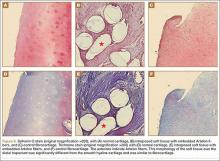
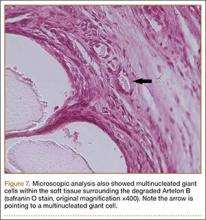
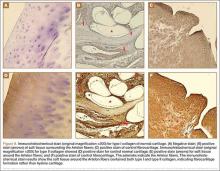
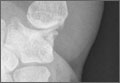
User login
Case Report: A 48-Year-Old Woman With Acute Abdomen
Case
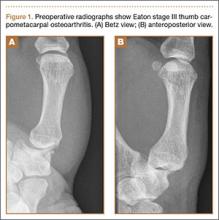
A 48-year-old woman presented to the ED with significant periumbilical abdominal pain and left lower extremity pain, which she rated an “8” on a scale of 1 to 10. She stated that the pain worsened with movement and change in position. The claudication in the patient’s left lower extremity began a few weeks prior to presentation, at which time she had received medical attention, including ankle brachial index testing that showed an abnormal value in the left lower extremity. The patient noted that when the abdominal pain began, the pain in her leg became more frequent and of higher intensity, with intermittent numbness. She reported some nausea, paresthesia, and sensory changes to the left lower extremity; however, she denied diarrhea, headache, fever, back pain, urinary symptoms, chest pain, and shortness of breath.
Regarding social history, the patient admitted to smoking half a pack of cigarettes a day and drinking alcohol socially. She denied any significant family history of disease. The patient’s own medical history included colon cancer, claudication, and multiple abdominal surgeries. The patient had been diagnosed with stage II colon cancer 4 years earlier, for which she had undergone a colon resection.
During the physical examination, the patient was diaphoretic, uncomfortable, and in severe distress. Her vital signs were: blood pressure, 146/77 mm Hg; respiratory rate, 18 breaths/minute; heart rate, 129 beats/minute; and temperature within normal limits. Oxygen saturation was 94% on room air.
The abdominal examination revealed a distended abdomen that was severely tender to palpation, with rigidity, guarding, and rebound tenderness. Examination of the lower extremities revealed an absent palpable dorsalis pedis pulse to the left lower extremity, but dorsalis pedis pulse and posterior tibial pulse in the left lower extremity were appreciated by Doppler. The right lower extremity had palpable 2+ dorsalis pedis and posterior tibial pulses.
The patient was immediately started on fentanyl and intravenous (IV) fluids; she was also given IV ondansetron and promethazine for nausea. Her pain was refractory to treatment, and required multiple doses of hydromorphone. Laboratory evaluation revealed leukocytosis with a white blood cell (WBC) count of 15.1 thou/cmm.
Computed tomography angiography (CTA) with runoff was ordered to evaluate lower extremity vasculature and perfusion, as well as abdominal vasculature and intra-abdominal organ pathology. The CTA revealed 99% stenosis in the left iliac artery; multiple areas of stenosis within the abdominal vasculature, including the superior mesenteric artery (SMA) and inferior mesenteric artery (IMA); and a small ventral hernia slightly left of the umbilicus but without evidence of obstruction. The patient remained stable while in the ED, and an emergent vascular surgery consultation was ordered. She was transferred to surgical services.
Mesenteric Ischemia
Mesenteric ischemia is a condition in which the intestine does not receive adequate blood supply, resulting in inflammation and injury. Cases of the disease may be acute or chronic. Acute mesenteric ischemia (AMI) may be occlusive or nonocclusive. Occlusive AMI is most commonly caused by embolic or thrombotic occlusion of one or more mesenteric arteries. Nonocclusive AMI (NMI) is most commonly due to primary splanchnic vasoconstriction.1 It can also be seen in patients on high-dose vasopressor agents. Chronic mesenteric ischemia indicates continuous intestinal hypoperfusion that is often associated with meals and referred to as postprandial or intestinal angina.
Mesenteric ischemia is associated with poor outcomes, having a mortality rate ranging from 40% to 70%.2 It is imperative that diagnosis and treatment commence rapidly to avoid potentially catastrophic complications such as transmural bowel infarction. Although visceral ischemia is rare, occurring in only 2 to 3 per 100,000, the high mortality rate makes prompt and accurate diagnosis essential to decreasing morbidity and mortality.3
Symptoms and Signs
The classical presentation of mesenteric ischemia is sudden onset of abdominal pain out of proportion to physical examination findings; however, peritoneal signs are also not uncommon later in the disease process. The most common presenting symptoms are abdominal pain, nausea, and diarrhea. Laboratory findings associated with mesenteric ischemia include leukocytosis, metabolic acidosis, elevated lactate, and an elevated D-dimer.2
Early recognition is crucial given the significant risk of bowel necrosis. Signs of peritonitis are frequently present late in the disease course; signs such as nausea, vomiting, and constipation are more frequent. Patients may also have complications such as ileus, gastrointestinal bleeding, and pancreatitis, which may mask the diagnosis of AMI.4
Prompt diagnosis and treatment are paramount. Acute AMI should especially be considered in patients who are over age 60 years, have a history of atrial fibrillation, claudication, hypercoagulable states or a previous history of atherosclerotic disease, myocardial infarction, and a history of postprandial abdominal pain and weight loss.
Laboratory Evaluation
The most common laboratory abnormalities in AMI are hemoconcentration, leukocytosis, elevated lactic acid, metabolic acidosis, and a high anion gap. Elevated amylase and creatinine phosphokinase are also frequently observed but are not specific for AMI. Hyperphosphatemia and hyperkalemia are frequently late signs and are associated with bowel infarction. Findings on plain abdominal radiographs are nonspecific and should not be utilized in the workups. Barium enemas also have no place in diagnosis, as this may reduce perfusion to the bowel wall and cause perforation.5 Leukocytosis and high lactate levels appear to be present in the majority of patients, though these are not specific for acute mesenteric ischemia.4
Imaging Studies
In the past, catheter-based angiography was considered the gold standard for diagnosis. However, the more readily available CTA is emerging as the primary imaging modality to diagnose mesenteric ischemia.3 Both CT and contrast angiography play a major role in the diagnosis. In addition to mesenteric ischemia, CT also allows for identification of nonvascular causes of abdominal pain. Contrast angiography has an important role in early diagnosis and is helpful in treatment planning as well as operative interventions.4
While CTA is the most frequently used technique in suspected AMI, contrast-enhanced three-dimensional magnetic resonance angiography (MRA) is also widely used. However, the inferior mesenteric artery and other splanchnic vessel periphery are currently better assessed with CTA due to the higher special and temporal resolution of the former. Both CTA and MRA are excellent screening techniques for AMI due to various causes.6
Duplex Doppler sonography has also been suggested as a screening tool in patients with suspected mesenteric ischemia, but this modality has multiple limitations, including failure to obtain adequate Doppler signal due to bowel gas or vessel wall calcification. Since significant disease is often common in the SMA and the celiac arteries of asymptomatic elderly patients, this modality should be considered when examining patients with suspected mesenteric ischemia.7
Treatment
Endovascular intervention or catheter-directed vasodilator therapy can be started immediately postangiography. The role of endovascular therapy in AMI is controversial. In NMI, a catheter-directed vasodilator infusion continues to be the treatment of choice in patients without peritonitis. Catheter-directed thrombolysis and percutaneous angioplasty have also been investigated in the treatment of AMI.4
The goal of surgical care is the removal of necrotic and nonsalvageable bowel and the prevention of further infarction. Stenting of the affected arteries may be utilized. An exploratory laparotomy remains the gold standard for assessment of bowel viability. Multiorgan failure poses a great risk in patients with AMI and mortality remains high.4 The most preferred surgical revascularization technique in embolic AMI remains the balloon catheter thromboembolectomy—with or without patch angioplasty of the superior mesenteric artery.
Prevention therapy should be utilized aggressively for AMI; patients with atrial fibrillation should be started on anticoagulants. Elective and timely revascularization may be undertaken in patients with chronic claudication and AMI secondary to atherosclerotic disease. In addition, patients should be advised not to smoke.4
Upon diagnosis of AMI, aggressive IV fluid resuscitation with crystalloids should be administered starting with volumes as high as 100 mL/kg to correct any metabolic derangements. A broad-spectrum antibiotic should also be started as early as possible. If no contraindications to anticoagulation exist, therapeutic IV heparin sodium should be administered to maintain an activated partial thromboplastin time at twice the normal value.5 The patient in this case was started on IV heparin and broad-spectrum antibiotics. In an optimized hemodynamic status, attempts to reduce acute vasospasm in AMI can be made with an IV glucagon infusion, starting at 1 mcg/kg/minute. The presence of peritoneal signs indicates bowel infarction and mandates an emergency laparotomy.5 As noted in the patient’s history, she was not on any anticoagulants on presentation and was a smoker.
Conclusion
The causes of abdominal pain range from benign to life threatening; therefore, it is imperative for clinicians to obtain a thorough history and physical examination of patients presenting with abdominal pain, and to consider a vascular etiology in the differential diagnosis. This case is unique in that the patient had multiple areas of stenosis within the abdomen, including the SMA and IMA, and either an acute or chronic occlusion, and claudication of her left lower extremity.
Dr Orlik is a resident, department of emergency medicine, Akron General Medical Center, Ohio. Mr Bosman is an undergraduate research fellow, department of emergency medicine, Akron General Medical Center, Ohio. Dr Simon is the emergency medicine research director, department of emergency medicine, Akron General Medical Center, Northeast Ohio Medical University.
- Tendler DA, Lamont JT. Nonocclusive mesenteric ischemia. UpToDate. http://www.uptodate.com/contents/nonocclusive-mesenteric-ischemia?source=search_result&search=Acute+Mesenteric+Ischemia&selectedTitle=2~72. Accessed March 27, 2015.
- Bobadilla JL. Mesenteric ischemia. Surg Clin North Am. 2013;93(4):925-940, ix.
- van den Heijkant TC, Aerts BA, Teijink JA, Buurman WA, Luyer MD. Challenges in diagnosing mesenteric ischemia. World J Gastroenterol. 2013;19(9):1338-1341.
- Park WM, Gloviczki P, Cherry KJ jR, et al. Contemporary management of acute mesenteric ischemia: Factors associated with survival. J Vasc Surg. 2002;35(3):445-452.
- Oldenburg AW, Lau LL, Rodenberg TJ, Edmonds HJ, Burger CD. Acute mesenteric ischemia: a clinical review. Arch Intern Med. 2004;164(10):1054-1062.
- Shih MC, Hagspiel, KD. CTA and MRA in mesenteric ischemia: part 1, Role in diagnosis and differential diagnosis. AJR Am J Roentgenol. 2007;188(2):452-461.
- Roobottom CA, Dubbins PA. Significant disease of the celiac and superior mesenteric arteries in asymptomatic patients: predictive value of Doppler sonography. AJR
Case
A 48-year-old woman presented to the ED with significant periumbilical abdominal pain and left lower extremity pain, which she rated an “8” on a scale of 1 to 10. She stated that the pain worsened with movement and change in position. The claudication in the patient’s left lower extremity began a few weeks prior to presentation, at which time she had received medical attention, including ankle brachial index testing that showed an abnormal value in the left lower extremity. The patient noted that when the abdominal pain began, the pain in her leg became more frequent and of higher intensity, with intermittent numbness. She reported some nausea, paresthesia, and sensory changes to the left lower extremity; however, she denied diarrhea, headache, fever, back pain, urinary symptoms, chest pain, and shortness of breath.
Regarding social history, the patient admitted to smoking half a pack of cigarettes a day and drinking alcohol socially. She denied any significant family history of disease. The patient’s own medical history included colon cancer, claudication, and multiple abdominal surgeries. The patient had been diagnosed with stage II colon cancer 4 years earlier, for which she had undergone a colon resection.
During the physical examination, the patient was diaphoretic, uncomfortable, and in severe distress. Her vital signs were: blood pressure, 146/77 mm Hg; respiratory rate, 18 breaths/minute; heart rate, 129 beats/minute; and temperature within normal limits. Oxygen saturation was 94% on room air.
The abdominal examination revealed a distended abdomen that was severely tender to palpation, with rigidity, guarding, and rebound tenderness. Examination of the lower extremities revealed an absent palpable dorsalis pedis pulse to the left lower extremity, but dorsalis pedis pulse and posterior tibial pulse in the left lower extremity were appreciated by Doppler. The right lower extremity had palpable 2+ dorsalis pedis and posterior tibial pulses.
The patient was immediately started on fentanyl and intravenous (IV) fluids; she was also given IV ondansetron and promethazine for nausea. Her pain was refractory to treatment, and required multiple doses of hydromorphone. Laboratory evaluation revealed leukocytosis with a white blood cell (WBC) count of 15.1 thou/cmm.
Computed tomography angiography (CTA) with runoff was ordered to evaluate lower extremity vasculature and perfusion, as well as abdominal vasculature and intra-abdominal organ pathology. The CTA revealed 99% stenosis in the left iliac artery; multiple areas of stenosis within the abdominal vasculature, including the superior mesenteric artery (SMA) and inferior mesenteric artery (IMA); and a small ventral hernia slightly left of the umbilicus but without evidence of obstruction. The patient remained stable while in the ED, and an emergent vascular surgery consultation was ordered. She was transferred to surgical services.
Mesenteric Ischemia
Mesenteric ischemia is a condition in which the intestine does not receive adequate blood supply, resulting in inflammation and injury. Cases of the disease may be acute or chronic. Acute mesenteric ischemia (AMI) may be occlusive or nonocclusive. Occlusive AMI is most commonly caused by embolic or thrombotic occlusion of one or more mesenteric arteries. Nonocclusive AMI (NMI) is most commonly due to primary splanchnic vasoconstriction.1 It can also be seen in patients on high-dose vasopressor agents. Chronic mesenteric ischemia indicates continuous intestinal hypoperfusion that is often associated with meals and referred to as postprandial or intestinal angina.
Mesenteric ischemia is associated with poor outcomes, having a mortality rate ranging from 40% to 70%.2 It is imperative that diagnosis and treatment commence rapidly to avoid potentially catastrophic complications such as transmural bowel infarction. Although visceral ischemia is rare, occurring in only 2 to 3 per 100,000, the high mortality rate makes prompt and accurate diagnosis essential to decreasing morbidity and mortality.3
Symptoms and Signs
The classical presentation of mesenteric ischemia is sudden onset of abdominal pain out of proportion to physical examination findings; however, peritoneal signs are also not uncommon later in the disease process. The most common presenting symptoms are abdominal pain, nausea, and diarrhea. Laboratory findings associated with mesenteric ischemia include leukocytosis, metabolic acidosis, elevated lactate, and an elevated D-dimer.2
Early recognition is crucial given the significant risk of bowel necrosis. Signs of peritonitis are frequently present late in the disease course; signs such as nausea, vomiting, and constipation are more frequent. Patients may also have complications such as ileus, gastrointestinal bleeding, and pancreatitis, which may mask the diagnosis of AMI.4
Prompt diagnosis and treatment are paramount. Acute AMI should especially be considered in patients who are over age 60 years, have a history of atrial fibrillation, claudication, hypercoagulable states or a previous history of atherosclerotic disease, myocardial infarction, and a history of postprandial abdominal pain and weight loss.
Laboratory Evaluation
The most common laboratory abnormalities in AMI are hemoconcentration, leukocytosis, elevated lactic acid, metabolic acidosis, and a high anion gap. Elevated amylase and creatinine phosphokinase are also frequently observed but are not specific for AMI. Hyperphosphatemia and hyperkalemia are frequently late signs and are associated with bowel infarction. Findings on plain abdominal radiographs are nonspecific and should not be utilized in the workups. Barium enemas also have no place in diagnosis, as this may reduce perfusion to the bowel wall and cause perforation.5 Leukocytosis and high lactate levels appear to be present in the majority of patients, though these are not specific for acute mesenteric ischemia.4
Imaging Studies
In the past, catheter-based angiography was considered the gold standard for diagnosis. However, the more readily available CTA is emerging as the primary imaging modality to diagnose mesenteric ischemia.3 Both CT and contrast angiography play a major role in the diagnosis. In addition to mesenteric ischemia, CT also allows for identification of nonvascular causes of abdominal pain. Contrast angiography has an important role in early diagnosis and is helpful in treatment planning as well as operative interventions.4
While CTA is the most frequently used technique in suspected AMI, contrast-enhanced three-dimensional magnetic resonance angiography (MRA) is also widely used. However, the inferior mesenteric artery and other splanchnic vessel periphery are currently better assessed with CTA due to the higher special and temporal resolution of the former. Both CTA and MRA are excellent screening techniques for AMI due to various causes.6
Duplex Doppler sonography has also been suggested as a screening tool in patients with suspected mesenteric ischemia, but this modality has multiple limitations, including failure to obtain adequate Doppler signal due to bowel gas or vessel wall calcification. Since significant disease is often common in the SMA and the celiac arteries of asymptomatic elderly patients, this modality should be considered when examining patients with suspected mesenteric ischemia.7
Treatment
Endovascular intervention or catheter-directed vasodilator therapy can be started immediately postangiography. The role of endovascular therapy in AMI is controversial. In NMI, a catheter-directed vasodilator infusion continues to be the treatment of choice in patients without peritonitis. Catheter-directed thrombolysis and percutaneous angioplasty have also been investigated in the treatment of AMI.4
The goal of surgical care is the removal of necrotic and nonsalvageable bowel and the prevention of further infarction. Stenting of the affected arteries may be utilized. An exploratory laparotomy remains the gold standard for assessment of bowel viability. Multiorgan failure poses a great risk in patients with AMI and mortality remains high.4 The most preferred surgical revascularization technique in embolic AMI remains the balloon catheter thromboembolectomy—with or without patch angioplasty of the superior mesenteric artery.
Prevention therapy should be utilized aggressively for AMI; patients with atrial fibrillation should be started on anticoagulants. Elective and timely revascularization may be undertaken in patients with chronic claudication and AMI secondary to atherosclerotic disease. In addition, patients should be advised not to smoke.4
Upon diagnosis of AMI, aggressive IV fluid resuscitation with crystalloids should be administered starting with volumes as high as 100 mL/kg to correct any metabolic derangements. A broad-spectrum antibiotic should also be started as early as possible. If no contraindications to anticoagulation exist, therapeutic IV heparin sodium should be administered to maintain an activated partial thromboplastin time at twice the normal value.5 The patient in this case was started on IV heparin and broad-spectrum antibiotics. In an optimized hemodynamic status, attempts to reduce acute vasospasm in AMI can be made with an IV glucagon infusion, starting at 1 mcg/kg/minute. The presence of peritoneal signs indicates bowel infarction and mandates an emergency laparotomy.5 As noted in the patient’s history, she was not on any anticoagulants on presentation and was a smoker.
Conclusion
The causes of abdominal pain range from benign to life threatening; therefore, it is imperative for clinicians to obtain a thorough history and physical examination of patients presenting with abdominal pain, and to consider a vascular etiology in the differential diagnosis. This case is unique in that the patient had multiple areas of stenosis within the abdomen, including the SMA and IMA, and either an acute or chronic occlusion, and claudication of her left lower extremity.
Dr Orlik is a resident, department of emergency medicine, Akron General Medical Center, Ohio. Mr Bosman is an undergraduate research fellow, department of emergency medicine, Akron General Medical Center, Ohio. Dr Simon is the emergency medicine research director, department of emergency medicine, Akron General Medical Center, Northeast Ohio Medical University.
Case
A 48-year-old woman presented to the ED with significant periumbilical abdominal pain and left lower extremity pain, which she rated an “8” on a scale of 1 to 10. She stated that the pain worsened with movement and change in position. The claudication in the patient’s left lower extremity began a few weeks prior to presentation, at which time she had received medical attention, including ankle brachial index testing that showed an abnormal value in the left lower extremity. The patient noted that when the abdominal pain began, the pain in her leg became more frequent and of higher intensity, with intermittent numbness. She reported some nausea, paresthesia, and sensory changes to the left lower extremity; however, she denied diarrhea, headache, fever, back pain, urinary symptoms, chest pain, and shortness of breath.
Regarding social history, the patient admitted to smoking half a pack of cigarettes a day and drinking alcohol socially. She denied any significant family history of disease. The patient’s own medical history included colon cancer, claudication, and multiple abdominal surgeries. The patient had been diagnosed with stage II colon cancer 4 years earlier, for which she had undergone a colon resection.
During the physical examination, the patient was diaphoretic, uncomfortable, and in severe distress. Her vital signs were: blood pressure, 146/77 mm Hg; respiratory rate, 18 breaths/minute; heart rate, 129 beats/minute; and temperature within normal limits. Oxygen saturation was 94% on room air.
The abdominal examination revealed a distended abdomen that was severely tender to palpation, with rigidity, guarding, and rebound tenderness. Examination of the lower extremities revealed an absent palpable dorsalis pedis pulse to the left lower extremity, but dorsalis pedis pulse and posterior tibial pulse in the left lower extremity were appreciated by Doppler. The right lower extremity had palpable 2+ dorsalis pedis and posterior tibial pulses.
The patient was immediately started on fentanyl and intravenous (IV) fluids; she was also given IV ondansetron and promethazine for nausea. Her pain was refractory to treatment, and required multiple doses of hydromorphone. Laboratory evaluation revealed leukocytosis with a white blood cell (WBC) count of 15.1 thou/cmm.
Computed tomography angiography (CTA) with runoff was ordered to evaluate lower extremity vasculature and perfusion, as well as abdominal vasculature and intra-abdominal organ pathology. The CTA revealed 99% stenosis in the left iliac artery; multiple areas of stenosis within the abdominal vasculature, including the superior mesenteric artery (SMA) and inferior mesenteric artery (IMA); and a small ventral hernia slightly left of the umbilicus but without evidence of obstruction. The patient remained stable while in the ED, and an emergent vascular surgery consultation was ordered. She was transferred to surgical services.
Mesenteric Ischemia
Mesenteric ischemia is a condition in which the intestine does not receive adequate blood supply, resulting in inflammation and injury. Cases of the disease may be acute or chronic. Acute mesenteric ischemia (AMI) may be occlusive or nonocclusive. Occlusive AMI is most commonly caused by embolic or thrombotic occlusion of one or more mesenteric arteries. Nonocclusive AMI (NMI) is most commonly due to primary splanchnic vasoconstriction.1 It can also be seen in patients on high-dose vasopressor agents. Chronic mesenteric ischemia indicates continuous intestinal hypoperfusion that is often associated with meals and referred to as postprandial or intestinal angina.
Mesenteric ischemia is associated with poor outcomes, having a mortality rate ranging from 40% to 70%.2 It is imperative that diagnosis and treatment commence rapidly to avoid potentially catastrophic complications such as transmural bowel infarction. Although visceral ischemia is rare, occurring in only 2 to 3 per 100,000, the high mortality rate makes prompt and accurate diagnosis essential to decreasing morbidity and mortality.3
Symptoms and Signs
The classical presentation of mesenteric ischemia is sudden onset of abdominal pain out of proportion to physical examination findings; however, peritoneal signs are also not uncommon later in the disease process. The most common presenting symptoms are abdominal pain, nausea, and diarrhea. Laboratory findings associated with mesenteric ischemia include leukocytosis, metabolic acidosis, elevated lactate, and an elevated D-dimer.2
Early recognition is crucial given the significant risk of bowel necrosis. Signs of peritonitis are frequently present late in the disease course; signs such as nausea, vomiting, and constipation are more frequent. Patients may also have complications such as ileus, gastrointestinal bleeding, and pancreatitis, which may mask the diagnosis of AMI.4
Prompt diagnosis and treatment are paramount. Acute AMI should especially be considered in patients who are over age 60 years, have a history of atrial fibrillation, claudication, hypercoagulable states or a previous history of atherosclerotic disease, myocardial infarction, and a history of postprandial abdominal pain and weight loss.
Laboratory Evaluation
The most common laboratory abnormalities in AMI are hemoconcentration, leukocytosis, elevated lactic acid, metabolic acidosis, and a high anion gap. Elevated amylase and creatinine phosphokinase are also frequently observed but are not specific for AMI. Hyperphosphatemia and hyperkalemia are frequently late signs and are associated with bowel infarction. Findings on plain abdominal radiographs are nonspecific and should not be utilized in the workups. Barium enemas also have no place in diagnosis, as this may reduce perfusion to the bowel wall and cause perforation.5 Leukocytosis and high lactate levels appear to be present in the majority of patients, though these are not specific for acute mesenteric ischemia.4
Imaging Studies
In the past, catheter-based angiography was considered the gold standard for diagnosis. However, the more readily available CTA is emerging as the primary imaging modality to diagnose mesenteric ischemia.3 Both CT and contrast angiography play a major role in the diagnosis. In addition to mesenteric ischemia, CT also allows for identification of nonvascular causes of abdominal pain. Contrast angiography has an important role in early diagnosis and is helpful in treatment planning as well as operative interventions.4
While CTA is the most frequently used technique in suspected AMI, contrast-enhanced three-dimensional magnetic resonance angiography (MRA) is also widely used. However, the inferior mesenteric artery and other splanchnic vessel periphery are currently better assessed with CTA due to the higher special and temporal resolution of the former. Both CTA and MRA are excellent screening techniques for AMI due to various causes.6
Duplex Doppler sonography has also been suggested as a screening tool in patients with suspected mesenteric ischemia, but this modality has multiple limitations, including failure to obtain adequate Doppler signal due to bowel gas or vessel wall calcification. Since significant disease is often common in the SMA and the celiac arteries of asymptomatic elderly patients, this modality should be considered when examining patients with suspected mesenteric ischemia.7
Treatment
Endovascular intervention or catheter-directed vasodilator therapy can be started immediately postangiography. The role of endovascular therapy in AMI is controversial. In NMI, a catheter-directed vasodilator infusion continues to be the treatment of choice in patients without peritonitis. Catheter-directed thrombolysis and percutaneous angioplasty have also been investigated in the treatment of AMI.4
The goal of surgical care is the removal of necrotic and nonsalvageable bowel and the prevention of further infarction. Stenting of the affected arteries may be utilized. An exploratory laparotomy remains the gold standard for assessment of bowel viability. Multiorgan failure poses a great risk in patients with AMI and mortality remains high.4 The most preferred surgical revascularization technique in embolic AMI remains the balloon catheter thromboembolectomy—with or without patch angioplasty of the superior mesenteric artery.
Prevention therapy should be utilized aggressively for AMI; patients with atrial fibrillation should be started on anticoagulants. Elective and timely revascularization may be undertaken in patients with chronic claudication and AMI secondary to atherosclerotic disease. In addition, patients should be advised not to smoke.4
Upon diagnosis of AMI, aggressive IV fluid resuscitation with crystalloids should be administered starting with volumes as high as 100 mL/kg to correct any metabolic derangements. A broad-spectrum antibiotic should also be started as early as possible. If no contraindications to anticoagulation exist, therapeutic IV heparin sodium should be administered to maintain an activated partial thromboplastin time at twice the normal value.5 The patient in this case was started on IV heparin and broad-spectrum antibiotics. In an optimized hemodynamic status, attempts to reduce acute vasospasm in AMI can be made with an IV glucagon infusion, starting at 1 mcg/kg/minute. The presence of peritoneal signs indicates bowel infarction and mandates an emergency laparotomy.5 As noted in the patient’s history, she was not on any anticoagulants on presentation and was a smoker.
