User login
Case Studies in Toxicology: Double Take—Is Re-exposure Necessary to Explain Delayed Recurrent Opioid Toxicity?
Case
A previously healthy 10-month-old girl was brought to the ED by her mother, who noted that the child had been excessively drowsy throughout the day. She reported that her husband had dropped an unknown amount of his morphine sulfate extended-release 60-mg tablets and oxycodone 10-mg/acetaminophen 325-mg tablets on the floor 5 days earlier. Although unsure of how many tablets he had dropped, the father believed he had located all of them. The mother, however, found some of the tablets around the crib in their daughter’s room.
When the child arrived to the ED, her vital signs were: blood pressure, 95/60 mm Hg; heart rate, 102 beats/minute; respiratory rate (RR), 18 breaths/minute; and temperature, 98.4°F. Oxygen saturation was 98% on room air. On physical examination, the child was lethargic, her pupils were less than 1 mm in diameter, and her bowel sounds were absent. After the administration of intravenous (IV) naloxone 0.4 mg, the patient became less drowsy and her RR normalized. Approximately 1 hour later, though, the child again became lethargic; she was given a repeat dose of IV naloxone 0.4 mg, and a naloxone infusion was initiated at 0.3 mg/h. Over approximately 20 hours, the infusion was tapered and discontinued. Three hours after the infusion was stopped, the child’s vital signs and behavior were both normal. After a social worker and representative from the Administration for Children’s Services reviewed the patient’s case, she was discharged home with her parents.
Less than 1 hour later, however, the mother returned to the ED with the child, who was again unresponsive. Although the girl’s RR was normal, she had pinpoint pupils. After she was given IV naloxone 0.4 mg, the child awoke and remained responsive for 20 minutes before returning to a somnolent state. Another IV dose of naloxone 0.4 mg was administered, which showed partial improvement in responsiveness. A naloxone infusion was then initiated and titrated up to 1 mg/h to maintain wakefulness and ventilation. In the pediatric intensive care unit, the child required titration of the naloxone infusion to 2 mg/h to which she responded well. Over the next 12 hours, the infusion was tapered off and the child was discharged home with her parents.
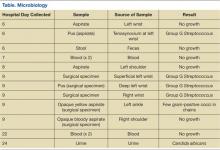
Blood samples from both the initial visit and the return visit were sent for toxicologic analysis by gas chromatography-mass spectrometry (GC-MS). Serum from the first visit contained morphine at a concentration of 3,000 ng/mL; serum from the second visit contained morphine at 420 ng/mL. Both samples were negative for oxycodone or any of the other substances checked on the extended GC-MS screen.
What is the toxicologic differential?
Although this patient’s extreme somnolence was suspected to be opioid-induced, and was confirmed by an appropriate response to naloxone, children may present to the ED somnolent for a variety of unknown reasons. Even with a fairly clear history, the clinician should also consider metabolic, neurological, infectious, traumatic, and psychiatric causes of altered mental status.1 The toxicologic causes of altered mental status are expansive and include the effects of many medications used therapeutically or in overdose. Opioids, benzodiazepines, barbiturates, α-2 agonists (eg, clonidine), sleep aids (eg, zolpidem, diphenhydramine), and ethanol are common causes of induced an altered mental status. When taking a toxicologic history, it is important to inquire not only about the patient’s medications but also the medications of other members of the household to which the patient may have access. This includes not only prescription medications but also over-the-counter, complementary, and herbal preparations.
Why did this child have delayed recurrent opioid toxicity?
When used as directed, opioids cause analgesia and euphoria. Analgesia is mediated by agonism at the μ- , κ-, and δ-opioid receptors throughout the brain and spinal cord. The majority of morphine’s analgesic activity comes from activation of the μ-opioid receptors.2 In overdose, opioids classically cause a toxidrome characterized by miosis, coma, decreased bowel sounds, and respiratory depression. These signs can give clues to a patient’s exposure.
Supportive care is the cornerstone of treatment for patients with opioid toxicity, and maintaining the airway and monitoring the respiratory status are extremely important. When ventilation decreases due to the actions of opioids (typically denoted by a RR of <12 breaths/minute in adults, but may be marked by a reduction in depth of breathing as well), the use of an opioid antagonist is appropriate.4 The most commonly used antagonist is naloxone, an antidote with antagonism at all opioid receptor subtypes.5
In patients who are not dependent on opioids, IV naloxone 0.4 mg is an appropriate initial dose—regardless of patient size or specifics of the exposure. Patients with opioid dependency (eg, patients taking opioids for chronic pain or palliative care, or in those with suspected or confirmed opioid abuse), should receive smaller initial doses of naloxone (eg, 0.04 mg); the dose should be titrated up to effect to avoid precipitating acute opioid withdrawal. The goal of opioid antagonism is to allow the patient to breathe spontaneously and at an appropriate rate and depth without precipitating withdrawal. The duration of action of naloxone is 20 to 90 minutes in adults.
Patients presenting with heroin overdose should be monitored for at least 2 hours after naloxone administration (some suggest 3 hours) to determine whether or not additional dosing will be necessary. After oral opioid exposures, particularly with extended-release or long-acting formulations, longer periods of observation are required (this is unrelated to the naloxone pharmacokinetics, but rather to the slow rise in blood levels from some of these formulations). If repeated opioid toxicity occurs in adults, a naloxone infusion may be helpful to reduce the need for repetitive re-dosing. Initially, an hourly infusion equal to two-thirds of the dose of naloxone that reversed the patient’s respiratory depression is suggested6
Naloxone is eliminated by conjugation with glucuronic acid before is it excreted from the body. Due to decreased hepatic conjugation and prolonged metabolization of drugs in pediatric patients, naloxone may have a longer half-life in children—especially neonates and infants7; in children, the half-life of naloxone may extend up to three times that of adults.8 This extended half-life can lead to a false sense of assurance that a child is free of opioid effects 120 minutes after receiving naloxone—the time by which an adult patient would likely be without significant systemic effects of naloxone—when in fact the effect of naloxone has not yet sufficiently waned. This in turn may prompt discharge before sufficient time has passed to exclude recrudescence of opioid toxicity: The presence of persistent opioid agonist concentrations in the blood, even at consequential amounts, remains masked by the persistent presence of naloxone.
The goal of opioid antagonism is to allow the patient to breathe spontaneously and at an appropriate rate and depth without precipitating withdrawal. In this patient, it is not surprising that the the ingestion of an extended-relief form of morphine should produce a prolonged opioid effect. At therapeutic concentrations in children (~10 ng/mL), the half-life of morphine is slightly longer than in adults (~3 hours vs 2 hours) and is likely even longer with very high serum concentrations. It is metabolized to morphine 6-glucuronide, which is active and longer lasting than the parent compound. This may account for additional clinical effects beyond the time that the serum morphine concentration falls, and is particularly relevant following immediate-release morphine overdose.
In this case it is also important to consider whether or not the patient was re-exposed to an opioid between the first and second ED visit. The dramatically elevated initial serum morphine concentrations and the relatively appropriate fall in magnitude of the second sample suggest that the recurrence of respiratory depression was not the result of re-exposure. The patient’s recurrent effects, even a day out from exposure, can be explained by the immediate-release morphine exposure and the discharge prior to waning of the naloxone. In children with opioid toxicity, another potential option, though not directly studied, is to administer the long-acting opioid antagonist naltrexone to the patient prior to discharge.
Case Conclusion
When used appropriately and under the correct circumstances, naloxone is safe and effective for the reversal of opioid toxicity. As with any antidote, patients must be appropriately monitored for any adverse effects or recurrence of toxicity. Moreover, the clinician should be mindful of the pharmacokinetic differences between adults and young children and the possibility of a later-than-expected recurrence of opioid toxicity in pediatric patients.
This case is a reminder of the importance of safe medication storage. Infants and young children who are crawling and exploring their environment are especially vulnerable to toxicity from medications found on the floor. Regardless of age, quick recognition of opioid-induced respiratory depression and appropriate use of naloxone can help to decrease the morbidity associated with excessive opioid exposures in all patients.
Dr Berman is a senior medical toxicology fellow at North Shore-Long Island Jewish Medical Center, New York. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board. Dr Majlesi is the director of medical toxicology at Staten Island University Hospital, New York.
- Lehman RK, Mink J. Altered mental status. Clin Pediatr Emerg Med. 2008;9:68-75.
- Chang SH, Maney KM, Phillips JP, Langford RM, Mehta V. A comparison of the respiratory effects of oxycodone versus morphine: a randomised, double-blind, placebo-controlled investigation. Anaesthesia. 2010;65(10):1007-1012.
- Holstege CP, Borek HA. Toxidromes. Crit Care Clin. 2012;28(4):479-498.
- Hoffman JR, Schriger DL, Luo JS. The empiric use of naloxone in patients with altered mental status: a reappraisal. Ann Emerg Men. 1991;20(3):246-252.
- Howland MA, Nelson LS. Chapter A6. Opioid antagonists. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:579-585.
- Goldfrank L, Weisman RS, Errick JK, Lo MW. A dosing nomogram for continuous infusion intravenous naloxone. Ann Emerg Med. 1986;15(5):566-570.
- Moreland TA, Brice JE, Walker CH, Parija AC. Naloxone pharmacokinetics in the newborn. Br J Clin Pharmacol. 1980;9(6):609-612.
- Ngai SH, Berkowitz BA, Yang JC, et al. Pharmacokinetics of naloxone in rats and in man: basis for its potency and short duration of action. Anesthesiology. 1976;44(5):398-401.
Case
A previously healthy 10-month-old girl was brought to the ED by her mother, who noted that the child had been excessively drowsy throughout the day. She reported that her husband had dropped an unknown amount of his morphine sulfate extended-release 60-mg tablets and oxycodone 10-mg/acetaminophen 325-mg tablets on the floor 5 days earlier. Although unsure of how many tablets he had dropped, the father believed he had located all of them. The mother, however, found some of the tablets around the crib in their daughter’s room.
When the child arrived to the ED, her vital signs were: blood pressure, 95/60 mm Hg; heart rate, 102 beats/minute; respiratory rate (RR), 18 breaths/minute; and temperature, 98.4°F. Oxygen saturation was 98% on room air. On physical examination, the child was lethargic, her pupils were less than 1 mm in diameter, and her bowel sounds were absent. After the administration of intravenous (IV) naloxone 0.4 mg, the patient became less drowsy and her RR normalized. Approximately 1 hour later, though, the child again became lethargic; she was given a repeat dose of IV naloxone 0.4 mg, and a naloxone infusion was initiated at 0.3 mg/h. Over approximately 20 hours, the infusion was tapered and discontinued. Three hours after the infusion was stopped, the child’s vital signs and behavior were both normal. After a social worker and representative from the Administration for Children’s Services reviewed the patient’s case, she was discharged home with her parents.
Less than 1 hour later, however, the mother returned to the ED with the child, who was again unresponsive. Although the girl’s RR was normal, she had pinpoint pupils. After she was given IV naloxone 0.4 mg, the child awoke and remained responsive for 20 minutes before returning to a somnolent state. Another IV dose of naloxone 0.4 mg was administered, which showed partial improvement in responsiveness. A naloxone infusion was then initiated and titrated up to 1 mg/h to maintain wakefulness and ventilation. In the pediatric intensive care unit, the child required titration of the naloxone infusion to 2 mg/h to which she responded well. Over the next 12 hours, the infusion was tapered off and the child was discharged home with her parents.
Blood samples from both the initial visit and the return visit were sent for toxicologic analysis by gas chromatography-mass spectrometry (GC-MS). Serum from the first visit contained morphine at a concentration of 3,000 ng/mL; serum from the second visit contained morphine at 420 ng/mL. Both samples were negative for oxycodone or any of the other substances checked on the extended GC-MS screen.
What is the toxicologic differential?
Although this patient’s extreme somnolence was suspected to be opioid-induced, and was confirmed by an appropriate response to naloxone, children may present to the ED somnolent for a variety of unknown reasons. Even with a fairly clear history, the clinician should also consider metabolic, neurological, infectious, traumatic, and psychiatric causes of altered mental status.1 The toxicologic causes of altered mental status are expansive and include the effects of many medications used therapeutically or in overdose. Opioids, benzodiazepines, barbiturates, α-2 agonists (eg, clonidine), sleep aids (eg, zolpidem, diphenhydramine), and ethanol are common causes of induced an altered mental status. When taking a toxicologic history, it is important to inquire not only about the patient’s medications but also the medications of other members of the household to which the patient may have access. This includes not only prescription medications but also over-the-counter, complementary, and herbal preparations.
Why did this child have delayed recurrent opioid toxicity?
When used as directed, opioids cause analgesia and euphoria. Analgesia is mediated by agonism at the μ- , κ-, and δ-opioid receptors throughout the brain and spinal cord. The majority of morphine’s analgesic activity comes from activation of the μ-opioid receptors.2 In overdose, opioids classically cause a toxidrome characterized by miosis, coma, decreased bowel sounds, and respiratory depression. These signs can give clues to a patient’s exposure.
Supportive care is the cornerstone of treatment for patients with opioid toxicity, and maintaining the airway and monitoring the respiratory status are extremely important. When ventilation decreases due to the actions of opioids (typically denoted by a RR of <12 breaths/minute in adults, but may be marked by a reduction in depth of breathing as well), the use of an opioid antagonist is appropriate.4 The most commonly used antagonist is naloxone, an antidote with antagonism at all opioid receptor subtypes.5
In patients who are not dependent on opioids, IV naloxone 0.4 mg is an appropriate initial dose—regardless of patient size or specifics of the exposure. Patients with opioid dependency (eg, patients taking opioids for chronic pain or palliative care, or in those with suspected or confirmed opioid abuse), should receive smaller initial doses of naloxone (eg, 0.04 mg); the dose should be titrated up to effect to avoid precipitating acute opioid withdrawal. The goal of opioid antagonism is to allow the patient to breathe spontaneously and at an appropriate rate and depth without precipitating withdrawal. The duration of action of naloxone is 20 to 90 minutes in adults.
Patients presenting with heroin overdose should be monitored for at least 2 hours after naloxone administration (some suggest 3 hours) to determine whether or not additional dosing will be necessary. After oral opioid exposures, particularly with extended-release or long-acting formulations, longer periods of observation are required (this is unrelated to the naloxone pharmacokinetics, but rather to the slow rise in blood levels from some of these formulations). If repeated opioid toxicity occurs in adults, a naloxone infusion may be helpful to reduce the need for repetitive re-dosing. Initially, an hourly infusion equal to two-thirds of the dose of naloxone that reversed the patient’s respiratory depression is suggested6
Naloxone is eliminated by conjugation with glucuronic acid before is it excreted from the body. Due to decreased hepatic conjugation and prolonged metabolization of drugs in pediatric patients, naloxone may have a longer half-life in children—especially neonates and infants7; in children, the half-life of naloxone may extend up to three times that of adults.8 This extended half-life can lead to a false sense of assurance that a child is free of opioid effects 120 minutes after receiving naloxone—the time by which an adult patient would likely be without significant systemic effects of naloxone—when in fact the effect of naloxone has not yet sufficiently waned. This in turn may prompt discharge before sufficient time has passed to exclude recrudescence of opioid toxicity: The presence of persistent opioid agonist concentrations in the blood, even at consequential amounts, remains masked by the persistent presence of naloxone.
The goal of opioid antagonism is to allow the patient to breathe spontaneously and at an appropriate rate and depth without precipitating withdrawal. In this patient, it is not surprising that the the ingestion of an extended-relief form of morphine should produce a prolonged opioid effect. At therapeutic concentrations in children (~10 ng/mL), the half-life of morphine is slightly longer than in adults (~3 hours vs 2 hours) and is likely even longer with very high serum concentrations. It is metabolized to morphine 6-glucuronide, which is active and longer lasting than the parent compound. This may account for additional clinical effects beyond the time that the serum morphine concentration falls, and is particularly relevant following immediate-release morphine overdose.
In this case it is also important to consider whether or not the patient was re-exposed to an opioid between the first and second ED visit. The dramatically elevated initial serum morphine concentrations and the relatively appropriate fall in magnitude of the second sample suggest that the recurrence of respiratory depression was not the result of re-exposure. The patient’s recurrent effects, even a day out from exposure, can be explained by the immediate-release morphine exposure and the discharge prior to waning of the naloxone. In children with opioid toxicity, another potential option, though not directly studied, is to administer the long-acting opioid antagonist naltrexone to the patient prior to discharge.
Case Conclusion
When used appropriately and under the correct circumstances, naloxone is safe and effective for the reversal of opioid toxicity. As with any antidote, patients must be appropriately monitored for any adverse effects or recurrence of toxicity. Moreover, the clinician should be mindful of the pharmacokinetic differences between adults and young children and the possibility of a later-than-expected recurrence of opioid toxicity in pediatric patients.
This case is a reminder of the importance of safe medication storage. Infants and young children who are crawling and exploring their environment are especially vulnerable to toxicity from medications found on the floor. Regardless of age, quick recognition of opioid-induced respiratory depression and appropriate use of naloxone can help to decrease the morbidity associated with excessive opioid exposures in all patients.
Dr Berman is a senior medical toxicology fellow at North Shore-Long Island Jewish Medical Center, New York. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board. Dr Majlesi is the director of medical toxicology at Staten Island University Hospital, New York.
Case
A previously healthy 10-month-old girl was brought to the ED by her mother, who noted that the child had been excessively drowsy throughout the day. She reported that her husband had dropped an unknown amount of his morphine sulfate extended-release 60-mg tablets and oxycodone 10-mg/acetaminophen 325-mg tablets on the floor 5 days earlier. Although unsure of how many tablets he had dropped, the father believed he had located all of them. The mother, however, found some of the tablets around the crib in their daughter’s room.
When the child arrived to the ED, her vital signs were: blood pressure, 95/60 mm Hg; heart rate, 102 beats/minute; respiratory rate (RR), 18 breaths/minute; and temperature, 98.4°F. Oxygen saturation was 98% on room air. On physical examination, the child was lethargic, her pupils were less than 1 mm in diameter, and her bowel sounds were absent. After the administration of intravenous (IV) naloxone 0.4 mg, the patient became less drowsy and her RR normalized. Approximately 1 hour later, though, the child again became lethargic; she was given a repeat dose of IV naloxone 0.4 mg, and a naloxone infusion was initiated at 0.3 mg/h. Over approximately 20 hours, the infusion was tapered and discontinued. Three hours after the infusion was stopped, the child’s vital signs and behavior were both normal. After a social worker and representative from the Administration for Children’s Services reviewed the patient’s case, she was discharged home with her parents.
Less than 1 hour later, however, the mother returned to the ED with the child, who was again unresponsive. Although the girl’s RR was normal, she had pinpoint pupils. After she was given IV naloxone 0.4 mg, the child awoke and remained responsive for 20 minutes before returning to a somnolent state. Another IV dose of naloxone 0.4 mg was administered, which showed partial improvement in responsiveness. A naloxone infusion was then initiated and titrated up to 1 mg/h to maintain wakefulness and ventilation. In the pediatric intensive care unit, the child required titration of the naloxone infusion to 2 mg/h to which she responded well. Over the next 12 hours, the infusion was tapered off and the child was discharged home with her parents.
Blood samples from both the initial visit and the return visit were sent for toxicologic analysis by gas chromatography-mass spectrometry (GC-MS). Serum from the first visit contained morphine at a concentration of 3,000 ng/mL; serum from the second visit contained morphine at 420 ng/mL. Both samples were negative for oxycodone or any of the other substances checked on the extended GC-MS screen.
What is the toxicologic differential?
Although this patient’s extreme somnolence was suspected to be opioid-induced, and was confirmed by an appropriate response to naloxone, children may present to the ED somnolent for a variety of unknown reasons. Even with a fairly clear history, the clinician should also consider metabolic, neurological, infectious, traumatic, and psychiatric causes of altered mental status.1 The toxicologic causes of altered mental status are expansive and include the effects of many medications used therapeutically or in overdose. Opioids, benzodiazepines, barbiturates, α-2 agonists (eg, clonidine), sleep aids (eg, zolpidem, diphenhydramine), and ethanol are common causes of induced an altered mental status. When taking a toxicologic history, it is important to inquire not only about the patient’s medications but also the medications of other members of the household to which the patient may have access. This includes not only prescription medications but also over-the-counter, complementary, and herbal preparations.
Why did this child have delayed recurrent opioid toxicity?
When used as directed, opioids cause analgesia and euphoria. Analgesia is mediated by agonism at the μ- , κ-, and δ-opioid receptors throughout the brain and spinal cord. The majority of morphine’s analgesic activity comes from activation of the μ-opioid receptors.2 In overdose, opioids classically cause a toxidrome characterized by miosis, coma, decreased bowel sounds, and respiratory depression. These signs can give clues to a patient’s exposure.
Supportive care is the cornerstone of treatment for patients with opioid toxicity, and maintaining the airway and monitoring the respiratory status are extremely important. When ventilation decreases due to the actions of opioids (typically denoted by a RR of <12 breaths/minute in adults, but may be marked by a reduction in depth of breathing as well), the use of an opioid antagonist is appropriate.4 The most commonly used antagonist is naloxone, an antidote with antagonism at all opioid receptor subtypes.5
In patients who are not dependent on opioids, IV naloxone 0.4 mg is an appropriate initial dose—regardless of patient size or specifics of the exposure. Patients with opioid dependency (eg, patients taking opioids for chronic pain or palliative care, or in those with suspected or confirmed opioid abuse), should receive smaller initial doses of naloxone (eg, 0.04 mg); the dose should be titrated up to effect to avoid precipitating acute opioid withdrawal. The goal of opioid antagonism is to allow the patient to breathe spontaneously and at an appropriate rate and depth without precipitating withdrawal. The duration of action of naloxone is 20 to 90 minutes in adults.
Patients presenting with heroin overdose should be monitored for at least 2 hours after naloxone administration (some suggest 3 hours) to determine whether or not additional dosing will be necessary. After oral opioid exposures, particularly with extended-release or long-acting formulations, longer periods of observation are required (this is unrelated to the naloxone pharmacokinetics, but rather to the slow rise in blood levels from some of these formulations). If repeated opioid toxicity occurs in adults, a naloxone infusion may be helpful to reduce the need for repetitive re-dosing. Initially, an hourly infusion equal to two-thirds of the dose of naloxone that reversed the patient’s respiratory depression is suggested6
Naloxone is eliminated by conjugation with glucuronic acid before is it excreted from the body. Due to decreased hepatic conjugation and prolonged metabolization of drugs in pediatric patients, naloxone may have a longer half-life in children—especially neonates and infants7; in children, the half-life of naloxone may extend up to three times that of adults.8 This extended half-life can lead to a false sense of assurance that a child is free of opioid effects 120 minutes after receiving naloxone—the time by which an adult patient would likely be without significant systemic effects of naloxone—when in fact the effect of naloxone has not yet sufficiently waned. This in turn may prompt discharge before sufficient time has passed to exclude recrudescence of opioid toxicity: The presence of persistent opioid agonist concentrations in the blood, even at consequential amounts, remains masked by the persistent presence of naloxone.
The goal of opioid antagonism is to allow the patient to breathe spontaneously and at an appropriate rate and depth without precipitating withdrawal. In this patient, it is not surprising that the the ingestion of an extended-relief form of morphine should produce a prolonged opioid effect. At therapeutic concentrations in children (~10 ng/mL), the half-life of morphine is slightly longer than in adults (~3 hours vs 2 hours) and is likely even longer with very high serum concentrations. It is metabolized to morphine 6-glucuronide, which is active and longer lasting than the parent compound. This may account for additional clinical effects beyond the time that the serum morphine concentration falls, and is particularly relevant following immediate-release morphine overdose.
In this case it is also important to consider whether or not the patient was re-exposed to an opioid between the first and second ED visit. The dramatically elevated initial serum morphine concentrations and the relatively appropriate fall in magnitude of the second sample suggest that the recurrence of respiratory depression was not the result of re-exposure. The patient’s recurrent effects, even a day out from exposure, can be explained by the immediate-release morphine exposure and the discharge prior to waning of the naloxone. In children with opioid toxicity, another potential option, though not directly studied, is to administer the long-acting opioid antagonist naltrexone to the patient prior to discharge.
Case Conclusion
When used appropriately and under the correct circumstances, naloxone is safe and effective for the reversal of opioid toxicity. As with any antidote, patients must be appropriately monitored for any adverse effects or recurrence of toxicity. Moreover, the clinician should be mindful of the pharmacokinetic differences between adults and young children and the possibility of a later-than-expected recurrence of opioid toxicity in pediatric patients.
This case is a reminder of the importance of safe medication storage. Infants and young children who are crawling and exploring their environment are especially vulnerable to toxicity from medications found on the floor. Regardless of age, quick recognition of opioid-induced respiratory depression and appropriate use of naloxone can help to decrease the morbidity associated with excessive opioid exposures in all patients.
Dr Berman is a senior medical toxicology fellow at North Shore-Long Island Jewish Medical Center, New York. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board. Dr Majlesi is the director of medical toxicology at Staten Island University Hospital, New York.
- Lehman RK, Mink J. Altered mental status. Clin Pediatr Emerg Med. 2008;9:68-75.
- Chang SH, Maney KM, Phillips JP, Langford RM, Mehta V. A comparison of the respiratory effects of oxycodone versus morphine: a randomised, double-blind, placebo-controlled investigation. Anaesthesia. 2010;65(10):1007-1012.
- Holstege CP, Borek HA. Toxidromes. Crit Care Clin. 2012;28(4):479-498.
- Hoffman JR, Schriger DL, Luo JS. The empiric use of naloxone in patients with altered mental status: a reappraisal. Ann Emerg Men. 1991;20(3):246-252.
- Howland MA, Nelson LS. Chapter A6. Opioid antagonists. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:579-585.
- Goldfrank L, Weisman RS, Errick JK, Lo MW. A dosing nomogram for continuous infusion intravenous naloxone. Ann Emerg Med. 1986;15(5):566-570.
- Moreland TA, Brice JE, Walker CH, Parija AC. Naloxone pharmacokinetics in the newborn. Br J Clin Pharmacol. 1980;9(6):609-612.
- Ngai SH, Berkowitz BA, Yang JC, et al. Pharmacokinetics of naloxone in rats and in man: basis for its potency and short duration of action. Anesthesiology. 1976;44(5):398-401.
- Lehman RK, Mink J. Altered mental status. Clin Pediatr Emerg Med. 2008;9:68-75.
- Chang SH, Maney KM, Phillips JP, Langford RM, Mehta V. A comparison of the respiratory effects of oxycodone versus morphine: a randomised, double-blind, placebo-controlled investigation. Anaesthesia. 2010;65(10):1007-1012.
- Holstege CP, Borek HA. Toxidromes. Crit Care Clin. 2012;28(4):479-498.
- Hoffman JR, Schriger DL, Luo JS. The empiric use of naloxone in patients with altered mental status: a reappraisal. Ann Emerg Men. 1991;20(3):246-252.
- Howland MA, Nelson LS. Chapter A6. Opioid antagonists. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:579-585.
- Goldfrank L, Weisman RS, Errick JK, Lo MW. A dosing nomogram for continuous infusion intravenous naloxone. Ann Emerg Med. 1986;15(5):566-570.
- Moreland TA, Brice JE, Walker CH, Parija AC. Naloxone pharmacokinetics in the newborn. Br J Clin Pharmacol. 1980;9(6):609-612.
- Ngai SH, Berkowitz BA, Yang JC, et al. Pharmacokinetics of naloxone in rats and in man: basis for its potency and short duration of action. Anesthesiology. 1976;44(5):398-401.
Intragrade Intramedullary Nailing of an Open Tibial Shaft Fracture in a Patient With Concomitant Ipsilateral Total Knee Arthroplasty
Fracture of the tibial shaft below an ipsilateral total knee arthroplasty (TKA) is an infrequently occurring injury pattern that presents a unique treatment scenario. The high predilection for open wounds associated with these diaphyseal fractures further complicates the treatment algorithm.1,2 The standard principles of treatment for open tibial shaft fractures entail open fracture débridement followed by adequate fracture reduction and stable skeletal fixation in a manner that limits adverse complications of this injury, which include nonunion, malunion, infection, soft-tissue compromise, and reoperation.3,4
Antegrade intramedullary (IM) tibial nailing has become standard treatment for tibial shaft fractures.5-7 This minimally invasive method of fixation limits damage to the soft-tissue envelope, provides superior neutralization of the mechanical forces to provide a template for biologic fracture healing, and allows the best options for revision procedures in the event of inadequate healing. This case report examines treatment options for an open tibial shaft fracture of an ipsilateral TKA, complicating the standard treatment of antegrade tibial nailing. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 66-year-old woman became light-headed and fell down a flight of stairs at her home. She was taken to the local emergency room where she presented with left leg pain, deformity, and a skin wound. The wound was dressed with sterile gauze and the extremity immobilized in a temporary plaster splint after which the patient was transferred to our level I trauma center. The accident occurred shortly after dawn, and she received definitive evaluation at the level I trauma center before noon the same day, making the time from injury to evaluation less than 6 hours.
The patient’s medical history was significant for depressive and anxiety disorders, fibromyalgia, hypertension, peripheral vascular disease, and lymphedema. Her surgical history was significant for a remote left TKA and remote open reduction with internal fixation of a left lateral malleolus fracture. She was prescribed antidepressant and anti-anxiolytic medications, narcotic medication, and antihypertensive therapy. She smoked 1 pack of cigarettes per day for approximately 20 years and denied alcohol consumption or illicit drug use. Her body mass index was 37.5, and she ambulated independently in the community.
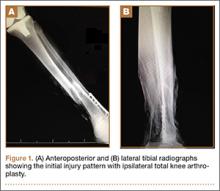
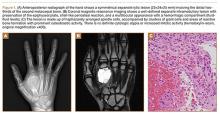
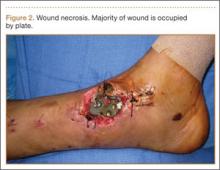
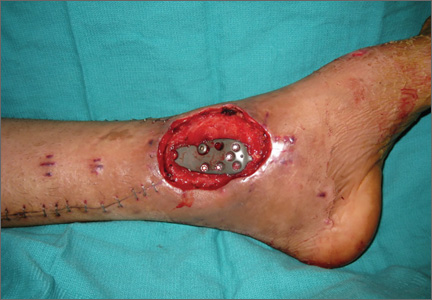
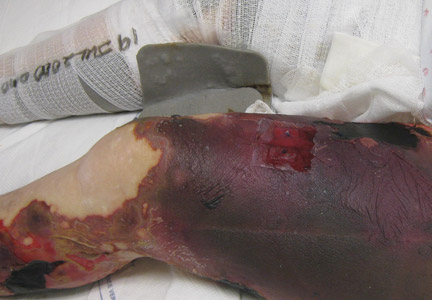
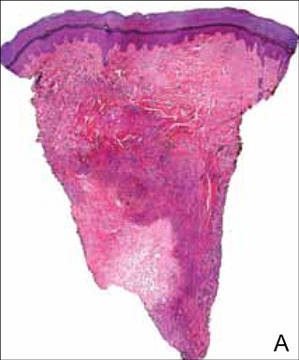
Upon presentation at our hospital, the patient was hemodynamically stable with no discernable systemic compromise from the extremity injury. An examination of the left lower extremity showed a large longitudinal skin wound over the anteromedial surface of the lower leg measuring roughly 10 cm in length with obvious periosteal stripping and protrusion of the proximal fracture segment. Neurologic motor and sensory function was intact in the lower extremities and pulses were strong. Lower leg compartments were soft. Radiographic imaging confirmed a short oblique fracture of the distal third of the tibial diaphysis. The left TKA was intact with no signs of component loosening or periprosthetic fracture (Figures 1A, 1B).
The patient urgently received broad-spectrum antibiotics with intravenous (IV) cefazolin and IV gentamicin as well as tetanus vaccination. Her fracture was temporarily stabilized in a long-leg splint before she was transported to the operating room. Based upon the characteristics of the patient and the open fracture, we had an extensive discussion with the patient regarding the severity of her injury and treatment options, including nonoperative treatment, operative irrigation and débridement with skeletal stabilization, or below-knee amputation. The patient was adamant that limb salvage be attempted despite adequate understanding that she was exposing herself to risk of multiple reoperations from potential complications, as well as systemic medical compromise. Thus, we considered possible techniques for internal fixation of the tibial shaft fracture and treatment of the open wound.
Two primary technical concerns were addressed in the preoperative planning phase: the first was the need for primary closure of the open wound. This patient had a large wound over the anteromedial surface of the distal third of the tibia with scant soft-tissue coverage. Consequently, skin graft alone would not be adequate. While a muscle flap is another option, it would be prone to failure because of the patient’s age and comorbidities, including hypertension, peripheral vascular disease, lymphedema, and tobacco use. Therefore, we hoped to achieve primary closure. Our second major concern was that the method of fixation must be biomechanically sound without impeding our first goal of primary wound closure. In the setting of an ipsilateral TKA, standard antegrade IM nail fixation would not be possible. While we considered plate fixation, it is biomechanically less stable than an IM nail, and we had great concerns about wound complications. External fixation—uniplanar and mutliplanar (eg, Ilizarov)—was limited by issues of long-term fracture stability and risk of pin-site infection. Both methods appeared less desirable compared with IM nail fixation. Thus, we devised an innovative technique to implant an IM nail into the tibial canal.
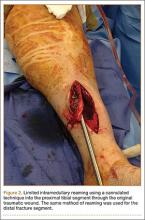
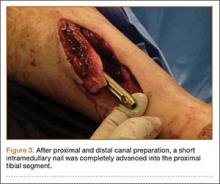
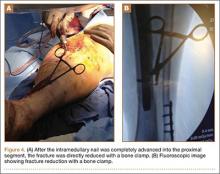
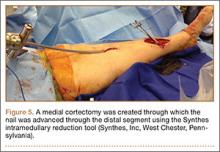
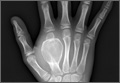
The operative procedure first entailed standard open fracture care comprising débridement of nonviable soft tissue from the traumatic anteromedial tibial wound, curettage of the fractured bone ends, and irrigation with pulse-jet lavage. Then, we turned to reduction and internal fixation of the bony injury. The large traumatic wound was not extended and was used as the primary surgical approach to permit introduction of the IM nail into the canal. Through the traumatic wound, we performed limited reaming of the proximal and distal fracture segments. Using a cannulated technique over guide wires, we reamed to 11 mm (Figure 2). The tourniquet was not used during the IM reaming. We determined the maximum nail length (approximately 22 cm) by measuring the distance from the fracture to the bone interface with the tibial component. We used a 10×200-mm femoral retrograde Synthes nail (Synthes, Inc, West Chester, Pennsylvania) for the procedure, although we considered an IM humerus nail. Through the traumatic wound, the nail was advanced in its entirety into the proximal tibial segment (Figure 3). The fracture was reduced anatomically and held with a bone tenaculum (Figures 4A, 4B). A medial cortical window proximal to the proximal extent of the IM nail was created through which the Synthes IM reduction tool (aluminum femoral finger) was advanced to impact the IM nail antegrade through the fracture site into the distal segment (Figure 5). After placement of the nail was complete, the excised fragment of bone was reinserted into the cortical window. The Synthes IM reduction tool was chosen for its diameter, length, and, most important, its relative flexibility. While maintaining reduction of the fracture, cross-locking of the nail was performed at the distal and proximal ends with perfect circle technique through stab incisions. Length, alignment, and rotation of the affected tibia were deemed symmetric to the contralateral side based on preoperative clinical measurements. Final fluoroscopic images showed appropriate alignment and proper implant placement.
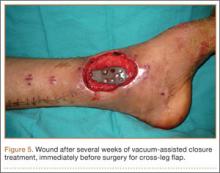
Following open reduction and internal fixation of the fracture, the traumatic and surgical wounds were closed in a layered fashion. A subcutaneous drain and an incisional vacuum-assisted closure (VAC) device were applied to the closed traumatic wound, and a second subcutaneous drain was placed at the site of the cortical window. The patient tolerated the procedure well without perioperative complications.
In the acute period after surgery, the patient’s neurologic and vascular status remained stable. Her muscular compartments remained soft and compressible on physical examination, and her pain was well controlled. The incisional VAC and the 2 Hemovac drains were removed within a few days of the operation. Intravenous cefazolin was continued through her hospital stay and she was transitioned to oral cephalexin at discharge as recommended by our infectious disease colleagues to complete a 10-day course of antibiotic therapy.
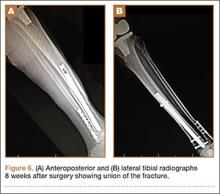
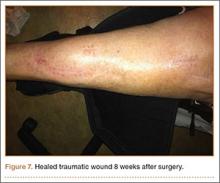
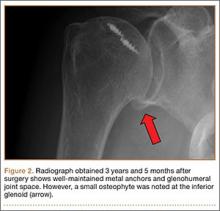
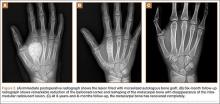
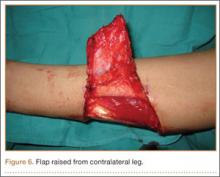
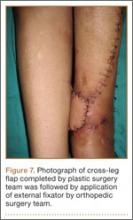
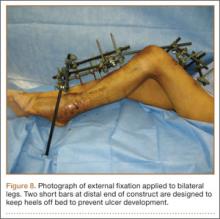
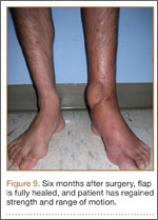
At the time of discharge—within 1 week of her initial injury—the patient’s wounds were dry and she was ambulatory with a walker. She was instructed to remain non-weight-bearing and to keep her wounds clean and dry with follow-up in 2 weeks. Over 6 to 8 weeks after surgery, the patient’s weight-bearing status was gradually advanced to full weight-bearing, and she achieved union of the fracture and uneventful healing of the traumatic wound (Figures 6A, 6B, 7).
Discussion
We have presented a case of an open distal-third tibial shaft fracture in a 66-year-old obese woman with an ipsilateral TKA. Open fracture of the tibial shaft is potentially limb-threatening because of the challenging management of the bone and soft-tissue injury. The presence of an ipsilateral TKA adds a degree of complexity. From a biomechanical standpoint, the lower interdigitation of cortical bone, coupled with weight-bearing of the lower extremity, subjects the tibia diaphysis to issues of rotation, length, and angular control.8 Due to the diaphyseal nature of the fracture, consisting of cortical bone with comparably lower vascularity and a small soft-tissue envelope, these fractures heal very slowly and often take as many as 6 to 9 months to achieve union.9,10 Furthermore, as was the case here, short oblique fractures of the tibial shaft often occur under bending stresses that also cause significant damage to the tibial soft-tissue envelope and periosteum, as indicated by the open wound. This disruption deprives the fracture and soft tissues of important vascular supply that is critical to healing and to avoiding infection and soft-tissue necrosis.11-13 The effects of treatment may magnify these biomechanical and biologic consequences. Ideal fixation serves to minimize potential complications by neutralizing the biomechanical forces to permit fracture healing while also limiting the amount of soft-tissue trauma and tension. Because the challenges associated with treatment of open tibial shaft fractures make it a limb-threatening injury in a patient with poor peripheral circulation, it is appropriate to consider primary amputation.14
If circumstances warrant an attempt at limb salvage, IM nailing with static interlocking screws would typically be the standard of care for treatment of an open fracture of the tibia shaft. This provides stable internal fixation that controls tibial alignment in 6° of freedom and neutralizes bending forces with less strain on the implant because of the IM position.15,16 In addition to superior neutralization of the biomechanical forces, IM nailing is also a minimally invasive approach that limits further trauma to the periosteum and soft-tissue envelope surrounding the fracture site. This optimizes biologic fracture healing and minimizes complications of malunion, infection, and nonunion.17-19 Moreover, by limiting further damage to the surrounding soft tissue, there is a diminished need for a plastic surgery procedure to reestablish soft-tissue integrity overlying the fracture site. This is particularly advantageous in patients with medical comorbidities that make skin grafts and muscle flaps less likely to succeed. For these reasons, IM nailing was our preferred method of fixation in our patient; however, the presence of an ipsilateral TKA made this standard treatment through an antegrade approach impossible.
Consequently, we considered other methods of fixation, including internal fixation with plate application or external fixation with a multiplanar construct, such as an Ilizarov frame. Some orthopedists consider plate application a superior technique for achieving fracture union because it results in interfragmentary compression, which promotes primary healing. Interestingly, some would argue that the absolute stability provided by the plate may be too rigid a construct to enable optimal fracture healing biology if compression is not achieved.20 However, to allow primary healing to complete fracture union, absolute stability with rigid and strong fixation must be provided. In the tibial shaft, with large bending forces and rotational moments, this is difficult to achieve with plate fixation alone.8 Furthermore, plate application often requires relatively extensive soft-tissue dissection and may impede biologic factors in healing of the bone and soft tissue, increasing the likelihood of infection.21 Finally, adequate plate fixation would significantly increase the soft-tissue volume at this location, further compromising the soft tissues and impeding our goal of primary wound closure.
A uniplanar or mutliplanar external fixator would be an appealing option for definitive fixation because of minimal additional soft-tissue damage that is created during its application. However, it is difficult to achieve adequate stability to encourage either primary, or more commonly, secondary healing in the adult or elderly population.22 An Ilizarov frame is a multiplanar external construct, which allows reconstructive applications because of multiple points of fixation in bone.23 However, the multiple fixation points result in burdensome size of the implant for the patient and requires patient compliance to minimize risk of pin-site infection, which is magnified in a patient with multiple medical comorbid conditions. Furthermore, when comparing treatment options that aim to minimize additional soft-tissue trauma at the site of injury, there is little evidence to show a lower risk of infection at the open fracture site compared with IM nailing.24,25 Thus, in our patient, customary treatment of an open tibial shaft fracture using antegrade IM nailing was not possible, while plate application and external fixation, though potential treatment options, would be relatively contraindicated due to a higher likelihood of failure.
Consequently, primary amputation may be the most appropriate treatment option in a patient with multiple comorbid medical conditions, including peripheral vascular disease. Primary amputation prevents morbidity and mortality associated with complications related to the aforementioned treatment options, as well as limiting risks associated with multiple reoperations.14,25 Studies illustrate that patient functional outcomes after primary amputation are equal to and, in some cases, superior to those patients undergoing limb salvage procedures for open tibial shaft fractures.26-28
Despite the appropriateness of primary amputation in this case, the patient requested limb salvage. Therefore, other innovative treatment options were explored to achieve our goals of primary wound closure and stable internal fixation. Previous case reports have examined retrograde IM nailing as a means of rigidly fixing tibial shaft fractures in the setting of poor soft tissues or ipsilateral knee arthroplasty.29-31 However, the retrograde approach to IM nailing requires passage of reamers through the subtalar and ankle joints, leading to associated arthritis in these joints or, more commonly, rigidity because the final nail position often crosses these joints in addition to the fracture site. Therefore, a novel approach for IM nailing was performed using the large open-fracture wound. Through the traumatic wound, open-fracture débridement was first performed, followed by placement of a nail into the medullary canal with little additional disruption of the surrounding periosteum or soft tissue.
