User login
Distinguishing cellulitis from its mimics
More than 10% of patients labeled as having cellulitis do not have cellulitis.1 This is unfortunate, as it leads to excessive and incorrect use of antibiotics and to delays in appropriate therapy.2 However, it is not surprising, given the number of conditions that bear a striking similarity to cellulitis. A familiarity with the features of true cellulitis and with the handful of conditions that can bear a striking similarity to it is the way out of this potential diagnostic quagmire.
WHAT CELLULITIS IS—AND IS NOT
The key characteristics of cellulitis are redness, warmth, tenderness, and swelling of the skin. A history of trauma and pain in the affected area and evidence of leukocytosis3 suggest cellulitis. A symmetric or diffusely scattered pattern indicates a condition other than cellulitis, which is overwhelmingly unilateral, with smooth, indistinct borders4,5 Other factors pointing to cellulitis are underlying immunosuppression, a more rapid progression, previous episodes, systemic symptoms (eg, fever, leukocytosis), new medications, new travel or outdoor exposure, and comorbidities such as diabetes and peripheral vascular disease. A long-standing, slowly progressive course and a history of unsuccessful treatment with antibiotics are strong indicators of a condition other than cellulitis.
Consultation with a dermatologist is recommended to narrow the differential diagnosis. The dermatologist can determine if biopsy is necessary, as many dermatoses that mimic cellulitis can be diagnosed by visual recognition alone.
STASIS DERMATITIS
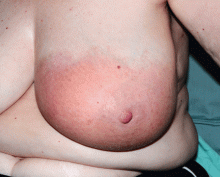
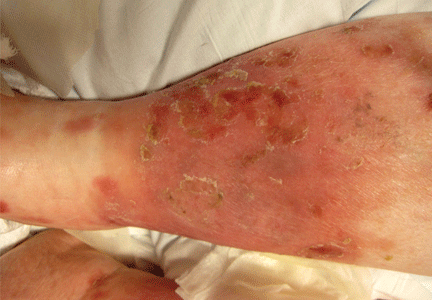
The most common mimic of cellulitis is stasis dermatitis (Figure 1).2 Patients can present with ill-defined, bilateral, pitting edema of the lower extremities, typically with erythema, hyperpigmentation, serous drainage, and superficial desquamation.3,6,7
The inciting factor is chronic venous insufficiency, leading to interstitial edema, extravasation of red blood cells, and decreased tissue oxygenation. This process causes micro-vascular changes and microthrombi that up-regulate transforming growth factor beta and fibroblastic growth factor.7 If the process is allowed to continue, stasis dermatitis may progress to lipodermatosclerosis.
Tip: Stasis dermatitis is generally bilateral, the process will have been ongoing for years, there is often pitting edema, and the legs should be nontender.
LIPODERMATOSCLEROSIS
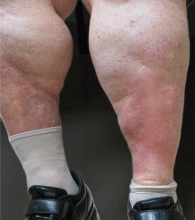
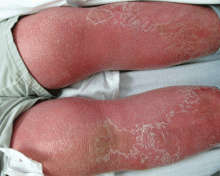
Lipodermatosclerosis is a sclerosing panniculitis classically described as an “inverted champagne bottle” or “inverted bowling pin” appearance of the leg, ie, the diameter of the leg is sharply narrowed directly below the calf (Figure 2).
There is an acute and a chronic phase. The acute phase is characterized by inflammation and erythema, and the chronic phase is characterized by fibrosis.8 The acute phase presents with severe lower-extremity pain above the medial malleolus, erythema, edema, and warmth; there is no sharp demarcation between affected and unaffected skin.9,10 This phase can be difficult to distinguish from cellulitis, so the history plays a key role. Known venous insufficiency, cutaneous changes of stasis dermatitis, and the absence of systemic symptoms all point to lipodermatosclerosis.
The chronic phase is characterized by unilateral or bilateral, indurated, sclerotic plaques with a “bound-down” appearance (ie, they appear as if tethered—or bound—to the subcutaneous tissue) affecting the skin from below the knee to the ankle; there is a sharp demarcation between affected and unaffected skin.9–11 The skin is often bronze or brown secondary to hemosiderin deposits. There can be prominent varicosities and scattered ulcerations depending on the course of the disease.
This condition is thought to be the result of long-standing chronic venous insufficiency.7,8,9,11 It is proposed that venous incompetence leads to extravasation of interstitial fluid and red blood cells, decreased diffusion of oxygen to the tissues, and eventual tissue and endothelial damage. As the endothelium is damaged, microthrombi formation and infarction ensue, stimulating fibroblasts to form granulation tissue.
Tip: The history helps to distinguish acute lipodermatosclerosis from cellulitis. Chroniclipodermatoslcerosis will have been ongoing for years, the legs should be nontender, the skin will be bound-down, and the diameter of the leg will sharply decrease from knee to ankle.
CONTACT DERMATITIS
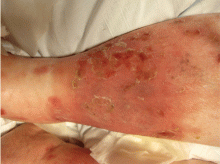
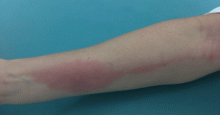
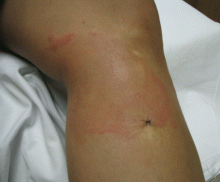
Allergic and irritant forms of contact dermatitis are often mistaken for cellulitis. Irritant contact dermatitis (Figure 3) presents with erythematous patches and plaques with well-defined borders, often in a geometric distribution where the skin was exposed to an irritant.12 Allergic contact dermatitis is a delayed hypersensitivity dermatitis that can be secondary to something ingested, applied to the skin, or airborne (Figure 4). It presents as erythematous macules, papules, and plaques that may have serous drainage or vesiculation. Lesions of allergic contact dermatitis are usually confined to the site of contact with the allergen, but they can infrequently be found at distant sites, in which case it is considered systemic contact dermatitis.3,5 Depending on the severity of the allergy, patients may complain of intense pain and pruritus.3
Additionally, chronic, nonhealing leg ulcers may have a confounding allergic contact dermatitis.7 Although patients may believe they are helping the ulcer heal by applying topical antibiotics or other lubricants, they may in fact be impeding the healing process. Always inquire as to what the patient is applying if he or she has leg ulceration with surrounding edema and erythema that has not resolved with conventional treatments.13,14
Tip: The key to distinguishing contact dermatitis from cellulitis is the history. For example, ask about recent changes in medications, soaps, and laundry detergents, new hobbies, or recent surgeries. The involved site is often confined to the area where the allergen contacted the skin, except in cases of exposure to an airborne allergen.
LYMPHEDEMA
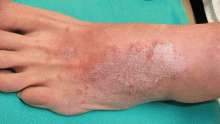
Lymphedema is characterized by localized edema of an affected extremity, with induration, erythema, and secondary cutaneous changes such as hyperkeratosis, dyspigmentation, and wart-like architecture (Figure 5).
Primary lymphedema appears in the setting of congenital abnormalities, whereas secondary lymphedema results from an interruption of a previously functioning lymphatic system (eg, after radical mastectomy).
Patients often present with unilateral nonpitting edema and erythema in the absence of systemic symptoms.12 Many patients presenting with lower-extremity lymphedema are overweight or obese, as the weight they carry causes obstruction of the inguinal lymphatics.6
The pathophysiology is not clearly delineated but is thought to be a consequence of decreased oxygenation of tissue secondary to extravasated lymph. As the oxygen is compromised, macrophages and fibroblasts are recruited, resulting in fibrosis.6
Patients with lymphedema are more susceptible to superficial and deep skin infections, as the natural defense system in the epidermis and papillary dermis is compromised by impaired lymphatic drainage.15
To differentiate uncomplicated lymphedema from a secondary cutaneous infection, the clinician should take into account the presence or absence of warmth, pain, increased erythema, and systemic symptoms (Figure 6).
Tip: Primary lymphedema will most likely present in childhood with no inciting factors and will require a full workup. Obtaining a history should make secondary lymphedema a relatively straightforward diagnosis: Has the patient undergone lymph node dissection? Has the patient had an injury in the affected leg? Lymphedema is overwhelmingly unilateral and nonpitting, and is often seen in overweight people (if no precipitating factor is present).
EOSINOPHILIC CELLULITIS
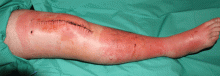
Eosinophilic cellulitis, or Wells syndrome, was first described in 1971 as a granulomatous dermatitis.16 It is a recurrent hypersensitivity reaction to a drug, to a vaccine, or to an insect bite, or to a viral or fungal infection that presents on the extremities as localized erythema, edema, and induration with sharp borders and a green or gray hue (Figure 7).17–19 The lesions commonly progress to firm, indurated plaques that resemble morphea. The plaques may take weeks or years to resolve, but they do so without scarring.12,17,20,21
As patients tend to have recurrent bouts of eosinophilic cellulitis, they may have lesions in different stages of healing. Patients tend to report itching and burning that precedes the onset of plaques.22 The complete blood count typically shows a transient hypereosinophilia.12,16,17,23–25
Tip: This diagnosis often requires biopsy for confirmation, but helpful clues are a history of recurrent episodes, the color of the lesions, and peripheral eosinophilia.
PAPULAR URTICARIA
Papular urticaria is a dermal hypersensitivity reaction to an insect bite, most commonly from a flea or mosquito.26 Patients are often children, as their immune system may be hypersensitive. But children often develop tolerance before puberty.27
The presentation may vary, from numerous urticarial papules near the site of a bite, to generalized, large, indurated, erythematous plaques reminiscent of cellulitis (Figure 8).5,26 The lesions usually develop within hours of a bite and persist for an average of 1 to 2 weeks.28 The areas typically affected are the head and neck or the upper or lower extremities; the palms, soles, and trunk are usually spared.27
Patients most often complain of intense itching.12 The pathogenesis is proposed to be mediated by the immune complex, and tissue biopsy study shows increased eosinophils. The eosinophils stimulate mast cells, causing release of histamine, leading to increased vascular permeability, edema, and erythema.28,29
Tip: Biopsy may be necessary to confirm the diagnosis, though often the history may be sufficient. The patient may or may not recall a bite, so probe into recent activities such as outdoor sports or contact with a new pet. The papules and plaques are generally very pruritic but not painful.
DERMATOLOGY CONSULT
If the clinical presentation and history do not correlate, or if the skin condition has been treated with antibiotics yet has failed to respond, the possibility of other cutaneous dermatoses should be entertained. A dermatology consult can help determine the diagnosis, the need for further evaluation, and the best treatment course.
- Hepburn MJ, Dooley DP, Ellis MW. Alternative diagnoses that often mimic cellulitis. Am Fam Physician 2003; 67:2471.
- David CV, Chira S, Eells SJ, et al. Diagnostic accuracy in patients admitted to hospitals with cellulitis. Dermatol Online J 2011; 17:1.
- Bailey E, Kroshinsky D. Cellulitis: diagnosis and management. Dermatol Ther 2011; 24:229–239.
- Stevens DL, Bisno AL, Chambers HF, et al; Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis 2005; 41:1373–1406.
- Lio PA. The many faces of cellulitis. Arch Dis Child Educ Pract Ed 2009; 94:50–54.
- Yosipovitch G, DeVore A, Dawn A. Obesity and the skin: skin physiology and skin manifestations of obesity. J Am Acad Dermatol 2007; 56:901–916.
- Farage MA, Miller KW, Berardesca E, Maibach HI. Clinical implications of aging skin: cutaneous disorders in the elderly. Am J Clin Dermatol 2009; 10:73–86.
- Kirsner RS, Pardes JB, Eaglstein WH, Falanga V. The clinical spectrum of lipodermatosclerosis. J Am Acad Dermatol 1993; 28:623–627.
- Miteva M, Romanelli P, Kirsner RS. Lipodermatosclerosis. Dermatol Ther 2010; 23:375–388.
- Barron GS, Jacob SE, Kirsner RS. Dermatologic complications of chronic venous disease: medical management and beyond. Ann Vasc Surg 2007; 21:652–662.
- Bruce AJ, Bennett DD, Lohse CM, Rooke TW, Davis MD. Lipodermatosclerosis: review of cases evaluated at Mayo Clinic. J Am Acad Dermatol 2002; 46:187–192.
- Falagas ME, Vergidis PI. Narrative review: diseases that masquerade as infectious cellulitis. Ann Intern Med 2005; 142:47–55.
- Wilson CL, Cameron J, Powell SM, Cherry G, Ryan TJ. High incidence of contact dermatitis in leg-ulcer patients—implications for management. Clin Exp Dermatol 1991; 16:250–253.
- Wolf R. The lanolin paradox. Dermatology 1996; 192:198–202.
- Keeley VL. Lymphoedema and cellulitis: chicken or egg? Br J Dermatol 2008; 158:1175–1176.
- Wells GC. Recurrent granulomatous dermatitis with eosinophilia. Trans St Johns Hosp Dermatol Soc 1971; 57:46–56.
- Ferreli C, Pinna AL, Atzori L, Aste N. Eosinophilic cellulitis (Well’s syndrome): a new case description. J Eur Acad Dermatol Venereol 1999; 13:41–45.
- Ladoyanni E, Vlachou C, Thushara R, Snead D. A patient with Wells’ syndrome. Clin Exp Dermatol 2010; 35:e3–e4.
- Moon HS, Park K, Lee JH, Son SJ. Eosinophilic cellulitis in an infant. Int J Dermatol 2010; 49:592–593.
- Walker P, Long D, James C, Marshman G. Exaggerated insect bite reaction exacerbated by a pyogenic infection in a patient with chronic lymphocytic leukaemia. Australas J Dermatol 2007; 48:165–169.
- Laliwala NM, Kulshrestha R, Singh R, Balasubramaniam P. A case of eosinophilic cellulitis of the hand mimicking bacterial cellulitis. J Hand Surg Eur Vol 2009; 34:410–411.
- Chung CL, Cusack CA. Wells syndrome: an enigmatic and therapeutically challenging disease. J Drugs Dermatol 2006; 5:908–911.
- Melski JW. Wells’ syndrome, insect bites, and eosinophils. Dermatol Clin 1990; 8:287–293.
- Spigel GT, Winkelmann RK. Wells’ syndrome. Recurrent granulomatous dermatitis with eosinophilia. Arch Dermatol 1979; 115:611–613.
- Clark DP, Anderson PC. Eosinophilic cellulitis caused by arthropod bites. Int J Dermatol 1988; 27:411–412.
- Howard R, Frieden IJ. Papular urticaria in children. Pediatr Dermatol 1996; 13:246–249.
- Hernandez RG, Cohen BA. Insect bite-induced hypersensitivity and the SCRATCH principles: a new approach to papular urticaria. Pediatrics 2006; 118:e189–e196.
- Heng MC, Kloss SG, Haberfelde GC. Pathogenesis of papular urticaria. J Am Acad Dermatol 1984; 10:1030–1034.
- Kossard S, Hamann I, Wilkinson B. Defining urticarial dermatitis: a subset of dermal hypersensitivity reaction pattern. Arch Dermatol 2006; 142:29–34.
More than 10% of patients labeled as having cellulitis do not have cellulitis.1 This is unfortunate, as it leads to excessive and incorrect use of antibiotics and to delays in appropriate therapy.2 However, it is not surprising, given the number of conditions that bear a striking similarity to cellulitis. A familiarity with the features of true cellulitis and with the handful of conditions that can bear a striking similarity to it is the way out of this potential diagnostic quagmire.
WHAT CELLULITIS IS—AND IS NOT
The key characteristics of cellulitis are redness, warmth, tenderness, and swelling of the skin. A history of trauma and pain in the affected area and evidence of leukocytosis3 suggest cellulitis. A symmetric or diffusely scattered pattern indicates a condition other than cellulitis, which is overwhelmingly unilateral, with smooth, indistinct borders4,5 Other factors pointing to cellulitis are underlying immunosuppression, a more rapid progression, previous episodes, systemic symptoms (eg, fever, leukocytosis), new medications, new travel or outdoor exposure, and comorbidities such as diabetes and peripheral vascular disease. A long-standing, slowly progressive course and a history of unsuccessful treatment with antibiotics are strong indicators of a condition other than cellulitis.
Consultation with a dermatologist is recommended to narrow the differential diagnosis. The dermatologist can determine if biopsy is necessary, as many dermatoses that mimic cellulitis can be diagnosed by visual recognition alone.
STASIS DERMATITIS
The most common mimic of cellulitis is stasis dermatitis (Figure 1).2 Patients can present with ill-defined, bilateral, pitting edema of the lower extremities, typically with erythema, hyperpigmentation, serous drainage, and superficial desquamation.3,6,7
The inciting factor is chronic venous insufficiency, leading to interstitial edema, extravasation of red blood cells, and decreased tissue oxygenation. This process causes micro-vascular changes and microthrombi that up-regulate transforming growth factor beta and fibroblastic growth factor.7 If the process is allowed to continue, stasis dermatitis may progress to lipodermatosclerosis.
Tip: Stasis dermatitis is generally bilateral, the process will have been ongoing for years, there is often pitting edema, and the legs should be nontender.
LIPODERMATOSCLEROSIS
Lipodermatosclerosis is a sclerosing panniculitis classically described as an “inverted champagne bottle” or “inverted bowling pin” appearance of the leg, ie, the diameter of the leg is sharply narrowed directly below the calf (Figure 2).
There is an acute and a chronic phase. The acute phase is characterized by inflammation and erythema, and the chronic phase is characterized by fibrosis.8 The acute phase presents with severe lower-extremity pain above the medial malleolus, erythema, edema, and warmth; there is no sharp demarcation between affected and unaffected skin.9,10 This phase can be difficult to distinguish from cellulitis, so the history plays a key role. Known venous insufficiency, cutaneous changes of stasis dermatitis, and the absence of systemic symptoms all point to lipodermatosclerosis.
The chronic phase is characterized by unilateral or bilateral, indurated, sclerotic plaques with a “bound-down” appearance (ie, they appear as if tethered—or bound—to the subcutaneous tissue) affecting the skin from below the knee to the ankle; there is a sharp demarcation between affected and unaffected skin.9–11 The skin is often bronze or brown secondary to hemosiderin deposits. There can be prominent varicosities and scattered ulcerations depending on the course of the disease.
This condition is thought to be the result of long-standing chronic venous insufficiency.7,8,9,11 It is proposed that venous incompetence leads to extravasation of interstitial fluid and red blood cells, decreased diffusion of oxygen to the tissues, and eventual tissue and endothelial damage. As the endothelium is damaged, microthrombi formation and infarction ensue, stimulating fibroblasts to form granulation tissue.
Tip: The history helps to distinguish acute lipodermatosclerosis from cellulitis. Chroniclipodermatoslcerosis will have been ongoing for years, the legs should be nontender, the skin will be bound-down, and the diameter of the leg will sharply decrease from knee to ankle.
CONTACT DERMATITIS
Allergic and irritant forms of contact dermatitis are often mistaken for cellulitis. Irritant contact dermatitis (Figure 3) presents with erythematous patches and plaques with well-defined borders, often in a geometric distribution where the skin was exposed to an irritant.12 Allergic contact dermatitis is a delayed hypersensitivity dermatitis that can be secondary to something ingested, applied to the skin, or airborne (Figure 4). It presents as erythematous macules, papules, and plaques that may have serous drainage or vesiculation. Lesions of allergic contact dermatitis are usually confined to the site of contact with the allergen, but they can infrequently be found at distant sites, in which case it is considered systemic contact dermatitis.3,5 Depending on the severity of the allergy, patients may complain of intense pain and pruritus.3
Additionally, chronic, nonhealing leg ulcers may have a confounding allergic contact dermatitis.7 Although patients may believe they are helping the ulcer heal by applying topical antibiotics or other lubricants, they may in fact be impeding the healing process. Always inquire as to what the patient is applying if he or she has leg ulceration with surrounding edema and erythema that has not resolved with conventional treatments.13,14
Tip: The key to distinguishing contact dermatitis from cellulitis is the history. For example, ask about recent changes in medications, soaps, and laundry detergents, new hobbies, or recent surgeries. The involved site is often confined to the area where the allergen contacted the skin, except in cases of exposure to an airborne allergen.
LYMPHEDEMA
Lymphedema is characterized by localized edema of an affected extremity, with induration, erythema, and secondary cutaneous changes such as hyperkeratosis, dyspigmentation, and wart-like architecture (Figure 5).
Primary lymphedema appears in the setting of congenital abnormalities, whereas secondary lymphedema results from an interruption of a previously functioning lymphatic system (eg, after radical mastectomy).
Patients often present with unilateral nonpitting edema and erythema in the absence of systemic symptoms.12 Many patients presenting with lower-extremity lymphedema are overweight or obese, as the weight they carry causes obstruction of the inguinal lymphatics.6
The pathophysiology is not clearly delineated but is thought to be a consequence of decreased oxygenation of tissue secondary to extravasated lymph. As the oxygen is compromised, macrophages and fibroblasts are recruited, resulting in fibrosis.6
Patients with lymphedema are more susceptible to superficial and deep skin infections, as the natural defense system in the epidermis and papillary dermis is compromised by impaired lymphatic drainage.15
To differentiate uncomplicated lymphedema from a secondary cutaneous infection, the clinician should take into account the presence or absence of warmth, pain, increased erythema, and systemic symptoms (Figure 6).
