User login
Gadolinium Intermediate Elimination and Persistent Symptoms After Magnetic Resonance Imaging Contrast Agent Exposure
Gadolinium Intermediate Elimination and Persistent Symptoms After Magnetic Resonance Imaging Contrast Agent Exposure
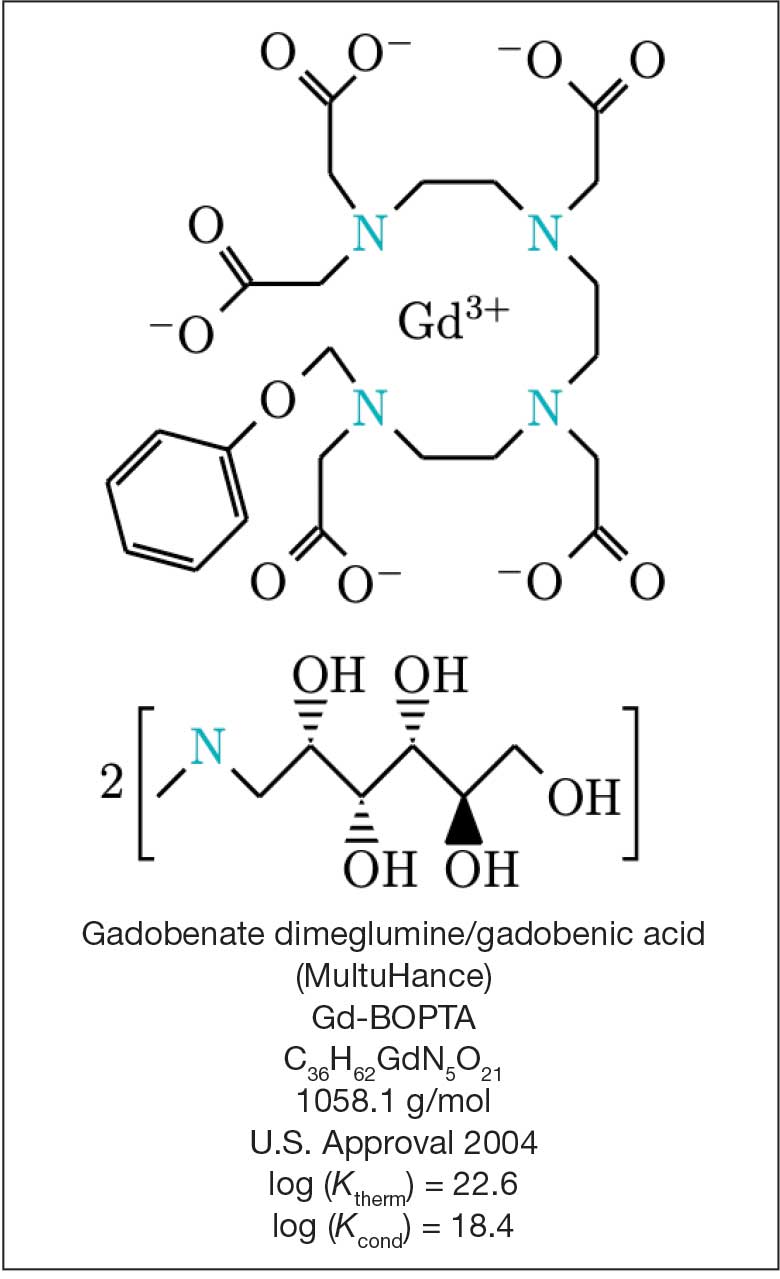
Magnetic resonance image (MRI) contrast agents can induce profound complications, including gadolinium encephalopathy, kidney injury, gadolinium-associated plaques, and progressive systemic fibrosis, which can be fatal.1-10 About 50% of MRIs use gadolinium-based contrast (Gd3+), a toxic rare earth metal ion that enhances imaging but requires binding with pharmaceutical ligands to reduce toxicity and promote renal elimination (Figure 1). Despite these measures, Gd3+ can persist in the body, including the brain.11,12 Wastewater treatment fails to remove these agents, making Gd3+ a growing pollutant in water and the food chain.13-15 Because Gd3+ is a rare earth metal ion in the milieu intérieur, there is an urgent need to study its biological and long-term effects (Appendix 1).
Case Presentation
A 65-year-old Vietnam-era veteran presented to nephrology at the Raymond G. Murphy Veterans Affairs Medical Center (RGMVAMC) in Albuquerque, New Mexico, for evaluation of gadolinium-induced symptoms. His medical history included metabolic syndrome, hypertension, hyperlipidemia, hypogonadism, cervical spondylosis, and an elevated prostate-specific antigen, previously assessed with a contrast-enhanced MRI in 2019 (Gadobenic acid, 19 mL). Surgical history included cervical fusion and ankle hardware.
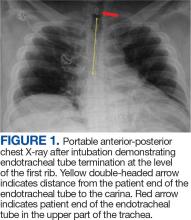
The patient had a scheduled MRI 25 days earlier, following an elevated prostate specific antigen test result, prompting urologic surveillance and concern for malignancy. In preparation for the contrast-enhanced MRI, his right arm was cannulated with a line primed with gadobenic acid contrast. Though the technician stated the infusion had not started, the patient’s symptoms began shortly after entry into the scanner, before any programmed pulse sequences. The patient experienced claustrophobia, diaphoresis, palpitations, xerostomia, dysgeusia, shortness of breath, and a sensation of heat in his groin, chest, “kidneys,” and lower back. The MRI was terminated prematurely in response to the patient’s acute symptomatology. The patient continued experiencing new symptoms intermittently during the following week, including lightheadedness, headaches, right clavicular pain, raspy voice, edema, and a sense of doom.
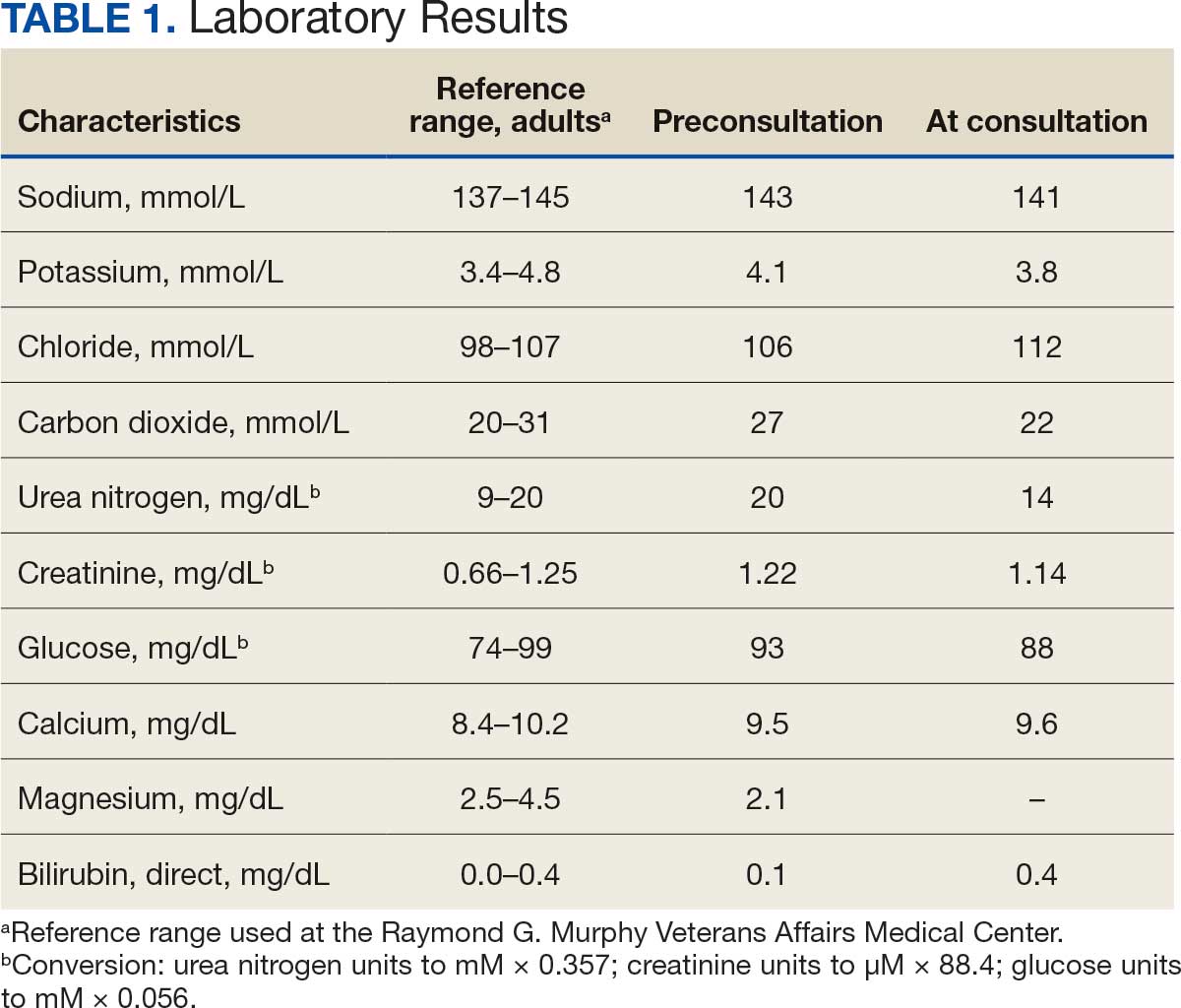
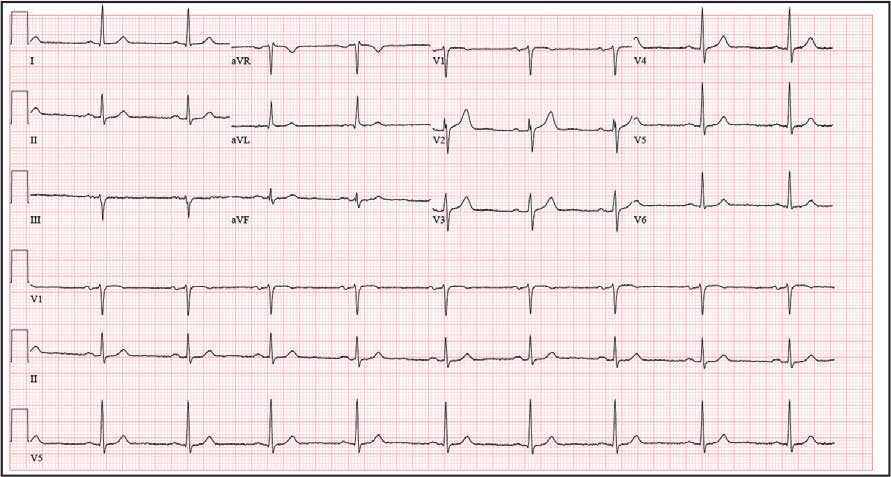
The patient presented to the RGMVAMC emergency department (ED) 8 days after the MRI with worsening symptoms and was hospitalized for 10 days. During this time, he was referred to nephrology for outpatient evaluation. While awaiting his nephrology appointment, the patient presented to the RGMVAMC ED 20 days after the initial episode with ongoing symptoms. “I thought I was dying,” he said. Laboratory results and a 12-lead electrocardiogram showed a finely static background, wide P waves (> 80 ms) with notching in lead II, sinusoidal P waves in V1, R transition in V2, RR’ in V2, ST flat in lead III, and sinus bradycardia (Table 1 and Appendix 2).
The patient’s medical and surgical histories were reviewed at the nephrology evaluation 25 days following the MRI. He reported that household water was sourced from a well and that he filtered his drinking water with a reverse osmosis system. He served in the US Army for 10 years as an engineer specializing in mechanical systems, power generation, and vehicles. Following Army retirement, the patient served in the US Air Force Reserves for 15 years, working as a crew chief in pneudraulics. The patient reported stopping tobacco use 1 year before and also reported regular use of a broad array of prescription medications and dietary supplements, including dexamethasone (4 mg twice daily), fluticasone nasal spray (50 mcg per nostril, twice daily), ibuprofen (400 mg twice daily, as needed), loratadine (10 mg daily), aspirin (81 mg daily), and metoprolol succinate (50 mg nightly). In addition, he reported consistent use of cholecalciferol (3000 IU daily), another supplemental vitamin D preparation, chelated magnesium glycinate (3 tablets daily for bone issues), turmeric (1 tablet daily), a multivitamin (Living Green Liquid Gel, daily), and a mega-B complex.
Physical examination revealed a well-nourished, tall man with hypertension (145/87 mmHg) and bilateral lower extremity edema. Oral examination showed poor dentition, including missing molars (#1-3, #14-16, #17-19, #30-31), with the anterior teeth replaced by bridges supported by dental implants. The review of systems was otherwise unremarkable, with nocturia noted before the consultation.
Serum and urine gadolinium testing, (Mayo Clinic Laboratories) revealed gadolinium levels of 0.3 mcg/24 h in the urine and 0.1 ng/mL in the serum. Nonzero values indicated detectable gadolinium, suggesting retention. The patient had a prior gadolinium exposure during a 2019 MRI (about 1340 days before) and suspected a repeat exposure on day 0, although the MRI technician stated that no contrast was administered. Given his elevated vitamin D levels, the patient was advised to minimize dietary supplements, particularly vitamin D, to avoid confounding symptoms. The plan included monitoring symptoms and a follow-up evaluation with repeat laboratory tests on day 116.
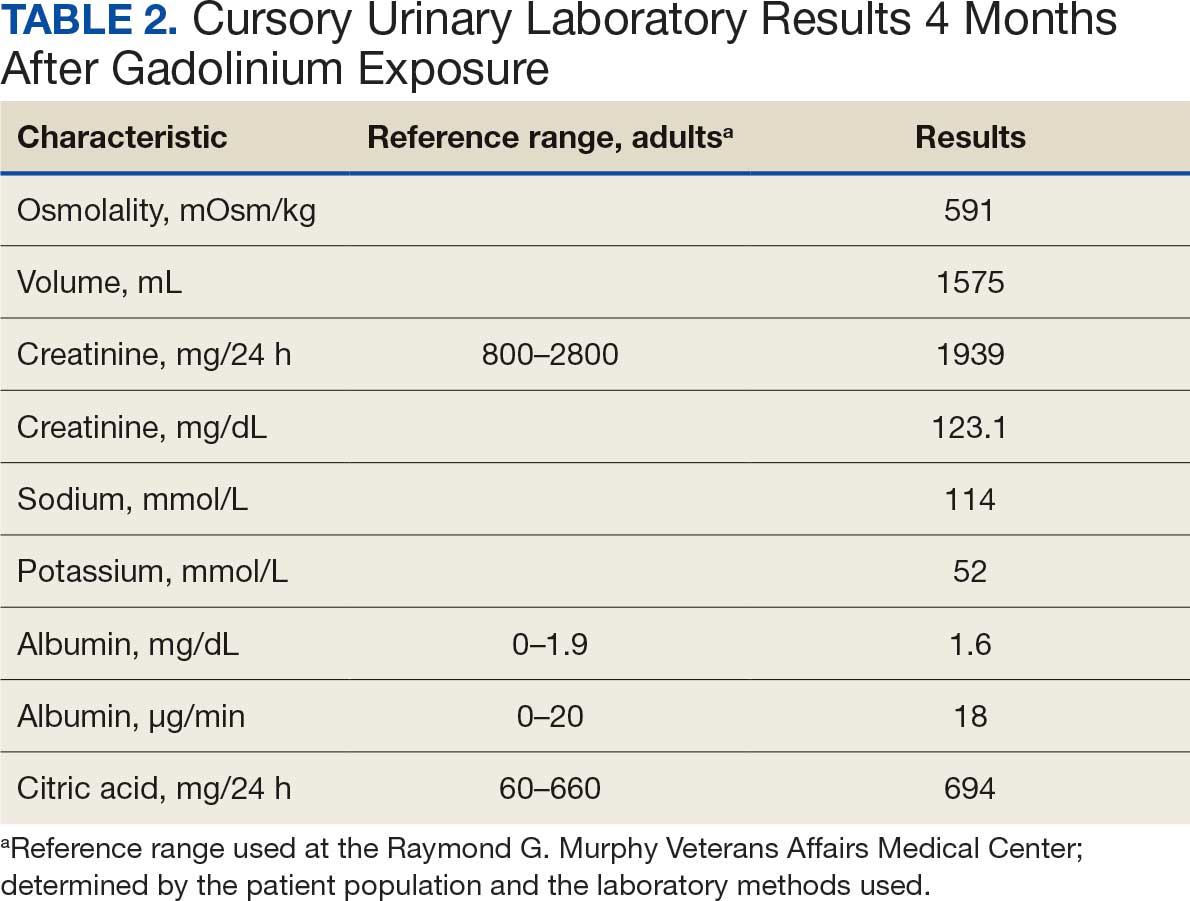
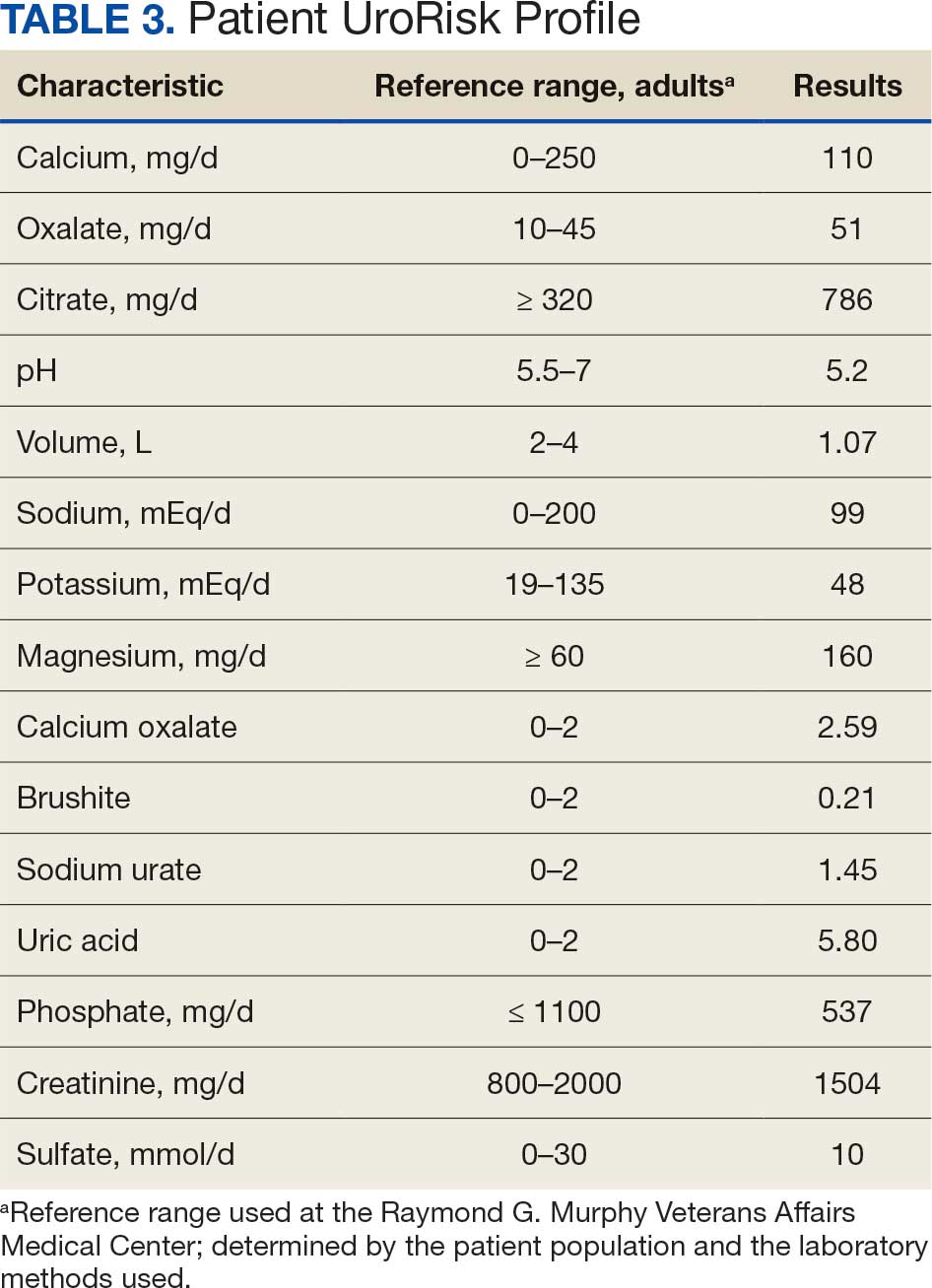
At the nephrology follow-up 4 months postexposure, the patient's symptoms had primarily abated, with a marked reduction in the previously noted metallic dysgeusia. Physical examination remained consistent with prior findings. He was afebrile (97.7 °F) with a blood pressure of 111/72 mmHg, a pulse of 63 beats per minute, and an oxygen saturation of 98% on ambient air. Laboratory analysis revealed serum and urine gadolinium levels below detectable thresholds (< 0.1 ng/mL and < 0.1 mcg/24 h). A 24-hour creatinine clearance, calculated from a urine volume of 1300 mL, measured at an optimal 106 mL/min, indicating preserved renal function (Tables 2 and 3). Of note, his 24-hour oxalate was above the reference range, with a urine pH below the reference range and a high supersaturation index for calcium oxalate.
Discussion
Use of enhanced MRI has increased in the Veterans Health Administration (Figure 2). A growing range of indications for enhanced procedures (eg, cardiac MRI) has contributed to this rise. The market has grown with new gadolinium-based contrast agents, such as gadopiclenol. However, reliance on untested assumptions about the safety of newer agents and need for robust clinical trials pose potential risks to patient safety.