Conclusion
The causes of abdominal pain range from benign to life threatening; therefore, it is imperative for clinicians to obtain a thorough history and physical examination of patients presenting with abdominal pain, and to consider a vascular etiology in the differential diagnosis. This case is unique in that the patient had multiple areas of stenosis within the abdomen, including the SMA and IMA, and either an acute or chronic occlusion, and claudication of her left lower extremity.
Dr Orlik is a resident, department of emergency medicine, Akron General Medical Center, Ohio. Mr Bosman is an undergraduate research fellow, department of emergency medicine, Akron General Medical Center, Ohio. Dr Simon is the emergency medicine research director, department of emergency medicine, Akron General Medical Center, Northeast Ohio Medical University.
- Tendler DA, Lamont JT. Nonocclusive mesenteric ischemia. UpToDate. http://www.uptodate.com/contents/nonocclusive-mesenteric-ischemia?source=search_result&search=Acute+Mesenteric+Ischemia&selectedTitle=2~72. Accessed March 27, 2015.
- Bobadilla JL. Mesenteric ischemia. Surg Clin North Am. 2013;93(4):925-940, ix.
- van den Heijkant TC, Aerts BA, Teijink JA, Buurman WA, Luyer MD. Challenges in diagnosing mesenteric ischemia. World J Gastroenterol. 2013;19(9):1338-1341.
- Park WM, Gloviczki P, Cherry KJ jR, et al. Contemporary management of acute mesenteric ischemia: Factors associated with survival. J Vasc Surg. 2002;35(3):445-452.
- Oldenburg AW, Lau LL, Rodenberg TJ, Edmonds HJ, Burger CD. Acute mesenteric ischemia: a clinical review. Arch Intern Med. 2004;164(10):1054-1062.
- Shih MC, Hagspiel, KD. CTA and MRA in mesenteric ischemia: part 1, Role in diagnosis and differential diagnosis. AJR Am J Roentgenol. 2007;188(2):452-461.
- Roobottom CA, Dubbins PA. Significant disease of the celiac and superior mesenteric arteries in asymptomatic patients: predictive value of Doppler sonography. AJR
- Tendler DA, Lamont JT. Nonocclusive mesenteric ischemia. UpToDate. http://www.uptodate.com/contents/nonocclusive-mesenteric-ischemia?source=search_result&search=Acute+Mesenteric+Ischemia&selectedTitle=2~72. Accessed March 27, 2015.
- Bobadilla JL. Mesenteric ischemia. Surg Clin North Am. 2013;93(4):925-940, ix.
- van den Heijkant TC, Aerts BA, Teijink JA, Buurman WA, Luyer MD. Challenges in diagnosing mesenteric ischemia. World J Gastroenterol. 2013;19(9):1338-1341.
- Park WM, Gloviczki P, Cherry KJ jR, et al. Contemporary management of acute mesenteric ischemia: Factors associated with survival. J Vasc Surg. 2002;35(3):445-452.
- Oldenburg AW, Lau LL, Rodenberg TJ, Edmonds HJ, Burger CD. Acute mesenteric ischemia: a clinical review. Arch Intern Med. 2004;164(10):1054-1062.
- Shih MC, Hagspiel, KD. CTA and MRA in mesenteric ischemia: part 1, Role in diagnosis and differential diagnosis. AJR Am J Roentgenol. 2007;188(2):452-461.
- Roobottom CA, Dubbins PA. Significant disease of the celiac and superior mesenteric arteries in asymptomatic patients: predictive value of Doppler sonography. AJR
Successful Surgical Treatment of an Intraneural Ganglion of the Common Peroneal Nerve
Intraneural ganglion cysts of peripheral nerves occurring within the epineural sheath are rare.1-7 Case reports exist primarily within the neurosurgical literature, but very little in the orthopedic literature describes this condition. The peripheral nerve most commonly affected by an intraneural ganglion is the common peroneal nerve (CPN).2,8,9 Such ganglia most often afflict middle-aged men with a history of micro- or macro-trauma and present with typical clinical manifestations of calf pain and progressive symptoms of ipsilateral foot drop and lower leg paresthesia.2-5,10-12 The mechanism by which these ganglia form is not well understood and, as a result, treatment options are debated.6 Recent development of a “unified articular theory,” suggests that such intraneural ganglia of the CPN are fed by a small, recurrent articular branch of the CPN.6,12,13 Cadaveric studies indicate that this branch originates from the deep peroneal nerve, just millimeters distal to the bifurcation of the CPN, and extends to the superior tibiofibular joint, providing direct access for cyst fluid to enter the CPN following the path of least resistance.7,8,12,14 Therefore, according to the unified articular theory, the recommended treatment involves division of the articular branch, allowing the ganglion to be decompressed.6
We present a case of a 41-year-old man with an intraneural ganglion cyst of the CPN who was successfully treated, according to the recommendations of the unified articular theory. It is important for orthopedic surgeons to read about and recognize this condition, because knowledge of the operative technique outlined in our report allows it to be treated quite effectively. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
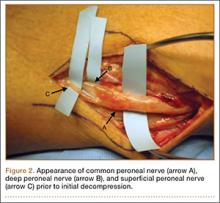
A 41-year-old man presented with a 2-month history of traumatic left lateral knee pain with numbness and weakness to the left foot and ankle. Initial examination showed a mild restriction of lumbosacral range of motion, with no complaints of lower back pain. Sciatic root stretch signs were negative. Strength testing of the lower extremities revealed 3+/5 strength of ankle dorsiflexion and great toe extension on the left side. There was a mild alteration in sensation to light touch on the lateral side of the left foot. Tenderness, without swelling, was present around the left fibular head. There was a positive Tinel sign over the peroneal nerve at the level of the fibular neck.
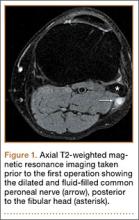
The patient was initially treated with anti-inflammatories and activity modification. An electromyogram (EMG)/nerve conduction study of the lower extremity showed a left peroneal nerve neurapraxia at the level of the fibular head. Noncontrast magnetic resonance imaging (MRI) of the left knee showed a “slightly prominent vein coursing posterior to the fibular head near the expected location of the common peroneal nerve,” according to the radiologist’s notes (Figure 1). The patient exhibited improvement with use of anti-inflammatories over several months. There was an increase in his ankle dorsiflexion strength to 4/5 and improvement in his pain and numbness.
Approximately 7 months after his initial presentation, the patient developed a marked worsening—increased numbness and weakness to ankle dorsiflexion—of his original symptoms. A repeat EMG/nerve conduction study of the lower extremity showed a persistent peroneal nerve neuropathy with a persistent denervation of the extensor hallucis longus, tibialis anterior, and extensor digitorum brevis muscles.
Because of continuing symptoms and increasing pain, the patient had surgery 8 months after his initial presentation. At that time, a markedly thickened peroneal nerve was identified. An incision in the epineural sheath released a clear gelatinous fluid consistent with a ganglion cyst. Through the epineural incision, the nerve was decompressed by manually “milking” the fluid from within the sheath. Approximately 30 mL of mucinous fluid was obtained and sent to pathology. No cells were identified.
Postoperatively, the patient noted a marked improvement in his pain. By 2 weeks postoperatively, the numbness in his foot had resolved. At 6 weeks after surgery, the strength of his tibialis anterior and extensor hallucis longus muscles had improved from 3+ to 4-, and he was free of pain.
At 2 months postoperatively, the patient redeveloped pain and numbness, and noted progressive weakness of his left foot and ankle. A repeat MRI of the left knee showed a dilated tubular structure corresponding to the course of the CPN. Comparison of this MRI with the initial MRI showed that the “prominent vein” was actually the dilated CPN.
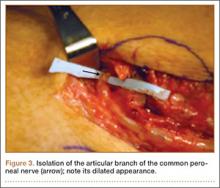
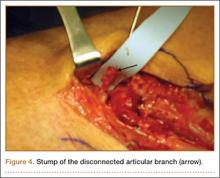
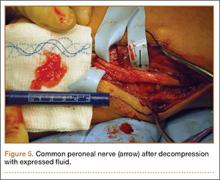
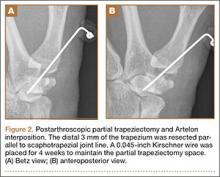
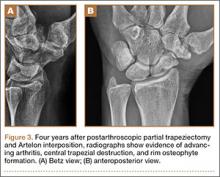
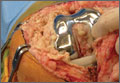
He was taken to the operating room again 5 months after his first operation. At this time, the CPN was again noted to be markedly dilated (Figure 2). The nerve was explored and a recurrent branch to the proximal tibiofibular joint was identified and divided (Figures 3, 4). Through the divided branch, the CPN could be decompressed by manually “milking” the nerve in a proximal-to-distal direction, expressing clear gelatinous fluid consistent with a ganglion cyst (Figure 5). Pathology of the excised portion of the recurrent nerve was consistent with an intraneural ganglion cyst.
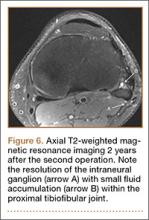
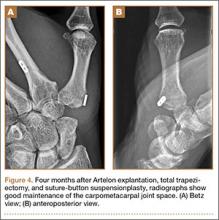
By 2 weeks postoperatively, the numbness of the patient’s left foot had completely resolved, as did his pain. By 3 months after surgery, his extensor hallucis longus strength was 5/5, and ankle dorsiflexion was 4-/5. At 6 months, his ankle dorsiflexion strength was 5/5, and he was completely asymptomatic. At 2 years postoperatively, he remained completely asymptomatic. A follow-up MRI of the left knee showed a ganglion cyst present at the proximal tibiofibular joint with resolution of the intraneural ganglion cyst within the CPN (Figure 6).
Discussion
Intraneural ganglia of peripheral nerves are relatively rare, most commonly occurring in the CPN.6,8,9 A literature search reveals that this condition is only sparsely reported in orthopedic journals. This report, therefore, describes this rare, yet curable, condition. As noted, without appropriate intervention, the condition has a high likelihood of recurrence with only a brief interruption of symptoms.6,8,9,12
The operative technique delineated in this report relies heavily on research demonstrating that peroneal intraneural ganglia develop from the superior tibiofibular joint and gain access to the CPN via the recurrent articular branch.8,13 Research indicates that such ganglia preferentially proceed proximally along the deep portion of the CPN, within the epineurium.6 This hypothesis was corroborated in our case by the swollen appearance of the CPN proximal to its bifurcation.
Currently, there is no consensus on treatment of intraneural ganglion cysts of the CPN. However, evidence suggests that disconnection of the recurrent branch of the CPN may be important in successfully treating the condition.6,9,14 This unified articular theory was initially proposed by Spinner and colleagues12 in 2003 and recommends that surgical treatment focus on the articular branch as the source of cyst fluid.6,9,12,14 This theory by Spinner and coauthors12,14 was substantiated in our case: Once the articular branch was disconnected, cyst fluid was easily expressed via antegrade massage through the disconnected end. Pathologic analysis of a portion of the detached articular branch is also recommended to rule out other cystic lesions, such as cystic shwannomas.14
The history of the unified articular theory began in the mid-1990s, when Dr. Robert Spinner, board certified in both orthopedic and neurologic surgery, began researching causes of intraneural ganglion cysts. At the time, such ganglia were often treated by radical resection of the nerve and the cyst. Based on his review of literature, and his own cases, Spinner15 developed the theory that, just as with extraneural ganglia, these cysts are fed by fluid from the joint. According to Spinner,9 the sources of such connections were very small articular nerve branches that connect the nerve to the joint. His research led him to the original citation of such an intraneural ganglion of the ulnar nerve, first described by Dr. M. Beauchene, a French physician, in 1810.16 Spinner also discovered that Beauchene’s original dissection specimen had been preserved and was displayed in a medical museum in Paris. When Spinner went to France to view the specimen, he indeed found an intraneural ganglion of the ulnar nerve. On closer inspection, Spinner also discovered a small articular nerve branch containing a “hollow lumen” that would have been capable of allowing the passage of fluid into the nerve and leading to the development of a cyst.16
In our case, in the first operation, a simple incisional decompression of the CPN was performed. Unfortunately, the ganglion cyst quickly recurred, as did the patient’s symptoms. In the second surgical procedure, the articular branch connecting the peroneal nerve to the proximal tibiofibular joint was incised and disconnected from the nerve. This allowed the nerve to be decompressed and prevented a recurrence of the ganglion cyst within the nerve with complete resolution of the patient’s symptoms. This difference alone most likely accounts for the rapid recurrence of symptoms after the initial operation, since the fluid was simply drained, but the source was not detached, allowing the ganglion to recur.6,12,14 This is similar in theory to excising the attachment of a ganglion cyst at the wrist from the underlying joint capsule rather than performing a needle aspiration or puncturing of the cyst.12
Regarding the imaging techniques used to identify intraneural ganglia, it is essential that the surgeon be aware of the unified articular theory and the likely presence of an articular branch. Such branches are extremely small and may be easily missed on imaging and intraoperatively.17,18 MRI is the best method to image these cysts because of its superior ability to visualize soft-tissue lesions.18,19 Intraneural ganglion cysts typically appear as homogenous, lobulated, well-circumscribed masses that are hyperintense on T2-weighted MRI.3,19 Gadolinium may also offer diagnostic utility, because these masses do not enhance with its use on T1-weighted MRI.3,17,19 By employing these techniques, one may easily view most of the ganglion cyst. To image the small articular branch, Spinner and colleagues17 recommend thin-section images with high–spatial resolution T2-imaging. They also advocate obtaining multiple image views and planes to increase the likelihood of successful imaging.17
The applications of the unified articular theory also extend beyond intraneural ganglia of the CPN. While the CPN is the most common location for intraneural ganglion occurrence,6,17,20 cases have also been described of intraneural ganglion cysts of the tibial nerve at the proximal tibiofibular joint, as well as via the posterior tibial and medial plantar nerves at the subtalar joint within the tarsal tunnel.11,18-23 Most cases involving the posterior tibial and medial plantar nerves were found in patients presenting with signs of tarsal tunnel syndrome.22,23 Intraneural ganglia have also been found within the superficial peroneal nerve arising from the inferior tibiofibular joint.20 In certain cases, these ganglia have also been noted to connect to the joint via a small articular branch.19,22 In 1 case of an intraneural ganglion of the tibial nerve at the superior tibiofibular joint, initial conservative surgery led to early recurrence of symptoms.19 Just as in our case, the patient returned to the operating room and, after isolation and ligation of an articular branch, the patient experienced long-term resolution of both the symptoms and the cyst.19
Given the overwhelming evidence in support of the unified articular theory, we agree with the recommendation by Spinner and colleagues19 to search for an articular branch both via preoperative imaging and during the operation itself in all cases of intraneural ganglia. Assuming the mechanism of cyst formation is the same in most cases of intraneural ganglia, one could reasonably apply the same surgical techniques used in our case to the management of all intraneural ganglia, drastically reducing recurrence rates.
Conclusion
Based on research and corroborated by this case, the key to successful operative treatment of a common peroneal intraneural ganglion is division of the recurrent articular branch, which connects the proximal tibiofibular joint to the CPN.6,9,11,12,14 Evidence has shown that disconnecting the articular branch and disrupting the source of the intraneural ganglion can resolve the condition and dramatically diminish the chance of recurrence.6,8,12,14 This has become known as the unified articular theory.6,12,14 Reports also suggest that, without disconnecting this articular branch, intraneural ganglion recurrence rates may be higher than 30%.6,12,14,19 This case, therefore, supports the findings of previous authors9-11,14 and provides an example of successful utilization of the treatment protocol delineated by Spinner and colleagues.10,11
1. Coakley FV, Finlay DB, Harper WM, Allen MJ. Direct and indirect MRI findings in ganglion cysts of the common peroneal nerve. Clin Radiol. 1995;50(3):168-169.
2. Coleman SH, Beredjeklian PK, Weiland AJ. Intraneural ganglion cyst of the peroneal nerve accompanied by complete foot drop. A case report. Am J Sports Med. 2001;29(2):238-241.
3. Dubuisson AS, Stevenaert A. Recurrent ganglion cyst of the peroneal nerve: radiological and operative observations. Case report. J Neurosurg. 1996;84(2):280-283.
4. Lee YS, Kim JE, Kwak JH, Wang IW, Lee BK. Foot drop secondary to peroneal intraneural cyst arising from tibiofibular joint. Knee Surg Sports Traumatol Arthrosc. 2013;21(9):2063-2065.
5. Leijten FS, Arts WF, Puylaert JB. Ultrasound diagnosis of an intraneural ganglion cyst of the peroneal nerve. Case report. J Neurosurg. 1992;76(3):538-540.
6. Spinner RJ, Desy NM, Rock MG, Amrami KK. Peroneal intraneural ganglia. Part I. Techniques for successful diagnosis and treatment. Neurosurg Focus. 2007;22(6):E16.
7. Spinner RJ, Desy NM, Amrami KK. Cystic transverse limb of the articular branch: a pathognomonic sign for peroneal intraneural ganglia at the superior tibiofibular joint. Neurosurgery. 2006;59(1):157-166.
8. Spinner RJ, Carmichael SW, Wang H, Parisi TJ, Skinner JA, Amrami KK. Patterns of intraneural ganglion cyst descent. Clin Anat. 2008;21(3):233-245.
9. Spinner RJ, Atkinson JL, Scheithauer BW, et al. Peroneal intraneural ganglia: the importance of the articular branch. Clinical series. J Neurosurg. 2003;99(2):319-329.
10. Spillane RM, Whitman GJ, Chew FS. Peroneal nerve ganglion cyst. AJR Am J Roentgenol. 1996;166(3):682.
11. Spinner RJ, Hébert-Blouin MN, Amrami KK, Rock MG. Peroneal and tibial intraneural ganglion cysts in the knee region: a technical note. Neurosurgery. 2010;67(3 Suppl Operative):ons71-78.
12. Spinner RJ, Atkinson JL, Tiel RL. Peroneal intraneural ganglia: the importance of the articular branch. A unifying theory. J Neurosurg. 2003;99(2):330-343.
13. Spinner RJ, Amrami KK, Wolanskyj AP, et al. Dynamic phases of peroneal and tibial intraneural ganglia formation: a new dimension added to the unifying articular theory. J Neurosurg. 2007;107(2):296-307.
14. Spinner RJ, Desy NM, Rock MG, Amrami KK. Peroneal intraneural ganglia. Part II. Lessons learned and pitfalls to avoid for successful diagnosis and treatment. Neurosurg Focus. 2007;22(6):E27.
15. Spinner RJ; Mayo Clinic. 200-year-old mystery solved: intraneural ganglion cyst [video]. YouTube. www.youtube.com/watch?v=5Xk4kq-qygg. Published October 13, 2008. Accessed February 23, 2015.
16. Spinner RJ, Vincent JF, Wolanskyj AP, Scheithauer BW. Intraneural ganglion cyst: a 200-year-old mystery solved. Clin Anat. 2008;21(7):611-618.
17. Spinner RJ, Dellon AL, Rosson GD, Anderson SR, Amrami KK. Tibial intraneural ganglia in the tarsal tunnel: Is there a joint connection? J Foot Ankle Surg. 2007;46(1):27-31.
18. Spinner RJ, Amrami KK, Rock MG. The use of MR arthrography to document an occult joint communication in a recurrent peroneal intraneural ganglion. Skeletal Radiol. 2006;35(3):172-179.
19. Spinner RJ, Atkinson JL, Harper CM Jr, Wenger DE. Recurrent intraneural ganglion cyst of the tibial nerve. Case report. J Neurosurg. 2000;92(2):334-337.20. Stamatis ED, Manidakis NE, Patouras PP. Intraneural ganglion of the superficial peroneal nerve: a case report. J Foot Ankle Surg. 2010;49(4):400.e1-4.
21. Patel P, Schucany WG. A rare case of intraneural ganglion cyst involving the tibial nerve. Proc (Bayl Univ Med Cent). 2012;25(2):132-135.
22. Høgh J. Benign cystic lesions of peripheral nerves. Int Orthop. 1988;12(4):269-271.
23. Poppi M, Giuliani G, Pozzati E, Acciarri N, Forti A. Tarsal tunnel syndrome secondary to intraneural ganglion. J Neurol Neurosurg Psychiatr. 1989;52(8):1014-1015.
Intraneural ganglion cysts of peripheral nerves occurring within the epineural sheath are rare.1-7 Case reports exist primarily within the neurosurgical literature, but very little in the orthopedic literature describes this condition. The peripheral nerve most commonly affected by an intraneural ganglion is the common peroneal nerve (CPN).2,8,9 Such ganglia most often afflict middle-aged men with a history of micro- or macro-trauma and present with typical clinical manifestations of calf pain and progressive symptoms of ipsilateral foot drop and lower leg paresthesia.2-5,10-12 The mechanism by which these ganglia form is not well understood and, as a result, treatment options are debated.6 Recent development of a “unified articular theory,” suggests that such intraneural ganglia of the CPN are fed by a small, recurrent articular branch of the CPN.6,12,13 Cadaveric studies indicate that this branch originates from the deep peroneal nerve, just millimeters distal to the bifurcation of the CPN, and extends to the superior tibiofibular joint, providing direct access for cyst fluid to enter the CPN following the path of least resistance.7,8,12,14 Therefore, according to the unified articular theory, the recommended treatment involves division of the articular branch, allowing the ganglion to be decompressed.6
We present a case of a 41-year-old man with an intraneural ganglion cyst of the CPN who was successfully treated, according to the recommendations of the unified articular theory. It is important for orthopedic surgeons to read about and recognize this condition, because knowledge of the operative technique outlined in our report allows it to be treated quite effectively. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 41-year-old man presented with a 2-month history of traumatic left lateral knee pain with numbness and weakness to the left foot and ankle. Initial examination showed a mild restriction of lumbosacral range of motion, with no complaints of lower back pain. Sciatic root stretch signs were negative. Strength testing of the lower extremities revealed 3+/5 strength of ankle dorsiflexion and great toe extension on the left side. There was a mild alteration in sensation to light touch on the lateral side of the left foot. Tenderness, without swelling, was present around the left fibular head. There was a positive Tinel sign over the peroneal nerve at the level of the fibular neck.
The patient was initially treated with anti-inflammatories and activity modification. An electromyogram (EMG)/nerve conduction study of the lower extremity showed a left peroneal nerve neurapraxia at the level of the fibular head. Noncontrast magnetic resonance imaging (MRI) of the left knee showed a “slightly prominent vein coursing posterior to the fibular head near the expected location of the common peroneal nerve,” according to the radiologist’s notes (Figure 1). The patient exhibited improvement with use of anti-inflammatories over several months. There was an increase in his ankle dorsiflexion strength to 4/5 and improvement in his pain and numbness.
Approximately 7 months after his initial presentation, the patient developed a marked worsening—increased numbness and weakness to ankle dorsiflexion—of his original symptoms. A repeat EMG/nerve conduction study of the lower extremity showed a persistent peroneal nerve neuropathy with a persistent denervation of the extensor hallucis longus, tibialis anterior, and extensor digitorum brevis muscles.
Because of continuing symptoms and increasing pain, the patient had surgery 8 months after his initial presentation. At that time, a markedly thickened peroneal nerve was identified. An incision in the epineural sheath released a clear gelatinous fluid consistent with a ganglion cyst. Through the epineural incision, the nerve was decompressed by manually “milking” the fluid from within the sheath. Approximately 30 mL of mucinous fluid was obtained and sent to pathology. No cells were identified.
Postoperatively, the patient noted a marked improvement in his pain. By 2 weeks postoperatively, the numbness in his foot had resolved. At 6 weeks after surgery, the strength of his tibialis anterior and extensor hallucis longus muscles had improved from 3+ to 4-, and he was free of pain.
At 2 months postoperatively, the patient redeveloped pain and numbness, and noted progressive weakness of his left foot and ankle. A repeat MRI of the left knee showed a dilated tubular structure corresponding to the course of the CPN. Comparison of this MRI with the initial MRI showed that the “prominent vein” was actually the dilated CPN.
He was taken to the operating room again 5 months after his first operation. At this time, the CPN was again noted to be markedly dilated (Figure 2). The nerve was explored and a recurrent branch to the proximal tibiofibular joint was identified and divided (Figures 3, 4). Through the divided branch, the CPN could be decompressed by manually “milking” the nerve in a proximal-to-distal direction, expressing clear gelatinous fluid consistent with a ganglion cyst (Figure 5). Pathology of the excised portion of the recurrent nerve was consistent with an intraneural ganglion cyst.
By 2 weeks postoperatively, the numbness of the patient’s left foot had completely resolved, as did his pain. By 3 months after surgery, his extensor hallucis longus strength was 5/5, and ankle dorsiflexion was 4-/5. At 6 months, his ankle dorsiflexion strength was 5/5, and he was completely asymptomatic. At 2 years postoperatively, he remained completely asymptomatic. A follow-up MRI of the left knee showed a ganglion cyst present at the proximal tibiofibular joint with resolution of the intraneural ganglion cyst within the CPN (Figure 6).
Discussion
Intraneural ganglia of peripheral nerves are relatively rare, most commonly occurring in the CPN.6,8,9 A literature search reveals that this condition is only sparsely reported in orthopedic journals. This report, therefore, describes this rare, yet curable, condition. As noted, without appropriate intervention, the condition has a high likelihood of recurrence with only a brief interruption of symptoms.6,8,9,12
The operative technique delineated in this report relies heavily on research demonstrating that peroneal intraneural ganglia develop from the superior tibiofibular joint and gain access to the CPN via the recurrent articular branch.8,13 Research indicates that such ganglia preferentially proceed proximally along the deep portion of the CPN, within the epineurium.6 This hypothesis was corroborated in our case by the swollen appearance of the CPN proximal to its bifurcation.
