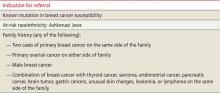
User login
Diagnostic puzzler: Hypertension in teen
A review of the patient’s blood pressure since admission indicated consistently elevated systolic and diastolic pressures, on average, of 170/105 mm Hg. Additionally, his serum potassium levels ranged from 2.4 to 3.1 mmol/L (normal 3.5-5.1 mmol/L).
A detailed medical history and review of previous records revealed that an initial diagnosis of hypertension had been made during a routine sports physical at age 14, although the patient was cleared for full activity. Over the previous year, the patient said he had gained weight—much of it in the abdomen—and experienced sleep disturbances, increasing fatigability, muscle weakness, hair loss, and declining performance in his high school sports. In addition, he had noticed increased facial flushing and sweating.
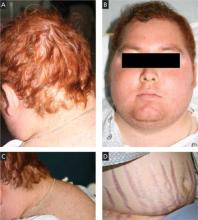
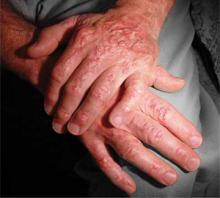
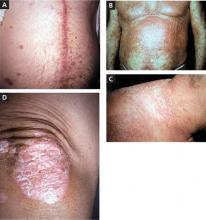
On physical exam, we noted an obese male (height 5’5’’, weight 191.5 lb, BMI 30.86 kg/m2) with the following vital signs: temperature 97.9°F, pulse 105 bpm, and blood pressure 177/111 mm Hg. Pertinent physical exam findings outside of his surgical site included diffusely thinning hair, moon facies, facial plethora, increased supraclavicular and dorsocervical fat pads, thoracic and abdominal striae, thinned skin overlying his upper and lower extremities, and lower extremity edema ( FIGURE 1 ). All other exam findings were within normal limits.
Initial blood chemistry lab results revealed hyperglycemia (290 mg/dL; random normal, <200 mg/dL), hypokalemia (3.0 mmol/L; normal, 3.5-5.4 mmol/L), hypochloremia (96 mmol/L; normal, 98-107 mmol/L), and a mean corpuscular volume of 101.2 fL (normal, 80-100 fL). The patient also had a white blood cell (WBC) count of 14,400/mcL with a predominance of neutrophils and 6 bands, and a high sensitivity C-reactive protein level of 7.5 mg/dL (normal, <0.748 mg/dL).
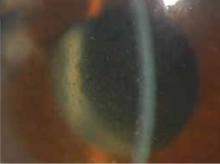
FIGURE 1
The signs were all there
Our patient exhibited thinning hair (A), moon facies (B), dorsocervical fat pads (“buffalo hump”) (C), and abdominal striae (D).
WHAT ADDITIONAL TESTING WOULD YOU ORDER?
WHAT IS YOUR PRESUMPTIVE DIAGNOSIS?
Suspecting Cushing’s syndrome, we ordered cortisol and ACTH
Based on our initial findings, we ordered a 24-hour urinary free cortisol and plasma adrenocorticotropic hormone (ACTH) level—both of which had to be sent to outside laboratories. We also ordered a computed tomography (CT) scan of the patient’s adrenal glands and magnetic resonance imaging (MRI) of his pituitary gland.
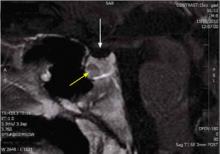
The CT scan revealed mild hyperplasia of the adrenal glands bilaterally; the MRI demonstrated a 7 × 6 × 6 mm pituitary microadenoma ( FIGURE 2 ) in the anterior portion of the gland. In addition, a 6 × 6 × 1 mm lesion was noted—thought to be a Rathke’s cleft (Pars intermedia) cyst by the reviewing radiologist.
The patient’s initial cortisol and ACTH lab work revealed a urinary cortisol level of 5089.2 mcg/24 h (normal, 3-55 mcg/24 h) and an ACTH level of 216 pg/mL (normal, 9-57 pg/mL for ages 3-17 years).
We diagnosed Cushing’s syndrome in this patient.
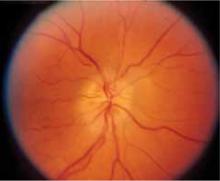
FIGURE 2
MRI reveals pituitary microadenoma
The patient had a microadenoma in the anterior portion of the pituitary gland (yellow arrow), and a lesion believed to be a Rathke’s cleft cyst (white arrow).
Differentiating between ACTH-dependent and -independent Cushing’s syndrome
Cushing’s syndrome is a constellation of signs and symptoms caused by an overproduction of cortisol, which results in a variety of abnormalities in the hypothalamic-pituitary-adrenal axis. In general, the syndrome is differentiated as either ACTH-dependent or ACTH-independent, based on the underlying cause.1 Examples of ACTH-dependent Cushing’s syndrome include pituitary adenoma (formally classified as Cushing’s disease) and ectopic ACTH or corticotrophin-releasing hormone-producing tumors. Examples of ACTH-independent Cushing’s syndrome include adrenal adenoma or carcinoma and exogenous glucocorticoid therapy.2
Clinical manifestations include obesity, hypertension (usually with some degree of concurrent hypokalemia), skin abnormalities (eg, plethora, hirsutism, violaceous striae), musculoskeletal weakness, neuropsychiatric symptoms (eg, depression), gonadal dysfunction, and metabolic derangements, including glucose intolerance, diabetes, and hyperlipidemia. In children, a near universal decrease in linear growth secondary to hypercortisolism is seen.3
Investigating a suspected case of Cushing’s syndrome can be divided into 2 stages: confirming the diagnosis and establishing the etiology. The following tests can be used to make the diagnosis: 24-hour urinary free cortisol, low-dose dexamethasone suppression, and late-night salivary cortisol. Several of these tests require late-night administration that necessitates hospital admission. These tests are typically followed by a CT scan of the patient’s adrenal glands and/or an MRI of the patient’s pituitary gland to evaluate the etiology. Additionally, as demonstrated by the patient described here, ongoing issues with hypertension, metabolic abnormalities, and hyperglycemia may require intensive intervention and management.4
Don't be fooled
Potential complications in diagnosing the syndrome, however, can cloud an accurate diagnosis—especially early in the disease process. In addition to biochemical similarities between Cushing’s syndrome and obesity, depression, and alcoholism, ACTH-dependent Cushing’s syndrome can undergo cyclical or intermittent activity and can remain in remission for years.1 Also, ectopic ACTH-secreting tumors may, by virtue of their small size and location, go undetected.
Don’t try to lower BP through usual means
Hypertension, a hallmark finding in approximately 80% of adults and 47% of children with Cushing’s syndrome,5 stems from hypercortisol-driven pathologic changes in the mechanisms controlling plasma volume, peripheral vascular resistance, and cardiac output. In addition, these cortisol-driven changes have a direct effect on mineralocorticoid and glucocorticoid receptors within the central nervous system. Secondary effects such as insulin resistance and the development of sleep apnea further complicate the management of this generally treatment-resistant hypertension. Lastly, specific mechanisms such as the cross-reactivity of excess glucocorticoids with mineralocorticoid receptors acting on targets within the kidney, can also lead to metabolic derangements, such as profound hypokalemia and metabolic alkalosis.
Thus, controlling hypertension and the metabolic changes seen in Cushing’s syndrome often requires addressing the underlying hypercortisolism rather than achieving normotension and normal serum electrolytes through the usual means.5
Treatment puts our patient back on track
Our patient was transferred to a tertiary care hospital for further management and consultation with endocrinology and neurosurgery. He was started on high-dose ketoconazole, an imidazole-derivative antifungal medication that acts to inhibit adrenal steroidogenesis and has been used successfully in patients with Cushing’s syndrome.6,7 (Ketoconazole is typically dosed at 400-1200 mg/d7 and can be used for >6 months to 1 year, or temporarily in advance of surgery.)
Our patient underwent successful transsphenoidal adenectomy by neurosurgery, and his blood pressure, serum electrolytes, and serum glucose returned to normal levels. He is about to begin his senior year in high school.
CORRESPONDENCE Michael Barna, MD, Department of Family Medicine, Naval Hospital Camp Pendleton, Box 555191, Camp Pendleton, CA 92055; michael.barna@med.navy.mil
1. Carroll TB, Aron DC, Findling JW, et al. Glucocorticoids and adrenal androgens. In: Gardner D, Shoback D, eds. Greenspan’s Basic & Clinical Endocrinology. 9th ed. New York: McGraw-Hill; 2011:285–327.
2. Trainer PJ, Grossman A. The diagnosis and differential diagnosis of Cushing’s syndrome. Clin Endocrinol (Oxf). 1991;34:317-330.
3. Voutilainen R, Leisti S, Perheentupa J. Growth in Cushing syndrome. Eur J Pediatr. 1985;144:141-145.
4. Giordano R, Picu A, Marinazzo E, et al. Metabolic and cardiovascular outcomes in patients with Cushing’s syndrome of different aetiologies during active disease and 1 year after remission. Clin Endocrinol (Oxf). 2011;75:354-360.
5. Cicala MV, Mantero F. Hypertension in Cushing’s syndrome: from pathogenesis to treatment. Neuroendocrinology. 2010;92(suppl 1):44-49.
6. Atkinson A. The treatment of Cushing’s syndrome. Clin Endocrinol (Oxf). 1991;34:507-513.
7. Tabarin A, Navarranne A, Guerin J, et al. Use of ketoconazole in the treatment of Cushing’s disease and ectopic ACTH syndrome. Clin Endocrinol (Oxf). 1991;34:63-69.
A review of the patient’s blood pressure since admission indicated consistently elevated systolic and diastolic pressures, on average, of 170/105 mm Hg. Additionally, his serum potassium levels ranged from 2.4 to 3.1 mmol/L (normal 3.5-5.1 mmol/L).
A detailed medical history and review of previous records revealed that an initial diagnosis of hypertension had been made during a routine sports physical at age 14, although the patient was cleared for full activity. Over the previous year, the patient said he had gained weight—much of it in the abdomen—and experienced sleep disturbances, increasing fatigability, muscle weakness, hair loss, and declining performance in his high school sports. In addition, he had noticed increased facial flushing and sweating.
On physical exam, we noted an obese male (height 5’5’’, weight 191.5 lb, BMI 30.86 kg/m2) with the following vital signs: temperature 97.9°F, pulse 105 bpm, and blood pressure 177/111 mm Hg. Pertinent physical exam findings outside of his surgical site included diffusely thinning hair, moon facies, facial plethora, increased supraclavicular and dorsocervical fat pads, thoracic and abdominal striae, thinned skin overlying his upper and lower extremities, and lower extremity edema ( FIGURE 1 ). All other exam findings were within normal limits.
Initial blood chemistry lab results revealed hyperglycemia (290 mg/dL; random normal, <200 mg/dL), hypokalemia (3.0 mmol/L; normal, 3.5-5.4 mmol/L), hypochloremia (96 mmol/L; normal, 98-107 mmol/L), and a mean corpuscular volume of 101.2 fL (normal, 80-100 fL). The patient also had a white blood cell (WBC) count of 14,400/mcL with a predominance of neutrophils and 6 bands, and a high sensitivity C-reactive protein level of 7.5 mg/dL (normal, <0.748 mg/dL).
FIGURE 1
The signs were all there
Our patient exhibited thinning hair (A), moon facies (B), dorsocervical fat pads (“buffalo hump”) (C), and abdominal striae (D).
WHAT ADDITIONAL TESTING WOULD YOU ORDER?
WHAT IS YOUR PRESUMPTIVE DIAGNOSIS?
Suspecting Cushing’s syndrome, we ordered cortisol and ACTH
Based on our initial findings, we ordered a 24-hour urinary free cortisol and plasma adrenocorticotropic hormone (ACTH) level—both of which had to be sent to outside laboratories. We also ordered a computed tomography (CT) scan of the patient’s adrenal glands and magnetic resonance imaging (MRI) of his pituitary gland.
The CT scan revealed mild hyperplasia of the adrenal glands bilaterally; the MRI demonstrated a 7 × 6 × 6 mm pituitary microadenoma ( FIGURE 2 ) in the anterior portion of the gland. In addition, a 6 × 6 × 1 mm lesion was noted—thought to be a Rathke’s cleft (Pars intermedia) cyst by the reviewing radiologist.
The patient’s initial cortisol and ACTH lab work revealed a urinary cortisol level of 5089.2 mcg/24 h (normal, 3-55 mcg/24 h) and an ACTH level of 216 pg/mL (normal, 9-57 pg/mL for ages 3-17 years).
We diagnosed Cushing’s syndrome in this patient.
FIGURE 2
MRI reveals pituitary microadenoma
The patient had a microadenoma in the anterior portion of the pituitary gland (yellow arrow), and a lesion believed to be a Rathke’s cleft cyst (white arrow).
Differentiating between ACTH-dependent and -independent Cushing’s syndrome
Cushing’s syndrome is a constellation of signs and symptoms caused by an overproduction of cortisol, which results in a variety of abnormalities in the hypothalamic-pituitary-adrenal axis. In general, the syndrome is differentiated as either ACTH-dependent or ACTH-independent, based on the underlying cause.1 Examples of ACTH-dependent Cushing’s syndrome include pituitary adenoma (formally classified as Cushing’s disease) and ectopic ACTH or corticotrophin-releasing hormone-producing tumors. Examples of ACTH-independent Cushing’s syndrome include adrenal adenoma or carcinoma and exogenous glucocorticoid therapy.2
Clinical manifestations include obesity, hypertension (usually with some degree of concurrent hypokalemia), skin abnormalities (eg, plethora, hirsutism, violaceous striae), musculoskeletal weakness, neuropsychiatric symptoms (eg, depression), gonadal dysfunction, and metabolic derangements, including glucose intolerance, diabetes, and hyperlipidemia. In children, a near universal decrease in linear growth secondary to hypercortisolism is seen.3
Investigating a suspected case of Cushing’s syndrome can be divided into 2 stages: confirming the diagnosis and establishing the etiology. The following tests can be used to make the diagnosis: 24-hour urinary free cortisol, low-dose dexamethasone suppression, and late-night salivary cortisol. Several of these tests require late-night administration that necessitates hospital admission. These tests are typically followed by a CT scan of the patient’s adrenal glands and/or an MRI of the patient’s pituitary gland to evaluate the etiology. Additionally, as demonstrated by the patient described here, ongoing issues with hypertension, metabolic abnormalities, and hyperglycemia may require intensive intervention and management.4
Don't be fooled
Potential complications in diagnosing the syndrome, however, can cloud an accurate diagnosis—especially early in the disease process. In addition to biochemical similarities between Cushing’s syndrome and obesity, depression, and alcoholism, ACTH-dependent Cushing’s syndrome can undergo cyclical or intermittent activity and can remain in remission for years.1 Also, ectopic ACTH-secreting tumors may, by virtue of their small size and location, go undetected.
Don’t try to lower BP through usual means
Hypertension, a hallmark finding in approximately 80% of adults and 47% of children with Cushing’s syndrome,5 stems from hypercortisol-driven pathologic changes in the mechanisms controlling plasma volume, peripheral vascular resistance, and cardiac output. In addition, these cortisol-driven changes have a direct effect on mineralocorticoid and glucocorticoid receptors within the central nervous system. Secondary effects such as insulin resistance and the development of sleep apnea further complicate the management of this generally treatment-resistant hypertension. Lastly, specific mechanisms such as the cross-reactivity of excess glucocorticoids with mineralocorticoid receptors acting on targets within the kidney, can also lead to metabolic derangements, such as profound hypokalemia and metabolic alkalosis.
Thus, controlling hypertension and the metabolic changes seen in Cushing’s syndrome often requires addressing the underlying hypercortisolism rather than achieving normotension and normal serum electrolytes through the usual means.5
Treatment puts our patient back on track
Our patient was transferred to a tertiary care hospital for further management and consultation with endocrinology and neurosurgery. He was started on high-dose ketoconazole, an imidazole-derivative antifungal medication that acts to inhibit adrenal steroidogenesis and has been used successfully in patients with Cushing’s syndrome.6,7 (Ketoconazole is typically dosed at 400-1200 mg/d7 and can be used for >6 months to 1 year, or temporarily in advance of surgery.)
Our patient underwent successful transsphenoidal adenectomy by neurosurgery, and his blood pressure, serum electrolytes, and serum glucose returned to normal levels. He is about to begin his senior year in high school.
CORRESPONDENCE Michael Barna, MD, Department of Family Medicine, Naval Hospital Camp Pendleton, Box 555191, Camp Pendleton, CA 92055; michael.barna@med.navy.mil
A review of the patient’s blood pressure since admission indicated consistently elevated systolic and diastolic pressures, on average, of 170/105 mm Hg. Additionally, his serum potassium levels ranged from 2.4 to 3.1 mmol/L (normal 3.5-5.1 mmol/L).
A detailed medical history and review of previous records revealed that an initial diagnosis of hypertension had been made during a routine sports physical at age 14, although the patient was cleared for full activity. Over the previous year, the patient said he had gained weight—much of it in the abdomen—and experienced sleep disturbances, increasing fatigability, muscle weakness, hair loss, and declining performance in his high school sports. In addition, he had noticed increased facial flushing and sweating.
On physical exam, we noted an obese male (height 5’5’’, weight 191.5 lb, BMI 30.86 kg/m2) with the following vital signs: temperature 97.9°F, pulse 105 bpm, and blood pressure 177/111 mm Hg. Pertinent physical exam findings outside of his surgical site included diffusely thinning hair, moon facies, facial plethora, increased supraclavicular and dorsocervical fat pads, thoracic and abdominal striae, thinned skin overlying his upper and lower extremities, and lower extremity edema ( FIGURE 1 ). All other exam findings were within normal limits.
Initial blood chemistry lab results revealed hyperglycemia (290 mg/dL; random normal, <200 mg/dL), hypokalemia (3.0 mmol/L; normal, 3.5-5.4 mmol/L), hypochloremia (96 mmol/L; normal, 98-107 mmol/L), and a mean corpuscular volume of 101.2 fL (normal, 80-100 fL). The patient also had a white blood cell (WBC) count of 14,400/mcL with a predominance of neutrophils and 6 bands, and a high sensitivity C-reactive protein level of 7.5 mg/dL (normal, <0.748 mg/dL).
FIGURE 1
The signs were all there
Our patient exhibited thinning hair (A), moon facies (B), dorsocervical fat pads (“buffalo hump”) (C), and abdominal striae (D).
WHAT ADDITIONAL TESTING WOULD YOU ORDER?
WHAT IS YOUR PRESUMPTIVE DIAGNOSIS?
Suspecting Cushing’s syndrome, we ordered cortisol and ACTH
Based on our initial findings, we ordered a 24-hour urinary free cortisol and plasma adrenocorticotropic hormone (ACTH) level—both of which had to be sent to outside laboratories. We also ordered a computed tomography (CT) scan of the patient’s adrenal glands and magnetic resonance imaging (MRI) of his pituitary gland.
The CT scan revealed mild hyperplasia of the adrenal glands bilaterally; the MRI demonstrated a 7 × 6 × 6 mm pituitary microadenoma ( FIGURE 2 ) in the anterior portion of the gland. In addition, a 6 × 6 × 1 mm lesion was noted—thought to be a Rathke’s cleft (Pars intermedia) cyst by the reviewing radiologist.
The patient’s initial cortisol and ACTH lab work revealed a urinary cortisol level of 5089.2 mcg/24 h (normal, 3-55 mcg/24 h) and an ACTH level of 216 pg/mL (normal, 9-57 pg/mL for ages 3-17 years).
We diagnosed Cushing’s syndrome in this patient.
FIGURE 2
MRI reveals pituitary microadenoma
The patient had a microadenoma in the anterior portion of the pituitary gland (yellow arrow), and a lesion believed to be a Rathke’s cleft cyst (white arrow).
Differentiating between ACTH-dependent and -independent Cushing’s syndrome
Cushing’s syndrome is a constellation of signs and symptoms caused by an overproduction of cortisol, which results in a variety of abnormalities in the hypothalamic-pituitary-adrenal axis. In general, the syndrome is differentiated as either ACTH-dependent or ACTH-independent, based on the underlying cause.1 Examples of ACTH-dependent Cushing’s syndrome include pituitary adenoma (formally classified as Cushing’s disease) and ectopic ACTH or corticotrophin-releasing hormone-producing tumors. Examples of ACTH-independent Cushing’s syndrome include adrenal adenoma or carcinoma and exogenous glucocorticoid therapy.2
Clinical manifestations include obesity, hypertension (usually with some degree of concurrent hypokalemia), skin abnormalities (eg, plethora, hirsutism, violaceous striae), musculoskeletal weakness, neuropsychiatric symptoms (eg, depression), gonadal dysfunction, and metabolic derangements, including glucose intolerance, diabetes, and hyperlipidemia. In children, a near universal decrease in linear growth secondary to hypercortisolism is seen.3
Investigating a suspected case of Cushing’s syndrome can be divided into 2 stages: confirming the diagnosis and establishing the etiology. The following tests can be used to make the diagnosis: 24-hour urinary free cortisol, low-dose dexamethasone suppression, and late-night salivary cortisol. Several of these tests require late-night administration that necessitates hospital admission. These tests are typically followed by a CT scan of the patient’s adrenal glands and/or an MRI of the patient’s pituitary gland to evaluate the etiology. Additionally, as demonstrated by the patient described here, ongoing issues with hypertension, metabolic abnormalities, and hyperglycemia may require intensive intervention and management.4
Don't be fooled
Potential complications in diagnosing the syndrome, however, can cloud an accurate diagnosis—especially early in the disease process. In addition to biochemical similarities between Cushing’s syndrome and obesity, depression, and alcoholism, ACTH-dependent Cushing’s syndrome can undergo cyclical or intermittent activity and can remain in remission for years.1 Also, ectopic ACTH-secreting tumors may, by virtue of their small size and location, go undetected.
Don’t try to lower BP through usual means
Hypertension, a hallmark finding in approximately 80% of adults and 47% of children with Cushing’s syndrome,5 stems from hypercortisol-driven pathologic changes in the mechanisms controlling plasma volume, peripheral vascular resistance, and cardiac output. In addition, these cortisol-driven changes have a direct effect on mineralocorticoid and glucocorticoid receptors within the central nervous system. Secondary effects such as insulin resistance and the development of sleep apnea further complicate the management of this generally treatment-resistant hypertension. Lastly, specific mechanisms such as the cross-reactivity of excess glucocorticoids with mineralocorticoid receptors acting on targets within the kidney, can also lead to metabolic derangements, such as profound hypokalemia and metabolic alkalosis.
Thus, controlling hypertension and the metabolic changes seen in Cushing’s syndrome often requires addressing the underlying hypercortisolism rather than achieving normotension and normal serum electrolytes through the usual means.5
Treatment puts our patient back on track
Our patient was transferred to a tertiary care hospital for further management and consultation with endocrinology and neurosurgery. He was started on high-dose ketoconazole, an imidazole-derivative antifungal medication that acts to inhibit adrenal steroidogenesis and has been used successfully in patients with Cushing’s syndrome.6,7 (Ketoconazole is typically dosed at 400-1200 mg/d7 and can be used for >6 months to 1 year, or temporarily in advance of surgery.)
Our patient underwent successful transsphenoidal adenectomy by neurosurgery, and his blood pressure, serum electrolytes, and serum glucose returned to normal levels. He is about to begin his senior year in high school.
CORRESPONDENCE Michael Barna, MD, Department of Family Medicine, Naval Hospital Camp Pendleton, Box 555191, Camp Pendleton, CA 92055; michael.barna@med.navy.mil
1. Carroll TB, Aron DC, Findling JW, et al. Glucocorticoids and adrenal androgens. In: Gardner D, Shoback D, eds. Greenspan’s Basic & Clinical Endocrinology. 9th ed. New York: McGraw-Hill; 2011:285–327.
2. Trainer PJ, Grossman A. The diagnosis and differential diagnosis of Cushing’s syndrome. Clin Endocrinol (Oxf). 1991;34:317-330.
3. Voutilainen R, Leisti S, Perheentupa J. Growth in Cushing syndrome. Eur J Pediatr. 1985;144:141-145.
4. Giordano R, Picu A, Marinazzo E, et al. Metabolic and cardiovascular outcomes in patients with Cushing’s syndrome of different aetiologies during active disease and 1 year after remission. Clin Endocrinol (Oxf). 2011;75:354-360.
5. Cicala MV, Mantero F. Hypertension in Cushing’s syndrome: from pathogenesis to treatment. Neuroendocrinology. 2010;92(suppl 1):44-49.
6. Atkinson A. The treatment of Cushing’s syndrome. Clin Endocrinol (Oxf). 1991;34:507-513.
7. Tabarin A, Navarranne A, Guerin J, et al. Use of ketoconazole in the treatment of Cushing’s disease and ectopic ACTH syndrome. Clin Endocrinol (Oxf). 1991;34:63-69.
1. Carroll TB, Aron DC, Findling JW, et al. Glucocorticoids and adrenal androgens. In: Gardner D, Shoback D, eds. Greenspan’s Basic & Clinical Endocrinology. 9th ed. New York: McGraw-Hill; 2011:285–327.
2. Trainer PJ, Grossman A. The diagnosis and differential diagnosis of Cushing’s syndrome. Clin Endocrinol (Oxf). 1991;34:317-330.
3. Voutilainen R, Leisti S, Perheentupa J. Growth in Cushing syndrome. Eur J Pediatr. 1985;144:141-145.
4. Giordano R, Picu A, Marinazzo E, et al. Metabolic and cardiovascular outcomes in patients with Cushing’s syndrome of different aetiologies during active disease and 1 year after remission. Clin Endocrinol (Oxf). 2011;75:354-360.
5. Cicala MV, Mantero F. Hypertension in Cushing’s syndrome: from pathogenesis to treatment. Neuroendocrinology. 2010;92(suppl 1):44-49.
6. Atkinson A. The treatment of Cushing’s syndrome. Clin Endocrinol (Oxf). 1991;34:507-513.
7. Tabarin A, Navarranne A, Guerin J, et al. Use of ketoconazole in the treatment of Cushing’s disease and ectopic ACTH syndrome. Clin Endocrinol (Oxf). 1991;34:63-69.
Managing incontinence: A 2-visit approach
• Advise the patient to complete a 3-day voiding diary, which will help you categorize and determine the cause and severity of her incontinence. B
• Recommend Kegel exercises for stress incontinence; provide instruction in technique and/or a referral for pelvic floor physical therapy for instruction. A
• Try an anticholinergic medication with indications for urinary incontinence for women with primarily urge-type incontinence. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
PATIENT HANDOUT
Voiding diary: What it’s for, how to fill it out
Visit #1: Simple steps to take right away
When a patient confides that she suffers from urinary incontinence, even if it’s near the end of her visit, there are 3 critical steps that can be taken without delay:
- Collect a urine sample for urinalysis (if the patient is capable of providing a clean-catch sample and did not already do so at the start of the visit).
- Ask her to keep a voiding diary for 3 days, using the clip-and-copy patient handout. (Tech-savvy patients may prefer to use the voiding diary app available at no charge at http://itunes.apple.com/us/app/bladder-pal/id435763473?mt=8.) Review the instructions for filling out the voiding diary with the patient, or have a nurse or medical assistant do so. Call the patient’s attention to the numerical (1-4) formula recommended for approximating the quantity of urine lost in an episode of incontinence, as this is often the component of the diary that patients have the most trouble with. 7
- Schedule a follow-up visit. If the urinalysis shows that the patient has a urinary tract infection, initiate treatment as soon as possible. Treatment should be completed by the time she arrives for visit#2 so that her incontinence can be reassessed in the absence of infection.
Tell your patient to bring the completed voiding diary to this visit and to avoid emptying her bladder before she sees you. When the patient checks in, she should be reminded again not to urinate until you have completed the examination.
What to do during Visit #2
This visit will begin with a targeted assessment to determine what type of incontinence the patient has and whether you should initiate treatment or refer her to a specialist.
Start with a targeted history and review of the voiding diary
Ask the patient about timing, quantity, and the overall circumstances of typical incontinence episodes, as well as the incidents that she finds most troubling. A medication history should be included, with information not only about what she’s taking, but also about when she takes her medications. A careful review of the voiding diary will provide additional information, including fluid intake quantity and quality (eg, caffeine or alcohol) and urinary frequency, quantity, and timing.
The initial goal is to classify her incontinence as urge (occurring simultaneously with, or right after, a strong feeling of the need to void); stress (the result of an acute increase in abdominal pressure, typically associated with a physical act such as coughing, sneezing, bending over, heavy lifting, or starting to run); or mixed, which involves some episodes of both.8 “High-yield” questions like the ones that follow are particularly helpful in pinpointing the type of incontinence and guiding treatment decisions:
- Do you worry about leakage when you cough or sneeze? (Stress incontinence.)
- When your bladder is full, do you typically have to stop what you’re doing and go right away, or can you wait until a convenient time to find a bathroom? (Urge incontinence.)
And, for patients who report both stress and urge episodes (mixed incontinence):
- Which is more upsetting to you: leakage that occurs when you cough or sneeze or the inability to wait until it’s convenient to get to a bathroom?
A pelvic exam and cough stress test
Next, perform a pelvic examination and assess the following:
External genitalia. Look for signs of chronic urine exposure (eg, erythema, skin breakdown) and urethral abnormalities.
Cystoceles or rectoceles. Ask the patient to bear down strongly to allow you to visualize cystoceles and rectoceles, which usually present as a significant bulging of the tissues of the anterior or posterior vaginal walls. Reassure her that urine leakage and flatus are completely acceptable—and expected—during this part of the exam.
Pelvic mass. Palpate for evidence of a pelvic mass, which can cause urinary obstruction and lead to overflow incontinence.
Kegel exercises. Ask her to perform a Kegel maneuver. If she’s doing it correctly, her perineal body should be easily seen moving upward toward her clitoris and inward toward her introitus. If you place a finger in the vagina and ask her to begin Kegel exercises, you’ll feel the tightening of the pelvic muscles if she’s doing it right. When she does this on her own, she can insert her own finger into the vagina to ensure that her technique is correct or simply practice stopping the flow of urine.
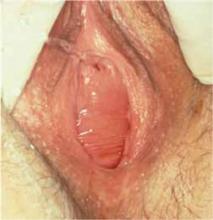
After completing the pelvic exam, perform a cough stress test. With the patient in the lithotomy position, ask her to cough while you hold the labia apart, both to observe for any leakage of urine and to approximate the volume leaked, if any ( FIGURE ). To encourage her to bear down with ample force, remind her that here, too, both leakage of urine and passing of flatus are expected.
Postvoid residual (PVr). After the pelvic exam and cough stress test, the patient should urinate and the PVR should be promptly measured. If you have doubts about her ability to provide a clean-catch urine sample (or your clinic does not have a bladder scanning ultrasound), consider obtaining the PVR via a straight catheterization. A normal PVR is 0 to 200 cc.
By now you should have the information you need to determine whether to treat the incontinence yourself or refer the patient to a urologist or urogynecologist.
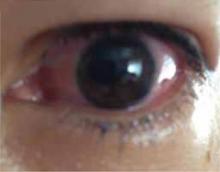
FIGURE
Positive cough stress test
A physician separates the labia to observe urine leakage during a cough stress test.
Treat these conditions yourself
Dx: Uncomplicated stress incontinence. The patient has a positive cough stress test, and most urinary incontinence episodes she recorded are stress type. Urinalysis and PVR are normal, with no evidence of pelvic organ prolapse on exam.
Well-timed pelvic floor (Kegel) exercises have been shown to significantly reduce symptoms of both stress and urge incontinence when they’re done regularly, starting with 4 times a day and increased slowly to 8 times twice a day.9 Review Kegel technique and advise the patient to perform this maneuver and hold the contraction, if possible, whenever she is about to cough, laugh, sneeze, or otherwise exert herself. Tell her to practice Kegel exercises until they become second nature. If she continues to have trouble, she may need a referral to pelvic floor physical therapy to learn to do them effectively. (You can go to http://www.apta.org/apta/findapt/index.aspx?navID=10737422525 to find a therapist specializing in women’s health in your area.)
Dx: Uncomplicated urge incontinence. The patient has a normal cough stress test and most of her urinary incontinence episodes are the urge type. Her pelvic exam and PVR are within normal limits.
Prescribe an anticholinergic bladder agent. Medication with antimuscarinic properties has been shown to decrease urge incontinence and significantly improve patient-reported measures of quality of life.10
A reasonable approach is to start her on an inexpensive generic medication ( TABLE )11,12 and follow up in one month. If the medication helps and does not cause adverse effects, she can be maintained on it; if it’s effective but she has significant adverse effects (eg, constipation, dry mouth, blurred vision, urinary retention, as well as confusion and sedation in the elderly), the patient can be switched to a more expensive brand-name drug with fewer adverse effects.
Behavioral training, including timed voiding and Kegel exercises, has been shown to significantly decrease symptoms of urge incontinence.9 Timed voiding can be especially helpful for nocturnal urge incontinence; advise the patient to set an alarm for an hour before the usual time she awakens with a sense of urgency, and to empty her bladder before it is so full that she leaks.
Point out, too, that not every urinary urge requires immediately running to the toilet. A randomized, placebo-controlled trial found that patients who were taught to respond to visual cues, such as a toilet, by walking past it, then sitting down, relaxing, and contracting their pelvic muscles repeatedly had diminished urgency. Their actions also inhibited detrusor contractions, and often prevented urine loss. When the training was combined with one or 2 lessons in proper Kegel technique—reinforced at several visits—weekly incontinence episodes were reduced by 80%. The controls, who had the same number of clinic visits but did not receive specific education on urgency-reduction strategies, reduced incontinence episodes by 40%.13
Dx: Fluid overuse. The patient is drinking an excessive quantity of fluid, as evidenced by urine volume >2100 cc. (The International Continence Society defines polyuria as >40 mL/kg per day.14 )
Advise the patient to decrease her overall fluid intake, particularly within several hours of bedtime. Studies of tea and coffee consumption and incontinence have had conflicting results,15,16 and data on caffeinated soda consumption and incontinence are lacking. Nonetheless, patients should be advised that there is a possible association between caffeinated beverages and urinary incontinence. (In one small study, caffeine was found to cause a significant increase in detrusor pressure.17 ) Giving women one-time general instructions on fluid intake modification has been shown to significantly decrease incontinence episodes.18
Dx: Badly timed medication intake. This patient is taking one or more drugs that may contribute to incontinence, such as alpha-blockers or diuretics, either shortly before going to bed or before activities that make a bathroom visit inconvenient or impossible.
Adjust her medication regimen to minimize nocturia or urinary urgency during times of peak activity—eg, taking a diuretic early in the day instead of in the afternoon or evening, if possible. Keep in mind, however, that this strategy is based primarily on expert opinion, as very little evidence exists to show that medication of any type has a significant effect on urinary continence.19
Dx: Lower extremity edema with postural diuresis. The patient has nocturia, with larger total urine output at night than during the day. She may or may not have some leakage.
Women with lower extremity edema due to a variety of medical causes often experience postural diuresis overnight. If the patient is already on a diuretic, the problem can often be ameliorated by taking it early in the day instead of in the afternoon or evening; if she’s not, prescribe a small dose of a diuretic, to be taken in the morning. This therapeutic intervention has not been rigorously studied, but is relatively easy to implement and worth a try for patients with heart failure or other causes of pedal edema.
Dx: Constipation associated with autonomic dysfunction. Because the rectum and bladder are controlled by the same sacral segments of the spinal cord and share many autonomic ganglia, problems in one compartment often affect another. In a patient for whom incontinence is a minor, or occasional, problem while constipation is a major complaint, the optimal approach is to treat the constipation first and see if the urinary incontinence also resolves.
A trial of polyethylene glycol is a good place to start, particularly if there is no evidence of another correctable cause of the incontinence. Studies have shown that successful treatment of constipation often results in significant improvement of urinary urgency and frequency.20
TABLE
Anticholinergic medications for urge incontinence11,12
| Medication | Dosing | Comments |
|---|---|---|
| Oxybutynin | 2.5-5 mg bid to tid (≤5 mg qid) | Significant adverse effects, including constipation, dry mouth, blurred vision, urinary retention; confusion and sedation, particularly in elderly |
| Oxybutynin ER | 5-10 mg/d; increase weekly by 5-mg/d increments to ≤30 mg/d | |
| Oxybutynin transdermal patch (Oxytrol) | 1 patch 2x/wk on abdomen, hips, or buttocks (dose=3.9 mg/d) | Adverse effects may be less frequent than with oral medication due to the avoidance of metabolites |
| Oxybutynin transdermal gel (Gelnique) | 1 gel pack/d on abdomen, thighs, or shoulders (dose=100 mg in 1-g gel pack) | |
| Tolterodine (Detrol) | 1-2 mg bid (immediate release) or 2-4 mg/d (ER) | Similar effects and efficacy as oxybutynin, but adverse effects are decreased due to greater uroselectivity on muscarinic receptors |
| Darifenacin hydrobromide (Enablex) | 7.5 mg/d; increase to 15 mg/d after 2 wk if necessary | |
| Solifenacin (VESIcare) | 5-10 mg/d | |
| Fesoteridine (Toviaz) | 4-8 mg/d | |
| Trospium (Sanctura) | 20 mg bid (immediate release) or 60 mg/d (ER) | |
| ER, extended release. | ||
These conditions typically warrant a referral
Dx: Hematuria. Urinalysis with hematuria but no evidence of a urinary tract infection should raise a red flag, regardless of other findings. The patient should be referred for further urologic evaluation, including cystoscopy, although you may want to repeat the urinalysis with another clean-catch specimen first. Straight catheterization is not recommended in such a case, as a traumatized urethra can be a source of hematuria.
Dx: Stress incontinence with an anatomic abnormality. There are 2 options for a patient who has stress incontinence with an obvious cystocele on exam: Fit her with a pessary (or refer her to a physician who has this capability), or provide a referral to a urogynecologist for corrective surgery. Which option to choose should be a collaborative decision between patient and physician. For most women, it makes sense to try the pessary first.
Dx: Urinary retention. A patient with a high normal or elevated PVR (>100-200 cc) and no obvious pelvic organ prolapse needs a work-up for urinary retention. There is a broad differential that can be divided into neurologic, obstructive, and pharmacologic etiologies.21 A referral is indicated so that a urologist can oversee the work-up.
Particularly worrisome causes of urinary retention are multiple sclerosis, which is more likely in a relatively young, otherwise healthy woman, and a pelvic mass. Medications are also a likely cause. The list of drugs that can induce urinary retention is extensive, and includes anticholinergics, antidepressants (tricyclics and some heterocyclics), antihistamines, and muscle relaxants, among others. If you’re unable to find a likely cause, a referral is indicated so that a urologist can oversee the work-up.
CORRESPONDENCE Abigail Lowther, MD, University of Michigan Department of Family Medicine, 24 Frank Lloyd Wright Drive, Lobby H, Ann Arbor, MI 48106-5795; abigaill@med.umich.edu
1. Hunskaar S, Lose G, Sykes D, et al. The prevalence of urinary incontinence in women in four European countries. BJU Int 2004;93:324-330.
2. Roberts RO, Jacobsen SJ, Rhodes T, et al. Urinary incontinence in a community-based cohort: prevalence and healthcare-seeking. J Am Geriatr Soc 1998;46:467-472.
3. Markland AD, Richter HE, Chyng-Wen F, et al. Prevalence and trends of urinary incontinence in adults in the United States, 2001-2008. J Urol 2011;186:589-593.
4. Kinchen KS, Burgio K, Diokno AC, et al. Factors associated with women’s decisions to seek treatment for urinary incontinence. J Womens Health (Larchmt) 2003;12:687-698.
5. O’Donnell M, Viktrup L, Hunskaar S. The role of general practitioners in the initial management of women with urinary incontinence in France, Germany, Spain and the UK. Eur J Gen Pract 2007;13:20-26.
6. Wenger NS, Roth CP, Shekelle PG, et al. A practice-based intervention to improve primary care for falls, urinary incontinence, and dementia. J Am Geriatr Soc 2009;57:547-555.
7. Miller JM, Ashton-Miller JA, Carchidi LT, et al. On the lack of correlation between self-report and urine loss measured with standing provocation test in older stress-incontinent women. J Womens Health 1999;8:157-162.
8. Deng DY. Urinary incontinence in women. Med Clin North Am 2011;95:101-109.
9. Nygaard IE, Kreder KJ, Lepic MM, et al. Efficacy of pelvic floor muscle exercises in women with stress, urge, and mixed urinary incontinence. Am J Obstet Gynecol 1996;174:120-125.
10. Van Kerrebroeck PE, Kelleher CJ, Coyne KS, et al. Correlations among improvements in urgency urinary incontinence, health-related quality of life, and perception of bladder-related problems in incontinent subjects with overactive bladder treated with tolterodine or placebo. Health Qual Life Outcomes 2009;7:13-18.
11. Vaughan CP, Goode PS, Burgio KL, et al. Urinary incontinence in older adults. Mt Sinai J Med 2011;78:558-570.
12. Davila GW, Starkman JS, Dmochowski RR. Transdermal oxybutynin for overactive bladder. Urol Clin North Am 2006;33:455-463.
13. Burgio KL, Locher JL, Goode PS, et al. Behavioral vs drug treatment for urge urinary incontinence in older women: a randomized controlled trial. JAMA 1998;280:1995-2000.
14. Homma Y. Lower urinary tract symptomatology: its definition and confusion. J Urol 2008;15:35-43.
15. Hannestad YS, Rortveit G, Daltveit AK, et al. Are smoking and other lifestyle factors associated with female urinary incontinence? The Norwegian EPINCONT Study. BJOG. 2003;110:247-254.
16. Hirayama F, Lee AH. Green tea drinking is inversely associated with urinary incontinence in middle-aged and older women. Neurourol Urodyn 2011;30:1262-1265.
17. Creighton SM, Stanton SL. Caffeine: does it affect your bladder? Br J Urol 1990;66:613-614
18. Zimmern P, Litman HJ, Mueller E, et al. Effect of fluid management on fluid intake and urge incontinence in a trial for overactive bladder in women. BJU Int. 2010;105:1680-1685.
19. Tsakiris P, Oelke M, Michel MC. Drug-induced urinary incontinence. Drugs Aging 2008;25:541-549.
20. Wyman JF, Burgio KL, Newman DK. Practical aspects of lifestyle modifications and behavioural interventions in the treatment of overactive bladder and urgency urinary incontinence. Int J Clin Pract 2009;63:1177-1191.
21. Selius B, Subedi R. Urinary retention in adults: diagnosis and initial management. Am Fam Physician 2008;77:643-650.
• Advise the patient to complete a 3-day voiding diary, which will help you categorize and determine the cause and severity of her incontinence. B
• Recommend Kegel exercises for stress incontinence; provide instruction in technique and/or a referral for pelvic floor physical therapy for instruction. A
• Try an anticholinergic medication with indications for urinary incontinence for women with primarily urge-type incontinence. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
PATIENT HANDOUT
Voiding diary: What it’s for, how to fill it out
Visit #1: Simple steps to take right away
When a patient confides that she suffers from urinary incontinence, even if it’s near the end of her visit, there are 3 critical steps that can be taken without delay:
- Collect a urine sample for urinalysis (if the patient is capable of providing a clean-catch sample and did not already do so at the start of the visit).
- Ask her to keep a voiding diary for 3 days, using the clip-and-copy patient handout. (Tech-savvy patients may prefer to use the voiding diary app available at no charge at http://itunes.apple.com/us/app/bladder-pal/id435763473?mt=8.) Review the instructions for filling out the voiding diary with the patient, or have a nurse or medical assistant do so. Call the patient’s attention to the numerical (1-4) formula recommended for approximating the quantity of urine lost in an episode of incontinence, as this is often the component of the diary that patients have the most trouble with. 7
- Schedule a follow-up visit. If the urinalysis shows that the patient has a urinary tract infection, initiate treatment as soon as possible. Treatment should be completed by the time she arrives for visit#2 so that her incontinence can be reassessed in the absence of infection.
Tell your patient to bring the completed voiding diary to this visit and to avoid emptying her bladder before she sees you. When the patient checks in, she should be reminded again not to urinate until you have completed the examination.
What to do during Visit #2
This visit will begin with a targeted assessment to determine what type of incontinence the patient has and whether you should initiate treatment or refer her to a specialist.
Start with a targeted history and review of the voiding diary
Ask the patient about timing, quantity, and the overall circumstances of typical incontinence episodes, as well as the incidents that she finds most troubling. A medication history should be included, with information not only about what she’s taking, but also about when she takes her medications. A careful review of the voiding diary will provide additional information, including fluid intake quantity and quality (eg, caffeine or alcohol) and urinary frequency, quantity, and timing.
The initial goal is to classify her incontinence as urge (occurring simultaneously with, or right after, a strong feeling of the need to void); stress (the result of an acute increase in abdominal pressure, typically associated with a physical act such as coughing, sneezing, bending over, heavy lifting, or starting to run); or mixed, which involves some episodes of both.8 “High-yield” questions like the ones that follow are particularly helpful in pinpointing the type of incontinence and guiding treatment decisions:
- Do you worry about leakage when you cough or sneeze? (Stress incontinence.)
- When your bladder is full, do you typically have to stop what you’re doing and go right away, or can you wait until a convenient time to find a bathroom? (Urge incontinence.)
And, for patients who report both stress and urge episodes (mixed incontinence):
- Which is more upsetting to you: leakage that occurs when you cough or sneeze or the inability to wait until it’s convenient to get to a bathroom?
A pelvic exam and cough stress test
Next, perform a pelvic examination and assess the following:
External genitalia. Look for signs of chronic urine exposure (eg, erythema, skin breakdown) and urethral abnormalities.
Cystoceles or rectoceles. Ask the patient to bear down strongly to allow you to visualize cystoceles and rectoceles, which usually present as a significant bulging of the tissues of the anterior or posterior vaginal walls. Reassure her that urine leakage and flatus are completely acceptable—and expected—during this part of the exam.
Pelvic mass. Palpate for evidence of a pelvic mass, which can cause urinary obstruction and lead to overflow incontinence.
Kegel exercises. Ask her to perform a Kegel maneuver. If she’s doing it correctly, her perineal body should be easily seen moving upward toward her clitoris and inward toward her introitus. If you place a finger in the vagina and ask her to begin Kegel exercises, you’ll feel the tightening of the pelvic muscles if she’s doing it right. When she does this on her own, she can insert her own finger into the vagina to ensure that her technique is correct or simply practice stopping the flow of urine.
After completing the pelvic exam, perform a cough stress test. With the patient in the lithotomy position, ask her to cough while you hold the labia apart, both to observe for any leakage of urine and to approximate the volume leaked, if any ( FIGURE ). To encourage her to bear down with ample force, remind her that here, too, both leakage of urine and passing of flatus are expected.
Postvoid residual (PVr). After the pelvic exam and cough stress test, the patient should urinate and the PVR should be promptly measured. If you have doubts about her ability to provide a clean-catch urine sample (or your clinic does not have a bladder scanning ultrasound), consider obtaining the PVR via a straight catheterization. A normal PVR is 0 to 200 cc.
By now you should have the information you need to determine whether to treat the incontinence yourself or refer the patient to a urologist or urogynecologist.
FIGURE
Positive cough stress test
A physician separates the labia to observe urine leakage during a cough stress test.
Treat these conditions yourself
Dx: Uncomplicated stress incontinence. The patient has a positive cough stress test, and most urinary incontinence episodes she recorded are stress type. Urinalysis and PVR are normal, with no evidence of pelvic organ prolapse on exam.
Well-timed pelvic floor (Kegel) exercises have been shown to significantly reduce symptoms of both stress and urge incontinence when they’re done regularly, starting with 4 times a day and increased slowly to 8 times twice a day.9 Review Kegel technique and advise the patient to perform this maneuver and hold the contraction, if possible, whenever she is about to cough, laugh, sneeze, or otherwise exert herself. Tell her to practice Kegel exercises until they become second nature. If she continues to have trouble, she may need a referral to pelvic floor physical therapy to learn to do them effectively. (You can go to http://www.apta.org/apta/findapt/index.aspx?navID=10737422525 to find a therapist specializing in women’s health in your area.)
Dx: Uncomplicated urge incontinence. The patient has a normal cough stress test and most of her urinary incontinence episodes are the urge type. Her pelvic exam and PVR are within normal limits.
Prescribe an anticholinergic bladder agent. Medication with antimuscarinic properties has been shown to decrease urge incontinence and significantly improve patient-reported measures of quality of life.10
A reasonable approach is to start her on an inexpensive generic medication ( TABLE )11,12 and follow up in one month. If the medication helps and does not cause adverse effects, she can be maintained on it; if it’s effective but she has significant adverse effects (eg, constipation, dry mouth, blurred vision, urinary retention, as well as confusion and sedation in the elderly), the patient can be switched to a more expensive brand-name drug with fewer adverse effects.
Behavioral training, including timed voiding and Kegel exercises, has been shown to significantly decrease symptoms of urge incontinence.9 Timed voiding can be especially helpful for nocturnal urge incontinence; advise the patient to set an alarm for an hour before the usual time she awakens with a sense of urgency, and to empty her bladder before it is so full that she leaks.
Point out, too, that not every urinary urge requires immediately running to the toilet. A randomized, placebo-controlled trial found that patients who were taught to respond to visual cues, such as a toilet, by walking past it, then sitting down, relaxing, and contracting their pelvic muscles repeatedly had diminished urgency. Their actions also inhibited detrusor contractions, and often prevented urine loss. When the training was combined with one or 2 lessons in proper Kegel technique—reinforced at several visits—weekly incontinence episodes were reduced by 80%. The controls, who had the same number of clinic visits but did not receive specific education on urgency-reduction strategies, reduced incontinence episodes by 40%.13
Dx: Fluid overuse. The patient is drinking an excessive quantity of fluid, as evidenced by urine volume >2100 cc. (The International Continence Society defines polyuria as >40 mL/kg per day.14 )
Advise the patient to decrease her overall fluid intake, particularly within several hours of bedtime. Studies of tea and coffee consumption and incontinence have had conflicting results,15,16 and data on caffeinated soda consumption and incontinence are lacking. Nonetheless, patients should be advised that there is a possible association between caffeinated beverages and urinary incontinence. (In one small study, caffeine was found to cause a significant increase in detrusor pressure.17 ) Giving women one-time general instructions on fluid intake modification has been shown to significantly decrease incontinence episodes.18
Dx: Badly timed medication intake. This patient is taking one or more drugs that may contribute to incontinence, such as alpha-blockers or diuretics, either shortly before going to bed or before activities that make a bathroom visit inconvenient or impossible.
Adjust her medication regimen to minimize nocturia or urinary urgency during times of peak activity—eg, taking a diuretic early in the day instead of in the afternoon or evening, if possible. Keep in mind, however, that this strategy is based primarily on expert opinion, as very little evidence exists to show that medication of any type has a significant effect on urinary continence.19
Dx: Lower extremity edema with postural diuresis. The patient has nocturia, with larger total urine output at night than during the day. She may or may not have some leakage.
Women with lower extremity edema due to a variety of medical causes often experience postural diuresis overnight. If the patient is already on a diuretic, the problem can often be ameliorated by taking it early in the day instead of in the afternoon or evening; if she’s not, prescribe a small dose of a diuretic, to be taken in the morning. This therapeutic intervention has not been rigorously studied, but is relatively easy to implement and worth a try for patients with heart failure or other causes of pedal edema.
Dx: Constipation associated with autonomic dysfunction. Because the rectum and bladder are controlled by the same sacral segments of the spinal cord and share many autonomic ganglia, problems in one compartment often affect another. In a patient for whom incontinence is a minor, or occasional, problem while constipation is a major complaint, the optimal approach is to treat the constipation first and see if the urinary incontinence also resolves.
A trial of polyethylene glycol is a good place to start, particularly if there is no evidence of another correctable cause of the incontinence. Studies have shown that successful treatment of constipation often results in significant improvement of urinary urgency and frequency.20
TABLE
Anticholinergic medications for urge incontinence11,12
| Medication | Dosing | Comments |
|---|---|---|
| Oxybutynin | 2.5-5 mg bid to tid (≤5 mg qid) | Significant adverse effects, including constipation, dry mouth, blurred vision, urinary retention; confusion and sedation, particularly in elderly |
| Oxybutynin ER | 5-10 mg/d; increase weekly by 5-mg/d increments to ≤30 mg/d | |
| Oxybutynin transdermal patch (Oxytrol) | 1 patch 2x/wk on abdomen, hips, or buttocks (dose=3.9 mg/d) | Adverse effects may be less frequent than with oral medication due to the avoidance of metabolites |
| Oxybutynin transdermal gel (Gelnique) | 1 gel pack/d on abdomen, thighs, or shoulders (dose=100 mg in 1-g gel pack) | |
| Tolterodine (Detrol) | 1-2 mg bid (immediate release) or 2-4 mg/d (ER) | Similar effects and efficacy as oxybutynin, but adverse effects are decreased due to greater uroselectivity on muscarinic receptors |
| Darifenacin hydrobromide (Enablex) | 7.5 mg/d; increase to 15 mg/d after 2 wk if necessary | |
| Solifenacin (VESIcare) | 5-10 mg/d | |
| Fesoteridine (Toviaz) | 4-8 mg/d | |
| Trospium (Sanctura) | 20 mg bid (immediate release) or 60 mg/d (ER) | |
| ER, extended release. | ||
These conditions typically warrant a referral
Dx: Hematuria. Urinalysis with hematuria but no evidence of a urinary tract infection should raise a red flag, regardless of other findings. The patient should be referred for further urologic evaluation, including cystoscopy, although you may want to repeat the urinalysis with another clean-catch specimen first. Straight catheterization is not recommended in such a case, as a traumatized urethra can be a source of hematuria.
Dx: Stress incontinence with an anatomic abnormality. There are 2 options for a patient who has stress incontinence with an obvious cystocele on exam: Fit her with a pessary (or refer her to a physician who has this capability), or provide a referral to a urogynecologist for corrective surgery. Which option to choose should be a collaborative decision between patient and physician. For most women, it makes sense to try the pessary first.
Dx: Urinary retention. A patient with a high normal or elevated PVR (>100-200 cc) and no obvious pelvic organ prolapse needs a work-up for urinary retention. There is a broad differential that can be divided into neurologic, obstructive, and pharmacologic etiologies.21 A referral is indicated so that a urologist can oversee the work-up.
Particularly worrisome causes of urinary retention are multiple sclerosis, which is more likely in a relatively young, otherwise healthy woman, and a pelvic mass. Medications are also a likely cause. The list of drugs that can induce urinary retention is extensive, and includes anticholinergics, antidepressants (tricyclics and some heterocyclics), antihistamines, and muscle relaxants, among others. If you’re unable to find a likely cause, a referral is indicated so that a urologist can oversee the work-up.
CORRESPONDENCE Abigail Lowther, MD, University of Michigan Department of Family Medicine, 24 Frank Lloyd Wright Drive, Lobby H, Ann Arbor, MI 48106-5795; abigaill@med.umich.edu
• Advise the patient to complete a 3-day voiding diary, which will help you categorize and determine the cause and severity of her incontinence. B
• Recommend Kegel exercises for stress incontinence; provide instruction in technique and/or a referral for pelvic floor physical therapy for instruction. A
• Try an anticholinergic medication with indications for urinary incontinence for women with primarily urge-type incontinence. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
PATIENT HANDOUT
Voiding diary: What it’s for, how to fill it out
Visit #1: Simple steps to take right away
When a patient confides that she suffers from urinary incontinence, even if it’s near the end of her visit, there are 3 critical steps that can be taken without delay:
- Collect a urine sample for urinalysis (if the patient is capable of providing a clean-catch sample and did not already do so at the start of the visit).
- Ask her to keep a voiding diary for 3 days, using the clip-and-copy patient handout. (Tech-savvy patients may prefer to use the voiding diary app available at no charge at http://itunes.apple.com/us/app/bladder-pal/id435763473?mt=8.) Review the instructions for filling out the voiding diary with the patient, or have a nurse or medical assistant do so. Call the patient’s attention to the numerical (1-4) formula recommended for approximating the quantity of urine lost in an episode of incontinence, as this is often the component of the diary that patients have the most trouble with. 7
- Schedule a follow-up visit. If the urinalysis shows that the patient has a urinary tract infection, initiate treatment as soon as possible. Treatment should be completed by the time she arrives for visit#2 so that her incontinence can be reassessed in the absence of infection.
Tell your patient to bring the completed voiding diary to this visit and to avoid emptying her bladder before she sees you. When the patient checks in, she should be reminded again not to urinate until you have completed the examination.
What to do during Visit #2
This visit will begin with a targeted assessment to determine what type of incontinence the patient has and whether you should initiate treatment or refer her to a specialist.
Start with a targeted history and review of the voiding diary
Ask the patient about timing, quantity, and the overall circumstances of typical incontinence episodes, as well as the incidents that she finds most troubling. A medication history should be included, with information not only about what she’s taking, but also about when she takes her medications. A careful review of the voiding diary will provide additional information, including fluid intake quantity and quality (eg, caffeine or alcohol) and urinary frequency, quantity, and timing.
The initial goal is to classify her incontinence as urge (occurring simultaneously with, or right after, a strong feeling of the need to void); stress (the result of an acute increase in abdominal pressure, typically associated with a physical act such as coughing, sneezing, bending over, heavy lifting, or starting to run); or mixed, which involves some episodes of both.8 “High-yield” questions like the ones that follow are particularly helpful in pinpointing the type of incontinence and guiding treatment decisions:
- Do you worry about leakage when you cough or sneeze? (Stress incontinence.)
- When your bladder is full, do you typically have to stop what you’re doing and go right away, or can you wait until a convenient time to find a bathroom? (Urge incontinence.)
And, for patients who report both stress and urge episodes (mixed incontinence):
- Which is more upsetting to you: leakage that occurs when you cough or sneeze or the inability to wait until it’s convenient to get to a bathroom?
A pelvic exam and cough stress test
Next, perform a pelvic examination and assess the following:
External genitalia. Look for signs of chronic urine exposure (eg, erythema, skin breakdown) and urethral abnormalities.
Cystoceles or rectoceles. Ask the patient to bear down strongly to allow you to visualize cystoceles and rectoceles, which usually present as a significant bulging of the tissues of the anterior or posterior vaginal walls. Reassure her that urine leakage and flatus are completely acceptable—and expected—during this part of the exam.
Pelvic mass. Palpate for evidence of a pelvic mass, which can cause urinary obstruction and lead to overflow incontinence.
Kegel exercises. Ask her to perform a Kegel maneuver. If she’s doing it correctly, her perineal body should be easily seen moving upward toward her clitoris and inward toward her introitus. If you place a finger in the vagina and ask her to begin Kegel exercises, you’ll feel the tightening of the pelvic muscles if she’s doing it right. When she does this on her own, she can insert her own finger into the vagina to ensure that her technique is correct or simply practice stopping the flow of urine.
After completing the pelvic exam, perform a cough stress test. With the patient in the lithotomy position, ask her to cough while you hold the labia apart, both to observe for any leakage of urine and to approximate the volume leaked, if any ( FIGURE ). To encourage her to bear down with ample force, remind her that here, too, both leakage of urine and passing of flatus are expected.
Postvoid residual (PVr). After the pelvic exam and cough stress test, the patient should urinate and the PVR should be promptly measured. If you have doubts about her ability to provide a clean-catch urine sample (or your clinic does not have a bladder scanning ultrasound), consider obtaining the PVR via a straight catheterization. A normal PVR is 0 to 200 cc.
By now you should have the information you need to determine whether to treat the incontinence yourself or refer the patient to a urologist or urogynecologist.
FIGURE
Positive cough stress test
A physician separates the labia to observe urine leakage during a cough stress test.
Treat these conditions yourself
Dx: Uncomplicated stress incontinence. The patient has a positive cough stress test, and most urinary incontinence episodes she recorded are stress type. Urinalysis and PVR are normal, with no evidence of pelvic organ prolapse on exam.
Well-timed pelvic floor (Kegel) exercises have been shown to significantly reduce symptoms of both stress and urge incontinence when they’re done regularly, starting with 4 times a day and increased slowly to 8 times twice a day.9 Review Kegel technique and advise the patient to perform this maneuver and hold the contraction, if possible, whenever she is about to cough, laugh, sneeze, or otherwise exert herself. Tell her to practice Kegel exercises until they become second nature. If she continues to have trouble, she may need a referral to pelvic floor physical therapy to learn to do them effectively. (You can go to http://www.apta.org/apta/findapt/index.aspx?navID=10737422525 to find a therapist specializing in women’s health in your area.)
Dx: Uncomplicated urge incontinence. The patient has a normal cough stress test and most of her urinary incontinence episodes are the urge type. Her pelvic exam and PVR are within normal limits.
Prescribe an anticholinergic bladder agent. Medication with antimuscarinic properties has been shown to decrease urge incontinence and significantly improve patient-reported measures of quality of life.10
A reasonable approach is to start her on an inexpensive generic medication ( TABLE )11,12 and follow up in one month. If the medication helps and does not cause adverse effects, she can be maintained on it; if it’s effective but she has significant adverse effects (eg, constipation, dry mouth, blurred vision, urinary retention, as well as confusion and sedation in the elderly), the patient can be switched to a more expensive brand-name drug with fewer adverse effects.
Behavioral training, including timed voiding and Kegel exercises, has been shown to significantly decrease symptoms of urge incontinence.9 Timed voiding can be especially helpful for nocturnal urge incontinence; advise the patient to set an alarm for an hour before the usual time she awakens with a sense of urgency, and to empty her bladder before it is so full that she leaks.
Point out, too, that not every urinary urge requires immediately running to the toilet. A randomized, placebo-controlled trial found that patients who were taught to respond to visual cues, such as a toilet, by walking past it, then sitting down, relaxing, and contracting their pelvic muscles repeatedly had diminished urgency. Their actions also inhibited detrusor contractions, and often prevented urine loss. When the training was combined with one or 2 lessons in proper Kegel technique—reinforced at several visits—weekly incontinence episodes were reduced by 80%. The controls, who had the same number of clinic visits but did not receive specific education on urgency-reduction strategies, reduced incontinence episodes by 40%.13
Dx: Fluid overuse. The patient is drinking an excessive quantity of fluid, as evidenced by urine volume >2100 cc. (The International Continence Society defines polyuria as >40 mL/kg per day.14 )
Advise the patient to decrease her overall fluid intake, particularly within several hours of bedtime. Studies of tea and coffee consumption and incontinence have had conflicting results,15,16 and data on caffeinated soda consumption and incontinence are lacking. Nonetheless, patients should be advised that there is a possible association between caffeinated beverages and urinary incontinence. (In one small study, caffeine was found to cause a significant increase in detrusor pressure.17 ) Giving women one-time general instructions on fluid intake modification has been shown to significantly decrease incontinence episodes.18
Dx: Badly timed medication intake. This patient is taking one or more drugs that may contribute to incontinence, such as alpha-blockers or diuretics, either shortly before going to bed or before activities that make a bathroom visit inconvenient or impossible.
Adjust her medication regimen to minimize nocturia or urinary urgency during times of peak activity—eg, taking a diuretic early in the day instead of in the afternoon or evening, if possible. Keep in mind, however, that this strategy is based primarily on expert opinion, as very little evidence exists to show that medication of any type has a significant effect on urinary continence.19
Dx: Lower extremity edema with postural diuresis. The patient has nocturia, with larger total urine output at night than during the day. She may or may not have some leakage.
Women with lower extremity edema due to a variety of medical causes often experience postural diuresis overnight. If the patient is already on a diuretic, the problem can often be ameliorated by taking it early in the day instead of in the afternoon or evening; if she’s not, prescribe a small dose of a diuretic, to be taken in the morning. This therapeutic intervention has not been rigorously studied, but is relatively easy to implement and worth a try for patients with heart failure or other causes of pedal edema.
Dx: Constipation associated with autonomic dysfunction. Because the rectum and bladder are controlled by the same sacral segments of the spinal cord and share many autonomic ganglia, problems in one compartment often affect another. In a patient for whom incontinence is a minor, or occasional, problem while constipation is a major complaint, the optimal approach is to treat the constipation first and see if the urinary incontinence also resolves.
A trial of polyethylene glycol is a good place to start, particularly if there is no evidence of another correctable cause of the incontinence. Studies have shown that successful treatment of constipation often results in significant improvement of urinary urgency and frequency.20
TABLE
Anticholinergic medications for urge incontinence11,12
| Medication | Dosing | Comments |
|---|---|---|
| Oxybutynin | 2.5-5 mg bid to tid (≤5 mg qid) | Significant adverse effects, including constipation, dry mouth, blurred vision, urinary retention; confusion and sedation, particularly in elderly |
| Oxybutynin ER | 5-10 mg/d; increase weekly by 5-mg/d increments to ≤30 mg/d | |
| Oxybutynin transdermal patch (Oxytrol) | 1 patch 2x/wk on abdomen, hips, or buttocks (dose=3.9 mg/d) | Adverse effects may be less frequent than with oral medication due to the avoidance of metabolites |
| Oxybutynin transdermal gel (Gelnique) | 1 gel pack/d on abdomen, thighs, or shoulders (dose=100 mg in 1-g gel pack) | |
| Tolterodine (Detrol) | 1-2 mg bid (immediate release) or 2-4 mg/d (ER) | Similar effects and efficacy as oxybutynin, but adverse effects are decreased due to greater uroselectivity on muscarinic receptors |
| Darifenacin hydrobromide (Enablex) | 7.5 mg/d; increase to 15 mg/d after 2 wk if necessary | |
| Solifenacin (VESIcare) | 5-10 mg/d | |
| Fesoteridine (Toviaz) | 4-8 mg/d | |
| Trospium (Sanctura) | 20 mg bid (immediate release) or 60 mg/d (ER) | |
| ER, extended release. | ||
These conditions typically warrant a referral
Dx: Hematuria. Urinalysis with hematuria but no evidence of a urinary tract infection should raise a red flag, regardless of other findings. The patient should be referred for further urologic evaluation, including cystoscopy, although you may want to repeat the urinalysis with another clean-catch specimen first. Straight catheterization is not recommended in such a case, as a traumatized urethra can be a source of hematuria.
Dx: Stress incontinence with an anatomic abnormality. There are 2 options for a patient who has stress incontinence with an obvious cystocele on exam: Fit her with a pessary (or refer her to a physician who has this capability), or provide a referral to a urogynecologist for corrective surgery. Which option to choose should be a collaborative decision between patient and physician. For most women, it makes sense to try the pessary first.
Dx: Urinary retention. A patient with a high normal or elevated PVR (>100-200 cc) and no obvious pelvic organ prolapse needs a work-up for urinary retention. There is a broad differential that can be divided into neurologic, obstructive, and pharmacologic etiologies.21 A referral is indicated so that a urologist can oversee the work-up.
Particularly worrisome causes of urinary retention are multiple sclerosis, which is more likely in a relatively young, otherwise healthy woman, and a pelvic mass. Medications are also a likely cause. The list of drugs that can induce urinary retention is extensive, and includes anticholinergics, antidepressants (tricyclics and some heterocyclics), antihistamines, and muscle relaxants, among others. If you’re unable to find a likely cause, a referral is indicated so that a urologist can oversee the work-up.
CORRESPONDENCE Abigail Lowther, MD, University of Michigan Department of Family Medicine, 24 Frank Lloyd Wright Drive, Lobby H, Ann Arbor, MI 48106-5795; abigaill@med.umich.edu
1. Hunskaar S, Lose G, Sykes D, et al. The prevalence of urinary incontinence in women in four European countries. BJU Int 2004;93:324-330.
2. Roberts RO, Jacobsen SJ, Rhodes T, et al. Urinary incontinence in a community-based cohort: prevalence and healthcare-seeking. J Am Geriatr Soc 1998;46:467-472.
3. Markland AD, Richter HE, Chyng-Wen F, et al. Prevalence and trends of urinary incontinence in adults in the United States, 2001-2008. J Urol 2011;186:589-593.
4. Kinchen KS, Burgio K, Diokno AC, et al. Factors associated with women’s decisions to seek treatment for urinary incontinence. J Womens Health (Larchmt) 2003;12:687-698.
5. O’Donnell M, Viktrup L, Hunskaar S. The role of general practitioners in the initial management of women with urinary incontinence in France, Germany, Spain and the UK. Eur J Gen Pract 2007;13:20-26.
6. Wenger NS, Roth CP, Shekelle PG, et al. A practice-based intervention to improve primary care for falls, urinary incontinence, and dementia. J Am Geriatr Soc 2009;57:547-555.
7. Miller JM, Ashton-Miller JA, Carchidi LT, et al. On the lack of correlation between self-report and urine loss measured with standing provocation test in older stress-incontinent women. J Womens Health 1999;8:157-162.
8. Deng DY. Urinary incontinence in women. Med Clin North Am 2011;95:101-109.
9. Nygaard IE, Kreder KJ, Lepic MM, et al. Efficacy of pelvic floor muscle exercises in women with stress, urge, and mixed urinary incontinence. Am J Obstet Gynecol 1996;174:120-125.
10. Van Kerrebroeck PE, Kelleher CJ, Coyne KS, et al. Correlations among improvements in urgency urinary incontinence, health-related quality of life, and perception of bladder-related problems in incontinent subjects with overactive bladder treated with tolterodine or placebo. Health Qual Life Outcomes 2009;7:13-18.
11. Vaughan CP, Goode PS, Burgio KL, et al. Urinary incontinence in older adults. Mt Sinai J Med 2011;78:558-570.
12. Davila GW, Starkman JS, Dmochowski RR. Transdermal oxybutynin for overactive bladder. Urol Clin North Am 2006;33:455-463.
13. Burgio KL, Locher JL, Goode PS, et al. Behavioral vs drug treatment for urge urinary incontinence in older women: a randomized controlled trial. JAMA 1998;280:1995-2000.
14. Homma Y. Lower urinary tract symptomatology: its definition and confusion. J Urol 2008;15:35-43.
15. Hannestad YS, Rortveit G, Daltveit AK, et al. Are smoking and other lifestyle factors associated with female urinary incontinence? The Norwegian EPINCONT Study. BJOG. 2003;110:247-254.
16. Hirayama F, Lee AH. Green tea drinking is inversely associated with urinary incontinence in middle-aged and older women. Neurourol Urodyn 2011;30:1262-1265.
17. Creighton SM, Stanton SL. Caffeine: does it affect your bladder? Br J Urol 1990;66:613-614
18. Zimmern P, Litman HJ, Mueller E, et al. Effect of fluid management on fluid intake and urge incontinence in a trial for overactive bladder in women. BJU Int. 2010;105:1680-1685.
19. Tsakiris P, Oelke M, Michel MC. Drug-induced urinary incontinence. Drugs Aging 2008;25:541-549.
20. Wyman JF, Burgio KL, Newman DK. Practical aspects of lifestyle modifications and behavioural interventions in the treatment of overactive bladder and urgency urinary incontinence. Int J Clin Pract 2009;63:1177-1191.
21. Selius B, Subedi R. Urinary retention in adults: diagnosis and initial management. Am Fam Physician 2008;77:643-650.
1. Hunskaar S, Lose G, Sykes D, et al. The prevalence of urinary incontinence in women in four European countries. BJU Int 2004;93:324-330.
2. Roberts RO, Jacobsen SJ, Rhodes T, et al. Urinary incontinence in a community-based cohort: prevalence and healthcare-seeking. J Am Geriatr Soc 1998;46:467-472.
3. Markland AD, Richter HE, Chyng-Wen F, et al. Prevalence and trends of urinary incontinence in adults in the United States, 2001-2008. J Urol 2011;186:589-593.
4. Kinchen KS, Burgio K, Diokno AC, et al. Factors associated with women’s decisions to seek treatment for urinary incontinence. J Womens Health (Larchmt) 2003;12:687-698.
5. O’Donnell M, Viktrup L, Hunskaar S. The role of general practitioners in the initial management of women with urinary incontinence in France, Germany, Spain and the UK. Eur J Gen Pract 2007;13:20-26.
6. Wenger NS, Roth CP, Shekelle PG, et al. A practice-based intervention to improve primary care for falls, urinary incontinence, and dementia. J Am Geriatr Soc 2009;57:547-555.
7. Miller JM, Ashton-Miller JA, Carchidi LT, et al. On the lack of correlation between self-report and urine loss measured with standing provocation test in older stress-incontinent women. J Womens Health 1999;8:157-162.
