User login
Duodenal Switch May Excel at Type 2 Diabetes Resolution
MADISON, WIS. – Total complication rates are high but comparable over the long term between duodenal switch surgery and Roux-en-Y gastric bypass, according to a propensity matched analysis of 309 superobese patients.
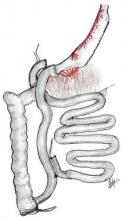
"Duodenal switch is a valid alternative to the Roux-en-Y gastric bypass, especially if significant comorbid illnesses are present, particularly diabetes," Dr. Robert B. Dorman said.
His conclusion is drawn from a study that focused on the long-term outcomes of 178 consecutive patients who underwent duodenal switch (DS) surgery and 139 propensity matched patients undergoing Roux-en-Y gastric bypass (RYGB). In addition to a chart review, the University of Minnesota Bariatric Surgery Outcomes Survey tool was used to prospectively track patients’ weight, comorbid illnesses, adverse outcomes, readmissions, and general health status. Mean follow-up was 3.7 years in the DS group and 6.2 years in the RYGB group.
There were five deaths in the DS group (postop day 38 and months 5, 7, 16, and 66) and three deaths in the RYGB group (postop months 3, 7, and 72), leaving 173 patients and 136 patients, respectively, in the analysis, Dr. Dorman said at the annual meeting of the Central Surgical Association.
Notably, weight loss in the two groups was comparable, decreasing from an average body mass index of 52 kg/m2 to 31 kg/m2 in the DS group and from 51 kg/m2 to 34 kg/m2 in the RYGB group, said Dr. Dorman, a general surgery resident at the University of Minnesota, Minneapolis.
Resolution of type 2 diabetes, hypertension, and hyperlipidemia was greatest among DS patients at 82%, 67%, and 81%, respectively, compared with 64%, 39%, and 55%, respectively, among RYGB patients.
DS patients, however, experienced significantly more loose stools, bloating, and heartburn than did RYGB patients, who had significantly more constipation. Nausea and emesis were comparable between the two groups.
With regard to complications, DS patients were significantly more likely to visit the emergency department (ED) than were RYGB patients (40% vs. 25%; P value less than .01) and to experience hair loss (67% vs. 41%; P less than .01), Dr. Dorman said.
There was also a nonsignificant trend for DS patients to be readmitted more often than RYGB patients (25.4% vs. 23.5%) and to have more gastrointestinal leaks (1.7% vs. 0%), abdominal reoperations (29% vs. 23%), total parenteral nutrition/tube feeds (7.6% vs. 3%), and infusion therapy (28.5% vs. 23.5%). The RYGB patients, however, underwent more endoscopy (22% vs. 14%).
Dr. Dorman said providers should explain to patients the adverse symptoms they can expect following duodenal switch, but noted that the investigators "still feel DS should be limited to surgeons and centers with experience."
Invited discussant Dr. James Wallace, a bariatric and general surgeon from the Medical College of Wisconsin, Milwaukee, described the 40% rate of ED visits in the DS group as "extreme," and questioned the use of nutritional, vitamin, and protein supplementation – particularly in light of the observed hair loss.
"I commend the authors for their excellent surgical outcomes with the duodenal switch – much better than others have reported in the literature – but I’m unconvinced that the incremental improvement in weight loss and resolution of metabolic derangements justifies the increased nutritional risk of the duodenal switch," he said.
Dr. Dorman responded that the ED visits may represent a "knee-jerk reflex" on the part of DS patients when they experience a complication. He added that the university has instituted more frequent checks and phone calls, particularly to high-risk DS patients, and has partnered with their transplant clinic’s infusion center to provide IV fluids. Nutritional support data in this study was insufficient to report on for all patients, but supplementary nutrition is implemented based on factors such as vitamin and albumin levels or difficulty with eating, according to Dr. Dorman.
Invited discussant Dr. Bradley Needleman, director of the bariatric surgery program at Ohio State University in Columbus, said he was most fascinated by the lack of a significant difference in weight loss between the two groups and asked how this finding would influence patient consultations.
Dr. Dorman said a recent case-matched study at their institution also found no significant difference in weight loss between the two procedures (Ann. Surg. 2012;255:287-93), although a recent prospective randomized European study reported that weight loss was significantly greater with duodenal switch surgery than with gastric bypass (Ann. Intern. Med. 2011;155:281-91).
"It seems to be a finding that exists only within our institution and that should be taken into account when we discuss with our own patients which operations they should undergo," Dr. Dorman said. "I think that duodenal switch in a patient with diabetes and BMI over 45-50 [kg/m2] is certainly a valid operation at an experienced center, as long as we understand the symptoms they may experience afterward."
Duodenal switch should remain a valid alternative because RYGB has its own inherent downfalls – notably, high marginal ulcer and stricture rates, as indicated in the current study by the trend toward significantly greater use of endoscopic procedures in the RYGB patients, said Dr. Sayeed Ikramuddin. Also, the group has now twice shown in matched patient populations the superiority by which the duodenal switch provides resolution of type 2 diabetes when compared with RYGB, added Dr. Ikramuddin, senior author and director of gastrointestinal surgery at the University of Minnesota.
"Lastly, the Roux-en-Y gastric bypass has a high long-term failure rate resulting in patients regaining their weight," Dr. Ikramuddin said in an interview. "This is a phenomenon not as common among duodenal switch patients, likely due to the more malabsorptive nature of the operation."
When asked what contraindications exist for duodenal switch surgery, Dr. Dorman replied that the only absolute contraindications are women of reproductive age because of concerns of nutritional malabsorption and patients unwilling to commit to follow-up. A patient with significant gastroesophageal reflux disease would most likely be offered RYGB, he said, noting that DS surgery had been performed on 5%-10% of their last 100 GI patients.
Dr. Dorman reported no relevant conflicts of interest.
MADISON, WIS. – Total complication rates are high but comparable over the long term between duodenal switch surgery and Roux-en-Y gastric bypass, according to a propensity matched analysis of 309 superobese patients.
"Duodenal switch is a valid alternative to the Roux-en-Y gastric bypass, especially if significant comorbid illnesses are present, particularly diabetes," Dr. Robert B. Dorman said.
His conclusion is drawn from a study that focused on the long-term outcomes of 178 consecutive patients who underwent duodenal switch (DS) surgery and 139 propensity matched patients undergoing Roux-en-Y gastric bypass (RYGB). In addition to a chart review, the University of Minnesota Bariatric Surgery Outcomes Survey tool was used to prospectively track patients’ weight, comorbid illnesses, adverse outcomes, readmissions, and general health status. Mean follow-up was 3.7 years in the DS group and 6.2 years in the RYGB group.
There were five deaths in the DS group (postop day 38 and months 5, 7, 16, and 66) and three deaths in the RYGB group (postop months 3, 7, and 72), leaving 173 patients and 136 patients, respectively, in the analysis, Dr. Dorman said at the annual meeting of the Central Surgical Association.
Notably, weight loss in the two groups was comparable, decreasing from an average body mass index of 52 kg/m2 to 31 kg/m2 in the DS group and from 51 kg/m2 to 34 kg/m2 in the RYGB group, said Dr. Dorman, a general surgery resident at the University of Minnesota, Minneapolis.
Resolution of type 2 diabetes, hypertension, and hyperlipidemia was greatest among DS patients at 82%, 67%, and 81%, respectively, compared with 64%, 39%, and 55%, respectively, among RYGB patients.
DS patients, however, experienced significantly more loose stools, bloating, and heartburn than did RYGB patients, who had significantly more constipation. Nausea and emesis were comparable between the two groups.
With regard to complications, DS patients were significantly more likely to visit the emergency department (ED) than were RYGB patients (40% vs. 25%; P value less than .01) and to experience hair loss (67% vs. 41%; P less than .01), Dr. Dorman said.
There was also a nonsignificant trend for DS patients to be readmitted more often than RYGB patients (25.4% vs. 23.5%) and to have more gastrointestinal leaks (1.7% vs. 0%), abdominal reoperations (29% vs. 23%), total parenteral nutrition/tube feeds (7.6% vs. 3%), and infusion therapy (28.5% vs. 23.5%). The RYGB patients, however, underwent more endoscopy (22% vs. 14%).
Dr. Dorman said providers should explain to patients the adverse symptoms they can expect following duodenal switch, but noted that the investigators "still feel DS should be limited to surgeons and centers with experience."
Invited discussant Dr. James Wallace, a bariatric and general surgeon from the Medical College of Wisconsin, Milwaukee, described the 40% rate of ED visits in the DS group as "extreme," and questioned the use of nutritional, vitamin, and protein supplementation – particularly in light of the observed hair loss.
"I commend the authors for their excellent surgical outcomes with the duodenal switch – much better than others have reported in the literature – but I’m unconvinced that the incremental improvement in weight loss and resolution of metabolic derangements justifies the increased nutritional risk of the duodenal switch," he said.
Dr. Dorman responded that the ED visits may represent a "knee-jerk reflex" on the part of DS patients when they experience a complication. He added that the university has instituted more frequent checks and phone calls, particularly to high-risk DS patients, and has partnered with their transplant clinic’s infusion center to provide IV fluids. Nutritional support data in this study was insufficient to report on for all patients, but supplementary nutrition is implemented based on factors such as vitamin and albumin levels or difficulty with eating, according to Dr. Dorman.
Invited discussant Dr. Bradley Needleman, director of the bariatric surgery program at Ohio State University in Columbus, said he was most fascinated by the lack of a significant difference in weight loss between the two groups and asked how this finding would influence patient consultations.
Dr. Dorman said a recent case-matched study at their institution also found no significant difference in weight loss between the two procedures (Ann. Surg. 2012;255:287-93), although a recent prospective randomized European study reported that weight loss was significantly greater with duodenal switch surgery than with gastric bypass (Ann. Intern. Med. 2011;155:281-91).
"It seems to be a finding that exists only within our institution and that should be taken into account when we discuss with our own patients which operations they should undergo," Dr. Dorman said. "I think that duodenal switch in a patient with diabetes and BMI over 45-50 [kg/m2] is certainly a valid operation at an experienced center, as long as we understand the symptoms they may experience afterward."
Duodenal switch should remain a valid alternative because RYGB has its own inherent downfalls – notably, high marginal ulcer and stricture rates, as indicated in the current study by the trend toward significantly greater use of endoscopic procedures in the RYGB patients, said Dr. Sayeed Ikramuddin. Also, the group has now twice shown in matched patient populations the superiority by which the duodenal switch provides resolution of type 2 diabetes when compared with RYGB, added Dr. Ikramuddin, senior author and director of gastrointestinal surgery at the University of Minnesota.
"Lastly, the Roux-en-Y gastric bypass has a high long-term failure rate resulting in patients regaining their weight," Dr. Ikramuddin said in an interview. "This is a phenomenon not as common among duodenal switch patients, likely due to the more malabsorptive nature of the operation."
When asked what contraindications exist for duodenal switch surgery, Dr. Dorman replied that the only absolute contraindications are women of reproductive age because of concerns of nutritional malabsorption and patients unwilling to commit to follow-up. A patient with significant gastroesophageal reflux disease would most likely be offered RYGB, he said, noting that DS surgery had been performed on 5%-10% of their last 100 GI patients.
Dr. Dorman reported no relevant conflicts of interest.
MADISON, WIS. – Total complication rates are high but comparable over the long term between duodenal switch surgery and Roux-en-Y gastric bypass, according to a propensity matched analysis of 309 superobese patients.
"Duodenal switch is a valid alternative to the Roux-en-Y gastric bypass, especially if significant comorbid illnesses are present, particularly diabetes," Dr. Robert B. Dorman said.
His conclusion is drawn from a study that focused on the long-term outcomes of 178 consecutive patients who underwent duodenal switch (DS) surgery and 139 propensity matched patients undergoing Roux-en-Y gastric bypass (RYGB). In addition to a chart review, the University of Minnesota Bariatric Surgery Outcomes Survey tool was used to prospectively track patients’ weight, comorbid illnesses, adverse outcomes, readmissions, and general health status. Mean follow-up was 3.7 years in the DS group and 6.2 years in the RYGB group.
There were five deaths in the DS group (postop day 38 and months 5, 7, 16, and 66) and three deaths in the RYGB group (postop months 3, 7, and 72), leaving 173 patients and 136 patients, respectively, in the analysis, Dr. Dorman said at the annual meeting of the Central Surgical Association.
Notably, weight loss in the two groups was comparable, decreasing from an average body mass index of 52 kg/m2 to 31 kg/m2 in the DS group and from 51 kg/m2 to 34 kg/m2 in the RYGB group, said Dr. Dorman, a general surgery resident at the University of Minnesota, Minneapolis.
Resolution of type 2 diabetes, hypertension, and hyperlipidemia was greatest among DS patients at 82%, 67%, and 81%, respectively, compared with 64%, 39%, and 55%, respectively, among RYGB patients.
DS patients, however, experienced significantly more loose stools, bloating, and heartburn than did RYGB patients, who had significantly more constipation. Nausea and emesis were comparable between the two groups.
With regard to complications, DS patients were significantly more likely to visit the emergency department (ED) than were RYGB patients (40% vs. 25%; P value less than .01) and to experience hair loss (67% vs. 41%; P less than .01), Dr. Dorman said.
There was also a nonsignificant trend for DS patients to be readmitted more often than RYGB patients (25.4% vs. 23.5%) and to have more gastrointestinal leaks (1.7% vs. 0%), abdominal reoperations (29% vs. 23%), total parenteral nutrition/tube feeds (7.6% vs. 3%), and infusion therapy (28.5% vs. 23.5%). The RYGB patients, however, underwent more endoscopy (22% vs. 14%).
Dr. Dorman said providers should explain to patients the adverse symptoms they can expect following duodenal switch, but noted that the investigators "still feel DS should be limited to surgeons and centers with experience."
Invited discussant Dr. James Wallace, a bariatric and general surgeon from the Medical College of Wisconsin, Milwaukee, described the 40% rate of ED visits in the DS group as "extreme," and questioned the use of nutritional, vitamin, and protein supplementation – particularly in light of the observed hair loss.
"I commend the authors for their excellent surgical outcomes with the duodenal switch – much better than others have reported in the literature – but I’m unconvinced that the incremental improvement in weight loss and resolution of metabolic derangements justifies the increased nutritional risk of the duodenal switch," he said.
Dr. Dorman responded that the ED visits may represent a "knee-jerk reflex" on the part of DS patients when they experience a complication. He added that the university has instituted more frequent checks and phone calls, particularly to high-risk DS patients, and has partnered with their transplant clinic’s infusion center to provide IV fluids. Nutritional support data in this study was insufficient to report on for all patients, but supplementary nutrition is implemented based on factors such as vitamin and albumin levels or difficulty with eating, according to Dr. Dorman.
Invited discussant Dr. Bradley Needleman, director of the bariatric surgery program at Ohio State University in Columbus, said he was most fascinated by the lack of a significant difference in weight loss between the two groups and asked how this finding would influence patient consultations.
Dr. Dorman said a recent case-matched study at their institution also found no significant difference in weight loss between the two procedures (Ann. Surg. 2012;255:287-93), although a recent prospective randomized European study reported that weight loss was significantly greater with duodenal switch surgery than with gastric bypass (Ann. Intern. Med. 2011;155:281-91).
"It seems to be a finding that exists only within our institution and that should be taken into account when we discuss with our own patients which operations they should undergo," Dr. Dorman said. "I think that duodenal switch in a patient with diabetes and BMI over 45-50 [kg/m2] is certainly a valid operation at an experienced center, as long as we understand the symptoms they may experience afterward."
Duodenal switch should remain a valid alternative because RYGB has its own inherent downfalls – notably, high marginal ulcer and stricture rates, as indicated in the current study by the trend toward significantly greater use of endoscopic procedures in the RYGB patients, said Dr. Sayeed Ikramuddin. Also, the group has now twice shown in matched patient populations the superiority by which the duodenal switch provides resolution of type 2 diabetes when compared with RYGB, added Dr. Ikramuddin, senior author and director of gastrointestinal surgery at the University of Minnesota.
"Lastly, the Roux-en-Y gastric bypass has a high long-term failure rate resulting in patients regaining their weight," Dr. Ikramuddin said in an interview. "This is a phenomenon not as common among duodenal switch patients, likely due to the more malabsorptive nature of the operation."
When asked what contraindications exist for duodenal switch surgery, Dr. Dorman replied that the only absolute contraindications are women of reproductive age because of concerns of nutritional malabsorption and patients unwilling to commit to follow-up. A patient with significant gastroesophageal reflux disease would most likely be offered RYGB, he said, noting that DS surgery had been performed on 5%-10% of their last 100 GI patients.
Dr. Dorman reported no relevant conflicts of interest.
FROM THE ANNUAL MEETING OF THE CENTRAL SURGICAL ASSOCIATION
Major Finding: Resolution of type 2 diabetes, hypertension, and hyperlipidemia was greatest among duodenal switch patients, at 82%, 67%, and 81%, vs. 64%, 39%, and 55% among Roux-en-Y gastric bypass patients.
Data Source: Data were taken from a chart review and prospective survey of 309 superobese patients.
Disclosures: Dr. Dorman reported no relevant conflicts of interest.
ONLINE EXCLUSIVE: How the School of Medicine at Stanford University Is Addressing Women Physicians and Leadership
Whenever Hannah Valantine, MD, needs reassurance that women leadership interventions at Stanford University’s School of Medicine are working, she looks at the numbers.
In the span of five to six years, the medical school increased the percentage of women at each faculty rank so that it now surpasses national averages as calculated by the Association of American Medical Colleges. Indeed, the percentage of women at the full professor rank jumped from 14.5 percent to 22 percent.
“We really are making progress,” says Dr. Valantine, full professor of medicine and the medical school’s senior associate dean for diversity and leadership.
With structural elements such as tenure clock extension, extended maternity and family leave, onsite childcare, early stage research funding support, and mentoring in place, Dr. Valantine is turning her attention to the next round of interventions, which focus more on psychological and social factors impairing women’s advancement.
She will use a National Institutes of Health grant to develop interventions for the phenomenon of stereotype threat, which is the fear that one's behavior will confirm an existing stereotype about one’s social group. This fear may lead to an impairment of performance.
Over the next six months, Dr. Valantine and her team will also conduct several pilot programs involving map career customization, a model that encourages people to chart their career over the next 5 to 10 to 20 years, taking into consideration their life outside of work. The intent is to help individuals identify their priorities and goals and how they change over time, and also help supervisors better match the ebbs and flows of a person’s life to the workplace and identify and develop aspiring leaders.
Stanford’s medical school is organized around teams of doctors that care for groups of patients. Each team must achieve excellence in four academic missions: clinical care, education, research, and administration. The map career customization pilot programs are aimed at helping doctors within the team plan their career path around these four missions and then putting the individual plans together in a team context in order to meet the team’s goals, says Dr. Valantine.
“This way the work and the four missions are entirely covered,” she says. “We create a vibrant academic environment where we create new things and have time to think and integrate our life and work… It’s a little countercultural, but I think people are crying out for that and I think it stands a great chance of making the culture change.”
Stanford’s burgeoning efforts in map career customization have intrigued SHM board member Janet Nagamine, RN, MD, FHM, a hospitalist at Kaiser Permanente Medical Center in Santa Clara, Calif., and Stanford alum.
She hopes to collaborate with Dr. Valantine and incorporate in hospital medicine the interventions that Stanford is doing while conducting studies and developing workforce planning initiatives specific to hospitalists. The goal is to create a hospital medicine model that replicates Stanford’s success in cultivating women physician leaders.
“We make this false assumption that your career is going to look the same throughout your life and that’s just not realistic,” Dr. Nagamine says.
Whenever Hannah Valantine, MD, needs reassurance that women leadership interventions at Stanford University’s School of Medicine are working, she looks at the numbers.
In the span of five to six years, the medical school increased the percentage of women at each faculty rank so that it now surpasses national averages as calculated by the Association of American Medical Colleges. Indeed, the percentage of women at the full professor rank jumped from 14.5 percent to 22 percent.
“We really are making progress,” says Dr. Valantine, full professor of medicine and the medical school’s senior associate dean for diversity and leadership.
With structural elements such as tenure clock extension, extended maternity and family leave, onsite childcare, early stage research funding support, and mentoring in place, Dr. Valantine is turning her attention to the next round of interventions, which focus more on psychological and social factors impairing women’s advancement.
She will use a National Institutes of Health grant to develop interventions for the phenomenon of stereotype threat, which is the fear that one's behavior will confirm an existing stereotype about one’s social group. This fear may lead to an impairment of performance.
Over the next six months, Dr. Valantine and her team will also conduct several pilot programs involving map career customization, a model that encourages people to chart their career over the next 5 to 10 to 20 years, taking into consideration their life outside of work. The intent is to help individuals identify their priorities and goals and how they change over time, and also help supervisors better match the ebbs and flows of a person’s life to the workplace and identify and develop aspiring leaders.
Stanford’s medical school is organized around teams of doctors that care for groups of patients. Each team must achieve excellence in four academic missions: clinical care, education, research, and administration. The map career customization pilot programs are aimed at helping doctors within the team plan their career path around these four missions and then putting the individual plans together in a team context in order to meet the team’s goals, says Dr. Valantine.
“This way the work and the four missions are entirely covered,” she says. “We create a vibrant academic environment where we create new things and have time to think and integrate our life and work… It’s a little countercultural, but I think people are crying out for that and I think it stands a great chance of making the culture change.”
Stanford’s burgeoning efforts in map career customization have intrigued SHM board member Janet Nagamine, RN, MD, FHM, a hospitalist at Kaiser Permanente Medical Center in Santa Clara, Calif., and Stanford alum.
She hopes to collaborate with Dr. Valantine and incorporate in hospital medicine the interventions that Stanford is doing while conducting studies and developing workforce planning initiatives specific to hospitalists. The goal is to create a hospital medicine model that replicates Stanford’s success in cultivating women physician leaders.
“We make this false assumption that your career is going to look the same throughout your life and that’s just not realistic,” Dr. Nagamine says.
Whenever Hannah Valantine, MD, needs reassurance that women leadership interventions at Stanford University’s School of Medicine are working, she looks at the numbers.
In the span of five to six years, the medical school increased the percentage of women at each faculty rank so that it now surpasses national averages as calculated by the Association of American Medical Colleges. Indeed, the percentage of women at the full professor rank jumped from 14.5 percent to 22 percent.
“We really are making progress,” says Dr. Valantine, full professor of medicine and the medical school’s senior associate dean for diversity and leadership.
With structural elements such as tenure clock extension, extended maternity and family leave, onsite childcare, early stage research funding support, and mentoring in place, Dr. Valantine is turning her attention to the next round of interventions, which focus more on psychological and social factors impairing women’s advancement.
She will use a National Institutes of Health grant to develop interventions for the phenomenon of stereotype threat, which is the fear that one's behavior will confirm an existing stereotype about one’s social group. This fear may lead to an impairment of performance.
Over the next six months, Dr. Valantine and her team will also conduct several pilot programs involving map career customization, a model that encourages people to chart their career over the next 5 to 10 to 20 years, taking into consideration their life outside of work. The intent is to help individuals identify their priorities and goals and how they change over time, and also help supervisors better match the ebbs and flows of a person’s life to the workplace and identify and develop aspiring leaders.
Stanford’s medical school is organized around teams of doctors that care for groups of patients. Each team must achieve excellence in four academic missions: clinical care, education, research, and administration. The map career customization pilot programs are aimed at helping doctors within the team plan their career path around these four missions and then putting the individual plans together in a team context in order to meet the team’s goals, says Dr. Valantine.
“This way the work and the four missions are entirely covered,” she says. “We create a vibrant academic environment where we create new things and have time to think and integrate our life and work… It’s a little countercultural, but I think people are crying out for that and I think it stands a great chance of making the culture change.”
Stanford’s burgeoning efforts in map career customization have intrigued SHM board member Janet Nagamine, RN, MD, FHM, a hospitalist at Kaiser Permanente Medical Center in Santa Clara, Calif., and Stanford alum.
She hopes to collaborate with Dr. Valantine and incorporate in hospital medicine the interventions that Stanford is doing while conducting studies and developing workforce planning initiatives specific to hospitalists. The goal is to create a hospital medicine model that replicates Stanford’s success in cultivating women physician leaders.
“We make this false assumption that your career is going to look the same throughout your life and that’s just not realistic,” Dr. Nagamine says.
CDI: The Scope of the Problem
Clostridium difficile is a gram‐positive, spore‐forming, toxin‐producing, anaerobic bacillus that was established as the causative pathogen of most cases of antibiotic‐associated colitis in 1978. 1, 2 The spectrum of possible clinical presentations of C. difficile range from asymptomatic colonization, uncomplicated diarrhea, severe pseudomembranous colitis, paralytic ileus, to sepsis and death, with a mortality rate upwards of 80% in fulminant cases requiring colectomy. 3
Vegetative C. difficile cells die rapidly on dry surfaces, but they have been found to remain viable for up to 6 hours on moist surfaces in room air. 4 Spores shed from the gastrointestinal (GI) tract, however, are highly resistant to common hospital disinfectants, and can survive in the environment for many months. 2 C. difficile spores are primarily transmitted from patient to patient on the hands or equipment of healthcare workers. 2 Once spores are ingested and reach the GI tract, they germinate in the vegetative form. 2, 5 In the GI tract, C. difficile causes disease by the production of toxins, primarily toxins A and B, both of which cause severe inflammation. 5 Toxin A attracts neutrophils and monocytes, and toxin B breaks down colonic epithelial cells. 5 Both of these mechanisms lead to colitis, formation of pseudomembranes, and watery diarrhea. 5
After alteration of the healthy colonic bacterial flora, the immune response to C. difficile toxins appears to play a major role in determining host susceptibility to C. difficile infection (CDI). 5, 6 Those with antitoxin immunity are more likely to become symptomless carriers than patients without preexisting immunity. 3 More than 60% of healthy adults have protective immunity against a primary CDI, demonstrated by detectable serum IgG and IgA to both toxins A and B, as a consequence of childhood immunity or frequent exposure to C. difficile in the environment. 3 After a primary episode of CDI, many patients acquire protective immunity against C. difficile toxins, seen as significantly higher serum concentrations of IgM against C. difficile toxin by the third day from onset of diarrhea, and significantly higher serum concentrations of IgG against toxin A by the 12th day. 7 Patients who experience recurrent CDI lack development of this protective immunity to C. difficile. 6, 7
CDI INCIDENCE IS ON THE RISE
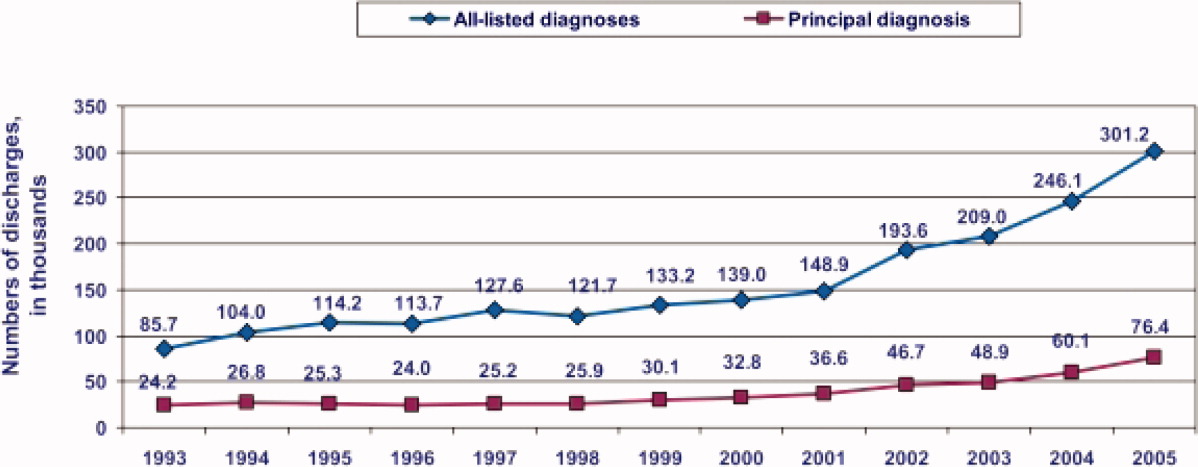
During the past decade, rates of CDI have increased steadily to levels not previously seen. A report published by the Agency of Healthcare Research and Quality demonstrated that the number of CDI diagnoses on hospital discharge more than doubled in the United States from 139,000 to 301,200 between 2000 and 2005 (Figure 1). 8 Examination of a more recent Nationwide Inpatient Sample (NIS) indicates continuation of this trend, with nearly 350,000 CDI diagnoses recorded upon discharge from acute care hospitals in 2008. 9 Of note, in 2006 the state of Ohio mandated CDI reporting from both hospitals and nursing homes. It was estimated there were more than 18,000 cases of CDI during this year, of which more than 60% were diagnosed in nursing homes. 10 Based on the 2008 NIS data and the data from Ohio, it is conceivable there were as many as 1 million cases of CDI in the US in 2008.
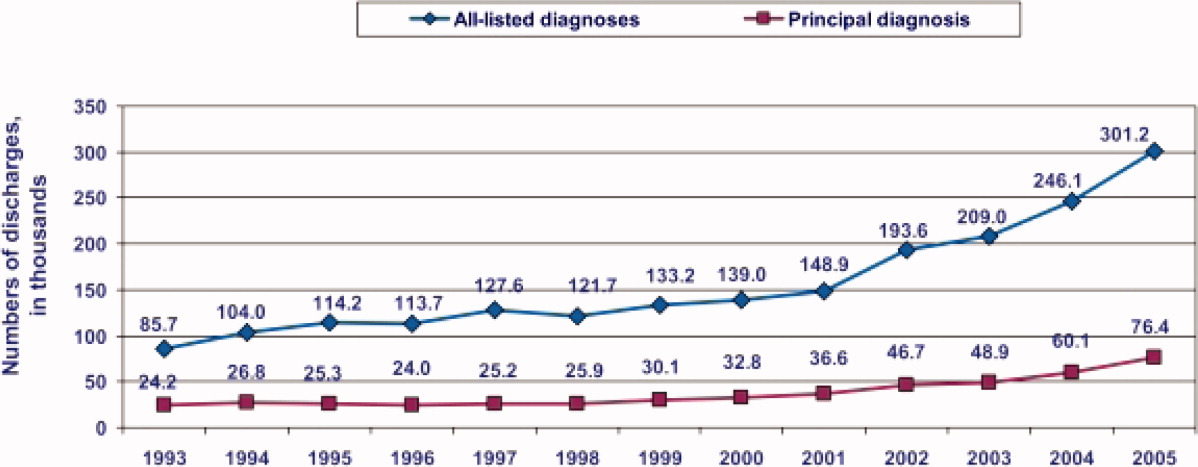
8
This increased incidence of CDI contrasts with several other healthcare‐associated infections, which have declined in incidence over the last decade. 1113 C. difficile is the most common causative agent of healthcare‐associated infections in some areas. A cohort study of common infections among inpatients at 30 community hospitals in the Duke Infection Control Outreach Network conducted between January 1, 2008 and June 30, 2009 found the incidence of CDI cases was 0.26 cases per 1000 patient‐days, which was higher than the incidence of methicillin‐resistant Staphylococcus aureus (MRSA) at 0.22 cases per 1000 patient‐days. 14 Another study utilizing the NIS data found that, while vancomycin‐resistant enterococcus and pseudomonas infections remained stable, CDI increased in many areas of the country and was more common than MRSA in some areas. 15
HYPERVIRULENT STRAIN OF C. DIFFICILE
In the early 2000s, an epidemic and hypervirulent strain of C. difficile emerged in North America and Europe that altered the epidemiology of CDI. 16 Due to multiple different methods for molecular typing of C. difficile, this strain has several names depending on the method of typing performed. The most common names for this strain are BI (REA typing), NAP1 (pulsed field gel electrophoresis), and 027 (PCR‐ribotyping). This strain has become the predominant strain of C. difficile in some areas, accounting for more than 80% of CDI cases in some areas. 3
The characteristics of this particular strain epidemic in North America typically include:
-
A deletion in the tcd gene that downregulates toxin production, which renders the gene nonfunctional in the epidemic strain. Some in vitro data have demonstrated that this epidemic strain produces 16‐fold higher concentrations of toxin A and 23‐fold higher concentrations of toxin B than nonepidemic strains of C. difficile. 17
-
Production of a third toxin, called binary toxin CDT. The role of this toxin in the pathogenesis of CDI is not clear, but the presence of this toxin has been associated with more severe CDI‐related diarrhea. 2, 16
-
High‐level resistance to fluoroquinolones, including moxifloxacin and gatifloxacin. 5, 16 It has been theorized that increasing use of fluoroquinolones during the past decade may have provided a selective advantage for the BI/NAP1/027 strain to predominate. 2
-
Production of more spores than other strains of C. difficile. 17, 18 This may increase its ability to contaminate the environment and be transmitted in a healthcare facility.
CDI SEVERITY IS INCREASING
Paralleling the increased prevalence of CDI, C. difficile infections are generally becoming more severe. In Sherbrooke, Quebec, Canada, which experienced a dramatic outbreak of CDI associated with increased CDI severity, the cumulative 1‐year attributable mortality was nearly 37% (60 of 161 CDI cases) in a hospital case review of nonsurgical admissions between January 2003 and June 2004. 19 In St Louis, Missouri in 2003, a 5.7% 180‐day mortality rate was reported in an endemic setting. 20 Among the 24% of patients readmitted within 180 days of discharge (4207 of 17,492) in this retrospective case review, patients with CDI were more than twice as likely as non‐CDI patients to be readmitted to the hospital (52% vs 23%, N = 4207). 20 Furthermore, patients with CDI were significantly more likely to require discharge to a long‐term care facility (32%) than non‐CDI controls (23%). 19
Based on NIS data for CDI‐related hospitalizations between 2000 and 2005, the crude, age‐adjusted case‐fatality rate rose from 1.2% in 2000 to 2.2% in 2004. 21 This increase was mirrored by a doubling of CDI cases admitted for hospitalization during the same 6‐year period. 21 According to the investigators, these findings indirectly confirm that the doubling in CDI deaths is attributable to an increase in C. difficile virulence. 21 A 6‐month prospective surveillance of CDI patient outcomes in 29 Canadian hospitals was conducted by the Canadian Nosocomial Infection Surveillance Program (CNISP) beginning in November 2004. 22 At 30 days after onset of CDI, the percentage of deaths directly or indirectly attributable to CDI was 5.7%, which represented an almost 4‐fold increase over CDI‐attributable deaths recorded in the 1997 CNISP survey. 22 Overall 30‐day mortality was retrospectively analyzed among patients with CDI in a St Louis, Missouri 1200‐bed teaching hospital intensive care unit (ICU) over a 2‐year period (20042005). 23 The 30‐day crude mortality among 278 patients admitted to the ICU with CDI was 37% (n = 102), and mortality directly attributable to CDI in these critically ill patients was 6%. 23 The number of deaths in the United States due to CDI increased sharply from 793 patients in 1999 to 6225 patients in 2006. 24 In 2006, it ranked among the top 20 causes of death for those aged 65 years and older. 24
INCREASE IN TREATMENT FAILURES
In addition to being more severe, there have been several reports of increases in CDI treatment failures and/or increases in recurrent CDI. 6 Recent studies indicate there may be more metronidazole treatment failures regardless of whether the infecting strain is the BI/NAP1/027 strain, despite a lack of laboratory evidence indicating resistance to metronidazole. 2529 Regardless of the initial therapy chosen, patients must be carefully monitored to ensure they are responding appropriately to treatment and their condition is not deteriorating. 29 Some of the original trials of CDI treatments found relapse rates as low as 5% to 15%. 30 More recent data indicate relapse occurs after 30% of initial CDI episodes, and as frequent as 65% if the patient has had multiple prior CDI episodes. 3, 6, 31
COMMUNITY‐ASSOCIATED CDI
The epidemiology of community‐associated CDI may also be changing. Virulent strains, which cause more severe disease in high‐risk patients, may also cause more frequent, severe disease in populations previously thought to be at low risk. Some studies have found an increase in community‐associated CDI in otherwise healthy individuals with little or no exposure to a healthcare facility. Although antimicrobial exposure remains the most important risk factor for community‐associated CDI, antimicrobial exposure is less common in community‐associated CDI than healthcare‐associated CDI. 3235
In a Canadian study, the rate of diagnosed community‐acquired CDI cases was stable at about 22 cases per 100,000 patient‐years per calendar year between 1998 and 2002, but rose steadily for the next 2 years to 53 cases per 100,000 patient‐years in 2004. 33 Similar results were seen in the United Kingdom, with an exponential increase from fewer than 1 case per 100,000 person‐years in 1994 to 22 cases per 100,000 person‐years in 2004. 32 There are currently no comprehensive longitudinal studies in the United States investigating the incidence of purely community‐acquired CDI where a patient had no prior hospital exposure. However, regional surveys have reported an incidence of community‐acquired CDI of 12 cases per 100,000 person‐years during 1992 to 1994, 36 7.6 cases per 100,000 person‐years in 2005, 37 and 6.9 cases per 100,000 person‐years in 2006. 34, 37
One patient population generally thought to be at low risk for CDI that may be at increased risk for severe CDI is pregnant women. In one study 419 infectious disease consultants who responded to a survey conducted by the Emerging Infections Network had seen or were aware of 55 cases of CDI in peripartum women. 38 There were 21 cases with complications, including 10 relapses and 5 cases of toxic megacolon. 38 In a prior report of severe CDI among 10 peripartum women, 3 women died and 3 infants (2 were twins) were stillborn. 38 This data emphasizes why clinicians must have a high index of suspicion for CDI, and should be aware of the potential for severe outcomes, even in patients traditionally considered to be at low risk. 38
ECONOMIC IMPACT OF CDI
The economic burden of CDI in the United States is staggering, with estimates ranging from $1.1 to $3.2 billion annually (Table 1). 3941 These estimates are based on the cost of caring for patients with CDI in acute care facilities and are primarily driven by increased length of stay in the hospital due to CDI. These data also predate the emergence of the BI/NAP1/027 strain. Therefore, the costs of CDI are likely higher than these estimates due to the increases in CDI severity seen since these studies were performed. It is important to note that these studies did not include patients diagnosed and treated in nursing homes or the community, nor the increase in costs due to discharge to a long‐term care facility. 39
| Study | Patient Population | Estimated Attributable Cost per Episode* | Increase in LOS, days | Estimated Annual Attributable Cost, US |
|---|---|---|---|---|
| ||||
| Kyne et al 40 | Two medical wards (n = 40) | $3669 | 3.6 | $1.1 billion |
| Dubberke et al 39 | Nonsurgical patients∥ (n = 439) | $2454$3240 | 3.0 | $897 million$1.3 billion# |
| O'Brien et al 41 | Massachusetts discharge database (n = 3692)** | Primary diagnosis: $10,212; secondary diagnosis: $13,675 | Primary diagnosis: 6.4; secondary diagnosis: 2.9 | $3.2 billion |
SUMMARY
C. difficile infections are becoming more prevalent and more severe. The issue is sufficiently serious that healthcare‐onset CDI has recently been called a major public health threat. 42 For this reason, efforts to combat virulent C. difficile should include good antimicrobial stewardship, effective infection control, and control of environmental factors that promote transmission. 35 Healthcare professionals who oversee the care of inpatients should act as catalysts for improvement by taking a leadership role in the multidisciplinary approach needed to reduce the morbidity, mortality, and cost burden for patients and the healthcare system.
- . Narrative review: the new epidemic of Clostridium difficile‐associated enteric disease. Ann Intern Med. 2006;145(10): 758–764.
- Association for Professionals in Infection Control and Epidemiology, Inc (APIC). Guide to the elimination of Clostridium difficile in healthcare settings. Available at: http://www.apic.org/Content/NavigationMenu/PracticeGuidance/APICEliminationGuides/C.diff_Elimination_guide_logo.pdf. 2008. Accessed October 8, 2011.
- . Clostridium difficile and the disease it causes. Methods Mol Biol. 2010;646:9–35.
- , , . Vegetative Clostridium difficile survives in room air on moist surfaces and in gastric contents with reduced acidity: a potential mechanism to explain the association between proton pump inhibitors and C. difficile‐associated diarrhea?Antimicrob Agents Chemother. 2007;51(8): 2883–2887.
- , . Clostridium difficile‐associated disease: new challenges from an established pathogen. Cleve Clin J Med. 2006;73(2): 187–197.
- . A 76‐year‐old man with recurrent Clostridium difficile‐associated diarrhea: review of C difficile infection. JAMA. 2009;301(9): 954–962.
- , , , . Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet. 2001;357(9251): 189–193.
- , .Clostridium difficile‐associated disease in US hospitals, 1993–2005. Healthcare Cost and Utilization Project. Statistical Brief #50. April 2008. Available at: http://www.ncbi.nlm.nih.gov/books/NBK56038/pdf/sb50.pdf. Accessed December 12, 2011.
- Agency of Healthcare Research and Quality. Healthcare Cost and Utilization Project Database. Available at: http://www.hcup‐us.ahrq.gov/nisoverview.jsp. Accessed April 2011.
- , , , et al. Clostridium difficile infection in Ohio hospitals and nursing homes during 2006. Infect Control Hosp Epidemiol. 2009;30(6): 526–533.
- , , , et al. Trends in catheter‐associated urinary tract infections in adult intensive care units—United States, 1990–2007. Infect Control Hosp Epidemiol. 2011;32(8): 748–756.
- , , , et al. Methicillin‐resistant Staphylococcus aureus central line‐associated bloodstream infections in US intensive care units, 1997–2007. JAMA. 2009;301(7): 727–736.
- , , , et al. An intervention to decrease catheter‐related bloodstream infections in the ICU. N Engl J Med. 2006;355(26): 2725–2732.
- , , , .The impact of hospital‐onset healthcare facility associated (HO‐HCFA) Clostridium difficile infection (CDI) in community hospitals: surpassing methicillin‐resistant Staphylococcus aureus (MRSA) as the new superbug [abstract 386]. Presented at: The Fifth Decennial International Conference on Healthcare‐Associated Infections (ICHAI). March 20, 2010; Atlanta, GA.
- , , . Growth and geographic variation in hospitalizations with resistant infections, United States, 2000–2005. Emerg Infect Dis. 2008;14(11): 1756–1758.
- , , . An epidemic, toxin gene‐variant strain of Clostridium difficile. New Engl J Med. 2005;353(23): 2433–2441.
- , , , et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366(9491): 1079–1084.
- , , , et al. Increased sporulation rate of epidemic Clostridium difficile type 027/NAP1. J Clin Microbiol. 2008;46(4): 1530–1533.
- , , . Mortality attributable to nosocomial Clostridium difficile‐ associated disease during an epidemic caused by a hypervirulent strain in Quebec. Can Med Assoc J. 2005;173(9): 1037–1042.
- , , , et al. Attributable outcomes of endemic Clostridium difficile‐associated disease in nonsurgical patients. Emerg Infect Dis. 2008;14:1031–1038.
- , , . Increase in adult Clostridium difficile‐related hospitalizations and case‐fatality rate, United States, 2000–2005. Emerg Infect Dis. 2008;14(6): 929–931.
- , , , et al. Health care‐associated Clostridium difficile infection in adults admitted to acute care hospitals in Canada: a Canadian Nosocomial Infection Surveillance Program study. Clin Infect Dis. 2009:48(5);568–576.
- , , , et al. Analysis of 30‐day mortality for Clostridium difficile‐associated disease in the ICU setting. Cheat. 2007;132(2): 418–424.
- , , , et al. Deaths: final data for 2006. Natl Vital Stat Rep. 2009;57(14): 1–134.
- , , . Factors associated with failure of metronidazole in Clostridium difficile‐associated disease. J Clin Gastroenterol. 2004;38(5): 414–418.
- , , , et al. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis. 2005;40(11): 1586–1590.
- , , , et al. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40(11): 1591–1597.
- , , , et al. Outcome of metronidazole therapy for Clostridium difficile disease and correlation with a scoring system. J Infect. 2007;55(6): 495–501.
- Centers for Disease Control and Prevention. Information about the current strain of Clostridium difficile. Available at: http://www.cdc.gov/HAI/organisms/cdiff/Cdiff‐current‐strain.html. Last updated: January 25, 2011. Accessed October 9, 2011.
- , , , et al. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile‐associated diarrhea. Clin Infect Dis. 1996;22(5): 813–818.
- , , . Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97(7): 1769–1775.
- , , , . Use of gastric acid‐suppressive agents and the risk of community‐acquired Clostridium difficile‐associated disease. JAMA. 2005;294(23): 2989–2995.
- , , , , . Patterns of antibiotic use and risk of hospital admission because of Clostridium difficile infection. Can Med Assoc J. 2008;179(8): 767–772.
- Centers for Disease Control and Prevention. Severe Clostridium difficile‐associated disease in populations previously at low risk—four states, 2005. MMWR. 2005;54(47): 1201–1205.
- . Clostridium difficile‐associated disease: an emerging threat to patient safety: insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy. 2006;26(3): 299–311.
- , , , et al. Antibiotics and Clostridium difficile in the ambulatory care setting. Clin Ther. 2000;22(1): 91–102.
- Centers for Disease Control and Prevention. Surveillance for community‐associated C. difficile—Connecticut, 2006. MMWR. 2008;57:340–343.
- , O', , et al. Clostridium difficile‐associated diarrhea: an emerging threat to pregnant women. Am J Obstet Gynecol. 2008;198(6):635.e1–635.e6.
- , , , et al. Short‐ and long‐term attributable costs of Clostridium difficile‐associated disease in nonsurgical inpatients. Clin Infect Dis. 2008;46(4): 497–504.
- , , , . Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infect Dis. 2002;34(3): 346–353.
- , , , . The emerging infectious challenge of Clostridium difficile‐associated disease in Massachusetts hospitals: clinical and economic consequences. Infect Control Hosp Epidemiol. 2007;28(11): 1219–1227.
- , , , , . National Clostridium difficile infection (CDI) related hospitalizations approaches MRSA related hospitalizations. The need for antibiotic stewardship program [poster 94]. Presented at: The SHEA 2011 Annual Scientific Meeting. April 2, 2011; Dallas, TX.
Clostridium difficile is a gram‐positive, spore‐forming, toxin‐producing, anaerobic bacillus that was established as the causative pathogen of most cases of antibiotic‐associated colitis in 1978. 1, 2 The spectrum of possible clinical presentations of C. difficile range from asymptomatic colonization, uncomplicated diarrhea, severe pseudomembranous colitis, paralytic ileus, to sepsis and death, with a mortality rate upwards of 80% in fulminant cases requiring colectomy. 3
Vegetative C. difficile cells die rapidly on dry surfaces, but they have been found to remain viable for up to 6 hours on moist surfaces in room air. 4 Spores shed from the gastrointestinal (GI) tract, however, are highly resistant to common hospital disinfectants, and can survive in the environment for many months. 2 C. difficile spores are primarily transmitted from patient to patient on the hands or equipment of healthcare workers. 2 Once spores are ingested and reach the GI tract, they germinate in the vegetative form. 2, 5 In the GI tract, C. difficile causes disease by the production of toxins, primarily toxins A and B, both of which cause severe inflammation. 5 Toxin A attracts neutrophils and monocytes, and toxin B breaks down colonic epithelial cells. 5 Both of these mechanisms lead to colitis, formation of pseudomembranes, and watery diarrhea. 5
After alteration of the healthy colonic bacterial flora, the immune response to C. difficile toxins appears to play a major role in determining host susceptibility to C. difficile infection (CDI). 5, 6 Those with antitoxin immunity are more likely to become symptomless carriers than patients without preexisting immunity. 3 More than 60% of healthy adults have protective immunity against a primary CDI, demonstrated by detectable serum IgG and IgA to both toxins A and B, as a consequence of childhood immunity or frequent exposure to C. difficile in the environment. 3 After a primary episode of CDI, many patients acquire protective immunity against C. difficile toxins, seen as significantly higher serum concentrations of IgM against C. difficile toxin by the third day from onset of diarrhea, and significantly higher serum concentrations of IgG against toxin A by the 12th day. 7 Patients who experience recurrent CDI lack development of this protective immunity to C. difficile. 6, 7
CDI INCIDENCE IS ON THE RISE
During the past decade, rates of CDI have increased steadily to levels not previously seen. A report published by the Agency of Healthcare Research and Quality demonstrated that the number of CDI diagnoses on hospital discharge more than doubled in the United States from 139,000 to 301,200 between 2000 and 2005 (Figure 1). 8 Examination of a more recent Nationwide Inpatient Sample (NIS) indicates continuation of this trend, with nearly 350,000 CDI diagnoses recorded upon discharge from acute care hospitals in 2008. 9 Of note, in 2006 the state of Ohio mandated CDI reporting from both hospitals and nursing homes. It was estimated there were more than 18,000 cases of CDI during this year, of which more than 60% were diagnosed in nursing homes. 10 Based on the 2008 NIS data and the data from Ohio, it is conceivable there were as many as 1 million cases of CDI in the US in 2008.