Currently, there is no consensus on treatment of intraneural ganglion cysts of the CPN. However, evidence suggests that disconnection of the recurrent branch of the CPN may be important in successfully treating the condition.6,9,14 This unified articular theory was initially proposed by Spinner and colleagues12 in 2003 and recommends that surgical treatment focus on the articular branch as the source of cyst fluid.6,9,12,14 This theory by Spinner and coauthors12,14 was substantiated in our case: Once the articular branch was disconnected, cyst fluid was easily expressed via antegrade massage through the disconnected end. Pathologic analysis of a portion of the detached articular branch is also recommended to rule out other cystic lesions, such as cystic shwannomas.14
The history of the unified articular theory began in the mid-1990s, when Dr. Robert Spinner, board certified in both orthopedic and neurologic surgery, began researching causes of intraneural ganglion cysts. At the time, such ganglia were often treated by radical resection of the nerve and the cyst. Based on his review of literature, and his own cases, Spinner15 developed the theory that, just as with extraneural ganglia, these cysts are fed by fluid from the joint. According to Spinner,9 the sources of such connections were very small articular nerve branches that connect the nerve to the joint. His research led him to the original citation of such an intraneural ganglion of the ulnar nerve, first described by Dr. M. Beauchene, a French physician, in 1810.16 Spinner also discovered that Beauchene’s original dissection specimen had been preserved and was displayed in a medical museum in Paris. When Spinner went to France to view the specimen, he indeed found an intraneural ganglion of the ulnar nerve. On closer inspection, Spinner also discovered a small articular nerve branch containing a “hollow lumen” that would have been capable of allowing the passage of fluid into the nerve and leading to the development of a cyst.16
In our case, in the first operation, a simple incisional decompression of the CPN was performed. Unfortunately, the ganglion cyst quickly recurred, as did the patient’s symptoms. In the second surgical procedure, the articular branch connecting the peroneal nerve to the proximal tibiofibular joint was incised and disconnected from the nerve. This allowed the nerve to be decompressed and prevented a recurrence of the ganglion cyst within the nerve with complete resolution of the patient’s symptoms. This difference alone most likely accounts for the rapid recurrence of symptoms after the initial operation, since the fluid was simply drained, but the source was not detached, allowing the ganglion to recur.6,12,14 This is similar in theory to excising the attachment of a ganglion cyst at the wrist from the underlying joint capsule rather than performing a needle aspiration or puncturing of the cyst.12
Regarding the imaging techniques used to identify intraneural ganglia, it is essential that the surgeon be aware of the unified articular theory and the likely presence of an articular branch. Such branches are extremely small and may be easily missed on imaging and intraoperatively.17,18 MRI is the best method to image these cysts because of its superior ability to visualize soft-tissue lesions.18,19 Intraneural ganglion cysts typically appear as homogenous, lobulated, well-circumscribed masses that are hyperintense on T2-weighted MRI.3,19 Gadolinium may also offer diagnostic utility, because these masses do not enhance with its use on T1-weighted MRI.3,17,19 By employing these techniques, one may easily view most of the ganglion cyst. To image the small articular branch, Spinner and colleagues17 recommend thin-section images with high–spatial resolution T2-imaging. They also advocate obtaining multiple image views and planes to increase the likelihood of successful imaging.17
The applications of the unified articular theory also extend beyond intraneural ganglia of the CPN. While the CPN is the most common location for intraneural ganglion occurrence,6,17,20 cases have also been described of intraneural ganglion cysts of the tibial nerve at the proximal tibiofibular joint, as well as via the posterior tibial and medial plantar nerves at the subtalar joint within the tarsal tunnel.11,18-23 Most cases involving the posterior tibial and medial plantar nerves were found in patients presenting with signs of tarsal tunnel syndrome.22,23 Intraneural ganglia have also been found within the superficial peroneal nerve arising from the inferior tibiofibular joint.20 In certain cases, these ganglia have also been noted to connect to the joint via a small articular branch.19,22 In 1 case of an intraneural ganglion of the tibial nerve at the superior tibiofibular joint, initial conservative surgery led to early recurrence of symptoms.19 Just as in our case, the patient returned to the operating room and, after isolation and ligation of an articular branch, the patient experienced long-term resolution of both the symptoms and the cyst.19
Given the overwhelming evidence in support of the unified articular theory, we agree with the recommendation by Spinner and colleagues19 to search for an articular branch both via preoperative imaging and during the operation itself in all cases of intraneural ganglia. Assuming the mechanism of cyst formation is the same in most cases of intraneural ganglia, one could reasonably apply the same surgical techniques used in our case to the management of all intraneural ganglia, drastically reducing recurrence rates.
Conclusion
Based on research and corroborated by this case, the key to successful operative treatment of a common peroneal intraneural ganglion is division of the recurrent articular branch, which connects the proximal tibiofibular joint to the CPN.6,9,11,12,14 Evidence has shown that disconnecting the articular branch and disrupting the source of the intraneural ganglion can resolve the condition and dramatically diminish the chance of recurrence.6,8,12,14 This has become known as the unified articular theory.6,12,14 Reports also suggest that, without disconnecting this articular branch, intraneural ganglion recurrence rates may be higher than 30%.6,12,14,19 This case, therefore, supports the findings of previous authors9-11,14 and provides an example of successful utilization of the treatment protocol delineated by Spinner and colleagues.10,11
Intraneural ganglion cysts of peripheral nerves occurring within the epineural sheath are rare.1-7 Case reports exist primarily within the neurosurgical literature, but very little in the orthopedic literature describes this condition. The peripheral nerve most commonly affected by an intraneural ganglion is the common peroneal nerve (CPN).2,8,9 Such ganglia most often afflict middle-aged men with a history of micro- or macro-trauma and present with typical clinical manifestations of calf pain and progressive symptoms of ipsilateral foot drop and lower leg paresthesia.2-5,10-12 The mechanism by which these ganglia form is not well understood and, as a result, treatment options are debated.6 Recent development of a “unified articular theory,” suggests that such intraneural ganglia of the CPN are fed by a small, recurrent articular branch of the CPN.6,12,13 Cadaveric studies indicate that this branch originates from the deep peroneal nerve, just millimeters distal to the bifurcation of the CPN, and extends to the superior tibiofibular joint, providing direct access for cyst fluid to enter the CPN following the path of least resistance.7,8,12,14 Therefore, according to the unified articular theory, the recommended treatment involves division of the articular branch, allowing the ganglion to be decompressed.6
We present a case of a 41-year-old man with an intraneural ganglion cyst of the CPN who was successfully treated, according to the recommendations of the unified articular theory. It is important for orthopedic surgeons to read about and recognize this condition, because knowledge of the operative technique outlined in our report allows it to be treated quite effectively. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 41-year-old man presented with a 2-month history of traumatic left lateral knee pain with numbness and weakness to the left foot and ankle. Initial examination showed a mild restriction of lumbosacral range of motion, with no complaints of lower back pain. Sciatic root stretch signs were negative. Strength testing of the lower extremities revealed 3+/5 strength of ankle dorsiflexion and great toe extension on the left side. There was a mild alteration in sensation to light touch on the lateral side of the left foot. Tenderness, without swelling, was present around the left fibular head. There was a positive Tinel sign over the peroneal nerve at the level of the fibular neck.
The patient was initially treated with anti-inflammatories and activity modification. An electromyogram (EMG)/nerve conduction study of the lower extremity showed a left peroneal nerve neurapraxia at the level of the fibular head. Noncontrast magnetic resonance imaging (MRI) of the left knee showed a “slightly prominent vein coursing posterior to the fibular head near the expected location of the common peroneal nerve,” according to the radiologist’s notes (Figure 1). The patient exhibited improvement with use of anti-inflammatories over several months. There was an increase in his ankle dorsiflexion strength to 4/5 and improvement in his pain and numbness.
Approximately 7 months after his initial presentation, the patient developed a marked worsening—increased numbness and weakness to ankle dorsiflexion—of his original symptoms. A repeat EMG/nerve conduction study of the lower extremity showed a persistent peroneal nerve neuropathy with a persistent denervation of the extensor hallucis longus, tibialis anterior, and extensor digitorum brevis muscles.
Because of continuing symptoms and increasing pain, the patient had surgery 8 months after his initial presentation. At that time, a markedly thickened peroneal nerve was identified. An incision in the epineural sheath released a clear gelatinous fluid consistent with a ganglion cyst. Through the epineural incision, the nerve was decompressed by manually “milking” the fluid from within the sheath. Approximately 30 mL of mucinous fluid was obtained and sent to pathology. No cells were identified.
Postoperatively, the patient noted a marked improvement in his pain. By 2 weeks postoperatively, the numbness in his foot had resolved. At 6 weeks after surgery, the strength of his tibialis anterior and extensor hallucis longus muscles had improved from 3+ to 4-, and he was free of pain.
At 2 months postoperatively, the patient redeveloped pain and numbness, and noted progressive weakness of his left foot and ankle. A repeat MRI of the left knee showed a dilated tubular structure corresponding to the course of the CPN. Comparison of this MRI with the initial MRI showed that the “prominent vein” was actually the dilated CPN.
He was taken to the operating room again 5 months after his first operation. At this time, the CPN was again noted to be markedly dilated (Figure 2). The nerve was explored and a recurrent branch to the proximal tibiofibular joint was identified and divided (Figures 3, 4). Through the divided branch, the CPN could be decompressed by manually “milking” the nerve in a proximal-to-distal direction, expressing clear gelatinous fluid consistent with a ganglion cyst (Figure 5). Pathology of the excised portion of the recurrent nerve was consistent with an intraneural ganglion cyst.
By 2 weeks postoperatively, the numbness of the patient’s left foot had completely resolved, as did his pain. By 3 months after surgery, his extensor hallucis longus strength was 5/5, and ankle dorsiflexion was 4-/5. At 6 months, his ankle dorsiflexion strength was 5/5, and he was completely asymptomatic. At 2 years postoperatively, he remained completely asymptomatic. A follow-up MRI of the left knee showed a ganglion cyst present at the proximal tibiofibular joint with resolution of the intraneural ganglion cyst within the CPN (Figure 6).
Discussion
Intraneural ganglia of peripheral nerves are relatively rare, most commonly occurring in the CPN.6,8,9 A literature search reveals that this condition is only sparsely reported in orthopedic journals. This report, therefore, describes this rare, yet curable, condition. As noted, without appropriate intervention, the condition has a high likelihood of recurrence with only a brief interruption of symptoms.6,8,9,12
The operative technique delineated in this report relies heavily on research demonstrating that peroneal intraneural ganglia develop from the superior tibiofibular joint and gain access to the CPN via the recurrent articular branch.8,13 Research indicates that such ganglia preferentially proceed proximally along the deep portion of the CPN, within the epineurium.6 This hypothesis was corroborated in our case by the swollen appearance of the CPN proximal to its bifurcation.
Currently, there is no consensus on treatment of intraneural ganglion cysts of the CPN. However, evidence suggests that disconnection of the recurrent branch of the CPN may be important in successfully treating the condition.6,9,14 This unified articular theory was initially proposed by Spinner and colleagues12 in 2003 and recommends that surgical treatment focus on the articular branch as the source of cyst fluid.6,9,12,14 This theory by Spinner and coauthors12,14 was substantiated in our case: Once the articular branch was disconnected, cyst fluid was easily expressed via antegrade massage through the disconnected end. Pathologic analysis of a portion of the detached articular branch is also recommended to rule out other cystic lesions, such as cystic shwannomas.14
The history of the unified articular theory began in the mid-1990s, when Dr. Robert Spinner, board certified in both orthopedic and neurologic surgery, began researching causes of intraneural ganglion cysts. At the time, such ganglia were often treated by radical resection of the nerve and the cyst. Based on his review of literature, and his own cases, Spinner15 developed the theory that, just as with extraneural ganglia, these cysts are fed by fluid from the joint. According to Spinner,9 the sources of such connections were very small articular nerve branches that connect the nerve to the joint. His research led him to the original citation of such an intraneural ganglion of the ulnar nerve, first described by Dr. M. Beauchene, a French physician, in 1810.16 Spinner also discovered that Beauchene’s original dissection specimen had been preserved and was displayed in a medical museum in Paris. When Spinner went to France to view the specimen, he indeed found an intraneural ganglion of the ulnar nerve. On closer inspection, Spinner also discovered a small articular nerve branch containing a “hollow lumen” that would have been capable of allowing the passage of fluid into the nerve and leading to the development of a cyst.16
In our case, in the first operation, a simple incisional decompression of the CPN was performed. Unfortunately, the ganglion cyst quickly recurred, as did the patient’s symptoms. In the second surgical procedure, the articular branch connecting the peroneal nerve to the proximal tibiofibular joint was incised and disconnected from the nerve. This allowed the nerve to be decompressed and prevented a recurrence of the ganglion cyst within the nerve with complete resolution of the patient’s symptoms. This difference alone most likely accounts for the rapid recurrence of symptoms after the initial operation, since the fluid was simply drained, but the source was not detached, allowing the ganglion to recur.6,12,14 This is similar in theory to excising the attachment of a ganglion cyst at the wrist from the underlying joint capsule rather than performing a needle aspiration or puncturing of the cyst.12
Regarding the imaging techniques used to identify intraneural ganglia, it is essential that the surgeon be aware of the unified articular theory and the likely presence of an articular branch. Such branches are extremely small and may be easily missed on imaging and intraoperatively.17,18 MRI is the best method to image these cysts because of its superior ability to visualize soft-tissue lesions.18,19 Intraneural ganglion cysts typically appear as homogenous, lobulated, well-circumscribed masses that are hyperintense on T2-weighted MRI.3,19 Gadolinium may also offer diagnostic utility, because these masses do not enhance with its use on T1-weighted MRI.3,17,19 By employing these techniques, one may easily view most of the ganglion cyst. To image the small articular branch, Spinner and colleagues17 recommend thin-section images with high–spatial resolution T2-imaging. They also advocate obtaining multiple image views and planes to increase the likelihood of successful imaging.17
The applications of the unified articular theory also extend beyond intraneural ganglia of the CPN. While the CPN is the most common location for intraneural ganglion occurrence,6,17,20 cases have also been described of intraneural ganglion cysts of the tibial nerve at the proximal tibiofibular joint, as well as via the posterior tibial and medial plantar nerves at the subtalar joint within the tarsal tunnel.11,18-23 Most cases involving the posterior tibial and medial plantar nerves were found in patients presenting with signs of tarsal tunnel syndrome.22,23 Intraneural ganglia have also been found within the superficial peroneal nerve arising from the inferior tibiofibular joint.20 In certain cases, these ganglia have also been noted to connect to the joint via a small articular branch.19,22 In 1 case of an intraneural ganglion of the tibial nerve at the superior tibiofibular joint, initial conservative surgery led to early recurrence of symptoms.19 Just as in our case, the patient returned to the operating room and, after isolation and ligation of an articular branch, the patient experienced long-term resolution of both the symptoms and the cyst.19
Given the overwhelming evidence in support of the unified articular theory, we agree with the recommendation by Spinner and colleagues19 to search for an articular branch both via preoperative imaging and during the operation itself in all cases of intraneural ganglia. Assuming the mechanism of cyst formation is the same in most cases of intraneural ganglia, one could reasonably apply the same surgical techniques used in our case to the management of all intraneural ganglia, drastically reducing recurrence rates.
Conclusion
Based on research and corroborated by this case, the key to successful operative treatment of a common peroneal intraneural ganglion is division of the recurrent articular branch, which connects the proximal tibiofibular joint to the CPN.6,9,11,12,14 Evidence has shown that disconnecting the articular branch and disrupting the source of the intraneural ganglion can resolve the condition and dramatically diminish the chance of recurrence.6,8,12,14 This has become known as the unified articular theory.6,12,14 Reports also suggest that, without disconnecting this articular branch, intraneural ganglion recurrence rates may be higher than 30%.6,12,14,19 This case, therefore, supports the findings of previous authors9-11,14 and provides an example of successful utilization of the treatment protocol delineated by Spinner and colleagues.10,11
1. Coakley FV, Finlay DB, Harper WM, Allen MJ. Direct and indirect MRI findings in ganglion cysts of the common peroneal nerve. Clin Radiol. 1995;50(3):168-169.
2. Coleman SH, Beredjeklian PK, Weiland AJ. Intraneural ganglion cyst of the peroneal nerve accompanied by complete foot drop. A case report. Am J Sports Med. 2001;29(2):238-241.
3. Dubuisson AS, Stevenaert A. Recurrent ganglion cyst of the peroneal nerve: radiological and operative observations. Case report. J Neurosurg. 1996;84(2):280-283.
4. Lee YS, Kim JE, Kwak JH, Wang IW, Lee BK. Foot drop secondary to peroneal intraneural cyst arising from tibiofibular joint. Knee Surg Sports Traumatol Arthrosc. 2013;21(9):2063-2065.
5. Leijten FS, Arts WF, Puylaert JB. Ultrasound diagnosis of an intraneural ganglion cyst of the peroneal nerve. Case report. J Neurosurg. 1992;76(3):538-540.
6. Spinner RJ, Desy NM, Rock MG, Amrami KK. Peroneal intraneural ganglia. Part I. Techniques for successful diagnosis and treatment. Neurosurg Focus. 2007;22(6):E16.
7. Spinner RJ, Desy NM, Amrami KK. Cystic transverse limb of the articular branch: a pathognomonic sign for peroneal intraneural ganglia at the superior tibiofibular joint. Neurosurgery. 2006;59(1):157-166.
8. Spinner RJ, Carmichael SW, Wang H, Parisi TJ, Skinner JA, Amrami KK. Patterns of intraneural ganglion cyst descent. Clin Anat. 2008;21(3):233-245.
9. Spinner RJ, Atkinson JL, Scheithauer BW, et al. Peroneal intraneural ganglia: the importance of the articular branch. Clinical series. J Neurosurg. 2003;99(2):319-329.
10. Spillane RM, Whitman GJ, Chew FS. Peroneal nerve ganglion cyst. AJR Am J Roentgenol. 1996;166(3):682.
11. Spinner RJ, Hébert-Blouin MN, Amrami KK, Rock MG. Peroneal and tibial intraneural ganglion cysts in the knee region: a technical note. Neurosurgery. 2010;67(3 Suppl Operative):ons71-78.
12. Spinner RJ, Atkinson JL, Tiel RL. Peroneal intraneural ganglia: the importance of the articular branch. A unifying theory. J Neurosurg. 2003;99(2):330-343.
13. Spinner RJ, Amrami KK, Wolanskyj AP, et al. Dynamic phases of peroneal and tibial intraneural ganglia formation: a new dimension added to the unifying articular theory. J Neurosurg. 2007;107(2):296-307.
14. Spinner RJ, Desy NM, Rock MG, Amrami KK. Peroneal intraneural ganglia. Part II. Lessons learned and pitfalls to avoid for successful diagnosis and treatment. Neurosurg Focus. 2007;22(6):E27.
15. Spinner RJ; Mayo Clinic. 200-year-old mystery solved: intraneural ganglion cyst [video]. YouTube. www.youtube.com/watch?v=5Xk4kq-qygg. Published October 13, 2008. Accessed February 23, 2015.
16. Spinner RJ, Vincent JF, Wolanskyj AP, Scheithauer BW. Intraneural ganglion cyst: a 200-year-old mystery solved. Clin Anat. 2008;21(7):611-618.
17. Spinner RJ, Dellon AL, Rosson GD, Anderson SR, Amrami KK. Tibial intraneural ganglia in the tarsal tunnel: Is there a joint connection? J Foot Ankle Surg. 2007;46(1):27-31.
18. Spinner RJ, Amrami KK, Rock MG. The use of MR arthrography to document an occult joint communication in a recurrent peroneal intraneural ganglion. Skeletal Radiol. 2006;35(3):172-179.
19. Spinner RJ, Atkinson JL, Harper CM Jr, Wenger DE. Recurrent intraneural ganglion cyst of the tibial nerve. Case report. J Neurosurg. 2000;92(2):334-337.20. Stamatis ED, Manidakis NE, Patouras PP. Intraneural ganglion of the superficial peroneal nerve: a case report. J Foot Ankle Surg. 2010;49(4):400.e1-4.
21. Patel P, Schucany WG. A rare case of intraneural ganglion cyst involving the tibial nerve. Proc (Bayl Univ Med Cent). 2012;25(2):132-135.
22. Høgh J. Benign cystic lesions of peripheral nerves. Int Orthop. 1988;12(4):269-271.
23. Poppi M, Giuliani G, Pozzati E, Acciarri N, Forti A. Tarsal tunnel syndrome secondary to intraneural ganglion. J Neurol Neurosurg Psychiatr. 1989;52(8):1014-1015.
1. Coakley FV, Finlay DB, Harper WM, Allen MJ. Direct and indirect MRI findings in ganglion cysts of the common peroneal nerve. Clin Radiol. 1995;50(3):168-169.
2. Coleman SH, Beredjeklian PK, Weiland AJ. Intraneural ganglion cyst of the peroneal nerve accompanied by complete foot drop. A case report. Am J Sports Med. 2001;29(2):238-241.
3. Dubuisson AS, Stevenaert A. Recurrent ganglion cyst of the peroneal nerve: radiological and operative observations. Case report. J Neurosurg. 1996;84(2):280-283.
4. Lee YS, Kim JE, Kwak JH, Wang IW, Lee BK. Foot drop secondary to peroneal intraneural cyst arising from tibiofibular joint. Knee Surg Sports Traumatol Arthrosc. 2013;21(9):2063-2065.
5. Leijten FS, Arts WF, Puylaert JB. Ultrasound diagnosis of an intraneural ganglion cyst of the peroneal nerve. Case report. J Neurosurg. 1992;76(3):538-540.
6. Spinner RJ, Desy NM, Rock MG, Amrami KK. Peroneal intraneural ganglia. Part I. Techniques for successful diagnosis and treatment. Neurosurg Focus. 2007;22(6):E16.
7. Spinner RJ, Desy NM, Amrami KK. Cystic transverse limb of the articular branch: a pathognomonic sign for peroneal intraneural ganglia at the superior tibiofibular joint. Neurosurgery. 2006;59(1):157-166.
8. Spinner RJ, Carmichael SW, Wang H, Parisi TJ, Skinner JA, Amrami KK. Patterns of intraneural ganglion cyst descent. Clin Anat. 2008;21(3):233-245.
9. Spinner RJ, Atkinson JL, Scheithauer BW, et al. Peroneal intraneural ganglia: the importance of the articular branch. Clinical series. J Neurosurg. 2003;99(2):319-329.
10. Spillane RM, Whitman GJ, Chew FS. Peroneal nerve ganglion cyst. AJR Am J Roentgenol. 1996;166(3):682.
11. Spinner RJ, Hébert-Blouin MN, Amrami KK, Rock MG. Peroneal and tibial intraneural ganglion cysts in the knee region: a technical note. Neurosurgery. 2010;67(3 Suppl Operative):ons71-78.
12. Spinner RJ, Atkinson JL, Tiel RL. Peroneal intraneural ganglia: the importance of the articular branch. A unifying theory. J Neurosurg. 2003;99(2):330-343.
13. Spinner RJ, Amrami KK, Wolanskyj AP, et al. Dynamic phases of peroneal and tibial intraneural ganglia formation: a new dimension added to the unifying articular theory. J Neurosurg. 2007;107(2):296-307.
14. Spinner RJ, Desy NM, Rock MG, Amrami KK. Peroneal intraneural ganglia. Part II. Lessons learned and pitfalls to avoid for successful diagnosis and treatment. Neurosurg Focus. 2007;22(6):E27.
15. Spinner RJ; Mayo Clinic. 200-year-old mystery solved: intraneural ganglion cyst [video]. YouTube. www.youtube.com/watch?v=5Xk4kq-qygg. Published October 13, 2008. Accessed February 23, 2015.
16. Spinner RJ, Vincent JF, Wolanskyj AP, Scheithauer BW. Intraneural ganglion cyst: a 200-year-old mystery solved. Clin Anat. 2008;21(7):611-618.
17. Spinner RJ, Dellon AL, Rosson GD, Anderson SR, Amrami KK. Tibial intraneural ganglia in the tarsal tunnel: Is there a joint connection? J Foot Ankle Surg. 2007;46(1):27-31.
18. Spinner RJ, Amrami KK, Rock MG. The use of MR arthrography to document an occult joint communication in a recurrent peroneal intraneural ganglion. Skeletal Radiol. 2006;35(3):172-179.
19. Spinner RJ, Atkinson JL, Harper CM Jr, Wenger DE. Recurrent intraneural ganglion cyst of the tibial nerve. Case report. J Neurosurg. 2000;92(2):334-337.20. Stamatis ED, Manidakis NE, Patouras PP. Intraneural ganglion of the superficial peroneal nerve: a case report. J Foot Ankle Surg. 2010;49(4):400.e1-4.
21. Patel P, Schucany WG. A rare case of intraneural ganglion cyst involving the tibial nerve. Proc (Bayl Univ Med Cent). 2012;25(2):132-135.
22. Høgh J. Benign cystic lesions of peripheral nerves. Int Orthop. 1988;12(4):269-271.
23. Poppi M, Giuliani G, Pozzati E, Acciarri N, Forti A. Tarsal tunnel syndrome secondary to intraneural ganglion. J Neurol Neurosurg Psychiatr. 1989;52(8):1014-1015.
Failure of Artelon Interposition Arthroplasty After Partial Trapeziectomy: A Case Report With Histologic and Immunohistochemical Analysis
Osteoarthritis (OA) of the first carpometacarpal (CMC) joint is a common disabling condition that mostly affects women over 45 years of age.1 Surgical intervention is usually indicated in advanced stage OA of the first CMC joint that has failed conservative treatment. Several surgical techniques have been described, including partial or total trapeziectomy, interposition arthroplasty with or without ligament reconstruction,2,3 metacarpal osteotomy,4 hematoma and distraction arthroplasty,5 total joint arthroplasty, arthrodesis, and suspensionplasty.6 However, no single surgical procedure has proved to be superior.7
The Artelon implant (Artelon, Nashville, Tennessee) is a T-shaped spacer composed of a biocompatible and biodegradable polycaprolactone-based polyurethane urea polymer. The developers of the implant first presented its use in CMC OA in 2005.8 The device, an endoprosthetic replacement for the CMC joint, was designed to work through 2 modes of action: stabilization of the CMC joint by augmentation of the joint capsule and by formation of a new articular surface at the trapeziometacarpal interface. The interposed biomaterial has been described as preventing bony impingement and allowing time for replacement with a newly formed articular surface as it undergoes slow and controlled degradation.8
We present a patient with recurrent CMC pain and disability 4 years after arthroscopic hemitrapeziectomy and Artelon interposition and discuss the associated histologic findings. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 53-year-old man presented with painful disability of right thumb of several months’ duration. Clinical and radiographic evaluation supported the diagnosis of right thumb CMC joint Eaton stage III arthritis (Figures 1A, 1B). Surgical intervention was indicated after a failed course of conservative treatment, including splinting, nonsteroidal anti-inflammatory medications, activity modification, and corticosteroid injection. Preoperatively, the patient reported a visual analog scale (VAS) score of 8 with activity and 5 at rest, and a Disabilities of the Arm, Shoulder, and Hand (DASH) score of 72.5.
Arthroscopic débridement, hemitrapeziectomy, and interposition arthroplasty with the Artelon spacer were performed. Using standard thumb arthroscopy, 3 mm of the distal trapezium was excised and shaped parallel to scaphotrapezial joint. The wings of the standard-sized Artelon spacer were removed, and the central (articulating) portion was rolled into a tube and inserted through the 1R portal (directly radial to the abductor pollicis longus tendon) into the trapezial space. The Artelon spacer was unrolled within the joint to cover the remaining trapezium and was stabilized with the placement of a 0.045-inch Kirschner wire through the metacarpal, the spacer, and the remaining trapezium. The patient used a thumb spica splint for 4 weeks.
The postoperative radiographs showed a smooth and adequate hemitrapeziectomy with good alignment and implant position (Figures 2A, 2B). Four weeks after surgery, the Kirschner wire and cast were removed and physical therapy was initiated. The patient’s CMC pain gradually subsided. At the 3-month postoperative visit, the patient’s VAS score was 3 with activity and 1 at rest, with a DASH score of 28. His key pinch strength was 12 lb, compared with 20 lb on the contralateral side. At 6 months, the patient’s VAS score was 1 with activity and 0 at rest, with a DASH score of 12. His key pinch strength was 18 lb, compared with 22 lb on the contralateral side. At his 2-year postoperative visit, the patient was doing well with the exception of some mild residual pain when he opened tight jars. His VAS score was 1 with activity and 0 at rest, with a DASH score of 3. His key pinch strength was 20 lb, compared with 23 lb on the contralateral side. Radiographs showed good maintenance of the CMC space.
Four years postoperatively, the patient presented with worsening right CMC pain with decrease in pinch strength that interfered with his activities of daily living. His VAS score was 9 with activity and 6 at rest, with a DASH score of 70. On examination, pinch strength was 16 lb, compared with 22 lb on the contralateral side. Radiographs showed advancing arthritis with new osteophyte formation and irregular contour of distal trapezium (Figures 3A, 3B). The symptoms were refractory to conservative measures and continued to interfere with his activities of daily living. Revision surgical intervention was indicated and pursued in the form of an open CMC arthroplasty.