Tip: Primary lymphedema will most likely present in childhood with no inciting factors and will require a full workup. Obtaining a history should make secondary lymphedema a relatively straightforward diagnosis: Has the patient undergone lymph node dissection? Has the patient had an injury in the affected leg? Lymphedema is overwhelmingly unilateral and nonpitting, and is often seen in overweight people (if no precipitating factor is present).
EOSINOPHILIC CELLULITIS
Eosinophilic cellulitis, or Wells syndrome, was first described in 1971 as a granulomatous dermatitis.16 It is a recurrent hypersensitivity reaction to a drug, to a vaccine, or to an insect bite, or to a viral or fungal infection that presents on the extremities as localized erythema, edema, and induration with sharp borders and a green or gray hue (Figure 7).17–19 The lesions commonly progress to firm, indurated plaques that resemble morphea. The plaques may take weeks or years to resolve, but they do so without scarring.12,17,20,21
As patients tend to have recurrent bouts of eosinophilic cellulitis, they may have lesions in different stages of healing. Patients tend to report itching and burning that precedes the onset of plaques.22 The complete blood count typically shows a transient hypereosinophilia.12,16,17,23–25
Tip: This diagnosis often requires biopsy for confirmation, but helpful clues are a history of recurrent episodes, the color of the lesions, and peripheral eosinophilia.
PAPULAR URTICARIA
Papular urticaria is a dermal hypersensitivity reaction to an insect bite, most commonly from a flea or mosquito.26 Patients are often children, as their immune system may be hypersensitive. But children often develop tolerance before puberty.27
The presentation may vary, from numerous urticarial papules near the site of a bite, to generalized, large, indurated, erythematous plaques reminiscent of cellulitis (Figure 8).5,26 The lesions usually develop within hours of a bite and persist for an average of 1 to 2 weeks.28 The areas typically affected are the head and neck or the upper or lower extremities; the palms, soles, and trunk are usually spared.27
Patients most often complain of intense itching.12 The pathogenesis is proposed to be mediated by the immune complex, and tissue biopsy study shows increased eosinophils. The eosinophils stimulate mast cells, causing release of histamine, leading to increased vascular permeability, edema, and erythema.28,29
Tip: Biopsy may be necessary to confirm the diagnosis, though often the history may be sufficient. The patient may or may not recall a bite, so probe into recent activities such as outdoor sports or contact with a new pet. The papules and plaques are generally very pruritic but not painful.
DERMATOLOGY CONSULT
If the clinical presentation and history do not correlate, or if the skin condition has been treated with antibiotics yet has failed to respond, the possibility of other cutaneous dermatoses should be entertained. A dermatology consult can help determine the diagnosis, the need for further evaluation, and the best treatment course.
More than 10% of patients labeled as having cellulitis do not have cellulitis.1 This is unfortunate, as it leads to excessive and incorrect use of antibiotics and to delays in appropriate therapy.2 However, it is not surprising, given the number of conditions that bear a striking similarity to cellulitis. A familiarity with the features of true cellulitis and with the handful of conditions that can bear a striking similarity to it is the way out of this potential diagnostic quagmire.
WHAT CELLULITIS IS—AND IS NOT
The key characteristics of cellulitis are redness, warmth, tenderness, and swelling of the skin. A history of trauma and pain in the affected area and evidence of leukocytosis3 suggest cellulitis. A symmetric or diffusely scattered pattern indicates a condition other than cellulitis, which is overwhelmingly unilateral, with smooth, indistinct borders4,5 Other factors pointing to cellulitis are underlying immunosuppression, a more rapid progression, previous episodes, systemic symptoms (eg, fever, leukocytosis), new medications, new travel or outdoor exposure, and comorbidities such as diabetes and peripheral vascular disease. A long-standing, slowly progressive course and a history of unsuccessful treatment with antibiotics are strong indicators of a condition other than cellulitis.
Consultation with a dermatologist is recommended to narrow the differential diagnosis. The dermatologist can determine if biopsy is necessary, as many dermatoses that mimic cellulitis can be diagnosed by visual recognition alone.
STASIS DERMATITIS
The most common mimic of cellulitis is stasis dermatitis (Figure 1).2 Patients can present with ill-defined, bilateral, pitting edema of the lower extremities, typically with erythema, hyperpigmentation, serous drainage, and superficial desquamation.3,6,7
The inciting factor is chronic venous insufficiency, leading to interstitial edema, extravasation of red blood cells, and decreased tissue oxygenation. This process causes micro-vascular changes and microthrombi that up-regulate transforming growth factor beta and fibroblastic growth factor.7 If the process is allowed to continue, stasis dermatitis may progress to lipodermatosclerosis.
Tip: Stasis dermatitis is generally bilateral, the process will have been ongoing for years, there is often pitting edema, and the legs should be nontender.
LIPODERMATOSCLEROSIS
Lipodermatosclerosis is a sclerosing panniculitis classically described as an “inverted champagne bottle” or “inverted bowling pin” appearance of the leg, ie, the diameter of the leg is sharply narrowed directly below the calf (Figure 2).
There is an acute and a chronic phase. The acute phase is characterized by inflammation and erythema, and the chronic phase is characterized by fibrosis.8 The acute phase presents with severe lower-extremity pain above the medial malleolus, erythema, edema, and warmth; there is no sharp demarcation between affected and unaffected skin.9,10 This phase can be difficult to distinguish from cellulitis, so the history plays a key role. Known venous insufficiency, cutaneous changes of stasis dermatitis, and the absence of systemic symptoms all point to lipodermatosclerosis.
The chronic phase is characterized by unilateral or bilateral, indurated, sclerotic plaques with a “bound-down” appearance (ie, they appear as if tethered—or bound—to the subcutaneous tissue) affecting the skin from below the knee to the ankle; there is a sharp demarcation between affected and unaffected skin.9–11 The skin is often bronze or brown secondary to hemosiderin deposits. There can be prominent varicosities and scattered ulcerations depending on the course of the disease.
This condition is thought to be the result of long-standing chronic venous insufficiency.7,8,9,11 It is proposed that venous incompetence leads to extravasation of interstitial fluid and red blood cells, decreased diffusion of oxygen to the tissues, and eventual tissue and endothelial damage. As the endothelium is damaged, microthrombi formation and infarction ensue, stimulating fibroblasts to form granulation tissue.
Tip: The history helps to distinguish acute lipodermatosclerosis from cellulitis. Chroniclipodermatoslcerosis will have been ongoing for years, the legs should be nontender, the skin will be bound-down, and the diameter of the leg will sharply decrease from knee to ankle.
CONTACT DERMATITIS
Allergic and irritant forms of contact dermatitis are often mistaken for cellulitis. Irritant contact dermatitis (Figure 3) presents with erythematous patches and plaques with well-defined borders, often in a geometric distribution where the skin was exposed to an irritant.12 Allergic contact dermatitis is a delayed hypersensitivity dermatitis that can be secondary to something ingested, applied to the skin, or airborne (Figure 4). It presents as erythematous macules, papules, and plaques that may have serous drainage or vesiculation. Lesions of allergic contact dermatitis are usually confined to the site of contact with the allergen, but they can infrequently be found at distant sites, in which case it is considered systemic contact dermatitis.3,5 Depending on the severity of the allergy, patients may complain of intense pain and pruritus.3
Additionally, chronic, nonhealing leg ulcers may have a confounding allergic contact dermatitis.7 Although patients may believe they are helping the ulcer heal by applying topical antibiotics or other lubricants, they may in fact be impeding the healing process. Always inquire as to what the patient is applying if he or she has leg ulceration with surrounding edema and erythema that has not resolved with conventional treatments.13,14
Tip: The key to distinguishing contact dermatitis from cellulitis is the history. For example, ask about recent changes in medications, soaps, and laundry detergents, new hobbies, or recent surgeries. The involved site is often confined to the area where the allergen contacted the skin, except in cases of exposure to an airborne allergen.
LYMPHEDEMA
Lymphedema is characterized by localized edema of an affected extremity, with induration, erythema, and secondary cutaneous changes such as hyperkeratosis, dyspigmentation, and wart-like architecture (Figure 5).
Primary lymphedema appears in the setting of congenital abnormalities, whereas secondary lymphedema results from an interruption of a previously functioning lymphatic system (eg, after radical mastectomy).
Patients often present with unilateral nonpitting edema and erythema in the absence of systemic symptoms.12 Many patients presenting with lower-extremity lymphedema are overweight or obese, as the weight they carry causes obstruction of the inguinal lymphatics.6
The pathophysiology is not clearly delineated but is thought to be a consequence of decreased oxygenation of tissue secondary to extravasated lymph. As the oxygen is compromised, macrophages and fibroblasts are recruited, resulting in fibrosis.6
Patients with lymphedema are more susceptible to superficial and deep skin infections, as the natural defense system in the epidermis and papillary dermis is compromised by impaired lymphatic drainage.15
To differentiate uncomplicated lymphedema from a secondary cutaneous infection, the clinician should take into account the presence or absence of warmth, pain, increased erythema, and systemic symptoms (Figure 6).
Tip: Primary lymphedema will most likely present in childhood with no inciting factors and will require a full workup. Obtaining a history should make secondary lymphedema a relatively straightforward diagnosis: Has the patient undergone lymph node dissection? Has the patient had an injury in the affected leg? Lymphedema is overwhelmingly unilateral and nonpitting, and is often seen in overweight people (if no precipitating factor is present).
EOSINOPHILIC CELLULITIS
Eosinophilic cellulitis, or Wells syndrome, was first described in 1971 as a granulomatous dermatitis.16 It is a recurrent hypersensitivity reaction to a drug, to a vaccine, or to an insect bite, or to a viral or fungal infection that presents on the extremities as localized erythema, edema, and induration with sharp borders and a green or gray hue (Figure 7).17–19 The lesions commonly progress to firm, indurated plaques that resemble morphea. The plaques may take weeks or years to resolve, but they do so without scarring.12,17,20,21
As patients tend to have recurrent bouts of eosinophilic cellulitis, they may have lesions in different stages of healing. Patients tend to report itching and burning that precedes the onset of plaques.22 The complete blood count typically shows a transient hypereosinophilia.12,16,17,23–25
Tip: This diagnosis often requires biopsy for confirmation, but helpful clues are a history of recurrent episodes, the color of the lesions, and peripheral eosinophilia.
PAPULAR URTICARIA
Papular urticaria is a dermal hypersensitivity reaction to an insect bite, most commonly from a flea or mosquito.26 Patients are often children, as their immune system may be hypersensitive. But children often develop tolerance before puberty.27
The presentation may vary, from numerous urticarial papules near the site of a bite, to generalized, large, indurated, erythematous plaques reminiscent of cellulitis (Figure 8).5,26 The lesions usually develop within hours of a bite and persist for an average of 1 to 2 weeks.28 The areas typically affected are the head and neck or the upper or lower extremities; the palms, soles, and trunk are usually spared.27
Patients most often complain of intense itching.12 The pathogenesis is proposed to be mediated by the immune complex, and tissue biopsy study shows increased eosinophils. The eosinophils stimulate mast cells, causing release of histamine, leading to increased vascular permeability, edema, and erythema.28,29
Tip: Biopsy may be necessary to confirm the diagnosis, though often the history may be sufficient. The patient may or may not recall a bite, so probe into recent activities such as outdoor sports or contact with a new pet. The papules and plaques are generally very pruritic but not painful.
DERMATOLOGY CONSULT
If the clinical presentation and history do not correlate, or if the skin condition has been treated with antibiotics yet has failed to respond, the possibility of other cutaneous dermatoses should be entertained. A dermatology consult can help determine the diagnosis, the need for further evaluation, and the best treatment course.
- Hepburn MJ, Dooley DP, Ellis MW. Alternative diagnoses that often mimic cellulitis. Am Fam Physician 2003; 67:2471.
- David CV, Chira S, Eells SJ, et al. Diagnostic accuracy in patients admitted to hospitals with cellulitis. Dermatol Online J 2011; 17:1.
- Bailey E, Kroshinsky D. Cellulitis: diagnosis and management. Dermatol Ther 2011; 24:229–239.
- Stevens DL, Bisno AL, Chambers HF, et al; Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis 2005; 41:1373–1406.
- Lio PA. The many faces of cellulitis. Arch Dis Child Educ Pract Ed 2009; 94:50–54.
- Yosipovitch G, DeVore A, Dawn A. Obesity and the skin: skin physiology and skin manifestations of obesity. J Am Acad Dermatol 2007; 56:901–916.
- Farage MA, Miller KW, Berardesca E, Maibach HI. Clinical implications of aging skin: cutaneous disorders in the elderly. Am J Clin Dermatol 2009; 10:73–86.
- Kirsner RS, Pardes JB, Eaglstein WH, Falanga V. The clinical spectrum of lipodermatosclerosis. J Am Acad Dermatol 1993; 28:623–627.
- Miteva M, Romanelli P, Kirsner RS. Lipodermatosclerosis. Dermatol Ther 2010; 23:375–388.
- Barron GS, Jacob SE, Kirsner RS. Dermatologic complications of chronic venous disease: medical management and beyond. Ann Vasc Surg 2007; 21:652–662.
- Bruce AJ, Bennett DD, Lohse CM, Rooke TW, Davis MD. Lipodermatosclerosis: review of cases evaluated at Mayo Clinic. J Am Acad Dermatol 2002; 46:187–192.
- Falagas ME, Vergidis PI. Narrative review: diseases that masquerade as infectious cellulitis. Ann Intern Med 2005; 142:47–55.
- Wilson CL, Cameron J, Powell SM, Cherry G, Ryan TJ. High incidence of contact dermatitis in leg-ulcer patients—implications for management. Clin Exp Dermatol 1991; 16:250–253.
- Wolf R. The lanolin paradox. Dermatology 1996; 192:198–202.
- Keeley VL. Lymphoedema and cellulitis: chicken or egg? Br J Dermatol 2008; 158:1175–1176.
- Wells GC. Recurrent granulomatous dermatitis with eosinophilia. Trans St Johns Hosp Dermatol Soc 1971; 57:46–56.
- Ferreli C, Pinna AL, Atzori L, Aste N. Eosinophilic cellulitis (Well’s syndrome): a new case description. J Eur Acad Dermatol Venereol 1999; 13:41–45.
- Ladoyanni E, Vlachou C, Thushara R, Snead D. A patient with Wells’ syndrome. Clin Exp Dermatol 2010; 35:e3–e4.
- Moon HS, Park K, Lee JH, Son SJ. Eosinophilic cellulitis in an infant. Int J Dermatol 2010; 49:592–593.
- Walker P, Long D, James C, Marshman G. Exaggerated insect bite reaction exacerbated by a pyogenic infection in a patient with chronic lymphocytic leukaemia. Australas J Dermatol 2007; 48:165–169.
- Laliwala NM, Kulshrestha R, Singh R, Balasubramaniam P. A case of eosinophilic cellulitis of the hand mimicking bacterial cellulitis. J Hand Surg Eur Vol 2009; 34:410–411.
- Chung CL, Cusack CA. Wells syndrome: an enigmatic and therapeutically challenging disease. J Drugs Dermatol 2006; 5:908–911.
- Melski JW. Wells’ syndrome, insect bites, and eosinophils. Dermatol Clin 1990; 8:287–293.
- Spigel GT, Winkelmann RK. Wells’ syndrome. Recurrent granulomatous dermatitis with eosinophilia. Arch Dermatol 1979; 115:611–613.
- Clark DP, Anderson PC. Eosinophilic cellulitis caused by arthropod bites. Int J Dermatol 1988; 27:411–412.
- Howard R, Frieden IJ. Papular urticaria in children. Pediatr Dermatol 1996; 13:246–249.
- Hernandez RG, Cohen BA. Insect bite-induced hypersensitivity and the SCRATCH principles: a new approach to papular urticaria. Pediatrics 2006; 118:e189–e196.
- Heng MC, Kloss SG, Haberfelde GC. Pathogenesis of papular urticaria. J Am Acad Dermatol 1984; 10:1030–1034.
- Kossard S, Hamann I, Wilkinson B. Defining urticarial dermatitis: a subset of dermal hypersensitivity reaction pattern. Arch Dermatol 2006; 142:29–34.
- Hepburn MJ, Dooley DP, Ellis MW. Alternative diagnoses that often mimic cellulitis. Am Fam Physician 2003; 67:2471.
- David CV, Chira S, Eells SJ, et al. Diagnostic accuracy in patients admitted to hospitals with cellulitis. Dermatol Online J 2011; 17:1.
- Bailey E, Kroshinsky D. Cellulitis: diagnosis and management. Dermatol Ther 2011; 24:229–239.
- Stevens DL, Bisno AL, Chambers HF, et al; Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis 2005; 41:1373–1406.
- Lio PA. The many faces of cellulitis. Arch Dis Child Educ Pract Ed 2009; 94:50–54.
- Yosipovitch G, DeVore A, Dawn A. Obesity and the skin: skin physiology and skin manifestations of obesity. J Am Acad Dermatol 2007; 56:901–916.
- Farage MA, Miller KW, Berardesca E, Maibach HI. Clinical implications of aging skin: cutaneous disorders in the elderly. Am J Clin Dermatol 2009; 10:73–86.
- Kirsner RS, Pardes JB, Eaglstein WH, Falanga V. The clinical spectrum of lipodermatosclerosis. J Am Acad Dermatol 1993; 28:623–627.
- Miteva M, Romanelli P, Kirsner RS. Lipodermatosclerosis. Dermatol Ther 2010; 23:375–388.
- Barron GS, Jacob SE, Kirsner RS. Dermatologic complications of chronic venous disease: medical management and beyond. Ann Vasc Surg 2007; 21:652–662.
- Bruce AJ, Bennett DD, Lohse CM, Rooke TW, Davis MD. Lipodermatosclerosis: review of cases evaluated at Mayo Clinic. J Am Acad Dermatol 2002; 46:187–192.
- Falagas ME, Vergidis PI. Narrative review: diseases that masquerade as infectious cellulitis. Ann Intern Med 2005; 142:47–55.
- Wilson CL, Cameron J, Powell SM, Cherry G, Ryan TJ. High incidence of contact dermatitis in leg-ulcer patients—implications for management. Clin Exp Dermatol 1991; 16:250–253.
- Wolf R. The lanolin paradox. Dermatology 1996; 192:198–202.
- Keeley VL. Lymphoedema and cellulitis: chicken or egg? Br J Dermatol 2008; 158:1175–1176.
- Wells GC. Recurrent granulomatous dermatitis with eosinophilia. Trans St Johns Hosp Dermatol Soc 1971; 57:46–56.
- Ferreli C, Pinna AL, Atzori L, Aste N. Eosinophilic cellulitis (Well’s syndrome): a new case description. J Eur Acad Dermatol Venereol 1999; 13:41–45.
- Ladoyanni E, Vlachou C, Thushara R, Snead D. A patient with Wells’ syndrome. Clin Exp Dermatol 2010; 35:e3–e4.
- Moon HS, Park K, Lee JH, Son SJ. Eosinophilic cellulitis in an infant. Int J Dermatol 2010; 49:592–593.
- Walker P, Long D, James C, Marshman G. Exaggerated insect bite reaction exacerbated by a pyogenic infection in a patient with chronic lymphocytic leukaemia. Australas J Dermatol 2007; 48:165–169.
- Laliwala NM, Kulshrestha R, Singh R, Balasubramaniam P. A case of eosinophilic cellulitis of the hand mimicking bacterial cellulitis. J Hand Surg Eur Vol 2009; 34:410–411.
- Chung CL, Cusack CA. Wells syndrome: an enigmatic and therapeutically challenging disease. J Drugs Dermatol 2006; 5:908–911.
- Melski JW. Wells’ syndrome, insect bites, and eosinophils. Dermatol Clin 1990; 8:287–293.
- Spigel GT, Winkelmann RK. Wells’ syndrome. Recurrent granulomatous dermatitis with eosinophilia. Arch Dermatol 1979; 115:611–613.
- Clark DP, Anderson PC. Eosinophilic cellulitis caused by arthropod bites. Int J Dermatol 1988; 27:411–412.
- Howard R, Frieden IJ. Papular urticaria in children. Pediatr Dermatol 1996; 13:246–249.
- Hernandez RG, Cohen BA. Insect bite-induced hypersensitivity and the SCRATCH principles: a new approach to papular urticaria. Pediatrics 2006; 118:e189–e196.
- Heng MC, Kloss SG, Haberfelde GC. Pathogenesis of papular urticaria. J Am Acad Dermatol 1984; 10:1030–1034.
- Kossard S, Hamann I, Wilkinson B. Defining urticarial dermatitis: a subset of dermal hypersensitivity reaction pattern. Arch Dermatol 2006; 142:29–34.
KEY POINTS
- Cellulitis is rarely bilateral.
- Patients with cellulitis often have systemic symptoms, such as fever and leukocytosis.
- A chronic course points to a diagnosis other than cellulitis.
- Plaques with a “bound-down” appearance or dark pigmentation point to a chronic disease rather than cellulitis.
