User login
Isolated Radiopalmar Dislocation of Fifth Carpometacarpal Joint: A Rare Presentation
Isolated dislocation of the carpometacarpal (CMC) joint of the hand is a rare injury. While the dislocation can be dorsal or palmar, dorsal dislocation is more common. Palmar dislocations can be either ulnopalmar or radiopalmar. There are very few reports of isolated radiopalmar dislocations of the fifth CMC joint in the English-language literature.1-3 We present a case of delayed presentation and management of radiopalmar dislocation of the fifth CMC joint. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
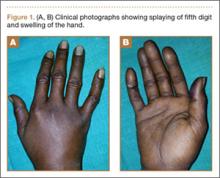
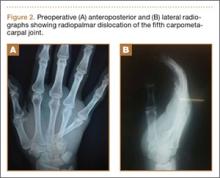
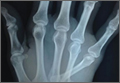
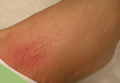
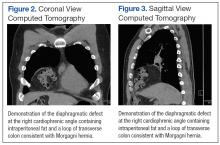
A 42-year-old man presented with polytrauma to our emergency department. He was stabilized initially, and open fractures were treated by débridement and external fixator application. During an examination 3 days after admission, swelling was noted in the right hand. On further study, there was splaying of the fifth digit and tenderness over the fourth and fifth CMC joints (Figure 1). No abnormal mobility or crepitus could be elicited. Plain radiographs of the right hand in anteroposterior and lateral views revealed radiopalmar dislocation of the fifth CMC joint (Figure 2). It was decided to reduce the dislocation immediately after the patient was declared fit for surgery.
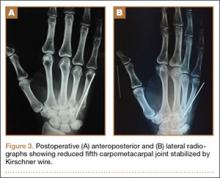
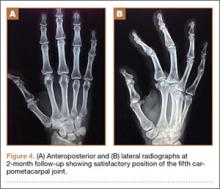
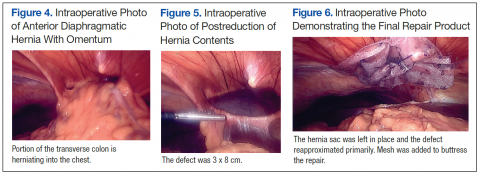
Under axillary block, closed reduction was unsuccessful. Open reduction of the fifth CMC joint was performed through a dorsal incision. The base of the fifth metacarpal bone was found to be stripped of soft-tissue attachments and lying in a radiopalmar location. Reduction, which was checked under image intensifier, was found to be satisfactory (Figure 3). Reduction was stabilized by passing a smooth Kirschner wire (K-wire) from the fifth metacarpal to the hamate bone. After achieving hemostasis, the wound was closed in layers and a below-elbow splint was applied. The perioperative period was uneventful, and sutures were removed on postoperative day 10. The K-wire was removed after 4 weeks, and radiographs showed satisfactory position of the fifth CMC joint. Gentle active and passive mobilization of fingers and wrist were started. The patient had regained good function of the wrist and fingers 2 months after surgery (Figure 4).
Discussion
Carpometacarpal joint dislocations are uncommon injuries and account for less than 1% of hand injuries.4 They are classified as dorsal and volar (palmar) dislocations. Dorsal dislocations of the CMC joints occur more frequently than do volar dislocations, mainly affecting the fourth and fifth digits.5 Isolated volar or palmar dislocation of the fifth CMC joint is an uncommon injury that was first reported in 1918 by McWhorter.6 In 1968, Nalebuff7 classified the volar dislocations into 2 groups according to the direction of the displacement of the fifth metacarpal base: radiopalmar and ulnopalmar. Berg and Murphy8 found the hook of the hamate to deviate the metacarpal bone to either the ulnar or radial side. Tearing of all ligament and tendon attachments of the base of the fifth metacarpal results in radiopalmar dislocation.7 The attachments of ligaments and tendons remain intact in the ulnopalmar dislocation.7
The clinical features of this injury are pain and swelling about the base of the fifth metacarpal and axial deformity of the little finger with apparent shortening. The deep motor branch of the ulnar nerve lies volar to the fifth CMC joint as it courses around the hook of the hamate. It is vulnerable to injury in both dorsal9,10 and volar11 CMC dislocations. For radiologic evaluation, in addition to standard anteroposterior and lateral radiographs, a lateral view in 30º pronation of the hand can provide an improved view of the fifth CMC joint, as suggested by Bora and Didizian.12
The treatment of ulnopalmar dislocation has evolved. Ulnopalmar dislocations have been successfully treated by closed reduction without fixation,8 and by open reduction and K-wire fixation.3,7,13
Radiopalmar dislocations are inherently unstable because of the tearing of all ligament and tendon attachments of the base of the fifth metacarpal.7 In our case of radiopalmar dislocation, diagnosis was delayed and attempts at closed reduction were unsuccessful. Therefore, it was treated by open reduction and K-wire fixation. In our case, open reduction and K-wire fixation for radiopalmar dislocation of the fifth CMC joint provided promising results.
Conclusion
Radiopalmar dislocation of the fifth CMC joint is a rare injury, and very few cases have been reported in the English-language literature. We report one such case, which was successfully treated with open reduction and K-wire fixation.
1. Buzby BF. Palmar carpometacarpal dislocation of the fifth metacarpal. Ann Surg. 1934;100:555-557.
2. Chen VT. Dislocation of carpometacarpal joint of the little finger. J Hand Surg. 1987;12(2):260-263.
3. Dennyson WG, Stother IG. Carpometacarpal dislocation of the little finger. Hand. 1976;8(2):161-164.
4. Domingo A, Font L, Saz L, Arandes JM. Isolated radial palmar dislocation of the fifth carpometacarpal joint with ulnar neuropathy associated: successful treatment with closed reduction and internal fixation. Eur J Orthop Surg Traumatol. 19(2):101-107.
5. Fisher MR, Rogers LF, Hendrix RW. Systematic approach to identifying fourth and fifth carpometacarpal joint dislocations. AJR Am J Roentgenol. 1983;140(2):319-324.
6. McWhorter GL. Isolated and complete dislocation of the fifth carpometacarpal joint: open operation. Surg Clin Chic. 1918;2:793-796.
7. Nalebuff EA. Isolated anterior carpometacarpal dislocation of the fifth finger: classification and case report. J Trauma. 1968;8(6):1119-1123.
8. Berg EE, Murphy DF. Ulnopalmar dislocation of the fifth carpometacarpal joint – successful closed reduction: review of the literature and anatomic reevaluation. J Hand Surg Am. 1986;11(4):521-525.
9. Peterson P, Sacks S. Fracture-dislocation of the base of the fifth metacarpal associated with injury to the deep motor branch of the ulnar nerve: a case report. J Hand Surg Am. 1986;11(4):525-528.
10. Young TB. Dorsal dislocation of the metacarpal base of the little and ring fingers with ulnar nerve branch compression. Injury. 1987;18(1):65-66.
11. O’Rourke PJ, Quinlan W. Fracture dislocation of the fifth metacarpal resulting in compression of the deep branch of the ulnar nerve. J Hand Surg Br. 1993;18(2):190-191.
12. Bora FW Jr, Didizian NH. The treatment of injuries to the carpometacarpal joint of the little finger. J Bone Joint Surg Am. 1974;56(7):1459-1463.
13. Tountas AA, Kwok JM. Isolated volar dislocation of the fifth carpometacarpal joint. Case report. Clin Orthop Relat Res. 1984;187:172-175.
Isolated dislocation of the carpometacarpal (CMC) joint of the hand is a rare injury. While the dislocation can be dorsal or palmar, dorsal dislocation is more common. Palmar dislocations can be either ulnopalmar or radiopalmar. There are very few reports of isolated radiopalmar dislocations of the fifth CMC joint in the English-language literature.1-3 We present a case of delayed presentation and management of radiopalmar dislocation of the fifth CMC joint. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 42-year-old man presented with polytrauma to our emergency department. He was stabilized initially, and open fractures were treated by débridement and external fixator application. During an examination 3 days after admission, swelling was noted in the right hand. On further study, there was splaying of the fifth digit and tenderness over the fourth and fifth CMC joints (Figure 1). No abnormal mobility or crepitus could be elicited. Plain radiographs of the right hand in anteroposterior and lateral views revealed radiopalmar dislocation of the fifth CMC joint (Figure 2). It was decided to reduce the dislocation immediately after the patient was declared fit for surgery.
Under axillary block, closed reduction was unsuccessful. Open reduction of the fifth CMC joint was performed through a dorsal incision. The base of the fifth metacarpal bone was found to be stripped of soft-tissue attachments and lying in a radiopalmar location. Reduction, which was checked under image intensifier, was found to be satisfactory (Figure 3). Reduction was stabilized by passing a smooth Kirschner wire (K-wire) from the fifth metacarpal to the hamate bone. After achieving hemostasis, the wound was closed in layers and a below-elbow splint was applied. The perioperative period was uneventful, and sutures were removed on postoperative day 10. The K-wire was removed after 4 weeks, and radiographs showed satisfactory position of the fifth CMC joint. Gentle active and passive mobilization of fingers and wrist were started. The patient had regained good function of the wrist and fingers 2 months after surgery (Figure 4).
Discussion
Carpometacarpal joint dislocations are uncommon injuries and account for less than 1% of hand injuries.4 They are classified as dorsal and volar (palmar) dislocations. Dorsal dislocations of the CMC joints occur more frequently than do volar dislocations, mainly affecting the fourth and fifth digits.5 Isolated volar or palmar dislocation of the fifth CMC joint is an uncommon injury that was first reported in 1918 by McWhorter.6 In 1968, Nalebuff7 classified the volar dislocations into 2 groups according to the direction of the displacement of the fifth metacarpal base: radiopalmar and ulnopalmar. Berg and Murphy8 found the hook of the hamate to deviate the metacarpal bone to either the ulnar or radial side. Tearing of all ligament and tendon attachments of the base of the fifth metacarpal results in radiopalmar dislocation.7 The attachments of ligaments and tendons remain intact in the ulnopalmar dislocation.7
The clinical features of this injury are pain and swelling about the base of the fifth metacarpal and axial deformity of the little finger with apparent shortening. The deep motor branch of the ulnar nerve lies volar to the fifth CMC joint as it courses around the hook of the hamate. It is vulnerable to injury in both dorsal9,10 and volar11 CMC dislocations. For radiologic evaluation, in addition to standard anteroposterior and lateral radiographs, a lateral view in 30º pronation of the hand can provide an improved view of the fifth CMC joint, as suggested by Bora and Didizian.12
The treatment of ulnopalmar dislocation has evolved. Ulnopalmar dislocations have been successfully treated by closed reduction without fixation,8 and by open reduction and K-wire fixation.3,7,13
Radiopalmar dislocations are inherently unstable because of the tearing of all ligament and tendon attachments of the base of the fifth metacarpal.7 In our case of radiopalmar dislocation, diagnosis was delayed and attempts at closed reduction were unsuccessful. Therefore, it was treated by open reduction and K-wire fixation. In our case, open reduction and K-wire fixation for radiopalmar dislocation of the fifth CMC joint provided promising results.
Conclusion
Radiopalmar dislocation of the fifth CMC joint is a rare injury, and very few cases have been reported in the English-language literature. We report one such case, which was successfully treated with open reduction and K-wire fixation.
Isolated dislocation of the carpometacarpal (CMC) joint of the hand is a rare injury. While the dislocation can be dorsal or palmar, dorsal dislocation is more common. Palmar dislocations can be either ulnopalmar or radiopalmar. There are very few reports of isolated radiopalmar dislocations of the fifth CMC joint in the English-language literature.1-3 We present a case of delayed presentation and management of radiopalmar dislocation of the fifth CMC joint. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 42-year-old man presented with polytrauma to our emergency department. He was stabilized initially, and open fractures were treated by débridement and external fixator application. During an examination 3 days after admission, swelling was noted in the right hand. On further study, there was splaying of the fifth digit and tenderness over the fourth and fifth CMC joints (Figure 1). No abnormal mobility or crepitus could be elicited. Plain radiographs of the right hand in anteroposterior and lateral views revealed radiopalmar dislocation of the fifth CMC joint (Figure 2). It was decided to reduce the dislocation immediately after the patient was declared fit for surgery.
Under axillary block, closed reduction was unsuccessful. Open reduction of the fifth CMC joint was performed through a dorsal incision. The base of the fifth metacarpal bone was found to be stripped of soft-tissue attachments and lying in a radiopalmar location. Reduction, which was checked under image intensifier, was found to be satisfactory (Figure 3). Reduction was stabilized by passing a smooth Kirschner wire (K-wire) from the fifth metacarpal to the hamate bone. After achieving hemostasis, the wound was closed in layers and a below-elbow splint was applied. The perioperative period was uneventful, and sutures were removed on postoperative day 10. The K-wire was removed after 4 weeks, and radiographs showed satisfactory position of the fifth CMC joint. Gentle active and passive mobilization of fingers and wrist were started. The patient had regained good function of the wrist and fingers 2 months after surgery (Figure 4).
Discussion
Carpometacarpal joint dislocations are uncommon injuries and account for less than 1% of hand injuries.4 They are classified as dorsal and volar (palmar) dislocations. Dorsal dislocations of the CMC joints occur more frequently than do volar dislocations, mainly affecting the fourth and fifth digits.5 Isolated volar or palmar dislocation of the fifth CMC joint is an uncommon injury that was first reported in 1918 by McWhorter.6 In 1968, Nalebuff7 classified the volar dislocations into 2 groups according to the direction of the displacement of the fifth metacarpal base: radiopalmar and ulnopalmar. Berg and Murphy8 found the hook of the hamate to deviate the metacarpal bone to either the ulnar or radial side. Tearing of all ligament and tendon attachments of the base of the fifth metacarpal results in radiopalmar dislocation.7 The attachments of ligaments and tendons remain intact in the ulnopalmar dislocation.7
The clinical features of this injury are pain and swelling about the base of the fifth metacarpal and axial deformity of the little finger with apparent shortening. The deep motor branch of the ulnar nerve lies volar to the fifth CMC joint as it courses around the hook of the hamate. It is vulnerable to injury in both dorsal9,10 and volar11 CMC dislocations. For radiologic evaluation, in addition to standard anteroposterior and lateral radiographs, a lateral view in 30º pronation of the hand can provide an improved view of the fifth CMC joint, as suggested by Bora and Didizian.12
The treatment of ulnopalmar dislocation has evolved. Ulnopalmar dislocations have been successfully treated by closed reduction without fixation,8 and by open reduction and K-wire fixation.3,7,13
Radiopalmar dislocations are inherently unstable because of the tearing of all ligament and tendon attachments of the base of the fifth metacarpal.7 In our case of radiopalmar dislocation, diagnosis was delayed and attempts at closed reduction were unsuccessful. Therefore, it was treated by open reduction and K-wire fixation. In our case, open reduction and K-wire fixation for radiopalmar dislocation of the fifth CMC joint provided promising results.
Conclusion
Radiopalmar dislocation of the fifth CMC joint is a rare injury, and very few cases have been reported in the English-language literature. We report one such case, which was successfully treated with open reduction and K-wire fixation.
1. Buzby BF. Palmar carpometacarpal dislocation of the fifth metacarpal. Ann Surg. 1934;100:555-557.
2. Chen VT. Dislocation of carpometacarpal joint of the little finger. J Hand Surg. 1987;12(2):260-263.
3. Dennyson WG, Stother IG. Carpometacarpal dislocation of the little finger. Hand. 1976;8(2):161-164.
4. Domingo A, Font L, Saz L, Arandes JM. Isolated radial palmar dislocation of the fifth carpometacarpal joint with ulnar neuropathy associated: successful treatment with closed reduction and internal fixation. Eur J Orthop Surg Traumatol. 19(2):101-107.
5. Fisher MR, Rogers LF, Hendrix RW. Systematic approach to identifying fourth and fifth carpometacarpal joint dislocations. AJR Am J Roentgenol. 1983;140(2):319-324.
6. McWhorter GL. Isolated and complete dislocation of the fifth carpometacarpal joint: open operation. Surg Clin Chic. 1918;2:793-796.
7. Nalebuff EA. Isolated anterior carpometacarpal dislocation of the fifth finger: classification and case report. J Trauma. 1968;8(6):1119-1123.
8. Berg EE, Murphy DF. Ulnopalmar dislocation of the fifth carpometacarpal joint – successful closed reduction: review of the literature and anatomic reevaluation. J Hand Surg Am. 1986;11(4):521-525.
9. Peterson P, Sacks S. Fracture-dislocation of the base of the fifth metacarpal associated with injury to the deep motor branch of the ulnar nerve: a case report. J Hand Surg Am. 1986;11(4):525-528.
10. Young TB. Dorsal dislocation of the metacarpal base of the little and ring fingers with ulnar nerve branch compression. Injury. 1987;18(1):65-66.
11. O’Rourke PJ, Quinlan W. Fracture dislocation of the fifth metacarpal resulting in compression of the deep branch of the ulnar nerve. J Hand Surg Br. 1993;18(2):190-191.
12. Bora FW Jr, Didizian NH. The treatment of injuries to the carpometacarpal joint of the little finger. J Bone Joint Surg Am. 1974;56(7):1459-1463.
13. Tountas AA, Kwok JM. Isolated volar dislocation of the fifth carpometacarpal joint. Case report. Clin Orthop Relat Res. 1984;187:172-175.
1. Buzby BF. Palmar carpometacarpal dislocation of the fifth metacarpal. Ann Surg. 1934;100:555-557.
2. Chen VT. Dislocation of carpometacarpal joint of the little finger. J Hand Surg. 1987;12(2):260-263.
3. Dennyson WG, Stother IG. Carpometacarpal dislocation of the little finger. Hand. 1976;8(2):161-164.
4. Domingo A, Font L, Saz L, Arandes JM. Isolated radial palmar dislocation of the fifth carpometacarpal joint with ulnar neuropathy associated: successful treatment with closed reduction and internal fixation. Eur J Orthop Surg Traumatol. 19(2):101-107.
5. Fisher MR, Rogers LF, Hendrix RW. Systematic approach to identifying fourth and fifth carpometacarpal joint dislocations. AJR Am J Roentgenol. 1983;140(2):319-324.
6. McWhorter GL. Isolated and complete dislocation of the fifth carpometacarpal joint: open operation. Surg Clin Chic. 1918;2:793-796.
7. Nalebuff EA. Isolated anterior carpometacarpal dislocation of the fifth finger: classification and case report. J Trauma. 1968;8(6):1119-1123.
8. Berg EE, Murphy DF. Ulnopalmar dislocation of the fifth carpometacarpal joint – successful closed reduction: review of the literature and anatomic reevaluation. J Hand Surg Am. 1986;11(4):521-525.
9. Peterson P, Sacks S. Fracture-dislocation of the base of the fifth metacarpal associated with injury to the deep motor branch of the ulnar nerve: a case report. J Hand Surg Am. 1986;11(4):525-528.
10. Young TB. Dorsal dislocation of the metacarpal base of the little and ring fingers with ulnar nerve branch compression. Injury. 1987;18(1):65-66.
11. O’Rourke PJ, Quinlan W. Fracture dislocation of the fifth metacarpal resulting in compression of the deep branch of the ulnar nerve. J Hand Surg Br. 1993;18(2):190-191.
12. Bora FW Jr, Didizian NH. The treatment of injuries to the carpometacarpal joint of the little finger. J Bone Joint Surg Am. 1974;56(7):1459-1463.
13. Tountas AA, Kwok JM. Isolated volar dislocation of the fifth carpometacarpal joint. Case report. Clin Orthop Relat Res. 1984;187:172-175.
Nonoperative Management of Multiple Hand Enchondromas in Ollier Disease With Progressive Ossification
Ollier disease, or multiple enchondromatosis, is a rare nonfamilial condition characterized by multiple cartilaginous tumors often beginning in early childhood. There is significant variation in disease distribution, location, size, number of lesions, and behavior, but the tumors are often located unilaterally.1 Enchondromas are most commonly found in the metacarpals, metatarsals, and phalanges, and develop from metaphyseal bone in close proximity to the physis. They frequently present as painless masses or are incidentally noted during the evaluation of another musculoskeletal condition. Radiographically, enchondromas of the hands and feet appear as oval radiolucencies with thinned, sclerotic rims. The lesions have varying degrees of mineralization and endosteal scalloping, and may expand the bone.2 Enchondromas usually enlarge until skeletal maturity and have been observed to ossify spontaneously.1,3 The clinical course of Ollier disease is variable, and a number of cases of significant hand deformity and malignant transformation have been reported.4-6
In this case report, we present a mild form of Ollier disease isolated to the patient’s left hand, which we followed for 8 years, demonstrating part of the natural history of these lesions. We discuss the patient’s clinical features, radiologic findings, diagnosis, treatment, prognosis, and follow-up, as well as review the literature. The patient and the patient’s family provided written informed consent for print and electronic publication of this case report.
Case Report
A 10-year-old, right-handed girl was referred to our department for the evaluation of left-hand masses. At age 3 years, the patient underwent a chondroma excision from the middle phalanx of her middle finger on her left hand. No operative or pathology report was available from this surgery, and the patient tolerated the procedure well without any complications. At the time of presentation, the masses did not cause any pain, motor or sensory dysfunction, or any systemic symptoms. No history of recent or distant trauma was elicited. The patient’s medical and family history was unremarkable.
On physical examination, there was a firm, immobile, nontender palpable mass over the dorsal aspect of the distal second metacarpal bone of the left hand. The mass extended medially between the second and third metacarpals. A second small, firm, nontender left-hand mass was palpated over the volar aspect of her proximal phalanx on her index finger. She was neurovascularly intact with full active range of motion of the metacarpophalangeal and proximal and distal interphalangeal joints. There was no angular deformity of the digits. Plain radiographs taken at the time of initial presentation showed a 2.3×1.7-cm radiolucent lesion located in the metaphysis and diaphysis of the second metacarpal of the left hand (Figures 1A-1C). The lesion had varying degrees of mineralization with cortical thinning and expansion in the volar, dorsal, radial, and ulnar directions, consistent with a chondroid lesion. The second and third lesions were oval radiolucencies with sclerotic rims located at the metaphyseal-diaphyseal junction of the proximal phalanx of the index finger and middle phalanx of the middle finger, respectively. No fractures were identified in the radiographs, and the physes were open at this time. The patient was diagnosed with multiple enchondromatosis, or Ollier disease.
Our case showed 1 episode of pain and tenderness to palpation at the second proximal phalanx approximately 6 months after initial presentation. We attributed the pain and tenderness to a small pathologic fracture but did not see radiographic evidence of this. We elected to provide a trial of supportive measures, such as splinting and buddy taping, and to monitor the pain with a tentative plan of open biopsy with curettage and bone grafting if the pain persisted or evidence of fracture was seen on radiographs. The pain and tenderness to palpation resolved at a follow-up visit, and the surgery was deferred.
The patient was treated nonoperatively at initial presentation given the lack of significant cosmetic deformity or functional compromise and was advised close follow-up at 3 and 6 months. Given the absence of disease progression, annual checks (ie, clinical examination and radiographs) in a skeletally immature patient were decided on after consultation with the patient and parent. The family was educated about the possibility of pathologic fracture from minimal trauma to the hand versus the small risk of iatrogenic physeal injury with surgical curettage and bone grafting. No protective splinting was offered. A favorable prognosis and reassurance was provided to the patient and family, given the absence of symptoms, low suspicion and risk of malignant transformation, and stability of the lesion. Serial radiographs showed gradual increases in the lesions’ sizes but were consistent with the stable growth of the metacarpal and phalanges. With the patient nearing skeletal maturity, no pathologic fractures were identified on radiography during follow-up, and the risks of surgery lessened with growth; however, the continued absence of symptoms led to the mutual decision to continue observation.
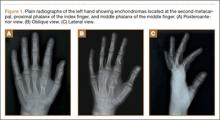
Nearly 8 years after initial presentation, plain radiographs showed closed physes and partially ossified bone masses (Figures 2A-2C). The metacarpal lesion measured 3.2×1.5 cm, and the cortex appeared thickened and regular. The proximal phalanx lesion had a thickened cortex without periosteal reaction, and the middle phalanx lesion appeared to be completely healed. The patient has been asymptomatic for many years, and she has retained complete function of her left hand without any growth retardation, angular deformity, or pathologic fracture. A small but potential risk of malignant transformation was discussed with the patient and her family, as was the need for lifetime follow-up. We intend to follow the enchondromas clinically and radiographically every 2 years and obtain new radiographs if the mass presents with new clinical findings, such as enlargement or pain, for surveillance of tumor transformation. If the patient desired or symptoms developed, curettage and bone grafting would be offered, and the surgical tissue would be sent for pathologic analysis. A bone scan that was obtained at the request of the patient, when she was 21 years old, showed no other sites of disease besides the fingers.
Discussion
Multiple enchondromatosis was first described by Ollier at the turn of the 19th century and has been estimated to affect one in every 100,000 persons.1 The low prevalence and variable manifestations of Ollier disease lead clinicians to handle the disease and its complications, namely skeletal deformity and malignant transformation, on a case-by-case approach. Additionally, the prognosis of Ollier disease with malignant transformation is quite variable, with studies reporting the estimated incidence as 5% to 50%.7 Muramatsu and colleagues6 reported that the occurrence of malignant transformation of multiple enchondromas limited to the bones of the hand was extremely rare, with only 12 cases of malignant transformation. Enchondromas of the pelvis, scapula, and long bones of the extremities have increased risks and rates of secondary transformation to chondrosarcoma.8
A recent large European multicenter retrospective study investigating the clinical characteristics and behavior of enchondromas in 144 patients with Ollier disease has provided new information regarding this rare disease.7 Verdegaal and colleagues7 divided patients into 3 categories depending on their distribution of enchondromas. The development of chondrosarcoma was notably different between individuals with enchondromas limited to the small bones of the hands and feet (15%, group I) versus individuals with enchondromas limited to the long bones and flat bones (43%, group II) or individuals with enchondromas of the short, long, and flat bones (46%, group III).7 The only location found to be statistically significant for the development of chondrosarcoma was the pelvis.
The clinical findings associated with risk of malignant transformation of enchondromas are increasing size of the lesion and onset of pain and tenderness. Dahlin and Salvador9 reported that only 60% of patients with chondrosarcoma of the hand experience pain. The absence of pain may lead to a delay in patient presentation to the clinician.5,6 Radiographic findings of malignant transformation include the classic features of temporal increases in the lesion’s size after skeletal maturity and cortical destruction associated with soft-tissue invasion. However, both findings are nonspecific for differentiating enchondromas from grade 1 chondrosarcomas as described by Geirnaerdt and colleagues.10
Sassoon and colleagues11 reported on a series of hand enchondromas treated operatively. Subgroup analysis between pathologic fractures treated primarily or in delayed fashion showed similar outcomes for achieving full motion and similar number of complications; however, they noted that the delayed group required 7 more weeks of immobilization. Additionally, review of the whole series showed 1 episode of metacarpal shortening and 1 occurrence of angular malalignment. In our patient, we were concerned about introducing an iatrogenic cosmetic deformity, and we believed a pathologic fracture could be managed expectantly. Overall, patients without pathologic fracture treated surgically experienced a complication rate of 12%, whereas patients with a fracture had a complication rate of 20%.11 The majority of patients with multiple enchondromatosis treated with surgical curettage and grafting had successful outcomes, with 86% of patients regaining full motion, but the recurrence rate was 21%.11 Patients with expansile lesions regained less motion than patients with nonexpansile lesions. There was a single lesion believed preoperatively to be an enchondroma, but it underwent malignant transformation, as confirmed on intraoperative pathology. This patient had Maffucci syndrome and was treated with an amputation through the metacarpophalangeal joint.
There are 3 options for treating hand enchondromas: observation, curettage alone, or curettage with bone grafting. There is no consensus about conservative management, timing of intervention, or risk of pathologic fracture. Each patient is treated individually with attention to reason for presentation, number of lesions, associated pain, deformity, or pathologic fracture. Operative criteria include high risk of pathologic fracture based on location of enchondroma, cortical thinning, and previous pathologic fracture with resulting angular deformity. Nonoperative management may increase the risk of pathologic fracture, particularly in patients involved in aggressive contact sports, but the physician may offer protective splinting or counsel the patient on activity modification. Our case provides a study of the natural history of multiple enchondromatosis and shows mild increases in the lesions’ sizes during the 8-year follow-up. This was an expected finding given the patient’s immature skeleton. The lesions’ cortices continued to ossify after the physes closed and now provides an excellent comparison for the identification of future malignant changes.
Histologic analysis of biopsied or surgically treated lesions contributes to the differentiation between benign hand enchondromas and chondrosarcoma. Pathologic findings must be correlated with clinical and radiographic findings because hand enchondromas contain cytologic features of chondrosarcoma.12 In a series of 55 patients with chondrosarcoma, Liu and colleagues8 reported no cases from the hand. Verdegaal and colleagues7 reported a total of 13 chondrosarcomas in the metacarpals and hand phalanges in 97 group I and III patients. Five of these lesions were grade 1, 2 were grade 2, 1 was grade 3, and 5 lesions were unknown.
For patients with multiple enchondromatosis limited to the hands, prognosis is relatively good with respect to risk of secondary chondrosarcoma transformation, metastasis of secondary chondrosarcoma, and death. Verdegaal and colleagues7 reported the rate of secondary transformation in the hand to be 15%. Patil and colleagues13 reported no distant metastases in 23 patients with hand chondrosarcoma at mean follow-up of 8.5 years (range, 2-19 years), although none of their patients had Ollier disease. Verdegaal and colleagues7 reported 7 of the 8 deaths in their study were related to development of pulmonary metastases; however, none originated from chondrosarcomas in the hand. Additionally, there were no disease-related deaths in 29 group I patients. Herget and colleagues,14 in summarizing the literature, postulated that the overall survival rate of patients with secondary chondrosarcoma at 5 years is approximately 90%.
In our case, the patient, who had 3 enchondromas isolated to the left hand, can be categorized in group I. Thus, this case highlights the natural history of a patient with hand enchondromas and demonstrates that enchondromatosis of the short tubular bones of the hands can mature and ossify.
1. Silve C, Jüppner H. Ollier disease. Orphanet J Rare Dis. 2006;1:37-42.
2. Baert A. Encyclopedia of Diagnostic Imaging. Vol. 1. Berlin, Germany: Springer; 2008.
3. Takigawa K. Chondroma of the bones of the hand. A review of 110 cases. J Bone Joint Surg Am. 1971;53(8):1591-1600.
4. Mosher J. Multiple enchondromatosis of the hand. A case report. J Bone Joint Surg Am. 1976;58(5):717-719.
5. Goto T, Motoi T, Komiya K, et al. Chondrosarcoma of the hand secondary to multiple enchondromatosis; report of two cases. Arch Orthop Trauma Surg. 2003;123(1):42-47.
6. Muramatsu K, Kawakami Y, Tani Y, Taguchi T. Malignant transformation of multiple enchondromas in the hand: case report. J Hand Surg Am. 2011;36(2):304-307.
7. Verdegaal SH, Bovee JV, Pansuriya TC, et al. Incidence, predictive factors, and prognosis of chondrosarcoma in patients with Ollier disease and Maffucci syndrome: an international multicenter study of 161 patients. Oncologist. 2011;16(12):1771-1779.
8. Liu J, Hudkins PG, Swee RG, Unni KK. Bone sarcomas associated with Ollier’s disease. Cancer. 1987;59(7):1376-1385.
9. Dahlin D, Salvador AH. Chondrosarcomas of bones of the hands and feet—a study of 30 cases. Cancer. 1974;34(3):755-760.
