User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'medstat-accordion-set article-series')]
Digital Danger: How Cyberattacks Put Patients at Risk
On September 27, 2024, UMC Health System in Lubbock, Texas, experienced an IT outage because of a cybersecurity incident that temporarily diverted patients to other healthcare facilities. So far, in 2024, there have been 386 cyberattacks on healthcare organizations. These high-impact ransomware attacks disrupt and delay patient care.
In recent years, many healthcare systems, including Scripps Health, Universal Health Services, Vastaamo, Sky Lakes, and the University of Vermont, have paid millions — even tens of millions — to recover data after a cyberattack or data breach. When healthcare systems come under cyber fire, the impact extends far past disrupting workflows and compromising data, patient safety can be also be compromised, vital information may be lost, and imaging and lab results can go missing or be held for ransom, making physicians’ job difficult or impossible.
In fact, cyberattacks on hospitals are far more common than you may realize. A new report issued by Ponemon and Proofpoint found that 92% of healthcare organizations have experienced a cyberattack in the past 12 months. Even more sobering is that about half of the organizations affected suffered disruptions in patient care.
Healthcare Systems = ‘Soft Targets’
Healthcare systems are a “soft target” for hackers for several reasons, pointed out Matthew Radolec, vice president, incident response and cloud operations at Varonis, a data security company. “One, they’re usually an amalgamation of many healthcare systems that are interconnected,” said Radolec. “A lot of hospitals are connected to other hospitals or connected to educational institutions, which means their computer vulnerabilities are shared ... and if they have an issue, it could very easily spread to your network.”
Another factor is the cost of securing data. “[With hospitals], they’ll say that a dollar spent on security is a dollar not spent on patient care,” said Radolec. “So the idea of investing in security is really tough from a budget standpoint…they’re choosing between a new MRI machine or better antivirus, backups, or data security.”
Because of the wealth of private data and healthcare information they maintain, hospitals are considered “high impact” for cybercriminals. Attackers know that if they get a foothold in a hospital, it’s more likely to pay — and pay quickly, Radolec told this news organization. Hospitals are also likely to have cyber insurance to help cover the cost of having their data stolen, encrypted, and ransomed.
The 2024 Microsoft Digital Defense Report also found that the bad actors are more sophisticated and better resourced and can challenge even the best cybersecurity. Improved defenses may not be good enough, and the sheer volume of attacks must be met with effective deterrence and government solutions that impose consequences for cybercriminals.
Vulnerable Users
Whether through a phishing email or text, password attack, or web attack, “the moment a ‘threat actor’ gets into your institution and gets credentials ... that’s the Nirvana state of a threat actor,” warned Ryan Witt, chair of the healthcare customer advisory board and vice president of Industry Solutions at Proofpoint, a cybersecurity platform. “They have those credentials and will go into deep reconnaissance mode. It often takes healthcare up to 6 months to even ascertain whether somebody’s actually in the network.” During that time, the hacker is learning how the institution works, what job functions matter, and how best to plan their attack.
“Attackers are getting in because they’re buying databases of usernames and passwords. And they’re trying them by the millions,” added Radolec. “For a sophisticated actor, all it takes is time and motivation. They have the skills. It’s just a matter of how persistent they want to be.”
Certain hospital staff are also more likely to be targeted by cyberhackers than others. “About 10% of a healthcare organization’s user base is much more vulnerable for all sorts of reasons — how they work, the value of their job title and job function, and therefore their access to systems,” said Witt.
High-profile staff are more likely to be targeted than those in lower-level positions; the so-called “CEO attack” is typical. However, staff in other hospital departments are also subject to cybercriminals, including hospice departments/hospice organizations and research arms of hospitals.
The Impact of Cyberattacks on Patients
Physicians and healthcare execs may have considered cybersecurity more of a compliance issue than a true threat to patients in the past. But this attitude is rapidly changing. “We are starting to see a very clear connection between a cyber event and how it can impact patient care and patient safety,” said Witt.
According to the Proofpoint report, cyber breaches can severely affect patient care. In 2024:
- 56% of respondents saw a delay in patient tests/procedures
- 53% experienced increased patient complications from medical procedures
- 52% noted a longer patient length of stay
- 44% saw an increase in patient transfers to other facilities
- 28% had an increase in mortality rate
What Hospitals and Physicians Can Do
Fortunately, hospitals can take measures to better protect their data and their patients. One strategy is segmenting networks to reduce the amount of data or systems one person or system can access. Educating staff about the dangers of phishing and spoofing emails also help protect organizations from ransomware attacks. Having staff avoid reusing passwords and updating logins and passwords frequently helps.
Most hospitals also need more robust security controls. Physicians and healthcare facilities must also embrace the cybersecurity controls found in other industries, said Witt. “Multifactor authentication is one of those things that can cause us frustration,” he said. “The controls can seem onerous, but they’re really valuable overall…and should become standard practice.”
Doctors can also prepare for a ransomware attack and protect patients by practicing some “old-school” medicine, like using paper systems and maintaining good patient notes — often, those notes are synced locally as well as offsite, so you’d be able to access them even during a data breach. “It’s smart to write prescriptions on pads sometimes,” said Radolec. “Don’t forget how to do those things because that will make you more resilient in the event of a ransomware attack.”
A Continuing Threat
Cyberattacks will continue. “When you look at the high likelihood [of success] and the soft target, you end up with ... a perfect storm,” said Radolec. “Hospitals have a lot of vulnerabilities. They have to keep operations going just to receive income, but also to deliver care to people.”
That means that the burden is on healthcare organizations — including physicians, nurses, staff, and C-level execs — to help keep the “security” in cybersecurity. “We are all part of the cybersecurity defense,” said Witt. Helping to maintain that defense has become a critical aspect of caring for patients.
A version of this article first appeared on Medscape.com.
On September 27, 2024, UMC Health System in Lubbock, Texas, experienced an IT outage because of a cybersecurity incident that temporarily diverted patients to other healthcare facilities. So far, in 2024, there have been 386 cyberattacks on healthcare organizations. These high-impact ransomware attacks disrupt and delay patient care.
In recent years, many healthcare systems, including Scripps Health, Universal Health Services, Vastaamo, Sky Lakes, and the University of Vermont, have paid millions — even tens of millions — to recover data after a cyberattack or data breach. When healthcare systems come under cyber fire, the impact extends far past disrupting workflows and compromising data, patient safety can be also be compromised, vital information may be lost, and imaging and lab results can go missing or be held for ransom, making physicians’ job difficult or impossible.
In fact, cyberattacks on hospitals are far more common than you may realize. A new report issued by Ponemon and Proofpoint found that 92% of healthcare organizations have experienced a cyberattack in the past 12 months. Even more sobering is that about half of the organizations affected suffered disruptions in patient care.
Healthcare Systems = ‘Soft Targets’
Healthcare systems are a “soft target” for hackers for several reasons, pointed out Matthew Radolec, vice president, incident response and cloud operations at Varonis, a data security company. “One, they’re usually an amalgamation of many healthcare systems that are interconnected,” said Radolec. “A lot of hospitals are connected to other hospitals or connected to educational institutions, which means their computer vulnerabilities are shared ... and if they have an issue, it could very easily spread to your network.”
Another factor is the cost of securing data. “[With hospitals], they’ll say that a dollar spent on security is a dollar not spent on patient care,” said Radolec. “So the idea of investing in security is really tough from a budget standpoint…they’re choosing between a new MRI machine or better antivirus, backups, or data security.”
Because of the wealth of private data and healthcare information they maintain, hospitals are considered “high impact” for cybercriminals. Attackers know that if they get a foothold in a hospital, it’s more likely to pay — and pay quickly, Radolec told this news organization. Hospitals are also likely to have cyber insurance to help cover the cost of having their data stolen, encrypted, and ransomed.
The 2024 Microsoft Digital Defense Report also found that the bad actors are more sophisticated and better resourced and can challenge even the best cybersecurity. Improved defenses may not be good enough, and the sheer volume of attacks must be met with effective deterrence and government solutions that impose consequences for cybercriminals.
Vulnerable Users
Whether through a phishing email or text, password attack, or web attack, “the moment a ‘threat actor’ gets into your institution and gets credentials ... that’s the Nirvana state of a threat actor,” warned Ryan Witt, chair of the healthcare customer advisory board and vice president of Industry Solutions at Proofpoint, a cybersecurity platform. “They have those credentials and will go into deep reconnaissance mode. It often takes healthcare up to 6 months to even ascertain whether somebody’s actually in the network.” During that time, the hacker is learning how the institution works, what job functions matter, and how best to plan their attack.
“Attackers are getting in because they’re buying databases of usernames and passwords. And they’re trying them by the millions,” added Radolec. “For a sophisticated actor, all it takes is time and motivation. They have the skills. It’s just a matter of how persistent they want to be.”
Certain hospital staff are also more likely to be targeted by cyberhackers than others. “About 10% of a healthcare organization’s user base is much more vulnerable for all sorts of reasons — how they work, the value of their job title and job function, and therefore their access to systems,” said Witt.
High-profile staff are more likely to be targeted than those in lower-level positions; the so-called “CEO attack” is typical. However, staff in other hospital departments are also subject to cybercriminals, including hospice departments/hospice organizations and research arms of hospitals.
The Impact of Cyberattacks on Patients
Physicians and healthcare execs may have considered cybersecurity more of a compliance issue than a true threat to patients in the past. But this attitude is rapidly changing. “We are starting to see a very clear connection between a cyber event and how it can impact patient care and patient safety,” said Witt.
According to the Proofpoint report, cyber breaches can severely affect patient care. In 2024:
- 56% of respondents saw a delay in patient tests/procedures
- 53% experienced increased patient complications from medical procedures
- 52% noted a longer patient length of stay
- 44% saw an increase in patient transfers to other facilities
- 28% had an increase in mortality rate
What Hospitals and Physicians Can Do
Fortunately, hospitals can take measures to better protect their data and their patients. One strategy is segmenting networks to reduce the amount of data or systems one person or system can access. Educating staff about the dangers of phishing and spoofing emails also help protect organizations from ransomware attacks. Having staff avoid reusing passwords and updating logins and passwords frequently helps.
Most hospitals also need more robust security controls. Physicians and healthcare facilities must also embrace the cybersecurity controls found in other industries, said Witt. “Multifactor authentication is one of those things that can cause us frustration,” he said. “The controls can seem onerous, but they’re really valuable overall…and should become standard practice.”
Doctors can also prepare for a ransomware attack and protect patients by practicing some “old-school” medicine, like using paper systems and maintaining good patient notes — often, those notes are synced locally as well as offsite, so you’d be able to access them even during a data breach. “It’s smart to write prescriptions on pads sometimes,” said Radolec. “Don’t forget how to do those things because that will make you more resilient in the event of a ransomware attack.”
A Continuing Threat
Cyberattacks will continue. “When you look at the high likelihood [of success] and the soft target, you end up with ... a perfect storm,” said Radolec. “Hospitals have a lot of vulnerabilities. They have to keep operations going just to receive income, but also to deliver care to people.”
That means that the burden is on healthcare organizations — including physicians, nurses, staff, and C-level execs — to help keep the “security” in cybersecurity. “We are all part of the cybersecurity defense,” said Witt. Helping to maintain that defense has become a critical aspect of caring for patients.
A version of this article first appeared on Medscape.com.
On September 27, 2024, UMC Health System in Lubbock, Texas, experienced an IT outage because of a cybersecurity incident that temporarily diverted patients to other healthcare facilities. So far, in 2024, there have been 386 cyberattacks on healthcare organizations. These high-impact ransomware attacks disrupt and delay patient care.
In recent years, many healthcare systems, including Scripps Health, Universal Health Services, Vastaamo, Sky Lakes, and the University of Vermont, have paid millions — even tens of millions — to recover data after a cyberattack or data breach. When healthcare systems come under cyber fire, the impact extends far past disrupting workflows and compromising data, patient safety can be also be compromised, vital information may be lost, and imaging and lab results can go missing or be held for ransom, making physicians’ job difficult or impossible.
In fact, cyberattacks on hospitals are far more common than you may realize. A new report issued by Ponemon and Proofpoint found that 92% of healthcare organizations have experienced a cyberattack in the past 12 months. Even more sobering is that about half of the organizations affected suffered disruptions in patient care.
Healthcare Systems = ‘Soft Targets’
Healthcare systems are a “soft target” for hackers for several reasons, pointed out Matthew Radolec, vice president, incident response and cloud operations at Varonis, a data security company. “One, they’re usually an amalgamation of many healthcare systems that are interconnected,” said Radolec. “A lot of hospitals are connected to other hospitals or connected to educational institutions, which means their computer vulnerabilities are shared ... and if they have an issue, it could very easily spread to your network.”
Another factor is the cost of securing data. “[With hospitals], they’ll say that a dollar spent on security is a dollar not spent on patient care,” said Radolec. “So the idea of investing in security is really tough from a budget standpoint…they’re choosing between a new MRI machine or better antivirus, backups, or data security.”
Because of the wealth of private data and healthcare information they maintain, hospitals are considered “high impact” for cybercriminals. Attackers know that if they get a foothold in a hospital, it’s more likely to pay — and pay quickly, Radolec told this news organization. Hospitals are also likely to have cyber insurance to help cover the cost of having their data stolen, encrypted, and ransomed.
The 2024 Microsoft Digital Defense Report also found that the bad actors are more sophisticated and better resourced and can challenge even the best cybersecurity. Improved defenses may not be good enough, and the sheer volume of attacks must be met with effective deterrence and government solutions that impose consequences for cybercriminals.
Vulnerable Users
Whether through a phishing email or text, password attack, or web attack, “the moment a ‘threat actor’ gets into your institution and gets credentials ... that’s the Nirvana state of a threat actor,” warned Ryan Witt, chair of the healthcare customer advisory board and vice president of Industry Solutions at Proofpoint, a cybersecurity platform. “They have those credentials and will go into deep reconnaissance mode. It often takes healthcare up to 6 months to even ascertain whether somebody’s actually in the network.” During that time, the hacker is learning how the institution works, what job functions matter, and how best to plan their attack.
“Attackers are getting in because they’re buying databases of usernames and passwords. And they’re trying them by the millions,” added Radolec. “For a sophisticated actor, all it takes is time and motivation. They have the skills. It’s just a matter of how persistent they want to be.”
Certain hospital staff are also more likely to be targeted by cyberhackers than others. “About 10% of a healthcare organization’s user base is much more vulnerable for all sorts of reasons — how they work, the value of their job title and job function, and therefore their access to systems,” said Witt.
High-profile staff are more likely to be targeted than those in lower-level positions; the so-called “CEO attack” is typical. However, staff in other hospital departments are also subject to cybercriminals, including hospice departments/hospice organizations and research arms of hospitals.
The Impact of Cyberattacks on Patients
Physicians and healthcare execs may have considered cybersecurity more of a compliance issue than a true threat to patients in the past. But this attitude is rapidly changing. “We are starting to see a very clear connection between a cyber event and how it can impact patient care and patient safety,” said Witt.
According to the Proofpoint report, cyber breaches can severely affect patient care. In 2024:
- 56% of respondents saw a delay in patient tests/procedures
- 53% experienced increased patient complications from medical procedures
- 52% noted a longer patient length of stay
- 44% saw an increase in patient transfers to other facilities
- 28% had an increase in mortality rate
What Hospitals and Physicians Can Do
Fortunately, hospitals can take measures to better protect their data and their patients. One strategy is segmenting networks to reduce the amount of data or systems one person or system can access. Educating staff about the dangers of phishing and spoofing emails also help protect organizations from ransomware attacks. Having staff avoid reusing passwords and updating logins and passwords frequently helps.
Most hospitals also need more robust security controls. Physicians and healthcare facilities must also embrace the cybersecurity controls found in other industries, said Witt. “Multifactor authentication is one of those things that can cause us frustration,” he said. “The controls can seem onerous, but they’re really valuable overall…and should become standard practice.”
Doctors can also prepare for a ransomware attack and protect patients by practicing some “old-school” medicine, like using paper systems and maintaining good patient notes — often, those notes are synced locally as well as offsite, so you’d be able to access them even during a data breach. “It’s smart to write prescriptions on pads sometimes,” said Radolec. “Don’t forget how to do those things because that will make you more resilient in the event of a ransomware attack.”
A Continuing Threat
Cyberattacks will continue. “When you look at the high likelihood [of success] and the soft target, you end up with ... a perfect storm,” said Radolec. “Hospitals have a lot of vulnerabilities. They have to keep operations going just to receive income, but also to deliver care to people.”
That means that the burden is on healthcare organizations — including physicians, nurses, staff, and C-level execs — to help keep the “security” in cybersecurity. “We are all part of the cybersecurity defense,” said Witt. Helping to maintain that defense has become a critical aspect of caring for patients.
A version of this article first appeared on Medscape.com.
When Your Malpractice Insurer Investigates You: What to Know
When psychiatrist Paul Sartain, MD (not his real name), received a letter from his state’s medical board, he was concerned. A patient’s family complained that he made sexual advances to a young woman he treated for psychotic depression.
“There was absolutely no evidence, and the claims were vague,” he said. “I think the family was angry at me and with the system — the woman had not gotten better.” Sartain reviewed his medical records and then called his malpractice insurer.
The insurer asked about his involvement with the patient’s case, if there was anything credible to the patient’s complaint, and if he had thorough documentation. Then, the carrier offered Sartain his choice of several attorneys who could represent him. The medical board ultimately closed the case with no findings against him, and the patient’s family never sued him.
“If I’m wrongly accused, I’m defended (by the carrier). If I had stolen money or had a sexual relationship with the patient, then you’re acting outside the bounds of what is protected (by the carrier),” he said.
How Medical Board and Malpractice Insurer Investigations Differ
Medical board complaints differ from malpractice claims, in which patients seek damages. The investigation process also varies.
When a patient reports a doctor to a state medical board, they may also sue the doctor for monetary damages in civil court. The medical board responds to patient complaints made directly to them, but it also may also initiate its own investigations. Those can be prompted by a malpractice claim resolution, with a court verdict against the doctor, or a settlement recorded in the National Practitioner Data Bank.
Malpractice insurers may offer limited legal representation for medical board investigations, requiring the doctor to report the medical board issue to them before the doctor takes any action. Often, they will cover up to $50,000 in defense costs but not cover any subsequent medical board fines or required classes or medical board fees.
When a doctor contacts the carrier about a medical board investigation, the carrier may ask for the medical board document and the medical records, said Alex Keoskey, a partner in Frier Levitt’s life sciences group.
The carrier may want to ask about the patient, staff members involved, the doctor’s background, if there have been previous medical board investigations or lawsuits against this doctor, and the doctor’s opinion of the allegations. The doctor should be transparent with the carrier, Keoskey said.
Some carriers conduct more in-depth investigations, examining record-keeping, prescription practices, patient consent processes, and continuing medical education status. That’s because the medical board may inquire about these as well should its own investigation expand.
Not all carriers explore cases like these, even if reimbursing for defense costs, said Karen Frisella, director of professional liability claims at BETA Healthcare Group in California. In her experience, a licensing investigation usually follows a claim resolution that was already worked up by the carrier. If a complaint was made directly to the licensing board without an accompanying liability claim, the carrier’s ability to initiate an investigation on the incident depends on the policy terms or coverage available.
“Typically, a professional liability policy requires that the insured report a claim to trigger coverage. The carrier can’t unilaterally decide to open a claim,” she said. A licensing board investigation is not a claim by definition and therefore does not provide a mechanism for the carrier to open a liability claim file, she added.
If the medical board ultimately restricts the doctor’s license or puts the doctor on probation, that becomes public, and the underwriting department may then look into it.
Malpractice insurers routinely monitor licensing board discipline notices. A reprimand or restrictions on a doctor’s license could trigger a review of the physician’s future insurability and lead to higher premiums or even nonrenewal, Frisella said.
If a carrier investigates a reported claim and determines there are issues with the care rendered, whether there is an accompanying medical board action, that also can affect underwriting decisions, Frisella said.
Who Is Your Attorney Really Working for?
The doctor should understand whose interests the attorney represents. In a medical board claim, the attorney — even if defense is paid by the carrier — represents the doctor.
Frisella said her organization provides pass-through coverage, meaning it reimburses the doctor for medical board defense costs. “Because the carrier isn’t directing the medical board defense, it is not generally privy to the work product.”
If a patient files a malpractice claim, however, the attorney ultimately represents the insurance company.
“The panel counsel who works for the insurer does not work for the doctor, and that’s always important to remember,” Keoskey said. While the attorney will do their best to aggressively defend the doctor, “he’s going to protect the insurer’s interest before the doctor’s.”
Physicians who find any conflict of interest with their insurer should seek counsel.
Such conflicts could include:
- Disagreements over the case’s ultimate worth. For example, a physician might want a case to settle for less than their carrier is willing to pay.
- The legal judgment may exceed the carrier’s policy limits, or there are punitive damages or allegations of criminal acts that the insurer does not cover.
In these cases, the insurance company should recommend the doctor get personal counsel. They will send a reservation of rights letter saying they will defend the doctor for now, but if the facts show the doctor committed some type of misconduct, they may decline coverage, said Keoskey. Some states, including California, require that the carrier pay for this independent counsel.
Unless there is a conflict of interest, though, having a personal attorney just makes the situation more complicated, said Frisella.
A version of this article first appeared on Medscape.com.
When psychiatrist Paul Sartain, MD (not his real name), received a letter from his state’s medical board, he was concerned. A patient’s family complained that he made sexual advances to a young woman he treated for psychotic depression.