- Stasis dermatitis is the most common mimic of cellulitis.
An argument for reviving the disappearing skill of cardiac auscultation
Bedside clinical diagnosis is an increasingly underappreciated art and skill. For example, contemporary medical students, residents, fellows, and cardiologists have been shown to lack competency in cardiac auscultation,1,2 despite warnings from older physicians trained in an era when the physical examination was valued.3,4
However, echocardiography has given physicians the ability to visually evaluate cardiac function noninvasively and quickly. With advanced technology, does this modern decline in auscultatory skills matter? And specifically, can inexpert cardiac auscultation lead to the inadequate evaluation of valvular heart disease and subsequently to an incorrect recommendation for surgery?
Although the ill consequences for patient care would be difficult to prove, we strongly believe, on the basis of our experiences in a busy cardiovascular surgery clinic in a tertiary care center, that the answer to both questions is yes.
Here, we present three recent scenarios from the clinic of a senior cardiac surgeon who regards the skillful use of his stethoscope as being as important as the echocardiogram. These scenarios highlight how the clinical examination can complement echocardiography in the evaluation of valvular heart disease and how it can affect important management decisions.
SCENARIO 1: SEVERE AORTIC INSUFFICIENCY?
A 53-year-old woman with Turner syndrome (gonadal dysgenesis) suffered an acute ascending aortic dissection requiring resuspension of the aortic valve and replacement of the ascending aorta. Her postoperative course was complicated by pneumonia, respiratory failure, and prolonged mechanical ventilation requiring tracheostomy.
Three months after she completed her convalescence at a skilled nursing facility, she presented to her cardiologist with progressive shortness of breath that severely limited her activity. Echocardiography showed moderately severe aortic insufficiency, and she was referred for aortic valve replacement.
At the cardiac surgery clinic, she reported a further decline in her functional status, with dyspnea during minimal exertion. On physical examination, however, there was no evidence of significant aortic incompetence, ie, no widened pulse pressure, left ventricular heave, or diastolic murmur. A cardiologist specializing in echocardiography reviewed the echocardiogram from the referring physician and found that the appearance was more consistent with mild to moderate aortic insufficiency.
Because her profound symptoms were out of proportion with the degree of aortic insufficiency that was observed, further workup including pulmonary function testing was pursued to find another cause; she was subsequently found to have significant tracheal stenosis, likely related to her tracheostomy. Surgery to remove scar tissue resulted in marked improvement of her symptoms.
SCENARIO 2: SEVERE MITRAL REGURGITATION?
A 67-year-old man who had undergone homograft aortic valve replacement 13 years ago underwent routine echocardiography at another hospital. The test showed a large regurgitant jet and backward flow in the pulmonary veins, indicating moderate to severe mitral regurgitation. Also noted was a mildly decreased ejection fraction of 45%. Because of these findings, he was referred for consideration of mitral valve surgery.
At presentation, he had essentially no symptoms and had a very active lifestyle that included regular biking and running. A physical examination that included auscultation in the left lateral decubitus position noted only a soft systolic ejection murmur at the left upper sternal border.
In view of these findings, repeat echocardiography was ordered and revealed mild mitral regurgitation with normal left atrial and ventricular dimensions, as well as normal left ventricular systolic function. These findings were markedly different from those obtained at the other hospital. The murmur was thought to likely represent flow across the base of the homograft valve. These results confirmed our clinical suspicion that there was no indication for mitral valve surgery.
SCENARIO 3: NORMAL HEART VALVES?
A 62-year-old woman presented to her local cardiologist with a 3-month history of worsening shortness of breath and fatigue. She had an abnormal nuclear stress test that led to left heart catheterization, which revealed a 60% to 70% stenosis of the left main coronary artery. She was promptly referred for coronary artery bypass grafting.
The report from her referring cardiologist indicated normal findings on her cardiac physical examination. However, when we examined her, we noted an accentuation of the first heart sound, with an opening snap and a low-pitched mid-diastolic rumble heard best at the apex, in addition to a systolic ejection murmur, diminished second heart sound, and late-peaking carotid upstroke. Echocardiography revealed significant mitral stenosis, with a mitral valve area of 1.05 cm2, as well as moderately severe aortic stenosis. These findings were consistent with rheumatic heart disease, and upon questioning, the patient reported that she had received that diagnosis in her 30s while teaching in China.
In light of the findings on physical examination and imaging, the patient underwent mitral and aortic valve replacement in addition to the coronary bypass procedure for which she had originally been referred.
A SELF-FULFILLING PROPHECY
These vignettes illustrate the importance of a detailed physical examination—particularly cardiac auscultation—in the clinical evaluation of structural heart disease.
In the first two, there were significant inconsistencies between the auscultatory and echocardiographic findings, and information obtained from careful cardiac auscultation ultimately directed further testing and led to the correct diagnosis. The third scenario is particularly worrisome in our opinion, as it not only represents a lack of auscultatory skills, but probably a failure to listen at all. Further, in this patient’s case, failure to diagnose significant valvular disease would likely have meant a need for reoperation at a later date.
Although this is clearly unacceptable, in our experience it is not uncommon. As the skill of auscultation is lost, less and less information is obtained, and the abandonment of auscultation becomes a self-fulfilling prophecy.
AUSCULTATION SAVES MONEY
While these cases show the diagnostic capability of cardiac auscultation, they also show that auscultation has another virtue: it can save money. With skyrocketing health care costs, cost-effectiveness of care is increasingly important. In fact, the modern physician is called to the commitment of the just distribution of finite resources as a principle of medical professionalism.5 Physicians skilled in cardiac auscultation will be better able to distinguish patients who do not have significant disease and, therefore, will provide more appropriate care by decreasing the mindless use of expensive imaging.
Physicians, especially cardiologists, who are not worried about the loss of auscultatory skills are likely those who do not know how to properly auscultate the heart and, therefore, do not appreciate the vital information it may provide. Dependent on echocardiography, they fail to recognize its numerous limitations, particularly in a real-world setting where core echocardiography laboratories are not commonplace. Furthermore, the use of sophisticated hand-held echocardiography machines, often by inexperienced and untrained operators, is on the rise.
Echocardiography: Still an imperfect science
Many variables contribute to the echocardiographic assessment of severity in valvular heart disease. These include jet size and character, which may be affected by inappropriate gain settings, Nyquist limits, wall filters, ultrasound beam angulations, and regurgitant orifice area calculations. Other factors potentially affecting echocardiographic reproducibility include variability between machines, sonographers, and interpreters, as well as differences in medications, loading conditions, and blood pressure.6,7 This potential for variability in echocardiography underlines the importance of auscultation, particularly at tertiary referral centers, where many patients are evaluated and treated on the basis of testing at other facilities. Although echocardiography has rightfully become the cornerstone of diagnosing valvular heart disease, we may often forget that it is an imperfect science.
Well-honed cardiac auscultatory skills are still an essential part of medical practice and are an indispensable complement to echocardiography. For this reason, medical schools and training programs in cardiology should encourage a renaissance in the art of cardiac auscultation and bedside clinical diagnosis, which we believe will ultimately improve patient care. Excellent resources are available for teaching auscultation, including Web sites and audiovisual software. And there may even be a wise old doctor still around for advice.
Acknowledgment: We would like to thank Jane Owenby for her assistance in the preparation of this manuscript.
- Mangione S. Cardiac auscultatory skills of physicians-in-training: a comparison of three English-speaking countries. Am J Med 2001; 110:210–216.
- Vukanovic-Criley JM, Criley S, Warde CM, et al. Competency in cardiac examination skills in medical students, trainees, physicians, and faculty: a multicenter study. Arch Intern Med 2006; 166:610–616.
- Fred HL. Hyposkillia: deficiency of clinical skills. Tex Heart Inst J 2005; 32:255–257.
- Grais IM. Bedside skills: a 50-year personal retrospective. Tex Heart Inst J 2010; 37:629–632.
- ABIM Foundation. Medical professionalism in the new millennium: a physician charter. Ann Intern Med 2002; 136:243–246.
- Gottdiener JS, Panza JA, St John Sutton M, Bannon P, Kushner H, Weissman NJ. Testing the test: the reliability of echocardiography in the sequential assessment of valvular regurgitation. Am Heart J 2002; 144:115–121.
- Fan PH, Anayiotos A, Nanda NC, Yoganathan AP, Cape EG. Intramachine and intermachine variability in transesophageal color Doppler images of pulsatile jets. In vitro studies. Circulation 1994; 89:2141–2149.
Bedside clinical diagnosis is an increasingly underappreciated art and skill. For example, contemporary medical students, residents, fellows, and cardiologists have been shown to lack competency in cardiac auscultation,1,2 despite warnings from older physicians trained in an era when the physical examination was valued.3,4
However, echocardiography has given physicians the ability to visually evaluate cardiac function noninvasively and quickly. With advanced technology, does this modern decline in auscultatory skills matter? And specifically, can inexpert cardiac auscultation lead to the inadequate evaluation of valvular heart disease and subsequently to an incorrect recommendation for surgery?
Although the ill consequences for patient care would be difficult to prove, we strongly believe, on the basis of our experiences in a busy cardiovascular surgery clinic in a tertiary care center, that the answer to both questions is yes.
Here, we present three recent scenarios from the clinic of a senior cardiac surgeon who regards the skillful use of his stethoscope as being as important as the echocardiogram. These scenarios highlight how the clinical examination can complement echocardiography in the evaluation of valvular heart disease and how it can affect important management decisions.
SCENARIO 1: SEVERE AORTIC INSUFFICIENCY?
A 53-year-old woman with Turner syndrome (gonadal dysgenesis) suffered an acute ascending aortic dissection requiring resuspension of the aortic valve and replacement of the ascending aorta. Her postoperative course was complicated by pneumonia, respiratory failure, and prolonged mechanical ventilation requiring tracheostomy.
Three months after she completed her convalescence at a skilled nursing facility, she presented to her cardiologist with progressive shortness of breath that severely limited her activity. Echocardiography showed moderately severe aortic insufficiency, and she was referred for aortic valve replacement.
At the cardiac surgery clinic, she reported a further decline in her functional status, with dyspnea during minimal exertion. On physical examination, however, there was no evidence of significant aortic incompetence, ie, no widened pulse pressure, left ventricular heave, or diastolic murmur. A cardiologist specializing in echocardiography reviewed the echocardiogram from the referring physician and found that the appearance was more consistent with mild to moderate aortic insufficiency.
Because her profound symptoms were out of proportion with the degree of aortic insufficiency that was observed, further workup including pulmonary function testing was pursued to find another cause; she was subsequently found to have significant tracheal stenosis, likely related to her tracheostomy. Surgery to remove scar tissue resulted in marked improvement of her symptoms.
SCENARIO 2: SEVERE MITRAL REGURGITATION?
A 67-year-old man who had undergone homograft aortic valve replacement 13 years ago underwent routine echocardiography at another hospital. The test showed a large regurgitant jet and backward flow in the pulmonary veins, indicating moderate to severe mitral regurgitation. Also noted was a mildly decreased ejection fraction of 45%. Because of these findings, he was referred for consideration of mitral valve surgery.
At presentation, he had essentially no symptoms and had a very active lifestyle that included regular biking and running. A physical examination that included auscultation in the left lateral decubitus position noted only a soft systolic ejection murmur at the left upper sternal border.
In view of these findings, repeat echocardiography was ordered and revealed mild mitral regurgitation with normal left atrial and ventricular dimensions, as well as normal left ventricular systolic function. These findings were markedly different from those obtained at the other hospital. The murmur was thought to likely represent flow across the base of the homograft valve. These results confirmed our clinical suspicion that there was no indication for mitral valve surgery.
SCENARIO 3: NORMAL HEART VALVES?
A 62-year-old woman presented to her local cardiologist with a 3-month history of worsening shortness of breath and fatigue. She had an abnormal nuclear stress test that led to left heart catheterization, which revealed a 60% to 70% stenosis of the left main coronary artery. She was promptly referred for coronary artery bypass grafting.
The report from her referring cardiologist indicated normal findings on her cardiac physical examination. However, when we examined her, we noted an accentuation of the first heart sound, with an opening snap and a low-pitched mid-diastolic rumble heard best at the apex, in addition to a systolic ejection murmur, diminished second heart sound, and late-peaking carotid upstroke. Echocardiography revealed significant mitral stenosis, with a mitral valve area of 1.05 cm2, as well as moderately severe aortic stenosis. These findings were consistent with rheumatic heart disease, and upon questioning, the patient reported that she had received that diagnosis in her 30s while teaching in China.
In light of the findings on physical examination and imaging, the patient underwent mitral and aortic valve replacement in addition to the coronary bypass procedure for which she had originally been referred.
A SELF-FULFILLING PROPHECY
These vignettes illustrate the importance of a detailed physical examination—particularly cardiac auscultation—in the clinical evaluation of structural heart disease.
In the first two, there were significant inconsistencies between the auscultatory and echocardiographic findings, and information obtained from careful cardiac auscultation ultimately directed further testing and led to the correct diagnosis. The third scenario is particularly worrisome in our opinion, as it not only represents a lack of auscultatory skills, but probably a failure to listen at all. Further, in this patient’s case, failure to diagnose significant valvular disease would likely have meant a need for reoperation at a later date.
Although this is clearly unacceptable, in our experience it is not uncommon. As the skill of auscultation is lost, less and less information is obtained, and the abandonment of auscultation becomes a self-fulfilling prophecy.
AUSCULTATION SAVES MONEY
While these cases show the diagnostic capability of cardiac auscultation, they also show that auscultation has another virtue: it can save money. With skyrocketing health care costs, cost-effectiveness of care is increasingly important. In fact, the modern physician is called to the commitment of the just distribution of finite resources as a principle of medical professionalism.5 Physicians skilled in cardiac auscultation will be better able to distinguish patients who do not have significant disease and, therefore, will provide more appropriate care by decreasing the mindless use of expensive imaging.
Physicians, especially cardiologists, who are not worried about the loss of auscultatory skills are likely those who do not know how to properly auscultate the heart and, therefore, do not appreciate the vital information it may provide. Dependent on echocardiography, they fail to recognize its numerous limitations, particularly in a real-world setting where core echocardiography laboratories are not commonplace. Furthermore, the use of sophisticated hand-held echocardiography machines, often by inexperienced and untrained operators, is on the rise.
Echocardiography: Still an imperfect science
Many variables contribute to the echocardiographic assessment of severity in valvular heart disease. These include jet size and character, which may be affected by inappropriate gain settings, Nyquist limits, wall filters, ultrasound beam angulations, and regurgitant orifice area calculations. Other factors potentially affecting echocardiographic reproducibility include variability between machines, sonographers, and interpreters, as well as differences in medications, loading conditions, and blood pressure.6,7 This potential for variability in echocardiography underlines the importance of auscultation, particularly at tertiary referral centers, where many patients are evaluated and treated on the basis of testing at other facilities. Although echocardiography has rightfully become the cornerstone of diagnosing valvular heart disease, we may often forget that it is an imperfect science.
Well-honed cardiac auscultatory skills are still an essential part of medical practice and are an indispensable complement to echocardiography. For this reason, medical schools and training programs in cardiology should encourage a renaissance in the art of cardiac auscultation and bedside clinical diagnosis, which we believe will ultimately improve patient care. Excellent resources are available for teaching auscultation, including Web sites and audiovisual software. And there may even be a wise old doctor still around for advice.
Acknowledgment: We would like to thank Jane Owenby for her assistance in the preparation of this manuscript.
Bedside clinical diagnosis is an increasingly underappreciated art and skill. For example, contemporary medical students, residents, fellows, and cardiologists have been shown to lack competency in cardiac auscultation,1,2 despite warnings from older physicians trained in an era when the physical examination was valued.3,4
However, echocardiography has given physicians the ability to visually evaluate cardiac function noninvasively and quickly. With advanced technology, does this modern decline in auscultatory skills matter? And specifically, can inexpert cardiac auscultation lead to the inadequate evaluation of valvular heart disease and subsequently to an incorrect recommendation for surgery?
Although the ill consequences for patient care would be difficult to prove, we strongly believe, on the basis of our experiences in a busy cardiovascular surgery clinic in a tertiary care center, that the answer to both questions is yes.
Here, we present three recent scenarios from the clinic of a senior cardiac surgeon who regards the skillful use of his stethoscope as being as important as the echocardiogram. These scenarios highlight how the clinical examination can complement echocardiography in the evaluation of valvular heart disease and how it can affect important management decisions.
SCENARIO 1: SEVERE AORTIC INSUFFICIENCY?
A 53-year-old woman with Turner syndrome (gonadal dysgenesis) suffered an acute ascending aortic dissection requiring resuspension of the aortic valve and replacement of the ascending aorta. Her postoperative course was complicated by pneumonia, respiratory failure, and prolonged mechanical ventilation requiring tracheostomy.
Three months after she completed her convalescence at a skilled nursing facility, she presented to her cardiologist with progressive shortness of breath that severely limited her activity. Echocardiography showed moderately severe aortic insufficiency, and she was referred for aortic valve replacement.
At the cardiac surgery clinic, she reported a further decline in her functional status, with dyspnea during minimal exertion. On physical examination, however, there was no evidence of significant aortic incompetence, ie, no widened pulse pressure, left ventricular heave, or diastolic murmur. A cardiologist specializing in echocardiography reviewed the echocardiogram from the referring physician and found that the appearance was more consistent with mild to moderate aortic insufficiency.
Because her profound symptoms were out of proportion with the degree of aortic insufficiency that was observed, further workup including pulmonary function testing was pursued to find another cause; she was subsequently found to have significant tracheal stenosis, likely related to her tracheostomy. Surgery to remove scar tissue resulted in marked improvement of her symptoms.
SCENARIO 2: SEVERE MITRAL REGURGITATION?
A 67-year-old man who had undergone homograft aortic valve replacement 13 years ago underwent routine echocardiography at another hospital. The test showed a large regurgitant jet and backward flow in the pulmonary veins, indicating moderate to severe mitral regurgitation. Also noted was a mildly decreased ejection fraction of 45%. Because of these findings, he was referred for consideration of mitral valve surgery.
At presentation, he had essentially no symptoms and had a very active lifestyle that included regular biking and running. A physical examination that included auscultation in the left lateral decubitus position noted only a soft systolic ejection murmur at the left upper sternal border.
In view of these findings, repeat echocardiography was ordered and revealed mild mitral regurgitation with normal left atrial and ventricular dimensions, as well as normal left ventricular systolic function. These findings were markedly different from those obtained at the other hospital. The murmur was thought to likely represent flow across the base of the homograft valve. These results confirmed our clinical suspicion that there was no indication for mitral valve surgery.
SCENARIO 3: NORMAL HEART VALVES?
A 62-year-old woman presented to her local cardiologist with a 3-month history of worsening shortness of breath and fatigue. She had an abnormal nuclear stress test that led to left heart catheterization, which revealed a 60% to 70% stenosis of the left main coronary artery. She was promptly referred for coronary artery bypass grafting.
The report from her referring cardiologist indicated normal findings on her cardiac physical examination. However, when we examined her, we noted an accentuation of the first heart sound, with an opening snap and a low-pitched mid-diastolic rumble heard best at the apex, in addition to a systolic ejection murmur, diminished second heart sound, and late-peaking carotid upstroke. Echocardiography revealed significant mitral stenosis, with a mitral valve area of 1.05 cm2, as well as moderately severe aortic stenosis. These findings were consistent with rheumatic heart disease, and upon questioning, the patient reported that she had received that diagnosis in her 30s while teaching in China.
In light of the findings on physical examination and imaging, the patient underwent mitral and aortic valve replacement in addition to the coronary bypass procedure for which she had originally been referred.
A SELF-FULFILLING PROPHECY
These vignettes illustrate the importance of a detailed physical examination—particularly cardiac auscultation—in the clinical evaluation of structural heart disease.
In the first two, there were significant inconsistencies between the auscultatory and echocardiographic findings, and information obtained from careful cardiac auscultation ultimately directed further testing and led to the correct diagnosis. The third scenario is particularly worrisome in our opinion, as it not only represents a lack of auscultatory skills, but probably a failure to listen at all. Further, in this patient’s case, failure to diagnose significant valvular disease would likely have meant a need for reoperation at a later date.
Although this is clearly unacceptable, in our experience it is not uncommon. As the skill of auscultation is lost, less and less information is obtained, and the abandonment of auscultation becomes a self-fulfilling prophecy.
AUSCULTATION SAVES MONEY
While these cases show the diagnostic capability of cardiac auscultation, they also show that auscultation has another virtue: it can save money. With skyrocketing health care costs, cost-effectiveness of care is increasingly important. In fact, the modern physician is called to the commitment of the just distribution of finite resources as a principle of medical professionalism.5 Physicians skilled in cardiac auscultation will be better able to distinguish patients who do not have significant disease and, therefore, will provide more appropriate care by decreasing the mindless use of expensive imaging.
Physicians, especially cardiologists, who are not worried about the loss of auscultatory skills are likely those who do not know how to properly auscultate the heart and, therefore, do not appreciate the vital information it may provide. Dependent on echocardiography, they fail to recognize its numerous limitations, particularly in a real-world setting where core echocardiography laboratories are not commonplace. Furthermore, the use of sophisticated hand-held echocardiography machines, often by inexperienced and untrained operators, is on the rise.