10. Geirnaerdt MJ, Hermans J, Bloem JL, et al. Usefulness of radiography in differentiating enchondroma from central grade I chondrosarcoma. AJR Am J Roentgenol. 1997;169(4):1097-1104.
11. Sassoon AA, Fitz-Gibbon PD, Harmsen WS, Moran SL. Enchondromas of the hand: factors affecting recurrence, healing, motion, and malignant transformation. J Hand Surg Am. 2012;37(6):1229-1234.
12. Ogose A, Unni KK, Swee R, May GK, Rowland CM, Sim FH. Chondrosarcoma of small bones of the hands and feet. Cancer. 1997;80(1):50-59.
13. Patil S, de Silva MV, Crossan J, Reid R. Chondrosarcoma of small bones of the hand. J Hand Surg Br. 2003;28(6):602-608.
14. Herget GW, Strohm P, Rottenburger C, et al. Insights in Enchondroma, Enchondromatosis and the risk of secondary Chondrosarcoma. Review of the literature with an emphasis on the clinical behaviour, radiology, malignant transformation and the follow up. Neoplasma. 2014;61(4):365-378.
Ollier disease, or multiple enchondromatosis, is a rare nonfamilial condition characterized by multiple cartilaginous tumors often beginning in early childhood. There is significant variation in disease distribution, location, size, number of lesions, and behavior, but the tumors are often located unilaterally.1 Enchondromas are most commonly found in the metacarpals, metatarsals, and phalanges, and develop from metaphyseal bone in close proximity to the physis. They frequently present as painless masses or are incidentally noted during the evaluation of another musculoskeletal condition. Radiographically, enchondromas of the hands and feet appear as oval radiolucencies with thinned, sclerotic rims. The lesions have varying degrees of mineralization and endosteal scalloping, and may expand the bone.2 Enchondromas usually enlarge until skeletal maturity and have been observed to ossify spontaneously.1,3 The clinical course of Ollier disease is variable, and a number of cases of significant hand deformity and malignant transformation have been reported.4-6
In this case report, we present a mild form of Ollier disease isolated to the patient’s left hand, which we followed for 8 years, demonstrating part of the natural history of these lesions. We discuss the patient’s clinical features, radiologic findings, diagnosis, treatment, prognosis, and follow-up, as well as review the literature. The patient and the patient’s family provided written informed consent for print and electronic publication of this case report.
Case Report
A 10-year-old, right-handed girl was referred to our department for the evaluation of left-hand masses. At age 3 years, the patient underwent a chondroma excision from the middle phalanx of her middle finger on her left hand. No operative or pathology report was available from this surgery, and the patient tolerated the procedure well without any complications. At the time of presentation, the masses did not cause any pain, motor or sensory dysfunction, or any systemic symptoms. No history of recent or distant trauma was elicited. The patient’s medical and family history was unremarkable.
On physical examination, there was a firm, immobile, nontender palpable mass over the dorsal aspect of the distal second metacarpal bone of the left hand. The mass extended medially between the second and third metacarpals. A second small, firm, nontender left-hand mass was palpated over the volar aspect of her proximal phalanx on her index finger. She was neurovascularly intact with full active range of motion of the metacarpophalangeal and proximal and distal interphalangeal joints. There was no angular deformity of the digits. Plain radiographs taken at the time of initial presentation showed a 2.3×1.7-cm radiolucent lesion located in the metaphysis and diaphysis of the second metacarpal of the left hand (Figures 1A-1C). The lesion had varying degrees of mineralization with cortical thinning and expansion in the volar, dorsal, radial, and ulnar directions, consistent with a chondroid lesion. The second and third lesions were oval radiolucencies with sclerotic rims located at the metaphyseal-diaphyseal junction of the proximal phalanx of the index finger and middle phalanx of the middle finger, respectively. No fractures were identified in the radiographs, and the physes were open at this time. The patient was diagnosed with multiple enchondromatosis, or Ollier disease.
Our case showed 1 episode of pain and tenderness to palpation at the second proximal phalanx approximately 6 months after initial presentation. We attributed the pain and tenderness to a small pathologic fracture but did not see radiographic evidence of this. We elected to provide a trial of supportive measures, such as splinting and buddy taping, and to monitor the pain with a tentative plan of open biopsy with curettage and bone grafting if the pain persisted or evidence of fracture was seen on radiographs. The pain and tenderness to palpation resolved at a follow-up visit, and the surgery was deferred.
The patient was treated nonoperatively at initial presentation given the lack of significant cosmetic deformity or functional compromise and was advised close follow-up at 3 and 6 months. Given the absence of disease progression, annual checks (ie, clinical examination and radiographs) in a skeletally immature patient were decided on after consultation with the patient and parent. The family was educated about the possibility of pathologic fracture from minimal trauma to the hand versus the small risk of iatrogenic physeal injury with surgical curettage and bone grafting. No protective splinting was offered. A favorable prognosis and reassurance was provided to the patient and family, given the absence of symptoms, low suspicion and risk of malignant transformation, and stability of the lesion. Serial radiographs showed gradual increases in the lesions’ sizes but were consistent with the stable growth of the metacarpal and phalanges. With the patient nearing skeletal maturity, no pathologic fractures were identified on radiography during follow-up, and the risks of surgery lessened with growth; however, the continued absence of symptoms led to the mutual decision to continue observation.
Nearly 8 years after initial presentation, plain radiographs showed closed physes and partially ossified bone masses (Figures 2A-2C). The metacarpal lesion measured 3.2×1.5 cm, and the cortex appeared thickened and regular. The proximal phalanx lesion had a thickened cortex without periosteal reaction, and the middle phalanx lesion appeared to be completely healed. The patient has been asymptomatic for many years, and she has retained complete function of her left hand without any growth retardation, angular deformity, or pathologic fracture. A small but potential risk of malignant transformation was discussed with the patient and her family, as was the need for lifetime follow-up. We intend to follow the enchondromas clinically and radiographically every 2 years and obtain new radiographs if the mass presents with new clinical findings, such as enlargement or pain, for surveillance of tumor transformation. If the patient desired or symptoms developed, curettage and bone grafting would be offered, and the surgical tissue would be sent for pathologic analysis. A bone scan that was obtained at the request of the patient, when she was 21 years old, showed no other sites of disease besides the fingers.
Discussion
Multiple enchondromatosis was first described by Ollier at the turn of the 19th century and has been estimated to affect one in every 100,000 persons.1 The low prevalence and variable manifestations of Ollier disease lead clinicians to handle the disease and its complications, namely skeletal deformity and malignant transformation, on a case-by-case approach. Additionally, the prognosis of Ollier disease with malignant transformation is quite variable, with studies reporting the estimated incidence as 5% to 50%.7 Muramatsu and colleagues6 reported that the occurrence of malignant transformation of multiple enchondromas limited to the bones of the hand was extremely rare, with only 12 cases of malignant transformation. Enchondromas of the pelvis, scapula, and long bones of the extremities have increased risks and rates of secondary transformation to chondrosarcoma.8
A recent large European multicenter retrospective study investigating the clinical characteristics and behavior of enchondromas in 144 patients with Ollier disease has provided new information regarding this rare disease.7 Verdegaal and colleagues7 divided patients into 3 categories depending on their distribution of enchondromas. The development of chondrosarcoma was notably different between individuals with enchondromas limited to the small bones of the hands and feet (15%, group I) versus individuals with enchondromas limited to the long bones and flat bones (43%, group II) or individuals with enchondromas of the short, long, and flat bones (46%, group III).7 The only location found to be statistically significant for the development of chondrosarcoma was the pelvis.
The clinical findings associated with risk of malignant transformation of enchondromas are increasing size of the lesion and onset of pain and tenderness. Dahlin and Salvador9 reported that only 60% of patients with chondrosarcoma of the hand experience pain. The absence of pain may lead to a delay in patient presentation to the clinician.5,6 Radiographic findings of malignant transformation include the classic features of temporal increases in the lesion’s size after skeletal maturity and cortical destruction associated with soft-tissue invasion. However, both findings are nonspecific for differentiating enchondromas from grade 1 chondrosarcomas as described by Geirnaerdt and colleagues.10
Sassoon and colleagues11 reported on a series of hand enchondromas treated operatively. Subgroup analysis between pathologic fractures treated primarily or in delayed fashion showed similar outcomes for achieving full motion and similar number of complications; however, they noted that the delayed group required 7 more weeks of immobilization. Additionally, review of the whole series showed 1 episode of metacarpal shortening and 1 occurrence of angular malalignment. In our patient, we were concerned about introducing an iatrogenic cosmetic deformity, and we believed a pathologic fracture could be managed expectantly. Overall, patients without pathologic fracture treated surgically experienced a complication rate of 12%, whereas patients with a fracture had a complication rate of 20%.11 The majority of patients with multiple enchondromatosis treated with surgical curettage and grafting had successful outcomes, with 86% of patients regaining full motion, but the recurrence rate was 21%.11 Patients with expansile lesions regained less motion than patients with nonexpansile lesions. There was a single lesion believed preoperatively to be an enchondroma, but it underwent malignant transformation, as confirmed on intraoperative pathology. This patient had Maffucci syndrome and was treated with an amputation through the metacarpophalangeal joint.
There are 3 options for treating hand enchondromas: observation, curettage alone, or curettage with bone grafting. There is no consensus about conservative management, timing of intervention, or risk of pathologic fracture. Each patient is treated individually with attention to reason for presentation, number of lesions, associated pain, deformity, or pathologic fracture. Operative criteria include high risk of pathologic fracture based on location of enchondroma, cortical thinning, and previous pathologic fracture with resulting angular deformity. Nonoperative management may increase the risk of pathologic fracture, particularly in patients involved in aggressive contact sports, but the physician may offer protective splinting or counsel the patient on activity modification. Our case provides a study of the natural history of multiple enchondromatosis and shows mild increases in the lesions’ sizes during the 8-year follow-up. This was an expected finding given the patient’s immature skeleton. The lesions’ cortices continued to ossify after the physes closed and now provides an excellent comparison for the identification of future malignant changes.
Histologic analysis of biopsied or surgically treated lesions contributes to the differentiation between benign hand enchondromas and chondrosarcoma. Pathologic findings must be correlated with clinical and radiographic findings because hand enchondromas contain cytologic features of chondrosarcoma.12 In a series of 55 patients with chondrosarcoma, Liu and colleagues8 reported no cases from the hand. Verdegaal and colleagues7 reported a total of 13 chondrosarcomas in the metacarpals and hand phalanges in 97 group I and III patients. Five of these lesions were grade 1, 2 were grade 2, 1 was grade 3, and 5 lesions were unknown.
For patients with multiple enchondromatosis limited to the hands, prognosis is relatively good with respect to risk of secondary chondrosarcoma transformation, metastasis of secondary chondrosarcoma, and death. Verdegaal and colleagues7 reported the rate of secondary transformation in the hand to be 15%. Patil and colleagues13 reported no distant metastases in 23 patients with hand chondrosarcoma at mean follow-up of 8.5 years (range, 2-19 years), although none of their patients had Ollier disease. Verdegaal and colleagues7 reported 7 of the 8 deaths in their study were related to development of pulmonary metastases; however, none originated from chondrosarcomas in the hand. Additionally, there were no disease-related deaths in 29 group I patients. Herget and colleagues,14 in summarizing the literature, postulated that the overall survival rate of patients with secondary chondrosarcoma at 5 years is approximately 90%.
In our case, the patient, who had 3 enchondromas isolated to the left hand, can be categorized in group I. Thus, this case highlights the natural history of a patient with hand enchondromas and demonstrates that enchondromatosis of the short tubular bones of the hands can mature and ossify.
Ollier disease, or multiple enchondromatosis, is a rare nonfamilial condition characterized by multiple cartilaginous tumors often beginning in early childhood. There is significant variation in disease distribution, location, size, number of lesions, and behavior, but the tumors are often located unilaterally.1 Enchondromas are most commonly found in the metacarpals, metatarsals, and phalanges, and develop from metaphyseal bone in close proximity to the physis. They frequently present as painless masses or are incidentally noted during the evaluation of another musculoskeletal condition. Radiographically, enchondromas of the hands and feet appear as oval radiolucencies with thinned, sclerotic rims. The lesions have varying degrees of mineralization and endosteal scalloping, and may expand the bone.2 Enchondromas usually enlarge until skeletal maturity and have been observed to ossify spontaneously.1,3 The clinical course of Ollier disease is variable, and a number of cases of significant hand deformity and malignant transformation have been reported.4-6
In this case report, we present a mild form of Ollier disease isolated to the patient’s left hand, which we followed for 8 years, demonstrating part of the natural history of these lesions. We discuss the patient’s clinical features, radiologic findings, diagnosis, treatment, prognosis, and follow-up, as well as review the literature. The patient and the patient’s family provided written informed consent for print and electronic publication of this case report.
Case Report
A 10-year-old, right-handed girl was referred to our department for the evaluation of left-hand masses. At age 3 years, the patient underwent a chondroma excision from the middle phalanx of her middle finger on her left hand. No operative or pathology report was available from this surgery, and the patient tolerated the procedure well without any complications. At the time of presentation, the masses did not cause any pain, motor or sensory dysfunction, or any systemic symptoms. No history of recent or distant trauma was elicited. The patient’s medical and family history was unremarkable.
On physical examination, there was a firm, immobile, nontender palpable mass over the dorsal aspect of the distal second metacarpal bone of the left hand. The mass extended medially between the second and third metacarpals. A second small, firm, nontender left-hand mass was palpated over the volar aspect of her proximal phalanx on her index finger. She was neurovascularly intact with full active range of motion of the metacarpophalangeal and proximal and distal interphalangeal joints. There was no angular deformity of the digits. Plain radiographs taken at the time of initial presentation showed a 2.3×1.7-cm radiolucent lesion located in the metaphysis and diaphysis of the second metacarpal of the left hand (Figures 1A-1C). The lesion had varying degrees of mineralization with cortical thinning and expansion in the volar, dorsal, radial, and ulnar directions, consistent with a chondroid lesion. The second and third lesions were oval radiolucencies with sclerotic rims located at the metaphyseal-diaphyseal junction of the proximal phalanx of the index finger and middle phalanx of the middle finger, respectively. No fractures were identified in the radiographs, and the physes were open at this time. The patient was diagnosed with multiple enchondromatosis, or Ollier disease.
Our case showed 1 episode of pain and tenderness to palpation at the second proximal phalanx approximately 6 months after initial presentation. We attributed the pain and tenderness to a small pathologic fracture but did not see radiographic evidence of this. We elected to provide a trial of supportive measures, such as splinting and buddy taping, and to monitor the pain with a tentative plan of open biopsy with curettage and bone grafting if the pain persisted or evidence of fracture was seen on radiographs. The pain and tenderness to palpation resolved at a follow-up visit, and the surgery was deferred.
The patient was treated nonoperatively at initial presentation given the lack of significant cosmetic deformity or functional compromise and was advised close follow-up at 3 and 6 months. Given the absence of disease progression, annual checks (ie, clinical examination and radiographs) in a skeletally immature patient were decided on after consultation with the patient and parent. The family was educated about the possibility of pathologic fracture from minimal trauma to the hand versus the small risk of iatrogenic physeal injury with surgical curettage and bone grafting. No protective splinting was offered. A favorable prognosis and reassurance was provided to the patient and family, given the absence of symptoms, low suspicion and risk of malignant transformation, and stability of the lesion. Serial radiographs showed gradual increases in the lesions’ sizes but were consistent with the stable growth of the metacarpal and phalanges. With the patient nearing skeletal maturity, no pathologic fractures were identified on radiography during follow-up, and the risks of surgery lessened with growth; however, the continued absence of symptoms led to the mutual decision to continue observation.
Nearly 8 years after initial presentation, plain radiographs showed closed physes and partially ossified bone masses (Figures 2A-2C). The metacarpal lesion measured 3.2×1.5 cm, and the cortex appeared thickened and regular. The proximal phalanx lesion had a thickened cortex without periosteal reaction, and the middle phalanx lesion appeared to be completely healed. The patient has been asymptomatic for many years, and she has retained complete function of her left hand without any growth retardation, angular deformity, or pathologic fracture. A small but potential risk of malignant transformation was discussed with the patient and her family, as was the need for lifetime follow-up. We intend to follow the enchondromas clinically and radiographically every 2 years and obtain new radiographs if the mass presents with new clinical findings, such as enlargement or pain, for surveillance of tumor transformation. If the patient desired or symptoms developed, curettage and bone grafting would be offered, and the surgical tissue would be sent for pathologic analysis. A bone scan that was obtained at the request of the patient, when she was 21 years old, showed no other sites of disease besides the fingers.
Discussion
Multiple enchondromatosis was first described by Ollier at the turn of the 19th century and has been estimated to affect one in every 100,000 persons.1 The low prevalence and variable manifestations of Ollier disease lead clinicians to handle the disease and its complications, namely skeletal deformity and malignant transformation, on a case-by-case approach. Additionally, the prognosis of Ollier disease with malignant transformation is quite variable, with studies reporting the estimated incidence as 5% to 50%.7 Muramatsu and colleagues6 reported that the occurrence of malignant transformation of multiple enchondromas limited to the bones of the hand was extremely rare, with only 12 cases of malignant transformation. Enchondromas of the pelvis, scapula, and long bones of the extremities have increased risks and rates of secondary transformation to chondrosarcoma.8
A recent large European multicenter retrospective study investigating the clinical characteristics and behavior of enchondromas in 144 patients with Ollier disease has provided new information regarding this rare disease.7 Verdegaal and colleagues7 divided patients into 3 categories depending on their distribution of enchondromas. The development of chondrosarcoma was notably different between individuals with enchondromas limited to the small bones of the hands and feet (15%, group I) versus individuals with enchondromas limited to the long bones and flat bones (43%, group II) or individuals with enchondromas of the short, long, and flat bones (46%, group III).7 The only location found to be statistically significant for the development of chondrosarcoma was the pelvis.
The clinical findings associated with risk of malignant transformation of enchondromas are increasing size of the lesion and onset of pain and tenderness. Dahlin and Salvador9 reported that only 60% of patients with chondrosarcoma of the hand experience pain. The absence of pain may lead to a delay in patient presentation to the clinician.5,6 Radiographic findings of malignant transformation include the classic features of temporal increases in the lesion’s size after skeletal maturity and cortical destruction associated with soft-tissue invasion. However, both findings are nonspecific for differentiating enchondromas from grade 1 chondrosarcomas as described by Geirnaerdt and colleagues.10
Sassoon and colleagues11 reported on a series of hand enchondromas treated operatively. Subgroup analysis between pathologic fractures treated primarily or in delayed fashion showed similar outcomes for achieving full motion and similar number of complications; however, they noted that the delayed group required 7 more weeks of immobilization. Additionally, review of the whole series showed 1 episode of metacarpal shortening and 1 occurrence of angular malalignment. In our patient, we were concerned about introducing an iatrogenic cosmetic deformity, and we believed a pathologic fracture could be managed expectantly. Overall, patients without pathologic fracture treated surgically experienced a complication rate of 12%, whereas patients with a fracture had a complication rate of 20%.11 The majority of patients with multiple enchondromatosis treated with surgical curettage and grafting had successful outcomes, with 86% of patients regaining full motion, but the recurrence rate was 21%.11 Patients with expansile lesions regained less motion than patients with nonexpansile lesions. There was a single lesion believed preoperatively to be an enchondroma, but it underwent malignant transformation, as confirmed on intraoperative pathology. This patient had Maffucci syndrome and was treated with an amputation through the metacarpophalangeal joint.
There are 3 options for treating hand enchondromas: observation, curettage alone, or curettage with bone grafting. There is no consensus about conservative management, timing of intervention, or risk of pathologic fracture. Each patient is treated individually with attention to reason for presentation, number of lesions, associated pain, deformity, or pathologic fracture. Operative criteria include high risk of pathologic fracture based on location of enchondroma, cortical thinning, and previous pathologic fracture with resulting angular deformity. Nonoperative management may increase the risk of pathologic fracture, particularly in patients involved in aggressive contact sports, but the physician may offer protective splinting or counsel the patient on activity modification. Our case provides a study of the natural history of multiple enchondromatosis and shows mild increases in the lesions’ sizes during the 8-year follow-up. This was an expected finding given the patient’s immature skeleton. The lesions’ cortices continued to ossify after the physes closed and now provides an excellent comparison for the identification of future malignant changes.
Histologic analysis of biopsied or surgically treated lesions contributes to the differentiation between benign hand enchondromas and chondrosarcoma. Pathologic findings must be correlated with clinical and radiographic findings because hand enchondromas contain cytologic features of chondrosarcoma.12 In a series of 55 patients with chondrosarcoma, Liu and colleagues8 reported no cases from the hand. Verdegaal and colleagues7 reported a total of 13 chondrosarcomas in the metacarpals and hand phalanges in 97 group I and III patients. Five of these lesions were grade 1, 2 were grade 2, 1 was grade 3, and 5 lesions were unknown.
For patients with multiple enchondromatosis limited to the hands, prognosis is relatively good with respect to risk of secondary chondrosarcoma transformation, metastasis of secondary chondrosarcoma, and death. Verdegaal and colleagues7 reported the rate of secondary transformation in the hand to be 15%. Patil and colleagues13 reported no distant metastases in 23 patients with hand chondrosarcoma at mean follow-up of 8.5 years (range, 2-19 years), although none of their patients had Ollier disease. Verdegaal and colleagues7 reported 7 of the 8 deaths in their study were related to development of pulmonary metastases; however, none originated from chondrosarcomas in the hand. Additionally, there were no disease-related deaths in 29 group I patients. Herget and colleagues,14 in summarizing the literature, postulated that the overall survival rate of patients with secondary chondrosarcoma at 5 years is approximately 90%.
In our case, the patient, who had 3 enchondromas isolated to the left hand, can be categorized in group I. Thus, this case highlights the natural history of a patient with hand enchondromas and demonstrates that enchondromatosis of the short tubular bones of the hands can mature and ossify.
1. Silve C, Jüppner H. Ollier disease. Orphanet J Rare Dis. 2006;1:37-42.
2. Baert A. Encyclopedia of Diagnostic Imaging. Vol. 1. Berlin, Germany: Springer; 2008.
3. Takigawa K. Chondroma of the bones of the hand. A review of 110 cases. J Bone Joint Surg Am. 1971;53(8):1591-1600.
4. Mosher J. Multiple enchondromatosis of the hand. A case report. J Bone Joint Surg Am. 1976;58(5):717-719.
5. Goto T, Motoi T, Komiya K, et al. Chondrosarcoma of the hand secondary to multiple enchondromatosis; report of two cases. Arch Orthop Trauma Surg. 2003;123(1):42-47.
6. Muramatsu K, Kawakami Y, Tani Y, Taguchi T. Malignant transformation of multiple enchondromas in the hand: case report. J Hand Surg Am. 2011;36(2):304-307.
7. Verdegaal SH, Bovee JV, Pansuriya TC, et al. Incidence, predictive factors, and prognosis of chondrosarcoma in patients with Ollier disease and Maffucci syndrome: an international multicenter study of 161 patients. Oncologist. 2011;16(12):1771-1779.
8. Liu J, Hudkins PG, Swee RG, Unni KK. Bone sarcomas associated with Ollier’s disease. Cancer. 1987;59(7):1376-1385.
9. Dahlin D, Salvador AH. Chondrosarcomas of bones of the hands and feet—a study of 30 cases. Cancer. 1974;34(3):755-760.
10. Geirnaerdt MJ, Hermans J, Bloem JL, et al. Usefulness of radiography in differentiating enchondroma from central grade I chondrosarcoma. AJR Am J Roentgenol. 1997;169(4):1097-1104.
11. Sassoon AA, Fitz-Gibbon PD, Harmsen WS, Moran SL. Enchondromas of the hand: factors affecting recurrence, healing, motion, and malignant transformation. J Hand Surg Am. 2012;37(6):1229-1234.
12. Ogose A, Unni KK, Swee R, May GK, Rowland CM, Sim FH. Chondrosarcoma of small bones of the hands and feet. Cancer. 1997;80(1):50-59.
13. Patil S, de Silva MV, Crossan J, Reid R. Chondrosarcoma of small bones of the hand. J Hand Surg Br. 2003;28(6):602-608.
14. Herget GW, Strohm P, Rottenburger C, et al. Insights in Enchondroma, Enchondromatosis and the risk of secondary Chondrosarcoma. Review of the literature with an emphasis on the clinical behaviour, radiology, malignant transformation and the follow up. Neoplasma. 2014;61(4):365-378.
1. Silve C, Jüppner H. Ollier disease. Orphanet J Rare Dis. 2006;1:37-42.
2. Baert A. Encyclopedia of Diagnostic Imaging. Vol. 1. Berlin, Germany: Springer; 2008.
3. Takigawa K. Chondroma of the bones of the hand. A review of 110 cases. J Bone Joint Surg Am. 1971;53(8):1591-1600.
4. Mosher J. Multiple enchondromatosis of the hand. A case report. J Bone Joint Surg Am. 1976;58(5):717-719.
5. Goto T, Motoi T, Komiya K, et al. Chondrosarcoma of the hand secondary to multiple enchondromatosis; report of two cases. Arch Orthop Trauma Surg. 2003;123(1):42-47.
6. Muramatsu K, Kawakami Y, Tani Y, Taguchi T. Malignant transformation of multiple enchondromas in the hand: case report. J Hand Surg Am. 2011;36(2):304-307.
7. Verdegaal SH, Bovee JV, Pansuriya TC, et al. Incidence, predictive factors, and prognosis of chondrosarcoma in patients with Ollier disease and Maffucci syndrome: an international multicenter study of 161 patients. Oncologist. 2011;16(12):1771-1779.
8. Liu J, Hudkins PG, Swee RG, Unni KK. Bone sarcomas associated with Ollier’s disease. Cancer. 1987;59(7):1376-1385.
9. Dahlin D, Salvador AH. Chondrosarcomas of bones of the hands and feet—a study of 30 cases. Cancer. 1974;34(3):755-760.
10. Geirnaerdt MJ, Hermans J, Bloem JL, et al. Usefulness of radiography in differentiating enchondroma from central grade I chondrosarcoma. AJR Am J Roentgenol. 1997;169(4):1097-1104.
11. Sassoon AA, Fitz-Gibbon PD, Harmsen WS, Moran SL. Enchondromas of the hand: factors affecting recurrence, healing, motion, and malignant transformation. J Hand Surg Am. 2012;37(6):1229-1234.
12. Ogose A, Unni KK, Swee R, May GK, Rowland CM, Sim FH. Chondrosarcoma of small bones of the hands and feet. Cancer. 1997;80(1):50-59.
13. Patil S, de Silva MV, Crossan J, Reid R. Chondrosarcoma of small bones of the hand. J Hand Surg Br. 2003;28(6):602-608.
14. Herget GW, Strohm P, Rottenburger C, et al. Insights in Enchondroma, Enchondromatosis and the risk of secondary Chondrosarcoma. Review of the literature with an emphasis on the clinical behaviour, radiology, malignant transformation and the follow up. Neoplasma. 2014;61(4):365-378.
Fibromyalgia • anxiety/depression • urinary retention • Dx?
THE CASE
A 72-year-old woman came to our internal medicine department clinic for a follow-up appointment for her fibromyalgia. Thirteen months earlier, she had sought care at our facility not only for fibromyalgia, but for insomnia, anxiety, depression, and urinary incontinence. At the time, we prescribed amitriptyline 10 mg/d—for her pain and depression—as well as clonazepam 10 mg/d and paracetamol 650 mg, as needed.
When she came in for the follow-up, she indicated that for the past 8 months, she’d been experiencing urinary retention that required her to self-catheterize 2 to 3 times a day. She said she hadn’t used other medicines or herbal products during this time.
The patient had visited her family physician several times over the previous few months, and had been referred to a urologist. During an episode of acute urinary retention, she went to the emergency department (ED), where the ED physician performed urinary catheterization and referred her to the hospital’s Urology Department. After 48 hours, she was evaluated by a urologist, who diagnosed chronic urinary retention related to a hypercontractile bladder, without any particular cause. She was advised to continue to catheterize herself when needed. She was also prescribed pyridostigmine bromide, but she stopped taking it because of abdominal pain and bloating.
Two months prior to her visit with us, the patient suffered a second acute urinary retention episode and returned to the ED. Urinary catheterization was performed for 72 hours. At her next visit to her urologist, she was told to continue self-catheterization and was prescribed silodosin 8 mg/d.
THE DIAGNOSIS
Based on the patient’s history, we suspected the urinary retention was secondary to the anticholinergic effects of amitriptyline. We were able to determine that the patient’s urinary retention was likely the result of an adverse drug reaction (ADR) by using the causality algorithm of the Spanish Pharmacovigilance System, which suggests the following criteria:1 a) a positive time sequence (ie, onset of symptoms closely followed administration of the medication), b) the existence of an ADR that is well known and consistent with the mechanism of action of the drug,2 c) symptoms that resolve after suspending the drug; d) no repeat exposure (to the adverse effects of amitriptyline) due to ethical reasons; and e) the absence of an alternative explanation for the symptoms.3
DISCUSSION
Although indicated for depression, amitriptyline is also used for other conditions, including nocturnal enuresis and chronic neuropathic pain.4 Amitriptyline exhibits anticholinergic effects that can cause symptoms related to the nervous system (agitation, disorientation, sleepiness, delirium, cognitive impairment), ocular system (blurred vision, dry eye, accommodation disturbances, increased intraocular pressure), cardiovascular system (tachycardia), gastrointestinal tract (dry mouth, paralytic ileus, constipation), urinary system (urinary retention); and skin and mucosal membranes (dryness).5,6 Anticholinergic effects can also induce hyperthermia or increase the risk of falls.5,6
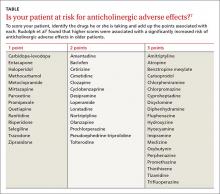
Anticholinergic medications can cause ADRs in high-risk older patients and thus are usually considered inappropriate for this patient population.6 The Anticholinergic Risk Scale (ARS) can be used to categorize medications based on their potential for anticholinergic adverse effects (TABLE).7 Amitriptyline is included in the group with the highest risk of ADRs. Amitriptyline is also included in the list of drugs that should be avoided in older adults, according to the 2012 American Geriatrics Society Beers Criteria.8
Our patient. We instructed her to stop taking amitriptyline, and her urinary retention disappeared within 48 hours. Two months later, she remained asymptomatic.
THE TAKEAWAY
Although many medications are known to cause adverse events, they can be missed when clinicians fail to pinpoint exactly when a new sign, symptom, or health problem appeared. This often leads to a chain reaction of unnecessary explorations, harmful treatment, patient suffering, and unjustified costs.9-11 Our patient had seen 4 different health care providers (a family physician, urologist, and 2 ED physicians) before we saw her and ultimately made the diagnosis. Family physicians can prevent anticholinergic ADRs by using a scale, such as the ARS, before prescribing a medication.
1. Meyboom RH, Royer RJ. Causality classification at pharmacovigilance centres in the European community. Pharmacoepidemiol Drug Saf. 1992;1:87–97.
2. Agencia Española de Medicamentos y Productos Sanitarios (AEMPS). Ficha Técnica Tryptizol. Agencia Española de Medicamentosy Productos Sanitarios (AEMPS) Web site. Available at: http://www.aemps.gob.es/cima/pdfs/en/ft/51064/FT_51064.pdf. Accessed July 24, 2015.
3. Agencia Española de Medicamentos y Productos Sanitarios (AEMPS). ¿Qué es el Sistema Español de Farmacovigilancia de medicamentos de Uso Humano? Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) Web site. Available at: http://www.aemps.gob.es/vigilancia/medicamentosUsoHumano/SEFV-H/home.htm. Accessed July 6, 2015.