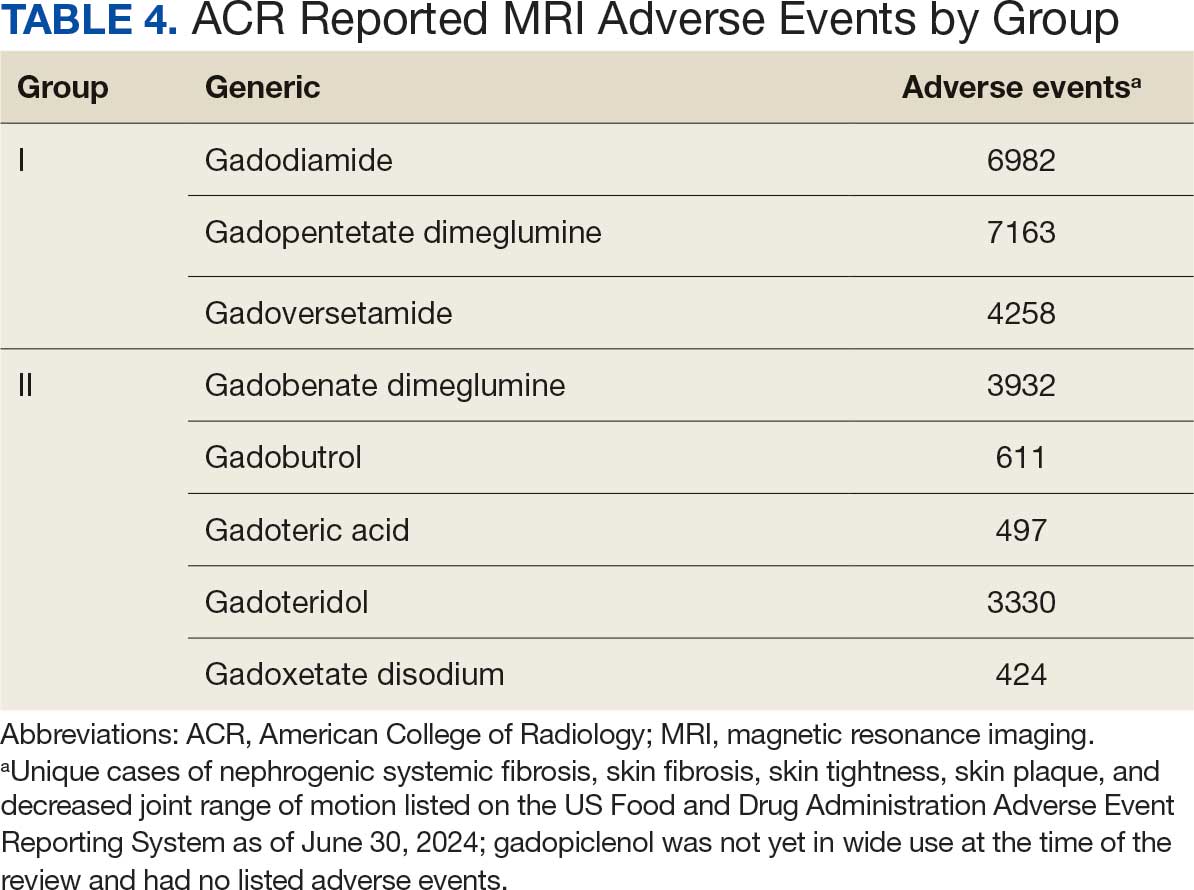
Without prospective evidence, the American College of Radiology (ACR) classifies gadolinium-based contrast agents into 3 groups: Group 1, associated with the highest number of nephrogenic systemic fibrosis cases; Group 2, linked to few, if any, unconfounded cases; and Group 3, where data on nephrogenic systemic fibrosis risk have been limited. As of April 2024, the ACR reclassified Group 3 agents (Ablavar/Vasovist/Angiomark and Primovist/Eovist) into Group 2. Curiously, Vueway and Elucirem were approved in late 2022 and should clearly be categorized as Group 3 (Table 4).There were 19 cases of nephrogenic systemic fibrosis or similar manifestations, 8 of which were unconfounded by other factors. These patients had been exposed to gadobutrol, often combined with other agents. Gadobutrol—like other Group 2 agents—has been associated with nephrogenic systemic fibrosis.16,17 Despite US Food and Drug Administration (FDA) documentation of rising reports, many clinicians remain unaware that nephrogenic systemic fibrosis is increasingly linked to Group 2 agents classified by the ACR.18 While declines in reported cases of nephrogenic systemic fibrosis may suggest reduced incidence, this trend may reflect diminished clinical vigilance and underreporting, particularly given emerging evidence implicating even Group 2 gadolinium-based contrast agents in delayed and underrecognized presentations. This information has yet to permeate the medical community, particularly among nephrologists. Considering these cases, revisiting the ACR guidelines may be prudent.
To address this growing concern, clinicians must adopt stricter vigilance and actively pursue updated information to mitigate patient risks tied to these contrast agents.
There exists an illusion of knowledge in disregarding the confounded exposures of MRI contrast agents. Ten distinct brands of contrast agents have been approved for clinical use. With repeated imaging, patients are often exposed to varying formulations of gadolinium-based agents. Yet investigators commonly discard these data points when assessing risk. By doing so, they assume—without evidence—that some formulations are inherently less likely to provoke adverse effects (AEs) than others. This untested presumption becomes perilous, especially given the limited understanding of the mechanisms underlying gadolinium-induced pathologies. As Aldous Huxley warned, “Facts do not cease to exist because they are ignored.”19
Gadolinium Persistence
Contrary to expectations, gadolinium persists in the body far longer than initially presumed. Symptoms associated with gadolinium exposure (SAGE) encapsulate the chronic, often enigmatic maladies tied to MRI contrast agents.20 The prolonged retention of this rare earth metal offers a compelling hypothesis for the etiology of SAGE. It has been hypothesized that Lewis base-rich metabolites increase susceptibility to gadolinium-based contrast agent complications.21
The blood and urine concentration elimination curves of gadolinium are exponential and categorized as fast, intermediate, and long-term.1 For urinary elimination, the function of the curves is exponential. The quantity of gadolinium in the urine at a time (t) after exposure (D[Gd](t)) is equal to the product of the amount of gadolinium in the sample (urine or blood) at the end of the fast elimination period (D[Gd](t0)) and the exponential decay with k being a rate constant.
To the authors’ knowledge, we are the only research team currently investigating the rate constant for the intermediate- and long-term phase gadolinium elimination. The Retention and Toxicity of Gadolinium-based Contrast Agents study was approved by the University of New Mexico Health Sciences Center Institutional Review Board on May 27, 2020 (IRB ID 19-660). The data for the patient in this case were compared with preliminary results for patients with exposure-to-measurement intervals < 100 days.
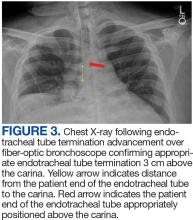
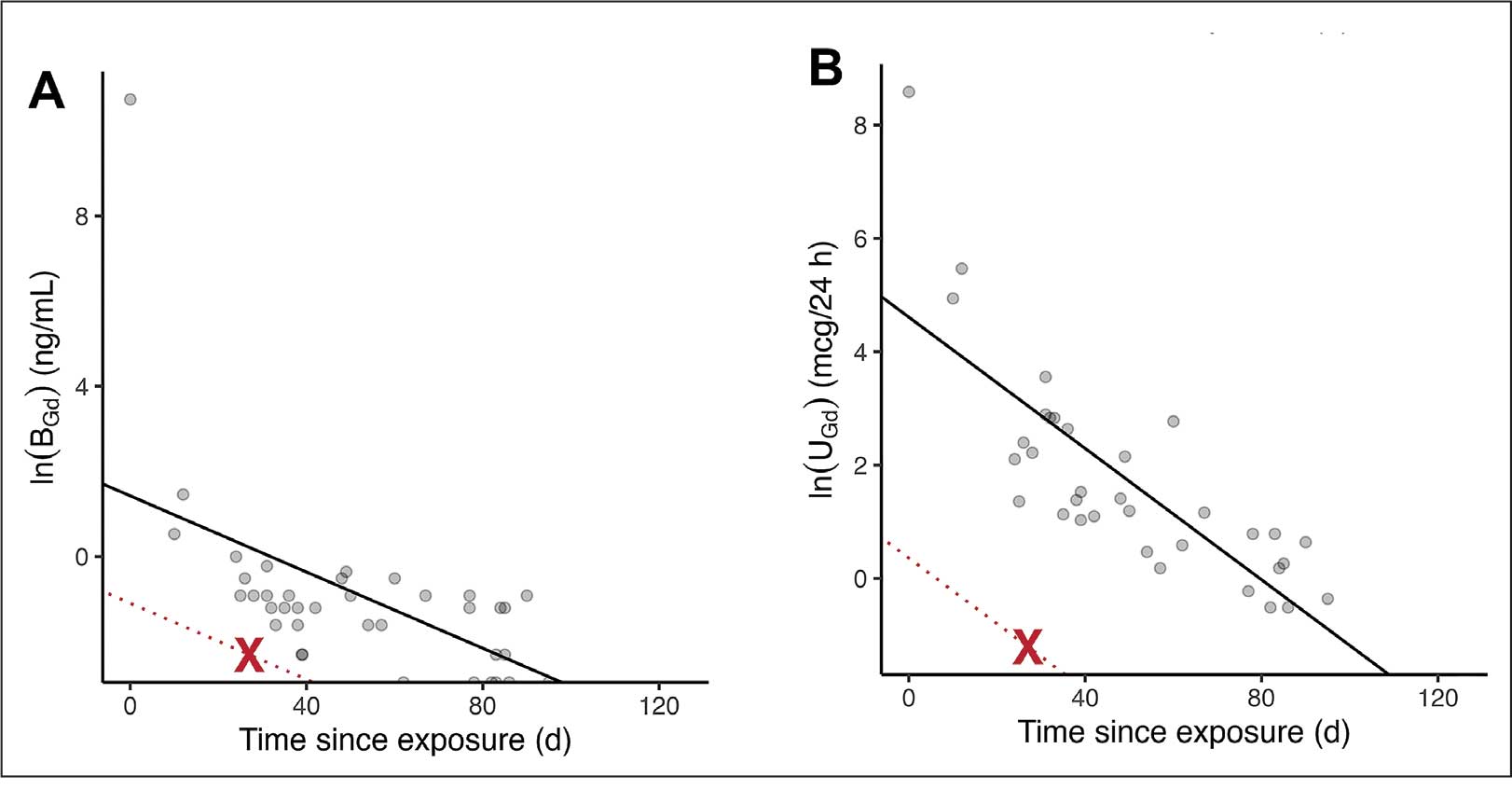
The patient in this case presented with detectable gadolinium levels in urine and serum shortly after an attempted contrast-enhanced MRI procedure (Figure 3). The presence of detectable gadolinium levels in the patient’s urine and serum suggests a likely exposure to a contrast agent about 27 days before his consultation. While the technician reported that no contrast was administered during the attempted MRI, it remains possible that a small amount was introduced during cannulation, potentially triggering the patient’s symptoms. Linear modeling of semilogarithmic plots for participants exposed to contrast agents within 100 days (urine: P = 1.8 × 10ˉ8, adjusted r² = 0.62; blood: P = .005, adjusted r² = 0.21) provided clearance rates (k values) for urine and blood. Extrapolating from these models to the presumed exposure date, the intercepts estimate that the patient received between 0.5% and 8% of a standard contrast dose.
MRI contrast agents can cause skin disease. Systemic fibrosis is considered one of the most severe AEs. Skin pathophysiology involving myeloid cells is driven by elevated levels of monocyte chemoattractant protein-1, which recruits circulating fibroblasts via the C-C chemokine receptor 2.22,23 This occurs alongside activation of NADPH oxidase Nox4.4,24,25 Intracellular gadolinium-rich nanoparticles likely serve as catalysts for this reactive cascade.2,18,22,26,27 These particles assemble around intracellular lipid droplets and ferrule them in spiculated rare earth-rich shells that compromise cellular architecture.2,18,21,22,26,27 Frequently sequestered within endosomal compartments, they disrupt vesicular integrity and threaten cellular homeostasis. Interference with degradative systems such as the endolysosomal axis perturbs energy-recycling pathways—an insidious disturbance, particularly in cells with high metabolic demand. Skin-related symptoms are among the most frequently reported AEs, according to the FDA AE reporting system.18
Studies indicate repeated exposure to MRI contrast agents can lead to permanent gadolinium retention in the brain and other vital organs. Intravenous (IV) contrast agents cross the blood-brain barrier rapidly, while intrathecal administration has been linked to significant and lasting neurologic effects.18
Gadolinium is chemically bound to pharmaceutical ligands to enhance renal clearance and reduce toxicity. However, available data from human samples suggest potential ligand exchanges with undefined physiologic substances. This exchange may facilitate gadolinium precipitation and accumulation within cells into spiculated nanoparticles. Transmission electron microscopy reveals the formation of unilamellar bodies associated with mitochondriopathy and cellular damage, particularly in renal proximal tubules.2,18,22,26,27 It is proposed that intracellular nanoparticle formation represents a key mechanism driving the systemic symptoms observed in patients.1,2,18, 22,26,27
Any hypothesis based on free soluble gadolinium—or concept derived from it—should be discarded. The high affinity of pharmaceutical ligands for gadolinium suggests that the cationic rare earth metal remains predominantly in a ligand-bound, soluble form. It is hypothesized that gadolinium undergoes ligand exchange with physiologic substances, directly leading to nanoparticle formation. Current data demonstrate gadolinium precipitation according to the Le Chatelier’s principle. Since precipitated gadolinium does not readily re-equilibrate with pharmaceutical ligands, repeated administration of different contrast agent brands may contribute to nanoparticle growth.26
Meanwhile, a growing number of patients are turning to chelation therapy, a largely untested treatment. The premise of chelation therapy is rooted in several unproven assumptions.18,21 First, it assumes that clinically significant amounts of gadolinium persist in compartments such as the extracellular space, where they can be effectively chelated and cleared. Second, it presumes that free gadolinium is the primary driver of chronic symptoms, an assertion that remains scientifically unsubstantiated. Finally, chelation proponents overlook the potential harm caused by depleting essential physiological metals during the process, assuming without evidence that the scant removal of gadolinium outweighs the risk of physiological mineral depletion.
These assumptions underpin an unproven remedy that demands critical scrutiny. Recent findings reveal that gadolinium deposits in the skin and kidney often take the form of intracellular nanoparticles, directly challenging the foundation of chelation therapy. Chelation advocates must demonstrate that these intracellular gadolinium deposits neither trigger cellular toxicity nor initiate a cytokine cascade. Chelation supporters must prove that the systemic response to these foreign particles is unrelated to the symptoms reported by patients. Until then, the validity of chelation therapy remains highly questionable.
The causality of the symptoms, mainly whether IV gadolinium was administered, was examined. The null hypothesis stated that the patient was not exposed to gadolinium. However, this hypothesis was contradicted by the detection of gadolinium in the serum and urine 27 days after the potential exposure.
Two plausible explanations exist for the nonzero gadolinium levels detected in the serum and urine. The first possibility is that minute quantities of gadolinium were introduced during cannulation, with the amount being sufficient to persist in measurable concentrations 27 days postexposure. The second possibility is that the gadolinium originated from an MRI contrast agent administered 4 years earlier. In this scenario, gadolinium stored in organ reservoirs such as bone, liver, or kidneys may have been mobilized into the extracellular fluid compartment due to the administration of high-dose steroids 20 days after the recent contrast-enhanced MRI procedure attempt. Coyte et al reported elevated gadolinium levels in the serum, cord blood, breast milk, and placenta of pregnant women with prior exposure to MRI contrast agents.28 These findings suggest that gadolinium, stored in organs such as bone may be remobilized by variables affecting bone remodeling (eg, high-dose steroids).
Significantly, the patient exhibited elevated urinary oxalate levels. Previous research has found that oxalic acid reacts rapidly with MRI contrast agents, forming digadolinium trioxalate. While the gadolinium-rich nanoparticles identified in tissues such as the skin and kidney (including the human kidney) are amorphous, these in vitro findings establish a proof-of-concept: the intracellular environment facilitates gadolinium dissociation from pharmaceutical chelates.
Furthermore, in vitro experiments show that proteins and lysosomal pH promote this dissociation, underscoring how human metabolic conditions—particularly oxalic acid concentration—may drive intracellular gadolinium deposition.
Patient Perspective
“They put something into my body that they cannot get out.” This stark realization underpins the patient’s profound concern about gadolinium-based contrast agents and their potential long-term effects. Reflecting on his experience, the patient expressed deep fears about the unknown future impacts: “I’m concerned about my kidneys, I’m concerned about my heart, and I’m concerned about my brain. I don’t know how this stuff is going to affect me in the future.”
He drew an unsettling parallel between gadolinium and heavy metals: “Heavy metal is poison. The body does not produce this kind of stuff on its own.” His reaction to the procedure left a lasting impression, prompting him to question the logic of using a substance that cannot be purged: “Why would you put something into someone’s body that you cannot extract? Nobody—nobody—should experience what I went through.”
The patient emphasized the lack of clear research on long-term outcomes, which compounds his anxiety: “If there was research that said, ‘Well, this is only going to affect these organs for this long,’ OK, I might be able to accept that. But there is no research like that. Nobody can tell me what’s going to happen in 5 years.”
Strengths and Limitations
A significant strength of this approach is the ability to track gadolinium elimination and symptom resolution over time, supported by unique access to intermediate and long-term clearance data from our ongoing research protocol. The investigators were equipped to back-extrapolate the exposure, which provided a rare opportunity to correlate gadolinium levels with clinical outcomes. The primary limitation is the lack of a defined clinical case definition for gadolinium toxicity and limited mechanistic understanding of SAGE, which hinders diagnosis and management.
Metabolites, proteins, and lipids rich in Lewis bases could initiate this process as substrates for intracellular gadolinium sedimentation. Future studies should investigate whether metabolic conditions such as oxalate burden or altered parathyroid hormone levels modulate gadolinium compartmentalization and tissue retention. If gadolinium-rich nanoparticle formation and accumulation disrupt cellular equilibrium, it underscores an urgent need to understand the implications of long-term gadolinium retention. The research team continues to gather evidence that the gadolinium cation remains chelated from the moment MRI contrast agents are administered through to the formation of intracellular nanoparticles. Retained gadolinium nanoparticles may act as a nidus, triggering cellular signaling cascades that lead to multisymptomatic illnesses. Intracellular and insoluble retained gadolinium challenges proponents of untested chelation therapies.
Conclusions
This case highlights emerging clinical and ethical concerns surrounding gadolinium-based contrast agent use. Clinicians may benefit from considering gadolinium retention as a contributor to persistent, unexplained symptoms—particularly in patients with recent imaging exposure. As contrast use continues to rise within federal health systems, regulatory and administrative stakeholders would do well to re-examine current safety frameworks. Informed consent should reflect what is known: gadolinium can remain in the body long after administration, potentially indefinitely. The long-term consequences of cumulative exposure remain poorly defined, but the presence of a lanthanide element in human tissue warrants greater attention from researchers and regulators alike. Interest in alternative imaging modalities and long-term safety monitoring would mark progress toward more transparent, accountable care.
Jackson DB, MacIntyre T, Duarte-Miramontes V, et al. Gadolinium deposition disease: a case report and the prevalence of enhanced MRI procedures within the Veterans Health Administration. Fed Pract. 2022;39:218-225. doi:10.12788/fp.0258
Do C, DeAguero J, Brearley A, et al. Gadolinium-based contrast agent use, their safety, and practice evolution. Kidney360. 2020;1:561-568.doi:10.34067/kid.0000272019
Leyba K, Wagner B. Gadolinium-based contrast agents: why nephrologists need to be concerned. Curr Opin Nephrol Hypertens. 2019;28:154-162. doi:10.1097/MNH.0000000000000475
Wagner B, Drel V, Gorin Y. Pathophysiology of gadolinium-associated systemic fibrosis. Am J Physiol Renal Physiol. 2016;311:F1-F11. doi:10.1152/ajprenal.00166.2016
Maramattom BV, Manno EM, Wijdicks EF, et al. Gadolinium encephalopathy in a patient with renal failure. Neurology. 2005;64:1276-1278.doi:10.1212/01.WNL.0000156805.45547.6E
Sam AD II, Morasch MD, Collins J, et al. Safety of gadolinium contrast angiography in patients with chronic renal insufficiency. J Vasc Surg. 2003;38:313-318. doi:10.1016/s0741-5214(03)00315-x
Schenker MP, Solomon JA, Roberts DA. Gadolinium arteriography complicated by acute pancreatitis and acute renal failure. J Vasc Interv Radiol. 2001;12:393. doi:10.1016/s1051-0443(07)61925-3
Gemery J, Idelson B, Reid S, et al. Acute renal failure after arteriography with a gadolinium-based contrast agent. AJR Am J Roentgenol. 1998;171:1277-1278. doi:10.2214/ajr.171.5.9798860
Akgun H, Gonlusen G, Cartwright J Jr, et al. Are gadolinium-based contrast media nephrotoxic? A renal biopsy study. Arch Pathol Lab Med. 2006;130:1354-1357. doi:10.5858/2006-130-1354-AGCMNA
Gathings RM, Reddy R, Santa Cruz D, et al. Gadolinium-associated plaques: a new, distinctive clinical entity. JAMA Dermatol. 2015;151:316-319. doi:10.1001/jamadermatol.2014.2660
McDonald RJ, McDonald JS, Kallmes DF, et al. Gadolinium deposition in human brain tissues after contrast-enhanced MR imaging in adult patients without intracranial abnormalities. Radiology. 2017;285(2):546-554. doi:10.1148/radiol.2017161595
Kanda T, Ishii K, Kawaguchi H, et al. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology. 2014;270(3):834-841. doi:10.1148/radiol.13131669
Schmidt K, Bau M, Merschel G, et al. Anthropogenic gadolinium in tap water and in tap water-based beverages from fast-food franchises in six major cities in Germany. Sci Total Environ. 2019;687:1401-1408. doi:10.1016/j.scitotenv.2019.07.075
Kulaksız S, Bau M. Anthropogenic gadolinium as a microcontaminant in tap water used as drinking water in urban areas and megacities. Appl Geochem. 2011;26:1877-1885.
