User login
Onychomatricoma: An Often Misdiagnosed Tumor of the Nails
Changes in the appearance of the nail apparatus can be produced by a variety of conditions. Onychomatricoma is an unusual benign tumor with specific clinical characteristics that was first described more than 2 decades ago.1 It is often and easily misdiagnosed because the condition rarely has been described. We report a case of onychomatricoma in a 54-year-old Colombian man who presented with a deformity of the nail plate on the right index finger that corresponded with the classic description of onychomatricoma. We emphasize the importance of reporting such lesions to prevent misdiagnosis and delay of proper treatment.
Case Report
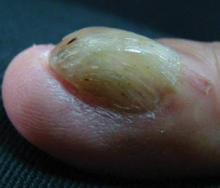
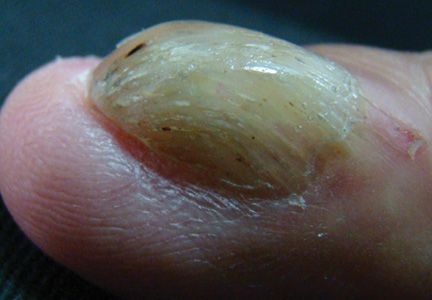
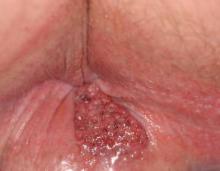
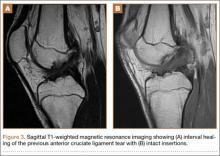
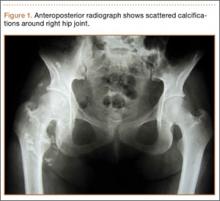
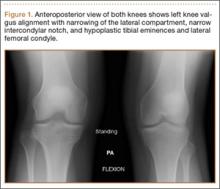
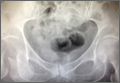
A 54-year-old Colombian man presented with nail dystrophy involving the right index finger of 2 years’ duration. He did not recall any trauma prior to the onset of the nail abnormalities. Several topical treatments had previously been ineffective. Physical examination revealed a longitudinally banded thickening of the lateral half of the nail plate on the right index finger with yellowish brown discoloration, transverse overcurvature of the nail, longitudinal white lines, and splinter hemorrhages (Figure 1). Direct microscopy and fungal culture were performed to diagnose or rule out onychomycosis.
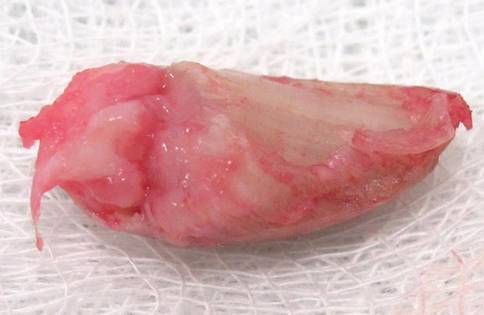
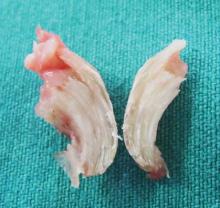
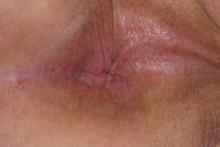
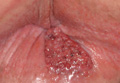
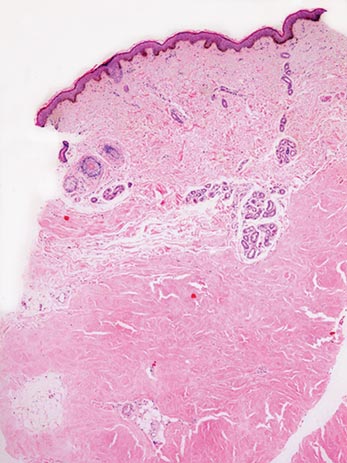
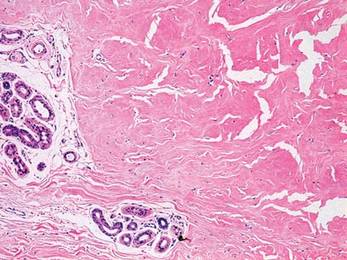
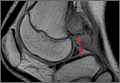
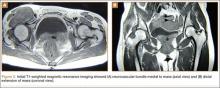
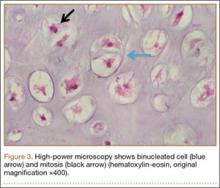
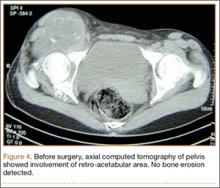
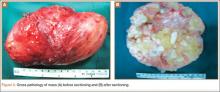
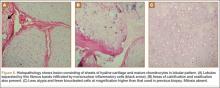
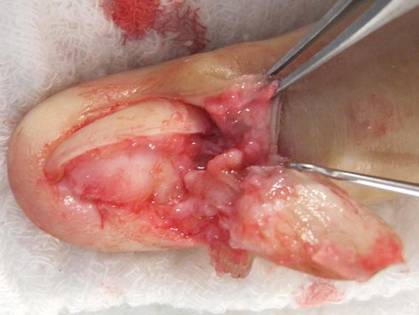
A clinical diagnosis of onychomatricoma was made, and the lesion was surgically removed and sent for histopathologic study (Figure 2). The radial half of the nail plate was avulsed, and the proximal part of the removed nail plate contained a large, firmly attached, filamentous tumor arising from the nail matrix (Figure 3) with multiple fine filiform projections (Figure 4). The nail bed was cleaned with a curette to remove any debris, the ulnar half of the nail plate and nail bed was left in place, and the radial border was reconstructed. Histology confirmed the clinical diagnosis (Figure 5). No recurrences of the tumor were seen 36 months following surgery.
|  |
Comment
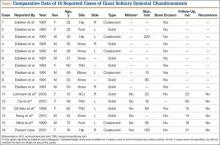
Since the original report of this tumor,1 fewer than 10 cases of onychomatricoma have been reported in Latin America,2-5 with no more than 80 cases reported worldwide.6 Clinicians and academicians are becoming interested in the topic, which will result in better recognition and more reports in the literature.
The clinical differential diagnosis of onycho-matricoma is extensive,7,8 but onychomatricoma has characteristic clinical and histopathologic features that allow its separation from other nail disorders and subungual tumors (Table).9 There are 4 cardinal clinical signs that suggest a diagnosis of onychomatricoma: (1) banded or diffuse thickening of the nail plate of variable widths; (2) yellowish discoloration of the involved nail plate, often showing fine splinter hemorrhages in the proximal nail portion; (3) transverse overcurvature of the nail; and (4) exposure of a filamentous tufted tumor emerging from the matrix in a funnel-shaped nail by avulsion.1
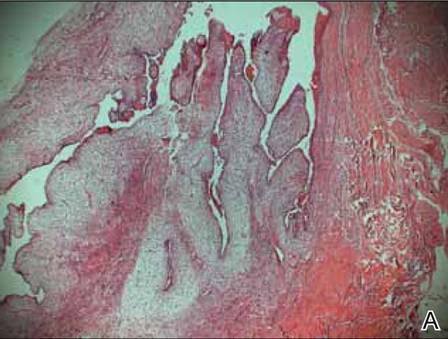 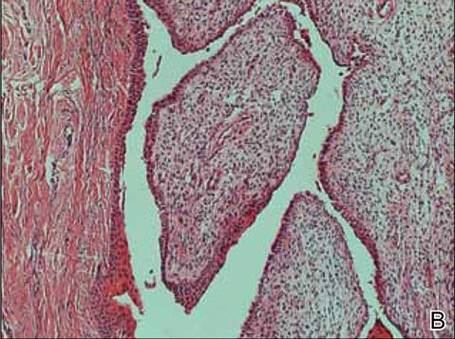 |
Histologic findings of onychomatricoma typically demonstrate a fibroepithelial tumor with a biphasic growth pattern mimicking normal nail matrix histology, including a proximal zone, which corresponds to the base of the fibroepithelial tumor, and a distal zone, which is composed of multiple epithelial digitations that extend into the small cavities present in the attached nail.10-12 Nevertheless, the anatomic tumor location, the often fragmented aspect of the tissue specimen, and the choice of the section planes may change the typical histologic features seen in onychomatricoma.13 Stromal prominence, cellularity, and atypia may vary in individual cases.10-12
The etiology of onychomatricoma is not yet known. Although it has been suggested that onychomatricoma could be an epithelial and connective tissue hamartoma simulating the nail matrix structure,1,10 the more recent concept of an epithelial onychogenic tumor with onychogenic mesenchyme will help to clarify its etiology because new histopathologic and immunohistochemical features suggest a neoplastic nature.14 On the other hand, predisposing factors such as trauma to the nail plate and onychomycosis may play a role,7 as the thumbs, index fingers, and great toes are more susceptible to onychomycosis and accidental trauma.
Conclusion
Our patient fulfilled the criteria of onychomatricoma.1 Onychomatricoma should be kept in mind in the differential diagnosis of subungual or periungual tumors to avoid misdiagnosis and erroneous treatments.
1. Baran R, Kint A. Onychomatrixoma: filamentous tufted tumor in the matrix of a funnel-shaped nail: a new entity (report of three cases). Br J Dermatol. 1992;126:510-515.
2. Estrada-Chavez G, Vega-Memije ME, Toussaint-Caire S, et al. Giant onychomatricoma: report of two cases with rare clinical presentation. Int J Dermatol. 2007;46: 634-636.
3. Soto R, Wortsman X, Corredoira Y. Onychomatricoma: clinical and sonographic findings. Arch Dermatol. 2009;145:1461-1462.
4. Tavares GT, Chiacchio NG, Chiacchio ND, et al. Onychomatricoma: a tumor unknown to dermatologists. An Bras Dermatol. 2015;90:265-267.
5. Fernández-Sánchez M, Saeb-Lima M, Charli-Joseph Y, et al. Onychomatricoma: an infrequent nail tumor. Indian J Dermatol Venereol Leprol. 2012;78:382-383.
6. Tavares G, Di-Chiacchio N, Di-Santis E, et al. Onycho-matricoma: epidemiological and clinical findings in a large series of 30 cases [published online ahead of print May 12, 2015]. Br J Dermatol. doi:10.1111/bjd.13900.
7. Rashid RM, Swan J. Onychomatricoma: benign sporadic nail lesion or much more? Dermatol Online J. 2006;12:4.
8. Goutos I, Furniss D, Smith GD. Onychomatricoma: an unusual case of ungual pathology. case report and review of the literature. J Plast Reconstr Aesthet Surg. 2010;63:54-57.
9. Fraga GR, Patterson JW, McHargue CA. Onychomatricoma: report of a case and its comparison with fibrokeratoma of the nailbed. Am J Dermatopathol. 2001;23:36-40.
10. Perrin C, Goettmann S, Baran R. Onychomatricoma: clinical and histopathologic findings in 12 cases. J Am Acad Dermatol. 1998;39:560-564.
11. Gaertner EM, Gordon M, Reed T. Onychomatricoma: case report of an unusual subungual tumor with literature review. J Cutan Pathol. 2009;36(suppl 1):S66-S69.
12. Perrin C, Baran R, Pisani A, et al. The onychomatricoma: additional histologic criteria and immunohistochemical study. Am J Dermatopathol. 2002;24:199-203.
13. Perrin C, Baran R, Balaguer T, et al. Onychomatricoma: new clinical and histological features. a review of 19 tumors. Am J Dermatopathol. 2010;32:1-8.
14. Perrin C, Langbein L, Schweizer J, et al. Onychomatricoma in the light of the microanatomy of the normal nail unit. Am J Dermatopathol. 2011;33:131-139.
Changes in the appearance of the nail apparatus can be produced by a variety of conditions. Onychomatricoma is an unusual benign tumor with specific clinical characteristics that was first described more than 2 decades ago.1 It is often and easily misdiagnosed because the condition rarely has been described. We report a case of onychomatricoma in a 54-year-old Colombian man who presented with a deformity of the nail plate on the right index finger that corresponded with the classic description of onychomatricoma. We emphasize the importance of reporting such lesions to prevent misdiagnosis and delay of proper treatment.
Case Report
A 54-year-old Colombian man presented with nail dystrophy involving the right index finger of 2 years’ duration. He did not recall any trauma prior to the onset of the nail abnormalities. Several topical treatments had previously been ineffective. Physical examination revealed a longitudinally banded thickening of the lateral half of the nail plate on the right index finger with yellowish brown discoloration, transverse overcurvature of the nail, longitudinal white lines, and splinter hemorrhages (Figure 1). Direct microscopy and fungal culture were performed to diagnose or rule out onychomycosis.
A clinical diagnosis of onychomatricoma was made, and the lesion was surgically removed and sent for histopathologic study (Figure 2). The radial half of the nail plate was avulsed, and the proximal part of the removed nail plate contained a large, firmly attached, filamentous tumor arising from the nail matrix (Figure 3) with multiple fine filiform projections (Figure 4). The nail bed was cleaned with a curette to remove any debris, the ulnar half of the nail plate and nail bed was left in place, and the radial border was reconstructed. Histology confirmed the clinical diagnosis (Figure 5). No recurrences of the tumor were seen 36 months following surgery.
|  |
Comment
Since the original report of this tumor,1 fewer than 10 cases of onychomatricoma have been reported in Latin America,2-5 with no more than 80 cases reported worldwide.6 Clinicians and academicians are becoming interested in the topic, which will result in better recognition and more reports in the literature.
The clinical differential diagnosis of onycho-matricoma is extensive,7,8 but onychomatricoma has characteristic clinical and histopathologic features that allow its separation from other nail disorders and subungual tumors (Table).9 There are 4 cardinal clinical signs that suggest a diagnosis of onychomatricoma: (1) banded or diffuse thickening of the nail plate of variable widths; (2) yellowish discoloration of the involved nail plate, often showing fine splinter hemorrhages in the proximal nail portion; (3) transverse overcurvature of the nail; and (4) exposure of a filamentous tufted tumor emerging from the matrix in a funnel-shaped nail by avulsion.1
  |
Histologic findings of onychomatricoma typically demonstrate a fibroepithelial tumor with a biphasic growth pattern mimicking normal nail matrix histology, including a proximal zone, which corresponds to the base of the fibroepithelial tumor, and a distal zone, which is composed of multiple epithelial digitations that extend into the small cavities present in the attached nail.10-12 Nevertheless, the anatomic tumor location, the often fragmented aspect of the tissue specimen, and the choice of the section planes may change the typical histologic features seen in onychomatricoma.13 Stromal prominence, cellularity, and atypia may vary in individual cases.10-12
The etiology of onychomatricoma is not yet known. Although it has been suggested that onychomatricoma could be an epithelial and connective tissue hamartoma simulating the nail matrix structure,1,10 the more recent concept of an epithelial onychogenic tumor with onychogenic mesenchyme will help to clarify its etiology because new histopathologic and immunohistochemical features suggest a neoplastic nature.14 On the other hand, predisposing factors such as trauma to the nail plate and onychomycosis may play a role,7 as the thumbs, index fingers, and great toes are more susceptible to onychomycosis and accidental trauma.
Conclusion
Our patient fulfilled the criteria of onychomatricoma.1 Onychomatricoma should be kept in mind in the differential diagnosis of subungual or periungual tumors to avoid misdiagnosis and erroneous treatments.
Changes in the appearance of the nail apparatus can be produced by a variety of conditions. Onychomatricoma is an unusual benign tumor with specific clinical characteristics that was first described more than 2 decades ago.1 It is often and easily misdiagnosed because the condition rarely has been described. We report a case of onychomatricoma in a 54-year-old Colombian man who presented with a deformity of the nail plate on the right index finger that corresponded with the classic description of onychomatricoma. We emphasize the importance of reporting such lesions to prevent misdiagnosis and delay of proper treatment.
Case Report
A 54-year-old Colombian man presented with nail dystrophy involving the right index finger of 2 years’ duration. He did not recall any trauma prior to the onset of the nail abnormalities. Several topical treatments had previously been ineffective. Physical examination revealed a longitudinally banded thickening of the lateral half of the nail plate on the right index finger with yellowish brown discoloration, transverse overcurvature of the nail, longitudinal white lines, and splinter hemorrhages (Figure 1). Direct microscopy and fungal culture were performed to diagnose or rule out onychomycosis.
A clinical diagnosis of onychomatricoma was made, and the lesion was surgically removed and sent for histopathologic study (Figure 2). The radial half of the nail plate was avulsed, and the proximal part of the removed nail plate contained a large, firmly attached, filamentous tumor arising from the nail matrix (Figure 3) with multiple fine filiform projections (Figure 4). The nail bed was cleaned with a curette to remove any debris, the ulnar half of the nail plate and nail bed was left in place, and the radial border was reconstructed. Histology confirmed the clinical diagnosis (Figure 5). No recurrences of the tumor were seen 36 months following surgery.
|  |
Comment
Since the original report of this tumor,1 fewer than 10 cases of onychomatricoma have been reported in Latin America,2-5 with no more than 80 cases reported worldwide.6 Clinicians and academicians are becoming interested in the topic, which will result in better recognition and more reports in the literature.
The clinical differential diagnosis of onycho-matricoma is extensive,7,8 but onychomatricoma has characteristic clinical and histopathologic features that allow its separation from other nail disorders and subungual tumors (Table).9 There are 4 cardinal clinical signs that suggest a diagnosis of onychomatricoma: (1) banded or diffuse thickening of the nail plate of variable widths; (2) yellowish discoloration of the involved nail plate, often showing fine splinter hemorrhages in the proximal nail portion; (3) transverse overcurvature of the nail; and (4) exposure of a filamentous tufted tumor emerging from the matrix in a funnel-shaped nail by avulsion.1
  |
Histologic findings of onychomatricoma typically demonstrate a fibroepithelial tumor with a biphasic growth pattern mimicking normal nail matrix histology, including a proximal zone, which corresponds to the base of the fibroepithelial tumor, and a distal zone, which is composed of multiple epithelial digitations that extend into the small cavities present in the attached nail.10-12 Nevertheless, the anatomic tumor location, the often fragmented aspect of the tissue specimen, and the choice of the section planes may change the typical histologic features seen in onychomatricoma.13 Stromal prominence, cellularity, and atypia may vary in individual cases.10-12
The etiology of onychomatricoma is not yet known. Although it has been suggested that onychomatricoma could be an epithelial and connective tissue hamartoma simulating the nail matrix structure,1,10 the more recent concept of an epithelial onychogenic tumor with onychogenic mesenchyme will help to clarify its etiology because new histopathologic and immunohistochemical features suggest a neoplastic nature.14 On the other hand, predisposing factors such as trauma to the nail plate and onychomycosis may play a role,7 as the thumbs, index fingers, and great toes are more susceptible to onychomycosis and accidental trauma.
Conclusion
Our patient fulfilled the criteria of onychomatricoma.1 Onychomatricoma should be kept in mind in the differential diagnosis of subungual or periungual tumors to avoid misdiagnosis and erroneous treatments.
1. Baran R, Kint A. Onychomatrixoma: filamentous tufted tumor in the matrix of a funnel-shaped nail: a new entity (report of three cases). Br J Dermatol. 1992;126:510-515.
2. Estrada-Chavez G, Vega-Memije ME, Toussaint-Caire S, et al. Giant onychomatricoma: report of two cases with rare clinical presentation. Int J Dermatol. 2007;46: 634-636.
3. Soto R, Wortsman X, Corredoira Y. Onychomatricoma: clinical and sonographic findings. Arch Dermatol. 2009;145:1461-1462.
4. Tavares GT, Chiacchio NG, Chiacchio ND, et al. Onychomatricoma: a tumor unknown to dermatologists. An Bras Dermatol. 2015;90:265-267.
5. Fernández-Sánchez M, Saeb-Lima M, Charli-Joseph Y, et al. Onychomatricoma: an infrequent nail tumor. Indian J Dermatol Venereol Leprol. 2012;78:382-383.
6. Tavares G, Di-Chiacchio N, Di-Santis E, et al. Onycho-matricoma: epidemiological and clinical findings in a large series of 30 cases [published online ahead of print May 12, 2015]. Br J Dermatol. doi:10.1111/bjd.13900.
7. Rashid RM, Swan J. Onychomatricoma: benign sporadic nail lesion or much more? Dermatol Online J. 2006;12:4.
8. Goutos I, Furniss D, Smith GD. Onychomatricoma: an unusual case of ungual pathology. case report and review of the literature. J Plast Reconstr Aesthet Surg. 2010;63:54-57.
9. Fraga GR, Patterson JW, McHargue CA. Onychomatricoma: report of a case and its comparison with fibrokeratoma of the nailbed. Am J Dermatopathol. 2001;23:36-40.
10. Perrin C, Goettmann S, Baran R. Onychomatricoma: clinical and histopathologic findings in 12 cases. J Am Acad Dermatol. 1998;39:560-564.
11. Gaertner EM, Gordon M, Reed T. Onychomatricoma: case report of an unusual subungual tumor with literature review. J Cutan Pathol. 2009;36(suppl 1):S66-S69.
12. Perrin C, Baran R, Pisani A, et al. The onychomatricoma: additional histologic criteria and immunohistochemical study. Am J Dermatopathol. 2002;24:199-203.
13. Perrin C, Baran R, Balaguer T, et al. Onychomatricoma: new clinical and histological features. a review of 19 tumors. Am J Dermatopathol. 2010;32:1-8.
14. Perrin C, Langbein L, Schweizer J, et al. Onychomatricoma in the light of the microanatomy of the normal nail unit. Am J Dermatopathol. 2011;33:131-139.
1. Baran R, Kint A. Onychomatrixoma: filamentous tufted tumor in the matrix of a funnel-shaped nail: a new entity (report of three cases). Br J Dermatol. 1992;126:510-515.
2. Estrada-Chavez G, Vega-Memije ME, Toussaint-Caire S, et al. Giant onychomatricoma: report of two cases with rare clinical presentation. Int J Dermatol. 2007;46: 634-636.
3. Soto R, Wortsman X, Corredoira Y. Onychomatricoma: clinical and sonographic findings. Arch Dermatol. 2009;145:1461-1462.
4. Tavares GT, Chiacchio NG, Chiacchio ND, et al. Onychomatricoma: a tumor unknown to dermatologists. An Bras Dermatol. 2015;90:265-267.
5. Fernández-Sánchez M, Saeb-Lima M, Charli-Joseph Y, et al. Onychomatricoma: an infrequent nail tumor. Indian J Dermatol Venereol Leprol. 2012;78:382-383.
6. Tavares G, Di-Chiacchio N, Di-Santis E, et al. Onycho-matricoma: epidemiological and clinical findings in a large series of 30 cases [published online ahead of print May 12, 2015]. Br J Dermatol. doi:10.1111/bjd.13900.
7. Rashid RM, Swan J. Onychomatricoma: benign sporadic nail lesion or much more? Dermatol Online J. 2006;12:4.
8. Goutos I, Furniss D, Smith GD. Onychomatricoma: an unusual case of ungual pathology. case report and review of the literature. J Plast Reconstr Aesthet Surg. 2010;63:54-57.
9. Fraga GR, Patterson JW, McHargue CA. Onychomatricoma: report of a case and its comparison with fibrokeratoma of the nailbed. Am J Dermatopathol. 2001;23:36-40.
10. Perrin C, Goettmann S, Baran R. Onychomatricoma: clinical and histopathologic findings in 12 cases. J Am Acad Dermatol. 1998;39:560-564.
11. Gaertner EM, Gordon M, Reed T. Onychomatricoma: case report of an unusual subungual tumor with literature review. J Cutan Pathol. 2009;36(suppl 1):S66-S69.
12. Perrin C, Baran R, Pisani A, et al. The onychomatricoma: additional histologic criteria and immunohistochemical study. Am J Dermatopathol. 2002;24:199-203.
13. Perrin C, Baran R, Balaguer T, et al. Onychomatricoma: new clinical and histological features. a review of 19 tumors. Am J Dermatopathol. 2010;32:1-8.
14. Perrin C, Langbein L, Schweizer J, et al. Onychomatricoma in the light of the microanatomy of the normal nail unit. Am J Dermatopathol. 2011;33:131-139.
Practice Points
- Onychomatricoma has been described mostly in white individuals, but it can occur in all races and ethnic groups.
- Onychomatricoma should be kept in mind in the differential diagnosis of subungual or periungual tumors.
- Treatment of onychomatricoma is complete surgical excision.
Epidermodysplasia Verruciformis: Successful Treatment With Squaric Acid Dibutylester
Epidermodysplasia verruciformis (EV) is an uncommon autosomal-recessive inherited disorder characterized by disseminated cutaneous warts in predisposed patients who are highly susceptible to genus â-papillomavirus infections. Squaric acid dibutylester (SADBE) is a contact sensitizer agent that has gained general acceptance over the years for the treatment of a variety of dermatologic diseases, including alopecia areata and cutaneous warts. We report the case of a 40-year-old woman with a balanced chromosomal translocation and lymphocytopenia who presented with the sole clinical finding of refractory multiple flat warts that had been present for 25 years. After failed attempts at therapy with oral isotretinoin, cryotherapy with topical trichloroacetic acid, and topical tretinoin, the lesions were successfully eradicated with topical SADBE with prior sensitization.
Case Report
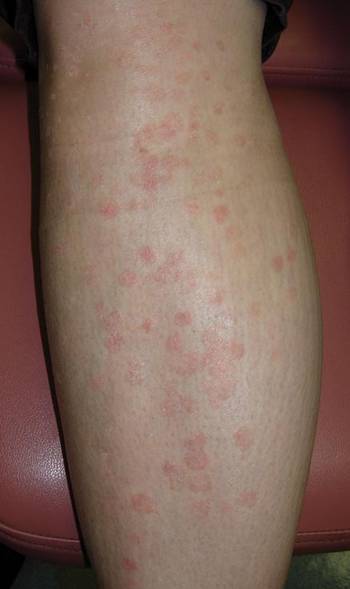
A 40-year-old woman presented with multiple flat warts on the bilateral arms and legs of 25 years’ duration (Figure 1) that had been unsuccessfully treated by an outside physician with imiquimod cream 5% and tazarotene gel 0.1%. Her medical history was remarkable for recurrent upper respiratory tract infections, urinary tract infections, yeast infections, and otitis media. She also reported a history of 6 spontaneous miscarriages that had been attributed to a balanced chromosomal translocation between chromosomes 12 and 14.
  |
Laboratory evaluation revealed leukopenia, lymphopenia, and hypogammaglobulinemia, with a white blood cell count of 3600/μL (reference range, 4500–11,000/mL), a lymphocyte count of 12.1% (20%–45%), absolute CD4 count of 77 cells/μL (490–1740 cells/μL), absolute CD8 count of 56 cells/mL (180–1170 cells/μL), and serum IgM level of 17 mg/dL (48–271 mg/dL). Human immunodeficiency virus (HIV) titers were negative.
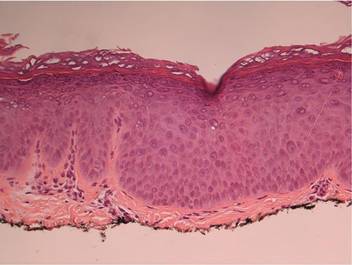
On physical examination numerous pink, flat-topped papules were noted on the forehead and bilateral arms and legs. Histologic analysis of a tangential plane biopsy of a lesion on the right leg revealed hyperkeratosis of the stratum corneum and epidermal hyperplasia (Figure 2). The epidermis also showed focal papillomatosis with areas of hypergranulosis and viropathic changes; these findings were consistent with a diagnosis of verruca plana. Human papillomavirus (HPV) DNA typing by polymerase chain reaction from the verrucous lesions showed HPV type 20, which has been associated with EV. Based on the patient’s clinical findings and HPV subtype, she was diagnosed with atypical EV.
Subsequent treatment with liquid nitrogen, tretinoin cream 0.1%, and topical trichloroacetic acid 50% failed. She received oral isotretinoin at a dosage of 80 mg daily for 9 months, but the lesions persisted and she developed alopecia and ankle stiffness; therefore, the isotretinoin was discontinued. Candida antigen testing revealed that the patient was anergic, and SADBE sensitization was subsequently initiated. Squaric acid dibutylester was utilized as a sensitizing agent, and it was formulated as 2% and 0.2% solutions in acetone, supplied in 20-mL tinted glass bottles.
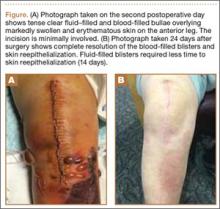
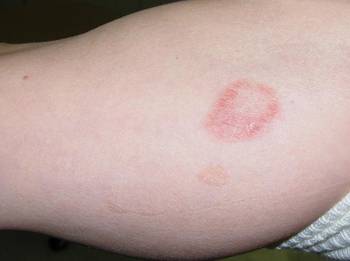
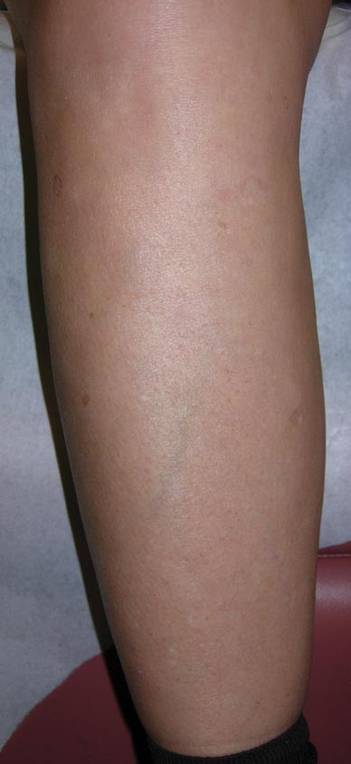
Squaric acid dibutylester solution 2% under occlusion was applied to a test area on the right forearm. Three days later, results indicated prominent erythema and inflammation at the application site. Two weeks later, a chronic dermatitic response was noted at the test site (Figure 3). Squaric acid dibutylester 0.2% was then applied to an affected area on the right shin and was kept under occlusion for 48 hours. One month later, no notable changes in the lesions were observed, and no further treatments were performed. Three months later, the patient returned for evaluation and it was noted that the flat warts on the right shin that had been treated with SADBE 0.2% 4 months prior had resolved (Figure 4). Subsequently, it was noted that all of the lesions had regressed, even those that had not been treated with SADBE.
Comment
Epidermodysplasia verruciformis is a rare genodermatosis caused by a group of phylogenetically related viruses1 belonging to the β-papillomavirus genus.2,3 It is characterized by a combination of pityriasis versicolor–like lesions, reddish verrucalike plaques, and seborrheic keratosis–like plaques,1,4 preferentially on sun-exposed areas.5 The lesions undergo malignant transformation in 30% to 60% of patients,3,6 especially into squamous cell carcinomas.7 The most frequent HPV types found in EV skin lesions are 5, 8, 9, 12, 14, 15, 17, and 19 to 25; types 5 and 8 are found in 90% of cutaneous squamous cell carcinomas in EV patients.2 Human papillomavirus type 20, the type identified in our patient, has been isolated from warts in EV patients,1,2 though it is not the most common type. It has been shown that more than one HPV type could be present concurrently in the same EV patient,1 which necessitates close follow-up for skin cancer evaluation in all EV patients, as oncogenic strains may be present in some lesions.
  |
Epidermodysplasia verruciformis has no particular predisposition for race or geographic location.1,7 It usually is inherited in an autosomal-recessive fashion1,4,7 and has been linked to mutations in 2 EV genes located on chromosome 17: EVER1/TCM6 and EVER2/TCM8.8 However, approximately 25% of EV cases are not associated with these gene mutations,5,9 as demonstrated in our patient. Autosomal-dominant or X-linked mutations also have been reported.10 In our case, a chromosomal abnormality in the form of a balanced chromosomal translocation was present, which is unique. A connection between EV and balanced chromosomal translocation cannot be excluded and warrants further investigation.
Epidermodysplasia verruciformis has been associated with decreased cell-mediated immunity.1,7 However, nonimmunologic factors likely contribute considering the rarity of EV-like eruptions in immunodeficiency disorders11 as well as its frequent coinfection with HPV type 312 and its association with EVER1/TCM6 and EVER2/TCM8.8 Epidermodysplasia verruciformis–like lesions have been reported in several immunosuppressed states, including HIV infection,13 combined variable immunodeficiency syndrome,14 IgM deficiency,15 and CD4+ T-cell lymphocytopenia.11 Our patient’s findings fit the latter diagnostic criteria, as she had a chronically low CD4 count of 77 cells/μL, negative HIV titers, and absence of alternative explanation to the lymphopenia. Thus, we could consider her as having EV, as a low CD4 count is a known association. Her immunodeficient state could possibly be attributed to the chromosomal translocation; however, the genetic loci surrounding the chromosomal translocation have not been identified to date, leaving this hypothesis unsubstantiated. Nevertheless, in our otherwise healthy patient, no explanation was found as to why a cell-mediated deficiency would selectively favor a cutaneous HPV infection. According to Zavattaro et al,5 a possible cause could be the presence of additional genetic or environmental factors in the patient that predisposed her to this particular infection.
Every patient with EV requires close lifelong observation for skin cancer and education regarding strict sun avoidance and protection.1 Treatment options for the lesions include topical therapies with imiquimod 5%, immunomodulators, and salicylic acid16,17; oral isotretinoin18; and combinations of acitretin and interferon alfa.19 Physical ablative procedures also have been proposed, including cryotherapy with liquid nitrogen, electrosurgery, surgical excision, and laser therapies.20
Topical immunotherapy with SADBE initially was used to treat refractory alopecia areata and also has been described in the treatment of recalcitrant warts.21-24 Historically, 2,4-dinitrochlorobenzene was used for contact immunotherapy in wart management but is now avoided due to its mutagenic potential.25 Squaric acid dibutylester and diphenylcyclopropenone currently are the favored contact sensitizers, with a resolution rate of 60% reported in refractory warts.26
Topical immunotherapy involves sensitization of the patient with high-concentration (2%) SADBE on a small surface area until an eczematous dermatitis appears. The rash indicates sensitization has been achieved, and then a lower-concentration SADBE is applied to the warts. Observation of mild contact dermatitis should not be an indication to stop treatment, as this effect is an integral part of therapeutic response. No serious side effects were reported to SADBE; erythema, desquamation, edema, itching, and burning were described.23
The mechanism of action of SADBE is not clear. The most common proposed theory is the induction of a type IV hypersensitivity reaction in the warts, leading to their destruction. Other authors suggest that wart resolution is caused by a nonspecific inflammatory reaction. An argument in favor of the latter hypothesis is the spontaneous regression of untreated warts in patients treated with SADBE at a remote site, suggesting a mechanism of action beyond a simple cell-mediated process.23
Epidermodysplasia verruciformis should be included in the differential diagnosis for any eruptive, warty, papular, and plaque-type lesions that appear in immunocompromised individuals. Moreover, the diagnosis of idiopathic CD4+ T-cell lymphocytopenia should be considered in any patient with a CD4 count deficit presenting with widespread viral, fungal, or mycobacterial infection with negative HIV test. Appropriate evaluation of the absolute CD4+ counts also should be performed. In our case, it was hypothesized that the patient’s balanced chromosomal translocation was related to her lymphopenia and EV, though this correlation has yet to be confirmed. However, it is notable that her son carried the same translocation and has a normal white blood cell count and no evidence of flat warts. This case demonstrates the success of contact immunotherapy in treating these widespread and often recalcitrant lesions.
1. Vohra S, Sharma NL, Shanker V, et al. Autosomal dominant epidermodysplasia verruciformis: a clinicotherapeutic experience in two cases. Indian J Dermatol Venereol Leprol. 2010;76:557-561.
2. Dell’Oste V, Azzimonti B, De Andrea M, et al. High beta-HPV DNA loads and strong seroreactivity are present in epidermodysplasia verruciformis. J Invest Dermatol. 2009;129:1026-1034.
3. Orth G. Epidermodysplasia verruciformis: a model for understanding the oncogenicity of human papillomavirus. Ciba Found Symp. 1986;120:157-174.
4. Michael KM, Waterboer T, Pfister H, et al. Seroreactivity of 38 human papillomavirus types in epidermodysplasia verruciformis patients, relatives, and controls. J Invest Dermatol. 2010;130:841-848.
5. Zavattaro E, Azzimonti B, Mondini M, et al. Identification of defective Fas function and variation of the perforin gene in an epidermodysplasia verruciformis patient lacking EVER1 and EVER2 mutations. J Invest Dermatol. 2008;128:732-735.
6. Majewski S, Jabłońska S. Epidermodysplasia verruciformis as a model of human papillomavirus-induced genetic cancer of the skin. Arch Dermatol. 1995;131:1312-1318.
7. Robati RM, Marefat A, Saeedi M, et al. Four familial cases of epidermodysplasia verruciformis: mother and three sons. Dermatol Online J. 2009;15:8.
8. Ramoz N, Rueda LA, Bouadjar B, et al. Mutations in two adjacent novel genes are associated with epidermodysplasia verruciformis. Nat Genet. 2002;32:579-581.
9. Azzimonti B, Mondini M, De Andrea M, et al. CD8+ T-cell lymphocytopenia and lack of EVER mutations in a patient with clinically and virologically typical epidermodysplasia verruciformis. Arch Dermatol. 2005;141:1323-1325.
10. Androphy EJ, Dvoretzky I, Lowy DR. X-linked inheritance of epidermodysplasia verruciformis. genetic and virologic studies of a kindred. Arch Dermatol. 1985;121:864-868.
11. Tobin E, Rohwedder A, Holland SM, et al. Recurrent ‘sterile’ verrucous cyst abscesses and epidermodysplasia verruciformis-like eruption associated with idiopathic CD4 lymphopenia. Br J Dermatol. 2003;149:627-633.
12. Obalek S, Favre M, Szymanczyk J, et al. Human papillomavirus (HPV) types specific of epidermodysplasia verruciformis detected in warts induced by HPV3 or HPV3-related types in immunosuppressed patients. J Invest Dermatol. 1992;98:936-941.
13. Berk DR, Bruckner AL, Lu D. Epidermodysplasia verruciform-like lesions in an HIV patient. Dermatol Online J. 2009;15:1.