4. Parfitt K, ed. Martindale: The Complete Drug Reference. 32nd ed. London, UK: Pharmaceutical Press;1999:273-276.
5. Rang HP, Dale MM, Ritter JM. Farmacología. 4th ed. Barcelona, Spain: Ediciones Harcourt, S.A. Impresión Mateu Cromo, S.A.;2000:123-128,594-600.
6. Ness J, Hoth A, Barnett MJ, et al. Anticholinergic medications in community-dwelling older veterans: prevalence of anticholinergic symptoms, symptom burden, and adverse drug events. Am J Geriatr Pharmacother. 2006;4:42-51.
7. Rudolph JL, Salow MJ, Angelini MC, et al. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med. 2008;168:508-513.
8. American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616-631.
9. CSM Update. Br Med J (Clin Res Ed). 1985;291:1638.
10. Palop Larrea V, Sempere i Verdú E, Martínez-Mir I. Anamnesis farmacológica y reacciones adversas a medicamentos. Aten Primaria. 2000;25:666,668.
11. Rochon PA, Gurwitz JH. Optimising drug treatment for elderly people: the prescribing cascade. BMJ. 1997;315:1096-1099.
THE CASE
A 72-year-old woman came to our internal medicine department clinic for a follow-up appointment for her fibromyalgia. Thirteen months earlier, she had sought care at our facility not only for fibromyalgia, but for insomnia, anxiety, depression, and urinary incontinence. At the time, we prescribed amitriptyline 10 mg/d—for her pain and depression—as well as clonazepam 10 mg/d and paracetamol 650 mg, as needed.
When she came in for the follow-up, she indicated that for the past 8 months, she’d been experiencing urinary retention that required her to self-catheterize 2 to 3 times a day. She said she hadn’t used other medicines or herbal products during this time.
The patient had visited her family physician several times over the previous few months, and had been referred to a urologist. During an episode of acute urinary retention, she went to the emergency department (ED), where the ED physician performed urinary catheterization and referred her to the hospital’s Urology Department. After 48 hours, she was evaluated by a urologist, who diagnosed chronic urinary retention related to a hypercontractile bladder, without any particular cause. She was advised to continue to catheterize herself when needed. She was also prescribed pyridostigmine bromide, but she stopped taking it because of abdominal pain and bloating.
Two months prior to her visit with us, the patient suffered a second acute urinary retention episode and returned to the ED. Urinary catheterization was performed for 72 hours. At her next visit to her urologist, she was told to continue self-catheterization and was prescribed silodosin 8 mg/d.
THE DIAGNOSIS
Based on the patient’s history, we suspected the urinary retention was secondary to the anticholinergic effects of amitriptyline. We were able to determine that the patient’s urinary retention was likely the result of an adverse drug reaction (ADR) by using the causality algorithm of the Spanish Pharmacovigilance System, which suggests the following criteria:1 a) a positive time sequence (ie, onset of symptoms closely followed administration of the medication), b) the existence of an ADR that is well known and consistent with the mechanism of action of the drug,2 c) symptoms that resolve after suspending the drug; d) no repeat exposure (to the adverse effects of amitriptyline) due to ethical reasons; and e) the absence of an alternative explanation for the symptoms.3
DISCUSSION
Although indicated for depression, amitriptyline is also used for other conditions, including nocturnal enuresis and chronic neuropathic pain.4 Amitriptyline exhibits anticholinergic effects that can cause symptoms related to the nervous system (agitation, disorientation, sleepiness, delirium, cognitive impairment), ocular system (blurred vision, dry eye, accommodation disturbances, increased intraocular pressure), cardiovascular system (tachycardia), gastrointestinal tract (dry mouth, paralytic ileus, constipation), urinary system (urinary retention); and skin and mucosal membranes (dryness).5,6 Anticholinergic effects can also induce hyperthermia or increase the risk of falls.5,6
Anticholinergic medications can cause ADRs in high-risk older patients and thus are usually considered inappropriate for this patient population.6 The Anticholinergic Risk Scale (ARS) can be used to categorize medications based on their potential for anticholinergic adverse effects (TABLE).7 Amitriptyline is included in the group with the highest risk of ADRs. Amitriptyline is also included in the list of drugs that should be avoided in older adults, according to the 2012 American Geriatrics Society Beers Criteria.8
Our patient. We instructed her to stop taking amitriptyline, and her urinary retention disappeared within 48 hours. Two months later, she remained asymptomatic.
THE TAKEAWAY
Although many medications are known to cause adverse events, they can be missed when clinicians fail to pinpoint exactly when a new sign, symptom, or health problem appeared. This often leads to a chain reaction of unnecessary explorations, harmful treatment, patient suffering, and unjustified costs.9-11 Our patient had seen 4 different health care providers (a family physician, urologist, and 2 ED physicians) before we saw her and ultimately made the diagnosis. Family physicians can prevent anticholinergic ADRs by using a scale, such as the ARS, before prescribing a medication.
THE CASE
A 72-year-old woman came to our internal medicine department clinic for a follow-up appointment for her fibromyalgia. Thirteen months earlier, she had sought care at our facility not only for fibromyalgia, but for insomnia, anxiety, depression, and urinary incontinence. At the time, we prescribed amitriptyline 10 mg/d—for her pain and depression—as well as clonazepam 10 mg/d and paracetamol 650 mg, as needed.
When she came in for the follow-up, she indicated that for the past 8 months, she’d been experiencing urinary retention that required her to self-catheterize 2 to 3 times a day. She said she hadn’t used other medicines or herbal products during this time.
The patient had visited her family physician several times over the previous few months, and had been referred to a urologist. During an episode of acute urinary retention, she went to the emergency department (ED), where the ED physician performed urinary catheterization and referred her to the hospital’s Urology Department. After 48 hours, she was evaluated by a urologist, who diagnosed chronic urinary retention related to a hypercontractile bladder, without any particular cause. She was advised to continue to catheterize herself when needed. She was also prescribed pyridostigmine bromide, but she stopped taking it because of abdominal pain and bloating.
Two months prior to her visit with us, the patient suffered a second acute urinary retention episode and returned to the ED. Urinary catheterization was performed for 72 hours. At her next visit to her urologist, she was told to continue self-catheterization and was prescribed silodosin 8 mg/d.
THE DIAGNOSIS
Based on the patient’s history, we suspected the urinary retention was secondary to the anticholinergic effects of amitriptyline. We were able to determine that the patient’s urinary retention was likely the result of an adverse drug reaction (ADR) by using the causality algorithm of the Spanish Pharmacovigilance System, which suggests the following criteria:1 a) a positive time sequence (ie, onset of symptoms closely followed administration of the medication), b) the existence of an ADR that is well known and consistent with the mechanism of action of the drug,2 c) symptoms that resolve after suspending the drug; d) no repeat exposure (to the adverse effects of amitriptyline) due to ethical reasons; and e) the absence of an alternative explanation for the symptoms.3
DISCUSSION
Although indicated for depression, amitriptyline is also used for other conditions, including nocturnal enuresis and chronic neuropathic pain.4 Amitriptyline exhibits anticholinergic effects that can cause symptoms related to the nervous system (agitation, disorientation, sleepiness, delirium, cognitive impairment), ocular system (blurred vision, dry eye, accommodation disturbances, increased intraocular pressure), cardiovascular system (tachycardia), gastrointestinal tract (dry mouth, paralytic ileus, constipation), urinary system (urinary retention); and skin and mucosal membranes (dryness).5,6 Anticholinergic effects can also induce hyperthermia or increase the risk of falls.5,6
Anticholinergic medications can cause ADRs in high-risk older patients and thus are usually considered inappropriate for this patient population.6 The Anticholinergic Risk Scale (ARS) can be used to categorize medications based on their potential for anticholinergic adverse effects (TABLE).7 Amitriptyline is included in the group with the highest risk of ADRs. Amitriptyline is also included in the list of drugs that should be avoided in older adults, according to the 2012 American Geriatrics Society Beers Criteria.8
Our patient. We instructed her to stop taking amitriptyline, and her urinary retention disappeared within 48 hours. Two months later, she remained asymptomatic.
THE TAKEAWAY
Although many medications are known to cause adverse events, they can be missed when clinicians fail to pinpoint exactly when a new sign, symptom, or health problem appeared. This often leads to a chain reaction of unnecessary explorations, harmful treatment, patient suffering, and unjustified costs.9-11 Our patient had seen 4 different health care providers (a family physician, urologist, and 2 ED physicians) before we saw her and ultimately made the diagnosis. Family physicians can prevent anticholinergic ADRs by using a scale, such as the ARS, before prescribing a medication.
1. Meyboom RH, Royer RJ. Causality classification at pharmacovigilance centres in the European community. Pharmacoepidemiol Drug Saf. 1992;1:87–97.
2. Agencia Española de Medicamentos y Productos Sanitarios (AEMPS). Ficha Técnica Tryptizol. Agencia Española de Medicamentosy Productos Sanitarios (AEMPS) Web site. Available at: http://www.aemps.gob.es/cima/pdfs/en/ft/51064/FT_51064.pdf. Accessed July 24, 2015.
3. Agencia Española de Medicamentos y Productos Sanitarios (AEMPS). ¿Qué es el Sistema Español de Farmacovigilancia de medicamentos de Uso Humano? Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) Web site. Available at: http://www.aemps.gob.es/vigilancia/medicamentosUsoHumano/SEFV-H/home.htm. Accessed July 6, 2015.
4. Parfitt K, ed. Martindale: The Complete Drug Reference. 32nd ed. London, UK: Pharmaceutical Press;1999:273-276.
5. Rang HP, Dale MM, Ritter JM. Farmacología. 4th ed. Barcelona, Spain: Ediciones Harcourt, S.A. Impresión Mateu Cromo, S.A.;2000:123-128,594-600.
6. Ness J, Hoth A, Barnett MJ, et al. Anticholinergic medications in community-dwelling older veterans: prevalence of anticholinergic symptoms, symptom burden, and adverse drug events. Am J Geriatr Pharmacother. 2006;4:42-51.
7. Rudolph JL, Salow MJ, Angelini MC, et al. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med. 2008;168:508-513.
8. American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616-631.
9. CSM Update. Br Med J (Clin Res Ed). 1985;291:1638.
10. Palop Larrea V, Sempere i Verdú E, Martínez-Mir I. Anamnesis farmacológica y reacciones adversas a medicamentos. Aten Primaria. 2000;25:666,668.
11. Rochon PA, Gurwitz JH. Optimising drug treatment for elderly people: the prescribing cascade. BMJ. 1997;315:1096-1099.
1. Meyboom RH, Royer RJ. Causality classification at pharmacovigilance centres in the European community. Pharmacoepidemiol Drug Saf. 1992;1:87–97.
2. Agencia Española de Medicamentos y Productos Sanitarios (AEMPS). Ficha Técnica Tryptizol. Agencia Española de Medicamentosy Productos Sanitarios (AEMPS) Web site. Available at: http://www.aemps.gob.es/cima/pdfs/en/ft/51064/FT_51064.pdf. Accessed July 24, 2015.
3. Agencia Española de Medicamentos y Productos Sanitarios (AEMPS). ¿Qué es el Sistema Español de Farmacovigilancia de medicamentos de Uso Humano? Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) Web site. Available at: http://www.aemps.gob.es/vigilancia/medicamentosUsoHumano/SEFV-H/home.htm. Accessed July 6, 2015.
4. Parfitt K, ed. Martindale: The Complete Drug Reference. 32nd ed. London, UK: Pharmaceutical Press;1999:273-276.
5. Rang HP, Dale MM, Ritter JM. Farmacología. 4th ed. Barcelona, Spain: Ediciones Harcourt, S.A. Impresión Mateu Cromo, S.A.;2000:123-128,594-600.
6. Ness J, Hoth A, Barnett MJ, et al. Anticholinergic medications in community-dwelling older veterans: prevalence of anticholinergic symptoms, symptom burden, and adverse drug events. Am J Geriatr Pharmacother. 2006;4:42-51.
7. Rudolph JL, Salow MJ, Angelini MC, et al. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med. 2008;168:508-513.
8. American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616-631.
9. CSM Update. Br Med J (Clin Res Ed). 1985;291:1638.
10. Palop Larrea V, Sempere i Verdú E, Martínez-Mir I. Anamnesis farmacológica y reacciones adversas a medicamentos. Aten Primaria. 2000;25:666,668.
11. Rochon PA, Gurwitz JH. Optimising drug treatment for elderly people: the prescribing cascade. BMJ. 1997;315:1096-1099.
Hot flashes and night sweats • amenorrhea • positive home pregnancy test • Dx?
THE CASE
A 25-year-old G2P2 woman came to our family practice clinic because she had multiple positive home pregnancy test results despite having undergone a sterilization procedure 4 years earlier. She said that 9 months ago, she had begun to experience hot flashes and night sweats that were getting progressively worse. Her menstrual cycles had been regular until 6 months earlier, when her bleeding became very light and irregular (2- to 6-week cycles with only one day of menstruation). Then 3 months ago, she stopped menstruating.
She’d had 2 uncomplicated pregnancies with normal vaginal deliveries 3 and 4 years ago, and had undergone a transcervical sterilization procedure after delivering her second child. Her medical history included hypothyroidism diagnosed at age 15, moderate persistent asthma, and seasonal allergies. She was taking levothyroxine 250 mcg/d, inhaled fluticasone/salmeterol, albuterol, and intranasal mometasone.
Transvaginal ultrasound failed to identify an intrauterine or ectopic pregnancy, and the patient’s ovaries were not visualized (uterine anatomy was normal with an endometrial stripe of 5.7 mm). The result of a serum human chorionic gonadotropin (hCG) test was 6.73 mIU/ mL. (In a nonpregnant, premenopausal woman, hCG is typically undetectable.) Subsequent serial hCG measurements remained low (6.72-7.09 mIU/mL), but persistent. Given these low hCG levels, it was imperative to rule out an intrauterine or ectopic pregnancy. A urine hCG was negative.
THE DIAGNOSIS
Because of our patient’s vasomotor symptoms, we ordered additional laboratory studies, which revealed an elevated follicle-stimulating hormone (FSH) level (66.08 mIU/mL and 42.2 mIU/mL taken one year apart; normal, 1.98-9.58 mIU/mL in a premenopausal female), an elevated luteinizing hormone (LH) level (46.1 mIU/mL; normal, 2.58-15.5 mIU/mL in a premenopausal female), a low thyroid-stimulating hormone (TSH) level (0.445 mIU/mL; normal, 0.465-4.65 mIU/mL), and a normal prolactin level (12.5 mIU/mL). Based on these results, we diagnosed primary ovarian insufficiency (POI).
DISCUSSION
POI, formerly known as premature ovarian failure, is defined as 4 to 6 months of amenorrhea or oligomenorrhea in a woman younger than 40 with an elevated FSH on 2 occasions, at least 4 weeks apart.1-3
The etiology of POI is broad. It can be caused by a failure of the pituitary gland or hypothalamus to secrete regulating hormones to stimulate the ovaries. Possible genetic causes include Turner’s syndrome, fragile X permutation, and other autosomal disorders that cause follicle dysfunction or destruction.1 Infections such as mumps, varicella, and tuberculosis are known to affect ovary function, as well.1,4 In addition, women who are exposed to chemotherapy or radiation are at higher risk for developing POI.1
Because POI and autoimmune disorders tend to occur together, consider screening any patient with POI for disorders such as hypothyroidism and Addison’s disease. A serum analysis to evaluate for autoantibodies against steroid-producing cells may be a potential marker for POI in patients with an autoimmune disease that affects the adrenal glands or thyroid. However, patients with isolated Addison’s disease, autoimmune hypothyroidism, or diabetes mellitus in the absence of POI do not appear to have steroid-specific antibodies.2 In our patient’s case, her hypothyroidism may have placed her at higher risk of having a second organ system adversely affected by her immune system.
What causes a false-positive pregnancy test? This case is unique because our patient reported multiple positive home pregnancy test results and had persistently low serum hCG levels. While she had symptoms that suggested menopause (hot flashes, oligomenorrhea that progressed to amenorrhea), she believed these symptoms were related to pregnancy. In addition to pregnancy, an elevated serum hCG measurement can be due to various malignancies, molar pregnancy, pituitary production of hCG, elevated LH, cross-reactivity with multiple animal exposures (due to the production of human anti-animal antibodies that react with testing), and recent mononucleosis infection.5
Other potential causes for false-positive urine pregnancy test results include tuboovarian abscess,6 adenomyosis,7 and cancers that produce hCG, such as colon, pancreatic, lung, liver, and urothelial bladder carcinoma.8,9 Urine with significant proteinuria can also cause a positive pregnancy test result.10
Our patient likely had a false-positive hCG due to elevated LH, secondary to POI, that demonstrated cross-reactivity on the hCG assay. The similarity in the chemical structure of the beta subunits of hCG and LH have been reported as false-positive tests in the absence of pregnancy.5
Because home pregnancy tests are designed to detect pregnancy as early as possible, they typically feature a high sensitivity by detecting very low levels of hCG, which leads to more frequent false-positive results. It is possible that different assay methods could account for the discrepancy between our patient’s positive home pregnancy tests and our negative laboratory urine pregnancy test.
Our patient and her husband were both counseled regarding her POI diagnosis. We conducted further studies to establish a possible etiology. She was found to have a normal karyotype of 46, XX, which ruled out Turner’s syndrome. Testing for permutations of the FMR1 gene was negative for fragile X syndrome, and antibody testing for thyroid and adrenal glands was negative for autoimmune disease.
Hormone therapy and supplemental calcium and vitamin D are recommended for women with POI to help prevent bone loss and other negative effects of low estrogen.11 We did not take this tack with our patient, however, because she decided she wanted to pursue a tubal ligation reversal in order to get pregnant. So instead, we decreased her dose of levothyroxine to 150 mcg (since her TSH was low) and we referred her to the Reproductive Endocrinology Department.
THE TAKEAWAY
Although many cases of POI have no discernible etiology, it is important to rule out malignancies, failure of pituitary production, genetic causes, infections, and other possible causes. Hormone therapy and prophylactic doses of calcium and vitamin D are recommended for patients diagnosed with POI.
1. Welt CK. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol (Oxf). 2008;68:499-509.
2. Betterle C, Rossi A, Dalla Pria S, et al. Premature ovarian failure: autoimmunity and natural history. Clin Endocrinol (Oxf). 1993;39:35-43.
3. Fox H. The pathology of premature ovarian failure. J Pathol. 1992;167:357-363.
4. Panay N, Kalu E. Management of premature ovarian failure. Best Practice & Research Clinical Obstetrics and Gynaecology. 2009;23;129-140.
5. Braunstein GD. False-positive serum human chorionic gonadotropin results: causes, characteristics, and recognition. Am J Obstet Gynecol. 2002;187:217-224.
6. Levsky ME, Handler JA, Suarez RD, et al. False-positive urine beta-HCG in a woman with a tubo-ovarian abscess. J Emerg Med. 2001;21:407-409.
7. Er TK, Chiang CH, Cheng BH, et al. False-positive urine pregnancy test in a woman with adenomysosis. Am J Emerg Med. 2009;27:1019.e5-7.
8. Rajabi B, Khoury J, Brewer C, et al. Urothelial bladder carcinoma with choriocarcinomatous differentiation presenting with a false-positive pregnancy test. Am J Med Sci. 2013;346:256-258.
9. Marcillac I, Troalen F, Bidart JM, et al. Free human chorionic gonadotropin beta subunit in gonadal and nongonadal neoplasms. Cancer Res. 1992;52:3901-3907.
10. Kountz DS, Kolander SA, Rozovsky A. False positive urinary pregnancy test in the nephrotic syndrome. N Engl J Med. 1989;321:1416.
11. National Institute of Health, National Institute of Child Health and Human Development. What are the treatments for POI? National Institute of Child Health and Human Development Web site. Available at: https://www.nichd.nih.gov/health/topics/poi/conditioninfo/Pages/treatments.aspx. Accessed August 5, 2015.
THE CASE
A 25-year-old G2P2 woman came to our family practice clinic because she had multiple positive home pregnancy test results despite having undergone a sterilization procedure 4 years earlier. She said that 9 months ago, she had begun to experience hot flashes and night sweats that were getting progressively worse. Her menstrual cycles had been regular until 6 months earlier, when her bleeding became very light and irregular (2- to 6-week cycles with only one day of menstruation). Then 3 months ago, she stopped menstruating.
She’d had 2 uncomplicated pregnancies with normal vaginal deliveries 3 and 4 years ago, and had undergone a transcervical sterilization procedure after delivering her second child. Her medical history included hypothyroidism diagnosed at age 15, moderate persistent asthma, and seasonal allergies. She was taking levothyroxine 250 mcg/d, inhaled fluticasone/salmeterol, albuterol, and intranasal mometasone.
Transvaginal ultrasound failed to identify an intrauterine or ectopic pregnancy, and the patient’s ovaries were not visualized (uterine anatomy was normal with an endometrial stripe of 5.7 mm). The result of a serum human chorionic gonadotropin (hCG) test was 6.73 mIU/ mL. (In a nonpregnant, premenopausal woman, hCG is typically undetectable.) Subsequent serial hCG measurements remained low (6.72-7.09 mIU/mL), but persistent. Given these low hCG levels, it was imperative to rule out an intrauterine or ectopic pregnancy. A urine hCG was negative.
THE DIAGNOSIS
Because of our patient’s vasomotor symptoms, we ordered additional laboratory studies, which revealed an elevated follicle-stimulating hormone (FSH) level (66.08 mIU/mL and 42.2 mIU/mL taken one year apart; normal, 1.98-9.58 mIU/mL in a premenopausal female), an elevated luteinizing hormone (LH) level (46.1 mIU/mL; normal, 2.58-15.5 mIU/mL in a premenopausal female), a low thyroid-stimulating hormone (TSH) level (0.445 mIU/mL; normal, 0.465-4.65 mIU/mL), and a normal prolactin level (12.5 mIU/mL). Based on these results, we diagnosed primary ovarian insufficiency (POI).
DISCUSSION
POI, formerly known as premature ovarian failure, is defined as 4 to 6 months of amenorrhea or oligomenorrhea in a woman younger than 40 with an elevated FSH on 2 occasions, at least 4 weeks apart.1-3
The etiology of POI is broad. It can be caused by a failure of the pituitary gland or hypothalamus to secrete regulating hormones to stimulate the ovaries. Possible genetic causes include Turner’s syndrome, fragile X permutation, and other autosomal disorders that cause follicle dysfunction or destruction.1 Infections such as mumps, varicella, and tuberculosis are known to affect ovary function, as well.1,4 In addition, women who are exposed to chemotherapy or radiation are at higher risk for developing POI.1
Because POI and autoimmune disorders tend to occur together, consider screening any patient with POI for disorders such as hypothyroidism and Addison’s disease. A serum analysis to evaluate for autoantibodies against steroid-producing cells may be a potential marker for POI in patients with an autoimmune disease that affects the adrenal glands or thyroid. However, patients with isolated Addison’s disease, autoimmune hypothyroidism, or diabetes mellitus in the absence of POI do not appear to have steroid-specific antibodies.2 In our patient’s case, her hypothyroidism may have placed her at higher risk of having a second organ system adversely affected by her immune system.
What causes a false-positive pregnancy test? This case is unique because our patient reported multiple positive home pregnancy test results and had persistently low serum hCG levels. While she had symptoms that suggested menopause (hot flashes, oligomenorrhea that progressed to amenorrhea), she believed these symptoms were related to pregnancy. In addition to pregnancy, an elevated serum hCG measurement can be due to various malignancies, molar pregnancy, pituitary production of hCG, elevated LH, cross-reactivity with multiple animal exposures (due to the production of human anti-animal antibodies that react with testing), and recent mononucleosis infection.5
Other potential causes for false-positive urine pregnancy test results include tuboovarian abscess,6 adenomyosis,7 and cancers that produce hCG, such as colon, pancreatic, lung, liver, and urothelial bladder carcinoma.8,9 Urine with significant proteinuria can also cause a positive pregnancy test result.10
Our patient likely had a false-positive hCG due to elevated LH, secondary to POI, that demonstrated cross-reactivity on the hCG assay. The similarity in the chemical structure of the beta subunits of hCG and LH have been reported as false-positive tests in the absence of pregnancy.5
Because home pregnancy tests are designed to detect pregnancy as early as possible, they typically feature a high sensitivity by detecting very low levels of hCG, which leads to more frequent false-positive results. It is possible that different assay methods could account for the discrepancy between our patient’s positive home pregnancy tests and our negative laboratory urine pregnancy test.
Our patient and her husband were both counseled regarding her POI diagnosis. We conducted further studies to establish a possible etiology. She was found to have a normal karyotype of 46, XX, which ruled out Turner’s syndrome. Testing for permutations of the FMR1 gene was negative for fragile X syndrome, and antibody testing for thyroid and adrenal glands was negative for autoimmune disease.
Hormone therapy and supplemental calcium and vitamin D are recommended for women with POI to help prevent bone loss and other negative effects of low estrogen.11 We did not take this tack with our patient, however, because she decided she wanted to pursue a tubal ligation reversal in order to get pregnant. So instead, we decreased her dose of levothyroxine to 150 mcg (since her TSH was low) and we referred her to the Reproductive Endocrinology Department.
THE TAKEAWAY
Although many cases of POI have no discernible etiology, it is important to rule out malignancies, failure of pituitary production, genetic causes, infections, and other possible causes. Hormone therapy and prophylactic doses of calcium and vitamin D are recommended for patients diagnosed with POI.
THE CASE
A 25-year-old G2P2 woman came to our family practice clinic because she had multiple positive home pregnancy test results despite having undergone a sterilization procedure 4 years earlier. She said that 9 months ago, she had begun to experience hot flashes and night sweats that were getting progressively worse. Her menstrual cycles had been regular until 6 months earlier, when her bleeding became very light and irregular (2- to 6-week cycles with only one day of menstruation). Then 3 months ago, she stopped menstruating.
She’d had 2 uncomplicated pregnancies with normal vaginal deliveries 3 and 4 years ago, and had undergone a transcervical sterilization procedure after delivering her second child. Her medical history included hypothyroidism diagnosed at age 15, moderate persistent asthma, and seasonal allergies. She was taking levothyroxine 250 mcg/d, inhaled fluticasone/salmeterol, albuterol, and intranasal mometasone.
Transvaginal ultrasound failed to identify an intrauterine or ectopic pregnancy, and the patient’s ovaries were not visualized (uterine anatomy was normal with an endometrial stripe of 5.7 mm). The result of a serum human chorionic gonadotropin (hCG) test was 6.73 mIU/ mL. (In a nonpregnant, premenopausal woman, hCG is typically undetectable.) Subsequent serial hCG measurements remained low (6.72-7.09 mIU/mL), but persistent. Given these low hCG levels, it was imperative to rule out an intrauterine or ectopic pregnancy. A urine hCG was negative.
THE DIAGNOSIS
Because of our patient’s vasomotor symptoms, we ordered additional laboratory studies, which revealed an elevated follicle-stimulating hormone (FSH) level (66.08 mIU/mL and 42.2 mIU/mL taken one year apart; normal, 1.98-9.58 mIU/mL in a premenopausal female), an elevated luteinizing hormone (LH) level (46.1 mIU/mL; normal, 2.58-15.5 mIU/mL in a premenopausal female), a low thyroid-stimulating hormone (TSH) level (0.445 mIU/mL; normal, 0.465-4.65 mIU/mL), and a normal prolactin level (12.5 mIU/mL). Based on these results, we diagnosed primary ovarian insufficiency (POI).
DISCUSSION
POI, formerly known as premature ovarian failure, is defined as 4 to 6 months of amenorrhea or oligomenorrhea in a woman younger than 40 with an elevated FSH on 2 occasions, at least 4 weeks apart.1-3
The etiology of POI is broad. It can be caused by a failure of the pituitary gland or hypothalamus to secrete regulating hormones to stimulate the ovaries. Possible genetic causes include Turner’s syndrome, fragile X permutation, and other autosomal disorders that cause follicle dysfunction or destruction.1 Infections such as mumps, varicella, and tuberculosis are known to affect ovary function, as well.1,4 In addition, women who are exposed to chemotherapy or radiation are at higher risk for developing POI.1
Because POI and autoimmune disorders tend to occur together, consider screening any patient with POI for disorders such as hypothyroidism and Addison’s disease. A serum analysis to evaluate for autoantibodies against steroid-producing cells may be a potential marker for POI in patients with an autoimmune disease that affects the adrenal glands or thyroid. However, patients with isolated Addison’s disease, autoimmune hypothyroidism, or diabetes mellitus in the absence of POI do not appear to have steroid-specific antibodies.2 In our patient’s case, her hypothyroidism may have placed her at higher risk of having a second organ system adversely affected by her immune system.
What causes a false-positive pregnancy test? This case is unique because our patient reported multiple positive home pregnancy test results and had persistently low serum hCG levels. While she had symptoms that suggested menopause (hot flashes, oligomenorrhea that progressed to amenorrhea), she believed these symptoms were related to pregnancy. In addition to pregnancy, an elevated serum hCG measurement can be due to various malignancies, molar pregnancy, pituitary production of hCG, elevated LH, cross-reactivity with multiple animal exposures (due to the production of human anti-animal antibodies that react with testing), and recent mononucleosis infection.5
Other potential causes for false-positive urine pregnancy test results include tuboovarian abscess,6 adenomyosis,7 and cancers that produce hCG, such as colon, pancreatic, lung, liver, and urothelial bladder carcinoma.8,9 Urine with significant proteinuria can also cause a positive pregnancy test result.10
Our patient likely had a false-positive hCG due to elevated LH, secondary to POI, that demonstrated cross-reactivity on the hCG assay. The similarity in the chemical structure of the beta subunits of hCG and LH have been reported as false-positive tests in the absence of pregnancy.5
Because home pregnancy tests are designed to detect pregnancy as early as possible, they typically feature a high sensitivity by detecting very low levels of hCG, which leads to more frequent false-positive results. It is possible that different assay methods could account for the discrepancy between our patient’s positive home pregnancy tests and our negative laboratory urine pregnancy test.
Our patient and her husband were both counseled regarding her POI diagnosis. We conducted further studies to establish a possible etiology. She was found to have a normal karyotype of 46, XX, which ruled out Turner’s syndrome. Testing for permutations of the FMR1 gene was negative for fragile X syndrome, and antibody testing for thyroid and adrenal glands was negative for autoimmune disease.
Hormone therapy and supplemental calcium and vitamin D are recommended for women with POI to help prevent bone loss and other negative effects of low estrogen.11 We did not take this tack with our patient, however, because she decided she wanted to pursue a tubal ligation reversal in order to get pregnant. So instead, we decreased her dose of levothyroxine to 150 mcg (since her TSH was low) and we referred her to the Reproductive Endocrinology Department.
THE TAKEAWAY
Although many cases of POI have no discernible etiology, it is important to rule out malignancies, failure of pituitary production, genetic causes, infections, and other possible causes. Hormone therapy and prophylactic doses of calcium and vitamin D are recommended for patients diagnosed with POI.
1. Welt CK. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol (Oxf). 2008;68:499-509.
2. Betterle C, Rossi A, Dalla Pria S, et al. Premature ovarian failure: autoimmunity and natural history. Clin Endocrinol (Oxf). 1993;39:35-43.
3. Fox H. The pathology of premature ovarian failure. J Pathol. 1992;167:357-363.
4. Panay N, Kalu E. Management of premature ovarian failure. Best Practice & Research Clinical Obstetrics and Gynaecology. 2009;23;129-140.
5. Braunstein GD. False-positive serum human chorionic gonadotropin results: causes, characteristics, and recognition. Am J Obstet Gynecol. 2002;187:217-224.
6. Levsky ME, Handler JA, Suarez RD, et al. False-positive urine beta-HCG in a woman with a tubo-ovarian abscess. J Emerg Med. 2001;21:407-409.