Brunjes R, Hofmann T. Anthropogenic gadolinium in freshwater and drinking water systems. Water Res. 2020;182:115966. doi:10.1016/j.watres.2020.115966
Endrikat J, Gutberlet M, Hoffmann KT, et al. Clinical safety of gadobutrol: review of over 25 years of use exceeding 100 million administrations. Invest Radiol. 2024;59(9):605-613. doi:10.1097/RLI.0000000000001072
Elmholdt TR, Jørgensen B, Ramsing M, et al. Two cases of nephrogenic systemic fibrosis after exposure to the macrocyclic compound gadobutrol. NDT Plus. 2010;3(3):285-287. doi:10.1093/ndtplus/sfq028
Cunningham A, Kirk M, Hong E, et al. The safety of magnetic resonance imaging contrast agents. Front Toxicol. 2024;6:1376587. doi:10.3389/ftox.2024.1376587
Huxley A. Complete Essays. Volume II, 1926-1929. Chicago; 2000:227.
McDonald RJ, Weinreb JC, Davenport MS. Symptoms associated with gadolinium exposure (SAGE): a suggested term. Radiology. 2022;302(2):270-273. doi:10.1148/radiol.2021211349
Henderson IM, Benevidez AD, Mowry CD, et al. Precipitation of gadolinium from magnetic resonance imaging contrast agents may be the Brass tacks of toxicity. Magn Reson Imaging. 2025;119:110383. doi:10.1016/j.mri.2025.110383
Do C, Drel V, Tan C, et al. Nephrogenic systemic fibrosis is mediated by myeloid C-C chemokine receptor 2. J Invest Dermatol. 2019;139(10):2134-2143. doi:10.1016/j.jid.2019.03.1145
Drel VR, Tan C, Barnes JL, et al. Centrality of bone marrow in the severity of gadolinium-based contrast-induced systemic fibrosis. FASEB J. 2016;30(9):3026-3038. doi:10.1096/fj.201500188R
Bruno F, DeAguero J, Do C, et al. Overlapping roles of NADPH oxidase 4 for diabetic and gadolinium-based contrast agent-induced systemic fibrosis. Am J Physiol Renal Physiol. 2021;320(4):F617-F627. doi:10.1152/ajprenal.00456.2020
Wagner B, Tan C, Barnes JL, et al. Nephrogenic systemic fibrosis: evidence for oxidative stress and bone marrow-derived fibrocytes in skin, liver, and heart lesions using a 5/6 nephrectomy rodent model. Am J Pathol. 2012;181(6):1941-1952. doi:10.1016/j.ajpath.2012.08.026
DeAguero J, Howard T, Kusewitt D, et al. The onset of rare earth metallosis begins with renal gadolinium-rich nanoparticles from magnetic resonance imaging contrast agent exposure. Sci Rep. 2023;13(1):2025. doi:10.1038/s41598-023-28666-1
Do C, Ford B, Lee DY, et al. Gadolinium-based contrast agents: Stimulators of myeloid-induced renal fibrosis and major metabolic disruptors. Toxicol Appl Pharmacol. 2019;375:32-45. doi:10.1016/j.taap.2019.05.009
Coyte RM, Darrah T, Olesik J, et al. Gadolinium during human pregnancy following administration of gadolinium chelate before pregnancy. Birth Defects Res. 2023;115(14):1264-1273. doi:10.1002/bdr2.2209
Magnetic resonance image (MRI) contrast agents can induce profound complications, including gadolinium encephalopathy, kidney injury, gadolinium-associated plaques, and progressive systemic fibrosis, which can be fatal.1-10 About 50% of MRIs use gadolinium-based contrast (Gd3+), a toxic rare earth metal ion that enhances imaging but requires binding with pharmaceutical ligands to reduce toxicity and promote renal elimination (Figure 1). Despite these measures, Gd3+ can persist in the body, including the brain.11,12 Wastewater treatment fails to remove these agents, making Gd3+ a growing pollutant in water and the food chain.13-15 Because Gd3+ is a rare earth metal ion in the milieu intérieur, there is an urgent need to study its biological and long-term effects (Appendix 1).
Case Presentation
A 65-year-old Vietnam-era veteran presented to nephrology at the Raymond G. Murphy Veterans Affairs Medical Center (RGMVAMC) in Albuquerque, New Mexico, for evaluation of gadolinium-induced symptoms. His medical history included metabolic syndrome, hypertension, hyperlipidemia, hypogonadism, cervical spondylosis, and an elevated prostate-specific antigen, previously assessed with a contrast-enhanced MRI in 2019 (Gadobenic acid, 19 mL). Surgical history included cervical fusion and ankle hardware.
The patient had a scheduled MRI 25 days earlier, following an elevated prostate specific antigen test result, prompting urologic surveillance and concern for malignancy. In preparation for the contrast-enhanced MRI, his right arm was cannulated with a line primed with gadobenic acid contrast. Though the technician stated the infusion had not started, the patient’s symptoms began shortly after entry into the scanner, before any programmed pulse sequences. The patient experienced claustrophobia, diaphoresis, palpitations, xerostomia, dysgeusia, shortness of breath, and a sensation of heat in his groin, chest, “kidneys,” and lower back. The MRI was terminated prematurely in response to the patient’s acute symptomatology. The patient continued experiencing new symptoms intermittently during the following week, including lightheadedness, headaches, right clavicular pain, raspy voice, edema, and a sense of doom.


The patient presented to the RGMVAMC emergency department (ED) 8 days after the MRI with worsening symptoms and was hospitalized for 10 days. During this time, he was referred to nephrology for outpatient evaluation. While awaiting his nephrology appointment, the patient presented to the RGMVAMC ED 20 days after the initial episode with ongoing symptoms. “I thought I was dying,” he said. Laboratory results and a 12-lead electrocardiogram showed a finely static background, wide P waves (> 80 ms) with notching in lead II, sinusoidal P waves in V1, R transition in V2, RR’ in V2, ST flat in lead III, and sinus bradycardia (Table 1 and Appendix 2).
The patient’s medical and surgical histories were reviewed at the nephrology evaluation 25 days following the MRI. He reported that household water was sourced from a well and that he filtered his drinking water with a reverse osmosis system. He served in the US Army for 10 years as an engineer specializing in mechanical systems, power generation, and vehicles. Following Army retirement, the patient served in the US Air Force Reserves for 15 years, working as a crew chief in pneudraulics. The patient reported stopping tobacco use 1 year before and also reported regular use of a broad array of prescription medications and dietary supplements, including dexamethasone (4 mg twice daily), fluticasone nasal spray (50 mcg per nostril, twice daily), ibuprofen (400 mg twice daily, as needed), loratadine (10 mg daily), aspirin (81 mg daily), and metoprolol succinate (50 mg nightly). In addition, he reported consistent use of cholecalciferol (3000 IU daily), another supplemental vitamin D preparation, chelated magnesium glycinate (3 tablets daily for bone issues), turmeric (1 tablet daily), a multivitamin (Living Green Liquid Gel, daily), and a mega-B complex.
Physical examination revealed a well-nourished, tall man with hypertension (145/87 mmHg) and bilateral lower extremity edema. Oral examination showed poor dentition, including missing molars (#1-3, #14-16, #17-19, #30-31), with the anterior teeth replaced by bridges supported by dental implants. The review of systems was otherwise unremarkable, with nocturia noted before the consultation.

Serum and urine gadolinium testing, (Mayo Clinic Laboratories) revealed gadolinium levels of 0.3 mcg/24 h in the urine and 0.1 ng/mL in the serum. Nonzero values indicated detectable gadolinium, suggesting retention. The patient had a prior gadolinium exposure during a 2019 MRI (about 1340 days before) and suspected a repeat exposure on day 0, although the MRI technician stated that no contrast was administered. Given his elevated vitamin D levels, the patient was advised to minimize dietary supplements, particularly vitamin D, to avoid confounding symptoms. The plan included monitoring symptoms and a follow-up evaluation with repeat laboratory tests on day 116.
At the nephrology follow-up 4 months postexposure, the patient's symptoms had primarily abated, with a marked reduction in the previously noted metallic dysgeusia. Physical examination remained consistent with prior findings. He was afebrile (97.7 °F) with a blood pressure of 111/72 mmHg, a pulse of 63 beats per minute, and an oxygen saturation of 98% on ambient air. Laboratory analysis revealed serum and urine gadolinium levels below detectable thresholds (< 0.1 ng/mL and < 0.1 mcg/24 h). A 24-hour creatinine clearance, calculated from a urine volume of 1300 mL, measured at an optimal 106 mL/min, indicating preserved renal function (Tables 2 and 3). Of note, his 24-hour oxalate was above the reference range, with a urine pH below the reference range and a high supersaturation index for calcium oxalate.
Discussion
Use of enhanced MRI has increased in the Veterans Health Administration (Figure 2). A growing range of indications for enhanced procedures (eg, cardiac MRI) has contributed to this rise. The market has grown with new gadolinium-based contrast agents, such as gadopiclenol. However, reliance on untested assumptions about the safety of newer agents and need for robust clinical trials pose potential risks to patient safety.
Without prospective evidence, the American College of Radiology (ACR) classifies gadolinium-based contrast agents into 3 groups: Group 1, associated with the highest number of nephrogenic systemic fibrosis cases; Group 2, linked to few, if any, unconfounded cases; and Group 3, where data on nephrogenic systemic fibrosis risk have been limited. As of April 2024, the ACR reclassified Group 3 agents (Ablavar/Vasovist/Angiomark and Primovist/Eovist) into Group 2. Curiously, Vueway and Elucirem were approved in late 2022 and should clearly be categorized as Group 3 (Table 4).There were 19 cases of nephrogenic systemic fibrosis or similar manifestations, 8 of which were unconfounded by other factors. These patients had been exposed to gadobutrol, often combined with other agents. Gadobutrol—like other Group 2 agents—has been associated with nephrogenic systemic fibrosis.16,17 Despite US Food and Drug Administration (FDA) documentation of rising reports, many clinicians remain unaware that nephrogenic systemic fibrosis is increasingly linked to Group 2 agents classified by the ACR.18 While declines in reported cases of nephrogenic systemic fibrosis may suggest reduced incidence, this trend may reflect diminished clinical vigilance and underreporting, particularly given emerging evidence implicating even Group 2 gadolinium-based contrast agents in delayed and underrecognized presentations. This information has yet to permeate the medical community, particularly among nephrologists. Considering these cases, revisiting the ACR guidelines may be prudent.

To address this growing concern, clinicians must adopt stricter vigilance and actively pursue updated information to mitigate patient risks tied to these contrast agents.
There exists an illusion of knowledge in disregarding the confounded exposures of MRI contrast agents. Ten distinct brands of contrast agents have been approved for clinical use. With repeated imaging, patients are often exposed to varying formulations of gadolinium-based agents. Yet investigators commonly discard these data points when assessing risk. By doing so, they assume—without evidence—that some formulations are inherently less likely to provoke adverse effects (AEs) than others. This untested presumption becomes perilous, especially given the limited understanding of the mechanisms underlying gadolinium-induced pathologies. As Aldous Huxley warned, “Facts do not cease to exist because they are ignored.”19
Gadolinium Persistence
Contrary to expectations, gadolinium persists in the body far longer than initially presumed. Symptoms associated with gadolinium exposure (SAGE) encapsulate the chronic, often enigmatic maladies tied to MRI contrast agents.20 The prolonged retention of this rare earth metal offers a compelling hypothesis for the etiology of SAGE. It has been hypothesized that Lewis base-rich metabolites increase susceptibility to gadolinium-based contrast agent complications.21
The blood and urine concentration elimination curves of gadolinium are exponential and categorized as fast, intermediate, and long-term.1 For urinary elimination, the function of the curves is exponential. The quantity of gadolinium in the urine at a time (t) after exposure (D[Gd](t)) is equal to the product of the amount of gadolinium in the sample (urine or blood) at the end of the fast elimination period (D[Gd](t0)) and the exponential decay with k being a rate constant.
To the authors’ knowledge, we are the only research team currently investigating the rate constant for the intermediate- and long-term phase gadolinium elimination. The Retention and Toxicity of Gadolinium-based Contrast Agents study was approved by the University of New Mexico Health Sciences Center Institutional Review Board on May 27, 2020 (IRB ID 19-660). The data for the patient in this case were compared with preliminary results for patients with exposure-to-measurement intervals < 100 days.
The patient in this case presented with detectable gadolinium levels in urine and serum shortly after an attempted contrast-enhanced MRI procedure (Figure 3). The presence of detectable gadolinium levels in the patient’s urine and serum suggests a likely exposure to a contrast agent about 27 days before his consultation. While the technician reported that no contrast was administered during the attempted MRI, it remains possible that a small amount was introduced during cannulation, potentially triggering the patient’s symptoms. Linear modeling of semilogarithmic plots for participants exposed to contrast agents within 100 days (urine: P = 1.8 × 10ˉ8, adjusted r² = 0.62; blood: P = .005, adjusted r² = 0.21) provided clearance rates (k values) for urine and blood. Extrapolating from these models to the presumed exposure date, the intercepts estimate that the patient received between 0.5% and 8% of a standard contrast dose.

MRI contrast agents can cause skin disease. Systemic fibrosis is considered one of the most severe AEs. Skin pathophysiology involving myeloid cells is driven by elevated levels of monocyte chemoattractant protein-1, which recruits circulating fibroblasts via the C-C chemokine receptor 2.22,23 This occurs alongside activation of NADPH oxidase Nox4.4,24,25 Intracellular gadolinium-rich nanoparticles likely serve as catalysts for this reactive cascade.2,18,22,26,27 These particles assemble around intracellular lipid droplets and ferrule them in spiculated rare earth-rich shells that compromise cellular architecture.2,18,21,22,26,27 Frequently sequestered within endosomal compartments, they disrupt vesicular integrity and threaten cellular homeostasis. Interference with degradative systems such as the endolysosomal axis perturbs energy-recycling pathways—an insidious disturbance, particularly in cells with high metabolic demand. Skin-related symptoms are among the most frequently reported AEs, according to the FDA AE reporting system.18
Studies indicate repeated exposure to MRI contrast agents can lead to permanent gadolinium retention in the brain and other vital organs. Intravenous (IV) contrast agents cross the blood-brain barrier rapidly, while intrathecal administration has been linked to significant and lasting neurologic effects.18
Gadolinium is chemically bound to pharmaceutical ligands to enhance renal clearance and reduce toxicity. However, available data from human samples suggest potential ligand exchanges with undefined physiologic substances. This exchange may facilitate gadolinium precipitation and accumulation within cells into spiculated nanoparticles. Transmission electron microscopy reveals the formation of unilamellar bodies associated with mitochondriopathy and cellular damage, particularly in renal proximal tubules.2,18,22,26,27 It is proposed that intracellular nanoparticle formation represents a key mechanism driving the systemic symptoms observed in patients.1,2,18, 22,26,27
Any hypothesis based on free soluble gadolinium—or concept derived from it—should be discarded. The high affinity of pharmaceutical ligands for gadolinium suggests that the cationic rare earth metal remains predominantly in a ligand-bound, soluble form. It is hypothesized that gadolinium undergoes ligand exchange with physiologic substances, directly leading to nanoparticle formation. Current data demonstrate gadolinium precipitation according to the Le Chatelier’s principle. Since precipitated gadolinium does not readily re-equilibrate with pharmaceutical ligands, repeated administration of different contrast agent brands may contribute to nanoparticle growth.26
Meanwhile, a growing number of patients are turning to chelation therapy, a largely untested treatment. The premise of chelation therapy is rooted in several unproven assumptions.18,21 First, it assumes that clinically significant amounts of gadolinium persist in compartments such as the extracellular space, where they can be effectively chelated and cleared. Second, it presumes that free gadolinium is the primary driver of chronic symptoms, an assertion that remains scientifically unsubstantiated. Finally, chelation proponents overlook the potential harm caused by depleting essential physiological metals during the process, assuming without evidence that the scant removal of gadolinium outweighs the risk of physiological mineral depletion.

These assumptions underpin an unproven remedy that demands critical scrutiny. Recent findings reveal that gadolinium deposits in the skin and kidney often take the form of intracellular nanoparticles, directly challenging the foundation of chelation therapy. Chelation advocates must demonstrate that these intracellular gadolinium deposits neither trigger cellular toxicity nor initiate a cytokine cascade. Chelation supporters must prove that the systemic response to these foreign particles is unrelated to the symptoms reported by patients. Until then, the validity of chelation therapy remains highly questionable.
The causality of the symptoms, mainly whether IV gadolinium was administered, was examined. The null hypothesis stated that the patient was not exposed to gadolinium. However, this hypothesis was contradicted by the detection of gadolinium in the serum and urine 27 days after the potential exposure.
Two plausible explanations exist for the nonzero gadolinium levels detected in the serum and urine. The first possibility is that minute quantities of gadolinium were introduced during cannulation, with the amount being sufficient to persist in measurable concentrations 27 days postexposure. The second possibility is that the gadolinium originated from an MRI contrast agent administered 4 years earlier. In this scenario, gadolinium stored in organ reservoirs such as bone, liver, or kidneys may have been mobilized into the extracellular fluid compartment due to the administration of high-dose steroids 20 days after the recent contrast-enhanced MRI procedure attempt. Coyte et al reported elevated gadolinium levels in the serum, cord blood, breast milk, and placenta of pregnant women with prior exposure to MRI contrast agents.28 These findings suggest that gadolinium, stored in organs such as bone may be remobilized by variables affecting bone remodeling (eg, high-dose steroids).
Significantly, the patient exhibited elevated urinary oxalate levels. Previous research has found that oxalic acid reacts rapidly with MRI contrast agents, forming digadolinium trioxalate. While the gadolinium-rich nanoparticles identified in tissues such as the skin and kidney (including the human kidney) are amorphous, these in vitro findings establish a proof-of-concept: the intracellular environment facilitates gadolinium dissociation from pharmaceutical chelates.

Furthermore, in vitro experiments show that proteins and lysosomal pH promote this dissociation, underscoring how human metabolic conditions—particularly oxalic acid concentration—may drive intracellular gadolinium deposition.
Patient Perspective
“They put something into my body that they cannot get out.” This stark realization underpins the patient’s profound concern about gadolinium-based contrast agents and their potential long-term effects. Reflecting on his experience, the patient expressed deep fears about the unknown future impacts: “I’m concerned about my kidneys, I’m concerned about my heart, and I’m concerned about my brain. I don’t know how this stuff is going to affect me in the future.”
He drew an unsettling parallel between gadolinium and heavy metals: “Heavy metal is poison. The body does not produce this kind of stuff on its own.” His reaction to the procedure left a lasting impression, prompting him to question the logic of using a substance that cannot be purged: “Why would you put something into someone’s body that you cannot extract? Nobody—nobody—should experience what I went through.”
The patient emphasized the lack of clear research on long-term outcomes, which compounds his anxiety: “If there was research that said, ‘Well, this is only going to affect these organs for this long,’ OK, I might be able to accept that. But there is no research like that. Nobody can tell me what’s going to happen in 5 years.”
Strengths and Limitations
A significant strength of this approach is the ability to track gadolinium elimination and symptom resolution over time, supported by unique access to intermediate and long-term clearance data from our ongoing research protocol. The investigators were equipped to back-extrapolate the exposure, which provided a rare opportunity to correlate gadolinium levels with clinical outcomes. The primary limitation is the lack of a defined clinical case definition for gadolinium toxicity and limited mechanistic understanding of SAGE, which hinders diagnosis and management.
Metabolites, proteins, and lipids rich in Lewis bases could initiate this process as substrates for intracellular gadolinium sedimentation. Future studies should investigate whether metabolic conditions such as oxalate burden or altered parathyroid hormone levels modulate gadolinium compartmentalization and tissue retention. If gadolinium-rich nanoparticle formation and accumulation disrupt cellular equilibrium, it underscores an urgent need to understand the implications of long-term gadolinium retention. The research team continues to gather evidence that the gadolinium cation remains chelated from the moment MRI contrast agents are administered through to the formation of intracellular nanoparticles. Retained gadolinium nanoparticles may act as a nidus, triggering cellular signaling cascades that lead to multisymptomatic illnesses. Intracellular and insoluble retained gadolinium challenges proponents of untested chelation therapies.
Conclusions
This case highlights emerging clinical and ethical concerns surrounding gadolinium-based contrast agent use. Clinicians may benefit from considering gadolinium retention as a contributor to persistent, unexplained symptoms—particularly in patients with recent imaging exposure. As contrast use continues to rise within federal health systems, regulatory and administrative stakeholders would do well to re-examine current safety frameworks. Informed consent should reflect what is known: gadolinium can remain in the body long after administration, potentially indefinitely. The long-term consequences of cumulative exposure remain poorly defined, but the presence of a lanthanide element in human tissue warrants greater attention from researchers and regulators alike. Interest in alternative imaging modalities and long-term safety monitoring would mark progress toward more transparent, accountable care.


Magnetic resonance image (MRI) contrast agents can induce profound complications, including gadolinium encephalopathy, kidney injury, gadolinium-associated plaques, and progressive systemic fibrosis, which can be fatal.1-10 About 50% of MRIs use gadolinium-based contrast (Gd3+), a toxic rare earth metal ion that enhances imaging but requires binding with pharmaceutical ligands to reduce toxicity and promote renal elimination (Figure 1). Despite these measures, Gd3+ can persist in the body, including the brain.11,12 Wastewater treatment fails to remove these agents, making Gd3+ a growing pollutant in water and the food chain.13-15 Because Gd3+ is a rare earth metal ion in the milieu intérieur, there is an urgent need to study its biological and long-term effects (Appendix 1).
Case Presentation
A 65-year-old Vietnam-era veteran presented to nephrology at the Raymond G. Murphy Veterans Affairs Medical Center (RGMVAMC) in Albuquerque, New Mexico, for evaluation of gadolinium-induced symptoms. His medical history included metabolic syndrome, hypertension, hyperlipidemia, hypogonadism, cervical spondylosis, and an elevated prostate-specific antigen, previously assessed with a contrast-enhanced MRI in 2019 (Gadobenic acid, 19 mL). Surgical history included cervical fusion and ankle hardware.