8
This increased incidence of CDI contrasts with several other healthcare‐associated infections, which have declined in incidence over the last decade. 1113 C. difficile is the most common causative agent of healthcare‐associated infections in some areas. A cohort study of common infections among inpatients at 30 community hospitals in the Duke Infection Control Outreach Network conducted between January 1, 2008 and June 30, 2009 found the incidence of CDI cases was 0.26 cases per 1000 patient‐days, which was higher than the incidence of methicillin‐resistant Staphylococcus aureus (MRSA) at 0.22 cases per 1000 patient‐days. 14 Another study utilizing the NIS data found that, while vancomycin‐resistant enterococcus and pseudomonas infections remained stable, CDI increased in many areas of the country and was more common than MRSA in some areas. 15
HYPERVIRULENT STRAIN OF C. DIFFICILE
In the early 2000s, an epidemic and hypervirulent strain of C. difficile emerged in North America and Europe that altered the epidemiology of CDI. 16 Due to multiple different methods for molecular typing of C. difficile, this strain has several names depending on the method of typing performed. The most common names for this strain are BI (REA typing), NAP1 (pulsed field gel electrophoresis), and 027 (PCR‐ribotyping). This strain has become the predominant strain of C. difficile in some areas, accounting for more than 80% of CDI cases in some areas. 3
The characteristics of this particular strain epidemic in North America typically include:
-
A deletion in the tcd gene that downregulates toxin production, which renders the gene nonfunctional in the epidemic strain. Some in vitro data have demonstrated that this epidemic strain produces 16‐fold higher concentrations of toxin A and 23‐fold higher concentrations of toxin B than nonepidemic strains of C. difficile. 17
-
Production of a third toxin, called binary toxin CDT. The role of this toxin in the pathogenesis of CDI is not clear, but the presence of this toxin has been associated with more severe CDI‐related diarrhea. 2, 16
-
High‐level resistance to fluoroquinolones, including moxifloxacin and gatifloxacin. 5, 16 It has been theorized that increasing use of fluoroquinolones during the past decade may have provided a selective advantage for the BI/NAP1/027 strain to predominate. 2
-
Production of more spores than other strains of C. difficile. 17, 18 This may increase its ability to contaminate the environment and be transmitted in a healthcare facility.
CDI SEVERITY IS INCREASING
Paralleling the increased prevalence of CDI, C. difficile infections are generally becoming more severe. In Sherbrooke, Quebec, Canada, which experienced a dramatic outbreak of CDI associated with increased CDI severity, the cumulative 1‐year attributable mortality was nearly 37% (60 of 161 CDI cases) in a hospital case review of nonsurgical admissions between January 2003 and June 2004. 19 In St Louis, Missouri in 2003, a 5.7% 180‐day mortality rate was reported in an endemic setting. 20 Among the 24% of patients readmitted within 180 days of discharge (4207 of 17,492) in this retrospective case review, patients with CDI were more than twice as likely as non‐CDI patients to be readmitted to the hospital (52% vs 23%, N = 4207). 20 Furthermore, patients with CDI were significantly more likely to require discharge to a long‐term care facility (32%) than non‐CDI controls (23%). 19
Based on NIS data for CDI‐related hospitalizations between 2000 and 2005, the crude, age‐adjusted case‐fatality rate rose from 1.2% in 2000 to 2.2% in 2004. 21 This increase was mirrored by a doubling of CDI cases admitted for hospitalization during the same 6‐year period. 21 According to the investigators, these findings indirectly confirm that the doubling in CDI deaths is attributable to an increase in C. difficile virulence. 21 A 6‐month prospective surveillance of CDI patient outcomes in 29 Canadian hospitals was conducted by the Canadian Nosocomial Infection Surveillance Program (CNISP) beginning in November 2004. 22 At 30 days after onset of CDI, the percentage of deaths directly or indirectly attributable to CDI was 5.7%, which represented an almost 4‐fold increase over CDI‐attributable deaths recorded in the 1997 CNISP survey. 22 Overall 30‐day mortality was retrospectively analyzed among patients with CDI in a St Louis, Missouri 1200‐bed teaching hospital intensive care unit (ICU) over a 2‐year period (20042005). 23 The 30‐day crude mortality among 278 patients admitted to the ICU with CDI was 37% (n = 102), and mortality directly attributable to CDI in these critically ill patients was 6%. 23 The number of deaths in the United States due to CDI increased sharply from 793 patients in 1999 to 6225 patients in 2006. 24 In 2006, it ranked among the top 20 causes of death for those aged 65 years and older. 24
INCREASE IN TREATMENT FAILURES
In addition to being more severe, there have been several reports of increases in CDI treatment failures and/or increases in recurrent CDI. 6 Recent studies indicate there may be more metronidazole treatment failures regardless of whether the infecting strain is the BI/NAP1/027 strain, despite a lack of laboratory evidence indicating resistance to metronidazole. 2529 Regardless of the initial therapy chosen, patients must be carefully monitored to ensure they are responding appropriately to treatment and their condition is not deteriorating. 29 Some of the original trials of CDI treatments found relapse rates as low as 5% to 15%. 30 More recent data indicate relapse occurs after 30% of initial CDI episodes, and as frequent as 65% if the patient has had multiple prior CDI episodes. 3, 6, 31
COMMUNITY‐ASSOCIATED CDI
The epidemiology of community‐associated CDI may also be changing. Virulent strains, which cause more severe disease in high‐risk patients, may also cause more frequent, severe disease in populations previously thought to be at low risk. Some studies have found an increase in community‐associated CDI in otherwise healthy individuals with little or no exposure to a healthcare facility. Although antimicrobial exposure remains the most important risk factor for community‐associated CDI, antimicrobial exposure is less common in community‐associated CDI than healthcare‐associated CDI. 3235
In a Canadian study, the rate of diagnosed community‐acquired CDI cases was stable at about 22 cases per 100,000 patient‐years per calendar year between 1998 and 2002, but rose steadily for the next 2 years to 53 cases per 100,000 patient‐years in 2004. 33 Similar results were seen in the United Kingdom, with an exponential increase from fewer than 1 case per 100,000 person‐years in 1994 to 22 cases per 100,000 person‐years in 2004. 32 There are currently no comprehensive longitudinal studies in the United States investigating the incidence of purely community‐acquired CDI where a patient had no prior hospital exposure. However, regional surveys have reported an incidence of community‐acquired CDI of 12 cases per 100,000 person‐years during 1992 to 1994, 36 7.6 cases per 100,000 person‐years in 2005, 37 and 6.9 cases per 100,000 person‐years in 2006. 34, 37
One patient population generally thought to be at low risk for CDI that may be at increased risk for severe CDI is pregnant women. In one study 419 infectious disease consultants who responded to a survey conducted by the Emerging Infections Network had seen or were aware of 55 cases of CDI in peripartum women. 38 There were 21 cases with complications, including 10 relapses and 5 cases of toxic megacolon. 38 In a prior report of severe CDI among 10 peripartum women, 3 women died and 3 infants (2 were twins) were stillborn. 38 This data emphasizes why clinicians must have a high index of suspicion for CDI, and should be aware of the potential for severe outcomes, even in patients traditionally considered to be at low risk. 38
ECONOMIC IMPACT OF CDI
The economic burden of CDI in the United States is staggering, with estimates ranging from $1.1 to $3.2 billion annually (Table 1). 3941 These estimates are based on the cost of caring for patients with CDI in acute care facilities and are primarily driven by increased length of stay in the hospital due to CDI. These data also predate the emergence of the BI/NAP1/027 strain. Therefore, the costs of CDI are likely higher than these estimates due to the increases in CDI severity seen since these studies were performed. It is important to note that these studies did not include patients diagnosed and treated in nursing homes or the community, nor the increase in costs due to discharge to a long‐term care facility. 39
| Study | Patient Population | Estimated Attributable Cost per Episode* | Increase in LOS, days | Estimated Annual Attributable Cost, US |
|---|---|---|---|---|
| ||||
| Kyne et al 40 | Two medical wards (n = 40) | $3669 | 3.6 | $1.1 billion |
| Dubberke et al 39 | Nonsurgical patients∥ (n = 439) | $2454$3240 | 3.0 | $897 million$1.3 billion# |
| O'Brien et al 41 | Massachusetts discharge database (n = 3692)** | Primary diagnosis: $10,212; secondary diagnosis: $13,675 | Primary diagnosis: 6.4; secondary diagnosis: 2.9 | $3.2 billion |
SUMMARY
C. difficile infections are becoming more prevalent and more severe. The issue is sufficiently serious that healthcare‐onset CDI has recently been called a major public health threat. 42 For this reason, efforts to combat virulent C. difficile should include good antimicrobial stewardship, effective infection control, and control of environmental factors that promote transmission. 35 Healthcare professionals who oversee the care of inpatients should act as catalysts for improvement by taking a leadership role in the multidisciplinary approach needed to reduce the morbidity, mortality, and cost burden for patients and the healthcare system.
Clostridium difficile is a gram‐positive, spore‐forming, toxin‐producing, anaerobic bacillus that was established as the causative pathogen of most cases of antibiotic‐associated colitis in 1978. 1, 2 The spectrum of possible clinical presentations of C. difficile range from asymptomatic colonization, uncomplicated diarrhea, severe pseudomembranous colitis, paralytic ileus, to sepsis and death, with a mortality rate upwards of 80% in fulminant cases requiring colectomy. 3
Vegetative C. difficile cells die rapidly on dry surfaces, but they have been found to remain viable for up to 6 hours on moist surfaces in room air. 4 Spores shed from the gastrointestinal (GI) tract, however, are highly resistant to common hospital disinfectants, and can survive in the environment for many months. 2 C. difficile spores are primarily transmitted from patient to patient on the hands or equipment of healthcare workers. 2 Once spores are ingested and reach the GI tract, they germinate in the vegetative form. 2, 5 In the GI tract, C. difficile causes disease by the production of toxins, primarily toxins A and B, both of which cause severe inflammation. 5 Toxin A attracts neutrophils and monocytes, and toxin B breaks down colonic epithelial cells. 5 Both of these mechanisms lead to colitis, formation of pseudomembranes, and watery diarrhea. 5
After alteration of the healthy colonic bacterial flora, the immune response to C. difficile toxins appears to play a major role in determining host susceptibility to C. difficile infection (CDI). 5, 6 Those with antitoxin immunity are more likely to become symptomless carriers than patients without preexisting immunity. 3 More than 60% of healthy adults have protective immunity against a primary CDI, demonstrated by detectable serum IgG and IgA to both toxins A and B, as a consequence of childhood immunity or frequent exposure to C. difficile in the environment. 3 After a primary episode of CDI, many patients acquire protective immunity against C. difficile toxins, seen as significantly higher serum concentrations of IgM against C. difficile toxin by the third day from onset of diarrhea, and significantly higher serum concentrations of IgG against toxin A by the 12th day. 7 Patients who experience recurrent CDI lack development of this protective immunity to C. difficile. 6, 7
CDI INCIDENCE IS ON THE RISE
During the past decade, rates of CDI have increased steadily to levels not previously seen. A report published by the Agency of Healthcare Research and Quality demonstrated that the number of CDI diagnoses on hospital discharge more than doubled in the United States from 139,000 to 301,200 between 2000 and 2005 (Figure 1). 8 Examination of a more recent Nationwide Inpatient Sample (NIS) indicates continuation of this trend, with nearly 350,000 CDI diagnoses recorded upon discharge from acute care hospitals in 2008. 9 Of note, in 2006 the state of Ohio mandated CDI reporting from both hospitals and nursing homes. It was estimated there were more than 18,000 cases of CDI during this year, of which more than 60% were diagnosed in nursing homes. 10 Based on the 2008 NIS data and the data from Ohio, it is conceivable there were as many as 1 million cases of CDI in the US in 2008.