7. Er TK, Chiang CH, Cheng BH, et al. False-positive urine pregnancy test in a woman with adenomysosis. Am J Emerg Med. 2009;27:1019.e5-7.
8. Rajabi B, Khoury J, Brewer C, et al. Urothelial bladder carcinoma with choriocarcinomatous differentiation presenting with a false-positive pregnancy test. Am J Med Sci. 2013;346:256-258.
9. Marcillac I, Troalen F, Bidart JM, et al. Free human chorionic gonadotropin beta subunit in gonadal and nongonadal neoplasms. Cancer Res. 1992;52:3901-3907.
10. Kountz DS, Kolander SA, Rozovsky A. False positive urinary pregnancy test in the nephrotic syndrome. N Engl J Med. 1989;321:1416.
11. National Institute of Health, National Institute of Child Health and Human Development. What are the treatments for POI? National Institute of Child Health and Human Development Web site. Available at: https://www.nichd.nih.gov/health/topics/poi/conditioninfo/Pages/treatments.aspx. Accessed August 5, 2015.
1. Welt CK. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol (Oxf). 2008;68:499-509.
2. Betterle C, Rossi A, Dalla Pria S, et al. Premature ovarian failure: autoimmunity and natural history. Clin Endocrinol (Oxf). 1993;39:35-43.
3. Fox H. The pathology of premature ovarian failure. J Pathol. 1992;167:357-363.
4. Panay N, Kalu E. Management of premature ovarian failure. Best Practice & Research Clinical Obstetrics and Gynaecology. 2009;23;129-140.
5. Braunstein GD. False-positive serum human chorionic gonadotropin results: causes, characteristics, and recognition. Am J Obstet Gynecol. 2002;187:217-224.
6. Levsky ME, Handler JA, Suarez RD, et al. False-positive urine beta-HCG in a woman with a tubo-ovarian abscess. J Emerg Med. 2001;21:407-409.
7. Er TK, Chiang CH, Cheng BH, et al. False-positive urine pregnancy test in a woman with adenomysosis. Am J Emerg Med. 2009;27:1019.e5-7.
8. Rajabi B, Khoury J, Brewer C, et al. Urothelial bladder carcinoma with choriocarcinomatous differentiation presenting with a false-positive pregnancy test. Am J Med Sci. 2013;346:256-258.
9. Marcillac I, Troalen F, Bidart JM, et al. Free human chorionic gonadotropin beta subunit in gonadal and nongonadal neoplasms. Cancer Res. 1992;52:3901-3907.
10. Kountz DS, Kolander SA, Rozovsky A. False positive urinary pregnancy test in the nephrotic syndrome. N Engl J Med. 1989;321:1416.
11. National Institute of Health, National Institute of Child Health and Human Development. What are the treatments for POI? National Institute of Child Health and Human Development Web site. Available at: https://www.nichd.nih.gov/health/topics/poi/conditioninfo/Pages/treatments.aspx. Accessed August 5, 2015.
Case Report: A Bittersweet Death
Case
A 32-year-old Hispanic man presented to the ED with complications associated with diabetes mellitus (DM), the symptoms of which started approximately 3 days prior to arrival. The patient reported feelings of fatigue, dry mouth, increased thirst, and frequent urination. He denied sweating, nausea, chest pain, shortness of breath, diarrhea, or blood in his urine; he also denied blurry vision or dizziness.
During history intake, the patient informed the emergency physician (EP) that he had been diagnosed with DM and hyperglycemia earlier that day by his primary care physician, who had immediately referred the patient to the ED for urgent management. The patient’s own medical history was noncontributory; however, his father’s history was notable for DM and chronic renal failure. The patient further stated that he was not on any medications. Regarding his social history, he denied cigarette smoking and noted only occasional alcohol consumption.
The patient’s vital signs on presentation were: blood pressure (BP), 116/74 mm Hg; heart rate, 113 beats/minute; respiratory rate, 26 breaths/minute; and temperature, 97.8°F. Oxygen saturation was 97% on room air. On physical examination, the patient was severely anxious, with tachycardia and respiratory distress. He was obese, with a body mass index of 30.9 kg/m2 (height, 5 feet, 4 inches; weight, 180 lb).
The patient was started on an intravenous (IV) bolus of 0.9% normal saline (2 L at 20 mL/kg). After a consultation with endocrinology, he was then given a maintenance dose of normal saline IV at 250 cc/h and an IV insulin drip at 0.1 U/kg/h following a bolus of 8 units of insulin IV. His glucose levels were carefully monitored via hourly finger-stick glucose testing.
Although the patient’s condition stabilized, he collapsed while walking to the bathroom. He had agonal respirations and no pulse. Resuscitation efforts were started with bag-valve-mask ventilation, along with emergent advanced cardiac life support (ACLS) treatment, the protocol of which included epinephrine administration (x2) IV push 5 minutes apart, 2 ampules of sodium bicarbonate (50 mEq each) IV push, and calcium gluconate 10% (x1) 10 mL (1 g) IV push. A pulse was re-established, and the patient was intubated.
The patient was diagnosed with diabetic ketoacidosis (DKA) and admitted to the intensive care unit where repeat laboratory evaluation was ordered. Additional pharmacological management included IV administration of dopamine, norepinephrine, phenylephrine, vasopressin, antibiotics (azithromycin, meropenem, and vancomycin), pantoprazole, and subcutaneous heparin.
During treatment, the patient coded a second time and was revived according to ACLS protocols. Shortly thereafter, he coded a third time, but resuscitation efforts failed. Pathology reported no biological cause of death, and the coroner closed the case as death due to DM-related complications.
Diabetic Ketoacidosis
Diabetic ketoacidosis is a major complication of DM.4 Although the condition usually occurs in type 1 DM, it can also develop in type 2 DM. Diabetic ketoacidosis may be an inciting event leading to the eventual diagnosis of DM, but can also develop during a concurrent illness such as a urinary tract infection or an eating disorder.5 Risk factors for DKA include patients with type 1 or type 2 DM, a family history of DM, obesity, and nonwhite patients whose ethnic background places them at increased risk.6 Hispanic, black, and African American patients are at a greater risk of developing DKA and are more likely to develop “ketosis-prone” type 2 DM.7
Patients who do not fit into the definitive categories of type 1 or 2 DM can be classified under ketosis-prone DM.7,8 Diabetic ketoacidosis acts as the inciting event for the disease and evolves into severe β-cell dysfunction, hence blurring the lines between the archetypal DM categories. Fifty percent of ketosis-prone DM patients are A-β+ (absent autoantibodies, present β-cell function), which indicates that the dysfunction can be partially reversed. Reversal of the condition is largely based on long-term β-cell reserves, which are dependent on tight glycemic control and insulin dependence. Higher incidences of the A-β+ variant of ketosis-prone diabetes are seen in the male population and are often unprovoked.9-11
Diabetic ketoacidosis is the result of either a decrease or absence of insulin in the body (Table 2).4 Without insulin modulating exogenous glucose intake and endogenous glucose production (via glucagon, glycogenolysis, and gluconeogenesis), high levels of glucose are found in the circulation, leading to prominent hyperglycemia (>250 mg/dL or >13.8 mmol/L).6 This environment causes the body to switch from carbohydrate metabolism to fatty acid metabolism. As a result, acidic ketone bodies such β-hydroxybutyrate and acetoacetate are produced. These physiological changes in the body cause the signs and symptoms typically found in DKA.
Signs and Symptoms
Over a period of 24 hours, symptoms such as nausea, vomiting, increased thirst, and polyuria develop due to dehydration caused by osmotic diuresis and glucosuria.5 Patients may also present with hypotension and tachycardia. Confusion, deep gasping breaths or Kussmaul respirations, and metabolic acidosis result from hyperventilation and failure to compensate for the increased serum concentration of ketone bodies. Ketone production leads to a fruit-like odor in the patient’s breath and ketonuria in the urinalysis. In DKA, laboratory values will indicate metabolic acidosis and abnormal serum electrolytes. In both DM and DKA, increased urea and creatinine due to dehydration, increased ketones, and the presence of diabetic nephropathy are useful indicators of impaired kidney function.12
Management and Treatment
Diabetic ketoacidosis can be managed and reversed, especially when recognized and treated early.6,13 Dehydration in DKA can be corrected with IV fluid replacement. Normal saline (0.9%) can be started at 15 to 20 mL/kg/h or 1 L/h. As the patient’s vital signs stabilize, IV fluids can be titrated to a lower dose of 250 to 500 mL/h. Monitoring BP and electrolytes are key at this point as alterations in sodium levels and glucose levels may require switching to half-normal saline and/or dextrose.
The hyperglycemic state of patients with DKA is managed by IV insulin. An initial bolus of 0.1 U/kg/h can be given, but should only be administered when potassium levels are greater than 3.3 mmol/L.14 If adequate perfusion can be maintained, then 0.14 U/kg/h can be used instead of a bolus. Glucose levels must be monitored; once the levels decrease to approximately 200 mg/dL, the infusion rate of insulin should be titrated down to 0.05 to 0.1 U/kg/h. Dextrose is then added to maintain glucose levels at approximately 150 to 200 mg/dL.
Electrolytes, especially potassium, must be monitored closely in patients with DKA. Insulin leads to the shift of potassium into cells. The lack of insulin keeps potassium in the extracellular space. Due to osmotic diuresis, potassium is lost in the urine, leading to hypokalemia. Potassium levels in patients with DKA should be maintained at a level between 4 to 5 mmol/L. Patients with potassium levels between 3.3 to 5.2 mmol/L can be started on IV potassium between 20 to 30 mmol/h. If the patient is severely hypokalemic (<3.3 mmol/L), insulin should be withheld, and only IV potassium should be given at a rate of 20 to 30 mmol/h.
Bicarbonate levels can also be managed as acidosis can lead to both neurological and cardiac complications. If the patient’s pH is less than 6.9, the American Diabetes Association recommends starting 100 mmol of sodium bicarbonate in 400 mL sterile water (in addition to potassium chloride at 200 mL/h) for 2 hours. Dosing should be repeated every 2 hours until the patient’s pH is greater than 6.9.
In uncomplicated cases of DKA, the condition is resolved when a patient’s pH is greater than 7.3; glucose level is less than 200 mg/dL; and bicarbonate level is greater than or equal to 18 mmol/L. After patients become hemodynamically stable, they can be discharged and managed at home with a combination of intermediate- or long-acting insulin as well as short- or rapid-acting insulin.
Complications and Mortality
Diabetic ketoacidosis can cause sudden and fluctuating changes in the body. Therefore, it is very important to monitor a patient’s laboratory values very carefully and frequently to avoid any pitfalls. Since patients can present with hyponatremia due to the osmotic draw of glucose in the blood,13 sodium levels may have to be corrected. The corrected serum sodium can be calculated by adding 1.6 mmol/L for every 100 mg/dL of glucose (when finger-stick readings are above 200 mg/dL).15 Patients with DKA can also present with leukocytosis (even in the absence of infection) and hypertriglyceridemia (due to impaired lipoprotein lipase).15 Serum creatine may be elevated due to blood acetoacetate levels.15
Interestingly, there are other acute conditions that can mimic DKA.15 For example, chronic ethanol abuse can lead to ketoacidosis. Unlike DKA, however, alcoholic ketoacidosis does not have profound hyperglycemia, which can help differentiate the two during initial assessment.
Complications due to DKA can arise comprising the patient’s health, including hypoglycemia, hypokalemia, rhabdomyolysis, acute renal failure, pulmonary edema, and shock.16 Cerebral edema is seen in up to 1% of DKA patients,15 the cause of which may be due to the severity of the acidosis, high glucose levels, and rapid hydration. Even when cerebral edema is reduced, patients are often neurologically impaired. Mortality rates from DKA deaths due to cerebral edema can be as high as 24%.13 In the United States, over 100,000 patients with DM per year are admitted to the hospital for DKA, and 9% of patients with DM suffer from DKA-related complications postdischarge.15 With current treatment protocols, mortality rates for DKA-associated deaths are now down to 1%.6,15
Diabetes ketoacidosis-related deaths are usually the result of the following: a triad of DKA symptoms (hyperglycemia, hyperketonemia, and metabolic acidosis), another underlying comorbid condition (eg, myocardial infarction, sepsis, acute respiratory distress syndrome), or the release of biological markers (ie, catecholamines).14,15,17 Thus, as previously stated, the management of potassium levels is important as both hyperkalemia and hypokalemia can lead to fatal arrthythmias.15
Direct mortality from DKA has dropped significantly over the past 20 years, from 8% to less than 1%.6 The US Centers for Disease Control and Prevention has observed a downward trend in death and estimates that 2,417 patients died in 2009 due to DKA,18 and recent postmortem studies have revealed new insights into DKA-related deaths.19 Blood and vitreous acetone concentrations are strong indicators for predeath hyperglycemia and ketosis (if there are no underlying comorbid and/or pharmacological provocations). Blood acetone levels greater than 0.01 g/dL antemortem are suggestive of DKA. It is recommended that these tests should be performed in sudden deaths which have no biological or anatomical cause of death. Postmortem diagnosis of DKA is made with the following criteria: history of DM, increased vitreous glucose concentrations, and elevated blood/vitreous/urine acetone concentrations (>200 mg/dL). If results of the abovementioned parameters are inconclusive, measurement of lactic acid postmortem is thought to further support a diagnosis of DKA.19
Patient Counseling and Education
Approximately 33% of patients whose death was associated with DKA had no personal history of DM.19 This statistic emphasizes the importance of taking a thorough history, physical examination, blood glucose evaluation, and educating patients about the signs and symptoms of DM and DKA.
Patient counseling and education are important, especially in patients whose racial/ethnic background places them at increased risk of developing DM (eg, patients of black or African American, American Indian, Alaskan Native, Asian American, Hispanic, Native Hawaiian, or Pacific Islander descent).20,21 Strategies for preventive management include advocating regular glucose monitoring as well as dietary and lifestyle modifications. In patients with DM, successful management of the condition and its comorbidities can help prevent DKA and associated mortality.
Conclusion
As this case demonstrates, despite prompt diagnosis and management, patients with DKA—especially those with uncontrolled, undiagnosed, or advanced DM—are associated with fatal outcomes. In many cases, however, DKA can be successfully managed and reversed, especially when the condition is recognized early. Management includes not only IV therapy to adjust fluid and insulin levels, but also restoring electrolyte balance (especially potassium and bicarbonate). Frequent and careful evaluation of laboratory values is vital to the successful treatment of DKA, as there are numerous pitfalls and complications that the emergency physician can encounter. Patients who either have or are at an increased risk of developing DM or DKA may benefit from preventive measures, including regular glucose monitoring and appropriate diet and lifestyle modifications.
Mr Hassan-Ali is a fourth-year medical student at Windsor University School of Medicine, St Kitts, West Indies. Dr Raziuddin is an internist and an emergency medicine physician at Weiss Memorial, Thorek Memorial, and Westlake Hospitals, Chicago, Illinois.
- Kitabchi AH, Umpierrez GE, Murphy MB, et al. Management of hyperglycemic crises in patients with diabetes. Diabetes Care. 2001;24(1):131-153.
- Farinda A. Lab values, normal adult: laboratory reference ranges in healthy adults. 2015. Medscape Web site. http://emedicine.medscape.com/article/2172316-overview. Updated May 14, 2014. Accessed August 14, 2015.
- Young D. Implementation of SI units for clinical laboratory data. Ann Intern Med. 1987;106(1):114-129.
- Maitra A. The endocrine system. In: Kumar V, Abbas AK, Aster JC. Robbins and Cotran Pathologic Basis of Disease. 9th ed. New York, NY: Elsevier Saunders; 2015:1105-1120.
- Powers AC. Diabetes mellitus: management and therapies. In: Kasper DL, Fauci AS, Longo DL, Hauser SL, Jameson JL, Loscalzo J. Harrison’s Principles of Internal Medicine. 19th ed. New York, NY; McGraw-Hill Medical Publishing Division; 2015:2407-2422.
- Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335-1343.
- Umpierrez GE, Smiley D, Kitabchi AE. Narrative review: ketosis-prone type 2 diabetes mellitus. Ann Intern Med. 2006;144(5):350-357.
- Umpierrez G, Smiley D, Gosmanov A, Thomason D. Ketosis-prone type 2 diabetes: effect of hyperglycemia on beta-cell function and skeletal muscle insulin signaling. Endocr Pract. 2007;13(3):283-290.
- Mauvais-Jarvis F, Sobngwi E, Porcher R, et al. Ketosis-prone type 2 diabetes in patients of sub-Saharan African origin: clinical pathophysiology and natural history of beta-cell dysfunction and insulin resistance. Diabetes. 2004;53(3):645-653.
- Umpierrez GE, Casals MM, Gebhart SP, Mixon PS, Clark WS, Phillips LS. Diabetic ketoacidosis in obese African-Americans. Diabetes. 1995;44(7):790-795.
- Piñero-Piloña A, Raskin P. Idiopathic type 1 diabetes. J Diabetes Complications. 2001;15(6):328-335.
- Kemperman FA, Weber JA, Gorgels J, van Zanten AP, Krediet RT, Arisz L. The influence of ketoacids on plasma creatinine assays in diabetic ketoacidosis. J Intern Med. 2000;248(6):511-517.
- Westerberg DP. Diabetic ketoacidosis: evaluation and treatment. Am Fam Physician. 2013;87(5):337-346.
- Trachtenbarg DE. Diabetic ketoacidosis. Am Fam Physician. 2005;71(9):1705-1714.
- Umpierrez GE, Murphy MB, Kitabchi AE. Diabetic ketoacidosis and hyperglycemic hyperosmolar ayndrome. Diabetes Spectrum. 2002;15(1):28-36.
- Wolfsdorf J, Glaser N, Sperling MA; American Diabetes Association. Diabetic ketoacidosis in infants, children, and adolescents: A consensus statement from the American Diabetes Association. Diabetes Care. 2006;29(5):1150-1159.
- Rosenbloom AL. Sudden death of a young woman attributed to diabetic ketoacidosis. J Forensic Leg Med. 2013;20(8):1063-1065.
- Centers for Disease Control and Prevention. Number of deaths for hyperglycemic crises as underlying cause, United States, 1980-2009. http://www.cdc.gov/diabetes/statistics/mortalitydka/fnumberofdka.htm. Updated November 19, 2013. Accessed August 14, 2015.
- Ali Z, Levine B, Ripple M, Fowler DR. Diabetic ketoacidosis: a silent death. Am J Forensic Med Pathol. 2012;33(3):189-193.
- US Department of Health and Human Services Office of Minority Health. Diabetes and Hispanic Americans. http://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=63. Updated June 15, 2013. Accessed August 14, 2015.
- US Department of Health and Human Services Office of Minority Health. Profile: Native Hawaiian/Other Pacific Islanders. http://minorityhealth.hhs.gov/omh/browse.aspx?lvl=3&lvlid=65. Updated January 15, 2015. Accessed August 14, 2015.
Case
A 32-year-old Hispanic man presented to the ED with complications associated with diabetes mellitus (DM), the symptoms of which started approximately 3 days prior to arrival. The patient reported feelings of fatigue, dry mouth, increased thirst, and frequent urination. He denied sweating, nausea, chest pain, shortness of breath, diarrhea, or blood in his urine; he also denied blurry vision or dizziness.
During history intake, the patient informed the emergency physician (EP) that he had been diagnosed with DM and hyperglycemia earlier that day by his primary care physician, who had immediately referred the patient to the ED for urgent management. The patient’s own medical history was noncontributory; however, his father’s history was notable for DM and chronic renal failure. The patient further stated that he was not on any medications. Regarding his social history, he denied cigarette smoking and noted only occasional alcohol consumption.
The patient’s vital signs on presentation were: blood pressure (BP), 116/74 mm Hg; heart rate, 113 beats/minute; respiratory rate, 26 breaths/minute; and temperature, 97.8°F. Oxygen saturation was 97% on room air. On physical examination, the patient was severely anxious, with tachycardia and respiratory distress. He was obese, with a body mass index of 30.9 kg/m2 (height, 5 feet, 4 inches; weight, 180 lb).
The patient was started on an intravenous (IV) bolus of 0.9% normal saline (2 L at 20 mL/kg). After a consultation with endocrinology, he was then given a maintenance dose of normal saline IV at 250 cc/h and an IV insulin drip at 0.1 U/kg/h following a bolus of 8 units of insulin IV. His glucose levels were carefully monitored via hourly finger-stick glucose testing.
Although the patient’s condition stabilized, he collapsed while walking to the bathroom. He had agonal respirations and no pulse. Resuscitation efforts were started with bag-valve-mask ventilation, along with emergent advanced cardiac life support (ACLS) treatment, the protocol of which included epinephrine administration (x2) IV push 5 minutes apart, 2 ampules of sodium bicarbonate (50 mEq each) IV push, and calcium gluconate 10% (x1) 10 mL (1 g) IV push. A pulse was re-established, and the patient was intubated.
The patient was diagnosed with diabetic ketoacidosis (DKA) and admitted to the intensive care unit where repeat laboratory evaluation was ordered. Additional pharmacological management included IV administration of dopamine, norepinephrine, phenylephrine, vasopressin, antibiotics (azithromycin, meropenem, and vancomycin), pantoprazole, and subcutaneous heparin.
During treatment, the patient coded a second time and was revived according to ACLS protocols. Shortly thereafter, he coded a third time, but resuscitation efforts failed. Pathology reported no biological cause of death, and the coroner closed the case as death due to DM-related complications.
Diabetic Ketoacidosis
Diabetic ketoacidosis is a major complication of DM.4 Although the condition usually occurs in type 1 DM, it can also develop in type 2 DM. Diabetic ketoacidosis may be an inciting event leading to the eventual diagnosis of DM, but can also develop during a concurrent illness such as a urinary tract infection or an eating disorder.5 Risk factors for DKA include patients with type 1 or type 2 DM, a family history of DM, obesity, and nonwhite patients whose ethnic background places them at increased risk.6 Hispanic, black, and African American patients are at a greater risk of developing DKA and are more likely to develop “ketosis-prone” type 2 DM.7
Patients who do not fit into the definitive categories of type 1 or 2 DM can be classified under ketosis-prone DM.7,8 Diabetic ketoacidosis acts as the inciting event for the disease and evolves into severe β-cell dysfunction, hence blurring the lines between the archetypal DM categories. Fifty percent of ketosis-prone DM patients are A-β+ (absent autoantibodies, present β-cell function), which indicates that the dysfunction can be partially reversed. Reversal of the condition is largely based on long-term β-cell reserves, which are dependent on tight glycemic control and insulin dependence. Higher incidences of the A-β+ variant of ketosis-prone diabetes are seen in the male population and are often unprovoked.9-11
Diabetic ketoacidosis is the result of either a decrease or absence of insulin in the body (Table 2).4 Without insulin modulating exogenous glucose intake and endogenous glucose production (via glucagon, glycogenolysis, and gluconeogenesis), high levels of glucose are found in the circulation, leading to prominent hyperglycemia (>250 mg/dL or >13.8 mmol/L).6 This environment causes the body to switch from carbohydrate metabolism to fatty acid metabolism. As a result, acidic ketone bodies such β-hydroxybutyrate and acetoacetate are produced. These physiological changes in the body cause the signs and symptoms typically found in DKA.
Signs and Symptoms
Over a period of 24 hours, symptoms such as nausea, vomiting, increased thirst, and polyuria develop due to dehydration caused by osmotic diuresis and glucosuria.5 Patients may also present with hypotension and tachycardia. Confusion, deep gasping breaths or Kussmaul respirations, and metabolic acidosis result from hyperventilation and failure to compensate for the increased serum concentration of ketone bodies. Ketone production leads to a fruit-like odor in the patient’s breath and ketonuria in the urinalysis. In DKA, laboratory values will indicate metabolic acidosis and abnormal serum electrolytes. In both DM and DKA, increased urea and creatinine due to dehydration, increased ketones, and the presence of diabetic nephropathy are useful indicators of impaired kidney function.12
Management and Treatment
Diabetic ketoacidosis can be managed and reversed, especially when recognized and treated early.6,13 Dehydration in DKA can be corrected with IV fluid replacement. Normal saline (0.9%) can be started at 15 to 20 mL/kg/h or 1 L/h. As the patient’s vital signs stabilize, IV fluids can be titrated to a lower dose of 250 to 500 mL/h. Monitoring BP and electrolytes are key at this point as alterations in sodium levels and glucose levels may require switching to half-normal saline and/or dextrose.
The hyperglycemic state of patients with DKA is managed by IV insulin. An initial bolus of 0.1 U/kg/h can be given, but should only be administered when potassium levels are greater than 3.3 mmol/L.14 If adequate perfusion can be maintained, then 0.14 U/kg/h can be used instead of a bolus. Glucose levels must be monitored; once the levels decrease to approximately 200 mg/dL, the infusion rate of insulin should be titrated down to 0.05 to 0.1 U/kg/h. Dextrose is then added to maintain glucose levels at approximately 150 to 200 mg/dL.
Electrolytes, especially potassium, must be monitored closely in patients with DKA. Insulin leads to the shift of potassium into cells. The lack of insulin keeps potassium in the extracellular space. Due to osmotic diuresis, potassium is lost in the urine, leading to hypokalemia. Potassium levels in patients with DKA should be maintained at a level between 4 to 5 mmol/L. Patients with potassium levels between 3.3 to 5.2 mmol/L can be started on IV potassium between 20 to 30 mmol/h. If the patient is severely hypokalemic (<3.3 mmol/L), insulin should be withheld, and only IV potassium should be given at a rate of 20 to 30 mmol/h.
Bicarbonate levels can also be managed as acidosis can lead to both neurological and cardiac complications. If the patient’s pH is less than 6.9, the American Diabetes Association recommends starting 100 mmol of sodium bicarbonate in 400 mL sterile water (in addition to potassium chloride at 200 mL/h) for 2 hours. Dosing should be repeated every 2 hours until the patient’s pH is greater than 6.9.
In uncomplicated cases of DKA, the condition is resolved when a patient’s pH is greater than 7.3; glucose level is less than 200 mg/dL; and bicarbonate level is greater than or equal to 18 mmol/L. After patients become hemodynamically stable, they can be discharged and managed at home with a combination of intermediate- or long-acting insulin as well as short- or rapid-acting insulin.
Complications and Mortality
Diabetic ketoacidosis can cause sudden and fluctuating changes in the body. Therefore, it is very important to monitor a patient’s laboratory values very carefully and frequently to avoid any pitfalls. Since patients can present with hyponatremia due to the osmotic draw of glucose in the blood,13 sodium levels may have to be corrected. The corrected serum sodium can be calculated by adding 1.6 mmol/L for every 100 mg/dL of glucose (when finger-stick readings are above 200 mg/dL).15 Patients with DKA can also present with leukocytosis (even in the absence of infection) and hypertriglyceridemia (due to impaired lipoprotein lipase).15 Serum creatine may be elevated due to blood acetoacetate levels.15
Interestingly, there are other acute conditions that can mimic DKA.15 For example, chronic ethanol abuse can lead to ketoacidosis. Unlike DKA, however, alcoholic ketoacidosis does not have profound hyperglycemia, which can help differentiate the two during initial assessment.
Complications due to DKA can arise comprising the patient’s health, including hypoglycemia, hypokalemia, rhabdomyolysis, acute renal failure, pulmonary edema, and shock.16 Cerebral edema is seen in up to 1% of DKA patients,15 the cause of which may be due to the severity of the acidosis, high glucose levels, and rapid hydration. Even when cerebral edema is reduced, patients are often neurologically impaired. Mortality rates from DKA deaths due to cerebral edema can be as high as 24%.13 In the United States, over 100,000 patients with DM per year are admitted to the hospital for DKA, and 9% of patients with DM suffer from DKA-related complications postdischarge.15 With current treatment protocols, mortality rates for DKA-associated deaths are now down to 1%.6,15
Diabetes ketoacidosis-related deaths are usually the result of the following: a triad of DKA symptoms (hyperglycemia, hyperketonemia, and metabolic acidosis), another underlying comorbid condition (eg, myocardial infarction, sepsis, acute respiratory distress syndrome), or the release of biological markers (ie, catecholamines).14,15,17 Thus, as previously stated, the management of potassium levels is important as both hyperkalemia and hypokalemia can lead to fatal arrthythmias.15
Direct mortality from DKA has dropped significantly over the past 20 years, from 8% to less than 1%.6 The US Centers for Disease Control and Prevention has observed a downward trend in death and estimates that 2,417 patients died in 2009 due to DKA,18 and recent postmortem studies have revealed new insights into DKA-related deaths.19 Blood and vitreous acetone concentrations are strong indicators for predeath hyperglycemia and ketosis (if there are no underlying comorbid and/or pharmacological provocations). Blood acetone levels greater than 0.01 g/dL antemortem are suggestive of DKA. It is recommended that these tests should be performed in sudden deaths which have no biological or anatomical cause of death. Postmortem diagnosis of DKA is made with the following criteria: history of DM, increased vitreous glucose concentrations, and elevated blood/vitreous/urine acetone concentrations (>200 mg/dL). If results of the abovementioned parameters are inconclusive, measurement of lactic acid postmortem is thought to further support a diagnosis of DKA.19
Patient Counseling and Education
Approximately 33% of patients whose death was associated with DKA had no personal history of DM.19 This statistic emphasizes the importance of taking a thorough history, physical examination, blood glucose evaluation, and educating patients about the signs and symptoms of DM and DKA.
Patient counseling and education are important, especially in patients whose racial/ethnic background places them at increased risk of developing DM (eg, patients of black or African American, American Indian, Alaskan Native, Asian American, Hispanic, Native Hawaiian, or Pacific Islander descent).20,21 Strategies for preventive management include advocating regular glucose monitoring as well as dietary and lifestyle modifications. In patients with DM, successful management of the condition and its comorbidities can help prevent DKA and associated mortality.
Conclusion
As this case demonstrates, despite prompt diagnosis and management, patients with DKA—especially those with uncontrolled, undiagnosed, or advanced DM—are associated with fatal outcomes. In many cases, however, DKA can be successfully managed and reversed, especially when the condition is recognized early. Management includes not only IV therapy to adjust fluid and insulin levels, but also restoring electrolyte balance (especially potassium and bicarbonate). Frequent and careful evaluation of laboratory values is vital to the successful treatment of DKA, as there are numerous pitfalls and complications that the emergency physician can encounter. Patients who either have or are at an increased risk of developing DM or DKA may benefit from preventive measures, including regular glucose monitoring and appropriate diet and lifestyle modifications.
Mr Hassan-Ali is a fourth-year medical student at Windsor University School of Medicine, St Kitts, West Indies. Dr Raziuddin is an internist and an emergency medicine physician at Weiss Memorial, Thorek Memorial, and Westlake Hospitals, Chicago, Illinois.
Case
A 32-year-old Hispanic man presented to the ED with complications associated with diabetes mellitus (DM), the symptoms of which started approximately 3 days prior to arrival. The patient reported feelings of fatigue, dry mouth, increased thirst, and frequent urination. He denied sweating, nausea, chest pain, shortness of breath, diarrhea, or blood in his urine; he also denied blurry vision or dizziness.
During history intake, the patient informed the emergency physician (EP) that he had been diagnosed with DM and hyperglycemia earlier that day by his primary care physician, who had immediately referred the patient to the ED for urgent management. The patient’s own medical history was noncontributory; however, his father’s history was notable for DM and chronic renal failure. The patient further stated that he was not on any medications. Regarding his social history, he denied cigarette smoking and noted only occasional alcohol consumption.
