User login
New approach for treating PNH

Investigators have identified a novel strategy for treating paroxysmal nocturnal hemoglobinuria (PNH), according to a paper published in Blood.
In patients with PNH, defective expression of regulatory proteins on the surface of red blood cells leaves the cells vulnerable to attack by the complement immune system.
This can lead to hemolysis, which results in severe anemia and contributes to a high risk of thrombosis.
Eculizumab is the only approved therapeutic for PNH. The drug reduces hemolysis and can provide patients with relief from blood transfusions.
However, eculizumab is costly (currently more than $400,000 per year per patient), and one third of PNH patients who receive eculizumab continue to require blood transfusions to manage their anemia.
Investigators previously discovered that this non-response is due to fragments of complement C3 proteins on the surface of red blood cells, which are eventually attacked by immune cells.
Therefore, John Lambris, PhD, of the University of Pennsylvania, and his colleagues hypothesized that using small molecules to inhibit the complement cascade at the level of C3 proteins might be an effective strategy for treating PNH.
The team thought this method would prevent both hemolysis and immune cell recognition, and it might be more cost-effective than the current antibody-based treatment.
So they investigated the effect of a C3 inhibitor called Cp40 and its long-acting form, PEG-Cp40, on self-attack and resulting hemolysis using human PNH cells. Both compounds effectively inhibited hemolysis and efficiently prevented deposition of C3 fragments on PNH red blood cells.
In non-human primates, a single injection of PEG-Cp40 had an elimination half-life of more than 5 days. However, the investigators found evidence to suggest the drug may affect plasma levels of C3.
“We think these 2 compounds are excellent and potentially cost-effective candidates for further clinical investigation,” Dr Lambris said.
He hopes the compounds will be tested in clinical trials by 2015. Dr Lambris and his colleague, Daniel Ricklin, PhD, are the inventors of patents and patent applications owned by the University of Pennsylvania that describe the use of complement inhibitors for therapeutic purposes.
And Dr Lambris is a founder and equity holder of Amyndas Pharmaceuticals, which has exclusively licensed the Cp40 and PEG-Cp40 technologies from the university and is developing complement inhibitors for clinical applications. ![]()

Investigators have identified a novel strategy for treating paroxysmal nocturnal hemoglobinuria (PNH), according to a paper published in Blood.
In patients with PNH, defective expression of regulatory proteins on the surface of red blood cells leaves the cells vulnerable to attack by the complement immune system.
This can lead to hemolysis, which results in severe anemia and contributes to a high risk of thrombosis.
Eculizumab is the only approved therapeutic for PNH. The drug reduces hemolysis and can provide patients with relief from blood transfusions.
However, eculizumab is costly (currently more than $400,000 per year per patient), and one third of PNH patients who receive eculizumab continue to require blood transfusions to manage their anemia.
Investigators previously discovered that this non-response is due to fragments of complement C3 proteins on the surface of red blood cells, which are eventually attacked by immune cells.
Therefore, John Lambris, PhD, of the University of Pennsylvania, and his colleagues hypothesized that using small molecules to inhibit the complement cascade at the level of C3 proteins might be an effective strategy for treating PNH.
The team thought this method would prevent both hemolysis and immune cell recognition, and it might be more cost-effective than the current antibody-based treatment.
So they investigated the effect of a C3 inhibitor called Cp40 and its long-acting form, PEG-Cp40, on self-attack and resulting hemolysis using human PNH cells. Both compounds effectively inhibited hemolysis and efficiently prevented deposition of C3 fragments on PNH red blood cells.
In non-human primates, a single injection of PEG-Cp40 had an elimination half-life of more than 5 days. However, the investigators found evidence to suggest the drug may affect plasma levels of C3.
“We think these 2 compounds are excellent and potentially cost-effective candidates for further clinical investigation,” Dr Lambris said.
He hopes the compounds will be tested in clinical trials by 2015. Dr Lambris and his colleague, Daniel Ricklin, PhD, are the inventors of patents and patent applications owned by the University of Pennsylvania that describe the use of complement inhibitors for therapeutic purposes.
And Dr Lambris is a founder and equity holder of Amyndas Pharmaceuticals, which has exclusively licensed the Cp40 and PEG-Cp40 technologies from the university and is developing complement inhibitors for clinical applications. ![]()

Investigators have identified a novel strategy for treating paroxysmal nocturnal hemoglobinuria (PNH), according to a paper published in Blood.
In patients with PNH, defective expression of regulatory proteins on the surface of red blood cells leaves the cells vulnerable to attack by the complement immune system.
This can lead to hemolysis, which results in severe anemia and contributes to a high risk of thrombosis.
Eculizumab is the only approved therapeutic for PNH. The drug reduces hemolysis and can provide patients with relief from blood transfusions.
However, eculizumab is costly (currently more than $400,000 per year per patient), and one third of PNH patients who receive eculizumab continue to require blood transfusions to manage their anemia.
Investigators previously discovered that this non-response is due to fragments of complement C3 proteins on the surface of red blood cells, which are eventually attacked by immune cells.
Therefore, John Lambris, PhD, of the University of Pennsylvania, and his colleagues hypothesized that using small molecules to inhibit the complement cascade at the level of C3 proteins might be an effective strategy for treating PNH.
The team thought this method would prevent both hemolysis and immune cell recognition, and it might be more cost-effective than the current antibody-based treatment.
So they investigated the effect of a C3 inhibitor called Cp40 and its long-acting form, PEG-Cp40, on self-attack and resulting hemolysis using human PNH cells. Both compounds effectively inhibited hemolysis and efficiently prevented deposition of C3 fragments on PNH red blood cells.
In non-human primates, a single injection of PEG-Cp40 had an elimination half-life of more than 5 days. However, the investigators found evidence to suggest the drug may affect plasma levels of C3.
“We think these 2 compounds are excellent and potentially cost-effective candidates for further clinical investigation,” Dr Lambris said.
He hopes the compounds will be tested in clinical trials by 2015. Dr Lambris and his colleague, Daniel Ricklin, PhD, are the inventors of patents and patent applications owned by the University of Pennsylvania that describe the use of complement inhibitors for therapeutic purposes.
And Dr Lambris is a founder and equity holder of Amyndas Pharmaceuticals, which has exclusively licensed the Cp40 and PEG-Cp40 technologies from the university and is developing complement inhibitors for clinical applications. ![]()
Letters to the Editor
After reading the letter to the editor from Neil Goldfarb, we are concerned that the focus of our study[1] was misinterpreted. Upon reviewing the methodology for Leapfrog's Hospital Safety Score in May 2013, we were surprised to find that Leapfrog uses 2 separate scoring methodologies, depending on whether the hospital participates in the Leapfrog Hospital Survey. Survey participants are scored from 26 measures, whereas nonparticipants are scored from only 18 measures3 of which are imputed from other data sourceswith recalibrated weightings for each measure. Measuring and publicly disclosing hospital information are paramount to improving safety and quality, and we applaud Leapfrog for taking a leading role in this. However, our report demonstrated that Leapfrog's Hospital Safety Score, which was attained through 2 separate methodologies, may result in unintended inconsistency or misinterpretation.
We believe Mr. Goldfarb misunderstood our notion of statistical significance. In the report, we acknowledged that the mean score differences between participating and nonparticipating hospitals in our sample were not statistically significant, possibly due to small sample size. However, this was not the focus of our report. Utilizing a mean imputation approach, we rescored the nonparticipating hospitals in our sample as if they had participated in the Leapfrog Hospital Survey. The differences between the original nonparticipant scores and their respective participant estimations were not statistically significant. However, due to the cutoff points Leapfrog uses to assign letter grades, these differences resulted in a letter grade change for many of the nonparticipating hospitals in our sample.
We wish to clarify that a hospital's choice to participate or not to participate in the Leapfrog Hospital Survey is not a reflection of their willingness to promote patient safety. Hospitals voluntarily report data to numerous private organizations and are required to report hundreds of quality and safety measures to government agencies. The 26 (or 18) measures included in Leapfrog's Hospital Safety Score are merely a fraction of the measures hospitals already report.
Finally, we regret that our brief report has been mischaracterized by Neil Goldfarb as being clearly biased against the work of the Leapfrog Group. This is far from our intent. Throughout the manuscript, we repeatedly acknowledge Leapfrog's contribution in patient safety improvement; our work does not intend to discredit Leapfrog's hard‐earned reputation. We provide a recommendation that Leapfrog produce 2 separate reports for participating and nonparticipating hospitals to maintain clarity. Our research has followed academic protocol, has undergone a stringent peer‐review process, and included full disclosure of any potential conflicts of interest. We hope our analysis will contribute to the continuing improvement of Leapfrog's hospital patient safety reporting.
- , , , . Hospital patient safety grades may misrepresent hospital performance. J Hosp Med. 2014;9(2):111–115.
After reading the letter to the editor from Neil Goldfarb, we are concerned that the focus of our study[1] was misinterpreted. Upon reviewing the methodology for Leapfrog's Hospital Safety Score in May 2013, we were surprised to find that Leapfrog uses 2 separate scoring methodologies, depending on whether the hospital participates in the Leapfrog Hospital Survey. Survey participants are scored from 26 measures, whereas nonparticipants are scored from only 18 measures3 of which are imputed from other data sourceswith recalibrated weightings for each measure. Measuring and publicly disclosing hospital information are paramount to improving safety and quality, and we applaud Leapfrog for taking a leading role in this. However, our report demonstrated that Leapfrog's Hospital Safety Score, which was attained through 2 separate methodologies, may result in unintended inconsistency or misinterpretation.
We believe Mr. Goldfarb misunderstood our notion of statistical significance. In the report, we acknowledged that the mean score differences between participating and nonparticipating hospitals in our sample were not statistically significant, possibly due to small sample size. However, this was not the focus of our report. Utilizing a mean imputation approach, we rescored the nonparticipating hospitals in our sample as if they had participated in the Leapfrog Hospital Survey. The differences between the original nonparticipant scores and their respective participant estimations were not statistically significant. However, due to the cutoff points Leapfrog uses to assign letter grades, these differences resulted in a letter grade change for many of the nonparticipating hospitals in our sample.
We wish to clarify that a hospital's choice to participate or not to participate in the Leapfrog Hospital Survey is not a reflection of their willingness to promote patient safety. Hospitals voluntarily report data to numerous private organizations and are required to report hundreds of quality and safety measures to government agencies. The 26 (or 18) measures included in Leapfrog's Hospital Safety Score are merely a fraction of the measures hospitals already report.