8
This increased incidence of CDI contrasts with several other healthcare‐associated infections, which have declined in incidence over the last decade. 1113 C. difficile is the most common causative agent of healthcare‐associated infections in some areas. A cohort study of common infections among inpatients at 30 community hospitals in the Duke Infection Control Outreach Network conducted between January 1, 2008 and June 30, 2009 found the incidence of CDI cases was 0.26 cases per 1000 patient‐days, which was higher than the incidence of methicillin‐resistant Staphylococcus aureus (MRSA) at 0.22 cases per 1000 patient‐days. 14 Another study utilizing the NIS data found that, while vancomycin‐resistant enterococcus and pseudomonas infections remained stable, CDI increased in many areas of the country and was more common than MRSA in some areas. 15
HYPERVIRULENT STRAIN OF C. DIFFICILE
In the early 2000s, an epidemic and hypervirulent strain of C. difficile emerged in North America and Europe that altered the epidemiology of CDI. 16 Due to multiple different methods for molecular typing of C. difficile, this strain has several names depending on the method of typing performed. The most common names for this strain are BI (REA typing), NAP1 (pulsed field gel electrophoresis), and 027 (PCR‐ribotyping). This strain has become the predominant strain of C. difficile in some areas, accounting for more than 80% of CDI cases in some areas. 3
The characteristics of this particular strain epidemic in North America typically include:
-
A deletion in the tcd gene that downregulates toxin production, which renders the gene nonfunctional in the epidemic strain. Some in vitro data have demonstrated that this epidemic strain produces 16‐fold higher concentrations of toxin A and 23‐fold higher concentrations of toxin B than nonepidemic strains of C. difficile. 17
-
Production of a third toxin, called binary toxin CDT. The role of this toxin in the pathogenesis of CDI is not clear, but the presence of this toxin has been associated with more severe CDI‐related diarrhea. 2, 16
-
High‐level resistance to fluoroquinolones, including moxifloxacin and gatifloxacin. 5, 16 It has been theorized that increasing use of fluoroquinolones during the past decade may have provided a selective advantage for the BI/NAP1/027 strain to predominate. 2
-
Production of more spores than other strains of C. difficile. 17, 18 This may increase its ability to contaminate the environment and be transmitted in a healthcare facility.
CDI SEVERITY IS INCREASING
Paralleling the increased prevalence of CDI, C. difficile infections are generally becoming more severe. In Sherbrooke, Quebec, Canada, which experienced a dramatic outbreak of CDI associated with increased CDI severity, the cumulative 1‐year attributable mortality was nearly 37% (60 of 161 CDI cases) in a hospital case review of nonsurgical admissions between January 2003 and June 2004. 19 In St Louis, Missouri in 2003, a 5.7% 180‐day mortality rate was reported in an endemic setting. 20 Among the 24% of patients readmitted within 180 days of discharge (4207 of 17,492) in this retrospective case review, patients with CDI were more than twice as likely as non‐CDI patients to be readmitted to the hospital (52% vs 23%, N = 4207). 20 Furthermore, patients with CDI were significantly more likely to require discharge to a long‐term care facility (32%) than non‐CDI controls (23%). 19
Based on NIS data for CDI‐related hospitalizations between 2000 and 2005, the crude, age‐adjusted case‐fatality rate rose from 1.2% in 2000 to 2.2% in 2004. 21 This increase was mirrored by a doubling of CDI cases admitted for hospitalization during the same 6‐year period. 21 According to the investigators, these findings indirectly confirm that the doubling in CDI deaths is attributable to an increase in C. difficile virulence. 21 A 6‐month prospective surveillance of CDI patient outcomes in 29 Canadian hospitals was conducted by the Canadian Nosocomial Infection Surveillance Program (CNISP) beginning in November 2004. 22 At 30 days after onset of CDI, the percentage of deaths directly or indirectly attributable to CDI was 5.7%, which represented an almost 4‐fold increase over CDI‐attributable deaths recorded in the 1997 CNISP survey. 22 Overall 30‐day mortality was retrospectively analyzed among patients with CDI in a St Louis, Missouri 1200‐bed teaching hospital intensive care unit (ICU) over a 2‐year period (20042005). 23 The 30‐day crude mortality among 278 patients admitted to the ICU with CDI was 37% (n = 102), and mortality directly attributable to CDI in these critically ill patients was 6%. 23 The number of deaths in the United States due to CDI increased sharply from 793 patients in 1999 to 6225 patients in 2006. 24 In 2006, it ranked among the top 20 causes of death for those aged 65 years and older. 24
INCREASE IN TREATMENT FAILURES
In addition to being more severe, there have been several reports of increases in CDI treatment failures and/or increases in recurrent CDI. 6 Recent studies indicate there may be more metronidazole treatment failures regardless of whether the infecting strain is the BI/NAP1/027 strain, despite a lack of laboratory evidence indicating resistance to metronidazole. 2529 Regardless of the initial therapy chosen, patients must be carefully monitored to ensure they are responding appropriately to treatment and their condition is not deteriorating. 29 Some of the original trials of CDI treatments found relapse rates as low as 5% to 15%. 30 More recent data indicate relapse occurs after 30% of initial CDI episodes, and as frequent as 65% if the patient has had multiple prior CDI episodes. 3, 6, 31
COMMUNITY‐ASSOCIATED CDI
The epidemiology of community‐associated CDI may also be changing. Virulent strains, which cause more severe disease in high‐risk patients, may also cause more frequent, severe disease in populations previously thought to be at low risk. Some studies have found an increase in community‐associated CDI in otherwise healthy individuals with little or no exposure to a healthcare facility. Although antimicrobial exposure remains the most important risk factor for community‐associated CDI, antimicrobial exposure is less common in community‐associated CDI than healthcare‐associated CDI. 3235
In a Canadian study, the rate of diagnosed community‐acquired CDI cases was stable at about 22 cases per 100,000 patient‐years per calendar year between 1998 and 2002, but rose steadily for the next 2 years to 53 cases per 100,000 patient‐years in 2004. 33 Similar results were seen in the United Kingdom, with an exponential increase from fewer than 1 case per 100,000 person‐years in 1994 to 22 cases per 100,000 person‐years in 2004. 32 There are currently no comprehensive longitudinal studies in the United States investigating the incidence of purely community‐acquired CDI where a patient had no prior hospital exposure. However, regional surveys have reported an incidence of community‐acquired CDI of 12 cases per 100,000 person‐years during 1992 to 1994, 36 7.6 cases per 100,000 person‐years in 2005, 37 and 6.9 cases per 100,000 person‐years in 2006. 34, 37
One patient population generally thought to be at low risk for CDI that may be at increased risk for severe CDI is pregnant women. In one study 419 infectious disease consultants who responded to a survey conducted by the Emerging Infections Network had seen or were aware of 55 cases of CDI in peripartum women. 38 There were 21 cases with complications, including 10 relapses and 5 cases of toxic megacolon. 38 In a prior report of severe CDI among 10 peripartum women, 3 women died and 3 infants (2 were twins) were stillborn. 38 This data emphasizes why clinicians must have a high index of suspicion for CDI, and should be aware of the potential for severe outcomes, even in patients traditionally considered to be at low risk. 38
ECONOMIC IMPACT OF CDI
The economic burden of CDI in the United States is staggering, with estimates ranging from $1.1 to $3.2 billion annually (Table 1). 3941 These estimates are based on the cost of caring for patients with CDI in acute care facilities and are primarily driven by increased length of stay in the hospital due to CDI. These data also predate the emergence of the BI/NAP1/027 strain. Therefore, the costs of CDI are likely higher than these estimates due to the increases in CDI severity seen since these studies were performed. It is important to note that these studies did not include patients diagnosed and treated in nursing homes or the community, nor the increase in costs due to discharge to a long‐term care facility. 39
| Study | Patient Population | Estimated Attributable Cost per Episode* | Increase in LOS, days | Estimated Annual Attributable Cost, US |
|---|---|---|---|---|
| ||||
| Kyne et al 40 | Two medical wards (n = 40) | $3669 | 3.6 | $1.1 billion |
| Dubberke et al 39 | Nonsurgical patients∥ (n = 439) | $2454$3240 | 3.0 | $897 million$1.3 billion# |
| O'Brien et al 41 | Massachusetts discharge database (n = 3692)** | Primary diagnosis: $10,212; secondary diagnosis: $13,675 | Primary diagnosis: 6.4; secondary diagnosis: 2.9 | $3.2 billion |
SUMMARY
C. difficile infections are becoming more prevalent and more severe. The issue is sufficiently serious that healthcare‐onset CDI has recently been called a major public health threat. 42 For this reason, efforts to combat virulent C. difficile should include good antimicrobial stewardship, effective infection control, and control of environmental factors that promote transmission. 35 Healthcare professionals who oversee the care of inpatients should act as catalysts for improvement by taking a leadership role in the multidisciplinary approach needed to reduce the morbidity, mortality, and cost burden for patients and the healthcare system.
- . Narrative review: the new epidemic of Clostridium difficile‐associated enteric disease. Ann Intern Med. 2006;145(10): 758–764.
- Association for Professionals in Infection Control and Epidemiology, Inc (APIC). Guide to the elimination of Clostridium difficile in healthcare settings. Available at: http://www.apic.org/Content/NavigationMenu/PracticeGuidance/APICEliminationGuides/C.diff_Elimination_guide_logo.pdf. 2008. Accessed October 8, 2011.
- . Clostridium difficile and the disease it causes. Methods Mol Biol. 2010;646:9–35.
- , , . Vegetative Clostridium difficile survives in room air on moist surfaces and in gastric contents with reduced acidity: a potential mechanism to explain the association between proton pump inhibitors and C. difficile‐associated diarrhea?Antimicrob Agents Chemother. 2007;51(8): 2883–2887.
- , . Clostridium difficile‐associated disease: new challenges from an established pathogen. Cleve Clin J Med. 2006;73(2): 187–197.
- . A 76‐year‐old man with recurrent Clostridium difficile‐associated diarrhea: review of C difficile infection. JAMA. 2009;301(9): 954–962.
- , , , . Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet. 2001;357(9251): 189–193.
- , .Clostridium difficile‐associated disease in US hospitals, 1993–2005. Healthcare Cost and Utilization Project. Statistical Brief #50. April 2008. Available at: http://www.ncbi.nlm.nih.gov/books/NBK56038/pdf/sb50.pdf. Accessed December 12, 2011.
- Agency of Healthcare Research and Quality. Healthcare Cost and Utilization Project Database. Available at: http://www.hcup‐us.ahrq.gov/nisoverview.jsp. Accessed April 2011.
- , , , et al. Clostridium difficile infection in Ohio hospitals and nursing homes during 2006. Infect Control Hosp Epidemiol. 2009;30(6): 526–533.
- , , , et al. Trends in catheter‐associated urinary tract infections in adult intensive care units—United States, 1990–2007. Infect Control Hosp Epidemiol. 2011;32(8): 748–756.
- , , , et al. Methicillin‐resistant Staphylococcus aureus central line‐associated bloodstream infections in US intensive care units, 1997–2007. JAMA. 2009;301(7): 727–736.
- , , , et al. An intervention to decrease catheter‐related bloodstream infections in the ICU. N Engl J Med. 2006;355(26): 2725–2732.
- , , , .The impact of hospital‐onset healthcare facility associated (HO‐HCFA) Clostridium difficile infection (CDI) in community hospitals: surpassing methicillin‐resistant Staphylococcus aureus (MRSA) as the new superbug [abstract 386]. Presented at: The Fifth Decennial International Conference on Healthcare‐Associated Infections (ICHAI). March 20, 2010; Atlanta, GA.
- , , . Growth and geographic variation in hospitalizations with resistant infections, United States, 2000–2005. Emerg Infect Dis. 2008;14(11): 1756–1758.
- , , . An epidemic, toxin gene‐variant strain of Clostridium difficile. New Engl J Med. 2005;353(23): 2433–2441.
- , , , et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366(9491): 1079–1084.
- , , , et al. Increased sporulation rate of epidemic Clostridium difficile type 027/NAP1. J Clin Microbiol. 2008;46(4): 1530–1533.
- , , . Mortality attributable to nosocomial Clostridium difficile‐ associated disease during an epidemic caused by a hypervirulent strain in Quebec. Can Med Assoc J. 2005;173(9): 1037–1042.
- , , , et al. Attributable outcomes of endemic Clostridium difficile‐associated disease in nonsurgical patients. Emerg Infect Dis. 2008;14:1031–1038.
- , , . Increase in adult Clostridium difficile‐related hospitalizations and case‐fatality rate, United States, 2000–2005. Emerg Infect Dis. 2008;14(6): 929–931.
- , , , et al. Health care‐associated Clostridium difficile infection in adults admitted to acute care hospitals in Canada: a Canadian Nosocomial Infection Surveillance Program study. Clin Infect Dis. 2009:48(5);568–576.
- , , , et al. Analysis of 30‐day mortality for Clostridium difficile‐associated disease in the ICU setting. Cheat. 2007;132(2): 418–424.
- , , , et al. Deaths: final data for 2006. Natl Vital Stat Rep. 2009;57(14): 1–134.
- , , . Factors associated with failure of metronidazole in Clostridium difficile‐associated disease. J Clin Gastroenterol. 2004;38(5): 414–418.
- , , , et al. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis. 2005;40(11): 1586–1590.
- , , , et al. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40(11): 1591–1597.
- , , , et al. Outcome of metronidazole therapy for Clostridium difficile disease and correlation with a scoring system. J Infect. 2007;55(6): 495–501.
- Centers for Disease Control and Prevention. Information about the current strain of Clostridium difficile. Available at: http://www.cdc.gov/HAI/organisms/cdiff/Cdiff‐current‐strain.html. Last updated: January 25, 2011. Accessed October 9, 2011.
- , , , et al. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile‐associated diarrhea. Clin Infect Dis. 1996;22(5): 813–818.
- , , . Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97(7): 1769–1775.
- , , , . Use of gastric acid‐suppressive agents and the risk of community‐acquired Clostridium difficile‐associated disease. JAMA. 2005;294(23): 2989–2995.
- , , , , . Patterns of antibiotic use and risk of hospital admission because of Clostridium difficile infection. Can Med Assoc J. 2008;179(8): 767–772.
- Centers for Disease Control and Prevention. Severe Clostridium difficile‐associated disease in populations previously at low risk—four states, 2005. MMWR. 2005;54(47): 1201–1205.
- . Clostridium difficile‐associated disease: an emerging threat to patient safety: insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy. 2006;26(3): 299–311.
- , , , et al. Antibiotics and Clostridium difficile in the ambulatory care setting. Clin Ther. 2000;22(1): 91–102.
- Centers for Disease Control and Prevention. Surveillance for community‐associated C. difficile—Connecticut, 2006. MMWR. 2008;57:340–343.
- , O', , et al. Clostridium difficile‐associated diarrhea: an emerging threat to pregnant women. Am J Obstet Gynecol. 2008;198(6):635.e1–635.e6.
- , , , et al. Short‐ and long‐term attributable costs of Clostridium difficile‐associated disease in nonsurgical inpatients. Clin Infect Dis. 2008;46(4): 497–504.
- , , , . Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infect Dis. 2002;34(3): 346–353.
- , , , . The emerging infectious challenge of Clostridium difficile‐associated disease in Massachusetts hospitals: clinical and economic consequences. Infect Control Hosp Epidemiol. 2007;28(11): 1219–1227.
- , , , , . National Clostridium difficile infection (CDI) related hospitalizations approaches MRSA related hospitalizations. The need for antibiotic stewardship program [poster 94]. Presented at: The SHEA 2011 Annual Scientific Meeting. April 2, 2011; Dallas, TX.
- . Narrative review: the new epidemic of Clostridium difficile‐associated enteric disease. Ann Intern Med. 2006;145(10): 758–764.
- Association for Professionals in Infection Control and Epidemiology, Inc (APIC). Guide to the elimination of Clostridium difficile in healthcare settings. Available at: http://www.apic.org/Content/NavigationMenu/PracticeGuidance/APICEliminationGuides/C.diff_Elimination_guide_logo.pdf. 2008. Accessed October 8, 2011.
- . Clostridium difficile and the disease it causes. Methods Mol Biol. 2010;646:9–35.
- , , . Vegetative Clostridium difficile survives in room air on moist surfaces and in gastric contents with reduced acidity: a potential mechanism to explain the association between proton pump inhibitors and C. difficile‐associated diarrhea?Antimicrob Agents Chemother. 2007;51(8): 2883–2887.
- , . Clostridium difficile‐associated disease: new challenges from an established pathogen. Cleve Clin J Med. 2006;73(2): 187–197.
- . A 76‐year‐old man with recurrent Clostridium difficile‐associated diarrhea: review of C difficile infection. JAMA. 2009;301(9): 954–962.
- , , , . Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet. 2001;357(9251): 189–193.
- , .Clostridium difficile‐associated disease in US hospitals, 1993–2005. Healthcare Cost and Utilization Project. Statistical Brief #50. April 2008. Available at: http://www.ncbi.nlm.nih.gov/books/NBK56038/pdf/sb50.pdf. Accessed December 12, 2011.
- Agency of Healthcare Research and Quality. Healthcare Cost and Utilization Project Database. Available at: http://www.hcup‐us.ahrq.gov/nisoverview.jsp. Accessed April 2011.
- , , , et al. Clostridium difficile infection in Ohio hospitals and nursing homes during 2006. Infect Control Hosp Epidemiol. 2009;30(6): 526–533.
- , , , et al. Trends in catheter‐associated urinary tract infections in adult intensive care units—United States, 1990–2007. Infect Control Hosp Epidemiol. 2011;32(8): 748–756.
- , , , et al. Methicillin‐resistant Staphylococcus aureus central line‐associated bloodstream infections in US intensive care units, 1997–2007. JAMA. 2009;301(7): 727–736.
- , , , et al. An intervention to decrease catheter‐related bloodstream infections in the ICU. N Engl J Med. 2006;355(26): 2725–2732.
- , , , .The impact of hospital‐onset healthcare facility associated (HO‐HCFA) Clostridium difficile infection (CDI) in community hospitals: surpassing methicillin‐resistant Staphylococcus aureus (MRSA) as the new superbug [abstract 386]. Presented at: The Fifth Decennial International Conference on Healthcare‐Associated Infections (ICHAI). March 20, 2010; Atlanta, GA.
- , , . Growth and geographic variation in hospitalizations with resistant infections, United States, 2000–2005. Emerg Infect Dis. 2008;14(11): 1756–1758.
- , , . An epidemic, toxin gene‐variant strain of Clostridium difficile. New Engl J Med. 2005;353(23): 2433–2441.
- , , , et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366(9491): 1079–1084.
- , , , et al. Increased sporulation rate of epidemic Clostridium difficile type 027/NAP1. J Clin Microbiol. 2008;46(4): 1530–1533.
- , , . Mortality attributable to nosocomial Clostridium difficile‐ associated disease during an epidemic caused by a hypervirulent strain in Quebec. Can Med Assoc J. 2005;173(9): 1037–1042.
- , , , et al. Attributable outcomes of endemic Clostridium difficile‐associated disease in nonsurgical patients. Emerg Infect Dis. 2008;14:1031–1038.
- , , . Increase in adult Clostridium difficile‐related hospitalizations and case‐fatality rate, United States, 2000–2005. Emerg Infect Dis. 2008;14(6): 929–931.
- , , , et al. Health care‐associated Clostridium difficile infection in adults admitted to acute care hospitals in Canada: a Canadian Nosocomial Infection Surveillance Program study. Clin Infect Dis. 2009:48(5);568–576.
- , , , et al. Analysis of 30‐day mortality for Clostridium difficile‐associated disease in the ICU setting. Cheat. 2007;132(2): 418–424.
- , , , et al. Deaths: final data for 2006. Natl Vital Stat Rep. 2009;57(14): 1–134.
- , , . Factors associated with failure of metronidazole in Clostridium difficile‐associated disease. J Clin Gastroenterol. 2004;38(5): 414–418.
- , , , et al. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis. 2005;40(11): 1586–1590.
- , , , et al. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005;40(11): 1591–1597.
- , , , et al. Outcome of metronidazole therapy for Clostridium difficile disease and correlation with a scoring system. J Infect. 2007;55(6): 495–501.
- Centers for Disease Control and Prevention. Information about the current strain of Clostridium difficile. Available at: http://www.cdc.gov/HAI/organisms/cdiff/Cdiff‐current‐strain.html. Last updated: January 25, 2011. Accessed October 9, 2011.
- , , , et al. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile‐associated diarrhea. Clin Infect Dis. 1996;22(5): 813–818.
- , , . Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97(7): 1769–1775.
- , , , . Use of gastric acid‐suppressive agents and the risk of community‐acquired Clostridium difficile‐associated disease. JAMA. 2005;294(23): 2989–2995.
- , , , , . Patterns of antibiotic use and risk of hospital admission because of Clostridium difficile infection. Can Med Assoc J. 2008;179(8): 767–772.
- Centers for Disease Control and Prevention. Severe Clostridium difficile‐associated disease in populations previously at low risk—four states, 2005. MMWR. 2005;54(47): 1201–1205.
- . Clostridium difficile‐associated disease: an emerging threat to patient safety: insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy. 2006;26(3): 299–311.
- , , , et al. Antibiotics and Clostridium difficile in the ambulatory care setting. Clin Ther. 2000;22(1): 91–102.
- Centers for Disease Control and Prevention. Surveillance for community‐associated C. difficile—Connecticut, 2006. MMWR. 2008;57:340–343.
- , O', , et al. Clostridium difficile‐associated diarrhea: an emerging threat to pregnant women. Am J Obstet Gynecol. 2008;198(6):635.e1–635.e6.
- , , , et al. Short‐ and long‐term attributable costs of Clostridium difficile‐associated disease in nonsurgical inpatients. Clin Infect Dis. 2008;46(4): 497–504.
- , , , . Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infect Dis. 2002;34(3): 346–353.
- , , , . The emerging infectious challenge of Clostridium difficile‐associated disease in Massachusetts hospitals: clinical and economic consequences. Infect Control Hosp Epidemiol. 2007;28(11): 1219–1227.
- , , , , . National Clostridium difficile infection (CDI) related hospitalizations approaches MRSA related hospitalizations. The need for antibiotic stewardship program [poster 94]. Presented at: The SHEA 2011 Annual Scientific Meeting. April 2, 2011; Dallas, TX.
Management of Initial CDI
The incidence and severity of Clostridium difficile infections (CDI) have increased steadily over the past decade, paralleling the emergence of an epidemic strain of C. difficile in North America, the North American pulsed field type 1 (NAP1), restriction‐endonuclease analysis type BI, polymerase‐chain‐reaction ribotype 027, commonly referred to as NAP1/BI/027. The reduced responsiveness of CDI to standard antibiotic therapy, and increased death rate attributable to CDI, present a significant challenge to clinicians. 1 This is a brief review of clinical strategies for effective management of initial CDI for hospital‐based physicians.
Effective management of CDI requires a multidisciplinary effort that includes identification of patients at risk, rapid implementation of contact isolation for patients suspected of having CDI, and implementation of early and appropriate treatment based on current clinical evidence. 2 Early recognition is, in large part, based on suspecting CDI anytime a patient develops antibiotic‐associated diarrhea. An understanding of traditional and emerging risk factors for CDI can help clinicians identify this serious condition early.
CASE STUDY
A.L. is an 87‐year‐old woman in a rehabilitation facility who is recovering from left hip replacement surgery performed 3 weeks ago. Her past medical history is positive for heart failure, atrial fibrillation, type 2 diabetes mellitus, and chronic obstructive pulmonary disease. During her hospitalization, she was treated preoperatively with cephalexin for prophylaxis. She was recently started on ciprofloxacin for a urinary tract infection, and has been taking this for 6 days.
During morning rounds, her nurse reports that A.L. has had diarrhea for 2 days. She is currently afebrile, and her white blood cell (WBC) count is 11,400/L. Ciprofloxacin was discontinued and a stool test for C. difficile toxin was ordered.
RISK FACTORS FOR CDI
The risk of developing CDI depends on 3 groups of factors: impairment of colonization resistance, risk of exposure to toxigenic C. difficile or its spores, and host health and immune status.
Impairment of Colonization Resistance
C. difficile is extremely common in the general environment. However, balanced intestinal microflora normally confer colonization resistance, a host factor that limits the proliferation of pathogenic microorganisms such as C. difficile. 3 While colonization of C. difficile occurs in the community in only 1% to 4% of healthy adults, the rate of colonization in hospitalized adults is much higher, approximately 20% to 30%. 4 Loss of normal resistance to C. difficile in adults is most commonly a consequence of antimicrobial therapy, which disrupts the intestinal microflora. The propensity of different antimicrobial agents to increase the risk for CDI varies due to differences in the complex relationship of their luminal concentrations, activity against C. difficile, and effects on the normal intestinal microflora. 4 Almost every available antibiotic has been associated with CDI; however, broad‐spectrum agents with antianaerobic activity appear to cause the greatest risk (Table 1). 5 Second‐ and third‐generation cephalosporins and fluoroquinolones are the most problematic because of their frequent use and high levels of resistance among strains of C. difficile to these agents. 1, 69 Regimens with multiple antibiotics and/or longer treatment courses are also associated with an increased risk. 9
| Very Commonly Related | Less Commonly Related | Uncommonly Related |
|---|---|---|
| Clindamycin | Other penicillins | Aminoglycosides |
| Cephalosporins | Sulfonamides | Bacitracin |
| Fluoroquinolones | Trimethoprim | Metronidazole |
| Ampicillin | Cotrimoxazole | Teicoplanin |
| Amoxicillin | Macrolides | Rifampin |
| Chloramphenicol | ||
| Tetracyclines | ||
| Carbapenems | ||
| Daptomycin | ||
| Tigecycline | ||
Clinicians should be aware that while CDI usually presents during or shortly after initiation of the causative antimicrobial, onset may be delayed for 2 or 3 months. 3 Healthcare professionals should consider CDI in patients who present with diarrhea and have a history of recent antimicrobial treatment in a hospital or as an outpatient. Other factors that may disrupt intestinal flora and lead to colonization by C. difficile include:
-
Bowel preparation for colonoscopy or surgery.
-
Cytotoxic chemotherapy.
-
Colitis caused by inflammatory bowel disease.
Risk of Exposure to Toxigenic C. difficile or Its Spores
C. difficile spores, which are highly resistant to drying, temperature fluxes, and many common disinfectants, contaminate the patient care environment in hospitals and other healthcare facilities. They are viable for long periods and may be transmitted from the hands or fomites of healthcare personnel to patients. It is not surprising that this leads to a major infection control challenge. 3
Host Health and Immune Status
A healthy immune response to C. difficile and toxins A and B is associated with milder forms of the condition. Many patients colonized by pathogenic strains of C. difficile do not have symptoms; this carrier state is associated with high circulating titers of immunoglobulin G (IgG) antitoxin. Conversely, several important individual factors that increase the risk for CDI include advancing age, hospital admission, longer duration of hospital stay, severe underlying disease, impairment of immune function, suppression of gastric acid secretion (eg, with proton pump inhibitors), enteral feedings (especially with use of a post‐pyloric tube), and mechanical ventilation. 1, 10
RISK FACTORS FOR RECURRENT CDI
The incidence of recurrent CDI within 60 to 90 days of initial CDI resolution following a course of treatment with metronidazole, vancomycin, or both is mostly 19% to 29%, but was 50% in 1 report. 11 The risk for CDI recurrence increases with each recurrent episode 12: Patients with 1 prior episode of recurrent CDI have a >40% risk for an additional recurrence, and those with 2 or more episodes have a >60% risk. 13, 14 Two likely mechanisms that predispose patients to recurrent CDI are an inadequate immune response to C. difficile toxins and persistent disruption of the normal colonic flora due to therapy with metronidazole, vancomycin, or other concomitant antibiotics. 15 Recurrent CDI is seldom due to resistance of vegetative cells of C. difficile to vancomycin or metronidazole. 14 Two other important factors associated with recurrence are infection with a hypervirulent strain of C. difficile, and the fact that the current hospital population generally consists of older and sicker patients who have been treated with many broad‐spectrum antibiotics. 16
Specific patient risk factors associated with recurrent CDI include 14, 17, 18:
-
Previous history of recurrence.
-
Increased age (>65).
-
Severe underlying disease.
-
Renal impairment.
-
Conditions or treatments that lead to immunocompromise.
-
Hospital admission (especially prolonged hospital stay).
-
Use of additional antibiotics.
As noted above, ongoing treatment with antibiotics plays an important role in the risk for recurrence. Hu and colleagues found that concomitant antibiotic use after a diagnosis of CDI was associated with a 10‐fold increased risk for recurrence (odds ratio [OR], 10.0; 95% confidence interval [CI], 1.5‐68.3). 12 Johnson and colleagues found that the rate of sustained response to CDI therapy, without subsequent recurrence, was higher in patients able to stop all other antibiotics and be treated with only fidaxomicin or vancomycin than it was in a group of patients treated with 1 of these agents plus an additional antibiotic (91.9% and 76.1%, respectively). 19 In general, patients who require concomitant antibiotics have more comorbidities and are sicker, so the entire difference cannot be attributed to antibiotics. However, clinicians should carefully consider the ongoing need for antibiotics if CDI is suspected or confirmed.
Continued exposure to C. difficile in the hospital or home environment often leads to reinfection when vancomycin and metronidazole concentrations have decreased. Data show that at least half of clinical recurrences are reinfection with a different strain, and half are due to persisting intestinal infection with the original infecting strain. 20 Therefore, patients with CDI should be educated about appropriate hygiene at home.
CLINICAL MANIFESTATIONS OF CDI
CDI has a wide range of clinical manifestations, ranging from a mild and self‐limited diarrheal illness to fulminant, life‐threatening colitis. The onset of symptoms usually occurs within 3 to 7 days of antibiotic exposure, but may not arise for up to 10 weeks after stopping antibiotics. 5 CDI is associated with watery diarrhea that is often accompanied by cramping abdominal pain and low‐grade fever. Systemic symptoms generally increase with the degree of colitis. Patients with severe disease may also progress to having an ileus, or toxic megacolon or acute abdomen. 5
Up to 20% of critically ill patients have ileus or toxic megacolon, and therefore may not present with diarrhea; this, combined with a limited ability to communicate among some critically ill patients makes early diagnosis of CDI in this patient population extremely challenging. Therefore, physicians and other clinical staff must be vigilant about evaluating patients for the presence of CDI based on physical exam and laboratory findings. For example, fever, abdominal pain, and abdominal distention are likely to be present in patients with severe colitis. In addition, patients often have significant leukocytosis (often >20,000 cells/mm 3) with bandemia. In advanced cases, an elevated serum lactate dehydrogenase may be seenthis is a nonspecific finding for gastrointestinal disease, but provides a clue to the presence of CDI. Because these findings often precede multiorgan dysfunction, the presence of CDI must be determined quickly and appropriate treatment initiated. 5
PRINCIPLES OF DIAGNOSIS
C. difficile infection should be suspected in patients with antimicrobial‐associated diarrhea. Confirmatory testing should be performed, but only on watery or loose stools because the rate of symptomless colonization with C. difficile in hospitalized patients is high; a positive result on a normal stool sample proves only that the patient is colonized with C. difficile, but not necessarily infected. 14 A notable exception is when CDI is suspected in a patient with ileus; as many laboratories will not accept solid stool for C. difficile testing, the clinician should notify the laboratory about the specific request and reasons for suspecting CDI. 21 Stool testing for eradication of C. difficile during or after therapy is not advised, as many successfully treated patients will continue to shed the organism and its spores. 2 This symptomless carriage does not require additional treatment.
CASE STUDY CONTINUED
A.L. was empirically started on oral metronidazole, 500 mg 3 times a day, pending results of the stool C. difficile test.
On rounds the following day, diarrhea was less frequent (decreased from 9 to 4 loose bowel movements in 24 hours). She reported mild abdominal discomfort and nausea, but was tolerating oral intake. Her temperature was 100.8F.
Her lab results returned later in the afternoon were:
-
WBC: 18,600 cells/L.
-
Potassium: 3.2 mEq/mL.
-
Creatinine: 2.4 mg/dL (up from 1.1).
-
Stool C. difficile test: negative.
There are a variety of tests for C. difficile, each with advantages and disadvantages (Table 2). 2, 3, 21 Factors to consider when selecting a diagnostic test include turnaround time, sensitivity, specificity, cost, whether there is an ongoing outbreak, and the availability of particular tests. 21 Recent guidelines for CDI management jointly developed by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Disease Society of America (IDSA) note that stool culture is the gold standard, but recognize the clinical limitations of its long turnaround time. 2 Enzyme immunoassay (EIA) is noted to be a suboptimal alternative approach for diagnosis than the cell cytotoxin assay. 2 Therefore, because EIA is most often used in clinical practice, it is important to be aware that a high clinical suspicion for CDI may warrant empiric therapy or repeat testing with a more sensitive test in a patient with an initial negative test result. 3, 21
| Test | Advantage(s) | Disadvantage(s) |
|---|---|---|
| Toxin testing | ||
| Enzyme immunoassay | Rapid, simple, | Least sensitive method, some detect only toxin A (some strains only produce toxin B) |
| Cell cytotoxin assay | More sensitive than enzyme immunoassay | Labor intensive; requires 72 hr for a final result, special equipment |
| Organism identification | ||
| Detection of glutamate dehydrogenase | Rapid, sensitive, may prove useful as a triage or screening tool | Not specific, toxin testing required to verify diagnosis; may not be 100% sensitive |
| Polymerase chain reaction | Rapid, sensitive, detects presence of toxin gene | Cost, special equipment needed |
| Stool culture | Most sensitive test available when performed appropriately | May be associated with false‐positive results if isolate is not tested for toxin; labor‐intensive; requires 72 hr for results |
CURRENT STRATEGIES FOR CDI MANAGEMENT
While current guidelines recommend that treatment of CDI be based on disease severity, 2, 22 determination of severity is challenging, in part, because standard definitions are lacking and because the illness varies along a continuum of symptoms. 5 In general, CDI can be categorized as: mild to moderate, severe, and severe disease with complications. Mild to moderate CDI is characterized by diarrhea and abdominal cramping, only without systemic symptoms. 5, 21 Severe CDI is distinguished by abundant diarrhea, severe abdominal pain/distension, leukocytosis, fever, or other systemic symptoms (Table 3). 5, 21, 2326 Patients with severe disease and other complications may present with a wide range of gastrointestinal symptoms accompanied by paralytic ileus, toxic megacolon, or other life‐threatening conditions. 5, 21 CDI may progress in severity rapidly, even after initiation of treatment, so ongoing assessment of the patient's condition and disease category is important. 5, 21
| Severe diarrhea (>10 bowel movements/day) |
| Leukocytosis |
| WBC >15,000 associated with severe CDI |
| WBC >25,000 associated with increased fatality |
| High or rising (50% increase) serum creatinine, or creatinine >2 mg/dL |
| Low serum albumin (<2.5 mg/dL) |
| Severe abdominal distension, pain |
| Ileus or toxic megacolon |
| Colonic thickening on CT scan |
| Ascites on CT scan |
| Pseudomembranes on endoscopy |
| Hemodynamic instability |
| Organ failure |
In 2010, SHEA/IDSA published evidence‐based guidelines for managing CDI based on severity of illness (summarized in Table 4). 2 The first and most important step in the effective management of CDI in all patients, regardless of severity, is to discontinue the causative antibiotic(s). 1 Data show that when all antibiotics are stopped, about 25% of patients with mild CDI who are otherwise healthy have resolution of diarrhea within 48 hours 27; most importantly, recurrent CDI is unlikely. Many hospitalized patients, especially in the intensive care setting, have serious concomitant infections, and therefore it may not be appropriate to discontinue the inciting antibiotic. In these patients, the regimen and available culture and sensitivity results should be thoughtfully reviewed, and change made when possible to a more narrow‐spectrum regimen less likely to cause or exacerbate CDI (Table 1).
| Clinical Severity | Supportive Laboratory Data | Recommended Treatment |
|---|---|---|
| ||
| Mild to moderate | WBC 15,000 cells/L) or serum creatinine <1.5 times premorbid level | Metronidazole 500 mg orally 3 times per day for 10‐14 days |
| Severe | WBC 15,000 cells/L) or serum creatinine 1.5 times premorbid level | Vancomycin 125 mg orally 4 times per day for 10‐14 days |
| Severe, complicated | Hypotension or shock, ileus and/or megacolon; organ failure (eg, ARDS); coagulopathy | Vancomycin 500 mg 4 times per day orally or by nasogastric tube plus metronidazole 500 mg IV every 8 hr |
The next step, as discussed above, is to send a stool sample for C. difficile testing. Based on the patient's clinical circumstances, the decision must be made whether or not to begin empiric therapy. In general, beginning treatment without testing for C. difficile is not recommended, because, at most, only about a third of hospitalized patients with diarrhea have CDI, even in an epidemic setting. 21 If a patient is severely ill or has a rapidly deteriorating clinical course and is at high risk for CDI, empiric therapy may be appropriate while awaiting test results. 21
In patients with severe CDI and complications, reduced or absent bowel motility can reduce the amount of orally administered vancomycin that reaches the site of infection. Intracolonic administration of vancomycin may be indicated in these cases, or when oral therapy cannot be tolerated. 28 Higher doses of oral vancomycin may also be used, with the goal of increasing fecal concentrations, however this strategy has not been studied. 5 In all patients, antiperistaltic agents are usually avoided because of unproven concerns that they might mask symptoms and/or increase the risk for toxic megacolon. 2
Fidaxomicin, a new macrolide antibiotic in the macrocyclic group, has a narrow‐spectrum and excellent activity against C. difficile. The US Food and Drug Administration approved fidaxomicin for treatment of adults for C. difficileassociated diarrhea (CDAD) in May 2011. 29 Approval was based on 2 phase III trials involving 1105 patients with CDAD in which fidaxomicin was shown to have similar initial clinical efficacy and safety as vancomycin. 30 In addition, more patients treated with fidaxomicin had a sustained response 25 days following discontinuation of treatment than patients treated with vancomycin. 31 The recommended dosage of fidaxomicin is one 200‐mg tablet orally twice daily for 10 days. 31
In a phase III trial (N = 596) of fidaxomicin (200 mg orally every 12 hours) versus vancomycin (125 mg orally every 6 hours) for 10 days, fidaxomicin was shown to be noninferior to vancomycin in achieving an initial clinical response and significantly better at preventing recurrent CDI. 32 The rates of initial clinical response, the primary endpoint, and rates of recurrent CDI are shown in Table 5. The significant difference in recurrence may be explained by the fact that metronidazole and vancomycin impact commensal microflora populations that normally mediate competitive exclusion of C. difficile. Compared with vancomycin, fidaxomicin has less effect on the composition of the fecal microbiota, in particular some clostridial clusters and Bifidobacterium. 33 While acquisition costs for this new antibiotic are a consideration, they may be offset by a reduction in recurrent CDI, especially in high‐risk patients.
| Modified Intention‐to‐Treat Population | Per‐Protocol Population | |||
|---|---|---|---|---|
| Fidaxomicin n/N (%) | Vancomycin n/N (%) | Fidaxomicin n/N (%) | Vancomycin n/N (%) | |
| ||||
| Rates of initial clinical response | ||||
| Total population | 253/287 (88.2) | 265/309 (85.8) | 244/265 (92.1) | 254/283 (89.8) |
| Non‐NAP1/BI/027 strain type | 117/125 (93.6) | 121/132 (91.7) | 115/119 (96.6) | 119/126 (94.4) |
| Use of concomitant systemic antimicrobial therapy | 67/83 (80.7) | 72/94 (76.6) | 63/71 (88.7) | 67/80 (83.8) |
| Rates of recurrence of C. difficile infection | ||||
| Total population | 39/253 (15.4)* | 67/265 (25.3)* | 28/211 (13.3) | 53/221 (24.0) |
| Non‐NAP1/BI/027 strain type | 12/117 (10.3) | 34/121 (28.1) | 8/103 (7.8) | 27/106 (25.5) |
| Use of concomitant systemic antimicrobial therapy | 14/81 (17.3) | 25/90 (27.8) | 8/56 (14.3) | 20/65 (30.8) |
FOCUS ON FULMINANT/REFRACTORY CDI
Management of CDI that is fulminant and/or refractory can be extremely challenging. 3 In clinical practice, these conditions often overlap. Clinical characteristics that may help identify fulminant CDI include abdominal pain and tenderness, colonic distension, and signs of sepsis. Diarrhea may be absent or minimal due to ileus. Furthermore, a diagnosis of CDI is easy to miss, as these symptoms are also consistent with ischemic bowel or a perforated viscus. 3 Gentle, flexible sigmoidoscopy or colonoscopy (without bowel preparation and with minimal air insufflation) may be extremely valuable to allow for immediate identification of pseudomembranous colitis, which speeds appropriate medical and surgical management. 3
First‐line treatment for fulminant or refractory CDI is oral or intragastric vancomycin 500 mg every 6 hours. 2, 3 Intravenous (IV) metronidazole 500 mg every 6 hours should be added to the treatment regimen in those with ileus or megacolon. For patients with complete ileus, vancomycin can be administered rectally as 500 mg in 100 mL of normal saline every 6 hours. 3 Normal pooled IV immunoglobulin has been used with mixed success, in patients with fulminant and/or refractory CDI, in an attempt to avert surgery or death by providing passive immunotherapy against C. difficile toxins A and B. 34 In a study of monoclonal antibodies to toxins A and B that included 200 patients, recurrence rates among those with the epidemic NAP1/BI/027 strain were 8% for the antibody group and 32% for the placebo group (P = 0.06); in a subset with more than 1 previous episode of CDI, recurrence rates were 7% and 38%, respectively (P = 0.006). 35
Some patients with fulminant or refractory CDI are best managed with subtotal colectomy, which may be lifesaving. 36 However, the optimal timing of surgery is difficult to establish, and the decision to proceed with this course can be difficult because patients with severe CDI are typically poor surgical candidates. 3 Delaying surgery until the development of a systemic inflammatory response syndrome with concomitant severe disease, such as multisystem organ failure, immunocompromise, or hemodynamic instability, usually results in a poor outcome. 37, 38 The best approach is to get a surgical consult early, when the course of CDI starts to deteriorate, so that management decisions can be based on input from a multidisciplinary team of clinicians. 3
CASE STUDY CONTINUED
A.L. was subsequently transferred to an acute care facility. Her therapy for C. difficile was switched to oral vancomycin, 125 mg 4 times daily. On arrival in the emergency department, the following findings were noted:
-
No further diarrhea, but abdominal distention noted.
-
Temperature: 101.4F.
-
Fall in blood pressure (BP) to 90/48 mmHg during transfer, which responded to a fluid bolus, and increased to 108/74.
-
WBC: 27,000/L.
-
Abdominal computed tomography (CT) showed thickening of descending and sigmoid colon, and the rectum.
-
Proctoscopy confirmed pseudomembranous colitis.
Oral vancomycin was increased to 500 mg 4 times daily, and IV metronidazole 500 mg every 8 hours was added. C. difficile infection responded to this regimen and she was subsequently discharged to home care.
- .Narrative review: the new epidemic of Clostridium difficile‐associated enteric disease.Ann Intern Med.2006;145(10):758–764.
- ,,, et al.Clinical practice guidelines for Clostridium diffcile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA).Infect Control Hosp Epidemiol.2010;31(5):431–455.
- .A 76‐year‐old man with recurrent Clostridium difficile–associated diarrhea: review of C difficile infection.JAMA.2009;301(9):954–962.
- .Antibiotic‐associated diarrhea.N Engl J Med.2002;346(5):334–339.
- ,.Clostridium difficile infection in the intensive care unit.Infect Dis Clin North Am.2009;23(3):727–743.
- ,,, et al.A predominantly clonal multi‐institutional outbreak of Clostridium difficile‐associated diarrhea with high morbidity and mortality.N Engl J Med.2005;353:2442–2449.
- ,,.Antimicrobial therapy of Clostridium difficile‐associated diarrhea.Med Clin North Am.2006;90(6):1141–1163.
- ,,,,.Patterns of antibiotic use and risk of hospital admission because of Clostridium difficile.Can Med Assoc J.2008;179(8):767–772.
- ,,, et al.Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile‐associated diarrhea: a cohort study during an epidemic in Quebec.Clin Infect Dis.2005;41(9):1254–1260.
- .Clostridium difficile and the disease it causes.Methods Mol Biol.2010;646:9–35.
- ,,.Treatment of Clostridium difficile‐associated disease: old therapies and new.Lancet Infect Dis.2005;5(9):549–557.
- , , , et al. Prospective derviation and validation of a clinical prediction rule for recurrent Clostridium difficile infection. Gastroenterology. 2009;136:1206–1214.
- ,,, et al.A randomized placebo‐controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease.JAMA.1994;271(24):1913–1918.
- ,,.Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease.Am J Gastroenterol.2002;97(7):1769–1775.
- .Recurrent Clostridium difficile infection: a review of risk factors, treatments, and outcomes.J Infect.2009;58(6):403–410.
- ,.Clostridium difficile: recent epidemiologic findings and advances in therapy.Pharmacotherapy.2007;27(7):1029–1039.
- ,,, et al.Risk factors for early recurrent Clostridium difficile‐associated diarrheas.Clin Infect Dis.1998;26(4):954–959.
- ,,,.Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea.Lancet.2001;357(9251):189–193.
- ,,,,,.Randomized clinical trial in Clostridium difficile infection confirms superiority of fidaxomicin over vancomycin [abstract 828630]. Presented at: Digestive Disease Week 2010; May 4,2010; New Orleans, LA.
- ,,,.Recurrence of symptoms in Clostridium difficile infection—relapse or reinfection?J Hosp Infect.1998;38(2):93–100.
- ,.Clostridium difficile‐associated disease: new challenges from an established pathogen.Cleve Clin J Med.2006;73(2):187–197.
- ,,,.A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile–associated diarrhea, stratified by disease severity.Clin Infect Dis.2007;45(3):302–307.
- ,,, et al.Clostridium difficile–associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity.Can Med Assoc J.2004;171(5):466–472.
- ,.Clinical recognition and diagnosis of Clostridium difficile infection.Clin Infect Dis.2008;46(suppl 1):S12–S18.
- ,,,.Clinical risk factors for severe Clostridium difficile‐associated disease.Emerg Infect Dis.2009;15(3):415–422.
- ,,.European Society of Clinical Microbiology and Infectious Diseases (ESCMID): treatment guidance document for Clostridium difficile infection (CDI).Clin Microbiol Infect.2009;15(12):1067–1079.
- ,,, et al.Prospective randomised trial of metronidazole versus vancomycin for Clostridium difficile–associated diarrhoea and colitis.Lancet.1983;2(8358):1043–1046.
- ,,.Adjunctive intracolonic vancomycin for severe Clostridium difficile colitis: case series and review of the literature.Clin Infect Dis.2002;35(6):690–696.
- United States Food and Drug Administration. FDA approves treatment for Clostridium difficile infection. Available at: www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm257024.htm. Last updated: May, 27, 2011. Accessed June 15,2011.
- ,,, et al.Efficacy and safety of fidaxomicin (FDX) vs. vancomycin (VAN) in Clostridium difficile infection (CDI) in 2 randomized controlled trials (RCT) with 1105 patients [abstract 1417]. Presented at: The 48th Annual IDSA Meeting; October 21–24,2010; Vancouver, BC, Canada.
- Dificid [package insert].San Diego, CA:Optimer Pharmaceuticals, Inc;2011.
- ,,, et al.Fidaxomicin versus vancomycin for Clostridium difficile infection.N Engl J Med.2011;364(5):422–431.
- ,,, et al.A new macrocyclic antibiotic, fidaxomicin (OPT‐80), causes less alteration to the bowel microbiota of Clostridium difficile‐infected patients than does vancomycin.Microbiology.2010;156(pt 11):3354–3359.
- ,,,.Intravenous immunoglobulin for the treatment of severe Clostridium difficile colitis: an observational study and review of the literature.J Hosp Med.2010;5(1):E1–E9.
- ,,, et al.Treatment with monoclonal antibodies against Clostridium difficile toxins.N Engl J Med.2010;362(3):197–205.
- ,.Surgical aspects of fulminant Clostridium difficile colitis.Am J Surg.2010;200(1):131–135.
- ,,, et al.Clostridium difficile colitis in the critically ill.Dis Colon Rectum.1996;39(6):619–623.
- ,,.Early surgical intervention for fulminant pseudomembranous colitis.Am Surg.74(1):20–26.
The incidence and severity of Clostridium difficile infections (CDI) have increased steadily over the past decade, paralleling the emergence of an epidemic strain of C. difficile in North America, the North American pulsed field type 1 (NAP1), restriction‐endonuclease analysis type BI, polymerase‐chain‐reaction ribotype 027, commonly referred to as NAP1/BI/027. The reduced responsiveness of CDI to standard antibiotic therapy, and increased death rate attributable to CDI, present a significant challenge to clinicians. 1 This is a brief review of clinical strategies for effective management of initial CDI for hospital‐based physicians.
Effective management of CDI requires a multidisciplinary effort that includes identification of patients at risk, rapid implementation of contact isolation for patients suspected of having CDI, and implementation of early and appropriate treatment based on current clinical evidence. 2 Early recognition is, in large part, based on suspecting CDI anytime a patient develops antibiotic‐associated diarrhea. An understanding of traditional and emerging risk factors for CDI can help clinicians identify this serious condition early.
CASE STUDY
A.L. is an 87‐year‐old woman in a rehabilitation facility who is recovering from left hip replacement surgery performed 3 weeks ago. Her past medical history is positive for heart failure, atrial fibrillation, type 2 diabetes mellitus, and chronic obstructive pulmonary disease. During her hospitalization, she was treated preoperatively with cephalexin for prophylaxis. She was recently started on ciprofloxacin for a urinary tract infection, and has been taking this for 6 days.
During morning rounds, her nurse reports that A.L. has had diarrhea for 2 days. She is currently afebrile, and her white blood cell (WBC) count is 11,400/L. Ciprofloxacin was discontinued and a stool test for C. difficile toxin was ordered.
RISK FACTORS FOR CDI
The risk of developing CDI depends on 3 groups of factors: impairment of colonization resistance, risk of exposure to toxigenic C. difficile or its spores, and host health and immune status.
Impairment of Colonization Resistance
C. difficile is extremely common in the general environment. However, balanced intestinal microflora normally confer colonization resistance, a host factor that limits the proliferation of pathogenic microorganisms such as C. difficile. 3 While colonization of C. difficile occurs in the community in only 1% to 4% of healthy adults, the rate of colonization in hospitalized adults is much higher, approximately 20% to 30%. 4 Loss of normal resistance to C. difficile in adults is most commonly a consequence of antimicrobial therapy, which disrupts the intestinal microflora. The propensity of different antimicrobial agents to increase the risk for CDI varies due to differences in the complex relationship of their luminal concentrations, activity against C. difficile, and effects on the normal intestinal microflora. 4 Almost every available antibiotic has been associated with CDI; however, broad‐spectrum agents with antianaerobic activity appear to cause the greatest risk (Table 1). 5 Second‐ and third‐generation cephalosporins and fluoroquinolones are the most problematic because of their frequent use and high levels of resistance among strains of C. difficile to these agents. 1, 69 Regimens with multiple antibiotics and/or longer treatment courses are also associated with an increased risk. 9
| Very Commonly Related | Less Commonly Related | Uncommonly Related |
|---|---|---|
| Clindamycin | Other penicillins | Aminoglycosides |
| Cephalosporins | Sulfonamides | Bacitracin |
| Fluoroquinolones | Trimethoprim | Metronidazole |
| Ampicillin | Cotrimoxazole | Teicoplanin |
| Amoxicillin | Macrolides | Rifampin |
| Chloramphenicol | ||
| Tetracyclines | ||
| Carbapenems | ||
| Daptomycin | ||
| Tigecycline | ||
Clinicians should be aware that while CDI usually presents during or shortly after initiation of the causative antimicrobial, onset may be delayed for 2 or 3 months. 3 Healthcare professionals should consider CDI in patients who present with diarrhea and have a history of recent antimicrobial treatment in a hospital or as an outpatient. Other factors that may disrupt intestinal flora and lead to colonization by C. difficile include:
-
Bowel preparation for colonoscopy or surgery.
-
Cytotoxic chemotherapy.
-
Colitis caused by inflammatory bowel disease.
Risk of Exposure to Toxigenic C. difficile or Its Spores
C. difficile spores, which are highly resistant to drying, temperature fluxes, and many common disinfectants, contaminate the patient care environment in hospitals and other healthcare facilities. They are viable for long periods and may be transmitted from the hands or fomites of healthcare personnel to patients. It is not surprising that this leads to a major infection control challenge. 3
Host Health and Immune Status
A healthy immune response to C. difficile and toxins A and B is associated with milder forms of the condition. Many patients colonized by pathogenic strains of C. difficile do not have symptoms; this carrier state is associated with high circulating titers of immunoglobulin G (IgG) antitoxin. Conversely, several important individual factors that increase the risk for CDI include advancing age, hospital admission, longer duration of hospital stay, severe underlying disease, impairment of immune function, suppression of gastric acid secretion (eg, with proton pump inhibitors), enteral feedings (especially with use of a post‐pyloric tube), and mechanical ventilation. 1, 10
RISK FACTORS FOR RECURRENT CDI
The incidence of recurrent CDI within 60 to 90 days of initial CDI resolution following a course of treatment with metronidazole, vancomycin, or both is mostly 19% to 29%, but was 50% in 1 report. 11 The risk for CDI recurrence increases with each recurrent episode 12: Patients with 1 prior episode of recurrent CDI have a >40% risk for an additional recurrence, and those with 2 or more episodes have a >60% risk. 13, 14 Two likely mechanisms that predispose patients to recurrent CDI are an inadequate immune response to C. difficile toxins and persistent disruption of the normal colonic flora due to therapy with metronidazole, vancomycin, or other concomitant antibiotics. 15 Recurrent CDI is seldom due to resistance of vegetative cells of C. difficile to vancomycin or metronidazole. 14 Two other important factors associated with recurrence are infection with a hypervirulent strain of C. difficile, and the fact that the current hospital population generally consists of older and sicker patients who have been treated with many broad‐spectrum antibiotics. 16
Specific patient risk factors associated with recurrent CDI include 14, 17, 18:
-
Previous history of recurrence.
-
Increased age (>65).
-
Severe underlying disease.
-
Renal impairment.
-
Conditions or treatments that lead to immunocompromise.
-
Hospital admission (especially prolonged hospital stay).
-
Use of additional antibiotics.
As noted above, ongoing treatment with antibiotics plays an important role in the risk for recurrence. Hu and colleagues found that concomitant antibiotic use after a diagnosis of CDI was associated with a 10‐fold increased risk for recurrence (odds ratio [OR], 10.0; 95% confidence interval [CI], 1.5‐68.3). 12 Johnson and colleagues found that the rate of sustained response to CDI therapy, without subsequent recurrence, was higher in patients able to stop all other antibiotics and be treated with only fidaxomicin or vancomycin than it was in a group of patients treated with 1 of these agents plus an additional antibiotic (91.9% and 76.1%, respectively). 19 In general, patients who require concomitant antibiotics have more comorbidities and are sicker, so the entire difference cannot be attributed to antibiotics. However, clinicians should carefully consider the ongoing need for antibiotics if CDI is suspected or confirmed.
Continued exposure to C. difficile in the hospital or home environment often leads to reinfection when vancomycin and metronidazole concentrations have decreased. Data show that at least half of clinical recurrences are reinfection with a different strain, and half are due to persisting intestinal infection with the original infecting strain. 20 Therefore, patients with CDI should be educated about appropriate hygiene at home.
CLINICAL MANIFESTATIONS OF CDI
CDI has a wide range of clinical manifestations, ranging from a mild and self‐limited diarrheal illness to fulminant, life‐threatening colitis. The onset of symptoms usually occurs within 3 to 7 days of antibiotic exposure, but may not arise for up to 10 weeks after stopping antibiotics. 5 CDI is associated with watery diarrhea that is often accompanied by cramping abdominal pain and low‐grade fever. Systemic symptoms generally increase with the degree of colitis. Patients with severe disease may also progress to having an ileus, or toxic megacolon or acute abdomen. 5
Up to 20% of critically ill patients have ileus or toxic megacolon, and therefore may not present with diarrhea; this, combined with a limited ability to communicate among some critically ill patients makes early diagnosis of CDI in this patient population extremely challenging. Therefore, physicians and other clinical staff must be vigilant about evaluating patients for the presence of CDI based on physical exam and laboratory findings. For example, fever, abdominal pain, and abdominal distention are likely to be present in patients with severe colitis. In addition, patients often have significant leukocytosis (often >20,000 cells/mm 3) with bandemia. In advanced cases, an elevated serum lactate dehydrogenase may be seenthis is a nonspecific finding for gastrointestinal disease, but provides a clue to the presence of CDI. Because these findings often precede multiorgan dysfunction, the presence of CDI must be determined quickly and appropriate treatment initiated. 5
PRINCIPLES OF DIAGNOSIS
C. difficile infection should be suspected in patients with antimicrobial‐associated diarrhea. Confirmatory testing should be performed, but only on watery or loose stools because the rate of symptomless colonization with C. difficile in hospitalized patients is high; a positive result on a normal stool sample proves only that the patient is colonized with C. difficile, but not necessarily infected. 14 A notable exception is when CDI is suspected in a patient with ileus; as many laboratories will not accept solid stool for C. difficile testing, the clinician should notify the laboratory about the specific request and reasons for suspecting CDI. 21 Stool testing for eradication of C. difficile during or after therapy is not advised, as many successfully treated patients will continue to shed the organism and its spores. 2 This symptomless carriage does not require additional treatment.
CASE STUDY CONTINUED
A.L. was empirically started on oral metronidazole, 500 mg 3 times a day, pending results of the stool C. difficile test.
On rounds the following day, diarrhea was less frequent (decreased from 9 to 4 loose bowel movements in 24 hours). She reported mild abdominal discomfort and nausea, but was tolerating oral intake. Her temperature was 100.8F.
Her lab results returned later in the afternoon were:
-
WBC: 18,600 cells/L.
-
Potassium: 3.2 mEq/mL.
-
Creatinine: 2.4 mg/dL (up from 1.1).
-
Stool C. difficile test: negative.
There are a variety of tests for C. difficile, each with advantages and disadvantages (Table 2). 2, 3, 21 Factors to consider when selecting a diagnostic test include turnaround time, sensitivity, specificity, cost, whether there is an ongoing outbreak, and the availability of particular tests. 21 Recent guidelines for CDI management jointly developed by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Disease Society of America (IDSA) note that stool culture is the gold standard, but recognize the clinical limitations of its long turnaround time. 2 Enzyme immunoassay (EIA) is noted to be a suboptimal alternative approach for diagnosis than the cell cytotoxin assay. 2 Therefore, because EIA is most often used in clinical practice, it is important to be aware that a high clinical suspicion for CDI may warrant empiric therapy or repeat testing with a more sensitive test in a patient with an initial negative test result. 3, 21
| Test | Advantage(s) | Disadvantage(s) |
|---|---|---|
| Toxin testing | ||
| Enzyme immunoassay | Rapid, simple, | Least sensitive method, some detect only toxin A (some strains only produce toxin B) |
| Cell cytotoxin assay | More sensitive than enzyme immunoassay | Labor intensive; requires 72 hr for a final result, special equipment |
| Organism identification | ||
| Detection of glutamate dehydrogenase | Rapid, sensitive, may prove useful as a triage or screening tool | Not specific, toxin testing required to verify diagnosis; may not be 100% sensitive |
| Polymerase chain reaction | Rapid, sensitive, detects presence of toxin gene | Cost, special equipment needed |
| Stool culture | Most sensitive test available when performed appropriately | May be associated with false‐positive results if isolate is not tested for toxin; labor‐intensive; requires 72 hr for results |
CURRENT STRATEGIES FOR CDI MANAGEMENT
While current guidelines recommend that treatment of CDI be based on disease severity, 2, 22 determination of severity is challenging, in part, because standard definitions are lacking and because the illness varies along a continuum of symptoms. 5 In general, CDI can be categorized as: mild to moderate, severe, and severe disease with complications. Mild to moderate CDI is characterized by diarrhea and abdominal cramping, only without systemic symptoms. 5, 21 Severe CDI is distinguished by abundant diarrhea, severe abdominal pain/distension, leukocytosis, fever, or other systemic symptoms (Table 3). 5, 21, 2326 Patients with severe disease and other complications may present with a wide range of gastrointestinal symptoms accompanied by paralytic ileus, toxic megacolon, or other life‐threatening conditions. 5, 21 CDI may progress in severity rapidly, even after initiation of treatment, so ongoing assessment of the patient's condition and disease category is important. 5, 21
| Severe diarrhea (>10 bowel movements/day) |
| Leukocytosis |
| WBC >15,000 associated with severe CDI |
| WBC >25,000 associated with increased fatality |
| High or rising (50% increase) serum creatinine, or creatinine >2 mg/dL |
| Low serum albumin (<2.5 mg/dL) |
| Severe abdominal distension, pain |
| Ileus or toxic megacolon |
| Colonic thickening on CT scan |
| Ascites on CT scan |
| Pseudomembranes on endoscopy |
| Hemodynamic instability |
| Organ failure |
In 2010, SHEA/IDSA published evidence‐based guidelines for managing CDI based on severity of illness (summarized in Table 4). 2 The first and most important step in the effective management of CDI in all patients, regardless of severity, is to discontinue the causative antibiotic(s). 1 Data show that when all antibiotics are stopped, about 25% of patients with mild CDI who are otherwise healthy have resolution of diarrhea within 48 hours 27; most importantly, recurrent CDI is unlikely. Many hospitalized patients, especially in the intensive care setting, have serious concomitant infections, and therefore it may not be appropriate to discontinue the inciting antibiotic. In these patients, the regimen and available culture and sensitivity results should be thoughtfully reviewed, and change made when possible to a more narrow‐spectrum regimen less likely to cause or exacerbate CDI (Table 1).
| Clinical Severity | Supportive Laboratory Data | Recommended Treatment |
|---|---|---|
| ||
| Mild to moderate | WBC 15,000 cells/L) or serum creatinine <1.5 times premorbid level | Metronidazole 500 mg orally 3 times per day for 10‐14 days |
| Severe | WBC 15,000 cells/L) or serum creatinine 1.5 times premorbid level | Vancomycin 125 mg orally 4 times per day for 10‐14 days |
| Severe, complicated | Hypotension or shock, ileus and/or megacolon; organ failure (eg, ARDS); coagulopathy | Vancomycin 500 mg 4 times per day orally or by nasogastric tube plus metronidazole 500 mg IV every 8 hr |
The next step, as discussed above, is to send a stool sample for C. difficile testing. Based on the patient's clinical circumstances, the decision must be made whether or not to begin empiric therapy. In general, beginning treatment without testing for C. difficile is not recommended, because, at most, only about a third of hospitalized patients with diarrhea have CDI, even in an epidemic setting. 21 If a patient is severely ill or has a rapidly deteriorating clinical course and is at high risk for CDI, empiric therapy may be appropriate while awaiting test results. 21
In patients with severe CDI and complications, reduced or absent bowel motility can reduce the amount of orally administered vancomycin that reaches the site of infection. Intracolonic administration of vancomycin may be indicated in these cases, or when oral therapy cannot be tolerated. 28 Higher doses of oral vancomycin may also be used, with the goal of increasing fecal concentrations, however this strategy has not been studied. 5 In all patients, antiperistaltic agents are usually avoided because of unproven concerns that they might mask symptoms and/or increase the risk for toxic megacolon. 2
Fidaxomicin, a new macrolide antibiotic in the macrocyclic group, has a narrow‐spectrum and excellent activity against C. difficile. The US Food and Drug Administration approved fidaxomicin for treatment of adults for C. difficileassociated diarrhea (CDAD) in May 2011. 29 Approval was based on 2 phase III trials involving 1105 patients with CDAD in which fidaxomicin was shown to have similar initial clinical efficacy and safety as vancomycin. 30 In addition, more patients treated with fidaxomicin had a sustained response 25 days following discontinuation of treatment than patients treated with vancomycin. 31 The recommended dosage of fidaxomicin is one 200‐mg tablet orally twice daily for 10 days. 31
In a phase III trial (N = 596) of fidaxomicin (200 mg orally every 12 hours) versus vancomycin (125 mg orally every 6 hours) for 10 days, fidaxomicin was shown to be noninferior to vancomycin in achieving an initial clinical response and significantly better at preventing recurrent CDI. 32 The rates of initial clinical response, the primary endpoint, and rates of recurrent CDI are shown in Table 5. The significant difference in recurrence may be explained by the fact that metronidazole and vancomycin impact commensal microflora populations that normally mediate competitive exclusion of C. difficile. Compared with vancomycin, fidaxomicin has less effect on the composition of the fecal microbiota, in particular some clostridial clusters and Bifidobacterium. 33 While acquisition costs for this new antibiotic are a consideration, they may be offset by a reduction in recurrent CDI, especially in high‐risk patients.
| Modified Intention‐to‐Treat Population | Per‐Protocol Population | |||
|---|---|---|---|---|
| Fidaxomicin n/N (%) | Vancomycin n/N (%) | Fidaxomicin n/N (%) | Vancomycin n/N (%) | |
| ||||
| Rates of initial clinical response | ||||
| Total population | 253/287 (88.2) | 265/309 (85.8) | 244/265 (92.1) | 254/283 (89.8) |
| Non‐NAP1/BI/027 strain type | 117/125 (93.6) | 121/132 (91.7) | 115/119 (96.6) | 119/126 (94.4) |
| Use of concomitant systemic antimicrobial therapy | 67/83 (80.7) | 72/94 (76.6) | 63/71 (88.7) | 67/80 (83.8) |
| Rates of recurrence of C. difficile infection | ||||
| Total population | 39/253 (15.4)* | 67/265 (25.3)* | 28/211 (13.3) | 53/221 (24.0) |
| Non‐NAP1/BI/027 strain type | 12/117 (10.3) | 34/121 (28.1) | 8/103 (7.8) | 27/106 (25.5) |
| Use of concomitant systemic antimicrobial therapy | 14/81 (17.3) | 25/90 (27.8) | 8/56 (14.3) | 20/65 (30.8) |
FOCUS ON FULMINANT/REFRACTORY CDI
Management of CDI that is fulminant and/or refractory can be extremely challenging. 3 In clinical practice, these conditions often overlap. Clinical characteristics that may help identify fulminant CDI include abdominal pain and tenderness, colonic distension, and signs of sepsis. Diarrhea may be absent or minimal due to ileus. Furthermore, a diagnosis of CDI is easy to miss, as these symptoms are also consistent with ischemic bowel or a perforated viscus. 3 Gentle, flexible sigmoidoscopy or colonoscopy (without bowel preparation and with minimal air insufflation) may be extremely valuable to allow for immediate identification of pseudomembranous colitis, which speeds appropriate medical and surgical management. 3
First‐line treatment for fulminant or refractory CDI is oral or intragastric vancomycin 500 mg every 6 hours. 2, 3 Intravenous (IV) metronidazole 500 mg every 6 hours should be added to the treatment regimen in those with ileus or megacolon. For patients with complete ileus, vancomycin can be administered rectally as 500 mg in 100 mL of normal saline every 6 hours. 3 Normal pooled IV immunoglobulin has been used with mixed success, in patients with fulminant and/or refractory CDI, in an attempt to avert surgery or death by providing passive immunotherapy against C. difficile toxins A and B. 34 In a study of monoclonal antibodies to toxins A and B that included 200 patients, recurrence rates among those with the epidemic NAP1/BI/027 strain were 8% for the antibody group and 32% for the placebo group (P = 0.06); in a subset with more than 1 previous episode of CDI, recurrence rates were 7% and 38%, respectively (P = 0.006). 35
Some patients with fulminant or refractory CDI are best managed with subtotal colectomy, which may be lifesaving. 36 However, the optimal timing of surgery is difficult to establish, and the decision to proceed with this course can be difficult because patients with severe CDI are typically poor surgical candidates. 3 Delaying surgery until the development of a systemic inflammatory response syndrome with concomitant severe disease, such as multisystem organ failure, immunocompromise, or hemodynamic instability, usually results in a poor outcome. 37, 38 The best approach is to get a surgical consult early, when the course of CDI starts to deteriorate, so that management decisions can be based on input from a multidisciplinary team of clinicians. 3
CASE STUDY CONTINUED
A.L. was subsequently transferred to an acute care facility. Her therapy for C. difficile was switched to oral vancomycin, 125 mg 4 times daily. On arrival in the emergency department, the following findings were noted:
-
No further diarrhea, but abdominal distention noted.
-
Temperature: 101.4F.
-
Fall in blood pressure (BP) to 90/48 mmHg during transfer, which responded to a fluid bolus, and increased to 108/74.
-
WBC: 27,000/L.
-
Abdominal computed tomography (CT) showed thickening of descending and sigmoid colon, and the rectum.
-
Proctoscopy confirmed pseudomembranous colitis.
Oral vancomycin was increased to 500 mg 4 times daily, and IV metronidazole 500 mg every 8 hours was added. C. difficile infection responded to this regimen and she was subsequently discharged to home care.
The incidence and severity of Clostridium difficile infections (CDI) have increased steadily over the past decade, paralleling the emergence of an epidemic strain of C. difficile in North America, the North American pulsed field type 1 (NAP1), restriction‐endonuclease analysis type BI, polymerase‐chain‐reaction ribotype 027, commonly referred to as NAP1/BI/027. The reduced responsiveness of CDI to standard antibiotic therapy, and increased death rate attributable to CDI, present a significant challenge to clinicians. 1 This is a brief review of clinical strategies for effective management of initial CDI for hospital‐based physicians.
Effective management of CDI requires a multidisciplinary effort that includes identification of patients at risk, rapid implementation of contact isolation for patients suspected of having CDI, and implementation of early and appropriate treatment based on current clinical evidence. 2 Early recognition is, in large part, based on suspecting CDI anytime a patient develops antibiotic‐associated diarrhea. An understanding of traditional and emerging risk factors for CDI can help clinicians identify this serious condition early.
CASE STUDY
A.L. is an 87‐year‐old woman in a rehabilitation facility who is recovering from left hip replacement surgery performed 3 weeks ago. Her past medical history is positive for heart failure, atrial fibrillation, type 2 diabetes mellitus, and chronic obstructive pulmonary disease. During her hospitalization, she was treated preoperatively with cephalexin for prophylaxis. She was recently started on ciprofloxacin for a urinary tract infection, and has been taking this for 6 days.
During morning rounds, her nurse reports that A.L. has had diarrhea for 2 days. She is currently afebrile, and her white blood cell (WBC) count is 11,400/L. Ciprofloxacin was discontinued and a stool test for C. difficile toxin was ordered.
RISK FACTORS FOR CDI
The risk of developing CDI depends on 3 groups of factors: impairment of colonization resistance, risk of exposure to toxigenic C. difficile or its spores, and host health and immune status.
Impairment of Colonization Resistance
C. difficile is extremely common in the general environment. However, balanced intestinal microflora normally confer colonization resistance, a host factor that limits the proliferation of pathogenic microorganisms such as C. difficile. 3 While colonization of C. difficile occurs in the community in only 1% to 4% of healthy adults, the rate of colonization in hospitalized adults is much higher, approximately 20% to 30%. 4 Loss of normal resistance to C. difficile in adults is most commonly a consequence of antimicrobial therapy, which disrupts the intestinal microflora. The propensity of different antimicrobial agents to increase the risk for CDI varies due to differences in the complex relationship of their luminal concentrations, activity against C. difficile, and effects on the normal intestinal microflora. 4 Almost every available antibiotic has been associated with CDI; however, broad‐spectrum agents with antianaerobic activity appear to cause the greatest risk (Table 1). 5 Second‐ and third‐generation cephalosporins and fluoroquinolones are the most problematic because of their frequent use and high levels of resistance among strains of C. difficile to these agents. 1, 69 Regimens with multiple antibiotics and/or longer treatment courses are also associated with an increased risk. 9
| Very Commonly Related | Less Commonly Related | Uncommonly Related |
|---|---|---|
| Clindamycin | Other penicillins | Aminoglycosides |
| Cephalosporins | Sulfonamides | Bacitracin |
| Fluoroquinolones | Trimethoprim | Metronidazole |
| Ampicillin | Cotrimoxazole | Teicoplanin |
| Amoxicillin | Macrolides | Rifampin |
| Chloramphenicol | ||
| Tetracyclines | ||
| Carbapenems | ||
| Daptomycin | ||
| Tigecycline | ||
Clinicians should be aware that while CDI usually presents during or shortly after initiation of the causative antimicrobial, onset may be delayed for 2 or 3 months. 3 Healthcare professionals should consider CDI in patients who present with diarrhea and have a history of recent antimicrobial treatment in a hospital or as an outpatient. Other factors that may disrupt intestinal flora and lead to colonization by C. difficile include:
-
Bowel preparation for colonoscopy or surgery.
-
Cytotoxic chemotherapy.
-
Colitis caused by inflammatory bowel disease.
Risk of Exposure to Toxigenic C. difficile or Its Spores
C. difficile spores, which are highly resistant to drying, temperature fluxes, and many common disinfectants, contaminate the patient care environment in hospitals and other healthcare facilities. They are viable for long periods and may be transmitted from the hands or fomites of healthcare personnel to patients. It is not surprising that this leads to a major infection control challenge. 3
Host Health and Immune Status
A healthy immune response to C. difficile and toxins A and B is associated with milder forms of the condition. Many patients colonized by pathogenic strains of C. difficile do not have symptoms; this carrier state is associated with high circulating titers of immunoglobulin G (IgG) antitoxin. Conversely, several important individual factors that increase the risk for CDI include advancing age, hospital admission, longer duration of hospital stay, severe underlying disease, impairment of immune function, suppression of gastric acid secretion (eg, with proton pump inhibitors), enteral feedings (especially with use of a post‐pyloric tube), and mechanical ventilation. 1, 10
RISK FACTORS FOR RECURRENT CDI
The incidence of recurrent CDI within 60 to 90 days of initial CDI resolution following a course of treatment with metronidazole, vancomycin, or both is mostly 19% to 29%, but was 50% in 1 report. 11 The risk for CDI recurrence increases with each recurrent episode 12: Patients with 1 prior episode of recurrent CDI have a >40% risk for an additional recurrence, and those with 2 or more episodes have a >60% risk. 13, 14 Two likely mechanisms that predispose patients to recurrent CDI are an inadequate immune response to C. difficile toxins and persistent disruption of the normal colonic flora due to therapy with metronidazole, vancomycin, or other concomitant antibiotics. 15 Recurrent CDI is seldom due to resistance of vegetative cells of C. difficile to vancomycin or metronidazole. 14 Two other important factors associated with recurrence are infection with a hypervirulent strain of C. difficile, and the fact that the current hospital population generally consists of older and sicker patients who have been treated with many broad‐spectrum antibiotics. 16
Specific patient risk factors associated with recurrent CDI include 14, 17, 18:
-
Previous history of recurrence.
-
Increased age (>65).
-
Severe underlying disease.
-
Renal impairment.
-
Conditions or treatments that lead to immunocompromise.
-
Hospital admission (especially prolonged hospital stay).
-
Use of additional antibiotics.
As noted above, ongoing treatment with antibiotics plays an important role in the risk for recurrence. Hu and colleagues found that concomitant antibiotic use after a diagnosis of CDI was associated with a 10‐fold increased risk for recurrence (odds ratio [OR], 10.0; 95% confidence interval [CI], 1.5‐68.3). 12 Johnson and colleagues found that the rate of sustained response to CDI therapy, without subsequent recurrence, was higher in patients able to stop all other antibiotics and be treated with only fidaxomicin or vancomycin than it was in a group of patients treated with 1 of these agents plus an additional antibiotic (91.9% and 76.1%, respectively). 19 In general, patients who require concomitant antibiotics have more comorbidities and are sicker, so the entire difference cannot be attributed to antibiotics. However, clinicians should carefully consider the ongoing need for antibiotics if CDI is suspected or confirmed.
Continued exposure to C. difficile in the hospital or home environment often leads to reinfection when vancomycin and metronidazole concentrations have decreased. Data show that at least half of clinical recurrences are reinfection with a different strain, and half are due to persisting intestinal infection with the original infecting strain. 20 Therefore, patients with CDI should be educated about appropriate hygiene at home.
CLINICAL MANIFESTATIONS OF CDI
CDI has a wide range of clinical manifestations, ranging from a mild and self‐limited diarrheal illness to fulminant, life‐threatening colitis. The onset of symptoms usually occurs within 3 to 7 days of antibiotic exposure, but may not arise for up to 10 weeks after stopping antibiotics. 5 CDI is associated with watery diarrhea that is often accompanied by cramping abdominal pain and low‐grade fever. Systemic symptoms generally increase with the degree of colitis. Patients with severe disease may also progress to having an ileus, or toxic megacolon or acute abdomen. 5
Up to 20% of critically ill patients have ileus or toxic megacolon, and therefore may not present with diarrhea; this, combined with a limited ability to communicate among some critically ill patients makes early diagnosis of CDI in this patient population extremely challenging. Therefore, physicians and other clinical staff must be vigilant about evaluating patients for the presence of CDI based on physical exam and laboratory findings. For example, fever, abdominal pain, and abdominal distention are likely to be present in patients with severe colitis. In addition, patients often have significant leukocytosis (often >20,000 cells/mm 3) with bandemia. In advanced cases, an elevated serum lactate dehydrogenase may be seenthis is a nonspecific finding for gastrointestinal disease, but provides a clue to the presence of CDI. Because these findings often precede multiorgan dysfunction, the presence of CDI must be determined quickly and appropriate treatment initiated. 5
PRINCIPLES OF DIAGNOSIS
C. difficile infection should be suspected in patients with antimicrobial‐associated diarrhea. Confirmatory testing should be performed, but only on watery or loose stools because the rate of symptomless colonization with C. difficile in hospitalized patients is high; a positive result on a normal stool sample proves only that the patient is colonized with C. difficile, but not necessarily infected. 14 A notable exception is when CDI is suspected in a patient with ileus; as many laboratories will not accept solid stool for C. difficile testing, the clinician should notify the laboratory about the specific request and reasons for suspecting CDI. 21 Stool testing for eradication of C. difficile during or after therapy is not advised, as many successfully treated patients will continue to shed the organism and its spores. 2 This symptomless carriage does not require additional treatment.
CASE STUDY CONTINUED
A.L. was empirically started on oral metronidazole, 500 mg 3 times a day, pending results of the stool C. difficile test.
On rounds the following day, diarrhea was less frequent (decreased from 9 to 4 loose bowel movements in 24 hours). She reported mild abdominal discomfort and nausea, but was tolerating oral intake. Her temperature was 100.8F.
Her lab results returned later in the afternoon were:
-
WBC: 18,600 cells/L.
-
Potassium: 3.2 mEq/mL.
-
Creatinine: 2.4 mg/dL (up from 1.1).
-
Stool C. difficile test: negative.
There are a variety of tests for C. difficile, each with advantages and disadvantages (Table 2). 2, 3, 21 Factors to consider when selecting a diagnostic test include turnaround time, sensitivity, specificity, cost, whether there is an ongoing outbreak, and the availability of particular tests. 21 Recent guidelines for CDI management jointly developed by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Disease Society of America (IDSA) note that stool culture is the gold standard, but recognize the clinical limitations of its long turnaround time. 2 Enzyme immunoassay (EIA) is noted to be a suboptimal alternative approach for diagnosis than the cell cytotoxin assay. 2 Therefore, because EIA is most often used in clinical practice, it is important to be aware that a high clinical suspicion for CDI may warrant empiric therapy or repeat testing with a more sensitive test in a patient with an initial negative test result. 3, 21
| Test | Advantage(s) | Disadvantage(s) |
|---|---|---|
| Toxin testing | ||
| Enzyme immunoassay | Rapid, simple, | Least sensitive method, some detect only toxin A (some strains only produce toxin B) |
| Cell cytotoxin assay | More sensitive than enzyme immunoassay | Labor intensive; requires 72 hr for a final result, special equipment |
| Organism identification | ||
| Detection of glutamate dehydrogenase | Rapid, sensitive, may prove useful as a triage or screening tool | Not specific, toxin testing required to verify diagnosis; may not be 100% sensitive |
| Polymerase chain reaction | Rapid, sensitive, detects presence of toxin gene | Cost, special equipment needed |
| Stool culture | Most sensitive test available when performed appropriately | May be associated with false‐positive results if isolate is not tested for toxin; labor‐intensive; requires 72 hr for results |
CURRENT STRATEGIES FOR CDI MANAGEMENT
While current guidelines recommend that treatment of CDI be based on disease severity, 2, 22 determination of severity is challenging, in part, because standard definitions are lacking and because the illness varies along a continuum of symptoms. 5 In general, CDI can be categorized as: mild to moderate, severe, and severe disease with complications. Mild to moderate CDI is characterized by diarrhea and abdominal cramping, only without systemic symptoms. 5, 21 Severe CDI is distinguished by abundant diarrhea, severe abdominal pain/distension, leukocytosis, fever, or other systemic symptoms (Table 3). 5, 21, 2326 Patients with severe disease and other complications may present with a wide range of gastrointestinal symptoms accompanied by paralytic ileus, toxic megacolon, or other life‐threatening conditions. 5, 21 CDI may progress in severity rapidly, even after initiation of treatment, so ongoing assessment of the patient's condition and disease category is important. 5, 21
| Severe diarrhea (>10 bowel movements/day) |
| Leukocytosis |
| WBC >15,000 associated with severe CDI |
| WBC >25,000 associated with increased fatality |
| High or rising (50% increase) serum creatinine, or creatinine >2 mg/dL |
| Low serum albumin (<2.5 mg/dL) |
| Severe abdominal distension, pain |
| Ileus or toxic megacolon |
| Colonic thickening on CT scan |
| Ascites on CT scan |
| Pseudomembranes on endoscopy |
| Hemodynamic instability |
| Organ failure |
In 2010, SHEA/IDSA published evidence‐based guidelines for managing CDI based on severity of illness (summarized in Table 4). 2 The first and most important step in the effective management of CDI in all patients, regardless of severity, is to discontinue the causative antibiotic(s). 1 Data show that when all antibiotics are stopped, about 25% of patients with mild CDI who are otherwise healthy have resolution of diarrhea within 48 hours 27; most importantly, recurrent CDI is unlikely. Many hospitalized patients, especially in the intensive care setting, have serious concomitant infections, and therefore it may not be appropriate to discontinue the inciting antibiotic. In these patients, the regimen and available culture and sensitivity results should be thoughtfully reviewed, and change made when possible to a more narrow‐spectrum regimen less likely to cause or exacerbate CDI (Table 1).
| Clinical Severity | Supportive Laboratory Data | Recommended Treatment |
|---|---|---|
| ||
| Mild to moderate | WBC 15,000 cells/L) or serum creatinine <1.5 times premorbid level | Metronidazole 500 mg orally 3 times per day for 10‐14 days |
| Severe | WBC 15,000 cells/L) or serum creatinine 1.5 times premorbid level | Vancomycin 125 mg orally 4 times per day for 10‐14 days |
| Severe, complicated | Hypotension or shock, ileus and/or megacolon; organ failure (eg, ARDS); coagulopathy | Vancomycin 500 mg 4 times per day orally or by nasogastric tube plus metronidazole 500 mg IV every 8 hr |
The next step, as discussed above, is to send a stool sample for C. difficile testing. Based on the patient's clinical circumstances, the decision must be made whether or not to begin empiric therapy. In general, beginning treatment without testing for C. difficile is not recommended, because, at most, only about a third of hospitalized patients with diarrhea have CDI, even in an epidemic setting. 21 If a patient is severely ill or has a rapidly deteriorating clinical course and is at high risk for CDI, empiric therapy may be appropriate while awaiting test results. 21
In patients with severe CDI and complications, reduced or absent bowel motility can reduce the amount of orally administered vancomycin that reaches the site of infection. Intracolonic administration of vancomycin may be indicated in these cases, or when oral therapy cannot be tolerated. 28 Higher doses of oral vancomycin may also be used, with the goal of increasing fecal concentrations, however this strategy has not been studied. 5 In all patients, antiperistaltic agents are usually avoided because of unproven concerns that they might mask symptoms and/or increase the risk for toxic megacolon. 2
Fidaxomicin, a new macrolide antibiotic in the macrocyclic group, has a narrow‐spectrum and excellent activity against C. difficile. The US Food and Drug Administration approved fidaxomicin for treatment of adults for C. difficileassociated diarrhea (CDAD) in May 2011. 29 Approval was based on 2 phase III trials involving 1105 patients with CDAD in which fidaxomicin was shown to have similar initial clinical efficacy and safety as vancomycin. 30 In addition, more patients treated with fidaxomicin had a sustained response 25 days following discontinuation of treatment than patients treated with vancomycin. 31 The recommended dosage of fidaxomicin is one 200‐mg tablet orally twice daily for 10 days. 31
In a phase III trial (N = 596) of fidaxomicin (200 mg orally every 12 hours) versus vancomycin (125 mg orally every 6 hours) for 10 days, fidaxomicin was shown to be noninferior to vancomycin in achieving an initial clinical response and significantly better at preventing recurrent CDI. 32 The rates of initial clinical response, the primary endpoint, and rates of recurrent CDI are shown in Table 5. The significant difference in recurrence may be explained by the fact that metronidazole and vancomycin impact commensal microflora populations that normally mediate competitive exclusion of C. difficile. Compared with vancomycin, fidaxomicin has less effect on the composition of the fecal microbiota, in particular some clostridial clusters and Bifidobacterium. 33 While acquisition costs for this new antibiotic are a consideration, they may be offset by a reduction in recurrent CDI, especially in high‐risk patients.
| Modified Intention‐to‐Treat Population | Per‐Protocol Population | |||
|---|---|---|---|---|
| Fidaxomicin n/N (%) | Vancomycin n/N (%) | Fidaxomicin n/N (%) | Vancomycin n/N (%) | |
| ||||
| Rates of initial clinical response | ||||
| Total population | 253/287 (88.2) | 265/309 (85.8) | 244/265 (92.1) | 254/283 (89.8) |
| Non‐NAP1/BI/027 strain type | 117/125 (93.6) | 121/132 (91.7) | 115/119 (96.6) | 119/126 (94.4) |
| Use of concomitant systemic antimicrobial therapy | 67/83 (80.7) | 72/94 (76.6) | 63/71 (88.7) | 67/80 (83.8) |
| Rates of recurrence of C. difficile infection | ||||
| Total population | 39/253 (15.4)* | 67/265 (25.3)* | 28/211 (13.3) | 53/221 (24.0) |
| Non‐NAP1/BI/027 strain type | 12/117 (10.3) | 34/121 (28.1) | 8/103 (7.8) | 27/106 (25.5) |
| Use of concomitant systemic antimicrobial therapy | 14/81 (17.3) | 25/90 (27.8) | 8/56 (14.3) | 20/65 (30.8) |
FOCUS ON FULMINANT/REFRACTORY CDI
Management of CDI that is fulminant and/or refractory can be extremely challenging. 3 In clinical practice, these conditions often overlap. Clinical characteristics that may help identify fulminant CDI include abdominal pain and tenderness, colonic distension, and signs of sepsis. Diarrhea may be absent or minimal due to ileus. Furthermore, a diagnosis of CDI is easy to miss, as these symptoms are also consistent with ischemic bowel or a perforated viscus. 3 Gentle, flexible sigmoidoscopy or colonoscopy (without bowel preparation and with minimal air insufflation) may be extremely valuable to allow for immediate identification of pseudomembranous colitis, which speeds appropriate medical and surgical management. 3
First‐line treatment for fulminant or refractory CDI is oral or intragastric vancomycin 500 mg every 6 hours. 2, 3 Intravenous (IV) metronidazole 500 mg every 6 hours should be added to the treatment regimen in those with ileus or megacolon. For patients with complete ileus, vancomycin can be administered rectally as 500 mg in 100 mL of normal saline every 6 hours. 3 Normal pooled IV immunoglobulin has been used with mixed success, in patients with fulminant and/or refractory CDI, in an attempt to avert surgery or death by providing passive immunotherapy against C. difficile toxins A and B. 34 In a study of monoclonal antibodies to toxins A and B that included 200 patients, recurrence rates among those with the epidemic NAP1/BI/027 strain were 8% for the antibody group and 32% for the placebo group (P = 0.06); in a subset with more than 1 previous episode of CDI, recurrence rates were 7% and 38%, respectively (P = 0.006). 35
Some patients with fulminant or refractory CDI are best managed with subtotal colectomy, which may be lifesaving. 36 However, the optimal timing of surgery is difficult to establish, and the decision to proceed with this course can be difficult because patients with severe CDI are typically poor surgical candidates. 3 Delaying surgery until the development of a systemic inflammatory response syndrome with concomitant severe disease, such as multisystem organ failure, immunocompromise, or hemodynamic instability, usually results in a poor outcome. 37, 38 The best approach is to get a surgical consult early, when the course of CDI starts to deteriorate, so that management decisions can be based on input from a multidisciplinary team of clinicians. 3
CASE STUDY CONTINUED
A.L. was subsequently transferred to an acute care facility. Her therapy for C. difficile was switched to oral vancomycin, 125 mg 4 times daily. On arrival in the emergency department, the following findings were noted:
-
No further diarrhea, but abdominal distention noted.
-
Temperature: 101.4F.
-
Fall in blood pressure (BP) to 90/48 mmHg during transfer, which responded to a fluid bolus, and increased to 108/74.
-
WBC: 27,000/L.
-
Abdominal computed tomography (CT) showed thickening of descending and sigmoid colon, and the rectum.
-
Proctoscopy confirmed pseudomembranous colitis.
Oral vancomycin was increased to 500 mg 4 times daily, and IV metronidazole 500 mg every 8 hours was added. C. difficile infection responded to this regimen and she was subsequently discharged to home care.
- .Narrative review: the new epidemic of Clostridium difficile‐associated enteric disease.Ann Intern Med.2006;145(10):758–764.
- ,,, et al.Clinical practice guidelines for Clostridium diffcile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA).Infect Control Hosp Epidemiol.2010;31(5):431–455.
- .A 76‐year‐old man with recurrent Clostridium difficile–associated diarrhea: review of C difficile infection.JAMA.2009;301(9):954–962.
- .Antibiotic‐associated diarrhea.N Engl J Med.2002;346(5):334–339.
- ,.Clostridium difficile infection in the intensive care unit.Infect Dis Clin North Am.2009;23(3):727–743.
- ,,, et al.A predominantly clonal multi‐institutional outbreak of Clostridium difficile‐associated diarrhea with high morbidity and mortality.N Engl J Med.2005;353:2442–2449.
- ,,.Antimicrobial therapy of Clostridium difficile‐associated diarrhea.Med Clin North Am.2006;90(6):1141–1163.
- ,,,,.Patterns of antibiotic use and risk of hospital admission because of Clostridium difficile.Can Med Assoc J.2008;179(8):767–772.
- ,,, et al.Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile‐associated diarrhea: a cohort study during an epidemic in Quebec.Clin Infect Dis.2005;41(9):1254–1260.
- .Clostridium difficile and the disease it causes.Methods Mol Biol.2010;646:9–35.
- ,,.Treatment of Clostridium difficile‐associated disease: old therapies and new.Lancet Infect Dis.2005;5(9):549–557.
- , , , et al. Prospective derviation and validation of a clinical prediction rule for recurrent Clostridium difficile infection. Gastroenterology. 2009;136:1206–1214.
- ,,, et al.A randomized placebo‐controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease.JAMA.1994;271(24):1913–1918.
- ,,.Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease.Am J Gastroenterol.2002;97(7):1769–1775.
- .Recurrent Clostridium difficile infection: a review of risk factors, treatments, and outcomes.J Infect.2009;58(6):403–410.
- ,.Clostridium difficile: recent epidemiologic findings and advances in therapy.Pharmacotherapy.2007;27(7):1029–1039.
- ,,, et al.Risk factors for early recurrent Clostridium difficile‐associated diarrheas.Clin Infect Dis.1998;26(4):954–959.
- ,,,.Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea.Lancet.2001;357(9251):189–193.
- ,,,,,.Randomized clinical trial in Clostridium difficile infection confirms superiority of fidaxomicin over vancomycin [abstract 828630]. Presented at: Digestive Disease Week 2010; May 4,2010; New Orleans, LA.
- ,,,.Recurrence of symptoms in Clostridium difficile infection—relapse or reinfection?J Hosp Infect.1998;38(2):93–100.
- ,.Clostridium difficile‐associated disease: new challenges from an established pathogen.Cleve Clin J Med.2006;73(2):187–197.
- ,,,.A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile–associated diarrhea, stratified by disease severity.Clin Infect Dis.2007;45(3):302–307.
- ,,, et al.Clostridium difficile–associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity.Can Med Assoc J.2004;171(5):466–472.
- ,.Clinical recognition and diagnosis of Clostridium difficile infection.Clin Infect Dis.2008;46(suppl 1):S12–S18.
- ,,,.Clinical risk factors for severe Clostridium difficile‐associated disease.Emerg Infect Dis.2009;15(3):415–422.
- ,,.European Society of Clinical Microbiology and Infectious Diseases (ESCMID): treatment guidance document for Clostridium difficile infection (CDI).Clin Microbiol Infect.2009;15(12):1067–1079.
- ,,, et al.Prospective randomised trial of metronidazole versus vancomycin for Clostridium difficile–associated diarrhoea and colitis.Lancet.1983;2(8358):1043–1046.
- ,,.Adjunctive intracolonic vancomycin for severe Clostridium difficile colitis: case series and review of the literature.Clin Infect Dis.2002;35(6):690–696.
- United States Food and Drug Administration. FDA approves treatment for Clostridium difficile infection. Available at: www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm257024.htm. Last updated: May, 27, 2011. Accessed June 15,2011.
- ,,, et al.Efficacy and safety of fidaxomicin (FDX) vs. vancomycin (VAN) in Clostridium difficile infection (CDI) in 2 randomized controlled trials (RCT) with 1105 patients [abstract 1417]. Presented at: The 48th Annual IDSA Meeting; October 21–24,2010; Vancouver, BC, Canada.
- Dificid [package insert].San Diego, CA:Optimer Pharmaceuticals, Inc;2011.
- ,,, et al.Fidaxomicin versus vancomycin for Clostridium difficile infection.N Engl J Med.2011;364(5):422–431.
- ,,, et al.A new macrocyclic antibiotic, fidaxomicin (OPT‐80), causes less alteration to the bowel microbiota of Clostridium difficile‐infected patients than does vancomycin.Microbiology.2010;156(pt 11):3354–3359.
- ,,,.Intravenous immunoglobulin for the treatment of severe Clostridium difficile colitis: an observational study and review of the literature.J Hosp Med.2010;5(1):E1–E9.
- ,,, et al.Treatment with monoclonal antibodies against Clostridium difficile toxins.N Engl J Med.2010;362(3):197–205.
- ,.Surgical aspects of fulminant Clostridium difficile colitis.Am J Surg.2010;200(1):131–135.
- ,,, et al.Clostridium difficile colitis in the critically ill.Dis Colon Rectum.1996;39(6):619–623.
- ,,.Early surgical intervention for fulminant pseudomembranous colitis.Am Surg.74(1):20–26.
- .Narrative review: the new epidemic of Clostridium difficile‐associated enteric disease.Ann Intern Med.2006;145(10):758–764.
- ,,, et al.Clinical practice guidelines for Clostridium diffcile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA).Infect Control Hosp Epidemiol.2010;31(5):431–455.
- .A 76‐year‐old man with recurrent Clostridium difficile–associated diarrhea: review of C difficile infection.JAMA.2009;301(9):954–962.
- .Antibiotic‐associated diarrhea.N Engl J Med.2002;346(5):334–339.
- ,.Clostridium difficile infection in the intensive care unit.Infect Dis Clin North Am.2009;23(3):727–743.
- ,,, et al.A predominantly clonal multi‐institutional outbreak of Clostridium difficile‐associated diarrhea with high morbidity and mortality.N Engl J Med.2005;353:2442–2449.
- ,,.Antimicrobial therapy of Clostridium difficile‐associated diarrhea.Med Clin North Am.2006;90(6):1141–1163.
- ,,,,.Patterns of antibiotic use and risk of hospital admission because of Clostridium difficile.Can Med Assoc J.2008;179(8):767–772.
- ,,, et al.Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile‐associated diarrhea: a cohort study during an epidemic in Quebec.Clin Infect Dis.2005;41(9):1254–1260.
- .Clostridium difficile and the disease it causes.Methods Mol Biol.2010;646:9–35.
- ,,.Treatment of Clostridium difficile‐associated disease: old therapies and new.Lancet Infect Dis.2005;5(9):549–557.
- , , , et al. Prospective derviation and validation of a clinical prediction rule for recurrent Clostridium difficile infection. Gastroenterology. 2009;136:1206–1214.
- ,,, et al.A randomized placebo‐controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease.JAMA.1994;271(24):1913–1918.
- ,,.Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease.Am J Gastroenterol.2002;97(7):1769–1775.
- .Recurrent Clostridium difficile infection: a review of risk factors, treatments, and outcomes.J Infect.2009;58(6):403–410.
- ,.Clostridium difficile: recent epidemiologic findings and advances in therapy.Pharmacotherapy.2007;27(7):1029–1039.
- ,,, et al.Risk factors for early recurrent Clostridium difficile‐associated diarrheas.Clin Infect Dis.1998;26(4):954–959.
- ,,,.Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea.Lancet.2001;357(9251):189–193.
- ,,,,,.Randomized clinical trial in Clostridium difficile infection confirms superiority of fidaxomicin over vancomycin [abstract 828630]. Presented at: Digestive Disease Week 2010; May 4,2010; New Orleans, LA.
- ,,,.Recurrence of symptoms in Clostridium difficile infection—relapse or reinfection?J Hosp Infect.1998;38(2):93–100.
- ,.Clostridium difficile‐associated disease: new challenges from an established pathogen.Cleve Clin J Med.2006;73(2):187–197.
- ,,,.A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile–associated diarrhea, stratified by disease severity.Clin Infect Dis.2007;45(3):302–307.
- ,,, et al.Clostridium difficile–associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity.Can Med Assoc J.2004;171(5):466–472.
- ,.Clinical recognition and diagnosis of Clostridium difficile infection.Clin Infect Dis.2008;46(suppl 1):S12–S18.
- ,,,.Clinical risk factors for severe Clostridium difficile‐associated disease.Emerg Infect Dis.2009;15(3):415–422.
- ,,.European Society of Clinical Microbiology and Infectious Diseases (ESCMID): treatment guidance document for Clostridium difficile infection (CDI).Clin Microbiol Infect.2009;15(12):1067–1079.
- ,,, et al.Prospective randomised trial of metronidazole versus vancomycin for Clostridium difficile–associated diarrhoea and colitis.Lancet.1983;2(8358):1043–1046.
- ,,.Adjunctive intracolonic vancomycin for severe Clostridium difficile colitis: case series and review of the literature.Clin Infect Dis.2002;35(6):690–696.
- United States Food and Drug Administration. FDA approves treatment for Clostridium difficile infection. Available at: www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm257024.htm. Last updated: May, 27, 2011. Accessed June 15,2011.
- ,,, et al.Efficacy and safety of fidaxomicin (FDX) vs. vancomycin (VAN) in Clostridium difficile infection (CDI) in 2 randomized controlled trials (RCT) with 1105 patients [abstract 1417]. Presented at: The 48th Annual IDSA Meeting; October 21–24,2010; Vancouver, BC, Canada.
- Dificid [package insert].San Diego, CA:Optimer Pharmaceuticals, Inc;2011.
- ,,, et al.Fidaxomicin versus vancomycin for Clostridium difficile infection.N Engl J Med.2011;364(5):422–431.
- ,,, et al.A new macrocyclic antibiotic, fidaxomicin (OPT‐80), causes less alteration to the bowel microbiota of Clostridium difficile‐infected patients than does vancomycin.Microbiology.2010;156(pt 11):3354–3359.
- ,,,.Intravenous immunoglobulin for the treatment of severe Clostridium difficile colitis: an observational study and review of the literature.J Hosp Med.2010;5(1):E1–E9.
- ,,, et al.Treatment with monoclonal antibodies against Clostridium difficile toxins.N Engl J Med.2010;362(3):197–205.
- ,.Surgical aspects of fulminant Clostridium difficile colitis.Am J Surg.2010;200(1):131–135.
- ,,, et al.Clostridium difficile colitis in the critically ill.Dis Colon Rectum.1996;39(6):619–623.
- ,,.Early surgical intervention for fulminant pseudomembranous colitis.Am Surg.74(1):20–26.
Meeting the Challenge of Recurrent CDI
As noted elsewhere in this supplement, recent studies show a high rate of recurrent Clostridium difficile infection (CDI) despite the availability of evidence‐based guidelines for CDI treatment. 1, 2 Treatment with vancomycin has been associated with recurrence in 25% or more of patients. The risk for recurrence increases with each episode, and is greater than 60% in patients with more than 2 episodes. 1, 3, 4 Identification of patients at risk for recurrence is critical to early diagnosis and prompt treatment. 5 Because recurrent CDI is so widespread, it should be common practice to educate patients about this complication, including:
-
The risk for continued exposure to C. difficile in the hospital or home environment and strategies for appropriate hygiene to minimize reinfection.
-
When to contact a healthcare professional for recurrent symptoms. 1
CASE STUDY CONTINUED
In the previous article in this supplement, a case study for patient A.L. was presented. A.L. is an 87‐year‐old woman who developed initial inpatient CDI in a rehabilitation facility. She was empirically started on oral metronidazole, 500 mg 3 times a day. Her symptoms improved but she became febrile with an elevated white blood cell (WBC) count. She was transferred to an acute care facility where her treatment was switched to oral vancomycin 125 mg 4 times daily. A proctoscopic exam confirmed pseudomembranous colitis, and her treatment regimen was increased to oral vancomycin 500 mg 4 times daily plus intravenous (IV) metronidazole 500 mg every 8 hours. The CDI responded to therapy and she was discharged to home care.
Ten days after her hospital discharge, A.L. noted a change in the character of her stools, which became looser and increased in frequency, accompanied by a foul odor that she recalls was present during her previous episode. The following day, she developed frank watery stools occurring every 1‐2 hours and had 2 incontinent episodes. Her family brought her to the emergency department because she was also light‐headed, confused, and had increased abdominal cramping. Intravenous fluid resuscitation was started, along with oral vancomycin, 125 mg every 6 hours for 14 days, followed by a vancomycin taper and pulse, which consisted of 125 mg twice daily for 7 days, once daily for 7 days, once every other day for 7 days, then every third day. Stool was sent to the lab for C. difficile testing in the middle of the taper regimen. The result was negative. Ten days after tapering the oral vancomycin regimen to every third day, she developed loose stools with the same odor, followed by increased frequency and frank watery stools.
This case illustrates several aspects of recurrent CDI. First, the recurrent episode may be severe, as evidenced by the need for IV fluid resuscitation in addition to specific anti‐C. difficile treatment. Second, although the patient was treated appropriately with standard 4 times daily vancomycin followed by a taper, subsequent recurrences may still occur, usually near the end of the taper/pulse (as in this case) or shortly after finishing the regimen. Finally, test of cure stool testing (either toxin testing, culture, or polymerase chain reaction [PCR]) may be misleading and is NOT recommended for managing recurrent CDI. Although subsequent management of this case was not addressed, repeating the standard vancomycin regimen, followed again by a taper/pulse would be a realistic option, as vancomycin resistance in C. difficile has not been reported and the patient would be expected to respond. Other management options should also be considered and are discussed below.
SHEA/IDSA RECOMMENDATIONS
There is no strong evidence to support a particular treatment strategy for recurrent CDI. 5 The Society for Healthcare Epidemiology of America/Infectious Diseases Society of America (SHEA/IDSA) guidelines 6 recommends the following:
-
When severe or complicated CDI is suspected, initiate empiric treatment as soon as the diagnosis is suspected (C‐III).
-
Treatment of the first recurrence is usually with the same regimen as for the initial episode (A‐II), but should be stratified by disease severity (C‐III).
-
Do not use metronidazole beyond first recurrence or for long‐term chronic therapy (B‐II).
-
Treatment of second or later recurrences with vancomycin using a taper and/or pulse regimen is the preferred next strategy (B‐III).
-
No recommendations can be made regarding prevention of recurrent CDI in patients requiring continued antimicrobial therapy (C‐III).
The vancomycin taper/pulse regimen is one of the most widely used regimens for treatment of recurrent CDI. 5 A tapered oral vancomycin regimen consists of a stepwise decrease in dose over a period of time. Intermittent or pulsed vancomycin therapy consists of administering the drug every few days.
A standard course of antibiotic therapy eradicates vegetative cells of C. difficile, but is not effective against spores. Administering antibiotics over an extended time period at decreasing doses (tapered regimen) or intermittent delivery (pulsed regimen) gradually clears C. difficile by eradicating cells as the spores germinate. 5 Thus, a taper/pulse regimen of vancomycin, in theory, leads to a decreased rate of recurrence and may aid restoration of the normal microflora. 5
Evidence for efficacy of the tapered dosage regimen is based on a post hoc analysis of patients treated for recurrence in 2 trials of probiotic treatment with Saccharomyces boulardii. When standard‐dose oral vancomycin (125 mg 4 times daily) was compared with high‐dose vancomycin (500 mg twice daily for 7 to 14 days), recurrence rates were not statistically different. However, a tapered regimen of vancomycin resulted in significantly fewer recurrences (31%, P = 0.01), as did a pulsed dose of vancomycin (14.3%, P = 0.02). 4 One empiric pulsed‐dose regimen consists of oral vancomycin, 125 mg every 6 hours for 14 days, followed by tapering to 125 mg every 12 hours for 7 days, then 125 mg once daily for another 7 days, followed in turn by pulse‐dosed vancomycin (125 mg once every 2 days for 4 doses, then once every 3 days for 5 doses, or longer). 1 Prolonged courses of metronidazole are not recommended because of potential adverse effects, including peripheral neuropathy. 1
Management of patients with multiple recurrences of CDI is difficult, and no regimens are supported by adequate clinical evidence. 5 Various strategies have been tried, including probiotics, antibiotics, toxin binders, and immune‐based treatments. 1 The strategy behind use of probiotics is to augment colonization resistance. The probiotic S. boulardii, 1 g daily for 4 weeks, decreased recurrence compared with placebo in a small study of 60 patients when given during and after standard treatment (ie, metronidazole or vancomycin). In patients receiving high‐dose vancomycin plus S. boulardii, 3 of 18 (16.7%) had a recurrence compared with 7 of 14 (50%) receiving high‐dose vancomycin plus placebo (P = 0.05). 7 However, a larger follow‐up study did not show a significant overall benefit of S. boulardii over placebo. 1, 7 In addition, there have been a few case reports of systemic infections in immunocompromised patients treated with probiotics. 8 Overall, the results of studies with probiotics, including Lactobacilli, have been inconsistent.
Another approach to restoring a normal gastrointestinal microflora is fecal transplantation, where a small amount of fresh feces from a healthy donor (ideally someone who lives with the patient), is suspended in saline, filtered, and administered through a nasogastric tube, by colonoscope, or by enema. In a recent case series of 18 patients, this approach showed a 94% success rate. 9
Another potential strategy to prevent recurrence is to block colonization of pathogenic C. difficile strains by administration of nontoxigenic and nonpathogenic strains of C. difficile. Researchers have identified a nontoxigenic strain that is being developed as a targeted biotherapeutic probiotic for human use. 1 Because patients with recurrent CDI lack a strong immune response to C. difficile toxins, IV immunoglobulin (IVIG) has been used empirically to provide passive immunotherapy. It has shown benefit in some case series of patients with multiple recurrences. 1, 10, 11
Other antibiotics have also been investigated in conjunction with vancomycin for recurrent infection. Rifaximin has good in vitro activity against C. difficile, and is not absorbed from the gastrointestinal tract. Oral rifaximin, 400 to 800 mg daily for 14 days following discontinuation of vancomycin, was shown to prevent further recurrence in 7 of 8 patients with a history of 4 to 8 CDI recurrences. 1, 12 It is important to note that rifaximin resistance has been reported in clinical isolates of C. difficile, and may be more common than initially thought, particularly among epidemic strains. 13
Fidaxomicin, a narrow‐spectrum macrocyclic antibiotic, was also compared with vancomycin in 2 multicenter, randomized, double‐blind Phase 3 clinical trials of 1105 adults with confirmed CDI. 14 Patients were treated with either oral fidaxomicin (200 mg every 12 hours) or oral vancomycin (125 mg every 6 hours) for 10 days. 15, 16 The clinical cure rate with fidaxomicin was comparable to vancomycin in both studies. 14 In the more recent study, 59.8% of subjects (N = 535) were receiving concomitant antibiotics during CDI treatment; among this group, treatment with fidaxomicin was associated with a significantly lower recurrence rate than treatment with vancomycin (17.6% vs 29.5%, P = 0.027). 15 In addition, there was a sustained clinical response. Global cure, also a secondary endpoint, was defined as patients who were cured and did not have a recurrence during a subsequent 4‐week period, compared with treatment with vancomycin (67.5% vs 53.4%, P = 0.020). 15 These results confirm the findings from the first fidaxomicin Phase 3 study 16 and suggest that even when concomitant antibiotics are administered, fidaxomicin may be more effective than vancomycin in preventing CDI recurrence.
SUMMARY
Because hospitalists take a leadership role and often coordinate care for patients with CDI, they can take an active role to ensure that clinicians are aware of evidence‐based treatments for recurrent CDI, and the importance of routine follow‐up and persistence. The most important considerations in managing patients with recurrent CDI are to:
-
Continue to try new or previous approaches, beginning with those that are evidence‐based, followed by options that have been shown to work but are not backed by strong clinical evidence.
-
Provide consistent follow‐up and ongoing support.
-
Be sympatheticbecause this condition has significant detrimental impact on quality of life.
- . A 76‐year‐old man with recurrent Clostridium difficile‐associated diarrhea: review of C difficile infection. JAMA. 2009;301(9):954–962.
- . Clostridium difficile and the disease it causes. Methods Mol Biol. 2010;646:9–35.
- , , , et al. A randomized placebo‐controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA. 1994;271(24):1913–1918.
- , , . Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97(7):1769–1775.
- . Recurrent Clostridium difficile infection: a review of risk factors, treatments, and outcomes. J Infect. 2009;58(6):403–410.
- , , , et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431–455.
- , , , et al. The search for a better treatment for recurrent Clostridium difficile disease: use of high‐dose vancomycin combined with Saccharomyces boulardii. Clin Infect Dis. 2000;31(4):1012–1017.
- , , , et al. Saccharomyces cerevisiae fungemia after Saccharomyces boulardii treatment in immunocompromised patients. J Clin Gastroenterol. 2003;36(1):41–43.
- , , . Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis. 2003;36(5):580–585.
- , , , et al. Treatment with intravenously administered gamma globulin of chronic relapsing colitis induced by Clostridium difficile toxin. J Pediatr. 1991;118(4 pt 1):633–637.
- . Descriptive study of intravenous immunoglobulin for the treatment of recurrent Clostridium difficile diarrhoea. J Antimicrob Chemother. 2004;53(5):882–884.
- , , , , . Interruption of recurrent Clostridium difficile‐associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clin Infect Dis. 2007;44(6):846–848.
- , , , et al. Rifampin and rifaximin resistance in clinical isolates of Clostridium difficile. Antimicrob Agents Chemother. 2008;52(8):2813–2817.
- , , , , , . Efficacy and safety of fidaxomicin (FDX) vs. vancomycin (VAN) in Clostridium difficile infection (CDI) in 2 randomized controlled trials (RCT) with 1105 patients [abstract 1417]. Presented at: The 48th Annual IDSA Meeting; October 21–24, 2010; Vancouver, BC, Canada.
- , , , , , . Randomized clinical trial in Clostridium difficile infection confirms superiority of fidaxomicin over vancomycin [abstract 828630]. Presented at: Digestive Disease Week 2010; May 4, 2010; New Orleans, LA.
- , , , et al for the OPT‐80–003 Clinical Study Group. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364(5):422–431.
As noted elsewhere in this supplement, recent studies show a high rate of recurrent Clostridium difficile infection (CDI) despite the availability of evidence‐based guidelines for CDI treatment. 1, 2 Treatment with vancomycin has been associated with recurrence in 25% or more of patients. The risk for recurrence increases with each episode, and is greater than 60% in patients with more than 2 episodes. 1, 3, 4 Identification of patients at risk for recurrence is critical to early diagnosis and prompt treatment. 5 Because recurrent CDI is so widespread, it should be common practice to educate patients about this complication, including:
-
The risk for continued exposure to C. difficile in the hospital or home environment and strategies for appropriate hygiene to minimize reinfection.
-
When to contact a healthcare professional for recurrent symptoms. 1
CASE STUDY CONTINUED
In the previous article in this supplement, a case study for patient A.L. was presented. A.L. is an 87‐year‐old woman who developed initial inpatient CDI in a rehabilitation facility. She was empirically started on oral metronidazole, 500 mg 3 times a day. Her symptoms improved but she became febrile with an elevated white blood cell (WBC) count. She was transferred to an acute care facility where her treatment was switched to oral vancomycin 125 mg 4 times daily. A proctoscopic exam confirmed pseudomembranous colitis, and her treatment regimen was increased to oral vancomycin 500 mg 4 times daily plus intravenous (IV) metronidazole 500 mg every 8 hours. The CDI responded to therapy and she was discharged to home care.
Ten days after her hospital discharge, A.L. noted a change in the character of her stools, which became looser and increased in frequency, accompanied by a foul odor that she recalls was present during her previous episode. The following day, she developed frank watery stools occurring every 1‐2 hours and had 2 incontinent episodes. Her family brought her to the emergency department because she was also light‐headed, confused, and had increased abdominal cramping. Intravenous fluid resuscitation was started, along with oral vancomycin, 125 mg every 6 hours for 14 days, followed by a vancomycin taper and pulse, which consisted of 125 mg twice daily for 7 days, once daily for 7 days, once every other day for 7 days, then every third day. Stool was sent to the lab for C. difficile testing in the middle of the taper regimen. The result was negative. Ten days after tapering the oral vancomycin regimen to every third day, she developed loose stools with the same odor, followed by increased frequency and frank watery stools.
This case illustrates several aspects of recurrent CDI. First, the recurrent episode may be severe, as evidenced by the need for IV fluid resuscitation in addition to specific anti‐C. difficile treatment. Second, although the patient was treated appropriately with standard 4 times daily vancomycin followed by a taper, subsequent recurrences may still occur, usually near the end of the taper/pulse (as in this case) or shortly after finishing the regimen. Finally, test of cure stool testing (either toxin testing, culture, or polymerase chain reaction [PCR]) may be misleading and is NOT recommended for managing recurrent CDI. Although subsequent management of this case was not addressed, repeating the standard vancomycin regimen, followed again by a taper/pulse would be a realistic option, as vancomycin resistance in C. difficile has not been reported and the patient would be expected to respond. Other management options should also be considered and are discussed below.
SHEA/IDSA RECOMMENDATIONS
There is no strong evidence to support a particular treatment strategy for recurrent CDI. 5 The Society for Healthcare Epidemiology of America/Infectious Diseases Society of America (SHEA/IDSA) guidelines 6 recommends the following:
-
When severe or complicated CDI is suspected, initiate empiric treatment as soon as the diagnosis is suspected (C‐III).
-
Treatment of the first recurrence is usually with the same regimen as for the initial episode (A‐II), but should be stratified by disease severity (C‐III).
-
Do not use metronidazole beyond first recurrence or for long‐term chronic therapy (B‐II).
-
Treatment of second or later recurrences with vancomycin using a taper and/or pulse regimen is the preferred next strategy (B‐III).
-
No recommendations can be made regarding prevention of recurrent CDI in patients requiring continued antimicrobial therapy (C‐III).
The vancomycin taper/pulse regimen is one of the most widely used regimens for treatment of recurrent CDI. 5 A tapered oral vancomycin regimen consists of a stepwise decrease in dose over a period of time. Intermittent or pulsed vancomycin therapy consists of administering the drug every few days.
A standard course of antibiotic therapy eradicates vegetative cells of C. difficile, but is not effective against spores. Administering antibiotics over an extended time period at decreasing doses (tapered regimen) or intermittent delivery (pulsed regimen) gradually clears C. difficile by eradicating cells as the spores germinate. 5 Thus, a taper/pulse regimen of vancomycin, in theory, leads to a decreased rate of recurrence and may aid restoration of the normal microflora. 5
Evidence for efficacy of the tapered dosage regimen is based on a post hoc analysis of patients treated for recurrence in 2 trials of probiotic treatment with Saccharomyces boulardii. When standard‐dose oral vancomycin (125 mg 4 times daily) was compared with high‐dose vancomycin (500 mg twice daily for 7 to 14 days), recurrence rates were not statistically different. However, a tapered regimen of vancomycin resulted in significantly fewer recurrences (31%, P = 0.01), as did a pulsed dose of vancomycin (14.3%, P = 0.02). 4 One empiric pulsed‐dose regimen consists of oral vancomycin, 125 mg every 6 hours for 14 days, followed by tapering to 125 mg every 12 hours for 7 days, then 125 mg once daily for another 7 days, followed in turn by pulse‐dosed vancomycin (125 mg once every 2 days for 4 doses, then once every 3 days for 5 doses, or longer). 1 Prolonged courses of metronidazole are not recommended because of potential adverse effects, including peripheral neuropathy. 1
Management of patients with multiple recurrences of CDI is difficult, and no regimens are supported by adequate clinical evidence. 5 Various strategies have been tried, including probiotics, antibiotics, toxin binders, and immune‐based treatments. 1 The strategy behind use of probiotics is to augment colonization resistance. The probiotic S. boulardii, 1 g daily for 4 weeks, decreased recurrence compared with placebo in a small study of 60 patients when given during and after standard treatment (ie, metronidazole or vancomycin). In patients receiving high‐dose vancomycin plus S. boulardii, 3 of 18 (16.7%) had a recurrence compared with 7 of 14 (50%) receiving high‐dose vancomycin plus placebo (P = 0.05). 7 However, a larger follow‐up study did not show a significant overall benefit of S. boulardii over placebo. 1, 7 In addition, there have been a few case reports of systemic infections in immunocompromised patients treated with probiotics. 8 Overall, the results of studies with probiotics, including Lactobacilli, have been inconsistent.
Another approach to restoring a normal gastrointestinal microflora is fecal transplantation, where a small amount of fresh feces from a healthy donor (ideally someone who lives with the patient), is suspended in saline, filtered, and administered through a nasogastric tube, by colonoscope, or by enema. In a recent case series of 18 patients, this approach showed a 94% success rate. 9
Another potential strategy to prevent recurrence is to block colonization of pathogenic C. difficile strains by administration of nontoxigenic and nonpathogenic strains of C. difficile. Researchers have identified a nontoxigenic strain that is being developed as a targeted biotherapeutic probiotic for human use. 1 Because patients with recurrent CDI lack a strong immune response to C. difficile toxins, IV immunoglobulin (IVIG) has been used empirically to provide passive immunotherapy. It has shown benefit in some case series of patients with multiple recurrences. 1, 10, 11
Other antibiotics have also been investigated in conjunction with vancomycin for recurrent infection. Rifaximin has good in vitro activity against C. difficile, and is not absorbed from the gastrointestinal tract. Oral rifaximin, 400 to 800 mg daily for 14 days following discontinuation of vancomycin, was shown to prevent further recurrence in 7 of 8 patients with a history of 4 to 8 CDI recurrences. 1, 12 It is important to note that rifaximin resistance has been reported in clinical isolates of C. difficile, and may be more common than initially thought, particularly among epidemic strains. 13
Fidaxomicin, a narrow‐spectrum macrocyclic antibiotic, was also compared with vancomycin in 2 multicenter, randomized, double‐blind Phase 3 clinical trials of 1105 adults with confirmed CDI. 14 Patients were treated with either oral fidaxomicin (200 mg every 12 hours) or oral vancomycin (125 mg every 6 hours) for 10 days. 15, 16 The clinical cure rate with fidaxomicin was comparable to vancomycin in both studies. 14 In the more recent study, 59.8% of subjects (N = 535) were receiving concomitant antibiotics during CDI treatment; among this group, treatment with fidaxomicin was associated with a significantly lower recurrence rate than treatment with vancomycin (17.6% vs 29.5%, P = 0.027). 15 In addition, there was a sustained clinical response. Global cure, also a secondary endpoint, was defined as patients who were cured and did not have a recurrence during a subsequent 4‐week period, compared with treatment with vancomycin (67.5% vs 53.4%, P = 0.020). 15 These results confirm the findings from the first fidaxomicin Phase 3 study 16 and suggest that even when concomitant antibiotics are administered, fidaxomicin may be more effective than vancomycin in preventing CDI recurrence.
SUMMARY
Because hospitalists take a leadership role and often coordinate care for patients with CDI, they can take an active role to ensure that clinicians are aware of evidence‐based treatments for recurrent CDI, and the importance of routine follow‐up and persistence. The most important considerations in managing patients with recurrent CDI are to:
-
Continue to try new or previous approaches, beginning with those that are evidence‐based, followed by options that have been shown to work but are not backed by strong clinical evidence.
-
Provide consistent follow‐up and ongoing support.
-
Be sympatheticbecause this condition has significant detrimental impact on quality of life.
As noted elsewhere in this supplement, recent studies show a high rate of recurrent Clostridium difficile infection (CDI) despite the availability of evidence‐based guidelines for CDI treatment. 1, 2 Treatment with vancomycin has been associated with recurrence in 25% or more of patients. The risk for recurrence increases with each episode, and is greater than 60% in patients with more than 2 episodes. 1, 3, 4 Identification of patients at risk for recurrence is critical to early diagnosis and prompt treatment. 5 Because recurrent CDI is so widespread, it should be common practice to educate patients about this complication, including:
-
The risk for continued exposure to C. difficile in the hospital or home environment and strategies for appropriate hygiene to minimize reinfection.
-
When to contact a healthcare professional for recurrent symptoms. 1
CASE STUDY CONTINUED
In the previous article in this supplement, a case study for patient A.L. was presented. A.L. is an 87‐year‐old woman who developed initial inpatient CDI in a rehabilitation facility. She was empirically started on oral metronidazole, 500 mg 3 times a day. Her symptoms improved but she became febrile with an elevated white blood cell (WBC) count. She was transferred to an acute care facility where her treatment was switched to oral vancomycin 125 mg 4 times daily. A proctoscopic exam confirmed pseudomembranous colitis, and her treatment regimen was increased to oral vancomycin 500 mg 4 times daily plus intravenous (IV) metronidazole 500 mg every 8 hours. The CDI responded to therapy and she was discharged to home care.
Ten days after her hospital discharge, A.L. noted a change in the character of her stools, which became looser and increased in frequency, accompanied by a foul odor that she recalls was present during her previous episode. The following day, she developed frank watery stools occurring every 1‐2 hours and had 2 incontinent episodes. Her family brought her to the emergency department because she was also light‐headed, confused, and had increased abdominal cramping. Intravenous fluid resuscitation was started, along with oral vancomycin, 125 mg every 6 hours for 14 days, followed by a vancomycin taper and pulse, which consisted of 125 mg twice daily for 7 days, once daily for 7 days, once every other day for 7 days, then every third day. Stool was sent to the lab for C. difficile testing in the middle of the taper regimen. The result was negative. Ten days after tapering the oral vancomycin regimen to every third day, she developed loose stools with the same odor, followed by increased frequency and frank watery stools.
This case illustrates several aspects of recurrent CDI. First, the recurrent episode may be severe, as evidenced by the need for IV fluid resuscitation in addition to specific anti‐C. difficile treatment. Second, although the patient was treated appropriately with standard 4 times daily vancomycin followed by a taper, subsequent recurrences may still occur, usually near the end of the taper/pulse (as in this case) or shortly after finishing the regimen. Finally, test of cure stool testing (either toxin testing, culture, or polymerase chain reaction [PCR]) may be misleading and is NOT recommended for managing recurrent CDI. Although subsequent management of this case was not addressed, repeating the standard vancomycin regimen, followed again by a taper/pulse would be a realistic option, as vancomycin resistance in C. difficile has not been reported and the patient would be expected to respond. Other management options should also be considered and are discussed below.
SHEA/IDSA RECOMMENDATIONS
There is no strong evidence to support a particular treatment strategy for recurrent CDI. 5 The Society for Healthcare Epidemiology of America/Infectious Diseases Society of America (SHEA/IDSA) guidelines 6 recommends the following:
-
When severe or complicated CDI is suspected, initiate empiric treatment as soon as the diagnosis is suspected (C‐III).
-
Treatment of the first recurrence is usually with the same regimen as for the initial episode (A‐II), but should be stratified by disease severity (C‐III).
-
Do not use metronidazole beyond first recurrence or for long‐term chronic therapy (B‐II).
-
Treatment of second or later recurrences with vancomycin using a taper and/or pulse regimen is the preferred next strategy (B‐III).
-
No recommendations can be made regarding prevention of recurrent CDI in patients requiring continued antimicrobial therapy (C‐III).
The vancomycin taper/pulse regimen is one of the most widely used regimens for treatment of recurrent CDI. 5 A tapered oral vancomycin regimen consists of a stepwise decrease in dose over a period of time. Intermittent or pulsed vancomycin therapy consists of administering the drug every few days.
A standard course of antibiotic therapy eradicates vegetative cells of C. difficile, but is not effective against spores. Administering antibiotics over an extended time period at decreasing doses (tapered regimen) or intermittent delivery (pulsed regimen) gradually clears C. difficile by eradicating cells as the spores germinate. 5 Thus, a taper/pulse regimen of vancomycin, in theory, leads to a decreased rate of recurrence and may aid restoration of the normal microflora. 5
Evidence for efficacy of the tapered dosage regimen is based on a post hoc analysis of patients treated for recurrence in 2 trials of probiotic treatment with Saccharomyces boulardii. When standard‐dose oral vancomycin (125 mg 4 times daily) was compared with high‐dose vancomycin (500 mg twice daily for 7 to 14 days), recurrence rates were not statistically different. However, a tapered regimen of vancomycin resulted in significantly fewer recurrences (31%, P = 0.01), as did a pulsed dose of vancomycin (14.3%, P = 0.02). 4 One empiric pulsed‐dose regimen consists of oral vancomycin, 125 mg every 6 hours for 14 days, followed by tapering to 125 mg every 12 hours for 7 days, then 125 mg once daily for another 7 days, followed in turn by pulse‐dosed vancomycin (125 mg once every 2 days for 4 doses, then once every 3 days for 5 doses, or longer). 1 Prolonged courses of metronidazole are not recommended because of potential adverse effects, including peripheral neuropathy. 1
Management of patients with multiple recurrences of CDI is difficult, and no regimens are supported by adequate clinical evidence. 5 Various strategies have been tried, including probiotics, antibiotics, toxin binders, and immune‐based treatments. 1 The strategy behind use of probiotics is to augment colonization resistance. The probiotic S. boulardii, 1 g daily for 4 weeks, decreased recurrence compared with placebo in a small study of 60 patients when given during and after standard treatment (ie, metronidazole or vancomycin). In patients receiving high‐dose vancomycin plus S. boulardii, 3 of 18 (16.7%) had a recurrence compared with 7 of 14 (50%) receiving high‐dose vancomycin plus placebo (P = 0.05). 7 However, a larger follow‐up study did not show a significant overall benefit of S. boulardii over placebo. 1, 7 In addition, there have been a few case reports of systemic infections in immunocompromised patients treated with probiotics. 8 Overall, the results of studies with probiotics, including Lactobacilli, have been inconsistent.
Another approach to restoring a normal gastrointestinal microflora is fecal transplantation, where a small amount of fresh feces from a healthy donor (ideally someone who lives with the patient), is suspended in saline, filtered, and administered through a nasogastric tube, by colonoscope, or by enema. In a recent case series of 18 patients, this approach showed a 94% success rate. 9
Another potential strategy to prevent recurrence is to block colonization of pathogenic C. difficile strains by administration of nontoxigenic and nonpathogenic strains of C. difficile. Researchers have identified a nontoxigenic strain that is being developed as a targeted biotherapeutic probiotic for human use. 1 Because patients with recurrent CDI lack a strong immune response to C. difficile toxins, IV immunoglobulin (IVIG) has been used empirically to provide passive immunotherapy. It has shown benefit in some case series of patients with multiple recurrences. 1, 10, 11
Other antibiotics have also been investigated in conjunction with vancomycin for recurrent infection. Rifaximin has good in vitro activity against C. difficile, and is not absorbed from the gastrointestinal tract. Oral rifaximin, 400 to 800 mg daily for 14 days following discontinuation of vancomycin, was shown to prevent further recurrence in 7 of 8 patients with a history of 4 to 8 CDI recurrences. 1, 12 It is important to note that rifaximin resistance has been reported in clinical isolates of C. difficile, and may be more common than initially thought, particularly among epidemic strains. 13
Fidaxomicin, a narrow‐spectrum macrocyclic antibiotic, was also compared with vancomycin in 2 multicenter, randomized, double‐blind Phase 3 clinical trials of 1105 adults with confirmed CDI. 14 Patients were treated with either oral fidaxomicin (200 mg every 12 hours) or oral vancomycin (125 mg every 6 hours) for 10 days. 15, 16 The clinical cure rate with fidaxomicin was comparable to vancomycin in both studies. 14 In the more recent study, 59.8% of subjects (N = 535) were receiving concomitant antibiotics during CDI treatment; among this group, treatment with fidaxomicin was associated with a significantly lower recurrence rate than treatment with vancomycin (17.6% vs 29.5%, P = 0.027). 15 In addition, there was a sustained clinical response. Global cure, also a secondary endpoint, was defined as patients who were cured and did not have a recurrence during a subsequent 4‐week period, compared with treatment with vancomycin (67.5% vs 53.4%, P = 0.020). 15 These results confirm the findings from the first fidaxomicin Phase 3 study 16 and suggest that even when concomitant antibiotics are administered, fidaxomicin may be more effective than vancomycin in preventing CDI recurrence.
SUMMARY
Because hospitalists take a leadership role and often coordinate care for patients with CDI, they can take an active role to ensure that clinicians are aware of evidence‐based treatments for recurrent CDI, and the importance of routine follow‐up and persistence. The most important considerations in managing patients with recurrent CDI are to:
-
Continue to try new or previous approaches, beginning with those that are evidence‐based, followed by options that have been shown to work but are not backed by strong clinical evidence.
-
Provide consistent follow‐up and ongoing support.
-
Be sympatheticbecause this condition has significant detrimental impact on quality of life.
- . A 76‐year‐old man with recurrent Clostridium difficile‐associated diarrhea: review of C difficile infection. JAMA. 2009;301(9):954–962.
- . Clostridium difficile and the disease it causes. Methods Mol Biol. 2010;646:9–35.
- , , , et al. A randomized placebo‐controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA. 1994;271(24):1913–1918.
- , , . Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97(7):1769–1775.
- . Recurrent Clostridium difficile infection: a review of risk factors, treatments, and outcomes. J Infect. 2009;58(6):403–410.
- , , , et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431–455.
- , , , et al. The search for a better treatment for recurrent Clostridium difficile disease: use of high‐dose vancomycin combined with Saccharomyces boulardii. Clin Infect Dis. 2000;31(4):1012–1017.
- , , , et al. Saccharomyces cerevisiae fungemia after Saccharomyces boulardii treatment in immunocompromised patients. J Clin Gastroenterol. 2003;36(1):41–43.
- , , . Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis. 2003;36(5):580–585.
- , , , et al. Treatment with intravenously administered gamma globulin of chronic relapsing colitis induced by Clostridium difficile toxin. J Pediatr. 1991;118(4 pt 1):633–637.
- . Descriptive study of intravenous immunoglobulin for the treatment of recurrent Clostridium difficile diarrhoea. J Antimicrob Chemother. 2004;53(5):882–884.
- , , , , . Interruption of recurrent Clostridium difficile‐associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clin Infect Dis. 2007;44(6):846–848.
- , , , et al. Rifampin and rifaximin resistance in clinical isolates of Clostridium difficile. Antimicrob Agents Chemother. 2008;52(8):2813–2817.
- , , , , , . Efficacy and safety of fidaxomicin (FDX) vs. vancomycin (VAN) in Clostridium difficile infection (CDI) in 2 randomized controlled trials (RCT) with 1105 patients [abstract 1417]. Presented at: The 48th Annual IDSA Meeting; October 21–24, 2010; Vancouver, BC, Canada.
- , , , , , . Randomized clinical trial in Clostridium difficile infection confirms superiority of fidaxomicin over vancomycin [abstract 828630]. Presented at: Digestive Disease Week 2010; May 4, 2010; New Orleans, LA.
- , , , et al for the OPT‐80–003 Clinical Study Group. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364(5):422–431.
- . A 76‐year‐old man with recurrent Clostridium difficile‐associated diarrhea: review of C difficile infection. JAMA. 2009;301(9):954–962.
- . Clostridium difficile and the disease it causes. Methods Mol Biol. 2010;646:9–35.
- , , , et al. A randomized placebo‐controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA. 1994;271(24):1913–1918.
- , , . Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97(7):1769–1775.
- . Recurrent Clostridium difficile infection: a review of risk factors, treatments, and outcomes. J Infect. 2009;58(6):403–410.
- , , , et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431–455.
- , , , et al. The search for a better treatment for recurrent Clostridium difficile disease: use of high‐dose vancomycin combined with Saccharomyces boulardii. Clin Infect Dis. 2000;31(4):1012–1017.
- , , , et al. Saccharomyces cerevisiae fungemia after Saccharomyces boulardii treatment in immunocompromised patients. J Clin Gastroenterol. 2003;36(1):41–43.
- , , . Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis. 2003;36(5):580–585.
- , , , et al. Treatment with intravenously administered gamma globulin of chronic relapsing colitis induced by Clostridium difficile toxin. J Pediatr. 1991;118(4 pt 1):633–637.
- . Descriptive study of intravenous immunoglobulin for the treatment of recurrent Clostridium difficile diarrhoea. J Antimicrob Chemother. 2004;53(5):882–884.
- , , , , . Interruption of recurrent Clostridium difficile‐associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clin Infect Dis. 2007;44(6):846–848.
- , , , et al. Rifampin and rifaximin resistance in clinical isolates of Clostridium difficile. Antimicrob Agents Chemother. 2008;52(8):2813–2817.
- , , , , , . Efficacy and safety of fidaxomicin (FDX) vs. vancomycin (VAN) in Clostridium difficile infection (CDI) in 2 randomized controlled trials (RCT) with 1105 patients [abstract 1417]. Presented at: The 48th Annual IDSA Meeting; October 21–24, 2010; Vancouver, BC, Canada.
- , , , , , . Randomized clinical trial in Clostridium difficile infection confirms superiority of fidaxomicin over vancomycin [abstract 828630]. Presented at: Digestive Disease Week 2010; May 4, 2010; New Orleans, LA.
- , , , et al for the OPT‐80–003 Clinical Study Group. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364(5):422–431.
Strategies for Prevention of CDI
Infection control is a critical component of an overall management strategy for Clostridium difficile infection (CDI). In fact, preventing patients from acquiring this nosocomial condition in the healthcare setting has been identified as the most essential component.1 In 2008, the Society for Healthcare Epidemiology of America/Infectious Diseases Society of America (SHEA/IDSA) published a compendium of strategies to prevent healthcare‐associated infections, including CDI. This guideline includes graded recommendations and provides helpful strategies for applying them in a healthcare facility. An effective and comprehensive preventive program to reduce the incidence and impact of CDI requires several key components:2
-
Communication of responsibilities and accountability.
-
Application of special recommendations if the incidence of CDI is not adequately controlled with the basic recommendations (Table 2).2
| Recommendation | Grade* |
|---|---|
| |
| Contact precautions for patients with CDI until 48 hr after diarrhea resolves | A‐I for gloves |
| A‐II for hand hygiene | |
| B‐III for gowns | |
| B‐III for single‐patient room | |
| Ensure adequate disinfection of equipment and environment | B‐II for environment |
| B‐III for equipment | |
| Laboratory‐based alert system to notify clinical and infection prevention and control personnel if patient diagnosed with CDI | B‐III for alert system |
| Conduct CDI surveillance and feedback data to units and hospital administrators | B‐III for CDI surveillance |
| Educate healthcare personnel, housekeeping personnel, and hospital administration about CDI | B‐III for hospital staff education |
| Educate patients and their families about CDI, as appropriate | B‐III for patient education |
| Measure hand hygiene and contact precaution compliance | B‐III for monitoring compliance |
| Recommendations | Grade* |
|---|---|
| |
| Initiate an antimicrobial stewardship program | A‐II |
| Use diluted sodium hypochlorite for environmental disinfection if current practices deemed adequate | B‐II |
| Intensify efforts at hand hygiene and contact precaution compliance | B‐III |
| Preferentially use soap and water when performing hand hygiene after caring for a patient with CDI | B‐III |
| Place patients in contact precautions while C. difficile testing is pending | B‐III |
| Prolong contact precautions until discharge | B‐III |
| Assess the adequacy of room cleaning | B‐III |
Many healthcare providers are involved in patient care, and therefore each of these departmentsincluding administration, the medical staff, the infection control department, nursing, pharmacy, the clinical laboratory, and environmental controlmust be supportive of, and accountable for, implementing strategies to prevent CDI. Hospital administration must ensure that nursing, environmental services, and infection prevention and control have adequate support. The department of infection prevention and control should take the lead role in designing, implementing, and monitoring the CDI prevention program, including the education of hospital staff.
Clinical staff must comply with infection prevention and control policies, and have a high index of suspicion for rapid identification of patients with CDI, so they can be placed under contact precautions and started on treatment quickly. Nursing and physician leaders must hold personnel accountable for adhering to infection prevention and control policies. Finally, environmental services play a key role and must ensure that housekeeping personnel are appropriately trained and monitored to ensure they are following effective cleaning policies and procedures.
TRANSMISSION OF CDI
Healthcare workers are a primary mode of C. difficile transmission. C. difficile spores end up on multiple hospital surfaces and contaminate healthcare worker hands and medical devices (stethoscopes, thermometers, etc) used on multiple patients. One study found that after caring for a patient with CDI, 59% of healthcare workers had hand contamination regardless of whether or not they actually touched the patient.3 Many studies have shown that patients in adjacent rooms are at equal or higher risk of acquiring CDI as patients admitted to the same room.4, 5 Although a recent study found that admission to an intensive care unit room that previously housed a patient for CDI was a risk factor for developing CDI, 89% of patients who actually developed CDI did not have this risk factor.6 This indicates that most C. difficile acquisitions came from healthcare workers.
CONTACT PRECAUTIONS AND STRICT HAND HYGIENE ARE KEY
The combination of appropriate contact precautions and strict hand hygiene has been reported to reduce the incidence of CDI by as much as 80%.1, 7, 8 The CDI prevention recommendation with the strongest level of evidence is the donning of gloves when caring for a patient with CDI (Table 1).9
The optimal method of hand hygiene after caring for a patient with CDI is a matter of some confusion. Alcohol‐based hand sanitizers did not reduce the amount of C. difficile spores on the hands of volunteers contaminated with a known quantity of C. difficile spores.10 However, studies have not found an increase in CDI with use of alcohol‐based hand sanitizers or a decrease in CDI with use of soap and water.11 In addition, several of these studies have found the use of alcohol‐based hand hygiene products to be associated with decreases in methicillin‐resistant Staphylococcus aureus or vancomycin‐resistant enterococcus. For these reasons, in non‐outbreak settings, hand hygiene with alcohol‐based hand sanitizers, in addition to wearing gloves as a component of contact precautions, is considered an acceptable method of hand hygiene after caring for a patient with CDI.11 In outbreak settings, however, preferential use of soap and water is recommended after caring for a patient with CDI because of the theoretical increase in risk of C. difficile transmission based on the volunteer hand contamination studies.2, 11, 12
DISINFECTION OF EQUIPMENT AND ENVIRONMENT
Environmental services staff must be educated about the incidence, transmission of, and impact of CDI, as well as strategies effective for C. difficile spores, which are resistant to standard cleaning products and may persist in patient rooms for many months.1 During CDI outbreaks, rooms should be cleaned with a chlorine‐based disinfectant (either an Environmental Protection Agency‐approved disinfectant with known sporicidal activity or a 1:10 dilution of household bleach), which rapidly destroys C. difficile spores.1 The sporicidal solution should have a contact time of at least 10 minutes.2 Efforts to control spores in the environment and prevent transmission are even more important considering recent data demonstrating that hypervirulent C. difficile strains may have increased sporulation, which in combination with increased toxin production, pose a major management challenge.13 Identification and removal of other sources of C. difficile, including replacement of electronic rectal thermometers with disposable thermometers, can also reduce the incidence of CDI.12
ANTIMICROBIAL STEWARDSHIP AND RESTRICTION
Interventions to ensure appropriate use of antibiotics, including antimicrobial stewardship programs and antibiotic restriction programs, are also effective. A study during an outbreak of a hypervirulent strain of C. difficile showed that an antimicrobial stewardship program reduced the incidence of CDI by 60%.14 In this study, the antimicrobial stewardship program focused on shifting antimicrobial selection to antimicrobials that were associated with a lower risk of CDI at their institution whenever possible. Reducing unnecessary antimicrobial use was stressed as well. Formal restrictions were not instituted; rather, clinicians received education and pocket guides to assist in antimicrobial selection.
Several studies have found respiratory fluoroquinolones, such as gatifloxacin or moxifloxacin, to be associated with the highest risk of CDI during outbreaks due to the BI/NAP1/027 strain.15, 16 Interestingly, this antimicrobial stewardship program recommended respiratory fluoroquinolones over cephalosporins for community‐acquired pneumonia, as cephalosporins historically have been strongly associated with CDI. Nevertheless, the incidence of CDI decreased after initiation of the antimicrobial stewardship program, despite increased use of respiratory fluoroquinolones. The antimicrobial stewardship program was implemented prior to the identification of the fluoroquinolone‐resistant epidemic strain. This shows that herd protection against CDI can occur by improvements in overall antimicrobial prescribing practices by decreasing the total number of patients at risk for CDI. This, in turn, will decrease the number of patients who develop CDI and contribute to the spread of C. difficile. In addition to using education to improve antimicrobial prescribing, several studies have found that restriction of specific antimicrobials associated with CDI (for example, clindamycin or fluoroquinolones) can result in a decrease in CDI.1, 1720
INSIGHTS ABOUT OPPORTUNITIES FOR IMPROVEMENT
Results of a recent point prevalence survey conducted by the Association for Professionals in Infection Control and Epidemiology, Inc (APIC) provide important insights into knowledge and clinical practice gaps related to early diagnosis and prevention of CDI.21 More than 12,000 APIC members were asked to provide a 1‐day snapshot of patients identified with CDI or colonization at their institutions. Responses from 648 (12.5%) acute care hospitals in the United States, representing 47 states, indicate a clear need to improve infection control practices.21 The following recommendations are based on recent evidence:
-
Patients should be placed in contact isolation at the first suspicion of CDI, and kept in isolation for up to 2 days after diarrhea resolves because contamination persists in the environment that long.2 Of note, this differs from the SHEA/IDSA Clinical Practice Guidelines for Clostridium difficile Infection in Adults, which state: Maintain contact precautions for the duration of diarrhea.12 The Centers for Disease Control and Prevention (CDC) currently recommends contact precautions for the duration of illness when caring for patients with CDI.22
-
Bleach solution should be used for routine and terminal cleaning during CDI outbreaks, as recommended by SHEA/IDSA and the CDC.
-
Hand washing with soap and water is more effective than alcohol‐based hand sanitizers for removal of spores. However, appropriate donning and removal of gloves prevents hand contamination with C. difficile spores, likely explaining why hand washing with soap and water has not been associated with a decrease in CDI compared with alcohol‐based products.
-
A formal program to educate environmental services personnel should be implemented to ensure they understand their critical role on the infection control team and effective strategies for cleaning.
SPECIAL APPROACHES
When basic approaches are not effective to reduce the incidence of CDI, the SHEA has recommendations for special approaches, which should be implemented as appropriate for each institution (summarized in Table 2).2 Strategies for prevention of CDI are also available from the CDC and the Institute for Healthcare Improvement (IHI).23, 24
SUMMARY
Effective management and prevention of CDI requires a multidisciplinary approach that includes leaders in hospital administration, clinicians, the infection control department, nursing, pharmacy, and the clinical laboratory, as well as environmental services. All of these professionals must be accountable and take an active role in implementing and complying with evidence‐based strategies to ensure that patients at risk are identified early and managed appropriately, and that effective strategies for prevention are in place. Hospitalists, as front‐line caregivers, physician leaders in their hospitals, and coordinators of patient care, can play a key role in these regards. Care when deciding when and which antimicrobial to use to treat non‐CDI infections; being attuned to symptoms that may be due to CDI, and prompt diagnosis and treatment of CDI; adhering to infection control policies; awareness of cleaning practices; and also being an active member of the infection control committee are all ways that hospitalists may take active roles in preventing CDI.
- ,.Clostridium difficile infection in the intensive care unit.Infect Dis Clin North Am.2009;23(3):727–743.
- ,,.Strategies to prevent Clostridium difficile infections in acute care hospitals.Infect Control Hosp Epidemiol.2008;29(suppl 1):S81–S92.
- ,,,.Nosocomial acquisition of Clostridium difficile infection.N Engl J Med.1989;320(4):204–210.
- ,,, et al.Acquisition of Clostridium difficile by hospitalized patients: evidence for colonized new admissions as a source of infection.J Infect Dis.1992;166:561–567.
- ,.The role of physical proximity in nosocomial diarrhea.Clin Infect Dis.2000;31(3):717–722.
- ,,, et al.Evaluation of hospital room assignment and acquisition of Clostridium difficile infection.Infect Control Hosp Epidemiol.2011;32(3):201–206.
- ,,,,.Effectiveness of infection control program in controlling nosocomial Clostridium difficile.Am J Infect Control.1998;26(6):588–593.
- ,,, et al.Control of an outbreak of infection with the hypervirulent Clostridium difficile BI strain in a university hospital using a comprehensive “bundle” approach.Clin Infect Dis.2007;45(10):1266–1273.
- ,,, et al.Epidemics of diarrhea caused by a clindamycin‐resistant strain of Clostridium difficile in four hospitals.N Engl J Med.1999;341(22):1645–1651.
- ,,,,.Hand hygiene with soap and water is superior to alcohol rub and antiseptic wipes for removal of Clostridium difficile.Infect Control Hosp Epidemiol.2009;30(10):939–944.
- ,,.Measures to control and prevent Clostridium difficile infection.Clin Infect Dis.2008;46(suppl 1):S43–S49.
- ,,, et al.Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA).Infect Control Hosp Epidemiol.2010;31(5):431–455.
- ,,, et al.Human hypervirulent Clostridium difficile strains exhibit increased sporulation as well as robust toxin production.J Bacteriol.2010;192(19):4904–4911.
- ,,,,.Impact of a reduction in the use of high‐risk antibiotics on the course of an epidemic of Clostridium difficile‐associated disease caused by the hypervirulent NAP1/027 strain.Clin Infect Dis.2007;45(suppl 2):S112–S121.
- ,,, et al.Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile‐associated diarrhea: a cohort study during an epidemic in Quebec, Canada.Clin Infect Dis.2005;41(9):1254–1260.
- ,,,, et al.A predominantly clonal multi‐institutional outbreak of Clostridium difficile‐associated diarrhea with high morbidity and mortality.N Engl J Med.2005;353:2442–2449.
- ,,, et al.Hospital‐wide restriction of clindamycin: effect on the incidence of Clostridium difficile‐associated diarrhea and cost.Ann Intern Med.1998;128:989–995.
- ,,, et al.Interventions to improve antibiotic prescribing practices for hospital inpatients.Cochrane Database Syst Rev.2005CD003543.
- ,.Impact of changes in antibiotic policy on Clostridium difficile‐associated diarrhoea (CDAD) over a five‐year period in a district general hospital.J Hosp Infect.2003;54:104–108.
- ,,, et al.Antibiotic prescribing policy and Clostridium difficile diarrhoea.Q J Med.2004;97:423–429.
- Association for Professionals in Infection Control and Epidemiology (APIC). Guide to the elimination of Clostridium difficile in healthcare settings. Available at: http://www.apic.org/Content/NavigationMenu/PracticeGuidance/APICEliminationGuides/C.diff_Elimination_guide_logo.pdf. Accessed August 9,2011.
- Centers for Disease Control and Prevention. Frequently asked questions about Clostridium difficile for healthcare providers. Available at: http://www.cdc.gov/HAI/organisms/cdiff/Cdiff_faqs_HCP.html. Accessed August 9,2011.
- Centers for Disease Control and Prevention. Information about the current strain of Clostridium difficile. Available at: http://www.cdc.gov/HAI/organisms/cdiff/Cdiff‐current‐strain.html. Accessed June 15,2011.
- Institute for Healthcare Improvement. Available at: http://www.ihi.org. Accessed July 26,2011.
Infection control is a critical component of an overall management strategy for Clostridium difficile infection (CDI). In fact, preventing patients from acquiring this nosocomial condition in the healthcare setting has been identified as the most essential component.1 In 2008, the Society for Healthcare Epidemiology of America/Infectious Diseases Society of America (SHEA/IDSA) published a compendium of strategies to prevent healthcare‐associated infections, including CDI. This guideline includes graded recommendations and provides helpful strategies for applying them in a healthcare facility. An effective and comprehensive preventive program to reduce the incidence and impact of CDI requires several key components:2
-
Communication of responsibilities and accountability.
-
Application of special recommendations if the incidence of CDI is not adequately controlled with the basic recommendations (Table 2).2
| Recommendation | Grade* |
|---|---|
| |
| Contact precautions for patients with CDI until 48 hr after diarrhea resolves | A‐I for gloves |
| A‐II for hand hygiene | |
| B‐III for gowns | |
| B‐III for single‐patient room | |
| Ensure adequate disinfection of equipment and environment | B‐II for environment |
| B‐III for equipment | |
| Laboratory‐based alert system to notify clinical and infection prevention and control personnel if patient diagnosed with CDI | B‐III for alert system |
| Conduct CDI surveillance and feedback data to units and hospital administrators | B‐III for CDI surveillance |
| Educate healthcare personnel, housekeeping personnel, and hospital administration about CDI | B‐III for hospital staff education |
| Educate patients and their families about CDI, as appropriate | B‐III for patient education |
| Measure hand hygiene and contact precaution compliance | B‐III for monitoring compliance |
| Recommendations | Grade* |
|---|---|
| |
| Initiate an antimicrobial stewardship program | A‐II |
| Use diluted sodium hypochlorite for environmental disinfection if current practices deemed adequate | B‐II |
| Intensify efforts at hand hygiene and contact precaution compliance | B‐III |
| Preferentially use soap and water when performing hand hygiene after caring for a patient with CDI | B‐III |
| Place patients in contact precautions while C. difficile testing is pending | B‐III |
| Prolong contact precautions until discharge | B‐III |
| Assess the adequacy of room cleaning | B‐III |
Many healthcare providers are involved in patient care, and therefore each of these departmentsincluding administration, the medical staff, the infection control department, nursing, pharmacy, the clinical laboratory, and environmental controlmust be supportive of, and accountable for, implementing strategies to prevent CDI. Hospital administration must ensure that nursing, environmental services, and infection prevention and control have adequate support. The department of infection prevention and control should take the lead role in designing, implementing, and monitoring the CDI prevention program, including the education of hospital staff.
Clinical staff must comply with infection prevention and control policies, and have a high index of suspicion for rapid identification of patients with CDI, so they can be placed under contact precautions and started on treatment quickly. Nursing and physician leaders must hold personnel accountable for adhering to infection prevention and control policies. Finally, environmental services play a key role and must ensure that housekeeping personnel are appropriately trained and monitored to ensure they are following effective cleaning policies and procedures.
TRANSMISSION OF CDI
Healthcare workers are a primary mode of C. difficile transmission. C. difficile spores end up on multiple hospital surfaces and contaminate healthcare worker hands and medical devices (stethoscopes, thermometers, etc) used on multiple patients. One study found that after caring for a patient with CDI, 59% of healthcare workers had hand contamination regardless of whether or not they actually touched the patient.3 Many studies have shown that patients in adjacent rooms are at equal or higher risk of acquiring CDI as patients admitted to the same room.4, 5 Although a recent study found that admission to an intensive care unit room that previously housed a patient for CDI was a risk factor for developing CDI, 89% of patients who actually developed CDI did not have this risk factor.6 This indicates that most C. difficile acquisitions came from healthcare workers.
CONTACT PRECAUTIONS AND STRICT HAND HYGIENE ARE KEY
The combination of appropriate contact precautions and strict hand hygiene has been reported to reduce the incidence of CDI by as much as 80%.1, 7, 8 The CDI prevention recommendation with the strongest level of evidence is the donning of gloves when caring for a patient with CDI (Table 1).9
The optimal method of hand hygiene after caring for a patient with CDI is a matter of some confusion. Alcohol‐based hand sanitizers did not reduce the amount of C. difficile spores on the hands of volunteers contaminated with a known quantity of C. difficile spores.10 However, studies have not found an increase in CDI with use of alcohol‐based hand sanitizers or a decrease in CDI with use of soap and water.11 In addition, several of these studies have found the use of alcohol‐based hand hygiene products to be associated with decreases in methicillin‐resistant Staphylococcus aureus or vancomycin‐resistant enterococcus. For these reasons, in non‐outbreak settings, hand hygiene with alcohol‐based hand sanitizers, in addition to wearing gloves as a component of contact precautions, is considered an acceptable method of hand hygiene after caring for a patient with CDI.11 In outbreak settings, however, preferential use of soap and water is recommended after caring for a patient with CDI because of the theoretical increase in risk of C. difficile transmission based on the volunteer hand contamination studies.2, 11, 12
DISINFECTION OF EQUIPMENT AND ENVIRONMENT
Environmental services staff must be educated about the incidence, transmission of, and impact of CDI, as well as strategies effective for C. difficile spores, which are resistant to standard cleaning products and may persist in patient rooms for many months.1 During CDI outbreaks, rooms should be cleaned with a chlorine‐based disinfectant (either an Environmental Protection Agency‐approved disinfectant with known sporicidal activity or a 1:10 dilution of household bleach), which rapidly destroys C. difficile spores.1 The sporicidal solution should have a contact time of at least 10 minutes.2 Efforts to control spores in the environment and prevent transmission are even more important considering recent data demonstrating that hypervirulent C. difficile strains may have increased sporulation, which in combination with increased toxin production, pose a major management challenge.13 Identification and removal of other sources of C. difficile, including replacement of electronic rectal thermometers with disposable thermometers, can also reduce the incidence of CDI.12
ANTIMICROBIAL STEWARDSHIP AND RESTRICTION
Interventions to ensure appropriate use of antibiotics, including antimicrobial stewardship programs and antibiotic restriction programs, are also effective. A study during an outbreak of a hypervirulent strain of C. difficile showed that an antimicrobial stewardship program reduced the incidence of CDI by 60%.14 In this study, the antimicrobial stewardship program focused on shifting antimicrobial selection to antimicrobials that were associated with a lower risk of CDI at their institution whenever possible. Reducing unnecessary antimicrobial use was stressed as well. Formal restrictions were not instituted; rather, clinicians received education and pocket guides to assist in antimicrobial selection.
Several studies have found respiratory fluoroquinolones, such as gatifloxacin or moxifloxacin, to be associated with the highest risk of CDI during outbreaks due to the BI/NAP1/027 strain.15, 16 Interestingly, this antimicrobial stewardship program recommended respiratory fluoroquinolones over cephalosporins for community‐acquired pneumonia, as cephalosporins historically have been strongly associated with CDI. Nevertheless, the incidence of CDI decreased after initiation of the antimicrobial stewardship program, despite increased use of respiratory fluoroquinolones. The antimicrobial stewardship program was implemented prior to the identification of the fluoroquinolone‐resistant epidemic strain. This shows that herd protection against CDI can occur by improvements in overall antimicrobial prescribing practices by decreasing the total number of patients at risk for CDI. This, in turn, will decrease the number of patients who develop CDI and contribute to the spread of C. difficile. In addition to using education to improve antimicrobial prescribing, several studies have found that restriction of specific antimicrobials associated with CDI (for example, clindamycin or fluoroquinolones) can result in a decrease in CDI.1, 1720
INSIGHTS ABOUT OPPORTUNITIES FOR IMPROVEMENT
Results of a recent point prevalence survey conducted by the Association for Professionals in Infection Control and Epidemiology, Inc (APIC) provide important insights into knowledge and clinical practice gaps related to early diagnosis and prevention of CDI.21 More than 12,000 APIC members were asked to provide a 1‐day snapshot of patients identified with CDI or colonization at their institutions. Responses from 648 (12.5%) acute care hospitals in the United States, representing 47 states, indicate a clear need to improve infection control practices.21 The following recommendations are based on recent evidence:
-
Patients should be placed in contact isolation at the first suspicion of CDI, and kept in isolation for up to 2 days after diarrhea resolves because contamination persists in the environment that long.2 Of note, this differs from the SHEA/IDSA Clinical Practice Guidelines for Clostridium difficile Infection in Adults, which state: Maintain contact precautions for the duration of diarrhea.12 The Centers for Disease Control and Prevention (CDC) currently recommends contact precautions for the duration of illness when caring for patients with CDI.22
-
Bleach solution should be used for routine and terminal cleaning during CDI outbreaks, as recommended by SHEA/IDSA and the CDC.
-
Hand washing with soap and water is more effective than alcohol‐based hand sanitizers for removal of spores. However, appropriate donning and removal of gloves prevents hand contamination with C. difficile spores, likely explaining why hand washing with soap and water has not been associated with a decrease in CDI compared with alcohol‐based products.
-
A formal program to educate environmental services personnel should be implemented to ensure they understand their critical role on the infection control team and effective strategies for cleaning.
SPECIAL APPROACHES
When basic approaches are not effective to reduce the incidence of CDI, the SHEA has recommendations for special approaches, which should be implemented as appropriate for each institution (summarized in Table 2).2 Strategies for prevention of CDI are also available from the CDC and the Institute for Healthcare Improvement (IHI).23, 24
SUMMARY
Effective management and prevention of CDI requires a multidisciplinary approach that includes leaders in hospital administration, clinicians, the infection control department, nursing, pharmacy, and the clinical laboratory, as well as environmental services. All of these professionals must be accountable and take an active role in implementing and complying with evidence‐based strategies to ensure that patients at risk are identified early and managed appropriately, and that effective strategies for prevention are in place. Hospitalists, as front‐line caregivers, physician leaders in their hospitals, and coordinators of patient care, can play a key role in these regards. Care when deciding when and which antimicrobial to use to treat non‐CDI infections; being attuned to symptoms that may be due to CDI, and prompt diagnosis and treatment of CDI; adhering to infection control policies; awareness of cleaning practices; and also being an active member of the infection control committee are all ways that hospitalists may take active roles in preventing CDI.
Infection control is a critical component of an overall management strategy for Clostridium difficile infection (CDI). In fact, preventing patients from acquiring this nosocomial condition in the healthcare setting has been identified as the most essential component.1 In 2008, the Society for Healthcare Epidemiology of America/Infectious Diseases Society of America (SHEA/IDSA) published a compendium of strategies to prevent healthcare‐associated infections, including CDI. This guideline includes graded recommendations and provides helpful strategies for applying them in a healthcare facility. An effective and comprehensive preventive program to reduce the incidence and impact of CDI requires several key components:2
-
Communication of responsibilities and accountability.
-
Application of special recommendations if the incidence of CDI is not adequately controlled with the basic recommendations (Table 2).2
| Recommendation | Grade* |
|---|---|
| |
| Contact precautions for patients with CDI until 48 hr after diarrhea resolves | A‐I for gloves |
| A‐II for hand hygiene | |
| B‐III for gowns | |
| B‐III for single‐patient room | |
| Ensure adequate disinfection of equipment and environment | B‐II for environment |
| B‐III for equipment | |
| Laboratory‐based alert system to notify clinical and infection prevention and control personnel if patient diagnosed with CDI | B‐III for alert system |
| Conduct CDI surveillance and feedback data to units and hospital administrators | B‐III for CDI surveillance |
| Educate healthcare personnel, housekeeping personnel, and hospital administration about CDI | B‐III for hospital staff education |
| Educate patients and their families about CDI, as appropriate | B‐III for patient education |
| Measure hand hygiene and contact precaution compliance | B‐III for monitoring compliance |
| Recommendations | Grade* |
|---|---|
| |
| Initiate an antimicrobial stewardship program | A‐II |
| Use diluted sodium hypochlorite for environmental disinfection if current practices deemed adequate | B‐II |
| Intensify efforts at hand hygiene and contact precaution compliance | B‐III |
| Preferentially use soap and water when performing hand hygiene after caring for a patient with CDI | B‐III |
| Place patients in contact precautions while C. difficile testing is pending | B‐III |
| Prolong contact precautions until discharge | B‐III |
| Assess the adequacy of room cleaning | B‐III |
Many healthcare providers are involved in patient care, and therefore each of these departmentsincluding administration, the medical staff, the infection control department, nursing, pharmacy, the clinical laboratory, and environmental controlmust be supportive of, and accountable for, implementing strategies to prevent CDI. Hospital administration must ensure that nursing, environmental services, and infection prevention and control have adequate support. The department of infection prevention and control should take the lead role in designing, implementing, and monitoring the CDI prevention program, including the education of hospital staff.
Clinical staff must comply with infection prevention and control policies, and have a high index of suspicion for rapid identification of patients with CDI, so they can be placed under contact precautions and started on treatment quickly. Nursing and physician leaders must hold personnel accountable for adhering to infection prevention and control policies. Finally, environmental services play a key role and must ensure that housekeeping personnel are appropriately trained and monitored to ensure they are following effective cleaning policies and procedures.
TRANSMISSION OF CDI
Healthcare workers are a primary mode of C. difficile transmission. C. difficile spores end up on multiple hospital surfaces and contaminate healthcare worker hands and medical devices (stethoscopes, thermometers, etc) used on multiple patients. One study found that after caring for a patient with CDI, 59% of healthcare workers had hand contamination regardless of whether or not they actually touched the patient.3 Many studies have shown that patients in adjacent rooms are at equal or higher risk of acquiring CDI as patients admitted to the same room.4, 5 Although a recent study found that admission to an intensive care unit room that previously housed a patient for CDI was a risk factor for developing CDI, 89% of patients who actually developed CDI did not have this risk factor.6 This indicates that most C. difficile acquisitions came from healthcare workers.
CONTACT PRECAUTIONS AND STRICT HAND HYGIENE ARE KEY
The combination of appropriate contact precautions and strict hand hygiene has been reported to reduce the incidence of CDI by as much as 80%.1, 7, 8 The CDI prevention recommendation with the strongest level of evidence is the donning of gloves when caring for a patient with CDI (Table 1).9
The optimal method of hand hygiene after caring for a patient with CDI is a matter of some confusion. Alcohol‐based hand sanitizers did not reduce the amount of C. difficile spores on the hands of volunteers contaminated with a known quantity of C. difficile spores.10 However, studies have not found an increase in CDI with use of alcohol‐based hand sanitizers or a decrease in CDI with use of soap and water.11 In addition, several of these studies have found the use of alcohol‐based hand hygiene products to be associated with decreases in methicillin‐resistant Staphylococcus aureus or vancomycin‐resistant enterococcus. For these reasons, in non‐outbreak settings, hand hygiene with alcohol‐based hand sanitizers, in addition to wearing gloves as a component of contact precautions, is considered an acceptable method of hand hygiene after caring for a patient with CDI.11 In outbreak settings, however, preferential use of soap and water is recommended after caring for a patient with CDI because of the theoretical increase in risk of C. difficile transmission based on the volunteer hand contamination studies.2, 11, 12
DISINFECTION OF EQUIPMENT AND ENVIRONMENT
Environmental services staff must be educated about the incidence, transmission of, and impact of CDI, as well as strategies effective for C. difficile spores, which are resistant to standard cleaning products and may persist in patient rooms for many months.1 During CDI outbreaks, rooms should be cleaned with a chlorine‐based disinfectant (either an Environmental Protection Agency‐approved disinfectant with known sporicidal activity or a 1:10 dilution of household bleach), which rapidly destroys C. difficile spores.1 The sporicidal solution should have a contact time of at least 10 minutes.2 Efforts to control spores in the environment and prevent transmission are even more important considering recent data demonstrating that hypervirulent C. difficile strains may have increased sporulation, which in combination with increased toxin production, pose a major management challenge.13 Identification and removal of other sources of C. difficile, including replacement of electronic rectal thermometers with disposable thermometers, can also reduce the incidence of CDI.12
ANTIMICROBIAL STEWARDSHIP AND RESTRICTION
Interventions to ensure appropriate use of antibiotics, including antimicrobial stewardship programs and antibiotic restriction programs, are also effective. A study during an outbreak of a hypervirulent strain of C. difficile showed that an antimicrobial stewardship program reduced the incidence of CDI by 60%.14 In this study, the antimicrobial stewardship program focused on shifting antimicrobial selection to antimicrobials that were associated with a lower risk of CDI at their institution whenever possible. Reducing unnecessary antimicrobial use was stressed as well. Formal restrictions were not instituted; rather, clinicians received education and pocket guides to assist in antimicrobial selection.
Several studies have found respiratory fluoroquinolones, such as gatifloxacin or moxifloxacin, to be associated with the highest risk of CDI during outbreaks due to the BI/NAP1/027 strain.15, 16 Interestingly, this antimicrobial stewardship program recommended respiratory fluoroquinolones over cephalosporins for community‐acquired pneumonia, as cephalosporins historically have been strongly associated with CDI. Nevertheless, the incidence of CDI decreased after initiation of the antimicrobial stewardship program, despite increased use of respiratory fluoroquinolones. The antimicrobial stewardship program was implemented prior to the identification of the fluoroquinolone‐resistant epidemic strain. This shows that herd protection against CDI can occur by improvements in overall antimicrobial prescribing practices by decreasing the total number of patients at risk for CDI. This, in turn, will decrease the number of patients who develop CDI and contribute to the spread of C. difficile. In addition to using education to improve antimicrobial prescribing, several studies have found that restriction of specific antimicrobials associated with CDI (for example, clindamycin or fluoroquinolones) can result in a decrease in CDI.1, 1720
INSIGHTS ABOUT OPPORTUNITIES FOR IMPROVEMENT
Results of a recent point prevalence survey conducted by the Association for Professionals in Infection Control and Epidemiology, Inc (APIC) provide important insights into knowledge and clinical practice gaps related to early diagnosis and prevention of CDI.21 More than 12,000 APIC members were asked to provide a 1‐day snapshot of patients identified with CDI or colonization at their institutions. Responses from 648 (12.5%) acute care hospitals in the United States, representing 47 states, indicate a clear need to improve infection control practices.21 The following recommendations are based on recent evidence:
-
Patients should be placed in contact isolation at the first suspicion of CDI, and kept in isolation for up to 2 days after diarrhea resolves because contamination persists in the environment that long.2 Of note, this differs from the SHEA/IDSA Clinical Practice Guidelines for Clostridium difficile Infection in Adults, which state: Maintain contact precautions for the duration of diarrhea.12 The Centers for Disease Control and Prevention (CDC) currently recommends contact precautions for the duration of illness when caring for patients with CDI.22
-
Bleach solution should be used for routine and terminal cleaning during CDI outbreaks, as recommended by SHEA/IDSA and the CDC.
-
Hand washing with soap and water is more effective than alcohol‐based hand sanitizers for removal of spores. However, appropriate donning and removal of gloves prevents hand contamination with C. difficile spores, likely explaining why hand washing with soap and water has not been associated with a decrease in CDI compared with alcohol‐based products.
-
A formal program to educate environmental services personnel should be implemented to ensure they understand their critical role on the infection control team and effective strategies for cleaning.
SPECIAL APPROACHES
When basic approaches are not effective to reduce the incidence of CDI, the SHEA has recommendations for special approaches, which should be implemented as appropriate for each institution (summarized in Table 2).2 Strategies for prevention of CDI are also available from the CDC and the Institute for Healthcare Improvement (IHI).23, 24
SUMMARY
Effective management and prevention of CDI requires a multidisciplinary approach that includes leaders in hospital administration, clinicians, the infection control department, nursing, pharmacy, and the clinical laboratory, as well as environmental services. All of these professionals must be accountable and take an active role in implementing and complying with evidence‐based strategies to ensure that patients at risk are identified early and managed appropriately, and that effective strategies for prevention are in place. Hospitalists, as front‐line caregivers, physician leaders in their hospitals, and coordinators of patient care, can play a key role in these regards. Care when deciding when and which antimicrobial to use to treat non‐CDI infections; being attuned to symptoms that may be due to CDI, and prompt diagnosis and treatment of CDI; adhering to infection control policies; awareness of cleaning practices; and also being an active member of the infection control committee are all ways that hospitalists may take active roles in preventing CDI.
- ,.Clostridium difficile infection in the intensive care unit.Infect Dis Clin North Am.2009;23(3):727–743.
- ,,.Strategies to prevent Clostridium difficile infections in acute care hospitals.Infect Control Hosp Epidemiol.2008;29(suppl 1):S81–S92.
- ,,,.Nosocomial acquisition of Clostridium difficile infection.N Engl J Med.1989;320(4):204–210.
- ,,, et al.Acquisition of Clostridium difficile by hospitalized patients: evidence for colonized new admissions as a source of infection.J Infect Dis.1992;166:561–567.
- ,.The role of physical proximity in nosocomial diarrhea.Clin Infect Dis.2000;31(3):717–722.
- ,,, et al.Evaluation of hospital room assignment and acquisition of Clostridium difficile infection.Infect Control Hosp Epidemiol.2011;32(3):201–206.
- ,,,,.Effectiveness of infection control program in controlling nosocomial Clostridium difficile.Am J Infect Control.1998;26(6):588–593.
- ,,, et al.Control of an outbreak of infection with the hypervirulent Clostridium difficile BI strain in a university hospital using a comprehensive “bundle” approach.Clin Infect Dis.2007;45(10):1266–1273.
- ,,, et al.Epidemics of diarrhea caused by a clindamycin‐resistant strain of Clostridium difficile in four hospitals.N Engl J Med.1999;341(22):1645–1651.
- ,,,,.Hand hygiene with soap and water is superior to alcohol rub and antiseptic wipes for removal of Clostridium difficile.Infect Control Hosp Epidemiol.2009;30(10):939–944.
- ,,.Measures to control and prevent Clostridium difficile infection.Clin Infect Dis.2008;46(suppl 1):S43–S49.
- ,,, et al.Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA).Infect Control Hosp Epidemiol.2010;31(5):431–455.
- ,,, et al.Human hypervirulent Clostridium difficile strains exhibit increased sporulation as well as robust toxin production.J Bacteriol.2010;192(19):4904–4911.
- ,,,,.Impact of a reduction in the use of high‐risk antibiotics on the course of an epidemic of Clostridium difficile‐associated disease caused by the hypervirulent NAP1/027 strain.Clin Infect Dis.2007;45(suppl 2):S112–S121.
- ,,, et al.Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile‐associated diarrhea: a cohort study during an epidemic in Quebec, Canada.Clin Infect Dis.2005;41(9):1254–1260.
- ,,,, et al.A predominantly clonal multi‐institutional outbreak of Clostridium difficile‐associated diarrhea with high morbidity and mortality.N Engl J Med.2005;353:2442–2449.
- ,,, et al.Hospital‐wide restriction of clindamycin: effect on the incidence of Clostridium difficile‐associated diarrhea and cost.Ann Intern Med.1998;128:989–995.
- ,,, et al.Interventions to improve antibiotic prescribing practices for hospital inpatients.Cochrane Database Syst Rev.2005CD003543.
- ,.Impact of changes in antibiotic policy on Clostridium difficile‐associated diarrhoea (CDAD) over a five‐year period in a district general hospital.J Hosp Infect.2003;54:104–108.
- ,,, et al.Antibiotic prescribing policy and Clostridium difficile diarrhoea.Q J Med.2004;97:423–429.
- Association for Professionals in Infection Control and Epidemiology (APIC). Guide to the elimination of Clostridium difficile in healthcare settings. Available at: http://www.apic.org/Content/NavigationMenu/PracticeGuidance/APICEliminationGuides/C.diff_Elimination_guide_logo.pdf. Accessed August 9,2011.
- Centers for Disease Control and Prevention. Frequently asked questions about Clostridium difficile for healthcare providers. Available at: http://www.cdc.gov/HAI/organisms/cdiff/Cdiff_faqs_HCP.html. Accessed August 9,2011.
- Centers for Disease Control and Prevention. Information about the current strain of Clostridium difficile. Available at: http://www.cdc.gov/HAI/organisms/cdiff/Cdiff‐current‐strain.html. Accessed June 15,2011.
- Institute for Healthcare Improvement. Available at: http://www.ihi.org. Accessed July 26,2011.
- ,.Clostridium difficile infection in the intensive care unit.Infect Dis Clin North Am.2009;23(3):727–743.
- ,,.Strategies to prevent Clostridium difficile infections in acute care hospitals.Infect Control Hosp Epidemiol.2008;29(suppl 1):S81–S92.
- ,,,.Nosocomial acquisition of Clostridium difficile infection.N Engl J Med.1989;320(4):204–210.
- ,,, et al.Acquisition of Clostridium difficile by hospitalized patients: evidence for colonized new admissions as a source of infection.J Infect Dis.1992;166:561–567.
- ,.The role of physical proximity in nosocomial diarrhea.Clin Infect Dis.2000;31(3):717–722.
- ,,, et al.Evaluation of hospital room assignment and acquisition of Clostridium difficile infection.Infect Control Hosp Epidemiol.2011;32(3):201–206.
- ,,,,.Effectiveness of infection control program in controlling nosocomial Clostridium difficile.Am J Infect Control.1998;26(6):588–593.
- ,,, et al.Control of an outbreak of infection with the hypervirulent Clostridium difficile BI strain in a university hospital using a comprehensive “bundle” approach.Clin Infect Dis.2007;45(10):1266–1273.
- ,,, et al.Epidemics of diarrhea caused by a clindamycin‐resistant strain of Clostridium difficile in four hospitals.N Engl J Med.1999;341(22):1645–1651.
- ,,,,.Hand hygiene with soap and water is superior to alcohol rub and antiseptic wipes for removal of Clostridium difficile.Infect Control Hosp Epidemiol.2009;30(10):939–944.
- ,,.Measures to control and prevent Clostridium difficile infection.Clin Infect Dis.2008;46(suppl 1):S43–S49.
- ,,, et al.Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA).Infect Control Hosp Epidemiol.2010;31(5):431–455.
- ,,, et al.Human hypervirulent Clostridium difficile strains exhibit increased sporulation as well as robust toxin production.J Bacteriol.2010;192(19):4904–4911.
- ,,,,.Impact of a reduction in the use of high‐risk antibiotics on the course of an epidemic of Clostridium difficile‐associated disease caused by the hypervirulent NAP1/027 strain.Clin Infect Dis.2007;45(suppl 2):S112–S121.
- ,,, et al.Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile‐associated diarrhea: a cohort study during an epidemic in Quebec, Canada.Clin Infect Dis.2005;41(9):1254–1260.
- ,,,, et al.A predominantly clonal multi‐institutional outbreak of Clostridium difficile‐associated diarrhea with high morbidity and mortality.N Engl J Med.2005;353:2442–2449.
- ,,, et al.Hospital‐wide restriction of clindamycin: effect on the incidence of Clostridium difficile‐associated diarrhea and cost.Ann Intern Med.1998;128:989–995.
- ,,, et al.Interventions to improve antibiotic prescribing practices for hospital inpatients.Cochrane Database Syst Rev.2005CD003543.
- ,.Impact of changes in antibiotic policy on Clostridium difficile‐associated diarrhoea (CDAD) over a five‐year period in a district general hospital.J Hosp Infect.2003;54:104–108.
- ,,, et al.Antibiotic prescribing policy and Clostridium difficile diarrhoea.Q J Med.2004;97:423–429.
- Association for Professionals in Infection Control and Epidemiology (APIC). Guide to the elimination of Clostridium difficile in healthcare settings. Available at: http://www.apic.org/Content/NavigationMenu/PracticeGuidance/APICEliminationGuides/C.diff_Elimination_guide_logo.pdf. Accessed August 9,2011.
- Centers for Disease Control and Prevention. Frequently asked questions about Clostridium difficile for healthcare providers. Available at: http://www.cdc.gov/HAI/organisms/cdiff/Cdiff_faqs_HCP.html. Accessed August 9,2011.
- Centers for Disease Control and Prevention. Information about the current strain of Clostridium difficile. Available at: http://www.cdc.gov/HAI/organisms/cdiff/Cdiff‐current‐strain.html. Accessed June 15,2011.
- Institute for Healthcare Improvement. Available at: http://www.ihi.org. Accessed July 26,2011.
Improving Sleep in Hospitalized Patients
In recent years, the philosophy of major hospitals has become more patient‐centered with increased focus on outcomes, safety, and patient satisfaction. To this end, many hospitals are looking for innovative ways not only to optimize quality of care, but also to improve patient satisfaction.
Sleep is a domain in which the goals of improving patient outcomes and satisfaction can be mutually achieved. Poor sleep has become a prevalent problem, and a single night of complete sleep loss can result in the undesirable consequences of daytime sleepiness, lethargy, irritability, confusion, and poor short‐term memory.1, 2 Literature has also suggested that chronic partial sleep loss can have significant consequences for safety, mood stability, neurological and medical functioning, and quality of life.38 The importance of acknowledging the relationship between sleep and a patient's level of functioning is magnified in the context of hospitalized patients, particularly those undergoing neurological inpatient care. Changes in level of alertness due to sleep loss can have serious implications for these patients, as they can lead to unnecessary testing and decreased participation with rehabilitative services.
Among the potential causes of sleep deprivation in hospitalized patients are poor pain control, lights, activities of others, and increased noise levels. The effect that increased noise has on patients has been evaluated in a variety of hospital settings, most notably in pediatric and adult intensive care units and nursing homes.9, 10 Noise has been shown to increase blood pressure, heart rate, respiratory rate, and body temperature. It has also been associated with failure to thrive, impaired immune function, delayed wound healing, and increased stress levels.11
The majority of literature regarding sleep disturbance in the hospital has focused on sleep disruption in the intensive care unit, where interventions associated with sleep loss are required to deliver the appropriate standard level of care.1218 However, few evidence‐based strategies to promote sleep quality in hospitalized patients have been evaluated.16, 1823 In this study, we aimed to examine sleep among neurological and neurosurgical inpatients, identify specific sleep‐disruptive factors, and assess patient satisfaction regarding their sleep. We implemented a sleep‐promoting protocol with the hypothesis that improvement of modifiable sleep‐disruptive factors would improve sleep and patient satisfaction.
METHODS
Study Design
This prospective, observational study was designed and implemented by an interdisciplinary team of physicians, neuroscience nurses, and hospital administrators.
Patient Selection
The study was performed on a Neurology and Neurosurgery unit, with both private and semi‐private rooms, at a large, urban, tertiary teaching hospital from February 2009 through June 2010. During enrollment periods, all patients on the unit were screened daily for eligibility. Eligible patients were medically stable and capable of giving verbal consent. Patients who were less than 16 years of age, encephalopathic, aphasic, or non‐English speaking were excluded. Eligible patients were asked for consent to participate in the study. After consultation with the hospital's institutional review board (IRB) committee, written consent was waived in this observational, quality improvement study.
Study Timeline
The study comprised 4 phases (Figure 1). In Phase 1, we collected baseline data on patients in the unit. Data were collected in the form of sleep surveys, Press Ganey surveys, and noise meter recordings. The baseline phase (Phase 1) lasted 10 weeks from February to April 2009. We then implemented a novel sleep‐promoting intervention called Basic Sleep Rounds (Phase 2, May to August 2009). After discontinuing Basic Sleep Rounds, data were collected for the washout phase (Phase 3, September 2009 to February 2010). An enhanced version of the sleep‐promoting intervention called Deluxe Sleep Rounds was then instituted (Phase 4, March to June 2010). In Phases 2 and 4, sleep rounds were implemented for 2 weeks before data collection to ensure uniform application of Sleep Rounds.
Sleep Promoting Interventions
Prior to implementing Basic Sleep Rounds in Phase 2, a nursing in‐service was performed where staff were educated about sleep in the hospital and about the planned interventions, and posters promoting sleep were hung on the unit. Basic Sleep Rounds were performed during Phase 2 by the patient's bedside nurse or the unit charge nurse. This occurred for all patients on the unit at approximately 23:00 nightly using the Basic Sleep Rounds checklist, which formalized simple hospital functions, such as lights out, television off, room temperature adjustment, and a final restroom usage (Figure 2). For Phase 4, a team of undergraduate volunteers was organized to assist with the delivery of Sleep Rounds. In this phase (Deluxe Sleep Rounds), nurses performed Basic Sleep Rounds by completing the checklist, and undergraduate volunteers offered patients any of the following sleep amenities: warm blanket, warm milk, white‐noise machine, hypoallergenic lotion, or room spritzer.24, 25