Possible complications of this novel method for IM nail passage warrant discussion. First, potentially unfavorable aspects associated with IM reaming include impairment of endosteal blood circulation in the subacute postoperative period.32-34 If the patient develops complications, such as deep infection, nonunion, hardware failure, or periprosthetic fracture, treatment options that require removal of the nail would be very difficult to execute because this nail was passed “intragrade,” or through the fracture site, not from the knee or the calcaneus. However, unique to this case of intragrade nailing, complications associated with the proximal cortical window may occur. In particular, unintended cortical fracture may happen during impaction of the nail into the distal segment of the fracture after reduction. However, this complication may be avoided with the use of a 1-cm wide and 2-cm long window and the use of the malleable aluminum femoral finger (Synthes). Furthermore, use of a femoral nail is recommended because the Herzog curve of a tibial nail cannot be inserted in the proximal tibial segment using an “intragrade” nailing technique. However, fracture may occur intraoperatively or during rehabilitation after surgery because the cortical window creates a region of high stress distal to the tibial arthroplasty component. Likewise, the area of bone between the proximal extent of the IM nail and tibial component of the TKA represents an area of high stress susceptible to periprosthetic fracture.
Conclusion
We have presented a case of a high-energy open distal tibial diaphyseal fracture in a 66-year-old woman with medical comorbidities and treatment complicated by the presence of an ipsilateral TKA. Intramedullary nailing has become the standard of care for open fractures of the tibial diaphysis because of the high rate of union with little additional soft-tissue damage at the fracture site. Despite these advantages, the ipsilateral TKA complicated the placement of an antegrade tibial nail. An alternative treatment, such as an external fixation using an Ilizarov frame, would present equally challenging treatment aspects, including patient compliance, with little proven benefit over an IM nail. Application of a plate would be less desirable because of increased risk of infection at the fracture site, soft-tissue and periosteum disruption, and muscle necrosis compared with other treatment options. Primary amputation was an appropriate consideration for this patient given her comorbid medical circumstances, but the patient refused this treatment option. Therefore, we created a novel approach to place an IM nail, using the traumatic wound to achieve access to the medullary canal proximally and distally.
1. Patzakis MJ, Wilkins J. Factors influencing infection rate in open fracture wounds. Clin Orthop. 1989;243:36-40.
2. Court-Brown CM, McBirnie J. The epidemiology of tibial fractures. J Bone Joint Surg Br. 1995;77(3):417-421.
3. Puno RM, Teynor JT, Nagano J, Gustilo RB. Critical analysis of results of treatment of 201 tibial shaft fractures. Clin Orthop. 1986;212:113-121.
4. Melvin JS, Dombroski DG, Torbert JT, Kovach SJ, Esterhal JL, Mehta S. Open tibial shaft fractures: I. Evaluation and initial wound management. J Am Acad Orthop Surg. 2010;18(1):10-19.
5. Bhandari M, Guyatt GH, Swiontkowski MF, Schemitsch EH. Treatment of open fractures of the shaft of the tibia. J Bone Joint Surg Br. 2001;83(1):62-68.
6. SPRINT Investigators, Bhandari M, Guyatt G, Tornetta P 3rd, et al. Study to prospectively evaluate reamed intramedually nails in patients with tibial fractures (S.P.R.I.N.T.): study rationale and design. BMC Musculoskelet Disord. 2008;9:91.
7. Study to Prospectively Evaluate Reamed Intramedullary Nails in Patients with Tibial Fractures Investigators, Bhandari M, Guyatt G, Tornetta P 3rd, et al. Randomized trial of reamed and unreamed intramedullary nailing of tibial shaft fractures. J Bone Joint Surg Am. 2008;90(12):2567-2578.
8. Burr DB, Milgrom C, Fyhrie D, et al. In vivo measurement of human tibial strains during vigorous activity. Bone. 1996;18(5):405-410.
9. Edwards P. Fracture of the shaft of the tibia: 492 consecutive cases in adults: Importance of soft tissue injury. Acta Orthop Scand (Suppl). 1965;76(suppl 76):1-82.
10. Papakostidis C, Kanakaris NK, Pretel J, Faour O, Morell DJ, Giannoudis PV. Prevalence of complications of open tibial shaft fractures stratified as per the Gustilo–Anderson classification. Injury. 2011;42(12):1408-1415.
11. Gustilo RB, Mendoza RM, Williams DN. Problems in the management of type III (severe) open fractures: a new classification of type III open fractures. J Trauma. 1984;24(8):742-746.
12. DeLong WG Jr, Born CT, Wei SY, Petrik ME, Ponzio R, Schwab CW. Aggressive treatment of 119 open fracture wounds. J Trauma. 1999;46(6):1049-1054.
13. Tielinen L, Lindahl JE, Tukiainen EJ. Acute unreamed intramedullary nailing and soft tissue reconstruction with muscle flaps for the treatment of severe open tibial shaft fractures. Injury. 2007;38(8):906-912.
14. Georgiadis GM, Behrens FF, Joyce MJ, Earle AS, Simmons AL. Open tibial fractures with severe soft-tissue loss. Limb salvage compared with below-the-knee amputation. J Bone Joint Surg Am. 1993;75(10):1431-1441.
15. Hansen M, Mehler D, Hessmann MH, Blum J, Rommens PM. Intramedullary stabilization of extraarticular proximal tibial fractures: a biomechanical comparison of intramedullary and extramedullary implants including a new proximal tibia nail (PTN). J Orthop Trauma. 2007;21(10):701-709.
16. Hoegel FW, Hoffmann S, Weninger P, Bühren V, Augat P. Biomechanical comparison of locked plate osteosynthesis, reamed and unreamed nailing in conventional interlocking technique, and unreamed angle stable nailing in distal tibia fractures. J Trauma Acute Care Surg. 2012;73(4):933-938.
17. Brumback RJ, Reilly JP, Poka A, Lakatos RP, Bathon GH, Burgess AR. Intramedullary nailing of femoral shaft fractures. Part 1: Decision-making errors with interlocking fixation. J Bone Joint Surg Am. 1988;70(10):1441-1452.
18. Hooper GJ, Keddell RG, Penny ID. Conservative management or closed nailing for tibial shaft fractures. A randomised prospective trial. J Bone Joint Surg Br. 1991;73(1):83-85.
19. Karladani AH, Granhed H, Edshage B, Jerre R, Styf J. Displaced tibial shaft fractures: a prospective randomized study of closed intramedullary nailing versus cast treatment in 53 patients. Acta Orthop Scand. 2000;71(12):160-167.
20. Kenwright J, Richardson JB, Goodship AE, et al. Effect of controlled axial micromovement on healing of tibial fractures. Lancet. 1986;22(8517):1185-1187.
21. Im GI, Tae SK. Distal metaphyseal fractures of tibia: a prospective randomized trial of closed reduction and intramedullary nail versus open reduction and plate and screws fixation. J Trauma. 2005;59(5):1219-1223.
22. Henley MB, Chapman JR, Agel J, Harvey EJ, Whorton AM, Swiontkowski MF. Treatment of type II, IIIA, and IIIB open fractures of the tibial shaft: a prospective comparison of unreamed interlocking intramedullary nails and half-pin external fixators. J Orthop Trauma. 1998;12(1):1-7.
23. Ramos T, Ekholm C, Eriksson BI, Karlsson J, Nistor L. The Ilizarov external fixator - a useful alternative for the treatment of proximal tibial fractures. A prospective observational study of 30 consecutive patients. BMC Musculoskelet Disord. 2013;14:11.
24. Bhandari M, Guyatt GH, Swiontkowski MF, Schemitsch EH. Treatment of open fractures of the shaft of the tibia. J Bone Joint Surg Br. 2001;83(1):62-68.
25. Webb LX, Bosse MJ, Castillo RC, MacKenzie EJ; LEAP Study Group. Analysis of surgeon-controlled variables in the treatment of limb-threatening type-III open tibial diaphyseal fractures. J Bone Joint Surg Am. 2007;89(5):923-928.
26. Bondurant FJ, Cotler HB, Buckle R, Miller-Crotchett P, Browner BD. The medical and economic impact of severely injured lower extremities. J Trauma. 1988;28(8):1270-1273.
27. Bosse MJ, MacKenzie EJ, Kellam JF, et al. An analysis of outcomes of reconstruction or amputation of leg-threatening injuries. N Engl J Med. 2002;347(24):1924-1931.
28. MacKenzie EJ, Bosse MJ, Pollak AN, et al. Long-term persistence of disability following severe lower-limb trauma. Results of a seven-year follow-up. J Bone Joint Surg Am. 2005;87(8):1801-1809.
29. Doulens KM, Joshi AB, Wagner RA. Tibial fracture after total knee arthroplasty treated with retrograde intramedullary fixation. Am J Orthop. 2007;36(7):E111-E113.
30. Zafra-Jiménez JA, Pretell-Mazzini J, Resines-Erasun C. Distal tibial fracture below a total knee arthroplasty: retrograde intramedullary nailing as an alternative method of treatment: a case report. J Orthop Trauma. 2011;25(7):e74-e76.
31. Loosen S, Preuss S, Zelle BA, Pape HC, Tarken IS. Multimorbid patients with poor soft tissue conditions: Treatment of distal tibia fractures with retrograde intramedullary nailing. Unfallchirurg. 2012;116(6):553-558.
32. Kessler SB, Hallfeldt KJ, Perren SM, Schweiberer L. The effects of reaming and intramedullary nailing on fracture healing. Clin Orthop. 1986;212:18-25.
33. Klein MP, Rahn BA, Frigg R, Kessler S, Perren SM. Reaming versus non-reaming in medullary nailing: interference with cortical circulation of the canine tibia. Arch Orthop Trauma Surg. 1990;109(6):314-316.
34. Reichert IL, McCarthy ID, Hughes SP. The acute vascular response to intramedullary reaming. Microsphere estimation of blood flow in the intact ovine tibia. J Bone Joint Surg Br. 1995;77(3):490-493.
Fracture of the tibial shaft below an ipsilateral total knee arthroplasty (TKA) is an infrequently occurring injury pattern that presents a unique treatment scenario. The high predilection for open wounds associated with these diaphyseal fractures further complicates the treatment algorithm.1,2 The standard principles of treatment for open tibial shaft fractures entail open fracture débridement followed by adequate fracture reduction and stable skeletal fixation in a manner that limits adverse complications of this injury, which include nonunion, malunion, infection, soft-tissue compromise, and reoperation.3,4
Antegrade intramedullary (IM) tibial nailing has become standard treatment for tibial shaft fractures.5-7 This minimally invasive method of fixation limits damage to the soft-tissue envelope, provides superior neutralization of the mechanical forces to provide a template for biologic fracture healing, and allows the best options for revision procedures in the event of inadequate healing. This case report examines treatment options for an open tibial shaft fracture of an ipsilateral TKA, complicating the standard treatment of antegrade tibial nailing. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 66-year-old woman became light-headed and fell down a flight of stairs at her home. She was taken to the local emergency room where she presented with left leg pain, deformity, and a skin wound. The wound was dressed with sterile gauze and the extremity immobilized in a temporary plaster splint after which the patient was transferred to our level I trauma center. The accident occurred shortly after dawn, and she received definitive evaluation at the level I trauma center before noon the same day, making the time from injury to evaluation less than 6 hours.
The patient’s medical history was significant for depressive and anxiety disorders, fibromyalgia, hypertension, peripheral vascular disease, and lymphedema. Her surgical history was significant for a remote left TKA and remote open reduction with internal fixation of a left lateral malleolus fracture. She was prescribed antidepressant and anti-anxiolytic medications, narcotic medication, and antihypertensive therapy. She smoked 1 pack of cigarettes per day for approximately 20 years and denied alcohol consumption or illicit drug use. Her body mass index was 37.5, and she ambulated independently in the community.
Upon presentation at our hospital, the patient was hemodynamically stable with no discernable systemic compromise from the extremity injury. An examination of the left lower extremity showed a large longitudinal skin wound over the anteromedial surface of the lower leg measuring roughly 10 cm in length with obvious periosteal stripping and protrusion of the proximal fracture segment. Neurologic motor and sensory function was intact in the lower extremities and pulses were strong. Lower leg compartments were soft. Radiographic imaging confirmed a short oblique fracture of the distal third of the tibial diaphysis. The left TKA was intact with no signs of component loosening or periprosthetic fracture (Figures 1A, 1B).
The patient urgently received broad-spectrum antibiotics with intravenous (IV) cefazolin and IV gentamicin as well as tetanus vaccination. Her fracture was temporarily stabilized in a long-leg splint before she was transported to the operating room. Based upon the characteristics of the patient and the open fracture, we had an extensive discussion with the patient regarding the severity of her injury and treatment options, including nonoperative treatment, operative irrigation and débridement with skeletal stabilization, or below-knee amputation. The patient was adamant that limb salvage be attempted despite adequate understanding that she was exposing herself to risk of multiple reoperations from potential complications, as well as systemic medical compromise. Thus, we considered possible techniques for internal fixation of the tibial shaft fracture and treatment of the open wound.
Two primary technical concerns were addressed in the preoperative planning phase: the first was the need for primary closure of the open wound. This patient had a large wound over the anteromedial surface of the distal third of the tibia with scant soft-tissue coverage. Consequently, skin graft alone would not be adequate. While a muscle flap is another option, it would be prone to failure because of the patient’s age and comorbidities, including hypertension, peripheral vascular disease, lymphedema, and tobacco use. Therefore, we hoped to achieve primary closure. Our second major concern was that the method of fixation must be biomechanically sound without impeding our first goal of primary wound closure. In the setting of an ipsilateral TKA, standard antegrade IM nail fixation would not be possible. While we considered plate fixation, it is biomechanically less stable than an IM nail, and we had great concerns about wound complications. External fixation—uniplanar and mutliplanar (eg, Ilizarov)—was limited by issues of long-term fracture stability and risk of pin-site infection. Both methods appeared less desirable compared with IM nail fixation. Thus, we devised an innovative technique to implant an IM nail into the tibial canal.
The operative procedure first entailed standard open fracture care comprising débridement of nonviable soft tissue from the traumatic anteromedial tibial wound, curettage of the fractured bone ends, and irrigation with pulse-jet lavage. Then, we turned to reduction and internal fixation of the bony injury. The large traumatic wound was not extended and was used as the primary surgical approach to permit introduction of the IM nail into the canal. Through the traumatic wound, we performed limited reaming of the proximal and distal fracture segments. Using a cannulated technique over guide wires, we reamed to 11 mm (Figure 2). The tourniquet was not used during the IM reaming. We determined the maximum nail length (approximately 22 cm) by measuring the distance from the fracture to the bone interface with the tibial component. We used a 10×200-mm femoral retrograde Synthes nail (Synthes, Inc, West Chester, Pennsylvania) for the procedure, although we considered an IM humerus nail. Through the traumatic wound, the nail was advanced in its entirety into the proximal tibial segment (Figure 3). The fracture was reduced anatomically and held with a bone tenaculum (Figures 4A, 4B). A medial cortical window proximal to the proximal extent of the IM nail was created through which the Synthes IM reduction tool (aluminum femoral finger) was advanced to impact the IM nail antegrade through the fracture site into the distal segment (Figure 5). After placement of the nail was complete, the excised fragment of bone was reinserted into the cortical window. The Synthes IM reduction tool was chosen for its diameter, length, and, most important, its relative flexibility. While maintaining reduction of the fracture, cross-locking of the nail was performed at the distal and proximal ends with perfect circle technique through stab incisions. Length, alignment, and rotation of the affected tibia were deemed symmetric to the contralateral side based on preoperative clinical measurements. Final fluoroscopic images showed appropriate alignment and proper implant placement.
Following open reduction and internal fixation of the fracture, the traumatic and surgical wounds were closed in a layered fashion. A subcutaneous drain and an incisional vacuum-assisted closure (VAC) device were applied to the closed traumatic wound, and a second subcutaneous drain was placed at the site of the cortical window. The patient tolerated the procedure well without perioperative complications.
In the acute period after surgery, the patient’s neurologic and vascular status remained stable. Her muscular compartments remained soft and compressible on physical examination, and her pain was well controlled. The incisional VAC and the 2 Hemovac drains were removed within a few days of the operation. Intravenous cefazolin was continued through her hospital stay and she was transitioned to oral cephalexin at discharge as recommended by our infectious disease colleagues to complete a 10-day course of antibiotic therapy.
At the time of discharge—within 1 week of her initial injury—the patient’s wounds were dry and she was ambulatory with a walker. She was instructed to remain non-weight-bearing and to keep her wounds clean and dry with follow-up in 2 weeks. Over 6 to 8 weeks after surgery, the patient’s weight-bearing status was gradually advanced to full weight-bearing, and she achieved union of the fracture and uneventful healing of the traumatic wound (Figures 6A, 6B, 7).
Discussion
We have presented a case of an open distal-third tibial shaft fracture in a 66-year-old obese woman with an ipsilateral TKA. Open fracture of the tibial shaft is potentially limb-threatening because of the challenging management of the bone and soft-tissue injury. The presence of an ipsilateral TKA adds a degree of complexity. From a biomechanical standpoint, the lower interdigitation of cortical bone, coupled with weight-bearing of the lower extremity, subjects the tibia diaphysis to issues of rotation, length, and angular control.8 Due to the diaphyseal nature of the fracture, consisting of cortical bone with comparably lower vascularity and a small soft-tissue envelope, these fractures heal very slowly and often take as many as 6 to 9 months to achieve union.9,10 Furthermore, as was the case here, short oblique fractures of the tibial shaft often occur under bending stresses that also cause significant damage to the tibial soft-tissue envelope and periosteum, as indicated by the open wound. This disruption deprives the fracture and soft tissues of important vascular supply that is critical to healing and to avoiding infection and soft-tissue necrosis.11-13 The effects of treatment may magnify these biomechanical and biologic consequences. Ideal fixation serves to minimize potential complications by neutralizing the biomechanical forces to permit fracture healing while also limiting the amount of soft-tissue trauma and tension. Because the challenges associated with treatment of open tibial shaft fractures make it a limb-threatening injury in a patient with poor peripheral circulation, it is appropriate to consider primary amputation.14
If circumstances warrant an attempt at limb salvage, IM nailing with static interlocking screws would typically be the standard of care for treatment of an open fracture of the tibia shaft. This provides stable internal fixation that controls tibial alignment in 6° of freedom and neutralizes bending forces with less strain on the implant because of the IM position.15,16 In addition to superior neutralization of the biomechanical forces, IM nailing is also a minimally invasive approach that limits further trauma to the periosteum and soft-tissue envelope surrounding the fracture site. This optimizes biologic fracture healing and minimizes complications of malunion, infection, and nonunion.17-19 Moreover, by limiting further damage to the surrounding soft tissue, there is a diminished need for a plastic surgery procedure to reestablish soft-tissue integrity overlying the fracture site. This is particularly advantageous in patients with medical comorbidities that make skin grafts and muscle flaps less likely to succeed. For these reasons, IM nailing was our preferred method of fixation in our patient; however, the presence of an ipsilateral TKA made this standard treatment through an antegrade approach impossible.
Consequently, we considered other methods of fixation, including internal fixation with plate application or external fixation with a multiplanar construct, such as an Ilizarov frame. Some orthopedists consider plate application a superior technique for achieving fracture union because it results in interfragmentary compression, which promotes primary healing. Interestingly, some would argue that the absolute stability provided by the plate may be too rigid a construct to enable optimal fracture healing biology if compression is not achieved.20 However, to allow primary healing to complete fracture union, absolute stability with rigid and strong fixation must be provided. In the tibial shaft, with large bending forces and rotational moments, this is difficult to achieve with plate fixation alone.8 Furthermore, plate application often requires relatively extensive soft-tissue dissection and may impede biologic factors in healing of the bone and soft tissue, increasing the likelihood of infection.21 Finally, adequate plate fixation would significantly increase the soft-tissue volume at this location, further compromising the soft tissues and impeding our goal of primary wound closure.
A uniplanar or mutliplanar external fixator would be an appealing option for definitive fixation because of minimal additional soft-tissue damage that is created during its application. However, it is difficult to achieve adequate stability to encourage either primary, or more commonly, secondary healing in the adult or elderly population.22 An Ilizarov frame is a multiplanar external construct, which allows reconstructive applications because of multiple points of fixation in bone.23 However, the multiple fixation points result in burdensome size of the implant for the patient and requires patient compliance to minimize risk of pin-site infection, which is magnified in a patient with multiple medical comorbid conditions. Furthermore, when comparing treatment options that aim to minimize additional soft-tissue trauma at the site of injury, there is little evidence to show a lower risk of infection at the open fracture site compared with IM nailing.24,25 Thus, in our patient, customary treatment of an open tibial shaft fracture using antegrade IM nailing was not possible, while plate application and external fixation, though potential treatment options, would be relatively contraindicated due to a higher likelihood of failure.
Consequently, primary amputation may be the most appropriate treatment option in a patient with multiple comorbid medical conditions, including peripheral vascular disease. Primary amputation prevents morbidity and mortality associated with complications related to the aforementioned treatment options, as well as limiting risks associated with multiple reoperations.14,25 Studies illustrate that patient functional outcomes after primary amputation are equal to and, in some cases, superior to those patients undergoing limb salvage procedures for open tibial shaft fractures.26-28
Despite the appropriateness of primary amputation in this case, the patient requested limb salvage. Therefore, other innovative treatment options were explored to achieve our goals of primary wound closure and stable internal fixation. Previous case reports have examined retrograde IM nailing as a means of rigidly fixing tibial shaft fractures in the setting of poor soft tissues or ipsilateral knee arthroplasty.29-31 However, the retrograde approach to IM nailing requires passage of reamers through the subtalar and ankle joints, leading to associated arthritis in these joints or, more commonly, rigidity because the final nail position often crosses these joints in addition to the fracture site. Therefore, a novel approach for IM nailing was performed using the large open-fracture wound. Through the traumatic wound, open-fracture débridement was first performed, followed by placement of a nail into the medullary canal with little additional disruption of the surrounding periosteum or soft tissue.
Possible complications of this novel method for IM nail passage warrant discussion. First, potentially unfavorable aspects associated with IM reaming include impairment of endosteal blood circulation in the subacute postoperative period.32-34 If the patient develops complications, such as deep infection, nonunion, hardware failure, or periprosthetic fracture, treatment options that require removal of the nail would be very difficult to execute because this nail was passed “intragrade,” or through the fracture site, not from the knee or the calcaneus. However, unique to this case of intragrade nailing, complications associated with the proximal cortical window may occur. In particular, unintended cortical fracture may happen during impaction of the nail into the distal segment of the fracture after reduction. However, this complication may be avoided with the use of a 1-cm wide and 2-cm long window and the use of the malleable aluminum femoral finger (Synthes). Furthermore, use of a femoral nail is recommended because the Herzog curve of a tibial nail cannot be inserted in the proximal tibial segment using an “intragrade” nailing technique. However, fracture may occur intraoperatively or during rehabilitation after surgery because the cortical window creates a region of high stress distal to the tibial arthroplasty component. Likewise, the area of bone between the proximal extent of the IM nail and tibial component of the TKA represents an area of high stress susceptible to periprosthetic fracture.
Conclusion
We have presented a case of a high-energy open distal tibial diaphyseal fracture in a 66-year-old woman with medical comorbidities and treatment complicated by the presence of an ipsilateral TKA. Intramedullary nailing has become the standard of care for open fractures of the tibial diaphysis because of the high rate of union with little additional soft-tissue damage at the fracture site. Despite these advantages, the ipsilateral TKA complicated the placement of an antegrade tibial nail. An alternative treatment, such as an external fixation using an Ilizarov frame, would present equally challenging treatment aspects, including patient compliance, with little proven benefit over an IM nail. Application of a plate would be less desirable because of increased risk of infection at the fracture site, soft-tissue and periosteum disruption, and muscle necrosis compared with other treatment options. Primary amputation was an appropriate consideration for this patient given her comorbid medical circumstances, but the patient refused this treatment option. Therefore, we created a novel approach to place an IM nail, using the traumatic wound to achieve access to the medullary canal proximally and distally.
Fracture of the tibial shaft below an ipsilateral total knee arthroplasty (TKA) is an infrequently occurring injury pattern that presents a unique treatment scenario. The high predilection for open wounds associated with these diaphyseal fractures further complicates the treatment algorithm.1,2 The standard principles of treatment for open tibial shaft fractures entail open fracture débridement followed by adequate fracture reduction and stable skeletal fixation in a manner that limits adverse complications of this injury, which include nonunion, malunion, infection, soft-tissue compromise, and reoperation.3,4
Antegrade intramedullary (IM) tibial nailing has become standard treatment for tibial shaft fractures.5-7 This minimally invasive method of fixation limits damage to the soft-tissue envelope, provides superior neutralization of the mechanical forces to provide a template for biologic fracture healing, and allows the best options for revision procedures in the event of inadequate healing. This case report examines treatment options for an open tibial shaft fracture of an ipsilateral TKA, complicating the standard treatment of antegrade tibial nailing. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 66-year-old woman became light-headed and fell down a flight of stairs at her home. She was taken to the local emergency room where she presented with left leg pain, deformity, and a skin wound. The wound was dressed with sterile gauze and the extremity immobilized in a temporary plaster splint after which the patient was transferred to our level I trauma center. The accident occurred shortly after dawn, and she received definitive evaluation at the level I trauma center before noon the same day, making the time from injury to evaluation less than 6 hours.
The patient’s medical history was significant for depressive and anxiety disorders, fibromyalgia, hypertension, peripheral vascular disease, and lymphedema. Her surgical history was significant for a remote left TKA and remote open reduction with internal fixation of a left lateral malleolus fracture. She was prescribed antidepressant and anti-anxiolytic medications, narcotic medication, and antihypertensive therapy. She smoked 1 pack of cigarettes per day for approximately 20 years and denied alcohol consumption or illicit drug use. Her body mass index was 37.5, and she ambulated independently in the community.
Upon presentation at our hospital, the patient was hemodynamically stable with no discernable systemic compromise from the extremity injury. An examination of the left lower extremity showed a large longitudinal skin wound over the anteromedial surface of the lower leg measuring roughly 10 cm in length with obvious periosteal stripping and protrusion of the proximal fracture segment. Neurologic motor and sensory function was intact in the lower extremities and pulses were strong. Lower leg compartments were soft. Radiographic imaging confirmed a short oblique fracture of the distal third of the tibial diaphysis. The left TKA was intact with no signs of component loosening or periprosthetic fracture (Figures 1A, 1B).
The patient urgently received broad-spectrum antibiotics with intravenous (IV) cefazolin and IV gentamicin as well as tetanus vaccination. Her fracture was temporarily stabilized in a long-leg splint before she was transported to the operating room. Based upon the characteristics of the patient and the open fracture, we had an extensive discussion with the patient regarding the severity of her injury and treatment options, including nonoperative treatment, operative irrigation and débridement with skeletal stabilization, or below-knee amputation. The patient was adamant that limb salvage be attempted despite adequate understanding that she was exposing herself to risk of multiple reoperations from potential complications, as well as systemic medical compromise. Thus, we considered possible techniques for internal fixation of the tibial shaft fracture and treatment of the open wound.
Two primary technical concerns were addressed in the preoperative planning phase: the first was the need for primary closure of the open wound. This patient had a large wound over the anteromedial surface of the distal third of the tibia with scant soft-tissue coverage. Consequently, skin graft alone would not be adequate. While a muscle flap is another option, it would be prone to failure because of the patient’s age and comorbidities, including hypertension, peripheral vascular disease, lymphedema, and tobacco use. Therefore, we hoped to achieve primary closure. Our second major concern was that the method of fixation must be biomechanically sound without impeding our first goal of primary wound closure. In the setting of an ipsilateral TKA, standard antegrade IM nail fixation would not be possible. While we considered plate fixation, it is biomechanically less stable than an IM nail, and we had great concerns about wound complications. External fixation—uniplanar and mutliplanar (eg, Ilizarov)—was limited by issues of long-term fracture stability and risk of pin-site infection. Both methods appeared less desirable compared with IM nail fixation. Thus, we devised an innovative technique to implant an IM nail into the tibial canal.
The operative procedure first entailed standard open fracture care comprising débridement of nonviable soft tissue from the traumatic anteromedial tibial wound, curettage of the fractured bone ends, and irrigation with pulse-jet lavage. Then, we turned to reduction and internal fixation of the bony injury. The large traumatic wound was not extended and was used as the primary surgical approach to permit introduction of the IM nail into the canal. Through the traumatic wound, we performed limited reaming of the proximal and distal fracture segments. Using a cannulated technique over guide wires, we reamed to 11 mm (Figure 2). The tourniquet was not used during the IM reaming. We determined the maximum nail length (approximately 22 cm) by measuring the distance from the fracture to the bone interface with the tibial component. We used a 10×200-mm femoral retrograde Synthes nail (Synthes, Inc, West Chester, Pennsylvania) for the procedure, although we considered an IM humerus nail. Through the traumatic wound, the nail was advanced in its entirety into the proximal tibial segment (Figure 3). The fracture was reduced anatomically and held with a bone tenaculum (Figures 4A, 4B). A medial cortical window proximal to the proximal extent of the IM nail was created through which the Synthes IM reduction tool (aluminum femoral finger) was advanced to impact the IM nail antegrade through the fracture site into the distal segment (Figure 5). After placement of the nail was complete, the excised fragment of bone was reinserted into the cortical window. The Synthes IM reduction tool was chosen for its diameter, length, and, most important, its relative flexibility. While maintaining reduction of the fracture, cross-locking of the nail was performed at the distal and proximal ends with perfect circle technique through stab incisions. Length, alignment, and rotation of the affected tibia were deemed symmetric to the contralateral side based on preoperative clinical measurements. Final fluoroscopic images showed appropriate alignment and proper implant placement.
Following open reduction and internal fixation of the fracture, the traumatic and surgical wounds were closed in a layered fashion. A subcutaneous drain and an incisional vacuum-assisted closure (VAC) device were applied to the closed traumatic wound, and a second subcutaneous drain was placed at the site of the cortical window. The patient tolerated the procedure well without perioperative complications.
In the acute period after surgery, the patient’s neurologic and vascular status remained stable. Her muscular compartments remained soft and compressible on physical examination, and her pain was well controlled. The incisional VAC and the 2 Hemovac drains were removed within a few days of the operation. Intravenous cefazolin was continued through her hospital stay and she was transitioned to oral cephalexin at discharge as recommended by our infectious disease colleagues to complete a 10-day course of antibiotic therapy.
At the time of discharge—within 1 week of her initial injury—the patient’s wounds were dry and she was ambulatory with a walker. She was instructed to remain non-weight-bearing and to keep her wounds clean and dry with follow-up in 2 weeks. Over 6 to 8 weeks after surgery, the patient’s weight-bearing status was gradually advanced to full weight-bearing, and she achieved union of the fracture and uneventful healing of the traumatic wound (Figures 6A, 6B, 7).
Discussion
We have presented a case of an open distal-third tibial shaft fracture in a 66-year-old obese woman with an ipsilateral TKA. Open fracture of the tibial shaft is potentially limb-threatening because of the challenging management of the bone and soft-tissue injury. The presence of an ipsilateral TKA adds a degree of complexity. From a biomechanical standpoint, the lower interdigitation of cortical bone, coupled with weight-bearing of the lower extremity, subjects the tibia diaphysis to issues of rotation, length, and angular control.8 Due to the diaphyseal nature of the fracture, consisting of cortical bone with comparably lower vascularity and a small soft-tissue envelope, these fractures heal very slowly and often take as many as 6 to 9 months to achieve union.9,10 Furthermore, as was the case here, short oblique fractures of the tibial shaft often occur under bending stresses that also cause significant damage to the tibial soft-tissue envelope and periosteum, as indicated by the open wound. This disruption deprives the fracture and soft tissues of important vascular supply that is critical to healing and to avoiding infection and soft-tissue necrosis.11-13 The effects of treatment may magnify these biomechanical and biologic consequences. Ideal fixation serves to minimize potential complications by neutralizing the biomechanical forces to permit fracture healing while also limiting the amount of soft-tissue trauma and tension. Because the challenges associated with treatment of open tibial shaft fractures make it a limb-threatening injury in a patient with poor peripheral circulation, it is appropriate to consider primary amputation.14
If circumstances warrant an attempt at limb salvage, IM nailing with static interlocking screws would typically be the standard of care for treatment of an open fracture of the tibia shaft. This provides stable internal fixation that controls tibial alignment in 6° of freedom and neutralizes bending forces with less strain on the implant because of the IM position.15,16 In addition to superior neutralization of the biomechanical forces, IM nailing is also a minimally invasive approach that limits further trauma to the periosteum and soft-tissue envelope surrounding the fracture site. This optimizes biologic fracture healing and minimizes complications of malunion, infection, and nonunion.17-19 Moreover, by limiting further damage to the surrounding soft tissue, there is a diminished need for a plastic surgery procedure to reestablish soft-tissue integrity overlying the fracture site. This is particularly advantageous in patients with medical comorbidities that make skin grafts and muscle flaps less likely to succeed. For these reasons, IM nailing was our preferred method of fixation in our patient; however, the presence of an ipsilateral TKA made this standard treatment through an antegrade approach impossible.
Consequently, we considered other methods of fixation, including internal fixation with plate application or external fixation with a multiplanar construct, such as an Ilizarov frame. Some orthopedists consider plate application a superior technique for achieving fracture union because it results in interfragmentary compression, which promotes primary healing. Interestingly, some would argue that the absolute stability provided by the plate may be too rigid a construct to enable optimal fracture healing biology if compression is not achieved.20 However, to allow primary healing to complete fracture union, absolute stability with rigid and strong fixation must be provided. In the tibial shaft, with large bending forces and rotational moments, this is difficult to achieve with plate fixation alone.8 Furthermore, plate application often requires relatively extensive soft-tissue dissection and may impede biologic factors in healing of the bone and soft tissue, increasing the likelihood of infection.21 Finally, adequate plate fixation would significantly increase the soft-tissue volume at this location, further compromising the soft tissues and impeding our goal of primary wound closure.
A uniplanar or mutliplanar external fixator would be an appealing option for definitive fixation because of minimal additional soft-tissue damage that is created during its application. However, it is difficult to achieve adequate stability to encourage either primary, or more commonly, secondary healing in the adult or elderly population.22 An Ilizarov frame is a multiplanar external construct, which allows reconstructive applications because of multiple points of fixation in bone.23 However, the multiple fixation points result in burdensome size of the implant for the patient and requires patient compliance to minimize risk of pin-site infection, which is magnified in a patient with multiple medical comorbid conditions. Furthermore, when comparing treatment options that aim to minimize additional soft-tissue trauma at the site of injury, there is little evidence to show a lower risk of infection at the open fracture site compared with IM nailing.24,25 Thus, in our patient, customary treatment of an open tibial shaft fracture using antegrade IM nailing was not possible, while plate application and external fixation, though potential treatment options, would be relatively contraindicated due to a higher likelihood of failure.
Consequently, primary amputation may be the most appropriate treatment option in a patient with multiple comorbid medical conditions, including peripheral vascular disease. Primary amputation prevents morbidity and mortality associated with complications related to the aforementioned treatment options, as well as limiting risks associated with multiple reoperations.14,25 Studies illustrate that patient functional outcomes after primary amputation are equal to and, in some cases, superior to those patients undergoing limb salvage procedures for open tibial shaft fractures.26-28
Despite the appropriateness of primary amputation in this case, the patient requested limb salvage. Therefore, other innovative treatment options were explored to achieve our goals of primary wound closure and stable internal fixation. Previous case reports have examined retrograde IM nailing as a means of rigidly fixing tibial shaft fractures in the setting of poor soft tissues or ipsilateral knee arthroplasty.29-31 However, the retrograde approach to IM nailing requires passage of reamers through the subtalar and ankle joints, leading to associated arthritis in these joints or, more commonly, rigidity because the final nail position often crosses these joints in addition to the fracture site. Therefore, a novel approach for IM nailing was performed using the large open-fracture wound. Through the traumatic wound, open-fracture débridement was first performed, followed by placement of a nail into the medullary canal with little additional disruption of the surrounding periosteum or soft tissue.
Possible complications of this novel method for IM nail passage warrant discussion. First, potentially unfavorable aspects associated with IM reaming include impairment of endosteal blood circulation in the subacute postoperative period.32-34 If the patient develops complications, such as deep infection, nonunion, hardware failure, or periprosthetic fracture, treatment options that require removal of the nail would be very difficult to execute because this nail was passed “intragrade,” or through the fracture site, not from the knee or the calcaneus. However, unique to this case of intragrade nailing, complications associated with the proximal cortical window may occur. In particular, unintended cortical fracture may happen during impaction of the nail into the distal segment of the fracture after reduction. However, this complication may be avoided with the use of a 1-cm wide and 2-cm long window and the use of the malleable aluminum femoral finger (Synthes). Furthermore, use of a femoral nail is recommended because the Herzog curve of a tibial nail cannot be inserted in the proximal tibial segment using an “intragrade” nailing technique. However, fracture may occur intraoperatively or during rehabilitation after surgery because the cortical window creates a region of high stress distal to the tibial arthroplasty component. Likewise, the area of bone between the proximal extent of the IM nail and tibial component of the TKA represents an area of high stress susceptible to periprosthetic fracture.
Conclusion
We have presented a case of a high-energy open distal tibial diaphyseal fracture in a 66-year-old woman with medical comorbidities and treatment complicated by the presence of an ipsilateral TKA. Intramedullary nailing has become the standard of care for open fractures of the tibial diaphysis because of the high rate of union with little additional soft-tissue damage at the fracture site. Despite these advantages, the ipsilateral TKA complicated the placement of an antegrade tibial nail. An alternative treatment, such as an external fixation using an Ilizarov frame, would present equally challenging treatment aspects, including patient compliance, with little proven benefit over an IM nail. Application of a plate would be less desirable because of increased risk of infection at the fracture site, soft-tissue and periosteum disruption, and muscle necrosis compared with other treatment options. Primary amputation was an appropriate consideration for this patient given her comorbid medical circumstances, but the patient refused this treatment option. Therefore, we created a novel approach to place an IM nail, using the traumatic wound to achieve access to the medullary canal proximally and distally.
1. Patzakis MJ, Wilkins J. Factors influencing infection rate in open fracture wounds. Clin Orthop. 1989;243:36-40.
2. Court-Brown CM, McBirnie J. The epidemiology of tibial fractures. J Bone Joint Surg Br. 1995;77(3):417-421.
3. Puno RM, Teynor JT, Nagano J, Gustilo RB. Critical analysis of results of treatment of 201 tibial shaft fractures. Clin Orthop. 1986;212:113-121.
4. Melvin JS, Dombroski DG, Torbert JT, Kovach SJ, Esterhal JL, Mehta S. Open tibial shaft fractures: I. Evaluation and initial wound management. J Am Acad Orthop Surg. 2010;18(1):10-19.
5. Bhandari M, Guyatt GH, Swiontkowski MF, Schemitsch EH. Treatment of open fractures of the shaft of the tibia. J Bone Joint Surg Br. 2001;83(1):62-68.
6. SPRINT Investigators, Bhandari M, Guyatt G, Tornetta P 3rd, et al. Study to prospectively evaluate reamed intramedually nails in patients with tibial fractures (S.P.R.I.N.T.): study rationale and design. BMC Musculoskelet Disord. 2008;9:91.
7. Study to Prospectively Evaluate Reamed Intramedullary Nails in Patients with Tibial Fractures Investigators, Bhandari M, Guyatt G, Tornetta P 3rd, et al. Randomized trial of reamed and unreamed intramedullary nailing of tibial shaft fractures. J Bone Joint Surg Am. 2008;90(12):2567-2578.
8. Burr DB, Milgrom C, Fyhrie D, et al. In vivo measurement of human tibial strains during vigorous activity. Bone. 1996;18(5):405-410.
9. Edwards P. Fracture of the shaft of the tibia: 492 consecutive cases in adults: Importance of soft tissue injury. Acta Orthop Scand (Suppl). 1965;76(suppl 76):1-82.
10. Papakostidis C, Kanakaris NK, Pretel J, Faour O, Morell DJ, Giannoudis PV. Prevalence of complications of open tibial shaft fractures stratified as per the Gustilo–Anderson classification. Injury. 2011;42(12):1408-1415.
11. Gustilo RB, Mendoza RM, Williams DN. Problems in the management of type III (severe) open fractures: a new classification of type III open fractures. J Trauma. 1984;24(8):742-746.
12. DeLong WG Jr, Born CT, Wei SY, Petrik ME, Ponzio R, Schwab CW. Aggressive treatment of 119 open fracture wounds. J Trauma. 1999;46(6):1049-1054.
13. Tielinen L, Lindahl JE, Tukiainen EJ. Acute unreamed intramedullary nailing and soft tissue reconstruction with muscle flaps for the treatment of severe open tibial shaft fractures. Injury. 2007;38(8):906-912.
14. Georgiadis GM, Behrens FF, Joyce MJ, Earle AS, Simmons AL. Open tibial fractures with severe soft-tissue loss. Limb salvage compared with below-the-knee amputation. J Bone Joint Surg Am. 1993;75(10):1431-1441.
15. Hansen M, Mehler D, Hessmann MH, Blum J, Rommens PM. Intramedullary stabilization of extraarticular proximal tibial fractures: a biomechanical comparison of intramedullary and extramedullary implants including a new proximal tibia nail (PTN). J Orthop Trauma. 2007;21(10):701-709.
16. Hoegel FW, Hoffmann S, Weninger P, Bühren V, Augat P. Biomechanical comparison of locked plate osteosynthesis, reamed and unreamed nailing in conventional interlocking technique, and unreamed angle stable nailing in distal tibia fractures. J Trauma Acute Care Surg. 2012;73(4):933-938.
17. Brumback RJ, Reilly JP, Poka A, Lakatos RP, Bathon GH, Burgess AR. Intramedullary nailing of femoral shaft fractures. Part 1: Decision-making errors with interlocking fixation. J Bone Joint Surg Am. 1988;70(10):1441-1452.
18. Hooper GJ, Keddell RG, Penny ID. Conservative management or closed nailing for tibial shaft fractures. A randomised prospective trial. J Bone Joint Surg Br. 1991;73(1):83-85.
19. Karladani AH, Granhed H, Edshage B, Jerre R, Styf J. Displaced tibial shaft fractures: a prospective randomized study of closed intramedullary nailing versus cast treatment in 53 patients. Acta Orthop Scand. 2000;71(12):160-167.
20. Kenwright J, Richardson JB, Goodship AE, et al. Effect of controlled axial micromovement on healing of tibial fractures. Lancet. 1986;22(8517):1185-1187.
21. Im GI, Tae SK. Distal metaphyseal fractures of tibia: a prospective randomized trial of closed reduction and intramedullary nail versus open reduction and plate and screws fixation. J Trauma. 2005;59(5):1219-1223.
22. Henley MB, Chapman JR, Agel J, Harvey EJ, Whorton AM, Swiontkowski MF. Treatment of type II, IIIA, and IIIB open fractures of the tibial shaft: a prospective comparison of unreamed interlocking intramedullary nails and half-pin external fixators. J Orthop Trauma. 1998;12(1):1-7.
23. Ramos T, Ekholm C, Eriksson BI, Karlsson J, Nistor L. The Ilizarov external fixator - a useful alternative for the treatment of proximal tibial fractures. A prospective observational study of 30 consecutive patients. BMC Musculoskelet Disord. 2013;14:11.
24. Bhandari M, Guyatt GH, Swiontkowski MF, Schemitsch EH. Treatment of open fractures of the shaft of the tibia. J Bone Joint Surg Br. 2001;83(1):62-68.
25. Webb LX, Bosse MJ, Castillo RC, MacKenzie EJ; LEAP Study Group. Analysis of surgeon-controlled variables in the treatment of limb-threatening type-III open tibial diaphyseal fractures. J Bone Joint Surg Am. 2007;89(5):923-928.
26. Bondurant FJ, Cotler HB, Buckle R, Miller-Crotchett P, Browner BD. The medical and economic impact of severely injured lower extremities. J Trauma. 1988;28(8):1270-1273.