Finally, we regret that our brief report has been mischaracterized by Neil Goldfarb as being clearly biased against the work of the Leapfrog Group. This is far from our intent. Throughout the manuscript, we repeatedly acknowledge Leapfrog's contribution in patient safety improvement; our work does not intend to discredit Leapfrog's hard‐earned reputation. We provide a recommendation that Leapfrog produce 2 separate reports for participating and nonparticipating hospitals to maintain clarity. Our research has followed academic protocol, has undergone a stringent peer‐review process, and included full disclosure of any potential conflicts of interest. We hope our analysis will contribute to the continuing improvement of Leapfrog's hospital patient safety reporting.
After reading the letter to the editor from Neil Goldfarb, we are concerned that the focus of our study[1] was misinterpreted. Upon reviewing the methodology for Leapfrog's Hospital Safety Score in May 2013, we were surprised to find that Leapfrog uses 2 separate scoring methodologies, depending on whether the hospital participates in the Leapfrog Hospital Survey. Survey participants are scored from 26 measures, whereas nonparticipants are scored from only 18 measures3 of which are imputed from other data sourceswith recalibrated weightings for each measure. Measuring and publicly disclosing hospital information are paramount to improving safety and quality, and we applaud Leapfrog for taking a leading role in this. However, our report demonstrated that Leapfrog's Hospital Safety Score, which was attained through 2 separate methodologies, may result in unintended inconsistency or misinterpretation.
We believe Mr. Goldfarb misunderstood our notion of statistical significance. In the report, we acknowledged that the mean score differences between participating and nonparticipating hospitals in our sample were not statistically significant, possibly due to small sample size. However, this was not the focus of our report. Utilizing a mean imputation approach, we rescored the nonparticipating hospitals in our sample as if they had participated in the Leapfrog Hospital Survey. The differences between the original nonparticipant scores and their respective participant estimations were not statistically significant. However, due to the cutoff points Leapfrog uses to assign letter grades, these differences resulted in a letter grade change for many of the nonparticipating hospitals in our sample.
We wish to clarify that a hospital's choice to participate or not to participate in the Leapfrog Hospital Survey is not a reflection of their willingness to promote patient safety. Hospitals voluntarily report data to numerous private organizations and are required to report hundreds of quality and safety measures to government agencies. The 26 (or 18) measures included in Leapfrog's Hospital Safety Score are merely a fraction of the measures hospitals already report.
Finally, we regret that our brief report has been mischaracterized by Neil Goldfarb as being clearly biased against the work of the Leapfrog Group. This is far from our intent. Throughout the manuscript, we repeatedly acknowledge Leapfrog's contribution in patient safety improvement; our work does not intend to discredit Leapfrog's hard‐earned reputation. We provide a recommendation that Leapfrog produce 2 separate reports for participating and nonparticipating hospitals to maintain clarity. Our research has followed academic protocol, has undergone a stringent peer‐review process, and included full disclosure of any potential conflicts of interest. We hope our analysis will contribute to the continuing improvement of Leapfrog's hospital patient safety reporting.
- , , , . Hospital patient safety grades may misrepresent hospital performance. J Hosp Med. 2014;9(2):111–115.
- , , , . Hospital patient safety grades may misrepresent hospital performance. J Hosp Med. 2014;9(2):111–115.
Letters to the Editor
As the Executive Director of a purchaser coalition that has been promoting hospital participation in the Leapfrog Hospital Survey in our region, I found the brief report from Hwang and colleagues, Hospital Patient Safety Grades May Misrepresent Hospital Performance,[1] troubling. Putting aside the methodological vagaries and the lack of statistical significance to the findings, the authors have a clear bias against the work of the Leapfrog Group. As acknowledged in the disclosures, their institution does not participate in the Leapfrog Hospital Survey. What is not acknowledged is that their institution has not performed particularly well on the hospital safety score.
The authors note in their introduction that according to Leapfrog, 4 to 6 days are required for a hospital to compile the necessary survey data with an additional 90‐minute time commitment to enter the data, and state that this is a significant time commitment for many hospitals. Although it undoubtedly is a significant time commitment, apparently more than 1400 hospitals have found the time and made a commitment to measuring and publicly disclosing information that will help consumers, purchasers, and health plans identify and select safer, higher‐quality care providers. In addition, many studies have shown that public reporting helps to drive providers to improve. In the 12 years since To Err Is Human[2] was published, nothing suggests that the number of deaths associated with medical errors has diminished; in fact, a recent study suggested that over 400,000 deaths may occur annually due to errors.[3] In light of these ongoing safety concerns, is a commitment of 4 to 6 days really too large an investment?
It is time that America's hospitals stopped whining about the burden of public reporting and recognized that their customers have a right to, and are starting to demand, better data on quality, safety, and costs of care. If the Hospital Safety Score is indeed biased against nonreporting hospitals (and I remain unconvinced from this poorly designed study that it is), the main message of the article should have been that hospitals need to start reporting their data, not that the Leapfrog Group needs to change its methodology.
- , , , et al. Hospital patient safety grades may misrepresent hospital performance. J Hosp Med. 2014;9(2):111–115.
- Kohn LT, Corrigan JM, Donaldson MS, eds; Institute of Medicine. To Err Is Human: Building a Safer Health System. Washington, DC: National Academy Press; 2000.
- . A new, evidence‐based estimate of patient harms associated with hospital care. J Patient Saf. 2013;9:122–128.
As the Executive Director of a purchaser coalition that has been promoting hospital participation in the Leapfrog Hospital Survey in our region, I found the brief report from Hwang and colleagues, Hospital Patient Safety Grades May Misrepresent Hospital Performance,[1] troubling. Putting aside the methodological vagaries and the lack of statistical significance to the findings, the authors have a clear bias against the work of the Leapfrog Group. As acknowledged in the disclosures, their institution does not participate in the Leapfrog Hospital Survey. What is not acknowledged is that their institution has not performed particularly well on the hospital safety score.
The authors note in their introduction that according to Leapfrog, 4 to 6 days are required for a hospital to compile the necessary survey data with an additional 90‐minute time commitment to enter the data, and state that this is a significant time commitment for many hospitals. Although it undoubtedly is a significant time commitment, apparently more than 1400 hospitals have found the time and made a commitment to measuring and publicly disclosing information that will help consumers, purchasers, and health plans identify and select safer, higher‐quality care providers. In addition, many studies have shown that public reporting helps to drive providers to improve. In the 12 years since To Err Is Human[2] was published, nothing suggests that the number of deaths associated with medical errors has diminished; in fact, a recent study suggested that over 400,000 deaths may occur annually due to errors.[3] In light of these ongoing safety concerns, is a commitment of 4 to 6 days really too large an investment?
It is time that America's hospitals stopped whining about the burden of public reporting and recognized that their customers have a right to, and are starting to demand, better data on quality, safety, and costs of care. If the Hospital Safety Score is indeed biased against nonreporting hospitals (and I remain unconvinced from this poorly designed study that it is), the main message of the article should have been that hospitals need to start reporting their data, not that the Leapfrog Group needs to change its methodology.
As the Executive Director of a purchaser coalition that has been promoting hospital participation in the Leapfrog Hospital Survey in our region, I found the brief report from Hwang and colleagues, Hospital Patient Safety Grades May Misrepresent Hospital Performance,[1] troubling. Putting aside the methodological vagaries and the lack of statistical significance to the findings, the authors have a clear bias against the work of the Leapfrog Group. As acknowledged in the disclosures, their institution does not participate in the Leapfrog Hospital Survey. What is not acknowledged is that their institution has not performed particularly well on the hospital safety score.
The authors note in their introduction that according to Leapfrog, 4 to 6 days are required for a hospital to compile the necessary survey data with an additional 90‐minute time commitment to enter the data, and state that this is a significant time commitment for many hospitals. Although it undoubtedly is a significant time commitment, apparently more than 1400 hospitals have found the time and made a commitment to measuring and publicly disclosing information that will help consumers, purchasers, and health plans identify and select safer, higher‐quality care providers. In addition, many studies have shown that public reporting helps to drive providers to improve. In the 12 years since To Err Is Human[2] was published, nothing suggests that the number of deaths associated with medical errors has diminished; in fact, a recent study suggested that over 400,000 deaths may occur annually due to errors.[3] In light of these ongoing safety concerns, is a commitment of 4 to 6 days really too large an investment?
It is time that America's hospitals stopped whining about the burden of public reporting and recognized that their customers have a right to, and are starting to demand, better data on quality, safety, and costs of care. If the Hospital Safety Score is indeed biased against nonreporting hospitals (and I remain unconvinced from this poorly designed study that it is), the main message of the article should have been that hospitals need to start reporting their data, not that the Leapfrog Group needs to change its methodology.
- , , , et al. Hospital patient safety grades may misrepresent hospital performance. J Hosp Med. 2014;9(2):111–115.
- Kohn LT, Corrigan JM, Donaldson MS, eds; Institute of Medicine. To Err Is Human: Building a Safer Health System. Washington, DC: National Academy Press; 2000.
- . A new, evidence‐based estimate of patient harms associated with hospital care. J Patient Saf. 2013;9:122–128.
- , , , et al. Hospital patient safety grades may misrepresent hospital performance. J Hosp Med. 2014;9(2):111–115.
- Kohn LT, Corrigan JM, Donaldson MS, eds; Institute of Medicine. To Err Is Human: Building a Safer Health System. Washington, DC: National Academy Press; 2000.
- . A new, evidence‐based estimate of patient harms associated with hospital care. J Patient Saf. 2013;9:122–128.
Global Health Hospitalists Share a Passion for Their Work
Global health hospitalists are passionate about their work. The Hospitalist asked them to expand on the reasons they choose this work.
“Working in Haiti has been the most compelling work in my life,” says Michelle Morse, MD, MPH, an instructor in medicine at Harvard Medical School and deputy chief medical officer for Partners in Health (PIH) in Boston. She has worked with the Navajo Nation in conjunction with PIH’s Community Outreach and Patient Empowerment (COPE) program. The sharing of information is “bi-directional,” Dr. Morse says.
Her Haitian colleagues, she says, have developed “transformative” systems improvements, and she’s found that her own diagnostic and physical exam skills have strengthened because of her work abroad.
“You really have to think bigger than your group of patients and bigger than your community, and think about the whole system to make things better around the world,” she says. “I think that is a fundamental part of becoming a physician.”
UCSF clinical fellow Varun Verma, MD, says he was tired of working in “fragmented volunteer assignments” with relief organizations. Three-month clinical rotations, in which he essentially functions as a teaching attending, have solved the “filling in” feeling he’d grown weary of.