Additionally, during the Basic and Deluxe intervention phases, noise‐sensitive traffic lights (Talk Light Too;
Data Collection
A survey was designed to evaluate sleep quality, estimate sleep quantity, identify sleep disruptors, and assess patient satisfaction (see Supporting Figure 1 in the online version of this article). The survey was given to all eligible participants on the morning after their second night in the unit. This time point was chosen to account for potentially confounding first night effects, and to ensure that enrolled patients spent a full night in the unit.
To better evaluate one of the sleep disruptors, a subset of the survey participants had noise meters placed in their rooms. Every morning, a member of the team would visit all eligible patients to ask if they were willing to participate in this portion of the study. Data recorded between 8:00 PM and 8:00 AM on the second night of each participant's stay were later used for analysis. Noise was recorded in decibels using a Vernier Sound Level Meter, attached to a LabQuest data collection device (
Scores from Press Ganey surveys were also analyzed. These surveys were mailed to patients shortly after hospital discharge, and subsequently processed by Press Ganey Associates, Inc (
Data Analysis
Most datasets were not described by a normal distribution, thus most data are presented as medians with interquartile ranges (IQR), and comparisons between datasets were made using the MannWhitney U test. Press Ganey data are presented as means with standard errors of the mean, as distributed by Press Ganey. P < 0.05 was considered significant for all data comparisons.
RESULTS
Basic demographic data were available on all participants from whom both noise and survey data were collected. As in Table 1, these participants were demographically similar (P < 0.05) with regards to age, sex, and ethnic background. For unknown reasons, neurosurgery patients comprised the majority of participants in Phases 1 and 3, and neurology patients comprised the majority in Phase 2. This difference was not significant.
| Demographic | Phase 1 (n = 32) | Phase 2 (n = 33) | Phase 3 (n = 30) |
|---|---|---|---|
| Average age | 49 1 | 43 3 | 46 3 |
| % Female | 71% | 71% | 57% |
| % Neurology | 42% | 65% | 37% |
| % White | 67% | 77% | 73% |
Sleep Survey
A total of 253 sleep surveys were collected in all 4 phases. Data generated from these surveys are demonstrated in Table 2. On a 7‐point scale (1 being the best score, corresponding to the answer none, and 7 the worst, corresponding to extreme), the median scores for overall difficulty sleeping were not significantly different in Phases 1, 2, and 4. In Phase 3, the median score was 4 (moderate), significantly worse than in the other 3 phases (0.002 < P < 0.01). Despite the reported difficulty sleeping during Phase 3, the median number of hours of sleep and awakenings in Phases 1, 2, 3, and 4 were not significantly different. Sleep latency was scored on a 6‐point scale (1 being the best, corresponding to 010 min, and 6 the worst, corresponding to greater than 45 minutes). Similar sleep latency was reported in Phases 1, 3, and 4. However, median sleep latency in Phase 2 was 1 (010 min), significantly shorter than in the other phases (0.001 < P < 0.02). Despite similar survey results throughout most of the phases, there was a significant improvement in sleep latency in the Basic Sleep Rounds phase (Phase 2), and a significant worsening in overall difficulty sleeping in the washout phase (Phase 3).
| Survey Question | Phase 1 | Phase 2 | Phase 3 | Phase 4 |
|---|---|---|---|---|
| ||||
| 1. How much difficulty did you have sleeping last night? | 3 | 2 | 4* | 3 |
| IQR 4 N = 100 | IQR 4 N = 78 | IQR 3 N = 75 | IQR 2 N = 22 | |
| 2. How many hours did you sleep last night? | 6 hr | 6 hr | 5 hr | 5 hr |
| IQR 4 N = 98 | IQR 3 N = 77 | IQR 3 N = 72 | IQR 2 N = 22 | |
| 3. About how many times did you wake up during the night while you were trying to sleep? | 3 | 3 | 4 | 3 |
| IQR 3 N = 101 | IQR 3 N = 77 | IQR 3 N = 73 | IQR 3 N = 22 | |
| 4. How long did it take you to go to sleep last night? | 3 (1620 min) | 1 (010 min) | 2 (1115 min) | 2 (1115 min) |
| IQR 3 N = 101 | IQR 2 N = 77 | IQR 4 N = 75 | IQR 3 N = 22 | |
Participants also ranked each of the 7 queried disruptive factors on a 7‐point scale with regards to degree of sleep interruption. Even though less than half of the participants were in shared rooms, the presence of a roommate among those with roommates was the only sleep disrupter that ranked differently among the 4 phases. In Phases 1 and 2, when asked how much their sleep was disturbed by roommates, the median response was 1 (none), IQR = 1 (N = 41 and 31, respectively). In Phase 4, the median was 2 (a little), IQR = 2 (N = 6), but not significantly different. Answers in Phase 3 were significantly different, with a median of 3 (mild), IQR = 3 (N = 30) (0.005 < P < 0.006). Because there were no other statistically significant differences among individual sleep disruptors as compared by phases, survey data from all 4 phases for these factors was also analyzed collectively. Pain and staff interruptions (IQR = 3, N = 252 and IQR = 2, N = 253, respectively) were reported as the most disturbing factors, each with a median of 2 (a little). All remaining factors had a median score of 1 (none): noise inside the room (IQR = 2, N = 253), noise outside of the room (IQR = 1, N = 253), temperature (IQR = 1, N = 253), noise outside of the building (IQR = 0, N = 252), and light (IQR = 0, N = 252).
Noise Meter Recordings
Noise data were recorded from 95 participants in Phases 1 through 3, yielding high‐quality data suitable for analysis from 63 participants (11 in Phase 1, 24 in Phase 2, and 28 in Phase 3). Recorded noise ranged from 35 to 80 dB. As shown in Supporting Figure 2 in the online version of this article, raw data were plotted as decibels as a function of time. Noise levels were then analyzed in aggregate and for each of four 3‐hour time blocks (8 PM11 PM, 11 PM 2 AM,, 2 AM 5 AM, and 5 AM8 AM). Median noise levels during the entire 12‐hour period increased significantly between the first 3 phases of the study (P < 0.001): 38.6 dB (IQR 5.4) in Phase 1; 40.6 dB (IQR 5.3) in Phase 2; and 43.5 (IQR 7) in Phase 3. As in Supporting Table 1 in the online version of this article, within each phase, the median noise levels were significantly less during the 11 PM2 AM and 2 AM5 AM periods, as compared to the 8 PM11 PM and 5 AM8 AM periods (P < 0.001). Due to equipment dysfunction, noise data were not available for Phase 4.
Press Ganey Survey
A total of 457 Press Ganey surveys were collected. According to these surveys, patients' mean raw score of noise, on a scale from 1 to 100 (100 representing the best score), ranged from a low of 59.5 7.2 (January 2010; N = 21) to a high of 82.1 5.2 (April 2009; N = 21). Figure 3 illustrates the monthly trend of the mean score for noise compared to the national average compiled from other large hospitals around the country. It demonstrates that during the phases in which Sleep Rounds were performed (Phases 2 and 4), patients' perceptions of noise were improved.