8. Deng DY. Urinary incontinence in women. Med Clin North Am 2011;95:101-109.
9. Nygaard IE, Kreder KJ, Lepic MM, et al. Efficacy of pelvic floor muscle exercises in women with stress, urge, and mixed urinary incontinence. Am J Obstet Gynecol 1996;174:120-125.
10. Van Kerrebroeck PE, Kelleher CJ, Coyne KS, et al. Correlations among improvements in urgency urinary incontinence, health-related quality of life, and perception of bladder-related problems in incontinent subjects with overactive bladder treated with tolterodine or placebo. Health Qual Life Outcomes 2009;7:13-18.
11. Vaughan CP, Goode PS, Burgio KL, et al. Urinary incontinence in older adults. Mt Sinai J Med 2011;78:558-570.
12. Davila GW, Starkman JS, Dmochowski RR. Transdermal oxybutynin for overactive bladder. Urol Clin North Am 2006;33:455-463.
13. Burgio KL, Locher JL, Goode PS, et al. Behavioral vs drug treatment for urge urinary incontinence in older women: a randomized controlled trial. JAMA 1998;280:1995-2000.
14. Homma Y. Lower urinary tract symptomatology: its definition and confusion. J Urol 2008;15:35-43.
15. Hannestad YS, Rortveit G, Daltveit AK, et al. Are smoking and other lifestyle factors associated with female urinary incontinence? The Norwegian EPINCONT Study. BJOG. 2003;110:247-254.
16. Hirayama F, Lee AH. Green tea drinking is inversely associated with urinary incontinence in middle-aged and older women. Neurourol Urodyn 2011;30:1262-1265.
17. Creighton SM, Stanton SL. Caffeine: does it affect your bladder? Br J Urol 1990;66:613-614
18. Zimmern P, Litman HJ, Mueller E, et al. Effect of fluid management on fluid intake and urge incontinence in a trial for overactive bladder in women. BJU Int. 2010;105:1680-1685.
19. Tsakiris P, Oelke M, Michel MC. Drug-induced urinary incontinence. Drugs Aging 2008;25:541-549.
20. Wyman JF, Burgio KL, Newman DK. Practical aspects of lifestyle modifications and behavioural interventions in the treatment of overactive bladder and urgency urinary incontinence. Int J Clin Pract 2009;63:1177-1191.
21. Selius B, Subedi R. Urinary retention in adults: diagnosis and initial management. Am Fam Physician 2008;77:643-650.
Eye pain: 5 cases to test your skill
Your patient comes in complaining of acute eye pain. Is it an ophthalmic emergency? Benign? Something in between?
The varying degrees of severity are reflected in the cases presented here, and in the multiple-choice quiz that follows. After selecting your answer to each of the questions, turn the page to find out if you were right.
CASE 1 A 74-year-old man presents with left eye pain that began when he lowered the shades in his bedroom to get ready for a nap. The pain worsened rapidly, he reports, and now—a half hour later—he has a headache, nausea, and blurred vision in the affected eye.
The patient has a history of hypertension, which is controlled with hydrochlorothiazide, but takes no other drugs. He denies any eye trauma. An eye exam reveals that the pupil is dilated and reacts poorly to light; you note corneal haziness and edema, as well ( FIGURE 1; vertical line is the slit lamp beam).
FIGURE 1
What’s your diagnosis?
- angle-closure glaucoma
- macular degeneration
- open-angle glaucoma
- retinal detachment.
CASE 2 A 20-year-old man was playing touch football while wearing contact lenses. Soon after, the game, he felt a stinging sensation in his right eye—”like sand under the eyelid,” he said. Exposure to sunlight also produced eye pain. The patient had tried flushing his eye with water, but experienced little pain relief.
An eye exam reveals a grayish-white spot in the line of vision ( FIGURE 2 ), with no sign of a penetrating injury.
FIGURE 2
What’s your next step?
- Prescribe antibiotic eye drops and send the patient home.
- Provide an emergent ophthalmology referral for foreign body extraction.
- Send the patient home with an eye patch to relieve the photophobia.
- Attempt to conduct a visual acuity test and penlight exam without anesthetic; if necessary, apply anesthetic drops and fluorescein stain.
CASE 3 A 17-year-old woman is brought in by her mother because of pain and vision loss in her right eye. The pain started as soon as she awoke; 2 hours later, the vision in the affected eye became blurry. Soon after, she saw spots of light, followed by complete loss of vision. She denies any trauma and says she has never experienced anything like this before.
The patient’s visual acuity is 20/200 in her right eye, and 20/40 in the left. You follow up with a funduscopic exam of the eye, which shows edema of the optic disc, blurring of the disc margins, and distended veins ( FIGURE 3 ).
FIGURE 3
What diagnostic test should be next?
- fluorescein angiography
- intraocular pressure measurement
- magnetic resonance imaging (MRI) of the brain
- visual fields assessment.
CASE 4 A 31-year-old woman with a history of sarcoidosis comes in because of spontaneous redness ( FIGURE 4 ) and pain in her left eye, which began early that day. She denies any eye trauma, eye surgery, or recent cold, and has no discharge or crust around the eye.
FIGURE 4
What’s your diagnosis?
- anterior uveitis
- conjunctivitis
- corneal abrasion
- posterior uveitis.
CASE 5 A 21-year-old college student complains of pain in her left eye that started a day ago. She wears contact lenses and, upon questioning, tells you that she sometimes keeps them in all night.
The patient reports that she used a friend’s steroid eye drops twice yesterday, but the drops didn’t help. The pain is worse today. It feels as if there’s something in her eye, she says, and she finds it hard to open her eye. Penlight examination ( FIGURE 5 ) reveals a branching opacity on the cornea.
FIGURE 5
What’s your next step?
- Advise the patient to stop wearing her contact lenses overnight, give her a cycloplegic, and follow up in 2 days.
- Patch the patient’s left eye, prescribe antibiotics, and tell her to return tomorrow.
- Prescribe steroid eye drops and follow up in 2 days.
- Provide an urgent referral to an ophthalmologist.
CASE 1 The answer is A: angle-closure glaucoma.
Glaucoma is the world’s leading cause of blindness,1,2 and angle-closure glaucoma comprises 10% of cases.2 In this condition, the anterior chamber angle—formed by the iris and the cornea—is narrow. When the iris dilates in low light (eg, the darkened room in which the patient had planned to nap), it folds into the narrowed angle, preventing the flow of aqueous humor and leading to an increase in intraocular pressure.3
Signs and symptoms
Patients with acute angle-closure glaucoma often develop decreased vision, halos, headaches, severe eye pain, nausea, and vomiting. If the intraocular pressure increases quickly, symptoms can have an acute and dramatic onset; if pressure builds slowly, patients may have limited or no symptoms.4
Physical findings
An eye exam will reveal conjunctival erythema, corneal edema and cloudiness, iris irregularity, a shallow anterior chamber (visible with a slit lamp), a dilated pupil (4-6 mm) that reacts sluggishly to light, and increased ocular pressure. The cupping of the optic disc seen in this patient’s retinal exam ( FIGURE 6 ) is more commonly associated with chronic open-angle glaucoma.
FIGURE 6
Management
Acute angle-closure glaucoma is an ophthalmologic emergency, and a referral to a specialist is crucial. If no ophthalmologist can see the patient within an hour of the presentation of symptoms, you’ll need to initiate topical treatment to reduce intraocular pressure and ensure that he or she is seen by a specialist as soon as possible.5 First-line treatments for acute angle-closure glaucoma are listed in TABLE 1 .5,6
In severe cases, topical therapy alone may be ineffective. If there are no contraindications, systemic medications such as acetazolamide, mannitol, glycerol, or isosorbide may be needed. Eye pressure should be checked 30 to 60 minutes after initiating systemic therapy and the patient closely monitored for adverse effects (eg, anaphylaxis, convulsions [acetazolamide]; hypotension [mannitol, isosorbide]).5
When the patient does see an ophthalmologist, the evaluation will include assessment of visual acuity, pupillary evaluation, measurement of intraocular pressure, slit lamp examination, gonioscopy to measure the angle of the anterior chamber, and a fundus exam without pupil dilation.5 Treatment may include a surgical procedure, such as paracentesis of the eye, peripheral iridotomy, or iridectomy, and chronic administration of topical medication.7
TABLE 1
Treatments for acute angle-closure glaucoma5,6
| Medication | Quantity |
|---|---|
| Topical | |
| 0.5% timolol maleate | 1 drop in affected eye 2x/d |
| 1% apraclonidine | 1-2 drops 3x/d |
| 2% pilocarpine | 1 drop 4x/d |
| Oral | |
| Acetazolamide | 500 mg 2x/d |
| Glycerol 50% solution | 1-1.8 g/kg |
| Isosorbide solution | 1.5 g/kg |
| Intravenous | |
| Acetazolamide | 250 mg 4x/d |
| Mannitol | 0.25-2 g/kg (one-time dose) |
CASE 2 The answer is D: Attempt to conduct a visual acuity test and penlight exam without anesthetic; if necessary, apply anesthetic drops and fluorescein stain.
This patient likely has a corneal ulcer or abrasion caused by a contact lens. Corneal ulcers—which constitute a loss of the corneal epithelium—can be caused by trauma or a foreign body, or develop spontaneously.
Signs and symptoms
Because of the rich innervation of the corneal epithelium by the trigeminal nerve, the cornea is highly sensitive to pain. Patients with corneal abrasions typically experience eye pain, photophobia, tearing, a foreign body sensation, and discomfort when driving, working, or reading. If a patient has damage to the trigeminal nerve, however (eg, from trauma, a tumor, or a herpes infection), corneal injuries may be painless.8
In some cases, corneal abrasions may be so painful that patients become disruptive while waiting to be seen. Thus, any patient suspected of having a corneal ulcer or abrasion should be ushered into a quiet, dark room and instructed to keep his or her eyes closed until the examination.
The patient history should focus on recent trauma, type of work, and use of protective eyewear, which may provide clues to the development of penetrating injuries, retained foreign bodies, or abrasions caused by a foreign body. Ask about the use of contact lenses, as well; corneal injury caused by contacts is associated with specific bacterial infections, most commonly staphylococcal organisms and Pseudomonas.9
Physical findings
Attempt to test visual acuity without topical anesthetics. If the patient can’t tolerate the test, a single drop of topical anesthetic (proparacaine 0.5%) may help. Visual acuity provides clues to the location of the injury. If acuity is close to baseline, the corneal abrasion is likely peripheral to the visual axis. Decreased visual acuity indicates either that the abrasion involves the central axis area or that there is corneal edema.9
Evaluation of the pupillary reflex and optic fundus should follow. Reactive miosis may be present with corneal abrasions. A large, nonreactive pupil may be a sign of injury to the pupillary sphincter from blunt or penetrating injury—an ophthalmologic emergency that requires urgent referral. The eyelid should be flipped and examined for foreign bodies. 9
Some abrasions can be seen with the naked eye, so a penlight exam should be performed before applying fluorescein stain. If a corneal lesion is suspicious for herpesvirus infection ( SEE CASE 5 ), fluorescein should not be applied, as it can interfere with the antibody test.10 Refer the patient to an ophthalmologist instead.
If there’s no evidence of infection, apply fluorescein and examine the cornea with a Wood’s lamp. A thin line or several vertical lines are suggestive of a foreign body in the cornea or under the eyelid, whereas round defects are often due to contact lenses.9 Physical findings associated with specific types of corneal abrasions or ulcers are detailed in TABLE 2 .9,11,12
TABLE 2
Corneal abrasion or ulcers: The differential diagnosis9,11,12
| Diagnosis | Physical findings | Management |
|---|---|---|
| Penetrating trauma |
|
|
| Infected corneal abrasion | Grayish edge near abrasions or ulcers |
|
| Retained foreign body |
|
|
| Herpesvirus infection | Branching pattern |
|
| Spontaneous erosions |
|
|
| *Gel-like extrusion of ocular contents seen with fluorescein. †Ointment is preferable to drops. If a contact lens caused the abrasion, a solution that covers Pseudomonas should be used. | ||
Management
Patients with corneal abrasions or ulcers should receive topical antibiotics to prevent infection. Ointments (erythromycin ointment 4 times daily for 3-5 days) are preferable to drops, but may be harder to obtain.9 If a patient must use drops, sulfacetamide 10%, polymyxin/trimethoprim, ciprofloxacin, or ofloxacin can be used, with the same frequency and duration.
Aminoglycosides are toxic to the corneal epithelium and should be avoided, except in abrasions caused by contacts.9 Because of the likelihood of pseudomonal keratitis in cases involving contact lenses, antibiotics covering Pseudomonas, such as ofloxacin, ciprofloxacin, or tobramycin, should be used.9
Pain control is achieved with cycloplegics11 like cyclopentolate 0.5% to 1% or a one-day course of systemic opioids. For children, over-the-counter analgesics for mild pain and mild opioids for severe pain may be used.
Chronic use of topical anesthetics should be avoided in patients of any age. Although they relieve pain, frequent use can lead to delayed healing, ulcerations, perforations, scarring, or even blindness.8
Patching has not been found to improve healing or comfort;13 instead, it delays healing.14,15 The “pirate patch,” which hovers over the eye, does not keep the eyelid down and therefore is not recommended.9
Follow up within 24 hours of initiating treatment to assure that the abrasion is healing. If it appears to be getting worse or is simply not improving, an immediate referral to an ophthalmologist is needed. Abrasions caused by contact with potentially infected material (eg, farm equipment, tree branches, or soil) require daily monitoring until they heal.11
CASE 3 The answer is C: Order an MRI of the brain.
This patient has optic neuritis, caused by inflammation of the optic nerve and disruption of the nerve’s myelin sheath. It predominantly affects young adults, and is more common in women than in men.16 The incidence of optic neuritis is higher among Asians, black South Africans, and children under the age of 15.17,18
Signs and symptoms
Optic neuritis is characterized by monocular (90%) or binocular (10%) complete or partial vision loss, photopsia (flashes of light), and eye pain. Up to 60% of pediatric patients present with blurred vision, bilateral involvement,19 and no pain, while adults predominantly have pain and unilateral vision loss.20 Optic neuritis is often a presenting symptom of multiple sclerosis (MS).16
Physical findings
Physical exam findings in optic neuritis include a sluggish direct light reflex, loss of visual acuity and color vision, as well as acute eye pain.18 Ophthalmoscopic exam may reveal papillitis with edema of the optic disc.21 In the Optic Neuritis Treatment Trial (ONTT), however, only one-third of patients presented with papillitis and swelling of the optic disc.22
MRI of the brain with gadolinium contrast is generally used to confirm the diagnosis. On MRI, 95% of patients with optic neuritis have signs of inflammation of the optic nerve and/or white matter changes consistent with MS (periventricular and ovoid demyelination).23,24
Patients with evidence of demyelination should also be evaluated for MS and other demyelinating disorders. In the ONTT trial, the risk of developing MS within 15 years of an optic neuritis diagnosis was as low as 25% (95% confidence interval [CI], 18%-32%) for patients with no lesions on a baseline brain MRI and as high as 72% (95% CI, 63%-81%) for those with one or more lesions on a baseline MRI, according to the study’s final follow-up.22
Management
The recommended treatment for optic neuritis is intravenous (IV) methylprednisolone 250 mg every 6 hours for 3 to 5 days, followed by oral prednisone at 1 mg/kg/d for 7 to 10 days. Vision usually returns slowly over the course of several months to a year. Ophthalmology consultation should be considered to rule out other causes of optic neuritis.25
CASE 4 The answer is A: anterior uveitis.
Uveitis is often associated with systemic disease or infection, and diagnosis is typically suspected based on a history of conditions such as sarcoidosis, juvenile idiopathic arthritis, Kawasaki’s disease, Sjögren’s syndrome, toxoplasmosis, human immunodeficiency virus (HIV), tuberculosis (TB), syphilis, herpes simplex, and herpes zoster.26
Signs and symptoms
Signs and symptoms vary depending on the part of the uveal tract that’s involved. Anterior uveitis, or iritis, is associated with pain, photophobia, redness, and a varying degree of vision loss. Posterior and intermediate uveitis are less likely to be associated with pain, but can be accompanied by decreased visual acuity and floaters.27
Physical findings
Visual acuity in patients with uveitis can range from normal to varying degrees of vision loss. Redness around the iris can be seen; conjunctival infection is most marked around the circumference of the corneal limbus rather than more peripherally, as seen in conjunctivitis. On slit lamp examination, the beam of light can be seen in the aqueous humor due to protein and leukocyte accumulation—a phenomenon known as “flare.” The pupillary light reflex may be abnormal, and the pupillary opening may be irregular rather than round due to anterior and posterior synechia.28
Management
Patients should be referred to an ophthalmologist for management of the immediate condition and to prevent or treat complications such as vision loss, optic nerve damage, and glaucoma. Acute management includes topical steroids, such as prednisolone acetate ophthalmic 1% 2 to 4 times daily, as well as treatment of the underlying condition. Long-term management varies, depending on the cause of the uveitis.26,29
If the etiology is unknown, a workup should be considered to identify inflammatory and infectious disorders that might be causing uveitis. Chest radiograph is a good beginning to look for evidence of sarcoidosis or TB; serologic testing for syphilis, HIV, and lupus may also be considered.26,29
CASE 5 The answer is D: Provide an urgent referral to an ophthalmologist.
This patient has viral keratitis caused by herpes. While the pain and foreign body sensation are the same for bacterial and viral keratitis, herpesvirus is distinguishable by the branching opacity that develops on the cornea.
Varicella zoster is the most common cause of viral keratitis, although it can also be caused by herpes simplex and adeno-virus. Because a person who is infected with herpes has the virus for life, however, multiple attacks are possible. Reactivation is associated with stress and a weakened immune system, but may occur spontaneously, as well. Patients who wear contact lenses are no more likely than those who don’t to be infected with the herpesvirus.
Bacterial keratitis is often associated with contact lenses, particularly when they’re continually worn, but also with normal wear.30 Immunosuppression, dry ocular surfaces, and topical corticosteroid use may predispose patients to bacterial keratitis, as well.12 Staphylococcus aureus, Pseudomonas aeruginosa, coagulase-negative Staphylococcus, diphtheroids, and Streptococcus pneumoniae are the most common pathogens.31
Signs and symptoms
Patients with keratitis typically complain of eye pain, a sensation of having a foreign body in the eye, photophobia, tearing, and vision changes; a mucopurulent discharge is sometimes present, as well.3 The condition is easily distinguished from conjunctivitis, which typically does not involve eye pain or vision changes.
Physical findings
Visual acuity may be affected if the lesion or corneal edema involves the visual axis. Physical exam in a patient with bacterial keratitis sometimes shows a gray or white corneal opacity, along with corneal erythema. As already noted, a penlight exam will reveal a branching opacity in patients with herpes keratitis.30
Management
Patients with keratitis should be referred immediately to an ophthalmologist32 for a slit lamp evaluation, treatment, and close follow-up.
Corneal cultures can be difficult to obtain, but before prescribing antibiotics, an attempt to collect samples should be made. This can be done—after the administration of topical anesthesia—with a sterile calcium alginate swab. Gently swab the cornea and then inoculate the appropriate gels or mediums. Avoid contact with lashes and eyelids to prevent culture contamination.32
When herpesvirus is suspected, start the patient on an antiviral agent such as trifluridine ophthalmic (1%) 9 times a day, vidarabine ophthalmic (3%) 5 times daily, or 400 mg oral acyclovir 5 times a day. Patients with bacterial keratitis should be started on antibiotic eye drops with Pseudomonas coverage, such as ofloxacin (0.3%), ciprofloxacin (0.3%), or tobramycin (0.3%), 6 to 8 times a day.9
CORRESPONDENCE Uyen Michelle Le, MD, 967 Galindo Court, Milpitas, CA 95035; mlkala21@aol.com
1. Foster PJ, Johnson GJ. Glaucoma in China: how big is the problem? Br J Ophthalmol. 2001;85:1277-1282.
2. American Academy of Ophthalmology. Preferred Practice Pattern Guidelines. Primary angle closure PPP - October 2010. Available at: http://one.aao.org/CE/PracticeGuidelines/PPP_Content.aspx?cid=92bea8f6-5459-49a6-9233-4528343dc4c3. Accessed July 12, 2012.
3. Sau SM, Gazzard G, Friedman DS. Interventions for angle-closure glaucoma: an evidence-based update. Ophthalmology. 2003;110:1878-1879, 1930.
4. Leibowitz HM. The red eye. N Engl J Med. 2000;343:345-351.
5. Shields SR. Managing eye disease in primary care. Part 3. When to refer for ophthalmologic care. Postgrad Med. 2000;108:99-106.
6. Awasthi P. Srivastava SN. Role of oral glycerol in glaucoma. Br J Ophthalmol. 1965;49:660-666.
7. Quigley HA. Glaucoma. Lancet. 2011;377:1367-1377.
8. Peyman GA, Rahimy MH, Fenandes ML. Effects of morphine on corneal sensitivity and epithelial wound healing: implications for topical ophthalmic analgesia. Br J Ophthalmol. 1994;78:138-141.
9. Schein OD. Contact lens abrasions and the nonophthalmologist. Am J Emerg Med. 1993;11:606-608.
10. Goldschmidt P. Effects of topical anaesthetics and fluorescein on the real-time PCR used for the diagnosis of herpesviruses and acanthamoeba keratitis. Br J Ophthalmol. 2006;90:1354-1356.
11. Benson WH, Snyder IS, Granus V, et al. Tetanus prophylaxis following ocular injuries. J Emerg Med. 1993;11:677-683.
12. DeBroff BM, Donahue SP, Caputo BJ, et al. Clinical characteristics of corneal foreign bodies and their associated culture results. CLAO J. 1994;20:128-130.
13. Turner A, Rabiu M. Patching for corneal abrasion. Cochrane Database Syst Rev. 2006;(2):CD004764.-
14. Kaiser PK. A comparison of pressure patching versus no patching for corneal abrasions due to trauma or foreign body removal. Corneal Abrasion Patching Study Group. Ophthalmology. 1995;102:1936-1942.
15. Clemons CS, Cohen EJ, Arentset JJ, et al. Pseudomonas ulcers following patching of corneal abrasions associated with contact lens wear. CLAO J. 1987;13:161-164.
16. Balcer LJ. Clinical practice. Optic neuritis. N Engl J Med. 2006;354:1273-1280.
17. De la Cruz J, Kupersmith MJ. Clinical profile of simultaneous bilateral optic neuritis in adults. Br J Ophthalmol. 2006;90:551-554.
18. Hwang JM, Lee YJ, Kim MK. Optic neuritis in Asian children. J Ped Ophthalmol Strabismus. 2002;39:26-32.
19. Lana-Peixoto MA, Andreade GC. The clinical profile of childhood optic neuritis. Arq Neuropsiquiatr. 2001;59(2-B):311-317.
20. Boomer JA, Siatkowski RM. Optic neuritis is adults and children. Semin Ophthalmol. 2003;18:174-180.
21. Lucchinetti CF, Kiers L, O’Duffy A, et al. Risk factors for developing multiple sclerosis after childhood optic neuritis. Neurology. 1997;49:1413-1418.
22. Optic Neuritis Study Group. Multiple sclerosis risk after optic neuritis: final optic neuritis treatment trial follow-up. Arch Neurol. 2008;65:727-732.
23. Wray SH. Optic neuritis. In: Principles and Practice of Ophthalmology. Albert DM, Jakobiec FA, eds. WB Saunders; Philadelphia, Pa: 1994.
24. Hickman SJ, Toosy AT, Miszkiel KA, et al. Visual recovery following acute optic neuritis—a clinical, electrophysiological and magnetic resonance imaging study. J Neurol. 2004;251:996-1005.
25. Sellebjerg F, Nielsen HS, Frederiksen JL, et al. A randomized, controlled trial of oral high-dose methylprednisolone in acute optic neuritis. Neurology. 1999;52:1479.-
26. Rosenbaum JT, Wernick R. The utility of routine screening of patients with uveitis for systemic lupus erythematosus or tuberculosis. A Bayesian analysis. Arch Ophthalmol. 1990;108:1291-1293.
27. Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509-516.
28. Darrel RW, Wagener HP, Kurland LT. Epidemiology of uveitis. Incidence and prevalence in a small urban community. Arch Ophthalmol. 1962;68:502-514.
29. Rosenbaum JT, Rahn DW. Prevalence of Lyme disease among patients with uveitis. Am J Ophthalmol. 1991;112:462-463.
30. Limberg MB. A review of bacterial keratitis and bacterial conjunctivitis. Am J Ophthalmol. 1991;112(4 suppl):2S-9S.
31. Hindman HB, Patel SB, Jun AS. Rationale for adjunctive topical corticosteroids in bacterial keratitis. Arch Ophthalmol. 2009;127:97-102.
32. Kaye SB, Rao PG, Smith G, et al. Simplifying collection of corneal specimens in cases of suspected bacterial keratitis. J Clin Microbiol. 2003;41:3192-3197.
Your patient comes in complaining of acute eye pain. Is it an ophthalmic emergency? Benign? Something in between?
The varying degrees of severity are reflected in the cases presented here, and in the multiple-choice quiz that follows. After selecting your answer to each of the questions, turn the page to find out if you were right.
CASE 1 A 74-year-old man presents with left eye pain that began when he lowered the shades in his bedroom to get ready for a nap. The pain worsened rapidly, he reports, and now—a half hour later—he has a headache, nausea, and blurred vision in the affected eye.
The patient has a history of hypertension, which is controlled with hydrochlorothiazide, but takes no other drugs. He denies any eye trauma. An eye exam reveals that the pupil is dilated and reacts poorly to light; you note corneal haziness and edema, as well ( FIGURE 1; vertical line is the slit lamp beam).
FIGURE 1
What’s your diagnosis?
- angle-closure glaucoma
- macular degeneration
- open-angle glaucoma
- retinal detachment.
CASE 2 A 20-year-old man was playing touch football while wearing contact lenses. Soon after, the game, he felt a stinging sensation in his right eye—”like sand under the eyelid,” he said. Exposure to sunlight also produced eye pain. The patient had tried flushing his eye with water, but experienced little pain relief.
An eye exam reveals a grayish-white spot in the line of vision ( FIGURE 2 ), with no sign of a penetrating injury.
FIGURE 2
What’s your next step?
- Prescribe antibiotic eye drops and send the patient home.
- Provide an emergent ophthalmology referral for foreign body extraction.
- Send the patient home with an eye patch to relieve the photophobia.
- Attempt to conduct a visual acuity test and penlight exam without anesthetic; if necessary, apply anesthetic drops and fluorescein stain.
CASE 3 A 17-year-old woman is brought in by her mother because of pain and vision loss in her right eye. The pain started as soon as she awoke; 2 hours later, the vision in the affected eye became blurry. Soon after, she saw spots of light, followed by complete loss of vision. She denies any trauma and says she has never experienced anything like this before.
The patient’s visual acuity is 20/200 in her right eye, and 20/40 in the left. You follow up with a funduscopic exam of the eye, which shows edema of the optic disc, blurring of the disc margins, and distended veins ( FIGURE 3 ).
FIGURE 3
What diagnostic test should be next?
- fluorescein angiography
- intraocular pressure measurement
- magnetic resonance imaging (MRI) of the brain
- visual fields assessment.
CASE 4 A 31-year-old woman with a history of sarcoidosis comes in because of spontaneous redness ( FIGURE 4 ) and pain in her left eye, which began early that day. She denies any eye trauma, eye surgery, or recent cold, and has no discharge or crust around the eye.
FIGURE 4
What’s your diagnosis?
- anterior uveitis
- conjunctivitis
- corneal abrasion
- posterior uveitis.
CASE 5 A 21-year-old college student complains of pain in her left eye that started a day ago. She wears contact lenses and, upon questioning, tells you that she sometimes keeps them in all night.
The patient reports that she used a friend’s steroid eye drops twice yesterday, but the drops didn’t help. The pain is worse today. It feels as if there’s something in her eye, she says, and she finds it hard to open her eye. Penlight examination ( FIGURE 5 ) reveals a branching opacity on the cornea.
FIGURE 5
What’s your next step?
- Advise the patient to stop wearing her contact lenses overnight, give her a cycloplegic, and follow up in 2 days.
- Patch the patient’s left eye, prescribe antibiotics, and tell her to return tomorrow.
- Prescribe steroid eye drops and follow up in 2 days.
- Provide an urgent referral to an ophthalmologist.
CASE 1 The answer is A: angle-closure glaucoma.
Glaucoma is the world’s leading cause of blindness,1,2 and angle-closure glaucoma comprises 10% of cases.2 In this condition, the anterior chamber angle—formed by the iris and the cornea—is narrow. When the iris dilates in low light (eg, the darkened room in which the patient had planned to nap), it folds into the narrowed angle, preventing the flow of aqueous humor and leading to an increase in intraocular pressure.3
Signs and symptoms
Patients with acute angle-closure glaucoma often develop decreased vision, halos, headaches, severe eye pain, nausea, and vomiting. If the intraocular pressure increases quickly, symptoms can have an acute and dramatic onset; if pressure builds slowly, patients may have limited or no symptoms.4
Physical findings
An eye exam will reveal conjunctival erythema, corneal edema and cloudiness, iris irregularity, a shallow anterior chamber (visible with a slit lamp), a dilated pupil (4-6 mm) that reacts sluggishly to light, and increased ocular pressure. The cupping of the optic disc seen in this patient’s retinal exam ( FIGURE 6 ) is more commonly associated with chronic open-angle glaucoma.
FIGURE 6
Management
Acute angle-closure glaucoma is an ophthalmologic emergency, and a referral to a specialist is crucial. If no ophthalmologist can see the patient within an hour of the presentation of symptoms, you’ll need to initiate topical treatment to reduce intraocular pressure and ensure that he or she is seen by a specialist as soon as possible.5 First-line treatments for acute angle-closure glaucoma are listed in TABLE 1 .5,6
In severe cases, topical therapy alone may be ineffective. If there are no contraindications, systemic medications such as acetazolamide, mannitol, glycerol, or isosorbide may be needed. Eye pressure should be checked 30 to 60 minutes after initiating systemic therapy and the patient closely monitored for adverse effects (eg, anaphylaxis, convulsions [acetazolamide]; hypotension [mannitol, isosorbide]).5
When the patient does see an ophthalmologist, the evaluation will include assessment of visual acuity, pupillary evaluation, measurement of intraocular pressure, slit lamp examination, gonioscopy to measure the angle of the anterior chamber, and a fundus exam without pupil dilation.5 Treatment may include a surgical procedure, such as paracentesis of the eye, peripheral iridotomy, or iridectomy, and chronic administration of topical medication.7
TABLE 1
Treatments for acute angle-closure glaucoma5,6
| Medication | Quantity |
|---|---|
| Topical | |
| 0.5% timolol maleate | 1 drop in affected eye 2x/d |
| 1% apraclonidine | 1-2 drops 3x/d |
| 2% pilocarpine | 1 drop 4x/d |
| Oral | |
| Acetazolamide | 500 mg 2x/d |
| Glycerol 50% solution | 1-1.8 g/kg |
| Isosorbide solution | 1.5 g/kg |
| Intravenous | |
| Acetazolamide | 250 mg 4x/d |
| Mannitol | 0.25-2 g/kg (one-time dose) |
CASE 2 The answer is D: Attempt to conduct a visual acuity test and penlight exam without anesthetic; if necessary, apply anesthetic drops and fluorescein stain.
This patient likely has a corneal ulcer or abrasion caused by a contact lens. Corneal ulcers—which constitute a loss of the corneal epithelium—can be caused by trauma or a foreign body, or develop spontaneously.
Signs and symptoms
Because of the rich innervation of the corneal epithelium by the trigeminal nerve, the cornea is highly sensitive to pain. Patients with corneal abrasions typically experience eye pain, photophobia, tearing, a foreign body sensation, and discomfort when driving, working, or reading. If a patient has damage to the trigeminal nerve, however (eg, from trauma, a tumor, or a herpes infection), corneal injuries may be painless.8
In some cases, corneal abrasions may be so painful that patients become disruptive while waiting to be seen. Thus, any patient suspected of having a corneal ulcer or abrasion should be ushered into a quiet, dark room and instructed to keep his or her eyes closed until the examination.
The patient history should focus on recent trauma, type of work, and use of protective eyewear, which may provide clues to the development of penetrating injuries, retained foreign bodies, or abrasions caused by a foreign body. Ask about the use of contact lenses, as well; corneal injury caused by contacts is associated with specific bacterial infections, most commonly staphylococcal organisms and Pseudomonas.9
Physical findings
Attempt to test visual acuity without topical anesthetics. If the patient can’t tolerate the test, a single drop of topical anesthetic (proparacaine 0.5%) may help. Visual acuity provides clues to the location of the injury. If acuity is close to baseline, the corneal abrasion is likely peripheral to the visual axis. Decreased visual acuity indicates either that the abrasion involves the central axis area or that there is corneal edema.9
Evaluation of the pupillary reflex and optic fundus should follow. Reactive miosis may be present with corneal abrasions. A large, nonreactive pupil may be a sign of injury to the pupillary sphincter from blunt or penetrating injury—an ophthalmologic emergency that requires urgent referral. The eyelid should be flipped and examined for foreign bodies. 9
Some abrasions can be seen with the naked eye, so a penlight exam should be performed before applying fluorescein stain. If a corneal lesion is suspicious for herpesvirus infection ( SEE CASE 5 ), fluorescein should not be applied, as it can interfere with the antibody test.10 Refer the patient to an ophthalmologist instead.
If there’s no evidence of infection, apply fluorescein and examine the cornea with a Wood’s lamp. A thin line or several vertical lines are suggestive of a foreign body in the cornea or under the eyelid, whereas round defects are often due to contact lenses.9 Physical findings associated with specific types of corneal abrasions or ulcers are detailed in TABLE 2 .9,11,12
TABLE 2
Corneal abrasion or ulcers: The differential diagnosis9,11,12
| Diagnosis | Physical findings | Management |
|---|---|---|
| Penetrating trauma |
|
|
| Infected corneal abrasion | Grayish edge near abrasions or ulcers |
|
| Retained foreign body |
|
|
| Herpesvirus infection | Branching pattern |
|
| Spontaneous erosions |
|
|
| *Gel-like extrusion of ocular contents seen with fluorescein. †Ointment is preferable to drops. If a contact lens caused the abrasion, a solution that covers Pseudomonas should be used. | ||
Management
Patients with corneal abrasions or ulcers should receive topical antibiotics to prevent infection. Ointments (erythromycin ointment 4 times daily for 3-5 days) are preferable to drops, but may be harder to obtain.9 If a patient must use drops, sulfacetamide 10%, polymyxin/trimethoprim, ciprofloxacin, or ofloxacin can be used, with the same frequency and duration.
Aminoglycosides are toxic to the corneal epithelium and should be avoided, except in abrasions caused by contacts.9 Because of the likelihood of pseudomonal keratitis in cases involving contact lenses, antibiotics covering Pseudomonas, such as ofloxacin, ciprofloxacin, or tobramycin, should be used.9
Pain control is achieved with cycloplegics11 like cyclopentolate 0.5% to 1% or a one-day course of systemic opioids. For children, over-the-counter analgesics for mild pain and mild opioids for severe pain may be used.
Chronic use of topical anesthetics should be avoided in patients of any age. Although they relieve pain, frequent use can lead to delayed healing, ulcerations, perforations, scarring, or even blindness.8
Patching has not been found to improve healing or comfort;13 instead, it delays healing.14,15 The “pirate patch,” which hovers over the eye, does not keep the eyelid down and therefore is not recommended.9
Follow up within 24 hours of initiating treatment to assure that the abrasion is healing. If it appears to be getting worse or is simply not improving, an immediate referral to an ophthalmologist is needed. Abrasions caused by contact with potentially infected material (eg, farm equipment, tree branches, or soil) require daily monitoring until they heal.11
CASE 3 The answer is C: Order an MRI of the brain.
This patient has optic neuritis, caused by inflammation of the optic nerve and disruption of the nerve’s myelin sheath. It predominantly affects young adults, and is more common in women than in men.16 The incidence of optic neuritis is higher among Asians, black South Africans, and children under the age of 15.17,18
Signs and symptoms
Optic neuritis is characterized by monocular (90%) or binocular (10%) complete or partial vision loss, photopsia (flashes of light), and eye pain. Up to 60% of pediatric patients present with blurred vision, bilateral involvement,19 and no pain, while adults predominantly have pain and unilateral vision loss.20 Optic neuritis is often a presenting symptom of multiple sclerosis (MS).16
Physical findings
Physical exam findings in optic neuritis include a sluggish direct light reflex, loss of visual acuity and color vision, as well as acute eye pain.18 Ophthalmoscopic exam may reveal papillitis with edema of the optic disc.21 In the Optic Neuritis Treatment Trial (ONTT), however, only one-third of patients presented with papillitis and swelling of the optic disc.22
MRI of the brain with gadolinium contrast is generally used to confirm the diagnosis. On MRI, 95% of patients with optic neuritis have signs of inflammation of the optic nerve and/or white matter changes consistent with MS (periventricular and ovoid demyelination).23,24
Patients with evidence of demyelination should also be evaluated for MS and other demyelinating disorders. In the ONTT trial, the risk of developing MS within 15 years of an optic neuritis diagnosis was as low as 25% (95% confidence interval [CI], 18%-32%) for patients with no lesions on a baseline brain MRI and as high as 72% (95% CI, 63%-81%) for those with one or more lesions on a baseline MRI, according to the study’s final follow-up.22
Management
The recommended treatment for optic neuritis is intravenous (IV) methylprednisolone 250 mg every 6 hours for 3 to 5 days, followed by oral prednisone at 1 mg/kg/d for 7 to 10 days. Vision usually returns slowly over the course of several months to a year. Ophthalmology consultation should be considered to rule out other causes of optic neuritis.25
CASE 4 The answer is A: anterior uveitis.
Uveitis is often associated with systemic disease or infection, and diagnosis is typically suspected based on a history of conditions such as sarcoidosis, juvenile idiopathic arthritis, Kawasaki’s disease, Sjögren’s syndrome, toxoplasmosis, human immunodeficiency virus (HIV), tuberculosis (TB), syphilis, herpes simplex, and herpes zoster.26
Signs and symptoms
Signs and symptoms vary depending on the part of the uveal tract that’s involved. Anterior uveitis, or iritis, is associated with pain, photophobia, redness, and a varying degree of vision loss. Posterior and intermediate uveitis are less likely to be associated with pain, but can be accompanied by decreased visual acuity and floaters.27
Physical findings
Visual acuity in patients with uveitis can range from normal to varying degrees of vision loss. Redness around the iris can be seen; conjunctival infection is most marked around the circumference of the corneal limbus rather than more peripherally, as seen in conjunctivitis. On slit lamp examination, the beam of light can be seen in the aqueous humor due to protein and leukocyte accumulation—a phenomenon known as “flare.” The pupillary light reflex may be abnormal, and the pupillary opening may be irregular rather than round due to anterior and posterior synechia.28
Management
Patients should be referred to an ophthalmologist for management of the immediate condition and to prevent or treat complications such as vision loss, optic nerve damage, and glaucoma. Acute management includes topical steroids, such as prednisolone acetate ophthalmic 1% 2 to 4 times daily, as well as treatment of the underlying condition. Long-term management varies, depending on the cause of the uveitis.26,29
If the etiology is unknown, a workup should be considered to identify inflammatory and infectious disorders that might be causing uveitis. Chest radiograph is a good beginning to look for evidence of sarcoidosis or TB; serologic testing for syphilis, HIV, and lupus may also be considered.26,29
CASE 5 The answer is D: Provide an urgent referral to an ophthalmologist.
This patient has viral keratitis caused by herpes. While the pain and foreign body sensation are the same for bacterial and viral keratitis, herpesvirus is distinguishable by the branching opacity that develops on the cornea.
Varicella zoster is the most common cause of viral keratitis, although it can also be caused by herpes simplex and adeno-virus. Because a person who is infected with herpes has the virus for life, however, multiple attacks are possible. Reactivation is associated with stress and a weakened immune system, but may occur spontaneously, as well. Patients who wear contact lenses are no more likely than those who don’t to be infected with the herpesvirus.
Bacterial keratitis is often associated with contact lenses, particularly when they’re continually worn, but also with normal wear.30 Immunosuppression, dry ocular surfaces, and topical corticosteroid use may predispose patients to bacterial keratitis, as well.12 Staphylococcus aureus, Pseudomonas aeruginosa, coagulase-negative Staphylococcus, diphtheroids, and Streptococcus pneumoniae are the most common pathogens.31
Signs and symptoms
Patients with keratitis typically complain of eye pain, a sensation of having a foreign body in the eye, photophobia, tearing, and vision changes; a mucopurulent discharge is sometimes present, as well.3 The condition is easily distinguished from conjunctivitis, which typically does not involve eye pain or vision changes.
Physical findings
Visual acuity may be affected if the lesion or corneal edema involves the visual axis. Physical exam in a patient with bacterial keratitis sometimes shows a gray or white corneal opacity, along with corneal erythema. As already noted, a penlight exam will reveal a branching opacity in patients with herpes keratitis.30
Management
Patients with keratitis should be referred immediately to an ophthalmologist32 for a slit lamp evaluation, treatment, and close follow-up.
Corneal cultures can be difficult to obtain, but before prescribing antibiotics, an attempt to collect samples should be made. This can be done—after the administration of topical anesthesia—with a sterile calcium alginate swab. Gently swab the cornea and then inoculate the appropriate gels or mediums. Avoid contact with lashes and eyelids to prevent culture contamination.32
When herpesvirus is suspected, start the patient on an antiviral agent such as trifluridine ophthalmic (1%) 9 times a day, vidarabine ophthalmic (3%) 5 times daily, or 400 mg oral acyclovir 5 times a day. Patients with bacterial keratitis should be started on antibiotic eye drops with Pseudomonas coverage, such as ofloxacin (0.3%), ciprofloxacin (0.3%), or tobramycin (0.3%), 6 to 8 times a day.9
CORRESPONDENCE Uyen Michelle Le, MD, 967 Galindo Court, Milpitas, CA 95035; mlkala21@aol.com
Your patient comes in complaining of acute eye pain. Is it an ophthalmic emergency? Benign? Something in between?
The varying degrees of severity are reflected in the cases presented here, and in the multiple-choice quiz that follows. After selecting your answer to each of the questions, turn the page to find out if you were right.
CASE 1 A 74-year-old man presents with left eye pain that began when he lowered the shades in his bedroom to get ready for a nap. The pain worsened rapidly, he reports, and now—a half hour later—he has a headache, nausea, and blurred vision in the affected eye.
The patient has a history of hypertension, which is controlled with hydrochlorothiazide, but takes no other drugs. He denies any eye trauma. An eye exam reveals that the pupil is dilated and reacts poorly to light; you note corneal haziness and edema, as well ( FIGURE 1; vertical line is the slit lamp beam).
FIGURE 1
What’s your diagnosis?
- angle-closure glaucoma
- macular degeneration
- open-angle glaucoma
- retinal detachment.
CASE 2 A 20-year-old man was playing touch football while wearing contact lenses. Soon after, the game, he felt a stinging sensation in his right eye—”like sand under the eyelid,” he said. Exposure to sunlight also produced eye pain. The patient had tried flushing his eye with water, but experienced little pain relief.
An eye exam reveals a grayish-white spot in the line of vision ( FIGURE 2 ), with no sign of a penetrating injury.
FIGURE 2
What’s your next step?
- Prescribe antibiotic eye drops and send the patient home.
- Provide an emergent ophthalmology referral for foreign body extraction.
- Send the patient home with an eye patch to relieve the photophobia.
- Attempt to conduct a visual acuity test and penlight exam without anesthetic; if necessary, apply anesthetic drops and fluorescein stain.
CASE 3 A 17-year-old woman is brought in by her mother because of pain and vision loss in her right eye. The pain started as soon as she awoke; 2 hours later, the vision in the affected eye became blurry. Soon after, she saw spots of light, followed by complete loss of vision. She denies any trauma and says she has never experienced anything like this before.
The patient’s visual acuity is 20/200 in her right eye, and 20/40 in the left. You follow up with a funduscopic exam of the eye, which shows edema of the optic disc, blurring of the disc margins, and distended veins ( FIGURE 3 ).
FIGURE 3
What diagnostic test should be next?
- fluorescein angiography
- intraocular pressure measurement
- magnetic resonance imaging (MRI) of the brain
- visual fields assessment.
CASE 4 A 31-year-old woman with a history of sarcoidosis comes in because of spontaneous redness ( FIGURE 4 ) and pain in her left eye, which began early that day. She denies any eye trauma, eye surgery, or recent cold, and has no discharge or crust around the eye.
FIGURE 4
What’s your diagnosis?
- anterior uveitis
- conjunctivitis
- corneal abrasion
- posterior uveitis.
CASE 5 A 21-year-old college student complains of pain in her left eye that started a day ago. She wears contact lenses and, upon questioning, tells you that she sometimes keeps them in all night.
The patient reports that she used a friend’s steroid eye drops twice yesterday, but the drops didn’t help. The pain is worse today. It feels as if there’s something in her eye, she says, and she finds it hard to open her eye. Penlight examination ( FIGURE 5 ) reveals a branching opacity on the cornea.
FIGURE 5
What’s your next step?
- Advise the patient to stop wearing her contact lenses overnight, give her a cycloplegic, and follow up in 2 days.
- Patch the patient’s left eye, prescribe antibiotics, and tell her to return tomorrow.
- Prescribe steroid eye drops and follow up in 2 days.
- Provide an urgent referral to an ophthalmologist.
CASE 1 The answer is A: angle-closure glaucoma.
Glaucoma is the world’s leading cause of blindness,1,2 and angle-closure glaucoma comprises 10% of cases.2 In this condition, the anterior chamber angle—formed by the iris and the cornea—is narrow. When the iris dilates in low light (eg, the darkened room in which the patient had planned to nap), it folds into the narrowed angle, preventing the flow of aqueous humor and leading to an increase in intraocular pressure.3
Signs and symptoms
Patients with acute angle-closure glaucoma often develop decreased vision, halos, headaches, severe eye pain, nausea, and vomiting. If the intraocular pressure increases quickly, symptoms can have an acute and dramatic onset; if pressure builds slowly, patients may have limited or no symptoms.4
Physical findings
An eye exam will reveal conjunctival erythema, corneal edema and cloudiness, iris irregularity, a shallow anterior chamber (visible with a slit lamp), a dilated pupil (4-6 mm) that reacts sluggishly to light, and increased ocular pressure. The cupping of the optic disc seen in this patient’s retinal exam ( FIGURE 6 ) is more commonly associated with chronic open-angle glaucoma.
FIGURE 6
Management
Acute angle-closure glaucoma is an ophthalmologic emergency, and a referral to a specialist is crucial. If no ophthalmologist can see the patient within an hour of the presentation of symptoms, you’ll need to initiate topical treatment to reduce intraocular pressure and ensure that he or she is seen by a specialist as soon as possible.5 First-line treatments for acute angle-closure glaucoma are listed in TABLE 1 .5,6
In severe cases, topical therapy alone may be ineffective. If there are no contraindications, systemic medications such as acetazolamide, mannitol, glycerol, or isosorbide may be needed. Eye pressure should be checked 30 to 60 minutes after initiating systemic therapy and the patient closely monitored for adverse effects (eg, anaphylaxis, convulsions [acetazolamide]; hypotension [mannitol, isosorbide]).5
When the patient does see an ophthalmologist, the evaluation will include assessment of visual acuity, pupillary evaluation, measurement of intraocular pressure, slit lamp examination, gonioscopy to measure the angle of the anterior chamber, and a fundus exam without pupil dilation.5 Treatment may include a surgical procedure, such as paracentesis of the eye, peripheral iridotomy, or iridectomy, and chronic administration of topical medication.7
TABLE 1
Treatments for acute angle-closure glaucoma5,6
| Medication | Quantity |
|---|---|
| Topical | |
| 0.5% timolol maleate | 1 drop in affected eye 2x/d |
| 1% apraclonidine | 1-2 drops 3x/d |
| 2% pilocarpine | 1 drop 4x/d |
| Oral | |
| Acetazolamide | 500 mg 2x/d |
| Glycerol 50% solution | 1-1.8 g/kg |
| Isosorbide solution | 1.5 g/kg |
| Intravenous | |
| Acetazolamide | 250 mg 4x/d |
| Mannitol | 0.25-2 g/kg (one-time dose) |
CASE 2 The answer is D: Attempt to conduct a visual acuity test and penlight exam without anesthetic; if necessary, apply anesthetic drops and fluorescein stain.
This patient likely has a corneal ulcer or abrasion caused by a contact lens. Corneal ulcers—which constitute a loss of the corneal epithelium—can be caused by trauma or a foreign body, or develop spontaneously.
Signs and symptoms
Because of the rich innervation of the corneal epithelium by the trigeminal nerve, the cornea is highly sensitive to pain. Patients with corneal abrasions typically experience eye pain, photophobia, tearing, a foreign body sensation, and discomfort when driving, working, or reading. If a patient has damage to the trigeminal nerve, however (eg, from trauma, a tumor, or a herpes infection), corneal injuries may be painless.8
In some cases, corneal abrasions may be so painful that patients become disruptive while waiting to be seen. Thus, any patient suspected of having a corneal ulcer or abrasion should be ushered into a quiet, dark room and instructed to keep his or her eyes closed until the examination.
The patient history should focus on recent trauma, type of work, and use of protective eyewear, which may provide clues to the development of penetrating injuries, retained foreign bodies, or abrasions caused by a foreign body. Ask about the use of contact lenses, as well; corneal injury caused by contacts is associated with specific bacterial infections, most commonly staphylococcal organisms and Pseudomonas.9
Physical findings
Attempt to test visual acuity without topical anesthetics. If the patient can’t tolerate the test, a single drop of topical anesthetic (proparacaine 0.5%) may help. Visual acuity provides clues to the location of the injury. If acuity is close to baseline, the corneal abrasion is likely peripheral to the visual axis. Decreased visual acuity indicates either that the abrasion involves the central axis area or that there is corneal edema.9
Evaluation of the pupillary reflex and optic fundus should follow. Reactive miosis may be present with corneal abrasions. A large, nonreactive pupil may be a sign of injury to the pupillary sphincter from blunt or penetrating injury—an ophthalmologic emergency that requires urgent referral. The eyelid should be flipped and examined for foreign bodies. 9
Some abrasions can be seen with the naked eye, so a penlight exam should be performed before applying fluorescein stain. If a corneal lesion is suspicious for herpesvirus infection ( SEE CASE 5 ), fluorescein should not be applied, as it can interfere with the antibody test.10 Refer the patient to an ophthalmologist instead.
If there’s no evidence of infection, apply fluorescein and examine the cornea with a Wood’s lamp. A thin line or several vertical lines are suggestive of a foreign body in the cornea or under the eyelid, whereas round defects are often due to contact lenses.9 Physical findings associated with specific types of corneal abrasions or ulcers are detailed in TABLE 2 .9,11,12
TABLE 2
Corneal abrasion or ulcers: The differential diagnosis9,11,12
| Diagnosis | Physical findings | Management |
|---|---|---|
| Penetrating trauma |
|
|
| Infected corneal abrasion | Grayish edge near abrasions or ulcers |
|
| Retained foreign body |
|
|
| Herpesvirus infection | Branching pattern |
|
| Spontaneous erosions |
|
|
| *Gel-like extrusion of ocular contents seen with fluorescein. †Ointment is preferable to drops. If a contact lens caused the abrasion, a solution that covers Pseudomonas should be used. | ||
Management
Patients with corneal abrasions or ulcers should receive topical antibiotics to prevent infection. Ointments (erythromycin ointment 4 times daily for 3-5 days) are preferable to drops, but may be harder to obtain.9 If a patient must use drops, sulfacetamide 10%, polymyxin/trimethoprim, ciprofloxacin, or ofloxacin can be used, with the same frequency and duration.
Aminoglycosides are toxic to the corneal epithelium and should be avoided, except in abrasions caused by contacts.9 Because of the likelihood of pseudomonal keratitis in cases involving contact lenses, antibiotics covering Pseudomonas, such as ofloxacin, ciprofloxacin, or tobramycin, should be used.9
Pain control is achieved with cycloplegics11 like cyclopentolate 0.5% to 1% or a one-day course of systemic opioids. For children, over-the-counter analgesics for mild pain and mild opioids for severe pain may be used.
Chronic use of topical anesthetics should be avoided in patients of any age. Although they relieve pain, frequent use can lead to delayed healing, ulcerations, perforations, scarring, or even blindness.8
Patching has not been found to improve healing or comfort;13 instead, it delays healing.14,15 The “pirate patch,” which hovers over the eye, does not keep the eyelid down and therefore is not recommended.9
Follow up within 24 hours of initiating treatment to assure that the abrasion is healing. If it appears to be getting worse or is simply not improving, an immediate referral to an ophthalmologist is needed. Abrasions caused by contact with potentially infected material (eg, farm equipment, tree branches, or soil) require daily monitoring until they heal.11
CASE 3 The answer is C: Order an MRI of the brain.
This patient has optic neuritis, caused by inflammation of the optic nerve and disruption of the nerve’s myelin sheath. It predominantly affects young adults, and is more common in women than in men.16 The incidence of optic neuritis is higher among Asians, black South Africans, and children under the age of 15.17,18
Signs and symptoms
Optic neuritis is characterized by monocular (90%) or binocular (10%) complete or partial vision loss, photopsia (flashes of light), and eye pain. Up to 60% of pediatric patients present with blurred vision, bilateral involvement,19 and no pain, while adults predominantly have pain and unilateral vision loss.20 Optic neuritis is often a presenting symptom of multiple sclerosis (MS).16
Physical findings
Physical exam findings in optic neuritis include a sluggish direct light reflex, loss of visual acuity and color vision, as well as acute eye pain.18 Ophthalmoscopic exam may reveal papillitis with edema of the optic disc.21 In the Optic Neuritis Treatment Trial (ONTT), however, only one-third of patients presented with papillitis and swelling of the optic disc.22
MRI of the brain with gadolinium contrast is generally used to confirm the diagnosis. On MRI, 95% of patients with optic neuritis have signs of inflammation of the optic nerve and/or white matter changes consistent with MS (periventricular and ovoid demyelination).23,24
Patients with evidence of demyelination should also be evaluated for MS and other demyelinating disorders. In the ONTT trial, the risk of developing MS within 15 years of an optic neuritis diagnosis was as low as 25% (95% confidence interval [CI], 18%-32%) for patients with no lesions on a baseline brain MRI and as high as 72% (95% CI, 63%-81%) for those with one or more lesions on a baseline MRI, according to the study’s final follow-up.22
Management
The recommended treatment for optic neuritis is intravenous (IV) methylprednisolone 250 mg every 6 hours for 3 to 5 days, followed by oral prednisone at 1 mg/kg/d for 7 to 10 days. Vision usually returns slowly over the course of several months to a year. Ophthalmology consultation should be considered to rule out other causes of optic neuritis.25
CASE 4 The answer is A: anterior uveitis.
Uveitis is often associated with systemic disease or infection, and diagnosis is typically suspected based on a history of conditions such as sarcoidosis, juvenile idiopathic arthritis, Kawasaki’s disease, Sjögren’s syndrome, toxoplasmosis, human immunodeficiency virus (HIV), tuberculosis (TB), syphilis, herpes simplex, and herpes zoster.26
Signs and symptoms
Signs and symptoms vary depending on the part of the uveal tract that’s involved. Anterior uveitis, or iritis, is associated with pain, photophobia, redness, and a varying degree of vision loss. Posterior and intermediate uveitis are less likely to be associated with pain, but can be accompanied by decreased visual acuity and floaters.27
Physical findings
Visual acuity in patients with uveitis can range from normal to varying degrees of vision loss. Redness around the iris can be seen; conjunctival infection is most marked around the circumference of the corneal limbus rather than more peripherally, as seen in conjunctivitis. On slit lamp examination, the beam of light can be seen in the aqueous humor due to protein and leukocyte accumulation—a phenomenon known as “flare.” The pupillary light reflex may be abnormal, and the pupillary opening may be irregular rather than round due to anterior and posterior synechia.28
Management
Patients should be referred to an ophthalmologist for management of the immediate condition and to prevent or treat complications such as vision loss, optic nerve damage, and glaucoma. Acute management includes topical steroids, such as prednisolone acetate ophthalmic 1% 2 to 4 times daily, as well as treatment of the underlying condition. Long-term management varies, depending on the cause of the uveitis.26,29
If the etiology is unknown, a workup should be considered to identify inflammatory and infectious disorders that might be causing uveitis. Chest radiograph is a good beginning to look for evidence of sarcoidosis or TB; serologic testing for syphilis, HIV, and lupus may also be considered.26,29
CASE 5 The answer is D: Provide an urgent referral to an ophthalmologist.
This patient has viral keratitis caused by herpes. While the pain and foreign body sensation are the same for bacterial and viral keratitis, herpesvirus is distinguishable by the branching opacity that develops on the cornea.
Varicella zoster is the most common cause of viral keratitis, although it can also be caused by herpes simplex and adeno-virus. Because a person who is infected with herpes has the virus for life, however, multiple attacks are possible. Reactivation is associated with stress and a weakened immune system, but may occur spontaneously, as well. Patients who wear contact lenses are no more likely than those who don’t to be infected with the herpesvirus.
Bacterial keratitis is often associated with contact lenses, particularly when they’re continually worn, but also with normal wear.30 Immunosuppression, dry ocular surfaces, and topical corticosteroid use may predispose patients to bacterial keratitis, as well.12 Staphylococcus aureus, Pseudomonas aeruginosa, coagulase-negative Staphylococcus, diphtheroids, and Streptococcus pneumoniae are the most common pathogens.31
Signs and symptoms
Patients with keratitis typically complain of eye pain, a sensation of having a foreign body in the eye, photophobia, tearing, and vision changes; a mucopurulent discharge is sometimes present, as well.3 The condition is easily distinguished from conjunctivitis, which typically does not involve eye pain or vision changes.
Physical findings
Visual acuity may be affected if the lesion or corneal edema involves the visual axis. Physical exam in a patient with bacterial keratitis sometimes shows a gray or white corneal opacity, along with corneal erythema. As already noted, a penlight exam will reveal a branching opacity in patients with herpes keratitis.30
Management
Patients with keratitis should be referred immediately to an ophthalmologist32 for a slit lamp evaluation, treatment, and close follow-up.
Corneal cultures can be difficult to obtain, but before prescribing antibiotics, an attempt to collect samples should be made. This can be done—after the administration of topical anesthesia—with a sterile calcium alginate swab. Gently swab the cornea and then inoculate the appropriate gels or mediums. Avoid contact with lashes and eyelids to prevent culture contamination.32
When herpesvirus is suspected, start the patient on an antiviral agent such as trifluridine ophthalmic (1%) 9 times a day, vidarabine ophthalmic (3%) 5 times daily, or 400 mg oral acyclovir 5 times a day. Patients with bacterial keratitis should be started on antibiotic eye drops with Pseudomonas coverage, such as ofloxacin (0.3%), ciprofloxacin (0.3%), or tobramycin (0.3%), 6 to 8 times a day.9
CORRESPONDENCE Uyen Michelle Le, MD, 967 Galindo Court, Milpitas, CA 95035; mlkala21@aol.com
1. Foster PJ, Johnson GJ. Glaucoma in China: how big is the problem? Br J Ophthalmol. 2001;85:1277-1282.
2. American Academy of Ophthalmology. Preferred Practice Pattern Guidelines. Primary angle closure PPP - October 2010. Available at: http://one.aao.org/CE/PracticeGuidelines/PPP_Content.aspx?cid=92bea8f6-5459-49a6-9233-4528343dc4c3. Accessed July 12, 2012.
3. Sau SM, Gazzard G, Friedman DS. Interventions for angle-closure glaucoma: an evidence-based update. Ophthalmology. 2003;110:1878-1879, 1930.
4. Leibowitz HM. The red eye. N Engl J Med. 2000;343:345-351.
5. Shields SR. Managing eye disease in primary care. Part 3. When to refer for ophthalmologic care. Postgrad Med. 2000;108:99-106.
6. Awasthi P. Srivastava SN. Role of oral glycerol in glaucoma. Br J Ophthalmol. 1965;49:660-666.
7. Quigley HA. Glaucoma. Lancet. 2011;377:1367-1377.
8. Peyman GA, Rahimy MH, Fenandes ML. Effects of morphine on corneal sensitivity and epithelial wound healing: implications for topical ophthalmic analgesia. Br J Ophthalmol. 1994;78:138-141.
9. Schein OD. Contact lens abrasions and the nonophthalmologist. Am J Emerg Med. 1993;11:606-608.
10. Goldschmidt P. Effects of topical anaesthetics and fluorescein on the real-time PCR used for the diagnosis of herpesviruses and acanthamoeba keratitis. Br J Ophthalmol. 2006;90:1354-1356.
11. Benson WH, Snyder IS, Granus V, et al. Tetanus prophylaxis following ocular injuries. J Emerg Med. 1993;11:677-683.
12. DeBroff BM, Donahue SP, Caputo BJ, et al. Clinical characteristics of corneal foreign bodies and their associated culture results. CLAO J. 1994;20:128-130.
13. Turner A, Rabiu M. Patching for corneal abrasion. Cochrane Database Syst Rev. 2006;(2):CD004764.-
14. Kaiser PK. A comparison of pressure patching versus no patching for corneal abrasions due to trauma or foreign body removal. Corneal Abrasion Patching Study Group. Ophthalmology. 1995;102:1936-1942.
15. Clemons CS, Cohen EJ, Arentset JJ, et al. Pseudomonas ulcers following patching of corneal abrasions associated with contact lens wear. CLAO J. 1987;13:161-164.
16. Balcer LJ. Clinical practice. Optic neuritis. N Engl J Med. 2006;354:1273-1280.
17. De la Cruz J, Kupersmith MJ. Clinical profile of simultaneous bilateral optic neuritis in adults. Br J Ophthalmol. 2006;90:551-554.
18. Hwang JM, Lee YJ, Kim MK. Optic neuritis in Asian children. J Ped Ophthalmol Strabismus. 2002;39:26-32.
19. Lana-Peixoto MA, Andreade GC. The clinical profile of childhood optic neuritis. Arq Neuropsiquiatr. 2001;59(2-B):311-317.
20. Boomer JA, Siatkowski RM. Optic neuritis is adults and children. Semin Ophthalmol. 2003;18:174-180.
21. Lucchinetti CF, Kiers L, O’Duffy A, et al. Risk factors for developing multiple sclerosis after childhood optic neuritis. Neurology. 1997;49:1413-1418.
22. Optic Neuritis Study Group. Multiple sclerosis risk after optic neuritis: final optic neuritis treatment trial follow-up. Arch Neurol. 2008;65:727-732.
23. Wray SH. Optic neuritis. In: Principles and Practice of Ophthalmology. Albert DM, Jakobiec FA, eds. WB Saunders; Philadelphia, Pa: 1994.
24. Hickman SJ, Toosy AT, Miszkiel KA, et al. Visual recovery following acute optic neuritis—a clinical, electrophysiological and magnetic resonance imaging study. J Neurol. 2004;251:996-1005.
25. Sellebjerg F, Nielsen HS, Frederiksen JL, et al. A randomized, controlled trial of oral high-dose methylprednisolone in acute optic neuritis. Neurology. 1999;52:1479.-
26. Rosenbaum JT, Wernick R. The utility of routine screening of patients with uveitis for systemic lupus erythematosus or tuberculosis. A Bayesian analysis. Arch Ophthalmol. 1990;108:1291-1293.
27. Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509-516.
28. Darrel RW, Wagener HP, Kurland LT. Epidemiology of uveitis. Incidence and prevalence in a small urban community. Arch Ophthalmol. 1962;68:502-514.
29. Rosenbaum JT, Rahn DW. Prevalence of Lyme disease among patients with uveitis. Am J Ophthalmol. 1991;112:462-463.
30. Limberg MB. A review of bacterial keratitis and bacterial conjunctivitis. Am J Ophthalmol. 1991;112(4 suppl):2S-9S.
31. Hindman HB, Patel SB, Jun AS. Rationale for adjunctive topical corticosteroids in bacterial keratitis. Arch Ophthalmol. 2009;127:97-102.
32. Kaye SB, Rao PG, Smith G, et al. Simplifying collection of corneal specimens in cases of suspected bacterial keratitis. J Clin Microbiol. 2003;41:3192-3197.
1. Foster PJ, Johnson GJ. Glaucoma in China: how big is the problem? Br J Ophthalmol. 2001;85:1277-1282.
2. American Academy of Ophthalmology. Preferred Practice Pattern Guidelines. Primary angle closure PPP - October 2010. Available at: http://one.aao.org/CE/PracticeGuidelines/PPP_Content.aspx?cid=92bea8f6-5459-49a6-9233-4528343dc4c3. Accessed July 12, 2012.
3. Sau SM, Gazzard G, Friedman DS. Interventions for angle-closure glaucoma: an evidence-based update. Ophthalmology. 2003;110:1878-1879, 1930.
4. Leibowitz HM. The red eye. N Engl J Med. 2000;343:345-351.
5. Shields SR. Managing eye disease in primary care. Part 3. When to refer for ophthalmologic care. Postgrad Med. 2000;108:99-106.
6. Awasthi P. Srivastava SN. Role of oral glycerol in glaucoma. Br J Ophthalmol. 1965;49:660-666.
7. Quigley HA. Glaucoma. Lancet. 2011;377:1367-1377.
8. Peyman GA, Rahimy MH, Fenandes ML. Effects of morphine on corneal sensitivity and epithelial wound healing: implications for topical ophthalmic analgesia. Br J Ophthalmol. 1994;78:138-141.
9. Schein OD. Contact lens abrasions and the nonophthalmologist. Am J Emerg Med. 1993;11:606-608.
10. Goldschmidt P. Effects of topical anaesthetics and fluorescein on the real-time PCR used for the diagnosis of herpesviruses and acanthamoeba keratitis. Br J Ophthalmol. 2006;90:1354-1356.
11. Benson WH, Snyder IS, Granus V, et al. Tetanus prophylaxis following ocular injuries. J Emerg Med. 1993;11:677-683.
12. DeBroff BM, Donahue SP, Caputo BJ, et al. Clinical characteristics of corneal foreign bodies and their associated culture results. CLAO J. 1994;20:128-130.
13. Turner A, Rabiu M. Patching for corneal abrasion. Cochrane Database Syst Rev. 2006;(2):CD004764.-
14. Kaiser PK. A comparison of pressure patching versus no patching for corneal abrasions due to trauma or foreign body removal. Corneal Abrasion Patching Study Group. Ophthalmology. 1995;102:1936-1942.
15. Clemons CS, Cohen EJ, Arentset JJ, et al. Pseudomonas ulcers following patching of corneal abrasions associated with contact lens wear. CLAO J. 1987;13:161-164.
16. Balcer LJ. Clinical practice. Optic neuritis. N Engl J Med. 2006;354:1273-1280.
17. De la Cruz J, Kupersmith MJ. Clinical profile of simultaneous bilateral optic neuritis in adults. Br J Ophthalmol. 2006;90:551-554.
18. Hwang JM, Lee YJ, Kim MK. Optic neuritis in Asian children. J Ped Ophthalmol Strabismus. 2002;39:26-32.
19. Lana-Peixoto MA, Andreade GC. The clinical profile of childhood optic neuritis. Arq Neuropsiquiatr. 2001;59(2-B):311-317.
20. Boomer JA, Siatkowski RM. Optic neuritis is adults and children. Semin Ophthalmol. 2003;18:174-180.
21. Lucchinetti CF, Kiers L, O’Duffy A, et al. Risk factors for developing multiple sclerosis after childhood optic neuritis. Neurology. 1997;49:1413-1418.
22. Optic Neuritis Study Group. Multiple sclerosis risk after optic neuritis: final optic neuritis treatment trial follow-up. Arch Neurol. 2008;65:727-732.
23. Wray SH. Optic neuritis. In: Principles and Practice of Ophthalmology. Albert DM, Jakobiec FA, eds. WB Saunders; Philadelphia, Pa: 1994.
24. Hickman SJ, Toosy AT, Miszkiel KA, et al. Visual recovery following acute optic neuritis—a clinical, electrophysiological and magnetic resonance imaging study. J Neurol. 2004;251:996-1005.
25. Sellebjerg F, Nielsen HS, Frederiksen JL, et al. A randomized, controlled trial of oral high-dose methylprednisolone in acute optic neuritis. Neurology. 1999;52:1479.-
26. Rosenbaum JT, Wernick R. The utility of routine screening of patients with uveitis for systemic lupus erythematosus or tuberculosis. A Bayesian analysis. Arch Ophthalmol. 1990;108:1291-1293.
27. Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509-516.
28. Darrel RW, Wagener HP, Kurland LT. Epidemiology of uveitis. Incidence and prevalence in a small urban community. Arch Ophthalmol. 1962;68:502-514.
29. Rosenbaum JT, Rahn DW. Prevalence of Lyme disease among patients with uveitis. Am J Ophthalmol. 1991;112:462-463.
30. Limberg MB. A review of bacterial keratitis and bacterial conjunctivitis. Am J Ophthalmol. 1991;112(4 suppl):2S-9S.
31. Hindman HB, Patel SB, Jun AS. Rationale for adjunctive topical corticosteroids in bacterial keratitis. Arch Ophthalmol. 2009;127:97-102.
32. Kaye SB, Rao PG, Smith G, et al. Simplifying collection of corneal specimens in cases of suspected bacterial keratitis. J Clin Microbiol. 2003;41:3192-3197.
Next steps when BP won’t come down
• Review the family history of patients who do not respond to appropriate antihypertensive therapy, targeting hypertension and inherited disorders associated with high blood pressure (BP). B
• Include obstructive sleep apnea in the differential diagnosis of patients with resistant hypertension, particularly if they’re obese. B
• Include a thorough medication history in a work-up for resistant hypertension, as a number of drugs can cause or exacerbate high BP. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
What to include in the workup
Whether you’re doing an initial evaluation of a patient with high blood pressure (BP) or examining a patient with resistant hypertension, the history should focus on the duration of hypertension, previous BP levels, and comorbid conditions. It is also important to take a targeted family history, inquiring about hypertension as well as genetic disorders that increase the likelihood of secondary hypertension.
Inherited diseases associated with secondary hypertension include polycystic kidney disease, multiple endocrine neoplasia type 2 (MEN2), and von Hippel-Lindau syndrome.12,13 All are inherited in an autosomal dominant pattern. Patients with von Hippel-Lindau syndrome may present with multiple tumors, which can develop in the eyes, brain, adrenal glands, pancreas, liver, spinal cord, kidneys, or other parts of the body. Pheochromocytoma is a manifestation of both MEN2 and von Hippel-Lindau syndrome, and some specialists recommend that everyone with a family history of either condition undergo screening for pheochromocytoma.14
Table
Secondary hypertension: What you’ll see, what to test for8-11
| Secondary cause* | Signs and symptoms | Screening tests |
|---|---|---|
| Renal disease | Depends on underlying cause (eg, diabetes, polycystic kidney disease, glomerulonephritis) | Serum creatinine, urinalysis, renal ultrasound |
| Renal artery stenosis | Abdominal or flank bruits | Renal ultrasound, MRA, CT angiography |
| Primary hyperaldosteronism | Muscle cramps | PA/PRA |
| Pheochromocytoma | Paroxysms of palpitations, diaphoresis, headaches | Plasma metanephrine and normetanephrine |
| Cushing’s syndrome | Rapid weight gain, truncal obesity, abdominal striae | Measurement of 24-hour urinary free cortisol |
| OSA† | Obesity, daytime somnolence, nighttime snoring | Overnight polysomnography |
| Coarctation of the aorta‡ | Murmur of anterior and posterior thorax; claudication and weak femoral pulses | Echocardiography |
| CT, computed tomography; MRA, magnetic resonance angiography; OSA, obstructive sleep apnea; PA/PRA, plasma aldosterone-plasma renin activity. *Secondary hypertension may also be drug-induced, related to pregnancy (hypertension complicates up to 15% of pregnancies), or associated with inherited syndromes. †Highly prevalent in obese patients. ‡Higher prevalence in childhood hypertension; rarely diagnosed in adulthood. | ||
BP measurement is key
The physical examination should start with a calculation of body mass index, as well as a careful measurement of BP. The patient should be seated quietly in a chair for ≥5 minutes, with both feet on the floor and the arm being tested supported at heart level.