“Here at St. Thérèse Hospital [in Hinche, Haiti], they do not need us to take care of patients on a moment-to-moment basis. There are Haitian clinicians for that,” he says. “Part of our job is to do medical teaching of residents and try to involve everyone in quality improvement projects. It’s sometimes challenging discussing best practices of managing conditions, given the resources at hand, but I find that the Haitian doctors are always interested in learning how we do things in the U.S.”
Evan Lyon, MD, assistant professor of medicine in the section of hospital medicine, supervises clinical fellows in the department of medicine at the University of Chicago. He believes hospitalists who take on global health assignments gain a deeper appreciation for assessing patients’ social histories.
“There’s no better way to deepen your learning of physical exam and history-taking skills than to be out here on the edge and have to rely on those skills,” he says. “Back in the states, you might order an echocardiogram before you listen to the patient’s heart. I think all of us have a different relationship to labs, testing, and X-rays when we return. But the deepest influence for me has been around understanding patients’ social histories and their social context, which is a neglected piece of American medicine.”
Sharing resources and knowledge is what drives Marwa Shoeb MD, MS, assistant professor in the division of hospital medicine at UCSF. “I see this as an extension of our daily work,” she says. “We are just taking it to a different context.”
Gretchen Henkel is a freelance writer in southern California.
Global health hospitalists are passionate about their work. The Hospitalist asked them to expand on the reasons they choose this work.
“Working in Haiti has been the most compelling work in my life,” says Michelle Morse, MD, MPH, an instructor in medicine at Harvard Medical School and deputy chief medical officer for Partners in Health (PIH) in Boston. She has worked with the Navajo Nation in conjunction with PIH’s Community Outreach and Patient Empowerment (COPE) program. The sharing of information is “bi-directional,” Dr. Morse says.
Her Haitian colleagues, she says, have developed “transformative” systems improvements, and she’s found that her own diagnostic and physical exam skills have strengthened because of her work abroad.
“You really have to think bigger than your group of patients and bigger than your community, and think about the whole system to make things better around the world,” she says. “I think that is a fundamental part of becoming a physician.”
UCSF clinical fellow Varun Verma, MD, says he was tired of working in “fragmented volunteer assignments” with relief organizations. Three-month clinical rotations, in which he essentially functions as a teaching attending, have solved the “filling in” feeling he’d grown weary of.
“Here at St. Thérèse Hospital [in Hinche, Haiti], they do not need us to take care of patients on a moment-to-moment basis. There are Haitian clinicians for that,” he says. “Part of our job is to do medical teaching of residents and try to involve everyone in quality improvement projects. It’s sometimes challenging discussing best practices of managing conditions, given the resources at hand, but I find that the Haitian doctors are always interested in learning how we do things in the U.S.”
Evan Lyon, MD, assistant professor of medicine in the section of hospital medicine, supervises clinical fellows in the department of medicine at the University of Chicago. He believes hospitalists who take on global health assignments gain a deeper appreciation for assessing patients’ social histories.
“There’s no better way to deepen your learning of physical exam and history-taking skills than to be out here on the edge and have to rely on those skills,” he says. “Back in the states, you might order an echocardiogram before you listen to the patient’s heart. I think all of us have a different relationship to labs, testing, and X-rays when we return. But the deepest influence for me has been around understanding patients’ social histories and their social context, which is a neglected piece of American medicine.”
Sharing resources and knowledge is what drives Marwa Shoeb MD, MS, assistant professor in the division of hospital medicine at UCSF. “I see this as an extension of our daily work,” she says. “We are just taking it to a different context.”
Gretchen Henkel is a freelance writer in southern California.
Global health hospitalists are passionate about their work. The Hospitalist asked them to expand on the reasons they choose this work.
“Working in Haiti has been the most compelling work in my life,” says Michelle Morse, MD, MPH, an instructor in medicine at Harvard Medical School and deputy chief medical officer for Partners in Health (PIH) in Boston. She has worked with the Navajo Nation in conjunction with PIH’s Community Outreach and Patient Empowerment (COPE) program. The sharing of information is “bi-directional,” Dr. Morse says.
Her Haitian colleagues, she says, have developed “transformative” systems improvements, and she’s found that her own diagnostic and physical exam skills have strengthened because of her work abroad.
“You really have to think bigger than your group of patients and bigger than your community, and think about the whole system to make things better around the world,” she says. “I think that is a fundamental part of becoming a physician.”
UCSF clinical fellow Varun Verma, MD, says he was tired of working in “fragmented volunteer assignments” with relief organizations. Three-month clinical rotations, in which he essentially functions as a teaching attending, have solved the “filling in” feeling he’d grown weary of.
“Here at St. Thérèse Hospital [in Hinche, Haiti], they do not need us to take care of patients on a moment-to-moment basis. There are Haitian clinicians for that,” he says. “Part of our job is to do medical teaching of residents and try to involve everyone in quality improvement projects. It’s sometimes challenging discussing best practices of managing conditions, given the resources at hand, but I find that the Haitian doctors are always interested in learning how we do things in the U.S.”
Evan Lyon, MD, assistant professor of medicine in the section of hospital medicine, supervises clinical fellows in the department of medicine at the University of Chicago. He believes hospitalists who take on global health assignments gain a deeper appreciation for assessing patients’ social histories.
“There’s no better way to deepen your learning of physical exam and history-taking skills than to be out here on the edge and have to rely on those skills,” he says. “Back in the states, you might order an echocardiogram before you listen to the patient’s heart. I think all of us have a different relationship to labs, testing, and X-rays when we return. But the deepest influence for me has been around understanding patients’ social histories and their social context, which is a neglected piece of American medicine.”
Sharing resources and knowledge is what drives Marwa Shoeb MD, MS, assistant professor in the division of hospital medicine at UCSF. “I see this as an extension of our daily work,” she says. “We are just taking it to a different context.”
Gretchen Henkel is a freelance writer in southern California.
Brett Hendel-Paterson, MD, Discusses Advantages of Needs Assessments
Listen to more of our interview with Dr. Hendel-Paterson, as he discusses the advantages of a good needs assessment.
Listen to more of our interview with Dr. Hendel-Paterson, as he discusses the advantages of a good needs assessment.
Listen to more of our interview with Dr. Hendel-Paterson, as he discusses the advantages of a good needs assessment.
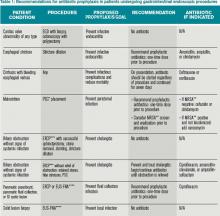
What Patients Undergoing Gastrointestinal Endoscopic Procedures Should Receive Antibiotic Prophylaxis?
Case
You are asked to admit two patients. The first is a 75-year-old male with a prosthetic aortic valve on warfarin who presents with bright red blood per rectum and is scheduled for colonoscopy. The second patient is a 35-year-old female with biliary obstruction due to choledocholithiasis; she is afebrile with normal vital signs and no leukocytosis. She underwent endoscopic retrograde cholangiopancreatography (ERCP), which did not resolve her biliary obstruction. Should you prescribe prophylactic antibiotics for either patient?
Overview
Providers are often confused regarding which patients undergoing gastrointestinal (GI) endoscopic procedures should receive antibiotic prophylaxis. To answer this question, it is important to understand the goal of prophylactic antibiotics. Are we trying to prevent infective endocarditis or a localized infection?
There are few large, prospective, randomized controlled trials that have examined the need for antibiotic prophylaxis with GI endoscopic procedures. Guidelines from professional societies are mainly based on expert opinion, evidence from retrospective case studies, and meta-analysis reviews.
Review of the Data
Infective endocarditis resulting from GI endoscopy has been a concern of physicians for decades. The American Heart Association (AHA) first published its recommendations for antibiotic prophylaxis of GI tract procedures in 1965. The most recent antibacterial prophylaxis guidelines, published in 2007, have simplified recommendations and greatly scaled back the indications for antibiotics. The new guidelines conclude that frequent bacteremia from daily activities is more likely to precipitate endocarditis than a single dental, GI, or genitourinary tract procedure.1
The American Society for Gastrointestinal Endoscopy (ASGE) reports that 14.2 million colonoscopies, 2.8 million flexible sigmoidoscopies, and nearly as many upper endoscopies are performed in the U.S. each year, but only 15 cases of endocarditis have been reported with a temporal association to a procedure.2
The British Society of Gastroenterology (BSG) found, after reviewing the histories of patients with infective endocarditis from 1983 through 2006, that there is not enough evidence to warrant antibiotic prophylaxis prior to endoscopy. They noted less than one case of endocarditis after GI endoscopy per year as well as significant variation in the time interval between the procedure and symptoms. The BSG also recognized that antibiotic prophylaxis does not always protect against infection and that clinical factors unrelated to the endoscopy may play a significant role in the development of endocarditis.3
Upper GI Endoscopy, Colonoscopy with Biopsy, and Esophageal Dilatation. Administering antibiotics to prevent infective endocarditis is not recommended for patients undergoing routine procedures such as endoscopy with biopsy and colonoscopy with polypectomy. Likewise, patients with a history of prosthetic heart valves, valve repair with prosthetic material, endocarditis, congenital heart disease, or cardiac transplant with valvulopathy do not need prophylactic antibiotics before GI endoscopic procedures. However, for patients who are being treated for an active GI infection, antibiotic coverage for enterococcus may be warranted given the increased risk of developing endocarditis. The AHA acknowledges there are no published studies to support the efficacy of antibiotics to prevent enterococcal endocarditis in patients in this clinical setting.1
Unlike routine endoscopy, esophageal dilation is associated with an increased rate of bacteremia (12%-100%).4 Streptococcus viridans has been found in blood cultures up to 79% of the time after esophageal dilation.5 Patients with malignant strictures have higher rates of bacteremia than those with benign strictures (52.9% versus 15.7%). Patients treated with multiple passes with the esophageal dilator compared to those treated with a single dilation have a higher risk of bacteremia.6 All patients undergoing esophageal stricture dilation should receive pre-procedural prophylactic antibiotics.7
Patients with bleeding esophageal varices also have high rates of bacteremia. Up to 20% of patients with cirrhosis and GI bleeding on admission develop an infection within 48 hours of presentation.8 There is evidence that the bacteremia may actually be related to the variceal bleeding rather than the procedure.9 Patients with bleeding esophageal varices treated with antibiotics have improved outcomes, including a decrease in mortality.10 Therefore, all patients with bleeding esophageal varices should be placed on antibiotic therapy regardless of whether an endoscopic intervention is planned.