14. Vu J, Wallace GR, Singh R, et al. Common variable immunodeficiency syndrome associated with epidermodysplasia verruciformis. Am J Clin Dermatol. 2007;8:307-310.
15. Gul U, Soylu S, Yavuzer R. Epidermodysplasia verruciformis associated with IgM deficiency. Indian J Dermatol Venereol Leprol. 2007;73:420-422.
16. De Oliveira WR, Festa Neto C, Rady PL, et al. Clinical aspects of epidermodysplasia verruciformis. J Eur Acad Dermatol Venereol. 2003;17:394-398.
17. Jablonska S, Majewski S. Epidermodysplasia verruciformis: what’s new? J Eur Acad Dermatol Venereol. 2003;17:381-382.
18. Rallis E, Papatheodorou G, Bimpakis E, et al. Systemic low-dose isotretinoin maintains remission status in epidermodysplasia verruciformis. J Eur Acad Dermatol Venereol. 2008;22:523-525.
19. Anadolu R, Oskay T, Erdem C, et al. Treatment of epidermodysplasia verruciformis with a combination of acitretin and interferon alfa-2a. J Am Acad Dermatol. 2001;45:296-299.
20. Fang F, Zhao L, Jiang MJ, et al. Epidermodysplasia verruciformis with severe hand and foot deformity successfully treated with surgical excision. J Plast Reconstr Aesthet Surg. 2008;61:338-341.
21. Huang W, Morrell D. Successful treatment of recalcitrant warts with topical squaric acid in immunosuppressed child. Pediatr Dermatol. 2008;25:275-276.
22. Hama N, Hatamochi A, Hayashi S, et al. Usefulness of topical immunotherapy with squaric acid dibutylester for refractory common warts on the face and neck. J Dermatol. 2009;36:660-662.
23. Micali G, Nasca MR, Tedeschi A, et al. Use of squaric acid dibutylester (SADBE) for cutaneous warts in children. Pediatr Dermatol. 2000;17:315-318.
24. Mastrolonardo M, Lopalco PL, Diaferio A. Topical immunotherapy with contact sensitizers: a model to study the natural history of delayedhypersensitivity. Contact Dermatitis. 2002;47:210-214.
25. Lewis HM. Topical immunotherapy of refractory warts. Cutis. 1973;12:863-869.
26. Weisshaar E, Neumann HJ, Gollnick H. Successful treatment of disseminated facial verrucae with contact immunotherapy. Eur J Dermatol. 1998;8:488-491.
Epidermodysplasia verruciformis (EV) is an uncommon autosomal-recessive inherited disorder characterized by disseminated cutaneous warts in predisposed patients who are highly susceptible to genus â-papillomavirus infections. Squaric acid dibutylester (SADBE) is a contact sensitizer agent that has gained general acceptance over the years for the treatment of a variety of dermatologic diseases, including alopecia areata and cutaneous warts. We report the case of a 40-year-old woman with a balanced chromosomal translocation and lymphocytopenia who presented with the sole clinical finding of refractory multiple flat warts that had been present for 25 years. After failed attempts at therapy with oral isotretinoin, cryotherapy with topical trichloroacetic acid, and topical tretinoin, the lesions were successfully eradicated with topical SADBE with prior sensitization.
Case Report
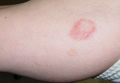
A 40-year-old woman presented with multiple flat warts on the bilateral arms and legs of 25 years’ duration (Figure 1) that had been unsuccessfully treated by an outside physician with imiquimod cream 5% and tazarotene gel 0.1%. Her medical history was remarkable for recurrent upper respiratory tract infections, urinary tract infections, yeast infections, and otitis media. She also reported a history of 6 spontaneous miscarriages that had been attributed to a balanced chromosomal translocation between chromosomes 12 and 14.
  |
Laboratory evaluation revealed leukopenia, lymphopenia, and hypogammaglobulinemia, with a white blood cell count of 3600/μL (reference range, 4500–11,000/mL), a lymphocyte count of 12.1% (20%–45%), absolute CD4 count of 77 cells/μL (490–1740 cells/μL), absolute CD8 count of 56 cells/mL (180–1170 cells/μL), and serum IgM level of 17 mg/dL (48–271 mg/dL). Human immunodeficiency virus (HIV) titers were negative.
On physical examination numerous pink, flat-topped papules were noted on the forehead and bilateral arms and legs. Histologic analysis of a tangential plane biopsy of a lesion on the right leg revealed hyperkeratosis of the stratum corneum and epidermal hyperplasia (Figure 2). The epidermis also showed focal papillomatosis with areas of hypergranulosis and viropathic changes; these findings were consistent with a diagnosis of verruca plana. Human papillomavirus (HPV) DNA typing by polymerase chain reaction from the verrucous lesions showed HPV type 20, which has been associated with EV. Based on the patient’s clinical findings and HPV subtype, she was diagnosed with atypical EV.
Subsequent treatment with liquid nitrogen, tretinoin cream 0.1%, and topical trichloroacetic acid 50% failed. She received oral isotretinoin at a dosage of 80 mg daily for 9 months, but the lesions persisted and she developed alopecia and ankle stiffness; therefore, the isotretinoin was discontinued. Candida antigen testing revealed that the patient was anergic, and SADBE sensitization was subsequently initiated. Squaric acid dibutylester was utilized as a sensitizing agent, and it was formulated as 2% and 0.2% solutions in acetone, supplied in 20-mL tinted glass bottles.
Squaric acid dibutylester solution 2% under occlusion was applied to a test area on the right forearm. Three days later, results indicated prominent erythema and inflammation at the application site. Two weeks later, a chronic dermatitic response was noted at the test site (Figure 3). Squaric acid dibutylester 0.2% was then applied to an affected area on the right shin and was kept under occlusion for 48 hours. One month later, no notable changes in the lesions were observed, and no further treatments were performed. Three months later, the patient returned for evaluation and it was noted that the flat warts on the right shin that had been treated with SADBE 0.2% 4 months prior had resolved (Figure 4). Subsequently, it was noted that all of the lesions had regressed, even those that had not been treated with SADBE.
Comment
Epidermodysplasia verruciformis is a rare genodermatosis caused by a group of phylogenetically related viruses1 belonging to the β-papillomavirus genus.2,3 It is characterized by a combination of pityriasis versicolor–like lesions, reddish verrucalike plaques, and seborrheic keratosis–like plaques,1,4 preferentially on sun-exposed areas.5 The lesions undergo malignant transformation in 30% to 60% of patients,3,6 especially into squamous cell carcinomas.7 The most frequent HPV types found in EV skin lesions are 5, 8, 9, 12, 14, 15, 17, and 19 to 25; types 5 and 8 are found in 90% of cutaneous squamous cell carcinomas in EV patients.2 Human papillomavirus type 20, the type identified in our patient, has been isolated from warts in EV patients,1,2 though it is not the most common type. It has been shown that more than one HPV type could be present concurrently in the same EV patient,1 which necessitates close follow-up for skin cancer evaluation in all EV patients, as oncogenic strains may be present in some lesions.
  |
Epidermodysplasia verruciformis has no particular predisposition for race or geographic location.1,7 It usually is inherited in an autosomal-recessive fashion1,4,7 and has been linked to mutations in 2 EV genes located on chromosome 17: EVER1/TCM6 and EVER2/TCM8.8 However, approximately 25% of EV cases are not associated with these gene mutations,5,9 as demonstrated in our patient. Autosomal-dominant or X-linked mutations also have been reported.10 In our case, a chromosomal abnormality in the form of a balanced chromosomal translocation was present, which is unique. A connection between EV and balanced chromosomal translocation cannot be excluded and warrants further investigation.
Epidermodysplasia verruciformis has been associated with decreased cell-mediated immunity.1,7 However, nonimmunologic factors likely contribute considering the rarity of EV-like eruptions in immunodeficiency disorders11 as well as its frequent coinfection with HPV type 312 and its association with EVER1/TCM6 and EVER2/TCM8.8 Epidermodysplasia verruciformis–like lesions have been reported in several immunosuppressed states, including HIV infection,13 combined variable immunodeficiency syndrome,14 IgM deficiency,15 and CD4+ T-cell lymphocytopenia.11 Our patient’s findings fit the latter diagnostic criteria, as she had a chronically low CD4 count of 77 cells/μL, negative HIV titers, and absence of alternative explanation to the lymphopenia. Thus, we could consider her as having EV, as a low CD4 count is a known association. Her immunodeficient state could possibly be attributed to the chromosomal translocation; however, the genetic loci surrounding the chromosomal translocation have not been identified to date, leaving this hypothesis unsubstantiated. Nevertheless, in our otherwise healthy patient, no explanation was found as to why a cell-mediated deficiency would selectively favor a cutaneous HPV infection. According to Zavattaro et al,5 a possible cause could be the presence of additional genetic or environmental factors in the patient that predisposed her to this particular infection.
Every patient with EV requires close lifelong observation for skin cancer and education regarding strict sun avoidance and protection.1 Treatment options for the lesions include topical therapies with imiquimod 5%, immunomodulators, and salicylic acid16,17; oral isotretinoin18; and combinations of acitretin and interferon alfa.19 Physical ablative procedures also have been proposed, including cryotherapy with liquid nitrogen, electrosurgery, surgical excision, and laser therapies.20
Topical immunotherapy with SADBE initially was used to treat refractory alopecia areata and also has been described in the treatment of recalcitrant warts.21-24 Historically, 2,4-dinitrochlorobenzene was used for contact immunotherapy in wart management but is now avoided due to its mutagenic potential.25 Squaric acid dibutylester and diphenylcyclopropenone currently are the favored contact sensitizers, with a resolution rate of 60% reported in refractory warts.26
Topical immunotherapy involves sensitization of the patient with high-concentration (2%) SADBE on a small surface area until an eczematous dermatitis appears. The rash indicates sensitization has been achieved, and then a lower-concentration SADBE is applied to the warts. Observation of mild contact dermatitis should not be an indication to stop treatment, as this effect is an integral part of therapeutic response. No serious side effects were reported to SADBE; erythema, desquamation, edema, itching, and burning were described.23
The mechanism of action of SADBE is not clear. The most common proposed theory is the induction of a type IV hypersensitivity reaction in the warts, leading to their destruction. Other authors suggest that wart resolution is caused by a nonspecific inflammatory reaction. An argument in favor of the latter hypothesis is the spontaneous regression of untreated warts in patients treated with SADBE at a remote site, suggesting a mechanism of action beyond a simple cell-mediated process.23
Epidermodysplasia verruciformis should be included in the differential diagnosis for any eruptive, warty, papular, and plaque-type lesions that appear in immunocompromised individuals. Moreover, the diagnosis of idiopathic CD4+ T-cell lymphocytopenia should be considered in any patient with a CD4 count deficit presenting with widespread viral, fungal, or mycobacterial infection with negative HIV test. Appropriate evaluation of the absolute CD4+ counts also should be performed. In our case, it was hypothesized that the patient’s balanced chromosomal translocation was related to her lymphopenia and EV, though this correlation has yet to be confirmed. However, it is notable that her son carried the same translocation and has a normal white blood cell count and no evidence of flat warts. This case demonstrates the success of contact immunotherapy in treating these widespread and often recalcitrant lesions.
Epidermodysplasia verruciformis (EV) is an uncommon autosomal-recessive inherited disorder characterized by disseminated cutaneous warts in predisposed patients who are highly susceptible to genus â-papillomavirus infections. Squaric acid dibutylester (SADBE) is a contact sensitizer agent that has gained general acceptance over the years for the treatment of a variety of dermatologic diseases, including alopecia areata and cutaneous warts. We report the case of a 40-year-old woman with a balanced chromosomal translocation and lymphocytopenia who presented with the sole clinical finding of refractory multiple flat warts that had been present for 25 years. After failed attempts at therapy with oral isotretinoin, cryotherapy with topical trichloroacetic acid, and topical tretinoin, the lesions were successfully eradicated with topical SADBE with prior sensitization.
Case Report
A 40-year-old woman presented with multiple flat warts on the bilateral arms and legs of 25 years’ duration (Figure 1) that had been unsuccessfully treated by an outside physician with imiquimod cream 5% and tazarotene gel 0.1%. Her medical history was remarkable for recurrent upper respiratory tract infections, urinary tract infections, yeast infections, and otitis media. She also reported a history of 6 spontaneous miscarriages that had been attributed to a balanced chromosomal translocation between chromosomes 12 and 14.
  |
Laboratory evaluation revealed leukopenia, lymphopenia, and hypogammaglobulinemia, with a white blood cell count of 3600/μL (reference range, 4500–11,000/mL), a lymphocyte count of 12.1% (20%–45%), absolute CD4 count of 77 cells/μL (490–1740 cells/μL), absolute CD8 count of 56 cells/mL (180–1170 cells/μL), and serum IgM level of 17 mg/dL (48–271 mg/dL). Human immunodeficiency virus (HIV) titers were negative.
On physical examination numerous pink, flat-topped papules were noted on the forehead and bilateral arms and legs. Histologic analysis of a tangential plane biopsy of a lesion on the right leg revealed hyperkeratosis of the stratum corneum and epidermal hyperplasia (Figure 2). The epidermis also showed focal papillomatosis with areas of hypergranulosis and viropathic changes; these findings were consistent with a diagnosis of verruca plana. Human papillomavirus (HPV) DNA typing by polymerase chain reaction from the verrucous lesions showed HPV type 20, which has been associated with EV. Based on the patient’s clinical findings and HPV subtype, she was diagnosed with atypical EV.
Subsequent treatment with liquid nitrogen, tretinoin cream 0.1%, and topical trichloroacetic acid 50% failed. She received oral isotretinoin at a dosage of 80 mg daily for 9 months, but the lesions persisted and she developed alopecia and ankle stiffness; therefore, the isotretinoin was discontinued. Candida antigen testing revealed that the patient was anergic, and SADBE sensitization was subsequently initiated. Squaric acid dibutylester was utilized as a sensitizing agent, and it was formulated as 2% and 0.2% solutions in acetone, supplied in 20-mL tinted glass bottles.
Squaric acid dibutylester solution 2% under occlusion was applied to a test area on the right forearm. Three days later, results indicated prominent erythema and inflammation at the application site. Two weeks later, a chronic dermatitic response was noted at the test site (Figure 3). Squaric acid dibutylester 0.2% was then applied to an affected area on the right shin and was kept under occlusion for 48 hours. One month later, no notable changes in the lesions were observed, and no further treatments were performed. Three months later, the patient returned for evaluation and it was noted that the flat warts on the right shin that had been treated with SADBE 0.2% 4 months prior had resolved (Figure 4). Subsequently, it was noted that all of the lesions had regressed, even those that had not been treated with SADBE.
Comment
Epidermodysplasia verruciformis is a rare genodermatosis caused by a group of phylogenetically related viruses1 belonging to the β-papillomavirus genus.2,3 It is characterized by a combination of pityriasis versicolor–like lesions, reddish verrucalike plaques, and seborrheic keratosis–like plaques,1,4 preferentially on sun-exposed areas.5 The lesions undergo malignant transformation in 30% to 60% of patients,3,6 especially into squamous cell carcinomas.7 The most frequent HPV types found in EV skin lesions are 5, 8, 9, 12, 14, 15, 17, and 19 to 25; types 5 and 8 are found in 90% of cutaneous squamous cell carcinomas in EV patients.2 Human papillomavirus type 20, the type identified in our patient, has been isolated from warts in EV patients,1,2 though it is not the most common type. It has been shown that more than one HPV type could be present concurrently in the same EV patient,1 which necessitates close follow-up for skin cancer evaluation in all EV patients, as oncogenic strains may be present in some lesions.
  |
Epidermodysplasia verruciformis has no particular predisposition for race or geographic location.1,7 It usually is inherited in an autosomal-recessive fashion1,4,7 and has been linked to mutations in 2 EV genes located on chromosome 17: EVER1/TCM6 and EVER2/TCM8.8 However, approximately 25% of EV cases are not associated with these gene mutations,5,9 as demonstrated in our patient. Autosomal-dominant or X-linked mutations also have been reported.10 In our case, a chromosomal abnormality in the form of a balanced chromosomal translocation was present, which is unique. A connection between EV and balanced chromosomal translocation cannot be excluded and warrants further investigation.
Epidermodysplasia verruciformis has been associated with decreased cell-mediated immunity.1,7 However, nonimmunologic factors likely contribute considering the rarity of EV-like eruptions in immunodeficiency disorders11 as well as its frequent coinfection with HPV type 312 and its association with EVER1/TCM6 and EVER2/TCM8.8 Epidermodysplasia verruciformis–like lesions have been reported in several immunosuppressed states, including HIV infection,13 combined variable immunodeficiency syndrome,14 IgM deficiency,15 and CD4+ T-cell lymphocytopenia.11 Our patient’s findings fit the latter diagnostic criteria, as she had a chronically low CD4 count of 77 cells/μL, negative HIV titers, and absence of alternative explanation to the lymphopenia. Thus, we could consider her as having EV, as a low CD4 count is a known association. Her immunodeficient state could possibly be attributed to the chromosomal translocation; however, the genetic loci surrounding the chromosomal translocation have not been identified to date, leaving this hypothesis unsubstantiated. Nevertheless, in our otherwise healthy patient, no explanation was found as to why a cell-mediated deficiency would selectively favor a cutaneous HPV infection. According to Zavattaro et al,5 a possible cause could be the presence of additional genetic or environmental factors in the patient that predisposed her to this particular infection.
Every patient with EV requires close lifelong observation for skin cancer and education regarding strict sun avoidance and protection.1 Treatment options for the lesions include topical therapies with imiquimod 5%, immunomodulators, and salicylic acid16,17; oral isotretinoin18; and combinations of acitretin and interferon alfa.19 Physical ablative procedures also have been proposed, including cryotherapy with liquid nitrogen, electrosurgery, surgical excision, and laser therapies.20
Topical immunotherapy with SADBE initially was used to treat refractory alopecia areata and also has been described in the treatment of recalcitrant warts.21-24 Historically, 2,4-dinitrochlorobenzene was used for contact immunotherapy in wart management but is now avoided due to its mutagenic potential.25 Squaric acid dibutylester and diphenylcyclopropenone currently are the favored contact sensitizers, with a resolution rate of 60% reported in refractory warts.26
Topical immunotherapy involves sensitization of the patient with high-concentration (2%) SADBE on a small surface area until an eczematous dermatitis appears. The rash indicates sensitization has been achieved, and then a lower-concentration SADBE is applied to the warts. Observation of mild contact dermatitis should not be an indication to stop treatment, as this effect is an integral part of therapeutic response. No serious side effects were reported to SADBE; erythema, desquamation, edema, itching, and burning were described.23
The mechanism of action of SADBE is not clear. The most common proposed theory is the induction of a type IV hypersensitivity reaction in the warts, leading to their destruction. Other authors suggest that wart resolution is caused by a nonspecific inflammatory reaction. An argument in favor of the latter hypothesis is the spontaneous regression of untreated warts in patients treated with SADBE at a remote site, suggesting a mechanism of action beyond a simple cell-mediated process.23
Epidermodysplasia verruciformis should be included in the differential diagnosis for any eruptive, warty, papular, and plaque-type lesions that appear in immunocompromised individuals. Moreover, the diagnosis of idiopathic CD4+ T-cell lymphocytopenia should be considered in any patient with a CD4 count deficit presenting with widespread viral, fungal, or mycobacterial infection with negative HIV test. Appropriate evaluation of the absolute CD4+ counts also should be performed. In our case, it was hypothesized that the patient’s balanced chromosomal translocation was related to her lymphopenia and EV, though this correlation has yet to be confirmed. However, it is notable that her son carried the same translocation and has a normal white blood cell count and no evidence of flat warts. This case demonstrates the success of contact immunotherapy in treating these widespread and often recalcitrant lesions.
1. Vohra S, Sharma NL, Shanker V, et al. Autosomal dominant epidermodysplasia verruciformis: a clinicotherapeutic experience in two cases. Indian J Dermatol Venereol Leprol. 2010;76:557-561.
2. Dell’Oste V, Azzimonti B, De Andrea M, et al. High beta-HPV DNA loads and strong seroreactivity are present in epidermodysplasia verruciformis. J Invest Dermatol. 2009;129:1026-1034.
3. Orth G. Epidermodysplasia verruciformis: a model for understanding the oncogenicity of human papillomavirus. Ciba Found Symp. 1986;120:157-174.
4. Michael KM, Waterboer T, Pfister H, et al. Seroreactivity of 38 human papillomavirus types in epidermodysplasia verruciformis patients, relatives, and controls. J Invest Dermatol. 2010;130:841-848.
5. Zavattaro E, Azzimonti B, Mondini M, et al. Identification of defective Fas function and variation of the perforin gene in an epidermodysplasia verruciformis patient lacking EVER1 and EVER2 mutations. J Invest Dermatol. 2008;128:732-735.
6. Majewski S, Jabłońska S. Epidermodysplasia verruciformis as a model of human papillomavirus-induced genetic cancer of the skin. Arch Dermatol. 1995;131:1312-1318.
7. Robati RM, Marefat A, Saeedi M, et al. Four familial cases of epidermodysplasia verruciformis: mother and three sons. Dermatol Online J. 2009;15:8.
8. Ramoz N, Rueda LA, Bouadjar B, et al. Mutations in two adjacent novel genes are associated with epidermodysplasia verruciformis. Nat Genet. 2002;32:579-581.
9. Azzimonti B, Mondini M, De Andrea M, et al. CD8+ T-cell lymphocytopenia and lack of EVER mutations in a patient with clinically and virologically typical epidermodysplasia verruciformis. Arch Dermatol. 2005;141:1323-1325.
10. Androphy EJ, Dvoretzky I, Lowy DR. X-linked inheritance of epidermodysplasia verruciformis. genetic and virologic studies of a kindred. Arch Dermatol. 1985;121:864-868.
11. Tobin E, Rohwedder A, Holland SM, et al. Recurrent ‘sterile’ verrucous cyst abscesses and epidermodysplasia verruciformis-like eruption associated with idiopathic CD4 lymphopenia. Br J Dermatol. 2003;149:627-633.
12. Obalek S, Favre M, Szymanczyk J, et al. Human papillomavirus (HPV) types specific of epidermodysplasia verruciformis detected in warts induced by HPV3 or HPV3-related types in immunosuppressed patients. J Invest Dermatol. 1992;98:936-941.
13. Berk DR, Bruckner AL, Lu D. Epidermodysplasia verruciform-like lesions in an HIV patient. Dermatol Online J. 2009;15:1.
14. Vu J, Wallace GR, Singh R, et al. Common variable immunodeficiency syndrome associated with epidermodysplasia verruciformis. Am J Clin Dermatol. 2007;8:307-310.
15. Gul U, Soylu S, Yavuzer R. Epidermodysplasia verruciformis associated with IgM deficiency. Indian J Dermatol Venereol Leprol. 2007;73:420-422.
16. De Oliveira WR, Festa Neto C, Rady PL, et al. Clinical aspects of epidermodysplasia verruciformis. J Eur Acad Dermatol Venereol. 2003;17:394-398.
17. Jablonska S, Majewski S. Epidermodysplasia verruciformis: what’s new? J Eur Acad Dermatol Venereol. 2003;17:381-382.
18. Rallis E, Papatheodorou G, Bimpakis E, et al. Systemic low-dose isotretinoin maintains remission status in epidermodysplasia verruciformis. J Eur Acad Dermatol Venereol. 2008;22:523-525.
19. Anadolu R, Oskay T, Erdem C, et al. Treatment of epidermodysplasia verruciformis with a combination of acitretin and interferon alfa-2a. J Am Acad Dermatol. 2001;45:296-299.
20. Fang F, Zhao L, Jiang MJ, et al. Epidermodysplasia verruciformis with severe hand and foot deformity successfully treated with surgical excision. J Plast Reconstr Aesthet Surg. 2008;61:338-341.
21. Huang W, Morrell D. Successful treatment of recalcitrant warts with topical squaric acid in immunosuppressed child. Pediatr Dermatol. 2008;25:275-276.
22. Hama N, Hatamochi A, Hayashi S, et al. Usefulness of topical immunotherapy with squaric acid dibutylester for refractory common warts on the face and neck. J Dermatol. 2009;36:660-662.
23. Micali G, Nasca MR, Tedeschi A, et al. Use of squaric acid dibutylester (SADBE) for cutaneous warts in children. Pediatr Dermatol. 2000;17:315-318.
24. Mastrolonardo M, Lopalco PL, Diaferio A. Topical immunotherapy with contact sensitizers: a model to study the natural history of delayedhypersensitivity. Contact Dermatitis. 2002;47:210-214.
25. Lewis HM. Topical immunotherapy of refractory warts. Cutis. 1973;12:863-869.
26. Weisshaar E, Neumann HJ, Gollnick H. Successful treatment of disseminated facial verrucae with contact immunotherapy. Eur J Dermatol. 1998;8:488-491.
1. Vohra S, Sharma NL, Shanker V, et al. Autosomal dominant epidermodysplasia verruciformis: a clinicotherapeutic experience in two cases. Indian J Dermatol Venereol Leprol. 2010;76:557-561.
2. Dell’Oste V, Azzimonti B, De Andrea M, et al. High beta-HPV DNA loads and strong seroreactivity are present in epidermodysplasia verruciformis. J Invest Dermatol. 2009;129:1026-1034.
3. Orth G. Epidermodysplasia verruciformis: a model for understanding the oncogenicity of human papillomavirus. Ciba Found Symp. 1986;120:157-174.
4. Michael KM, Waterboer T, Pfister H, et al. Seroreactivity of 38 human papillomavirus types in epidermodysplasia verruciformis patients, relatives, and controls. J Invest Dermatol. 2010;130:841-848.
5. Zavattaro E, Azzimonti B, Mondini M, et al. Identification of defective Fas function and variation of the perforin gene in an epidermodysplasia verruciformis patient lacking EVER1 and EVER2 mutations. J Invest Dermatol. 2008;128:732-735.
6. Majewski S, Jabłońska S. Epidermodysplasia verruciformis as a model of human papillomavirus-induced genetic cancer of the skin. Arch Dermatol. 1995;131:1312-1318.
7. Robati RM, Marefat A, Saeedi M, et al. Four familial cases of epidermodysplasia verruciformis: mother and three sons. Dermatol Online J. 2009;15:8.
8. Ramoz N, Rueda LA, Bouadjar B, et al. Mutations in two adjacent novel genes are associated with epidermodysplasia verruciformis. Nat Genet. 2002;32:579-581.
9. Azzimonti B, Mondini M, De Andrea M, et al. CD8+ T-cell lymphocytopenia and lack of EVER mutations in a patient with clinically and virologically typical epidermodysplasia verruciformis. Arch Dermatol. 2005;141:1323-1325.
10. Androphy EJ, Dvoretzky I, Lowy DR. X-linked inheritance of epidermodysplasia verruciformis. genetic and virologic studies of a kindred. Arch Dermatol. 1985;121:864-868.
11. Tobin E, Rohwedder A, Holland SM, et al. Recurrent ‘sterile’ verrucous cyst abscesses and epidermodysplasia verruciformis-like eruption associated with idiopathic CD4 lymphopenia. Br J Dermatol. 2003;149:627-633.
12. Obalek S, Favre M, Szymanczyk J, et al. Human papillomavirus (HPV) types specific of epidermodysplasia verruciformis detected in warts induced by HPV3 or HPV3-related types in immunosuppressed patients. J Invest Dermatol. 1992;98:936-941.
13. Berk DR, Bruckner AL, Lu D. Epidermodysplasia verruciform-like lesions in an HIV patient. Dermatol Online J. 2009;15:1.
14. Vu J, Wallace GR, Singh R, et al. Common variable immunodeficiency syndrome associated with epidermodysplasia verruciformis. Am J Clin Dermatol. 2007;8:307-310.
15. Gul U, Soylu S, Yavuzer R. Epidermodysplasia verruciformis associated with IgM deficiency. Indian J Dermatol Venereol Leprol. 2007;73:420-422.
16. De Oliveira WR, Festa Neto C, Rady PL, et al. Clinical aspects of epidermodysplasia verruciformis. J Eur Acad Dermatol Venereol. 2003;17:394-398.
17. Jablonska S, Majewski S. Epidermodysplasia verruciformis: what’s new? J Eur Acad Dermatol Venereol. 2003;17:381-382.
18. Rallis E, Papatheodorou G, Bimpakis E, et al. Systemic low-dose isotretinoin maintains remission status in epidermodysplasia verruciformis. J Eur Acad Dermatol Venereol. 2008;22:523-525.
19. Anadolu R, Oskay T, Erdem C, et al. Treatment of epidermodysplasia verruciformis with a combination of acitretin and interferon alfa-2a. J Am Acad Dermatol. 2001;45:296-299.
20. Fang F, Zhao L, Jiang MJ, et al. Epidermodysplasia verruciformis with severe hand and foot deformity successfully treated with surgical excision. J Plast Reconstr Aesthet Surg. 2008;61:338-341.
21. Huang W, Morrell D. Successful treatment of recalcitrant warts with topical squaric acid in immunosuppressed child. Pediatr Dermatol. 2008;25:275-276.
22. Hama N, Hatamochi A, Hayashi S, et al. Usefulness of topical immunotherapy with squaric acid dibutylester for refractory common warts on the face and neck. J Dermatol. 2009;36:660-662.
23. Micali G, Nasca MR, Tedeschi A, et al. Use of squaric acid dibutylester (SADBE) for cutaneous warts in children. Pediatr Dermatol. 2000;17:315-318.
24. Mastrolonardo M, Lopalco PL, Diaferio A. Topical immunotherapy with contact sensitizers: a model to study the natural history of delayedhypersensitivity. Contact Dermatitis. 2002;47:210-214.
25. Lewis HM. Topical immunotherapy of refractory warts. Cutis. 1973;12:863-869.
26. Weisshaar E, Neumann HJ, Gollnick H. Successful treatment of disseminated facial verrucae with contact immunotherapy. Eur J Dermatol. 1998;8:488-491.
Practice Points
- Epidermodysplasia verruciformis (EV) is a rare immune deficiency. Associated warts are difficult to treat.
- Topical immunotherapy with squaric acid dibutylester (SADBE) has successfully treated long-standing warts in an EV patient.
- Consider immunotherapy with a contact sensitizer such as SADBE to treat resistant warts, even in immune deficiency patients.
Evaluating Endoleaks in the Dermatology Office
Endoleaks are common complications following endovascular aneurysm repairs (EVARs) that may occur any time after surgery. There are 5 types of endoleaks with various etiologies. A type V endoleak (also known as endotension) is not considered a true endoleak but instead is characterized by continued aneurysm expansion without a leak, which is demonstrated via imaging tests.1 Type V endoleaks typically require open aneurysm repair.2 We report the case of a 69-year-old woman who presented to our dermatology office for treatment of a suspected lipoma overlying the right mid sternum that was confirmed to be a type V endoleak via computed tomography angiography.
Case Report
A 69-year-old woman was referred to our dermatology office by her primary care physician for evaluation of a subcutaneous mass overlying the right mid sternum, which was a suspected lipoma. The patient reported that the mass had been present for approximately 2 weeks and was enlarging but otherwise asymptomatic. Her medical history was remarkable for hypertension, an ascending aortic aneurysm, and a subsequent aortic valve replacement approximately 2.5 years prior. Her current medications included amlodipine, lisinopril, nebivolol, ibuprofen, and aspirin. She denied use of alcohol, tobacco, or illicit drugs. A review of systems was noncontributory.
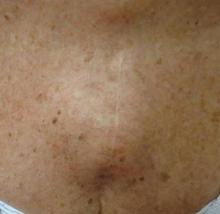
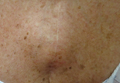
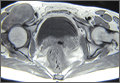
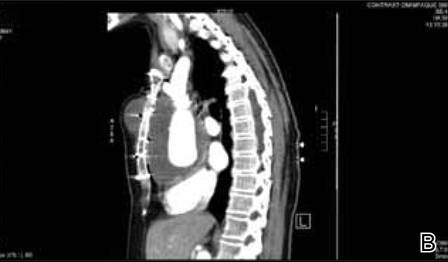
Physical examination revealed a single 3.5×4.5-cm, soft, nonmobile subcutaneous mass located at the site of the thoracotomy scar (Figure 1). The mass appeared to have a central attachment to the sternum. No erythema, swelling, or exudate was noted, and the patient denied tenderness on palpation. The diagnosis of lipoma was questioned, and the patient was referred for ultrasonography and computed tomography angiography. Ultrasonography showed a nonspecific chest wall mass with internal blood flow, and computed tomography angiography showed a large, low-attenuation collection of blood around the entire circumference of the ascending aorta, extending from the aortic root to the arch of the aorta. There was extension of the collection of blood through either the sternocostal junction or the sternotomy defect into the subcutaneous tissue anterior to the sternum (Figure 2). Findings were most consistent with a type V endoleak, and the patient was referred to a cardiothoracic surgeon for treatment. We later learned that our patient died during surgery attempting to repair the aneurysm approximately 2 weeks after her presentation to our office.
Comment
An endoleak is a common complication following an EVAR that is characterized by persistent blood flow within the aneurysm sac. Endoleaks have been described as the Achilles’ heel of EVARs.1 The goal of an EVAR is to create a complete seal so that the flow of blood completely excludes the aneurysm, thus ultimately preventing an aneurysm rupture. An endoleak results when there is failure to obtain a complete seal due to a variety of different mechanisms. White et al3 first described and classified endoleaks in 1997. The initial terminology used to classify endoleaks was based on timing (primary or secondary/late) and location (graft related/perigraft or non–graft related/retrograde). Today, endoleaks are classified into 5 types, 3 of which are considered true endoleaks and 2 of which are not.4 Type I endoleaks result from a failure to create an adequate seal at one of the attachments of the graft to the vessel wall. Type II endoleaks are due to retrograde flow through collateral vessels into the aneurysm sac. They are much more common than type I, occurring in 10% to 25% of abdominal endograft cases. The last true endoleak, type III, occurs due to device failure in the form of disjunction of the components of the graft system (type IIIa) or a defect in the graft fabric (type IIIb). Type IV and type V endoleaks are not considered to be true endoleaks. Type IV endoleaks are due to the porosity of the graft material and have virtually been eliminated by changes in graft materials to decrease porosity. Type V endoleaks are characterized by continued blood flow into the aneurysm without any evidence of a leak on any imaging modality. Type V endoleaks are poorly understood but are believed to be due to pulsation of the graft wall, which is transmitted through the perivascular space to the aneurysm wall.4
  |
Treatment of type V endoleaks is controversial. It is important to characterize the endoleak by various imaging modalities, and if a type V endoleak is confirmed, an open aneurysm repair often is required.2 A case of nonsurgical management of a type V endoleak has been described but is rare.5 In this case, the patient was referred to our dermatology office by her primary care physician for what appeared to be a benign lipoma, but it proved to be a type V endoleak on further examination. It is imperative that dermatologists are aware of endoleaks as common complications of EVARs, as they can be life threatening and usually require surgical intervention.
Conclusion
Endoleaks are common complications of EVARs. Dermatologists may encounter endoleaks that have been misdiagnosed as benign subcutaneous masses such as lipomas. It is imperative that dermatologists are aware of endoleaks, and patients who present with subcutaneous thoracic masses with a history of aneurysm repair require imaging, including computed tomography angiography, and referral to a cardiothoracic surgeon if appropriate.
1. Rosen RJ, Green RM. Endoleak management following endovascular aneurysm repair. J Vasc Interv Radiol. 2008;19(suppl 6):S37-S43.
2. Stavropoulos SW, Charagundla SR. Imaging techniques for detection and management of endoleaks after endovascular aortic aneurysm repair. Radiology. 2007;243:641-655.
3. White GH, Yu W, May J, et al. Endoleak as a complication of endoluminal grafting of abdominal aortic aneurysms: classification, incidence, diagnosis and management. J Endovascular Surg. 1997;4:152-168.
4. Veith FJ, Baum BA, Ohki T, et al. Nature and significance of endoleaks and endotension: summary of opinions expressed at an international conference. J Vasc Surg. 2002;35:1029-1035.
5. Mennander A, Pimenoff G, Heikkinen M, et al. Nonoperative approach to endotension. J Vasc Surg. 2005;42:194-198.
Endoleaks are common complications following endovascular aneurysm repairs (EVARs) that may occur any time after surgery. There are 5 types of endoleaks with various etiologies. A type V endoleak (also known as endotension) is not considered a true endoleak but instead is characterized by continued aneurysm expansion without a leak, which is demonstrated via imaging tests.1 Type V endoleaks typically require open aneurysm repair.2 We report the case of a 69-year-old woman who presented to our dermatology office for treatment of a suspected lipoma overlying the right mid sternum that was confirmed to be a type V endoleak via computed tomography angiography.
Case Report
A 69-year-old woman was referred to our dermatology office by her primary care physician for evaluation of a subcutaneous mass overlying the right mid sternum, which was a suspected lipoma. The patient reported that the mass had been present for approximately 2 weeks and was enlarging but otherwise asymptomatic. Her medical history was remarkable for hypertension, an ascending aortic aneurysm, and a subsequent aortic valve replacement approximately 2.5 years prior. Her current medications included amlodipine, lisinopril, nebivolol, ibuprofen, and aspirin. She denied use of alcohol, tobacco, or illicit drugs. A review of systems was noncontributory.
Physical examination revealed a single 3.5×4.5-cm, soft, nonmobile subcutaneous mass located at the site of the thoracotomy scar (Figure 1). The mass appeared to have a central attachment to the sternum. No erythema, swelling, or exudate was noted, and the patient denied tenderness on palpation. The diagnosis of lipoma was questioned, and the patient was referred for ultrasonography and computed tomography angiography. Ultrasonography showed a nonspecific chest wall mass with internal blood flow, and computed tomography angiography showed a large, low-attenuation collection of blood around the entire circumference of the ascending aorta, extending from the aortic root to the arch of the aorta. There was extension of the collection of blood through either the sternocostal junction or the sternotomy defect into the subcutaneous tissue anterior to the sternum (Figure 2). Findings were most consistent with a type V endoleak, and the patient was referred to a cardiothoracic surgeon for treatment. We later learned that our patient died during surgery attempting to repair the aneurysm approximately 2 weeks after her presentation to our office.