“There was absolutely no evidence, and the claims were vague,” he said. “I think the family was angry at me and with the system — the woman had not gotten better.” Sartain reviewed his medical records and then called his malpractice insurer.
The insurer asked about his involvement with the patient’s case, if there was anything credible to the patient’s complaint, and if he had thorough documentation. Then, the carrier offered Sartain his choice of several attorneys who could represent him. The medical board ultimately closed the case with no findings against him, and the patient’s family never sued him.
“If I’m wrongly accused, I’m defended (by the carrier). If I had stolen money or had a sexual relationship with the patient, then you’re acting outside the bounds of what is protected (by the carrier),” he said.
How Medical Board and Malpractice Insurer Investigations Differ
Medical board complaints differ from malpractice claims, in which patients seek damages. The investigation process also varies.
When a patient reports a doctor to a state medical board, they may also sue the doctor for monetary damages in civil court. The medical board responds to patient complaints made directly to them, but it also may also initiate its own investigations. Those can be prompted by a malpractice claim resolution, with a court verdict against the doctor, or a settlement recorded in the National Practitioner Data Bank.
Malpractice insurers may offer limited legal representation for medical board investigations, requiring the doctor to report the medical board issue to them before the doctor takes any action. Often, they will cover up to $50,000 in defense costs but not cover any subsequent medical board fines or required classes or medical board fees.
When a doctor contacts the carrier about a medical board investigation, the carrier may ask for the medical board document and the medical records, said Alex Keoskey, a partner in Frier Levitt’s life sciences group.
The carrier may want to ask about the patient, staff members involved, the doctor’s background, if there have been previous medical board investigations or lawsuits against this doctor, and the doctor’s opinion of the allegations. The doctor should be transparent with the carrier, Keoskey said.
Some carriers conduct more in-depth investigations, examining record-keeping, prescription practices, patient consent processes, and continuing medical education status. That’s because the medical board may inquire about these as well should its own investigation expand.
Not all carriers explore cases like these, even if reimbursing for defense costs, said Karen Frisella, director of professional liability claims at BETA Healthcare Group in California. In her experience, a licensing investigation usually follows a claim resolution that was already worked up by the carrier. If a complaint was made directly to the licensing board without an accompanying liability claim, the carrier’s ability to initiate an investigation on the incident depends on the policy terms or coverage available.
“Typically, a professional liability policy requires that the insured report a claim to trigger coverage. The carrier can’t unilaterally decide to open a claim,” she said. A licensing board investigation is not a claim by definition and therefore does not provide a mechanism for the carrier to open a liability claim file, she added.
If the medical board ultimately restricts the doctor’s license or puts the doctor on probation, that becomes public, and the underwriting department may then look into it.
Malpractice insurers routinely monitor licensing board discipline notices. A reprimand or restrictions on a doctor’s license could trigger a review of the physician’s future insurability and lead to higher premiums or even nonrenewal, Frisella said.
If a carrier investigates a reported claim and determines there are issues with the care rendered, whether there is an accompanying medical board action, that also can affect underwriting decisions, Frisella said.
Who Is Your Attorney Really Working for?
The doctor should understand whose interests the attorney represents. In a medical board claim, the attorney — even if defense is paid by the carrier — represents the doctor.
Frisella said her organization provides pass-through coverage, meaning it reimburses the doctor for medical board defense costs. “Because the carrier isn’t directing the medical board defense, it is not generally privy to the work product.”
If a patient files a malpractice claim, however, the attorney ultimately represents the insurance company.
“The panel counsel who works for the insurer does not work for the doctor, and that’s always important to remember,” Keoskey said. While the attorney will do their best to aggressively defend the doctor, “he’s going to protect the insurer’s interest before the doctor’s.”
Physicians who find any conflict of interest with their insurer should seek counsel.
Such conflicts could include:
- Disagreements over the case’s ultimate worth. For example, a physician might want a case to settle for less than their carrier is willing to pay.
- The legal judgment may exceed the carrier’s policy limits, or there are punitive damages or allegations of criminal acts that the insurer does not cover.
In these cases, the insurance company should recommend the doctor get personal counsel. They will send a reservation of rights letter saying they will defend the doctor for now, but if the facts show the doctor committed some type of misconduct, they may decline coverage, said Keoskey. Some states, including California, require that the carrier pay for this independent counsel.
Unless there is a conflict of interest, though, having a personal attorney just makes the situation more complicated, said Frisella.
A version of this article first appeared on Medscape.com.
When psychiatrist Paul Sartain, MD (not his real name), received a letter from his state’s medical board, he was concerned. A patient’s family complained that he made sexual advances to a young woman he treated for psychotic depression.
“There was absolutely no evidence, and the claims were vague,” he said. “I think the family was angry at me and with the system — the woman had not gotten better.” Sartain reviewed his medical records and then called his malpractice insurer.
The insurer asked about his involvement with the patient’s case, if there was anything credible to the patient’s complaint, and if he had thorough documentation. Then, the carrier offered Sartain his choice of several attorneys who could represent him. The medical board ultimately closed the case with no findings against him, and the patient’s family never sued him.
“If I’m wrongly accused, I’m defended (by the carrier). If I had stolen money or had a sexual relationship with the patient, then you’re acting outside the bounds of what is protected (by the carrier),” he said.
How Medical Board and Malpractice Insurer Investigations Differ
Medical board complaints differ from malpractice claims, in which patients seek damages. The investigation process also varies.
When a patient reports a doctor to a state medical board, they may also sue the doctor for monetary damages in civil court. The medical board responds to patient complaints made directly to them, but it also may also initiate its own investigations. Those can be prompted by a malpractice claim resolution, with a court verdict against the doctor, or a settlement recorded in the National Practitioner Data Bank.
Malpractice insurers may offer limited legal representation for medical board investigations, requiring the doctor to report the medical board issue to them before the doctor takes any action. Often, they will cover up to $50,000 in defense costs but not cover any subsequent medical board fines or required classes or medical board fees.
When a doctor contacts the carrier about a medical board investigation, the carrier may ask for the medical board document and the medical records, said Alex Keoskey, a partner in Frier Levitt’s life sciences group.
The carrier may want to ask about the patient, staff members involved, the doctor’s background, if there have been previous medical board investigations or lawsuits against this doctor, and the doctor’s opinion of the allegations. The doctor should be transparent with the carrier, Keoskey said.
Some carriers conduct more in-depth investigations, examining record-keeping, prescription practices, patient consent processes, and continuing medical education status. That’s because the medical board may inquire about these as well should its own investigation expand.
Not all carriers explore cases like these, even if reimbursing for defense costs, said Karen Frisella, director of professional liability claims at BETA Healthcare Group in California. In her experience, a licensing investigation usually follows a claim resolution that was already worked up by the carrier. If a complaint was made directly to the licensing board without an accompanying liability claim, the carrier’s ability to initiate an investigation on the incident depends on the policy terms or coverage available.
“Typically, a professional liability policy requires that the insured report a claim to trigger coverage. The carrier can’t unilaterally decide to open a claim,” she said. A licensing board investigation is not a claim by definition and therefore does not provide a mechanism for the carrier to open a liability claim file, she added.
If the medical board ultimately restricts the doctor’s license or puts the doctor on probation, that becomes public, and the underwriting department may then look into it.
Malpractice insurers routinely monitor licensing board discipline notices. A reprimand or restrictions on a doctor’s license could trigger a review of the physician’s future insurability and lead to higher premiums or even nonrenewal, Frisella said.
If a carrier investigates a reported claim and determines there are issues with the care rendered, whether there is an accompanying medical board action, that also can affect underwriting decisions, Frisella said.
Who Is Your Attorney Really Working for?
The doctor should understand whose interests the attorney represents. In a medical board claim, the attorney — even if defense is paid by the carrier — represents the doctor.
Frisella said her organization provides pass-through coverage, meaning it reimburses the doctor for medical board defense costs. “Because the carrier isn’t directing the medical board defense, it is not generally privy to the work product.”
If a patient files a malpractice claim, however, the attorney ultimately represents the insurance company.
“The panel counsel who works for the insurer does not work for the doctor, and that’s always important to remember,” Keoskey said. While the attorney will do their best to aggressively defend the doctor, “he’s going to protect the insurer’s interest before the doctor’s.”
Physicians who find any conflict of interest with their insurer should seek counsel.
Such conflicts could include:
- Disagreements over the case’s ultimate worth. For example, a physician might want a case to settle for less than their carrier is willing to pay.
- The legal judgment may exceed the carrier’s policy limits, or there are punitive damages or allegations of criminal acts that the insurer does not cover.
In these cases, the insurance company should recommend the doctor get personal counsel. They will send a reservation of rights letter saying they will defend the doctor for now, but if the facts show the doctor committed some type of misconduct, they may decline coverage, said Keoskey. Some states, including California, require that the carrier pay for this independent counsel.
Unless there is a conflict of interest, though, having a personal attorney just makes the situation more complicated, said Frisella.
A version of this article first appeared on Medscape.com.
The Rise of Sham Peer Reviews
While a medical peer review occurs once a patient, fellow doctor, or staff member reports that a physician failed to treat a patient up to standards or acted improperly, a “sham peer review” is undertaken for ulterior motives.
They’re typically undertaken due to professional competition or institutional politics rather than to promote quality care or uphold professional standards.
Physicians should be concerned. In a soon-to-be-published Medscape report on peer reviews, 56% of US physicians surveyed expressed higher levels of concern that a peer review could be misused to punish a physician for reasons unrelated to the matter being reviewed.
This is a troublesome issue, and many doctors may not be aware of it or how often it occurs.
“The biggest misconception about sham peer reviews is a denial of how pervasive they are,” said Andy Schlafly, general counsel for the Association of American Physicians and Surgeons (AAPS), which offers a free legal consultation service for physicians facing a sham peer review. “Many hospital administrations are as dangerous to good physicians as street gangs can be in a crime-ridden neighborhood.”
“Physicians should become aware of whether sham peer reviews are prevalent at their hospital and, if so, those physicians should look to practice somewhere else,” Schlafly said in an interview.
Unfortunately, there are limited data on how often this happens. When it does, it can be a career killer, said Lawrence Huntoon, MD, PhD, who has run the AAPS sham peer review hotline for over 20 years.
The physicians at the most risk for a sham peer review tend to be those who work for large hospital systems — as this is one way for hospitals to get rid of the doctors they don’t want to retain on staff, Huntoon said.
“Hospitals want a model whereby every physician on the medical staff is an employee,” Huntoon added. “This gives them complete power and control over these physicians, including the way they practice and how many patients they see per day, which, for some, is 20-50 a day to generate sufficient revenue.”
Complaints are generally filed via incident reporting software.
“The complaint could be that the physician is ‘disruptive,’ which can include facial expression, tone of voice, and body language — for example, ‘I found his facial expression demeaning’ or ‘I found her tone condescending’ — and this can be used to prosecute a doctor,” Huntoon said.
After the complaint is filed, the leaders of a hospital’s peer review committee meet to discuss the incident, followed by a panel of fellow physicians convened to review the matter. Once the date for a meeting is set, the accused doctor is allowed to testify, offer evidence, and have attorney representation.
The entire experience can take a physician by surprise.
“A sham peer review is difficult to prepare for because no physician thinks this is going to happen to them,” said Laurie L. York, a medical law attorney in Austin, Texas.
York added that there may also be a misperception of what is actually happening.
“When a physician becomes aware of an investigation, it initially may look like a regular peer review, and the physician may feel there has been a ‘misunderstanding’ that they can make right by explaining things,” York said. “The window of opportunity to shut down a sham peer review happens quickly. That’s why the physician needs the help of an experienced attorney as early in the process as possible.”
If You’re a Victim of a Sham Peer Review
Be vigilant. The most important thing you should think about when it comes to sham peer reviews is that this can, indeed, happen to you, Huntoon said. “I’ve written articles to help educate physicians about the tactics that are used,” he said. “You need to be educated and read medical staff bylaws to know your rights before something bad happens.”
Stay in your job. No matter what, if you’re under review, do not resign your position, no matter how difficult this may be. “A resignation during a sham peer review triggers an adverse report to the National Practitioner Data Bank [NPDB],” Schlafly said. The NPDB is a flagging system created by Congress to improve healthcare quality and reduce healthcare fraud and abuse. “A resignation also waives the physician’s right to contest the unfair review. In addition, leverage to negotiate a favorable settlement is lost if the physician simply resigns.”
Get a lawyer on board early. This is the only way to protect your rights. “Don’t wait a year to get an attorney involved,” Huntoon said. But this also can’t be any lawyer. It’s critical to find someone who specializes in sham peer reviews, so be sure to ask about their experience in handling peer review matters in hospitals and how knowledgeable they are about databank reporting requirements. “Sometimes, doctors will hire a malpractice attorney with no knowledge of what happens with sham peer reviews, and they may give bad advice,” he said. “Others may hire an employment attorney and that attorney will be up on employment law but has no experience with peer review matters in hospitals.”
Given the seriousness of a sham peer review, following these guidelines can help.
Contact the AAPA right away. There are things that can be done early on like getting a withdrawal of the request for corrective action as well as obtaining a preliminary injunction. Preparing for the fallout that may occur can be just as challenging.
“After this situation, the doctor is damaged goods,” Huntoon said. “What hospital will want to hire damaged goods to be part of their medical staff? Finding employment is going to be challenging and opening your own practice may also be difficult because the insurers have access to data bank reports.”
Ultimately, the best advice Huntoon can offer is to do your best to stay one step ahead of any work issues that could even lead to a sham peer review.
“Try and shield yourself from a sham peer review and be prepared should it happen,” he said. “I’ve seen careers end in the blink of an eye — wrongfully.”
A version of this article first appeared on Medscape.com.
While a medical peer review occurs once a patient, fellow doctor, or staff member reports that a physician failed to treat a patient up to standards or acted improperly, a “sham peer review” is undertaken for ulterior motives.
They’re typically undertaken due to professional competition or institutional politics rather than to promote quality care or uphold professional standards.
Physicians should be concerned. In a soon-to-be-published Medscape report on peer reviews, 56% of US physicians surveyed expressed higher levels of concern that a peer review could be misused to punish a physician for reasons unrelated to the matter being reviewed.
This is a troublesome issue, and many doctors may not be aware of it or how often it occurs.
“The biggest misconception about sham peer reviews is a denial of how pervasive they are,” said Andy Schlafly, general counsel for the Association of American Physicians and Surgeons (AAPS), which offers a free legal consultation service for physicians facing a sham peer review. “Many hospital administrations are as dangerous to good physicians as street gangs can be in a crime-ridden neighborhood.”
“Physicians should become aware of whether sham peer reviews are prevalent at their hospital and, if so, those physicians should look to practice somewhere else,” Schlafly said in an interview.
Unfortunately, there are limited data on how often this happens. When it does, it can be a career killer, said Lawrence Huntoon, MD, PhD, who has run the AAPS sham peer review hotline for over 20 years.
The physicians at the most risk for a sham peer review tend to be those who work for large hospital systems — as this is one way for hospitals to get rid of the doctors they don’t want to retain on staff, Huntoon said.
“Hospitals want a model whereby every physician on the medical staff is an employee,” Huntoon added. “This gives them complete power and control over these physicians, including the way they practice and how many patients they see per day, which, for some, is 20-50 a day to generate sufficient revenue.”
Complaints are generally filed via incident reporting software.
“The complaint could be that the physician is ‘disruptive,’ which can include facial expression, tone of voice, and body language — for example, ‘I found his facial expression demeaning’ or ‘I found her tone condescending’ — and this can be used to prosecute a doctor,” Huntoon said.
After the complaint is filed, the leaders of a hospital’s peer review committee meet to discuss the incident, followed by a panel of fellow physicians convened to review the matter. Once the date for a meeting is set, the accused doctor is allowed to testify, offer evidence, and have attorney representation.
The entire experience can take a physician by surprise.
“A sham peer review is difficult to prepare for because no physician thinks this is going to happen to them,” said Laurie L. York, a medical law attorney in Austin, Texas.
York added that there may also be a misperception of what is actually happening.
“When a physician becomes aware of an investigation, it initially may look like a regular peer review, and the physician may feel there has been a ‘misunderstanding’ that they can make right by explaining things,” York said. “The window of opportunity to shut down a sham peer review happens quickly. That’s why the physician needs the help of an experienced attorney as early in the process as possible.”
If You’re a Victim of a Sham Peer Review
Be vigilant. The most important thing you should think about when it comes to sham peer reviews is that this can, indeed, happen to you, Huntoon said. “I’ve written articles to help educate physicians about the tactics that are used,” he said. “You need to be educated and read medical staff bylaws to know your rights before something bad happens.”
Stay in your job. No matter what, if you’re under review, do not resign your position, no matter how difficult this may be. “A resignation during a sham peer review triggers an adverse report to the National Practitioner Data Bank [NPDB],” Schlafly said. The NPDB is a flagging system created by Congress to improve healthcare quality and reduce healthcare fraud and abuse. “A resignation also waives the physician’s right to contest the unfair review. In addition, leverage to negotiate a favorable settlement is lost if the physician simply resigns.”
Get a lawyer on board early. This is the only way to protect your rights. “Don’t wait a year to get an attorney involved,” Huntoon said. But this also can’t be any lawyer. It’s critical to find someone who specializes in sham peer reviews, so be sure to ask about their experience in handling peer review matters in hospitals and how knowledgeable they are about databank reporting requirements. “Sometimes, doctors will hire a malpractice attorney with no knowledge of what happens with sham peer reviews, and they may give bad advice,” he said. “Others may hire an employment attorney and that attorney will be up on employment law but has no experience with peer review matters in hospitals.”
Given the seriousness of a sham peer review, following these guidelines can help.
Contact the AAPA right away. There are things that can be done early on like getting a withdrawal of the request for corrective action as well as obtaining a preliminary injunction. Preparing for the fallout that may occur can be just as challenging.
“After this situation, the doctor is damaged goods,” Huntoon said. “What hospital will want to hire damaged goods to be part of their medical staff? Finding employment is going to be challenging and opening your own practice may also be difficult because the insurers have access to data bank reports.”
Ultimately, the best advice Huntoon can offer is to do your best to stay one step ahead of any work issues that could even lead to a sham peer review.
“Try and shield yourself from a sham peer review and be prepared should it happen,” he said. “I’ve seen careers end in the blink of an eye — wrongfully.”
A version of this article first appeared on Medscape.com.
While a medical peer review occurs once a patient, fellow doctor, or staff member reports that a physician failed to treat a patient up to standards or acted improperly, a “sham peer review” is undertaken for ulterior motives.
They’re typically undertaken due to professional competition or institutional politics rather than to promote quality care or uphold professional standards.
Physicians should be concerned. In a soon-to-be-published Medscape report on peer reviews, 56% of US physicians surveyed expressed higher levels of concern that a peer review could be misused to punish a physician for reasons unrelated to the matter being reviewed.
This is a troublesome issue, and many doctors may not be aware of it or how often it occurs.
“The biggest misconception about sham peer reviews is a denial of how pervasive they are,” said Andy Schlafly, general counsel for the Association of American Physicians and Surgeons (AAPS), which offers a free legal consultation service for physicians facing a sham peer review. “Many hospital administrations are as dangerous to good physicians as street gangs can be in a crime-ridden neighborhood.”
“Physicians should become aware of whether sham peer reviews are prevalent at their hospital and, if so, those physicians should look to practice somewhere else,” Schlafly said in an interview.
Unfortunately, there are limited data on how often this happens. When it does, it can be a career killer, said Lawrence Huntoon, MD, PhD, who has run the AAPS sham peer review hotline for over 20 years.
The physicians at the most risk for a sham peer review tend to be those who work for large hospital systems — as this is one way for hospitals to get rid of the doctors they don’t want to retain on staff, Huntoon said.
“Hospitals want a model whereby every physician on the medical staff is an employee,” Huntoon added. “This gives them complete power and control over these physicians, including the way they practice and how many patients they see per day, which, for some, is 20-50 a day to generate sufficient revenue.”
Complaints are generally filed via incident reporting software.
“The complaint could be that the physician is ‘disruptive,’ which can include facial expression, tone of voice, and body language — for example, ‘I found his facial expression demeaning’ or ‘I found her tone condescending’ — and this can be used to prosecute a doctor,” Huntoon said.
After the complaint is filed, the leaders of a hospital’s peer review committee meet to discuss the incident, followed by a panel of fellow physicians convened to review the matter. Once the date for a meeting is set, the accused doctor is allowed to testify, offer evidence, and have attorney representation.
The entire experience can take a physician by surprise.
“A sham peer review is difficult to prepare for because no physician thinks this is going to happen to them,” said Laurie L. York, a medical law attorney in Austin, Texas.
York added that there may also be a misperception of what is actually happening.
“When a physician becomes aware of an investigation, it initially may look like a regular peer review, and the physician may feel there has been a ‘misunderstanding’ that they can make right by explaining things,” York said. “The window of opportunity to shut down a sham peer review happens quickly. That’s why the physician needs the help of an experienced attorney as early in the process as possible.”
If You’re a Victim of a Sham Peer Review
Be vigilant. The most important thing you should think about when it comes to sham peer reviews is that this can, indeed, happen to you, Huntoon said. “I’ve written articles to help educate physicians about the tactics that are used,” he said. “You need to be educated and read medical staff bylaws to know your rights before something bad happens.”