Percutaneous Endoscopic Gastrostomy (PEG) Placement. Prophylactic antibiotics are recommended before placement of a PEG. The indication for prophylactic antibiotics is to prevent a gastrostomy site infection, not infective endocarditis. Gastrostomy site infection is unfortunately a fairly common infection, affecting 4% to 30% of patients who undergo PEG tube placement. There is significant evidence that antibiotics are beneficial in preventing peristomal infections. A meta-analysis showed that only eight patients need to be treated with prophylactic antibiotics to prevent a single peristomal infection.11 Since these infections are believed to be caused by contamination from the oropharynx, physicians should consider prophylaxis against pathogens from the oral flora.12
More recently, it has been noted that methicillin-resistant Staphylococcus aureus (MRSA) is increasingly cultured from infection sites.13 In centers with endemic MRSA, patients should be screened and then undergo decontamination prior to the PEG placement in positive cases.
Endoscopic Ultrasound with Fine Needle Aspiration (EUS-FNA). Antibiotic prophylaxis before EUS-FNA of a solid lesion in an organ is generally thought to be unnecessary because the risk of bacteremia with this procedure is low, comparable to routine GI endoscopy with biopsy. The recommendation for prophylactic antibiotics before biopsy of a cystic lesion is different. There is concern that puncturing cystic lesions may create a new infected fluid collection.2 A systematic review of more than 10,000 patients undergoing EUS-FNA with a full range of target organs revealed that, overall, 11.2% of patients experienced a fever and 4.7% of patients had a peri-procedural infection. While it was not possible in this study to determine which patients received prophylactic antibiotics, 93.7% of patients with pancreatic cystic lesions were reported to have been treated with antibiotics.14
A separate, single-center, retrospective trial produced different results. This study examined a population of 253 patients who underwent 266 EUS-FNA of pancreatic cysts and found that prophylactic antibiotics were associated with more adverse events and were not protective for the 3% of the patients with infectious symptoms.15 Despite the conflicting data, guidelines at this time recommend prophylactic antibiotics before drainage of a sterile pancreatic fluid collection that communicates with the pancreatic duct and also for aspiration of cystic lesions along the GI tract and the mediastinum.2
Endoscopic Retrograde Cholangiopancreatography (ERCP). In patients undergoing ERCP, the routine use of prophylactic antibiotics has not been found to be effective in decreasing the risk of post-procedure cholangitis.16 Guidelines recommend the use of prophylactic antibiotics only in those patients in which the ERCP may not completely resolve the biliary obstruction.2 In these patients, the thought is that ERCP can precipitate infection by disturbing bacteria already present in the biliary tree, especially with increased intrabiliary pressure at the time of contrast dye injection.17
Patients with incomplete biliary drainage, including those with primary sclerosing cholangitis (PSC), hilar cholangiocarcinoma, persistent biliary that were not extracted, and strictures that continue to obstruct despite attempted intervention, are thought to be at elevated risk of developing cholangitis post-ERCP. These patients should be placed on prophylactic antibiotics at the time of the procedure to cover biliary flora such as enteric gram negatives and enterococci. Antibiotics should be continued until the biliary obstruction is resolved.2
Additional Populations to Consider. Previously, the International Society for Peritoneal Dialysis recommended that patients on peritoneal dialysis receive prophylactic antibiotics and empty their abdomen of dialysate prior to colonoscopy. This recommendation has been removed from the 2010 guidelines.18 There is also no indication that patients with synthetic vascular grafts or cardiac devices should receive prophylactic antibiotics prior to routine GI endoscopy.19 The American Academy of Orthopaedic Surgeons no longer recommends that patients with joint replacements receive antibiotic prophylaxis prior to GI endoscopy.20
Back to the Case
The older gentleman with a prosthetic valve undergoing colonoscopy should not receive prophylactic antibiotics, because even in the setting of valvulopathy, colonoscopy does not pose a significant risk for infective endocarditis. The young patient with severe choledocholithiasis should be placed on prophylactic antibiotics because she has continued biliary obstruction, which could result in a cholangitis after ERCP.
Bottom Line
Prophylactic antibiotics are not recommended for any patient undergoing routine endoscopy or colonoscopy. They are indicated for patients with bleeding esophageal varices and for patients who undergo esophageal stricture dilation, PEG placement, or pseudocyst or cyst drainage, and those with continued biliary obstruction undergoing ERCP as summarized in Table 1.
Drs. Ritter, Jupiter, Carbo, and Li are hospitalists at Beth Israel Deaconess Medical Center and Harvard Medical School faculty in Boston.
References
- Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116(15):1736-1754.
- Banerjee S, Shen B, Baron TH, et al. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2008;67(6):791-798.
- Allison MC, Sandoe JA, Tighe R, Simpson IA, Hall RJ, Elliott TS. Antibiotic prophylaxis in gastrointestinal endoscopy. Gut. 2009;58(6):869-880.
- Nelson DB. Infectious disease complications of GI endoscopy: Part I, endogenous infections. Gastrointest Endosc. 2003;57(4):546-556.
- Zuccaro G Jr., Richter JE, Rice TW, et al. Viridans streptococcal bacteremia after esophageal stricture dilation. Gastrointest Endosc. 1998;48(6):568-573.
- Nelson DB, Sanderson SJ, Azar MM. Bacteremia with esophageal dilation. Gastrointest Endosc.1998;48(6):563-567.
- Hirota WK, Petersen K, Baron TH, et al. Guidelines for antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2003;58(4):475-482.
- Ho H, Zuckerman MJ, Wassem C. A prospective controlled study of the risk of bacteremia in emergency sclerotherapy of esophageal varices. Gastroenterology. 1991;101(6):1642-1648.
- Rolando N, Gimson A, Philpott-Howard J, et al. Infectious sequelae after endoscopic sclerotherapy of oesophageal varices: Role of antibiotic prophylaxis. J Hepatol. 1993;18(3):290-294.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46(3):922-938.
- Jafri NS, Mahid SS, Minor KS, Idstein SR, Hornung CA, Galandiuk S. Meta-analysis: Antibiotic prophylaxis to prevent peristomal infection following percutaneous endoscopic gastrostomy. Aliment Pharmacol Ther. 2007;25(6):647-656.
- Chuang CH, Hung KH, Chen JR, et al. Airway infection predisposes to peristomal infection after percutaneous endoscopic gastrostomy with high concordance between sputum and wound isolates. J Gastrointest Surg. 2010;14(1):45-51.
- Chaudhary KA, Smith OJ, Cuddy PG, Clarkston WK. PEG site infections: The emergence of methicillin resistant Staphylococcus aureus as a major pathogen. Am J Gastroenterol. 2002;97(7):1713-1716.
- Wang KX, Ben QW, Jin ZD, et al. Assessment of morbidity and mortality associated with EUS-guided FNA: A systematic review. Gastrointest Endosc. 2011;73(2):283-290.
- Guarner-Argente C, Shah P, Buchner A, Ahmad NA, Kochman ML, Ginsberg GG. Use of antimicrobials for EUS-guided FNA of pancreatic cysts: A retrospective, comparative analysis. Gastrointest Endosc. 2011;74(1):81-86.
- Bai Y, Gao F, Gao J, Zou DW, Li ZS. Prophylactic antibiotics cannot prevent endoscopic retrograde cholangiopancreatography-induced cholangitis: A meta-analysis. Pancreas. 2009;38(2):126-130.
- Cotton PB, Connor P, Rawls E, Romagnuolo J. Infection after ERCP, and antibiotic prophylaxis: A sequential quality-improvement approach over 11 years. Gastrointest Endosc. 2008;67(3):471-475.
- Li PK, Szeto CC, Piraino B, et al. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int. 2010;30(4):393-423.
- Baddour LM, Bettmann MA, Bolger AF, et al. Nonvalvular cardiovascular device-related infections. Circulation. 2003;108(16):2015-2031.
- Rethman MP, Watters W III, Abt E, et al. The American Academy of Orthopaedic Surgeons and the American Dental Association clinical practice guideline on the prevention of orthopaedic implant infection in patients undergoing dental procedures. J Bone Joint Surg Am. 2013;95(8):745-747.
Case
You are asked to admit two patients. The first is a 75-year-old male with a prosthetic aortic valve on warfarin who presents with bright red blood per rectum and is scheduled for colonoscopy. The second patient is a 35-year-old female with biliary obstruction due to choledocholithiasis; she is afebrile with normal vital signs and no leukocytosis. She underwent endoscopic retrograde cholangiopancreatography (ERCP), which did not resolve her biliary obstruction. Should you prescribe prophylactic antibiotics for either patient?
Overview
Providers are often confused regarding which patients undergoing gastrointestinal (GI) endoscopic procedures should receive antibiotic prophylaxis. To answer this question, it is important to understand the goal of prophylactic antibiotics. Are we trying to prevent infective endocarditis or a localized infection?
There are few large, prospective, randomized controlled trials that have examined the need for antibiotic prophylaxis with GI endoscopic procedures. Guidelines from professional societies are mainly based on expert opinion, evidence from retrospective case studies, and meta-analysis reviews.
Review of the Data
Infective endocarditis resulting from GI endoscopy has been a concern of physicians for decades. The American Heart Association (AHA) first published its recommendations for antibiotic prophylaxis of GI tract procedures in 1965. The most recent antibacterial prophylaxis guidelines, published in 2007, have simplified recommendations and greatly scaled back the indications for antibiotics. The new guidelines conclude that frequent bacteremia from daily activities is more likely to precipitate endocarditis than a single dental, GI, or genitourinary tract procedure.1
The American Society for Gastrointestinal Endoscopy (ASGE) reports that 14.2 million colonoscopies, 2.8 million flexible sigmoidoscopies, and nearly as many upper endoscopies are performed in the U.S. each year, but only 15 cases of endocarditis have been reported with a temporal association to a procedure.2
The British Society of Gastroenterology (BSG) found, after reviewing the histories of patients with infective endocarditis from 1983 through 2006, that there is not enough evidence to warrant antibiotic prophylaxis prior to endoscopy. They noted less than one case of endocarditis after GI endoscopy per year as well as significant variation in the time interval between the procedure and symptoms. The BSG also recognized that antibiotic prophylaxis does not always protect against infection and that clinical factors unrelated to the endoscopy may play a significant role in the development of endocarditis.3
Upper GI Endoscopy, Colonoscopy with Biopsy, and Esophageal Dilatation. Administering antibiotics to prevent infective endocarditis is not recommended for patients undergoing routine procedures such as endoscopy with biopsy and colonoscopy with polypectomy. Likewise, patients with a history of prosthetic heart valves, valve repair with prosthetic material, endocarditis, congenital heart disease, or cardiac transplant with valvulopathy do not need prophylactic antibiotics before GI endoscopic procedures. However, for patients who are being treated for an active GI infection, antibiotic coverage for enterococcus may be warranted given the increased risk of developing endocarditis. The AHA acknowledges there are no published studies to support the efficacy of antibiotics to prevent enterococcal endocarditis in patients in this clinical setting.1
Unlike routine endoscopy, esophageal dilation is associated with an increased rate of bacteremia (12%-100%).4 Streptococcus viridans has been found in blood cultures up to 79% of the time after esophageal dilation.5 Patients with malignant strictures have higher rates of bacteremia than those with benign strictures (52.9% versus 15.7%). Patients treated with multiple passes with the esophageal dilator compared to those treated with a single dilation have a higher risk of bacteremia.6 All patients undergoing esophageal stricture dilation should receive pre-procedural prophylactic antibiotics.7
Patients with bleeding esophageal varices also have high rates of bacteremia. Up to 20% of patients with cirrhosis and GI bleeding on admission develop an infection within 48 hours of presentation.8 There is evidence that the bacteremia may actually be related to the variceal bleeding rather than the procedure.9 Patients with bleeding esophageal varices treated with antibiotics have improved outcomes, including a decrease in mortality.10 Therefore, all patients with bleeding esophageal varices should be placed on antibiotic therapy regardless of whether an endoscopic intervention is planned.
