User login
Sodium deoxycholate and triamcinolone: A good mix?
In September 2023, Goldman et al. published a communication in Dermatologic Surgery describing their use of subcutaneous sodium deoxycholate injection (SDOC), with or without triamcinolone acetonide, for reduction of submental fat..
As they note, “patients experience a variable degree of edema and discomfort following subcutaneous injection,” of SDOC, something that I and others have also observed in our practices.
In their double-blind study of 20 patients with a baseline Clinician-Reported Submental Fat Rating Scale of 2 or 3 out of 4, 5 patients were randomized to receive SDOC as recommended in the label, while 15 received SDOC plus triamcinolone. In the latter group, 2 mL of SDOC was mixed with 0.5 mL of 40 mg/mL of triamcinolone acetate, then administered in up to 50 injections in the submentum spaced 1.0 cm apart at 0.25 mL per injection. Three treatments were administered 1 month apart.
For both groups, volumes between 5 mL and 8 mL per treatment were delivered. There were no significant differences in efficacy 30, 60, and 90 days after the final injection between the two groups. However, at day 180, the group that received only SDOC had a significantly greater reduction in submental fat, which the authors wrote indicated that the addition of triamcinolone “may mildly diminish the fat reduction effects” at that time point.
Subcutaneous SDOC (deoxycholic acid) injections for reduction of submental fullness was approved by the Food and Drug Administration in 2015 for improving the appearance of moderate to severe convexity or fullness associated with submental fat in adults. (I was involved in the clinical trials.) We found that in the trial, for optimal efficacy, most patients require two to four treatments spread at least a month apart, with patients who had larger treatment areas requiring up to six treatments.
While the clinical trial treatments were spaced 4 weeks apart, post approval, we found that patients would sometimes report further efficacy even 2-3 months post injection. Since not everyone wants to go around with edema every month for 2-4 consecutive months, spacing the treatments farther apart allows patients more time to heal and coordinate the recovery appearance around their work and social schedules.
In my practice, very rarely have we seen minimal to moderate prolonged edema, particularly in younger patients, beyond 1 month post injection. Most people have the most noticeable edema — the “bull-frog” appearance — for the first 1-3 days, with some minor fullness that appears to be almost back to baseline at 1 week. In some of these patients with prolonged submental fullness, it looks fuller than it appeared pretreatment even months afterwards.
While rare, like the study authors, I have found intralesional triamcinolone to be helpful at reducing this persistent fullness should it occur. It is likely to be reducing any persistent inflammation or posttreatment fibrosis in these patients.
Unlike the study authors, I do not combine SDOC and triamcinolone injections at the time of treatment. Rather, I consider injecting triamcinolone if submental fullness is greater than at baseline or edema persists after SDOC treatment. It is rare that I’ve had to do this, as most cases self-resolve, but I have used triamcinolone 10 mg/mL, up to 1cc total, injected 6-8 weeks apart one to three times to the affected area and found it to be effective if fullness has persisted beyond 6 months. Liposuction may also be an option, if needed, if fullness/edema persists.
Overall, SDOC is an effective treatment for small pockets of subcutaneous fat. Approved for submental fullness, it is now sometimes used off-label for other parts of the body, such as bra fat, small pockets of the abdomen, and lipomas. While some inflammation after treatment is expected — and desired — to achieve an effective outcome of fat apoptosis, intralesional triamcinolone is an interesting tool to utilize should inflammation or posttreatment fullness persist.
Dr. Wesley practices dermatology in Beverly Hills, California. Write to her at dermnews@mdedge.com. She was an investigator in clinical trials of Kybella.
In September 2023, Goldman et al. published a communication in Dermatologic Surgery describing their use of subcutaneous sodium deoxycholate injection (SDOC), with or without triamcinolone acetonide, for reduction of submental fat..
As they note, “patients experience a variable degree of edema and discomfort following subcutaneous injection,” of SDOC, something that I and others have also observed in our practices.
In their double-blind study of 20 patients with a baseline Clinician-Reported Submental Fat Rating Scale of 2 or 3 out of 4, 5 patients were randomized to receive SDOC as recommended in the label, while 15 received SDOC plus triamcinolone. In the latter group, 2 mL of SDOC was mixed with 0.5 mL of 40 mg/mL of triamcinolone acetate, then administered in up to 50 injections in the submentum spaced 1.0 cm apart at 0.25 mL per injection. Three treatments were administered 1 month apart.
For both groups, volumes between 5 mL and 8 mL per treatment were delivered. There were no significant differences in efficacy 30, 60, and 90 days after the final injection between the two groups. However, at day 180, the group that received only SDOC had a significantly greater reduction in submental fat, which the authors wrote indicated that the addition of triamcinolone “may mildly diminish the fat reduction effects” at that time point.
Subcutaneous SDOC (deoxycholic acid) injections for reduction of submental fullness was approved by the Food and Drug Administration in 2015 for improving the appearance of moderate to severe convexity or fullness associated with submental fat in adults. (I was involved in the clinical trials.) We found that in the trial, for optimal efficacy, most patients require two to four treatments spread at least a month apart, with patients who had larger treatment areas requiring up to six treatments.
While the clinical trial treatments were spaced 4 weeks apart, post approval, we found that patients would sometimes report further efficacy even 2-3 months post injection. Since not everyone wants to go around with edema every month for 2-4 consecutive months, spacing the treatments farther apart allows patients more time to heal and coordinate the recovery appearance around their work and social schedules.
In my practice, very rarely have we seen minimal to moderate prolonged edema, particularly in younger patients, beyond 1 month post injection. Most people have the most noticeable edema — the “bull-frog” appearance — for the first 1-3 days, with some minor fullness that appears to be almost back to baseline at 1 week. In some of these patients with prolonged submental fullness, it looks fuller than it appeared pretreatment even months afterwards.
While rare, like the study authors, I have found intralesional triamcinolone to be helpful at reducing this persistent fullness should it occur. It is likely to be reducing any persistent inflammation or posttreatment fibrosis in these patients.
Unlike the study authors, I do not combine SDOC and triamcinolone injections at the time of treatment. Rather, I consider injecting triamcinolone if submental fullness is greater than at baseline or edema persists after SDOC treatment. It is rare that I’ve had to do this, as most cases self-resolve, but I have used triamcinolone 10 mg/mL, up to 1cc total, injected 6-8 weeks apart one to three times to the affected area and found it to be effective if fullness has persisted beyond 6 months. Liposuction may also be an option, if needed, if fullness/edema persists.
Overall, SDOC is an effective treatment for small pockets of subcutaneous fat. Approved for submental fullness, it is now sometimes used off-label for other parts of the body, such as bra fat, small pockets of the abdomen, and lipomas. While some inflammation after treatment is expected — and desired — to achieve an effective outcome of fat apoptosis, intralesional triamcinolone is an interesting tool to utilize should inflammation or posttreatment fullness persist.
Dr. Wesley practices dermatology in Beverly Hills, California. Write to her at dermnews@mdedge.com. She was an investigator in clinical trials of Kybella.
In September 2023, Goldman et al. published a communication in Dermatologic Surgery describing their use of subcutaneous sodium deoxycholate injection (SDOC), with or without triamcinolone acetonide, for reduction of submental fat..
As they note, “patients experience a variable degree of edema and discomfort following subcutaneous injection,” of SDOC, something that I and others have also observed in our practices.
In their double-blind study of 20 patients with a baseline Clinician-Reported Submental Fat Rating Scale of 2 or 3 out of 4, 5 patients were randomized to receive SDOC as recommended in the label, while 15 received SDOC plus triamcinolone. In the latter group, 2 mL of SDOC was mixed with 0.5 mL of 40 mg/mL of triamcinolone acetate, then administered in up to 50 injections in the submentum spaced 1.0 cm apart at 0.25 mL per injection. Three treatments were administered 1 month apart.
For both groups, volumes between 5 mL and 8 mL per treatment were delivered. There were no significant differences in efficacy 30, 60, and 90 days after the final injection between the two groups. However, at day 180, the group that received only SDOC had a significantly greater reduction in submental fat, which the authors wrote indicated that the addition of triamcinolone “may mildly diminish the fat reduction effects” at that time point.
Subcutaneous SDOC (deoxycholic acid) injections for reduction of submental fullness was approved by the Food and Drug Administration in 2015 for improving the appearance of moderate to severe convexity or fullness associated with submental fat in adults. (I was involved in the clinical trials.) We found that in the trial, for optimal efficacy, most patients require two to four treatments spread at least a month apart, with patients who had larger treatment areas requiring up to six treatments.
While the clinical trial treatments were spaced 4 weeks apart, post approval, we found that patients would sometimes report further efficacy even 2-3 months post injection. Since not everyone wants to go around with edema every month for 2-4 consecutive months, spacing the treatments farther apart allows patients more time to heal and coordinate the recovery appearance around their work and social schedules.
In my practice, very rarely have we seen minimal to moderate prolonged edema, particularly in younger patients, beyond 1 month post injection. Most people have the most noticeable edema — the “bull-frog” appearance — for the first 1-3 days, with some minor fullness that appears to be almost back to baseline at 1 week. In some of these patients with prolonged submental fullness, it looks fuller than it appeared pretreatment even months afterwards.
While rare, like the study authors, I have found intralesional triamcinolone to be helpful at reducing this persistent fullness should it occur. It is likely to be reducing any persistent inflammation or posttreatment fibrosis in these patients.
Unlike the study authors, I do not combine SDOC and triamcinolone injections at the time of treatment. Rather, I consider injecting triamcinolone if submental fullness is greater than at baseline or edema persists after SDOC treatment. It is rare that I’ve had to do this, as most cases self-resolve, but I have used triamcinolone 10 mg/mL, up to 1cc total, injected 6-8 weeks apart one to three times to the affected area and found it to be effective if fullness has persisted beyond 6 months. Liposuction may also be an option, if needed, if fullness/edema persists.
Overall, SDOC is an effective treatment for small pockets of subcutaneous fat. Approved for submental fullness, it is now sometimes used off-label for other parts of the body, such as bra fat, small pockets of the abdomen, and lipomas. While some inflammation after treatment is expected — and desired — to achieve an effective outcome of fat apoptosis, intralesional triamcinolone is an interesting tool to utilize should inflammation or posttreatment fullness persist.
Dr. Wesley practices dermatology in Beverly Hills, California. Write to her at dermnews@mdedge.com. She was an investigator in clinical trials of Kybella.
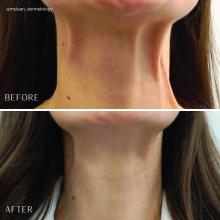
Treatment of the neck and lower face with botulinum toxin
.
The neck and the lower face are covered by thin layers of a vertical muscle, the anterior and posterior platysma muscle that is innervated by the cervical branch of the facial nerve. This muscle superficially blends with the muscles of the lower face, including the depressor anguli oris, depressor labii inferioris, mentalis, risorius, and orbicularis oris muscles. The inferior portion blends with the pectoralis and anterior deltoid muscles and lifts the skin of the neck.
Treatment of the platysma muscle and bands with botulinum toxin is an effective treatment for aging and sagging of the lower face and neck. Although treatment techniques differ and there are currently no standardized guidelines, the treatment starts by having the patient contract the neck muscles (I have them sit upright, with their head completely straight and say “E” with force). After evaluating the tension of the muscle, the muscle should be grasped and pulled away from the neck. Botulinum toxin is injected perpendicular to the muscle, with a dose of approximately 2 units, 2 cm apart along the vertical muscle. Approximately 20-40 units are used for the anterior and lateral bands.
To balance the opposing forces of the depressors of the lower face and improve jowling and downturning of the mouth, 10-20 units are also injected subdermally 1 cm above and 1 cm below the mandibular border.
Understanding the anatomy of the face and neck is crucial to proper injection. Side effects from improper injection include dysphagia, dysphonia, asymmetric smile, and weakness of the neck muscles. It is also important to set realistic expectations and address other components of neck aging, including actinic damage, as well as submental and jowl fat. The manufacturer of onabotulinumtoxinA (Botox Cosmetic) recently announced positive results of a second phase 3 clinical trial evaluating onabotulinumtoxinA for the treatment of moderate to severe platysma prominence. Results of the multicenter, randomized, double blind, placebo-controlled study evaluated the safety and efficacy of one treatment versus placebo in 426 adults with moderate to severe platysmal prominence. The results showed statistically significant improvement of platysma prominence from baseline, based on investigator and patient assessments, with no new safety signals, according to the company. The company expects to submit phase 3 data to the Food and Drug Administration by the end of this year and if approved, it will be the first neurotoxin approved for the treatment of platysmal bands.
Dr. Talakoub is in private practice in McLean, Va. Write to her at dermnews@mdedge.com. She had no relevant disclosures.
References
Brandt FS, Bellman B. Dermatol Surg. 1998 Nov;24(11):1232-4.
Matarasso A et al. Plast Reconstr Surg. 1999 Feb;103(2):645-52.
Rohrich RJ et al. Plast Reconstr Surg Glob Open. 2020 Jun 23;8(6):e2812.