The patient had a scheduled MRI 25 days earlier, following an elevated prostate specific antigen test result, prompting urologic surveillance and concern for malignancy. In preparation for the contrast-enhanced MRI, his right arm was cannulated with a line primed with gadobenic acid contrast. Though the technician stated the infusion had not started, the patient’s symptoms began shortly after entry into the scanner, before any programmed pulse sequences. The patient experienced claustrophobia, diaphoresis, palpitations, xerostomia, dysgeusia, shortness of breath, and a sensation of heat in his groin, chest, “kidneys,” and lower back. The MRI was terminated prematurely in response to the patient’s acute symptomatology. The patient continued experiencing new symptoms intermittently during the following week, including lightheadedness, headaches, right clavicular pain, raspy voice, edema, and a sense of doom.


The patient presented to the RGMVAMC emergency department (ED) 8 days after the MRI with worsening symptoms and was hospitalized for 10 days. During this time, he was referred to nephrology for outpatient evaluation. While awaiting his nephrology appointment, the patient presented to the RGMVAMC ED 20 days after the initial episode with ongoing symptoms. “I thought I was dying,” he said. Laboratory results and a 12-lead electrocardiogram showed a finely static background, wide P waves (> 80 ms) with notching in lead II, sinusoidal P waves in V1, R transition in V2, RR’ in V2, ST flat in lead III, and sinus bradycardia (Table 1 and Appendix 2).
The patient’s medical and surgical histories were reviewed at the nephrology evaluation 25 days following the MRI. He reported that household water was sourced from a well and that he filtered his drinking water with a reverse osmosis system. He served in the US Army for 10 years as an engineer specializing in mechanical systems, power generation, and vehicles. Following Army retirement, the patient served in the US Air Force Reserves for 15 years, working as a crew chief in pneudraulics. The patient reported stopping tobacco use 1 year before and also reported regular use of a broad array of prescription medications and dietary supplements, including dexamethasone (4 mg twice daily), fluticasone nasal spray (50 mcg per nostril, twice daily), ibuprofen (400 mg twice daily, as needed), loratadine (10 mg daily), aspirin (81 mg daily), and metoprolol succinate (50 mg nightly). In addition, he reported consistent use of cholecalciferol (3000 IU daily), another supplemental vitamin D preparation, chelated magnesium glycinate (3 tablets daily for bone issues), turmeric (1 tablet daily), a multivitamin (Living Green Liquid Gel, daily), and a mega-B complex.
Physical examination revealed a well-nourished, tall man with hypertension (145/87 mmHg) and bilateral lower extremity edema. Oral examination showed poor dentition, including missing molars (#1-3, #14-16, #17-19, #30-31), with the anterior teeth replaced by bridges supported by dental implants. The review of systems was otherwise unremarkable, with nocturia noted before the consultation.

Serum and urine gadolinium testing, (Mayo Clinic Laboratories) revealed gadolinium levels of 0.3 mcg/24 h in the urine and 0.1 ng/mL in the serum. Nonzero values indicated detectable gadolinium, suggesting retention. The patient had a prior gadolinium exposure during a 2019 MRI (about 1340 days before) and suspected a repeat exposure on day 0, although the MRI technician stated that no contrast was administered. Given his elevated vitamin D levels, the patient was advised to minimize dietary supplements, particularly vitamin D, to avoid confounding symptoms. The plan included monitoring symptoms and a follow-up evaluation with repeat laboratory tests on day 116.
At the nephrology follow-up 4 months postexposure, the patient's symptoms had primarily abated, with a marked reduction in the previously noted metallic dysgeusia. Physical examination remained consistent with prior findings. He was afebrile (97.7 °F) with a blood pressure of 111/72 mmHg, a pulse of 63 beats per minute, and an oxygen saturation of 98% on ambient air. Laboratory analysis revealed serum and urine gadolinium levels below detectable thresholds (< 0.1 ng/mL and < 0.1 mcg/24 h). A 24-hour creatinine clearance, calculated from a urine volume of 1300 mL, measured at an optimal 106 mL/min, indicating preserved renal function (Tables 2 and 3). Of note, his 24-hour oxalate was above the reference range, with a urine pH below the reference range and a high supersaturation index for calcium oxalate.
Discussion
Use of enhanced MRI has increased in the Veterans Health Administration (Figure 2). A growing range of indications for enhanced procedures (eg, cardiac MRI) has contributed to this rise. The market has grown with new gadolinium-based contrast agents, such as gadopiclenol. However, reliance on untested assumptions about the safety of newer agents and need for robust clinical trials pose potential risks to patient safety.
Without prospective evidence, the American College of Radiology (ACR) classifies gadolinium-based contrast agents into 3 groups: Group 1, associated with the highest number of nephrogenic systemic fibrosis cases; Group 2, linked to few, if any, unconfounded cases; and Group 3, where data on nephrogenic systemic fibrosis risk have been limited. As of April 2024, the ACR reclassified Group 3 agents (Ablavar/Vasovist/Angiomark and Primovist/Eovist) into Group 2. Curiously, Vueway and Elucirem were approved in late 2022 and should clearly be categorized as Group 3 (Table 4).There were 19 cases of nephrogenic systemic fibrosis or similar manifestations, 8 of which were unconfounded by other factors. These patients had been exposed to gadobutrol, often combined with other agents. Gadobutrol—like other Group 2 agents—has been associated with nephrogenic systemic fibrosis.16,17 Despite US Food and Drug Administration (FDA) documentation of rising reports, many clinicians remain unaware that nephrogenic systemic fibrosis is increasingly linked to Group 2 agents classified by the ACR.18 While declines in reported cases of nephrogenic systemic fibrosis may suggest reduced incidence, this trend may reflect diminished clinical vigilance and underreporting, particularly given emerging evidence implicating even Group 2 gadolinium-based contrast agents in delayed and underrecognized presentations. This information has yet to permeate the medical community, particularly among nephrologists. Considering these cases, revisiting the ACR guidelines may be prudent.

To address this growing concern, clinicians must adopt stricter vigilance and actively pursue updated information to mitigate patient risks tied to these contrast agents.
There exists an illusion of knowledge in disregarding the confounded exposures of MRI contrast agents. Ten distinct brands of contrast agents have been approved for clinical use. With repeated imaging, patients are often exposed to varying formulations of gadolinium-based agents. Yet investigators commonly discard these data points when assessing risk. By doing so, they assume—without evidence—that some formulations are inherently less likely to provoke adverse effects (AEs) than others. This untested presumption becomes perilous, especially given the limited understanding of the mechanisms underlying gadolinium-induced pathologies. As Aldous Huxley warned, “Facts do not cease to exist because they are ignored.”19
Gadolinium Persistence
Contrary to expectations, gadolinium persists in the body far longer than initially presumed. Symptoms associated with gadolinium exposure (SAGE) encapsulate the chronic, often enigmatic maladies tied to MRI contrast agents.20 The prolonged retention of this rare earth metal offers a compelling hypothesis for the etiology of SAGE. It has been hypothesized that Lewis base-rich metabolites increase susceptibility to gadolinium-based contrast agent complications.21
The blood and urine concentration elimination curves of gadolinium are exponential and categorized as fast, intermediate, and long-term.1 For urinary elimination, the function of the curves is exponential. The quantity of gadolinium in the urine at a time (t) after exposure (D[Gd](t)) is equal to the product of the amount of gadolinium in the sample (urine or blood) at the end of the fast elimination period (D[Gd](t0)) and the exponential decay with k being a rate constant.
To the authors’ knowledge, we are the only research team currently investigating the rate constant for the intermediate- and long-term phase gadolinium elimination. The Retention and Toxicity of Gadolinium-based Contrast Agents study was approved by the University of New Mexico Health Sciences Center Institutional Review Board on May 27, 2020 (IRB ID 19-660). The data for the patient in this case were compared with preliminary results for patients with exposure-to-measurement intervals < 100 days.
The patient in this case presented with detectable gadolinium levels in urine and serum shortly after an attempted contrast-enhanced MRI procedure (Figure 3). The presence of detectable gadolinium levels in the patient’s urine and serum suggests a likely exposure to a contrast agent about 27 days before his consultation. While the technician reported that no contrast was administered during the attempted MRI, it remains possible that a small amount was introduced during cannulation, potentially triggering the patient’s symptoms. Linear modeling of semilogarithmic plots for participants exposed to contrast agents within 100 days (urine: P = 1.8 × 10ˉ8, adjusted r² = 0.62; blood: P = .005, adjusted r² = 0.21) provided clearance rates (k values) for urine and blood. Extrapolating from these models to the presumed exposure date, the intercepts estimate that the patient received between 0.5% and 8% of a standard contrast dose.

MRI contrast agents can cause skin disease. Systemic fibrosis is considered one of the most severe AEs. Skin pathophysiology involving myeloid cells is driven by elevated levels of monocyte chemoattractant protein-1, which recruits circulating fibroblasts via the C-C chemokine receptor 2.22,23 This occurs alongside activation of NADPH oxidase Nox4.4,24,25 Intracellular gadolinium-rich nanoparticles likely serve as catalysts for this reactive cascade.2,18,22,26,27 These particles assemble around intracellular lipid droplets and ferrule them in spiculated rare earth-rich shells that compromise cellular architecture.2,18,21,22,26,27 Frequently sequestered within endosomal compartments, they disrupt vesicular integrity and threaten cellular homeostasis. Interference with degradative systems such as the endolysosomal axis perturbs energy-recycling pathways—an insidious disturbance, particularly in cells with high metabolic demand. Skin-related symptoms are among the most frequently reported AEs, according to the FDA AE reporting system.18
Studies indicate repeated exposure to MRI contrast agents can lead to permanent gadolinium retention in the brain and other vital organs. Intravenous (IV) contrast agents cross the blood-brain barrier rapidly, while intrathecal administration has been linked to significant and lasting neurologic effects.18
Gadolinium is chemically bound to pharmaceutical ligands to enhance renal clearance and reduce toxicity. However, available data from human samples suggest potential ligand exchanges with undefined physiologic substances. This exchange may facilitate gadolinium precipitation and accumulation within cells into spiculated nanoparticles. Transmission electron microscopy reveals the formation of unilamellar bodies associated with mitochondriopathy and cellular damage, particularly in renal proximal tubules.2,18,22,26,27 It is proposed that intracellular nanoparticle formation represents a key mechanism driving the systemic symptoms observed in patients.1,2,18, 22,26,27
Any hypothesis based on free soluble gadolinium—or concept derived from it—should be discarded. The high affinity of pharmaceutical ligands for gadolinium suggests that the cationic rare earth metal remains predominantly in a ligand-bound, soluble form. It is hypothesized that gadolinium undergoes ligand exchange with physiologic substances, directly leading to nanoparticle formation. Current data demonstrate gadolinium precipitation according to the Le Chatelier’s principle. Since precipitated gadolinium does not readily re-equilibrate with pharmaceutical ligands, repeated administration of different contrast agent brands may contribute to nanoparticle growth.26
Meanwhile, a growing number of patients are turning to chelation therapy, a largely untested treatment. The premise of chelation therapy is rooted in several unproven assumptions.18,21 First, it assumes that clinically significant amounts of gadolinium persist in compartments such as the extracellular space, where they can be effectively chelated and cleared. Second, it presumes that free gadolinium is the primary driver of chronic symptoms, an assertion that remains scientifically unsubstantiated. Finally, chelation proponents overlook the potential harm caused by depleting essential physiological metals during the process, assuming without evidence that the scant removal of gadolinium outweighs the risk of physiological mineral depletion.

These assumptions underpin an unproven remedy that demands critical scrutiny. Recent findings reveal that gadolinium deposits in the skin and kidney often take the form of intracellular nanoparticles, directly challenging the foundation of chelation therapy. Chelation advocates must demonstrate that these intracellular gadolinium deposits neither trigger cellular toxicity nor initiate a cytokine cascade. Chelation supporters must prove that the systemic response to these foreign particles is unrelated to the symptoms reported by patients. Until then, the validity of chelation therapy remains highly questionable.
The causality of the symptoms, mainly whether IV gadolinium was administered, was examined. The null hypothesis stated that the patient was not exposed to gadolinium. However, this hypothesis was contradicted by the detection of gadolinium in the serum and urine 27 days after the potential exposure.
Two plausible explanations exist for the nonzero gadolinium levels detected in the serum and urine. The first possibility is that minute quantities of gadolinium were introduced during cannulation, with the amount being sufficient to persist in measurable concentrations 27 days postexposure. The second possibility is that the gadolinium originated from an MRI contrast agent administered 4 years earlier. In this scenario, gadolinium stored in organ reservoirs such as bone, liver, or kidneys may have been mobilized into the extracellular fluid compartment due to the administration of high-dose steroids 20 days after the recent contrast-enhanced MRI procedure attempt. Coyte et al reported elevated gadolinium levels in the serum, cord blood, breast milk, and placenta of pregnant women with prior exposure to MRI contrast agents.28 These findings suggest that gadolinium, stored in organs such as bone may be remobilized by variables affecting bone remodeling (eg, high-dose steroids).
Significantly, the patient exhibited elevated urinary oxalate levels. Previous research has found that oxalic acid reacts rapidly with MRI contrast agents, forming digadolinium trioxalate. While the gadolinium-rich nanoparticles identified in tissues such as the skin and kidney (including the human kidney) are amorphous, these in vitro findings establish a proof-of-concept: the intracellular environment facilitates gadolinium dissociation from pharmaceutical chelates.

Furthermore, in vitro experiments show that proteins and lysosomal pH promote this dissociation, underscoring how human metabolic conditions—particularly oxalic acid concentration—may drive intracellular gadolinium deposition.
Patient Perspective
“They put something into my body that they cannot get out.” This stark realization underpins the patient’s profound concern about gadolinium-based contrast agents and their potential long-term effects. Reflecting on his experience, the patient expressed deep fears about the unknown future impacts: “I’m concerned about my kidneys, I’m concerned about my heart, and I’m concerned about my brain. I don’t know how this stuff is going to affect me in the future.”
He drew an unsettling parallel between gadolinium and heavy metals: “Heavy metal is poison. The body does not produce this kind of stuff on its own.” His reaction to the procedure left a lasting impression, prompting him to question the logic of using a substance that cannot be purged: “Why would you put something into someone’s body that you cannot extract? Nobody—nobody—should experience what I went through.”
The patient emphasized the lack of clear research on long-term outcomes, which compounds his anxiety: “If there was research that said, ‘Well, this is only going to affect these organs for this long,’ OK, I might be able to accept that. But there is no research like that. Nobody can tell me what’s going to happen in 5 years.”
Strengths and Limitations
A significant strength of this approach is the ability to track gadolinium elimination and symptom resolution over time, supported by unique access to intermediate and long-term clearance data from our ongoing research protocol. The investigators were equipped to back-extrapolate the exposure, which provided a rare opportunity to correlate gadolinium levels with clinical outcomes. The primary limitation is the lack of a defined clinical case definition for gadolinium toxicity and limited mechanistic understanding of SAGE, which hinders diagnosis and management.
Metabolites, proteins, and lipids rich in Lewis bases could initiate this process as substrates for intracellular gadolinium sedimentation. Future studies should investigate whether metabolic conditions such as oxalate burden or altered parathyroid hormone levels modulate gadolinium compartmentalization and tissue retention. If gadolinium-rich nanoparticle formation and accumulation disrupt cellular equilibrium, it underscores an urgent need to understand the implications of long-term gadolinium retention. The research team continues to gather evidence that the gadolinium cation remains chelated from the moment MRI contrast agents are administered through to the formation of intracellular nanoparticles. Retained gadolinium nanoparticles may act as a nidus, triggering cellular signaling cascades that lead to multisymptomatic illnesses. Intracellular and insoluble retained gadolinium challenges proponents of untested chelation therapies.
Conclusions
This case highlights emerging clinical and ethical concerns surrounding gadolinium-based contrast agent use. Clinicians may benefit from considering gadolinium retention as a contributor to persistent, unexplained symptoms—particularly in patients with recent imaging exposure. As contrast use continues to rise within federal health systems, regulatory and administrative stakeholders would do well to re-examine current safety frameworks. Informed consent should reflect what is known: gadolinium can remain in the body long after administration, potentially indefinitely. The long-term consequences of cumulative exposure remain poorly defined, but the presence of a lanthanide element in human tissue warrants greater attention from researchers and regulators alike. Interest in alternative imaging modalities and long-term safety monitoring would mark progress toward more transparent, accountable care.


Jackson DB, MacIntyre T, Duarte-Miramontes V, et al. Gadolinium deposition disease: a case report and the prevalence of enhanced MRI procedures within the Veterans Health Administration. Fed Pract. 2022;39:218-225. doi:10.12788/fp.0258
Do C, DeAguero J, Brearley A, et al. Gadolinium-based contrast agent use, their safety, and practice evolution. Kidney360. 2020;1:561-568.doi:10.34067/kid.0000272019
Leyba K, Wagner B. Gadolinium-based contrast agents: why nephrologists need to be concerned. Curr Opin Nephrol Hypertens. 2019;28:154-162. doi:10.1097/MNH.0000000000000475
Wagner B, Drel V, Gorin Y. Pathophysiology of gadolinium-associated systemic fibrosis. Am J Physiol Renal Physiol. 2016;311:F1-F11. doi:10.1152/ajprenal.00166.2016
Maramattom BV, Manno EM, Wijdicks EF, et al. Gadolinium encephalopathy in a patient with renal failure. Neurology. 2005;64:1276-1278.doi:10.1212/01.WNL.0000156805.45547.6E
Sam AD II, Morasch MD, Collins J, et al. Safety of gadolinium contrast angiography in patients with chronic renal insufficiency. J Vasc Surg. 2003;38:313-318. doi:10.1016/s0741-5214(03)00315-x
Schenker MP, Solomon JA, Roberts DA. Gadolinium arteriography complicated by acute pancreatitis and acute renal failure. J Vasc Interv Radiol. 2001;12:393. doi:10.1016/s1051-0443(07)61925-3
Gemery J, Idelson B, Reid S, et al. Acute renal failure after arteriography with a gadolinium-based contrast agent. AJR Am J Roentgenol. 1998;171:1277-1278. doi:10.2214/ajr.171.5.9798860
Akgun H, Gonlusen G, Cartwright J Jr, et al. Are gadolinium-based contrast media nephrotoxic? A renal biopsy study. Arch Pathol Lab Med. 2006;130:1354-1357. doi:10.5858/2006-130-1354-AGCMNA
Gathings RM, Reddy R, Santa Cruz D, et al. Gadolinium-associated plaques: a new, distinctive clinical entity. JAMA Dermatol. 2015;151:316-319. doi:10.1001/jamadermatol.2014.2660
McDonald RJ, McDonald JS, Kallmes DF, et al. Gadolinium deposition in human brain tissues after contrast-enhanced MR imaging in adult patients without intracranial abnormalities. Radiology. 2017;285(2):546-554. doi:10.1148/radiol.2017161595
Kanda T, Ishii K, Kawaguchi H, et al. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology. 2014;270(3):834-841. doi:10.1148/radiol.13131669
Schmidt K, Bau M, Merschel G, et al. Anthropogenic gadolinium in tap water and in tap water-based beverages from fast-food franchises in six major cities in Germany. Sci Total Environ. 2019;687:1401-1408. doi:10.1016/j.scitotenv.2019.07.075
Kulaksız S, Bau M. Anthropogenic gadolinium as a microcontaminant in tap water used as drinking water in urban areas and megacities. Appl Geochem. 2011;26:1877-1885.
Brunjes R, Hofmann T. Anthropogenic gadolinium in freshwater and drinking water systems. Water Res. 2020;182:115966. doi:10.1016/j.watres.2020.115966
Endrikat J, Gutberlet M, Hoffmann KT, et al. Clinical safety of gadobutrol: review of over 25 years of use exceeding 100 million administrations. Invest Radiol. 2024;59(9):605-613. doi:10.1097/RLI.0000000000001072
Elmholdt TR, Jørgensen B, Ramsing M, et al. Two cases of nephrogenic systemic fibrosis after exposure to the macrocyclic compound gadobutrol. NDT Plus. 2010;3(3):285-287. doi:10.1093/ndtplus/sfq028
Cunningham A, Kirk M, Hong E, et al. The safety of magnetic resonance imaging contrast agents. Front Toxicol. 2024;6:1376587. doi:10.3389/ftox.2024.1376587
Huxley A. Complete Essays. Volume II, 1926-1929. Chicago; 2000:227.