Comment
An endoleak is a common complication following an EVAR that is characterized by persistent blood flow within the aneurysm sac. Endoleaks have been described as the Achilles’ heel of EVARs.1 The goal of an EVAR is to create a complete seal so that the flow of blood completely excludes the aneurysm, thus ultimately preventing an aneurysm rupture. An endoleak results when there is failure to obtain a complete seal due to a variety of different mechanisms. White et al3 first described and classified endoleaks in 1997. The initial terminology used to classify endoleaks was based on timing (primary or secondary/late) and location (graft related/perigraft or non–graft related/retrograde). Today, endoleaks are classified into 5 types, 3 of which are considered true endoleaks and 2 of which are not.4 Type I endoleaks result from a failure to create an adequate seal at one of the attachments of the graft to the vessel wall. Type II endoleaks are due to retrograde flow through collateral vessels into the aneurysm sac. They are much more common than type I, occurring in 10% to 25% of abdominal endograft cases. The last true endoleak, type III, occurs due to device failure in the form of disjunction of the components of the graft system (type IIIa) or a defect in the graft fabric (type IIIb). Type IV and type V endoleaks are not considered to be true endoleaks. Type IV endoleaks are due to the porosity of the graft material and have virtually been eliminated by changes in graft materials to decrease porosity. Type V endoleaks are characterized by continued blood flow into the aneurysm without any evidence of a leak on any imaging modality. Type V endoleaks are poorly understood but are believed to be due to pulsation of the graft wall, which is transmitted through the perivascular space to the aneurysm wall.4
  |
Treatment of type V endoleaks is controversial. It is important to characterize the endoleak by various imaging modalities, and if a type V endoleak is confirmed, an open aneurysm repair often is required.2 A case of nonsurgical management of a type V endoleak has been described but is rare.5 In this case, the patient was referred to our dermatology office by her primary care physician for what appeared to be a benign lipoma, but it proved to be a type V endoleak on further examination. It is imperative that dermatologists are aware of endoleaks as common complications of EVARs, as they can be life threatening and usually require surgical intervention.
Conclusion
Endoleaks are common complications of EVARs. Dermatologists may encounter endoleaks that have been misdiagnosed as benign subcutaneous masses such as lipomas. It is imperative that dermatologists are aware of endoleaks, and patients who present with subcutaneous thoracic masses with a history of aneurysm repair require imaging, including computed tomography angiography, and referral to a cardiothoracic surgeon if appropriate.
Endoleaks are common complications following endovascular aneurysm repairs (EVARs) that may occur any time after surgery. There are 5 types of endoleaks with various etiologies. A type V endoleak (also known as endotension) is not considered a true endoleak but instead is characterized by continued aneurysm expansion without a leak, which is demonstrated via imaging tests.1 Type V endoleaks typically require open aneurysm repair.2 We report the case of a 69-year-old woman who presented to our dermatology office for treatment of a suspected lipoma overlying the right mid sternum that was confirmed to be a type V endoleak via computed tomography angiography.
Case Report
A 69-year-old woman was referred to our dermatology office by her primary care physician for evaluation of a subcutaneous mass overlying the right mid sternum, which was a suspected lipoma. The patient reported that the mass had been present for approximately 2 weeks and was enlarging but otherwise asymptomatic. Her medical history was remarkable for hypertension, an ascending aortic aneurysm, and a subsequent aortic valve replacement approximately 2.5 years prior. Her current medications included amlodipine, lisinopril, nebivolol, ibuprofen, and aspirin. She denied use of alcohol, tobacco, or illicit drugs. A review of systems was noncontributory.
Physical examination revealed a single 3.5×4.5-cm, soft, nonmobile subcutaneous mass located at the site of the thoracotomy scar (Figure 1). The mass appeared to have a central attachment to the sternum. No erythema, swelling, or exudate was noted, and the patient denied tenderness on palpation. The diagnosis of lipoma was questioned, and the patient was referred for ultrasonography and computed tomography angiography. Ultrasonography showed a nonspecific chest wall mass with internal blood flow, and computed tomography angiography showed a large, low-attenuation collection of blood around the entire circumference of the ascending aorta, extending from the aortic root to the arch of the aorta. There was extension of the collection of blood through either the sternocostal junction or the sternotomy defect into the subcutaneous tissue anterior to the sternum (Figure 2). Findings were most consistent with a type V endoleak, and the patient was referred to a cardiothoracic surgeon for treatment. We later learned that our patient died during surgery attempting to repair the aneurysm approximately 2 weeks after her presentation to our office.
Comment
An endoleak is a common complication following an EVAR that is characterized by persistent blood flow within the aneurysm sac. Endoleaks have been described as the Achilles’ heel of EVARs.1 The goal of an EVAR is to create a complete seal so that the flow of blood completely excludes the aneurysm, thus ultimately preventing an aneurysm rupture. An endoleak results when there is failure to obtain a complete seal due to a variety of different mechanisms. White et al3 first described and classified endoleaks in 1997. The initial terminology used to classify endoleaks was based on timing (primary or secondary/late) and location (graft related/perigraft or non–graft related/retrograde). Today, endoleaks are classified into 5 types, 3 of which are considered true endoleaks and 2 of which are not.4 Type I endoleaks result from a failure to create an adequate seal at one of the attachments of the graft to the vessel wall. Type II endoleaks are due to retrograde flow through collateral vessels into the aneurysm sac. They are much more common than type I, occurring in 10% to 25% of abdominal endograft cases. The last true endoleak, type III, occurs due to device failure in the form of disjunction of the components of the graft system (type IIIa) or a defect in the graft fabric (type IIIb). Type IV and type V endoleaks are not considered to be true endoleaks. Type IV endoleaks are due to the porosity of the graft material and have virtually been eliminated by changes in graft materials to decrease porosity. Type V endoleaks are characterized by continued blood flow into the aneurysm without any evidence of a leak on any imaging modality. Type V endoleaks are poorly understood but are believed to be due to pulsation of the graft wall, which is transmitted through the perivascular space to the aneurysm wall.4
  |
Treatment of type V endoleaks is controversial. It is important to characterize the endoleak by various imaging modalities, and if a type V endoleak is confirmed, an open aneurysm repair often is required.2 A case of nonsurgical management of a type V endoleak has been described but is rare.5 In this case, the patient was referred to our dermatology office by her primary care physician for what appeared to be a benign lipoma, but it proved to be a type V endoleak on further examination. It is imperative that dermatologists are aware of endoleaks as common complications of EVARs, as they can be life threatening and usually require surgical intervention.
Conclusion
Endoleaks are common complications of EVARs. Dermatologists may encounter endoleaks that have been misdiagnosed as benign subcutaneous masses such as lipomas. It is imperative that dermatologists are aware of endoleaks, and patients who present with subcutaneous thoracic masses with a history of aneurysm repair require imaging, including computed tomography angiography, and referral to a cardiothoracic surgeon if appropriate.
1. Rosen RJ, Green RM. Endoleak management following endovascular aneurysm repair. J Vasc Interv Radiol. 2008;19(suppl 6):S37-S43.
2. Stavropoulos SW, Charagundla SR. Imaging techniques for detection and management of endoleaks after endovascular aortic aneurysm repair. Radiology. 2007;243:641-655.
3. White GH, Yu W, May J, et al. Endoleak as a complication of endoluminal grafting of abdominal aortic aneurysms: classification, incidence, diagnosis and management. J Endovascular Surg. 1997;4:152-168.
4. Veith FJ, Baum BA, Ohki T, et al. Nature and significance of endoleaks and endotension: summary of opinions expressed at an international conference. J Vasc Surg. 2002;35:1029-1035.
5. Mennander A, Pimenoff G, Heikkinen M, et al. Nonoperative approach to endotension. J Vasc Surg. 2005;42:194-198.
1. Rosen RJ, Green RM. Endoleak management following endovascular aneurysm repair. J Vasc Interv Radiol. 2008;19(suppl 6):S37-S43.
2. Stavropoulos SW, Charagundla SR. Imaging techniques for detection and management of endoleaks after endovascular aortic aneurysm repair. Radiology. 2007;243:641-655.
3. White GH, Yu W, May J, et al. Endoleak as a complication of endoluminal grafting of abdominal aortic aneurysms: classification, incidence, diagnosis and management. J Endovascular Surg. 1997;4:152-168.
4. Veith FJ, Baum BA, Ohki T, et al. Nature and significance of endoleaks and endotension: summary of opinions expressed at an international conference. J Vasc Surg. 2002;35:1029-1035.
5. Mennander A, Pimenoff G, Heikkinen M, et al. Nonoperative approach to endotension. J Vasc Surg. 2005;42:194-198.
Practice Points
- An endoleak should be considered in any patient with a thoracic subcutaneous mass and history of aneurysm repair.
- Order imaging when an endoleak is suspected, including computed tomography angiography. Endoleaks can result in substantial morbidity and mortality if they are not recognized and treated appropriately.
- Dermatologists should be familiar with and able to recognize endoleaks, as patients may present to a dermatologist for evaluation of a subcutaneous mass that proves to be an endoleak.
Perianal North American Blastomycosis
Cutaneous North American blastomycosis is a deep fungal infection caused by Blastomyces dermatitidis, a thermally dimorphic fungus that is endemic to the Great Lakes region as well as the Mississippi and Ohio River valleys where it thrives in moist acidic soil enriched with organic material.1,2 In humans, the annual incidence rate is estimated to be 0.6 cases per million,3 though it may be as high as 42 cases per 100,000 in endemic areas.4 Infection typically results from the inhalation of conidia and manifests as either acute or chronic pneumonia.5 Most patients with acute disease present with nonspecific flulike symptoms and a nonproductive cough.
Dissemination occurs in approximately 25% of cases,6 most commonly affecting the skin. Other potential sites of dissemination include bone, the genitourinary tract, and the central nervous system. Cutaneous lesions, which may be either verrucous or ulcerative plaques, often occur on or around orifices contiguous to the respiratory tract.7 Verrucous lesions tend to have an irregular shape with well-defined borders and surface crusting. Ulcerative lesions have heaped-up borders and often have an exudative base.8 The differential diagnosis of cutaneous North American blastomycosis lesions includes squamous cell carcinoma, giant keratoacanthoma, verrucae, basal cell carcinoma, scrofuloderma, lupus vulgaris, nocardiosis, syphilis, bromoderma, iododerma, granuloma inguinale, tuberculosis verrucosa cutis, mycetoma, and actinomycosis.7,8
Although periorificial cutaneous manifestations of disseminated blastomycosis are common, perianal lesions are rare. The differential diagnosis of perianal verrucous plaques includes condyloma acuminatum, squamous cell carcinoma, adenocarcinoma, Buschke-Löwenstein tumor, actinomycosis, and localized fungal infections such as blastomycosis.9
Case Report
A 57-year-old man presented with a palpable perianal mass that produced small amounts of blood in his underwear and on toilet paper. The patient reported no history of hemorrhoids, anoreceptive intercourse, or sexually transmitted disease. Four months prior to presentation, he had a prolonged upper respiratory tract illness with a subjective fever and productive cough of 2 months’ duration. The patient described himself as an avid outdoorsman who worked at a summer resort and spent a great deal of time in the forests of central Wisconsin last autumn. Physical examination revealed a well-demarcated, firm, moist plaque with a verrucous surface that measured 3.5×2.7 cm and extended from the anal verge to the perianal skin (Figure 1).
Potassium hydroxide preparation of a biopsy specimen (Figure 2), a punch biopsy of the lesion (Figure 3), and Gomori methenamine-silver staining (Figure 4) revealed scattered yeast spores, some demonstrating broad-based budding, with pseudoepitheliomatous hyperplasia, dermal neutrophils, and intraepithelial microabscesses. The patient’s urine was positive for Blastomyces antigen (1.04 ng/mL). Chest radiography demonstrated a localized infiltrate in the right hilum with possible mass effect. Computed tomography showed a consolidative opacity measuring 4.0×3.4 cm in the upper lobe of the right lung (Figure 5).
 | 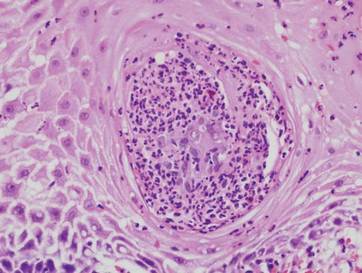 |
The patient was diagnosed with cutaneous North American blastomycosis and prescribed a 6-month course of oral itraconazole 200 mg twice daily. At his 3-month follow-up visit, the perianal plaque hadalmost completely resolved (Figure 6). However, because the patient had increasing lower extremity edema, subjective hearing loss, and abnormal liver function tests, itraconazole treatment was discontinued and replaced with oral fluconazole 400 mg daily for the next 3 months. The right hilar mass had visibly improved on follow-up chest radiography 2 months after the patient started antifungal therapy with itraconazole and had resolved within another 3 months of treatment.
 | 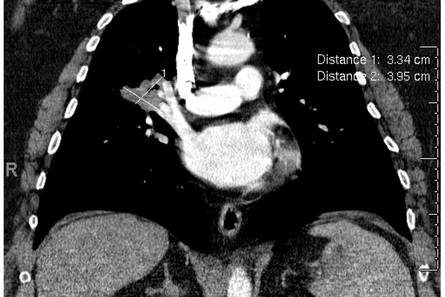
|
Comment
Cutaneous blastomycosis results most often from the hematogenous spread of B dermatitidis from the lungs and rarely from direct inoculation.5,10 Skin lesions tend to occur on exposed areas, such as the face, scalp, hands, wrists, feet, and ankles.7,11-13 Dissemination to the perianal skin is rare, though it has been reported in 2 other patients; both patients, similar to our patient, had evidence of pulmonary involvement at some point in their clinical course.9,14
Diagnosis is based on identification of B dermatitidis by microscopy or culture. Potassium hydroxide preparation of biopsy specimens typically shows broad-based budding yeast.13 Characteristic findings of histopathologic studies include pseudo-epitheliomatous hyperplasia, intraepidermal abscesses, and a dermal infiltrate of polymorphonuclear leukocytes.15 On fungal culture, B dermatitidis is slow growing and may require a 2- to 4-week incubation period. Serologic tests are available, but sensitivity is low, at 9%, 28%, and 77% for complement fixation, immunodiffusion, and enzyme immunoassay, respectively.16
Conclusion
North American blastomycosis should be considered in patients who have verrucous or ulcerative perianal lesions and have lived in or traveled to endemic regions, especially if they have recent or ongoing pulmonary symptoms. Potassium hydroxide preparation and fungal staining of biopsy specimens can aid in diagnosis.
Acknowledgment
The authors thank the Marshfield Clinic Research Foundation’s Office of Scientific Writing and Publication (Marshfield, Wisconsin) for editorial assistance in the preparation of this manuscript.
1. Klein BS, Vergeront JM, Davis JP. Epidemiologic aspects of blastomycosis, the enigmatic systemic mycosis. Semin Respir Infect. 1986;1:29-39.
2. Klein BS, Vergeront JM, Weeks RJ, et al. Isolation of Blastomyces dermatitidis in soil associated with a large outbreak of blastomycosis in Wisconsin. N Engl J Med. 1986;314:529-534.
3. Reingold AL, Lu XD, Plikaytis BD, et al. Systemic mycoses in the United States, 1980-1982. J Med Vet Mycol. 1986;24:433-436.
4. Centers for Disease Control and Prevention (CDC). Blastomycosis—Wisconsin, 1986-1995. MMWR Morb Mortal Wkly Rep. 1996;45:601-603.
5. Smith JA, Kauffman CA. Blastomycosis. Proc Am Thorac Soc. 2010;7:173-180.
6. Goldman M, Johnson PC, Sarosi GA. Fungal pneumonias. the endemic mycoses. Clin Chest Med. 1999;20:507-519.
7. Mercurio MG, Elewski BE. Cutaneous blastomycosis. Cutis. 1992;50:422-424.
8. Saccente M, Woods GL. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev. 2010;23:367-381.
9. Ricciardi R, Alavi K, Filice GA, et al. Blastomyces dermatitidis of the perianal skin: report of a case. Dis Colon Rectum. 2007;50:118-121.
10. Gray NA, Baddour LM. Cutaneous inoculation blastomycosis [published online ahead of print April 17, 2002]. Clin Infect Dis. 2002;34:e44-e49.
11. Kisso B, Mahmoud F, Thakkar JR. Blastomycosis presenting as recurrent tender cutaneous nodules. S D Med. 2006;59:255-259.
12. Mandell GL, Bennett JE, Dolin R. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, PA: Churchill Livingstone/Elsevier; 2010.
13. Mason AR, Cortes GY, Cook J, et al. Cutaneous blastomycosis: a diagnostic challenge. Int J Dermatol. 2008;47:824-830.
14. Linn JE. Pseudo-epitheliomatous lesions of the perirectal tissue: report of a case of squamous epithelioma due to blastomycosis. South Med J. 1958;51:1101-1104.
15. Woofter MJ, Cripps DJ, Warner TF. Verrucous plaques on the face. North American blastomycosis. Arch Dermatol. 2000;136:547, 550.
16. Klein BS, Vergeront JM, Kaufman L, et al. Serological tests for blastomycosis: assessments during a large point-source outbreak in Wisconsin. J Infect Dis. 1987;155:262-268.
Cutaneous North American blastomycosis is a deep fungal infection caused by Blastomyces dermatitidis, a thermally dimorphic fungus that is endemic to the Great Lakes region as well as the Mississippi and Ohio River valleys where it thrives in moist acidic soil enriched with organic material.1,2 In humans, the annual incidence rate is estimated to be 0.6 cases per million,3 though it may be as high as 42 cases per 100,000 in endemic areas.4 Infection typically results from the inhalation of conidia and manifests as either acute or chronic pneumonia.5 Most patients with acute disease present with nonspecific flulike symptoms and a nonproductive cough.
Dissemination occurs in approximately 25% of cases,6 most commonly affecting the skin. Other potential sites of dissemination include bone, the genitourinary tract, and the central nervous system. Cutaneous lesions, which may be either verrucous or ulcerative plaques, often occur on or around orifices contiguous to the respiratory tract.7 Verrucous lesions tend to have an irregular shape with well-defined borders and surface crusting. Ulcerative lesions have heaped-up borders and often have an exudative base.8 The differential diagnosis of cutaneous North American blastomycosis lesions includes squamous cell carcinoma, giant keratoacanthoma, verrucae, basal cell carcinoma, scrofuloderma, lupus vulgaris, nocardiosis, syphilis, bromoderma, iododerma, granuloma inguinale, tuberculosis verrucosa cutis, mycetoma, and actinomycosis.7,8
Although periorificial cutaneous manifestations of disseminated blastomycosis are common, perianal lesions are rare. The differential diagnosis of perianal verrucous plaques includes condyloma acuminatum, squamous cell carcinoma, adenocarcinoma, Buschke-Löwenstein tumor, actinomycosis, and localized fungal infections such as blastomycosis.9
Case Report
A 57-year-old man presented with a palpable perianal mass that produced small amounts of blood in his underwear and on toilet paper. The patient reported no history of hemorrhoids, anoreceptive intercourse, or sexually transmitted disease. Four months prior to presentation, he had a prolonged upper respiratory tract illness with a subjective fever and productive cough of 2 months’ duration. The patient described himself as an avid outdoorsman who worked at a summer resort and spent a great deal of time in the forests of central Wisconsin last autumn. Physical examination revealed a well-demarcated, firm, moist plaque with a verrucous surface that measured 3.5×2.7 cm and extended from the anal verge to the perianal skin (Figure 1).
Potassium hydroxide preparation of a biopsy specimen (Figure 2), a punch biopsy of the lesion (Figure 3), and Gomori methenamine-silver staining (Figure 4) revealed scattered yeast spores, some demonstrating broad-based budding, with pseudoepitheliomatous hyperplasia, dermal neutrophils, and intraepithelial microabscesses. The patient’s urine was positive for Blastomyces antigen (1.04 ng/mL). Chest radiography demonstrated a localized infiltrate in the right hilum with possible mass effect. Computed tomography showed a consolidative opacity measuring 4.0×3.4 cm in the upper lobe of the right lung (Figure 5).
 |  |
The patient was diagnosed with cutaneous North American blastomycosis and prescribed a 6-month course of oral itraconazole 200 mg twice daily. At his 3-month follow-up visit, the perianal plaque hadalmost completely resolved (Figure 6). However, because the patient had increasing lower extremity edema, subjective hearing loss, and abnormal liver function tests, itraconazole treatment was discontinued and replaced with oral fluconazole 400 mg daily for the next 3 months. The right hilar mass had visibly improved on follow-up chest radiography 2 months after the patient started antifungal therapy with itraconazole and had resolved within another 3 months of treatment.
 | 
|
Comment
Cutaneous blastomycosis results most often from the hematogenous spread of B dermatitidis from the lungs and rarely from direct inoculation.5,10 Skin lesions tend to occur on exposed areas, such as the face, scalp, hands, wrists, feet, and ankles.7,11-13 Dissemination to the perianal skin is rare, though it has been reported in 2 other patients; both patients, similar to our patient, had evidence of pulmonary involvement at some point in their clinical course.9,14
Diagnosis is based on identification of B dermatitidis by microscopy or culture. Potassium hydroxide preparation of biopsy specimens typically shows broad-based budding yeast.13 Characteristic findings of histopathologic studies include pseudo-epitheliomatous hyperplasia, intraepidermal abscesses, and a dermal infiltrate of polymorphonuclear leukocytes.15 On fungal culture, B dermatitidis is slow growing and may require a 2- to 4-week incubation period. Serologic tests are available, but sensitivity is low, at 9%, 28%, and 77% for complement fixation, immunodiffusion, and enzyme immunoassay, respectively.16
Conclusion
North American blastomycosis should be considered in patients who have verrucous or ulcerative perianal lesions and have lived in or traveled to endemic regions, especially if they have recent or ongoing pulmonary symptoms. Potassium hydroxide preparation and fungal staining of biopsy specimens can aid in diagnosis.
Acknowledgment
The authors thank the Marshfield Clinic Research Foundation’s Office of Scientific Writing and Publication (Marshfield, Wisconsin) for editorial assistance in the preparation of this manuscript.
Cutaneous North American blastomycosis is a deep fungal infection caused by Blastomyces dermatitidis, a thermally dimorphic fungus that is endemic to the Great Lakes region as well as the Mississippi and Ohio River valleys where it thrives in moist acidic soil enriched with organic material.1,2 In humans, the annual incidence rate is estimated to be 0.6 cases per million,3 though it may be as high as 42 cases per 100,000 in endemic areas.4 Infection typically results from the inhalation of conidia and manifests as either acute or chronic pneumonia.5 Most patients with acute disease present with nonspecific flulike symptoms and a nonproductive cough.
Dissemination occurs in approximately 25% of cases,6 most commonly affecting the skin. Other potential sites of dissemination include bone, the genitourinary tract, and the central nervous system. Cutaneous lesions, which may be either verrucous or ulcerative plaques, often occur on or around orifices contiguous to the respiratory tract.7 Verrucous lesions tend to have an irregular shape with well-defined borders and surface crusting. Ulcerative lesions have heaped-up borders and often have an exudative base.8 The differential diagnosis of cutaneous North American blastomycosis lesions includes squamous cell carcinoma, giant keratoacanthoma, verrucae, basal cell carcinoma, scrofuloderma, lupus vulgaris, nocardiosis, syphilis, bromoderma, iododerma, granuloma inguinale, tuberculosis verrucosa cutis, mycetoma, and actinomycosis.7,8
Although periorificial cutaneous manifestations of disseminated blastomycosis are common, perianal lesions are rare. The differential diagnosis of perianal verrucous plaques includes condyloma acuminatum, squamous cell carcinoma, adenocarcinoma, Buschke-Löwenstein tumor, actinomycosis, and localized fungal infections such as blastomycosis.9
Case Report
A 57-year-old man presented with a palpable perianal mass that produced small amounts of blood in his underwear and on toilet paper. The patient reported no history of hemorrhoids, anoreceptive intercourse, or sexually transmitted disease. Four months prior to presentation, he had a prolonged upper respiratory tract illness with a subjective fever and productive cough of 2 months’ duration. The patient described himself as an avid outdoorsman who worked at a summer resort and spent a great deal of time in the forests of central Wisconsin last autumn. Physical examination revealed a well-demarcated, firm, moist plaque with a verrucous surface that measured 3.5×2.7 cm and extended from the anal verge to the perianal skin (Figure 1).
Potassium hydroxide preparation of a biopsy specimen (Figure 2), a punch biopsy of the lesion (Figure 3), and Gomori methenamine-silver staining (Figure 4) revealed scattered yeast spores, some demonstrating broad-based budding, with pseudoepitheliomatous hyperplasia, dermal neutrophils, and intraepithelial microabscesses. The patient’s urine was positive for Blastomyces antigen (1.04 ng/mL). Chest radiography demonstrated a localized infiltrate in the right hilum with possible mass effect. Computed tomography showed a consolidative opacity measuring 4.0×3.4 cm in the upper lobe of the right lung (Figure 5).
 |  |
The patient was diagnosed with cutaneous North American blastomycosis and prescribed a 6-month course of oral itraconazole 200 mg twice daily. At his 3-month follow-up visit, the perianal plaque hadalmost completely resolved (Figure 6). However, because the patient had increasing lower extremity edema, subjective hearing loss, and abnormal liver function tests, itraconazole treatment was discontinued and replaced with oral fluconazole 400 mg daily for the next 3 months. The right hilar mass had visibly improved on follow-up chest radiography 2 months after the patient started antifungal therapy with itraconazole and had resolved within another 3 months of treatment.
 | 
|
Comment
Cutaneous blastomycosis results most often from the hematogenous spread of B dermatitidis from the lungs and rarely from direct inoculation.5,10 Skin lesions tend to occur on exposed areas, such as the face, scalp, hands, wrists, feet, and ankles.7,11-13 Dissemination to the perianal skin is rare, though it has been reported in 2 other patients; both patients, similar to our patient, had evidence of pulmonary involvement at some point in their clinical course.9,14
Diagnosis is based on identification of B dermatitidis by microscopy or culture. Potassium hydroxide preparation of biopsy specimens typically shows broad-based budding yeast.13 Characteristic findings of histopathologic studies include pseudo-epitheliomatous hyperplasia, intraepidermal abscesses, and a dermal infiltrate of polymorphonuclear leukocytes.15 On fungal culture, B dermatitidis is slow growing and may require a 2- to 4-week incubation period. Serologic tests are available, but sensitivity is low, at 9%, 28%, and 77% for complement fixation, immunodiffusion, and enzyme immunoassay, respectively.16
Conclusion
North American blastomycosis should be considered in patients who have verrucous or ulcerative perianal lesions and have lived in or traveled to endemic regions, especially if they have recent or ongoing pulmonary symptoms. Potassium hydroxide preparation and fungal staining of biopsy specimens can aid in diagnosis.
Acknowledgment
The authors thank the Marshfield Clinic Research Foundation’s Office of Scientific Writing and Publication (Marshfield, Wisconsin) for editorial assistance in the preparation of this manuscript.
1. Klein BS, Vergeront JM, Davis JP. Epidemiologic aspects of blastomycosis, the enigmatic systemic mycosis. Semin Respir Infect. 1986;1:29-39.
2. Klein BS, Vergeront JM, Weeks RJ, et al. Isolation of Blastomyces dermatitidis in soil associated with a large outbreak of blastomycosis in Wisconsin. N Engl J Med. 1986;314:529-534.
3. Reingold AL, Lu XD, Plikaytis BD, et al. Systemic mycoses in the United States, 1980-1982. J Med Vet Mycol. 1986;24:433-436.
4. Centers for Disease Control and Prevention (CDC). Blastomycosis—Wisconsin, 1986-1995. MMWR Morb Mortal Wkly Rep. 1996;45:601-603.
5. Smith JA, Kauffman CA. Blastomycosis. Proc Am Thorac Soc. 2010;7:173-180.
6. Goldman M, Johnson PC, Sarosi GA. Fungal pneumonias. the endemic mycoses. Clin Chest Med. 1999;20:507-519.
7. Mercurio MG, Elewski BE. Cutaneous blastomycosis. Cutis. 1992;50:422-424.
8. Saccente M, Woods GL. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev. 2010;23:367-381.
9. Ricciardi R, Alavi K, Filice GA, et al. Blastomyces dermatitidis of the perianal skin: report of a case. Dis Colon Rectum. 2007;50:118-121.
10. Gray NA, Baddour LM. Cutaneous inoculation blastomycosis [published online ahead of print April 17, 2002]. Clin Infect Dis. 2002;34:e44-e49.
11. Kisso B, Mahmoud F, Thakkar JR. Blastomycosis presenting as recurrent tender cutaneous nodules. S D Med. 2006;59:255-259.
12. Mandell GL, Bennett JE, Dolin R. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, PA: Churchill Livingstone/Elsevier; 2010.
13. Mason AR, Cortes GY, Cook J, et al. Cutaneous blastomycosis: a diagnostic challenge. Int J Dermatol. 2008;47:824-830.
14. Linn JE. Pseudo-epitheliomatous lesions of the perirectal tissue: report of a case of squamous epithelioma due to blastomycosis. South Med J. 1958;51:1101-1104.
15. Woofter MJ, Cripps DJ, Warner TF. Verrucous plaques on the face. North American blastomycosis. Arch Dermatol. 2000;136:547, 550.
16. Klein BS, Vergeront JM, Kaufman L, et al. Serological tests for blastomycosis: assessments during a large point-source outbreak in Wisconsin. J Infect Dis. 1987;155:262-268.
1. Klein BS, Vergeront JM, Davis JP. Epidemiologic aspects of blastomycosis, the enigmatic systemic mycosis. Semin Respir Infect. 1986;1:29-39.
2. Klein BS, Vergeront JM, Weeks RJ, et al. Isolation of Blastomyces dermatitidis in soil associated with a large outbreak of blastomycosis in Wisconsin. N Engl J Med. 1986;314:529-534.
3. Reingold AL, Lu XD, Plikaytis BD, et al. Systemic mycoses in the United States, 1980-1982. J Med Vet Mycol. 1986;24:433-436.
4. Centers for Disease Control and Prevention (CDC). Blastomycosis—Wisconsin, 1986-1995. MMWR Morb Mortal Wkly Rep. 1996;45:601-603.
5. Smith JA, Kauffman CA. Blastomycosis. Proc Am Thorac Soc. 2010;7:173-180.
6. Goldman M, Johnson PC, Sarosi GA. Fungal pneumonias. the endemic mycoses. Clin Chest Med. 1999;20:507-519.
7. Mercurio MG, Elewski BE. Cutaneous blastomycosis. Cutis. 1992;50:422-424.
8. Saccente M, Woods GL. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev. 2010;23:367-381.
9. Ricciardi R, Alavi K, Filice GA, et al. Blastomyces dermatitidis of the perianal skin: report of a case. Dis Colon Rectum. 2007;50:118-121.
10. Gray NA, Baddour LM. Cutaneous inoculation blastomycosis [published online ahead of print April 17, 2002]. Clin Infect Dis. 2002;34:e44-e49.
11. Kisso B, Mahmoud F, Thakkar JR. Blastomycosis presenting as recurrent tender cutaneous nodules. S D Med. 2006;59:255-259.
12. Mandell GL, Bennett JE, Dolin R. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, PA: Churchill Livingstone/Elsevier; 2010.
13. Mason AR, Cortes GY, Cook J, et al. Cutaneous blastomycosis: a diagnostic challenge. Int J Dermatol. 2008;47:824-830.
14. Linn JE. Pseudo-epitheliomatous lesions of the perirectal tissue: report of a case of squamous epithelioma due to blastomycosis. South Med J. 1958;51:1101-1104.
15. Woofter MJ, Cripps DJ, Warner TF. Verrucous plaques on the face. North American blastomycosis. Arch Dermatol. 2000;136:547, 550.
16. Klein BS, Vergeront JM, Kaufman L, et al. Serological tests for blastomycosis: assessments during a large point-source outbreak in Wisconsin. J Infect Dis. 1987;155:262-268.
Practice Points
- Cutaneous North American blastomycosis usually occurs in a periorificial distribution.
- The perianal region should be included in the periorificial regions considered in North American blastomycosis infections.
Nodular Scleroderma in a Patient With Chronic Hepatitis C Virus Infection: A Coexistent or Causal Infection?
Case Report
A 63-year-old woman was referred to our clinic for evaluation of multiple papules and nodules on the neck and trunk that had been present for 2 years. Three years prior to presentation she had been diagnosed with systemic sclerosis (SSc) after developing progressive diffuse cutaneous sclerosis, Raynaud phenomenon with digital pitted scarring, esophageal dysmotility, myositis, pericardial effusion, and interstitial lung disease. Serologic test results were positive for anti-Scl-70 antibodies. Antinuclear antibody test results were negative for anti–double-stranded DNA, anti-nRNP, anti-Ro/La, anti-Sm, and anti-Jo-1 antibodies. The patient was treated with prednisolone 7.5 mg daily, nifedipine 15 mg daily, valsartan 80 mg daily, manidipine 20 mg daily, omeprazole 20 mg daily, and beraprost 80 mg daily. One year later, numerous asymptomatic flesh-colored papules and nodules developed on the neck, chest, abdomen, and back. There was no history of trauma or surgery at any of the affected sites.
On further investigation, anti–hepatitis C virus (HCV) antibodies were identified and confirmed by HCV ribonucleic acid polymerase chain reaction at the same time that the diagnosis of SSc was established. Hepatitis C virus genotype 3a was noted, and the patient’s viral load was 378,000 IU/mL. Therefore, a diagnosis of chronic HCV infection was established. The patient was initially unable to receive medical treatment due to lack of finances. A year and a half following the diagnosis of HCV infection, with worsening liver function tests and increasing viral load (1,369,113 IU/mL), the patient began therapy with peginterferon alfa-2b 80 mg weekly and ribavirin 800 mg daily. However, the medications were discontinued after 2 months when she developed severe hemolytic anemia related to ribavirin.
On physical examination, the patient was noted to have a masklike facies with a pinched nose and constricted opening of the mouth. Her skin was tightened and stiff extending from the fingers to the proximal extremities. Numerous well-circumscribed, flesh-colored, firm papules and nodules ranging from 2 to 20 mm in diameter were present on the neck (Figure 1), chest, abdomen (Figure 2), and back.
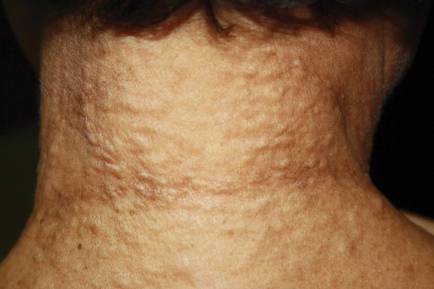 | 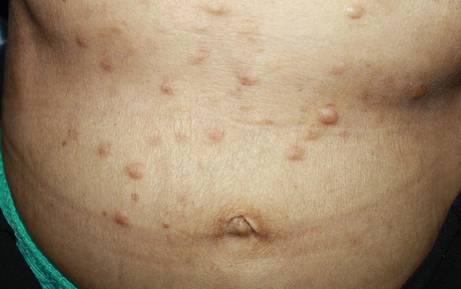 |
Two 4-mm punch biopsy samples obtained from a papule on the neck and a nodule on the abdomen revealed homogenized collagen bundles with scattered plump fibroblasts in the lower reticular dermis. Clinicopathologic correlation of the biopsy findings with the cutaneous examination resulted in a diagnosis of nodular scleroderma (Figures 3 and 4).
|
The patient began treatment with intralesional injections of triamcinolone 5 to 10 mg/mL for nodules as well as an ultrapotent corticosteroid cream, clobetasol propionate 0.05%, for small papules. Injections were performed at 4- to 8-week intervals and resulted in modest clinical improvement.
Comment
Scleroderma may be present only in the skin (morphea) or as a systemic disease (systemic scleroderma). Rarely, cutaneous involvement can exhibit a nodular or hypertrophic morphology, which has been described in the literature as nodular or keloidal scleroderma in a patient with known SSc1-10 and as nodular or keloidal morphea in localized cutaneous scleroderma.3,11-13
Histopathology
The distinction between the terms nodular scleroderma and keloidal scleroderma is not clear, and they are not necessarily interchangeable. To provide clarity, we find it useful to delineate specific histologic findings associated with the diagnoses of keloid, scleroderma, and the uncommon keloid/scleroderma overlap. The histopathologic findings of keloids include a fibrotic dermis and broad dispersed bundles of eosinophilic hyalinized collagen. The histopathologic findings of scleroderma include broad sclerotic bands of collagen throughout the dermis with loss of perieccrine fat. In the overlapping keloid/scleroderma condition, which is a variant of scleroderma, hyalinized collagen fibers and keloidal collagen appear in the same specimen.3,4
To distinguish these conditions, Barzilai et al5 proposed that only cases showing both clinical and histologic characteristics of a keloid should be referred to as keloidal morphea/scleroderma. They further stated that the terms nodular morphea or nodular scleroderma ought to be used only for cases that are indistinguishable histologically from scleroderma. The term morphea is appropriate when only a limited amount of skin disease is present, while scleroderma implies association with systemic disease.5 Likely, there is a histologic continuum in this variant of scleroderma, in which nodular morphea/scleroderma exists at one end and keloidal morphea/scleroderma exists at the other end.5,13
In the case of our patient, papulonodular lesions developed 1 year after the diagnosis of SSc was made, and the histopathologic examination revealed classic findings of scleroderma. As a result, our patient is most appropriately classified as having nodular scleroderma.
