User login
Applying Education Theory to Vascular Training
Citing a revolution in the way surgeons learn their craft, Dr. Erica L. Mitchell and Dr. Sonal Arora present an analysis of vascular training to identify key learning points and needs as residents move from novice to expert. Their report is in the August issue of the Journal of Vascular Surgery.
A shift toward competency-based training programs is now reflecting a growing emphasis on outcomes-based medical education, according to Dr. Mitchell and Dr. Arora. They discuss how pedagogy and adult learning tools can be applied to vascular training and the development of technical expertise (J Vasc Surg 2012;56:530-7).
"Surgical educators should use training and assessment methods soundly based in educational principles to develop and deliver curricula that will allow trainees to acquire the skills befitting the modern vascular surgeon," they concluded.
Find the original article by clicking here.
Citing a revolution in the way surgeons learn their craft, Dr. Erica L. Mitchell and Dr. Sonal Arora present an analysis of vascular training to identify key learning points and needs as residents move from novice to expert. Their report is in the August issue of the Journal of Vascular Surgery.
A shift toward competency-based training programs is now reflecting a growing emphasis on outcomes-based medical education, according to Dr. Mitchell and Dr. Arora. They discuss how pedagogy and adult learning tools can be applied to vascular training and the development of technical expertise (J Vasc Surg 2012;56:530-7).
"Surgical educators should use training and assessment methods soundly based in educational principles to develop and deliver curricula that will allow trainees to acquire the skills befitting the modern vascular surgeon," they concluded.
Find the original article by clicking here.
Citing a revolution in the way surgeons learn their craft, Dr. Erica L. Mitchell and Dr. Sonal Arora present an analysis of vascular training to identify key learning points and needs as residents move from novice to expert. Their report is in the August issue of the Journal of Vascular Surgery.
A shift toward competency-based training programs is now reflecting a growing emphasis on outcomes-based medical education, according to Dr. Mitchell and Dr. Arora. They discuss how pedagogy and adult learning tools can be applied to vascular training and the development of technical expertise (J Vasc Surg 2012;56:530-7).
"Surgical educators should use training and assessment methods soundly based in educational principles to develop and deliver curricula that will allow trainees to acquire the skills befitting the modern vascular surgeon," they concluded.
Find the original article by clicking here.
FROM THE JOURNAL OF VASCULAR SURGERY
Labs Find Evidence of Cancer Stem Cells
In an era of targeted cancer therapies, laboratory scientists working with mice may have found the ultimate target – a reservoir of stem cells that drive cancers to grow and metastasize.
Separate reports in the journals Science and Nature document the presence of cancer stem cells in intestinal adenomas (Science 2012 Aug. 1 [doi:10.1126/science.1224676]), squamous skin cancer, (Nature 2012 Aug. 1 [doi:10.1038/nature11344]), and glioblastoma multiforme (Nature 2012 Aug. 1 [doi:10.1038/nature11287]).
In the last study, mice with these highly lethal brain tumors were given temozolomide (Temodar), an approved treatment in humans, along with ganciclovir, an antiviral. Despite a transient therapeutic response to chemotherapy, the cancers continued to grow, driven by "a relatively quiescent subset of endogenous glioma cells, with properties similar to those proposed for cancer stem cells," the authors wrote.
Whether these reports will resolve controversy over the existence of stem cells or lead to clinically meaningful treatments remains to be seen. There is no doubt, however, that they will lead to further investigation.
In an era of targeted cancer therapies, laboratory scientists working with mice may have found the ultimate target – a reservoir of stem cells that drive cancers to grow and metastasize.
Separate reports in the journals Science and Nature document the presence of cancer stem cells in intestinal adenomas (Science 2012 Aug. 1 [doi:10.1126/science.1224676]), squamous skin cancer, (Nature 2012 Aug. 1 [doi:10.1038/nature11344]), and glioblastoma multiforme (Nature 2012 Aug. 1 [doi:10.1038/nature11287]).
In the last study, mice with these highly lethal brain tumors were given temozolomide (Temodar), an approved treatment in humans, along with ganciclovir, an antiviral. Despite a transient therapeutic response to chemotherapy, the cancers continued to grow, driven by "a relatively quiescent subset of endogenous glioma cells, with properties similar to those proposed for cancer stem cells," the authors wrote.
Whether these reports will resolve controversy over the existence of stem cells or lead to clinically meaningful treatments remains to be seen. There is no doubt, however, that they will lead to further investigation.
In an era of targeted cancer therapies, laboratory scientists working with mice may have found the ultimate target – a reservoir of stem cells that drive cancers to grow and metastasize.
Separate reports in the journals Science and Nature document the presence of cancer stem cells in intestinal adenomas (Science 2012 Aug. 1 [doi:10.1126/science.1224676]), squamous skin cancer, (Nature 2012 Aug. 1 [doi:10.1038/nature11344]), and glioblastoma multiforme (Nature 2012 Aug. 1 [doi:10.1038/nature11287]).
In the last study, mice with these highly lethal brain tumors were given temozolomide (Temodar), an approved treatment in humans, along with ganciclovir, an antiviral. Despite a transient therapeutic response to chemotherapy, the cancers continued to grow, driven by "a relatively quiescent subset of endogenous glioma cells, with properties similar to those proposed for cancer stem cells," the authors wrote.
Whether these reports will resolve controversy over the existence of stem cells or lead to clinically meaningful treatments remains to be seen. There is no doubt, however, that they will lead to further investigation.
Drug-Drug Interactions Added to Hepatitis C Drug Label
New information about interactions between boceprevir and several other drugs has been added to the prescribing information for the antiviral drug, the Food and Drug Administration announced Aug. 1.