The patient’s vital signs on presentation were: blood pressure (BP), 116/74 mm Hg; heart rate, 113 beats/minute; respiratory rate, 26 breaths/minute; and temperature, 97.8°F. Oxygen saturation was 97% on room air. On physical examination, the patient was severely anxious, with tachycardia and respiratory distress. He was obese, with a body mass index of 30.9 kg/m2 (height, 5 feet, 4 inches; weight, 180 lb).
The patient was started on an intravenous (IV) bolus of 0.9% normal saline (2 L at 20 mL/kg). After a consultation with endocrinology, he was then given a maintenance dose of normal saline IV at 250 cc/h and an IV insulin drip at 0.1 U/kg/h following a bolus of 8 units of insulin IV. His glucose levels were carefully monitored via hourly finger-stick glucose testing.
Although the patient’s condition stabilized, he collapsed while walking to the bathroom. He had agonal respirations and no pulse. Resuscitation efforts were started with bag-valve-mask ventilation, along with emergent advanced cardiac life support (ACLS) treatment, the protocol of which included epinephrine administration (x2) IV push 5 minutes apart, 2 ampules of sodium bicarbonate (50 mEq each) IV push, and calcium gluconate 10% (x1) 10 mL (1 g) IV push. A pulse was re-established, and the patient was intubated.
The patient was diagnosed with diabetic ketoacidosis (DKA) and admitted to the intensive care unit where repeat laboratory evaluation was ordered. Additional pharmacological management included IV administration of dopamine, norepinephrine, phenylephrine, vasopressin, antibiotics (azithromycin, meropenem, and vancomycin), pantoprazole, and subcutaneous heparin.
During treatment, the patient coded a second time and was revived according to ACLS protocols. Shortly thereafter, he coded a third time, but resuscitation efforts failed. Pathology reported no biological cause of death, and the coroner closed the case as death due to DM-related complications.
Diabetic Ketoacidosis
Diabetic ketoacidosis is a major complication of DM.4 Although the condition usually occurs in type 1 DM, it can also develop in type 2 DM. Diabetic ketoacidosis may be an inciting event leading to the eventual diagnosis of DM, but can also develop during a concurrent illness such as a urinary tract infection or an eating disorder.5 Risk factors for DKA include patients with type 1 or type 2 DM, a family history of DM, obesity, and nonwhite patients whose ethnic background places them at increased risk.6 Hispanic, black, and African American patients are at a greater risk of developing DKA and are more likely to develop “ketosis-prone” type 2 DM.7
Patients who do not fit into the definitive categories of type 1 or 2 DM can be classified under ketosis-prone DM.7,8 Diabetic ketoacidosis acts as the inciting event for the disease and evolves into severe β-cell dysfunction, hence blurring the lines between the archetypal DM categories. Fifty percent of ketosis-prone DM patients are A-β+ (absent autoantibodies, present β-cell function), which indicates that the dysfunction can be partially reversed. Reversal of the condition is largely based on long-term β-cell reserves, which are dependent on tight glycemic control and insulin dependence. Higher incidences of the A-β+ variant of ketosis-prone diabetes are seen in the male population and are often unprovoked.9-11
Diabetic ketoacidosis is the result of either a decrease or absence of insulin in the body (Table 2).4 Without insulin modulating exogenous glucose intake and endogenous glucose production (via glucagon, glycogenolysis, and gluconeogenesis), high levels of glucose are found in the circulation, leading to prominent hyperglycemia (>250 mg/dL or >13.8 mmol/L).6 This environment causes the body to switch from carbohydrate metabolism to fatty acid metabolism. As a result, acidic ketone bodies such β-hydroxybutyrate and acetoacetate are produced. These physiological changes in the body cause the signs and symptoms typically found in DKA.
Signs and Symptoms
Over a period of 24 hours, symptoms such as nausea, vomiting, increased thirst, and polyuria develop due to dehydration caused by osmotic diuresis and glucosuria.5 Patients may also present with hypotension and tachycardia. Confusion, deep gasping breaths or Kussmaul respirations, and metabolic acidosis result from hyperventilation and failure to compensate for the increased serum concentration of ketone bodies. Ketone production leads to a fruit-like odor in the patient’s breath and ketonuria in the urinalysis. In DKA, laboratory values will indicate metabolic acidosis and abnormal serum electrolytes. In both DM and DKA, increased urea and creatinine due to dehydration, increased ketones, and the presence of diabetic nephropathy are useful indicators of impaired kidney function.12
Management and Treatment
Diabetic ketoacidosis can be managed and reversed, especially when recognized and treated early.6,13 Dehydration in DKA can be corrected with IV fluid replacement. Normal saline (0.9%) can be started at 15 to 20 mL/kg/h or 1 L/h. As the patient’s vital signs stabilize, IV fluids can be titrated to a lower dose of 250 to 500 mL/h. Monitoring BP and electrolytes are key at this point as alterations in sodium levels and glucose levels may require switching to half-normal saline and/or dextrose.
The hyperglycemic state of patients with DKA is managed by IV insulin. An initial bolus of 0.1 U/kg/h can be given, but should only be administered when potassium levels are greater than 3.3 mmol/L.14 If adequate perfusion can be maintained, then 0.14 U/kg/h can be used instead of a bolus. Glucose levels must be monitored; once the levels decrease to approximately 200 mg/dL, the infusion rate of insulin should be titrated down to 0.05 to 0.1 U/kg/h. Dextrose is then added to maintain glucose levels at approximately 150 to 200 mg/dL.
Electrolytes, especially potassium, must be monitored closely in patients with DKA. Insulin leads to the shift of potassium into cells. The lack of insulin keeps potassium in the extracellular space. Due to osmotic diuresis, potassium is lost in the urine, leading to hypokalemia. Potassium levels in patients with DKA should be maintained at a level between 4 to 5 mmol/L. Patients with potassium levels between 3.3 to 5.2 mmol/L can be started on IV potassium between 20 to 30 mmol/h. If the patient is severely hypokalemic (<3.3 mmol/L), insulin should be withheld, and only IV potassium should be given at a rate of 20 to 30 mmol/h.
Bicarbonate levels can also be managed as acidosis can lead to both neurological and cardiac complications. If the patient’s pH is less than 6.9, the American Diabetes Association recommends starting 100 mmol of sodium bicarbonate in 400 mL sterile water (in addition to potassium chloride at 200 mL/h) for 2 hours. Dosing should be repeated every 2 hours until the patient’s pH is greater than 6.9.
In uncomplicated cases of DKA, the condition is resolved when a patient’s pH is greater than 7.3; glucose level is less than 200 mg/dL; and bicarbonate level is greater than or equal to 18 mmol/L. After patients become hemodynamically stable, they can be discharged and managed at home with a combination of intermediate- or long-acting insulin as well as short- or rapid-acting insulin.
Complications and Mortality
Diabetic ketoacidosis can cause sudden and fluctuating changes in the body. Therefore, it is very important to monitor a patient’s laboratory values very carefully and frequently to avoid any pitfalls. Since patients can present with hyponatremia due to the osmotic draw of glucose in the blood,13 sodium levels may have to be corrected. The corrected serum sodium can be calculated by adding 1.6 mmol/L for every 100 mg/dL of glucose (when finger-stick readings are above 200 mg/dL).15 Patients with DKA can also present with leukocytosis (even in the absence of infection) and hypertriglyceridemia (due to impaired lipoprotein lipase).15 Serum creatine may be elevated due to blood acetoacetate levels.15
Interestingly, there are other acute conditions that can mimic DKA.15 For example, chronic ethanol abuse can lead to ketoacidosis. Unlike DKA, however, alcoholic ketoacidosis does not have profound hyperglycemia, which can help differentiate the two during initial assessment.
Complications due to DKA can arise comprising the patient’s health, including hypoglycemia, hypokalemia, rhabdomyolysis, acute renal failure, pulmonary edema, and shock.16 Cerebral edema is seen in up to 1% of DKA patients,15 the cause of which may be due to the severity of the acidosis, high glucose levels, and rapid hydration. Even when cerebral edema is reduced, patients are often neurologically impaired. Mortality rates from DKA deaths due to cerebral edema can be as high as 24%.13 In the United States, over 100,000 patients with DM per year are admitted to the hospital for DKA, and 9% of patients with DM suffer from DKA-related complications postdischarge.15 With current treatment protocols, mortality rates for DKA-associated deaths are now down to 1%.6,15
Diabetes ketoacidosis-related deaths are usually the result of the following: a triad of DKA symptoms (hyperglycemia, hyperketonemia, and metabolic acidosis), another underlying comorbid condition (eg, myocardial infarction, sepsis, acute respiratory distress syndrome), or the release of biological markers (ie, catecholamines).14,15,17 Thus, as previously stated, the management of potassium levels is important as both hyperkalemia and hypokalemia can lead to fatal arrthythmias.15
Direct mortality from DKA has dropped significantly over the past 20 years, from 8% to less than 1%.6 The US Centers for Disease Control and Prevention has observed a downward trend in death and estimates that 2,417 patients died in 2009 due to DKA,18 and recent postmortem studies have revealed new insights into DKA-related deaths.19 Blood and vitreous acetone concentrations are strong indicators for predeath hyperglycemia and ketosis (if there are no underlying comorbid and/or pharmacological provocations). Blood acetone levels greater than 0.01 g/dL antemortem are suggestive of DKA. It is recommended that these tests should be performed in sudden deaths which have no biological or anatomical cause of death. Postmortem diagnosis of DKA is made with the following criteria: history of DM, increased vitreous glucose concentrations, and elevated blood/vitreous/urine acetone concentrations (>200 mg/dL). If results of the abovementioned parameters are inconclusive, measurement of lactic acid postmortem is thought to further support a diagnosis of DKA.19
Patient Counseling and Education
Approximately 33% of patients whose death was associated with DKA had no personal history of DM.19 This statistic emphasizes the importance of taking a thorough history, physical examination, blood glucose evaluation, and educating patients about the signs and symptoms of DM and DKA.
Patient counseling and education are important, especially in patients whose racial/ethnic background places them at increased risk of developing DM (eg, patients of black or African American, American Indian, Alaskan Native, Asian American, Hispanic, Native Hawaiian, or Pacific Islander descent).20,21 Strategies for preventive management include advocating regular glucose monitoring as well as dietary and lifestyle modifications. In patients with DM, successful management of the condition and its comorbidities can help prevent DKA and associated mortality.
Conclusion
As this case demonstrates, despite prompt diagnosis and management, patients with DKA—especially those with uncontrolled, undiagnosed, or advanced DM—are associated with fatal outcomes. In many cases, however, DKA can be successfully managed and reversed, especially when the condition is recognized early. Management includes not only IV therapy to adjust fluid and insulin levels, but also restoring electrolyte balance (especially potassium and bicarbonate). Frequent and careful evaluation of laboratory values is vital to the successful treatment of DKA, as there are numerous pitfalls and complications that the emergency physician can encounter. Patients who either have or are at an increased risk of developing DM or DKA may benefit from preventive measures, including regular glucose monitoring and appropriate diet and lifestyle modifications.
Mr Hassan-Ali is a fourth-year medical student at Windsor University School of Medicine, St Kitts, West Indies. Dr Raziuddin is an internist and an emergency medicine physician at Weiss Memorial, Thorek Memorial, and Westlake Hospitals, Chicago, Illinois.
- Kitabchi AH, Umpierrez GE, Murphy MB, et al. Management of hyperglycemic crises in patients with diabetes. Diabetes Care. 2001;24(1):131-153.
- Farinda A. Lab values, normal adult: laboratory reference ranges in healthy adults. 2015. Medscape Web site. http://emedicine.medscape.com/article/2172316-overview. Updated May 14, 2014. Accessed August 14, 2015.
- Young D. Implementation of SI units for clinical laboratory data. Ann Intern Med. 1987;106(1):114-129.
- Maitra A. The endocrine system. In: Kumar V, Abbas AK, Aster JC. Robbins and Cotran Pathologic Basis of Disease. 9th ed. New York, NY: Elsevier Saunders; 2015:1105-1120.
- Powers AC. Diabetes mellitus: management and therapies. In: Kasper DL, Fauci AS, Longo DL, Hauser SL, Jameson JL, Loscalzo J. Harrison’s Principles of Internal Medicine. 19th ed. New York, NY; McGraw-Hill Medical Publishing Division; 2015:2407-2422.
- Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335-1343.
- Umpierrez GE, Smiley D, Kitabchi AE. Narrative review: ketosis-prone type 2 diabetes mellitus. Ann Intern Med. 2006;144(5):350-357.
- Umpierrez G, Smiley D, Gosmanov A, Thomason D. Ketosis-prone type 2 diabetes: effect of hyperglycemia on beta-cell function and skeletal muscle insulin signaling. Endocr Pract. 2007;13(3):283-290.
- Mauvais-Jarvis F, Sobngwi E, Porcher R, et al. Ketosis-prone type 2 diabetes in patients of sub-Saharan African origin: clinical pathophysiology and natural history of beta-cell dysfunction and insulin resistance. Diabetes. 2004;53(3):645-653.
- Umpierrez GE, Casals MM, Gebhart SP, Mixon PS, Clark WS, Phillips LS. Diabetic ketoacidosis in obese African-Americans. Diabetes. 1995;44(7):790-795.
- Piñero-Piloña A, Raskin P. Idiopathic type 1 diabetes. J Diabetes Complications. 2001;15(6):328-335.
- Kemperman FA, Weber JA, Gorgels J, van Zanten AP, Krediet RT, Arisz L. The influence of ketoacids on plasma creatinine assays in diabetic ketoacidosis. J Intern Med. 2000;248(6):511-517.
- Westerberg DP. Diabetic ketoacidosis: evaluation and treatment. Am Fam Physician. 2013;87(5):337-346.
- Trachtenbarg DE. Diabetic ketoacidosis. Am Fam Physician. 2005;71(9):1705-1714.
- Umpierrez GE, Murphy MB, Kitabchi AE. Diabetic ketoacidosis and hyperglycemic hyperosmolar ayndrome. Diabetes Spectrum. 2002;15(1):28-36.
- Wolfsdorf J, Glaser N, Sperling MA; American Diabetes Association. Diabetic ketoacidosis in infants, children, and adolescents: A consensus statement from the American Diabetes Association. Diabetes Care. 2006;29(5):1150-1159.
- Rosenbloom AL. Sudden death of a young woman attributed to diabetic ketoacidosis. J Forensic Leg Med. 2013;20(8):1063-1065.
- Centers for Disease Control and Prevention. Number of deaths for hyperglycemic crises as underlying cause, United States, 1980-2009. http://www.cdc.gov/diabetes/statistics/mortalitydka/fnumberofdka.htm. Updated November 19, 2013. Accessed August 14, 2015.
- Ali Z, Levine B, Ripple M, Fowler DR. Diabetic ketoacidosis: a silent death. Am J Forensic Med Pathol. 2012;33(3):189-193.
- US Department of Health and Human Services Office of Minority Health. Diabetes and Hispanic Americans. http://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=63. Updated June 15, 2013. Accessed August 14, 2015.
- US Department of Health and Human Services Office of Minority Health. Profile: Native Hawaiian/Other Pacific Islanders. http://minorityhealth.hhs.gov/omh/browse.aspx?lvl=3&lvlid=65. Updated January 15, 2015. Accessed August 14, 2015.
- Kitabchi AH, Umpierrez GE, Murphy MB, et al. Management of hyperglycemic crises in patients with diabetes. Diabetes Care. 2001;24(1):131-153.
- Farinda A. Lab values, normal adult: laboratory reference ranges in healthy adults. 2015. Medscape Web site. http://emedicine.medscape.com/article/2172316-overview. Updated May 14, 2014. Accessed August 14, 2015.
- Young D. Implementation of SI units for clinical laboratory data. Ann Intern Med. 1987;106(1):114-129.
- Maitra A. The endocrine system. In: Kumar V, Abbas AK, Aster JC. Robbins and Cotran Pathologic Basis of Disease. 9th ed. New York, NY: Elsevier Saunders; 2015:1105-1120.
- Powers AC. Diabetes mellitus: management and therapies. In: Kasper DL, Fauci AS, Longo DL, Hauser SL, Jameson JL, Loscalzo J. Harrison’s Principles of Internal Medicine. 19th ed. New York, NY; McGraw-Hill Medical Publishing Division; 2015:2407-2422.
- Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335-1343.
- Umpierrez GE, Smiley D, Kitabchi AE. Narrative review: ketosis-prone type 2 diabetes mellitus. Ann Intern Med. 2006;144(5):350-357.
- Umpierrez G, Smiley D, Gosmanov A, Thomason D. Ketosis-prone type 2 diabetes: effect of hyperglycemia on beta-cell function and skeletal muscle insulin signaling. Endocr Pract. 2007;13(3):283-290.
- Mauvais-Jarvis F, Sobngwi E, Porcher R, et al. Ketosis-prone type 2 diabetes in patients of sub-Saharan African origin: clinical pathophysiology and natural history of beta-cell dysfunction and insulin resistance. Diabetes. 2004;53(3):645-653.
- Umpierrez GE, Casals MM, Gebhart SP, Mixon PS, Clark WS, Phillips LS. Diabetic ketoacidosis in obese African-Americans. Diabetes. 1995;44(7):790-795.
- Piñero-Piloña A, Raskin P. Idiopathic type 1 diabetes. J Diabetes Complications. 2001;15(6):328-335.
- Kemperman FA, Weber JA, Gorgels J, van Zanten AP, Krediet RT, Arisz L. The influence of ketoacids on plasma creatinine assays in diabetic ketoacidosis. J Intern Med. 2000;248(6):511-517.
- Westerberg DP. Diabetic ketoacidosis: evaluation and treatment. Am Fam Physician. 2013;87(5):337-346.
- Trachtenbarg DE. Diabetic ketoacidosis. Am Fam Physician. 2005;71(9):1705-1714.
- Umpierrez GE, Murphy MB, Kitabchi AE. Diabetic ketoacidosis and hyperglycemic hyperosmolar ayndrome. Diabetes Spectrum. 2002;15(1):28-36.
- Wolfsdorf J, Glaser N, Sperling MA; American Diabetes Association. Diabetic ketoacidosis in infants, children, and adolescents: A consensus statement from the American Diabetes Association. Diabetes Care. 2006;29(5):1150-1159.
- Rosenbloom AL. Sudden death of a young woman attributed to diabetic ketoacidosis. J Forensic Leg Med. 2013;20(8):1063-1065.
- Centers for Disease Control and Prevention. Number of deaths for hyperglycemic crises as underlying cause, United States, 1980-2009. http://www.cdc.gov/diabetes/statistics/mortalitydka/fnumberofdka.htm. Updated November 19, 2013. Accessed August 14, 2015.
- Ali Z, Levine B, Ripple M, Fowler DR. Diabetic ketoacidosis: a silent death. Am J Forensic Med Pathol. 2012;33(3):189-193.
- US Department of Health and Human Services Office of Minority Health. Diabetes and Hispanic Americans. http://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=63. Updated June 15, 2013. Accessed August 14, 2015.
- US Department of Health and Human Services Office of Minority Health. Profile: Native Hawaiian/Other Pacific Islanders. http://minorityhealth.hhs.gov/omh/browse.aspx?lvl=3&lvlid=65. Updated January 15, 2015. Accessed August 14, 2015.
Erythema Induratum of Bazin Presenting as Peripheral Neuropathy
Case Report
A 54-year-old Hispanic woman with a history of type 2 diabetes mellitus and hyperlipidemia presented with recurrent painful plaques and nodules on the bilateral lower extremities and a severe burning sensation on the feet of 3 years’ duration. The patient denied experiencing any associated fevers, chills, night sweats, weight loss, joint aches, cough, or shortness of breath. She had a history of pustular psoriasis and reported a positive purified protein derivative (PPD)(tuberculin) skin test approximately 40 years prior.
She presented with a 4×4-cm, poorly defined, tender, indurated plaque on the middle of the left shin and a 2×3-cm, red-brown plaque on the dorsal aspect of the left foot (Figure 1). No lymphadenopathy or any other abnormalities were noted. The clinical differential diagnosis included various panniculitides, such as erythema nodosum, erythema induratum of Bazin (EIB), and lupus panniculitis, as well as other conditions, including polyarteritis nodosa, sarcoidosis, Sweet disease, deep fungal and mycobacterial infections, and cutaneous lymphoma.
  |
| Figure 1. Erythematous indurated plaques on the middle of the left shin (A) and on the dorsal aspect of the left foot (B). |
Two skin biopsies taken for histopathologic evaluation revealed primarily granulomatous lobular panniculitis with foci of microthrombi and vasculitis (Figure 2). These findings were consistent with nodular vasculitis. Acid-fast bacillus and Gomori methenamine-silver stains were negative for mycobacterial or fungal organisms. Tissue cultures also were negative. Results from a complete blood cell count, chemistry panel, thyroid and liver function tests, hepatitis panel, and rapid plasma reagin test were unremarkable. Immunologic markers, including antinuclear antibody, antineutrophil cytoplasmic antibody, rheumatoid factor, and cryoglobulins, also revealed no abnormalities. A chest radiograph showed scarring in the suprahilar region of the upper lobe of the left lung consistent with a prior case of pulmonary tuberculosis, and exposure to Mycobacterium tuberculosis was confirmed with an IFN-γ release assay (IGRA) result of 1.49 IU/mL (>0.34 IU/mL indicates positive test). These findings from clinical and histopathologic examination as well as laboratory tests were consistent with a diagnosis of EIB.
   |
Figure 2. Granulomatous lobular panniculitis (A)(H&E, original magnification ×40) with foci of microthrombi and vasculitis (B and C)(both H&E, original magnifications ×200). |
Standard antituberculosis therapy with rifampin, isoniazid, pyrazinamide, and ethambutol (RIPE) was simplified to rifampin and isoniazid due to her inability to tolerate the full regimen because of gastrointestinal tract upset and diarrhea. After 6 months of therapy, a repeat IGRA decreased to 0.43 IU/mL, and the painful plaques and nodules on the lower extremities and burning sensation in the feet completely resolved.
Comment
Our case of EIB associated with peripheral neuropathy is a unique presentation of lesions on the pretibial area of the bilateral legs and dorsal aspect of the feet. We confirmed the presence of latent tuberculosis infection with a chest radiograph and an IGRA. Symptoms of peripheral neuropathy resolved after antituberculosis treatment, which suggests an immune-mediated mechanism of neuronal damage from circulating tuberculosis antigens.
Pathogenesis
Although erythema induratum was first described by Ernest Bazin in 1861, it was not until the early 1900s that the link between tuberculosis and erythema induratum was made by French dermatologists.1,2 Around the same time, similar cases of erythema induratum were discovered in England with no evidence of tuberculosis, which led to the distinct classification of erythema induratum of Whitfield (EIW). This classification described these nontuberculoid cases.1,2 In 1945, Montgomery et al3 in the United States coined the term nodular vasculitis for EIW and categorized its clinical features and histopathology as separate from EIB.3 Today, some authors use EIB, EIW, and nodular vasculitis interchangeably and believe they all are the same entity.2 We use EIB for all cases related to tuberculosis and nodular vasulitis when referring to all other etiologies, including nontuberculoid bacterial infections, chronic hepatitis B and C virus, thrombophlebitis, hypothyroidism, and rheumatoid arthritis.4,5
Erythema induratum of Bazin, lichen scrofulosorum, and papulonecrotic tuberculids are considered tuberculid diseases and are thought to be caused by hypersensitivity reactions to mycobacterial antigens rather than local mycobacterial infections. Tuberculids are believed to be a reaction to an id reaction of circulating mycobacterial antigens in the setting of latent or active tuberculosis infection. The basis for this view is that mycobacteria cannot be cultured or visualized from lesions in tuberculid diseases.6 Cutaneous tuberculosis, such as scrofuloderma, miliary tuberculosis, tuberculosis chancre, lupus vulgaris, and gummatous tuberculosis, differ from EIB and other tuberculid diseases in that mycobacteria can be cultured and visualized on histologic examination with Ziehl-Neelsen staining.6 The pathology of cutaneous tuberculosis results from a mycobacterial infection of the skin, and cutaneous tuberculosis diseases are categorized as multibacillary or paucibacillary based on the number of organisms visualized in biopsies.
The absence of M tuberculosis organisms in skin lesions has led some to doubt the causal relationship between M tuberculosis and EIB.7 However, the advent of polymerase chain reaction (PCR) and specific DNA primers for M tuberculosis has allowed for the detection of M tuberculosis DNA in biopsy specimens, which has further established the relationship between tuberculosis and EIB.5 Some authors have suggested that the absence of mycobacteria in EIB and other tuberculid lesions may be due to small numbers of bacilli in the lesions or early destruction of mycobacteria organisms before biopsy.8,9 These authors consider cutaneous tuberculosis and tuberculids as diseases on the same spectrum, with tuberculids representing one extreme in which there are few mycobacteria organisms present in the lesions.
Presentation
Erythema induratum commonly affects middle-aged women and presents with recurrent crops of tender nodules on the lower extremities.10-13 Nodules often are most commonly found on the lower calves but also can present on the arms, thighs, feet, or buttocks.10 Our patient’s presentation was atypical in that lesions were distributed on the pretibial area of the legs and dorsal aspect of the feet. Obesity and venous insufficiency of the lower extremities are believed to be predisposing factors to the development of EIB nodules.2 The nodules develop over several weeks and heal over several months with possible ulceration and hyperpigmented scarring.10,11 Ulcerated nodules often are irregular and shallow with an overlying crust and a bluish border.11,13 Nodules often are precipitated by cold weather or venostasis.1,11,12
Silva et al14 reported a case of EIB on the lower legs associated with a burning sensation on the feet and paresthesia; all known causes of peripheral neuropathy were excluded by a comprehensive laboratory workup. The burning sensation on the feet resolved after several weeks of antituberculosis therapy. Our patient also presented with a burning sensation on the feet that remarkably improved after 6 months of antituberculosis therapy. Peripheral neuropathy could have been a consequence of diabetes mellitus in our patient, though neuronal damage also could be a consequence of hypersensitivity to tuberculosis antigens. Silva et al14 proposed that macrophages activated by M tuberculosis antigens produce lytic enzymes that can cause tissue necrosis and nerve damage if released into surrounding tissue.
Diagnosis
The diagnosis of EIB is made based on clinical presentation, evidence of prior or current tuberculosis infection, histopathologic findings, and response to antituberculosis therapy.15 Evidence of active or latent tuberculosis infection typically is gathered by patient history, chest radiograph, tuberculin skin tests, interferon-releasing assays, and PCR of skin biopsies. Tuberculin skin tests in patients with EIB result in reactive induration that is typically more than 20 mm.8 In vitro T-lymphocyte proliferation assays in response to PPD have further supported the suggestion that there is a markedly enhanced T-lymphocyte response to M tuberculosis antigens in patients with EIB.16
IFN-γ release assays have provided useful methods for the detection of latent tuberculosis infection.17 The IGRA is effective when the tuberculin skin test yields a suspected false-negative or in the context of prior bacille Calmette-Guérin vaccination.17 IFN-γ release assays also may be preferred to tuberculin skin tests because it provides less discomfort to the patient in the event of a positive hypersensitive reaction to the PPD.
Before the advent of IGRAs, PCR was used to detect M tuberculosis DNA in skin biopsies and to confirm the diagnosis of EIB. Some researchers believe PCR can be an important tool for confirming a diagnosis of EIB, especially in cases and countries where results from the Mantoux test do not have great value.15,18 A PCR assay for detecting M tuberculosis DNA in blood and urine samples also was found helpful in confirming a diagnosis of EIB when skin biopsies were unavailable.8,19 However, PCR has been shown to have low sensitivity for the diagnosis of EIB because of its ability to detect M tuberculosis DNA ranging from 0% to 77% of skin biopsy specimens.20,21 Therefore, a negative PCR for the detection of M tuberculosis DNA in nodules does not exclude a diagnosis of erythema induratum.
Treatment
The mainstay of EIB treatment is a multidrug antituberculosis regimen.5,8,10-12 Our patient was successfully treated with rifampin and isoniazid and a repeat IGRA was used as a laboratory marker of response to therapy. Single-drug therapy with isoniazid has been shown to result in greater likelihood of EIB relapse in comparison to multidrug regimens.22 Other treatments include potassium iodide and gold, but they are not well-studied.23-25 Treatment of venous insufficiency with bed rest and nonsteroidal anti-inflammatory drugs for pain also may be helpful.2 In cases of nodular vasulitis that are not associated with tuberculosis infection, treatment should be targeted at the underlying cause of the immune response. For example, a case of nodular vasculitis associated with hepatitis C virus did not respond to antituberculosis multidrug therapy, but skin lesions did improve with pegylated interferon and ribavirin.4
1. Segura S, Pujol RM, Trindade F, et al. Vasculitis in erythema induratum of Bazin: a histopathologic study of 101 biopsy specimens from 86 patients. J Am Acad Dermatol. 2008;59:839-851.
2. Requena L, Sánchez Yus E. Panniculitis. part II. mostly lobular panniculitis. J Am Acad Dermatol. 2001;45:325-361.
3. Montgomery H, O’Leary PA, Barker NW. Nodular vascular disease of the legs: erythema induratum and allied conditions. JAMA. 1945;128:335-342.
4. Fernandes SS, Carvalho J, Leite S, et al. Erythema induratum and chronic hepatitis C infection [published online ahead of print February 23, 2009]. J Clin Virol. 2009;44:333-336.
5. Gilchrist H, Patterson JW. Erythema nodosum and erythema induratum (nodular vasculitis): diagnosis and management. Dermatol Ther. 2010;23:320-327.
6. Frankel A, Penrose C, Emer J. Cutaneous tuberculosis: a practical case report and review for the dermatologist. J Clin Aesthet Dermatol. 2009;2:19-27.
7. Schneider JW, Jordaan HF, Geiger DH, et al. Erythema induratum of Bazin. a clinicopathological study of 20 cases and detection of Mycobacterium tuberculosis DNA in skin lesions by polymerase chain reaction. Am J Dermatopathol. 1995;17:350-356.
8. Lighter J, Tse DB, Li Y, et al. Erythema induratum of Bazin in a child: evidence for a cell-mediated hyper-response to Mycobacterium tuberculosis. Pediatr Infect Dis J. 2009;28:326-328.
9. Bravo FG, Gotuzzo E. Cutaneous tuberculosis. Clin Dermatol. 2007;25:173-180.
10. Rademaker M, Lowe DG, Munro DD. Erythema induratum (Bazin’s disease). J Am Acad Dermatol. 1989;21 (4, pt 1):740-745.
11. Sharon V, Goodarzi H, Chambers CJ, et al. Erythema induratum of Bazin. Dermatol Online J. 2010;16:1.
12. Feiwel M, Munro DD. Diagnosis and treatment of erythema induratum (Bazin). Br Med J. 1965;1:1109-1111.
13. Lebel M, Lassonde M. Erythema induratum of Bazin. J Am Acad Dermatol. 1986;14(5, pt 1):738-742.
14. Silva MT, Antunes SL, Rolla VC, et al. Distal painful peripheral neuropathy associated with erythema induratum of Bazin. Eur J Neurol. 2006;13:e5-e6.
15. Jacinto SS, Nograles KB. Erythema induratum of bazin: role of polymerase chain reaction in diagnosis. Int J Dermatol. 2003;42:380-381.
16. Ollert MW, Thomas P, Korting HC, et al. Erythema induratum of Bazin. evidence of T-lymphocyte hyperresponsiveness to purified protein derivative of tuberculin: report of two cases and treatment. Arch Dermatol. 1993;129:469-473.
17. Angus J, Roberts C, Kulkarni K, et al. Usefulness of the QuantiFERON test in the confirmation of latent tuberculosis in association with erythema induratum [published online ahead of print October 10, 2007]. Br J Dermatol. 2007;157:1293-1294.