The intraoperative findings revealed degradation and disorganization of the Artelon implant within the central portion of the remaining distal trapezium. Rim osteophytes, especially along the ulnar aspect, were noted. Total trapeziectomy and débridement within the CMC space and suture-button suspensionplasty were performed.8 Slight degenerative changes of the distal scaphoid were also noted. The incision was irrigated, closed, and stabilized in a thumb spica splint (Figures 4A, 4B).
The harvested trapezium was immediately immersed in buffered formalin. The bone tissue was decalcified, dehydrated, embedded in paraffin, and sectioned in the coronal plane. The sections were stained with safranin O and trichrome, and light microscopic analysis was performed. Central erosion of distal trapezium without smooth resurfacing soft-tissue formation was noted grossly (Figure 5A) and microscopically (Figures 5B, 5C). The histologic morphology of the soft tissue over the distal trapezium was significantly different when compared with the smooth hyaline cartilage at the preserved trapezio-trapezoidal joint (Figures 6A-6F). Microscopic analysis also showed multinucleated giant cells within the soft tissue surrounding the degraded Artelon B (Figure 7).
Immunohistochemical analysis was performed to identify type I and type II collagen using the Histostain-Plus,3rd Gen IHC Detection Kit (Invitrogen Corporation, Camarillo, California) (Figures 8A-8F).9 The immunohistochemical stain was used to identify new hyaline cartilage formation that may have been induced by the Artelon as the resurfacing articulation. Hyaline cartilage contains mainly type II collagen, and collagen types VI, IX, X, XI, XII, and XIV all contribute to the mature matrix.10 Little type I collagen is found in hyaline cartilage. The results showed that the soft tissue over the distal trapezium with embedded Artelon fiber contained both type I and type II collagen. There was no visible hyaline cartilage formation induced by the Artelon. Both morphologic analysis and immunohistochemical staining revealed that the soft-tissue growth into the Artelon spacer on the distal trapezium consisted primarily of fibrocartilaginous tissue, which is composed mainly of type I collagen with some type II collagen.
Two weeks after total surgical excision of the Artelon implant, total trapeziectomy and suture-button suspensionplasty, the sutures were removed and physical therapy was initiated. Radiographs showed good alignment and position of thumb metacarpal with good maintenance of the implant and CMC space. Four months postoperatively, the patient reported that he was doing well without pain and without interference in his activities of daily living. On examination, the patient exhibited no pain with the CMC grind maneuver. Radial abduction of the right thumb was 85° and palmar abduction was 90° (compared with 100° and 90° of the left thumb), obtained by measuring the angle between thumb and index finger, respectively. Opposition was to the small finger metacarpophalangeal joint. Grip strength was 72 lb and pinch strength was 20 lb (compared with 70 lb and 24 lb, respectively, on the contralateral side).
Discussion
The use of Artelon as an endoprosthetic spacer to treat osteoarthritis in the CMC joint of the thumb appears to stabilize and resurface the joint while avoiding total trapeziectomy.8 Nilsson and colleagues8 presented a prospective study concluding that the Artelon CMC spacer provided better pinch strength when compared with a traditional abductor pollicis longus suspensionplasty procedure. This study also suggested incorporation of the device in the surface of the adjacent bone with no signs of foreign-body reaction. The synthetic material was shown to be safe and biocompatible in vitro and in animal studies.11-13
This case report describes the gross and histologic findings after continued pain led to explantation 4 years after arthroscopic partial trapeziectomy and insertion of the spacer. Intraoperative findings at this stage showed lack of incorporation of the Artelon material, central destruction of distal trapezium, and no evidence of smooth articular surface formation. Our histologic analysis showed only poorly organized fibrocartilage within the CMC space rather than a smooth articular surface. These histologic findings may correlate more with Jörheim and colleagues’14 matched cohort study, which showed that short-term outcomes after treatment with the Artelon implant were not clinically superior to those of tendon suspension-interposition arthroplasties. Multinucleated giant cells were also seen in our specimens. Choung and Tan15 presented a case report of foreign-body reaction to the Artelon spacer with histologic findings. The foreign body–type reactions associated with Artelon resulted in multinucleated giant cells in their specimens. Recently, several case reports have described similar foreign-body reactions.16 Nilsson and coauthors17 presented a randomized, controlled, multicenter study of 109 patients. They reported the Artelon CMC spacer did not result in superior results compared with tendon interposition arthroplasty. In a study by Gretzer and colleagues,18 the authors suggested that chronic inflammation may result from unstable Artelon fixation instead of the foreign-body reaction.
It is possible that the central erosion of the distal trapezium seen in our case may have resulted from chronic inflammation caused by foreign-body reaction and/or an unstably fixed spacer. The spacer was transfixed to the remaining trapezium in the CMC joint with a Kirschner wire followed by immobilization for 4 weeks. Poor soft-tissue integration of the Artelon spacer may have led to unintended motion and chronic inflammation, which may have also resulted in erosion between the Artelon spacer and the trapezium, leading to central destruction of the distal trapezium. Lastly, the byproducts formed by the degradation of the spacer may have resulted in erosion of the remaining trapezium.
Conclusion
The Artelon CMC spacer used in this patient provided comparable, but not superior, clinical results to other procedures. Histologically, the new articular surface in our patient was formed with rugged fibrocartilage instead of the expected smooth cartilaginous surface. The chronic inflammatory reaction may have resulted from foreign-body reaction, unstable implant fixation, or poor soft-tissue integration. This inflammatory reaction may have contributed to the patient’s recurrence of symptoms. These findings support recent clinical data that suggest the use of the Artelon spacer may not provide superior results to other surgical options for the treatment of CMC joint arthritis.
1. Dahaghin S, Bierma-Zeinstra SM, Ginai AZ, Pols HA, Hazes JM, Koes BW. Prevalence and pattern of radiographic hand osteoarthritis and association with pain and disability (the Rotterdam study). Ann Rheum Dis. 2005;64(5):682-687.
2. Eaton RG, Glickel SZ, Littler JW. Tendon interposition arthroplasty for degenerative arthritis of the trapeziometacarpal joint of the thumb. J Hand Surg. 1985;10(5):645-654.
3. Gibbons CE, Gosal HS, Choudri AH, Magnussen PA. Trapeziectomy for basal thumb joint osteoarthritis: 3- to 19-year follow-up. Int Orthop. 1999;23(4):216-218.
4. Gwynne-Jones DP, Penny ID, Sewell SA, Hughes TH. Basal thumb metacarpal osteotomy for trapeziometacarpal osteoarthritis. J Orthop Surg (Hong Kong). 2006;14(1):58-63.
5. Gray KV, Meals RA. Hematoma and distraction arthroplasty for thumb basal joint osteoarthritis: minimum 6.5-year follow-up evaluation. J Hand Surg Am. 2007;32(1):23-29.
6. Cox CA, Zlotolow DA, Yao J. Suture button suspensionplasty after arthroscopic hemitrapeziectomy for treatment of thumb carpometacarpal arthritis. Arthroscopy. 2010;26(10):1395-1403.
7. Vermeulen GM, Slijper H, Feitz R, Hovius SE, Moojen TM, Selles RW. Surgical management of primary thumb carpometacarpal osteoarthritis: a systematic review. J Hand Surg Am. 2011;36(1):157-169.
8. Nilsson A, Liljensten E, Bergström C, Sollerman C. Results from a degradable TMC joint Spacer (Artelon) compared with tendon arthroplasty. J Hand Surg Am. 2005;30(2):380-389.
9. Histostain®-Plus, 3rd Gen IHC Detection Kit [product information]. Invitrogen website. http://tools.invitrogen.com/content/sfs/manuals/859073_Rev1108.pdf. Revised November 2008. Accessed February 27, 2015.
10. Eyre D. Collagen of articular cartilage. Arthritis Res. 2002;4(1):30-35.
11. Gisselfält K, Edberg B, Flodin P. Synthesis and properties of degradable poly(urethane urea)s to be used for ligament reconstructions. Biomacromolecules. 2002;3(5):951-958.
12. Liljensten E, Gisselfält K, Edberg B, et al. Studies of polyurethane urea bands for ACL reconstruction. J Mater Sci Mater Med. 2002;13(4):351-359.
13. Gretzer C, Gisselfält K, Liljensten E, Rydén L, Thomsen P. Adhesion, apoptosis and cytokine release of human mononuclear cells cultured on degradable poly(urethane urea), polystyrene and titanium in vitro. Biomaterials. 2003;24(17):2843-2852.
14. Jörheim M, Isaxon I, Flondell M, Kalén P, Atroshi I. Short-term outcomes of trapeziometacarpal artelon implant compared with tendon suspension interposition arthroplasty for osteoarthritis: a matched cohort study. J Hand Surg Am. 2009;34(8):1381-1387.
15. Choung EW, Tan V. Foreign-body reaction to the Artelon CMC joint spacer: case report. J Hand Surg Am. 2008;33(9):1617-1620.
16. Robinson PM, Muir LT. Foreign body reaction associated with Artelon: report of three cases. J Hand Surg Am. 2011;36(1):116-120.
17. Nilsson A, Wiig M, Alnehill H, et al. The Artelon CMC spacer compared with tendon interposition arthroplasty. Acta Orthop. 2010;81(2):237-244.
18. Gretzer C, Emanuelsson L, Liljensten E, Thomsen P. The inflammatory cell influx and cytokines changes during transition from acute inflammation to fibrous repair around implanted materials. J Biomater Sci Polym Ed. 2006;17(6):669-687.
Osteoarthritis (OA) of the first carpometacarpal (CMC) joint is a common disabling condition that mostly affects women over 45 years of age.1 Surgical intervention is usually indicated in advanced stage OA of the first CMC joint that has failed conservative treatment. Several surgical techniques have been described, including partial or total trapeziectomy, interposition arthroplasty with or without ligament reconstruction,2,3 metacarpal osteotomy,4 hematoma and distraction arthroplasty,5 total joint arthroplasty, arthrodesis, and suspensionplasty.6 However, no single surgical procedure has proved to be superior.7
The Artelon implant (Artelon, Nashville, Tennessee) is a T-shaped spacer composed of a biocompatible and biodegradable polycaprolactone-based polyurethane urea polymer. The developers of the implant first presented its use in CMC OA in 2005.8 The device, an endoprosthetic replacement for the CMC joint, was designed to work through 2 modes of action: stabilization of the CMC joint by augmentation of the joint capsule and by formation of a new articular surface at the trapeziometacarpal interface. The interposed biomaterial has been described as preventing bony impingement and allowing time for replacement with a newly formed articular surface as it undergoes slow and controlled degradation.8
We present a patient with recurrent CMC pain and disability 4 years after arthroscopic hemitrapeziectomy and Artelon interposition and discuss the associated histologic findings. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 53-year-old man presented with painful disability of right thumb of several months’ duration. Clinical and radiographic evaluation supported the diagnosis of right thumb CMC joint Eaton stage III arthritis (Figures 1A, 1B). Surgical intervention was indicated after a failed course of conservative treatment, including splinting, nonsteroidal anti-inflammatory medications, activity modification, and corticosteroid injection. Preoperatively, the patient reported a visual analog scale (VAS) score of 8 with activity and 5 at rest, and a Disabilities of the Arm, Shoulder, and Hand (DASH) score of 72.5.
Arthroscopic débridement, hemitrapeziectomy, and interposition arthroplasty with the Artelon spacer were performed. Using standard thumb arthroscopy, 3 mm of the distal trapezium was excised and shaped parallel to scaphotrapezial joint. The wings of the standard-sized Artelon spacer were removed, and the central (articulating) portion was rolled into a tube and inserted through the 1R portal (directly radial to the abductor pollicis longus tendon) into the trapezial space. The Artelon spacer was unrolled within the joint to cover the remaining trapezium and was stabilized with the placement of a 0.045-inch Kirschner wire through the metacarpal, the spacer, and the remaining trapezium. The patient used a thumb spica splint for 4 weeks.
The postoperative radiographs showed a smooth and adequate hemitrapeziectomy with good alignment and implant position (Figures 2A, 2B). Four weeks after surgery, the Kirschner wire and cast were removed and physical therapy was initiated. The patient’s CMC pain gradually subsided. At the 3-month postoperative visit, the patient’s VAS score was 3 with activity and 1 at rest, with a DASH score of 28. His key pinch strength was 12 lb, compared with 20 lb on the contralateral side. At 6 months, the patient’s VAS score was 1 with activity and 0 at rest, with a DASH score of 12. His key pinch strength was 18 lb, compared with 22 lb on the contralateral side. At his 2-year postoperative visit, the patient was doing well with the exception of some mild residual pain when he opened tight jars. His VAS score was 1 with activity and 0 at rest, with a DASH score of 3. His key pinch strength was 20 lb, compared with 23 lb on the contralateral side. Radiographs showed good maintenance of the CMC space.
Four years postoperatively, the patient presented with worsening right CMC pain with decrease in pinch strength that interfered with his activities of daily living. His VAS score was 9 with activity and 6 at rest, with a DASH score of 70. On examination, pinch strength was 16 lb, compared with 22 lb on the contralateral side. Radiographs showed advancing arthritis with new osteophyte formation and irregular contour of distal trapezium (Figures 3A, 3B). The symptoms were refractory to conservative measures and continued to interfere with his activities of daily living. Revision surgical intervention was indicated and pursued in the form of an open CMC arthroplasty.
The intraoperative findings revealed degradation and disorganization of the Artelon implant within the central portion of the remaining distal trapezium. Rim osteophytes, especially along the ulnar aspect, were noted. Total trapeziectomy and débridement within the CMC space and suture-button suspensionplasty were performed.8 Slight degenerative changes of the distal scaphoid were also noted. The incision was irrigated, closed, and stabilized in a thumb spica splint (Figures 4A, 4B).
The harvested trapezium was immediately immersed in buffered formalin. The bone tissue was decalcified, dehydrated, embedded in paraffin, and sectioned in the coronal plane. The sections were stained with safranin O and trichrome, and light microscopic analysis was performed. Central erosion of distal trapezium without smooth resurfacing soft-tissue formation was noted grossly (Figure 5A) and microscopically (Figures 5B, 5C). The histologic morphology of the soft tissue over the distal trapezium was significantly different when compared with the smooth hyaline cartilage at the preserved trapezio-trapezoidal joint (Figures 6A-6F). Microscopic analysis also showed multinucleated giant cells within the soft tissue surrounding the degraded Artelon B (Figure 7).
Immunohistochemical analysis was performed to identify type I and type II collagen using the Histostain-Plus,3rd Gen IHC Detection Kit (Invitrogen Corporation, Camarillo, California) (Figures 8A-8F).9 The immunohistochemical stain was used to identify new hyaline cartilage formation that may have been induced by the Artelon as the resurfacing articulation. Hyaline cartilage contains mainly type II collagen, and collagen types VI, IX, X, XI, XII, and XIV all contribute to the mature matrix.10 Little type I collagen is found in hyaline cartilage. The results showed that the soft tissue over the distal trapezium with embedded Artelon fiber contained both type I and type II collagen. There was no visible hyaline cartilage formation induced by the Artelon. Both morphologic analysis and immunohistochemical staining revealed that the soft-tissue growth into the Artelon spacer on the distal trapezium consisted primarily of fibrocartilaginous tissue, which is composed mainly of type I collagen with some type II collagen.
Two weeks after total surgical excision of the Artelon implant, total trapeziectomy and suture-button suspensionplasty, the sutures were removed and physical therapy was initiated. Radiographs showed good alignment and position of thumb metacarpal with good maintenance of the implant and CMC space. Four months postoperatively, the patient reported that he was doing well without pain and without interference in his activities of daily living. On examination, the patient exhibited no pain with the CMC grind maneuver. Radial abduction of the right thumb was 85° and palmar abduction was 90° (compared with 100° and 90° of the left thumb), obtained by measuring the angle between thumb and index finger, respectively. Opposition was to the small finger metacarpophalangeal joint. Grip strength was 72 lb and pinch strength was 20 lb (compared with 70 lb and 24 lb, respectively, on the contralateral side).
Discussion
The use of Artelon as an endoprosthetic spacer to treat osteoarthritis in the CMC joint of the thumb appears to stabilize and resurface the joint while avoiding total trapeziectomy.8 Nilsson and colleagues8 presented a prospective study concluding that the Artelon CMC spacer provided better pinch strength when compared with a traditional abductor pollicis longus suspensionplasty procedure. This study also suggested incorporation of the device in the surface of the adjacent bone with no signs of foreign-body reaction. The synthetic material was shown to be safe and biocompatible in vitro and in animal studies.11-13
This case report describes the gross and histologic findings after continued pain led to explantation 4 years after arthroscopic partial trapeziectomy and insertion of the spacer. Intraoperative findings at this stage showed lack of incorporation of the Artelon material, central destruction of distal trapezium, and no evidence of smooth articular surface formation. Our histologic analysis showed only poorly organized fibrocartilage within the CMC space rather than a smooth articular surface. These histologic findings may correlate more with Jörheim and colleagues’14 matched cohort study, which showed that short-term outcomes after treatment with the Artelon implant were not clinically superior to those of tendon suspension-interposition arthroplasties. Multinucleated giant cells were also seen in our specimens. Choung and Tan15 presented a case report of foreign-body reaction to the Artelon spacer with histologic findings. The foreign body–type reactions associated with Artelon resulted in multinucleated giant cells in their specimens. Recently, several case reports have described similar foreign-body reactions.16 Nilsson and coauthors17 presented a randomized, controlled, multicenter study of 109 patients. They reported the Artelon CMC spacer did not result in superior results compared with tendon interposition arthroplasty. In a study by Gretzer and colleagues,18 the authors suggested that chronic inflammation may result from unstable Artelon fixation instead of the foreign-body reaction.
It is possible that the central erosion of the distal trapezium seen in our case may have resulted from chronic inflammation caused by foreign-body reaction and/or an unstably fixed spacer. The spacer was transfixed to the remaining trapezium in the CMC joint with a Kirschner wire followed by immobilization for 4 weeks. Poor soft-tissue integration of the Artelon spacer may have led to unintended motion and chronic inflammation, which may have also resulted in erosion between the Artelon spacer and the trapezium, leading to central destruction of the distal trapezium. Lastly, the byproducts formed by the degradation of the spacer may have resulted in erosion of the remaining trapezium.
Conclusion
The Artelon CMC spacer used in this patient provided comparable, but not superior, clinical results to other procedures. Histologically, the new articular surface in our patient was formed with rugged fibrocartilage instead of the expected smooth cartilaginous surface. The chronic inflammatory reaction may have resulted from foreign-body reaction, unstable implant fixation, or poor soft-tissue integration. This inflammatory reaction may have contributed to the patient’s recurrence of symptoms. These findings support recent clinical data that suggest the use of the Artelon spacer may not provide superior results to other surgical options for the treatment of CMC joint arthritis.
Osteoarthritis (OA) of the first carpometacarpal (CMC) joint is a common disabling condition that mostly affects women over 45 years of age.1 Surgical intervention is usually indicated in advanced stage OA of the first CMC joint that has failed conservative treatment. Several surgical techniques have been described, including partial or total trapeziectomy, interposition arthroplasty with or without ligament reconstruction,2,3 metacarpal osteotomy,4 hematoma and distraction arthroplasty,5 total joint arthroplasty, arthrodesis, and suspensionplasty.6 However, no single surgical procedure has proved to be superior.7
The Artelon implant (Artelon, Nashville, Tennessee) is a T-shaped spacer composed of a biocompatible and biodegradable polycaprolactone-based polyurethane urea polymer. The developers of the implant first presented its use in CMC OA in 2005.8 The device, an endoprosthetic replacement for the CMC joint, was designed to work through 2 modes of action: stabilization of the CMC joint by augmentation of the joint capsule and by formation of a new articular surface at the trapeziometacarpal interface. The interposed biomaterial has been described as preventing bony impingement and allowing time for replacement with a newly formed articular surface as it undergoes slow and controlled degradation.8
We present a patient with recurrent CMC pain and disability 4 years after arthroscopic hemitrapeziectomy and Artelon interposition and discuss the associated histologic findings. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 53-year-old man presented with painful disability of right thumb of several months’ duration. Clinical and radiographic evaluation supported the diagnosis of right thumb CMC joint Eaton stage III arthritis (Figures 1A, 1B). Surgical intervention was indicated after a failed course of conservative treatment, including splinting, nonsteroidal anti-inflammatory medications, activity modification, and corticosteroid injection. Preoperatively, the patient reported a visual analog scale (VAS) score of 8 with activity and 5 at rest, and a Disabilities of the Arm, Shoulder, and Hand (DASH) score of 72.5.
Arthroscopic débridement, hemitrapeziectomy, and interposition arthroplasty with the Artelon spacer were performed. Using standard thumb arthroscopy, 3 mm of the distal trapezium was excised and shaped parallel to scaphotrapezial joint. The wings of the standard-sized Artelon spacer were removed, and the central (articulating) portion was rolled into a tube and inserted through the 1R portal (directly radial to the abductor pollicis longus tendon) into the trapezial space. The Artelon spacer was unrolled within the joint to cover the remaining trapezium and was stabilized with the placement of a 0.045-inch Kirschner wire through the metacarpal, the spacer, and the remaining trapezium. The patient used a thumb spica splint for 4 weeks.
The postoperative radiographs showed a smooth and adequate hemitrapeziectomy with good alignment and implant position (Figures 2A, 2B). Four weeks after surgery, the Kirschner wire and cast were removed and physical therapy was initiated. The patient’s CMC pain gradually subsided. At the 3-month postoperative visit, the patient’s VAS score was 3 with activity and 1 at rest, with a DASH score of 28. His key pinch strength was 12 lb, compared with 20 lb on the contralateral side. At 6 months, the patient’s VAS score was 1 with activity and 0 at rest, with a DASH score of 12. His key pinch strength was 18 lb, compared with 22 lb on the contralateral side. At his 2-year postoperative visit, the patient was doing well with the exception of some mild residual pain when he opened tight jars. His VAS score was 1 with activity and 0 at rest, with a DASH score of 3. His key pinch strength was 20 lb, compared with 23 lb on the contralateral side. Radiographs showed good maintenance of the CMC space.
Four years postoperatively, the patient presented with worsening right CMC pain with decrease in pinch strength that interfered with his activities of daily living. His VAS score was 9 with activity and 6 at rest, with a DASH score of 70. On examination, pinch strength was 16 lb, compared with 22 lb on the contralateral side. Radiographs showed advancing arthritis with new osteophyte formation and irregular contour of distal trapezium (Figures 3A, 3B). The symptoms were refractory to conservative measures and continued to interfere with his activities of daily living. Revision surgical intervention was indicated and pursued in the form of an open CMC arthroplasty.
The intraoperative findings revealed degradation and disorganization of the Artelon implant within the central portion of the remaining distal trapezium. Rim osteophytes, especially along the ulnar aspect, were noted. Total trapeziectomy and débridement within the CMC space and suture-button suspensionplasty were performed.8 Slight degenerative changes of the distal scaphoid were also noted. The incision was irrigated, closed, and stabilized in a thumb spica splint (Figures 4A, 4B).
The harvested trapezium was immediately immersed in buffered formalin. The bone tissue was decalcified, dehydrated, embedded in paraffin, and sectioned in the coronal plane. The sections were stained with safranin O and trichrome, and light microscopic analysis was performed. Central erosion of distal trapezium without smooth resurfacing soft-tissue formation was noted grossly (Figure 5A) and microscopically (Figures 5B, 5C). The histologic morphology of the soft tissue over the distal trapezium was significantly different when compared with the smooth hyaline cartilage at the preserved trapezio-trapezoidal joint (Figures 6A-6F). Microscopic analysis also showed multinucleated giant cells within the soft tissue surrounding the degraded Artelon B (Figure 7).
Immunohistochemical analysis was performed to identify type I and type II collagen using the Histostain-Plus,3rd Gen IHC Detection Kit (Invitrogen Corporation, Camarillo, California) (Figures 8A-8F).9 The immunohistochemical stain was used to identify new hyaline cartilage formation that may have been induced by the Artelon as the resurfacing articulation. Hyaline cartilage contains mainly type II collagen, and collagen types VI, IX, X, XI, XII, and XIV all contribute to the mature matrix.10 Little type I collagen is found in hyaline cartilage. The results showed that the soft tissue over the distal trapezium with embedded Artelon fiber contained both type I and type II collagen. There was no visible hyaline cartilage formation induced by the Artelon. Both morphologic analysis and immunohistochemical staining revealed that the soft-tissue growth into the Artelon spacer on the distal trapezium consisted primarily of fibrocartilaginous tissue, which is composed mainly of type I collagen with some type II collagen.
Two weeks after total surgical excision of the Artelon implant, total trapeziectomy and suture-button suspensionplasty, the sutures were removed and physical therapy was initiated. Radiographs showed good alignment and position of thumb metacarpal with good maintenance of the implant and CMC space. Four months postoperatively, the patient reported that he was doing well without pain and without interference in his activities of daily living. On examination, the patient exhibited no pain with the CMC grind maneuver. Radial abduction of the right thumb was 85° and palmar abduction was 90° (compared with 100° and 90° of the left thumb), obtained by measuring the angle between thumb and index finger, respectively. Opposition was to the small finger metacarpophalangeal joint. Grip strength was 72 lb and pinch strength was 20 lb (compared with 70 lb and 24 lb, respectively, on the contralateral side).
Discussion
The use of Artelon as an endoprosthetic spacer to treat osteoarthritis in the CMC joint of the thumb appears to stabilize and resurface the joint while avoiding total trapeziectomy.8 Nilsson and colleagues8 presented a prospective study concluding that the Artelon CMC spacer provided better pinch strength when compared with a traditional abductor pollicis longus suspensionplasty procedure. This study also suggested incorporation of the device in the surface of the adjacent bone with no signs of foreign-body reaction. The synthetic material was shown to be safe and biocompatible in vitro and in animal studies.11-13
This case report describes the gross and histologic findings after continued pain led to explantation 4 years after arthroscopic partial trapeziectomy and insertion of the spacer. Intraoperative findings at this stage showed lack of incorporation of the Artelon material, central destruction of distal trapezium, and no evidence of smooth articular surface formation. Our histologic analysis showed only poorly organized fibrocartilage within the CMC space rather than a smooth articular surface. These histologic findings may correlate more with Jörheim and colleagues’14 matched cohort study, which showed that short-term outcomes after treatment with the Artelon implant were not clinically superior to those of tendon suspension-interposition arthroplasties. Multinucleated giant cells were also seen in our specimens. Choung and Tan15 presented a case report of foreign-body reaction to the Artelon spacer with histologic findings. The foreign body–type reactions associated with Artelon resulted in multinucleated giant cells in their specimens. Recently, several case reports have described similar foreign-body reactions.16 Nilsson and coauthors17 presented a randomized, controlled, multicenter study of 109 patients. They reported the Artelon CMC spacer did not result in superior results compared with tendon interposition arthroplasty. In a study by Gretzer and colleagues,18 the authors suggested that chronic inflammation may result from unstable Artelon fixation instead of the foreign-body reaction.
It is possible that the central erosion of the distal trapezium seen in our case may have resulted from chronic inflammation caused by foreign-body reaction and/or an unstably fixed spacer. The spacer was transfixed to the remaining trapezium in the CMC joint with a Kirschner wire followed by immobilization for 4 weeks. Poor soft-tissue integration of the Artelon spacer may have led to unintended motion and chronic inflammation, which may have also resulted in erosion between the Artelon spacer and the trapezium, leading to central destruction of the distal trapezium. Lastly, the byproducts formed by the degradation of the spacer may have resulted in erosion of the remaining trapezium.