27. Bosse MJ, MacKenzie EJ, Kellam JF, et al. An analysis of outcomes of reconstruction or amputation of leg-threatening injuries. N Engl J Med. 2002;347(24):1924-1931.
28. MacKenzie EJ, Bosse MJ, Pollak AN, et al. Long-term persistence of disability following severe lower-limb trauma. Results of a seven-year follow-up. J Bone Joint Surg Am. 2005;87(8):1801-1809.
29. Doulens KM, Joshi AB, Wagner RA. Tibial fracture after total knee arthroplasty treated with retrograde intramedullary fixation. Am J Orthop. 2007;36(7):E111-E113.
30. Zafra-Jiménez JA, Pretell-Mazzini J, Resines-Erasun C. Distal tibial fracture below a total knee arthroplasty: retrograde intramedullary nailing as an alternative method of treatment: a case report. J Orthop Trauma. 2011;25(7):e74-e76.
31. Loosen S, Preuss S, Zelle BA, Pape HC, Tarken IS. Multimorbid patients with poor soft tissue conditions: Treatment of distal tibia fractures with retrograde intramedullary nailing. Unfallchirurg. 2012;116(6):553-558.
32. Kessler SB, Hallfeldt KJ, Perren SM, Schweiberer L. The effects of reaming and intramedullary nailing on fracture healing. Clin Orthop. 1986;212:18-25.
33. Klein MP, Rahn BA, Frigg R, Kessler S, Perren SM. Reaming versus non-reaming in medullary nailing: interference with cortical circulation of the canine tibia. Arch Orthop Trauma Surg. 1990;109(6):314-316.
34. Reichert IL, McCarthy ID, Hughes SP. The acute vascular response to intramedullary reaming. Microsphere estimation of blood flow in the intact ovine tibia. J Bone Joint Surg Br. 1995;77(3):490-493.
1. Patzakis MJ, Wilkins J. Factors influencing infection rate in open fracture wounds. Clin Orthop. 1989;243:36-40.
2. Court-Brown CM, McBirnie J. The epidemiology of tibial fractures. J Bone Joint Surg Br. 1995;77(3):417-421.
3. Puno RM, Teynor JT, Nagano J, Gustilo RB. Critical analysis of results of treatment of 201 tibial shaft fractures. Clin Orthop. 1986;212:113-121.
4. Melvin JS, Dombroski DG, Torbert JT, Kovach SJ, Esterhal JL, Mehta S. Open tibial shaft fractures: I. Evaluation and initial wound management. J Am Acad Orthop Surg. 2010;18(1):10-19.
5. Bhandari M, Guyatt GH, Swiontkowski MF, Schemitsch EH. Treatment of open fractures of the shaft of the tibia. J Bone Joint Surg Br. 2001;83(1):62-68.
6. SPRINT Investigators, Bhandari M, Guyatt G, Tornetta P 3rd, et al. Study to prospectively evaluate reamed intramedually nails in patients with tibial fractures (S.P.R.I.N.T.): study rationale and design. BMC Musculoskelet Disord. 2008;9:91.
7. Study to Prospectively Evaluate Reamed Intramedullary Nails in Patients with Tibial Fractures Investigators, Bhandari M, Guyatt G, Tornetta P 3rd, et al. Randomized trial of reamed and unreamed intramedullary nailing of tibial shaft fractures. J Bone Joint Surg Am. 2008;90(12):2567-2578.
8. Burr DB, Milgrom C, Fyhrie D, et al. In vivo measurement of human tibial strains during vigorous activity. Bone. 1996;18(5):405-410.
9. Edwards P. Fracture of the shaft of the tibia: 492 consecutive cases in adults: Importance of soft tissue injury. Acta Orthop Scand (Suppl). 1965;76(suppl 76):1-82.
10. Papakostidis C, Kanakaris NK, Pretel J, Faour O, Morell DJ, Giannoudis PV. Prevalence of complications of open tibial shaft fractures stratified as per the Gustilo–Anderson classification. Injury. 2011;42(12):1408-1415.
11. Gustilo RB, Mendoza RM, Williams DN. Problems in the management of type III (severe) open fractures: a new classification of type III open fractures. J Trauma. 1984;24(8):742-746.
12. DeLong WG Jr, Born CT, Wei SY, Petrik ME, Ponzio R, Schwab CW. Aggressive treatment of 119 open fracture wounds. J Trauma. 1999;46(6):1049-1054.
13. Tielinen L, Lindahl JE, Tukiainen EJ. Acute unreamed intramedullary nailing and soft tissue reconstruction with muscle flaps for the treatment of severe open tibial shaft fractures. Injury. 2007;38(8):906-912.
14. Georgiadis GM, Behrens FF, Joyce MJ, Earle AS, Simmons AL. Open tibial fractures with severe soft-tissue loss. Limb salvage compared with below-the-knee amputation. J Bone Joint Surg Am. 1993;75(10):1431-1441.
15. Hansen M, Mehler D, Hessmann MH, Blum J, Rommens PM. Intramedullary stabilization of extraarticular proximal tibial fractures: a biomechanical comparison of intramedullary and extramedullary implants including a new proximal tibia nail (PTN). J Orthop Trauma. 2007;21(10):701-709.
16. Hoegel FW, Hoffmann S, Weninger P, Bühren V, Augat P. Biomechanical comparison of locked plate osteosynthesis, reamed and unreamed nailing in conventional interlocking technique, and unreamed angle stable nailing in distal tibia fractures. J Trauma Acute Care Surg. 2012;73(4):933-938.
17. Brumback RJ, Reilly JP, Poka A, Lakatos RP, Bathon GH, Burgess AR. Intramedullary nailing of femoral shaft fractures. Part 1: Decision-making errors with interlocking fixation. J Bone Joint Surg Am. 1988;70(10):1441-1452.
18. Hooper GJ, Keddell RG, Penny ID. Conservative management or closed nailing for tibial shaft fractures. A randomised prospective trial. J Bone Joint Surg Br. 1991;73(1):83-85.
19. Karladani AH, Granhed H, Edshage B, Jerre R, Styf J. Displaced tibial shaft fractures: a prospective randomized study of closed intramedullary nailing versus cast treatment in 53 patients. Acta Orthop Scand. 2000;71(12):160-167.
20. Kenwright J, Richardson JB, Goodship AE, et al. Effect of controlled axial micromovement on healing of tibial fractures. Lancet. 1986;22(8517):1185-1187.
21. Im GI, Tae SK. Distal metaphyseal fractures of tibia: a prospective randomized trial of closed reduction and intramedullary nail versus open reduction and plate and screws fixation. J Trauma. 2005;59(5):1219-1223.
22. Henley MB, Chapman JR, Agel J, Harvey EJ, Whorton AM, Swiontkowski MF. Treatment of type II, IIIA, and IIIB open fractures of the tibial shaft: a prospective comparison of unreamed interlocking intramedullary nails and half-pin external fixators. J Orthop Trauma. 1998;12(1):1-7.
23. Ramos T, Ekholm C, Eriksson BI, Karlsson J, Nistor L. The Ilizarov external fixator - a useful alternative for the treatment of proximal tibial fractures. A prospective observational study of 30 consecutive patients. BMC Musculoskelet Disord. 2013;14:11.
24. Bhandari M, Guyatt GH, Swiontkowski MF, Schemitsch EH. Treatment of open fractures of the shaft of the tibia. J Bone Joint Surg Br. 2001;83(1):62-68.
25. Webb LX, Bosse MJ, Castillo RC, MacKenzie EJ; LEAP Study Group. Analysis of surgeon-controlled variables in the treatment of limb-threatening type-III open tibial diaphyseal fractures. J Bone Joint Surg Am. 2007;89(5):923-928.
26. Bondurant FJ, Cotler HB, Buckle R, Miller-Crotchett P, Browner BD. The medical and economic impact of severely injured lower extremities. J Trauma. 1988;28(8):1270-1273.
27. Bosse MJ, MacKenzie EJ, Kellam JF, et al. An analysis of outcomes of reconstruction or amputation of leg-threatening injuries. N Engl J Med. 2002;347(24):1924-1931.
28. MacKenzie EJ, Bosse MJ, Pollak AN, et al. Long-term persistence of disability following severe lower-limb trauma. Results of a seven-year follow-up. J Bone Joint Surg Am. 2005;87(8):1801-1809.
29. Doulens KM, Joshi AB, Wagner RA. Tibial fracture after total knee arthroplasty treated with retrograde intramedullary fixation. Am J Orthop. 2007;36(7):E111-E113.
30. Zafra-Jiménez JA, Pretell-Mazzini J, Resines-Erasun C. Distal tibial fracture below a total knee arthroplasty: retrograde intramedullary nailing as an alternative method of treatment: a case report. J Orthop Trauma. 2011;25(7):e74-e76.
31. Loosen S, Preuss S, Zelle BA, Pape HC, Tarken IS. Multimorbid patients with poor soft tissue conditions: Treatment of distal tibia fractures with retrograde intramedullary nailing. Unfallchirurg. 2012;116(6):553-558.
32. Kessler SB, Hallfeldt KJ, Perren SM, Schweiberer L. The effects of reaming and intramedullary nailing on fracture healing. Clin Orthop. 1986;212:18-25.
33. Klein MP, Rahn BA, Frigg R, Kessler S, Perren SM. Reaming versus non-reaming in medullary nailing: interference with cortical circulation of the canine tibia. Arch Orthop Trauma Surg. 1990;109(6):314-316.
34. Reichert IL, McCarthy ID, Hughes SP. The acute vascular response to intramedullary reaming. Microsphere estimation of blood flow in the intact ovine tibia. J Bone Joint Surg Br. 1995;77(3):490-493.
Glenoid Damage From Articular Protrusion of Metal Suture Anchor After Arthroscopic Rotator Cuff Repair
Complications with the use of anchor screws in shoulder surgery have been well-documented1,2 and can be divided into 3 categories: insertion (eg, incomplete seating, inadequate insertion, and migration), biologic (eg, large tacks producing synovitis and bone reaction), and, less commonly, mechanical (eg, intra- and extra-articular bone pull-out with migration) complications.
Prominent hardware, including suture anchors, as a cause of arthritis and joint damage has been well-documented in shoulder surgery.3,4 For example, anchors placed on the glenoid rim have been implicated in severe cartilage loss if they protrude above the level of the glenoid rim.3 However, to the authors’ knowledge, prominent anchor placement after rotator cuff repair has not been reported as a cause of arthritis unless the anchor dislodges into the glenohumeral joint. The authors present a case in which a suture anchor used for rotator cuff repair protruded through the humeral head, resulting in glenohumeral arthritis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 61-year-old woman presented with complaints of persistent right shoulder pain for 5 months after a fall from a bicycle. She had taken nonsteroidal anti-inflammatory medication without pain relief. On presentation, she had no atrophy or deformity, was neurologically intact for sensation and reflexes, and had full range of motion (ROM) but a painful arc. She had tenderness over the greater tuberosity and positive Neer and Hawkins-Kennedy impingement signs. She had pain but no weakness to resisted abduction or to resisted external rotation with the arms at the sides.
Preoperative conventional radiographs of the shoulder were normal. A gadolinium-enhanced magnetic resonance arthrogram showed a high-grade articular partial tear of the supraspinatus, which was judged to be at least two-thirds of the tendon width. Because nonoperative methods had failed, the patient elected operative intervention for this tear.
Diagnostic arthroscopy (with the patient in a lateral decubitus position) showed a normal joint except for a high-grade, 8×8-mm, greater than 6 mm deep, partial tear of the articular side of the supraspinatus tendon. The subacromial space had moderate to severe bursal tissue inflammation but no full-thickness component to the rotator cuff tear. A bursectomy, coracoacromial ligament release, and partial anterolateral acromioplasty were performed.
A transtendinous technique was used to repair this high-grade tear. For an anatomically rigid repair, we used 3 suture anchors with a straight configuration because each metal anchor has only 1 suture. According to the standard arthroscopic transtendinous repair technique, the suture anchors were placed through the rotator cuff tendon (at the lateral articular margin at the medial extent of the footprint) after localization of the angle with a spinal needle. A shuttle relay was used to pass the sutures, and the knot was pulled into the subacromial space, cinching the rotator cuff on top of the suture anchors and reestablishing the contact of the tendon to the footprint. We used two 2.4-mm FASTak suture anchors (Arthrex, Naples, Florida) and one 3.5-mm Corkscrew suture anchor (Arthrex). This process was repeated for the remaining suture limbs. The placement of the suture anchors adequately reduced the articular part of the cuff to the footprint.
After surgery, the patient had no complications, and radiographs taken the next day suggested no abnormalities (Figure 1A). The shoulder was immobilized for 4 weeks after surgery, and passive, gentle ROM exercise was supervised by a physical therapist twice a week during this period. After the first 4 weeks, an active ROM program was begun. However, shortly after initiating motion in the shoulder, the patient complained of a recurrence of pain that she described as a sharp and grinding sensation.
The patient was reevaluated 8 weeks after surgery. Her pain was worsening, and she was having difficulty regaining ROM. Conventional radiographs showed the tip of the metal anchor protruding through the articular cartilage of the humeral head (Figure 1B). The patient was informed of the findings, and immediate surgery was performed to remove the anchor.
Arthroscopic examination showed extensive damage to the glenoid cartilage (Figure 1C) and an intra-articularly intact rotator cuff repair. The cartilage damage was located in the posterior and inferior half of the glenoid, which is related to the forward flexion of the arm; the depth of the cartilage defect was approximately 2 mm. Under the image intensifier, an empty suture anchor driver was inserted into the previous screw insertion hole, and the anchor was screwed back out and removed.
After surgery, the patient’s arm was placed in a sling, and an ROM program began 4 weeks later. The sensation of grinding was eliminated, and her pain gradually improved. Three years after surgery, she had no pain, no weakness, and full ROM without limitations (Figure 2).
Discussion
Protrusion and migration of suture anchors in shoulder surgery has been documented extensively.3,4 Zuckerman and Matsen4 divided these complications into 4 groups: (1) incorrect placement, (2) migration after placement, (3) loosening, and (4) device breakage. These complications may be frequently related to surgical technique, and all these studies describe backward migration of the anchor out of the drill hole. In the current case, the anchor tip penetrated the articular surface of the humeral head, not because of anchor migration but because the anchor was inserted too far. To the authors’ knowledge, there is only 1 reported case of anchor protrusion through the humeral head; it involved a different type of anchor insertion system.5 In that case, there was only mild cartilage damage to the glenoid, and the patient recovered after removal of the anchors.
Several factors contributed to the improper insertion of the anchor in the current patient. First, repairing a high-grade articular side defect or partial articular supraspinatus tendon avulsion lesion can be technically challenging because rotator cuff tissue obscures the view when inserting the anchor. Second, the anchor was inserted too medially on the greater tuberosity, which made the distance from the tuberosity to the joint shorter. Wong and colleagues5 performed an analysis of the angle of insertion that would be safe using a PEEK PushLock SP system (Arthrex), but they emphasized that the angle depends on the configuration of the particular insertion system. The current case also shows that the surgeon should be cognizant of the fact that penetration of the humeral head by the anchor can occur if the surgeon is unaware of the distance from the anchor to the laser line on the insertion device or of the distance from the tuberosity to the articular surface of the humeral head.
The current case also shows that the type of anchor and delivery system may contribute to this complication. Double-loaded suture anchors can decrease the number of anchors needed for secure fixation. Bioabsorbable anchors can be used for this purpose, but they may be technically more difficult to use for repairing partial tears of the rotator cuff. Better visualization of the laser line on the anchor may be facilitated by using a probe from an anterior portal to hold the cuff up while the anchor is inserted.
This case has shown the importance of obtaining postoperative radiographic studies in patients who have metal anchors placed during shoulder surgery, especially if they complain of continued pain, new pain, crepitus, or grinding. When conventional radiography is insufficient for locating the anchor or its proximity to the joint line, computed tomography can be helpful.1
Conclusion
Removing failed suture anchors can be challenging, especially when they protrude into the joint on the humeral side.1,6 The best way to prevent this complication is through careful technique. The anchors should not be inserted beyond the depth of the laser line on the anchors, and every attempt should be made to make sure the laser line is visible at the time of anchor insertion. Postoperative radiographs should be considered for patients with metal anchors in the shoulder, especially if the patient continues to have symptoms or develops new symptoms in the shoulder after surgery.
1. Park HB, Keyurapan E, Gill HS, Selhi HS, McFarland EG. Suture anchors and tacks for shoulder surgery. Part II: The prevention and treatment of complications. Am J Sports Med. 2006;34(1):136-144.
2. McFarland EG, Park HB, Keyurapan E, Gill HS, Selhi HS. Suture anchors and tacks for shoulder surgery. Part I: Biology and biomechanics. Am J Sports Med. 2005;33(12):1918-1923.
3. Rhee YG, Lee DH, Chun IH, Bae SC. Glenohumeral arthropathy after arthroscopic anterior shoulder stabilization. Arthroscopy. 2004;20(4):402-406.
4. Zuckerman JD, Matsen FA III. Complications about the glenohumeral joint related to the use of screws and staples. J Bone Joint Surg Am. 1984;66(2):175-180.
5. Wong AS, Kokkalis ZT, Schmidt CC. Proper insertion angle is essential to prevent intra-articular protrusion of a knotless suture anchor in shoulder rotator cuff repair. Arthroscopy. 2010;26(2):286-290.
6. Grutter PW, McFarland EG, Zikria BA, Dai Z, Petersen SA. Techniques for suture anchor removal in shoulder surgery. Am J Sports Med. 2010;38(8):1706-1710.
Complications with the use of anchor screws in shoulder surgery have been well-documented1,2 and can be divided into 3 categories: insertion (eg, incomplete seating, inadequate insertion, and migration), biologic (eg, large tacks producing synovitis and bone reaction), and, less commonly, mechanical (eg, intra- and extra-articular bone pull-out with migration) complications.
Prominent hardware, including suture anchors, as a cause of arthritis and joint damage has been well-documented in shoulder surgery.3,4 For example, anchors placed on the glenoid rim have been implicated in severe cartilage loss if they protrude above the level of the glenoid rim.3 However, to the authors’ knowledge, prominent anchor placement after rotator cuff repair has not been reported as a cause of arthritis unless the anchor dislodges into the glenohumeral joint. The authors present a case in which a suture anchor used for rotator cuff repair protruded through the humeral head, resulting in glenohumeral arthritis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 61-year-old woman presented with complaints of persistent right shoulder pain for 5 months after a fall from a bicycle. She had taken nonsteroidal anti-inflammatory medication without pain relief. On presentation, she had no atrophy or deformity, was neurologically intact for sensation and reflexes, and had full range of motion (ROM) but a painful arc. She had tenderness over the greater tuberosity and positive Neer and Hawkins-Kennedy impingement signs. She had pain but no weakness to resisted abduction or to resisted external rotation with the arms at the sides.
Preoperative conventional radiographs of the shoulder were normal. A gadolinium-enhanced magnetic resonance arthrogram showed a high-grade articular partial tear of the supraspinatus, which was judged to be at least two-thirds of the tendon width. Because nonoperative methods had failed, the patient elected operative intervention for this tear.
Diagnostic arthroscopy (with the patient in a lateral decubitus position) showed a normal joint except for a high-grade, 8×8-mm, greater than 6 mm deep, partial tear of the articular side of the supraspinatus tendon. The subacromial space had moderate to severe bursal tissue inflammation but no full-thickness component to the rotator cuff tear. A bursectomy, coracoacromial ligament release, and partial anterolateral acromioplasty were performed.
A transtendinous technique was used to repair this high-grade tear. For an anatomically rigid repair, we used 3 suture anchors with a straight configuration because each metal anchor has only 1 suture. According to the standard arthroscopic transtendinous repair technique, the suture anchors were placed through the rotator cuff tendon (at the lateral articular margin at the medial extent of the footprint) after localization of the angle with a spinal needle. A shuttle relay was used to pass the sutures, and the knot was pulled into the subacromial space, cinching the rotator cuff on top of the suture anchors and reestablishing the contact of the tendon to the footprint. We used two 2.4-mm FASTak suture anchors (Arthrex, Naples, Florida) and one 3.5-mm Corkscrew suture anchor (Arthrex). This process was repeated for the remaining suture limbs. The placement of the suture anchors adequately reduced the articular part of the cuff to the footprint.
After surgery, the patient had no complications, and radiographs taken the next day suggested no abnormalities (Figure 1A). The shoulder was immobilized for 4 weeks after surgery, and passive, gentle ROM exercise was supervised by a physical therapist twice a week during this period. After the first 4 weeks, an active ROM program was begun. However, shortly after initiating motion in the shoulder, the patient complained of a recurrence of pain that she described as a sharp and grinding sensation.
The patient was reevaluated 8 weeks after surgery. Her pain was worsening, and she was having difficulty regaining ROM. Conventional radiographs showed the tip of the metal anchor protruding through the articular cartilage of the humeral head (Figure 1B). The patient was informed of the findings, and immediate surgery was performed to remove the anchor.
Arthroscopic examination showed extensive damage to the glenoid cartilage (Figure 1C) and an intra-articularly intact rotator cuff repair. The cartilage damage was located in the posterior and inferior half of the glenoid, which is related to the forward flexion of the arm; the depth of the cartilage defect was approximately 2 mm. Under the image intensifier, an empty suture anchor driver was inserted into the previous screw insertion hole, and the anchor was screwed back out and removed.
After surgery, the patient’s arm was placed in a sling, and an ROM program began 4 weeks later. The sensation of grinding was eliminated, and her pain gradually improved. Three years after surgery, she had no pain, no weakness, and full ROM without limitations (Figure 2).
Discussion
Protrusion and migration of suture anchors in shoulder surgery has been documented extensively.3,4 Zuckerman and Matsen4 divided these complications into 4 groups: (1) incorrect placement, (2) migration after placement, (3) loosening, and (4) device breakage. These complications may be frequently related to surgical technique, and all these studies describe backward migration of the anchor out of the drill hole. In the current case, the anchor tip penetrated the articular surface of the humeral head, not because of anchor migration but because the anchor was inserted too far. To the authors’ knowledge, there is only 1 reported case of anchor protrusion through the humeral head; it involved a different type of anchor insertion system.5 In that case, there was only mild cartilage damage to the glenoid, and the patient recovered after removal of the anchors.
Several factors contributed to the improper insertion of the anchor in the current patient. First, repairing a high-grade articular side defect or partial articular supraspinatus tendon avulsion lesion can be technically challenging because rotator cuff tissue obscures the view when inserting the anchor. Second, the anchor was inserted too medially on the greater tuberosity, which made the distance from the tuberosity to the joint shorter. Wong and colleagues5 performed an analysis of the angle of insertion that would be safe using a PEEK PushLock SP system (Arthrex), but they emphasized that the angle depends on the configuration of the particular insertion system. The current case also shows that the surgeon should be cognizant of the fact that penetration of the humeral head by the anchor can occur if the surgeon is unaware of the distance from the anchor to the laser line on the insertion device or of the distance from the tuberosity to the articular surface of the humeral head.
The current case also shows that the type of anchor and delivery system may contribute to this complication. Double-loaded suture anchors can decrease the number of anchors needed for secure fixation. Bioabsorbable anchors can be used for this purpose, but they may be technically more difficult to use for repairing partial tears of the rotator cuff. Better visualization of the laser line on the anchor may be facilitated by using a probe from an anterior portal to hold the cuff up while the anchor is inserted.
This case has shown the importance of obtaining postoperative radiographic studies in patients who have metal anchors placed during shoulder surgery, especially if they complain of continued pain, new pain, crepitus, or grinding. When conventional radiography is insufficient for locating the anchor or its proximity to the joint line, computed tomography can be helpful.1
Conclusion
Removing failed suture anchors can be challenging, especially when they protrude into the joint on the humeral side.1,6 The best way to prevent this complication is through careful technique. The anchors should not be inserted beyond the depth of the laser line on the anchors, and every attempt should be made to make sure the laser line is visible at the time of anchor insertion. Postoperative radiographs should be considered for patients with metal anchors in the shoulder, especially if the patient continues to have symptoms or develops new symptoms in the shoulder after surgery.
Complications with the use of anchor screws in shoulder surgery have been well-documented1,2 and can be divided into 3 categories: insertion (eg, incomplete seating, inadequate insertion, and migration), biologic (eg, large tacks producing synovitis and bone reaction), and, less commonly, mechanical (eg, intra- and extra-articular bone pull-out with migration) complications.
Prominent hardware, including suture anchors, as a cause of arthritis and joint damage has been well-documented in shoulder surgery.3,4 For example, anchors placed on the glenoid rim have been implicated in severe cartilage loss if they protrude above the level of the glenoid rim.3 However, to the authors’ knowledge, prominent anchor placement after rotator cuff repair has not been reported as a cause of arthritis unless the anchor dislodges into the glenohumeral joint. The authors present a case in which a suture anchor used for rotator cuff repair protruded through the humeral head, resulting in glenohumeral arthritis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 61-year-old woman presented with complaints of persistent right shoulder pain for 5 months after a fall from a bicycle. She had taken nonsteroidal anti-inflammatory medication without pain relief. On presentation, she had no atrophy or deformity, was neurologically intact for sensation and reflexes, and had full range of motion (ROM) but a painful arc. She had tenderness over the greater tuberosity and positive Neer and Hawkins-Kennedy impingement signs. She had pain but no weakness to resisted abduction or to resisted external rotation with the arms at the sides.
Preoperative conventional radiographs of the shoulder were normal. A gadolinium-enhanced magnetic resonance arthrogram showed a high-grade articular partial tear of the supraspinatus, which was judged to be at least two-thirds of the tendon width. Because nonoperative methods had failed, the patient elected operative intervention for this tear.
Diagnostic arthroscopy (with the patient in a lateral decubitus position) showed a normal joint except for a high-grade, 8×8-mm, greater than 6 mm deep, partial tear of the articular side of the supraspinatus tendon. The subacromial space had moderate to severe bursal tissue inflammation but no full-thickness component to the rotator cuff tear. A bursectomy, coracoacromial ligament release, and partial anterolateral acromioplasty were performed.
A transtendinous technique was used to repair this high-grade tear. For an anatomically rigid repair, we used 3 suture anchors with a straight configuration because each metal anchor has only 1 suture. According to the standard arthroscopic transtendinous repair technique, the suture anchors were placed through the rotator cuff tendon (at the lateral articular margin at the medial extent of the footprint) after localization of the angle with a spinal needle. A shuttle relay was used to pass the sutures, and the knot was pulled into the subacromial space, cinching the rotator cuff on top of the suture anchors and reestablishing the contact of the tendon to the footprint. We used two 2.4-mm FASTak suture anchors (Arthrex, Naples, Florida) and one 3.5-mm Corkscrew suture anchor (Arthrex). This process was repeated for the remaining suture limbs. The placement of the suture anchors adequately reduced the articular part of the cuff to the footprint.
After surgery, the patient had no complications, and radiographs taken the next day suggested no abnormalities (Figure 1A). The shoulder was immobilized for 4 weeks after surgery, and passive, gentle ROM exercise was supervised by a physical therapist twice a week during this period. After the first 4 weeks, an active ROM program was begun. However, shortly after initiating motion in the shoulder, the patient complained of a recurrence of pain that she described as a sharp and grinding sensation.
The patient was reevaluated 8 weeks after surgery. Her pain was worsening, and she was having difficulty regaining ROM. Conventional radiographs showed the tip of the metal anchor protruding through the articular cartilage of the humeral head (Figure 1B). The patient was informed of the findings, and immediate surgery was performed to remove the anchor.
Arthroscopic examination showed extensive damage to the glenoid cartilage (Figure 1C) and an intra-articularly intact rotator cuff repair. The cartilage damage was located in the posterior and inferior half of the glenoid, which is related to the forward flexion of the arm; the depth of the cartilage defect was approximately 2 mm. Under the image intensifier, an empty suture anchor driver was inserted into the previous screw insertion hole, and the anchor was screwed back out and removed.
After surgery, the patient’s arm was placed in a sling, and an ROM program began 4 weeks later. The sensation of grinding was eliminated, and her pain gradually improved. Three years after surgery, she had no pain, no weakness, and full ROM without limitations (Figure 2).
Discussion
Protrusion and migration of suture anchors in shoulder surgery has been documented extensively.3,4 Zuckerman and Matsen4 divided these complications into 4 groups: (1) incorrect placement, (2) migration after placement, (3) loosening, and (4) device breakage. These complications may be frequently related to surgical technique, and all these studies describe backward migration of the anchor out of the drill hole. In the current case, the anchor tip penetrated the articular surface of the humeral head, not because of anchor migration but because the anchor was inserted too far. To the authors’ knowledge, there is only 1 reported case of anchor protrusion through the humeral head; it involved a different type of anchor insertion system.5 In that case, there was only mild cartilage damage to the glenoid, and the patient recovered after removal of the anchors.
Several factors contributed to the improper insertion of the anchor in the current patient. First, repairing a high-grade articular side defect or partial articular supraspinatus tendon avulsion lesion can be technically challenging because rotator cuff tissue obscures the view when inserting the anchor. Second, the anchor was inserted too medially on the greater tuberosity, which made the distance from the tuberosity to the joint shorter. Wong and colleagues5 performed an analysis of the angle of insertion that would be safe using a PEEK PushLock SP system (Arthrex), but they emphasized that the angle depends on the configuration of the particular insertion system. The current case also shows that the surgeon should be cognizant of the fact that penetration of the humeral head by the anchor can occur if the surgeon is unaware of the distance from the anchor to the laser line on the insertion device or of the distance from the tuberosity to the articular surface of the humeral head.
The current case also shows that the type of anchor and delivery system may contribute to this complication. Double-loaded suture anchors can decrease the number of anchors needed for secure fixation. Bioabsorbable anchors can be used for this purpose, but they may be technically more difficult to use for repairing partial tears of the rotator cuff. Better visualization of the laser line on the anchor may be facilitated by using a probe from an anterior portal to hold the cuff up while the anchor is inserted.
This case has shown the importance of obtaining postoperative radiographic studies in patients who have metal anchors placed during shoulder surgery, especially if they complain of continued pain, new pain, crepitus, or grinding. When conventional radiography is insufficient for locating the anchor or its proximity to the joint line, computed tomography can be helpful.1
Conclusion
Removing failed suture anchors can be challenging, especially when they protrude into the joint on the humeral side.1,6 The best way to prevent this complication is through careful technique. The anchors should not be inserted beyond the depth of the laser line on the anchors, and every attempt should be made to make sure the laser line is visible at the time of anchor insertion. Postoperative radiographs should be considered for patients with metal anchors in the shoulder, especially if the patient continues to have symptoms or develops new symptoms in the shoulder after surgery.
1. Park HB, Keyurapan E, Gill HS, Selhi HS, McFarland EG. Suture anchors and tacks for shoulder surgery. Part II: The prevention and treatment of complications. Am J Sports Med. 2006;34(1):136-144.
2. McFarland EG, Park HB, Keyurapan E, Gill HS, Selhi HS. Suture anchors and tacks for shoulder surgery. Part I: Biology and biomechanics. Am J Sports Med. 2005;33(12):1918-1923.
3. Rhee YG, Lee DH, Chun IH, Bae SC. Glenohumeral arthropathy after arthroscopic anterior shoulder stabilization. Arthroscopy. 2004;20(4):402-406.
4. Zuckerman JD, Matsen FA III. Complications about the glenohumeral joint related to the use of screws and staples. J Bone Joint Surg Am. 1984;66(2):175-180.
5. Wong AS, Kokkalis ZT, Schmidt CC. Proper insertion angle is essential to prevent intra-articular protrusion of a knotless suture anchor in shoulder rotator cuff repair. Arthroscopy. 2010;26(2):286-290.
6. Grutter PW, McFarland EG, Zikria BA, Dai Z, Petersen SA. Techniques for suture anchor removal in shoulder surgery. Am J Sports Med. 2010;38(8):1706-1710.
1. Park HB, Keyurapan E, Gill HS, Selhi HS, McFarland EG. Suture anchors and tacks for shoulder surgery. Part II: The prevention and treatment of complications. Am J Sports Med. 2006;34(1):136-144.
2. McFarland EG, Park HB, Keyurapan E, Gill HS, Selhi HS. Suture anchors and tacks for shoulder surgery. Part I: Biology and biomechanics. Am J Sports Med. 2005;33(12):1918-1923.
3. Rhee YG, Lee DH, Chun IH, Bae SC. Glenohumeral arthropathy after arthroscopic anterior shoulder stabilization. Arthroscopy. 2004;20(4):402-406.
4. Zuckerman JD, Matsen FA III. Complications about the glenohumeral joint related to the use of screws and staples. J Bone Joint Surg Am. 1984;66(2):175-180.
5. Wong AS, Kokkalis ZT, Schmidt CC. Proper insertion angle is essential to prevent intra-articular protrusion of a knotless suture anchor in shoulder rotator cuff repair. Arthroscopy. 2010;26(2):286-290.
6. Grutter PW, McFarland EG, Zikria BA, Dai Z, Petersen SA. Techniques for suture anchor removal in shoulder surgery. Am J Sports Med. 2010;38(8):1706-1710.
Harrington Rod Revision After Failed Total Hip Arthroplasty Due to Missed Acetabular Metastasis
We report the case of a patient who was treated with total hip arthroplasty (THA) for osteoarthritis but was found to have a large acetabular defect caused by pulmonary metastasis. She was promptly referred to our orthopedic oncology clinic for revision because she had experienced no improvement in her symptoms. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 61-year-old woman was referred to us for evaluation of a large right supra-acetabular lesion after undergoing a right THA at another hospital 3 weeks earlier. Preoperative radiographs showed severe osteoarthritis of the right hip but there was no diagnosis of an acetabular lesion in her medical history. During the operation, the surgeon noted poor acetabulum bone quality and sent acetabular reamings for histopathologic analysis, which revealed adenocarcinoma. The arthroplasty was completed in a normal fashion, and the patient was discharged. Postoperatively, her pain did not resolve, and her functional status deteriorated from ambulating with a walker to very limited activity and weight-bearing.
When the patient came to our clinic, we learned she underwent a lobectomy in 2011 for lung cancer resulting from her 40-pack-year history of smoking and had a strong family history of breast cancer. She also had a history of coronary artery disease, hypertension, hyperlipidemia, morbid obesity, and depression. We obtained plain films and a computed tomography (CT) scan that showed a 6.5×7.1×6.5-cm lytic lesion arising from the right acetabulum with cortical penetration and an extraosseous soft-tissue component. Two smaller 10-mm to 12-mm lesions were also found superior and medial to the large lesion. These radiographs and CT images are shown in Figures 1-3.
We discussed nonoperative and operative options for treatment with the patient and her family, and she elected to undergo palliative surgical curettage and fixation. Significant bone erosion of the acetabulum and a resultant lack of mechanical support for the acetabular cup were found intraoperatively. An unusual surgical approach was selected in order to minimize morbidity and avoid performing a revision acetabular component if the cup was found to be stable from the standpoint of osseointegration. We approached from the superior side of the ilium, removing the abductors in the superperiosteal fashion extending down from the supra-acetabular ilium, sparing the hip capsule. When the acetabular component was exposed and stressed under fluoroscopy, there was no evidence of loosening. We decided to reconstruct the mechanical defect without revision of the acetabular component and to leave the screw in place. After partial excision of the right supra-acetabular ilium, specimens were sent to pathology. We placed five 4.8-mm and four 4.0-mm threaded Steinmann pins intraosseously through the iliac wing to abut the acetabular cup. In this way, the Steinmann pins provided a stable roof to the cup for weight-bearing and scaffolding for methylmethacrylate cement impregnated with tobramycin. A postoperative radiograph of the patient’s pelvis is shown in Figure 4.
Immediately after her surgery, the patient was bearing weight as tolerated and participating in physical therapy 3 times a day. Two months postoperatively, she was able to walk 1 block with use of a walker, and her pain was controlled with oral pain medication. At her 1-year visit, she was walking without pain for prolonged distances. She had a mild limp but did not need ambulatory aids. She had full range of motion, was able to perform all of her desired activities, and was quite pleased with her result. One-year postoperative radiographs (Figure 5) show stable placement of her acetabular cup with her pins and cement in an unchanged position without recurrence of her destructive lesion. There was no evidence of progression of her cancer, although she had some heterotopic bone in her lateral soft tissues.
Discussion
Many cases have been reported in the literature of metastases to the pelvis and acetabulum; almost 10% of bone metastases are in the pelvis.1 Although many are seen on radiographs, pelvic metastases, especially if they involve the acetabulum, can present with hip pain, decreased joint range of motion, and reduced ambulatory function, all symptoms that are similar to osteoarthritis. While the presence of metastases indicates late-stage disease, many patients still live for years with hip symptoms before succumbing to cancer.1 Palliative treatment initially consists of protected weight-bearing, analgesics, antineoplastic medications ,and radiation. When these first-line therapies fail, palliative operative treatment can be considered, with goals to maintain stability and to preserve mobility, independence, and comfort.2 Patients should be offered this only if there is a reasonable chance that structural stability can be achieved via reconstruction and if the patient will live long enough to realize the functional improvement.3 Harrington4 described patterns of acetabular metastases and surgical treatments in his classic series of 58 patients. For class II and III lesions, he concluded it was necessary to provide additional structural support to the acetabular component of a THA, either in the form of a protrusion shell or with Steinmann pins and bone cement.4 Antiprotrusion cages combined with arthroplasty have been used with modest success for cases where implant bone integration is unlikely.5-6 Several studies since Harrington have shown that constructs with cement reinforced with Steinmann pins can provide reduced pain and improved mobility with a low failure rate for the remainder of the patient’s life.7-9
In addition, a few cases have been reported of metastases to endoprostheses, which were implanted long before the diagnosis of cancer.10 To an unsuspecting surgeon, the lytic periprosthetic metastases may look like osteolysis or pseudotumor. Fabbri and colleagues11 presented 4 cases showing how sarcoma around a joint endoprosthesis can easily be mistaken for pseudotumor. A patient considering primary or revision THA for bone loss caused by osteolysis would be given different options than if the bone loss were secondary to metastases. Revision techniques in the setting of acetabular osteolysis include acetabular liner exchanges, cementless hemispherical components and jumbo cups, structural allografts, metal augments, impaction grafting, and acetabular cages and cup-cage constructs. Rarely are “Harrington” reconstructions performed for this reason.12
This case is unusual because the diagnosis of metastatic disease was missed and THA was performed under the presumptive diagnosis of osteoarthritis. While a malignant process was recognized intraoperatively, the joint replacement was completed nonetheless, with revision surgery inevitably occurring within a few weeks. Our patient’s history of lung cancer reinforces the importance of preoperative history taking, and the missed diagnosis highlights the need for clinicians to maintain a broad differential, even in seemingly simple arthritis cases. Proper preoperative imaging, biopsies, and cultures are also paramount. Lesions that are painful, involve the whole cortex, appear soon after implementation, and are rapidly progressing should raise concern for malignancy.10 If there is concern for osteolysis, quantitative CT with 3-dimensional reconstructions can help visualize the lesions and help in planning surgery.13 Had a timely diagnosis been made, the proper reconstruction could have been planned before the index procedure, and our patient could have been spared the pain, risk, and morbidity of a second operation.
The second lesson of this case is that, as long as the cup was stable, the etiology of the hip pain was lack of mechanical support. Once corrected, the total hip functioned as planned. A minimally invasive approach that allowed for observation of the cup without exposing the entire hip saved a patient a significant amount of morbidity and led to an acceptable outcome.
1. Ho L, Ahlmann ER, Menendez LR. Modified Harrington reconstruction for advanced periacetabular metastatic disease. J Surg Oncol. 2010;101(2):170-174.
2. Papagelopoulos PJ, Mavrogenis AF, Soucacos PN. Evaluation and treatment of pelvic metastases. Injury. 2007;38(4):509-520.
3. Allan DG, Bell RS, Davis A, Langer F. Complex acetabular reconstruction for metastatic tumor. J Arthroplasty. 1995;10(3):301-306.
4. Harrington KD. The management of acetabular insufficiency secondary to metastatic malignant disease. J Bone Joint Surg Am. 1981;63(4):653-64.
5. Hoell S, Dedy N, Gosheger G, Dieckmann R, Daniilidis K, Hardes J. The Burch-Schneider cage for reconstruction after metastatic destruction of the acetabulum: outcome and complications. Arch Orthop Trauma Surg. 2012;132(3):405-410.
6. Clayer M. The survivorship of protrusio cages for metastatic disease involving the acetabulum. Clin Orthop. 2010;468(11):2980-2984.
7. Marco RA, Sheth DS, Boland PJ, Wunder JS, Siegel JA, Healey JH. Functional and oncological outcome of acetabular reconstruction for the treatment of metastatic disease. J Bone Joint Surg Am. 2000;82(5):642-651.
8. Tillman RM, Myers GJ, Abudu AT, Carter SR, Grimer RJ. The three-pin modified ‘Harrington’ procedure for advanced metastatic destruction of the acetabulum. J Bone Joint Surg Br. 2008;90(1):84-87.
9. Walker RH. Pelvic reconstruction/total hip arthroplasty for metastatic acetabular insufficiency. Clin Orthop. 1993;294:170-175.
10. Dramis A, Desai AS, Board TN, Hekal WE, Panezai JR. Periprosthetic osteolysis due to metastatic renal cell carcinoma: a case report. Cases J. 2008;1(1):297.
11. Fabbri N, Rustemi E, Masetti C, et al. Severe osteolysis and soft tissue mass around total hip arthroplasty: description of four cases and review of the literature with respect to clinico-radiographic and pathologic differential diagnosis. Eur J Radiol. 2011;77(1):43-50.
12. Deirmengian GK, Zmistowski B, O’Neil JT, Hozack WJ. Management of acetabular bone loss in revision total hip arthroplasty. J Bone Joint Surg Am. 2011;93(19):1842-1852.
13. Kitamura N, Leung SB, Engh CA Sr. Characteristics of pelvic osteolysis on computed tomography after total hip arthroplasty. Clin Orthop. 2005;441:291-297.
We report the case of a patient who was treated with total hip arthroplasty (THA) for osteoarthritis but was found to have a large acetabular defect caused by pulmonary metastasis. She was promptly referred to our orthopedic oncology clinic for revision because she had experienced no improvement in her symptoms. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 61-year-old woman was referred to us for evaluation of a large right supra-acetabular lesion after undergoing a right THA at another hospital 3 weeks earlier. Preoperative radiographs showed severe osteoarthritis of the right hip but there was no diagnosis of an acetabular lesion in her medical history. During the operation, the surgeon noted poor acetabulum bone quality and sent acetabular reamings for histopathologic analysis, which revealed adenocarcinoma. The arthroplasty was completed in a normal fashion, and the patient was discharged. Postoperatively, her pain did not resolve, and her functional status deteriorated from ambulating with a walker to very limited activity and weight-bearing.
When the patient came to our clinic, we learned she underwent a lobectomy in 2011 for lung cancer resulting from her 40-pack-year history of smoking and had a strong family history of breast cancer. She also had a history of coronary artery disease, hypertension, hyperlipidemia, morbid obesity, and depression. We obtained plain films and a computed tomography (CT) scan that showed a 6.5×7.1×6.5-cm lytic lesion arising from the right acetabulum with cortical penetration and an extraosseous soft-tissue component. Two smaller 10-mm to 12-mm lesions were also found superior and medial to the large lesion. These radiographs and CT images are shown in Figures 1-3.