Stay in your job. No matter what, if you’re under review, do not resign your position, no matter how difficult this may be. “A resignation during a sham peer review triggers an adverse report to the National Practitioner Data Bank [NPDB],” Schlafly said. The NPDB is a flagging system created by Congress to improve healthcare quality and reduce healthcare fraud and abuse. “A resignation also waives the physician’s right to contest the unfair review. In addition, leverage to negotiate a favorable settlement is lost if the physician simply resigns.”
Get a lawyer on board early. This is the only way to protect your rights. “Don’t wait a year to get an attorney involved,” Huntoon said. But this also can’t be any lawyer. It’s critical to find someone who specializes in sham peer reviews, so be sure to ask about their experience in handling peer review matters in hospitals and how knowledgeable they are about databank reporting requirements. “Sometimes, doctors will hire a malpractice attorney with no knowledge of what happens with sham peer reviews, and they may give bad advice,” he said. “Others may hire an employment attorney and that attorney will be up on employment law but has no experience with peer review matters in hospitals.”
Given the seriousness of a sham peer review, following these guidelines can help.
Contact the AAPA right away. There are things that can be done early on like getting a withdrawal of the request for corrective action as well as obtaining a preliminary injunction. Preparing for the fallout that may occur can be just as challenging.
“After this situation, the doctor is damaged goods,” Huntoon said. “What hospital will want to hire damaged goods to be part of their medical staff? Finding employment is going to be challenging and opening your own practice may also be difficult because the insurers have access to data bank reports.”
Ultimately, the best advice Huntoon can offer is to do your best to stay one step ahead of any work issues that could even lead to a sham peer review.
“Try and shield yourself from a sham peer review and be prepared should it happen,” he said. “I’ve seen careers end in the blink of an eye — wrongfully.”
A version of this article first appeared on Medscape.com.
The Bad News Behind the Rise in Locum Tenens
I’ve worked locum tenens off and on since 1982. Flexible schedules allowed me to write several books, pursue a parallel career as a medical journalist, lead medical missions in the Philippines, and develop modest expertise as an underwater photographer.
But the recent rise in locum tenens practitioners signals trouble for medicine.
A Multibillion-Dollar Industry
Roughly 52,000 US doctors work locum tenens full or part time. In annual reports by CHG Healthcare, two thirds of healthcare facilities surveyed report using locums and more than half expect to maintain or increase their use in 2024.
Another measure of the industry’s growth is that membership of The National Association of Locum Tenens Organizations (NALTO), formed in 2001 to lead this fledgling industry, has doubled since 2019. Currently, NALTO has 148 member agencies.
Why Locums?
What used to be the preserve of older physicians transitioning to retirement is now becoming a career choice. According to the 2024 Survey of Locum Tenens Physicians and Advanced Practice Professionals by AMN Healthcare, 81% of respondents said they started taking locum tenens assignments immediately after finishing medical training or in mid-career. What entices doctors to move from place to place, repeatedly adapt to new facilities and electronic medical records, live in cheap hotels, and work without paid vacations, health insurance, or retirement benefits?
Supplemental income is one reason. But the elephant in the room is clearly burnout. Rates of burnout in practicing doctors and physicians-in-training have exceeded 50%. Burnout results in medical errors, malpractice suits, and increased healthcare costs.
A recent Doximity poll of 7590 physicians revealed that 63% would not want their children to pursue a medical career. And in a Medscape survey of 7000 physicians, a third of docs under 40 would not choose medicine again if they had a do-over. If a career in medicine brings high income and privileged status, why do so many physicians regret it and discourage their children from taking the same path?
Where Is Marcus Welby, MD?
Private practice is an endangered species that no one is trying to save. According to a 2022 AMA survey, 44% of physicians owned their practices compared with 76% of physicians in the 1980s. Even fewer younger physicians are choosing private practice. Among physicians under 45 years of age, only 32% owned their practices. Most physicians are now employees, not employers. They have lost control over their duties and work hours.
In 2022, barely 13% of physicians were in solo practice. The iconic Dr Marcus Welby of the 1970s TV series has transmuted from an idealized physician to an implausible figure. (My medical students have never heard of him.)
Hospitals and health systems have purchased many private medical groups. Private-equity companies own close to 1000 physician practices and staff up to 40% of emergency rooms. For these firms, profits are paramount.
Canary in a Coal Mine
Locum tenens offers physicians unprecedented flexibility where they work, when they work, and how much they work. It provides an escape from overwhelming and unsatisfying clinical practice. While some physicians have fled to nonclinical careers, locums physicians can practice medicine without the burdens of administration, hospital politics, and ever-increasing overhead.
The locum tenens paradox is that its successful growth indicates a deteriorating traditional healthcare model. Locum tenens is not the problem, but it’s also not the solution. At best, locums is a pair of crutches that helps the current system limp along.
Healthcare is increasingly controlled by those who prioritize profit, not patients. If physicians become nothing more than complicit cogs in a dysfunctional system, burnout will fester. The profession will fail to attract the best and the brightest, the doctor shortage will increase, and the quality of patient care will decline. Everyone will suffer.
It’s already happening.
Andrew Wilner is an associate professor of neurology at the University of Tennessee Health Science Center, Memphis. He reported conflicts of interest from Accordant Health Services.
A version of this article first appeared on Medscape.com.
I’ve worked locum tenens off and on since 1982. Flexible schedules allowed me to write several books, pursue a parallel career as a medical journalist, lead medical missions in the Philippines, and develop modest expertise as an underwater photographer.
But the recent rise in locum tenens practitioners signals trouble for medicine.
A Multibillion-Dollar Industry
Roughly 52,000 US doctors work locum tenens full or part time. In annual reports by CHG Healthcare, two thirds of healthcare facilities surveyed report using locums and more than half expect to maintain or increase their use in 2024.
Another measure of the industry’s growth is that membership of The National Association of Locum Tenens Organizations (NALTO), formed in 2001 to lead this fledgling industry, has doubled since 2019. Currently, NALTO has 148 member agencies.
Why Locums?
What used to be the preserve of older physicians transitioning to retirement is now becoming a career choice. According to the 2024 Survey of Locum Tenens Physicians and Advanced Practice Professionals by AMN Healthcare, 81% of respondents said they started taking locum tenens assignments immediately after finishing medical training or in mid-career. What entices doctors to move from place to place, repeatedly adapt to new facilities and electronic medical records, live in cheap hotels, and work without paid vacations, health insurance, or retirement benefits?
Supplemental income is one reason. But the elephant in the room is clearly burnout. Rates of burnout in practicing doctors and physicians-in-training have exceeded 50%. Burnout results in medical errors, malpractice suits, and increased healthcare costs.
A recent Doximity poll of 7590 physicians revealed that 63% would not want their children to pursue a medical career. And in a Medscape survey of 7000 physicians, a third of docs under 40 would not choose medicine again if they had a do-over. If a career in medicine brings high income and privileged status, why do so many physicians regret it and discourage their children from taking the same path?
Where Is Marcus Welby, MD?
Private practice is an endangered species that no one is trying to save. According to a 2022 AMA survey, 44% of physicians owned their practices compared with 76% of physicians in the 1980s. Even fewer younger physicians are choosing private practice. Among physicians under 45 years of age, only 32% owned their practices. Most physicians are now employees, not employers. They have lost control over their duties and work hours.
In 2022, barely 13% of physicians were in solo practice. The iconic Dr Marcus Welby of the 1970s TV series has transmuted from an idealized physician to an implausible figure. (My medical students have never heard of him.)
Hospitals and health systems have purchased many private medical groups. Private-equity companies own close to 1000 physician practices and staff up to 40% of emergency rooms. For these firms, profits are paramount.
Canary in a Coal Mine
Locum tenens offers physicians unprecedented flexibility where they work, when they work, and how much they work. It provides an escape from overwhelming and unsatisfying clinical practice. While some physicians have fled to nonclinical careers, locums physicians can practice medicine without the burdens of administration, hospital politics, and ever-increasing overhead.
The locum tenens paradox is that its successful growth indicates a deteriorating traditional healthcare model. Locum tenens is not the problem, but it’s also not the solution. At best, locums is a pair of crutches that helps the current system limp along.
Healthcare is increasingly controlled by those who prioritize profit, not patients. If physicians become nothing more than complicit cogs in a dysfunctional system, burnout will fester. The profession will fail to attract the best and the brightest, the doctor shortage will increase, and the quality of patient care will decline. Everyone will suffer.
It’s already happening.
Andrew Wilner is an associate professor of neurology at the University of Tennessee Health Science Center, Memphis. He reported conflicts of interest from Accordant Health Services.
A version of this article first appeared on Medscape.com.
I’ve worked locum tenens off and on since 1982. Flexible schedules allowed me to write several books, pursue a parallel career as a medical journalist, lead medical missions in the Philippines, and develop modest expertise as an underwater photographer.
But the recent rise in locum tenens practitioners signals trouble for medicine.
A Multibillion-Dollar Industry
Roughly 52,000 US doctors work locum tenens full or part time. In annual reports by CHG Healthcare, two thirds of healthcare facilities surveyed report using locums and more than half expect to maintain or increase their use in 2024.
Another measure of the industry’s growth is that membership of The National Association of Locum Tenens Organizations (NALTO), formed in 2001 to lead this fledgling industry, has doubled since 2019. Currently, NALTO has 148 member agencies.
Why Locums?
What used to be the preserve of older physicians transitioning to retirement is now becoming a career choice. According to the 2024 Survey of Locum Tenens Physicians and Advanced Practice Professionals by AMN Healthcare, 81% of respondents said they started taking locum tenens assignments immediately after finishing medical training or in mid-career. What entices doctors to move from place to place, repeatedly adapt to new facilities and electronic medical records, live in cheap hotels, and work without paid vacations, health insurance, or retirement benefits?
Supplemental income is one reason. But the elephant in the room is clearly burnout. Rates of burnout in practicing doctors and physicians-in-training have exceeded 50%. Burnout results in medical errors, malpractice suits, and increased healthcare costs.
A recent Doximity poll of 7590 physicians revealed that 63% would not want their children to pursue a medical career. And in a Medscape survey of 7000 physicians, a third of docs under 40 would not choose medicine again if they had a do-over. If a career in medicine brings high income and privileged status, why do so many physicians regret it and discourage their children from taking the same path?
Where Is Marcus Welby, MD?
Private practice is an endangered species that no one is trying to save. According to a 2022 AMA survey, 44% of physicians owned their practices compared with 76% of physicians in the 1980s. Even fewer younger physicians are choosing private practice. Among physicians under 45 years of age, only 32% owned their practices. Most physicians are now employees, not employers. They have lost control over their duties and work hours.
In 2022, barely 13% of physicians were in solo practice. The iconic Dr Marcus Welby of the 1970s TV series has transmuted from an idealized physician to an implausible figure. (My medical students have never heard of him.)
Hospitals and health systems have purchased many private medical groups. Private-equity companies own close to 1000 physician practices and staff up to 40% of emergency rooms. For these firms, profits are paramount.
Canary in a Coal Mine
Locum tenens offers physicians unprecedented flexibility where they work, when they work, and how much they work. It provides an escape from overwhelming and unsatisfying clinical practice. While some physicians have fled to nonclinical careers, locums physicians can practice medicine without the burdens of administration, hospital politics, and ever-increasing overhead.
The locum tenens paradox is that its successful growth indicates a deteriorating traditional healthcare model. Locum tenens is not the problem, but it’s also not the solution. At best, locums is a pair of crutches that helps the current system limp along.
Healthcare is increasingly controlled by those who prioritize profit, not patients. If physicians become nothing more than complicit cogs in a dysfunctional system, burnout will fester. The profession will fail to attract the best and the brightest, the doctor shortage will increase, and the quality of patient care will decline. Everyone will suffer.
It’s already happening.
Andrew Wilner is an associate professor of neurology at the University of Tennessee Health Science Center, Memphis. He reported conflicts of interest from Accordant Health Services.
A version of this article first appeared on Medscape.com.
Men Wanted: New Efforts to Attract Male Nurses
Only 12% of the nurses providing patient care at hospitals and health clinics today are men. Although the percentage of nurses has increased — men made up just 2.7% of nurses in 1970 — nursing is still considered a “pink collar” profession, a female-dominated field.
“We’ve made strides over the last couple of decades, but [the number of men pursuing nursing careers] is leveling out,” said Jason Dunne, DNP, MSN, RN, chief academic officer at the Arizona College of Nursing, Phoenix. “There continues to be persistent gender stereotypes that [have] discouraged men from entering the profession.”
“The nursing shortage is very real,” Dunne said. “We need to be highly focused on the shortage and look at opportunities to bring diversity into the profession, and one big way to solve it is bringing more men into nursing.”
Representation Matters
Colleges recognize the need to diversify their nursing student population and have turned their attention to increasing the number of men attending informational sessions and career days. Dunne believes, “There is a general lack of awareness of nursing as a career choice [for men].”
The Nursing Consortium of Florida hosts a “Day in the Life of a Nurse” program to introduce high school students to nursing careers, and the University of Virginia School of Nursing invites male nursing students to speak at educational events to promote workforce diversity.
“When I was growing up, the males wouldn’t have been included in those sessions,” said Melissa Gilbert Gomes, PhD, APRN, PMHNP-BC, FNAP, FAAN, associate dean for diversity, equity, and inclusion at the University of Virginia School of Nursing, Charlottesville, Virginia. “It was nice to see their interest and to have a male student there for them to ask questions and to help them see that this could be a place for them.”
Nursing schools have also engaged in other efforts to encourage more men to consider nursing careers, from highlighting male nurses in marketing materials and engaging with men at career fairs to updating course curriculum to include content on men’s health and connecting male nursing students with men in nursing faculty or clinical settings.
Focusing on nursing as a lucrative career choice could also attract more men to the profession. On average, male registered nurses (RNs) make $7300 per year more than their female counterparts due to the gender pay gap. The median wage for male RNs in acute care, cardiology, and perioperative specialties is $90,000 annually.
At the University of Virginia School of Nursing, which the American Association for Men in Nursing (AAMN) named “Best School for Men in Nursing” in 2023, 20% of nursing students are men.
The school has a Men Advancing Nursing club and is in the process of chartering a new AAMN chapter. The goal, according to Gomes, is to create an environment where male nursing students feel represented and supported.
“Valuing the perspective that men bring [to nursing] is important,” she said. “Coming together [and] having that camaraderie and intrinsic motivation to specifically speak to areas that impact men ... is important.”
Promoting Patient Care
Highlighting the diversity of career options within the nursing profession is also essential. RNs can pursue careers in specialties ranging from pediatrics, orthopedics, and occupational health to anesthesia, cardiology, and nephrology. The specialty with the highest number of male RNs tends to be acute care, which encompasses emergency/trauma and medical-surgical.
John Schmidt, DNP, MSN, BSN, faculty member and program lead for the acute care nurse practitioner program at Purdue Global School of Nursing, refers to these specialties as having a high excitement factor.
“Men gravitate to nursing to help people,” he said. “In critical care, there is instant gratification. You see patients get better. It’s the same in the [intensive care unit] and the emergency department. We take care of them and can see how we made a difference.”
When hospitals and health systems create environments that support men in nursing, patients also benefit. Research shows that patients often prefer nurses of the same gender, and a more diverse healthcare workforce has been linked to improved patient outcomes. Reducing gender inequities among nursing staff could also improve job satisfaction and retention rates for men in nursing.
“When you’re in a vulnerable space as a patient ... it’s important to know that your care provider understands you [and] having men as nurses is a part of that,” said Gomes. “Even though patients might not be used to having a male nurse at the bedside, once they have the experience, it challenges preconceived notions [and] that connection is important.”
Hospitals must proactively support men in nursing to achieve the benefits of greater gender diversity in the nursing workforce. Male nurses have fewer role models and report higher levels of loneliness, isolation, and role strain.
Groups such as NYC Men in Nursing and mentorship programs such as Men in Nursing at RUSH University College of Nursing and RUSH University Medical Center, and the North Carolina Healthcare Association Diverse Healthcare Leaders Mentorship Program were designed to provide coaching, education, and networking opportunities and connect men in nursing.
Male nurses, Dunne added, must be role models and must take the lead in changing the conversations about gender roles in nursing. Establishing support systems and mentorship opportunities is instrumental in inspiring men to pursue nursing careers and creating visibility into the profession and “would create a level of parity for men in the profession and encourage them to want to stay in nursing as a long-term career.”
He told this news organization that creating scholarships for men enrolled in nursing school, increasing the involvement of male nurse leaders in recruitment efforts, and updating curriculum to ensure men are reflected in the materials is also essential.
“We’ve got to be willing and open to having the conversations to end the stereotypes that have plagued the profession,” said Dunne. “And we’ve got to push men in nursing to be front and center so folks see that there are opportunities for men in nursing.”
A version of this article appeared on Medscape.com.
Only 12% of the nurses providing patient care at hospitals and health clinics today are men. Although the percentage of nurses has increased — men made up just 2.7% of nurses in 1970 — nursing is still considered a “pink collar” profession, a female-dominated field.
“We’ve made strides over the last couple of decades, but [the number of men pursuing nursing careers] is leveling out,” said Jason Dunne, DNP, MSN, RN, chief academic officer at the Arizona College of Nursing, Phoenix. “There continues to be persistent gender stereotypes that [have] discouraged men from entering the profession.”
“The nursing shortage is very real,” Dunne said. “We need to be highly focused on the shortage and look at opportunities to bring diversity into the profession, and one big way to solve it is bringing more men into nursing.”
Representation Matters
Colleges recognize the need to diversify their nursing student population and have turned their attention to increasing the number of men attending informational sessions and career days. Dunne believes, “There is a general lack of awareness of nursing as a career choice [for men].”
The Nursing Consortium of Florida hosts a “Day in the Life of a Nurse” program to introduce high school students to nursing careers, and the University of Virginia School of Nursing invites male nursing students to speak at educational events to promote workforce diversity.
“When I was growing up, the males wouldn’t have been included in those sessions,” said Melissa Gilbert Gomes, PhD, APRN, PMHNP-BC, FNAP, FAAN, associate dean for diversity, equity, and inclusion at the University of Virginia School of Nursing, Charlottesville, Virginia. “It was nice to see their interest and to have a male student there for them to ask questions and to help them see that this could be a place for them.”
Nursing schools have also engaged in other efforts to encourage more men to consider nursing careers, from highlighting male nurses in marketing materials and engaging with men at career fairs to updating course curriculum to include content on men’s health and connecting male nursing students with men in nursing faculty or clinical settings.
Focusing on nursing as a lucrative career choice could also attract more men to the profession. On average, male registered nurses (RNs) make $7300 per year more than their female counterparts due to the gender pay gap. The median wage for male RNs in acute care, cardiology, and perioperative specialties is $90,000 annually.
At the University of Virginia School of Nursing, which the American Association for Men in Nursing (AAMN) named “Best School for Men in Nursing” in 2023, 20% of nursing students are men.
The school has a Men Advancing Nursing club and is in the process of chartering a new AAMN chapter. The goal, according to Gomes, is to create an environment where male nursing students feel represented and supported.
“Valuing the perspective that men bring [to nursing] is important,” she said. “Coming together [and] having that camaraderie and intrinsic motivation to specifically speak to areas that impact men ... is important.”
Promoting Patient Care
Highlighting the diversity of career options within the nursing profession is also essential. RNs can pursue careers in specialties ranging from pediatrics, orthopedics, and occupational health to anesthesia, cardiology, and nephrology. The specialty with the highest number of male RNs tends to be acute care, which encompasses emergency/trauma and medical-surgical.
John Schmidt, DNP, MSN, BSN, faculty member and program lead for the acute care nurse practitioner program at Purdue Global School of Nursing, refers to these specialties as having a high excitement factor.
“Men gravitate to nursing to help people,” he said. “In critical care, there is instant gratification. You see patients get better. It’s the same in the [intensive care unit] and the emergency department. We take care of them and can see how we made a difference.”
When hospitals and health systems create environments that support men in nursing, patients also benefit. Research shows that patients often prefer nurses of the same gender, and a more diverse healthcare workforce has been linked to improved patient outcomes. Reducing gender inequities among nursing staff could also improve job satisfaction and retention rates for men in nursing.
“When you’re in a vulnerable space as a patient ... it’s important to know that your care provider understands you [and] having men as nurses is a part of that,” said Gomes. “Even though patients might not be used to having a male nurse at the bedside, once they have the experience, it challenges preconceived notions [and] that connection is important.”
Hospitals must proactively support men in nursing to achieve the benefits of greater gender diversity in the nursing workforce. Male nurses have fewer role models and report higher levels of loneliness, isolation, and role strain.
Groups such as NYC Men in Nursing and mentorship programs such as Men in Nursing at RUSH University College of Nursing and RUSH University Medical Center, and the North Carolina Healthcare Association Diverse Healthcare Leaders Mentorship Program were designed to provide coaching, education, and networking opportunities and connect men in nursing.
Male nurses, Dunne added, must be role models and must take the lead in changing the conversations about gender roles in nursing. Establishing support systems and mentorship opportunities is instrumental in inspiring men to pursue nursing careers and creating visibility into the profession and “would create a level of parity for men in the profession and encourage them to want to stay in nursing as a long-term career.”
He told this news organization that creating scholarships for men enrolled in nursing school, increasing the involvement of male nurse leaders in recruitment efforts, and updating curriculum to ensure men are reflected in the materials is also essential.
“We’ve got to be willing and open to having the conversations to end the stereotypes that have plagued the profession,” said Dunne. “And we’ve got to push men in nursing to be front and center so folks see that there are opportunities for men in nursing.”