Clinical Features
Nodular scleroderma mostly affects young and middle-aged women and is clinically characterized by solitary or multiple firm, long-lasting papules or nodules on the upper trunk and chest, neck, and proximal extremities.1-4,6
Etiology and Pathogenesis
The triggers and cellular mechanisms of nodular scleroderma are unclear. Some authors have implicated matricellular protein and growth factors such as tenascin, connective tissue growth factor, and epidermal growth factor in nodule formation.7,8,11 Yamamoto et al9 cited chemical exposure to a silica-containing abrasive as the cause of nodular scleroderma in a worker.
Possible HCV Association
Some reports have indicated an association between nodular scleroderma and pathogens such as acid-fast bacteria10 and HCV.6 Of note, many extrahepatic conditions have been associated with HCV infection, such as membranoproliferative glomerulonephritis, cutaneous vasculitis, lichen planus, and porphyria cutanea tarda.14
The association of HCV infection with systemic autoimmune disease (SAD) has been described in a number of instances; cryoglobulinemia has most commonly been linked to HCV.15 Although the association between HCV and other SADs is less clear, there is growing interest in a possible relationship between them. To that end, physicians of the HISPAMEC (Hispanoamerican Study Group of Autoimmune Manifestations Associated With Hepatitis C Virus) study group described the clinical and immunologic characteristics of 1020 patients with SAD and associated chronic HCV infection. The 3 most frequent SADs (>90% of cases) were Sjögren syndrome, rheumatoid arthritis, and systemic lupus erythematosus.16 However, the strength of association differs for each SAD based on existing descriptions.16,17 Less commonly, there may be a causal relationship between HCV infection and SSc. It should be noted that most of these data are based on small series and case reports.6,16-19
The role of HCV in the pathogenesis of systemic scleroderma and other autoimmune diseases is unknown. It is also possible that the replication of HCV outside the liver, particularly in mononuclear cells, may suppress immune tolerance in genetically predisposed individuals.20
Conclusion
Nodular scleroderma associated with HCV infection is a rare entity. At present, it cannot be determined whether there is an etiopathologic association between HCV infection and SSc or whether the simultaneous diagnosis may be coincidental. Routine determination of HCV serology in scleroderma patients may help to clarify this issue.
1. Krell JM, Solomon AR, Glavey CM, et al. Nodular scleroderma. J Am Acad Dermatol. 1995;32:343-345.
2. Cannick L 3rd, Douglas G, Crater S, et al. Nodular scleroderma: case report and literature review. J Rheumatol. 2003;30:2500-2502.
3. Rencic A, Brinster NK, Nousari CH. Keloid morphea and nodular scleroderma: two distinct clinical variants of scleroderma? J Cutan Med Surg. 2003;7:20-24.
4. Wriston CC, Rubin AI, Elenitsas R, et al. Nodular scleroderma: a report of 2 cases. Am J Dermatopathol. 2008;30:385-388.
5. Barzilai A, Lyakhovitsky A, Horowitz A, et al. Keloid-like scleroderma. Am J Dermatopathol. 2003;25:327-330.
6. Melani L, Caproni M, Cardinali C, et al. A case of nodular scleroderma. J Dermatol. 2005;32:1028-1031.
7. Mizutani H, Taniguchi H, Sakakura T, et al. Nodular scleroderma: focally increased tenascin expression differing from that in the surrounding scleroderma skin. J Dermatol. 1995;22:267-271.
8. Yamamoto T, Sawada Y, Katayama I, et al. Nodular scleroderma: increased expression of connective tissue growth factor. Dermatology. 2005;211:218-223.
9. Yamamoto T, Furuse Y, Katayama I, et al. Nodular scleroderma in a worker using a silica-containing abrasive. J Dermatol. 1994;21:751-754.
10. Cantwell AR Jr, Rowe L, Kelso DW. Nodular scleroderma and pleomorphic acid-fast bacteria. Arch Dermatol. 1980;116:1283-1290.
11. Yamamoto T, Sakashita S, Sawada Y, et al. Possible role of epidermal growth factor in the lesional skin of nodular morphea. Acta Derm Venereol. 1998;78:312-313.
12. Jain K, Dayal S, Jain VK, et al. Blaschko linear nodular morphea with dermal mucinosis. Arch Dermatol. 2007;143:953-955.
13. Kauer F, Simon JC, Sticherling M. Nodular morphea. Dermatology. 2009;218:63-66.
14. Gumber SC, Chopra S. Hepatitis C: a multifaceted disease. review of extrahepatic manifestations. Ann Intern Med. 1995;123:615-620.
15. Ferri C, Greco F, Longombardo G, et al. Antibodies to hepatitis C virus in patients with mixed cryoglobulinemia. Arthritis Rheum. 1991;34:1606-1610.
16. Ramos-Casals M, Munoz S, Medina F, et al. Systemic autoimmune diseases in patients with hepatitis C virus infection: characterization of 1020 cases (The HISPAMEC Registry). J Rheumatol. 2009;36:1442-1448.
17. Ramos-Casals M, Jara LJ, Medina F, et al. Systemic autoimmune diseases co-existing with chronic hepatitis C virus infection (the HISPAMEC Registry): patterns of clinical and immunological expression in 180 cases. J Intern Med. 2005;257:549-557.
18. Abu-Shakra M, Sukenik S, Buskila D. Systemic sclerosis: another rheumatic disease associated with hepatitis C virus infection. Clin Rheumatol. 2000;19:378-380.
19. Yamamoto M, Yamamoto T, Tsuboi R. Discoid lupus erythematosus in a patient with scleroderma and hepatitis C virus infection. Rheumatol Int. 2010;30:969-971.
20. Abu-Shakra M, Shoenfeld Y. Chronic infections and autoimmunity. Immunol Ser. 1992;55:285-313.
Case Report
A 63-year-old woman was referred to our clinic for evaluation of multiple papules and nodules on the neck and trunk that had been present for 2 years. Three years prior to presentation she had been diagnosed with systemic sclerosis (SSc) after developing progressive diffuse cutaneous sclerosis, Raynaud phenomenon with digital pitted scarring, esophageal dysmotility, myositis, pericardial effusion, and interstitial lung disease. Serologic test results were positive for anti-Scl-70 antibodies. Antinuclear antibody test results were negative for anti–double-stranded DNA, anti-nRNP, anti-Ro/La, anti-Sm, and anti-Jo-1 antibodies. The patient was treated with prednisolone 7.5 mg daily, nifedipine 15 mg daily, valsartan 80 mg daily, manidipine 20 mg daily, omeprazole 20 mg daily, and beraprost 80 mg daily. One year later, numerous asymptomatic flesh-colored papules and nodules developed on the neck, chest, abdomen, and back. There was no history of trauma or surgery at any of the affected sites.
On further investigation, anti–hepatitis C virus (HCV) antibodies were identified and confirmed by HCV ribonucleic acid polymerase chain reaction at the same time that the diagnosis of SSc was established. Hepatitis C virus genotype 3a was noted, and the patient’s viral load was 378,000 IU/mL. Therefore, a diagnosis of chronic HCV infection was established. The patient was initially unable to receive medical treatment due to lack of finances. A year and a half following the diagnosis of HCV infection, with worsening liver function tests and increasing viral load (1,369,113 IU/mL), the patient began therapy with peginterferon alfa-2b 80 mg weekly and ribavirin 800 mg daily. However, the medications were discontinued after 2 months when she developed severe hemolytic anemia related to ribavirin.
On physical examination, the patient was noted to have a masklike facies with a pinched nose and constricted opening of the mouth. Her skin was tightened and stiff extending from the fingers to the proximal extremities. Numerous well-circumscribed, flesh-colored, firm papules and nodules ranging from 2 to 20 mm in diameter were present on the neck (Figure 1), chest, abdomen (Figure 2), and back.
 |  |
Two 4-mm punch biopsy samples obtained from a papule on the neck and a nodule on the abdomen revealed homogenized collagen bundles with scattered plump fibroblasts in the lower reticular dermis. Clinicopathologic correlation of the biopsy findings with the cutaneous examination resulted in a diagnosis of nodular scleroderma (Figures 3 and 4).
|
The patient began treatment with intralesional injections of triamcinolone 5 to 10 mg/mL for nodules as well as an ultrapotent corticosteroid cream, clobetasol propionate 0.05%, for small papules. Injections were performed at 4- to 8-week intervals and resulted in modest clinical improvement.
Comment
Scleroderma may be present only in the skin (morphea) or as a systemic disease (systemic scleroderma). Rarely, cutaneous involvement can exhibit a nodular or hypertrophic morphology, which has been described in the literature as nodular or keloidal scleroderma in a patient with known SSc1-10 and as nodular or keloidal morphea in localized cutaneous scleroderma.3,11-13
Histopathology
The distinction between the terms nodular scleroderma and keloidal scleroderma is not clear, and they are not necessarily interchangeable. To provide clarity, we find it useful to delineate specific histologic findings associated with the diagnoses of keloid, scleroderma, and the uncommon keloid/scleroderma overlap. The histopathologic findings of keloids include a fibrotic dermis and broad dispersed bundles of eosinophilic hyalinized collagen. The histopathologic findings of scleroderma include broad sclerotic bands of collagen throughout the dermis with loss of perieccrine fat. In the overlapping keloid/scleroderma condition, which is a variant of scleroderma, hyalinized collagen fibers and keloidal collagen appear in the same specimen.3,4
To distinguish these conditions, Barzilai et al5 proposed that only cases showing both clinical and histologic characteristics of a keloid should be referred to as keloidal morphea/scleroderma. They further stated that the terms nodular morphea or nodular scleroderma ought to be used only for cases that are indistinguishable histologically from scleroderma. The term morphea is appropriate when only a limited amount of skin disease is present, while scleroderma implies association with systemic disease.5 Likely, there is a histologic continuum in this variant of scleroderma, in which nodular morphea/scleroderma exists at one end and keloidal morphea/scleroderma exists at the other end.5,13
In the case of our patient, papulonodular lesions developed 1 year after the diagnosis of SSc was made, and the histopathologic examination revealed classic findings of scleroderma. As a result, our patient is most appropriately classified as having nodular scleroderma.
Clinical Features
Nodular scleroderma mostly affects young and middle-aged women and is clinically characterized by solitary or multiple firm, long-lasting papules or nodules on the upper trunk and chest, neck, and proximal extremities.1-4,6
Etiology and Pathogenesis
The triggers and cellular mechanisms of nodular scleroderma are unclear. Some authors have implicated matricellular protein and growth factors such as tenascin, connective tissue growth factor, and epidermal growth factor in nodule formation.7,8,11 Yamamoto et al9 cited chemical exposure to a silica-containing abrasive as the cause of nodular scleroderma in a worker.
Possible HCV Association
Some reports have indicated an association between nodular scleroderma and pathogens such as acid-fast bacteria10 and HCV.6 Of note, many extrahepatic conditions have been associated with HCV infection, such as membranoproliferative glomerulonephritis, cutaneous vasculitis, lichen planus, and porphyria cutanea tarda.14
The association of HCV infection with systemic autoimmune disease (SAD) has been described in a number of instances; cryoglobulinemia has most commonly been linked to HCV.15 Although the association between HCV and other SADs is less clear, there is growing interest in a possible relationship between them. To that end, physicians of the HISPAMEC (Hispanoamerican Study Group of Autoimmune Manifestations Associated With Hepatitis C Virus) study group described the clinical and immunologic characteristics of 1020 patients with SAD and associated chronic HCV infection. The 3 most frequent SADs (>90% of cases) were Sjögren syndrome, rheumatoid arthritis, and systemic lupus erythematosus.16 However, the strength of association differs for each SAD based on existing descriptions.16,17 Less commonly, there may be a causal relationship between HCV infection and SSc. It should be noted that most of these data are based on small series and case reports.6,16-19
The role of HCV in the pathogenesis of systemic scleroderma and other autoimmune diseases is unknown. It is also possible that the replication of HCV outside the liver, particularly in mononuclear cells, may suppress immune tolerance in genetically predisposed individuals.20
Conclusion
Nodular scleroderma associated with HCV infection is a rare entity. At present, it cannot be determined whether there is an etiopathologic association between HCV infection and SSc or whether the simultaneous diagnosis may be coincidental. Routine determination of HCV serology in scleroderma patients may help to clarify this issue.
Case Report
A 63-year-old woman was referred to our clinic for evaluation of multiple papules and nodules on the neck and trunk that had been present for 2 years. Three years prior to presentation she had been diagnosed with systemic sclerosis (SSc) after developing progressive diffuse cutaneous sclerosis, Raynaud phenomenon with digital pitted scarring, esophageal dysmotility, myositis, pericardial effusion, and interstitial lung disease. Serologic test results were positive for anti-Scl-70 antibodies. Antinuclear antibody test results were negative for anti–double-stranded DNA, anti-nRNP, anti-Ro/La, anti-Sm, and anti-Jo-1 antibodies. The patient was treated with prednisolone 7.5 mg daily, nifedipine 15 mg daily, valsartan 80 mg daily, manidipine 20 mg daily, omeprazole 20 mg daily, and beraprost 80 mg daily. One year later, numerous asymptomatic flesh-colored papules and nodules developed on the neck, chest, abdomen, and back. There was no history of trauma or surgery at any of the affected sites.
On further investigation, anti–hepatitis C virus (HCV) antibodies were identified and confirmed by HCV ribonucleic acid polymerase chain reaction at the same time that the diagnosis of SSc was established. Hepatitis C virus genotype 3a was noted, and the patient’s viral load was 378,000 IU/mL. Therefore, a diagnosis of chronic HCV infection was established. The patient was initially unable to receive medical treatment due to lack of finances. A year and a half following the diagnosis of HCV infection, with worsening liver function tests and increasing viral load (1,369,113 IU/mL), the patient began therapy with peginterferon alfa-2b 80 mg weekly and ribavirin 800 mg daily. However, the medications were discontinued after 2 months when she developed severe hemolytic anemia related to ribavirin.
On physical examination, the patient was noted to have a masklike facies with a pinched nose and constricted opening of the mouth. Her skin was tightened and stiff extending from the fingers to the proximal extremities. Numerous well-circumscribed, flesh-colored, firm papules and nodules ranging from 2 to 20 mm in diameter were present on the neck (Figure 1), chest, abdomen (Figure 2), and back.
 |  |
Two 4-mm punch biopsy samples obtained from a papule on the neck and a nodule on the abdomen revealed homogenized collagen bundles with scattered plump fibroblasts in the lower reticular dermis. Clinicopathologic correlation of the biopsy findings with the cutaneous examination resulted in a diagnosis of nodular scleroderma (Figures 3 and 4).
|
The patient began treatment with intralesional injections of triamcinolone 5 to 10 mg/mL for nodules as well as an ultrapotent corticosteroid cream, clobetasol propionate 0.05%, for small papules. Injections were performed at 4- to 8-week intervals and resulted in modest clinical improvement.
Comment
Scleroderma may be present only in the skin (morphea) or as a systemic disease (systemic scleroderma). Rarely, cutaneous involvement can exhibit a nodular or hypertrophic morphology, which has been described in the literature as nodular or keloidal scleroderma in a patient with known SSc1-10 and as nodular or keloidal morphea in localized cutaneous scleroderma.3,11-13
Histopathology
The distinction between the terms nodular scleroderma and keloidal scleroderma is not clear, and they are not necessarily interchangeable. To provide clarity, we find it useful to delineate specific histologic findings associated with the diagnoses of keloid, scleroderma, and the uncommon keloid/scleroderma overlap. The histopathologic findings of keloids include a fibrotic dermis and broad dispersed bundles of eosinophilic hyalinized collagen. The histopathologic findings of scleroderma include broad sclerotic bands of collagen throughout the dermis with loss of perieccrine fat. In the overlapping keloid/scleroderma condition, which is a variant of scleroderma, hyalinized collagen fibers and keloidal collagen appear in the same specimen.3,4
To distinguish these conditions, Barzilai et al5 proposed that only cases showing both clinical and histologic characteristics of a keloid should be referred to as keloidal morphea/scleroderma. They further stated that the terms nodular morphea or nodular scleroderma ought to be used only for cases that are indistinguishable histologically from scleroderma. The term morphea is appropriate when only a limited amount of skin disease is present, while scleroderma implies association with systemic disease.5 Likely, there is a histologic continuum in this variant of scleroderma, in which nodular morphea/scleroderma exists at one end and keloidal morphea/scleroderma exists at the other end.5,13
In the case of our patient, papulonodular lesions developed 1 year after the diagnosis of SSc was made, and the histopathologic examination revealed classic findings of scleroderma. As a result, our patient is most appropriately classified as having nodular scleroderma.
Clinical Features
Nodular scleroderma mostly affects young and middle-aged women and is clinically characterized by solitary or multiple firm, long-lasting papules or nodules on the upper trunk and chest, neck, and proximal extremities.1-4,6
Etiology and Pathogenesis
The triggers and cellular mechanisms of nodular scleroderma are unclear. Some authors have implicated matricellular protein and growth factors such as tenascin, connective tissue growth factor, and epidermal growth factor in nodule formation.7,8,11 Yamamoto et al9 cited chemical exposure to a silica-containing abrasive as the cause of nodular scleroderma in a worker.
Possible HCV Association
Some reports have indicated an association between nodular scleroderma and pathogens such as acid-fast bacteria10 and HCV.6 Of note, many extrahepatic conditions have been associated with HCV infection, such as membranoproliferative glomerulonephritis, cutaneous vasculitis, lichen planus, and porphyria cutanea tarda.14
The association of HCV infection with systemic autoimmune disease (SAD) has been described in a number of instances; cryoglobulinemia has most commonly been linked to HCV.15 Although the association between HCV and other SADs is less clear, there is growing interest in a possible relationship between them. To that end, physicians of the HISPAMEC (Hispanoamerican Study Group of Autoimmune Manifestations Associated With Hepatitis C Virus) study group described the clinical and immunologic characteristics of 1020 patients with SAD and associated chronic HCV infection. The 3 most frequent SADs (>90% of cases) were Sjögren syndrome, rheumatoid arthritis, and systemic lupus erythematosus.16 However, the strength of association differs for each SAD based on existing descriptions.16,17 Less commonly, there may be a causal relationship between HCV infection and SSc. It should be noted that most of these data are based on small series and case reports.6,16-19
The role of HCV in the pathogenesis of systemic scleroderma and other autoimmune diseases is unknown. It is also possible that the replication of HCV outside the liver, particularly in mononuclear cells, may suppress immune tolerance in genetically predisposed individuals.20
Conclusion
Nodular scleroderma associated with HCV infection is a rare entity. At present, it cannot be determined whether there is an etiopathologic association between HCV infection and SSc or whether the simultaneous diagnosis may be coincidental. Routine determination of HCV serology in scleroderma patients may help to clarify this issue.
1. Krell JM, Solomon AR, Glavey CM, et al. Nodular scleroderma. J Am Acad Dermatol. 1995;32:343-345.
2. Cannick L 3rd, Douglas G, Crater S, et al. Nodular scleroderma: case report and literature review. J Rheumatol. 2003;30:2500-2502.
3. Rencic A, Brinster NK, Nousari CH. Keloid morphea and nodular scleroderma: two distinct clinical variants of scleroderma? J Cutan Med Surg. 2003;7:20-24.
4. Wriston CC, Rubin AI, Elenitsas R, et al. Nodular scleroderma: a report of 2 cases. Am J Dermatopathol. 2008;30:385-388.
5. Barzilai A, Lyakhovitsky A, Horowitz A, et al. Keloid-like scleroderma. Am J Dermatopathol. 2003;25:327-330.
6. Melani L, Caproni M, Cardinali C, et al. A case of nodular scleroderma. J Dermatol. 2005;32:1028-1031.
7. Mizutani H, Taniguchi H, Sakakura T, et al. Nodular scleroderma: focally increased tenascin expression differing from that in the surrounding scleroderma skin. J Dermatol. 1995;22:267-271.
8. Yamamoto T, Sawada Y, Katayama I, et al. Nodular scleroderma: increased expression of connective tissue growth factor. Dermatology. 2005;211:218-223.
9. Yamamoto T, Furuse Y, Katayama I, et al. Nodular scleroderma in a worker using a silica-containing abrasive. J Dermatol. 1994;21:751-754.
10. Cantwell AR Jr, Rowe L, Kelso DW. Nodular scleroderma and pleomorphic acid-fast bacteria. Arch Dermatol. 1980;116:1283-1290.
11. Yamamoto T, Sakashita S, Sawada Y, et al. Possible role of epidermal growth factor in the lesional skin of nodular morphea. Acta Derm Venereol. 1998;78:312-313.
12. Jain K, Dayal S, Jain VK, et al. Blaschko linear nodular morphea with dermal mucinosis. Arch Dermatol. 2007;143:953-955.
13. Kauer F, Simon JC, Sticherling M. Nodular morphea. Dermatology. 2009;218:63-66.
14. Gumber SC, Chopra S. Hepatitis C: a multifaceted disease. review of extrahepatic manifestations. Ann Intern Med. 1995;123:615-620.
15. Ferri C, Greco F, Longombardo G, et al. Antibodies to hepatitis C virus in patients with mixed cryoglobulinemia. Arthritis Rheum. 1991;34:1606-1610.
16. Ramos-Casals M, Munoz S, Medina F, et al. Systemic autoimmune diseases in patients with hepatitis C virus infection: characterization of 1020 cases (The HISPAMEC Registry). J Rheumatol. 2009;36:1442-1448.
17. Ramos-Casals M, Jara LJ, Medina F, et al. Systemic autoimmune diseases co-existing with chronic hepatitis C virus infection (the HISPAMEC Registry): patterns of clinical and immunological expression in 180 cases. J Intern Med. 2005;257:549-557.
18. Abu-Shakra M, Sukenik S, Buskila D. Systemic sclerosis: another rheumatic disease associated with hepatitis C virus infection. Clin Rheumatol. 2000;19:378-380.
19. Yamamoto M, Yamamoto T, Tsuboi R. Discoid lupus erythematosus in a patient with scleroderma and hepatitis C virus infection. Rheumatol Int. 2010;30:969-971.
20. Abu-Shakra M, Shoenfeld Y. Chronic infections and autoimmunity. Immunol Ser. 1992;55:285-313.
1. Krell JM, Solomon AR, Glavey CM, et al. Nodular scleroderma. J Am Acad Dermatol. 1995;32:343-345.
2. Cannick L 3rd, Douglas G, Crater S, et al. Nodular scleroderma: case report and literature review. J Rheumatol. 2003;30:2500-2502.
3. Rencic A, Brinster NK, Nousari CH. Keloid morphea and nodular scleroderma: two distinct clinical variants of scleroderma? J Cutan Med Surg. 2003;7:20-24.
4. Wriston CC, Rubin AI, Elenitsas R, et al. Nodular scleroderma: a report of 2 cases. Am J Dermatopathol. 2008;30:385-388.
5. Barzilai A, Lyakhovitsky A, Horowitz A, et al. Keloid-like scleroderma. Am J Dermatopathol. 2003;25:327-330.
6. Melani L, Caproni M, Cardinali C, et al. A case of nodular scleroderma. J Dermatol. 2005;32:1028-1031.
7. Mizutani H, Taniguchi H, Sakakura T, et al. Nodular scleroderma: focally increased tenascin expression differing from that in the surrounding scleroderma skin. J Dermatol. 1995;22:267-271.
8. Yamamoto T, Sawada Y, Katayama I, et al. Nodular scleroderma: increased expression of connective tissue growth factor. Dermatology. 2005;211:218-223.
9. Yamamoto T, Furuse Y, Katayama I, et al. Nodular scleroderma in a worker using a silica-containing abrasive. J Dermatol. 1994;21:751-754.
10. Cantwell AR Jr, Rowe L, Kelso DW. Nodular scleroderma and pleomorphic acid-fast bacteria. Arch Dermatol. 1980;116:1283-1290.
11. Yamamoto T, Sakashita S, Sawada Y, et al. Possible role of epidermal growth factor in the lesional skin of nodular morphea. Acta Derm Venereol. 1998;78:312-313.
12. Jain K, Dayal S, Jain VK, et al. Blaschko linear nodular morphea with dermal mucinosis. Arch Dermatol. 2007;143:953-955.
13. Kauer F, Simon JC, Sticherling M. Nodular morphea. Dermatology. 2009;218:63-66.
14. Gumber SC, Chopra S. Hepatitis C: a multifaceted disease. review of extrahepatic manifestations. Ann Intern Med. 1995;123:615-620.
15. Ferri C, Greco F, Longombardo G, et al. Antibodies to hepatitis C virus in patients with mixed cryoglobulinemia. Arthritis Rheum. 1991;34:1606-1610.
16. Ramos-Casals M, Munoz S, Medina F, et al. Systemic autoimmune diseases in patients with hepatitis C virus infection: characterization of 1020 cases (The HISPAMEC Registry). J Rheumatol. 2009;36:1442-1448.
17. Ramos-Casals M, Jara LJ, Medina F, et al. Systemic autoimmune diseases co-existing with chronic hepatitis C virus infection (the HISPAMEC Registry): patterns of clinical and immunological expression in 180 cases. J Intern Med. 2005;257:549-557.
18. Abu-Shakra M, Sukenik S, Buskila D. Systemic sclerosis: another rheumatic disease associated with hepatitis C virus infection. Clin Rheumatol. 2000;19:378-380.
19. Yamamoto M, Yamamoto T, Tsuboi R. Discoid lupus erythematosus in a patient with scleroderma and hepatitis C virus infection. Rheumatol Int. 2010;30:969-971.
20. Abu-Shakra M, Shoenfeld Y. Chronic infections and autoimmunity. Immunol Ser. 1992;55:285-313.
Practice Points
- Nodular scleroderma is a rare form of cutaneous scleroderma that can occur in association with systemic scleroderma or localized morphea.
- The clinical features are characterized by solitary or multiple, firm, long-lasting papules or nodules on the neck, upper trunk, and proximal extremities.
- The pathogenesis is still unclear. Some reports have suggested that matricellular protein and growth factor, acid-fast bacteria, organic solvents, or the hepatitis C virus may be involved.
Intrinsic Healing of the Anterior Cruciate Ligament in an Adolescent
The anterior cruciate ligament (ACL) restrains anterior translation of the tibia on the femur and controls rotation of the knee. The natural primary healing potential of the ACL has been extremely poor in clinical and experimental studies, and primary suture repair has not provided stability to the joint in most patients.1-8 This has led surgeons to reconstruct the ACL, rather than to attempt nonoperative treatment. Anterior cruciate ligament reconstruction is recommended to help patients maintain activities that place shear and torque forces on the knee or to ameliorate persistent pain due to instability.9 Reconstruction of the ACL in adults is one of the most common procedures performed by orthopedic surgeons. However, reconstruction in the ACL-deficient adolescent remains a controversial subject, with debates surrounding operative timing and surgical technique.
This case report presents a skeletally immature patient who suffered a complete traumatic rupture of his ACL, which intrinsically healed. The patient had a protracted treatment course, complicated by an open tibial fracture with delayed union. He responded to a progressive rehabilitation program and has made a good functional recovery. Review of the literature has demonstrated limited evidence of intrinsic ACL healing, none of which has been shown to occur in a skeletally immature patient. The patient’s mother provided written informed consent for print and electronic publication of this case report.
Case Report
A 12-year-old boy was brought to our level I trauma center by ambulance after being hit by a car while riding a motorized scooter. He presented with a grade IIIB open tibial fracture and a distal fibula fracture of his left lower extremity and was taken to the operating room that night for irrigation and débridement, percutaneous fixation of the fibula, and intramedullary flexible nail fixation of the tibia. On postoperative day 1, he had increasing pain and, once his splint was removed, his compartments were found to be very tense. He was taken emergently to the operating room for 4 compartment fasciotomies of the left lower extremity with wound vacuum-assisted closure (VAC) placement. This was changed on hospital day 4 and was removed with definitive closure on day 7. Examination under anesthesia prior to the final wound VAC change was performed given the patient’s complaints during physical therapy. This showed anterior and posterior ligamentous instability of the knee, and he was placed in a knee immobilizer. He was discharged on hospital day 11.
At 2-week follow-up, the patient was doing well, except that he was nonadherent with the knee immobilizer and unable to fully extend his left knee. On examination, a posterior drawer sign was noted; therefore, the patient was referred for magnetic resonance imaging (MRI) to evaluate his ligaments. His MRI, 9 weeks after injury, showed: (1) complete tears of both the anterior and posterior cruciate ligaments (PCLs) (Figures 1A, 1B); (2) medial meniscus and lateral meniscus tears; (3) 2.0-cm plate-like avulsion fracture of the posterolateral femoral metaphysis involving the insertion of the lateral head of the gastrocnemius muscle, fibular collateral ligament, and popliteus muscle (Figure 2); and (4) left posterior lateral tibial plateau contusion.
The patient was started on a 6-week course of physical therapy with active and active-assisted extension exercises. At follow-up approximately 3½ months after injury, he was found to have a 35º flexion contracture with pain at the end extension. Unfortunately, his tibial fracture showed minimal signs of healing, and the decision was made to delay surgical intervention on the knee until the tibial fracture had healed. He was given a knee orthotic to wear at night to help regain his knee extension.
Six months after injury, the patient underwent open removal of the avulsed bony fragment, posterior knee capsule release, and autograft of the delayed union tibial fracture. He was placed in a straight leg cast postoperatively and was discharged home on postoperative day 2. He transitioned to a knee immobilizer after 2 weeks. Six weeks after the last surgery, he had range of motion of 0º to 130º. Ligamentous examination at this time showed anterior and posterior drawer signs, positive Lachman test, and dial test with 90º of external rotation. He was placed in physical therapy for a total of 10 weeks to work on his quadriceps muscle strength and 15º extension lag.
On 13-month postinjury radiographs, the patient was noted to have adequate healing of his tibial fracture, and ligamentous reconstruction was discussed. At this time, the patient did not have any instability or pain in the knee. Examination demonstrated a very mild effusion of the left knee. Range of motion determined by goniometer was from -3º to 140º, and Lachman test was positive but with solid 2+ endpoint. He also had a positive posterior drawer sign with no endpoint, positive sag sign of his tibia, and positive active quadriceps test of the left leg. His dial test showed some increased external rotation at 90º but was equivocal at 30º when compared with the contralateral knee, demonstrating involvement of the posterolateral corner.
Sixteen months after injury, repeat MRI to further evaluate the posterolateral corner showed: (1) complete medial and lateral meniscal healing without evidence of residual or recurrent tear, and (2) interval healing of the remote ACL and PCL tears with intact insertions (Figures 3A, 3B). This scan showed an end-to-end continuous ACL with homogeneous signal and disappearance of the secondary signs. Physical examination at this time showed a very firm endpoint on Lachman test but some laxity with his posterior drawer. Given these findings, the patient was given a brace and continued in physical therapy to strengthen his quadriceps muscle. By 20 months after injury, he had returned to competitive hockey and had no complaints of pain or instability. His physical examination showed full range of motion in a ligamentously stable knee with firm endpoint. The patient’s condition was unchanged at 29-month follow-up.
Discussion
There is a body of evidence that states a completely ruptured ACL does not heal.3,6,10 In animal models, the ACL has been shown to have poor healing potential.3,11 Some studies have suggested this is secondary to poor blood supply. Blood supply to the ACL is derived from a periligamentous, then endoligamentous, arterial network with a less vascularized area in the middle third of the ACL. Additionally, there is no blood supply from the tibia or femur, meaning the areas of attachment of the ligament are poorly vascularized.12 With a minimal blood supply to the ACL, the supply of undifferentiated mesenchymal cells from the surrounding tissue during the initial healing process is limited. In vitro cell cultures of these cells have showed a reduced potential for proliferation and migration.9 Cells of the ACL have a lower response to growth factors than human medial collateral ligament cells, further suggesting a decreased reparative capacity.7 Joint fluid has been shown to inhibit the proliferation of these cells, further reducing their regenerative potential.13 Additionally, biomechanical factors that alter signaling pathways, sites of ligament reattachment, and injury to proprioceptive structures have been shown to negatively influence the healing response.14-18
Review of the literature on healing of ACLs includes 2 case reports, totaling 3 patients, and 3 level IV therapeutic studies involving 74 patients total.10,19-22 In most cases, the authors of these studies have indicated a nonoperative treatment protocol with bracing and a specific rehabilitation program. Malanga and colleagues10 demonstrated that an ACL torn from its attachment on the femur, with the majority of the ligament in good condition and no compromise in the length, healed back onto the femur. Kurosaka and coauthors20 described case reports of isolated distal or proximal midsubstance tears that have healed spontaneously. However, none of the patients described in the literature were under the age of 20 years.
Treatment for pediatric patients with open physes causes some debate. Nonoperative management of ACL deficiency in adolescents is generally not recommended because the continued instability of the joint leads to intra-articular injury, functional impairment, and joint degeneration.23-25 A recent systematic review found only 1 study that showed no increase in secondary intra-articular injury when surgery was delayed until skeletal maturity.26
Our patient was a 12-year-old boy whose traumatic knee injury with multiple ruptured ligaments healed over the course of 20 months. It is likely that bracing associated with the patient’s second surgery and delayed union of his tibial fracture allowed healing tissue to be protected from excessive stress until it remodeled with sufficient strength. Most would assume that healing would occur early, during the first 6 to 9 months; however, our patient regained his stability between 8 and 13 months. It is possible that the hostile healing environment of the ACL, including the low blood supply, poor response to growth factors, and biomechanical environment, as described previously, played a factor in this delay.7,9,12,13
It is important to recognize that our patient tore his ACL during a traumatic motorized scooter rollover collision, not the more common noncontact twisting injury. Additionally, given the patient’s knee surgery that was performed 6 months after the initial injury, it is possible that intra-articular scar formation contributed to his healing capacity. While this patient did not undergo arthroscopy to visualize the tear in the ACL, or its reconstitution, recent evidence suggests that the accuracy of MRI in diagnosing pediatric ACL injuries is excellent.27,28 The diagnostic accuracy with new MRI machines has sensitivity and specificity approaching 100%.29 Additionally, the patient’s subjective and objective improvements argue for a change in anatomy over a change in the quality of his examination.
Conclusion
The goal of ACL reconstruction in adolescents is to provide long-term stability to the knee while minimizing the risk of growth disturbance. This goal was achieved in our patient through the in situ healing of his ACL. Intrinsic reconstitution of a torn ACL is rare, and it is difficult to speculate which patients may have some healing potential. While this patient was an extreme example, his case demonstrated that protection of the knee from undue stress could favorably alter the environment of the knee to allow for healing of ACL tears. Such information could be valuable in managing select pediatric patients with open physes and ACL injuries nonoperatively, sparing them from the risks associated with surgical treatment. While we do not recommend nonoperative treatment for patients with acute tears of the ACL, we believe more investigation into the healing potential of the ACL, and potential pathways to augment this, is warranted.
1. Noyes FR, Mooar PA, Matthews DS, Butler DL. The symptomatic anterior cruciate-deficient knee. Part I: the long-term functional disability in athletically active individuals. J Bone Joint Surg Am. 1983;65(2):154-162.
2. Nagineni CN, Amiel D, Green MH, Berchuck M, Akeson WH. Characterization of the intrinsic properties of the anterior cruciate and medial collateral ligament cells: an in vitro cell culture study. J Orthop Res. 1992;10(4):465-475.
3. Hefti FL, Kress A, Fasel J, Morscher EW. Healing of the transected anterior cruciate ligament in the rabbit. J Bone Joint Surg Am. 1991;73(3):373-383.
4. Andersson C, Odensten M, Good L, Gillquist J. Surgical or non-surgical treatment of acute rupture of the anterior cruciate ligament. A randomized study with long-term follow-up. J Bone Joint Surg Am. 1989;71(7):965-974.
5. Tang Z, Yang L, Wang Y, et al. Contributions of different intraarticular tissues to the acute phase elevation of synovial fluid MMP-2 following rat ACL rupture. J Orthop Res. 2009;27(2):243-248.
6. Woo SL, Chan SS, Yamaji T. Biomechanics of knee ligament healing, repair and reconstruction. J Biomech. 1997;30(5):431-439.
7. Yoshida M, Fujii K. Differences in cellular properties and responses to growth factors between human ACL and MCL cells. J Orthop Sci. 1999;4(4):293-298.
8. Taylor DC, Posner M, Curl WW, Feagin JA. Isolated tears of the anterior cruciate ligament: over 30-year follow-up of patients treated with arthrotomy and primary repair. Am J Sports Med. 2009;37(1):65-71.
9. Noyes FR, Matthews DS, Mooar PA, Grood ES. The symptomatic anterior cruciate-deficient knee. Part II: the results of rehabilitation, activity modification, and counseling on functional disability. J Bone Joint Surg Am. 1983;65(2):163-174.
10. Malanga GA, Giradi J, Nadler SF. The spontaneous healing of a torn anterior cruciate ligament. Clin J Sport Med. 2001;11(2):118-120.
11. O’Donoghue DH, Rockwood CA Jr, Frank GR, Jack SC, Kenyon R. Repair of the anterior cruciate ligament in dogs. J Bone Joint Surg Am. 1966;48(3):503-519.
12. Guenoun D, Le Corroller T, Amous Z, Pauly V, Sbihi A, Champsaur P. The contribution of MRI to the diagnosis of traumatic tears of the anterior cruciate ligament. Diagn Intervent Imaging. 2012;93(5):331-341.
13. Andrish J, Holmes R. Effects of synovial fluid on fibroblasts in tissue culture. Clin Orthop Relat Res. 1979;(138):279-283.
14. Zimny ML, Schutte M, Dabezies E. Mechanoreceptors in the human anterior cruciate ligament. Anat Rec. 1986;214(2):204-209.
15. Bush-Joseph CA, Cummings JF, Buseck M, et al. Effect of tibial attachment location on the healing of the anterior cruciate ligament freeze model. J Orthop Res. 1996;14(4):534-541.
16. Sung KL, Whittemore DE, Yang L, Amiel D, Akeson WH. Signal pathways and ligament cell adhesiveness. J Orthop Res. 1996;14(5):729-735.
17. Deie M, Ochi M, Ikuta Y. High intrinsic healing potential of human anterior cruciate ligament. Organ culture experiments. Acta Orthop Scand. 1995;66(1):28-32.