Unfortunately, reliability on the office BP measurement can be a confounding factor in the diagnosis of hypertension. “White coat hypertension”—in which BP is persistently elevated in the office and persistently normal in nonclinical settings—should be considered in patients who have high BP but no other signs or symptoms, and ambulatory monitoring used to rule out hypertension.15,16
Physicians also need to consider the opposite effect: Masked hypertension, characterized by normal office readings and elevated ambulatory readings, is more serious, of course, with patients at higher risk for end organ damage from unrecognized hypertension.17,18 Asking patients who self-monitor what type of BP readings they’re getting can be helpful in identifying masked hypertension. Ambulatory monitoring may be used to identify this condition, as well.
Other components in the physical workup include a fundoscopic exam; assessment of the thorax for murmurs and the abdomen for enlarged kidneys, masses, and abnormal aortic pulsation; auscultation for abdominal and carotid bruits; palpation of the thyroid gland; and palpation of the lower extremities for edema and pulses.
Include these tests in the workup
Routine tests for a patient with hypertension include:
- electrocardiogram
- blood glucose and hematocrit
- serum potassium, creatinine, and fasting lipid profiles
- urinalysis with measurement of microalbumin.
Microalbuminuria, a sensitive marker of early renal disease, is defined as a urinary albumin excretion between 30 and 300 mg/d.19 The albumin-creatinine ratio (30-300 mcg/mg), measured in spot urine specimens, is a more convenient way to detect it.20
Suspicious findings prompt further testing. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) recommends specific testing—much of it detailed below—if any aspect of the initial evaluation raises suspicion of a secondary cause or the patient has hypertension that’s of sudden onset or hard to control.21 (According to the National Heart, Lung, and Blood Institute, JNC 8 is due for release later this year.)
Kidney disease may be a consequence or a cause
The overall prevalence of hypertension in patients with renal disease is 60%,22 but varies according to the type of nephropathy. Eighty-seven percent of patients with diabetic nephropathy also have hypertension, and hypertension and diabetes are the 2 most common causes of end-stage renal disease.23,24
A combination of 2 or more drugs is usually needed to achieve the target BP of <130/80 mm Hg in patients with diabetes.21 ACE inhibitors and angiotensin receptor blockers have been found to slow the progression of diabetic nephropathy.25-27
Is renal artery stenosis to blame?
Renal artery stenosis is the most common form of secondary hypertension that’s reversible, affecting about 0.2% to 3.1% of hypertensive patients.5,6,28 The condition leads to renal ischemia, thereby stimulating the renin-angiotensin-aldosterone axis and causing secondary hyperaldosteronism.
In younger patients, especially women between 15 and 50 years of age, fibromuscular disease is the most common cause of renovascular hypertension.29,30 In older patients, atherosclerosis (which accounts for 90% of renovascular hypertension) is more likely.29,30
The choice of initial imaging tests includes duplex renal ultrasonography, magnetic resonance angiography (MRA), and spiral computed tomographic angiography. Contrast angiography is the gold standard, but it carries a risk of contrast-induced nephropathy. Duplex ultrasonography and MRA do not use iodinated contrast media, and are safe for patients with chronic kidney disease.8
Treatment. Percutaneous transluminal renal artery angioplasty is a treatment option for patients with renal artery stenosis. Angioplasty achieves higher cure rates for patients with fibromuscular dysplasia than for those with atherosclerotic renal artery stenosis.31 Most patients referred for renal artery revascularization have atherosclerosis. Because they’re generally older individuals with comorbidities, the benefits of stent revascularization for this group is controversial. Such patients require antihypertensive therapy with drugs that block the renin-angiotensin system.32
Endocrine disorders must be ruled out
Primary hyperaldosteronism is thought to be present in 0.3% to 1.4% of patients with hypertension.5,6 The prevalence varies widely from one source to another, however, and may be as high as 5% to 20% among patients with resistant hypertension.33,34
Hyperaldosteronism is related to either an aldosterone-secreting adrenal adenoma (in about 40% of cases) or bilateral adrenal hyperplasia (in the remaining 60%), and leads to increased sodium reabsorption and, typically, to a loss of potassium.35
Renin-secreting tumor, which usually arises from the juxtaglomerular cells of the kidney, is a rare cause of hyperaldosteronism. Extrarenal renin-secreting tumors have also been reported.36
What should raise your suspicion. Suspect hyperaldosteronism in patients who have both hypertension and hypokalemia, but keep in mind that not all patients with hyperaldosteronism have low serum potassium.37 Further laboratory evaluation should include a simultaneous measurement of plasma aldosterone (PA) and plasma renin activity (PRA). Patients with hyperaldosteronism will have elevated PA and suppressed PRA.
Testing considerations. It is important to ensure that the PA/PRA test is performed in the morning, with the patient in an upright position.36 He or she should be on a high sodium diet in preparation for the test, consuming 2 to 3 grams of sodium per meal for ≥2 days.37
In patients with a positive PA/PRA ratio (≥20), a 24-hour urinary aldosterone excretion test should be performed. A finding >12 to 14 mcg, along with a PRA <1.0 ng/mL per hour, confirms the diagnosis of primary hyperaldosteronism.18,37 Computed tomography or magnetic resonance imaging of the adrenal glands will distinguish between aldosterone-producing adenoma and bilateral adrenal hyperplasia.
Treatment. Laparoscopic adrenalectomy is the accepted surgical treatment of primary hyperaldosteronism.37 Patients with bilateral disease due to idiopathic hyperaldosteronism are not candidates for surgery and should be treated medically, with potassium-sparing diuretics such as spironolactone.
Cushing’s syndrome is marked by rapid weight gain
High BP may be a manifestation of Cushing’s syndrome, which affects 0.1% to 0.5% of patients with hypertension.5-7 Other signs and symptoms of Cushing’s syndrome include fatigue, weakness, hirsutism, amenorrhea, moon facies, dorsal hump, purple striae, truncal obesity, and hypokalemia. Rapid weight gain is the most common manifestation, and typically the one for which patients seek medical attention.38
The most widely used screening test for Cushing’s syndrome is a 24-hour urine collection measuring urinary-free cortisol.9 Normal urinary cortisol excretion is 20 to 100 mcg/dL in 24 hours; most patients with Cushing’s syndrome produce >250 mcg/dL in that time frame.9
Once hypercortisolism is established, determination of the cause is the next step. A serum adrenocorticotropic hormone (ACTH) level is needed to localize it. Normal (9-52 pg/mL) or elevated ACTH indicates a pituitary or ectopic source, while low levels of ACTH are an indication of an adrenal source.9,39
Treatment. Surgical resection of the tumor is often curative. For pituitary tumors (Cushing’s disease), transsphenoidal resection is the standard of care.39 For adrenal adenomas, unilateral adrenalectomy is the best option.39
Pheochromocytomas: Most are adrenal, sporadic, and benign
Pheochromocytomas—neuroendocrine, catecholamine-secreting tumors that develop from the adrenal medulla—are another cause of secondary hypertension. Catecholamines include norepinephrine and epinephrine and, rarely, dopamine secreted either intermittently or continuously. The prevalence of pheochromocytoma is 0.1% to 0.3% among patients with hypertension.5,6,28 A “rule of 10” (90:10 ratio) is often applied to pheochromocytomas because of the following:
- 90% of pheochromocytomas are located in the adrenal glands; the remaining 10% are extra-adrenal and can occur anywhere along the sympathetic chain40
- 90% are sporadic; 10% are familial41
- 90% are benign; 10% are malignant40
- 90% are found in adults; 10% affect children.42
Signs and symptoms of pheochromocytomas include palpitations, headache, dyspnea, diaphoresis, and flushing, as well as paroxysmal hypertension.40 Measurement of 24-hour urinary catecholamines and their metabolites has been the screening test of choice,43 but recent evidence suggests that measurement of plasma metanephrine and normetanephrine is a far more sensitive screen.10
Treatment. Surgical resection is the treatment of choice. Alpha blockade is started 7 to 10 days preoperatively;44,45 a beta-blocker is added only after an adequate alpha blockade has been established, as unopposed alpha stimulation could precipitate a hypertensive crisis. Laparoscopic adrenalectomy is routinely performed for small (<5 cm) pheochromocytomas.46,47
Don’t forget these (relatively) common secondary causes
Obstructive sleep apnea (OSA) is one of the most common conditions associated with resistant hypertension.48 Signs and symptoms include snoring, daytime somnolence, and obesity (body mass index ≥30 kg/m2).
OSA involves upper airway collapse during inspiration, causing intermittent hypoxemia with resultant sympathetic nervous system activation.11 The underlying mechanism of OSA-induced hypertension is strongly related to sympathetic activation.49 Overnight polysomnography is required for diagnosis.11
Continuous positive airway pressure is the treatment of choice for patients unable to lose weight.11
Pregnancy-induced hypertension is the most common medical problem encountered in pregnancy. It occurs in up to 15% of pregnancies, either during the pregnancy itself or postpartum. Postpartum hypertension may be related to preexisting chronic hypertension or to the persistence of gestational hypertension or preeclampsia, which usually occurs after 20 weeks’ gestation and is characterized by the presence of hypertension and proteinuria.50 Methyldopa and labetalol are commonly used treatments for hypertension during pregnancy.51
Drug-induced hypertension. Several drugs can cause or exacerbate hypertension, rendering it resistant to therapy. A careful review of the patient’s medication regimen is essential. Generally, drug-induced hypertension falls into 2 broad categories based on mechanism of action: volume overload and sympathetic activity.52,53
Corticosteroids can elevate BP in a dose-dependent manner, as a result of volume overload. Glycyrrhizic acid, the main ingredient in licorice, produces a state of excess mineralocorticoid, with a similar effect. Estrogen-containing oral contraceptives can cause an increased synthesis of angiotensin in the liver, resulting in greater aldosterone secretion and volume overload.
Drugs that stimulate sympathetic activity include cocaine, ephedrine, amphetamine, phenylephrine, phenylpropanolamine, caffeine, and alcohol. Nonsteroidal anti-inflammatory drugs may interfere with the action of ACE inhibitors and cause renal vasoconstriction, leading to sodium and water retention and hypertension.54
Discontinuation of the medication in question is preferable. In many cases, an agent that does not affect BP can be found to replace it.
If the patient is a child
Hypertension is uncommon in young people. However, coarctation of the aorta, a congenital narrowing associated with secondary hypertension, is typically diagnosed in childhood. In rare cases, the condition remains undetected well into adulthood.55 Clinical signs include weak femoral pulses, visible pulsations in the neck, a systolic murmur of the anterior and posterior thorax, and elevated BP in the upper extremities with low BP in the lower extremities.
Thus, once hypertension is confirmed in a young patient, BP should be measured in both arms and legs.56 Delayed or absent femoral pulses and a difference in systolic BP of ≥20 mm Hg between the arms and legs provide evidence of aortic coarctation.57 In adults, stenting is the initial treatment for this condition because of the morbidity associated with surgery.57 Stenting is an option for children with this condition, as well.58
CORRESPONDENCE Bernard M. Karnath, MD, University of Texas Medical Branch at Galveston, 301 University Boulevard, Galveston, TX 77555; bmkarnat@utmb.edu
1. Middleton K, Hing E, Xu J. National hospital ambulatory medical care survey: 2005 outpatient department summary. Adv Data. 2007;389:1-34.
2. Ong KL, Cheung BM, Man YB, et al. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999-2004. Hypertension. 2007;49:69-75.
3. Fields LE, Burt VL, Cutler JA, et al. The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension. 2004;44:398-404.
4. Wang TJ, Vasan RS. Epidemiology of uncontrolled hypertension in the United States. Circulation. 2005;112:1651-1662.
5. Anderson GH, Jr, Blakeman N, Streeten DH. The effect of age on prevalence of secondary forms of hypertension in 4429 consecutively referred patients. J Hypertens. 1994;12:609-615.
6. Sinclair AM, Isles CG, Brown I, et al. Secondary hypertension in a blood pressure clinic. Arch Intern Med. 1987;147:1289-1293.
7. Dosh SA. The diagnosis of essential and secondary hypertension in adults. J Fam Pract. 2001;50:707-712.
8. Eardley KS, Lipkin GW. Atherosclerotic renal artery stenosis: is it worth diagnosing?J Hum Hypertens. 1999;13:217-220.
9. Boscaro M, Barzon L, Fallo F, et al. Cushing’s syndrome. Lancet. 2001;357:783-791.
10. Unger N, Pitt C, Schmidt IL. Diagnostic value of various biochemical parameters for the diagnosis of pheochromocytoma in patients with adrenal mass. Eur J Endocrinol. 2006;154:409-417.
11. Prisant LM, Dillard TA, Blanchard AR. Obstructive sleep apnea syndrome. J Clin Hypertens. 2006;8:746-750.
12. Marini F, Falchetti A, Del Monte F, et al. Multiple endocrine neoplasia type 2. Orphanet J Rare Dis. 2006;1:45.-
13. Bryant J, Farmer J, Kessler LJ, et al. Pheochromocytoma: the expanding genetic differential diagnosis. J Natl Cancer Inst. 2003;95:1196-1204.
14. Neumann HP, Berger DP, Sigmund G, et al. Pheochromocytomas, multiple endocrine neoplasia type 2, and von Hippel-Lindau disease. N Engl J Med. 1993;329:1531-1538.
15. Mancia G, Bertinieri G, Grassi G, et al. Effects of blood-pressure measurement by the doctor on patient’s blood pressure and heart rate. Lancet. 1983;2:695-698.
16. Pickering TG, James GD, Boddie C. How common is white coat hypertension? JAMA. 1988;259:225-228.
17. Kotsis V, Stabouli S, Toumanidis S, et al. Target organ damage in “white coat hypertension” and “masked hypertension”. Am J Hypertens. 2008;21:393-399.
18. Pickering TG, Davidson K, Gerin W, et al. Masked hypertension. Hypertension. 2002;40:795-796.
19. Volpe M. Microalbuminuria screening in patients with hypertension: recommendations for clinical practice. Int J Clin Pract. 2008;62:97-108.
20. Keane WF, Eknoyan G. Proteinuria, albuminuria, risk, assessment, detection, elimination (PARADE): a position paper of the National Kidney Foundation. Am J Kidney Dis. 1999;33:1004-1010.
21. Chobanian AV, Bakris GL, Black HR, et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206-1252.
22. Ridao N, Luño J, García de Vinuesa S, et al. Prevalence of hypertension in renal disease. Nephrol Dial Transplant. 2001;16(suppl 1):S70-S73.
23. Foley RN, Collins AJ. End-stage renal disease in the United States: an update from the United States Renal Data System. J Am Soc Nephrol. 2007;18:2644-2648.
24. Parmar MS. Chronic renal disease. BMJ. 2002;325:85-90.
25. Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861-869.
26. Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851-860.
27. Menne J, Izzo JL, Jr, Ito S, et al. Prevention of microalbuminuria in patients with type 2 diabetes and hypertension. J Hypertens. 2012;30:811-818.
28. Rossi H, Kim A, Prinz RA. Primary hyperaldosteronism in the era of laparoscopic adrenalectomy. Am Surg. 2002;68:253-256.
29. Safian RD, Textor SC. Renal artery stenosis. N Engl J Med. 2001;244:431-442.
30. Slovut DP, Olin JW. Fibromuscular dysplasia. N Engl J Med. 2004;350:1862-1871.
31. Bonelli FS, McKusick MA, Textor SC. Renal artery angioplasty: technical results and clinical outcome in 320 patients. Mayo Clin Proc. 1995;70:1041-1052.
32. Textor SC. Renovascular hypertension in 2007: where are we now? Curr Cardiol Rep. 2007;9:453-461.
33. Calhoun DA. Is there an unrecognized epidemic of primary aldosteronism? Pro. Hypertension. 2007;50:447-453.
34. Young WF, Jr. Minireview: primary aldosteronism—changing concepts in diagnosis and treatment. Endocrinology. 2003;144:2208-2213.
35. Young WF. Primary aldosteronism: renaissance of a syndrome. Clin Endocrinol (Oxf). 2007;66:607-618.
36. Pursell RN, Quinlan PM. Secondary hypertension due to a renin-producing teratoma. Am J Hypertens. 2003;16:592-595.
37. Ganguly A. Primary aldosteronism. N Engl J Med. 1998;339:1828-1834.
38. Muller M, Longo Mazzuco T, Martinie M, et al. Diagnosis of Cushing’s syndrome: a retrospective evaluation of clinical practice. Eur J Intern Med. 2006;17:334-338.
39. Norton JA, Li M, Gillary J, et al. Cushing’s syndrome. Curr Probl Surg. 2001;38:488-545.
40. Lenders JW, Eisenhofer G, Mannelli M, et al. Phaeochromocytoma. Lancet. 2005;366:665-675.
41. Bryant J, Farmer J, Kessler LJ, et al. Pheochromocytoma: the expanding genetic differential diagnosis. J Natl Cancer Inst. 2003;95:1196-1204.
42. Sullivan J, Groshong T, Tobias JD. Presenting signs and symptoms of pheochromocytoma in pediatric-aged patients. Clin Pediatr. 2005;44:715-719.
43. Young WF, Jr. Pheochromocytoma: issues in diagnosis and treatment. Compr Ther. 1997;23:319-326.
44. Kocak S, Aydintug S, Canakci N. Alpha blockade in preoperative preparation of patients with pheochromocytomas. Int Surg. 2002;87:191-194.
45. Russell WJ, Metcalfe IR, Tonkin AL, et al. The preoperative management of phaeochromocytoma. Anaesth Intensive Care. 1998;26:196-200.
46. Kalady MF, McKinlay R, Olson JA, Jr, et al. Laparoscopic adrenalectomy for pheochromocytoma. A comparison to aldosteronoma and incidentaloma. Surg Endosc. 2004;18:621-625.
47. Naya Y, Ichikawa T, Suzuki H, et al. Efficacy and safety of laparoscopic surgery for pheochromocytoma. Int J Urol. 2005;12:128-133.
48. Pedrosa RP, Drager LF, Gonzaga CC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension. 2011;5:811-817.
49. Sharabi Y, Dagan Y, Grossman E. Sleep apnea as a risk factor for hypertension. Curr Opin Nephrol Hypertens. 2004;13:359-364.
50. James PR, Nelson-Piercy C. Management of hypertension before, during, and after pregnancy. Heart. 2004;90:1499-1504.
51. Solomon CG, Seely EW. Hypertension in pregnancy. Endocrinol Metab Clin North Am. 2011;40:847-863.
52. Grossman E, Messerli FH. Drug-induced hypertension: an unappreciated cause of secondary hypertension. Am J Med. 2012;125:14-22.
53. Rossi GP, Seccia TM, Maniero C, et al. Drug-related hypertension and resistance to antihypertensive treatment: a call for action. J Hypertens. 2011;29:2295-2309.
54. Grossman E, Messerli FH. Secondary hypertension: interfering substances. J Clin Hypertens. 2008;10:556-566.
55. Cicek D, Haberal C, Ozkan S, et al. A severe coarctation of aorta in a 52-year-old male: a case report. Int J Med Sci. 2010;7:340-341.
56. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 suppl 4th Report):S555-S576.
57. Rao PS. Coarctation of the aorta. Curr Cardiol Rep. 2005;7:425-434.
58. Rao PS. Stents in the management of aortic coarctation in young children. JACC Cardiovasc Interv. 2009;2:884-886.
• Review the family history of patients who do not respond to appropriate antihypertensive therapy, targeting hypertension and inherited disorders associated with high blood pressure (BP). B
• Include obstructive sleep apnea in the differential diagnosis of patients with resistant hypertension, particularly if they’re obese. B
• Include a thorough medication history in a work-up for resistant hypertension, as a number of drugs can cause or exacerbate high BP. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
What to include in the workup
Whether you’re doing an initial evaluation of a patient with high blood pressure (BP) or examining a patient with resistant hypertension, the history should focus on the duration of hypertension, previous BP levels, and comorbid conditions. It is also important to take a targeted family history, inquiring about hypertension as well as genetic disorders that increase the likelihood of secondary hypertension.
Inherited diseases associated with secondary hypertension include polycystic kidney disease, multiple endocrine neoplasia type 2 (MEN2), and von Hippel-Lindau syndrome.12,13 All are inherited in an autosomal dominant pattern. Patients with von Hippel-Lindau syndrome may present with multiple tumors, which can develop in the eyes, brain, adrenal glands, pancreas, liver, spinal cord, kidneys, or other parts of the body. Pheochromocytoma is a manifestation of both MEN2 and von Hippel-Lindau syndrome, and some specialists recommend that everyone with a family history of either condition undergo screening for pheochromocytoma.14
Table
Secondary hypertension: What you’ll see, what to test for8-11
| Secondary cause* | Signs and symptoms | Screening tests |
|---|---|---|
| Renal disease | Depends on underlying cause (eg, diabetes, polycystic kidney disease, glomerulonephritis) | Serum creatinine, urinalysis, renal ultrasound |
| Renal artery stenosis | Abdominal or flank bruits | Renal ultrasound, MRA, CT angiography |
| Primary hyperaldosteronism | Muscle cramps | PA/PRA |
| Pheochromocytoma | Paroxysms of palpitations, diaphoresis, headaches | Plasma metanephrine and normetanephrine |
| Cushing’s syndrome | Rapid weight gain, truncal obesity, abdominal striae | Measurement of 24-hour urinary free cortisol |
| OSA† | Obesity, daytime somnolence, nighttime snoring | Overnight polysomnography |
| Coarctation of the aorta‡ | Murmur of anterior and posterior thorax; claudication and weak femoral pulses | Echocardiography |
| CT, computed tomography; MRA, magnetic resonance angiography; OSA, obstructive sleep apnea; PA/PRA, plasma aldosterone-plasma renin activity. *Secondary hypertension may also be drug-induced, related to pregnancy (hypertension complicates up to 15% of pregnancies), or associated with inherited syndromes. †Highly prevalent in obese patients. ‡Higher prevalence in childhood hypertension; rarely diagnosed in adulthood. | ||
BP measurement is key
The physical examination should start with a calculation of body mass index, as well as a careful measurement of BP. The patient should be seated quietly in a chair for ≥5 minutes, with both feet on the floor and the arm being tested supported at heart level.
Unfortunately, reliability on the office BP measurement can be a confounding factor in the diagnosis of hypertension. “White coat hypertension”—in which BP is persistently elevated in the office and persistently normal in nonclinical settings—should be considered in patients who have high BP but no other signs or symptoms, and ambulatory monitoring used to rule out hypertension.15,16
Physicians also need to consider the opposite effect: Masked hypertension, characterized by normal office readings and elevated ambulatory readings, is more serious, of course, with patients at higher risk for end organ damage from unrecognized hypertension.17,18 Asking patients who self-monitor what type of BP readings they’re getting can be helpful in identifying masked hypertension. Ambulatory monitoring may be used to identify this condition, as well.
Other components in the physical workup include a fundoscopic exam; assessment of the thorax for murmurs and the abdomen for enlarged kidneys, masses, and abnormal aortic pulsation; auscultation for abdominal and carotid bruits; palpation of the thyroid gland; and palpation of the lower extremities for edema and pulses.
Include these tests in the workup
Routine tests for a patient with hypertension include:
- electrocardiogram
- blood glucose and hematocrit
- serum potassium, creatinine, and fasting lipid profiles
- urinalysis with measurement of microalbumin.
Microalbuminuria, a sensitive marker of early renal disease, is defined as a urinary albumin excretion between 30 and 300 mg/d.19 The albumin-creatinine ratio (30-300 mcg/mg), measured in spot urine specimens, is a more convenient way to detect it.20
Suspicious findings prompt further testing. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) recommends specific testing—much of it detailed below—if any aspect of the initial evaluation raises suspicion of a secondary cause or the patient has hypertension that’s of sudden onset or hard to control.21 (According to the National Heart, Lung, and Blood Institute, JNC 8 is due for release later this year.)
Kidney disease may be a consequence or a cause
The overall prevalence of hypertension in patients with renal disease is 60%,22 but varies according to the type of nephropathy. Eighty-seven percent of patients with diabetic nephropathy also have hypertension, and hypertension and diabetes are the 2 most common causes of end-stage renal disease.23,24
A combination of 2 or more drugs is usually needed to achieve the target BP of <130/80 mm Hg in patients with diabetes.21 ACE inhibitors and angiotensin receptor blockers have been found to slow the progression of diabetic nephropathy.25-27
Is renal artery stenosis to blame?
Renal artery stenosis is the most common form of secondary hypertension that’s reversible, affecting about 0.2% to 3.1% of hypertensive patients.5,6,28 The condition leads to renal ischemia, thereby stimulating the renin-angiotensin-aldosterone axis and causing secondary hyperaldosteronism.
In younger patients, especially women between 15 and 50 years of age, fibromuscular disease is the most common cause of renovascular hypertension.29,30 In older patients, atherosclerosis (which accounts for 90% of renovascular hypertension) is more likely.29,30
The choice of initial imaging tests includes duplex renal ultrasonography, magnetic resonance angiography (MRA), and spiral computed tomographic angiography. Contrast angiography is the gold standard, but it carries a risk of contrast-induced nephropathy. Duplex ultrasonography and MRA do not use iodinated contrast media, and are safe for patients with chronic kidney disease.8
Treatment. Percutaneous transluminal renal artery angioplasty is a treatment option for patients with renal artery stenosis. Angioplasty achieves higher cure rates for patients with fibromuscular dysplasia than for those with atherosclerotic renal artery stenosis.31 Most patients referred for renal artery revascularization have atherosclerosis. Because they’re generally older individuals with comorbidities, the benefits of stent revascularization for this group is controversial. Such patients require antihypertensive therapy with drugs that block the renin-angiotensin system.32
Endocrine disorders must be ruled out
Primary hyperaldosteronism is thought to be present in 0.3% to 1.4% of patients with hypertension.5,6 The prevalence varies widely from one source to another, however, and may be as high as 5% to 20% among patients with resistant hypertension.33,34
Hyperaldosteronism is related to either an aldosterone-secreting adrenal adenoma (in about 40% of cases) or bilateral adrenal hyperplasia (in the remaining 60%), and leads to increased sodium reabsorption and, typically, to a loss of potassium.35
Renin-secreting tumor, which usually arises from the juxtaglomerular cells of the kidney, is a rare cause of hyperaldosteronism. Extrarenal renin-secreting tumors have also been reported.36
What should raise your suspicion. Suspect hyperaldosteronism in patients who have both hypertension and hypokalemia, but keep in mind that not all patients with hyperaldosteronism have low serum potassium.37 Further laboratory evaluation should include a simultaneous measurement of plasma aldosterone (PA) and plasma renin activity (PRA). Patients with hyperaldosteronism will have elevated PA and suppressed PRA.
Testing considerations. It is important to ensure that the PA/PRA test is performed in the morning, with the patient in an upright position.36 He or she should be on a high sodium diet in preparation for the test, consuming 2 to 3 grams of sodium per meal for ≥2 days.37
In patients with a positive PA/PRA ratio (≥20), a 24-hour urinary aldosterone excretion test should be performed. A finding >12 to 14 mcg, along with a PRA <1.0 ng/mL per hour, confirms the diagnosis of primary hyperaldosteronism.18,37 Computed tomography or magnetic resonance imaging of the adrenal glands will distinguish between aldosterone-producing adenoma and bilateral adrenal hyperplasia.
Treatment. Laparoscopic adrenalectomy is the accepted surgical treatment of primary hyperaldosteronism.37 Patients with bilateral disease due to idiopathic hyperaldosteronism are not candidates for surgery and should be treated medically, with potassium-sparing diuretics such as spironolactone.
Cushing’s syndrome is marked by rapid weight gain
High BP may be a manifestation of Cushing’s syndrome, which affects 0.1% to 0.5% of patients with hypertension.5-7 Other signs and symptoms of Cushing’s syndrome include fatigue, weakness, hirsutism, amenorrhea, moon facies, dorsal hump, purple striae, truncal obesity, and hypokalemia. Rapid weight gain is the most common manifestation, and typically the one for which patients seek medical attention.38
The most widely used screening test for Cushing’s syndrome is a 24-hour urine collection measuring urinary-free cortisol.9 Normal urinary cortisol excretion is 20 to 100 mcg/dL in 24 hours; most patients with Cushing’s syndrome produce >250 mcg/dL in that time frame.9
Once hypercortisolism is established, determination of the cause is the next step. A serum adrenocorticotropic hormone (ACTH) level is needed to localize it. Normal (9-52 pg/mL) or elevated ACTH indicates a pituitary or ectopic source, while low levels of ACTH are an indication of an adrenal source.9,39
Treatment. Surgical resection of the tumor is often curative. For pituitary tumors (Cushing’s disease), transsphenoidal resection is the standard of care.39 For adrenal adenomas, unilateral adrenalectomy is the best option.39
Pheochromocytomas: Most are adrenal, sporadic, and benign
Pheochromocytomas—neuroendocrine, catecholamine-secreting tumors that develop from the adrenal medulla—are another cause of secondary hypertension. Catecholamines include norepinephrine and epinephrine and, rarely, dopamine secreted either intermittently or continuously. The prevalence of pheochromocytoma is 0.1% to 0.3% among patients with hypertension.5,6,28 A “rule of 10” (90:10 ratio) is often applied to pheochromocytomas because of the following:
- 90% of pheochromocytomas are located in the adrenal glands; the remaining 10% are extra-adrenal and can occur anywhere along the sympathetic chain40
- 90% are sporadic; 10% are familial41
- 90% are benign; 10% are malignant40
- 90% are found in adults; 10% affect children.42
Signs and symptoms of pheochromocytomas include palpitations, headache, dyspnea, diaphoresis, and flushing, as well as paroxysmal hypertension.40 Measurement of 24-hour urinary catecholamines and their metabolites has been the screening test of choice,43 but recent evidence suggests that measurement of plasma metanephrine and normetanephrine is a far more sensitive screen.10
Treatment. Surgical resection is the treatment of choice. Alpha blockade is started 7 to 10 days preoperatively;44,45 a beta-blocker is added only after an adequate alpha blockade has been established, as unopposed alpha stimulation could precipitate a hypertensive crisis. Laparoscopic adrenalectomy is routinely performed for small (<5 cm) pheochromocytomas.46,47
Don’t forget these (relatively) common secondary causes
Obstructive sleep apnea (OSA) is one of the most common conditions associated with resistant hypertension.48 Signs and symptoms include snoring, daytime somnolence, and obesity (body mass index ≥30 kg/m2).
OSA involves upper airway collapse during inspiration, causing intermittent hypoxemia with resultant sympathetic nervous system activation.11 The underlying mechanism of OSA-induced hypertension is strongly related to sympathetic activation.49 Overnight polysomnography is required for diagnosis.11
Continuous positive airway pressure is the treatment of choice for patients unable to lose weight.11
Pregnancy-induced hypertension is the most common medical problem encountered in pregnancy. It occurs in up to 15% of pregnancies, either during the pregnancy itself or postpartum. Postpartum hypertension may be related to preexisting chronic hypertension or to the persistence of gestational hypertension or preeclampsia, which usually occurs after 20 weeks’ gestation and is characterized by the presence of hypertension and proteinuria.50 Methyldopa and labetalol are commonly used treatments for hypertension during pregnancy.51
Drug-induced hypertension. Several drugs can cause or exacerbate hypertension, rendering it resistant to therapy. A careful review of the patient’s medication regimen is essential. Generally, drug-induced hypertension falls into 2 broad categories based on mechanism of action: volume overload and sympathetic activity.52,53
Corticosteroids can elevate BP in a dose-dependent manner, as a result of volume overload. Glycyrrhizic acid, the main ingredient in licorice, produces a state of excess mineralocorticoid, with a similar effect. Estrogen-containing oral contraceptives can cause an increased synthesis of angiotensin in the liver, resulting in greater aldosterone secretion and volume overload.
Drugs that stimulate sympathetic activity include cocaine, ephedrine, amphetamine, phenylephrine, phenylpropanolamine, caffeine, and alcohol. Nonsteroidal anti-inflammatory drugs may interfere with the action of ACE inhibitors and cause renal vasoconstriction, leading to sodium and water retention and hypertension.54
Discontinuation of the medication in question is preferable. In many cases, an agent that does not affect BP can be found to replace it.
If the patient is a child
Hypertension is uncommon in young people. However, coarctation of the aorta, a congenital narrowing associated with secondary hypertension, is typically diagnosed in childhood. In rare cases, the condition remains undetected well into adulthood.55 Clinical signs include weak femoral pulses, visible pulsations in the neck, a systolic murmur of the anterior and posterior thorax, and elevated BP in the upper extremities with low BP in the lower extremities.
Thus, once hypertension is confirmed in a young patient, BP should be measured in both arms and legs.56 Delayed or absent femoral pulses and a difference in systolic BP of ≥20 mm Hg between the arms and legs provide evidence of aortic coarctation.57 In adults, stenting is the initial treatment for this condition because of the morbidity associated with surgery.57 Stenting is an option for children with this condition, as well.58
CORRESPONDENCE Bernard M. Karnath, MD, University of Texas Medical Branch at Galveston, 301 University Boulevard, Galveston, TX 77555; bmkarnat@utmb.edu
• Review the family history of patients who do not respond to appropriate antihypertensive therapy, targeting hypertension and inherited disorders associated with high blood pressure (BP). B
• Include obstructive sleep apnea in the differential diagnosis of patients with resistant hypertension, particularly if they’re obese. B
• Include a thorough medication history in a work-up for resistant hypertension, as a number of drugs can cause or exacerbate high BP. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
What to include in the workup
Whether you’re doing an initial evaluation of a patient with high blood pressure (BP) or examining a patient with resistant hypertension, the history should focus on the duration of hypertension, previous BP levels, and comorbid conditions. It is also important to take a targeted family history, inquiring about hypertension as well as genetic disorders that increase the likelihood of secondary hypertension.
Inherited diseases associated with secondary hypertension include polycystic kidney disease, multiple endocrine neoplasia type 2 (MEN2), and von Hippel-Lindau syndrome.12,13 All are inherited in an autosomal dominant pattern. Patients with von Hippel-Lindau syndrome may present with multiple tumors, which can develop in the eyes, brain, adrenal glands, pancreas, liver, spinal cord, kidneys, or other parts of the body. Pheochromocytoma is a manifestation of both MEN2 and von Hippel-Lindau syndrome, and some specialists recommend that everyone with a family history of either condition undergo screening for pheochromocytoma.14
Table
Secondary hypertension: What you’ll see, what to test for8-11
| Secondary cause* | Signs and symptoms | Screening tests |
|---|---|---|
| Renal disease | Depends on underlying cause (eg, diabetes, polycystic kidney disease, glomerulonephritis) | Serum creatinine, urinalysis, renal ultrasound |
| Renal artery stenosis | Abdominal or flank bruits | Renal ultrasound, MRA, CT angiography |
| Primary hyperaldosteronism | Muscle cramps | PA/PRA |
| Pheochromocytoma | Paroxysms of palpitations, diaphoresis, headaches | Plasma metanephrine and normetanephrine |
| Cushing’s syndrome | Rapid weight gain, truncal obesity, abdominal striae | Measurement of 24-hour urinary free cortisol |
| OSA† | Obesity, daytime somnolence, nighttime snoring | Overnight polysomnography |
| Coarctation of the aorta‡ | Murmur of anterior and posterior thorax; claudication and weak femoral pulses | Echocardiography |
| CT, computed tomography; MRA, magnetic resonance angiography; OSA, obstructive sleep apnea; PA/PRA, plasma aldosterone-plasma renin activity. *Secondary hypertension may also be drug-induced, related to pregnancy (hypertension complicates up to 15% of pregnancies), or associated with inherited syndromes. †Highly prevalent in obese patients. ‡Higher prevalence in childhood hypertension; rarely diagnosed in adulthood. | ||
BP measurement is key
The physical examination should start with a calculation of body mass index, as well as a careful measurement of BP. The patient should be seated quietly in a chair for ≥5 minutes, with both feet on the floor and the arm being tested supported at heart level.
Unfortunately, reliability on the office BP measurement can be a confounding factor in the diagnosis of hypertension. “White coat hypertension”—in which BP is persistently elevated in the office and persistently normal in nonclinical settings—should be considered in patients who have high BP but no other signs or symptoms, and ambulatory monitoring used to rule out hypertension.15,16
Physicians also need to consider the opposite effect: Masked hypertension, characterized by normal office readings and elevated ambulatory readings, is more serious, of course, with patients at higher risk for end organ damage from unrecognized hypertension.17,18 Asking patients who self-monitor what type of BP readings they’re getting can be helpful in identifying masked hypertension. Ambulatory monitoring may be used to identify this condition, as well.
Other components in the physical workup include a fundoscopic exam; assessment of the thorax for murmurs and the abdomen for enlarged kidneys, masses, and abnormal aortic pulsation; auscultation for abdominal and carotid bruits; palpation of the thyroid gland; and palpation of the lower extremities for edema and pulses.
Include these tests in the workup
Routine tests for a patient with hypertension include:
- electrocardiogram
- blood glucose and hematocrit
- serum potassium, creatinine, and fasting lipid profiles
- urinalysis with measurement of microalbumin.
Microalbuminuria, a sensitive marker of early renal disease, is defined as a urinary albumin excretion between 30 and 300 mg/d.19 The albumin-creatinine ratio (30-300 mcg/mg), measured in spot urine specimens, is a more convenient way to detect it.20
Suspicious findings prompt further testing. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) recommends specific testing—much of it detailed below—if any aspect of the initial evaluation raises suspicion of a secondary cause or the patient has hypertension that’s of sudden onset or hard to control.21 (According to the National Heart, Lung, and Blood Institute, JNC 8 is due for release later this year.)
Kidney disease may be a consequence or a cause
The overall prevalence of hypertension in patients with renal disease is 60%,22 but varies according to the type of nephropathy. Eighty-seven percent of patients with diabetic nephropathy also have hypertension, and hypertension and diabetes are the 2 most common causes of end-stage renal disease.23,24
A combination of 2 or more drugs is usually needed to achieve the target BP of <130/80 mm Hg in patients with diabetes.21 ACE inhibitors and angiotensin receptor blockers have been found to slow the progression of diabetic nephropathy.25-27
Is renal artery stenosis to blame?
Renal artery stenosis is the most common form of secondary hypertension that’s reversible, affecting about 0.2% to 3.1% of hypertensive patients.5,6,28 The condition leads to renal ischemia, thereby stimulating the renin-angiotensin-aldosterone axis and causing secondary hyperaldosteronism.
In younger patients, especially women between 15 and 50 years of age, fibromuscular disease is the most common cause of renovascular hypertension.29,30 In older patients, atherosclerosis (which accounts for 90% of renovascular hypertension) is more likely.29,30
The choice of initial imaging tests includes duplex renal ultrasonography, magnetic resonance angiography (MRA), and spiral computed tomographic angiography. Contrast angiography is the gold standard, but it carries a risk of contrast-induced nephropathy. Duplex ultrasonography and MRA do not use iodinated contrast media, and are safe for patients with chronic kidney disease.8
Treatment. Percutaneous transluminal renal artery angioplasty is a treatment option for patients with renal artery stenosis. Angioplasty achieves higher cure rates for patients with fibromuscular dysplasia than for those with atherosclerotic renal artery stenosis.31 Most patients referred for renal artery revascularization have atherosclerosis. Because they’re generally older individuals with comorbidities, the benefits of stent revascularization for this group is controversial. Such patients require antihypertensive therapy with drugs that block the renin-angiotensin system.32
Endocrine disorders must be ruled out
Primary hyperaldosteronism is thought to be present in 0.3% to 1.4% of patients with hypertension.5,6 The prevalence varies widely from one source to another, however, and may be as high as 5% to 20% among patients with resistant hypertension.33,34
Hyperaldosteronism is related to either an aldosterone-secreting adrenal adenoma (in about 40% of cases) or bilateral adrenal hyperplasia (in the remaining 60%), and leads to increased sodium reabsorption and, typically, to a loss of potassium.35
Renin-secreting tumor, which usually arises from the juxtaglomerular cells of the kidney, is a rare cause of hyperaldosteronism. Extrarenal renin-secreting tumors have also been reported.36
What should raise your suspicion. Suspect hyperaldosteronism in patients who have both hypertension and hypokalemia, but keep in mind that not all patients with hyperaldosteronism have low serum potassium.37 Further laboratory evaluation should include a simultaneous measurement of plasma aldosterone (PA) and plasma renin activity (PRA). Patients with hyperaldosteronism will have elevated PA and suppressed PRA.
Testing considerations. It is important to ensure that the PA/PRA test is performed in the morning, with the patient in an upright position.36 He or she should be on a high sodium diet in preparation for the test, consuming 2 to 3 grams of sodium per meal for ≥2 days.37
In patients with a positive PA/PRA ratio (≥20), a 24-hour urinary aldosterone excretion test should be performed. A finding >12 to 14 mcg, along with a PRA <1.0 ng/mL per hour, confirms the diagnosis of primary hyperaldosteronism.18,37 Computed tomography or magnetic resonance imaging of the adrenal glands will distinguish between aldosterone-producing adenoma and bilateral adrenal hyperplasia.
Treatment. Laparoscopic adrenalectomy is the accepted surgical treatment of primary hyperaldosteronism.37 Patients with bilateral disease due to idiopathic hyperaldosteronism are not candidates for surgery and should be treated medically, with potassium-sparing diuretics such as spironolactone.
Cushing’s syndrome is marked by rapid weight gain
High BP may be a manifestation of Cushing’s syndrome, which affects 0.1% to 0.5% of patients with hypertension.5-7 Other signs and symptoms of Cushing’s syndrome include fatigue, weakness, hirsutism, amenorrhea, moon facies, dorsal hump, purple striae, truncal obesity, and hypokalemia. Rapid weight gain is the most common manifestation, and typically the one for which patients seek medical attention.38
The most widely used screening test for Cushing’s syndrome is a 24-hour urine collection measuring urinary-free cortisol.9 Normal urinary cortisol excretion is 20 to 100 mcg/dL in 24 hours; most patients with Cushing’s syndrome produce >250 mcg/dL in that time frame.9
Once hypercortisolism is established, determination of the cause is the next step. A serum adrenocorticotropic hormone (ACTH) level is needed to localize it. Normal (9-52 pg/mL) or elevated ACTH indicates a pituitary or ectopic source, while low levels of ACTH are an indication of an adrenal source.9,39
Treatment. Surgical resection of the tumor is often curative. For pituitary tumors (Cushing’s disease), transsphenoidal resection is the standard of care.39 For adrenal adenomas, unilateral adrenalectomy is the best option.39
Pheochromocytomas: Most are adrenal, sporadic, and benign
Pheochromocytomas—neuroendocrine, catecholamine-secreting tumors that develop from the adrenal medulla—are another cause of secondary hypertension. Catecholamines include norepinephrine and epinephrine and, rarely, dopamine secreted either intermittently or continuously. The prevalence of pheochromocytoma is 0.1% to 0.3% among patients with hypertension.5,6,28 A “rule of 10” (90:10 ratio) is often applied to pheochromocytomas because of the following:
- 90% of pheochromocytomas are located in the adrenal glands; the remaining 10% are extra-adrenal and can occur anywhere along the sympathetic chain40
- 90% are sporadic; 10% are familial41
- 90% are benign; 10% are malignant40
- 90% are found in adults; 10% affect children.42
Signs and symptoms of pheochromocytomas include palpitations, headache, dyspnea, diaphoresis, and flushing, as well as paroxysmal hypertension.40 Measurement of 24-hour urinary catecholamines and their metabolites has been the screening test of choice,43 but recent evidence suggests that measurement of plasma metanephrine and normetanephrine is a far more sensitive screen.10
Treatment. Surgical resection is the treatment of choice. Alpha blockade is started 7 to 10 days preoperatively;44,45 a beta-blocker is added only after an adequate alpha blockade has been established, as unopposed alpha stimulation could precipitate a hypertensive crisis. Laparoscopic adrenalectomy is routinely performed for small (<5 cm) pheochromocytomas.46,47
Don’t forget these (relatively) common secondary causes
Obstructive sleep apnea (OSA) is one of the most common conditions associated with resistant hypertension.48 Signs and symptoms include snoring, daytime somnolence, and obesity (body mass index ≥30 kg/m2).
OSA involves upper airway collapse during inspiration, causing intermittent hypoxemia with resultant sympathetic nervous system activation.11 The underlying mechanism of OSA-induced hypertension is strongly related to sympathetic activation.49 Overnight polysomnography is required for diagnosis.11
Continuous positive airway pressure is the treatment of choice for patients unable to lose weight.11
Pregnancy-induced hypertension is the most common medical problem encountered in pregnancy. It occurs in up to 15% of pregnancies, either during the pregnancy itself or postpartum. Postpartum hypertension may be related to preexisting chronic hypertension or to the persistence of gestational hypertension or preeclampsia, which usually occurs after 20 weeks’ gestation and is characterized by the presence of hypertension and proteinuria.50 Methyldopa and labetalol are commonly used treatments for hypertension during pregnancy.51
Drug-induced hypertension. Several drugs can cause or exacerbate hypertension, rendering it resistant to therapy. A careful review of the patient’s medication regimen is essential. Generally, drug-induced hypertension falls into 2 broad categories based on mechanism of action: volume overload and sympathetic activity.52,53
Corticosteroids can elevate BP in a dose-dependent manner, as a result of volume overload. Glycyrrhizic acid, the main ingredient in licorice, produces a state of excess mineralocorticoid, with a similar effect. Estrogen-containing oral contraceptives can cause an increased synthesis of angiotensin in the liver, resulting in greater aldosterone secretion and volume overload.
Drugs that stimulate sympathetic activity include cocaine, ephedrine, amphetamine, phenylephrine, phenylpropanolamine, caffeine, and alcohol. Nonsteroidal anti-inflammatory drugs may interfere with the action of ACE inhibitors and cause renal vasoconstriction, leading to sodium and water retention and hypertension.54
Discontinuation of the medication in question is preferable. In many cases, an agent that does not affect BP can be found to replace it.
If the patient is a child
Hypertension is uncommon in young people. However, coarctation of the aorta, a congenital narrowing associated with secondary hypertension, is typically diagnosed in childhood. In rare cases, the condition remains undetected well into adulthood.55 Clinical signs include weak femoral pulses, visible pulsations in the neck, a systolic murmur of the anterior and posterior thorax, and elevated BP in the upper extremities with low BP in the lower extremities.
Thus, once hypertension is confirmed in a young patient, BP should be measured in both arms and legs.56 Delayed or absent femoral pulses and a difference in systolic BP of ≥20 mm Hg between the arms and legs provide evidence of aortic coarctation.57 In adults, stenting is the initial treatment for this condition because of the morbidity associated with surgery.57 Stenting is an option for children with this condition, as well.58
CORRESPONDENCE Bernard M. Karnath, MD, University of Texas Medical Branch at Galveston, 301 University Boulevard, Galveston, TX 77555; bmkarnat@utmb.edu
1. Middleton K, Hing E, Xu J. National hospital ambulatory medical care survey: 2005 outpatient department summary. Adv Data. 2007;389:1-34.
2. Ong KL, Cheung BM, Man YB, et al. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999-2004. Hypertension. 2007;49:69-75.
3. Fields LE, Burt VL, Cutler JA, et al. The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension. 2004;44:398-404.
4. Wang TJ, Vasan RS. Epidemiology of uncontrolled hypertension in the United States. Circulation. 2005;112:1651-1662.
5. Anderson GH, Jr, Blakeman N, Streeten DH. The effect of age on prevalence of secondary forms of hypertension in 4429 consecutively referred patients. J Hypertens. 1994;12:609-615.
6. Sinclair AM, Isles CG, Brown I, et al. Secondary hypertension in a blood pressure clinic. Arch Intern Med. 1987;147:1289-1293.
7. Dosh SA. The diagnosis of essential and secondary hypertension in adults. J Fam Pract. 2001;50:707-712.
8. Eardley KS, Lipkin GW. Atherosclerotic renal artery stenosis: is it worth diagnosing?J Hum Hypertens. 1999;13:217-220.
9. Boscaro M, Barzon L, Fallo F, et al. Cushing’s syndrome. Lancet. 2001;357:783-791.
10. Unger N, Pitt C, Schmidt IL. Diagnostic value of various biochemical parameters for the diagnosis of pheochromocytoma in patients with adrenal mass. Eur J Endocrinol. 2006;154:409-417.
11. Prisant LM, Dillard TA, Blanchard AR. Obstructive sleep apnea syndrome. J Clin Hypertens. 2006;8:746-750.
12. Marini F, Falchetti A, Del Monte F, et al. Multiple endocrine neoplasia type 2. Orphanet J Rare Dis. 2006;1:45.-
13. Bryant J, Farmer J, Kessler LJ, et al. Pheochromocytoma: the expanding genetic differential diagnosis. J Natl Cancer Inst. 2003;95:1196-1204.
14. Neumann HP, Berger DP, Sigmund G, et al. Pheochromocytomas, multiple endocrine neoplasia type 2, and von Hippel-Lindau disease. N Engl J Med. 1993;329:1531-1538.
15. Mancia G, Bertinieri G, Grassi G, et al. Effects of blood-pressure measurement by the doctor on patient’s blood pressure and heart rate. Lancet. 1983;2:695-698.
16. Pickering TG, James GD, Boddie C. How common is white coat hypertension? JAMA. 1988;259:225-228.
17. Kotsis V, Stabouli S, Toumanidis S, et al. Target organ damage in “white coat hypertension” and “masked hypertension”. Am J Hypertens. 2008;21:393-399.
18. Pickering TG, Davidson K, Gerin W, et al. Masked hypertension. Hypertension. 2002;40:795-796.
19. Volpe M. Microalbuminuria screening in patients with hypertension: recommendations for clinical practice. Int J Clin Pract. 2008;62:97-108.
20. Keane WF, Eknoyan G. Proteinuria, albuminuria, risk, assessment, detection, elimination (PARADE): a position paper of the National Kidney Foundation. Am J Kidney Dis. 1999;33:1004-1010.
21. Chobanian AV, Bakris GL, Black HR, et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206-1252.
22. Ridao N, Luño J, García de Vinuesa S, et al. Prevalence of hypertension in renal disease. Nephrol Dial Transplant. 2001;16(suppl 1):S70-S73.
23. Foley RN, Collins AJ. End-stage renal disease in the United States: an update from the United States Renal Data System. J Am Soc Nephrol. 2007;18:2644-2648.
24. Parmar MS. Chronic renal disease. BMJ. 2002;325:85-90.
25. Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861-869.
26. Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851-860.
27. Menne J, Izzo JL, Jr, Ito S, et al. Prevention of microalbuminuria in patients with type 2 diabetes and hypertension. J Hypertens. 2012;30:811-818.
28. Rossi H, Kim A, Prinz RA. Primary hyperaldosteronism in the era of laparoscopic adrenalectomy. Am Surg. 2002;68:253-256.
29. Safian RD, Textor SC. Renal artery stenosis. N Engl J Med. 2001;244:431-442.
30. Slovut DP, Olin JW. Fibromuscular dysplasia. N Engl J Med. 2004;350:1862-1871.
31. Bonelli FS, McKusick MA, Textor SC. Renal artery angioplasty: technical results and clinical outcome in 320 patients. Mayo Clin Proc. 1995;70:1041-1052.
32. Textor SC. Renovascular hypertension in 2007: where are we now? Curr Cardiol Rep. 2007;9:453-461.
33. Calhoun DA. Is there an unrecognized epidemic of primary aldosteronism? Pro. Hypertension. 2007;50:447-453.
34. Young WF, Jr. Minireview: primary aldosteronism—changing concepts in diagnosis and treatment. Endocrinology. 2003;144:2208-2213.
35. Young WF. Primary aldosteronism: renaissance of a syndrome. Clin Endocrinol (Oxf). 2007;66:607-618.
36. Pursell RN, Quinlan PM. Secondary hypertension due to a renin-producing teratoma. Am J Hypertens. 2003;16:592-595.
37. Ganguly A. Primary aldosteronism. N Engl J Med. 1998;339:1828-1834.
38. Muller M, Longo Mazzuco T, Martinie M, et al. Diagnosis of Cushing’s syndrome: a retrospective evaluation of clinical practice. Eur J Intern Med. 2006;17:334-338.
39. Norton JA, Li M, Gillary J, et al. Cushing’s syndrome. Curr Probl Surg. 2001;38:488-545.
40. Lenders JW, Eisenhofer G, Mannelli M, et al. Phaeochromocytoma. Lancet. 2005;366:665-675.
41. Bryant J, Farmer J, Kessler LJ, et al. Pheochromocytoma: the expanding genetic differential diagnosis. J Natl Cancer Inst. 2003;95:1196-1204.
42. Sullivan J, Groshong T, Tobias JD. Presenting signs and symptoms of pheochromocytoma in pediatric-aged patients. Clin Pediatr. 2005;44:715-719.
43. Young WF, Jr. Pheochromocytoma: issues in diagnosis and treatment. Compr Ther. 1997;23:319-326.
44. Kocak S, Aydintug S, Canakci N. Alpha blockade in preoperative preparation of patients with pheochromocytomas. Int Surg. 2002;87:191-194.
45. Russell WJ, Metcalfe IR, Tonkin AL, et al. The preoperative management of phaeochromocytoma. Anaesth Intensive Care. 1998;26:196-200.
46. Kalady MF, McKinlay R, Olson JA, Jr, et al. Laparoscopic adrenalectomy for pheochromocytoma. A comparison to aldosteronoma and incidentaloma. Surg Endosc. 2004;18:621-625.
47. Naya Y, Ichikawa T, Suzuki H, et al. Efficacy and safety of laparoscopic surgery for pheochromocytoma. Int J Urol. 2005;12:128-133.
48. Pedrosa RP, Drager LF, Gonzaga CC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension. 2011;5:811-817.
49. Sharabi Y, Dagan Y, Grossman E. Sleep apnea as a risk factor for hypertension. Curr Opin Nephrol Hypertens. 2004;13:359-364.
50. James PR, Nelson-Piercy C. Management of hypertension before, during, and after pregnancy. Heart. 2004;90:1499-1504.
51. Solomon CG, Seely EW. Hypertension in pregnancy. Endocrinol Metab Clin North Am. 2011;40:847-863.
52. Grossman E, Messerli FH. Drug-induced hypertension: an unappreciated cause of secondary hypertension. Am J Med. 2012;125:14-22.
53. Rossi GP, Seccia TM, Maniero C, et al. Drug-related hypertension and resistance to antihypertensive treatment: a call for action. J Hypertens. 2011;29:2295-2309.
54. Grossman E, Messerli FH. Secondary hypertension: interfering substances. J Clin Hypertens. 2008;10:556-566.
55. Cicek D, Haberal C, Ozkan S, et al. A severe coarctation of aorta in a 52-year-old male: a case report. Int J Med Sci. 2010;7:340-341.
56. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 suppl 4th Report):S555-S576.
57. Rao PS. Coarctation of the aorta. Curr Cardiol Rep. 2005;7:425-434.
58. Rao PS. Stents in the management of aortic coarctation in young children. JACC Cardiovasc Interv. 2009;2:884-886.
1. Middleton K, Hing E, Xu J. National hospital ambulatory medical care survey: 2005 outpatient department summary. Adv Data. 2007;389:1-34.
2. Ong KL, Cheung BM, Man YB, et al. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999-2004. Hypertension. 2007;49:69-75.
3. Fields LE, Burt VL, Cutler JA, et al. The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension. 2004;44:398-404.
4. Wang TJ, Vasan RS. Epidemiology of uncontrolled hypertension in the United States. Circulation. 2005;112:1651-1662.
5. Anderson GH, Jr, Blakeman N, Streeten DH. The effect of age on prevalence of secondary forms of hypertension in 4429 consecutively referred patients. J Hypertens. 1994;12:609-615.
6. Sinclair AM, Isles CG, Brown I, et al. Secondary hypertension in a blood pressure clinic. Arch Intern Med. 1987;147:1289-1293.
7. Dosh SA. The diagnosis of essential and secondary hypertension in adults. J Fam Pract. 2001;50:707-712.
8. Eardley KS, Lipkin GW. Atherosclerotic renal artery stenosis: is it worth diagnosing?J Hum Hypertens. 1999;13:217-220.
9. Boscaro M, Barzon L, Fallo F, et al. Cushing’s syndrome. Lancet. 2001;357:783-791.
10. Unger N, Pitt C, Schmidt IL. Diagnostic value of various biochemical parameters for the diagnosis of pheochromocytoma in patients with adrenal mass. Eur J Endocrinol. 2006;154:409-417.
11. Prisant LM, Dillard TA, Blanchard AR. Obstructive sleep apnea syndrome. J Clin Hypertens. 2006;8:746-750.
12. Marini F, Falchetti A, Del Monte F, et al. Multiple endocrine neoplasia type 2. Orphanet J Rare Dis. 2006;1:45.-
13. Bryant J, Farmer J, Kessler LJ, et al. Pheochromocytoma: the expanding genetic differential diagnosis. J Natl Cancer Inst. 2003;95:1196-1204.
14. Neumann HP, Berger DP, Sigmund G, et al. Pheochromocytomas, multiple endocrine neoplasia type 2, and von Hippel-Lindau disease. N Engl J Med. 1993;329:1531-1538.
15. Mancia G, Bertinieri G, Grassi G, et al. Effects of blood-pressure measurement by the doctor on patient’s blood pressure and heart rate. Lancet. 1983;2:695-698.
16. Pickering TG, James GD, Boddie C. How common is white coat hypertension? JAMA. 1988;259:225-228.
17. Kotsis V, Stabouli S, Toumanidis S, et al. Target organ damage in “white coat hypertension” and “masked hypertension”. Am J Hypertens. 2008;21:393-399.
18. Pickering TG, Davidson K, Gerin W, et al. Masked hypertension. Hypertension. 2002;40:795-796.
19. Volpe M. Microalbuminuria screening in patients with hypertension: recommendations for clinical practice. Int J Clin Pract. 2008;62:97-108.
20. Keane WF, Eknoyan G. Proteinuria, albuminuria, risk, assessment, detection, elimination (PARADE): a position paper of the National Kidney Foundation. Am J Kidney Dis. 1999;33:1004-1010.
21. Chobanian AV, Bakris GL, Black HR, et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206-1252.
22. Ridao N, Luño J, García de Vinuesa S, et al. Prevalence of hypertension in renal disease. Nephrol Dial Transplant. 2001;16(suppl 1):S70-S73.
23. Foley RN, Collins AJ. End-stage renal disease in the United States: an update from the United States Renal Data System. J Am Soc Nephrol. 2007;18:2644-2648.
24. Parmar MS. Chronic renal disease. BMJ. 2002;325:85-90.
25. Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861-869.
26. Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851-860.
27. Menne J, Izzo JL, Jr, Ito S, et al. Prevention of microalbuminuria in patients with type 2 diabetes and hypertension. J Hypertens. 2012;30:811-818.
28. Rossi H, Kim A, Prinz RA. Primary hyperaldosteronism in the era of laparoscopic adrenalectomy. Am Surg. 2002;68:253-256.
29. Safian RD, Textor SC. Renal artery stenosis. N Engl J Med. 2001;244:431-442.
30. Slovut DP, Olin JW. Fibromuscular dysplasia. N Engl J Med. 2004;350:1862-1871.
31. Bonelli FS, McKusick MA, Textor SC. Renal artery angioplasty: technical results and clinical outcome in 320 patients. Mayo Clin Proc. 1995;70:1041-1052.
32. Textor SC. Renovascular hypertension in 2007: where are we now? Curr Cardiol Rep. 2007;9:453-461.
33. Calhoun DA. Is there an unrecognized epidemic of primary aldosteronism? Pro. Hypertension. 2007;50:447-453.
34. Young WF, Jr. Minireview: primary aldosteronism—changing concepts in diagnosis and treatment. Endocrinology. 2003;144:2208-2213.
35. Young WF. Primary aldosteronism: renaissance of a syndrome. Clin Endocrinol (Oxf). 2007;66:607-618.
36. Pursell RN, Quinlan PM. Secondary hypertension due to a renin-producing teratoma. Am J Hypertens. 2003;16:592-595.
37. Ganguly A. Primary aldosteronism. N Engl J Med. 1998;339:1828-1834.
38. Muller M, Longo Mazzuco T, Martinie M, et al. Diagnosis of Cushing’s syndrome: a retrospective evaluation of clinical practice. Eur J Intern Med. 2006;17:334-338.
39. Norton JA, Li M, Gillary J, et al. Cushing’s syndrome. Curr Probl Surg. 2001;38:488-545.
40. Lenders JW, Eisenhofer G, Mannelli M, et al. Phaeochromocytoma. Lancet. 2005;366:665-675.
41. Bryant J, Farmer J, Kessler LJ, et al. Pheochromocytoma: the expanding genetic differential diagnosis. J Natl Cancer Inst. 2003;95:1196-1204.
42. Sullivan J, Groshong T, Tobias JD. Presenting signs and symptoms of pheochromocytoma in pediatric-aged patients. Clin Pediatr. 2005;44:715-719.
43. Young WF, Jr. Pheochromocytoma: issues in diagnosis and treatment. Compr Ther. 1997;23:319-326.
44. Kocak S, Aydintug S, Canakci N. Alpha blockade in preoperative preparation of patients with pheochromocytomas. Int Surg. 2002;87:191-194.
45. Russell WJ, Metcalfe IR, Tonkin AL, et al. The preoperative management of phaeochromocytoma. Anaesth Intensive Care. 1998;26:196-200.
46. Kalady MF, McKinlay R, Olson JA, Jr, et al. Laparoscopic adrenalectomy for pheochromocytoma. A comparison to aldosteronoma and incidentaloma. Surg Endosc. 2004;18:621-625.
47. Naya Y, Ichikawa T, Suzuki H, et al. Efficacy and safety of laparoscopic surgery for pheochromocytoma. Int J Urol. 2005;12:128-133.
48. Pedrosa RP, Drager LF, Gonzaga CC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension. 2011;5:811-817.
49. Sharabi Y, Dagan Y, Grossman E. Sleep apnea as a risk factor for hypertension. Curr Opin Nephrol Hypertens. 2004;13:359-364.
50. James PR, Nelson-Piercy C. Management of hypertension before, during, and after pregnancy. Heart. 2004;90:1499-1504.
51. Solomon CG, Seely EW. Hypertension in pregnancy. Endocrinol Metab Clin North Am. 2011;40:847-863.
52. Grossman E, Messerli FH. Drug-induced hypertension: an unappreciated cause of secondary hypertension. Am J Med. 2012;125:14-22.
53. Rossi GP, Seccia TM, Maniero C, et al. Drug-related hypertension and resistance to antihypertensive treatment: a call for action. J Hypertens. 2011;29:2295-2309.
54. Grossman E, Messerli FH. Secondary hypertension: interfering substances. J Clin Hypertens. 2008;10:556-566.
55. Cicek D, Haberal C, Ozkan S, et al. A severe coarctation of aorta in a 52-year-old male: a case report. Int J Med Sci. 2010;7:340-341.
56. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 suppl 4th Report):S555-S576.
57. Rao PS. Coarctation of the aorta. Curr Cardiol Rep. 2005;7:425-434.
58. Rao PS. Stents in the management of aortic coarctation in young children. JACC Cardiovasc Interv. 2009;2:884-886.
Emergency contraception: An underutilized resource
• Offer emergency contraception (EC) to any woman who reports contraceptive failure or unprotected intercourse within the last 5 days; no clinical exam is necessary. B
• Prescribe a progestin-only EC or ulipristal acetate, both of which are more effective and have fewer adverse effects than an estrogen-progestin combination. A
• Consider giving sexually active teens <17 years an advance prescription for EC, as it is not available over the counter to this age group. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The average American woman will spend more than 30 years of her life trying to prevent pregnancy—not always successfully. Each year, half of the approximately 6 million pregnancies in the United States are unintended.1 Emergency contraception (EC) gives a woman a second chance to prevent pregnancy after a contraceptive failure or unprotected sex. But all too often, it isn’t offered and she doesn’t request it.
Lack of knowledge about EC continues to be a barrier to its use. Some women have heard about the “morning after pill,” but may not know that EC can be effective for up to 5 days after intercourse—or even that it’s available in this country.2 Others are unaware that it is possible to prevent pregnancy after intercourse,2 and mistakenly believe that EC drugs are abortifacients. In fact, they work primarily by interfering with ovulation and have not been found to prevent implantation or to disrupt an existing pregnancy.3-5
Providers also contribute to the limited use of EC, often because they’re unfamiliar with the options or uncomfortable discussing them with patients, particularly sexually active teens.2
This update can help you clear up misconceptions about EC with your patients. It also provides evidence-based information about the various types of EC, a review of issues affecting accessibility, and a telephone triage protocol to guide your response to women seeking postcoital contraception.
EC today: Plan B and beyond
Hormonal EC was first studied in the 1920s, when researchers found that estrogenic ovarian extracts interfered with pregnancy in animals. The first regimen was a high-dose estrogen-only formulation. In 1974, a combined estrogen-progestin replaced it. Known as the Yuzpe method for the physician who discovered it,6 this regimen used a widely available brand of combined estrogen-progestin oral contraceptive pills. The standard dose consisted of 100 mcg ethinyl estradiol (EE) and 0.5 mg levonorgestrel (LNG) taken 12 hours apart.2,7
Although the Yuzpe method is still in use, progestin-only EC—Plan B as well as generic (Next Choice) and single-dose (Plan B One-Step) LNG formulations—has become the standard of care because it has greater efficacy and fewer adverse effects.2 There are 2 additional options: the copper intrauterine device (IUD), which is highly effective both as EC and as a long-term contraceptive,6 and ulipristal acetate (UPA), which received US Food and Drug Administration (FDA) approval in 2010. This second-generation antiprogestin, sold under the brand name Ella, is well tolerated and highly effective.8
EC efficacy: What the evidence shows
EC is most likely to work when used within 24 hours, but remains effective—albeit to varying degrees—for up to 120 hours (TABLE).2,5,8,9 Thus, which EC is best for a particular patient depends, in part, on timing.
TABLE
Emergency contraception: Comparing methods*2,5,8,9
| EC method | Dose and timing | Benefits | Adverse effects/ drawbacks |
|---|---|---|---|
| Estrogen-progestin OCs | 100 mcg EE and 0.5 mg LNG, taken 12 h apart First dose within 72 h | Easily accessible and widely available; patient may use OCs she already has at home | Higher rates of adverse effects, including nausea, vomiting, headache; less effective than other methods |
| Progestin-only (Plan B, Next Choice, others) | 1.5 mg LNG within 72 h (available in divided doses or in a single tablet; 2 tablets may be taken as a single dose) | Available OTC for patients ≥17 y; more effective and fewer adverse effects than estrogen-progestin Convenience of single dose | Prescription required for patients <17 y Approved for use within 72 h; effectiveness diminishes thereafter |
| UPA (Ella) | 30 mg UPA, taken ≤120 h† | More effective than LNG; fewer adverse effects than estrogen-progestin Efficacy remains high ≤5 days Convenience of single dose | Prescription required; not available at all pharmacies Not studied in breastfeeding‡ |
| Copper IUD | Insert ≤120 h | Extremely effective Provides immediate, long-term contraception | Insertion requires staff training; higher cost than oral EC |
| EC, emergency contraception; EE, ethinyl estradiol; IUD, intrauterine device; LNG, levonorgestrel; OCs, oral contraceptives; OTC, over the counter; UPA, ulipristal acetate. *Low doses of mifepristone (<25-50 mg)—approved as an abortifacient in much larger doses—may also be used as EC. †Dosage should be repeated if vomiting occurs within 3 hours. ‡Advise patients to avoid breastfeeding for 36 hours | |||
Copper IUDs have the highest success rate: Studies have found the copper IUD to be >99% effective in preventing pregnancy when inserted within 5 days of unprotected intercourse.9,10 The copper ions it contains have a toxic effect on sperm, and impair the potential for fertilization; the device may also make the endometrium inhospitable to implantation.9,10
A just-published systematic review of 42 studies in 6 countries over a period of more than 30 years yielded similar results: Among more than 7000 women who had the IUDs inserted after unprotected intercourse, the pregnancy rate was 0.09%.11
But an IUD is appropriate only for women who want long-term contraception and would otherwise qualify for IUD insertion. By comparison, hormonal EC is not as effective and generally works best when used within a shorter time frame.
Progestin alone vs estrogen-progestin combo. To compare hormonal contraception, many researchers use a “prevented fraction”—an estimated percentage of pregnancies averted by treatment. A large World Health Organization-sponsored study found that the efficacy of progestin-only EC is superior to that of the estrogen-progestin combination, with prevented fractions of 85% and 57%, respectively. The progestin-only EC was also associated with significantly fewer adverse effects.12
In more recent studies, the prevented fraction for progestin-only EC has been found to range from 60% to 94%, while a meta-analysis of studies assessing estrogen-progestin EC r evealed a prevented fraction of ≥74%.2
Although there is evidence suggesting that progestin-only EC may work for up to 5 days,13,14 it has FDA approval only for use within 72 hours of intercourse.13 A time-sensitive analysis showed that when it was used within 12 hours of intercourse, the pregnancy rate was 0.5%. The rate increased steadily to 4.1% when the progestin-based EC was taken 61 to 72 hours after intercourse, and rose by an additional 50% after an additional 12-hour delay.15
Hormonal EC is only effective before ovulation occurs. Once luteinizing hormone (LH) starts to rise, it is ineffective. However, the likelihood of pregnancy drops precipitously after ovulation, and there is no risk of pregnancy in the luteal phase, with or without EC.
One pill or 2? Both Plan B and the generic Next Choice are sold as 2-dose regimens, with one 0.75-mg tablet taken within 72 hours and the second taken 12 hours later. Plan B One-Step, which consists of a single 1.5-mg tablet, is clinically equivalent to the 2-dose formula,16 but is more convenient and may improve adherence. Notably, though, one large randomized controlled trial (RCT) in China found that the 2-pill regimen was significantly more effective in preventing pregnancy in women who had further acts of unprotected intercourse after treatment.17
UPA has a 5-day window. UPA has FDA approval for use within 120 hours of unprotected intercourse and has been found to be more effective than progestin-only EC, especially when used on Day 4 or 5 (72-120 hours).8 Adverse effects are mild to moderate, similar to those of LNG, and may include headache, abdominal pain, nausea, dysmenorrhea, fatigue, and dizziness.8
The medication binds to progesterone receptors, acting as an antagonist as well as a partial agonist. The mechanism of action depends on the phase of the woman’s cycle. Taken during the midfollicular phase, UPA inhibits follicle development.18 When used in the advanced follicular phase, just prior to ovulation, it delays LH peak and postpones ovulation.19
In one small study in which women were randomized to either UPA or placebo, researchers found that the drug delayed ovulation for ≥5 days in about 60% of those who took it; in comparison, ovulation occurred by Day 5 in every woman in the placebo group.19
How accessible is EC?
EC has a tumultuous history in the United States,20 and accessibility depends on a variety of factors—age among them.
Plan B, for instance, is subject to a 2-tier system. It was approved in 1999 as a prescription-only product and has been available over the counter (OTC) to women 17 years and older since 2009. Younger women can get it only by prescription.21
Nonetheless, Plan B made the news again last year, when US Health and Human Services Secretary Kathleen Sebelius overruled an FDA decision to give teens younger than 17 OTC access.22 Thus, the age restriction remains in place, although there is no medical evidence to support it.23 Other forms of EC, including UPA, are available to all women only by prescription.
Accessibility of EC also may vary from one part of the country to another. Some states have enacted laws with conscience clauses that allow pharmacists to refuse to dispense EC. Others have worked to increase access by authorizing pharmacists to initiate and dispense EC on their own, provided they work in collaboration with a doctor or other licensed prescriber. As of 2011, 9 states—Alaska, California, Hawaii, Maine, Massachusetts, New Hampshire, New Mexico, Vermont, and Washington—had such agreements in place.24
Cost is another potential barrier. The cost of oral EC varies from about $10 to $70, plus the cost of a doctor visit for a teen who needs a prescription. Obtaining the copper IUD without insurance coverage would cost hundreds of dollars, to cover the price of insertion as well as the device.5
Increasing access: What you can do
In view of the barriers that adolescents face in obtaining EC, the American College of Obstetricians and Gynecologists and the American Academy of Pediatrics, among other organizations, recommend that physicians give advance prescriptions to teens under the age of 17. 2,25
But how likely are they to actually buy the medication and use it on an emergency basis?