Echocardiography: Still an imperfect science
Many variables contribute to the echocardiographic assessment of severity in valvular heart disease. These include jet size and character, which may be affected by inappropriate gain settings, Nyquist limits, wall filters, ultrasound beam angulations, and regurgitant orifice area calculations. Other factors potentially affecting echocardiographic reproducibility include variability between machines, sonographers, and interpreters, as well as differences in medications, loading conditions, and blood pressure.6,7 This potential for variability in echocardiography underlines the importance of auscultation, particularly at tertiary referral centers, where many patients are evaluated and treated on the basis of testing at other facilities. Although echocardiography has rightfully become the cornerstone of diagnosing valvular heart disease, we may often forget that it is an imperfect science.
Well-honed cardiac auscultatory skills are still an essential part of medical practice and are an indispensable complement to echocardiography. For this reason, medical schools and training programs in cardiology should encourage a renaissance in the art of cardiac auscultation and bedside clinical diagnosis, which we believe will ultimately improve patient care. Excellent resources are available for teaching auscultation, including Web sites and audiovisual software. And there may even be a wise old doctor still around for advice.
Acknowledgment: We would like to thank Jane Owenby for her assistance in the preparation of this manuscript.
- Mangione S. Cardiac auscultatory skills of physicians-in-training: a comparison of three English-speaking countries. Am J Med 2001; 110:210–216.
- Vukanovic-Criley JM, Criley S, Warde CM, et al. Competency in cardiac examination skills in medical students, trainees, physicians, and faculty: a multicenter study. Arch Intern Med 2006; 166:610–616.
- Fred HL. Hyposkillia: deficiency of clinical skills. Tex Heart Inst J 2005; 32:255–257.
- Grais IM. Bedside skills: a 50-year personal retrospective. Tex Heart Inst J 2010; 37:629–632.
- ABIM Foundation. Medical professionalism in the new millennium: a physician charter. Ann Intern Med 2002; 136:243–246.
- Gottdiener JS, Panza JA, St John Sutton M, Bannon P, Kushner H, Weissman NJ. Testing the test: the reliability of echocardiography in the sequential assessment of valvular regurgitation. Am Heart J 2002; 144:115–121.
- Fan PH, Anayiotos A, Nanda NC, Yoganathan AP, Cape EG. Intramachine and intermachine variability in transesophageal color Doppler images of pulsatile jets. In vitro studies. Circulation 1994; 89:2141–2149.
- Mangione S. Cardiac auscultatory skills of physicians-in-training: a comparison of three English-speaking countries. Am J Med 2001; 110:210–216.
- Vukanovic-Criley JM, Criley S, Warde CM, et al. Competency in cardiac examination skills in medical students, trainees, physicians, and faculty: a multicenter study. Arch Intern Med 2006; 166:610–616.
- Fred HL. Hyposkillia: deficiency of clinical skills. Tex Heart Inst J 2005; 32:255–257.
- Grais IM. Bedside skills: a 50-year personal retrospective. Tex Heart Inst J 2010; 37:629–632.
- ABIM Foundation. Medical professionalism in the new millennium: a physician charter. Ann Intern Med 2002; 136:243–246.
- Gottdiener JS, Panza JA, St John Sutton M, Bannon P, Kushner H, Weissman NJ. Testing the test: the reliability of echocardiography in the sequential assessment of valvular regurgitation. Am Heart J 2002; 144:115–121.
- Fan PH, Anayiotos A, Nanda NC, Yoganathan AP, Cape EG. Intramachine and intermachine variability in transesophageal color Doppler images of pulsatile jets. In vitro studies. Circulation 1994; 89:2141–2149.
The stethoscope as metaphor
“Those who advise that all stethoscopes should be ‘scrapped’ may be influenced by the fact that they do not know how to use their own.”
From Pulmonary Tuberculosis, 1921, by Sir James Kingston Fowler (1852–1934) of the Brompton Hospital, England
The commentary by Clark et al in this issue1 is a timely reminder of an important problem in modern medicine: the demise of the bedside. My only divergence from the authors is in their conclusion, since my Mediterranean pessimism leads me to believe that theirs is just a gallant attempt at rearguard action for a battle that, unfortunately, has long been lost.
More than half a century ago, Paul Wood warned us against the “danger of losing our clinical heritage and pinning too much faith in figures thrown out by machines,” thundering that “medicine must suffer if this tendency is not checked.”2 Well, that tendency was not checked, and medicine (and our wallets) have indeed suffered.
Still, technology is not the enemy. The misuse of technology is the problem.
Like Dr. Clark and his colleagues, I’ve seen many cases in which technology unguided by bedside skills took physicians down a path where tests begot tests and where, at the end, there was usually a surgeon, and often a lawyer. Sometimes even an undertaker. The deaths of Jonathan Larson (writer-composer of the musical Rent) and of his namesake, actor Jonathan (John) Ritter—who both succumbed to undiagnosed aortic dissection—make me wonder whether their pulses were ever checked.
Editorials have lamented the “hyposkillia” of our times,3 and the usual suspects have been already rounded up: our overreliance on tests, our ever-increasing fascination with the machine (what Erich Fromm called the necrophilia of our times),4 the loss of bedside teaching, and lastly, the lure of compensation. But one important player has so far gone unnoticed, despite being probably the major offender. In fact, it may even be responsible for the other disturbing trend in modern medicine: the loss of empathy.5
I’m referring to the disappearance of the humanities in both the undergraduate and the graduate curriculum. This is actually new. If we look, for example, at the great bedside diagnosticians of the past, we find that they were passionately interested in everything human. Most, if not all, were indeed humanists—lovers of the arts and literature, travelers and historians, poets and painters, curious of any field that could enrich the human spirit. Charcot, who single-handedly invented neurology, was not only a superb scientist, but also a talented artist who drew and painted (skills he considered fundamental for bedside observation) plus a bona-fide Beethoven fanatic who spent Thursday evenings on music, strictly forbidding any medical talk. Laënnec himself was a poet and musician who modeled his stethoscope after the flutes he made. And Charles Bell (of Bell palsy, phenomenon, and law) was a well-respected painter who soldiered with Wellington and left us incredible sketches of the Waterloo wounded and maimed. Even Osler, the pinnacle of 19th century humanistic medicine, believed so strongly in the value of a liberal education as to provide students with a list of 10 books (ranging from Plutarch and Montaigne to Marcus Aurelius and Shakespeare) to read for half an hour before going to sleep. Addressing the Classical Association just before his death, he lamented the “grievous damage” that had been done by regarding the humanities and science in any other light than complementary, while in reality they are “twin berries on one stem.”6
Until the 1870s, medicine was in fact a spin-off of the humanities. A solid humanistic education was deemed essential for admission to medical school. Then the German victory in the Franco-Prussian War shifted the axis from Paris to Berlin, and medicine went the German way. Never as touchy-feely as the French, and definitely more comfortable in the laboratory than at the bedside, the Germans produced giants like Koch, Virchow, and Roentgen, who gradually moved medicine away from the bedside and into the lab. In fact, medicine even adopted the uniform of the laboratory—the infamous white coat now banned by the British National Health system as a dirty carrier of bacteria.
Finding herself at a crossroads, America went the German way, mostly because of Flexner (himself the son of German immigrants), whose 1910 report totally changed the face of medical education. The “two cultures” were born—science was “in” and the humanities “out.”7
The result is what Lewis Thomas called the “baleful and malign” influence of the modern medical school on liberal-arts education.8 Michael Crichton put it even more bluntly. Explaining why he dropped out of medicine, he wrote, “My classmates [at Harvard] tended to think that literature, music, and art were irrelevant distractions. They held these “cultural” matters in the same intellectual contempt that a physicist holds astrology. Everything outside medicine was just a waste of time.”9
And since then, things have only worsened.10
Yet the link between humanities and the bedside remains crucial. I have had the privilege of meeting most of the clinicians who still contribute to physical diagnosis, and they are almost all humanists.
So why should the humanities nurture the bedside? For one, they may increase our tolerance of ambiguity, a trait sorely lacking in modern medicine. This makes sense, since decoding feeble sounds emanating from chests, palpating indistinct organs, and detecting bedside nuances are all painful reminders of the ambiguous in our craft, not to mention in life. And if unprepared by a humanistic education to deal with the uncertain, students may easily opt for the “certainties” of the laboratory or radiology suite.11 Once again, Osler comes to our rescue.
“A distressing feature in the life which you are about to enter” he told the graduating class of the University of Pennsylvania in 1889, “is the uncertainty which pertains not alone to our science and arts but to the very hopes and fears which make us men. In seeking absolute truth we aim at the unattainable, and must be content with finding broken portions.”12
The stethoscope is too closely bound with the doctor’s image not to be a metaphor for something larger. To me, it’s a metaphor for medicine as both an art and a science, wherein the humanities are—and of right ought to be—a fundamental part of the education. Hence, if we want to rekindle the bedside, we must rekindle the humanities. After all, this is what both Lewis Thomas8 and Sherwin Nuland13 have urged us to do. My hunch is that this would need to be done sooner rather than later, because if it is possible to make a scientist out of a humanist (it was done for centuries), it might be considerably harder to make a humanist out of a scientist. The experience of the past few decades seems to support this conclusion.
The alternative is a future full of tricorders and technicians, but sorely lacking in healers.
- Clark D, Ahmed MI, Dell’Italia LJ, Fan P, McGiffin DC. An argument for retrieving the disappearing skill of cardiac auscultation. Cleve Clin J Med 2012; 79:536–544.
- Wood PH. Diseases of the Heart and Circulation. London: Eyre and Spottiswoode; 1950.
- Fred HL. Hyposkillia: deficiency of clinical skills. Tex Heart Inst J 2005; 32:255–257.
- Fromm E. To Have or To Be? New York, NY: Harper & Row; 1976.
- Hojat M, Mangione S, Nasca TJ, Gonnella JS, Magee M. Empathy scores in medical school and ratings of empathic behavior in residency training 3 years later. J Soc Psychol 2005; 145:663–672.
- Osler W. The old humanities and the new science: The presidential address delivered before the Classical Association at Oxford, May, 1919. Br Med J 1919; 2:1–7.
- Snow CP. The Two Cultures and the Scientific Revolution. London, England: Cambridge University Press; 1959.
- Thomas L. Notes of a biology-watcher. How to fix the premedical curriculum. N Engl J Med 1978; 298:1180–1181.
- Crichton M. Travels. New York, NY: Alfred A. Knopf, Inc; 1988:69.
- Gunderman RB, Kanter SL. Perspective: “How to fix the premedical curriculum” revisited. Acad Med 2008; 83:1158–1161.
- Nevalainen M, Kuikka L, Sjoberg L, Eriksson J, Pitkala K. Tolerance of uncertainty and fears of making mistakes among fifth-year medical students. Fam Med 2012; 44:240–246.
- Osler W. Aequanimitas, with other addresses to medical students, nurses, and practitioners of medicine. May 1, 1889. www.medicalarchives.jhmi.edu/osler/aequessay.htm. Accessed June 26, 2012.
- Nuland SB. Where is Wisdom? Restraint in a Time of Biomedical Miracles. The Great Lectures Library. Chautauqua Institution; 2006.
“Those who advise that all stethoscopes should be ‘scrapped’ may be influenced by the fact that they do not know how to use their own.”
From Pulmonary Tuberculosis, 1921, by Sir James Kingston Fowler (1852–1934) of the Brompton Hospital, England
The commentary by Clark et al in this issue1 is a timely reminder of an important problem in modern medicine: the demise of the bedside. My only divergence from the authors is in their conclusion, since my Mediterranean pessimism leads me to believe that theirs is just a gallant attempt at rearguard action for a battle that, unfortunately, has long been lost.
More than half a century ago, Paul Wood warned us against the “danger of losing our clinical heritage and pinning too much faith in figures thrown out by machines,” thundering that “medicine must suffer if this tendency is not checked.”2 Well, that tendency was not checked, and medicine (and our wallets) have indeed suffered.
Still, technology is not the enemy. The misuse of technology is the problem.
Like Dr. Clark and his colleagues, I’ve seen many cases in which technology unguided by bedside skills took physicians down a path where tests begot tests and where, at the end, there was usually a surgeon, and often a lawyer. Sometimes even an undertaker. The deaths of Jonathan Larson (writer-composer of the musical Rent) and of his namesake, actor Jonathan (John) Ritter—who both succumbed to undiagnosed aortic dissection—make me wonder whether their pulses were ever checked.
Editorials have lamented the “hyposkillia” of our times,3 and the usual suspects have been already rounded up: our overreliance on tests, our ever-increasing fascination with the machine (what Erich Fromm called the necrophilia of our times),4 the loss of bedside teaching, and lastly, the lure of compensation. But one important player has so far gone unnoticed, despite being probably the major offender. In fact, it may even be responsible for the other disturbing trend in modern medicine: the loss of empathy.5
I’m referring to the disappearance of the humanities in both the undergraduate and the graduate curriculum. This is actually new. If we look, for example, at the great bedside diagnosticians of the past, we find that they were passionately interested in everything human. Most, if not all, were indeed humanists—lovers of the arts and literature, travelers and historians, poets and painters, curious of any field that could enrich the human spirit. Charcot, who single-handedly invented neurology, was not only a superb scientist, but also a talented artist who drew and painted (skills he considered fundamental for bedside observation) plus a bona-fide Beethoven fanatic who spent Thursday evenings on music, strictly forbidding any medical talk. Laënnec himself was a poet and musician who modeled his stethoscope after the flutes he made. And Charles Bell (of Bell palsy, phenomenon, and law) was a well-respected painter who soldiered with Wellington and left us incredible sketches of the Waterloo wounded and maimed. Even Osler, the pinnacle of 19th century humanistic medicine, believed so strongly in the value of a liberal education as to provide students with a list of 10 books (ranging from Plutarch and Montaigne to Marcus Aurelius and Shakespeare) to read for half an hour before going to sleep. Addressing the Classical Association just before his death, he lamented the “grievous damage” that had been done by regarding the humanities and science in any other light than complementary, while in reality they are “twin berries on one stem.”6
Until the 1870s, medicine was in fact a spin-off of the humanities. A solid humanistic education was deemed essential for admission to medical school. Then the German victory in the Franco-Prussian War shifted the axis from Paris to Berlin, and medicine went the German way. Never as touchy-feely as the French, and definitely more comfortable in the laboratory than at the bedside, the Germans produced giants like Koch, Virchow, and Roentgen, who gradually moved medicine away from the bedside and into the lab. In fact, medicine even adopted the uniform of the laboratory—the infamous white coat now banned by the British National Health system as a dirty carrier of bacteria.
Finding herself at a crossroads, America went the German way, mostly because of Flexner (himself the son of German immigrants), whose 1910 report totally changed the face of medical education. The “two cultures” were born—science was “in” and the humanities “out.”7
The result is what Lewis Thomas called the “baleful and malign” influence of the modern medical school on liberal-arts education.8 Michael Crichton put it even more bluntly. Explaining why he dropped out of medicine, he wrote, “My classmates [at Harvard] tended to think that literature, music, and art were irrelevant distractions. They held these “cultural” matters in the same intellectual contempt that a physicist holds astrology. Everything outside medicine was just a waste of time.”9
And since then, things have only worsened.10
Yet the link between humanities and the bedside remains crucial. I have had the privilege of meeting most of the clinicians who still contribute to physical diagnosis, and they are almost all humanists.
So why should the humanities nurture the bedside? For one, they may increase our tolerance of ambiguity, a trait sorely lacking in modern medicine. This makes sense, since decoding feeble sounds emanating from chests, palpating indistinct organs, and detecting bedside nuances are all painful reminders of the ambiguous in our craft, not to mention in life. And if unprepared by a humanistic education to deal with the uncertain, students may easily opt for the “certainties” of the laboratory or radiology suite.11 Once again, Osler comes to our rescue.
“A distressing feature in the life which you are about to enter” he told the graduating class of the University of Pennsylvania in 1889, “is the uncertainty which pertains not alone to our science and arts but to the very hopes and fears which make us men. In seeking absolute truth we aim at the unattainable, and must be content with finding broken portions.”12
The stethoscope is too closely bound with the doctor’s image not to be a metaphor for something larger. To me, it’s a metaphor for medicine as both an art and a science, wherein the humanities are—and of right ought to be—a fundamental part of the education. Hence, if we want to rekindle the bedside, we must rekindle the humanities. After all, this is what both Lewis Thomas8 and Sherwin Nuland13 have urged us to do. My hunch is that this would need to be done sooner rather than later, because if it is possible to make a scientist out of a humanist (it was done for centuries), it might be considerably harder to make a humanist out of a scientist. The experience of the past few decades seems to support this conclusion.
The alternative is a future full of tricorders and technicians, but sorely lacking in healers.
“Those who advise that all stethoscopes should be ‘scrapped’ may be influenced by the fact that they do not know how to use their own.”
From Pulmonary Tuberculosis, 1921, by Sir James Kingston Fowler (1852–1934) of the Brompton Hospital, England
The commentary by Clark et al in this issue1 is a timely reminder of an important problem in modern medicine: the demise of the bedside. My only divergence from the authors is in their conclusion, since my Mediterranean pessimism leads me to believe that theirs is just a gallant attempt at rearguard action for a battle that, unfortunately, has long been lost.
More than half a century ago, Paul Wood warned us against the “danger of losing our clinical heritage and pinning too much faith in figures thrown out by machines,” thundering that “medicine must suffer if this tendency is not checked.”2 Well, that tendency was not checked, and medicine (and our wallets) have indeed suffered.
Still, technology is not the enemy. The misuse of technology is the problem.
Like Dr. Clark and his colleagues, I’ve seen many cases in which technology unguided by bedside skills took physicians down a path where tests begot tests and where, at the end, there was usually a surgeon, and often a lawyer. Sometimes even an undertaker. The deaths of Jonathan Larson (writer-composer of the musical Rent) and of his namesake, actor Jonathan (John) Ritter—who both succumbed to undiagnosed aortic dissection—make me wonder whether their pulses were ever checked.
Editorials have lamented the “hyposkillia” of our times,3 and the usual suspects have been already rounded up: our overreliance on tests, our ever-increasing fascination with the machine (what Erich Fromm called the necrophilia of our times),4 the loss of bedside teaching, and lastly, the lure of compensation. But one important player has so far gone unnoticed, despite being probably the major offender. In fact, it may even be responsible for the other disturbing trend in modern medicine: the loss of empathy.5
I’m referring to the disappearance of the humanities in both the undergraduate and the graduate curriculum. This is actually new. If we look, for example, at the great bedside diagnosticians of the past, we find that they were passionately interested in everything human. Most, if not all, were indeed humanists—lovers of the arts and literature, travelers and historians, poets and painters, curious of any field that could enrich the human spirit. Charcot, who single-handedly invented neurology, was not only a superb scientist, but also a talented artist who drew and painted (skills he considered fundamental for bedside observation) plus a bona-fide Beethoven fanatic who spent Thursday evenings on music, strictly forbidding any medical talk. Laënnec himself was a poet and musician who modeled his stethoscope after the flutes he made. And Charles Bell (of Bell palsy, phenomenon, and law) was a well-respected painter who soldiered with Wellington and left us incredible sketches of the Waterloo wounded and maimed. Even Osler, the pinnacle of 19th century humanistic medicine, believed so strongly in the value of a liberal education as to provide students with a list of 10 books (ranging from Plutarch and Montaigne to Marcus Aurelius and Shakespeare) to read for half an hour before going to sleep. Addressing the Classical Association just before his death, he lamented the “grievous damage” that had been done by regarding the humanities and science in any other light than complementary, while in reality they are “twin berries on one stem.”6
Until the 1870s, medicine was in fact a spin-off of the humanities. A solid humanistic education was deemed essential for admission to medical school. Then the German victory in the Franco-Prussian War shifted the axis from Paris to Berlin, and medicine went the German way. Never as touchy-feely as the French, and definitely more comfortable in the laboratory than at the bedside, the Germans produced giants like Koch, Virchow, and Roentgen, who gradually moved medicine away from the bedside and into the lab. In fact, medicine even adopted the uniform of the laboratory—the infamous white coat now banned by the British National Health system as a dirty carrier of bacteria.
Finding herself at a crossroads, America went the German way, mostly because of Flexner (himself the son of German immigrants), whose 1910 report totally changed the face of medical education. The “two cultures” were born—science was “in” and the humanities “out.”7
The result is what Lewis Thomas called the “baleful and malign” influence of the modern medical school on liberal-arts education.8 Michael Crichton put it even more bluntly. Explaining why he dropped out of medicine, he wrote, “My classmates [at Harvard] tended to think that literature, music, and art were irrelevant distractions. They held these “cultural” matters in the same intellectual contempt that a physicist holds astrology. Everything outside medicine was just a waste of time.”9
And since then, things have only worsened.10
Yet the link between humanities and the bedside remains crucial. I have had the privilege of meeting most of the clinicians who still contribute to physical diagnosis, and they are almost all humanists.