18. Voloshin I, Bronstein RD, DeHaven KE. Spontaneous healing of a patellar tendon anterior cruciate ligament graft. A case report. Am J Sports Med. 2002;30(5):751-753.
19. Costa-Paz M, Ayerza MA, Tanoira I, Astoul J, Muscolo DL. Spontaneous healing in complete ACL ruptures: a clinical and MRI study. Clin Orthop Relat Res. 2012;470(4):979-985.
20. Kurosaka M, Yoshiya S, Mizuno T, Mizuno K. Spontaneous healing of a tear of the anterior cruciate ligament. A report of two cases. J Bone Joint Surg Am. 1998;80(8):1200-1203.
21. Fujimoto E, Sumen Y, Ochi M, Ikuta Y. Spontaneous healing of acute anterior cruciate ligament (ACL) injuries - conservative treatment using an extension block soft brace without anterior stabilization. Arch Orthop Trauma Surg. 2002;122(4):212-216.
22. Ihara H, Miwa M, Deya K, Torisu K. MRI of anterior cruciate ligament healing. J Comput Assist Tomogr. 1996;20(2):317-321.
23. Graf BK, Lange RH, Fujisaki CK, Landry GL, Saluja RK. Anterior cruciate ligament tears in skeletally immature patients: meniscal pathology at presentation and after attempted conservative treatment. Arthroscopy. 1992;8(2):229-233.
24. Kannus P, Jarvinen M. Knee ligament injuries in adolescents. Eight year follow-up of conservative management. J Bone Joint Surg Br. 1988;70(5):772-776.
25. Pressman AE, Letts RM, Jarvis JG. Anterior cruciate ligament tears in children: an analysis of operative versus nonoperative treatment. J Pediatr Orthop. 1997;17(4):505-511.
26. Vavken P, Murray MM. Treating anterior cruciate ligament tears in skeletally immature patients. Arthroscopy. 2011;27(5):704-716.
27. Lee K, Siegel MJ, Lau DM, Hildebolt CF, Matava MJ. Anterior cruciate ligament tears: MR imaging-based diagnosis in a pediatric population. Radiology. 1999;213(3):697-704.
28. Major NM, Beard LN Jr, Helms CA. Accuracy of MR imaging of the knee in adolescents. AJR Am J Roentgenol. 2003;180(1):17-19.
29. Sampson MJ, Jackson MP, Moran CJ, Shine S, Moran R, Eustace SJ. Three Tesla MRI for the diagnosis of meniscal and anterior cruciate ligament pathology: a comparison to arthroscopic findings. Clin Radiol. 2008;63(10):1106-1111.
The anterior cruciate ligament (ACL) restrains anterior translation of the tibia on the femur and controls rotation of the knee. The natural primary healing potential of the ACL has been extremely poor in clinical and experimental studies, and primary suture repair has not provided stability to the joint in most patients.1-8 This has led surgeons to reconstruct the ACL, rather than to attempt nonoperative treatment. Anterior cruciate ligament reconstruction is recommended to help patients maintain activities that place shear and torque forces on the knee or to ameliorate persistent pain due to instability.9 Reconstruction of the ACL in adults is one of the most common procedures performed by orthopedic surgeons. However, reconstruction in the ACL-deficient adolescent remains a controversial subject, with debates surrounding operative timing and surgical technique.
This case report presents a skeletally immature patient who suffered a complete traumatic rupture of his ACL, which intrinsically healed. The patient had a protracted treatment course, complicated by an open tibial fracture with delayed union. He responded to a progressive rehabilitation program and has made a good functional recovery. Review of the literature has demonstrated limited evidence of intrinsic ACL healing, none of which has been shown to occur in a skeletally immature patient. The patient’s mother provided written informed consent for print and electronic publication of this case report.
Case Report
A 12-year-old boy was brought to our level I trauma center by ambulance after being hit by a car while riding a motorized scooter. He presented with a grade IIIB open tibial fracture and a distal fibula fracture of his left lower extremity and was taken to the operating room that night for irrigation and débridement, percutaneous fixation of the fibula, and intramedullary flexible nail fixation of the tibia. On postoperative day 1, he had increasing pain and, once his splint was removed, his compartments were found to be very tense. He was taken emergently to the operating room for 4 compartment fasciotomies of the left lower extremity with wound vacuum-assisted closure (VAC) placement. This was changed on hospital day 4 and was removed with definitive closure on day 7. Examination under anesthesia prior to the final wound VAC change was performed given the patient’s complaints during physical therapy. This showed anterior and posterior ligamentous instability of the knee, and he was placed in a knee immobilizer. He was discharged on hospital day 11.
At 2-week follow-up, the patient was doing well, except that he was nonadherent with the knee immobilizer and unable to fully extend his left knee. On examination, a posterior drawer sign was noted; therefore, the patient was referred for magnetic resonance imaging (MRI) to evaluate his ligaments. His MRI, 9 weeks after injury, showed: (1) complete tears of both the anterior and posterior cruciate ligaments (PCLs) (Figures 1A, 1B); (2) medial meniscus and lateral meniscus tears; (3) 2.0-cm plate-like avulsion fracture of the posterolateral femoral metaphysis involving the insertion of the lateral head of the gastrocnemius muscle, fibular collateral ligament, and popliteus muscle (Figure 2); and (4) left posterior lateral tibial plateau contusion.
The patient was started on a 6-week course of physical therapy with active and active-assisted extension exercises. At follow-up approximately 3½ months after injury, he was found to have a 35º flexion contracture with pain at the end extension. Unfortunately, his tibial fracture showed minimal signs of healing, and the decision was made to delay surgical intervention on the knee until the tibial fracture had healed. He was given a knee orthotic to wear at night to help regain his knee extension.
Six months after injury, the patient underwent open removal of the avulsed bony fragment, posterior knee capsule release, and autograft of the delayed union tibial fracture. He was placed in a straight leg cast postoperatively and was discharged home on postoperative day 2. He transitioned to a knee immobilizer after 2 weeks. Six weeks after the last surgery, he had range of motion of 0º to 130º. Ligamentous examination at this time showed anterior and posterior drawer signs, positive Lachman test, and dial test with 90º of external rotation. He was placed in physical therapy for a total of 10 weeks to work on his quadriceps muscle strength and 15º extension lag.
On 13-month postinjury radiographs, the patient was noted to have adequate healing of his tibial fracture, and ligamentous reconstruction was discussed. At this time, the patient did not have any instability or pain in the knee. Examination demonstrated a very mild effusion of the left knee. Range of motion determined by goniometer was from -3º to 140º, and Lachman test was positive but with solid 2+ endpoint. He also had a positive posterior drawer sign with no endpoint, positive sag sign of his tibia, and positive active quadriceps test of the left leg. His dial test showed some increased external rotation at 90º but was equivocal at 30º when compared with the contralateral knee, demonstrating involvement of the posterolateral corner.
Sixteen months after injury, repeat MRI to further evaluate the posterolateral corner showed: (1) complete medial and lateral meniscal healing without evidence of residual or recurrent tear, and (2) interval healing of the remote ACL and PCL tears with intact insertions (Figures 3A, 3B). This scan showed an end-to-end continuous ACL with homogeneous signal and disappearance of the secondary signs. Physical examination at this time showed a very firm endpoint on Lachman test but some laxity with his posterior drawer. Given these findings, the patient was given a brace and continued in physical therapy to strengthen his quadriceps muscle. By 20 months after injury, he had returned to competitive hockey and had no complaints of pain or instability. His physical examination showed full range of motion in a ligamentously stable knee with firm endpoint. The patient’s condition was unchanged at 29-month follow-up.
Discussion
There is a body of evidence that states a completely ruptured ACL does not heal.3,6,10 In animal models, the ACL has been shown to have poor healing potential.3,11 Some studies have suggested this is secondary to poor blood supply. Blood supply to the ACL is derived from a periligamentous, then endoligamentous, arterial network with a less vascularized area in the middle third of the ACL. Additionally, there is no blood supply from the tibia or femur, meaning the areas of attachment of the ligament are poorly vascularized.12 With a minimal blood supply to the ACL, the supply of undifferentiated mesenchymal cells from the surrounding tissue during the initial healing process is limited. In vitro cell cultures of these cells have showed a reduced potential for proliferation and migration.9 Cells of the ACL have a lower response to growth factors than human medial collateral ligament cells, further suggesting a decreased reparative capacity.7 Joint fluid has been shown to inhibit the proliferation of these cells, further reducing their regenerative potential.13 Additionally, biomechanical factors that alter signaling pathways, sites of ligament reattachment, and injury to proprioceptive structures have been shown to negatively influence the healing response.14-18
Review of the literature on healing of ACLs includes 2 case reports, totaling 3 patients, and 3 level IV therapeutic studies involving 74 patients total.10,19-22 In most cases, the authors of these studies have indicated a nonoperative treatment protocol with bracing and a specific rehabilitation program. Malanga and colleagues10 demonstrated that an ACL torn from its attachment on the femur, with the majority of the ligament in good condition and no compromise in the length, healed back onto the femur. Kurosaka and coauthors20 described case reports of isolated distal or proximal midsubstance tears that have healed spontaneously. However, none of the patients described in the literature were under the age of 20 years.
Treatment for pediatric patients with open physes causes some debate. Nonoperative management of ACL deficiency in adolescents is generally not recommended because the continued instability of the joint leads to intra-articular injury, functional impairment, and joint degeneration.23-25 A recent systematic review found only 1 study that showed no increase in secondary intra-articular injury when surgery was delayed until skeletal maturity.26
Our patient was a 12-year-old boy whose traumatic knee injury with multiple ruptured ligaments healed over the course of 20 months. It is likely that bracing associated with the patient’s second surgery and delayed union of his tibial fracture allowed healing tissue to be protected from excessive stress until it remodeled with sufficient strength. Most would assume that healing would occur early, during the first 6 to 9 months; however, our patient regained his stability between 8 and 13 months. It is possible that the hostile healing environment of the ACL, including the low blood supply, poor response to growth factors, and biomechanical environment, as described previously, played a factor in this delay.7,9,12,13
It is important to recognize that our patient tore his ACL during a traumatic motorized scooter rollover collision, not the more common noncontact twisting injury. Additionally, given the patient’s knee surgery that was performed 6 months after the initial injury, it is possible that intra-articular scar formation contributed to his healing capacity. While this patient did not undergo arthroscopy to visualize the tear in the ACL, or its reconstitution, recent evidence suggests that the accuracy of MRI in diagnosing pediatric ACL injuries is excellent.27,28 The diagnostic accuracy with new MRI machines has sensitivity and specificity approaching 100%.29 Additionally, the patient’s subjective and objective improvements argue for a change in anatomy over a change in the quality of his examination.
Conclusion
The goal of ACL reconstruction in adolescents is to provide long-term stability to the knee while minimizing the risk of growth disturbance. This goal was achieved in our patient through the in situ healing of his ACL. Intrinsic reconstitution of a torn ACL is rare, and it is difficult to speculate which patients may have some healing potential. While this patient was an extreme example, his case demonstrated that protection of the knee from undue stress could favorably alter the environment of the knee to allow for healing of ACL tears. Such information could be valuable in managing select pediatric patients with open physes and ACL injuries nonoperatively, sparing them from the risks associated with surgical treatment. While we do not recommend nonoperative treatment for patients with acute tears of the ACL, we believe more investigation into the healing potential of the ACL, and potential pathways to augment this, is warranted.
The anterior cruciate ligament (ACL) restrains anterior translation of the tibia on the femur and controls rotation of the knee. The natural primary healing potential of the ACL has been extremely poor in clinical and experimental studies, and primary suture repair has not provided stability to the joint in most patients.1-8 This has led surgeons to reconstruct the ACL, rather than to attempt nonoperative treatment. Anterior cruciate ligament reconstruction is recommended to help patients maintain activities that place shear and torque forces on the knee or to ameliorate persistent pain due to instability.9 Reconstruction of the ACL in adults is one of the most common procedures performed by orthopedic surgeons. However, reconstruction in the ACL-deficient adolescent remains a controversial subject, with debates surrounding operative timing and surgical technique.
This case report presents a skeletally immature patient who suffered a complete traumatic rupture of his ACL, which intrinsically healed. The patient had a protracted treatment course, complicated by an open tibial fracture with delayed union. He responded to a progressive rehabilitation program and has made a good functional recovery. Review of the literature has demonstrated limited evidence of intrinsic ACL healing, none of which has been shown to occur in a skeletally immature patient. The patient’s mother provided written informed consent for print and electronic publication of this case report.
Case Report
A 12-year-old boy was brought to our level I trauma center by ambulance after being hit by a car while riding a motorized scooter. He presented with a grade IIIB open tibial fracture and a distal fibula fracture of his left lower extremity and was taken to the operating room that night for irrigation and débridement, percutaneous fixation of the fibula, and intramedullary flexible nail fixation of the tibia. On postoperative day 1, he had increasing pain and, once his splint was removed, his compartments were found to be very tense. He was taken emergently to the operating room for 4 compartment fasciotomies of the left lower extremity with wound vacuum-assisted closure (VAC) placement. This was changed on hospital day 4 and was removed with definitive closure on day 7. Examination under anesthesia prior to the final wound VAC change was performed given the patient’s complaints during physical therapy. This showed anterior and posterior ligamentous instability of the knee, and he was placed in a knee immobilizer. He was discharged on hospital day 11.
At 2-week follow-up, the patient was doing well, except that he was nonadherent with the knee immobilizer and unable to fully extend his left knee. On examination, a posterior drawer sign was noted; therefore, the patient was referred for magnetic resonance imaging (MRI) to evaluate his ligaments. His MRI, 9 weeks after injury, showed: (1) complete tears of both the anterior and posterior cruciate ligaments (PCLs) (Figures 1A, 1B); (2) medial meniscus and lateral meniscus tears; (3) 2.0-cm plate-like avulsion fracture of the posterolateral femoral metaphysis involving the insertion of the lateral head of the gastrocnemius muscle, fibular collateral ligament, and popliteus muscle (Figure 2); and (4) left posterior lateral tibial plateau contusion.
The patient was started on a 6-week course of physical therapy with active and active-assisted extension exercises. At follow-up approximately 3½ months after injury, he was found to have a 35º flexion contracture with pain at the end extension. Unfortunately, his tibial fracture showed minimal signs of healing, and the decision was made to delay surgical intervention on the knee until the tibial fracture had healed. He was given a knee orthotic to wear at night to help regain his knee extension.
Six months after injury, the patient underwent open removal of the avulsed bony fragment, posterior knee capsule release, and autograft of the delayed union tibial fracture. He was placed in a straight leg cast postoperatively and was discharged home on postoperative day 2. He transitioned to a knee immobilizer after 2 weeks. Six weeks after the last surgery, he had range of motion of 0º to 130º. Ligamentous examination at this time showed anterior and posterior drawer signs, positive Lachman test, and dial test with 90º of external rotation. He was placed in physical therapy for a total of 10 weeks to work on his quadriceps muscle strength and 15º extension lag.
On 13-month postinjury radiographs, the patient was noted to have adequate healing of his tibial fracture, and ligamentous reconstruction was discussed. At this time, the patient did not have any instability or pain in the knee. Examination demonstrated a very mild effusion of the left knee. Range of motion determined by goniometer was from -3º to 140º, and Lachman test was positive but with solid 2+ endpoint. He also had a positive posterior drawer sign with no endpoint, positive sag sign of his tibia, and positive active quadriceps test of the left leg. His dial test showed some increased external rotation at 90º but was equivocal at 30º when compared with the contralateral knee, demonstrating involvement of the posterolateral corner.
Sixteen months after injury, repeat MRI to further evaluate the posterolateral corner showed: (1) complete medial and lateral meniscal healing without evidence of residual or recurrent tear, and (2) interval healing of the remote ACL and PCL tears with intact insertions (Figures 3A, 3B). This scan showed an end-to-end continuous ACL with homogeneous signal and disappearance of the secondary signs. Physical examination at this time showed a very firm endpoint on Lachman test but some laxity with his posterior drawer. Given these findings, the patient was given a brace and continued in physical therapy to strengthen his quadriceps muscle. By 20 months after injury, he had returned to competitive hockey and had no complaints of pain or instability. His physical examination showed full range of motion in a ligamentously stable knee with firm endpoint. The patient’s condition was unchanged at 29-month follow-up.
Discussion
There is a body of evidence that states a completely ruptured ACL does not heal.3,6,10 In animal models, the ACL has been shown to have poor healing potential.3,11 Some studies have suggested this is secondary to poor blood supply. Blood supply to the ACL is derived from a periligamentous, then endoligamentous, arterial network with a less vascularized area in the middle third of the ACL. Additionally, there is no blood supply from the tibia or femur, meaning the areas of attachment of the ligament are poorly vascularized.12 With a minimal blood supply to the ACL, the supply of undifferentiated mesenchymal cells from the surrounding tissue during the initial healing process is limited. In vitro cell cultures of these cells have showed a reduced potential for proliferation and migration.9 Cells of the ACL have a lower response to growth factors than human medial collateral ligament cells, further suggesting a decreased reparative capacity.7 Joint fluid has been shown to inhibit the proliferation of these cells, further reducing their regenerative potential.13 Additionally, biomechanical factors that alter signaling pathways, sites of ligament reattachment, and injury to proprioceptive structures have been shown to negatively influence the healing response.14-18
Review of the literature on healing of ACLs includes 2 case reports, totaling 3 patients, and 3 level IV therapeutic studies involving 74 patients total.10,19-22 In most cases, the authors of these studies have indicated a nonoperative treatment protocol with bracing and a specific rehabilitation program. Malanga and colleagues10 demonstrated that an ACL torn from its attachment on the femur, with the majority of the ligament in good condition and no compromise in the length, healed back onto the femur. Kurosaka and coauthors20 described case reports of isolated distal or proximal midsubstance tears that have healed spontaneously. However, none of the patients described in the literature were under the age of 20 years.
Treatment for pediatric patients with open physes causes some debate. Nonoperative management of ACL deficiency in adolescents is generally not recommended because the continued instability of the joint leads to intra-articular injury, functional impairment, and joint degeneration.23-25 A recent systematic review found only 1 study that showed no increase in secondary intra-articular injury when surgery was delayed until skeletal maturity.26
Our patient was a 12-year-old boy whose traumatic knee injury with multiple ruptured ligaments healed over the course of 20 months. It is likely that bracing associated with the patient’s second surgery and delayed union of his tibial fracture allowed healing tissue to be protected from excessive stress until it remodeled with sufficient strength. Most would assume that healing would occur early, during the first 6 to 9 months; however, our patient regained his stability between 8 and 13 months. It is possible that the hostile healing environment of the ACL, including the low blood supply, poor response to growth factors, and biomechanical environment, as described previously, played a factor in this delay.7,9,12,13
It is important to recognize that our patient tore his ACL during a traumatic motorized scooter rollover collision, not the more common noncontact twisting injury. Additionally, given the patient’s knee surgery that was performed 6 months after the initial injury, it is possible that intra-articular scar formation contributed to his healing capacity. While this patient did not undergo arthroscopy to visualize the tear in the ACL, or its reconstitution, recent evidence suggests that the accuracy of MRI in diagnosing pediatric ACL injuries is excellent.27,28 The diagnostic accuracy with new MRI machines has sensitivity and specificity approaching 100%.29 Additionally, the patient’s subjective and objective improvements argue for a change in anatomy over a change in the quality of his examination.
Conclusion
The goal of ACL reconstruction in adolescents is to provide long-term stability to the knee while minimizing the risk of growth disturbance. This goal was achieved in our patient through the in situ healing of his ACL. Intrinsic reconstitution of a torn ACL is rare, and it is difficult to speculate which patients may have some healing potential. While this patient was an extreme example, his case demonstrated that protection of the knee from undue stress could favorably alter the environment of the knee to allow for healing of ACL tears. Such information could be valuable in managing select pediatric patients with open physes and ACL injuries nonoperatively, sparing them from the risks associated with surgical treatment. While we do not recommend nonoperative treatment for patients with acute tears of the ACL, we believe more investigation into the healing potential of the ACL, and potential pathways to augment this, is warranted.
1. Noyes FR, Mooar PA, Matthews DS, Butler DL. The symptomatic anterior cruciate-deficient knee. Part I: the long-term functional disability in athletically active individuals. J Bone Joint Surg Am. 1983;65(2):154-162.
2. Nagineni CN, Amiel D, Green MH, Berchuck M, Akeson WH. Characterization of the intrinsic properties of the anterior cruciate and medial collateral ligament cells: an in vitro cell culture study. J Orthop Res. 1992;10(4):465-475.
3. Hefti FL, Kress A, Fasel J, Morscher EW. Healing of the transected anterior cruciate ligament in the rabbit. J Bone Joint Surg Am. 1991;73(3):373-383.
4. Andersson C, Odensten M, Good L, Gillquist J. Surgical or non-surgical treatment of acute rupture of the anterior cruciate ligament. A randomized study with long-term follow-up. J Bone Joint Surg Am. 1989;71(7):965-974.
5. Tang Z, Yang L, Wang Y, et al. Contributions of different intraarticular tissues to the acute phase elevation of synovial fluid MMP-2 following rat ACL rupture. J Orthop Res. 2009;27(2):243-248.
6. Woo SL, Chan SS, Yamaji T. Biomechanics of knee ligament healing, repair and reconstruction. J Biomech. 1997;30(5):431-439.
7. Yoshida M, Fujii K. Differences in cellular properties and responses to growth factors between human ACL and MCL cells. J Orthop Sci. 1999;4(4):293-298.
8. Taylor DC, Posner M, Curl WW, Feagin JA. Isolated tears of the anterior cruciate ligament: over 30-year follow-up of patients treated with arthrotomy and primary repair. Am J Sports Med. 2009;37(1):65-71.
9. Noyes FR, Matthews DS, Mooar PA, Grood ES. The symptomatic anterior cruciate-deficient knee. Part II: the results of rehabilitation, activity modification, and counseling on functional disability. J Bone Joint Surg Am. 1983;65(2):163-174.
10. Malanga GA, Giradi J, Nadler SF. The spontaneous healing of a torn anterior cruciate ligament. Clin J Sport Med. 2001;11(2):118-120.
11. O’Donoghue DH, Rockwood CA Jr, Frank GR, Jack SC, Kenyon R. Repair of the anterior cruciate ligament in dogs. J Bone Joint Surg Am. 1966;48(3):503-519.
12. Guenoun D, Le Corroller T, Amous Z, Pauly V, Sbihi A, Champsaur P. The contribution of MRI to the diagnosis of traumatic tears of the anterior cruciate ligament. Diagn Intervent Imaging. 2012;93(5):331-341.
13. Andrish J, Holmes R. Effects of synovial fluid on fibroblasts in tissue culture. Clin Orthop Relat Res. 1979;(138):279-283.
14. Zimny ML, Schutte M, Dabezies E. Mechanoreceptors in the human anterior cruciate ligament. Anat Rec. 1986;214(2):204-209.
15. Bush-Joseph CA, Cummings JF, Buseck M, et al. Effect of tibial attachment location on the healing of the anterior cruciate ligament freeze model. J Orthop Res. 1996;14(4):534-541.
16. Sung KL, Whittemore DE, Yang L, Amiel D, Akeson WH. Signal pathways and ligament cell adhesiveness. J Orthop Res. 1996;14(5):729-735.
17. Deie M, Ochi M, Ikuta Y. High intrinsic healing potential of human anterior cruciate ligament. Organ culture experiments. Acta Orthop Scand. 1995;66(1):28-32.
18. Voloshin I, Bronstein RD, DeHaven KE. Spontaneous healing of a patellar tendon anterior cruciate ligament graft. A case report. Am J Sports Med. 2002;30(5):751-753.
19. Costa-Paz M, Ayerza MA, Tanoira I, Astoul J, Muscolo DL. Spontaneous healing in complete ACL ruptures: a clinical and MRI study. Clin Orthop Relat Res. 2012;470(4):979-985.
20. Kurosaka M, Yoshiya S, Mizuno T, Mizuno K. Spontaneous healing of a tear of the anterior cruciate ligament. A report of two cases. J Bone Joint Surg Am. 1998;80(8):1200-1203.
21. Fujimoto E, Sumen Y, Ochi M, Ikuta Y. Spontaneous healing of acute anterior cruciate ligament (ACL) injuries - conservative treatment using an extension block soft brace without anterior stabilization. Arch Orthop Trauma Surg. 2002;122(4):212-216.
22. Ihara H, Miwa M, Deya K, Torisu K. MRI of anterior cruciate ligament healing. J Comput Assist Tomogr. 1996;20(2):317-321.
23. Graf BK, Lange RH, Fujisaki CK, Landry GL, Saluja RK. Anterior cruciate ligament tears in skeletally immature patients: meniscal pathology at presentation and after attempted conservative treatment. Arthroscopy. 1992;8(2):229-233.
24. Kannus P, Jarvinen M. Knee ligament injuries in adolescents. Eight year follow-up of conservative management. J Bone Joint Surg Br. 1988;70(5):772-776.
25. Pressman AE, Letts RM, Jarvis JG. Anterior cruciate ligament tears in children: an analysis of operative versus nonoperative treatment. J Pediatr Orthop. 1997;17(4):505-511.
26. Vavken P, Murray MM. Treating anterior cruciate ligament tears in skeletally immature patients. Arthroscopy. 2011;27(5):704-716.
27. Lee K, Siegel MJ, Lau DM, Hildebolt CF, Matava MJ. Anterior cruciate ligament tears: MR imaging-based diagnosis in a pediatric population. Radiology. 1999;213(3):697-704.
28. Major NM, Beard LN Jr, Helms CA. Accuracy of MR imaging of the knee in adolescents. AJR Am J Roentgenol. 2003;180(1):17-19.
29. Sampson MJ, Jackson MP, Moran CJ, Shine S, Moran R, Eustace SJ. Three Tesla MRI for the diagnosis of meniscal and anterior cruciate ligament pathology: a comparison to arthroscopic findings. Clin Radiol. 2008;63(10):1106-1111.
1. Noyes FR, Mooar PA, Matthews DS, Butler DL. The symptomatic anterior cruciate-deficient knee. Part I: the long-term functional disability in athletically active individuals. J Bone Joint Surg Am. 1983;65(2):154-162.
2. Nagineni CN, Amiel D, Green MH, Berchuck M, Akeson WH. Characterization of the intrinsic properties of the anterior cruciate and medial collateral ligament cells: an in vitro cell culture study. J Orthop Res. 1992;10(4):465-475.
3. Hefti FL, Kress A, Fasel J, Morscher EW. Healing of the transected anterior cruciate ligament in the rabbit. J Bone Joint Surg Am. 1991;73(3):373-383.
4. Andersson C, Odensten M, Good L, Gillquist J. Surgical or non-surgical treatment of acute rupture of the anterior cruciate ligament. A randomized study with long-term follow-up. J Bone Joint Surg Am. 1989;71(7):965-974.
5. Tang Z, Yang L, Wang Y, et al. Contributions of different intraarticular tissues to the acute phase elevation of synovial fluid MMP-2 following rat ACL rupture. J Orthop Res. 2009;27(2):243-248.
6. Woo SL, Chan SS, Yamaji T. Biomechanics of knee ligament healing, repair and reconstruction. J Biomech. 1997;30(5):431-439.
7. Yoshida M, Fujii K. Differences in cellular properties and responses to growth factors between human ACL and MCL cells. J Orthop Sci. 1999;4(4):293-298.
8. Taylor DC, Posner M, Curl WW, Feagin JA. Isolated tears of the anterior cruciate ligament: over 30-year follow-up of patients treated with arthrotomy and primary repair. Am J Sports Med. 2009;37(1):65-71.
9. Noyes FR, Matthews DS, Mooar PA, Grood ES. The symptomatic anterior cruciate-deficient knee. Part II: the results of rehabilitation, activity modification, and counseling on functional disability. J Bone Joint Surg Am. 1983;65(2):163-174.
10. Malanga GA, Giradi J, Nadler SF. The spontaneous healing of a torn anterior cruciate ligament. Clin J Sport Med. 2001;11(2):118-120.
11. O’Donoghue DH, Rockwood CA Jr, Frank GR, Jack SC, Kenyon R. Repair of the anterior cruciate ligament in dogs. J Bone Joint Surg Am. 1966;48(3):503-519.
12. Guenoun D, Le Corroller T, Amous Z, Pauly V, Sbihi A, Champsaur P. The contribution of MRI to the diagnosis of traumatic tears of the anterior cruciate ligament. Diagn Intervent Imaging. 2012;93(5):331-341.
13. Andrish J, Holmes R. Effects of synovial fluid on fibroblasts in tissue culture. Clin Orthop Relat Res. 1979;(138):279-283.
14. Zimny ML, Schutte M, Dabezies E. Mechanoreceptors in the human anterior cruciate ligament. Anat Rec. 1986;214(2):204-209.
15. Bush-Joseph CA, Cummings JF, Buseck M, et al. Effect of tibial attachment location on the healing of the anterior cruciate ligament freeze model. J Orthop Res. 1996;14(4):534-541.
16. Sung KL, Whittemore DE, Yang L, Amiel D, Akeson WH. Signal pathways and ligament cell adhesiveness. J Orthop Res. 1996;14(5):729-735.
17. Deie M, Ochi M, Ikuta Y. High intrinsic healing potential of human anterior cruciate ligament. Organ culture experiments. Acta Orthop Scand. 1995;66(1):28-32.
18. Voloshin I, Bronstein RD, DeHaven KE. Spontaneous healing of a patellar tendon anterior cruciate ligament graft. A case report. Am J Sports Med. 2002;30(5):751-753.
19. Costa-Paz M, Ayerza MA, Tanoira I, Astoul J, Muscolo DL. Spontaneous healing in complete ACL ruptures: a clinical and MRI study. Clin Orthop Relat Res. 2012;470(4):979-985.
20. Kurosaka M, Yoshiya S, Mizuno T, Mizuno K. Spontaneous healing of a tear of the anterior cruciate ligament. A report of two cases. J Bone Joint Surg Am. 1998;80(8):1200-1203.
21. Fujimoto E, Sumen Y, Ochi M, Ikuta Y. Spontaneous healing of acute anterior cruciate ligament (ACL) injuries - conservative treatment using an extension block soft brace without anterior stabilization. Arch Orthop Trauma Surg. 2002;122(4):212-216.
22. Ihara H, Miwa M, Deya K, Torisu K. MRI of anterior cruciate ligament healing. J Comput Assist Tomogr. 1996;20(2):317-321.
23. Graf BK, Lange RH, Fujisaki CK, Landry GL, Saluja RK. Anterior cruciate ligament tears in skeletally immature patients: meniscal pathology at presentation and after attempted conservative treatment. Arthroscopy. 1992;8(2):229-233.
24. Kannus P, Jarvinen M. Knee ligament injuries in adolescents. Eight year follow-up of conservative management. J Bone Joint Surg Br. 1988;70(5):772-776.
25. Pressman AE, Letts RM, Jarvis JG. Anterior cruciate ligament tears in children: an analysis of operative versus nonoperative treatment. J Pediatr Orthop. 1997;17(4):505-511.
26. Vavken P, Murray MM. Treating anterior cruciate ligament tears in skeletally immature patients. Arthroscopy. 2011;27(5):704-716.
27. Lee K, Siegel MJ, Lau DM, Hildebolt CF, Matava MJ. Anterior cruciate ligament tears: MR imaging-based diagnosis in a pediatric population. Radiology. 1999;213(3):697-704.
28. Major NM, Beard LN Jr, Helms CA. Accuracy of MR imaging of the knee in adolescents. AJR Am J Roentgenol. 2003;180(1):17-19.
29. Sampson MJ, Jackson MP, Moran CJ, Shine S, Moran R, Eustace SJ. Three Tesla MRI for the diagnosis of meniscal and anterior cruciate ligament pathology: a comparison to arthroscopic findings. Clin Radiol. 2008;63(10):1106-1111.
Fracture Blisters After Primary Total Knee Arthroplasty
Fracture blisters are a relatively uncommon complication of high-energy fractures, with an incidence of 2.9%.1 In the lower extremity, fracture blisters almost always occur distal to the knee.1 Histologically, the blisters represent an injury to the dermoepidermal junction.2 On physical examination, there are tense blood- and/or clear fluid–filled bullae overlying markedly swollen and edematous soft tissue,1 resembling a second-degree burn.3 Infection may develop after fracture blisters,1 and this is perhaps the most dreaded complication of total knee arthroplasty (TKA). The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 71-year-old man with end-stage osteoarthritis of the right knee underwent an elective TKA with cemented components (Legion PS; Smith & Nephew). His medical history included venous insufficiency, type 2 diabetes mellitus, chronic obstructive sleep apnea, hypertension, morbid obesity (body mass index, 50), and a previous uneventful left TKA. Tourniquet time was 78 minutes and estimated blood loss was 100 mL. An intra-articular drain was used and was removed on the first postoperative day. After wound closure, a soft splint bandage consisting of 2 to 3 layers of cotton and bias wrap was applied. Deep vein thrombosis (DVT) prophylaxis with enoxaparin 40 mg once daily was started on the first postoperative day.
Upon removal of the surgical dressings on the second postoperative day, the anterior leg was found to have a combination of tense clear fluid– and blood-filled blisters on markedly swollen and erythematous skin. The incision was minimally involved (Figure A). There was diffuse 2+ pitting edema with hyperesthesia in the affected skin distal to the knee. Prior to these findings, the patient had complained of increasing pain in his operative leg, but there was no escalation in analgesic requirements. There was no evidence of compartment syndrome on serial examinations. An ultrasound of the lower extremity was negative for DVT. Plain films did not show iatrogenic fractures. There was no intraoperative vascular injury, and the foot pulses remained unchanged between the time the patient was in the preoperative holding unit, the postanesthesia care unit, and the orthopedic ward. The operative leg was treated with elevation and loosely applied Kerlix roll gauze (Kendall, Covidien), but active blister formation continued for another 2 days. A 10-day prophylactic course of trimethoprim/sulfamethoxazole was initiated on the third postoperative day after the blisters started to rupture. The patient was allowed to bear weight as tolerated, but his physical therapy (PT) course was limited by pain and fear “of losing his leg.” He declined several PT sessions and was hesitant to use continuous passive motion. The patient was discharged to a short-term rehabilitation facility with weekly outpatient follow-up. On the second postoperative week, his fluid-filled blisters completely reepithelialized, but the blood-filled blisters required an additional week for reepithelialization (Figure B). While the patient’s knee was stiff because of limited PT participation, it was not until the second postoperative week when most of the fracture blisters had healed that he was able to resume an intensive knee exercise program, avoiding the need for manipulation under anesthesia.
Discussion
Giordano and colleagues2 identified 2 types of fracture blisters: clear fluid– and blood-filled. While both types involved disruption of the dermoepidermal junction, greater disruption and complete absence of dermal epithelial cells was observed in the hemorrhagic type. Clinical follow-up of the patients in the study by Giordano and colleagues2 showed that the mean time for reepithelialization was 12 days for fluid-filled blisters and 16 days for blood-filled blisters. These findings are similar to what we observed in our case report. In particular, the fluid-filled blisters healed in 2 weeks, whereas the blood-filled blisters required 3 weeks to heal.
The etiology of the fracture blisters in this patient is likely multifactorial and related to age, obesity, venous insufficiency, and diabetes mellitus. Farage and colleagues4 described a series of progressive degenerative changes in the aging skin, including vascular atrophy and degradation of dermal connective tissue, leading to compromised skin competence. The integrity of the dermis can be further reduced in patients with diabetes through glycosylation of collagen fibrils.5 Compared with age-matched normal controls, patients with insulin-dependent diabetes have a reduced threshold to suction-induced blister formation.6 Obesity is another potential contributing factor, with multiple studies showing significantly impaired venous flow in obese patients.7,8 Taken together, soft-tissue swelling after surgery in the setting of chronic venous insufficiency and compromised skin due to advanced age and diabetes may lead to markedly elevated interstitial pressure. One mechanism to relieve such abnormally high pressure is the formation of fracture blisters.1
Surgical risk factors that could have contributed to the complication in this case include the surgical skin preparation solution (ChloraPrep; CareFusion), use of adhesive antimicrobial drape (Ioban, 3M), tourniquet time, dressing choice, and DVT prophylaxis regimen. While the skin preparation solution is an unlikely culprit since the presentation is not consistent with contact dermatitis, inappropriate strapping or removal of the adhesive drape could result in stretch injury of the skin, shearing the dermoepidermal junction and causing tension blisters.9 There were no intraoperative complications and the tourniquet time was appropriate (78 minutes). Postoperatively, no compressive or adhesive dressings were used. With regards to DVT prophylaxis, the patient received a single dose of enoxaparin on the first postoperative day. While heparin-induced hemorrhagic blisters have been reported,10 I do not feel that the use of enoxaparin was a contributing factor. Heparin-induced blisters have been described as systemic blisters,10 whereas the blisters in this case were confined to the operative extremity. The patient was not taking any nutritional supplements (eg, fish oil, vitamin E) that could have increased his risk of bleeding. Throughout his hospital stay, he was hemodynamically stable and did not require blood transfusion.
Management of fracture blisters is controversial, and there is no consensus on appropriate soft-tissue handling. In this patient, the blisters were left intact. Blister fluid has been shown to be sterile, containing growth factors, opsonins, and activated neutrophils that aid in healing and infection prevention.1 Giordano and Koval11 found no difference in the outcome of 3 soft-tissue treatment techniques: (1) aspiration of the blister, (2) deroofing of the blister followed by application of a topical antibiotic cream or coverage with nonadherent dressing, or (3) keeping the blister intact and covered with loose dressing or exposed to air. In contrast, Strauss and colleagues12 found that deroofing the fracture blister to healthy tissue followed by twice-daily application of silver sulfadiazine antibiotic cream promoted reepithelialization and resulted in better cosmetic appearance and higher patient satisfaction.
The optimal dressing for fracture blisters remains elusive. Madden and colleagues13 showed that the use of occlusive nonadherent dressing was associated with significantly faster healing and less pain compared with semiocclusive, antibiotic-impregnated dressings. In another study, Varela and colleagues1 found no differences in blister healing between patients treated with either (1) dry dressing and casting, (2) Silvadene dressing (King Pharmaceuticals), or (3) whirlpool débridement and Silvadene dressing.
