, the Federation of American Hospitals, an organization representing over 1,000 providers of health care, is working on a study it hopes will help persuade lawmakers to forgo the proposed cuts.
In this seemingly never-ending flux of our new health care system, it appears that we hospitalists, for the moment, are faring quite well. And, relatively unburdened by these forces of flux, we are free to focus our energies on top-notch patient care.
Dr. Hester is a hospitalist with Baltimore-Washington Medical Center who has a passion for empowering patients to partner in their health care. She is the creator of the Patient Whiz, a patient-engagement app for iOS.
Online reviews
The last time I gave the talk, "Help! I’ve Been Yelped!" to physicians, there was a full house, a sometimes defiant, sometimes incredulous but always engaged full house. Most physicians don’t like Yelp and other online doctor rating sites because of the potential for negative reviews.
In past columns, I’ve written about these sites and how to respond to negative reviews and comments. Now, I’m going to share data on the use of online reviews and why they are important.
We live in a digital world that values reviews. We compare hotels on TripAdvisor.com before booking them and read reviews on Amazon.com before ordering products. We "like" or "dislike" Facebook pages and give thumbs up or thumbs down to videos on YouTube. We even rate physicians’ comments on medical question-and-answer sites such as HealthTap.com.
But how much do all of these online ratings really matter? A 2012 Nielsen report that surveyed more than 28,000 Internet users in 56 countries found that online consumer reviews are the second-most-trusted source of brand information, following only recommendations from family and friends. In other words, we trust online reviews and use them to make our own decisions.
The same is true when it comes to shopping for a doctor. According to an Internet-based survey of 2,137 adults published in the February issue of JAMA, 59% of respondents said that online doctor ratings were either "somewhat important" or "very important" when choosing a physician (2014;11:734-5).
Similarly, the "2013 Industry View Report" by Software Advice found that 62% of respondents said they read online reviews when seeking a new doctor. Although HealthGrades.com was the most commonly used site, Yelp.com was the most trusted. Forty-four percent of those respondents considered Yelp the most trustworthy review site followed by Health Grades (31%), Vitals.com (17%), and ZocDoc.com (7%).
Whether or not we trust Yelp and other online review sites, our patients do. In the JAMA survey, 35% of respondents said that they selected a physician based on good ratings, while 37% said that they avoided a physician with negative reviews. The 2013 Industry View Report also found that 45% of respondents ranked "quality of care" as the most important type of information sought about a doctor. And since many patients equate service with quality, reviews that focus on service matter.
This isn’t an entirely bad thing. If we really listen to what patients are saying, their comments can help us to improve service and communication. And, in some instances, it can lead to stronger doctor-patient relationships. Like many other industries, health care is moving toward transparency, and doctor rating sites are a key component of that.
Dr. Jeffrey Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego and a volunteer clinical assistant professor at the University of California, San Diego. He has published numerous scientific articles and is a member and fellow of the American Academy of Dermatology, and a member of the Telemedicine Association and the American Medical Association, among others. He is board certified in dermatology as well as medicine and surgery in the state of California. Dr. Benabio has a special interest in the uses of social media for education and building dermatology practice. He is the founder of The Derm Blog, an educational website that has had more than 2 million unique visitors. Dr. Benabio is also a founding member and the skin care expert for Livestrong.com, a health and wellness website of Lance Armstrong’s the Livestrong Foundation. Dr. Benabio is @Dermdoc on Twitter.
The last time I gave the talk, "Help! I’ve Been Yelped!" to physicians, there was a full house, a sometimes defiant, sometimes incredulous but always engaged full house. Most physicians don’t like Yelp and other online doctor rating sites because of the potential for negative reviews.
In past columns, I’ve written about these sites and how to respond to negative reviews and comments. Now, I’m going to share data on the use of online reviews and why they are important.
We live in a digital world that values reviews. We compare hotels on TripAdvisor.com before booking them and read reviews on Amazon.com before ordering products. We "like" or "dislike" Facebook pages and give thumbs up or thumbs down to videos on YouTube. We even rate physicians’ comments on medical question-and-answer sites such as HealthTap.com.
But how much do all of these online ratings really matter? A 2012 Nielsen report that surveyed more than 28,000 Internet users in 56 countries found that online consumer reviews are the second-most-trusted source of brand information, following only recommendations from family and friends. In other words, we trust online reviews and use them to make our own decisions.
The same is true when it comes to shopping for a doctor. According to an Internet-based survey of 2,137 adults published in the February issue of JAMA, 59% of respondents said that online doctor ratings were either "somewhat important" or "very important" when choosing a physician (2014;11:734-5).
Similarly, the "2013 Industry View Report" by Software Advice found that 62% of respondents said they read online reviews when seeking a new doctor. Although HealthGrades.com was the most commonly used site, Yelp.com was the most trusted. Forty-four percent of those respondents considered Yelp the most trustworthy review site followed by Health Grades (31%), Vitals.com (17%), and ZocDoc.com (7%).
Whether or not we trust Yelp and other online review sites, our patients do. In the JAMA survey, 35% of respondents said that they selected a physician based on good ratings, while 37% said that they avoided a physician with negative reviews. The 2013 Industry View Report also found that 45% of respondents ranked "quality of care" as the most important type of information sought about a doctor. And since many patients equate service with quality, reviews that focus on service matter.
This isn’t an entirely bad thing. If we really listen to what patients are saying, their comments can help us to improve service and communication. And, in some instances, it can lead to stronger doctor-patient relationships. Like many other industries, health care is moving toward transparency, and doctor rating sites are a key component of that.
Dr. Jeffrey Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego and a volunteer clinical assistant professor at the University of California, San Diego. He has published numerous scientific articles and is a member and fellow of the American Academy of Dermatology, and a member of the Telemedicine Association and the American Medical Association, among others. He is board certified in dermatology as well as medicine and surgery in the state of California. Dr. Benabio has a special interest in the uses of social media for education and building dermatology practice. He is the founder of The Derm Blog, an educational website that has had more than 2 million unique visitors. Dr. Benabio is also a founding member and the skin care expert for Livestrong.com, a health and wellness website of Lance Armstrong’s the Livestrong Foundation. Dr. Benabio is @Dermdoc on Twitter.
The last time I gave the talk, "Help! I’ve Been Yelped!" to physicians, there was a full house, a sometimes defiant, sometimes incredulous but always engaged full house. Most physicians don’t like Yelp and other online doctor rating sites because of the potential for negative reviews.
In past columns, I’ve written about these sites and how to respond to negative reviews and comments. Now, I’m going to share data on the use of online reviews and why they are important.
We live in a digital world that values reviews. We compare hotels on TripAdvisor.com before booking them and read reviews on Amazon.com before ordering products. We "like" or "dislike" Facebook pages and give thumbs up or thumbs down to videos on YouTube. We even rate physicians’ comments on medical question-and-answer sites such as HealthTap.com.
But how much do all of these online ratings really matter? A 2012 Nielsen report that surveyed more than 28,000 Internet users in 56 countries found that online consumer reviews are the second-most-trusted source of brand information, following only recommendations from family and friends. In other words, we trust online reviews and use them to make our own decisions.
The same is true when it comes to shopping for a doctor. According to an Internet-based survey of 2,137 adults published in the February issue of JAMA, 59% of respondents said that online doctor ratings were either "somewhat important" or "very important" when choosing a physician (2014;11:734-5).
Similarly, the "2013 Industry View Report" by Software Advice found that 62% of respondents said they read online reviews when seeking a new doctor. Although HealthGrades.com was the most commonly used site, Yelp.com was the most trusted. Forty-four percent of those respondents considered Yelp the most trustworthy review site followed by Health Grades (31%), Vitals.com (17%), and ZocDoc.com (7%).
Whether or not we trust Yelp and other online review sites, our patients do. In the JAMA survey, 35% of respondents said that they selected a physician based on good ratings, while 37% said that they avoided a physician with negative reviews. The 2013 Industry View Report also found that 45% of respondents ranked "quality of care" as the most important type of information sought about a doctor. And since many patients equate service with quality, reviews that focus on service matter.
This isn’t an entirely bad thing. If we really listen to what patients are saying, their comments can help us to improve service and communication. And, in some instances, it can lead to stronger doctor-patient relationships. Like many other industries, health care is moving toward transparency, and doctor rating sites are a key component of that.
Dr. Jeffrey Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego and a volunteer clinical assistant professor at the University of California, San Diego. He has published numerous scientific articles and is a member and fellow of the American Academy of Dermatology, and a member of the Telemedicine Association and the American Medical Association, among others. He is board certified in dermatology as well as medicine and surgery in the state of California. Dr. Benabio has a special interest in the uses of social media for education and building dermatology practice. He is the founder of The Derm Blog, an educational website that has had more than 2 million unique visitors. Dr. Benabio is also a founding member and the skin care expert for Livestrong.com, a health and wellness website of Lance Armstrong’s the Livestrong Foundation. Dr. Benabio is @Dermdoc on Twitter.
Cervical cancer screening
Numerous screening methods for cervical cancer have been proposed internationally by various professional societies, including Pap cytology alone, cytology with human papillomavirus testing as triage (HPV testing for atypical squamous cells of unknown significance [ASCUS] on cytology), cytology with HPV cotesting (cytology and HPV testing obtained together), HPV testing alone, or HPV testing followed by Pap cytology triage (cytology in patients who are positive for high-risk oncogenic subtypes of HPV). Recommendations for use of cervical cytology and HPV testing continue to vary among professional societies, with variable adoption of these guidelines by providers as well. (Am. J. Prev. Med. 2013;45:175-81).
In 2012, updated cervical cancer screening recommendations were published by ASCCP (the American Society for Colposcopy and Cervical Pathology) (Am. J. Clin. Pathol. 2012;137:516-42); the USPSTF (U.S. Preventive Services Task Force ); and ACOG (the American College of Obstetricians and Gynecologists) (Obstet. Gynecol. 2009;114:1409-20).
These most recent guidelines show a greater degree of harmony across these governing bodies than did prior guidelines. All three professional societies recommend initiating screening at age 21 years and ceasing screening at age 65 years with an adequate screening history. All groups recommend against HPV cotesting in women under 30 years of age; however, after age 30 years, ASCCP and ACOG recommend HPV cotesting every 5 years as the preferred method of cervical cancer screening, while USPSTF suggests this only as an "option." Primary HPV testing without concurrent cytology for cervical cancer screening is not currently recommended by ASCCP and USPSTF and is not addressed by ACOG.
Efficacy of screening modalities
The rationale behind these screening recommendations depends on the efficacy of both cervical cytology and HPV testing to identify preinvasive cases or invasive cervical cancer. Multiple studies have addressed the sensitivity and specificity of cytology in cervical cancer screening. Overall, the sensitivity of Pap cytology is low at approximately 51%, while specificity is high at 96%-98% (Ann. Intern. Med. 2000;132:810-9; Vaccine 2008;26 Suppl. 10:K29-41). Since the initiation of cervical cytology for cancer screening, serial annual screening has compensated for the overall poor sensitivity of the test. Two consecutive annual Pap tests can increase overall sensitivity for detection of cervical cancer to 76%, and three consecutive annual Pap tests can increase overall sensitivity to 88%.
Unlike Pap cytology, HPV testing has a high sensitivity, ranging from 81%-97% in detection of cervical cancer (N. Engl. J. Med. 2007;357:1579-88). As a result, HPV testing does not rely on serial testing for accuracy and has a high negative predictive value, making negative results very reassuring. However, HPV testing has a slightly lower specificity of 94%, which results in a higher number of false positives. Furthermore, many patients who screen positive for high-risk HPV subtypes may have transient HPV infections, which are not clinically significant, and may not cause invasive cervical cancer.
Several randomized studies have compared Pap cytology to HPV testing for use in cervical cancer screening. A Canadian study randomized more than 10,000 women to either Pap cytology or HPV testing to detect cervical intraepithelial neoplasia (CIN) 2 or higher grade cervical lesions (Int. J. Cancer. 2006;119:615-23). Findings showed a sensitivity of 55.4% for Pap cytology vs. 94.6% for HPV testing. Pap cytology had a specificity of 96.8% while HPV testing had a specificity of 94.1%. The negative predictive value of HPV testing was 100%.
Swedescreen, a Swedish study of more 12,000 women (J. Med. Virol. 2007;79:1169-75), and POBASCAM, a large Dutch study of more than 18,000 women (Lancet 2007;370:1764-72), both compared HPV testing combined with Pap cytology (cotesting) to cytology alone. Both studies found that patients screened with Pap cytology alone had more CIN2 or greater lesions in follow-up than did patients screened with cytology in combination with HPV testing (relative risk, 0.53-0.58 for CIN 2+ and RR 0.45-0.53 for CIN 3+) (J. Natl. Cancer Inst. 2009;101:88-99).
Because of the higher sensitivity of HPV testing compared with Pap cytology, some have advocated the use of HPV testing as primary screening with cytology triage rather than the reverse (cytology with HPV triage), which is more commonly used today. A Finnish study showed that primary HPV testing with cytology performed only in patients who screened positive for high risk oncogenic subtypes of HPV was more sensitive than was conventional cytology in identifying cervical dysplasia and cancer. Additionally, in women over age 35 years, HPV testing combined with Pap cytology triage was more specific than cytology alone, and decreased colposcopy referrals and follow-up tests, making this screening option cost effective (J. Natl. Cancer Inst. 2009;101:1612-23). Nowhere else in medicine is a more specific test used prior to a more sensitive test when screening for disease; the screening test is typically the more sensitive, while the confirmatory test is the more specific.
HPV vaccination and effects on screening
Currently, given that the HPV vaccines available do not protect women from all oncogenic HPV types, the ASCCP, USPSTF, and ACOG all recommend screening vaccinated women in an identical fashion to unvaccinated women. Increasing vaccination rates will likely have an impact on the efficacy of the various cervical cancer screening modalities. Vaccination will result in a reduction in the prevalence of cytologic abnormalities. As disease prevalence decreases and screening intervals increase based on current guidelines, the positive predictive value of Pap cytology also will decline, resulting in more false-positive diagnoses and possibly unnecessary procedures and patient stress (Vaccine 2013;31:5495-9). As prevalence of disease decreases, Pap cytology has the potential to become less reliable. While the positive predictive value of HPV testing also declines with decreasing disease prevalence, HPV testing is more reproducible than interpretation of Pap cytology, so the extent of increasing false-positive results may be less (Vaccine 2006;24 Suppl 3:S3/171-7).
Future directions
HPV testing as primary screening for cervical cancer is not currently recommended. However, in the post-HPV vaccination era, this may become an increasingly reasonable approach, particularly in conjunction with Pap cytology used to triage patients who test positive for high-risk HPV subtypes. HPV testing has much greater sensitivity than Pap cytology does and can better identify patients who are likely to have a cytologic abnormality. In this group of patients with greater disease prevalence, the slightly higher specificity of Pap cytology can then be used to identify precancerous lesions and guide treatment. Once this group of patients with higher lesion prevalence than the general population has been identified through HPV testing, Pap cytology can then be used and will perform better than in a lower prevalence population.
The importance of Pap cytology and HPV testing in cervical cancer screening continues to evolve, particularly in the current era of HPV vaccination. The combination of HPV testing followed by Pap cytology has potential for becoming a highly effective screening strategy; however, the optimal administration of these tests is yet to be determined. As current screening modalities improve and new technologies emerge, ongoing work is needed to identify the most effective screening method for cervical cancer.
Dr. Wysham is currently a fellow in the department of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Kim is the department of gynecologic oncology at UNC-Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at UNC-Chapel Hill.
Numerous screening methods for cervical cancer have been proposed internationally by various professional societies, including Pap cytology alone, cytology with human papillomavirus testing as triage (HPV testing for atypical squamous cells of unknown significance [ASCUS] on cytology), cytology with HPV cotesting (cytology and HPV testing obtained together), HPV testing alone, or HPV testing followed by Pap cytology triage (cytology in patients who are positive for high-risk oncogenic subtypes of HPV). Recommendations for use of cervical cytology and HPV testing continue to vary among professional societies, with variable adoption of these guidelines by providers as well. (Am. J. Prev. Med. 2013;45:175-81).
In 2012, updated cervical cancer screening recommendations were published by ASCCP (the American Society for Colposcopy and Cervical Pathology) (Am. J. Clin. Pathol. 2012;137:516-42); the USPSTF (U.S. Preventive Services Task Force ); and ACOG (the American College of Obstetricians and Gynecologists) (Obstet. Gynecol. 2009;114:1409-20).
These most recent guidelines show a greater degree of harmony across these governing bodies than did prior guidelines. All three professional societies recommend initiating screening at age 21 years and ceasing screening at age 65 years with an adequate screening history. All groups recommend against HPV cotesting in women under 30 years of age; however, after age 30 years, ASCCP and ACOG recommend HPV cotesting every 5 years as the preferred method of cervical cancer screening, while USPSTF suggests this only as an "option." Primary HPV testing without concurrent cytology for cervical cancer screening is not currently recommended by ASCCP and USPSTF and is not addressed by ACOG.
Efficacy of screening modalities
The rationale behind these screening recommendations depends on the efficacy of both cervical cytology and HPV testing to identify preinvasive cases or invasive cervical cancer. Multiple studies have addressed the sensitivity and specificity of cytology in cervical cancer screening. Overall, the sensitivity of Pap cytology is low at approximately 51%, while specificity is high at 96%-98% (Ann. Intern. Med. 2000;132:810-9; Vaccine 2008;26 Suppl. 10:K29-41). Since the initiation of cervical cytology for cancer screening, serial annual screening has compensated for the overall poor sensitivity of the test. Two consecutive annual Pap tests can increase overall sensitivity for detection of cervical cancer to 76%, and three consecutive annual Pap tests can increase overall sensitivity to 88%.