Boceprevir (Victrelis), a protease inhibitor approved for treating hepatitis C in 2011, interacts with cyclosporine, tacrolimus (Prograf), escitalopram (Lexapro), atorvastatin (Lipitor), and pravastatin (Pravachol), according to the FDA statement.
The new information states that, when administered with boceprevir, exposure to atorvastatin increases. When the two drugs are used together, the lowest effective dose of atorvastatin should be used, not to exceed a daily dose of 40 mg, according to the FDA.
Dose adjustments of cyclosporine should be anticipated when it is given with boceprevir, and "should be guided by close monitoring of cyclosporine blood concentrations, and frequent assessments of renal function and cyclosporine-related side effects."
When administered with boceprevir, exposure of escitalopram "was slightly decreased," the statement said. Although selective serotonin reuptake inhibitors (SSRIs) such as escitalopram have a wide therapeutic index, it may be necessary to adjust the dosage when it is administered with boceprevir.
Coadministration of boceprevir with pravastatin increases exposure to pravastatin, but pravastatin can be started at the recommended dosage when coadministered with boceprevir. "Close clinical monitoring is warranted," the statement said.
Giving tacrolimus and boceprevir together "requires significant dose reduction and prolongation of the dosing interval for tacrolimus, with close monitoring of tacrolimus blood concentrations and frequent assessments of renal function and tacrolimus-related side effects," the statement said.
Boceprevir is manufactured in a capsule formulation by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., and is taken by mouth three times a day.
The drug-drug interaction data are from in vivo drug interaction trials, which the company conducted as part of its postmarketing commitments.
At a meeting in April 2011, an FDA advisory panel enthusiastically supported the approval of boceprevir for treating hepatitis C infection because of the antiviral’s efficacy but emphasized that postmarketing studies on interactions with other drugs, including antidepressants, were needed.
Serious adverse events associated with boceprevir should be reported to MedWatch or by phone at 800-332-1088.
New information about interactions between boceprevir and several other drugs has been added to the prescribing information for the antiviral drug, the Food and Drug Administration announced Aug. 1.
Boceprevir (Victrelis), a protease inhibitor approved for treating hepatitis C in 2011, interacts with cyclosporine, tacrolimus (Prograf), escitalopram (Lexapro), atorvastatin (Lipitor), and pravastatin (Pravachol), according to the FDA statement.
The new information states that, when administered with boceprevir, exposure to atorvastatin increases. When the two drugs are used together, the lowest effective dose of atorvastatin should be used, not to exceed a daily dose of 40 mg, according to the FDA.
Dose adjustments of cyclosporine should be anticipated when it is given with boceprevir, and "should be guided by close monitoring of cyclosporine blood concentrations, and frequent assessments of renal function and cyclosporine-related side effects."
When administered with boceprevir, exposure of escitalopram "was slightly decreased," the statement said. Although selective serotonin reuptake inhibitors (SSRIs) such as escitalopram have a wide therapeutic index, it may be necessary to adjust the dosage when it is administered with boceprevir.
Coadministration of boceprevir with pravastatin increases exposure to pravastatin, but pravastatin can be started at the recommended dosage when coadministered with boceprevir. "Close clinical monitoring is warranted," the statement said.
Giving tacrolimus and boceprevir together "requires significant dose reduction and prolongation of the dosing interval for tacrolimus, with close monitoring of tacrolimus blood concentrations and frequent assessments of renal function and tacrolimus-related side effects," the statement said.
Boceprevir is manufactured in a capsule formulation by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., and is taken by mouth three times a day.
The drug-drug interaction data are from in vivo drug interaction trials, which the company conducted as part of its postmarketing commitments.
At a meeting in April 2011, an FDA advisory panel enthusiastically supported the approval of boceprevir for treating hepatitis C infection because of the antiviral’s efficacy but emphasized that postmarketing studies on interactions with other drugs, including antidepressants, were needed.
Serious adverse events associated with boceprevir should be reported to MedWatch or by phone at 800-332-1088.
New information about interactions between boceprevir and several other drugs has been added to the prescribing information for the antiviral drug, the Food and Drug Administration announced Aug. 1.
Boceprevir (Victrelis), a protease inhibitor approved for treating hepatitis C in 2011, interacts with cyclosporine, tacrolimus (Prograf), escitalopram (Lexapro), atorvastatin (Lipitor), and pravastatin (Pravachol), according to the FDA statement.
The new information states that, when administered with boceprevir, exposure to atorvastatin increases. When the two drugs are used together, the lowest effective dose of atorvastatin should be used, not to exceed a daily dose of 40 mg, according to the FDA.
Dose adjustments of cyclosporine should be anticipated when it is given with boceprevir, and "should be guided by close monitoring of cyclosporine blood concentrations, and frequent assessments of renal function and cyclosporine-related side effects."
When administered with boceprevir, exposure of escitalopram "was slightly decreased," the statement said. Although selective serotonin reuptake inhibitors (SSRIs) such as escitalopram have a wide therapeutic index, it may be necessary to adjust the dosage when it is administered with boceprevir.
Coadministration of boceprevir with pravastatin increases exposure to pravastatin, but pravastatin can be started at the recommended dosage when coadministered with boceprevir. "Close clinical monitoring is warranted," the statement said.
Giving tacrolimus and boceprevir together "requires significant dose reduction and prolongation of the dosing interval for tacrolimus, with close monitoring of tacrolimus blood concentrations and frequent assessments of renal function and tacrolimus-related side effects," the statement said.
Boceprevir is manufactured in a capsule formulation by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., and is taken by mouth three times a day.
The drug-drug interaction data are from in vivo drug interaction trials, which the company conducted as part of its postmarketing commitments.