.
The neck and the lower face are covered by thin layers of a vertical muscle, the anterior and posterior platysma muscle that is innervated by the cervical branch of the facial nerve. This muscle superficially blends with the muscles of the lower face, including the depressor anguli oris, depressor labii inferioris, mentalis, risorius, and orbicularis oris muscles. The inferior portion blends with the pectoralis and anterior deltoid muscles and lifts the skin of the neck.
Treatment of the platysma muscle and bands with botulinum toxin is an effective treatment for aging and sagging of the lower face and neck. Although treatment techniques differ and there are currently no standardized guidelines, the treatment starts by having the patient contract the neck muscles (I have them sit upright, with their head completely straight and say “E” with force). After evaluating the tension of the muscle, the muscle should be grasped and pulled away from the neck. Botulinum toxin is injected perpendicular to the muscle, with a dose of approximately 2 units, 2 cm apart along the vertical muscle. Approximately 20-40 units are used for the anterior and lateral bands.
To balance the opposing forces of the depressors of the lower face and improve jowling and downturning of the mouth, 10-20 units are also injected subdermally 1 cm above and 1 cm below the mandibular border.
Understanding the anatomy of the face and neck is crucial to proper injection. Side effects from improper injection include dysphagia, dysphonia, asymmetric smile, and weakness of the neck muscles. It is also important to set realistic expectations and address other components of neck aging, including actinic damage, as well as submental and jowl fat. The manufacturer of onabotulinumtoxinA (Botox Cosmetic) recently announced positive results of a second phase 3 clinical trial evaluating onabotulinumtoxinA for the treatment of moderate to severe platysma prominence. Results of the multicenter, randomized, double blind, placebo-controlled study evaluated the safety and efficacy of one treatment versus placebo in 426 adults with moderate to severe platysmal prominence. The results showed statistically significant improvement of platysma prominence from baseline, based on investigator and patient assessments, with no new safety signals, according to the company. The company expects to submit phase 3 data to the Food and Drug Administration by the end of this year and if approved, it will be the first neurotoxin approved for the treatment of platysmal bands.
Dr. Talakoub is in private practice in McLean, Va. Write to her at dermnews@mdedge.com. She had no relevant disclosures.
References
Brandt FS, Bellman B. Dermatol Surg. 1998 Nov;24(11):1232-4.
Matarasso A et al. Plast Reconstr Surg. 1999 Feb;103(2):645-52.
Rohrich RJ et al. Plast Reconstr Surg Glob Open. 2020 Jun 23;8(6):e2812.
.
The neck and the lower face are covered by thin layers of a vertical muscle, the anterior and posterior platysma muscle that is innervated by the cervical branch of the facial nerve. This muscle superficially blends with the muscles of the lower face, including the depressor anguli oris, depressor labii inferioris, mentalis, risorius, and orbicularis oris muscles. The inferior portion blends with the pectoralis and anterior deltoid muscles and lifts the skin of the neck.
Treatment of the platysma muscle and bands with botulinum toxin is an effective treatment for aging and sagging of the lower face and neck. Although treatment techniques differ and there are currently no standardized guidelines, the treatment starts by having the patient contract the neck muscles (I have them sit upright, with their head completely straight and say “E” with force). After evaluating the tension of the muscle, the muscle should be grasped and pulled away from the neck. Botulinum toxin is injected perpendicular to the muscle, with a dose of approximately 2 units, 2 cm apart along the vertical muscle. Approximately 20-40 units are used for the anterior and lateral bands.
To balance the opposing forces of the depressors of the lower face and improve jowling and downturning of the mouth, 10-20 units are also injected subdermally 1 cm above and 1 cm below the mandibular border.
Understanding the anatomy of the face and neck is crucial to proper injection. Side effects from improper injection include dysphagia, dysphonia, asymmetric smile, and weakness of the neck muscles. It is also important to set realistic expectations and address other components of neck aging, including actinic damage, as well as submental and jowl fat. The manufacturer of onabotulinumtoxinA (Botox Cosmetic) recently announced positive results of a second phase 3 clinical trial evaluating onabotulinumtoxinA for the treatment of moderate to severe platysma prominence. Results of the multicenter, randomized, double blind, placebo-controlled study evaluated the safety and efficacy of one treatment versus placebo in 426 adults with moderate to severe platysmal prominence. The results showed statistically significant improvement of platysma prominence from baseline, based on investigator and patient assessments, with no new safety signals, according to the company. The company expects to submit phase 3 data to the Food and Drug Administration by the end of this year and if approved, it will be the first neurotoxin approved for the treatment of platysmal bands.
Dr. Talakoub is in private practice in McLean, Va. Write to her at dermnews@mdedge.com. She had no relevant disclosures.
References
Brandt FS, Bellman B. Dermatol Surg. 1998 Nov;24(11):1232-4.
Matarasso A et al. Plast Reconstr Surg. 1999 Feb;103(2):645-52.
Rohrich RJ et al. Plast Reconstr Surg Glob Open. 2020 Jun 23;8(6):e2812.
The role of social media in aesthetic trends
Recently, but I had never heard it before. Not too long afterwards, patients were asking me about it in the office, using the same terminology, and I had several calls about it in one day. When I asked one trusted patient where she’d heard this term, which seemed to be trending, she told me that she had seen it on Instagram, as an ad or a “suggested for you” post.
Whether it’s a different name or term for a cosmetic procedure or laser we use that I’ve never heard before – such as “lip flip” or trap tox (also known as “Barbie Botox”) – many of these trendy terms spread like wildfire on social media. Some of the terms may be marketing tools started and spread by doctors who perform aesthetic procedures, something I don’t recommend as it only creates confusion for patients and practitioners, similar to the confusion consumers face regarding the plethora of over-the-counter skin care options and the marketing terms used for them. Other terms and trends are also started by nonphysician or non–professionally trained providers, sometimes leading to an unsafe or misleading term for an aesthetic procedure.
Over the past few years, several articles about the impact of social media in aesthetics have been published. In one recent paper, published in 2022, Boen and Jerdan noted that 72% of people in the United States use social media, up from 5% of American adults in 2005. In the United States, they note, “YouTube is the most popular platform with 73% of adult users, followed by Facebook (69%), Instagram (37%), SnapChat (24%), and Twitter (22%). Of the sites used daily, Facebook has the most activity (74%), followed by Instagram (64%), SnapChat (63%), YouTube (51%), and Twitter (42%).” They argue that the pros of social media in aesthetic medicine include its use as an educational tool by medical professionals to educate and provide accurate information about cosmetic procedures, and that “providing factual and evidence-based medical information to the public can help to counteract the abundant misinformation that is out there.” The cons include misinformation, no credentialing verification of the provider of the information – essentially anyone can be an “influencer” – as well as the addictive nature of social media for the consumer.
Along the same lines, younger patients tend to rely more on social media in choosing treatments and providers, further perpetuating any anxiety created from misinformation and unrealistic expectations from nonmedical influencers regarding procedures, filters used on photographs, photo editing, etc., in achieving an aesthetic result.
Physicians, particularly fellowship-trained aesthetic and surgical dermatologists, plastic and reconstructive surgeons, oculoplastic surgeons, and ENT facial plastic surgeons, who have the most training, knowledge, and expertise about aesthetic procedures, often have the least amount of time to devote to education via social media, compared with nonmedical influencers. Unless sponsored, they are also not being compensated for using it as an educational tool, except for potential indirect compensation from using it as a marketing tool for themselves and their practices. In contrast, nonmedical influencers often have many followers and time to create content, and in some cases, this is their full-time job.
All in all, most authors agree that social media has been associated with an increased acceptance of cosmetic surgery and procedures. Whether it be a trend seen on social media, or viewing one’s appearance in a filtered or photoediting app, or seeing an image of how another person looks (similar to how people in magazines, films and on television, were viewed in the past), social media has piqued people’s interest in aesthetics. It remains a balance for interested physicians to help keep information about cosmetic procedures presented in a healthy, interesting, professional, and accurate manner, and in a non–time-consuming way.
Dr. Wesley practices dermatology in Beverly Hills, Calif. Write to her at dermnews@mdedge.com. She had no relevant disclosures.
References
Boen M and Jerdan K. Clin Dermatol. 2022 Jan-Feb;40(1):45-8.
Chen J et al. JAMA Facial Plast Surg. 2019 Sep 1;21(5):361-7.
Chopan M et al. Plast Reconstr Surg. 2019 Apr;143(4):1259-65.
Recently, but I had never heard it before. Not too long afterwards, patients were asking me about it in the office, using the same terminology, and I had several calls about it in one day. When I asked one trusted patient where she’d heard this term, which seemed to be trending, she told me that she had seen it on Instagram, as an ad or a “suggested for you” post.
Whether it’s a different name or term for a cosmetic procedure or laser we use that I’ve never heard before – such as “lip flip” or trap tox (also known as “Barbie Botox”) – many of these trendy terms spread like wildfire on social media. Some of the terms may be marketing tools started and spread by doctors who perform aesthetic procedures, something I don’t recommend as it only creates confusion for patients and practitioners, similar to the confusion consumers face regarding the plethora of over-the-counter skin care options and the marketing terms used for them. Other terms and trends are also started by nonphysician or non–professionally trained providers, sometimes leading to an unsafe or misleading term for an aesthetic procedure.
Over the past few years, several articles about the impact of social media in aesthetics have been published. In one recent paper, published in 2022, Boen and Jerdan noted that 72% of people in the United States use social media, up from 5% of American adults in 2005. In the United States, they note, “YouTube is the most popular platform with 73% of adult users, followed by Facebook (69%), Instagram (37%), SnapChat (24%), and Twitter (22%). Of the sites used daily, Facebook has the most activity (74%), followed by Instagram (64%), SnapChat (63%), YouTube (51%), and Twitter (42%).” They argue that the pros of social media in aesthetic medicine include its use as an educational tool by medical professionals to educate and provide accurate information about cosmetic procedures, and that “providing factual and evidence-based medical information to the public can help to counteract the abundant misinformation that is out there.” The cons include misinformation, no credentialing verification of the provider of the information – essentially anyone can be an “influencer” – as well as the addictive nature of social media for the consumer.
Along the same lines, younger patients tend to rely more on social media in choosing treatments and providers, further perpetuating any anxiety created from misinformation and unrealistic expectations from nonmedical influencers regarding procedures, filters used on photographs, photo editing, etc., in achieving an aesthetic result.
Physicians, particularly fellowship-trained aesthetic and surgical dermatologists, plastic and reconstructive surgeons, oculoplastic surgeons, and ENT facial plastic surgeons, who have the most training, knowledge, and expertise about aesthetic procedures, often have the least amount of time to devote to education via social media, compared with nonmedical influencers. Unless sponsored, they are also not being compensated for using it as an educational tool, except for potential indirect compensation from using it as a marketing tool for themselves and their practices. In contrast, nonmedical influencers often have many followers and time to create content, and in some cases, this is their full-time job.
All in all, most authors agree that social media has been associated with an increased acceptance of cosmetic surgery and procedures. Whether it be a trend seen on social media, or viewing one’s appearance in a filtered or photoediting app, or seeing an image of how another person looks (similar to how people in magazines, films and on television, were viewed in the past), social media has piqued people’s interest in aesthetics. It remains a balance for interested physicians to help keep information about cosmetic procedures presented in a healthy, interesting, professional, and accurate manner, and in a non–time-consuming way.
Dr. Wesley practices dermatology in Beverly Hills, Calif. Write to her at dermnews@mdedge.com. She had no relevant disclosures.
References
Boen M and Jerdan K. Clin Dermatol. 2022 Jan-Feb;40(1):45-8.
Chen J et al. JAMA Facial Plast Surg. 2019 Sep 1;21(5):361-7.
Chopan M et al. Plast Reconstr Surg. 2019 Apr;143(4):1259-65.
Recently, but I had never heard it before. Not too long afterwards, patients were asking me about it in the office, using the same terminology, and I had several calls about it in one day. When I asked one trusted patient where she’d heard this term, which seemed to be trending, she told me that she had seen it on Instagram, as an ad or a “suggested for you” post.
Whether it’s a different name or term for a cosmetic procedure or laser we use that I’ve never heard before – such as “lip flip” or trap tox (also known as “Barbie Botox”) – many of these trendy terms spread like wildfire on social media. Some of the terms may be marketing tools started and spread by doctors who perform aesthetic procedures, something I don’t recommend as it only creates confusion for patients and practitioners, similar to the confusion consumers face regarding the plethora of over-the-counter skin care options and the marketing terms used for them. Other terms and trends are also started by nonphysician or non–professionally trained providers, sometimes leading to an unsafe or misleading term for an aesthetic procedure.