McDonald RJ, Weinreb JC, Davenport MS. Symptoms associated with gadolinium exposure (SAGE): a suggested term. Radiology. 2022;302(2):270-273. doi:10.1148/radiol.2021211349
Henderson IM, Benevidez AD, Mowry CD, et al. Precipitation of gadolinium from magnetic resonance imaging contrast agents may be the Brass tacks of toxicity. Magn Reson Imaging. 2025;119:110383. doi:10.1016/j.mri.2025.110383
Do C, Drel V, Tan C, et al. Nephrogenic systemic fibrosis is mediated by myeloid C-C chemokine receptor 2. J Invest Dermatol. 2019;139(10):2134-2143. doi:10.1016/j.jid.2019.03.1145
Drel VR, Tan C, Barnes JL, et al. Centrality of bone marrow in the severity of gadolinium-based contrast-induced systemic fibrosis. FASEB J. 2016;30(9):3026-3038. doi:10.1096/fj.201500188R
Bruno F, DeAguero J, Do C, et al. Overlapping roles of NADPH oxidase 4 for diabetic and gadolinium-based contrast agent-induced systemic fibrosis. Am J Physiol Renal Physiol. 2021;320(4):F617-F627. doi:10.1152/ajprenal.00456.2020
Wagner B, Tan C, Barnes JL, et al. Nephrogenic systemic fibrosis: evidence for oxidative stress and bone marrow-derived fibrocytes in skin, liver, and heart lesions using a 5/6 nephrectomy rodent model. Am J Pathol. 2012;181(6):1941-1952. doi:10.1016/j.ajpath.2012.08.026
DeAguero J, Howard T, Kusewitt D, et al. The onset of rare earth metallosis begins with renal gadolinium-rich nanoparticles from magnetic resonance imaging contrast agent exposure. Sci Rep. 2023;13(1):2025. doi:10.1038/s41598-023-28666-1
Do C, Ford B, Lee DY, et al. Gadolinium-based contrast agents: Stimulators of myeloid-induced renal fibrosis and major metabolic disruptors. Toxicol Appl Pharmacol. 2019;375:32-45. doi:10.1016/j.taap.2019.05.009
Coyte RM, Darrah T, Olesik J, et al. Gadolinium during human pregnancy following administration of gadolinium chelate before pregnancy. Birth Defects Res. 2023;115(14):1264-1273. doi:10.1002/bdr2.2209
Jackson DB, MacIntyre T, Duarte-Miramontes V, et al. Gadolinium deposition disease: a case report and the prevalence of enhanced MRI procedures within the Veterans Health Administration. Fed Pract. 2022;39:218-225. doi:10.12788/fp.0258
Do C, DeAguero J, Brearley A, et al. Gadolinium-based contrast agent use, their safety, and practice evolution. Kidney360. 2020;1:561-568.doi:10.34067/kid.0000272019
Leyba K, Wagner B. Gadolinium-based contrast agents: why nephrologists need to be concerned. Curr Opin Nephrol Hypertens. 2019;28:154-162. doi:10.1097/MNH.0000000000000475
Wagner B, Drel V, Gorin Y. Pathophysiology of gadolinium-associated systemic fibrosis. Am J Physiol Renal Physiol. 2016;311:F1-F11. doi:10.1152/ajprenal.00166.2016
Maramattom BV, Manno EM, Wijdicks EF, et al. Gadolinium encephalopathy in a patient with renal failure. Neurology. 2005;64:1276-1278.doi:10.1212/01.WNL.0000156805.45547.6E
Sam AD II, Morasch MD, Collins J, et al. Safety of gadolinium contrast angiography in patients with chronic renal insufficiency. J Vasc Surg. 2003;38:313-318. doi:10.1016/s0741-5214(03)00315-x
Schenker MP, Solomon JA, Roberts DA. Gadolinium arteriography complicated by acute pancreatitis and acute renal failure. J Vasc Interv Radiol. 2001;12:393. doi:10.1016/s1051-0443(07)61925-3
Gemery J, Idelson B, Reid S, et al. Acute renal failure after arteriography with a gadolinium-based contrast agent. AJR Am J Roentgenol. 1998;171:1277-1278. doi:10.2214/ajr.171.5.9798860
Akgun H, Gonlusen G, Cartwright J Jr, et al. Are gadolinium-based contrast media nephrotoxic? A renal biopsy study. Arch Pathol Lab Med. 2006;130:1354-1357. doi:10.5858/2006-130-1354-AGCMNA
Gathings RM, Reddy R, Santa Cruz D, et al. Gadolinium-associated plaques: a new, distinctive clinical entity. JAMA Dermatol. 2015;151:316-319. doi:10.1001/jamadermatol.2014.2660
McDonald RJ, McDonald JS, Kallmes DF, et al. Gadolinium deposition in human brain tissues after contrast-enhanced MR imaging in adult patients without intracranial abnormalities. Radiology. 2017;285(2):546-554. doi:10.1148/radiol.2017161595
Kanda T, Ishii K, Kawaguchi H, et al. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology. 2014;270(3):834-841. doi:10.1148/radiol.13131669
Schmidt K, Bau M, Merschel G, et al. Anthropogenic gadolinium in tap water and in tap water-based beverages from fast-food franchises in six major cities in Germany. Sci Total Environ. 2019;687:1401-1408. doi:10.1016/j.scitotenv.2019.07.075
Kulaksız S, Bau M. Anthropogenic gadolinium as a microcontaminant in tap water used as drinking water in urban areas and megacities. Appl Geochem. 2011;26:1877-1885.
Brunjes R, Hofmann T. Anthropogenic gadolinium in freshwater and drinking water systems. Water Res. 2020;182:115966. doi:10.1016/j.watres.2020.115966
Endrikat J, Gutberlet M, Hoffmann KT, et al. Clinical safety of gadobutrol: review of over 25 years of use exceeding 100 million administrations. Invest Radiol. 2024;59(9):605-613. doi:10.1097/RLI.0000000000001072
Elmholdt TR, Jørgensen B, Ramsing M, et al. Two cases of nephrogenic systemic fibrosis after exposure to the macrocyclic compound gadobutrol. NDT Plus. 2010;3(3):285-287. doi:10.1093/ndtplus/sfq028
Cunningham A, Kirk M, Hong E, et al. The safety of magnetic resonance imaging contrast agents. Front Toxicol. 2024;6:1376587. doi:10.3389/ftox.2024.1376587
Huxley A. Complete Essays. Volume II, 1926-1929. Chicago; 2000:227.
McDonald RJ, Weinreb JC, Davenport MS. Symptoms associated with gadolinium exposure (SAGE): a suggested term. Radiology. 2022;302(2):270-273. doi:10.1148/radiol.2021211349
Henderson IM, Benevidez AD, Mowry CD, et al. Precipitation of gadolinium from magnetic resonance imaging contrast agents may be the Brass tacks of toxicity. Magn Reson Imaging. 2025;119:110383. doi:10.1016/j.mri.2025.110383
Do C, Drel V, Tan C, et al. Nephrogenic systemic fibrosis is mediated by myeloid C-C chemokine receptor 2. J Invest Dermatol. 2019;139(10):2134-2143. doi:10.1016/j.jid.2019.03.1145
Drel VR, Tan C, Barnes JL, et al. Centrality of bone marrow in the severity of gadolinium-based contrast-induced systemic fibrosis. FASEB J. 2016;30(9):3026-3038. doi:10.1096/fj.201500188R
Bruno F, DeAguero J, Do C, et al. Overlapping roles of NADPH oxidase 4 for diabetic and gadolinium-based contrast agent-induced systemic fibrosis. Am J Physiol Renal Physiol. 2021;320(4):F617-F627. doi:10.1152/ajprenal.00456.2020
Wagner B, Tan C, Barnes JL, et al. Nephrogenic systemic fibrosis: evidence for oxidative stress and bone marrow-derived fibrocytes in skin, liver, and heart lesions using a 5/6 nephrectomy rodent model. Am J Pathol. 2012;181(6):1941-1952. doi:10.1016/j.ajpath.2012.08.026
DeAguero J, Howard T, Kusewitt D, et al. The onset of rare earth metallosis begins with renal gadolinium-rich nanoparticles from magnetic resonance imaging contrast agent exposure. Sci Rep. 2023;13(1):2025. doi:10.1038/s41598-023-28666-1
Do C, Ford B, Lee DY, et al. Gadolinium-based contrast agents: Stimulators of myeloid-induced renal fibrosis and major metabolic disruptors. Toxicol Appl Pharmacol. 2019;375:32-45. doi:10.1016/j.taap.2019.05.009
Coyte RM, Darrah T, Olesik J, et al. Gadolinium during human pregnancy following administration of gadolinium chelate before pregnancy. Birth Defects Res. 2023;115(14):1264-1273. doi:10.1002/bdr2.2209
Gadolinium Intermediate Elimination and Persistent Symptoms After Magnetic Resonance Imaging Contrast Agent Exposure
Gadolinium Intermediate Elimination and Persistent Symptoms After Magnetic Resonance Imaging Contrast Agent Exposure
A Case Report of Unanticipated Difficult Intubation Due to Posterior Tracheal Angulation
A Case Report of Unanticipated Difficult Intubation Due to Posterior Tracheal Angulation
Tracheal deviation mostly occurs from mechanical compression of the trachea, and can be caused by a variety of clinical conditions, including trauma,¹ pharyngeal abscess,² neck hematoma,³ thyroid enlargement,4 and kyphoscoliosis.5 These conditions often result in lateral tracheal deviation, which can be associated with tracheal compression and reduction in tracheal caliber.
Anterior-posterior (A-P) tracheal deviation has rarely been reported. Kyphoscoliosis, scarring after a tracheostomy, or innominate vein compression are probable causes of A-P tracheal deviation and can be associated with tracheal narrowing and vascular fistula formation. This report describes a case of difficult endotracheal tube (ETT) advancement secondary to unexpected acute posterior tracheal deviation encountered during cardiopulmonary resuscitation (CPR). A waiver of patient consent was obtained from the Human Research Protection Program at the US Department of Veterans Affairs (VA) Puget Sound Health Care System.
Case Presentation
A 50-year-old male with a history of chronic cerebral venous sinus thrombosis and taking enoxaparin, presented to the emergency department for recurrent headaches. He experienced sudden cardiac arrest, and CPR in the form of chest compression and bag mask ventilation was immediately initiated. With the patient's head in an extended position and using a video laryngoscope, a Cormack–Lehane grade 1 view of the glottic opening was obtained and the trachea was intubated with an 8 mm (internal diameter) polyvinyl chloride ETT. Tracheal intubation was confirmed by utilizing continuous EtCO2 monitoring. The ETT was secured at 22 cm measured at the teeth.
After about 40 minutes of CPR, spontaneous circulation restarted and a portable A-P chest X-ray with the head in a neutral position indicated the ETT tip was at the level of the first rib (Figure 1). This finding, along with a persistent air leak, prompted blind advancement of the ETT to 26 cm at the teeth, but resistance to advancement was noted. A subsequent chest computed tomography (CT) with the head in a neutral position revealed the ETT remained inappropriately positioned with the tip measured 8.2 cm above the carina (Figure 2A). Concurrently, a sagittal CT view demonstrated significant posterior deviation of the mid and lower trachea. This deviation was determined to be the most likely cause of the difficulty encountered in advancing the ETT. No masses or lesions contributing to the acute tracheal angulation could be identified. Comparing CT imaging from 2 months prior, the trachea was of normal caliber and ordinarily aligned with the vertebral column (Figure 2B).
With the patient in Fowler position with the head midline, a flexible fiber-optic bronchoscopy was performed. Acute, almost 90-degree tracheal angulation was encountered and navigated by retroflexion of the flexible bronchoscope. Once the posterior tracheal wall was encountered, retroflexion was relaxed and the carina was visualized. The bronchoscope tip was placed near the carina, and the ETT was advanced over the fiber-optic bronchoscope to terminate 3 cm above the carina. A subsequent chest X-ray confirmed appropriate ETT position (Figure 3).
Discussion
Tracheal deviation in the A-P dimension resulting in difficult tracheal intubation has rarely been reported. Previous reports have described anatomical lesions contributing to similar tracheal deviation, such as retro-tracheal thyroid tissue, pronounced cervical lordosis, and severe kyphoscoliosis with destructive cervical fusion.5-8 In a study of the anatomical correlation of double lumen tube placement while using positron emission tomography CT, Cameron et al evaluated the size and angulation of the glottis and proximal trachea using calibrated CT measurements and an online digital protractor and note nearly perfect alignment of the pharynx and glottis.9 However, the trachea turned posteriorly relative to the glottis, resulting in an overall posterior angle of the proximal trachea compared to the glottis of 30.4 to 50.1 degrees, with no sex differences. The need to maneuver similar proximal tracheal angulation during endotracheal intubation has been reported as a cause of difficult intubation.10
In this case, the posterior angulation was not encountered in the proximal trachea but rather in the more distal trachea. The extreme A-P tracheal deviation was not associated with any identifiable masses or lesions. A CT performed 2 months prior demonstrated normal tracheal anatomy, and there was no interval history of neck trauma or tracheal obstruction suggestive of a likely cause for this deviation. This change in the patient’s tracheal anatomy was only discovered after CPR had been performed and as part of the workup for cardiac arrest. Iatrogenic injuries are known to occur during CPR. Common CPR-related airway injuries include tracheal mucosal injury from traumatic intubation and bony injuries to the chest wall from compressions.11 Laryngeal cartilage damage from intubation may also occur, but tracheal displacement following CPR has not been previously reported.11
This case of tracheal deviation is unlikely to be related to patient positioning, as the A-P deviation persisted in 3 separate head and neck alignments. First, during indirect laryngoscopy, performed in a standard sniffing position. Second, during the CT, performed in the supine position, with no head support. The acute A-P deviation seen in Figure 2 was clearly noted in this position. Lastly, flexible fiber-optic bronchoscopy was performed in a semiupright position with the head supported on a pillow. A-P deviation was encountered and navigated in this position during flexible fiber-optic guided ETT repositioning.
Using magnetic resonance imaging, alterations in the alignment of pharyngeal and tracheal axes have been described with changes in neck positioning; however, tracheal deviation has not been described with changes in head and neck alignment.12 Although the clinical presentation in this case was consistent with prior reports, we were unable to identify any previously reported anatomic cause for the tracheal deviation.5,6,8 Initial glottic visualization with a video laryngoscope was unremarkable, but resistance to sufficient ETT advancement past the vocal cords and a persistent air leak due to cuff herniation through the glottic opening was noticeable. The ETT was maneuvered to an appropriate position in the trachea using a flexible fiber-optic bronchoscope. The acute angulation of the trachea that was appreciated on bronchoscopy did not result in kinking of the ETT both initially and after in-situ thermosoftening of the polyvinyl chloride tube.13 Previously reported instances of A-P tracheal deviation have outlined the necessity of using alternative techniques to establish a patent airway, including the use of a laryngeal mask airway and a cuffless ETT with saline-soaked gauze packing.5,8 In 1 reported case, awake fiber-optic intubation was performed when difficult tracheal intubation was anticipated due to known A-P tracheal deviation.6
Failure of ETT advancement can be due to obstruction from the arytenoids and at the level of the vocal cords.14 When the ETT has been visualized to have traversed the vocal cords, tracheal A-P deviation should be considered as a cause of difficult ETT advancement. If an adequate endotracheal airway cannot be established, prompt consideration should be given to placement of a supraglottic airway. Early fiber-optic bronchoscopy should be used to establish the diagnosis and assist with proper ETT positioning.
Conclusions
This case illustrates the rare occurrence of A-P tracheal deviation leading to difficult intubation during CPR. The findings underscore the importance of considering A-P deviation as a potential cause of airway complications in emergency settings, especially in patients with previously normal tracheal anatomy. The successful use of flexible fiber-optic bronchoscopy in this case provides a valuable technique for addressing acute tracheal angulation. This report contributes to the limited literature on A-P tracheal deviation and serves as a reminder for clinicians to maintain a high index of suspicion for unusual airway challenges during critical interventions.
Creasy JD, Chiles C, Routh WD, et al. Overview of traumatic injury of the thoracic aorta. Radiogr Rev Publ Radiol Soc N Am Inc. 1997;17:27-45. doi:10.1148/radiographics.17.1.9017797
Yee AM, Christensen DN, Waterbrook AL, et al. Parapharyngeal abscess with tracheal deviation. Intern Emerg Med. 2017;12:1077-1078.doi:10.1007/s11739-017-1634-8
Querney J, Singh SI, Sebbag I. Tracheal deviation with phrenic nerve palsy after brachial plexus block. Anaesth Rep. 2021;9:41-43. doi:10.1002/anr3.12100
Geissler B, Wagner T, Dorn R, et al. Extensive sterile abscess in an invasive fibrous thyroiditis (Riedel’s thyroiditis) caused by an occlusive vasculitis. J Endocrinol Invest. 2001;24:111-115. doi:10.1007/BF03343824
Kim HJ, Choi YS, Park SH, et al. Difficult endotracheal intubation secondary to tracheal deviation and stenosis in a patient with severe kyphoscoliosis: a case report. Korean J Anesthesiol. 2016;69:386-389. doi:10.4097/kjae.2016.69.4.386
Crabb IJ. Anterior deviation of the trachea. Anaesthesia. 2001;56:284-286.doi:10.1046/j.1365-2044.2001.01918-17.x
De Cassai A, Boscolo A, Rose K, et al. Predictive parameters of difficult intubation in thyroid surgery: a meta-analysis. Minerva Anestesiol. 2020;86:317-326. doi:10.23736/S0375-9393.19.14127-2
Davies R. Difficult tracheal intubation secondary to a tracheal diverticulum and a 90 degree deviation in the trachea. Anaesthesia. 2000;55:923-925. doi:10.1046/j.1365-2044.2000.01664-18.x
Cameron RB, Peacock WJ, Chang XG, et al. Double lumen endobronchial tube intubation: lessons learned from anatomy. BMC Anesthesiol. 2024;24:150. doi:10.1186/s12871-024-02517-6
Walls RM, Samuels-Kalow M, Perkins A. A new maneuver for endotracheal tube insertion during difficult GlideScope intubation. J Emerg Med. 2010;39:86-88. doi:10.1016/j.jemermed.2009.11.005
Buschmann CT, Tsokos M. Frequent and rare complications of resuscitation attempts. Intensive Care Med. 2009;35:397-404. doi:10.1007/s00134-008-1255-9
Greenland KB, Edwards MJ, Hutton NJ, et al. Changes in airway configuration with different head and neck positions using magnetic resonance imaging of normal airways: a new concept with possible clinical applications. Br J Anaesth. 2010;105:683-690. doi:10.1093/bja/aeq239
Takasugi Y, Futagawa K, Umeda T, et al. Thermophysical Properties of Thermosoftening Nasotracheal Tubes. Anesth Prog. 2018;65:100-105. doi:10.2344/anpr-65-02-06
Phelan MP. Use of the endotracheal bougie introducer for difficult intubations. Am J Emerg Med. 2004;22:479-482. doi:10.1016/j.ajem.2004.07.017
Tracheal deviation mostly occurs from mechanical compression of the trachea, and can be caused by a variety of clinical conditions, including trauma,¹ pharyngeal abscess,² neck hematoma,³ thyroid enlargement,4 and kyphoscoliosis.5 These conditions often result in lateral tracheal deviation, which can be associated with tracheal compression and reduction in tracheal caliber.
Anterior-posterior (A-P) tracheal deviation has rarely been reported. Kyphoscoliosis, scarring after a tracheostomy, or innominate vein compression are probable causes of A-P tracheal deviation and can be associated with tracheal narrowing and vascular fistula formation. This report describes a case of difficult endotracheal tube (ETT) advancement secondary to unexpected acute posterior tracheal deviation encountered during cardiopulmonary resuscitation (CPR). A waiver of patient consent was obtained from the Human Research Protection Program at the US Department of Veterans Affairs (VA) Puget Sound Health Care System.
Case Presentation
A 50-year-old male with a history of chronic cerebral venous sinus thrombosis and taking enoxaparin, presented to the emergency department for recurrent headaches. He experienced sudden cardiac arrest, and CPR in the form of chest compression and bag mask ventilation was immediately initiated. With the patient's head in an extended position and using a video laryngoscope, a Cormack–Lehane grade 1 view of the glottic opening was obtained and the trachea was intubated with an 8 mm (internal diameter) polyvinyl chloride ETT. Tracheal intubation was confirmed by utilizing continuous EtCO2 monitoring. The ETT was secured at 22 cm measured at the teeth.
After about 40 minutes of CPR, spontaneous circulation restarted and a portable A-P chest X-ray with the head in a neutral position indicated the ETT tip was at the level of the first rib (Figure 1). This finding, along with a persistent air leak, prompted blind advancement of the ETT to 26 cm at the teeth, but resistance to advancement was noted. A subsequent chest computed tomography (CT) with the head in a neutral position revealed the ETT remained inappropriately positioned with the tip measured 8.2 cm above the carina (Figure 2A). Concurrently, a sagittal CT view demonstrated significant posterior deviation of the mid and lower trachea. This deviation was determined to be the most likely cause of the difficulty encountered in advancing the ETT. No masses or lesions contributing to the acute tracheal angulation could be identified. Comparing CT imaging from 2 months prior, the trachea was of normal caliber and ordinarily aligned with the vertebral column (Figure 2B).
With the patient in Fowler position with the head midline, a flexible fiber-optic bronchoscopy was performed. Acute, almost 90-degree tracheal angulation was encountered and navigated by retroflexion of the flexible bronchoscope. Once the posterior tracheal wall was encountered, retroflexion was relaxed and the carina was visualized. The bronchoscope tip was placed near the carina, and the ETT was advanced over the fiber-optic bronchoscope to terminate 3 cm above the carina. A subsequent chest X-ray confirmed appropriate ETT position (Figure 3).
Discussion
Tracheal deviation in the A-P dimension resulting in difficult tracheal intubation has rarely been reported. Previous reports have described anatomical lesions contributing to similar tracheal deviation, such as retro-tracheal thyroid tissue, pronounced cervical lordosis, and severe kyphoscoliosis with destructive cervical fusion.5-8 In a study of the anatomical correlation of double lumen tube placement while using positron emission tomography CT, Cameron et al evaluated the size and angulation of the glottis and proximal trachea using calibrated CT measurements and an online digital protractor and note nearly perfect alignment of the pharynx and glottis.9 However, the trachea turned posteriorly relative to the glottis, resulting in an overall posterior angle of the proximal trachea compared to the glottis of 30.4 to 50.1 degrees, with no sex differences. The need to maneuver similar proximal tracheal angulation during endotracheal intubation has been reported as a cause of difficult intubation.10
In this case, the posterior angulation was not encountered in the proximal trachea but rather in the more distal trachea. The extreme A-P tracheal deviation was not associated with any identifiable masses or lesions. A CT performed 2 months prior demonstrated normal tracheal anatomy, and there was no interval history of neck trauma or tracheal obstruction suggestive of a likely cause for this deviation. This change in the patient’s tracheal anatomy was only discovered after CPR had been performed and as part of the workup for cardiac arrest. Iatrogenic injuries are known to occur during CPR. Common CPR-related airway injuries include tracheal mucosal injury from traumatic intubation and bony injuries to the chest wall from compressions.11 Laryngeal cartilage damage from intubation may also occur, but tracheal displacement following CPR has not been previously reported.11
This case of tracheal deviation is unlikely to be related to patient positioning, as the A-P deviation persisted in 3 separate head and neck alignments. First, during indirect laryngoscopy, performed in a standard sniffing position. Second, during the CT, performed in the supine position, with no head support. The acute A-P deviation seen in Figure 2 was clearly noted in this position. Lastly, flexible fiber-optic bronchoscopy was performed in a semiupright position with the head supported on a pillow. A-P deviation was encountered and navigated in this position during flexible fiber-optic guided ETT repositioning.