A 2007 Cochrane review found that giving women advance prescriptions for EC did not reduce pregnancy or abortion rates.26 Other studies have found that EC use is highest among women with the lowest risk of pregnancy—those who are already using contraception and are less likely to have unprotected intercourse. Those at the highest risk for unintended pregnancy were found to be less likely to use EC after every episode of unprotected intercourse.23,26 One RCT demonstrated that rates of pregnancy and sexually transmitted infection were not significantly increased by advance provision of EC, leading the researchers to conclude that it was therefore unreasonable to restrict access.27
While it is prudent to make women aware that EC is available should they need it, the focus should be on the fact that consistent use of a reliable form of contraception—an IUD or hormonal contraception, in particular—gives them the best chance of preventing an unwanted pregnancy.
What to do when that call comes in
When a woman calls to report a contraceptive failure or tells you she has had unprotected intercourse, start by finding out how recently it occurred. Subsequent questions and actions that can be used by triage nurses or physicians on call are detailed in the easy-to-use EC telephone triage protocol (FIGURE)28 on page 395. Whether you prescribe oral EC or schedule an appointment to insert a copper IUD within the next few days, there are a number of key points to keep in mind.
Initiate EC as soon as possible, but make it available to any woman who requests it for up to 5 days after unprotected intercourse.
Advise patients that oral EC is safe for most women—even those with contraindications to oral contraceptives. No physical examination is necessary, and there’s usually no need for a pregnancy test.2 The one exception: A woman who has not had a period in the past 30 days should be given a pregnancy test before taking UPA.2
Offer EC at any time in the cycle. Although EC works primarily in the preovulatory phase, it should be offered regardless of the phase of the patient’s menstrual cycle. That’s because of the possibility of late ovulation, as well as the difficulty in accurately determining the phase of a woman’s cycle based on a history alone.
Make EC available to any woman who has been sexually abused. At many emergency departments, EC is not routinely offered to women who come in after being raped, although it clearly should be.29
FIGURE
Telephone triage protocol for emergency contraception
EC, emergency contraception; IUD, intrauterine device; LNG, levonorgestrel; Rx, prescription; UPA, ulipristal acetate.
Adapted from: Reproductive Health Access Project. http://www.reproductiveaccess.org/contraception/tel_triage_ec.htm.28
Patient counseling about EC
Advise patients for whom you prescribe oral EC that the medication delays ovulation, which means they could be at risk for pregnancy later in the cycle. Stress the need to use an alternative means of contraception (a barrier method is recommended for women taking UPA) until their next menses and to come in for a pregnancy test if their period is more than a week late.
Point out, too, that EC can be used more than once within the same cycle, if necessary. That said, even a single request for EC should result in a discussion of effective, longer-term contraception, including the possibility of an IUD.
CORRESPONDENCE Sarina Schrager, MD, MS, University of Wisconsin School of Public Health, Department of Family Medicine, 1100 Delaplaine Court, Madison, WI 53715; sbschrag@wisc.edu
1. Guttmacher Institute. National Reproductive Health profile. Available at: http://www.guttmacher.org/datacenter/profiles/print/US.jsp. Accessed November 3, 2011.
2. American College of Obstetricians and Gynecologists ACOG practice bulletin No. 112. Emergency contraception. Obstet Gynecol. 2010;115:1100–1109.
3. Belluck P. No abortion role seen for morning-after pill. New York Times. June 6, 2012; A1.
4. Trussell J, Raymond E. Emergency contraception: a last chance to prevent unintended pregnancy. Princeton, NJ: Office of Population Research at Princeton University; June 2012. Available at: http://ec.princeton.edu/questions/ec-review.pdf. Accessed June 20, 2012.
5. Planned Parenthood. Morning-after pill (emergency contraception). Available at: http://www.plannedparenthood.org/health-topics/emergency-contraception-morning-after-pill-4363.asp. Accessed June 7, 2012.
6. Ellertson C. History and efficacy of emergency contraception: beyond Coca-Cola. Fam Plann Perspect. 1996;28:44-48.
7. Yuzpe AA, Thurlow HJ, Ramzy I, et al. Post coital contraception—a pilot study. J Reprod Med. 1974;13:53-58.
8. Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet. 2010;375:555-562.
9. Belden P, Harper CC, Speidel J. The copper IUD for emergency contraception, a neglected option. Contraception. 2012;85:338-339.
10. Wu S, Godfrey EM, Wojdyla D, et al. Copper T380A intrauterine device for emergency contraception: a prospective, multicentre, cohort clinical trial. Br J Obstet Gynaecol. 2010;117:1205-1210.
11. Cleland K, Zhu H, Goldstuck N, et al. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod. 2012 May 8 [Epub ahead of print].
12. World Health Organization’s Task Force on postovulatory methods of fertility regulation. Randomised controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Lancet. 1998;352:428-433.
13. US Food and Drug Administration. Plan B: questions and answers. Updated December 14, 2006. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm109783.htm. Accessed June 7, 2012.
14. Trussell J, Rodriguez G, Ellertson C. A meta-analysis of efficacy for the Yuzpe method (estrogen-progestin). Contraception. 1999;59:147-151.
15. Piaggio G, von Hertzen H, Grimes DA, et al. Task Force on Postovulatory Methods of Fertility. Timing of emergency contraception with levonorgestrel or the Yuzpe regimen. Lancet. 1999;353:721.
16. Cheng L, Gülmezoglu AM, Piaggio GGP, et al. Interventions for emergency contraception. Cochrane Database Syst Rev. 2008;(2):CD001324.
17. Ngai SW, Fan S, Li S, et al. A randomized trial to compare 24 h versus 12 h double dose regimen of levonorgestrel for emergency contraception. Hum Reprod. 2005;20:307-311.
18. Stratton P, Hartog B, Hajizadeh N, et al. A single mid-follicular dose of CDB-2914, a new antiprogestin, inhibits folliculogenesis and endometrial differentiation in normally cycling women. Hum Reprod. 2000;5:1092-1099.
19. Brache V, Cochon L, Jesam C, et al. Immediate pre-ovulatory administration of 30 mg ulipristal acetate significantly delays follicular rupture. Hum Reprod. 2010;25:2256-2263.
20. Kliff S. Plan B’s complicated history. Available at: http://www.thedailybeast.com/newsweek/2009/08/24/plan-b-s-complicated-history.html/. Accessed June 7, 2012.
21. US Food and Drug Administration Updated FDA action on Plan B (levonorgestrel) tablets. April 22, 2009. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2009/ucm149568.htm. Accessed June 7, 2012.
22. US Department of Health and Human Services. A statement by U.S. Department of Health and Human Services Secretary Kathleen Sebelius. December 7, 2011. Available at: http://www.hhs.gov/news/press/2011pres/12/20111207a.html. Accessed June 7, 2012.
23. Duffy K, Gold M. Adolescents and emergency contraception: update 2011. Curr Opin Obstet Gynecol. 2011;23:328-333.
24. National Conference of State Legislatures. Emergency contraception state laws. Updated July 2011. Available at: http://www.ncsl.org/issues-research/health/emergency-contraception-state-laws.aspx. Accessed May 31, 2012.
25. Cash S. New AAP policy advises on emergency contraception use. AAP News. 2005;26:1.
26. Polis CB, Grimes DA, Schaffer K, et al. Advance provision of EC for pregnancy prevention. Cochrane Database Syst Rev. 2010;(2):CD005497.
27. Raine T, Harper CC, Rocca CH, et al. Direct access to emergency contraception through pharmacies and effect on unintended pregnancy and STIs: a randomized controlled trial. JAMA. 2005;293:54-62.
28. Reproductive Health Access Project. Telephone triage protocol for emergency contraception. Available at: http://www.reproductiveaccess.org/contraception/tel_triage_ec.htm. Accessed May 31, 2012.
29. American College of Obstetricians and Gynecologists. Sexual assault. Committee Opinion No. 499. Obstet Gynecol. 2011;118:296-399.
• Offer emergency contraception (EC) to any woman who reports contraceptive failure or unprotected intercourse within the last 5 days; no clinical exam is necessary. B
• Prescribe a progestin-only EC or ulipristal acetate, both of which are more effective and have fewer adverse effects than an estrogen-progestin combination. A
• Consider giving sexually active teens <17 years an advance prescription for EC, as it is not available over the counter to this age group. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The average American woman will spend more than 30 years of her life trying to prevent pregnancy—not always successfully. Each year, half of the approximately 6 million pregnancies in the United States are unintended.1 Emergency contraception (EC) gives a woman a second chance to prevent pregnancy after a contraceptive failure or unprotected sex. But all too often, it isn’t offered and she doesn’t request it.
Lack of knowledge about EC continues to be a barrier to its use. Some women have heard about the “morning after pill,” but may not know that EC can be effective for up to 5 days after intercourse—or even that it’s available in this country.2 Others are unaware that it is possible to prevent pregnancy after intercourse,2 and mistakenly believe that EC drugs are abortifacients. In fact, they work primarily by interfering with ovulation and have not been found to prevent implantation or to disrupt an existing pregnancy.3-5
Providers also contribute to the limited use of EC, often because they’re unfamiliar with the options or uncomfortable discussing them with patients, particularly sexually active teens.2
This update can help you clear up misconceptions about EC with your patients. It also provides evidence-based information about the various types of EC, a review of issues affecting accessibility, and a telephone triage protocol to guide your response to women seeking postcoital contraception.
EC today: Plan B and beyond
Hormonal EC was first studied in the 1920s, when researchers found that estrogenic ovarian extracts interfered with pregnancy in animals. The first regimen was a high-dose estrogen-only formulation. In 1974, a combined estrogen-progestin replaced it. Known as the Yuzpe method for the physician who discovered it,6 this regimen used a widely available brand of combined estrogen-progestin oral contraceptive pills. The standard dose consisted of 100 mcg ethinyl estradiol (EE) and 0.5 mg levonorgestrel (LNG) taken 12 hours apart.2,7
Although the Yuzpe method is still in use, progestin-only EC—Plan B as well as generic (Next Choice) and single-dose (Plan B One-Step) LNG formulations—has become the standard of care because it has greater efficacy and fewer adverse effects.2 There are 2 additional options: the copper intrauterine device (IUD), which is highly effective both as EC and as a long-term contraceptive,6 and ulipristal acetate (UPA), which received US Food and Drug Administration (FDA) approval in 2010. This second-generation antiprogestin, sold under the brand name Ella, is well tolerated and highly effective.8
EC efficacy: What the evidence shows
EC is most likely to work when used within 24 hours, but remains effective—albeit to varying degrees—for up to 120 hours (TABLE).2,5,8,9 Thus, which EC is best for a particular patient depends, in part, on timing.
TABLE
Emergency contraception: Comparing methods*2,5,8,9
| EC method | Dose and timing | Benefits | Adverse effects/ drawbacks |
|---|---|---|---|
| Estrogen-progestin OCs | 100 mcg EE and 0.5 mg LNG, taken 12 h apart First dose within 72 h | Easily accessible and widely available; patient may use OCs she already has at home | Higher rates of adverse effects, including nausea, vomiting, headache; less effective than other methods |
| Progestin-only (Plan B, Next Choice, others) | 1.5 mg LNG within 72 h (available in divided doses or in a single tablet; 2 tablets may be taken as a single dose) | Available OTC for patients ≥17 y; more effective and fewer adverse effects than estrogen-progestin Convenience of single dose | Prescription required for patients <17 y Approved for use within 72 h; effectiveness diminishes thereafter |
| UPA (Ella) | 30 mg UPA, taken ≤120 h† | More effective than LNG; fewer adverse effects than estrogen-progestin Efficacy remains high ≤5 days Convenience of single dose | Prescription required; not available at all pharmacies Not studied in breastfeeding‡ |
| Copper IUD | Insert ≤120 h | Extremely effective Provides immediate, long-term contraception | Insertion requires staff training; higher cost than oral EC |
| EC, emergency contraception; EE, ethinyl estradiol; IUD, intrauterine device; LNG, levonorgestrel; OCs, oral contraceptives; OTC, over the counter; UPA, ulipristal acetate. *Low doses of mifepristone (<25-50 mg)—approved as an abortifacient in much larger doses—may also be used as EC. †Dosage should be repeated if vomiting occurs within 3 hours. ‡Advise patients to avoid breastfeeding for 36 hours | |||
Copper IUDs have the highest success rate: Studies have found the copper IUD to be >99% effective in preventing pregnancy when inserted within 5 days of unprotected intercourse.9,10 The copper ions it contains have a toxic effect on sperm, and impair the potential for fertilization; the device may also make the endometrium inhospitable to implantation.9,10
A just-published systematic review of 42 studies in 6 countries over a period of more than 30 years yielded similar results: Among more than 7000 women who had the IUDs inserted after unprotected intercourse, the pregnancy rate was 0.09%.11
But an IUD is appropriate only for women who want long-term contraception and would otherwise qualify for IUD insertion. By comparison, hormonal EC is not as effective and generally works best when used within a shorter time frame.
Progestin alone vs estrogen-progestin combo. To compare hormonal contraception, many researchers use a “prevented fraction”—an estimated percentage of pregnancies averted by treatment. A large World Health Organization-sponsored study found that the efficacy of progestin-only EC is superior to that of the estrogen-progestin combination, with prevented fractions of 85% and 57%, respectively. The progestin-only EC was also associated with significantly fewer adverse effects.12
In more recent studies, the prevented fraction for progestin-only EC has been found to range from 60% to 94%, while a meta-analysis of studies assessing estrogen-progestin EC r evealed a prevented fraction of ≥74%.2
Although there is evidence suggesting that progestin-only EC may work for up to 5 days,13,14 it has FDA approval only for use within 72 hours of intercourse.13 A time-sensitive analysis showed that when it was used within 12 hours of intercourse, the pregnancy rate was 0.5%. The rate increased steadily to 4.1% when the progestin-based EC was taken 61 to 72 hours after intercourse, and rose by an additional 50% after an additional 12-hour delay.15
Hormonal EC is only effective before ovulation occurs. Once luteinizing hormone (LH) starts to rise, it is ineffective. However, the likelihood of pregnancy drops precipitously after ovulation, and there is no risk of pregnancy in the luteal phase, with or without EC.
One pill or 2? Both Plan B and the generic Next Choice are sold as 2-dose regimens, with one 0.75-mg tablet taken within 72 hours and the second taken 12 hours later. Plan B One-Step, which consists of a single 1.5-mg tablet, is clinically equivalent to the 2-dose formula,16 but is more convenient and may improve adherence. Notably, though, one large randomized controlled trial (RCT) in China found that the 2-pill regimen was significantly more effective in preventing pregnancy in women who had further acts of unprotected intercourse after treatment.17
UPA has a 5-day window. UPA has FDA approval for use within 120 hours of unprotected intercourse and has been found to be more effective than progestin-only EC, especially when used on Day 4 or 5 (72-120 hours).8 Adverse effects are mild to moderate, similar to those of LNG, and may include headache, abdominal pain, nausea, dysmenorrhea, fatigue, and dizziness.8
The medication binds to progesterone receptors, acting as an antagonist as well as a partial agonist. The mechanism of action depends on the phase of the woman’s cycle. Taken during the midfollicular phase, UPA inhibits follicle development.18 When used in the advanced follicular phase, just prior to ovulation, it delays LH peak and postpones ovulation.19
In one small study in which women were randomized to either UPA or placebo, researchers found that the drug delayed ovulation for ≥5 days in about 60% of those who took it; in comparison, ovulation occurred by Day 5 in every woman in the placebo group.19
How accessible is EC?
EC has a tumultuous history in the United States,20 and accessibility depends on a variety of factors—age among them.
Plan B, for instance, is subject to a 2-tier system. It was approved in 1999 as a prescription-only product and has been available over the counter (OTC) to women 17 years and older since 2009. Younger women can get it only by prescription.21
Nonetheless, Plan B made the news again last year, when US Health and Human Services Secretary Kathleen Sebelius overruled an FDA decision to give teens younger than 17 OTC access.22 Thus, the age restriction remains in place, although there is no medical evidence to support it.23 Other forms of EC, including UPA, are available to all women only by prescription.
Accessibility of EC also may vary from one part of the country to another. Some states have enacted laws with conscience clauses that allow pharmacists to refuse to dispense EC. Others have worked to increase access by authorizing pharmacists to initiate and dispense EC on their own, provided they work in collaboration with a doctor or other licensed prescriber. As of 2011, 9 states—Alaska, California, Hawaii, Maine, Massachusetts, New Hampshire, New Mexico, Vermont, and Washington—had such agreements in place.24
Cost is another potential barrier. The cost of oral EC varies from about $10 to $70, plus the cost of a doctor visit for a teen who needs a prescription. Obtaining the copper IUD without insurance coverage would cost hundreds of dollars, to cover the price of insertion as well as the device.5
Increasing access: What you can do
In view of the barriers that adolescents face in obtaining EC, the American College of Obstetricians and Gynecologists and the American Academy of Pediatrics, among other organizations, recommend that physicians give advance prescriptions to teens under the age of 17. 2,25
But how likely are they to actually buy the medication and use it on an emergency basis?
A 2007 Cochrane review found that giving women advance prescriptions for EC did not reduce pregnancy or abortion rates.26 Other studies have found that EC use is highest among women with the lowest risk of pregnancy—those who are already using contraception and are less likely to have unprotected intercourse. Those at the highest risk for unintended pregnancy were found to be less likely to use EC after every episode of unprotected intercourse.23,26 One RCT demonstrated that rates of pregnancy and sexually transmitted infection were not significantly increased by advance provision of EC, leading the researchers to conclude that it was therefore unreasonable to restrict access.27
While it is prudent to make women aware that EC is available should they need it, the focus should be on the fact that consistent use of a reliable form of contraception—an IUD or hormonal contraception, in particular—gives them the best chance of preventing an unwanted pregnancy.
What to do when that call comes in
When a woman calls to report a contraceptive failure or tells you she has had unprotected intercourse, start by finding out how recently it occurred. Subsequent questions and actions that can be used by triage nurses or physicians on call are detailed in the easy-to-use EC telephone triage protocol (FIGURE)28 on page 395. Whether you prescribe oral EC or schedule an appointment to insert a copper IUD within the next few days, there are a number of key points to keep in mind.
Initiate EC as soon as possible, but make it available to any woman who requests it for up to 5 days after unprotected intercourse.
Advise patients that oral EC is safe for most women—even those with contraindications to oral contraceptives. No physical examination is necessary, and there’s usually no need for a pregnancy test.2 The one exception: A woman who has not had a period in the past 30 days should be given a pregnancy test before taking UPA.2
Offer EC at any time in the cycle. Although EC works primarily in the preovulatory phase, it should be offered regardless of the phase of the patient’s menstrual cycle. That’s because of the possibility of late ovulation, as well as the difficulty in accurately determining the phase of a woman’s cycle based on a history alone.
Make EC available to any woman who has been sexually abused. At many emergency departments, EC is not routinely offered to women who come in after being raped, although it clearly should be.29
FIGURE
Telephone triage protocol for emergency contraception
EC, emergency contraception; IUD, intrauterine device; LNG, levonorgestrel; Rx, prescription; UPA, ulipristal acetate.
Adapted from: Reproductive Health Access Project. http://www.reproductiveaccess.org/contraception/tel_triage_ec.htm.28
Patient counseling about EC
Advise patients for whom you prescribe oral EC that the medication delays ovulation, which means they could be at risk for pregnancy later in the cycle. Stress the need to use an alternative means of contraception (a barrier method is recommended for women taking UPA) until their next menses and to come in for a pregnancy test if their period is more than a week late.
Point out, too, that EC can be used more than once within the same cycle, if necessary. That said, even a single request for EC should result in a discussion of effective, longer-term contraception, including the possibility of an IUD.
CORRESPONDENCE Sarina Schrager, MD, MS, University of Wisconsin School of Public Health, Department of Family Medicine, 1100 Delaplaine Court, Madison, WI 53715; sbschrag@wisc.edu
• Offer emergency contraception (EC) to any woman who reports contraceptive failure or unprotected intercourse within the last 5 days; no clinical exam is necessary. B
• Prescribe a progestin-only EC or ulipristal acetate, both of which are more effective and have fewer adverse effects than an estrogen-progestin combination. A
• Consider giving sexually active teens <17 years an advance prescription for EC, as it is not available over the counter to this age group. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The average American woman will spend more than 30 years of her life trying to prevent pregnancy—not always successfully. Each year, half of the approximately 6 million pregnancies in the United States are unintended.1 Emergency contraception (EC) gives a woman a second chance to prevent pregnancy after a contraceptive failure or unprotected sex. But all too often, it isn’t offered and she doesn’t request it.
Lack of knowledge about EC continues to be a barrier to its use. Some women have heard about the “morning after pill,” but may not know that EC can be effective for up to 5 days after intercourse—or even that it’s available in this country.2 Others are unaware that it is possible to prevent pregnancy after intercourse,2 and mistakenly believe that EC drugs are abortifacients. In fact, they work primarily by interfering with ovulation and have not been found to prevent implantation or to disrupt an existing pregnancy.3-5
Providers also contribute to the limited use of EC, often because they’re unfamiliar with the options or uncomfortable discussing them with patients, particularly sexually active teens.2
This update can help you clear up misconceptions about EC with your patients. It also provides evidence-based information about the various types of EC, a review of issues affecting accessibility, and a telephone triage protocol to guide your response to women seeking postcoital contraception.
EC today: Plan B and beyond
Hormonal EC was first studied in the 1920s, when researchers found that estrogenic ovarian extracts interfered with pregnancy in animals. The first regimen was a high-dose estrogen-only formulation. In 1974, a combined estrogen-progestin replaced it. Known as the Yuzpe method for the physician who discovered it,6 this regimen used a widely available brand of combined estrogen-progestin oral contraceptive pills. The standard dose consisted of 100 mcg ethinyl estradiol (EE) and 0.5 mg levonorgestrel (LNG) taken 12 hours apart.2,7
Although the Yuzpe method is still in use, progestin-only EC—Plan B as well as generic (Next Choice) and single-dose (Plan B One-Step) LNG formulations—has become the standard of care because it has greater efficacy and fewer adverse effects.2 There are 2 additional options: the copper intrauterine device (IUD), which is highly effective both as EC and as a long-term contraceptive,6 and ulipristal acetate (UPA), which received US Food and Drug Administration (FDA) approval in 2010. This second-generation antiprogestin, sold under the brand name Ella, is well tolerated and highly effective.8
EC efficacy: What the evidence shows
EC is most likely to work when used within 24 hours, but remains effective—albeit to varying degrees—for up to 120 hours (TABLE).2,5,8,9 Thus, which EC is best for a particular patient depends, in part, on timing.
TABLE
Emergency contraception: Comparing methods*2,5,8,9
| EC method | Dose and timing | Benefits | Adverse effects/ drawbacks |
|---|---|---|---|
| Estrogen-progestin OCs | 100 mcg EE and 0.5 mg LNG, taken 12 h apart First dose within 72 h | Easily accessible and widely available; patient may use OCs she already has at home | Higher rates of adverse effects, including nausea, vomiting, headache; less effective than other methods |
| Progestin-only (Plan B, Next Choice, others) | 1.5 mg LNG within 72 h (available in divided doses or in a single tablet; 2 tablets may be taken as a single dose) | Available OTC for patients ≥17 y; more effective and fewer adverse effects than estrogen-progestin Convenience of single dose | Prescription required for patients <17 y Approved for use within 72 h; effectiveness diminishes thereafter |
| UPA (Ella) | 30 mg UPA, taken ≤120 h† | More effective than LNG; fewer adverse effects than estrogen-progestin Efficacy remains high ≤5 days Convenience of single dose | Prescription required; not available at all pharmacies Not studied in breastfeeding‡ |
| Copper IUD | Insert ≤120 h | Extremely effective Provides immediate, long-term contraception | Insertion requires staff training; higher cost than oral EC |
| EC, emergency contraception; EE, ethinyl estradiol; IUD, intrauterine device; LNG, levonorgestrel; OCs, oral contraceptives; OTC, over the counter; UPA, ulipristal acetate. *Low doses of mifepristone (<25-50 mg)—approved as an abortifacient in much larger doses—may also be used as EC. †Dosage should be repeated if vomiting occurs within 3 hours. ‡Advise patients to avoid breastfeeding for 36 hours | |||
Copper IUDs have the highest success rate: Studies have found the copper IUD to be >99% effective in preventing pregnancy when inserted within 5 days of unprotected intercourse.9,10 The copper ions it contains have a toxic effect on sperm, and impair the potential for fertilization; the device may also make the endometrium inhospitable to implantation.9,10
A just-published systematic review of 42 studies in 6 countries over a period of more than 30 years yielded similar results: Among more than 7000 women who had the IUDs inserted after unprotected intercourse, the pregnancy rate was 0.09%.11
But an IUD is appropriate only for women who want long-term contraception and would otherwise qualify for IUD insertion. By comparison, hormonal EC is not as effective and generally works best when used within a shorter time frame.
Progestin alone vs estrogen-progestin combo. To compare hormonal contraception, many researchers use a “prevented fraction”—an estimated percentage of pregnancies averted by treatment. A large World Health Organization-sponsored study found that the efficacy of progestin-only EC is superior to that of the estrogen-progestin combination, with prevented fractions of 85% and 57%, respectively. The progestin-only EC was also associated with significantly fewer adverse effects.12
In more recent studies, the prevented fraction for progestin-only EC has been found to range from 60% to 94%, while a meta-analysis of studies assessing estrogen-progestin EC r evealed a prevented fraction of ≥74%.2
Although there is evidence suggesting that progestin-only EC may work for up to 5 days,13,14 it has FDA approval only for use within 72 hours of intercourse.13 A time-sensitive analysis showed that when it was used within 12 hours of intercourse, the pregnancy rate was 0.5%. The rate increased steadily to 4.1% when the progestin-based EC was taken 61 to 72 hours after intercourse, and rose by an additional 50% after an additional 12-hour delay.15
Hormonal EC is only effective before ovulation occurs. Once luteinizing hormone (LH) starts to rise, it is ineffective. However, the likelihood of pregnancy drops precipitously after ovulation, and there is no risk of pregnancy in the luteal phase, with or without EC.
One pill or 2? Both Plan B and the generic Next Choice are sold as 2-dose regimens, with one 0.75-mg tablet taken within 72 hours and the second taken 12 hours later. Plan B One-Step, which consists of a single 1.5-mg tablet, is clinically equivalent to the 2-dose formula,16 but is more convenient and may improve adherence. Notably, though, one large randomized controlled trial (RCT) in China found that the 2-pill regimen was significantly more effective in preventing pregnancy in women who had further acts of unprotected intercourse after treatment.17
UPA has a 5-day window. UPA has FDA approval for use within 120 hours of unprotected intercourse and has been found to be more effective than progestin-only EC, especially when used on Day 4 or 5 (72-120 hours).8 Adverse effects are mild to moderate, similar to those of LNG, and may include headache, abdominal pain, nausea, dysmenorrhea, fatigue, and dizziness.8
The medication binds to progesterone receptors, acting as an antagonist as well as a partial agonist. The mechanism of action depends on the phase of the woman’s cycle. Taken during the midfollicular phase, UPA inhibits follicle development.18 When used in the advanced follicular phase, just prior to ovulation, it delays LH peak and postpones ovulation.19
In one small study in which women were randomized to either UPA or placebo, researchers found that the drug delayed ovulation for ≥5 days in about 60% of those who took it; in comparison, ovulation occurred by Day 5 in every woman in the placebo group.19
How accessible is EC?
EC has a tumultuous history in the United States,20 and accessibility depends on a variety of factors—age among them.
Plan B, for instance, is subject to a 2-tier system. It was approved in 1999 as a prescription-only product and has been available over the counter (OTC) to women 17 years and older since 2009. Younger women can get it only by prescription.21
Nonetheless, Plan B made the news again last year, when US Health and Human Services Secretary Kathleen Sebelius overruled an FDA decision to give teens younger than 17 OTC access.22 Thus, the age restriction remains in place, although there is no medical evidence to support it.23 Other forms of EC, including UPA, are available to all women only by prescription.
Accessibility of EC also may vary from one part of the country to another. Some states have enacted laws with conscience clauses that allow pharmacists to refuse to dispense EC. Others have worked to increase access by authorizing pharmacists to initiate and dispense EC on their own, provided they work in collaboration with a doctor or other licensed prescriber. As of 2011, 9 states—Alaska, California, Hawaii, Maine, Massachusetts, New Hampshire, New Mexico, Vermont, and Washington—had such agreements in place.24
Cost is another potential barrier. The cost of oral EC varies from about $10 to $70, plus the cost of a doctor visit for a teen who needs a prescription. Obtaining the copper IUD without insurance coverage would cost hundreds of dollars, to cover the price of insertion as well as the device.5
Increasing access: What you can do
In view of the barriers that adolescents face in obtaining EC, the American College of Obstetricians and Gynecologists and the American Academy of Pediatrics, among other organizations, recommend that physicians give advance prescriptions to teens under the age of 17. 2,25
But how likely are they to actually buy the medication and use it on an emergency basis?
A 2007 Cochrane review found that giving women advance prescriptions for EC did not reduce pregnancy or abortion rates.26 Other studies have found that EC use is highest among women with the lowest risk of pregnancy—those who are already using contraception and are less likely to have unprotected intercourse. Those at the highest risk for unintended pregnancy were found to be less likely to use EC after every episode of unprotected intercourse.23,26 One RCT demonstrated that rates of pregnancy and sexually transmitted infection were not significantly increased by advance provision of EC, leading the researchers to conclude that it was therefore unreasonable to restrict access.27
While it is prudent to make women aware that EC is available should they need it, the focus should be on the fact that consistent use of a reliable form of contraception—an IUD or hormonal contraception, in particular—gives them the best chance of preventing an unwanted pregnancy.
What to do when that call comes in
When a woman calls to report a contraceptive failure or tells you she has had unprotected intercourse, start by finding out how recently it occurred. Subsequent questions and actions that can be used by triage nurses or physicians on call are detailed in the easy-to-use EC telephone triage protocol (FIGURE)28 on page 395. Whether you prescribe oral EC or schedule an appointment to insert a copper IUD within the next few days, there are a number of key points to keep in mind.
Initiate EC as soon as possible, but make it available to any woman who requests it for up to 5 days after unprotected intercourse.
Advise patients that oral EC is safe for most women—even those with contraindications to oral contraceptives. No physical examination is necessary, and there’s usually no need for a pregnancy test.2 The one exception: A woman who has not had a period in the past 30 days should be given a pregnancy test before taking UPA.2
Offer EC at any time in the cycle. Although EC works primarily in the preovulatory phase, it should be offered regardless of the phase of the patient’s menstrual cycle. That’s because of the possibility of late ovulation, as well as the difficulty in accurately determining the phase of a woman’s cycle based on a history alone.
Make EC available to any woman who has been sexually abused. At many emergency departments, EC is not routinely offered to women who come in after being raped, although it clearly should be.29
FIGURE
Telephone triage protocol for emergency contraception
EC, emergency contraception; IUD, intrauterine device; LNG, levonorgestrel; Rx, prescription; UPA, ulipristal acetate.
Adapted from: Reproductive Health Access Project. http://www.reproductiveaccess.org/contraception/tel_triage_ec.htm.28
Patient counseling about EC
Advise patients for whom you prescribe oral EC that the medication delays ovulation, which means they could be at risk for pregnancy later in the cycle. Stress the need to use an alternative means of contraception (a barrier method is recommended for women taking UPA) until their next menses and to come in for a pregnancy test if their period is more than a week late.
Point out, too, that EC can be used more than once within the same cycle, if necessary. That said, even a single request for EC should result in a discussion of effective, longer-term contraception, including the possibility of an IUD.
CORRESPONDENCE Sarina Schrager, MD, MS, University of Wisconsin School of Public Health, Department of Family Medicine, 1100 Delaplaine Court, Madison, WI 53715; sbschrag@wisc.edu
1. Guttmacher Institute. National Reproductive Health profile. Available at: http://www.guttmacher.org/datacenter/profiles/print/US.jsp. Accessed November 3, 2011.
2. American College of Obstetricians and Gynecologists ACOG practice bulletin No. 112. Emergency contraception. Obstet Gynecol. 2010;115:1100–1109.
3. Belluck P. No abortion role seen for morning-after pill. New York Times. June 6, 2012; A1.
4. Trussell J, Raymond E. Emergency contraception: a last chance to prevent unintended pregnancy. Princeton, NJ: Office of Population Research at Princeton University; June 2012. Available at: http://ec.princeton.edu/questions/ec-review.pdf. Accessed June 20, 2012.
5. Planned Parenthood. Morning-after pill (emergency contraception). Available at: http://www.plannedparenthood.org/health-topics/emergency-contraception-morning-after-pill-4363.asp. Accessed June 7, 2012.
6. Ellertson C. History and efficacy of emergency contraception: beyond Coca-Cola. Fam Plann Perspect. 1996;28:44-48.
7. Yuzpe AA, Thurlow HJ, Ramzy I, et al. Post coital contraception—a pilot study. J Reprod Med. 1974;13:53-58.
8. Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet. 2010;375:555-562.
9. Belden P, Harper CC, Speidel J. The copper IUD for emergency contraception, a neglected option. Contraception. 2012;85:338-339.
10. Wu S, Godfrey EM, Wojdyla D, et al. Copper T380A intrauterine device for emergency contraception: a prospective, multicentre, cohort clinical trial. Br J Obstet Gynaecol. 2010;117:1205-1210.
11. Cleland K, Zhu H, Goldstuck N, et al. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod. 2012 May 8 [Epub ahead of print].
12. World Health Organization’s Task Force on postovulatory methods of fertility regulation. Randomised controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Lancet. 1998;352:428-433.
13. US Food and Drug Administration. Plan B: questions and answers. Updated December 14, 2006. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm109783.htm. Accessed June 7, 2012.
14. Trussell J, Rodriguez G, Ellertson C. A meta-analysis of efficacy for the Yuzpe method (estrogen-progestin). Contraception. 1999;59:147-151.
15. Piaggio G, von Hertzen H, Grimes DA, et al. Task Force on Postovulatory Methods of Fertility. Timing of emergency contraception with levonorgestrel or the Yuzpe regimen. Lancet. 1999;353:721.
16. Cheng L, Gülmezoglu AM, Piaggio GGP, et al. Interventions for emergency contraception. Cochrane Database Syst Rev. 2008;(2):CD001324.
17. Ngai SW, Fan S, Li S, et al. A randomized trial to compare 24 h versus 12 h double dose regimen of levonorgestrel for emergency contraception. Hum Reprod. 2005;20:307-311.
18. Stratton P, Hartog B, Hajizadeh N, et al. A single mid-follicular dose of CDB-2914, a new antiprogestin, inhibits folliculogenesis and endometrial differentiation in normally cycling women. Hum Reprod. 2000;5:1092-1099.
19. Brache V, Cochon L, Jesam C, et al. Immediate pre-ovulatory administration of 30 mg ulipristal acetate significantly delays follicular rupture. Hum Reprod. 2010;25:2256-2263.
20. Kliff S. Plan B’s complicated history. Available at: http://www.thedailybeast.com/newsweek/2009/08/24/plan-b-s-complicated-history.html/. Accessed June 7, 2012.
21. US Food and Drug Administration Updated FDA action on Plan B (levonorgestrel) tablets. April 22, 2009. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2009/ucm149568.htm. Accessed June 7, 2012.
22. US Department of Health and Human Services. A statement by U.S. Department of Health and Human Services Secretary Kathleen Sebelius. December 7, 2011. Available at: http://www.hhs.gov/news/press/2011pres/12/20111207a.html. Accessed June 7, 2012.
23. Duffy K, Gold M. Adolescents and emergency contraception: update 2011. Curr Opin Obstet Gynecol. 2011;23:328-333.
24. National Conference of State Legislatures. Emergency contraception state laws. Updated July 2011. Available at: http://www.ncsl.org/issues-research/health/emergency-contraception-state-laws.aspx. Accessed May 31, 2012.
25. Cash S. New AAP policy advises on emergency contraception use. AAP News. 2005;26:1.
26. Polis CB, Grimes DA, Schaffer K, et al. Advance provision of EC for pregnancy prevention. Cochrane Database Syst Rev. 2010;(2):CD005497.
27. Raine T, Harper CC, Rocca CH, et al. Direct access to emergency contraception through pharmacies and effect on unintended pregnancy and STIs: a randomized controlled trial. JAMA. 2005;293:54-62.
28. Reproductive Health Access Project. Telephone triage protocol for emergency contraception. Available at: http://www.reproductiveaccess.org/contraception/tel_triage_ec.htm. Accessed May 31, 2012.
29. American College of Obstetricians and Gynecologists. Sexual assault. Committee Opinion No. 499. Obstet Gynecol. 2011;118:296-399.
1. Guttmacher Institute. National Reproductive Health profile. Available at: http://www.guttmacher.org/datacenter/profiles/print/US.jsp. Accessed November 3, 2011.
2. American College of Obstetricians and Gynecologists ACOG practice bulletin No. 112. Emergency contraception. Obstet Gynecol. 2010;115:1100–1109.
3. Belluck P. No abortion role seen for morning-after pill. New York Times. June 6, 2012; A1.
4. Trussell J, Raymond E. Emergency contraception: a last chance to prevent unintended pregnancy. Princeton, NJ: Office of Population Research at Princeton University; June 2012. Available at: http://ec.princeton.edu/questions/ec-review.pdf. Accessed June 20, 2012.
5. Planned Parenthood. Morning-after pill (emergency contraception). Available at: http://www.plannedparenthood.org/health-topics/emergency-contraception-morning-after-pill-4363.asp. Accessed June 7, 2012.
6. Ellertson C. History and efficacy of emergency contraception: beyond Coca-Cola. Fam Plann Perspect. 1996;28:44-48.
7. Yuzpe AA, Thurlow HJ, Ramzy I, et al. Post coital contraception—a pilot study. J Reprod Med. 1974;13:53-58.
8. Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet. 2010;375:555-562.
9. Belden P, Harper CC, Speidel J. The copper IUD for emergency contraception, a neglected option. Contraception. 2012;85:338-339.
10. Wu S, Godfrey EM, Wojdyla D, et al. Copper T380A intrauterine device for emergency contraception: a prospective, multicentre, cohort clinical trial. Br J Obstet Gynaecol. 2010;117:1205-1210.
11. Cleland K, Zhu H, Goldstuck N, et al. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod. 2012 May 8 [Epub ahead of print].
12. World Health Organization’s Task Force on postovulatory methods of fertility regulation. Randomised controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Lancet. 1998;352:428-433.
13. US Food and Drug Administration. Plan B: questions and answers. Updated December 14, 2006. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm109783.htm. Accessed June 7, 2012.
14. Trussell J, Rodriguez G, Ellertson C. A meta-analysis of efficacy for the Yuzpe method (estrogen-progestin). Contraception. 1999;59:147-151.
15. Piaggio G, von Hertzen H, Grimes DA, et al. Task Force on Postovulatory Methods of Fertility. Timing of emergency contraception with levonorgestrel or the Yuzpe regimen. Lancet. 1999;353:721.
16. Cheng L, Gülmezoglu AM, Piaggio GGP, et al. Interventions for emergency contraception. Cochrane Database Syst Rev. 2008;(2):CD001324.
17. Ngai SW, Fan S, Li S, et al. A randomized trial to compare 24 h versus 12 h double dose regimen of levonorgestrel for emergency contraception. Hum Reprod. 2005;20:307-311.
18. Stratton P, Hartog B, Hajizadeh N, et al. A single mid-follicular dose of CDB-2914, a new antiprogestin, inhibits folliculogenesis and endometrial differentiation in normally cycling women. Hum Reprod. 2000;5:1092-1099.
19. Brache V, Cochon L, Jesam C, et al. Immediate pre-ovulatory administration of 30 mg ulipristal acetate significantly delays follicular rupture. Hum Reprod. 2010;25:2256-2263.
20. Kliff S. Plan B’s complicated history. Available at: http://www.thedailybeast.com/newsweek/2009/08/24/plan-b-s-complicated-history.html/. Accessed June 7, 2012.
21. US Food and Drug Administration Updated FDA action on Plan B (levonorgestrel) tablets. April 22, 2009. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2009/ucm149568.htm. Accessed June 7, 2012.
22. US Department of Health and Human Services. A statement by U.S. Department of Health and Human Services Secretary Kathleen Sebelius. December 7, 2011. Available at: http://www.hhs.gov/news/press/2011pres/12/20111207a.html. Accessed June 7, 2012.
23. Duffy K, Gold M. Adolescents and emergency contraception: update 2011. Curr Opin Obstet Gynecol. 2011;23:328-333.
24. National Conference of State Legislatures. Emergency contraception state laws. Updated July 2011. Available at: http://www.ncsl.org/issues-research/health/emergency-contraception-state-laws.aspx. Accessed May 31, 2012.
25. Cash S. New AAP policy advises on emergency contraception use. AAP News. 2005;26:1.
26. Polis CB, Grimes DA, Schaffer K, et al. Advance provision of EC for pregnancy prevention. Cochrane Database Syst Rev. 2010;(2):CD005497.
27. Raine T, Harper CC, Rocca CH, et al. Direct access to emergency contraception through pharmacies and effect on unintended pregnancy and STIs: a randomized controlled trial. JAMA. 2005;293:54-62.
28. Reproductive Health Access Project. Telephone triage protocol for emergency contraception. Available at: http://www.reproductiveaccess.org/contraception/tel_triage_ec.htm. Accessed May 31, 2012.
29. American College of Obstetricians and Gynecologists. Sexual assault. Committee Opinion No. 499. Obstet Gynecol. 2011;118:296-399.
Managing psoriasis: What’s best for your patient?
• Prescribe high-potency (Class 1) topical steroids for use on thicker, chronic plaques and low-potency (Class 7) steroids for the face, intertriginous areas, and the groin. A
• Recommend folate supplementation (1-5 mg/d) for patients being treated with methotrexate, as it may reduce the drug’s hematologic and gastrointestinal adverse effects without decreasing efficacy. A
• Advise patients scheduled to begin biologic therapy to get standard vaccinations—eg, pneumococcal, influenza, hepatitis A and B—before treatment is initiated and to avoid live and live-attenuated vaccines thereafter. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Psoriasis is a systemic inflammatory disorder altered by environmental and genetic factors that presents as scaly erythematous plaques and affects 2% to 3% of the population.1 Eighty percent have mild-to-moderate cutaneous disease; disabling arthritis occurs in as many as 42% of cases.1,2 The devastating effects on quality of life— including social stigmatization, pain, physical disability, and psychological distress—are comparable to the effects of conditions like cancer and depression.2 There is no definitive cure, and patients are left with a lifetime of waxing and waning symptoms.
Helping to create an individualized treatment plan tailored to disease type and extent, comorbidities, and needs of the patient can directly impact the quality of his or her life.3 For those with localized disease, topical therapy is a suitable first choice. Phototherapy is generally the first-line treatment for patients with extensive psoriasis or disabling symptoms. When phototherapy is not feasible or is ineffective, systemic treatments with conventional oral agents or biologics are indicated.
Discussions about therapeutic options should include expected results and duration of remission, cost, convenience, adverse effects, insurance coverage, and safety concerns. The patient’s preferences should be taken into account in the treatment plan you create.3 A guiding principle: The optimal protocol is the one the patient is motivated to adhere to.
The limits of topical therapy
Topical treatments are safe and effective when used properly, as monotherapy for localized disease and adjunctive therapy for resistant lesions. Monotherapy with topical agents is not recommended for sites that have a significant impact on quality of life, such as the palms and soles, and is not practical for patients with extensive disease (>10% of body surface).4
More potent topicals, such as corticosteroids, are recommended for acute flares; less potent agents with fewer adverse effects, such as calcipotriene, are typically used for maintenance ( TABLE 1 ).5-8 Topical agents can be used either long term or intermittently. Here, too, the most effective treatment is the one the patient will actually apply.5
TABLE 1
A look at nonsteroidal topical treatments5-8
| Class | Examples | Adverse effects | Comments |
|---|---|---|---|
| Vitamin D analogues | Calcipotriol (calcipotriene), calcitriol | Burning, pruritus, edema, peeling, dryness, erythema | Combining with beta-methasone dipropionate increases efficacy |
| Retinoids | Tazarotene, tretinoin | Teratogenic, photosensitivity, irritation | Increased efficacy when combined with NB-UVB (and less UV exposure); increased efficacy, duration of remission, and reduction in steroid-induced atrophy when used with steroids |
| Calcineurin inhibitors | Tacrolimus, pimecrolimus | Burning and pruritus Black box warning for risk of malignancies* | No clinical evidence of increased cancer risk |
| Others | Emollients, salicylic acid, anthralin, coal tar | Severe skin irritation, staining of clothes, odor with anthralin and tar | Salicylic acid works well with steroids and topical immunomodulators, but is not compatible with calcipotriene |
| *Lymphoma seen with oral therapy. UVB, ultraviolet B; NB-UVB, narrow-band ultraviolet B. | |||
Steroid selection is based on site, severity
Corticosteroids are antiproliferative, immunosuppressive, anti-inflammatory, and vasoconstrictive, and divided into 7 classes. Low-potency agents (Class 7) are used on thinner skin like the face, intertriginous areas, and groin; high-potency steroids (Class 1) are reserved for thicker, chronic plaques.9 As a general rule, Class 1 steroids can be safely used for 2 to 4 weeks, with increased risk of both cutaneous effects and systemic absorption if used continuously for longer periods.5 The optimal end point for less potent agents is not known.
Cutaneous adverse effects are more common than systemic ones and include skin atrophy, telangiectasia, striae distensae, acne, folliculitis, and purpura.
Systemic adverse effects include Cushing’s syndrome, osteonecrosis of the femoral head, cataracts, and glaucoma.5 The greatest risk of systemic effects is associated with prolonged use of high-potency corticosteroids over large surfaces or under occlusion with dressings or plastic wrap. Patients should be transitioned to the lowest potency possible to maintain efficacy, use corticosteroids intermittently, or combine them with nonsteroidal agents to avoid unwanted effects.
Topicals aren’t working? Move on to phototherapy
Phototherapy is an option for patients with extensive disease or skin manifestations that are recalcitrant to topicals. It is efficacious, cost-effective, and lacks systemic immunosuppression. Ultraviolet (UV) A and B act on Langerhans cells, cytokines, and adhesion molecules, inhibiting epidermal proliferation and angiogenesis.10
Broadband UVB (BB-UVB) was first used during the 1950s, with crude tar or anthralin, but is rarely used today.
Narrow-band UVB (NB-UVB) (311-313 nm), developed in the 1980s, has largely replaced BB-UVB. In addition to providing more rapid clearing and resolution of psoriasis compared with BB-UVB, NB-UVB may have less phototoxicity.11 Between 20 and 25 NB-UVB treatments, 2 to 3 times a week (in the office or at home) are usually required for significant improvement.
Photocarcinogenesis is a concern but numerous studies, including a review of 3867 patients treated with NB-UVB with a median 5.5-year follow-up, found no significant association with cutaneous malignancies.12 NB-UVB is considered safe during pregnancy and used as first-line therapy for pregnant patients.13
Targeted UVB therapy using a 308-nm excimer laser, another option, selectively targets psoriatic lesions, leaving normal skin untreated. This makes supraerythemogenic doses possible, which increases UVB’s efficacy. Long-term adverse effects and duration of remission have not been clearly established.14
Psoralen and UVA (PUVA), which uses oral or topical psoralens to sensitize the skin to UVA, has a slightly higher efficacy than NB-UVB, but with increased risk of squamous cell carcinoma (SCC) and possibly melanoma.15 Clearing can occur within 24 treatments, with remissions lasting 3 to 6 months;16 monthly maintenance has not been found to lengthen remission.13
Common adverse effects include erythema that peaks 48 to 96 hours after a treatment, pruritus, xerosis, irregular pigmentation, and gastrointestinal symptoms that can be reduced by decreasing psoralen and/or UVA doses.13 High cumulative doses of oral PUVA (>200 treatments) is associated with a dose-related increased risk of nonmelanoma skin cancer, particularly SCC, in the Caucasian population. This increased risk has not been demonstrated in patients treated with PUVA bath therapy, which is more common in Scandinavian countries.17 There is no consensus regarding the risk of melanoma.15
A careful risk-benefit analysis is needed before initiating phototherapy in patients who take photosensitizing drugs, are immunosuppressed, or have a photosensitivity disorder or a history of melanoma, atypical nevi, multiple melanoma risk factors, or multiple nonmelanoma skin cancers.13 Regardless of the type of UV therapy administered, eye protection with goggles is required to decrease the risk of UV-related cataract formation, and genital shielding is needed to prevent increased risk of tumors.13 Photoaging is a long-term effect.
CASE When questioned further about the challenges that Tom has had with controlling his symptoms, he admitted to being noncompliant. As a busy executive, he said he didn’t have time to use the topical corticosteroids regularly. Phototherapy could alleviate his cutaneous symptoms, but would not address his symptoms that were consistent with psoriatic arthritis.
Pairing therapeutic modalities decreases exposure
Combining therapeutic modalities like emollients and topical or oral retinoids with NB-UVB improves efficacy while reducing the number of treatment sessions and cumulative UVB dosage. If calcipotriene is used, it should be applied after phototherapy because it is degraded upon UVB exposure.18 Acitretin should be started 2 weeks prior to initiation of phototherapy, and its use accompanied by a 25% reduction in initial UV dosage.13
PUVA may also be combined with topical calcipotriene or retinoids.19 In both cases, the addition of the other agent typically decreases the duration of phototherapy, improves the clinical response, and reduces the risk of cancer.13,20
Extensive disease? Consider a traditional systemic agent
Traditional systemic therapy is used to treat extensive disease ( FIGURE ), psoriasis refractory to topical agents, and debilitating disease on the palms, soles, or scalp. Biologics are a recent alternative, but traditional systemics have been utilized longer and have a more longstanding adverse effect and safety profile, are administered orally, and are much less expensive than biologics. Monitoring patients on systemic therapy is necessary ( TABLE 2 ).21-24
Methotrexate (MTX), a competitive inhibitor of dihydrofolate reductase, is the most commonly prescribed traditional systemic psoriasis treatment.21 It is administered in a single weekly dose via tablet, parenteral solution, or intramuscular (IM) or subcutaneous (SC) injection.25 A test dose (2.5 or 5 mg) is given initially and complete blood cell count is monitored within one week to evaluate for potential bone marrow toxicity. If none is observed, the dose may be increased to control the disease while minimizing adverse effects.21
Common adverse effects of MTX, such as nausea, vomiting, stomatitis, and fatigue, may be minimized by IM or SC administration, splitting the dose, or providing folate supplementation.21-24 Given in doses of 1 to 5 mg/d, folate may reduce adverse hematologic, gastrointestinal, and hepatic effects without decreasing efficacy.22
The major severe toxicities are myelosuppression, hepatotoxicity, and pulmonary fibrosis.24 MTX-induced hepatotoxicity is similar to nonalcoholic fatty liver disease (NAFLD) and is thought to exacerbate preexisting NAFLD, which is common in patients with metabolic syndrome. A liver biopsy or serum assays for liver fibrosis (amino-terminal peptide of pro-collagen III) may be warranted during therapy.24
MTX is an abortifacient and teratogen, so contraception during treatment and for up to 3 months thereafter is mandatory for women of childbearing age.26 Men should be advised that MTX decreases sperm count. (For more on methotrexate, see: “When a fetus survives methotrexate exposure,” at http://www.jfponline.com/Pages.asp?AID=10299).
Cyclosporine (CSA), an oral calcineurin inhibitor, is a potent immunosuppressant that rapidly clears psoriasis.27 Because duration of use correlates with permanent nephrotoxicity, hypertension, and potential increased risk of SCC and lymphoma, intermittent 12-week courses are recommended. Calcium channel blockers are the preferred treatment for CSA-induced hypertension because of their effect on smooth muscle vasodilation.21
Oral retinoids. Acitretin modulates epidermal proliferation and is anti-inflammatory. Because it lacks immunosuppression, acitretin is generally considered the treatment of choice in HIV patients with severe psoriasis.28 Acitretin is teratogenic and contraindicated in women who plan to become pregnant or who are unwilling to use adequate contraception for 3 years after discontinuing the drug.21
CASE Given the significant percentage of body surface area involved and symptoms consistent with psoriatic arthritis, Tom required an aggressive therapeutic regimen. His history of nonalcoholic fatty liver and social drinking precluded the use of methotrexate. A biologic therapy was the next therapeutic choice that could relieve both his cutaneous and joint symptoms.
FIGURE
4 psoriasis patients, 4 different presentations
A. The linear erythematous, scaly plaque along this patient’s cardiac bypass scar demonstrates koebnerization of plaque psoriasis.
B. Diffuse erythematous scaly papules coalesce into plaques on this patient’s anterior chest, arms, abdomen, and periumbilicus.
C. The pinpoint pustules on an erythematous base on this patient’s lateral neck, shoulders, and upper back are characteristic of pustular psoriasis.
D. This patient has erythematous plaques with overlying silvery scale on the elbow—a classic
TABLE 2
Traditional systemic therapy21-24
| Methotrexate (MTX) | Cyclosporine (CSA)* | Acitretin | |
|---|---|---|---|
| Dosing | ≤30 mg in one weekly dose (PO, IM, or SC) | 2.5-5.0 mg/kg/d in 2 divided doses for 12 wk, then 12-wk nontreatment period Dose decreased (by 0.5-1.0 mg/kg) with disease clearance or when hypertension or nephrotoxicity are detected | 10-50 mg/d given as a single dose Lower doses (25 mg/d) used to minimize adverse effects and in combination regimens When added to UV, light dose should be reduced 30%-50% |
| Contraindications |
|
|
|
| Baseline monitoring† |
|
|
|
| BP, blood pressure; BUN, blood urea nitrogen; CBC, complete blood cell count; CR, creatinine; H&P, history and physical; IM, intramuscular; LFTs, liver function tests; PO, by mouth; PPD, purified protein derivative; PUVA, psoralen and ultraviolet A; SC, subcutaneous; UV, ultraviolet; UVB, ultraviolet B. *Avoid live vaccinations; caution required with major infection and poorly controlled diabetes. †Ongoing monitoring for MTX: BUN, CBC, CR, LFTs; possible liver biopsy (for high-risk patients or cumulative dose >3.5-4 g); CSA: BP, BUN, CBC, CR, LFTs; lipd profile; magnesium, uric acid, potassium tests; pregnancy testing; Acitretin: CBC, LFTs, lipid profile, renal function test, pregnancy testing. | |||
Biologics require lab work and a detailed medication list
Before beginning biologic therapy for a patient, the National Psoriasis Foundation29 recommends obtaining a complete history, physical, medication list, future plans (ie, pregnancy or travel to locations requiring vaccinations), and baseline labs to identify possible risk factors and/or contraindications. Periodic evaluation to monitor development of new symptoms, including infection and malignancy ( TABLE 3 ),24,30-33 is needed, as well.
TABLE 3
Is your patient a candidate for biologics?24,30-33
| Agent (Drug class) | Alefacept (T-cell inhibitor) | Adalimumab (TNF-inhibitor) | Etanercept (TNF-inhibitor) | Infliximab (TNF-inhibitor) | Ustekinumab (IL-12/23 inhibitor) |
|---|---|---|---|---|---|
| Dosing | 15 mg IM/wk for 12 wk, then 12-wk nontreatment period | 80 mg SC the first wk, 40 mg the 2nd wk, followed by 40 mg every other wk | 50 mg SC twice/wk for 3 mo, then 50 mg/wk | 5 mg/kg IV infusion to start, repeat at 2 and 6 wk, then q6-8 wk | 45 mg SC (for patients <100 kg); 90 mg (for patients >100 kg) to start, repeat at 4 wk, followed by q12 wk for maintenance |
| Contra-indications | HIV |
|
|
| Active TB |
| Baseline monitoring | CD4 count |
|
|
| PPD |
| Ongoing monitoring | Biweekly CD4 count; hold dose for counts <250 |
|
|
| Consider a yearly PPD |
| CBC, complete blood cell count; CHF, congestive heart failure; HIV, human immunodeficiency virus; H&P, history and physical; IL-12/23, interleukin-12, interleukin-23; LFTs, liver function test; MS, multiple sclerosis; NYHA, New York Heart Association; PPD, purified protein derivative; TB, tuberculosis; TNF, tumor necrosis factor. *For this patient population, adalimumab and etanercept have a (theoretical) risk. | |||||
Biologic therapy is contraindicated in patients with active serious infection. If patients develop infections requiring antibiotics while being treated, holding the biologic until infection resolution is advised.34 Standard vaccinations (eg, pneumococcal, hepatitis A and B, influenza, diphtheria, tetanus) are recommended before initiation of immunosuppressive therapy. After therapy starts, patients should avoid live and live-attenuated vaccines (varicella, mumps, measles, and rubella, oral typhoid, yellow fever, herpes zoster, intranasal influenza).35
Currently, none of the biologics are indicated for use in children or adolescents with psoriasis, despite epidemiologic data suggesting that one-third of adults with psoriasis developed it during childhood, in a form severe enough to warrant the use of systemic medications.34 The FDA is currently reviewing the possibility of indicating etanercept for pediatric psoriasis patients. All biologics are category B for pregnancy as there is no evidence that they negatively affect pregnancy.24
T-cell inhibitor. Alefacept binds CD2 on memory-effector T lymphocytes, inhibiting activation. Weekly intramuscular injections of alefacept for 12 weeks can clear lesions with long remissions.30
TNF-inhibitors. The TNF-inhibitors have been available for more than 10 years, predominantly for inflammatory bowel disease (IBD) and rheumatoid arthritis (RA), and more than 1.5 million patients have used adalimumab, etanercept, and infliximab for these disorders. Safety data, especially long term, are mostly derived from patients with IBD or RA, who have often combined TNF-inhibitors with additional immunosuppressive therapies. Thus, for psoriasis patients, who typically use biologics as monotherapy, the risk may be overestimated.24
TNF-inhibitors increase the risk for infection, most commonly of the upper respiratory tract, and, rarely, have been associated with opportunistic infections. Numerous cases of TB reactivation and an increased incidence of disseminated cases have been associated with TNF-inhibitors, so screening is recommended.24
The impact of TNF inhibition on congestive heart failure (CHF) is not well understood. Studies have variously shown that TNF-inhibitors have no effect on CHF morbidity or mortality, increase CHF mortality, or improve left ventricular function. TNF-inhibitors should be avoided in patients with severe CHF (New York Heart Association class III or IV). In milder CHF patients with worsening of symptoms, treatment should be discontinued.36
The increased risk of malignancy, especially lymphoma, is a concern, as there have been numerous case reports of lymphoma occurring with TNF-inhibitors. Psoriasis patients in general have an increased risk of lymphoma that confounds data interpretation.31 A number of case reports and a large observational study have shown patients receiving TNF-inhibitors may be at a greater risk for developing melanoma and nonmelanoma skin cancer.32
Ustekinumab, an interleukin 12/23 inhibitor, is a human monoclonal antibody that is absorbed and eliminated slowly, making dosing injections every 12 weeks convenient with efficacy maintained for at least one year.33 Because of its relative novelty, few studies are published regarding long-term safety. A recent head-to-head trial compared the efficacy and safety of ustekinumab with etanercept and found superior efficacy with ustekinumab, with comparable adverse events.37,38 Similar concerns exist with ustekinumab as with TNF-inhibitors, including infection, malignancy, CHF, and TB.33
CASE Tom denied having a personal family history of multiple sclerosis, or any demyelinating disorder. Nor did he have a history of cancer, tuberculosis exposure, CHF, or hepatitis. A purified protein derivative (PPD) was negative, as was his hepatitis panel, and his complete blood count with differential and metabolic panel were within normal limits.
Tom was started on the TNF-inhibitor adalimumab, after undergoing patient education and training and receiving instructions to stop the medication if he developed a major illness or infection. He received a loading dose of 80 mg SC, followed by 40 mg every other week. He tolerated the treatment well and 70% of his cutaneous symptoms cleared after 12 weeks of therapy; his joint pain also was reduced.
Tom is followed regularly in the clinic, with labs every 4 to 6 months. He is maintained on the injections and happy with the results. At each visit, weight loss and decreased beer intake are encouraged, both of which have been shown to reduce psoriasis severity. Although the beta-blockers and ACE inhibitors he takes are known to exacerbate psoriasis, the medications are necessary to treat Tom’s coronary artery disease.
CORRESPONDENCE Elizabeth Uhlenhake, MD, 18100 Oakwood Boulevard, Suite 300, Dearborn, MI 48124; eulenha@neomed.edu
1. Gelfand JM, Weinstein R, Porter SB, et al. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141:1537-1541.
2. Fortune DG, Richards HL, Griffiths CE. Psychologic factors in psoriasis: consequences, mechanisms, and interventions. Dermatol Clin. 2005;23:681-694.
3. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
4. Pettey AA, Balkrishnan R, Rapp SR, et al. Patients with palmoplantar psoriasis have more physical disability and discomfort than patients with other forms of psoriasis: implications for clinical practice. J Am Acad Dermatol. 2003;49:271-275.
5. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
6. Kaufmann R, Bibby AJ, Bissonnette R, et al. A new calcipotriol/betamethasone dipropionate formulation (Daivobet) is an effective once-daily treatment for psoriasis vulgaris. Dermatology. 2002;205:389-393.
7. Koo JY, Martin D. Investigator-masked comparison of tazarotene gel q.d. plus mometasone furoate cream q.d. vs. mometasone furoate cream b.i.d. in the treatment of plaque psoriasis. Int J Dermatol. 2001;40:210-212.
8. Berger TG, Duvic M, Van Voorhees AS, et al. The use of topical calcineurin inhibitors in dermatology: safety concerns. Report of the American Academy of Dermatology Association Task Force. J Am Acad Dermatol. 2006;54:818-823.
9. Cornell RC, Stoughton RB. Correlation of the vasoconstriction assay and clinical activity in psoriasis. Arch Dermatol. 1985;121:63-67.
10. Lui H. Phototherapy of psoriasis: update with practical pearls. J Cutan Med Surg. 2002;6(suppl):17-21.
11. Walters IB, Burack LH, Coven TR, et al. Suberythemogenic narrow-band UVB is markedly more effective than conventional UVB in treatment of psoriasis vulgaris. J Am Acad Dermatol. 1999;40:893-900.
12. Stern RS, Laird N. The carcinogenic risk of treatments for severe psoriasis. Photochemotherapy follow-up study. Cancer. 1994;73:2759-2764.
13. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
14. Trehan M, Taylor CR. High-dose 308-nm excimer laser for the treatment of psoriasis. J Am Acad Dermatol. 2002;46:732-737.
15. Morison WL, Baughman RD, Day RM, et al. Consensus workshop on the toxic effects of long-term PUVA therapy. Arch Dermatol. 1998;134:595-598.
16. Spuls PI, Witkamp L, Bossuyt PM, et al. A systematic review of five systemic treatments for severe psoriasis. Br J Dermatol. 1997;137:943-949.
17. Murase JE, Lee EE, Koo J. Effect of ethnicity on the risk of developing nonmelanoma skin cancer following long-term PUVA therapy. Int J Dermatol. 2005;44:1016-1021.
18. Lowe NJ, Prystowsky JH, Bourget T, et al. Acitretin plus UVB therapy for psoriasis. Comparisons with placebo plus UVB and acitretin alone. J Am Acad Dermatol. 1991;24:591-594.
19. Torras H, Aliaga A, Lopez-Estebaranz JL, et al. A combination therapy of calcipotriol cream and PUVA reduces the UVA dose and improves the response of psoriasis vulgaris. J Dermatolog Treat. 2004;15:98-103.
20. Tanew A, Guggenbichler A, Honigsmann H, et al. Photochemotherapy for severe psoriasis without or in combination with acitretin: a randomized, double-blind comparison study. J Am Acad Dermatol. 1991;25:682-684.
21. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
22. Strober BE, Menon K. Folate supplementation during methotrexate therapy for patients with psoriasis. J Am Acad Dermatol. 2005;53:652-659.
23. Taler SJ, Textor SC, Canzanello VJ, et al. Cyclosporine-induced hypertension: incidence, pathogenesis and management. Drug Saf. 1999;20:437-449.
24. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
25. Heydendael VM, Spuls PI, Opmeer BC, et al. Methotrexate versus cyclosporine in moderate-to-severe chronic plaque psoriasis. N Engl J Med. 2003;349:658-665.
26. Lloyd ME, Carr M, McElhatton P, et al. The effects of methotrexate on pregnancy, fertility and lactation. QJM. 1999;92:551-563.
27. Ellis CN, Fradin MS, Messana JM, et al. Cyclosporine for plaque-type psoriasis. Results of a multidose, double-blind trial. N Engl J Med. 1991;324:277-284.
28. Buccheri L, Katchen BR, Karter AJ, et al. Acitretin therapy is effective for psoriasis associated with human immunodeficiency virus infection. Arch Dermatol. 1997;133:711-715.
29. Lebwohl M, Bagel J, Gelfand JM, et al. From the Medical Board of the National Psoriasis Foundation: monitoring and vaccinations in patients treated with biologics for psoriasis. J Am Acad Dermatol. 2008;58:94-105.
30. Gordon KB, Vaishnaw AK, O’Gorman J, et al. Treatment of psoriasis with alefacept: correlation of clinical improvement with reductions of memory T-cell counts. Arch Dermatol. 2003;139:1563-1570.
31. Brown SL, Greene MH, Gershon SK, et al. Tumor necrosis factor antagonist therapy and lymphoma development: twenty-six cases reported to the Food and Drug Administration. Arthritis Rheum. 2002;46:3151-3158.
32. Fulchiero GJ, Jr, Salvaggio H, Drabick JJ, et al. Eruptive latent metastatic melanomas after initiation of antitumor necrosis factor therapies. J Am Acad Dermatol. 2007;56(suppl):S65-S67.
33. Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371:1665-1674.
34. US Food and Drug Administration. FDA approves new drug to treat psoriasis. Sept. 25, 2009. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm183851.htm. Accessed June 15, 2012.
35. Duchini A, Goss JA, Karpen S, et al. Vaccinations for adult solid-organ transplant recipients: current recommendations and protocols. Clin Microbiol Rev. 2003;16:357-364.
36. Mann DL, McMurray JJ, Packer M, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation. 2004;109:1594-1602.
37. Griffiths CE, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118-128.
38. Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684.
• Prescribe high-potency (Class 1) topical steroids for use on thicker, chronic plaques and low-potency (Class 7) steroids for the face, intertriginous areas, and the groin. A
• Recommend folate supplementation (1-5 mg/d) for patients being treated with methotrexate, as it may reduce the drug’s hematologic and gastrointestinal adverse effects without decreasing efficacy. A
• Advise patients scheduled to begin biologic therapy to get standard vaccinations—eg, pneumococcal, influenza, hepatitis A and B—before treatment is initiated and to avoid live and live-attenuated vaccines thereafter. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Psoriasis is a systemic inflammatory disorder altered by environmental and genetic factors that presents as scaly erythematous plaques and affects 2% to 3% of the population.1 Eighty percent have mild-to-moderate cutaneous disease; disabling arthritis occurs in as many as 42% of cases.1,2 The devastating effects on quality of life— including social stigmatization, pain, physical disability, and psychological distress—are comparable to the effects of conditions like cancer and depression.2 There is no definitive cure, and patients are left with a lifetime of waxing and waning symptoms.
Helping to create an individualized treatment plan tailored to disease type and extent, comorbidities, and needs of the patient can directly impact the quality of his or her life.3 For those with localized disease, topical therapy is a suitable first choice. Phototherapy is generally the first-line treatment for patients with extensive psoriasis or disabling symptoms. When phototherapy is not feasible or is ineffective, systemic treatments with conventional oral agents or biologics are indicated.
Discussions about therapeutic options should include expected results and duration of remission, cost, convenience, adverse effects, insurance coverage, and safety concerns. The patient’s preferences should be taken into account in the treatment plan you create.3 A guiding principle: The optimal protocol is the one the patient is motivated to adhere to.
The limits of topical therapy
Topical treatments are safe and effective when used properly, as monotherapy for localized disease and adjunctive therapy for resistant lesions. Monotherapy with topical agents is not recommended for sites that have a significant impact on quality of life, such as the palms and soles, and is not practical for patients with extensive disease (>10% of body surface).4
More potent topicals, such as corticosteroids, are recommended for acute flares; less potent agents with fewer adverse effects, such as calcipotriene, are typically used for maintenance ( TABLE 1 ).5-8 Topical agents can be used either long term or intermittently. Here, too, the most effective treatment is the one the patient will actually apply.5
TABLE 1
A look at nonsteroidal topical treatments5-8
| Class | Examples | Adverse effects | Comments |
|---|---|---|---|
| Vitamin D analogues | Calcipotriol (calcipotriene), calcitriol | Burning, pruritus, edema, peeling, dryness, erythema | Combining with beta-methasone dipropionate increases efficacy |
| Retinoids | Tazarotene, tretinoin | Teratogenic, photosensitivity, irritation | Increased efficacy when combined with NB-UVB (and less UV exposure); increased efficacy, duration of remission, and reduction in steroid-induced atrophy when used with steroids |
| Calcineurin inhibitors | Tacrolimus, pimecrolimus | Burning and pruritus Black box warning for risk of malignancies* | No clinical evidence of increased cancer risk |
| Others | Emollients, salicylic acid, anthralin, coal tar | Severe skin irritation, staining of clothes, odor with anthralin and tar | Salicylic acid works well with steroids and topical immunomodulators, but is not compatible with calcipotriene |
| *Lymphoma seen with oral therapy. UVB, ultraviolet B; NB-UVB, narrow-band ultraviolet B. | |||
Steroid selection is based on site, severity
Corticosteroids are antiproliferative, immunosuppressive, anti-inflammatory, and vasoconstrictive, and divided into 7 classes. Low-potency agents (Class 7) are used on thinner skin like the face, intertriginous areas, and groin; high-potency steroids (Class 1) are reserved for thicker, chronic plaques.9 As a general rule, Class 1 steroids can be safely used for 2 to 4 weeks, with increased risk of both cutaneous effects and systemic absorption if used continuously for longer periods.5 The optimal end point for less potent agents is not known.
Cutaneous adverse effects are more common than systemic ones and include skin atrophy, telangiectasia, striae distensae, acne, folliculitis, and purpura.
Systemic adverse effects include Cushing’s syndrome, osteonecrosis of the femoral head, cataracts, and glaucoma.5 The greatest risk of systemic effects is associated with prolonged use of high-potency corticosteroids over large surfaces or under occlusion with dressings or plastic wrap. Patients should be transitioned to the lowest potency possible to maintain efficacy, use corticosteroids intermittently, or combine them with nonsteroidal agents to avoid unwanted effects.
Topicals aren’t working? Move on to phototherapy
Phototherapy is an option for patients with extensive disease or skin manifestations that are recalcitrant to topicals. It is efficacious, cost-effective, and lacks systemic immunosuppression. Ultraviolet (UV) A and B act on Langerhans cells, cytokines, and adhesion molecules, inhibiting epidermal proliferation and angiogenesis.10
Broadband UVB (BB-UVB) was first used during the 1950s, with crude tar or anthralin, but is rarely used today.
Narrow-band UVB (NB-UVB) (311-313 nm), developed in the 1980s, has largely replaced BB-UVB. In addition to providing more rapid clearing and resolution of psoriasis compared with BB-UVB, NB-UVB may have less phototoxicity.11 Between 20 and 25 NB-UVB treatments, 2 to 3 times a week (in the office or at home) are usually required for significant improvement.