Percutaneous Endoscopic Gastrostomy (PEG) Placement. Prophylactic antibiotics are recommended before placement of a PEG. The indication for prophylactic antibiotics is to prevent a gastrostomy site infection, not infective endocarditis. Gastrostomy site infection is unfortunately a fairly common infection, affecting 4% to 30% of patients who undergo PEG tube placement. There is significant evidence that antibiotics are beneficial in preventing peristomal infections. A meta-analysis showed that only eight patients need to be treated with prophylactic antibiotics to prevent a single peristomal infection.11 Since these infections are believed to be caused by contamination from the oropharynx, physicians should consider prophylaxis against pathogens from the oral flora.12
More recently, it has been noted that methicillin-resistant Staphylococcus aureus (MRSA) is increasingly cultured from infection sites.13 In centers with endemic MRSA, patients should be screened and then undergo decontamination prior to the PEG placement in positive cases.
Endoscopic Ultrasound with Fine Needle Aspiration (EUS-FNA). Antibiotic prophylaxis before EUS-FNA of a solid lesion in an organ is generally thought to be unnecessary because the risk of bacteremia with this procedure is low, comparable to routine GI endoscopy with biopsy. The recommendation for prophylactic antibiotics before biopsy of a cystic lesion is different. There is concern that puncturing cystic lesions may create a new infected fluid collection.2 A systematic review of more than 10,000 patients undergoing EUS-FNA with a full range of target organs revealed that, overall, 11.2% of patients experienced a fever and 4.7% of patients had a peri-procedural infection. While it was not possible in this study to determine which patients received prophylactic antibiotics, 93.7% of patients with pancreatic cystic lesions were reported to have been treated with antibiotics.14
A separate, single-center, retrospective trial produced different results. This study examined a population of 253 patients who underwent 266 EUS-FNA of pancreatic cysts and found that prophylactic antibiotics were associated with more adverse events and were not protective for the 3% of the patients with infectious symptoms.15 Despite the conflicting data, guidelines at this time recommend prophylactic antibiotics before drainage of a sterile pancreatic fluid collection that communicates with the pancreatic duct and also for aspiration of cystic lesions along the GI tract and the mediastinum.2
Endoscopic Retrograde Cholangiopancreatography (ERCP). In patients undergoing ERCP, the routine use of prophylactic antibiotics has not been found to be effective in decreasing the risk of post-procedure cholangitis.16 Guidelines recommend the use of prophylactic antibiotics only in those patients in which the ERCP may not completely resolve the biliary obstruction.2 In these patients, the thought is that ERCP can precipitate infection by disturbing bacteria already present in the biliary tree, especially with increased intrabiliary pressure at the time of contrast dye injection.17
Patients with incomplete biliary drainage, including those with primary sclerosing cholangitis (PSC), hilar cholangiocarcinoma, persistent biliary that were not extracted, and strictures that continue to obstruct despite attempted intervention, are thought to be at elevated risk of developing cholangitis post-ERCP. These patients should be placed on prophylactic antibiotics at the time of the procedure to cover biliary flora such as enteric gram negatives and enterococci. Antibiotics should be continued until the biliary obstruction is resolved.2
Additional Populations to Consider. Previously, the International Society for Peritoneal Dialysis recommended that patients on peritoneal dialysis receive prophylactic antibiotics and empty their abdomen of dialysate prior to colonoscopy. This recommendation has been removed from the 2010 guidelines.18 There is also no indication that patients with synthetic vascular grafts or cardiac devices should receive prophylactic antibiotics prior to routine GI endoscopy.19 The American Academy of Orthopaedic Surgeons no longer recommends that patients with joint replacements receive antibiotic prophylaxis prior to GI endoscopy.20
Back to the Case
The older gentleman with a prosthetic valve undergoing colonoscopy should not receive prophylactic antibiotics, because even in the setting of valvulopathy, colonoscopy does not pose a significant risk for infective endocarditis. The young patient with severe choledocholithiasis should be placed on prophylactic antibiotics because she has continued biliary obstruction, which could result in a cholangitis after ERCP.
Bottom Line
Prophylactic antibiotics are not recommended for any patient undergoing routine endoscopy or colonoscopy. They are indicated for patients with bleeding esophageal varices and for patients who undergo esophageal stricture dilation, PEG placement, or pseudocyst or cyst drainage, and those with continued biliary obstruction undergoing ERCP as summarized in Table 1.
Drs. Ritter, Jupiter, Carbo, and Li are hospitalists at Beth Israel Deaconess Medical Center and Harvard Medical School faculty in Boston.
References
- Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116(15):1736-1754.
- Banerjee S, Shen B, Baron TH, et al. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2008;67(6):791-798.
- Allison MC, Sandoe JA, Tighe R, Simpson IA, Hall RJ, Elliott TS. Antibiotic prophylaxis in gastrointestinal endoscopy. Gut. 2009;58(6):869-880.
- Nelson DB. Infectious disease complications of GI endoscopy: Part I, endogenous infections. Gastrointest Endosc. 2003;57(4):546-556.
- Zuccaro G Jr., Richter JE, Rice TW, et al. Viridans streptococcal bacteremia after esophageal stricture dilation. Gastrointest Endosc. 1998;48(6):568-573.
- Nelson DB, Sanderson SJ, Azar MM. Bacteremia with esophageal dilation. Gastrointest Endosc.1998;48(6):563-567.
- Hirota WK, Petersen K, Baron TH, et al. Guidelines for antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2003;58(4):475-482.
- Ho H, Zuckerman MJ, Wassem C. A prospective controlled study of the risk of bacteremia in emergency sclerotherapy of esophageal varices. Gastroenterology. 1991;101(6):1642-1648.
- Rolando N, Gimson A, Philpott-Howard J, et al. Infectious sequelae after endoscopic sclerotherapy of oesophageal varices: Role of antibiotic prophylaxis. J Hepatol. 1993;18(3):290-294.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46(3):922-938.
- Jafri NS, Mahid SS, Minor KS, Idstein SR, Hornung CA, Galandiuk S. Meta-analysis: Antibiotic prophylaxis to prevent peristomal infection following percutaneous endoscopic gastrostomy. Aliment Pharmacol Ther. 2007;25(6):647-656.
- Chuang CH, Hung KH, Chen JR, et al. Airway infection predisposes to peristomal infection after percutaneous endoscopic gastrostomy with high concordance between sputum and wound isolates. J Gastrointest Surg. 2010;14(1):45-51.
- Chaudhary KA, Smith OJ, Cuddy PG, Clarkston WK. PEG site infections: The emergence of methicillin resistant Staphylococcus aureus as a major pathogen. Am J Gastroenterol. 2002;97(7):1713-1716.
- Wang KX, Ben QW, Jin ZD, et al. Assessment of morbidity and mortality associated with EUS-guided FNA: A systematic review. Gastrointest Endosc. 2011;73(2):283-290.
- Guarner-Argente C, Shah P, Buchner A, Ahmad NA, Kochman ML, Ginsberg GG. Use of antimicrobials for EUS-guided FNA of pancreatic cysts: A retrospective, comparative analysis. Gastrointest Endosc. 2011;74(1):81-86.
- Bai Y, Gao F, Gao J, Zou DW, Li ZS. Prophylactic antibiotics cannot prevent endoscopic retrograde cholangiopancreatography-induced cholangitis: A meta-analysis. Pancreas. 2009;38(2):126-130.
- Cotton PB, Connor P, Rawls E, Romagnuolo J. Infection after ERCP, and antibiotic prophylaxis: A sequential quality-improvement approach over 11 years. Gastrointest Endosc. 2008;67(3):471-475.
- Li PK, Szeto CC, Piraino B, et al. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int. 2010;30(4):393-423.
- Baddour LM, Bettmann MA, Bolger AF, et al. Nonvalvular cardiovascular device-related infections. Circulation. 2003;108(16):2015-2031.
- Rethman MP, Watters W III, Abt E, et al. The American Academy of Orthopaedic Surgeons and the American Dental Association clinical practice guideline on the prevention of orthopaedic implant infection in patients undergoing dental procedures. J Bone Joint Surg Am. 2013;95(8):745-747.
Case
You are asked to admit two patients. The first is a 75-year-old male with a prosthetic aortic valve on warfarin who presents with bright red blood per rectum and is scheduled for colonoscopy. The second patient is a 35-year-old female with biliary obstruction due to choledocholithiasis; she is afebrile with normal vital signs and no leukocytosis. She underwent endoscopic retrograde cholangiopancreatography (ERCP), which did not resolve her biliary obstruction. Should you prescribe prophylactic antibiotics for either patient?
Overview
Providers are often confused regarding which patients undergoing gastrointestinal (GI) endoscopic procedures should receive antibiotic prophylaxis. To answer this question, it is important to understand the goal of prophylactic antibiotics. Are we trying to prevent infective endocarditis or a localized infection?
There are few large, prospective, randomized controlled trials that have examined the need for antibiotic prophylaxis with GI endoscopic procedures. Guidelines from professional societies are mainly based on expert opinion, evidence from retrospective case studies, and meta-analysis reviews.