Using magnetic resonance imaging, alterations in the alignment of pharyngeal and tracheal axes have been described with changes in neck positioning; however, tracheal deviation has not been described with changes in head and neck alignment.12 Although the clinical presentation in this case was consistent with prior reports, we were unable to identify any previously reported anatomic cause for the tracheal deviation.5,6,8 Initial glottic visualization with a video laryngoscope was unremarkable, but resistance to sufficient ETT advancement past the vocal cords and a persistent air leak due to cuff herniation through the glottic opening was noticeable. The ETT was maneuvered to an appropriate position in the trachea using a flexible fiber-optic bronchoscope. The acute angulation of the trachea that was appreciated on bronchoscopy did not result in kinking of the ETT both initially and after in-situ thermosoftening of the polyvinyl chloride tube.13 Previously reported instances of A-P tracheal deviation have outlined the necessity of using alternative techniques to establish a patent airway, including the use of a laryngeal mask airway and a cuffless ETT with saline-soaked gauze packing.5,8 In 1 reported case, awake fiber-optic intubation was performed when difficult tracheal intubation was anticipated due to known A-P tracheal deviation.6
Failure of ETT advancement can be due to obstruction from the arytenoids and at the level of the vocal cords.14 When the ETT has been visualized to have traversed the vocal cords, tracheal A-P deviation should be considered as a cause of difficult ETT advancement. If an adequate endotracheal airway cannot be established, prompt consideration should be given to placement of a supraglottic airway. Early fiber-optic bronchoscopy should be used to establish the diagnosis and assist with proper ETT positioning.
Conclusions
This case illustrates the rare occurrence of A-P tracheal deviation leading to difficult intubation during CPR. The findings underscore the importance of considering A-P deviation as a potential cause of airway complications in emergency settings, especially in patients with previously normal tracheal anatomy. The successful use of flexible fiber-optic bronchoscopy in this case provides a valuable technique for addressing acute tracheal angulation. This report contributes to the limited literature on A-P tracheal deviation and serves as a reminder for clinicians to maintain a high index of suspicion for unusual airway challenges during critical interventions.
Tracheal deviation mostly occurs from mechanical compression of the trachea, and can be caused by a variety of clinical conditions, including trauma,¹ pharyngeal abscess,² neck hematoma,³ thyroid enlargement,4 and kyphoscoliosis.5 These conditions often result in lateral tracheal deviation, which can be associated with tracheal compression and reduction in tracheal caliber.
Anterior-posterior (A-P) tracheal deviation has rarely been reported. Kyphoscoliosis, scarring after a tracheostomy, or innominate vein compression are probable causes of A-P tracheal deviation and can be associated with tracheal narrowing and vascular fistula formation. This report describes a case of difficult endotracheal tube (ETT) advancement secondary to unexpected acute posterior tracheal deviation encountered during cardiopulmonary resuscitation (CPR). A waiver of patient consent was obtained from the Human Research Protection Program at the US Department of Veterans Affairs (VA) Puget Sound Health Care System.
Case Presentation
A 50-year-old male with a history of chronic cerebral venous sinus thrombosis and taking enoxaparin, presented to the emergency department for recurrent headaches. He experienced sudden cardiac arrest, and CPR in the form of chest compression and bag mask ventilation was immediately initiated. With the patient's head in an extended position and using a video laryngoscope, a Cormack–Lehane grade 1 view of the glottic opening was obtained and the trachea was intubated with an 8 mm (internal diameter) polyvinyl chloride ETT. Tracheal intubation was confirmed by utilizing continuous EtCO2 monitoring. The ETT was secured at 22 cm measured at the teeth.
After about 40 minutes of CPR, spontaneous circulation restarted and a portable A-P chest X-ray with the head in a neutral position indicated the ETT tip was at the level of the first rib (Figure 1). This finding, along with a persistent air leak, prompted blind advancement of the ETT to 26 cm at the teeth, but resistance to advancement was noted. A subsequent chest computed tomography (CT) with the head in a neutral position revealed the ETT remained inappropriately positioned with the tip measured 8.2 cm above the carina (Figure 2A). Concurrently, a sagittal CT view demonstrated significant posterior deviation of the mid and lower trachea. This deviation was determined to be the most likely cause of the difficulty encountered in advancing the ETT. No masses or lesions contributing to the acute tracheal angulation could be identified. Comparing CT imaging from 2 months prior, the trachea was of normal caliber and ordinarily aligned with the vertebral column (Figure 2B).
With the patient in Fowler position with the head midline, a flexible fiber-optic bronchoscopy was performed. Acute, almost 90-degree tracheal angulation was encountered and navigated by retroflexion of the flexible bronchoscope. Once the posterior tracheal wall was encountered, retroflexion was relaxed and the carina was visualized. The bronchoscope tip was placed near the carina, and the ETT was advanced over the fiber-optic bronchoscope to terminate 3 cm above the carina. A subsequent chest X-ray confirmed appropriate ETT position (Figure 3).
Discussion
Tracheal deviation in the A-P dimension resulting in difficult tracheal intubation has rarely been reported. Previous reports have described anatomical lesions contributing to similar tracheal deviation, such as retro-tracheal thyroid tissue, pronounced cervical lordosis, and severe kyphoscoliosis with destructive cervical fusion.5-8 In a study of the anatomical correlation of double lumen tube placement while using positron emission tomography CT, Cameron et al evaluated the size and angulation of the glottis and proximal trachea using calibrated CT measurements and an online digital protractor and note nearly perfect alignment of the pharynx and glottis.9 However, the trachea turned posteriorly relative to the glottis, resulting in an overall posterior angle of the proximal trachea compared to the glottis of 30.4 to 50.1 degrees, with no sex differences. The need to maneuver similar proximal tracheal angulation during endotracheal intubation has been reported as a cause of difficult intubation.10
In this case, the posterior angulation was not encountered in the proximal trachea but rather in the more distal trachea. The extreme A-P tracheal deviation was not associated with any identifiable masses or lesions. A CT performed 2 months prior demonstrated normal tracheal anatomy, and there was no interval history of neck trauma or tracheal obstruction suggestive of a likely cause for this deviation. This change in the patient’s tracheal anatomy was only discovered after CPR had been performed and as part of the workup for cardiac arrest. Iatrogenic injuries are known to occur during CPR. Common CPR-related airway injuries include tracheal mucosal injury from traumatic intubation and bony injuries to the chest wall from compressions.11 Laryngeal cartilage damage from intubation may also occur, but tracheal displacement following CPR has not been previously reported.11
This case of tracheal deviation is unlikely to be related to patient positioning, as the A-P deviation persisted in 3 separate head and neck alignments. First, during indirect laryngoscopy, performed in a standard sniffing position. Second, during the CT, performed in the supine position, with no head support. The acute A-P deviation seen in Figure 2 was clearly noted in this position. Lastly, flexible fiber-optic bronchoscopy was performed in a semiupright position with the head supported on a pillow. A-P deviation was encountered and navigated in this position during flexible fiber-optic guided ETT repositioning.
Using magnetic resonance imaging, alterations in the alignment of pharyngeal and tracheal axes have been described with changes in neck positioning; however, tracheal deviation has not been described with changes in head and neck alignment.12 Although the clinical presentation in this case was consistent with prior reports, we were unable to identify any previously reported anatomic cause for the tracheal deviation.5,6,8 Initial glottic visualization with a video laryngoscope was unremarkable, but resistance to sufficient ETT advancement past the vocal cords and a persistent air leak due to cuff herniation through the glottic opening was noticeable. The ETT was maneuvered to an appropriate position in the trachea using a flexible fiber-optic bronchoscope. The acute angulation of the trachea that was appreciated on bronchoscopy did not result in kinking of the ETT both initially and after in-situ thermosoftening of the polyvinyl chloride tube.13 Previously reported instances of A-P tracheal deviation have outlined the necessity of using alternative techniques to establish a patent airway, including the use of a laryngeal mask airway and a cuffless ETT with saline-soaked gauze packing.5,8 In 1 reported case, awake fiber-optic intubation was performed when difficult tracheal intubation was anticipated due to known A-P tracheal deviation.6
Failure of ETT advancement can be due to obstruction from the arytenoids and at the level of the vocal cords.14 When the ETT has been visualized to have traversed the vocal cords, tracheal A-P deviation should be considered as a cause of difficult ETT advancement. If an adequate endotracheal airway cannot be established, prompt consideration should be given to placement of a supraglottic airway. Early fiber-optic bronchoscopy should be used to establish the diagnosis and assist with proper ETT positioning.
Conclusions
This case illustrates the rare occurrence of A-P tracheal deviation leading to difficult intubation during CPR. The findings underscore the importance of considering A-P deviation as a potential cause of airway complications in emergency settings, especially in patients with previously normal tracheal anatomy. The successful use of flexible fiber-optic bronchoscopy in this case provides a valuable technique for addressing acute tracheal angulation. This report contributes to the limited literature on A-P tracheal deviation and serves as a reminder for clinicians to maintain a high index of suspicion for unusual airway challenges during critical interventions.
Creasy JD, Chiles C, Routh WD, et al. Overview of traumatic injury of the thoracic aorta. Radiogr Rev Publ Radiol Soc N Am Inc. 1997;17:27-45. doi:10.1148/radiographics.17.1.9017797
Yee AM, Christensen DN, Waterbrook AL, et al. Parapharyngeal abscess with tracheal deviation. Intern Emerg Med. 2017;12:1077-1078.doi:10.1007/s11739-017-1634-8
Querney J, Singh SI, Sebbag I. Tracheal deviation with phrenic nerve palsy after brachial plexus block. Anaesth Rep. 2021;9:41-43. doi:10.1002/anr3.12100
Geissler B, Wagner T, Dorn R, et al. Extensive sterile abscess in an invasive fibrous thyroiditis (Riedel’s thyroiditis) caused by an occlusive vasculitis. J Endocrinol Invest. 2001;24:111-115. doi:10.1007/BF03343824
Kim HJ, Choi YS, Park SH, et al. Difficult endotracheal intubation secondary to tracheal deviation and stenosis in a patient with severe kyphoscoliosis: a case report. Korean J Anesthesiol. 2016;69:386-389. doi:10.4097/kjae.2016.69.4.386
Crabb IJ. Anterior deviation of the trachea. Anaesthesia. 2001;56:284-286.doi:10.1046/j.1365-2044.2001.01918-17.x
De Cassai A, Boscolo A, Rose K, et al. Predictive parameters of difficult intubation in thyroid surgery: a meta-analysis. Minerva Anestesiol. 2020;86:317-326. doi:10.23736/S0375-9393.19.14127-2
Davies R. Difficult tracheal intubation secondary to a tracheal diverticulum and a 90 degree deviation in the trachea. Anaesthesia. 2000;55:923-925. doi:10.1046/j.1365-2044.2000.01664-18.x
Cameron RB, Peacock WJ, Chang XG, et al. Double lumen endobronchial tube intubation: lessons learned from anatomy. BMC Anesthesiol. 2024;24:150. doi:10.1186/s12871-024-02517-6
Walls RM, Samuels-Kalow M, Perkins A. A new maneuver for endotracheal tube insertion during difficult GlideScope intubation. J Emerg Med. 2010;39:86-88. doi:10.1016/j.jemermed.2009.11.005
Buschmann CT, Tsokos M. Frequent and rare complications of resuscitation attempts. Intensive Care Med. 2009;35:397-404. doi:10.1007/s00134-008-1255-9
Greenland KB, Edwards MJ, Hutton NJ, et al. Changes in airway configuration with different head and neck positions using magnetic resonance imaging of normal airways: a new concept with possible clinical applications. Br J Anaesth. 2010;105:683-690. doi:10.1093/bja/aeq239
Takasugi Y, Futagawa K, Umeda T, et al. Thermophysical Properties of Thermosoftening Nasotracheal Tubes. Anesth Prog. 2018;65:100-105. doi:10.2344/anpr-65-02-06
Phelan MP. Use of the endotracheal bougie introducer for difficult intubations. Am J Emerg Med. 2004;22:479-482. doi:10.1016/j.ajem.2004.07.017
Creasy JD, Chiles C, Routh WD, et al. Overview of traumatic injury of the thoracic aorta. Radiogr Rev Publ Radiol Soc N Am Inc. 1997;17:27-45. doi:10.1148/radiographics.17.1.9017797
Yee AM, Christensen DN, Waterbrook AL, et al. Parapharyngeal abscess with tracheal deviation. Intern Emerg Med. 2017;12:1077-1078.doi:10.1007/s11739-017-1634-8
Querney J, Singh SI, Sebbag I. Tracheal deviation with phrenic nerve palsy after brachial plexus block. Anaesth Rep. 2021;9:41-43. doi:10.1002/anr3.12100
Geissler B, Wagner T, Dorn R, et al. Extensive sterile abscess in an invasive fibrous thyroiditis (Riedel’s thyroiditis) caused by an occlusive vasculitis. J Endocrinol Invest. 2001;24:111-115. doi:10.1007/BF03343824
Kim HJ, Choi YS, Park SH, et al. Difficult endotracheal intubation secondary to tracheal deviation and stenosis in a patient with severe kyphoscoliosis: a case report. Korean J Anesthesiol. 2016;69:386-389. doi:10.4097/kjae.2016.69.4.386
Crabb IJ. Anterior deviation of the trachea. Anaesthesia. 2001;56:284-286.doi:10.1046/j.1365-2044.2001.01918-17.x
De Cassai A, Boscolo A, Rose K, et al. Predictive parameters of difficult intubation in thyroid surgery: a meta-analysis. Minerva Anestesiol. 2020;86:317-326. doi:10.23736/S0375-9393.19.14127-2
Davies R. Difficult tracheal intubation secondary to a tracheal diverticulum and a 90 degree deviation in the trachea. Anaesthesia. 2000;55:923-925. doi:10.1046/j.1365-2044.2000.01664-18.x
Cameron RB, Peacock WJ, Chang XG, et al. Double lumen endobronchial tube intubation: lessons learned from anatomy. BMC Anesthesiol. 2024;24:150. doi:10.1186/s12871-024-02517-6
Walls RM, Samuels-Kalow M, Perkins A. A new maneuver for endotracheal tube insertion during difficult GlideScope intubation. J Emerg Med. 2010;39:86-88. doi:10.1016/j.jemermed.2009.11.005
Buschmann CT, Tsokos M. Frequent and rare complications of resuscitation attempts. Intensive Care Med. 2009;35:397-404. doi:10.1007/s00134-008-1255-9
Greenland KB, Edwards MJ, Hutton NJ, et al. Changes in airway configuration with different head and neck positions using magnetic resonance imaging of normal airways: a new concept with possible clinical applications. Br J Anaesth. 2010;105:683-690. doi:10.1093/bja/aeq239
Takasugi Y, Futagawa K, Umeda T, et al. Thermophysical Properties of Thermosoftening Nasotracheal Tubes. Anesth Prog. 2018;65:100-105. doi:10.2344/anpr-65-02-06
Phelan MP. Use of the endotracheal bougie introducer for difficult intubations. Am J Emerg Med. 2004;22:479-482. doi:10.1016/j.ajem.2004.07.017
A Case Report of Unanticipated Difficult Intubation Due to Posterior Tracheal Angulation
A Case Report of Unanticipated Difficult Intubation Due to Posterior Tracheal Angulation
A Case Report on Bortezomib-Induced Hypotension: Rare Adverse Effect in Proteasome Inhibitor Therapy
Case Presentation
A 75-year-old man with chronic kidney disease, hypertension and diabetes mellitus presented with acute kidney injury (creatinine 5.2 from baseline 4.2) and a two-week history of increased urinary frequency. Labs revealed high anion gap metabolic acidosis, proteinuria, hematuria, pyuria, and acute on chronic anemia. He was diagnosed with kappa light chain nephropathy and multiple myeloma with 32% plasma cells on bone marrow biopsy. He began treatment with bortezomib, cyclophosphamide, and dexamethasone (Cy- BorD). Three days after cyclophosphamide and five days after bortezomib, the patient developed persistent hypotension with systolic BP in the 50s, unresponsive to fluids and Trendelenburg position. Due to end-stage renal disease with anuria, fluid resuscitation was limited. He required norepinephrine and was transferred to the ICU. Given instability, hemodialysis was deferred, and continuous renal replacement therapy was initiated. Shock evaluation included a CT abdomen showing enteritis versus ileus; however, infectious workup was negative. Cardiogenic shock was ruled out with a serial echocardiogram showing normal ejection fractions of 59-67% without significant valvular disease. The workup for adrenal insufficiency was negative. After the exclusion of other potential causes of shock, severe refractory hypotension was attributed to bortezomib toxicity.Hypotension is a known adverse effect of bortezomib. Orthostatic hypotension may occur in 8 to 9% of patients, and rarely, patients may experience heart failure, conduction disorders and arrhythmias, or cardiogenic shock. The pathologic mechanism of this toxicity is still poorly understood. Proposed mechanisms include direct endothelial toxicity as evidenced by thrombotic microangiopathy or impairment of sympathetic and parasympathetic nerve fibres. Most commonly, patients experience neurotoxicity, which may manifest as autonomic dysfunction or peripheral neuropathy. Cardiovascular complications are typically reversible. Our patient’s cardiac function remained within normal limits; therefore, his persistent hypotension was felt to be the result of direct toxicity from bortezomib rather than cardiogenic shock. Ultimately, blood pressure did improve, and vasopressors were discontinued. However, he continued to have orthostatic hypotension and continued to require supportive fludrocortisone, midodrine, and pyridostigmine. Goals of care have been discussed, and he wished to continue pursuing restorative care, with a plan for transition to carfilzomib versus daratumumab outpatient.
Case Presentation
A 75-year-old man with chronic kidney disease, hypertension and diabetes mellitus presented with acute kidney injury (creatinine 5.2 from baseline 4.2) and a two-week history of increased urinary frequency. Labs revealed high anion gap metabolic acidosis, proteinuria, hematuria, pyuria, and acute on chronic anemia. He was diagnosed with kappa light chain nephropathy and multiple myeloma with 32% plasma cells on bone marrow biopsy. He began treatment with bortezomib, cyclophosphamide, and dexamethasone (Cy- BorD). Three days after cyclophosphamide and five days after bortezomib, the patient developed persistent hypotension with systolic BP in the 50s, unresponsive to fluids and Trendelenburg position. Due to end-stage renal disease with anuria, fluid resuscitation was limited. He required norepinephrine and was transferred to the ICU. Given instability, hemodialysis was deferred, and continuous renal replacement therapy was initiated. Shock evaluation included a CT abdomen showing enteritis versus ileus; however, infectious workup was negative. Cardiogenic shock was ruled out with a serial echocardiogram showing normal ejection fractions of 59-67% without significant valvular disease. The workup for adrenal insufficiency was negative. After the exclusion of other potential causes of shock, severe refractory hypotension was attributed to bortezomib toxicity.Hypotension is a known adverse effect of bortezomib. Orthostatic hypotension may occur in 8 to 9% of patients, and rarely, patients may experience heart failure, conduction disorders and arrhythmias, or cardiogenic shock. The pathologic mechanism of this toxicity is still poorly understood. Proposed mechanisms include direct endothelial toxicity as evidenced by thrombotic microangiopathy or impairment of sympathetic and parasympathetic nerve fibres. Most commonly, patients experience neurotoxicity, which may manifest as autonomic dysfunction or peripheral neuropathy. Cardiovascular complications are typically reversible. Our patient’s cardiac function remained within normal limits; therefore, his persistent hypotension was felt to be the result of direct toxicity from bortezomib rather than cardiogenic shock. Ultimately, blood pressure did improve, and vasopressors were discontinued. However, he continued to have orthostatic hypotension and continued to require supportive fludrocortisone, midodrine, and pyridostigmine. Goals of care have been discussed, and he wished to continue pursuing restorative care, with a plan for transition to carfilzomib versus daratumumab outpatient.
Case Presentation
A 75-year-old man with chronic kidney disease, hypertension and diabetes mellitus presented with acute kidney injury (creatinine 5.2 from baseline 4.2) and a two-week history of increased urinary frequency. Labs revealed high anion gap metabolic acidosis, proteinuria, hematuria, pyuria, and acute on chronic anemia. He was diagnosed with kappa light chain nephropathy and multiple myeloma with 32% plasma cells on bone marrow biopsy. He began treatment with bortezomib, cyclophosphamide, and dexamethasone (Cy- BorD). Three days after cyclophosphamide and five days after bortezomib, the patient developed persistent hypotension with systolic BP in the 50s, unresponsive to fluids and Trendelenburg position. Due to end-stage renal disease with anuria, fluid resuscitation was limited. He required norepinephrine and was transferred to the ICU. Given instability, hemodialysis was deferred, and continuous renal replacement therapy was initiated. Shock evaluation included a CT abdomen showing enteritis versus ileus; however, infectious workup was negative. Cardiogenic shock was ruled out with a serial echocardiogram showing normal ejection fractions of 59-67% without significant valvular disease. The workup for adrenal insufficiency was negative. After the exclusion of other potential causes of shock, severe refractory hypotension was attributed to bortezomib toxicity.Hypotension is a known adverse effect of bortezomib. Orthostatic hypotension may occur in 8 to 9% of patients, and rarely, patients may experience heart failure, conduction disorders and arrhythmias, or cardiogenic shock. The pathologic mechanism of this toxicity is still poorly understood. Proposed mechanisms include direct endothelial toxicity as evidenced by thrombotic microangiopathy or impairment of sympathetic and parasympathetic nerve fibres. Most commonly, patients experience neurotoxicity, which may manifest as autonomic dysfunction or peripheral neuropathy. Cardiovascular complications are typically reversible. Our patient’s cardiac function remained within normal limits; therefore, his persistent hypotension was felt to be the result of direct toxicity from bortezomib rather than cardiogenic shock. Ultimately, blood pressure did improve, and vasopressors were discontinued. However, he continued to have orthostatic hypotension and continued to require supportive fludrocortisone, midodrine, and pyridostigmine. Goals of care have been discussed, and he wished to continue pursuing restorative care, with a plan for transition to carfilzomib versus daratumumab outpatient.