Unlike Pap cytology, HPV testing has a high sensitivity, ranging from 81%-97% in detection of cervical cancer (N. Engl. J. Med. 2007;357:1579-88). As a result, HPV testing does not rely on serial testing for accuracy and has a high negative predictive value, making negative results very reassuring. However, HPV testing has a slightly lower specificity of 94%, which results in a higher number of false positives. Furthermore, many patients who screen positive for high-risk HPV subtypes may have transient HPV infections, which are not clinically significant, and may not cause invasive cervical cancer.
Several randomized studies have compared Pap cytology to HPV testing for use in cervical cancer screening. A Canadian study randomized more than 10,000 women to either Pap cytology or HPV testing to detect cervical intraepithelial neoplasia (CIN) 2 or higher grade cervical lesions (Int. J. Cancer. 2006;119:615-23). Findings showed a sensitivity of 55.4% for Pap cytology vs. 94.6% for HPV testing. Pap cytology had a specificity of 96.8% while HPV testing had a specificity of 94.1%. The negative predictive value of HPV testing was 100%.
Swedescreen, a Swedish study of more 12,000 women (J. Med. Virol. 2007;79:1169-75), and POBASCAM, a large Dutch study of more than 18,000 women (Lancet 2007;370:1764-72), both compared HPV testing combined with Pap cytology (cotesting) to cytology alone. Both studies found that patients screened with Pap cytology alone had more CIN2 or greater lesions in follow-up than did patients screened with cytology in combination with HPV testing (relative risk, 0.53-0.58 for CIN 2+ and RR 0.45-0.53 for CIN 3+) (J. Natl. Cancer Inst. 2009;101:88-99).
Because of the higher sensitivity of HPV testing compared with Pap cytology, some have advocated the use of HPV testing as primary screening with cytology triage rather than the reverse (cytology with HPV triage), which is more commonly used today. A Finnish study showed that primary HPV testing with cytology performed only in patients who screened positive for high risk oncogenic subtypes of HPV was more sensitive than was conventional cytology in identifying cervical dysplasia and cancer. Additionally, in women over age 35 years, HPV testing combined with Pap cytology triage was more specific than cytology alone, and decreased colposcopy referrals and follow-up tests, making this screening option cost effective (J. Natl. Cancer Inst. 2009;101:1612-23). Nowhere else in medicine is a more specific test used prior to a more sensitive test when screening for disease; the screening test is typically the more sensitive, while the confirmatory test is the more specific.
HPV vaccination and effects on screening
Currently, given that the HPV vaccines available do not protect women from all oncogenic HPV types, the ASCCP, USPSTF, and ACOG all recommend screening vaccinated women in an identical fashion to unvaccinated women. Increasing vaccination rates will likely have an impact on the efficacy of the various cervical cancer screening modalities. Vaccination will result in a reduction in the prevalence of cytologic abnormalities. As disease prevalence decreases and screening intervals increase based on current guidelines, the positive predictive value of Pap cytology also will decline, resulting in more false-positive diagnoses and possibly unnecessary procedures and patient stress (Vaccine 2013;31:5495-9). As prevalence of disease decreases, Pap cytology has the potential to become less reliable. While the positive predictive value of HPV testing also declines with decreasing disease prevalence, HPV testing is more reproducible than interpretation of Pap cytology, so the extent of increasing false-positive results may be less (Vaccine 2006;24 Suppl 3:S3/171-7).
Future directions
HPV testing as primary screening for cervical cancer is not currently recommended. However, in the post-HPV vaccination era, this may become an increasingly reasonable approach, particularly in conjunction with Pap cytology used to triage patients who test positive for high-risk HPV subtypes. HPV testing has much greater sensitivity than Pap cytology does and can better identify patients who are likely to have a cytologic abnormality. In this group of patients with greater disease prevalence, the slightly higher specificity of Pap cytology can then be used to identify precancerous lesions and guide treatment. Once this group of patients with higher lesion prevalence than the general population has been identified through HPV testing, Pap cytology can then be used and will perform better than in a lower prevalence population.
The importance of Pap cytology and HPV testing in cervical cancer screening continues to evolve, particularly in the current era of HPV vaccination. The combination of HPV testing followed by Pap cytology has potential for becoming a highly effective screening strategy; however, the optimal administration of these tests is yet to be determined. As current screening modalities improve and new technologies emerge, ongoing work is needed to identify the most effective screening method for cervical cancer.
Dr. Wysham is currently a fellow in the department of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Kim is the department of gynecologic oncology at UNC-Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at UNC-Chapel Hill.
Numerous screening methods for cervical cancer have been proposed internationally by various professional societies, including Pap cytology alone, cytology with human papillomavirus testing as triage (HPV testing for atypical squamous cells of unknown significance [ASCUS] on cytology), cytology with HPV cotesting (cytology and HPV testing obtained together), HPV testing alone, or HPV testing followed by Pap cytology triage (cytology in patients who are positive for high-risk oncogenic subtypes of HPV). Recommendations for use of cervical cytology and HPV testing continue to vary among professional societies, with variable adoption of these guidelines by providers as well. (Am. J. Prev. Med. 2013;45:175-81).
In 2012, updated cervical cancer screening recommendations were published by ASCCP (the American Society for Colposcopy and Cervical Pathology) (Am. J. Clin. Pathol. 2012;137:516-42); the USPSTF (U.S. Preventive Services Task Force ); and ACOG (the American College of Obstetricians and Gynecologists) (Obstet. Gynecol. 2009;114:1409-20).
These most recent guidelines show a greater degree of harmony across these governing bodies than did prior guidelines. All three professional societies recommend initiating screening at age 21 years and ceasing screening at age 65 years with an adequate screening history. All groups recommend against HPV cotesting in women under 30 years of age; however, after age 30 years, ASCCP and ACOG recommend HPV cotesting every 5 years as the preferred method of cervical cancer screening, while USPSTF suggests this only as an "option." Primary HPV testing without concurrent cytology for cervical cancer screening is not currently recommended by ASCCP and USPSTF and is not addressed by ACOG.
Efficacy of screening modalities
The rationale behind these screening recommendations depends on the efficacy of both cervical cytology and HPV testing to identify preinvasive cases or invasive cervical cancer. Multiple studies have addressed the sensitivity and specificity of cytology in cervical cancer screening. Overall, the sensitivity of Pap cytology is low at approximately 51%, while specificity is high at 96%-98% (Ann. Intern. Med. 2000;132:810-9; Vaccine 2008;26 Suppl. 10:K29-41). Since the initiation of cervical cytology for cancer screening, serial annual screening has compensated for the overall poor sensitivity of the test. Two consecutive annual Pap tests can increase overall sensitivity for detection of cervical cancer to 76%, and three consecutive annual Pap tests can increase overall sensitivity to 88%.
Unlike Pap cytology, HPV testing has a high sensitivity, ranging from 81%-97% in detection of cervical cancer (N. Engl. J. Med. 2007;357:1579-88). As a result, HPV testing does not rely on serial testing for accuracy and has a high negative predictive value, making negative results very reassuring. However, HPV testing has a slightly lower specificity of 94%, which results in a higher number of false positives. Furthermore, many patients who screen positive for high-risk HPV subtypes may have transient HPV infections, which are not clinically significant, and may not cause invasive cervical cancer.
Several randomized studies have compared Pap cytology to HPV testing for use in cervical cancer screening. A Canadian study randomized more than 10,000 women to either Pap cytology or HPV testing to detect cervical intraepithelial neoplasia (CIN) 2 or higher grade cervical lesions (Int. J. Cancer. 2006;119:615-23). Findings showed a sensitivity of 55.4% for Pap cytology vs. 94.6% for HPV testing. Pap cytology had a specificity of 96.8% while HPV testing had a specificity of 94.1%. The negative predictive value of HPV testing was 100%.
Swedescreen, a Swedish study of more 12,000 women (J. Med. Virol. 2007;79:1169-75), and POBASCAM, a large Dutch study of more than 18,000 women (Lancet 2007;370:1764-72), both compared HPV testing combined with Pap cytology (cotesting) to cytology alone. Both studies found that patients screened with Pap cytology alone had more CIN2 or greater lesions in follow-up than did patients screened with cytology in combination with HPV testing (relative risk, 0.53-0.58 for CIN 2+ and RR 0.45-0.53 for CIN 3+) (J. Natl. Cancer Inst. 2009;101:88-99).
Because of the higher sensitivity of HPV testing compared with Pap cytology, some have advocated the use of HPV testing as primary screening with cytology triage rather than the reverse (cytology with HPV triage), which is more commonly used today. A Finnish study showed that primary HPV testing with cytology performed only in patients who screened positive for high risk oncogenic subtypes of HPV was more sensitive than was conventional cytology in identifying cervical dysplasia and cancer. Additionally, in women over age 35 years, HPV testing combined with Pap cytology triage was more specific than cytology alone, and decreased colposcopy referrals and follow-up tests, making this screening option cost effective (J. Natl. Cancer Inst. 2009;101:1612-23). Nowhere else in medicine is a more specific test used prior to a more sensitive test when screening for disease; the screening test is typically the more sensitive, while the confirmatory test is the more specific.
HPV vaccination and effects on screening
Currently, given that the HPV vaccines available do not protect women from all oncogenic HPV types, the ASCCP, USPSTF, and ACOG all recommend screening vaccinated women in an identical fashion to unvaccinated women. Increasing vaccination rates will likely have an impact on the efficacy of the various cervical cancer screening modalities. Vaccination will result in a reduction in the prevalence of cytologic abnormalities. As disease prevalence decreases and screening intervals increase based on current guidelines, the positive predictive value of Pap cytology also will decline, resulting in more false-positive diagnoses and possibly unnecessary procedures and patient stress (Vaccine 2013;31:5495-9). As prevalence of disease decreases, Pap cytology has the potential to become less reliable. While the positive predictive value of HPV testing also declines with decreasing disease prevalence, HPV testing is more reproducible than interpretation of Pap cytology, so the extent of increasing false-positive results may be less (Vaccine 2006;24 Suppl 3:S3/171-7).
Future directions
HPV testing as primary screening for cervical cancer is not currently recommended. However, in the post-HPV vaccination era, this may become an increasingly reasonable approach, particularly in conjunction with Pap cytology used to triage patients who test positive for high-risk HPV subtypes. HPV testing has much greater sensitivity than Pap cytology does and can better identify patients who are likely to have a cytologic abnormality. In this group of patients with greater disease prevalence, the slightly higher specificity of Pap cytology can then be used to identify precancerous lesions and guide treatment. Once this group of patients with higher lesion prevalence than the general population has been identified through HPV testing, Pap cytology can then be used and will perform better than in a lower prevalence population.
The importance of Pap cytology and HPV testing in cervical cancer screening continues to evolve, particularly in the current era of HPV vaccination. The combination of HPV testing followed by Pap cytology has potential for becoming a highly effective screening strategy; however, the optimal administration of these tests is yet to be determined. As current screening modalities improve and new technologies emerge, ongoing work is needed to identify the most effective screening method for cervical cancer.
Dr. Wysham is currently a fellow in the department of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Kim is the department of gynecologic oncology at UNC-Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at UNC-Chapel Hill.
Preventing Weight Loss in Patients With Dementia
The link between dementia and weight loss has been established: Weight loss is associated with even mild dementia, increasing with advancing disease severity and duration. But with a large multicountry study, researchers from the National Institutes of Health in Bethesda, Maryland, King’s College London and Newcastle University, both in the United Kingdom, and Universidad Nacional Pedro Henriquez Ureña in Santo Domingo, Dominican Republic, add new information about the universality of the association.
The researchers surveyed 16,538 older adults, asking them or a caregiver whether the patient had lost ≥ 10 pounds in the previous 3 months. The prevalence of weight loss ranged from 2% in China to 26% in the Dominican Republic and was lowest for participants with no dementia and highest in those with a Clinical Dementia Rating Scale Severity of two-thirds in all countries. The association increased and strengthened linearly through stages of dementia severity in all the study countries.
Weight loss in people with dementia can lead to further morbidity, worse prognosis, and death, the researchers note. They call for more studies, but in the meantime, they emphasize that treating and preventing weight loss is critical, particularly for institutionalized dementia patients.
One intervention that could help is giving patients nutritionally complete oral supplements, say researchers from The Royal Berkshire Hospital NHS Foundation Trust and the University of Reading, both in the United Kingdom. They reviewed 12 studies involving 1,824 patients. Most interventions, which ranged from 3 weeks to 1 year, were compared with a normal diet and care.
Two findings had to do with how weight loss is measured in older people. Skin-fold thickness and arm muscle circumference are not affected by supplement use, the researchers found. They add that those anthropometric measurements have a low level of reproducibility and are not an accurate method for obtaining evidence of changes in body composition (mid-arm muscle circumference is calculated from the skin-fold measurements). Further, measuring by body mass index (BMI) is less accurate in older patients, they note, when determining fat mass and subsequent nutritional status due to changes in height and age-related redistribution of fat mass.
However, the studies were short enough to allow BMI and weight measurements to detect the influence of the supplements. And the conclusion was that the nutritional supplement drinks had positive effects on weight gain and BMI. Overall, the consumption was “fairly good”; however, consumption was lowest in one of the longest studies. That might have been due to changes in staff behavior—increased vigilance and verbal prompting, common in the shorter studies, may have dropped off in longer studies.
Supplement use was significantly associated with improved overall energy intake and a small but statistically significant change in weight and BMI (P < .0001). However, where the control was a macro- or micronutrient supplement, findings were less positive. That may indicate that comparisons of nutritional supplements to vitamin/mineral tablets and high protein-calorie shots needs more research, the researchers conclude.
Sources
Albanese E, Taylor C, Siervo M, Stewart R, Prince MJ, Acosta D. Alzheimer’s Dement. 2013;9(6):649-656.
doi: 10.1016/j.jalz.2012.11.014.
Allen VJ, Methven L, Gosney MA. Clin Nutr. 2013;32(6):950-957.
doi: 10.1016/j.clnu.2013.03.015.
The link between dementia and weight loss has been established: Weight loss is associated with even mild dementia, increasing with advancing disease severity and duration. But with a large multicountry study, researchers from the National Institutes of Health in Bethesda, Maryland, King’s College London and Newcastle University, both in the United Kingdom, and Universidad Nacional Pedro Henriquez Ureña in Santo Domingo, Dominican Republic, add new information about the universality of the association.
The researchers surveyed 16,538 older adults, asking them or a caregiver whether the patient had lost ≥ 10 pounds in the previous 3 months. The prevalence of weight loss ranged from 2% in China to 26% in the Dominican Republic and was lowest for participants with no dementia and highest in those with a Clinical Dementia Rating Scale Severity of two-thirds in all countries. The association increased and strengthened linearly through stages of dementia severity in all the study countries.
Weight loss in people with dementia can lead to further morbidity, worse prognosis, and death, the researchers note. They call for more studies, but in the meantime, they emphasize that treating and preventing weight loss is critical, particularly for institutionalized dementia patients.
One intervention that could help is giving patients nutritionally complete oral supplements, say researchers from The Royal Berkshire Hospital NHS Foundation Trust and the University of Reading, both in the United Kingdom. They reviewed 12 studies involving 1,824 patients. Most interventions, which ranged from 3 weeks to 1 year, were compared with a normal diet and care.
Two findings had to do with how weight loss is measured in older people. Skin-fold thickness and arm muscle circumference are not affected by supplement use, the researchers found. They add that those anthropometric measurements have a low level of reproducibility and are not an accurate method for obtaining evidence of changes in body composition (mid-arm muscle circumference is calculated from the skin-fold measurements). Further, measuring by body mass index (BMI) is less accurate in older patients, they note, when determining fat mass and subsequent nutritional status due to changes in height and age-related redistribution of fat mass.
However, the studies were short enough to allow BMI and weight measurements to detect the influence of the supplements. And the conclusion was that the nutritional supplement drinks had positive effects on weight gain and BMI. Overall, the consumption was “fairly good”; however, consumption was lowest in one of the longest studies. That might have been due to changes in staff behavior—increased vigilance and verbal prompting, common in the shorter studies, may have dropped off in longer studies.
Supplement use was significantly associated with improved overall energy intake and a small but statistically significant change in weight and BMI (P < .0001). However, where the control was a macro- or micronutrient supplement, findings were less positive. That may indicate that comparisons of nutritional supplements to vitamin/mineral tablets and high protein-calorie shots needs more research, the researchers conclude.
Sources
Albanese E, Taylor C, Siervo M, Stewart R, Prince MJ, Acosta D. Alzheimer’s Dement. 2013;9(6):649-656.
doi: 10.1016/j.jalz.2012.11.014.
Allen VJ, Methven L, Gosney MA. Clin Nutr. 2013;32(6):950-957.
doi: 10.1016/j.clnu.2013.03.015.
The link between dementia and weight loss has been established: Weight loss is associated with even mild dementia, increasing with advancing disease severity and duration. But with a large multicountry study, researchers from the National Institutes of Health in Bethesda, Maryland, King’s College London and Newcastle University, both in the United Kingdom, and Universidad Nacional Pedro Henriquez Ureña in Santo Domingo, Dominican Republic, add new information about the universality of the association.
The researchers surveyed 16,538 older adults, asking them or a caregiver whether the patient had lost ≥ 10 pounds in the previous 3 months. The prevalence of weight loss ranged from 2% in China to 26% in the Dominican Republic and was lowest for participants with no dementia and highest in those with a Clinical Dementia Rating Scale Severity of two-thirds in all countries. The association increased and strengthened linearly through stages of dementia severity in all the study countries.
Weight loss in people with dementia can lead to further morbidity, worse prognosis, and death, the researchers note. They call for more studies, but in the meantime, they emphasize that treating and preventing weight loss is critical, particularly for institutionalized dementia patients.