Photocarcinogenesis is a concern but numerous studies, including a review of 3867 patients treated with NB-UVB with a median 5.5-year follow-up, found no significant association with cutaneous malignancies.12 NB-UVB is considered safe during pregnancy and used as first-line therapy for pregnant patients.13
Targeted UVB therapy using a 308-nm excimer laser, another option, selectively targets psoriatic lesions, leaving normal skin untreated. This makes supraerythemogenic doses possible, which increases UVB’s efficacy. Long-term adverse effects and duration of remission have not been clearly established.14
Psoralen and UVA (PUVA), which uses oral or topical psoralens to sensitize the skin to UVA, has a slightly higher efficacy than NB-UVB, but with increased risk of squamous cell carcinoma (SCC) and possibly melanoma.15 Clearing can occur within 24 treatments, with remissions lasting 3 to 6 months;16 monthly maintenance has not been found to lengthen remission.13
Common adverse effects include erythema that peaks 48 to 96 hours after a treatment, pruritus, xerosis, irregular pigmentation, and gastrointestinal symptoms that can be reduced by decreasing psoralen and/or UVA doses.13 High cumulative doses of oral PUVA (>200 treatments) is associated with a dose-related increased risk of nonmelanoma skin cancer, particularly SCC, in the Caucasian population. This increased risk has not been demonstrated in patients treated with PUVA bath therapy, which is more common in Scandinavian countries.17 There is no consensus regarding the risk of melanoma.15
A careful risk-benefit analysis is needed before initiating phototherapy in patients who take photosensitizing drugs, are immunosuppressed, or have a photosensitivity disorder or a history of melanoma, atypical nevi, multiple melanoma risk factors, or multiple nonmelanoma skin cancers.13 Regardless of the type of UV therapy administered, eye protection with goggles is required to decrease the risk of UV-related cataract formation, and genital shielding is needed to prevent increased risk of tumors.13 Photoaging is a long-term effect.
CASE When questioned further about the challenges that Tom has had with controlling his symptoms, he admitted to being noncompliant. As a busy executive, he said he didn’t have time to use the topical corticosteroids regularly. Phototherapy could alleviate his cutaneous symptoms, but would not address his symptoms that were consistent with psoriatic arthritis.
Pairing therapeutic modalities decreases exposure
Combining therapeutic modalities like emollients and topical or oral retinoids with NB-UVB improves efficacy while reducing the number of treatment sessions and cumulative UVB dosage. If calcipotriene is used, it should be applied after phototherapy because it is degraded upon UVB exposure.18 Acitretin should be started 2 weeks prior to initiation of phototherapy, and its use accompanied by a 25% reduction in initial UV dosage.13
PUVA may also be combined with topical calcipotriene or retinoids.19 In both cases, the addition of the other agent typically decreases the duration of phototherapy, improves the clinical response, and reduces the risk of cancer.13,20
Extensive disease? Consider a traditional systemic agent
Traditional systemic therapy is used to treat extensive disease ( FIGURE ), psoriasis refractory to topical agents, and debilitating disease on the palms, soles, or scalp. Biologics are a recent alternative, but traditional systemics have been utilized longer and have a more longstanding adverse effect and safety profile, are administered orally, and are much less expensive than biologics. Monitoring patients on systemic therapy is necessary ( TABLE 2 ).21-24
Methotrexate (MTX), a competitive inhibitor of dihydrofolate reductase, is the most commonly prescribed traditional systemic psoriasis treatment.21 It is administered in a single weekly dose via tablet, parenteral solution, or intramuscular (IM) or subcutaneous (SC) injection.25 A test dose (2.5 or 5 mg) is given initially and complete blood cell count is monitored within one week to evaluate for potential bone marrow toxicity. If none is observed, the dose may be increased to control the disease while minimizing adverse effects.21
Common adverse effects of MTX, such as nausea, vomiting, stomatitis, and fatigue, may be minimized by IM or SC administration, splitting the dose, or providing folate supplementation.21-24 Given in doses of 1 to 5 mg/d, folate may reduce adverse hematologic, gastrointestinal, and hepatic effects without decreasing efficacy.22
The major severe toxicities are myelosuppression, hepatotoxicity, and pulmonary fibrosis.24 MTX-induced hepatotoxicity is similar to nonalcoholic fatty liver disease (NAFLD) and is thought to exacerbate preexisting NAFLD, which is common in patients with metabolic syndrome. A liver biopsy or serum assays for liver fibrosis (amino-terminal peptide of pro-collagen III) may be warranted during therapy.24
MTX is an abortifacient and teratogen, so contraception during treatment and for up to 3 months thereafter is mandatory for women of childbearing age.26 Men should be advised that MTX decreases sperm count. (For more on methotrexate, see: “When a fetus survives methotrexate exposure,” at http://www.jfponline.com/Pages.asp?AID=10299).
Cyclosporine (CSA), an oral calcineurin inhibitor, is a potent immunosuppressant that rapidly clears psoriasis.27 Because duration of use correlates with permanent nephrotoxicity, hypertension, and potential increased risk of SCC and lymphoma, intermittent 12-week courses are recommended. Calcium channel blockers are the preferred treatment for CSA-induced hypertension because of their effect on smooth muscle vasodilation.21
Oral retinoids. Acitretin modulates epidermal proliferation and is anti-inflammatory. Because it lacks immunosuppression, acitretin is generally considered the treatment of choice in HIV patients with severe psoriasis.28 Acitretin is teratogenic and contraindicated in women who plan to become pregnant or who are unwilling to use adequate contraception for 3 years after discontinuing the drug.21
CASE Given the significant percentage of body surface area involved and symptoms consistent with psoriatic arthritis, Tom required an aggressive therapeutic regimen. His history of nonalcoholic fatty liver and social drinking precluded the use of methotrexate. A biologic therapy was the next therapeutic choice that could relieve both his cutaneous and joint symptoms.
FIGURE
4 psoriasis patients, 4 different presentations
A. The linear erythematous, scaly plaque along this patient’s cardiac bypass scar demonstrates koebnerization of plaque psoriasis.
B. Diffuse erythematous scaly papules coalesce into plaques on this patient’s anterior chest, arms, abdomen, and periumbilicus.
C. The pinpoint pustules on an erythematous base on this patient’s lateral neck, shoulders, and upper back are characteristic of pustular psoriasis.
D. This patient has erythematous plaques with overlying silvery scale on the elbow—a classic
TABLE 2
Traditional systemic therapy21-24
| Methotrexate (MTX) | Cyclosporine (CSA)* | Acitretin | |
|---|---|---|---|
| Dosing | ≤30 mg in one weekly dose (PO, IM, or SC) | 2.5-5.0 mg/kg/d in 2 divided doses for 12 wk, then 12-wk nontreatment period Dose decreased (by 0.5-1.0 mg/kg) with disease clearance or when hypertension or nephrotoxicity are detected | 10-50 mg/d given as a single dose Lower doses (25 mg/d) used to minimize adverse effects and in combination regimens When added to UV, light dose should be reduced 30%-50% |
| Contraindications |
|
|
|
| Baseline monitoring† |
|
|
|
| BP, blood pressure; BUN, blood urea nitrogen; CBC, complete blood cell count; CR, creatinine; H&P, history and physical; IM, intramuscular; LFTs, liver function tests; PO, by mouth; PPD, purified protein derivative; PUVA, psoralen and ultraviolet A; SC, subcutaneous; UV, ultraviolet; UVB, ultraviolet B. *Avoid live vaccinations; caution required with major infection and poorly controlled diabetes. †Ongoing monitoring for MTX: BUN, CBC, CR, LFTs; possible liver biopsy (for high-risk patients or cumulative dose >3.5-4 g); CSA: BP, BUN, CBC, CR, LFTs; lipd profile; magnesium, uric acid, potassium tests; pregnancy testing; Acitretin: CBC, LFTs, lipid profile, renal function test, pregnancy testing. | |||
Biologics require lab work and a detailed medication list
Before beginning biologic therapy for a patient, the National Psoriasis Foundation29 recommends obtaining a complete history, physical, medication list, future plans (ie, pregnancy or travel to locations requiring vaccinations), and baseline labs to identify possible risk factors and/or contraindications. Periodic evaluation to monitor development of new symptoms, including infection and malignancy ( TABLE 3 ),24,30-33 is needed, as well.
TABLE 3
Is your patient a candidate for biologics?24,30-33
| Agent (Drug class) | Alefacept (T-cell inhibitor) | Adalimumab (TNF-inhibitor) | Etanercept (TNF-inhibitor) | Infliximab (TNF-inhibitor) | Ustekinumab (IL-12/23 inhibitor) |
|---|---|---|---|---|---|
| Dosing | 15 mg IM/wk for 12 wk, then 12-wk nontreatment period | 80 mg SC the first wk, 40 mg the 2nd wk, followed by 40 mg every other wk | 50 mg SC twice/wk for 3 mo, then 50 mg/wk | 5 mg/kg IV infusion to start, repeat at 2 and 6 wk, then q6-8 wk | 45 mg SC (for patients <100 kg); 90 mg (for patients >100 kg) to start, repeat at 4 wk, followed by q12 wk for maintenance |
| Contra-indications | HIV |
|
|
| Active TB |
| Baseline monitoring | CD4 count |
|
|
| PPD |
| Ongoing monitoring | Biweekly CD4 count; hold dose for counts <250 |
|
|
| Consider a yearly PPD |
| CBC, complete blood cell count; CHF, congestive heart failure; HIV, human immunodeficiency virus; H&P, history and physical; IL-12/23, interleukin-12, interleukin-23; LFTs, liver function test; MS, multiple sclerosis; NYHA, New York Heart Association; PPD, purified protein derivative; TB, tuberculosis; TNF, tumor necrosis factor. *For this patient population, adalimumab and etanercept have a (theoretical) risk. | |||||
Biologic therapy is contraindicated in patients with active serious infection. If patients develop infections requiring antibiotics while being treated, holding the biologic until infection resolution is advised.34 Standard vaccinations (eg, pneumococcal, hepatitis A and B, influenza, diphtheria, tetanus) are recommended before initiation of immunosuppressive therapy. After therapy starts, patients should avoid live and live-attenuated vaccines (varicella, mumps, measles, and rubella, oral typhoid, yellow fever, herpes zoster, intranasal influenza).35
Currently, none of the biologics are indicated for use in children or adolescents with psoriasis, despite epidemiologic data suggesting that one-third of adults with psoriasis developed it during childhood, in a form severe enough to warrant the use of systemic medications.34 The FDA is currently reviewing the possibility of indicating etanercept for pediatric psoriasis patients. All biologics are category B for pregnancy as there is no evidence that they negatively affect pregnancy.24
T-cell inhibitor. Alefacept binds CD2 on memory-effector T lymphocytes, inhibiting activation. Weekly intramuscular injections of alefacept for 12 weeks can clear lesions with long remissions.30
TNF-inhibitors. The TNF-inhibitors have been available for more than 10 years, predominantly for inflammatory bowel disease (IBD) and rheumatoid arthritis (RA), and more than 1.5 million patients have used adalimumab, etanercept, and infliximab for these disorders. Safety data, especially long term, are mostly derived from patients with IBD or RA, who have often combined TNF-inhibitors with additional immunosuppressive therapies. Thus, for psoriasis patients, who typically use biologics as monotherapy, the risk may be overestimated.24
TNF-inhibitors increase the risk for infection, most commonly of the upper respiratory tract, and, rarely, have been associated with opportunistic infections. Numerous cases of TB reactivation and an increased incidence of disseminated cases have been associated with TNF-inhibitors, so screening is recommended.24
The impact of TNF inhibition on congestive heart failure (CHF) is not well understood. Studies have variously shown that TNF-inhibitors have no effect on CHF morbidity or mortality, increase CHF mortality, or improve left ventricular function. TNF-inhibitors should be avoided in patients with severe CHF (New York Heart Association class III or IV). In milder CHF patients with worsening of symptoms, treatment should be discontinued.36
The increased risk of malignancy, especially lymphoma, is a concern, as there have been numerous case reports of lymphoma occurring with TNF-inhibitors. Psoriasis patients in general have an increased risk of lymphoma that confounds data interpretation.31 A number of case reports and a large observational study have shown patients receiving TNF-inhibitors may be at a greater risk for developing melanoma and nonmelanoma skin cancer.32
Ustekinumab, an interleukin 12/23 inhibitor, is a human monoclonal antibody that is absorbed and eliminated slowly, making dosing injections every 12 weeks convenient with efficacy maintained for at least one year.33 Because of its relative novelty, few studies are published regarding long-term safety. A recent head-to-head trial compared the efficacy and safety of ustekinumab with etanercept and found superior efficacy with ustekinumab, with comparable adverse events.37,38 Similar concerns exist with ustekinumab as with TNF-inhibitors, including infection, malignancy, CHF, and TB.33
CASE Tom denied having a personal family history of multiple sclerosis, or any demyelinating disorder. Nor did he have a history of cancer, tuberculosis exposure, CHF, or hepatitis. A purified protein derivative (PPD) was negative, as was his hepatitis panel, and his complete blood count with differential and metabolic panel were within normal limits.
Tom was started on the TNF-inhibitor adalimumab, after undergoing patient education and training and receiving instructions to stop the medication if he developed a major illness or infection. He received a loading dose of 80 mg SC, followed by 40 mg every other week. He tolerated the treatment well and 70% of his cutaneous symptoms cleared after 12 weeks of therapy; his joint pain also was reduced.
Tom is followed regularly in the clinic, with labs every 4 to 6 months. He is maintained on the injections and happy with the results. At each visit, weight loss and decreased beer intake are encouraged, both of which have been shown to reduce psoriasis severity. Although the beta-blockers and ACE inhibitors he takes are known to exacerbate psoriasis, the medications are necessary to treat Tom’s coronary artery disease.
CORRESPONDENCE Elizabeth Uhlenhake, MD, 18100 Oakwood Boulevard, Suite 300, Dearborn, MI 48124; eulenha@neomed.edu
• Prescribe high-potency (Class 1) topical steroids for use on thicker, chronic plaques and low-potency (Class 7) steroids for the face, intertriginous areas, and the groin. A
• Recommend folate supplementation (1-5 mg/d) for patients being treated with methotrexate, as it may reduce the drug’s hematologic and gastrointestinal adverse effects without decreasing efficacy. A
• Advise patients scheduled to begin biologic therapy to get standard vaccinations—eg, pneumococcal, influenza, hepatitis A and B—before treatment is initiated and to avoid live and live-attenuated vaccines thereafter. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Psoriasis is a systemic inflammatory disorder altered by environmental and genetic factors that presents as scaly erythematous plaques and affects 2% to 3% of the population.1 Eighty percent have mild-to-moderate cutaneous disease; disabling arthritis occurs in as many as 42% of cases.1,2 The devastating effects on quality of life— including social stigmatization, pain, physical disability, and psychological distress—are comparable to the effects of conditions like cancer and depression.2 There is no definitive cure, and patients are left with a lifetime of waxing and waning symptoms.
Helping to create an individualized treatment plan tailored to disease type and extent, comorbidities, and needs of the patient can directly impact the quality of his or her life.3 For those with localized disease, topical therapy is a suitable first choice. Phototherapy is generally the first-line treatment for patients with extensive psoriasis or disabling symptoms. When phototherapy is not feasible or is ineffective, systemic treatments with conventional oral agents or biologics are indicated.
Discussions about therapeutic options should include expected results and duration of remission, cost, convenience, adverse effects, insurance coverage, and safety concerns. The patient’s preferences should be taken into account in the treatment plan you create.3 A guiding principle: The optimal protocol is the one the patient is motivated to adhere to.
The limits of topical therapy
Topical treatments are safe and effective when used properly, as monotherapy for localized disease and adjunctive therapy for resistant lesions. Monotherapy with topical agents is not recommended for sites that have a significant impact on quality of life, such as the palms and soles, and is not practical for patients with extensive disease (>10% of body surface).4
More potent topicals, such as corticosteroids, are recommended for acute flares; less potent agents with fewer adverse effects, such as calcipotriene, are typically used for maintenance ( TABLE 1 ).5-8 Topical agents can be used either long term or intermittently. Here, too, the most effective treatment is the one the patient will actually apply.5
TABLE 1
A look at nonsteroidal topical treatments5-8
| Class | Examples | Adverse effects | Comments |
|---|---|---|---|
| Vitamin D analogues | Calcipotriol (calcipotriene), calcitriol | Burning, pruritus, edema, peeling, dryness, erythema | Combining with beta-methasone dipropionate increases efficacy |
| Retinoids | Tazarotene, tretinoin | Teratogenic, photosensitivity, irritation | Increased efficacy when combined with NB-UVB (and less UV exposure); increased efficacy, duration of remission, and reduction in steroid-induced atrophy when used with steroids |
| Calcineurin inhibitors | Tacrolimus, pimecrolimus | Burning and pruritus Black box warning for risk of malignancies* | No clinical evidence of increased cancer risk |
| Others | Emollients, salicylic acid, anthralin, coal tar | Severe skin irritation, staining of clothes, odor with anthralin and tar | Salicylic acid works well with steroids and topical immunomodulators, but is not compatible with calcipotriene |
| *Lymphoma seen with oral therapy. UVB, ultraviolet B; NB-UVB, narrow-band ultraviolet B. | |||
Steroid selection is based on site, severity
Corticosteroids are antiproliferative, immunosuppressive, anti-inflammatory, and vasoconstrictive, and divided into 7 classes. Low-potency agents (Class 7) are used on thinner skin like the face, intertriginous areas, and groin; high-potency steroids (Class 1) are reserved for thicker, chronic plaques.9 As a general rule, Class 1 steroids can be safely used for 2 to 4 weeks, with increased risk of both cutaneous effects and systemic absorption if used continuously for longer periods.5 The optimal end point for less potent agents is not known.
Cutaneous adverse effects are more common than systemic ones and include skin atrophy, telangiectasia, striae distensae, acne, folliculitis, and purpura.
Systemic adverse effects include Cushing’s syndrome, osteonecrosis of the femoral head, cataracts, and glaucoma.5 The greatest risk of systemic effects is associated with prolonged use of high-potency corticosteroids over large surfaces or under occlusion with dressings or plastic wrap. Patients should be transitioned to the lowest potency possible to maintain efficacy, use corticosteroids intermittently, or combine them with nonsteroidal agents to avoid unwanted effects.
Topicals aren’t working? Move on to phototherapy
Phototherapy is an option for patients with extensive disease or skin manifestations that are recalcitrant to topicals. It is efficacious, cost-effective, and lacks systemic immunosuppression. Ultraviolet (UV) A and B act on Langerhans cells, cytokines, and adhesion molecules, inhibiting epidermal proliferation and angiogenesis.10
Broadband UVB (BB-UVB) was first used during the 1950s, with crude tar or anthralin, but is rarely used today.
Narrow-band UVB (NB-UVB) (311-313 nm), developed in the 1980s, has largely replaced BB-UVB. In addition to providing more rapid clearing and resolution of psoriasis compared with BB-UVB, NB-UVB may have less phototoxicity.11 Between 20 and 25 NB-UVB treatments, 2 to 3 times a week (in the office or at home) are usually required for significant improvement.
Photocarcinogenesis is a concern but numerous studies, including a review of 3867 patients treated with NB-UVB with a median 5.5-year follow-up, found no significant association with cutaneous malignancies.12 NB-UVB is considered safe during pregnancy and used as first-line therapy for pregnant patients.13
Targeted UVB therapy using a 308-nm excimer laser, another option, selectively targets psoriatic lesions, leaving normal skin untreated. This makes supraerythemogenic doses possible, which increases UVB’s efficacy. Long-term adverse effects and duration of remission have not been clearly established.14
Psoralen and UVA (PUVA), which uses oral or topical psoralens to sensitize the skin to UVA, has a slightly higher efficacy than NB-UVB, but with increased risk of squamous cell carcinoma (SCC) and possibly melanoma.15 Clearing can occur within 24 treatments, with remissions lasting 3 to 6 months;16 monthly maintenance has not been found to lengthen remission.13
Common adverse effects include erythema that peaks 48 to 96 hours after a treatment, pruritus, xerosis, irregular pigmentation, and gastrointestinal symptoms that can be reduced by decreasing psoralen and/or UVA doses.13 High cumulative doses of oral PUVA (>200 treatments) is associated with a dose-related increased risk of nonmelanoma skin cancer, particularly SCC, in the Caucasian population. This increased risk has not been demonstrated in patients treated with PUVA bath therapy, which is more common in Scandinavian countries.17 There is no consensus regarding the risk of melanoma.15
A careful risk-benefit analysis is needed before initiating phototherapy in patients who take photosensitizing drugs, are immunosuppressed, or have a photosensitivity disorder or a history of melanoma, atypical nevi, multiple melanoma risk factors, or multiple nonmelanoma skin cancers.13 Regardless of the type of UV therapy administered, eye protection with goggles is required to decrease the risk of UV-related cataract formation, and genital shielding is needed to prevent increased risk of tumors.13 Photoaging is a long-term effect.
CASE When questioned further about the challenges that Tom has had with controlling his symptoms, he admitted to being noncompliant. As a busy executive, he said he didn’t have time to use the topical corticosteroids regularly. Phototherapy could alleviate his cutaneous symptoms, but would not address his symptoms that were consistent with psoriatic arthritis.
Pairing therapeutic modalities decreases exposure
Combining therapeutic modalities like emollients and topical or oral retinoids with NB-UVB improves efficacy while reducing the number of treatment sessions and cumulative UVB dosage. If calcipotriene is used, it should be applied after phototherapy because it is degraded upon UVB exposure.18 Acitretin should be started 2 weeks prior to initiation of phototherapy, and its use accompanied by a 25% reduction in initial UV dosage.13
PUVA may also be combined with topical calcipotriene or retinoids.19 In both cases, the addition of the other agent typically decreases the duration of phototherapy, improves the clinical response, and reduces the risk of cancer.13,20
Extensive disease? Consider a traditional systemic agent
Traditional systemic therapy is used to treat extensive disease ( FIGURE ), psoriasis refractory to topical agents, and debilitating disease on the palms, soles, or scalp. Biologics are a recent alternative, but traditional systemics have been utilized longer and have a more longstanding adverse effect and safety profile, are administered orally, and are much less expensive than biologics. Monitoring patients on systemic therapy is necessary ( TABLE 2 ).21-24
Methotrexate (MTX), a competitive inhibitor of dihydrofolate reductase, is the most commonly prescribed traditional systemic psoriasis treatment.21 It is administered in a single weekly dose via tablet, parenteral solution, or intramuscular (IM) or subcutaneous (SC) injection.25 A test dose (2.5 or 5 mg) is given initially and complete blood cell count is monitored within one week to evaluate for potential bone marrow toxicity. If none is observed, the dose may be increased to control the disease while minimizing adverse effects.21
Common adverse effects of MTX, such as nausea, vomiting, stomatitis, and fatigue, may be minimized by IM or SC administration, splitting the dose, or providing folate supplementation.21-24 Given in doses of 1 to 5 mg/d, folate may reduce adverse hematologic, gastrointestinal, and hepatic effects without decreasing efficacy.22
The major severe toxicities are myelosuppression, hepatotoxicity, and pulmonary fibrosis.24 MTX-induced hepatotoxicity is similar to nonalcoholic fatty liver disease (NAFLD) and is thought to exacerbate preexisting NAFLD, which is common in patients with metabolic syndrome. A liver biopsy or serum assays for liver fibrosis (amino-terminal peptide of pro-collagen III) may be warranted during therapy.24
MTX is an abortifacient and teratogen, so contraception during treatment and for up to 3 months thereafter is mandatory for women of childbearing age.26 Men should be advised that MTX decreases sperm count. (For more on methotrexate, see: “When a fetus survives methotrexate exposure,” at http://www.jfponline.com/Pages.asp?AID=10299).
Cyclosporine (CSA), an oral calcineurin inhibitor, is a potent immunosuppressant that rapidly clears psoriasis.27 Because duration of use correlates with permanent nephrotoxicity, hypertension, and potential increased risk of SCC and lymphoma, intermittent 12-week courses are recommended. Calcium channel blockers are the preferred treatment for CSA-induced hypertension because of their effect on smooth muscle vasodilation.21
Oral retinoids. Acitretin modulates epidermal proliferation and is anti-inflammatory. Because it lacks immunosuppression, acitretin is generally considered the treatment of choice in HIV patients with severe psoriasis.28 Acitretin is teratogenic and contraindicated in women who plan to become pregnant or who are unwilling to use adequate contraception for 3 years after discontinuing the drug.21
CASE Given the significant percentage of body surface area involved and symptoms consistent with psoriatic arthritis, Tom required an aggressive therapeutic regimen. His history of nonalcoholic fatty liver and social drinking precluded the use of methotrexate. A biologic therapy was the next therapeutic choice that could relieve both his cutaneous and joint symptoms.
FIGURE
4 psoriasis patients, 4 different presentations
A. The linear erythematous, scaly plaque along this patient’s cardiac bypass scar demonstrates koebnerization of plaque psoriasis.
B. Diffuse erythematous scaly papules coalesce into plaques on this patient’s anterior chest, arms, abdomen, and periumbilicus.
C. The pinpoint pustules on an erythematous base on this patient’s lateral neck, shoulders, and upper back are characteristic of pustular psoriasis.
D. This patient has erythematous plaques with overlying silvery scale on the elbow—a classic
TABLE 2
Traditional systemic therapy21-24
| Methotrexate (MTX) | Cyclosporine (CSA)* | Acitretin | |
|---|---|---|---|
| Dosing | ≤30 mg in one weekly dose (PO, IM, or SC) | 2.5-5.0 mg/kg/d in 2 divided doses for 12 wk, then 12-wk nontreatment period Dose decreased (by 0.5-1.0 mg/kg) with disease clearance or when hypertension or nephrotoxicity are detected | 10-50 mg/d given as a single dose Lower doses (25 mg/d) used to minimize adverse effects and in combination regimens When added to UV, light dose should be reduced 30%-50% |
| Contraindications |
|
|
|
| Baseline monitoring† |
|
|
|
| BP, blood pressure; BUN, blood urea nitrogen; CBC, complete blood cell count; CR, creatinine; H&P, history and physical; IM, intramuscular; LFTs, liver function tests; PO, by mouth; PPD, purified protein derivative; PUVA, psoralen and ultraviolet A; SC, subcutaneous; UV, ultraviolet; UVB, ultraviolet B. *Avoid live vaccinations; caution required with major infection and poorly controlled diabetes. †Ongoing monitoring for MTX: BUN, CBC, CR, LFTs; possible liver biopsy (for high-risk patients or cumulative dose >3.5-4 g); CSA: BP, BUN, CBC, CR, LFTs; lipd profile; magnesium, uric acid, potassium tests; pregnancy testing; Acitretin: CBC, LFTs, lipid profile, renal function test, pregnancy testing. | |||
Biologics require lab work and a detailed medication list
Before beginning biologic therapy for a patient, the National Psoriasis Foundation29 recommends obtaining a complete history, physical, medication list, future plans (ie, pregnancy or travel to locations requiring vaccinations), and baseline labs to identify possible risk factors and/or contraindications. Periodic evaluation to monitor development of new symptoms, including infection and malignancy ( TABLE 3 ),24,30-33 is needed, as well.
TABLE 3
Is your patient a candidate for biologics?24,30-33
| Agent (Drug class) | Alefacept (T-cell inhibitor) | Adalimumab (TNF-inhibitor) | Etanercept (TNF-inhibitor) | Infliximab (TNF-inhibitor) | Ustekinumab (IL-12/23 inhibitor) |
|---|---|---|---|---|---|
| Dosing | 15 mg IM/wk for 12 wk, then 12-wk nontreatment period | 80 mg SC the first wk, 40 mg the 2nd wk, followed by 40 mg every other wk | 50 mg SC twice/wk for 3 mo, then 50 mg/wk | 5 mg/kg IV infusion to start, repeat at 2 and 6 wk, then q6-8 wk | 45 mg SC (for patients <100 kg); 90 mg (for patients >100 kg) to start, repeat at 4 wk, followed by q12 wk for maintenance |
| Contra-indications | HIV |
|
|
| Active TB |
| Baseline monitoring | CD4 count |
|
|
| PPD |
| Ongoing monitoring | Biweekly CD4 count; hold dose for counts <250 |
|
|
| Consider a yearly PPD |
| CBC, complete blood cell count; CHF, congestive heart failure; HIV, human immunodeficiency virus; H&P, history and physical; IL-12/23, interleukin-12, interleukin-23; LFTs, liver function test; MS, multiple sclerosis; NYHA, New York Heart Association; PPD, purified protein derivative; TB, tuberculosis; TNF, tumor necrosis factor. *For this patient population, adalimumab and etanercept have a (theoretical) risk. | |||||
Biologic therapy is contraindicated in patients with active serious infection. If patients develop infections requiring antibiotics while being treated, holding the biologic until infection resolution is advised.34 Standard vaccinations (eg, pneumococcal, hepatitis A and B, influenza, diphtheria, tetanus) are recommended before initiation of immunosuppressive therapy. After therapy starts, patients should avoid live and live-attenuated vaccines (varicella, mumps, measles, and rubella, oral typhoid, yellow fever, herpes zoster, intranasal influenza).35
Currently, none of the biologics are indicated for use in children or adolescents with psoriasis, despite epidemiologic data suggesting that one-third of adults with psoriasis developed it during childhood, in a form severe enough to warrant the use of systemic medications.34 The FDA is currently reviewing the possibility of indicating etanercept for pediatric psoriasis patients. All biologics are category B for pregnancy as there is no evidence that they negatively affect pregnancy.24
T-cell inhibitor. Alefacept binds CD2 on memory-effector T lymphocytes, inhibiting activation. Weekly intramuscular injections of alefacept for 12 weeks can clear lesions with long remissions.30
TNF-inhibitors. The TNF-inhibitors have been available for more than 10 years, predominantly for inflammatory bowel disease (IBD) and rheumatoid arthritis (RA), and more than 1.5 million patients have used adalimumab, etanercept, and infliximab for these disorders. Safety data, especially long term, are mostly derived from patients with IBD or RA, who have often combined TNF-inhibitors with additional immunosuppressive therapies. Thus, for psoriasis patients, who typically use biologics as monotherapy, the risk may be overestimated.24
TNF-inhibitors increase the risk for infection, most commonly of the upper respiratory tract, and, rarely, have been associated with opportunistic infections. Numerous cases of TB reactivation and an increased incidence of disseminated cases have been associated with TNF-inhibitors, so screening is recommended.24
The impact of TNF inhibition on congestive heart failure (CHF) is not well understood. Studies have variously shown that TNF-inhibitors have no effect on CHF morbidity or mortality, increase CHF mortality, or improve left ventricular function. TNF-inhibitors should be avoided in patients with severe CHF (New York Heart Association class III or IV). In milder CHF patients with worsening of symptoms, treatment should be discontinued.36
The increased risk of malignancy, especially lymphoma, is a concern, as there have been numerous case reports of lymphoma occurring with TNF-inhibitors. Psoriasis patients in general have an increased risk of lymphoma that confounds data interpretation.31 A number of case reports and a large observational study have shown patients receiving TNF-inhibitors may be at a greater risk for developing melanoma and nonmelanoma skin cancer.32
Ustekinumab, an interleukin 12/23 inhibitor, is a human monoclonal antibody that is absorbed and eliminated slowly, making dosing injections every 12 weeks convenient with efficacy maintained for at least one year.33 Because of its relative novelty, few studies are published regarding long-term safety. A recent head-to-head trial compared the efficacy and safety of ustekinumab with etanercept and found superior efficacy with ustekinumab, with comparable adverse events.37,38 Similar concerns exist with ustekinumab as with TNF-inhibitors, including infection, malignancy, CHF, and TB.33
CASE Tom denied having a personal family history of multiple sclerosis, or any demyelinating disorder. Nor did he have a history of cancer, tuberculosis exposure, CHF, or hepatitis. A purified protein derivative (PPD) was negative, as was his hepatitis panel, and his complete blood count with differential and metabolic panel were within normal limits.
Tom was started on the TNF-inhibitor adalimumab, after undergoing patient education and training and receiving instructions to stop the medication if he developed a major illness or infection. He received a loading dose of 80 mg SC, followed by 40 mg every other week. He tolerated the treatment well and 70% of his cutaneous symptoms cleared after 12 weeks of therapy; his joint pain also was reduced.
Tom is followed regularly in the clinic, with labs every 4 to 6 months. He is maintained on the injections and happy with the results. At each visit, weight loss and decreased beer intake are encouraged, both of which have been shown to reduce psoriasis severity. Although the beta-blockers and ACE inhibitors he takes are known to exacerbate psoriasis, the medications are necessary to treat Tom’s coronary artery disease.
CORRESPONDENCE Elizabeth Uhlenhake, MD, 18100 Oakwood Boulevard, Suite 300, Dearborn, MI 48124; eulenha@neomed.edu
1. Gelfand JM, Weinstein R, Porter SB, et al. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141:1537-1541.
2. Fortune DG, Richards HL, Griffiths CE. Psychologic factors in psoriasis: consequences, mechanisms, and interventions. Dermatol Clin. 2005;23:681-694.
3. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
4. Pettey AA, Balkrishnan R, Rapp SR, et al. Patients with palmoplantar psoriasis have more physical disability and discomfort than patients with other forms of psoriasis: implications for clinical practice. J Am Acad Dermatol. 2003;49:271-275.
5. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
6. Kaufmann R, Bibby AJ, Bissonnette R, et al. A new calcipotriol/betamethasone dipropionate formulation (Daivobet) is an effective once-daily treatment for psoriasis vulgaris. Dermatology. 2002;205:389-393.
7. Koo JY, Martin D. Investigator-masked comparison of tazarotene gel q.d. plus mometasone furoate cream q.d. vs. mometasone furoate cream b.i.d. in the treatment of plaque psoriasis. Int J Dermatol. 2001;40:210-212.
8. Berger TG, Duvic M, Van Voorhees AS, et al. The use of topical calcineurin inhibitors in dermatology: safety concerns. Report of the American Academy of Dermatology Association Task Force. J Am Acad Dermatol. 2006;54:818-823.
9. Cornell RC, Stoughton RB. Correlation of the vasoconstriction assay and clinical activity in psoriasis. Arch Dermatol. 1985;121:63-67.
10. Lui H. Phototherapy of psoriasis: update with practical pearls. J Cutan Med Surg. 2002;6(suppl):17-21.
11. Walters IB, Burack LH, Coven TR, et al. Suberythemogenic narrow-band UVB is markedly more effective than conventional UVB in treatment of psoriasis vulgaris. J Am Acad Dermatol. 1999;40:893-900.
12. Stern RS, Laird N. The carcinogenic risk of treatments for severe psoriasis. Photochemotherapy follow-up study. Cancer. 1994;73:2759-2764.
13. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
14. Trehan M, Taylor CR. High-dose 308-nm excimer laser for the treatment of psoriasis. J Am Acad Dermatol. 2002;46:732-737.
15. Morison WL, Baughman RD, Day RM, et al. Consensus workshop on the toxic effects of long-term PUVA therapy. Arch Dermatol. 1998;134:595-598.
16. Spuls PI, Witkamp L, Bossuyt PM, et al. A systematic review of five systemic treatments for severe psoriasis. Br J Dermatol. 1997;137:943-949.
17. Murase JE, Lee EE, Koo J. Effect of ethnicity on the risk of developing nonmelanoma skin cancer following long-term PUVA therapy. Int J Dermatol. 2005;44:1016-1021.
18. Lowe NJ, Prystowsky JH, Bourget T, et al. Acitretin plus UVB therapy for psoriasis. Comparisons with placebo plus UVB and acitretin alone. J Am Acad Dermatol. 1991;24:591-594.
19. Torras H, Aliaga A, Lopez-Estebaranz JL, et al. A combination therapy of calcipotriol cream and PUVA reduces the UVA dose and improves the response of psoriasis vulgaris. J Dermatolog Treat. 2004;15:98-103.
20. Tanew A, Guggenbichler A, Honigsmann H, et al. Photochemotherapy for severe psoriasis without or in combination with acitretin: a randomized, double-blind comparison study. J Am Acad Dermatol. 1991;25:682-684.
21. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
22. Strober BE, Menon K. Folate supplementation during methotrexate therapy for patients with psoriasis. J Am Acad Dermatol. 2005;53:652-659.
23. Taler SJ, Textor SC, Canzanello VJ, et al. Cyclosporine-induced hypertension: incidence, pathogenesis and management. Drug Saf. 1999;20:437-449.
24. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
25. Heydendael VM, Spuls PI, Opmeer BC, et al. Methotrexate versus cyclosporine in moderate-to-severe chronic plaque psoriasis. N Engl J Med. 2003;349:658-665.
26. Lloyd ME, Carr M, McElhatton P, et al. The effects of methotrexate on pregnancy, fertility and lactation. QJM. 1999;92:551-563.
27. Ellis CN, Fradin MS, Messana JM, et al. Cyclosporine for plaque-type psoriasis. Results of a multidose, double-blind trial. N Engl J Med. 1991;324:277-284.
28. Buccheri L, Katchen BR, Karter AJ, et al. Acitretin therapy is effective for psoriasis associated with human immunodeficiency virus infection. Arch Dermatol. 1997;133:711-715.
29. Lebwohl M, Bagel J, Gelfand JM, et al. From the Medical Board of the National Psoriasis Foundation: monitoring and vaccinations in patients treated with biologics for psoriasis. J Am Acad Dermatol. 2008;58:94-105.
30. Gordon KB, Vaishnaw AK, O’Gorman J, et al. Treatment of psoriasis with alefacept: correlation of clinical improvement with reductions of memory T-cell counts. Arch Dermatol. 2003;139:1563-1570.
31. Brown SL, Greene MH, Gershon SK, et al. Tumor necrosis factor antagonist therapy and lymphoma development: twenty-six cases reported to the Food and Drug Administration. Arthritis Rheum. 2002;46:3151-3158.
32. Fulchiero GJ, Jr, Salvaggio H, Drabick JJ, et al. Eruptive latent metastatic melanomas after initiation of antitumor necrosis factor therapies. J Am Acad Dermatol. 2007;56(suppl):S65-S67.
33. Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371:1665-1674.
34. US Food and Drug Administration. FDA approves new drug to treat psoriasis. Sept. 25, 2009. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm183851.htm. Accessed June 15, 2012.
35. Duchini A, Goss JA, Karpen S, et al. Vaccinations for adult solid-organ transplant recipients: current recommendations and protocols. Clin Microbiol Rev. 2003;16:357-364.
36. Mann DL, McMurray JJ, Packer M, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation. 2004;109:1594-1602.
37. Griffiths CE, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118-128.
38. Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684.
1. Gelfand JM, Weinstein R, Porter SB, et al. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141:1537-1541.
2. Fortune DG, Richards HL, Griffiths CE. Psychologic factors in psoriasis: consequences, mechanisms, and interventions. Dermatol Clin. 2005;23:681-694.
3. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
4. Pettey AA, Balkrishnan R, Rapp SR, et al. Patients with palmoplantar psoriasis have more physical disability and discomfort than patients with other forms of psoriasis: implications for clinical practice. J Am Acad Dermatol. 2003;49:271-275.
5. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
6. Kaufmann R, Bibby AJ, Bissonnette R, et al. A new calcipotriol/betamethasone dipropionate formulation (Daivobet) is an effective once-daily treatment for psoriasis vulgaris. Dermatology. 2002;205:389-393.
7. Koo JY, Martin D. Investigator-masked comparison of tazarotene gel q.d. plus mometasone furoate cream q.d. vs. mometasone furoate cream b.i.d. in the treatment of plaque psoriasis. Int J Dermatol. 2001;40:210-212.
8. Berger TG, Duvic M, Van Voorhees AS, et al. The use of topical calcineurin inhibitors in dermatology: safety concerns. Report of the American Academy of Dermatology Association Task Force. J Am Acad Dermatol. 2006;54:818-823.
9. Cornell RC, Stoughton RB. Correlation of the vasoconstriction assay and clinical activity in psoriasis. Arch Dermatol. 1985;121:63-67.
10. Lui H. Phototherapy of psoriasis: update with practical pearls. J Cutan Med Surg. 2002;6(suppl):17-21.
11. Walters IB, Burack LH, Coven TR, et al. Suberythemogenic narrow-band UVB is markedly more effective than conventional UVB in treatment of psoriasis vulgaris. J Am Acad Dermatol. 1999;40:893-900.
12. Stern RS, Laird N. The carcinogenic risk of treatments for severe psoriasis. Photochemotherapy follow-up study. Cancer. 1994;73:2759-2764.
13. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
14. Trehan M, Taylor CR. High-dose 308-nm excimer laser for the treatment of psoriasis. J Am Acad Dermatol. 2002;46:732-737.
15. Morison WL, Baughman RD, Day RM, et al. Consensus workshop on the toxic effects of long-term PUVA therapy. Arch Dermatol. 1998;134:595-598.
16. Spuls PI, Witkamp L, Bossuyt PM, et al. A systematic review of five systemic treatments for severe psoriasis. Br J Dermatol. 1997;137:943-949.
17. Murase JE, Lee EE, Koo J. Effect of ethnicity on the risk of developing nonmelanoma skin cancer following long-term PUVA therapy. Int J Dermatol. 2005;44:1016-1021.
18. Lowe NJ, Prystowsky JH, Bourget T, et al. Acitretin plus UVB therapy for psoriasis. Comparisons with placebo plus UVB and acitretin alone. J Am Acad Dermatol. 1991;24:591-594.
19. Torras H, Aliaga A, Lopez-Estebaranz JL, et al. A combination therapy of calcipotriol cream and PUVA reduces the UVA dose and improves the response of psoriasis vulgaris. J Dermatolog Treat. 2004;15:98-103.
20. Tanew A, Guggenbichler A, Honigsmann H, et al. Photochemotherapy for severe psoriasis without or in combination with acitretin: a randomized, double-blind comparison study. J Am Acad Dermatol. 1991;25:682-684.
21. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
22. Strober BE, Menon K. Folate supplementation during methotrexate therapy for patients with psoriasis. J Am Acad Dermatol. 2005;53:652-659.
23. Taler SJ, Textor SC, Canzanello VJ, et al. Cyclosporine-induced hypertension: incidence, pathogenesis and management. Drug Saf. 1999;20:437-449.
24. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
25. Heydendael VM, Spuls PI, Opmeer BC, et al. Methotrexate versus cyclosporine in moderate-to-severe chronic plaque psoriasis. N Engl J Med. 2003;349:658-665.
26. Lloyd ME, Carr M, McElhatton P, et al. The effects of methotrexate on pregnancy, fertility and lactation. QJM. 1999;92:551-563.
27. Ellis CN, Fradin MS, Messana JM, et al. Cyclosporine for plaque-type psoriasis. Results of a multidose, double-blind trial. N Engl J Med. 1991;324:277-284.
28. Buccheri L, Katchen BR, Karter AJ, et al. Acitretin therapy is effective for psoriasis associated with human immunodeficiency virus infection. Arch Dermatol. 1997;133:711-715.
29. Lebwohl M, Bagel J, Gelfand JM, et al. From the Medical Board of the National Psoriasis Foundation: monitoring and vaccinations in patients treated with biologics for psoriasis. J Am Acad Dermatol. 2008;58:94-105.
30. Gordon KB, Vaishnaw AK, O’Gorman J, et al. Treatment of psoriasis with alefacept: correlation of clinical improvement with reductions of memory T-cell counts. Arch Dermatol. 2003;139:1563-1570.
31. Brown SL, Greene MH, Gershon SK, et al. Tumor necrosis factor antagonist therapy and lymphoma development: twenty-six cases reported to the Food and Drug Administration. Arthritis Rheum. 2002;46:3151-3158.
32. Fulchiero GJ, Jr, Salvaggio H, Drabick JJ, et al. Eruptive latent metastatic melanomas after initiation of antitumor necrosis factor therapies. J Am Acad Dermatol. 2007;56(suppl):S65-S67.
33. Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371:1665-1674.
34. US Food and Drug Administration. FDA approves new drug to treat psoriasis. Sept. 25, 2009. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm183851.htm. Accessed June 15, 2012.
35. Duchini A, Goss JA, Karpen S, et al. Vaccinations for adult solid-organ transplant recipients: current recommendations and protocols. Clin Microbiol Rev. 2003;16:357-364.
36. Mann DL, McMurray JJ, Packer M, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation. 2004;109:1594-1602.
37. Griffiths CE, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118-128.
38. Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684.
Managing psoriasis: What’s best for your patient?
• Prescribe high-potency (Class 1) topical steroids for use on thicker, chronic plaques and low-potency (Class 7) steroids for the face, intertriginous areas, and the groin. A
• Recommend folate supplementation (1-5 mg/d) for patients being treated with methotrexate, as it may reduce the drug’s hematologic and gastrointestinal adverse effects without decreasing efficacy. A
• Advise patients scheduled to begin biologic therapy to get standard vaccinations—eg, pneumococcal, influenza, hepatitis A and B—before treatment is initiated and to avoid live and live-attenuated vaccines thereafter. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Psoriasis is a systemic inflammatory disorder altered by environmental and genetic factors that presents as scaly erythematous plaques and affects 2% to 3% of the population.1 Eighty percent have mild-to-moderate cutaneous disease; disabling arthritis occurs in as many as 42% of cases.1,2 The devastating effects on quality of life— including social stigmatization, pain, physical disability, and psychological distress—are comparable to the effects of conditions like cancer and depression.2 There is no definitive cure, and patients are left with a lifetime of waxing and waning symptoms.
Helping to create an individualized treatment plan tailored to disease type and extent, comorbidities, and needs of the patient can directly impact the quality of his or her life.3 For those with localized disease, topical therapy is a suitable first choice. Phototherapy is generally the first-line treatment for patients with extensive psoriasis or disabling symptoms. When phototherapy is not feasible or is ineffective, systemic treatments with conventional oral agents or biologics are indicated.
Discussions about therapeutic options should include expected results and duration of remission, cost, convenience, adverse effects, insurance coverage, and safety concerns. The patient’s preferences should be taken into account in the treatment plan you create.3 A guiding principle: The optimal protocol is the one the patient is motivated to adhere to.
The limits of topical therapy
Topical treatments are safe and effective when used properly, as monotherapy for localized disease and adjunctive therapy for resistant lesions. Monotherapy with topical agents is not recommended for sites that have a significant impact on quality of life, such as the palms and soles, and is not practical for patients with extensive disease (>10% of body surface).4
More potent topicals, such as corticosteroids, are recommended for acute flares; less potent agents with fewer adverse effects, such as calcipotriene, are typically used for maintenance ( TABLE 1 ).5-8 Topical agents can be used either long term or intermittently. Here, too, the most effective treatment is the one the patient will actually apply.5
TABLE 1
A look at nonsteroidal topical treatments5-8
| Class | Examples | Adverse effects | Comments |
|---|---|---|---|
| Vitamin D analogues | Calcipotriol (calcipotriene), calcitriol | Burning, pruritus, edema, peeling, dryness, erythema | Combining with beta-methasone dipropionate increases efficacy |
| Retinoids | Tazarotene, tretinoin | Teratogenic, photosensitivity, irritation | Increased efficacy when combined with NB-UVB (and less UV exposure); increased efficacy, duration of remission, and reduction in steroid-induced atrophy when used with steroids |
| Calcineurin inhibitors | Tacrolimus, pimecrolimus | Burning and pruritus Black box warning for risk of malignancies* | No clinical evidence of increased cancer risk |
| Others | Emollients, salicylic acid, anthralin, coal tar | Severe skin irritation, staining of clothes, odor with anthralin and tar | Salicylic acid works well with steroids and topical immunomodulators, but is not compatible with calcipotriene |
| *Lymphoma seen with oral therapy. UVB, ultraviolet B; NB-UVB, narrow-band ultraviolet B. | |||
Steroid selection is based on site, severity
Corticosteroids are antiproliferative, immunosuppressive, anti-inflammatory, and vasoconstrictive, and divided into 7 classes. Low-potency agents (Class 7) are used on thinner skin like the face, intertriginous areas, and groin; high-potency steroids (Class 1) are reserved for thicker, chronic plaques.9 As a general rule, Class 1 steroids can be safely used for 2 to 4 weeks, with increased risk of both cutaneous effects and systemic absorption if used continuously for longer periods.5 The optimal end point for less potent agents is not known.
Cutaneous adverse effects are more common than systemic ones and include skin atrophy, telangiectasia, striae distensae, acne, folliculitis, and purpura.
Systemic adverse effects include Cushing’s syndrome, osteonecrosis of the femoral head, cataracts, and glaucoma.5 The greatest risk of systemic effects is associated with prolonged use of high-potency corticosteroids over large surfaces or under occlusion with dressings or plastic wrap. Patients should be transitioned to the lowest potency possible to maintain efficacy, use corticosteroids intermittently, or combine them with nonsteroidal agents to avoid unwanted effects.
Topicals aren’t working? Move on to phototherapy
Phototherapy is an option for patients with extensive disease or skin manifestations that are recalcitrant to topicals. It is efficacious, cost-effective, and lacks systemic immunosuppression. Ultraviolet (UV) A and B act on Langerhans cells, cytokines, and adhesion molecules, inhibiting epidermal proliferation and angiogenesis.10
Broadband UVB (BB-UVB) was first used during the 1950s, with crude tar or anthralin, but is rarely used today.
Narrow-band UVB (NB-UVB) (311-313 nm), developed in the 1980s, has largely replaced BB-UVB. In addition to providing more rapid clearing and resolution of psoriasis compared with BB-UVB, NB-UVB may have less phototoxicity.11 Between 20 and 25 NB-UVB treatments, 2 to 3 times a week (in the office or at home) are usually required for significant improvement.
Photocarcinogenesis is a concern but numerous studies, including a review of 3867 patients treated with NB-UVB with a median 5.5-year follow-up, found no significant association with cutaneous malignancies.12 NB-UVB is considered safe during pregnancy and used as first-line therapy for pregnant patients.13
Targeted UVB therapy using a 308-nm excimer laser, another option, selectively targets psoriatic lesions, leaving normal skin untreated. This makes supraerythemogenic doses possible, which increases UVB’s efficacy. Long-term adverse effects and duration of remission have not been clearly established.14
Psoralen and UVA (PUVA), which uses oral or topical psoralens to sensitize the skin to UVA, has a slightly higher efficacy than NB-UVB, but with increased risk of squamous cell carcinoma (SCC) and possibly melanoma.15 Clearing can occur within 24 treatments, with remissions lasting 3 to 6 months;16 monthly maintenance has not been found to lengthen remission.13
Common adverse effects include erythema that peaks 48 to 96 hours after a treatment, pruritus, xerosis, irregular pigmentation, and gastrointestinal symptoms that can be reduced by decreasing psoralen and/or UVA doses.13 High cumulative doses of oral PUVA (>200 treatments) is associated with a dose-related increased risk of nonmelanoma skin cancer, particularly SCC, in the Caucasian population. This increased risk has not been demonstrated in patients treated with PUVA bath therapy, which is more common in Scandinavian countries.17 There is no consensus regarding the risk of melanoma.15
A careful risk-benefit analysis is needed before initiating phototherapy in patients who take photosensitizing drugs, are immunosuppressed, or have a photosensitivity disorder or a history of melanoma, atypical nevi, multiple melanoma risk factors, or multiple nonmelanoma skin cancers.13 Regardless of the type of UV therapy administered, eye protection with goggles is required to decrease the risk of UV-related cataract formation, and genital shielding is needed to prevent increased risk of tumors.13 Photoaging is a long-term effect.
CASE When questioned further about the challenges that Tom has had with controlling his symptoms, he admitted to being noncompliant. As a busy executive, he said he didn’t have time to use the topical corticosteroids regularly. Phototherapy could alleviate his cutaneous symptoms, but would not address his symptoms that were consistent with psoriatic arthritis.
Pairing therapeutic modalities decreases exposure
Combining therapeutic modalities like emollients and topical or oral retinoids with NB-UVB improves efficacy while reducing the number of treatment sessions and cumulative UVB dosage. If calcipotriene is used, it should be applied after phototherapy because it is degraded upon UVB exposure.18 Acitretin should be started 2 weeks prior to initiation of phototherapy, and its use accompanied by a 25% reduction in initial UV dosage.13
PUVA may also be combined with topical calcipotriene or retinoids.19 In both cases, the addition of the other agent typically decreases the duration of phototherapy, improves the clinical response, and reduces the risk of cancer.13,20
Extensive disease? Consider a traditional systemic agent
Traditional systemic therapy is used to treat extensive disease ( FIGURE ), psoriasis refractory to topical agents, and debilitating disease on the palms, soles, or scalp. Biologics are a recent alternative, but traditional systemics have been utilized longer and have a more longstanding adverse effect and safety profile, are administered orally, and are much less expensive than biologics. Monitoring patients on systemic therapy is necessary ( TABLE 2 ).21-24
Methotrexate (MTX), a competitive inhibitor of dihydrofolate reductase, is the most commonly prescribed traditional systemic psoriasis treatment.21 It is administered in a single weekly dose via tablet, parenteral solution, or intramuscular (IM) or subcutaneous (SC) injection.25 A test dose (2.5 or 5 mg) is given initially and complete blood cell count is monitored within one week to evaluate for potential bone marrow toxicity. If none is observed, the dose may be increased to control the disease while minimizing adverse effects.21
Common adverse effects of MTX, such as nausea, vomiting, stomatitis, and fatigue, may be minimized by IM or SC administration, splitting the dose, or providing folate supplementation.21-24 Given in doses of 1 to 5 mg/d, folate may reduce adverse hematologic, gastrointestinal, and hepatic effects without decreasing efficacy.22
The major severe toxicities are myelosuppression, hepatotoxicity, and pulmonary fibrosis.24 MTX-induced hepatotoxicity is similar to nonalcoholic fatty liver disease (NAFLD) and is thought to exacerbate preexisting NAFLD, which is common in patients with metabolic syndrome. A liver biopsy or serum assays for liver fibrosis (amino-terminal peptide of pro-collagen III) may be warranted during therapy.24
MTX is an abortifacient and teratogen, so contraception during treatment and for up to 3 months thereafter is mandatory for women of childbearing age.26 Men should be advised that MTX decreases sperm count. (For more on methotrexate, see: “When a fetus survives methotrexate exposure,” at http://www.jfponline.com/Pages.asp?AID=10299).
Cyclosporine (CSA), an oral calcineurin inhibitor, is a potent immunosuppressant that rapidly clears psoriasis.27 Because duration of use correlates with permanent nephrotoxicity, hypertension, and potential increased risk of SCC and lymphoma, intermittent 12-week courses are recommended. Calcium channel blockers are the preferred treatment for CSA-induced hypertension because of their effect on smooth muscle vasodilation.21
Oral retinoids. Acitretin modulates epidermal proliferation and is anti-inflammatory. Because it lacks immunosuppression, acitretin is generally considered the treatment of choice in HIV patients with severe psoriasis.28 Acitretin is teratogenic and contraindicated in women who plan to become pregnant or who are unwilling to use adequate contraception for 3 years after discontinuing the drug.21
CASE Given the significant percentage of body surface area involved and symptoms consistent with psoriatic arthritis, Tom required an aggressive therapeutic regimen. His history of nonalcoholic fatty liver and social drinking precluded the use of methotrexate. A biologic therapy was the next therapeutic choice that could relieve both his cutaneous and joint symptoms.
FIGURE
4 psoriasis patients, 4 different presentations
A. The linear erythematous, scaly plaque along this patient’s cardiac bypass scar demonstrates koebnerization of plaque psoriasis.
B. Diffuse erythematous scaly papules coalesce into plaques on this patient’s anterior chest, arms, abdomen, and periumbilicus.
C. The pinpoint pustules on an erythematous base on this patient’s lateral neck, shoulders, and upper back are characteristic of pustular psoriasis.
D. This patient has erythematous plaques with overlying silvery scale on the elbow—a classic
TABLE 2
Traditional systemic therapy21-24
| Methotrexate (MTX) | Cyclosporine (CSA)* | Acitretin | |
|---|---|---|---|
| Dosing | ≤30 mg in one weekly dose (PO, IM, or SC) | 2.5-5.0 mg/kg/d in 2 divided doses for 12 wk, then 12-wk nontreatment period Dose decreased (by 0.5-1.0 mg/kg) with disease clearance or when hypertension or nephrotoxicity are detected | 10-50 mg/d given as a single dose Lower doses (25 mg/d) used to minimize adverse effects and in combination regimens When added to UV, light dose should be reduced 30%-50% |
| Contraindications |
|
|
|
| Baseline monitoring† |
|
|
|
| BP, blood pressure; BUN, blood urea nitrogen; CBC, complete blood cell count; CR, creatinine; H&P, history and physical; IM, intramuscular; LFTs, liver function tests; PO, by mouth; PPD, purified protein derivative; PUVA, psoralen and ultraviolet A; SC, subcutaneous; UV, ultraviolet; UVB, ultraviolet B. *Avoid live vaccinations; caution required with major infection and poorly controlled diabetes. †Ongoing monitoring for MTX: BUN, CBC, CR, LFTs; possible liver biopsy (for high-risk patients or cumulative dose >3.5-4 g); CSA: BP, BUN, CBC, CR, LFTs; lipd profile; magnesium, uric acid, potassium tests; pregnancy testing; Acitretin: CBC, LFTs, lipid profile, renal function test, pregnancy testing. | |||
Biologics require lab work and a detailed medication list
Before beginning biologic therapy for a patient, the National Psoriasis Foundation29 recommends obtaining a complete history, physical, medication list, future plans (ie, pregnancy or travel to locations requiring vaccinations), and baseline labs to identify possible risk factors and/or contraindications. Periodic evaluation to monitor development of new symptoms, including infection and malignancy ( TABLE 3 ),24,30-33 is needed, as well.
TABLE 3
Is your patient a candidate for biologics?24,30-33
| Agent (Drug class) | Alefacept (T-cell inhibitor) | Adalimumab (TNF-inhibitor) | Etanercept (TNF-inhibitor) | Infliximab (TNF-inhibitor) | Ustekinumab (IL-12/23 inhibitor) |
|---|---|---|---|---|---|
| Dosing | 15 mg IM/wk for 12 wk, then 12-wk nontreatment period | 80 mg SC the first wk, 40 mg the 2nd wk, followed by 40 mg every other wk | 50 mg SC twice/wk for 3 mo, then 50 mg/wk | 5 mg/kg IV infusion to start, repeat at 2 and 6 wk, then q6-8 wk | 45 mg SC (for patients <100 kg); 90 mg (for patients >100 kg) to start, repeat at 4 wk, followed by q12 wk for maintenance |
| Contra-indications | HIV |
|
|
| Active TB |
| Baseline monitoring | CD4 count |
|
|
| PPD |
| Ongoing monitoring | Biweekly CD4 count; hold dose for counts <250 |
|
|
| Consider a yearly PPD |
| CBC, complete blood cell count; CHF, congestive heart failure; HIV, human immunodeficiency virus; H&P, history and physical; IL-12/23, interleukin-12, interleukin-23; LFTs, liver function test; MS, multiple sclerosis; NYHA, New York Heart Association; PPD, purified protein derivative; TB, tuberculosis; TNF, tumor necrosis factor. *For this patient population, adalimumab and etanercept have a (theoretical) risk. | |||||
Biologic therapy is contraindicated in patients with active serious infection. If patients develop infections requiring antibiotics while being treated, holding the biologic until infection resolution is advised.34 Standard vaccinations (eg, pneumococcal, hepatitis A and B, influenza, diphtheria, tetanus) are recommended before initiation of immunosuppressive therapy. After therapy starts, patients should avoid live and live-attenuated vaccines (varicella, mumps, measles, and rubella, oral typhoid, yellow fever, herpes zoster, intranasal influenza).35
Currently, none of the biologics are indicated for use in children or adolescents with psoriasis, despite epidemiologic data suggesting that one-third of adults with psoriasis developed it during childhood, in a form severe enough to warrant the use of systemic medications.34 The FDA is currently reviewing the possibility of indicating etanercept for pediatric psoriasis patients. All biologics are category B for pregnancy as there is no evidence that they negatively affect pregnancy.24
T-cell inhibitor. Alefacept binds CD2 on memory-effector T lymphocytes, inhibiting activation. Weekly intramuscular injections of alefacept for 12 weeks can clear lesions with long remissions.30
TNF-inhibitors. The TNF-inhibitors have been available for more than 10 years, predominantly for inflammatory bowel disease (IBD) and rheumatoid arthritis (RA), and more than 1.5 million patients have used adalimumab, etanercept, and infliximab for these disorders. Safety data, especially long term, are mostly derived from patients with IBD or RA, who have often combined TNF-inhibitors with additional immunosuppressive therapies. Thus, for psoriasis patients, who typically use biologics as monotherapy, the risk may be overestimated.24
TNF-inhibitors increase the risk for infection, most commonly of the upper respiratory tract, and, rarely, have been associated with opportunistic infections. Numerous cases of TB reactivation and an increased incidence of disseminated cases have been associated with TNF-inhibitors, so screening is recommended.24
The impact of TNF inhibition on congestive heart failure (CHF) is not well understood. Studies have variously shown that TNF-inhibitors have no effect on CHF morbidity or mortality, increase CHF mortality, or improve left ventricular function. TNF-inhibitors should be avoided in patients with severe CHF (New York Heart Association class III or IV). In milder CHF patients with worsening of symptoms, treatment should be discontinued.36
The increased risk of malignancy, especially lymphoma, is a concern, as there have been numerous case reports of lymphoma occurring with TNF-inhibitors. Psoriasis patients in general have an increased risk of lymphoma that confounds data interpretation.31 A number of case reports and a large observational study have shown patients receiving TNF-inhibitors may be at a greater risk for developing melanoma and nonmelanoma skin cancer.32
Ustekinumab, an interleukin 12/23 inhibitor, is a human monoclonal antibody that is absorbed and eliminated slowly, making dosing injections every 12 weeks convenient with efficacy maintained for at least one year.33 Because of its relative novelty, few studies are published regarding long-term safety. A recent head-to-head trial compared the efficacy and safety of ustekinumab with etanercept and found superior efficacy with ustekinumab, with comparable adverse events.37,38 Similar concerns exist with ustekinumab as with TNF-inhibitors, including infection, malignancy, CHF, and TB.33
CASE Tom denied having a personal family history of multiple sclerosis, or any demyelinating disorder. Nor did he have a history of cancer, tuberculosis exposure, CHF, or hepatitis. A purified protein derivative (PPD) was negative, as was his hepatitis panel, and his complete blood count with differential and metabolic panel were within normal limits.
Tom was started on the TNF-inhibitor adalimumab, after undergoing patient education and training and receiving instructions to stop the medication if he developed a major illness or infection. He received a loading dose of 80 mg SC, followed by 40 mg every other week. He tolerated the treatment well and 70% of his cutaneous symptoms cleared after 12 weeks of therapy; his joint pain also was reduced.
Tom is followed regularly in the clinic, with labs every 4 to 6 months. He is maintained on the injections and happy with the results. At each visit, weight loss and decreased beer intake are encouraged, both of which have been shown to reduce psoriasis severity. Although the beta-blockers and ACE inhibitors he takes are known to exacerbate psoriasis, the medications are necessary to treat Tom’s coronary artery disease.
CORRESPONDENCE Elizabeth Uhlenhake, MD, 18100 Oakwood Boulevard, Suite 300, Dearborn, MI 48124; eulenha@neomed.edu
1. Gelfand JM, Weinstein R, Porter SB, et al. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141:1537-1541.
2. Fortune DG, Richards HL, Griffiths CE. Psychologic factors in psoriasis: consequences, mechanisms, and interventions. Dermatol Clin. 2005;23:681-694.
3. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
4. Pettey AA, Balkrishnan R, Rapp SR, et al. Patients with palmoplantar psoriasis have more physical disability and discomfort than patients with other forms of psoriasis: implications for clinical practice. J Am Acad Dermatol. 2003;49:271-275.
5. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
6. Kaufmann R, Bibby AJ, Bissonnette R, et al. A new calcipotriol/betamethasone dipropionate formulation (Daivobet) is an effective once-daily treatment for psoriasis vulgaris. Dermatology. 2002;205:389-393.
7. Koo JY, Martin D. Investigator-masked comparison of tazarotene gel q.d. plus mometasone furoate cream q.d. vs. mometasone furoate cream b.i.d. in the treatment of plaque psoriasis. Int J Dermatol. 2001;40:210-212.
8. Berger TG, Duvic M, Van Voorhees AS, et al. The use of topical calcineurin inhibitors in dermatology: safety concerns. Report of the American Academy of Dermatology Association Task Force. J Am Acad Dermatol. 2006;54:818-823.
9. Cornell RC, Stoughton RB. Correlation of the vasoconstriction assay and clinical activity in psoriasis. Arch Dermatol. 1985;121:63-67.
10. Lui H. Phototherapy of psoriasis: update with practical pearls. J Cutan Med Surg. 2002;6(suppl):17-21.
11. Walters IB, Burack LH, Coven TR, et al. Suberythemogenic narrow-band UVB is markedly more effective than conventional UVB in treatment of psoriasis vulgaris. J Am Acad Dermatol. 1999;40:893-900.
12. Stern RS, Laird N. The carcinogenic risk of treatments for severe psoriasis. Photochemotherapy follow-up study. Cancer. 1994;73:2759-2764.
13. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
14. Trehan M, Taylor CR. High-dose 308-nm excimer laser for the treatment of psoriasis. J Am Acad Dermatol. 2002;46:732-737.
15. Morison WL, Baughman RD, Day RM, et al. Consensus workshop on the toxic effects of long-term PUVA therapy. Arch Dermatol. 1998;134:595-598.
16. Spuls PI, Witkamp L, Bossuyt PM, et al. A systematic review of five systemic treatments for severe psoriasis. Br J Dermatol. 1997;137:943-949.
17. Murase JE, Lee EE, Koo J. Effect of ethnicity on the risk of developing nonmelanoma skin cancer following long-term PUVA therapy. Int J Dermatol. 2005;44:1016-1021.
18. Lowe NJ, Prystowsky JH, Bourget T, et al. Acitretin plus UVB therapy for psoriasis. Comparisons with placebo plus UVB and acitretin alone. J Am Acad Dermatol. 1991;24:591-594.
19. Torras H, Aliaga A, Lopez-Estebaranz JL, et al. A combination therapy of calcipotriol cream and PUVA reduces the UVA dose and improves the response of psoriasis vulgaris. J Dermatolog Treat. 2004;15:98-103.
20. Tanew A, Guggenbichler A, Honigsmann H, et al. Photochemotherapy for severe psoriasis without or in combination with acitretin: a randomized, double-blind comparison study. J Am Acad Dermatol. 1991;25:682-684.
21. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
22. Strober BE, Menon K. Folate supplementation during methotrexate therapy for patients with psoriasis. J Am Acad Dermatol. 2005;53:652-659.
23. Taler SJ, Textor SC, Canzanello VJ, et al. Cyclosporine-induced hypertension: incidence, pathogenesis and management. Drug Saf. 1999;20:437-449.
24. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
25. Heydendael VM, Spuls PI, Opmeer BC, et al. Methotrexate versus cyclosporine in moderate-to-severe chronic plaque psoriasis. N Engl J Med. 2003;349:658-665.
26. Lloyd ME, Carr M, McElhatton P, et al. The effects of methotrexate on pregnancy, fertility and lactation. QJM. 1999;92:551-563.
27. Ellis CN, Fradin MS, Messana JM, et al. Cyclosporine for plaque-type psoriasis. Results of a multidose, double-blind trial. N Engl J Med. 1991;324:277-284.
28. Buccheri L, Katchen BR, Karter AJ, et al. Acitretin therapy is effective for psoriasis associated with human immunodeficiency virus infection. Arch Dermatol. 1997;133:711-715.
29. Lebwohl M, Bagel J, Gelfand JM, et al. From the Medical Board of the National Psoriasis Foundation: monitoring and vaccinations in patients treated with biologics for psoriasis. J Am Acad Dermatol. 2008;58:94-105.
30. Gordon KB, Vaishnaw AK, O’Gorman J, et al. Treatment of psoriasis with alefacept: correlation of clinical improvement with reductions of memory T-cell counts. Arch Dermatol. 2003;139:1563-1570.
31. Brown SL, Greene MH, Gershon SK, et al. Tumor necrosis factor antagonist therapy and lymphoma development: twenty-six cases reported to the Food and Drug Administration. Arthritis Rheum. 2002;46:3151-3158.
32. Fulchiero GJ, Jr, Salvaggio H, Drabick JJ, et al. Eruptive latent metastatic melanomas after initiation of antitumor necrosis factor therapies. J Am Acad Dermatol. 2007;56(suppl):S65-S67.
33. Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371:1665-1674.
34. US Food and Drug Administration. FDA approves new drug to treat psoriasis. Sept. 25, 2009. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm183851.htm. Accessed June 15, 2012.
35. Duchini A, Goss JA, Karpen S, et al. Vaccinations for adult solid-organ transplant recipients: current recommendations and protocols. Clin Microbiol Rev. 2003;16:357-364.
36. Mann DL, McMurray JJ, Packer M, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation. 2004;109:1594-1602.
37. Griffiths CE, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118-128.
38. Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684.
• Prescribe high-potency (Class 1) topical steroids for use on thicker, chronic plaques and low-potency (Class 7) steroids for the face, intertriginous areas, and the groin. A
• Recommend folate supplementation (1-5 mg/d) for patients being treated with methotrexate, as it may reduce the drug’s hematologic and gastrointestinal adverse effects without decreasing efficacy. A
• Advise patients scheduled to begin biologic therapy to get standard vaccinations—eg, pneumococcal, influenza, hepatitis A and B—before treatment is initiated and to avoid live and live-attenuated vaccines thereafter. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Psoriasis is a systemic inflammatory disorder altered by environmental and genetic factors that presents as scaly erythematous plaques and affects 2% to 3% of the population.1 Eighty percent have mild-to-moderate cutaneous disease; disabling arthritis occurs in as many as 42% of cases.1,2 The devastating effects on quality of life— including social stigmatization, pain, physical disability, and psychological distress—are comparable to the effects of conditions like cancer and depression.2 There is no definitive cure, and patients are left with a lifetime of waxing and waning symptoms.
Helping to create an individualized treatment plan tailored to disease type and extent, comorbidities, and needs of the patient can directly impact the quality of his or her life.3 For those with localized disease, topical therapy is a suitable first choice. Phototherapy is generally the first-line treatment for patients with extensive psoriasis or disabling symptoms. When phototherapy is not feasible or is ineffective, systemic treatments with conventional oral agents or biologics are indicated.
Discussions about therapeutic options should include expected results and duration of remission, cost, convenience, adverse effects, insurance coverage, and safety concerns. The patient’s preferences should be taken into account in the treatment plan you create.3 A guiding principle: The optimal protocol is the one the patient is motivated to adhere to.
The limits of topical therapy
Topical treatments are safe and effective when used properly, as monotherapy for localized disease and adjunctive therapy for resistant lesions. Monotherapy with topical agents is not recommended for sites that have a significant impact on quality of life, such as the palms and soles, and is not practical for patients with extensive disease (>10% of body surface).4
More potent topicals, such as corticosteroids, are recommended for acute flares; less potent agents with fewer adverse effects, such as calcipotriene, are typically used for maintenance ( TABLE 1 ).5-8 Topical agents can be used either long term or intermittently. Here, too, the most effective treatment is the one the patient will actually apply.5
TABLE 1
A look at nonsteroidal topical treatments5-8
| Class | Examples | Adverse effects | Comments |
|---|---|---|---|
| Vitamin D analogues | Calcipotriol (calcipotriene), calcitriol | Burning, pruritus, edema, peeling, dryness, erythema | Combining with beta-methasone dipropionate increases efficacy |
| Retinoids | Tazarotene, tretinoin | Teratogenic, photosensitivity, irritation | Increased efficacy when combined with NB-UVB (and less UV exposure); increased efficacy, duration of remission, and reduction in steroid-induced atrophy when used with steroids |
| Calcineurin inhibitors | Tacrolimus, pimecrolimus | Burning and pruritus Black box warning for risk of malignancies* | No clinical evidence of increased cancer risk |
| Others | Emollients, salicylic acid, anthralin, coal tar | Severe skin irritation, staining of clothes, odor with anthralin and tar | Salicylic acid works well with steroids and topical immunomodulators, but is not compatible with calcipotriene |
| *Lymphoma seen with oral therapy. UVB, ultraviolet B; NB-UVB, narrow-band ultraviolet B. | |||
Steroid selection is based on site, severity
Corticosteroids are antiproliferative, immunosuppressive, anti-inflammatory, and vasoconstrictive, and divided into 7 classes. Low-potency agents (Class 7) are used on thinner skin like the face, intertriginous areas, and groin; high-potency steroids (Class 1) are reserved for thicker, chronic plaques.9 As a general rule, Class 1 steroids can be safely used for 2 to 4 weeks, with increased risk of both cutaneous effects and systemic absorption if used continuously for longer periods.5 The optimal end point for less potent agents is not known.