Diagnostic Challenges of Persistent Hypoglycemia in a Patient with Gastrointestinal Stromal Tumors
Background
Gastrointestinal stromal tumors (GISTs) are rare neoplasms of the gastrointestinal (GI) tract, accounting for approximately 1–2% of GI cancers. Hypoglycemia in patients with GIST is an uncommon and diagnostically challenging presentation, often involving a broad differential diagnosis. This case report explores the diagnostic difficulties encountered in managing persistent hypoglycemia in a patient with a history of advanced GIST.
Case Presentation
An 80-year-old male with a history of stage IV GIST, diagnosed in 2010, presented with persistent symptomatic hypoglycemia. His medical history included extensive abdominal disease, managed with multiple interventions: esophagogastrostomy, left lateral liver resection, a Whipple procedure, and Y-90 radioembolization. He received adjuvant imatinib therapy, which was discontinued in April 2024 due to significant adverse effects, including anasarca. In 2025, the patient developed progressive hypoglycemia, ultimately requiring continuous D10 infusion to maintain euglycemia, prompting an endocrinology evaluation. The initial diagnostic workup included cortisol, insulin, C-peptide levels, and IGF-1/IGF-2 ratio ruling out insulinoma, adrenal insufficiency, and GISTrelated paraneoplastic syndrome. Imaging studies, including PET and CT, showed no radiological evidence of recurrent GIST. Treatment with octreotide infusion resulted in minimal improvement, whereas daily corticosteroid therapy significantly alleviated the patient’s symptoms. The etiology of hypoglycemia remains elusive, with potential causes under consideration including Y-90 radioembolization-induced damage to glucagon-producing cells, immunotherapy-related adverse effects, or radiologically occult GIST. Insulin autoantibody testing is pending, and the case remains under active investigation, highlighting the diagnostic complexity of hypoglycemia in advanced GIST.
Discussion
Hypoglycemia in the context of GIST is a rare and poorly understood phenomenon. Potential mechanisms include paraneoplastic syndromes, such as non-islet cell tumor hypoglycemia (NICTH) mediated by IGF-2, or treatment-related effects, such as radiation-induced pancreatic or hepatic dysfunction. In this case, the absence of detectable IGF-2 abnormalities and negative imaging complicates the diagnosis. The lack of response to octreotide indicates that somatostatin receptor-mediated pathways may not be involved. The discontinuation of imatinib and prior Y-90 radioembolization further broadens the differential, as both could contribute to metabolic dysregulation.
Conclusions
This case illustrates the need for a systematic and multidisciplinary approach to evaluate hypoglycemia in patients with advanced GIST.
Background
Gastrointestinal stromal tumors (GISTs) are rare neoplasms of the gastrointestinal (GI) tract, accounting for approximately 1–2% of GI cancers. Hypoglycemia in patients with GIST is an uncommon and diagnostically challenging presentation, often involving a broad differential diagnosis. This case report explores the diagnostic difficulties encountered in managing persistent hypoglycemia in a patient with a history of advanced GIST.
Case Presentation
An 80-year-old male with a history of stage IV GIST, diagnosed in 2010, presented with persistent symptomatic hypoglycemia. His medical history included extensive abdominal disease, managed with multiple interventions: esophagogastrostomy, left lateral liver resection, a Whipple procedure, and Y-90 radioembolization. He received adjuvant imatinib therapy, which was discontinued in April 2024 due to significant adverse effects, including anasarca. In 2025, the patient developed progressive hypoglycemia, ultimately requiring continuous D10 infusion to maintain euglycemia, prompting an endocrinology evaluation. The initial diagnostic workup included cortisol, insulin, C-peptide levels, and IGF-1/IGF-2 ratio ruling out insulinoma, adrenal insufficiency, and GISTrelated paraneoplastic syndrome. Imaging studies, including PET and CT, showed no radiological evidence of recurrent GIST. Treatment with octreotide infusion resulted in minimal improvement, whereas daily corticosteroid therapy significantly alleviated the patient’s symptoms. The etiology of hypoglycemia remains elusive, with potential causes under consideration including Y-90 radioembolization-induced damage to glucagon-producing cells, immunotherapy-related adverse effects, or radiologically occult GIST. Insulin autoantibody testing is pending, and the case remains under active investigation, highlighting the diagnostic complexity of hypoglycemia in advanced GIST.
Discussion
Hypoglycemia in the context of GIST is a rare and poorly understood phenomenon. Potential mechanisms include paraneoplastic syndromes, such as non-islet cell tumor hypoglycemia (NICTH) mediated by IGF-2, or treatment-related effects, such as radiation-induced pancreatic or hepatic dysfunction. In this case, the absence of detectable IGF-2 abnormalities and negative imaging complicates the diagnosis. The lack of response to octreotide indicates that somatostatin receptor-mediated pathways may not be involved. The discontinuation of imatinib and prior Y-90 radioembolization further broadens the differential, as both could contribute to metabolic dysregulation.
Conclusions
This case illustrates the need for a systematic and multidisciplinary approach to evaluate hypoglycemia in patients with advanced GIST.
Background
Gastrointestinal stromal tumors (GISTs) are rare neoplasms of the gastrointestinal (GI) tract, accounting for approximately 1–2% of GI cancers. Hypoglycemia in patients with GIST is an uncommon and diagnostically challenging presentation, often involving a broad differential diagnosis. This case report explores the diagnostic difficulties encountered in managing persistent hypoglycemia in a patient with a history of advanced GIST.
Case Presentation
An 80-year-old male with a history of stage IV GIST, diagnosed in 2010, presented with persistent symptomatic hypoglycemia. His medical history included extensive abdominal disease, managed with multiple interventions: esophagogastrostomy, left lateral liver resection, a Whipple procedure, and Y-90 radioembolization. He received adjuvant imatinib therapy, which was discontinued in April 2024 due to significant adverse effects, including anasarca. In 2025, the patient developed progressive hypoglycemia, ultimately requiring continuous D10 infusion to maintain euglycemia, prompting an endocrinology evaluation. The initial diagnostic workup included cortisol, insulin, C-peptide levels, and IGF-1/IGF-2 ratio ruling out insulinoma, adrenal insufficiency, and GISTrelated paraneoplastic syndrome. Imaging studies, including PET and CT, showed no radiological evidence of recurrent GIST. Treatment with octreotide infusion resulted in minimal improvement, whereas daily corticosteroid therapy significantly alleviated the patient’s symptoms. The etiology of hypoglycemia remains elusive, with potential causes under consideration including Y-90 radioembolization-induced damage to glucagon-producing cells, immunotherapy-related adverse effects, or radiologically occult GIST. Insulin autoantibody testing is pending, and the case remains under active investigation, highlighting the diagnostic complexity of hypoglycemia in advanced GIST.
Discussion
Hypoglycemia in the context of GIST is a rare and poorly understood phenomenon. Potential mechanisms include paraneoplastic syndromes, such as non-islet cell tumor hypoglycemia (NICTH) mediated by IGF-2, or treatment-related effects, such as radiation-induced pancreatic or hepatic dysfunction. In this case, the absence of detectable IGF-2 abnormalities and negative imaging complicates the diagnosis. The lack of response to octreotide indicates that somatostatin receptor-mediated pathways may not be involved. The discontinuation of imatinib and prior Y-90 radioembolization further broadens the differential, as both could contribute to metabolic dysregulation.
Conclusions
This case illustrates the need for a systematic and multidisciplinary approach to evaluate hypoglycemia in patients with advanced GIST.
Checkpoint Inhibitor-Associated Optic Neuritis: A Rare irAE With Reversible Vision Loss
Background
Immune-related adverse events (irAEs) associated with checkpoint inhibitors can involve virtually any organ system. Optic neuritis is a rare but potentially reversible toxicity, with limited reports in the literature.
Case Presentation
A 57-year-old male with Stage IV poorly-differentiated neuroendocrine carcinoma presented with progressive bilateral vision loss following a near-complete response to four cycles of atezolizumab, carboplatin, and etoposide chemotherapy, and one cycle of maintenance atezolizumab. Symptoms began in the right eye and progressed to the left over 12 days. Neurological and ophthalmological evaluations included brain and orbital MRI, autoimmune panels, and infectious workup, all of which were unrevealing. The clinical picture remained consistent with isolated, immunemediated optic neuritis.
Discussion
High-dose intravenous methylprednisolone was initiated, resulting in gradual improvement and partial visual recovery by day four. An oral prednisone taper was prescribed for continued treatment. This is the second reported case of isolated optic neuritis associated with PD-L1 inhibitor therapy and the second with negative imaging findings. The rarity of this irAE and the absence of radiographic abnormalities may delay diagnosis and treatment.
Conclusions
Checkpoint-inhibitor-induced optic neuritis should be considered in patients with visual symptoms on immunotherapy, even in the setting of negative imaging. Early recognition and corticosteroid therapy are critical in preserving visual function.
Background
Immune-related adverse events (irAEs) associated with checkpoint inhibitors can involve virtually any organ system. Optic neuritis is a rare but potentially reversible toxicity, with limited reports in the literature.
Case Presentation
A 57-year-old male with Stage IV poorly-differentiated neuroendocrine carcinoma presented with progressive bilateral vision loss following a near-complete response to four cycles of atezolizumab, carboplatin, and etoposide chemotherapy, and one cycle of maintenance atezolizumab. Symptoms began in the right eye and progressed to the left over 12 days. Neurological and ophthalmological evaluations included brain and orbital MRI, autoimmune panels, and infectious workup, all of which were unrevealing. The clinical picture remained consistent with isolated, immunemediated optic neuritis.
Discussion
High-dose intravenous methylprednisolone was initiated, resulting in gradual improvement and partial visual recovery by day four. An oral prednisone taper was prescribed for continued treatment. This is the second reported case of isolated optic neuritis associated with PD-L1 inhibitor therapy and the second with negative imaging findings. The rarity of this irAE and the absence of radiographic abnormalities may delay diagnosis and treatment.
Conclusions
Checkpoint-inhibitor-induced optic neuritis should be considered in patients with visual symptoms on immunotherapy, even in the setting of negative imaging. Early recognition and corticosteroid therapy are critical in preserving visual function.
Background
Immune-related adverse events (irAEs) associated with checkpoint inhibitors can involve virtually any organ system. Optic neuritis is a rare but potentially reversible toxicity, with limited reports in the literature.
Case Presentation
A 57-year-old male with Stage IV poorly-differentiated neuroendocrine carcinoma presented with progressive bilateral vision loss following a near-complete response to four cycles of atezolizumab, carboplatin, and etoposide chemotherapy, and one cycle of maintenance atezolizumab. Symptoms began in the right eye and progressed to the left over 12 days. Neurological and ophthalmological evaluations included brain and orbital MRI, autoimmune panels, and infectious workup, all of which were unrevealing. The clinical picture remained consistent with isolated, immunemediated optic neuritis.
Discussion
High-dose intravenous methylprednisolone was initiated, resulting in gradual improvement and partial visual recovery by day four. An oral prednisone taper was prescribed for continued treatment. This is the second reported case of isolated optic neuritis associated with PD-L1 inhibitor therapy and the second with negative imaging findings. The rarity of this irAE and the absence of radiographic abnormalities may delay diagnosis and treatment.
Conclusions
Checkpoint-inhibitor-induced optic neuritis should be considered in patients with visual symptoms on immunotherapy, even in the setting of negative imaging. Early recognition and corticosteroid therapy are critical in preserving visual function.
An Uncommon Presentation of Marginal Zone Lymphoma Involving the Sciatic Foramen
Background
Marginal zone lymphoma (MZL) is an indolent B-cell non-Hodgkin lymphoma most commonly arising in mucosal, nodal, or splenic tissues. While extranodal presentations are recognized, involvement of the sciatic foramen is exceedingly rare. We present a unique case of stage IV MZL with primary involvement of the left sciatic foramen, identified incidentally during urologic evaluation.
Case Presentation
A 74-year-old male patient was referred for hematologic evaluation after imaging revealed a left sciatic foraminal mass during work-up for elevated PSA. CT abdomen/pelvis revealed a 4.7 cm mass in the left sciatic foramen. Follow-up PET-CT confirmed hypermetabolic activity in the mass, with additional areas of uptake in the right ilium and pleural- pericardial regions. The patient was asymptomatic and denied B-symptoms. CT-guided biopsy of the sciatic mass revealed low-grade B-cell lymphoma. Flow cytometry showed a CD20-positive, CD5-negative, CD10-negative, lambda light chain–restricted population consistent with marginal zone lymphoma. Laboratory studies demonstrated iron deficiency anemia, with otherwise unremarkable counts and chemistries. He was started on monotherapy with rituximab for four cycles. He tolerated treatment well. Interval PET imaging in April 2025 showed stable disease in the sciatic foramen and mild improvement in pleural- pericardial uptake. He is planned to start obinutuzumab in the upcoming month.
Discussion
This case illustrates a rare anatomic presentation of MZL, likely representing primary sciatic foramen involvement. The presence of additional PETavid lesions complicates staging, raising consideration of stage I vs. III/IV disease. Biopsy was limited to the sciatic lesion, and no bone marrow sampling was performed. Given the patient’s excellent performance status, absence of symptoms, and low tumor burden, single-agent rituximab was chosen initially in accordance with NCCN guidelines.
Conclusions
Sciatic foramen involvement by MZL is an extremely rare occurrence and may mimic more common soft tissue or neurogenic tumors radiographically. This case underscores the importance of biopsy for diagnosis and the value of multidisciplinary care. In the veteran population, such incidental findings on imaging warrant comprehensive evaluation, particularly in atypical anatomical sites.
Background
Marginal zone lymphoma (MZL) is an indolent B-cell non-Hodgkin lymphoma most commonly arising in mucosal, nodal, or splenic tissues. While extranodal presentations are recognized, involvement of the sciatic foramen is exceedingly rare. We present a unique case of stage IV MZL with primary involvement of the left sciatic foramen, identified incidentally during urologic evaluation.
Case Presentation
A 74-year-old male patient was referred for hematologic evaluation after imaging revealed a left sciatic foraminal mass during work-up for elevated PSA. CT abdomen/pelvis revealed a 4.7 cm mass in the left sciatic foramen. Follow-up PET-CT confirmed hypermetabolic activity in the mass, with additional areas of uptake in the right ilium and pleural- pericardial regions. The patient was asymptomatic and denied B-symptoms. CT-guided biopsy of the sciatic mass revealed low-grade B-cell lymphoma. Flow cytometry showed a CD20-positive, CD5-negative, CD10-negative, lambda light chain–restricted population consistent with marginal zone lymphoma. Laboratory studies demonstrated iron deficiency anemia, with otherwise unremarkable counts and chemistries. He was started on monotherapy with rituximab for four cycles. He tolerated treatment well. Interval PET imaging in April 2025 showed stable disease in the sciatic foramen and mild improvement in pleural- pericardial uptake. He is planned to start obinutuzumab in the upcoming month.
Discussion
This case illustrates a rare anatomic presentation of MZL, likely representing primary sciatic foramen involvement. The presence of additional PETavid lesions complicates staging, raising consideration of stage I vs. III/IV disease. Biopsy was limited to the sciatic lesion, and no bone marrow sampling was performed. Given the patient’s excellent performance status, absence of symptoms, and low tumor burden, single-agent rituximab was chosen initially in accordance with NCCN guidelines.
Conclusions
Sciatic foramen involvement by MZL is an extremely rare occurrence and may mimic more common soft tissue or neurogenic tumors radiographically. This case underscores the importance of biopsy for diagnosis and the value of multidisciplinary care. In the veteran population, such incidental findings on imaging warrant comprehensive evaluation, particularly in atypical anatomical sites.
Background
Marginal zone lymphoma (MZL) is an indolent B-cell non-Hodgkin lymphoma most commonly arising in mucosal, nodal, or splenic tissues. While extranodal presentations are recognized, involvement of the sciatic foramen is exceedingly rare. We present a unique case of stage IV MZL with primary involvement of the left sciatic foramen, identified incidentally during urologic evaluation.
Case Presentation
A 74-year-old male patient was referred for hematologic evaluation after imaging revealed a left sciatic foraminal mass during work-up for elevated PSA. CT abdomen/pelvis revealed a 4.7 cm mass in the left sciatic foramen. Follow-up PET-CT confirmed hypermetabolic activity in the mass, with additional areas of uptake in the right ilium and pleural- pericardial regions. The patient was asymptomatic and denied B-symptoms. CT-guided biopsy of the sciatic mass revealed low-grade B-cell lymphoma. Flow cytometry showed a CD20-positive, CD5-negative, CD10-negative, lambda light chain–restricted population consistent with marginal zone lymphoma. Laboratory studies demonstrated iron deficiency anemia, with otherwise unremarkable counts and chemistries. He was started on monotherapy with rituximab for four cycles. He tolerated treatment well. Interval PET imaging in April 2025 showed stable disease in the sciatic foramen and mild improvement in pleural- pericardial uptake. He is planned to start obinutuzumab in the upcoming month.
Discussion
This case illustrates a rare anatomic presentation of MZL, likely representing primary sciatic foramen involvement. The presence of additional PETavid lesions complicates staging, raising consideration of stage I vs. III/IV disease. Biopsy was limited to the sciatic lesion, and no bone marrow sampling was performed. Given the patient’s excellent performance status, absence of symptoms, and low tumor burden, single-agent rituximab was chosen initially in accordance with NCCN guidelines.
Conclusions
Sciatic foramen involvement by MZL is an extremely rare occurrence and may mimic more common soft tissue or neurogenic tumors radiographically. This case underscores the importance of biopsy for diagnosis and the value of multidisciplinary care. In the veteran population, such incidental findings on imaging warrant comprehensive evaluation, particularly in atypical anatomical sites.
Targeted Syncope Workup in Hypercoagulable Patients
Background
Syncope presents a common diagnostic challenge due to its broad differential, ranging from benign to life-threatening conditions. Despite guidelines emphasizing a history- and physical examination- driven approach, nearly $33 billion is spent annually on syncope evaluations, often without yielding conclusive diagnoses. Here, we present a case of syncope secondary to cerebral venous sinus thrombosis, underscoring the importance of cerebrovascular imaging in select high-risk patient populations.
Case Presentation
An 80-year-old male with chronic sinusitis and history of pulmonary embolism (managed with thrombolysis and apixaban) presented due to an episode of transient loss of consciousness followed by nausea and vomiting. He denied any preceding symptoms but his wife did notice his arm move up for a few seconds. He didn’t have any headaches or post-ictal state. Physical exam showed normal orthostatic vital signs, symmetric blood pressure and radial pulses bilaterally. An extensive neurological exam was done and was unremarkable for any focal deficits including no vision changes. Initial evaluation including electrocardiogram, telemetry monitoring, transthoracic echocardiogram, and electroencephalography showed no significant abnormalities.
Chest CT angiography revealed right-sided segmental pulmonary emboli, unchanged from prior imaging. Head CT did not show any acute intracranial findings, and CT angiography demonstrated no vascular abnormality. Ultimately, an MRI brain revealed a left sigmoid sinus filling defect, suggestive of cerebral venous sinus thrombosis (CVST), in addition to chronic sinusitis. As CVST occurred while on apixaban, anticoagulation was switched to enoxaparin. He did not experience any recurrent symptoms during admission.
Conclusions
This case highlights the need for a patient- specific approach to syncope evaluation. Early neurovascular imaging may aid in prompt diagnosis and prevent unnecessary testing. CVST is a rare manifestation of venous thromboembolism and may present with symptoms mimicking vasovagal syncope, such as nausea and transient loss of consciousness. Typical symptoms of CVST include headaches, vomiting, vision changes, focal deficits, seizures, mental status changes, stupor or coma. Risk factors include prior thrombosis, hypercoagulable states like pregnancy or malignancy, obesity, OCPs, and chronic sinusitis. Noncontrast CT may miss CVST, and advanced neuroimaging is necessary for diagnosis in high-risk patients with thrombotic risk factors or symptoms suggestive of elevated intracranial pressure.
Background
Syncope presents a common diagnostic challenge due to its broad differential, ranging from benign to life-threatening conditions. Despite guidelines emphasizing a history- and physical examination- driven approach, nearly $33 billion is spent annually on syncope evaluations, often without yielding conclusive diagnoses. Here, we present a case of syncope secondary to cerebral venous sinus thrombosis, underscoring the importance of cerebrovascular imaging in select high-risk patient populations.
Case Presentation
An 80-year-old male with chronic sinusitis and history of pulmonary embolism (managed with thrombolysis and apixaban) presented due to an episode of transient loss of consciousness followed by nausea and vomiting. He denied any preceding symptoms but his wife did notice his arm move up for a few seconds. He didn’t have any headaches or post-ictal state. Physical exam showed normal orthostatic vital signs, symmetric blood pressure and radial pulses bilaterally. An extensive neurological exam was done and was unremarkable for any focal deficits including no vision changes. Initial evaluation including electrocardiogram, telemetry monitoring, transthoracic echocardiogram, and electroencephalography showed no significant abnormalities.
Chest CT angiography revealed right-sided segmental pulmonary emboli, unchanged from prior imaging. Head CT did not show any acute intracranial findings, and CT angiography demonstrated no vascular abnormality. Ultimately, an MRI brain revealed a left sigmoid sinus filling defect, suggestive of cerebral venous sinus thrombosis (CVST), in addition to chronic sinusitis. As CVST occurred while on apixaban, anticoagulation was switched to enoxaparin. He did not experience any recurrent symptoms during admission.