At a meeting in April 2011, an FDA advisory panel enthusiastically supported the approval of boceprevir for treating hepatitis C infection because of the antiviral’s efficacy but emphasized that postmarketing studies on interactions with other drugs, including antidepressants, were needed.
Serious adverse events associated with boceprevir should be reported to MedWatch or by phone at 800-332-1088.
Report: Pharmacist-Led Interventions Don’t Reduce Medication Errors Post-Discharge
At first blush, some hospitalists might see it as bad news that a recent report found a pharmacist-assisted medication reconciliation ("med rec") intervention did not significantly reduce clinically important medication errors after discharge. But a deeper reading of the study tells a different story, says a hospitalist who worked on the report.
"This is the latest in our growing understanding of the roles of certain interventions on transitions of care," says Jeffrey Schnipper, MD, MPH, FHM, director of clinical research and an associate physician in the general medicine division at Brigham and Women's Hospitalist Service in Boston, and co-author of the study "Effect of a Pharmacist Intervention on Clinically Important Medication Errors after Hospital Discharge." "What I don't want to have happen is for people to read this article ... and say, 'Oh, pharmacists don't make a difference.' They absolutely make a difference. This is a more nuanced issue of who do they have the biggest impact with, and 'On top of what other interventions are you doing this?'"
The researchers set out to determine whether a pharmacist-delivered intervention on patients with low health literacy (including a post-discharge telephone call) would lower adverse drug events and other clinically important medication errors. They concluded that it did not (unadjusted incidence rate ratio, 0.92 [95% CI, 0.77 to 1.10]).
Dr. Schnipper says the impact was likely muted because the patients studied had higher health-literacy levels than researchers expected. Also, because most follow-up phone calls occurred within a few days of discharge, the intervention failed to capture any events that happened in the 30 days after discharge.
He also notes that the institutions that participated in the study have already implemented multiple med-rec interventions over the past few years. Hospitals that have not focused intently on the issue could find much larger gains from implementing pharmacist-led programs.
"If you're a hospital that has not been fixated on improving medication safety and transitions of care, I think pharmacists are huge," Dr. Schnipper says. "The key, then, is to focus them on the highest-risk patients."
At first blush, some hospitalists might see it as bad news that a recent report found a pharmacist-assisted medication reconciliation ("med rec") intervention did not significantly reduce clinically important medication errors after discharge. But a deeper reading of the study tells a different story, says a hospitalist who worked on the report.
"This is the latest in our growing understanding of the roles of certain interventions on transitions of care," says Jeffrey Schnipper, MD, MPH, FHM, director of clinical research and an associate physician in the general medicine division at Brigham and Women's Hospitalist Service in Boston, and co-author of the study "Effect of a Pharmacist Intervention on Clinically Important Medication Errors after Hospital Discharge." "What I don't want to have happen is for people to read this article ... and say, 'Oh, pharmacists don't make a difference.' They absolutely make a difference. This is a more nuanced issue of who do they have the biggest impact with, and 'On top of what other interventions are you doing this?'"
The researchers set out to determine whether a pharmacist-delivered intervention on patients with low health literacy (including a post-discharge telephone call) would lower adverse drug events and other clinically important medication errors. They concluded that it did not (unadjusted incidence rate ratio, 0.92 [95% CI, 0.77 to 1.10]).
Dr. Schnipper says the impact was likely muted because the patients studied had higher health-literacy levels than researchers expected. Also, because most follow-up phone calls occurred within a few days of discharge, the intervention failed to capture any events that happened in the 30 days after discharge.
He also notes that the institutions that participated in the study have already implemented multiple med-rec interventions over the past few years. Hospitals that have not focused intently on the issue could find much larger gains from implementing pharmacist-led programs.
"If you're a hospital that has not been fixated on improving medication safety and transitions of care, I think pharmacists are huge," Dr. Schnipper says. "The key, then, is to focus them on the highest-risk patients."
At first blush, some hospitalists might see it as bad news that a recent report found a pharmacist-assisted medication reconciliation ("med rec") intervention did not significantly reduce clinically important medication errors after discharge. But a deeper reading of the study tells a different story, says a hospitalist who worked on the report.
"This is the latest in our growing understanding of the roles of certain interventions on transitions of care," says Jeffrey Schnipper, MD, MPH, FHM, director of clinical research and an associate physician in the general medicine division at Brigham and Women's Hospitalist Service in Boston, and co-author of the study "Effect of a Pharmacist Intervention on Clinically Important Medication Errors after Hospital Discharge." "What I don't want to have happen is for people to read this article ... and say, 'Oh, pharmacists don't make a difference.' They absolutely make a difference. This is a more nuanced issue of who do they have the biggest impact with, and 'On top of what other interventions are you doing this?'"
The researchers set out to determine whether a pharmacist-delivered intervention on patients with low health literacy (including a post-discharge telephone call) would lower adverse drug events and other clinically important medication errors. They concluded that it did not (unadjusted incidence rate ratio, 0.92 [95% CI, 0.77 to 1.10]).
Dr. Schnipper says the impact was likely muted because the patients studied had higher health-literacy levels than researchers expected. Also, because most follow-up phone calls occurred within a few days of discharge, the intervention failed to capture any events that happened in the 30 days after discharge.
He also notes that the institutions that participated in the study have already implemented multiple med-rec interventions over the past few years. Hospitals that have not focused intently on the issue could find much larger gains from implementing pharmacist-led programs.
"If you're a hospital that has not been fixated on improving medication safety and transitions of care, I think pharmacists are huge," Dr. Schnipper says. "The key, then, is to focus them on the highest-risk patients."
Insurers Promote Collaborative Approach to 30-Day Readmission Reductions
Although Medicare's looming financial penalties for hospitals with excessive readmissions might seem like a blunt weapon, private health plans often have the flexibility to negotiate with partnering hospitals around incentives for readmissions prevention.