Cutaneous adverse effects are more common than systemic ones and include skin atrophy, telangiectasia, striae distensae, acne, folliculitis, and purpura.
Systemic adverse effects include Cushing’s syndrome, osteonecrosis of the femoral head, cataracts, and glaucoma.5 The greatest risk of systemic effects is associated with prolonged use of high-potency corticosteroids over large surfaces or under occlusion with dressings or plastic wrap. Patients should be transitioned to the lowest potency possible to maintain efficacy, use corticosteroids intermittently, or combine them with nonsteroidal agents to avoid unwanted effects.
Topicals aren’t working? Move on to phototherapy
Phototherapy is an option for patients with extensive disease or skin manifestations that are recalcitrant to topicals. It is efficacious, cost-effective, and lacks systemic immunosuppression. Ultraviolet (UV) A and B act on Langerhans cells, cytokines, and adhesion molecules, inhibiting epidermal proliferation and angiogenesis.10
Broadband UVB (BB-UVB) was first used during the 1950s, with crude tar or anthralin, but is rarely used today.
Narrow-band UVB (NB-UVB) (311-313 nm), developed in the 1980s, has largely replaced BB-UVB. In addition to providing more rapid clearing and resolution of psoriasis compared with BB-UVB, NB-UVB may have less phototoxicity.11 Between 20 and 25 NB-UVB treatments, 2 to 3 times a week (in the office or at home) are usually required for significant improvement.
Photocarcinogenesis is a concern but numerous studies, including a review of 3867 patients treated with NB-UVB with a median 5.5-year follow-up, found no significant association with cutaneous malignancies.12 NB-UVB is considered safe during pregnancy and used as first-line therapy for pregnant patients.13
Targeted UVB therapy using a 308-nm excimer laser, another option, selectively targets psoriatic lesions, leaving normal skin untreated. This makes supraerythemogenic doses possible, which increases UVB’s efficacy. Long-term adverse effects and duration of remission have not been clearly established.14
Psoralen and UVA (PUVA), which uses oral or topical psoralens to sensitize the skin to UVA, has a slightly higher efficacy than NB-UVB, but with increased risk of squamous cell carcinoma (SCC) and possibly melanoma.15 Clearing can occur within 24 treatments, with remissions lasting 3 to 6 months;16 monthly maintenance has not been found to lengthen remission.13
Common adverse effects include erythema that peaks 48 to 96 hours after a treatment, pruritus, xerosis, irregular pigmentation, and gastrointestinal symptoms that can be reduced by decreasing psoralen and/or UVA doses.13 High cumulative doses of oral PUVA (>200 treatments) is associated with a dose-related increased risk of nonmelanoma skin cancer, particularly SCC, in the Caucasian population. This increased risk has not been demonstrated in patients treated with PUVA bath therapy, which is more common in Scandinavian countries.17 There is no consensus regarding the risk of melanoma.15
A careful risk-benefit analysis is needed before initiating phototherapy in patients who take photosensitizing drugs, are immunosuppressed, or have a photosensitivity disorder or a history of melanoma, atypical nevi, multiple melanoma risk factors, or multiple nonmelanoma skin cancers.13 Regardless of the type of UV therapy administered, eye protection with goggles is required to decrease the risk of UV-related cataract formation, and genital shielding is needed to prevent increased risk of tumors.13 Photoaging is a long-term effect.
CASE When questioned further about the challenges that Tom has had with controlling his symptoms, he admitted to being noncompliant. As a busy executive, he said he didn’t have time to use the topical corticosteroids regularly. Phototherapy could alleviate his cutaneous symptoms, but would not address his symptoms that were consistent with psoriatic arthritis.
Pairing therapeutic modalities decreases exposure
Combining therapeutic modalities like emollients and topical or oral retinoids with NB-UVB improves efficacy while reducing the number of treatment sessions and cumulative UVB dosage. If calcipotriene is used, it should be applied after phototherapy because it is degraded upon UVB exposure.18 Acitretin should be started 2 weeks prior to initiation of phototherapy, and its use accompanied by a 25% reduction in initial UV dosage.13
PUVA may also be combined with topical calcipotriene or retinoids.19 In both cases, the addition of the other agent typically decreases the duration of phototherapy, improves the clinical response, and reduces the risk of cancer.13,20
Extensive disease? Consider a traditional systemic agent
Traditional systemic therapy is used to treat extensive disease ( FIGURE ), psoriasis refractory to topical agents, and debilitating disease on the palms, soles, or scalp. Biologics are a recent alternative, but traditional systemics have been utilized longer and have a more longstanding adverse effect and safety profile, are administered orally, and are much less expensive than biologics. Monitoring patients on systemic therapy is necessary ( TABLE 2 ).21-24
Methotrexate (MTX), a competitive inhibitor of dihydrofolate reductase, is the most commonly prescribed traditional systemic psoriasis treatment.21 It is administered in a single weekly dose via tablet, parenteral solution, or intramuscular (IM) or subcutaneous (SC) injection.25 A test dose (2.5 or 5 mg) is given initially and complete blood cell count is monitored within one week to evaluate for potential bone marrow toxicity. If none is observed, the dose may be increased to control the disease while minimizing adverse effects.21
Common adverse effects of MTX, such as nausea, vomiting, stomatitis, and fatigue, may be minimized by IM or SC administration, splitting the dose, or providing folate supplementation.21-24 Given in doses of 1 to 5 mg/d, folate may reduce adverse hematologic, gastrointestinal, and hepatic effects without decreasing efficacy.22
The major severe toxicities are myelosuppression, hepatotoxicity, and pulmonary fibrosis.24 MTX-induced hepatotoxicity is similar to nonalcoholic fatty liver disease (NAFLD) and is thought to exacerbate preexisting NAFLD, which is common in patients with metabolic syndrome. A liver biopsy or serum assays for liver fibrosis (amino-terminal peptide of pro-collagen III) may be warranted during therapy.24
MTX is an abortifacient and teratogen, so contraception during treatment and for up to 3 months thereafter is mandatory for women of childbearing age.26 Men should be advised that MTX decreases sperm count. (For more on methotrexate, see: “When a fetus survives methotrexate exposure,” at http://www.jfponline.com/Pages.asp?AID=10299).
Cyclosporine (CSA), an oral calcineurin inhibitor, is a potent immunosuppressant that rapidly clears psoriasis.27 Because duration of use correlates with permanent nephrotoxicity, hypertension, and potential increased risk of SCC and lymphoma, intermittent 12-week courses are recommended. Calcium channel blockers are the preferred treatment for CSA-induced hypertension because of their effect on smooth muscle vasodilation.21
Oral retinoids. Acitretin modulates epidermal proliferation and is anti-inflammatory. Because it lacks immunosuppression, acitretin is generally considered the treatment of choice in HIV patients with severe psoriasis.28 Acitretin is teratogenic and contraindicated in women who plan to become pregnant or who are unwilling to use adequate contraception for 3 years after discontinuing the drug.21
CASE Given the significant percentage of body surface area involved and symptoms consistent with psoriatic arthritis, Tom required an aggressive therapeutic regimen. His history of nonalcoholic fatty liver and social drinking precluded the use of methotrexate. A biologic therapy was the next therapeutic choice that could relieve both his cutaneous and joint symptoms.
FIGURE
4 psoriasis patients, 4 different presentations
A. The linear erythematous, scaly plaque along this patient’s cardiac bypass scar demonstrates koebnerization of plaque psoriasis.
B. Diffuse erythematous scaly papules coalesce into plaques on this patient’s anterior chest, arms, abdomen, and periumbilicus.
C. The pinpoint pustules on an erythematous base on this patient’s lateral neck, shoulders, and upper back are characteristic of pustular psoriasis.
D. This patient has erythematous plaques with overlying silvery scale on the elbow—a classic
TABLE 2
Traditional systemic therapy21-24
| Methotrexate (MTX) | Cyclosporine (CSA)* | Acitretin | |
|---|---|---|---|
| Dosing | ≤30 mg in one weekly dose (PO, IM, or SC) | 2.5-5.0 mg/kg/d in 2 divided doses for 12 wk, then 12-wk nontreatment period Dose decreased (by 0.5-1.0 mg/kg) with disease clearance or when hypertension or nephrotoxicity are detected | 10-50 mg/d given as a single dose Lower doses (25 mg/d) used to minimize adverse effects and in combination regimens When added to UV, light dose should be reduced 30%-50% |
| Contraindications |
|
|
|
| Baseline monitoring† |
|
|
|
| BP, blood pressure; BUN, blood urea nitrogen; CBC, complete blood cell count; CR, creatinine; H&P, history and physical; IM, intramuscular; LFTs, liver function tests; PO, by mouth; PPD, purified protein derivative; PUVA, psoralen and ultraviolet A; SC, subcutaneous; UV, ultraviolet; UVB, ultraviolet B. *Avoid live vaccinations; caution required with major infection and poorly controlled diabetes. †Ongoing monitoring for MTX: BUN, CBC, CR, LFTs; possible liver biopsy (for high-risk patients or cumulative dose >3.5-4 g); CSA: BP, BUN, CBC, CR, LFTs; lipd profile; magnesium, uric acid, potassium tests; pregnancy testing; Acitretin: CBC, LFTs, lipid profile, renal function test, pregnancy testing. | |||
Biologics require lab work and a detailed medication list
Before beginning biologic therapy for a patient, the National Psoriasis Foundation29 recommends obtaining a complete history, physical, medication list, future plans (ie, pregnancy or travel to locations requiring vaccinations), and baseline labs to identify possible risk factors and/or contraindications. Periodic evaluation to monitor development of new symptoms, including infection and malignancy ( TABLE 3 ),24,30-33 is needed, as well.
TABLE 3
Is your patient a candidate for biologics?24,30-33
| Agent (Drug class) | Alefacept (T-cell inhibitor) | Adalimumab (TNF-inhibitor) | Etanercept (TNF-inhibitor) | Infliximab (TNF-inhibitor) | Ustekinumab (IL-12/23 inhibitor) |
|---|---|---|---|---|---|
| Dosing | 15 mg IM/wk for 12 wk, then 12-wk nontreatment period | 80 mg SC the first wk, 40 mg the 2nd wk, followed by 40 mg every other wk | 50 mg SC twice/wk for 3 mo, then 50 mg/wk | 5 mg/kg IV infusion to start, repeat at 2 and 6 wk, then q6-8 wk | 45 mg SC (for patients <100 kg); 90 mg (for patients >100 kg) to start, repeat at 4 wk, followed by q12 wk for maintenance |
| Contra-indications | HIV |
|
|
| Active TB |
| Baseline monitoring | CD4 count |
|
|
| PPD |
| Ongoing monitoring | Biweekly CD4 count; hold dose for counts <250 |
|
|
| Consider a yearly PPD |
| CBC, complete blood cell count; CHF, congestive heart failure; HIV, human immunodeficiency virus; H&P, history and physical; IL-12/23, interleukin-12, interleukin-23; LFTs, liver function test; MS, multiple sclerosis; NYHA, New York Heart Association; PPD, purified protein derivative; TB, tuberculosis; TNF, tumor necrosis factor. *For this patient population, adalimumab and etanercept have a (theoretical) risk. | |||||
Biologic therapy is contraindicated in patients with active serious infection. If patients develop infections requiring antibiotics while being treated, holding the biologic until infection resolution is advised.34 Standard vaccinations (eg, pneumococcal, hepatitis A and B, influenza, diphtheria, tetanus) are recommended before initiation of immunosuppressive therapy. After therapy starts, patients should avoid live and live-attenuated vaccines (varicella, mumps, measles, and rubella, oral typhoid, yellow fever, herpes zoster, intranasal influenza).35
Currently, none of the biologics are indicated for use in children or adolescents with psoriasis, despite epidemiologic data suggesting that one-third of adults with psoriasis developed it during childhood, in a form severe enough to warrant the use of systemic medications.34 The FDA is currently reviewing the possibility of indicating etanercept for pediatric psoriasis patients. All biologics are category B for pregnancy as there is no evidence that they negatively affect pregnancy.24
T-cell inhibitor. Alefacept binds CD2 on memory-effector T lymphocytes, inhibiting activation. Weekly intramuscular injections of alefacept for 12 weeks can clear lesions with long remissions.30
TNF-inhibitors. The TNF-inhibitors have been available for more than 10 years, predominantly for inflammatory bowel disease (IBD) and rheumatoid arthritis (RA), and more than 1.5 million patients have used adalimumab, etanercept, and infliximab for these disorders. Safety data, especially long term, are mostly derived from patients with IBD or RA, who have often combined TNF-inhibitors with additional immunosuppressive therapies. Thus, for psoriasis patients, who typically use biologics as monotherapy, the risk may be overestimated.24
TNF-inhibitors increase the risk for infection, most commonly of the upper respiratory tract, and, rarely, have been associated with opportunistic infections. Numerous cases of TB reactivation and an increased incidence of disseminated cases have been associated with TNF-inhibitors, so screening is recommended.24
The impact of TNF inhibition on congestive heart failure (CHF) is not well understood. Studies have variously shown that TNF-inhibitors have no effect on CHF morbidity or mortality, increase CHF mortality, or improve left ventricular function. TNF-inhibitors should be avoided in patients with severe CHF (New York Heart Association class III or IV). In milder CHF patients with worsening of symptoms, treatment should be discontinued.36
The increased risk of malignancy, especially lymphoma, is a concern, as there have been numerous case reports of lymphoma occurring with TNF-inhibitors. Psoriasis patients in general have an increased risk of lymphoma that confounds data interpretation.31 A number of case reports and a large observational study have shown patients receiving TNF-inhibitors may be at a greater risk for developing melanoma and nonmelanoma skin cancer.32
Ustekinumab, an interleukin 12/23 inhibitor, is a human monoclonal antibody that is absorbed and eliminated slowly, making dosing injections every 12 weeks convenient with efficacy maintained for at least one year.33 Because of its relative novelty, few studies are published regarding long-term safety. A recent head-to-head trial compared the efficacy and safety of ustekinumab with etanercept and found superior efficacy with ustekinumab, with comparable adverse events.37,38 Similar concerns exist with ustekinumab as with TNF-inhibitors, including infection, malignancy, CHF, and TB.33
CASE Tom denied having a personal family history of multiple sclerosis, or any demyelinating disorder. Nor did he have a history of cancer, tuberculosis exposure, CHF, or hepatitis. A purified protein derivative (PPD) was negative, as was his hepatitis panel, and his complete blood count with differential and metabolic panel were within normal limits.
Tom was started on the TNF-inhibitor adalimumab, after undergoing patient education and training and receiving instructions to stop the medication if he developed a major illness or infection. He received a loading dose of 80 mg SC, followed by 40 mg every other week. He tolerated the treatment well and 70% of his cutaneous symptoms cleared after 12 weeks of therapy; his joint pain also was reduced.
Tom is followed regularly in the clinic, with labs every 4 to 6 months. He is maintained on the injections and happy with the results. At each visit, weight loss and decreased beer intake are encouraged, both of which have been shown to reduce psoriasis severity. Although the beta-blockers and ACE inhibitors he takes are known to exacerbate psoriasis, the medications are necessary to treat Tom’s coronary artery disease.
CORRESPONDENCE Elizabeth Uhlenhake, MD, 18100 Oakwood Boulevard, Suite 300, Dearborn, MI 48124; eulenha@neomed.edu
• Prescribe high-potency (Class 1) topical steroids for use on thicker, chronic plaques and low-potency (Class 7) steroids for the face, intertriginous areas, and the groin. A
• Recommend folate supplementation (1-5 mg/d) for patients being treated with methotrexate, as it may reduce the drug’s hematologic and gastrointestinal adverse effects without decreasing efficacy. A
• Advise patients scheduled to begin biologic therapy to get standard vaccinations—eg, pneumococcal, influenza, hepatitis A and B—before treatment is initiated and to avoid live and live-attenuated vaccines thereafter. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Psoriasis is a systemic inflammatory disorder altered by environmental and genetic factors that presents as scaly erythematous plaques and affects 2% to 3% of the population.1 Eighty percent have mild-to-moderate cutaneous disease; disabling arthritis occurs in as many as 42% of cases.1,2 The devastating effects on quality of life— including social stigmatization, pain, physical disability, and psychological distress—are comparable to the effects of conditions like cancer and depression.2 There is no definitive cure, and patients are left with a lifetime of waxing and waning symptoms.
Helping to create an individualized treatment plan tailored to disease type and extent, comorbidities, and needs of the patient can directly impact the quality of his or her life.3 For those with localized disease, topical therapy is a suitable first choice. Phototherapy is generally the first-line treatment for patients with extensive psoriasis or disabling symptoms. When phototherapy is not feasible or is ineffective, systemic treatments with conventional oral agents or biologics are indicated.
Discussions about therapeutic options should include expected results and duration of remission, cost, convenience, adverse effects, insurance coverage, and safety concerns. The patient’s preferences should be taken into account in the treatment plan you create.3 A guiding principle: The optimal protocol is the one the patient is motivated to adhere to.
The limits of topical therapy
Topical treatments are safe and effective when used properly, as monotherapy for localized disease and adjunctive therapy for resistant lesions. Monotherapy with topical agents is not recommended for sites that have a significant impact on quality of life, such as the palms and soles, and is not practical for patients with extensive disease (>10% of body surface).4
More potent topicals, such as corticosteroids, are recommended for acute flares; less potent agents with fewer adverse effects, such as calcipotriene, are typically used for maintenance ( TABLE 1 ).5-8 Topical agents can be used either long term or intermittently. Here, too, the most effective treatment is the one the patient will actually apply.5
TABLE 1
A look at nonsteroidal topical treatments5-8
| Class | Examples | Adverse effects | Comments |
|---|---|---|---|
| Vitamin D analogues | Calcipotriol (calcipotriene), calcitriol | Burning, pruritus, edema, peeling, dryness, erythema | Combining with beta-methasone dipropionate increases efficacy |
| Retinoids | Tazarotene, tretinoin | Teratogenic, photosensitivity, irritation | Increased efficacy when combined with NB-UVB (and less UV exposure); increased efficacy, duration of remission, and reduction in steroid-induced atrophy when used with steroids |
| Calcineurin inhibitors | Tacrolimus, pimecrolimus | Burning and pruritus Black box warning for risk of malignancies* | No clinical evidence of increased cancer risk |
| Others | Emollients, salicylic acid, anthralin, coal tar | Severe skin irritation, staining of clothes, odor with anthralin and tar | Salicylic acid works well with steroids and topical immunomodulators, but is not compatible with calcipotriene |
| *Lymphoma seen with oral therapy. UVB, ultraviolet B; NB-UVB, narrow-band ultraviolet B. | |||
Steroid selection is based on site, severity
Corticosteroids are antiproliferative, immunosuppressive, anti-inflammatory, and vasoconstrictive, and divided into 7 classes. Low-potency agents (Class 7) are used on thinner skin like the face, intertriginous areas, and groin; high-potency steroids (Class 1) are reserved for thicker, chronic plaques.9 As a general rule, Class 1 steroids can be safely used for 2 to 4 weeks, with increased risk of both cutaneous effects and systemic absorption if used continuously for longer periods.5 The optimal end point for less potent agents is not known.
Cutaneous adverse effects are more common than systemic ones and include skin atrophy, telangiectasia, striae distensae, acne, folliculitis, and purpura.
Systemic adverse effects include Cushing’s syndrome, osteonecrosis of the femoral head, cataracts, and glaucoma.5 The greatest risk of systemic effects is associated with prolonged use of high-potency corticosteroids over large surfaces or under occlusion with dressings or plastic wrap. Patients should be transitioned to the lowest potency possible to maintain efficacy, use corticosteroids intermittently, or combine them with nonsteroidal agents to avoid unwanted effects.
Topicals aren’t working? Move on to phototherapy
Phototherapy is an option for patients with extensive disease or skin manifestations that are recalcitrant to topicals. It is efficacious, cost-effective, and lacks systemic immunosuppression. Ultraviolet (UV) A and B act on Langerhans cells, cytokines, and adhesion molecules, inhibiting epidermal proliferation and angiogenesis.10
Broadband UVB (BB-UVB) was first used during the 1950s, with crude tar or anthralin, but is rarely used today.
Narrow-band UVB (NB-UVB) (311-313 nm), developed in the 1980s, has largely replaced BB-UVB. In addition to providing more rapid clearing and resolution of psoriasis compared with BB-UVB, NB-UVB may have less phototoxicity.11 Between 20 and 25 NB-UVB treatments, 2 to 3 times a week (in the office or at home) are usually required for significant improvement.
Photocarcinogenesis is a concern but numerous studies, including a review of 3867 patients treated with NB-UVB with a median 5.5-year follow-up, found no significant association with cutaneous malignancies.12 NB-UVB is considered safe during pregnancy and used as first-line therapy for pregnant patients.13
Targeted UVB therapy using a 308-nm excimer laser, another option, selectively targets psoriatic lesions, leaving normal skin untreated. This makes supraerythemogenic doses possible, which increases UVB’s efficacy. Long-term adverse effects and duration of remission have not been clearly established.14
Psoralen and UVA (PUVA), which uses oral or topical psoralens to sensitize the skin to UVA, has a slightly higher efficacy than NB-UVB, but with increased risk of squamous cell carcinoma (SCC) and possibly melanoma.15 Clearing can occur within 24 treatments, with remissions lasting 3 to 6 months;16 monthly maintenance has not been found to lengthen remission.13
Common adverse effects include erythema that peaks 48 to 96 hours after a treatment, pruritus, xerosis, irregular pigmentation, and gastrointestinal symptoms that can be reduced by decreasing psoralen and/or UVA doses.13 High cumulative doses of oral PUVA (>200 treatments) is associated with a dose-related increased risk of nonmelanoma skin cancer, particularly SCC, in the Caucasian population. This increased risk has not been demonstrated in patients treated with PUVA bath therapy, which is more common in Scandinavian countries.17 There is no consensus regarding the risk of melanoma.15
A careful risk-benefit analysis is needed before initiating phototherapy in patients who take photosensitizing drugs, are immunosuppressed, or have a photosensitivity disorder or a history of melanoma, atypical nevi, multiple melanoma risk factors, or multiple nonmelanoma skin cancers.13 Regardless of the type of UV therapy administered, eye protection with goggles is required to decrease the risk of UV-related cataract formation, and genital shielding is needed to prevent increased risk of tumors.13 Photoaging is a long-term effect.
CASE When questioned further about the challenges that Tom has had with controlling his symptoms, he admitted to being noncompliant. As a busy executive, he said he didn’t have time to use the topical corticosteroids regularly. Phototherapy could alleviate his cutaneous symptoms, but would not address his symptoms that were consistent with psoriatic arthritis.
Pairing therapeutic modalities decreases exposure
Combining therapeutic modalities like emollients and topical or oral retinoids with NB-UVB improves efficacy while reducing the number of treatment sessions and cumulative UVB dosage. If calcipotriene is used, it should be applied after phototherapy because it is degraded upon UVB exposure.18 Acitretin should be started 2 weeks prior to initiation of phototherapy, and its use accompanied by a 25% reduction in initial UV dosage.13
PUVA may also be combined with topical calcipotriene or retinoids.19 In both cases, the addition of the other agent typically decreases the duration of phototherapy, improves the clinical response, and reduces the risk of cancer.13,20
Extensive disease? Consider a traditional systemic agent
Traditional systemic therapy is used to treat extensive disease ( FIGURE ), psoriasis refractory to topical agents, and debilitating disease on the palms, soles, or scalp. Biologics are a recent alternative, but traditional systemics have been utilized longer and have a more longstanding adverse effect and safety profile, are administered orally, and are much less expensive than biologics. Monitoring patients on systemic therapy is necessary ( TABLE 2 ).21-24
Methotrexate (MTX), a competitive inhibitor of dihydrofolate reductase, is the most commonly prescribed traditional systemic psoriasis treatment.21 It is administered in a single weekly dose via tablet, parenteral solution, or intramuscular (IM) or subcutaneous (SC) injection.25 A test dose (2.5 or 5 mg) is given initially and complete blood cell count is monitored within one week to evaluate for potential bone marrow toxicity. If none is observed, the dose may be increased to control the disease while minimizing adverse effects.21
Common adverse effects of MTX, such as nausea, vomiting, stomatitis, and fatigue, may be minimized by IM or SC administration, splitting the dose, or providing folate supplementation.21-24 Given in doses of 1 to 5 mg/d, folate may reduce adverse hematologic, gastrointestinal, and hepatic effects without decreasing efficacy.22
The major severe toxicities are myelosuppression, hepatotoxicity, and pulmonary fibrosis.24 MTX-induced hepatotoxicity is similar to nonalcoholic fatty liver disease (NAFLD) and is thought to exacerbate preexisting NAFLD, which is common in patients with metabolic syndrome. A liver biopsy or serum assays for liver fibrosis (amino-terminal peptide of pro-collagen III) may be warranted during therapy.24
MTX is an abortifacient and teratogen, so contraception during treatment and for up to 3 months thereafter is mandatory for women of childbearing age.26 Men should be advised that MTX decreases sperm count. (For more on methotrexate, see: “When a fetus survives methotrexate exposure,” at http://www.jfponline.com/Pages.asp?AID=10299).
Cyclosporine (CSA), an oral calcineurin inhibitor, is a potent immunosuppressant that rapidly clears psoriasis.27 Because duration of use correlates with permanent nephrotoxicity, hypertension, and potential increased risk of SCC and lymphoma, intermittent 12-week courses are recommended. Calcium channel blockers are the preferred treatment for CSA-induced hypertension because of their effect on smooth muscle vasodilation.21
Oral retinoids. Acitretin modulates epidermal proliferation and is anti-inflammatory. Because it lacks immunosuppression, acitretin is generally considered the treatment of choice in HIV patients with severe psoriasis.28 Acitretin is teratogenic and contraindicated in women who plan to become pregnant or who are unwilling to use adequate contraception for 3 years after discontinuing the drug.21
CASE Given the significant percentage of body surface area involved and symptoms consistent with psoriatic arthritis, Tom required an aggressive therapeutic regimen. His history of nonalcoholic fatty liver and social drinking precluded the use of methotrexate. A biologic therapy was the next therapeutic choice that could relieve both his cutaneous and joint symptoms.
FIGURE
4 psoriasis patients, 4 different presentations
A. The linear erythematous, scaly plaque along this patient’s cardiac bypass scar demonstrates koebnerization of plaque psoriasis.
B. Diffuse erythematous scaly papules coalesce into plaques on this patient’s anterior chest, arms, abdomen, and periumbilicus.
C. The pinpoint pustules on an erythematous base on this patient’s lateral neck, shoulders, and upper back are characteristic of pustular psoriasis.
D. This patient has erythematous plaques with overlying silvery scale on the elbow—a classic
TABLE 2
Traditional systemic therapy21-24
| Methotrexate (MTX) | Cyclosporine (CSA)* | Acitretin | |
|---|---|---|---|
| Dosing | ≤30 mg in one weekly dose (PO, IM, or SC) | 2.5-5.0 mg/kg/d in 2 divided doses for 12 wk, then 12-wk nontreatment period Dose decreased (by 0.5-1.0 mg/kg) with disease clearance or when hypertension or nephrotoxicity are detected | 10-50 mg/d given as a single dose Lower doses (25 mg/d) used to minimize adverse effects and in combination regimens When added to UV, light dose should be reduced 30%-50% |
| Contraindications |
|
|
|
| Baseline monitoring† |
|
|
|
| BP, blood pressure; BUN, blood urea nitrogen; CBC, complete blood cell count; CR, creatinine; H&P, history and physical; IM, intramuscular; LFTs, liver function tests; PO, by mouth; PPD, purified protein derivative; PUVA, psoralen and ultraviolet A; SC, subcutaneous; UV, ultraviolet; UVB, ultraviolet B. *Avoid live vaccinations; caution required with major infection and poorly controlled diabetes. †Ongoing monitoring for MTX: BUN, CBC, CR, LFTs; possible liver biopsy (for high-risk patients or cumulative dose >3.5-4 g); CSA: BP, BUN, CBC, CR, LFTs; lipd profile; magnesium, uric acid, potassium tests; pregnancy testing; Acitretin: CBC, LFTs, lipid profile, renal function test, pregnancy testing. | |||
Biologics require lab work and a detailed medication list
Before beginning biologic therapy for a patient, the National Psoriasis Foundation29 recommends obtaining a complete history, physical, medication list, future plans (ie, pregnancy or travel to locations requiring vaccinations), and baseline labs to identify possible risk factors and/or contraindications. Periodic evaluation to monitor development of new symptoms, including infection and malignancy ( TABLE 3 ),24,30-33 is needed, as well.
TABLE 3
Is your patient a candidate for biologics?24,30-33
| Agent (Drug class) | Alefacept (T-cell inhibitor) | Adalimumab (TNF-inhibitor) | Etanercept (TNF-inhibitor) | Infliximab (TNF-inhibitor) | Ustekinumab (IL-12/23 inhibitor) |
|---|---|---|---|---|---|
| Dosing | 15 mg IM/wk for 12 wk, then 12-wk nontreatment period | 80 mg SC the first wk, 40 mg the 2nd wk, followed by 40 mg every other wk | 50 mg SC twice/wk for 3 mo, then 50 mg/wk | 5 mg/kg IV infusion to start, repeat at 2 and 6 wk, then q6-8 wk | 45 mg SC (for patients <100 kg); 90 mg (for patients >100 kg) to start, repeat at 4 wk, followed by q12 wk for maintenance |
| Contra-indications | HIV |
|
|
| Active TB |
| Baseline monitoring | CD4 count |
|
|
| PPD |
| Ongoing monitoring | Biweekly CD4 count; hold dose for counts <250 |
|
|
| Consider a yearly PPD |
| CBC, complete blood cell count; CHF, congestive heart failure; HIV, human immunodeficiency virus; H&P, history and physical; IL-12/23, interleukin-12, interleukin-23; LFTs, liver function test; MS, multiple sclerosis; NYHA, New York Heart Association; PPD, purified protein derivative; TB, tuberculosis; TNF, tumor necrosis factor. *For this patient population, adalimumab and etanercept have a (theoretical) risk. | |||||
Biologic therapy is contraindicated in patients with active serious infection. If patients develop infections requiring antibiotics while being treated, holding the biologic until infection resolution is advised.34 Standard vaccinations (eg, pneumococcal, hepatitis A and B, influenza, diphtheria, tetanus) are recommended before initiation of immunosuppressive therapy. After therapy starts, patients should avoid live and live-attenuated vaccines (varicella, mumps, measles, and rubella, oral typhoid, yellow fever, herpes zoster, intranasal influenza).35
Currently, none of the biologics are indicated for use in children or adolescents with psoriasis, despite epidemiologic data suggesting that one-third of adults with psoriasis developed it during childhood, in a form severe enough to warrant the use of systemic medications.34 The FDA is currently reviewing the possibility of indicating etanercept for pediatric psoriasis patients. All biologics are category B for pregnancy as there is no evidence that they negatively affect pregnancy.24
T-cell inhibitor. Alefacept binds CD2 on memory-effector T lymphocytes, inhibiting activation. Weekly intramuscular injections of alefacept for 12 weeks can clear lesions with long remissions.30
TNF-inhibitors. The TNF-inhibitors have been available for more than 10 years, predominantly for inflammatory bowel disease (IBD) and rheumatoid arthritis (RA), and more than 1.5 million patients have used adalimumab, etanercept, and infliximab for these disorders. Safety data, especially long term, are mostly derived from patients with IBD or RA, who have often combined TNF-inhibitors with additional immunosuppressive therapies. Thus, for psoriasis patients, who typically use biologics as monotherapy, the risk may be overestimated.24
TNF-inhibitors increase the risk for infection, most commonly of the upper respiratory tract, and, rarely, have been associated with opportunistic infections. Numerous cases of TB reactivation and an increased incidence of disseminated cases have been associated with TNF-inhibitors, so screening is recommended.24
The impact of TNF inhibition on congestive heart failure (CHF) is not well understood. Studies have variously shown that TNF-inhibitors have no effect on CHF morbidity or mortality, increase CHF mortality, or improve left ventricular function. TNF-inhibitors should be avoided in patients with severe CHF (New York Heart Association class III or IV). In milder CHF patients with worsening of symptoms, treatment should be discontinued.36
The increased risk of malignancy, especially lymphoma, is a concern, as there have been numerous case reports of lymphoma occurring with TNF-inhibitors. Psoriasis patients in general have an increased risk of lymphoma that confounds data interpretation.31 A number of case reports and a large observational study have shown patients receiving TNF-inhibitors may be at a greater risk for developing melanoma and nonmelanoma skin cancer.32
Ustekinumab, an interleukin 12/23 inhibitor, is a human monoclonal antibody that is absorbed and eliminated slowly, making dosing injections every 12 weeks convenient with efficacy maintained for at least one year.33 Because of its relative novelty, few studies are published regarding long-term safety. A recent head-to-head trial compared the efficacy and safety of ustekinumab with etanercept and found superior efficacy with ustekinumab, with comparable adverse events.37,38 Similar concerns exist with ustekinumab as with TNF-inhibitors, including infection, malignancy, CHF, and TB.33
CASE Tom denied having a personal family history of multiple sclerosis, or any demyelinating disorder. Nor did he have a history of cancer, tuberculosis exposure, CHF, or hepatitis. A purified protein derivative (PPD) was negative, as was his hepatitis panel, and his complete blood count with differential and metabolic panel were within normal limits.
Tom was started on the TNF-inhibitor adalimumab, after undergoing patient education and training and receiving instructions to stop the medication if he developed a major illness or infection. He received a loading dose of 80 mg SC, followed by 40 mg every other week. He tolerated the treatment well and 70% of his cutaneous symptoms cleared after 12 weeks of therapy; his joint pain also was reduced.
Tom is followed regularly in the clinic, with labs every 4 to 6 months. He is maintained on the injections and happy with the results. At each visit, weight loss and decreased beer intake are encouraged, both of which have been shown to reduce psoriasis severity. Although the beta-blockers and ACE inhibitors he takes are known to exacerbate psoriasis, the medications are necessary to treat Tom’s coronary artery disease.
CORRESPONDENCE Elizabeth Uhlenhake, MD, 18100 Oakwood Boulevard, Suite 300, Dearborn, MI 48124; eulenha@neomed.edu
1. Gelfand JM, Weinstein R, Porter SB, et al. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141:1537-1541.
2. Fortune DG, Richards HL, Griffiths CE. Psychologic factors in psoriasis: consequences, mechanisms, and interventions. Dermatol Clin. 2005;23:681-694.
3. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
4. Pettey AA, Balkrishnan R, Rapp SR, et al. Patients with palmoplantar psoriasis have more physical disability and discomfort than patients with other forms of psoriasis: implications for clinical practice. J Am Acad Dermatol. 2003;49:271-275.
5. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
6. Kaufmann R, Bibby AJ, Bissonnette R, et al. A new calcipotriol/betamethasone dipropionate formulation (Daivobet) is an effective once-daily treatment for psoriasis vulgaris. Dermatology. 2002;205:389-393.
7. Koo JY, Martin D. Investigator-masked comparison of tazarotene gel q.d. plus mometasone furoate cream q.d. vs. mometasone furoate cream b.i.d. in the treatment of plaque psoriasis. Int J Dermatol. 2001;40:210-212.
8. Berger TG, Duvic M, Van Voorhees AS, et al. The use of topical calcineurin inhibitors in dermatology: safety concerns. Report of the American Academy of Dermatology Association Task Force. J Am Acad Dermatol. 2006;54:818-823.
9. Cornell RC, Stoughton RB. Correlation of the vasoconstriction assay and clinical activity in psoriasis. Arch Dermatol. 1985;121:63-67.
10. Lui H. Phototherapy of psoriasis: update with practical pearls. J Cutan Med Surg. 2002;6(suppl):17-21.
11. Walters IB, Burack LH, Coven TR, et al. Suberythemogenic narrow-band UVB is markedly more effective than conventional UVB in treatment of psoriasis vulgaris. J Am Acad Dermatol. 1999;40:893-900.
12. Stern RS, Laird N. The carcinogenic risk of treatments for severe psoriasis. Photochemotherapy follow-up study. Cancer. 1994;73:2759-2764.
13. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
14. Trehan M, Taylor CR. High-dose 308-nm excimer laser for the treatment of psoriasis. J Am Acad Dermatol. 2002;46:732-737.
15. Morison WL, Baughman RD, Day RM, et al. Consensus workshop on the toxic effects of long-term PUVA therapy. Arch Dermatol. 1998;134:595-598.
16. Spuls PI, Witkamp L, Bossuyt PM, et al. A systematic review of five systemic treatments for severe psoriasis. Br J Dermatol. 1997;137:943-949.
17. Murase JE, Lee EE, Koo J. Effect of ethnicity on the risk of developing nonmelanoma skin cancer following long-term PUVA therapy. Int J Dermatol. 2005;44:1016-1021.
18. Lowe NJ, Prystowsky JH, Bourget T, et al. Acitretin plus UVB therapy for psoriasis. Comparisons with placebo plus UVB and acitretin alone. J Am Acad Dermatol. 1991;24:591-594.
19. Torras H, Aliaga A, Lopez-Estebaranz JL, et al. A combination therapy of calcipotriol cream and PUVA reduces the UVA dose and improves the response of psoriasis vulgaris. J Dermatolog Treat. 2004;15:98-103.
20. Tanew A, Guggenbichler A, Honigsmann H, et al. Photochemotherapy for severe psoriasis without or in combination with acitretin: a randomized, double-blind comparison study. J Am Acad Dermatol. 1991;25:682-684.
21. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
22. Strober BE, Menon K. Folate supplementation during methotrexate therapy for patients with psoriasis. J Am Acad Dermatol. 2005;53:652-659.
23. Taler SJ, Textor SC, Canzanello VJ, et al. Cyclosporine-induced hypertension: incidence, pathogenesis and management. Drug Saf. 1999;20:437-449.
24. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
25. Heydendael VM, Spuls PI, Opmeer BC, et al. Methotrexate versus cyclosporine in moderate-to-severe chronic plaque psoriasis. N Engl J Med. 2003;349:658-665.
26. Lloyd ME, Carr M, McElhatton P, et al. The effects of methotrexate on pregnancy, fertility and lactation. QJM. 1999;92:551-563.
27. Ellis CN, Fradin MS, Messana JM, et al. Cyclosporine for plaque-type psoriasis. Results of a multidose, double-blind trial. N Engl J Med. 1991;324:277-284.
28. Buccheri L, Katchen BR, Karter AJ, et al. Acitretin therapy is effective for psoriasis associated with human immunodeficiency virus infection. Arch Dermatol. 1997;133:711-715.
29. Lebwohl M, Bagel J, Gelfand JM, et al. From the Medical Board of the National Psoriasis Foundation: monitoring and vaccinations in patients treated with biologics for psoriasis. J Am Acad Dermatol. 2008;58:94-105.
30. Gordon KB, Vaishnaw AK, O’Gorman J, et al. Treatment of psoriasis with alefacept: correlation of clinical improvement with reductions of memory T-cell counts. Arch Dermatol. 2003;139:1563-1570.
31. Brown SL, Greene MH, Gershon SK, et al. Tumor necrosis factor antagonist therapy and lymphoma development: twenty-six cases reported to the Food and Drug Administration. Arthritis Rheum. 2002;46:3151-3158.
32. Fulchiero GJ, Jr, Salvaggio H, Drabick JJ, et al. Eruptive latent metastatic melanomas after initiation of antitumor necrosis factor therapies. J Am Acad Dermatol. 2007;56(suppl):S65-S67.
33. Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371:1665-1674.
34. US Food and Drug Administration. FDA approves new drug to treat psoriasis. Sept. 25, 2009. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm183851.htm. Accessed June 15, 2012.
35. Duchini A, Goss JA, Karpen S, et al. Vaccinations for adult solid-organ transplant recipients: current recommendations and protocols. Clin Microbiol Rev. 2003;16:357-364.
36. Mann DL, McMurray JJ, Packer M, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation. 2004;109:1594-1602.
37. Griffiths CE, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118-128.
38. Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684.
1. Gelfand JM, Weinstein R, Porter SB, et al. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141:1537-1541.
2. Fortune DG, Richards HL, Griffiths CE. Psychologic factors in psoriasis: consequences, mechanisms, and interventions. Dermatol Clin. 2005;23:681-694.
3. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
4. Pettey AA, Balkrishnan R, Rapp SR, et al. Patients with palmoplantar psoriasis have more physical disability and discomfort than patients with other forms of psoriasis: implications for clinical practice. J Am Acad Dermatol. 2003;49:271-275.
5. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
6. Kaufmann R, Bibby AJ, Bissonnette R, et al. A new calcipotriol/betamethasone dipropionate formulation (Daivobet) is an effective once-daily treatment for psoriasis vulgaris. Dermatology. 2002;205:389-393.
7. Koo JY, Martin D. Investigator-masked comparison of tazarotene gel q.d. plus mometasone furoate cream q.d. vs. mometasone furoate cream b.i.d. in the treatment of plaque psoriasis. Int J Dermatol. 2001;40:210-212.
8. Berger TG, Duvic M, Van Voorhees AS, et al. The use of topical calcineurin inhibitors in dermatology: safety concerns. Report of the American Academy of Dermatology Association Task Force. J Am Acad Dermatol. 2006;54:818-823.
9. Cornell RC, Stoughton RB. Correlation of the vasoconstriction assay and clinical activity in psoriasis. Arch Dermatol. 1985;121:63-67.
10. Lui H. Phototherapy of psoriasis: update with practical pearls. J Cutan Med Surg. 2002;6(suppl):17-21.
11. Walters IB, Burack LH, Coven TR, et al. Suberythemogenic narrow-band UVB is markedly more effective than conventional UVB in treatment of psoriasis vulgaris. J Am Acad Dermatol. 1999;40:893-900.
12. Stern RS, Laird N. The carcinogenic risk of treatments for severe psoriasis. Photochemotherapy follow-up study. Cancer. 1994;73:2759-2764.
13. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114-135.
14. Trehan M, Taylor CR. High-dose 308-nm excimer laser for the treatment of psoriasis. J Am Acad Dermatol. 2002;46:732-737.
15. Morison WL, Baughman RD, Day RM, et al. Consensus workshop on the toxic effects of long-term PUVA therapy. Arch Dermatol. 1998;134:595-598.
16. Spuls PI, Witkamp L, Bossuyt PM, et al. A systematic review of five systemic treatments for severe psoriasis. Br J Dermatol. 1997;137:943-949.
17. Murase JE, Lee EE, Koo J. Effect of ethnicity on the risk of developing nonmelanoma skin cancer following long-term PUVA therapy. Int J Dermatol. 2005;44:1016-1021.
18. Lowe NJ, Prystowsky JH, Bourget T, et al. Acitretin plus UVB therapy for psoriasis. Comparisons with placebo plus UVB and acitretin alone. J Am Acad Dermatol. 1991;24:591-594.
19. Torras H, Aliaga A, Lopez-Estebaranz JL, et al. A combination therapy of calcipotriol cream and PUVA reduces the UVA dose and improves the response of psoriasis vulgaris. J Dermatolog Treat. 2004;15:98-103.
20. Tanew A, Guggenbichler A, Honigsmann H, et al. Photochemotherapy for severe psoriasis without or in combination with acitretin: a randomized, double-blind comparison study. J Am Acad Dermatol. 1991;25:682-684.
21. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
22. Strober BE, Menon K. Folate supplementation during methotrexate therapy for patients with psoriasis. J Am Acad Dermatol. 2005;53:652-659.
23. Taler SJ, Textor SC, Canzanello VJ, et al. Cyclosporine-induced hypertension: incidence, pathogenesis and management. Drug Saf. 1999;20:437-449.
24. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
25. Heydendael VM, Spuls PI, Opmeer BC, et al. Methotrexate versus cyclosporine in moderate-to-severe chronic plaque psoriasis. N Engl J Med. 2003;349:658-665.
26. Lloyd ME, Carr M, McElhatton P, et al. The effects of methotrexate on pregnancy, fertility and lactation. QJM. 1999;92:551-563.
27. Ellis CN, Fradin MS, Messana JM, et al. Cyclosporine for plaque-type psoriasis. Results of a multidose, double-blind trial. N Engl J Med. 1991;324:277-284.
28. Buccheri L, Katchen BR, Karter AJ, et al. Acitretin therapy is effective for psoriasis associated with human immunodeficiency virus infection. Arch Dermatol. 1997;133:711-715.
29. Lebwohl M, Bagel J, Gelfand JM, et al. From the Medical Board of the National Psoriasis Foundation: monitoring and vaccinations in patients treated with biologics for psoriasis. J Am Acad Dermatol. 2008;58:94-105.
30. Gordon KB, Vaishnaw AK, O’Gorman J, et al. Treatment of psoriasis with alefacept: correlation of clinical improvement with reductions of memory T-cell counts. Arch Dermatol. 2003;139:1563-1570.
31. Brown SL, Greene MH, Gershon SK, et al. Tumor necrosis factor antagonist therapy and lymphoma development: twenty-six cases reported to the Food and Drug Administration. Arthritis Rheum. 2002;46:3151-3158.
32. Fulchiero GJ, Jr, Salvaggio H, Drabick JJ, et al. Eruptive latent metastatic melanomas after initiation of antitumor necrosis factor therapies. J Am Acad Dermatol. 2007;56(suppl):S65-S67.
33. Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371:1665-1674.
34. US Food and Drug Administration. FDA approves new drug to treat psoriasis. Sept. 25, 2009. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm183851.htm. Accessed June 15, 2012.
35. Duchini A, Goss JA, Karpen S, et al. Vaccinations for adult solid-organ transplant recipients: current recommendations and protocols. Clin Microbiol Rev. 2003;16:357-364.
36. Mann DL, McMurray JJ, Packer M, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation. 2004;109:1594-1602.
37. Griffiths CE, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118-128.
38. Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684.
When bed bugs bite
• Provide symptomatic relief for bed bug bites with antihistamines or corticosteroids. C
• Advise patients experiencing an infestation to consider the CDC’s recommended integrated pest management program (eg, heat treatment, vacuuming, nonchemical pesticides) to increase the likelihood of successful extermination. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Bed bugs, Cimex spp, are a re-emerging public health problem in the United States. First recorded by the ancient Greeks,1 bed bugs have plagued societies for centuries. In the United States, infestations peaked in intensity in the 1920s and 1930s, then were largely eliminated as a significant concern after World War II, thanks to synthetic, residual pesticides.2 By the mid-1990s, bed bugs were so uncommon that specimens could not be obtained for medical education purposes.3
That has all changed.
Although commonly perceived as disproportionately affecting the underprivileged,4 bed bugs are equal-opportunity pests, infesting the most posh hotels, retailers, and theaters.5,6 According to one news report summarizing data from a national pest control firm, US cities with the highest infestation rates are, in descending order: Cincinnati, Columbus, Chicago, Denver, Detroit, Washington DC, New York, Philadelphia, Dayton, and Baltimore.7 Although bed bugs are not known to transmit infectious diseases, they are responsible for significant dermatitis, allergic reactions, and psychological distress.
Bed bug biology and behavior
The bed bug life cycle has 7 stages. All but the egg stage require blood meals before the arthropod can molt to the next stage. Bed bugs are attracted to their hosts by body heat and exhaled carbon dioxide, and they feed only through the skin. This makes baiting and trapping challenging, although it’s a common extermination strategy for other domestic pests. Also, unlike cockroaches, flies, or other pests, bed bug infestations are not associated with hygienic deficiencies. Improved housekeeping does not significantly affect their populations; bed bugs feed on household inhabitants, not their spilled or improperly stored food. However, clutter does increase their chances of finding refuge.
Interestingly, researchers recently discovered that bed bugs are themselves hosts to the endosymbiotic bacterium, Wolbachia.8,9 This genus is found in many invertebrates and appears to be essential for normal bed bug fertility and reproduction. Targeting the bacteria may inhibit the ability of bed bugs to breed—something we’ll discuss a bit later.
Clinical assessment
Patients with bed bug bites complain about intensely pruritic lesions. These are typically erythematous and indurated and may be hemorrhagic. The pattern of bites is often linear, and 3 bites in a row are common, sometimes referred to as “breakfast, lunch, and dinner.” Patients typically have no recollection of being bitten, as bed bugs feed on sleeping hosts, and their bite is usually painless.
Clues to bed bugs as the source. Scabies mites also cause linear pruritic lesions, but bed bug lesions differ in appearance and distribution. Scabies lesions are subtle, appearing as burrows and excoriations, in contrast to the more prominent erythematous papule seen with bed bugs and other arthropod bites. Scabies tend to occur in skin folds, finger webbing, genitals, and areas where clothing is tight, such as beltlines. In contrast, bed bugs tend to attack easily accessible, exposed areas. Areas covered with loose clothing are less affected, and areas covered by tight clothing are essentially spared. Multiple members of the household are often affected.
Flea bite? Bed bug bites may be virtually indistinguishable from those of other arthropods such as fleas, spiders, or mosquitoes. While the linear 3-bite pattern may suggest bed bug exposure, it is not pathognomonic. Capturing the arthropod or finding evidence of infestation (discussed in a bit) is needed to confirm a bed bug as the source of the bite.
The etiology of pruritic papules is broad. Besides arthropod bites, include conditions such as papular eczema, papular pruritic eruption, and eosinophilic folliculitis in the differential diagnosis.
Potential for complications
As with any break in the skin, secondary infection is a risk, although it is rarely a complication of the bite. If infection occurs, it is more likely due to scratching. Bed bug bites are allergenic, and they have also been implicated in asthma exacerbations and even anaphylaxis.10,11 In severe infestations, anemia from the extensive blood-meals can occur.12
Experimental studies have found that >45 human pathogens—ranging from viruses to methicillin-resistant Staphylococcus aureus to helminths—can survive ingestion by bed bugs, but none have shown pathogens to be transmitted to humans by bed bugs.11 Fortunately, bed bugs do not appear to be competent as vectors, although prospective studies are ongoing.13 In addition to allergic manifestations, bed bug bites have been associated with significant, even incapacitating, psychiatric problems such as anxiety, obsession, and depression to the point of suicide.14
Treating symptoms and cause
Management of bed bugs consists of symptomatic treatment of the bites and elimination of the infestation—treating both patients and their environments.15
Treating patients
Treating bed bug bites mainly involves providing symptomatic relief with antipruritic agents (antihistamines, topical or oral corticosteroids, over-the-counter topical anesthetics).16 When, rarely, a bite becomes infected, antibiotics may be indicated. Address psychological distress associated with an infestation. Counseling with cognitive behavioral therapy is effective most of the time, although some cases may warrant short-term psychopharmacotherapy.
Symptomatic relief will be short-lived, however, without remediation of the underlying infestation. If the bugs remain, the biting will continue.
Treating the environment
Every object and location in which bed bugs may have taken refuge must be treated. The first step in eradicating an infestation is to find it. In light infestations, evidence may be limited. However, they are dirty bugs. Significant amounts of litter, including molted exoskeletons, dark feces, and eggs, are found wherever there is an infestation. These signs of infestation may be found on mattresses or box springs, or in the bottom of bureau drawers and the corners of rooms. Anywhere just out of reach of the vacuum cleaner can harbor their detritus. Some success has been reported using bed bug detectors/monitors.17 Bed bug-sniffing dogs have been trained and employed in both identifying infestations and monitoring the efficacy of eradication interventions.
A number of extermination methods have been used. The most commonly used chemicals are permethrins, the same agents that have proven effective in antimalarial bed net programs. This agent is applied to the environment, not to the patient. Generally, at least 2 applications are required. Although as recently as 1990 no bed bug resistance to permethrin had been reported,18 there is now widespread resistance.19 Efforts at developing new agents are progressing.
Besides resistance, toxicity to humans is a concern. The Centers for Disease Control and Prevention (CDC) has reported both morbidity and mortality from chemical pesticides used in bed bug extermination efforts.20,21
Physical methods have also been applied.
Thermal treatment (heating or steaming to >48°C [120°F] for one hour or freezing to -20°C [-4°F] for one hour) has proven effective.22 Books, clothing, and other small items may be placed in an oven or freezer (as long as specified temperatures are met); steamers are useful for treating furniture and baseboards. If an oven is used, diligent attention must be paid to avoid too high a temperature, which could create a fire hazard. Let patients know that, even at 120°F, some book bindings and slipcovers could be damaged.
Desiccant dusts such as silica gel and diatomaceous earth, applied along the baseboards and the back of bookshelves, have also demonstrated efficacy.23 As with chemical pesticides, it is important to follow directions when using desiccant dusts to minimize potential health hazards.
The CDC recommends a comprehensive, integrated pest management program to control bed bugs. This program includes a number of methods, such as removing clutter and sealing cracks and crevices where bed bugs take refuge, applying heat treatment, vacuuming, using nonchemical pesticides, and cautiously applying effective chemical pesticides.17 An approach such as this is labor- and time-intensive, and can be costly.
Given the inadequacies of current strategies in controlling infestations, new approaches are needed. One such approach may be xenointoxication, in which patients take an oral arthropodicidal agent, making the blood meal toxic to the parasite and decimating the population. Although there are no literature reports of its application to bed bugs, the technique, using ivermectin, has been successfully applied to other ectoparasites, including scabies,24,25 lice,25,26 and the medically important arthropod vectors Triatoma27 and Anopheles.28 The TABLE shows dosing recommendations for 3 of these indications.
In vitro studies demonstrate that Cimex is susceptible to this same class of agents,29 so there is reason for optimism. Future studies will reveal the viability of this approach. Another potential approach to bed bug control is targeting the Wolbachia endosymbionts. Elimination of these bacteria has been associated with a significant decrease in parasite reproduction9; this strategy has also been efficacious in treating human filarial infections.30
TABLE
Xenointoxication with ivermectin has proven effective against several ectoparasite infestations24-28
| Ectoparasite | Condition | Ivermectin dosing |
|---|---|---|
| Sarcoptes scabiei | Scabies | 0.2 mg/kg, single dose |
| Pediculus capitis | Head lice | 0.2 mg/kg every 10 days x 2 doses |
| Pediculus corpora | Body lice | 0.2 mg/kg every 7 days x 3 doses |
In vitro research has shown that bed bugs are also susceptible to this class of antiparasitic drugs.
Preventing infestation
Bed bugs depend largely on humans for their dissemination. They take refuge in or near their host’s bed during the day, and when the bed or other object in which they are hiding is moved, they are transplanted to a new location. They also migrate directly to adjacent apartments, hotel rooms, etc, along plumbing and wiring or through cracks.16 Bed bugs are effective at hiding, and can survive for up to a year without feeding.31 This contributes to the frequent failure of elimination efforts and the presence of bed bugs in hotels, furnished apartments, theaters, shopping centers, airplanes, newly purchased houses, and other places.
Avoiding bites while in an infested facility is difficult, if not impossible. But people can take steps to decrease the likelihood of bringing them home. Although there are no strong evidence-based guidelines on preventing infestation, pest control experts make a number of recommendations,32 which you can pass on to your patients.
Protect luggage when traveling. When staying in hotels, for instance, patients should keep suitcases tightly closed when not in use. Protection is further enhanced by placing suitcases in a sealed plastic bag; “contractor” trash bags available at hardware stores are large and durable. Keeping suitcases in the bathroom rather than the sleeping quarters also decreases the possibility of stowaways, as bed bugs typically shelter within a few feet of their host’s sleeping place.
Immediate laundering of clothes upon returning home from a trip, and storing suitcases outside the living quarters can decrease risk, too.33 There are commercially available suitcase heaters that raise the temperature of the suitcase and its contents to insecticidal levels, but they are fairly cost-prohibitive.
Screen items brought into the home. Used items, especially furniture, may harbor bed bugs. Fumigating used furniture was once common; it is still a good idea before second-hand items are brought into the house. Cardboard boxes in which used items are commonly stored or transported can shelter bed bugs, too.33
Deprive bed bugs of hiding places. Decluttering one’s sleeping quarters decreases the number of places bed bugs can hide. This tactic diminishes the likelihood of an infestation becoming firmly established before being discovered. Intervention early in the course of infestation, when it is limited to a single room, increases the likelihood of successful elimination.33
Mattress and box-spring encasements can prevent bed bug infestations by blocking movement of the bugs into and out of their shelters. If encasements are placed during an infestation, it is important to keep them in place for an extended period, given that bed bugs can survive for up to a year without feeding. Also effective is caulking and sealing molding, joints, and cracks wider than the thickness of a credit card in the room and in furniture.33
Vacuuming is part of the CDC’s recommendation for household pest control. But vacuum cleaners can also transfer bed bugs from infested to uninfested rooms. During an infestation, it’s important to empty vacuum bags immediately. And sharing vacuum cleaners between dwellings is best avoided.
A need for better solutions
Although bed bugs are not competent as vectors for the transmission of infectious diseases, they are responsible for significant dermatitis, allergic reactions, and psychological distress. Treatment of symptoms is effective in the short-term, but current methods of eliminating infestation are cumbersome, toxic, and are seldom completely successful. New strategies are desperately needed. The CDC Web page (http://www.cdc.gov/nceh/ehs/topics/bedbugs.htm) is regularly updated, and is a good source of information as new approaches are developed.
CORRESPONDENCE Mark K. Huntington, MD, PhD, Center for Family Medicine, 1115 East Twentieth Street, Sioux Falls, SD 57105; mark.huntington@usd.edu
1. Usinger RI. Monograph of the Cimicidae (Hemiptera-Heteroptera). Vol VII. College Park, Md: Entomological Society of America; 1966.
2. Berg R. Bed bugs: the pesticide dilemma. J Environ Health. 2010;72:32-35.
3. Snetsinger R. Bed bugs & other bugs. In: Hedges S, ed. Mallis’ Handbook of Pest Control. 8th ed. Cleveland, Ohio: GIE Media; 1997:392–424.
4. Eddy C, Jones SC. Bed bugs, public health, and social justice: part 1, a call to action. J Environ Health. 2011;73:8-14.
5. Hurst S, Humphreys M. Bedbugs: not back by popular demand. Dimens Crit Care Nurs. 2011;30:94-96.
6. Anderson A. The decade of bedbugs and fear. Environ Health Insights. 2011;5:53-54.
7. America’s 10 most infested cities. The Daily Beast; August 24, 2010. Available at: http://www.thedailybeast.com/articles/2010/08/24/bedbug-outbreak-which-cities-are-most-infested.html. Accessed August 28, 2011.
8. Hosokawa T, Koga R, Kikuchi Y, et al. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc Natl Acad Sci U S A. 2010;107:769-774.
9. Sakamoto JM, Rasgon JL. Geographic distribution of Wolbachia infections in Cimex lectularius (Heteroptera: Cimicidae). J Med Entomol. 2006;43:696-700.
10. Abou Gamra EM, el Shayed FA, Morsy TA, et al. The relation between Cimex lectularius antigen and bronchial asthma in Egypt. J Egypt Soc Parasitol. 1991;21:735-746.
11. Delaunay P, Blanc V, Del Giudice P, et al. Bedbugs and infectious diseases. Clin Infect Dis. 2011;52:200-210.
12. Pritchard MJ, Hwang SW. Cases: severe anemia from bedbugs. CMAJ. 2009;181:287-288.
13. Delaunay P. Cimex lectularius or bed bug: vector of infectious agents and pathogenic role. Available at: http://www.clinicaltrials.gov. Identifier: NCT01089465. Accessed November 29, 2011.
14. Rieder E, Hamalian G, Ying P. Psychiatric implications of bedbugs. Presented at: 164th Annual Meeting of the American Psychiatric Association; May 14-18, 2011; Honolulu, Hawaii. Abstract NR01-51.
15. Roos TC, Alam M, Roos S, et al. Pharmacotherapy of ectoparasitic infections. Drugs. 2001;61:1067-1088.
16. Goddard J, deShazo R. Bed bugs (Cimex lectularius) and clinical consequences of their bites. JAMA. 2009;301:1358-1366.
17. Centers for Disease Control and Prevention and Environmental Protection Agency. Joint statement on bed bug control in the United States from the U.S. Centers for Disease Control and Prevention (CDC) and the U.S. Environmental Protection Agency (EPA). Atlanta, Ga: US Department of Health and Human Services; 2010. Available at: http://www.cdc.gov/nceh/ehs/Publications/Bed_Bugs_CDC-EPA_Statement.htm. Accessed June 15, 2012.
18. Axtell RC, Arends JJ. Ecology and management of arthropod pests of poultry. Annu Rev Entomol. 1990;35:101-126.
19. Moore DJ, Miller DM. Field evaluations of insecticide treatment regimens for control of the common bed bug, Cimex lectularius (L.). Pest Manag Sci. 2009;65:332-338.
20. CDC. Acute illnesses associated with insecticides used to control bed bugs—seven states, 2003–2010. MMWR Morb Mortal Wkly Rep. 2011;60:1269-1274.
21. Tawatsin A, Thavara U, Chompoosri J, et al. Insecticide resistance in bedbugs in Thailand and laboratory evaluation of insecticides for the control of Cimex hemipterus and Cimex lectularius (Hemiptera: Cimicidae). J Med Entomol. 2011;48:1023-1030.
22. Benoit JB, Lopez-Martinez G, Teets NM, et al. Responses of the bed bug, Cimex lectularius, to temperature extremes and dehydration: levels of tolerance, rapid cold hardening and expression of heat shock proteins. Med Vet Entomol. 2009;23:418-425.
23. Benoit JB, Phillips SA, Croxall TJ, et al. Addition of alarm pheromone components improves the effectiveness of desiccant dusts against Cimex lectularius. J Med Entomol. 2009;46:572-579.
24. Heukelbach J, Feldmeier H. Scabies. Lancet. 2006;367:1767-1774.
25. Fox LM. Ivermectin: uses and impact 20 years on. Curr Opin Infect Dis. 2006;19:588-593.
26. Foucault C, Ranque S, Badiaga S, et al. Oral ivermectin in the treatment of body lice. J Infect Dis. 2006;193:474-476.
27. Dias JC, Schofield CJ, Machado EM, et al. Ticks, ivermectin, and experimental Chagas disease. Mem Inst Oswaldo Cruz. 2005;100:829-832.
28. Kobylinski KC, Sylla M, Chapman PL, et al. Ivermectin mass drug administration to humans disrupts malaria parasite transmission in Senegalese villages. Am J Trop Med Hyg. 2011;85:3-5.
29. Ostlind DA, Cifelli S, Conroy JA, etal. A novel Cimex lectularius– rodent assay for the detection of systemic ectoparasiticide activity. Southwest Entomol. 2001;26:181-186.
30. Tamarozzi F, Halliday A, Gentil K, et al. Onchocerciasis: the role of Wolbachia bacterial endosymbionts in parasite biology, disease pathogenesis, and treatment. Clin Microbiol Rev. 2011;24:459-468.
31. Kells SA, Hahn J. Prevention and control of bed bugs in residences. Available at: http://www.extension.umn.edu/distribution/housingandclothing/dk1022.html. Accessed November 29, 2011.
32. Got bedbugs? Your hotel might! Available at: http://www.smartertravel.com/travel-advice/avoiding-bedbugs-every-traveler-nightmare.html?id=4726511. Published April 25, 2010. Accessed November 29, 2011.
33. US Environmental Protection Agency. Bed bug information. Available at: http://www.epa.gov/bedbugs/index.html. Accessed November 29, 2011.
• Provide symptomatic relief for bed bug bites with antihistamines or corticosteroids. C
• Advise patients experiencing an infestation to consider the CDC’s recommended integrated pest management program (eg, heat treatment, vacuuming, nonchemical pesticides) to increase the likelihood of successful extermination. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Bed bugs, Cimex spp, are a re-emerging public health problem in the United States. First recorded by the ancient Greeks,1 bed bugs have plagued societies for centuries. In the United States, infestations peaked in intensity in the 1920s and 1930s, then were largely eliminated as a significant concern after World War II, thanks to synthetic, residual pesticides.2 By the mid-1990s, bed bugs were so uncommon that specimens could not be obtained for medical education purposes.3
That has all changed.
Although commonly perceived as disproportionately affecting the underprivileged,4 bed bugs are equal-opportunity pests, infesting the most posh hotels, retailers, and theaters.5,6 According to one news report summarizing data from a national pest control firm, US cities with the highest infestation rates are, in descending order: Cincinnati, Columbus, Chicago, Denver, Detroit, Washington DC, New York, Philadelphia, Dayton, and Baltimore.7 Although bed bugs are not known to transmit infectious diseases, they are responsible for significant dermatitis, allergic reactions, and psychological distress.
Bed bug biology and behavior
The bed bug life cycle has 7 stages. All but the egg stage require blood meals before the arthropod can molt to the next stage. Bed bugs are attracted to their hosts by body heat and exhaled carbon dioxide, and they feed only through the skin. This makes baiting and trapping challenging, although it’s a common extermination strategy for other domestic pests. Also, unlike cockroaches, flies, or other pests, bed bug infestations are not associated with hygienic deficiencies. Improved housekeeping does not significantly affect their populations; bed bugs feed on household inhabitants, not their spilled or improperly stored food. However, clutter does increase their chances of finding refuge.
Interestingly, researchers recently discovered that bed bugs are themselves hosts to the endosymbiotic bacterium, Wolbachia.8,9 This genus is found in many invertebrates and appears to be essential for normal bed bug fertility and reproduction. Targeting the bacteria may inhibit the ability of bed bugs to breed—something we’ll discuss a bit later.
Clinical assessment
Patients with bed bug bites complain about intensely pruritic lesions. These are typically erythematous and indurated and may be hemorrhagic. The pattern of bites is often linear, and 3 bites in a row are common, sometimes referred to as “breakfast, lunch, and dinner.” Patients typically have no recollection of being bitten, as bed bugs feed on sleeping hosts, and their bite is usually painless.
Clues to bed bugs as the source. Scabies mites also cause linear pruritic lesions, but bed bug lesions differ in appearance and distribution. Scabies lesions are subtle, appearing as burrows and excoriations, in contrast to the more prominent erythematous papule seen with bed bugs and other arthropod bites. Scabies tend to occur in skin folds, finger webbing, genitals, and areas where clothing is tight, such as beltlines. In contrast, bed bugs tend to attack easily accessible, exposed areas. Areas covered with loose clothing are less affected, and areas covered by tight clothing are essentially spared. Multiple members of the household are often affected.
Flea bite? Bed bug bites may be virtually indistinguishable from those of other arthropods such as fleas, spiders, or mosquitoes. While the linear 3-bite pattern may suggest bed bug exposure, it is not pathognomonic. Capturing the arthropod or finding evidence of infestation (discussed in a bit) is needed to confirm a bed bug as the source of the bite.
The etiology of pruritic papules is broad. Besides arthropod bites, include conditions such as papular eczema, papular pruritic eruption, and eosinophilic folliculitis in the differential diagnosis.
Potential for complications
As with any break in the skin, secondary infection is a risk, although it is rarely a complication of the bite. If infection occurs, it is more likely due to scratching. Bed bug bites are allergenic, and they have also been implicated in asthma exacerbations and even anaphylaxis.10,11 In severe infestations, anemia from the extensive blood-meals can occur.12
Experimental studies have found that >45 human pathogens—ranging from viruses to methicillin-resistant Staphylococcus aureus to helminths—can survive ingestion by bed bugs, but none have shown pathogens to be transmitted to humans by bed bugs.11 Fortunately, bed bugs do not appear to be competent as vectors, although prospective studies are ongoing.13 In addition to allergic manifestations, bed bug bites have been associated with significant, even incapacitating, psychiatric problems such as anxiety, obsession, and depression to the point of suicide.14
Treating symptoms and cause
Management of bed bugs consists of symptomatic treatment of the bites and elimination of the infestation—treating both patients and their environments.15
Treating patients
Treating bed bug bites mainly involves providing symptomatic relief with antipruritic agents (antihistamines, topical or oral corticosteroids, over-the-counter topical anesthetics).16 When, rarely, a bite becomes infected, antibiotics may be indicated. Address psychological distress associated with an infestation. Counseling with cognitive behavioral therapy is effective most of the time, although some cases may warrant short-term psychopharmacotherapy.
Symptomatic relief will be short-lived, however, without remediation of the underlying infestation. If the bugs remain, the biting will continue.
Treating the environment
Every object and location in which bed bugs may have taken refuge must be treated. The first step in eradicating an infestation is to find it. In light infestations, evidence may be limited. However, they are dirty bugs. Significant amounts of litter, including molted exoskeletons, dark feces, and eggs, are found wherever there is an infestation. These signs of infestation may be found on mattresses or box springs, or in the bottom of bureau drawers and the corners of rooms. Anywhere just out of reach of the vacuum cleaner can harbor their detritus. Some success has been reported using bed bug detectors/monitors.17 Bed bug-sniffing dogs have been trained and employed in both identifying infestations and monitoring the efficacy of eradication interventions.
A number of extermination methods have been used. The most commonly used chemicals are permethrins, the same agents that have proven effective in antimalarial bed net programs. This agent is applied to the environment, not to the patient. Generally, at least 2 applications are required. Although as recently as 1990 no bed bug resistance to permethrin had been reported,18 there is now widespread resistance.19 Efforts at developing new agents are progressing.
Besides resistance, toxicity to humans is a concern. The Centers for Disease Control and Prevention (CDC) has reported both morbidity and mortality from chemical pesticides used in bed bug extermination efforts.20,21
Physical methods have also been applied.
Thermal treatment (heating or steaming to >48°C [120°F] for one hour or freezing to -20°C [-4°F] for one hour) has proven effective.22 Books, clothing, and other small items may be placed in an oven or freezer (as long as specified temperatures are met); steamers are useful for treating furniture and baseboards. If an oven is used, diligent attention must be paid to avoid too high a temperature, which could create a fire hazard. Let patients know that, even at 120°F, some book bindings and slipcovers could be damaged.
Desiccant dusts such as silica gel and diatomaceous earth, applied along the baseboards and the back of bookshelves, have also demonstrated efficacy.23 As with chemical pesticides, it is important to follow directions when using desiccant dusts to minimize potential health hazards.
The CDC recommends a comprehensive, integrated pest management program to control bed bugs. This program includes a number of methods, such as removing clutter and sealing cracks and crevices where bed bugs take refuge, applying heat treatment, vacuuming, using nonchemical pesticides, and cautiously applying effective chemical pesticides.17 An approach such as this is labor- and time-intensive, and can be costly.
Given the inadequacies of current strategies in controlling infestations, new approaches are needed. One such approach may be xenointoxication, in which patients take an oral arthropodicidal agent, making the blood meal toxic to the parasite and decimating the population. Although there are no literature reports of its application to bed bugs, the technique, using ivermectin, has been successfully applied to other ectoparasites, including scabies,24,25 lice,25,26 and the medically important arthropod vectors Triatoma27 and Anopheles.28 The TABLE shows dosing recommendations for 3 of these indications.
In vitro studies demonstrate that Cimex is susceptible to this same class of agents,29 so there is reason for optimism. Future studies will reveal the viability of this approach. Another potential approach to bed bug control is targeting the Wolbachia endosymbionts. Elimination of these bacteria has been associated with a significant decrease in parasite reproduction9; this strategy has also been efficacious in treating human filarial infections.30
TABLE
Xenointoxication with ivermectin has proven effective against several ectoparasite infestations24-28
| Ectoparasite | Condition | Ivermectin dosing |
|---|---|---|
| Sarcoptes scabiei | Scabies | 0.2 mg/kg, single dose |
| Pediculus capitis | Head lice | 0.2 mg/kg every 10 days x 2 doses |
| Pediculus corpora | Body lice | 0.2 mg/kg every 7 days x 3 doses |
In vitro research has shown that bed bugs are also susceptible to this class of antiparasitic drugs.
Preventing infestation
Bed bugs depend largely on humans for their dissemination. They take refuge in or near their host’s bed during the day, and when the bed or other object in which they are hiding is moved, they are transplanted to a new location. They also migrate directly to adjacent apartments, hotel rooms, etc, along plumbing and wiring or through cracks.16 Bed bugs are effective at hiding, and can survive for up to a year without feeding.31 This contributes to the frequent failure of elimination efforts and the presence of bed bugs in hotels, furnished apartments, theaters, shopping centers, airplanes, newly purchased houses, and other places.
Avoiding bites while in an infested facility is difficult, if not impossible. But people can take steps to decrease the likelihood of bringing them home. Although there are no strong evidence-based guidelines on preventing infestation, pest control experts make a number of recommendations,32 which you can pass on to your patients.