18. Seckin D, Hízel N, Demirhan B, et al. The diagnostic value of polymerase chain reaction in erythema induratum of Bazin. Br J Dermatol. 1997;137:1011-1012.
19. Cannas A, Goletti D, Girardi E, et al. Mycobacterium tuberculosis DNA detection in soluble fraction of urine from pulmonary tuberculosis patients. Int J Tuberc Lung Dis. 2008;12:146-151.
20. Tan SH, Tan BH, Goh CL, et al. Detection of Mycobacterium tuberculosis DNA using polymerase chain reaction in cutaneous tuberculosis and tuberculids. Int J Dermatol. 1999;38:122-127.
21. Baselga E, Margall N, Barnadas MA, et al. Detection of Mycobacterium tuberculosis DNA in lobular granulomatous panniculitis (erythema induratum-nodular vasculitis). Arch Dermatol. 1997;133:457-462.
22. Cho KH, Lee DY, Kim CW. Erythema induratum of Bazin. Int J Dermatol. 1996;35:802-808.
23. Schulz EJ, Whiting DA. Treatment of erythema nodosum and nodular vasculitis with potassium iodide. Br J Dermatol. 1976;94:75-78.
24. Horio T, Imamura S, Danno K, et al. Potassium iodide in the treatment of erythema nodosum and nodular vasculitis. Arch Dermatol. 1981;117:29-31.
25. Shaffer N, Kerdel FA. Nodular vasculitis (erythema induratum): treatment with auranofin. J Am Acad Dermatol. 1991;25(2, pt 2):426-429.
Case Report
A 54-year-old Hispanic woman with a history of type 2 diabetes mellitus and hyperlipidemia presented with recurrent painful plaques and nodules on the bilateral lower extremities and a severe burning sensation on the feet of 3 years’ duration. The patient denied experiencing any associated fevers, chills, night sweats, weight loss, joint aches, cough, or shortness of breath. She had a history of pustular psoriasis and reported a positive purified protein derivative (PPD)(tuberculin) skin test approximately 40 years prior.
She presented with a 4×4-cm, poorly defined, tender, indurated plaque on the middle of the left shin and a 2×3-cm, red-brown plaque on the dorsal aspect of the left foot (Figure 1). No lymphadenopathy or any other abnormalities were noted. The clinical differential diagnosis included various panniculitides, such as erythema nodosum, erythema induratum of Bazin (EIB), and lupus panniculitis, as well as other conditions, including polyarteritis nodosa, sarcoidosis, Sweet disease, deep fungal and mycobacterial infections, and cutaneous lymphoma.
  |
| Figure 1. Erythematous indurated plaques on the middle of the left shin (A) and on the dorsal aspect of the left foot (B). |
Two skin biopsies taken for histopathologic evaluation revealed primarily granulomatous lobular panniculitis with foci of microthrombi and vasculitis (Figure 2). These findings were consistent with nodular vasculitis. Acid-fast bacillus and Gomori methenamine-silver stains were negative for mycobacterial or fungal organisms. Tissue cultures also were negative. Results from a complete blood cell count, chemistry panel, thyroid and liver function tests, hepatitis panel, and rapid plasma reagin test were unremarkable. Immunologic markers, including antinuclear antibody, antineutrophil cytoplasmic antibody, rheumatoid factor, and cryoglobulins, also revealed no abnormalities. A chest radiograph showed scarring in the suprahilar region of the upper lobe of the left lung consistent with a prior case of pulmonary tuberculosis, and exposure to Mycobacterium tuberculosis was confirmed with an IFN-γ release assay (IGRA) result of 1.49 IU/mL (>0.34 IU/mL indicates positive test). These findings from clinical and histopathologic examination as well as laboratory tests were consistent with a diagnosis of EIB.
   |
Figure 2. Granulomatous lobular panniculitis (A)(H&E, original magnification ×40) with foci of microthrombi and vasculitis (B and C)(both H&E, original magnifications ×200). |
Standard antituberculosis therapy with rifampin, isoniazid, pyrazinamide, and ethambutol (RIPE) was simplified to rifampin and isoniazid due to her inability to tolerate the full regimen because of gastrointestinal tract upset and diarrhea. After 6 months of therapy, a repeat IGRA decreased to 0.43 IU/mL, and the painful plaques and nodules on the lower extremities and burning sensation in the feet completely resolved.
Comment
Our case of EIB associated with peripheral neuropathy is a unique presentation of lesions on the pretibial area of the bilateral legs and dorsal aspect of the feet. We confirmed the presence of latent tuberculosis infection with a chest radiograph and an IGRA. Symptoms of peripheral neuropathy resolved after antituberculosis treatment, which suggests an immune-mediated mechanism of neuronal damage from circulating tuberculosis antigens.
Pathogenesis
Although erythema induratum was first described by Ernest Bazin in 1861, it was not until the early 1900s that the link between tuberculosis and erythema induratum was made by French dermatologists.1,2 Around the same time, similar cases of erythema induratum were discovered in England with no evidence of tuberculosis, which led to the distinct classification of erythema induratum of Whitfield (EIW). This classification described these nontuberculoid cases.1,2 In 1945, Montgomery et al3 in the United States coined the term nodular vasculitis for EIW and categorized its clinical features and histopathology as separate from EIB.3 Today, some authors use EIB, EIW, and nodular vasculitis interchangeably and believe they all are the same entity.2 We use EIB for all cases related to tuberculosis and nodular vasulitis when referring to all other etiologies, including nontuberculoid bacterial infections, chronic hepatitis B and C virus, thrombophlebitis, hypothyroidism, and rheumatoid arthritis.4,5
Erythema induratum of Bazin, lichen scrofulosorum, and papulonecrotic tuberculids are considered tuberculid diseases and are thought to be caused by hypersensitivity reactions to mycobacterial antigens rather than local mycobacterial infections. Tuberculids are believed to be a reaction to an id reaction of circulating mycobacterial antigens in the setting of latent or active tuberculosis infection. The basis for this view is that mycobacteria cannot be cultured or visualized from lesions in tuberculid diseases.6 Cutaneous tuberculosis, such as scrofuloderma, miliary tuberculosis, tuberculosis chancre, lupus vulgaris, and gummatous tuberculosis, differ from EIB and other tuberculid diseases in that mycobacteria can be cultured and visualized on histologic examination with Ziehl-Neelsen staining.6 The pathology of cutaneous tuberculosis results from a mycobacterial infection of the skin, and cutaneous tuberculosis diseases are categorized as multibacillary or paucibacillary based on the number of organisms visualized in biopsies.
The absence of M tuberculosis organisms in skin lesions has led some to doubt the causal relationship between M tuberculosis and EIB.7 However, the advent of polymerase chain reaction (PCR) and specific DNA primers for M tuberculosis has allowed for the detection of M tuberculosis DNA in biopsy specimens, which has further established the relationship between tuberculosis and EIB.5 Some authors have suggested that the absence of mycobacteria in EIB and other tuberculid lesions may be due to small numbers of bacilli in the lesions or early destruction of mycobacteria organisms before biopsy.8,9 These authors consider cutaneous tuberculosis and tuberculids as diseases on the same spectrum, with tuberculids representing one extreme in which there are few mycobacteria organisms present in the lesions.
Presentation
Erythema induratum commonly affects middle-aged women and presents with recurrent crops of tender nodules on the lower extremities.10-13 Nodules often are most commonly found on the lower calves but also can present on the arms, thighs, feet, or buttocks.10 Our patient’s presentation was atypical in that lesions were distributed on the pretibial area of the legs and dorsal aspect of the feet. Obesity and venous insufficiency of the lower extremities are believed to be predisposing factors to the development of EIB nodules.2 The nodules develop over several weeks and heal over several months with possible ulceration and hyperpigmented scarring.10,11 Ulcerated nodules often are irregular and shallow with an overlying crust and a bluish border.11,13 Nodules often are precipitated by cold weather or venostasis.1,11,12
Silva et al14 reported a case of EIB on the lower legs associated with a burning sensation on the feet and paresthesia; all known causes of peripheral neuropathy were excluded by a comprehensive laboratory workup. The burning sensation on the feet resolved after several weeks of antituberculosis therapy. Our patient also presented with a burning sensation on the feet that remarkably improved after 6 months of antituberculosis therapy. Peripheral neuropathy could have been a consequence of diabetes mellitus in our patient, though neuronal damage also could be a consequence of hypersensitivity to tuberculosis antigens. Silva et al14 proposed that macrophages activated by M tuberculosis antigens produce lytic enzymes that can cause tissue necrosis and nerve damage if released into surrounding tissue.
Diagnosis
The diagnosis of EIB is made based on clinical presentation, evidence of prior or current tuberculosis infection, histopathologic findings, and response to antituberculosis therapy.15 Evidence of active or latent tuberculosis infection typically is gathered by patient history, chest radiograph, tuberculin skin tests, interferon-releasing assays, and PCR of skin biopsies. Tuberculin skin tests in patients with EIB result in reactive induration that is typically more than 20 mm.8 In vitro T-lymphocyte proliferation assays in response to PPD have further supported the suggestion that there is a markedly enhanced T-lymphocyte response to M tuberculosis antigens in patients with EIB.16
IFN-γ release assays have provided useful methods for the detection of latent tuberculosis infection.17 The IGRA is effective when the tuberculin skin test yields a suspected false-negative or in the context of prior bacille Calmette-Guérin vaccination.17 IFN-γ release assays also may be preferred to tuberculin skin tests because it provides less discomfort to the patient in the event of a positive hypersensitive reaction to the PPD.
Before the advent of IGRAs, PCR was used to detect M tuberculosis DNA in skin biopsies and to confirm the diagnosis of EIB. Some researchers believe PCR can be an important tool for confirming a diagnosis of EIB, especially in cases and countries where results from the Mantoux test do not have great value.15,18 A PCR assay for detecting M tuberculosis DNA in blood and urine samples also was found helpful in confirming a diagnosis of EIB when skin biopsies were unavailable.8,19 However, PCR has been shown to have low sensitivity for the diagnosis of EIB because of its ability to detect M tuberculosis DNA ranging from 0% to 77% of skin biopsy specimens.20,21 Therefore, a negative PCR for the detection of M tuberculosis DNA in nodules does not exclude a diagnosis of erythema induratum.
Treatment
The mainstay of EIB treatment is a multidrug antituberculosis regimen.5,8,10-12 Our patient was successfully treated with rifampin and isoniazid and a repeat IGRA was used as a laboratory marker of response to therapy. Single-drug therapy with isoniazid has been shown to result in greater likelihood of EIB relapse in comparison to multidrug regimens.22 Other treatments include potassium iodide and gold, but they are not well-studied.23-25 Treatment of venous insufficiency with bed rest and nonsteroidal anti-inflammatory drugs for pain also may be helpful.2 In cases of nodular vasulitis that are not associated with tuberculosis infection, treatment should be targeted at the underlying cause of the immune response. For example, a case of nodular vasculitis associated with hepatitis C virus did not respond to antituberculosis multidrug therapy, but skin lesions did improve with pegylated interferon and ribavirin.4
Case Report
A 54-year-old Hispanic woman with a history of type 2 diabetes mellitus and hyperlipidemia presented with recurrent painful plaques and nodules on the bilateral lower extremities and a severe burning sensation on the feet of 3 years’ duration. The patient denied experiencing any associated fevers, chills, night sweats, weight loss, joint aches, cough, or shortness of breath. She had a history of pustular psoriasis and reported a positive purified protein derivative (PPD)(tuberculin) skin test approximately 40 years prior.
She presented with a 4×4-cm, poorly defined, tender, indurated plaque on the middle of the left shin and a 2×3-cm, red-brown plaque on the dorsal aspect of the left foot (Figure 1). No lymphadenopathy or any other abnormalities were noted. The clinical differential diagnosis included various panniculitides, such as erythema nodosum, erythema induratum of Bazin (EIB), and lupus panniculitis, as well as other conditions, including polyarteritis nodosa, sarcoidosis, Sweet disease, deep fungal and mycobacterial infections, and cutaneous lymphoma.
  |
| Figure 1. Erythematous indurated plaques on the middle of the left shin (A) and on the dorsal aspect of the left foot (B). |
Two skin biopsies taken for histopathologic evaluation revealed primarily granulomatous lobular panniculitis with foci of microthrombi and vasculitis (Figure 2). These findings were consistent with nodular vasculitis. Acid-fast bacillus and Gomori methenamine-silver stains were negative for mycobacterial or fungal organisms. Tissue cultures also were negative. Results from a complete blood cell count, chemistry panel, thyroid and liver function tests, hepatitis panel, and rapid plasma reagin test were unremarkable. Immunologic markers, including antinuclear antibody, antineutrophil cytoplasmic antibody, rheumatoid factor, and cryoglobulins, also revealed no abnormalities. A chest radiograph showed scarring in the suprahilar region of the upper lobe of the left lung consistent with a prior case of pulmonary tuberculosis, and exposure to Mycobacterium tuberculosis was confirmed with an IFN-γ release assay (IGRA) result of 1.49 IU/mL (>0.34 IU/mL indicates positive test). These findings from clinical and histopathologic examination as well as laboratory tests were consistent with a diagnosis of EIB.
   |
Figure 2. Granulomatous lobular panniculitis (A)(H&E, original magnification ×40) with foci of microthrombi and vasculitis (B and C)(both H&E, original magnifications ×200). |
Standard antituberculosis therapy with rifampin, isoniazid, pyrazinamide, and ethambutol (RIPE) was simplified to rifampin and isoniazid due to her inability to tolerate the full regimen because of gastrointestinal tract upset and diarrhea. After 6 months of therapy, a repeat IGRA decreased to 0.43 IU/mL, and the painful plaques and nodules on the lower extremities and burning sensation in the feet completely resolved.
Comment
Our case of EIB associated with peripheral neuropathy is a unique presentation of lesions on the pretibial area of the bilateral legs and dorsal aspect of the feet. We confirmed the presence of latent tuberculosis infection with a chest radiograph and an IGRA. Symptoms of peripheral neuropathy resolved after antituberculosis treatment, which suggests an immune-mediated mechanism of neuronal damage from circulating tuberculosis antigens.
Pathogenesis
Although erythema induratum was first described by Ernest Bazin in 1861, it was not until the early 1900s that the link between tuberculosis and erythema induratum was made by French dermatologists.1,2 Around the same time, similar cases of erythema induratum were discovered in England with no evidence of tuberculosis, which led to the distinct classification of erythema induratum of Whitfield (EIW). This classification described these nontuberculoid cases.1,2 In 1945, Montgomery et al3 in the United States coined the term nodular vasculitis for EIW and categorized its clinical features and histopathology as separate from EIB.3 Today, some authors use EIB, EIW, and nodular vasculitis interchangeably and believe they all are the same entity.2 We use EIB for all cases related to tuberculosis and nodular vasulitis when referring to all other etiologies, including nontuberculoid bacterial infections, chronic hepatitis B and C virus, thrombophlebitis, hypothyroidism, and rheumatoid arthritis.4,5
Erythema induratum of Bazin, lichen scrofulosorum, and papulonecrotic tuberculids are considered tuberculid diseases and are thought to be caused by hypersensitivity reactions to mycobacterial antigens rather than local mycobacterial infections. Tuberculids are believed to be a reaction to an id reaction of circulating mycobacterial antigens in the setting of latent or active tuberculosis infection. The basis for this view is that mycobacteria cannot be cultured or visualized from lesions in tuberculid diseases.6 Cutaneous tuberculosis, such as scrofuloderma, miliary tuberculosis, tuberculosis chancre, lupus vulgaris, and gummatous tuberculosis, differ from EIB and other tuberculid diseases in that mycobacteria can be cultured and visualized on histologic examination with Ziehl-Neelsen staining.6 The pathology of cutaneous tuberculosis results from a mycobacterial infection of the skin, and cutaneous tuberculosis diseases are categorized as multibacillary or paucibacillary based on the number of organisms visualized in biopsies.
The absence of M tuberculosis organisms in skin lesions has led some to doubt the causal relationship between M tuberculosis and EIB.7 However, the advent of polymerase chain reaction (PCR) and specific DNA primers for M tuberculosis has allowed for the detection of M tuberculosis DNA in biopsy specimens, which has further established the relationship between tuberculosis and EIB.5 Some authors have suggested that the absence of mycobacteria in EIB and other tuberculid lesions may be due to small numbers of bacilli in the lesions or early destruction of mycobacteria organisms before biopsy.8,9 These authors consider cutaneous tuberculosis and tuberculids as diseases on the same spectrum, with tuberculids representing one extreme in which there are few mycobacteria organisms present in the lesions.
Presentation
Erythema induratum commonly affects middle-aged women and presents with recurrent crops of tender nodules on the lower extremities.10-13 Nodules often are most commonly found on the lower calves but also can present on the arms, thighs, feet, or buttocks.10 Our patient’s presentation was atypical in that lesions were distributed on the pretibial area of the legs and dorsal aspect of the feet. Obesity and venous insufficiency of the lower extremities are believed to be predisposing factors to the development of EIB nodules.2 The nodules develop over several weeks and heal over several months with possible ulceration and hyperpigmented scarring.10,11 Ulcerated nodules often are irregular and shallow with an overlying crust and a bluish border.11,13 Nodules often are precipitated by cold weather or venostasis.1,11,12
Silva et al14 reported a case of EIB on the lower legs associated with a burning sensation on the feet and paresthesia; all known causes of peripheral neuropathy were excluded by a comprehensive laboratory workup. The burning sensation on the feet resolved after several weeks of antituberculosis therapy. Our patient also presented with a burning sensation on the feet that remarkably improved after 6 months of antituberculosis therapy. Peripheral neuropathy could have been a consequence of diabetes mellitus in our patient, though neuronal damage also could be a consequence of hypersensitivity to tuberculosis antigens. Silva et al14 proposed that macrophages activated by M tuberculosis antigens produce lytic enzymes that can cause tissue necrosis and nerve damage if released into surrounding tissue.
Diagnosis
The diagnosis of EIB is made based on clinical presentation, evidence of prior or current tuberculosis infection, histopathologic findings, and response to antituberculosis therapy.15 Evidence of active or latent tuberculosis infection typically is gathered by patient history, chest radiograph, tuberculin skin tests, interferon-releasing assays, and PCR of skin biopsies. Tuberculin skin tests in patients with EIB result in reactive induration that is typically more than 20 mm.8 In vitro T-lymphocyte proliferation assays in response to PPD have further supported the suggestion that there is a markedly enhanced T-lymphocyte response to M tuberculosis antigens in patients with EIB.16
IFN-γ release assays have provided useful methods for the detection of latent tuberculosis infection.17 The IGRA is effective when the tuberculin skin test yields a suspected false-negative or in the context of prior bacille Calmette-Guérin vaccination.17 IFN-γ release assays also may be preferred to tuberculin skin tests because it provides less discomfort to the patient in the event of a positive hypersensitive reaction to the PPD.
Before the advent of IGRAs, PCR was used to detect M tuberculosis DNA in skin biopsies and to confirm the diagnosis of EIB. Some researchers believe PCR can be an important tool for confirming a diagnosis of EIB, especially in cases and countries where results from the Mantoux test do not have great value.15,18 A PCR assay for detecting M tuberculosis DNA in blood and urine samples also was found helpful in confirming a diagnosis of EIB when skin biopsies were unavailable.8,19 However, PCR has been shown to have low sensitivity for the diagnosis of EIB because of its ability to detect M tuberculosis DNA ranging from 0% to 77% of skin biopsy specimens.20,21 Therefore, a negative PCR for the detection of M tuberculosis DNA in nodules does not exclude a diagnosis of erythema induratum.
Treatment
The mainstay of EIB treatment is a multidrug antituberculosis regimen.5,8,10-12 Our patient was successfully treated with rifampin and isoniazid and a repeat IGRA was used as a laboratory marker of response to therapy. Single-drug therapy with isoniazid has been shown to result in greater likelihood of EIB relapse in comparison to multidrug regimens.22 Other treatments include potassium iodide and gold, but they are not well-studied.23-25 Treatment of venous insufficiency with bed rest and nonsteroidal anti-inflammatory drugs for pain also may be helpful.2 In cases of nodular vasulitis that are not associated with tuberculosis infection, treatment should be targeted at the underlying cause of the immune response. For example, a case of nodular vasculitis associated with hepatitis C virus did not respond to antituberculosis multidrug therapy, but skin lesions did improve with pegylated interferon and ribavirin.4
1. Segura S, Pujol RM, Trindade F, et al. Vasculitis in erythema induratum of Bazin: a histopathologic study of 101 biopsy specimens from 86 patients. J Am Acad Dermatol. 2008;59:839-851.
2. Requena L, Sánchez Yus E. Panniculitis. part II. mostly lobular panniculitis. J Am Acad Dermatol. 2001;45:325-361.
3. Montgomery H, O’Leary PA, Barker NW. Nodular vascular disease of the legs: erythema induratum and allied conditions. JAMA. 1945;128:335-342.
4. Fernandes SS, Carvalho J, Leite S, et al. Erythema induratum and chronic hepatitis C infection [published online ahead of print February 23, 2009]. J Clin Virol. 2009;44:333-336.
5. Gilchrist H, Patterson JW. Erythema nodosum and erythema induratum (nodular vasculitis): diagnosis and management. Dermatol Ther. 2010;23:320-327.
6. Frankel A, Penrose C, Emer J. Cutaneous tuberculosis: a practical case report and review for the dermatologist. J Clin Aesthet Dermatol. 2009;2:19-27.
7. Schneider JW, Jordaan HF, Geiger DH, et al. Erythema induratum of Bazin. a clinicopathological study of 20 cases and detection of Mycobacterium tuberculosis DNA in skin lesions by polymerase chain reaction. Am J Dermatopathol. 1995;17:350-356.
8. Lighter J, Tse DB, Li Y, et al. Erythema induratum of Bazin in a child: evidence for a cell-mediated hyper-response to Mycobacterium tuberculosis. Pediatr Infect Dis J. 2009;28:326-328.
9. Bravo FG, Gotuzzo E. Cutaneous tuberculosis. Clin Dermatol. 2007;25:173-180.
10. Rademaker M, Lowe DG, Munro DD. Erythema induratum (Bazin’s disease). J Am Acad Dermatol. 1989;21 (4, pt 1):740-745.
11. Sharon V, Goodarzi H, Chambers CJ, et al. Erythema induratum of Bazin. Dermatol Online J. 2010;16:1.
12. Feiwel M, Munro DD. Diagnosis and treatment of erythema induratum (Bazin). Br Med J. 1965;1:1109-1111.
13. Lebel M, Lassonde M. Erythema induratum of Bazin. J Am Acad Dermatol. 1986;14(5, pt 1):738-742.
14. Silva MT, Antunes SL, Rolla VC, et al. Distal painful peripheral neuropathy associated with erythema induratum of Bazin. Eur J Neurol. 2006;13:e5-e6.
15. Jacinto SS, Nograles KB. Erythema induratum of bazin: role of polymerase chain reaction in diagnosis. Int J Dermatol. 2003;42:380-381.
16. Ollert MW, Thomas P, Korting HC, et al. Erythema induratum of Bazin. evidence of T-lymphocyte hyperresponsiveness to purified protein derivative of tuberculin: report of two cases and treatment. Arch Dermatol. 1993;129:469-473.
17. Angus J, Roberts C, Kulkarni K, et al. Usefulness of the QuantiFERON test in the confirmation of latent tuberculosis in association with erythema induratum [published online ahead of print October 10, 2007]. Br J Dermatol. 2007;157:1293-1294.
18. Seckin D, Hízel N, Demirhan B, et al. The diagnostic value of polymerase chain reaction in erythema induratum of Bazin. Br J Dermatol. 1997;137:1011-1012.
19. Cannas A, Goletti D, Girardi E, et al. Mycobacterium tuberculosis DNA detection in soluble fraction of urine from pulmonary tuberculosis patients. Int J Tuberc Lung Dis. 2008;12:146-151.
20. Tan SH, Tan BH, Goh CL, et al. Detection of Mycobacterium tuberculosis DNA using polymerase chain reaction in cutaneous tuberculosis and tuberculids. Int J Dermatol. 1999;38:122-127.
21. Baselga E, Margall N, Barnadas MA, et al. Detection of Mycobacterium tuberculosis DNA in lobular granulomatous panniculitis (erythema induratum-nodular vasculitis). Arch Dermatol. 1997;133:457-462.
22. Cho KH, Lee DY, Kim CW. Erythema induratum of Bazin. Int J Dermatol. 1996;35:802-808.
23. Schulz EJ, Whiting DA. Treatment of erythema nodosum and nodular vasculitis with potassium iodide. Br J Dermatol. 1976;94:75-78.
24. Horio T, Imamura S, Danno K, et al. Potassium iodide in the treatment of erythema nodosum and nodular vasculitis. Arch Dermatol. 1981;117:29-31.
25. Shaffer N, Kerdel FA. Nodular vasculitis (erythema induratum): treatment with auranofin. J Am Acad Dermatol. 1991;25(2, pt 2):426-429.
1. Segura S, Pujol RM, Trindade F, et al. Vasculitis in erythema induratum of Bazin: a histopathologic study of 101 biopsy specimens from 86 patients. J Am Acad Dermatol. 2008;59:839-851.
2. Requena L, Sánchez Yus E. Panniculitis. part II. mostly lobular panniculitis. J Am Acad Dermatol. 2001;45:325-361.
3. Montgomery H, O’Leary PA, Barker NW. Nodular vascular disease of the legs: erythema induratum and allied conditions. JAMA. 1945;128:335-342.
4. Fernandes SS, Carvalho J, Leite S, et al. Erythema induratum and chronic hepatitis C infection [published online ahead of print February 23, 2009]. J Clin Virol. 2009;44:333-336.
5. Gilchrist H, Patterson JW. Erythema nodosum and erythema induratum (nodular vasculitis): diagnosis and management. Dermatol Ther. 2010;23:320-327.
6. Frankel A, Penrose C, Emer J. Cutaneous tuberculosis: a practical case report and review for the dermatologist. J Clin Aesthet Dermatol. 2009;2:19-27.
7. Schneider JW, Jordaan HF, Geiger DH, et al. Erythema induratum of Bazin. a clinicopathological study of 20 cases and detection of Mycobacterium tuberculosis DNA in skin lesions by polymerase chain reaction. Am J Dermatopathol. 1995;17:350-356.
8. Lighter J, Tse DB, Li Y, et al. Erythema induratum of Bazin in a child: evidence for a cell-mediated hyper-response to Mycobacterium tuberculosis. Pediatr Infect Dis J. 2009;28:326-328.
9. Bravo FG, Gotuzzo E. Cutaneous tuberculosis. Clin Dermatol. 2007;25:173-180.
10. Rademaker M, Lowe DG, Munro DD. Erythema induratum (Bazin’s disease). J Am Acad Dermatol. 1989;21 (4, pt 1):740-745.
11. Sharon V, Goodarzi H, Chambers CJ, et al. Erythema induratum of Bazin. Dermatol Online J. 2010;16:1.
12. Feiwel M, Munro DD. Diagnosis and treatment of erythema induratum (Bazin). Br Med J. 1965;1:1109-1111.
13. Lebel M, Lassonde M. Erythema induratum of Bazin. J Am Acad Dermatol. 1986;14(5, pt 1):738-742.
14. Silva MT, Antunes SL, Rolla VC, et al. Distal painful peripheral neuropathy associated with erythema induratum of Bazin. Eur J Neurol. 2006;13:e5-e6.
15. Jacinto SS, Nograles KB. Erythema induratum of bazin: role of polymerase chain reaction in diagnosis. Int J Dermatol. 2003;42:380-381.
16. Ollert MW, Thomas P, Korting HC, et al. Erythema induratum of Bazin. evidence of T-lymphocyte hyperresponsiveness to purified protein derivative of tuberculin: report of two cases and treatment. Arch Dermatol. 1993;129:469-473.
17. Angus J, Roberts C, Kulkarni K, et al. Usefulness of the QuantiFERON test in the confirmation of latent tuberculosis in association with erythema induratum [published online ahead of print October 10, 2007]. Br J Dermatol. 2007;157:1293-1294.
18. Seckin D, Hízel N, Demirhan B, et al. The diagnostic value of polymerase chain reaction in erythema induratum of Bazin. Br J Dermatol. 1997;137:1011-1012.
19. Cannas A, Goletti D, Girardi E, et al. Mycobacterium tuberculosis DNA detection in soluble fraction of urine from pulmonary tuberculosis patients. Int J Tuberc Lung Dis. 2008;12:146-151.
20. Tan SH, Tan BH, Goh CL, et al. Detection of Mycobacterium tuberculosis DNA using polymerase chain reaction in cutaneous tuberculosis and tuberculids. Int J Dermatol. 1999;38:122-127.
21. Baselga E, Margall N, Barnadas MA, et al. Detection of Mycobacterium tuberculosis DNA in lobular granulomatous panniculitis (erythema induratum-nodular vasculitis). Arch Dermatol. 1997;133:457-462.
22. Cho KH, Lee DY, Kim CW. Erythema induratum of Bazin. Int J Dermatol. 1996;35:802-808.
23. Schulz EJ, Whiting DA. Treatment of erythema nodosum and nodular vasculitis with potassium iodide. Br J Dermatol. 1976;94:75-78.
24. Horio T, Imamura S, Danno K, et al. Potassium iodide in the treatment of erythema nodosum and nodular vasculitis. Arch Dermatol. 1981;117:29-31.
25. Shaffer N, Kerdel FA. Nodular vasculitis (erythema induratum): treatment with auranofin. J Am Acad Dermatol. 1991;25(2, pt 2):426-429.
Practice Points
- Erythema induratum of Bazin (EIB) is a type of nodular vasculitis related to tuberculosis and commonly presents with plaques and nodules on the lower extremities.
- Peripheral neuropathy may manifest as a result of a hypersensitivity reaction to tuberculosis antigens causing tissue and nerve damage.
- Treatment of EIB and other cases of nodular vasculitis should be directed at the underlying cause of the immune response.
Recorrection Osteotomies and Total Knee Arthroplasties After Failed Bilateral High Tibial Osteotomies
High tibial osteotomy has proved successful in treating unicompartmental arthritis in young, active patients.1-3 However, this procedure fails over time because the other compartments deteriorate.4 The next step is conversion of the osteotomy to total knee arthroplasty (TKA). Conversion results vary, with several authors reporting poor outcomes5-9 and others reporting outcomes equal to those of primary TKA.10-14
The long-term success of TKA depends on proper restoration of the mechanical axis and soft-tissue balancing.15 Preexisting extra-articular deformity may adversely affect outcomes. A deformity of more than 15° may make it difficult to obtain intra-articular correction of an extra-articular deformity through soft-tissue balancing alone.16
In this article, we report the unique case of a patient whose bilateral high tibial osteotomies failed because of excessive extra-articular deformity. TKAs were performed consecutively, in 2 separate settings. Each TKA was combined with a recorrection tibial osteotomy in a single operation, allowing for re-creation of normal knee alignment with ligament balance. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 58-year-old man (weight, 250 pounds; body mass index, 30) underwent staged bilateral medial opening wedge osteotomies using distraction osteogenesis. A uniplanar external fixator was used for fixation on each knee. Before surgery, anatomical axis was 2° (right knee) and –1° (left knee) (Figure 1A), and tibial slope was 9° (right) and 8° (left) (Figures 1B, 1C). The procedures were performed 10 months apart. After surgery, anatomical alignment was 17° valgus (right knee) and 12° valgus (left knee) (Figure 2), and tibial slope was 20° (right) and 13° (left).