Review of the Data
Infective endocarditis resulting from GI endoscopy has been a concern of physicians for decades. The American Heart Association (AHA) first published its recommendations for antibiotic prophylaxis of GI tract procedures in 1965. The most recent antibacterial prophylaxis guidelines, published in 2007, have simplified recommendations and greatly scaled back the indications for antibiotics. The new guidelines conclude that frequent bacteremia from daily activities is more likely to precipitate endocarditis than a single dental, GI, or genitourinary tract procedure.1
The American Society for Gastrointestinal Endoscopy (ASGE) reports that 14.2 million colonoscopies, 2.8 million flexible sigmoidoscopies, and nearly as many upper endoscopies are performed in the U.S. each year, but only 15 cases of endocarditis have been reported with a temporal association to a procedure.2
The British Society of Gastroenterology (BSG) found, after reviewing the histories of patients with infective endocarditis from 1983 through 2006, that there is not enough evidence to warrant antibiotic prophylaxis prior to endoscopy. They noted less than one case of endocarditis after GI endoscopy per year as well as significant variation in the time interval between the procedure and symptoms. The BSG also recognized that antibiotic prophylaxis does not always protect against infection and that clinical factors unrelated to the endoscopy may play a significant role in the development of endocarditis.3
Upper GI Endoscopy, Colonoscopy with Biopsy, and Esophageal Dilatation. Administering antibiotics to prevent infective endocarditis is not recommended for patients undergoing routine procedures such as endoscopy with biopsy and colonoscopy with polypectomy. Likewise, patients with a history of prosthetic heart valves, valve repair with prosthetic material, endocarditis, congenital heart disease, or cardiac transplant with valvulopathy do not need prophylactic antibiotics before GI endoscopic procedures. However, for patients who are being treated for an active GI infection, antibiotic coverage for enterococcus may be warranted given the increased risk of developing endocarditis. The AHA acknowledges there are no published studies to support the efficacy of antibiotics to prevent enterococcal endocarditis in patients in this clinical setting.1
Unlike routine endoscopy, esophageal dilation is associated with an increased rate of bacteremia (12%-100%).4 Streptococcus viridans has been found in blood cultures up to 79% of the time after esophageal dilation.5 Patients with malignant strictures have higher rates of bacteremia than those with benign strictures (52.9% versus 15.7%). Patients treated with multiple passes with the esophageal dilator compared to those treated with a single dilation have a higher risk of bacteremia.6 All patients undergoing esophageal stricture dilation should receive pre-procedural prophylactic antibiotics.7
Patients with bleeding esophageal varices also have high rates of bacteremia. Up to 20% of patients with cirrhosis and GI bleeding on admission develop an infection within 48 hours of presentation.8 There is evidence that the bacteremia may actually be related to the variceal bleeding rather than the procedure.9 Patients with bleeding esophageal varices treated with antibiotics have improved outcomes, including a decrease in mortality.10 Therefore, all patients with bleeding esophageal varices should be placed on antibiotic therapy regardless of whether an endoscopic intervention is planned.
Percutaneous Endoscopic Gastrostomy (PEG) Placement. Prophylactic antibiotics are recommended before placement of a PEG. The indication for prophylactic antibiotics is to prevent a gastrostomy site infection, not infective endocarditis. Gastrostomy site infection is unfortunately a fairly common infection, affecting 4% to 30% of patients who undergo PEG tube placement. There is significant evidence that antibiotics are beneficial in preventing peristomal infections. A meta-analysis showed that only eight patients need to be treated with prophylactic antibiotics to prevent a single peristomal infection.11 Since these infections are believed to be caused by contamination from the oropharynx, physicians should consider prophylaxis against pathogens from the oral flora.12
More recently, it has been noted that methicillin-resistant Staphylococcus aureus (MRSA) is increasingly cultured from infection sites.13 In centers with endemic MRSA, patients should be screened and then undergo decontamination prior to the PEG placement in positive cases.
Endoscopic Ultrasound with Fine Needle Aspiration (EUS-FNA). Antibiotic prophylaxis before EUS-FNA of a solid lesion in an organ is generally thought to be unnecessary because the risk of bacteremia with this procedure is low, comparable to routine GI endoscopy with biopsy. The recommendation for prophylactic antibiotics before biopsy of a cystic lesion is different. There is concern that puncturing cystic lesions may create a new infected fluid collection.2 A systematic review of more than 10,000 patients undergoing EUS-FNA with a full range of target organs revealed that, overall, 11.2% of patients experienced a fever and 4.7% of patients had a peri-procedural infection. While it was not possible in this study to determine which patients received prophylactic antibiotics, 93.7% of patients with pancreatic cystic lesions were reported to have been treated with antibiotics.14
A separate, single-center, retrospective trial produced different results. This study examined a population of 253 patients who underwent 266 EUS-FNA of pancreatic cysts and found that prophylactic antibiotics were associated with more adverse events and were not protective for the 3% of the patients with infectious symptoms.15 Despite the conflicting data, guidelines at this time recommend prophylactic antibiotics before drainage of a sterile pancreatic fluid collection that communicates with the pancreatic duct and also for aspiration of cystic lesions along the GI tract and the mediastinum.2
Endoscopic Retrograde Cholangiopancreatography (ERCP). In patients undergoing ERCP, the routine use of prophylactic antibiotics has not been found to be effective in decreasing the risk of post-procedure cholangitis.16 Guidelines recommend the use of prophylactic antibiotics only in those patients in which the ERCP may not completely resolve the biliary obstruction.2 In these patients, the thought is that ERCP can precipitate infection by disturbing bacteria already present in the biliary tree, especially with increased intrabiliary pressure at the time of contrast dye injection.17
Patients with incomplete biliary drainage, including those with primary sclerosing cholangitis (PSC), hilar cholangiocarcinoma, persistent biliary that were not extracted, and strictures that continue to obstruct despite attempted intervention, are thought to be at elevated risk of developing cholangitis post-ERCP. These patients should be placed on prophylactic antibiotics at the time of the procedure to cover biliary flora such as enteric gram negatives and enterococci. Antibiotics should be continued until the biliary obstruction is resolved.2
Additional Populations to Consider. Previously, the International Society for Peritoneal Dialysis recommended that patients on peritoneal dialysis receive prophylactic antibiotics and empty their abdomen of dialysate prior to colonoscopy. This recommendation has been removed from the 2010 guidelines.18 There is also no indication that patients with synthetic vascular grafts or cardiac devices should receive prophylactic antibiotics prior to routine GI endoscopy.19 The American Academy of Orthopaedic Surgeons no longer recommends that patients with joint replacements receive antibiotic prophylaxis prior to GI endoscopy.20
Back to the Case
The older gentleman with a prosthetic valve undergoing colonoscopy should not receive prophylactic antibiotics, because even in the setting of valvulopathy, colonoscopy does not pose a significant risk for infective endocarditis. The young patient with severe choledocholithiasis should be placed on prophylactic antibiotics because she has continued biliary obstruction, which could result in a cholangitis after ERCP.
Bottom Line
Prophylactic antibiotics are not recommended for any patient undergoing routine endoscopy or colonoscopy. They are indicated for patients with bleeding esophageal varices and for patients who undergo esophageal stricture dilation, PEG placement, or pseudocyst or cyst drainage, and those with continued biliary obstruction undergoing ERCP as summarized in Table 1.
Drs. Ritter, Jupiter, Carbo, and Li are hospitalists at Beth Israel Deaconess Medical Center and Harvard Medical School faculty in Boston.
References
- Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116(15):1736-1754.
- Banerjee S, Shen B, Baron TH, et al. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2008;67(6):791-798.
- Allison MC, Sandoe JA, Tighe R, Simpson IA, Hall RJ, Elliott TS. Antibiotic prophylaxis in gastrointestinal endoscopy. Gut. 2009;58(6):869-880.
- Nelson DB. Infectious disease complications of GI endoscopy: Part I, endogenous infections. Gastrointest Endosc. 2003;57(4):546-556.
- Zuccaro G Jr., Richter JE, Rice TW, et al. Viridans streptococcal bacteremia after esophageal stricture dilation. Gastrointest Endosc. 1998;48(6):568-573.
- Nelson DB, Sanderson SJ, Azar MM. Bacteremia with esophageal dilation. Gastrointest Endosc.1998;48(6):563-567.
- Hirota WK, Petersen K, Baron TH, et al. Guidelines for antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2003;58(4):475-482.
- Ho H, Zuckerman MJ, Wassem C. A prospective controlled study of the risk of bacteremia in emergency sclerotherapy of esophageal varices. Gastroenterology. 1991;101(6):1642-1648.
- Rolando N, Gimson A, Philpott-Howard J, et al. Infectious sequelae after endoscopic sclerotherapy of oesophageal varices: Role of antibiotic prophylaxis. J Hepatol. 1993;18(3):290-294.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46(3):922-938.
- Jafri NS, Mahid SS, Minor KS, Idstein SR, Hornung CA, Galandiuk S. Meta-analysis: Antibiotic prophylaxis to prevent peristomal infection following percutaneous endoscopic gastrostomy. Aliment Pharmacol Ther. 2007;25(6):647-656.
- Chuang CH, Hung KH, Chen JR, et al. Airway infection predisposes to peristomal infection after percutaneous endoscopic gastrostomy with high concordance between sputum and wound isolates. J Gastrointest Surg. 2010;14(1):45-51.
- Chaudhary KA, Smith OJ, Cuddy PG, Clarkston WK. PEG site infections: The emergence of methicillin resistant Staphylococcus aureus as a major pathogen. Am J Gastroenterol. 2002;97(7):1713-1716.
- Wang KX, Ben QW, Jin ZD, et al. Assessment of morbidity and mortality associated with EUS-guided FNA: A systematic review. Gastrointest Endosc. 2011;73(2):283-290.
- Guarner-Argente C, Shah P, Buchner A, Ahmad NA, Kochman ML, Ginsberg GG. Use of antimicrobials for EUS-guided FNA of pancreatic cysts: A retrospective, comparative analysis. Gastrointest Endosc. 2011;74(1):81-86.
- Bai Y, Gao F, Gao J, Zou DW, Li ZS. Prophylactic antibiotics cannot prevent endoscopic retrograde cholangiopancreatography-induced cholangitis: A meta-analysis. Pancreas. 2009;38(2):126-130.
- Cotton PB, Connor P, Rawls E, Romagnuolo J. Infection after ERCP, and antibiotic prophylaxis: A sequential quality-improvement approach over 11 years. Gastrointest Endosc. 2008;67(3):471-475.
- Li PK, Szeto CC, Piraino B, et al. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int. 2010;30(4):393-423.
- Baddour LM, Bettmann MA, Bolger AF, et al. Nonvalvular cardiovascular device-related infections. Circulation. 2003;108(16):2015-2031.
- Rethman MP, Watters W III, Abt E, et al. The American Academy of Orthopaedic Surgeons and the American Dental Association clinical practice guideline on the prevention of orthopaedic implant infection in patients undergoing dental procedures. J Bone Joint Surg Am. 2013;95(8):745-747.