Over the past few years, several articles about the impact of social media in aesthetics have been published. In one recent paper, published in 2022, Boen and Jerdan noted that 72% of people in the United States use social media, up from 5% of American adults in 2005. In the United States, they note, “YouTube is the most popular platform with 73% of adult users, followed by Facebook (69%), Instagram (37%), SnapChat (24%), and Twitter (22%). Of the sites used daily, Facebook has the most activity (74%), followed by Instagram (64%), SnapChat (63%), YouTube (51%), and Twitter (42%).” They argue that the pros of social media in aesthetic medicine include its use as an educational tool by medical professionals to educate and provide accurate information about cosmetic procedures, and that “providing factual and evidence-based medical information to the public can help to counteract the abundant misinformation that is out there.” The cons include misinformation, no credentialing verification of the provider of the information – essentially anyone can be an “influencer” – as well as the addictive nature of social media for the consumer.
Along the same lines, younger patients tend to rely more on social media in choosing treatments and providers, further perpetuating any anxiety created from misinformation and unrealistic expectations from nonmedical influencers regarding procedures, filters used on photographs, photo editing, etc., in achieving an aesthetic result.
Physicians, particularly fellowship-trained aesthetic and surgical dermatologists, plastic and reconstructive surgeons, oculoplastic surgeons, and ENT facial plastic surgeons, who have the most training, knowledge, and expertise about aesthetic procedures, often have the least amount of time to devote to education via social media, compared with nonmedical influencers. Unless sponsored, they are also not being compensated for using it as an educational tool, except for potential indirect compensation from using it as a marketing tool for themselves and their practices. In contrast, nonmedical influencers often have many followers and time to create content, and in some cases, this is their full-time job.
All in all, most authors agree that social media has been associated with an increased acceptance of cosmetic surgery and procedures. Whether it be a trend seen on social media, or viewing one’s appearance in a filtered or photoediting app, or seeing an image of how another person looks (similar to how people in magazines, films and on television, were viewed in the past), social media has piqued people’s interest in aesthetics. It remains a balance for interested physicians to help keep information about cosmetic procedures presented in a healthy, interesting, professional, and accurate manner, and in a non–time-consuming way.
Dr. Wesley practices dermatology in Beverly Hills, Calif. Write to her at dermnews@mdedge.com. She had no relevant disclosures.
References
Boen M and Jerdan K. Clin Dermatol. 2022 Jan-Feb;40(1):45-8.
Chen J et al. JAMA Facial Plast Surg. 2019 Sep 1;21(5):361-7.
Chopan M et al. Plast Reconstr Surg. 2019 Apr;143(4):1259-65.
Going into solo practice? An expert shares tips
SAN DIEGO – When the Boston-based cosmetic dermatology practice that employed Catherine M. DiGiorgio, MD, MS, was sold to a private equity firm a few years ago, she found herself at a crossroads: Stay and work for a large corporation, or open a solo practice?
She opted to start her own practice in Boston, “because I didn’t want to work for a large corporation, and I want to provide the best care for my patients in a more intimate manner,” Dr. DiGiorgio, a board-certified laser and cosmetic dermatologist, said at the annual Masters of Aesthetics Symposium.
The decision also tested her mettle. “I spoke to several colleagues and friends, and I was terrified,” she said. “I was like: ‘I don’t even know where to start.’ ”
On the heels of opening a new office in a matter of weeks, she offered the following tips and questions to consider when launching a solo dermatology practice:
Select a location. “That’s your first decision,” she said. “Where in the city are you going to open? Are you going to a new city, or are you moving back home? Don’t be afraid to start from scratch, and don’t be afraid to start a [solo] practice if you already have a patient base.”
Will you lease or purchase your space? After she secured a bank loan, Dr. DiGiorgio chose to lease the space for her new practice, “because you can kind of see where things go, get all the kinks out and figure out how to build things in a space that you don’t own. Then, when you’re ready and you have grown, you can invest more into your practice.”
Will you accept insurance? She built her practice around the direct specialty care model, which emphasizes the patient-physician relationship and removes third-party payors. “It’s not a concierge practice, but it’s a transparent, reasonable fee schedule for medical dermatology,” she explained. “I’ve done 100% cosmetics for about 5 years now, [but] I do medical dermatology for a fee. On my website I have a full price list on how much a full skin check is, [and] how much biopsies are. It’s completely transparent. Patients can submit to their insurance for reimbursement, but we don’t guarantee that they’re going to be reimbursed.”
Where will your patients come from? Will you advertise? Do you have physicians in the area who will refer to you if you’re a board-certified dermatologist? She emphasized the importance of “learning how to present yourself” on a website dedicated to your own practice. “Instagram, Facebook, and social media are great, but you don’t own those pages,” noted Dr. DiGiorgio, who served as the program cochair of the 2023 annual meeting of the American Society for Laser Medicine and Surgery and was recently elected to serve on the board of directors for the American Society for Dermatology Surgery. “You don’t own one of those pictures that are posted on your social media page. They can disappear in a second. If that happens, how are people going to find you?”
Are you going to hire more physicians in the future? That will influence the size of the new office and the floor plan.
Lawyer up. Hiring a health care attorney can “help you navigate transitioning from whatever position you’re in to opening up your own practice, as well as setting up the regulatory paperwork necessary for your new practice. You’ll also need a real estate attorney to help once you have selected a place, to help you navigate through that process,” she said, such as figuring out if the elevator in the building meets the Americans With Disabilities Act (ADA) requirements.
Create a mission statement. That way, “you know why you’re doing this, and it stays with you as you’re getting through the hard roadblocks.”
Find an architect, contractor, or designer. “If you’re building out a space from scratch, you’re going to need an architect,” she said. “Along with that architect will come a full-on contracting firm. I ended up hiring everyone individually, because I’m trying to spend as little money as possible.” She also hired a designer to help select furnishings and create the office atmosphere.
Secure a building permit ASAP. “It’s almost better to have the city permit before you sign the lease, because the permits can take a year, and you don’t want to pay rent on an empty space for a year if you don’t have a permit or if there are other hoops to go through,” Dr. DiGiorgio said.
Find an agent to help you set up medical malpractice insurance, liability insurance, and worker’s compensation insurance. “Make sure you read all the paperwork, because it can be very intricate,” she said.
Find an accountant. That person can help set up a bookkeeping process.
What equipment and devices will you need? That depends largely on the patient population a physician serves. Dr. DiGiorgio noted that eligible small businesses may take a tax credit of up to $5,000 per year for accommodations made to comply with the ADA. “That’s a nice feature, so that you can purchase ADA compliant items like a larger exam chair and custom reception areas.”
Dr. DiGiorgio reported having no relevant disclosures.
SAN DIEGO – When the Boston-based cosmetic dermatology practice that employed Catherine M. DiGiorgio, MD, MS, was sold to a private equity firm a few years ago, she found herself at a crossroads: Stay and work for a large corporation, or open a solo practice?
She opted to start her own practice in Boston, “because I didn’t want to work for a large corporation, and I want to provide the best care for my patients in a more intimate manner,” Dr. DiGiorgio, a board-certified laser and cosmetic dermatologist, said at the annual Masters of Aesthetics Symposium.
The decision also tested her mettle. “I spoke to several colleagues and friends, and I was terrified,” she said. “I was like: ‘I don’t even know where to start.’ ”
On the heels of opening a new office in a matter of weeks, she offered the following tips and questions to consider when launching a solo dermatology practice:
Select a location. “That’s your first decision,” she said. “Where in the city are you going to open? Are you going to a new city, or are you moving back home? Don’t be afraid to start from scratch, and don’t be afraid to start a [solo] practice if you already have a patient base.”
Will you lease or purchase your space? After she secured a bank loan, Dr. DiGiorgio chose to lease the space for her new practice, “because you can kind of see where things go, get all the kinks out and figure out how to build things in a space that you don’t own. Then, when you’re ready and you have grown, you can invest more into your practice.”
Will you accept insurance? She built her practice around the direct specialty care model, which emphasizes the patient-physician relationship and removes third-party payors. “It’s not a concierge practice, but it’s a transparent, reasonable fee schedule for medical dermatology,” she explained. “I’ve done 100% cosmetics for about 5 years now, [but] I do medical dermatology for a fee. On my website I have a full price list on how much a full skin check is, [and] how much biopsies are. It’s completely transparent. Patients can submit to their insurance for reimbursement, but we don’t guarantee that they’re going to be reimbursed.”
Where will your patients come from? Will you advertise? Do you have physicians in the area who will refer to you if you’re a board-certified dermatologist? She emphasized the importance of “learning how to present yourself” on a website dedicated to your own practice. “Instagram, Facebook, and social media are great, but you don’t own those pages,” noted Dr. DiGiorgio, who served as the program cochair of the 2023 annual meeting of the American Society for Laser Medicine and Surgery and was recently elected to serve on the board of directors for the American Society for Dermatology Surgery. “You don’t own one of those pictures that are posted on your social media page. They can disappear in a second. If that happens, how are people going to find you?”
Are you going to hire more physicians in the future? That will influence the size of the new office and the floor plan.
Lawyer up. Hiring a health care attorney can “help you navigate transitioning from whatever position you’re in to opening up your own practice, as well as setting up the regulatory paperwork necessary for your new practice. You’ll also need a real estate attorney to help once you have selected a place, to help you navigate through that process,” she said, such as figuring out if the elevator in the building meets the Americans With Disabilities Act (ADA) requirements.
Create a mission statement. That way, “you know why you’re doing this, and it stays with you as you’re getting through the hard roadblocks.”
Find an architect, contractor, or designer. “If you’re building out a space from scratch, you’re going to need an architect,” she said. “Along with that architect will come a full-on contracting firm. I ended up hiring everyone individually, because I’m trying to spend as little money as possible.” She also hired a designer to help select furnishings and create the office atmosphere.
Secure a building permit ASAP. “It’s almost better to have the city permit before you sign the lease, because the permits can take a year, and you don’t want to pay rent on an empty space for a year if you don’t have a permit or if there are other hoops to go through,” Dr. DiGiorgio said.
Find an agent to help you set up medical malpractice insurance, liability insurance, and worker’s compensation insurance. “Make sure you read all the paperwork, because it can be very intricate,” she said.
Find an accountant. That person can help set up a bookkeeping process.
What equipment and devices will you need? That depends largely on the patient population a physician serves. Dr. DiGiorgio noted that eligible small businesses may take a tax credit of up to $5,000 per year for accommodations made to comply with the ADA. “That’s a nice feature, so that you can purchase ADA compliant items like a larger exam chair and custom reception areas.”
Dr. DiGiorgio reported having no relevant disclosures.
SAN DIEGO – When the Boston-based cosmetic dermatology practice that employed Catherine M. DiGiorgio, MD, MS, was sold to a private equity firm a few years ago, she found herself at a crossroads: Stay and work for a large corporation, or open a solo practice?
She opted to start her own practice in Boston, “because I didn’t want to work for a large corporation, and I want to provide the best care for my patients in a more intimate manner,” Dr. DiGiorgio, a board-certified laser and cosmetic dermatologist, said at the annual Masters of Aesthetics Symposium.
The decision also tested her mettle. “I spoke to several colleagues and friends, and I was terrified,” she said. “I was like: ‘I don’t even know where to start.’ ”
On the heels of opening a new office in a matter of weeks, she offered the following tips and questions to consider when launching a solo dermatology practice:
Select a location. “That’s your first decision,” she said. “Where in the city are you going to open? Are you going to a new city, or are you moving back home? Don’t be afraid to start from scratch, and don’t be afraid to start a [solo] practice if you already have a patient base.”
Will you lease or purchase your space? After she secured a bank loan, Dr. DiGiorgio chose to lease the space for her new practice, “because you can kind of see where things go, get all the kinks out and figure out how to build things in a space that you don’t own. Then, when you’re ready and you have grown, you can invest more into your practice.”
Will you accept insurance? She built her practice around the direct specialty care model, which emphasizes the patient-physician relationship and removes third-party payors. “It’s not a concierge practice, but it’s a transparent, reasonable fee schedule for medical dermatology,” she explained. “I’ve done 100% cosmetics for about 5 years now, [but] I do medical dermatology for a fee. On my website I have a full price list on how much a full skin check is, [and] how much biopsies are. It’s completely transparent. Patients can submit to their insurance for reimbursement, but we don’t guarantee that they’re going to be reimbursed.”
Where will your patients come from? Will you advertise? Do you have physicians in the area who will refer to you if you’re a board-certified dermatologist? She emphasized the importance of “learning how to present yourself” on a website dedicated to your own practice. “Instagram, Facebook, and social media are great, but you don’t own those pages,” noted Dr. DiGiorgio, who served as the program cochair of the 2023 annual meeting of the American Society for Laser Medicine and Surgery and was recently elected to serve on the board of directors for the American Society for Dermatology Surgery. “You don’t own one of those pictures that are posted on your social media page. They can disappear in a second. If that happens, how are people going to find you?”
Are you going to hire more physicians in the future? That will influence the size of the new office and the floor plan.
Lawyer up. Hiring a health care attorney can “help you navigate transitioning from whatever position you’re in to opening up your own practice, as well as setting up the regulatory paperwork necessary for your new practice. You’ll also need a real estate attorney to help once you have selected a place, to help you navigate through that process,” she said, such as figuring out if the elevator in the building meets the Americans With Disabilities Act (ADA) requirements.