DISCUSSION
The major conclusions of this study are: 1) hospitalized patients suffer from poor sleep quality and quantity; 2) implementation of simple measures such as Sleep Rounds to change standard practice within the hospital is feasible and effective; and 3) despite an increase in measured noise, patients' perception of their sleep and of noise levels was improved by these measures. This study developed and tested a sleep promotion program that could easily be implemented on any inpatient floor. Our Sleep Rounds checklist outlines a novel, but simple approach to sleep health by hospital providers, with the immediate goal of improving sleep among inpatients and the ultimate goal of improving outcomes.
Our study confirms that sleep disruption is prevalent among patients admitted to general hospital wards. In this study, patients reported a median of 5 hours of sleep, 3 awakenings, and sleep latency of 1115 minutes. Although not alarmingly low, 5 hours is only 60% of the recommended 8 hours of sleep for healthy individuals each night and 72% of the 6.9 hours of sleep reported by the average American each night.26 Poor pain control, frequent staff interactions, and the presence of roommates were rated as most problematic by the patients we surveyed. Interestingly, patients rated noise, temperature, and light as less problematic sleep disruptors.
Although we did not detect a statistically significant improvement in total sleep time or number of awakenings, there was a significant improvement in sleep latency during Phase 2 of the study when Basic Sleep Rounds were performed. In Phase 3 (washout phase), there was less active participation by the nursing staff in sleep hygiene promotion, and patients' perception of sleep quality was significantly worse than it was in other phases. These results suggest that the perception of sleep quality and quantity could have been enhanced by both our Basic (Phase 2) and Deluxe (Phase 4) Sleep Rounds interventions.
We were able to achieve appropriate noise levels at night (40 dB) during this study, even before our intervention began.27 Noise levels increased 2 dB between Phases 1 and 2, and another 3 dB in Phase 3. Although the changes in decibel level were statistically significant, a change of 23 dB is barely perceptible.28 Interestingly, despite the increase in measured noise throughout the study, Press Ganey results showed a trend towards perceived improvement in noise levels just before implementation of the first intervention. This may be attributable to an increased awareness of noise created by consenting patients and placing noise meters in their rooms. Perception of noise worsened significantly during the washout phase, suggesting that abandonment of Sleep Rounds was associated with less concern about noise.
Prior to initiating this study, an educational in‐service was conducted for the nursing team regarding the purpose and overall aims of this project. This may have raised awareness of the importance of sleep before collection of Phase 1 data, and had the unintended effect of an increased focus on sleep even before Sleep Rounds began. Other limitations of the study include lack of objective sleep data, nonrandomized design, inability to demonstrate causality, generalizability of results, inability to control for comorbidity including baseline sleep hygiene, limited patient numbers, inability to blind patients and team members, and difficulty obtaining accurate and complete noise data on all patients enrolled.
This study suggests that although it remains difficult for patients to sleep well in the hospital, it is possible to improve sleep and patients' perception of their sleep while they are hospitalized. Further studies are warranted to systematically evaluate interventions aimed at improving and overcoming the identified sleep disruptors without compromising patient care. However, we believe that Sleep Rounds could be associated with improvements in inpatient sleep hygiene and patient satisfaction, and could ultimately benefit patient outcomes.
Acknowledgements
The authors thank JoEllen Robinson, Jane Hill, and the nursing staff of Meyer 8 for their invaluable contributions to this project.
- .Sleep in acute care settings: an integrative review.J Nurs Scholarsh.2000;32(1):31–38.
- ,.Nursing standard‐of‐practice protocol: sleep disturbances in elderly patients. The NICHE Faculty.Geriatr Nurs.1995;16(5):238–243.
- ,,, et al.Sleep patterns and mortality among elderly patients in a geriatric hospital.Gerontology.2000;46(6):318–322.
- ,,.Sleep, insomnia and falls in elderly patients.Sleep Med.2008;9(suppl 1):S18–S22.
- ,,,,,.Behavioural and physiological reactivity to noise in the newborn.J Paediatr Child Health.2004;40(5–6):275–281.
- ,,.Sleep problems as a risk factor for falls in a sample of community‐dwelling adults aged 64–99 years.J Am Geriatr Soc.2000;48(10):1234–1240.
- ,,, et al.Reducing dangerous nighttime events in persons with dementia by using a nighttime monitoring system.Alzheimers Dement.2009;5(5):419–426.
- ,.Neurocognitive consequences of sleep deprivation.Semin Neurol.2005;25(1):117–129.
- ,,,,.The nursing home at night: effects of an intervention on noise, light, and sleep.J Am Geriatr Soc.1999;47(4):430–438.
- ,,,,.Sleep in hospitalized elders: a pilot study.Geriatr Nurs.2010;31(4):263–271.
- ,.Interactive relationships between hospital patients' noise‐induced stress and other stress with sleep.Heart Lung.2001;30(4):237–243.
- ,,.The impact of noise on patients' sleep and the effectiveness of noise reduction strategies in intensive care units.Crit Care.2009;13(2):208.
- ,,,,,.Sleep in critically ill patients requiring mechanical ventilation.Chest.2000;117(3):809–818.
- ,.Adverse effects of sleep deprivation in the ICU.Crit Care Clin.2008;24(3):461–476, v–vi.
- ,,,,.Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit.Am J Respir Crit Care Med.2001;163(2):451–457.
- ,.Responses of premature infants to routine nursing interventions and noise in the NICU.Nurs Res.1995;44(3):179–185.
- ,,,,.Noise, stress, and annoyance in a pediatric intensive care unit.Crit Care Med.2003;31(1):113–119.
- ,,,,.Effects of guidelines implementation in a surgical intensive care unit to control nighttime light and noise levels.Crit Care Med.2000;28(7):2242–2247.
- ,,,,.Noise control: a nursing team's approach to sleep promotion.Am J Nurs.2004;104(2):40–48; quiz 48–49.
- ,,, et al.Environmental noise sources and interventions to minimize them: a tale of 2 hospitals.J Nurs Care Qual.2008;23(3):216–224; quiz 225–216.
- ,,, et al.Interventions to reduce decibel levels on patient care units.Am Surg.1998;64(9):894–899.
- ,,,.Examining the feasibility of implementing specific nursing interventions to promote sleep in hospitalized elderly patients.Geriatr Nurs.2008;29(3):197–206.
- ,,,.Applicability of two brief evidence‐based interventions to improve sleep quality in inpatient mental health care.Int J Ment Health Nurs.2011;20(5)319–327.
- .Sleep deprivation in critical care units.Crit Care Nurs Q.2003;26(3):179–189; quiz 190–171.
- ,,,.Sleep promotion in hospitalized elders.Medsurg Nurs.2003;12(5):279–289; quiz 290.
- 2005 NSF Sleep in America Poll.Washington, DC:National Sleep Foundation;2005.
- ,,.Guidelines for Community Noise.Geneva, Switzerland:World Health Organization;1999.
- PhysicsArchives.com.2010. Available at: http://physicsarchives.com/index.php/courses/219. Accessed May 15, 2011.
In recent years, the philosophy of major hospitals has become more patient‐centered with increased focus on outcomes, safety, and patient satisfaction. To this end, many hospitals are looking for innovative ways not only to optimize quality of care, but also to improve patient satisfaction.
Sleep is a domain in which the goals of improving patient outcomes and satisfaction can be mutually achieved. Poor sleep has become a prevalent problem, and a single night of complete sleep loss can result in the undesirable consequences of daytime sleepiness, lethargy, irritability, confusion, and poor short‐term memory.1, 2 Literature has also suggested that chronic partial sleep loss can have significant consequences for safety, mood stability, neurological and medical functioning, and quality of life.38 The importance of acknowledging the relationship between sleep and a patient's level of functioning is magnified in the context of hospitalized patients, particularly those undergoing neurological inpatient care. Changes in level of alertness due to sleep loss can have serious implications for these patients, as they can lead to unnecessary testing and decreased participation with rehabilitative services.
Among the potential causes of sleep deprivation in hospitalized patients are poor pain control, lights, activities of others, and increased noise levels. The effect that increased noise has on patients has been evaluated in a variety of hospital settings, most notably in pediatric and adult intensive care units and nursing homes.9, 10 Noise has been shown to increase blood pressure, heart rate, respiratory rate, and body temperature. It has also been associated with failure to thrive, impaired immune function, delayed wound healing, and increased stress levels.11
The majority of literature regarding sleep disturbance in the hospital has focused on sleep disruption in the intensive care unit, where interventions associated with sleep loss are required to deliver the appropriate standard level of care.1218 However, few evidence‐based strategies to promote sleep quality in hospitalized patients have been evaluated.16, 1823 In this study, we aimed to examine sleep among neurological and neurosurgical inpatients, identify specific sleep‐disruptive factors, and assess patient satisfaction regarding their sleep. We implemented a sleep‐promoting protocol with the hypothesis that improvement of modifiable sleep‐disruptive factors would improve sleep and patient satisfaction.
METHODS
Study Design
This prospective, observational study was designed and implemented by an interdisciplinary team of physicians, neuroscience nurses, and hospital administrators.
Patient Selection
The study was performed on a Neurology and Neurosurgery unit, with both private and semi‐private rooms, at a large, urban, tertiary teaching hospital from February 2009 through June 2010. During enrollment periods, all patients on the unit were screened daily for eligibility. Eligible patients were medically stable and capable of giving verbal consent. Patients who were less than 16 years of age, encephalopathic, aphasic, or non‐English speaking were excluded. Eligible patients were asked for consent to participate in the study. After consultation with the hospital's institutional review board (IRB) committee, written consent was waived in this observational, quality improvement study.
Study Timeline
The study comprised 4 phases (Figure 1). In Phase 1, we collected baseline data on patients in the unit. Data were collected in the form of sleep surveys, Press Ganey surveys, and noise meter recordings. The baseline phase (Phase 1) lasted 10 weeks from February to April 2009. We then implemented a novel sleep‐promoting intervention called Basic Sleep Rounds (Phase 2, May to August 2009). After discontinuing Basic Sleep Rounds, data were collected for the washout phase (Phase 3, September 2009 to February 2010). An enhanced version of the sleep‐promoting intervention called Deluxe Sleep Rounds was then instituted (Phase 4, March to June 2010). In Phases 2 and 4, sleep rounds were implemented for 2 weeks before data collection to ensure uniform application of Sleep Rounds.