We discussed nonoperative and operative options for treatment with the patient and her family, and she elected to undergo palliative surgical curettage and fixation. Significant bone erosion of the acetabulum and a resultant lack of mechanical support for the acetabular cup were found intraoperatively. An unusual surgical approach was selected in order to minimize morbidity and avoid performing a revision acetabular component if the cup was found to be stable from the standpoint of osseointegration. We approached from the superior side of the ilium, removing the abductors in the superperiosteal fashion extending down from the supra-acetabular ilium, sparing the hip capsule. When the acetabular component was exposed and stressed under fluoroscopy, there was no evidence of loosening. We decided to reconstruct the mechanical defect without revision of the acetabular component and to leave the screw in place. After partial excision of the right supra-acetabular ilium, specimens were sent to pathology. We placed five 4.8-mm and four 4.0-mm threaded Steinmann pins intraosseously through the iliac wing to abut the acetabular cup. In this way, the Steinmann pins provided a stable roof to the cup for weight-bearing and scaffolding for methylmethacrylate cement impregnated with tobramycin. A postoperative radiograph of the patient’s pelvis is shown in Figure 4.
Immediately after her surgery, the patient was bearing weight as tolerated and participating in physical therapy 3 times a day. Two months postoperatively, she was able to walk 1 block with use of a walker, and her pain was controlled with oral pain medication. At her 1-year visit, she was walking without pain for prolonged distances. She had a mild limp but did not need ambulatory aids. She had full range of motion, was able to perform all of her desired activities, and was quite pleased with her result. One-year postoperative radiographs (Figure 5) show stable placement of her acetabular cup with her pins and cement in an unchanged position without recurrence of her destructive lesion. There was no evidence of progression of her cancer, although she had some heterotopic bone in her lateral soft tissues.
Discussion
Many cases have been reported in the literature of metastases to the pelvis and acetabulum; almost 10% of bone metastases are in the pelvis.1 Although many are seen on radiographs, pelvic metastases, especially if they involve the acetabulum, can present with hip pain, decreased joint range of motion, and reduced ambulatory function, all symptoms that are similar to osteoarthritis. While the presence of metastases indicates late-stage disease, many patients still live for years with hip symptoms before succumbing to cancer.1 Palliative treatment initially consists of protected weight-bearing, analgesics, antineoplastic medications ,and radiation. When these first-line therapies fail, palliative operative treatment can be considered, with goals to maintain stability and to preserve mobility, independence, and comfort.2 Patients should be offered this only if there is a reasonable chance that structural stability can be achieved via reconstruction and if the patient will live long enough to realize the functional improvement.3 Harrington4 described patterns of acetabular metastases and surgical treatments in his classic series of 58 patients. For class II and III lesions, he concluded it was necessary to provide additional structural support to the acetabular component of a THA, either in the form of a protrusion shell or with Steinmann pins and bone cement.4 Antiprotrusion cages combined with arthroplasty have been used with modest success for cases where implant bone integration is unlikely.5-6 Several studies since Harrington have shown that constructs with cement reinforced with Steinmann pins can provide reduced pain and improved mobility with a low failure rate for the remainder of the patient’s life.7-9
In addition, a few cases have been reported of metastases to endoprostheses, which were implanted long before the diagnosis of cancer.10 To an unsuspecting surgeon, the lytic periprosthetic metastases may look like osteolysis or pseudotumor. Fabbri and colleagues11 presented 4 cases showing how sarcoma around a joint endoprosthesis can easily be mistaken for pseudotumor. A patient considering primary or revision THA for bone loss caused by osteolysis would be given different options than if the bone loss were secondary to metastases. Revision techniques in the setting of acetabular osteolysis include acetabular liner exchanges, cementless hemispherical components and jumbo cups, structural allografts, metal augments, impaction grafting, and acetabular cages and cup-cage constructs. Rarely are “Harrington” reconstructions performed for this reason.12
This case is unusual because the diagnosis of metastatic disease was missed and THA was performed under the presumptive diagnosis of osteoarthritis. While a malignant process was recognized intraoperatively, the joint replacement was completed nonetheless, with revision surgery inevitably occurring within a few weeks. Our patient’s history of lung cancer reinforces the importance of preoperative history taking, and the missed diagnosis highlights the need for clinicians to maintain a broad differential, even in seemingly simple arthritis cases. Proper preoperative imaging, biopsies, and cultures are also paramount. Lesions that are painful, involve the whole cortex, appear soon after implementation, and are rapidly progressing should raise concern for malignancy.10 If there is concern for osteolysis, quantitative CT with 3-dimensional reconstructions can help visualize the lesions and help in planning surgery.13 Had a timely diagnosis been made, the proper reconstruction could have been planned before the index procedure, and our patient could have been spared the pain, risk, and morbidity of a second operation.
The second lesson of this case is that, as long as the cup was stable, the etiology of the hip pain was lack of mechanical support. Once corrected, the total hip functioned as planned. A minimally invasive approach that allowed for observation of the cup without exposing the entire hip saved a patient a significant amount of morbidity and led to an acceptable outcome.
We report the case of a patient who was treated with total hip arthroplasty (THA) for osteoarthritis but was found to have a large acetabular defect caused by pulmonary metastasis. She was promptly referred to our orthopedic oncology clinic for revision because she had experienced no improvement in her symptoms. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 61-year-old woman was referred to us for evaluation of a large right supra-acetabular lesion after undergoing a right THA at another hospital 3 weeks earlier. Preoperative radiographs showed severe osteoarthritis of the right hip but there was no diagnosis of an acetabular lesion in her medical history. During the operation, the surgeon noted poor acetabulum bone quality and sent acetabular reamings for histopathologic analysis, which revealed adenocarcinoma. The arthroplasty was completed in a normal fashion, and the patient was discharged. Postoperatively, her pain did not resolve, and her functional status deteriorated from ambulating with a walker to very limited activity and weight-bearing.
When the patient came to our clinic, we learned she underwent a lobectomy in 2011 for lung cancer resulting from her 40-pack-year history of smoking and had a strong family history of breast cancer. She also had a history of coronary artery disease, hypertension, hyperlipidemia, morbid obesity, and depression. We obtained plain films and a computed tomography (CT) scan that showed a 6.5×7.1×6.5-cm lytic lesion arising from the right acetabulum with cortical penetration and an extraosseous soft-tissue component. Two smaller 10-mm to 12-mm lesions were also found superior and medial to the large lesion. These radiographs and CT images are shown in Figures 1-3.
We discussed nonoperative and operative options for treatment with the patient and her family, and she elected to undergo palliative surgical curettage and fixation. Significant bone erosion of the acetabulum and a resultant lack of mechanical support for the acetabular cup were found intraoperatively. An unusual surgical approach was selected in order to minimize morbidity and avoid performing a revision acetabular component if the cup was found to be stable from the standpoint of osseointegration. We approached from the superior side of the ilium, removing the abductors in the superperiosteal fashion extending down from the supra-acetabular ilium, sparing the hip capsule. When the acetabular component was exposed and stressed under fluoroscopy, there was no evidence of loosening. We decided to reconstruct the mechanical defect without revision of the acetabular component and to leave the screw in place. After partial excision of the right supra-acetabular ilium, specimens were sent to pathology. We placed five 4.8-mm and four 4.0-mm threaded Steinmann pins intraosseously through the iliac wing to abut the acetabular cup. In this way, the Steinmann pins provided a stable roof to the cup for weight-bearing and scaffolding for methylmethacrylate cement impregnated with tobramycin. A postoperative radiograph of the patient’s pelvis is shown in Figure 4.
Immediately after her surgery, the patient was bearing weight as tolerated and participating in physical therapy 3 times a day. Two months postoperatively, she was able to walk 1 block with use of a walker, and her pain was controlled with oral pain medication. At her 1-year visit, she was walking without pain for prolonged distances. She had a mild limp but did not need ambulatory aids. She had full range of motion, was able to perform all of her desired activities, and was quite pleased with her result. One-year postoperative radiographs (Figure 5) show stable placement of her acetabular cup with her pins and cement in an unchanged position without recurrence of her destructive lesion. There was no evidence of progression of her cancer, although she had some heterotopic bone in her lateral soft tissues.
Discussion
Many cases have been reported in the literature of metastases to the pelvis and acetabulum; almost 10% of bone metastases are in the pelvis.1 Although many are seen on radiographs, pelvic metastases, especially if they involve the acetabulum, can present with hip pain, decreased joint range of motion, and reduced ambulatory function, all symptoms that are similar to osteoarthritis. While the presence of metastases indicates late-stage disease, many patients still live for years with hip symptoms before succumbing to cancer.1 Palliative treatment initially consists of protected weight-bearing, analgesics, antineoplastic medications ,and radiation. When these first-line therapies fail, palliative operative treatment can be considered, with goals to maintain stability and to preserve mobility, independence, and comfort.2 Patients should be offered this only if there is a reasonable chance that structural stability can be achieved via reconstruction and if the patient will live long enough to realize the functional improvement.3 Harrington4 described patterns of acetabular metastases and surgical treatments in his classic series of 58 patients. For class II and III lesions, he concluded it was necessary to provide additional structural support to the acetabular component of a THA, either in the form of a protrusion shell or with Steinmann pins and bone cement.4 Antiprotrusion cages combined with arthroplasty have been used with modest success for cases where implant bone integration is unlikely.5-6 Several studies since Harrington have shown that constructs with cement reinforced with Steinmann pins can provide reduced pain and improved mobility with a low failure rate for the remainder of the patient’s life.7-9
In addition, a few cases have been reported of metastases to endoprostheses, which were implanted long before the diagnosis of cancer.10 To an unsuspecting surgeon, the lytic periprosthetic metastases may look like osteolysis or pseudotumor. Fabbri and colleagues11 presented 4 cases showing how sarcoma around a joint endoprosthesis can easily be mistaken for pseudotumor. A patient considering primary or revision THA for bone loss caused by osteolysis would be given different options than if the bone loss were secondary to metastases. Revision techniques in the setting of acetabular osteolysis include acetabular liner exchanges, cementless hemispherical components and jumbo cups, structural allografts, metal augments, impaction grafting, and acetabular cages and cup-cage constructs. Rarely are “Harrington” reconstructions performed for this reason.12
This case is unusual because the diagnosis of metastatic disease was missed and THA was performed under the presumptive diagnosis of osteoarthritis. While a malignant process was recognized intraoperatively, the joint replacement was completed nonetheless, with revision surgery inevitably occurring within a few weeks. Our patient’s history of lung cancer reinforces the importance of preoperative history taking, and the missed diagnosis highlights the need for clinicians to maintain a broad differential, even in seemingly simple arthritis cases. Proper preoperative imaging, biopsies, and cultures are also paramount. Lesions that are painful, involve the whole cortex, appear soon after implementation, and are rapidly progressing should raise concern for malignancy.10 If there is concern for osteolysis, quantitative CT with 3-dimensional reconstructions can help visualize the lesions and help in planning surgery.13 Had a timely diagnosis been made, the proper reconstruction could have been planned before the index procedure, and our patient could have been spared the pain, risk, and morbidity of a second operation.
The second lesson of this case is that, as long as the cup was stable, the etiology of the hip pain was lack of mechanical support. Once corrected, the total hip functioned as planned. A minimally invasive approach that allowed for observation of the cup without exposing the entire hip saved a patient a significant amount of morbidity and led to an acceptable outcome.
1. Ho L, Ahlmann ER, Menendez LR. Modified Harrington reconstruction for advanced periacetabular metastatic disease. J Surg Oncol. 2010;101(2):170-174.
2. Papagelopoulos PJ, Mavrogenis AF, Soucacos PN. Evaluation and treatment of pelvic metastases. Injury. 2007;38(4):509-520.
3. Allan DG, Bell RS, Davis A, Langer F. Complex acetabular reconstruction for metastatic tumor. J Arthroplasty. 1995;10(3):301-306.
4. Harrington KD. The management of acetabular insufficiency secondary to metastatic malignant disease. J Bone Joint Surg Am. 1981;63(4):653-64.
5. Hoell S, Dedy N, Gosheger G, Dieckmann R, Daniilidis K, Hardes J. The Burch-Schneider cage for reconstruction after metastatic destruction of the acetabulum: outcome and complications. Arch Orthop Trauma Surg. 2012;132(3):405-410.
6. Clayer M. The survivorship of protrusio cages for metastatic disease involving the acetabulum. Clin Orthop. 2010;468(11):2980-2984.
7. Marco RA, Sheth DS, Boland PJ, Wunder JS, Siegel JA, Healey JH. Functional and oncological outcome of acetabular reconstruction for the treatment of metastatic disease. J Bone Joint Surg Am. 2000;82(5):642-651.
8. Tillman RM, Myers GJ, Abudu AT, Carter SR, Grimer RJ. The three-pin modified ‘Harrington’ procedure for advanced metastatic destruction of the acetabulum. J Bone Joint Surg Br. 2008;90(1):84-87.
9. Walker RH. Pelvic reconstruction/total hip arthroplasty for metastatic acetabular insufficiency. Clin Orthop. 1993;294:170-175.
10. Dramis A, Desai AS, Board TN, Hekal WE, Panezai JR. Periprosthetic osteolysis due to metastatic renal cell carcinoma: a case report. Cases J. 2008;1(1):297.
11. Fabbri N, Rustemi E, Masetti C, et al. Severe osteolysis and soft tissue mass around total hip arthroplasty: description of four cases and review of the literature with respect to clinico-radiographic and pathologic differential diagnosis. Eur J Radiol. 2011;77(1):43-50.
12. Deirmengian GK, Zmistowski B, O’Neil JT, Hozack WJ. Management of acetabular bone loss in revision total hip arthroplasty. J Bone Joint Surg Am. 2011;93(19):1842-1852.
13. Kitamura N, Leung SB, Engh CA Sr. Characteristics of pelvic osteolysis on computed tomography after total hip arthroplasty. Clin Orthop. 2005;441:291-297.
1. Ho L, Ahlmann ER, Menendez LR. Modified Harrington reconstruction for advanced periacetabular metastatic disease. J Surg Oncol. 2010;101(2):170-174.
2. Papagelopoulos PJ, Mavrogenis AF, Soucacos PN. Evaluation and treatment of pelvic metastases. Injury. 2007;38(4):509-520.
3. Allan DG, Bell RS, Davis A, Langer F. Complex acetabular reconstruction for metastatic tumor. J Arthroplasty. 1995;10(3):301-306.
4. Harrington KD. The management of acetabular insufficiency secondary to metastatic malignant disease. J Bone Joint Surg Am. 1981;63(4):653-64.
5. Hoell S, Dedy N, Gosheger G, Dieckmann R, Daniilidis K, Hardes J. The Burch-Schneider cage for reconstruction after metastatic destruction of the acetabulum: outcome and complications. Arch Orthop Trauma Surg. 2012;132(3):405-410.
6. Clayer M. The survivorship of protrusio cages for metastatic disease involving the acetabulum. Clin Orthop. 2010;468(11):2980-2984.
7. Marco RA, Sheth DS, Boland PJ, Wunder JS, Siegel JA, Healey JH. Functional and oncological outcome of acetabular reconstruction for the treatment of metastatic disease. J Bone Joint Surg Am. 2000;82(5):642-651.
8. Tillman RM, Myers GJ, Abudu AT, Carter SR, Grimer RJ. The three-pin modified ‘Harrington’ procedure for advanced metastatic destruction of the acetabulum. J Bone Joint Surg Br. 2008;90(1):84-87.
9. Walker RH. Pelvic reconstruction/total hip arthroplasty for metastatic acetabular insufficiency. Clin Orthop. 1993;294:170-175.
10. Dramis A, Desai AS, Board TN, Hekal WE, Panezai JR. Periprosthetic osteolysis due to metastatic renal cell carcinoma: a case report. Cases J. 2008;1(1):297.
11. Fabbri N, Rustemi E, Masetti C, et al. Severe osteolysis and soft tissue mass around total hip arthroplasty: description of four cases and review of the literature with respect to clinico-radiographic and pathologic differential diagnosis. Eur J Radiol. 2011;77(1):43-50.
12. Deirmengian GK, Zmistowski B, O’Neil JT, Hozack WJ. Management of acetabular bone loss in revision total hip arthroplasty. J Bone Joint Surg Am. 2011;93(19):1842-1852.
13. Kitamura N, Leung SB, Engh CA Sr. Characteristics of pelvic osteolysis on computed tomography after total hip arthroplasty. Clin Orthop. 2005;441:291-297.
Atypical Presentation of Fat Embolism Syndrome After Gunshot Wound to the Foot
Fat embolism syndrome (FES) is a rare complication reported primarily after long bone fractures, with an incidence of 0.3% to 2.2%.1-3 It is most commonly caused by trauma and is thought to result from movement of bone fragments or to occur during intramedullary reaming.1 Both of these factors lead to a distortion of the bone marrow cavity, allowing marrow and fat to enter the circulatory system.1
Although the true pathophysiology remains poorly understood, it is possible that, once in systemic circulation, the fat particles become lodged in the vascular system, inciting an inflammatory response, leading to organ dysfunction via mechanical or biochemical processes.4 Typically, the diagnosis is made after clinical features are observed, including hypoxemia, petechial rash, and cerebral signs not related to a head injury or other conditions.5,6
Although FES is an uncommon complication after traumatic injuries, mortality after FES in a recent study was reported to be 10%.1 FES is most commonly seen after fractures of the femur and tibia, although cases have been described involving fractures of the radius, ulna, and humerus.1,3 We present an atypical case of cerebral FES after multiple fractures of the foot; to our knowledge, such a case has not been reported in the English-language literature. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 42-year-old man was hunting with his son when he was accidentally shot in the left foot with a .270-caliber rifle bullet at close range. The patient sought care at a local hospital and, in the ensuing 3 hours, his mentation appeared normal. He reported pain and numbness distal to the injury in the tibial nerve distribution, but he remained vascularly intact, alert, and oriented. He was given 7 mg of hydromorphone hydrochloride over 2 hours for pain control and was transferred to our hospital via ambulance approximately 6 hours after injury. Upon arrival, he was noted to be extremely sedated and obtunded, responding only to pain with spontaneous eye opening. He was unable to follow commands. He was given
1.2 mg of naloxone intravenously to reverse what was presumed to be acute opioid intoxication; however, his mental status did not improve.
On examination, the patient was noted to have a small entrance wound through the Achilles tendon (Figures 1A, 1B) and an exit wound on the plantar aspect of the foot near the heads of the first and second metatarsals (Figures 1C, 1D) with minimal bleeding and no gross contamination. There was significant edema on the medial and proximal aspects of the left foot, 3+ dorsalis pedis pulse, and a capillary refill of 4 seconds. Radiographs showed traumatic fracture deformities of the calcaneus, navicular, medial cuneiform, and first and second metatarsal bases, as well as an intra-articular fracture deformity of the left talus extending to the talar dome (Figures 2A-2C). Neurologic examination could not be reliably obtained because of the patient’s mental status. He was determined to be unstable for immediate surgery, and his left leg was splinted pending neurologic evaluation.
The patient’s oxygen saturation was 94%, and his temperature was 38.2°C (100.76°F). Although his heart rate was in the 90s upon arrival, he became tachycardic over the next 4 hours, with heart rate ranging from the 110s to 130s; he remained tachycardic for approximately 72 hours. Laboratory values upon arrival showed a hemoglobin value of 12.8 g/dL and platelets of 249,000/μL. He developed anemia and thrombocytopenia within 72 hours of the injury, with a low of 6.6 g/dL and 88,000/μL, respectively, by postinjury day 4. Computed tomography of the head, electroencephalography, urine drug screen, and lumbar puncture were unremarkable. The patient never became hypoxemic. Within 14 hours after injury, he was completely comatose with extensor posturing. In the intensive care unit (ICU), the patient was intubated for airway protection.
The next day, the patient underwent magnetic resonance imaging (MRI) of the brain, which showed innumerable tiny infarcts throughout cerebral hemispheres, cerebellum, and brainstem in a characteristic “starfield” pattern on T2-weighted images (Figure 3). This was radiographically consistent with fat emboli related to the left lower extremity gunshot wound. An echocardiogram showed small right-to-left shunt and a possible intrapulmonary shunt, although this was never confirmed. The echocardiogram was technically challenging secondary to his persistent tachycardia. He also developed a subtle petechial rash (Figure 4A).
The patient underwent percutaneous gastrostomy-tube placement for nutrition on postinjury day 4 and remained intubated, unable to protect his airway, and nonresponsive with extensor posturing (Figure 4B). He was also taken to the operating room for spanning external fixator placement on postinjury day 3 to restore calcaneal height and length as well as foot stability (Figures 5A, 5B).
The patient was treated with supportive care and was discharged from the hospital in a comatose state on hospital day 17 to a rehabilitation facility. He began to emerge from the coma 6 weeks after injury, and his external fixator was removed and a cast applied to his lower extremity. His entrance and exit wounds healed as expected. Initial agitation was treated with propranolol and quetiapine. Because he continued to have difficulty with spasticity and increased tone, he was given botulinum toxin type A injections in the pectoral muscles, biceps, and forearms. He made continued and rapid improvement in response to intensive multidisciplinary therapy and returned home 4½ months after injury. Eight months after the injury, he is now walking independently with a cane and independent with his activities of daily living. Unfortunately, he has substantial pain in his foot, which appears to be a combination of both neuropathic and posttraumatic arthrosis causes. He is undergoing consultation for a possible amputation. Radiographs show consolidation of the hind and midfoot fractures with retained bullet fragments (Figures 6A-6C). He continues to receive multidisciplinary care to address cognitive limitations and is making progress.
Discussion
FES is a life-threatening disease affecting multiple organ systems.7 Classically, the pulmonary, central nervous, and dermatologic systems are affected.5,6,8 While FES is most recognizable after long bone fractures and orthopedic procedures involving the intramedullary canal, to our knowledge, FES after gunshot wound and concomitant fractures of the foot has never been reported.
The syndrome is defined by major and minor criteria as outlined by Gurd.5 Major criteria include hypoxia, deteriorating mental status, and petechiae. This case represents a somewhat atypical presentation of FES, because dermatologic manifestations and pulmonary compromise were subtle. The minor criteria consisting of tachycardia, fever, anemia, and thrombocytopenia were present in our patient, although at different phases during the progression of the syndrome. This emphasizes the difficulty in diagnosing FES because the symptoms do not occur simultaneously.
In the classic syndrome, after an initial asymptomatic interval of 12 to 72 hours, pulmonary, neurologic, and/or dermatologic changes usually ensue.9 Altered mental status, including headache, confusion, stupor, coma, rigidity, or convulsions, has been documented in 86% of patients.10 In our case, the neurologic symptoms presented earlier, at around 6 hours after injury, and respiratory symptoms, including hypoxia, tachypnea, and dyspnea, reported in 75% of cases,2,11 did not occur at all. In fact, continued intubation was only required in this case for neuromuscular airway protection. Classic dermatologic manifestations, a reddish-brown nonpalpable petechial rash diffusely covering the upper torso and extremities, normally appears within 12 to 36 hours.12,13 Nevertheless, in our case, these findings were subtle compared with others previously reported.14,15 In fact, despite being seen by numerous physicians, including neurologists and ICU intensivists, only the orthopedists’ notes made reference to this modest finding (Figure 4A).
Further complicating the diagnosis is that, during the onset of symptoms, patients are typically victims of polytrauma and/or routinely given narcotics to help with significant pain. Therefore, it is appropriate to rule out opioid overdose and other metabolic sources of mental-status change. This can be done fairly expeditiously with laboratory testing and narcotic reversal. After these have been eliminated, FES should be considered in a patient with rapid neurologic deterioration, because a delay in treatment can affect outcomes.2,4,16
Because continuous showering of emboli to the brain and other organs occurs without fracture stabilization, rapid diagnosis with high clinical suspicion of FES is essential and can be aided immensely with MRI. In fact, MRI is the most sensitive test for this diagnosis and correlates with clinical severity of brain injury.17 T2-weighted images show regions of high-signal intensity and “starfield” pattern, which are sensitive markers for FES (Figure 3).18 These tests can be done concomitantly with a well-splinted extremity, and definitive stabilization should be carried out promptly because early splinting and fixation of orthopedic fractures improves outcomes.17
Perhaps the most important reason to make an expeditious diagnosis is to help counsel families, who are undoubtedly in shock and disbelief. Recovery times can vary widely, with the patient often continuing to regain cognitive and motor function over the course of months to years.2 Without knowledge of signs of improvement in neurologic outcome, families cannot be accurately counseled regarding potential for recovery. The practicing orthopedist should be aware of this disorder, because initial neurologic deterioration may seem hopeless. Furthermore, supportive care should be initiated early with multidisciplinary teams and extensive rehabilitation because these offer the best outcomes in patients with FES.4,18 Although our patient continues to have cognitive impairment, his recovery in the preceding 8 months has been aided by rapid diagnosis and multidisciplinary care and should offer hope to other patients faced with this situation.
1. Akhtar S. Fat embolism. Anesthesiol Clin. 2009;27(3):533-550.
2. Müller C, Rahn BA, Pfister U, Meinig RP. The incidence, pathogenesis, diagnosis, and treatment of fat embolism. Orthop Rev. 1994;23(2):107-117.
3. Stein PD, Yaekoub AY, Matta F, Kleerekoper M. Fat embolism syndrome. Am J Med Sci. 2008;336(6):472-477.
4. Parisi DM, Koval K, Egol K. Fat embolism syndrome. Am J Orthop. 2002;31(9):507-512.
5. Gurd AR. Fat embolism: an aid to diagnosis. J Bone Joint Surb Br. 1970;52(4):732-737.
6. Lee SC, Yoon JY, Nam CH, Kim TK, Jung KA, Lee DW. Cerebral fat embolism syndrome after simultaneous bilateral total knee arthroplasty: a case series. J Arthroplasty. 2012;27(3):409-414.
7. Gurd AR, Wilson RI. Fat-embolism syndrome. Lancet. 1972;2(7770):
231-232.
8. Habashi NM, Andrews PL, Scalea TM. Therapeutic aspects of fat embolism syndrome. Injury. 2006;37(Suppl 4):S68-S73.
9. Weiss W, Bardana D, Yen D. Delayed presentation of fat embolism syndrome after intramedullary nailing of a fractured femur: a case report. J Trauma. 2009;66(3):E42-E45.
10. Byrick RJ. Fat embolism and postoperative coagulopathy. Can J Anaesth. 2001;48(7):618-621.
11. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
12. Burgher LW. Fat embolism syndrome. Chest. 1981;79(2):131-132.
13. Burgher LW, Dines DE, Linscheid RL, Didier EP. Fat embolism and the adult respiratory distress syndrome. Mayo Clin Proc. 1974;49(2):107-109.
14. Liu DD, Hsieh NK, Chen HI. Histopathological and biochemical changes following fat embolism with administration of corn oil micelles: a new animal model for fat embolism syndrome. J Bone Joint Surg Br. 2008;90(11):
1517-1521.
15. Liu HK, Chen WC. Images in clinical medicine. Fat embolism syndrome. N Engl J Med. 2011;364(18):1761.
16. Pinney SJ, Keating JF, Meek RN. Fat embolism syndrome in isolated femoral fractures: does timing of nailing influence incidence? Injury. 1998;29(2):
131-133.
17. Takahashi M, Suzuki R, Osakabe Y, et al. Magnetic resonance imaging findings in cerebral fat embolism: correlation with clinical manifestations. J Trauma. 1999;46(2):324-327.
18. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
Fat embolism syndrome (FES) is a rare complication reported primarily after long bone fractures, with an incidence of 0.3% to 2.2%.1-3 It is most commonly caused by trauma and is thought to result from movement of bone fragments or to occur during intramedullary reaming.1 Both of these factors lead to a distortion of the bone marrow cavity, allowing marrow and fat to enter the circulatory system.1
Although the true pathophysiology remains poorly understood, it is possible that, once in systemic circulation, the fat particles become lodged in the vascular system, inciting an inflammatory response, leading to organ dysfunction via mechanical or biochemical processes.4 Typically, the diagnosis is made after clinical features are observed, including hypoxemia, petechial rash, and cerebral signs not related to a head injury or other conditions.5,6
Although FES is an uncommon complication after traumatic injuries, mortality after FES in a recent study was reported to be 10%.1 FES is most commonly seen after fractures of the femur and tibia, although cases have been described involving fractures of the radius, ulna, and humerus.1,3 We present an atypical case of cerebral FES after multiple fractures of the foot; to our knowledge, such a case has not been reported in the English-language literature. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 42-year-old man was hunting with his son when he was accidentally shot in the left foot with a .270-caliber rifle bullet at close range. The patient sought care at a local hospital and, in the ensuing 3 hours, his mentation appeared normal. He reported pain and numbness distal to the injury in the tibial nerve distribution, but he remained vascularly intact, alert, and oriented. He was given 7 mg of hydromorphone hydrochloride over 2 hours for pain control and was transferred to our hospital via ambulance approximately 6 hours after injury. Upon arrival, he was noted to be extremely sedated and obtunded, responding only to pain with spontaneous eye opening. He was unable to follow commands. He was given
1.2 mg of naloxone intravenously to reverse what was presumed to be acute opioid intoxication; however, his mental status did not improve.
On examination, the patient was noted to have a small entrance wound through the Achilles tendon (Figures 1A, 1B) and an exit wound on the plantar aspect of the foot near the heads of the first and second metatarsals (Figures 1C, 1D) with minimal bleeding and no gross contamination. There was significant edema on the medial and proximal aspects of the left foot, 3+ dorsalis pedis pulse, and a capillary refill of 4 seconds. Radiographs showed traumatic fracture deformities of the calcaneus, navicular, medial cuneiform, and first and second metatarsal bases, as well as an intra-articular fracture deformity of the left talus extending to the talar dome (Figures 2A-2C). Neurologic examination could not be reliably obtained because of the patient’s mental status. He was determined to be unstable for immediate surgery, and his left leg was splinted pending neurologic evaluation.
The patient’s oxygen saturation was 94%, and his temperature was 38.2°C (100.76°F). Although his heart rate was in the 90s upon arrival, he became tachycardic over the next 4 hours, with heart rate ranging from the 110s to 130s; he remained tachycardic for approximately 72 hours. Laboratory values upon arrival showed a hemoglobin value of 12.8 g/dL and platelets of 249,000/μL. He developed anemia and thrombocytopenia within 72 hours of the injury, with a low of 6.6 g/dL and 88,000/μL, respectively, by postinjury day 4. Computed tomography of the head, electroencephalography, urine drug screen, and lumbar puncture were unremarkable. The patient never became hypoxemic. Within 14 hours after injury, he was completely comatose with extensor posturing. In the intensive care unit (ICU), the patient was intubated for airway protection.
The next day, the patient underwent magnetic resonance imaging (MRI) of the brain, which showed innumerable tiny infarcts throughout cerebral hemispheres, cerebellum, and brainstem in a characteristic “starfield” pattern on T2-weighted images (Figure 3). This was radiographically consistent with fat emboli related to the left lower extremity gunshot wound. An echocardiogram showed small right-to-left shunt and a possible intrapulmonary shunt, although this was never confirmed. The echocardiogram was technically challenging secondary to his persistent tachycardia. He also developed a subtle petechial rash (Figure 4A).
The patient underwent percutaneous gastrostomy-tube placement for nutrition on postinjury day 4 and remained intubated, unable to protect his airway, and nonresponsive with extensor posturing (Figure 4B). He was also taken to the operating room for spanning external fixator placement on postinjury day 3 to restore calcaneal height and length as well as foot stability (Figures 5A, 5B).
The patient was treated with supportive care and was discharged from the hospital in a comatose state on hospital day 17 to a rehabilitation facility. He began to emerge from the coma 6 weeks after injury, and his external fixator was removed and a cast applied to his lower extremity. His entrance and exit wounds healed as expected. Initial agitation was treated with propranolol and quetiapine. Because he continued to have difficulty with spasticity and increased tone, he was given botulinum toxin type A injections in the pectoral muscles, biceps, and forearms. He made continued and rapid improvement in response to intensive multidisciplinary therapy and returned home 4½ months after injury. Eight months after the injury, he is now walking independently with a cane and independent with his activities of daily living. Unfortunately, he has substantial pain in his foot, which appears to be a combination of both neuropathic and posttraumatic arthrosis causes. He is undergoing consultation for a possible amputation. Radiographs show consolidation of the hind and midfoot fractures with retained bullet fragments (Figures 6A-6C). He continues to receive multidisciplinary care to address cognitive limitations and is making progress.
Discussion
FES is a life-threatening disease affecting multiple organ systems.7 Classically, the pulmonary, central nervous, and dermatologic systems are affected.5,6,8 While FES is most recognizable after long bone fractures and orthopedic procedures involving the intramedullary canal, to our knowledge, FES after gunshot wound and concomitant fractures of the foot has never been reported.
The syndrome is defined by major and minor criteria as outlined by Gurd.5 Major criteria include hypoxia, deteriorating mental status, and petechiae. This case represents a somewhat atypical presentation of FES, because dermatologic manifestations and pulmonary compromise were subtle. The minor criteria consisting of tachycardia, fever, anemia, and thrombocytopenia were present in our patient, although at different phases during the progression of the syndrome. This emphasizes the difficulty in diagnosing FES because the symptoms do not occur simultaneously.
In the classic syndrome, after an initial asymptomatic interval of 12 to 72 hours, pulmonary, neurologic, and/or dermatologic changes usually ensue.9 Altered mental status, including headache, confusion, stupor, coma, rigidity, or convulsions, has been documented in 86% of patients.10 In our case, the neurologic symptoms presented earlier, at around 6 hours after injury, and respiratory symptoms, including hypoxia, tachypnea, and dyspnea, reported in 75% of cases,2,11 did not occur at all. In fact, continued intubation was only required in this case for neuromuscular airway protection. Classic dermatologic manifestations, a reddish-brown nonpalpable petechial rash diffusely covering the upper torso and extremities, normally appears within 12 to 36 hours.12,13 Nevertheless, in our case, these findings were subtle compared with others previously reported.14,15 In fact, despite being seen by numerous physicians, including neurologists and ICU intensivists, only the orthopedists’ notes made reference to this modest finding (Figure 4A).
Further complicating the diagnosis is that, during the onset of symptoms, patients are typically victims of polytrauma and/or routinely given narcotics to help with significant pain. Therefore, it is appropriate to rule out opioid overdose and other metabolic sources of mental-status change. This can be done fairly expeditiously with laboratory testing and narcotic reversal. After these have been eliminated, FES should be considered in a patient with rapid neurologic deterioration, because a delay in treatment can affect outcomes.2,4,16
Because continuous showering of emboli to the brain and other organs occurs without fracture stabilization, rapid diagnosis with high clinical suspicion of FES is essential and can be aided immensely with MRI. In fact, MRI is the most sensitive test for this diagnosis and correlates with clinical severity of brain injury.17 T2-weighted images show regions of high-signal intensity and “starfield” pattern, which are sensitive markers for FES (Figure 3).18 These tests can be done concomitantly with a well-splinted extremity, and definitive stabilization should be carried out promptly because early splinting and fixation of orthopedic fractures improves outcomes.17
Perhaps the most important reason to make an expeditious diagnosis is to help counsel families, who are undoubtedly in shock and disbelief. Recovery times can vary widely, with the patient often continuing to regain cognitive and motor function over the course of months to years.2 Without knowledge of signs of improvement in neurologic outcome, families cannot be accurately counseled regarding potential for recovery. The practicing orthopedist should be aware of this disorder, because initial neurologic deterioration may seem hopeless. Furthermore, supportive care should be initiated early with multidisciplinary teams and extensive rehabilitation because these offer the best outcomes in patients with FES.4,18 Although our patient continues to have cognitive impairment, his recovery in the preceding 8 months has been aided by rapid diagnosis and multidisciplinary care and should offer hope to other patients faced with this situation.
Fat embolism syndrome (FES) is a rare complication reported primarily after long bone fractures, with an incidence of 0.3% to 2.2%.1-3 It is most commonly caused by trauma and is thought to result from movement of bone fragments or to occur during intramedullary reaming.1 Both of these factors lead to a distortion of the bone marrow cavity, allowing marrow and fat to enter the circulatory system.1
Although the true pathophysiology remains poorly understood, it is possible that, once in systemic circulation, the fat particles become lodged in the vascular system, inciting an inflammatory response, leading to organ dysfunction via mechanical or biochemical processes.4 Typically, the diagnosis is made after clinical features are observed, including hypoxemia, petechial rash, and cerebral signs not related to a head injury or other conditions.5,6
Although FES is an uncommon complication after traumatic injuries, mortality after FES in a recent study was reported to be 10%.1 FES is most commonly seen after fractures of the femur and tibia, although cases have been described involving fractures of the radius, ulna, and humerus.1,3 We present an atypical case of cerebral FES after multiple fractures of the foot; to our knowledge, such a case has not been reported in the English-language literature. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 42-year-old man was hunting with his son when he was accidentally shot in the left foot with a .270-caliber rifle bullet at close range. The patient sought care at a local hospital and, in the ensuing 3 hours, his mentation appeared normal. He reported pain and numbness distal to the injury in the tibial nerve distribution, but he remained vascularly intact, alert, and oriented. He was given 7 mg of hydromorphone hydrochloride over 2 hours for pain control and was transferred to our hospital via ambulance approximately 6 hours after injury. Upon arrival, he was noted to be extremely sedated and obtunded, responding only to pain with spontaneous eye opening. He was unable to follow commands. He was given
1.2 mg of naloxone intravenously to reverse what was presumed to be acute opioid intoxication; however, his mental status did not improve.
On examination, the patient was noted to have a small entrance wound through the Achilles tendon (Figures 1A, 1B) and an exit wound on the plantar aspect of the foot near the heads of the first and second metatarsals (Figures 1C, 1D) with minimal bleeding and no gross contamination. There was significant edema on the medial and proximal aspects of the left foot, 3+ dorsalis pedis pulse, and a capillary refill of 4 seconds. Radiographs showed traumatic fracture deformities of the calcaneus, navicular, medial cuneiform, and first and second metatarsal bases, as well as an intra-articular fracture deformity of the left talus extending to the talar dome (Figures 2A-2C). Neurologic examination could not be reliably obtained because of the patient’s mental status. He was determined to be unstable for immediate surgery, and his left leg was splinted pending neurologic evaluation.
The patient’s oxygen saturation was 94%, and his temperature was 38.2°C (100.76°F). Although his heart rate was in the 90s upon arrival, he became tachycardic over the next 4 hours, with heart rate ranging from the 110s to 130s; he remained tachycardic for approximately 72 hours. Laboratory values upon arrival showed a hemoglobin value of 12.8 g/dL and platelets of 249,000/μL. He developed anemia and thrombocytopenia within 72 hours of the injury, with a low of 6.6 g/dL and 88,000/μL, respectively, by postinjury day 4. Computed tomography of the head, electroencephalography, urine drug screen, and lumbar puncture were unremarkable. The patient never became hypoxemic. Within 14 hours after injury, he was completely comatose with extensor posturing. In the intensive care unit (ICU), the patient was intubated for airway protection.
The next day, the patient underwent magnetic resonance imaging (MRI) of the brain, which showed innumerable tiny infarcts throughout cerebral hemispheres, cerebellum, and brainstem in a characteristic “starfield” pattern on T2-weighted images (Figure 3). This was radiographically consistent with fat emboli related to the left lower extremity gunshot wound. An echocardiogram showed small right-to-left shunt and a possible intrapulmonary shunt, although this was never confirmed. The echocardiogram was technically challenging secondary to his persistent tachycardia. He also developed a subtle petechial rash (Figure 4A).
The patient underwent percutaneous gastrostomy-tube placement for nutrition on postinjury day 4 and remained intubated, unable to protect his airway, and nonresponsive with extensor posturing (Figure 4B). He was also taken to the operating room for spanning external fixator placement on postinjury day 3 to restore calcaneal height and length as well as foot stability (Figures 5A, 5B).
The patient was treated with supportive care and was discharged from the hospital in a comatose state on hospital day 17 to a rehabilitation facility. He began to emerge from the coma 6 weeks after injury, and his external fixator was removed and a cast applied to his lower extremity. His entrance and exit wounds healed as expected. Initial agitation was treated with propranolol and quetiapine. Because he continued to have difficulty with spasticity and increased tone, he was given botulinum toxin type A injections in the pectoral muscles, biceps, and forearms. He made continued and rapid improvement in response to intensive multidisciplinary therapy and returned home 4½ months after injury. Eight months after the injury, he is now walking independently with a cane and independent with his activities of daily living. Unfortunately, he has substantial pain in his foot, which appears to be a combination of both neuropathic and posttraumatic arthrosis causes. He is undergoing consultation for a possible amputation. Radiographs show consolidation of the hind and midfoot fractures with retained bullet fragments (Figures 6A-6C). He continues to receive multidisciplinary care to address cognitive limitations and is making progress.
Discussion
FES is a life-threatening disease affecting multiple organ systems.7 Classically, the pulmonary, central nervous, and dermatologic systems are affected.5,6,8 While FES is most recognizable after long bone fractures and orthopedic procedures involving the intramedullary canal, to our knowledge, FES after gunshot wound and concomitant fractures of the foot has never been reported.
The syndrome is defined by major and minor criteria as outlined by Gurd.5 Major criteria include hypoxia, deteriorating mental status, and petechiae. This case represents a somewhat atypical presentation of FES, because dermatologic manifestations and pulmonary compromise were subtle. The minor criteria consisting of tachycardia, fever, anemia, and thrombocytopenia were present in our patient, although at different phases during the progression of the syndrome. This emphasizes the difficulty in diagnosing FES because the symptoms do not occur simultaneously.
In the classic syndrome, after an initial asymptomatic interval of 12 to 72 hours, pulmonary, neurologic, and/or dermatologic changes usually ensue.9 Altered mental status, including headache, confusion, stupor, coma, rigidity, or convulsions, has been documented in 86% of patients.10 In our case, the neurologic symptoms presented earlier, at around 6 hours after injury, and respiratory symptoms, including hypoxia, tachypnea, and dyspnea, reported in 75% of cases,2,11 did not occur at all. In fact, continued intubation was only required in this case for neuromuscular airway protection. Classic dermatologic manifestations, a reddish-brown nonpalpable petechial rash diffusely covering the upper torso and extremities, normally appears within 12 to 36 hours.12,13 Nevertheless, in our case, these findings were subtle compared with others previously reported.14,15 In fact, despite being seen by numerous physicians, including neurologists and ICU intensivists, only the orthopedists’ notes made reference to this modest finding (Figure 4A).
Further complicating the diagnosis is that, during the onset of symptoms, patients are typically victims of polytrauma and/or routinely given narcotics to help with significant pain. Therefore, it is appropriate to rule out opioid overdose and other metabolic sources of mental-status change. This can be done fairly expeditiously with laboratory testing and narcotic reversal. After these have been eliminated, FES should be considered in a patient with rapid neurologic deterioration, because a delay in treatment can affect outcomes.2,4,16
Because continuous showering of emboli to the brain and other organs occurs without fracture stabilization, rapid diagnosis with high clinical suspicion of FES is essential and can be aided immensely with MRI. In fact, MRI is the most sensitive test for this diagnosis and correlates with clinical severity of brain injury.17 T2-weighted images show regions of high-signal intensity and “starfield” pattern, which are sensitive markers for FES (Figure 3).18 These tests can be done concomitantly with a well-splinted extremity, and definitive stabilization should be carried out promptly because early splinting and fixation of orthopedic fractures improves outcomes.17
Perhaps the most important reason to make an expeditious diagnosis is to help counsel families, who are undoubtedly in shock and disbelief. Recovery times can vary widely, with the patient often continuing to regain cognitive and motor function over the course of months to years.2 Without knowledge of signs of improvement in neurologic outcome, families cannot be accurately counseled regarding potential for recovery. The practicing orthopedist should be aware of this disorder, because initial neurologic deterioration may seem hopeless. Furthermore, supportive care should be initiated early with multidisciplinary teams and extensive rehabilitation because these offer the best outcomes in patients with FES.4,18 Although our patient continues to have cognitive impairment, his recovery in the preceding 8 months has been aided by rapid diagnosis and multidisciplinary care and should offer hope to other patients faced with this situation.