Protect luggage when traveling. When staying in hotels, for instance, patients should keep suitcases tightly closed when not in use. Protection is further enhanced by placing suitcases in a sealed plastic bag; “contractor” trash bags available at hardware stores are large and durable. Keeping suitcases in the bathroom rather than the sleeping quarters also decreases the possibility of stowaways, as bed bugs typically shelter within a few feet of their host’s sleeping place.
Immediate laundering of clothes upon returning home from a trip, and storing suitcases outside the living quarters can decrease risk, too.33 There are commercially available suitcase heaters that raise the temperature of the suitcase and its contents to insecticidal levels, but they are fairly cost-prohibitive.
Screen items brought into the home. Used items, especially furniture, may harbor bed bugs. Fumigating used furniture was once common; it is still a good idea before second-hand items are brought into the house. Cardboard boxes in which used items are commonly stored or transported can shelter bed bugs, too.33
Deprive bed bugs of hiding places. Decluttering one’s sleeping quarters decreases the number of places bed bugs can hide. This tactic diminishes the likelihood of an infestation becoming firmly established before being discovered. Intervention early in the course of infestation, when it is limited to a single room, increases the likelihood of successful elimination.33
Mattress and box-spring encasements can prevent bed bug infestations by blocking movement of the bugs into and out of their shelters. If encasements are placed during an infestation, it is important to keep them in place for an extended period, given that bed bugs can survive for up to a year without feeding. Also effective is caulking and sealing molding, joints, and cracks wider than the thickness of a credit card in the room and in furniture.33
Vacuuming is part of the CDC’s recommendation for household pest control. But vacuum cleaners can also transfer bed bugs from infested to uninfested rooms. During an infestation, it’s important to empty vacuum bags immediately. And sharing vacuum cleaners between dwellings is best avoided.
A need for better solutions
Although bed bugs are not competent as vectors for the transmission of infectious diseases, they are responsible for significant dermatitis, allergic reactions, and psychological distress. Treatment of symptoms is effective in the short-term, but current methods of eliminating infestation are cumbersome, toxic, and are seldom completely successful. New strategies are desperately needed. The CDC Web page (http://www.cdc.gov/nceh/ehs/topics/bedbugs.htm) is regularly updated, and is a good source of information as new approaches are developed.
CORRESPONDENCE Mark K. Huntington, MD, PhD, Center for Family Medicine, 1115 East Twentieth Street, Sioux Falls, SD 57105; mark.huntington@usd.edu
• Provide symptomatic relief for bed bug bites with antihistamines or corticosteroids. C
• Advise patients experiencing an infestation to consider the CDC’s recommended integrated pest management program (eg, heat treatment, vacuuming, nonchemical pesticides) to increase the likelihood of successful extermination. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Bed bugs, Cimex spp, are a re-emerging public health problem in the United States. First recorded by the ancient Greeks,1 bed bugs have plagued societies for centuries. In the United States, infestations peaked in intensity in the 1920s and 1930s, then were largely eliminated as a significant concern after World War II, thanks to synthetic, residual pesticides.2 By the mid-1990s, bed bugs were so uncommon that specimens could not be obtained for medical education purposes.3
That has all changed.
Although commonly perceived as disproportionately affecting the underprivileged,4 bed bugs are equal-opportunity pests, infesting the most posh hotels, retailers, and theaters.5,6 According to one news report summarizing data from a national pest control firm, US cities with the highest infestation rates are, in descending order: Cincinnati, Columbus, Chicago, Denver, Detroit, Washington DC, New York, Philadelphia, Dayton, and Baltimore.7 Although bed bugs are not known to transmit infectious diseases, they are responsible for significant dermatitis, allergic reactions, and psychological distress.
Bed bug biology and behavior
The bed bug life cycle has 7 stages. All but the egg stage require blood meals before the arthropod can molt to the next stage. Bed bugs are attracted to their hosts by body heat and exhaled carbon dioxide, and they feed only through the skin. This makes baiting and trapping challenging, although it’s a common extermination strategy for other domestic pests. Also, unlike cockroaches, flies, or other pests, bed bug infestations are not associated with hygienic deficiencies. Improved housekeeping does not significantly affect their populations; bed bugs feed on household inhabitants, not their spilled or improperly stored food. However, clutter does increase their chances of finding refuge.
Interestingly, researchers recently discovered that bed bugs are themselves hosts to the endosymbiotic bacterium, Wolbachia.8,9 This genus is found in many invertebrates and appears to be essential for normal bed bug fertility and reproduction. Targeting the bacteria may inhibit the ability of bed bugs to breed—something we’ll discuss a bit later.
Clinical assessment
Patients with bed bug bites complain about intensely pruritic lesions. These are typically erythematous and indurated and may be hemorrhagic. The pattern of bites is often linear, and 3 bites in a row are common, sometimes referred to as “breakfast, lunch, and dinner.” Patients typically have no recollection of being bitten, as bed bugs feed on sleeping hosts, and their bite is usually painless.
Clues to bed bugs as the source. Scabies mites also cause linear pruritic lesions, but bed bug lesions differ in appearance and distribution. Scabies lesions are subtle, appearing as burrows and excoriations, in contrast to the more prominent erythematous papule seen with bed bugs and other arthropod bites. Scabies tend to occur in skin folds, finger webbing, genitals, and areas where clothing is tight, such as beltlines. In contrast, bed bugs tend to attack easily accessible, exposed areas. Areas covered with loose clothing are less affected, and areas covered by tight clothing are essentially spared. Multiple members of the household are often affected.
Flea bite? Bed bug bites may be virtually indistinguishable from those of other arthropods such as fleas, spiders, or mosquitoes. While the linear 3-bite pattern may suggest bed bug exposure, it is not pathognomonic. Capturing the arthropod or finding evidence of infestation (discussed in a bit) is needed to confirm a bed bug as the source of the bite.
The etiology of pruritic papules is broad. Besides arthropod bites, include conditions such as papular eczema, papular pruritic eruption, and eosinophilic folliculitis in the differential diagnosis.
Potential for complications
As with any break in the skin, secondary infection is a risk, although it is rarely a complication of the bite. If infection occurs, it is more likely due to scratching. Bed bug bites are allergenic, and they have also been implicated in asthma exacerbations and even anaphylaxis.10,11 In severe infestations, anemia from the extensive blood-meals can occur.12
Experimental studies have found that >45 human pathogens—ranging from viruses to methicillin-resistant Staphylococcus aureus to helminths—can survive ingestion by bed bugs, but none have shown pathogens to be transmitted to humans by bed bugs.11 Fortunately, bed bugs do not appear to be competent as vectors, although prospective studies are ongoing.13 In addition to allergic manifestations, bed bug bites have been associated with significant, even incapacitating, psychiatric problems such as anxiety, obsession, and depression to the point of suicide.14
Treating symptoms and cause
Management of bed bugs consists of symptomatic treatment of the bites and elimination of the infestation—treating both patients and their environments.15
Treating patients
Treating bed bug bites mainly involves providing symptomatic relief with antipruritic agents (antihistamines, topical or oral corticosteroids, over-the-counter topical anesthetics).16 When, rarely, a bite becomes infected, antibiotics may be indicated. Address psychological distress associated with an infestation. Counseling with cognitive behavioral therapy is effective most of the time, although some cases may warrant short-term psychopharmacotherapy.
Symptomatic relief will be short-lived, however, without remediation of the underlying infestation. If the bugs remain, the biting will continue.
Treating the environment
Every object and location in which bed bugs may have taken refuge must be treated. The first step in eradicating an infestation is to find it. In light infestations, evidence may be limited. However, they are dirty bugs. Significant amounts of litter, including molted exoskeletons, dark feces, and eggs, are found wherever there is an infestation. These signs of infestation may be found on mattresses or box springs, or in the bottom of bureau drawers and the corners of rooms. Anywhere just out of reach of the vacuum cleaner can harbor their detritus. Some success has been reported using bed bug detectors/monitors.17 Bed bug-sniffing dogs have been trained and employed in both identifying infestations and monitoring the efficacy of eradication interventions.
A number of extermination methods have been used. The most commonly used chemicals are permethrins, the same agents that have proven effective in antimalarial bed net programs. This agent is applied to the environment, not to the patient. Generally, at least 2 applications are required. Although as recently as 1990 no bed bug resistance to permethrin had been reported,18 there is now widespread resistance.19 Efforts at developing new agents are progressing.
Besides resistance, toxicity to humans is a concern. The Centers for Disease Control and Prevention (CDC) has reported both morbidity and mortality from chemical pesticides used in bed bug extermination efforts.20,21
Physical methods have also been applied.
Thermal treatment (heating or steaming to >48°C [120°F] for one hour or freezing to -20°C [-4°F] for one hour) has proven effective.22 Books, clothing, and other small items may be placed in an oven or freezer (as long as specified temperatures are met); steamers are useful for treating furniture and baseboards. If an oven is used, diligent attention must be paid to avoid too high a temperature, which could create a fire hazard. Let patients know that, even at 120°F, some book bindings and slipcovers could be damaged.
Desiccant dusts such as silica gel and diatomaceous earth, applied along the baseboards and the back of bookshelves, have also demonstrated efficacy.23 As with chemical pesticides, it is important to follow directions when using desiccant dusts to minimize potential health hazards.
The CDC recommends a comprehensive, integrated pest management program to control bed bugs. This program includes a number of methods, such as removing clutter and sealing cracks and crevices where bed bugs take refuge, applying heat treatment, vacuuming, using nonchemical pesticides, and cautiously applying effective chemical pesticides.17 An approach such as this is labor- and time-intensive, and can be costly.
Given the inadequacies of current strategies in controlling infestations, new approaches are needed. One such approach may be xenointoxication, in which patients take an oral arthropodicidal agent, making the blood meal toxic to the parasite and decimating the population. Although there are no literature reports of its application to bed bugs, the technique, using ivermectin, has been successfully applied to other ectoparasites, including scabies,24,25 lice,25,26 and the medically important arthropod vectors Triatoma27 and Anopheles.28 The TABLE shows dosing recommendations for 3 of these indications.
In vitro studies demonstrate that Cimex is susceptible to this same class of agents,29 so there is reason for optimism. Future studies will reveal the viability of this approach. Another potential approach to bed bug control is targeting the Wolbachia endosymbionts. Elimination of these bacteria has been associated with a significant decrease in parasite reproduction9; this strategy has also been efficacious in treating human filarial infections.30
TABLE
Xenointoxication with ivermectin has proven effective against several ectoparasite infestations24-28
| Ectoparasite | Condition | Ivermectin dosing |
|---|---|---|
| Sarcoptes scabiei | Scabies | 0.2 mg/kg, single dose |
| Pediculus capitis | Head lice | 0.2 mg/kg every 10 days x 2 doses |
| Pediculus corpora | Body lice | 0.2 mg/kg every 7 days x 3 doses |
In vitro research has shown that bed bugs are also susceptible to this class of antiparasitic drugs.
Preventing infestation
Bed bugs depend largely on humans for their dissemination. They take refuge in or near their host’s bed during the day, and when the bed or other object in which they are hiding is moved, they are transplanted to a new location. They also migrate directly to adjacent apartments, hotel rooms, etc, along plumbing and wiring or through cracks.16 Bed bugs are effective at hiding, and can survive for up to a year without feeding.31 This contributes to the frequent failure of elimination efforts and the presence of bed bugs in hotels, furnished apartments, theaters, shopping centers, airplanes, newly purchased houses, and other places.
Avoiding bites while in an infested facility is difficult, if not impossible. But people can take steps to decrease the likelihood of bringing them home. Although there are no strong evidence-based guidelines on preventing infestation, pest control experts make a number of recommendations,32 which you can pass on to your patients.
Protect luggage when traveling. When staying in hotels, for instance, patients should keep suitcases tightly closed when not in use. Protection is further enhanced by placing suitcases in a sealed plastic bag; “contractor” trash bags available at hardware stores are large and durable. Keeping suitcases in the bathroom rather than the sleeping quarters also decreases the possibility of stowaways, as bed bugs typically shelter within a few feet of their host’s sleeping place.
Immediate laundering of clothes upon returning home from a trip, and storing suitcases outside the living quarters can decrease risk, too.33 There are commercially available suitcase heaters that raise the temperature of the suitcase and its contents to insecticidal levels, but they are fairly cost-prohibitive.
Screen items brought into the home. Used items, especially furniture, may harbor bed bugs. Fumigating used furniture was once common; it is still a good idea before second-hand items are brought into the house. Cardboard boxes in which used items are commonly stored or transported can shelter bed bugs, too.33
Deprive bed bugs of hiding places. Decluttering one’s sleeping quarters decreases the number of places bed bugs can hide. This tactic diminishes the likelihood of an infestation becoming firmly established before being discovered. Intervention early in the course of infestation, when it is limited to a single room, increases the likelihood of successful elimination.33
Mattress and box-spring encasements can prevent bed bug infestations by blocking movement of the bugs into and out of their shelters. If encasements are placed during an infestation, it is important to keep them in place for an extended period, given that bed bugs can survive for up to a year without feeding. Also effective is caulking and sealing molding, joints, and cracks wider than the thickness of a credit card in the room and in furniture.33
Vacuuming is part of the CDC’s recommendation for household pest control. But vacuum cleaners can also transfer bed bugs from infested to uninfested rooms. During an infestation, it’s important to empty vacuum bags immediately. And sharing vacuum cleaners between dwellings is best avoided.
A need for better solutions
Although bed bugs are not competent as vectors for the transmission of infectious diseases, they are responsible for significant dermatitis, allergic reactions, and psychological distress. Treatment of symptoms is effective in the short-term, but current methods of eliminating infestation are cumbersome, toxic, and are seldom completely successful. New strategies are desperately needed. The CDC Web page (http://www.cdc.gov/nceh/ehs/topics/bedbugs.htm) is regularly updated, and is a good source of information as new approaches are developed.
CORRESPONDENCE Mark K. Huntington, MD, PhD, Center for Family Medicine, 1115 East Twentieth Street, Sioux Falls, SD 57105; mark.huntington@usd.edu
1. Usinger RI. Monograph of the Cimicidae (Hemiptera-Heteroptera). Vol VII. College Park, Md: Entomological Society of America; 1966.
2. Berg R. Bed bugs: the pesticide dilemma. J Environ Health. 2010;72:32-35.
3. Snetsinger R. Bed bugs & other bugs. In: Hedges S, ed. Mallis’ Handbook of Pest Control. 8th ed. Cleveland, Ohio: GIE Media; 1997:392–424.
4. Eddy C, Jones SC. Bed bugs, public health, and social justice: part 1, a call to action. J Environ Health. 2011;73:8-14.
5. Hurst S, Humphreys M. Bedbugs: not back by popular demand. Dimens Crit Care Nurs. 2011;30:94-96.
6. Anderson A. The decade of bedbugs and fear. Environ Health Insights. 2011;5:53-54.
7. America’s 10 most infested cities. The Daily Beast; August 24, 2010. Available at: http://www.thedailybeast.com/articles/2010/08/24/bedbug-outbreak-which-cities-are-most-infested.html. Accessed August 28, 2011.
8. Hosokawa T, Koga R, Kikuchi Y, et al. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc Natl Acad Sci U S A. 2010;107:769-774.
9. Sakamoto JM, Rasgon JL. Geographic distribution of Wolbachia infections in Cimex lectularius (Heteroptera: Cimicidae). J Med Entomol. 2006;43:696-700.
10. Abou Gamra EM, el Shayed FA, Morsy TA, et al. The relation between Cimex lectularius antigen and bronchial asthma in Egypt. J Egypt Soc Parasitol. 1991;21:735-746.
11. Delaunay P, Blanc V, Del Giudice P, et al. Bedbugs and infectious diseases. Clin Infect Dis. 2011;52:200-210.
12. Pritchard MJ, Hwang SW. Cases: severe anemia from bedbugs. CMAJ. 2009;181:287-288.
13. Delaunay P. Cimex lectularius or bed bug: vector of infectious agents and pathogenic role. Available at: http://www.clinicaltrials.gov. Identifier: NCT01089465. Accessed November 29, 2011.
14. Rieder E, Hamalian G, Ying P. Psychiatric implications of bedbugs. Presented at: 164th Annual Meeting of the American Psychiatric Association; May 14-18, 2011; Honolulu, Hawaii. Abstract NR01-51.
15. Roos TC, Alam M, Roos S, et al. Pharmacotherapy of ectoparasitic infections. Drugs. 2001;61:1067-1088.
16. Goddard J, deShazo R. Bed bugs (Cimex lectularius) and clinical consequences of their bites. JAMA. 2009;301:1358-1366.
17. Centers for Disease Control and Prevention and Environmental Protection Agency. Joint statement on bed bug control in the United States from the U.S. Centers for Disease Control and Prevention (CDC) and the U.S. Environmental Protection Agency (EPA). Atlanta, Ga: US Department of Health and Human Services; 2010. Available at: http://www.cdc.gov/nceh/ehs/Publications/Bed_Bugs_CDC-EPA_Statement.htm. Accessed June 15, 2012.
18. Axtell RC, Arends JJ. Ecology and management of arthropod pests of poultry. Annu Rev Entomol. 1990;35:101-126.
19. Moore DJ, Miller DM. Field evaluations of insecticide treatment regimens for control of the common bed bug, Cimex lectularius (L.). Pest Manag Sci. 2009;65:332-338.
20. CDC. Acute illnesses associated with insecticides used to control bed bugs—seven states, 2003–2010. MMWR Morb Mortal Wkly Rep. 2011;60:1269-1274.
21. Tawatsin A, Thavara U, Chompoosri J, et al. Insecticide resistance in bedbugs in Thailand and laboratory evaluation of insecticides for the control of Cimex hemipterus and Cimex lectularius (Hemiptera: Cimicidae). J Med Entomol. 2011;48:1023-1030.
22. Benoit JB, Lopez-Martinez G, Teets NM, et al. Responses of the bed bug, Cimex lectularius, to temperature extremes and dehydration: levels of tolerance, rapid cold hardening and expression of heat shock proteins. Med Vet Entomol. 2009;23:418-425.
23. Benoit JB, Phillips SA, Croxall TJ, et al. Addition of alarm pheromone components improves the effectiveness of desiccant dusts against Cimex lectularius. J Med Entomol. 2009;46:572-579.
24. Heukelbach J, Feldmeier H. Scabies. Lancet. 2006;367:1767-1774.
25. Fox LM. Ivermectin: uses and impact 20 years on. Curr Opin Infect Dis. 2006;19:588-593.
26. Foucault C, Ranque S, Badiaga S, et al. Oral ivermectin in the treatment of body lice. J Infect Dis. 2006;193:474-476.
27. Dias JC, Schofield CJ, Machado EM, et al. Ticks, ivermectin, and experimental Chagas disease. Mem Inst Oswaldo Cruz. 2005;100:829-832.
28. Kobylinski KC, Sylla M, Chapman PL, et al. Ivermectin mass drug administration to humans disrupts malaria parasite transmission in Senegalese villages. Am J Trop Med Hyg. 2011;85:3-5.
29. Ostlind DA, Cifelli S, Conroy JA, etal. A novel Cimex lectularius– rodent assay for the detection of systemic ectoparasiticide activity. Southwest Entomol. 2001;26:181-186.
30. Tamarozzi F, Halliday A, Gentil K, et al. Onchocerciasis: the role of Wolbachia bacterial endosymbionts in parasite biology, disease pathogenesis, and treatment. Clin Microbiol Rev. 2011;24:459-468.
31. Kells SA, Hahn J. Prevention and control of bed bugs in residences. Available at: http://www.extension.umn.edu/distribution/housingandclothing/dk1022.html. Accessed November 29, 2011.
32. Got bedbugs? Your hotel might! Available at: http://www.smartertravel.com/travel-advice/avoiding-bedbugs-every-traveler-nightmare.html?id=4726511. Published April 25, 2010. Accessed November 29, 2011.
33. US Environmental Protection Agency. Bed bug information. Available at: http://www.epa.gov/bedbugs/index.html. Accessed November 29, 2011.
1. Usinger RI. Monograph of the Cimicidae (Hemiptera-Heteroptera). Vol VII. College Park, Md: Entomological Society of America; 1966.
2. Berg R. Bed bugs: the pesticide dilemma. J Environ Health. 2010;72:32-35.
3. Snetsinger R. Bed bugs & other bugs. In: Hedges S, ed. Mallis’ Handbook of Pest Control. 8th ed. Cleveland, Ohio: GIE Media; 1997:392–424.
4. Eddy C, Jones SC. Bed bugs, public health, and social justice: part 1, a call to action. J Environ Health. 2011;73:8-14.
5. Hurst S, Humphreys M. Bedbugs: not back by popular demand. Dimens Crit Care Nurs. 2011;30:94-96.
6. Anderson A. The decade of bedbugs and fear. Environ Health Insights. 2011;5:53-54.
7. America’s 10 most infested cities. The Daily Beast; August 24, 2010. Available at: http://www.thedailybeast.com/articles/2010/08/24/bedbug-outbreak-which-cities-are-most-infested.html. Accessed August 28, 2011.
8. Hosokawa T, Koga R, Kikuchi Y, et al. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc Natl Acad Sci U S A. 2010;107:769-774.
9. Sakamoto JM, Rasgon JL. Geographic distribution of Wolbachia infections in Cimex lectularius (Heteroptera: Cimicidae). J Med Entomol. 2006;43:696-700.
10. Abou Gamra EM, el Shayed FA, Morsy TA, et al. The relation between Cimex lectularius antigen and bronchial asthma in Egypt. J Egypt Soc Parasitol. 1991;21:735-746.
11. Delaunay P, Blanc V, Del Giudice P, et al. Bedbugs and infectious diseases. Clin Infect Dis. 2011;52:200-210.
12. Pritchard MJ, Hwang SW. Cases: severe anemia from bedbugs. CMAJ. 2009;181:287-288.
13. Delaunay P. Cimex lectularius or bed bug: vector of infectious agents and pathogenic role. Available at: http://www.clinicaltrials.gov. Identifier: NCT01089465. Accessed November 29, 2011.
14. Rieder E, Hamalian G, Ying P. Psychiatric implications of bedbugs. Presented at: 164th Annual Meeting of the American Psychiatric Association; May 14-18, 2011; Honolulu, Hawaii. Abstract NR01-51.
15. Roos TC, Alam M, Roos S, et al. Pharmacotherapy of ectoparasitic infections. Drugs. 2001;61:1067-1088.
16. Goddard J, deShazo R. Bed bugs (Cimex lectularius) and clinical consequences of their bites. JAMA. 2009;301:1358-1366.
17. Centers for Disease Control and Prevention and Environmental Protection Agency. Joint statement on bed bug control in the United States from the U.S. Centers for Disease Control and Prevention (CDC) and the U.S. Environmental Protection Agency (EPA). Atlanta, Ga: US Department of Health and Human Services; 2010. Available at: http://www.cdc.gov/nceh/ehs/Publications/Bed_Bugs_CDC-EPA_Statement.htm. Accessed June 15, 2012.
18. Axtell RC, Arends JJ. Ecology and management of arthropod pests of poultry. Annu Rev Entomol. 1990;35:101-126.
19. Moore DJ, Miller DM. Field evaluations of insecticide treatment regimens for control of the common bed bug, Cimex lectularius (L.). Pest Manag Sci. 2009;65:332-338.
20. CDC. Acute illnesses associated with insecticides used to control bed bugs—seven states, 2003–2010. MMWR Morb Mortal Wkly Rep. 2011;60:1269-1274.
21. Tawatsin A, Thavara U, Chompoosri J, et al. Insecticide resistance in bedbugs in Thailand and laboratory evaluation of insecticides for the control of Cimex hemipterus and Cimex lectularius (Hemiptera: Cimicidae). J Med Entomol. 2011;48:1023-1030.
22. Benoit JB, Lopez-Martinez G, Teets NM, et al. Responses of the bed bug, Cimex lectularius, to temperature extremes and dehydration: levels of tolerance, rapid cold hardening and expression of heat shock proteins. Med Vet Entomol. 2009;23:418-425.
23. Benoit JB, Phillips SA, Croxall TJ, et al. Addition of alarm pheromone components improves the effectiveness of desiccant dusts against Cimex lectularius. J Med Entomol. 2009;46:572-579.
24. Heukelbach J, Feldmeier H. Scabies. Lancet. 2006;367:1767-1774.
25. Fox LM. Ivermectin: uses and impact 20 years on. Curr Opin Infect Dis. 2006;19:588-593.
26. Foucault C, Ranque S, Badiaga S, et al. Oral ivermectin in the treatment of body lice. J Infect Dis. 2006;193:474-476.
27. Dias JC, Schofield CJ, Machado EM, et al. Ticks, ivermectin, and experimental Chagas disease. Mem Inst Oswaldo Cruz. 2005;100:829-832.
28. Kobylinski KC, Sylla M, Chapman PL, et al. Ivermectin mass drug administration to humans disrupts malaria parasite transmission in Senegalese villages. Am J Trop Med Hyg. 2011;85:3-5.
29. Ostlind DA, Cifelli S, Conroy JA, etal. A novel Cimex lectularius– rodent assay for the detection of systemic ectoparasiticide activity. Southwest Entomol. 2001;26:181-186.
30. Tamarozzi F, Halliday A, Gentil K, et al. Onchocerciasis: the role of Wolbachia bacterial endosymbionts in parasite biology, disease pathogenesis, and treatment. Clin Microbiol Rev. 2011;24:459-468.
31. Kells SA, Hahn J. Prevention and control of bed bugs in residences. Available at: http://www.extension.umn.edu/distribution/housingandclothing/dk1022.html. Accessed November 29, 2011.
32. Got bedbugs? Your hotel might! Available at: http://www.smartertravel.com/travel-advice/avoiding-bedbugs-every-traveler-nightmare.html?id=4726511. Published April 25, 2010. Accessed November 29, 2011.
33. US Environmental Protection Agency. Bed bug information. Available at: http://www.epa.gov/bedbugs/index.html. Accessed November 29, 2011.
Reducing the risk of breast cancer: A personalized approach
• Use a validated breast cancer risk assessment tool for any woman with a suspicious family history, precancerous breast lesions, or reproductive risk factors. C
• Recommend a semi-annual clinical breast exam and an annual mammogram for women at high risk for invasive breast cancer. C
• Discuss chemoprevention with a selective estrogen-receptor modifier or aromatase inhibitor with women at high risk for breast cancer and low risk for adverse events. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Identifying patients at risk
Among the known risk factors for breast cancer, some are modifiable (use of oral contraceptives and alcohol consumption, for example); others, such as family history and age at which menopause occurs, are not (TABLE 1).4-7 Aging itself confers the greatest risk: The incidence of breast cancer comes close to doubling at each 10-year interval before menopause and continues to climb, but more slowly, thereafter.8,9
TABLE 1
Risk factors for breast cancer4-7
| Nonmodifiable | Age, atypical hyperplasia, chest wall radiation (between the ages of 10-30 y), early menarche, family history, late menopause, race, sex |
| Modifiable | Alcohol consumption, hormone therapy (for menopausal symptoms, oral contraceptives), obesity, parity (first child after age 35, nulliparity) |
Estrogen exposure: The risk is cumulative
A number of studies have linked early onset of menarche (<12 years of age) and late menopause (>55 years) to an increase in breast cancer risk. Nulliparity, or having a first child after age 35, is also associated with greater risk; oophorectomy prior to age 50 may reduce the risk by as much as 40%.4,5,10-13
The mammogram shows a malignancy in the superior portion of the breast (arrow). Oral contraceptive use is an additional risk, but the effect slowly diminishes in the 10 years after cessation.4,5 Postmenopausal hormone replacement therapy—specifically, oral conjugated equine estrogen and medroxyprogesterone acetate—was found by the Woman’s Health Initiative to increase breast cancer risk.6
Other nongenetic risk factors include:
Atypical findings on breast biopsy. Evidence of atypical ductal hyperplasia (ADH) or lobular hyperplasia (ALH) is associated with a 4-fold increase in risk.7
Environmental exposure. Radiation, especially to the chest wall (typically as a treatment for Hodgkin’s lymphoma) increases a woman’s risk for breast cancer, particularly if the exposure occurred when she was between the ages of 10 and 30.14
Lifestyle factors. Obesity, particularly in postmenopausal women, and alcohol consumption of more than a drink or two per day are both associated with an increased risk.4
Genetic mutations and breast cancer risk
An estimated 5% to 10% of breast cancers are inherited.5 Genetic susceptibility is generally transmitted as an autosomal dominant trait.
There are 2 known breast cancer genes, BRCA1 and BRCA2, located on the long arm of chromosomes 17 and 13, respectively. The genes themselves encode tumor suppressor proteins. Mutations in these genes impair the DNA repair process, resulting in increased risk.8
The chance of carrying a mutation in either BRCA1 or BRCA2 is estimated at one in 500 to 800 in women of Northern/Western European descent. Among Ashkenazi Jews, however, the frequency is about one in 50.5
A thorough family history that takes into account both the number of affected relatives and their age at diagnosis (TABLE 2)8,15 is helpful in determining whether a patient is at low, high, or very high risk of carrying a genetic mutation. Women who have no first-degree relative with breast cancer—or a relative who was diagnosed with breast cancer after age 50—are at low risk, while those with at least one first-degree relative diagnosed with breast cancer before the age of 50 would be categorized as high risk.
A woman with a family history of early-onset breast or ovarian cancer or a relative who developed both breast and ovarian cancer, bilateral breast cancer, or male breast cancer would be classified as very high risk for a genetic mutation, as would a patient with 2 or more family members affected by breast or ovarian cancer.
Ashkenazi Jewish heritage and a relative who was diagnosed with ovarian or breast cancer indicate an increased likelihood of a BRCA mutation, as well.8 (Other genetic conditions, with mutations that are distinct from the BRCA genes, have also been linked to breast cancer, but occur less frequently.)
BRCA gene testing can confirm very high risk status, prompting the initiation of preventive measures and facilitating early detection. Such testing can also identify—and relieve the anxiety of—noncarriers in high-risk families. Recently published guidelines from the US Preventive Services Task Force (USPSTF) support testing in women with suspicious family histories with a grade B recommendation, indicating that there is at least fair evidence that testing improves important health outcomes and that the benefits of testing outweigh the harms.15
The downside of specific BRCA gene testing for patients who find that they do not have this genetic mutation may include a false sense of security and the failure to identify any other genetic mutations. Patients who learn that they do carry a BRCA gene mutation could face psychosocial or economic harm associated with aggressive surveillance and surgical intervention.5
TABLE 2
Genetic counseling for patients at high risk8,15
Tools can quantify 5-year, 10-year, and lifetime risk
A number of breast cancer risk assessment tools have been developed to help clinicians individualize patient care. None provides the basis for an all-encompassing approach to breast cancer risk or a comprehensive patient discussion of preventive strategies. We have found that, when used in combination, 2 or more predictive models can complement each other and guide the development of a targeted risk reduction approach.
When to use a predictive tool
It is not necessary to use a predictive model for patients at low risk for breast cancer. The tools detailed in TABLE 3 5,14,16-23 are better suited to women who have a suspicious family history, a history of precancerous breast lesions, or known reproductive risks. Although each model has limitations, it is important that you have a working knowledge of circumstances that favor one tool over another. For instance, the Gail model, the most widely used, can help determine if a particular patient is a candidate for chemoprevention.16-20 Others, such as the Tyrer-Cuzick model14,21,22 and the Claus model,14,23 are useful in deciding whether a patient is a candidate for breast magnetic resonance imaging (MRI) as an adjunct to mammography screening. Another useful tool is the BRCAPRO, which is used primarily by genetic counselors to assess the likelihood that a patient carries a BRCA1 or BRCA2 mutation and would benefit from genetic testing.4,5
TABLE 3
Breast cancer risk assessment tools: What you need to know5,14,16-23
| Tool | Intended use | Criteria considered | Results | Limitations | Validation | How to access |
|---|---|---|---|---|---|---|
| Gail model | Assess eligibility for chemoprevention in women >35 years | Reproductive history, history of breast biopsies, first-degree relatives with breast cancer | Estimates 5-year and lifetime risk for invasive breast cancer | Can overestimate risk in patients with previous biopsy and atypical hyperplasia results and family history | Validated in independent projects; widely used to define excess risk; modified model for minorities validated | Available at http://www.cancer.gov/bcrisktool/ |
| Tyrer-Cuzick* model | Assess need for breast MRI | Hormonal and reproductive history, history of breast biopsies, number and age of onset of first- and second-degree relatives with breast cancer | Estimates 10-year and lifetime risk for invasive breast cancer | Potential for significant overestimation of risk in patients with atypical hyperplasia findings on breast biopsy | Not validated | Go to http://www.ems-trials.org/riskevaluator Click on “software downloads” to select the appropriate version |
| Claus model | Assess need for breast MRI | Age of onset of first- and second-degree relatives with history of breast cancer | Estimates incremental 10-year and lifetime risk for invasive breast cancer | Looks only at family history, without considering hormonal or reproductive risk factors | Validation does not extend to minorities | Tables found in Cancer (1994;73:643-651) available at no charge from http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1097-0142/issues |
| BRCAPRO | Determine whether genetic testing is indicated | Family history of breast and ovarian cancer | Estimates likelihood of genetic mutation | Time-consuming; requires highly detailed family history | Validation does not extend to minorities | Not widely available; used primarily by genetic counselors |
| *Also known as the IBIS model. IBIS, International Breast Cancer Intervention Study; MRI, magnetic resonance imaging. | ||||||
Managing patients at all risk levels
Although patients with average, high, or very high risk will all be managed differently, evidence suggests that lifestyle modification as needed, imaging, and chemoprevention, in some cases, can reduce the likelihood of breast cancer for women at all levels of risk.24
For women with an average risk (a 5-year Gail model score ≤1.66% and no significant family history),19 a discussion of the benefits and risks, as well as the limitations, of annual screening mammography beginning at age 40 vs age 50 is in order. Several major organizations, the American College of Obstetricians and Gynecologists25 and American Cancer Society (ACS)16 among them, have guidelines that support annual mammography beginning at 40 years but do not specify at what age to discontinue screening. In contrast, the USPSTF26 recommends biennial mammography between the ages of 50 and 74 years (See “The mammography controvrsy: When should you screen?” J Fam Pract. 2011;60:524-531).
How to proceed? Talk to patients in the 40- to 50-year age range about the benefits and risks of earlier, more frequent screening vs waiting until 50 to start mammography and opting for screening every 2 years. Breast health awareness and the role of clinical breast exams also should be included in a balanced discussion of early detection of breast cancer. A review of the patient’s reproductive status and use of hormone preparations is appropriate, as well.4,5
Patients at high risk (a Gail model score >1.66%; a history of ADH, ALH, or lobular carcinoma in situ; or a family history of breast cancer)3 should be advised to have a clinical breast exam every 6 months and annual mammograms. High-risk patients should also be offered the option of chemoprevention with tamoxifen, raloxifene,27,28 or exemestane29 if the benefits of treatment outweigh the risk of potential adverse effects. The merits of MRI breast surveillance have not been defined for women with this level of risk.14
For very high-risk patients (those with a family history that strongly suggests a genetic predisposition, a confirmed gene mutation, evidence of hereditary breast and ovarian cancer, or a personal history of chest wall irradiation between the ages of 10 and 30 years), a discussion of more aggressive risk-reduction strategies is recommended.4 A clinical breast exam and mammogram should be performed beginning at age 25—or 5 to 10 years before the earliest age at which a first-degree relative was diagnosed.
Starting at age 30, patients at very high risk should undergo annual mammography and breast MRI, either simultaneously or staggered every 6 months, along with a twice-yearly clinical breast exam.14 Breast health awareness and lifestyle modification should be emphasized, and the benefits and risks of chemoprevention should be discussed. Surgical risk-reduction strategies, such as prophylactic mastectomy and oophorectomy, should also be discussed, along with the offer of a referral to a surgeon for consultation.5
What to tell patients about chemoprevention
The USPSTF has issued a grade B recommendation to a discussion of chemoprevention for women who are at high risk for breast cancer and low risk for an adverse event.30 Counseling a patient regarding the risks and benefits of chemoprevention will depend on her age, comorbidities, whether or not she has had a hysterectomy, and her willingness to take the suggested medication.
Selective estrogen receptor modulators (SERMs). The American Society of Clinical Oncology Clinical Practice Guideline Update has reviewed the benefits and potential adverse effects of the SERMs tamoxifen and raloxifene. The Society supports the use of tamoxifen in pre- and postmenopausal women for breast cancer risk reduction; it also supports the use of raloxifene for postmenopausal women, the only patient population for which raloxifene has been approved.27
In a review of 7 placebo-controlled, randomized clinical trials and one head-to-head trial, both drugs reduced the risk for invasive, estrogen receptor–positive breast cancer by about 40% compared with placebo. Breast cancer deaths, however, did not decrease.31
Both tamoxifen and raloxifene were found to increase bone mineral density and reduce fracture risk.31 Thromboembolic events—which occurred less frequently with raloxifene than tamoxifen—was the chief adverse effect, with an incidence of 0.4% to 0.7%. In addition, fewer cases of endometrial cancer were reported with raloxifene compared with tamoxifen, making raloxifene the preferred treatment for postmenopausal women with an intact uterus.31
The National Surgical Adjuvant Breast and Bowel Project STAR study—one of the trials included in the review—initially reported that tamoxifen and raloxifene were equivalent in reducing breast cancer risk in postmenopausal women at increased risk.28 In an updated analysis based on 81 months of use, however, tamoxifen resulted in a 50% reduction in the incidence of breast cancer vs a reduction of 38% for raloxifene.32
The greater reduction in breast cancer risk seen with tamoxifen comes at a potential cost. Tamoxifen was found to have a worse adverse effect profile, leading to a higher risk for endometrial hyperplasia and hysterectomy, as well as thromboembolic events. The difference in all-cause mortality, however, was not statistically significant.32
Aromatase inhibitor therapy. The National Cancer Institute of Canada recently published a major chemoprevention trial, evaluating the effectiveness of aromatase inhibition in breast cancer risk reduction.29 This randomized, double-blind trial of exemestane vs placebo included more than 4500 women with a median follow-up of 3 years, and found that the exemestane reduced the incidence of invasive breast cancer in postmenopausal women at moderate risk by 65% (hazard ratio=0.35; 95% confidence interval, 0.18-0.70; P=.002).29
IBIS-II, a multicenter study in the United Kingdom, randomly assigned 6000 women at increased risk for breast cancer to placebo or anastrozole, an alternative aromatase inhibitor. This trial is ongoing, and breast cancer incidence is the primary endpoint.33 Aromatase inhibitors have not been approved by the US Food and Drug Administration for breast cancer prevention.34
Imaging strategies for those at risk
Although there is evidence that mammography performed on postmenopausal women can reduce breast cancer mortality by 25%, there are known limitations to this detection method.14
One drawback is that in premenopausal women, breast density lowers mammography’s sensitivity. In addition, several studies have found that mammography has a low sensitivity for detecting tumors in patients with a BRCA mutation. This has led to the use of other imaging modalities, especially MRI, for women with a family history that suggests a genetic predisposition.
The first study to demonstrate the superior sensitivity of MRI for detecting invasive breast cancer compared with clinical breast exam and mammography was published in 2004.35 A few years later, the ACS issued guidelines that call for surveillance with MRI as an adjunct to mammography, starting at age 30, for women whose family history, carrier status, or history of chest wall radiation puts them at very high risk (ie, a lifetime risk >20%-25%).14
The ACS found insufficient evidence to recommend for or against breast MRI for women with a lifetime risk of 15% to 20% (or documented high-risk lesions such as lobular carcinoma in situ, ALH, or ADH). Mammographic density, which in itself is a strong risk factor for the development of breast cancer, was not determined to be an indication for MRI screening. In deciding whether MRI is indicated for any high-risk patient, the cost, quality of imaging, and lower specificity must be considered.14
Weighing the benefits of surgery
For women who have a strong family history of breast cancer or are known carriers of a BRCA1 or BRCA gene mutation, the already high risk of developing breast cancer increases as they age. Prophylactic surgery—risk-reduction mastectomy (RRM) and/or bilateral salpingo-oophorectomy (RRSO)—has been found to lower the risk.5,36,37
RRM can reduce the risk of breast cancer by as much as 90% for such patients;38,39 RRSO yields similar results, reducing the risk of ovarian cancer by 80% to 95% and the risk of breast cancer by 40% to 59%, provided the surgery is performed before the patient is 40 years old.36,37
These potential benefits must be weighed against the harm associated with surgically induced menopause, with the attendant risks of cardiovascular disease, osteoporosis, and menopausal symptoms.40 Notably, hormone therapy use after RRSO in women with a gene mutation has not been found to increase the risk of breast cancer. In fact, it may be associated with a decreased risk.5 In general, short-term use of low-dose estrogen—up to the age of 51 or 52 years—is considered to be safe for this population,41,42 but long-term data on breast cancer risk are lacking.
CORRESPONDENCE
Marcia G. Ko, MD, Mayo Clinic, 13737 North 92nd Street, Scottsdale, AZ 85369; ko.marcia@mayo.edu
1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300.
2. Smith R. Risk-based screening for breast cancer: is there a practical strategy? Semin Breast Dis. 1999;2:280-291.
3. Guerra CE, Sherman M, Armstrong K. Diffusion of breast cancer risk assessment in primary care. J Am Board Fam Med. 2009;22:272-279.
4. Pruthi S, Brandt KR, Degnim AC, et al. A multidisciplinary approach to the management of breast cancer, part 1: prevention and diagnosis. Mayo Clin Proc. 2007;82:999-1012.
5. Pruthi S, Gostout BS, Lindor NM. Identification and management of women with BRCA mutations or hereditary predisposition for breast and ovarian cancer. Mayo Clin Proc. 2010;85:1111-1120.
6. Rossouw J, Anderson G, Prentice R, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
7. Hartmann LC, Sellers TA, Frost MH, et al. Benign breast disease and the risk of breast cancer. N Engl J Med. 2005;353:229-237.
8. Daly MB, Axilbund JE, Buys S, et al. Genetic/familial high-risk assessment: breast and ovarian. J Natl Compr Canc Netw. 2010;8:562-594.
9. McPherson K, Steel CM, Dixon JM. ABC of breast diseases. BMJ. 2000;321:624-628.
10. Brinton LA, Schairer C, Hoover RN, et al. Menstrual factors and risk of breast cancer. Cancer Invest. 1988;6:245-254.
11. Apter D, Reinilä M, Vihko R. Some endocrine characteristics of early menarche, a risk factor for breast cancer, are preserved into adulthood. Int J Cancer. 1989;44:783-787.
12. Rebbeck TR, Lynch HT, Neuhausen SL, et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med. 2002;346:1616-1622.
13. Narod SA, Brunet JS, Ghadirian P, et al. Tamoxifen and risk of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers: a case-control study. Lancet. 2000;356:1876-1881.
14. Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
15. U.S. Preventive Services Task Force. Genetic risk assessment and BRCA mutation testing for breast and ovarian cancer susceptibility. Ann Intern Med. 2005;143:355-361.
16. Gail M, Benichou J. Validation studies on a model for breast cancer risk. J Natl Cancer Inst. 1994;86:573-575.
17. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 2005;97:1652-1662.
18. Pankratz VS, Hartmann LC, Degnim AC, et al. Assessment of the accuracy of the Gail model in women with atypical hyperplasia. J Clin Oncol. 2008;26:5374-5379.
19. Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879-1886.
20. Vogel VG, Costantino JP, Wickerham DL, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: preventing breast cancer. Cancer Prev Res (Phila). 2010;3:696-706.
21. Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23:1111-1130.
22. Boughey JC, Hartmann LC, Anderson SS, et al. Evaluation of the Tyrer-Cuzick (International Breast Cancer Intervention Study) model for breast cancer risk prediction in women with atypical hyperplasia. J Clin Oncol. 2010;28:3591-3596.
23. Claus EB, Risch N, Thompson W. Autosomal dominant inheritance of early-onset breast cancer. Cancer. 1994;73:643-651.
24. Cummings SR, Tice JA, Bauer S, et al. Prevention of breast cancer in postmenopausal women. J Natl Cancer Inst. 2009;101:384-398.
25. American College of Obstetricians-Gynecologists. Practice bulletin no. 122: Breast cancer screening. Obstet Gynecol. 2011;118(2 pt 1):372-382.
26. U.S. Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716-726.
27. Visvanathan K, Chlebowski R, Hurley P, et al. American Society of Clinical Oncology clinical practice guideline update on the use of pharmacologic interventions including tamoxifen, raloxifene, and aromatase inhibition for breast cancer risk reduction. J Clin Oncol. 2009;27:3235-3258.
28. Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
29. Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381-2391.
30. U.S. Preventive Services Task Force. Chemoprevention of breast cancer. Ann Intern Med. 2002;137:56-58.
31. Nelson HD, Fu R, Griffin JC, et al. Systematic review: comparative effectiveness of medications to reduce risk for primary breast cancer. Ann Intern Med. 2009;151:703-715.
32. Vogel VG, Costantino JP, Wickerham DL, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: preventing breast cancer. Cancer Prev Res (Phila). 2010;3:696-706.
33. Dunn BK, Ryan A. Phase 3 trials of aromatase inhibitors for breast cancer prevention. Ann N Y Acad Sci. 2009;1155:141-161.
34. National Cancer Institute. Hormone therapy for breast cancer. Reviewed April 11, 2012. Available at: http://www.cancer.gov/cancertopics/factsheet/Therapy/hormone-therapy-breast. Accessed May 18, 2012.
35. Kriege M, Brekelmans CTM, Boetes C, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351:427-437.
36. Domchek SM, Friebel TM, Singer CF, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304:967-975.
37. Eisen A, Lubinski J, Klijn J, et al. Breast cancer risk following bilateral oophorectomy in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2005;23:7491-7496.
38. Rebbeck TR, Friebel T, Lynch HT, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2004;22:1055-1062.
39. Hartmann LC, Schaid DJ, Woods JE, et al. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N Engl J Med. 1999;340:77-84.
40. Shuster LT, Gostout BS, Grossardt BR, Rocca WA. Prophylactic oophorectomy in premenopausal women and long-term health. Menopause Int. 2008;14:111-116.
41. Armstrong K, Schwartz JS, Randall T, et al. Hormone replacement therapy and life expectancy after prophylactic oophorectomy in women with BRCA1/2 mutations: a decision analysis. J Clin Oncol. 2004;22:1045-1054.
42. Eisen A, Lubinski J, Gronwald J, et al. Hormone therapy and the risk of breast cancer in BRCA1 mutation carriers. J Natl Cancer Inst. 2008;100:1361-1367.
;
• Use a validated breast cancer risk assessment tool for any woman with a suspicious family history, precancerous breast lesions, or reproductive risk factors. C
• Recommend a semi-annual clinical breast exam and an annual mammogram for women at high risk for invasive breast cancer. C
• Discuss chemoprevention with a selective estrogen-receptor modifier or aromatase inhibitor with women at high risk for breast cancer and low risk for adverse events. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Identifying patients at risk
Among the known risk factors for breast cancer, some are modifiable (use of oral contraceptives and alcohol consumption, for example); others, such as family history and age at which menopause occurs, are not (TABLE 1).4-7 Aging itself confers the greatest risk: The incidence of breast cancer comes close to doubling at each 10-year interval before menopause and continues to climb, but more slowly, thereafter.8,9
TABLE 1
Risk factors for breast cancer4-7
| Nonmodifiable | Age, atypical hyperplasia, chest wall radiation (between the ages of 10-30 y), early menarche, family history, late menopause, race, sex |
| Modifiable | Alcohol consumption, hormone therapy (for menopausal symptoms, oral contraceptives), obesity, parity (first child after age 35, nulliparity) |
Estrogen exposure: The risk is cumulative
A number of studies have linked early onset of menarche (<12 years of age) and late menopause (>55 years) to an increase in breast cancer risk. Nulliparity, or having a first child after age 35, is also associated with greater risk; oophorectomy prior to age 50 may reduce the risk by as much as 40%.4,5,10-13

The mammogram shows a malignancy in the superior portion of the breast (arrow). Oral contraceptive use is an additional risk, but the effect slowly diminishes in the 10 years after cessation.4,5 Postmenopausal hormone replacement therapy—specifically, oral conjugated equine estrogen and medroxyprogesterone acetate—was found by the Woman’s Health Initiative to increase breast cancer risk.6
Other nongenetic risk factors include:
Atypical findings on breast biopsy. Evidence of atypical ductal hyperplasia (ADH) or lobular hyperplasia (ALH) is associated with a 4-fold increase in risk.7
Environmental exposure. Radiation, especially to the chest wall (typically as a treatment for Hodgkin’s lymphoma) increases a woman’s risk for breast cancer, particularly if the exposure occurred when she was between the ages of 10 and 30.14
Lifestyle factors. Obesity, particularly in postmenopausal women, and alcohol consumption of more than a drink or two per day are both associated with an increased risk.4
Genetic mutations and breast cancer risk
An estimated 5% to 10% of breast cancers are inherited.5 Genetic susceptibility is generally transmitted as an autosomal dominant trait.
There are 2 known breast cancer genes, BRCA1 and BRCA2, located on the long arm of chromosomes 17 and 13, respectively. The genes themselves encode tumor suppressor proteins. Mutations in these genes impair the DNA repair process, resulting in increased risk.8
The chance of carrying a mutation in either BRCA1 or BRCA2 is estimated at one in 500 to 800 in women of Northern/Western European descent. Among Ashkenazi Jews, however, the frequency is about one in 50.5
A thorough family history that takes into account both the number of affected relatives and their age at diagnosis (TABLE 2)8,15 is helpful in determining whether a patient is at low, high, or very high risk of carrying a genetic mutation. Women who have no first-degree relative with breast cancer—or a relative who was diagnosed with breast cancer after age 50—are at low risk, while those with at least one first-degree relative diagnosed with breast cancer before the age of 50 would be categorized as high risk.
A woman with a family history of early-onset breast or ovarian cancer or a relative who developed both breast and ovarian cancer, bilateral breast cancer, or male breast cancer would be classified as very high risk for a genetic mutation, as would a patient with 2 or more family members affected by breast or ovarian cancer.
Ashkenazi Jewish heritage and a relative who was diagnosed with ovarian or breast cancer indicate an increased likelihood of a BRCA mutation, as well.8 (Other genetic conditions, with mutations that are distinct from the BRCA genes, have also been linked to breast cancer, but occur less frequently.)
BRCA gene testing can confirm very high risk status, prompting the initiation of preventive measures and facilitating early detection. Such testing can also identify—and relieve the anxiety of—noncarriers in high-risk families. Recently published guidelines from the US Preventive Services Task Force (USPSTF) support testing in women with suspicious family histories with a grade B recommendation, indicating that there is at least fair evidence that testing improves important health outcomes and that the benefits of testing outweigh the harms.15
The downside of specific BRCA gene testing for patients who find that they do not have this genetic mutation may include a false sense of security and the failure to identify any other genetic mutations. Patients who learn that they do carry a BRCA gene mutation could face psychosocial or economic harm associated with aggressive surveillance and surgical intervention.5
TABLE 2
Genetic counseling for patients at high risk8,15
Tools can quantify 5-year, 10-year, and lifetime risk
A number of breast cancer risk assessment tools have been developed to help clinicians individualize patient care. None provides the basis for an all-encompassing approach to breast cancer risk or a comprehensive patient discussion of preventive strategies. We have found that, when used in combination, 2 or more predictive models can complement each other and guide the development of a targeted risk reduction approach.
When to use a predictive tool
It is not necessary to use a predictive model for patients at low risk for breast cancer. The tools detailed in TABLE 3 5,14,16-23 are better suited to women who have a suspicious family history, a history of precancerous breast lesions, or known reproductive risks. Although each model has limitations, it is important that you have a working knowledge of circumstances that favor one tool over another. For instance, the Gail model, the most widely used, can help determine if a particular patient is a candidate for chemoprevention.16-20 Others, such as the Tyrer-Cuzick model14,21,22 and the Claus model,14,23 are useful in deciding whether a patient is a candidate for breast magnetic resonance imaging (MRI) as an adjunct to mammography screening. Another useful tool is the BRCAPRO, which is used primarily by genetic counselors to assess the likelihood that a patient carries a BRCA1 or BRCA2 mutation and would benefit from genetic testing.4,5
TABLE 3
Breast cancer risk assessment tools: What you need to know5,14,16-23
| Tool | Intended use | Criteria considered | Results | Limitations | Validation | How to access |
|---|---|---|---|---|---|---|
| Gail model | Assess eligibility for chemoprevention in women >35 years | Reproductive history, history of breast biopsies, first-degree relatives with breast cancer | Estimates 5-year and lifetime risk for invasive breast cancer | Can overestimate risk in patients with previous biopsy and atypical hyperplasia results and family history | Validated in independent projects; widely used to define excess risk; modified model for minorities validated | Available at http://www.cancer.gov/bcrisktool/ |
| Tyrer-Cuzick* model | Assess need for breast MRI | Hormonal and reproductive history, history of breast biopsies, number and age of onset of first- and second-degree relatives with breast cancer | Estimates 10-year and lifetime risk for invasive breast cancer | Potential for significant overestimation of risk in patients with atypical hyperplasia findings on breast biopsy | Not validated | Go to http://www.ems-trials.org/riskevaluator Click on “software downloads” to select the appropriate version |
| Claus model | Assess need for breast MRI | Age of onset of first- and second-degree relatives with history of breast cancer | Estimates incremental 10-year and lifetime risk for invasive breast cancer | Looks only at family history, without considering hormonal or reproductive risk factors | Validation does not extend to minorities | Tables found in Cancer (1994;73:643-651) available at no charge from http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1097-0142/issues |
| BRCAPRO | Determine whether genetic testing is indicated | Family history of breast and ovarian cancer | Estimates likelihood of genetic mutation | Time-consuming; requires highly detailed family history | Validation does not extend to minorities | Not widely available; used primarily by genetic counselors |
| *Also known as the IBIS model. IBIS, International Breast Cancer Intervention Study; MRI, magnetic resonance imaging. | ||||||
Managing patients at all risk levels
Although patients with average, high, or very high risk will all be managed differently, evidence suggests that lifestyle modification as needed, imaging, and chemoprevention, in some cases, can reduce the likelihood of breast cancer for women at all levels of risk.24
For women with an average risk (a 5-year Gail model score ≤1.66% and no significant family history),19 a discussion of the benefits and risks, as well as the limitations, of annual screening mammography beginning at age 40 vs age 50 is in order. Several major organizations, the American College of Obstetricians and Gynecologists25 and American Cancer Society (ACS)16 among them, have guidelines that support annual mammography beginning at 40 years but do not specify at what age to discontinue screening. In contrast, the USPSTF26 recommends biennial mammography between the ages of 50 and 74 years (See “The mammography controvrsy: When should you screen?” J Fam Pract. 2011;60:524-531).
How to proceed? Talk to patients in the 40- to 50-year age range about the benefits and risks of earlier, more frequent screening vs waiting until 50 to start mammography and opting for screening every 2 years. Breast health awareness and the role of clinical breast exams also should be included in a balanced discussion of early detection of breast cancer. A review of the patient’s reproductive status and use of hormone preparations is appropriate, as well.4,5
Patients at high risk (a Gail model score >1.66%; a history of ADH, ALH, or lobular carcinoma in situ; or a family history of breast cancer)3 should be advised to have a clinical breast exam every 6 months and annual mammograms. High-risk patients should also be offered the option of chemoprevention with tamoxifen, raloxifene,27,28 or exemestane29 if the benefits of treatment outweigh the risk of potential adverse effects. The merits of MRI breast surveillance have not been defined for women with this level of risk.14
For very high-risk patients (those with a family history that strongly suggests a genetic predisposition, a confirmed gene mutation, evidence of hereditary breast and ovarian cancer, or a personal history of chest wall irradiation between the ages of 10 and 30 years), a discussion of more aggressive risk-reduction strategies is recommended.4 A clinical breast exam and mammogram should be performed beginning at age 25—or 5 to 10 years before the earliest age at which a first-degree relative was diagnosed.
Starting at age 30, patients at very high risk should undergo annual mammography and breast MRI, either simultaneously or staggered every 6 months, along with a twice-yearly clinical breast exam.14 Breast health awareness and lifestyle modification should be emphasized, and the benefits and risks of chemoprevention should be discussed. Surgical risk-reduction strategies, such as prophylactic mastectomy and oophorectomy, should also be discussed, along with the offer of a referral to a surgeon for consultation.5
What to tell patients about chemoprevention
The USPSTF has issued a grade B recommendation to a discussion of chemoprevention for women who are at high risk for breast cancer and low risk for an adverse event.30 Counseling a patient regarding the risks and benefits of chemoprevention will depend on her age, comorbidities, whether or not she has had a hysterectomy, and her willingness to take the suggested medication.
Selective estrogen receptor modulators (SERMs). The American Society of Clinical Oncology Clinical Practice Guideline Update has reviewed the benefits and potential adverse effects of the SERMs tamoxifen and raloxifene. The Society supports the use of tamoxifen in pre- and postmenopausal women for breast cancer risk reduction; it also supports the use of raloxifene for postmenopausal women, the only patient population for which raloxifene has been approved.27
In a review of 7 placebo-controlled, randomized clinical trials and one head-to-head trial, both drugs reduced the risk for invasive, estrogen receptor–positive breast cancer by about 40% compared with placebo. Breast cancer deaths, however, did not decrease.31
Both tamoxifen and raloxifene were found to increase bone mineral density and reduce fracture risk.31 Thromboembolic events—which occurred less frequently with raloxifene than tamoxifen—was the chief adverse effect, with an incidence of 0.4% to 0.7%. In addition, fewer cases of endometrial cancer were reported with raloxifene compared with tamoxifen, making raloxifene the preferred treatment for postmenopausal women with an intact uterus.31
The National Surgical Adjuvant Breast and Bowel Project STAR study—one of the trials included in the review—initially reported that tamoxifen and raloxifene were equivalent in reducing breast cancer risk in postmenopausal women at increased risk.28 In an updated analysis based on 81 months of use, however, tamoxifen resulted in a 50% reduction in the incidence of breast cancer vs a reduction of 38% for raloxifene.32
The greater reduction in breast cancer risk seen with tamoxifen comes at a potential cost. Tamoxifen was found to have a worse adverse effect profile, leading to a higher risk for endometrial hyperplasia and hysterectomy, as well as thromboembolic events. The difference in all-cause mortality, however, was not statistically significant.32
Aromatase inhibitor therapy. The National Cancer Institute of Canada recently published a major chemoprevention trial, evaluating the effectiveness of aromatase inhibition in breast cancer risk reduction.29 This randomized, double-blind trial of exemestane vs placebo included more than 4500 women with a median follow-up of 3 years, and found that the exemestane reduced the incidence of invasive breast cancer in postmenopausal women at moderate risk by 65% (hazard ratio=0.35; 95% confidence interval, 0.18-0.70; P=.002).29
IBIS-II, a multicenter study in the United Kingdom, randomly assigned 6000 women at increased risk for breast cancer to placebo or anastrozole, an alternative aromatase inhibitor. This trial is ongoing, and breast cancer incidence is the primary endpoint.33 Aromatase inhibitors have not been approved by the US Food and Drug Administration for breast cancer prevention.34
Imaging strategies for those at risk
Although there is evidence that mammography performed on postmenopausal women can reduce breast cancer mortality by 25%, there are known limitations to this detection method.14
One drawback is that in premenopausal women, breast density lowers mammography’s sensitivity. In addition, several studies have found that mammography has a low sensitivity for detecting tumors in patients with a BRCA mutation. This has led to the use of other imaging modalities, especially MRI, for women with a family history that suggests a genetic predisposition.
The first study to demonstrate the superior sensitivity of MRI for detecting invasive breast cancer compared with clinical breast exam and mammography was published in 2004.35 A few years later, the ACS issued guidelines that call for surveillance with MRI as an adjunct to mammography, starting at age 30, for women whose family history, carrier status, or history of chest wall radiation puts them at very high risk (ie, a lifetime risk >20%-25%).14
The ACS found insufficient evidence to recommend for or against breast MRI for women with a lifetime risk of 15% to 20% (or documented high-risk lesions such as lobular carcinoma in situ, ALH, or ADH). Mammographic density, which in itself is a strong risk factor for the development of breast cancer, was not determined to be an indication for MRI screening. In deciding whether MRI is indicated for any high-risk patient, the cost, quality of imaging, and lower specificity must be considered.14
Weighing the benefits of surgery
For women who have a strong family history of breast cancer or are known carriers of a BRCA1 or BRCA gene mutation, the already high risk of developing breast cancer increases as they age. Prophylactic surgery—risk-reduction mastectomy (RRM) and/or bilateral salpingo-oophorectomy (RRSO)—has been found to lower the risk.5,36,37
RRM can reduce the risk of breast cancer by as much as 90% for such patients;38,39 RRSO yields similar results, reducing the risk of ovarian cancer by 80% to 95% and the risk of breast cancer by 40% to 59%, provided the surgery is performed before the patient is 40 years old.36,37
These potential benefits must be weighed against the harm associated with surgically induced menopause, with the attendant risks of cardiovascular disease, osteoporosis, and menopausal symptoms.40 Notably, hormone therapy use after RRSO in women with a gene mutation has not been found to increase the risk of breast cancer. In fact, it may be associated with a decreased risk.5 In general, short-term use of low-dose estrogen—up to the age of 51 or 52 years—is considered to be safe for this population,41,42 but long-term data on breast cancer risk are lacking.
CORRESPONDENCE
Marcia G. Ko, MD, Mayo Clinic, 13737 North 92nd Street, Scottsdale, AZ 85369; ko.marcia@mayo.edu
• Use a validated breast cancer risk assessment tool for any woman with a suspicious family history, precancerous breast lesions, or reproductive risk factors. C
• Recommend a semi-annual clinical breast exam and an annual mammogram for women at high risk for invasive breast cancer. C
• Discuss chemoprevention with a selective estrogen-receptor modifier or aromatase inhibitor with women at high risk for breast cancer and low risk for adverse events. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Identifying patients at risk
Among the known risk factors for breast cancer, some are modifiable (use of oral contraceptives and alcohol consumption, for example); others, such as family history and age at which menopause occurs, are not (TABLE 1).4-7 Aging itself confers the greatest risk: The incidence of breast cancer comes close to doubling at each 10-year interval before menopause and continues to climb, but more slowly, thereafter.8,9
TABLE 1
Risk factors for breast cancer4-7
| Nonmodifiable | Age, atypical hyperplasia, chest wall radiation (between the ages of 10-30 y), early menarche, family history, late menopause, race, sex |
| Modifiable | Alcohol consumption, hormone therapy (for menopausal symptoms, oral contraceptives), obesity, parity (first child after age 35, nulliparity) |
Estrogen exposure: The risk is cumulative
A number of studies have linked early onset of menarche (<12 years of age) and late menopause (>55 years) to an increase in breast cancer risk. Nulliparity, or having a first child after age 35, is also associated with greater risk; oophorectomy prior to age 50 may reduce the risk by as much as 40%.4,5,10-13

The mammogram shows a malignancy in the superior portion of the breast (arrow). Oral contraceptive use is an additional risk, but the effect slowly diminishes in the 10 years after cessation.4,5 Postmenopausal hormone replacement therapy—specifically, oral conjugated equine estrogen and medroxyprogesterone acetate—was found by the Woman’s Health Initiative to increase breast cancer risk.6
Other nongenetic risk factors include:
Atypical findings on breast biopsy. Evidence of atypical ductal hyperplasia (ADH) or lobular hyperplasia (ALH) is associated with a 4-fold increase in risk.7
Environmental exposure. Radiation, especially to the chest wall (typically as a treatment for Hodgkin’s lymphoma) increases a woman’s risk for breast cancer, particularly if the exposure occurred when she was between the ages of 10 and 30.14
Lifestyle factors. Obesity, particularly in postmenopausal women, and alcohol consumption of more than a drink or two per day are both associated with an increased risk.4
Genetic mutations and breast cancer risk
An estimated 5% to 10% of breast cancers are inherited.5 Genetic susceptibility is generally transmitted as an autosomal dominant trait.
There are 2 known breast cancer genes, BRCA1 and BRCA2, located on the long arm of chromosomes 17 and 13, respectively. The genes themselves encode tumor suppressor proteins. Mutations in these genes impair the DNA repair process, resulting in increased risk.8
The chance of carrying a mutation in either BRCA1 or BRCA2 is estimated at one in 500 to 800 in women of Northern/Western European descent. Among Ashkenazi Jews, however, the frequency is about one in 50.5
A thorough family history that takes into account both the number of affected relatives and their age at diagnosis (TABLE 2)8,15 is helpful in determining whether a patient is at low, high, or very high risk of carrying a genetic mutation. Women who have no first-degree relative with breast cancer—or a relative who was diagnosed with breast cancer after age 50—are at low risk, while those with at least one first-degree relative diagnosed with breast cancer before the age of 50 would be categorized as high risk.
A woman with a family history of early-onset breast or ovarian cancer or a relative who developed both breast and ovarian cancer, bilateral breast cancer, or male breast cancer would be classified as very high risk for a genetic mutation, as would a patient with 2 or more family members affected by breast or ovarian cancer.
Ashkenazi Jewish heritage and a relative who was diagnosed with ovarian or breast cancer indicate an increased likelihood of a BRCA mutation, as well.8 (Other genetic conditions, with mutations that are distinct from the BRCA genes, have also been linked to breast cancer, but occur less frequently.)
BRCA gene testing can confirm very high risk status, prompting the initiation of preventive measures and facilitating early detection. Such testing can also identify—and relieve the anxiety of—noncarriers in high-risk families. Recently published guidelines from the US Preventive Services Task Force (USPSTF) support testing in women with suspicious family histories with a grade B recommendation, indicating that there is at least fair evidence that testing improves important health outcomes and that the benefits of testing outweigh the harms.15
The downside of specific BRCA gene testing for patients who find that they do not have this genetic mutation may include a false sense of security and the failure to identify any other genetic mutations. Patients who learn that they do carry a BRCA gene mutation could face psychosocial or economic harm associated with aggressive surveillance and surgical intervention.5
TABLE 2
Genetic counseling for patients at high risk8,15
Tools can quantify 5-year, 10-year, and lifetime risk
A number of breast cancer risk assessment tools have been developed to help clinicians individualize patient care. None provides the basis for an all-encompassing approach to breast cancer risk or a comprehensive patient discussion of preventive strategies. We have found that, when used in combination, 2 or more predictive models can complement each other and guide the development of a targeted risk reduction approach.
When to use a predictive tool
It is not necessary to use a predictive model for patients at low risk for breast cancer. The tools detailed in TABLE 3 5,14,16-23 are better suited to women who have a suspicious family history, a history of precancerous breast lesions, or known reproductive risks. Although each model has limitations, it is important that you have a working knowledge of circumstances that favor one tool over another. For instance, the Gail model, the most widely used, can help determine if a particular patient is a candidate for chemoprevention.16-20 Others, such as the Tyrer-Cuzick model14,21,22 and the Claus model,14,23 are useful in deciding whether a patient is a candidate for breast magnetic resonance imaging (MRI) as an adjunct to mammography screening. Another useful tool is the BRCAPRO, which is used primarily by genetic counselors to assess the likelihood that a patient carries a BRCA1 or BRCA2 mutation and would benefit from genetic testing.4,5
TABLE 3
Breast cancer risk assessment tools: What you need to know5,14,16-23
| Tool | Intended use | Criteria considered | Results | Limitations | Validation | How to access |
|---|---|---|---|---|---|---|
| Gail model | Assess eligibility for chemoprevention in women >35 years | Reproductive history, history of breast biopsies, first-degree relatives with breast cancer | Estimates 5-year and lifetime risk for invasive breast cancer | Can overestimate risk in patients with previous biopsy and atypical hyperplasia results and family history | Validated in independent projects; widely used to define excess risk; modified model for minorities validated | Available at http://www.cancer.gov/bcrisktool/ |
| Tyrer-Cuzick* model | Assess need for breast MRI | Hormonal and reproductive history, history of breast biopsies, number and age of onset of first- and second-degree relatives with breast cancer | Estimates 10-year and lifetime risk for invasive breast cancer | Potential for significant overestimation of risk in patients with atypical hyperplasia findings on breast biopsy | Not validated | Go to http://www.ems-trials.org/riskevaluator Click on “software downloads” to select the appropriate version |
| Claus model | Assess need for breast MRI | Age of onset of first- and second-degree relatives with history of breast cancer | Estimates incremental 10-year and lifetime risk for invasive breast cancer | Looks only at family history, without considering hormonal or reproductive risk factors | Validation does not extend to minorities | Tables found in Cancer (1994;73:643-651) available at no charge from http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1097-0142/issues |
| BRCAPRO | Determine whether genetic testing is indicated | Family history of breast and ovarian cancer | Estimates likelihood of genetic mutation | Time-consuming; requires highly detailed family history | Validation does not extend to minorities | Not widely available; used primarily by genetic counselors |
| *Also known as the IBIS model. IBIS, International Breast Cancer Intervention Study; MRI, magnetic resonance imaging. | ||||||
Managing patients at all risk levels
Although patients with average, high, or very high risk will all be managed differently, evidence suggests that lifestyle modification as needed, imaging, and chemoprevention, in some cases, can reduce the likelihood of breast cancer for women at all levels of risk.24
For women with an average risk (a 5-year Gail model score ≤1.66% and no significant family history),19 a discussion of the benefits and risks, as well as the limitations, of annual screening mammography beginning at age 40 vs age 50 is in order. Several major organizations, the American College of Obstetricians and Gynecologists25 and American Cancer Society (ACS)16 among them, have guidelines that support annual mammography beginning at 40 years but do not specify at what age to discontinue screening. In contrast, the USPSTF26 recommends biennial mammography between the ages of 50 and 74 years (See “The mammography controvrsy: When should you screen?” J Fam Pract. 2011;60:524-531).
How to proceed? Talk to patients in the 40- to 50-year age range about the benefits and risks of earlier, more frequent screening vs waiting until 50 to start mammography and opting for screening every 2 years. Breast health awareness and the role of clinical breast exams also should be included in a balanced discussion of early detection of breast cancer. A review of the patient’s reproductive status and use of hormone preparations is appropriate, as well.4,5
Patients at high risk (a Gail model score >1.66%; a history of ADH, ALH, or lobular carcinoma in situ; or a family history of breast cancer)3 should be advised to have a clinical breast exam every 6 months and annual mammograms. High-risk patients should also be offered the option of chemoprevention with tamoxifen, raloxifene,27,28 or exemestane29 if the benefits of treatment outweigh the risk of potential adverse effects. The merits of MRI breast surveillance have not been defined for women with this level of risk.14
For very high-risk patients (those with a family history that strongly suggests a genetic predisposition, a confirmed gene mutation, evidence of hereditary breast and ovarian cancer, or a personal history of chest wall irradiation between the ages of 10 and 30 years), a discussion of more aggressive risk-reduction strategies is recommended.4 A clinical breast exam and mammogram should be performed beginning at age 25—or 5 to 10 years before the earliest age at which a first-degree relative was diagnosed.
Starting at age 30, patients at very high risk should undergo annual mammography and breast MRI, either simultaneously or staggered every 6 months, along with a twice-yearly clinical breast exam.14 Breast health awareness and lifestyle modification should be emphasized, and the benefits and risks of chemoprevention should be discussed. Surgical risk-reduction strategies, such as prophylactic mastectomy and oophorectomy, should also be discussed, along with the offer of a referral to a surgeon for consultation.5
What to tell patients about chemoprevention
The USPSTF has issued a grade B recommendation to a discussion of chemoprevention for women who are at high risk for breast cancer and low risk for an adverse event.30 Counseling a patient regarding the risks and benefits of chemoprevention will depend on her age, comorbidities, whether or not she has had a hysterectomy, and her willingness to take the suggested medication.