The patient received mild relief of his arthritis symptoms. Fifty-six months after the index operation, he decided to undergo conversion of the right high tibial osteotomy to TKA because of progressive painful arthritis of the knee. Excessive valgus alignment caused by the initial osteotomy raised concerns about being able to correct the extra-articular deformity intra-articularly while maintaining kinematic ligament balance. For this reason, a recorrection osteotomy was performed concurrently with the TKA. A posterior cruciate ligament–retaining (PCL-retaining) knee design (NexGen, Zimmer) was selected.
The procedure began with bone cuts for the TKA. Initial cuts were made on the femur. The tibial cut was made in valgus corresponding to the preoperative valgus deformity. The tibial recorrection osteotomy was made at the level of the original osteotomy site. A stemmed tibial component was used to cross the osteotomy site, correcting the valgus deformity and providing stability at the osteotomy site. A 3.5-mm locking compression T-plate (Synthes) was medially placed to prevent loss of correction and control rotation of the osteotomy during healing. The patient began range of motion on postoperative day 1. Continuous passive motion was not used. Protective weight-bearing continued for 6 weeks. After 6 weeks, and once there was radiograph evidence of healing at the osteotomy site, full weight-bearing was allowed.
After 4 months, the patient decided to undergo a similar procedure on the left knee. Postoperative rehabilitation was the same. A year after the bilateral TKAs, the patient maintained a Knee Society Score of 95 and a functional score of 90. After surgery, anatomical alignment was 6° (right knee) and 3° (left knee) (Figure 3), and tibial slope was 6° (right) and 7° (left) (Figures 4A, 4B). In each knee, the PCL was preserved with ligament balance.
Discussion
Clinical outcomes of TKA after high tibial osteotomy vary. Windsor and colleagues9 reported that knee arthroplasties after tibial osteotomy were less successful than primary TKAs. In small studies, both Staeheli and colleagues17 and Katz and colleagues5 found that TKA outcomes after osteotomy were satisfactory compared with outcomes of primary TKA without previous osteotomy. A meta-analysis by Ramappa and colleagues18 showed no difference in outcomes between TKAs with and without previous osteotomy. In addition, there were no differences in outcomes between TKAs performed after opening wedge versus closing wedge osteotomies.19
An arthritic knee compartment is unloaded when a high tibial osteotomy produces an extra-articular deformity. Neyret and colleagues7 reported difficulties in correcting angulations of 9° or more through soft-tissue release. Cameron and Welsh16 suggested pre–knee arthroplasty correction of the extra-articular deformity for malalignments of more than 15°. In cases of severe malalignment produced by an osteotomy, Katz and colleagues5 also suggested that a second osteotomy be performed to correct alignment before TKA.
For TKA after high tibial osteotomy, a neutral plateau resection removes more bone medially than laterally, creating medial laxity. Without correction of the tibial deformity, lateral release (or, as Krackow and Holtgrewe20 advocated, medial advancement) is required for ligament stability. Both technically demanding options may not provide complete stability throughout the arc of motion. In addition, neither corrects for rotational or sagittal deformities (the concern with correcting an extra-articular deformity with intra-articular ligament balancing).
Another option is valgus tibial resection, which maintains native ligament balance at the cost of excessive valgus alignment. In the low-demand patient, a condylar constrained implant provides a means of correcting the malalignment with knee stability.8,13,17 The increased restraint produces greater forces at the implant–bone interface and may risk early loosening.
The case presented here represents a unique situation of failed bilateral high tibial osteotomies with excessive valgus malalignment. In a similar situation, Papagelopoulos and colleagues21 suggested correcting fracture deformities before or at time of knee arthroplasty. Yoshina and colleagues22 reported using a stemmed tibial component with TKA in treating nonunion of a high tibial osteotomy. As mentioned, Katz and colleagues5 and Neyret and colleagues7 suggested preoperative correction of the osteotomy in cases of severe malalignment. Others have suggested combining recorrection osteotomy and knee arthroplasty in either consecutive operations or a single operation.23-26 Wolff and colleagues27 and Uchinou and colleagues28 described recorrection osteotomy performed concurrent with TKA. The present article is the first to report a case involving concurrent bilateral recorrection osteotomy and TKA.
In one setting, the recorrection osteotomy is performed after the bony cuts are made for the TKA. The initial tibial plateau resection is performed in valgus at the same degree of malalignment as the osteotomy. This allows the plane of the tibial resection to parallel the floor once the recorrection is finished. With use of a tibial stem crossing the osteotomy site and a derotation plate, adequate fixation of the osteotomy is obtained. The recorrection osteotomy prevents the ligaments from overlengthening, allows the native ligament balance of the knee, and preserves the PCL—which lets the surgeon obtain ligament balance for the TKA throughout the arc of motion, avoiding midstance instabilities and achieving knee alignment rotationally and in the coronal and sagittal planes.
The TKA used in the present case was a PCL-retaining design. Both posterior-stabilized and PCL-retaining designs are reasonable options for use in combination with recorrection osteotomy. A stemmed tibial component is needed to cross the osteotomy site. In our patient’s case, use of a PCL-retaining design was based on surgeon preference and experience.
Patella infera has been noted as a problem in studies on converting high tibial osteotomy to TKA.9,12,29 A postulated cause is scarring of the infrapatellar tendon after high tibial osteotomy. In addition, a higher incidence of lateral retinacular release has been identified.9-11 Patella infera did not occur in either knee in the present case, and lateral release was not required.
Our patient’s lateral radiographs (Figures 4A, 4B) showed persistence of the osteotomy plane anterior to the tibia. The osteotomy healed posteriorly but not completely anteriorly. This raises the issue of risk for nonunion when recorrection osteotomy is performed with TKA. Use of a stemmed tibial implant with a derotation plate provides the benefit of intramedullary fixation for the recorrection osteotomy. If the recorrection osteotomy were performed in a separate setting before TKA, plate fixation would be the primary fixation option. Should nonunion occur at the recorrection osteotomy site, revision of the tibial plateau with a new stemmed implant would be required in combination with plate fixation. Madelaine and colleagues30 reported on a series of 15 severe varus knees treated with both osteotomy and TKA. Two nonunions occurred. Fixation was a staple in one case and a cement wedge in the other. Risk for nonunion may be reduced with the combination of stemmed tibial implant and internal fixation with a derotation plate. Protective weight-bearing is recommended for the first 6 postoperative weeks.
Conclusion
Ligament imbalances produced by high tibial osteotomy and exacerbated by conversion to TKA are difficult to address. In this report, we have described successful single-stage high tibial osteotomy recorrection and TKA performed bilaterally in separate settings. With use of a stemmed tibial component and a derotation plate, solid fixation was obtained with an excellent clinical outcome. The malalignment was corrected while ligament balance was maintained for a PCL-retaining TKA design.
1. Billings A, Scott DF, Camargo MP, Hofmann AA. High tibial osteotomy with a calibrated osteotomy guide, rigid internal fixation, and early motion. Long-term follow-up. J Bone Joint Surg Am. 2000;82(1):70-79.
2. Coventry MB, Ilstrup DM, Wallrichs SL. Proximal tibial osteotomy. A critical long-term study of eighty-seven cases. J Bone Joint Surg Am. 1993;75(2):196-201.
3. Rinonapoli E, Mancini GB, Corvaglia A, Musiello S. Tibial osteotomy for varus gonarthrosis. A 10- to 21-year followup study. Clin Orthop Relat Res. 1998;(353):185-193.
4. Ritter MA, Fechtman RA. Proximal tibial osteotomy. A survivorship analysis. J Arthroplasty. 1988;3(4):309-311.
5. Katz MM, Hungerford DS, Krackow KA, Lennox DW. Results of total knee arthroplasty after failed proximal tibial osteotomy for osteoarthritis. J Bone Joint Surg Am. 1987;69(2):225-233.
6. Mont MA, Antonaides S, Krackow KA, Hungerford DS. Total knee arthroplasty after failed high tibial osteotomy. A comparison with a matched group. Clin Orthop Relat Res. 1994;299:125-130.
7. Neyret P, Deroche P, Deschamps G, Dejour H. Total knee replacement after valgus tibial osteotomy. Technical problems [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1992;78(7):438-448.
8. Parvizi J, Hanssen AD, Spangehl MJ. Total knee arthroplasty following proximal tibial osteotomy: risk factors for failure. J Bone Joint Surg Am. 2004;86(3):474-479.
9. Windsor RE, Insall JN, Vince KG. Technical considerations of total knee arthroplasty after proximal tibial osteotomy. J Bone Joint Surg Am. 1988;70(4):547-555.
10. Amendola A, Rorabeck CH, Bourne RB, Apyan PM. Total knee arthroplasty following high tibial osteotomy for osteoarthritis. J Arthroplasty. 1989;(4 suppl):S11-S17.
11. Kazakos KJ, Chatzipapas C, Verettas D, Galanis V, Xarchas KC, Psillakis I. Mid-term results of total knee arthroplasty after high tibial osteotomy. Arch Orthop Trauma Surg. 2008;128(2):167-173.
12. Meding JB, Keating EM, Ritter MA, Faris PM. Total knee arthroplasty after high tibial osteotomy. A comparison study in patients who had bilateral total knee replacement. J Bone Joint Surg Am. 2000;82(9):1252-1259.
13. Niinimaki T, Eskelinen A, Ohtonen P, Puhto AP, Mann BS, Leppilahti J. Total knee arthroplasty after high tibial osteotomy: a registry-based case–control study of 1,036 knees. Arch Orthop Trauma Surg. 2014;134(1):73-77.
14. van Raaij TM, Reijman M, Furlan AD, Verhaar JA. Total knee arthroplasty after high tibial osteotomy. A systematic review. BMC Musculoskelet Disord. 2009;10:88-98.
15. Lotke PA, Ecker ML. Influence of positioning of prosthesis in total knee replacement. J Bone Joint Surg Am. 1977;59(1):77-79.
16. Cameron HU, Welsh RP. Potential complications of total knee replacement following tibial osteotomy. Orthop Rev. 1988;17(1):39-43.
17. Staeheli JW, Cass JR, Morrey BF. Condylar total knee arthroplasty after failed proximal tibial osteotomy. J Bone Joint Surg Am. 1987;69(1):28-31.
18. Ramappa M, Anand S, Jennings A. Total knee replacement following high tibial osteotomy versus total knee replacement without high tibial osteotomy: a systematic review and meta analysis. Arch Orthop Trauma Surg. 2013;133(11):1587-1593.
19. Preston S, Howard J, Naudie D, Somerville L, McAuley J. Total knee arthroplasty after high tibial osteotomy: no differences between medial and lateral osteotomy approaches. Clin Orthop Relat Res. 2014;472(1):105-110.
20. Krackow KA, Holtgrewe JL. Experience with a new technique for managing severely overcorrected valgus high tibial osteotomy at total knee arthroplasty. Clin Orthop Relat Res. 1990;(258):213-224.
21. Papagelopoulos PJ, Karachalios T, Themistocleous GS, Papadopoulos ECh, Savvidou OD, Rand JA. Total knee arthroplasty in patients with pre-existing fracture deformity. Orthopaedics. 2007;30(5):373-378.
22. Yoshina N, Takai S, Watanabe Y, Nakamura S, Kubo T. Total knee arthroplasty with long stem for treatment of nonunion after high tibial osteotomy. J Arthroplasty. 2004;19(4):528-531.
23. Mont MA, Alexander N, Krackow KA, Hungerford DS. Total knee arthroplasty after failed high tibial osteotomy. Orthop Clin North Am. 1994;25(3):515-525.
24. Scott WN. Insall & Scott’s Surgery of the Knee. Vol 1. 4th ed. Philadelphia, PA: Churchill Livingstone Elsevier; 2006.
25. Gill T, Schemitsch EH, Brick GW, Thornhill TS. Revision total knee arthroplasty after failed unicompartmental knee arthroplasty or high tibial osteotomy. Clin Orthop Relat Res. 1995;(321):10-18.
26. Figgie HE 3rd, Goldberg VM, Heiple KG, Moller HS 3rd, Gordon NH. The influence of tibial-patellofemoral location on function of the knee in patients with the posterior stabilized condylar knee prosthesis. J Bone Joint Surg Am. 1986;68(7):1035-1040.
27. Wolff AM, Hungerford DS, Pepe CL. The effect of extraarticular varus and valgus deformity on total knee arthroplasty. Clin Orthop Relat Res. 1994;(271):35-51.
28. Uchinou S, Yano H, Shimizu K, Masumi S. A severely overcorrected high tibial osteotomy: revision by osteotomy and a long stem component. Acta Orthop Scand. 1996;67(2):193-194.
29. Noda T, Yasuda S, Nagano K, Takahara Y, Namba Y, Inoue H. Clinico-radiological study of total knee arthroplasty after high tibial osteotomy. J Orthop Sci. 2000;5(1):25-36.
30. Madelaine A, Villa V, Yela C, et al. Results and complications of single-stage total knee arthroplasty and high tibial osteotomy. Int Orthop. 2014;38(10):2091-2098.
High tibial osteotomy has proved successful in treating unicompartmental arthritis in young, active patients.1-3 However, this procedure fails over time because the other compartments deteriorate.4 The next step is conversion of the osteotomy to total knee arthroplasty (TKA). Conversion results vary, with several authors reporting poor outcomes5-9 and others reporting outcomes equal to those of primary TKA.10-14
The long-term success of TKA depends on proper restoration of the mechanical axis and soft-tissue balancing.15 Preexisting extra-articular deformity may adversely affect outcomes. A deformity of more than 15° may make it difficult to obtain intra-articular correction of an extra-articular deformity through soft-tissue balancing alone.16
In this article, we report the unique case of a patient whose bilateral high tibial osteotomies failed because of excessive extra-articular deformity. TKAs were performed consecutively, in 2 separate settings. Each TKA was combined with a recorrection tibial osteotomy in a single operation, allowing for re-creation of normal knee alignment with ligament balance. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 58-year-old man (weight, 250 pounds; body mass index, 30) underwent staged bilateral medial opening wedge osteotomies using distraction osteogenesis. A uniplanar external fixator was used for fixation on each knee. Before surgery, anatomical axis was 2° (right knee) and –1° (left knee) (Figure 1A), and tibial slope was 9° (right) and 8° (left) (Figures 1B, 1C). The procedures were performed 10 months apart. After surgery, anatomical alignment was 17° valgus (right knee) and 12° valgus (left knee) (Figure 2), and tibial slope was 20° (right) and 13° (left).
The patient received mild relief of his arthritis symptoms. Fifty-six months after the index operation, he decided to undergo conversion of the right high tibial osteotomy to TKA because of progressive painful arthritis of the knee. Excessive valgus alignment caused by the initial osteotomy raised concerns about being able to correct the extra-articular deformity intra-articularly while maintaining kinematic ligament balance. For this reason, a recorrection osteotomy was performed concurrently with the TKA. A posterior cruciate ligament–retaining (PCL-retaining) knee design (NexGen, Zimmer) was selected.
The procedure began with bone cuts for the TKA. Initial cuts were made on the femur. The tibial cut was made in valgus corresponding to the preoperative valgus deformity. The tibial recorrection osteotomy was made at the level of the original osteotomy site. A stemmed tibial component was used to cross the osteotomy site, correcting the valgus deformity and providing stability at the osteotomy site. A 3.5-mm locking compression T-plate (Synthes) was medially placed to prevent loss of correction and control rotation of the osteotomy during healing. The patient began range of motion on postoperative day 1. Continuous passive motion was not used. Protective weight-bearing continued for 6 weeks. After 6 weeks, and once there was radiograph evidence of healing at the osteotomy site, full weight-bearing was allowed.
After 4 months, the patient decided to undergo a similar procedure on the left knee. Postoperative rehabilitation was the same. A year after the bilateral TKAs, the patient maintained a Knee Society Score of 95 and a functional score of 90. After surgery, anatomical alignment was 6° (right knee) and 3° (left knee) (Figure 3), and tibial slope was 6° (right) and 7° (left) (Figures 4A, 4B). In each knee, the PCL was preserved with ligament balance.
Discussion
Clinical outcomes of TKA after high tibial osteotomy vary. Windsor and colleagues9 reported that knee arthroplasties after tibial osteotomy were less successful than primary TKAs. In small studies, both Staeheli and colleagues17 and Katz and colleagues5 found that TKA outcomes after osteotomy were satisfactory compared with outcomes of primary TKA without previous osteotomy. A meta-analysis by Ramappa and colleagues18 showed no difference in outcomes between TKAs with and without previous osteotomy. In addition, there were no differences in outcomes between TKAs performed after opening wedge versus closing wedge osteotomies.19
An arthritic knee compartment is unloaded when a high tibial osteotomy produces an extra-articular deformity. Neyret and colleagues7 reported difficulties in correcting angulations of 9° or more through soft-tissue release. Cameron and Welsh16 suggested pre–knee arthroplasty correction of the extra-articular deformity for malalignments of more than 15°. In cases of severe malalignment produced by an osteotomy, Katz and colleagues5 also suggested that a second osteotomy be performed to correct alignment before TKA.
For TKA after high tibial osteotomy, a neutral plateau resection removes more bone medially than laterally, creating medial laxity. Without correction of the tibial deformity, lateral release (or, as Krackow and Holtgrewe20 advocated, medial advancement) is required for ligament stability. Both technically demanding options may not provide complete stability throughout the arc of motion. In addition, neither corrects for rotational or sagittal deformities (the concern with correcting an extra-articular deformity with intra-articular ligament balancing).
Another option is valgus tibial resection, which maintains native ligament balance at the cost of excessive valgus alignment. In the low-demand patient, a condylar constrained implant provides a means of correcting the malalignment with knee stability.8,13,17 The increased restraint produces greater forces at the implant–bone interface and may risk early loosening.
The case presented here represents a unique situation of failed bilateral high tibial osteotomies with excessive valgus malalignment. In a similar situation, Papagelopoulos and colleagues21 suggested correcting fracture deformities before or at time of knee arthroplasty. Yoshina and colleagues22 reported using a stemmed tibial component with TKA in treating nonunion of a high tibial osteotomy. As mentioned, Katz and colleagues5 and Neyret and colleagues7 suggested preoperative correction of the osteotomy in cases of severe malalignment. Others have suggested combining recorrection osteotomy and knee arthroplasty in either consecutive operations or a single operation.23-26 Wolff and colleagues27 and Uchinou and colleagues28 described recorrection osteotomy performed concurrent with TKA. The present article is the first to report a case involving concurrent bilateral recorrection osteotomy and TKA.
In one setting, the recorrection osteotomy is performed after the bony cuts are made for the TKA. The initial tibial plateau resection is performed in valgus at the same degree of malalignment as the osteotomy. This allows the plane of the tibial resection to parallel the floor once the recorrection is finished. With use of a tibial stem crossing the osteotomy site and a derotation plate, adequate fixation of the osteotomy is obtained. The recorrection osteotomy prevents the ligaments from overlengthening, allows the native ligament balance of the knee, and preserves the PCL—which lets the surgeon obtain ligament balance for the TKA throughout the arc of motion, avoiding midstance instabilities and achieving knee alignment rotationally and in the coronal and sagittal planes.
The TKA used in the present case was a PCL-retaining design. Both posterior-stabilized and PCL-retaining designs are reasonable options for use in combination with recorrection osteotomy. A stemmed tibial component is needed to cross the osteotomy site. In our patient’s case, use of a PCL-retaining design was based on surgeon preference and experience.
Patella infera has been noted as a problem in studies on converting high tibial osteotomy to TKA.9,12,29 A postulated cause is scarring of the infrapatellar tendon after high tibial osteotomy. In addition, a higher incidence of lateral retinacular release has been identified.9-11 Patella infera did not occur in either knee in the present case, and lateral release was not required.
Our patient’s lateral radiographs (Figures 4A, 4B) showed persistence of the osteotomy plane anterior to the tibia. The osteotomy healed posteriorly but not completely anteriorly. This raises the issue of risk for nonunion when recorrection osteotomy is performed with TKA. Use of a stemmed tibial implant with a derotation plate provides the benefit of intramedullary fixation for the recorrection osteotomy. If the recorrection osteotomy were performed in a separate setting before TKA, plate fixation would be the primary fixation option. Should nonunion occur at the recorrection osteotomy site, revision of the tibial plateau with a new stemmed implant would be required in combination with plate fixation. Madelaine and colleagues30 reported on a series of 15 severe varus knees treated with both osteotomy and TKA. Two nonunions occurred. Fixation was a staple in one case and a cement wedge in the other. Risk for nonunion may be reduced with the combination of stemmed tibial implant and internal fixation with a derotation plate. Protective weight-bearing is recommended for the first 6 postoperative weeks.
Conclusion
Ligament imbalances produced by high tibial osteotomy and exacerbated by conversion to TKA are difficult to address. In this report, we have described successful single-stage high tibial osteotomy recorrection and TKA performed bilaterally in separate settings. With use of a stemmed tibial component and a derotation plate, solid fixation was obtained with an excellent clinical outcome. The malalignment was corrected while ligament balance was maintained for a PCL-retaining TKA design.
High tibial osteotomy has proved successful in treating unicompartmental arthritis in young, active patients.1-3 However, this procedure fails over time because the other compartments deteriorate.4 The next step is conversion of the osteotomy to total knee arthroplasty (TKA). Conversion results vary, with several authors reporting poor outcomes5-9 and others reporting outcomes equal to those of primary TKA.10-14
The long-term success of TKA depends on proper restoration of the mechanical axis and soft-tissue balancing.15 Preexisting extra-articular deformity may adversely affect outcomes. A deformity of more than 15° may make it difficult to obtain intra-articular correction of an extra-articular deformity through soft-tissue balancing alone.16
In this article, we report the unique case of a patient whose bilateral high tibial osteotomies failed because of excessive extra-articular deformity. TKAs were performed consecutively, in 2 separate settings. Each TKA was combined with a recorrection tibial osteotomy in a single operation, allowing for re-creation of normal knee alignment with ligament balance. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 58-year-old man (weight, 250 pounds; body mass index, 30) underwent staged bilateral medial opening wedge osteotomies using distraction osteogenesis. A uniplanar external fixator was used for fixation on each knee. Before surgery, anatomical axis was 2° (right knee) and –1° (left knee) (Figure 1A), and tibial slope was 9° (right) and 8° (left) (Figures 1B, 1C). The procedures were performed 10 months apart. After surgery, anatomical alignment was 17° valgus (right knee) and 12° valgus (left knee) (Figure 2), and tibial slope was 20° (right) and 13° (left).
The patient received mild relief of his arthritis symptoms. Fifty-six months after the index operation, he decided to undergo conversion of the right high tibial osteotomy to TKA because of progressive painful arthritis of the knee. Excessive valgus alignment caused by the initial osteotomy raised concerns about being able to correct the extra-articular deformity intra-articularly while maintaining kinematic ligament balance. For this reason, a recorrection osteotomy was performed concurrently with the TKA. A posterior cruciate ligament–retaining (PCL-retaining) knee design (NexGen, Zimmer) was selected.
The procedure began with bone cuts for the TKA. Initial cuts were made on the femur. The tibial cut was made in valgus corresponding to the preoperative valgus deformity. The tibial recorrection osteotomy was made at the level of the original osteotomy site. A stemmed tibial component was used to cross the osteotomy site, correcting the valgus deformity and providing stability at the osteotomy site. A 3.5-mm locking compression T-plate (Synthes) was medially placed to prevent loss of correction and control rotation of the osteotomy during healing. The patient began range of motion on postoperative day 1. Continuous passive motion was not used. Protective weight-bearing continued for 6 weeks. After 6 weeks, and once there was radiograph evidence of healing at the osteotomy site, full weight-bearing was allowed.
After 4 months, the patient decided to undergo a similar procedure on the left knee. Postoperative rehabilitation was the same. A year after the bilateral TKAs, the patient maintained a Knee Society Score of 95 and a functional score of 90. After surgery, anatomical alignment was 6° (right knee) and 3° (left knee) (Figure 3), and tibial slope was 6° (right) and 7° (left) (Figures 4A, 4B). In each knee, the PCL was preserved with ligament balance.
Discussion
Clinical outcomes of TKA after high tibial osteotomy vary. Windsor and colleagues9 reported that knee arthroplasties after tibial osteotomy were less successful than primary TKAs. In small studies, both Staeheli and colleagues17 and Katz and colleagues5 found that TKA outcomes after osteotomy were satisfactory compared with outcomes of primary TKA without previous osteotomy. A meta-analysis by Ramappa and colleagues18 showed no difference in outcomes between TKAs with and without previous osteotomy. In addition, there were no differences in outcomes between TKAs performed after opening wedge versus closing wedge osteotomies.19
An arthritic knee compartment is unloaded when a high tibial osteotomy produces an extra-articular deformity. Neyret and colleagues7 reported difficulties in correcting angulations of 9° or more through soft-tissue release. Cameron and Welsh16 suggested pre–knee arthroplasty correction of the extra-articular deformity for malalignments of more than 15°. In cases of severe malalignment produced by an osteotomy, Katz and colleagues5 also suggested that a second osteotomy be performed to correct alignment before TKA.
For TKA after high tibial osteotomy, a neutral plateau resection removes more bone medially than laterally, creating medial laxity. Without correction of the tibial deformity, lateral release (or, as Krackow and Holtgrewe20 advocated, medial advancement) is required for ligament stability. Both technically demanding options may not provide complete stability throughout the arc of motion. In addition, neither corrects for rotational or sagittal deformities (the concern with correcting an extra-articular deformity with intra-articular ligament balancing).
Another option is valgus tibial resection, which maintains native ligament balance at the cost of excessive valgus alignment. In the low-demand patient, a condylar constrained implant provides a means of correcting the malalignment with knee stability.8,13,17 The increased restraint produces greater forces at the implant–bone interface and may risk early loosening.
The case presented here represents a unique situation of failed bilateral high tibial osteotomies with excessive valgus malalignment. In a similar situation, Papagelopoulos and colleagues21 suggested correcting fracture deformities before or at time of knee arthroplasty. Yoshina and colleagues22 reported using a stemmed tibial component with TKA in treating nonunion of a high tibial osteotomy. As mentioned, Katz and colleagues5 and Neyret and colleagues7 suggested preoperative correction of the osteotomy in cases of severe malalignment. Others have suggested combining recorrection osteotomy and knee arthroplasty in either consecutive operations or a single operation.23-26 Wolff and colleagues27 and Uchinou and colleagues28 described recorrection osteotomy performed concurrent with TKA. The present article is the first to report a case involving concurrent bilateral recorrection osteotomy and TKA.
In one setting, the recorrection osteotomy is performed after the bony cuts are made for the TKA. The initial tibial plateau resection is performed in valgus at the same degree of malalignment as the osteotomy. This allows the plane of the tibial resection to parallel the floor once the recorrection is finished. With use of a tibial stem crossing the osteotomy site and a derotation plate, adequate fixation of the osteotomy is obtained. The recorrection osteotomy prevents the ligaments from overlengthening, allows the native ligament balance of the knee, and preserves the PCL—which lets the surgeon obtain ligament balance for the TKA throughout the arc of motion, avoiding midstance instabilities and achieving knee alignment rotationally and in the coronal and sagittal planes.
The TKA used in the present case was a PCL-retaining design. Both posterior-stabilized and PCL-retaining designs are reasonable options for use in combination with recorrection osteotomy. A stemmed tibial component is needed to cross the osteotomy site. In our patient’s case, use of a PCL-retaining design was based on surgeon preference and experience.
Patella infera has been noted as a problem in studies on converting high tibial osteotomy to TKA.9,12,29 A postulated cause is scarring of the infrapatellar tendon after high tibial osteotomy. In addition, a higher incidence of lateral retinacular release has been identified.9-11 Patella infera did not occur in either knee in the present case, and lateral release was not required.
Our patient’s lateral radiographs (Figures 4A, 4B) showed persistence of the osteotomy plane anterior to the tibia. The osteotomy healed posteriorly but not completely anteriorly. This raises the issue of risk for nonunion when recorrection osteotomy is performed with TKA. Use of a stemmed tibial implant with a derotation plate provides the benefit of intramedullary fixation for the recorrection osteotomy. If the recorrection osteotomy were performed in a separate setting before TKA, plate fixation would be the primary fixation option. Should nonunion occur at the recorrection osteotomy site, revision of the tibial plateau with a new stemmed implant would be required in combination with plate fixation. Madelaine and colleagues30 reported on a series of 15 severe varus knees treated with both osteotomy and TKA. Two nonunions occurred. Fixation was a staple in one case and a cement wedge in the other. Risk for nonunion may be reduced with the combination of stemmed tibial implant and internal fixation with a derotation plate. Protective weight-bearing is recommended for the first 6 postoperative weeks.
Conclusion
Ligament imbalances produced by high tibial osteotomy and exacerbated by conversion to TKA are difficult to address. In this report, we have described successful single-stage high tibial osteotomy recorrection and TKA performed bilaterally in separate settings. With use of a stemmed tibial component and a derotation plate, solid fixation was obtained with an excellent clinical outcome. The malalignment was corrected while ligament balance was maintained for a PCL-retaining TKA design.
1. Billings A, Scott DF, Camargo MP, Hofmann AA. High tibial osteotomy with a calibrated osteotomy guide, rigid internal fixation, and early motion. Long-term follow-up. J Bone Joint Surg Am. 2000;82(1):70-79.
2. Coventry MB, Ilstrup DM, Wallrichs SL. Proximal tibial osteotomy. A critical long-term study of eighty-seven cases. J Bone Joint Surg Am. 1993;75(2):196-201.
3. Rinonapoli E, Mancini GB, Corvaglia A, Musiello S. Tibial osteotomy for varus gonarthrosis. A 10- to 21-year followup study. Clin Orthop Relat Res. 1998;(353):185-193.
4. Ritter MA, Fechtman RA. Proximal tibial osteotomy. A survivorship analysis. J Arthroplasty. 1988;3(4):309-311.
5. Katz MM, Hungerford DS, Krackow KA, Lennox DW. Results of total knee arthroplasty after failed proximal tibial osteotomy for osteoarthritis. J Bone Joint Surg Am. 1987;69(2):225-233.
6. Mont MA, Antonaides S, Krackow KA, Hungerford DS. Total knee arthroplasty after failed high tibial osteotomy. A comparison with a matched group. Clin Orthop Relat Res. 1994;299:125-130.
7. Neyret P, Deroche P, Deschamps G, Dejour H. Total knee replacement after valgus tibial osteotomy. Technical problems [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1992;78(7):438-448.
8. Parvizi J, Hanssen AD, Spangehl MJ. Total knee arthroplasty following proximal tibial osteotomy: risk factors for failure. J Bone Joint Surg Am. 2004;86(3):474-479.
9. Windsor RE, Insall JN, Vince KG. Technical considerations of total knee arthroplasty after proximal tibial osteotomy. J Bone Joint Surg Am. 1988;70(4):547-555.
10. Amendola A, Rorabeck CH, Bourne RB, Apyan PM. Total knee arthroplasty following high tibial osteotomy for osteoarthritis. J Arthroplasty. 1989;(4 suppl):S11-S17.
11. Kazakos KJ, Chatzipapas C, Verettas D, Galanis V, Xarchas KC, Psillakis I. Mid-term results of total knee arthroplasty after high tibial osteotomy. Arch Orthop Trauma Surg. 2008;128(2):167-173.
12. Meding JB, Keating EM, Ritter MA, Faris PM. Total knee arthroplasty after high tibial osteotomy. A comparison study in patients who had bilateral total knee replacement. J Bone Joint Surg Am. 2000;82(9):1252-1259.
13. Niinimaki T, Eskelinen A, Ohtonen P, Puhto AP, Mann BS, Leppilahti J. Total knee arthroplasty after high tibial osteotomy: a registry-based case–control study of 1,036 knees. Arch Orthop Trauma Surg. 2014;134(1):73-77.
14. van Raaij TM, Reijman M, Furlan AD, Verhaar JA. Total knee arthroplasty after high tibial osteotomy. A systematic review. BMC Musculoskelet Disord. 2009;10:88-98.