Create a mission statement. That way, “you know why you’re doing this, and it stays with you as you’re getting through the hard roadblocks.”
Find an architect, contractor, or designer. “If you’re building out a space from scratch, you’re going to need an architect,” she said. “Along with that architect will come a full-on contracting firm. I ended up hiring everyone individually, because I’m trying to spend as little money as possible.” She also hired a designer to help select furnishings and create the office atmosphere.
Secure a building permit ASAP. “It’s almost better to have the city permit before you sign the lease, because the permits can take a year, and you don’t want to pay rent on an empty space for a year if you don’t have a permit or if there are other hoops to go through,” Dr. DiGiorgio said.
Find an agent to help you set up medical malpractice insurance, liability insurance, and worker’s compensation insurance. “Make sure you read all the paperwork, because it can be very intricate,” she said.
Find an accountant. That person can help set up a bookkeeping process.
What equipment and devices will you need? That depends largely on the patient population a physician serves. Dr. DiGiorgio noted that eligible small businesses may take a tax credit of up to $5,000 per year for accommodations made to comply with the ADA. “That’s a nice feature, so that you can purchase ADA compliant items like a larger exam chair and custom reception areas.”
Dr. DiGiorgio reported having no relevant disclosures.
AT MOAS 2023
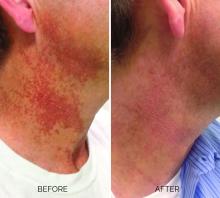
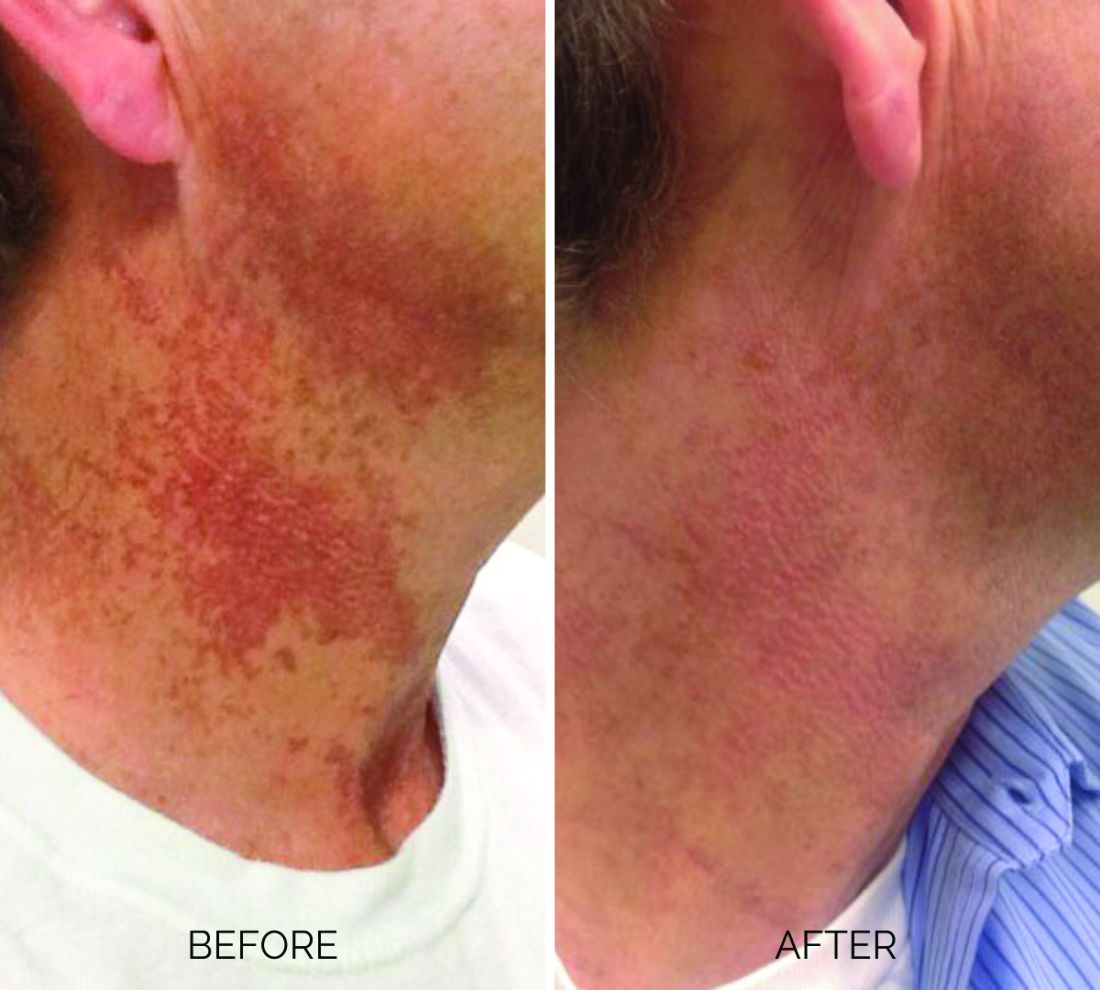
Treating poikiloderma
and is one of the most frustrating dermatologic problems to treat.
Poikiloderma is an area of mottled pigmentation (hyper and hypo) with telangiectasias and atrophy often present on the V of the chest, lateral neck, and lateral face. It is always present in sun-exposed areas but shaded areas of the neck, such as the area under the chin, are spared. Cumulative UV radiation is the predominant underlying cause; however, postmenopausal hormonal changes and contact sensitization with perfumes and cosmetics can exacerbate the condition.
Breaking down the subtypes will help direct the treatment options. There are two main types of poikiloderma – telangiectatic and hyperpigmented – and of course, an overlap between the two. Choosing which subtype is dominant is based primarily on clinical presentation and dermoscopic findings. Atrophy is ubiquitous, thus collagen remodeling is a necessary treatment for both.
In my clinical practice, the pigmentation component of poikiloderma in all skin types is pretreated and posttreated with topical hydroquinone and/or oral tranexamic acid to avoid recurrence after any laser treatment. In the majority of my patients with poikiloderma, I first treat the pigmentation with hydroquinone and tranexamic acid (if the patient is a candidate) to minimize the pigment as much as possible and then treat the telangiectasias with lasers. I try to avoid laser treatment of the hyperpigmentation if at all possible.
Telangiectatic poikiloderma is characterized by a linear and reticular dilated network of vessels. Laser treatment options include IPL, V-beam, and KTP lasers. Multiple treatments are usually necessary and if the patient has concomitant flushing and burning symptoms associated with poikiloderma, topical rosacea treatments such as topical oxymetazoline, as well as avoidance of fragrance, and strict use of a broad spectrum mineral sunscreen, should be initiated prior to laser treatments.
Hyperpigmented poikiloderma is characterized by mottled hyperpigmentation caused by the increased melanin irregularly distributed in the basal layer of the epidermis and melanophages within the dermis. The best treatment for this is with 1,927-nm fractionated resurfacing modalities. Although IPL has been used in this area and is often recommended in the literature for the lentigines, in my experience, the results are transient and it is much harder to blend the color of the skin with the surrounding area of the neck, lateral chest, shoulders, and arms. The 1,927-nm fractionated laser allows for a smoother transition and blending of the skin and also helps with some collagen remodeling of the dermis.
Atrophy is visualized under dermoscopy as a white polka dot–like print with flattened, atrophic epidermis and an elastotic papillary dermis in between the hyperemic telangiectatic network. With every case of poikiloderma, there is some atrophy present; therefore, I combine platelet rich plasma (PRP), PRP with microneedling, or very light treatments with the Fraxel dual (1927/1550) laser to help improve architectural changes of the dermis.
As with any condition of the chest and neck, there is a very fine line between treatment efficacy and complications. All treatments, particularly lasers, should be used with considerable caution and test spots and with the expectation that the treatment will mitigate, not resolve the condition. Sun avoidance, use of daily mineral SPF, and avoidance of fragrance should be emphasized. If expectations are set properly, patients are often satisfied with small improvements as this condition can be very troubling and difficult to treat.
Dr. Talakoub is in private practice in McLean, Va. Write to her at dermnews@mdedge.com. She had no relevant disclosures.
References
Geronemus R. Arch Dermatol. 1990 Apr;126(4):547-8.
Goldman MP and Weiss RA. Plast Reconstr Surg. 2001 May;107(6):1376-81.
Katoulis AC and Stavrianeas NG. Poikiloderma of Civatte. In: Rigopoulos D, Katoulis AC, editors. Hyperpigmentation (Boca Raton, Fla.: CRC Press, 2017). Chapter 12.
and is one of the most frustrating dermatologic problems to treat.
Poikiloderma is an area of mottled pigmentation (hyper and hypo) with telangiectasias and atrophy often present on the V of the chest, lateral neck, and lateral face. It is always present in sun-exposed areas but shaded areas of the neck, such as the area under the chin, are spared. Cumulative UV radiation is the predominant underlying cause; however, postmenopausal hormonal changes and contact sensitization with perfumes and cosmetics can exacerbate the condition.
Breaking down the subtypes will help direct the treatment options. There are two main types of poikiloderma – telangiectatic and hyperpigmented – and of course, an overlap between the two. Choosing which subtype is dominant is based primarily on clinical presentation and dermoscopic findings. Atrophy is ubiquitous, thus collagen remodeling is a necessary treatment for both.
In my clinical practice, the pigmentation component of poikiloderma in all skin types is pretreated and posttreated with topical hydroquinone and/or oral tranexamic acid to avoid recurrence after any laser treatment. In the majority of my patients with poikiloderma, I first treat the pigmentation with hydroquinone and tranexamic acid (if the patient is a candidate) to minimize the pigment as much as possible and then treat the telangiectasias with lasers. I try to avoid laser treatment of the hyperpigmentation if at all possible.
Telangiectatic poikiloderma is characterized by a linear and reticular dilated network of vessels. Laser treatment options include IPL, V-beam, and KTP lasers. Multiple treatments are usually necessary and if the patient has concomitant flushing and burning symptoms associated with poikiloderma, topical rosacea treatments such as topical oxymetazoline, as well as avoidance of fragrance, and strict use of a broad spectrum mineral sunscreen, should be initiated prior to laser treatments.
Hyperpigmented poikiloderma is characterized by mottled hyperpigmentation caused by the increased melanin irregularly distributed in the basal layer of the epidermis and melanophages within the dermis. The best treatment for this is with 1,927-nm fractionated resurfacing modalities. Although IPL has been used in this area and is often recommended in the literature for the lentigines, in my experience, the results are transient and it is much harder to blend the color of the skin with the surrounding area of the neck, lateral chest, shoulders, and arms. The 1,927-nm fractionated laser allows for a smoother transition and blending of the skin and also helps with some collagen remodeling of the dermis.
Atrophy is visualized under dermoscopy as a white polka dot–like print with flattened, atrophic epidermis and an elastotic papillary dermis in between the hyperemic telangiectatic network. With every case of poikiloderma, there is some atrophy present; therefore, I combine platelet rich plasma (PRP), PRP with microneedling, or very light treatments with the Fraxel dual (1927/1550) laser to help improve architectural changes of the dermis.
As with any condition of the chest and neck, there is a very fine line between treatment efficacy and complications. All treatments, particularly lasers, should be used with considerable caution and test spots and with the expectation that the treatment will mitigate, not resolve the condition. Sun avoidance, use of daily mineral SPF, and avoidance of fragrance should be emphasized. If expectations are set properly, patients are often satisfied with small improvements as this condition can be very troubling and difficult to treat.
Dr. Talakoub is in private practice in McLean, Va. Write to her at dermnews@mdedge.com. She had no relevant disclosures.
References
Geronemus R. Arch Dermatol. 1990 Apr;126(4):547-8.
Goldman MP and Weiss RA. Plast Reconstr Surg. 2001 May;107(6):1376-81.
Katoulis AC and Stavrianeas NG. Poikiloderma of Civatte. In: Rigopoulos D, Katoulis AC, editors. Hyperpigmentation (Boca Raton, Fla.: CRC Press, 2017). Chapter 12.
and is one of the most frustrating dermatologic problems to treat.
Poikiloderma is an area of mottled pigmentation (hyper and hypo) with telangiectasias and atrophy often present on the V of the chest, lateral neck, and lateral face. It is always present in sun-exposed areas but shaded areas of the neck, such as the area under the chin, are spared. Cumulative UV radiation is the predominant underlying cause; however, postmenopausal hormonal changes and contact sensitization with perfumes and cosmetics can exacerbate the condition.
Breaking down the subtypes will help direct the treatment options. There are two main types of poikiloderma – telangiectatic and hyperpigmented – and of course, an overlap between the two. Choosing which subtype is dominant is based primarily on clinical presentation and dermoscopic findings. Atrophy is ubiquitous, thus collagen remodeling is a necessary treatment for both.