One intervention that could help is giving patients nutritionally complete oral supplements, say researchers from The Royal Berkshire Hospital NHS Foundation Trust and the University of Reading, both in the United Kingdom. They reviewed 12 studies involving 1,824 patients. Most interventions, which ranged from 3 weeks to 1 year, were compared with a normal diet and care.
Two findings had to do with how weight loss is measured in older people. Skin-fold thickness and arm muscle circumference are not affected by supplement use, the researchers found. They add that those anthropometric measurements have a low level of reproducibility and are not an accurate method for obtaining evidence of changes in body composition (mid-arm muscle circumference is calculated from the skin-fold measurements). Further, measuring by body mass index (BMI) is less accurate in older patients, they note, when determining fat mass and subsequent nutritional status due to changes in height and age-related redistribution of fat mass.
However, the studies were short enough to allow BMI and weight measurements to detect the influence of the supplements. And the conclusion was that the nutritional supplement drinks had positive effects on weight gain and BMI. Overall, the consumption was “fairly good”; however, consumption was lowest in one of the longest studies. That might have been due to changes in staff behavior—increased vigilance and verbal prompting, common in the shorter studies, may have dropped off in longer studies.
Supplement use was significantly associated with improved overall energy intake and a small but statistically significant change in weight and BMI (P < .0001). However, where the control was a macro- or micronutrient supplement, findings were less positive. That may indicate that comparisons of nutritional supplements to vitamin/mineral tablets and high protein-calorie shots needs more research, the researchers conclude.
Sources
Albanese E, Taylor C, Siervo M, Stewart R, Prince MJ, Acosta D. Alzheimer’s Dement. 2013;9(6):649-656.
doi: 10.1016/j.jalz.2012.11.014.
Allen VJ, Methven L, Gosney MA. Clin Nutr. 2013;32(6):950-957.
doi: 10.1016/j.clnu.2013.03.015.
FDA approves IV formulation of antifungal agent

The US Food and Drug Administration has approved an intravenous formulation of posaconazole (Noxafil), which is expected to be available at wholesalers in mid-April.
The antifungal agent is already available as delayed-release tablets and in an oral suspension formulation.
In any formulation, posaconazole is indicated for prophylaxis of invasive Aspergillus and Candida infections in immunocompromised patients who are at high risk of developing these infections.
This includes patients who have developed graft-vs-host disease after hematopoietic stem cell transplant and patients with hematologic malignancies who have prolonged neutropenia resulting from chemotherapy.
Posaconazole injection is indicated for use in patients 18 years of age and older. The delayed-release tablets and oral suspension are indicated for patients 13 years of age and older.
Posaconazole injection is administered with a loading dose of 300 mg (one 300 mg vial) twice a day on the first day of therapy, then 300 mg once a day thereafter. It is given through a central venous line by slow intravenous infusion over approximately 90 minutes.
Once combined with a mixture of intravenous solution (150 mL of 5% dextrose in water or sodium chloride 0.9%), posaconazole injection should be administered immediately. If not used immediately, the solution can be stored up to 24 hours if refrigerated at 2-8 degrees C (36-46 degrees F).
Co-administration of drugs that can decrease the plasma concentration of posaconazole should be avoided unless the benefit outweighs the risk. If such drugs are necessary, patients should be monitored closely for breakthrough fungal infections.
In clinical trials, the adverse reactions reported for posaconazole injection were generally similar to those reported in trials of posaconazole oral suspension. The most frequently reported adverse reactions with an onset during the posaconazole intravenous phase of dosing 300 mg once-daily therapy were diarrhea (32%), hypokalemia (22%), fever (21%), and nausea (19%).
Patients who are allergic to posaconazole or other azole antifungal medicines should not receive posaconazole. The drug should not be given along with sirolimus, pimozide, quinidine, atorvastatin, lovastatin, simvastatin, or ergot alkaloids.
Drugs such as cyclosporine and tacrolimus require dose adjustments and frequent blood monitoring when administered with posaconazole. Serious side effects, including nephrotoxicity, leukoencephalopathy, and death, have been reported in patients with increased cyclosporine or tacrolimus blood levels.
Healthcare professionals should use caution when administering posaconazole to patients at risk of developing an irregular heart rhythm, as the drug has been shown to prolong the QT interval, and cases of potentially fatal irregular heart rhythm (torsades de pointes) have been reported in patients taking posaconazole.
For more details, see the complete prescribing information. Posaconazole is marketed as Noxafil by Merck. ![]()

The US Food and Drug Administration has approved an intravenous formulation of posaconazole (Noxafil), which is expected to be available at wholesalers in mid-April.
The antifungal agent is already available as delayed-release tablets and in an oral suspension formulation.
In any formulation, posaconazole is indicated for prophylaxis of invasive Aspergillus and Candida infections in immunocompromised patients who are at high risk of developing these infections.
This includes patients who have developed graft-vs-host disease after hematopoietic stem cell transplant and patients with hematologic malignancies who have prolonged neutropenia resulting from chemotherapy.
Posaconazole injection is indicated for use in patients 18 years of age and older. The delayed-release tablets and oral suspension are indicated for patients 13 years of age and older.
Posaconazole injection is administered with a loading dose of 300 mg (one 300 mg vial) twice a day on the first day of therapy, then 300 mg once a day thereafter. It is given through a central venous line by slow intravenous infusion over approximately 90 minutes.
Once combined with a mixture of intravenous solution (150 mL of 5% dextrose in water or sodium chloride 0.9%), posaconazole injection should be administered immediately. If not used immediately, the solution can be stored up to 24 hours if refrigerated at 2-8 degrees C (36-46 degrees F).
Co-administration of drugs that can decrease the plasma concentration of posaconazole should be avoided unless the benefit outweighs the risk. If such drugs are necessary, patients should be monitored closely for breakthrough fungal infections.
In clinical trials, the adverse reactions reported for posaconazole injection were generally similar to those reported in trials of posaconazole oral suspension. The most frequently reported adverse reactions with an onset during the posaconazole intravenous phase of dosing 300 mg once-daily therapy were diarrhea (32%), hypokalemia (22%), fever (21%), and nausea (19%).
Patients who are allergic to posaconazole or other azole antifungal medicines should not receive posaconazole. The drug should not be given along with sirolimus, pimozide, quinidine, atorvastatin, lovastatin, simvastatin, or ergot alkaloids.
Drugs such as cyclosporine and tacrolimus require dose adjustments and frequent blood monitoring when administered with posaconazole. Serious side effects, including nephrotoxicity, leukoencephalopathy, and death, have been reported in patients with increased cyclosporine or tacrolimus blood levels.
Healthcare professionals should use caution when administering posaconazole to patients at risk of developing an irregular heart rhythm, as the drug has been shown to prolong the QT interval, and cases of potentially fatal irregular heart rhythm (torsades de pointes) have been reported in patients taking posaconazole.
For more details, see the complete prescribing information. Posaconazole is marketed as Noxafil by Merck. ![]()

The US Food and Drug Administration has approved an intravenous formulation of posaconazole (Noxafil), which is expected to be available at wholesalers in mid-April.
The antifungal agent is already available as delayed-release tablets and in an oral suspension formulation.
In any formulation, posaconazole is indicated for prophylaxis of invasive Aspergillus and Candida infections in immunocompromised patients who are at high risk of developing these infections.
This includes patients who have developed graft-vs-host disease after hematopoietic stem cell transplant and patients with hematologic malignancies who have prolonged neutropenia resulting from chemotherapy.
Posaconazole injection is indicated for use in patients 18 years of age and older. The delayed-release tablets and oral suspension are indicated for patients 13 years of age and older.
Posaconazole injection is administered with a loading dose of 300 mg (one 300 mg vial) twice a day on the first day of therapy, then 300 mg once a day thereafter. It is given through a central venous line by slow intravenous infusion over approximately 90 minutes.
Once combined with a mixture of intravenous solution (150 mL of 5% dextrose in water or sodium chloride 0.9%), posaconazole injection should be administered immediately. If not used immediately, the solution can be stored up to 24 hours if refrigerated at 2-8 degrees C (36-46 degrees F).
Co-administration of drugs that can decrease the plasma concentration of posaconazole should be avoided unless the benefit outweighs the risk. If such drugs are necessary, patients should be monitored closely for breakthrough fungal infections.
In clinical trials, the adverse reactions reported for posaconazole injection were generally similar to those reported in trials of posaconazole oral suspension. The most frequently reported adverse reactions with an onset during the posaconazole intravenous phase of dosing 300 mg once-daily therapy were diarrhea (32%), hypokalemia (22%), fever (21%), and nausea (19%).
Patients who are allergic to posaconazole or other azole antifungal medicines should not receive posaconazole. The drug should not be given along with sirolimus, pimozide, quinidine, atorvastatin, lovastatin, simvastatin, or ergot alkaloids.
Drugs such as cyclosporine and tacrolimus require dose adjustments and frequent blood monitoring when administered with posaconazole. Serious side effects, including nephrotoxicity, leukoencephalopathy, and death, have been reported in patients with increased cyclosporine or tacrolimus blood levels.
Healthcare professionals should use caution when administering posaconazole to patients at risk of developing an irregular heart rhythm, as the drug has been shown to prolong the QT interval, and cases of potentially fatal irregular heart rhythm (torsades de pointes) have been reported in patients taking posaconazole.
For more details, see the complete prescribing information. Posaconazole is marketed as Noxafil by Merck. ![]()
High cost of eculizumab needs explaining, NICE says

Credit: Bill Branson
The UK’s National Institute for Health and Care Excellence (NICE) has asked the manufacturer of eculizumab (Soliris) to explain the high cost of the drug.
Research has suggested that eculizumab can be effective against atypical hemolytic uremic syndrome (aHUS), a rare disease that often proves difficult to treat.
So the National Health Service (NHS) has made eculizumab available for these patients on an interim basis, pending NICE appraisal.
However, an advisory committee for NICE has estimated that routine use of eculizumab would cost the NHS about £58 million in the first year, and costs would exceed £80 million in 5 years.
Therefore, in its draft guidance for eculizumab, the committee has asked the drug’s manufacturer, Alexion Pharma, to explain its costs.
“[The committee has] asked for clarification from the company on aspects of the manufacturing, research, and development costs of a medicinal product for the treatment of a very rare condition,” said Sir Andrew Dillon, Chief Executive at NICE.
“It has also asked NHS England for clarification on treatment costs for a highly specialized technology in the context of a highly specialized service. The information provided will be considered at the next meeting of the evaluation committee in April.”
The committee will also consider comments on its draft guidance at the meeting. The guidance is available for public comment until midday on March 25.
About aHUS
Estimated to affect more than 200 people in England, aHUS is a chronic condition that causes severe inflammation of blood vessels and thrombus formation in small blood vessels throughout the body.
Patients with aHUS can experience significant kidney impairment, thrombosis, heart failure, and brain injury. In about 70% of patients, aHUS is associated with an underlying genetic or acquired abnormality of proteins in the complement immune system.
Before eculizumab became available, plasma therapy (infusion and/or exchange) was the main treatment for aHUS. However, not all patients with aHUS respond to plasma therapy. And up to 40% of patients may die or progress to end-stage renal failure and require dialysis with the first clinical aHUS manifestation, despite the use of plasma therapy.
Some patients may be eligible for a kidney or combined kidney-liver transplantation. However, there is a high risk of organ rejection following recurrent disease.
Eculizumab in aHUS: Treatment and cost
Eculizumab inhibits the disease process by blocking pro-thrombotic and pro-inflammatory processes that can lead to cellular damage in small blood vessels throughout the body, renal failure, and damage to other organs.
Eculizumab is given intravenously in adults as initial treatment at a dose of 900 mg for 4 weeks, then as maintenance treatment at a dose of 1200 mg on week 5 and then every 12 to 16 days. The summary of product characteristics for eculizumab states that treatment should be continued for the patient’s lifetime, unless discontinuation is clinically indicated.
Eculizumab costs £3150 per 30 mL vial, excluding tax, according to the British National Formulary.
“Alexion insisted that its information about the overall cost of eculizumab be kept confidential, and so NICE is unable to share these details of the Alexion submission with stakeholders,” Dillon said.
However, to allow consultees and commentators to properly engage in the consultation process, NICE has prepared an estimate of the possible budget impact eculizumab might have, using information available in the public domain.
This is based on a treatment cost of £340,200 per adult patient in the first year (based on the acquisition cost of the drug and the recommended dosing for an adult), and assumes a patient cohort of 170, as estimated by NHS England in its interim commissioning policy.
Assuming all of these patients receive eculizumab, the budget impact for the first year would be £57.8 million. If an additional 20 new patients are treated the following year (based on a worldwide incidence of 0.4 million), the budget impact will rise to £62.5 million. That is assuming all new patients are treated and all existing patients continue to be treated at the maintenance cost of £327,600 per year.
Using the same assumptions, the budget impact will rise to £69 million in year 3 (190 existing and 20 new patients), £75 million in year 4 (210 existing and 20 new patients) and £82 million in year 5 (230 existing and 20 new patients). ![]()

Credit: Bill Branson
The UK’s National Institute for Health and Care Excellence (NICE) has asked the manufacturer of eculizumab (Soliris) to explain the high cost of the drug.
Research has suggested that eculizumab can be effective against atypical hemolytic uremic syndrome (aHUS), a rare disease that often proves difficult to treat.
So the National Health Service (NHS) has made eculizumab available for these patients on an interim basis, pending NICE appraisal.
However, an advisory committee for NICE has estimated that routine use of eculizumab would cost the NHS about £58 million in the first year, and costs would exceed £80 million in 5 years.
Therefore, in its draft guidance for eculizumab, the committee has asked the drug’s manufacturer, Alexion Pharma, to explain its costs.
“[The committee has] asked for clarification from the company on aspects of the manufacturing, research, and development costs of a medicinal product for the treatment of a very rare condition,” said Sir Andrew Dillon, Chief Executive at NICE.
“It has also asked NHS England for clarification on treatment costs for a highly specialized technology in the context of a highly specialized service. The information provided will be considered at the next meeting of the evaluation committee in April.”
The committee will also consider comments on its draft guidance at the meeting. The guidance is available for public comment until midday on March 25.
About aHUS
Estimated to affect more than 200 people in England, aHUS is a chronic condition that causes severe inflammation of blood vessels and thrombus formation in small blood vessels throughout the body.
Patients with aHUS can experience significant kidney impairment, thrombosis, heart failure, and brain injury. In about 70% of patients, aHUS is associated with an underlying genetic or acquired abnormality of proteins in the complement immune system.
Before eculizumab became available, plasma therapy (infusion and/or exchange) was the main treatment for aHUS. However, not all patients with aHUS respond to plasma therapy. And up to 40% of patients may die or progress to end-stage renal failure and require dialysis with the first clinical aHUS manifestation, despite the use of plasma therapy.
Some patients may be eligible for a kidney or combined kidney-liver transplantation. However, there is a high risk of organ rejection following recurrent disease.
Eculizumab in aHUS: Treatment and cost
Eculizumab inhibits the disease process by blocking pro-thrombotic and pro-inflammatory processes that can lead to cellular damage in small blood vessels throughout the body, renal failure, and damage to other organs.
Eculizumab is given intravenously in adults as initial treatment at a dose of 900 mg for 4 weeks, then as maintenance treatment at a dose of 1200 mg on week 5 and then every 12 to 16 days. The summary of product characteristics for eculizumab states that treatment should be continued for the patient’s lifetime, unless discontinuation is clinically indicated.
Eculizumab costs £3150 per 30 mL vial, excluding tax, according to the British National Formulary.
“Alexion insisted that its information about the overall cost of eculizumab be kept confidential, and so NICE is unable to share these details of the Alexion submission with stakeholders,” Dillon said.
However, to allow consultees and commentators to properly engage in the consultation process, NICE has prepared an estimate of the possible budget impact eculizumab might have, using information available in the public domain.
This is based on a treatment cost of £340,200 per adult patient in the first year (based on the acquisition cost of the drug and the recommended dosing for an adult), and assumes a patient cohort of 170, as estimated by NHS England in its interim commissioning policy.
Assuming all of these patients receive eculizumab, the budget impact for the first year would be £57.8 million. If an additional 20 new patients are treated the following year (based on a worldwide incidence of 0.4 million), the budget impact will rise to £62.5 million. That is assuming all new patients are treated and all existing patients continue to be treated at the maintenance cost of £327,600 per year.
Using the same assumptions, the budget impact will rise to £69 million in year 3 (190 existing and 20 new patients), £75 million in year 4 (210 existing and 20 new patients) and £82 million in year 5 (230 existing and 20 new patients). ![]()

Credit: Bill Branson
The UK’s National Institute for Health and Care Excellence (NICE) has asked the manufacturer of eculizumab (Soliris) to explain the high cost of the drug.
Research has suggested that eculizumab can be effective against atypical hemolytic uremic syndrome (aHUS), a rare disease that often proves difficult to treat.
So the National Health Service (NHS) has made eculizumab available for these patients on an interim basis, pending NICE appraisal.
However, an advisory committee for NICE has estimated that routine use of eculizumab would cost the NHS about £58 million in the first year, and costs would exceed £80 million in 5 years.
Therefore, in its draft guidance for eculizumab, the committee has asked the drug’s manufacturer, Alexion Pharma, to explain its costs.
“[The committee has] asked for clarification from the company on aspects of the manufacturing, research, and development costs of a medicinal product for the treatment of a very rare condition,” said Sir Andrew Dillon, Chief Executive at NICE.
“It has also asked NHS England for clarification on treatment costs for a highly specialized technology in the context of a highly specialized service. The information provided will be considered at the next meeting of the evaluation committee in April.”
The committee will also consider comments on its draft guidance at the meeting. The guidance is available for public comment until midday on March 25.
About aHUS
Estimated to affect more than 200 people in England, aHUS is a chronic condition that causes severe inflammation of blood vessels and thrombus formation in small blood vessels throughout the body.