So why should the humanities nurture the bedside? For one, they may increase our tolerance of ambiguity, a trait sorely lacking in modern medicine. This makes sense, since decoding feeble sounds emanating from chests, palpating indistinct organs, and detecting bedside nuances are all painful reminders of the ambiguous in our craft, not to mention in life. And if unprepared by a humanistic education to deal with the uncertain, students may easily opt for the “certainties” of the laboratory or radiology suite.11 Once again, Osler comes to our rescue.
“A distressing feature in the life which you are about to enter” he told the graduating class of the University of Pennsylvania in 1889, “is the uncertainty which pertains not alone to our science and arts but to the very hopes and fears which make us men. In seeking absolute truth we aim at the unattainable, and must be content with finding broken portions.”12
The stethoscope is too closely bound with the doctor’s image not to be a metaphor for something larger. To me, it’s a metaphor for medicine as both an art and a science, wherein the humanities are—and of right ought to be—a fundamental part of the education. Hence, if we want to rekindle the bedside, we must rekindle the humanities. After all, this is what both Lewis Thomas8 and Sherwin Nuland13 have urged us to do. My hunch is that this would need to be done sooner rather than later, because if it is possible to make a scientist out of a humanist (it was done for centuries), it might be considerably harder to make a humanist out of a scientist. The experience of the past few decades seems to support this conclusion.
The alternative is a future full of tricorders and technicians, but sorely lacking in healers.
- Clark D, Ahmed MI, Dell’Italia LJ, Fan P, McGiffin DC. An argument for retrieving the disappearing skill of cardiac auscultation. Cleve Clin J Med 2012; 79:536–544.
- Wood PH. Diseases of the Heart and Circulation. London: Eyre and Spottiswoode; 1950.
- Fred HL. Hyposkillia: deficiency of clinical skills. Tex Heart Inst J 2005; 32:255–257.
- Fromm E. To Have or To Be? New York, NY: Harper & Row; 1976.
- Hojat M, Mangione S, Nasca TJ, Gonnella JS, Magee M. Empathy scores in medical school and ratings of empathic behavior in residency training 3 years later. J Soc Psychol 2005; 145:663–672.
- Osler W. The old humanities and the new science: The presidential address delivered before the Classical Association at Oxford, May, 1919. Br Med J 1919; 2:1–7.
- Snow CP. The Two Cultures and the Scientific Revolution. London, England: Cambridge University Press; 1959.
- Thomas L. Notes of a biology-watcher. How to fix the premedical curriculum. N Engl J Med 1978; 298:1180–1181.
- Crichton M. Travels. New York, NY: Alfred A. Knopf, Inc; 1988:69.
- Gunderman RB, Kanter SL. Perspective: “How to fix the premedical curriculum” revisited. Acad Med 2008; 83:1158–1161.
- Nevalainen M, Kuikka L, Sjoberg L, Eriksson J, Pitkala K. Tolerance of uncertainty and fears of making mistakes among fifth-year medical students. Fam Med 2012; 44:240–246.
- Osler W. Aequanimitas, with other addresses to medical students, nurses, and practitioners of medicine. May 1, 1889. www.medicalarchives.jhmi.edu/osler/aequessay.htm. Accessed June 26, 2012.
- Nuland SB. Where is Wisdom? Restraint in a Time of Biomedical Miracles. The Great Lectures Library. Chautauqua Institution; 2006.
- Clark D, Ahmed MI, Dell’Italia LJ, Fan P, McGiffin DC. An argument for retrieving the disappearing skill of cardiac auscultation. Cleve Clin J Med 2012; 79:536–544.
- Wood PH. Diseases of the Heart and Circulation. London: Eyre and Spottiswoode; 1950.
- Fred HL. Hyposkillia: deficiency of clinical skills. Tex Heart Inst J 2005; 32:255–257.
- Fromm E. To Have or To Be? New York, NY: Harper & Row; 1976.
- Hojat M, Mangione S, Nasca TJ, Gonnella JS, Magee M. Empathy scores in medical school and ratings of empathic behavior in residency training 3 years later. J Soc Psychol 2005; 145:663–672.
- Osler W. The old humanities and the new science: The presidential address delivered before the Classical Association at Oxford, May, 1919. Br Med J 1919; 2:1–7.
- Snow CP. The Two Cultures and the Scientific Revolution. London, England: Cambridge University Press; 1959.
- Thomas L. Notes of a biology-watcher. How to fix the premedical curriculum. N Engl J Med 1978; 298:1180–1181.
- Crichton M. Travels. New York, NY: Alfred A. Knopf, Inc; 1988:69.
- Gunderman RB, Kanter SL. Perspective: “How to fix the premedical curriculum” revisited. Acad Med 2008; 83:1158–1161.
- Nevalainen M, Kuikka L, Sjoberg L, Eriksson J, Pitkala K. Tolerance of uncertainty and fears of making mistakes among fifth-year medical students. Fam Med 2012; 44:240–246.
- Osler W. Aequanimitas, with other addresses to medical students, nurses, and practitioners of medicine. May 1, 1889. www.medicalarchives.jhmi.edu/osler/aequessay.htm. Accessed June 26, 2012.
- Nuland SB. Where is Wisdom? Restraint in a Time of Biomedical Miracles. The Great Lectures Library. Chautauqua Institution; 2006.
Regularizing the approach to the irregularly irregular
Atrial fibrillation is the most common chronic rapid arrhythmia requiring the attention of internists and cardiologists. Patients with this arrhythmia have higher rates of morbidity and death than similar patients with normal sinus rhythm, and they do so for a number of reasons.
Patients with atrial fibrillation have a slew of comorbidities, including hypertensive and ischemic heart disease. Patients undergoing cardiac surgery have a dramatically higher risk of a postoperative bout of atrial fibrillation. The main concerns are the risk of stroke and the symptoms of heart failure and fatigue (often with exercise intolerance).
Information from registries of patients with atrial fibrillation has permitted the development of prognosticators of stroke risk. The CHADS2 score (congestive heart failure, hypertension, age > 75, diabetes, and prior stroke or transient ischemic attack) is an amazingly simple way to identify patients with atrial fibrillation who are at highest risk of stroke. This in turn has allowed stratification of patients for entrance into various anticoagulation studies. And perhaps surprisingly, when many factors are considered, nothing turns out to be dramatically better than warfarin (Coumadin)—if the international normalized ratio (INR) can be appropriately controlled.
Not many options are available to prevent atrial fibrillation. Postoperative atrial fibrillation may be prevented with high-dose steroids or colchicine (Colcrys), but this is often a self-limited, situational event. Chronic or recurrent intermittent atrial fibrillation is not readily prevented in most patients, and many symptomatic patients, as discussed by Dr. Bruce Lindsay in this issue, may benefit from drug therapy or radiofrequency ablation.
Studies suggest that trying to convert atrial fibrillation to normal sinus rhythm (vs controlling the rate) may not be worth the effort and the risk in many patients with asymptomatic atrial fibrillation. Furthermore, in patients with symptomatic atrial fibrillation, determining the cause of symptoms is difficult. For example, it may not always be easily determined if fatigue in an elderly patient with chronic atrial fibrillation is due to mild rate-related congestive heart failure, decreased left ventricular output due to the loss of the atrial “kick,” chronic ischemia, or the sedating effect of a beta-blocker given in an effort to control the tachycardia.
Despite many large, well-done studies comparing antiarrhythmic drugs, ablation techniques, and anticoagulants, patients will still benefit most from an experienced clinician’s reflective, individualized assessment before embarking on algorithm-driven long-term therapy. We have more choices, more data, and more management algorithms, but there is still no panacea for patients with atrial fibrillation.
Atrial fibrillation is the most common chronic rapid arrhythmia requiring the attention of internists and cardiologists. Patients with this arrhythmia have higher rates of morbidity and death than similar patients with normal sinus rhythm, and they do so for a number of reasons.
Patients with atrial fibrillation have a slew of comorbidities, including hypertensive and ischemic heart disease. Patients undergoing cardiac surgery have a dramatically higher risk of a postoperative bout of atrial fibrillation. The main concerns are the risk of stroke and the symptoms of heart failure and fatigue (often with exercise intolerance).
Information from registries of patients with atrial fibrillation has permitted the development of prognosticators of stroke risk. The CHADS2 score (congestive heart failure, hypertension, age > 75, diabetes, and prior stroke or transient ischemic attack) is an amazingly simple way to identify patients with atrial fibrillation who are at highest risk of stroke. This in turn has allowed stratification of patients for entrance into various anticoagulation studies. And perhaps surprisingly, when many factors are considered, nothing turns out to be dramatically better than warfarin (Coumadin)—if the international normalized ratio (INR) can be appropriately controlled.
Not many options are available to prevent atrial fibrillation. Postoperative atrial fibrillation may be prevented with high-dose steroids or colchicine (Colcrys), but this is often a self-limited, situational event. Chronic or recurrent intermittent atrial fibrillation is not readily prevented in most patients, and many symptomatic patients, as discussed by Dr. Bruce Lindsay in this issue, may benefit from drug therapy or radiofrequency ablation.
Studies suggest that trying to convert atrial fibrillation to normal sinus rhythm (vs controlling the rate) may not be worth the effort and the risk in many patients with asymptomatic atrial fibrillation. Furthermore, in patients with symptomatic atrial fibrillation, determining the cause of symptoms is difficult. For example, it may not always be easily determined if fatigue in an elderly patient with chronic atrial fibrillation is due to mild rate-related congestive heart failure, decreased left ventricular output due to the loss of the atrial “kick,” chronic ischemia, or the sedating effect of a beta-blocker given in an effort to control the tachycardia.
Despite many large, well-done studies comparing antiarrhythmic drugs, ablation techniques, and anticoagulants, patients will still benefit most from an experienced clinician’s reflective, individualized assessment before embarking on algorithm-driven long-term therapy. We have more choices, more data, and more management algorithms, but there is still no panacea for patients with atrial fibrillation.
Atrial fibrillation is the most common chronic rapid arrhythmia requiring the attention of internists and cardiologists. Patients with this arrhythmia have higher rates of morbidity and death than similar patients with normal sinus rhythm, and they do so for a number of reasons.
Patients with atrial fibrillation have a slew of comorbidities, including hypertensive and ischemic heart disease. Patients undergoing cardiac surgery have a dramatically higher risk of a postoperative bout of atrial fibrillation. The main concerns are the risk of stroke and the symptoms of heart failure and fatigue (often with exercise intolerance).
Information from registries of patients with atrial fibrillation has permitted the development of prognosticators of stroke risk. The CHADS2 score (congestive heart failure, hypertension, age > 75, diabetes, and prior stroke or transient ischemic attack) is an amazingly simple way to identify patients with atrial fibrillation who are at highest risk of stroke. This in turn has allowed stratification of patients for entrance into various anticoagulation studies. And perhaps surprisingly, when many factors are considered, nothing turns out to be dramatically better than warfarin (Coumadin)—if the international normalized ratio (INR) can be appropriately controlled.
Not many options are available to prevent atrial fibrillation. Postoperative atrial fibrillation may be prevented with high-dose steroids or colchicine (Colcrys), but this is often a self-limited, situational event. Chronic or recurrent intermittent atrial fibrillation is not readily prevented in most patients, and many symptomatic patients, as discussed by Dr. Bruce Lindsay in this issue, may benefit from drug therapy or radiofrequency ablation.
Studies suggest that trying to convert atrial fibrillation to normal sinus rhythm (vs controlling the rate) may not be worth the effort and the risk in many patients with asymptomatic atrial fibrillation. Furthermore, in patients with symptomatic atrial fibrillation, determining the cause of symptoms is difficult. For example, it may not always be easily determined if fatigue in an elderly patient with chronic atrial fibrillation is due to mild rate-related congestive heart failure, decreased left ventricular output due to the loss of the atrial “kick,” chronic ischemia, or the sedating effect of a beta-blocker given in an effort to control the tachycardia.
Despite many large, well-done studies comparing antiarrhythmic drugs, ablation techniques, and anticoagulants, patients will still benefit most from an experienced clinician’s reflective, individualized assessment before embarking on algorithm-driven long-term therapy. We have more choices, more data, and more management algorithms, but there is still no panacea for patients with atrial fibrillation.
Atrial fibrillation: New drugs, devices, and procedures
Although many developments have occurred in the last decade for managing atrial fibrillation, challenges remain. New and emerging alternatives to warfarin (Coumadin) for anticoagulation therapy prevent stroke marginally better and pose slightly less risk of hemorrhage, but they have important drawbacks.
The antiarrhythmic drug dronedarone (Multaq) has been found to offer only temporary benefit for persistent atrial fibrillation, and significant risks have emerged.
Radiofrequency ablation is gaining prominence, but repeat procedures are sometimes necessary.
An investigational device can be implanted via percutaneous catheter in the left atrial appendage to prevent embolization. It is too soon to know its eventual role in clinical practice.
This article reviews the results of clinical trials of these new treatments and discusses their role in clinical practice.
CHALLENGES OF ANTICOAGULATION
The main focus of managing atrial fibrillation is on alleviating symptoms, by either rate control or rhythm control. The other focus is on preventing stroke—a devastating outcome—with anticoagulation therapy.
For deciding whether to give warfarin to patients with atrial fibrillation, the six-point CHADS2 score is a crude but effective way of assessing the risk of stroke based on the following risk factors: congestive heart failure, hypertension, age 75 years or older, and diabetes (1 point each); or a history of stroke or transient ischemic attack (2 points).1 Warfarin is given if patients have a score of at least 2 points.
Warfarin has a narrow therapeutic window, with a higher risk of ischemic stroke if the international normalized ratio (INR) is less than 2.0,2 and a higher risk of intracranial hemorrhage if the INR is more than 3.0.3 Keeping the INR in the therapeutic range is difficult because of variations in diet, concurrent medications, and other factors.
The percent of time that the INR is within the therapeutic range predicts the risk of adverse events. Connolly et al4 showed that the cumulative risk of stroke, myocardial infarction, systemic embolism, or vascular death was no better with warfarin than with clopidogrel (Plavix) plus aspirin if the INR was in the therapeutic range less than 65% of the time, but the risk was significantly less if the INR was in the therapeutic range more than 65% of the time.
Also, comparing warfarin with the combination of aspirin and clopidogrel, Verheugt5 found that the rates of stroke of any kind, of disabling and fatal stroke, and of stroke per major bleed were lower in patients taking warfarin. Although many physicians prefer aspirin plus clopidogrel because of concerns about bleeding with warfarin, the rates of major bleeding were about the same in the two groups.
In a trial in patients for whom warfarin was “unsuitable,”6 the combination of aspirin plus clopidogrel was associated with a lower rate of stroke than aspirin alone (2.4% per year vs 3.3% per year, relative risk 0.762) but a higher rate of major bleeding events (2.0% per year vs 1.3% per year, relative risk 1.57).
NEW ALTERNATIVES TO WARFARIN
Because of the problems with warfarin, alternatives have been sought for many years. Several new oral anticoagulants are available or are being developed,7 including the factor Xa inhibitors rivaroxaban (Xarelto) and apixaban (Eliquis) and the direct factor II (thrombin) inhibitor dabigatran (Pradaxa) (Table 1).
Dabigatran’s advantages and drawbacks
Dabigatran has been on the market for more than a year and has gained rapid acceptance. The dosage is 150 mg twice a day, or 75 mg twice a day if renal function is impaired. Cleared by the kidneys, it has a half-life of 12 to 17 hours; 75% is cleared within 24 hours. For a patient who needs surgery that poses a low risk of bleeding, the general recommendation is to stop dabigatran the night before the surgical procedure. For operations with a greater risk of bleeding, many surgeons recommend stopping the drug 3 or 4 days before.
Advantages of dabigatran include that it is not influenced by diet and that the onset of therapeutic benefit is within 1 hour. Although some drugs affect dabigatran, drug interactions are more troublesome with warfarin.
A serious concern about dabigatran and the other new agents is that if a bleeding problem arises, the effects of these drugs are not reversible by administration of fresh frozen plasma. Dabigatran is reversible by dialysis; however, if a patient is also hypotensive, dialysis is not an option, and simply waiting for the drug to clear is the only choice.
Another drawback is that therapeutic levels cannot be monitored. If a patient taking warfarin requires cardioversion, the INR is carefully monitored for several weeks beforehand to reduce the risk of stroke. With dabigatran, there is no way to know if a patient is actually taking the drug as prescribed.
Clinical trials show that alternatives are marginally better than warfarin
In the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial,8 dabigatran was associated with a significantly lower incidence of intracranial hemorrhage, combined strokes, and systemic embolization than warfarin. The incidence of major bleeds was slightly lower with dabigatran. Although dabigatran performed better, the differences were small and would not require patients to change from warfarin if they are already doing well.
Apixaban and rivaroxaban are other alternatives to warfarin, with different mechanisms of action and metabolism. Although rivaroxaban’s half-life is similar to that of apixaban and dabigatran, it is being marketed as allowing once-daily dosing instead of twice-daily.
Recent randomized controlled clinical trials of the new drugs include:
- The Apixaban Versus Acetylsalicylic Acid (ASA) to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment (AVERROES) trial,9 which compared apixaban and aspirin
- The Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial comparing apixaban and warfarin10
- The Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF),11 comparing rivaroxaban and warfarin
- RE-LY,8 comparing dabigatran and warfarin.
In the ARISTOTLE,10 ROCKET AF,11 and RE-LY trials,8 the time that the warfarin patients’ INRs were in the therapeutic range varied from 55% to 68%. This seems low and is a problem when trying to compare therapies, but is probably about as high as one can expect in the real world.
In AVERROES,9 the combined rate of stroke and embolism was higher with aspirin than with apixaban. In the other trials, the rates were slightly better with the new drugs than with warfarin, and the rates of major hemorrhage and hemorrhagic stroke were only slightly higher with warfarin than with the new drugs. Because the differences in benefits and risks are so small, the main advantage of the newer drugs will probably be for patients who have difficulty staying in the therapeutic INR range on warfarin.
RATE CONTROL VS RESTORATION OF SINUS RHYTHM
Evidence is insufficient to determine the risk of very-long-term asymptomatic atrial fibrillation in patients on appropriate anticoagulation. Rate control is an option for asymptomatic patients but provides no change in quality of life and no definitive reduction in the risk of stroke. The main argument for restoring normal sinus rhythm in patients with mild to moderate symptoms is that it improves exercise capacity. The need for anticoagulation persists when patients are converted to sinus rhythm because the risk of recurrent atrial fibrillation remains high.
For patients with symptomatic atrial fibrillation, rate control is sometimes achieved with beta-blockers or calcium channel blockers. Rate control may be augmented with the addition of digoxin, but when used alone digoxin generally does not control the rate of atrial fibrillation. However, in many cases of atrial fibrillation, symptoms are not rate-related, and cardioversion to normal sinus rhythm should be attempted. In such cases, the symptoms may be attributable to a loss of atrial transport function.
Patients with the following risk factors should be admitted to the hospital to start antiarrhythmic drugs:
- Borderline or a long QTc interval at baseline (> 450 msec)
- Treatment with dofetilide (Tikosyn) because of its effects on the QT interval
- Heart failure or poor left-ventricular function
- Sinus node dysfunction
- Significant atrioventricular conduction disease.
Selecting an antiarrhythmic drug
Any of the antiarrhythmic drugs listed in Table 2 can be used for a patient with lone atrial fibrillation (ie, not caused by underlying heart disease). The choice of drug should be determined by whether coronary artery disease or renal failure is present as well. Liver disease or chronic obstructive pulmonary disease also may affect this decision.
Benefits of dronedarone are mixed
In a randomized trial of dronedarone vs placebo in patients with atrial fibrillation, the rate of death and the rate of first hospitalization due to a cardiovascular event at 21 months were significantly lower with dronedarone.12 No difference was found between the two groups in the rate of death from all causes, but fewer people died of cardiovascular causes in the dronedarone group. More patients taking dronedarone developed bradycardia, QT-interval prolongation, nausea, diarrhea, rash, or a higher serum creatinine level. Gastrointestinal side effects are often a problem with dronedarone: 20% to 30% of patients cannot tolerate the drug.
Dronedarone may cause a small rise in creatinine, and although this effect should be monitored, by itself it should not be interpreted as impairment of renal function. In a study in healthy people,13 dronedarone caused a 10% to 15% increase in serum creatinine, but the glomerular filtration rate was unchanged, as were renal plasma flow and anion secretion.
Another trial, in patients with severe heart failure, found that patients taking dronedarone had higher rates of hospitalization and overall mortality, raising serious concern about the safety of this drug in patients with advanced heart failure.14
Singh et al15 pooled the data from two multicenter, randomized trials that compared dronedarone with placebo for maintaining sinus rhythm in patients with atrial fibrillation or flutter. The mean time to the recurrence of atrial fibrillation was 116 days with dronedarone and 53 days with placebo. Other trials also showed longer times to recurrence and lower recurrence rates with dronedarone. Although the differences were statistically significant, they may not be clinically meaningful for patients.
Dronedarone is structurally similar to amiodarone (Cordarone), but the two drugs work differently. A meta-analysis of clinical trials16 found that amiodarone recipients had a lower rate of recurrence of atrial fibrillation than did those receiving dronedarone.