Sleep Promoting Interventions
Prior to implementing Basic Sleep Rounds in Phase 2, a nursing in‐service was performed where staff were educated about sleep in the hospital and about the planned interventions, and posters promoting sleep were hung on the unit. Basic Sleep Rounds were performed during Phase 2 by the patient's bedside nurse or the unit charge nurse. This occurred for all patients on the unit at approximately 23:00 nightly using the Basic Sleep Rounds checklist, which formalized simple hospital functions, such as lights out, television off, room temperature adjustment, and a final restroom usage (Figure 2). For Phase 4, a team of undergraduate volunteers was organized to assist with the delivery of Sleep Rounds. In this phase (Deluxe Sleep Rounds), nurses performed Basic Sleep Rounds by completing the checklist, and undergraduate volunteers offered patients any of the following sleep amenities: warm blanket, warm milk, white‐noise machine, hypoallergenic lotion, or room spritzer.24, 25

Additionally, during the Basic and Deluxe intervention phases, noise‐sensitive traffic lights (Talk Light Too;
Data Collection
A survey was designed to evaluate sleep quality, estimate sleep quantity, identify sleep disruptors, and assess patient satisfaction (see Supporting Figure 1 in the online version of this article). The survey was given to all eligible participants on the morning after their second night in the unit. This time point was chosen to account for potentially confounding first night effects, and to ensure that enrolled patients spent a full night in the unit.
To better evaluate one of the sleep disruptors, a subset of the survey participants had noise meters placed in their rooms. Every morning, a member of the team would visit all eligible patients to ask if they were willing to participate in this portion of the study. Data recorded between 8:00 PM and 8:00 AM on the second night of each participant's stay were later used for analysis. Noise was recorded in decibels using a Vernier Sound Level Meter, attached to a LabQuest data collection device (
Scores from Press Ganey surveys were also analyzed. These surveys were mailed to patients shortly after hospital discharge, and subsequently processed by Press Ganey Associates, Inc (
Data Analysis
Most datasets were not described by a normal distribution, thus most data are presented as medians with interquartile ranges (IQR), and comparisons between datasets were made using the MannWhitney U test. Press Ganey data are presented as means with standard errors of the mean, as distributed by Press Ganey. P < 0.05 was considered significant for all data comparisons.
RESULTS
Basic demographic data were available on all participants from whom both noise and survey data were collected. As in Table 1, these participants were demographically similar (P < 0.05) with regards to age, sex, and ethnic background. For unknown reasons, neurosurgery patients comprised the majority of participants in Phases 1 and 3, and neurology patients comprised the majority in Phase 2. This difference was not significant.
| Demographic | Phase 1 (n = 32) | Phase 2 (n = 33) | Phase 3 (n = 30) |
|---|---|---|---|
| Average age | 49 1 | 43 3 | 46 3 |
| % Female | 71% | 71% | 57% |
| % Neurology | 42% | 65% | 37% |
| % White | 67% | 77% | 73% |
Sleep Survey
A total of 253 sleep surveys were collected in all 4 phases. Data generated from these surveys are demonstrated in Table 2. On a 7‐point scale (1 being the best score, corresponding to the answer none, and 7 the worst, corresponding to extreme), the median scores for overall difficulty sleeping were not significantly different in Phases 1, 2, and 4. In Phase 3, the median score was 4 (moderate), significantly worse than in the other 3 phases (0.002 < P < 0.01). Despite the reported difficulty sleeping during Phase 3, the median number of hours of sleep and awakenings in Phases 1, 2, 3, and 4 were not significantly different. Sleep latency was scored on a 6‐point scale (1 being the best, corresponding to 010 min, and 6 the worst, corresponding to greater than 45 minutes). Similar sleep latency was reported in Phases 1, 3, and 4. However, median sleep latency in Phase 2 was 1 (010 min), significantly shorter than in the other phases (0.001 < P < 0.02). Despite similar survey results throughout most of the phases, there was a significant improvement in sleep latency in the Basic Sleep Rounds phase (Phase 2), and a significant worsening in overall difficulty sleeping in the washout phase (Phase 3).
| Survey Question | Phase 1 | Phase 2 | Phase 3 | Phase 4 |
|---|---|---|---|---|
| ||||
| 1. How much difficulty did you have sleeping last night? | 3 | 2 | 4* | 3 |
| IQR 4 N = 100 | IQR 4 N = 78 | IQR 3 N = 75 | IQR 2 N = 22 | |
| 2. How many hours did you sleep last night? | 6 hr | 6 hr | 5 hr | 5 hr |
| IQR 4 N = 98 | IQR 3 N = 77 | IQR 3 N = 72 | IQR 2 N = 22 | |
| 3. About how many times did you wake up during the night while you were trying to sleep? | 3 | 3 | 4 | 3 |
| IQR 3 N = 101 | IQR 3 N = 77 | IQR 3 N = 73 | IQR 3 N = 22 | |
| 4. How long did it take you to go to sleep last night? | 3 (1620 min) | 1 (010 min) | 2 (1115 min) | 2 (1115 min) |
| IQR 3 N = 101 | IQR 2 N = 77 | IQR 4 N = 75 | IQR 3 N = 22 | |
Participants also ranked each of the 7 queried disruptive factors on a 7‐point scale with regards to degree of sleep interruption. Even though less than half of the participants were in shared rooms, the presence of a roommate among those with roommates was the only sleep disrupter that ranked differently among the 4 phases. In Phases 1 and 2, when asked how much their sleep was disturbed by roommates, the median response was 1 (none), IQR = 1 (N = 41 and 31, respectively). In Phase 4, the median was 2 (a little), IQR = 2 (N = 6), but not significantly different. Answers in Phase 3 were significantly different, with a median of 3 (mild), IQR = 3 (N = 30) (0.005 < P < 0.006). Because there were no other statistically significant differences among individual sleep disruptors as compared by phases, survey data from all 4 phases for these factors was also analyzed collectively. Pain and staff interruptions (IQR = 3, N = 252 and IQR = 2, N = 253, respectively) were reported as the most disturbing factors, each with a median of 2 (a little). All remaining factors had a median score of 1 (none): noise inside the room (IQR = 2, N = 253), noise outside of the room (IQR = 1, N = 253), temperature (IQR = 1, N = 253), noise outside of the building (IQR = 0, N = 252), and light (IQR = 0, N = 252).
Noise Meter Recordings
Noise data were recorded from 95 participants in Phases 1 through 3, yielding high‐quality data suitable for analysis from 63 participants (11 in Phase 1, 24 in Phase 2, and 28 in Phase 3). Recorded noise ranged from 35 to 80 dB. As shown in Supporting Figure 2 in the online version of this article, raw data were plotted as decibels as a function of time. Noise levels were then analyzed in aggregate and for each of four 3‐hour time blocks (8 PM11 PM, 11 PM 2 AM,, 2 AM 5 AM, and 5 AM8 AM). Median noise levels during the entire 12‐hour period increased significantly between the first 3 phases of the study (P < 0.001): 38.6 dB (IQR 5.4) in Phase 1; 40.6 dB (IQR 5.3) in Phase 2; and 43.5 (IQR 7) in Phase 3. As in Supporting Table 1 in the online version of this article, within each phase, the median noise levels were significantly less during the 11 PM2 AM and 2 AM5 AM periods, as compared to the 8 PM11 PM and 5 AM8 AM periods (P < 0.001). Due to equipment dysfunction, noise data were not available for Phase 4.
Press Ganey Survey
A total of 457 Press Ganey surveys were collected. According to these surveys, patients' mean raw score of noise, on a scale from 1 to 100 (100 representing the best score), ranged from a low of 59.5 7.2 (January 2010; N = 21) to a high of 82.1 5.2 (April 2009; N = 21). Figure 3 illustrates the monthly trend of the mean score for noise compared to the national average compiled from other large hospitals around the country. It demonstrates that during the phases in which Sleep Rounds were performed (Phases 2 and 4), patients' perceptions of noise were improved.

DISCUSSION
The major conclusions of this study are: 1) hospitalized patients suffer from poor sleep quality and quantity; 2) implementation of simple measures such as Sleep Rounds to change standard practice within the hospital is feasible and effective; and 3) despite an increase in measured noise, patients' perception of their sleep and of noise levels was improved by these measures. This study developed and tested a sleep promotion program that could easily be implemented on any inpatient floor. Our Sleep Rounds checklist outlines a novel, but simple approach to sleep health by hospital providers, with the immediate goal of improving sleep among inpatients and the ultimate goal of improving outcomes.
Our study confirms that sleep disruption is prevalent among patients admitted to general hospital wards. In this study, patients reported a median of 5 hours of sleep, 3 awakenings, and sleep latency of 1115 minutes. Although not alarmingly low, 5 hours is only 60% of the recommended 8 hours of sleep for healthy individuals each night and 72% of the 6.9 hours of sleep reported by the average American each night.26 Poor pain control, frequent staff interactions, and the presence of roommates were rated as most problematic by the patients we surveyed. Interestingly, patients rated noise, temperature, and light as less problematic sleep disruptors.
Although we did not detect a statistically significant improvement in total sleep time or number of awakenings, there was a significant improvement in sleep latency during Phase 2 of the study when Basic Sleep Rounds were performed. In Phase 3 (washout phase), there was less active participation by the nursing staff in sleep hygiene promotion, and patients' perception of sleep quality was significantly worse than it was in other phases. These results suggest that the perception of sleep quality and quantity could have been enhanced by both our Basic (Phase 2) and Deluxe (Phase 4) Sleep Rounds interventions.
We were able to achieve appropriate noise levels at night (40 dB) during this study, even before our intervention began.27 Noise levels increased 2 dB between Phases 1 and 2, and another 3 dB in Phase 3. Although the changes in decibel level were statistically significant, a change of 23 dB is barely perceptible.28 Interestingly, despite the increase in measured noise throughout the study, Press Ganey results showed a trend towards perceived improvement in noise levels just before implementation of the first intervention. This may be attributable to an increased awareness of noise created by consenting patients and placing noise meters in their rooms. Perception of noise worsened significantly during the washout phase, suggesting that abandonment of Sleep Rounds was associated with less concern about noise.
Prior to initiating this study, an educational in‐service was conducted for the nursing team regarding the purpose and overall aims of this project. This may have raised awareness of the importance of sleep before collection of Phase 1 data, and had the unintended effect of an increased focus on sleep even before Sleep Rounds began. Other limitations of the study include lack of objective sleep data, nonrandomized design, inability to demonstrate causality, generalizability of results, inability to control for comorbidity including baseline sleep hygiene, limited patient numbers, inability to blind patients and team members, and difficulty obtaining accurate and complete noise data on all patients enrolled.
This study suggests that although it remains difficult for patients to sleep well in the hospital, it is possible to improve sleep and patients' perception of their sleep while they are hospitalized. Further studies are warranted to systematically evaluate interventions aimed at improving and overcoming the identified sleep disruptors without compromising patient care. However, we believe that Sleep Rounds could be associated with improvements in inpatient sleep hygiene and patient satisfaction, and could ultimately benefit patient outcomes.
Acknowledgements
The authors thank JoEllen Robinson, Jane Hill, and the nursing staff of Meyer 8 for their invaluable contributions to this project.
In recent years, the philosophy of major hospitals has become more patient‐centered with increased focus on outcomes, safety, and patient satisfaction. To this end, many hospitals are looking for innovative ways not only to optimize quality of care, but also to improve patient satisfaction.
Sleep is a domain in which the goals of improving patient outcomes and satisfaction can be mutually achieved. Poor sleep has become a prevalent problem, and a single night of complete sleep loss can result in the undesirable consequences of daytime sleepiness, lethargy, irritability, confusion, and poor short‐term memory.1, 2 Literature has also suggested that chronic partial sleep loss can have significant consequences for safety, mood stability, neurological and medical functioning, and quality of life.38 The importance of acknowledging the relationship between sleep and a patient's level of functioning is magnified in the context of hospitalized patients, particularly those undergoing neurological inpatient care. Changes in level of alertness due to sleep loss can have serious implications for these patients, as they can lead to unnecessary testing and decreased participation with rehabilitative services.
Among the potential causes of sleep deprivation in hospitalized patients are poor pain control, lights, activities of others, and increased noise levels. The effect that increased noise has on patients has been evaluated in a variety of hospital settings, most notably in pediatric and adult intensive care units and nursing homes.9, 10 Noise has been shown to increase blood pressure, heart rate, respiratory rate, and body temperature. It has also been associated with failure to thrive, impaired immune function, delayed wound healing, and increased stress levels.11
The majority of literature regarding sleep disturbance in the hospital has focused on sleep disruption in the intensive care unit, where interventions associated with sleep loss are required to deliver the appropriate standard level of care.1218 However, few evidence‐based strategies to promote sleep quality in hospitalized patients have been evaluated.16, 1823 In this study, we aimed to examine sleep among neurological and neurosurgical inpatients, identify specific sleep‐disruptive factors, and assess patient satisfaction regarding their sleep. We implemented a sleep‐promoting protocol with the hypothesis that improvement of modifiable sleep‐disruptive factors would improve sleep and patient satisfaction.
METHODS
Study Design
This prospective, observational study was designed and implemented by an interdisciplinary team of physicians, neuroscience nurses, and hospital administrators.
Patient Selection
The study was performed on a Neurology and Neurosurgery unit, with both private and semi‐private rooms, at a large, urban, tertiary teaching hospital from February 2009 through June 2010. During enrollment periods, all patients on the unit were screened daily for eligibility. Eligible patients were medically stable and capable of giving verbal consent. Patients who were less than 16 years of age, encephalopathic, aphasic, or non‐English speaking were excluded. Eligible patients were asked for consent to participate in the study. After consultation with the hospital's institutional review board (IRB) committee, written consent was waived in this observational, quality improvement study.
Study Timeline
The study comprised 4 phases (Figure 1). In Phase 1, we collected baseline data on patients in the unit. Data were collected in the form of sleep surveys, Press Ganey surveys, and noise meter recordings. The baseline phase (Phase 1) lasted 10 weeks from February to April 2009. We then implemented a novel sleep‐promoting intervention called Basic Sleep Rounds (Phase 2, May to August 2009). After discontinuing Basic Sleep Rounds, data were collected for the washout phase (Phase 3, September 2009 to February 2010). An enhanced version of the sleep‐promoting intervention called Deluxe Sleep Rounds was then instituted (Phase 4, March to June 2010). In Phases 2 and 4, sleep rounds were implemented for 2 weeks before data collection to ensure uniform application of Sleep Rounds.

Sleep Promoting Interventions
Prior to implementing Basic Sleep Rounds in Phase 2, a nursing in‐service was performed where staff were educated about sleep in the hospital and about the planned interventions, and posters promoting sleep were hung on the unit. Basic Sleep Rounds were performed during Phase 2 by the patient's bedside nurse or the unit charge nurse. This occurred for all patients on the unit at approximately 23:00 nightly using the Basic Sleep Rounds checklist, which formalized simple hospital functions, such as lights out, television off, room temperature adjustment, and a final restroom usage (Figure 2). For Phase 4, a team of undergraduate volunteers was organized to assist with the delivery of Sleep Rounds. In this phase (Deluxe Sleep Rounds), nurses performed Basic Sleep Rounds by completing the checklist, and undergraduate volunteers offered patients any of the following sleep amenities: warm blanket, warm milk, white‐noise machine, hypoallergenic lotion, or room spritzer.24, 25

Additionally, during the Basic and Deluxe intervention phases, noise‐sensitive traffic lights (Talk Light Too;
Data Collection
A survey was designed to evaluate sleep quality, estimate sleep quantity, identify sleep disruptors, and assess patient satisfaction (see Supporting Figure 1 in the online version of this article). The survey was given to all eligible participants on the morning after their second night in the unit. This time point was chosen to account for potentially confounding first night effects, and to ensure that enrolled patients spent a full night in the unit.
To better evaluate one of the sleep disruptors, a subset of the survey participants had noise meters placed in their rooms. Every morning, a member of the team would visit all eligible patients to ask if they were willing to participate in this portion of the study. Data recorded between 8:00 PM and 8:00 AM on the second night of each participant's stay were later used for analysis. Noise was recorded in decibels using a Vernier Sound Level Meter, attached to a LabQuest data collection device (
Scores from Press Ganey surveys were also analyzed. These surveys were mailed to patients shortly after hospital discharge, and subsequently processed by Press Ganey Associates, Inc (
Data Analysis
Most datasets were not described by a normal distribution, thus most data are presented as medians with interquartile ranges (IQR), and comparisons between datasets were made using the MannWhitney U test. Press Ganey data are presented as means with standard errors of the mean, as distributed by Press Ganey. P < 0.05 was considered significant for all data comparisons.
RESULTS
Basic demographic data were available on all participants from whom both noise and survey data were collected. As in Table 1, these participants were demographically similar (P < 0.05) with regards to age, sex, and ethnic background. For unknown reasons, neurosurgery patients comprised the majority of participants in Phases 1 and 3, and neurology patients comprised the majority in Phase 2. This difference was not significant.
| Demographic | Phase 1 (n = 32) | Phase 2 (n = 33) | Phase 3 (n = 30) |
|---|---|---|---|
| Average age | 49 1 | 43 3 | 46 3 |
| % Female | 71% | 71% | 57% |
| % Neurology | 42% | 65% | 37% |
| % White | 67% | 77% | 73% |
Sleep Survey
A total of 253 sleep surveys were collected in all 4 phases. Data generated from these surveys are demonstrated in Table 2. On a 7‐point scale (1 being the best score, corresponding to the answer none, and 7 the worst, corresponding to extreme), the median scores for overall difficulty sleeping were not significantly different in Phases 1, 2, and 4. In Phase 3, the median score was 4 (moderate), significantly worse than in the other 3 phases (0.002 < P < 0.01). Despite the reported difficulty sleeping during Phase 3, the median number of hours of sleep and awakenings in Phases 1, 2, 3, and 4 were not significantly different. Sleep latency was scored on a 6‐point scale (1 being the best, corresponding to 010 min, and 6 the worst, corresponding to greater than 45 minutes). Similar sleep latency was reported in Phases 1, 3, and 4. However, median sleep latency in Phase 2 was 1 (010 min), significantly shorter than in the other phases (0.001 < P < 0.02). Despite similar survey results throughout most of the phases, there was a significant improvement in sleep latency in the Basic Sleep Rounds phase (Phase 2), and a significant worsening in overall difficulty sleeping in the washout phase (Phase 3).
| Survey Question | Phase 1 | Phase 2 | Phase 3 | Phase 4 |
|---|---|---|---|---|
| ||||
| 1. How much difficulty did you have sleeping last night? | 3 | 2 | 4* | 3 |
| IQR 4 N = 100 | IQR 4 N = 78 | IQR 3 N = 75 | IQR 2 N = 22 | |
| 2. How many hours did you sleep last night? | 6 hr | 6 hr | 5 hr | 5 hr |
| IQR 4 N = 98 | IQR 3 N = 77 | IQR 3 N = 72 | IQR 2 N = 22 | |
| 3. About how many times did you wake up during the night while you were trying to sleep? | 3 | 3 | 4 | 3 |
| IQR 3 N = 101 | IQR 3 N = 77 | IQR 3 N = 73 | IQR 3 N = 22 | |
| 4. How long did it take you to go to sleep last night? | 3 (1620 min) | 1 (010 min) | 2 (1115 min) | 2 (1115 min) |
| IQR 3 N = 101 | IQR 2 N = 77 | IQR 4 N = 75 | IQR 3 N = 22 | |
Participants also ranked each of the 7 queried disruptive factors on a 7‐point scale with regards to degree of sleep interruption. Even though less than half of the participants were in shared rooms, the presence of a roommate among those with roommates was the only sleep disrupter that ranked differently among the 4 phases. In Phases 1 and 2, when asked how much their sleep was disturbed by roommates, the median response was 1 (none), IQR = 1 (N = 41 and 31, respectively). In Phase 4, the median was 2 (a little), IQR = 2 (N = 6), but not significantly different. Answers in Phase 3 were significantly different, with a median of 3 (mild), IQR = 3 (N = 30) (0.005 < P < 0.006). Because there were no other statistically significant differences among individual sleep disruptors as compared by phases, survey data from all 4 phases for these factors was also analyzed collectively. Pain and staff interruptions (IQR = 3, N = 252 and IQR = 2, N = 253, respectively) were reported as the most disturbing factors, each with a median of 2 (a little). All remaining factors had a median score of 1 (none): noise inside the room (IQR = 2, N = 253), noise outside of the room (IQR = 1, N = 253), temperature (IQR = 1, N = 253), noise outside of the building (IQR = 0, N = 252), and light (IQR = 0, N = 252).
Noise Meter Recordings
Noise data were recorded from 95 participants in Phases 1 through 3, yielding high‐quality data suitable for analysis from 63 participants (11 in Phase 1, 24 in Phase 2, and 28 in Phase 3). Recorded noise ranged from 35 to 80 dB. As shown in Supporting Figure 2 in the online version of this article, raw data were plotted as decibels as a function of time. Noise levels were then analyzed in aggregate and for each of four 3‐hour time blocks (8 PM11 PM, 11 PM 2 AM,, 2 AM 5 AM, and 5 AM8 AM). Median noise levels during the entire 12‐hour period increased significantly between the first 3 phases of the study (P < 0.001): 38.6 dB (IQR 5.4) in Phase 1; 40.6 dB (IQR 5.3) in Phase 2; and 43.5 (IQR 7) in Phase 3. As in Supporting Table 1 in the online version of this article, within each phase, the median noise levels were significantly less during the 11 PM2 AM and 2 AM5 AM periods, as compared to the 8 PM11 PM and 5 AM8 AM periods (P < 0.001). Due to equipment dysfunction, noise data were not available for Phase 4.
Press Ganey Survey
A total of 457 Press Ganey surveys were collected. According to these surveys, patients' mean raw score of noise, on a scale from 1 to 100 (100 representing the best score), ranged from a low of 59.5 7.2 (January 2010; N = 21) to a high of 82.1 5.2 (April 2009; N = 21). Figure 3 illustrates the monthly trend of the mean score for noise compared to the national average compiled from other large hospitals around the country. It demonstrates that during the phases in which Sleep Rounds were performed (Phases 2 and 4), patients' perceptions of noise were improved.

DISCUSSION
The major conclusions of this study are: 1) hospitalized patients suffer from poor sleep quality and quantity; 2) implementation of simple measures such as Sleep Rounds to change standard practice within the hospital is feasible and effective; and 3) despite an increase in measured noise, patients' perception of their sleep and of noise levels was improved by these measures. This study developed and tested a sleep promotion program that could easily be implemented on any inpatient floor. Our Sleep Rounds checklist outlines a novel, but simple approach to sleep health by hospital providers, with the immediate goal of improving sleep among inpatients and the ultimate goal of improving outcomes.
Our study confirms that sleep disruption is prevalent among patients admitted to general hospital wards. In this study, patients reported a median of 5 hours of sleep, 3 awakenings, and sleep latency of 1115 minutes. Although not alarmingly low, 5 hours is only 60% of the recommended 8 hours of sleep for healthy individuals each night and 72% of the 6.9 hours of sleep reported by the average American each night.26 Poor pain control, frequent staff interactions, and the presence of roommates were rated as most problematic by the patients we surveyed. Interestingly, patients rated noise, temperature, and light as less problematic sleep disruptors.
Although we did not detect a statistically significant improvement in total sleep time or number of awakenings, there was a significant improvement in sleep latency during Phase 2 of the study when Basic Sleep Rounds were performed. In Phase 3 (washout phase), there was less active participation by the nursing staff in sleep hygiene promotion, and patients' perception of sleep quality was significantly worse than it was in other phases. These results suggest that the perception of sleep quality and quantity could have been enhanced by both our Basic (Phase 2) and Deluxe (Phase 4) Sleep Rounds interventions.
We were able to achieve appropriate noise levels at night (40 dB) during this study, even before our intervention began.27 Noise levels increased 2 dB between Phases 1 and 2, and another 3 dB in Phase 3. Although the changes in decibel level were statistically significant, a change of 23 dB is barely perceptible.28 Interestingly, despite the increase in measured noise throughout the study, Press Ganey results showed a trend towards perceived improvement in noise levels just before implementation of the first intervention. This may be attributable to an increased awareness of noise created by consenting patients and placing noise meters in their rooms. Perception of noise worsened significantly during the washout phase, suggesting that abandonment of Sleep Rounds was associated with less concern about noise.
Prior to initiating this study, an educational in‐service was conducted for the nursing team regarding the purpose and overall aims of this project. This may have raised awareness of the importance of sleep before collection of Phase 1 data, and had the unintended effect of an increased focus on sleep even before Sleep Rounds began. Other limitations of the study include lack of objective sleep data, nonrandomized design, inability to demonstrate causality, generalizability of results, inability to control for comorbidity including baseline sleep hygiene, limited patient numbers, inability to blind patients and team members, and difficulty obtaining accurate and complete noise data on all patients enrolled.
This study suggests that although it remains difficult for patients to sleep well in the hospital, it is possible to improve sleep and patients' perception of their sleep while they are hospitalized. Further studies are warranted to systematically evaluate interventions aimed at improving and overcoming the identified sleep disruptors without compromising patient care. However, we believe that Sleep Rounds could be associated with improvements in inpatient sleep hygiene and patient satisfaction, and could ultimately benefit patient outcomes.
Acknowledgements
The authors thank JoEllen Robinson, Jane Hill, and the nursing staff of Meyer 8 for their invaluable contributions to this project.
- .Sleep in acute care settings: an integrative review.J Nurs Scholarsh.2000;32(1):31–38.
- ,.Nursing standard‐of‐practice protocol: sleep disturbances in elderly patients. The NICHE Faculty.Geriatr Nurs.1995;16(5):238–243.
- ,,, et al.Sleep patterns and mortality among elderly patients in a geriatric hospital.Gerontology.2000;46(6):318–322.
- ,,.Sleep, insomnia and falls in elderly patients.Sleep Med.2008;9(suppl 1):S18–S22.
- ,,,,,.Behavioural and physiological reactivity to noise in the newborn.J Paediatr Child Health.2004;40(5–6):275–281.
- ,,.Sleep problems as a risk factor for falls in a sample of community‐dwelling adults aged 64–99 years.J Am Geriatr Soc.2000;48(10):1234–1240.
- ,,, et al.Reducing dangerous nighttime events in persons with dementia by using a nighttime monitoring system.Alzheimers Dement.2009;5(5):419–426.
- ,.Neurocognitive consequences of sleep deprivation.Semin Neurol.2005;25(1):117–129.
- ,,,,.The nursing home at night: effects of an intervention on noise, light, and sleep.J Am Geriatr Soc.1999;47(4):430–438.
- ,,,,.Sleep in hospitalized elders: a pilot study.Geriatr Nurs.2010;31(4):263–271.
- ,.Interactive relationships between hospital patients' noise‐induced stress and other stress with sleep.Heart Lung.2001;30(4):237–243.
- ,,.The impact of noise on patients' sleep and the effectiveness of noise reduction strategies in intensive care units.Crit Care.2009;13(2):208.
- ,,,,,.Sleep in critically ill patients requiring mechanical ventilation.Chest.2000;117(3):809–818.
- ,.Adverse effects of sleep deprivation in the ICU.Crit Care Clin.2008;24(3):461–476, v–vi.
- ,,,,.Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit.Am J Respir Crit Care Med.2001;163(2):451–457.
- ,.Responses of premature infants to routine nursing interventions and noise in the NICU.Nurs Res.1995;44(3):179–185.
- ,,,,.Noise, stress, and annoyance in a pediatric intensive care unit.Crit Care Med.2003;31(1):113–119.
- ,,,,.Effects of guidelines implementation in a surgical intensive care unit to control nighttime light and noise levels.Crit Care Med.2000;28(7):2242–2247.
- ,,,,.Noise control: a nursing team's approach to sleep promotion.Am J Nurs.2004;104(2):40–48; quiz 48–49.
- ,,, et al.Environmental noise sources and interventions to minimize them: a tale of 2 hospitals.J Nurs Care Qual.2008;23(3):216–224; quiz 225–216.
- ,,, et al.Interventions to reduce decibel levels on patient care units.Am Surg.1998;64(9):894–899.
- ,,,.Examining the feasibility of implementing specific nursing interventions to promote sleep in hospitalized elderly patients.Geriatr Nurs.2008;29(3):197–206.
- ,,,.Applicability of two brief evidence‐based interventions to improve sleep quality in inpatient mental health care.Int J Ment Health Nurs.2011;20(5)319–327.
- .Sleep deprivation in critical care units.Crit Care Nurs Q.2003;26(3):179–189; quiz 190–171.
- ,,,.Sleep promotion in hospitalized elders.Medsurg Nurs.2003;12(5):279–289; quiz 290.
- 2005 NSF Sleep in America Poll.Washington, DC:National Sleep Foundation;2005.
- ,,.Guidelines for Community Noise.Geneva, Switzerland:World Health Organization;1999.
- PhysicsArchives.com.2010. Available at: http://physicsarchives.com/index.php/courses/219. Accessed May 15, 2011.
- .Sleep in acute care settings: an integrative review.J Nurs Scholarsh.2000;32(1):31–38.
- ,.Nursing standard‐of‐practice protocol: sleep disturbances in elderly patients. The NICHE Faculty.Geriatr Nurs.1995;16(5):238–243.
- ,,, et al.Sleep patterns and mortality among elderly patients in a geriatric hospital.Gerontology.2000;46(6):318–322.
- ,,.Sleep, insomnia and falls in elderly patients.Sleep Med.2008;9(suppl 1):S18–S22.
- ,,,,,.Behavioural and physiological reactivity to noise in the newborn.J Paediatr Child Health.2004;40(5–6):275–281.
- ,,.Sleep problems as a risk factor for falls in a sample of community‐dwelling adults aged 64–99 years.J Am Geriatr Soc.2000;48(10):1234–1240.
- ,,, et al.Reducing dangerous nighttime events in persons with dementia by using a nighttime monitoring system.Alzheimers Dement.2009;5(5):419–426.
- ,.Neurocognitive consequences of sleep deprivation.Semin Neurol.2005;25(1):117–129.
- ,,,,.The nursing home at night: effects of an intervention on noise, light, and sleep.J Am Geriatr Soc.1999;47(4):430–438.
- ,,,,.Sleep in hospitalized elders: a pilot study.Geriatr Nurs.2010;31(4):263–271.
- ,.Interactive relationships between hospital patients' noise‐induced stress and other stress with sleep.Heart Lung.2001;30(4):237–243.
- ,,.The impact of noise on patients' sleep and the effectiveness of noise reduction strategies in intensive care units.Crit Care.2009;13(2):208.
- ,,,,,.Sleep in critically ill patients requiring mechanical ventilation.Chest.2000;117(3):809–818.
- ,.Adverse effects of sleep deprivation in the ICU.Crit Care Clin.2008;24(3):461–476, v–vi.
- ,,,,.Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit.Am J Respir Crit Care Med.2001;163(2):451–457.
- ,.Responses of premature infants to routine nursing interventions and noise in the NICU.Nurs Res.1995;44(3):179–185.
- ,,,,.Noise, stress, and annoyance in a pediatric intensive care unit.Crit Care Med.2003;31(1):113–119.
- ,,,,.Effects of guidelines implementation in a surgical intensive care unit to control nighttime light and noise levels.Crit Care Med.2000;28(7):2242–2247.
- ,,,,.Noise control: a nursing team's approach to sleep promotion.Am J Nurs.2004;104(2):40–48; quiz 48–49.
- ,,, et al.Environmental noise sources and interventions to minimize them: a tale of 2 hospitals.J Nurs Care Qual.2008;23(3):216–224; quiz 225–216.
- ,,, et al.Interventions to reduce decibel levels on patient care units.Am Surg.1998;64(9):894–899.
- ,,,.Examining the feasibility of implementing specific nursing interventions to promote sleep in hospitalized elderly patients.Geriatr Nurs.2008;29(3):197–206.
- ,,,.Applicability of two brief evidence‐based interventions to improve sleep quality in inpatient mental health care.Int J Ment Health Nurs.2011;20(5)319–327.
- .Sleep deprivation in critical care units.Crit Care Nurs Q.2003;26(3):179–189; quiz 190–171.
- ,,,.Sleep promotion in hospitalized elders.Medsurg Nurs.2003;12(5):279–289; quiz 290.
- 2005 NSF Sleep in America Poll.Washington, DC:National Sleep Foundation;2005.
- ,,.Guidelines for Community Noise.Geneva, Switzerland:World Health Organization;1999.
- PhysicsArchives.com.2010. Available at: http://physicsarchives.com/index.php/courses/219. Accessed May 15, 2011.
Overcome by Weakness
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
This icon represents the patient's case. Each paragraph that follows represents the discussant's thoughts.
An 89‐year‐old man presented to the emergency department with progressive fatigue, confusion, and generalized weakness over 2 months, worsening in the prior few days.
Four categories of disease account for most cases of confusion in the elderly: metabolic derangements; infection (both within and outside of the central nervous system); structural brain disorder (eg, bleed or tumor); and toxins (generally medications). It will be important early on to determine if weakness refers to true loss of motor function, reflecting a neuromuscular lesion.
At baseline, the patient had normal cognition, ambulated without assistance, and was independent in activities of daily living. Over the preceding 2 months, general functional decline, unsteady gait, balance problems, and word‐finding difficulty developed. He also needed a front‐wheel walker to avoid falling. One month prior to presentation, the patient's children noticed he was markedly fatigued and was requiring a nightly sedative‐hypnotic in order to fall asleep.
He denied any recent travel, sick contacts, or recent illness. He denied vertigo, dizziness, or syncope. He reported occasional urinary incontinence which he attributed to being too weak to get to the bathroom promptly.
This rapid progression over 2 months is not consistent with the time course of the more common neurodegenerative causes of dementia, such as Alzheimer's or Parkinson's disease. In Parkinson's, cognitive impairment is a late feature, occurring years after gait and motor disturbances develop. Normal pressure hydrocephalus, which causes the classic triad of incontinence, ataxia, and confusion, would also be unlikely to develop so abruptly. Although we do not think of vascular (multi‐infarct) dementia as having such a short time course, on occasion a seemingly rapid presentation is the postscript to a more insidious progression that has been underway for years. A subdural hematoma, which may have occurred with any of his falls, must also be considered, as should neoplastic and paraneoplastic processes.
His past medical history included paroxysmal atrial fibrillation, diabetes mellitus, hypertension, hyperlipidemia, coronary artery disease complicated by prior myocardial infarction for which he underwent coronary artery bypass grafting 7 years prior, mild aortic sclerosis and insufficiency, mild mitral regurgitation, anemia, recurrent low‐grade bladder cancer treated with serial local resections over the last 8 years, low‐grade prostate cancer which had not required treatment, hypothyroidism, chronic kidney disease, and lumbar spinal stenosis.
His atrial fibrillation and valvular disease put him at risk for thrombotic and infective embolic phenomena causing multiple cerebral infarcts. He has all the requisite underlying conditions for vascular dementia. Untreated hypothyroidism could explain his decline and sedation. Prostate and bladder cancers would be unusual causes of subacute central nervous system (CNS) disease. Finally, his chronic kidney disease may have progressed to uremia.
One year prior to admission, the patient developed bilateral shoulder pain, right‐sided headache with loss of vision in his right eye, fevers, and an elevated erythrocyte sedimentation rate (ESR). Although temporal artery biopsy specimens did not reveal arterial inflammation, he was started on high‐dose prednisone for polymyalgia rheumatica and giant cell arteritis (GCA); he experienced improvement in his ESR and in all symptoms, with the exception of permanent right eye blindness. Maintenance prednisone was continued for disease suppression.
Even without confirmatory biopsy results, the clinical case for GCA was compelling and the rationale for starting steroids strong; his sustained response over 1 year further supports the diagnosis. GCA is almost always confined to extracranial vessels, and altered sensorium would be an unusual manifestation. His extended treatment with prednisone expands the list of CNS and systemic infections, particularly opportunistic ones, for which he is now at risk.
Outpatient medications were prednisone at doses fluctuating between 10 and 20 mg daily, furosemide 20 mg daily, amiodarone 200 mg daily, levothyroxine 50 mcg daily, alendronate 70 mg weekly, eszopiclone 1 mg nightly, losartan 50 mg daily, and warfarin. The patient was an accomplished professor and had published a book 1 year prior to admission. He quit smoking over 30 years ago, and he occasionally drank wine. He denied any drug use.
Three months prior to the current presentation, the patient was hospitalized for right upper‐lobe pneumonia for which he received a course of doxycycline, and his symptoms improved. Follow‐up chest x‐ray, 4 weeks later (2 months prior to admission), showed only slight improvement of the right upper‐lobe opacity.
Leading possibilities for the persistent lung opacity are cancer and untreated infection. After 3 decades of being tobacco‐free, his smoking‐related risk of cancer is low, but remains above baseline population risk. There are at least 4 ways untreated lung cancer may render patients confused: direct metastases to the brain, carcinomatous or lymphomatous meningitis, paraneoplastic phenomenon (eg, limbic encephalitis), and metabolic derangements (eg, syndrome of inappropriate antidiuretic hormone secretion, hypercalcemia).
The upper‐lobe infiltrate that failed to improve with doxycycline could also reflect an aspiration pneumonia that evolved into an abscess, or an infection with mycobacteria or endemic fungi.
In the emergency department, the patient's temperature was 38.5C, blood pressure 139/56 mmHg, heart rate 92 beats per minute, respiratory rate 18 breaths per minute, and oxygen saturation while breathing ambient room air was 98%.
He was alert and well‐appearing. Jugular venous pressure was normal. The thyroid was normal. He had rhonchi in his right anterior upper chest and right lower lung base. Cardiac exam demonstrated a regular rhythm, with a 3/6 systolic murmur at the second right intercostal space that radiated to the carotids, and a 2/6 nonradiating holosystolic murmur at the apex. Abdomen was soft with no organomegaly or masses. There was no lymphadenopathy, and his extremities showed no clubbing or edema. There were multiple contusions in various stages of healing on his legs.
He was confused, had word‐finding difficulty, and frequently would lose his train of thought, stopping in mid‐sentence. He had no dysarthria. Cranial nerves were normal, except for reduced visual acuity and diminished pupillary response to light in his right pupil, which had been previously documented. Finger‐to‐nose testing was slow bilaterally, but was more sluggish on the right. Rapid alternating hand movements were intact. He was unable to perform heel‐to‐shin testing. Sensation was intact. Plantar reflexes were flexor bilaterally. Strength in his limbs was preserved both distally and proximally, and deep tendon reflexes were normal. However, he was unable to sit up or stand on his own due to weakness.
The fever on prednisone is a red flag for infection. The infection may be the primary diagnosis (eg, meningoencephalitis) or may reflect an additional superimposed insult (eg, urinary tract infection) on the underlying encephalopathy. Two murmurs in a febrile patient with the multifocal CNS findings suggest endocarditis. The abnormalities on chest examination could indicate a lung infection complicated by hematogenous spread to the brain, such as a lung abscess (secondary to the aspiration event), tuberculosis (TB), or endemic fungal infection.
Serum chemistries were normal, and the serum creatinine was 1.1 mg/dL. White blood cell count was 20,100 per mm3 with 90% neutrophils, 9% lymphocytes, and 1% monocytes. Hemoglobin was 13.7 g/dL, platelet count was 464,000 per mm3. Thyroid stimulating hormone (TSH) was 6.0 IU/mL (normal, <5.5). International normalized ratio (INR) was 2.2. Urinalysis was normal. Transaminases, bilirubin, and alkaline phosphatase were normal. Lactate was 1.9 mmol/L.
Electrocardiogram (EKG) was unchanged from his baseline. ESR was >120 mm/hr (the maximum reportable value); his ESR measurements had been gradually rising during the previous 4 months. Chest x‐ray demonstrated a right upper‐lobe opacity, slightly more pronounced in comparison with chest x‐ray 2 months earlier.
His fever, leukocytosis, elevated ESR, and thrombocytosis all reflect severe inflammation. While infection and then malignancy remain the primary considerations, a third category of inflammatory diseaseautoimmunitywarrants mention. For instance, Wegener's granulomatosis can cause pulmonary and CNS disease in the elderly.
Intravenous ceftriaxone and oral doxycycline were administered. Chest computed tomography (CT) (Figure 1) demonstrated dense right upper‐lobe mass‐like consolidation with associated adenopathy and pleural effusion; in addition, several nodules were present in the left and right lower lobes, the largest of which was 10 mm. CT of the chest 10 months prior to current admission had been normal. CT of the brain, performed without contrast, demonstrated multiple areas of abnormal vasogenic edema with suggestion of underlying masses.
The imaging provides evidence of a combined pulmonaryCNS syndrome. It is far more common for disease to originate in the lungs (a common portal of entry and environmental exposure) and spread to the brain than vice versa. The list of diseases and pathogens that affect the lungs and spread to the brain includes: primary lung cancer, lymphoma, bacteria, mycobacteria, fungi, molds (eg, Aspergillus), Wegener's granulomatosis, and lymphomatoid granulomatosis. Bacterial lung abscess, such as that caused by Streptococcus milleri group, may spread to the brain. Nocardia, a ubiquitous soil organism, infects immunocompromised patients and causes a similar pattern. Actinomycosis is an atypical infection that may mimic cancer, particularly in the lungs; while head and neck disease is characteristic, CNS involvement is less so. Overall, the imaging does not specifically pinpoint 1 entity, but infection remains heavily favored over malignancy, with autoimmunity a distant third.
Respiratory cultures showed normal respiratory flora. Blood cultures grew no organisms. Two samples of induced sputum were negative for acid‐fast bacilli (AFB) on smear examination. Forty‐eight hours after a purified protein derivative (PPD) skin test was placed, there was 0 mm of induration. Magnetic resonance imaging (MRI) of the brain (Figure 2) demonstrated 8 ring‐enhancing supratentorial lesions at the graywhite junction.

Negative blood cultures substantially lower the probability of bacterial endocarditis; there are no epidemiologic risk factors for the rare causes of culture‐negative endocarditis (eg, farm exposure, homelessness). Two negative smears for AFB with dense pulmonary or cavitary disease signify a low probability of tuberculosis.
In the setting of depressed cell‐mediated immunity (eg, human immunodeficiency virus [HIV] infection or chronic prednisone use), multiple ring‐enhancing CNS lesions are a classic appearance of toxoplasmosis, but they also are typical of bacterial brain abscesses and Nocardia. Brain metastases are usually solid, but as central necrosis develops, peripheral enhancement may appear. The diffuse distribution and the localization at the graywhite junction further support a hematogenously disseminated process, but do not differentiate infection from metastases.
Transthoracic echocardiogram demonstrated normal left ventricular ejection fraction, clinically insignificant aortic sclerosis and mitral regurgitation, and no evidence of vegetations. Results of a CT‐guided fine‐needle aspiration of the lung were nondiagnostic, showing necropurulent material and benign lung parenchyma with fibrosis. A core biopsy of the lung showed alveolar tissue with patchy mild deposition of fibrinous material and rare scattered acute and chronic inflammatory cells without granulomas. Pleural fluid cytology showed reactive mesothelial cells with mixed inflammatory cells. There were no fungal elements or malignant cells.
The failure to detect malignancy after 2 biopsies and 1 thoracentesis lowers the suspicion of cancer, and thereby bolsters the probability of atypical infections which may elude diagnosis on routine cultures and biopsy. A detailed history, with attention to geographic exposures, is warranted to see which endemic mycosis would put him most at risk. Based on his California residency, disseminated coccidiomycosis or the ubiquitous Cryptococcus are conceivable. Nocardia remains a strong consideration because of his chronic immunosuppression and the lung‐CNS pattern.
Fungal stains and cultures from the biopsies and pleural fluid were negative. Serum antibodies to coccidiomycosis and serum cryptococcal antigen tests were negative. On the eighth hospital day, the microbiology lab reported a few acid‐fast bacilli from a third induced sputum sample. RNA amplification testing for Mycobacterium tuberculosis was negative.
Due to his continued decline, the patient met with the palliative care team and expressed his desire to go home with hospice. While arrangements were being made, he died later that day in the hospital.
There is reasonable evidence that tuberculosis is not the culprit pathogen here: negative PPD, 2 negative sputa in the setting of a massive necrotic lesion, and a negative RNA amplification test. Nontuberculous mycobacteria such as Mycobacterium avium complex (MAC) and M. kansasii may cause disease similar to TB, but they are usually not this difficult to identify. Nocardia is classically a weakly acid‐fast positive bacteria and fits this patient's clinical picture best.
Four colonies of Nocardia (not further speciated) were identified postmortem from the patient's sputum.
DISCUSSION
Nocardia species are ubiquitous soil‐dwelling, Gram‐positive, branching rods which are weakly positive with acid‐fast staining.1 Almost all Nocardia infections occur in patients with immune systems compromised by chronic disease (HIV, malignancy, alcoholism, chronic lung or kidney disease) or by medications. Corticosteroid treatment is the most frequent risk factor. In cases of nocardiosis in patients taking steroids, the median daily prednisone dose was 25 mg (range, 1080 mg) for a median duration of 3 months.2, 3
Nocardia should be considered in any patient with unexplained pulmonary, CNS, or cutaneous disease and appropriate risk factors. Pulmonary disease is most common, seen in approximately two‐thirds of patients, and is typically bilateral. Chest radiographic findings include infiltrates (59%), nodules (35%), effusions, and cavities.2 Up to half of all cases of pulmonary nocardiosis are associated with hematogenous dissemination, most commonly to the CNS, where manifestations include incidentally discovered asymptomatic lesions, headache, confusion, and focal neurologic deficits; meningitis is rare.1 CNS involvement and severe predisposing illness are adverse prognostic markers.
Diagnosis of nocardiosis is typically delayed by 6 weeks to 1 year.4, 5 This has been attributed to its rarity, its nonspecific and indolent presentation, its slow growth, and the difficulty isolating Nocardia from clinical specimens. Although Nocardia may disseminate widely to almost any site, isolation of Nocardia from blood cultures is rare. Clinicians must rely on sputum or tissue samples to demonstrate the characteristic Gram‐positive rods which stain weakly on acid‐fast preparations. Polymerase chain reaction (PCR)‐based tests improve the yield but are not routinely available.
The standard antibiotic for the treatment of Nocardia infections is trimethoprim‐sulfamethoxazole (TMP‐SMX) which has excellent CNS penetration. In patients with pulmonary disease or CNS dissemination, a second parenteral antimicrobial (usually amikacin or imipenem) is typically added to TMP‐SMX, and treatment is extended to 12 months or longer.6, 7 Prophylaxis with TMP‐SMX, which is usually prescribed to prevent Pneumocystis jirovecii in susceptible hosts, also reduces the incidence of Nocardia.2, 3, 6 Nocardia's restricted susceptibility pattern presents a challenge for hospitalists, as TMP‐SMX and aminoglycosides are rarely administered empirically for cases of suspected pneumonia or atypical pulmonary infections (other than P. jirovecii).
When confronted with the pattern of simultaneous pulmonary and CNS lesions, hospitalists must consider infections (lung abscess, mycobacteria, fungi, Nocardia), malignancies, and autoimmune conditions (sarcoidosis, Wegener's granulomatosis). This patient's weakness was a direct result of his weakened immune system, which allowed this weakly acid‐fast organism to flourish. Only by recognizing the possibility of nocardiosis (eg, a patient receiving steroids who develops pulmonary and CNS lesions) is there hope for early diagnosis and treatment.
TEACHING POINTS
-
Suspect disseminated nocardiosis in immunocompromised patients with unexplained pulmonary disease and CNS disease characterized by multiple ring‐enhancing abscesses.
-
Corticosteroid treatment is the most common risk factor for Nocardia infections. Patients taking prednisone at doses in excess of 10 mg daily for greater than 3 months should receive P. jirovecii prophylaxis with TMP‐SMX, which also reduces the incidence of Nocardia.
-
Prolonged courses of TMP‐SMX combined with at least 1 other agent for at least 612 months are typically required to treat disseminated Nocardia.
Acknowledgements
Disclosure: Dr Thomas E. Baudendistel is a former Deputy Editor at the Journal of Hospital Medicine and received a stipend for this work.
- ,.Nocardia species: host‐parasite relationships.Clin Microbiol Rev.1994;7:213–264.
- ,,,,,.Nocardiosis at the turn of the century.Medicine (Baltimore).2009;88:250–261.
- ,.A case series and focused review of nocardiosis: clinical and microbiologic aspects.Medicine (Baltimore).2004;83:300–313.
- ,,, et al.Pulmonary nocardiosis: risk factors and outcomes.Respirology.2007;12:394–400.
- ,.Infection with Nocardia species in Queensland. A review of 102 clinical isolates.Med J Aust.1992;156:692–697.
- .Nocardia in solid organ transplant recipients.Am J Transplant.2009;9:S70–S77.
- .Nocardiosis.Clin Infect Dis.1996;22:891–905.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
This icon represents the patient's case. Each paragraph that follows represents the discussant's thoughts.
An 89‐year‐old man presented to the emergency department with progressive fatigue, confusion, and generalized weakness over 2 months, worsening in the prior few days.
Four categories of disease account for most cases of confusion in the elderly: metabolic derangements; infection (both within and outside of the central nervous system); structural brain disorder (eg, bleed or tumor); and toxins (generally medications). It will be important early on to determine if weakness refers to true loss of motor function, reflecting a neuromuscular lesion.
At baseline, the patient had normal cognition, ambulated without assistance, and was independent in activities of daily living. Over the preceding 2 months, general functional decline, unsteady gait, balance problems, and word‐finding difficulty developed. He also needed a front‐wheel walker to avoid falling. One month prior to presentation, the patient's children noticed he was markedly fatigued and was requiring a nightly sedative‐hypnotic in order to fall asleep.
He denied any recent travel, sick contacts, or recent illness. He denied vertigo, dizziness, or syncope. He reported occasional urinary incontinence which he attributed to being too weak to get to the bathroom promptly.
This rapid progression over 2 months is not consistent with the time course of the more common neurodegenerative causes of dementia, such as Alzheimer's or Parkinson's disease. In Parkinson's, cognitive impairment is a late feature, occurring years after gait and motor disturbances develop. Normal pressure hydrocephalus, which causes the classic triad of incontinence, ataxia, and confusion, would also be unlikely to develop so abruptly. Although we do not think of vascular (multi‐infarct) dementia as having such a short time course, on occasion a seemingly rapid presentation is the postscript to a more insidious progression that has been underway for years. A subdural hematoma, which may have occurred with any of his falls, must also be considered, as should neoplastic and paraneoplastic processes.
His past medical history included paroxysmal atrial fibrillation, diabetes mellitus, hypertension, hyperlipidemia, coronary artery disease complicated by prior myocardial infarction for which he underwent coronary artery bypass grafting 7 years prior, mild aortic sclerosis and insufficiency, mild mitral regurgitation, anemia, recurrent low‐grade bladder cancer treated with serial local resections over the last 8 years, low‐grade prostate cancer which had not required treatment, hypothyroidism, chronic kidney disease, and lumbar spinal stenosis.
His atrial fibrillation and valvular disease put him at risk for thrombotic and infective embolic phenomena causing multiple cerebral infarcts. He has all the requisite underlying conditions for vascular dementia. Untreated hypothyroidism could explain his decline and sedation. Prostate and bladder cancers would be unusual causes of subacute central nervous system (CNS) disease. Finally, his chronic kidney disease may have progressed to uremia.
One year prior to admission, the patient developed bilateral shoulder pain, right‐sided headache with loss of vision in his right eye, fevers, and an elevated erythrocyte sedimentation rate (ESR). Although temporal artery biopsy specimens did not reveal arterial inflammation, he was started on high‐dose prednisone for polymyalgia rheumatica and giant cell arteritis (GCA); he experienced improvement in his ESR and in all symptoms, with the exception of permanent right eye blindness. Maintenance prednisone was continued for disease suppression.
Even without confirmatory biopsy results, the clinical case for GCA was compelling and the rationale for starting steroids strong; his sustained response over 1 year further supports the diagnosis. GCA is almost always confined to extracranial vessels, and altered sensorium would be an unusual manifestation. His extended treatment with prednisone expands the list of CNS and systemic infections, particularly opportunistic ones, for which he is now at risk.
Outpatient medications were prednisone at doses fluctuating between 10 and 20 mg daily, furosemide 20 mg daily, amiodarone 200 mg daily, levothyroxine 50 mcg daily, alendronate 70 mg weekly, eszopiclone 1 mg nightly, losartan 50 mg daily, and warfarin. The patient was an accomplished professor and had published a book 1 year prior to admission. He quit smoking over 30 years ago, and he occasionally drank wine. He denied any drug use.
Three months prior to the current presentation, the patient was hospitalized for right upper‐lobe pneumonia for which he received a course of doxycycline, and his symptoms improved. Follow‐up chest x‐ray, 4 weeks later (2 months prior to admission), showed only slight improvement of the right upper‐lobe opacity.
Leading possibilities for the persistent lung opacity are cancer and untreated infection. After 3 decades of being tobacco‐free, his smoking‐related risk of cancer is low, but remains above baseline population risk. There are at least 4 ways untreated lung cancer may render patients confused: direct metastases to the brain, carcinomatous or lymphomatous meningitis, paraneoplastic phenomenon (eg, limbic encephalitis), and metabolic derangements (eg, syndrome of inappropriate antidiuretic hormone secretion, hypercalcemia).
The upper‐lobe infiltrate that failed to improve with doxycycline could also reflect an aspiration pneumonia that evolved into an abscess, or an infection with mycobacteria or endemic fungi.
In the emergency department, the patient's temperature was 38.5C, blood pressure 139/56 mmHg, heart rate 92 beats per minute, respiratory rate 18 breaths per minute, and oxygen saturation while breathing ambient room air was 98%.
He was alert and well‐appearing. Jugular venous pressure was normal. The thyroid was normal. He had rhonchi in his right anterior upper chest and right lower lung base. Cardiac exam demonstrated a regular rhythm, with a 3/6 systolic murmur at the second right intercostal space that radiated to the carotids, and a 2/6 nonradiating holosystolic murmur at the apex. Abdomen was soft with no organomegaly or masses. There was no lymphadenopathy, and his extremities showed no clubbing or edema. There were multiple contusions in various stages of healing on his legs.
He was confused, had word‐finding difficulty, and frequently would lose his train of thought, stopping in mid‐sentence. He had no dysarthria. Cranial nerves were normal, except for reduced visual acuity and diminished pupillary response to light in his right pupil, which had been previously documented. Finger‐to‐nose testing was slow bilaterally, but was more sluggish on the right. Rapid alternating hand movements were intact. He was unable to perform heel‐to‐shin testing. Sensation was intact. Plantar reflexes were flexor bilaterally. Strength in his limbs was preserved both distally and proximally, and deep tendon reflexes were normal. However, he was unable to sit up or stand on his own due to weakness.
The fever on prednisone is a red flag for infection. The infection may be the primary diagnosis (eg, meningoencephalitis) or may reflect an additional superimposed insult (eg, urinary tract infection) on the underlying encephalopathy. Two murmurs in a febrile patient with the multifocal CNS findings suggest endocarditis. The abnormalities on chest examination could indicate a lung infection complicated by hematogenous spread to the brain, such as a lung abscess (secondary to the aspiration event), tuberculosis (TB), or endemic fungal infection.
Serum chemistries were normal, and the serum creatinine was 1.1 mg/dL. White blood cell count was 20,100 per mm3 with 90% neutrophils, 9% lymphocytes, and 1% monocytes. Hemoglobin was 13.7 g/dL, platelet count was 464,000 per mm3. Thyroid stimulating hormone (TSH) was 6.0 IU/mL (normal, <5.5). International normalized ratio (INR) was 2.2. Urinalysis was normal. Transaminases, bilirubin, and alkaline phosphatase were normal. Lactate was 1.9 mmol/L.
Electrocardiogram (EKG) was unchanged from his baseline. ESR was >120 mm/hr (the maximum reportable value); his ESR measurements had been gradually rising during the previous 4 months. Chest x‐ray demonstrated a right upper‐lobe opacity, slightly more pronounced in comparison with chest x‐ray 2 months earlier.
His fever, leukocytosis, elevated ESR, and thrombocytosis all reflect severe inflammation. While infection and then malignancy remain the primary considerations, a third category of inflammatory diseaseautoimmunitywarrants mention. For instance, Wegener's granulomatosis can cause pulmonary and CNS disease in the elderly.
Intravenous ceftriaxone and oral doxycycline were administered. Chest computed tomography (CT) (Figure 1) demonstrated dense right upper‐lobe mass‐like consolidation with associated adenopathy and pleural effusion; in addition, several nodules were present in the left and right lower lobes, the largest of which was 10 mm. CT of the chest 10 months prior to current admission had been normal. CT of the brain, performed without contrast, demonstrated multiple areas of abnormal vasogenic edema with suggestion of underlying masses.