A version of this article appeared on Medscape.com.
Only 12% of the nurses providing patient care at hospitals and health clinics today are men. Although the percentage of nurses has increased — men made up just 2.7% of nurses in 1970 — nursing is still considered a “pink collar” profession, a female-dominated field.
“We’ve made strides over the last couple of decades, but [the number of men pursuing nursing careers] is leveling out,” said Jason Dunne, DNP, MSN, RN, chief academic officer at the Arizona College of Nursing, Phoenix. “There continues to be persistent gender stereotypes that [have] discouraged men from entering the profession.”
“The nursing shortage is very real,” Dunne said. “We need to be highly focused on the shortage and look at opportunities to bring diversity into the profession, and one big way to solve it is bringing more men into nursing.”
Representation Matters
Colleges recognize the need to diversify their nursing student population and have turned their attention to increasing the number of men attending informational sessions and career days. Dunne believes, “There is a general lack of awareness of nursing as a career choice [for men].”
The Nursing Consortium of Florida hosts a “Day in the Life of a Nurse” program to introduce high school students to nursing careers, and the University of Virginia School of Nursing invites male nursing students to speak at educational events to promote workforce diversity.
“When I was growing up, the males wouldn’t have been included in those sessions,” said Melissa Gilbert Gomes, PhD, APRN, PMHNP-BC, FNAP, FAAN, associate dean for diversity, equity, and inclusion at the University of Virginia School of Nursing, Charlottesville, Virginia. “It was nice to see their interest and to have a male student there for them to ask questions and to help them see that this could be a place for them.”
Nursing schools have also engaged in other efforts to encourage more men to consider nursing careers, from highlighting male nurses in marketing materials and engaging with men at career fairs to updating course curriculum to include content on men’s health and connecting male nursing students with men in nursing faculty or clinical settings.
Focusing on nursing as a lucrative career choice could also attract more men to the profession. On average, male registered nurses (RNs) make $7300 per year more than their female counterparts due to the gender pay gap. The median wage for male RNs in acute care, cardiology, and perioperative specialties is $90,000 annually.
At the University of Virginia School of Nursing, which the American Association for Men in Nursing (AAMN) named “Best School for Men in Nursing” in 2023, 20% of nursing students are men.
The school has a Men Advancing Nursing club and is in the process of chartering a new AAMN chapter. The goal, according to Gomes, is to create an environment where male nursing students feel represented and supported.
“Valuing the perspective that men bring [to nursing] is important,” she said. “Coming together [and] having that camaraderie and intrinsic motivation to specifically speak to areas that impact men ... is important.”
Promoting Patient Care
Highlighting the diversity of career options within the nursing profession is also essential. RNs can pursue careers in specialties ranging from pediatrics, orthopedics, and occupational health to anesthesia, cardiology, and nephrology. The specialty with the highest number of male RNs tends to be acute care, which encompasses emergency/trauma and medical-surgical.
John Schmidt, DNP, MSN, BSN, faculty member and program lead for the acute care nurse practitioner program at Purdue Global School of Nursing, refers to these specialties as having a high excitement factor.
“Men gravitate to nursing to help people,” he said. “In critical care, there is instant gratification. You see patients get better. It’s the same in the [intensive care unit] and the emergency department. We take care of them and can see how we made a difference.”
When hospitals and health systems create environments that support men in nursing, patients also benefit. Research shows that patients often prefer nurses of the same gender, and a more diverse healthcare workforce has been linked to improved patient outcomes. Reducing gender inequities among nursing staff could also improve job satisfaction and retention rates for men in nursing.
“When you’re in a vulnerable space as a patient ... it’s important to know that your care provider understands you [and] having men as nurses is a part of that,” said Gomes. “Even though patients might not be used to having a male nurse at the bedside, once they have the experience, it challenges preconceived notions [and] that connection is important.”
Hospitals must proactively support men in nursing to achieve the benefits of greater gender diversity in the nursing workforce. Male nurses have fewer role models and report higher levels of loneliness, isolation, and role strain.
Groups such as NYC Men in Nursing and mentorship programs such as Men in Nursing at RUSH University College of Nursing and RUSH University Medical Center, and the North Carolina Healthcare Association Diverse Healthcare Leaders Mentorship Program were designed to provide coaching, education, and networking opportunities and connect men in nursing.
Male nurses, Dunne added, must be role models and must take the lead in changing the conversations about gender roles in nursing. Establishing support systems and mentorship opportunities is instrumental in inspiring men to pursue nursing careers and creating visibility into the profession and “would create a level of parity for men in the profession and encourage them to want to stay in nursing as a long-term career.”
He told this news organization that creating scholarships for men enrolled in nursing school, increasing the involvement of male nurse leaders in recruitment efforts, and updating curriculum to ensure men are reflected in the materials is also essential.
“We’ve got to be willing and open to having the conversations to end the stereotypes that have plagued the profession,” said Dunne. “And we’ve got to push men in nursing to be front and center so folks see that there are opportunities for men in nursing.”
A version of this article appeared on Medscape.com.
Lawmakers Rush to Stave Off Doctor Pay Cuts as Medicare Finalizes 2025 Rates
Federal lawmakers are rushing to soften the blow of Medicare’s 2025 effective pay cut for doctors in 2025, introducing a bill that could limit the cut. But they have little time to act.
In 2025, the conversion factor used to calculate payment to doctors and hospitals caring for Medicare patients will drop to $32.35, a nearly 3% decrease from the current level.
Congress likely will act before the cuts take effect, said Rep. Larry Bucshon, MD (R-IN), who specialized in cardiothoracic surgery before joining Congress. Lawmakers in past years have typically tinkered with the Medicare physician fee schedule at the last minute, tucking in fixes to December legislative packages and spending bills.
“I’m pretty optimistic that a good portion of the fee cuts will be mitigated and they won’t go through,” Bucshon told this news organization in an interview.
Bruce A. Scott, MD, president of the American Medical Association (AMA) said in a statement that CMS’ release of the final fee schedule on November 1 should trigger serious work on a change to the 2025 Medicare physician fee schedule.
“The fee schedule rule released [on November 1] starts the clock — with January 1 looming,” Scott said. “A legislative remedy will require hard work and compromise. The 66 million patients who rely on Medicare are counting on that.”
Both Bucshon and Scott also joined many lawmakers and medical associations in calling on Congress for a larger overhaul of the Medicare physician fee schedule, well beyond whatever temporary adjustment may be made in the months ahead to avoid or soften the 2025 cuts.
The physician fee schedule sets formulas and rules regarding how the largest US buyer of health services pays the almost 1.3 million clinicians who bill Medicare. Of these, 51% are physicians. The physician fee schedule also covers payments for nurse practitioners, physician assistants, physical therapists, and other health professionals.
Last Major Overhaul Unpopular
There’s broad dissatisfaction with Congress’ last major overhaul of the Medicare physician fee schedule. The 2015 Medicare Access and CHIP Reauthorization Act (MACRA) aimed to shift clinicians toward programs tying pay increases to quality measures. But the implementation of that aim through the Merit-based Incentive Payment System is widely considered a disappointment.
MACRA was intended to end the need for annual “doc fixes,” as Congress’ last-minute Medicare adjustments are known. Seventeen such tweaks passed before MACRA took effect.
But MACRA did not include a broad-based inflation adjuster, and some clinicians’ incomes are lagging as inflation rates — and practice costs — have risen. Scott said the Medicare Economic Index, which is a measure used to gauge increases in practice costs for clinicians, is expected to rise by 3.5%.
“To put it bluntly, Medicare plans to pay us less while costs go up. You don’t have to be an economist to know that is an unsustainable trend, though one that has been going on for decades,” Scott said. “For physician practices operating on small margins already, this means it is harder to acquire new equipment, harder to retain staff, harder to take on new Medicare patients, and harder to keep the doors open, particularly in rural and underserved areas.”
In a statement, Jen Brull, MD, president of the American Academy of Family Physicians, noted that this likely will be the fifth year in a row that Congress will need to do a patch to prevent cuts in pay to clinicians.
Bucshon, who will retire from the House in January, said he expects Congress to pass legislation tying Medicare payment rates to inflation — eventually.
“People want to find a way to fix this problem, but also do it in a way that does not cut benefits to anyone, and that’s the key,” Bucshon said. “We’re going to have to find a way to make sure that providers are properly reimbursed.”
A version of this article first appeared on Medscape.com.
Federal lawmakers are rushing to soften the blow of Medicare’s 2025 effective pay cut for doctors in 2025, introducing a bill that could limit the cut. But they have little time to act.
In 2025, the conversion factor used to calculate payment to doctors and hospitals caring for Medicare patients will drop to $32.35, a nearly 3% decrease from the current level.
Congress likely will act before the cuts take effect, said Rep. Larry Bucshon, MD (R-IN), who specialized in cardiothoracic surgery before joining Congress. Lawmakers in past years have typically tinkered with the Medicare physician fee schedule at the last minute, tucking in fixes to December legislative packages and spending bills.
“I’m pretty optimistic that a good portion of the fee cuts will be mitigated and they won’t go through,” Bucshon told this news organization in an interview.
Bruce A. Scott, MD, president of the American Medical Association (AMA) said in a statement that CMS’ release of the final fee schedule on November 1 should trigger serious work on a change to the 2025 Medicare physician fee schedule.
“The fee schedule rule released [on November 1] starts the clock — with January 1 looming,” Scott said. “A legislative remedy will require hard work and compromise. The 66 million patients who rely on Medicare are counting on that.”
Both Bucshon and Scott also joined many lawmakers and medical associations in calling on Congress for a larger overhaul of the Medicare physician fee schedule, well beyond whatever temporary adjustment may be made in the months ahead to avoid or soften the 2025 cuts.
The physician fee schedule sets formulas and rules regarding how the largest US buyer of health services pays the almost 1.3 million clinicians who bill Medicare. Of these, 51% are physicians. The physician fee schedule also covers payments for nurse practitioners, physician assistants, physical therapists, and other health professionals.
Last Major Overhaul Unpopular
There’s broad dissatisfaction with Congress’ last major overhaul of the Medicare physician fee schedule. The 2015 Medicare Access and CHIP Reauthorization Act (MACRA) aimed to shift clinicians toward programs tying pay increases to quality measures. But the implementation of that aim through the Merit-based Incentive Payment System is widely considered a disappointment.
MACRA was intended to end the need for annual “doc fixes,” as Congress’ last-minute Medicare adjustments are known. Seventeen such tweaks passed before MACRA took effect.
But MACRA did not include a broad-based inflation adjuster, and some clinicians’ incomes are lagging as inflation rates — and practice costs — have risen. Scott said the Medicare Economic Index, which is a measure used to gauge increases in practice costs for clinicians, is expected to rise by 3.5%.
“To put it bluntly, Medicare plans to pay us less while costs go up. You don’t have to be an economist to know that is an unsustainable trend, though one that has been going on for decades,” Scott said. “For physician practices operating on small margins already, this means it is harder to acquire new equipment, harder to retain staff, harder to take on new Medicare patients, and harder to keep the doors open, particularly in rural and underserved areas.”
In a statement, Jen Brull, MD, president of the American Academy of Family Physicians, noted that this likely will be the fifth year in a row that Congress will need to do a patch to prevent cuts in pay to clinicians.
Bucshon, who will retire from the House in January, said he expects Congress to pass legislation tying Medicare payment rates to inflation — eventually.
“People want to find a way to fix this problem, but also do it in a way that does not cut benefits to anyone, and that’s the key,” Bucshon said. “We’re going to have to find a way to make sure that providers are properly reimbursed.”
A version of this article first appeared on Medscape.com.
Federal lawmakers are rushing to soften the blow of Medicare’s 2025 effective pay cut for doctors in 2025, introducing a bill that could limit the cut. But they have little time to act.
In 2025, the conversion factor used to calculate payment to doctors and hospitals caring for Medicare patients will drop to $32.35, a nearly 3% decrease from the current level.
Congress likely will act before the cuts take effect, said Rep. Larry Bucshon, MD (R-IN), who specialized in cardiothoracic surgery before joining Congress. Lawmakers in past years have typically tinkered with the Medicare physician fee schedule at the last minute, tucking in fixes to December legislative packages and spending bills.
“I’m pretty optimistic that a good portion of the fee cuts will be mitigated and they won’t go through,” Bucshon told this news organization in an interview.
Bruce A. Scott, MD, president of the American Medical Association (AMA) said in a statement that CMS’ release of the final fee schedule on November 1 should trigger serious work on a change to the 2025 Medicare physician fee schedule.
“The fee schedule rule released [on November 1] starts the clock — with January 1 looming,” Scott said. “A legislative remedy will require hard work and compromise. The 66 million patients who rely on Medicare are counting on that.”
Both Bucshon and Scott also joined many lawmakers and medical associations in calling on Congress for a larger overhaul of the Medicare physician fee schedule, well beyond whatever temporary adjustment may be made in the months ahead to avoid or soften the 2025 cuts.
The physician fee schedule sets formulas and rules regarding how the largest US buyer of health services pays the almost 1.3 million clinicians who bill Medicare. Of these, 51% are physicians. The physician fee schedule also covers payments for nurse practitioners, physician assistants, physical therapists, and other health professionals.
Last Major Overhaul Unpopular
There’s broad dissatisfaction with Congress’ last major overhaul of the Medicare physician fee schedule. The 2015 Medicare Access and CHIP Reauthorization Act (MACRA) aimed to shift clinicians toward programs tying pay increases to quality measures. But the implementation of that aim through the Merit-based Incentive Payment System is widely considered a disappointment.
MACRA was intended to end the need for annual “doc fixes,” as Congress’ last-minute Medicare adjustments are known. Seventeen such tweaks passed before MACRA took effect.
But MACRA did not include a broad-based inflation adjuster, and some clinicians’ incomes are lagging as inflation rates — and practice costs — have risen. Scott said the Medicare Economic Index, which is a measure used to gauge increases in practice costs for clinicians, is expected to rise by 3.5%.
“To put it bluntly, Medicare plans to pay us less while costs go up. You don’t have to be an economist to know that is an unsustainable trend, though one that has been going on for decades,” Scott said. “For physician practices operating on small margins already, this means it is harder to acquire new equipment, harder to retain staff, harder to take on new Medicare patients, and harder to keep the doors open, particularly in rural and underserved areas.”
In a statement, Jen Brull, MD, president of the American Academy of Family Physicians, noted that this likely will be the fifth year in a row that Congress will need to do a patch to prevent cuts in pay to clinicians.
Bucshon, who will retire from the House in January, said he expects Congress to pass legislation tying Medicare payment rates to inflation — eventually.
“People want to find a way to fix this problem, but also do it in a way that does not cut benefits to anyone, and that’s the key,” Bucshon said. “We’re going to have to find a way to make sure that providers are properly reimbursed.”
A version of this article first appeared on Medscape.com.
RA Prevention: A Decade of Trials Provides Insights on What’s to Come
With the discovery of autoantibodies and other risk factors for rheumatoid arthritis (RA), researchers developed clinical trials to see whether the disease can be prevented entirely. In the past 10 years, a number of these trials have concluded, with variable results.
While some trials demonstrated no effect at all, others showed that medical intervention can delay the onset of disease in certain populations and even reduce the rates of progression to RA. These completed trials also offer researchers the chance to identify opportunities to improve RA prevention trials moving forward.
“We’re looking at all that data and trying to figure out what the next step is going to be,” said Kevin Deane, MD, PhD, a professor of medicine and a rheumatologist at the University of Colorado School of Medicine, Aurora.
Key lessons include the need for improved risk stratification tools and better understanding of RA pathogenesis, he said.
The Research So Far
All RA prevention trials except for one have been completed and/or published within the past decade, bringing valuable insights to the field. (See chart below.)
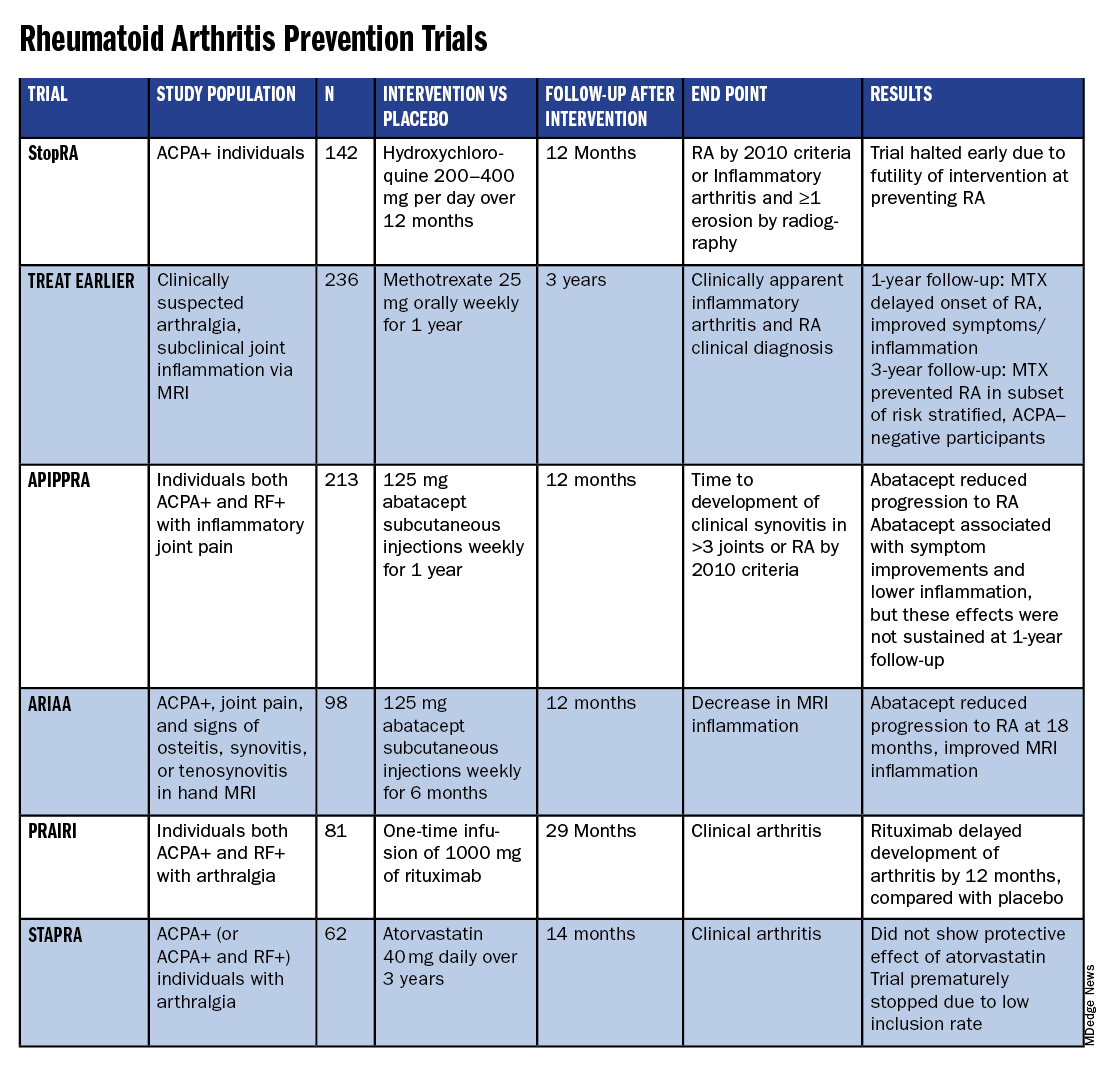
Atorvastatin (STAPRA) and hydroxychloroquine (StopRA) proved ineffective in preventing the onset of RA, and both trials were stopped early. Rituximab and methotrexate (MTX) both delayed the onset of RA, but the effect disappeared by the end of the follow-up periods.
However, the 2-year results from the TREAT EARLIER trial showed that compared with patients given placebo, those given MTX showed improved MRI-detected joint inflammation, physical functioning, and reported symptoms.
The 4-year analysis of the trial further risk stratified participants and found that MTX showed a preventive effect in anti–citrullinated protein antibody (ACPA)–negative participants at an increased risk for RA.
Abatacept also showed promise in preventing RA in two separate trials. In the ARIAA trial, compared with placebo, 6 months of treatment with abatacept reduced MRI inflammation and symptoms and lowered the rates of progression to RA. This treatment effect lessened during the 1-year follow-up period, but the difference between the two groups was still significant at 18 months.
In the APIPPRA trial, 12 months of treatment with abatacept improved subclinical inflammation and quality-of-life measures in participants and reduced the rates of progression to RA through another 12 months of observation. However, during this post-treatment follow-up period, the treatment effect began to diminish.
While there have been some promising findings — not only in disease prevention but also in disease modification — these studies all looked at different patient groups, noted Kulveer Mankia, MA, DM, an associate professor and consulting rheumatologist at the Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds in England.
“You have disparate, different inclusion criteria in different studies, all of which take years to complete,” he said. For example, while the TREAT EARLIER trial recruited patients with joint pain and subclinical joint inflammation via MRI, regardless of autoantibody status, the APIPPRA trial enrolled patients that were both ACPA+ and rheumatoid factor (RF)+ with joint pain.
“You’re left extrapolating as to whether [these interventions] will work in different at-risk populations,” he said.
Even with specific inclusion criteria in each study, there can still be heterogeneity in risk within a study group, Deane said. In the TREAT EARLIER study, 18%-20% of participants ultimately developed RA over the study period, which is lower than expected.