"We have arrangements with private insurance companies where we put at risk future compensation, based on achieving negotiated readmissions results," says Mark Carley, vice president of managed care and network development for Centura Health, a 13-hospital system in Colorado.
Payors, including United Healthcare, have developed their own readmissions programs and reporting mechanisms, although each program’s incentives are a little different, Carley says. Target rates are negotiated based on each hospital's readmissions in the previous 12-month period and national averages. The plan can also provide helpful data on its beneficiaries and other forms of assistance, because it wants to see the hospital hit the target, he adds. "If the target has been set too high, they may be willing to renegotiate."
But the plan doesn't tell the hospital how to reach that target.
"Where the complexity comes in is how we as a system implement internal policies and procedures to improve our care coordination, discharge processes, follow-up, and communication with downstream providers," says Carley. Centura Health's approach to readmissions has included close study of past performance data in search of opportunities for improvement, fine-tuning of the discharge planning process, and follow-up phone calls to patients and providers.
"In addition, we are working with post-acute providers to provide smoother transitions in the discharge process," he says.
Although Medicare's looming financial penalties for hospitals with excessive readmissions might seem like a blunt weapon, private health plans often have the flexibility to negotiate with partnering hospitals around incentives for readmissions prevention.
"We have arrangements with private insurance companies where we put at risk future compensation, based on achieving negotiated readmissions results," says Mark Carley, vice president of managed care and network development for Centura Health, a 13-hospital system in Colorado.
Payors, including United Healthcare, have developed their own readmissions programs and reporting mechanisms, although each program’s incentives are a little different, Carley says. Target rates are negotiated based on each hospital's readmissions in the previous 12-month period and national averages. The plan can also provide helpful data on its beneficiaries and other forms of assistance, because it wants to see the hospital hit the target, he adds. "If the target has been set too high, they may be willing to renegotiate."
But the plan doesn't tell the hospital how to reach that target.
"Where the complexity comes in is how we as a system implement internal policies and procedures to improve our care coordination, discharge processes, follow-up, and communication with downstream providers," says Carley. Centura Health's approach to readmissions has included close study of past performance data in search of opportunities for improvement, fine-tuning of the discharge planning process, and follow-up phone calls to patients and providers.
"In addition, we are working with post-acute providers to provide smoother transitions in the discharge process," he says.
Although Medicare's looming financial penalties for hospitals with excessive readmissions might seem like a blunt weapon, private health plans often have the flexibility to negotiate with partnering hospitals around incentives for readmissions prevention.
"We have arrangements with private insurance companies where we put at risk future compensation, based on achieving negotiated readmissions results," says Mark Carley, vice president of managed care and network development for Centura Health, a 13-hospital system in Colorado.
Payors, including United Healthcare, have developed their own readmissions programs and reporting mechanisms, although each program’s incentives are a little different, Carley says. Target rates are negotiated based on each hospital's readmissions in the previous 12-month period and national averages. The plan can also provide helpful data on its beneficiaries and other forms of assistance, because it wants to see the hospital hit the target, he adds. "If the target has been set too high, they may be willing to renegotiate."
But the plan doesn't tell the hospital how to reach that target.
"Where the complexity comes in is how we as a system implement internal policies and procedures to improve our care coordination, discharge processes, follow-up, and communication with downstream providers," says Carley. Centura Health's approach to readmissions has included close study of past performance data in search of opportunities for improvement, fine-tuning of the discharge planning process, and follow-up phone calls to patients and providers.
"In addition, we are working with post-acute providers to provide smoother transitions in the discharge process," he says.
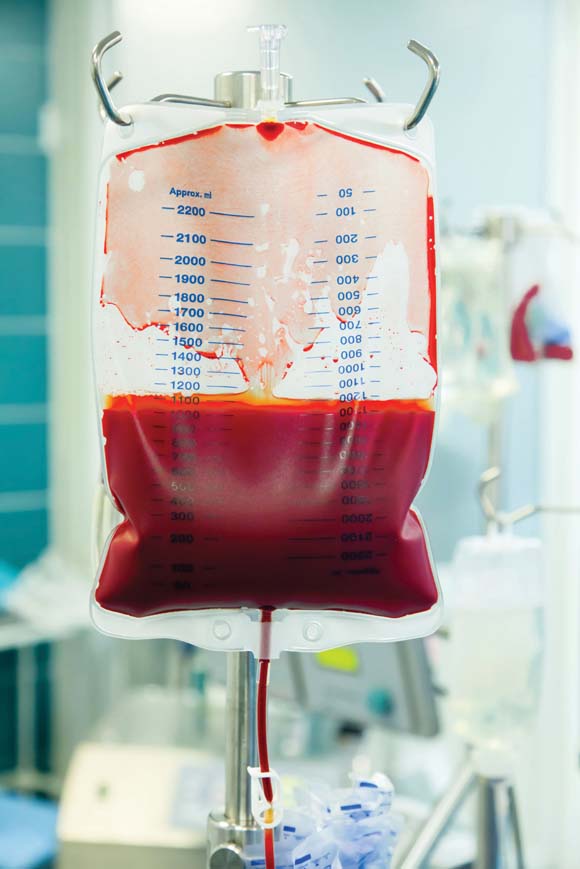
Transfusion Rates Vary Widely at Academic Hospitals
Wide variations in perioperative blood transfusion rates among patients undergoing major noncardiac procedures across U.S. hospitals highlight the need to further investigate evidence-based "transfusion triggers" in this population of surgical patients, according to a study published ahead of print in Annals of Surgery.