1. Akhtar S. Fat embolism. Anesthesiol Clin. 2009;27(3):533-550.
2. Müller C, Rahn BA, Pfister U, Meinig RP. The incidence, pathogenesis, diagnosis, and treatment of fat embolism. Orthop Rev. 1994;23(2):107-117.
3. Stein PD, Yaekoub AY, Matta F, Kleerekoper M. Fat embolism syndrome. Am J Med Sci. 2008;336(6):472-477.
4. Parisi DM, Koval K, Egol K. Fat embolism syndrome. Am J Orthop. 2002;31(9):507-512.
5. Gurd AR. Fat embolism: an aid to diagnosis. J Bone Joint Surb Br. 1970;52(4):732-737.
6. Lee SC, Yoon JY, Nam CH, Kim TK, Jung KA, Lee DW. Cerebral fat embolism syndrome after simultaneous bilateral total knee arthroplasty: a case series. J Arthroplasty. 2012;27(3):409-414.
7. Gurd AR, Wilson RI. Fat-embolism syndrome. Lancet. 1972;2(7770):
231-232.
8. Habashi NM, Andrews PL, Scalea TM. Therapeutic aspects of fat embolism syndrome. Injury. 2006;37(Suppl 4):S68-S73.
9. Weiss W, Bardana D, Yen D. Delayed presentation of fat embolism syndrome after intramedullary nailing of a fractured femur: a case report. J Trauma. 2009;66(3):E42-E45.
10. Byrick RJ. Fat embolism and postoperative coagulopathy. Can J Anaesth. 2001;48(7):618-621.
11. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
12. Burgher LW. Fat embolism syndrome. Chest. 1981;79(2):131-132.
13. Burgher LW, Dines DE, Linscheid RL, Didier EP. Fat embolism and the adult respiratory distress syndrome. Mayo Clin Proc. 1974;49(2):107-109.
14. Liu DD, Hsieh NK, Chen HI. Histopathological and biochemical changes following fat embolism with administration of corn oil micelles: a new animal model for fat embolism syndrome. J Bone Joint Surg Br. 2008;90(11):
1517-1521.
15. Liu HK, Chen WC. Images in clinical medicine. Fat embolism syndrome. N Engl J Med. 2011;364(18):1761.
16. Pinney SJ, Keating JF, Meek RN. Fat embolism syndrome in isolated femoral fractures: does timing of nailing influence incidence? Injury. 1998;29(2):
131-133.
17. Takahashi M, Suzuki R, Osakabe Y, et al. Magnetic resonance imaging findings in cerebral fat embolism: correlation with clinical manifestations. J Trauma. 1999;46(2):324-327.
18. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
1. Akhtar S. Fat embolism. Anesthesiol Clin. 2009;27(3):533-550.
2. Müller C, Rahn BA, Pfister U, Meinig RP. The incidence, pathogenesis, diagnosis, and treatment of fat embolism. Orthop Rev. 1994;23(2):107-117.
3. Stein PD, Yaekoub AY, Matta F, Kleerekoper M. Fat embolism syndrome. Am J Med Sci. 2008;336(6):472-477.
4. Parisi DM, Koval K, Egol K. Fat embolism syndrome. Am J Orthop. 2002;31(9):507-512.
5. Gurd AR. Fat embolism: an aid to diagnosis. J Bone Joint Surb Br. 1970;52(4):732-737.
6. Lee SC, Yoon JY, Nam CH, Kim TK, Jung KA, Lee DW. Cerebral fat embolism syndrome after simultaneous bilateral total knee arthroplasty: a case series. J Arthroplasty. 2012;27(3):409-414.
7. Gurd AR, Wilson RI. Fat-embolism syndrome. Lancet. 1972;2(7770):
231-232.
8. Habashi NM, Andrews PL, Scalea TM. Therapeutic aspects of fat embolism syndrome. Injury. 2006;37(Suppl 4):S68-S73.
9. Weiss W, Bardana D, Yen D. Delayed presentation of fat embolism syndrome after intramedullary nailing of a fractured femur: a case report. J Trauma. 2009;66(3):E42-E45.
10. Byrick RJ. Fat embolism and postoperative coagulopathy. Can J Anaesth. 2001;48(7):618-621.
11. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
12. Burgher LW. Fat embolism syndrome. Chest. 1981;79(2):131-132.
13. Burgher LW, Dines DE, Linscheid RL, Didier EP. Fat embolism and the adult respiratory distress syndrome. Mayo Clin Proc. 1974;49(2):107-109.
14. Liu DD, Hsieh NK, Chen HI. Histopathological and biochemical changes following fat embolism with administration of corn oil micelles: a new animal model for fat embolism syndrome. J Bone Joint Surg Br. 2008;90(11):
1517-1521.
15. Liu HK, Chen WC. Images in clinical medicine. Fat embolism syndrome. N Engl J Med. 2011;364(18):1761.
16. Pinney SJ, Keating JF, Meek RN. Fat embolism syndrome in isolated femoral fractures: does timing of nailing influence incidence? Injury. 1998;29(2):
131-133.
17. Takahashi M, Suzuki R, Osakabe Y, et al. Magnetic resonance imaging findings in cerebral fat embolism: correlation with clinical manifestations. J Trauma. 1999;46(2):324-327.
18. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
Aneurysmal Bone Cyst Involving the Metacarpal Bone in a Child
Less than 5% of aneurysmal bone cysts (ABCs) are located in the hand,1 and only a few cases have been reported in the literature.2-7 Unfortunately, it is impossible to predict when an ABC will exhibit aggressive behavior.4,8 Aneurysmal bone cysts and giant cell bone tumors have been considered benign9 lesions that can behave in a locally aggressive fashion.1 Optimal treatment has not been established because treatment is variable depending on the condition of the lesion. Several authors have recommended more radical treatment modalities, such as en bloc resection or excision diaphysectomy followed by strut bone grafting, which had a relatively low rate of recurrence. A relatively low rate of recurrence and other complications indicate that those techniques would serve as a good strategy for patients with expansile hand ABCs in terms of safety, simplicity, and reduced number of reoperations.3,7,10
This article reports a case of an ABC of the second metacarpal bone of the right hand in a 12-year-old boy treated with curettage and autologous morselized iliac bone grafting. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a right hand–dominant 12-year-old-boy, who noticed the development of a lump in the dorsum of his right hand. On examination, we found a large, firm swelling of the dorsum of his right hand over the second metacarpal. Radiographic examination showed a symmetrical expansile lytic lesion (22×24×25 mm) involving the entire second metacarpal bone (Figure 1A). Magnetic resonance imaging (MRI) showed a well-defined expansile intramedullary lesion with preservation of the epiphyseal plate, shell-like periosteal reaction, and a multilocular appearance with a hemorrhagic compartment (fluid-fluid levels) (Figure 1B).
At surgery, we found a blood-filled cyst, and the cortex was very thin. The lesion extended to the distal two-thirds of the bone to the level of the physeal plate. We had considered using allograft or other bone substitutes. However, we did not have confidence in the bone-induction potential and power of osteogenesis of bone substitutes or allograft compared with autologous bone graft. Consequently, we performed autologous bone grafting, despite its being an invasive procedure, on the immature iliac crest. We performed thorough curettage of the intramedullary material without damaging the physeal plate, followed by impact morselized autologous bone grafting. Histologic examination confirmed that the final diagnosis was identical to the provisional diagnosis shown on MRI (Figure 1C). A thumb spica cast was applied for 4 weeks after surgery, and regular follow-up radiographs were taken for 3 years and 6 months until confirmation of complete normalization of the lesion without recurrence (Figures 2A-2C).
Discussion
Primary ABCs in the small tubular bones of the hands are rare. Less than 5% of aneurysmal cysts are located in the hand.1 Only a few small cases of this condition have been reported in the literature.2-7 Radiographic examination showed that, in all cases, the lesion was both expansile and completely lucent.7 Although radiographic finding of ABC in short tubular bone characteristically shows central symmetry with expansion into the diaphysis and subarticular bone, the appearance of an ABC on radiographs and angiograms is usually not diagnostic.8 Even though fluid-fluid levels are highly suggestive of ABC, only pathologic study confirms the diagnosis. MRI may be a good tool for postsurgery follow-up. On the basis of these ideas, we performed histological examination and confirmed the diagnosis of ABC of the metacarpus by radiograph and MRI.
The goals in the treatment of primary ABCs are preservation of function and avoidance of recurrence. Unfortunately, it is impossible to predict the possible aggressive behavior in ABCs. Active or aggressive character in certain localizations of ABC in children requires either curettage, which has a considerable recurrence rate, or radical segmental excision, which raises complex reconstructive challenges. Frassica and colleagues7 reported no recurrences in 3 patients treated by complete excision and bone grafting. Curettage and bone grafting in 7 cases were associated with 4 recurrences.7
Because optimal treatment has not been established,3 current recommendations vary, depending on the condition of the lesion. Several authors recommend more radical treatment modalities, such as en bloc resection, excision diaphysectomy, cryotherapy, and strut bone grafting, and a relatively low rate of recurrence and other complications indicates that those techniques would serve as a good strategy for patients with expansile ABCs in the hand.3,7,10 On the other hand, successful results with less aggressive procedures, such as curettage and autologous bone grafting, have been reported.4,5,8
In pediatric patients, surgery to preserve the growth plate is recommended.5 Ropars and colleagues4 suggested that aggressive treatment approaches, such as cryotherapy and resection with reconstruction, should be used only in cases when the articular surface is involved, when full-bone invasion of the phalanx or metacarpal has occurred, or in cases of more than 1 recurrence.
In conclusion, despite the high risk of recurrence of ABC treated with curettage with bone grafting, the findings of the present case show that ABC of the metacarpal bone in children can be treated successfully with curettage followed by morselized autologous bone grafting without recurrence.
1. Athanasian EA. Aneurysmal bone cyst and giant cell tumor of bone of the hand and distal radius. Hand Clin. 2004;20(3):269-281, vi.
2. Tarazona-Velutini P, Romo-Rodriguez R, Saleme-Cruz J. Aneurysmatic bone cyst in the proximal phalanx of a finger. Case report and literature review. Acta Ortop Mex. 2012;26(4):245-249.
3. Jafari D, Jamshidi K, Najdmazhar F, Shariatzade H, Liaghat O. Expansile aneurysmal bone cyst in the tubular bones of the hand treated with en bloc excision and autograft reconstruction: a report of 12 cases. J Hand Surg Eur Vol. 2011;36(8):648-655.
4. Ropars M, Kaila R, Briggs T, Cannon S. Aneurysmal bone cysts of the metacarpals and phalanges of the hand. A 6 case series and literature review. Chir Main. 2007;26(4-5):214-217.
5. Sproule JA, Salmo E, Mortimer G, O’Sullivan M. Aneursymal bone cyst of the proximal phalanx of the thumb in a child. Hand Surg. 2002;7(1):147-150.
6. Schwartz GB, Hammerman MZ. Aneurysmal bone cyst of the fifth metacarpal. Orthop Rev. 1989;18(12):1309-1314.
7. Frassica FJ, Amadio PC, Wold LE, Beabout JW. Aneurysmal bone cyst: clinicopathologic features and treatment of ten cases involving the hand. J Hand Surg Am. 1988;13(5):676-683.
8. Louahem D, Kouyoumdjian P, Ghanem I, et al. Active aneurysmal bone cysts in children: possible evolution after biopsy. J Child Orthop. 2012;6(4):333-338.
9. Lindfors NC. Treatment of a recurrent aneurysmal bone cyst with bioactive glass in a child allows for good bone remodelling and growth. Bone. 2009;45(2):398-400.
10. Salon A, Rémi J, Brunelle F, Drapé JL, Glorion Ch. Total replacement of a middle phalanx by free non-vascularized chondral graft, after failure of sclerotherapy for treatment of an aneurysmal bone cyst. Chir Main. 2005;24(3-4):187-192.
Less than 5% of aneurysmal bone cysts (ABCs) are located in the hand,1 and only a few cases have been reported in the literature.2-7 Unfortunately, it is impossible to predict when an ABC will exhibit aggressive behavior.4,8 Aneurysmal bone cysts and giant cell bone tumors have been considered benign9 lesions that can behave in a locally aggressive fashion.1 Optimal treatment has not been established because treatment is variable depending on the condition of the lesion. Several authors have recommended more radical treatment modalities, such as en bloc resection or excision diaphysectomy followed by strut bone grafting, which had a relatively low rate of recurrence. A relatively low rate of recurrence and other complications indicate that those techniques would serve as a good strategy for patients with expansile hand ABCs in terms of safety, simplicity, and reduced number of reoperations.3,7,10
This article reports a case of an ABC of the second metacarpal bone of the right hand in a 12-year-old boy treated with curettage and autologous morselized iliac bone grafting. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a right hand–dominant 12-year-old-boy, who noticed the development of a lump in the dorsum of his right hand. On examination, we found a large, firm swelling of the dorsum of his right hand over the second metacarpal. Radiographic examination showed a symmetrical expansile lytic lesion (22×24×25 mm) involving the entire second metacarpal bone (Figure 1A). Magnetic resonance imaging (MRI) showed a well-defined expansile intramedullary lesion with preservation of the epiphyseal plate, shell-like periosteal reaction, and a multilocular appearance with a hemorrhagic compartment (fluid-fluid levels) (Figure 1B).
At surgery, we found a blood-filled cyst, and the cortex was very thin. The lesion extended to the distal two-thirds of the bone to the level of the physeal plate. We had considered using allograft or other bone substitutes. However, we did not have confidence in the bone-induction potential and power of osteogenesis of bone substitutes or allograft compared with autologous bone graft. Consequently, we performed autologous bone grafting, despite its being an invasive procedure, on the immature iliac crest. We performed thorough curettage of the intramedullary material without damaging the physeal plate, followed by impact morselized autologous bone grafting. Histologic examination confirmed that the final diagnosis was identical to the provisional diagnosis shown on MRI (Figure 1C). A thumb spica cast was applied for 4 weeks after surgery, and regular follow-up radiographs were taken for 3 years and 6 months until confirmation of complete normalization of the lesion without recurrence (Figures 2A-2C).
Discussion
Primary ABCs in the small tubular bones of the hands are rare. Less than 5% of aneurysmal cysts are located in the hand.1 Only a few small cases of this condition have been reported in the literature.2-7 Radiographic examination showed that, in all cases, the lesion was both expansile and completely lucent.7 Although radiographic finding of ABC in short tubular bone characteristically shows central symmetry with expansion into the diaphysis and subarticular bone, the appearance of an ABC on radiographs and angiograms is usually not diagnostic.8 Even though fluid-fluid levels are highly suggestive of ABC, only pathologic study confirms the diagnosis. MRI may be a good tool for postsurgery follow-up. On the basis of these ideas, we performed histological examination and confirmed the diagnosis of ABC of the metacarpus by radiograph and MRI.
The goals in the treatment of primary ABCs are preservation of function and avoidance of recurrence. Unfortunately, it is impossible to predict the possible aggressive behavior in ABCs. Active or aggressive character in certain localizations of ABC in children requires either curettage, which has a considerable recurrence rate, or radical segmental excision, which raises complex reconstructive challenges. Frassica and colleagues7 reported no recurrences in 3 patients treated by complete excision and bone grafting. Curettage and bone grafting in 7 cases were associated with 4 recurrences.7
Because optimal treatment has not been established,3 current recommendations vary, depending on the condition of the lesion. Several authors recommend more radical treatment modalities, such as en bloc resection, excision diaphysectomy, cryotherapy, and strut bone grafting, and a relatively low rate of recurrence and other complications indicates that those techniques would serve as a good strategy for patients with expansile ABCs in the hand.3,7,10 On the other hand, successful results with less aggressive procedures, such as curettage and autologous bone grafting, have been reported.4,5,8
In pediatric patients, surgery to preserve the growth plate is recommended.5 Ropars and colleagues4 suggested that aggressive treatment approaches, such as cryotherapy and resection with reconstruction, should be used only in cases when the articular surface is involved, when full-bone invasion of the phalanx or metacarpal has occurred, or in cases of more than 1 recurrence.
In conclusion, despite the high risk of recurrence of ABC treated with curettage with bone grafting, the findings of the present case show that ABC of the metacarpal bone in children can be treated successfully with curettage followed by morselized autologous bone grafting without recurrence.
Less than 5% of aneurysmal bone cysts (ABCs) are located in the hand,1 and only a few cases have been reported in the literature.2-7 Unfortunately, it is impossible to predict when an ABC will exhibit aggressive behavior.4,8 Aneurysmal bone cysts and giant cell bone tumors have been considered benign9 lesions that can behave in a locally aggressive fashion.1 Optimal treatment has not been established because treatment is variable depending on the condition of the lesion. Several authors have recommended more radical treatment modalities, such as en bloc resection or excision diaphysectomy followed by strut bone grafting, which had a relatively low rate of recurrence. A relatively low rate of recurrence and other complications indicate that those techniques would serve as a good strategy for patients with expansile hand ABCs in terms of safety, simplicity, and reduced number of reoperations.3,7,10
This article reports a case of an ABC of the second metacarpal bone of the right hand in a 12-year-old boy treated with curettage and autologous morselized iliac bone grafting. The patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a right hand–dominant 12-year-old-boy, who noticed the development of a lump in the dorsum of his right hand. On examination, we found a large, firm swelling of the dorsum of his right hand over the second metacarpal. Radiographic examination showed a symmetrical expansile lytic lesion (22×24×25 mm) involving the entire second metacarpal bone (Figure 1A). Magnetic resonance imaging (MRI) showed a well-defined expansile intramedullary lesion with preservation of the epiphyseal plate, shell-like periosteal reaction, and a multilocular appearance with a hemorrhagic compartment (fluid-fluid levels) (Figure 1B).
At surgery, we found a blood-filled cyst, and the cortex was very thin. The lesion extended to the distal two-thirds of the bone to the level of the physeal plate. We had considered using allograft or other bone substitutes. However, we did not have confidence in the bone-induction potential and power of osteogenesis of bone substitutes or allograft compared with autologous bone graft. Consequently, we performed autologous bone grafting, despite its being an invasive procedure, on the immature iliac crest. We performed thorough curettage of the intramedullary material without damaging the physeal plate, followed by impact morselized autologous bone grafting. Histologic examination confirmed that the final diagnosis was identical to the provisional diagnosis shown on MRI (Figure 1C). A thumb spica cast was applied for 4 weeks after surgery, and regular follow-up radiographs were taken for 3 years and 6 months until confirmation of complete normalization of the lesion without recurrence (Figures 2A-2C).
Discussion
Primary ABCs in the small tubular bones of the hands are rare. Less than 5% of aneurysmal cysts are located in the hand.1 Only a few small cases of this condition have been reported in the literature.2-7 Radiographic examination showed that, in all cases, the lesion was both expansile and completely lucent.7 Although radiographic finding of ABC in short tubular bone characteristically shows central symmetry with expansion into the diaphysis and subarticular bone, the appearance of an ABC on radiographs and angiograms is usually not diagnostic.8 Even though fluid-fluid levels are highly suggestive of ABC, only pathologic study confirms the diagnosis. MRI may be a good tool for postsurgery follow-up. On the basis of these ideas, we performed histological examination and confirmed the diagnosis of ABC of the metacarpus by radiograph and MRI.
The goals in the treatment of primary ABCs are preservation of function and avoidance of recurrence. Unfortunately, it is impossible to predict the possible aggressive behavior in ABCs. Active or aggressive character in certain localizations of ABC in children requires either curettage, which has a considerable recurrence rate, or radical segmental excision, which raises complex reconstructive challenges. Frassica and colleagues7 reported no recurrences in 3 patients treated by complete excision and bone grafting. Curettage and bone grafting in 7 cases were associated with 4 recurrences.7
Because optimal treatment has not been established,3 current recommendations vary, depending on the condition of the lesion. Several authors recommend more radical treatment modalities, such as en bloc resection, excision diaphysectomy, cryotherapy, and strut bone grafting, and a relatively low rate of recurrence and other complications indicates that those techniques would serve as a good strategy for patients with expansile ABCs in the hand.3,7,10 On the other hand, successful results with less aggressive procedures, such as curettage and autologous bone grafting, have been reported.4,5,8
In pediatric patients, surgery to preserve the growth plate is recommended.5 Ropars and colleagues4 suggested that aggressive treatment approaches, such as cryotherapy and resection with reconstruction, should be used only in cases when the articular surface is involved, when full-bone invasion of the phalanx or metacarpal has occurred, or in cases of more than 1 recurrence.
In conclusion, despite the high risk of recurrence of ABC treated with curettage with bone grafting, the findings of the present case show that ABC of the metacarpal bone in children can be treated successfully with curettage followed by morselized autologous bone grafting without recurrence.
1. Athanasian EA. Aneurysmal bone cyst and giant cell tumor of bone of the hand and distal radius. Hand Clin. 2004;20(3):269-281, vi.
2. Tarazona-Velutini P, Romo-Rodriguez R, Saleme-Cruz J. Aneurysmatic bone cyst in the proximal phalanx of a finger. Case report and literature review. Acta Ortop Mex. 2012;26(4):245-249.
3. Jafari D, Jamshidi K, Najdmazhar F, Shariatzade H, Liaghat O. Expansile aneurysmal bone cyst in the tubular bones of the hand treated with en bloc excision and autograft reconstruction: a report of 12 cases. J Hand Surg Eur Vol. 2011;36(8):648-655.
4. Ropars M, Kaila R, Briggs T, Cannon S. Aneurysmal bone cysts of the metacarpals and phalanges of the hand. A 6 case series and literature review. Chir Main. 2007;26(4-5):214-217.
5. Sproule JA, Salmo E, Mortimer G, O’Sullivan M. Aneursymal bone cyst of the proximal phalanx of the thumb in a child. Hand Surg. 2002;7(1):147-150.
6. Schwartz GB, Hammerman MZ. Aneurysmal bone cyst of the fifth metacarpal. Orthop Rev. 1989;18(12):1309-1314.
7. Frassica FJ, Amadio PC, Wold LE, Beabout JW. Aneurysmal bone cyst: clinicopathologic features and treatment of ten cases involving the hand. J Hand Surg Am. 1988;13(5):676-683.
8. Louahem D, Kouyoumdjian P, Ghanem I, et al. Active aneurysmal bone cysts in children: possible evolution after biopsy. J Child Orthop. 2012;6(4):333-338.
9. Lindfors NC. Treatment of a recurrent aneurysmal bone cyst with bioactive glass in a child allows for good bone remodelling and growth. Bone. 2009;45(2):398-400.
10. Salon A, Rémi J, Brunelle F, Drapé JL, Glorion Ch. Total replacement of a middle phalanx by free non-vascularized chondral graft, after failure of sclerotherapy for treatment of an aneurysmal bone cyst. Chir Main. 2005;24(3-4):187-192.
1. Athanasian EA. Aneurysmal bone cyst and giant cell tumor of bone of the hand and distal radius. Hand Clin. 2004;20(3):269-281, vi.
2. Tarazona-Velutini P, Romo-Rodriguez R, Saleme-Cruz J. Aneurysmatic bone cyst in the proximal phalanx of a finger. Case report and literature review. Acta Ortop Mex. 2012;26(4):245-249.
3. Jafari D, Jamshidi K, Najdmazhar F, Shariatzade H, Liaghat O. Expansile aneurysmal bone cyst in the tubular bones of the hand treated with en bloc excision and autograft reconstruction: a report of 12 cases. J Hand Surg Eur Vol. 2011;36(8):648-655.
4. Ropars M, Kaila R, Briggs T, Cannon S. Aneurysmal bone cysts of the metacarpals and phalanges of the hand. A 6 case series and literature review. Chir Main. 2007;26(4-5):214-217.
5. Sproule JA, Salmo E, Mortimer G, O’Sullivan M. Aneursymal bone cyst of the proximal phalanx of the thumb in a child. Hand Surg. 2002;7(1):147-150.
6. Schwartz GB, Hammerman MZ. Aneurysmal bone cyst of the fifth metacarpal. Orthop Rev. 1989;18(12):1309-1314.
7. Frassica FJ, Amadio PC, Wold LE, Beabout JW. Aneurysmal bone cyst: clinicopathologic features and treatment of ten cases involving the hand. J Hand Surg Am. 1988;13(5):676-683.
8. Louahem D, Kouyoumdjian P, Ghanem I, et al. Active aneurysmal bone cysts in children: possible evolution after biopsy. J Child Orthop. 2012;6(4):333-338.
9. Lindfors NC. Treatment of a recurrent aneurysmal bone cyst with bioactive glass in a child allows for good bone remodelling and growth. Bone. 2009;45(2):398-400.
10. Salon A, Rémi J, Brunelle F, Drapé JL, Glorion Ch. Total replacement of a middle phalanx by free non-vascularized chondral graft, after failure of sclerotherapy for treatment of an aneurysmal bone cyst. Chir Main. 2005;24(3-4):187-192.
Use of Cross-Leg Flap for Wound Complications Resulting From Open Pilon Fracture
Soft-tissue complications are a known problem in the treatment of pilon fractures of the distal end of the tibia. These fractures typically occur as the result of a high-energy mechanism, and axial load and shear forces often lead to a severe soft-tissue injury. In many cases, these injuries may require additional procedures to provide adequate soft-tissue coverage. These procedures can include use of either a rotational muscle flap or a free flap transfer. In some cases, however, these flaps are not possible secondary to vascular compromise.
In this article, we report the case of a pilon fracture combined with severe soft-tissue injury and vascular compromise of the leg. A cross-leg fasciocutaneous flap was performed as a salvage procedure for coverage of the soft-tissue defect. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 23-year-old man sustained a left grade III open pilon fracture after a fall off a cherry picker. He was initially treated with irrigation and débridement of the open anteromedial wound, wound closure, application of external fixation, and open reduction and internal fixation (ORIF) of the concomitant comminuted fibular fracture. Operative fixation of the pilon was performed 3 weeks after injury, once skin and soft tissues were in acceptable condition (Figure 1). Skin closure was performed with 2-0 Vicryl sutures (Ethicon, Inc, Somerville, New Jersey) followed by 3-0 nylon skin sutures and No. 2 nylon retention sutures to reduce tension at the incision.
On postoperative day 17, the patient was found to have skin necrosis with exposed hardware over the medial laceration that had resulted from the open fracture (Figure 2). The wound measured 7×6 cm. The plastic surgery team was consulted, and a soft-tissue flap was recommended. Preoperative computed tomography angiogram (Figure 3) revealed 1 vessel runoff in the leg, constituting the peroneal artery, and a conventional angiogram confirmed this finding (Figure 4). Despite these findings, the patient was taken to the operating room 4 weeks after initial injury to try to find a vessel compatible with anastomosis. Intraoperative wound exploration confirmed no patent blood supply for local soft-tissue flap coverage. Therefore, the wound was irrigated and débrided, and a vacuum-assisted closure (VAC) dressing was applied despite exposed hardware and bone. A decision was then made to attempt a cross-leg flap as a salvage procedure, and VAC dressing therapy was continued for several weeks to prepare the recipient site (Figure 5).
Seven weeks after injury, the patient was taken to the operating room by the orthopedic surgery and plastic surgery teams. After débridement, a fasciocutaneous flap was raised from the middle third of the contralateral leg (Figure 6) based on a posterior tibial artery perforator. The flap, which measured 7×7 cm (sufficient to cover the defect), was raised from lateral to medial from the posterior aspect of the leg with the pedicle located on the medial aspect of the right leg. Flap placement was facilitated by flexing the left knee to 80°. The flap was sutured into place with 4-0 Vicryl deep sutures followed by 4-0 nylon and superficial sutures in an interrupted fashion (Figure 7). Rigid external fixation was then applied to both extremities, bridging them together in optimal position (Figure 8). This construct included 2 short bars that would elevate the patient’s heels off the bed to reduce the chance of heel decubiti. Although including the feet in the external fixator construct may help prevent equinus contracture, we splinted the ankles in neutral position immediately after surgery so that we could begin early range-of-motion (ROM) exercises of the ankles to prevent stiffness. Ankle ROM exercises were started once the flap incorporated, 3 weeks after placement of the external fixator. Lacking medical insurance coverage, the patient could not be admitted to a rehabilitation facility or receive home care. He lived independently and had no help at home, so he had to remain hospitalized after placement of the external fixator. While hospitalized, the surgical site was treated with frequent dressing changes, including use of bacitracin and nonadherent dressing.
After flap coverage and 4 weeks of bed rest, a base clamping test confirmed the flap was incorporated into the recipient bed. The patient was then returned to the operating room for removal of the external fixator and skin grafting of the donor site. After surgery, he was started on physical therapy, including exercises for bilateral hip, knee, and ankle ROM and strengthening of the lower extremities. Four months after initial injury, the fracture was healed, based on bone consolidation, seen on radiographs, that is consistent with other pilon fractures treated at our institution. Six months after external fixator removal, the patient was able to ambulate independently with minimal discomfort (Figure 9). Passive and active ankle ROM was 20° of dorsiflexion and 25° of plantarflexion, compared with 25° of dorsiflexion and 45° of plantarflexion on the contralateral extremity. Subtalar motion had some stiffness with a 10° arc, compared with a 25° arc on the contralateral extremity. On simple manual testing, the patient had 5/5 motor strength with dorsiflexion, plantarflexion, inversion, and eversion. He returned to full duty as a landscaper about 1 year after initial injury and had no recurrence of wound complications or infection.
Discussion
Fractures of the distal tibia are commonly known as pilon or plafond fractures. They represent up to 10% of all tibial fractures. The injury consists of an intra-articular fracture of the tibiotalar joint with varying degrees of proximal extension into the tibial metaphysis. The etiology is an axial load on the tibia with or without a rotational force.1 Treatment is challenging. The literature includes many reports of wound and soft-tissue complications after ORIF. In 1969, Rüedi and Allgöwer2 published recommendations that have become the standard for treatment of pilon fractures. Twelve percent of the 84 fractures included in their study were associated with wound complications. In 2004, Sirkin and colleagues3 suggested that wound problems associated with ORIF of pilon fractures may be caused by attempts at immediate fixation through swollen soft tissue. They postulated that staging the procedure and waiting for decreased soft-tissue swelling may reduce the incidence of wound complications. In their series, only 2.9% of closed pilon fractures and only 9.1% of open fractures had any wound complications, and none of their patients required skin grafts, rotation flaps, or free tissue transfers.
However, soft-tissue complications still remain a significant threat in the treatment of pilon fracture, and cases that require additional procedures for soft-tissue coverage are common. In some cases, wound necrosis may lead to below-knee amputation.4 There are several coverage options, including local rotational flaps using the soleus muscle5,6 as well as free flaps using the latissimus dorsi, gracilis, or rectus abdominis muscles.7 These options require a sufficient blood supply to the region.
Many high-energy pilon fractures may be associated with vascular injury, and therefore flap survival may be compromised. We have reported such a case in the present article. Our patient’s preoperative angiogram indicated he had 1-vessel runoff to the distal leg—a situation incompatible with free tissue transfer. It is not clear whether this finding is secondary to trauma to the leg or is caused by an anatomical anomaly. Nevertheless, the poor vascularity posed a challenge to providing soft-tissue coverage. Cross-finger8 and cross-foot9 flaps have been described in upper and lower extremity injuries. In 2006, Zhao and colleagues10 reported on 5 patients with tibia and/or hardware exposure after operative fixation of tibia fractures. These patients had poor local soft tissue around the wound and therefore underwent cross-leg flap for coverage. It is not clear where the soft-tissue defects were located and whether any studies were performed to assess the local blood flow.
From our patient’s case, we learned that multiple factors should be considered when assessing such high-energy injuries. First, respecting the soft tissues is of paramount importance. Our initial management on presentation consisted of irrigation and débridement of the wound, fixation of the fibula, and application of an external fixator to allow for soft-tissue healing before definitive fixation of the pilon. Although ultimately the patient required soft-tissue coverage, soft-tissue healing and viability are important in preventing unnecessary soft-tissue procedures, and therefore we would not have handled our initial treatment differently.
Patient selection is also important. The ideal candidate for a cross-leg flap is a young, healthy person who is compliant and has a strong support system to help with activities of daily living. Unfortunately, because of financial issues and lack of home support, our patient remained hospitalized during his treatment course. For a patient who has support, it is possible to be discharged either home or to a rehabilitation facility once flap viability has been confirmed after surgery.
Another consideration is type of immobilization. Immobilization options include casting, use of Kirschner wires (K-wires), and use of rigid external fixation. For cross-leg flaps, external fixation is superior to casting and K-wires, as it provides a more rigid construct and easier access to the flap for serial evaluation. Further, it is easier for the patient to maintain personal hygiene, and it can provide heel rises to avoid pressure ulcers.
Conclusion
To our knowledge, there have been no reports of using a cross-leg flap for wound complications in high-energy pilon fractures. As already mentioned, many of these fractures may be associated with severe soft-tissue injury and may need flap coverage. A cross-leg flap with external fixation of both legs provides a limb salvage option with satisfactory patient outcomes.
1. McCann PA, Jackson M, Mitchell ST, Atkins RM. Complications of definitive open reduction and internal fixation of pilon fractures of the distal tibia. Int Orthop. 2011;35(3):413-418.
2. Rüedi TP, Allgöwer M. Fractures of the lower end of the tibia into the ankle joint. Injury. 1969;1:92-99.
3. Sirkin M, Sanders R, DiPasquale T, Herscovici D Jr. A staged protocol for soft tissue management in the treatment of complex pilon fractures. J Orthop Trauma. 2004;18(8 suppl):S32-S38.
4. Boraiah S, Kemp TJ, Erwteman A, Lucas PA, Asprinio DE. Outcome following open reduction and internal fixation of open pilon fractures. J Bone Joint Surg Am. 2010;92(2):346-352.
5. Cheng C, Li X, Abudu S. Repairing postoperative soft tissue defects of tibia and ankle open fractures with muscle flap pedicled with medial half of soleus [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009;23(12):1440-1442.
6. Yunus A, Yusuf A, Chen G. Repair of soft tissue defect by reverse soleus muscle flap after pilon fracture fixation [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2007;21(9):925-927.
7. Conroy J, Agarwal M, Giannoudis PV, Matthews SJ. Early internal fixation and soft tissue cover of severe open tibial pilon fractures. Int Orthop. 2003;27(6):343-347.
8. Megerle K, Palm-Bröking K, Germann G. The cross-finger flap [in German]. Oper Orthop Traumatol. 2008;20(2):97-102.
9. Largey A, Faline A, Hebrard W, Hamoui M, Canovas F. Management of massive traumatic compound defects of the foot. Orthop Traumatol Surg Res. 2009;95(4):301-304.
10. Zhao L, Wan L, Wang S. Clinical studies on maintenance of cross-leg position through internal fixation with Kirschner wire after cross-leg flap procedure. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2006;20(12):1211-1213.
Soft-tissue complications are a known problem in the treatment of pilon fractures of the distal end of the tibia. These fractures typically occur as the result of a high-energy mechanism, and axial load and shear forces often lead to a severe soft-tissue injury. In many cases, these injuries may require additional procedures to provide adequate soft-tissue coverage. These procedures can include use of either a rotational muscle flap or a free flap transfer. In some cases, however, these flaps are not possible secondary to vascular compromise.
In this article, we report the case of a pilon fracture combined with severe soft-tissue injury and vascular compromise of the leg. A cross-leg fasciocutaneous flap was performed as a salvage procedure for coverage of the soft-tissue defect. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 23-year-old man sustained a left grade III open pilon fracture after a fall off a cherry picker. He was initially treated with irrigation and débridement of the open anteromedial wound, wound closure, application of external fixation, and open reduction and internal fixation (ORIF) of the concomitant comminuted fibular fracture. Operative fixation of the pilon was performed 3 weeks after injury, once skin and soft tissues were in acceptable condition (Figure 1). Skin closure was performed with 2-0 Vicryl sutures (Ethicon, Inc, Somerville, New Jersey) followed by 3-0 nylon skin sutures and No. 2 nylon retention sutures to reduce tension at the incision.
On postoperative day 17, the patient was found to have skin necrosis with exposed hardware over the medial laceration that had resulted from the open fracture (Figure 2). The wound measured 7×6 cm. The plastic surgery team was consulted, and a soft-tissue flap was recommended. Preoperative computed tomography angiogram (Figure 3) revealed 1 vessel runoff in the leg, constituting the peroneal artery, and a conventional angiogram confirmed this finding (Figure 4). Despite these findings, the patient was taken to the operating room 4 weeks after initial injury to try to find a vessel compatible with anastomosis. Intraoperative wound exploration confirmed no patent blood supply for local soft-tissue flap coverage. Therefore, the wound was irrigated and débrided, and a vacuum-assisted closure (VAC) dressing was applied despite exposed hardware and bone. A decision was then made to attempt a cross-leg flap as a salvage procedure, and VAC dressing therapy was continued for several weeks to prepare the recipient site (Figure 5).
Seven weeks after injury, the patient was taken to the operating room by the orthopedic surgery and plastic surgery teams. After débridement, a fasciocutaneous flap was raised from the middle third of the contralateral leg (Figure 6) based on a posterior tibial artery perforator. The flap, which measured 7×7 cm (sufficient to cover the defect), was raised from lateral to medial from the posterior aspect of the leg with the pedicle located on the medial aspect of the right leg. Flap placement was facilitated by flexing the left knee to 80°. The flap was sutured into place with 4-0 Vicryl deep sutures followed by 4-0 nylon and superficial sutures in an interrupted fashion (Figure 7). Rigid external fixation was then applied to both extremities, bridging them together in optimal position (Figure 8). This construct included 2 short bars that would elevate the patient’s heels off the bed to reduce the chance of heel decubiti. Although including the feet in the external fixator construct may help prevent equinus contracture, we splinted the ankles in neutral position immediately after surgery so that we could begin early range-of-motion (ROM) exercises of the ankles to prevent stiffness. Ankle ROM exercises were started once the flap incorporated, 3 weeks after placement of the external fixator. Lacking medical insurance coverage, the patient could not be admitted to a rehabilitation facility or receive home care. He lived independently and had no help at home, so he had to remain hospitalized after placement of the external fixator. While hospitalized, the surgical site was treated with frequent dressing changes, including use of bacitracin and nonadherent dressing.
After flap coverage and 4 weeks of bed rest, a base clamping test confirmed the flap was incorporated into the recipient bed. The patient was then returned to the operating room for removal of the external fixator and skin grafting of the donor site. After surgery, he was started on physical therapy, including exercises for bilateral hip, knee, and ankle ROM and strengthening of the lower extremities. Four months after initial injury, the fracture was healed, based on bone consolidation, seen on radiographs, that is consistent with other pilon fractures treated at our institution. Six months after external fixator removal, the patient was able to ambulate independently with minimal discomfort (Figure 9). Passive and active ankle ROM was 20° of dorsiflexion and 25° of plantarflexion, compared with 25° of dorsiflexion and 45° of plantarflexion on the contralateral extremity. Subtalar motion had some stiffness with a 10° arc, compared with a 25° arc on the contralateral extremity. On simple manual testing, the patient had 5/5 motor strength with dorsiflexion, plantarflexion, inversion, and eversion. He returned to full duty as a landscaper about 1 year after initial injury and had no recurrence of wound complications or infection.
Discussion
Fractures of the distal tibia are commonly known as pilon or plafond fractures. They represent up to 10% of all tibial fractures. The injury consists of an intra-articular fracture of the tibiotalar joint with varying degrees of proximal extension into the tibial metaphysis. The etiology is an axial load on the tibia with or without a rotational force.1 Treatment is challenging. The literature includes many reports of wound and soft-tissue complications after ORIF. In 1969, Rüedi and Allgöwer2 published recommendations that have become the standard for treatment of pilon fractures. Twelve percent of the 84 fractures included in their study were associated with wound complications. In 2004, Sirkin and colleagues3 suggested that wound problems associated with ORIF of pilon fractures may be caused by attempts at immediate fixation through swollen soft tissue. They postulated that staging the procedure and waiting for decreased soft-tissue swelling may reduce the incidence of wound complications. In their series, only 2.9% of closed pilon fractures and only 9.1% of open fractures had any wound complications, and none of their patients required skin grafts, rotation flaps, or free tissue transfers.
However, soft-tissue complications still remain a significant threat in the treatment of pilon fracture, and cases that require additional procedures for soft-tissue coverage are common. In some cases, wound necrosis may lead to below-knee amputation.4 There are several coverage options, including local rotational flaps using the soleus muscle5,6 as well as free flaps using the latissimus dorsi, gracilis, or rectus abdominis muscles.7 These options require a sufficient blood supply to the region.
Many high-energy pilon fractures may be associated with vascular injury, and therefore flap survival may be compromised. We have reported such a case in the present article. Our patient’s preoperative angiogram indicated he had 1-vessel runoff to the distal leg—a situation incompatible with free tissue transfer. It is not clear whether this finding is secondary to trauma to the leg or is caused by an anatomical anomaly. Nevertheless, the poor vascularity posed a challenge to providing soft-tissue coverage. Cross-finger8 and cross-foot9 flaps have been described in upper and lower extremity injuries. In 2006, Zhao and colleagues10 reported on 5 patients with tibia and/or hardware exposure after operative fixation of tibia fractures. These patients had poor local soft tissue around the wound and therefore underwent cross-leg flap for coverage. It is not clear where the soft-tissue defects were located and whether any studies were performed to assess the local blood flow.
From our patient’s case, we learned that multiple factors should be considered when assessing such high-energy injuries. First, respecting the soft tissues is of paramount importance. Our initial management on presentation consisted of irrigation and débridement of the wound, fixation of the fibula, and application of an external fixator to allow for soft-tissue healing before definitive fixation of the pilon. Although ultimately the patient required soft-tissue coverage, soft-tissue healing and viability are important in preventing unnecessary soft-tissue procedures, and therefore we would not have handled our initial treatment differently.
Patient selection is also important. The ideal candidate for a cross-leg flap is a young, healthy person who is compliant and has a strong support system to help with activities of daily living. Unfortunately, because of financial issues and lack of home support, our patient remained hospitalized during his treatment course. For a patient who has support, it is possible to be discharged either home or to a rehabilitation facility once flap viability has been confirmed after surgery.
Another consideration is type of immobilization. Immobilization options include casting, use of Kirschner wires (K-wires), and use of rigid external fixation. For cross-leg flaps, external fixation is superior to casting and K-wires, as it provides a more rigid construct and easier access to the flap for serial evaluation. Further, it is easier for the patient to maintain personal hygiene, and it can provide heel rises to avoid pressure ulcers.
Conclusion
To our knowledge, there have been no reports of using a cross-leg flap for wound complications in high-energy pilon fractures. As already mentioned, many of these fractures may be associated with severe soft-tissue injury and may need flap coverage. A cross-leg flap with external fixation of both legs provides a limb salvage option with satisfactory patient outcomes.
Soft-tissue complications are a known problem in the treatment of pilon fractures of the distal end of the tibia. These fractures typically occur as the result of a high-energy mechanism, and axial load and shear forces often lead to a severe soft-tissue injury. In many cases, these injuries may require additional procedures to provide adequate soft-tissue coverage. These procedures can include use of either a rotational muscle flap or a free flap transfer. In some cases, however, these flaps are not possible secondary to vascular compromise.