“While it seemed like a pretty high-risk group, it wasn’t as high risk as we thought,” he said, “and that’s why we’ve gone back to the drawing board.”
Risk Stratification Efforts
There are now two ongoing joint efforts by the American College of Rheumatology (ACR) and the European Alliance of Associations for Rheumatology (EULAR) to define these populations and “bring some consensus to the field,” Mankia said.
The first aims to create a unanimous risk stratification tool for future RA prevention studies. The proposed system, devised for individuals with new joint symptoms who are at a risk for RA, was presented at the EULAR 2024 annual meeting and will be further discussed at the upcoming ACR 2024 annual meeting in Washington, DC.
The system uses a point system based on six criteria — three lab tests and three criteria commonly assessed in clinical practice:
- Morning stiffness
- Patient-reported joint swelling
- Difficulty making a fist
- Increased C-reactive protein
- RF positivity
- ACPA positivity
These criteria were picked so that the risk stratification tool can be used without imaging; however, the inclusion of MRI can further refine the score.
The ACR-EULAR task force that created the tool has emphasized that this criterion is specifically designed for research purposes and should not be used in clinical practice. Using this stratification tool should allow future clinical studies to group patients by similar risk, Deane said.
“Not that all studies have to look at exactly the same people, but each study should have similar risk stratification,” he said.
The second ACR-EULAR joint effort is taking a population-based approach to risk stratification, Deane said, to better predict RA risk in individuals without common symptoms like joint pain.
The aim is to create something analogous to the Framingham Risk Score in predicting cardiovascular disease, in which simple variables like total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, and smoking status can be used to calculate an individual’s 10-year risk for CVD, Deane explained.
The second approach could also identify patients earlier in the progression to RA, which may be easier to treat than later stages of disease.
Understanding RA Origins
However, treating an earlier stage of disease might require a different approach. Up to this point, medical interventions for RA prevention used drugs approved to treat RA, but inventions during the pre-RA stage — before any joint symptoms appear — might require targeting different immunologic pathways.
“The general concept is if there is a pre-RA stage when joints are not involved, that means all the immunologic abnormalities are probably happening somewhere else in the body,” he said. “The big question is: Where is that, and how exactly is that happening?”
One theory is that RA begins to develop in mucosal sites, such as the intestines or lungs, before it involves synovial joints.
“In the absence of resolution, these localized immune processes transition into a systemic process that targets the joints, either by direct effects of microbiota, molecular mimicry, and/or immune amplification,” wrote Deane and coauthors in a recent review article in Annals of the Rheumatic Diseases. “This, in turn, leads to inappropriate engagement of a range of effector mechanisms in both synovium and periarticular sites.”
Following this logic, the progression of the at-risk stage of RA could be considered a continuum along which there are multiple possible points for intervention. It’s also probable that the disease can develop through multiple pathways, Deane said.
“If you look at all the people who get rheumatoid arthritis, there’s probably no way those could have the same exact pathways,” he said. “There’s probably going to be different endotypes and understanding that is going to help us prevent disease in a better way.”
Looking Forward
Beyond improving risk stratification and understanding RA pathogenesis, researchers are also considering novel therapeutic approaches for future trials. Glucagon-like peptide 1 (GLP-1) receptor agonists could be worth exploring in RA prevention and treatment, said Jeffrey A. Sparks, MD, MMSc, a rheumatologist at Brigham and Women’s Hospital, Boston, Massachusetts.
These drugs — initially developed for diabetes — have already shown anti-inflammatory effects, and one study suggested that GLP-1s lowered the risk for major adverse cardiovascular events and all-cause mortality in individuals with immune-mediated inflammatory diseases. Obesity is a known risk factor for RA, so weight loss aided by GLP-1 drugs could also help reduce risk in certain patients. Clinical trials are needed to explore GLP-1s for both RA prevention and treatment, he said.
While prevention trials up to this point have used one-time, time-limited interventions, longer durations of medication or multiple rounds of therapy may be more efficacious. Even for trials that demonstrated the intervention arms had less progression to RA, this effect diminished once participants stopped the medication. In the ARIAA and APIPPRA trials using abatacept, “it wasn’t like we hit a reset button and [patients] just permanently now did not get rheumatoid arthritis,” Deane said, suggesting that alternative approaches should be explored.
“Future studies need to look at potentially longer doses of drug or lower doses of drug, or some combination that might be effective,” he said.
Deane received honoraria from Bristol-Myers Squibb, Thermo Fisher, and Werfen and grant funding from Janssen Research and Development and Gilead Sciences. Mankia received grant support from Gilead, Lilly, AstraZeneca, and Serac Life Sciences and honoraria or consultant fees from AbbVie, UCB, Lilly, Galapagos, DeepCure, Serac Life Sciences, AstraZeneca, and Zura Bio. Sparks received research support from Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, and Sonoma Biotherapeutics. He consulted for AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Gilead, Inova Diagnostics, Janssen, Merck, Mustang, Optum, Pfizer, ReCor Medical, Sana, Sobi, and UCB.
A version of this article first appeared on Medscape.com.
With the discovery of autoantibodies and other risk factors for rheumatoid arthritis (RA), researchers developed clinical trials to see whether the disease can be prevented entirely. In the past 10 years, a number of these trials have concluded, with variable results.
While some trials demonstrated no effect at all, others showed that medical intervention can delay the onset of disease in certain populations and even reduce the rates of progression to RA. These completed trials also offer researchers the chance to identify opportunities to improve RA prevention trials moving forward.
“We’re looking at all that data and trying to figure out what the next step is going to be,” said Kevin Deane, MD, PhD, a professor of medicine and a rheumatologist at the University of Colorado School of Medicine, Aurora.
Key lessons include the need for improved risk stratification tools and better understanding of RA pathogenesis, he said.
The Research So Far
All RA prevention trials except for one have been completed and/or published within the past decade, bringing valuable insights to the field. (See chart below.)

Atorvastatin (STAPRA) and hydroxychloroquine (StopRA) proved ineffective in preventing the onset of RA, and both trials were stopped early. Rituximab and methotrexate (MTX) both delayed the onset of RA, but the effect disappeared by the end of the follow-up periods.
However, the 2-year results from the TREAT EARLIER trial showed that compared with patients given placebo, those given MTX showed improved MRI-detected joint inflammation, physical functioning, and reported symptoms.
The 4-year analysis of the trial further risk stratified participants and found that MTX showed a preventive effect in anti–citrullinated protein antibody (ACPA)–negative participants at an increased risk for RA.
Abatacept also showed promise in preventing RA in two separate trials. In the ARIAA trial, compared with placebo, 6 months of treatment with abatacept reduced MRI inflammation and symptoms and lowered the rates of progression to RA. This treatment effect lessened during the 1-year follow-up period, but the difference between the two groups was still significant at 18 months.
In the APIPPRA trial, 12 months of treatment with abatacept improved subclinical inflammation and quality-of-life measures in participants and reduced the rates of progression to RA through another 12 months of observation. However, during this post-treatment follow-up period, the treatment effect began to diminish.
While there have been some promising findings — not only in disease prevention but also in disease modification — these studies all looked at different patient groups, noted Kulveer Mankia, MA, DM, an associate professor and consulting rheumatologist at the Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds in England.
“You have disparate, different inclusion criteria in different studies, all of which take years to complete,” he said. For example, while the TREAT EARLIER trial recruited patients with joint pain and subclinical joint inflammation via MRI, regardless of autoantibody status, the APIPPRA trial enrolled patients that were both ACPA+ and rheumatoid factor (RF)+ with joint pain.
“You’re left extrapolating as to whether [these interventions] will work in different at-risk populations,” he said.
Even with specific inclusion criteria in each study, there can still be heterogeneity in risk within a study group, Deane said. In the TREAT EARLIER study, 18%-20% of participants ultimately developed RA over the study period, which is lower than expected.
“While it seemed like a pretty high-risk group, it wasn’t as high risk as we thought,” he said, “and that’s why we’ve gone back to the drawing board.”
Risk Stratification Efforts
There are now two ongoing joint efforts by the American College of Rheumatology (ACR) and the European Alliance of Associations for Rheumatology (EULAR) to define these populations and “bring some consensus to the field,” Mankia said.
The first aims to create a unanimous risk stratification tool for future RA prevention studies. The proposed system, devised for individuals with new joint symptoms who are at a risk for RA, was presented at the EULAR 2024 annual meeting and will be further discussed at the upcoming ACR 2024 annual meeting in Washington, DC.
The system uses a point system based on six criteria — three lab tests and three criteria commonly assessed in clinical practice:
- Morning stiffness
- Patient-reported joint swelling
- Difficulty making a fist
- Increased C-reactive protein
- RF positivity
- ACPA positivity
These criteria were picked so that the risk stratification tool can be used without imaging; however, the inclusion of MRI can further refine the score.
The ACR-EULAR task force that created the tool has emphasized that this criterion is specifically designed for research purposes and should not be used in clinical practice. Using this stratification tool should allow future clinical studies to group patients by similar risk, Deane said.
“Not that all studies have to look at exactly the same people, but each study should have similar risk stratification,” he said.
The second ACR-EULAR joint effort is taking a population-based approach to risk stratification, Deane said, to better predict RA risk in individuals without common symptoms like joint pain.
The aim is to create something analogous to the Framingham Risk Score in predicting cardiovascular disease, in which simple variables like total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, and smoking status can be used to calculate an individual’s 10-year risk for CVD, Deane explained.
The second approach could also identify patients earlier in the progression to RA, which may be easier to treat than later stages of disease.
Understanding RA Origins
However, treating an earlier stage of disease might require a different approach. Up to this point, medical interventions for RA prevention used drugs approved to treat RA, but inventions during the pre-RA stage — before any joint symptoms appear — might require targeting different immunologic pathways.
“The general concept is if there is a pre-RA stage when joints are not involved, that means all the immunologic abnormalities are probably happening somewhere else in the body,” he said. “The big question is: Where is that, and how exactly is that happening?”
One theory is that RA begins to develop in mucosal sites, such as the intestines or lungs, before it involves synovial joints.
“In the absence of resolution, these localized immune processes transition into a systemic process that targets the joints, either by direct effects of microbiota, molecular mimicry, and/or immune amplification,” wrote Deane and coauthors in a recent review article in Annals of the Rheumatic Diseases. “This, in turn, leads to inappropriate engagement of a range of effector mechanisms in both synovium and periarticular sites.”
Following this logic, the progression of the at-risk stage of RA could be considered a continuum along which there are multiple possible points for intervention. It’s also probable that the disease can develop through multiple pathways, Deane said.
“If you look at all the people who get rheumatoid arthritis, there’s probably no way those could have the same exact pathways,” he said. “There’s probably going to be different endotypes and understanding that is going to help us prevent disease in a better way.”
Looking Forward
Beyond improving risk stratification and understanding RA pathogenesis, researchers are also considering novel therapeutic approaches for future trials. Glucagon-like peptide 1 (GLP-1) receptor agonists could be worth exploring in RA prevention and treatment, said Jeffrey A. Sparks, MD, MMSc, a rheumatologist at Brigham and Women’s Hospital, Boston, Massachusetts.
These drugs — initially developed for diabetes — have already shown anti-inflammatory effects, and one study suggested that GLP-1s lowered the risk for major adverse cardiovascular events and all-cause mortality in individuals with immune-mediated inflammatory diseases. Obesity is a known risk factor for RA, so weight loss aided by GLP-1 drugs could also help reduce risk in certain patients. Clinical trials are needed to explore GLP-1s for both RA prevention and treatment, he said.
While prevention trials up to this point have used one-time, time-limited interventions, longer durations of medication or multiple rounds of therapy may be more efficacious. Even for trials that demonstrated the intervention arms had less progression to RA, this effect diminished once participants stopped the medication. In the ARIAA and APIPPRA trials using abatacept, “it wasn’t like we hit a reset button and [patients] just permanently now did not get rheumatoid arthritis,” Deane said, suggesting that alternative approaches should be explored.
“Future studies need to look at potentially longer doses of drug or lower doses of drug, or some combination that might be effective,” he said.
Deane received honoraria from Bristol-Myers Squibb, Thermo Fisher, and Werfen and grant funding from Janssen Research and Development and Gilead Sciences. Mankia received grant support from Gilead, Lilly, AstraZeneca, and Serac Life Sciences and honoraria or consultant fees from AbbVie, UCB, Lilly, Galapagos, DeepCure, Serac Life Sciences, AstraZeneca, and Zura Bio. Sparks received research support from Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, and Sonoma Biotherapeutics. He consulted for AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Gilead, Inova Diagnostics, Janssen, Merck, Mustang, Optum, Pfizer, ReCor Medical, Sana, Sobi, and UCB.
A version of this article first appeared on Medscape.com.
With the discovery of autoantibodies and other risk factors for rheumatoid arthritis (RA), researchers developed clinical trials to see whether the disease can be prevented entirely. In the past 10 years, a number of these trials have concluded, with variable results.
While some trials demonstrated no effect at all, others showed that medical intervention can delay the onset of disease in certain populations and even reduce the rates of progression to RA. These completed trials also offer researchers the chance to identify opportunities to improve RA prevention trials moving forward.
“We’re looking at all that data and trying to figure out what the next step is going to be,” said Kevin Deane, MD, PhD, a professor of medicine and a rheumatologist at the University of Colorado School of Medicine, Aurora.
Key lessons include the need for improved risk stratification tools and better understanding of RA pathogenesis, he said.
The Research So Far
All RA prevention trials except for one have been completed and/or published within the past decade, bringing valuable insights to the field. (See chart below.)

Atorvastatin (STAPRA) and hydroxychloroquine (StopRA) proved ineffective in preventing the onset of RA, and both trials were stopped early. Rituximab and methotrexate (MTX) both delayed the onset of RA, but the effect disappeared by the end of the follow-up periods.
However, the 2-year results from the TREAT EARLIER trial showed that compared with patients given placebo, those given MTX showed improved MRI-detected joint inflammation, physical functioning, and reported symptoms.
The 4-year analysis of the trial further risk stratified participants and found that MTX showed a preventive effect in anti–citrullinated protein antibody (ACPA)–negative participants at an increased risk for RA.
Abatacept also showed promise in preventing RA in two separate trials. In the ARIAA trial, compared with placebo, 6 months of treatment with abatacept reduced MRI inflammation and symptoms and lowered the rates of progression to RA. This treatment effect lessened during the 1-year follow-up period, but the difference between the two groups was still significant at 18 months.
In the APIPPRA trial, 12 months of treatment with abatacept improved subclinical inflammation and quality-of-life measures in participants and reduced the rates of progression to RA through another 12 months of observation. However, during this post-treatment follow-up period, the treatment effect began to diminish.
While there have been some promising findings — not only in disease prevention but also in disease modification — these studies all looked at different patient groups, noted Kulveer Mankia, MA, DM, an associate professor and consulting rheumatologist at the Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds in England.
“You have disparate, different inclusion criteria in different studies, all of which take years to complete,” he said. For example, while the TREAT EARLIER trial recruited patients with joint pain and subclinical joint inflammation via MRI, regardless of autoantibody status, the APIPPRA trial enrolled patients that were both ACPA+ and rheumatoid factor (RF)+ with joint pain.
“You’re left extrapolating as to whether [these interventions] will work in different at-risk populations,” he said.
Even with specific inclusion criteria in each study, there can still be heterogeneity in risk within a study group, Deane said. In the TREAT EARLIER study, 18%-20% of participants ultimately developed RA over the study period, which is lower than expected.
“While it seemed like a pretty high-risk group, it wasn’t as high risk as we thought,” he said, “and that’s why we’ve gone back to the drawing board.”
Risk Stratification Efforts
There are now two ongoing joint efforts by the American College of Rheumatology (ACR) and the European Alliance of Associations for Rheumatology (EULAR) to define these populations and “bring some consensus to the field,” Mankia said.
The first aims to create a unanimous risk stratification tool for future RA prevention studies. The proposed system, devised for individuals with new joint symptoms who are at a risk for RA, was presented at the EULAR 2024 annual meeting and will be further discussed at the upcoming ACR 2024 annual meeting in Washington, DC.
The system uses a point system based on six criteria — three lab tests and three criteria commonly assessed in clinical practice:
- Morning stiffness
- Patient-reported joint swelling
- Difficulty making a fist
- Increased C-reactive protein
- RF positivity
- ACPA positivity
These criteria were picked so that the risk stratification tool can be used without imaging; however, the inclusion of MRI can further refine the score.
The ACR-EULAR task force that created the tool has emphasized that this criterion is specifically designed for research purposes and should not be used in clinical practice. Using this stratification tool should allow future clinical studies to group patients by similar risk, Deane said.
“Not that all studies have to look at exactly the same people, but each study should have similar risk stratification,” he said.
The second ACR-EULAR joint effort is taking a population-based approach to risk stratification, Deane said, to better predict RA risk in individuals without common symptoms like joint pain.
The aim is to create something analogous to the Framingham Risk Score in predicting cardiovascular disease, in which simple variables like total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, and smoking status can be used to calculate an individual’s 10-year risk for CVD, Deane explained.
The second approach could also identify patients earlier in the progression to RA, which may be easier to treat than later stages of disease.
Understanding RA Origins
However, treating an earlier stage of disease might require a different approach. Up to this point, medical interventions for RA prevention used drugs approved to treat RA, but inventions during the pre-RA stage — before any joint symptoms appear — might require targeting different immunologic pathways.
“The general concept is if there is a pre-RA stage when joints are not involved, that means all the immunologic abnormalities are probably happening somewhere else in the body,” he said. “The big question is: Where is that, and how exactly is that happening?”
One theory is that RA begins to develop in mucosal sites, such as the intestines or lungs, before it involves synovial joints.
“In the absence of resolution, these localized immune processes transition into a systemic process that targets the joints, either by direct effects of microbiota, molecular mimicry, and/or immune amplification,” wrote Deane and coauthors in a recent review article in Annals of the Rheumatic Diseases. “This, in turn, leads to inappropriate engagement of a range of effector mechanisms in both synovium and periarticular sites.”
Following this logic, the progression of the at-risk stage of RA could be considered a continuum along which there are multiple possible points for intervention. It’s also probable that the disease can develop through multiple pathways, Deane said.
“If you look at all the people who get rheumatoid arthritis, there’s probably no way those could have the same exact pathways,” he said. “There’s probably going to be different endotypes and understanding that is going to help us prevent disease in a better way.”
Looking Forward
Beyond improving risk stratification and understanding RA pathogenesis, researchers are also considering novel therapeutic approaches for future trials. Glucagon-like peptide 1 (GLP-1) receptor agonists could be worth exploring in RA prevention and treatment, said Jeffrey A. Sparks, MD, MMSc, a rheumatologist at Brigham and Women’s Hospital, Boston, Massachusetts.
These drugs — initially developed for diabetes — have already shown anti-inflammatory effects, and one study suggested that GLP-1s lowered the risk for major adverse cardiovascular events and all-cause mortality in individuals with immune-mediated inflammatory diseases. Obesity is a known risk factor for RA, so weight loss aided by GLP-1 drugs could also help reduce risk in certain patients. Clinical trials are needed to explore GLP-1s for both RA prevention and treatment, he said.
While prevention trials up to this point have used one-time, time-limited interventions, longer durations of medication or multiple rounds of therapy may be more efficacious. Even for trials that demonstrated the intervention arms had less progression to RA, this effect diminished once participants stopped the medication. In the ARIAA and APIPPRA trials using abatacept, “it wasn’t like we hit a reset button and [patients] just permanently now did not get rheumatoid arthritis,” Deane said, suggesting that alternative approaches should be explored.
“Future studies need to look at potentially longer doses of drug or lower doses of drug, or some combination that might be effective,” he said.
Deane received honoraria from Bristol-Myers Squibb, Thermo Fisher, and Werfen and grant funding from Janssen Research and Development and Gilead Sciences. Mankia received grant support from Gilead, Lilly, AstraZeneca, and Serac Life Sciences and honoraria or consultant fees from AbbVie, UCB, Lilly, Galapagos, DeepCure, Serac Life Sciences, AstraZeneca, and Zura Bio. Sparks received research support from Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, and Sonoma Biotherapeutics. He consulted for AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Gilead, Inova Diagnostics, Janssen, Merck, Mustang, Optum, Pfizer, ReCor Medical, Sana, Sobi, and UCB.
A version of this article first appeared on Medscape.com.
Novel Treatment Promising for Cutaneous Lupus in Phase 2 Trial
TOPLINE:
particularly in subacute and chronic cases.
METHODOLOGY:
- Researchers conducted a randomized phase 2 trial to evaluate the efficacy and safety of iberdomide in 288 patients with CLE (mean age, 45 years; 97% women). Iberdomide is a cereblon modulator, which results in degradation of two transcription factors of immune cell development and homeostasis — Ikaros and Aiolos — that have been implicated in the genetic predisposition of systemic lupus.
- CLE Disease Area and Severity Index Activity (CLASI-A) endpoints included mean percent change from baseline and ≥ 50% reduction from baseline (CLASI-50), which were evaluated in all patients with baseline CLASI-A scores ≥ 8 and by CLE subtypes (acute, subacute, and chronic).
- At baseline, 56% of patients had acute CLE, 29% had chronic CLE, and 16% had subacute CLE; 28% of patients had a baseline CLASI-A score ≥ 8.
- Patients were randomly assigned to receive oral iberdomide (0.45 mg, 0.30 mg, 0.15 mg, or placebo daily) for 24 weeks while continuing standard lupus medications. At week 24, patients on placebo were rerandomized to iberdomide 0.45 mg or 0.30 mg once a day, while those on iberdomide continued their assigned dose through week 52.