"In light of the increased risk of mortality and major complications associated with blood transfusion, the extensive variability in hospital transfusion practice in noncardiac surgery may represent an important opportunity to improve surgical outcomes," wrote Feng Qian, Ph.D., of the University of Rochester (N.Y.), and associates.
The researchers used the University HealthSystem Consortium hospital database to compare transfusion rates of allogeneic red blood cells, fresh frozen plasma, and platelets in patients undergoing elective primary total hip replacement (54,405 patients), colectomy (21,334), or pancreaticoduodenectomy (7,929) at 77 hospitals between June 2006 and September 2010. Most of the hospitals were teaching hospitals with at least 500 beds.
Transfusion rates varied widely before and after adjustment for comorbidities and other patient risk factors. Patients who were treated in hospitals with high rates of transfusions were about twice as likely to receive a blood transfusion as were patients at hospitals with average transfusion rates (Ann. Surg. 2012 July 13[doi:10.1097/SLA.0b013e31825ffc37]).
In hospitals where the transfusion rate for one procedure was high, transfusion rates also tended to be high for the other two procedures. There was some evidence indicating that a higher volume of surgical cases was associated with lower transfusion rates.
After adjusting for patient risk factors, the authors determined that transfusion rates for the different blood components among those undergoing a total hip replacement ranged from 1.3% to almost 75% (red blood cells), from 0.1% to 7.7% (fresh frozen plasma), and from 0.1% to 2% (platelets). Among colectomy patients, transfusion rates ranged from 1.9% to 47.8% (RBCs), from 1.4% to 17.7% (fresh frozen plasma), and from 1.3% to 6.2% (platelets). Among those undergoing a pancreaticoduodenectomy, the rates ranged from 3% to 78.6% (RBCs), from 1% to 47% (fresh frozen plasma), and from 1.4% to 12.6% (platelets).
The variability, the authors said, "reflects, in part, the complexity of the medical decision-making process underlying transfusion therapy." Because the data included patients from 90% of academic medical centers in the United States, the results provide "a broad and contemporary picture of transfusion practices in academic surgical centers" and "reflect transfusion practices that are being taught to the next generation of academic and private-practice clinicians during residency training," they noted.
To the best of their knowledge, the authors said, there are no large randomized studies that have compared liberal and restrictive transfusion strategies in noncardiac surgery patients, and they believe that such trials are "urgently needed to better define evidence-based transfusion triggers for patients undergoing noncardiac surgery."
The study was supported by a grant from the Agency for Healthcare and Quality Research and funding from the department of anesthesiology at the University of Rochester. No disclosures were reported by the authors.
Wide variations in perioperative blood transfusion rates among patients undergoing major noncardiac procedures across U.S. hospitals highlight the need to further investigate evidence-based "transfusion triggers" in this population of surgical patients, according to a study published ahead of print in Annals of Surgery.
"In light of the increased risk of mortality and major complications associated with blood transfusion, the extensive variability in hospital transfusion practice in noncardiac surgery may represent an important opportunity to improve surgical outcomes," wrote Feng Qian, Ph.D., of the University of Rochester (N.Y.), and associates.
The researchers used the University HealthSystem Consortium hospital database to compare transfusion rates of allogeneic red blood cells, fresh frozen plasma, and platelets in patients undergoing elective primary total hip replacement (54,405 patients), colectomy (21,334), or pancreaticoduodenectomy (7,929) at 77 hospitals between June 2006 and September 2010. Most of the hospitals were teaching hospitals with at least 500 beds.
Transfusion rates varied widely before and after adjustment for comorbidities and other patient risk factors. Patients who were treated in hospitals with high rates of transfusions were about twice as likely to receive a blood transfusion as were patients at hospitals with average transfusion rates (Ann. Surg. 2012 July 13[doi:10.1097/SLA.0b013e31825ffc37]).
In hospitals where the transfusion rate for one procedure was high, transfusion rates also tended to be high for the other two procedures. There was some evidence indicating that a higher volume of surgical cases was associated with lower transfusion rates.
After adjusting for patient risk factors, the authors determined that transfusion rates for the different blood components among those undergoing a total hip replacement ranged from 1.3% to almost 75% (red blood cells), from 0.1% to 7.7% (fresh frozen plasma), and from 0.1% to 2% (platelets). Among colectomy patients, transfusion rates ranged from 1.9% to 47.8% (RBCs), from 1.4% to 17.7% (fresh frozen plasma), and from 1.3% to 6.2% (platelets). Among those undergoing a pancreaticoduodenectomy, the rates ranged from 3% to 78.6% (RBCs), from 1% to 47% (fresh frozen plasma), and from 1.4% to 12.6% (platelets).
The variability, the authors said, "reflects, in part, the complexity of the medical decision-making process underlying transfusion therapy." Because the data included patients from 90% of academic medical centers in the United States, the results provide "a broad and contemporary picture of transfusion practices in academic surgical centers" and "reflect transfusion practices that are being taught to the next generation of academic and private-practice clinicians during residency training," they noted.
To the best of their knowledge, the authors said, there are no large randomized studies that have compared liberal and restrictive transfusion strategies in noncardiac surgery patients, and they believe that such trials are "urgently needed to better define evidence-based transfusion triggers for patients undergoing noncardiac surgery."
The study was supported by a grant from the Agency for Healthcare and Quality Research and funding from the department of anesthesiology at the University of Rochester. No disclosures were reported by the authors.
Wide variations in perioperative blood transfusion rates among patients undergoing major noncardiac procedures across U.S. hospitals highlight the need to further investigate evidence-based "transfusion triggers" in this population of surgical patients, according to a study published ahead of print in Annals of Surgery.
"In light of the increased risk of mortality and major complications associated with blood transfusion, the extensive variability in hospital transfusion practice in noncardiac surgery may represent an important opportunity to improve surgical outcomes," wrote Feng Qian, Ph.D., of the University of Rochester (N.Y.), and associates.