In this article, we report the case of a pilon fracture combined with severe soft-tissue injury and vascular compromise of the leg. A cross-leg fasciocutaneous flap was performed as a salvage procedure for coverage of the soft-tissue defect. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 23-year-old man sustained a left grade III open pilon fracture after a fall off a cherry picker. He was initially treated with irrigation and débridement of the open anteromedial wound, wound closure, application of external fixation, and open reduction and internal fixation (ORIF) of the concomitant comminuted fibular fracture. Operative fixation of the pilon was performed 3 weeks after injury, once skin and soft tissues were in acceptable condition (Figure 1). Skin closure was performed with 2-0 Vicryl sutures (Ethicon, Inc, Somerville, New Jersey) followed by 3-0 nylon skin sutures and No. 2 nylon retention sutures to reduce tension at the incision.
On postoperative day 17, the patient was found to have skin necrosis with exposed hardware over the medial laceration that had resulted from the open fracture (Figure 2). The wound measured 7×6 cm. The plastic surgery team was consulted, and a soft-tissue flap was recommended. Preoperative computed tomography angiogram (Figure 3) revealed 1 vessel runoff in the leg, constituting the peroneal artery, and a conventional angiogram confirmed this finding (Figure 4). Despite these findings, the patient was taken to the operating room 4 weeks after initial injury to try to find a vessel compatible with anastomosis. Intraoperative wound exploration confirmed no patent blood supply for local soft-tissue flap coverage. Therefore, the wound was irrigated and débrided, and a vacuum-assisted closure (VAC) dressing was applied despite exposed hardware and bone. A decision was then made to attempt a cross-leg flap as a salvage procedure, and VAC dressing therapy was continued for several weeks to prepare the recipient site (Figure 5).
Seven weeks after injury, the patient was taken to the operating room by the orthopedic surgery and plastic surgery teams. After débridement, a fasciocutaneous flap was raised from the middle third of the contralateral leg (Figure 6) based on a posterior tibial artery perforator. The flap, which measured 7×7 cm (sufficient to cover the defect), was raised from lateral to medial from the posterior aspect of the leg with the pedicle located on the medial aspect of the right leg. Flap placement was facilitated by flexing the left knee to 80°. The flap was sutured into place with 4-0 Vicryl deep sutures followed by 4-0 nylon and superficial sutures in an interrupted fashion (Figure 7). Rigid external fixation was then applied to both extremities, bridging them together in optimal position (Figure 8). This construct included 2 short bars that would elevate the patient’s heels off the bed to reduce the chance of heel decubiti. Although including the feet in the external fixator construct may help prevent equinus contracture, we splinted the ankles in neutral position immediately after surgery so that we could begin early range-of-motion (ROM) exercises of the ankles to prevent stiffness. Ankle ROM exercises were started once the flap incorporated, 3 weeks after placement of the external fixator. Lacking medical insurance coverage, the patient could not be admitted to a rehabilitation facility or receive home care. He lived independently and had no help at home, so he had to remain hospitalized after placement of the external fixator. While hospitalized, the surgical site was treated with frequent dressing changes, including use of bacitracin and nonadherent dressing.
After flap coverage and 4 weeks of bed rest, a base clamping test confirmed the flap was incorporated into the recipient bed. The patient was then returned to the operating room for removal of the external fixator and skin grafting of the donor site. After surgery, he was started on physical therapy, including exercises for bilateral hip, knee, and ankle ROM and strengthening of the lower extremities. Four months after initial injury, the fracture was healed, based on bone consolidation, seen on radiographs, that is consistent with other pilon fractures treated at our institution. Six months after external fixator removal, the patient was able to ambulate independently with minimal discomfort (Figure 9). Passive and active ankle ROM was 20° of dorsiflexion and 25° of plantarflexion, compared with 25° of dorsiflexion and 45° of plantarflexion on the contralateral extremity. Subtalar motion had some stiffness with a 10° arc, compared with a 25° arc on the contralateral extremity. On simple manual testing, the patient had 5/5 motor strength with dorsiflexion, plantarflexion, inversion, and eversion. He returned to full duty as a landscaper about 1 year after initial injury and had no recurrence of wound complications or infection.
Discussion
Fractures of the distal tibia are commonly known as pilon or plafond fractures. They represent up to 10% of all tibial fractures. The injury consists of an intra-articular fracture of the tibiotalar joint with varying degrees of proximal extension into the tibial metaphysis. The etiology is an axial load on the tibia with or without a rotational force.1 Treatment is challenging. The literature includes many reports of wound and soft-tissue complications after ORIF. In 1969, Rüedi and Allgöwer2 published recommendations that have become the standard for treatment of pilon fractures. Twelve percent of the 84 fractures included in their study were associated with wound complications. In 2004, Sirkin and colleagues3 suggested that wound problems associated with ORIF of pilon fractures may be caused by attempts at immediate fixation through swollen soft tissue. They postulated that staging the procedure and waiting for decreased soft-tissue swelling may reduce the incidence of wound complications. In their series, only 2.9% of closed pilon fractures and only 9.1% of open fractures had any wound complications, and none of their patients required skin grafts, rotation flaps, or free tissue transfers.
However, soft-tissue complications still remain a significant threat in the treatment of pilon fracture, and cases that require additional procedures for soft-tissue coverage are common. In some cases, wound necrosis may lead to below-knee amputation.4 There are several coverage options, including local rotational flaps using the soleus muscle5,6 as well as free flaps using the latissimus dorsi, gracilis, or rectus abdominis muscles.7 These options require a sufficient blood supply to the region.
Many high-energy pilon fractures may be associated with vascular injury, and therefore flap survival may be compromised. We have reported such a case in the present article. Our patient’s preoperative angiogram indicated he had 1-vessel runoff to the distal leg—a situation incompatible with free tissue transfer. It is not clear whether this finding is secondary to trauma to the leg or is caused by an anatomical anomaly. Nevertheless, the poor vascularity posed a challenge to providing soft-tissue coverage. Cross-finger8 and cross-foot9 flaps have been described in upper and lower extremity injuries. In 2006, Zhao and colleagues10 reported on 5 patients with tibia and/or hardware exposure after operative fixation of tibia fractures. These patients had poor local soft tissue around the wound and therefore underwent cross-leg flap for coverage. It is not clear where the soft-tissue defects were located and whether any studies were performed to assess the local blood flow.
From our patient’s case, we learned that multiple factors should be considered when assessing such high-energy injuries. First, respecting the soft tissues is of paramount importance. Our initial management on presentation consisted of irrigation and débridement of the wound, fixation of the fibula, and application of an external fixator to allow for soft-tissue healing before definitive fixation of the pilon. Although ultimately the patient required soft-tissue coverage, soft-tissue healing and viability are important in preventing unnecessary soft-tissue procedures, and therefore we would not have handled our initial treatment differently.
Patient selection is also important. The ideal candidate for a cross-leg flap is a young, healthy person who is compliant and has a strong support system to help with activities of daily living. Unfortunately, because of financial issues and lack of home support, our patient remained hospitalized during his treatment course. For a patient who has support, it is possible to be discharged either home or to a rehabilitation facility once flap viability has been confirmed after surgery.
Another consideration is type of immobilization. Immobilization options include casting, use of Kirschner wires (K-wires), and use of rigid external fixation. For cross-leg flaps, external fixation is superior to casting and K-wires, as it provides a more rigid construct and easier access to the flap for serial evaluation. Further, it is easier for the patient to maintain personal hygiene, and it can provide heel rises to avoid pressure ulcers.
Conclusion
To our knowledge, there have been no reports of using a cross-leg flap for wound complications in high-energy pilon fractures. As already mentioned, many of these fractures may be associated with severe soft-tissue injury and may need flap coverage. A cross-leg flap with external fixation of both legs provides a limb salvage option with satisfactory patient outcomes.
1. McCann PA, Jackson M, Mitchell ST, Atkins RM. Complications of definitive open reduction and internal fixation of pilon fractures of the distal tibia. Int Orthop. 2011;35(3):413-418.
2. Rüedi TP, Allgöwer M. Fractures of the lower end of the tibia into the ankle joint. Injury. 1969;1:92-99.
3. Sirkin M, Sanders R, DiPasquale T, Herscovici D Jr. A staged protocol for soft tissue management in the treatment of complex pilon fractures. J Orthop Trauma. 2004;18(8 suppl):S32-S38.
4. Boraiah S, Kemp TJ, Erwteman A, Lucas PA, Asprinio DE. Outcome following open reduction and internal fixation of open pilon fractures. J Bone Joint Surg Am. 2010;92(2):346-352.
5. Cheng C, Li X, Abudu S. Repairing postoperative soft tissue defects of tibia and ankle open fractures with muscle flap pedicled with medial half of soleus [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009;23(12):1440-1442.
6. Yunus A, Yusuf A, Chen G. Repair of soft tissue defect by reverse soleus muscle flap after pilon fracture fixation [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2007;21(9):925-927.
7. Conroy J, Agarwal M, Giannoudis PV, Matthews SJ. Early internal fixation and soft tissue cover of severe open tibial pilon fractures. Int Orthop. 2003;27(6):343-347.
8. Megerle K, Palm-Bröking K, Germann G. The cross-finger flap [in German]. Oper Orthop Traumatol. 2008;20(2):97-102.
9. Largey A, Faline A, Hebrard W, Hamoui M, Canovas F. Management of massive traumatic compound defects of the foot. Orthop Traumatol Surg Res. 2009;95(4):301-304.
10. Zhao L, Wan L, Wang S. Clinical studies on maintenance of cross-leg position through internal fixation with Kirschner wire after cross-leg flap procedure. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2006;20(12):1211-1213.
1. McCann PA, Jackson M, Mitchell ST, Atkins RM. Complications of definitive open reduction and internal fixation of pilon fractures of the distal tibia. Int Orthop. 2011;35(3):413-418.
2. Rüedi TP, Allgöwer M. Fractures of the lower end of the tibia into the ankle joint. Injury. 1969;1:92-99.
3. Sirkin M, Sanders R, DiPasquale T, Herscovici D Jr. A staged protocol for soft tissue management in the treatment of complex pilon fractures. J Orthop Trauma. 2004;18(8 suppl):S32-S38.
4. Boraiah S, Kemp TJ, Erwteman A, Lucas PA, Asprinio DE. Outcome following open reduction and internal fixation of open pilon fractures. J Bone Joint Surg Am. 2010;92(2):346-352.
5. Cheng C, Li X, Abudu S. Repairing postoperative soft tissue defects of tibia and ankle open fractures with muscle flap pedicled with medial half of soleus [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009;23(12):1440-1442.
6. Yunus A, Yusuf A, Chen G. Repair of soft tissue defect by reverse soleus muscle flap after pilon fracture fixation [in Chinese]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2007;21(9):925-927.
7. Conroy J, Agarwal M, Giannoudis PV, Matthews SJ. Early internal fixation and soft tissue cover of severe open tibial pilon fractures. Int Orthop. 2003;27(6):343-347.
8. Megerle K, Palm-Bröking K, Germann G. The cross-finger flap [in German]. Oper Orthop Traumatol. 2008;20(2):97-102.
9. Largey A, Faline A, Hebrard W, Hamoui M, Canovas F. Management of massive traumatic compound defects of the foot. Orthop Traumatol Surg Res. 2009;95(4):301-304.
10. Zhao L, Wan L, Wang S. Clinical studies on maintenance of cross-leg position through internal fixation with Kirschner wire after cross-leg flap procedure. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2006;20(12):1211-1213.
Arthritis, Infectious Tenosynovitis, and Tendon Rupture in a Patient With Rheumatoid Arthritis and Psoriasis
Compared with monoarticular arthritis, polyarticular arthritis may yield an initially narrower differential diagnosis that focuses on systemic inflammatory conditions, such as rheumatoid arthritis (RA). Approximately 15% to 30% of septic arthritis is polyarticular, of which about 45% is associated with underlying RA.1,2 Regardless of the number of joints involved, septic (infectious) arthritis is a valid consideration given the morbidity and mortality.
In a retrospective study in the United Kingdom (UK) between 1982 and 1991, the morbidity and mortality of septic arthritis was 31.6% and 11.5%, respectively, and 16% of the study population had RA.3 A review of the literature by Dubost and colleagues found that polyarticular septic arthritis (PASA) has a mortality of 31% to 42% compared with 4% to 8% for monoarticular septic arthritis, and RA was present in 67% of the PASA fatalities.1
Related: The Golden Era of Treatment in Rheumatology
Rheumatoid arthritis and its treatment predispose patients to septic arthritis. Septic arthritis in the UK general population is 0.42 per 100 patient-years for patients with RA on antitumor necrosis factor therapy.3,4 In a retrospective study in the U.S., the incidence of septic arthritis was 0.40 per 100 patient-years for patients with RA compared with 0.02 per 100 patient-years for patients without RA.5
Other complications of RA include infectious tenosynovitis and tendon rupture. The incidence and prevalence of infectious tenosynovitis and tendon rupture in RA are not firmly established in the literature.
We present a patient with RA and psoriasis who responded initially to acute management for RA but subsequently was diagnosed with culture-negative polyarticular arthritis and infectious tenosynovitis associated with beta hemolytic group G Streptococcus (GGS), a part of Streptococcus milleri (S. milleri). During surgery, he was also found to have bilateral extensor pollicus longus (EPL) tendon rupture. Given the possible morbidity, the authors believe this patient may be of interest to the medical community.
Case Presentation
A 69-year-old African American male presented with 3 to 4 days of swelling and pain of bilateral wrists, bilateral hands, and the left ankle with subjective, but resolved, fevers and chills. His medical history was significant for seropositive erosive RA, psoriasis, hypertension, hyperlipidemia, alcohol abuse, chronic tobacco use, osteoporosis, and glaucoma. He did not have diabetes, reported no IV drug abuse, and except for the immunosuppressive effects of his medications, was not otherwise immunocompromised.
For 2 years in the outpatient setting, the rheumatology clinic had been managing the patient’s rheumatoid factor (RF) positive and anti-cyclic citrullinated peptide (CCP) antibody positive erosive RA with etanercept 25 mg subcutaneously twice a week. The RA affected his hands, wrists, shoulders, and ankles bilaterally but was successfully controlled. The dermatology clinic was managing the patient’s psoriasis with calcipotriene cream 0.005% twice a week and clobetasol ointment 0.05% twice a week. Psoriatic plaques were noted on bilateral elbows, bilateral dorsal hands, and bilateral dorsal feet.
Initial Evaluation
At evaluation, the patient’s vital signs revealed a temperature of 36.3°C (97.3°F), pulse of 102 beats per minute, respiratory rate of 16 breaths per minute, oxygen saturation of 99% on room air, and blood pressure of 102/70 mm Hg. He was found to have edema, tenderness, and erythema of the wrists bilaterally and left metacarpophalangeal joints (MCPs) and edematous right MCPs and left medial ankle.
The patient had been nonadherent with etanercept for 5 monthsand restarted taking the medication only 2 weeks before presentation. He had noticed worsening arthritis for at least 1 month. His last RA flare was approximately 1 year before presentation. Additional symptoms included 4 days of nausea, nonbloody and nonbilious emesis, left lower quadrant pain, and diarrhea without melena or hematochezia.
Initial laboratory studies found 3.2 k/μL white blood cells (WBCs) with a differential of 11.9% lymphocytes, 4.2% monocytes, 83.3% neutrophils, 0.5% eosinophils, and 0.1% basophils; 165 k/μL platelets; 96 mm/h erythrocyte sedimentation rate (ESR); and 45 mg/dL C-reactive protein. The patient was diagnosed with viral gastroenteritis and RA flare and was admitted for inpatient management secondary to limited ability to care for himself.
Related: Infliximab-Induced Complications
The patient was started on prednisone 40 mg orally once a day (for 5 days) for empiric treatment of an RA flare and continued on etanercept. The inpatient rheumatology service was consulted. Further evaluation later that day found involvement of the proximal interphalangeal joints and elbows and tenderness of the tendons of the dorsal hand bilaterally. Over the next 2 days, the patient remained afebrile and WBCs were within normal limits. Edema, erythema, and tenderness of the involved joints somewhat improved, but tenderness along the tendons of the dorsal hand worsened, which concerned the managing teams for infectious tenosynovitis.
By day 4, the patient was afebrile and had a leukocytosis of 12.9 k/μLwith neutrophils 86.7%, but improvement of erythema, pain, and range of motion of involved joints and no tenderness to palpation of tendons was noted. The inpatient orthopedic surgery service evaluated the patient and did not find sufficient evidence necessitating surgical intervention.
Worsening Condition
On day 6, arthrocentesis of the left wrist was performed secondary to worsening of erythema and edema. The patient experienced new edema of the left shoulder and leukocytosis continued to trend upward (15.7 k/μL on day 6). Purulent aspirate (1.5 mL) was obtained from the fluctuance and tenosynovium of the left wrist. Empiric vancomycin 1 g IV twice daily and ceftriaxone 2 g IV daily were started and continued for 3 days. By this point in his hospital course, the patient had received 1 dose of etanercept. Prednisone and etanercept were previously discontinued because of the discovered infection. Blood cultures were drawn and had no growth (Table). Gastroenterology studies were limited to stool cultures and did not include colonoscopy. Leukocytosis began trending down.
On day 8, antibiotics were tailored to penicillin G 4 million units IV every 4 hours following growth of GGS from the sample of the left wrist. Subsequently, synovial fluid (3 mL) from the left shoulder was obtained following initiation of antibiotic therapy and had no growth. Magnetic resonance imaging (MRI) found tenosynovitis of the left ankle and right wrist.
On day 9, transthoracic echocardiography was performed and found no evidence of infectious endocarditis. Later that night, the patient was taken to surgery for incision and drainage/debridement of bilateral wrists and left ankle, synovectomy of right wrist, and aspiration of right shoulder. Findings included abscess in the left wrist and inflammatory synovitis and bilateral EPL tendon rupture consistent with RA. Pus from the left ankle had few gram-positive cocci in chains with no growth, and the specimens from both wrists grew GGS. Aspirate from the left ankle was an opaque yellow fluid with 14,900/mm3 WBC, 30,000/mm3 red blood cells (RBC), 97% neutrophils, 1% macrophages, 2% lymphocytes, and 0% monocytes. Aspirate from the right shoulder was an opaque bloody fluid with 10,100/mm3 WBC, 40,000/mm3 RBC, 95% neutrophils, 2% macrophages, 1% lymphocytes, and 1% monocytes. On day 10, sulfasalazine 500 mg twice a day was initiated for RA.
Following surgery and continued antibiotics, the patient’s leukocytosis resolved, and improvement was seen in all joints with decreased edema, erythema, and pain and increased range of motion. Postoperative recovery was complicated by ileus, urinary retention, and fungal (Candida albicans) urinary tract infection, all of which resolved without significant complications. The inpatient rheumatology service restarted prednisone at a lower dose of 20 mg. The patient became afebrile and sufficiently stable for transfer to a lower level of care with continued physical therapy and IV antibiotics for another 3 weeks.
Discussion
The patient had 2 underlying systemic inflammatory conditions: RA and psoriasis. The underlying chronic arthritis was likely caused by RA, not psoriatic arthritis (PsA). The patient met the 2010 American College of Rheumatology criteria but failed to meet the classification criteria for PsA.6,7 However, the clinical features of RA and PsA overlap. Rheumatoid factor and CCP can be positive laboratory findings in both RA and PsA.8-14 Tenosynovitis is found in about half of RA patients and PsA patients (P > .05).15 In its evaluation of the patient, the inpatient rheumatology service suspected that the patient may have had RA with components of PsA.
Rheumatoid arthritis complicates the diagnosis of septic arthritis. In a study by Nolla and colleagues, a mean of 7.3 days (range 3 to 18 days) elapsed before a diagnosis of septic arthritis was made in 10 patients with RA on corticosteroids.2 Consideration of risk factors such as increasing age, male sex, tobacco use, extra-articular manifestations of RA, positive RF, rheumatoid nodules, poor functional capacity, high ESR, leukopenia, comorbidities (chronic lung disease, alcoholism, organic brain disease, and diabetes), and the use of corticosteroids may expedite the diagnosis of infections in patients with RA.16 In this case, the patient had some of these risk factors: age, male sex, alcoholism, chronic tobacco use, positive RF, high ESR, and leukopenia (at presentation).
Related: Trend Toward Concomitant Supplements and Medications
The history of medication nonadherence of etanercept with progressively worsening arthritis and early clinical improvement (reduction in erythema, edema, and pain and temporary loss of signs of tenosynovitis on examination) while on prednisone suggested that the patient had a RA flare. The prednisone likely alleviated the inflammatory process but created an immunosuppressed state that allowed GGS to invade and possibly disseminate. Alternately, the patient may have been infected before presentation. The lack of a definitive time line for his case prevented the authors from forming conclusions about a possible causal relationship between the infection and medications. The subjective fevers before admission were nonspecific and could have been caused by RA, presumed gastroenteritis, or other undiagnosed infectious processes. The observed leukocytosis may have been initially corticosteroid-induced.17
Septic Arthritis
The suspicion of septic arthritis and infectious tenosynovitis substantially increased on day 6 with worsening symptoms, involvement of additional joints, and spiking fevers. Group G Streptococcus was obtained from the aspirate of the left wrist and from the surgical specimens from the bilateral wrists. The clinical presentation, MRI imaging studies, and surgical and nonsurgical specimens supported a diagnosis of GGS tenosynovitis. However, there was no clear evidence (ie, positive culture with identified organism) of septic arthritis, likely secondary to early septic arthritis and initiation of antibiotics before joint aspirations. The aspirate from the left ankle was yellow and opaque, but the culture was negative.
The pathogenic organism in the patient was GGS. Group G Streptococcus is normal flora of the oral cavity, gastrointestinal (GI) tract, upper respiratory tract, genital tract, and skin, which were all possible sources of seeding.18 Streptococcal species account for about 20% of septic arthritis, and GGS arthritis accounts for 4% to 19% of streptococcal arthritis.19-22 From a review of the literature, 2 cases of GGS tenosynovitis have been published.23,24 However, in an ultrasound study and MRI study, 49% and 43%, respectively, of patients with RA had tenosynovitis of the tendons of the hands.15,25
GGS Demographics
About three-quarters (71%) of patients with GGS arthritis are male.19 The analysis of the literature by Bronze and colleagues found that chronic joint disease and alcoholism are present in 34% and 14% of patients with GGS arthritis, respectively. One-quarter (23% from Dubost and colleagues) to one-third (32% from Schattner and colleagues) of patients with GGS arthritis have RA.19,26
Fever is present in less than half (43%) of patients with GGS arthritis.19 Positive synovial fluid is expected in 90% of patients.19 Leukocytosis and elevated ESR need not be present.27,28 The arthritis is polyarticular in one-quarter of patients (24% from Bronze and colleagues and 26% from Dubost and colleagues).19,26
Positive blood cultures can be expected in one-fourth (26%) of patients with GGS arthritis.19 The patient’s blood cultures were negative. Blood cultures drawn before initiation of antibiotics yielded no growth, so if the spread was hematogenous, the bacteremia was transient or intermittent. Before and after initiation of antibiotics, specimens from the shoulders did not grow colonies, whereas specimens from the wrists did. If the shoulders were truly infected, these findings and the notably later involvement of the shoulders suggest that the shoulders may have been seeded later in the hospital course.
Trenkner and colleagues proposed that GI abnormalities provide a portal of entry for GGS, which is under the umbrella of S. milleri.29S. milleri is associated with abscess formation, usually of the GI tract.30-32 In the study patient, the possible gastroenteritis may have provided such a portal of entry and subsequent seeding to the joints, and an abscess was found in the left wrist.
Tendon Rupture
Additionally, bilateral EPL tendon rupture likely occurred as a consequence of the inflammatory process from RA and infectious tenosynovitis in the patient. According to Zheng and colleagues, tenosynovitis is an inflammatory process of the synovial tendon sheath that may result in degeneration and rupture of the tendons and may contribute to bone erosions, development of joint deformities, and loss of functional capacity.33 In a histologic study of a ruptured EPL tendon from a patient with RA, Harris observed a chronic inflammatory cellular reaction.34 Harris also described a male with RA with unconfirmed bilateral EPL rupture.34 Björkman and colleague identified previous injury, RA, and local or systemic steroids as important etiologic factors for EPL tendon rupture.35
As in the case of this patient, the utilization of both medical and surgical therapy is not uncommon for treating GGS infection. Antibiotic therapy typically consists of penicillin (74%).26 Surgical intervention is necessary in 16% to 37% of patients.19,26 This patient required both penicillin and incision and drainage/debridement before significant clinical improvement was noted. Prognosis of GGS arthritis is favorable with 5% mortality.26
Conclusion
Septic arthritis and infectious tenosynovitis are readily treatable with low mortality if promptly identified. Identification can be masked by other medical conditions, such as RA and psoriasis, and their associated immunosuppressive treatment. Bilateral EPL tendon rupture may be a complication of RA, particularly with an underlying septic arthritis and infectious tenosynovitis.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Dubost JJ, Fis I, Denis P, et al. Polyarticular septic arthritis. Medicine (Baltimore). 1993;72(5):296-310.
2. Nolla JM, Gómez-Vaquero C, Fiter J, et al. Pyarthrosis in patients with rheumatoid arthritis: A detailed analysis of 10 cases and literature review. Semin Arthritis Rheum. 2000;30(2):121-126.
3.Weston VC, Jones AC, Bradbury N, Fawthrop F, Doherty M. Clinical features and outcome of septic arthritis in a single UK Health District 1982-1991. Ann Rheum Dis. 1999;58(4):214-219.
4. Galloway JB, Hyrich KL, Mercer LK, et al; BSR Biologics Register. Risk of septic arthritis in patients with rheumatoid arthritis and the effect of anti-TNF therapy: Results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2011;70(10):1810-1814.
5. Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: A population-based study. Arthritis Rheum. 2002;46(9):2287-2293.
6. Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569-2581.
7. Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H; CASPAR Study Group. Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis Rheum. 2006;54(8):2665-2673.
8. Gladman DD, Shuckett R, Russell ML, Thorne JC, Schachter RK. Psoriatic arthritis (PSA)—An analysis of 220 patients. Q J Med. 1987;62(238):127-141.
9. Bogliolo L, Alpini C, Caporali R, Scirè CA, Moratti R, Montecucco C. Antibodies to cyclic citrullinated peptides in psoriatic arthritis. J Rheumatol. 2005;32(3):511-515.
10. Vander Cruyssen B, Hoffman IE, Zmierczak H, et al. Anti-citrullinated peptide antibodies may occur in patients with psoriatic arthritis. Ann Rheum Dis. 2005;64(8):1145-1149.
11. Alenius GM, Berglin E, Rantapää Dahlgvist S. Antibodies against cyclic citrullinated peptide (CCP) in psoriatic patients with or without joint inflammation. Ann Rheum Dis. 2006;65(3):398-400.
12. Candia L, Marquez J, Gonzalez C, et al. Low frequency of anticyclic citrullinated peptide antibodies in psoriatic arthritis but not in cutaneous psoriasis. J Clin Rheumatol. 2006;12(5):226-229.
13. Inanc N, Dalkilic E, Kamali S, et al. Anti-CCP antibodies in rheumatoid arthritis and psoriatic arthritis. Clin Rheumatol. 2007;26(1):17-23.
14. Popescu C, Zofota S, Bojinca V, Ionescu R. Anti-cyclic citrullinated peptide antibodies in psoriatic arthritis—Cross-sectional study and literature review. J Med Life. 2013;6(4):376-382.
15. Schoellnast H, Deutschmann HA, Hermann J, et al. Psoriatic arthritis and rheumatoid arthritis: Findings in contrast-enhanced MRI. AJR Am J Roentgenol. 2006;187(2):351-357.
16. Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Predictors of infection in rheumatoid arthritis. Arthritis Rheum. 2002;46(9):2294-2300.
17. Shoenfeld Y, Gurewich Y, Gallant LA, Pinkhas J. Prednisone-induced leukocytosis. Influence of dosage, method and duration of administration on the degree of leukocytosis. Am J Med. 1981;71(5):773-778.
18. Gossling J. Occurrence and pathogenicity of the Streptococcus milleri group. Rev Infect Dis. 1988;10(2):257-285.
19. Dubost JJ, Soubrier M, De Champs C, Ristori JM, Sauvezie B. Streptococcal septic arthritis in adults. A study of 55 cases with a literature review. Joint Bone Spine. 2004;71(4):303-311.
20. Ryan MJ, Kavanagh R, Wall PG, Hazleman BL. Bacterial joint infections in England and Wales: Analysis of bacterial isolates over a four year period. Br J Rheumatol. 1997;36(3):370-373.
21. Morgan DS, Fisher D, Merianos A, Currie BJ. An 18 year clinical review of septic arthritis from tropical Australia. Epidemiol Infect. 1996;117(3):423-428.
22. Kaandorp CJ, Dinant HJ, van de Laar MA, Moens HJ, Prins AP, Dijkmans BA. Incidence and sources of native and prosthetic joint infection: A community based prospective survey. Ann Rheum Dis. 1997;56(8):470-475.
23. Bradlow A, Mitchell RG, Mowat AG. Group G streptococcal arthritis. Rheumatol Rehabil. 1982;21(4):206-210.
24. Meier JL, Gerster JC. Bursitis and tenosynovitis caused by group G streptococci. J Rheumatol. 1983;10(5):817-818.
25. Filippucci E, Gabba A, Di Geso L, Girolimetti R, Salaffi F, Grassi W. Hand tendon involvement in rheumatoid arthritis: An ultrasound study. Semin Arthritis Rheum. 2012;41(6):752-760.
26. Bronze MS, Whitby S, Schaberg DR. Group G streptococcal arthritis: Case report and review of the literature. Am J Med Sci. 1997;313(4):239-243.
27. Schattner A, Vosti KL. Bacterial arthritis due to beta-hemolytic streptococci of serogroups A, B, C, F, and G. Analysis of 23 cases and a review of the literature. Medicine (Baltimore). 1998;77(2):122-139.
28. Gaunt PN, Seal DV. Group G streptococcal infection of joints and joint prostheses. J Infect. 1986;13(2):115-123.
29. Trenkner SW, Braunstein EM, Lynn MD, Ike RW. Group G streptococcal arthritis and bowel disease: A rare enteropathic arthropathy. Gastrointest Radiol. 1987;12(3):265-267.
30. Bert F, Bariou-Lancelin M, Lambert-Zechovsky N. Clinical significance of bacteremia involving the “Streptococcus milleri” group: 51 cases and review. Clin Infect Dis. 1998;27(2):385-387.
31. Casariego E, Rodriguez A, Corredoira JC, et al. Prospective study of Streptococcus milleri bacteremia. Eur J Clin Microbiol Infect Dis. 1996;15(3):194-200.
32. Jacobs JA, Pietersen HG, Stobberingh EE, Soeters PB. Bacteremia involving the “Streptococcus milleri” group: Analysis of 19 cases. Clin Infect Dis. 1994;19(4):704-713.
33. Zheng S, Robinson E, Yeoman S, et al. MRI bone oedema predicts eight year tendon function at the wrist but not the requirement for orthopaedic surgery in rheumatoid arthritis. Ann Rheum Dis. 2006;65(5):607-611.
34. Harris R. Spontaneous rupture of the tendon of extensor pollicis longus as a complication of rheumatoid arthritis. Ann Rheum Dis. 1951;10(3):298-306.
35. Björkman A, Jörgsholm P. Rupture of the extensor pollicis longus tendon: A study of aetiological factors. Scand J Plast Reconstr Surg Hand Surg. 2004;38(1):32-35.
Compared with monoarticular arthritis, polyarticular arthritis may yield an initially narrower differential diagnosis that focuses on systemic inflammatory conditions, such as rheumatoid arthritis (RA). Approximately 15% to 30% of septic arthritis is polyarticular, of which about 45% is associated with underlying RA.1,2 Regardless of the number of joints involved, septic (infectious) arthritis is a valid consideration given the morbidity and mortality.
In a retrospective study in the United Kingdom (UK) between 1982 and 1991, the morbidity and mortality of septic arthritis was 31.6% and 11.5%, respectively, and 16% of the study population had RA.3 A review of the literature by Dubost and colleagues found that polyarticular septic arthritis (PASA) has a mortality of 31% to 42% compared with 4% to 8% for monoarticular septic arthritis, and RA was present in 67% of the PASA fatalities.1
Related: The Golden Era of Treatment in Rheumatology
Rheumatoid arthritis and its treatment predispose patients to septic arthritis. Septic arthritis in the UK general population is 0.42 per 100 patient-years for patients with RA on antitumor necrosis factor therapy.3,4 In a retrospective study in the U.S., the incidence of septic arthritis was 0.40 per 100 patient-years for patients with RA compared with 0.02 per 100 patient-years for patients without RA.5
Other complications of RA include infectious tenosynovitis and tendon rupture. The incidence and prevalence of infectious tenosynovitis and tendon rupture in RA are not firmly established in the literature.
We present a patient with RA and psoriasis who responded initially to acute management for RA but subsequently was diagnosed with culture-negative polyarticular arthritis and infectious tenosynovitis associated with beta hemolytic group G Streptococcus (GGS), a part of Streptococcus milleri (S. milleri). During surgery, he was also found to have bilateral extensor pollicus longus (EPL) tendon rupture. Given the possible morbidity, the authors believe this patient may be of interest to the medical community.
Case Presentation
A 69-year-old African American male presented with 3 to 4 days of swelling and pain of bilateral wrists, bilateral hands, and the left ankle with subjective, but resolved, fevers and chills. His medical history was significant for seropositive erosive RA, psoriasis, hypertension, hyperlipidemia, alcohol abuse, chronic tobacco use, osteoporosis, and glaucoma. He did not have diabetes, reported no IV drug abuse, and except for the immunosuppressive effects of his medications, was not otherwise immunocompromised.
For 2 years in the outpatient setting, the rheumatology clinic had been managing the patient’s rheumatoid factor (RF) positive and anti-cyclic citrullinated peptide (CCP) antibody positive erosive RA with etanercept 25 mg subcutaneously twice a week. The RA affected his hands, wrists, shoulders, and ankles bilaterally but was successfully controlled. The dermatology clinic was managing the patient’s psoriasis with calcipotriene cream 0.005% twice a week and clobetasol ointment 0.05% twice a week. Psoriatic plaques were noted on bilateral elbows, bilateral dorsal hands, and bilateral dorsal feet.
Initial Evaluation
At evaluation, the patient’s vital signs revealed a temperature of 36.3°C (97.3°F), pulse of 102 beats per minute, respiratory rate of 16 breaths per minute, oxygen saturation of 99% on room air, and blood pressure of 102/70 mm Hg. He was found to have edema, tenderness, and erythema of the wrists bilaterally and left metacarpophalangeal joints (MCPs) and edematous right MCPs and left medial ankle.
The patient had been nonadherent with etanercept for 5 monthsand restarted taking the medication only 2 weeks before presentation. He had noticed worsening arthritis for at least 1 month. His last RA flare was approximately 1 year before presentation. Additional symptoms included 4 days of nausea, nonbloody and nonbilious emesis, left lower quadrant pain, and diarrhea without melena or hematochezia.
Initial laboratory studies found 3.2 k/μL white blood cells (WBCs) with a differential of 11.9% lymphocytes, 4.2% monocytes, 83.3% neutrophils, 0.5% eosinophils, and 0.1% basophils; 165 k/μL platelets; 96 mm/h erythrocyte sedimentation rate (ESR); and 45 mg/dL C-reactive protein. The patient was diagnosed with viral gastroenteritis and RA flare and was admitted for inpatient management secondary to limited ability to care for himself.
Related: Infliximab-Induced Complications
The patient was started on prednisone 40 mg orally once a day (for 5 days) for empiric treatment of an RA flare and continued on etanercept. The inpatient rheumatology service was consulted. Further evaluation later that day found involvement of the proximal interphalangeal joints and elbows and tenderness of the tendons of the dorsal hand bilaterally. Over the next 2 days, the patient remained afebrile and WBCs were within normal limits. Edema, erythema, and tenderness of the involved joints somewhat improved, but tenderness along the tendons of the dorsal hand worsened, which concerned the managing teams for infectious tenosynovitis.
By day 4, the patient was afebrile and had a leukocytosis of 12.9 k/μLwith neutrophils 86.7%, but improvement of erythema, pain, and range of motion of involved joints and no tenderness to palpation of tendons was noted. The inpatient orthopedic surgery service evaluated the patient and did not find sufficient evidence necessitating surgical intervention.
Worsening Condition
On day 6, arthrocentesis of the left wrist was performed secondary to worsening of erythema and edema. The patient experienced new edema of the left shoulder and leukocytosis continued to trend upward (15.7 k/μL on day 6). Purulent aspirate (1.5 mL) was obtained from the fluctuance and tenosynovium of the left wrist. Empiric vancomycin 1 g IV twice daily and ceftriaxone 2 g IV daily were started and continued for 3 days. By this point in his hospital course, the patient had received 1 dose of etanercept. Prednisone and etanercept were previously discontinued because of the discovered infection. Blood cultures were drawn and had no growth (Table). Gastroenterology studies were limited to stool cultures and did not include colonoscopy. Leukocytosis began trending down.
On day 8, antibiotics were tailored to penicillin G 4 million units IV every 4 hours following growth of GGS from the sample of the left wrist. Subsequently, synovial fluid (3 mL) from the left shoulder was obtained following initiation of antibiotic therapy and had no growth. Magnetic resonance imaging (MRI) found tenosynovitis of the left ankle and right wrist.
On day 9, transthoracic echocardiography was performed and found no evidence of infectious endocarditis. Later that night, the patient was taken to surgery for incision and drainage/debridement of bilateral wrists and left ankle, synovectomy of right wrist, and aspiration of right shoulder. Findings included abscess in the left wrist and inflammatory synovitis and bilateral EPL tendon rupture consistent with RA. Pus from the left ankle had few gram-positive cocci in chains with no growth, and the specimens from both wrists grew GGS. Aspirate from the left ankle was an opaque yellow fluid with 14,900/mm3 WBC, 30,000/mm3 red blood cells (RBC), 97% neutrophils, 1% macrophages, 2% lymphocytes, and 0% monocytes. Aspirate from the right shoulder was an opaque bloody fluid with 10,100/mm3 WBC, 40,000/mm3 RBC, 95% neutrophils, 2% macrophages, 1% lymphocytes, and 1% monocytes. On day 10, sulfasalazine 500 mg twice a day was initiated for RA.
Following surgery and continued antibiotics, the patient’s leukocytosis resolved, and improvement was seen in all joints with decreased edema, erythema, and pain and increased range of motion. Postoperative recovery was complicated by ileus, urinary retention, and fungal (Candida albicans) urinary tract infection, all of which resolved without significant complications. The inpatient rheumatology service restarted prednisone at a lower dose of 20 mg. The patient became afebrile and sufficiently stable for transfer to a lower level of care with continued physical therapy and IV antibiotics for another 3 weeks.
Discussion
The patient had 2 underlying systemic inflammatory conditions: RA and psoriasis. The underlying chronic arthritis was likely caused by RA, not psoriatic arthritis (PsA). The patient met the 2010 American College of Rheumatology criteria but failed to meet the classification criteria for PsA.6,7 However, the clinical features of RA and PsA overlap. Rheumatoid factor and CCP can be positive laboratory findings in both RA and PsA.8-14 Tenosynovitis is found in about half of RA patients and PsA patients (P > .05).15 In its evaluation of the patient, the inpatient rheumatology service suspected that the patient may have had RA with components of PsA.
Rheumatoid arthritis complicates the diagnosis of septic arthritis. In a study by Nolla and colleagues, a mean of 7.3 days (range 3 to 18 days) elapsed before a diagnosis of septic arthritis was made in 10 patients with RA on corticosteroids.2 Consideration of risk factors such as increasing age, male sex, tobacco use, extra-articular manifestations of RA, positive RF, rheumatoid nodules, poor functional capacity, high ESR, leukopenia, comorbidities (chronic lung disease, alcoholism, organic brain disease, and diabetes), and the use of corticosteroids may expedite the diagnosis of infections in patients with RA.16 In this case, the patient had some of these risk factors: age, male sex, alcoholism, chronic tobacco use, positive RF, high ESR, and leukopenia (at presentation).
Related: Trend Toward Concomitant Supplements and Medications
The history of medication nonadherence of etanercept with progressively worsening arthritis and early clinical improvement (reduction in erythema, edema, and pain and temporary loss of signs of tenosynovitis on examination) while on prednisone suggested that the patient had a RA flare. The prednisone likely alleviated the inflammatory process but created an immunosuppressed state that allowed GGS to invade and possibly disseminate. Alternately, the patient may have been infected before presentation. The lack of a definitive time line for his case prevented the authors from forming conclusions about a possible causal relationship between the infection and medications. The subjective fevers before admission were nonspecific and could have been caused by RA, presumed gastroenteritis, or other undiagnosed infectious processes. The observed leukocytosis may have been initially corticosteroid-induced.17
Septic Arthritis
The suspicion of septic arthritis and infectious tenosynovitis substantially increased on day 6 with worsening symptoms, involvement of additional joints, and spiking fevers. Group G Streptococcus was obtained from the aspirate of the left wrist and from the surgical specimens from the bilateral wrists. The clinical presentation, MRI imaging studies, and surgical and nonsurgical specimens supported a diagnosis of GGS tenosynovitis. However, there was no clear evidence (ie, positive culture with identified organism) of septic arthritis, likely secondary to early septic arthritis and initiation of antibiotics before joint aspirations. The aspirate from the left ankle was yellow and opaque, but the culture was negative.
The pathogenic organism in the patient was GGS. Group G Streptococcus is normal flora of the oral cavity, gastrointestinal (GI) tract, upper respiratory tract, genital tract, and skin, which were all possible sources of seeding.18 Streptococcal species account for about 20% of septic arthritis, and GGS arthritis accounts for 4% to 19% of streptococcal arthritis.19-22 From a review of the literature, 2 cases of GGS tenosynovitis have been published.23,24 However, in an ultrasound study and MRI study, 49% and 43%, respectively, of patients with RA had tenosynovitis of the tendons of the hands.15,25
GGS Demographics
About three-quarters (71%) of patients with GGS arthritis are male.19 The analysis of the literature by Bronze and colleagues found that chronic joint disease and alcoholism are present in 34% and 14% of patients with GGS arthritis, respectively. One-quarter (23% from Dubost and colleagues) to one-third (32% from Schattner and colleagues) of patients with GGS arthritis have RA.19,26
Fever is present in less than half (43%) of patients with GGS arthritis.19 Positive synovial fluid is expected in 90% of patients.19 Leukocytosis and elevated ESR need not be present.27,28 The arthritis is polyarticular in one-quarter of patients (24% from Bronze and colleagues and 26% from Dubost and colleagues).19,26
Positive blood cultures can be expected in one-fourth (26%) of patients with GGS arthritis.19 The patient’s blood cultures were negative. Blood cultures drawn before initiation of antibiotics yielded no growth, so if the spread was hematogenous, the bacteremia was transient or intermittent. Before and after initiation of antibiotics, specimens from the shoulders did not grow colonies, whereas specimens from the wrists did. If the shoulders were truly infected, these findings and the notably later involvement of the shoulders suggest that the shoulders may have been seeded later in the hospital course.
Trenkner and colleagues proposed that GI abnormalities provide a portal of entry for GGS, which is under the umbrella of S. milleri.29S. milleri is associated with abscess formation, usually of the GI tract.30-32 In the study patient, the possible gastroenteritis may have provided such a portal of entry and subsequent seeding to the joints, and an abscess was found in the left wrist.