Patients with aHUS can experience significant kidney impairment, thrombosis, heart failure, and brain injury. In about 70% of patients, aHUS is associated with an underlying genetic or acquired abnormality of proteins in the complement immune system.
Before eculizumab became available, plasma therapy (infusion and/or exchange) was the main treatment for aHUS. However, not all patients with aHUS respond to plasma therapy. And up to 40% of patients may die or progress to end-stage renal failure and require dialysis with the first clinical aHUS manifestation, despite the use of plasma therapy.
Some patients may be eligible for a kidney or combined kidney-liver transplantation. However, there is a high risk of organ rejection following recurrent disease.
Eculizumab in aHUS: Treatment and cost
Eculizumab inhibits the disease process by blocking pro-thrombotic and pro-inflammatory processes that can lead to cellular damage in small blood vessels throughout the body, renal failure, and damage to other organs.
Eculizumab is given intravenously in adults as initial treatment at a dose of 900 mg for 4 weeks, then as maintenance treatment at a dose of 1200 mg on week 5 and then every 12 to 16 days. The summary of product characteristics for eculizumab states that treatment should be continued for the patient’s lifetime, unless discontinuation is clinically indicated.
Eculizumab costs £3150 per 30 mL vial, excluding tax, according to the British National Formulary.
“Alexion insisted that its information about the overall cost of eculizumab be kept confidential, and so NICE is unable to share these details of the Alexion submission with stakeholders,” Dillon said.
However, to allow consultees and commentators to properly engage in the consultation process, NICE has prepared an estimate of the possible budget impact eculizumab might have, using information available in the public domain.
This is based on a treatment cost of £340,200 per adult patient in the first year (based on the acquisition cost of the drug and the recommended dosing for an adult), and assumes a patient cohort of 170, as estimated by NHS England in its interim commissioning policy.
Assuming all of these patients receive eculizumab, the budget impact for the first year would be £57.8 million. If an additional 20 new patients are treated the following year (based on a worldwide incidence of 0.4 million), the budget impact will rise to £62.5 million. That is assuming all new patients are treated and all existing patients continue to be treated at the maintenance cost of £327,600 per year.
Using the same assumptions, the budget impact will rise to £69 million in year 3 (190 existing and 20 new patients), £75 million in year 4 (210 existing and 20 new patients) and £82 million in year 5 (230 existing and 20 new patients). ![]()
Study may explain why targeted treatment falls short in angiosarcoma
Heiser & Robert Ackland
Multiple mutations drive the development of angiosarcoma, according to a study published in Nature Genetics.
Researchers identified driver mutations in several genes associated with angiogenesis, including PTPRB and PLCG1.
They also found that PLCG1 mutations only occurred alongside mutations in PTPRB.
The investigators believe these findings may explain why angiosarcoma therapies directed at a single target fail to eradicate the disease.
Angiosarcoma is a rare cancer of the blood vessels that can occur spontaneously or develop after radiotherapy or chronic lymphedema.
Previous research indicated that aberrant angiogenesis, including somatic mutations in angiogenesis-signaling genes, drives angiosarcoma. So researchers developed drugs targeting pathways involved in angiogenesis, but these drugs have had little or no success.
“Because this cancer doesn’t respond well to traditional chemotherapy and radiotherapy, it makes sense to develop drugs that target pathways that control blood vessel formation,” said study author Peter Campbell, MD, PhD, of the Wellcome Trust Sanger Institute in the UK.
“We found 2 novel cancer genes that control blood vessel formation which are mutated in this cancer and which could be targeted for treatment of this highly aggressive cancer.”
To identify these genes, Dr Campbell and his colleagues performed whole-genome, whole-exome, and targeted sequencing in samples from patients with angiosarcoma.
Thirty-eight percent of the samples (15/39) carried mutations in genes that control angiogenesis, including PLCG1 and PTPRB.
The researchers identified 14 PTPRB mutations in 10 samples. This included 8 nonsense variants, 3 missense variants, 2 essential splice-site variants, and 1 frameshift insertion.
The investigators also discovered a recurrent mutation in PLCG1, a missense variant encoding p.Arg707Gln, which was present in 3 patient samples. All 3 PLCG1 mutations co-occurred with PTPRB mutations.
The researchers said this discovery may explain why drugs developed for a single target are ineffective in some angiosarcoma patients.
“Not only does our study change the way people view the biology of this tumor, it acts as a guide for future drug trials in angiosarcoma patients,” said study author Adrian Harris, MD, DPhil, of the University of Oxford in the UK.
He noted that researchers can use information from this study to determine if existing drugs could be effective against angiosarcoma. ![]()

Heiser & Robert Ackland
Multiple mutations drive the development of angiosarcoma, according to a study published in Nature Genetics.
Researchers identified driver mutations in several genes associated with angiogenesis, including PTPRB and PLCG1.
They also found that PLCG1 mutations only occurred alongside mutations in PTPRB.
The investigators believe these findings may explain why angiosarcoma therapies directed at a single target fail to eradicate the disease.
Angiosarcoma is a rare cancer of the blood vessels that can occur spontaneously or develop after radiotherapy or chronic lymphedema.
Previous research indicated that aberrant angiogenesis, including somatic mutations in angiogenesis-signaling genes, drives angiosarcoma. So researchers developed drugs targeting pathways involved in angiogenesis, but these drugs have had little or no success.
“Because this cancer doesn’t respond well to traditional chemotherapy and radiotherapy, it makes sense to develop drugs that target pathways that control blood vessel formation,” said study author Peter Campbell, MD, PhD, of the Wellcome Trust Sanger Institute in the UK.
“We found 2 novel cancer genes that control blood vessel formation which are mutated in this cancer and which could be targeted for treatment of this highly aggressive cancer.”
To identify these genes, Dr Campbell and his colleagues performed whole-genome, whole-exome, and targeted sequencing in samples from patients with angiosarcoma.
Thirty-eight percent of the samples (15/39) carried mutations in genes that control angiogenesis, including PLCG1 and PTPRB.
The researchers identified 14 PTPRB mutations in 10 samples. This included 8 nonsense variants, 3 missense variants, 2 essential splice-site variants, and 1 frameshift insertion.
The investigators also discovered a recurrent mutation in PLCG1, a missense variant encoding p.Arg707Gln, which was present in 3 patient samples. All 3 PLCG1 mutations co-occurred with PTPRB mutations.
The researchers said this discovery may explain why drugs developed for a single target are ineffective in some angiosarcoma patients.
“Not only does our study change the way people view the biology of this tumor, it acts as a guide for future drug trials in angiosarcoma patients,” said study author Adrian Harris, MD, DPhil, of the University of Oxford in the UK.
He noted that researchers can use information from this study to determine if existing drugs could be effective against angiosarcoma. ![]()

Heiser & Robert Ackland
Multiple mutations drive the development of angiosarcoma, according to a study published in Nature Genetics.
Researchers identified driver mutations in several genes associated with angiogenesis, including PTPRB and PLCG1.
They also found that PLCG1 mutations only occurred alongside mutations in PTPRB.
The investigators believe these findings may explain why angiosarcoma therapies directed at a single target fail to eradicate the disease.
Angiosarcoma is a rare cancer of the blood vessels that can occur spontaneously or develop after radiotherapy or chronic lymphedema.
Previous research indicated that aberrant angiogenesis, including somatic mutations in angiogenesis-signaling genes, drives angiosarcoma. So researchers developed drugs targeting pathways involved in angiogenesis, but these drugs have had little or no success.
“Because this cancer doesn’t respond well to traditional chemotherapy and radiotherapy, it makes sense to develop drugs that target pathways that control blood vessel formation,” said study author Peter Campbell, MD, PhD, of the Wellcome Trust Sanger Institute in the UK.
“We found 2 novel cancer genes that control blood vessel formation which are mutated in this cancer and which could be targeted for treatment of this highly aggressive cancer.”
To identify these genes, Dr Campbell and his colleagues performed whole-genome, whole-exome, and targeted sequencing in samples from patients with angiosarcoma.
Thirty-eight percent of the samples (15/39) carried mutations in genes that control angiogenesis, including PLCG1 and PTPRB.
The researchers identified 14 PTPRB mutations in 10 samples. This included 8 nonsense variants, 3 missense variants, 2 essential splice-site variants, and 1 frameshift insertion.
The investigators also discovered a recurrent mutation in PLCG1, a missense variant encoding p.Arg707Gln, which was present in 3 patient samples. All 3 PLCG1 mutations co-occurred with PTPRB mutations.
The researchers said this discovery may explain why drugs developed for a single target are ineffective in some angiosarcoma patients.
“Not only does our study change the way people view the biology of this tumor, it acts as a guide for future drug trials in angiosarcoma patients,” said study author Adrian Harris, MD, DPhil, of the University of Oxford in the UK.
He noted that researchers can use information from this study to determine if existing drugs could be effective against angiosarcoma. ![]()
How diabetes drugs can fight hematologic malignancies

Credit: PNAS
Researchers say they’ve discovered how a class of diabetes drugs known as biguanides exerts anticancer properties in certain malignancies.
The team identified a mitochondrial pathway that imbues cancer cells with the ability to survive in low-glucose environments.
By finding cancer cells with defects in this pathway or impaired glucose utilization, the researchers found they could predict which cancers would be sensitive to drugs that inhibit this pathway.
And follow-up experiments confirmed that lymphoma, leukemia, and myeloma tumors were among those sensitive to treatment.
Kivanç Birsoy, PhD, of the Whitehead Institute for Biomedical Research in Cambridge, Massachusetts, and his colleagues reported these findings in Nature.
To study how cancer cells survive in the kind of low-glucose environment found within cancerous tumors, the researchers developed a system that circulates low-nutrient media continuously around cells.
Of the 30 cancer cell lines the team tested within this system, most appeared unaffected by a lack of glucose. However, a few of the cells lines thrived and reproduced rapidly, while others struggled.
Specifically, a low-glucose environment prompted an increase in proliferation for the Burkitt lymphoma cell line Raji, as well as in medulloblastoma, lung, and stomach cancer cell lines.
However, the lymphoma cell lines U-937 and MC116, as well as the myeloma cell lines NCI-H929 and KMS-26, saw significant decreases in proliferation in a low-glucose environment. The leukemia cell line Jurkat was moderately sensitive to a low-glucose environment.
“No one really understood why cancer cells had these responses or whether they were important for the formation of the tumor,” said study author Richard Possemato, PhD, also of the Whitehead Institute.
To gain more insight, the researchers screened overly distressed cells for genes whose suppression improved or further hindered the cells’ survival rates. The screen flagged genes involved in glucose transportation and oxidative phosphorylation.
The team hypothesized that cancer cells with mutations in these genes are over-taxing their mitochondria under normal conditions. When placed in a harsh, low-glucose environment, the mitochondria are maxed out, and the cells suffer.
If true, the hypothesis would suggest that further impairing mitochondrial function with biguanides, which are known oxidative phosphorylation inhibitors, could push the mitochondria beyond their limits, to the detriment of the cancer cells.
The researchers first tested this hypothesis in vitro on cell lines with glucose utilization defects (NCI-H929, KMS-26, LP-1, L-363, MOLP-8, D341Med, and KMS-28BM) or mitochondrial DNA (mtDNA) mutations (U-937, BxPC3, Cal-62, HCC-1438, HCC-827, and NU-DHL-1).
They found that, in a low-glucose environment, cell lines with mtDNA mutations or impaired glucose utilization were 5 to 20 times more susceptible to phenformin, a more potent biguanide than metformin, when compared to control cancer cell lines or an immortalized B-cell line.
The team then tested phenformin in mice implanted with tumors derived from low-glucose-sensitive cancer cells. The drug inhibited the growth of tumors derived from cancer cells with mtDNA mutations (Cal-62 and U-937) or poor glucose consumption (KMS-26 and NCI-H929) but not from cells lacking these defects (NCI-H2171 and NCI-H82).
“These results show that mitochondrial DNA mutations and glucose import defects can be used as biomarkers for biguanide sensitivity to determine if a cancer patient might benefit from these drugs,” Dr Birsoy said.
“And this is the first time that anyone has shown that the direct cytotoxic effects of this class of drugs, including metformin and phenformin, on cancer cells are mediated through their effect on mitochondria.”
To confirm the accuracy of their proposed biomarkers, the researchers now want to analyze previous clinical trials to see if cancer patients with the proposed biomarkers fared better with metformin treatment than patients without the biomarkers. ![]()

Credit: PNAS
Researchers say they’ve discovered how a class of diabetes drugs known as biguanides exerts anticancer properties in certain malignancies.
The team identified a mitochondrial pathway that imbues cancer cells with the ability to survive in low-glucose environments.
By finding cancer cells with defects in this pathway or impaired glucose utilization, the researchers found they could predict which cancers would be sensitive to drugs that inhibit this pathway.
And follow-up experiments confirmed that lymphoma, leukemia, and myeloma tumors were among those sensitive to treatment.
Kivanç Birsoy, PhD, of the Whitehead Institute for Biomedical Research in Cambridge, Massachusetts, and his colleagues reported these findings in Nature.
To study how cancer cells survive in the kind of low-glucose environment found within cancerous tumors, the researchers developed a system that circulates low-nutrient media continuously around cells.
Of the 30 cancer cell lines the team tested within this system, most appeared unaffected by a lack of glucose. However, a few of the cells lines thrived and reproduced rapidly, while others struggled.
Specifically, a low-glucose environment prompted an increase in proliferation for the Burkitt lymphoma cell line Raji, as well as in medulloblastoma, lung, and stomach cancer cell lines.
However, the lymphoma cell lines U-937 and MC116, as well as the myeloma cell lines NCI-H929 and KMS-26, saw significant decreases in proliferation in a low-glucose environment. The leukemia cell line Jurkat was moderately sensitive to a low-glucose environment.
“No one really understood why cancer cells had these responses or whether they were important for the formation of the tumor,” said study author Richard Possemato, PhD, also of the Whitehead Institute.
To gain more insight, the researchers screened overly distressed cells for genes whose suppression improved or further hindered the cells’ survival rates. The screen flagged genes involved in glucose transportation and oxidative phosphorylation.
The team hypothesized that cancer cells with mutations in these genes are over-taxing their mitochondria under normal conditions. When placed in a harsh, low-glucose environment, the mitochondria are maxed out, and the cells suffer.
If true, the hypothesis would suggest that further impairing mitochondrial function with biguanides, which are known oxidative phosphorylation inhibitors, could push the mitochondria beyond their limits, to the detriment of the cancer cells.
The researchers first tested this hypothesis in vitro on cell lines with glucose utilization defects (NCI-H929, KMS-26, LP-1, L-363, MOLP-8, D341Med, and KMS-28BM) or mitochondrial DNA (mtDNA) mutations (U-937, BxPC3, Cal-62, HCC-1438, HCC-827, and NU-DHL-1).
They found that, in a low-glucose environment, cell lines with mtDNA mutations or impaired glucose utilization were 5 to 20 times more susceptible to phenformin, a more potent biguanide than metformin, when compared to control cancer cell lines or an immortalized B-cell line.
The team then tested phenformin in mice implanted with tumors derived from low-glucose-sensitive cancer cells. The drug inhibited the growth of tumors derived from cancer cells with mtDNA mutations (Cal-62 and U-937) or poor glucose consumption (KMS-26 and NCI-H929) but not from cells lacking these defects (NCI-H2171 and NCI-H82).
“These results show that mitochondrial DNA mutations and glucose import defects can be used as biomarkers for biguanide sensitivity to determine if a cancer patient might benefit from these drugs,” Dr Birsoy said.
“And this is the first time that anyone has shown that the direct cytotoxic effects of this class of drugs, including metformin and phenformin, on cancer cells are mediated through their effect on mitochondria.”
To confirm the accuracy of their proposed biomarkers, the researchers now want to analyze previous clinical trials to see if cancer patients with the proposed biomarkers fared better with metformin treatment than patients without the biomarkers. ![]()

Credit: PNAS
Researchers say they’ve discovered how a class of diabetes drugs known as biguanides exerts anticancer properties in certain malignancies.
The team identified a mitochondrial pathway that imbues cancer cells with the ability to survive in low-glucose environments.
By finding cancer cells with defects in this pathway or impaired glucose utilization, the researchers found they could predict which cancers would be sensitive to drugs that inhibit this pathway.
And follow-up experiments confirmed that lymphoma, leukemia, and myeloma tumors were among those sensitive to treatment.
Kivanç Birsoy, PhD, of the Whitehead Institute for Biomedical Research in Cambridge, Massachusetts, and his colleagues reported these findings in Nature.
To study how cancer cells survive in the kind of low-glucose environment found within cancerous tumors, the researchers developed a system that circulates low-nutrient media continuously around cells.
Of the 30 cancer cell lines the team tested within this system, most appeared unaffected by a lack of glucose. However, a few of the cells lines thrived and reproduced rapidly, while others struggled.
Specifically, a low-glucose environment prompted an increase in proliferation for the Burkitt lymphoma cell line Raji, as well as in medulloblastoma, lung, and stomach cancer cell lines.
However, the lymphoma cell lines U-937 and MC116, as well as the myeloma cell lines NCI-H929 and KMS-26, saw significant decreases in proliferation in a low-glucose environment. The leukemia cell line Jurkat was moderately sensitive to a low-glucose environment.
“No one really understood why cancer cells had these responses or whether they were important for the formation of the tumor,” said study author Richard Possemato, PhD, also of the Whitehead Institute.
To gain more insight, the researchers screened overly distressed cells for genes whose suppression improved or further hindered the cells’ survival rates. The screen flagged genes involved in glucose transportation and oxidative phosphorylation.
The team hypothesized that cancer cells with mutations in these genes are over-taxing their mitochondria under normal conditions. When placed in a harsh, low-glucose environment, the mitochondria are maxed out, and the cells suffer.