The researchers used the University HealthSystem Consortium hospital database to compare transfusion rates of allogeneic red blood cells, fresh frozen plasma, and platelets in patients undergoing elective primary total hip replacement (54,405 patients), colectomy (21,334), or pancreaticoduodenectomy (7,929) at 77 hospitals between June 2006 and September 2010. Most of the hospitals were teaching hospitals with at least 500 beds.
Transfusion rates varied widely before and after adjustment for comorbidities and other patient risk factors. Patients who were treated in hospitals with high rates of transfusions were about twice as likely to receive a blood transfusion as were patients at hospitals with average transfusion rates (Ann. Surg. 2012 July 13[doi:10.1097/SLA.0b013e31825ffc37]).
In hospitals where the transfusion rate for one procedure was high, transfusion rates also tended to be high for the other two procedures. There was some evidence indicating that a higher volume of surgical cases was associated with lower transfusion rates.
After adjusting for patient risk factors, the authors determined that transfusion rates for the different blood components among those undergoing a total hip replacement ranged from 1.3% to almost 75% (red blood cells), from 0.1% to 7.7% (fresh frozen plasma), and from 0.1% to 2% (platelets). Among colectomy patients, transfusion rates ranged from 1.9% to 47.8% (RBCs), from 1.4% to 17.7% (fresh frozen plasma), and from 1.3% to 6.2% (platelets). Among those undergoing a pancreaticoduodenectomy, the rates ranged from 3% to 78.6% (RBCs), from 1% to 47% (fresh frozen plasma), and from 1.4% to 12.6% (platelets).
The variability, the authors said, "reflects, in part, the complexity of the medical decision-making process underlying transfusion therapy." Because the data included patients from 90% of academic medical centers in the United States, the results provide "a broad and contemporary picture of transfusion practices in academic surgical centers" and "reflect transfusion practices that are being taught to the next generation of academic and private-practice clinicians during residency training," they noted.
To the best of their knowledge, the authors said, there are no large randomized studies that have compared liberal and restrictive transfusion strategies in noncardiac surgery patients, and they believe that such trials are "urgently needed to better define evidence-based transfusion triggers for patients undergoing noncardiac surgery."
The study was supported by a grant from the Agency for Healthcare and Quality Research and funding from the department of anesthesiology at the University of Rochester. No disclosures were reported by the authors.
FROM THE ANNALS OF SURGERY
Major Finding: Transfusion rates of red blood cells, fresh frozen plasma, and platelets among patients undergoing noncardiac procedures varied widely across different U.S. academic-affiliated hospitals.
Data Source: Data from a national database of academic medical centers were used to compare transfusions in patients undergoing one of three elective noncardiac surgical procedures at 77 academic hospitals between June 2006 and September 2010.
Disclosures: The study was supported by a grant from the Agency for Healthcare and Quality Research and funding from the department of anesthesiology at the University of Rochester (N.Y.). The authors reported no disclosures.
Understanding PTSD
Getting to Goal: How Thiazide-Type Diuretics, Following the Guidelines, and Improving Patient Adherence Can Help
An estimated 1 of every 3 Americans has hypertension, putting them at an increased risk for cardiovascular disease, heart failure, stroke, and kidney disease. Despite the availability of effective medications to control high blood pressure, only half of the patients with hypertension under treatment are meeting their blood pressure goals. To address these gaps in the quality of care patients receive, this supplement will focus on the following topics in hypertension management: key clinical trials and their influence on sequencing algorithms; the differences between thiazide-type diuretics; the use of thiazide-type diuretics in African American patients; and strategies to improve patient adherence to hypertensive therapy.
Webcast—October 2012
An estimated 1 of every 3 Americans has hypertension, putting them at an increased risk for cardiovascular disease, heart failure, stroke, and kidney disease. Despite the availability of effective medications to control high blood pressure, only half of the patients with hypertension under treatment are meeting their blood pressure goals. To address these gaps in the quality of care patients receive, this supplement will focus on the following topics in hypertension management: key clinical trials and their influence on sequencing algorithms; the differences between thiazide-type diuretics; the use of thiazide-type diuretics in African American patients; and strategies to improve patient adherence to hypertensive therapy.
Webcast—October 2012
An estimated 1 of every 3 Americans has hypertension, putting them at an increased risk for cardiovascular disease, heart failure, stroke, and kidney disease. Despite the availability of effective medications to control high blood pressure, only half of the patients with hypertension under treatment are meeting their blood pressure goals. To address these gaps in the quality of care patients receive, this supplement will focus on the following topics in hypertension management: key clinical trials and their influence on sequencing algorithms; the differences between thiazide-type diuretics; the use of thiazide-type diuretics in African American patients; and strategies to improve patient adherence to hypertensive therapy.
Webcast—October 2012
Synthetic legal intoxicating drugs
To the Editor: I greatly appreciate the well-presented article by Drs. Jerry, Collins, and Streem in your April 2012 issue.1
As a specialist in integrative addiction medicine, I have had first-hand experience with many of the medical concerns described by the authors, and I expect to learn more about optimal management strategies as we learn more as a profession.
The lone case report cited in the article suggests a relatively short time to onset of seizure of 30 minutes following intentional ingestion of synthetic cannabinoids (JWH-018).2
In the residential treatment (“rehab”) setting where I work, I am seeing a latency to seizure onset of 24 to 72 hours with patients reporting use of synthetic cannabinoids.
Given this experience to date, I have two questions for the authors regarding new-onset seizures.
Are the authors aware of this trend in patients who present to non-emergency-department treatment settings such as residential treatment facilities? And in these cases, what if any recommendations would the authors make regarding seizure prophylaxis in patients with no history of seizure?