Conclusion
The Artelon CMC spacer used in this patient provided comparable, but not superior, clinical results to other procedures. Histologically, the new articular surface in our patient was formed with rugged fibrocartilage instead of the expected smooth cartilaginous surface. The chronic inflammatory reaction may have resulted from foreign-body reaction, unstable implant fixation, or poor soft-tissue integration. This inflammatory reaction may have contributed to the patient’s recurrence of symptoms. These findings support recent clinical data that suggest the use of the Artelon spacer may not provide superior results to other surgical options for the treatment of CMC joint arthritis.
1. Dahaghin S, Bierma-Zeinstra SM, Ginai AZ, Pols HA, Hazes JM, Koes BW. Prevalence and pattern of radiographic hand osteoarthritis and association with pain and disability (the Rotterdam study). Ann Rheum Dis. 2005;64(5):682-687.
2. Eaton RG, Glickel SZ, Littler JW. Tendon interposition arthroplasty for degenerative arthritis of the trapeziometacarpal joint of the thumb. J Hand Surg. 1985;10(5):645-654.
3. Gibbons CE, Gosal HS, Choudri AH, Magnussen PA. Trapeziectomy for basal thumb joint osteoarthritis: 3- to 19-year follow-up. Int Orthop. 1999;23(4):216-218.
4. Gwynne-Jones DP, Penny ID, Sewell SA, Hughes TH. Basal thumb metacarpal osteotomy for trapeziometacarpal osteoarthritis. J Orthop Surg (Hong Kong). 2006;14(1):58-63.
5. Gray KV, Meals RA. Hematoma and distraction arthroplasty for thumb basal joint osteoarthritis: minimum 6.5-year follow-up evaluation. J Hand Surg Am. 2007;32(1):23-29.
6. Cox CA, Zlotolow DA, Yao J. Suture button suspensionplasty after arthroscopic hemitrapeziectomy for treatment of thumb carpometacarpal arthritis. Arthroscopy. 2010;26(10):1395-1403.
7. Vermeulen GM, Slijper H, Feitz R, Hovius SE, Moojen TM, Selles RW. Surgical management of primary thumb carpometacarpal osteoarthritis: a systematic review. J Hand Surg Am. 2011;36(1):157-169.
8. Nilsson A, Liljensten E, Bergström C, Sollerman C. Results from a degradable TMC joint Spacer (Artelon) compared with tendon arthroplasty. J Hand Surg Am. 2005;30(2):380-389.
9. Histostain®-Plus, 3rd Gen IHC Detection Kit [product information]. Invitrogen website. http://tools.invitrogen.com/content/sfs/manuals/859073_Rev1108.pdf. Revised November 2008. Accessed February 27, 2015.
10. Eyre D. Collagen of articular cartilage. Arthritis Res. 2002;4(1):30-35.
11. Gisselfält K, Edberg B, Flodin P. Synthesis and properties of degradable poly(urethane urea)s to be used for ligament reconstructions. Biomacromolecules. 2002;3(5):951-958.
12. Liljensten E, Gisselfält K, Edberg B, et al. Studies of polyurethane urea bands for ACL reconstruction. J Mater Sci Mater Med. 2002;13(4):351-359.
13. Gretzer C, Gisselfält K, Liljensten E, Rydén L, Thomsen P. Adhesion, apoptosis and cytokine release of human mononuclear cells cultured on degradable poly(urethane urea), polystyrene and titanium in vitro. Biomaterials. 2003;24(17):2843-2852.
14. Jörheim M, Isaxon I, Flondell M, Kalén P, Atroshi I. Short-term outcomes of trapeziometacarpal artelon implant compared with tendon suspension interposition arthroplasty for osteoarthritis: a matched cohort study. J Hand Surg Am. 2009;34(8):1381-1387.
15. Choung EW, Tan V. Foreign-body reaction to the Artelon CMC joint spacer: case report. J Hand Surg Am. 2008;33(9):1617-1620.
16. Robinson PM, Muir LT. Foreign body reaction associated with Artelon: report of three cases. J Hand Surg Am. 2011;36(1):116-120.
17. Nilsson A, Wiig M, Alnehill H, et al. The Artelon CMC spacer compared with tendon interposition arthroplasty. Acta Orthop. 2010;81(2):237-244.
18. Gretzer C, Emanuelsson L, Liljensten E, Thomsen P. The inflammatory cell influx and cytokines changes during transition from acute inflammation to fibrous repair around implanted materials. J Biomater Sci Polym Ed. 2006;17(6):669-687.
1. Dahaghin S, Bierma-Zeinstra SM, Ginai AZ, Pols HA, Hazes JM, Koes BW. Prevalence and pattern of radiographic hand osteoarthritis and association with pain and disability (the Rotterdam study). Ann Rheum Dis. 2005;64(5):682-687.
2. Eaton RG, Glickel SZ, Littler JW. Tendon interposition arthroplasty for degenerative arthritis of the trapeziometacarpal joint of the thumb. J Hand Surg. 1985;10(5):645-654.
3. Gibbons CE, Gosal HS, Choudri AH, Magnussen PA. Trapeziectomy for basal thumb joint osteoarthritis: 3- to 19-year follow-up. Int Orthop. 1999;23(4):216-218.
4. Gwynne-Jones DP, Penny ID, Sewell SA, Hughes TH. Basal thumb metacarpal osteotomy for trapeziometacarpal osteoarthritis. J Orthop Surg (Hong Kong). 2006;14(1):58-63.
5. Gray KV, Meals RA. Hematoma and distraction arthroplasty for thumb basal joint osteoarthritis: minimum 6.5-year follow-up evaluation. J Hand Surg Am. 2007;32(1):23-29.
6. Cox CA, Zlotolow DA, Yao J. Suture button suspensionplasty after arthroscopic hemitrapeziectomy for treatment of thumb carpometacarpal arthritis. Arthroscopy. 2010;26(10):1395-1403.
7. Vermeulen GM, Slijper H, Feitz R, Hovius SE, Moojen TM, Selles RW. Surgical management of primary thumb carpometacarpal osteoarthritis: a systematic review. J Hand Surg Am. 2011;36(1):157-169.
8. Nilsson A, Liljensten E, Bergström C, Sollerman C. Results from a degradable TMC joint Spacer (Artelon) compared with tendon arthroplasty. J Hand Surg Am. 2005;30(2):380-389.
9. Histostain®-Plus, 3rd Gen IHC Detection Kit [product information]. Invitrogen website. http://tools.invitrogen.com/content/sfs/manuals/859073_Rev1108.pdf. Revised November 2008. Accessed February 27, 2015.
10. Eyre D. Collagen of articular cartilage. Arthritis Res. 2002;4(1):30-35.
11. Gisselfält K, Edberg B, Flodin P. Synthesis and properties of degradable poly(urethane urea)s to be used for ligament reconstructions. Biomacromolecules. 2002;3(5):951-958.
12. Liljensten E, Gisselfält K, Edberg B, et al. Studies of polyurethane urea bands for ACL reconstruction. J Mater Sci Mater Med. 2002;13(4):351-359.
13. Gretzer C, Gisselfält K, Liljensten E, Rydén L, Thomsen P. Adhesion, apoptosis and cytokine release of human mononuclear cells cultured on degradable poly(urethane urea), polystyrene and titanium in vitro. Biomaterials. 2003;24(17):2843-2852.
14. Jörheim M, Isaxon I, Flondell M, Kalén P, Atroshi I. Short-term outcomes of trapeziometacarpal artelon implant compared with tendon suspension interposition arthroplasty for osteoarthritis: a matched cohort study. J Hand Surg Am. 2009;34(8):1381-1387.
15. Choung EW, Tan V. Foreign-body reaction to the Artelon CMC joint spacer: case report. J Hand Surg Am. 2008;33(9):1617-1620.
16. Robinson PM, Muir LT. Foreign body reaction associated with Artelon: report of three cases. J Hand Surg Am. 2011;36(1):116-120.
17. Nilsson A, Wiig M, Alnehill H, et al. The Artelon CMC spacer compared with tendon interposition arthroplasty. Acta Orthop. 2010;81(2):237-244.
18. Gretzer C, Emanuelsson L, Liljensten E, Thomsen P. The inflammatory cell influx and cytokines changes during transition from acute inflammation to fibrous repair around implanted materials. J Biomater Sci Polym Ed. 2006;17(6):669-687.
Massive Baker Cyst Resulting in Tibial Nerve Compression Neuropathy Secondary to Polyethylene Wear Disease
Symptomatic synovial cyst formation is a rare, late occurrence after total knee arthroplasty (TKA); these cysts are generally discovered by chance. If they enlarge, they can result in significant pain and disability. A few case reports have described the development of very large cysts that required revision knee surgery. In this patient, polyethylene wear disease after TKA resulted in a massive synovial cyst that extended into the posterior compartment of the leg, as well as a progressive peripheral neuropathy. Revision of a loose patella component and worn polyethylene liner with complete synovectomy, plus decompression of the cyst via needle aspiration, resulted in an excellent short-term outcome.
To the author’s knowledge, this is the first case report of peripheral neuropathy of the tibial nerve secondary to a massive Baker cyst after total knee replacement. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a 65-year-old woman with a complex medical history and multiple left knee surgeries, including a high tibial osteotomy and subsequent cemented TKA performed in the mid-1990s. She presented to the orthopedic department at a university hospital with complaints of knee pain 13 years after TKA. Observation was recommended; however, she was lost to follow-up.
The patient presented to her primary care physician (PCP) 16 years after TKA with a very large, painful mass in the back of her left leg. An ultrasound showed a large Baker cyst, and the patient was sent to interventional radiology. A few months later, she had an ultrasound-guided aspiration into the left calf, which produced 300 mL of thick synovial fluid. A cell count was not performed, but bacterial cultures were negative. Immediately after the aspiration, the pain was relieved.
Approximately 3 months after the aspiration, she presented again to her PCP with re-accumulation of fluid in the back of her left leg and severe leg pain. She was referred to a different orthopedic surgeon who determined that the risk of surgery was too great given her complex medical history.
The woman’s PCP referred the woman to our office 6 months after the aspiration. On presentation, her pain was localized to the posterior left leg. She reported the pain level as a constant 9 out of 10 on the visual analog scale, despite ingesting high doses of narcotics, including oxycontin and morphine. Her physical examination was remarkable for an ill-defined large calf mass. The posterior compartment of her left leg was firm and severely tender, similar to the characteristic findings seen in acute compartment syndrome.
Radiographs showed evidence of asymmetric polyethylene wear on the medial side of the knee (Figures 1A, 1B). Serum labs were ordered to evaluate for infection. C-reactive protein was mildly elevated at 5.5 mg/L (normal range, 0-5 mg/L); however, the erythrocyte sedimentation rate was normal at 12 mm/h (normal range, 0-20 mm/h). Magnetic resonance imaging of the left lower extremity with intravenous contrast showed the presence of a very large Baker cyst contained within the posterior compartment of the knee and a smaller surrounding cyst adjacent to the popliteal neurovascular bundle (Figure 2).
The Baker cyst was re-aspirated in the office. The automated synovial fluid cell count could not be performed because of high fluid viscosity. However, a manual review of the fluid specimen under light microscopy revealed proteinaceous, viscous tan-colored fluid containing no neutrophils and a few macrophages. Fluid cytology was also sent for review under polarizing light microscopy as described by Peterson and colleagues.1 Scattered fragments of polarizable foreign material were consistent with polyethylene debris (Figure 3).
The patient was counseled about the risks and benefits of surgery and was offered revision TKA with polyethylene liner exchange and synovectomy, only after complete cessation of smoking. She underwent serum nicotine monitoring to ensure tobacco cessation; however, she also reported the onset of a progressive sensory deficit over her left foot during this period. Although her medical history was remarkable for spinal stenosis, she noted a progressive decline in sensory function and new-onset paresthesia of her left foot.
An urgent consult to neurology was requested for nerve conduction studies. According to the electrodiagnostic study, the patient had a moderately severe left tibial neuropathy, likely at the popliteal fossa or distal to it. The nerve conduction study showed a chronic tibial nerve peripheral compressive mononeuropathy, and she was immediately scheduled for revision knee surgery with decompression of her Baker cyst to prevent further neurologic deficit.
During surgery, the knee joint exhibited hypertrophic synovitis with a characteristic pale-yellowish discoloration secondary to significant polyethylene wear disease (Figure 4). The polyethylene liner was severely worn with pitting, cracking, and delamination (Figure 5). While the patellar component was grossly loose, the tibial and femoral components were stable. After a complete synovectomy, the loose patellar component and tibial polyethylene liner were replaced. Osteolytic areas within the tibia underwent curettage and allograft impaction grafting. Lastly, decompression of the ruptured Baker cyst was performed via a 16-gauge needle placed in the posterior compartment of the left leg. The calf was gently squeezed with a “milking” maneuver, which yielded approximately 200 mL of thick, mucoid yellowish-brown synovial fluid resembling tapioca pudding (Figure 6).
Postoperatively, all intraoperative cultures were negative, and the patient was followed closely at 1 week, 2 weeks, 6 weeks, and 3 months after the surgery. At her latest follow-up, the posterior leg compartment remained decompressed and her progressive sensory deficit had nearly resolved. Moreover, the left leg and posterior knee pain completely resolved.
Discussion
A leading cause of TKA failure is attributed to aseptic loosening from polyethylene wear disease.2 Implanted high-molecular-weight polyethylene (HMWPE) liners are known to undergo a variety of mechanical wear patterns within the knee. Observed patterns include pitting, scratching, burnishing, scratching, and delamination, which can all liberate numerous fine polyethylene particles.3 This wear debris induces macrophage phagocytosis that triggers an inflammatory reaction within the knee joint and can lead to synovitis, repeat effusions and, ultimately, to aseptic loosening.
Prior to 1996, polyethylene used in total knee replacement underwent a sterilization process in air. This oxygen-rich environment led to the development of free radical formation within the HMWPE. Ultimately, this had a detrimental effect on the polyethylene, leading to the formation of increased wear debris.4
Subsequently, orthopedic companies have changed their sterilization and manufacturing methods. Polyethylene components now undergo a variety of processes to eliminate or reduce oxidation, free-radical formation, and mechanical wear debris. Now, sterilization typically takes place in an inert atmospheric environment. Modern HMWPE implants often undergo higher irradiation to induce mechanical cross-linking, followed by either a re-annealing or remelting step. In other cases, manufacturers “dope” their polyethylene with vitamin E to quench free radicals within the material. While these steps have reduced the number of in vitro wear particles, the problem of wear debris, subsequent osteolysis, and aseptic loosening has not been eliminated.1-5
Polyethylene wear debris within the synovial fluid or tissue of failed TKAs can be identified with scanning electron microscopy or by light microscopy utilizing polarized light.1 In this particular case, wear debris was confirmed within the synovial tissue and in the fluid of the Baker cyst by microscopic analysis.
Formation of a popliteal or Baker cyst as a result of polyethylene wear disease is an infrequent but known complication of TKA. Reports have demonstrated variable success in cyst eradication when revision surgery is performed on knees with synovial cysts. Most of these reports indicate that cyst formation tends to occur as a late complication (7 or more years) after TKA.6-12
Treatment options may include skillful observation with close follow-up or revision surgery. Polyethylene exchange with synovectomy when feasible, as well as component revision with or without excision of the synovial cyst, are surgical options.
Niki and colleagues13 described a gigantic popliteal synovial cyst caused by wear particles after TKA. In this report, the surgeon performed a synovectomy and polyethylene liner exchange with retention of prosthetic components. At 12-month follow-up, the patient was reported to be doing well.
Mavrogenis and coauthors14 reported a wear debris–induced pseudotumor in the popliteal fossa and calf after TKA. In this case, in addition to the synovectomy, the surgeon removed all prosthetic components and used a semi-constrained implant to revise the knee. At 30-month follow-up, the patient reported having a painless knee.
While case reports have indicated that revision TKA for large, painful synovial cysts is a reasonable treatment option in carefully selected patients, there is a paucity of literature on this subject. Moreover, the present case appears to be the first literature report of a tibial nerve compressive neuropathy secondary to a synovial cyst after TKA.
Conclusion
In this report, polyethylene wear disease after TKA resulted in a massive synovial cyst extending into the posterior compartment of the leg. A progressive peripheral neuropathy confirmed by electromyography was also discovered. The patient underwent revision of a loose patellar component and worn polyethylene liner with complete synovectomy plus decompression of the cyst via needle aspiration. This resulted in an excellent short-term outcome with resolution of pain and significant improvement of the peripheral neuropathy 3 months after surgery.
1. Peterson C, Benjamin JB, Szivek JA, Anderson PL, Shriki J, Wong M. Polyethylene particle morphology in synovial fluid of failed knee arthroplasty. Clin Orthop. 1999;359:167-175.
2. Sadoghi P, Liebensteiner M, Agreiter M, Leithner A, Böhler N, Labek G. Revision surgery after total joint arthroplasty: a complication-based analysis using worldwide arthroplasty registers. J Arthroplasty. 2013;28(8):1329-1332.
3. Calonius O, Saikko V. Analysis of polyethylene particles produced in different wear conditions in vitro. Clin Orthop. 2002;399:219-230.
4. Edwards BT, Leach PB, Zura R, Corpe RS, Young TR. Presentation of gamma-irradiated-in-air polyethylene wear in the form of a synovial cyst. J Long Term Eff Med Implants. 2003;13(5):413-417.
5. Bosco J, Benjamin J, Wallace D. Quantitative and qualitative analysis of polyethylene wear particles in synovial fluid of patients with total arthroplasty. A preliminary report. Clin Orthop. 1994;309:11-19.
6. Moretti B, Patella V, Mouhsine E, Pesce V, Spinarelli A, Garofalo R. Multilobulated popliteal cyst after a failed total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2007;15(2):212-216.
7. Segura J, Palanca D, Bueno AL, Seral B, Castiella T, Seral F. Baker’s pseudocyst in the prosthetic knee affected with aggressive granulomatosis caused by polyethylene wear. Chir Organi Mov. 1996;81(4):421-426.
8. Ghanem G, Ghanem I, Dagher F. Popliteal cyst in a patient with total knee arthroplasty: a case report and review of the literature. J Med Liban. 2001;49(6):347-350.
9. Hsu WH, Hsu RW, Huang TJ, Lee KF. Dissecting popliteal cyst resulting from a fragmented, dislodged metal part of the patellar component after total knee arthroplasty. J Arthroplasty. 2002;17(6):792-797.
10. Chan YS, Wang CJ, Shin CH. Two-stage operation for treatment of a large dissecting popliteal cyst after failed total knee arthroplasty. J Arthroplasty. 2000;15(8):1068-1072.
11. Dirschl DR, Lachiewicz PF. Dissecting popliteal cyst as the presenting symptom of a malfunctioning total knee arthroplasty. Report of four cases. J Arthroplasty. 1992;7(1):37-41.
12. Akisue T, Kurosaka M, Matsui N, et al. Paratibial cyst associated with wear debris after total knee arthroplasty. J Arthroplasty. 2001;16(3):389-393.
13. Niki Y, Matsumoto H, Otani T, Yoshimine F, Inokuchi W, Morisue H. Gigantic popliteal synovial cyst caused by wear particles after total knee arthroplasty. J Arthroplasty. 2003;18(8):1071-1075.
14. Mavrogenis AF, Nomikos GN, Sakellariou VI, Karaliotas GI, Kontovazenitis P, Papagelopoulos PJ. Wear debris pseudotumor following total knee arthroplasty: a case report. J Med Case Rep. 2009;3:9304.
Symptomatic synovial cyst formation is a rare, late occurrence after total knee arthroplasty (TKA); these cysts are generally discovered by chance. If they enlarge, they can result in significant pain and disability. A few case reports have described the development of very large cysts that required revision knee surgery. In this patient, polyethylene wear disease after TKA resulted in a massive synovial cyst that extended into the posterior compartment of the leg, as well as a progressive peripheral neuropathy. Revision of a loose patella component and worn polyethylene liner with complete synovectomy, plus decompression of the cyst via needle aspiration, resulted in an excellent short-term outcome.
To the author’s knowledge, this is the first case report of peripheral neuropathy of the tibial nerve secondary to a massive Baker cyst after total knee replacement. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a 65-year-old woman with a complex medical history and multiple left knee surgeries, including a high tibial osteotomy and subsequent cemented TKA performed in the mid-1990s. She presented to the orthopedic department at a university hospital with complaints of knee pain 13 years after TKA. Observation was recommended; however, she was lost to follow-up.
The patient presented to her primary care physician (PCP) 16 years after TKA with a very large, painful mass in the back of her left leg. An ultrasound showed a large Baker cyst, and the patient was sent to interventional radiology. A few months later, she had an ultrasound-guided aspiration into the left calf, which produced 300 mL of thick synovial fluid. A cell count was not performed, but bacterial cultures were negative. Immediately after the aspiration, the pain was relieved.
Approximately 3 months after the aspiration, she presented again to her PCP with re-accumulation of fluid in the back of her left leg and severe leg pain. She was referred to a different orthopedic surgeon who determined that the risk of surgery was too great given her complex medical history.
The woman’s PCP referred the woman to our office 6 months after the aspiration. On presentation, her pain was localized to the posterior left leg. She reported the pain level as a constant 9 out of 10 on the visual analog scale, despite ingesting high doses of narcotics, including oxycontin and morphine. Her physical examination was remarkable for an ill-defined large calf mass. The posterior compartment of her left leg was firm and severely tender, similar to the characteristic findings seen in acute compartment syndrome.
Radiographs showed evidence of asymmetric polyethylene wear on the medial side of the knee (Figures 1A, 1B). Serum labs were ordered to evaluate for infection. C-reactive protein was mildly elevated at 5.5 mg/L (normal range, 0-5 mg/L); however, the erythrocyte sedimentation rate was normal at 12 mm/h (normal range, 0-20 mm/h). Magnetic resonance imaging of the left lower extremity with intravenous contrast showed the presence of a very large Baker cyst contained within the posterior compartment of the knee and a smaller surrounding cyst adjacent to the popliteal neurovascular bundle (Figure 2).
The Baker cyst was re-aspirated in the office. The automated synovial fluid cell count could not be performed because of high fluid viscosity. However, a manual review of the fluid specimen under light microscopy revealed proteinaceous, viscous tan-colored fluid containing no neutrophils and a few macrophages. Fluid cytology was also sent for review under polarizing light microscopy as described by Peterson and colleagues.1 Scattered fragments of polarizable foreign material were consistent with polyethylene debris (Figure 3).
The patient was counseled about the risks and benefits of surgery and was offered revision TKA with polyethylene liner exchange and synovectomy, only after complete cessation of smoking. She underwent serum nicotine monitoring to ensure tobacco cessation; however, she also reported the onset of a progressive sensory deficit over her left foot during this period. Although her medical history was remarkable for spinal stenosis, she noted a progressive decline in sensory function and new-onset paresthesia of her left foot.
An urgent consult to neurology was requested for nerve conduction studies. According to the electrodiagnostic study, the patient had a moderately severe left tibial neuropathy, likely at the popliteal fossa or distal to it. The nerve conduction study showed a chronic tibial nerve peripheral compressive mononeuropathy, and she was immediately scheduled for revision knee surgery with decompression of her Baker cyst to prevent further neurologic deficit.
During surgery, the knee joint exhibited hypertrophic synovitis with a characteristic pale-yellowish discoloration secondary to significant polyethylene wear disease (Figure 4). The polyethylene liner was severely worn with pitting, cracking, and delamination (Figure 5). While the patellar component was grossly loose, the tibial and femoral components were stable. After a complete synovectomy, the loose patellar component and tibial polyethylene liner were replaced. Osteolytic areas within the tibia underwent curettage and allograft impaction grafting. Lastly, decompression of the ruptured Baker cyst was performed via a 16-gauge needle placed in the posterior compartment of the left leg. The calf was gently squeezed with a “milking” maneuver, which yielded approximately 200 mL of thick, mucoid yellowish-brown synovial fluid resembling tapioca pudding (Figure 6).
Postoperatively, all intraoperative cultures were negative, and the patient was followed closely at 1 week, 2 weeks, 6 weeks, and 3 months after the surgery. At her latest follow-up, the posterior leg compartment remained decompressed and her progressive sensory deficit had nearly resolved. Moreover, the left leg and posterior knee pain completely resolved.
Discussion
A leading cause of TKA failure is attributed to aseptic loosening from polyethylene wear disease.2 Implanted high-molecular-weight polyethylene (HMWPE) liners are known to undergo a variety of mechanical wear patterns within the knee. Observed patterns include pitting, scratching, burnishing, scratching, and delamination, which can all liberate numerous fine polyethylene particles.3 This wear debris induces macrophage phagocytosis that triggers an inflammatory reaction within the knee joint and can lead to synovitis, repeat effusions and, ultimately, to aseptic loosening.
Prior to 1996, polyethylene used in total knee replacement underwent a sterilization process in air. This oxygen-rich environment led to the development of free radical formation within the HMWPE. Ultimately, this had a detrimental effect on the polyethylene, leading to the formation of increased wear debris.4
Subsequently, orthopedic companies have changed their sterilization and manufacturing methods. Polyethylene components now undergo a variety of processes to eliminate or reduce oxidation, free-radical formation, and mechanical wear debris. Now, sterilization typically takes place in an inert atmospheric environment. Modern HMWPE implants often undergo higher irradiation to induce mechanical cross-linking, followed by either a re-annealing or remelting step. In other cases, manufacturers “dope” their polyethylene with vitamin E to quench free radicals within the material. While these steps have reduced the number of in vitro wear particles, the problem of wear debris, subsequent osteolysis, and aseptic loosening has not been eliminated.1-5
Polyethylene wear debris within the synovial fluid or tissue of failed TKAs can be identified with scanning electron microscopy or by light microscopy utilizing polarized light.1 In this particular case, wear debris was confirmed within the synovial tissue and in the fluid of the Baker cyst by microscopic analysis.
Formation of a popliteal or Baker cyst as a result of polyethylene wear disease is an infrequent but known complication of TKA. Reports have demonstrated variable success in cyst eradication when revision surgery is performed on knees with synovial cysts. Most of these reports indicate that cyst formation tends to occur as a late complication (7 or more years) after TKA.6-12
Treatment options may include skillful observation with close follow-up or revision surgery. Polyethylene exchange with synovectomy when feasible, as well as component revision with or without excision of the synovial cyst, are surgical options.
Niki and colleagues13 described a gigantic popliteal synovial cyst caused by wear particles after TKA. In this report, the surgeon performed a synovectomy and polyethylene liner exchange with retention of prosthetic components. At 12-month follow-up, the patient was reported to be doing well.
Mavrogenis and coauthors14 reported a wear debris–induced pseudotumor in the popliteal fossa and calf after TKA. In this case, in addition to the synovectomy, the surgeon removed all prosthetic components and used a semi-constrained implant to revise the knee. At 30-month follow-up, the patient reported having a painless knee.
While case reports have indicated that revision TKA for large, painful synovial cysts is a reasonable treatment option in carefully selected patients, there is a paucity of literature on this subject. Moreover, the present case appears to be the first literature report of a tibial nerve compressive neuropathy secondary to a synovial cyst after TKA.
Conclusion
In this report, polyethylene wear disease after TKA resulted in a massive synovial cyst extending into the posterior compartment of the leg. A progressive peripheral neuropathy confirmed by electromyography was also discovered. The patient underwent revision of a loose patellar component and worn polyethylene liner with complete synovectomy plus decompression of the cyst via needle aspiration. This resulted in an excellent short-term outcome with resolution of pain and significant improvement of the peripheral neuropathy 3 months after surgery.