Infection is perhaps the most dreaded complication of fracture blisters after TKA. Varela and colleagues1 showed that, while the fluid in intact blisters was a sterile transudate, polymicrobial colonization with skin flora often occurred soon after blister rupture and persisted until reepithelialization. Our patient received a 10-day course of prophylactic antibiotics and no superficial or deep infection developed; however, the real contribution of antibiotic prophylaxis to the absence of infection cannot be established based solely on 1 case.
Pain is another concern associated with fracture blisters. Our patient had significant pain that limited his ability to participate in PT, resulting in limited knee range of motion and eventual discharge to a short-term rehabilitation facility. Fortunately, after resolution of the fracture blisters, he was able to participate in an aggressive rehabilitation program. By 6 weeks after surgery, he had significant improvement in his knee motion, avoiding the need for manipulation under anesthesia.
Conclusion
This case represents the first reported fracture blisters after primary TKA. The risk of deep surgical site infection, a devastating complication after TKA, is perhaps the most frightening concern of this rare complication. While the etiology and the management are controversial, there is evidence to recommend prophylactic antibiotics after blister rupture and skin desquamation. The decision to withhold DVT prophylaxis should be based on individual patient risk factors and blister type (blood-filled vs clear fluid–filled). Patients should be encouraged to continue knee exercises during reepithelialization to avoid stiffness.
1. Varela CD, Vaughan TK, Carr JB, Slemmons BK. Fracture blisters: clinical and pathological aspects. J Orthop Trauma. 1993;7(5):417-427.
2. Giordano CP, Koval KJ, Zuckerman JD, Desai P. Fracture blisters. Clin Orthop Relat Res. 1994;(307):214-221.
3. Uebbing CM, Walsh M, Miller JB, Abraham M, Arnold C. Fracture blisters. West J Emerg Med. 2011;12(1):131-133.
4. Farage MA, Miller KW, Berardesca E, Maibach HI. Clinical implications of aging skin: cutaneous disorders in the elderly. Am J Clin Dermatol. 2009;10(2):73-86.
5. Quondamatteo F. Skin and diabetes mellitus: what do we know? Cell Tissue Res. 2014;355(1):1-21.
6. Bernstein JE, Levine LE, Medenica MM, Yung CW, Soltani K. Reduced threshold to suction-induced blister formation in insulin-dependent diabetics. J Am Acad Dermatol. 1983;8(6):790-791.
7. Willenberg T, Schumacher A, Amann-Vesti B, et al. Impact of obesity on venous hemodynamics of the lower limbs. J Vasc Surg. 2010;52(3):664-668.
8. van Rij AM, De Alwis CS, Jiang P, et al. Obesity and impaired venous function. Eur J Vasc Endovasc Surg. 2008;35(6):739-744.
9. Polatsch DB, Baskies MA, Hommen JP, Egol KA, Koval KJ. Tape blisters that develop after hip fracture surgery: a retrospective series and a review of the literature. Am J Orthop. 2004;33(9):452-456.
10. Roux J, Duong TA, Ingen-Housz-Oro S, et al. Heparin-induced hemorrhagic blisters. Eur J Dermatol. 2013;23(1):105-107.
11. Giordano CP, Koval KJ. Treatment of fracture blisters: a prospective study of 53 cases. J Orthop Trauma. 1995;9(2):171-176.
12. Strauss EJ, Petrucelli G, Bong M, Koval KJ, Egol KA. Blisters associated with lower-extremity fracture: results of a prospective treatment protocol. J Orthop Trauma. 2006;20(9):618-622.
13. Madden MR, Nolan E, Finkelstein JL, et al. Comparison of an occlusive and a semi-occlusive dressing and the effect of the wound exudate upon keratinocyte proliferation. J Trauma. 1989;29(7):924-930; discussion 930-931.
Fracture blisters are a relatively uncommon complication of high-energy fractures, with an incidence of 2.9%.1 In the lower extremity, fracture blisters almost always occur distal to the knee.1 Histologically, the blisters represent an injury to the dermoepidermal junction.2 On physical examination, there are tense blood- and/or clear fluid–filled bullae overlying markedly swollen and edematous soft tissue,1 resembling a second-degree burn.3 Infection may develop after fracture blisters,1 and this is perhaps the most dreaded complication of total knee arthroplasty (TKA). The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 71-year-old man with end-stage osteoarthritis of the right knee underwent an elective TKA with cemented components (Legion PS; Smith & Nephew). His medical history included venous insufficiency, type 2 diabetes mellitus, chronic obstructive sleep apnea, hypertension, morbid obesity (body mass index, 50), and a previous uneventful left TKA. Tourniquet time was 78 minutes and estimated blood loss was 100 mL. An intra-articular drain was used and was removed on the first postoperative day. After wound closure, a soft splint bandage consisting of 2 to 3 layers of cotton and bias wrap was applied. Deep vein thrombosis (DVT) prophylaxis with enoxaparin 40 mg once daily was started on the first postoperative day.
Upon removal of the surgical dressings on the second postoperative day, the anterior leg was found to have a combination of tense clear fluid– and blood-filled blisters on markedly swollen and erythematous skin. The incision was minimally involved (Figure A). There was diffuse 2+ pitting edema with hyperesthesia in the affected skin distal to the knee. Prior to these findings, the patient had complained of increasing pain in his operative leg, but there was no escalation in analgesic requirements. There was no evidence of compartment syndrome on serial examinations. An ultrasound of the lower extremity was negative for DVT. Plain films did not show iatrogenic fractures. There was no intraoperative vascular injury, and the foot pulses remained unchanged between the time the patient was in the preoperative holding unit, the postanesthesia care unit, and the orthopedic ward. The operative leg was treated with elevation and loosely applied Kerlix roll gauze (Kendall, Covidien), but active blister formation continued for another 2 days. A 10-day prophylactic course of trimethoprim/sulfamethoxazole was initiated on the third postoperative day after the blisters started to rupture. The patient was allowed to bear weight as tolerated, but his physical therapy (PT) course was limited by pain and fear “of losing his leg.” He declined several PT sessions and was hesitant to use continuous passive motion. The patient was discharged to a short-term rehabilitation facility with weekly outpatient follow-up. On the second postoperative week, his fluid-filled blisters completely reepithelialized, but the blood-filled blisters required an additional week for reepithelialization (Figure B). While the patient’s knee was stiff because of limited PT participation, it was not until the second postoperative week when most of the fracture blisters had healed that he was able to resume an intensive knee exercise program, avoiding the need for manipulation under anesthesia.
Discussion
Giordano and colleagues2 identified 2 types of fracture blisters: clear fluid– and blood-filled. While both types involved disruption of the dermoepidermal junction, greater disruption and complete absence of dermal epithelial cells was observed in the hemorrhagic type. Clinical follow-up of the patients in the study by Giordano and colleagues2 showed that the mean time for reepithelialization was 12 days for fluid-filled blisters and 16 days for blood-filled blisters. These findings are similar to what we observed in our case report. In particular, the fluid-filled blisters healed in 2 weeks, whereas the blood-filled blisters required 3 weeks to heal.
The etiology of the fracture blisters in this patient is likely multifactorial and related to age, obesity, venous insufficiency, and diabetes mellitus. Farage and colleagues4 described a series of progressive degenerative changes in the aging skin, including vascular atrophy and degradation of dermal connective tissue, leading to compromised skin competence. The integrity of the dermis can be further reduced in patients with diabetes through glycosylation of collagen fibrils.5 Compared with age-matched normal controls, patients with insulin-dependent diabetes have a reduced threshold to suction-induced blister formation.6 Obesity is another potential contributing factor, with multiple studies showing significantly impaired venous flow in obese patients.7,8 Taken together, soft-tissue swelling after surgery in the setting of chronic venous insufficiency and compromised skin due to advanced age and diabetes may lead to markedly elevated interstitial pressure. One mechanism to relieve such abnormally high pressure is the formation of fracture blisters.1
Surgical risk factors that could have contributed to the complication in this case include the surgical skin preparation solution (ChloraPrep; CareFusion), use of adhesive antimicrobial drape (Ioban, 3M), tourniquet time, dressing choice, and DVT prophylaxis regimen. While the skin preparation solution is an unlikely culprit since the presentation is not consistent with contact dermatitis, inappropriate strapping or removal of the adhesive drape could result in stretch injury of the skin, shearing the dermoepidermal junction and causing tension blisters.9 There were no intraoperative complications and the tourniquet time was appropriate (78 minutes). Postoperatively, no compressive or adhesive dressings were used. With regards to DVT prophylaxis, the patient received a single dose of enoxaparin on the first postoperative day. While heparin-induced hemorrhagic blisters have been reported,10 I do not feel that the use of enoxaparin was a contributing factor. Heparin-induced blisters have been described as systemic blisters,10 whereas the blisters in this case were confined to the operative extremity. The patient was not taking any nutritional supplements (eg, fish oil, vitamin E) that could have increased his risk of bleeding. Throughout his hospital stay, he was hemodynamically stable and did not require blood transfusion.
Management of fracture blisters is controversial, and there is no consensus on appropriate soft-tissue handling. In this patient, the blisters were left intact. Blister fluid has been shown to be sterile, containing growth factors, opsonins, and activated neutrophils that aid in healing and infection prevention.1 Giordano and Koval11 found no difference in the outcome of 3 soft-tissue treatment techniques: (1) aspiration of the blister, (2) deroofing of the blister followed by application of a topical antibiotic cream or coverage with nonadherent dressing, or (3) keeping the blister intact and covered with loose dressing or exposed to air. In contrast, Strauss and colleagues12 found that deroofing the fracture blister to healthy tissue followed by twice-daily application of silver sulfadiazine antibiotic cream promoted reepithelialization and resulted in better cosmetic appearance and higher patient satisfaction.
The optimal dressing for fracture blisters remains elusive. Madden and colleagues13 showed that the use of occlusive nonadherent dressing was associated with significantly faster healing and less pain compared with semiocclusive, antibiotic-impregnated dressings. In another study, Varela and colleagues1 found no differences in blister healing between patients treated with either (1) dry dressing and casting, (2) Silvadene dressing (King Pharmaceuticals), or (3) whirlpool débridement and Silvadene dressing.
Infection is perhaps the most dreaded complication of fracture blisters after TKA. Varela and colleagues1 showed that, while the fluid in intact blisters was a sterile transudate, polymicrobial colonization with skin flora often occurred soon after blister rupture and persisted until reepithelialization. Our patient received a 10-day course of prophylactic antibiotics and no superficial or deep infection developed; however, the real contribution of antibiotic prophylaxis to the absence of infection cannot be established based solely on 1 case.
Pain is another concern associated with fracture blisters. Our patient had significant pain that limited his ability to participate in PT, resulting in limited knee range of motion and eventual discharge to a short-term rehabilitation facility. Fortunately, after resolution of the fracture blisters, he was able to participate in an aggressive rehabilitation program. By 6 weeks after surgery, he had significant improvement in his knee motion, avoiding the need for manipulation under anesthesia.
Conclusion
This case represents the first reported fracture blisters after primary TKA. The risk of deep surgical site infection, a devastating complication after TKA, is perhaps the most frightening concern of this rare complication. While the etiology and the management are controversial, there is evidence to recommend prophylactic antibiotics after blister rupture and skin desquamation. The decision to withhold DVT prophylaxis should be based on individual patient risk factors and blister type (blood-filled vs clear fluid–filled). Patients should be encouraged to continue knee exercises during reepithelialization to avoid stiffness.
Fracture blisters are a relatively uncommon complication of high-energy fractures, with an incidence of 2.9%.1 In the lower extremity, fracture blisters almost always occur distal to the knee.1 Histologically, the blisters represent an injury to the dermoepidermal junction.2 On physical examination, there are tense blood- and/or clear fluid–filled bullae overlying markedly swollen and edematous soft tissue,1 resembling a second-degree burn.3 Infection may develop after fracture blisters,1 and this is perhaps the most dreaded complication of total knee arthroplasty (TKA). The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 71-year-old man with end-stage osteoarthritis of the right knee underwent an elective TKA with cemented components (Legion PS; Smith & Nephew). His medical history included venous insufficiency, type 2 diabetes mellitus, chronic obstructive sleep apnea, hypertension, morbid obesity (body mass index, 50), and a previous uneventful left TKA. Tourniquet time was 78 minutes and estimated blood loss was 100 mL. An intra-articular drain was used and was removed on the first postoperative day. After wound closure, a soft splint bandage consisting of 2 to 3 layers of cotton and bias wrap was applied. Deep vein thrombosis (DVT) prophylaxis with enoxaparin 40 mg once daily was started on the first postoperative day.
Upon removal of the surgical dressings on the second postoperative day, the anterior leg was found to have a combination of tense clear fluid– and blood-filled blisters on markedly swollen and erythematous skin. The incision was minimally involved (Figure A). There was diffuse 2+ pitting edema with hyperesthesia in the affected skin distal to the knee. Prior to these findings, the patient had complained of increasing pain in his operative leg, but there was no escalation in analgesic requirements. There was no evidence of compartment syndrome on serial examinations. An ultrasound of the lower extremity was negative for DVT. Plain films did not show iatrogenic fractures. There was no intraoperative vascular injury, and the foot pulses remained unchanged between the time the patient was in the preoperative holding unit, the postanesthesia care unit, and the orthopedic ward. The operative leg was treated with elevation and loosely applied Kerlix roll gauze (Kendall, Covidien), but active blister formation continued for another 2 days. A 10-day prophylactic course of trimethoprim/sulfamethoxazole was initiated on the third postoperative day after the blisters started to rupture. The patient was allowed to bear weight as tolerated, but his physical therapy (PT) course was limited by pain and fear “of losing his leg.” He declined several PT sessions and was hesitant to use continuous passive motion. The patient was discharged to a short-term rehabilitation facility with weekly outpatient follow-up. On the second postoperative week, his fluid-filled blisters completely reepithelialized, but the blood-filled blisters required an additional week for reepithelialization (Figure B). While the patient’s knee was stiff because of limited PT participation, it was not until the second postoperative week when most of the fracture blisters had healed that he was able to resume an intensive knee exercise program, avoiding the need for manipulation under anesthesia.
Discussion
Giordano and colleagues2 identified 2 types of fracture blisters: clear fluid– and blood-filled. While both types involved disruption of the dermoepidermal junction, greater disruption and complete absence of dermal epithelial cells was observed in the hemorrhagic type. Clinical follow-up of the patients in the study by Giordano and colleagues2 showed that the mean time for reepithelialization was 12 days for fluid-filled blisters and 16 days for blood-filled blisters. These findings are similar to what we observed in our case report. In particular, the fluid-filled blisters healed in 2 weeks, whereas the blood-filled blisters required 3 weeks to heal.
The etiology of the fracture blisters in this patient is likely multifactorial and related to age, obesity, venous insufficiency, and diabetes mellitus. Farage and colleagues4 described a series of progressive degenerative changes in the aging skin, including vascular atrophy and degradation of dermal connective tissue, leading to compromised skin competence. The integrity of the dermis can be further reduced in patients with diabetes through glycosylation of collagen fibrils.5 Compared with age-matched normal controls, patients with insulin-dependent diabetes have a reduced threshold to suction-induced blister formation.6 Obesity is another potential contributing factor, with multiple studies showing significantly impaired venous flow in obese patients.7,8 Taken together, soft-tissue swelling after surgery in the setting of chronic venous insufficiency and compromised skin due to advanced age and diabetes may lead to markedly elevated interstitial pressure. One mechanism to relieve such abnormally high pressure is the formation of fracture blisters.1
Surgical risk factors that could have contributed to the complication in this case include the surgical skin preparation solution (ChloraPrep; CareFusion), use of adhesive antimicrobial drape (Ioban, 3M), tourniquet time, dressing choice, and DVT prophylaxis regimen. While the skin preparation solution is an unlikely culprit since the presentation is not consistent with contact dermatitis, inappropriate strapping or removal of the adhesive drape could result in stretch injury of the skin, shearing the dermoepidermal junction and causing tension blisters.9 There were no intraoperative complications and the tourniquet time was appropriate (78 minutes). Postoperatively, no compressive or adhesive dressings were used. With regards to DVT prophylaxis, the patient received a single dose of enoxaparin on the first postoperative day. While heparin-induced hemorrhagic blisters have been reported,10 I do not feel that the use of enoxaparin was a contributing factor. Heparin-induced blisters have been described as systemic blisters,10 whereas the blisters in this case were confined to the operative extremity. The patient was not taking any nutritional supplements (eg, fish oil, vitamin E) that could have increased his risk of bleeding. Throughout his hospital stay, he was hemodynamically stable and did not require blood transfusion.
Management of fracture blisters is controversial, and there is no consensus on appropriate soft-tissue handling. In this patient, the blisters were left intact. Blister fluid has been shown to be sterile, containing growth factors, opsonins, and activated neutrophils that aid in healing and infection prevention.1 Giordano and Koval11 found no difference in the outcome of 3 soft-tissue treatment techniques: (1) aspiration of the blister, (2) deroofing of the blister followed by application of a topical antibiotic cream or coverage with nonadherent dressing, or (3) keeping the blister intact and covered with loose dressing or exposed to air. In contrast, Strauss and colleagues12 found that deroofing the fracture blister to healthy tissue followed by twice-daily application of silver sulfadiazine antibiotic cream promoted reepithelialization and resulted in better cosmetic appearance and higher patient satisfaction.
The optimal dressing for fracture blisters remains elusive. Madden and colleagues13 showed that the use of occlusive nonadherent dressing was associated with significantly faster healing and less pain compared with semiocclusive, antibiotic-impregnated dressings. In another study, Varela and colleagues1 found no differences in blister healing between patients treated with either (1) dry dressing and casting, (2) Silvadene dressing (King Pharmaceuticals), or (3) whirlpool débridement and Silvadene dressing.
Infection is perhaps the most dreaded complication of fracture blisters after TKA. Varela and colleagues1 showed that, while the fluid in intact blisters was a sterile transudate, polymicrobial colonization with skin flora often occurred soon after blister rupture and persisted until reepithelialization. Our patient received a 10-day course of prophylactic antibiotics and no superficial or deep infection developed; however, the real contribution of antibiotic prophylaxis to the absence of infection cannot be established based solely on 1 case.
Pain is another concern associated with fracture blisters. Our patient had significant pain that limited his ability to participate in PT, resulting in limited knee range of motion and eventual discharge to a short-term rehabilitation facility. Fortunately, after resolution of the fracture blisters, he was able to participate in an aggressive rehabilitation program. By 6 weeks after surgery, he had significant improvement in his knee motion, avoiding the need for manipulation under anesthesia.
Conclusion
This case represents the first reported fracture blisters after primary TKA. The risk of deep surgical site infection, a devastating complication after TKA, is perhaps the most frightening concern of this rare complication. While the etiology and the management are controversial, there is evidence to recommend prophylactic antibiotics after blister rupture and skin desquamation. The decision to withhold DVT prophylaxis should be based on individual patient risk factors and blister type (blood-filled vs clear fluid–filled). Patients should be encouraged to continue knee exercises during reepithelialization to avoid stiffness.
1. Varela CD, Vaughan TK, Carr JB, Slemmons BK. Fracture blisters: clinical and pathological aspects. J Orthop Trauma. 1993;7(5):417-427.
2. Giordano CP, Koval KJ, Zuckerman JD, Desai P. Fracture blisters. Clin Orthop Relat Res. 1994;(307):214-221.
3. Uebbing CM, Walsh M, Miller JB, Abraham M, Arnold C. Fracture blisters. West J Emerg Med. 2011;12(1):131-133.
4. Farage MA, Miller KW, Berardesca E, Maibach HI. Clinical implications of aging skin: cutaneous disorders in the elderly. Am J Clin Dermatol. 2009;10(2):73-86.
5. Quondamatteo F. Skin and diabetes mellitus: what do we know? Cell Tissue Res. 2014;355(1):1-21.
6. Bernstein JE, Levine LE, Medenica MM, Yung CW, Soltani K. Reduced threshold to suction-induced blister formation in insulin-dependent diabetics. J Am Acad Dermatol. 1983;8(6):790-791.
7. Willenberg T, Schumacher A, Amann-Vesti B, et al. Impact of obesity on venous hemodynamics of the lower limbs. J Vasc Surg. 2010;52(3):664-668.
8. van Rij AM, De Alwis CS, Jiang P, et al. Obesity and impaired venous function. Eur J Vasc Endovasc Surg. 2008;35(6):739-744.
9. Polatsch DB, Baskies MA, Hommen JP, Egol KA, Koval KJ. Tape blisters that develop after hip fracture surgery: a retrospective series and a review of the literature. Am J Orthop. 2004;33(9):452-456.
10. Roux J, Duong TA, Ingen-Housz-Oro S, et al. Heparin-induced hemorrhagic blisters. Eur J Dermatol. 2013;23(1):105-107.
11. Giordano CP, Koval KJ. Treatment of fracture blisters: a prospective study of 53 cases. J Orthop Trauma. 1995;9(2):171-176.
12. Strauss EJ, Petrucelli G, Bong M, Koval KJ, Egol KA. Blisters associated with lower-extremity fracture: results of a prospective treatment protocol. J Orthop Trauma. 2006;20(9):618-622.
13. Madden MR, Nolan E, Finkelstein JL, et al. Comparison of an occlusive and a semi-occlusive dressing and the effect of the wound exudate upon keratinocyte proliferation. J Trauma. 1989;29(7):924-930; discussion 930-931.
1. Varela CD, Vaughan TK, Carr JB, Slemmons BK. Fracture blisters: clinical and pathological aspects. J Orthop Trauma. 1993;7(5):417-427.
2. Giordano CP, Koval KJ, Zuckerman JD, Desai P. Fracture blisters. Clin Orthop Relat Res. 1994;(307):214-221.
3. Uebbing CM, Walsh M, Miller JB, Abraham M, Arnold C. Fracture blisters. West J Emerg Med. 2011;12(1):131-133.
4. Farage MA, Miller KW, Berardesca E, Maibach HI. Clinical implications of aging skin: cutaneous disorders in the elderly. Am J Clin Dermatol. 2009;10(2):73-86.
5. Quondamatteo F. Skin and diabetes mellitus: what do we know? Cell Tissue Res. 2014;355(1):1-21.
6. Bernstein JE, Levine LE, Medenica MM, Yung CW, Soltani K. Reduced threshold to suction-induced blister formation in insulin-dependent diabetics. J Am Acad Dermatol. 1983;8(6):790-791.
7. Willenberg T, Schumacher A, Amann-Vesti B, et al. Impact of obesity on venous hemodynamics of the lower limbs. J Vasc Surg. 2010;52(3):664-668.
8. van Rij AM, De Alwis CS, Jiang P, et al. Obesity and impaired venous function. Eur J Vasc Endovasc Surg. 2008;35(6):739-744.
9. Polatsch DB, Baskies MA, Hommen JP, Egol KA, Koval KJ. Tape blisters that develop after hip fracture surgery: a retrospective series and a review of the literature. Am J Orthop. 2004;33(9):452-456.
10. Roux J, Duong TA, Ingen-Housz-Oro S, et al. Heparin-induced hemorrhagic blisters. Eur J Dermatol. 2013;23(1):105-107.
11. Giordano CP, Koval KJ. Treatment of fracture blisters: a prospective study of 53 cases. J Orthop Trauma. 1995;9(2):171-176.
12. Strauss EJ, Petrucelli G, Bong M, Koval KJ, Egol KA. Blisters associated with lower-extremity fracture: results of a prospective treatment protocol. J Orthop Trauma. 2006;20(9):618-622.
13. Madden MR, Nolan E, Finkelstein JL, et al. Comparison of an occlusive and a semi-occlusive dressing and the effect of the wound exudate upon keratinocyte proliferation. J Trauma. 1989;29(7):924-930; discussion 930-931.
Giant Solitary Synovial Chondromatosis Mimicking Chondrosarcoma: Report of a Rare Histologic Presentation and Literature Review
Synovial chondromatosis (SCM) is a relatively rare benign lesion of the synovium.1 Its pathogenesis has been thought to be a chondral metaplasia of the subintimal layer of the intra- or extra-articular synovium.2 However, evidence supporting a neoplastic cause of the disease is emerging.3 When intra-articular, any joint can be affected, though large joints are more prone to the disease; the knee, hip, and elbow are the most common locations.4 The synovial layer of tendons or bursae can be the origin of extra-articular SCM.5
Synovial chondrosarcoma (SCS), an even rarer pathology, can be caused by malignant transformation of SCM or can appear de novo on a synovial background.6 Histologic differentiation from SCM might be difficult because of the high incidence of hypercellularity, cellular atypia, and binucleated cells.6 Some features, such as presence of a very large mass or erosion of the surrounding bones, have been indicated as possible signs of malignancy.3 An unusual presentation of SCM, giant solitary synovial chondromatosis (GSSCM), can be hard to distinguish from SCS because of the large volume and possible aggressive radiologic findings.7 Some histologic features, such as presence of necrosis and mitotic cells, have been suggested as distinctive criteria for malignancy.8
In this article, we present a case of benign GSSCM with a histologic feature that has not been considered typical for benign SCM. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old woman presented with a large mass over the right hip. The mass had been growing slowly for 2 years. One year before presentation, a radiograph showed a large hip mass with fluffy calcification (Figure 1), and magnetic resonance imaging (MRI) showed a large nonhomogeneous mass anterior to the hip capsule and extending into the hip joint back to the posterior part of the joint (Figures 2A, 2B). Open incisional biopsy was performed in a local hospital at the time, and the histologic analysis revealed presence of atypical binucleated cells and pleomorphism, in addition to some mitotic activity (0 to 1 per high-power field) (Figure 3). These findings suggested malignancy. The patient declined surgery up until the time she presented to our hospital, 1 year later.
Clinical examination findings on admission to our hospital were striking. The patient had a large mass in the groin region. It was fairly tender and firm to palpation, immobile, and close to the skin. Hip motion was mildly painful but obviously restricted.
The mass was restaged. New radiographs and MRI did not show any significant changes since the previous year, computed tomography (CT) did not show any bone erosion (Figure 4), and chest radiograph, CT, and whole-body bone scan did not demonstrate any signs of metastasis.
Given the clinical presentation and previous histopathologic findings, a diagnosis of GSSCM with possible malignant transformation was made. The patient was scheduled for surgery. During surgery, the tumor was exposed through the Smith-Petersen approach. The mass was extruding under the fascia between the femoral neurovascular bundle medially and iliopsoas muscle laterally. There was no adhesion of the surrounding structures, including the femoral neurovascular bundle, to the mass. The muscle was sitting on the anterolateral surface of the mass, which was considered located in the iliopsoas bursa but extending to the joint. In the vertical plane, the mass extended down to the subtrochanteric area. The entire solid extra-articular mass was excised en bloc, and hip capsulotomy was performed inferior to the area of emergence of the mass. The joint was occupied by a single solid cartilaginous mass molding around the femoral neck, filling the piriformis fossa and propagating to the posterior joint space. Obtaining enough exposure to the back of the joint required surgical hip dislocation. The visualized acetabular fossa revealed chondral fragments, which were excised. Bone erosion or significant osteoarthritis was not detected in any part of the joint. A nearly total synovectomy was performed, leaving the ascending retinacular vessels intact. Meticulous technique was used to avoid contaminating the extra-articular tissues. The wound was closed in the routine way after hip relocation.
The 16×9.5×9-cm mass (Figure 5A) had a conglomerated internal structure (Figure 5B). Multiple specimens from the intra- and extra-articular portions of the mass were sent for histopathologic analysis, which revealed clusters of mature chondrocytes arranged in a lobular pattern and separated by thin fibrous bands. Areas of calcification and ossification were appreciated as well (Figures 6A-6C). No necrosis, mitosis, or bone permeation was detected. These findings were compatible with typical SCM. Given these pathologic findings and the lack of clinical deterioration over the previous year, a diagnosis of GSSCM with extension along the iliopsoas and obturator externus bursae was made. The already-performed marginal excision was deemed sufficient treatment. At most recent follow-up, 38 months after surgery, the patient was pain-free and had good hip range of motion and no indication of recurrence.
Discussion
SCM is a benign disorder emerging from the synovium as a result of proliferative changes in the synovial membrane of the joints, tendon sheaths, or bursae, leading to the formation of numerous cartilaginous nodules, usually a few millimeters in diameter.8 In a rare presentation of the disease, the nodules may coalesce to form a large mass, or a single cartilaginous nodule may enlarge to form a mass. Edeiken and colleagues7 named this previously unrecognized SCM feature as GSSCM when there was a major single mass larger than 1 cm in diameter. There have been other SCM cases with multiple giant masses.9,10 In the English-language literature, we found 15 GSSCM cases, which include the first reported, by Edeiken and colleagues7 (Table). However, earlier SCM cases would be reclassified GSSCM according to their definition.11
The present case brings the total to 16. Nine of the 16 patients were male. Mean age at presentation was 41 years (range, 10-80 years). The knee was the most common GSSCM site (6 cases), followed by the temporomandibular and hip joints (3 each). Regarding gross pathology, 10 lesions were solid, and 6 (including the present one) were formed by conglomeration of the chondromatosis nodules. Lesions varied in size (16-200 mm), and 2 were primarily extra-articular (foot). One common issue with most of the cases was the initial diagnosis of chondrosarcoma. The exact surgical technique used was described for 6 cases (cases 11-16); the technique was marginal excision. In no case was recurrence 14 to 60 months after surgery reported.
This chondroproliferative process is potentially a diagnostic challenge, as distinguishing it from a chondrosarcoma, a more common lesion, could be difficult based on clinical and imaging findings, and, as is true for other chondral lesions, even histologic differentiation of the conditions might not be conclusive.12,13 Confusion in diagnosis was almost universal in this series of patients.
One important differentiating feature of benign and malignant skeletal lesions is the time course of the disease. Malignant tumors are expected to demonstrate rapid enlargement and local or systemic spread. Unfortunately, often SCS cannot be distinguished by this characteristic, as grade I or II chondrosarcoma is usually a slow-growing tumor and does not metastasize early.14 Although lack of recurrence is assuring, recurrence is not necessarily a sign of malignancy, as a considerable percentage of benign chondromatosis lesions recur.8
Radiologic differentiation between SCM and SCS is another challenge. Although bone erosion caused by a lesion not originating from bone is usually considered a sign of malignancy, GSSCM was reported as causing bone erosion in 5 of the 16 cases in our literature review.7,15 Our patient did not experience any bone erosion. However, lack of bone erosion is not a reliable criterion for excluding SCS, and bone erosion was noted in only 3 of the 9 SCS cases in the series reported by Bertoni and colleagues.6 Moreover, tumor size and propagation of tumor to surrounding tissue could be surprising in GSSCM. Large size (up to 20 cm) and extra-articular spread of a lesion originating in a joint are common findings.6,16 Our case was an obvious extension of a hip GSSCM to the iliopsoas and obturator externus bursa, which is the most common pattern of extracapsular spread of hip SCM.17 An interesting feature of the present case, however, was the relatively superficial location of the mass immediately under the fascia.
Calcified matrix is key in diagnosing a chondral lesion on imaging studies, but, in some cases, SCM does not demonstrate any radiographically detectable calcification at time of diagnosis.18 However, all the GSSCM cases reported to date had obvious calcified matrix.
The hypercellularity, cellular atypia, binucleated cells, and pleomorphism in the histologic examination of the present case are not features of malignancy in SCM.8 On the contrary, several other characteristics, including qualitative differences in the arrangement of chondrocytes (sheets rather than clusters), myxoid matrix, hypercellularity with crowding and spindling of the nuclei at the periphery, necrosis, and, most important, permeation of the trabecular bone with the filling up of marrow spaces, have been assumed to be indicative of malignancy.8 Furthermore, Davis and colleagues8 found no mitotic activity in the histopathologic investigation of 53 SCM cases. Even in 3 cases that developed malignant transformation to SCS, mitosis was not found in the initial biopsy specimens before transformation. This was compatible with the common opinion that SCM is not a neoplastic, but a metaplastic, process. Histopathologic data were available for only 8 of the previous 15 GSSCM cases. There were no reports of mitosis, and necrosis was found in only 1 case.16 In our patient’s case, however, the first biopsy did show remarkable mitotic activity. This was not the case for the second biopsy, when mature chondrocytes associated with marked calcification and ossification were prominent features (Figures 6A, 6B). We presume that, within a limited period during earlier stages of tissue maturation in SCM, mitotic activity might be a possible finding. Of note, none of the other aforementioned histologic criteria for malignancy was seen in the first or second biopsy in the present case (Figures 3, 6C).
The original idea that SCM originates from a metaplasia in the subintimal layer of the synovium, where the synovium is in direct contact with the articular cartilage, has been challenged. The high incidence of hypercellularity, binucleated cells, and cellular atypia was always an argument against a metaplastic origin for the disease. Evidence of clonal chromosomal changes, like translocation of chromosome 1218 and chromosome 5 and 6 abnormalities,19,20 in addition to other alterations,19,21 provide some evidence supporting a neoplastic rather than a metaplastic origin for SCM. Given the presence of mitosis in the present case, the lack of mitotic activity in SCM, as stated by other authors,22 is not a universal feature and cannot be used as an argument against a neoplastic origin for SCM.
Although mitotic activity is uncommon in SCM, the present case illustrates the possible presence of mitotic activity in GSSCM. The simple presence of mitotic activity, a common finding in some other chondral tumors,23,24 does not preclude the diagnosis of benign SCM, as suggested before,8 and correlation of the clinical and radiologic manifestations with histopathologic findings is crucial for a correct diagnosis.
1. Milgram JW. Synovial osteochondromatosis: a histopathological study of thirty cases. J Bone Joint Surg Am. 1977;59(6):792-801.
2. Trias A, Quintana O. Synovial chondrometaplasia: review of world literature and a study of 18 Canadian cases. Can J Surg. 1976;19(2):151-158.
3. Murphey MD, Vidal JA, Fanburg-Smith JC, Gajewski DA. Imaging of synovial chondromatosis with radiologic-pathologic correlation. Radiographics. 2007;27(5):1465-1488.
4. Milgram JW. Synovial osteochondromatosis in association with Legg-Calve-Perthes disease. Clin Orthop Relat Res. 1979;(145):179-182.
5. Sim FH, Dahlin DC, Ivins JC. Extra-articular synovial chondromatosis. J Bone Joint Surg Am. 1977;59(4):492-495.
6. Bertoni F, Unni KK, Beabout JW, Sim FH. Chondrosarcomas of the synovium. Cancer. 1991;67(1):155-162.
7. Edeiken J, Edeiken BS, Ayala AG, Raymond AK, Murray JA, Guo SQ. Giant solitary synovial chondromatosis. Skeletal Radiol. 1994;23(1):23-29.
8. Davis RI, Hamilton A, Biggart JD. Primary synovial chondromatosis: a clinicopathologic review and assessment of malignant potential. Hum Pathol. 1998;29(7):683-688.
9. Goel A, Cullen C, Paul AS, Freemont AJ. Multiple giant synovial chondromatosis of the knee. Knee. 2001;8(3):243-245.
10. Dogan A, Harman M, Uslu M, Bayram I, Akpinar F. Rocky form giant synovial chondromatosis: a case report. Knee Surg Sports Traumatol Arthrosc. 2006;14(5):465-468.
11. Eisenberg KS, Johnston JO. Synovial chondromatosis of the hip joint presenting as an intrapelvic mass: a case report. J Bone Joint Surg Am. 1972;54(1):176-178.
12. Lohmann CH, Köster G, Klinger HM, Kunze E. Giant synovial osteochondromatosis of the acromio-clavicular joint in a child. A case report and review of the literature. J Pediatr Orthop B. 2005;14(2):126-128.
13. Cai XY, Yang C, Chen MJ, Jiang B, Wang BL. Arthroscopically guided removal of large solitary synovial chondromatosis from the temporomandibular joint. Int J Oral Maxillofac Surg. 2010;39(12):1236-1239.
14. Gil-Salu JL, Lazaro R, Aldasoro J, Gonzalez-Darder JM. Giant solitary synovial chondromatosis of the temporomandibular joint with intracranial extension. Skull Base Surg. 1998;8(2):99-104.
15. Kang CH, Park JH, Lee DH, Kim CH, Park JM, Lee WS. Giant synovial chondromatosis of the knee mimicking a parosteal osteosarcoma: a case report. J Korean Bone Joint Tumor Soc. 2010;16(2):95-98.
16. Nihal A, Read CJ, Henderson DC, Malcolm AJ. Extra-articular giant solitary synovial chondromatosis of the foot: a case report and literature review. Foot Ankle Surg. 1999;5(1):29-32.
17. Robinson P, White LM, Kandel R, Bell RS, Wunder JS. Primary synovial osteochondromatosis of the hip: extracapsular patterns of spread. Skeletal Radiol. 2004;33(4):210-215.
18. Tallini G, Dorfman H, Brys P, et al. Correlation between clinicopathological features and karyotype in 100 cartilaginous and chordoid tumours. A report from the Chromosomes and Morphology (CHAMP) Collaborative Study Group. J Pathol. 2002;196(2):194-203.
19. Sah AP, Geller DS, Mankin HJ, et al. Malignant transformation of synovial chondromatosis of the shoulder to chondrosarcoma. A case report. J Bone Joint Surg Am. 2007;89(6):1321-1328.
20. Buddingh EP, Krallman P, Neff JR, Nelson M, Liu J, Bridge JA. Chromosome 6 abnormalities are recurrent in synovial chondromatosis. Cancer Genet Cytogenet. 2003;140(1):18-22.
21. Rizzo M, Ghert MA, Harrelson JM, Scully SP. Chondrosarcoma of bone: analysis of 108 cases and evaluation for predictors of outcome. Clin Orthop Relat Res. 2001;(391):224-233.
22. Davis RI, Foster H, Arthur K, Trewin S, Hamilton PW, Biggart DJ. Cell proliferation studies in primary synovial chondromatosis. J Pathol. 1998;184(1):18-23.