If true, the hypothesis would suggest that further impairing mitochondrial function with biguanides, which are known oxidative phosphorylation inhibitors, could push the mitochondria beyond their limits, to the detriment of the cancer cells.
The researchers first tested this hypothesis in vitro on cell lines with glucose utilization defects (NCI-H929, KMS-26, LP-1, L-363, MOLP-8, D341Med, and KMS-28BM) or mitochondrial DNA (mtDNA) mutations (U-937, BxPC3, Cal-62, HCC-1438, HCC-827, and NU-DHL-1).
They found that, in a low-glucose environment, cell lines with mtDNA mutations or impaired glucose utilization were 5 to 20 times more susceptible to phenformin, a more potent biguanide than metformin, when compared to control cancer cell lines or an immortalized B-cell line.
The team then tested phenformin in mice implanted with tumors derived from low-glucose-sensitive cancer cells. The drug inhibited the growth of tumors derived from cancer cells with mtDNA mutations (Cal-62 and U-937) or poor glucose consumption (KMS-26 and NCI-H929) but not from cells lacking these defects (NCI-H2171 and NCI-H82).
“These results show that mitochondrial DNA mutations and glucose import defects can be used as biomarkers for biguanide sensitivity to determine if a cancer patient might benefit from these drugs,” Dr Birsoy said.
“And this is the first time that anyone has shown that the direct cytotoxic effects of this class of drugs, including metformin and phenformin, on cancer cells are mediated through their effect on mitochondria.”
To confirm the accuracy of their proposed biomarkers, the researchers now want to analyze previous clinical trials to see if cancer patients with the proposed biomarkers fared better with metformin treatment than patients without the biomarkers. ![]()
Pathway Reduces Utilization and Disparities
Venous thromboembolism (VTE), including deep venous thrombosis (DVT) and pulmonary embolism (PE), is common, costly, and often fatal. Annual VTE incidence in the United States is over 1 million, including over 220,000 PE patients who have an average hospital length of stay (LOS) of 8 days, with a rising per‐patient cost of over $40,000.[1, 2] Nearly half of all PE readmissions occur within 30 days; recurrent DVT events are 21% more costly than the initial event.[3] Likewise, 30‐day PE mortality is 8%, with most deaths occurring within 1 hour of initial presentation.[4, 5]
Rapid implementation of therapeutic anticoagulation has reduced morbidity and mortality in VTE. Ineffective and untimely treatment increases disease progression, significant medication‐related adverse events, and cost. The Joint Commission recognized this risk and included National Patient Safety Goal 3.5.01 to reduce adverse events.[6] Appropriate use of anticoagulation was further emphasized by national quality initiatives through Joint Commission VTE core measures endorsed by the National Quality Forum and the Centers for Medicare and Medicaid Services.[7]
Many models of outpatient VTE care pathways exist. Early models focused on the feasibility of low‐molecular‐weight heparins (LMWH) in the ambulatory setting, with transition to long‐term warfarin. Focus shifted to comprehensive disease pathway implementation aimed at reducing healthcare resource utilization. These pathways have reduced cost and unnecessary hospital stays and minimized complications through enrolling low‐risk patients. To our knowledge, results of an interdisciplinary VTE care pathway have not been published from a large urban academic institution, where a substantial uninsured population exists.
Examining baseline VTE practices and care delivered at our institution provided critical knowledge in effectively developing a novel model of care. Prior to pathway development, acute VTE patients were typically admitted for initiation of therapeutic anticoagulation and appropriate overlap of injectable anticoagulants with warfarin. Significant healthcare disparities were seen among VTE patients at our institution: uninsured patients stayed in the hospital 2 additional days and accumulated twice the rate of 30‐day emergency department (ED) reutilization and cost than insured patients.[8] Discharged VTE patients were managed through a pharmacy‐run anticoagulation clinic pending primary care provider (PCP) follow‐up. We speculated many uninsured VTE patients lacked sufficient disease and treatment information, and lacked surveillance and timely access to medical care following hospitalization. We hypothesized that through (1) targeted education of patients and providers, (2) coordination of timely follow‐up for at‐risk patients, and (3) posthospital monitoring, we could achieve standardized care for all acute DVT and low‐risk PE patients. As a result, we aimed to decrease hospital LOS and produce fewer return visits and readmissions.
METHODS
Study Setting and Population
Acute medical VTE patients were targeted, where they were either discharged directly from the ED or admitted to a medicine service. Acute VTE was defined as primary or secondary diagnosis of new, lower extremity DVT, PE or concurrent DVT, and PE. Patients were identified and tracked by a professional research assistant (PRA) using our electronic medical record (EMR) search filter of all 120 discharge diagnoses for acute DVT and PE.
Our hospital is a 375‐bed, academic medical center in a metropolitan area of under 3 million people. ED volume is approximately 55,000 patients per year.
Exclusion Criteria
We excluded patients classified as surgical/postoperative/admitted to a surgery service, pregnant/postpartum/admitted to an obstetrical service, hospital direct admissions (including outside hospital transfers), and oncology service admissions. Clinically unstable patients requiring intensive care unit admission and/or thrombolytic therapy, and patients with upper extremity, recurrent, or catheter‐associated VTE were also excluded. To allow for comparative data, exclusion criteria were similar to those used in the historical, retrospective chart review performed previously at our institution.[8]
VTE Clinical Care Pathway
The pathway was developed as a quality improvement project through a multidisciplinary, collaborative effort, including pharmacists (inpatient and outpatient), administrative staff in the anticoagulation clinic, nurse leaders and educators, physician faculty (ED, inpatient and outpatient), case managers (inpatient and ED), and providers from local community health clinics, who provide the majority of follow‐up care for our uninsured patients.
We sought care standardization and system‐wide education for all acute, medical, lower‐extremity DVT and low‐risk PE patients, with a focus on coordination of transitional care. All pathway patients were provided education, lab testing, and outpatient medications including LMWH and warfarin. For patients lacking insurance, medications were provided through a medication assistance program at no cost to the patient. Timely outpatient clinic follow‐up and posthospital phone calls were targeted safety net features to facilitate timely hospital discharge and program success. We also aimed to meet nationally mandated quality of care measures and benchmarks. Funding for this project, obtained through a quality improvement (QI) grant from the hospital supported a PRA and educational materials.
The Colorado Multiple Institutional Review Board approved the protocol prior to study implementation. Specific elements of the care pathway have been outlined (see Supporting Information, Figure 1, in the online version of this article). The initial rollout of the program occurred as a pilot in the ED for patients presenting with DVT only to assess feasibility. Based on this success, the pathway team expanded the program to inpatients, including those with PE, and augmented the educational program.
Measures
Evaluation of the intervention was completed by real‐time chart extraction and phone interviews within 72 hours of hospital discharge and a chart review at 6 weeks following discharge. Chart review determined the number of follow‐up visits within 30 days to the anticoagulation clinic and episodes of recidivism. Study data (n=241) were obtained from February 1, 2011 to June 30, 2012 and compared to previously published retrospective data on VTE patients at our institution (n=234) from December 1, 2007 to April 4, 2009.8
We obtained patient demographics (age, gender, ethnicity, insurance category) and admission status from the EMR. We collected data on ED recidivism within 30 days (for VTE‐related issues), LOS, and readmissions within 30 days of discharge. We also collected total cost data for all VTE care from hospital administrative billing data including initial presentation and VTE‐related return visits to the ED and readmissions.
Outcomes
Descriptive information, including demographics, admission status and type of VTE event are summarized for the VTE care pathway. Pathway patients, stratified by payer status (uninsured vs insured), were compared to previously described historical controls.[8] Primary outcomes included comparisons of total costs, LOS, and 30‐day ED recidivism and hospital readmission rates. Further comparisons were made between insured and uninsured patients on these same outcomes.
Data Analysis
Data are presented as proportions or meanstandard deviation unless indicated otherwise. Categorical data were compared using the Fisher exact test or 2 test, where appropriate. Continuous variables were compared using the Student t test. All tests were 2‐tailed. Statistical analyses of the results were performed using GraphPad Prism 4.0 (GraphPad Software, San Diego, CA) and InStat 3.06 (GraphPad Software). A P value <0.05 was considered statistically significant for this study.
RESULTS
Care Pathway Cohort
We enrolled 241 medical patients with acute VTE during the 19‐month study period (Table 1). Of these, 107 (44.4%) presented with DVT alone, whereas the remaining 134 (55.6%) had PE. Eighty‐eight of the 241 VTE patients were uninsured (36.5%). Uninsured patients were younger on average (46.7 vs 55.5 years; P<0.0001) and more commonly presented with DVT only (58.0% vs 36.7%; P=0.036).
| Patients, N=241 | Uninsured, N=88 | Insured, N=153 | P Value | |
|---|---|---|---|---|
| ||||
| Mean age, y (SD) | 52.4 (15.8) | 46.7 (13.9) | 55.5 (16.1) | <0.0001 |
| Median age, y (IQR) | 53 (4263.5) | 56 (44.367) | 49 (35.358.5) | |
| Gender, male, n (%) | 113 (46.9) | 44 (50.0) | 69 (45.1) | 0.548 |
| Pulmonary embolism, n (%) | 134 (55.6) | 37 (42.0) | 97 (63.3) | 0.036 |
| All VTE, hospital admission, n (%) | 179 (74.3) | 58 (65.9) | 121 (79.1) | 0.032 |
| PE, hospital admission, n (%) | 132 (54.8) | 37 (42.0) | 95 (97.9) | 1.00 |
Utilizing the pathway, the majority of VTE patients (179; 74.3%) were admitted to the hospital. Among the uninsured, 58 of 88 (65.9%) patients were admitted compared to 121 of 153 (79.1%) among the insured (P=0.032). Among 107 DVT patients, 47 were admitted (43.9%), including 20 of 51 uninsured DVT patients (39.2%) compared to 27 of 56 insured DVT patients (48.2%). Nearly all PE patients (132 of 134; 98.5%) were admitted. Two insured PE patients were not admitted.
Care Pathway Versus Historical Cohort
Comparing VTE care pathway patients to historical VTE patients (prior to intervention), the age and gender, as well as number of VTE events, VTE type, and admission status were similar (Table 2).
| Outcome | Historical VTE, N=234 | Pathway VTE, N=241 | P Value |
|---|---|---|---|
| |||
| Age, y, mean | 53.1 | 52.4 | 0.64 |
| Male, n (%) | 125 (53.4) | 113 (47.0) | 0.46 |
| DVT (%) | 106 (45.3) | 107 (44.4) | 0.92 |
| Uninsured (%) | 38 (35.8) | 51 (47.7) | 0.93 |
| PE (%) | 128 (54.7) | 134 (55.6) | 0.92 |
| Uninsured (%) | 29 (22.7) | 38 (28.4) | 0.11 |
| Admitted (%) | 171 (73.1) | 179 (74.3) | 0.85 |
| DVT (%) | 43 (40.6) | 47 (43.9) | 0.91 |
| Uninsured (%) | 17 (39.6) | 20 (42.6) | 0.94 |
| PE (%) | 128 (100) | 132 (98.5) | 0.91 |
| Uninsured (%) | 29 (100) | 38 (100) | 0.32 |
| LOS, d, mean (SD) | 4.4 (3.8) | 3.1 (2.9) | <0.001 |
| Uninsured | 5.9 (5.1) | 3.1 (2.9) | <0.001 |
| Insured | 3.8 (3.1) | 3.1 (2.9) | 0.69 |
| ED revisit, n (%) | 26 (11.1) | 27 (11.2) | 0.974 |
| Uninsured, n (%) | 12 (17.9) | 12 (13.6) | 0.59 |
| Readmission, n (%) | 16 (9.4) | 10 (5.6) | 0.25 |
| Uninsured, n (%) | 5 (10.9) | 2 (3.4) | 0.24 |
| Total cost, $, mean (SD) | 7610 (9988) | 5295 (7975) | 0.005 |
| Uninsured | 9953 (14211) | 4304 (6596) | 0.001 |
| Insured | 6698 (7564) | 5875 (8650) | 0.36 |
| Cost, admitted, $, mean (SD) | 10324 (8988) | 7038 (8965) | 0.044 |
| Uninsured | 14420 (13351) | 6375 (7462) | 0.005 |
| Insured | 8843 (6565) | 7353 (9288) | 0.599 |
Average hospital LOS for an admitted care pathway patient was 3.1 days versus 4.4 days in an historical VTE patient (P=0.0001; Table 2). When stratified by insurance, uninsured pathway patients had a LOS of 3.1, decreased from a prepathway LOS of 5.9 days (P=0.0006), whereas this did not change among insured patients (3.1 from 3.8 days [P=0.688]).
For all VTE care pathway patients, 30‐day ED recidivism was 11.2%, similar to prepathway data (11.1%; Table 2). This was true regardless of insurance status. Thirty‐day readmission rates trended from 9.4% prepathway to 5.6% postpathway (P=0.254) (Table 2). Compared to historical VTE patients, uninsured pathway patients had readmission rates of 3.4% from 10.9% (P=0.237), whereas readmission rates for insured patients were 6.6% from 8.8% (P=0.686).
Average cost for a VTE care pathway patient was $5295 compared to an historical cost of $7610 per VTE patient (P <0.005). Among uninsured pathway patients, the cost of VTE care was $4304 compared to $9953 historically (P=0.001). Among insured pathway patients, the cost of VTE care was $5875 compared to an historical cost of $6698 (P=0.365).
The average VTE cost of care for an admitted pathway patient was $7038 versus $10,324 per admitted historical patient (P=0.044). For an admitted uninsured VTE pathway patient, cost was $6375 versus $14,420 per historical VTE patient (P=0.005). For an admitted insured VTE pathway patient, the cost was $7353 versus $8843 per historical VTE patient (P=0.599).
Patient satisfaction scores with the care pathway averaged 4.5 (15 Likert scale).
DISCUSSION
Development and implementation of a multidisciplinary VTE clinical care pathway at our institution represents success across multiple domains. As a QI project, we standardized care and delivered system‐wide education, and provided solutions to existing gaps in posthospital care. This pathway for a common, dangerous disease requiring high‐risk medications magnifies the importance of care delivered at vulnerable points. Results of our study are the first to our knowledge to mitigate healthcare disparities and reduce healthcare utilization through a care pathway across diverse populations. Hospital LOS for all VTE patients was significantly decreased, wile lowering hospital reutilization patterns, particularly among the uninsured. Hospital admission rates are now lower specifically for the uninsured patients, because ED and inpatient providers now have increased confidence in the follow‐up arrangements with the safety‐net clinics.
Many clinical care pathways for VTE are proven, safe, and cost‐effective.[9, 10, 11, 12] Outpatient DVT treatment delivers significant cost savings and averts unnecessary hospital stays.[13, 14] A hospital‐based program providing outpatient DVT treatment among inner‐city patients in New York demonstrated a lower incidence of adverse events and substantial cost savings, but excluded PE patients.[15] We intentionally sought to expand our VTE program by including both PE and vulnerable uninsured patients.
Lack of health insurance and routine primary care is a major challenge to successful implementation of any care pathway. Access to timely posthospital follow‐up care is far more limited in patients lacking private insurance.[16, 17] Uninsured patients are less likely to receive necessary medical care and more likely to have delayed care.[18, 19] Uninsured patients also have poorer short‐term health and are nearly 3 times more likely to have an ED revisit following hospital discharge than insured patients.[16, 20, 21] At our own institution, many discharged medical patients lack timely PCP follow‐up, especially the uninsured, leading to higher rates of hospital reutilization.[22] Interventions directed at the uninsured VTE patient to mitigate such disparities were specifically targeted. These included coordination of timely follow‐up care in community health clinics and provision of posthospital phone calls.
Efforts to improve transitional care for vulnerable patients have proven successful. Patients linked from the ED to community health clinics through scheduled follow‐up have improved frequency of follow‐up, receive routine care, and have reduced hospital utilization and rehospitalization.[23, 24, 25] Conversely, fewer care disparities are realized by patients within integrated systems such as the Veterans Administration.[26] Thus, the ultimate development of a VTE care pathway at our nonintegrated hospital required an innovative paradigm to deliver acute DVT and PE care. Through examining existing processes of our VTE care, we hypothesized that the main contributors of baseline care deficiencies included inadequate system‐wide education, fragmented care, and significant barriers to timely follow‐up.
Education of providers, patients, and system‐wide process change were key elements in pathway implementation. Provider educational opportunities concerning VTE disease and treatment were identified, including safe and effective outpatient management options. We anticipated provider reluctance prescribing potentially dangerous anticoagulation medications to otherwise stable patients who might lack close posthospital supervision (eg, ED clinicians accustomed to admitting patients and inpatient teams cautious in discharging patients). We postulated that patients received inadequate VTE education and lacked appropriate skills to effectively and safely manage their new disease and medications. The diverse educational components outlined within the pathway significantly contributed to improved provider confidence in their patients' follow‐up care as well as their patients' comprehension of their disease.
Timely posthospital care follow‐up for all VTE patients significantly impacted our pathway results. Historically, uninsured patients lacked primary care follow‐up, often waiting 3 months for an initial clinic visit. Through timely care coordination with local community health clinics, uninsured VTE care pathway patients discharged from our facility are routinely scheduled to be seen within 72 hours. Posthospital care is further addressed through follow‐up phone calls, which monitor patient understanding and care, and identify how and where potential medical needs are best met. Such calls increase patient satisfaction, resolve medication issues, and result in fewer ED return visits.[27] With our intervention, patient satisfaction scores averaged 4.5 (15 Likert scale), reflecting strong support for phone calls and overall experience.