Two safety warnings for dronedarone
In January 2011, the US Food and Drug Administration (FDA) issued an alert about cases of rare but severe liver injury in patients treated with dronedarone, including two cases of acute liver failure leading to liver transplantation.17
The Permanent Atrial Fibrillation Outcome Study Using Dronedarone on Top of Standard Therapy (PALLAS)18 compared dronedarone and placebo in patients with permanent atrial fibrillation. More people died or had serious cardiovascular adverse events in the dronedarone group. The study was stopped early after data monitoring showed that rates of death, stroke, and hospitalization for heart failure were two times higher in patients receiving dronedarone. This prompted the FDA to issue another safety alert in July 2011.
Interestingly, the PALLAS study did not set out to determine whether dronedarone controls atrial fibrillation, as the study patients had long-standing, persistent atrial fibrillation. The study was designed only to determine if the drug reduces the rate of adverse events; it clearly does not, and the study shows that dronedarone should not be used to control the heart rate in patients with persistent atrial fibrillation. Instead, its use is best restricted to patients with paroxysmal atrial fibrillation without significant cardiovascular disease.
ABLATION OF ATRIAL FIBRILLATION
Another way to try to restore sinus rhythm is to destroy or isolate the area that is generating the abnormal beats via a catheter-based procedure.
Radiofrequency ablation is generally tried in patients in whom one or two drugs have failed to control atrial fibrillation. Direct comparisons show that ablation is superior to drug therapy and is effective in about 75% of patients with paroxysmal atrial fibrillation vs 20% to 40% of patients on drug therapy. Ablation plus drug therapy is often more effective than either treatment alone.
Mechanisms of atrial fibrillation and ablation
In many cases, atrial fibrillation is stimulated by vagal and sympathetic inputs to the atrium that enter around the pulmonary veins and trigger electrical activations in the area, generating spiraling, reentering circuits. Focal atrial fibrillation also originates predominantly in the pulmonary veins. Ablation of tissue widely circumscribing the mouth of the pulmonary veins prevents the electrical signal from exiting into the atrium.
In about 11% to 37% of cases, atrial fibrillation originates elsewhere, eg, in the left atrium, in the superior vena cava, or in the vein of Marshall. Techniques have evolved to also ablate these regions.
Anticoagulation therapy is recommended before the procedure, and patients at low risk should continue it for a minimum of 2 months afterward. Patients with a higher CHADS2 score should receive anticoagulation therapy for at least 1 year. The consensus statement by the Heart Rhythm Society19 recommends that patients remain on warfarin or one of the newer anticoagulants if their CHADS2 score is 2 or higher. This is because patients have a significant risk of recurrence of atrial fibrillation after radiofrequency ablation, so if their stroke risk is high they should remain on anticoagulant therapy.
Ablation is usually effective, but it carries rare but serious risks
The efficacy of a single radiofrequency ablation procedure is in the range of 60% to 80% for paroxysmal atrial fibrillation and 40% to 60% for persistent atrial fibrillation. The Second International Ablation Registry20 shows a success rate of about 75% in patients with paroxysmal atrial fibrillation and about 65% in patients with persistent and permanent atrial fibrillation. Registry data are often more favorable because reporting is optional, but these results are consistent with those from experienced medical centers. Rates of suppression of atrial fibrillation are higher in patients who also take antiarrhythmic drugs, making a “hybrid” approach useful when ablation alone fails.
According to a worldwide survey, the risk of serious complications is 4.5%. These include stroke (0.23%), tamponade (1.3%), and pulmonary vein stenosis (< 0.29%). The esophagus lies just behind the right atrium, and burning through and creating a fistula between them occurs in about 0.04% of cases and is almost uniformly fatal.20
A second ablation procedure is sometimes indicated for the recurrence of atrial fibrillation, which is almost always caused by recovery of the pulmonary veins. Bhargava et al21 found that the success rate at Cleveland Clinic for a single procedure for paroxysmal atrial fibrillation was 77%, and that it was 92% after a repeat procedure. For persistent atrial fibrillation, success rates were 76% after the first procedure and 90% after the second. Even for long-standing persistent atrial fibrillation (ie, lasting more than 1 year), 80% success was achieved after two procedures. Patients who are less likely to have a successful ablation procedure are those with long-standing atrial fibrillation and coexisting heart disease, including severe valvular disease, although mitral regurgitation sometimes improves if sinus rhythm can be maintained.
The need for a second procedure
After ablation, patients should be closely monitored for a recurrence of atrial fibrillation. Continuous monitoring with implantable cardiac monitor loop recorders can detect unrecognized episodes of arrhythmia. Long-term follow-up is also required to track outcomes and quality of life.
According to the Heart Rhythm Society Task Force on Catheter and Surgical Ablation of Atrial Fibrillation,19 atrial fibrillation recurs after ablation in about 35% to 60% of patients in the first 3 months, with recurrence rates after 1 year ranging from 5% to 16%. The rate of success is determined by the skill of the surgeon, underlying heart disease, attention to follow-up, and how success is defined.
Freedom from recurrence early on is a good predictor that late recurrence is unlikely. Patients who only have a very early recurrence (within the first 4 weeks) are more likely to have long-term freedom from atrial fibrillation tha those who have recurrences after that time.22
In a study of 831 patients, Hussein et al23 found recurrence rates of 24% between months 3 to 13 following ablation and 9% after 12 months. At 55 months, 79% were free from atrial fibrillation without drugs, 11% were free of atrial fibrillation with medications, and 5% had refractory atrial fibrillation.
Recurrence—whether early or late—was more likely to occur in people with persistent vs paroxysmal atrial fibrillation. Other risk factors for late recurrence included older age and larger left atrial size (which is also a risk factor for recurrence on drug therapy). Although recurrent arrhythmia was most often atrial fibrillation, atrial flutter also occurred frequently (in 27% of patients with late recurrence). Three patients (4% of patients with late recurrence) developed atrial tachycardia.23
In patients with early recurrence, 81% underwent repeat ablation, all of whom had recovery of one or more pulmonary veins. After the second ablation, 21% had recurrence, 65% of whom were controlled by medications.23
Whether a patient should undergo subsequent ablation procedures depends on the severity of symptoms, the likelihood of success (based on an educated guess), and the patient’s willingness to undergo another procedure.
ATRIAL APPENDAGE OCCLUSION DEVICE UNDER INVESTIGATION
New devices are being investigated that occlude the left atrial appendage to try to prevent embolization.
The Watchman device, resembling an umbrella, is implanted via a percutaneous catheter in the left atrial appendage, closing it off to preclude a thrombus from forming in the appendage and embolizing to the body. Clinical trials showed that patients who received a device had a slightly lower risk of stroke than otherwise seen in clinical practice.24 Safety and efficacy are still being determined.
The device cannot be deployed in a patient with an existing thrombus because of the danger of dislodging the thrombus, allowing it to embolize.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the national Registry of Atrial Fibrillation. JAMA 2001; 285:2864–2870.
- Hylek EM, Skates SJ, Sheehan MA, Singer DE. An analysis of the lowest effective intensity of prophylactic anticoagulation for patients with nonrheumatic atrial fibrillation. N Engl J Med 1996; 335:540–546.
- Hylek EM, Singer DE. Risk factors for intracranial hemorrhage in outpatients taking warfarin. Ann Intern Med 1994; 120:897–902.
- Connolly SJ, Pogue J, Eikelboom J, et al; ACTIVE W Investigators. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation 2008; 118:2029–2037.
- Verheugt FWA. Who is ineligible for warfarin in atrial fibrillation? Lancet 2009; 374:510–511.
- ACTIVE Investigators; Connolly SJ, Pogue J, Hart RG. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med 2009; 360:2066–2078.
- Harenberg J. New anticoagulants in atrial fibrillation. Semin Thromb Hemost 2009; 35:574–585.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Connolly SJ, Eikelboom J, Joyner C, et al; AVERROES Steering Committee and Investigators. Apixaban in patients with atrial fibrillation. N Engl J Med 2011; 364:806–817.
- Granger CB, Alexander JH, McMurray JJV, et al; for the ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med August 28, 2011; 10.1056/nejmoa1107039.
- Patel MR, Mahaffey KW, Garg J, et al; the ROCKET AF Steering Committee. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Hohnloser SH, Crijns HJ, van Eickels M, et al; ATHENA Investigators. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med 2009; 360:688–678.
- Tschuppert Y, Buclin T, Rothuizen LE, et al. Effect of dronedarone on renal function in healthy subjects. Br J Clin Pharmacol 2007; 64:785–791.
- Kóber L, Torp-Pederson C, McMurray JJ, et al; Dronedarone Study Group. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med 2008; 358:2678–2687.
- Singh BN, Connolly SJ, Crijns HJ, et al; EURDIS and ADONIS Investigators. Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. N Engl J Med 2007; 357:987–999.
- Piccini JP, Hasselblad V, Peterson ED, Washam JB, Califf RM. Comparative efficacy of dronedarone and amiodarone for the maintenance of sinus rhythm in patients with atrial fibrillation. J Am Coll Cardiol 2009; 54:1089–1095.
- US Food and Drug Administration. FDA drug safety communication: severe liver injury associated with the use of dronedarone (marketed as Multaq). http://www.fda.gov/drugs/drugsafety/ucm240011.htm. Accessed July 5, 2012.
- Connolly SJ, Camm AJ, Halperin JL, et al; for the PALLAS Investigators. Dronedarone in high-risk permanent atrial fibrillation. N Engl J Med 2011; 365:2268–2276.
- HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. Heart Rhythm 2007; 4:1–46.
- Cappato R, Calkins H, Chen SA, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 2010; 3:32–38.
- Bhargava M, Di Biase L, Mohanty P, et al. Impact of type of atrial fibrillation and repeat catheter ablation on long-term freedom from atrial fibrillation: results from a multicenter study. Heart Rhythm 2009; 6:1403–1412.
- Themistoclakis S, Schweikert RA, Sliba WI, et al. Clinical predictors and relationship between early and late atrial tachyarrhythmias after pulmonary vein antrum isolation. Heart Rhythm 2008; 5:679–685.
- Hussein AA, Saliba WI, Martin DO, et al. Natural history and long-term outcomes of ablated atrial fibrillation. Circ Arrhythm Electrophysiol 2011; 4:271–278.
- Holmes DR, Reddy VY, Turi ZG, et al; for the PROTECT AF Investigators. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 2009; 374:534–542.
Although many developments have occurred in the last decade for managing atrial fibrillation, challenges remain. New and emerging alternatives to warfarin (Coumadin) for anticoagulation therapy prevent stroke marginally better and pose slightly less risk of hemorrhage, but they have important drawbacks.
The antiarrhythmic drug dronedarone (Multaq) has been found to offer only temporary benefit for persistent atrial fibrillation, and significant risks have emerged.
Radiofrequency ablation is gaining prominence, but repeat procedures are sometimes necessary.
An investigational device can be implanted via percutaneous catheter in the left atrial appendage to prevent embolization. It is too soon to know its eventual role in clinical practice.
This article reviews the results of clinical trials of these new treatments and discusses their role in clinical practice.
CHALLENGES OF ANTICOAGULATION
The main focus of managing atrial fibrillation is on alleviating symptoms, by either rate control or rhythm control. The other focus is on preventing stroke—a devastating outcome—with anticoagulation therapy.
For deciding whether to give warfarin to patients with atrial fibrillation, the six-point CHADS2 score is a crude but effective way of assessing the risk of stroke based on the following risk factors: congestive heart failure, hypertension, age 75 years or older, and diabetes (1 point each); or a history of stroke or transient ischemic attack (2 points).1 Warfarin is given if patients have a score of at least 2 points.
Warfarin has a narrow therapeutic window, with a higher risk of ischemic stroke if the international normalized ratio (INR) is less than 2.0,2 and a higher risk of intracranial hemorrhage if the INR is more than 3.0.3 Keeping the INR in the therapeutic range is difficult because of variations in diet, concurrent medications, and other factors.
The percent of time that the INR is within the therapeutic range predicts the risk of adverse events. Connolly et al4 showed that the cumulative risk of stroke, myocardial infarction, systemic embolism, or vascular death was no better with warfarin than with clopidogrel (Plavix) plus aspirin if the INR was in the therapeutic range less than 65% of the time, but the risk was significantly less if the INR was in the therapeutic range more than 65% of the time.
Also, comparing warfarin with the combination of aspirin and clopidogrel, Verheugt5 found that the rates of stroke of any kind, of disabling and fatal stroke, and of stroke per major bleed were lower in patients taking warfarin. Although many physicians prefer aspirin plus clopidogrel because of concerns about bleeding with warfarin, the rates of major bleeding were about the same in the two groups.
In a trial in patients for whom warfarin was “unsuitable,”6 the combination of aspirin plus clopidogrel was associated with a lower rate of stroke than aspirin alone (2.4% per year vs 3.3% per year, relative risk 0.762) but a higher rate of major bleeding events (2.0% per year vs 1.3% per year, relative risk 1.57).
NEW ALTERNATIVES TO WARFARIN
Because of the problems with warfarin, alternatives have been sought for many years. Several new oral anticoagulants are available or are being developed,7 including the factor Xa inhibitors rivaroxaban (Xarelto) and apixaban (Eliquis) and the direct factor II (thrombin) inhibitor dabigatran (Pradaxa) (Table 1).
Dabigatran’s advantages and drawbacks
Dabigatran has been on the market for more than a year and has gained rapid acceptance. The dosage is 150 mg twice a day, or 75 mg twice a day if renal function is impaired. Cleared by the kidneys, it has a half-life of 12 to 17 hours; 75% is cleared within 24 hours. For a patient who needs surgery that poses a low risk of bleeding, the general recommendation is to stop dabigatran the night before the surgical procedure. For operations with a greater risk of bleeding, many surgeons recommend stopping the drug 3 or 4 days before.
Advantages of dabigatran include that it is not influenced by diet and that the onset of therapeutic benefit is within 1 hour. Although some drugs affect dabigatran, drug interactions are more troublesome with warfarin.
A serious concern about dabigatran and the other new agents is that if a bleeding problem arises, the effects of these drugs are not reversible by administration of fresh frozen plasma. Dabigatran is reversible by dialysis; however, if a patient is also hypotensive, dialysis is not an option, and simply waiting for the drug to clear is the only choice.
Another drawback is that therapeutic levels cannot be monitored. If a patient taking warfarin requires cardioversion, the INR is carefully monitored for several weeks beforehand to reduce the risk of stroke. With dabigatran, there is no way to know if a patient is actually taking the drug as prescribed.
Clinical trials show that alternatives are marginally better than warfarin
In the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial,8 dabigatran was associated with a significantly lower incidence of intracranial hemorrhage, combined strokes, and systemic embolization than warfarin. The incidence of major bleeds was slightly lower with dabigatran. Although dabigatran performed better, the differences were small and would not require patients to change from warfarin if they are already doing well.
Apixaban and rivaroxaban are other alternatives to warfarin, with different mechanisms of action and metabolism. Although rivaroxaban’s half-life is similar to that of apixaban and dabigatran, it is being marketed as allowing once-daily dosing instead of twice-daily.
Recent randomized controlled clinical trials of the new drugs include:
- The Apixaban Versus Acetylsalicylic Acid (ASA) to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment (AVERROES) trial,9 which compared apixaban and aspirin
- The Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial comparing apixaban and warfarin10
- The Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF),11 comparing rivaroxaban and warfarin
- RE-LY,8 comparing dabigatran and warfarin.
In the ARISTOTLE,10 ROCKET AF,11 and RE-LY trials,8 the time that the warfarin patients’ INRs were in the therapeutic range varied from 55% to 68%. This seems low and is a problem when trying to compare therapies, but is probably about as high as one can expect in the real world.
In AVERROES,9 the combined rate of stroke and embolism was higher with aspirin than with apixaban. In the other trials, the rates were slightly better with the new drugs than with warfarin, and the rates of major hemorrhage and hemorrhagic stroke were only slightly higher with warfarin than with the new drugs. Because the differences in benefits and risks are so small, the main advantage of the newer drugs will probably be for patients who have difficulty staying in the therapeutic INR range on warfarin.
RATE CONTROL VS RESTORATION OF SINUS RHYTHM
Evidence is insufficient to determine the risk of very-long-term asymptomatic atrial fibrillation in patients on appropriate anticoagulation. Rate control is an option for asymptomatic patients but provides no change in quality of life and no definitive reduction in the risk of stroke. The main argument for restoring normal sinus rhythm in patients with mild to moderate symptoms is that it improves exercise capacity. The need for anticoagulation persists when patients are converted to sinus rhythm because the risk of recurrent atrial fibrillation remains high.
For patients with symptomatic atrial fibrillation, rate control is sometimes achieved with beta-blockers or calcium channel blockers. Rate control may be augmented with the addition of digoxin, but when used alone digoxin generally does not control the rate of atrial fibrillation. However, in many cases of atrial fibrillation, symptoms are not rate-related, and cardioversion to normal sinus rhythm should be attempted. In such cases, the symptoms may be attributable to a loss of atrial transport function.
Patients with the following risk factors should be admitted to the hospital to start antiarrhythmic drugs:
- Borderline or a long QTc interval at baseline (> 450 msec)
- Treatment with dofetilide (Tikosyn) because of its effects on the QT interval
- Heart failure or poor left-ventricular function
- Sinus node dysfunction
- Significant atrioventricular conduction disease.
Selecting an antiarrhythmic drug
Any of the antiarrhythmic drugs listed in Table 2 can be used for a patient with lone atrial fibrillation (ie, not caused by underlying heart disease). The choice of drug should be determined by whether coronary artery disease or renal failure is present as well. Liver disease or chronic obstructive pulmonary disease also may affect this decision.
Benefits of dronedarone are mixed
In a randomized trial of dronedarone vs placebo in patients with atrial fibrillation, the rate of death and the rate of first hospitalization due to a cardiovascular event at 21 months were significantly lower with dronedarone.12 No difference was found between the two groups in the rate of death from all causes, but fewer people died of cardiovascular causes in the dronedarone group. More patients taking dronedarone developed bradycardia, QT-interval prolongation, nausea, diarrhea, rash, or a higher serum creatinine level. Gastrointestinal side effects are often a problem with dronedarone: 20% to 30% of patients cannot tolerate the drug.
Dronedarone may cause a small rise in creatinine, and although this effect should be monitored, by itself it should not be interpreted as impairment of renal function. In a study in healthy people,13 dronedarone caused a 10% to 15% increase in serum creatinine, but the glomerular filtration rate was unchanged, as were renal plasma flow and anion secretion.
Another trial, in patients with severe heart failure, found that patients taking dronedarone had higher rates of hospitalization and overall mortality, raising serious concern about the safety of this drug in patients with advanced heart failure.14
Singh et al15 pooled the data from two multicenter, randomized trials that compared dronedarone with placebo for maintaining sinus rhythm in patients with atrial fibrillation or flutter. The mean time to the recurrence of atrial fibrillation was 116 days with dronedarone and 53 days with placebo. Other trials also showed longer times to recurrence and lower recurrence rates with dronedarone. Although the differences were statistically significant, they may not be clinically meaningful for patients.
Dronedarone is structurally similar to amiodarone (Cordarone), but the two drugs work differently. A meta-analysis of clinical trials16 found that amiodarone recipients had a lower rate of recurrence of atrial fibrillation than did those receiving dronedarone.
Two safety warnings for dronedarone
In January 2011, the US Food and Drug Administration (FDA) issued an alert about cases of rare but severe liver injury in patients treated with dronedarone, including two cases of acute liver failure leading to liver transplantation.17
The Permanent Atrial Fibrillation Outcome Study Using Dronedarone on Top of Standard Therapy (PALLAS)18 compared dronedarone and placebo in patients with permanent atrial fibrillation. More people died or had serious cardiovascular adverse events in the dronedarone group. The study was stopped early after data monitoring showed that rates of death, stroke, and hospitalization for heart failure were two times higher in patients receiving dronedarone. This prompted the FDA to issue another safety alert in July 2011.
Interestingly, the PALLAS study did not set out to determine whether dronedarone controls atrial fibrillation, as the study patients had long-standing, persistent atrial fibrillation. The study was designed only to determine if the drug reduces the rate of adverse events; it clearly does not, and the study shows that dronedarone should not be used to control the heart rate in patients with persistent atrial fibrillation. Instead, its use is best restricted to patients with paroxysmal atrial fibrillation without significant cardiovascular disease.
ABLATION OF ATRIAL FIBRILLATION
Another way to try to restore sinus rhythm is to destroy or isolate the area that is generating the abnormal beats via a catheter-based procedure.
Radiofrequency ablation is generally tried in patients in whom one or two drugs have failed to control atrial fibrillation. Direct comparisons show that ablation is superior to drug therapy and is effective in about 75% of patients with paroxysmal atrial fibrillation vs 20% to 40% of patients on drug therapy. Ablation plus drug therapy is often more effective than either treatment alone.
Mechanisms of atrial fibrillation and ablation
In many cases, atrial fibrillation is stimulated by vagal and sympathetic inputs to the atrium that enter around the pulmonary veins and trigger electrical activations in the area, generating spiraling, reentering circuits. Focal atrial fibrillation also originates predominantly in the pulmonary veins. Ablation of tissue widely circumscribing the mouth of the pulmonary veins prevents the electrical signal from exiting into the atrium.