Symptomatic synovial cyst formation is a rare, late occurrence after total knee arthroplasty (TKA); these cysts are generally discovered by chance. If they enlarge, they can result in significant pain and disability. A few case reports have described the development of very large cysts that required revision knee surgery. In this patient, polyethylene wear disease after TKA resulted in a massive synovial cyst that extended into the posterior compartment of the leg, as well as a progressive peripheral neuropathy. Revision of a loose patella component and worn polyethylene liner with complete synovectomy, plus decompression of the cyst via needle aspiration, resulted in an excellent short-term outcome.
To the author’s knowledge, this is the first case report of peripheral neuropathy of the tibial nerve secondary to a massive Baker cyst after total knee replacement. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient was a 65-year-old woman with a complex medical history and multiple left knee surgeries, including a high tibial osteotomy and subsequent cemented TKA performed in the mid-1990s. She presented to the orthopedic department at a university hospital with complaints of knee pain 13 years after TKA. Observation was recommended; however, she was lost to follow-up.
The patient presented to her primary care physician (PCP) 16 years after TKA with a very large, painful mass in the back of her left leg. An ultrasound showed a large Baker cyst, and the patient was sent to interventional radiology. A few months later, she had an ultrasound-guided aspiration into the left calf, which produced 300 mL of thick synovial fluid. A cell count was not performed, but bacterial cultures were negative. Immediately after the aspiration, the pain was relieved.
Approximately 3 months after the aspiration, she presented again to her PCP with re-accumulation of fluid in the back of her left leg and severe leg pain. She was referred to a different orthopedic surgeon who determined that the risk of surgery was too great given her complex medical history.
The woman’s PCP referred the woman to our office 6 months after the aspiration. On presentation, her pain was localized to the posterior left leg. She reported the pain level as a constant 9 out of 10 on the visual analog scale, despite ingesting high doses of narcotics, including oxycontin and morphine. Her physical examination was remarkable for an ill-defined large calf mass. The posterior compartment of her left leg was firm and severely tender, similar to the characteristic findings seen in acute compartment syndrome.
Radiographs showed evidence of asymmetric polyethylene wear on the medial side of the knee (Figures 1A, 1B). Serum labs were ordered to evaluate for infection. C-reactive protein was mildly elevated at 5.5 mg/L (normal range, 0-5 mg/L); however, the erythrocyte sedimentation rate was normal at 12 mm/h (normal range, 0-20 mm/h). Magnetic resonance imaging of the left lower extremity with intravenous contrast showed the presence of a very large Baker cyst contained within the posterior compartment of the knee and a smaller surrounding cyst adjacent to the popliteal neurovascular bundle (Figure 2).
The Baker cyst was re-aspirated in the office. The automated synovial fluid cell count could not be performed because of high fluid viscosity. However, a manual review of the fluid specimen under light microscopy revealed proteinaceous, viscous tan-colored fluid containing no neutrophils and a few macrophages. Fluid cytology was also sent for review under polarizing light microscopy as described by Peterson and colleagues.1 Scattered fragments of polarizable foreign material were consistent with polyethylene debris (Figure 3).
The patient was counseled about the risks and benefits of surgery and was offered revision TKA with polyethylene liner exchange and synovectomy, only after complete cessation of smoking. She underwent serum nicotine monitoring to ensure tobacco cessation; however, she also reported the onset of a progressive sensory deficit over her left foot during this period. Although her medical history was remarkable for spinal stenosis, she noted a progressive decline in sensory function and new-onset paresthesia of her left foot.
An urgent consult to neurology was requested for nerve conduction studies. According to the electrodiagnostic study, the patient had a moderately severe left tibial neuropathy, likely at the popliteal fossa or distal to it. The nerve conduction study showed a chronic tibial nerve peripheral compressive mononeuropathy, and she was immediately scheduled for revision knee surgery with decompression of her Baker cyst to prevent further neurologic deficit.
During surgery, the knee joint exhibited hypertrophic synovitis with a characteristic pale-yellowish discoloration secondary to significant polyethylene wear disease (Figure 4). The polyethylene liner was severely worn with pitting, cracking, and delamination (Figure 5). While the patellar component was grossly loose, the tibial and femoral components were stable. After a complete synovectomy, the loose patellar component and tibial polyethylene liner were replaced. Osteolytic areas within the tibia underwent curettage and allograft impaction grafting. Lastly, decompression of the ruptured Baker cyst was performed via a 16-gauge needle placed in the posterior compartment of the left leg. The calf was gently squeezed with a “milking” maneuver, which yielded approximately 200 mL of thick, mucoid yellowish-brown synovial fluid resembling tapioca pudding (Figure 6).
Postoperatively, all intraoperative cultures were negative, and the patient was followed closely at 1 week, 2 weeks, 6 weeks, and 3 months after the surgery. At her latest follow-up, the posterior leg compartment remained decompressed and her progressive sensory deficit had nearly resolved. Moreover, the left leg and posterior knee pain completely resolved.
Discussion
A leading cause of TKA failure is attributed to aseptic loosening from polyethylene wear disease.2 Implanted high-molecular-weight polyethylene (HMWPE) liners are known to undergo a variety of mechanical wear patterns within the knee. Observed patterns include pitting, scratching, burnishing, scratching, and delamination, which can all liberate numerous fine polyethylene particles.3 This wear debris induces macrophage phagocytosis that triggers an inflammatory reaction within the knee joint and can lead to synovitis, repeat effusions and, ultimately, to aseptic loosening.
Prior to 1996, polyethylene used in total knee replacement underwent a sterilization process in air. This oxygen-rich environment led to the development of free radical formation within the HMWPE. Ultimately, this had a detrimental effect on the polyethylene, leading to the formation of increased wear debris.4
Subsequently, orthopedic companies have changed their sterilization and manufacturing methods. Polyethylene components now undergo a variety of processes to eliminate or reduce oxidation, free-radical formation, and mechanical wear debris. Now, sterilization typically takes place in an inert atmospheric environment. Modern HMWPE implants often undergo higher irradiation to induce mechanical cross-linking, followed by either a re-annealing or remelting step. In other cases, manufacturers “dope” their polyethylene with vitamin E to quench free radicals within the material. While these steps have reduced the number of in vitro wear particles, the problem of wear debris, subsequent osteolysis, and aseptic loosening has not been eliminated.1-5
Polyethylene wear debris within the synovial fluid or tissue of failed TKAs can be identified with scanning electron microscopy or by light microscopy utilizing polarized light.1 In this particular case, wear debris was confirmed within the synovial tissue and in the fluid of the Baker cyst by microscopic analysis.
Formation of a popliteal or Baker cyst as a result of polyethylene wear disease is an infrequent but known complication of TKA. Reports have demonstrated variable success in cyst eradication when revision surgery is performed on knees with synovial cysts. Most of these reports indicate that cyst formation tends to occur as a late complication (7 or more years) after TKA.6-12
Treatment options may include skillful observation with close follow-up or revision surgery. Polyethylene exchange with synovectomy when feasible, as well as component revision with or without excision of the synovial cyst, are surgical options.
Niki and colleagues13 described a gigantic popliteal synovial cyst caused by wear particles after TKA. In this report, the surgeon performed a synovectomy and polyethylene liner exchange with retention of prosthetic components. At 12-month follow-up, the patient was reported to be doing well.
Mavrogenis and coauthors14 reported a wear debris–induced pseudotumor in the popliteal fossa and calf after TKA. In this case, in addition to the synovectomy, the surgeon removed all prosthetic components and used a semi-constrained implant to revise the knee. At 30-month follow-up, the patient reported having a painless knee.
While case reports have indicated that revision TKA for large, painful synovial cysts is a reasonable treatment option in carefully selected patients, there is a paucity of literature on this subject. Moreover, the present case appears to be the first literature report of a tibial nerve compressive neuropathy secondary to a synovial cyst after TKA.
Conclusion
In this report, polyethylene wear disease after TKA resulted in a massive synovial cyst extending into the posterior compartment of the leg. A progressive peripheral neuropathy confirmed by electromyography was also discovered. The patient underwent revision of a loose patellar component and worn polyethylene liner with complete synovectomy plus decompression of the cyst via needle aspiration. This resulted in an excellent short-term outcome with resolution of pain and significant improvement of the peripheral neuropathy 3 months after surgery.
1. Peterson C, Benjamin JB, Szivek JA, Anderson PL, Shriki J, Wong M. Polyethylene particle morphology in synovial fluid of failed knee arthroplasty. Clin Orthop. 1999;359:167-175.
2. Sadoghi P, Liebensteiner M, Agreiter M, Leithner A, Böhler N, Labek G. Revision surgery after total joint arthroplasty: a complication-based analysis using worldwide arthroplasty registers. J Arthroplasty. 2013;28(8):1329-1332.
3. Calonius O, Saikko V. Analysis of polyethylene particles produced in different wear conditions in vitro. Clin Orthop. 2002;399:219-230.
4. Edwards BT, Leach PB, Zura R, Corpe RS, Young TR. Presentation of gamma-irradiated-in-air polyethylene wear in the form of a synovial cyst. J Long Term Eff Med Implants. 2003;13(5):413-417.
5. Bosco J, Benjamin J, Wallace D. Quantitative and qualitative analysis of polyethylene wear particles in synovial fluid of patients with total arthroplasty. A preliminary report. Clin Orthop. 1994;309:11-19.
6. Moretti B, Patella V, Mouhsine E, Pesce V, Spinarelli A, Garofalo R. Multilobulated popliteal cyst after a failed total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2007;15(2):212-216.
7. Segura J, Palanca D, Bueno AL, Seral B, Castiella T, Seral F. Baker’s pseudocyst in the prosthetic knee affected with aggressive granulomatosis caused by polyethylene wear. Chir Organi Mov. 1996;81(4):421-426.
8. Ghanem G, Ghanem I, Dagher F. Popliteal cyst in a patient with total knee arthroplasty: a case report and review of the literature. J Med Liban. 2001;49(6):347-350.
9. Hsu WH, Hsu RW, Huang TJ, Lee KF. Dissecting popliteal cyst resulting from a fragmented, dislodged metal part of the patellar component after total knee arthroplasty. J Arthroplasty. 2002;17(6):792-797.
10. Chan YS, Wang CJ, Shin CH. Two-stage operation for treatment of a large dissecting popliteal cyst after failed total knee arthroplasty. J Arthroplasty. 2000;15(8):1068-1072.
11. Dirschl DR, Lachiewicz PF. Dissecting popliteal cyst as the presenting symptom of a malfunctioning total knee arthroplasty. Report of four cases. J Arthroplasty. 1992;7(1):37-41.
12. Akisue T, Kurosaka M, Matsui N, et al. Paratibial cyst associated with wear debris after total knee arthroplasty. J Arthroplasty. 2001;16(3):389-393.
13. Niki Y, Matsumoto H, Otani T, Yoshimine F, Inokuchi W, Morisue H. Gigantic popliteal synovial cyst caused by wear particles after total knee arthroplasty. J Arthroplasty. 2003;18(8):1071-1075.
14. Mavrogenis AF, Nomikos GN, Sakellariou VI, Karaliotas GI, Kontovazenitis P, Papagelopoulos PJ. Wear debris pseudotumor following total knee arthroplasty: a case report. J Med Case Rep. 2009;3:9304.
1. Peterson C, Benjamin JB, Szivek JA, Anderson PL, Shriki J, Wong M. Polyethylene particle morphology in synovial fluid of failed knee arthroplasty. Clin Orthop. 1999;359:167-175.
2. Sadoghi P, Liebensteiner M, Agreiter M, Leithner A, Böhler N, Labek G. Revision surgery after total joint arthroplasty: a complication-based analysis using worldwide arthroplasty registers. J Arthroplasty. 2013;28(8):1329-1332.
3. Calonius O, Saikko V. Analysis of polyethylene particles produced in different wear conditions in vitro. Clin Orthop. 2002;399:219-230.
4. Edwards BT, Leach PB, Zura R, Corpe RS, Young TR. Presentation of gamma-irradiated-in-air polyethylene wear in the form of a synovial cyst. J Long Term Eff Med Implants. 2003;13(5):413-417.
5. Bosco J, Benjamin J, Wallace D. Quantitative and qualitative analysis of polyethylene wear particles in synovial fluid of patients with total arthroplasty. A preliminary report. Clin Orthop. 1994;309:11-19.
6. Moretti B, Patella V, Mouhsine E, Pesce V, Spinarelli A, Garofalo R. Multilobulated popliteal cyst after a failed total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2007;15(2):212-216.
7. Segura J, Palanca D, Bueno AL, Seral B, Castiella T, Seral F. Baker’s pseudocyst in the prosthetic knee affected with aggressive granulomatosis caused by polyethylene wear. Chir Organi Mov. 1996;81(4):421-426.
8. Ghanem G, Ghanem I, Dagher F. Popliteal cyst in a patient with total knee arthroplasty: a case report and review of the literature. J Med Liban. 2001;49(6):347-350.
9. Hsu WH, Hsu RW, Huang TJ, Lee KF. Dissecting popliteal cyst resulting from a fragmented, dislodged metal part of the patellar component after total knee arthroplasty. J Arthroplasty. 2002;17(6):792-797.
10. Chan YS, Wang CJ, Shin CH. Two-stage operation for treatment of a large dissecting popliteal cyst after failed total knee arthroplasty. J Arthroplasty. 2000;15(8):1068-1072.
11. Dirschl DR, Lachiewicz PF. Dissecting popliteal cyst as the presenting symptom of a malfunctioning total knee arthroplasty. Report of four cases. J Arthroplasty. 1992;7(1):37-41.
12. Akisue T, Kurosaka M, Matsui N, et al. Paratibial cyst associated with wear debris after total knee arthroplasty. J Arthroplasty. 2001;16(3):389-393.
13. Niki Y, Matsumoto H, Otani T, Yoshimine F, Inokuchi W, Morisue H. Gigantic popliteal synovial cyst caused by wear particles after total knee arthroplasty. J Arthroplasty. 2003;18(8):1071-1075.
14. Mavrogenis AF, Nomikos GN, Sakellariou VI, Karaliotas GI, Kontovazenitis P, Papagelopoulos PJ. Wear debris pseudotumor following total knee arthroplasty: a case report. J Med Case Rep. 2009;3:9304.
Greater Auricular Nerve Palsy After Arthroscopic Anterior-Inferior and Posterior-Inferior Labral Tear Repair Using Beach-Chair Positioning and a Standard Universal Headrest
Anterior-inferior and posterior-inferior labral tears are common injuries treated with arthroscopic surgery1 typically performed with beach-chair2,3 or lateral decubitus1,2 positioning and variable headrest positioning. Iatrogenic nerve damage that occurs after arthroscopic shoulder surgery—including damage to the suprascapular, axillary, musculocutaneous, subscapular, and spinal accessory nerves—has recently been reported to be more common than previously recognized.2,4
Although iatrogenic nerve injuries are in general being recognized,1,2,4 reports of greater auricular nerve injuries are limited. The greater auricular nerve is a superficial cutaneous nerve that arises from the cervical plexus at the C2 and C3 spinal nerves, obliquely crosses the sternocleidomastoid muscle, and splits into anterior and posterior portions that innervate the skin over the mastoid process and parotid gland.5,6 In particular, as illustrated by Ginsberg and Eicher6 (Figure 1), its superficial anatomy lies very near where a headrest is positioned during arthroscopic surgery, and increased pressure on the nerve throughout arthroscopic shoulder surgery may lead to neurapraxia.6,7 In 2 case series, authors reported on a total of 5 patients who had greater auricular nerve palsy after uncomplicated shoulder surgery using beach-chair positioning and a horseshoe headrest.7,8 The authors attributed these palsies to the horseshoe headrest, which they believed was compressing the greater auricular nerve during the entire surgery.7,8 However, standard universal headrests, which are thought to distribute pressures that would theoretically place the greater auricular nerve at risk for palsy, previously had not been described as contributing to palsy of the greater auricular nerve.
In this article, we report on a case of greater auricular nerve palsy that occurred after the patient’s anterior-inferior and posterior-inferior labral tear was arthroscopically repaired using beach-chair positioning and a standard universal headrest. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old right-hand–dominant high school American football player was referred for orthopedic evaluation of left chronic glenohumeral instability after 6 months of physical therapy. Physical examination revealed a positive apprehension test with the shoulder abducted and externally rotated at 90° and a positive relocation test. The patient complained of pain and instability when his arm was placed in a cross-chest adducted position and a posteroinferiorly directed axial load was applied. Magnetic resonance arthrogram showed an anterior-inferior labral Bankart tear with a small Hill-Sachs lesion to the humeral head but did not clearly reveal the posterior-inferior labral tear. Because of persistent left shoulder instability with most overhead activities and continued pain, the patient decided to undergo left shoulder arthroscopic Bankart repair with inferior capsular shift and posterior-inferior labral repair with capsulorraphy. He had no significant past medical history or known drug allergies.
The patient was placed in the standard beach-chair position: upright at 45° to the floor, hips flexed at 60°, knees flexed at 30°.1 Pneumatic compression devices were placed on his lower extremities. His head was secured in neutral position to a standard universal headrest (model A-90026; Allen Medical Systems, Acton, Massachusetts) (Figures 2, 3). Care was taken to protect the deep neurovascular structures and bony prominences throughout. The patient was in this position for 122 minutes of the operation, from positioning and draping to wound closure and dressing application. Before draping, the anesthesiologist, head nurse, and circulating nurse ensured that head and neck were in neutral position. The anesthesiologist monitored positioning throughout the perioperative period to ensure head and neck were in neutral, and the head did not need to be repositioned during surgery. Standard preoperative intravenous antibiotics were given.
General anesthesia and postoperative interscalene block were used. Standard preparation and draping were performed. Three standard arthroscopic portal incisions were used: posterior, anterior, and anterosuperolateral. Findings included extensive labral pathology, small bony Hill-Sachs lesion to humeral head, small bony anterior glenoid deficiency, and deficient anterior-inferior and posterior-inferior labral remnant. These were repaired arthroscopically in a standard fashion using bioabsorbable suture anchors. There were no arthroscopic complications. After surgery, a standard well-fitted shoulder immobilizer was placed. The anesthesiologist provided interscalene regional analgesia (15 mL of bupivacaine 0.5%) in the recovery area after surgery.
Postoperative neurovascular examination in the recovery room revealed no discomfort. The patient was discharged the same day. At a scheduled 1-week follow-up, he complained of numbness and dysesthesia on the left side of the greater auricular nerve distribution. A diagnosis of greater auricular nerve palsy was made by physical examination; the symptoms were along the classic greater auricular nerve distribution affecting the lower face and ear (Figure 4). The patient had no pain, skin lesions, or soft-tissue swelling. Otolaryngology confirmed the diagnosis and recommended observation-only treatment of symptoms. Symptoms lessened over the next 3 months, and the altered sensation resolved without deficit by 6 months. In addition, by 6 months the patient had returned to full activities (including collision sports) pain-free and with normal left shoulder function. Because symptoms continued to improve, the patient was followed with clinical observation, and a formal work-up was not necessary.
Discussion
The most important finding in this case is the greater auricular nerve palsy that occurred after arthroscopic anterior-inferior and posterior-inferior labral repairs in beach-chair positioning. This greater auricular nerve palsy was the first encountered by Dr. Foad, who over 17 years in a primarily shoulder practice setting has used beach-chair positioning exclusively. Previous reports have described a palsy occurring after arthroscopic shoulder surgery using beach-chair positioning and a horseshoe headrest.7,8 Ng and Page7 discontinued and recommended against use of this headrest because of the complications of the palsy, and Park and Kim8 recommended a headrest redesign. We think the present case report is the first to describe a greater auricular nerve palsy that occurred after arthroscopic surgery using a standard universal headrest, which theoretically should prevent compression of the greater auricular nerve. Increased awareness of the possibility of greater auricular nerve palsy, even when proper precautions are taken,1 is therefore warranted.
Based on the location of our patient’s palsy, we think his paralysis was most likely the result of nerve compression by the headrest during the shoulder surgery. Other factors, though unlikely, may have played a role. These include operative time (increases duration of nerve compression) and head positioning. However, 122 minutes is not unusually long for a patient’s head to be in this position during a procedure, and over the past 10 years the same anesthesiologist, head nurse, and circulating nurse have routinely used the same beach-chair positioning and headrest for Dr. Foad’s patients. Second, the postoperative interscalene block theoretically could have caused the palsy, but we think this is unlikely, as the block is placed lower on the neck, at the C6 level, and the greater auricular nerve branches off the C2–C3 spinal nerves. As described by Rains and colleagues,9 other authors have reported transient neuropathies to the brachial plexus, which originates in the C5–C8 region, but not to the greater auricular nerve. Last, it cannot be ruled out that a variant of the greater auricular nerve could have played a role, given the variation in the greater auricular nerve.10,11 However, Brennan and colleagues10 reported that 2 of 25 neck dissections involved a variant in which the anterior division of the greater auricular nerve passed into the submandibular triangle and joined the mandibular region of the facial nerve. Stimulation of this nerve resulted in activity of the depressor of the lower lip, which was not the location of our patient’s palsy. In addition, our patient’s symptoms followed a classic nerve distribution of the greater auricular nerve (Figures 1, 4), which would seem to decrease the likelihood that a variant was the source of the palsy.
The superficial nature of the greater auricular nerve, which runs roughly parallel with the sternocleidomastoid muscle and innervates much of the superficial region of the skin over the mastoid, parotid gland, and mandible,5-7 theoretically places the nerve at risk for compressive forces from the headrest during arthroscopic shoulder surgery. Skyhar and colleagues3 first described beach-chair positioning as an alternative to lateral decubitus positioning, which had been reported to result in neurologic injury in about 10% of surgical cases.9 The theoretical advantages of beach-chair positioning are lack of traction needed and ease of setup, which would therefore decrease the possibility of neuropathy.1,3 However, as seen in this and other case reports,7,8 greater auricular nerve neuropathy should still be considered a possible complication, even when using beach-chair positioning.
Besides neuropathy after arthroscopic shoulder surgery, as described in previous case reports7,8 and in the present report, greater auricular nerve injury has been described as arising from other stimuli. Greater auricular nerve injury has arisen after perineural tumor metastasis,6 neuroma of greater auricular nerve after endolympathic shunt surgery,12 internal fixation of mandibular condyle,13 and carotid endarterectomy.14,15 Given the superficial nature of the greater auricular nerve, it may not be all that surprising that a palsy could also develop after headrest compression during arthroscopic shoulder surgery.
This case report brings to light a possible complication of greater auricular nerve palsy during arthroscopic shoulder surgery using beach-chair positioning and a standard universal headrest. Studies should now investigate whether greater auricular nerve palsy is more common than realized, especially with regard to specific headrests in beach-chair positioning. For now, though, Dr. Foad’s intention is not to change to a different headrest or discontinue beach-chair positioning but to draw attention to this rare complication. Additional attention should be given to the location of the headrest in relation to the greater auricular nerve, especially in cases in which operative time may be longer.
Conclusion
We have reported a greater auricular nerve palsy that occurred after arthroscopic shoulder surgery for an anterior-inferior and posterior-inferior labral tear. This is the first report of a greater auricular nerve palsy occurring with beach-chair positioning and a standard universal headrest. Symptoms resolved within 6 months. New studies should investigate the incidence of greater auricular nerve palsy after arthroscopic shoulder surgery.
1. Paxton ES, Backus J, Keener J, Brophy RH. Shoulder arthroscopy: basic principles of positioning, anesthesia, and portal anatomy. J Am Acad Orthop Surg. 2013;21(6):332-342.
2. Scully WF, Wilson DJ, Parada SA, Arrington ED. Iatrogenic nerve injuries in shoulder surgery. J Am Acad Orthop Surg. 2013;21(12):717-726.
3. Skyhar MJ, Altchek DW, Warren RF, Wickiewicz TL, O’Brien SJ. Shoulder arthroscopy with the patient in the beach-chair position. Arthroscopy. 1988;4(4):256-259.
4. Zhang J, Moore AE, Stringer MD. Iatrogenic upper limb nerve injuries: a systematic review. ANZ J Surg. 2011;81(4):227-236.
5. Alberti PW. The greater auricular nerve. Donor for facial nerve grafts: a note on its topographical anatomy. Arch Otolaryngol. 1962;76:422-424.
6. Ginsberg LE, Eicher SA. Great auricular nerve: anatomy and imaging in a case of perineural tumor spread. AJNR Am J Neuroradiol. 2000;21(3):568-571.
7. Ng AK, Page RS. Greater auricular nerve neuropraxia with beach chair positioning during shoulder surgery. Int J Shoulder Surg. 2010;4(2):48-50.
8. Park TS, Kim YS. Neuropraxia of the cutaneous nerve of the cervical plexus after shoulder arthroscopy. Arthroscopy. 2005;21(5):631.e1-e3.
9. Rains DD, Rooke GA, Wahl CJ. Pathomechanisms and complications related to patient positioning and anesthesia during shoulder arthroscopy. Arthroscopy. 2011;27(4):532-541.
10. Brennan PA, Al Gholmy M, Ounnas H, Zaki GA, Puxeddu R, Standring S. Communication of the anterior branch of the great auricular nerve with the marginal mandibular nerve: a prospective study of 25 neck dissections. Br J Oral Maxillofac Surg. 2010;48(6):431-433.
11. Sand T, Becser N. Neurophysiological and anatomical variability of the greater auricular nerve. Acta Neurol Scand. 1998;98(5):333-339.
12. Vorobeichik L, Fallucco MA, Hagan RR. Chronic daily headaches secondary to greater auricular and lesser occipital neuromas following endolymphatic shunt surgery. BMJ Case Rep. 2012;2012. pii: bcr-2012-007189. doi:10.1136/bcr-2012-007189.
13. Sverzut CE, Trivellato AE, Serra EC, Ferraz EP, Sverzut AT. Frey’s syndrome after condylar fracture: case report. Braz Dent J. 2004;15(2):159-162.
14. AbuRahma AF, Choueiri MA. Cranial and cervical nerve injuries after repeat carotid endarterectomy. J Vasc Surg. 2000;32(4):649-654.
15. Ballotta E, Da Giau G, Renon L, et al. Cranial and cervical nerve injuries after carotid endarterectomy: a prospective study. Surgery. 1999;125(1):85-91.
Anterior-inferior and posterior-inferior labral tears are common injuries treated with arthroscopic surgery1 typically performed with beach-chair2,3 or lateral decubitus1,2 positioning and variable headrest positioning. Iatrogenic nerve damage that occurs after arthroscopic shoulder surgery—including damage to the suprascapular, axillary, musculocutaneous, subscapular, and spinal accessory nerves—has recently been reported to be more common than previously recognized.2,4
Although iatrogenic nerve injuries are in general being recognized,1,2,4 reports of greater auricular nerve injuries are limited. The greater auricular nerve is a superficial cutaneous nerve that arises from the cervical plexus at the C2 and C3 spinal nerves, obliquely crosses the sternocleidomastoid muscle, and splits into anterior and posterior portions that innervate the skin over the mastoid process and parotid gland.5,6 In particular, as illustrated by Ginsberg and Eicher6 (Figure 1), its superficial anatomy lies very near where a headrest is positioned during arthroscopic surgery, and increased pressure on the nerve throughout arthroscopic shoulder surgery may lead to neurapraxia.6,7 In 2 case series, authors reported on a total of 5 patients who had greater auricular nerve palsy after uncomplicated shoulder surgery using beach-chair positioning and a horseshoe headrest.7,8 The authors attributed these palsies to the horseshoe headrest, which they believed was compressing the greater auricular nerve during the entire surgery.7,8 However, standard universal headrests, which are thought to distribute pressures that would theoretically place the greater auricular nerve at risk for palsy, previously had not been described as contributing to palsy of the greater auricular nerve.