The imaging provides evidence of a combined pulmonaryCNS syndrome. It is far more common for disease to originate in the lungs (a common portal of entry and environmental exposure) and spread to the brain than vice versa. The list of diseases and pathogens that affect the lungs and spread to the brain includes: primary lung cancer, lymphoma, bacteria, mycobacteria, fungi, molds (eg, Aspergillus), Wegener's granulomatosis, and lymphomatoid granulomatosis. Bacterial lung abscess, such as that caused by Streptococcus milleri group, may spread to the brain. Nocardia, a ubiquitous soil organism, infects immunocompromised patients and causes a similar pattern. Actinomycosis is an atypical infection that may mimic cancer, particularly in the lungs; while head and neck disease is characteristic, CNS involvement is less so. Overall, the imaging does not specifically pinpoint 1 entity, but infection remains heavily favored over malignancy, with autoimmunity a distant third.
Respiratory cultures showed normal respiratory flora. Blood cultures grew no organisms. Two samples of induced sputum were negative for acid‐fast bacilli (AFB) on smear examination. Forty‐eight hours after a purified protein derivative (PPD) skin test was placed, there was 0 mm of induration. Magnetic resonance imaging (MRI) of the brain (Figure 2) demonstrated 8 ring‐enhancing supratentorial lesions at the graywhite junction.

Negative blood cultures substantially lower the probability of bacterial endocarditis; there are no epidemiologic risk factors for the rare causes of culture‐negative endocarditis (eg, farm exposure, homelessness). Two negative smears for AFB with dense pulmonary or cavitary disease signify a low probability of tuberculosis.
In the setting of depressed cell‐mediated immunity (eg, human immunodeficiency virus [HIV] infection or chronic prednisone use), multiple ring‐enhancing CNS lesions are a classic appearance of toxoplasmosis, but they also are typical of bacterial brain abscesses and Nocardia. Brain metastases are usually solid, but as central necrosis develops, peripheral enhancement may appear. The diffuse distribution and the localization at the graywhite junction further support a hematogenously disseminated process, but do not differentiate infection from metastases.
Transthoracic echocardiogram demonstrated normal left ventricular ejection fraction, clinically insignificant aortic sclerosis and mitral regurgitation, and no evidence of vegetations. Results of a CT‐guided fine‐needle aspiration of the lung were nondiagnostic, showing necropurulent material and benign lung parenchyma with fibrosis. A core biopsy of the lung showed alveolar tissue with patchy mild deposition of fibrinous material and rare scattered acute and chronic inflammatory cells without granulomas. Pleural fluid cytology showed reactive mesothelial cells with mixed inflammatory cells. There were no fungal elements or malignant cells.
The failure to detect malignancy after 2 biopsies and 1 thoracentesis lowers the suspicion of cancer, and thereby bolsters the probability of atypical infections which may elude diagnosis on routine cultures and biopsy. A detailed history, with attention to geographic exposures, is warranted to see which endemic mycosis would put him most at risk. Based on his California residency, disseminated coccidiomycosis or the ubiquitous Cryptococcus are conceivable. Nocardia remains a strong consideration because of his chronic immunosuppression and the lung‐CNS pattern.
Fungal stains and cultures from the biopsies and pleural fluid were negative. Serum antibodies to coccidiomycosis and serum cryptococcal antigen tests were negative. On the eighth hospital day, the microbiology lab reported a few acid‐fast bacilli from a third induced sputum sample. RNA amplification testing for Mycobacterium tuberculosis was negative.
Due to his continued decline, the patient met with the palliative care team and expressed his desire to go home with hospice. While arrangements were being made, he died later that day in the hospital.
There is reasonable evidence that tuberculosis is not the culprit pathogen here: negative PPD, 2 negative sputa in the setting of a massive necrotic lesion, and a negative RNA amplification test. Nontuberculous mycobacteria such as Mycobacterium avium complex (MAC) and M. kansasii may cause disease similar to TB, but they are usually not this difficult to identify. Nocardia is classically a weakly acid‐fast positive bacteria and fits this patient's clinical picture best.
Four colonies of Nocardia (not further speciated) were identified postmortem from the patient's sputum.
DISCUSSION
Nocardia species are ubiquitous soil‐dwelling, Gram‐positive, branching rods which are weakly positive with acid‐fast staining.1 Almost all Nocardia infections occur in patients with immune systems compromised by chronic disease (HIV, malignancy, alcoholism, chronic lung or kidney disease) or by medications. Corticosteroid treatment is the most frequent risk factor. In cases of nocardiosis in patients taking steroids, the median daily prednisone dose was 25 mg (range, 1080 mg) for a median duration of 3 months.2, 3
Nocardia should be considered in any patient with unexplained pulmonary, CNS, or cutaneous disease and appropriate risk factors. Pulmonary disease is most common, seen in approximately two‐thirds of patients, and is typically bilateral. Chest radiographic findings include infiltrates (59%), nodules (35%), effusions, and cavities.2 Up to half of all cases of pulmonary nocardiosis are associated with hematogenous dissemination, most commonly to the CNS, where manifestations include incidentally discovered asymptomatic lesions, headache, confusion, and focal neurologic deficits; meningitis is rare.1 CNS involvement and severe predisposing illness are adverse prognostic markers.
Diagnosis of nocardiosis is typically delayed by 6 weeks to 1 year.4, 5 This has been attributed to its rarity, its nonspecific and indolent presentation, its slow growth, and the difficulty isolating Nocardia from clinical specimens. Although Nocardia may disseminate widely to almost any site, isolation of Nocardia from blood cultures is rare. Clinicians must rely on sputum or tissue samples to demonstrate the characteristic Gram‐positive rods which stain weakly on acid‐fast preparations. Polymerase chain reaction (PCR)‐based tests improve the yield but are not routinely available.
The standard antibiotic for the treatment of Nocardia infections is trimethoprim‐sulfamethoxazole (TMP‐SMX) which has excellent CNS penetration. In patients with pulmonary disease or CNS dissemination, a second parenteral antimicrobial (usually amikacin or imipenem) is typically added to TMP‐SMX, and treatment is extended to 12 months or longer.6, 7 Prophylaxis with TMP‐SMX, which is usually prescribed to prevent Pneumocystis jirovecii in susceptible hosts, also reduces the incidence of Nocardia.2, 3, 6 Nocardia's restricted susceptibility pattern presents a challenge for hospitalists, as TMP‐SMX and aminoglycosides are rarely administered empirically for cases of suspected pneumonia or atypical pulmonary infections (other than P. jirovecii).
When confronted with the pattern of simultaneous pulmonary and CNS lesions, hospitalists must consider infections (lung abscess, mycobacteria, fungi, Nocardia), malignancies, and autoimmune conditions (sarcoidosis, Wegener's granulomatosis). This patient's weakness was a direct result of his weakened immune system, which allowed this weakly acid‐fast organism to flourish. Only by recognizing the possibility of nocardiosis (eg, a patient receiving steroids who develops pulmonary and CNS lesions) is there hope for early diagnosis and treatment.
TEACHING POINTS
-
Suspect disseminated nocardiosis in immunocompromised patients with unexplained pulmonary disease and CNS disease characterized by multiple ring‐enhancing abscesses.
-
Corticosteroid treatment is the most common risk factor for Nocardia infections. Patients taking prednisone at doses in excess of 10 mg daily for greater than 3 months should receive P. jirovecii prophylaxis with TMP‐SMX, which also reduces the incidence of Nocardia.
-
Prolonged courses of TMP‐SMX combined with at least 1 other agent for at least 612 months are typically required to treat disseminated Nocardia.
Acknowledgements
Disclosure: Dr Thomas E. Baudendistel is a former Deputy Editor at the Journal of Hospital Medicine and received a stipend for this work.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
This icon represents the patient's case. Each paragraph that follows represents the discussant's thoughts.
An 89‐year‐old man presented to the emergency department with progressive fatigue, confusion, and generalized weakness over 2 months, worsening in the prior few days.
Four categories of disease account for most cases of confusion in the elderly: metabolic derangements; infection (both within and outside of the central nervous system); structural brain disorder (eg, bleed or tumor); and toxins (generally medications). It will be important early on to determine if weakness refers to true loss of motor function, reflecting a neuromuscular lesion.
At baseline, the patient had normal cognition, ambulated without assistance, and was independent in activities of daily living. Over the preceding 2 months, general functional decline, unsteady gait, balance problems, and word‐finding difficulty developed. He also needed a front‐wheel walker to avoid falling. One month prior to presentation, the patient's children noticed he was markedly fatigued and was requiring a nightly sedative‐hypnotic in order to fall asleep.
He denied any recent travel, sick contacts, or recent illness. He denied vertigo, dizziness, or syncope. He reported occasional urinary incontinence which he attributed to being too weak to get to the bathroom promptly.
This rapid progression over 2 months is not consistent with the time course of the more common neurodegenerative causes of dementia, such as Alzheimer's or Parkinson's disease. In Parkinson's, cognitive impairment is a late feature, occurring years after gait and motor disturbances develop. Normal pressure hydrocephalus, which causes the classic triad of incontinence, ataxia, and confusion, would also be unlikely to develop so abruptly. Although we do not think of vascular (multi‐infarct) dementia as having such a short time course, on occasion a seemingly rapid presentation is the postscript to a more insidious progression that has been underway for years. A subdural hematoma, which may have occurred with any of his falls, must also be considered, as should neoplastic and paraneoplastic processes.
His past medical history included paroxysmal atrial fibrillation, diabetes mellitus, hypertension, hyperlipidemia, coronary artery disease complicated by prior myocardial infarction for which he underwent coronary artery bypass grafting 7 years prior, mild aortic sclerosis and insufficiency, mild mitral regurgitation, anemia, recurrent low‐grade bladder cancer treated with serial local resections over the last 8 years, low‐grade prostate cancer which had not required treatment, hypothyroidism, chronic kidney disease, and lumbar spinal stenosis.
His atrial fibrillation and valvular disease put him at risk for thrombotic and infective embolic phenomena causing multiple cerebral infarcts. He has all the requisite underlying conditions for vascular dementia. Untreated hypothyroidism could explain his decline and sedation. Prostate and bladder cancers would be unusual causes of subacute central nervous system (CNS) disease. Finally, his chronic kidney disease may have progressed to uremia.
One year prior to admission, the patient developed bilateral shoulder pain, right‐sided headache with loss of vision in his right eye, fevers, and an elevated erythrocyte sedimentation rate (ESR). Although temporal artery biopsy specimens did not reveal arterial inflammation, he was started on high‐dose prednisone for polymyalgia rheumatica and giant cell arteritis (GCA); he experienced improvement in his ESR and in all symptoms, with the exception of permanent right eye blindness. Maintenance prednisone was continued for disease suppression.
Even without confirmatory biopsy results, the clinical case for GCA was compelling and the rationale for starting steroids strong; his sustained response over 1 year further supports the diagnosis. GCA is almost always confined to extracranial vessels, and altered sensorium would be an unusual manifestation. His extended treatment with prednisone expands the list of CNS and systemic infections, particularly opportunistic ones, for which he is now at risk.
Outpatient medications were prednisone at doses fluctuating between 10 and 20 mg daily, furosemide 20 mg daily, amiodarone 200 mg daily, levothyroxine 50 mcg daily, alendronate 70 mg weekly, eszopiclone 1 mg nightly, losartan 50 mg daily, and warfarin. The patient was an accomplished professor and had published a book 1 year prior to admission. He quit smoking over 30 years ago, and he occasionally drank wine. He denied any drug use.
Three months prior to the current presentation, the patient was hospitalized for right upper‐lobe pneumonia for which he received a course of doxycycline, and his symptoms improved. Follow‐up chest x‐ray, 4 weeks later (2 months prior to admission), showed only slight improvement of the right upper‐lobe opacity.
Leading possibilities for the persistent lung opacity are cancer and untreated infection. After 3 decades of being tobacco‐free, his smoking‐related risk of cancer is low, but remains above baseline population risk. There are at least 4 ways untreated lung cancer may render patients confused: direct metastases to the brain, carcinomatous or lymphomatous meningitis, paraneoplastic phenomenon (eg, limbic encephalitis), and metabolic derangements (eg, syndrome of inappropriate antidiuretic hormone secretion, hypercalcemia).
The upper‐lobe infiltrate that failed to improve with doxycycline could also reflect an aspiration pneumonia that evolved into an abscess, or an infection with mycobacteria or endemic fungi.
In the emergency department, the patient's temperature was 38.5C, blood pressure 139/56 mmHg, heart rate 92 beats per minute, respiratory rate 18 breaths per minute, and oxygen saturation while breathing ambient room air was 98%.
He was alert and well‐appearing. Jugular venous pressure was normal. The thyroid was normal. He had rhonchi in his right anterior upper chest and right lower lung base. Cardiac exam demonstrated a regular rhythm, with a 3/6 systolic murmur at the second right intercostal space that radiated to the carotids, and a 2/6 nonradiating holosystolic murmur at the apex. Abdomen was soft with no organomegaly or masses. There was no lymphadenopathy, and his extremities showed no clubbing or edema. There were multiple contusions in various stages of healing on his legs.
He was confused, had word‐finding difficulty, and frequently would lose his train of thought, stopping in mid‐sentence. He had no dysarthria. Cranial nerves were normal, except for reduced visual acuity and diminished pupillary response to light in his right pupil, which had been previously documented. Finger‐to‐nose testing was slow bilaterally, but was more sluggish on the right. Rapid alternating hand movements were intact. He was unable to perform heel‐to‐shin testing. Sensation was intact. Plantar reflexes were flexor bilaterally. Strength in his limbs was preserved both distally and proximally, and deep tendon reflexes were normal. However, he was unable to sit up or stand on his own due to weakness.
The fever on prednisone is a red flag for infection. The infection may be the primary diagnosis (eg, meningoencephalitis) or may reflect an additional superimposed insult (eg, urinary tract infection) on the underlying encephalopathy. Two murmurs in a febrile patient with the multifocal CNS findings suggest endocarditis. The abnormalities on chest examination could indicate a lung infection complicated by hematogenous spread to the brain, such as a lung abscess (secondary to the aspiration event), tuberculosis (TB), or endemic fungal infection.
Serum chemistries were normal, and the serum creatinine was 1.1 mg/dL. White blood cell count was 20,100 per mm3 with 90% neutrophils, 9% lymphocytes, and 1% monocytes. Hemoglobin was 13.7 g/dL, platelet count was 464,000 per mm3. Thyroid stimulating hormone (TSH) was 6.0 IU/mL (normal, <5.5). International normalized ratio (INR) was 2.2. Urinalysis was normal. Transaminases, bilirubin, and alkaline phosphatase were normal. Lactate was 1.9 mmol/L.
Electrocardiogram (EKG) was unchanged from his baseline. ESR was >120 mm/hr (the maximum reportable value); his ESR measurements had been gradually rising during the previous 4 months. Chest x‐ray demonstrated a right upper‐lobe opacity, slightly more pronounced in comparison with chest x‐ray 2 months earlier.
His fever, leukocytosis, elevated ESR, and thrombocytosis all reflect severe inflammation. While infection and then malignancy remain the primary considerations, a third category of inflammatory diseaseautoimmunitywarrants mention. For instance, Wegener's granulomatosis can cause pulmonary and CNS disease in the elderly.
Intravenous ceftriaxone and oral doxycycline were administered. Chest computed tomography (CT) (Figure 1) demonstrated dense right upper‐lobe mass‐like consolidation with associated adenopathy and pleural effusion; in addition, several nodules were present in the left and right lower lobes, the largest of which was 10 mm. CT of the chest 10 months prior to current admission had been normal. CT of the brain, performed without contrast, demonstrated multiple areas of abnormal vasogenic edema with suggestion of underlying masses.

The imaging provides evidence of a combined pulmonaryCNS syndrome. It is far more common for disease to originate in the lungs (a common portal of entry and environmental exposure) and spread to the brain than vice versa. The list of diseases and pathogens that affect the lungs and spread to the brain includes: primary lung cancer, lymphoma, bacteria, mycobacteria, fungi, molds (eg, Aspergillus), Wegener's granulomatosis, and lymphomatoid granulomatosis. Bacterial lung abscess, such as that caused by Streptococcus milleri group, may spread to the brain. Nocardia, a ubiquitous soil organism, infects immunocompromised patients and causes a similar pattern. Actinomycosis is an atypical infection that may mimic cancer, particularly in the lungs; while head and neck disease is characteristic, CNS involvement is less so. Overall, the imaging does not specifically pinpoint 1 entity, but infection remains heavily favored over malignancy, with autoimmunity a distant third.
Respiratory cultures showed normal respiratory flora. Blood cultures grew no organisms. Two samples of induced sputum were negative for acid‐fast bacilli (AFB) on smear examination. Forty‐eight hours after a purified protein derivative (PPD) skin test was placed, there was 0 mm of induration. Magnetic resonance imaging (MRI) of the brain (Figure 2) demonstrated 8 ring‐enhancing supratentorial lesions at the graywhite junction.