Tendon Rupture
Additionally, bilateral EPL tendon rupture likely occurred as a consequence of the inflammatory process from RA and infectious tenosynovitis in the patient. According to Zheng and colleagues, tenosynovitis is an inflammatory process of the synovial tendon sheath that may result in degeneration and rupture of the tendons and may contribute to bone erosions, development of joint deformities, and loss of functional capacity.33 In a histologic study of a ruptured EPL tendon from a patient with RA, Harris observed a chronic inflammatory cellular reaction.34 Harris also described a male with RA with unconfirmed bilateral EPL rupture.34 Björkman and colleague identified previous injury, RA, and local or systemic steroids as important etiologic factors for EPL tendon rupture.35
As in the case of this patient, the utilization of both medical and surgical therapy is not uncommon for treating GGS infection. Antibiotic therapy typically consists of penicillin (74%).26 Surgical intervention is necessary in 16% to 37% of patients.19,26 This patient required both penicillin and incision and drainage/debridement before significant clinical improvement was noted. Prognosis of GGS arthritis is favorable with 5% mortality.26
Conclusion
Septic arthritis and infectious tenosynovitis are readily treatable with low mortality if promptly identified. Identification can be masked by other medical conditions, such as RA and psoriasis, and their associated immunosuppressive treatment. Bilateral EPL tendon rupture may be a complication of RA, particularly with an underlying septic arthritis and infectious tenosynovitis.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Compared with monoarticular arthritis, polyarticular arthritis may yield an initially narrower differential diagnosis that focuses on systemic inflammatory conditions, such as rheumatoid arthritis (RA). Approximately 15% to 30% of septic arthritis is polyarticular, of which about 45% is associated with underlying RA.1,2 Regardless of the number of joints involved, septic (infectious) arthritis is a valid consideration given the morbidity and mortality.
In a retrospective study in the United Kingdom (UK) between 1982 and 1991, the morbidity and mortality of septic arthritis was 31.6% and 11.5%, respectively, and 16% of the study population had RA.3 A review of the literature by Dubost and colleagues found that polyarticular septic arthritis (PASA) has a mortality of 31% to 42% compared with 4% to 8% for monoarticular septic arthritis, and RA was present in 67% of the PASA fatalities.1
Related: The Golden Era of Treatment in Rheumatology
Rheumatoid arthritis and its treatment predispose patients to septic arthritis. Septic arthritis in the UK general population is 0.42 per 100 patient-years for patients with RA on antitumor necrosis factor therapy.3,4 In a retrospective study in the U.S., the incidence of septic arthritis was 0.40 per 100 patient-years for patients with RA compared with 0.02 per 100 patient-years for patients without RA.5
Other complications of RA include infectious tenosynovitis and tendon rupture. The incidence and prevalence of infectious tenosynovitis and tendon rupture in RA are not firmly established in the literature.
We present a patient with RA and psoriasis who responded initially to acute management for RA but subsequently was diagnosed with culture-negative polyarticular arthritis and infectious tenosynovitis associated with beta hemolytic group G Streptococcus (GGS), a part of Streptococcus milleri (S. milleri). During surgery, he was also found to have bilateral extensor pollicus longus (EPL) tendon rupture. Given the possible morbidity, the authors believe this patient may be of interest to the medical community.
Case Presentation
A 69-year-old African American male presented with 3 to 4 days of swelling and pain of bilateral wrists, bilateral hands, and the left ankle with subjective, but resolved, fevers and chills. His medical history was significant for seropositive erosive RA, psoriasis, hypertension, hyperlipidemia, alcohol abuse, chronic tobacco use, osteoporosis, and glaucoma. He did not have diabetes, reported no IV drug abuse, and except for the immunosuppressive effects of his medications, was not otherwise immunocompromised.
For 2 years in the outpatient setting, the rheumatology clinic had been managing the patient’s rheumatoid factor (RF) positive and anti-cyclic citrullinated peptide (CCP) antibody positive erosive RA with etanercept 25 mg subcutaneously twice a week. The RA affected his hands, wrists, shoulders, and ankles bilaterally but was successfully controlled. The dermatology clinic was managing the patient’s psoriasis with calcipotriene cream 0.005% twice a week and clobetasol ointment 0.05% twice a week. Psoriatic plaques were noted on bilateral elbows, bilateral dorsal hands, and bilateral dorsal feet.
Initial Evaluation
At evaluation, the patient’s vital signs revealed a temperature of 36.3°C (97.3°F), pulse of 102 beats per minute, respiratory rate of 16 breaths per minute, oxygen saturation of 99% on room air, and blood pressure of 102/70 mm Hg. He was found to have edema, tenderness, and erythema of the wrists bilaterally and left metacarpophalangeal joints (MCPs) and edematous right MCPs and left medial ankle.
The patient had been nonadherent with etanercept for 5 monthsand restarted taking the medication only 2 weeks before presentation. He had noticed worsening arthritis for at least 1 month. His last RA flare was approximately 1 year before presentation. Additional symptoms included 4 days of nausea, nonbloody and nonbilious emesis, left lower quadrant pain, and diarrhea without melena or hematochezia.
Initial laboratory studies found 3.2 k/μL white blood cells (WBCs) with a differential of 11.9% lymphocytes, 4.2% monocytes, 83.3% neutrophils, 0.5% eosinophils, and 0.1% basophils; 165 k/μL platelets; 96 mm/h erythrocyte sedimentation rate (ESR); and 45 mg/dL C-reactive protein. The patient was diagnosed with viral gastroenteritis and RA flare and was admitted for inpatient management secondary to limited ability to care for himself.
Related: Infliximab-Induced Complications
The patient was started on prednisone 40 mg orally once a day (for 5 days) for empiric treatment of an RA flare and continued on etanercept. The inpatient rheumatology service was consulted. Further evaluation later that day found involvement of the proximal interphalangeal joints and elbows and tenderness of the tendons of the dorsal hand bilaterally. Over the next 2 days, the patient remained afebrile and WBCs were within normal limits. Edema, erythema, and tenderness of the involved joints somewhat improved, but tenderness along the tendons of the dorsal hand worsened, which concerned the managing teams for infectious tenosynovitis.
By day 4, the patient was afebrile and had a leukocytosis of 12.9 k/μLwith neutrophils 86.7%, but improvement of erythema, pain, and range of motion of involved joints and no tenderness to palpation of tendons was noted. The inpatient orthopedic surgery service evaluated the patient and did not find sufficient evidence necessitating surgical intervention.
Worsening Condition
On day 6, arthrocentesis of the left wrist was performed secondary to worsening of erythema and edema. The patient experienced new edema of the left shoulder and leukocytosis continued to trend upward (15.7 k/μL on day 6). Purulent aspirate (1.5 mL) was obtained from the fluctuance and tenosynovium of the left wrist. Empiric vancomycin 1 g IV twice daily and ceftriaxone 2 g IV daily were started and continued for 3 days. By this point in his hospital course, the patient had received 1 dose of etanercept. Prednisone and etanercept were previously discontinued because of the discovered infection. Blood cultures were drawn and had no growth (Table). Gastroenterology studies were limited to stool cultures and did not include colonoscopy. Leukocytosis began trending down.
On day 8, antibiotics were tailored to penicillin G 4 million units IV every 4 hours following growth of GGS from the sample of the left wrist. Subsequently, synovial fluid (3 mL) from the left shoulder was obtained following initiation of antibiotic therapy and had no growth. Magnetic resonance imaging (MRI) found tenosynovitis of the left ankle and right wrist.
On day 9, transthoracic echocardiography was performed and found no evidence of infectious endocarditis. Later that night, the patient was taken to surgery for incision and drainage/debridement of bilateral wrists and left ankle, synovectomy of right wrist, and aspiration of right shoulder. Findings included abscess in the left wrist and inflammatory synovitis and bilateral EPL tendon rupture consistent with RA. Pus from the left ankle had few gram-positive cocci in chains with no growth, and the specimens from both wrists grew GGS. Aspirate from the left ankle was an opaque yellow fluid with 14,900/mm3 WBC, 30,000/mm3 red blood cells (RBC), 97% neutrophils, 1% macrophages, 2% lymphocytes, and 0% monocytes. Aspirate from the right shoulder was an opaque bloody fluid with 10,100/mm3 WBC, 40,000/mm3 RBC, 95% neutrophils, 2% macrophages, 1% lymphocytes, and 1% monocytes. On day 10, sulfasalazine 500 mg twice a day was initiated for RA.
Following surgery and continued antibiotics, the patient’s leukocytosis resolved, and improvement was seen in all joints with decreased edema, erythema, and pain and increased range of motion. Postoperative recovery was complicated by ileus, urinary retention, and fungal (Candida albicans) urinary tract infection, all of which resolved without significant complications. The inpatient rheumatology service restarted prednisone at a lower dose of 20 mg. The patient became afebrile and sufficiently stable for transfer to a lower level of care with continued physical therapy and IV antibiotics for another 3 weeks.
Discussion
The patient had 2 underlying systemic inflammatory conditions: RA and psoriasis. The underlying chronic arthritis was likely caused by RA, not psoriatic arthritis (PsA). The patient met the 2010 American College of Rheumatology criteria but failed to meet the classification criteria for PsA.6,7 However, the clinical features of RA and PsA overlap. Rheumatoid factor and CCP can be positive laboratory findings in both RA and PsA.8-14 Tenosynovitis is found in about half of RA patients and PsA patients (P > .05).15 In its evaluation of the patient, the inpatient rheumatology service suspected that the patient may have had RA with components of PsA.
Rheumatoid arthritis complicates the diagnosis of septic arthritis. In a study by Nolla and colleagues, a mean of 7.3 days (range 3 to 18 days) elapsed before a diagnosis of septic arthritis was made in 10 patients with RA on corticosteroids.2 Consideration of risk factors such as increasing age, male sex, tobacco use, extra-articular manifestations of RA, positive RF, rheumatoid nodules, poor functional capacity, high ESR, leukopenia, comorbidities (chronic lung disease, alcoholism, organic brain disease, and diabetes), and the use of corticosteroids may expedite the diagnosis of infections in patients with RA.16 In this case, the patient had some of these risk factors: age, male sex, alcoholism, chronic tobacco use, positive RF, high ESR, and leukopenia (at presentation).
Related: Trend Toward Concomitant Supplements and Medications
The history of medication nonadherence of etanercept with progressively worsening arthritis and early clinical improvement (reduction in erythema, edema, and pain and temporary loss of signs of tenosynovitis on examination) while on prednisone suggested that the patient had a RA flare. The prednisone likely alleviated the inflammatory process but created an immunosuppressed state that allowed GGS to invade and possibly disseminate. Alternately, the patient may have been infected before presentation. The lack of a definitive time line for his case prevented the authors from forming conclusions about a possible causal relationship between the infection and medications. The subjective fevers before admission were nonspecific and could have been caused by RA, presumed gastroenteritis, or other undiagnosed infectious processes. The observed leukocytosis may have been initially corticosteroid-induced.17
Septic Arthritis
The suspicion of septic arthritis and infectious tenosynovitis substantially increased on day 6 with worsening symptoms, involvement of additional joints, and spiking fevers. Group G Streptococcus was obtained from the aspirate of the left wrist and from the surgical specimens from the bilateral wrists. The clinical presentation, MRI imaging studies, and surgical and nonsurgical specimens supported a diagnosis of GGS tenosynovitis. However, there was no clear evidence (ie, positive culture with identified organism) of septic arthritis, likely secondary to early septic arthritis and initiation of antibiotics before joint aspirations. The aspirate from the left ankle was yellow and opaque, but the culture was negative.
The pathogenic organism in the patient was GGS. Group G Streptococcus is normal flora of the oral cavity, gastrointestinal (GI) tract, upper respiratory tract, genital tract, and skin, which were all possible sources of seeding.18 Streptococcal species account for about 20% of septic arthritis, and GGS arthritis accounts for 4% to 19% of streptococcal arthritis.19-22 From a review of the literature, 2 cases of GGS tenosynovitis have been published.23,24 However, in an ultrasound study and MRI study, 49% and 43%, respectively, of patients with RA had tenosynovitis of the tendons of the hands.15,25
GGS Demographics
About three-quarters (71%) of patients with GGS arthritis are male.19 The analysis of the literature by Bronze and colleagues found that chronic joint disease and alcoholism are present in 34% and 14% of patients with GGS arthritis, respectively. One-quarter (23% from Dubost and colleagues) to one-third (32% from Schattner and colleagues) of patients with GGS arthritis have RA.19,26
Fever is present in less than half (43%) of patients with GGS arthritis.19 Positive synovial fluid is expected in 90% of patients.19 Leukocytosis and elevated ESR need not be present.27,28 The arthritis is polyarticular in one-quarter of patients (24% from Bronze and colleagues and 26% from Dubost and colleagues).19,26
Positive blood cultures can be expected in one-fourth (26%) of patients with GGS arthritis.19 The patient’s blood cultures were negative. Blood cultures drawn before initiation of antibiotics yielded no growth, so if the spread was hematogenous, the bacteremia was transient or intermittent. Before and after initiation of antibiotics, specimens from the shoulders did not grow colonies, whereas specimens from the wrists did. If the shoulders were truly infected, these findings and the notably later involvement of the shoulders suggest that the shoulders may have been seeded later in the hospital course.
Trenkner and colleagues proposed that GI abnormalities provide a portal of entry for GGS, which is under the umbrella of S. milleri.29S. milleri is associated with abscess formation, usually of the GI tract.30-32 In the study patient, the possible gastroenteritis may have provided such a portal of entry and subsequent seeding to the joints, and an abscess was found in the left wrist.
Tendon Rupture
Additionally, bilateral EPL tendon rupture likely occurred as a consequence of the inflammatory process from RA and infectious tenosynovitis in the patient. According to Zheng and colleagues, tenosynovitis is an inflammatory process of the synovial tendon sheath that may result in degeneration and rupture of the tendons and may contribute to bone erosions, development of joint deformities, and loss of functional capacity.33 In a histologic study of a ruptured EPL tendon from a patient with RA, Harris observed a chronic inflammatory cellular reaction.34 Harris also described a male with RA with unconfirmed bilateral EPL rupture.34 Björkman and colleague identified previous injury, RA, and local or systemic steroids as important etiologic factors for EPL tendon rupture.35
As in the case of this patient, the utilization of both medical and surgical therapy is not uncommon for treating GGS infection. Antibiotic therapy typically consists of penicillin (74%).26 Surgical intervention is necessary in 16% to 37% of patients.19,26 This patient required both penicillin and incision and drainage/debridement before significant clinical improvement was noted. Prognosis of GGS arthritis is favorable with 5% mortality.26
Conclusion
Septic arthritis and infectious tenosynovitis are readily treatable with low mortality if promptly identified. Identification can be masked by other medical conditions, such as RA and psoriasis, and their associated immunosuppressive treatment. Bilateral EPL tendon rupture may be a complication of RA, particularly with an underlying septic arthritis and infectious tenosynovitis.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Dubost JJ, Fis I, Denis P, et al. Polyarticular septic arthritis. Medicine (Baltimore). 1993;72(5):296-310.
2. Nolla JM, Gómez-Vaquero C, Fiter J, et al. Pyarthrosis in patients with rheumatoid arthritis: A detailed analysis of 10 cases and literature review. Semin Arthritis Rheum. 2000;30(2):121-126.
3.Weston VC, Jones AC, Bradbury N, Fawthrop F, Doherty M. Clinical features and outcome of septic arthritis in a single UK Health District 1982-1991. Ann Rheum Dis. 1999;58(4):214-219.
4. Galloway JB, Hyrich KL, Mercer LK, et al; BSR Biologics Register. Risk of septic arthritis in patients with rheumatoid arthritis and the effect of anti-TNF therapy: Results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2011;70(10):1810-1814.
5. Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: A population-based study. Arthritis Rheum. 2002;46(9):2287-2293.
6. Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569-2581.
7. Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H; CASPAR Study Group. Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis Rheum. 2006;54(8):2665-2673.
8. Gladman DD, Shuckett R, Russell ML, Thorne JC, Schachter RK. Psoriatic arthritis (PSA)—An analysis of 220 patients. Q J Med. 1987;62(238):127-141.
9. Bogliolo L, Alpini C, Caporali R, Scirè CA, Moratti R, Montecucco C. Antibodies to cyclic citrullinated peptides in psoriatic arthritis. J Rheumatol. 2005;32(3):511-515.
10. Vander Cruyssen B, Hoffman IE, Zmierczak H, et al. Anti-citrullinated peptide antibodies may occur in patients with psoriatic arthritis. Ann Rheum Dis. 2005;64(8):1145-1149.
11. Alenius GM, Berglin E, Rantapää Dahlgvist S. Antibodies against cyclic citrullinated peptide (CCP) in psoriatic patients with or without joint inflammation. Ann Rheum Dis. 2006;65(3):398-400.
12. Candia L, Marquez J, Gonzalez C, et al. Low frequency of anticyclic citrullinated peptide antibodies in psoriatic arthritis but not in cutaneous psoriasis. J Clin Rheumatol. 2006;12(5):226-229.
13. Inanc N, Dalkilic E, Kamali S, et al. Anti-CCP antibodies in rheumatoid arthritis and psoriatic arthritis. Clin Rheumatol. 2007;26(1):17-23.
14. Popescu C, Zofota S, Bojinca V, Ionescu R. Anti-cyclic citrullinated peptide antibodies in psoriatic arthritis—Cross-sectional study and literature review. J Med Life. 2013;6(4):376-382.
15. Schoellnast H, Deutschmann HA, Hermann J, et al. Psoriatic arthritis and rheumatoid arthritis: Findings in contrast-enhanced MRI. AJR Am J Roentgenol. 2006;187(2):351-357.
16. Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Predictors of infection in rheumatoid arthritis. Arthritis Rheum. 2002;46(9):2294-2300.
17. Shoenfeld Y, Gurewich Y, Gallant LA, Pinkhas J. Prednisone-induced leukocytosis. Influence of dosage, method and duration of administration on the degree of leukocytosis. Am J Med. 1981;71(5):773-778.
18. Gossling J. Occurrence and pathogenicity of the Streptococcus milleri group. Rev Infect Dis. 1988;10(2):257-285.
19. Dubost JJ, Soubrier M, De Champs C, Ristori JM, Sauvezie B. Streptococcal septic arthritis in adults. A study of 55 cases with a literature review. Joint Bone Spine. 2004;71(4):303-311.
20. Ryan MJ, Kavanagh R, Wall PG, Hazleman BL. Bacterial joint infections in England and Wales: Analysis of bacterial isolates over a four year period. Br J Rheumatol. 1997;36(3):370-373.
21. Morgan DS, Fisher D, Merianos A, Currie BJ. An 18 year clinical review of septic arthritis from tropical Australia. Epidemiol Infect. 1996;117(3):423-428.
22. Kaandorp CJ, Dinant HJ, van de Laar MA, Moens HJ, Prins AP, Dijkmans BA. Incidence and sources of native and prosthetic joint infection: A community based prospective survey. Ann Rheum Dis. 1997;56(8):470-475.
23. Bradlow A, Mitchell RG, Mowat AG. Group G streptococcal arthritis. Rheumatol Rehabil. 1982;21(4):206-210.
24. Meier JL, Gerster JC. Bursitis and tenosynovitis caused by group G streptococci. J Rheumatol. 1983;10(5):817-818.
25. Filippucci E, Gabba A, Di Geso L, Girolimetti R, Salaffi F, Grassi W. Hand tendon involvement in rheumatoid arthritis: An ultrasound study. Semin Arthritis Rheum. 2012;41(6):752-760.
26. Bronze MS, Whitby S, Schaberg DR. Group G streptococcal arthritis: Case report and review of the literature. Am J Med Sci. 1997;313(4):239-243.
27. Schattner A, Vosti KL. Bacterial arthritis due to beta-hemolytic streptococci of serogroups A, B, C, F, and G. Analysis of 23 cases and a review of the literature. Medicine (Baltimore). 1998;77(2):122-139.
28. Gaunt PN, Seal DV. Group G streptococcal infection of joints and joint prostheses. J Infect. 1986;13(2):115-123.
29. Trenkner SW, Braunstein EM, Lynn MD, Ike RW. Group G streptococcal arthritis and bowel disease: A rare enteropathic arthropathy. Gastrointest Radiol. 1987;12(3):265-267.
30. Bert F, Bariou-Lancelin M, Lambert-Zechovsky N. Clinical significance of bacteremia involving the “Streptococcus milleri” group: 51 cases and review. Clin Infect Dis. 1998;27(2):385-387.
31. Casariego E, Rodriguez A, Corredoira JC, et al. Prospective study of Streptococcus milleri bacteremia. Eur J Clin Microbiol Infect Dis. 1996;15(3):194-200.
32. Jacobs JA, Pietersen HG, Stobberingh EE, Soeters PB. Bacteremia involving the “Streptococcus milleri” group: Analysis of 19 cases. Clin Infect Dis. 1994;19(4):704-713.
33. Zheng S, Robinson E, Yeoman S, et al. MRI bone oedema predicts eight year tendon function at the wrist but not the requirement for orthopaedic surgery in rheumatoid arthritis. Ann Rheum Dis. 2006;65(5):607-611.
34. Harris R. Spontaneous rupture of the tendon of extensor pollicis longus as a complication of rheumatoid arthritis. Ann Rheum Dis. 1951;10(3):298-306.
35. Björkman A, Jörgsholm P. Rupture of the extensor pollicis longus tendon: A study of aetiological factors. Scand J Plast Reconstr Surg Hand Surg. 2004;38(1):32-35.
1. Dubost JJ, Fis I, Denis P, et al. Polyarticular septic arthritis. Medicine (Baltimore). 1993;72(5):296-310.
2. Nolla JM, Gómez-Vaquero C, Fiter J, et al. Pyarthrosis in patients with rheumatoid arthritis: A detailed analysis of 10 cases and literature review. Semin Arthritis Rheum. 2000;30(2):121-126.
3.Weston VC, Jones AC, Bradbury N, Fawthrop F, Doherty M. Clinical features and outcome of septic arthritis in a single UK Health District 1982-1991. Ann Rheum Dis. 1999;58(4):214-219.
4. Galloway JB, Hyrich KL, Mercer LK, et al; BSR Biologics Register. Risk of septic arthritis in patients with rheumatoid arthritis and the effect of anti-TNF therapy: Results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2011;70(10):1810-1814.
5. Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: A population-based study. Arthritis Rheum. 2002;46(9):2287-2293.
6. Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569-2581.
7. Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H; CASPAR Study Group. Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis Rheum. 2006;54(8):2665-2673.
8. Gladman DD, Shuckett R, Russell ML, Thorne JC, Schachter RK. Psoriatic arthritis (PSA)—An analysis of 220 patients. Q J Med. 1987;62(238):127-141.
9. Bogliolo L, Alpini C, Caporali R, Scirè CA, Moratti R, Montecucco C. Antibodies to cyclic citrullinated peptides in psoriatic arthritis. J Rheumatol. 2005;32(3):511-515.
10. Vander Cruyssen B, Hoffman IE, Zmierczak H, et al. Anti-citrullinated peptide antibodies may occur in patients with psoriatic arthritis. Ann Rheum Dis. 2005;64(8):1145-1149.
11. Alenius GM, Berglin E, Rantapää Dahlgvist S. Antibodies against cyclic citrullinated peptide (CCP) in psoriatic patients with or without joint inflammation. Ann Rheum Dis. 2006;65(3):398-400.
12. Candia L, Marquez J, Gonzalez C, et al. Low frequency of anticyclic citrullinated peptide antibodies in psoriatic arthritis but not in cutaneous psoriasis. J Clin Rheumatol. 2006;12(5):226-229.
13. Inanc N, Dalkilic E, Kamali S, et al. Anti-CCP antibodies in rheumatoid arthritis and psoriatic arthritis. Clin Rheumatol. 2007;26(1):17-23.
14. Popescu C, Zofota S, Bojinca V, Ionescu R. Anti-cyclic citrullinated peptide antibodies in psoriatic arthritis—Cross-sectional study and literature review. J Med Life. 2013;6(4):376-382.
15. Schoellnast H, Deutschmann HA, Hermann J, et al. Psoriatic arthritis and rheumatoid arthritis: Findings in contrast-enhanced MRI. AJR Am J Roentgenol. 2006;187(2):351-357.
16. Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Predictors of infection in rheumatoid arthritis. Arthritis Rheum. 2002;46(9):2294-2300.
17. Shoenfeld Y, Gurewich Y, Gallant LA, Pinkhas J. Prednisone-induced leukocytosis. Influence of dosage, method and duration of administration on the degree of leukocytosis. Am J Med. 1981;71(5):773-778.
18. Gossling J. Occurrence and pathogenicity of the Streptococcus milleri group. Rev Infect Dis. 1988;10(2):257-285.
19. Dubost JJ, Soubrier M, De Champs C, Ristori JM, Sauvezie B. Streptococcal septic arthritis in adults. A study of 55 cases with a literature review. Joint Bone Spine. 2004;71(4):303-311.
20. Ryan MJ, Kavanagh R, Wall PG, Hazleman BL. Bacterial joint infections in England and Wales: Analysis of bacterial isolates over a four year period. Br J Rheumatol. 1997;36(3):370-373.
21. Morgan DS, Fisher D, Merianos A, Currie BJ. An 18 year clinical review of septic arthritis from tropical Australia. Epidemiol Infect. 1996;117(3):423-428.
22. Kaandorp CJ, Dinant HJ, van de Laar MA, Moens HJ, Prins AP, Dijkmans BA. Incidence and sources of native and prosthetic joint infection: A community based prospective survey. Ann Rheum Dis. 1997;56(8):470-475.
23. Bradlow A, Mitchell RG, Mowat AG. Group G streptococcal arthritis. Rheumatol Rehabil. 1982;21(4):206-210.
24. Meier JL, Gerster JC. Bursitis and tenosynovitis caused by group G streptococci. J Rheumatol. 1983;10(5):817-818.
25. Filippucci E, Gabba A, Di Geso L, Girolimetti R, Salaffi F, Grassi W. Hand tendon involvement in rheumatoid arthritis: An ultrasound study. Semin Arthritis Rheum. 2012;41(6):752-760.
26. Bronze MS, Whitby S, Schaberg DR. Group G streptococcal arthritis: Case report and review of the literature. Am J Med Sci. 1997;313(4):239-243.
27. Schattner A, Vosti KL. Bacterial arthritis due to beta-hemolytic streptococci of serogroups A, B, C, F, and G. Analysis of 23 cases and a review of the literature. Medicine (Baltimore). 1998;77(2):122-139.
28. Gaunt PN, Seal DV. Group G streptococcal infection of joints and joint prostheses. J Infect. 1986;13(2):115-123.
29. Trenkner SW, Braunstein EM, Lynn MD, Ike RW. Group G streptococcal arthritis and bowel disease: A rare enteropathic arthropathy. Gastrointest Radiol. 1987;12(3):265-267.
30. Bert F, Bariou-Lancelin M, Lambert-Zechovsky N. Clinical significance of bacteremia involving the “Streptococcus milleri” group: 51 cases and review. Clin Infect Dis. 1998;27(2):385-387.
31. Casariego E, Rodriguez A, Corredoira JC, et al. Prospective study of Streptococcus milleri bacteremia. Eur J Clin Microbiol Infect Dis. 1996;15(3):194-200.
32. Jacobs JA, Pietersen HG, Stobberingh EE, Soeters PB. Bacteremia involving the “Streptococcus milleri” group: Analysis of 19 cases. Clin Infect Dis. 1994;19(4):704-713.
33. Zheng S, Robinson E, Yeoman S, et al. MRI bone oedema predicts eight year tendon function at the wrist but not the requirement for orthopaedic surgery in rheumatoid arthritis. Ann Rheum Dis. 2006;65(5):607-611.
34. Harris R. Spontaneous rupture of the tendon of extensor pollicis longus as a complication of rheumatoid arthritis. Ann Rheum Dis. 1951;10(3):298-306.
35. Björkman A, Jörgsholm P. Rupture of the extensor pollicis longus tendon: A study of aetiological factors. Scand J Plast Reconstr Surg Hand Surg. 2004;38(1):32-35.
Angioimmunoblastic T-cell Lymphoma Presenting as Purpura Fulminans
Purpura fulminans is a hematologic emergency, with clinical skin necrosis and laboratory testing showing disseminated intravascular coagulation. The thrombotic occlusion usually affects small and medium-sized blood vessels and may involve any organ. Purpura fulminans has been implicated with sepsis, most commonly meningococcal infections; other infections such as Staphylococcus aureus, groups A and B β-hemolytic streptococci, Streptococcus pneumoniae, and Haemophilus influenzae; and as a sequela to benign childhood infections, such as varicella. Other associations with purpura fulminans include autoimmune disease and heritable or acquired deficiency of anticoagulant proteins, most commonly protein C. We present a rare case of purpura fulminans as the presenting sign of angioimmunoblastic T-cell lymphoma (AITL), an aggressive primary nodal peripheral T-cell lymphoma with a high mortality rate and nonspecific skin manifestations in roughly half of all patients involved.
Case Report
A 56-year-old woman presented with purpuric patches on the left foot (Figure 1A). Seven days after presentation the lesion progressed into ecchymotic geographic plaques and hemorrhagic bullae that spread upward and contralaterally, sparing the digits, trunk, head, neck, and mucous membranes. Ultimately, the involved skin became necrotic and involved 20% of the body surface area (Figure 1B). The lesions were painful with a burning sensation but were not pruritic. The patient also reported intermittent fevers, chills, myalgia, nausea, and shortness of breath. Enlarged lymph nodes were present in the right cervical chain. She denied new medications; stated she had been in good health prior to this episode; and had no history of spontaneous abortion, neurologic symptoms, or other serious illness.
  |
Computed tomography showed prominent diffuse mediastinal, mesenteric, retroperitoneal, and pelvic lymphadenopathy with involvement of the cervical and inguinal areas. Laboratory values showed thrombocytopenia and increased fibrin degradation products. Blood and tissue cultures were negative; the patient also had a negative viral serology, except for Epstein-Barr virus IgG titers (>1:2560). A skin biopsy of the left thigh demonstrated venules and capillaries in the mid and superficial dermis filled with fibrin thrombi without vasculitis (Figure 2). A lymph node biopsy was consistent with a diagnosis of AITL. The lymph node architecture was largely effaced by a polymorphous lymphoid infiltrate that predominantly expanded into paracortical areas and was associated with a prominent arborizing vascular proliferation. The infiltrate was composed of lymphocytes ranging in size from small to medium, with ample cytoplasm, coarsely clumped chromatin, and mildly irregular nuclear membranes. Large atypical lymphocytes with features of immunoblasts were easily identified. An associated inflammatory background composed of eosinophils, plasma cells, and histiocytes was present (Figure 3). The atypical lymphocytes stained positive for CD3and CD10 on immunohistochemistry. Additionally, a subset of large immunoblastlike lymphocytes was positive for Epstein-Barr–encoded small RNAs by in situ hybridization.
|
The patient was started on rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone. She received 2 cycles with positive response based on subsequent computed tomography and positron emission tomography scans that showed regression of her disease as well as the lack of formation of new skin lesions. She was transferred to a burn unit where she had continuing treatment and skin grafts. Despite 2 cycles of chemotherapy, broad-spectrum antibiotics, and daily wound care management, the patient died secondary to sepsis 6 months after presentation.
Comment
Angioimmunoblastic T-cell lymphoma is a primary nodal lymphoma with occasional cutaneous involvement. Cutaneous manifestations occur in roughly half of all patients with AITL1 and have mainly been described as erythematous macules and papules that can resemble a viral exanthem or a drug reaction.2 However, other skin manifestations include urticaria, papulovesicular lesions, nodules, erythroderma,3 and to a lesser degree purpura.4 The lesions have been noted to occur prior to, concurrent with, or anytime during the disease.3,5,6 This aggressive lymphoma has mortality rates ranging from 50% to 72%, and median survival ranges from 11 to 30 months.6
To arrive at the correct diagnosis of AITL, a nodal biopsy with immunochemistry is necessary. Classic findings on histopathology include effacement of normal architecture, marked vascular proliferation, and aggregates of atypical lymphoid cells. CD10 has been shown to be a good objective criterion for the diagnosis of AITL,4 with characteristic tumor cells expressing CD10. Nodal Epstein-Barr virus–positive lymphocytes often are present.2 Other T-cell lymphomas with primarily nodal presentation along with peripheral T-cell lymphoma include peripheral T-cell lymphoma unspecified type and anaplastic large cell lymphoma, according to the World Health Organization classification.7 Anaplastic large cell lymphoma is easily distinguished from AITL based on histopathology, immunostaining, and clinical presentation. Until recently, peripheral T-cell lymphoma unspecified type and reactive lymphoid hyperplasia presented a challenge to differentiate from AITL, especially in the early phases of the disease; however, the introduction of CD10 as a phenotypic marker has been instrumental in distinguishing AITL from other T-cell lymphomas with primary nodal involvement.1,4
The development of purpura fulminans and disseminated intravascular coagulation in a patient with AITL is rare. Although the exact mechanism for the thrombus formation in the skin has not been elucidated, purpura fulminans typically develops secondary to a severe infection. The exact incidence of purpura fulminans in the setting of AITL is unknown, but purpura as a cutaneous eruption has been associated as a clinical finding in AITL.6 Although our case may be a rare presentation of AITL, a prompt and accurate diagnosis can drastically change the prognosis of this aggressive disease.
1. Ferry JA. Angioimmunoblastic T-cell lymphoma. Adv Anat Pathol. 2002;9:273-279.
2. Brown HA, Macon WR, Kurtin PJ, et al. Cutaneous involvement by angioimmunoblastic T-cell lymphoma with remarkable heterogeneous Epstein-Barr virus expression. J Cutan Pathol. 2001;28:432-438.
3. Bernstein JE, Soltani K, Lorincz AL. Cutaneous manifestations of angioimmunoblastic lymphadenopathy. J Am Acad Dermatol. 1979;1:227-232.
4. Attygalle A, Al-Jehani R, Diss TC, et al. Neoplastic T cells in angioimmunoblastic T-cell lymphoma express CD10. Blood. 2002;99:627-633.
5. Jayaramna AG, Cassarino D, Advani R, et al. Cutaneous involvement by angioimmunoblastic T-cell lymphoma: a unique histologic presentation, mimicking an infectious etiology. J Cutan Pathol. 2006;33(suppl 2):6-11.
6. Martel P, Laroche L, Courville P, et al. Cutaneous involvement in patients with angioimmunoblastic lymphadenopathy with dysproteinemia: a clinical, immunohistological, and molecular analysis. Archives of Dermatology. 2000;136:881-886.
7. Jaffe ES, Harris NL, Stein H, et al, eds. Tumours of Haematopoietic and Lymphoid Tissues. 1st ed. Bethesda, MD: International Agency for Research on Cancer; 2001.
Purpura fulminans is a hematologic emergency, with clinical skin necrosis and laboratory testing showing disseminated intravascular coagulation. The thrombotic occlusion usually affects small and medium-sized blood vessels and may involve any organ. Purpura fulminans has been implicated with sepsis, most commonly meningococcal infections; other infections such as Staphylococcus aureus, groups A and B β-hemolytic streptococci, Streptococcus pneumoniae, and Haemophilus influenzae; and as a sequela to benign childhood infections, such as varicella. Other associations with purpura fulminans include autoimmune disease and heritable or acquired deficiency of anticoagulant proteins, most commonly protein C. We present a rare case of purpura fulminans as the presenting sign of angioimmunoblastic T-cell lymphoma (AITL), an aggressive primary nodal peripheral T-cell lymphoma with a high mortality rate and nonspecific skin manifestations in roughly half of all patients involved.
Case Report
A 56-year-old woman presented with purpuric patches on the left foot (Figure 1A). Seven days after presentation the lesion progressed into ecchymotic geographic plaques and hemorrhagic bullae that spread upward and contralaterally, sparing the digits, trunk, head, neck, and mucous membranes. Ultimately, the involved skin became necrotic and involved 20% of the body surface area (Figure 1B). The lesions were painful with a burning sensation but were not pruritic. The patient also reported intermittent fevers, chills, myalgia, nausea, and shortness of breath. Enlarged lymph nodes were present in the right cervical chain. She denied new medications; stated she had been in good health prior to this episode; and had no history of spontaneous abortion, neurologic symptoms, or other serious illness.
  |
Computed tomography showed prominent diffuse mediastinal, mesenteric, retroperitoneal, and pelvic lymphadenopathy with involvement of the cervical and inguinal areas. Laboratory values showed thrombocytopenia and increased fibrin degradation products. Blood and tissue cultures were negative; the patient also had a negative viral serology, except for Epstein-Barr virus IgG titers (>1:2560). A skin biopsy of the left thigh demonstrated venules and capillaries in the mid and superficial dermis filled with fibrin thrombi without vasculitis (Figure 2). A lymph node biopsy was consistent with a diagnosis of AITL. The lymph node architecture was largely effaced by a polymorphous lymphoid infiltrate that predominantly expanded into paracortical areas and was associated with a prominent arborizing vascular proliferation. The infiltrate was composed of lymphocytes ranging in size from small to medium, with ample cytoplasm, coarsely clumped chromatin, and mildly irregular nuclear membranes. Large atypical lymphocytes with features of immunoblasts were easily identified. An associated inflammatory background composed of eosinophils, plasma cells, and histiocytes was present (Figure 3). The atypical lymphocytes stained positive for CD3and CD10 on immunohistochemistry. Additionally, a subset of large immunoblastlike lymphocytes was positive for Epstein-Barr–encoded small RNAs by in situ hybridization.
|
The patient was started on rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone. She received 2 cycles with positive response based on subsequent computed tomography and positron emission tomography scans that showed regression of her disease as well as the lack of formation of new skin lesions. She was transferred to a burn unit where she had continuing treatment and skin grafts. Despite 2 cycles of chemotherapy, broad-spectrum antibiotics, and daily wound care management, the patient died secondary to sepsis 6 months after presentation.
Comment
Angioimmunoblastic T-cell lymphoma is a primary nodal lymphoma with occasional cutaneous involvement. Cutaneous manifestations occur in roughly half of all patients with AITL1 and have mainly been described as erythematous macules and papules that can resemble a viral exanthem or a drug reaction.2 However, other skin manifestations include urticaria, papulovesicular lesions, nodules, erythroderma,3 and to a lesser degree purpura.4 The lesions have been noted to occur prior to, concurrent with, or anytime during the disease.3,5,6 This aggressive lymphoma has mortality rates ranging from 50% to 72%, and median survival ranges from 11 to 30 months.6
To arrive at the correct diagnosis of AITL, a nodal biopsy with immunochemistry is necessary. Classic findings on histopathology include effacement of normal architecture, marked vascular proliferation, and aggregates of atypical lymphoid cells. CD10 has been shown to be a good objective criterion for the diagnosis of AITL,4 with characteristic tumor cells expressing CD10. Nodal Epstein-Barr virus–positive lymphocytes often are present.2 Other T-cell lymphomas with primarily nodal presentation along with peripheral T-cell lymphoma include peripheral T-cell lymphoma unspecified type and anaplastic large cell lymphoma, according to the World Health Organization classification.7 Anaplastic large cell lymphoma is easily distinguished from AITL based on histopathology, immunostaining, and clinical presentation. Until recently, peripheral T-cell lymphoma unspecified type and reactive lymphoid hyperplasia presented a challenge to differentiate from AITL, especially in the early phases of the disease; however, the introduction of CD10 as a phenotypic marker has been instrumental in distinguishing AITL from other T-cell lymphomas with primary nodal involvement.1,4
The development of purpura fulminans and disseminated intravascular coagulation in a patient with AITL is rare. Although the exact mechanism for the thrombus formation in the skin has not been elucidated, purpura fulminans typically develops secondary to a severe infection. The exact incidence of purpura fulminans in the setting of AITL is unknown, but purpura as a cutaneous eruption has been associated as a clinical finding in AITL.6 Although our case may be a rare presentation of AITL, a prompt and accurate diagnosis can drastically change the prognosis of this aggressive disease.
Purpura fulminans is a hematologic emergency, with clinical skin necrosis and laboratory testing showing disseminated intravascular coagulation. The thrombotic occlusion usually affects small and medium-sized blood vessels and may involve any organ. Purpura fulminans has been implicated with sepsis, most commonly meningococcal infections; other infections such as Staphylococcus aureus, groups A and B β-hemolytic streptococci, Streptococcus pneumoniae, and Haemophilus influenzae; and as a sequela to benign childhood infections, such as varicella. Other associations with purpura fulminans include autoimmune disease and heritable or acquired deficiency of anticoagulant proteins, most commonly protein C. We present a rare case of purpura fulminans as the presenting sign of angioimmunoblastic T-cell lymphoma (AITL), an aggressive primary nodal peripheral T-cell lymphoma with a high mortality rate and nonspecific skin manifestations in roughly half of all patients involved.
Case Report
A 56-year-old woman presented with purpuric patches on the left foot (Figure 1A). Seven days after presentation the lesion progressed into ecchymotic geographic plaques and hemorrhagic bullae that spread upward and contralaterally, sparing the digits, trunk, head, neck, and mucous membranes. Ultimately, the involved skin became necrotic and involved 20% of the body surface area (Figure 1B). The lesions were painful with a burning sensation but were not pruritic. The patient also reported intermittent fevers, chills, myalgia, nausea, and shortness of breath. Enlarged lymph nodes were present in the right cervical chain. She denied new medications; stated she had been in good health prior to this episode; and had no history of spontaneous abortion, neurologic symptoms, or other serious illness.
  |
Computed tomography showed prominent diffuse mediastinal, mesenteric, retroperitoneal, and pelvic lymphadenopathy with involvement of the cervical and inguinal areas. Laboratory values showed thrombocytopenia and increased fibrin degradation products. Blood and tissue cultures were negative; the patient also had a negative viral serology, except for Epstein-Barr virus IgG titers (>1:2560). A skin biopsy of the left thigh demonstrated venules and capillaries in the mid and superficial dermis filled with fibrin thrombi without vasculitis (Figure 2). A lymph node biopsy was consistent with a diagnosis of AITL. The lymph node architecture was largely effaced by a polymorphous lymphoid infiltrate that predominantly expanded into paracortical areas and was associated with a prominent arborizing vascular proliferation. The infiltrate was composed of lymphocytes ranging in size from small to medium, with ample cytoplasm, coarsely clumped chromatin, and mildly irregular nuclear membranes. Large atypical lymphocytes with features of immunoblasts were easily identified. An associated inflammatory background composed of eosinophils, plasma cells, and histiocytes was present (Figure 3). The atypical lymphocytes stained positive for CD3and CD10 on immunohistochemistry. Additionally, a subset of large immunoblastlike lymphocytes was positive for Epstein-Barr–encoded small RNAs by in situ hybridization.
|
The patient was started on rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone. She received 2 cycles with positive response based on subsequent computed tomography and positron emission tomography scans that showed regression of her disease as well as the lack of formation of new skin lesions. She was transferred to a burn unit where she had continuing treatment and skin grafts. Despite 2 cycles of chemotherapy, broad-spectrum antibiotics, and daily wound care management, the patient died secondary to sepsis 6 months after presentation.