In this article, we report on a case of greater auricular nerve palsy that occurred after the patient’s anterior-inferior and posterior-inferior labral tear was arthroscopically repaired using beach-chair positioning and a standard universal headrest. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old right-hand–dominant high school American football player was referred for orthopedic evaluation of left chronic glenohumeral instability after 6 months of physical therapy. Physical examination revealed a positive apprehension test with the shoulder abducted and externally rotated at 90° and a positive relocation test. The patient complained of pain and instability when his arm was placed in a cross-chest adducted position and a posteroinferiorly directed axial load was applied. Magnetic resonance arthrogram showed an anterior-inferior labral Bankart tear with a small Hill-Sachs lesion to the humeral head but did not clearly reveal the posterior-inferior labral tear. Because of persistent left shoulder instability with most overhead activities and continued pain, the patient decided to undergo left shoulder arthroscopic Bankart repair with inferior capsular shift and posterior-inferior labral repair with capsulorraphy. He had no significant past medical history or known drug allergies.
The patient was placed in the standard beach-chair position: upright at 45° to the floor, hips flexed at 60°, knees flexed at 30°.1 Pneumatic compression devices were placed on his lower extremities. His head was secured in neutral position to a standard universal headrest (model A-90026; Allen Medical Systems, Acton, Massachusetts) (Figures 2, 3). Care was taken to protect the deep neurovascular structures and bony prominences throughout. The patient was in this position for 122 minutes of the operation, from positioning and draping to wound closure and dressing application. Before draping, the anesthesiologist, head nurse, and circulating nurse ensured that head and neck were in neutral position. The anesthesiologist monitored positioning throughout the perioperative period to ensure head and neck were in neutral, and the head did not need to be repositioned during surgery. Standard preoperative intravenous antibiotics were given.
General anesthesia and postoperative interscalene block were used. Standard preparation and draping were performed. Three standard arthroscopic portal incisions were used: posterior, anterior, and anterosuperolateral. Findings included extensive labral pathology, small bony Hill-Sachs lesion to humeral head, small bony anterior glenoid deficiency, and deficient anterior-inferior and posterior-inferior labral remnant. These were repaired arthroscopically in a standard fashion using bioabsorbable suture anchors. There were no arthroscopic complications. After surgery, a standard well-fitted shoulder immobilizer was placed. The anesthesiologist provided interscalene regional analgesia (15 mL of bupivacaine 0.5%) in the recovery area after surgery.
Postoperative neurovascular examination in the recovery room revealed no discomfort. The patient was discharged the same day. At a scheduled 1-week follow-up, he complained of numbness and dysesthesia on the left side of the greater auricular nerve distribution. A diagnosis of greater auricular nerve palsy was made by physical examination; the symptoms were along the classic greater auricular nerve distribution affecting the lower face and ear (Figure 4). The patient had no pain, skin lesions, or soft-tissue swelling. Otolaryngology confirmed the diagnosis and recommended observation-only treatment of symptoms. Symptoms lessened over the next 3 months, and the altered sensation resolved without deficit by 6 months. In addition, by 6 months the patient had returned to full activities (including collision sports) pain-free and with normal left shoulder function. Because symptoms continued to improve, the patient was followed with clinical observation, and a formal work-up was not necessary.
Discussion
The most important finding in this case is the greater auricular nerve palsy that occurred after arthroscopic anterior-inferior and posterior-inferior labral repairs in beach-chair positioning. This greater auricular nerve palsy was the first encountered by Dr. Foad, who over 17 years in a primarily shoulder practice setting has used beach-chair positioning exclusively. Previous reports have described a palsy occurring after arthroscopic shoulder surgery using beach-chair positioning and a horseshoe headrest.7,8 Ng and Page7 discontinued and recommended against use of this headrest because of the complications of the palsy, and Park and Kim8 recommended a headrest redesign. We think the present case report is the first to describe a greater auricular nerve palsy that occurred after arthroscopic surgery using a standard universal headrest, which theoretically should prevent compression of the greater auricular nerve. Increased awareness of the possibility of greater auricular nerve palsy, even when proper precautions are taken,1 is therefore warranted.
Based on the location of our patient’s palsy, we think his paralysis was most likely the result of nerve compression by the headrest during the shoulder surgery. Other factors, though unlikely, may have played a role. These include operative time (increases duration of nerve compression) and head positioning. However, 122 minutes is not unusually long for a patient’s head to be in this position during a procedure, and over the past 10 years the same anesthesiologist, head nurse, and circulating nurse have routinely used the same beach-chair positioning and headrest for Dr. Foad’s patients. Second, the postoperative interscalene block theoretically could have caused the palsy, but we think this is unlikely, as the block is placed lower on the neck, at the C6 level, and the greater auricular nerve branches off the C2–C3 spinal nerves. As described by Rains and colleagues,9 other authors have reported transient neuropathies to the brachial plexus, which originates in the C5–C8 region, but not to the greater auricular nerve. Last, it cannot be ruled out that a variant of the greater auricular nerve could have played a role, given the variation in the greater auricular nerve.10,11 However, Brennan and colleagues10 reported that 2 of 25 neck dissections involved a variant in which the anterior division of the greater auricular nerve passed into the submandibular triangle and joined the mandibular region of the facial nerve. Stimulation of this nerve resulted in activity of the depressor of the lower lip, which was not the location of our patient’s palsy. In addition, our patient’s symptoms followed a classic nerve distribution of the greater auricular nerve (Figures 1, 4), which would seem to decrease the likelihood that a variant was the source of the palsy.
The superficial nature of the greater auricular nerve, which runs roughly parallel with the sternocleidomastoid muscle and innervates much of the superficial region of the skin over the mastoid, parotid gland, and mandible,5-7 theoretically places the nerve at risk for compressive forces from the headrest during arthroscopic shoulder surgery. Skyhar and colleagues3 first described beach-chair positioning as an alternative to lateral decubitus positioning, which had been reported to result in neurologic injury in about 10% of surgical cases.9 The theoretical advantages of beach-chair positioning are lack of traction needed and ease of setup, which would therefore decrease the possibility of neuropathy.1,3 However, as seen in this and other case reports,7,8 greater auricular nerve neuropathy should still be considered a possible complication, even when using beach-chair positioning.
Besides neuropathy after arthroscopic shoulder surgery, as described in previous case reports7,8 and in the present report, greater auricular nerve injury has been described as arising from other stimuli. Greater auricular nerve injury has arisen after perineural tumor metastasis,6 neuroma of greater auricular nerve after endolympathic shunt surgery,12 internal fixation of mandibular condyle,13 and carotid endarterectomy.14,15 Given the superficial nature of the greater auricular nerve, it may not be all that surprising that a palsy could also develop after headrest compression during arthroscopic shoulder surgery.
This case report brings to light a possible complication of greater auricular nerve palsy during arthroscopic shoulder surgery using beach-chair positioning and a standard universal headrest. Studies should now investigate whether greater auricular nerve palsy is more common than realized, especially with regard to specific headrests in beach-chair positioning. For now, though, Dr. Foad’s intention is not to change to a different headrest or discontinue beach-chair positioning but to draw attention to this rare complication. Additional attention should be given to the location of the headrest in relation to the greater auricular nerve, especially in cases in which operative time may be longer.
Conclusion
We have reported a greater auricular nerve palsy that occurred after arthroscopic shoulder surgery for an anterior-inferior and posterior-inferior labral tear. This is the first report of a greater auricular nerve palsy occurring with beach-chair positioning and a standard universal headrest. Symptoms resolved within 6 months. New studies should investigate the incidence of greater auricular nerve palsy after arthroscopic shoulder surgery.
Anterior-inferior and posterior-inferior labral tears are common injuries treated with arthroscopic surgery1 typically performed with beach-chair2,3 or lateral decubitus1,2 positioning and variable headrest positioning. Iatrogenic nerve damage that occurs after arthroscopic shoulder surgery—including damage to the suprascapular, axillary, musculocutaneous, subscapular, and spinal accessory nerves—has recently been reported to be more common than previously recognized.2,4
Although iatrogenic nerve injuries are in general being recognized,1,2,4 reports of greater auricular nerve injuries are limited. The greater auricular nerve is a superficial cutaneous nerve that arises from the cervical plexus at the C2 and C3 spinal nerves, obliquely crosses the sternocleidomastoid muscle, and splits into anterior and posterior portions that innervate the skin over the mastoid process and parotid gland.5,6 In particular, as illustrated by Ginsberg and Eicher6 (Figure 1), its superficial anatomy lies very near where a headrest is positioned during arthroscopic surgery, and increased pressure on the nerve throughout arthroscopic shoulder surgery may lead to neurapraxia.6,7 In 2 case series, authors reported on a total of 5 patients who had greater auricular nerve palsy after uncomplicated shoulder surgery using beach-chair positioning and a horseshoe headrest.7,8 The authors attributed these palsies to the horseshoe headrest, which they believed was compressing the greater auricular nerve during the entire surgery.7,8 However, standard universal headrests, which are thought to distribute pressures that would theoretically place the greater auricular nerve at risk for palsy, previously had not been described as contributing to palsy of the greater auricular nerve.
In this article, we report on a case of greater auricular nerve palsy that occurred after the patient’s anterior-inferior and posterior-inferior labral tear was arthroscopically repaired using beach-chair positioning and a standard universal headrest. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old right-hand–dominant high school American football player was referred for orthopedic evaluation of left chronic glenohumeral instability after 6 months of physical therapy. Physical examination revealed a positive apprehension test with the shoulder abducted and externally rotated at 90° and a positive relocation test. The patient complained of pain and instability when his arm was placed in a cross-chest adducted position and a posteroinferiorly directed axial load was applied. Magnetic resonance arthrogram showed an anterior-inferior labral Bankart tear with a small Hill-Sachs lesion to the humeral head but did not clearly reveal the posterior-inferior labral tear. Because of persistent left shoulder instability with most overhead activities and continued pain, the patient decided to undergo left shoulder arthroscopic Bankart repair with inferior capsular shift and posterior-inferior labral repair with capsulorraphy. He had no significant past medical history or known drug allergies.
The patient was placed in the standard beach-chair position: upright at 45° to the floor, hips flexed at 60°, knees flexed at 30°.1 Pneumatic compression devices were placed on his lower extremities. His head was secured in neutral position to a standard universal headrest (model A-90026; Allen Medical Systems, Acton, Massachusetts) (Figures 2, 3). Care was taken to protect the deep neurovascular structures and bony prominences throughout. The patient was in this position for 122 minutes of the operation, from positioning and draping to wound closure and dressing application. Before draping, the anesthesiologist, head nurse, and circulating nurse ensured that head and neck were in neutral position. The anesthesiologist monitored positioning throughout the perioperative period to ensure head and neck were in neutral, and the head did not need to be repositioned during surgery. Standard preoperative intravenous antibiotics were given.
General anesthesia and postoperative interscalene block were used. Standard preparation and draping were performed. Three standard arthroscopic portal incisions were used: posterior, anterior, and anterosuperolateral. Findings included extensive labral pathology, small bony Hill-Sachs lesion to humeral head, small bony anterior glenoid deficiency, and deficient anterior-inferior and posterior-inferior labral remnant. These were repaired arthroscopically in a standard fashion using bioabsorbable suture anchors. There were no arthroscopic complications. After surgery, a standard well-fitted shoulder immobilizer was placed. The anesthesiologist provided interscalene regional analgesia (15 mL of bupivacaine 0.5%) in the recovery area after surgery.
Postoperative neurovascular examination in the recovery room revealed no discomfort. The patient was discharged the same day. At a scheduled 1-week follow-up, he complained of numbness and dysesthesia on the left side of the greater auricular nerve distribution. A diagnosis of greater auricular nerve palsy was made by physical examination; the symptoms were along the classic greater auricular nerve distribution affecting the lower face and ear (Figure 4). The patient had no pain, skin lesions, or soft-tissue swelling. Otolaryngology confirmed the diagnosis and recommended observation-only treatment of symptoms. Symptoms lessened over the next 3 months, and the altered sensation resolved without deficit by 6 months. In addition, by 6 months the patient had returned to full activities (including collision sports) pain-free and with normal left shoulder function. Because symptoms continued to improve, the patient was followed with clinical observation, and a formal work-up was not necessary.
Discussion
The most important finding in this case is the greater auricular nerve palsy that occurred after arthroscopic anterior-inferior and posterior-inferior labral repairs in beach-chair positioning. This greater auricular nerve palsy was the first encountered by Dr. Foad, who over 17 years in a primarily shoulder practice setting has used beach-chair positioning exclusively. Previous reports have described a palsy occurring after arthroscopic shoulder surgery using beach-chair positioning and a horseshoe headrest.7,8 Ng and Page7 discontinued and recommended against use of this headrest because of the complications of the palsy, and Park and Kim8 recommended a headrest redesign. We think the present case report is the first to describe a greater auricular nerve palsy that occurred after arthroscopic surgery using a standard universal headrest, which theoretically should prevent compression of the greater auricular nerve. Increased awareness of the possibility of greater auricular nerve palsy, even when proper precautions are taken,1 is therefore warranted.
Based on the location of our patient’s palsy, we think his paralysis was most likely the result of nerve compression by the headrest during the shoulder surgery. Other factors, though unlikely, may have played a role. These include operative time (increases duration of nerve compression) and head positioning. However, 122 minutes is not unusually long for a patient’s head to be in this position during a procedure, and over the past 10 years the same anesthesiologist, head nurse, and circulating nurse have routinely used the same beach-chair positioning and headrest for Dr. Foad’s patients. Second, the postoperative interscalene block theoretically could have caused the palsy, but we think this is unlikely, as the block is placed lower on the neck, at the C6 level, and the greater auricular nerve branches off the C2–C3 spinal nerves. As described by Rains and colleagues,9 other authors have reported transient neuropathies to the brachial plexus, which originates in the C5–C8 region, but not to the greater auricular nerve. Last, it cannot be ruled out that a variant of the greater auricular nerve could have played a role, given the variation in the greater auricular nerve.10,11 However, Brennan and colleagues10 reported that 2 of 25 neck dissections involved a variant in which the anterior division of the greater auricular nerve passed into the submandibular triangle and joined the mandibular region of the facial nerve. Stimulation of this nerve resulted in activity of the depressor of the lower lip, which was not the location of our patient’s palsy. In addition, our patient’s symptoms followed a classic nerve distribution of the greater auricular nerve (Figures 1, 4), which would seem to decrease the likelihood that a variant was the source of the palsy.
The superficial nature of the greater auricular nerve, which runs roughly parallel with the sternocleidomastoid muscle and innervates much of the superficial region of the skin over the mastoid, parotid gland, and mandible,5-7 theoretically places the nerve at risk for compressive forces from the headrest during arthroscopic shoulder surgery. Skyhar and colleagues3 first described beach-chair positioning as an alternative to lateral decubitus positioning, which had been reported to result in neurologic injury in about 10% of surgical cases.9 The theoretical advantages of beach-chair positioning are lack of traction needed and ease of setup, which would therefore decrease the possibility of neuropathy.1,3 However, as seen in this and other case reports,7,8 greater auricular nerve neuropathy should still be considered a possible complication, even when using beach-chair positioning.
Besides neuropathy after arthroscopic shoulder surgery, as described in previous case reports7,8 and in the present report, greater auricular nerve injury has been described as arising from other stimuli. Greater auricular nerve injury has arisen after perineural tumor metastasis,6 neuroma of greater auricular nerve after endolympathic shunt surgery,12 internal fixation of mandibular condyle,13 and carotid endarterectomy.14,15 Given the superficial nature of the greater auricular nerve, it may not be all that surprising that a palsy could also develop after headrest compression during arthroscopic shoulder surgery.
This case report brings to light a possible complication of greater auricular nerve palsy during arthroscopic shoulder surgery using beach-chair positioning and a standard universal headrest. Studies should now investigate whether greater auricular nerve palsy is more common than realized, especially with regard to specific headrests in beach-chair positioning. For now, though, Dr. Foad’s intention is not to change to a different headrest or discontinue beach-chair positioning but to draw attention to this rare complication. Additional attention should be given to the location of the headrest in relation to the greater auricular nerve, especially in cases in which operative time may be longer.
Conclusion
We have reported a greater auricular nerve palsy that occurred after arthroscopic shoulder surgery for an anterior-inferior and posterior-inferior labral tear. This is the first report of a greater auricular nerve palsy occurring with beach-chair positioning and a standard universal headrest. Symptoms resolved within 6 months. New studies should investigate the incidence of greater auricular nerve palsy after arthroscopic shoulder surgery.
1. Paxton ES, Backus J, Keener J, Brophy RH. Shoulder arthroscopy: basic principles of positioning, anesthesia, and portal anatomy. J Am Acad Orthop Surg. 2013;21(6):332-342.
2. Scully WF, Wilson DJ, Parada SA, Arrington ED. Iatrogenic nerve injuries in shoulder surgery. J Am Acad Orthop Surg. 2013;21(12):717-726.
3. Skyhar MJ, Altchek DW, Warren RF, Wickiewicz TL, O’Brien SJ. Shoulder arthroscopy with the patient in the beach-chair position. Arthroscopy. 1988;4(4):256-259.
4. Zhang J, Moore AE, Stringer MD. Iatrogenic upper limb nerve injuries: a systematic review. ANZ J Surg. 2011;81(4):227-236.
5. Alberti PW. The greater auricular nerve. Donor for facial nerve grafts: a note on its topographical anatomy. Arch Otolaryngol. 1962;76:422-424.
6. Ginsberg LE, Eicher SA. Great auricular nerve: anatomy and imaging in a case of perineural tumor spread. AJNR Am J Neuroradiol. 2000;21(3):568-571.
7. Ng AK, Page RS. Greater auricular nerve neuropraxia with beach chair positioning during shoulder surgery. Int J Shoulder Surg. 2010;4(2):48-50.
8. Park TS, Kim YS. Neuropraxia of the cutaneous nerve of the cervical plexus after shoulder arthroscopy. Arthroscopy. 2005;21(5):631.e1-e3.
9. Rains DD, Rooke GA, Wahl CJ. Pathomechanisms and complications related to patient positioning and anesthesia during shoulder arthroscopy. Arthroscopy. 2011;27(4):532-541.
10. Brennan PA, Al Gholmy M, Ounnas H, Zaki GA, Puxeddu R, Standring S. Communication of the anterior branch of the great auricular nerve with the marginal mandibular nerve: a prospective study of 25 neck dissections. Br J Oral Maxillofac Surg. 2010;48(6):431-433.
11. Sand T, Becser N. Neurophysiological and anatomical variability of the greater auricular nerve. Acta Neurol Scand. 1998;98(5):333-339.
12. Vorobeichik L, Fallucco MA, Hagan RR. Chronic daily headaches secondary to greater auricular and lesser occipital neuromas following endolymphatic shunt surgery. BMJ Case Rep. 2012;2012. pii: bcr-2012-007189. doi:10.1136/bcr-2012-007189.
13. Sverzut CE, Trivellato AE, Serra EC, Ferraz EP, Sverzut AT. Frey’s syndrome after condylar fracture: case report. Braz Dent J. 2004;15(2):159-162.
14. AbuRahma AF, Choueiri MA. Cranial and cervical nerve injuries after repeat carotid endarterectomy. J Vasc Surg. 2000;32(4):649-654.
15. Ballotta E, Da Giau G, Renon L, et al. Cranial and cervical nerve injuries after carotid endarterectomy: a prospective study. Surgery. 1999;125(1):85-91.
1. Paxton ES, Backus J, Keener J, Brophy RH. Shoulder arthroscopy: basic principles of positioning, anesthesia, and portal anatomy. J Am Acad Orthop Surg. 2013;21(6):332-342.
2. Scully WF, Wilson DJ, Parada SA, Arrington ED. Iatrogenic nerve injuries in shoulder surgery. J Am Acad Orthop Surg. 2013;21(12):717-726.
3. Skyhar MJ, Altchek DW, Warren RF, Wickiewicz TL, O’Brien SJ. Shoulder arthroscopy with the patient in the beach-chair position. Arthroscopy. 1988;4(4):256-259.
4. Zhang J, Moore AE, Stringer MD. Iatrogenic upper limb nerve injuries: a systematic review. ANZ J Surg. 2011;81(4):227-236.
5. Alberti PW. The greater auricular nerve. Donor for facial nerve grafts: a note on its topographical anatomy. Arch Otolaryngol. 1962;76:422-424.
6. Ginsberg LE, Eicher SA. Great auricular nerve: anatomy and imaging in a case of perineural tumor spread. AJNR Am J Neuroradiol. 2000;21(3):568-571.
7. Ng AK, Page RS. Greater auricular nerve neuropraxia with beach chair positioning during shoulder surgery. Int J Shoulder Surg. 2010;4(2):48-50.
8. Park TS, Kim YS. Neuropraxia of the cutaneous nerve of the cervical plexus after shoulder arthroscopy. Arthroscopy. 2005;21(5):631.e1-e3.
9. Rains DD, Rooke GA, Wahl CJ. Pathomechanisms and complications related to patient positioning and anesthesia during shoulder arthroscopy. Arthroscopy. 2011;27(4):532-541.
10. Brennan PA, Al Gholmy M, Ounnas H, Zaki GA, Puxeddu R, Standring S. Communication of the anterior branch of the great auricular nerve with the marginal mandibular nerve: a prospective study of 25 neck dissections. Br J Oral Maxillofac Surg. 2010;48(6):431-433.
11. Sand T, Becser N. Neurophysiological and anatomical variability of the greater auricular nerve. Acta Neurol Scand. 1998;98(5):333-339.
12. Vorobeichik L, Fallucco MA, Hagan RR. Chronic daily headaches secondary to greater auricular and lesser occipital neuromas following endolymphatic shunt surgery. BMJ Case Rep. 2012;2012. pii: bcr-2012-007189. doi:10.1136/bcr-2012-007189.
13. Sverzut CE, Trivellato AE, Serra EC, Ferraz EP, Sverzut AT. Frey’s syndrome after condylar fracture: case report. Braz Dent J. 2004;15(2):159-162.
14. AbuRahma AF, Choueiri MA. Cranial and cervical nerve injuries after repeat carotid endarterectomy. J Vasc Surg. 2000;32(4):649-654.
15. Ballotta E, Da Giau G, Renon L, et al. Cranial and cervical nerve injuries after carotid endarterectomy: a prospective study. Surgery. 1999;125(1):85-91.
Pelvic pleomorphic rhabdomyosarcoma presenting as oliguria in a 61-year-old woman
Click on the PDF icon at the top of this introduction to read the full article.
Click on the PDF icon at the top of this introduction to read the full article.
Click on the PDF icon at the top of this introduction to read the full article.
Refeeding syndrome in a vegan patient with stage IV gastric cancer: a novel case
Click on the PDF icon at the top of this introduction to read the full article.
Click on the PDF icon at the top of this introduction to read the full article.
Click on the PDF icon at the top of this introduction to read the full article.
Trigeminal Trophic Syndrome With Histopathologic Correlation
Case Report
A 49-year-old woman presented to the dermatology department with a concern of itching distributed along the V1 branch of the trigeminal nerve on the left frontoparietal scalp following a herpes zoster (HZ) outbreak in the same dermatome 2 months prior. She initially presented to the emergency department 2 months earlier with vesicular lesions distributed along the V1 branch of the trigeminal nerve, along with facial swelling, periorbital edema, inability to open the left eye, and “excruciating” pain. Her left eye was “itchy” but no ophthalmologic pathology was noted on examination. She was diagnosed with HZ and was treated with valacyclovir and prednisone. Oxycodone-acetaminophen followed by hydromorphone was prescribed for the severe pain with limited benefit. After completing treatment with valacyclovir, oral gabapentin was added for additional pain management, with an initial dose of 100 mg 3 times daily.
At the current presentation, the patient reported profound pruritus in the left frontoparietal scalp region that was intractable and debilitating. Some improvement of the itching was achieved with scratching that resulted in deep ulcerations of the scalp with moderate associated pain. In addition to the prior HZ outbreak, her medical history was remarkable for recurrent lymphoma, uterine cancer, chronic bronchitis, depression, hypothyroidism, osteoarthritis, and primary varicella-zoster virus infection in childhood. Her current medications included oral gabapentin (600 mg 3 times daily), diphenhydramine, levothyroxine, simvastatin, and topical ointments for itching.
On dermatologic evaluation, the patient rated her pain as a 5 on a 10-point scale of intensity. Alopecia involving the left frontoparietal scalp with a 2×3-cm ulceration in a geometric pattern with surrounding erythema was noted (Figure 1A). There also was hyperpigmentation on the forehead distributed along the V1 branch of the trigeminal nerve (Figure 1B). The patient also had been seen in the pain clinic where examination revealed sensory loss to both light touch and sharp stimulus along the left V1 branch of the trigeminal nerve. Visual fields were full, ocular movements were intact, and the face was symmetric with lower cranial nerves intact.
 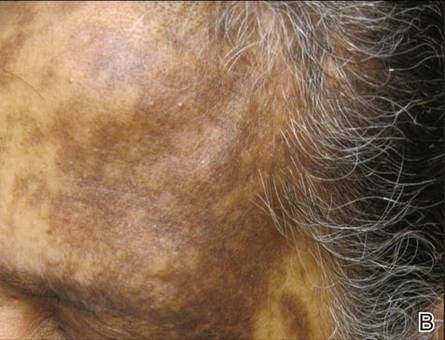 |
A diagnosis of trigeminal trophic syndrome (TTS) with chronic pain and pruritus due to a complex sensory neural disorder associated with HZ reactivation was made. Treatment included an increase in the dosage of oral gabapentin (1200 mg 3 times daily), oral oxycodone (5 mg every 4 to 6 hours as needed), and sphenopalatine ganglion block on the left side in an attempt to decrease pain and pruritus. At 6-week follow-up, the patient had no improvement in symptoms.
Three scalp punch biopsies were performed on presentation to the dermatology clinic including 2 from the affected area on the left frontoparietal scalp, and one from normal skin on the right side to assess the small nerve fibers affected. Protein gene product 9.5 (PGP 9.5) immunostaining was performed to assess epidermal nerve fiber density. The left scalp biopsies were consistent with a complete focal sensory neuropathy affecting sensory and autonomic axons (Figure 2A). The right scalp biopsy revealed well-innervated skin (Figure 2B).
  |
One year after the original HZ outbreak, the patient continued to have debilitating pruritus and pain in the affected dermatome. On physical examination at 1-year follow-up, the hyperpigmentation on the left side of the forehead showed minimal improvement. The ulcerations were healed, but excoriations were noted in the area. Having experienced some relief from titration of the dose of gabapentin 800 mg 3 times daily and doxepin 25 mg nightly at 1-year follow-up, the patient returned to work but remained highly distressed by her symptoms. Neurosurgery was consulted for possible balloon rhizotomy of the left trigeminal nerve, which she ultimately refused due to concerns about side effects.