Direct institutional annual cost savings realized with the VTE care pathway was $452,460. This occurred primarily as a result of nearly 50% fewer inpatient days required for admitted VTE patients. Indirect cost savings were further accomplished through increased availability of high‐demand outpatient anticoagulation visits given improved timely PCP follow‐up. Prior to pathway implementation, uninsured patients frequently had multiple, often unreimbursed, visits to this clinic while awaiting PCP follow‐up. Additional future cost savings may occur as healthcare reimbursement patterns are likely to include methods to penalize inefficient and high‐resource usage.
There are several limitations to our study. This was a single‐institution quality program with relatively small numbers. Comparison of pathway data with historical data provides an interval lag that may miss temporal changes in medical practice and disease trends. However, we believe the practice of VTE treatment changed minimally between the 2 time periods. We identified virtually the same number and type of patients in each cohort. Physician and PRA staff turnover complicated tracking patients and challenged continuous system‐wide education. However, we believe consistent education and feedback to PRA faculty throughout the study period minimized variability. Although we could not verify VTE presentations to outside hospitals other than by patient self‐report, it is likely that our patient population would have re‐presented to our institution for follow‐up VTE needs or bleeding concerns. As a result of timely follow‐up phone calls, the number of return visits to the hospital may have been magnified, because more educated patients may have overreacted to mild symptom changes. Prior to the intervention, discharged VTE patients may not have recognized signs and symptoms of worsening disease or may not have returned to our institution for follow‐up needs. Last, we did not control for comorbidities in either cohort, which may affect hospital utilization patterns, as younger patients may be less likely to be admitted or insured.
As a result of a comprehensive VTE clinical care pathway developed by key stakeholders, acute VTE patients who present to our hospital are therapeutically anticoagulated and monitored in a timely, uniform, and safe manner. We believe success reflects system‐wide education and standardization of care through reducing variation, including the high‐risk posthospital period. In an era of fragmented medical care, this program closes existing gaps in care and addresses the needs of vulnerable patients through strong collaboration and efficient coordination with local community health clinics. This is especially important in a dynamic healthcare landscape with an evolving payer mix that demands the medical establishment seek innovative ways to improve quality of care while reducing cost. Future research should explore etiologies and impacts of outcome variability based on insurance status, and identify other conditions and institutions demonstrating care disparities. Ultimately, implementation of this pathway provides strong evidence for improving care, meeting Joint Commission anticoagulation patient safety goals, and conserving limited resources for a common and deadly disease.
Acknowledgements
The authors thank Sancia Tonn, PRA, Carol Kemp‐Jackson from University of Colorado outpatient anticoagulation clinic, and the Metro Community Provider Network Clinics.
Disclosures
This project was funded by University of Colorado Hospital QI Small Grants Program. Preliminary results of this pathway were previously presented at the 2012 Society of Hospital Medicine Annual Meeting, San Diego, California, April 14, 2012.
- , , , et al. Economic burden of deep‐vein thrombosis, pulmonary embolism, and post‐thrombotic syndrome. Am J Health Syst Pharm. 2006;63(20 suppl 6):S5–S15.
- , , , et al. Recent trends in clinical outcomes and resource utilization for pulmonary embolism in the United States: findings from the nationwide inpatient sample. Chest. 2009;136(4):983–990.
- , . Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: an administrative claims analysis from 30 managed care organizations. J Manag Care Pharm. 2007;13(6):475–486.
- , , , , , . Early anticoagulation is associated with reduced mortality for acute pulmonary embolism. Chest. 2010;137(6):1382–1390.
- , , . Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet. 1999;353(9162):1386–1389.
- University of North Carolina Air Care website. Available at: http://www.unchealthcare.org/site/Nursing/servicelines/aircare/2009npsg. National Patient Safety Goal/NPSG.03.05.01. http://www.unchealthcare.org/site/Nursing/servicelines/aircare/. Accessed November 1, 2013.
- National consensus standards for the prevention and care of deep vein thrombosis (DVT) project between The Joint Commission and the National Quality Forum (NQF) 2008. Available at: http://www.jointcommission.org/venous_thromboembolism. Accessed November 1, 2013.
- , , , et al. Health Care disparities on venous thromboembolism based on insurance status in the United States. J Thromb Thrombolysis. 2011:32(4):393–398.
- , ; INNOVATE Investigators. Community‐based treatment of venous thromboembolism with a low‐molecular weight heparin and warfarin. J Thromb Thrombolysis. 2007;24(3):225–232.
- , , , et al. Implementation of a clinical pathway for emergency department out‐patient management of deep vein thrombosis. Ir Med J. 2010;103(8):246–248.
- . Outpatient‐based treatment protocols in the management of venous thromboembolic disease. Am J Manag Care. 2000;6(20 suppl):S1034–S1044.
- , , , et al. Cost savings and effectiveness of outpatient treatment with low molecular weight heparin of deep vein thrombosis in a community hospital. Can J Clin Pharmacol. 2004;11(1):e17–e27.
- , . Outpatient treatment of deep venous thrombosis: a clinical care pathway managed by the emergency department. Ann Emerg Med. 2001;37(3):251–258.
- , , . Effectiveness and economic impact associated with a program for outpatient management of acute deep vein thrombosis in a group model health maintenance organization. Arch Intern Med. 2000;160(19):2926–2932.
- , , , et al. Outpatient treatment of deep venous thrombosis in diverse inner‐city patients. Am J Med. 2001;110(6):458–462.
- , , , et al. Insurance status and access to urgent ambulatory care follow‐up appointments. JAMA. 2005;294(10):1248–1254.
- , . Follow‐up after hospital discharge: does insurance make a difference? J Health Care Poor Underserved. 1993;4(2):133–142.
- , , . Emergency department visits by persons recently discharged from U.S. hospitals. Natl Health Stat Report. 2008;(6):1–9.
- . Insurance coverage, medical care use, and short‐term health changes following an unintentional injury or the onset of a chronic condition. JAMA. 2007;297(10):1073–1084.
- , , . Health insurance and access to care for symptomatic conditions. Arch Intern Med. 2000;160(9):1269–1274.
- , , , et al. Delayed access to health care: risk factors, reasons, and consequences. Ann Intern Med. 1991;114(4):325–331.
- , , . Post‐hospitalization transitions: examining the effects of timing of primary care provider follow‐up. J Hosp Med. 2010;5:392–397.
- , , , et al. Medicaid patients seen at federally qualified health centers use hospital services less than those seen by private providers. Health Aff (Millwood). 2011;30(7):1335–1342.
- , , . Do public health clinics reduce rehospitalizations?: the urban diabetes study. J Health Care Poor Underserved. 2008;19(2):562–573.
- , , , et al. Impact of an internet‐based emergency department appointment system to access primary care at safety net community clinics. Ann Emerg Med. 2009;54(2):279–284.
- , , , et al. Racial disparities in patient safety indicator (psi) rates in the veterans health administration. In: Henriksen K, Battles JB, Keyes MA, Grady ML, eds. Advances in Patient Safety: New Directions and Alternative Approaches. Vol. 1: Assessment. Rockville, MD: Agency for Healthcare Research and Quality; 2008. Available at: http://www.ncbi.nlm.nih.gov/books/NBK43651, Accessed November 1, 2013.
- , , , et al. The impact of follow‐up telephone calls to patients after hospitalization. Dis Mon. 2002;48(4):239–248.
Venous thromboembolism (VTE), including deep venous thrombosis (DVT) and pulmonary embolism (PE), is common, costly, and often fatal. Annual VTE incidence in the United States is over 1 million, including over 220,000 PE patients who have an average hospital length of stay (LOS) of 8 days, with a rising per‐patient cost of over $40,000.[1, 2] Nearly half of all PE readmissions occur within 30 days; recurrent DVT events are 21% more costly than the initial event.[3] Likewise, 30‐day PE mortality is 8%, with most deaths occurring within 1 hour of initial presentation.[4, 5]
Rapid implementation of therapeutic anticoagulation has reduced morbidity and mortality in VTE. Ineffective and untimely treatment increases disease progression, significant medication‐related adverse events, and cost. The Joint Commission recognized this risk and included National Patient Safety Goal 3.5.01 to reduce adverse events.[6] Appropriate use of anticoagulation was further emphasized by national quality initiatives through Joint Commission VTE core measures endorsed by the National Quality Forum and the Centers for Medicare and Medicaid Services.[7]
Many models of outpatient VTE care pathways exist. Early models focused on the feasibility of low‐molecular‐weight heparins (LMWH) in the ambulatory setting, with transition to long‐term warfarin. Focus shifted to comprehensive disease pathway implementation aimed at reducing healthcare resource utilization. These pathways have reduced cost and unnecessary hospital stays and minimized complications through enrolling low‐risk patients. To our knowledge, results of an interdisciplinary VTE care pathway have not been published from a large urban academic institution, where a substantial uninsured population exists.
Examining baseline VTE practices and care delivered at our institution provided critical knowledge in effectively developing a novel model of care. Prior to pathway development, acute VTE patients were typically admitted for initiation of therapeutic anticoagulation and appropriate overlap of injectable anticoagulants with warfarin. Significant healthcare disparities were seen among VTE patients at our institution: uninsured patients stayed in the hospital 2 additional days and accumulated twice the rate of 30‐day emergency department (ED) reutilization and cost than insured patients.[8] Discharged VTE patients were managed through a pharmacy‐run anticoagulation clinic pending primary care provider (PCP) follow‐up. We speculated many uninsured VTE patients lacked sufficient disease and treatment information, and lacked surveillance and timely access to medical care following hospitalization. We hypothesized that through (1) targeted education of patients and providers, (2) coordination of timely follow‐up for at‐risk patients, and (3) posthospital monitoring, we could achieve standardized care for all acute DVT and low‐risk PE patients. As a result, we aimed to decrease hospital LOS and produce fewer return visits and readmissions.
METHODS
Study Setting and Population
Acute medical VTE patients were targeted, where they were either discharged directly from the ED or admitted to a medicine service. Acute VTE was defined as primary or secondary diagnosis of new, lower extremity DVT, PE or concurrent DVT, and PE. Patients were identified and tracked by a professional research assistant (PRA) using our electronic medical record (EMR) search filter of all 120 discharge diagnoses for acute DVT and PE.
Our hospital is a 375‐bed, academic medical center in a metropolitan area of under 3 million people. ED volume is approximately 55,000 patients per year.
Exclusion Criteria
We excluded patients classified as surgical/postoperative/admitted to a surgery service, pregnant/postpartum/admitted to an obstetrical service, hospital direct admissions (including outside hospital transfers), and oncology service admissions. Clinically unstable patients requiring intensive care unit admission and/or thrombolytic therapy, and patients with upper extremity, recurrent, or catheter‐associated VTE were also excluded. To allow for comparative data, exclusion criteria were similar to those used in the historical, retrospective chart review performed previously at our institution.[8]
VTE Clinical Care Pathway
The pathway was developed as a quality improvement project through a multidisciplinary, collaborative effort, including pharmacists (inpatient and outpatient), administrative staff in the anticoagulation clinic, nurse leaders and educators, physician faculty (ED, inpatient and outpatient), case managers (inpatient and ED), and providers from local community health clinics, who provide the majority of follow‐up care for our uninsured patients.
We sought care standardization and system‐wide education for all acute, medical, lower‐extremity DVT and low‐risk PE patients, with a focus on coordination of transitional care. All pathway patients were provided education, lab testing, and outpatient medications including LMWH and warfarin. For patients lacking insurance, medications were provided through a medication assistance program at no cost to the patient. Timely outpatient clinic follow‐up and posthospital phone calls were targeted safety net features to facilitate timely hospital discharge and program success. We also aimed to meet nationally mandated quality of care measures and benchmarks. Funding for this project, obtained through a quality improvement (QI) grant from the hospital supported a PRA and educational materials.
The Colorado Multiple Institutional Review Board approved the protocol prior to study implementation. Specific elements of the care pathway have been outlined (see Supporting Information, Figure 1, in the online version of this article). The initial rollout of the program occurred as a pilot in the ED for patients presenting with DVT only to assess feasibility. Based on this success, the pathway team expanded the program to inpatients, including those with PE, and augmented the educational program.
Measures
Evaluation of the intervention was completed by real‐time chart extraction and phone interviews within 72 hours of hospital discharge and a chart review at 6 weeks following discharge. Chart review determined the number of follow‐up visits within 30 days to the anticoagulation clinic and episodes of recidivism. Study data (n=241) were obtained from February 1, 2011 to June 30, 2012 and compared to previously published retrospective data on VTE patients at our institution (n=234) from December 1, 2007 to April 4, 2009.8
We obtained patient demographics (age, gender, ethnicity, insurance category) and admission status from the EMR. We collected data on ED recidivism within 30 days (for VTE‐related issues), LOS, and readmissions within 30 days of discharge. We also collected total cost data for all VTE care from hospital administrative billing data including initial presentation and VTE‐related return visits to the ED and readmissions.
Outcomes
Descriptive information, including demographics, admission status and type of VTE event are summarized for the VTE care pathway. Pathway patients, stratified by payer status (uninsured vs insured), were compared to previously described historical controls.[8] Primary outcomes included comparisons of total costs, LOS, and 30‐day ED recidivism and hospital readmission rates. Further comparisons were made between insured and uninsured patients on these same outcomes.
Data Analysis
Data are presented as proportions or meanstandard deviation unless indicated otherwise. Categorical data were compared using the Fisher exact test or 2 test, where appropriate. Continuous variables were compared using the Student t test. All tests were 2‐tailed. Statistical analyses of the results were performed using GraphPad Prism 4.0 (GraphPad Software, San Diego, CA) and InStat 3.06 (GraphPad Software). A P value <0.05 was considered statistically significant for this study.
RESULTS
Care Pathway Cohort
We enrolled 241 medical patients with acute VTE during the 19‐month study period (Table 1). Of these, 107 (44.4%) presented with DVT alone, whereas the remaining 134 (55.6%) had PE. Eighty‐eight of the 241 VTE patients were uninsured (36.5%). Uninsured patients were younger on average (46.7 vs 55.5 years; P<0.0001) and more commonly presented with DVT only (58.0% vs 36.7%; P=0.036).
| Patients, N=241 | Uninsured, N=88 | Insured, N=153 | P Value | |
|---|---|---|---|---|
| ||||
| Mean age, y (SD) | 52.4 (15.8) | 46.7 (13.9) | 55.5 (16.1) | <0.0001 |
| Median age, y (IQR) | 53 (4263.5) | 56 (44.367) | 49 (35.358.5) | |
| Gender, male, n (%) | 113 (46.9) | 44 (50.0) | 69 (45.1) | 0.548 |
| Pulmonary embolism, n (%) | 134 (55.6) | 37 (42.0) | 97 (63.3) | 0.036 |
| All VTE, hospital admission, n (%) | 179 (74.3) | 58 (65.9) | 121 (79.1) | 0.032 |
| PE, hospital admission, n (%) | 132 (54.8) | 37 (42.0) | 95 (97.9) | 1.00 |
Utilizing the pathway, the majority of VTE patients (179; 74.3%) were admitted to the hospital. Among the uninsured, 58 of 88 (65.9%) patients were admitted compared to 121 of 153 (79.1%) among the insured (P=0.032). Among 107 DVT patients, 47 were admitted (43.9%), including 20 of 51 uninsured DVT patients (39.2%) compared to 27 of 56 insured DVT patients (48.2%). Nearly all PE patients (132 of 134; 98.5%) were admitted. Two insured PE patients were not admitted.
Care Pathway Versus Historical Cohort
Comparing VTE care pathway patients to historical VTE patients (prior to intervention), the age and gender, as well as number of VTE events, VTE type, and admission status were similar (Table 2).
| Outcome | Historical VTE, N=234 | Pathway VTE, N=241 | P Value |
|---|---|---|---|
| |||
| Age, y, mean | 53.1 | 52.4 | 0.64 |
| Male, n (%) | 125 (53.4) | 113 (47.0) | 0.46 |
| DVT (%) | 106 (45.3) | 107 (44.4) | 0.92 |
| Uninsured (%) | 38 (35.8) | 51 (47.7) | 0.93 |
| PE (%) | 128 (54.7) | 134 (55.6) | 0.92 |
| Uninsured (%) | 29 (22.7) | 38 (28.4) | 0.11 |
| Admitted (%) | 171 (73.1) | 179 (74.3) | 0.85 |
| DVT (%) | 43 (40.6) | 47 (43.9) | 0.91 |
| Uninsured (%) | 17 (39.6) | 20 (42.6) | 0.94 |
| PE (%) | 128 (100) | 132 (98.5) | 0.91 |
| Uninsured (%) | 29 (100) | 38 (100) | 0.32 |
| LOS, d, mean (SD) | 4.4 (3.8) | 3.1 (2.9) | <0.001 |
| Uninsured | 5.9 (5.1) | 3.1 (2.9) | <0.001 |
| Insured | 3.8 (3.1) | 3.1 (2.9) | 0.69 |
| ED revisit, n (%) | 26 (11.1) | 27 (11.2) | 0.974 |
| Uninsured, n (%) | 12 (17.9) | 12 (13.6) | 0.59 |
| Readmission, n (%) | 16 (9.4) | 10 (5.6) | 0.25 |
| Uninsured, n (%) | 5 (10.9) | 2 (3.4) | 0.24 |
| Total cost, $, mean (SD) | 7610 (9988) | 5295 (7975) | 0.005 |
| Uninsured | 9953 (14211) | 4304 (6596) | 0.001 |
| Insured | 6698 (7564) | 5875 (8650) | 0.36 |
| Cost, admitted, $, mean (SD) | 10324 (8988) | 7038 (8965) | 0.044 |
| Uninsured | 14420 (13351) | 6375 (7462) | 0.005 |
| Insured | 8843 (6565) | 7353 (9288) | 0.599 |
Average hospital LOS for an admitted care pathway patient was 3.1 days versus 4.4 days in an historical VTE patient (P=0.0001; Table 2). When stratified by insurance, uninsured pathway patients had a LOS of 3.1, decreased from a prepathway LOS of 5.9 days (P=0.0006), whereas this did not change among insured patients (3.1 from 3.8 days [P=0.688]).