TAKEAWAY:
- Among patients with baseline CLASI-A ≥ 8, the mean change in CLASI-A score from baseline at week 24 was −66.7% for those on iberdomide 0.45 mg vs −54.2% for placebo (P = .295).
- At week 24, patients with subacute CLE showed a significantly greater mean percent change from baseline in CLASI-A with iberdomide 0.45 mg vs placebo (−90.5% vs −51.2%; P = .007), while no significant differences were observed with the 0.45-mg dose vs placebo in patients with chronic or acute CLE.
- Overall, CLASI-50 responses were not significantly different among those on 0.45 mg vs placebo (55.6% vs 44.6%). The proportions of patients achieving CLASI-50 at week 24 were significantly greater for iberdomide 0.45 mg vs placebo for those with subacute CLE (91.7% vs 52.9%; P = .035) and chronic CLE (62.1% vs 27.8%; P = .029), but not for those with baseline CLASI-A ≥ 8 (66.7% vs 50%).
- More than 80% of patients had treatment-emergent adverse events (TEAEs), which were mostly mild to moderate. Over 2 years, the most common were urinary tract infections, upper respiratory tract infections, neutropenia, and nasopharyngitis. TEAEs leading to iberdomide discontinuation in one or more patients were neutropenia (n = 7), rash (n = 7), increased hepatic enzymes (n = 4), and deep vein thrombosis (n = 3).
IN PRACTICE:
“Data from this phase 2 trial of iberdomide in patients with SLE suggest that a greater proportion of patients with subacute or chronic CLE who received the higher dose of 0.45 mg iberdomide achieved CLASI-50 vs placebo. For the overall population, CLASI-50 response was not significantly different between treatment groups at week 24, partly due to a high placebo response that may have been driven by patients with acute CLE,” the authors wrote.
SOURCE:
The study was led by Victoria P. Werth, MD, of the University of Pennsylvania and the Veteran’s Administration Medical Center, both in Philadelphia, and was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The study included small patient subgroups for different CLE subtypes, which may affect the generalizability of the findings. CLE subtype was determined by the investigator without additional photographic adjudication. Additionally, the use of background lupus medications could have influenced the placebo group’s response, limiting the ability to observe the treatment effect of iberdomide monotherapy.
DISCLOSURES:
The study was funded by Bristol-Myers Squibb. Six authors reported being employed by Bristol-Myers Squibb, and several others reported consultancy and research support from various sources including Bristol-Myers Squibb.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
particularly in subacute and chronic cases.
METHODOLOGY:
- Researchers conducted a randomized phase 2 trial to evaluate the efficacy and safety of iberdomide in 288 patients with CLE (mean age, 45 years; 97% women). Iberdomide is a cereblon modulator, which results in degradation of two transcription factors of immune cell development and homeostasis — Ikaros and Aiolos — that have been implicated in the genetic predisposition of systemic lupus.
- CLE Disease Area and Severity Index Activity (CLASI-A) endpoints included mean percent change from baseline and ≥ 50% reduction from baseline (CLASI-50), which were evaluated in all patients with baseline CLASI-A scores ≥ 8 and by CLE subtypes (acute, subacute, and chronic).
- At baseline, 56% of patients had acute CLE, 29% had chronic CLE, and 16% had subacute CLE; 28% of patients had a baseline CLASI-A score ≥ 8.
- Patients were randomly assigned to receive oral iberdomide (0.45 mg, 0.30 mg, 0.15 mg, or placebo daily) for 24 weeks while continuing standard lupus medications. At week 24, patients on placebo were rerandomized to iberdomide 0.45 mg or 0.30 mg once a day, while those on iberdomide continued their assigned dose through week 52.
TAKEAWAY:
- Among patients with baseline CLASI-A ≥ 8, the mean change in CLASI-A score from baseline at week 24 was −66.7% for those on iberdomide 0.45 mg vs −54.2% for placebo (P = .295).
- At week 24, patients with subacute CLE showed a significantly greater mean percent change from baseline in CLASI-A with iberdomide 0.45 mg vs placebo (−90.5% vs −51.2%; P = .007), while no significant differences were observed with the 0.45-mg dose vs placebo in patients with chronic or acute CLE.
- Overall, CLASI-50 responses were not significantly different among those on 0.45 mg vs placebo (55.6% vs 44.6%). The proportions of patients achieving CLASI-50 at week 24 were significantly greater for iberdomide 0.45 mg vs placebo for those with subacute CLE (91.7% vs 52.9%; P = .035) and chronic CLE (62.1% vs 27.8%; P = .029), but not for those with baseline CLASI-A ≥ 8 (66.7% vs 50%).
- More than 80% of patients had treatment-emergent adverse events (TEAEs), which were mostly mild to moderate. Over 2 years, the most common were urinary tract infections, upper respiratory tract infections, neutropenia, and nasopharyngitis. TEAEs leading to iberdomide discontinuation in one or more patients were neutropenia (n = 7), rash (n = 7), increased hepatic enzymes (n = 4), and deep vein thrombosis (n = 3).
IN PRACTICE:
“Data from this phase 2 trial of iberdomide in patients with SLE suggest that a greater proportion of patients with subacute or chronic CLE who received the higher dose of 0.45 mg iberdomide achieved CLASI-50 vs placebo. For the overall population, CLASI-50 response was not significantly different between treatment groups at week 24, partly due to a high placebo response that may have been driven by patients with acute CLE,” the authors wrote.
SOURCE:
The study was led by Victoria P. Werth, MD, of the University of Pennsylvania and the Veteran’s Administration Medical Center, both in Philadelphia, and was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The study included small patient subgroups for different CLE subtypes, which may affect the generalizability of the findings. CLE subtype was determined by the investigator without additional photographic adjudication. Additionally, the use of background lupus medications could have influenced the placebo group’s response, limiting the ability to observe the treatment effect of iberdomide monotherapy.
DISCLOSURES:
The study was funded by Bristol-Myers Squibb. Six authors reported being employed by Bristol-Myers Squibb, and several others reported consultancy and research support from various sources including Bristol-Myers Squibb.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
particularly in subacute and chronic cases.
METHODOLOGY:
- Researchers conducted a randomized phase 2 trial to evaluate the efficacy and safety of iberdomide in 288 patients with CLE (mean age, 45 years; 97% women). Iberdomide is a cereblon modulator, which results in degradation of two transcription factors of immune cell development and homeostasis — Ikaros and Aiolos — that have been implicated in the genetic predisposition of systemic lupus.
- CLE Disease Area and Severity Index Activity (CLASI-A) endpoints included mean percent change from baseline and ≥ 50% reduction from baseline (CLASI-50), which were evaluated in all patients with baseline CLASI-A scores ≥ 8 and by CLE subtypes (acute, subacute, and chronic).
- At baseline, 56% of patients had acute CLE, 29% had chronic CLE, and 16% had subacute CLE; 28% of patients had a baseline CLASI-A score ≥ 8.
- Patients were randomly assigned to receive oral iberdomide (0.45 mg, 0.30 mg, 0.15 mg, or placebo daily) for 24 weeks while continuing standard lupus medications. At week 24, patients on placebo were rerandomized to iberdomide 0.45 mg or 0.30 mg once a day, while those on iberdomide continued their assigned dose through week 52.
TAKEAWAY:
- Among patients with baseline CLASI-A ≥ 8, the mean change in CLASI-A score from baseline at week 24 was −66.7% for those on iberdomide 0.45 mg vs −54.2% for placebo (P = .295).
- At week 24, patients with subacute CLE showed a significantly greater mean percent change from baseline in CLASI-A with iberdomide 0.45 mg vs placebo (−90.5% vs −51.2%; P = .007), while no significant differences were observed with the 0.45-mg dose vs placebo in patients with chronic or acute CLE.
- Overall, CLASI-50 responses were not significantly different among those on 0.45 mg vs placebo (55.6% vs 44.6%). The proportions of patients achieving CLASI-50 at week 24 were significantly greater for iberdomide 0.45 mg vs placebo for those with subacute CLE (91.7% vs 52.9%; P = .035) and chronic CLE (62.1% vs 27.8%; P = .029), but not for those with baseline CLASI-A ≥ 8 (66.7% vs 50%).
- More than 80% of patients had treatment-emergent adverse events (TEAEs), which were mostly mild to moderate. Over 2 years, the most common were urinary tract infections, upper respiratory tract infections, neutropenia, and nasopharyngitis. TEAEs leading to iberdomide discontinuation in one or more patients were neutropenia (n = 7), rash (n = 7), increased hepatic enzymes (n = 4), and deep vein thrombosis (n = 3).
IN PRACTICE:
“Data from this phase 2 trial of iberdomide in patients with SLE suggest that a greater proportion of patients with subacute or chronic CLE who received the higher dose of 0.45 mg iberdomide achieved CLASI-50 vs placebo. For the overall population, CLASI-50 response was not significantly different between treatment groups at week 24, partly due to a high placebo response that may have been driven by patients with acute CLE,” the authors wrote.
SOURCE:
The study was led by Victoria P. Werth, MD, of the University of Pennsylvania and the Veteran’s Administration Medical Center, both in Philadelphia, and was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The study included small patient subgroups for different CLE subtypes, which may affect the generalizability of the findings. CLE subtype was determined by the investigator without additional photographic adjudication. Additionally, the use of background lupus medications could have influenced the placebo group’s response, limiting the ability to observe the treatment effect of iberdomide monotherapy.
DISCLOSURES:
The study was funded by Bristol-Myers Squibb. Six authors reported being employed by Bristol-Myers Squibb, and several others reported consultancy and research support from various sources including Bristol-Myers Squibb.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
What’s the Evidence Behind Popular Supplements in Rheumatology? Experts Weigh in
Many people with rheumatologic diseases try supplements for symptom relief. Here’s what you need to know about some common picks.
Dietary supplements were a $159 billion business in the United States in 2023, and many people with rheumatologic diseases are buying in. Research suggests more than 6 in 10 people with fibromyalgia, nearly 8 in 10 people with Sjögren’s disease, and more than 8 in 10 people with rheumatoid arthritis (RA) take dietary supplements.
Whatever the symptom — pain, swelling, or fatigue — you can probably find a supplement purporting to relieve it. But do these supplements work, and are they safe? A study review in RMD Open comprising 24 systematic reviews and 150 original articles suggests more high-quality research is needed on the effects of dietary supplements on rheumatologic diseases. Most studies have focused on RA or osteoarthritis (OA), where the evidence level is moderate at best.
“The studies in this space are usually not very high quality because there’s no money to support them, among other things, plus the products are disparate,” said Janet Funk, MD, MS, professor in the School of Nutritional Sciences and Wellness at the University of Arizona, Tucson. She recommended brushing up on supplements and finding out what patients are taking so you can offer advice and watch for drug-supplement interactions.
When asked for a medication list, many patients forget to report supplements, Funk said. “You have to prompt them specifically. I think some physicians have very negative views about supplements because so little data is known, and patients might pick up on that and decide not to report their use.” She recommended saying something like: “To give you the best possible care, I want to know everything you’re taking, including supplements. The things I’m prescribing could maybe interact with the things you’re taking, so I want to make sure I know about all of it so that together we can figure out if the combination of things is safe.”
The quality of dietary supplements varies, and they aren’t regulated like drugs by the Food and Drug Administration. Funk recommended selecting products verified by NSF or ConsumerLab. They test supplements to ensure the label reflects what’s inside.
This news organization scoured the literature and asked experts to weigh in on the evidence behind popular supplements in rheumatology today.
The Essential Nutrients
Vitamin supplements are a staple in many homes — but are they helpful? “Individual vitamin supplements will not provide any benefit unless the person is deficient in a specific vitamin or mineral,” according to Elena Philippou, PhD, RD, associate professor of nutrition-dietetics at the University of Nicosia in Cyprus, and Elena Nikiphorou, MBBS, a rheumatologist at King’s College London in England. For some patients, deficiency is a reality. A retrospective cohort study in The Journal of Clinical Medicine found that people with RA were 17% more likely than age-matched control individuals to have nutrient deficiencies, perhaps because symptoms like fatigue, pain, and nausea affect their eating habits. Here’s what the science says about common vitamin supplements.
Vitamin D. This hormone-like vitamin, which attaches to receptors on immune cells to tamp down inflammation, was the most popular dietary supplement among rheumatology patients in a recent study from the United Kingdom. Vitamin D deficiency is common in people with RA, lupus, Sjögren’s disease, ankylosing spondylitis, systemic sclerosis, and fibromyalgia. In some cases, vitamin D levels track with disease activity, research suggests. Corticosteroids can also make vitamin D deficiency more likely. Can supplements help?
In RA, evidence points to small improvements. A systematic review of 11 studies including 3049 patients published in Nutrition Reviews showed that vitamin D supplements significantly reduced patients’ pain and Disease Activity Score in 28 joints (DAS28) using both C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR).
The research is mixed on the benefits of vitamin D supplementation for fibromyalgia symptoms, according to a study review in SN Comprehensive Clinical Medicine that included two studies and 80 patients on supplementation. However, researchers said it’s still worth discussing the potential benefits of taking vitamin D.
“Vitamin D supplementation is important in the context of various rheumatic diseases to prevent or treat bone disease,” said Philippou and Nikiphorou. “People with rheumatic disease should speak to their healthcare provider and ask to check their blood vitamin D concentration.” The results can help you recommend a dose.
Folate. Patients on methotrexate should take folic acid supplements under the guidance of a healthcare provider, said Philippou and Nikiphorou. The reason: Methotrexate can deplete folic acid levels, increasing the risk for side effects. An analysis of adverse event reports published showed that methotrexate users who took folic acid (or tumor necrosis factor–alpha inhibitors) had a reduced risk for hepatotoxicity or myelosuppression. A commonly recommended dose is 1 mg/d.
Vitamin B12. In a 2024 perspective paper in Rheumatology International, researchers said physicians should assess vitamin B12 levels early in the diagnostic process of rheumatologic diseases. One reason: Many symptoms of pernicious anemia, like fatigue, mimic symptoms of rheumatologic diseases. The gastrointestinal (GI) effects of systemic sclerosis could bring on vitamin B12 deficiency. In a small study in The Journal of Clinical Rheumatology, 44 of 62 patients with systemic sclerosis had low vitamin B12 levels.
Vitamin E. Vitamin E deficiency is rare in healthy adults. However, some medical conditions, like inflammatory bowel disease and malabsorption disorders, can make vitamin E deficiency more likely. In RA, a vitamin E supplement could help reduce joint swelling and sensitivity, according to a systematic review of nine studies including 39,845 patients in The European Journal of Clinical Nutrition. Researchers credit the nutrient’s role in aiding intestinal repair. Use with caution, as this supplement can increase bleeding risk in doses over 1000 mg/d.
Vitamin A. Like vitamin E, vitamin A deficiency is rare in the United States. The risk of oversupplementing is higher than undersupplementing. However, vitamin A deficiency can happen in people with chronic pancreatic, liver, or GI problems. In people with deficiency, a vitamin A supplement can help relieve dry eye symptoms common in Sjögren’s disease, suggests a narrative review published in Nutrients. Vitamin A might help reduce ocular surface changes by supporting the production of proteins that protect the outermost surfaces of the eyes. The recommended daily allowance for vitamin A is 900 μg. High-dose supplements can cause toxicity, resulting in GI symptoms and problems like lethargy, drowsiness, increased intracranial pressure, and skin changes.
The Replacements
These substances are similar to naturally occurring compounds in our bodies. The question is whether ingesting them yields benefits.
Glucosamine and chondroitin. Glucosamine and chondroitin occur naturally in our bodies and help us form and protect connective tissues. In pill form, this combo is the most popular dietary supplement for OA, according to research in the journal Maturitas. But studies of its effectiveness yield mixed results. A systematic review of 25 studies published in Inflammopharmacology showed that, in patients with knee OA, supplementation with about 1500 mg of glucosamine per day reduced tibiofemoral joint space narrowing, while supplementation with about 800 mg/d of chondroitin reduced pain intensity and improved physical function, compared with placebo. The duo of glucosamine and chondroitin did not bring significant benefits, perhaps because more studies are needed. Most side effects were mild, but some literature points to the potential for glucosamine to increase warfarin’s blood-thinning effects.
Omega-3 fatty acids. Fish oil is a top-selling supplement, and it might be helpful in inflammatory rheumatologic diseases. A systematic review of 30 studies including 710 patients published in Arthritis Research & Therapy showed that omega-3 fatty acid supplements can improve pain, swollen and tender joint count, DAS28 scores, and Health Assessment Questionnaire scores in patients with RA, psoriatic arthritis, or ankylosing spondylitis. In patients with lupus, a study review that included five studies and 284 patients in The International Journal of Environmental Research and Public Health suggested omega-3 fatty acid supplements could improve ESR, CRP, disease activity, inflammatory markers, oxidative stress, lipid levels, and endothelial function.
Omega-3 fatty acids have anti-inflammatory effects that might explain their benefits. In patients with RA, for example, fish oil supplementation was associated with elevated blood levels of resolvins and protectins, which help quell inflammation, according to a study in Prostaglandins, Leukotrienes and Essential Fatty Acids.
Philippou and Nikiphorou recommended combining food and supplements: Eat oily fish at least twice a week, regularly consume plant-based sources of omega-3s — like chia seeds, flaxseeds, or walnuts — and consider a daily supplement that contains 2 g of omega-3s from docosahexaenoic acid and eicosapentaenoic acid. Most fish oil side effects are mild, like heartburn and bad breath. Fish oil can have blood-thinning effects at high doses, so special attention is needed for patients on anticoagulants.
Probiotics. Building up the good bacteria in your gut might help you fight the effects of rheumatologic diseases. A systematic review of 80 randomized controlled trials in BMC Medicine suggested that therapies targeting the gut microbiota might improve the symptoms or inflammatory factors in celiac disease, lupus, juvenile idiopathic arthritis, psoriasis, Sjögren’s disease, multiple sclerosis, systemic sclerosis, Crohn’s disease, and ulcerative colitis. Probiotics were also shown to relieve pain in fibromyalgia, but they didn’t affect scores on the Fibromyalgia Impact Questionnaire. Probiotics were not helpful in spondyloarthritis or RA. There were no adverse events. By improving the balance of bacteria in the gut, probiotics might inhibit pro-inflammatory factors and signaling pathways and regulate CD4+ T-cell differentiation, the researchers wrote.
Not all probiotic supplements are created equal. Effects can vary by microorganism and dose. Until more high-quality studies are published, Philippou and Nikiphorou recommend daily consumption of probiotic food sources such as yogurt, kefir, sauerkraut, kimchi, tempeh, miso, and kombucha, along with prebiotic food sources such as bananas, onion, artichokes, asparagus, oats, leeks, and garlic.
Collagen. An increasingly popular supplement for hair, skin, and nails, some collagen peptide or hydrolyzed collagen supplements come with claims about joint health, too. Inside our bodies, collagen helps build joints. As a supplement, the jury is still out. A systematic review of 19 studies in The International Journal of Rheumatic Diseases suggested more research is needed to determine whether collagen supplements are harmful or helpful in OA or RA. Studies haven’t shown adverse events, and doses typically range from 2.5 to 15 g/d.
Coenzyme Q10 (CoQ10). This antioxidant occurs naturally in our cells and is produced through microbial fermentation for use in dietary supplements. A study review of 20 articles including 483 patients in Clinical Nutrition ESPEN concluded that CoQ10 supplementation up to 300 mg/d was beneficial in RA, fibromyalgia, or antiphospholipid syndrome (APS).
In RA, CoQ10 supplementation improved disease activity index, ESR, and cytokine levels and decreased malondialdehyde. CoQ10 might protect against the overproduction of reactive oxygen species that can promote inflammation and joint damage, the researchers said. In fibromyalgia, CoQ10 was linked with improvements in pain, fatigue, sleep, tender points count, mood disorders, and scores on the Fibromyalgia Impact Questionnaire in most of the included studies. CoQ10 might help in fibromyalgia by improving mitochondrial dysfunction. In APS, CoQ10 improved endothelial function and decreased prothrombotic and pro-inflammatory mediators. CoQ10 might change the expression of genes that promote atherosclerosis. A few patients had GI side effects like nausea and diarrhea, but the supplements were generally well tolerated.
Melatonin. Commonly touted as a sleep aid, this hormone has immune and anti-inflammatory activities that could benefit people with rheumatologic diseases. A study review of 13 articles including 533 patients in Clinical Nutrition ESPEN concluded that melatonin can help improve sleep, pain, and mood in fibromyalgia, OA, and osteoporosis but not in RA. Side effects were minimal, but a few people experienced nausea, drowsiness, nightmares, or headaches. Doses of 5-6 mg/d are likely safe for most adults.
The Plant-Derived Antioxidants
Many supplements used in rheumatology are antioxidants derived from herbs, spices, or other plants. When plants encounter stressors, like temperature changes or hungry insects, their secondary metabolism revs up and creates compounds with biological properties. Some of these substances influence inflammatory pathways in the human body, said Luís Silva, PhD, a medicinal chemistry researcher at the Polytechnic Institute of Guarda in Portugal. “If it is possible to reduce these kinds of anti-inflammatory processes, it is also possible that we could help people with inflammatory diseases to a good life, or a better life.”