Negative blood cultures substantially lower the probability of bacterial endocarditis; there are no epidemiologic risk factors for the rare causes of culture‐negative endocarditis (eg, farm exposure, homelessness). Two negative smears for AFB with dense pulmonary or cavitary disease signify a low probability of tuberculosis.
In the setting of depressed cell‐mediated immunity (eg, human immunodeficiency virus [HIV] infection or chronic prednisone use), multiple ring‐enhancing CNS lesions are a classic appearance of toxoplasmosis, but they also are typical of bacterial brain abscesses and Nocardia. Brain metastases are usually solid, but as central necrosis develops, peripheral enhancement may appear. The diffuse distribution and the localization at the graywhite junction further support a hematogenously disseminated process, but do not differentiate infection from metastases.
Transthoracic echocardiogram demonstrated normal left ventricular ejection fraction, clinically insignificant aortic sclerosis and mitral regurgitation, and no evidence of vegetations. Results of a CT‐guided fine‐needle aspiration of the lung were nondiagnostic, showing necropurulent material and benign lung parenchyma with fibrosis. A core biopsy of the lung showed alveolar tissue with patchy mild deposition of fibrinous material and rare scattered acute and chronic inflammatory cells without granulomas. Pleural fluid cytology showed reactive mesothelial cells with mixed inflammatory cells. There were no fungal elements or malignant cells.
The failure to detect malignancy after 2 biopsies and 1 thoracentesis lowers the suspicion of cancer, and thereby bolsters the probability of atypical infections which may elude diagnosis on routine cultures and biopsy. A detailed history, with attention to geographic exposures, is warranted to see which endemic mycosis would put him most at risk. Based on his California residency, disseminated coccidiomycosis or the ubiquitous Cryptococcus are conceivable. Nocardia remains a strong consideration because of his chronic immunosuppression and the lung‐CNS pattern.
Fungal stains and cultures from the biopsies and pleural fluid were negative. Serum antibodies to coccidiomycosis and serum cryptococcal antigen tests were negative. On the eighth hospital day, the microbiology lab reported a few acid‐fast bacilli from a third induced sputum sample. RNA amplification testing for Mycobacterium tuberculosis was negative.
Due to his continued decline, the patient met with the palliative care team and expressed his desire to go home with hospice. While arrangements were being made, he died later that day in the hospital.
There is reasonable evidence that tuberculosis is not the culprit pathogen here: negative PPD, 2 negative sputa in the setting of a massive necrotic lesion, and a negative RNA amplification test. Nontuberculous mycobacteria such as Mycobacterium avium complex (MAC) and M. kansasii may cause disease similar to TB, but they are usually not this difficult to identify. Nocardia is classically a weakly acid‐fast positive bacteria and fits this patient's clinical picture best.
Four colonies of Nocardia (not further speciated) were identified postmortem from the patient's sputum.
DISCUSSION
Nocardia species are ubiquitous soil‐dwelling, Gram‐positive, branching rods which are weakly positive with acid‐fast staining.1 Almost all Nocardia infections occur in patients with immune systems compromised by chronic disease (HIV, malignancy, alcoholism, chronic lung or kidney disease) or by medications. Corticosteroid treatment is the most frequent risk factor. In cases of nocardiosis in patients taking steroids, the median daily prednisone dose was 25 mg (range, 1080 mg) for a median duration of 3 months.2, 3
Nocardia should be considered in any patient with unexplained pulmonary, CNS, or cutaneous disease and appropriate risk factors. Pulmonary disease is most common, seen in approximately two‐thirds of patients, and is typically bilateral. Chest radiographic findings include infiltrates (59%), nodules (35%), effusions, and cavities.2 Up to half of all cases of pulmonary nocardiosis are associated with hematogenous dissemination, most commonly to the CNS, where manifestations include incidentally discovered asymptomatic lesions, headache, confusion, and focal neurologic deficits; meningitis is rare.1 CNS involvement and severe predisposing illness are adverse prognostic markers.
Diagnosis of nocardiosis is typically delayed by 6 weeks to 1 year.4, 5 This has been attributed to its rarity, its nonspecific and indolent presentation, its slow growth, and the difficulty isolating Nocardia from clinical specimens. Although Nocardia may disseminate widely to almost any site, isolation of Nocardia from blood cultures is rare. Clinicians must rely on sputum or tissue samples to demonstrate the characteristic Gram‐positive rods which stain weakly on acid‐fast preparations. Polymerase chain reaction (PCR)‐based tests improve the yield but are not routinely available.
The standard antibiotic for the treatment of Nocardia infections is trimethoprim‐sulfamethoxazole (TMP‐SMX) which has excellent CNS penetration. In patients with pulmonary disease or CNS dissemination, a second parenteral antimicrobial (usually amikacin or imipenem) is typically added to TMP‐SMX, and treatment is extended to 12 months or longer.6, 7 Prophylaxis with TMP‐SMX, which is usually prescribed to prevent Pneumocystis jirovecii in susceptible hosts, also reduces the incidence of Nocardia.2, 3, 6 Nocardia's restricted susceptibility pattern presents a challenge for hospitalists, as TMP‐SMX and aminoglycosides are rarely administered empirically for cases of suspected pneumonia or atypical pulmonary infections (other than P. jirovecii).
When confronted with the pattern of simultaneous pulmonary and CNS lesions, hospitalists must consider infections (lung abscess, mycobacteria, fungi, Nocardia), malignancies, and autoimmune conditions (sarcoidosis, Wegener's granulomatosis). This patient's weakness was a direct result of his weakened immune system, which allowed this weakly acid‐fast organism to flourish. Only by recognizing the possibility of nocardiosis (eg, a patient receiving steroids who develops pulmonary and CNS lesions) is there hope for early diagnosis and treatment.
TEACHING POINTS
-
Suspect disseminated nocardiosis in immunocompromised patients with unexplained pulmonary disease and CNS disease characterized by multiple ring‐enhancing abscesses.
-
Corticosteroid treatment is the most common risk factor for Nocardia infections. Patients taking prednisone at doses in excess of 10 mg daily for greater than 3 months should receive P. jirovecii prophylaxis with TMP‐SMX, which also reduces the incidence of Nocardia.
-
Prolonged courses of TMP‐SMX combined with at least 1 other agent for at least 612 months are typically required to treat disseminated Nocardia.
Acknowledgements
Disclosure: Dr Thomas E. Baudendistel is a former Deputy Editor at the Journal of Hospital Medicine and received a stipend for this work.
- ,.Nocardia species: host‐parasite relationships.Clin Microbiol Rev.1994;7:213–264.
- ,,,,,.Nocardiosis at the turn of the century.Medicine (Baltimore).2009;88:250–261.
- ,.A case series and focused review of nocardiosis: clinical and microbiologic aspects.Medicine (Baltimore).2004;83:300–313.
- ,,, et al.Pulmonary nocardiosis: risk factors and outcomes.Respirology.2007;12:394–400.
- ,.Infection with Nocardia species in Queensland. A review of 102 clinical isolates.Med J Aust.1992;156:692–697.
- .Nocardia in solid organ transplant recipients.Am J Transplant.2009;9:S70–S77.
- .Nocardiosis.Clin Infect Dis.1996;22:891–905.
- ,.Nocardia species: host‐parasite relationships.Clin Microbiol Rev.1994;7:213–264.
- ,,,,,.Nocardiosis at the turn of the century.Medicine (Baltimore).2009;88:250–261.
- ,.A case series and focused review of nocardiosis: clinical and microbiologic aspects.Medicine (Baltimore).2004;83:300–313.
- ,,, et al.Pulmonary nocardiosis: risk factors and outcomes.Respirology.2007;12:394–400.
- ,.Infection with Nocardia species in Queensland. A review of 102 clinical isolates.Med J Aust.1992;156:692–697.
- .Nocardia in solid organ transplant recipients.Am J Transplant.2009;9:S70–S77.
- .Nocardiosis.Clin Infect Dis.1996;22:891–905.
Rounding up the usual suspects
A 76‐year‐old white male presented to his primary care physician with a 40‐pound weight loss and gradual decline in function over the prior 6 months. In addition, over the previous 2 months, he had begun to suffer a constant, non‐bloody, and non‐productive cough accompanied by night sweats. Associated complaints included a decline in physical activity, increased sleep needs, decreased appetite, irritability, and generalized body aches.
The patient, an elderly man, presents with a subacute, progressive systemic illness, which appears to have a pulmonary component. Broad disease categories meriting consideration include infections such as tuberculosis, endemic fungi, and infectious endocarditis; malignancies including bronchogenic carcinoma, as well as a variety of other neoplasms; and rheumatologic conditions including temporal arteritis/polymyalgia rheumatica and Wegener's granulomatosis. His complaints of anhedonia, somnolence, and irritability, while decidedly nonspecific, raise the possibility of central nervous system involvement.
His past medical history was notable for coronary artery disease, moderate aortic stenosis, hypertension, hyperlipidemia, and chronic sinusitis. Two years ago, he had unexplained kidney failure. Anti‐neutrophilic cytoplasmic antibodies (ANCA) were present, and indirect immunoflorescence revealed a peri‐nuclear (P‐ANCA) pattern on kidney biopsy. The patient had been empirically placed on azathioprine for presumed focal segmental glomerulosclerosis (FSGS), and his renal function remained stable at an estimated glomerular filtrate rate ranging from 15 to 30 mL/min/1.73 m2. His other medications included nifedipine, metoprolol, aspirin, isosorbide mononitrate, atorvastatin, calcitriol, and docusate. His family and social histories were unremarkable, including no history of tobacco. He had no pets and denied illicit drug use. He admitted to spending a considerable amount of time gardening, including working in his yard in bare feet.
The associations of focal segmental glomerulosclerosis, if indeed this diagnosis is correct, include lupus, vasculitis, and human immunodeficiency virus (HIV) infection. The nephrotic syndrome is a frequent manifestation of this entity, although, based on limited information, this patient does not appear to be clinically nephrotic. If possible, the biopsy pathology should be reviewed by a pathologist with interest in the kidney. The report of a positive P‐ANCA may not be particularly helpful here, given the frequency of false‐positive results, and in any event, P‐ANCAs have been associated with a host of conditions other than vasculitis.
The patient's gardening exposure, in bare feet no less, is intriguing. This potentially places him at risk for fungal infections including blastomycosis, histoplasmosis, cryptococcosis, and sporotrichosis. Gardening without shoes is a somewhat different enterprise in northeast Ohio than, say, Mississippi, and it will be helpful to know where this took place. Exposure in Appalachia or the South should prompt consideration of disseminated strongyloidiasis, given his azathioprine use.
Vital signs were as follows: blood pressure 151/76 mmHg, pulse 67 beats per minute, respiratory rate 20 breaths per minute, temperature 35.6C, and oxygen saturation 98% on room air. On examination, he appeared very thin but not in distress. Examination of the skin did not reveal rashes or lesions, and there was no lymphadenopathy. His thyroid was symmetric and normal in size. Lungs were clear to auscultation, and cardiac exam revealed a regular rate with a previously documented III/VI holosystolic murmur over the aortic auscultatory area. Abdominal exam revealed no organomegaly or tenderness. Joints were noted to be non‐inflamed, and extremities non‐edematous. Radial, brachial, popliteal, and dorsalis pedis pulses were normal bilaterally. A neurological exam revealed no focal deficits.
The physical examination does not help to substantively narrow or redirect the differential diagnosis. Although he appears to be tachypneic, this may simply reflect charting artifact. At this point, I would like to proceed with a number of basic diagnostic studies. In addition to complete blood count with differential, chemistries, and liver function panel, I would also obtain a thyroid stimulating hormone (TSH) assay, urinalysis, blood cultures, erythrocyte sedimentation rate/C‐reactive protein, a HIV enzyme‐linked immunosorbent assay (ELISA), chest radiograph, and a repeat ANCA panel. A purified protein derivative (PPD) skin test should be placed.
Blood chemistries were as follows: glucose 88 mg/dL, blood urea nitrogen (BUN) 48 mg/dL, creatinine 2.71 mg/dL, sodium 139 mmol/L, potassium 5.5 mmol/L, chloride 103 mmol/L, CO2 28 mmol/L, and anion gap 8 mmol/L. TSH, urinalysis, and PPD tests were unremarkable. His white blood cell count (WBC) was 33.62 K/L with 94% eosinophils and an absolute eosinophil count of 31.6 K/L. His platelet count was 189 K/L, hemoglobin 12.1 g/dL, and hematocrit 36.9%. A chest x‐ray revealed reticular opacities in the mid‐to‐lower lungs, and subsequent computed tomography (CT) scan of the chest demonstrated multiple bilateral indeterminate nodules and right axillary adenopathy.
The patient's strikingly elevated absolute eosinophil count is a very important clue that helps to significantly focus the diagnostic possibilities. In general, an eosinophilia this pronounced signifies one of several possibilities, including primary hypereosinophilic syndrome, ChurgStrauss syndrome, parasitic infection with an active tissue migration phase, eosinophilic leukemia, and perhaps chronic eosinophilic pneumonia. In addition, Wegener's granulomatosis still merits consideration, although an eosinophil count this high would certainly be unusual.
Of the above possibilities, ChurgStrauss seems less likely given his apparent absence of a history of asthma. Parasitic infections, particularly ascariasis but also strongyloidiasis, hookworm, and even visceral larva migrans are possible, although we have not been told whether geographical exposure exists to support the first 3 of these. Hypereosinophilic syndrome remains a strong consideration, although the patient does not yet clearly meet criteria for this diagnosis.
At this juncture, I would send stool and sputum for ova and parasite exam, and order Strongyloides serology, have the peripheral smear reviewed by a pathologist, await the repeat ANCA studies, and consider obtaining hematology consultation.
Tests for anti‐Smith, anti‐ribonuclear (RNP), anti‐SSA, anti‐SSB, anti‐centromere, anti‐Scl 70, and anti‐Jo antibodies were negative. Repeat ANCA testing was positive with P‐ANCA pattern on indirect immunofluorescence. His erythrocyte sedimentation rate and C‐reactive Protein (CRP) were mildly elevated at 29 mm/hr and 1.1 mg/dL, respectively. An immunodeficiency panel work‐up consisting of CD3, CD4, CD8, CD19, T‐cell, B‐cell, and natural killer (NK) cell differential counts demonstrated CD8 T‐cell depletion. Blood cultures demonstrated no growth at 72 hours. No definite M protein was identified on serum and urine protein electrophoresis. Strongyloides IgG was negative. HIV ELISA was negative. A serologic fungal battery to measure antibodies against Aspergillus, Blastomyces, Histoplasma, and Coccidiodes was negative. A microscopic examination of stool and sputum for ova and parasites was also negative. A peripheral blood smear showed anisocytosis and confirmed the elevated eosinophil count.
The preceding wealth of information helps to further refine the picture. The positive P‐ANCA by ELISA as well as immunofluorescence suggests this is a real phenomenon, and makes ChurgStrauss syndrome more likely, despite the absence of preceding or concurrent asthma. I am not aware of an association between P‐ANCA and hypereosinophilic syndrome, nor of a similar link to either chronic eosinophilic pneumonia or hematological malignancies. Although I would like to see 2 additional stool studies for ova and parasites performed by an experienced laboratory technician before discarding the diagnosis of parasitic infection entirely, I am increasingly suspicious that this patient has a prednisone‐deficient state, most likely ChurgStrauss syndrome. I am uncertain of the relationship between his more recent symptoms and his pre‐existing kidney disease, but proceeding to lung biopsy appears to be appropriate.
Bronchoscopic examination with accompanying bronchoalveolar lavage (BAL) and transbronchial biopsy were performed. The BAL showed many Aspergillus fumigatus as well as hemosiderin‐laden macrophages, and the biopsy demonstrated an eosinophilic infiltrate throughout the interstitia, alveolar spaces, and bronchiolar walls. However, the airways did not show features of asthma, capillaritis, vasculitis, or granulomas. A bone marrow biopsy showed no evidence of clonal hematologic disease.
The Aspergillus recovered from BAL, although unexpected, probably does not adequately explain the picture. I am not convinced that the patient has invasive aspergillosis, and although components of the case are consistent with allergic bronchopulmonary aspergillosis, the absence of an asthma history and the extreme degree of peripheral eosinophilia seem to speak against this diagnosis. The biopsy does not corroborate a vasculitic process, but the yield of transbronchial biopsy is relatively low in this setting, and the pulmonary vasculitides remain in play unless a more substantial biopsy specimen is obtained. It is worth noting that high‐dose corticosteroids are a risk factor for the conversion of Aspergillus colonization to invasive aspergillosis, and treatment with voriconazole would certainly be appropriate if prednisone was to be initiated.
I believe ChurgStrauss syndrome, hypereosinophilic syndrome, and chronic eosinophilic pneumonia remain the leading diagnostic possibilities, with the P‐ANCA likely serving as a red herring if the diagnosis turns out to be one of the latter entities. An open lung biopsy would be an appropriate next step, after first obtaining those additional ova and parasite exams for completeness.
An infectious diseases specialist recommended that the patient be discharged on voriconazole 300 mg PO bid for Aspergillus colonization with an underlying lung disease and likely allergic bronchopulmonary aspergillosis or invasive aspergillosis. Steroid therapy was contemplated but not initiated.
Three weeks later, the patient re‐presented with worsening of fatigue and cognitive deterioration marked by episodes of confusion and word‐finding difficulties. His WBC had increased to 45.67 K/L (94% eosinophils). He had now lost a total of 70 pounds, and an increase in generalized weakness was apparent. His blood pressure on presentation was 120/63 mmHg, pulse rate 75 beats per minute, respiratory rate 18 breaths per minute, temperature 35.8C, and oxygen saturation 97% on room air. He appeared cachectic, but not in overt distress. His skin, head, neck, chest, cardiac, abdominal, peripheral vascular, and neurological exam demonstrated no change from the last admission. A follow‐up chest x‐ray showed mild pulmonary edema and new poorly defined pulmonary nodules in the right upper lobe. A repeat CT scan of the thorax demonstrated interval progression of ground‐glass attenuation nodules, which were now more solid‐appearing and increased in number, and present in all lobes of the lung. A CT of the brain did not reveal acute processes such as intracranial hemorrhage, infarction, or mass lesions. Lumbar puncture was performed, with a normal opening pressure. Analysis of the clear and colorless cerebrospinal fluid (CSF) showed 1 red blood cell count (RBC)/L, 2 WBC/L with 92% lymphocytes, glucose 68 mg/dL, and protein 39 mg/dL. CSF fungal cultures, routine cultures, venereal disease reaction level (VDRL), and cryptococcal antigen were negative. CSF cytology did not demonstrate malignant cells. Multiple ova and parasite exams obtained from the previous admission were confirmed to be negative.
The patient's continued deterioration points to either ChurgStrauss syndrome or hypereosinophilic syndrome, I believe. His renal function and P‐ANCA (if related) support the former possibility, while the development of what now appear to be clear encephalopathic symptoms are more in favor of the latter. I would initiate steroid therapy while proceeding to an open lung biopsy in an effort to secure a definitive diagnosis, again under the cover of voriconazole, and would ask for hematology input if this had not already been obtained.
A video‐assisted right thoracoscopy with wedge resection of 2 visible nodules in the right lower lobe was performed. The biopsy conclusively diagnosed a peripheral T‐cell lymphoma. The patient's condition deteriorated, and ultimately he and his family chose a palliative approach.
COMMENTARY
Eosinophils are cells of myeloid lineage that contain cationic‐rich protein granules that mediate allergic response, reaction to parasitic infections, tissue inflammation, and immune modulation.1, 2 Eosinophilia (absolute eosinophil count 600 cells/L) suggests the possibility of a wide array of disorders. The degree of eosinophilia can be categorized as mild (6001500 cells/L), moderate (15005000 cells/L), or severe (>5000 cells/L).3 It may signify a reactive phenomenon (secondary) or, less commonly, either an underlying hematological neoplasm (primary) or an idiopathic process.2 Clinicians faced with an unexplained eosinophilia should seek the most frequent causes first.
Initial investigation should include a careful travel history; consideration of both prescription and over‐the‐counter medications, especially non‐steroidal anti‐inflammatory drugs (NSAIDs), with withdrawal of non‐essential agents; serology for Strongyloides stercoralis antibodies (and possibly other helminths, depending on potential exposure) should be assessed; and stool examinations for ova and parasites should be obtained. The possibility of a wide variety of other potential causes of eosinophilia (Table 1) should be entertained,413 and a careful search for end‐organ damage related to eosinophilic infiltration should be performed if eosinophilia is moderate or severe.1
| Differential Diagnoses | Comments |
|---|---|
| Asthma and common allergic diseases (atopic dermatitis, allergic rhinitis) | Levels >1500 cell/l are uncommon |
| Paraneoplastic eosinophilia | Associated with adenocarcinomas, Hodgkin disease, T‐cell lymphomas, and systemic mastocytosis |
| Drugs and drug‐associated eosinophilic syndromes | Commonly associated with antibiotics (especially B‐lactams) and anti‐epileptic drugs |
| Immunodeficiency disorders | Hyper‐IgE syndrome and Omenn syndrome are rare causes of eosinophilia |
| Adrenal insufficiency | Important consideration in the critical care setting because endogenous glucocorticoids are involved in the stimulation of eosinophil apoptosis |
| Organ‐specific eosinophilic disorders | Examples: acute and chronic eosinophilic pneumonia, gastrointestinal eosinophilic disorders (esophagitis, colitis) |
| Primary eosinophilia: clonal or idiopathic | Clonal eosinophilia has histologic, cytogenetic, or molecular evidence of an underlying myeloid malignancy |
| Helminthic infections | An active tissue migration phase may manifest with hypereosinophilia |
| Hypereosinophilic syndrome | Classic criteria: hypereosinophilia for at least 6 mo, exclusion of both secondary and clonal eosinophilia, and evidence of organ involvement |
| ChurgStrauss syndrome | Hypereosinophilia with asthma, systemic vasculitis, migratory pulmonary infiltrates, sinusitis, and extravascular eosinophils |
| Allergic bronchopulmonary aspergillosis (ABPA) | Major criteria: history of asthma, central bronchiectasis, immediate skin reactivity to Aspergillus, elevated total serum IgE (>1000 ng/mL), elevated IgE or IgG to Aspergillus |
Hypereosinophilia is defined as an eosinophil level greater than 1500 cells/L. These levels may be associated with end‐organ damage regardless of the underlying etiology, although the degree of eosinophilia frequently does not correlate closely with eosinophilic tissue infiltration. As a result, relatively modest degrees of peripheral eosinophilia may be seen in association with end‐organ damage, while severe eosinophilia may be tolerated well for prolonged periods in other cases.1 The most serious complications of hypereosinophilia are myocardial damage with ultimate development of cardiac fibrosis and refractory heart failure; pulmonary involvement with hypoxia; and involvement of both the central and peripheral nervous systems including stroke, encephalopathy, and mononeuritis multiplex. A number of studies should be considered to help evaluate for the possibility of end‐organ damage as well as to assess for the presence of primary and idiopathic causes of hypereosinophilia. These include peripheral blood smear looking particularly for dysplastic eosinophils or blasts, serum tryptase, serum vitamin B12, serum IgE, cardiac troponin levels, anti‐neutrophil cytoplasmic antibody, electrocardiography, echocardiography, pulmonary function tests, and thoracoabdominal CT scanning. Endoscopic studies with esophageal, duodenal, and colonic biopsy should be performed if eosinophilic gastroenteritis is suspected.1, 7, 10
While more modest degrees of eosinophilia are associated with a plethora of conditions, severe eosinophilia, especially that approaching the levels displayed by this patient, suggests a much more circumscribed differential diagnosis. This should prompt consideration of ChurgStrauss syndrome, parasitic infection with an active tissue migration phase, and hypereosinophilic syndrome (HES).4 HES has classically been characterized by hypereosinophilia for at least 6 months, exclusion of both secondary and clonal eosinophilia, and evidence of end‐organ involvement. More recently, however, a revised definition consisting of marked eosinophilia with reasonable exclusion of other causes has gained favor.1, 7, 10, 1416 While perhaps as many as 75% of cases of HES continue to be considered idiopathic at present, 2 subtypes have now been recognized, with important prognostic and therapeutic implications. Myeloproliferative HES has a strong male predominance, is frequently associated with elevated serum tryptase and B12 levels, often manifests with hepatosplenomegaly, and displays a characteristic gene mutation, FIP1L1/PDGFRA. Lymphocytic HES is typified by polyclonal eosinophilic expansion in response to elevated IL‐5 levels, is associated with less cardiac involvement and a somewhat more favorable prognosis in the absence of therapy, and has been associated with transformation into T‐cell lymphoma.1, 1417 We suspect, though we are unable to prove, that our patient was finally diagnosed at the end of a journey that began as lymphocytic HES and ultimately progressed to T‐cell lymphoma. T‐cell lymphoma has rarely been associated with profound eosinophilia. This appears to reflect disordered production of IL‐5, as was true of this patient, and many of these cases may represent transformed lymphocytic HES.14
Specific therapy exists for the myeloproliferative subtype of HES, consisting of the tyrosine kinase inhibitor imatinib, with excellent response in typical cases. Initial treatment of most other extreme eosinophilic syndromes not caused by parasitic infection, including lymphocytic and idiopathic HES as well as ChurgStrauss syndrome, consists of high‐dose corticosteroids, with a variety of other agents used as second‐line and steroid‐sparing treatments. The urgency of therapy is dictated by the presence and severity of end‐organ damage, and in some instances corticosteroids may need to be given before the diagnosis is fully secure. When S. stercoralis infection has not been ruled out, concurrent therapy with ivermectin should be given to prevent triggering Strongyloides hyperinfection. Hematology input is critical when HES is under serious consideration, with bone marrow examination, cytogenetic studies, T‐cell phenotyping and T‐cell receptor rearrangement studies essential in helping to establish the correct diagnosis.10, 17
The differential diagnosis of peripheral eosinophilia is broad and requires a thorough, stepwise approach. Although profound eosinophilia is usually caused by a limited number of diseases, this patient reminds us that Captain Renault's advice in the film Casablanca to round up the usual suspects does not always suffice, as the diagnosis of T‐cell lymphoma was not considered by either the clinicians or the discussant until lung biopsy results became available. Most patients with hypereosinophilia not caused by parasitic infection will ultimately require an invasive procedure to establish a diagnosis, which is essential before embarking on an often‐toxic course of therapy, as well as for providing an accurate prognosis.
TEACHING POINTS
-
The most common causes of eosinophilia include helminthic infections (the leading cause worldwide), asthma, allergic conditions (the leading cause in the United States), malignancies, and drugs.
-
Hypereosinophilia may lead to end‐organ damage. The most important etiologies include ChurgStrauss Syndrome, HES, or a helminthic infection in the larval migration phase.
-
The mainstay of therapy for most cases of HES is corticosteroids. The goal of therapy is to prevent, or ameliorate, end‐organ damage.
- ,.Practical approach to the patient with hypereosinophilia.J Allergy Clin Immunol.2010;126(1):39–44.
- ,,.Eosinophilia: secondary, clonal and idiopathic.Br J Haematol.2006;133(5):468–492.
- .Blood eosinophilia: a new paradigm in disease classification, diagnosis, and treatment.Mayo Clin Proc.2005;80(1):75–83.
- ,,,.Clinical manifestations and treatment of Churg‐Strauss syndrome.Rheum Dis Clin North Am.2010;36(3):527–543.
- ,,,.Relative eosinophilia and functional adrenal insufficiency in critically ill patients.Lancet.1999;353(9165):1675–1676.
- ,,,.Opposing effects of glucocorticoids on the rate of apoptosis in neutrophilic and eosinophilic granulocytes.J Immunol.1996;156(10):4422–4428.
- ,.Eosinophilic disorders.J Allergy Clin Immunol.2007;119(6):1291–1300; quiz 1301–1302.
- ,.Pulmonary eosinophilia.Clin Rev Allergy Immunol.2008;34(3):367–371.
- .Eosinophilic diseases of the gastrointestinal tract.Scand J Gastroenterol.2010;45(9):1013–1021.
- ,,.Hypereosinophilic syndrome and clonal eosinophilia: point‐of‐care diagnostic algorithm and treatment update.Mayo Clin Proc.2010;85(2):158–164.
- ,,,,,.Eosinophilia as a predictor of food allergy in atopic dermatitis.Allergy Asthma Proc.2010;31(2):e18–e24.
- ,,, et al.The American College of Rheumatology 1990 criteria for the classification of Churg‐Strauss syndrome (allergic granulomatosis and angiitis).Arthritis Rheum.1990;33(8):1094–1100.
- .Allergic bronchopulmonary aspergillosis. In: Adkinson NF, Yunginger JW, Busse WW, et al, eds. Middleton's Allergy Principles 2003:1353–1371.
- ,,, et al.TARC and IL‐5 expression correlates with tissue eosinophilia in peripheral T‐cell lymphomas.Leuk Res.2008;32(9):1431–1438.
- ,,.Hypereosinophilic syndrome and proliferative diseases.Acta Dermatovenerol Croat.2009;17(4):323–330.
- ,.The hypereosinophilic syndromes: current concepts and treatments.Br J Haematol.2009;145(3):271–285.
- ,,.Lymphocytic variant hypereosinophilic syndromes.Immunol Allergy Clin North Am.2007;27(3):389–413.
A 76‐year‐old white male presented to his primary care physician with a 40‐pound weight loss and gradual decline in function over the prior 6 months. In addition, over the previous 2 months, he had begun to suffer a constant, non‐bloody, and non‐productive cough accompanied by night sweats. Associated complaints included a decline in physical activity, increased sleep needs, decreased appetite, irritability, and generalized body aches.
The patient, an elderly man, presents with a subacute, progressive systemic illness, which appears to have a pulmonary component. Broad disease categories meriting consideration include infections such as tuberculosis, endemic fungi, and infectious endocarditis; malignancies including bronchogenic carcinoma, as well as a variety of other neoplasms; and rheumatologic conditions including temporal arteritis/polymyalgia rheumatica and Wegener's granulomatosis. His complaints of anhedonia, somnolence, and irritability, while decidedly nonspecific, raise the possibility of central nervous system involvement.
His past medical history was notable for coronary artery disease, moderate aortic stenosis, hypertension, hyperlipidemia, and chronic sinusitis. Two years ago, he had unexplained kidney failure. Anti‐neutrophilic cytoplasmic antibodies (ANCA) were present, and indirect immunoflorescence revealed a peri‐nuclear (P‐ANCA) pattern on kidney biopsy. The patient had been empirically placed on azathioprine for presumed focal segmental glomerulosclerosis (FSGS), and his renal function remained stable at an estimated glomerular filtrate rate ranging from 15 to 30 mL/min/1.73 m2. His other medications included nifedipine, metoprolol, aspirin, isosorbide mononitrate, atorvastatin, calcitriol, and docusate. His family and social histories were unremarkable, including no history of tobacco. He had no pets and denied illicit drug use. He admitted to spending a considerable amount of time gardening, including working in his yard in bare feet.
The associations of focal segmental glomerulosclerosis, if indeed this diagnosis is correct, include lupus, vasculitis, and human immunodeficiency virus (HIV) infection. The nephrotic syndrome is a frequent manifestation of this entity, although, based on limited information, this patient does not appear to be clinically nephrotic. If possible, the biopsy pathology should be reviewed by a pathologist with interest in the kidney. The report of a positive P‐ANCA may not be particularly helpful here, given the frequency of false‐positive results, and in any event, P‐ANCAs have been associated with a host of conditions other than vasculitis.
The patient's gardening exposure, in bare feet no less, is intriguing. This potentially places him at risk for fungal infections including blastomycosis, histoplasmosis, cryptococcosis, and sporotrichosis. Gardening without shoes is a somewhat different enterprise in northeast Ohio than, say, Mississippi, and it will be helpful to know where this took place. Exposure in Appalachia or the South should prompt consideration of disseminated strongyloidiasis, given his azathioprine use.
Vital signs were as follows: blood pressure 151/76 mmHg, pulse 67 beats per minute, respiratory rate 20 breaths per minute, temperature 35.6C, and oxygen saturation 98% on room air. On examination, he appeared very thin but not in distress. Examination of the skin did not reveal rashes or lesions, and there was no lymphadenopathy. His thyroid was symmetric and normal in size. Lungs were clear to auscultation, and cardiac exam revealed a regular rate with a previously documented III/VI holosystolic murmur over the aortic auscultatory area. Abdominal exam revealed no organomegaly or tenderness. Joints were noted to be non‐inflamed, and extremities non‐edematous. Radial, brachial, popliteal, and dorsalis pedis pulses were normal bilaterally. A neurological exam revealed no focal deficits.
The physical examination does not help to substantively narrow or redirect the differential diagnosis. Although he appears to be tachypneic, this may simply reflect charting artifact. At this point, I would like to proceed with a number of basic diagnostic studies. In addition to complete blood count with differential, chemistries, and liver function panel, I would also obtain a thyroid stimulating hormone (TSH) assay, urinalysis, blood cultures, erythrocyte sedimentation rate/C‐reactive protein, a HIV enzyme‐linked immunosorbent assay (ELISA), chest radiograph, and a repeat ANCA panel. A purified protein derivative (PPD) skin test should be placed.
Blood chemistries were as follows: glucose 88 mg/dL, blood urea nitrogen (BUN) 48 mg/dL, creatinine 2.71 mg/dL, sodium 139 mmol/L, potassium 5.5 mmol/L, chloride 103 mmol/L, CO2 28 mmol/L, and anion gap 8 mmol/L. TSH, urinalysis, and PPD tests were unremarkable. His white blood cell count (WBC) was 33.62 K/L with 94% eosinophils and an absolute eosinophil count of 31.6 K/L. His platelet count was 189 K/L, hemoglobin 12.1 g/dL, and hematocrit 36.9%. A chest x‐ray revealed reticular opacities in the mid‐to‐lower lungs, and subsequent computed tomography (CT) scan of the chest demonstrated multiple bilateral indeterminate nodules and right axillary adenopathy.
The patient's strikingly elevated absolute eosinophil count is a very important clue that helps to significantly focus the diagnostic possibilities. In general, an eosinophilia this pronounced signifies one of several possibilities, including primary hypereosinophilic syndrome, ChurgStrauss syndrome, parasitic infection with an active tissue migration phase, eosinophilic leukemia, and perhaps chronic eosinophilic pneumonia. In addition, Wegener's granulomatosis still merits consideration, although an eosinophil count this high would certainly be unusual.
Of the above possibilities, ChurgStrauss seems less likely given his apparent absence of a history of asthma. Parasitic infections, particularly ascariasis but also strongyloidiasis, hookworm, and even visceral larva migrans are possible, although we have not been told whether geographical exposure exists to support the first 3 of these. Hypereosinophilic syndrome remains a strong consideration, although the patient does not yet clearly meet criteria for this diagnosis.
At this juncture, I would send stool and sputum for ova and parasite exam, and order Strongyloides serology, have the peripheral smear reviewed by a pathologist, await the repeat ANCA studies, and consider obtaining hematology consultation.
Tests for anti‐Smith, anti‐ribonuclear (RNP), anti‐SSA, anti‐SSB, anti‐centromere, anti‐Scl 70, and anti‐Jo antibodies were negative. Repeat ANCA testing was positive with P‐ANCA pattern on indirect immunofluorescence. His erythrocyte sedimentation rate and C‐reactive Protein (CRP) were mildly elevated at 29 mm/hr and 1.1 mg/dL, respectively. An immunodeficiency panel work‐up consisting of CD3, CD4, CD8, CD19, T‐cell, B‐cell, and natural killer (NK) cell differential counts demonstrated CD8 T‐cell depletion. Blood cultures demonstrated no growth at 72 hours. No definite M protein was identified on serum and urine protein electrophoresis. Strongyloides IgG was negative. HIV ELISA was negative. A serologic fungal battery to measure antibodies against Aspergillus, Blastomyces, Histoplasma, and Coccidiodes was negative. A microscopic examination of stool and sputum for ova and parasites was also negative. A peripheral blood smear showed anisocytosis and confirmed the elevated eosinophil count.
The preceding wealth of information helps to further refine the picture. The positive P‐ANCA by ELISA as well as immunofluorescence suggests this is a real phenomenon, and makes ChurgStrauss syndrome more likely, despite the absence of preceding or concurrent asthma. I am not aware of an association between P‐ANCA and hypereosinophilic syndrome, nor of a similar link to either chronic eosinophilic pneumonia or hematological malignancies. Although I would like to see 2 additional stool studies for ova and parasites performed by an experienced laboratory technician before discarding the diagnosis of parasitic infection entirely, I am increasingly suspicious that this patient has a prednisone‐deficient state, most likely ChurgStrauss syndrome. I am uncertain of the relationship between his more recent symptoms and his pre‐existing kidney disease, but proceeding to lung biopsy appears to be appropriate.
Bronchoscopic examination with accompanying bronchoalveolar lavage (BAL) and transbronchial biopsy were performed. The BAL showed many Aspergillus fumigatus as well as hemosiderin‐laden macrophages, and the biopsy demonstrated an eosinophilic infiltrate throughout the interstitia, alveolar spaces, and bronchiolar walls. However, the airways did not show features of asthma, capillaritis, vasculitis, or granulomas. A bone marrow biopsy showed no evidence of clonal hematologic disease.
The Aspergillus recovered from BAL, although unexpected, probably does not adequately explain the picture. I am not convinced that the patient has invasive aspergillosis, and although components of the case are consistent with allergic bronchopulmonary aspergillosis, the absence of an asthma history and the extreme degree of peripheral eosinophilia seem to speak against this diagnosis. The biopsy does not corroborate a vasculitic process, but the yield of transbronchial biopsy is relatively low in this setting, and the pulmonary vasculitides remain in play unless a more substantial biopsy specimen is obtained. It is worth noting that high‐dose corticosteroids are a risk factor for the conversion of Aspergillus colonization to invasive aspergillosis, and treatment with voriconazole would certainly be appropriate if prednisone was to be initiated.
I believe ChurgStrauss syndrome, hypereosinophilic syndrome, and chronic eosinophilic pneumonia remain the leading diagnostic possibilities, with the P‐ANCA likely serving as a red herring if the diagnosis turns out to be one of the latter entities. An open lung biopsy would be an appropriate next step, after first obtaining those additional ova and parasite exams for completeness.
An infectious diseases specialist recommended that the patient be discharged on voriconazole 300 mg PO bid for Aspergillus colonization with an underlying lung disease and likely allergic bronchopulmonary aspergillosis or invasive aspergillosis. Steroid therapy was contemplated but not initiated.
Three weeks later, the patient re‐presented with worsening of fatigue and cognitive deterioration marked by episodes of confusion and word‐finding difficulties. His WBC had increased to 45.67 K/L (94% eosinophils). He had now lost a total of 70 pounds, and an increase in generalized weakness was apparent. His blood pressure on presentation was 120/63 mmHg, pulse rate 75 beats per minute, respiratory rate 18 breaths per minute, temperature 35.8C, and oxygen saturation 97% on room air. He appeared cachectic, but not in overt distress. His skin, head, neck, chest, cardiac, abdominal, peripheral vascular, and neurological exam demonstrated no change from the last admission. A follow‐up chest x‐ray showed mild pulmonary edema and new poorly defined pulmonary nodules in the right upper lobe. A repeat CT scan of the thorax demonstrated interval progression of ground‐glass attenuation nodules, which were now more solid‐appearing and increased in number, and present in all lobes of the lung. A CT of the brain did not reveal acute processes such as intracranial hemorrhage, infarction, or mass lesions. Lumbar puncture was performed, with a normal opening pressure. Analysis of the clear and colorless cerebrospinal fluid (CSF) showed 1 red blood cell count (RBC)/L, 2 WBC/L with 92% lymphocytes, glucose 68 mg/dL, and protein 39 mg/dL. CSF fungal cultures, routine cultures, venereal disease reaction level (VDRL), and cryptococcal antigen were negative. CSF cytology did not demonstrate malignant cells. Multiple ova and parasite exams obtained from the previous admission were confirmed to be negative.
The patient's continued deterioration points to either ChurgStrauss syndrome or hypereosinophilic syndrome, I believe. His renal function and P‐ANCA (if related) support the former possibility, while the development of what now appear to be clear encephalopathic symptoms are more in favor of the latter. I would initiate steroid therapy while proceeding to an open lung biopsy in an effort to secure a definitive diagnosis, again under the cover of voriconazole, and would ask for hematology input if this had not already been obtained.
A video‐assisted right thoracoscopy with wedge resection of 2 visible nodules in the right lower lobe was performed. The biopsy conclusively diagnosed a peripheral T‐cell lymphoma. The patient's condition deteriorated, and ultimately he and his family chose a palliative approach.
COMMENTARY
Eosinophils are cells of myeloid lineage that contain cationic‐rich protein granules that mediate allergic response, reaction to parasitic infections, tissue inflammation, and immune modulation.1, 2 Eosinophilia (absolute eosinophil count 600 cells/L) suggests the possibility of a wide array of disorders. The degree of eosinophilia can be categorized as mild (6001500 cells/L), moderate (15005000 cells/L), or severe (>5000 cells/L).3 It may signify a reactive phenomenon (secondary) or, less commonly, either an underlying hematological neoplasm (primary) or an idiopathic process.2 Clinicians faced with an unexplained eosinophilia should seek the most frequent causes first.
Initial investigation should include a careful travel history; consideration of both prescription and over‐the‐counter medications, especially non‐steroidal anti‐inflammatory drugs (NSAIDs), with withdrawal of non‐essential agents; serology for Strongyloides stercoralis antibodies (and possibly other helminths, depending on potential exposure) should be assessed; and stool examinations for ova and parasites should be obtained. The possibility of a wide variety of other potential causes of eosinophilia (Table 1) should be entertained,413 and a careful search for end‐organ damage related to eosinophilic infiltration should be performed if eosinophilia is moderate or severe.1
| Differential Diagnoses | Comments |
|---|---|
| Asthma and common allergic diseases (atopic dermatitis, allergic rhinitis) | Levels >1500 cell/l are uncommon |
| Paraneoplastic eosinophilia | Associated with adenocarcinomas, Hodgkin disease, T‐cell lymphomas, and systemic mastocytosis |
| Drugs and drug‐associated eosinophilic syndromes | Commonly associated with antibiotics (especially B‐lactams) and anti‐epileptic drugs |
| Immunodeficiency disorders | Hyper‐IgE syndrome and Omenn syndrome are rare causes of eosinophilia |
| Adrenal insufficiency | Important consideration in the critical care setting because endogenous glucocorticoids are involved in the stimulation of eosinophil apoptosis |
| Organ‐specific eosinophilic disorders | Examples: acute and chronic eosinophilic pneumonia, gastrointestinal eosinophilic disorders (esophagitis, colitis) |
| Primary eosinophilia: clonal or idiopathic | Clonal eosinophilia has histologic, cytogenetic, or molecular evidence of an underlying myeloid malignancy |
| Helminthic infections | An active tissue migration phase may manifest with hypereosinophilia |
| Hypereosinophilic syndrome | Classic criteria: hypereosinophilia for at least 6 mo, exclusion of both secondary and clonal eosinophilia, and evidence of organ involvement |
| ChurgStrauss syndrome | Hypereosinophilia with asthma, systemic vasculitis, migratory pulmonary infiltrates, sinusitis, and extravascular eosinophils |
| Allergic bronchopulmonary aspergillosis (ABPA) | Major criteria: history of asthma, central bronchiectasis, immediate skin reactivity to Aspergillus, elevated total serum IgE (>1000 ng/mL), elevated IgE or IgG to Aspergillus |
Hypereosinophilia is defined as an eosinophil level greater than 1500 cells/L. These levels may be associated with end‐organ damage regardless of the underlying etiology, although the degree of eosinophilia frequently does not correlate closely with eosinophilic tissue infiltration. As a result, relatively modest degrees of peripheral eosinophilia may be seen in association with end‐organ damage, while severe eosinophilia may be tolerated well for prolonged periods in other cases.1 The most serious complications of hypereosinophilia are myocardial damage with ultimate development of cardiac fibrosis and refractory heart failure; pulmonary involvement with hypoxia; and involvement of both the central and peripheral nervous systems including stroke, encephalopathy, and mononeuritis multiplex. A number of studies should be considered to help evaluate for the possibility of end‐organ damage as well as to assess for the presence of primary and idiopathic causes of hypereosinophilia. These include peripheral blood smear looking particularly for dysplastic eosinophils or blasts, serum tryptase, serum vitamin B12, serum IgE, cardiac troponin levels, anti‐neutrophil cytoplasmic antibody, electrocardiography, echocardiography, pulmonary function tests, and thoracoabdominal CT scanning. Endoscopic studies with esophageal, duodenal, and colonic biopsy should be performed if eosinophilic gastroenteritis is suspected.1, 7, 10
While more modest degrees of eosinophilia are associated with a plethora of conditions, severe eosinophilia, especially that approaching the levels displayed by this patient, suggests a much more circumscribed differential diagnosis. This should prompt consideration of ChurgStrauss syndrome, parasitic infection with an active tissue migration phase, and hypereosinophilic syndrome (HES).4 HES has classically been characterized by hypereosinophilia for at least 6 months, exclusion of both secondary and clonal eosinophilia, and evidence of end‐organ involvement. More recently, however, a revised definition consisting of marked eosinophilia with reasonable exclusion of other causes has gained favor.1, 7, 10, 1416 While perhaps as many as 75% of cases of HES continue to be considered idiopathic at present, 2 subtypes have now been recognized, with important prognostic and therapeutic implications. Myeloproliferative HES has a strong male predominance, is frequently associated with elevated serum tryptase and B12 levels, often manifests with hepatosplenomegaly, and displays a characteristic gene mutation, FIP1L1/PDGFRA. Lymphocytic HES is typified by polyclonal eosinophilic expansion in response to elevated IL‐5 levels, is associated with less cardiac involvement and a somewhat more favorable prognosis in the absence of therapy, and has been associated with transformation into T‐cell lymphoma.1, 1417 We suspect, though we are unable to prove, that our patient was finally diagnosed at the end of a journey that began as lymphocytic HES and ultimately progressed to T‐cell lymphoma. T‐cell lymphoma has rarely been associated with profound eosinophilia. This appears to reflect disordered production of IL‐5, as was true of this patient, and many of these cases may represent transformed lymphocytic HES.14
Specific therapy exists for the myeloproliferative subtype of HES, consisting of the tyrosine kinase inhibitor imatinib, with excellent response in typical cases. Initial treatment of most other extreme eosinophilic syndromes not caused by parasitic infection, including lymphocytic and idiopathic HES as well as ChurgStrauss syndrome, consists of high‐dose corticosteroids, with a variety of other agents used as second‐line and steroid‐sparing treatments. The urgency of therapy is dictated by the presence and severity of end‐organ damage, and in some instances corticosteroids may need to be given before the diagnosis is fully secure. When S. stercoralis infection has not been ruled out, concurrent therapy with ivermectin should be given to prevent triggering Strongyloides hyperinfection. Hematology input is critical when HES is under serious consideration, with bone marrow examination, cytogenetic studies, T‐cell phenotyping and T‐cell receptor rearrangement studies essential in helping to establish the correct diagnosis.10, 17
The differential diagnosis of peripheral eosinophilia is broad and requires a thorough, stepwise approach. Although profound eosinophilia is usually caused by a limited number of diseases, this patient reminds us that Captain Renault's advice in the film Casablanca to round up the usual suspects does not always suffice, as the diagnosis of T‐cell lymphoma was not considered by either the clinicians or the discussant until lung biopsy results became available. Most patients with hypereosinophilia not caused by parasitic infection will ultimately require an invasive procedure to establish a diagnosis, which is essential before embarking on an often‐toxic course of therapy, as well as for providing an accurate prognosis.
TEACHING POINTS
-
The most common causes of eosinophilia include helminthic infections (the leading cause worldwide), asthma, allergic conditions (the leading cause in the United States), malignancies, and drugs.
-
Hypereosinophilia may lead to end‐organ damage. The most important etiologies include ChurgStrauss Syndrome, HES, or a helminthic infection in the larval migration phase.
-
The mainstay of therapy for most cases of HES is corticosteroids. The goal of therapy is to prevent, or ameliorate, end‐organ damage.
A 76‐year‐old white male presented to his primary care physician with a 40‐pound weight loss and gradual decline in function over the prior 6 months. In addition, over the previous 2 months, he had begun to suffer a constant, non‐bloody, and non‐productive cough accompanied by night sweats. Associated complaints included a decline in physical activity, increased sleep needs, decreased appetite, irritability, and generalized body aches.
The patient, an elderly man, presents with a subacute, progressive systemic illness, which appears to have a pulmonary component. Broad disease categories meriting consideration include infections such as tuberculosis, endemic fungi, and infectious endocarditis; malignancies including bronchogenic carcinoma, as well as a variety of other neoplasms; and rheumatologic conditions including temporal arteritis/polymyalgia rheumatica and Wegener's granulomatosis. His complaints of anhedonia, somnolence, and irritability, while decidedly nonspecific, raise the possibility of central nervous system involvement.
His past medical history was notable for coronary artery disease, moderate aortic stenosis, hypertension, hyperlipidemia, and chronic sinusitis. Two years ago, he had unexplained kidney failure. Anti‐neutrophilic cytoplasmic antibodies (ANCA) were present, and indirect immunoflorescence revealed a peri‐nuclear (P‐ANCA) pattern on kidney biopsy. The patient had been empirically placed on azathioprine for presumed focal segmental glomerulosclerosis (FSGS), and his renal function remained stable at an estimated glomerular filtrate rate ranging from 15 to 30 mL/min/1.73 m2. His other medications included nifedipine, metoprolol, aspirin, isosorbide mononitrate, atorvastatin, calcitriol, and docusate. His family and social histories were unremarkable, including no history of tobacco. He had no pets and denied illicit drug use. He admitted to spending a considerable amount of time gardening, including working in his yard in bare feet.
The associations of focal segmental glomerulosclerosis, if indeed this diagnosis is correct, include lupus, vasculitis, and human immunodeficiency virus (HIV) infection. The nephrotic syndrome is a frequent manifestation of this entity, although, based on limited information, this patient does not appear to be clinically nephrotic. If possible, the biopsy pathology should be reviewed by a pathologist with interest in the kidney. The report of a positive P‐ANCA may not be particularly helpful here, given the frequency of false‐positive results, and in any event, P‐ANCAs have been associated with a host of conditions other than vasculitis.
The patient's gardening exposure, in bare feet no less, is intriguing. This potentially places him at risk for fungal infections including blastomycosis, histoplasmosis, cryptococcosis, and sporotrichosis. Gardening without shoes is a somewhat different enterprise in northeast Ohio than, say, Mississippi, and it will be helpful to know where this took place. Exposure in Appalachia or the South should prompt consideration of disseminated strongyloidiasis, given his azathioprine use.
Vital signs were as follows: blood pressure 151/76 mmHg, pulse 67 beats per minute, respiratory rate 20 breaths per minute, temperature 35.6C, and oxygen saturation 98% on room air. On examination, he appeared very thin but not in distress. Examination of the skin did not reveal rashes or lesions, and there was no lymphadenopathy. His thyroid was symmetric and normal in size. Lungs were clear to auscultation, and cardiac exam revealed a regular rate with a previously documented III/VI holosystolic murmur over the aortic auscultatory area. Abdominal exam revealed no organomegaly or tenderness. Joints were noted to be non‐inflamed, and extremities non‐edematous. Radial, brachial, popliteal, and dorsalis pedis pulses were normal bilaterally. A neurological exam revealed no focal deficits.
The physical examination does not help to substantively narrow or redirect the differential diagnosis. Although he appears to be tachypneic, this may simply reflect charting artifact. At this point, I would like to proceed with a number of basic diagnostic studies. In addition to complete blood count with differential, chemistries, and liver function panel, I would also obtain a thyroid stimulating hormone (TSH) assay, urinalysis, blood cultures, erythrocyte sedimentation rate/C‐reactive protein, a HIV enzyme‐linked immunosorbent assay (ELISA), chest radiograph, and a repeat ANCA panel. A purified protein derivative (PPD) skin test should be placed.
Blood chemistries were as follows: glucose 88 mg/dL, blood urea nitrogen (BUN) 48 mg/dL, creatinine 2.71 mg/dL, sodium 139 mmol/L, potassium 5.5 mmol/L, chloride 103 mmol/L, CO2 28 mmol/L, and anion gap 8 mmol/L. TSH, urinalysis, and PPD tests were unremarkable. His white blood cell count (WBC) was 33.62 K/L with 94% eosinophils and an absolute eosinophil count of 31.6 K/L. His platelet count was 189 K/L, hemoglobin 12.1 g/dL, and hematocrit 36.9%. A chest x‐ray revealed reticular opacities in the mid‐to‐lower lungs, and subsequent computed tomography (CT) scan of the chest demonstrated multiple bilateral indeterminate nodules and right axillary adenopathy.
The patient's strikingly elevated absolute eosinophil count is a very important clue that helps to significantly focus the diagnostic possibilities. In general, an eosinophilia this pronounced signifies one of several possibilities, including primary hypereosinophilic syndrome, ChurgStrauss syndrome, parasitic infection with an active tissue migration phase, eosinophilic leukemia, and perhaps chronic eosinophilic pneumonia. In addition, Wegener's granulomatosis still merits consideration, although an eosinophil count this high would certainly be unusual.
Of the above possibilities, ChurgStrauss seems less likely given his apparent absence of a history of asthma. Parasitic infections, particularly ascariasis but also strongyloidiasis, hookworm, and even visceral larva migrans are possible, although we have not been told whether geographical exposure exists to support the first 3 of these. Hypereosinophilic syndrome remains a strong consideration, although the patient does not yet clearly meet criteria for this diagnosis.
At this juncture, I would send stool and sputum for ova and parasite exam, and order Strongyloides serology, have the peripheral smear reviewed by a pathologist, await the repeat ANCA studies, and consider obtaining hematology consultation.
Tests for anti‐Smith, anti‐ribonuclear (RNP), anti‐SSA, anti‐SSB, anti‐centromere, anti‐Scl 70, and anti‐Jo antibodies were negative. Repeat ANCA testing was positive with P‐ANCA pattern on indirect immunofluorescence. His erythrocyte sedimentation rate and C‐reactive Protein (CRP) were mildly elevated at 29 mm/hr and 1.1 mg/dL, respectively. An immunodeficiency panel work‐up consisting of CD3, CD4, CD8, CD19, T‐cell, B‐cell, and natural killer (NK) cell differential counts demonstrated CD8 T‐cell depletion. Blood cultures demonstrated no growth at 72 hours. No definite M protein was identified on serum and urine protein electrophoresis. Strongyloides IgG was negative. HIV ELISA was negative. A serologic fungal battery to measure antibodies against Aspergillus, Blastomyces, Histoplasma, and Coccidiodes was negative. A microscopic examination of stool and sputum for ova and parasites was also negative. A peripheral blood smear showed anisocytosis and confirmed the elevated eosinophil count.
The preceding wealth of information helps to further refine the picture. The positive P‐ANCA by ELISA as well as immunofluorescence suggests this is a real phenomenon, and makes ChurgStrauss syndrome more likely, despite the absence of preceding or concurrent asthma. I am not aware of an association between P‐ANCA and hypereosinophilic syndrome, nor of a similar link to either chronic eosinophilic pneumonia or hematological malignancies. Although I would like to see 2 additional stool studies for ova and parasites performed by an experienced laboratory technician before discarding the diagnosis of parasitic infection entirely, I am increasingly suspicious that this patient has a prednisone‐deficient state, most likely ChurgStrauss syndrome. I am uncertain of the relationship between his more recent symptoms and his pre‐existing kidney disease, but proceeding to lung biopsy appears to be appropriate.
Bronchoscopic examination with accompanying bronchoalveolar lavage (BAL) and transbronchial biopsy were performed. The BAL showed many Aspergillus fumigatus as well as hemosiderin‐laden macrophages, and the biopsy demonstrated an eosinophilic infiltrate throughout the interstitia, alveolar spaces, and bronchiolar walls. However, the airways did not show features of asthma, capillaritis, vasculitis, or granulomas. A bone marrow biopsy showed no evidence of clonal hematologic disease.
The Aspergillus recovered from BAL, although unexpected, probably does not adequately explain the picture. I am not convinced that the patient has invasive aspergillosis, and although components of the case are consistent with allergic bronchopulmonary aspergillosis, the absence of an asthma history and the extreme degree of peripheral eosinophilia seem to speak against this diagnosis. The biopsy does not corroborate a vasculitic process, but the yield of transbronchial biopsy is relatively low in this setting, and the pulmonary vasculitides remain in play unless a more substantial biopsy specimen is obtained. It is worth noting that high‐dose corticosteroids are a risk factor for the conversion of Aspergillus colonization to invasive aspergillosis, and treatment with voriconazole would certainly be appropriate if prednisone was to be initiated.
I believe ChurgStrauss syndrome, hypereosinophilic syndrome, and chronic eosinophilic pneumonia remain the leading diagnostic possibilities, with the P‐ANCA likely serving as a red herring if the diagnosis turns out to be one of the latter entities. An open lung biopsy would be an appropriate next step, after first obtaining those additional ova and parasite exams for completeness.
An infectious diseases specialist recommended that the patient be discharged on voriconazole 300 mg PO bid for Aspergillus colonization with an underlying lung disease and likely allergic bronchopulmonary aspergillosis or invasive aspergillosis. Steroid therapy was contemplated but not initiated.
Three weeks later, the patient re‐presented with worsening of fatigue and cognitive deterioration marked by episodes of confusion and word‐finding difficulties. His WBC had increased to 45.67 K/L (94% eosinophils). He had now lost a total of 70 pounds, and an increase in generalized weakness was apparent. His blood pressure on presentation was 120/63 mmHg, pulse rate 75 beats per minute, respiratory rate 18 breaths per minute, temperature 35.8C, and oxygen saturation 97% on room air. He appeared cachectic, but not in overt distress. His skin, head, neck, chest, cardiac, abdominal, peripheral vascular, and neurological exam demonstrated no change from the last admission. A follow‐up chest x‐ray showed mild pulmonary edema and new poorly defined pulmonary nodules in the right upper lobe. A repeat CT scan of the thorax demonstrated interval progression of ground‐glass attenuation nodules, which were now more solid‐appearing and increased in number, and present in all lobes of the lung. A CT of the brain did not reveal acute processes such as intracranial hemorrhage, infarction, or mass lesions. Lumbar puncture was performed, with a normal opening pressure. Analysis of the clear and colorless cerebrospinal fluid (CSF) showed 1 red blood cell count (RBC)/L, 2 WBC/L with 92% lymphocytes, glucose 68 mg/dL, and protein 39 mg/dL. CSF fungal cultures, routine cultures, venereal disease reaction level (VDRL), and cryptococcal antigen were negative. CSF cytology did not demonstrate malignant cells. Multiple ova and parasite exams obtained from the previous admission were confirmed to be negative.
The patient's continued deterioration points to either ChurgStrauss syndrome or hypereosinophilic syndrome, I believe. His renal function and P‐ANCA (if related) support the former possibility, while the development of what now appear to be clear encephalopathic symptoms are more in favor of the latter. I would initiate steroid therapy while proceeding to an open lung biopsy in an effort to secure a definitive diagnosis, again under the cover of voriconazole, and would ask for hematology input if this had not already been obtained.
A video‐assisted right thoracoscopy with wedge resection of 2 visible nodules in the right lower lobe was performed. The biopsy conclusively diagnosed a peripheral T‐cell lymphoma. The patient's condition deteriorated, and ultimately he and his family chose a palliative approach.
COMMENTARY
Eosinophils are cells of myeloid lineage that contain cationic‐rich protein granules that mediate allergic response, reaction to parasitic infections, tissue inflammation, and immune modulation.1, 2 Eosinophilia (absolute eosinophil count 600 cells/L) suggests the possibility of a wide array of disorders. The degree of eosinophilia can be categorized as mild (6001500 cells/L), moderate (15005000 cells/L), or severe (>5000 cells/L).3 It may signify a reactive phenomenon (secondary) or, less commonly, either an underlying hematological neoplasm (primary) or an idiopathic process.2 Clinicians faced with an unexplained eosinophilia should seek the most frequent causes first.
Initial investigation should include a careful travel history; consideration of both prescription and over‐the‐counter medications, especially non‐steroidal anti‐inflammatory drugs (NSAIDs), with withdrawal of non‐essential agents; serology for Strongyloides stercoralis antibodies (and possibly other helminths, depending on potential exposure) should be assessed; and stool examinations for ova and parasites should be obtained. The possibility of a wide variety of other potential causes of eosinophilia (Table 1) should be entertained,413 and a careful search for end‐organ damage related to eosinophilic infiltration should be performed if eosinophilia is moderate or severe.1
| Differential Diagnoses | Comments |
|---|---|
| Asthma and common allergic diseases (atopic dermatitis, allergic rhinitis) | Levels >1500 cell/l are uncommon |
| Paraneoplastic eosinophilia | Associated with adenocarcinomas, Hodgkin disease, T‐cell lymphomas, and systemic mastocytosis |
| Drugs and drug‐associated eosinophilic syndromes | Commonly associated with antibiotics (especially B‐lactams) and anti‐epileptic drugs |
| Immunodeficiency disorders | Hyper‐IgE syndrome and Omenn syndrome are rare causes of eosinophilia |
| Adrenal insufficiency | Important consideration in the critical care setting because endogenous glucocorticoids are involved in the stimulation of eosinophil apoptosis |
| Organ‐specific eosinophilic disorders | Examples: acute and chronic eosinophilic pneumonia, gastrointestinal eosinophilic disorders (esophagitis, colitis) |
| Primary eosinophilia: clonal or idiopathic | Clonal eosinophilia has histologic, cytogenetic, or molecular evidence of an underlying myeloid malignancy |
| Helminthic infections | An active tissue migration phase may manifest with hypereosinophilia |
| Hypereosinophilic syndrome | Classic criteria: hypereosinophilia for at least 6 mo, exclusion of both secondary and clonal eosinophilia, and evidence of organ involvement |
| ChurgStrauss syndrome | Hypereosinophilia with asthma, systemic vasculitis, migratory pulmonary infiltrates, sinusitis, and extravascular eosinophils |
| Allergic bronchopulmonary aspergillosis (ABPA) | Major criteria: history of asthma, central bronchiectasis, immediate skin reactivity to Aspergillus, elevated total serum IgE (>1000 ng/mL), elevated IgE or IgG to Aspergillus |
Hypereosinophilia is defined as an eosinophil level greater than 1500 cells/L. These levels may be associated with end‐organ damage regardless of the underlying etiology, although the degree of eosinophilia frequently does not correlate closely with eosinophilic tissue infiltration. As a result, relatively modest degrees of peripheral eosinophilia may be seen in association with end‐organ damage, while severe eosinophilia may be tolerated well for prolonged periods in other cases.1 The most serious complications of hypereosinophilia are myocardial damage with ultimate development of cardiac fibrosis and refractory heart failure; pulmonary involvement with hypoxia; and involvement of both the central and peripheral nervous systems including stroke, encephalopathy, and mononeuritis multiplex. A number of studies should be considered to help evaluate for the possibility of end‐organ damage as well as to assess for the presence of primary and idiopathic causes of hypereosinophilia. These include peripheral blood smear looking particularly for dysplastic eosinophils or blasts, serum tryptase, serum vitamin B12, serum IgE, cardiac troponin levels, anti‐neutrophil cytoplasmic antibody, electrocardiography, echocardiography, pulmonary function tests, and thoracoabdominal CT scanning. Endoscopic studies with esophageal, duodenal, and colonic biopsy should be performed if eosinophilic gastroenteritis is suspected.1, 7, 10
While more modest degrees of eosinophilia are associated with a plethora of conditions, severe eosinophilia, especially that approaching the levels displayed by this patient, suggests a much more circumscribed differential diagnosis. This should prompt consideration of ChurgStrauss syndrome, parasitic infection with an active tissue migration phase, and hypereosinophilic syndrome (HES).4 HES has classically been characterized by hypereosinophilia for at least 6 months, exclusion of both secondary and clonal eosinophilia, and evidence of end‐organ involvement. More recently, however, a revised definition consisting of marked eosinophilia with reasonable exclusion of other causes has gained favor.1, 7, 10, 1416 While perhaps as many as 75% of cases of HES continue to be considered idiopathic at present, 2 subtypes have now been recognized, with important prognostic and therapeutic implications. Myeloproliferative HES has a strong male predominance, is frequently associated with elevated serum tryptase and B12 levels, often manifests with hepatosplenomegaly, and displays a characteristic gene mutation, FIP1L1/PDGFRA. Lymphocytic HES is typified by polyclonal eosinophilic expansion in response to elevated IL‐5 levels, is associated with less cardiac involvement and a somewhat more favorable prognosis in the absence of therapy, and has been associated with transformation into T‐cell lymphoma.1, 1417 We suspect, though we are unable to prove, that our patient was finally diagnosed at the end of a journey that began as lymphocytic HES and ultimately progressed to T‐cell lymphoma. T‐cell lymphoma has rarely been associated with profound eosinophilia. This appears to reflect disordered production of IL‐5, as was true of this patient, and many of these cases may represent transformed lymphocytic HES.14
Specific therapy exists for the myeloproliferative subtype of HES, consisting of the tyrosine kinase inhibitor imatinib, with excellent response in typical cases. Initial treatment of most other extreme eosinophilic syndromes not caused by parasitic infection, including lymphocytic and idiopathic HES as well as ChurgStrauss syndrome, consists of high‐dose corticosteroids, with a variety of other agents used as second‐line and steroid‐sparing treatments. The urgency of therapy is dictated by the presence and severity of end‐organ damage, and in some instances corticosteroids may need to be given before the diagnosis is fully secure. When S. stercoralis infection has not been ruled out, concurrent therapy with ivermectin should be given to prevent triggering Strongyloides hyperinfection. Hematology input is critical when HES is under serious consideration, with bone marrow examination, cytogenetic studies, T‐cell phenotyping and T‐cell receptor rearrangement studies essential in helping to establish the correct diagnosis.10, 17
The differential diagnosis of peripheral eosinophilia is broad and requires a thorough, stepwise approach. Although profound eosinophilia is usually caused by a limited number of diseases, this patient reminds us that Captain Renault's advice in the film Casablanca to round up the usual suspects does not always suffice, as the diagnosis of T‐cell lymphoma was not considered by either the clinicians or the discussant until lung biopsy results became available. Most patients with hypereosinophilia not caused by parasitic infection will ultimately require an invasive procedure to establish a diagnosis, which is essential before embarking on an often‐toxic course of therapy, as well as for providing an accurate prognosis.
TEACHING POINTS
-
The most common causes of eosinophilia include helminthic infections (the leading cause worldwide), asthma, allergic conditions (the leading cause in the United States), malignancies, and drugs.
-
Hypereosinophilia may lead to end‐organ damage. The most important etiologies include ChurgStrauss Syndrome, HES, or a helminthic infection in the larval migration phase.
-
The mainstay of therapy for most cases of HES is corticosteroids. The goal of therapy is to prevent, or ameliorate, end‐organ damage.
- ,.Practical approach to the patient with hypereosinophilia.J Allergy Clin Immunol.2010;126(1):39–44.
- ,,.Eosinophilia: secondary, clonal and idiopathic.Br J Haematol.2006;133(5):468–492.
- .Blood eosinophilia: a new paradigm in disease classification, diagnosis, and treatment.Mayo Clin Proc.2005;80(1):75–83.
- ,,,.Clinical manifestations and treatment of Churg‐Strauss syndrome.Rheum Dis Clin North Am.2010;36(3):527–543.
- ,,,.Relative eosinophilia and functional adrenal insufficiency in critically ill patients.Lancet.1999;353(9165):1675–1676.
- ,,,.Opposing effects of glucocorticoids on the rate of apoptosis in neutrophilic and eosinophilic granulocytes.J Immunol.1996;156(10):4422–4428.
- ,.Eosinophilic disorders.J Allergy Clin Immunol.2007;119(6):1291–1300; quiz 1301–1302.
- ,.Pulmonary eosinophilia.Clin Rev Allergy Immunol.2008;34(3):367–371.
- .Eosinophilic diseases of the gastrointestinal tract.Scand J Gastroenterol.2010;45(9):1013–1021.
- ,,.Hypereosinophilic syndrome and clonal eosinophilia: point‐of‐care diagnostic algorithm and treatment update.Mayo Clin Proc.2010;85(2):158–164.
- ,,,,,.Eosinophilia as a predictor of food allergy in atopic dermatitis.Allergy Asthma Proc.2010;31(2):e18–e24.
- ,,, et al.The American College of Rheumatology 1990 criteria for the classification of Churg‐Strauss syndrome (allergic granulomatosis and angiitis).Arthritis Rheum.1990;33(8):1094–1100.
- .Allergic bronchopulmonary aspergillosis. In: Adkinson NF, Yunginger JW, Busse WW, et al, eds. Middleton's Allergy Principles 2003:1353–1371.
- ,,, et al.TARC and IL‐5 expression correlates with tissue eosinophilia in peripheral T‐cell lymphomas.Leuk Res.2008;32(9):1431–1438.
- ,,.Hypereosinophilic syndrome and proliferative diseases.Acta Dermatovenerol Croat.2009;17(4):323–330.
- ,.The hypereosinophilic syndromes: current concepts and treatments.Br J Haematol.2009;145(3):271–285.
- ,,.Lymphocytic variant hypereosinophilic syndromes.Immunol Allergy Clin North Am.2007;27(3):389–413.
- ,.Practical approach to the patient with hypereosinophilia.J Allergy Clin Immunol.2010;126(1):39–44.
- ,,.Eosinophilia: secondary, clonal and idiopathic.Br J Haematol.2006;133(5):468–492.
- .Blood eosinophilia: a new paradigm in disease classification, diagnosis, and treatment.Mayo Clin Proc.2005;80(1):75–83.
- ,,,.Clinical manifestations and treatment of Churg‐Strauss syndrome.Rheum Dis Clin North Am.2010;36(3):527–543.
- ,,,.Relative eosinophilia and functional adrenal insufficiency in critically ill patients.Lancet.1999;353(9165):1675–1676.
- ,,,.Opposing effects of glucocorticoids on the rate of apoptosis in neutrophilic and eosinophilic granulocytes.J Immunol.1996;156(10):4422–4428.
- ,.Eosinophilic disorders.J Allergy Clin Immunol.2007;119(6):1291–1300; quiz 1301–1302.
- ,.Pulmonary eosinophilia.Clin Rev Allergy Immunol.2008;34(3):367–371.
- .Eosinophilic diseases of the gastrointestinal tract.Scand J Gastroenterol.2010;45(9):1013–1021.
- ,,.Hypereosinophilic syndrome and clonal eosinophilia: point‐of‐care diagnostic algorithm and treatment update.Mayo Clin Proc.2010;85(2):158–164.
- ,,,,,.Eosinophilia as a predictor of food allergy in atopic dermatitis.Allergy Asthma Proc.2010;31(2):e18–e24.
- ,,, et al.The American College of Rheumatology 1990 criteria for the classification of Churg‐Strauss syndrome (allergic granulomatosis and angiitis).Arthritis Rheum.1990;33(8):1094–1100.
- .Allergic bronchopulmonary aspergillosis. In: Adkinson NF, Yunginger JW, Busse WW, et al, eds. Middleton's Allergy Principles 2003:1353–1371.
- ,,, et al.TARC and IL‐5 expression correlates with tissue eosinophilia in peripheral T‐cell lymphomas.Leuk Res.2008;32(9):1431–1438.
- ,,.Hypereosinophilic syndrome and proliferative diseases.Acta Dermatovenerol Croat.2009;17(4):323–330.
- ,.The hypereosinophilic syndromes: current concepts and treatments.Br J Haematol.2009;145(3):271–285.
- ,,.Lymphocytic variant hypereosinophilic syndromes.Immunol Allergy Clin North Am.2007;27(3):389–413.
Vaccine Refusal
"The next patient is a 5-year-old who is unable to walk," reports the overnight resident at the morning sign-out. "He had a day of fever 4 days ago, leg pain beginning 2 days ago, and yesterday he awoke with refusal to bear weight."
As we get to the differential diagnosis, I try to expand on the list of possibilities rather than merely accept the working diagnosis of myositis developed in the emergency department.
We start with the common paradigm, "If you hear hoof beats behind you, you turn around expecting to see a horse, not a zebra. However, beware the hard-charging rhinoceros that will run you over if you don’t turn around fast enough." What is the rhinoceros in this situation? A septic hip. That needs to be ruled out emergently. If suspected, you get an immediate orthopedics consult and probably an aspiration of the hip, as destruction of the joint can occur in less than 24 hours. Physical exam had excluded this possibility. The hip was nontender.
The physical exam had revealed some mild tenderness of the calves.
"What if there were decreased or absent reflexes?" I ask. Several people jump on the diagnosis of Guillain-Barré syndrome. Correct, although I lament that only one of the four history and physicals obtained in the ED or by the admitting team had documented testing any reflexes. Teaching point made – do thorough physicals.
"What if," I asked, "you had detected weakness in the legs?" The room is silent as people consider the options. I hint, "It’s mostly of historical interest." After more silence, a medical student proffers polio. That is correct. A resident admits that she would never have thought of that diagnosis. Years of training had matured her book learning into a more honed clinical judgment. To a modern resident, polio isn’t a zebra, it’s nearly a unicorn.
I had the opportunity to check my e-mail prior to going on family-centered rounds. It contained two reminders that on that date in 1954 large-scale immunization with the Salk vaccine had begun.
The purpose of my column "Beyond the White Coat" is to provide updates, news, and perspective from the fields of law, philosophy, and the humanities that have an impact on clinical medicine. Historical events provide perspective. I was acutely aware that morning of the difference between what I faced and what one of my predecessors in the 1950s would have faced.
In that bygone era, the nightmare for many parents was putting an infant to bed with a fever, not knowing whether the baby would wake up paralyzed. Because of medical progress, I didn’t have to deliver such a grim diagnosis. I had good news to give. "Your child has a muscle inflammation that occasionally occurs after influenza and some other viruses. The muscle enzyme test this morning is improved from yesterday. The clinical exam is also better. He is now bearing weight. He’s going to be fine. I expect full recovery in another 1-3 days."
Parental refusal of vaccines remains a significant and growing problem. The Wall Street Journal last month carried yet another article about pediatricians who fire from their practice parents who refuse vaccines ("More Doctors Fire Vaccine Refusers," Feb. 15, 2012). The American Academy of Pediatrics has a policy statement discouraging such a response (Pediatrics 2005;115:1428-31), but many physicians disagree with that policy (Arch. Pediatr. Adolesc. Med. 2005;159:929-34). A wide variety of reasons are given to justify the practice. Other physicians, working locally such as in this Missouri article, are encouraging the virtue of accommodation ("Responding With Empathy to Parents Fears of Vaccinations," Missouri Medicine, Jan/Feb 2012). (Full disclosure: I’m proud to say I work with those two Missouri doctors.)
I have a research interest in parental refusals of care, as indicated in my September 2011 column ("A Parents Refusal and the Harm Principle," September 2011, p. 32). I won’t try to settle the vaccine controversy here. For me, this isn’t an issue of parental authority, parens patriae, and medical liability. The real motivation for spending the extra time educating and guiding parents who are worried about the safety of vaccines is rooted in my gratitude that I will never have to walk into a room and break the horrific news of polio to a family. It is a debt I owe those who came before me: the scientists, clinicians, parents, and children, who conquered polio. For those efforts, thank you.
P.S. My patient did get a flu shot before discharge.
Dr. Powell is associate professor of pediatrics at St. Louis University and a pediatric hospitalist at SSM Cardinal Glennon Children’s Medical Center, St. Louis.
"The next patient is a 5-year-old who is unable to walk," reports the overnight resident at the morning sign-out. "He had a day of fever 4 days ago, leg pain beginning 2 days ago, and yesterday he awoke with refusal to bear weight."
As we get to the differential diagnosis, I try to expand on the list of possibilities rather than merely accept the working diagnosis of myositis developed in the emergency department.
We start with the common paradigm, "If you hear hoof beats behind you, you turn around expecting to see a horse, not a zebra. However, beware the hard-charging rhinoceros that will run you over if you don’t turn around fast enough." What is the rhinoceros in this situation? A septic hip. That needs to be ruled out emergently. If suspected, you get an immediate orthopedics consult and probably an aspiration of the hip, as destruction of the joint can occur in less than 24 hours. Physical exam had excluded this possibility. The hip was nontender.
The physical exam had revealed some mild tenderness of the calves.
"What if there were decreased or absent reflexes?" I ask. Several people jump on the diagnosis of Guillain-Barré syndrome. Correct, although I lament that only one of the four history and physicals obtained in the ED or by the admitting team had documented testing any reflexes. Teaching point made – do thorough physicals.
"What if," I asked, "you had detected weakness in the legs?" The room is silent as people consider the options. I hint, "It’s mostly of historical interest." After more silence, a medical student proffers polio. That is correct. A resident admits that she would never have thought of that diagnosis. Years of training had matured her book learning into a more honed clinical judgment. To a modern resident, polio isn’t a zebra, it’s nearly a unicorn.
I had the opportunity to check my e-mail prior to going on family-centered rounds. It contained two reminders that on that date in 1954 large-scale immunization with the Salk vaccine had begun.
The purpose of my column "Beyond the White Coat" is to provide updates, news, and perspective from the fields of law, philosophy, and the humanities that have an impact on clinical medicine. Historical events provide perspective. I was acutely aware that morning of the difference between what I faced and what one of my predecessors in the 1950s would have faced.
In that bygone era, the nightmare for many parents was putting an infant to bed with a fever, not knowing whether the baby would wake up paralyzed. Because of medical progress, I didn’t have to deliver such a grim diagnosis. I had good news to give. "Your child has a muscle inflammation that occasionally occurs after influenza and some other viruses. The muscle enzyme test this morning is improved from yesterday. The clinical exam is also better. He is now bearing weight. He’s going to be fine. I expect full recovery in another 1-3 days."
Parental refusal of vaccines remains a significant and growing problem. The Wall Street Journal last month carried yet another article about pediatricians who fire from their practice parents who refuse vaccines ("More Doctors Fire Vaccine Refusers," Feb. 15, 2012). The American Academy of Pediatrics has a policy statement discouraging such a response (Pediatrics 2005;115:1428-31), but many physicians disagree with that policy (Arch. Pediatr. Adolesc. Med. 2005;159:929-34). A wide variety of reasons are given to justify the practice. Other physicians, working locally such as in this Missouri article, are encouraging the virtue of accommodation ("Responding With Empathy to Parents Fears of Vaccinations," Missouri Medicine, Jan/Feb 2012). (Full disclosure: I’m proud to say I work with those two Missouri doctors.)
I have a research interest in parental refusals of care, as indicated in my September 2011 column ("A Parents Refusal and the Harm Principle," September 2011, p. 32). I won’t try to settle the vaccine controversy here. For me, this isn’t an issue of parental authority, parens patriae, and medical liability. The real motivation for spending the extra time educating and guiding parents who are worried about the safety of vaccines is rooted in my gratitude that I will never have to walk into a room and break the horrific news of polio to a family. It is a debt I owe those who came before me: the scientists, clinicians, parents, and children, who conquered polio. For those efforts, thank you.
P.S. My patient did get a flu shot before discharge.
Dr. Powell is associate professor of pediatrics at St. Louis University and a pediatric hospitalist at SSM Cardinal Glennon Children’s Medical Center, St. Louis.
"The next patient is a 5-year-old who is unable to walk," reports the overnight resident at the morning sign-out. "He had a day of fever 4 days ago, leg pain beginning 2 days ago, and yesterday he awoke with refusal to bear weight."
As we get to the differential diagnosis, I try to expand on the list of possibilities rather than merely accept the working diagnosis of myositis developed in the emergency department.
We start with the common paradigm, "If you hear hoof beats behind you, you turn around expecting to see a horse, not a zebra. However, beware the hard-charging rhinoceros that will run you over if you don’t turn around fast enough." What is the rhinoceros in this situation? A septic hip. That needs to be ruled out emergently. If suspected, you get an immediate orthopedics consult and probably an aspiration of the hip, as destruction of the joint can occur in less than 24 hours. Physical exam had excluded this possibility. The hip was nontender.
The physical exam had revealed some mild tenderness of the calves.
"What if there were decreased or absent reflexes?" I ask. Several people jump on the diagnosis of Guillain-Barré syndrome. Correct, although I lament that only one of the four history and physicals obtained in the ED or by the admitting team had documented testing any reflexes. Teaching point made – do thorough physicals.
"What if," I asked, "you had detected weakness in the legs?" The room is silent as people consider the options. I hint, "It’s mostly of historical interest." After more silence, a medical student proffers polio. That is correct. A resident admits that she would never have thought of that diagnosis. Years of training had matured her book learning into a more honed clinical judgment. To a modern resident, polio isn’t a zebra, it’s nearly a unicorn.
I had the opportunity to check my e-mail prior to going on family-centered rounds. It contained two reminders that on that date in 1954 large-scale immunization with the Salk vaccine had begun.
The purpose of my column "Beyond the White Coat" is to provide updates, news, and perspective from the fields of law, philosophy, and the humanities that have an impact on clinical medicine. Historical events provide perspective. I was acutely aware that morning of the difference between what I faced and what one of my predecessors in the 1950s would have faced.
In that bygone era, the nightmare for many parents was putting an infant to bed with a fever, not knowing whether the baby would wake up paralyzed. Because of medical progress, I didn’t have to deliver such a grim diagnosis. I had good news to give. "Your child has a muscle inflammation that occasionally occurs after influenza and some other viruses. The muscle enzyme test this morning is improved from yesterday. The clinical exam is also better. He is now bearing weight. He’s going to be fine. I expect full recovery in another 1-3 days."
Parental refusal of vaccines remains a significant and growing problem. The Wall Street Journal last month carried yet another article about pediatricians who fire from their practice parents who refuse vaccines ("More Doctors Fire Vaccine Refusers," Feb. 15, 2012). The American Academy of Pediatrics has a policy statement discouraging such a response (Pediatrics 2005;115:1428-31), but many physicians disagree with that policy (Arch. Pediatr. Adolesc. Med. 2005;159:929-34). A wide variety of reasons are given to justify the practice. Other physicians, working locally such as in this Missouri article, are encouraging the virtue of accommodation ("Responding With Empathy to Parents Fears of Vaccinations," Missouri Medicine, Jan/Feb 2012). (Full disclosure: I’m proud to say I work with those two Missouri doctors.)
I have a research interest in parental refusals of care, as indicated in my September 2011 column ("A Parents Refusal and the Harm Principle," September 2011, p. 32). I won’t try to settle the vaccine controversy here. For me, this isn’t an issue of parental authority, parens patriae, and medical liability. The real motivation for spending the extra time educating and guiding parents who are worried about the safety of vaccines is rooted in my gratitude that I will never have to walk into a room and break the horrific news of polio to a family. It is a debt I owe those who came before me: the scientists, clinicians, parents, and children, who conquered polio. For those efforts, thank you.
P.S. My patient did get a flu shot before discharge.
Dr. Powell is associate professor of pediatrics at St. Louis University and a pediatric hospitalist at SSM Cardinal Glennon Children’s Medical Center, St. Louis.