HM14 to Feature Free Wi-Fi
At today’s meetings and conferences, wireless Internet access is a necessity for hospitalists who need to stay in touch with home and work, network with other conference-goers, and receive updates through the HM14 at Hand app. HM14 attendees will enjoy free Wi-Fi at the Mandalay Bay Convention Center in Las Vegas. To access, jot down the username (HM14) and the access code (hospitalist14), and log in with all your devices when you arrive.
Wi-Fi at HM14
Username: HM14
Access Code: hospitalist14
At today’s meetings and conferences, wireless Internet access is a necessity for hospitalists who need to stay in touch with home and work, network with other conference-goers, and receive updates through the HM14 at Hand app. HM14 attendees will enjoy free Wi-Fi at the Mandalay Bay Convention Center in Las Vegas. To access, jot down the username (HM14) and the access code (hospitalist14), and log in with all your devices when you arrive.
Wi-Fi at HM14
Username: HM14
Access Code: hospitalist14
At today’s meetings and conferences, wireless Internet access is a necessity for hospitalists who need to stay in touch with home and work, network with other conference-goers, and receive updates through the HM14 at Hand app. HM14 attendees will enjoy free Wi-Fi at the Mandalay Bay Convention Center in Las Vegas. To access, jot down the username (HM14) and the access code (hospitalist14), and log in with all your devices when you arrive.
Wi-Fi at HM14
Username: HM14
Access Code: hospitalist14
Problem Solving In Multi-Site Hospital Medicine Groups
Serving as the lead physician for a hospital medicine group (HMG) makes for challenging work. And the challenges and complexity only increase for anyone who serves as the physician leader for multiple practice sites in the same hospital system. In my November 2013 column on multi-site HMG leaders, I listed a few of the tricky issues they face and will mention a few more here.
Large-Small Friction
Unfortunately, tension between hospitalists at the big hospital and doctors at the small, “feeder” hospitals seems pretty common, and I think it’s due largely to high stress and a wide variation in workload, neither of which are in our direct control. At facilities where there is significant tension, I’m impressed by how vigorously the hospitalists at both the small and large hospitals argue that their own site faces the most stress and challenges. (This is a little like the endless debate about who works harder, those who work with residents and those who don’t.)
The hospitalists at the small site point out that they work with little or no subspecialty help and might even have to take night call from home while working during the day. Those at the big hospital say they are the ones with the very large scope of clinical practice and that, rather than making their life easier, the presence of lots of subspecialists makes for additional work coordinating care and communicating with all parties.
Where it exists, this tension is most evident during a transfer from one of the small hospitals to the large one. After all, one of the reasons to form a system of hospitals is so that nearly all patient needs can be met at one of the facilities in the system. Yet, for many reasons, the hospitalists at the large hospital are—sometimes—not as receptive to transfers as might be ideal. They might be short staffed or facing a high census or an unusually high number of admissions from their own ED. Or, perhaps, they’re concerned that the subspecialty services for which the patient is being transferred (e.g. to be scoped by a GI doctor) won’t be as helpful or prompt as needed. Or maybe they’ve felt “burned” by their colleagues at the small hospital for past transfers that didn’t seem necessary.
The result can be that the doctors at the smaller hospital complain that the “mother ship” hospitalists often are unfriendly and unreceptive to transfer requests. Although there may not be a definitive “cure” for this issue, there are several ways to help address the problem.
- In my last column, I mentioned the value of one or more in-person meetings between those who tend to be on the sending and receiving end of transfers, to establish some criteria regarding transfers that are appropriate and review the process of requesting a transfer and making the associated arrangements. In most cases there will be value in the parties meeting routinely—perhaps two to four times annually—to review how the system is working and address any difficulties.
- Periodic social meetings among the hospitalists at each site will help to form relationships that can make it less likely that any conversation about transfers will go in an unhelpful direction. Things can be very different when the people on each end of the phone call know each other personally.
- Record the phone calls between those seeking and accepting/declining each transfer. Scott Rissmiller, MD, the lead hospitalist for the 17 practice sites in Carolinas Healthcare, has said that having underperforming doctors listen to recordings of their phone calls about transfers has, in most cases he’s been involved with, proven to be a very effective way to encourage improvement.
Shared Staffing
The small hospitals in many systems sometimes struggle to find a way to provide economical night coverage. Hospitals below a certain size find it very difficult to justify a separate, in-house night provider. Some hospital systems have had success sharing night staffing, with the large hospital’s night hospitalist, nurse practitioner, or physician assistant providing telephone coverage for “cross cover” issues that arise after hours.
For example, when a nurse at the small hospital needs to contact a night hospitalist, staff will page the provider at the big hospital, and, in many cases, the issue can be managed effectively by phone. This works best when both hospitals are on the same electronic medical record, so that the responding provider can look through the record as needed.
The hospitalist at the small hospital typically stays on back-up call and is contacted if bedside attention is required.
Or, if the large and small hospitals are a short drive apart, the night hospitalist at the large facility might make the short drive to the small hospital when needed. In the case of emergencies (i.e., a code blue), the in-house night ED physician is relied on as the first responder.
Dr. Nelson has been a practicing hospitalist since 1988. He is co-founder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is co-director for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. Write to him at john.nelson@nelsonflores.com.
Serving as the lead physician for a hospital medicine group (HMG) makes for challenging work. And the challenges and complexity only increase for anyone who serves as the physician leader for multiple practice sites in the same hospital system. In my November 2013 column on multi-site HMG leaders, I listed a few of the tricky issues they face and will mention a few more here.
Large-Small Friction
Unfortunately, tension between hospitalists at the big hospital and doctors at the small, “feeder” hospitals seems pretty common, and I think it’s due largely to high stress and a wide variation in workload, neither of which are in our direct control. At facilities where there is significant tension, I’m impressed by how vigorously the hospitalists at both the small and large hospitals argue that their own site faces the most stress and challenges. (This is a little like the endless debate about who works harder, those who work with residents and those who don’t.)
The hospitalists at the small site point out that they work with little or no subspecialty help and might even have to take night call from home while working during the day. Those at the big hospital say they are the ones with the very large scope of clinical practice and that, rather than making their life easier, the presence of lots of subspecialists makes for additional work coordinating care and communicating with all parties.
Where it exists, this tension is most evident during a transfer from one of the small hospitals to the large one. After all, one of the reasons to form a system of hospitals is so that nearly all patient needs can be met at one of the facilities in the system. Yet, for many reasons, the hospitalists at the large hospital are—sometimes—not as receptive to transfers as might be ideal. They might be short staffed or facing a high census or an unusually high number of admissions from their own ED. Or, perhaps, they’re concerned that the subspecialty services for which the patient is being transferred (e.g. to be scoped by a GI doctor) won’t be as helpful or prompt as needed. Or maybe they’ve felt “burned” by their colleagues at the small hospital for past transfers that didn’t seem necessary.
The result can be that the doctors at the smaller hospital complain that the “mother ship” hospitalists often are unfriendly and unreceptive to transfer requests. Although there may not be a definitive “cure” for this issue, there are several ways to help address the problem.
- In my last column, I mentioned the value of one or more in-person meetings between those who tend to be on the sending and receiving end of transfers, to establish some criteria regarding transfers that are appropriate and review the process of requesting a transfer and making the associated arrangements. In most cases there will be value in the parties meeting routinely—perhaps two to four times annually—to review how the system is working and address any difficulties.
- Periodic social meetings among the hospitalists at each site will help to form relationships that can make it less likely that any conversation about transfers will go in an unhelpful direction. Things can be very different when the people on each end of the phone call know each other personally.
- Record the phone calls between those seeking and accepting/declining each transfer. Scott Rissmiller, MD, the lead hospitalist for the 17 practice sites in Carolinas Healthcare, has said that having underperforming doctors listen to recordings of their phone calls about transfers has, in most cases he’s been involved with, proven to be a very effective way to encourage improvement.
Shared Staffing
The small hospitals in many systems sometimes struggle to find a way to provide economical night coverage. Hospitals below a certain size find it very difficult to justify a separate, in-house night provider. Some hospital systems have had success sharing night staffing, with the large hospital’s night hospitalist, nurse practitioner, or physician assistant providing telephone coverage for “cross cover” issues that arise after hours.
For example, when a nurse at the small hospital needs to contact a night hospitalist, staff will page the provider at the big hospital, and, in many cases, the issue can be managed effectively by phone. This works best when both hospitals are on the same electronic medical record, so that the responding provider can look through the record as needed.
The hospitalist at the small hospital typically stays on back-up call and is contacted if bedside attention is required.
Or, if the large and small hospitals are a short drive apart, the night hospitalist at the large facility might make the short drive to the small hospital when needed. In the case of emergencies (i.e., a code blue), the in-house night ED physician is relied on as the first responder.
Dr. Nelson has been a practicing hospitalist since 1988. He is co-founder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is co-director for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. Write to him at john.nelson@nelsonflores.com.
Serving as the lead physician for a hospital medicine group (HMG) makes for challenging work. And the challenges and complexity only increase for anyone who serves as the physician leader for multiple practice sites in the same hospital system. In my November 2013 column on multi-site HMG leaders, I listed a few of the tricky issues they face and will mention a few more here.
Large-Small Friction
Unfortunately, tension between hospitalists at the big hospital and doctors at the small, “feeder” hospitals seems pretty common, and I think it’s due largely to high stress and a wide variation in workload, neither of which are in our direct control. At facilities where there is significant tension, I’m impressed by how vigorously the hospitalists at both the small and large hospitals argue that their own site faces the most stress and challenges. (This is a little like the endless debate about who works harder, those who work with residents and those who don’t.)
The hospitalists at the small site point out that they work with little or no subspecialty help and might even have to take night call from home while working during the day. Those at the big hospital say they are the ones with the very large scope of clinical practice and that, rather than making their life easier, the presence of lots of subspecialists makes for additional work coordinating care and communicating with all parties.
Where it exists, this tension is most evident during a transfer from one of the small hospitals to the large one. After all, one of the reasons to form a system of hospitals is so that nearly all patient needs can be met at one of the facilities in the system. Yet, for many reasons, the hospitalists at the large hospital are—sometimes—not as receptive to transfers as might be ideal. They might be short staffed or facing a high census or an unusually high number of admissions from their own ED. Or, perhaps, they’re concerned that the subspecialty services for which the patient is being transferred (e.g. to be scoped by a GI doctor) won’t be as helpful or prompt as needed. Or maybe they’ve felt “burned” by their colleagues at the small hospital for past transfers that didn’t seem necessary.