In about 11% to 37% of cases, atrial fibrillation originates elsewhere, eg, in the left atrium, in the superior vena cava, or in the vein of Marshall. Techniques have evolved to also ablate these regions.
Anticoagulation therapy is recommended before the procedure, and patients at low risk should continue it for a minimum of 2 months afterward. Patients with a higher CHADS2 score should receive anticoagulation therapy for at least 1 year. The consensus statement by the Heart Rhythm Society19 recommends that patients remain on warfarin or one of the newer anticoagulants if their CHADS2 score is 2 or higher. This is because patients have a significant risk of recurrence of atrial fibrillation after radiofrequency ablation, so if their stroke risk is high they should remain on anticoagulant therapy.
Ablation is usually effective, but it carries rare but serious risks
The efficacy of a single radiofrequency ablation procedure is in the range of 60% to 80% for paroxysmal atrial fibrillation and 40% to 60% for persistent atrial fibrillation. The Second International Ablation Registry20 shows a success rate of about 75% in patients with paroxysmal atrial fibrillation and about 65% in patients with persistent and permanent atrial fibrillation. Registry data are often more favorable because reporting is optional, but these results are consistent with those from experienced medical centers. Rates of suppression of atrial fibrillation are higher in patients who also take antiarrhythmic drugs, making a “hybrid” approach useful when ablation alone fails.
According to a worldwide survey, the risk of serious complications is 4.5%. These include stroke (0.23%), tamponade (1.3%), and pulmonary vein stenosis (< 0.29%). The esophagus lies just behind the right atrium, and burning through and creating a fistula between them occurs in about 0.04% of cases and is almost uniformly fatal.20
A second ablation procedure is sometimes indicated for the recurrence of atrial fibrillation, which is almost always caused by recovery of the pulmonary veins. Bhargava et al21 found that the success rate at Cleveland Clinic for a single procedure for paroxysmal atrial fibrillation was 77%, and that it was 92% after a repeat procedure. For persistent atrial fibrillation, success rates were 76% after the first procedure and 90% after the second. Even for long-standing persistent atrial fibrillation (ie, lasting more than 1 year), 80% success was achieved after two procedures. Patients who are less likely to have a successful ablation procedure are those with long-standing atrial fibrillation and coexisting heart disease, including severe valvular disease, although mitral regurgitation sometimes improves if sinus rhythm can be maintained.
The need for a second procedure
After ablation, patients should be closely monitored for a recurrence of atrial fibrillation. Continuous monitoring with implantable cardiac monitor loop recorders can detect unrecognized episodes of arrhythmia. Long-term follow-up is also required to track outcomes and quality of life.
According to the Heart Rhythm Society Task Force on Catheter and Surgical Ablation of Atrial Fibrillation,19 atrial fibrillation recurs after ablation in about 35% to 60% of patients in the first 3 months, with recurrence rates after 1 year ranging from 5% to 16%. The rate of success is determined by the skill of the surgeon, underlying heart disease, attention to follow-up, and how success is defined.
Freedom from recurrence early on is a good predictor that late recurrence is unlikely. Patients who only have a very early recurrence (within the first 4 weeks) are more likely to have long-term freedom from atrial fibrillation tha those who have recurrences after that time.22
In a study of 831 patients, Hussein et al23 found recurrence rates of 24% between months 3 to 13 following ablation and 9% after 12 months. At 55 months, 79% were free from atrial fibrillation without drugs, 11% were free of atrial fibrillation with medications, and 5% had refractory atrial fibrillation.
Recurrence—whether early or late—was more likely to occur in people with persistent vs paroxysmal atrial fibrillation. Other risk factors for late recurrence included older age and larger left atrial size (which is also a risk factor for recurrence on drug therapy). Although recurrent arrhythmia was most often atrial fibrillation, atrial flutter also occurred frequently (in 27% of patients with late recurrence). Three patients (4% of patients with late recurrence) developed atrial tachycardia.23
In patients with early recurrence, 81% underwent repeat ablation, all of whom had recovery of one or more pulmonary veins. After the second ablation, 21% had recurrence, 65% of whom were controlled by medications.23
Whether a patient should undergo subsequent ablation procedures depends on the severity of symptoms, the likelihood of success (based on an educated guess), and the patient’s willingness to undergo another procedure.
ATRIAL APPENDAGE OCCLUSION DEVICE UNDER INVESTIGATION
New devices are being investigated that occlude the left atrial appendage to try to prevent embolization.
The Watchman device, resembling an umbrella, is implanted via a percutaneous catheter in the left atrial appendage, closing it off to preclude a thrombus from forming in the appendage and embolizing to the body. Clinical trials showed that patients who received a device had a slightly lower risk of stroke than otherwise seen in clinical practice.24 Safety and efficacy are still being determined.
The device cannot be deployed in a patient with an existing thrombus because of the danger of dislodging the thrombus, allowing it to embolize.
Although many developments have occurred in the last decade for managing atrial fibrillation, challenges remain. New and emerging alternatives to warfarin (Coumadin) for anticoagulation therapy prevent stroke marginally better and pose slightly less risk of hemorrhage, but they have important drawbacks.
The antiarrhythmic drug dronedarone (Multaq) has been found to offer only temporary benefit for persistent atrial fibrillation, and significant risks have emerged.
Radiofrequency ablation is gaining prominence, but repeat procedures are sometimes necessary.
An investigational device can be implanted via percutaneous catheter in the left atrial appendage to prevent embolization. It is too soon to know its eventual role in clinical practice.
This article reviews the results of clinical trials of these new treatments and discusses their role in clinical practice.
CHALLENGES OF ANTICOAGULATION
The main focus of managing atrial fibrillation is on alleviating symptoms, by either rate control or rhythm control. The other focus is on preventing stroke—a devastating outcome—with anticoagulation therapy.
For deciding whether to give warfarin to patients with atrial fibrillation, the six-point CHADS2 score is a crude but effective way of assessing the risk of stroke based on the following risk factors: congestive heart failure, hypertension, age 75 years or older, and diabetes (1 point each); or a history of stroke or transient ischemic attack (2 points).1 Warfarin is given if patients have a score of at least 2 points.
Warfarin has a narrow therapeutic window, with a higher risk of ischemic stroke if the international normalized ratio (INR) is less than 2.0,2 and a higher risk of intracranial hemorrhage if the INR is more than 3.0.3 Keeping the INR in the therapeutic range is difficult because of variations in diet, concurrent medications, and other factors.
The percent of time that the INR is within the therapeutic range predicts the risk of adverse events. Connolly et al4 showed that the cumulative risk of stroke, myocardial infarction, systemic embolism, or vascular death was no better with warfarin than with clopidogrel (Plavix) plus aspirin if the INR was in the therapeutic range less than 65% of the time, but the risk was significantly less if the INR was in the therapeutic range more than 65% of the time.
Also, comparing warfarin with the combination of aspirin and clopidogrel, Verheugt5 found that the rates of stroke of any kind, of disabling and fatal stroke, and of stroke per major bleed were lower in patients taking warfarin. Although many physicians prefer aspirin plus clopidogrel because of concerns about bleeding with warfarin, the rates of major bleeding were about the same in the two groups.
In a trial in patients for whom warfarin was “unsuitable,”6 the combination of aspirin plus clopidogrel was associated with a lower rate of stroke than aspirin alone (2.4% per year vs 3.3% per year, relative risk 0.762) but a higher rate of major bleeding events (2.0% per year vs 1.3% per year, relative risk 1.57).
NEW ALTERNATIVES TO WARFARIN
Because of the problems with warfarin, alternatives have been sought for many years. Several new oral anticoagulants are available or are being developed,7 including the factor Xa inhibitors rivaroxaban (Xarelto) and apixaban (Eliquis) and the direct factor II (thrombin) inhibitor dabigatran (Pradaxa) (Table 1).
Dabigatran’s advantages and drawbacks
Dabigatran has been on the market for more than a year and has gained rapid acceptance. The dosage is 150 mg twice a day, or 75 mg twice a day if renal function is impaired. Cleared by the kidneys, it has a half-life of 12 to 17 hours; 75% is cleared within 24 hours. For a patient who needs surgery that poses a low risk of bleeding, the general recommendation is to stop dabigatran the night before the surgical procedure. For operations with a greater risk of bleeding, many surgeons recommend stopping the drug 3 or 4 days before.
Advantages of dabigatran include that it is not influenced by diet and that the onset of therapeutic benefit is within 1 hour. Although some drugs affect dabigatran, drug interactions are more troublesome with warfarin.
A serious concern about dabigatran and the other new agents is that if a bleeding problem arises, the effects of these drugs are not reversible by administration of fresh frozen plasma. Dabigatran is reversible by dialysis; however, if a patient is also hypotensive, dialysis is not an option, and simply waiting for the drug to clear is the only choice.
Another drawback is that therapeutic levels cannot be monitored. If a patient taking warfarin requires cardioversion, the INR is carefully monitored for several weeks beforehand to reduce the risk of stroke. With dabigatran, there is no way to know if a patient is actually taking the drug as prescribed.
Clinical trials show that alternatives are marginally better than warfarin
In the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial,8 dabigatran was associated with a significantly lower incidence of intracranial hemorrhage, combined strokes, and systemic embolization than warfarin. The incidence of major bleeds was slightly lower with dabigatran. Although dabigatran performed better, the differences were small and would not require patients to change from warfarin if they are already doing well.
Apixaban and rivaroxaban are other alternatives to warfarin, with different mechanisms of action and metabolism. Although rivaroxaban’s half-life is similar to that of apixaban and dabigatran, it is being marketed as allowing once-daily dosing instead of twice-daily.
Recent randomized controlled clinical trials of the new drugs include:
- The Apixaban Versus Acetylsalicylic Acid (ASA) to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment (AVERROES) trial,9 which compared apixaban and aspirin
- The Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial comparing apixaban and warfarin10
- The Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF),11 comparing rivaroxaban and warfarin
- RE-LY,8 comparing dabigatran and warfarin.
In the ARISTOTLE,10 ROCKET AF,11 and RE-LY trials,8 the time that the warfarin patients’ INRs were in the therapeutic range varied from 55% to 68%. This seems low and is a problem when trying to compare therapies, but is probably about as high as one can expect in the real world.
In AVERROES,9 the combined rate of stroke and embolism was higher with aspirin than with apixaban. In the other trials, the rates were slightly better with the new drugs than with warfarin, and the rates of major hemorrhage and hemorrhagic stroke were only slightly higher with warfarin than with the new drugs. Because the differences in benefits and risks are so small, the main advantage of the newer drugs will probably be for patients who have difficulty staying in the therapeutic INR range on warfarin.
RATE CONTROL VS RESTORATION OF SINUS RHYTHM
Evidence is insufficient to determine the risk of very-long-term asymptomatic atrial fibrillation in patients on appropriate anticoagulation. Rate control is an option for asymptomatic patients but provides no change in quality of life and no definitive reduction in the risk of stroke. The main argument for restoring normal sinus rhythm in patients with mild to moderate symptoms is that it improves exercise capacity. The need for anticoagulation persists when patients are converted to sinus rhythm because the risk of recurrent atrial fibrillation remains high.
For patients with symptomatic atrial fibrillation, rate control is sometimes achieved with beta-blockers or calcium channel blockers. Rate control may be augmented with the addition of digoxin, but when used alone digoxin generally does not control the rate of atrial fibrillation. However, in many cases of atrial fibrillation, symptoms are not rate-related, and cardioversion to normal sinus rhythm should be attempted. In such cases, the symptoms may be attributable to a loss of atrial transport function.
Patients with the following risk factors should be admitted to the hospital to start antiarrhythmic drugs:
- Borderline or a long QTc interval at baseline (> 450 msec)
- Treatment with dofetilide (Tikosyn) because of its effects on the QT interval
- Heart failure or poor left-ventricular function
- Sinus node dysfunction
- Significant atrioventricular conduction disease.
Selecting an antiarrhythmic drug
Any of the antiarrhythmic drugs listed in Table 2 can be used for a patient with lone atrial fibrillation (ie, not caused by underlying heart disease). The choice of drug should be determined by whether coronary artery disease or renal failure is present as well. Liver disease or chronic obstructive pulmonary disease also may affect this decision.
Benefits of dronedarone are mixed
In a randomized trial of dronedarone vs placebo in patients with atrial fibrillation, the rate of death and the rate of first hospitalization due to a cardiovascular event at 21 months were significantly lower with dronedarone.12 No difference was found between the two groups in the rate of death from all causes, but fewer people died of cardiovascular causes in the dronedarone group. More patients taking dronedarone developed bradycardia, QT-interval prolongation, nausea, diarrhea, rash, or a higher serum creatinine level. Gastrointestinal side effects are often a problem with dronedarone: 20% to 30% of patients cannot tolerate the drug.
Dronedarone may cause a small rise in creatinine, and although this effect should be monitored, by itself it should not be interpreted as impairment of renal function. In a study in healthy people,13 dronedarone caused a 10% to 15% increase in serum creatinine, but the glomerular filtration rate was unchanged, as were renal plasma flow and anion secretion.
Another trial, in patients with severe heart failure, found that patients taking dronedarone had higher rates of hospitalization and overall mortality, raising serious concern about the safety of this drug in patients with advanced heart failure.14
Singh et al15 pooled the data from two multicenter, randomized trials that compared dronedarone with placebo for maintaining sinus rhythm in patients with atrial fibrillation or flutter. The mean time to the recurrence of atrial fibrillation was 116 days with dronedarone and 53 days with placebo. Other trials also showed longer times to recurrence and lower recurrence rates with dronedarone. Although the differences were statistically significant, they may not be clinically meaningful for patients.
Dronedarone is structurally similar to amiodarone (Cordarone), but the two drugs work differently. A meta-analysis of clinical trials16 found that amiodarone recipients had a lower rate of recurrence of atrial fibrillation than did those receiving dronedarone.
Two safety warnings for dronedarone
In January 2011, the US Food and Drug Administration (FDA) issued an alert about cases of rare but severe liver injury in patients treated with dronedarone, including two cases of acute liver failure leading to liver transplantation.17
The Permanent Atrial Fibrillation Outcome Study Using Dronedarone on Top of Standard Therapy (PALLAS)18 compared dronedarone and placebo in patients with permanent atrial fibrillation. More people died or had serious cardiovascular adverse events in the dronedarone group. The study was stopped early after data monitoring showed that rates of death, stroke, and hospitalization for heart failure were two times higher in patients receiving dronedarone. This prompted the FDA to issue another safety alert in July 2011.
Interestingly, the PALLAS study did not set out to determine whether dronedarone controls atrial fibrillation, as the study patients had long-standing, persistent atrial fibrillation. The study was designed only to determine if the drug reduces the rate of adverse events; it clearly does not, and the study shows that dronedarone should not be used to control the heart rate in patients with persistent atrial fibrillation. Instead, its use is best restricted to patients with paroxysmal atrial fibrillation without significant cardiovascular disease.
ABLATION OF ATRIAL FIBRILLATION
Another way to try to restore sinus rhythm is to destroy or isolate the area that is generating the abnormal beats via a catheter-based procedure.
Radiofrequency ablation is generally tried in patients in whom one or two drugs have failed to control atrial fibrillation. Direct comparisons show that ablation is superior to drug therapy and is effective in about 75% of patients with paroxysmal atrial fibrillation vs 20% to 40% of patients on drug therapy. Ablation plus drug therapy is often more effective than either treatment alone.
Mechanisms of atrial fibrillation and ablation
In many cases, atrial fibrillation is stimulated by vagal and sympathetic inputs to the atrium that enter around the pulmonary veins and trigger electrical activations in the area, generating spiraling, reentering circuits. Focal atrial fibrillation also originates predominantly in the pulmonary veins. Ablation of tissue widely circumscribing the mouth of the pulmonary veins prevents the electrical signal from exiting into the atrium.
In about 11% to 37% of cases, atrial fibrillation originates elsewhere, eg, in the left atrium, in the superior vena cava, or in the vein of Marshall. Techniques have evolved to also ablate these regions.
Anticoagulation therapy is recommended before the procedure, and patients at low risk should continue it for a minimum of 2 months afterward. Patients with a higher CHADS2 score should receive anticoagulation therapy for at least 1 year. The consensus statement by the Heart Rhythm Society19 recommends that patients remain on warfarin or one of the newer anticoagulants if their CHADS2 score is 2 or higher. This is because patients have a significant risk of recurrence of atrial fibrillation after radiofrequency ablation, so if their stroke risk is high they should remain on anticoagulant therapy.
Ablation is usually effective, but it carries rare but serious risks
The efficacy of a single radiofrequency ablation procedure is in the range of 60% to 80% for paroxysmal atrial fibrillation and 40% to 60% for persistent atrial fibrillation. The Second International Ablation Registry20 shows a success rate of about 75% in patients with paroxysmal atrial fibrillation and about 65% in patients with persistent and permanent atrial fibrillation. Registry data are often more favorable because reporting is optional, but these results are consistent with those from experienced medical centers. Rates of suppression of atrial fibrillation are higher in patients who also take antiarrhythmic drugs, making a “hybrid” approach useful when ablation alone fails.
According to a worldwide survey, the risk of serious complications is 4.5%. These include stroke (0.23%), tamponade (1.3%), and pulmonary vein stenosis (< 0.29%). The esophagus lies just behind the right atrium, and burning through and creating a fistula between them occurs in about 0.04% of cases and is almost uniformly fatal.20
A second ablation procedure is sometimes indicated for the recurrence of atrial fibrillation, which is almost always caused by recovery of the pulmonary veins. Bhargava et al21 found that the success rate at Cleveland Clinic for a single procedure for paroxysmal atrial fibrillation was 77%, and that it was 92% after a repeat procedure. For persistent atrial fibrillation, success rates were 76% after the first procedure and 90% after the second. Even for long-standing persistent atrial fibrillation (ie, lasting more than 1 year), 80% success was achieved after two procedures. Patients who are less likely to have a successful ablation procedure are those with long-standing atrial fibrillation and coexisting heart disease, including severe valvular disease, although mitral regurgitation sometimes improves if sinus rhythm can be maintained.
The need for a second procedure
After ablation, patients should be closely monitored for a recurrence of atrial fibrillation. Continuous monitoring with implantable cardiac monitor loop recorders can detect unrecognized episodes of arrhythmia. Long-term follow-up is also required to track outcomes and quality of life.
According to the Heart Rhythm Society Task Force on Catheter and Surgical Ablation of Atrial Fibrillation,19 atrial fibrillation recurs after ablation in about 35% to 60% of patients in the first 3 months, with recurrence rates after 1 year ranging from 5% to 16%. The rate of success is determined by the skill of the surgeon, underlying heart disease, attention to follow-up, and how success is defined.
Freedom from recurrence early on is a good predictor that late recurrence is unlikely. Patients who only have a very early recurrence (within the first 4 weeks) are more likely to have long-term freedom from atrial fibrillation tha those who have recurrences after that time.22
In a study of 831 patients, Hussein et al23 found recurrence rates of 24% between months 3 to 13 following ablation and 9% after 12 months. At 55 months, 79% were free from atrial fibrillation without drugs, 11% were free of atrial fibrillation with medications, and 5% had refractory atrial fibrillation.
Recurrence—whether early or late—was more likely to occur in people with persistent vs paroxysmal atrial fibrillation. Other risk factors for late recurrence included older age and larger left atrial size (which is also a risk factor for recurrence on drug therapy). Although recurrent arrhythmia was most often atrial fibrillation, atrial flutter also occurred frequently (in 27% of patients with late recurrence). Three patients (4% of patients with late recurrence) developed atrial tachycardia.23
In patients with early recurrence, 81% underwent repeat ablation, all of whom had recovery of one or more pulmonary veins. After the second ablation, 21% had recurrence, 65% of whom were controlled by medications.23
Whether a patient should undergo subsequent ablation procedures depends on the severity of symptoms, the likelihood of success (based on an educated guess), and the patient’s willingness to undergo another procedure.
ATRIAL APPENDAGE OCCLUSION DEVICE UNDER INVESTIGATION
New devices are being investigated that occlude the left atrial appendage to try to prevent embolization.
The Watchman device, resembling an umbrella, is implanted via a percutaneous catheter in the left atrial appendage, closing it off to preclude a thrombus from forming in the appendage and embolizing to the body. Clinical trials showed that patients who received a device had a slightly lower risk of stroke than otherwise seen in clinical practice.24 Safety and efficacy are still being determined.
The device cannot be deployed in a patient with an existing thrombus because of the danger of dislodging the thrombus, allowing it to embolize.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the national Registry of Atrial Fibrillation. JAMA 2001; 285:2864–2870.
- Hylek EM, Skates SJ, Sheehan MA, Singer DE. An analysis of the lowest effective intensity of prophylactic anticoagulation for patients with nonrheumatic atrial fibrillation. N Engl J Med 1996; 335:540–546.
- Hylek EM, Singer DE. Risk factors for intracranial hemorrhage in outpatients taking warfarin. Ann Intern Med 1994; 120:897–902.