- Jerry J, Collins G, Streem D. Synthetic legal intoxicating drugs: the emerging ‘incense’ and ‘bath salt’ phenomenon. Cleve Clin J Med 2012; 79:258–264.
- Lapoint J, James LP, Moran CL, Nelson LS, Hoffman RS, Moran JH. Severe toxicity following synthetic cannabinoid ingestion. Clin Toxicol (Phila) 2011; 49:760–764.
To the Editor: I greatly appreciate the well-presented article by Drs. Jerry, Collins, and Streem in your April 2012 issue.1
As a specialist in integrative addiction medicine, I have had first-hand experience with many of the medical concerns described by the authors, and I expect to learn more about optimal management strategies as we learn more as a profession.
The lone case report cited in the article suggests a relatively short time to onset of seizure of 30 minutes following intentional ingestion of synthetic cannabinoids (JWH-018).2
In the residential treatment (“rehab”) setting where I work, I am seeing a latency to seizure onset of 24 to 72 hours with patients reporting use of synthetic cannabinoids.
Given this experience to date, I have two questions for the authors regarding new-onset seizures.
Are the authors aware of this trend in patients who present to non-emergency-department treatment settings such as residential treatment facilities? And in these cases, what if any recommendations would the authors make regarding seizure prophylaxis in patients with no history of seizure?
To the Editor: I greatly appreciate the well-presented article by Drs. Jerry, Collins, and Streem in your April 2012 issue.1
As a specialist in integrative addiction medicine, I have had first-hand experience with many of the medical concerns described by the authors, and I expect to learn more about optimal management strategies as we learn more as a profession.
The lone case report cited in the article suggests a relatively short time to onset of seizure of 30 minutes following intentional ingestion of synthetic cannabinoids (JWH-018).2
In the residential treatment (“rehab”) setting where I work, I am seeing a latency to seizure onset of 24 to 72 hours with patients reporting use of synthetic cannabinoids.
Given this experience to date, I have two questions for the authors regarding new-onset seizures.
Are the authors aware of this trend in patients who present to non-emergency-department treatment settings such as residential treatment facilities? And in these cases, what if any recommendations would the authors make regarding seizure prophylaxis in patients with no history of seizure?
- Jerry J, Collins G, Streem D. Synthetic legal intoxicating drugs: the emerging ‘incense’ and ‘bath salt’ phenomenon. Cleve Clin J Med 2012; 79:258–264.
- Lapoint J, James LP, Moran CL, Nelson LS, Hoffman RS, Moran JH. Severe toxicity following synthetic cannabinoid ingestion. Clin Toxicol (Phila) 2011; 49:760–764.
- Jerry J, Collins G, Streem D. Synthetic legal intoxicating drugs: the emerging ‘incense’ and ‘bath salt’ phenomenon. Cleve Clin J Med 2012; 79:258–264.
- Lapoint J, James LP, Moran CL, Nelson LS, Hoffman RS, Moran JH. Severe toxicity following synthetic cannabinoid ingestion. Clin Toxicol (Phila) 2011; 49:760–764.
In reply: Synthetic legal intoxicating drugs
In Reply: We thank Dr. Chandiramani for his thoughtful comments.
Only four cases of seizure-like activity associated with synthetic cannabinoids have been reported in the literature. In addition to the case reported in our paper,1 there was another in which a 19-year-old had two seizures soon after smoking a spice product, and the second seizure was witnessed by paramedics on the way to the hospital.2 Though this patient’s urine was not analyzed for synthetic cannabinoids, the spice product that was reportedly smoked by the patient was later sent to a laboratory for analysis and was found to contain four synthetic cannabinoids: JWH-018, JWH-081, JWH-250, and AM-2201.
In another case,3 seizure occurred after use of an incense product called “Spicy XXX,” but neither the incense sample nor the patient’s urine was tested for synthetic cannabinoids.
The final case reported in the literature involved a 25-year-old man who was brought to an emergency department by coworkers who had witnessed seizure-like activity.4 He was reported to have smoked an incense product about “45 minutes prior to presentation,”4 indicating that the seizure-like activity happened within that time frame. Two synthetic cannabinoids (JWH-018 and JWH-073) were detected in the patient’s urine.
In the case by Lapoint et al1 that we referred to in our paper,1 seizure activity recurred in the hospital and was successfully treated with lorazepam. The case reported by Schneir and Baumbacher2 described treatment of the second seizure with intranasal midazolam, with no recurrence of seizure activity.
In summary, the literature on seizure activity related to synthetic cannabinoids is sparse. When the time course has been documented in these few cases, seizures seem to occur “soon” after using these products,2 or from 45 minutes to 1 hour after use.1,4 Although benzodiazepines have been used to treat seizure activity, there have been no published reports of using medications to prevent seizures in individuals who have been using spice products. Furthermore, the routine employment of seizure prophylaxis of any kind would probably be premature at this point given the uncertainty of the actual seizure risk among all synthetic cannabinoid users. We would consider giving a benzodiazepine to prevent possible seizures after drug ingestion in cases in which prior seizures have occurred, in cases of extreme excitement or agitation, or in those with marked alterations of mental state.
- Lapoint J, James LP, Moran CL, Nelson LS, Hoffman RS, Moran JH. Severe toxicity following synthetic cannabinoid ingestion. Clin Toxicol (Phila) 2011; 49:760–764.
- Schneir AB, Baumbacher T. Convulsions associated with the use of a synthetic cannabinoid product. J Med Toxicol 2012; 8:62–64.
- Simmons JR, Skinner CG, Williams J, Kang CS, Schwartz MD, Wills BK. Intoxication from smoking “spice” (letter). Ann Emerg Med 2011; 57:187–188.