Conclusions
This case highlights the need for a patient- specific approach to syncope evaluation. Early neurovascular imaging may aid in prompt diagnosis and prevent unnecessary testing. CVST is a rare manifestation of venous thromboembolism and may present with symptoms mimicking vasovagal syncope, such as nausea and transient loss of consciousness. Typical symptoms of CVST include headaches, vomiting, vision changes, focal deficits, seizures, mental status changes, stupor or coma. Risk factors include prior thrombosis, hypercoagulable states like pregnancy or malignancy, obesity, OCPs, and chronic sinusitis. Noncontrast CT may miss CVST, and advanced neuroimaging is necessary for diagnosis in high-risk patients with thrombotic risk factors or symptoms suggestive of elevated intracranial pressure.
Background
Syncope presents a common diagnostic challenge due to its broad differential, ranging from benign to life-threatening conditions. Despite guidelines emphasizing a history- and physical examination- driven approach, nearly $33 billion is spent annually on syncope evaluations, often without yielding conclusive diagnoses. Here, we present a case of syncope secondary to cerebral venous sinus thrombosis, underscoring the importance of cerebrovascular imaging in select high-risk patient populations.
Case Presentation
An 80-year-old male with chronic sinusitis and history of pulmonary embolism (managed with thrombolysis and apixaban) presented due to an episode of transient loss of consciousness followed by nausea and vomiting. He denied any preceding symptoms but his wife did notice his arm move up for a few seconds. He didn’t have any headaches or post-ictal state. Physical exam showed normal orthostatic vital signs, symmetric blood pressure and radial pulses bilaterally. An extensive neurological exam was done and was unremarkable for any focal deficits including no vision changes. Initial evaluation including electrocardiogram, telemetry monitoring, transthoracic echocardiogram, and electroencephalography showed no significant abnormalities.
Chest CT angiography revealed right-sided segmental pulmonary emboli, unchanged from prior imaging. Head CT did not show any acute intracranial findings, and CT angiography demonstrated no vascular abnormality. Ultimately, an MRI brain revealed a left sigmoid sinus filling defect, suggestive of cerebral venous sinus thrombosis (CVST), in addition to chronic sinusitis. As CVST occurred while on apixaban, anticoagulation was switched to enoxaparin. He did not experience any recurrent symptoms during admission.
Conclusions
This case highlights the need for a patient- specific approach to syncope evaluation. Early neurovascular imaging may aid in prompt diagnosis and prevent unnecessary testing. CVST is a rare manifestation of venous thromboembolism and may present with symptoms mimicking vasovagal syncope, such as nausea and transient loss of consciousness. Typical symptoms of CVST include headaches, vomiting, vision changes, focal deficits, seizures, mental status changes, stupor or coma. Risk factors include prior thrombosis, hypercoagulable states like pregnancy or malignancy, obesity, OCPs, and chronic sinusitis. Noncontrast CT may miss CVST, and advanced neuroimaging is necessary for diagnosis in high-risk patients with thrombotic risk factors or symptoms suggestive of elevated intracranial pressure.
A Rare Delayed Presentation of Immune-Related Hepatitis in a Patient Treated With Pembrolizumab
Background
Immune checkpoint inhibitors, including pembrolizumab, are associated with a spectrum of immune-related adverse events (irAEs), including immune- mediated hepatitis. Typically, this toxicity manifests within the first 14 weeks of therapy. Delayed presentations beyond one year are exceedingly rare and pose diagnostic challenges.
Case Presentation
We report an elderly patient (over 90 years old) with stage IVa squamous cell carcinoma of the lung and high microsatellite instability (MSI) who had been receiving pembrolizumab since 2023. In 2024—13 months into therapy—he presented with subjective fevers, weakness, and altered mental status. Laboratory evaluation revealed cholestatic jaundice with AST 310 U/L, ALT 291 U/L, alkaline phosphatase 860 U/L, and total bilirubin 5.7 mg/dL. Infectious workup was negative. Imaging via MRCP showed multiple scattered hepatic cysts and a small pancreatic cyst, without biliary obstruction.
Further evaluation, including serologies for hepatitis B and C, CMV, HSV, autoimmune hepatitis panel, iron studies, and ceruloplasmin, was unremarkable except for mildly elevated alpha-1 antitrypsin. Scattered liver cysts were seen on an MRI. The overall findings were most consistent with immune-related hepatitis, as pembrolizumab is known to cause both hepatocellular and cholestatic patterns of liver injury.
The patient was started on high-dose prednisone, resulting in rapid clinical and biochemical improvement. Two weeks post-discharge, liver function tests (LFTs) had markedly improved (bilirubin 1.3, AST 19, ALT 40, ALP 193). Given the severity of transaminitis and hyperbilirubinemia (AST >8x ULN, bilirubin >3x ULN), pembrolizumab was permanently discontinued. LFTs normalized after completion of the steroid taper.
Conclusions
This case highlights a rare instance of delayed immune-related hepatitis occurring over a year after initiation of pembrolizumab, far beyond the typical window of onset. Clinicians should maintain a high index of suspicion for irAEs even in late stages of immunotherapy, particularly when common etiologies are excluded. Prompt recognition and corticosteroid treatment can lead to favorable outcomes, even in older patients.
Background
Immune checkpoint inhibitors, including pembrolizumab, are associated with a spectrum of immune-related adverse events (irAEs), including immune- mediated hepatitis. Typically, this toxicity manifests within the first 14 weeks of therapy. Delayed presentations beyond one year are exceedingly rare and pose diagnostic challenges.
Case Presentation
We report an elderly patient (over 90 years old) with stage IVa squamous cell carcinoma of the lung and high microsatellite instability (MSI) who had been receiving pembrolizumab since 2023. In 2024—13 months into therapy—he presented with subjective fevers, weakness, and altered mental status. Laboratory evaluation revealed cholestatic jaundice with AST 310 U/L, ALT 291 U/L, alkaline phosphatase 860 U/L, and total bilirubin 5.7 mg/dL. Infectious workup was negative. Imaging via MRCP showed multiple scattered hepatic cysts and a small pancreatic cyst, without biliary obstruction.
Further evaluation, including serologies for hepatitis B and C, CMV, HSV, autoimmune hepatitis panel, iron studies, and ceruloplasmin, was unremarkable except for mildly elevated alpha-1 antitrypsin. Scattered liver cysts were seen on an MRI. The overall findings were most consistent with immune-related hepatitis, as pembrolizumab is known to cause both hepatocellular and cholestatic patterns of liver injury.
The patient was started on high-dose prednisone, resulting in rapid clinical and biochemical improvement. Two weeks post-discharge, liver function tests (LFTs) had markedly improved (bilirubin 1.3, AST 19, ALT 40, ALP 193). Given the severity of transaminitis and hyperbilirubinemia (AST >8x ULN, bilirubin >3x ULN), pembrolizumab was permanently discontinued. LFTs normalized after completion of the steroid taper.
Conclusions
This case highlights a rare instance of delayed immune-related hepatitis occurring over a year after initiation of pembrolizumab, far beyond the typical window of onset. Clinicians should maintain a high index of suspicion for irAEs even in late stages of immunotherapy, particularly when common etiologies are excluded. Prompt recognition and corticosteroid treatment can lead to favorable outcomes, even in older patients.
Background
Immune checkpoint inhibitors, including pembrolizumab, are associated with a spectrum of immune-related adverse events (irAEs), including immune- mediated hepatitis. Typically, this toxicity manifests within the first 14 weeks of therapy. Delayed presentations beyond one year are exceedingly rare and pose diagnostic challenges.
Case Presentation
We report an elderly patient (over 90 years old) with stage IVa squamous cell carcinoma of the lung and high microsatellite instability (MSI) who had been receiving pembrolizumab since 2023. In 2024—13 months into therapy—he presented with subjective fevers, weakness, and altered mental status. Laboratory evaluation revealed cholestatic jaundice with AST 310 U/L, ALT 291 U/L, alkaline phosphatase 860 U/L, and total bilirubin 5.7 mg/dL. Infectious workup was negative. Imaging via MRCP showed multiple scattered hepatic cysts and a small pancreatic cyst, without biliary obstruction.
Further evaluation, including serologies for hepatitis B and C, CMV, HSV, autoimmune hepatitis panel, iron studies, and ceruloplasmin, was unremarkable except for mildly elevated alpha-1 antitrypsin. Scattered liver cysts were seen on an MRI. The overall findings were most consistent with immune-related hepatitis, as pembrolizumab is known to cause both hepatocellular and cholestatic patterns of liver injury.
The patient was started on high-dose prednisone, resulting in rapid clinical and biochemical improvement. Two weeks post-discharge, liver function tests (LFTs) had markedly improved (bilirubin 1.3, AST 19, ALT 40, ALP 193). Given the severity of transaminitis and hyperbilirubinemia (AST >8x ULN, bilirubin >3x ULN), pembrolizumab was permanently discontinued. LFTs normalized after completion of the steroid taper.
Conclusions
This case highlights a rare instance of delayed immune-related hepatitis occurring over a year after initiation of pembrolizumab, far beyond the typical window of onset. Clinicians should maintain a high index of suspicion for irAEs even in late stages of immunotherapy, particularly when common etiologies are excluded. Prompt recognition and corticosteroid treatment can lead to favorable outcomes, even in older patients.
An Unprecedented Case of AL and Apolipoprotein A-IV Renal Amyloidosis
Background
Whereas light chain (AL) amyloid is the most common cause of amyloidosis, Apolipoprotein A-IV (AApoAIV) amyloid is rare. Here we describe the first case of renal amyloidosis with pathology demonstrating concurrent deposition of AL and AApoAIV amyloid.
Case Presentation
A 79-year-old man presented with bilateral leg swelling and was diagnosed with nephrotic syndrome with preserved renal function. Serum protein electrophoresis and immunofixation showed no measurable monoclonal protein. Serum lambda free light chains were elevated at 97.72 mg/L, and kappa:lambda ratio was 0.22. Pathology from renal biopsy showed focal deposits of Congo red staining. Liquid chromatography tandem mass spectrometry identified both fibrillogenic ApoIV signal sequence peptides and a peptide profile consistent with AL amyloid deposition.
Further testing showed mild Bence Jones proteinuria, mildly elevated beta-2 microglobulin, elevated cardiac troponin T, normal NT-pro-B-type natriuretic peptide, and normal Factor X activity. Echocardiogram and FDG PET CT were unremarkable. Bone marrow biopsy demonstrated a lambda-restricted monotypic plasma cell population comprising 10% of plasma cells by immunohistochemistry. Although the Congo red stain was indeterminate, IGH::CCND1 fusion was detected by FISH.
Treatment was initiated with daratumumab, cyclophosphamide, bortezomib, and dexamethasone. Serum lambda light chains normalized after one cycle. Urine protein:creatinine ratio improved from 7.0 to 3.0 by the end of cycle 3. The patient will soon complete cycle 4 and undergo reassessment.
Discussion
While there are rare reports of AApoAIV renal amyloidosis and more prevalent cases of AL renal amyloidosis, the presence of both types of amyloid in the same patient has not been previously reported. AApoAIV renal amyloidosis has no established treatment and tends to present with rising serum creatinine as opposed to proteinuria. Given our patient’s clinical presentation with nephrotic syndrome and current light chain response to treatment, AL amyloidosis may be predominantly driving the disease.
Conclusions
Given the rarity of renal amyloidosis involving both AL and AApoAIV amyloid, the clinical course and optimal treatments are not established. The cumulative knowledge gained from individual case studies can serve as a basis for ongoing investigation and management of similar cases in the future.
Background
Whereas light chain (AL) amyloid is the most common cause of amyloidosis, Apolipoprotein A-IV (AApoAIV) amyloid is rare. Here we describe the first case of renal amyloidosis with pathology demonstrating concurrent deposition of AL and AApoAIV amyloid.
Case Presentation
A 79-year-old man presented with bilateral leg swelling and was diagnosed with nephrotic syndrome with preserved renal function. Serum protein electrophoresis and immunofixation showed no measurable monoclonal protein. Serum lambda free light chains were elevated at 97.72 mg/L, and kappa:lambda ratio was 0.22. Pathology from renal biopsy showed focal deposits of Congo red staining. Liquid chromatography tandem mass spectrometry identified both fibrillogenic ApoIV signal sequence peptides and a peptide profile consistent with AL amyloid deposition.
Further testing showed mild Bence Jones proteinuria, mildly elevated beta-2 microglobulin, elevated cardiac troponin T, normal NT-pro-B-type natriuretic peptide, and normal Factor X activity. Echocardiogram and FDG PET CT were unremarkable. Bone marrow biopsy demonstrated a lambda-restricted monotypic plasma cell population comprising 10% of plasma cells by immunohistochemistry. Although the Congo red stain was indeterminate, IGH::CCND1 fusion was detected by FISH.
Treatment was initiated with daratumumab, cyclophosphamide, bortezomib, and dexamethasone. Serum lambda light chains normalized after one cycle. Urine protein:creatinine ratio improved from 7.0 to 3.0 by the end of cycle 3. The patient will soon complete cycle 4 and undergo reassessment.
Discussion
While there are rare reports of AApoAIV renal amyloidosis and more prevalent cases of AL renal amyloidosis, the presence of both types of amyloid in the same patient has not been previously reported. AApoAIV renal amyloidosis has no established treatment and tends to present with rising serum creatinine as opposed to proteinuria. Given our patient’s clinical presentation with nephrotic syndrome and current light chain response to treatment, AL amyloidosis may be predominantly driving the disease.
Conclusions
Given the rarity of renal amyloidosis involving both AL and AApoAIV amyloid, the clinical course and optimal treatments are not established. The cumulative knowledge gained from individual case studies can serve as a basis for ongoing investigation and management of similar cases in the future.
Background
Whereas light chain (AL) amyloid is the most common cause of amyloidosis, Apolipoprotein A-IV (AApoAIV) amyloid is rare. Here we describe the first case of renal amyloidosis with pathology demonstrating concurrent deposition of AL and AApoAIV amyloid.
Case Presentation
A 79-year-old man presented with bilateral leg swelling and was diagnosed with nephrotic syndrome with preserved renal function. Serum protein electrophoresis and immunofixation showed no measurable monoclonal protein. Serum lambda free light chains were elevated at 97.72 mg/L, and kappa:lambda ratio was 0.22. Pathology from renal biopsy showed focal deposits of Congo red staining. Liquid chromatography tandem mass spectrometry identified both fibrillogenic ApoIV signal sequence peptides and a peptide profile consistent with AL amyloid deposition.
Further testing showed mild Bence Jones proteinuria, mildly elevated beta-2 microglobulin, elevated cardiac troponin T, normal NT-pro-B-type natriuretic peptide, and normal Factor X activity. Echocardiogram and FDG PET CT were unremarkable. Bone marrow biopsy demonstrated a lambda-restricted monotypic plasma cell population comprising 10% of plasma cells by immunohistochemistry. Although the Congo red stain was indeterminate, IGH::CCND1 fusion was detected by FISH.
Treatment was initiated with daratumumab, cyclophosphamide, bortezomib, and dexamethasone. Serum lambda light chains normalized after one cycle. Urine protein:creatinine ratio improved from 7.0 to 3.0 by the end of cycle 3. The patient will soon complete cycle 4 and undergo reassessment.
Discussion
While there are rare reports of AApoAIV renal amyloidosis and more prevalent cases of AL renal amyloidosis, the presence of both types of amyloid in the same patient has not been previously reported. AApoAIV renal amyloidosis has no established treatment and tends to present with rising serum creatinine as opposed to proteinuria. Given our patient’s clinical presentation with nephrotic syndrome and current light chain response to treatment, AL amyloidosis may be predominantly driving the disease.
Conclusions
Given the rarity of renal amyloidosis involving both AL and AApoAIV amyloid, the clinical course and optimal treatments are not established. The cumulative knowledge gained from individual case studies can serve as a basis for ongoing investigation and management of similar cases in the future.
Hypereosinophilic Syndrome With Eosinophilic Endomyocarditis: A Rare Cardiac Manifestation
Background
Hypereosinophilic syndrome (HES) is a rare condition caused by an overproduction of eosinophils leading to tissue infiltration and end-organ damage. HES can infiltrate the heart and lead to rare but severe cases of eosinophilic endomyocarditis, potentially causing heart failure, restrictive cardiomyopathy, and thromboembolic events.
Case Presentation
A 53-year-old female presented for abdominal pain but was found to have significant leukocytosis and eosinophilia with an absolute eosinophil count of 15.50×109/L. Further imaging with cardiac MRI showed early nodular subendocardial enhancement suggestive of eosinophilic endomyocarditis. Bone marrow biopsy was negative for clonal disorders and gastric biopsy was negative for eosinophils and H. pylori. Treatment with high-dose prednisone caused reduction in eosinophils and repeat cardiac MRI showed significant improvement in endomyocarditis.
Discussion
HES is a rare condition characterized by persistently elevated eosinophilia that can cause end organ damage, mainly affecting the heart, lungs, skin and GI system. It can be caused by primary, secondary, or idiopathic mechanisms. Primary HES often involves genetic mutations, whereas secondary HES arises due to infections or malignancies. Idiopathic HES is mainly a diagnosis of exclusion. Workup includes bone marrow biopsies and molecular testing to help differentiate between different causes and guide treatment. Eosinophilic endomyocarditis (EM) is a rare and severe complication of HES caused by eosinophilic infiltration of the myocardium. It is characterized by myocardial inflammation, fibrosis, edema, arrhythmias and heart failure if left untreated. EM is a major cause of mortality and morbidity among patients with HES. Cardiac MRI is helpful for early detection but endomyocardial biopsy is the gold standard for definitive diagnosis. Early treatment with corticosteroids can significantly reduce eosinophilic infiltration and improve outcomes. Given the severity of this rare manifestation of HES, further research is needed to help improve diagnostic and treatment strategies for EM.
Conclusions
HES is a rare condition that can cause damage affecting multiple organs with one such complication being eosinophilic endomyocarditis, a condition known to increase mortality and morbidity in those with HES. Early but accurate diagnosis and timely intervention with corticosteroids is necessary for improving the overall outcomes of those affected with this.
Background
Hypereosinophilic syndrome (HES) is a rare condition caused by an overproduction of eosinophils leading to tissue infiltration and end-organ damage. HES can infiltrate the heart and lead to rare but severe cases of eosinophilic endomyocarditis, potentially causing heart failure, restrictive cardiomyopathy, and thromboembolic events.
Case Presentation
A 53-year-old female presented for abdominal pain but was found to have significant leukocytosis and eosinophilia with an absolute eosinophil count of 15.50×109/L. Further imaging with cardiac MRI showed early nodular subendocardial enhancement suggestive of eosinophilic endomyocarditis. Bone marrow biopsy was negative for clonal disorders and gastric biopsy was negative for eosinophils and H. pylori. Treatment with high-dose prednisone caused reduction in eosinophils and repeat cardiac MRI showed significant improvement in endomyocarditis.
Discussion
HES is a rare condition characterized by persistently elevated eosinophilia that can cause end organ damage, mainly affecting the heart, lungs, skin and GI system. It can be caused by primary, secondary, or idiopathic mechanisms. Primary HES often involves genetic mutations, whereas secondary HES arises due to infections or malignancies. Idiopathic HES is mainly a diagnosis of exclusion. Workup includes bone marrow biopsies and molecular testing to help differentiate between different causes and guide treatment. Eosinophilic endomyocarditis (EM) is a rare and severe complication of HES caused by eosinophilic infiltration of the myocardium. It is characterized by myocardial inflammation, fibrosis, edema, arrhythmias and heart failure if left untreated. EM is a major cause of mortality and morbidity among patients with HES. Cardiac MRI is helpful for early detection but endomyocardial biopsy is the gold standard for definitive diagnosis. Early treatment with corticosteroids can significantly reduce eosinophilic infiltration and improve outcomes. Given the severity of this rare manifestation of HES, further research is needed to help improve diagnostic and treatment strategies for EM.
Conclusions
HES is a rare condition that can cause damage affecting multiple organs with one such complication being eosinophilic endomyocarditis, a condition known to increase mortality and morbidity in those with HES. Early but accurate diagnosis and timely intervention with corticosteroids is necessary for improving the overall outcomes of those affected with this.
Background
Hypereosinophilic syndrome (HES) is a rare condition caused by an overproduction of eosinophils leading to tissue infiltration and end-organ damage. HES can infiltrate the heart and lead to rare but severe cases of eosinophilic endomyocarditis, potentially causing heart failure, restrictive cardiomyopathy, and thromboembolic events.
Case Presentation
A 53-year-old female presented for abdominal pain but was found to have significant leukocytosis and eosinophilia with an absolute eosinophil count of 15.50×109/L. Further imaging with cardiac MRI showed early nodular subendocardial enhancement suggestive of eosinophilic endomyocarditis. Bone marrow biopsy was negative for clonal disorders and gastric biopsy was negative for eosinophils and H. pylori. Treatment with high-dose prednisone caused reduction in eosinophils and repeat cardiac MRI showed significant improvement in endomyocarditis.
Discussion
HES is a rare condition characterized by persistently elevated eosinophilia that can cause end organ damage, mainly affecting the heart, lungs, skin and GI system. It can be caused by primary, secondary, or idiopathic mechanisms. Primary HES often involves genetic mutations, whereas secondary HES arises due to infections or malignancies. Idiopathic HES is mainly a diagnosis of exclusion. Workup includes bone marrow biopsies and molecular testing to help differentiate between different causes and guide treatment. Eosinophilic endomyocarditis (EM) is a rare and severe complication of HES caused by eosinophilic infiltration of the myocardium. It is characterized by myocardial inflammation, fibrosis, edema, arrhythmias and heart failure if left untreated. EM is a major cause of mortality and morbidity among patients with HES. Cardiac MRI is helpful for early detection but endomyocardial biopsy is the gold standard for definitive diagnosis. Early treatment with corticosteroids can significantly reduce eosinophilic infiltration and improve outcomes. Given the severity of this rare manifestation of HES, further research is needed to help improve diagnostic and treatment strategies for EM.
Conclusions
HES is a rare condition that can cause damage affecting multiple organs with one such complication being eosinophilic endomyocarditis, a condition known to increase mortality and morbidity in those with HES. Early but accurate diagnosis and timely intervention with corticosteroids is necessary for improving the overall outcomes of those affected with this.