- Connolly SJ, Pogue J, Eikelboom J, et al; ACTIVE W Investigators. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation 2008; 118:2029–2037.
- Verheugt FWA. Who is ineligible for warfarin in atrial fibrillation? Lancet 2009; 374:510–511.
- ACTIVE Investigators; Connolly SJ, Pogue J, Hart RG. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med 2009; 360:2066–2078.
- Harenberg J. New anticoagulants in atrial fibrillation. Semin Thromb Hemost 2009; 35:574–585.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Connolly SJ, Eikelboom J, Joyner C, et al; AVERROES Steering Committee and Investigators. Apixaban in patients with atrial fibrillation. N Engl J Med 2011; 364:806–817.
- Granger CB, Alexander JH, McMurray JJV, et al; for the ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med August 28, 2011; 10.1056/nejmoa1107039.
- Patel MR, Mahaffey KW, Garg J, et al; the ROCKET AF Steering Committee. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Hohnloser SH, Crijns HJ, van Eickels M, et al; ATHENA Investigators. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med 2009; 360:688–678.
- Tschuppert Y, Buclin T, Rothuizen LE, et al. Effect of dronedarone on renal function in healthy subjects. Br J Clin Pharmacol 2007; 64:785–791.
- Kóber L, Torp-Pederson C, McMurray JJ, et al; Dronedarone Study Group. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med 2008; 358:2678–2687.
- Singh BN, Connolly SJ, Crijns HJ, et al; EURDIS and ADONIS Investigators. Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. N Engl J Med 2007; 357:987–999.
- Piccini JP, Hasselblad V, Peterson ED, Washam JB, Califf RM. Comparative efficacy of dronedarone and amiodarone for the maintenance of sinus rhythm in patients with atrial fibrillation. J Am Coll Cardiol 2009; 54:1089–1095.
- US Food and Drug Administration. FDA drug safety communication: severe liver injury associated with the use of dronedarone (marketed as Multaq). http://www.fda.gov/drugs/drugsafety/ucm240011.htm. Accessed July 5, 2012.
- Connolly SJ, Camm AJ, Halperin JL, et al; for the PALLAS Investigators. Dronedarone in high-risk permanent atrial fibrillation. N Engl J Med 2011; 365:2268–2276.
- HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. Heart Rhythm 2007; 4:1–46.
- Cappato R, Calkins H, Chen SA, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 2010; 3:32–38.
- Bhargava M, Di Biase L, Mohanty P, et al. Impact of type of atrial fibrillation and repeat catheter ablation on long-term freedom from atrial fibrillation: results from a multicenter study. Heart Rhythm 2009; 6:1403–1412.
- Themistoclakis S, Schweikert RA, Sliba WI, et al. Clinical predictors and relationship between early and late atrial tachyarrhythmias after pulmonary vein antrum isolation. Heart Rhythm 2008; 5:679–685.
- Hussein AA, Saliba WI, Martin DO, et al. Natural history and long-term outcomes of ablated atrial fibrillation. Circ Arrhythm Electrophysiol 2011; 4:271–278.
- Holmes DR, Reddy VY, Turi ZG, et al; for the PROTECT AF Investigators. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 2009; 374:534–542.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the national Registry of Atrial Fibrillation. JAMA 2001; 285:2864–2870.
- Hylek EM, Skates SJ, Sheehan MA, Singer DE. An analysis of the lowest effective intensity of prophylactic anticoagulation for patients with nonrheumatic atrial fibrillation. N Engl J Med 1996; 335:540–546.
- Hylek EM, Singer DE. Risk factors for intracranial hemorrhage in outpatients taking warfarin. Ann Intern Med 1994; 120:897–902.
- Connolly SJ, Pogue J, Eikelboom J, et al; ACTIVE W Investigators. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation 2008; 118:2029–2037.
- Verheugt FWA. Who is ineligible for warfarin in atrial fibrillation? Lancet 2009; 374:510–511.
- ACTIVE Investigators; Connolly SJ, Pogue J, Hart RG. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med 2009; 360:2066–2078.
- Harenberg J. New anticoagulants in atrial fibrillation. Semin Thromb Hemost 2009; 35:574–585.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Connolly SJ, Eikelboom J, Joyner C, et al; AVERROES Steering Committee and Investigators. Apixaban in patients with atrial fibrillation. N Engl J Med 2011; 364:806–817.
- Granger CB, Alexander JH, McMurray JJV, et al; for the ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med August 28, 2011; 10.1056/nejmoa1107039.
- Patel MR, Mahaffey KW, Garg J, et al; the ROCKET AF Steering Committee. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–891.
- Hohnloser SH, Crijns HJ, van Eickels M, et al; ATHENA Investigators. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med 2009; 360:688–678.
- Tschuppert Y, Buclin T, Rothuizen LE, et al. Effect of dronedarone on renal function in healthy subjects. Br J Clin Pharmacol 2007; 64:785–791.
- Kóber L, Torp-Pederson C, McMurray JJ, et al; Dronedarone Study Group. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med 2008; 358:2678–2687.
- Singh BN, Connolly SJ, Crijns HJ, et al; EURDIS and ADONIS Investigators. Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. N Engl J Med 2007; 357:987–999.
- Piccini JP, Hasselblad V, Peterson ED, Washam JB, Califf RM. Comparative efficacy of dronedarone and amiodarone for the maintenance of sinus rhythm in patients with atrial fibrillation. J Am Coll Cardiol 2009; 54:1089–1095.
- US Food and Drug Administration. FDA drug safety communication: severe liver injury associated with the use of dronedarone (marketed as Multaq). http://www.fda.gov/drugs/drugsafety/ucm240011.htm. Accessed July 5, 2012.
- Connolly SJ, Camm AJ, Halperin JL, et al; for the PALLAS Investigators. Dronedarone in high-risk permanent atrial fibrillation. N Engl J Med 2011; 365:2268–2276.
- HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. Heart Rhythm 2007; 4:1–46.
- Cappato R, Calkins H, Chen SA, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 2010; 3:32–38.
- Bhargava M, Di Biase L, Mohanty P, et al. Impact of type of atrial fibrillation and repeat catheter ablation on long-term freedom from atrial fibrillation: results from a multicenter study. Heart Rhythm 2009; 6:1403–1412.
- Themistoclakis S, Schweikert RA, Sliba WI, et al. Clinical predictors and relationship between early and late atrial tachyarrhythmias after pulmonary vein antrum isolation. Heart Rhythm 2008; 5:679–685.
- Hussein AA, Saliba WI, Martin DO, et al. Natural history and long-term outcomes of ablated atrial fibrillation. Circ Arrhythm Electrophysiol 2011; 4:271–278.
- Holmes DR, Reddy VY, Turi ZG, et al; for the PROTECT AF Investigators. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 2009; 374:534–542.
KEY POINTS
- Warfarin is as safe as—and more effective than—the combination of aspirin and clopidogrel (Plavix) if the international normalized ratio is in the therapeutic range 65% of the time or more.
- New anticoagulants are promising alternatives to warfarin, but they also pose risks. Patients who are doing well on warfarin need not change.
- Several antiarrhythmic drugs are available to control symptomatic atrial fibrillation. Dronedarone (Multaq) should only be considered for patients with paroxysmal atrial fibrillation without significant cardiovascular disease.
- Ablation is often effective in controlling atrial fibrillation, but recurrence is common. Early recurrence often subsides, but late recurrence often requires a repeat procedure.
Glucose Control and Avoidance of Hypoglycemia
Q: I am frustrated by the “always bring the blood sugars down slowly” philosophy, which I know is intended to avoid hypoglycemic symptoms. However, it often seems to be done at the expense of prolonged hyperglycemia, which is dangerous for patients’ long-term health and may cause more rapid beta-cell destruction. What’s the deal? There is evidence that rapid achievement of tight glucose control using intensive insulin therapy with multiple daily injections or insulin pumps in patients with newly diagnosed type 2 diabetes has favorable outcomes on recovery and maintenance of beta-cell function and prolonged glycemic remission, compared with treatment with oral hypoglycemic agents.1 However, this approach is time consuming and not practical in most primary care settings. Overcoming “clinical inertia” (the failure to initiate or intensify therapy when indicated) has been identified as a major barrier to achieving rapid glycemic control, to the detriment of the patient’s health. One recent study showed that more frequent follow-up with a multidisciplinary team and regular use of a computer-analyzed 7-point glucose profile resulted in more rapid and significantly better glycemic control with a lower A1C, compared to standard care.2 This approach is much more practical in a primary care setting. Additionally, we always treat our patients as individuals. There are very few maxims that are correct in all situations. Almost every answer to a clinical question begins with the qualifier “It depends….” The specifics of the individual case will clarify the appropriate answer. In regard to this particular question, the answer will vary by the clinical history of the patient. For example, for a pregnant patient with poor glycemic control, potential hospitalization and rapid titration of insulin would be the most judicious plan. In this case, quickly bringing glucose into tight control helps minimize risks to the developing fetus. However, if the patient is a frail 80-year-old with advanced cardiovascular disease, then slow and careful titration of medications would be the prudent course to meticulously avoid hypoglycemia. New guidelines from the American Diabetes Association and the European Association for the Study of Diabetes (ADA/EASD)3 are helpful in that they identify various clinical issues and give guidance on which medication regimens would be more appropriate for the specific clinical history. They categorize medications based on efficacy, weight gain, hypoglycemia, major side effects, and costs. Guidelines from the American Association of Clinical Endocrinologists/American College of Endocrinology (AACE/ACE)4 are also very useful, because they categorize treatment based on the A1C level, as well as potential for weight gain and hypoglycemia. For example, a patient with an A1C < 7.5% may be an appropriate candidate for monotherapy, while a symptomatic patient with an A1C > 9% would likely benefit from insulin therapy or triple oral agent therapy. First, it is helpful to set individual glycemic targets for your patient. The following factors can help you in determining A1C targets: • Psychosocial considerations (motivation, adherence to therapy, self-care capacity) • Resources or support systems (family support, community resources, living situation, etc) • Risk for hypoglycemia • Duration of diabetes • Life expectancy • Microvascular complications • Cardiovascular disease and coexisting conditions. For example, an older individual with poor motivation, lack of support systems, short life expectancy, and coexisting terminal cancer would have a less stringent A1C target of ≤ 8%, whereas a young, motivated individual with no complications or serious coexisting complications would have an A1C target of 6%. The new ADA/EASD guidelines list additional considerations for medication choices for various comorbidities, including coronary disease, heart failure, renal disease, liver dysfunction, and hypoglycemia. For each comorbidity listed, there are suggested medications that are preferred and those that should be avoided. If your goal is to avoid hypoglycemia, the ADA/EASD guidelines list medication choices that have low propensity to cause hypoglycemia (eg, metformin, pioglitazone, DPP-4 inhibitors, and GLP-1 receptor agonists). (Of note, special attention is given to medications that do not cause weight gain, such as GLP-1 receptor agonists, DPP-4 inhibitors, and metformin.) Finally, the consensus statement emphasizes the need for individualizing therapy. Many patients have multiple comorbidities and may have medication sensitivities, cost constraints, etc. All of these factors must be taken into consideration when making therapeutic choices. Keep in mind, “one size does not fit all” when it comes to diabetes therapy. The recent releases from both the ADA/EASD and AACE/ACE give us much more detailed guidance addressing medication choices in regard to efficacy, potential for hypoglycemia and weight gain, major side effects, and costs. As always, guidelines do not replace good clinical judgment, based on the patient sitting in front of you. REFERENCES 2. Pimazoni-Netto A, Rodbard D, Zanella MT; Diabetes Education and Control Group. Rapid improvement of glycemic control in type 2 diabetes using weekly intensive multifactorial interventions: structured glucose monitoring, patient education, and adjustment of therapy—a randomized controlled trial. Diabetes Technol Therapeutics. 2011;13(10):997-1004. 3. Inzucchi SE, Bergenstahl RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient centered approach. Diabetes Care. [Epub ahead of print; April 19, 2012]. 4. Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract. 2009;15(6):540-559. |
Q: I am frustrated by the “always bring the blood sugars down slowly” philosophy, which I know is intended to avoid hypoglycemic symptoms. However, it often seems to be done at the expense of prolonged hyperglycemia, which is dangerous for patients’ long-term health and may cause more rapid beta-cell destruction. What’s the deal? There is evidence that rapid achievement of tight glucose control using intensive insulin therapy with multiple daily injections or insulin pumps in patients with newly diagnosed type 2 diabetes has favorable outcomes on recovery and maintenance of beta-cell function and prolonged glycemic remission, compared with treatment with oral hypoglycemic agents.1 However, this approach is time consuming and not practical in most primary care settings. Overcoming “clinical inertia” (the failure to initiate or intensify therapy when indicated) has been identified as a major barrier to achieving rapid glycemic control, to the detriment of the patient’s health. One recent study showed that more frequent follow-up with a multidisciplinary team and regular use of a computer-analyzed 7-point glucose profile resulted in more rapid and significantly better glycemic control with a lower A1C, compared to standard care.2 This approach is much more practical in a primary care setting. Additionally, we always treat our patients as individuals. There are very few maxims that are correct in all situations. Almost every answer to a clinical question begins with the qualifier “It depends….” The specifics of the individual case will clarify the appropriate answer. In regard to this particular question, the answer will vary by the clinical history of the patient. For example, for a pregnant patient with poor glycemic control, potential hospitalization and rapid titration of insulin would be the most judicious plan. In this case, quickly bringing glucose into tight control helps minimize risks to the developing fetus. However, if the patient is a frail 80-year-old with advanced cardiovascular disease, then slow and careful titration of medications would be the prudent course to meticulously avoid hypoglycemia. New guidelines from the American Diabetes Association and the European Association for the Study of Diabetes (ADA/EASD)3 are helpful in that they identify various clinical issues and give guidance on which medication regimens would be more appropriate for the specific clinical history. They categorize medications based on efficacy, weight gain, hypoglycemia, major side effects, and costs. Guidelines from the American Association of Clinical Endocrinologists/American College of Endocrinology (AACE/ACE)4 are also very useful, because they categorize treatment based on the A1C level, as well as potential for weight gain and hypoglycemia. For example, a patient with an A1C < 7.5% may be an appropriate candidate for monotherapy, while a symptomatic patient with an A1C > 9% would likely benefit from insulin therapy or triple oral agent therapy. First, it is helpful to set individual glycemic targets for your patient. The following factors can help you in determining A1C targets: • Psychosocial considerations (motivation, adherence to therapy, self-care capacity) • Resources or support systems (family support, community resources, living situation, etc) • Risk for hypoglycemia • Duration of diabetes • Life expectancy • Microvascular complications • Cardiovascular disease and coexisting conditions. For example, an older individual with poor motivation, lack of support systems, short life expectancy, and coexisting terminal cancer would have a less stringent A1C target of ≤ 8%, whereas a young, motivated individual with no complications or serious coexisting complications would have an A1C target of 6%. The new ADA/EASD guidelines list additional considerations for medication choices for various comorbidities, including coronary disease, heart failure, renal disease, liver dysfunction, and hypoglycemia. For each comorbidity listed, there are suggested medications that are preferred and those that should be avoided. If your goal is to avoid hypoglycemia, the ADA/EASD guidelines list medication choices that have low propensity to cause hypoglycemia (eg, metformin, pioglitazone, DPP-4 inhibitors, and GLP-1 receptor agonists). (Of note, special attention is given to medications that do not cause weight gain, such as GLP-1 receptor agonists, DPP-4 inhibitors, and metformin.) Finally, the consensus statement emphasizes the need for individualizing therapy. Many patients have multiple comorbidities and may have medication sensitivities, cost constraints, etc. All of these factors must be taken into consideration when making therapeutic choices. Keep in mind, “one size does not fit all” when it comes to diabetes therapy. The recent releases from both the ADA/EASD and AACE/ACE give us much more detailed guidance addressing medication choices in regard to efficacy, potential for hypoglycemia and weight gain, major side effects, and costs. As always, guidelines do not replace good clinical judgment, based on the patient sitting in front of you. REFERENCES 2. Pimazoni-Netto A, Rodbard D, Zanella MT; Diabetes Education and Control Group. Rapid improvement of glycemic control in type 2 diabetes using weekly intensive multifactorial interventions: structured glucose monitoring, patient education, and adjustment of therapy—a randomized controlled trial. Diabetes Technol Therapeutics. 2011;13(10):997-1004. 3. Inzucchi SE, Bergenstahl RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient centered approach. Diabetes Care. [Epub ahead of print; April 19, 2012]. 4. Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract. 2009;15(6):540-559. |
Q: I am frustrated by the “always bring the blood sugars down slowly” philosophy, which I know is intended to avoid hypoglycemic symptoms. However, it often seems to be done at the expense of prolonged hyperglycemia, which is dangerous for patients’ long-term health and may cause more rapid beta-cell destruction. What’s the deal? There is evidence that rapid achievement of tight glucose control using intensive insulin therapy with multiple daily injections or insulin pumps in patients with newly diagnosed type 2 diabetes has favorable outcomes on recovery and maintenance of beta-cell function and prolonged glycemic remission, compared with treatment with oral hypoglycemic agents.1 However, this approach is time consuming and not practical in most primary care settings. Overcoming “clinical inertia” (the failure to initiate or intensify therapy when indicated) has been identified as a major barrier to achieving rapid glycemic control, to the detriment of the patient’s health. One recent study showed that more frequent follow-up with a multidisciplinary team and regular use of a computer-analyzed 7-point glucose profile resulted in more rapid and significantly better glycemic control with a lower A1C, compared to standard care.2 This approach is much more practical in a primary care setting. Additionally, we always treat our patients as individuals. There are very few maxims that are correct in all situations. Almost every answer to a clinical question begins with the qualifier “It depends….” The specifics of the individual case will clarify the appropriate answer. In regard to this particular question, the answer will vary by the clinical history of the patient. For example, for a pregnant patient with poor glycemic control, potential hospitalization and rapid titration of insulin would be the most judicious plan. In this case, quickly bringing glucose into tight control helps minimize risks to the developing fetus. However, if the patient is a frail 80-year-old with advanced cardiovascular disease, then slow and careful titration of medications would be the prudent course to meticulously avoid hypoglycemia. New guidelines from the American Diabetes Association and the European Association for the Study of Diabetes (ADA/EASD)3 are helpful in that they identify various clinical issues and give guidance on which medication regimens would be more appropriate for the specific clinical history. They categorize medications based on efficacy, weight gain, hypoglycemia, major side effects, and costs. Guidelines from the American Association of Clinical Endocrinologists/American College of Endocrinology (AACE/ACE)4 are also very useful, because they categorize treatment based on the A1C level, as well as potential for weight gain and hypoglycemia. For example, a patient with an A1C < 7.5% may be an appropriate candidate for monotherapy, while a symptomatic patient with an A1C > 9% would likely benefit from insulin therapy or triple oral agent therapy. First, it is helpful to set individual glycemic targets for your patient. The following factors can help you in determining A1C targets: • Psychosocial considerations (motivation, adherence to therapy, self-care capacity) • Resources or support systems (family support, community resources, living situation, etc) • Risk for hypoglycemia • Duration of diabetes • Life expectancy • Microvascular complications • Cardiovascular disease and coexisting conditions. For example, an older individual with poor motivation, lack of support systems, short life expectancy, and coexisting terminal cancer would have a less stringent A1C target of ≤ 8%, whereas a young, motivated individual with no complications or serious coexisting complications would have an A1C target of 6%. The new ADA/EASD guidelines list additional considerations for medication choices for various comorbidities, including coronary disease, heart failure, renal disease, liver dysfunction, and hypoglycemia. For each comorbidity listed, there are suggested medications that are preferred and those that should be avoided. If your goal is to avoid hypoglycemia, the ADA/EASD guidelines list medication choices that have low propensity to cause hypoglycemia (eg, metformin, pioglitazone, DPP-4 inhibitors, and GLP-1 receptor agonists). (Of note, special attention is given to medications that do not cause weight gain, such as GLP-1 receptor agonists, DPP-4 inhibitors, and metformin.) Finally, the consensus statement emphasizes the need for individualizing therapy. Many patients have multiple comorbidities and may have medication sensitivities, cost constraints, etc. All of these factors must be taken into consideration when making therapeutic choices. Keep in mind, “one size does not fit all” when it comes to diabetes therapy. The recent releases from both the ADA/EASD and AACE/ACE give us much more detailed guidance addressing medication choices in regard to efficacy, potential for hypoglycemia and weight gain, major side effects, and costs. As always, guidelines do not replace good clinical judgment, based on the patient sitting in front of you. REFERENCES 2. Pimazoni-Netto A, Rodbard D, Zanella MT; Diabetes Education and Control Group. Rapid improvement of glycemic control in type 2 diabetes using weekly intensive multifactorial interventions: structured glucose monitoring, patient education, and adjustment of therapy—a randomized controlled trial. Diabetes Technol Therapeutics. 2011;13(10):997-1004. 3. Inzucchi SE, Bergenstahl RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient centered approach. Diabetes Care. [Epub ahead of print; April 19, 2012]. 4. Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract. 2009;15(6):540-559. |