In my clinical practice, the pigmentation component of poikiloderma in all skin types is pretreated and posttreated with topical hydroquinone and/or oral tranexamic acid to avoid recurrence after any laser treatment. In the majority of my patients with poikiloderma, I first treat the pigmentation with hydroquinone and tranexamic acid (if the patient is a candidate) to minimize the pigment as much as possible and then treat the telangiectasias with lasers. I try to avoid laser treatment of the hyperpigmentation if at all possible.
Telangiectatic poikiloderma is characterized by a linear and reticular dilated network of vessels. Laser treatment options include IPL, V-beam, and KTP lasers. Multiple treatments are usually necessary and if the patient has concomitant flushing and burning symptoms associated with poikiloderma, topical rosacea treatments such as topical oxymetazoline, as well as avoidance of fragrance, and strict use of a broad spectrum mineral sunscreen, should be initiated prior to laser treatments.
Hyperpigmented poikiloderma is characterized by mottled hyperpigmentation caused by the increased melanin irregularly distributed in the basal layer of the epidermis and melanophages within the dermis. The best treatment for this is with 1,927-nm fractionated resurfacing modalities. Although IPL has been used in this area and is often recommended in the literature for the lentigines, in my experience, the results are transient and it is much harder to blend the color of the skin with the surrounding area of the neck, lateral chest, shoulders, and arms. The 1,927-nm fractionated laser allows for a smoother transition and blending of the skin and also helps with some collagen remodeling of the dermis.
Atrophy is visualized under dermoscopy as a white polka dot–like print with flattened, atrophic epidermis and an elastotic papillary dermis in between the hyperemic telangiectatic network. With every case of poikiloderma, there is some atrophy present; therefore, I combine platelet rich plasma (PRP), PRP with microneedling, or very light treatments with the Fraxel dual (1927/1550) laser to help improve architectural changes of the dermis.
As with any condition of the chest and neck, there is a very fine line between treatment efficacy and complications. All treatments, particularly lasers, should be used with considerable caution and test spots and with the expectation that the treatment will mitigate, not resolve the condition. Sun avoidance, use of daily mineral SPF, and avoidance of fragrance should be emphasized. If expectations are set properly, patients are often satisfied with small improvements as this condition can be very troubling and difficult to treat.
Dr. Talakoub is in private practice in McLean, Va. Write to her at dermnews@mdedge.com. She had no relevant disclosures.
References
Geronemus R. Arch Dermatol. 1990 Apr;126(4):547-8.
Goldman MP and Weiss RA. Plast Reconstr Surg. 2001 May;107(6):1376-81.
Katoulis AC and Stavrianeas NG. Poikiloderma of Civatte. In: Rigopoulos D, Katoulis AC, editors. Hyperpigmentation (Boca Raton, Fla.: CRC Press, 2017). Chapter 12.
Cleansing balms
A skin care trend, particularly in the Korean beauty product market and now worldwide, cleansing balms are a soft, yet solid variation of an oil-based cleanser. The solid oily component is combined with a surfactant or emulsifier. The cream balm texture melts into more of an oil texture once warmed with fingertips and applied to facial skin. The oils are effective at breaking down or attracting skin care products, oil, and grime on the skin surface. Once warm water is added, the oil emulsifies, and after it is wiped or rinsed off, what’s left behind is clean, hydrated skin.
They don’t tend to compromise the moisture barrier or disrupt skin pH, thus, resulting in less dry skin and have less potential to cause irritation. These products are particularly useful during drier, colder months, or in dry climates, and for those who have dry skin or eczema.
The popularity of cleansing balms has largely been based on their ability to remove makeup, similar to an oil cleanser, without the need to necessarily “double cleanse” with a regular cleanser afterward.
Alternatives to remove makeup besides cleansing balms, oil cleansers, and regular liquid water-based cleansers include micellar water (oil in water), chemical makeup removing cloths, and nonchemical makeup removing pads used with water. Micellar water is also gentle on the skin; it requires a cotton pad, tip, or cloth to remove makeup, without the need for water or washing. Both are effective, but it may be easier to remove makeup with cleansing balms, without the need for rubbing dry skin, than with micellar water. A study published in 2020 of 20 individuals reported that waterproof sunscreen was more effectively removed with a cleansing oil than a non–oil-based cleanser, with less irritation and dryness. Both were effective at removing non-waterproof sunscreen.
Both cleansing balms and oil-based cleansers need to be kept at room temperature (not in the refrigerator), since they may separate or solidify at low temperatures.
Most cleansing balms can be applied to dry skin, massaged, and rinsed off with warm water, but they are sometimes easier to remove with a wet cloth (typically either cotton or muslin). Many are nonirritating to the eyes, which is important when used to remove eye makeup and mascara on delicate skin. While many cleansing balms are noncomedogenic, residue from balms that are too thick or not rinsed off properly can contribute to comedones or milia. If residue is present after use, then “double-cleansing” with a water-based cleanser is reasonable, but not necessary for most users.
Did the development of Ponds cold cream mark the beginning of this trend? Yes and no. The creation of the first cold cream prototype has been attributed to the Greek physician, Galen (who lived in Rome), a combination of rose water, beeswax, and olive oil in 150 CE. While Ponds also has manufactured a cleansing balm, the original cold cream is a 50% moisturizer in a cleanser. So while similar in containing an oil, water, emulsifier, and thickener, and effective, it is more of a moisturizer and less of a solid oil/balm in its consistency.
Dr. Wesley practices dermatology in Beverly Hills, Calif. Write to her at dermnews@mdedge.com. She had no relevant disclosures.
A skin care trend, particularly in the Korean beauty product market and now worldwide, cleansing balms are a soft, yet solid variation of an oil-based cleanser. The solid oily component is combined with a surfactant or emulsifier. The cream balm texture melts into more of an oil texture once warmed with fingertips and applied to facial skin. The oils are effective at breaking down or attracting skin care products, oil, and grime on the skin surface. Once warm water is added, the oil emulsifies, and after it is wiped or rinsed off, what’s left behind is clean, hydrated skin.
They don’t tend to compromise the moisture barrier or disrupt skin pH, thus, resulting in less dry skin and have less potential to cause irritation. These products are particularly useful during drier, colder months, or in dry climates, and for those who have dry skin or eczema.
The popularity of cleansing balms has largely been based on their ability to remove makeup, similar to an oil cleanser, without the need to necessarily “double cleanse” with a regular cleanser afterward.
Alternatives to remove makeup besides cleansing balms, oil cleansers, and regular liquid water-based cleansers include micellar water (oil in water), chemical makeup removing cloths, and nonchemical makeup removing pads used with water. Micellar water is also gentle on the skin; it requires a cotton pad, tip, or cloth to remove makeup, without the need for water or washing. Both are effective, but it may be easier to remove makeup with cleansing balms, without the need for rubbing dry skin, than with micellar water. A study published in 2020 of 20 individuals reported that waterproof sunscreen was more effectively removed with a cleansing oil than a non–oil-based cleanser, with less irritation and dryness. Both were effective at removing non-waterproof sunscreen.
Both cleansing balms and oil-based cleansers need to be kept at room temperature (not in the refrigerator), since they may separate or solidify at low temperatures.
Most cleansing balms can be applied to dry skin, massaged, and rinsed off with warm water, but they are sometimes easier to remove with a wet cloth (typically either cotton or muslin). Many are nonirritating to the eyes, which is important when used to remove eye makeup and mascara on delicate skin. While many cleansing balms are noncomedogenic, residue from balms that are too thick or not rinsed off properly can contribute to comedones or milia. If residue is present after use, then “double-cleansing” with a water-based cleanser is reasonable, but not necessary for most users.
Did the development of Ponds cold cream mark the beginning of this trend? Yes and no. The creation of the first cold cream prototype has been attributed to the Greek physician, Galen (who lived in Rome), a combination of rose water, beeswax, and olive oil in 150 CE. While Ponds also has manufactured a cleansing balm, the original cold cream is a 50% moisturizer in a cleanser. So while similar in containing an oil, water, emulsifier, and thickener, and effective, it is more of a moisturizer and less of a solid oil/balm in its consistency.
Dr. Wesley practices dermatology in Beverly Hills, Calif. Write to her at dermnews@mdedge.com. She had no relevant disclosures.
A skin care trend, particularly in the Korean beauty product market and now worldwide, cleansing balms are a soft, yet solid variation of an oil-based cleanser. The solid oily component is combined with a surfactant or emulsifier. The cream balm texture melts into more of an oil texture once warmed with fingertips and applied to facial skin. The oils are effective at breaking down or attracting skin care products, oil, and grime on the skin surface. Once warm water is added, the oil emulsifies, and after it is wiped or rinsed off, what’s left behind is clean, hydrated skin.
They don’t tend to compromise the moisture barrier or disrupt skin pH, thus, resulting in less dry skin and have less potential to cause irritation. These products are particularly useful during drier, colder months, or in dry climates, and for those who have dry skin or eczema.
The popularity of cleansing balms has largely been based on their ability to remove makeup, similar to an oil cleanser, without the need to necessarily “double cleanse” with a regular cleanser afterward.
Alternatives to remove makeup besides cleansing balms, oil cleansers, and regular liquid water-based cleansers include micellar water (oil in water), chemical makeup removing cloths, and nonchemical makeup removing pads used with water. Micellar water is also gentle on the skin; it requires a cotton pad, tip, or cloth to remove makeup, without the need for water or washing. Both are effective, but it may be easier to remove makeup with cleansing balms, without the need for rubbing dry skin, than with micellar water. A study published in 2020 of 20 individuals reported that waterproof sunscreen was more effectively removed with a cleansing oil than a non–oil-based cleanser, with less irritation and dryness. Both were effective at removing non-waterproof sunscreen.
Both cleansing balms and oil-based cleansers need to be kept at room temperature (not in the refrigerator), since they may separate or solidify at low temperatures.
Most cleansing balms can be applied to dry skin, massaged, and rinsed off with warm water, but they are sometimes easier to remove with a wet cloth (typically either cotton or muslin). Many are nonirritating to the eyes, which is important when used to remove eye makeup and mascara on delicate skin. While many cleansing balms are noncomedogenic, residue from balms that are too thick or not rinsed off properly can contribute to comedones or milia. If residue is present after use, then “double-cleansing” with a water-based cleanser is reasonable, but not necessary for most users.
Did the development of Ponds cold cream mark the beginning of this trend? Yes and no. The creation of the first cold cream prototype has been attributed to the Greek physician, Galen (who lived in Rome), a combination of rose water, beeswax, and olive oil in 150 CE. While Ponds also has manufactured a cleansing balm, the original cold cream is a 50% moisturizer in a cleanser. So while similar in containing an oil, water, emulsifier, and thickener, and effective, it is more of a moisturizer and less of a solid oil/balm in its consistency.
Dr. Wesley practices dermatology in Beverly Hills, Calif. Write to her at dermnews@mdedge.com. She had no relevant disclosures.
Treatment of craniofacial hyperhidrosis
Hyperhidrosis has a significant impact on a person’s physical, psychological, and social aspects of life. The lack of treatment options and associated stigma limits access to care and treatment options.
Primary hyperhidrosis does not have an underlying cause; is symmetrical; can worsen with anxiety, fear, or stress; and may have a familial component. Palmar and axillary hyperhidrosis are the most common types of hyperhidrosis. The incidence of craniofacial hyperhidrosis has not been clearly defined but it is most commonly reported on the forehead, where the concentration of eccrine sweat glands is highest.
Treatment options for craniofacial hyperhidrosis include topical aluminum chloride, which blocks the eccrine sweat duct or causes eccrine cell atrophy. Although this option is a common treatment for palmar and axillary hyperhidrosis, use on the face has not been thoroughly studied, and may also cause skin irritation.
Topical and oral glycopyrrolate can be effective for all types of hyperhidrosis, but must be used daily and can have systemic side effects, with variable efficacy and longevity. Oral oxybutynin, beta-blockers, clonidine, and benzodiazepines have also been used with some limited studies available in patients with generalized hyperhidrosis.
Surgical treatments such as videothoracoscopy sympathectomy can be used in severe or recalcitrant cases of hyperhidrosis with good efficacy. However, surgical complications and inherent surgical risks limit these treatment options unless other modalities are exhausted.
OnabotulinumtoxinA is Food and Drug Administration approved for treating severe primary axillary hyperhidrosis, but is used off label for palmar, plantar, and craniofacial hyperhidrosis with great results and few side effects. Clinical pearls and guidelines for the use of botulinum toxin A in craniofacial hyperhidrosis were outlined by Wolosker and colleagues in a review article. As with any injection of neurotoxin, knowledge of the facial anatomy is critical to avoiding muscle paralysis.
double diluted. Treatment effects usually last 3 months, similar to cosmetic uses. Wolosker uses a dilution of 100 U botulinum toxin in 1.0 mL saline, which I find slightly more difficult to control and more likely to have loss of toxin.
In my experience, I have found the following dosing to be most effective with the least side effects for the following (dosages vary and can be titrated up to response):
- Upper lip: 6-10 U.
- Chin: 6-10 U.