For all VTE care pathway patients, 30‐day ED recidivism was 11.2%, similar to prepathway data (11.1%; Table 2). This was true regardless of insurance status. Thirty‐day readmission rates trended from 9.4% prepathway to 5.6% postpathway (P=0.254) (Table 2). Compared to historical VTE patients, uninsured pathway patients had readmission rates of 3.4% from 10.9% (P=0.237), whereas readmission rates for insured patients were 6.6% from 8.8% (P=0.686).
Average cost for a VTE care pathway patient was $5295 compared to an historical cost of $7610 per VTE patient (P <0.005). Among uninsured pathway patients, the cost of VTE care was $4304 compared to $9953 historically (P=0.001). Among insured pathway patients, the cost of VTE care was $5875 compared to an historical cost of $6698 (P=0.365).
The average VTE cost of care for an admitted pathway patient was $7038 versus $10,324 per admitted historical patient (P=0.044). For an admitted uninsured VTE pathway patient, cost was $6375 versus $14,420 per historical VTE patient (P=0.005). For an admitted insured VTE pathway patient, the cost was $7353 versus $8843 per historical VTE patient (P=0.599).
Patient satisfaction scores with the care pathway averaged 4.5 (15 Likert scale).
DISCUSSION
Development and implementation of a multidisciplinary VTE clinical care pathway at our institution represents success across multiple domains. As a QI project, we standardized care and delivered system‐wide education, and provided solutions to existing gaps in posthospital care. This pathway for a common, dangerous disease requiring high‐risk medications magnifies the importance of care delivered at vulnerable points. Results of our study are the first to our knowledge to mitigate healthcare disparities and reduce healthcare utilization through a care pathway across diverse populations. Hospital LOS for all VTE patients was significantly decreased, wile lowering hospital reutilization patterns, particularly among the uninsured. Hospital admission rates are now lower specifically for the uninsured patients, because ED and inpatient providers now have increased confidence in the follow‐up arrangements with the safety‐net clinics.
Many clinical care pathways for VTE are proven, safe, and cost‐effective.[9, 10, 11, 12] Outpatient DVT treatment delivers significant cost savings and averts unnecessary hospital stays.[13, 14] A hospital‐based program providing outpatient DVT treatment among inner‐city patients in New York demonstrated a lower incidence of adverse events and substantial cost savings, but excluded PE patients.[15] We intentionally sought to expand our VTE program by including both PE and vulnerable uninsured patients.
Lack of health insurance and routine primary care is a major challenge to successful implementation of any care pathway. Access to timely posthospital follow‐up care is far more limited in patients lacking private insurance.[16, 17] Uninsured patients are less likely to receive necessary medical care and more likely to have delayed care.[18, 19] Uninsured patients also have poorer short‐term health and are nearly 3 times more likely to have an ED revisit following hospital discharge than insured patients.[16, 20, 21] At our own institution, many discharged medical patients lack timely PCP follow‐up, especially the uninsured, leading to higher rates of hospital reutilization.[22] Interventions directed at the uninsured VTE patient to mitigate such disparities were specifically targeted. These included coordination of timely follow‐up care in community health clinics and provision of posthospital phone calls.
Efforts to improve transitional care for vulnerable patients have proven successful. Patients linked from the ED to community health clinics through scheduled follow‐up have improved frequency of follow‐up, receive routine care, and have reduced hospital utilization and rehospitalization.[23, 24, 25] Conversely, fewer care disparities are realized by patients within integrated systems such as the Veterans Administration.[26] Thus, the ultimate development of a VTE care pathway at our nonintegrated hospital required an innovative paradigm to deliver acute DVT and PE care. Through examining existing processes of our VTE care, we hypothesized that the main contributors of baseline care deficiencies included inadequate system‐wide education, fragmented care, and significant barriers to timely follow‐up.
Education of providers, patients, and system‐wide process change were key elements in pathway implementation. Provider educational opportunities concerning VTE disease and treatment were identified, including safe and effective outpatient management options. We anticipated provider reluctance prescribing potentially dangerous anticoagulation medications to otherwise stable patients who might lack close posthospital supervision (eg, ED clinicians accustomed to admitting patients and inpatient teams cautious in discharging patients). We postulated that patients received inadequate VTE education and lacked appropriate skills to effectively and safely manage their new disease and medications. The diverse educational components outlined within the pathway significantly contributed to improved provider confidence in their patients' follow‐up care as well as their patients' comprehension of their disease.
Timely posthospital care follow‐up for all VTE patients significantly impacted our pathway results. Historically, uninsured patients lacked primary care follow‐up, often waiting 3 months for an initial clinic visit. Through timely care coordination with local community health clinics, uninsured VTE care pathway patients discharged from our facility are routinely scheduled to be seen within 72 hours. Posthospital care is further addressed through follow‐up phone calls, which monitor patient understanding and care, and identify how and where potential medical needs are best met. Such calls increase patient satisfaction, resolve medication issues, and result in fewer ED return visits.[27] With our intervention, patient satisfaction scores averaged 4.5 (15 Likert scale), reflecting strong support for phone calls and overall experience.
Direct institutional annual cost savings realized with the VTE care pathway was $452,460. This occurred primarily as a result of nearly 50% fewer inpatient days required for admitted VTE patients. Indirect cost savings were further accomplished through increased availability of high‐demand outpatient anticoagulation visits given improved timely PCP follow‐up. Prior to pathway implementation, uninsured patients frequently had multiple, often unreimbursed, visits to this clinic while awaiting PCP follow‐up. Additional future cost savings may occur as healthcare reimbursement patterns are likely to include methods to penalize inefficient and high‐resource usage.
There are several limitations to our study. This was a single‐institution quality program with relatively small numbers. Comparison of pathway data with historical data provides an interval lag that may miss temporal changes in medical practice and disease trends. However, we believe the practice of VTE treatment changed minimally between the 2 time periods. We identified virtually the same number and type of patients in each cohort. Physician and PRA staff turnover complicated tracking patients and challenged continuous system‐wide education. However, we believe consistent education and feedback to PRA faculty throughout the study period minimized variability. Although we could not verify VTE presentations to outside hospitals other than by patient self‐report, it is likely that our patient population would have re‐presented to our institution for follow‐up VTE needs or bleeding concerns. As a result of timely follow‐up phone calls, the number of return visits to the hospital may have been magnified, because more educated patients may have overreacted to mild symptom changes. Prior to the intervention, discharged VTE patients may not have recognized signs and symptoms of worsening disease or may not have returned to our institution for follow‐up needs. Last, we did not control for comorbidities in either cohort, which may affect hospital utilization patterns, as younger patients may be less likely to be admitted or insured.
As a result of a comprehensive VTE clinical care pathway developed by key stakeholders, acute VTE patients who present to our hospital are therapeutically anticoagulated and monitored in a timely, uniform, and safe manner. We believe success reflects system‐wide education and standardization of care through reducing variation, including the high‐risk posthospital period. In an era of fragmented medical care, this program closes existing gaps in care and addresses the needs of vulnerable patients through strong collaboration and efficient coordination with local community health clinics. This is especially important in a dynamic healthcare landscape with an evolving payer mix that demands the medical establishment seek innovative ways to improve quality of care while reducing cost. Future research should explore etiologies and impacts of outcome variability based on insurance status, and identify other conditions and institutions demonstrating care disparities. Ultimately, implementation of this pathway provides strong evidence for improving care, meeting Joint Commission anticoagulation patient safety goals, and conserving limited resources for a common and deadly disease.
Acknowledgements
The authors thank Sancia Tonn, PRA, Carol Kemp‐Jackson from University of Colorado outpatient anticoagulation clinic, and the Metro Community Provider Network Clinics.
Disclosures
This project was funded by University of Colorado Hospital QI Small Grants Program. Preliminary results of this pathway were previously presented at the 2012 Society of Hospital Medicine Annual Meeting, San Diego, California, April 14, 2012.
Venous thromboembolism (VTE), including deep venous thrombosis (DVT) and pulmonary embolism (PE), is common, costly, and often fatal. Annual VTE incidence in the United States is over 1 million, including over 220,000 PE patients who have an average hospital length of stay (LOS) of 8 days, with a rising per‐patient cost of over $40,000.[1, 2] Nearly half of all PE readmissions occur within 30 days; recurrent DVT events are 21% more costly than the initial event.[3] Likewise, 30‐day PE mortality is 8%, with most deaths occurring within 1 hour of initial presentation.[4, 5]
Rapid implementation of therapeutic anticoagulation has reduced morbidity and mortality in VTE. Ineffective and untimely treatment increases disease progression, significant medication‐related adverse events, and cost. The Joint Commission recognized this risk and included National Patient Safety Goal 3.5.01 to reduce adverse events.[6] Appropriate use of anticoagulation was further emphasized by national quality initiatives through Joint Commission VTE core measures endorsed by the National Quality Forum and the Centers for Medicare and Medicaid Services.[7]
Many models of outpatient VTE care pathways exist. Early models focused on the feasibility of low‐molecular‐weight heparins (LMWH) in the ambulatory setting, with transition to long‐term warfarin. Focus shifted to comprehensive disease pathway implementation aimed at reducing healthcare resource utilization. These pathways have reduced cost and unnecessary hospital stays and minimized complications through enrolling low‐risk patients. To our knowledge, results of an interdisciplinary VTE care pathway have not been published from a large urban academic institution, where a substantial uninsured population exists.
Examining baseline VTE practices and care delivered at our institution provided critical knowledge in effectively developing a novel model of care. Prior to pathway development, acute VTE patients were typically admitted for initiation of therapeutic anticoagulation and appropriate overlap of injectable anticoagulants with warfarin. Significant healthcare disparities were seen among VTE patients at our institution: uninsured patients stayed in the hospital 2 additional days and accumulated twice the rate of 30‐day emergency department (ED) reutilization and cost than insured patients.[8] Discharged VTE patients were managed through a pharmacy‐run anticoagulation clinic pending primary care provider (PCP) follow‐up. We speculated many uninsured VTE patients lacked sufficient disease and treatment information, and lacked surveillance and timely access to medical care following hospitalization. We hypothesized that through (1) targeted education of patients and providers, (2) coordination of timely follow‐up for at‐risk patients, and (3) posthospital monitoring, we could achieve standardized care for all acute DVT and low‐risk PE patients. As a result, we aimed to decrease hospital LOS and produce fewer return visits and readmissions.
METHODS
Study Setting and Population
Acute medical VTE patients were targeted, where they were either discharged directly from the ED or admitted to a medicine service. Acute VTE was defined as primary or secondary diagnosis of new, lower extremity DVT, PE or concurrent DVT, and PE. Patients were identified and tracked by a professional research assistant (PRA) using our electronic medical record (EMR) search filter of all 120 discharge diagnoses for acute DVT and PE.
Our hospital is a 375‐bed, academic medical center in a metropolitan area of under 3 million people. ED volume is approximately 55,000 patients per year.
Exclusion Criteria
We excluded patients classified as surgical/postoperative/admitted to a surgery service, pregnant/postpartum/admitted to an obstetrical service, hospital direct admissions (including outside hospital transfers), and oncology service admissions. Clinically unstable patients requiring intensive care unit admission and/or thrombolytic therapy, and patients with upper extremity, recurrent, or catheter‐associated VTE were also excluded. To allow for comparative data, exclusion criteria were similar to those used in the historical, retrospective chart review performed previously at our institution.[8]
VTE Clinical Care Pathway
The pathway was developed as a quality improvement project through a multidisciplinary, collaborative effort, including pharmacists (inpatient and outpatient), administrative staff in the anticoagulation clinic, nurse leaders and educators, physician faculty (ED, inpatient and outpatient), case managers (inpatient and ED), and providers from local community health clinics, who provide the majority of follow‐up care for our uninsured patients.
We sought care standardization and system‐wide education for all acute, medical, lower‐extremity DVT and low‐risk PE patients, with a focus on coordination of transitional care. All pathway patients were provided education, lab testing, and outpatient medications including LMWH and warfarin. For patients lacking insurance, medications were provided through a medication assistance program at no cost to the patient. Timely outpatient clinic follow‐up and posthospital phone calls were targeted safety net features to facilitate timely hospital discharge and program success. We also aimed to meet nationally mandated quality of care measures and benchmarks. Funding for this project, obtained through a quality improvement (QI) grant from the hospital supported a PRA and educational materials.
The Colorado Multiple Institutional Review Board approved the protocol prior to study implementation. Specific elements of the care pathway have been outlined (see Supporting Information, Figure 1, in the online version of this article). The initial rollout of the program occurred as a pilot in the ED for patients presenting with DVT only to assess feasibility. Based on this success, the pathway team expanded the program to inpatients, including those with PE, and augmented the educational program.
Measures
Evaluation of the intervention was completed by real‐time chart extraction and phone interviews within 72 hours of hospital discharge and a chart review at 6 weeks following discharge. Chart review determined the number of follow‐up visits within 30 days to the anticoagulation clinic and episodes of recidivism. Study data (n=241) were obtained from February 1, 2011 to June 30, 2012 and compared to previously published retrospective data on VTE patients at our institution (n=234) from December 1, 2007 to April 4, 2009.8
We obtained patient demographics (age, gender, ethnicity, insurance category) and admission status from the EMR. We collected data on ED recidivism within 30 days (for VTE‐related issues), LOS, and readmissions within 30 days of discharge. We also collected total cost data for all VTE care from hospital administrative billing data including initial presentation and VTE‐related return visits to the ED and readmissions.
Outcomes
Descriptive information, including demographics, admission status and type of VTE event are summarized for the VTE care pathway. Pathway patients, stratified by payer status (uninsured vs insured), were compared to previously described historical controls.[8] Primary outcomes included comparisons of total costs, LOS, and 30‐day ED recidivism and hospital readmission rates. Further comparisons were made between insured and uninsured patients on these same outcomes.
Data Analysis
Data are presented as proportions or meanstandard deviation unless indicated otherwise. Categorical data were compared using the Fisher exact test or 2 test, where appropriate. Continuous variables were compared using the Student t test. All tests were 2‐tailed. Statistical analyses of the results were performed using GraphPad Prism 4.0 (GraphPad Software, San Diego, CA) and InStat 3.06 (GraphPad Software). A P value <0.05 was considered statistically significant for this study.
RESULTS
Care Pathway Cohort
We enrolled 241 medical patients with acute VTE during the 19‐month study period (Table 1). Of these, 107 (44.4%) presented with DVT alone, whereas the remaining 134 (55.6%) had PE. Eighty‐eight of the 241 VTE patients were uninsured (36.5%). Uninsured patients were younger on average (46.7 vs 55.5 years; P<0.0001) and more commonly presented with DVT only (58.0% vs 36.7%; P=0.036).
| Patients, N=241 | Uninsured, N=88 | Insured, N=153 | P Value | |
|---|---|---|---|---|
| ||||
| Mean age, y (SD) | 52.4 (15.8) | 46.7 (13.9) | 55.5 (16.1) | <0.0001 |
| Median age, y (IQR) | 53 (4263.5) | 56 (44.367) | 49 (35.358.5) | |
| Gender, male, n (%) | 113 (46.9) | 44 (50.0) | 69 (45.1) | 0.548 |
| Pulmonary embolism, n (%) | 134 (55.6) | 37 (42.0) | 97 (63.3) | 0.036 |
| All VTE, hospital admission, n (%) | 179 (74.3) | 58 (65.9) | 121 (79.1) | 0.032 |
| PE, hospital admission, n (%) | 132 (54.8) | 37 (42.0) | 95 (97.9) | 1.00 |
Utilizing the pathway, the majority of VTE patients (179; 74.3%) were admitted to the hospital. Among the uninsured, 58 of 88 (65.9%) patients were admitted compared to 121 of 153 (79.1%) among the insured (P=0.032). Among 107 DVT patients, 47 were admitted (43.9%), including 20 of 51 uninsured DVT patients (39.2%) compared to 27 of 56 insured DVT patients (48.2%). Nearly all PE patients (132 of 134; 98.5%) were admitted. Two insured PE patients were not admitted.
Care Pathway Versus Historical Cohort
Comparing VTE care pathway patients to historical VTE patients (prior to intervention), the age and gender, as well as number of VTE events, VTE type, and admission status were similar (Table 2).
| Outcome | Historical VTE, N=234 | Pathway VTE, N=241 | P Value |
|---|---|---|---|
| |||
| Age, y, mean | 53.1 | 52.4 | 0.64 |
| Male, n (%) | 125 (53.4) | 113 (47.0) | 0.46 |
| DVT (%) | 106 (45.3) | 107 (44.4) | 0.92 |
| Uninsured (%) | 38 (35.8) | 51 (47.7) | 0.93 |
| PE (%) | 128 (54.7) | 134 (55.6) | 0.92 |
| Uninsured (%) | 29 (22.7) | 38 (28.4) | 0.11 |
| Admitted (%) | 171 (73.1) | 179 (74.3) | 0.85 |
| DVT (%) | 43 (40.6) | 47 (43.9) | 0.91 |
| Uninsured (%) | 17 (39.6) | 20 (42.6) | 0.94 |
| PE (%) | 128 (100) | 132 (98.5) | 0.91 |
| Uninsured (%) | 29 (100) | 38 (100) | 0.32 |
| LOS, d, mean (SD) | 4.4 (3.8) | 3.1 (2.9) | <0.001 |
| Uninsured | 5.9 (5.1) | 3.1 (2.9) | <0.001 |
| Insured | 3.8 (3.1) | 3.1 (2.9) | 0.69 |
| ED revisit, n (%) | 26 (11.1) | 27 (11.2) | 0.974 |
| Uninsured, n (%) | 12 (17.9) | 12 (13.6) | 0.59 |
| Readmission, n (%) | 16 (9.4) | 10 (5.6) | 0.25 |
| Uninsured, n (%) | 5 (10.9) | 2 (3.4) | 0.24 |
| Total cost, $, mean (SD) | 7610 (9988) | 5295 (7975) | 0.005 |
| Uninsured | 9953 (14211) | 4304 (6596) | 0.001 |
| Insured | 6698 (7564) | 5875 (8650) | 0.36 |
| Cost, admitted, $, mean (SD) | 10324 (8988) | 7038 (8965) | 0.044 |
| Uninsured | 14420 (13351) | 6375 (7462) | 0.005 |
| Insured | 8843 (6565) | 7353 (9288) | 0.599 |
Average hospital LOS for an admitted care pathway patient was 3.1 days versus 4.4 days in an historical VTE patient (P=0.0001; Table 2). When stratified by insurance, uninsured pathway patients had a LOS of 3.1, decreased from a prepathway LOS of 5.9 days (P=0.0006), whereas this did not change among insured patients (3.1 from 3.8 days [P=0.688]).