23. Ishikawa E, Tsuboi K, Onizawa K, et al. Chondroblastoma of the temporal base with high mitotic activity. Neurol Med Chir (Tokyo). 2002;42(11):516-520.
24. Kirin I, Jurisic D, Mokrovic H, Stanec Z, Stalekar H. Chondromyxoid fibroma of the second metacarpal bone—a case report. Coll Antropol. 2011;35(3):929-931.
Synovial chondromatosis (SCM) is a relatively rare benign lesion of the synovium.1 Its pathogenesis has been thought to be a chondral metaplasia of the subintimal layer of the intra- or extra-articular synovium.2 However, evidence supporting a neoplastic cause of the disease is emerging.3 When intra-articular, any joint can be affected, though large joints are more prone to the disease; the knee, hip, and elbow are the most common locations.4 The synovial layer of tendons or bursae can be the origin of extra-articular SCM.5
Synovial chondrosarcoma (SCS), an even rarer pathology, can be caused by malignant transformation of SCM or can appear de novo on a synovial background.6 Histologic differentiation from SCM might be difficult because of the high incidence of hypercellularity, cellular atypia, and binucleated cells.6 Some features, such as presence of a very large mass or erosion of the surrounding bones, have been indicated as possible signs of malignancy.3 An unusual presentation of SCM, giant solitary synovial chondromatosis (GSSCM), can be hard to distinguish from SCS because of the large volume and possible aggressive radiologic findings.7 Some histologic features, such as presence of necrosis and mitotic cells, have been suggested as distinctive criteria for malignancy.8
In this article, we present a case of benign GSSCM with a histologic feature that has not been considered typical for benign SCM. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old woman presented with a large mass over the right hip. The mass had been growing slowly for 2 years. One year before presentation, a radiograph showed a large hip mass with fluffy calcification (Figure 1), and magnetic resonance imaging (MRI) showed a large nonhomogeneous mass anterior to the hip capsule and extending into the hip joint back to the posterior part of the joint (Figures 2A, 2B). Open incisional biopsy was performed in a local hospital at the time, and the histologic analysis revealed presence of atypical binucleated cells and pleomorphism, in addition to some mitotic activity (0 to 1 per high-power field) (Figure 3). These findings suggested malignancy. The patient declined surgery up until the time she presented to our hospital, 1 year later.
Clinical examination findings on admission to our hospital were striking. The patient had a large mass in the groin region. It was fairly tender and firm to palpation, immobile, and close to the skin. Hip motion was mildly painful but obviously restricted.
The mass was restaged. New radiographs and MRI did not show any significant changes since the previous year, computed tomography (CT) did not show any bone erosion (Figure 4), and chest radiograph, CT, and whole-body bone scan did not demonstrate any signs of metastasis.
Given the clinical presentation and previous histopathologic findings, a diagnosis of GSSCM with possible malignant transformation was made. The patient was scheduled for surgery. During surgery, the tumor was exposed through the Smith-Petersen approach. The mass was extruding under the fascia between the femoral neurovascular bundle medially and iliopsoas muscle laterally. There was no adhesion of the surrounding structures, including the femoral neurovascular bundle, to the mass. The muscle was sitting on the anterolateral surface of the mass, which was considered located in the iliopsoas bursa but extending to the joint. In the vertical plane, the mass extended down to the subtrochanteric area. The entire solid extra-articular mass was excised en bloc, and hip capsulotomy was performed inferior to the area of emergence of the mass. The joint was occupied by a single solid cartilaginous mass molding around the femoral neck, filling the piriformis fossa and propagating to the posterior joint space. Obtaining enough exposure to the back of the joint required surgical hip dislocation. The visualized acetabular fossa revealed chondral fragments, which were excised. Bone erosion or significant osteoarthritis was not detected in any part of the joint. A nearly total synovectomy was performed, leaving the ascending retinacular vessels intact. Meticulous technique was used to avoid contaminating the extra-articular tissues. The wound was closed in the routine way after hip relocation.
The 16×9.5×9-cm mass (Figure 5A) had a conglomerated internal structure (Figure 5B). Multiple specimens from the intra- and extra-articular portions of the mass were sent for histopathologic analysis, which revealed clusters of mature chondrocytes arranged in a lobular pattern and separated by thin fibrous bands. Areas of calcification and ossification were appreciated as well (Figures 6A-6C). No necrosis, mitosis, or bone permeation was detected. These findings were compatible with typical SCM. Given these pathologic findings and the lack of clinical deterioration over the previous year, a diagnosis of GSSCM with extension along the iliopsoas and obturator externus bursae was made. The already-performed marginal excision was deemed sufficient treatment. At most recent follow-up, 38 months after surgery, the patient was pain-free and had good hip range of motion and no indication of recurrence.
Discussion
SCM is a benign disorder emerging from the synovium as a result of proliferative changes in the synovial membrane of the joints, tendon sheaths, or bursae, leading to the formation of numerous cartilaginous nodules, usually a few millimeters in diameter.8 In a rare presentation of the disease, the nodules may coalesce to form a large mass, or a single cartilaginous nodule may enlarge to form a mass. Edeiken and colleagues7 named this previously unrecognized SCM feature as GSSCM when there was a major single mass larger than 1 cm in diameter. There have been other SCM cases with multiple giant masses.9,10 In the English-language literature, we found 15 GSSCM cases, which include the first reported, by Edeiken and colleagues7 (Table). However, earlier SCM cases would be reclassified GSSCM according to their definition.11
The present case brings the total to 16. Nine of the 16 patients were male. Mean age at presentation was 41 years (range, 10-80 years). The knee was the most common GSSCM site (6 cases), followed by the temporomandibular and hip joints (3 each). Regarding gross pathology, 10 lesions were solid, and 6 (including the present one) were formed by conglomeration of the chondromatosis nodules. Lesions varied in size (16-200 mm), and 2 were primarily extra-articular (foot). One common issue with most of the cases was the initial diagnosis of chondrosarcoma. The exact surgical technique used was described for 6 cases (cases 11-16); the technique was marginal excision. In no case was recurrence 14 to 60 months after surgery reported.
This chondroproliferative process is potentially a diagnostic challenge, as distinguishing it from a chondrosarcoma, a more common lesion, could be difficult based on clinical and imaging findings, and, as is true for other chondral lesions, even histologic differentiation of the conditions might not be conclusive.12,13 Confusion in diagnosis was almost universal in this series of patients.
One important differentiating feature of benign and malignant skeletal lesions is the time course of the disease. Malignant tumors are expected to demonstrate rapid enlargement and local or systemic spread. Unfortunately, often SCS cannot be distinguished by this characteristic, as grade I or II chondrosarcoma is usually a slow-growing tumor and does not metastasize early.14 Although lack of recurrence is assuring, recurrence is not necessarily a sign of malignancy, as a considerable percentage of benign chondromatosis lesions recur.8
Radiologic differentiation between SCM and SCS is another challenge. Although bone erosion caused by a lesion not originating from bone is usually considered a sign of malignancy, GSSCM was reported as causing bone erosion in 5 of the 16 cases in our literature review.7,15 Our patient did not experience any bone erosion. However, lack of bone erosion is not a reliable criterion for excluding SCS, and bone erosion was noted in only 3 of the 9 SCS cases in the series reported by Bertoni and colleagues.6 Moreover, tumor size and propagation of tumor to surrounding tissue could be surprising in GSSCM. Large size (up to 20 cm) and extra-articular spread of a lesion originating in a joint are common findings.6,16 Our case was an obvious extension of a hip GSSCM to the iliopsoas and obturator externus bursa, which is the most common pattern of extracapsular spread of hip SCM.17 An interesting feature of the present case, however, was the relatively superficial location of the mass immediately under the fascia.
Calcified matrix is key in diagnosing a chondral lesion on imaging studies, but, in some cases, SCM does not demonstrate any radiographically detectable calcification at time of diagnosis.18 However, all the GSSCM cases reported to date had obvious calcified matrix.
The hypercellularity, cellular atypia, binucleated cells, and pleomorphism in the histologic examination of the present case are not features of malignancy in SCM.8 On the contrary, several other characteristics, including qualitative differences in the arrangement of chondrocytes (sheets rather than clusters), myxoid matrix, hypercellularity with crowding and spindling of the nuclei at the periphery, necrosis, and, most important, permeation of the trabecular bone with the filling up of marrow spaces, have been assumed to be indicative of malignancy.8 Furthermore, Davis and colleagues8 found no mitotic activity in the histopathologic investigation of 53 SCM cases. Even in 3 cases that developed malignant transformation to SCS, mitosis was not found in the initial biopsy specimens before transformation. This was compatible with the common opinion that SCM is not a neoplastic, but a metaplastic, process. Histopathologic data were available for only 8 of the previous 15 GSSCM cases. There were no reports of mitosis, and necrosis was found in only 1 case.16 In our patient’s case, however, the first biopsy did show remarkable mitotic activity. This was not the case for the second biopsy, when mature chondrocytes associated with marked calcification and ossification were prominent features (Figures 6A, 6B). We presume that, within a limited period during earlier stages of tissue maturation in SCM, mitotic activity might be a possible finding. Of note, none of the other aforementioned histologic criteria for malignancy was seen in the first or second biopsy in the present case (Figures 3, 6C).
The original idea that SCM originates from a metaplasia in the subintimal layer of the synovium, where the synovium is in direct contact with the articular cartilage, has been challenged. The high incidence of hypercellularity, binucleated cells, and cellular atypia was always an argument against a metaplastic origin for the disease. Evidence of clonal chromosomal changes, like translocation of chromosome 1218 and chromosome 5 and 6 abnormalities,19,20 in addition to other alterations,19,21 provide some evidence supporting a neoplastic rather than a metaplastic origin for SCM. Given the presence of mitosis in the present case, the lack of mitotic activity in SCM, as stated by other authors,22 is not a universal feature and cannot be used as an argument against a neoplastic origin for SCM.
Although mitotic activity is uncommon in SCM, the present case illustrates the possible presence of mitotic activity in GSSCM. The simple presence of mitotic activity, a common finding in some other chondral tumors,23,24 does not preclude the diagnosis of benign SCM, as suggested before,8 and correlation of the clinical and radiologic manifestations with histopathologic findings is crucial for a correct diagnosis.
Synovial chondromatosis (SCM) is a relatively rare benign lesion of the synovium.1 Its pathogenesis has been thought to be a chondral metaplasia of the subintimal layer of the intra- or extra-articular synovium.2 However, evidence supporting a neoplastic cause of the disease is emerging.3 When intra-articular, any joint can be affected, though large joints are more prone to the disease; the knee, hip, and elbow are the most common locations.4 The synovial layer of tendons or bursae can be the origin of extra-articular SCM.5
Synovial chondrosarcoma (SCS), an even rarer pathology, can be caused by malignant transformation of SCM or can appear de novo on a synovial background.6 Histologic differentiation from SCM might be difficult because of the high incidence of hypercellularity, cellular atypia, and binucleated cells.6 Some features, such as presence of a very large mass or erosion of the surrounding bones, have been indicated as possible signs of malignancy.3 An unusual presentation of SCM, giant solitary synovial chondromatosis (GSSCM), can be hard to distinguish from SCS because of the large volume and possible aggressive radiologic findings.7 Some histologic features, such as presence of necrosis and mitotic cells, have been suggested as distinctive criteria for malignancy.8
In this article, we present a case of benign GSSCM with a histologic feature that has not been considered typical for benign SCM. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old woman presented with a large mass over the right hip. The mass had been growing slowly for 2 years. One year before presentation, a radiograph showed a large hip mass with fluffy calcification (Figure 1), and magnetic resonance imaging (MRI) showed a large nonhomogeneous mass anterior to the hip capsule and extending into the hip joint back to the posterior part of the joint (Figures 2A, 2B). Open incisional biopsy was performed in a local hospital at the time, and the histologic analysis revealed presence of atypical binucleated cells and pleomorphism, in addition to some mitotic activity (0 to 1 per high-power field) (Figure 3). These findings suggested malignancy. The patient declined surgery up until the time she presented to our hospital, 1 year later.
Clinical examination findings on admission to our hospital were striking. The patient had a large mass in the groin region. It was fairly tender and firm to palpation, immobile, and close to the skin. Hip motion was mildly painful but obviously restricted.
The mass was restaged. New radiographs and MRI did not show any significant changes since the previous year, computed tomography (CT) did not show any bone erosion (Figure 4), and chest radiograph, CT, and whole-body bone scan did not demonstrate any signs of metastasis.
Given the clinical presentation and previous histopathologic findings, a diagnosis of GSSCM with possible malignant transformation was made. The patient was scheduled for surgery. During surgery, the tumor was exposed through the Smith-Petersen approach. The mass was extruding under the fascia between the femoral neurovascular bundle medially and iliopsoas muscle laterally. There was no adhesion of the surrounding structures, including the femoral neurovascular bundle, to the mass. The muscle was sitting on the anterolateral surface of the mass, which was considered located in the iliopsoas bursa but extending to the joint. In the vertical plane, the mass extended down to the subtrochanteric area. The entire solid extra-articular mass was excised en bloc, and hip capsulotomy was performed inferior to the area of emergence of the mass. The joint was occupied by a single solid cartilaginous mass molding around the femoral neck, filling the piriformis fossa and propagating to the posterior joint space. Obtaining enough exposure to the back of the joint required surgical hip dislocation. The visualized acetabular fossa revealed chondral fragments, which were excised. Bone erosion or significant osteoarthritis was not detected in any part of the joint. A nearly total synovectomy was performed, leaving the ascending retinacular vessels intact. Meticulous technique was used to avoid contaminating the extra-articular tissues. The wound was closed in the routine way after hip relocation.
The 16×9.5×9-cm mass (Figure 5A) had a conglomerated internal structure (Figure 5B). Multiple specimens from the intra- and extra-articular portions of the mass were sent for histopathologic analysis, which revealed clusters of mature chondrocytes arranged in a lobular pattern and separated by thin fibrous bands. Areas of calcification and ossification were appreciated as well (Figures 6A-6C). No necrosis, mitosis, or bone permeation was detected. These findings were compatible with typical SCM. Given these pathologic findings and the lack of clinical deterioration over the previous year, a diagnosis of GSSCM with extension along the iliopsoas and obturator externus bursae was made. The already-performed marginal excision was deemed sufficient treatment. At most recent follow-up, 38 months after surgery, the patient was pain-free and had good hip range of motion and no indication of recurrence.
Discussion
SCM is a benign disorder emerging from the synovium as a result of proliferative changes in the synovial membrane of the joints, tendon sheaths, or bursae, leading to the formation of numerous cartilaginous nodules, usually a few millimeters in diameter.8 In a rare presentation of the disease, the nodules may coalesce to form a large mass, or a single cartilaginous nodule may enlarge to form a mass. Edeiken and colleagues7 named this previously unrecognized SCM feature as GSSCM when there was a major single mass larger than 1 cm in diameter. There have been other SCM cases with multiple giant masses.9,10 In the English-language literature, we found 15 GSSCM cases, which include the first reported, by Edeiken and colleagues7 (Table). However, earlier SCM cases would be reclassified GSSCM according to their definition.11
The present case brings the total to 16. Nine of the 16 patients were male. Mean age at presentation was 41 years (range, 10-80 years). The knee was the most common GSSCM site (6 cases), followed by the temporomandibular and hip joints (3 each). Regarding gross pathology, 10 lesions were solid, and 6 (including the present one) were formed by conglomeration of the chondromatosis nodules. Lesions varied in size (16-200 mm), and 2 were primarily extra-articular (foot). One common issue with most of the cases was the initial diagnosis of chondrosarcoma. The exact surgical technique used was described for 6 cases (cases 11-16); the technique was marginal excision. In no case was recurrence 14 to 60 months after surgery reported.
This chondroproliferative process is potentially a diagnostic challenge, as distinguishing it from a chondrosarcoma, a more common lesion, could be difficult based on clinical and imaging findings, and, as is true for other chondral lesions, even histologic differentiation of the conditions might not be conclusive.12,13 Confusion in diagnosis was almost universal in this series of patients.
One important differentiating feature of benign and malignant skeletal lesions is the time course of the disease. Malignant tumors are expected to demonstrate rapid enlargement and local or systemic spread. Unfortunately, often SCS cannot be distinguished by this characteristic, as grade I or II chondrosarcoma is usually a slow-growing tumor and does not metastasize early.14 Although lack of recurrence is assuring, recurrence is not necessarily a sign of malignancy, as a considerable percentage of benign chondromatosis lesions recur.8
Radiologic differentiation between SCM and SCS is another challenge. Although bone erosion caused by a lesion not originating from bone is usually considered a sign of malignancy, GSSCM was reported as causing bone erosion in 5 of the 16 cases in our literature review.7,15 Our patient did not experience any bone erosion. However, lack of bone erosion is not a reliable criterion for excluding SCS, and bone erosion was noted in only 3 of the 9 SCS cases in the series reported by Bertoni and colleagues.6 Moreover, tumor size and propagation of tumor to surrounding tissue could be surprising in GSSCM. Large size (up to 20 cm) and extra-articular spread of a lesion originating in a joint are common findings.6,16 Our case was an obvious extension of a hip GSSCM to the iliopsoas and obturator externus bursa, which is the most common pattern of extracapsular spread of hip SCM.17 An interesting feature of the present case, however, was the relatively superficial location of the mass immediately under the fascia.
Calcified matrix is key in diagnosing a chondral lesion on imaging studies, but, in some cases, SCM does not demonstrate any radiographically detectable calcification at time of diagnosis.18 However, all the GSSCM cases reported to date had obvious calcified matrix.
The hypercellularity, cellular atypia, binucleated cells, and pleomorphism in the histologic examination of the present case are not features of malignancy in SCM.8 On the contrary, several other characteristics, including qualitative differences in the arrangement of chondrocytes (sheets rather than clusters), myxoid matrix, hypercellularity with crowding and spindling of the nuclei at the periphery, necrosis, and, most important, permeation of the trabecular bone with the filling up of marrow spaces, have been assumed to be indicative of malignancy.8 Furthermore, Davis and colleagues8 found no mitotic activity in the histopathologic investigation of 53 SCM cases. Even in 3 cases that developed malignant transformation to SCS, mitosis was not found in the initial biopsy specimens before transformation. This was compatible with the common opinion that SCM is not a neoplastic, but a metaplastic, process. Histopathologic data were available for only 8 of the previous 15 GSSCM cases. There were no reports of mitosis, and necrosis was found in only 1 case.16 In our patient’s case, however, the first biopsy did show remarkable mitotic activity. This was not the case for the second biopsy, when mature chondrocytes associated with marked calcification and ossification were prominent features (Figures 6A, 6B). We presume that, within a limited period during earlier stages of tissue maturation in SCM, mitotic activity might be a possible finding. Of note, none of the other aforementioned histologic criteria for malignancy was seen in the first or second biopsy in the present case (Figures 3, 6C).
The original idea that SCM originates from a metaplasia in the subintimal layer of the synovium, where the synovium is in direct contact with the articular cartilage, has been challenged. The high incidence of hypercellularity, binucleated cells, and cellular atypia was always an argument against a metaplastic origin for the disease. Evidence of clonal chromosomal changes, like translocation of chromosome 1218 and chromosome 5 and 6 abnormalities,19,20 in addition to other alterations,19,21 provide some evidence supporting a neoplastic rather than a metaplastic origin for SCM. Given the presence of mitosis in the present case, the lack of mitotic activity in SCM, as stated by other authors,22 is not a universal feature and cannot be used as an argument against a neoplastic origin for SCM.
Although mitotic activity is uncommon in SCM, the present case illustrates the possible presence of mitotic activity in GSSCM. The simple presence of mitotic activity, a common finding in some other chondral tumors,23,24 does not preclude the diagnosis of benign SCM, as suggested before,8 and correlation of the clinical and radiologic manifestations with histopathologic findings is crucial for a correct diagnosis.
1. Milgram JW. Synovial osteochondromatosis: a histopathological study of thirty cases. J Bone Joint Surg Am. 1977;59(6):792-801.
2. Trias A, Quintana O. Synovial chondrometaplasia: review of world literature and a study of 18 Canadian cases. Can J Surg. 1976;19(2):151-158.
3. Murphey MD, Vidal JA, Fanburg-Smith JC, Gajewski DA. Imaging of synovial chondromatosis with radiologic-pathologic correlation. Radiographics. 2007;27(5):1465-1488.
4. Milgram JW. Synovial osteochondromatosis in association with Legg-Calve-Perthes disease. Clin Orthop Relat Res. 1979;(145):179-182.
5. Sim FH, Dahlin DC, Ivins JC. Extra-articular synovial chondromatosis. J Bone Joint Surg Am. 1977;59(4):492-495.
6. Bertoni F, Unni KK, Beabout JW, Sim FH. Chondrosarcomas of the synovium. Cancer. 1991;67(1):155-162.
7. Edeiken J, Edeiken BS, Ayala AG, Raymond AK, Murray JA, Guo SQ. Giant solitary synovial chondromatosis. Skeletal Radiol. 1994;23(1):23-29.
8. Davis RI, Hamilton A, Biggart JD. Primary synovial chondromatosis: a clinicopathologic review and assessment of malignant potential. Hum Pathol. 1998;29(7):683-688.
9. Goel A, Cullen C, Paul AS, Freemont AJ. Multiple giant synovial chondromatosis of the knee. Knee. 2001;8(3):243-245.
10. Dogan A, Harman M, Uslu M, Bayram I, Akpinar F. Rocky form giant synovial chondromatosis: a case report. Knee Surg Sports Traumatol Arthrosc. 2006;14(5):465-468.
11. Eisenberg KS, Johnston JO. Synovial chondromatosis of the hip joint presenting as an intrapelvic mass: a case report. J Bone Joint Surg Am. 1972;54(1):176-178.
12. Lohmann CH, Köster G, Klinger HM, Kunze E. Giant synovial osteochondromatosis of the acromio-clavicular joint in a child. A case report and review of the literature. J Pediatr Orthop B. 2005;14(2):126-128.
13. Cai XY, Yang C, Chen MJ, Jiang B, Wang BL. Arthroscopically guided removal of large solitary synovial chondromatosis from the temporomandibular joint. Int J Oral Maxillofac Surg. 2010;39(12):1236-1239.
14. Gil-Salu JL, Lazaro R, Aldasoro J, Gonzalez-Darder JM. Giant solitary synovial chondromatosis of the temporomandibular joint with intracranial extension. Skull Base Surg. 1998;8(2):99-104.
15. Kang CH, Park JH, Lee DH, Kim CH, Park JM, Lee WS. Giant synovial chondromatosis of the knee mimicking a parosteal osteosarcoma: a case report. J Korean Bone Joint Tumor Soc. 2010;16(2):95-98.
16. Nihal A, Read CJ, Henderson DC, Malcolm AJ. Extra-articular giant solitary synovial chondromatosis of the foot: a case report and literature review. Foot Ankle Surg. 1999;5(1):29-32.
17. Robinson P, White LM, Kandel R, Bell RS, Wunder JS. Primary synovial osteochondromatosis of the hip: extracapsular patterns of spread. Skeletal Radiol. 2004;33(4):210-215.
18. Tallini G, Dorfman H, Brys P, et al. Correlation between clinicopathological features and karyotype in 100 cartilaginous and chordoid tumours. A report from the Chromosomes and Morphology (CHAMP) Collaborative Study Group. J Pathol. 2002;196(2):194-203.
19. Sah AP, Geller DS, Mankin HJ, et al. Malignant transformation of synovial chondromatosis of the shoulder to chondrosarcoma. A case report. J Bone Joint Surg Am. 2007;89(6):1321-1328.
20. Buddingh EP, Krallman P, Neff JR, Nelson M, Liu J, Bridge JA. Chromosome 6 abnormalities are recurrent in synovial chondromatosis. Cancer Genet Cytogenet. 2003;140(1):18-22.
21. Rizzo M, Ghert MA, Harrelson JM, Scully SP. Chondrosarcoma of bone: analysis of 108 cases and evaluation for predictors of outcome. Clin Orthop Relat Res. 2001;(391):224-233.
22. Davis RI, Foster H, Arthur K, Trewin S, Hamilton PW, Biggart DJ. Cell proliferation studies in primary synovial chondromatosis. J Pathol. 1998;184(1):18-23.
23. Ishikawa E, Tsuboi K, Onizawa K, et al. Chondroblastoma of the temporal base with high mitotic activity. Neurol Med Chir (Tokyo). 2002;42(11):516-520.
24. Kirin I, Jurisic D, Mokrovic H, Stanec Z, Stalekar H. Chondromyxoid fibroma of the second metacarpal bone—a case report. Coll Antropol. 2011;35(3):929-931.
1. Milgram JW. Synovial osteochondromatosis: a histopathological study of thirty cases. J Bone Joint Surg Am. 1977;59(6):792-801.
2. Trias A, Quintana O. Synovial chondrometaplasia: review of world literature and a study of 18 Canadian cases. Can J Surg. 1976;19(2):151-158.
3. Murphey MD, Vidal JA, Fanburg-Smith JC, Gajewski DA. Imaging of synovial chondromatosis with radiologic-pathologic correlation. Radiographics. 2007;27(5):1465-1488.
4. Milgram JW. Synovial osteochondromatosis in association with Legg-Calve-Perthes disease. Clin Orthop Relat Res. 1979;(145):179-182.
5. Sim FH, Dahlin DC, Ivins JC. Extra-articular synovial chondromatosis. J Bone Joint Surg Am. 1977;59(4):492-495.
6. Bertoni F, Unni KK, Beabout JW, Sim FH. Chondrosarcomas of the synovium. Cancer. 1991;67(1):155-162.
7. Edeiken J, Edeiken BS, Ayala AG, Raymond AK, Murray JA, Guo SQ. Giant solitary synovial chondromatosis. Skeletal Radiol. 1994;23(1):23-29.
8. Davis RI, Hamilton A, Biggart JD. Primary synovial chondromatosis: a clinicopathologic review and assessment of malignant potential. Hum Pathol. 1998;29(7):683-688.
9. Goel A, Cullen C, Paul AS, Freemont AJ. Multiple giant synovial chondromatosis of the knee. Knee. 2001;8(3):243-245.
10. Dogan A, Harman M, Uslu M, Bayram I, Akpinar F. Rocky form giant synovial chondromatosis: a case report. Knee Surg Sports Traumatol Arthrosc. 2006;14(5):465-468.
11. Eisenberg KS, Johnston JO. Synovial chondromatosis of the hip joint presenting as an intrapelvic mass: a case report. J Bone Joint Surg Am. 1972;54(1):176-178.
12. Lohmann CH, Köster G, Klinger HM, Kunze E. Giant synovial osteochondromatosis of the acromio-clavicular joint in a child. A case report and review of the literature. J Pediatr Orthop B. 2005;14(2):126-128.
13. Cai XY, Yang C, Chen MJ, Jiang B, Wang BL. Arthroscopically guided removal of large solitary synovial chondromatosis from the temporomandibular joint. Int J Oral Maxillofac Surg. 2010;39(12):1236-1239.
14. Gil-Salu JL, Lazaro R, Aldasoro J, Gonzalez-Darder JM. Giant solitary synovial chondromatosis of the temporomandibular joint with intracranial extension. Skull Base Surg. 1998;8(2):99-104.
15. Kang CH, Park JH, Lee DH, Kim CH, Park JM, Lee WS. Giant synovial chondromatosis of the knee mimicking a parosteal osteosarcoma: a case report. J Korean Bone Joint Tumor Soc. 2010;16(2):95-98.
16. Nihal A, Read CJ, Henderson DC, Malcolm AJ. Extra-articular giant solitary synovial chondromatosis of the foot: a case report and literature review. Foot Ankle Surg. 1999;5(1):29-32.
17. Robinson P, White LM, Kandel R, Bell RS, Wunder JS. Primary synovial osteochondromatosis of the hip: extracapsular patterns of spread. Skeletal Radiol. 2004;33(4):210-215.
18. Tallini G, Dorfman H, Brys P, et al. Correlation between clinicopathological features and karyotype in 100 cartilaginous and chordoid tumours. A report from the Chromosomes and Morphology (CHAMP) Collaborative Study Group. J Pathol. 2002;196(2):194-203.
19. Sah AP, Geller DS, Mankin HJ, et al. Malignant transformation of synovial chondromatosis of the shoulder to chondrosarcoma. A case report. J Bone Joint Surg Am. 2007;89(6):1321-1328.
20. Buddingh EP, Krallman P, Neff JR, Nelson M, Liu J, Bridge JA. Chromosome 6 abnormalities are recurrent in synovial chondromatosis. Cancer Genet Cytogenet. 2003;140(1):18-22.
21. Rizzo M, Ghert MA, Harrelson JM, Scully SP. Chondrosarcoma of bone: analysis of 108 cases and evaluation for predictors of outcome. Clin Orthop Relat Res. 2001;(391):224-233.
22. Davis RI, Foster H, Arthur K, Trewin S, Hamilton PW, Biggart DJ. Cell proliferation studies in primary synovial chondromatosis. J Pathol. 1998;184(1):18-23.
23. Ishikawa E, Tsuboi K, Onizawa K, et al. Chondroblastoma of the temporal base with high mitotic activity. Neurol Med Chir (Tokyo). 2002;42(11):516-520.
24. Kirin I, Jurisic D, Mokrovic H, Stanec Z, Stalekar H. Chondromyxoid fibroma of the second metacarpal bone—a case report. Coll Antropol. 2011;35(3):929-931.
Congenital Absence of the Anterior Cruciate Ligament
Congenital absence of the anterior cruciate ligament (ACL) is a rare occurrence and has been seen most often in conjunction with conditions such as knee dislocation, knee dysplasia, proximal focal femoral deficiency, and fibular hemimelia.
We report on the incidental finding of ACL aplasia in a patient with a medial meniscal tear and history of leg-length discrepancy. Similar to earlier cases, this patient had hypertrophy of the meniscofemoral ligament of Humphrey, which likely provided stability. This case report emphasizes the importance of distinguishing between a stable and an unstable knee in congenital absence of the ACL. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 20-year-old woman presented for orthopedic evaluation with worsening medial left knee pain. Her pain was intermittent in nature, occurring about every 1 to 2 months and of 1 to 2 days’ duration. Onset was while using the elliptical machine, walking on uneven ground, or navigating stairs. She denied any buckling, catching, locking, instability, or swelling.
Her history was significant for a breech delivery and leg anisomelia, for which she had a contralateral distal femoral and proximal tibial percutaneous epiphysiodesis performed at age 10 years. Family history was negative for limb deformities.
Physical examination was notable for absence of global ligamentous laxity, overall valgus alignment of the left lower extremity, minimally decreased motion, trace effusion, positive medial joint line tenderness, positive McMurray test, and 1+ Lachman test with guarding on pivot shift testing.
Plain films showed valgus alignment with narrowing of the lateral compartment, narrow intercondylar notch, and hypoplasia of the tibial eminences and lateral femoral condyle (Figure 1). Magnetic resonance imaging showed a large tear in the posterior horn of the medial meniscus, hypertrophy of the meniscofemoral ligament of Humphrey (Figure 2A), and nonvisualization of the ACL with a small remnant (Figure 2B).
Arthroscopy showed complete absence of fibers of the ACL, hypertrophy of the meniscofemoral ligament of Humphrey, and a large posterior horn medial meniscal tear. A partial medial meniscectomy was performed. More than 2 years after surgery, the patient was doing very well without pain or instability, and was exercising regularly without difficulty.
Discussion
Our patient had left-sided congenital absence of the ACL with associated limb-length discrepancy of more than 2.5 cm. Isolated absence of the ACL has been described in a few case reports in the literature. Congenital ACL absence has most often been found in association with conditions such as knee dislocation (occurring with a frequency of .017/1000 births),1 knee dysplasia,2,3 fibular hemimelia,4 and proximal focal femoral deficiency.5 Johansson and Aparisi5,6 linked the finding of ACL absence with instability in those patients with known limb-length discrepancy and symptomatic instability. This report presents a patient who has congenital absence of the ACL in a foreshortened limb and torn medial meniscus. The classification of the patient’s cruciate dysplasia would be type I, as described by Manner and colleagues.7 The incidence of meniscal tears in association with congenital ACL absence is unknown. There have been reports of absence of the ACL associated with a ring meniscus,8 absence of both cruciate ligaments and menisci,9 and a bucket-handle tear of the medial meniscus.10
Gabos and colleagues4 recommend reconstructive surgery for patients with congenital absence of the ACL and symptomatic knee instability. Limb lengthening/shortening and realignment procedures have allowed patients such as ours to have functionally anatomic limbs and high activity levels. Surgical treatment is pursued to restore mechanical alignment and stability. Our patient had no symptoms of instability.
Similar to 3 of the 4 patients presented by Gabos and colleagues,4 our patient had marked hypertrophy of the meniscofemoral ligament of Humphrey. The report by Gabos and colleagues4 of this finding was the first in the literature. The hypertrophy of this ligament suggests it has a role in stabilizing the knee with a congenitally absent ACL. Our patient had no instability in her left knee but presented because of episodes of pain.
Of significant concern is the long-term outcome of patients with congenital ACL aplasia. Crawford and colleagues11 reported 11 patients with ACL deficiency and fibular hemimelia at a mean age of 37 years, showing similar functional outcomes to age-matched controls. However, there was no radiographic follow-up reported in regard to the development of osteoarthritis. To our knowledge, there have been no series published comparing surgical and nonsurgical treatment of congenital absence of the ACL. In the study by Gabos and colleagues,4 all patients were treated with reconstruction because these patients had symptomatic instability.
Conclusion
This report presents a patient whose symptoms improved after resection of her medial meniscal tear. This patient will be followed long-term to delineate her clinical course and to monitor for instability and/or development of osteoarthritis. Future studies should compare the treatment of congenital absence of the ACL with reconstruction and with conservative management.
1. Tachdjian MO. Pediatric Orthopedics. 2nd ed. Philadelphia: Saunders; 1990.
2. Thomas NP, Jackson AM, Aichroth PM. Congenital absence of the anterior cruciate ligament: A common component of knee dysplasia. J Bone Joint Surg Br. 1985;67(4):572-575.
3. Hejgaard N, Kjaerulff H. Congenital aplasia of the anterior cruciate ligament. Report of a case in a seven-year-old girl. Int Orthop. 1987;11(3):223-225.
4. Gabos PG, El Rassi G, Pahys J. Knee reconstruction in syndromes with congenital absence of the anterior cruciate ligament. J Pediatr Orthop. 2005;25(2):210-214.
5. Johansson E, Aparisi T. Missing cruciate ligament in congenital short femur. J Bone Joint Surg Am. 1983;65(8):1109-1115.
6. Johannson E, Aparisi T. Congenital absence of the cruciate ligaments. A case report and review of the literature. Clin Orthop Relat Res. 1982;162:108-111.
7. Manner HM, Radler C, Ganger R, Grill F. Dysplasia of the cruciate ligaments: radiographic assessment and classification. J Bone Joint Surg Am. 2006;88(1):130-137.
8. Noble J. Congenital absence of the anterior cruciate ligament associated with a ring meniscus. J Bone Joint Surg Am. 1975;57(8):1165-1166.
9. Tolo VT. Congenital absence of the menisci and cruciate ligaments of the knee. A case report. J Bone Joint Surg Am. 1981;63(6):1022-1024.
10. Kaelin A, Hulin PH, Carlioz H. Congenital aplasia of the cruciate ligaments. A report of six cases. J Bone Joint Surg Br. 1986;68(5):827-828.
11. Crawford DA, Tompkins BJ, Baird GO, Caskey PM. The long term function of the knee in patients with fibular hemimelia and anterior cruciate ligament deficiency. J Bone Joint Surg Br. 2012;94(3):328-333.
Congenital absence of the anterior cruciate ligament (ACL) is a rare occurrence and has been seen most often in conjunction with conditions such as knee dislocation, knee dysplasia, proximal focal femoral deficiency, and fibular hemimelia.
We report on the incidental finding of ACL aplasia in a patient with a medial meniscal tear and history of leg-length discrepancy. Similar to earlier cases, this patient had hypertrophy of the meniscofemoral ligament of Humphrey, which likely provided stability. This case report emphasizes the importance of distinguishing between a stable and an unstable knee in congenital absence of the ACL. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 20-year-old woman presented for orthopedic evaluation with worsening medial left knee pain. Her pain was intermittent in nature, occurring about every 1 to 2 months and of 1 to 2 days’ duration. Onset was while using the elliptical machine, walking on uneven ground, or navigating stairs. She denied any buckling, catching, locking, instability, or swelling.
Her history was significant for a breech delivery and leg anisomelia, for which she had a contralateral distal femoral and proximal tibial percutaneous epiphysiodesis performed at age 10 years. Family history was negative for limb deformities.
Physical examination was notable for absence of global ligamentous laxity, overall valgus alignment of the left lower extremity, minimally decreased motion, trace effusion, positive medial joint line tenderness, positive McMurray test, and 1+ Lachman test with guarding on pivot shift testing.
Plain films showed valgus alignment with narrowing of the lateral compartment, narrow intercondylar notch, and hypoplasia of the tibial eminences and lateral femoral condyle (Figure 1). Magnetic resonance imaging showed a large tear in the posterior horn of the medial meniscus, hypertrophy of the meniscofemoral ligament of Humphrey (Figure 2A), and nonvisualization of the ACL with a small remnant (Figure 2B).
Arthroscopy showed complete absence of fibers of the ACL, hypertrophy of the meniscofemoral ligament of Humphrey, and a large posterior horn medial meniscal tear. A partial medial meniscectomy was performed. More than 2 years after surgery, the patient was doing very well without pain or instability, and was exercising regularly without difficulty.