- Forehead: 15-30 U. (Avoid 1 cm above the brow unless risks of brow drop are reviewed and acceptable to the patient. In my experience patients would rather have a lower brow than obstructive sweating in their brow that can irritate the eyes, blur vision, and smudge skincare and makeup.)
- Nose: 10 U
- Cheeks: 10 U per side (staying very superficial with injections).
- Scalp: 30-50 U (using serial injections 1-2 cm apart in the area affected by hyperhidrosis).
Side effects include temporary erythema, bruising, and edema, as well as muscle paralysis and asymmetry if proper injection technique is not used, the dose is not diluted properly, or the injection is too deep.
There are scattered case studies of symbiotic techniques to help the penetration of botulinum toxin when treating craniofacial hyperhidrosis, including microneedling, radiofrequency, long-pulsed diode laser, and ultrasound. But the safety and efficacy of these procedures have not been properly evaluated.
In all of my patients with craniofacial hyperhidrosis treated with botulinum toxin, quality of life is significantly improved with almost no complications. Botulinum toxin is a safe, relatively quick in-office procedure to treat craniofacial hyperhidrosis that can be used to help patients – particularly those who experience anxiety or have social and occupational impairment related to their disease.
This procedure is cosmetic in nature, and therefore, not covered by insurance.
Dr. Talakoub is in private practice in McLean, Va. Write to her at dermnews@mdedge.com. She had no relevant disclosures.
References
Parashar K et al. Am J Clin Dermatol. 2023 Mar;24(2):187-98.
Doolittle J et al. Arch Dermatol Res. 2016 Dec;308(10):743-9.
Wolosker N et al. J Vasc Bras. 2020 Nov 16;19:e20190152.
Garcia-Souto F et al. Dermatol Ther. 2021 Jan;34(1):e14658.
Ebrahim H et al. J Clin Aesthet Dermatol. 2022 Sep;15(9):40-4.
Campanati A et al. Toxins (Basel). 2022 May 27;14(6):3727.
Hyperhidrosis has a significant impact on a person’s physical, psychological, and social aspects of life. The lack of treatment options and associated stigma limits access to care and treatment options.
Primary hyperhidrosis does not have an underlying cause; is symmetrical; can worsen with anxiety, fear, or stress; and may have a familial component. Palmar and axillary hyperhidrosis are the most common types of hyperhidrosis. The incidence of craniofacial hyperhidrosis has not been clearly defined but it is most commonly reported on the forehead, where the concentration of eccrine sweat glands is highest.
Treatment options for craniofacial hyperhidrosis include topical aluminum chloride, which blocks the eccrine sweat duct or causes eccrine cell atrophy. Although this option is a common treatment for palmar and axillary hyperhidrosis, use on the face has not been thoroughly studied, and may also cause skin irritation.
Topical and oral glycopyrrolate can be effective for all types of hyperhidrosis, but must be used daily and can have systemic side effects, with variable efficacy and longevity. Oral oxybutynin, beta-blockers, clonidine, and benzodiazepines have also been used with some limited studies available in patients with generalized hyperhidrosis.
Surgical treatments such as videothoracoscopy sympathectomy can be used in severe or recalcitrant cases of hyperhidrosis with good efficacy. However, surgical complications and inherent surgical risks limit these treatment options unless other modalities are exhausted.
OnabotulinumtoxinA is Food and Drug Administration approved for treating severe primary axillary hyperhidrosis, but is used off label for palmar, plantar, and craniofacial hyperhidrosis with great results and few side effects. Clinical pearls and guidelines for the use of botulinum toxin A in craniofacial hyperhidrosis were outlined by Wolosker and colleagues in a review article. As with any injection of neurotoxin, knowledge of the facial anatomy is critical to avoiding muscle paralysis.
double diluted. Treatment effects usually last 3 months, similar to cosmetic uses. Wolosker uses a dilution of 100 U botulinum toxin in 1.0 mL saline, which I find slightly more difficult to control and more likely to have loss of toxin.
In my experience, I have found the following dosing to be most effective with the least side effects for the following (dosages vary and can be titrated up to response):
- Upper lip: 6-10 U.
- Chin: 6-10 U.
- Forehead: 15-30 U. (Avoid 1 cm above the brow unless risks of brow drop are reviewed and acceptable to the patient. In my experience patients would rather have a lower brow than obstructive sweating in their brow that can irritate the eyes, blur vision, and smudge skincare and makeup.)
- Nose: 10 U
- Cheeks: 10 U per side (staying very superficial with injections).
- Scalp: 30-50 U (using serial injections 1-2 cm apart in the area affected by hyperhidrosis).
Side effects include temporary erythema, bruising, and edema, as well as muscle paralysis and asymmetry if proper injection technique is not used, the dose is not diluted properly, or the injection is too deep.
There are scattered case studies of symbiotic techniques to help the penetration of botulinum toxin when treating craniofacial hyperhidrosis, including microneedling, radiofrequency, long-pulsed diode laser, and ultrasound. But the safety and efficacy of these procedures have not been properly evaluated.
In all of my patients with craniofacial hyperhidrosis treated with botulinum toxin, quality of life is significantly improved with almost no complications. Botulinum toxin is a safe, relatively quick in-office procedure to treat craniofacial hyperhidrosis that can be used to help patients – particularly those who experience anxiety or have social and occupational impairment related to their disease.
This procedure is cosmetic in nature, and therefore, not covered by insurance.
Dr. Talakoub is in private practice in McLean, Va. Write to her at dermnews@mdedge.com. She had no relevant disclosures.
References
Parashar K et al. Am J Clin Dermatol. 2023 Mar;24(2):187-98.
Doolittle J et al. Arch Dermatol Res. 2016 Dec;308(10):743-9.
Wolosker N et al. J Vasc Bras. 2020 Nov 16;19:e20190152.
Garcia-Souto F et al. Dermatol Ther. 2021 Jan;34(1):e14658.
Ebrahim H et al. J Clin Aesthet Dermatol. 2022 Sep;15(9):40-4.
Campanati A et al. Toxins (Basel). 2022 May 27;14(6):3727.
Hyperhidrosis has a significant impact on a person’s physical, psychological, and social aspects of life. The lack of treatment options and associated stigma limits access to care and treatment options.
Primary hyperhidrosis does not have an underlying cause; is symmetrical; can worsen with anxiety, fear, or stress; and may have a familial component. Palmar and axillary hyperhidrosis are the most common types of hyperhidrosis. The incidence of craniofacial hyperhidrosis has not been clearly defined but it is most commonly reported on the forehead, where the concentration of eccrine sweat glands is highest.
Treatment options for craniofacial hyperhidrosis include topical aluminum chloride, which blocks the eccrine sweat duct or causes eccrine cell atrophy. Although this option is a common treatment for palmar and axillary hyperhidrosis, use on the face has not been thoroughly studied, and may also cause skin irritation.
Topical and oral glycopyrrolate can be effective for all types of hyperhidrosis, but must be used daily and can have systemic side effects, with variable efficacy and longevity. Oral oxybutynin, beta-blockers, clonidine, and benzodiazepines have also been used with some limited studies available in patients with generalized hyperhidrosis.
Surgical treatments such as videothoracoscopy sympathectomy can be used in severe or recalcitrant cases of hyperhidrosis with good efficacy. However, surgical complications and inherent surgical risks limit these treatment options unless other modalities are exhausted.
OnabotulinumtoxinA is Food and Drug Administration approved for treating severe primary axillary hyperhidrosis, but is used off label for palmar, plantar, and craniofacial hyperhidrosis with great results and few side effects. Clinical pearls and guidelines for the use of botulinum toxin A in craniofacial hyperhidrosis were outlined by Wolosker and colleagues in a review article. As with any injection of neurotoxin, knowledge of the facial anatomy is critical to avoiding muscle paralysis.
double diluted. Treatment effects usually last 3 months, similar to cosmetic uses. Wolosker uses a dilution of 100 U botulinum toxin in 1.0 mL saline, which I find slightly more difficult to control and more likely to have loss of toxin.
In my experience, I have found the following dosing to be most effective with the least side effects for the following (dosages vary and can be titrated up to response):
- Upper lip: 6-10 U.
- Chin: 6-10 U.
- Forehead: 15-30 U. (Avoid 1 cm above the brow unless risks of brow drop are reviewed and acceptable to the patient. In my experience patients would rather have a lower brow than obstructive sweating in their brow that can irritate the eyes, blur vision, and smudge skincare and makeup.)
- Nose: 10 U
- Cheeks: 10 U per side (staying very superficial with injections).
- Scalp: 30-50 U (using serial injections 1-2 cm apart in the area affected by hyperhidrosis).
Side effects include temporary erythema, bruising, and edema, as well as muscle paralysis and asymmetry if proper injection technique is not used, the dose is not diluted properly, or the injection is too deep.
There are scattered case studies of symbiotic techniques to help the penetration of botulinum toxin when treating craniofacial hyperhidrosis, including microneedling, radiofrequency, long-pulsed diode laser, and ultrasound. But the safety and efficacy of these procedures have not been properly evaluated.
In all of my patients with craniofacial hyperhidrosis treated with botulinum toxin, quality of life is significantly improved with almost no complications. Botulinum toxin is a safe, relatively quick in-office procedure to treat craniofacial hyperhidrosis that can be used to help patients – particularly those who experience anxiety or have social and occupational impairment related to their disease.
This procedure is cosmetic in nature, and therefore, not covered by insurance.
Dr. Talakoub is in private practice in McLean, Va. Write to her at dermnews@mdedge.com. She had no relevant disclosures.
References
Parashar K et al. Am J Clin Dermatol. 2023 Mar;24(2):187-98.
Doolittle J et al. Arch Dermatol Res. 2016 Dec;308(10):743-9.
Wolosker N et al. J Vasc Bras. 2020 Nov 16;19:e20190152.
Garcia-Souto F et al. Dermatol Ther. 2021 Jan;34(1):e14658.
Ebrahim H et al. J Clin Aesthet Dermatol. 2022 Sep;15(9):40-4.
Campanati A et al. Toxins (Basel). 2022 May 27;14(6):3727.
Facial lipoatrophy with semaglutide-related weight loss
Ozempic and Wegovy are two prescription drugs that have transformed the management of type 2 diabetes and obesity. Both are a form of semaglutide; the Food and Drug Administration approved Ozempic for treating type 2 diabetes in 2017, followed by Wegovy in 2021 for weight loss in adults with obesity or those who are overweight and have least one weight-related health condition, such as hypertension or hypercholesterolemia. Ozempic is not approved for weight loss, but it has been prescribed off label for that purpose.
An effective treatment, participants with overweight or obesity in one study experienced almost a mean 15% drop in body weight with subcutaneous semaglutide administered once a week versus about 2% with placebo after 68 weeks.
In 2022, high demand and global supply constraints gave rise to shortages of both medications. The FDA reported a Wegovy shortage in March 2022, followed by an Ozempic shortage in August. Social media attention and increased off-label prescribing, with some patients purporting to have had significant improvements with weight loss and their quality of life, including having their clothing fit better and being able to bend over and tie their shoes, increased attention on these medications to the point that off-label prescribing of both drugs for weight loss resulted in some patients with type 2 diabetes unable to receive their medication on time. In late January 2023, NBC reported that Ozempic prescriptions had “tripled from 2021 to 2022,” based on data from the prescription drug discount company SingleCare.
Semaglutide is designed to mimic a hormone that signals to the brain when a person is full and promotes the release of insulin. In turn, the medications can result in lower blood glucose levels, appetite suppression, and reduced caloric intake. Injected once weekly, the medication, a glucagonlike peptide–1 receptor agonist, specifically, activates GLP-1 receptors in the brain, increasing insulin secretion, decreasing glucagon secretion, and delaying gastric emptying (acting as an incretin mimetic).
‘Ozempic face’
Common adverse events with semaglutide can include nausea, vomiting, diarrhea, abdominal pain, constipation, and injection-site reactions. Rare, but more severe adverse events may include thyroid C-cell tumor (in animal studies), medullary thyroid cancer risk, hypersensitivity reaction, anaphylaxis, acute renal injury, chronic renal failure exacerbation, pancreatitis, and cholelithiasis.
A less severe but noticeable side effect that has gained attention is facial wasting and aging, reportedly coined “Ozempic face” by a dermatologist interviewed for an article published in January in The New York Times.
As of Feb. 9, TikTok videos from individuals describing their personal experiences, health care professionals, and others with the tag #ozempicface had 4.8 million views.
Theories as to why noticeable facial changes occur with these medications include: accelerated loss of facial pads that already tend to diminish or shift with normal aging, as well as the inability of skin elasticity to keep up with the loss of volume (fat), resulting in more prominent hanging skin and the appearance of “jowls.” Wan and colleagues have described the fat pad distribution in the face and the facial aging that occurs as a result of the loss and shifting of these fat pads over time.