The result can be that the doctors at the smaller hospital complain that the “mother ship” hospitalists often are unfriendly and unreceptive to transfer requests. Although there may not be a definitive “cure” for this issue, there are several ways to help address the problem.
- In my last column, I mentioned the value of one or more in-person meetings between those who tend to be on the sending and receiving end of transfers, to establish some criteria regarding transfers that are appropriate and review the process of requesting a transfer and making the associated arrangements. In most cases there will be value in the parties meeting routinely—perhaps two to four times annually—to review how the system is working and address any difficulties.
- Periodic social meetings among the hospitalists at each site will help to form relationships that can make it less likely that any conversation about transfers will go in an unhelpful direction. Things can be very different when the people on each end of the phone call know each other personally.
- Record the phone calls between those seeking and accepting/declining each transfer. Scott Rissmiller, MD, the lead hospitalist for the 17 practice sites in Carolinas Healthcare, has said that having underperforming doctors listen to recordings of their phone calls about transfers has, in most cases he’s been involved with, proven to be a very effective way to encourage improvement.
Shared Staffing
The small hospitals in many systems sometimes struggle to find a way to provide economical night coverage. Hospitals below a certain size find it very difficult to justify a separate, in-house night provider. Some hospital systems have had success sharing night staffing, with the large hospital’s night hospitalist, nurse practitioner, or physician assistant providing telephone coverage for “cross cover” issues that arise after hours.
For example, when a nurse at the small hospital needs to contact a night hospitalist, staff will page the provider at the big hospital, and, in many cases, the issue can be managed effectively by phone. This works best when both hospitals are on the same electronic medical record, so that the responding provider can look through the record as needed.
The hospitalist at the small hospital typically stays on back-up call and is contacted if bedside attention is required.
Or, if the large and small hospitals are a short drive apart, the night hospitalist at the large facility might make the short drive to the small hospital when needed. In the case of emergencies (i.e., a code blue), the in-house night ED physician is relied on as the first responder.
Dr. Nelson has been a practicing hospitalist since 1988. He is co-founder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is co-director for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. Write to him at john.nelson@nelsonflores.com.
Federal Grant Extends Anti-Infection Initiative
The American Hospital Association’s Health Research and Educational Trust (HRET) recently obtained a grant from the federal Agency for Healthcare Research and Quality to expand CUSP, the Comprehensive Unit-based Safety Program for reducing catheter-associated urinary tract infections (CAUTI) and other healthcare-associated infections, to nursing homes and skilled nursing facilities nationwide.
CUSP has posted a 40% reduction in central line-associated bloodstream infections (CLABSI) in 1,000 participating hospitals by providing education and support and an evidence-based protocol. The grant will be administered by HRET in partnership with others, including the University of Michigan Health System, the Association for Professionals in Infection Control and Epidemiology, and SHM.
Meanwhile, a study published in the American Journal of Infection Control found that rates of catheter-associated urinary tract infections in adult patients given urinary catheter placements dropped nationwide to 5.3% in 2010 from 9.4% in 2001.3 The retrospective analysis of data from the National Hospital Discharge Survey found that CAUTI-related mortality and associated length of hospital stay also declined during the same period.
Larry Beresford is a freelance writer in Alameda, Calif.
The American Hospital Association’s Health Research and Educational Trust (HRET) recently obtained a grant from the federal Agency for Healthcare Research and Quality to expand CUSP, the Comprehensive Unit-based Safety Program for reducing catheter-associated urinary tract infections (CAUTI) and other healthcare-associated infections, to nursing homes and skilled nursing facilities nationwide.
CUSP has posted a 40% reduction in central line-associated bloodstream infections (CLABSI) in 1,000 participating hospitals by providing education and support and an evidence-based protocol. The grant will be administered by HRET in partnership with others, including the University of Michigan Health System, the Association for Professionals in Infection Control and Epidemiology, and SHM.
Meanwhile, a study published in the American Journal of Infection Control found that rates of catheter-associated urinary tract infections in adult patients given urinary catheter placements dropped nationwide to 5.3% in 2010 from 9.4% in 2001.3 The retrospective analysis of data from the National Hospital Discharge Survey found that CAUTI-related mortality and associated length of hospital stay also declined during the same period.
Larry Beresford is a freelance writer in Alameda, Calif.
The American Hospital Association’s Health Research and Educational Trust (HRET) recently obtained a grant from the federal Agency for Healthcare Research and Quality to expand CUSP, the Comprehensive Unit-based Safety Program for reducing catheter-associated urinary tract infections (CAUTI) and other healthcare-associated infections, to nursing homes and skilled nursing facilities nationwide.
CUSP has posted a 40% reduction in central line-associated bloodstream infections (CLABSI) in 1,000 participating hospitals by providing education and support and an evidence-based protocol. The grant will be administered by HRET in partnership with others, including the University of Michigan Health System, the Association for Professionals in Infection Control and Epidemiology, and SHM.
Meanwhile, a study published in the American Journal of Infection Control found that rates of catheter-associated urinary tract infections in adult patients given urinary catheter placements dropped nationwide to 5.3% in 2010 from 9.4% in 2001.3 The retrospective analysis of data from the National Hospital Discharge Survey found that CAUTI-related mortality and associated length of hospital stay also declined during the same period.
Larry Beresford is a freelance writer in Alameda, Calif.
Patient Activation Measure Tool Helps Patients Avoid Hospital Readmissions
–Dr. Hibbard
A recent article in the Journal of Internal Medicine draws a strong link between readmission rates and the degree to which patients are activated—possessing the knowledge, skills, and confidence to manage their own health post-discharge.2 Co-author Judith Hibbard, DrPh, professor of health policy at the University of Oregon, is the lead inventor of the Patient Activation Measure (PAM), an eight-item tool that assigns patients to one of four levels of activation.
In a sample of 700 patients discharged from Boston Medical Center, those with the lowest levels of activation had 1.75 times the risk of 30-day readmissions, more ED visits, and greater utilization of health services, even after adjusting for severity of illness and demographics.
“Contrary to what some may assume, patients who demonstrate a lower level of activation do not fall into any specific racial, economic, or educational demographic,” Dr. Hibbard says, adding that providers should not expect to be able to reliably judge their patients’ ability to self-manage outside of the hospital. “We know that people who measure low tend to have little confidence in their ability to manage their own health. They feel overwhelmed, show poor problem-solving skills, don’t understand what professionals are telling them, and, as a result, may not pay close attention.”
Dr. Hibbard says higher activation scores reflect greater focus on personal health and the effort to monitor it—with more confidence.
The take-home message for hospitalists, she says, is to understand the importance of their patients’ activation level and to tailor interventions accordingly.
“Those with low activation may need more support,” such as post-discharge home visits instead of just a phone call. Low-activation patients should not be overwhelmed with information but should instead be given just a few prioritized key points, combined with the use of reinforcing communications techniques such as teach-back.
“Someone should sit with them and help negotiate their health behaviors,” she adds. “That’s how they get more activated. It doesn’t have to be a doctor going through these things. But just using the clinical lens to understand your patients is not enough.”
Larry Beresford is a freelance writer in Alameda, Calif.
–Dr. Hibbard
A recent article in the Journal of Internal Medicine draws a strong link between readmission rates and the degree to which patients are activated—possessing the knowledge, skills, and confidence to manage their own health post-discharge.2 Co-author Judith Hibbard, DrPh, professor of health policy at the University of Oregon, is the lead inventor of the Patient Activation Measure (PAM), an eight-item tool that assigns patients to one of four levels of activation.
In a sample of 700 patients discharged from Boston Medical Center, those with the lowest levels of activation had 1.75 times the risk of 30-day readmissions, more ED visits, and greater utilization of health services, even after adjusting for severity of illness and demographics.
“Contrary to what some may assume, patients who demonstrate a lower level of activation do not fall into any specific racial, economic, or educational demographic,” Dr. Hibbard says, adding that providers should not expect to be able to reliably judge their patients’ ability to self-manage outside of the hospital. “We know that people who measure low tend to have little confidence in their ability to manage their own health. They feel overwhelmed, show poor problem-solving skills, don’t understand what professionals are telling them, and, as a result, may not pay close attention.”
Dr. Hibbard says higher activation scores reflect greater focus on personal health and the effort to monitor it—with more confidence.
The take-home message for hospitalists, she says, is to understand the importance of their patients’ activation level and to tailor interventions accordingly.
“Those with low activation may need more support,” such as post-discharge home visits instead of just a phone call. Low-activation patients should not be overwhelmed with information but should instead be given just a few prioritized key points, combined with the use of reinforcing communications techniques such as teach-back.
“Someone should sit with them and help negotiate their health behaviors,” she adds. “That’s how they get more activated. It doesn’t have to be a doctor going through these things. But just using the clinical lens to understand your patients is not enough.”
Larry Beresford is a freelance writer in Alameda, Calif.
–Dr. Hibbard
A recent article in the Journal of Internal Medicine draws a strong link between readmission rates and the degree to which patients are activated—possessing the knowledge, skills, and confidence to manage their own health post-discharge.2 Co-author Judith Hibbard, DrPh, professor of health policy at the University of Oregon, is the lead inventor of the Patient Activation Measure (PAM), an eight-item tool that assigns patients to one of four levels of activation.
In a sample of 700 patients discharged from Boston Medical Center, those with the lowest levels of activation had 1.75 times the risk of 30-day readmissions, more ED visits, and greater utilization of health services, even after adjusting for severity of illness and demographics.
“Contrary to what some may assume, patients who demonstrate a lower level of activation do not fall into any specific racial, economic, or educational demographic,” Dr. Hibbard says, adding that providers should not expect to be able to reliably judge their patients’ ability to self-manage outside of the hospital. “We know that people who measure low tend to have little confidence in their ability to manage their own health. They feel overwhelmed, show poor problem-solving skills, don’t understand what professionals are telling them, and, as a result, may not pay close attention.”
Dr. Hibbard says higher activation scores reflect greater focus on personal health and the effort to monitor it—with more confidence.
The take-home message for hospitalists, she says, is to understand the importance of their patients’ activation level and to tailor interventions accordingly.
“Those with low activation may need more support,” such as post-discharge home visits instead of just a phone call. Low-activation patients should not be overwhelmed with information but should instead be given just a few prioritized key points, combined with the use of reinforcing communications techniques such as teach-back.
“Someone should sit with them and help negotiate their health behaviors,” she adds. “That’s how they get more activated. It doesn’t have to be a doctor going through these things. But just using the clinical lens to understand your patients is not enough.”
Larry Beresford is a freelance writer in Alameda, Calif.