15. Lotke PA, Ecker ML. Influence of positioning of prosthesis in total knee replacement. J Bone Joint Surg Am. 1977;59(1):77-79.
16. Cameron HU, Welsh RP. Potential complications of total knee replacement following tibial osteotomy. Orthop Rev. 1988;17(1):39-43.
17. Staeheli JW, Cass JR, Morrey BF. Condylar total knee arthroplasty after failed proximal tibial osteotomy. J Bone Joint Surg Am. 1987;69(1):28-31.
18. Ramappa M, Anand S, Jennings A. Total knee replacement following high tibial osteotomy versus total knee replacement without high tibial osteotomy: a systematic review and meta analysis. Arch Orthop Trauma Surg. 2013;133(11):1587-1593.
19. Preston S, Howard J, Naudie D, Somerville L, McAuley J. Total knee arthroplasty after high tibial osteotomy: no differences between medial and lateral osteotomy approaches. Clin Orthop Relat Res. 2014;472(1):105-110.
20. Krackow KA, Holtgrewe JL. Experience with a new technique for managing severely overcorrected valgus high tibial osteotomy at total knee arthroplasty. Clin Orthop Relat Res. 1990;(258):213-224.
21. Papagelopoulos PJ, Karachalios T, Themistocleous GS, Papadopoulos ECh, Savvidou OD, Rand JA. Total knee arthroplasty in patients with pre-existing fracture deformity. Orthopaedics. 2007;30(5):373-378.
22. Yoshina N, Takai S, Watanabe Y, Nakamura S, Kubo T. Total knee arthroplasty with long stem for treatment of nonunion after high tibial osteotomy. J Arthroplasty. 2004;19(4):528-531.
23. Mont MA, Alexander N, Krackow KA, Hungerford DS. Total knee arthroplasty after failed high tibial osteotomy. Orthop Clin North Am. 1994;25(3):515-525.
24. Scott WN. Insall & Scott’s Surgery of the Knee. Vol 1. 4th ed. Philadelphia, PA: Churchill Livingstone Elsevier; 2006.
25. Gill T, Schemitsch EH, Brick GW, Thornhill TS. Revision total knee arthroplasty after failed unicompartmental knee arthroplasty or high tibial osteotomy. Clin Orthop Relat Res. 1995;(321):10-18.
26. Figgie HE 3rd, Goldberg VM, Heiple KG, Moller HS 3rd, Gordon NH. The influence of tibial-patellofemoral location on function of the knee in patients with the posterior stabilized condylar knee prosthesis. J Bone Joint Surg Am. 1986;68(7):1035-1040.
27. Wolff AM, Hungerford DS, Pepe CL. The effect of extraarticular varus and valgus deformity on total knee arthroplasty. Clin Orthop Relat Res. 1994;(271):35-51.
28. Uchinou S, Yano H, Shimizu K, Masumi S. A severely overcorrected high tibial osteotomy: revision by osteotomy and a long stem component. Acta Orthop Scand. 1996;67(2):193-194.
29. Noda T, Yasuda S, Nagano K, Takahara Y, Namba Y, Inoue H. Clinico-radiological study of total knee arthroplasty after high tibial osteotomy. J Orthop Sci. 2000;5(1):25-36.
30. Madelaine A, Villa V, Yela C, et al. Results and complications of single-stage total knee arthroplasty and high tibial osteotomy. Int Orthop. 2014;38(10):2091-2098.
1. Billings A, Scott DF, Camargo MP, Hofmann AA. High tibial osteotomy with a calibrated osteotomy guide, rigid internal fixation, and early motion. Long-term follow-up. J Bone Joint Surg Am. 2000;82(1):70-79.
2. Coventry MB, Ilstrup DM, Wallrichs SL. Proximal tibial osteotomy. A critical long-term study of eighty-seven cases. J Bone Joint Surg Am. 1993;75(2):196-201.
3. Rinonapoli E, Mancini GB, Corvaglia A, Musiello S. Tibial osteotomy for varus gonarthrosis. A 10- to 21-year followup study. Clin Orthop Relat Res. 1998;(353):185-193.
4. Ritter MA, Fechtman RA. Proximal tibial osteotomy. A survivorship analysis. J Arthroplasty. 1988;3(4):309-311.
5. Katz MM, Hungerford DS, Krackow KA, Lennox DW. Results of total knee arthroplasty after failed proximal tibial osteotomy for osteoarthritis. J Bone Joint Surg Am. 1987;69(2):225-233.
6. Mont MA, Antonaides S, Krackow KA, Hungerford DS. Total knee arthroplasty after failed high tibial osteotomy. A comparison with a matched group. Clin Orthop Relat Res. 1994;299:125-130.
7. Neyret P, Deroche P, Deschamps G, Dejour H. Total knee replacement after valgus tibial osteotomy. Technical problems [in French]. Rev Chir Orthop Reparatrice Appar Mot. 1992;78(7):438-448.
8. Parvizi J, Hanssen AD, Spangehl MJ. Total knee arthroplasty following proximal tibial osteotomy: risk factors for failure. J Bone Joint Surg Am. 2004;86(3):474-479.
9. Windsor RE, Insall JN, Vince KG. Technical considerations of total knee arthroplasty after proximal tibial osteotomy. J Bone Joint Surg Am. 1988;70(4):547-555.
10. Amendola A, Rorabeck CH, Bourne RB, Apyan PM. Total knee arthroplasty following high tibial osteotomy for osteoarthritis. J Arthroplasty. 1989;(4 suppl):S11-S17.
11. Kazakos KJ, Chatzipapas C, Verettas D, Galanis V, Xarchas KC, Psillakis I. Mid-term results of total knee arthroplasty after high tibial osteotomy. Arch Orthop Trauma Surg. 2008;128(2):167-173.
12. Meding JB, Keating EM, Ritter MA, Faris PM. Total knee arthroplasty after high tibial osteotomy. A comparison study in patients who had bilateral total knee replacement. J Bone Joint Surg Am. 2000;82(9):1252-1259.
13. Niinimaki T, Eskelinen A, Ohtonen P, Puhto AP, Mann BS, Leppilahti J. Total knee arthroplasty after high tibial osteotomy: a registry-based case–control study of 1,036 knees. Arch Orthop Trauma Surg. 2014;134(1):73-77.
14. van Raaij TM, Reijman M, Furlan AD, Verhaar JA. Total knee arthroplasty after high tibial osteotomy. A systematic review. BMC Musculoskelet Disord. 2009;10:88-98.
15. Lotke PA, Ecker ML. Influence of positioning of prosthesis in total knee replacement. J Bone Joint Surg Am. 1977;59(1):77-79.
16. Cameron HU, Welsh RP. Potential complications of total knee replacement following tibial osteotomy. Orthop Rev. 1988;17(1):39-43.
17. Staeheli JW, Cass JR, Morrey BF. Condylar total knee arthroplasty after failed proximal tibial osteotomy. J Bone Joint Surg Am. 1987;69(1):28-31.
18. Ramappa M, Anand S, Jennings A. Total knee replacement following high tibial osteotomy versus total knee replacement without high tibial osteotomy: a systematic review and meta analysis. Arch Orthop Trauma Surg. 2013;133(11):1587-1593.
19. Preston S, Howard J, Naudie D, Somerville L, McAuley J. Total knee arthroplasty after high tibial osteotomy: no differences between medial and lateral osteotomy approaches. Clin Orthop Relat Res. 2014;472(1):105-110.
20. Krackow KA, Holtgrewe JL. Experience with a new technique for managing severely overcorrected valgus high tibial osteotomy at total knee arthroplasty. Clin Orthop Relat Res. 1990;(258):213-224.
21. Papagelopoulos PJ, Karachalios T, Themistocleous GS, Papadopoulos ECh, Savvidou OD, Rand JA. Total knee arthroplasty in patients with pre-existing fracture deformity. Orthopaedics. 2007;30(5):373-378.
22. Yoshina N, Takai S, Watanabe Y, Nakamura S, Kubo T. Total knee arthroplasty with long stem for treatment of nonunion after high tibial osteotomy. J Arthroplasty. 2004;19(4):528-531.
23. Mont MA, Alexander N, Krackow KA, Hungerford DS. Total knee arthroplasty after failed high tibial osteotomy. Orthop Clin North Am. 1994;25(3):515-525.
24. Scott WN. Insall & Scott’s Surgery of the Knee. Vol 1. 4th ed. Philadelphia, PA: Churchill Livingstone Elsevier; 2006.
25. Gill T, Schemitsch EH, Brick GW, Thornhill TS. Revision total knee arthroplasty after failed unicompartmental knee arthroplasty or high tibial osteotomy. Clin Orthop Relat Res. 1995;(321):10-18.
26. Figgie HE 3rd, Goldberg VM, Heiple KG, Moller HS 3rd, Gordon NH. The influence of tibial-patellofemoral location on function of the knee in patients with the posterior stabilized condylar knee prosthesis. J Bone Joint Surg Am. 1986;68(7):1035-1040.
27. Wolff AM, Hungerford DS, Pepe CL. The effect of extraarticular varus and valgus deformity on total knee arthroplasty. Clin Orthop Relat Res. 1994;(271):35-51.
28. Uchinou S, Yano H, Shimizu K, Masumi S. A severely overcorrected high tibial osteotomy: revision by osteotomy and a long stem component. Acta Orthop Scand. 1996;67(2):193-194.
29. Noda T, Yasuda S, Nagano K, Takahara Y, Namba Y, Inoue H. Clinico-radiological study of total knee arthroplasty after high tibial osteotomy. J Orthop Sci. 2000;5(1):25-36.
30. Madelaine A, Villa V, Yela C, et al. Results and complications of single-stage total knee arthroplasty and high tibial osteotomy. Int Orthop. 2014;38(10):2091-2098.
Drug-induced immune hemolytic anemia associated with albumin-bound paclitaxel
Click on the PDF icon at the top of this introduction to read the full article.
Click on the PDF icon at the top of this introduction to read the full article.
Click on the PDF icon at the top of this introduction to read the full article.
Life-threatening hypoglycemia resulting from a nonislet cell tumor
Nonislet cell tumor-induced hypoglycemia (NICTH), also known as Doege-Potter syndrome, is a rare paraneoplastic syndrome seen in association with various nonpancreatic tumors, benign and malignant, and comprising mesenchymal, vascular, or epithelial cell types. We report a case of recurrent life-threatening hypoglycemia from a large pelvic solitary fibrous tumor.
Click on the PDF icon at the top of this introduction to read the full article.
Nonislet cell tumor-induced hypoglycemia (NICTH), also known as Doege-Potter syndrome, is a rare paraneoplastic syndrome seen in association with various nonpancreatic tumors, benign and malignant, and comprising mesenchymal, vascular, or epithelial cell types. We report a case of recurrent life-threatening hypoglycemia from a large pelvic solitary fibrous tumor.
Click on the PDF icon at the top of this introduction to read the full article.
Nonislet cell tumor-induced hypoglycemia (NICTH), also known as Doege-Potter syndrome, is a rare paraneoplastic syndrome seen in association with various nonpancreatic tumors, benign and malignant, and comprising mesenchymal, vascular, or epithelial cell types. We report a case of recurrent life-threatening hypoglycemia from a large pelvic solitary fibrous tumor.
Click on the PDF icon at the top of this introduction to read the full article.
Colonic Dyspnea and the Morgagni Hernia: A Rare Adult Diagnosis
Congenital diaphragmatic hernias (CDHs) occur from a disruption in the muscular formation of the diaphragm, resulting in herniation of abdominal contents into the thoracic cavity. A rare diagnosis, most cases are identified in the pediatric and neonatal populations with an overall historical 50% mortality related to the diagnosis.1 More recent data published in the U.S. and Japan cite an overall survival rate of 67% to 80% secondary to improved understanding of the pathophysiology and subsequent enhancement of neonatal cardiopulmonary support adjuncts.2,3
Bochladek hernias (posterolateral space) are the most common presentation of CDH, accounting for > 90% of cases. First described by the Giovanni Batista Morgagni in On the Seats and Causes of Disease Investigated by Anatomy, the anteromedial sternocostal location is far less common and accounts for only 2% to 3% of cases.4,5 More commonly found on the right side of the diaphragm, despite protection from the liver, the right-sided space has been traditionally referred to as the Morgagni space. A left-sided defect is occasionally called the Larrey gap or space, after Napoleon’s surgeon who described the space as a potential location for pericardial drainage of tamponade.6,7
Related: Colonoscopy Bowel Preparation Instructions
There are a few congenital conditions, such as trisomy 21, Turner syndrome, Prader Willi syndrome, dextrocardia, and Tetralogy of Fallot, that have been associated with Morgagni hernias.7 Pulmonary hypertension and respiratory distress are the most common symptoms for neonatal patients; chest pain, sensations of tightness/fullness, reflux, and transient obstructive symptoms constitute the typical symptoms of adult patients with CDH. In this case study, the authors present a case of adult-onset Morgagni hernia as well as a review of the relevant literature.
Case Report
The patient was a 48-year-old man on active-duty who presented to the Naval Medical Center Portsmouth General Surgery clinic in Virginia with a 4-year history of gastroesophageal reflux-related symptoms. Specifically, he reported epigastric fullness, pyrosis, and discomfort that radiated toward his bilateral lower ribs for the previous 4 years. This discomfort was typically associated with the intake of solid food and was followed a few hours later by a loose bowel movement.
The patient was initially treated with antacids and proton pump inhibitors by his primary care physician, with only minimal relief. He also reported several months of chronic cough as well as intermittent episodes of “gasping air hunger” for about 6 years, which had been incidentally brought up during his separation physical examination. A chest X-ray performed during the workup revealed findings suggesting a right diaphragmatic hernia vs a bronchogenic cyst (Figure 1). A computed tomography (CT) of the thorax demonstrated a 3 x 8-cm hernia through the foramen of Morgagni containing a portion of the transverse colon along with intraperitoneal fat (Figures 2 and 3).
The patient underwent repair of this right Morgagni hernia via a laparoscopic approach. Intraoperative findings confirmed preoperative radiologic studies demonstrating colonic and omental contents within an easily reducible hernia sac (Figures 4 and 5). The hernia sac was left in vivo, and a combined direct hernia repair with mesh reinforcement was performed using Surgimesh XB (BG Medical, Barrington, IL) (Figure 6). The patient remained in the hospital for overnight observation and was discharged on postoperative day 1. The patient has since been seen in follow-up and is doing quite well with complete resolution of his reflux and pulmonary symptoms.
Discussion
A recent review of surgical literature revealed that over a 57-year period, 298 cases of Morgagni hernias have been described in adults.7 Although previous studies have postulated that a majority of adult patients are asymptomatic, more recent retrospective studies have found about a 70% symptomatic rate of patients with Morgagni hernias.7 The natural history of adult presentations lends itself to pulmonary (most common) or chronic upper gastrointestinal symptoms, although an acute presentation with potential volvulus and strangulation of the herniated contents has been described.7
Diagnosis is typically confirmed with a chest X-ray, although the CT scan has become more popular in the era of multimodal imaging.4,7 Multiple methods of repair have been described; however, thoracotomy has been the most widely used approach, and laparoscopy has gained popularity since the early 1990s.7 Mesh has been described in more than 60% of cases, and a laparoscopic repair has proven to have a low (< 5%) complication rate and short hospital stay.8,9 In particular, it has been suggested that a hernia defect larger than 20 to 30 cm2 should be repaired with a prosthetic adjunct, such as polypropylene, polytetrafluoroethylene, and bovine pericardium with a 1.5- to 2.5-cm mesh overlap.7,8
Related: Unusual Congenital Pulmonary Anomaly in an Adult Patient With Dyspnea
There is some controversy about the management of the hernia sac, with about 69% of surgeons choosing not to excise the sac due to concerns of intrathoracic or pericardial injury.7 In a separate study, 36 patients were evaluated retrospectively, and the hernia sac was not resected in any of the patients, with long-term follow-up revealing no evidence of recurrence.6
Conclusion
To allow for early intervention and avoidance of potentially life-threatening volvulus/strangulation, the medical practitioner has to be aware of this rare diagnosis when performing a workup for vague pulmonary and abdominal symptoms as described here. Disagreement exists over the method of repair and management of the hernia sac as well as the need for mesh buttressing of the defect. A well-planned surgical approach individualized to the patient’s anatomy, surgeon’s expertise, and hernia defect size will provide the best possible outcome with a low operative morbidity.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Holcomb GW, Murphy JP. Ashcraft’s Pediatric Surgery. 5th ed. Kansas City: Saunders Elsevier; 2010: 319-320.
2. Haroon, J, Chamberlain RS. An evidence-based review of the current treatment of congenital diaphragmatic hernia. Clin Pediatr (Phila). 2013;52(2):115-124.
3. Nagata K, Usui N, Kanamori Y, et al. The current profile and outcome of congenital diaphragmatic hernia: a nationwide survey in Japan. J Pediatr Surg. 2013;48(4):738-744.
4. Abraham V, Myla Y, Verghese S, Chandran BS. Morgagni-larrey hernia—a review of 20 cases. Indian J Surg. 2012;74(5):391-395.
5. Arora S, Haji A, Ng P. Adult Morgagni hernia: the need for clinical awareness, early diagnosis, and prompt surgical intervention. Ann R Coll Surg Engl. 2008;90(8):694-695.
6. Aghajanzadeh M, Khadem S, Khajeh Jahromi S, Gorabi HE, Ebrahimi H, Maafi AA. Clinical presentation and operative repair of Morgagni hernia. Interact Cardiovasc Thorac Surg. 2012;15(4):608-611.
7. Horton JD, Hofmann LJ, Hetz SP. Presentation and management of Morgagni hernias in adults: a review of 298 cases. Surg Endosc. 2008;22(6):1413-1420.
8. Terrosu G, Brizzolari M, Intini S, Cattin F, Bresadola V, De Anna D. Morgagni hernia: technical variation in the laparoscopic treatment. Ann Ital Chir. 2012;83(5):415-420.
9. Durak E, Gur S, Cokmez A, Atahan K, Zahtz E, Tarcan E. Laparoscopic repair of Morgagni hernia. Hernia. 2007;11(3):265-270.
Congenital diaphragmatic hernias (CDHs) occur from a disruption in the muscular formation of the diaphragm, resulting in herniation of abdominal contents into the thoracic cavity. A rare diagnosis, most cases are identified in the pediatric and neonatal populations with an overall historical 50% mortality related to the diagnosis.1 More recent data published in the U.S. and Japan cite an overall survival rate of 67% to 80% secondary to improved understanding of the pathophysiology and subsequent enhancement of neonatal cardiopulmonary support adjuncts.2,3
Bochladek hernias (posterolateral space) are the most common presentation of CDH, accounting for > 90% of cases. First described by the Giovanni Batista Morgagni in On the Seats and Causes of Disease Investigated by Anatomy, the anteromedial sternocostal location is far less common and accounts for only 2% to 3% of cases.4,5 More commonly found on the right side of the diaphragm, despite protection from the liver, the right-sided space has been traditionally referred to as the Morgagni space. A left-sided defect is occasionally called the Larrey gap or space, after Napoleon’s surgeon who described the space as a potential location for pericardial drainage of tamponade.6,7
Related: Colonoscopy Bowel Preparation Instructions
There are a few congenital conditions, such as trisomy 21, Turner syndrome, Prader Willi syndrome, dextrocardia, and Tetralogy of Fallot, that have been associated with Morgagni hernias.7 Pulmonary hypertension and respiratory distress are the most common symptoms for neonatal patients; chest pain, sensations of tightness/fullness, reflux, and transient obstructive symptoms constitute the typical symptoms of adult patients with CDH. In this case study, the authors present a case of adult-onset Morgagni hernia as well as a review of the relevant literature.
Case Report
The patient was a 48-year-old man on active-duty who presented to the Naval Medical Center Portsmouth General Surgery clinic in Virginia with a 4-year history of gastroesophageal reflux-related symptoms. Specifically, he reported epigastric fullness, pyrosis, and discomfort that radiated toward his bilateral lower ribs for the previous 4 years. This discomfort was typically associated with the intake of solid food and was followed a few hours later by a loose bowel movement.
The patient was initially treated with antacids and proton pump inhibitors by his primary care physician, with only minimal relief. He also reported several months of chronic cough as well as intermittent episodes of “gasping air hunger” for about 6 years, which had been incidentally brought up during his separation physical examination. A chest X-ray performed during the workup revealed findings suggesting a right diaphragmatic hernia vs a bronchogenic cyst (Figure 1). A computed tomography (CT) of the thorax demonstrated a 3 x 8-cm hernia through the foramen of Morgagni containing a portion of the transverse colon along with intraperitoneal fat (Figures 2 and 3).
The patient underwent repair of this right Morgagni hernia via a laparoscopic approach. Intraoperative findings confirmed preoperative radiologic studies demonstrating colonic and omental contents within an easily reducible hernia sac (Figures 4 and 5). The hernia sac was left in vivo, and a combined direct hernia repair with mesh reinforcement was performed using Surgimesh XB (BG Medical, Barrington, IL) (Figure 6). The patient remained in the hospital for overnight observation and was discharged on postoperative day 1. The patient has since been seen in follow-up and is doing quite well with complete resolution of his reflux and pulmonary symptoms.
Discussion
A recent review of surgical literature revealed that over a 57-year period, 298 cases of Morgagni hernias have been described in adults.7 Although previous studies have postulated that a majority of adult patients are asymptomatic, more recent retrospective studies have found about a 70% symptomatic rate of patients with Morgagni hernias.7 The natural history of adult presentations lends itself to pulmonary (most common) or chronic upper gastrointestinal symptoms, although an acute presentation with potential volvulus and strangulation of the herniated contents has been described.7
Diagnosis is typically confirmed with a chest X-ray, although the CT scan has become more popular in the era of multimodal imaging.4,7 Multiple methods of repair have been described; however, thoracotomy has been the most widely used approach, and laparoscopy has gained popularity since the early 1990s.7 Mesh has been described in more than 60% of cases, and a laparoscopic repair has proven to have a low (< 5%) complication rate and short hospital stay.8,9 In particular, it has been suggested that a hernia defect larger than 20 to 30 cm2 should be repaired with a prosthetic adjunct, such as polypropylene, polytetrafluoroethylene, and bovine pericardium with a 1.5- to 2.5-cm mesh overlap.7,8
Related: Unusual Congenital Pulmonary Anomaly in an Adult Patient With Dyspnea
There is some controversy about the management of the hernia sac, with about 69% of surgeons choosing not to excise the sac due to concerns of intrathoracic or pericardial injury.7 In a separate study, 36 patients were evaluated retrospectively, and the hernia sac was not resected in any of the patients, with long-term follow-up revealing no evidence of recurrence.6
Conclusion
To allow for early intervention and avoidance of potentially life-threatening volvulus/strangulation, the medical practitioner has to be aware of this rare diagnosis when performing a workup for vague pulmonary and abdominal symptoms as described here. Disagreement exists over the method of repair and management of the hernia sac as well as the need for mesh buttressing of the defect. A well-planned surgical approach individualized to the patient’s anatomy, surgeon’s expertise, and hernia defect size will provide the best possible outcome with a low operative morbidity.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Congenital diaphragmatic hernias (CDHs) occur from a disruption in the muscular formation of the diaphragm, resulting in herniation of abdominal contents into the thoracic cavity. A rare diagnosis, most cases are identified in the pediatric and neonatal populations with an overall historical 50% mortality related to the diagnosis.1 More recent data published in the U.S. and Japan cite an overall survival rate of 67% to 80% secondary to improved understanding of the pathophysiology and subsequent enhancement of neonatal cardiopulmonary support adjuncts.2,3
Bochladek hernias (posterolateral space) are the most common presentation of CDH, accounting for > 90% of cases. First described by the Giovanni Batista Morgagni in On the Seats and Causes of Disease Investigated by Anatomy, the anteromedial sternocostal location is far less common and accounts for only 2% to 3% of cases.4,5 More commonly found on the right side of the diaphragm, despite protection from the liver, the right-sided space has been traditionally referred to as the Morgagni space. A left-sided defect is occasionally called the Larrey gap or space, after Napoleon’s surgeon who described the space as a potential location for pericardial drainage of tamponade.6,7
Related: Colonoscopy Bowel Preparation Instructions
There are a few congenital conditions, such as trisomy 21, Turner syndrome, Prader Willi syndrome, dextrocardia, and Tetralogy of Fallot, that have been associated with Morgagni hernias.7 Pulmonary hypertension and respiratory distress are the most common symptoms for neonatal patients; chest pain, sensations of tightness/fullness, reflux, and transient obstructive symptoms constitute the typical symptoms of adult patients with CDH. In this case study, the authors present a case of adult-onset Morgagni hernia as well as a review of the relevant literature.
Case Report
The patient was a 48-year-old man on active-duty who presented to the Naval Medical Center Portsmouth General Surgery clinic in Virginia with a 4-year history of gastroesophageal reflux-related symptoms. Specifically, he reported epigastric fullness, pyrosis, and discomfort that radiated toward his bilateral lower ribs for the previous 4 years. This discomfort was typically associated with the intake of solid food and was followed a few hours later by a loose bowel movement.
The patient was initially treated with antacids and proton pump inhibitors by his primary care physician, with only minimal relief. He also reported several months of chronic cough as well as intermittent episodes of “gasping air hunger” for about 6 years, which had been incidentally brought up during his separation physical examination. A chest X-ray performed during the workup revealed findings suggesting a right diaphragmatic hernia vs a bronchogenic cyst (Figure 1). A computed tomography (CT) of the thorax demonstrated a 3 x 8-cm hernia through the foramen of Morgagni containing a portion of the transverse colon along with intraperitoneal fat (Figures 2 and 3).
The patient underwent repair of this right Morgagni hernia via a laparoscopic approach. Intraoperative findings confirmed preoperative radiologic studies demonstrating colonic and omental contents within an easily reducible hernia sac (Figures 4 and 5). The hernia sac was left in vivo, and a combined direct hernia repair with mesh reinforcement was performed using Surgimesh XB (BG Medical, Barrington, IL) (Figure 6). The patient remained in the hospital for overnight observation and was discharged on postoperative day 1. The patient has since been seen in follow-up and is doing quite well with complete resolution of his reflux and pulmonary symptoms.
Discussion
A recent review of surgical literature revealed that over a 57-year period, 298 cases of Morgagni hernias have been described in adults.7 Although previous studies have postulated that a majority of adult patients are asymptomatic, more recent retrospective studies have found about a 70% symptomatic rate of patients with Morgagni hernias.7 The natural history of adult presentations lends itself to pulmonary (most common) or chronic upper gastrointestinal symptoms, although an acute presentation with potential volvulus and strangulation of the herniated contents has been described.7
Diagnosis is typically confirmed with a chest X-ray, although the CT scan has become more popular in the era of multimodal imaging.4,7 Multiple methods of repair have been described; however, thoracotomy has been the most widely used approach, and laparoscopy has gained popularity since the early 1990s.7 Mesh has been described in more than 60% of cases, and a laparoscopic repair has proven to have a low (< 5%) complication rate and short hospital stay.8,9 In particular, it has been suggested that a hernia defect larger than 20 to 30 cm2 should be repaired with a prosthetic adjunct, such as polypropylene, polytetrafluoroethylene, and bovine pericardium with a 1.5- to 2.5-cm mesh overlap.7,8
Related: Unusual Congenital Pulmonary Anomaly in an Adult Patient With Dyspnea
There is some controversy about the management of the hernia sac, with about 69% of surgeons choosing not to excise the sac due to concerns of intrathoracic or pericardial injury.7 In a separate study, 36 patients were evaluated retrospectively, and the hernia sac was not resected in any of the patients, with long-term follow-up revealing no evidence of recurrence.6
Conclusion
To allow for early intervention and avoidance of potentially life-threatening volvulus/strangulation, the medical practitioner has to be aware of this rare diagnosis when performing a workup for vague pulmonary and abdominal symptoms as described here. Disagreement exists over the method of repair and management of the hernia sac as well as the need for mesh buttressing of the defect. A well-planned surgical approach individualized to the patient’s anatomy, surgeon’s expertise, and hernia defect size will provide the best possible outcome with a low operative morbidity.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Holcomb GW, Murphy JP. Ashcraft’s Pediatric Surgery. 5th ed. Kansas City: Saunders Elsevier; 2010: 319-320.
2. Haroon, J, Chamberlain RS. An evidence-based review of the current treatment of congenital diaphragmatic hernia. Clin Pediatr (Phila). 2013;52(2):115-124.
3. Nagata K, Usui N, Kanamori Y, et al. The current profile and outcome of congenital diaphragmatic hernia: a nationwide survey in Japan. J Pediatr Surg. 2013;48(4):738-744.
4. Abraham V, Myla Y, Verghese S, Chandran BS. Morgagni-larrey hernia—a review of 20 cases. Indian J Surg. 2012;74(5):391-395.
5. Arora S, Haji A, Ng P. Adult Morgagni hernia: the need for clinical awareness, early diagnosis, and prompt surgical intervention. Ann R Coll Surg Engl. 2008;90(8):694-695.
6. Aghajanzadeh M, Khadem S, Khajeh Jahromi S, Gorabi HE, Ebrahimi H, Maafi AA. Clinical presentation and operative repair of Morgagni hernia. Interact Cardiovasc Thorac Surg. 2012;15(4):608-611.
7. Horton JD, Hofmann LJ, Hetz SP. Presentation and management of Morgagni hernias in adults: a review of 298 cases. Surg Endosc. 2008;22(6):1413-1420.
8. Terrosu G, Brizzolari M, Intini S, Cattin F, Bresadola V, De Anna D. Morgagni hernia: technical variation in the laparoscopic treatment. Ann Ital Chir. 2012;83(5):415-420.
9. Durak E, Gur S, Cokmez A, Atahan K, Zahtz E, Tarcan E. Laparoscopic repair of Morgagni hernia. Hernia. 2007;11(3):265-270.
1. Holcomb GW, Murphy JP. Ashcraft’s Pediatric Surgery. 5th ed. Kansas City: Saunders Elsevier; 2010: 319-320.
2. Haroon, J, Chamberlain RS. An evidence-based review of the current treatment of congenital diaphragmatic hernia. Clin Pediatr (Phila). 2013;52(2):115-124.
3. Nagata K, Usui N, Kanamori Y, et al. The current profile and outcome of congenital diaphragmatic hernia: a nationwide survey in Japan. J Pediatr Surg. 2013;48(4):738-744.
4. Abraham V, Myla Y, Verghese S, Chandran BS. Morgagni-larrey hernia—a review of 20 cases. Indian J Surg. 2012;74(5):391-395.
5. Arora S, Haji A, Ng P. Adult Morgagni hernia: the need for clinical awareness, early diagnosis, and prompt surgical intervention. Ann R Coll Surg Engl. 2008;90(8):694-695.
6. Aghajanzadeh M, Khadem S, Khajeh Jahromi S, Gorabi HE, Ebrahimi H, Maafi AA. Clinical presentation and operative repair of Morgagni hernia. Interact Cardiovasc Thorac Surg. 2012;15(4):608-611.
7. Horton JD, Hofmann LJ, Hetz SP. Presentation and management of Morgagni hernias in adults: a review of 298 cases. Surg Endosc. 2008;22(6):1413-1420.
8. Terrosu G, Brizzolari M, Intini S, Cattin F, Bresadola V, De Anna D. Morgagni hernia: technical variation in the laparoscopic treatment. Ann Ital Chir. 2012;83(5):415-420.
9. Durak E, Gur S, Cokmez A, Atahan K, Zahtz E, Tarcan E. Laparoscopic repair of Morgagni hernia. Hernia. 2007;11(3):265-270.