In the same way that we use facial fillers to help treat and correct volume/fat loss associated with photoaging, facial fillers may be used to help restore volume where it’s been lost after weight loss. The sagging skin or loss of elasticity often associated with Ozempic-related weight loss or with rapid or noticeable weight loss in general, may or may not also require other interventions that include treatment with tissue tightening devices – such as radiofrequency energy, high-focused ultrasound energy, threads, and/or surgery – such as a face lift. The potential high cost of both off-label prescribing of these medications (especially without use of prescription health insurance) as well as treatment to correct any facial wasting has also received attention in news media and social media discussions of this topic.
Dr. Wesley practices dermatology in Beverly Hills, Calif. Write to her at dermnews@mdedge.com. She has no relevant disclosures.
*Correction 1/28/23: An earlier version of this story misstated the approval date of Wegovy. It was in 2021.
Ozempic and Wegovy are two prescription drugs that have transformed the management of type 2 diabetes and obesity. Both are a form of semaglutide; the Food and Drug Administration approved Ozempic for treating type 2 diabetes in 2017, followed by Wegovy in 2021 for weight loss in adults with obesity or those who are overweight and have least one weight-related health condition, such as hypertension or hypercholesterolemia. Ozempic is not approved for weight loss, but it has been prescribed off label for that purpose.
An effective treatment, participants with overweight or obesity in one study experienced almost a mean 15% drop in body weight with subcutaneous semaglutide administered once a week versus about 2% with placebo after 68 weeks.
In 2022, high demand and global supply constraints gave rise to shortages of both medications. The FDA reported a Wegovy shortage in March 2022, followed by an Ozempic shortage in August. Social media attention and increased off-label prescribing, with some patients purporting to have had significant improvements with weight loss and their quality of life, including having their clothing fit better and being able to bend over and tie their shoes, increased attention on these medications to the point that off-label prescribing of both drugs for weight loss resulted in some patients with type 2 diabetes unable to receive their medication on time. In late January 2023, NBC reported that Ozempic prescriptions had “tripled from 2021 to 2022,” based on data from the prescription drug discount company SingleCare.
Semaglutide is designed to mimic a hormone that signals to the brain when a person is full and promotes the release of insulin. In turn, the medications can result in lower blood glucose levels, appetite suppression, and reduced caloric intake. Injected once weekly, the medication, a glucagonlike peptide–1 receptor agonist, specifically, activates GLP-1 receptors in the brain, increasing insulin secretion, decreasing glucagon secretion, and delaying gastric emptying (acting as an incretin mimetic).
‘Ozempic face’
Common adverse events with semaglutide can include nausea, vomiting, diarrhea, abdominal pain, constipation, and injection-site reactions. Rare, but more severe adverse events may include thyroid C-cell tumor (in animal studies), medullary thyroid cancer risk, hypersensitivity reaction, anaphylaxis, acute renal injury, chronic renal failure exacerbation, pancreatitis, and cholelithiasis.
A less severe but noticeable side effect that has gained attention is facial wasting and aging, reportedly coined “Ozempic face” by a dermatologist interviewed for an article published in January in The New York Times.
As of Feb. 9, TikTok videos from individuals describing their personal experiences, health care professionals, and others with the tag #ozempicface had 4.8 million views.
Theories as to why noticeable facial changes occur with these medications include: accelerated loss of facial pads that already tend to diminish or shift with normal aging, as well as the inability of skin elasticity to keep up with the loss of volume (fat), resulting in more prominent hanging skin and the appearance of “jowls.” Wan and colleagues have described the fat pad distribution in the face and the facial aging that occurs as a result of the loss and shifting of these fat pads over time.
In the same way that we use facial fillers to help treat and correct volume/fat loss associated with photoaging, facial fillers may be used to help restore volume where it’s been lost after weight loss. The sagging skin or loss of elasticity often associated with Ozempic-related weight loss or with rapid or noticeable weight loss in general, may or may not also require other interventions that include treatment with tissue tightening devices – such as radiofrequency energy, high-focused ultrasound energy, threads, and/or surgery – such as a face lift. The potential high cost of both off-label prescribing of these medications (especially without use of prescription health insurance) as well as treatment to correct any facial wasting has also received attention in news media and social media discussions of this topic.
Dr. Wesley practices dermatology in Beverly Hills, Calif. Write to her at dermnews@mdedge.com. She has no relevant disclosures.
*Correction 1/28/23: An earlier version of this story misstated the approval date of Wegovy. It was in 2021.
Ozempic and Wegovy are two prescription drugs that have transformed the management of type 2 diabetes and obesity. Both are a form of semaglutide; the Food and Drug Administration approved Ozempic for treating type 2 diabetes in 2017, followed by Wegovy in 2021 for weight loss in adults with obesity or those who are overweight and have least one weight-related health condition, such as hypertension or hypercholesterolemia. Ozempic is not approved for weight loss, but it has been prescribed off label for that purpose.
An effective treatment, participants with overweight or obesity in one study experienced almost a mean 15% drop in body weight with subcutaneous semaglutide administered once a week versus about 2% with placebo after 68 weeks.
In 2022, high demand and global supply constraints gave rise to shortages of both medications. The FDA reported a Wegovy shortage in March 2022, followed by an Ozempic shortage in August. Social media attention and increased off-label prescribing, with some patients purporting to have had significant improvements with weight loss and their quality of life, including having their clothing fit better and being able to bend over and tie their shoes, increased attention on these medications to the point that off-label prescribing of both drugs for weight loss resulted in some patients with type 2 diabetes unable to receive their medication on time. In late January 2023, NBC reported that Ozempic prescriptions had “tripled from 2021 to 2022,” based on data from the prescription drug discount company SingleCare.
Semaglutide is designed to mimic a hormone that signals to the brain when a person is full and promotes the release of insulin. In turn, the medications can result in lower blood glucose levels, appetite suppression, and reduced caloric intake. Injected once weekly, the medication, a glucagonlike peptide–1 receptor agonist, specifically, activates GLP-1 receptors in the brain, increasing insulin secretion, decreasing glucagon secretion, and delaying gastric emptying (acting as an incretin mimetic).
‘Ozempic face’
Common adverse events with semaglutide can include nausea, vomiting, diarrhea, abdominal pain, constipation, and injection-site reactions. Rare, but more severe adverse events may include thyroid C-cell tumor (in animal studies), medullary thyroid cancer risk, hypersensitivity reaction, anaphylaxis, acute renal injury, chronic renal failure exacerbation, pancreatitis, and cholelithiasis.
A less severe but noticeable side effect that has gained attention is facial wasting and aging, reportedly coined “Ozempic face” by a dermatologist interviewed for an article published in January in The New York Times.
As of Feb. 9, TikTok videos from individuals describing their personal experiences, health care professionals, and others with the tag #ozempicface had 4.8 million views.
Theories as to why noticeable facial changes occur with these medications include: accelerated loss of facial pads that already tend to diminish or shift with normal aging, as well as the inability of skin elasticity to keep up with the loss of volume (fat), resulting in more prominent hanging skin and the appearance of “jowls.” Wan and colleagues have described the fat pad distribution in the face and the facial aging that occurs as a result of the loss and shifting of these fat pads over time.
In the same way that we use facial fillers to help treat and correct volume/fat loss associated with photoaging, facial fillers may be used to help restore volume where it’s been lost after weight loss. The sagging skin or loss of elasticity often associated with Ozempic-related weight loss or with rapid or noticeable weight loss in general, may or may not also require other interventions that include treatment with tissue tightening devices – such as radiofrequency energy, high-focused ultrasound energy, threads, and/or surgery – such as a face lift. The potential high cost of both off-label prescribing of these medications (especially without use of prescription health insurance) as well as treatment to correct any facial wasting has also received attention in news media and social media discussions of this topic.
Dr. Wesley practices dermatology in Beverly Hills, Calif. Write to her at dermnews@mdedge.com. She has no relevant disclosures.
*Correction 1/28/23: An earlier version of this story misstated the approval date of Wegovy. It was in 2021.
Buccal fat pad removal
The buccal fat pads, previously known as Bichat’s fat pads, were first described in 1802. Everyone has them and their size is predominantly related to genetics, but similar to other facial fat pockets, they can shrink or shift over time. Buccal fat pads are often resistant to weight loss and stubbornly persist because of a slower lipolytic rate than subcutaneous fat. Some patients with round facial shapes may seek removal of the midface volume to create a more angular cheek and jawline, which enhances the zygomatic prominence and mandibular angle, creating a more contoured face.
The buccal fat pad is a submuscular fat pad surrounded by a capsule that contains three lobes with four extensions. The anterior lobe rests in front of the anterior border of the masseter muscle. The intermediate lobe extends between the masseter and buccinator muscles. The posterior lobe extends between the temporal masticatory space. These pads range from 7-11 mL in volume and grow from ages 10 to 20 years, declining in size after age 20. Given their location in the central face, they contain a rich vascular supply and are surrounded by the facial nerves, salivary glands, the parotid gland, and muscles of mastication.
The aesthetic contour of the lower face is defined by the mandibular prominence, the masseter muscle, subcutaneous fat, and the buccal fat pad. Surgically, removal is a simple and safe procedure. Complications can include damage to the parotid gland, vessels, salivary duct, or facial nerve. Temporary numbness, swelling, and facial asymmetry are the most common complications.
Increasing popularity, controversy
Removal of the buccal fat pads has become popular because of celebrity media exposure, particularly among young women seeking a slim appearance to their face and jawlines. Although the procedure is relatively simple, there have been no long term studies evaluating the effects of buccal fat pad removal on facial aging.
The shrinking or shifting of fat that occurs with aging makes the removal of these fat pads in young women controversial because when removed, they cannot be effectively replaced. Shrinking of the fat pads with age, loss of midface volume, and solar elastosis can make the cheeks appear gaunt and “sucked in.”
An experienced surgeon will reduce and contour the fat pads – and will not completely remove them – to prevent a complete hollowing of the cheeks over time. Complete removal is not recommended and in men, overzealous removal in men can feminize the face.
In middle-aged men and women, the buccal fat pad can shift to the lower face and often drops below the angle of the mandible giving the appearance of jowls. Complete removal of the shifted buccal fat pad will help align the jawline; however, residual skin laxity is a complication and must be addressed to fully correct the jowls.
In my experience, the best approach to reducing buccal pads as an alternative to surgical removal is “melting” the buccal fat in a systematic, controlled manner over several sessions with either radiofrequency laser or deoxycholic acid injections. This slow, controlled method allows me to contour the cheeks appropriately in concordance with the patient’s anatomy. In younger patients or those with little skin laxity, I choose treatments with deoxycholic acid to remove the pads (which I also use to treat the jowls, as outlined in my 2020 column on treating the jowl overhang with deoxycholic acid).
In patients with more skin laxity, I perform sequential radiofrequency laser treatment over the fat pockets to simultaneously melt the fat pockets and tighten the overlying skin. Both of these methods often require three to six treatments. The controlled, cautious, treatments gradually shrink the fat pockets while preventing the overhollowing of the face.
Dr. Talakoub and Dr. Naissan O. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
References
Dubin B et al. Plast Reconstr Surg. 1989 Feb;83(2):257-64
Jackson IT. Plast Reconstr Surg. 1999 Jun;103(7):2059-60.
Matarasso A. Ann Plast Surg. 1991 May;26(5):413-8.
The buccal fat pads, previously known as Bichat’s fat pads, were first described in 1802. Everyone has them and their size is predominantly related to genetics, but similar to other facial fat pockets, they can shrink or shift over time. Buccal fat pads are often resistant to weight loss and stubbornly persist because of a slower lipolytic rate than subcutaneous fat. Some patients with round facial shapes may seek removal of the midface volume to create a more angular cheek and jawline, which enhances the zygomatic prominence and mandibular angle, creating a more contoured face.
The buccal fat pad is a submuscular fat pad surrounded by a capsule that contains three lobes with four extensions. The anterior lobe rests in front of the anterior border of the masseter muscle. The intermediate lobe extends between the masseter and buccinator muscles. The posterior lobe extends between the temporal masticatory space. These pads range from 7-11 mL in volume and grow from ages 10 to 20 years, declining in size after age 20. Given their location in the central face, they contain a rich vascular supply and are surrounded by the facial nerves, salivary glands, the parotid gland, and muscles of mastication.
The aesthetic contour of the lower face is defined by the mandibular prominence, the masseter muscle, subcutaneous fat, and the buccal fat pad. Surgically, removal is a simple and safe procedure. Complications can include damage to the parotid gland, vessels, salivary duct, or facial nerve. Temporary numbness, swelling, and facial asymmetry are the most common complications.
Increasing popularity, controversy
Removal of the buccal fat pads has become popular because of celebrity media exposure, particularly among young women seeking a slim appearance to their face and jawlines. Although the procedure is relatively simple, there have been no long term studies evaluating the effects of buccal fat pad removal on facial aging.
The shrinking or shifting of fat that occurs with aging makes the removal of these fat pads in young women controversial because when removed, they cannot be effectively replaced. Shrinking of the fat pads with age, loss of midface volume, and solar elastosis can make the cheeks appear gaunt and “sucked in.”