Selective estrogen receptor modulators (SERMs). The American Society of Clinical Oncology Clinical Practice Guideline Update has reviewed the benefits and potential adverse effects of the SERMs tamoxifen and raloxifene. The Society supports the use of tamoxifen in pre- and postmenopausal women for breast cancer risk reduction; it also supports the use of raloxifene for postmenopausal women, the only patient population for which raloxifene has been approved.27
In a review of 7 placebo-controlled, randomized clinical trials and one head-to-head trial, both drugs reduced the risk for invasive, estrogen receptor–positive breast cancer by about 40% compared with placebo. Breast cancer deaths, however, did not decrease.31
Both tamoxifen and raloxifene were found to increase bone mineral density and reduce fracture risk.31 Thromboembolic events—which occurred less frequently with raloxifene than tamoxifen—was the chief adverse effect, with an incidence of 0.4% to 0.7%. In addition, fewer cases of endometrial cancer were reported with raloxifene compared with tamoxifen, making raloxifene the preferred treatment for postmenopausal women with an intact uterus.31
The National Surgical Adjuvant Breast and Bowel Project STAR study—one of the trials included in the review—initially reported that tamoxifen and raloxifene were equivalent in reducing breast cancer risk in postmenopausal women at increased risk.28 In an updated analysis based on 81 months of use, however, tamoxifen resulted in a 50% reduction in the incidence of breast cancer vs a reduction of 38% for raloxifene.32
The greater reduction in breast cancer risk seen with tamoxifen comes at a potential cost. Tamoxifen was found to have a worse adverse effect profile, leading to a higher risk for endometrial hyperplasia and hysterectomy, as well as thromboembolic events. The difference in all-cause mortality, however, was not statistically significant.32
Aromatase inhibitor therapy. The National Cancer Institute of Canada recently published a major chemoprevention trial, evaluating the effectiveness of aromatase inhibition in breast cancer risk reduction.29 This randomized, double-blind trial of exemestane vs placebo included more than 4500 women with a median follow-up of 3 years, and found that the exemestane reduced the incidence of invasive breast cancer in postmenopausal women at moderate risk by 65% (hazard ratio=0.35; 95% confidence interval, 0.18-0.70; P=.002).29
IBIS-II, a multicenter study in the United Kingdom, randomly assigned 6000 women at increased risk for breast cancer to placebo or anastrozole, an alternative aromatase inhibitor. This trial is ongoing, and breast cancer incidence is the primary endpoint.33 Aromatase inhibitors have not been approved by the US Food and Drug Administration for breast cancer prevention.34
Imaging strategies for those at risk
Although there is evidence that mammography performed on postmenopausal women can reduce breast cancer mortality by 25%, there are known limitations to this detection method.14
One drawback is that in premenopausal women, breast density lowers mammography’s sensitivity. In addition, several studies have found that mammography has a low sensitivity for detecting tumors in patients with a BRCA mutation. This has led to the use of other imaging modalities, especially MRI, for women with a family history that suggests a genetic predisposition.
The first study to demonstrate the superior sensitivity of MRI for detecting invasive breast cancer compared with clinical breast exam and mammography was published in 2004.35 A few years later, the ACS issued guidelines that call for surveillance with MRI as an adjunct to mammography, starting at age 30, for women whose family history, carrier status, or history of chest wall radiation puts them at very high risk (ie, a lifetime risk >20%-25%).14
The ACS found insufficient evidence to recommend for or against breast MRI for women with a lifetime risk of 15% to 20% (or documented high-risk lesions such as lobular carcinoma in situ, ALH, or ADH). Mammographic density, which in itself is a strong risk factor for the development of breast cancer, was not determined to be an indication for MRI screening. In deciding whether MRI is indicated for any high-risk patient, the cost, quality of imaging, and lower specificity must be considered.14
Weighing the benefits of surgery
For women who have a strong family history of breast cancer or are known carriers of a BRCA1 or BRCA gene mutation, the already high risk of developing breast cancer increases as they age. Prophylactic surgery—risk-reduction mastectomy (RRM) and/or bilateral salpingo-oophorectomy (RRSO)—has been found to lower the risk.5,36,37
RRM can reduce the risk of breast cancer by as much as 90% for such patients;38,39 RRSO yields similar results, reducing the risk of ovarian cancer by 80% to 95% and the risk of breast cancer by 40% to 59%, provided the surgery is performed before the patient is 40 years old.36,37
These potential benefits must be weighed against the harm associated with surgically induced menopause, with the attendant risks of cardiovascular disease, osteoporosis, and menopausal symptoms.40 Notably, hormone therapy use after RRSO in women with a gene mutation has not been found to increase the risk of breast cancer. In fact, it may be associated with a decreased risk.5 In general, short-term use of low-dose estrogen—up to the age of 51 or 52 years—is considered to be safe for this population,41,42 but long-term data on breast cancer risk are lacking.
CORRESPONDENCE
Marcia G. Ko, MD, Mayo Clinic, 13737 North 92nd Street, Scottsdale, AZ 85369; ko.marcia@mayo.edu
1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300.
2. Smith R. Risk-based screening for breast cancer: is there a practical strategy? Semin Breast Dis. 1999;2:280-291.
3. Guerra CE, Sherman M, Armstrong K. Diffusion of breast cancer risk assessment in primary care. J Am Board Fam Med. 2009;22:272-279.
4. Pruthi S, Brandt KR, Degnim AC, et al. A multidisciplinary approach to the management of breast cancer, part 1: prevention and diagnosis. Mayo Clin Proc. 2007;82:999-1012.
5. Pruthi S, Gostout BS, Lindor NM. Identification and management of women with BRCA mutations or hereditary predisposition for breast and ovarian cancer. Mayo Clin Proc. 2010;85:1111-1120.
6. Rossouw J, Anderson G, Prentice R, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
7. Hartmann LC, Sellers TA, Frost MH, et al. Benign breast disease and the risk of breast cancer. N Engl J Med. 2005;353:229-237.
8. Daly MB, Axilbund JE, Buys S, et al. Genetic/familial high-risk assessment: breast and ovarian. J Natl Compr Canc Netw. 2010;8:562-594.
9. McPherson K, Steel CM, Dixon JM. ABC of breast diseases. BMJ. 2000;321:624-628.
10. Brinton LA, Schairer C, Hoover RN, et al. Menstrual factors and risk of breast cancer. Cancer Invest. 1988;6:245-254.
11. Apter D, Reinilä M, Vihko R. Some endocrine characteristics of early menarche, a risk factor for breast cancer, are preserved into adulthood. Int J Cancer. 1989;44:783-787.
12. Rebbeck TR, Lynch HT, Neuhausen SL, et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med. 2002;346:1616-1622.
13. Narod SA, Brunet JS, Ghadirian P, et al. Tamoxifen and risk of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers: a case-control study. Lancet. 2000;356:1876-1881.
14. Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
15. U.S. Preventive Services Task Force. Genetic risk assessment and BRCA mutation testing for breast and ovarian cancer susceptibility. Ann Intern Med. 2005;143:355-361.
16. Gail M, Benichou J. Validation studies on a model for breast cancer risk. J Natl Cancer Inst. 1994;86:573-575.
17. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 2005;97:1652-1662.
18. Pankratz VS, Hartmann LC, Degnim AC, et al. Assessment of the accuracy of the Gail model in women with atypical hyperplasia. J Clin Oncol. 2008;26:5374-5379.
19. Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879-1886.
20. Vogel VG, Costantino JP, Wickerham DL, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: preventing breast cancer. Cancer Prev Res (Phila). 2010;3:696-706.
21. Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23:1111-1130.
22. Boughey JC, Hartmann LC, Anderson SS, et al. Evaluation of the Tyrer-Cuzick (International Breast Cancer Intervention Study) model for breast cancer risk prediction in women with atypical hyperplasia. J Clin Oncol. 2010;28:3591-3596.
23. Claus EB, Risch N, Thompson W. Autosomal dominant inheritance of early-onset breast cancer. Cancer. 1994;73:643-651.
24. Cummings SR, Tice JA, Bauer S, et al. Prevention of breast cancer in postmenopausal women. J Natl Cancer Inst. 2009;101:384-398.
25. American College of Obstetricians-Gynecologists. Practice bulletin no. 122: Breast cancer screening. Obstet Gynecol. 2011;118(2 pt 1):372-382.
26. U.S. Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716-726.
27. Visvanathan K, Chlebowski R, Hurley P, et al. American Society of Clinical Oncology clinical practice guideline update on the use of pharmacologic interventions including tamoxifen, raloxifene, and aromatase inhibition for breast cancer risk reduction. J Clin Oncol. 2009;27:3235-3258.
28. Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
29. Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381-2391.
30. U.S. Preventive Services Task Force. Chemoprevention of breast cancer. Ann Intern Med. 2002;137:56-58.
31. Nelson HD, Fu R, Griffin JC, et al. Systematic review: comparative effectiveness of medications to reduce risk for primary breast cancer. Ann Intern Med. 2009;151:703-715.
32. Vogel VG, Costantino JP, Wickerham DL, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: preventing breast cancer. Cancer Prev Res (Phila). 2010;3:696-706.
33. Dunn BK, Ryan A. Phase 3 trials of aromatase inhibitors for breast cancer prevention. Ann N Y Acad Sci. 2009;1155:141-161.
34. National Cancer Institute. Hormone therapy for breast cancer. Reviewed April 11, 2012. Available at: http://www.cancer.gov/cancertopics/factsheet/Therapy/hormone-therapy-breast. Accessed May 18, 2012.
35. Kriege M, Brekelmans CTM, Boetes C, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351:427-437.
36. Domchek SM, Friebel TM, Singer CF, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304:967-975.
37. Eisen A, Lubinski J, Klijn J, et al. Breast cancer risk following bilateral oophorectomy in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2005;23:7491-7496.
38. Rebbeck TR, Friebel T, Lynch HT, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2004;22:1055-1062.
39. Hartmann LC, Schaid DJ, Woods JE, et al. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N Engl J Med. 1999;340:77-84.
40. Shuster LT, Gostout BS, Grossardt BR, Rocca WA. Prophylactic oophorectomy in premenopausal women and long-term health. Menopause Int. 2008;14:111-116.
41. Armstrong K, Schwartz JS, Randall T, et al. Hormone replacement therapy and life expectancy after prophylactic oophorectomy in women with BRCA1/2 mutations: a decision analysis. J Clin Oncol. 2004;22:1045-1054.
42. Eisen A, Lubinski J, Gronwald J, et al. Hormone therapy and the risk of breast cancer in BRCA1 mutation carriers. J Natl Cancer Inst. 2008;100:1361-1367.
1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300.
2. Smith R. Risk-based screening for breast cancer: is there a practical strategy? Semin Breast Dis. 1999;2:280-291.
3. Guerra CE, Sherman M, Armstrong K. Diffusion of breast cancer risk assessment in primary care. J Am Board Fam Med. 2009;22:272-279.
4. Pruthi S, Brandt KR, Degnim AC, et al. A multidisciplinary approach to the management of breast cancer, part 1: prevention and diagnosis. Mayo Clin Proc. 2007;82:999-1012.
5. Pruthi S, Gostout BS, Lindor NM. Identification and management of women with BRCA mutations or hereditary predisposition for breast and ovarian cancer. Mayo Clin Proc. 2010;85:1111-1120.
6. Rossouw J, Anderson G, Prentice R, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
7. Hartmann LC, Sellers TA, Frost MH, et al. Benign breast disease and the risk of breast cancer. N Engl J Med. 2005;353:229-237.
8. Daly MB, Axilbund JE, Buys S, et al. Genetic/familial high-risk assessment: breast and ovarian. J Natl Compr Canc Netw. 2010;8:562-594.
9. McPherson K, Steel CM, Dixon JM. ABC of breast diseases. BMJ. 2000;321:624-628.
10. Brinton LA, Schairer C, Hoover RN, et al. Menstrual factors and risk of breast cancer. Cancer Invest. 1988;6:245-254.
11. Apter D, Reinilä M, Vihko R. Some endocrine characteristics of early menarche, a risk factor for breast cancer, are preserved into adulthood. Int J Cancer. 1989;44:783-787.
12. Rebbeck TR, Lynch HT, Neuhausen SL, et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med. 2002;346:1616-1622.
13. Narod SA, Brunet JS, Ghadirian P, et al. Tamoxifen and risk of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers: a case-control study. Lancet. 2000;356:1876-1881.
14. Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
15. U.S. Preventive Services Task Force. Genetic risk assessment and BRCA mutation testing for breast and ovarian cancer susceptibility. Ann Intern Med. 2005;143:355-361.
16. Gail M, Benichou J. Validation studies on a model for breast cancer risk. J Natl Cancer Inst. 1994;86:573-575.
17. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 2005;97:1652-1662.
18. Pankratz VS, Hartmann LC, Degnim AC, et al. Assessment of the accuracy of the Gail model in women with atypical hyperplasia. J Clin Oncol. 2008;26:5374-5379.
19. Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879-1886.
20. Vogel VG, Costantino JP, Wickerham DL, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: preventing breast cancer. Cancer Prev Res (Phila). 2010;3:696-706.
21. Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23:1111-1130.
22. Boughey JC, Hartmann LC, Anderson SS, et al. Evaluation of the Tyrer-Cuzick (International Breast Cancer Intervention Study) model for breast cancer risk prediction in women with atypical hyperplasia. J Clin Oncol. 2010;28:3591-3596.
23. Claus EB, Risch N, Thompson W. Autosomal dominant inheritance of early-onset breast cancer. Cancer. 1994;73:643-651.
24. Cummings SR, Tice JA, Bauer S, et al. Prevention of breast cancer in postmenopausal women. J Natl Cancer Inst. 2009;101:384-398.
25. American College of Obstetricians-Gynecologists. Practice bulletin no. 122: Breast cancer screening. Obstet Gynecol. 2011;118(2 pt 1):372-382.
26. U.S. Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716-726.
27. Visvanathan K, Chlebowski R, Hurley P, et al. American Society of Clinical Oncology clinical practice guideline update on the use of pharmacologic interventions including tamoxifen, raloxifene, and aromatase inhibition for breast cancer risk reduction. J Clin Oncol. 2009;27:3235-3258.
28. Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727-2741.
29. Goss PE, Ingle JN, Alés-Martínez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381-2391.
30. U.S. Preventive Services Task Force. Chemoprevention of breast cancer. Ann Intern Med. 2002;137:56-58.
31. Nelson HD, Fu R, Griffin JC, et al. Systematic review: comparative effectiveness of medications to reduce risk for primary breast cancer. Ann Intern Med. 2009;151:703-715.
32. Vogel VG, Costantino JP, Wickerham DL, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: preventing breast cancer. Cancer Prev Res (Phila). 2010;3:696-706.
33. Dunn BK, Ryan A. Phase 3 trials of aromatase inhibitors for breast cancer prevention. Ann N Y Acad Sci. 2009;1155:141-161.
34. National Cancer Institute. Hormone therapy for breast cancer. Reviewed April 11, 2012. Available at: http://www.cancer.gov/cancertopics/factsheet/Therapy/hormone-therapy-breast. Accessed May 18, 2012.
35. Kriege M, Brekelmans CTM, Boetes C, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351:427-437.
36. Domchek SM, Friebel TM, Singer CF, et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA. 2010;304:967-975.
37. Eisen A, Lubinski J, Klijn J, et al. Breast cancer risk following bilateral oophorectomy in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2005;23:7491-7496.
38. Rebbeck TR, Friebel T, Lynch HT, et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2004;22:1055-1062.
39. Hartmann LC, Schaid DJ, Woods JE, et al. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N Engl J Med. 1999;340:77-84.
40. Shuster LT, Gostout BS, Grossardt BR, Rocca WA. Prophylactic oophorectomy in premenopausal women and long-term health. Menopause Int. 2008;14:111-116.
41. Armstrong K, Schwartz JS, Randall T, et al. Hormone replacement therapy and life expectancy after prophylactic oophorectomy in women with BRCA1/2 mutations: a decision analysis. J Clin Oncol. 2004;22:1045-1054.
42. Eisen A, Lubinski J, Gronwald J, et al. Hormone therapy and the risk of breast cancer in BRCA1 mutation carriers. J Natl Cancer Inst. 2008;100:1361-1367.
;
;
An anticoagulation option for nonvalvular atrial fibrillation
• Consider dabigatran as an alternative to warfarin for patients with nonvalvular paroxysmal or permanent atrial fibrillation and risk factors for stroke. A
• Avoid using dabigatran with patients who have a creatinine clearance <15 mL/min, a prosthetic heart valve, or hemodynamically significant valve disease. C
• Withhold dabigatran for at least 24 hours before planned surgery, or for a longer time if there is renal insufficiency or the procedure is high risk. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
There are an estimated 2.3 million cases of atrial fibrillation (AF) in the United States, and that number may increase to 5.6 million by the year 2050.1 The stasis of blood during AF, in addition to proinflammatory factors, predisposes patients to clot formation in the left atrium, especially in the left atrial appendage. In 5% of AF patients each year, such a thrombus dislodges and causes a stroke, a rate 2 to 7 times higher than that of people without AF.1-3 Patients with paroxysmal or permanent AF have similar risks of stroke.4
Stratifying stroke risk aids in treatment decisions. Multiple criteria have been devised to identify AF patients at a higher risk of stroke. The CHADS2 risk index, used extensively in clinical settings, stratifies risk according to a cumulative score based on a patient’s risk factors (TABLE 1).5 A joint 2006 guideline released by the American College of Cardiology, American Heart Association, and European Society of Cardiology,1 and a separate 2008 guideline by the American College of Chest Physicians6 recommend that patients with a CHADS2 score of ≥2 be treated with a vitamin K antagonist such as warfarin, while patients with a score of 1 may be treated with either antiplatelet or anticoagulant therapy.
The evidence behind the guidelines. These guidelines are based on a number of randomized clinical trials that demonstrated the superiority of dose-adjusted warfarin in preventing stroke compared with placebo: Stroke Prevention in Atrial Fibrillation (SPAF), Boston Area Anticoagulation Trial for Atrial Fibrillation (BAATAF), Copenhagen Atrial Fibrillation Aspirin Anticoagulation (AFASAK), Canadian Atrial Fibrillation Anticoagulation (CAFA), Stroke Prevention in Nonrheumatic Atrial Fibrillation (SPINAF), and European Atrial Fibrillation Trial (EAFT).7-12
Further support for anticoagulant therapy. In a meta-analysis conducted after release of the guidelines, dose-adjusted warfarin was associated with a 62% risk reduction for stroke vs placebo, and a 39% risk reduction vs antiplatelet agents.13 For high-risk patients in the SPAF III trial, dose-adjusted warfarin led to a 76% risk reduction of stroke and systemic embolism compared with combination therapy of aspirin and low-intensity fixed-dose warfarin.14 The Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE-W) trial was stopped prematurely when it demonstrated that, in patients with AF who have one or more risk factors for stroke, warfarin was superior to the combination of aspirin and clopidogrel in preventing a combined end point of stroke, non-CNS systemic embolism, myocardial infarction, and vascular death; secondary outcomes of stroke were also more favorable with warfarin.15 The results of all 3 studies were noted during a follow-up of 1 to 2 years. In clinical practice, patients must continue antithrombotic agents for a much longer period.
Disadvantages of long-term warfarin use. The main drawback of warfarin therapy is the need for frequent laboratory monitoring. It also interacts unfavorably with other drugs and with certain foods. These factors often lead to patient discontinuation of therapy or to inadequate anticoagulation even when patients are compliant.16 A meta-analysis of 67 clinical studies showed that, regardless of the setting of anticoagulation management with warfarin, the international normalization ratio (INR) was in the therapeutic range only 64% of the time.17 These issues with warfarin have increased interest in developing novel oral anticoagulants that have better drug profiles. An oral direct thrombin inhibitor, ximelagatran, was shown to be as effective as warfarin in the Stroke Prevention Using an Oral Thrombin Inhibitor in Atrial Fibrillation (SPORTIF) V trial,18 but it was associated with hepatotoxicity and did not receive US Food and Drug Administration (FDA) approval.
However, another thrombin inhibitor, dabigatran, was approved by the FDA for anticoagulation in nonvalvular AF, and has been incorporated into the ACCF/AHA/HRS guidelines as a therapeutic option.19 Since this article was submitted for publication, rivaroxaban, an oral factor Xa inhibitor, was approved by the FDA for anticoagulation in AF, based on results of the study Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET-AF).20
TABLE 1
CHADS2 score for stratifying risk of stroke in a patient with nonvalvular atrial fibrillation5
| Risk factor | Score |
|---|---|
| CHF (reduced EF%) | 1 |
| Hypertension | 1 |
| Age ≥75 years | 1 |
| Diabetes mellitus | 1 |
| Stroke/TIA | 2 |
| TOTAL | |
| CHADS2 score | Treatment considerations1,19,20 |
| 0 | Withhold treatment, or give aspirin |
| 1 | Give an antiplatelet or anticoagulant |
| ≥2 | Give an oral anticoagulant such as warfarin, dabigatran, or rivaroxaban |
| CHADS2, acronym comprising initial letters of risk factors listed; CHF, congestive heart failure; EF, ejection fraction; TIA, transient ischemic attack. | |
Dabigatran as an option for nonvalvular AF
Dabigatran’s approval was based on the clinical outcomes of the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) study.21 This multicenter randomized noninferiority trial compared warfarin with 2 doses of dabigatran (110 and 150 mg twice daily) in patients who had AF and a risk of stroke. A total of 18,113 patients with AF, a mean age of 71 years, and a mean CHADS2 score of 2.1 were randomly assigned in a blinded fashion to receive one of the dabigatran doses or, in nonblinded fashion, warfarin. The primary outcome was stroke or systemic embolism. The primary safety outcome was major bleeding defined as a reduction in the hemoglobin level of at least 20 g/L, a need for transfusion of at least 2 units of blood, or symptomatic bleeding in a critical area or organ. The mean follow-up period was 2 years.
The study showed that 110 mg dabigatran twice daily was statistically not inferior to warfarin in preventing stroke and systemic embolism (1.53% vs 1.69% per year; P<.001). In addition, this dose was associated with statistically lower rates of major bleeding (2.71% vs 3.36% per year; P=.003). However, dabigatran 150 mg twice daily was statistically superior to warfarin in reducing the risk of stroke and systemic embolism by 34% per year (1.11% vs 1.69%; P<.001) with rates of major bleeding similar to warfarin (3.11% vs 3.36% per year; P=.31). The beneficial effect of dabigatran was also seen in patients with higher CHADS2 scores of 3 to 6, who comprised one-third of the study population and were at higher risk of stroke. Interestingly, both doses of dabigatran were associated with lower rates of intracranial hemorrhage than was warfarin. The 110-mg dose of dabigatran, however, was not approved by the FDA.
A higher incidence of myocardial infarction (MI) occurred in the dabigatran group compared with warfarin, but it was not statistically significant.21,22 A recent meta-analysis of 7 randomized controlled trials, including RE-LY, found that dabigatran was significantly associated with a higher incidence of MI or acute coronary syndrome compared with heterogeneous control groups receiving placebo, warfarin, or enoxaparin (1.19% vs 0.79%, odds ratio, 1.33; P=.03).23
The exact reason for the difference is unknown. It may be due to a chance effect, given that the absolute number of events was small. Or warfarin may exert a protective effect against MI, as was seen in the WARIS II study, wherein warfarin, given alone or in combination with aspirin, was superior to aspirin in reducing the risk of reinfarction.24 However, a true adverse effect of dabigatran cannot be ruled out. If it proves to be the case, 2 more cases of MI can be expected to occur in 1000 patients treated with dabigatran, compared with warfarin, at 1 year.
In addition, there was a statistically significant higher incidence of major gastrointestinal hemorrhage with dabigatran 150 mg twice daily compared with warfarin. Most of these bleeding events occurred in the lower gastrointestinal tract. Here, too, the exact reason for the difference is unknown.
How dabigatran prevents thrombus formation
Dabigatran directly and competitively inhibits both free and fibrin-bound thrombin, thereby preventing thrombin-mediated effects on the coagulation cascade, including cleavage of fibrinogen to fibrin, activation of factors V, VIII, XI, and XII, and thrombin-induced platelet aggregation.25-28
The drug’s pharmacokinetic profile. Dabigatran is given as a prodrug, dabigatran etexilate. Serum esterase converts it to its active form. Peak concentration is reached within 2 to 3 hours of oral dosing, and its half-life is 12 to 17 hours. It is taken twice daily, mornings and evenings. The drug is excreted unchanged, primarily by the kidneys (~80%); the remainder is metabolized by the liver. Therefore, dabigatran is contraindicated in patients with severe renal dysfunction (creatinine clearance <15 mL/min). Compared with warfarin, dabigatran has a more predictable anticoagulant function, no need for laboratory monitoring, and less interaction with other drugs and foods (TABLE 2).29-32 No data are available regarding heterogenous genetic response to dabigatran.
TABLE 2
How warfarin and dabigatran compare pharmacologically29-32
| Attribute | Warfarin | Dabigatran |
|---|---|---|
| Administration | Oral | Oral |
| Mechanism of action | Inhibition of vitamin-K-dependent coagulation factors (II, VII, IX, X, and protein C and S) | Inhibition of thrombin |
| Oral bioavailability | 100% | 6.5% |
| Half-life | 20-60 hours | 12-17 hours |
| Metabolism | Hepatic | Renal (80%) |
| Time to onset | 24-72 hours | 1-2 hours |
| Protein binding | 99% | 35% |
| Antagonist | Vitamin K | None |
| Laboratory monitoring | Required | None required |
| Dose adjustment | Required for each individual | Reduction only for creatinine clearance of 15-30 mL/min |
| Interaction with diet | Interacts with foods rich in vitamin K (eg, cabbage, spinach) | No interaction with foods rich in vitamin K |
| Interaction with drugs | Interacts with amiodarone, antifungals, antibiotics, and alcohol, which may require dose adjustments of either warfarin or the concomitant agent | Dose adjustment of dabigatran may be required with ketoconazole and dronedarone |
Cost-effectiveness of dabigatran
The prescription cost of dabigatran is a lot higher than warfarin, although a recent study demonstrated its cost-effectiveness through a reduction in the need for laboratory monitoring and decreased complications due to over-and under-anticoagulation.33
Factors that come into play
Dabigatran is an alternative to warfarin for long-term anticoagulation in patients with nonvalvular AF who are at a higher risk of stroke with a CHADS2 score of ≥1 or systemic thromboembolism.18 While the main benefits of dabigatran are a quick onset of action, no need for laboratory monitoring, rare interactions with drugs and food, and a decrease in intracranial bleeding compared with warfarin, it did cause more gastrointestinal adverse effects, including bleeding, than warfarin in the RE-LY trial.
Dabigitran was also associated with a higher incidence of MI in RE-LY and an increased risk of MI or acute coronary syndrome in the meta-analysis, but the absolute risk increase in both cases was very small.21-23 Thus, for many patients, the choice of anticoagulant depends on individual preference and ability to comply with a twice-daily dosing regimen, availability of INR monitoring, and cost of treatment.34
Patients who should not receive dabigatran
Dabigatran is contraindicated for patients with a creatinine clearance <15 mL/min, a prosthetic valve, significant valve disease, a history of serious allergic reaction to the drug, or a high risk of bleeding (eg, from recurrent falls, bleeding peptic ulcer).35
Initiating dabigatran therapy
Start dabigatran at a dose of 150 mg twice daily if the creatinine clearance is >30 mL/min, or at 75 mg twice daily if creatinine clearance is 15 to 30 mL/min. In switching a patient from parenteral anticoagulation, you may start dabigatran ≤2 hours before the next scheduled dose of the parenteral agent (eg, low-molecular-weight heparin) or the termination of a continuously administered agent (eg, unfractionated heparin). For patients taking warfarin, withhold dabigatran until the INR is <2.29
Thrombin time is the most reliable measure of drug effect
Dabigatran has a variable and unpredictable effect on the INR, which should not be used to measure the drug’s anticoagulation effect. While therapeutic concentrations modestly elevate the INR, there have been some reports of significant INR elevation.29 However, lab results with the ecarin clotting test (ECT) or thrombin time (TT) correlate well with dabigatran serum concentrations. ECT is primarily a research tool and not commonly available in hospitals; TT, however, is readily available. Activated partial thromboplastin time (aPTT), also commonly available, is prolonged in a nonlinear fashion with dabigatran use. None of these tests has been systematically studied and correlated with clinical outcomes of dabigatran use.29
Adverse effects to watch for
In the RE-LY study, dyspepsia was the most commonly reported adverse effect of dabigatran (11%).21 As with warfarin, other adverse effects, such as dizziness, dyspnea, and fatigue, were reported for dabigatran. Unlike ximelgatran, there is no significant effect on liver enzymes. There is, however, a risk of major and minor bleeding complications.
Bleeding with dabigatran. In the event of a bleeding complication, discontinue dabigatran. There is no specific antidote for this drug; supportive therapy relies on surgical intervention and transfusion of fresh frozen plasma and packed cells. Maintaining adequate diuresis may enhance elimination of the drug. Given dabigatran’s low protein-binding potential, dialysis may be considered; however, data supporting this treatment decision are limited.29
Patients taking dual antiplatelet agents are at a higher risk of bleeding if they also receive either dabigatran or warfarin, although it is not known if one anticoagulant confers a higher risk than the other. In such patients, carefully weigh the risk of bleeding against the benefits of stroke prevention.
Discontinue dabigatran before surgery
Withhold dabigatran from patients scheduled for elective surgery (TABLE 3).29 For those with a high risk of bleeding, measure TT 6 to 12 hours before the procedure to ensure normalization of the value. An acceptable alternative measure, although less precise, is the aPTT. For emergency procedures, fresh frozen plasma may be used to acutely reverse the drug’s effect.
TABLE 3
Recommendations for withholding dabigatran before elective surgery29
| Renal function (creatinine clearance), mL/min | Estimated half-life (range), h | Discontinue dabigatran before surgery | |
|---|---|---|---|
| High risk of bleeding* | Standard risk | ||
| >50-80 | ~15 (12-17) | 2-3 days before | 24 hours before (2 doses) |
| 30-50 | ~18 (18-24) | 4 days before | At least 2 days (48 hours) before |
| <30 | ~27 (>24) | >5 days before | 2-5 days before |
| *Surgeries that confer a high risk of bleeding include, but are not limited to, cardiac surgery, neurosurgery, abdominal surgery, or procedures involving a major organ. Procedures involving spinal anesthesia or spinal tap may also be considered as having a high risk of bleeding | |||
CORRESPONDENCE Rajesh Kabra, MD, University of Tennessee Health Sciences Center, 1325 Eastmoreland Avenue, Suite 460, Memphis, TN 38104; rkabra@uthsc.edu
1. Fuster V, Rydén LE, Cannom DS, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines: Developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257-e354.
2. Kannel WB, Benjamin EJ. Status of the epidemiology of atrial fibrillation. Med Clin North Am. 2008;92:17-40, ix.
3. Hart RG, Halperin JL. Atrial fibrillation and thromboembolism: a decade of progress in stroke prevention. Ann Intern Med. 1999;131:688-695.
4. Hohnloser SH, Pajitnev D, Pogue J, et al. Incidence of stroke in paroxysmal versus sustained atrial fibrillation in patients taking oral anticoagulation or combined antiplatelet therapy: an ACTIVE W substudy. J Am Coll Cardiol. 2007;50:2156-2161.
5. Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864-2870.
6. Singer DE, Albers GW, Dalen JE, et al. Antithrombotic therapy in atrial fibrillation: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2008;133:546S-592S.
7. Stroke Prevention in Atrial Fibrillation Investigators. Stroke prevention in atrial fibrillation study. Final results. Circulation. 1991;84:527-539.
8. The Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators. The effect of low-dose warfarin on the risk of stroke in patients with nonrheumatic atrial fibrillation. N Engl J Med. 1990;323:1505-1511.
9. Petersen P, Boysen G, Godtfredsen J, et al. Placebo-controlled, randomized trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation. The Copenhagen AFASAK study. Lancet. 1989;1:175-179.
10. Connolly SJ, Laupacis A, Gent M, et al. Canadian Atrial Fibrillation Anticoagulation (CAFA) study. J Am Coll Cardiol. 1991;18:349-355.
11. Ezekowitz MD, Bridgers SL, James KE, et al. Warfarin in the prevention of stroke associated with nonrheumatic atrial fibrillation. Veterans Affairs Stroke Prevention in Nonrheumatic Atrial Fibrillation Investigators. N Engl J Med. 1992;327:1406-1412.
12. EAFT (European Atrial Fibrillation Trial) Study Group. Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. Lancet. 1993;342:1255-1262.
13. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857-867.
Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrillation: Stroke Prevention in Atrial Fibrillation III randomised clinical trial. Lancet. 1996;348:633-638.
15. Connolly S, Pogue J, Hart R, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006;367:1903-1912.
16. Connolly SJ, Pogue J, Eikelboom J, et al. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation. 2008;118:2029-2037.
17. Van Walraven C, Jennings A, Oake N, et al. Effect of study setting on anticoagulation control: a systematic review and metaregression. Chest. 2006;129:1155-1166.
18. Albers GW, Diener HC, Frison L, et al. Ximelagatran versus warfarin for stroke prevention in patients with nonvalvular atrial fibrillation (SPORTIF V study). JAMA. 2005;293:690-698.
19. Wann LS, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (update on dabigatran): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;57:1330-1337.
20. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-891.
21. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
22. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Newly identified events in the RE-LY trial. N Engl J Med. 2010;363:1875-1876.
23. Uchino K, Hernandez AV. Dabigatran association with higher risk of acute coronary events. Meta-analysis of noninferiority randomized controlled trials. Arch Intern Med. 2012;172:397-402.
24. Hurlen M, Abdelnoor M, Smith P, et al. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med. 2002;347:969-974.
25. Nutescu EA, Shapiro NL, Chevalier A. New anticoagulant agents: direct thrombin inhibitors. Cardiol Clin. 2008;26:169-187.
26. Alban S. Pharmacological strategies for inhibition of thrombin activity. Curr Pharm Des. 2008;14:1152-1175.
27. Mehta RS. Novel oral anticoagulants. Part II: direct thrombin inhibitors. Expert Rev Hematol. 2010;3:351-361.
28. Weber R, Diener HC, Weimar C. Prevention of cardioembolic stroke in patients with atrial fibrillation. Expert Rev Cardiovasc Ther. 2010;8:1405-1415.
29. Pradexa [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; 2010.
30. Strangier J. Clinical pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor of dabigatran etexilate. Clin Pharmacokinet. 2008;47:285-295.
31. Blech S, Ebner T, Ludwig-Schwellinger E, et al. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos. 2008;36:386-399.
32. Ma TK, Yan BP, Lam YY. Dabigatran etexilate versus warfarin as the oral anticoagulant of choice? A review of clinical data. Pharmacol Ther. 2011;129:185-194.
33. Freeman JV, Zhu RP, Owens DK, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med. 2011;154:1-11.
34. Gage BF. Can we rely on RE-LY? N Engl J Med. 2009;361:1200-1202.
35. Wann LS, Curtis AB, January CT, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (updating the 2006 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123:104-123.
• Consider dabigatran as an alternative to warfarin for patients with nonvalvular paroxysmal or permanent atrial fibrillation and risk factors for stroke. A
• Avoid using dabigatran with patients who have a creatinine clearance <15 mL/min, a prosthetic heart valve, or hemodynamically significant valve disease. C
• Withhold dabigatran for at least 24 hours before planned surgery, or for a longer time if there is renal insufficiency or the procedure is high risk. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
There are an estimated 2.3 million cases of atrial fibrillation (AF) in the United States, and that number may increase to 5.6 million by the year 2050.1 The stasis of blood during AF, in addition to proinflammatory factors, predisposes patients to clot formation in the left atrium, especially in the left atrial appendage. In 5% of AF patients each year, such a thrombus dislodges and causes a stroke, a rate 2 to 7 times higher than that of people without AF.1-3 Patients with paroxysmal or permanent AF have similar risks of stroke.4
Stratifying stroke risk aids in treatment decisions. Multiple criteria have been devised to identify AF patients at a higher risk of stroke. The CHADS2 risk index, used extensively in clinical settings, stratifies risk according to a cumulative score based on a patient’s risk factors (TABLE 1).5 A joint 2006 guideline released by the American College of Cardiology, American Heart Association, and European Society of Cardiology,1 and a separate 2008 guideline by the American College of Chest Physicians6 recommend that patients with a CHADS2 score of ≥2 be treated with a vitamin K antagonist such as warfarin, while patients with a score of 1 may be treated with either antiplatelet or anticoagulant therapy.
The evidence behind the guidelines. These guidelines are based on a number of randomized clinical trials that demonstrated the superiority of dose-adjusted warfarin in preventing stroke compared with placebo: Stroke Prevention in Atrial Fibrillation (SPAF), Boston Area Anticoagulation Trial for Atrial Fibrillation (BAATAF), Copenhagen Atrial Fibrillation Aspirin Anticoagulation (AFASAK), Canadian Atrial Fibrillation Anticoagulation (CAFA), Stroke Prevention in Nonrheumatic Atrial Fibrillation (SPINAF), and European Atrial Fibrillation Trial (EAFT).7-12
Further support for anticoagulant therapy. In a meta-analysis conducted after release of the guidelines, dose-adjusted warfarin was associated with a 62% risk reduction for stroke vs placebo, and a 39% risk reduction vs antiplatelet agents.13 For high-risk patients in the SPAF III trial, dose-adjusted warfarin led to a 76% risk reduction of stroke and systemic embolism compared with combination therapy of aspirin and low-intensity fixed-dose warfarin.14 The Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE-W) trial was stopped prematurely when it demonstrated that, in patients with AF who have one or more risk factors for stroke, warfarin was superior to the combination of aspirin and clopidogrel in preventing a combined end point of stroke, non-CNS systemic embolism, myocardial infarction, and vascular death; secondary outcomes of stroke were also more favorable with warfarin.15 The results of all 3 studies were noted during a follow-up of 1 to 2 years. In clinical practice, patients must continue antithrombotic agents for a much longer period.
Disadvantages of long-term warfarin use. The main drawback of warfarin therapy is the need for frequent laboratory monitoring. It also interacts unfavorably with other drugs and with certain foods. These factors often lead to patient discontinuation of therapy or to inadequate anticoagulation even when patients are compliant.16 A meta-analysis of 67 clinical studies showed that, regardless of the setting of anticoagulation management with warfarin, the international normalization ratio (INR) was in the therapeutic range only 64% of the time.17 These issues with warfarin have increased interest in developing novel oral anticoagulants that have better drug profiles. An oral direct thrombin inhibitor, ximelagatran, was shown to be as effective as warfarin in the Stroke Prevention Using an Oral Thrombin Inhibitor in Atrial Fibrillation (SPORTIF) V trial,18 but it was associated with hepatotoxicity and did not receive US Food and Drug Administration (FDA) approval.
However, another thrombin inhibitor, dabigatran, was approved by the FDA for anticoagulation in nonvalvular AF, and has been incorporated into the ACCF/AHA/HRS guidelines as a therapeutic option.19 Since this article was submitted for publication, rivaroxaban, an oral factor Xa inhibitor, was approved by the FDA for anticoagulation in AF, based on results of the study Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET-AF).20
TABLE 1
CHADS2 score for stratifying risk of stroke in a patient with nonvalvular atrial fibrillation5
| Risk factor | Score |
|---|---|
| CHF (reduced EF%) | 1 |
| Hypertension | 1 |
| Age ≥75 years | 1 |
| Diabetes mellitus | 1 |
| Stroke/TIA | 2 |
| TOTAL | |
| CHADS2 score | Treatment considerations1,19,20 |
| 0 | Withhold treatment, or give aspirin |
| 1 | Give an antiplatelet or anticoagulant |
| ≥2 | Give an oral anticoagulant such as warfarin, dabigatran, or rivaroxaban |
| CHADS2, acronym comprising initial letters of risk factors listed; CHF, congestive heart failure; EF, ejection fraction; TIA, transient ischemic attack. | |
Dabigatran as an option for nonvalvular AF
Dabigatran’s approval was based on the clinical outcomes of the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) study.21 This multicenter randomized noninferiority trial compared warfarin with 2 doses of dabigatran (110 and 150 mg twice daily) in patients who had AF and a risk of stroke. A total of 18,113 patients with AF, a mean age of 71 years, and a mean CHADS2 score of 2.1 were randomly assigned in a blinded fashion to receive one of the dabigatran doses or, in nonblinded fashion, warfarin. The primary outcome was stroke or systemic embolism. The primary safety outcome was major bleeding defined as a reduction in the hemoglobin level of at least 20 g/L, a need for transfusion of at least 2 units of blood, or symptomatic bleeding in a critical area or organ. The mean follow-up period was 2 years.
The study showed that 110 mg dabigatran twice daily was statistically not inferior to warfarin in preventing stroke and systemic embolism (1.53% vs 1.69% per year; P<.001). In addition, this dose was associated with statistically lower rates of major bleeding (2.71% vs 3.36% per year; P=.003). However, dabigatran 150 mg twice daily was statistically superior to warfarin in reducing the risk of stroke and systemic embolism by 34% per year (1.11% vs 1.69%; P<.001) with rates of major bleeding similar to warfarin (3.11% vs 3.36% per year; P=.31). The beneficial effect of dabigatran was also seen in patients with higher CHADS2 scores of 3 to 6, who comprised one-third of the study population and were at higher risk of stroke. Interestingly, both doses of dabigatran were associated with lower rates of intracranial hemorrhage than was warfarin. The 110-mg dose of dabigatran, however, was not approved by the FDA.
A higher incidence of myocardial infarction (MI) occurred in the dabigatran group compared with warfarin, but it was not statistically significant.21,22 A recent meta-analysis of 7 randomized controlled trials, including RE-LY, found that dabigatran was significantly associated with a higher incidence of MI or acute coronary syndrome compared with heterogeneous control groups receiving placebo, warfarin, or enoxaparin (1.19% vs 0.79%, odds ratio, 1.33; P=.03).23
The exact reason for the difference is unknown. It may be due to a chance effect, given that the absolute number of events was small. Or warfarin may exert a protective effect against MI, as was seen in the WARIS II study, wherein warfarin, given alone or in combination with aspirin, was superior to aspirin in reducing the risk of reinfarction.24 However, a true adverse effect of dabigatran cannot be ruled out. If it proves to be the case, 2 more cases of MI can be expected to occur in 1000 patients treated with dabigatran, compared with warfarin, at 1 year.
In addition, there was a statistically significant higher incidence of major gastrointestinal hemorrhage with dabigatran 150 mg twice daily compared with warfarin. Most of these bleeding events occurred in the lower gastrointestinal tract. Here, too, the exact reason for the difference is unknown.
How dabigatran prevents thrombus formation
Dabigatran directly and competitively inhibits both free and fibrin-bound thrombin, thereby preventing thrombin-mediated effects on the coagulation cascade, including cleavage of fibrinogen to fibrin, activation of factors V, VIII, XI, and XII, and thrombin-induced platelet aggregation.25-28
The drug’s pharmacokinetic profile. Dabigatran is given as a prodrug, dabigatran etexilate. Serum esterase converts it to its active form. Peak concentration is reached within 2 to 3 hours of oral dosing, and its half-life is 12 to 17 hours. It is taken twice daily, mornings and evenings. The drug is excreted unchanged, primarily by the kidneys (~80%); the remainder is metabolized by the liver. Therefore, dabigatran is contraindicated in patients with severe renal dysfunction (creatinine clearance <15 mL/min). Compared with warfarin, dabigatran has a more predictable anticoagulant function, no need for laboratory monitoring, and less interaction with other drugs and foods (TABLE 2).29-32 No data are available regarding heterogenous genetic response to dabigatran.
TABLE 2
How warfarin and dabigatran compare pharmacologically29-32
| Attribute | Warfarin | Dabigatran |
|---|---|---|
| Administration | Oral | Oral |
| Mechanism of action | Inhibition of vitamin-K-dependent coagulation factors (II, VII, IX, X, and protein C and S) | Inhibition of thrombin |
| Oral bioavailability | 100% | 6.5% |
| Half-life | 20-60 hours | 12-17 hours |
| Metabolism | Hepatic | Renal (80%) |
| Time to onset | 24-72 hours | 1-2 hours |
| Protein binding | 99% | 35% |
| Antagonist | Vitamin K | None |
| Laboratory monitoring | Required | None required |
| Dose adjustment | Required for each individual | Reduction only for creatinine clearance of 15-30 mL/min |
| Interaction with diet | Interacts with foods rich in vitamin K (eg, cabbage, spinach) | No interaction with foods rich in vitamin K |
| Interaction with drugs | Interacts with amiodarone, antifungals, antibiotics, and alcohol, which may require dose adjustments of either warfarin or the concomitant agent | Dose adjustment of dabigatran may be required with ketoconazole and dronedarone |
Cost-effectiveness of dabigatran
The prescription cost of dabigatran is a lot higher than warfarin, although a recent study demonstrated its cost-effectiveness through a reduction in the need for laboratory monitoring and decreased complications due to over-and under-anticoagulation.33
Factors that come into play
Dabigatran is an alternative to warfarin for long-term anticoagulation in patients with nonvalvular AF who are at a higher risk of stroke with a CHADS2 score of ≥1 or systemic thromboembolism.18 While the main benefits of dabigatran are a quick onset of action, no need for laboratory monitoring, rare interactions with drugs and food, and a decrease in intracranial bleeding compared with warfarin, it did cause more gastrointestinal adverse effects, including bleeding, than warfarin in the RE-LY trial.
Dabigitran was also associated with a higher incidence of MI in RE-LY and an increased risk of MI or acute coronary syndrome in the meta-analysis, but the absolute risk increase in both cases was very small.21-23 Thus, for many patients, the choice of anticoagulant depends on individual preference and ability to comply with a twice-daily dosing regimen, availability of INR monitoring, and cost of treatment.34
Patients who should not receive dabigatran
Dabigatran is contraindicated for patients with a creatinine clearance <15 mL/min, a prosthetic valve, significant valve disease, a history of serious allergic reaction to the drug, or a high risk of bleeding (eg, from recurrent falls, bleeding peptic ulcer).35
Initiating dabigatran therapy
Start dabigatran at a dose of 150 mg twice daily if the creatinine clearance is >30 mL/min, or at 75 mg twice daily if creatinine clearance is 15 to 30 mL/min. In switching a patient from parenteral anticoagulation, you may start dabigatran ≤2 hours before the next scheduled dose of the parenteral agent (eg, low-molecular-weight heparin) or the termination of a continuously administered agent (eg, unfractionated heparin). For patients taking warfarin, withhold dabigatran until the INR is <2.29
Thrombin time is the most reliable measure of drug effect
Dabigatran has a variable and unpredictable effect on the INR, which should not be used to measure the drug’s anticoagulation effect. While therapeutic concentrations modestly elevate the INR, there have been some reports of significant INR elevation.29 However, lab results with the ecarin clotting test (ECT) or thrombin time (TT) correlate well with dabigatran serum concentrations. ECT is primarily a research tool and not commonly available in hospitals; TT, however, is readily available. Activated partial thromboplastin time (aPTT), also commonly available, is prolonged in a nonlinear fashion with dabigatran use. None of these tests has been systematically studied and correlated with clinical outcomes of dabigatran use.29
Adverse effects to watch for
In the RE-LY study, dyspepsia was the most commonly reported adverse effect of dabigatran (11%).21 As with warfarin, other adverse effects, such as dizziness, dyspnea, and fatigue, were reported for dabigatran. Unlike ximelgatran, there is no significant effect on liver enzymes. There is, however, a risk of major and minor bleeding complications.
Bleeding with dabigatran. In the event of a bleeding complication, discontinue dabigatran. There is no specific antidote for this drug; supportive therapy relies on surgical intervention and transfusion of fresh frozen plasma and packed cells. Maintaining adequate diuresis may enhance elimination of the drug. Given dabigatran’s low protein-binding potential, dialysis may be considered; however, data supporting this treatment decision are limited.29
Patients taking dual antiplatelet agents are at a higher risk of bleeding if they also receive either dabigatran or warfarin, although it is not known if one anticoagulant confers a higher risk than the other. In such patients, carefully weigh the risk of bleeding against the benefits of stroke prevention.
Discontinue dabigatran before surgery
Withhold dabigatran from patients scheduled for elective surgery (TABLE 3).29 For those with a high risk of bleeding, measure TT 6 to 12 hours before the procedure to ensure normalization of the value. An acceptable alternative measure, although less precise, is the aPTT. For emergency procedures, fresh frozen plasma may be used to acutely reverse the drug’s effect.
TABLE 3
Recommendations for withholding dabigatran before elective surgery29
| Renal function (creatinine clearance), mL/min | Estimated half-life (range), h | Discontinue dabigatran before surgery | |
|---|---|---|---|
| High risk of bleeding* | Standard risk | ||
| >50-80 | ~15 (12-17) | 2-3 days before | 24 hours before (2 doses) |
| 30-50 | ~18 (18-24) | 4 days before | At least 2 days (48 hours) before |
| <30 | ~27 (>24) | >5 days before | 2-5 days before |
| *Surgeries that confer a high risk of bleeding include, but are not limited to, cardiac surgery, neurosurgery, abdominal surgery, or procedures involving a major organ. Procedures involving spinal anesthesia or spinal tap may also be considered as having a high risk of bleeding | |||
CORRESPONDENCE Rajesh Kabra, MD, University of Tennessee Health Sciences Center, 1325 Eastmoreland Avenue, Suite 460, Memphis, TN 38104; rkabra@uthsc.edu
• Consider dabigatran as an alternative to warfarin for patients with nonvalvular paroxysmal or permanent atrial fibrillation and risk factors for stroke. A
• Avoid using dabigatran with patients who have a creatinine clearance <15 mL/min, a prosthetic heart valve, or hemodynamically significant valve disease. C
• Withhold dabigatran for at least 24 hours before planned surgery, or for a longer time if there is renal insufficiency or the procedure is high risk. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
There are an estimated 2.3 million cases of atrial fibrillation (AF) in the United States, and that number may increase to 5.6 million by the year 2050.1 The stasis of blood during AF, in addition to proinflammatory factors, predisposes patients to clot formation in the left atrium, especially in the left atrial appendage. In 5% of AF patients each year, such a thrombus dislodges and causes a stroke, a rate 2 to 7 times higher than that of people without AF.1-3 Patients with paroxysmal or permanent AF have similar risks of stroke.4
Stratifying stroke risk aids in treatment decisions. Multiple criteria have been devised to identify AF patients at a higher risk of stroke. The CHADS2 risk index, used extensively in clinical settings, stratifies risk according to a cumulative score based on a patient’s risk factors (TABLE 1).5 A joint 2006 guideline released by the American College of Cardiology, American Heart Association, and European Society of Cardiology,1 and a separate 2008 guideline by the American College of Chest Physicians6 recommend that patients with a CHADS2 score of ≥2 be treated with a vitamin K antagonist such as warfarin, while patients with a score of 1 may be treated with either antiplatelet or anticoagulant therapy.
The evidence behind the guidelines. These guidelines are based on a number of randomized clinical trials that demonstrated the superiority of dose-adjusted warfarin in preventing stroke compared with placebo: Stroke Prevention in Atrial Fibrillation (SPAF), Boston Area Anticoagulation Trial for Atrial Fibrillation (BAATAF), Copenhagen Atrial Fibrillation Aspirin Anticoagulation (AFASAK), Canadian Atrial Fibrillation Anticoagulation (CAFA), Stroke Prevention in Nonrheumatic Atrial Fibrillation (SPINAF), and European Atrial Fibrillation Trial (EAFT).7-12
Further support for anticoagulant therapy. In a meta-analysis conducted after release of the guidelines, dose-adjusted warfarin was associated with a 62% risk reduction for stroke vs placebo, and a 39% risk reduction vs antiplatelet agents.13 For high-risk patients in the SPAF III trial, dose-adjusted warfarin led to a 76% risk reduction of stroke and systemic embolism compared with combination therapy of aspirin and low-intensity fixed-dose warfarin.14 The Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE-W) trial was stopped prematurely when it demonstrated that, in patients with AF who have one or more risk factors for stroke, warfarin was superior to the combination of aspirin and clopidogrel in preventing a combined end point of stroke, non-CNS systemic embolism, myocardial infarction, and vascular death; secondary outcomes of stroke were also more favorable with warfarin.15 The results of all 3 studies were noted during a follow-up of 1 to 2 years. In clinical practice, patients must continue antithrombotic agents for a much longer period.
Disadvantages of long-term warfarin use. The main drawback of warfarin therapy is the need for frequent laboratory monitoring. It also interacts unfavorably with other drugs and with certain foods. These factors often lead to patient discontinuation of therapy or to inadequate anticoagulation even when patients are compliant.16 A meta-analysis of 67 clinical studies showed that, regardless of the setting of anticoagulation management with warfarin, the international normalization ratio (INR) was in the therapeutic range only 64% of the time.17 These issues with warfarin have increased interest in developing novel oral anticoagulants that have better drug profiles. An oral direct thrombin inhibitor, ximelagatran, was shown to be as effective as warfarin in the Stroke Prevention Using an Oral Thrombin Inhibitor in Atrial Fibrillation (SPORTIF) V trial,18 but it was associated with hepatotoxicity and did not receive US Food and Drug Administration (FDA) approval.
However, another thrombin inhibitor, dabigatran, was approved by the FDA for anticoagulation in nonvalvular AF, and has been incorporated into the ACCF/AHA/HRS guidelines as a therapeutic option.19 Since this article was submitted for publication, rivaroxaban, an oral factor Xa inhibitor, was approved by the FDA for anticoagulation in AF, based on results of the study Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET-AF).20
TABLE 1
CHADS2 score for stratifying risk of stroke in a patient with nonvalvular atrial fibrillation5
| Risk factor | Score |
|---|---|
| CHF (reduced EF%) | 1 |
| Hypertension | 1 |
| Age ≥75 years | 1 |
| Diabetes mellitus | 1 |
| Stroke/TIA | 2 |
| TOTAL | |
| CHADS2 score | Treatment considerations1,19,20 |
| 0 | Withhold treatment, or give aspirin |
| 1 | Give an antiplatelet or anticoagulant |
| ≥2 | Give an oral anticoagulant such as warfarin, dabigatran, or rivaroxaban |
| CHADS2, acronym comprising initial letters of risk factors listed; CHF, congestive heart failure; EF, ejection fraction; TIA, transient ischemic attack. | |
Dabigatran as an option for nonvalvular AF
Dabigatran’s approval was based on the clinical outcomes of the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) study.21 This multicenter randomized noninferiority trial compared warfarin with 2 doses of dabigatran (110 and 150 mg twice daily) in patients who had AF and a risk of stroke. A total of 18,113 patients with AF, a mean age of 71 years, and a mean CHADS2 score of 2.1 were randomly assigned in a blinded fashion to receive one of the dabigatran doses or, in nonblinded fashion, warfarin. The primary outcome was stroke or systemic embolism. The primary safety outcome was major bleeding defined as a reduction in the hemoglobin level of at least 20 g/L, a need for transfusion of at least 2 units of blood, or symptomatic bleeding in a critical area or organ. The mean follow-up period was 2 years.
The study showed that 110 mg dabigatran twice daily was statistically not inferior to warfarin in preventing stroke and systemic embolism (1.53% vs 1.69% per year; P<.001). In addition, this dose was associated with statistically lower rates of major bleeding (2.71% vs 3.36% per year; P=.003). However, dabigatran 150 mg twice daily was statistically superior to warfarin in reducing the risk of stroke and systemic embolism by 34% per year (1.11% vs 1.69%; P<.001) with rates of major bleeding similar to warfarin (3.11% vs 3.36% per year; P=.31). The beneficial effect of dabigatran was also seen in patients with higher CHADS2 scores of 3 to 6, who comprised one-third of the study population and were at higher risk of stroke. Interestingly, both doses of dabigatran were associated with lower rates of intracranial hemorrhage than was warfarin. The 110-mg dose of dabigatran, however, was not approved by the FDA.
A higher incidence of myocardial infarction (MI) occurred in the dabigatran group compared with warfarin, but it was not statistically significant.21,22 A recent meta-analysis of 7 randomized controlled trials, including RE-LY, found that dabigatran was significantly associated with a higher incidence of MI or acute coronary syndrome compared with heterogeneous control groups receiving placebo, warfarin, or enoxaparin (1.19% vs 0.79%, odds ratio, 1.33; P=.03).23
The exact reason for the difference is unknown. It may be due to a chance effect, given that the absolute number of events was small. Or warfarin may exert a protective effect against MI, as was seen in the WARIS II study, wherein warfarin, given alone or in combination with aspirin, was superior to aspirin in reducing the risk of reinfarction.24 However, a true adverse effect of dabigatran cannot be ruled out. If it proves to be the case, 2 more cases of MI can be expected to occur in 1000 patients treated with dabigatran, compared with warfarin, at 1 year.
In addition, there was a statistically significant higher incidence of major gastrointestinal hemorrhage with dabigatran 150 mg twice daily compared with warfarin. Most of these bleeding events occurred in the lower gastrointestinal tract. Here, too, the exact reason for the difference is unknown.
How dabigatran prevents thrombus formation
Dabigatran directly and competitively inhibits both free and fibrin-bound thrombin, thereby preventing thrombin-mediated effects on the coagulation cascade, including cleavage of fibrinogen to fibrin, activation of factors V, VIII, XI, and XII, and thrombin-induced platelet aggregation.25-28
The drug’s pharmacokinetic profile. Dabigatran is given as a prodrug, dabigatran etexilate. Serum esterase converts it to its active form. Peak concentration is reached within 2 to 3 hours of oral dosing, and its half-life is 12 to 17 hours. It is taken twice daily, mornings and evenings. The drug is excreted unchanged, primarily by the kidneys (~80%); the remainder is metabolized by the liver. Therefore, dabigatran is contraindicated in patients with severe renal dysfunction (creatinine clearance <15 mL/min). Compared with warfarin, dabigatran has a more predictable anticoagulant function, no need for laboratory monitoring, and less interaction with other drugs and foods (TABLE 2).29-32 No data are available regarding heterogenous genetic response to dabigatran.
TABLE 2
How warfarin and dabigatran compare pharmacologically29-32
| Attribute | Warfarin | Dabigatran |
|---|---|---|
| Administration | Oral | Oral |
| Mechanism of action | Inhibition of vitamin-K-dependent coagulation factors (II, VII, IX, X, and protein C and S) | Inhibition of thrombin |
| Oral bioavailability | 100% | 6.5% |
| Half-life | 20-60 hours | 12-17 hours |
| Metabolism | Hepatic | Renal (80%) |
| Time to onset | 24-72 hours | 1-2 hours |
| Protein binding | 99% | 35% |
| Antagonist | Vitamin K | None |
| Laboratory monitoring | Required | None required |
| Dose adjustment | Required for each individual | Reduction only for creatinine clearance of 15-30 mL/min |
| Interaction with diet | Interacts with foods rich in vitamin K (eg, cabbage, spinach) | No interaction with foods rich in vitamin K |
| Interaction with drugs | Interacts with amiodarone, antifungals, antibiotics, and alcohol, which may require dose adjustments of either warfarin or the concomitant agent | Dose adjustment of dabigatran may be required with ketoconazole and dronedarone |
Cost-effectiveness of dabigatran
The prescription cost of dabigatran is a lot higher than warfarin, although a recent study demonstrated its cost-effectiveness through a reduction in the need for laboratory monitoring and decreased complications due to over-and under-anticoagulation.33
Factors that come into play
Dabigatran is an alternative to warfarin for long-term anticoagulation in patients with nonvalvular AF who are at a higher risk of stroke with a CHADS2 score of ≥1 or systemic thromboembolism.18 While the main benefits of dabigatran are a quick onset of action, no need for laboratory monitoring, rare interactions with drugs and food, and a decrease in intracranial bleeding compared with warfarin, it did cause more gastrointestinal adverse effects, including bleeding, than warfarin in the RE-LY trial.
Dabigitran was also associated with a higher incidence of MI in RE-LY and an increased risk of MI or acute coronary syndrome in the meta-analysis, but the absolute risk increase in both cases was very small.21-23 Thus, for many patients, the choice of anticoagulant depends on individual preference and ability to comply with a twice-daily dosing regimen, availability of INR monitoring, and cost of treatment.34
Patients who should not receive dabigatran
Dabigatran is contraindicated for patients with a creatinine clearance <15 mL/min, a prosthetic valve, significant valve disease, a history of serious allergic reaction to the drug, or a high risk of bleeding (eg, from recurrent falls, bleeding peptic ulcer).35
Initiating dabigatran therapy
Start dabigatran at a dose of 150 mg twice daily if the creatinine clearance is >30 mL/min, or at 75 mg twice daily if creatinine clearance is 15 to 30 mL/min. In switching a patient from parenteral anticoagulation, you may start dabigatran ≤2 hours before the next scheduled dose of the parenteral agent (eg, low-molecular-weight heparin) or the termination of a continuously administered agent (eg, unfractionated heparin). For patients taking warfarin, withhold dabigatran until the INR is <2.29
Thrombin time is the most reliable measure of drug effect
Dabigatran has a variable and unpredictable effect on the INR, which should not be used to measure the drug’s anticoagulation effect. While therapeutic concentrations modestly elevate the INR, there have been some reports of significant INR elevation.29 However, lab results with the ecarin clotting test (ECT) or thrombin time (TT) correlate well with dabigatran serum concentrations. ECT is primarily a research tool and not commonly available in hospitals; TT, however, is readily available. Activated partial thromboplastin time (aPTT), also commonly available, is prolonged in a nonlinear fashion with dabigatran use. None of these tests has been systematically studied and correlated with clinical outcomes of dabigatran use.29
Adverse effects to watch for
In the RE-LY study, dyspepsia was the most commonly reported adverse effect of dabigatran (11%).21 As with warfarin, other adverse effects, such as dizziness, dyspnea, and fatigue, were reported for dabigatran. Unlike ximelgatran, there is no significant effect on liver enzymes. There is, however, a risk of major and minor bleeding complications.
Bleeding with dabigatran. In the event of a bleeding complication, discontinue dabigatran. There is no specific antidote for this drug; supportive therapy relies on surgical intervention and transfusion of fresh frozen plasma and packed cells. Maintaining adequate diuresis may enhance elimination of the drug. Given dabigatran’s low protein-binding potential, dialysis may be considered; however, data supporting this treatment decision are limited.29
Patients taking dual antiplatelet agents are at a higher risk of bleeding if they also receive either dabigatran or warfarin, although it is not known if one anticoagulant confers a higher risk than the other. In such patients, carefully weigh the risk of bleeding against the benefits of stroke prevention.
Discontinue dabigatran before surgery
Withhold dabigatran from patients scheduled for elective surgery (TABLE 3).29 For those with a high risk of bleeding, measure TT 6 to 12 hours before the procedure to ensure normalization of the value. An acceptable alternative measure, although less precise, is the aPTT. For emergency procedures, fresh frozen plasma may be used to acutely reverse the drug’s effect.
TABLE 3
Recommendations for withholding dabigatran before elective surgery29
| Renal function (creatinine clearance), mL/min | Estimated half-life (range), h | Discontinue dabigatran before surgery | |
|---|---|---|---|
| High risk of bleeding* | Standard risk | ||
| >50-80 | ~15 (12-17) | 2-3 days before | 24 hours before (2 doses) |
| 30-50 | ~18 (18-24) | 4 days before | At least 2 days (48 hours) before |
| <30 | ~27 (>24) | >5 days before | 2-5 days before |
| *Surgeries that confer a high risk of bleeding include, but are not limited to, cardiac surgery, neurosurgery, abdominal surgery, or procedures involving a major organ. Procedures involving spinal anesthesia or spinal tap may also be considered as having a high risk of bleeding | |||
CORRESPONDENCE Rajesh Kabra, MD, University of Tennessee Health Sciences Center, 1325 Eastmoreland Avenue, Suite 460, Memphis, TN 38104; rkabra@uthsc.edu
1. Fuster V, Rydén LE, Cannom DS, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines: Developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257-e354.
2. Kannel WB, Benjamin EJ. Status of the epidemiology of atrial fibrillation. Med Clin North Am. 2008;92:17-40, ix.
3. Hart RG, Halperin JL. Atrial fibrillation and thromboembolism: a decade of progress in stroke prevention. Ann Intern Med. 1999;131:688-695.
4. Hohnloser SH, Pajitnev D, Pogue J, et al. Incidence of stroke in paroxysmal versus sustained atrial fibrillation in patients taking oral anticoagulation or combined antiplatelet therapy: an ACTIVE W substudy. J Am Coll Cardiol. 2007;50:2156-2161.
5. Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864-2870.
6. Singer DE, Albers GW, Dalen JE, et al. Antithrombotic therapy in atrial fibrillation: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2008;133:546S-592S.
7. Stroke Prevention in Atrial Fibrillation Investigators. Stroke prevention in atrial fibrillation study. Final results. Circulation. 1991;84:527-539.
8. The Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators. The effect of low-dose warfarin on the risk of stroke in patients with nonrheumatic atrial fibrillation. N Engl J Med. 1990;323:1505-1511.
9. Petersen P, Boysen G, Godtfredsen J, et al. Placebo-controlled, randomized trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation. The Copenhagen AFASAK study. Lancet. 1989;1:175-179.
10. Connolly SJ, Laupacis A, Gent M, et al. Canadian Atrial Fibrillation Anticoagulation (CAFA) study. J Am Coll Cardiol. 1991;18:349-355.
11. Ezekowitz MD, Bridgers SL, James KE, et al. Warfarin in the prevention of stroke associated with nonrheumatic atrial fibrillation. Veterans Affairs Stroke Prevention in Nonrheumatic Atrial Fibrillation Investigators. N Engl J Med. 1992;327:1406-1412.
12. EAFT (European Atrial Fibrillation Trial) Study Group. Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. Lancet. 1993;342:1255-1262.
13. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857-867.
Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrillation: Stroke Prevention in Atrial Fibrillation III randomised clinical trial. Lancet. 1996;348:633-638.
15. Connolly S, Pogue J, Hart R, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006;367:1903-1912.
16. Connolly SJ, Pogue J, Eikelboom J, et al. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation. 2008;118:2029-2037.
17. Van Walraven C, Jennings A, Oake N, et al. Effect of study setting on anticoagulation control: a systematic review and metaregression. Chest. 2006;129:1155-1166.
18. Albers GW, Diener HC, Frison L, et al. Ximelagatran versus warfarin for stroke prevention in patients with nonvalvular atrial fibrillation (SPORTIF V study). JAMA. 2005;293:690-698.
19. Wann LS, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (update on dabigatran): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;57:1330-1337.
20. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-891.
21. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
22. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Newly identified events in the RE-LY trial. N Engl J Med. 2010;363:1875-1876.
23. Uchino K, Hernandez AV. Dabigatran association with higher risk of acute coronary events. Meta-analysis of noninferiority randomized controlled trials. Arch Intern Med. 2012;172:397-402.
24. Hurlen M, Abdelnoor M, Smith P, et al. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med. 2002;347:969-974.
25. Nutescu EA, Shapiro NL, Chevalier A. New anticoagulant agents: direct thrombin inhibitors. Cardiol Clin. 2008;26:169-187.
26. Alban S. Pharmacological strategies for inhibition of thrombin activity. Curr Pharm Des. 2008;14:1152-1175.
27. Mehta RS. Novel oral anticoagulants. Part II: direct thrombin inhibitors. Expert Rev Hematol. 2010;3:351-361.
28. Weber R, Diener HC, Weimar C. Prevention of cardioembolic stroke in patients with atrial fibrillation. Expert Rev Cardiovasc Ther. 2010;8:1405-1415.
29. Pradexa [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; 2010.
30. Strangier J. Clinical pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor of dabigatran etexilate. Clin Pharmacokinet. 2008;47:285-295.
31. Blech S, Ebner T, Ludwig-Schwellinger E, et al. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos. 2008;36:386-399.
32. Ma TK, Yan BP, Lam YY. Dabigatran etexilate versus warfarin as the oral anticoagulant of choice? A review of clinical data. Pharmacol Ther. 2011;129:185-194.
33. Freeman JV, Zhu RP, Owens DK, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med. 2011;154:1-11.
34. Gage BF. Can we rely on RE-LY? N Engl J Med. 2009;361:1200-1202.
35. Wann LS, Curtis AB, January CT, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (updating the 2006 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123:104-123.
1. Fuster V, Rydén LE, Cannom DS, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines: Developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257-e354.
2. Kannel WB, Benjamin EJ. Status of the epidemiology of atrial fibrillation. Med Clin North Am. 2008;92:17-40, ix.
3. Hart RG, Halperin JL. Atrial fibrillation and thromboembolism: a decade of progress in stroke prevention. Ann Intern Med. 1999;131:688-695.
4. Hohnloser SH, Pajitnev D, Pogue J, et al. Incidence of stroke in paroxysmal versus sustained atrial fibrillation in patients taking oral anticoagulation or combined antiplatelet therapy: an ACTIVE W substudy. J Am Coll Cardiol. 2007;50:2156-2161.
5. Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864-2870.
6. Singer DE, Albers GW, Dalen JE, et al. Antithrombotic therapy in atrial fibrillation: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2008;133:546S-592S.
7. Stroke Prevention in Atrial Fibrillation Investigators. Stroke prevention in atrial fibrillation study. Final results. Circulation. 1991;84:527-539.
8. The Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators. The effect of low-dose warfarin on the risk of stroke in patients with nonrheumatic atrial fibrillation. N Engl J Med. 1990;323:1505-1511.
9. Petersen P, Boysen G, Godtfredsen J, et al. Placebo-controlled, randomized trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation. The Copenhagen AFASAK study. Lancet. 1989;1:175-179.
10. Connolly SJ, Laupacis A, Gent M, et al. Canadian Atrial Fibrillation Anticoagulation (CAFA) study. J Am Coll Cardiol. 1991;18:349-355.
11. Ezekowitz MD, Bridgers SL, James KE, et al. Warfarin in the prevention of stroke associated with nonrheumatic atrial fibrillation. Veterans Affairs Stroke Prevention in Nonrheumatic Atrial Fibrillation Investigators. N Engl J Med. 1992;327:1406-1412.
12. EAFT (European Atrial Fibrillation Trial) Study Group. Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. Lancet. 1993;342:1255-1262.
13. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857-867.
Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrillation: Stroke Prevention in Atrial Fibrillation III randomised clinical trial. Lancet. 1996;348:633-638.
15. Connolly S, Pogue J, Hart R, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006;367:1903-1912.
16. Connolly SJ, Pogue J, Eikelboom J, et al. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation. 2008;118:2029-2037.
17. Van Walraven C, Jennings A, Oake N, et al. Effect of study setting on anticoagulation control: a systematic review and metaregression. Chest. 2006;129:1155-1166.
18. Albers GW, Diener HC, Frison L, et al. Ximelagatran versus warfarin for stroke prevention in patients with nonvalvular atrial fibrillation (SPORTIF V study). JAMA. 2005;293:690-698.
19. Wann LS, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (update on dabigatran): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;57:1330-1337.
20. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-891.
21. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
22. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Newly identified events in the RE-LY trial. N Engl J Med. 2010;363:1875-1876.
23. Uchino K, Hernandez AV. Dabigatran association with higher risk of acute coronary events. Meta-analysis of noninferiority randomized controlled trials. Arch Intern Med. 2012;172:397-402.
24. Hurlen M, Abdelnoor M, Smith P, et al. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med. 2002;347:969-974.
25. Nutescu EA, Shapiro NL, Chevalier A. New anticoagulant agents: direct thrombin inhibitors. Cardiol Clin. 2008;26:169-187.
26. Alban S. Pharmacological strategies for inhibition of thrombin activity. Curr Pharm Des. 2008;14:1152-1175.
27. Mehta RS. Novel oral anticoagulants. Part II: direct thrombin inhibitors. Expert Rev Hematol. 2010;3:351-361.
28. Weber R, Diener HC, Weimar C. Prevention of cardioembolic stroke in patients with atrial fibrillation. Expert Rev Cardiovasc Ther. 2010;8:1405-1415.
29. Pradexa [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; 2010.
30. Strangier J. Clinical pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor of dabigatran etexilate. Clin Pharmacokinet. 2008;47:285-295.
31. Blech S, Ebner T, Ludwig-Schwellinger E, et al. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos. 2008;36:386-399.
32. Ma TK, Yan BP, Lam YY. Dabigatran etexilate versus warfarin as the oral anticoagulant of choice? A review of clinical data. Pharmacol Ther. 2011;129:185-194.
33. Freeman JV, Zhu RP, Owens DK, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med. 2011;154:1-11.
34. Gage BF. Can we rely on RE-LY? N Engl J Med. 2009;361:1200-1202.
35. Wann LS, Curtis AB, January CT, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (updating the 2006 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123:104-123.