Turmeric and curcumin. You might see this supplement labeled as turmeric, a golden spice in curry powder, or curcumin, an antioxidant compound known as a curcuminoid in turmeric. Curcuminoids might reduce inflammation by scavenging free radicals and inhibiting enzymes that make prostaglandins, Silva said.
Turmeric is the most popular herbal supplement for people with RA, according to Funk’s research. A study review of six publications including 539 patients in Frontiers in Immunology showed that curcumin supplements improved RA patients’ ESR, DAS, swollen joint count, and tender joint count. Turmeric could help patients with OA, too. Patients with OA who took 1000 mg/d of curcumin improved their pain and function, according to a systematic review including 12 studies and 1438 participants in the journal Nutrients. In lupus, small studies are promising but inconclusive, suggested a study review in Frontiers in Immunology.
Watch patients taking turmeric and methotrexate closely, Funk said. Both have been associated with liver problems. Some users also experience GI symptoms like diarrhea because turmeric doesn’t absorb well in the GI tract.
Milk thistle (silymarin). This flowering plant is often marketed as a liver-supporting supplement, but research also suggests promise in RA and OA. A systematic review of 12 studies in Current Rheumatology Reviews suggested that silymarin supplements might help relieve pain, reduce inflammation, and protect the cartilage matrix, synovial membrane, and cartilage cells in joints. This supplement might help via immunomodulatory, anti-inflammatory, antioxidant, and anti-apoptotic properties, the researchers said. Doses of 250-750 mg appear to be safe. Side effects such as gastroenteritis, diarrhea, bloating, and headache can occur.
Boswellia serrata. Sourced from the resin of a tree that grows in dry, mountainous regions of Asia and Africa, Boswellia serrata can help relieve joint pain and stiffness and improve joint function in OA, suggested a systematic review of seven trials involving 545 patients in BMC Complementary Medicine and Therapies. Users saw benefits when taking 100-250 mg/d for 4 weeks or more. Compounds in Boswellia serrata may inhibit 5-lipoxygenase, an enzyme involved in producing inflammatory leukotrienes. No adverse events were reported. In some studies, users have reported GI side effects.
Ginger. Ginger is a popular herbal supplement among people with RA, Funk’s research suggested. One small clinical trial involving 70 patients with RA in the journal Gene showed that taking 1500 mg/d of ginger for 12 weeks improved their DAS and boosted their expression of FoxP3 genes, which are linked with the function of regulatory T cells. A meta-analysis including three studies with 330 patients taking ginger published in the journal Nutrients suggested ginger can reduce pain and systemic inflammation in people with OA. Preclinical studies suggested phenolic compounds in this spicy root, such as gingerols, reduce inflammation through multiple mechanisms.
Funk’s research revealed wide variation in the quality of ginger supplements, reinforcing the importance of selecting an independently verified product. Research suggested a safe dose is up to 2-2.5 g/kg body weight.
Resveratrol. Found in red grapes and red wine, this compound is particularly good at blocking COX-2 enzymes, an important step in the inflammatory cascade, Silva said. “Because of their chemical structure, they have great affinity to these enzymes to lead to their inhibition,” he said. A study review of five articles including 481 patients in The European Journal of Rheumatology showed that people with OA, RA, or Takayasu arteritis who took 250-1000 mg/d of resveratrol saw improvements in pain, function, disease activity, joint swelling, and inflammation, with no side effects.
Cinnamon. This warming spice is gaining popularity as a supplement, reported the American Botanical Council. Cinnamon is often marketed as lowering blood sugar and supporting bone health. In a small study of 36 women with RA published in The Journal of the American College of Nutrition, participants who consumed 2 g/d of cinnamon powder had reduced DASs along with reduced pain and tender and swollen joint counts. Cinnamon may reduce pain by inhibiting prostaglandin and blunt inflammation by reducing the release of arachidonic acid from cell membranes, according to a study review in Frontiers in Pharmacology. GI problems and allergic reactions are among the most common side effects.
Funk, Nikiphorou, Philippou, and Silva all had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Many people with rheumatologic diseases try supplements for symptom relief. Here’s what you need to know about some common picks.
Dietary supplements were a $159 billion business in the United States in 2023, and many people with rheumatologic diseases are buying in. Research suggests more than 6 in 10 people with fibromyalgia, nearly 8 in 10 people with Sjögren’s disease, and more than 8 in 10 people with rheumatoid arthritis (RA) take dietary supplements.
Whatever the symptom — pain, swelling, or fatigue — you can probably find a supplement purporting to relieve it. But do these supplements work, and are they safe? A study review in RMD Open comprising 24 systematic reviews and 150 original articles suggests more high-quality research is needed on the effects of dietary supplements on rheumatologic diseases. Most studies have focused on RA or osteoarthritis (OA), where the evidence level is moderate at best.
“The studies in this space are usually not very high quality because there’s no money to support them, among other things, plus the products are disparate,” said Janet Funk, MD, MS, professor in the School of Nutritional Sciences and Wellness at the University of Arizona, Tucson. She recommended brushing up on supplements and finding out what patients are taking so you can offer advice and watch for drug-supplement interactions.
When asked for a medication list, many patients forget to report supplements, Funk said. “You have to prompt them specifically. I think some physicians have very negative views about supplements because so little data is known, and patients might pick up on that and decide not to report their use.” She recommended saying something like: “To give you the best possible care, I want to know everything you’re taking, including supplements. The things I’m prescribing could maybe interact with the things you’re taking, so I want to make sure I know about all of it so that together we can figure out if the combination of things is safe.”
The quality of dietary supplements varies, and they aren’t regulated like drugs by the Food and Drug Administration. Funk recommended selecting products verified by NSF or ConsumerLab. They test supplements to ensure the label reflects what’s inside.
This news organization scoured the literature and asked experts to weigh in on the evidence behind popular supplements in rheumatology today.
The Essential Nutrients
Vitamin supplements are a staple in many homes — but are they helpful? “Individual vitamin supplements will not provide any benefit unless the person is deficient in a specific vitamin or mineral,” according to Elena Philippou, PhD, RD, associate professor of nutrition-dietetics at the University of Nicosia in Cyprus, and Elena Nikiphorou, MBBS, a rheumatologist at King’s College London in England. For some patients, deficiency is a reality. A retrospective cohort study in The Journal of Clinical Medicine found that people with RA were 17% more likely than age-matched control individuals to have nutrient deficiencies, perhaps because symptoms like fatigue, pain, and nausea affect their eating habits. Here’s what the science says about common vitamin supplements.
Vitamin D. This hormone-like vitamin, which attaches to receptors on immune cells to tamp down inflammation, was the most popular dietary supplement among rheumatology patients in a recent study from the United Kingdom. Vitamin D deficiency is common in people with RA, lupus, Sjögren’s disease, ankylosing spondylitis, systemic sclerosis, and fibromyalgia. In some cases, vitamin D levels track with disease activity, research suggests. Corticosteroids can also make vitamin D deficiency more likely. Can supplements help?
In RA, evidence points to small improvements. A systematic review of 11 studies including 3049 patients published in Nutrition Reviews showed that vitamin D supplements significantly reduced patients’ pain and Disease Activity Score in 28 joints (DAS28) using both C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR).
The research is mixed on the benefits of vitamin D supplementation for fibromyalgia symptoms, according to a study review in SN Comprehensive Clinical Medicine that included two studies and 80 patients on supplementation. However, researchers said it’s still worth discussing the potential benefits of taking vitamin D.
“Vitamin D supplementation is important in the context of various rheumatic diseases to prevent or treat bone disease,” said Philippou and Nikiphorou. “People with rheumatic disease should speak to their healthcare provider and ask to check their blood vitamin D concentration.” The results can help you recommend a dose.
Folate. Patients on methotrexate should take folic acid supplements under the guidance of a healthcare provider, said Philippou and Nikiphorou. The reason: Methotrexate can deplete folic acid levels, increasing the risk for side effects. An analysis of adverse event reports published showed that methotrexate users who took folic acid (or tumor necrosis factor–alpha inhibitors) had a reduced risk for hepatotoxicity or myelosuppression. A commonly recommended dose is 1 mg/d.
Vitamin B12. In a 2024 perspective paper in Rheumatology International, researchers said physicians should assess vitamin B12 levels early in the diagnostic process of rheumatologic diseases. One reason: Many symptoms of pernicious anemia, like fatigue, mimic symptoms of rheumatologic diseases. The gastrointestinal (GI) effects of systemic sclerosis could bring on vitamin B12 deficiency. In a small study in The Journal of Clinical Rheumatology, 44 of 62 patients with systemic sclerosis had low vitamin B12 levels.
Vitamin E. Vitamin E deficiency is rare in healthy adults. However, some medical conditions, like inflammatory bowel disease and malabsorption disorders, can make vitamin E deficiency more likely. In RA, a vitamin E supplement could help reduce joint swelling and sensitivity, according to a systematic review of nine studies including 39,845 patients in The European Journal of Clinical Nutrition. Researchers credit the nutrient’s role in aiding intestinal repair. Use with caution, as this supplement can increase bleeding risk in doses over 1000 mg/d.
Vitamin A. Like vitamin E, vitamin A deficiency is rare in the United States. The risk of oversupplementing is higher than undersupplementing. However, vitamin A deficiency can happen in people with chronic pancreatic, liver, or GI problems. In people with deficiency, a vitamin A supplement can help relieve dry eye symptoms common in Sjögren’s disease, suggests a narrative review published in Nutrients. Vitamin A might help reduce ocular surface changes by supporting the production of proteins that protect the outermost surfaces of the eyes. The recommended daily allowance for vitamin A is 900 μg. High-dose supplements can cause toxicity, resulting in GI symptoms and problems like lethargy, drowsiness, increased intracranial pressure, and skin changes.
The Replacements
These substances are similar to naturally occurring compounds in our bodies. The question is whether ingesting them yields benefits.
Glucosamine and chondroitin. Glucosamine and chondroitin occur naturally in our bodies and help us form and protect connective tissues. In pill form, this combo is the most popular dietary supplement for OA, according to research in the journal Maturitas. But studies of its effectiveness yield mixed results. A systematic review of 25 studies published in Inflammopharmacology showed that, in patients with knee OA, supplementation with about 1500 mg of glucosamine per day reduced tibiofemoral joint space narrowing, while supplementation with about 800 mg/d of chondroitin reduced pain intensity and improved physical function, compared with placebo. The duo of glucosamine and chondroitin did not bring significant benefits, perhaps because more studies are needed. Most side effects were mild, but some literature points to the potential for glucosamine to increase warfarin’s blood-thinning effects.
Omega-3 fatty acids. Fish oil is a top-selling supplement, and it might be helpful in inflammatory rheumatologic diseases. A systematic review of 30 studies including 710 patients published in Arthritis Research & Therapy showed that omega-3 fatty acid supplements can improve pain, swollen and tender joint count, DAS28 scores, and Health Assessment Questionnaire scores in patients with RA, psoriatic arthritis, or ankylosing spondylitis. In patients with lupus, a study review that included five studies and 284 patients in The International Journal of Environmental Research and Public Health suggested omega-3 fatty acid supplements could improve ESR, CRP, disease activity, inflammatory markers, oxidative stress, lipid levels, and endothelial function.
Omega-3 fatty acids have anti-inflammatory effects that might explain their benefits. In patients with RA, for example, fish oil supplementation was associated with elevated blood levels of resolvins and protectins, which help quell inflammation, according to a study in Prostaglandins, Leukotrienes and Essential Fatty Acids.
Philippou and Nikiphorou recommended combining food and supplements: Eat oily fish at least twice a week, regularly consume plant-based sources of omega-3s — like chia seeds, flaxseeds, or walnuts — and consider a daily supplement that contains 2 g of omega-3s from docosahexaenoic acid and eicosapentaenoic acid. Most fish oil side effects are mild, like heartburn and bad breath. Fish oil can have blood-thinning effects at high doses, so special attention is needed for patients on anticoagulants.
Probiotics. Building up the good bacteria in your gut might help you fight the effects of rheumatologic diseases. A systematic review of 80 randomized controlled trials in BMC Medicine suggested that therapies targeting the gut microbiota might improve the symptoms or inflammatory factors in celiac disease, lupus, juvenile idiopathic arthritis, psoriasis, Sjögren’s disease, multiple sclerosis, systemic sclerosis, Crohn’s disease, and ulcerative colitis. Probiotics were also shown to relieve pain in fibromyalgia, but they didn’t affect scores on the Fibromyalgia Impact Questionnaire. Probiotics were not helpful in spondyloarthritis or RA. There were no adverse events. By improving the balance of bacteria in the gut, probiotics might inhibit pro-inflammatory factors and signaling pathways and regulate CD4+ T-cell differentiation, the researchers wrote.
Not all probiotic supplements are created equal. Effects can vary by microorganism and dose. Until more high-quality studies are published, Philippou and Nikiphorou recommend daily consumption of probiotic food sources such as yogurt, kefir, sauerkraut, kimchi, tempeh, miso, and kombucha, along with prebiotic food sources such as bananas, onion, artichokes, asparagus, oats, leeks, and garlic.
Collagen. An increasingly popular supplement for hair, skin, and nails, some collagen peptide or hydrolyzed collagen supplements come with claims about joint health, too. Inside our bodies, collagen helps build joints. As a supplement, the jury is still out. A systematic review of 19 studies in The International Journal of Rheumatic Diseases suggested more research is needed to determine whether collagen supplements are harmful or helpful in OA or RA. Studies haven’t shown adverse events, and doses typically range from 2.5 to 15 g/d.
Coenzyme Q10 (CoQ10). This antioxidant occurs naturally in our cells and is produced through microbial fermentation for use in dietary supplements. A study review of 20 articles including 483 patients in Clinical Nutrition ESPEN concluded that CoQ10 supplementation up to 300 mg/d was beneficial in RA, fibromyalgia, or antiphospholipid syndrome (APS).
In RA, CoQ10 supplementation improved disease activity index, ESR, and cytokine levels and decreased malondialdehyde. CoQ10 might protect against the overproduction of reactive oxygen species that can promote inflammation and joint damage, the researchers said. In fibromyalgia, CoQ10 was linked with improvements in pain, fatigue, sleep, tender points count, mood disorders, and scores on the Fibromyalgia Impact Questionnaire in most of the included studies. CoQ10 might help in fibromyalgia by improving mitochondrial dysfunction. In APS, CoQ10 improved endothelial function and decreased prothrombotic and pro-inflammatory mediators. CoQ10 might change the expression of genes that promote atherosclerosis. A few patients had GI side effects like nausea and diarrhea, but the supplements were generally well tolerated.
Melatonin. Commonly touted as a sleep aid, this hormone has immune and anti-inflammatory activities that could benefit people with rheumatologic diseases. A study review of 13 articles including 533 patients in Clinical Nutrition ESPEN concluded that melatonin can help improve sleep, pain, and mood in fibromyalgia, OA, and osteoporosis but not in RA. Side effects were minimal, but a few people experienced nausea, drowsiness, nightmares, or headaches. Doses of 5-6 mg/d are likely safe for most adults.
The Plant-Derived Antioxidants
Many supplements used in rheumatology are antioxidants derived from herbs, spices, or other plants. When plants encounter stressors, like temperature changes or hungry insects, their secondary metabolism revs up and creates compounds with biological properties. Some of these substances influence inflammatory pathways in the human body, said Luís Silva, PhD, a medicinal chemistry researcher at the Polytechnic Institute of Guarda in Portugal. “If it is possible to reduce these kinds of anti-inflammatory processes, it is also possible that we could help people with inflammatory diseases to a good life, or a better life.”
Turmeric and curcumin. You might see this supplement labeled as turmeric, a golden spice in curry powder, or curcumin, an antioxidant compound known as a curcuminoid in turmeric. Curcuminoids might reduce inflammation by scavenging free radicals and inhibiting enzymes that make prostaglandins, Silva said.
Turmeric is the most popular herbal supplement for people with RA, according to Funk’s research. A study review of six publications including 539 patients in Frontiers in Immunology showed that curcumin supplements improved RA patients’ ESR, DAS, swollen joint count, and tender joint count. Turmeric could help patients with OA, too. Patients with OA who took 1000 mg/d of curcumin improved their pain and function, according to a systematic review including 12 studies and 1438 participants in the journal Nutrients. In lupus, small studies are promising but inconclusive, suggested a study review in Frontiers in Immunology.
Watch patients taking turmeric and methotrexate closely, Funk said. Both have been associated with liver problems. Some users also experience GI symptoms like diarrhea because turmeric doesn’t absorb well in the GI tract.
Milk thistle (silymarin). This flowering plant is often marketed as a liver-supporting supplement, but research also suggests promise in RA and OA. A systematic review of 12 studies in Current Rheumatology Reviews suggested that silymarin supplements might help relieve pain, reduce inflammation, and protect the cartilage matrix, synovial membrane, and cartilage cells in joints. This supplement might help via immunomodulatory, anti-inflammatory, antioxidant, and anti-apoptotic properties, the researchers said. Doses of 250-750 mg appear to be safe. Side effects such as gastroenteritis, diarrhea, bloating, and headache can occur.
Boswellia serrata. Sourced from the resin of a tree that grows in dry, mountainous regions of Asia and Africa, Boswellia serrata can help relieve joint pain and stiffness and improve joint function in OA, suggested a systematic review of seven trials involving 545 patients in BMC Complementary Medicine and Therapies. Users saw benefits when taking 100-250 mg/d for 4 weeks or more. Compounds in Boswellia serrata may inhibit 5-lipoxygenase, an enzyme involved in producing inflammatory leukotrienes. No adverse events were reported. In some studies, users have reported GI side effects.
Ginger. Ginger is a popular herbal supplement among people with RA, Funk’s research suggested. One small clinical trial involving 70 patients with RA in the journal Gene showed that taking 1500 mg/d of ginger for 12 weeks improved their DAS and boosted their expression of FoxP3 genes, which are linked with the function of regulatory T cells. A meta-analysis including three studies with 330 patients taking ginger published in the journal Nutrients suggested ginger can reduce pain and systemic inflammation in people with OA. Preclinical studies suggested phenolic compounds in this spicy root, such as gingerols, reduce inflammation through multiple mechanisms.
Funk’s research revealed wide variation in the quality of ginger supplements, reinforcing the importance of selecting an independently verified product. Research suggested a safe dose is up to 2-2.5 g/kg body weight.
Resveratrol. Found in red grapes and red wine, this compound is particularly good at blocking COX-2 enzymes, an important step in the inflammatory cascade, Silva said. “Because of their chemical structure, they have great affinity to these enzymes to lead to their inhibition,” he said. A study review of five articles including 481 patients in The European Journal of Rheumatology showed that people with OA, RA, or Takayasu arteritis who took 250-1000 mg/d of resveratrol saw improvements in pain, function, disease activity, joint swelling, and inflammation, with no side effects.
Cinnamon. This warming spice is gaining popularity as a supplement, reported the American Botanical Council. Cinnamon is often marketed as lowering blood sugar and supporting bone health. In a small study of 36 women with RA published in The Journal of the American College of Nutrition, participants who consumed 2 g/d of cinnamon powder had reduced DASs along with reduced pain and tender and swollen joint counts. Cinnamon may reduce pain by inhibiting prostaglandin and blunt inflammation by reducing the release of arachidonic acid from cell membranes, according to a study review in Frontiers in Pharmacology. GI problems and allergic reactions are among the most common side effects.
Funk, Nikiphorou, Philippou, and Silva all had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Many people with rheumatologic diseases try supplements for symptom relief. Here’s what you need to know about some common picks.
Dietary supplements were a $159 billion business in the United States in 2023, and many people with rheumatologic diseases are buying in. Research suggests more than 6 in 10 people with fibromyalgia, nearly 8 in 10 people with Sjögren’s disease, and more than 8 in 10 people with rheumatoid arthritis (RA) take dietary supplements.
Whatever the symptom — pain, swelling, or fatigue — you can probably find a supplement purporting to relieve it. But do these supplements work, and are they safe? A study review in RMD Open comprising 24 systematic reviews and 150 original articles suggests more high-quality research is needed on the effects of dietary supplements on rheumatologic diseases. Most studies have focused on RA or osteoarthritis (OA), where the evidence level is moderate at best.
“The studies in this space are usually not very high quality because there’s no money to support them, among other things, plus the products are disparate,” said Janet Funk, MD, MS, professor in the School of Nutritional Sciences and Wellness at the University of Arizona, Tucson. She recommended brushing up on supplements and finding out what patients are taking so you can offer advice and watch for drug-supplement interactions.
When asked for a medication list, many patients forget to report supplements, Funk said. “You have to prompt them specifically. I think some physicians have very negative views about supplements because so little data is known, and patients might pick up on that and decide not to report their use.” She recommended saying something like: “To give you the best possible care, I want to know everything you’re taking, including supplements. The things I’m prescribing could maybe interact with the things you’re taking, so I want to make sure I know about all of it so that together we can figure out if the combination of things is safe.”
The quality of dietary supplements varies, and they aren’t regulated like drugs by the Food and Drug Administration. Funk recommended selecting products verified by NSF or ConsumerLab. They test supplements to ensure the label reflects what’s inside.
This news organization scoured the literature and asked experts to weigh in on the evidence behind popular supplements in rheumatology today.
The Essential Nutrients
Vitamin supplements are a staple in many homes — but are they helpful? “Individual vitamin supplements will not provide any benefit unless the person is deficient in a specific vitamin or mineral,” according to Elena Philippou, PhD, RD, associate professor of nutrition-dietetics at the University of Nicosia in Cyprus, and Elena Nikiphorou, MBBS, a rheumatologist at King’s College London in England. For some patients, deficiency is a reality. A retrospective cohort study in The Journal of Clinical Medicine found that people with RA were 17% more likely than age-matched control individuals to have nutrient deficiencies, perhaps because symptoms like fatigue, pain, and nausea affect their eating habits. Here’s what the science says about common vitamin supplements.