Comment
Angioimmunoblastic T-cell lymphoma is a primary nodal lymphoma with occasional cutaneous involvement. Cutaneous manifestations occur in roughly half of all patients with AITL1 and have mainly been described as erythematous macules and papules that can resemble a viral exanthem or a drug reaction.2 However, other skin manifestations include urticaria, papulovesicular lesions, nodules, erythroderma,3 and to a lesser degree purpura.4 The lesions have been noted to occur prior to, concurrent with, or anytime during the disease.3,5,6 This aggressive lymphoma has mortality rates ranging from 50% to 72%, and median survival ranges from 11 to 30 months.6
To arrive at the correct diagnosis of AITL, a nodal biopsy with immunochemistry is necessary. Classic findings on histopathology include effacement of normal architecture, marked vascular proliferation, and aggregates of atypical lymphoid cells. CD10 has been shown to be a good objective criterion for the diagnosis of AITL,4 with characteristic tumor cells expressing CD10. Nodal Epstein-Barr virus–positive lymphocytes often are present.2 Other T-cell lymphomas with primarily nodal presentation along with peripheral T-cell lymphoma include peripheral T-cell lymphoma unspecified type and anaplastic large cell lymphoma, according to the World Health Organization classification.7 Anaplastic large cell lymphoma is easily distinguished from AITL based on histopathology, immunostaining, and clinical presentation. Until recently, peripheral T-cell lymphoma unspecified type and reactive lymphoid hyperplasia presented a challenge to differentiate from AITL, especially in the early phases of the disease; however, the introduction of CD10 as a phenotypic marker has been instrumental in distinguishing AITL from other T-cell lymphomas with primary nodal involvement.1,4
The development of purpura fulminans and disseminated intravascular coagulation in a patient with AITL is rare. Although the exact mechanism for the thrombus formation in the skin has not been elucidated, purpura fulminans typically develops secondary to a severe infection. The exact incidence of purpura fulminans in the setting of AITL is unknown, but purpura as a cutaneous eruption has been associated as a clinical finding in AITL.6 Although our case may be a rare presentation of AITL, a prompt and accurate diagnosis can drastically change the prognosis of this aggressive disease.
1. Ferry JA. Angioimmunoblastic T-cell lymphoma. Adv Anat Pathol. 2002;9:273-279.
2. Brown HA, Macon WR, Kurtin PJ, et al. Cutaneous involvement by angioimmunoblastic T-cell lymphoma with remarkable heterogeneous Epstein-Barr virus expression. J Cutan Pathol. 2001;28:432-438.
3. Bernstein JE, Soltani K, Lorincz AL. Cutaneous manifestations of angioimmunoblastic lymphadenopathy. J Am Acad Dermatol. 1979;1:227-232.
4. Attygalle A, Al-Jehani R, Diss TC, et al. Neoplastic T cells in angioimmunoblastic T-cell lymphoma express CD10. Blood. 2002;99:627-633.
5. Jayaramna AG, Cassarino D, Advani R, et al. Cutaneous involvement by angioimmunoblastic T-cell lymphoma: a unique histologic presentation, mimicking an infectious etiology. J Cutan Pathol. 2006;33(suppl 2):6-11.
6. Martel P, Laroche L, Courville P, et al. Cutaneous involvement in patients with angioimmunoblastic lymphadenopathy with dysproteinemia: a clinical, immunohistological, and molecular analysis. Archives of Dermatology. 2000;136:881-886.
7. Jaffe ES, Harris NL, Stein H, et al, eds. Tumours of Haematopoietic and Lymphoid Tissues. 1st ed. Bethesda, MD: International Agency for Research on Cancer; 2001.
1. Ferry JA. Angioimmunoblastic T-cell lymphoma. Adv Anat Pathol. 2002;9:273-279.
2. Brown HA, Macon WR, Kurtin PJ, et al. Cutaneous involvement by angioimmunoblastic T-cell lymphoma with remarkable heterogeneous Epstein-Barr virus expression. J Cutan Pathol. 2001;28:432-438.
3. Bernstein JE, Soltani K, Lorincz AL. Cutaneous manifestations of angioimmunoblastic lymphadenopathy. J Am Acad Dermatol. 1979;1:227-232.
4. Attygalle A, Al-Jehani R, Diss TC, et al. Neoplastic T cells in angioimmunoblastic T-cell lymphoma express CD10. Blood. 2002;99:627-633.
5. Jayaramna AG, Cassarino D, Advani R, et al. Cutaneous involvement by angioimmunoblastic T-cell lymphoma: a unique histologic presentation, mimicking an infectious etiology. J Cutan Pathol. 2006;33(suppl 2):6-11.
6. Martel P, Laroche L, Courville P, et al. Cutaneous involvement in patients with angioimmunoblastic lymphadenopathy with dysproteinemia: a clinical, immunohistological, and molecular analysis. Archives of Dermatology. 2000;136:881-886.
7. Jaffe ES, Harris NL, Stein H, et al, eds. Tumours of Haematopoietic and Lymphoid Tissues. 1st ed. Bethesda, MD: International Agency for Research on Cancer; 2001.
Practice Points
- Angioimmunoblastic T-cell lymphoma (AITL) is a primary nodal lymphoma with occasional nonspecific cutaneous involvement that may be morbilliform, maculopapular, erythrodermic, or rarely purpuric.
- To arrive at the correct diagnosis of AITL, a nodal biopsy with immunochemistry is necessary.
- CD10 positivity is a good objective criterion for the diagnosis of AITL, and Epstein-Barr virus–positive lymphocytes are nearly always present.
Epithelioid Sarcoma Resembling Benign Fibrous Histiocytoma
Epithelioid sarcoma (ES) is a rare malignant soft tissue neoplasm that is most often encountered on the distal extremities of young adults.1 Epithelioid sarcoma is notorious for its tendency to mimic palisading granulomatous processes such as granuloma annulare. We report a case of ES on the right hand of a 23-year-old man that resembled a benign fibrous histiocytoma (dermatofibroma) on incisional biopsy. The typical histopathologic features of ES were identified after amputation of the hand and evaluation of the deeper regions of the tumor. The tendency for ES to mimic granulomatous processes is a common diagnostic pitfall, but the potential for its close resemblance to benign fibrous histiocytoma is less recognized.
|
Case Report
A 23-year-old man presented with a nonhealing lesion on the right palm. His medical history was remarkable for a giant cell tumor of the tendon sheath involving the right fifth finger that had been treated via excision at an outside institution 2 years prior. Clinical examination revealed a 0.8×0.6-cm painful, firm, ulcerated dermal nodule with a hemorrhagic crust on the palmar surface of the right hand (Figure 1A). The clinical differential diagnosis included melanoma, traumatized verruca vulgaris, thrombosed pyogenic granuloma, and foreign body. A shave biopsy demonstrated verrucous epidermal hyperplasia, but the specimen did not include the dermis. Cultures of the lesion were positive for Staphylococcus aureus, and antibiotic therapy was initiated. In light of the clinical findings and the patient’s history of a giant cell tumor, imaging studies were performed. Magnetic resonance angiography showed abnormal masslike infiltrative enhancement throughout the soft tissues surrounding the right fifth metacarpal bone. The differential included a recurrent giant cell tumor, fibromatosis, and other soft tissue neoplasms.
After several missed appointments and surgery cancellations, the patient returned 4 months later for an incisional biopsy. Physical examination revealed a persistent palmar ulcer that had grown to 1.4×1 cm in size, along with an indurated purple plaque wrapping around the ulnar aspect of the right hand (Figure 1B). The biopsy demonstrated a proliferation of spindled and ovoid cells with scant cytoplasm that surrounded sclerotic collagen bundles resembling a dermatofibroma (Figure 2A). Cytologic atypia and mitotic activity were absent (Figure 2B). Glass slides of the original biopsy, which ultimately led to the diagnosis of the giant cell tumor of the tendon sheath more than 2 years earlier, were obtained and showed similar features. The proliferating cells were strongly and diffusely immunoreactive for vimentin, CD34, and cancer antigen 125 (CA 125). Scattered tumor cells strongly expressed cytokeratins (CKs) AE1/AE3 and cell adhesion molecule 5.2 (Figure 3). Staining for CD99 and epithelial membrane antigen was diffuse but weak. Factor XIIIa, S-100, CK7, smooth muscle actin, muscle-specific actin (HHF35), CD31, CD68, and B-cell lymphoma 2 were negative within the proliferating cells. Based on the clinical examination and results of the immunohistochemical staining, a diagnosis of ES was favored.
|
After a negative metastatic workup, amputation of the right hand was performed. The amputation specimen showed a tumor that extended through the entire hand with encasement of large vessels and tendons. Although the more superficial regions were cytologically bland, deep-seated regions of the tumor exhibited greater cellularity, nuclear pleomorphism, and mitotic activity (Figure 4). There was no bone involvement. Right axillary sentinel lymph nodes were negative for metastasis. Eighteen months later the patient developed chest and back pain with dyspnea. Thorascopic surgery was performed for a left pleural effusion and metastases to the left parietal pleura and adjacent soft tissue were identified. The patient was subsequently lost to follow-up.
Comment
First described by Enzinger1 in 1970, ES is a rare malignant soft tissue neoplasm that most frequently arises on the hands, forearms, and pretibial soft tissues of young adults.1-3 It is an aggressive tumor characterized by frequent recurrences and a high metastatic rate, with lung and regional lymph nodes being favored metastatic sites.1-5 Periods of several months or even years often pass between the initial presentation and establishment of a correct diagnosis, as ES frequently is mistaken for other benign conditions. The tendency for ES to mimic granulomatous processes is a common diagnostic pitfall, but the potential for its close resemblance to benign fibrous histiocytoma is less recognized.6,7 In his original series of 62 cases, Enzinger1 noted that 17 patients were referred for treatment with a diagnosis of a benign fibrohistiocytic neoplasm, and other reports have described a resemblance to fibrous and fibrohistiocytic neoplasms.8-11 Mirra et al10 designated these tumors as fibromalike variants of ES. Additional subtypes of ES have subsequently been recognized, including those described as angiomatoid or angiosarcomalike, reflecting the potential of ES to resemble vascular tumors.12 A proximal type of ES also has been described. This lesion presents as a deep-seated tumor on the proximal limbs and is associated with more aggressive behavior. It lacks the granulomalike pattern and has more prominent epithelioid and rhabdoid histological presentation.13-15
Epithelioid sarcoma is a mesenchymal tumor that can display multidirectional differentiation that is primarily epithelial.16 The precise histogenesis of ES remains unclear, but studies have demonstrated a spectrum of differentiation that ranges from primitive myofibroblast or fibrohistiocytelike cells to those with well-developed epithelial properties.16,17 Epithelioid sarcoma characteristically coexpresses vimentin and low-molecular-weight CKs such as cell adhesion molecule 5.2. The tumor cells often are immunoreactive for epithelial membrane antigen and more than 50% of cases exhibit remarkable CD34 positivity.16 More recent studies have further refined the immunophenotype, demonstrating frequent expression of CK8 and CK19 but less commonly CK7, CK20, CK34bE12, and CK5/6.18-20 Additional studies reported that in 10 of 11 cases, ES was positive for CA 125 on immunohistochemical staining, and 3 of 5 patients also had elevated serum CA 125 levels.21,22 More recently, Hoshino et al23 showed elevated serum CA 125 levels in 5 of 7 patients with ES. Cancer antigen 125 is a high-molecular-weight glycoprotein commonly used in the identification of epithelial ovarian carcinomas; however, it also has been described in a number of other neoplasms including carcinomas of the breast, lungs, and colon and lymphoma.24-27 Although it appears that the addition of CA 125 to a panel of other immunohistochemical stains may be helpful in differentiating ES from other soft tissue sarcomas and serum CA 125 levels may help determine tumor burden, currently the number of cases studied is too small to definitively make that conclusion.21,23 In our case, the tumor cells were strongly and diffusely positive for CA 125. Serum CA 125 levels were not available.
Cytogenetic studies have failed to identify a consistent chromosomal abnormality in ES.5 Some analyses performed by comparative genomic hybridization on isolated cases and small case series indicate that the most frequent alterations involve 8q, 18q11, and 22q11.13,28,29 The tumor suppressor gene SMARCB1/INI1 (SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily B, member 1/integrase interactor 1) has been mapped to 22q11, and ES commonly shows absence of nuclear staining for this protein, indicating inactivation.13-15
Conclusion
Benign fibrohistiocytic proliferations should be included in the differential of histological mimickers of ES. Deep biopsies are essential to differentiate these benign tumors from fibrous histiocytomalike or fibromalike lesions of ES because superficial portions of ES may be well differentiated.
1. Enzinger FM. Epitheloid sarcoma. a sarcoma simulating a granuloma or a carcinoma. Cancer. 1970;26:1029-1041.
2. Spillane AJ, Thomas JM, Fisher C. Epithelioid sarcoma: the clinicopathological complexities of this rare soft tissue sarcoma. Ann Surg Oncol. 2000;7:218-225.
3. Chase DR, Enzinger FM. Epithelioid sarcoma. diagnosis, prognostic indicators, and treatment. Am J Surg Pathol. 1985;9:241-263.
4. Fisher C. Epithelioid sarcoma of Enzinger. Adv Anat Pathol. 2006;13:114-121.
5. Evans HL, Baer SC. Epithelioid sarcoma: a clinicopathologic and prognostic study of 26 cases. Semin Diagn Pathol. 1993;10:286-291.
6. Heenan PJ, Quirk CJ, Papadimitriou JM. Epithelioid sarcoma. a diagnostic problem. Am J Dermatopathol. 1986;8:95-104.
7. DiCaudo DJ, McCalmont TH, Wick MR. Selected diagnostic problems in neoplastic dermatopathology. Arch Pathol Lab Med. 2007;131:434-439.
8. Ormsby AH, Liou LS, Oriba HA, et al. Epithelioid sarcoma of the penis: report of an unusual case and review of the literature. Ann Diagn Pathol. 2000;4:88-94.
9. Lowentritt B, Parsons JK, Argani P, et al. Pediatric epithelioid sarcoma of the penis. J Urol. 2004;172:296-297.
10. Mirra JM, Kessler S, Bhuta S, et al. The fibroma-like variant of epithelioid sarcoma. a fibrohistiocytic/myoid cell lesion often confused with benign and malignant spindle cell tumors. Cancer. 1992;69:1382-1395.
11. Tan SH, Ong BH. Spindle cell variant of epithelioid sarcoma: an easily misdiagnosed tumour. Australas J Dermatol. 2001;42:139-141.
12. von Hochstetter AR, Grant JW, Meyer VE, et al. Angiomatoid variant of epithelioid sarcoma. the value of immunohistochemistry in the differential diagnosis. Chir Organi Mov. 1990;75(suppl 1):158-162.
13. Modena P, Lualdi E, Facchinetti F, et al. SMARCB1/INI1 tumor suppressor gene is frequently inactivated in epithelioid sarcomas. Cancer Res. 2005;65:4012-4019.
14. Lualdi E, Modena P, Debiec-Rychter M, et al. Molecular cytogenetic characterization of proximal-type epithelioid sarcoma. Genes Chromosomes Cancer. 2004;41:283-290.
15. Kosemehmetoglu K, Kaygusuz G, Bahrami A, et al. Intra-articular epithelioid sarcoma showing mixed classic and proximal-type features: report of 2 cases, with immunohistochemical and molecular cytogenetic INI-1 study. Am J Surg Pathol. 2011;35:891-897.
16. Armah HB, Parwani AV. Epithelioid sarcoma. Arch Pathol Lab Med. 2009;133:814-819.
17. Fisher C. Epithelioid sarcoma: the spectrum of ultrastructural differentiation in seven immunohistochemically defined cases. Hum Pathol. 1988;19:265-275.
18. Miettinen M, Fanburg-Smith JC, Virolainen M, et al. Epithelioid sarcoma: an immunohistochemical analysis of 112 classical and variant cases and a discussion of the differential diagnosis. Hum Pathol. 1999;30:934-942.
19. Humble SD, Prieto VG, Horenstein MG. Cytokeratin 7 and 20 expression in epithelioid sarcoma. J Cutan Pathol. 2003;30:242-246.
20. Lin L, Skacel M, Sigel JE, et al. Epithelioid sarcoma: an immunohistochemical analysis evaluating the utility of cytokeratin 5/6 in distinguishing superficial epithelioid sarcoma from spindled squamous cell carcinoma. J Cutan Pathol. 2003;30:114-117.
21. Kato H, Hatori M, Kokubun S, et al. CA125 expression in epithelioid sarcoma. Jpn J Clin Oncol. 2004;34:149-154.
22. Kato H, Hatori M, Watanabe M, et al. Epithelioid sarcomas with elevated serum CA125: report of two cases. Jpn J Clin Oncol. 2003;33:141-144.
23. Hoshino M, Kawashima H, Ogose A, et al. Serum CA 125 expression as a tumor marker for the diagnosis and monitoring the clinical course of epithelioid sarcoma [published online ahead of print September 16, 2009]. J Cancer Res Clin Oncol. 2010;136:457-464.
24. Lee AH, Paish EC, Marchio C, et al. The expression of Wilm’s tumour-1 and CA125 in invasive micropapillary carcinoma of the breast. Histopathology. 2007;51:824-828.
25. Homma S, Satoh H, Kagohashi K, et al. Production of CA125 by human lung cancer cell lines. Clin Exp Med. 2004;4:139-141.
26. Streppel MM, Vincent A, Mukherjee R, et al. Mucin 16 (cancer antigen 125) expression in human tissues and cell lines and correlation with clinical outcome in adenocarcinomas of the pancreas, esophagus, stomach, and colon. Hum Pathol. 2012;42:1755-1763.
27. Wei G, Yuping Z, Jun W, et al. CA125 expression in patients with non-Hodgkin’s lymphoma. Leuk Lymphoma. 2006; 47:1322-1326.
28. Feely MG, Fidler ME, Nelson M, et al. Cytogenetic findings in a case of epithelioid sarcoma and a review of the literature. Cancer Genet Cytogenet. 2000;119:155-157.
29. Lushnikova T, Knuutila S, Miettinen M. DNA copy number changes in epithelioid sarcoma and its variants: a comparative genomic hybridization study. Mod Pathol. 2000;13:1092-1096.
Epithelioid sarcoma (ES) is a rare malignant soft tissue neoplasm that is most often encountered on the distal extremities of young adults.1 Epithelioid sarcoma is notorious for its tendency to mimic palisading granulomatous processes such as granuloma annulare. We report a case of ES on the right hand of a 23-year-old man that resembled a benign fibrous histiocytoma (dermatofibroma) on incisional biopsy. The typical histopathologic features of ES were identified after amputation of the hand and evaluation of the deeper regions of the tumor. The tendency for ES to mimic granulomatous processes is a common diagnostic pitfall, but the potential for its close resemblance to benign fibrous histiocytoma is less recognized.
|
Case Report
A 23-year-old man presented with a nonhealing lesion on the right palm. His medical history was remarkable for a giant cell tumor of the tendon sheath involving the right fifth finger that had been treated via excision at an outside institution 2 years prior. Clinical examination revealed a 0.8×0.6-cm painful, firm, ulcerated dermal nodule with a hemorrhagic crust on the palmar surface of the right hand (Figure 1A). The clinical differential diagnosis included melanoma, traumatized verruca vulgaris, thrombosed pyogenic granuloma, and foreign body. A shave biopsy demonstrated verrucous epidermal hyperplasia, but the specimen did not include the dermis. Cultures of the lesion were positive for Staphylococcus aureus, and antibiotic therapy was initiated. In light of the clinical findings and the patient’s history of a giant cell tumor, imaging studies were performed. Magnetic resonance angiography showed abnormal masslike infiltrative enhancement throughout the soft tissues surrounding the right fifth metacarpal bone. The differential included a recurrent giant cell tumor, fibromatosis, and other soft tissue neoplasms.
After several missed appointments and surgery cancellations, the patient returned 4 months later for an incisional biopsy. Physical examination revealed a persistent palmar ulcer that had grown to 1.4×1 cm in size, along with an indurated purple plaque wrapping around the ulnar aspect of the right hand (Figure 1B). The biopsy demonstrated a proliferation of spindled and ovoid cells with scant cytoplasm that surrounded sclerotic collagen bundles resembling a dermatofibroma (Figure 2A). Cytologic atypia and mitotic activity were absent (Figure 2B). Glass slides of the original biopsy, which ultimately led to the diagnosis of the giant cell tumor of the tendon sheath more than 2 years earlier, were obtained and showed similar features. The proliferating cells were strongly and diffusely immunoreactive for vimentin, CD34, and cancer antigen 125 (CA 125). Scattered tumor cells strongly expressed cytokeratins (CKs) AE1/AE3 and cell adhesion molecule 5.2 (Figure 3). Staining for CD99 and epithelial membrane antigen was diffuse but weak. Factor XIIIa, S-100, CK7, smooth muscle actin, muscle-specific actin (HHF35), CD31, CD68, and B-cell lymphoma 2 were negative within the proliferating cells. Based on the clinical examination and results of the immunohistochemical staining, a diagnosis of ES was favored.

|
After a negative metastatic workup, amputation of the right hand was performed. The amputation specimen showed a tumor that extended through the entire hand with encasement of large vessels and tendons. Although the more superficial regions were cytologically bland, deep-seated regions of the tumor exhibited greater cellularity, nuclear pleomorphism, and mitotic activity (Figure 4). There was no bone involvement. Right axillary sentinel lymph nodes were negative for metastasis. Eighteen months later the patient developed chest and back pain with dyspnea. Thorascopic surgery was performed for a left pleural effusion and metastases to the left parietal pleura and adjacent soft tissue were identified. The patient was subsequently lost to follow-up.
Comment
First described by Enzinger1 in 1970, ES is a rare malignant soft tissue neoplasm that most frequently arises on the hands, forearms, and pretibial soft tissues of young adults.1-3 It is an aggressive tumor characterized by frequent recurrences and a high metastatic rate, with lung and regional lymph nodes being favored metastatic sites.1-5 Periods of several months or even years often pass between the initial presentation and establishment of a correct diagnosis, as ES frequently is mistaken for other benign conditions. The tendency for ES to mimic granulomatous processes is a common diagnostic pitfall, but the potential for its close resemblance to benign fibrous histiocytoma is less recognized.6,7 In his original series of 62 cases, Enzinger1 noted that 17 patients were referred for treatment with a diagnosis of a benign fibrohistiocytic neoplasm, and other reports have described a resemblance to fibrous and fibrohistiocytic neoplasms.8-11 Mirra et al10 designated these tumors as fibromalike variants of ES. Additional subtypes of ES have subsequently been recognized, including those described as angiomatoid or angiosarcomalike, reflecting the potential of ES to resemble vascular tumors.12 A proximal type of ES also has been described. This lesion presents as a deep-seated tumor on the proximal limbs and is associated with more aggressive behavior. It lacks the granulomalike pattern and has more prominent epithelioid and rhabdoid histological presentation.13-15
Epithelioid sarcoma is a mesenchymal tumor that can display multidirectional differentiation that is primarily epithelial.16 The precise histogenesis of ES remains unclear, but studies have demonstrated a spectrum of differentiation that ranges from primitive myofibroblast or fibrohistiocytelike cells to those with well-developed epithelial properties.16,17 Epithelioid sarcoma characteristically coexpresses vimentin and low-molecular-weight CKs such as cell adhesion molecule 5.2. The tumor cells often are immunoreactive for epithelial membrane antigen and more than 50% of cases exhibit remarkable CD34 positivity.16 More recent studies have further refined the immunophenotype, demonstrating frequent expression of CK8 and CK19 but less commonly CK7, CK20, CK34bE12, and CK5/6.18-20 Additional studies reported that in 10 of 11 cases, ES was positive for CA 125 on immunohistochemical staining, and 3 of 5 patients also had elevated serum CA 125 levels.21,22 More recently, Hoshino et al23 showed elevated serum CA 125 levels in 5 of 7 patients with ES. Cancer antigen 125 is a high-molecular-weight glycoprotein commonly used in the identification of epithelial ovarian carcinomas; however, it also has been described in a number of other neoplasms including carcinomas of the breast, lungs, and colon and lymphoma.24-27 Although it appears that the addition of CA 125 to a panel of other immunohistochemical stains may be helpful in differentiating ES from other soft tissue sarcomas and serum CA 125 levels may help determine tumor burden, currently the number of cases studied is too small to definitively make that conclusion.21,23 In our case, the tumor cells were strongly and diffusely positive for CA 125. Serum CA 125 levels were not available.
Cytogenetic studies have failed to identify a consistent chromosomal abnormality in ES.5 Some analyses performed by comparative genomic hybridization on isolated cases and small case series indicate that the most frequent alterations involve 8q, 18q11, and 22q11.13,28,29 The tumor suppressor gene SMARCB1/INI1 (SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily B, member 1/integrase interactor 1) has been mapped to 22q11, and ES commonly shows absence of nuclear staining for this protein, indicating inactivation.13-15
Conclusion
Benign fibrohistiocytic proliferations should be included in the differential of histological mimickers of ES. Deep biopsies are essential to differentiate these benign tumors from fibrous histiocytomalike or fibromalike lesions of ES because superficial portions of ES may be well differentiated.
Epithelioid sarcoma (ES) is a rare malignant soft tissue neoplasm that is most often encountered on the distal extremities of young adults.1 Epithelioid sarcoma is notorious for its tendency to mimic palisading granulomatous processes such as granuloma annulare. We report a case of ES on the right hand of a 23-year-old man that resembled a benign fibrous histiocytoma (dermatofibroma) on incisional biopsy. The typical histopathologic features of ES were identified after amputation of the hand and evaluation of the deeper regions of the tumor. The tendency for ES to mimic granulomatous processes is a common diagnostic pitfall, but the potential for its close resemblance to benign fibrous histiocytoma is less recognized.
|
Case Report
A 23-year-old man presented with a nonhealing lesion on the right palm. His medical history was remarkable for a giant cell tumor of the tendon sheath involving the right fifth finger that had been treated via excision at an outside institution 2 years prior. Clinical examination revealed a 0.8×0.6-cm painful, firm, ulcerated dermal nodule with a hemorrhagic crust on the palmar surface of the right hand (Figure 1A). The clinical differential diagnosis included melanoma, traumatized verruca vulgaris, thrombosed pyogenic granuloma, and foreign body. A shave biopsy demonstrated verrucous epidermal hyperplasia, but the specimen did not include the dermis. Cultures of the lesion were positive for Staphylococcus aureus, and antibiotic therapy was initiated. In light of the clinical findings and the patient’s history of a giant cell tumor, imaging studies were performed. Magnetic resonance angiography showed abnormal masslike infiltrative enhancement throughout the soft tissues surrounding the right fifth metacarpal bone. The differential included a recurrent giant cell tumor, fibromatosis, and other soft tissue neoplasms.
After several missed appointments and surgery cancellations, the patient returned 4 months later for an incisional biopsy. Physical examination revealed a persistent palmar ulcer that had grown to 1.4×1 cm in size, along with an indurated purple plaque wrapping around the ulnar aspect of the right hand (Figure 1B). The biopsy demonstrated a proliferation of spindled and ovoid cells with scant cytoplasm that surrounded sclerotic collagen bundles resembling a dermatofibroma (Figure 2A). Cytologic atypia and mitotic activity were absent (Figure 2B). Glass slides of the original biopsy, which ultimately led to the diagnosis of the giant cell tumor of the tendon sheath more than 2 years earlier, were obtained and showed similar features. The proliferating cells were strongly and diffusely immunoreactive for vimentin, CD34, and cancer antigen 125 (CA 125). Scattered tumor cells strongly expressed cytokeratins (CKs) AE1/AE3 and cell adhesion molecule 5.2 (Figure 3). Staining for CD99 and epithelial membrane antigen was diffuse but weak. Factor XIIIa, S-100, CK7, smooth muscle actin, muscle-specific actin (HHF35), CD31, CD68, and B-cell lymphoma 2 were negative within the proliferating cells. Based on the clinical examination and results of the immunohistochemical staining, a diagnosis of ES was favored.

|
After a negative metastatic workup, amputation of the right hand was performed. The amputation specimen showed a tumor that extended through the entire hand with encasement of large vessels and tendons. Although the more superficial regions were cytologically bland, deep-seated regions of the tumor exhibited greater cellularity, nuclear pleomorphism, and mitotic activity (Figure 4). There was no bone involvement. Right axillary sentinel lymph nodes were negative for metastasis. Eighteen months later the patient developed chest and back pain with dyspnea. Thorascopic surgery was performed for a left pleural effusion and metastases to the left parietal pleura and adjacent soft tissue were identified. The patient was subsequently lost to follow-up.
Comment
First described by Enzinger1 in 1970, ES is a rare malignant soft tissue neoplasm that most frequently arises on the hands, forearms, and pretibial soft tissues of young adults.1-3 It is an aggressive tumor characterized by frequent recurrences and a high metastatic rate, with lung and regional lymph nodes being favored metastatic sites.1-5 Periods of several months or even years often pass between the initial presentation and establishment of a correct diagnosis, as ES frequently is mistaken for other benign conditions. The tendency for ES to mimic granulomatous processes is a common diagnostic pitfall, but the potential for its close resemblance to benign fibrous histiocytoma is less recognized.6,7 In his original series of 62 cases, Enzinger1 noted that 17 patients were referred for treatment with a diagnosis of a benign fibrohistiocytic neoplasm, and other reports have described a resemblance to fibrous and fibrohistiocytic neoplasms.8-11 Mirra et al10 designated these tumors as fibromalike variants of ES. Additional subtypes of ES have subsequently been recognized, including those described as angiomatoid or angiosarcomalike, reflecting the potential of ES to resemble vascular tumors.12 A proximal type of ES also has been described. This lesion presents as a deep-seated tumor on the proximal limbs and is associated with more aggressive behavior. It lacks the granulomalike pattern and has more prominent epithelioid and rhabdoid histological presentation.13-15
Epithelioid sarcoma is a mesenchymal tumor that can display multidirectional differentiation that is primarily epithelial.16 The precise histogenesis of ES remains unclear, but studies have demonstrated a spectrum of differentiation that ranges from primitive myofibroblast or fibrohistiocytelike cells to those with well-developed epithelial properties.16,17 Epithelioid sarcoma characteristically coexpresses vimentin and low-molecular-weight CKs such as cell adhesion molecule 5.2. The tumor cells often are immunoreactive for epithelial membrane antigen and more than 50% of cases exhibit remarkable CD34 positivity.16 More recent studies have further refined the immunophenotype, demonstrating frequent expression of CK8 and CK19 but less commonly CK7, CK20, CK34bE12, and CK5/6.18-20 Additional studies reported that in 10 of 11 cases, ES was positive for CA 125 on immunohistochemical staining, and 3 of 5 patients also had elevated serum CA 125 levels.21,22 More recently, Hoshino et al23 showed elevated serum CA 125 levels in 5 of 7 patients with ES. Cancer antigen 125 is a high-molecular-weight glycoprotein commonly used in the identification of epithelial ovarian carcinomas; however, it also has been described in a number of other neoplasms including carcinomas of the breast, lungs, and colon and lymphoma.24-27 Although it appears that the addition of CA 125 to a panel of other immunohistochemical stains may be helpful in differentiating ES from other soft tissue sarcomas and serum CA 125 levels may help determine tumor burden, currently the number of cases studied is too small to definitively make that conclusion.21,23 In our case, the tumor cells were strongly and diffusely positive for CA 125. Serum CA 125 levels were not available.
Cytogenetic studies have failed to identify a consistent chromosomal abnormality in ES.5 Some analyses performed by comparative genomic hybridization on isolated cases and small case series indicate that the most frequent alterations involve 8q, 18q11, and 22q11.13,28,29 The tumor suppressor gene SMARCB1/INI1 (SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily B, member 1/integrase interactor 1) has been mapped to 22q11, and ES commonly shows absence of nuclear staining for this protein, indicating inactivation.13-15
Conclusion
Benign fibrohistiocytic proliferations should be included in the differential of histological mimickers of ES. Deep biopsies are essential to differentiate these benign tumors from fibrous histiocytomalike or fibromalike lesions of ES because superficial portions of ES may be well differentiated.
1. Enzinger FM. Epitheloid sarcoma. a sarcoma simulating a granuloma or a carcinoma. Cancer. 1970;26:1029-1041.
2. Spillane AJ, Thomas JM, Fisher C. Epithelioid sarcoma: the clinicopathological complexities of this rare soft tissue sarcoma. Ann Surg Oncol. 2000;7:218-225.
3. Chase DR, Enzinger FM. Epithelioid sarcoma. diagnosis, prognostic indicators, and treatment. Am J Surg Pathol. 1985;9:241-263.
4. Fisher C. Epithelioid sarcoma of Enzinger. Adv Anat Pathol. 2006;13:114-121.
5. Evans HL, Baer SC. Epithelioid sarcoma: a clinicopathologic and prognostic study of 26 cases. Semin Diagn Pathol. 1993;10:286-291.
6. Heenan PJ, Quirk CJ, Papadimitriou JM. Epithelioid sarcoma. a diagnostic problem. Am J Dermatopathol. 1986;8:95-104.
7. DiCaudo DJ, McCalmont TH, Wick MR. Selected diagnostic problems in neoplastic dermatopathology. Arch Pathol Lab Med. 2007;131:434-439.
8. Ormsby AH, Liou LS, Oriba HA, et al. Epithelioid sarcoma of the penis: report of an unusual case and review of the literature. Ann Diagn Pathol. 2000;4:88-94.
9. Lowentritt B, Parsons JK, Argani P, et al. Pediatric epithelioid sarcoma of the penis. J Urol. 2004;172:296-297.
10. Mirra JM, Kessler S, Bhuta S, et al. The fibroma-like variant of epithelioid sarcoma. a fibrohistiocytic/myoid cell lesion often confused with benign and malignant spindle cell tumors. Cancer. 1992;69:1382-1395.
11. Tan SH, Ong BH. Spindle cell variant of epithelioid sarcoma: an easily misdiagnosed tumour. Australas J Dermatol. 2001;42:139-141.
12. von Hochstetter AR, Grant JW, Meyer VE, et al. Angiomatoid variant of epithelioid sarcoma. the value of immunohistochemistry in the differential diagnosis. Chir Organi Mov. 1990;75(suppl 1):158-162.
13. Modena P, Lualdi E, Facchinetti F, et al. SMARCB1/INI1 tumor suppressor gene is frequently inactivated in epithelioid sarcomas. Cancer Res. 2005;65:4012-4019.
14. Lualdi E, Modena P, Debiec-Rychter M, et al. Molecular cytogenetic characterization of proximal-type epithelioid sarcoma. Genes Chromosomes Cancer. 2004;41:283-290.
15. Kosemehmetoglu K, Kaygusuz G, Bahrami A, et al. Intra-articular epithelioid sarcoma showing mixed classic and proximal-type features: report of 2 cases, with immunohistochemical and molecular cytogenetic INI-1 study. Am J Surg Pathol. 2011;35:891-897.
16. Armah HB, Parwani AV. Epithelioid sarcoma. Arch Pathol Lab Med. 2009;133:814-819.
17. Fisher C. Epithelioid sarcoma: the spectrum of ultrastructural differentiation in seven immunohistochemically defined cases. Hum Pathol. 1988;19:265-275.
18. Miettinen M, Fanburg-Smith JC, Virolainen M, et al. Epithelioid sarcoma: an immunohistochemical analysis of 112 classical and variant cases and a discussion of the differential diagnosis. Hum Pathol. 1999;30:934-942.
19. Humble SD, Prieto VG, Horenstein MG. Cytokeratin 7 and 20 expression in epithelioid sarcoma. J Cutan Pathol. 2003;30:242-246.
20. Lin L, Skacel M, Sigel JE, et al. Epithelioid sarcoma: an immunohistochemical analysis evaluating the utility of cytokeratin 5/6 in distinguishing superficial epithelioid sarcoma from spindled squamous cell carcinoma. J Cutan Pathol. 2003;30:114-117.
21. Kato H, Hatori M, Kokubun S, et al. CA125 expression in epithelioid sarcoma. Jpn J Clin Oncol. 2004;34:149-154.
22. Kato H, Hatori M, Watanabe M, et al. Epithelioid sarcomas with elevated serum CA125: report of two cases. Jpn J Clin Oncol. 2003;33:141-144.
23. Hoshino M, Kawashima H, Ogose A, et al. Serum CA 125 expression as a tumor marker for the diagnosis and monitoring the clinical course of epithelioid sarcoma [published online ahead of print September 16, 2009]. J Cancer Res Clin Oncol. 2010;136:457-464.
24. Lee AH, Paish EC, Marchio C, et al. The expression of Wilm’s tumour-1 and CA125 in invasive micropapillary carcinoma of the breast. Histopathology. 2007;51:824-828.
25. Homma S, Satoh H, Kagohashi K, et al. Production of CA125 by human lung cancer cell lines. Clin Exp Med. 2004;4:139-141.
26. Streppel MM, Vincent A, Mukherjee R, et al. Mucin 16 (cancer antigen 125) expression in human tissues and cell lines and correlation with clinical outcome in adenocarcinomas of the pancreas, esophagus, stomach, and colon. Hum Pathol. 2012;42:1755-1763.
27. Wei G, Yuping Z, Jun W, et al. CA125 expression in patients with non-Hodgkin’s lymphoma. Leuk Lymphoma. 2006; 47:1322-1326.
28. Feely MG, Fidler ME, Nelson M, et al. Cytogenetic findings in a case of epithelioid sarcoma and a review of the literature. Cancer Genet Cytogenet. 2000;119:155-157.
29. Lushnikova T, Knuutila S, Miettinen M. DNA copy number changes in epithelioid sarcoma and its variants: a comparative genomic hybridization study. Mod Pathol. 2000;13:1092-1096.
1. Enzinger FM. Epitheloid sarcoma. a sarcoma simulating a granuloma or a carcinoma. Cancer. 1970;26:1029-1041.
2. Spillane AJ, Thomas JM, Fisher C. Epithelioid sarcoma: the clinicopathological complexities of this rare soft tissue sarcoma. Ann Surg Oncol. 2000;7:218-225.
3. Chase DR, Enzinger FM. Epithelioid sarcoma. diagnosis, prognostic indicators, and treatment. Am J Surg Pathol. 1985;9:241-263.
4. Fisher C. Epithelioid sarcoma of Enzinger. Adv Anat Pathol. 2006;13:114-121.
5. Evans HL, Baer SC. Epithelioid sarcoma: a clinicopathologic and prognostic study of 26 cases. Semin Diagn Pathol. 1993;10:286-291.
6. Heenan PJ, Quirk CJ, Papadimitriou JM. Epithelioid sarcoma. a diagnostic problem. Am J Dermatopathol. 1986;8:95-104.
7. DiCaudo DJ, McCalmont TH, Wick MR. Selected diagnostic problems in neoplastic dermatopathology. Arch Pathol Lab Med. 2007;131:434-439.
8. Ormsby AH, Liou LS, Oriba HA, et al. Epithelioid sarcoma of the penis: report of an unusual case and review of the literature. Ann Diagn Pathol. 2000;4:88-94.
9. Lowentritt B, Parsons JK, Argani P, et al. Pediatric epithelioid sarcoma of the penis. J Urol. 2004;172:296-297.
10. Mirra JM, Kessler S, Bhuta S, et al. The fibroma-like variant of epithelioid sarcoma. a fibrohistiocytic/myoid cell lesion often confused with benign and malignant spindle cell tumors. Cancer. 1992;69:1382-1395.
11. Tan SH, Ong BH. Spindle cell variant of epithelioid sarcoma: an easily misdiagnosed tumour. Australas J Dermatol. 2001;42:139-141.
12. von Hochstetter AR, Grant JW, Meyer VE, et al. Angiomatoid variant of epithelioid sarcoma. the value of immunohistochemistry in the differential diagnosis. Chir Organi Mov. 1990;75(suppl 1):158-162.
13. Modena P, Lualdi E, Facchinetti F, et al. SMARCB1/INI1 tumor suppressor gene is frequently inactivated in epithelioid sarcomas. Cancer Res. 2005;65:4012-4019.
14. Lualdi E, Modena P, Debiec-Rychter M, et al. Molecular cytogenetic characterization of proximal-type epithelioid sarcoma. Genes Chromosomes Cancer. 2004;41:283-290.
15. Kosemehmetoglu K, Kaygusuz G, Bahrami A, et al. Intra-articular epithelioid sarcoma showing mixed classic and proximal-type features: report of 2 cases, with immunohistochemical and molecular cytogenetic INI-1 study. Am J Surg Pathol. 2011;35:891-897.
16. Armah HB, Parwani AV. Epithelioid sarcoma. Arch Pathol Lab Med. 2009;133:814-819.
17. Fisher C. Epithelioid sarcoma: the spectrum of ultrastructural differentiation in seven immunohistochemically defined cases. Hum Pathol. 1988;19:265-275.
18. Miettinen M, Fanburg-Smith JC, Virolainen M, et al. Epithelioid sarcoma: an immunohistochemical analysis of 112 classical and variant cases and a discussion of the differential diagnosis. Hum Pathol. 1999;30:934-942.
19. Humble SD, Prieto VG, Horenstein MG. Cytokeratin 7 and 20 expression in epithelioid sarcoma. J Cutan Pathol. 2003;30:242-246.
20. Lin L, Skacel M, Sigel JE, et al. Epithelioid sarcoma: an immunohistochemical analysis evaluating the utility of cytokeratin 5/6 in distinguishing superficial epithelioid sarcoma from spindled squamous cell carcinoma. J Cutan Pathol. 2003;30:114-117.
21. Kato H, Hatori M, Kokubun S, et al. CA125 expression in epithelioid sarcoma. Jpn J Clin Oncol. 2004;34:149-154.
22. Kato H, Hatori M, Watanabe M, et al. Epithelioid sarcomas with elevated serum CA125: report of two cases. Jpn J Clin Oncol. 2003;33:141-144.
23. Hoshino M, Kawashima H, Ogose A, et al. Serum CA 125 expression as a tumor marker for the diagnosis and monitoring the clinical course of epithelioid sarcoma [published online ahead of print September 16, 2009]. J Cancer Res Clin Oncol. 2010;136:457-464.
24. Lee AH, Paish EC, Marchio C, et al. The expression of Wilm’s tumour-1 and CA125 in invasive micropapillary carcinoma of the breast. Histopathology. 2007;51:824-828.
25. Homma S, Satoh H, Kagohashi K, et al. Production of CA125 by human lung cancer cell lines. Clin Exp Med. 2004;4:139-141.
26. Streppel MM, Vincent A, Mukherjee R, et al. Mucin 16 (cancer antigen 125) expression in human tissues and cell lines and correlation with clinical outcome in adenocarcinomas of the pancreas, esophagus, stomach, and colon. Hum Pathol. 2012;42:1755-1763.
27. Wei G, Yuping Z, Jun W, et al. CA125 expression in patients with non-Hodgkin’s lymphoma. Leuk Lymphoma. 2006; 47:1322-1326.
28. Feely MG, Fidler ME, Nelson M, et al. Cytogenetic findings in a case of epithelioid sarcoma and a review of the literature. Cancer Genet Cytogenet. 2000;119:155-157.
29. Lushnikova T, Knuutila S, Miettinen M. DNA copy number changes in epithelioid sarcoma and its variants: a comparative genomic hybridization study. Mod Pathol. 2000;13:1092-1096.
Practice Points
- Epithelioid sarcoma should be considered in the clinical differential diagnosis of nonhealing recurrent lesions of the distal extremities in a young adult.
- Histological presentation of epithelioid sarcoma can mimic a number of benign granulomatous and fibrohistiocytic processes, including benign fibrous histiocytoma.
- Deeper biopsies may be needed to demonstrate the overtly malignant morphology characteristic of epithelioid sarcoma.
- Inactivation of SMARCB1/INI1 is a common molecular aberration identified in epithelioid sarcoma and can be demonstrated immunohistochemically by absence of nuclear staining in tumor cells.