An experienced surgeon will reduce and contour the fat pads – and will not completely remove them – to prevent a complete hollowing of the cheeks over time. Complete removal is not recommended and in men, overzealous removal in men can feminize the face.
In middle-aged men and women, the buccal fat pad can shift to the lower face and often drops below the angle of the mandible giving the appearance of jowls. Complete removal of the shifted buccal fat pad will help align the jawline; however, residual skin laxity is a complication and must be addressed to fully correct the jowls.
In my experience, the best approach to reducing buccal pads as an alternative to surgical removal is “melting” the buccal fat in a systematic, controlled manner over several sessions with either radiofrequency laser or deoxycholic acid injections. This slow, controlled method allows me to contour the cheeks appropriately in concordance with the patient’s anatomy. In younger patients or those with little skin laxity, I choose treatments with deoxycholic acid to remove the pads (which I also use to treat the jowls, as outlined in my 2020 column on treating the jowl overhang with deoxycholic acid).
In patients with more skin laxity, I perform sequential radiofrequency laser treatment over the fat pockets to simultaneously melt the fat pockets and tighten the overlying skin. Both of these methods often require three to six treatments. The controlled, cautious, treatments gradually shrink the fat pockets while preventing the overhollowing of the face.
Dr. Talakoub and Dr. Naissan O. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
References
Dubin B et al. Plast Reconstr Surg. 1989 Feb;83(2):257-64
Jackson IT. Plast Reconstr Surg. 1999 Jun;103(7):2059-60.
Matarasso A. Ann Plast Surg. 1991 May;26(5):413-8.
The buccal fat pads, previously known as Bichat’s fat pads, were first described in 1802. Everyone has them and their size is predominantly related to genetics, but similar to other facial fat pockets, they can shrink or shift over time. Buccal fat pads are often resistant to weight loss and stubbornly persist because of a slower lipolytic rate than subcutaneous fat. Some patients with round facial shapes may seek removal of the midface volume to create a more angular cheek and jawline, which enhances the zygomatic prominence and mandibular angle, creating a more contoured face.
The buccal fat pad is a submuscular fat pad surrounded by a capsule that contains three lobes with four extensions. The anterior lobe rests in front of the anterior border of the masseter muscle. The intermediate lobe extends between the masseter and buccinator muscles. The posterior lobe extends between the temporal masticatory space. These pads range from 7-11 mL in volume and grow from ages 10 to 20 years, declining in size after age 20. Given their location in the central face, they contain a rich vascular supply and are surrounded by the facial nerves, salivary glands, the parotid gland, and muscles of mastication.
The aesthetic contour of the lower face is defined by the mandibular prominence, the masseter muscle, subcutaneous fat, and the buccal fat pad. Surgically, removal is a simple and safe procedure. Complications can include damage to the parotid gland, vessels, salivary duct, or facial nerve. Temporary numbness, swelling, and facial asymmetry are the most common complications.
Increasing popularity, controversy
Removal of the buccal fat pads has become popular because of celebrity media exposure, particularly among young women seeking a slim appearance to their face and jawlines. Although the procedure is relatively simple, there have been no long term studies evaluating the effects of buccal fat pad removal on facial aging.
The shrinking or shifting of fat that occurs with aging makes the removal of these fat pads in young women controversial because when removed, they cannot be effectively replaced. Shrinking of the fat pads with age, loss of midface volume, and solar elastosis can make the cheeks appear gaunt and “sucked in.”
An experienced surgeon will reduce and contour the fat pads – and will not completely remove them – to prevent a complete hollowing of the cheeks over time. Complete removal is not recommended and in men, overzealous removal in men can feminize the face.
In middle-aged men and women, the buccal fat pad can shift to the lower face and often drops below the angle of the mandible giving the appearance of jowls. Complete removal of the shifted buccal fat pad will help align the jawline; however, residual skin laxity is a complication and must be addressed to fully correct the jowls.
In my experience, the best approach to reducing buccal pads as an alternative to surgical removal is “melting” the buccal fat in a systematic, controlled manner over several sessions with either radiofrequency laser or deoxycholic acid injections. This slow, controlled method allows me to contour the cheeks appropriately in concordance with the patient’s anatomy. In younger patients or those with little skin laxity, I choose treatments with deoxycholic acid to remove the pads (which I also use to treat the jowls, as outlined in my 2020 column on treating the jowl overhang with deoxycholic acid).
In patients with more skin laxity, I perform sequential radiofrequency laser treatment over the fat pockets to simultaneously melt the fat pockets and tighten the overlying skin. Both of these methods often require three to six treatments. The controlled, cautious, treatments gradually shrink the fat pockets while preventing the overhollowing of the face.
Dr. Talakoub and Dr. Naissan O. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at dermnews@mdedge.com. They had no relevant disclosures.
References
Dubin B et al. Plast Reconstr Surg. 1989 Feb;83(2):257-64
Jackson IT. Plast Reconstr Surg. 1999 Jun;103(7):2059-60.
Matarasso A. Ann Plast Surg. 1991 May;26(5):413-8.
Hair supplements
in JAMA Dermatology in November 2022.
Drake and colleagues evaluated the safety and efficacy of nutritional supplements for treating hair loss. In a systematic database review from inception to Oct. 20, 2021, they evaluated and compiled the findings of all dietary and nutritional interventions for treatment of hair loss among individuals without a known baseline nutritional deficiency. Thirty articles were included, including 17 randomized clinical trials, 11 clinical trials, and 2 case series.
They found the highest-quality evidence showing the most potential benefit were for 12 of the 20 nutritional interventions in their review: Pumpkin seed oil capsules, omega-3 and -6 combined with antioxidants, tocotrienol, Pantogar, capsaicin and isoflavone, Viviscal (multiple formulations), Nourkrin, Nutrafol, apple nutraceutical, Lambdapil, total glucosides of paeony and compound glycyrrhizin tablets, and zinc. Vitamin D3, kimchi and cheonggukjang, and Forti5 had lower-quality evidence for disease course improvement. Adverse effects associated with the supplements were described as mild and rare.
In practice, for patients with nonscarring alopecia, I typically check screening labs for hair loss, in addition to the clinical exam, before starting treatment (including supplements), as addressing the underlying reason, if found, is always paramount. These labs are best performed when the patient is not taking biotin, as biotin has been shown numerous times to potentially be associated with endocrine lab abnormalities, most commonly thyroid-stimulating hormone, especially at higher doses, as well as troponin levels. Some over-the-counter hair supplements will contain much higher doses than the recommended 30 micrograms per day.
Separately, if ferritin levels are within normal range, but below 50 mcg/L, supplementation with Slow Fe or another slow-release iron supplement may also result in improved hair growth. Ferritin levels are typically rechecked 6 months after supplementation to see if levels of 50 mcg/L or above have been achieved.
Another point to consider before beginning supplementation is to educate patients about potential effects of supplementation, including increased hair growth in other areas besides the scalp. For some patients who are self-conscious about potential hirsutism, this could be an issue, whereas for others, this risk does not outweigh the benefit. Unwanted hair growth, should it occur, may also be addressed with hair removal methods including shaving, waxing, plucking, threading, depilatories, prescription eflornithine cream (Vaniqa), or laser hair removal if desired.
Our armamentarium for treating hair loss includes: addressing underlying systemic causes; topical treatments including topical minoxidil; oral supplements; platelet-rich plasma injections; prescription oral medications including finasteride in men or postmenopausal women or off-label oral minoxidil; and hair transplant surgery if warranted. Having this thorough review of the most common hair supplements currently available is extremely helpful and valuable in our specialty.
Dr. Wesley and Lily Talakoub, MD, are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. Write to them at dermnews@mdedge.com. This month’s column is by Dr. Wesley. She had no relevant disclosures.
in JAMA Dermatology in November 2022.
Drake and colleagues evaluated the safety and efficacy of nutritional supplements for treating hair loss. In a systematic database review from inception to Oct. 20, 2021, they evaluated and compiled the findings of all dietary and nutritional interventions for treatment of hair loss among individuals without a known baseline nutritional deficiency. Thirty articles were included, including 17 randomized clinical trials, 11 clinical trials, and 2 case series.
They found the highest-quality evidence showing the most potential benefit were for 12 of the 20 nutritional interventions in their review: Pumpkin seed oil capsules, omega-3 and -6 combined with antioxidants, tocotrienol, Pantogar, capsaicin and isoflavone, Viviscal (multiple formulations), Nourkrin, Nutrafol, apple nutraceutical, Lambdapil, total glucosides of paeony and compound glycyrrhizin tablets, and zinc. Vitamin D3, kimchi and cheonggukjang, and Forti5 had lower-quality evidence for disease course improvement. Adverse effects associated with the supplements were described as mild and rare.
In practice, for patients with nonscarring alopecia, I typically check screening labs for hair loss, in addition to the clinical exam, before starting treatment (including supplements), as addressing the underlying reason, if found, is always paramount. These labs are best performed when the patient is not taking biotin, as biotin has been shown numerous times to potentially be associated with endocrine lab abnormalities, most commonly thyroid-stimulating hormone, especially at higher doses, as well as troponin levels. Some over-the-counter hair supplements will contain much higher doses than the recommended 30 micrograms per day.
Separately, if ferritin levels are within normal range, but below 50 mcg/L, supplementation with Slow Fe or another slow-release iron supplement may also result in improved hair growth. Ferritin levels are typically rechecked 6 months after supplementation to see if levels of 50 mcg/L or above have been achieved.
Another point to consider before beginning supplementation is to educate patients about potential effects of supplementation, including increased hair growth in other areas besides the scalp. For some patients who are self-conscious about potential hirsutism, this could be an issue, whereas for others, this risk does not outweigh the benefit. Unwanted hair growth, should it occur, may also be addressed with hair removal methods including shaving, waxing, plucking, threading, depilatories, prescription eflornithine cream (Vaniqa), or laser hair removal if desired.
Our armamentarium for treating hair loss includes: addressing underlying systemic causes; topical treatments including topical minoxidil; oral supplements; platelet-rich plasma injections; prescription oral medications including finasteride in men or postmenopausal women or off-label oral minoxidil; and hair transplant surgery if warranted. Having this thorough review of the most common hair supplements currently available is extremely helpful and valuable in our specialty.
Dr. Wesley and Lily Talakoub, MD, are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. Write to them at dermnews@mdedge.com. This month’s column is by Dr. Wesley. She had no relevant disclosures.
in JAMA Dermatology in November 2022.
Drake and colleagues evaluated the safety and efficacy of nutritional supplements for treating hair loss. In a systematic database review from inception to Oct. 20, 2021, they evaluated and compiled the findings of all dietary and nutritional interventions for treatment of hair loss among individuals without a known baseline nutritional deficiency. Thirty articles were included, including 17 randomized clinical trials, 11 clinical trials, and 2 case series.
They found the highest-quality evidence showing the most potential benefit were for 12 of the 20 nutritional interventions in their review: Pumpkin seed oil capsules, omega-3 and -6 combined with antioxidants, tocotrienol, Pantogar, capsaicin and isoflavone, Viviscal (multiple formulations), Nourkrin, Nutrafol, apple nutraceutical, Lambdapil, total glucosides of paeony and compound glycyrrhizin tablets, and zinc. Vitamin D3, kimchi and cheonggukjang, and Forti5 had lower-quality evidence for disease course improvement. Adverse effects associated with the supplements were described as mild and rare.
In practice, for patients with nonscarring alopecia, I typically check screening labs for hair loss, in addition to the clinical exam, before starting treatment (including supplements), as addressing the underlying reason, if found, is always paramount. These labs are best performed when the patient is not taking biotin, as biotin has been shown numerous times to potentially be associated with endocrine lab abnormalities, most commonly thyroid-stimulating hormone, especially at higher doses, as well as troponin levels. Some over-the-counter hair supplements will contain much higher doses than the recommended 30 micrograms per day.
Separately, if ferritin levels are within normal range, but below 50 mcg/L, supplementation with Slow Fe or another slow-release iron supplement may also result in improved hair growth. Ferritin levels are typically rechecked 6 months after supplementation to see if levels of 50 mcg/L or above have been achieved.
Another point to consider before beginning supplementation is to educate patients about potential effects of supplementation, including increased hair growth in other areas besides the scalp. For some patients who are self-conscious about potential hirsutism, this could be an issue, whereas for others, this risk does not outweigh the benefit. Unwanted hair growth, should it occur, may also be addressed with hair removal methods including shaving, waxing, plucking, threading, depilatories, prescription eflornithine cream (Vaniqa), or laser hair removal if desired.
Our armamentarium for treating hair loss includes: addressing underlying systemic causes; topical treatments including topical minoxidil; oral supplements; platelet-rich plasma injections; prescription oral medications including finasteride in men or postmenopausal women or off-label oral minoxidil; and hair transplant surgery if warranted. Having this thorough review of the most common hair supplements currently available is extremely helpful and valuable in our specialty.
Dr. Wesley and Lily Talakoub, MD, are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. Write to them at dermnews@mdedge.com. This month’s column is by Dr. Wesley. She had no relevant disclosures.