Vitamin D. This hormone-like vitamin, which attaches to receptors on immune cells to tamp down inflammation, was the most popular dietary supplement among rheumatology patients in a recent study from the United Kingdom. Vitamin D deficiency is common in people with RA, lupus, Sjögren’s disease, ankylosing spondylitis, systemic sclerosis, and fibromyalgia. In some cases, vitamin D levels track with disease activity, research suggests. Corticosteroids can also make vitamin D deficiency more likely. Can supplements help?
In RA, evidence points to small improvements. A systematic review of 11 studies including 3049 patients published in Nutrition Reviews showed that vitamin D supplements significantly reduced patients’ pain and Disease Activity Score in 28 joints (DAS28) using both C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR).
The research is mixed on the benefits of vitamin D supplementation for fibromyalgia symptoms, according to a study review in SN Comprehensive Clinical Medicine that included two studies and 80 patients on supplementation. However, researchers said it’s still worth discussing the potential benefits of taking vitamin D.
“Vitamin D supplementation is important in the context of various rheumatic diseases to prevent or treat bone disease,” said Philippou and Nikiphorou. “People with rheumatic disease should speak to their healthcare provider and ask to check their blood vitamin D concentration.” The results can help you recommend a dose.
Folate. Patients on methotrexate should take folic acid supplements under the guidance of a healthcare provider, said Philippou and Nikiphorou. The reason: Methotrexate can deplete folic acid levels, increasing the risk for side effects. An analysis of adverse event reports published showed that methotrexate users who took folic acid (or tumor necrosis factor–alpha inhibitors) had a reduced risk for hepatotoxicity or myelosuppression. A commonly recommended dose is 1 mg/d.
Vitamin B12. In a 2024 perspective paper in Rheumatology International, researchers said physicians should assess vitamin B12 levels early in the diagnostic process of rheumatologic diseases. One reason: Many symptoms of pernicious anemia, like fatigue, mimic symptoms of rheumatologic diseases. The gastrointestinal (GI) effects of systemic sclerosis could bring on vitamin B12 deficiency. In a small study in The Journal of Clinical Rheumatology, 44 of 62 patients with systemic sclerosis had low vitamin B12 levels.
Vitamin E. Vitamin E deficiency is rare in healthy adults. However, some medical conditions, like inflammatory bowel disease and malabsorption disorders, can make vitamin E deficiency more likely. In RA, a vitamin E supplement could help reduce joint swelling and sensitivity, according to a systematic review of nine studies including 39,845 patients in The European Journal of Clinical Nutrition. Researchers credit the nutrient’s role in aiding intestinal repair. Use with caution, as this supplement can increase bleeding risk in doses over 1000 mg/d.
Vitamin A. Like vitamin E, vitamin A deficiency is rare in the United States. The risk of oversupplementing is higher than undersupplementing. However, vitamin A deficiency can happen in people with chronic pancreatic, liver, or GI problems. In people with deficiency, a vitamin A supplement can help relieve dry eye symptoms common in Sjögren’s disease, suggests a narrative review published in Nutrients. Vitamin A might help reduce ocular surface changes by supporting the production of proteins that protect the outermost surfaces of the eyes. The recommended daily allowance for vitamin A is 900 μg. High-dose supplements can cause toxicity, resulting in GI symptoms and problems like lethargy, drowsiness, increased intracranial pressure, and skin changes.
The Replacements
These substances are similar to naturally occurring compounds in our bodies. The question is whether ingesting them yields benefits.
Glucosamine and chondroitin. Glucosamine and chondroitin occur naturally in our bodies and help us form and protect connective tissues. In pill form, this combo is the most popular dietary supplement for OA, according to research in the journal Maturitas. But studies of its effectiveness yield mixed results. A systematic review of 25 studies published in Inflammopharmacology showed that, in patients with knee OA, supplementation with about 1500 mg of glucosamine per day reduced tibiofemoral joint space narrowing, while supplementation with about 800 mg/d of chondroitin reduced pain intensity and improved physical function, compared with placebo. The duo of glucosamine and chondroitin did not bring significant benefits, perhaps because more studies are needed. Most side effects were mild, but some literature points to the potential for glucosamine to increase warfarin’s blood-thinning effects.
Omega-3 fatty acids. Fish oil is a top-selling supplement, and it might be helpful in inflammatory rheumatologic diseases. A systematic review of 30 studies including 710 patients published in Arthritis Research & Therapy showed that omega-3 fatty acid supplements can improve pain, swollen and tender joint count, DAS28 scores, and Health Assessment Questionnaire scores in patients with RA, psoriatic arthritis, or ankylosing spondylitis. In patients with lupus, a study review that included five studies and 284 patients in The International Journal of Environmental Research and Public Health suggested omega-3 fatty acid supplements could improve ESR, CRP, disease activity, inflammatory markers, oxidative stress, lipid levels, and endothelial function.
Omega-3 fatty acids have anti-inflammatory effects that might explain their benefits. In patients with RA, for example, fish oil supplementation was associated with elevated blood levels of resolvins and protectins, which help quell inflammation, according to a study in Prostaglandins, Leukotrienes and Essential Fatty Acids.
Philippou and Nikiphorou recommended combining food and supplements: Eat oily fish at least twice a week, regularly consume plant-based sources of omega-3s — like chia seeds, flaxseeds, or walnuts — and consider a daily supplement that contains 2 g of omega-3s from docosahexaenoic acid and eicosapentaenoic acid. Most fish oil side effects are mild, like heartburn and bad breath. Fish oil can have blood-thinning effects at high doses, so special attention is needed for patients on anticoagulants.
Probiotics. Building up the good bacteria in your gut might help you fight the effects of rheumatologic diseases. A systematic review of 80 randomized controlled trials in BMC Medicine suggested that therapies targeting the gut microbiota might improve the symptoms or inflammatory factors in celiac disease, lupus, juvenile idiopathic arthritis, psoriasis, Sjögren’s disease, multiple sclerosis, systemic sclerosis, Crohn’s disease, and ulcerative colitis. Probiotics were also shown to relieve pain in fibromyalgia, but they didn’t affect scores on the Fibromyalgia Impact Questionnaire. Probiotics were not helpful in spondyloarthritis or RA. There were no adverse events. By improving the balance of bacteria in the gut, probiotics might inhibit pro-inflammatory factors and signaling pathways and regulate CD4+ T-cell differentiation, the researchers wrote.
Not all probiotic supplements are created equal. Effects can vary by microorganism and dose. Until more high-quality studies are published, Philippou and Nikiphorou recommend daily consumption of probiotic food sources such as yogurt, kefir, sauerkraut, kimchi, tempeh, miso, and kombucha, along with prebiotic food sources such as bananas, onion, artichokes, asparagus, oats, leeks, and garlic.
Collagen. An increasingly popular supplement for hair, skin, and nails, some collagen peptide or hydrolyzed collagen supplements come with claims about joint health, too. Inside our bodies, collagen helps build joints. As a supplement, the jury is still out. A systematic review of 19 studies in The International Journal of Rheumatic Diseases suggested more research is needed to determine whether collagen supplements are harmful or helpful in OA or RA. Studies haven’t shown adverse events, and doses typically range from 2.5 to 15 g/d.
Coenzyme Q10 (CoQ10). This antioxidant occurs naturally in our cells and is produced through microbial fermentation for use in dietary supplements. A study review of 20 articles including 483 patients in Clinical Nutrition ESPEN concluded that CoQ10 supplementation up to 300 mg/d was beneficial in RA, fibromyalgia, or antiphospholipid syndrome (APS).
In RA, CoQ10 supplementation improved disease activity index, ESR, and cytokine levels and decreased malondialdehyde. CoQ10 might protect against the overproduction of reactive oxygen species that can promote inflammation and joint damage, the researchers said. In fibromyalgia, CoQ10 was linked with improvements in pain, fatigue, sleep, tender points count, mood disorders, and scores on the Fibromyalgia Impact Questionnaire in most of the included studies. CoQ10 might help in fibromyalgia by improving mitochondrial dysfunction. In APS, CoQ10 improved endothelial function and decreased prothrombotic and pro-inflammatory mediators. CoQ10 might change the expression of genes that promote atherosclerosis. A few patients had GI side effects like nausea and diarrhea, but the supplements were generally well tolerated.
Melatonin. Commonly touted as a sleep aid, this hormone has immune and anti-inflammatory activities that could benefit people with rheumatologic diseases. A study review of 13 articles including 533 patients in Clinical Nutrition ESPEN concluded that melatonin can help improve sleep, pain, and mood in fibromyalgia, OA, and osteoporosis but not in RA. Side effects were minimal, but a few people experienced nausea, drowsiness, nightmares, or headaches. Doses of 5-6 mg/d are likely safe for most adults.
The Plant-Derived Antioxidants
Many supplements used in rheumatology are antioxidants derived from herbs, spices, or other plants. When plants encounter stressors, like temperature changes or hungry insects, their secondary metabolism revs up and creates compounds with biological properties. Some of these substances influence inflammatory pathways in the human body, said Luís Silva, PhD, a medicinal chemistry researcher at the Polytechnic Institute of Guarda in Portugal. “If it is possible to reduce these kinds of anti-inflammatory processes, it is also possible that we could help people with inflammatory diseases to a good life, or a better life.”
Turmeric and curcumin. You might see this supplement labeled as turmeric, a golden spice in curry powder, or curcumin, an antioxidant compound known as a curcuminoid in turmeric. Curcuminoids might reduce inflammation by scavenging free radicals and inhibiting enzymes that make prostaglandins, Silva said.
Turmeric is the most popular herbal supplement for people with RA, according to Funk’s research. A study review of six publications including 539 patients in Frontiers in Immunology showed that curcumin supplements improved RA patients’ ESR, DAS, swollen joint count, and tender joint count. Turmeric could help patients with OA, too. Patients with OA who took 1000 mg/d of curcumin improved their pain and function, according to a systematic review including 12 studies and 1438 participants in the journal Nutrients. In lupus, small studies are promising but inconclusive, suggested a study review in Frontiers in Immunology.
Watch patients taking turmeric and methotrexate closely, Funk said. Both have been associated with liver problems. Some users also experience GI symptoms like diarrhea because turmeric doesn’t absorb well in the GI tract.
Milk thistle (silymarin). This flowering plant is often marketed as a liver-supporting supplement, but research also suggests promise in RA and OA. A systematic review of 12 studies in Current Rheumatology Reviews suggested that silymarin supplements might help relieve pain, reduce inflammation, and protect the cartilage matrix, synovial membrane, and cartilage cells in joints. This supplement might help via immunomodulatory, anti-inflammatory, antioxidant, and anti-apoptotic properties, the researchers said. Doses of 250-750 mg appear to be safe. Side effects such as gastroenteritis, diarrhea, bloating, and headache can occur.
Boswellia serrata. Sourced from the resin of a tree that grows in dry, mountainous regions of Asia and Africa, Boswellia serrata can help relieve joint pain and stiffness and improve joint function in OA, suggested a systematic review of seven trials involving 545 patients in BMC Complementary Medicine and Therapies. Users saw benefits when taking 100-250 mg/d for 4 weeks or more. Compounds in Boswellia serrata may inhibit 5-lipoxygenase, an enzyme involved in producing inflammatory leukotrienes. No adverse events were reported. In some studies, users have reported GI side effects.
Ginger. Ginger is a popular herbal supplement among people with RA, Funk’s research suggested. One small clinical trial involving 70 patients with RA in the journal Gene showed that taking 1500 mg/d of ginger for 12 weeks improved their DAS and boosted their expression of FoxP3 genes, which are linked with the function of regulatory T cells. A meta-analysis including three studies with 330 patients taking ginger published in the journal Nutrients suggested ginger can reduce pain and systemic inflammation in people with OA. Preclinical studies suggested phenolic compounds in this spicy root, such as gingerols, reduce inflammation through multiple mechanisms.
Funk’s research revealed wide variation in the quality of ginger supplements, reinforcing the importance of selecting an independently verified product. Research suggested a safe dose is up to 2-2.5 g/kg body weight.
Resveratrol. Found in red grapes and red wine, this compound is particularly good at blocking COX-2 enzymes, an important step in the inflammatory cascade, Silva said. “Because of their chemical structure, they have great affinity to these enzymes to lead to their inhibition,” he said. A study review of five articles including 481 patients in The European Journal of Rheumatology showed that people with OA, RA, or Takayasu arteritis who took 250-1000 mg/d of resveratrol saw improvements in pain, function, disease activity, joint swelling, and inflammation, with no side effects.
Cinnamon. This warming spice is gaining popularity as a supplement, reported the American Botanical Council. Cinnamon is often marketed as lowering blood sugar and supporting bone health. In a small study of 36 women with RA published in The Journal of the American College of Nutrition, participants who consumed 2 g/d of cinnamon powder had reduced DASs along with reduced pain and tender and swollen joint counts. Cinnamon may reduce pain by inhibiting prostaglandin and blunt inflammation by reducing the release of arachidonic acid from cell membranes, according to a study review in Frontiers in Pharmacology. GI problems and allergic reactions are among the most common side effects.
Funk, Nikiphorou, Philippou, and Silva all had no relevant disclosures.
A version of this article first appeared on Medscape.com.
JIA Treatment Has Increasingly Involved New DMARDs Since 2001
TOPLINE:
The use of newer biologic or targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs) for treating juvenile idiopathic arthritis (JIA) rose sharply from 2001 to 2022, while the use of conventional synthetic DMARDs (csDMARDs) plummeted, with adalimumab becoming the most commonly used b/tsDMARD.
METHODOLOGY:
- Researchers performed a serial cross-sectional study using Merative MarketScan Commercial Claims and Encounters data from 2000 to 2022 to describe recent trends in DMARD use for children with JIA in the United States.
- They identified 20,258 new episodes of DMARD use among 13,696 children with JIA (median age, 14 years; 67.5% girls) who newly initiated at least one DMARD.
- Participants were required to have ≥ 365 days of continuous healthcare and pharmacy eligibility prior to the index date, defined as the date of DMARD initiation.
TAKEAWAY:
- The use of csDMARDs declined from 89.5% to 43.2% between 2001 and 2022 (P < .001 for trend), whereas the use of bDMARDs increased from 10.5% to 50.0% over the same period (P < .001).
- Methotrexate was the most commonly used DMARD throughout the study period ; however, as with other csDMARDs, its use declined from 42.1% in 2001 to 21.5% in 2022 (P < .001 ).
- Use of the tumor necrosis factor inhibitor adalimumab doubled from 7% in 2007 to 14% in 2008 and increased further up to 20.5% by 2022; adalimumab also became the most predominantly used b/tsDMARD after csDMARD monotherapy, accounting for 77.8% of prescriptions following csDMARDs in 2022.
- Even though the use of individual TNF inhibitors increased, their overall popularity fell in recent years as the use of newer b/tsDMARDs, such as ustekinumab and secukinumab, increased.
IN PRACTICE:
“These real-world treatment patterns give us insight into how selection of therapies for JIA has evolved with increasing availability of effective agents and help prepare for future studies on comparative DMARD safety and effectiveness,” the authors wrote.
SOURCE:
The study was led by Priyanka Yalamanchili, PharmD, MS, Center for Pharmacoepidemiology and Treatment Science, Institute for Health, Rutgers University, New Brunswick, New Jersey, and was published online October 22, 2024, in Arthritis & Rheumatology.
LIMITATIONS:
The dependence on commercial claims data may have limited the generalizability of the findings to other populations, such as those with public insurance or without insurance. The study did not have access to demographic data of the participants to investigate the presence of disparities in the use of DMARDs. Moreover, the lack of clinical details about the patients with JIA, including disease severity and specialty of prescribers, may have affected the interpretation of the results.
DISCLOSURES:
The study was supported by funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and several other institutes of the National Institutes of Health, as well as the Rheumatology Research Foundation and the Juvenile Diabetes Research Foundation. No conflicts of interest were reported by the authors.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The use of newer biologic or targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs) for treating juvenile idiopathic arthritis (JIA) rose sharply from 2001 to 2022, while the use of conventional synthetic DMARDs (csDMARDs) plummeted, with adalimumab becoming the most commonly used b/tsDMARD.
METHODOLOGY:
- Researchers performed a serial cross-sectional study using Merative MarketScan Commercial Claims and Encounters data from 2000 to 2022 to describe recent trends in DMARD use for children with JIA in the United States.
- They identified 20,258 new episodes of DMARD use among 13,696 children with JIA (median age, 14 years; 67.5% girls) who newly initiated at least one DMARD.
- Participants were required to have ≥ 365 days of continuous healthcare and pharmacy eligibility prior to the index date, defined as the date of DMARD initiation.
TAKEAWAY:
- The use of csDMARDs declined from 89.5% to 43.2% between 2001 and 2022 (P < .001 for trend), whereas the use of bDMARDs increased from 10.5% to 50.0% over the same period (P < .001).
- Methotrexate was the most commonly used DMARD throughout the study period ; however, as with other csDMARDs, its use declined from 42.1% in 2001 to 21.5% in 2022 (P < .001 ).
- Use of the tumor necrosis factor inhibitor adalimumab doubled from 7% in 2007 to 14% in 2008 and increased further up to 20.5% by 2022; adalimumab also became the most predominantly used b/tsDMARD after csDMARD monotherapy, accounting for 77.8% of prescriptions following csDMARDs in 2022.
- Even though the use of individual TNF inhibitors increased, their overall popularity fell in recent years as the use of newer b/tsDMARDs, such as ustekinumab and secukinumab, increased.
IN PRACTICE:
“These real-world treatment patterns give us insight into how selection of therapies for JIA has evolved with increasing availability of effective agents and help prepare for future studies on comparative DMARD safety and effectiveness,” the authors wrote.
SOURCE:
The study was led by Priyanka Yalamanchili, PharmD, MS, Center for Pharmacoepidemiology and Treatment Science, Institute for Health, Rutgers University, New Brunswick, New Jersey, and was published online October 22, 2024, in Arthritis & Rheumatology.
LIMITATIONS:
The dependence on commercial claims data may have limited the generalizability of the findings to other populations, such as those with public insurance or without insurance. The study did not have access to demographic data of the participants to investigate the presence of disparities in the use of DMARDs. Moreover, the lack of clinical details about the patients with JIA, including disease severity and specialty of prescribers, may have affected the interpretation of the results.
DISCLOSURES:
The study was supported by funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and several other institutes of the National Institutes of Health, as well as the Rheumatology Research Foundation and the Juvenile Diabetes Research Foundation. No conflicts of interest were reported by the authors.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
The use of newer biologic or targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs) for treating juvenile idiopathic arthritis (JIA) rose sharply from 2001 to 2022, while the use of conventional synthetic DMARDs (csDMARDs) plummeted, with adalimumab becoming the most commonly used b/tsDMARD.
METHODOLOGY:
- Researchers performed a serial cross-sectional study using Merative MarketScan Commercial Claims and Encounters data from 2000 to 2022 to describe recent trends in DMARD use for children with JIA in the United States.
- They identified 20,258 new episodes of DMARD use among 13,696 children with JIA (median age, 14 years; 67.5% girls) who newly initiated at least one DMARD.
- Participants were required to have ≥ 365 days of continuous healthcare and pharmacy eligibility prior to the index date, defined as the date of DMARD initiation.
TAKEAWAY:
- The use of csDMARDs declined from 89.5% to 43.2% between 2001 and 2022 (P < .001 for trend), whereas the use of bDMARDs increased from 10.5% to 50.0% over the same period (P < .001).
- Methotrexate was the most commonly used DMARD throughout the study period ; however, as with other csDMARDs, its use declined from 42.1% in 2001 to 21.5% in 2022 (P < .001 ).
- Use of the tumor necrosis factor inhibitor adalimumab doubled from 7% in 2007 to 14% in 2008 and increased further up to 20.5% by 2022; adalimumab also became the most predominantly used b/tsDMARD after csDMARD monotherapy, accounting for 77.8% of prescriptions following csDMARDs in 2022.
- Even though the use of individual TNF inhibitors increased, their overall popularity fell in recent years as the use of newer b/tsDMARDs, such as ustekinumab and secukinumab, increased.
IN PRACTICE:
“These real-world treatment patterns give us insight into how selection of therapies for JIA has evolved with increasing availability of effective agents and help prepare for future studies on comparative DMARD safety and effectiveness,” the authors wrote.
SOURCE:
The study was led by Priyanka Yalamanchili, PharmD, MS, Center for Pharmacoepidemiology and Treatment Science, Institute for Health, Rutgers University, New Brunswick, New Jersey, and was published online October 22, 2024, in Arthritis & Rheumatology.
LIMITATIONS:
The dependence on commercial claims data may have limited the generalizability of the findings to other populations, such as those with public insurance or without insurance. The study did not have access to demographic data of the participants to investigate the presence of disparities in the use of DMARDs. Moreover, the lack of clinical details about the patients with JIA, including disease severity and specialty of prescribers, may have affected the interpretation of the results.
DISCLOSURES:
The study was supported by funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and several other institutes of the National Institutes of Health, as well as the Rheumatology Research Foundation and the Juvenile Diabetes Research Foundation. No conflicts of interest were reported by the authors.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.