Discussion
Our patient had left-sided congenital absence of the ACL with associated limb-length discrepancy of more than 2.5 cm. Isolated absence of the ACL has been described in a few case reports in the literature. Congenital ACL absence has most often been found in association with conditions such as knee dislocation (occurring with a frequency of .017/1000 births),1 knee dysplasia,2,3 fibular hemimelia,4 and proximal focal femoral deficiency.5 Johansson and Aparisi5,6 linked the finding of ACL absence with instability in those patients with known limb-length discrepancy and symptomatic instability. This report presents a patient who has congenital absence of the ACL in a foreshortened limb and torn medial meniscus. The classification of the patient’s cruciate dysplasia would be type I, as described by Manner and colleagues.7 The incidence of meniscal tears in association with congenital ACL absence is unknown. There have been reports of absence of the ACL associated with a ring meniscus,8 absence of both cruciate ligaments and menisci,9 and a bucket-handle tear of the medial meniscus.10
Gabos and colleagues4 recommend reconstructive surgery for patients with congenital absence of the ACL and symptomatic knee instability. Limb lengthening/shortening and realignment procedures have allowed patients such as ours to have functionally anatomic limbs and high activity levels. Surgical treatment is pursued to restore mechanical alignment and stability. Our patient had no symptoms of instability.
Similar to 3 of the 4 patients presented by Gabos and colleagues,4 our patient had marked hypertrophy of the meniscofemoral ligament of Humphrey. The report by Gabos and colleagues4 of this finding was the first in the literature. The hypertrophy of this ligament suggests it has a role in stabilizing the knee with a congenitally absent ACL. Our patient had no instability in her left knee but presented because of episodes of pain.
Of significant concern is the long-term outcome of patients with congenital ACL aplasia. Crawford and colleagues11 reported 11 patients with ACL deficiency and fibular hemimelia at a mean age of 37 years, showing similar functional outcomes to age-matched controls. However, there was no radiographic follow-up reported in regard to the development of osteoarthritis. To our knowledge, there have been no series published comparing surgical and nonsurgical treatment of congenital absence of the ACL. In the study by Gabos and colleagues,4 all patients were treated with reconstruction because these patients had symptomatic instability.
Conclusion
This report presents a patient whose symptoms improved after resection of her medial meniscal tear. This patient will be followed long-term to delineate her clinical course and to monitor for instability and/or development of osteoarthritis. Future studies should compare the treatment of congenital absence of the ACL with reconstruction and with conservative management.
Congenital absence of the anterior cruciate ligament (ACL) is a rare occurrence and has been seen most often in conjunction with conditions such as knee dislocation, knee dysplasia, proximal focal femoral deficiency, and fibular hemimelia.
We report on the incidental finding of ACL aplasia in a patient with a medial meniscal tear and history of leg-length discrepancy. Similar to earlier cases, this patient had hypertrophy of the meniscofemoral ligament of Humphrey, which likely provided stability. This case report emphasizes the importance of distinguishing between a stable and an unstable knee in congenital absence of the ACL. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 20-year-old woman presented for orthopedic evaluation with worsening medial left knee pain. Her pain was intermittent in nature, occurring about every 1 to 2 months and of 1 to 2 days’ duration. Onset was while using the elliptical machine, walking on uneven ground, or navigating stairs. She denied any buckling, catching, locking, instability, or swelling.
Her history was significant for a breech delivery and leg anisomelia, for which she had a contralateral distal femoral and proximal tibial percutaneous epiphysiodesis performed at age 10 years. Family history was negative for limb deformities.
Physical examination was notable for absence of global ligamentous laxity, overall valgus alignment of the left lower extremity, minimally decreased motion, trace effusion, positive medial joint line tenderness, positive McMurray test, and 1+ Lachman test with guarding on pivot shift testing.
Plain films showed valgus alignment with narrowing of the lateral compartment, narrow intercondylar notch, and hypoplasia of the tibial eminences and lateral femoral condyle (Figure 1). Magnetic resonance imaging showed a large tear in the posterior horn of the medial meniscus, hypertrophy of the meniscofemoral ligament of Humphrey (Figure 2A), and nonvisualization of the ACL with a small remnant (Figure 2B).
Arthroscopy showed complete absence of fibers of the ACL, hypertrophy of the meniscofemoral ligament of Humphrey, and a large posterior horn medial meniscal tear. A partial medial meniscectomy was performed. More than 2 years after surgery, the patient was doing very well without pain or instability, and was exercising regularly without difficulty.
Discussion
Our patient had left-sided congenital absence of the ACL with associated limb-length discrepancy of more than 2.5 cm. Isolated absence of the ACL has been described in a few case reports in the literature. Congenital ACL absence has most often been found in association with conditions such as knee dislocation (occurring with a frequency of .017/1000 births),1 knee dysplasia,2,3 fibular hemimelia,4 and proximal focal femoral deficiency.5 Johansson and Aparisi5,6 linked the finding of ACL absence with instability in those patients with known limb-length discrepancy and symptomatic instability. This report presents a patient who has congenital absence of the ACL in a foreshortened limb and torn medial meniscus. The classification of the patient’s cruciate dysplasia would be type I, as described by Manner and colleagues.7 The incidence of meniscal tears in association with congenital ACL absence is unknown. There have been reports of absence of the ACL associated with a ring meniscus,8 absence of both cruciate ligaments and menisci,9 and a bucket-handle tear of the medial meniscus.10
Gabos and colleagues4 recommend reconstructive surgery for patients with congenital absence of the ACL and symptomatic knee instability. Limb lengthening/shortening and realignment procedures have allowed patients such as ours to have functionally anatomic limbs and high activity levels. Surgical treatment is pursued to restore mechanical alignment and stability. Our patient had no symptoms of instability.
Similar to 3 of the 4 patients presented by Gabos and colleagues,4 our patient had marked hypertrophy of the meniscofemoral ligament of Humphrey. The report by Gabos and colleagues4 of this finding was the first in the literature. The hypertrophy of this ligament suggests it has a role in stabilizing the knee with a congenitally absent ACL. Our patient had no instability in her left knee but presented because of episodes of pain.
Of significant concern is the long-term outcome of patients with congenital ACL aplasia. Crawford and colleagues11 reported 11 patients with ACL deficiency and fibular hemimelia at a mean age of 37 years, showing similar functional outcomes to age-matched controls. However, there was no radiographic follow-up reported in regard to the development of osteoarthritis. To our knowledge, there have been no series published comparing surgical and nonsurgical treatment of congenital absence of the ACL. In the study by Gabos and colleagues,4 all patients were treated with reconstruction because these patients had symptomatic instability.
Conclusion
This report presents a patient whose symptoms improved after resection of her medial meniscal tear. This patient will be followed long-term to delineate her clinical course and to monitor for instability and/or development of osteoarthritis. Future studies should compare the treatment of congenital absence of the ACL with reconstruction and with conservative management.
1. Tachdjian MO. Pediatric Orthopedics. 2nd ed. Philadelphia: Saunders; 1990.
2. Thomas NP, Jackson AM, Aichroth PM. Congenital absence of the anterior cruciate ligament: A common component of knee dysplasia. J Bone Joint Surg Br. 1985;67(4):572-575.
3. Hejgaard N, Kjaerulff H. Congenital aplasia of the anterior cruciate ligament. Report of a case in a seven-year-old girl. Int Orthop. 1987;11(3):223-225.
4. Gabos PG, El Rassi G, Pahys J. Knee reconstruction in syndromes with congenital absence of the anterior cruciate ligament. J Pediatr Orthop. 2005;25(2):210-214.
5. Johansson E, Aparisi T. Missing cruciate ligament in congenital short femur. J Bone Joint Surg Am. 1983;65(8):1109-1115.
6. Johannson E, Aparisi T. Congenital absence of the cruciate ligaments. A case report and review of the literature. Clin Orthop Relat Res. 1982;162:108-111.
7. Manner HM, Radler C, Ganger R, Grill F. Dysplasia of the cruciate ligaments: radiographic assessment and classification. J Bone Joint Surg Am. 2006;88(1):130-137.
8. Noble J. Congenital absence of the anterior cruciate ligament associated with a ring meniscus. J Bone Joint Surg Am. 1975;57(8):1165-1166.
9. Tolo VT. Congenital absence of the menisci and cruciate ligaments of the knee. A case report. J Bone Joint Surg Am. 1981;63(6):1022-1024.
10. Kaelin A, Hulin PH, Carlioz H. Congenital aplasia of the cruciate ligaments. A report of six cases. J Bone Joint Surg Br. 1986;68(5):827-828.
11. Crawford DA, Tompkins BJ, Baird GO, Caskey PM. The long term function of the knee in patients with fibular hemimelia and anterior cruciate ligament deficiency. J Bone Joint Surg Br. 2012;94(3):328-333.
1. Tachdjian MO. Pediatric Orthopedics. 2nd ed. Philadelphia: Saunders; 1990.
2. Thomas NP, Jackson AM, Aichroth PM. Congenital absence of the anterior cruciate ligament: A common component of knee dysplasia. J Bone Joint Surg Br. 1985;67(4):572-575.
3. Hejgaard N, Kjaerulff H. Congenital aplasia of the anterior cruciate ligament. Report of a case in a seven-year-old girl. Int Orthop. 1987;11(3):223-225.
4. Gabos PG, El Rassi G, Pahys J. Knee reconstruction in syndromes with congenital absence of the anterior cruciate ligament. J Pediatr Orthop. 2005;25(2):210-214.
5. Johansson E, Aparisi T. Missing cruciate ligament in congenital short femur. J Bone Joint Surg Am. 1983;65(8):1109-1115.
6. Johannson E, Aparisi T. Congenital absence of the cruciate ligaments. A case report and review of the literature. Clin Orthop Relat Res. 1982;162:108-111.
7. Manner HM, Radler C, Ganger R, Grill F. Dysplasia of the cruciate ligaments: radiographic assessment and classification. J Bone Joint Surg Am. 2006;88(1):130-137.
8. Noble J. Congenital absence of the anterior cruciate ligament associated with a ring meniscus. J Bone Joint Surg Am. 1975;57(8):1165-1166.
9. Tolo VT. Congenital absence of the menisci and cruciate ligaments of the knee. A case report. J Bone Joint Surg Am. 1981;63(6):1022-1024.
10. Kaelin A, Hulin PH, Carlioz H. Congenital aplasia of the cruciate ligaments. A report of six cases. J Bone Joint Surg Br. 1986;68(5):827-828.
11. Crawford DA, Tompkins BJ, Baird GO, Caskey PM. The long term function of the knee in patients with fibular hemimelia and anterior cruciate ligament deficiency. J Bone Joint Surg Br. 2012;94(3):328-333.
Iatrogenic Femoral Neck Fracture After Closed Reduction of Anterior Hip Dislocation in the Emergency Department
Anterior hip dislocations have been reported to account for approximately 5% to 10% of all hip dislocations.1 Epstein and Wiss2 originally divided anterior hip dislocations into superior (type I, including pubic or subspinous) and inferior (type II, including obturator and perineal) dislocations. This classification was further subdivided based on the presence of either no associated fracture (type A), fracture of the femoral head or neck (FNF; type B), or fracture of the acetabulum (type C).3 Of all anterior hip dislocations, it has been reported that the inferior or obturator type of dislocation is more common, constituting approximately 70% of all anterior dislocations.4 In 1943, Pringle5 described the mechanism of obturator dislocation as simultaneous abduction, flexion, and external rotation of the hip. Our literature search found only 2 case reports in non-English-language journals of a complete FNF associated with an attempted reduction of an anterior hip dislocation.6,7 Indentation fractures of the femoral head have been more commonly reported than FNFs, with a reported incidence of 35% to 55% after anterior dislocation.4,8 DeLee and colleagues8 also found that those patients with indentation fractures were at a higher risk for developing avascular necrosis of the femoral head in addition to being more likely to report poor or fair function of the hip 2 years after reduction.
There have been a number of different reduction maneuvers for anterior dislocation of hips published in the literature. Epstein and Harvey9 advocated reduction by traction in the line of the femur with the hip flexed and in gentle internal rotation and abduction while the patient was under general anesthesia. Toms and Williams,10 however, recommended adduction with gradual release of the longitudinal traction. Polesky and Polesky11 described a reduction method involving sharp internal rotation, which was found to be associated with FNF. The patient provided written informed consent for print and electronic publication of this case report, and approval was obtained from the Emory University Institutional Review Board.
Case Report
The patient was a 73-year-old woman, an independent ambulator with minimal antecedent hip pain, who, as a pedestrian, was struck by a heavy-duty pickup truck at low velocity. She was flown to our level I trauma center from an outside hospital. The patient arrived hemodynamically stable, with a Glasgow Coma Scale score of 15 and with major complaints of right shoulder and right hip pain. She had a positive Focused Assessment with Sonography for Trauma (FAST), and underwent a subsequent urgent chest, abdomen, and pelvis computed tomography (CT) scan for further investigation. CT showed a grade 1 liver laceration. Her anteroposterior (AP) pelvic radiograph and pelvic CT scan showed an anterior hip dislocation with the femoral head located adjacent to the obturator foramen (Figures 1, 2). The AP pelvic radiograph and pelvic CT scan were scrutinized extensively before reduction to rule out a possible FNF. Comparing the right and left femoral necks through multiple axial CT images showed no obvious differences between the 2 sides (Figures 3, 4). Her only other orthopedic injury was an inferior shoulder dislocation. It is not routine for the general surgery trauma team to obtain a pelvic CT scan prior to involvement of the orthopedic service and prompt reduction of a hip dislocation. Upon initial examination of her right hip, it was fixed in slight flexion and external rotation; she was neurovascularly intact.
After being cleared by the trauma service, the patient provided informed consent for closed reduction of the hip and shoulder under conscious sedation, performed by the emergency department (ED) staff. She received intravenous fentanyl and midazolam, and the reduction was attempted. The reduction maneuver was performed with gentle inline traction, adduction, and internal rotation and extension. There was an audible clunk, and the hip was thought to be reduced and stable. The right leg lower extremity was placed into a knee immobilizer and she remained neurovascularly intact. The shoulder was reduced. After the procedure, the patient had an episode of hypoxia requiring oxygenation via a bag valve mask by the ED staff. Postreduction radiographs confirmed reduction of the right shoulder; however, they also showed a FNF with the femoral head retained near the obturator foramen (Figures 5, 6). The patient and her family were informed of the fracture, and a total hip arthroplasty (THA) was recommended, given her pre-injury mild symptomatic osteoarthritis in the hip and her age. The patient was admitted to the intensive care unit for cardiopulmonary monitoring and was found to have a troponin leak on hospital day 1. She was evaluated by the cardiology service; serial electrocardiograms and troponins ruled out acute myocardial infarction. The patient was cleared for surgery on hospital day 4.
On hospital day 5, she underwent a right THA via a Kocher-Langenbeck approach. The patient’s femoral head was found to be anterior and laterally adjacent to her ischial tuberosity with an indentation fracture. The sciatic nerve was identified and found to be intact. A metal-on-polyethylene Stryker Accolade femoral component and Trident acetabular shell were implanted, and a posterior capsular repair was performed (Figure 7).
The patient tolerated the procedure well, and her postoperative course was uneventful. She was discharged to a subacute rehabilitation facility on postoperative day 3. The patient returned for her 2-week postoperative visit ambulating without assistance. At her last follow-up visit, approximately 6 weeks after surgery, she was a functionally independent community ambulator. Phone conversations with her private orthopedist at 6 months confirmed continued ambulation without problems.
Discussion
This case report of a complication that occurred in our institution has resulted in a change in our protocol for treatment of geriatric anterior hip dislocations. Our institution is a level I trauma center, and traumatic hip dislocations are relatively common, occurring usually in young patients with high-energy trauma. Although somewhat controversial, it is generally assumed that the incidence of avascular necrosis of the femoral head after dislocation of the hip is correlated with the time interval from dislocation to reduction of the hip. Therefore, our protocol for hip dislocations of the hip in young trauma patients is urgent reduction in the ED under appropriate analgesia and muscle relaxation.
In this case report, the patient was older than 65 years with radiographic evidence of possible impingement and postsurgical evidence of impingement of the femoral head in the obturator foremen (Figures 1, 2, 8). In addition, the patient was significantly osteopenic radiographically. An attempted reduction in the ED resulted in FNF requiring THA (Figures 5, 6, 9). After discussion of this complication in our institution’s morbidity and mortality conference, we have developed a protocol for the geriatric patient (older than 65 years) with a traumatic hip dislocation. These patients will undergo attempted reduction under controlled analgesia and muscle relaxation in the operating room (OR) with an attending surgeon present, ideally, an attending surgeon comfortable with arthroplasty in a terminally cleaned OR room. Our institution’s surgical site infection rate after total joint arthroplasty has significantly decreased with improved patient selection and the use of terminally cleaned OR rooms. Because our policy is to perform closed reduction of dislocated hips in an urgent manner, if there is not a terminally clean room or an arthroplasty-trained attending orthopedic surgeon available, then informed consent with discussion of the possibility of fracture requiring a subsequent arthroplasty should be obtained from the patient before the attempted reduction.
After review of the available literature, we believe that this case highlights some of the important treatment principles when treating anterior hip dislocations in the ED. The relatively high incidence of indentation fractures of the femoral head with obturator dislocations puts these fractures at higher risk for possible impingement around the obturator ring. This impingement, coupled with preexisting osteopenia, can predispose these dislocations to FNF, if appropriate analgesia and sedation are not obtained and gentle reduction is not performed. In addition, while it may not be time- or cost-effective to perform closed reduction on every hip dislocation, we bring geriatric patients with radiographic osteopenia to the OR for more controlled reductions. In the informed consent discussion, the possibility of FNF is mentioned, and the patient and family are told that an elective total hip replacement will be performed if this complication occurs.
We consider the following to be risk factors for closed reductions of anterior hip dislocations: (1) preexisting osteopenia on plain films, (2) age greater than 65 years, and (3) radiographic femoral head impingement on the surrounding bony pelvis. We continue to consider closed reduction of both anterior and posterior hip dislocations as urgent (within 6 hours from time of dislocation). This case adds to the existing literature on the risk of FNF with closed reduction of obturator hip dislocations, and we hope that it will encourage further study into the safest and most cost-effective reduction protocol.
1. Amihood, S. Anterior dislocation of the hip. Injury. 1975;7(2):107-110.
2. Epstein HC, Wiss DA. Traumatic anterior dislocation of the hip. Orthopedics. 1985;8(1):130, 132-134.
3. Epstein HC. Traumatic dislocations of the hip. Clin Orthop Relat Res. 1973(92):116-142.
4. Erb RE, Steele JR, Nance EP Jr, Edwards JR. Traumatic anterior dislocation of the hip: spectrum of plain film and CT findings. AJR Am J Roentgenol. 1995;165(5):1215-1219.
5. Pringle JH. Traumatic dislocation at the hip joint. An experimental study in the cadaver. Glasgow Med J. 1943;21:25-40.
6. Esenkaya I, Görgeç M. Traumatic anterior dislocation of the hip associated with ipsilateral femoral neck fracture: a case report. Acta Orthop Traumatol Turc. 2002;36(4):366-368.
7. Sadler AH, DiStefano M. Anterior dislocation of the hip with ipsilateral basicervical fracture. A case report. J Bone Joint Surg Am. 1985;67(2):326-329.
8. DeLee JC, Evans JA, Thomas J. Anterior dislocation of the hip and associated femoral-head fractures. J Bone Joint Surg Am. 1980;62(6):960-964.
9. Epstein HC, Harvey JP Jr. Traumatic anterior dislocations of the hip: management and results. An analysis of fifty-five cases. J Bone Joint Surg Am. 1972;54(7):1561-1562.
10. Toms AD, Williams S, White SH. Obturator dislocation of the hip. J Bone Joint Surg Br. 2001;83(1):113-115.
11. Polesky RE, Polesky FA. Intrapelvic dislocation of the femoral head following anterior dislocation of the hip. A case report. J Bone Joint Surg Am. 1972;54(5):1097-1098.
Anterior hip dislocations have been reported to account for approximately 5% to 10% of all hip dislocations.1 Epstein and Wiss2 originally divided anterior hip dislocations into superior (type I, including pubic or subspinous) and inferior (type II, including obturator and perineal) dislocations. This classification was further subdivided based on the presence of either no associated fracture (type A), fracture of the femoral head or neck (FNF; type B), or fracture of the acetabulum (type C).3 Of all anterior hip dislocations, it has been reported that the inferior or obturator type of dislocation is more common, constituting approximately 70% of all anterior dislocations.4 In 1943, Pringle5 described the mechanism of obturator dislocation as simultaneous abduction, flexion, and external rotation of the hip. Our literature search found only 2 case reports in non-English-language journals of a complete FNF associated with an attempted reduction of an anterior hip dislocation.6,7 Indentation fractures of the femoral head have been more commonly reported than FNFs, with a reported incidence of 35% to 55% after anterior dislocation.4,8 DeLee and colleagues8 also found that those patients with indentation fractures were at a higher risk for developing avascular necrosis of the femoral head in addition to being more likely to report poor or fair function of the hip 2 years after reduction.
There have been a number of different reduction maneuvers for anterior dislocation of hips published in the literature. Epstein and Harvey9 advocated reduction by traction in the line of the femur with the hip flexed and in gentle internal rotation and abduction while the patient was under general anesthesia. Toms and Williams,10 however, recommended adduction with gradual release of the longitudinal traction. Polesky and Polesky11 described a reduction method involving sharp internal rotation, which was found to be associated with FNF. The patient provided written informed consent for print and electronic publication of this case report, and approval was obtained from the Emory University Institutional Review Board.
Case Report
The patient was a 73-year-old woman, an independent ambulator with minimal antecedent hip pain, who, as a pedestrian, was struck by a heavy-duty pickup truck at low velocity. She was flown to our level I trauma center from an outside hospital. The patient arrived hemodynamically stable, with a Glasgow Coma Scale score of 15 and with major complaints of right shoulder and right hip pain. She had a positive Focused Assessment with Sonography for Trauma (FAST), and underwent a subsequent urgent chest, abdomen, and pelvis computed tomography (CT) scan for further investigation. CT showed a grade 1 liver laceration. Her anteroposterior (AP) pelvic radiograph and pelvic CT scan showed an anterior hip dislocation with the femoral head located adjacent to the obturator foramen (Figures 1, 2). The AP pelvic radiograph and pelvic CT scan were scrutinized extensively before reduction to rule out a possible FNF. Comparing the right and left femoral necks through multiple axial CT images showed no obvious differences between the 2 sides (Figures 3, 4). Her only other orthopedic injury was an inferior shoulder dislocation. It is not routine for the general surgery trauma team to obtain a pelvic CT scan prior to involvement of the orthopedic service and prompt reduction of a hip dislocation. Upon initial examination of her right hip, it was fixed in slight flexion and external rotation; she was neurovascularly intact.
After being cleared by the trauma service, the patient provided informed consent for closed reduction of the hip and shoulder under conscious sedation, performed by the emergency department (ED) staff. She received intravenous fentanyl and midazolam, and the reduction was attempted. The reduction maneuver was performed with gentle inline traction, adduction, and internal rotation and extension. There was an audible clunk, and the hip was thought to be reduced and stable. The right leg lower extremity was placed into a knee immobilizer and she remained neurovascularly intact. The shoulder was reduced. After the procedure, the patient had an episode of hypoxia requiring oxygenation via a bag valve mask by the ED staff. Postreduction radiographs confirmed reduction of the right shoulder; however, they also showed a FNF with the femoral head retained near the obturator foramen (Figures 5, 6). The patient and her family were informed of the fracture, and a total hip arthroplasty (THA) was recommended, given her pre-injury mild symptomatic osteoarthritis in the hip and her age. The patient was admitted to the intensive care unit for cardiopulmonary monitoring and was found to have a troponin leak on hospital day 1. She was evaluated by the cardiology service; serial electrocardiograms and troponins ruled out acute myocardial infarction. The patient was cleared for surgery on hospital day 4.
On hospital day 5, she underwent a right THA via a Kocher-Langenbeck approach. The patient’s femoral head was found to be anterior and laterally adjacent to her ischial tuberosity with an indentation fracture. The sciatic nerve was identified and found to be intact. A metal-on-polyethylene Stryker Accolade femoral component and Trident acetabular shell were implanted, and a posterior capsular repair was performed (Figure 7).
The patient tolerated the procedure well, and her postoperative course was uneventful. She was discharged to a subacute rehabilitation facility on postoperative day 3. The patient returned for her 2-week postoperative visit ambulating without assistance. At her last follow-up visit, approximately 6 weeks after surgery, she was a functionally independent community ambulator. Phone conversations with her private orthopedist at 6 months confirmed continued ambulation without problems.
Discussion
This case report of a complication that occurred in our institution has resulted in a change in our protocol for treatment of geriatric anterior hip dislocations. Our institution is a level I trauma center, and traumatic hip dislocations are relatively common, occurring usually in young patients with high-energy trauma. Although somewhat controversial, it is generally assumed that the incidence of avascular necrosis of the femoral head after dislocation of the hip is correlated with the time interval from dislocation to reduction of the hip. Therefore, our protocol for hip dislocations of the hip in young trauma patients is urgent reduction in the ED under appropriate analgesia and muscle relaxation.
In this case report, the patient was older than 65 years with radiographic evidence of possible impingement and postsurgical evidence of impingement of the femoral head in the obturator foremen (Figures 1, 2, 8). In addition, the patient was significantly osteopenic radiographically. An attempted reduction in the ED resulted in FNF requiring THA (Figures 5, 6, 9). After discussion of this complication in our institution’s morbidity and mortality conference, we have developed a protocol for the geriatric patient (older than 65 years) with a traumatic hip dislocation. These patients will undergo attempted reduction under controlled analgesia and muscle relaxation in the operating room (OR) with an attending surgeon present, ideally, an attending surgeon comfortable with arthroplasty in a terminally cleaned OR room. Our institution’s surgical site infection rate after total joint arthroplasty has significantly decreased with improved patient selection and the use of terminally cleaned OR rooms. Because our policy is to perform closed reduction of dislocated hips in an urgent manner, if there is not a terminally clean room or an arthroplasty-trained attending orthopedic surgeon available, then informed consent with discussion of the possibility of fracture requiring a subsequent arthroplasty should be obtained from the patient before the attempted reduction.
After review of the available literature, we believe that this case highlights some of the important treatment principles when treating anterior hip dislocations in the ED. The relatively high incidence of indentation fractures of the femoral head with obturator dislocations puts these fractures at higher risk for possible impingement around the obturator ring. This impingement, coupled with preexisting osteopenia, can predispose these dislocations to FNF, if appropriate analgesia and sedation are not obtained and gentle reduction is not performed. In addition, while it may not be time- or cost-effective to perform closed reduction on every hip dislocation, we bring geriatric patients with radiographic osteopenia to the OR for more controlled reductions. In the informed consent discussion, the possibility of FNF is mentioned, and the patient and family are told that an elective total hip replacement will be performed if this complication occurs.
We consider the following to be risk factors for closed reductions of anterior hip dislocations: (1) preexisting osteopenia on plain films, (2) age greater than 65 years, and (3) radiographic femoral head impingement on the surrounding bony pelvis. We continue to consider closed reduction of both anterior and posterior hip dislocations as urgent (within 6 hours from time of dislocation). This case adds to the existing literature on the risk of FNF with closed reduction of obturator hip dislocations, and we hope that it will encourage further study into the safest and most cost-effective reduction protocol.
Anterior hip dislocations have been reported to account for approximately 5% to 10% of all hip dislocations.1 Epstein and Wiss2 originally divided anterior hip dislocations into superior (type I, including pubic or subspinous) and inferior (type II, including obturator and perineal) dislocations. This classification was further subdivided based on the presence of either no associated fracture (type A), fracture of the femoral head or neck (FNF; type B), or fracture of the acetabulum (type C).3 Of all anterior hip dislocations, it has been reported that the inferior or obturator type of dislocation is more common, constituting approximately 70% of all anterior dislocations.4 In 1943, Pringle5 described the mechanism of obturator dislocation as simultaneous abduction, flexion, and external rotation of the hip. Our literature search found only 2 case reports in non-English-language journals of a complete FNF associated with an attempted reduction of an anterior hip dislocation.6,7 Indentation fractures of the femoral head have been more commonly reported than FNFs, with a reported incidence of 35% to 55% after anterior dislocation.4,8 DeLee and colleagues8 also found that those patients with indentation fractures were at a higher risk for developing avascular necrosis of the femoral head in addition to being more likely to report poor or fair function of the hip 2 years after reduction.
There have been a number of different reduction maneuvers for anterior dislocation of hips published in the literature. Epstein and Harvey9 advocated reduction by traction in the line of the femur with the hip flexed and in gentle internal rotation and abduction while the patient was under general anesthesia. Toms and Williams,10 however, recommended adduction with gradual release of the longitudinal traction. Polesky and Polesky11 described a reduction method involving sharp internal rotation, which was found to be associated with FNF. The patient provided written informed consent for print and electronic publication of this case report, and approval was obtained from the Emory University Institutional Review Board.
Case Report
The patient was a 73-year-old woman, an independent ambulator with minimal antecedent hip pain, who, as a pedestrian, was struck by a heavy-duty pickup truck at low velocity. She was flown to our level I trauma center from an outside hospital. The patient arrived hemodynamically stable, with a Glasgow Coma Scale score of 15 and with major complaints of right shoulder and right hip pain. She had a positive Focused Assessment with Sonography for Trauma (FAST), and underwent a subsequent urgent chest, abdomen, and pelvis computed tomography (CT) scan for further investigation. CT showed a grade 1 liver laceration. Her anteroposterior (AP) pelvic radiograph and pelvic CT scan showed an anterior hip dislocation with the femoral head located adjacent to the obturator foramen (Figures 1, 2). The AP pelvic radiograph and pelvic CT scan were scrutinized extensively before reduction to rule out a possible FNF. Comparing the right and left femoral necks through multiple axial CT images showed no obvious differences between the 2 sides (Figures 3, 4). Her only other orthopedic injury was an inferior shoulder dislocation. It is not routine for the general surgery trauma team to obtain a pelvic CT scan prior to involvement of the orthopedic service and prompt reduction of a hip dislocation. Upon initial examination of her right hip, it was fixed in slight flexion and external rotation; she was neurovascularly intact.
After being cleared by the trauma service, the patient provided informed consent for closed reduction of the hip and shoulder under conscious sedation, performed by the emergency department (ED) staff. She received intravenous fentanyl and midazolam, and the reduction was attempted. The reduction maneuver was performed with gentle inline traction, adduction, and internal rotation and extension. There was an audible clunk, and the hip was thought to be reduced and stable. The right leg lower extremity was placed into a knee immobilizer and she remained neurovascularly intact. The shoulder was reduced. After the procedure, the patient had an episode of hypoxia requiring oxygenation via a bag valve mask by the ED staff. Postreduction radiographs confirmed reduction of the right shoulder; however, they also showed a FNF with the femoral head retained near the obturator foramen (Figures 5, 6). The patient and her family were informed of the fracture, and a total hip arthroplasty (THA) was recommended, given her pre-injury mild symptomatic osteoarthritis in the hip and her age. The patient was admitted to the intensive care unit for cardiopulmonary monitoring and was found to have a troponin leak on hospital day 1. She was evaluated by the cardiology service; serial electrocardiograms and troponins ruled out acute myocardial infarction. The patient was cleared for surgery on hospital day 4.
On hospital day 5, she underwent a right THA via a Kocher-Langenbeck approach. The patient’s femoral head was found to be anterior and laterally adjacent to her ischial tuberosity with an indentation fracture. The sciatic nerve was identified and found to be intact. A metal-on-polyethylene Stryker Accolade femoral component and Trident acetabular shell were implanted, and a posterior capsular repair was performed (Figure 7).
The patient tolerated the procedure well, and her postoperative course was uneventful. She was discharged to a subacute rehabilitation facility on postoperative day 3. The patient returned for her 2-week postoperative visit ambulating without assistance. At her last follow-up visit, approximately 6 weeks after surgery, she was a functionally independent community ambulator. Phone conversations with her private orthopedist at 6 months confirmed continued ambulation without problems.
Discussion
This case report of a complication that occurred in our institution has resulted in a change in our protocol for treatment of geriatric anterior hip dislocations. Our institution is a level I trauma center, and traumatic hip dislocations are relatively common, occurring usually in young patients with high-energy trauma. Although somewhat controversial, it is generally assumed that the incidence of avascular necrosis of the femoral head after dislocation of the hip is correlated with the time interval from dislocation to reduction of the hip. Therefore, our protocol for hip dislocations of the hip in young trauma patients is urgent reduction in the ED under appropriate analgesia and muscle relaxation.
In this case report, the patient was older than 65 years with radiographic evidence of possible impingement and postsurgical evidence of impingement of the femoral head in the obturator foremen (Figures 1, 2, 8). In addition, the patient was significantly osteopenic radiographically. An attempted reduction in the ED resulted in FNF requiring THA (Figures 5, 6, 9). After discussion of this complication in our institution’s morbidity and mortality conference, we have developed a protocol for the geriatric patient (older than 65 years) with a traumatic hip dislocation. These patients will undergo attempted reduction under controlled analgesia and muscle relaxation in the operating room (OR) with an attending surgeon present, ideally, an attending surgeon comfortable with arthroplasty in a terminally cleaned OR room. Our institution’s surgical site infection rate after total joint arthroplasty has significantly decreased with improved patient selection and the use of terminally cleaned OR rooms. Because our policy is to perform closed reduction of dislocated hips in an urgent manner, if there is not a terminally clean room or an arthroplasty-trained attending orthopedic surgeon available, then informed consent with discussion of the possibility of fracture requiring a subsequent arthroplasty should be obtained from the patient before the attempted reduction.
After review of the available literature, we believe that this case highlights some of the important treatment principles when treating anterior hip dislocations in the ED. The relatively high incidence of indentation fractures of the femoral head with obturator dislocations puts these fractures at higher risk for possible impingement around the obturator ring. This impingement, coupled with preexisting osteopenia, can predispose these dislocations to FNF, if appropriate analgesia and sedation are not obtained and gentle reduction is not performed. In addition, while it may not be time- or cost-effective to perform closed reduction on every hip dislocation, we bring geriatric patients with radiographic osteopenia to the OR for more controlled reductions. In the informed consent discussion, the possibility of FNF is mentioned, and the patient and family are told that an elective total hip replacement will be performed if this complication occurs.
We consider the following to be risk factors for closed reductions of anterior hip dislocations: (1) preexisting osteopenia on plain films, (2) age greater than 65 years, and (3) radiographic femoral head impingement on the surrounding bony pelvis. We continue to consider closed reduction of both anterior and posterior hip dislocations as urgent (within 6 hours from time of dislocation). This case adds to the existing literature on the risk of FNF with closed reduction of obturator hip dislocations, and we hope that it will encourage further study into the safest and most cost-effective reduction protocol.
1. Amihood, S. Anterior dislocation of the hip. Injury. 1975;7(2):107-110.
2. Epstein HC, Wiss DA. Traumatic anterior dislocation of the hip. Orthopedics. 1985;8(1):130, 132-134.
3. Epstein HC. Traumatic dislocations of the hip. Clin Orthop Relat Res. 1973(92):116-142.
4. Erb RE, Steele JR, Nance EP Jr, Edwards JR. Traumatic anterior dislocation of the hip: spectrum of plain film and CT findings. AJR Am J Roentgenol. 1995;165(5):1215-1219.
5. Pringle JH. Traumatic dislocation at the hip joint. An experimental study in the cadaver. Glasgow Med J. 1943;21:25-40.
6. Esenkaya I, Görgeç M. Traumatic anterior dislocation of the hip associated with ipsilateral femoral neck fracture: a case report. Acta Orthop Traumatol Turc. 2002;36(4):366-368.
7. Sadler AH, DiStefano M. Anterior dislocation of the hip with ipsilateral basicervical fracture. A case report. J Bone Joint Surg Am. 1985;67(2):326-329.
8. DeLee JC, Evans JA, Thomas J. Anterior dislocation of the hip and associated femoral-head fractures. J Bone Joint Surg Am. 1980;62(6):960-964.
9. Epstein HC, Harvey JP Jr. Traumatic anterior dislocations of the hip: management and results. An analysis of fifty-five cases. J Bone Joint Surg Am. 1972;54(7):1561-1562.
10. Toms AD, Williams S, White SH. Obturator dislocation of the hip. J Bone Joint Surg Br. 2001;83(1):113-115.
11. Polesky RE, Polesky FA. Intrapelvic dislocation of the femoral head following anterior dislocation of the hip. A case report. J Bone Joint Surg Am. 1972;54(5):1097-1098.
1. Amihood, S. Anterior dislocation of the hip. Injury. 1975;7(2):107-110.
2. Epstein HC, Wiss DA. Traumatic anterior dislocation of the hip. Orthopedics. 1985;8(1):130, 132-134.
3. Epstein HC. Traumatic dislocations of the hip. Clin Orthop Relat Res. 1973(92):116-142.
4. Erb RE, Steele JR, Nance EP Jr, Edwards JR. Traumatic anterior dislocation of the hip: spectrum of plain film and CT findings. AJR Am J Roentgenol. 1995;165(5):1215-1219.
5. Pringle JH. Traumatic dislocation at the hip joint. An experimental study in the cadaver. Glasgow Med J. 1943;21:25-40.
6. Esenkaya I, Görgeç M. Traumatic anterior dislocation of the hip associated with ipsilateral femoral neck fracture: a case report. Acta Orthop Traumatol Turc. 2002;36(4):366-368.
7. Sadler AH, DiStefano M. Anterior dislocation of the hip with ipsilateral basicervical fracture. A case report. J Bone Joint Surg Am. 1985;67(2):326-329.
8. DeLee JC, Evans JA, Thomas J. Anterior dislocation of the hip and associated femoral-head fractures. J Bone Joint Surg Am. 1980;62(6):960-964.
9. Epstein HC, Harvey JP Jr. Traumatic anterior dislocations of the hip: management and results. An analysis of fifty-five cases. J Bone Joint Surg Am. 1972;54(7):1561-1562.
10. Toms AD, Williams S, White SH. Obturator dislocation of the hip. J Bone Joint Surg Br. 2001;83(1):113-115.
11. Polesky RE, Polesky FA. Intrapelvic dislocation of the femoral head following anterior dislocation of the hip. A case report. J Bone Joint Surg Am. 1972;54(5):1097-1098.