For all VTE care pathway patients, 30‐day ED recidivism was 11.2%, similar to prepathway data (11.1%; Table 2). This was true regardless of insurance status. Thirty‐day readmission rates trended from 9.4% prepathway to 5.6% postpathway (P=0.254) (Table 2). Compared to historical VTE patients, uninsured pathway patients had readmission rates of 3.4% from 10.9% (P=0.237), whereas readmission rates for insured patients were 6.6% from 8.8% (P=0.686).
Average cost for a VTE care pathway patient was $5295 compared to an historical cost of $7610 per VTE patient (P <0.005). Among uninsured pathway patients, the cost of VTE care was $4304 compared to $9953 historically (P=0.001). Among insured pathway patients, the cost of VTE care was $5875 compared to an historical cost of $6698 (P=0.365).
The average VTE cost of care for an admitted pathway patient was $7038 versus $10,324 per admitted historical patient (P=0.044). For an admitted uninsured VTE pathway patient, cost was $6375 versus $14,420 per historical VTE patient (P=0.005). For an admitted insured VTE pathway patient, the cost was $7353 versus $8843 per historical VTE patient (P=0.599).
Patient satisfaction scores with the care pathway averaged 4.5 (15 Likert scale).
DISCUSSION
Development and implementation of a multidisciplinary VTE clinical care pathway at our institution represents success across multiple domains. As a QI project, we standardized care and delivered system‐wide education, and provided solutions to existing gaps in posthospital care. This pathway for a common, dangerous disease requiring high‐risk medications magnifies the importance of care delivered at vulnerable points. Results of our study are the first to our knowledge to mitigate healthcare disparities and reduce healthcare utilization through a care pathway across diverse populations. Hospital LOS for all VTE patients was significantly decreased, wile lowering hospital reutilization patterns, particularly among the uninsured. Hospital admission rates are now lower specifically for the uninsured patients, because ED and inpatient providers now have increased confidence in the follow‐up arrangements with the safety‐net clinics.
Many clinical care pathways for VTE are proven, safe, and cost‐effective.[9, 10, 11, 12] Outpatient DVT treatment delivers significant cost savings and averts unnecessary hospital stays.[13, 14] A hospital‐based program providing outpatient DVT treatment among inner‐city patients in New York demonstrated a lower incidence of adverse events and substantial cost savings, but excluded PE patients.[15] We intentionally sought to expand our VTE program by including both PE and vulnerable uninsured patients.
Lack of health insurance and routine primary care is a major challenge to successful implementation of any care pathway. Access to timely posthospital follow‐up care is far more limited in patients lacking private insurance.[16, 17] Uninsured patients are less likely to receive necessary medical care and more likely to have delayed care.[18, 19] Uninsured patients also have poorer short‐term health and are nearly 3 times more likely to have an ED revisit following hospital discharge than insured patients.[16, 20, 21] At our own institution, many discharged medical patients lack timely PCP follow‐up, especially the uninsured, leading to higher rates of hospital reutilization.[22] Interventions directed at the uninsured VTE patient to mitigate such disparities were specifically targeted. These included coordination of timely follow‐up care in community health clinics and provision of posthospital phone calls.
Efforts to improve transitional care for vulnerable patients have proven successful. Patients linked from the ED to community health clinics through scheduled follow‐up have improved frequency of follow‐up, receive routine care, and have reduced hospital utilization and rehospitalization.[23, 24, 25] Conversely, fewer care disparities are realized by patients within integrated systems such as the Veterans Administration.[26] Thus, the ultimate development of a VTE care pathway at our nonintegrated hospital required an innovative paradigm to deliver acute DVT and PE care. Through examining existing processes of our VTE care, we hypothesized that the main contributors of baseline care deficiencies included inadequate system‐wide education, fragmented care, and significant barriers to timely follow‐up.
Education of providers, patients, and system‐wide process change were key elements in pathway implementation. Provider educational opportunities concerning VTE disease and treatment were identified, including safe and effective outpatient management options. We anticipated provider reluctance prescribing potentially dangerous anticoagulation medications to otherwise stable patients who might lack close posthospital supervision (eg, ED clinicians accustomed to admitting patients and inpatient teams cautious in discharging patients). We postulated that patients received inadequate VTE education and lacked appropriate skills to effectively and safely manage their new disease and medications. The diverse educational components outlined within the pathway significantly contributed to improved provider confidence in their patients' follow‐up care as well as their patients' comprehension of their disease.
Timely posthospital care follow‐up for all VTE patients significantly impacted our pathway results. Historically, uninsured patients lacked primary care follow‐up, often waiting 3 months for an initial clinic visit. Through timely care coordination with local community health clinics, uninsured VTE care pathway patients discharged from our facility are routinely scheduled to be seen within 72 hours. Posthospital care is further addressed through follow‐up phone calls, which monitor patient understanding and care, and identify how and where potential medical needs are best met. Such calls increase patient satisfaction, resolve medication issues, and result in fewer ED return visits.[27] With our intervention, patient satisfaction scores averaged 4.5 (15 Likert scale), reflecting strong support for phone calls and overall experience.
Direct institutional annual cost savings realized with the VTE care pathway was $452,460. This occurred primarily as a result of nearly 50% fewer inpatient days required for admitted VTE patients. Indirect cost savings were further accomplished through increased availability of high‐demand outpatient anticoagulation visits given improved timely PCP follow‐up. Prior to pathway implementation, uninsured patients frequently had multiple, often unreimbursed, visits to this clinic while awaiting PCP follow‐up. Additional future cost savings may occur as healthcare reimbursement patterns are likely to include methods to penalize inefficient and high‐resource usage.
There are several limitations to our study. This was a single‐institution quality program with relatively small numbers. Comparison of pathway data with historical data provides an interval lag that may miss temporal changes in medical practice and disease trends. However, we believe the practice of VTE treatment changed minimally between the 2 time periods. We identified virtually the same number and type of patients in each cohort. Physician and PRA staff turnover complicated tracking patients and challenged continuous system‐wide education. However, we believe consistent education and feedback to PRA faculty throughout the study period minimized variability. Although we could not verify VTE presentations to outside hospitals other than by patient self‐report, it is likely that our patient population would have re‐presented to our institution for follow‐up VTE needs or bleeding concerns. As a result of timely follow‐up phone calls, the number of return visits to the hospital may have been magnified, because more educated patients may have overreacted to mild symptom changes. Prior to the intervention, discharged VTE patients may not have recognized signs and symptoms of worsening disease or may not have returned to our institution for follow‐up needs. Last, we did not control for comorbidities in either cohort, which may affect hospital utilization patterns, as younger patients may be less likely to be admitted or insured.
As a result of a comprehensive VTE clinical care pathway developed by key stakeholders, acute VTE patients who present to our hospital are therapeutically anticoagulated and monitored in a timely, uniform, and safe manner. We believe success reflects system‐wide education and standardization of care through reducing variation, including the high‐risk posthospital period. In an era of fragmented medical care, this program closes existing gaps in care and addresses the needs of vulnerable patients through strong collaboration and efficient coordination with local community health clinics. This is especially important in a dynamic healthcare landscape with an evolving payer mix that demands the medical establishment seek innovative ways to improve quality of care while reducing cost. Future research should explore etiologies and impacts of outcome variability based on insurance status, and identify other conditions and institutions demonstrating care disparities. Ultimately, implementation of this pathway provides strong evidence for improving care, meeting Joint Commission anticoagulation patient safety goals, and conserving limited resources for a common and deadly disease.
Acknowledgements
The authors thank Sancia Tonn, PRA, Carol Kemp‐Jackson from University of Colorado outpatient anticoagulation clinic, and the Metro Community Provider Network Clinics.
Disclosures
This project was funded by University of Colorado Hospital QI Small Grants Program. Preliminary results of this pathway were previously presented at the 2012 Society of Hospital Medicine Annual Meeting, San Diego, California, April 14, 2012.
- , , , et al. Economic burden of deep‐vein thrombosis, pulmonary embolism, and post‐thrombotic syndrome. Am J Health Syst Pharm. 2006;63(20 suppl 6):S5–S15.
- , , , et al. Recent trends in clinical outcomes and resource utilization for pulmonary embolism in the United States: findings from the nationwide inpatient sample. Chest. 2009;136(4):983–990.
- , . Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: an administrative claims analysis from 30 managed care organizations. J Manag Care Pharm. 2007;13(6):475–486.
- , , , , , . Early anticoagulation is associated with reduced mortality for acute pulmonary embolism. Chest. 2010;137(6):1382–1390.
- , , . Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet. 1999;353(9162):1386–1389.
- University of North Carolina Air Care website. Available at: http://www.unchealthcare.org/site/Nursing/servicelines/aircare/2009npsg. National Patient Safety Goal/NPSG.03.05.01. http://www.unchealthcare.org/site/Nursing/servicelines/aircare/. Accessed November 1, 2013.
- National consensus standards for the prevention and care of deep vein thrombosis (DVT) project between The Joint Commission and the National Quality Forum (NQF) 2008. Available at: http://www.jointcommission.org/venous_thromboembolism. Accessed November 1, 2013.
- , , , et al. Health Care disparities on venous thromboembolism based on insurance status in the United States. J Thromb Thrombolysis. 2011:32(4):393–398.
- , ; INNOVATE Investigators. Community‐based treatment of venous thromboembolism with a low‐molecular weight heparin and warfarin. J Thromb Thrombolysis. 2007;24(3):225–232.
- , , , et al. Implementation of a clinical pathway for emergency department out‐patient management of deep vein thrombosis. Ir Med J. 2010;103(8):246–248.
- . Outpatient‐based treatment protocols in the management of venous thromboembolic disease. Am J Manag Care. 2000;6(20 suppl):S1034–S1044.
- , , , et al. Cost savings and effectiveness of outpatient treatment with low molecular weight heparin of deep vein thrombosis in a community hospital. Can J Clin Pharmacol. 2004;11(1):e17–e27.
- , . Outpatient treatment of deep venous thrombosis: a clinical care pathway managed by the emergency department. Ann Emerg Med. 2001;37(3):251–258.
- , , . Effectiveness and economic impact associated with a program for outpatient management of acute deep vein thrombosis in a group model health maintenance organization. Arch Intern Med. 2000;160(19):2926–2932.
- , , , et al. Outpatient treatment of deep venous thrombosis in diverse inner‐city patients. Am J Med. 2001;110(6):458–462.
- , , , et al. Insurance status and access to urgent ambulatory care follow‐up appointments. JAMA. 2005;294(10):1248–1254.
- , . Follow‐up after hospital discharge: does insurance make a difference? J Health Care Poor Underserved. 1993;4(2):133–142.
- , , . Emergency department visits by persons recently discharged from U.S. hospitals. Natl Health Stat Report. 2008;(6):1–9.
- . Insurance coverage, medical care use, and short‐term health changes following an unintentional injury or the onset of a chronic condition. JAMA. 2007;297(10):1073–1084.
- , , . Health insurance and access to care for symptomatic conditions. Arch Intern Med. 2000;160(9):1269–1274.
- , , , et al. Delayed access to health care: risk factors, reasons, and consequences. Ann Intern Med. 1991;114(4):325–331.
- , , . Post‐hospitalization transitions: examining the effects of timing of primary care provider follow‐up. J Hosp Med. 2010;5:392–397.
- , , , et al. Medicaid patients seen at federally qualified health centers use hospital services less than those seen by private providers. Health Aff (Millwood). 2011;30(7):1335–1342.
- , , . Do public health clinics reduce rehospitalizations?: the urban diabetes study. J Health Care Poor Underserved. 2008;19(2):562–573.
- , , , et al. Impact of an internet‐based emergency department appointment system to access primary care at safety net community clinics. Ann Emerg Med. 2009;54(2):279–284.
- , , , et al. Racial disparities in patient safety indicator (psi) rates in the veterans health administration. In: Henriksen K, Battles JB, Keyes MA, Grady ML, eds. Advances in Patient Safety: New Directions and Alternative Approaches. Vol. 1: Assessment. Rockville, MD: Agency for Healthcare Research and Quality; 2008. Available at: http://www.ncbi.nlm.nih.gov/books/NBK43651, Accessed November 1, 2013.
- , , , et al. The impact of follow‐up telephone calls to patients after hospitalization. Dis Mon. 2002;48(4):239–248.
- , , , et al. Economic burden of deep‐vein thrombosis, pulmonary embolism, and post‐thrombotic syndrome. Am J Health Syst Pharm. 2006;63(20 suppl 6):S5–S15.
- , , , et al. Recent trends in clinical outcomes and resource utilization for pulmonary embolism in the United States: findings from the nationwide inpatient sample. Chest. 2009;136(4):983–990.
- , . Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: an administrative claims analysis from 30 managed care organizations. J Manag Care Pharm. 2007;13(6):475–486.
- , , , , , . Early anticoagulation is associated with reduced mortality for acute pulmonary embolism. Chest. 2010;137(6):1382–1390.
- , , . Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet. 1999;353(9162):1386–1389.
- University of North Carolina Air Care website. Available at: http://www.unchealthcare.org/site/Nursing/servicelines/aircare/2009npsg. National Patient Safety Goal/NPSG.03.05.01. http://www.unchealthcare.org/site/Nursing/servicelines/aircare/. Accessed November 1, 2013.
- National consensus standards for the prevention and care of deep vein thrombosis (DVT) project between The Joint Commission and the National Quality Forum (NQF) 2008. Available at: http://www.jointcommission.org/venous_thromboembolism. Accessed November 1, 2013.
- , , , et al. Health Care disparities on venous thromboembolism based on insurance status in the United States. J Thromb Thrombolysis. 2011:32(4):393–398.
- , ; INNOVATE Investigators. Community‐based treatment of venous thromboembolism with a low‐molecular weight heparin and warfarin. J Thromb Thrombolysis. 2007;24(3):225–232.
- , , , et al. Implementation of a clinical pathway for emergency department out‐patient management of deep vein thrombosis. Ir Med J. 2010;103(8):246–248.
- . Outpatient‐based treatment protocols in the management of venous thromboembolic disease. Am J Manag Care. 2000;6(20 suppl):S1034–S1044.
- , , , et al. Cost savings and effectiveness of outpatient treatment with low molecular weight heparin of deep vein thrombosis in a community hospital. Can J Clin Pharmacol. 2004;11(1):e17–e27.
- , . Outpatient treatment of deep venous thrombosis: a clinical care pathway managed by the emergency department. Ann Emerg Med. 2001;37(3):251–258.
- , , . Effectiveness and economic impact associated with a program for outpatient management of acute deep vein thrombosis in a group model health maintenance organization. Arch Intern Med. 2000;160(19):2926–2932.
- , , , et al. Outpatient treatment of deep venous thrombosis in diverse inner‐city patients. Am J Med. 2001;110(6):458–462.
- , , , et al. Insurance status and access to urgent ambulatory care follow‐up appointments. JAMA. 2005;294(10):1248–1254.
- , . Follow‐up after hospital discharge: does insurance make a difference? J Health Care Poor Underserved. 1993;4(2):133–142.
- , , . Emergency department visits by persons recently discharged from U.S. hospitals. Natl Health Stat Report. 2008;(6):1–9.
- . Insurance coverage, medical care use, and short‐term health changes following an unintentional injury or the onset of a chronic condition. JAMA. 2007;297(10):1073–1084.
- , , . Health insurance and access to care for symptomatic conditions. Arch Intern Med. 2000;160(9):1269–1274.
- , , , et al. Delayed access to health care: risk factors, reasons, and consequences. Ann Intern Med. 1991;114(4):325–331.
- , , . Post‐hospitalization transitions: examining the effects of timing of primary care provider follow‐up. J Hosp Med. 2010;5:392–397.
- , , , et al. Medicaid patients seen at federally qualified health centers use hospital services less than those seen by private providers. Health Aff (Millwood). 2011;30(7):1335–1342.
- , , . Do public health clinics reduce rehospitalizations?: the urban diabetes study. J Health Care Poor Underserved. 2008;19(2):562–573.
- , , , et al. Impact of an internet‐based emergency department appointment system to access primary care at safety net community clinics. Ann Emerg Med. 2009;54(2):279–284.
- , , , et al. Racial disparities in patient safety indicator (psi) rates in the veterans health administration. In: Henriksen K, Battles JB, Keyes MA, Grady ML, eds. Advances in Patient Safety: New Directions and Alternative Approaches. Vol. 1: Assessment. Rockville, MD: Agency for Healthcare Research and Quality; 2008. Available at: http://www.ncbi.nlm.nih.gov/books/NBK43651, Accessed November 1, 2013.
- , , , et al. The impact of follow‐up telephone calls to patients after hospitalization. Dis Mon. 2002;48(4):239–248.
© 2014 Society of Hospital Medicine