- Simmons J, Cookman L, Kang C, Skinner C. Three cases of ‘spice’ exposure. Clin Toxicol (Phila) 2011; 49:431–433.
In Reply: We thank Dr. Chandiramani for his thoughtful comments.
Only four cases of seizure-like activity associated with synthetic cannabinoids have been reported in the literature. In addition to the case reported in our paper,1 there was another in which a 19-year-old had two seizures soon after smoking a spice product, and the second seizure was witnessed by paramedics on the way to the hospital.2 Though this patient’s urine was not analyzed for synthetic cannabinoids, the spice product that was reportedly smoked by the patient was later sent to a laboratory for analysis and was found to contain four synthetic cannabinoids: JWH-018, JWH-081, JWH-250, and AM-2201.
In another case,3 seizure occurred after use of an incense product called “Spicy XXX,” but neither the incense sample nor the patient’s urine was tested for synthetic cannabinoids.
The final case reported in the literature involved a 25-year-old man who was brought to an emergency department by coworkers who had witnessed seizure-like activity.4 He was reported to have smoked an incense product about “45 minutes prior to presentation,”4 indicating that the seizure-like activity happened within that time frame. Two synthetic cannabinoids (JWH-018 and JWH-073) were detected in the patient’s urine.
In the case by Lapoint et al1 that we referred to in our paper,1 seizure activity recurred in the hospital and was successfully treated with lorazepam. The case reported by Schneir and Baumbacher2 described treatment of the second seizure with intranasal midazolam, with no recurrence of seizure activity.
In summary, the literature on seizure activity related to synthetic cannabinoids is sparse. When the time course has been documented in these few cases, seizures seem to occur “soon” after using these products,2 or from 45 minutes to 1 hour after use.1,4 Although benzodiazepines have been used to treat seizure activity, there have been no published reports of using medications to prevent seizures in individuals who have been using spice products. Furthermore, the routine employment of seizure prophylaxis of any kind would probably be premature at this point given the uncertainty of the actual seizure risk among all synthetic cannabinoid users. We would consider giving a benzodiazepine to prevent possible seizures after drug ingestion in cases in which prior seizures have occurred, in cases of extreme excitement or agitation, or in those with marked alterations of mental state.
In Reply: We thank Dr. Chandiramani for his thoughtful comments.
Only four cases of seizure-like activity associated with synthetic cannabinoids have been reported in the literature. In addition to the case reported in our paper,1 there was another in which a 19-year-old had two seizures soon after smoking a spice product, and the second seizure was witnessed by paramedics on the way to the hospital.2 Though this patient’s urine was not analyzed for synthetic cannabinoids, the spice product that was reportedly smoked by the patient was later sent to a laboratory for analysis and was found to contain four synthetic cannabinoids: JWH-018, JWH-081, JWH-250, and AM-2201.
In another case,3 seizure occurred after use of an incense product called “Spicy XXX,” but neither the incense sample nor the patient’s urine was tested for synthetic cannabinoids.
The final case reported in the literature involved a 25-year-old man who was brought to an emergency department by coworkers who had witnessed seizure-like activity.4 He was reported to have smoked an incense product about “45 minutes prior to presentation,”4 indicating that the seizure-like activity happened within that time frame. Two synthetic cannabinoids (JWH-018 and JWH-073) were detected in the patient’s urine.
In the case by Lapoint et al1 that we referred to in our paper,1 seizure activity recurred in the hospital and was successfully treated with lorazepam. The case reported by Schneir and Baumbacher2 described treatment of the second seizure with intranasal midazolam, with no recurrence of seizure activity.
In summary, the literature on seizure activity related to synthetic cannabinoids is sparse. When the time course has been documented in these few cases, seizures seem to occur “soon” after using these products,2 or from 45 minutes to 1 hour after use.1,4 Although benzodiazepines have been used to treat seizure activity, there have been no published reports of using medications to prevent seizures in individuals who have been using spice products. Furthermore, the routine employment of seizure prophylaxis of any kind would probably be premature at this point given the uncertainty of the actual seizure risk among all synthetic cannabinoid users. We would consider giving a benzodiazepine to prevent possible seizures after drug ingestion in cases in which prior seizures have occurred, in cases of extreme excitement or agitation, or in those with marked alterations of mental state.
- Lapoint J, James LP, Moran CL, Nelson LS, Hoffman RS, Moran JH. Severe toxicity following synthetic cannabinoid ingestion. Clin Toxicol (Phila) 2011; 49:760–764.
- Schneir AB, Baumbacher T. Convulsions associated with the use of a synthetic cannabinoid product. J Med Toxicol 2012; 8:62–64.
- Simmons JR, Skinner CG, Williams J, Kang CS, Schwartz MD, Wills BK. Intoxication from smoking “spice” (letter). Ann Emerg Med 2011; 57:187–188.
- Simmons J, Cookman L, Kang C, Skinner C. Three cases of ‘spice’ exposure. Clin Toxicol (Phila) 2011; 49:431–433.
- Lapoint J, James LP, Moran CL, Nelson LS, Hoffman RS, Moran JH. Severe toxicity following synthetic cannabinoid ingestion. Clin Toxicol (Phila) 2011; 49:760–764.
- Schneir AB, Baumbacher T. Convulsions associated with the use of a synthetic cannabinoid product. J Med Toxicol 2012; 8:62–64.
- Simmons JR, Skinner CG, Williams J, Kang CS, Schwartz MD, Wills BK. Intoxication from smoking “spice” (letter). Ann Emerg Med 2011; 57:187–188.
- Simmons J, Cookman L, Kang C, Skinner C. Three cases of ‘spice’ exposure. Clin Toxicol (Phila) 2011; 49:431–433.