Comment
Trophic trigeminal syndrome is characterized by unilateral ulceration of the face with anesthesia, paresthesia, and a crescent-shaped erosion or ulcer.1,2 It is one of 2 causes of self-induced facial ulcerations, the other being factitial dermatitis.1,3,4 A 2008 retrospective medical chart review and report of 14 cases helped elucidate the epidemiology of TTS.2 In this case series, the female to male ratio was 6 to 1, and the mean age of TTS onset was 45 years (age range, 6–82 years). The cause of disease in most patients was iatrogenic and the latent period to onset ranged from days to almost one decade. Most patients self-manipulated the face (n=9), and most ulcers affected the second trigeminal division. Pain intensity was severe in most (n=6), and gabapentin offered relief in only 2 cases.2
The etiologies of TTS are wide ranging, and the differential diagnosis should be contemplated when patients present with facial ulcers. Most cases are iatrogenic secondary to trigeminal rhizotomy,5 alcohol injections into the gasserian ganglion, or electrocoagulation. Also common are cases caused by ischemic damage to the trigeminal ganglion6 or Wallenberg syndrome.7 More rare etiologies include trauma,7 craniotomy,7 astrocytoma, acoustic neuroma, meningioma,8 idiopathic causes, basal cell carcinoma, infectious diseases (eg, tertiary syphilis, recurrent herpes simplex virus, leishmaniasis, cutaneous tuberculosis, leprosy, HZ),9-11 or systemic disease (eg, Wegener granulomatosis, Horton arteritis).
Trigeminal trophic syndrome is rare and there is little agreement on a treatment algorithm. As in our case, a methodical trial-and-error approach is suggested while encouraging the patient not to abandon treatment when efforts are not fruitful. The most important treatment strategy is behavioral modification; patients must become aware of the role of self-manipulation and assiduously avoid it. Using occlusive dressings at the affected site also may be helpful3,12 Transcutaneous electrical nerve stimulation may lead to improvement, but relapse is common with treatment discontinuation. Therapies directed at reducing paresthesia (eg, carbamazepine, diazepam, amitriptyline, chlorpromazine, pimozide) are sometimes successful, but relapse is common.1,3 Transplantation of in vitro–cultured epidermal cells is a new experimental treatment that offers hope for future success.13 Facial reconstruction of the affected area may help patients who can restrain themselves from self-manipulation.4
Skin biopsy findings in our case revealed an interesting aspect of the disease process of TTS. Skin biopsies are helpful in ruling out malignancy and specific stains can be used to further elucidate disease or pathologic processes occurring in the skin. In TTS, no specific changes are seen on hematoxylin and eosin staining, revealing only nonspecific inflammatory changes.1,5 Strikingly, the pathology of affected skin in patients with postherpetic neuralgia often reveals distal nociceptive axon loss,9 as was seen in the skin biopsies from our patient’s left scalp. It has been proven by many researchers in many neuropathic pain conditions that the pathological signature of chronic neuropathic pain is reduction in the density of cutaneous nociceptive innervation.9 The most common method for visualizing cutaneous neuritis is using an immunohistochemical labeling method in which antibodies are directed against PGP 9.5. A pan-axonal neurofilament marker, PGP 9.5 allows for visualization of small sensory nerve endings in the skin. As nociceptive axons degenerate in neuropathic pain conditions, it is believed that initiation of proalgesic changes within remaining peripheral nerves and the central nervous system (CNS) occur. Another interesting aspect of our case was the patient’s persistent intractable itching and chronic pain 2 months following the initial HZ outbreak. Although pain and itching can be evoked by similar stimuli and injuries, it has been shown that both have separate neuronal pathways because they produce different conscious and reflex motor actions.14 For instance, pain causes a withdrawal reflex, while itching causes mechanical stimulation of the affected area. The act of itching is thought to have evolved to protect against threats by the act of dislodging the stimulus rather than withdrawing as seen in pain.14 It has been hypothesized that postherpetic itching (chronic pruritus following an HZ outbreak) is due to spontaneous firing of denervated CNS itch neurons.9
Postherpetic neuralgia–related pain seems to be most closely correlated with degeneration of varicella-zoster virus–infected primary afferent neurons. With deceased afferent neurons sending signals to the CNS and death or dysfunction of inhibitory interneurons in the dorsal horn of the spinal cord due to peripheral nerve injury, there is increased paradoxical electrical activity in specific CNS neurons. This CNS plasticity results in neuropathic pain and other altered sensory abnormalities in patients with TTS.9
Conclusion
We present a case of TTS distributed along the V1 branch of the trigeminal nerve on the left frontoparietal scalp following an HZ outbreak in a 49-year-old woman. Skin biopsies were consistent with this diagnosis, which revealed no neuronal innervation of the affected scalp despite intractable itching and chronic pain. Further research of TTS and postherpetic neuralgia is necessary to find appropriate treatment for patients with these conditions.
1. Kautz O, Bruckner-Tuderman L, Müller ML, et al. Trigeminal trophic syndrome with extensive ulceration following herpes zoster. Eur J Dermatol. 2009;19:61-63.
2. Garza I. The trigeminal trophic syndrome: an unusual cause of face pain, dysaesthesias, anaesthesia and skin/soft tissue lesions. Cephalalgia. 2008;28:980-985.
3. Farahani RM, Marsee DK, Baden LR, et al. Trigeminal trophic syndrome with features of oral CMV disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:15-18.
4. Tollefson TT, Kriet JD, Wang TD, et al. Self-induced nasal ulceration. Arch Facial Plast Surg. 2004;6:162-166.
5. Monrad SU, Terrell JE, Aronoff DM. The trigeminal trophic syndrome: an unusual cause of nasal ulceration. J Am Acad Dermatol. 2004;50:949-952.
6. Elloumi-Jellouli A, Ben Ammar S, Fenniche S, et al. Trigeminal trophic syndrome: a report of two cases with review of literature. Dermatol Online J. 2003;9:26.
7. Sadeghi P, Papay FA, Vidimos AT. Trigeminal trophic syndrome—report of four cases and review of the literature. Dermatol Surg. 2004;30:807-812.
8. Luksi´c I, Luksi´c I, Sestan-Crnek S, et al. Trigeminal trophic syndrome of all three nerve branches: an underrecognized complication after brain surgery. J Neurosurg. 2008;108:170-173.
9. Oaklander AL. Mechanisms of pain and itch caused by herpes zoster (shingles). J Pain. 2008;9(1 suppl 1):S10-S18.
10. Gawande A. The itch. The New Yorker. June 2008:58-67.
11. Oaklander AL, Cohen SP, Raju SV. Intractable postherpetic itch and cutaneous deafferentation after facial shingles. Pain. 2002;96:9-12.
12. Preston PW, Orpin SD, Tucker WF, et al. Successful use of a thermoplastic dressing in two cases of the trigeminal trophic syndrome. Clin Exp Dermatol. 2006;31:525-527.
13. Schwerdtner O, Damaskos T, Kage A, et al. Autologous epidermal cells can induce wound closure of neurotrophic ulceration caused by trigeminal trophic syndrome. Int J Oral Maxillofac Surg. 2005;34:443-445.
14. Oaklander AL, Siegel SM. Cutaneous innervation: form and function. J Am Acad Dermatol. 2005;53:1027-1037.
Case Report
A 49-year-old woman presented to the dermatology department with a concern of itching distributed along the V1 branch of the trigeminal nerve on the left frontoparietal scalp following a herpes zoster (HZ) outbreak in the same dermatome 2 months prior. She initially presented to the emergency department 2 months earlier with vesicular lesions distributed along the V1 branch of the trigeminal nerve, along with facial swelling, periorbital edema, inability to open the left eye, and “excruciating” pain. Her left eye was “itchy” but no ophthalmologic pathology was noted on examination. She was diagnosed with HZ and was treated with valacyclovir and prednisone. Oxycodone-acetaminophen followed by hydromorphone was prescribed for the severe pain with limited benefit. After completing treatment with valacyclovir, oral gabapentin was added for additional pain management, with an initial dose of 100 mg 3 times daily.
At the current presentation, the patient reported profound pruritus in the left frontoparietal scalp region that was intractable and debilitating. Some improvement of the itching was achieved with scratching that resulted in deep ulcerations of the scalp with moderate associated pain. In addition to the prior HZ outbreak, her medical history was remarkable for recurrent lymphoma, uterine cancer, chronic bronchitis, depression, hypothyroidism, osteoarthritis, and primary varicella-zoster virus infection in childhood. Her current medications included oral gabapentin (600 mg 3 times daily), diphenhydramine, levothyroxine, simvastatin, and topical ointments for itching.
On dermatologic evaluation, the patient rated her pain as a 5 on a 10-point scale of intensity. Alopecia involving the left frontoparietal scalp with a 2×3-cm ulceration in a geometric pattern with surrounding erythema was noted (Figure 1A). There also was hyperpigmentation on the forehead distributed along the V1 branch of the trigeminal nerve (Figure 1B). The patient also had been seen in the pain clinic where examination revealed sensory loss to both light touch and sharp stimulus along the left V1 branch of the trigeminal nerve. Visual fields were full, ocular movements were intact, and the face was symmetric with lower cranial nerves intact.
  |
A diagnosis of trigeminal trophic syndrome (TTS) with chronic pain and pruritus due to a complex sensory neural disorder associated with HZ reactivation was made. Treatment included an increase in the dosage of oral gabapentin (1200 mg 3 times daily), oral oxycodone (5 mg every 4 to 6 hours as needed), and sphenopalatine ganglion block on the left side in an attempt to decrease pain and pruritus. At 6-week follow-up, the patient had no improvement in symptoms.
Three scalp punch biopsies were performed on presentation to the dermatology clinic including 2 from the affected area on the left frontoparietal scalp, and one from normal skin on the right side to assess the small nerve fibers affected. Protein gene product 9.5 (PGP 9.5) immunostaining was performed to assess epidermal nerve fiber density. The left scalp biopsies were consistent with a complete focal sensory neuropathy affecting sensory and autonomic axons (Figure 2A). The right scalp biopsy revealed well-innervated skin (Figure 2B).
  |
One year after the original HZ outbreak, the patient continued to have debilitating pruritus and pain in the affected dermatome. On physical examination at 1-year follow-up, the hyperpigmentation on the left side of the forehead showed minimal improvement. The ulcerations were healed, but excoriations were noted in the area. Having experienced some relief from titration of the dose of gabapentin 800 mg 3 times daily and doxepin 25 mg nightly at 1-year follow-up, the patient returned to work but remained highly distressed by her symptoms. Neurosurgery was consulted for possible balloon rhizotomy of the left trigeminal nerve, which she ultimately refused due to concerns about side effects.
Comment
Trophic trigeminal syndrome is characterized by unilateral ulceration of the face with anesthesia, paresthesia, and a crescent-shaped erosion or ulcer.1,2 It is one of 2 causes of self-induced facial ulcerations, the other being factitial dermatitis.1,3,4 A 2008 retrospective medical chart review and report of 14 cases helped elucidate the epidemiology of TTS.2 In this case series, the female to male ratio was 6 to 1, and the mean age of TTS onset was 45 years (age range, 6–82 years). The cause of disease in most patients was iatrogenic and the latent period to onset ranged from days to almost one decade. Most patients self-manipulated the face (n=9), and most ulcers affected the second trigeminal division. Pain intensity was severe in most (n=6), and gabapentin offered relief in only 2 cases.2
The etiologies of TTS are wide ranging, and the differential diagnosis should be contemplated when patients present with facial ulcers. Most cases are iatrogenic secondary to trigeminal rhizotomy,5 alcohol injections into the gasserian ganglion, or electrocoagulation. Also common are cases caused by ischemic damage to the trigeminal ganglion6 or Wallenberg syndrome.7 More rare etiologies include trauma,7 craniotomy,7 astrocytoma, acoustic neuroma, meningioma,8 idiopathic causes, basal cell carcinoma, infectious diseases (eg, tertiary syphilis, recurrent herpes simplex virus, leishmaniasis, cutaneous tuberculosis, leprosy, HZ),9-11 or systemic disease (eg, Wegener granulomatosis, Horton arteritis).
Trigeminal trophic syndrome is rare and there is little agreement on a treatment algorithm. As in our case, a methodical trial-and-error approach is suggested while encouraging the patient not to abandon treatment when efforts are not fruitful. The most important treatment strategy is behavioral modification; patients must become aware of the role of self-manipulation and assiduously avoid it. Using occlusive dressings at the affected site also may be helpful3,12 Transcutaneous electrical nerve stimulation may lead to improvement, but relapse is common with treatment discontinuation. Therapies directed at reducing paresthesia (eg, carbamazepine, diazepam, amitriptyline, chlorpromazine, pimozide) are sometimes successful, but relapse is common.1,3 Transplantation of in vitro–cultured epidermal cells is a new experimental treatment that offers hope for future success.13 Facial reconstruction of the affected area may help patients who can restrain themselves from self-manipulation.4
Skin biopsy findings in our case revealed an interesting aspect of the disease process of TTS. Skin biopsies are helpful in ruling out malignancy and specific stains can be used to further elucidate disease or pathologic processes occurring in the skin. In TTS, no specific changes are seen on hematoxylin and eosin staining, revealing only nonspecific inflammatory changes.1,5 Strikingly, the pathology of affected skin in patients with postherpetic neuralgia often reveals distal nociceptive axon loss,9 as was seen in the skin biopsies from our patient’s left scalp. It has been proven by many researchers in many neuropathic pain conditions that the pathological signature of chronic neuropathic pain is reduction in the density of cutaneous nociceptive innervation.9 The most common method for visualizing cutaneous neuritis is using an immunohistochemical labeling method in which antibodies are directed against PGP 9.5. A pan-axonal neurofilament marker, PGP 9.5 allows for visualization of small sensory nerve endings in the skin. As nociceptive axons degenerate in neuropathic pain conditions, it is believed that initiation of proalgesic changes within remaining peripheral nerves and the central nervous system (CNS) occur. Another interesting aspect of our case was the patient’s persistent intractable itching and chronic pain 2 months following the initial HZ outbreak. Although pain and itching can be evoked by similar stimuli and injuries, it has been shown that both have separate neuronal pathways because they produce different conscious and reflex motor actions.14 For instance, pain causes a withdrawal reflex, while itching causes mechanical stimulation of the affected area. The act of itching is thought to have evolved to protect against threats by the act of dislodging the stimulus rather than withdrawing as seen in pain.14 It has been hypothesized that postherpetic itching (chronic pruritus following an HZ outbreak) is due to spontaneous firing of denervated CNS itch neurons.9
Postherpetic neuralgia–related pain seems to be most closely correlated with degeneration of varicella-zoster virus–infected primary afferent neurons. With deceased afferent neurons sending signals to the CNS and death or dysfunction of inhibitory interneurons in the dorsal horn of the spinal cord due to peripheral nerve injury, there is increased paradoxical electrical activity in specific CNS neurons. This CNS plasticity results in neuropathic pain and other altered sensory abnormalities in patients with TTS.9
Conclusion
We present a case of TTS distributed along the V1 branch of the trigeminal nerve on the left frontoparietal scalp following an HZ outbreak in a 49-year-old woman. Skin biopsies were consistent with this diagnosis, which revealed no neuronal innervation of the affected scalp despite intractable itching and chronic pain. Further research of TTS and postherpetic neuralgia is necessary to find appropriate treatment for patients with these conditions.
Case Report
A 49-year-old woman presented to the dermatology department with a concern of itching distributed along the V1 branch of the trigeminal nerve on the left frontoparietal scalp following a herpes zoster (HZ) outbreak in the same dermatome 2 months prior. She initially presented to the emergency department 2 months earlier with vesicular lesions distributed along the V1 branch of the trigeminal nerve, along with facial swelling, periorbital edema, inability to open the left eye, and “excruciating” pain. Her left eye was “itchy” but no ophthalmologic pathology was noted on examination. She was diagnosed with HZ and was treated with valacyclovir and prednisone. Oxycodone-acetaminophen followed by hydromorphone was prescribed for the severe pain with limited benefit. After completing treatment with valacyclovir, oral gabapentin was added for additional pain management, with an initial dose of 100 mg 3 times daily.
At the current presentation, the patient reported profound pruritus in the left frontoparietal scalp region that was intractable and debilitating. Some improvement of the itching was achieved with scratching that resulted in deep ulcerations of the scalp with moderate associated pain. In addition to the prior HZ outbreak, her medical history was remarkable for recurrent lymphoma, uterine cancer, chronic bronchitis, depression, hypothyroidism, osteoarthritis, and primary varicella-zoster virus infection in childhood. Her current medications included oral gabapentin (600 mg 3 times daily), diphenhydramine, levothyroxine, simvastatin, and topical ointments for itching.
On dermatologic evaluation, the patient rated her pain as a 5 on a 10-point scale of intensity. Alopecia involving the left frontoparietal scalp with a 2×3-cm ulceration in a geometric pattern with surrounding erythema was noted (Figure 1A). There also was hyperpigmentation on the forehead distributed along the V1 branch of the trigeminal nerve (Figure 1B). The patient also had been seen in the pain clinic where examination revealed sensory loss to both light touch and sharp stimulus along the left V1 branch of the trigeminal nerve. Visual fields were full, ocular movements were intact, and the face was symmetric with lower cranial nerves intact.
  |
A diagnosis of trigeminal trophic syndrome (TTS) with chronic pain and pruritus due to a complex sensory neural disorder associated with HZ reactivation was made. Treatment included an increase in the dosage of oral gabapentin (1200 mg 3 times daily), oral oxycodone (5 mg every 4 to 6 hours as needed), and sphenopalatine ganglion block on the left side in an attempt to decrease pain and pruritus. At 6-week follow-up, the patient had no improvement in symptoms.
Three scalp punch biopsies were performed on presentation to the dermatology clinic including 2 from the affected area on the left frontoparietal scalp, and one from normal skin on the right side to assess the small nerve fibers affected. Protein gene product 9.5 (PGP 9.5) immunostaining was performed to assess epidermal nerve fiber density. The left scalp biopsies were consistent with a complete focal sensory neuropathy affecting sensory and autonomic axons (Figure 2A). The right scalp biopsy revealed well-innervated skin (Figure 2B).
  |
One year after the original HZ outbreak, the patient continued to have debilitating pruritus and pain in the affected dermatome. On physical examination at 1-year follow-up, the hyperpigmentation on the left side of the forehead showed minimal improvement. The ulcerations were healed, but excoriations were noted in the area. Having experienced some relief from titration of the dose of gabapentin 800 mg 3 times daily and doxepin 25 mg nightly at 1-year follow-up, the patient returned to work but remained highly distressed by her symptoms. Neurosurgery was consulted for possible balloon rhizotomy of the left trigeminal nerve, which she ultimately refused due to concerns about side effects.
Comment
Trophic trigeminal syndrome is characterized by unilateral ulceration of the face with anesthesia, paresthesia, and a crescent-shaped erosion or ulcer.1,2 It is one of 2 causes of self-induced facial ulcerations, the other being factitial dermatitis.1,3,4 A 2008 retrospective medical chart review and report of 14 cases helped elucidate the epidemiology of TTS.2 In this case series, the female to male ratio was 6 to 1, and the mean age of TTS onset was 45 years (age range, 6–82 years). The cause of disease in most patients was iatrogenic and the latent period to onset ranged from days to almost one decade. Most patients self-manipulated the face (n=9), and most ulcers affected the second trigeminal division. Pain intensity was severe in most (n=6), and gabapentin offered relief in only 2 cases.2
The etiologies of TTS are wide ranging, and the differential diagnosis should be contemplated when patients present with facial ulcers. Most cases are iatrogenic secondary to trigeminal rhizotomy,5 alcohol injections into the gasserian ganglion, or electrocoagulation. Also common are cases caused by ischemic damage to the trigeminal ganglion6 or Wallenberg syndrome.7 More rare etiologies include trauma,7 craniotomy,7 astrocytoma, acoustic neuroma, meningioma,8 idiopathic causes, basal cell carcinoma, infectious diseases (eg, tertiary syphilis, recurrent herpes simplex virus, leishmaniasis, cutaneous tuberculosis, leprosy, HZ),9-11 or systemic disease (eg, Wegener granulomatosis, Horton arteritis).
Trigeminal trophic syndrome is rare and there is little agreement on a treatment algorithm. As in our case, a methodical trial-and-error approach is suggested while encouraging the patient not to abandon treatment when efforts are not fruitful. The most important treatment strategy is behavioral modification; patients must become aware of the role of self-manipulation and assiduously avoid it. Using occlusive dressings at the affected site also may be helpful3,12 Transcutaneous electrical nerve stimulation may lead to improvement, but relapse is common with treatment discontinuation. Therapies directed at reducing paresthesia (eg, carbamazepine, diazepam, amitriptyline, chlorpromazine, pimozide) are sometimes successful, but relapse is common.1,3 Transplantation of in vitro–cultured epidermal cells is a new experimental treatment that offers hope for future success.13 Facial reconstruction of the affected area may help patients who can restrain themselves from self-manipulation.4
Skin biopsy findings in our case revealed an interesting aspect of the disease process of TTS. Skin biopsies are helpful in ruling out malignancy and specific stains can be used to further elucidate disease or pathologic processes occurring in the skin. In TTS, no specific changes are seen on hematoxylin and eosin staining, revealing only nonspecific inflammatory changes.1,5 Strikingly, the pathology of affected skin in patients with postherpetic neuralgia often reveals distal nociceptive axon loss,9 as was seen in the skin biopsies from our patient’s left scalp. It has been proven by many researchers in many neuropathic pain conditions that the pathological signature of chronic neuropathic pain is reduction in the density of cutaneous nociceptive innervation.9 The most common method for visualizing cutaneous neuritis is using an immunohistochemical labeling method in which antibodies are directed against PGP 9.5. A pan-axonal neurofilament marker, PGP 9.5 allows for visualization of small sensory nerve endings in the skin. As nociceptive axons degenerate in neuropathic pain conditions, it is believed that initiation of proalgesic changes within remaining peripheral nerves and the central nervous system (CNS) occur. Another interesting aspect of our case was the patient’s persistent intractable itching and chronic pain 2 months following the initial HZ outbreak. Although pain and itching can be evoked by similar stimuli and injuries, it has been shown that both have separate neuronal pathways because they produce different conscious and reflex motor actions.14 For instance, pain causes a withdrawal reflex, while itching causes mechanical stimulation of the affected area. The act of itching is thought to have evolved to protect against threats by the act of dislodging the stimulus rather than withdrawing as seen in pain.14 It has been hypothesized that postherpetic itching (chronic pruritus following an HZ outbreak) is due to spontaneous firing of denervated CNS itch neurons.9
Postherpetic neuralgia–related pain seems to be most closely correlated with degeneration of varicella-zoster virus–infected primary afferent neurons. With deceased afferent neurons sending signals to the CNS and death or dysfunction of inhibitory interneurons in the dorsal horn of the spinal cord due to peripheral nerve injury, there is increased paradoxical electrical activity in specific CNS neurons. This CNS plasticity results in neuropathic pain and other altered sensory abnormalities in patients with TTS.9
Conclusion
We present a case of TTS distributed along the V1 branch of the trigeminal nerve on the left frontoparietal scalp following an HZ outbreak in a 49-year-old woman. Skin biopsies were consistent with this diagnosis, which revealed no neuronal innervation of the affected scalp despite intractable itching and chronic pain. Further research of TTS and postherpetic neuralgia is necessary to find appropriate treatment for patients with these conditions.
1. Kautz O, Bruckner-Tuderman L, Müller ML, et al. Trigeminal trophic syndrome with extensive ulceration following herpes zoster. Eur J Dermatol. 2009;19:61-63.
2. Garza I. The trigeminal trophic syndrome: an unusual cause of face pain, dysaesthesias, anaesthesia and skin/soft tissue lesions. Cephalalgia. 2008;28:980-985.
3. Farahani RM, Marsee DK, Baden LR, et al. Trigeminal trophic syndrome with features of oral CMV disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:15-18.
4. Tollefson TT, Kriet JD, Wang TD, et al. Self-induced nasal ulceration. Arch Facial Plast Surg. 2004;6:162-166.
5. Monrad SU, Terrell JE, Aronoff DM. The trigeminal trophic syndrome: an unusual cause of nasal ulceration. J Am Acad Dermatol. 2004;50:949-952.
6. Elloumi-Jellouli A, Ben Ammar S, Fenniche S, et al. Trigeminal trophic syndrome: a report of two cases with review of literature. Dermatol Online J. 2003;9:26.
7. Sadeghi P, Papay FA, Vidimos AT. Trigeminal trophic syndrome—report of four cases and review of the literature. Dermatol Surg. 2004;30:807-812.
8. Luksi´c I, Luksi´c I, Sestan-Crnek S, et al. Trigeminal trophic syndrome of all three nerve branches: an underrecognized complication after brain surgery. J Neurosurg. 2008;108:170-173.
9. Oaklander AL. Mechanisms of pain and itch caused by herpes zoster (shingles). J Pain. 2008;9(1 suppl 1):S10-S18.
10. Gawande A. The itch. The New Yorker. June 2008:58-67.
11. Oaklander AL, Cohen SP, Raju SV. Intractable postherpetic itch and cutaneous deafferentation after facial shingles. Pain. 2002;96:9-12.
12. Preston PW, Orpin SD, Tucker WF, et al. Successful use of a thermoplastic dressing in two cases of the trigeminal trophic syndrome. Clin Exp Dermatol. 2006;31:525-527.
13. Schwerdtner O, Damaskos T, Kage A, et al. Autologous epidermal cells can induce wound closure of neurotrophic ulceration caused by trigeminal trophic syndrome. Int J Oral Maxillofac Surg. 2005;34:443-445.
14. Oaklander AL, Siegel SM. Cutaneous innervation: form and function. J Am Acad Dermatol. 2005;53:1027-1037.
1. Kautz O, Bruckner-Tuderman L, Müller ML, et al. Trigeminal trophic syndrome with extensive ulceration following herpes zoster. Eur J Dermatol. 2009;19:61-63.
2. Garza I. The trigeminal trophic syndrome: an unusual cause of face pain, dysaesthesias, anaesthesia and skin/soft tissue lesions. Cephalalgia. 2008;28:980-985.
3. Farahani RM, Marsee DK, Baden LR, et al. Trigeminal trophic syndrome with features of oral CMV disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:15-18.
4. Tollefson TT, Kriet JD, Wang TD, et al. Self-induced nasal ulceration. Arch Facial Plast Surg. 2004;6:162-166.
5. Monrad SU, Terrell JE, Aronoff DM. The trigeminal trophic syndrome: an unusual cause of nasal ulceration. J Am Acad Dermatol. 2004;50:949-952.
6. Elloumi-Jellouli A, Ben Ammar S, Fenniche S, et al. Trigeminal trophic syndrome: a report of two cases with review of literature. Dermatol Online J. 2003;9:26.
7. Sadeghi P, Papay FA, Vidimos AT. Trigeminal trophic syndrome—report of four cases and review of the literature. Dermatol Surg. 2004;30:807-812.
8. Luksi´c I, Luksi´c I, Sestan-Crnek S, et al. Trigeminal trophic syndrome of all three nerve branches: an underrecognized complication after brain surgery. J Neurosurg. 2008;108:170-173.
9. Oaklander AL. Mechanisms of pain and itch caused by herpes zoster (shingles). J Pain. 2008;9(1 suppl 1):S10-S18.
10. Gawande A. The itch. The New Yorker. June 2008:58-67.
11. Oaklander AL, Cohen SP, Raju SV. Intractable postherpetic itch and cutaneous deafferentation after facial shingles. Pain. 2002;96:9-12.
12. Preston PW, Orpin SD, Tucker WF, et al. Successful use of a thermoplastic dressing in two cases of the trigeminal trophic syndrome. Clin Exp Dermatol. 2006;31:525-527.
13. Schwerdtner O, Damaskos T, Kage A, et al. Autologous epidermal cells can induce wound closure of neurotrophic ulceration caused by trigeminal trophic syndrome. Int J Oral Maxillofac Surg. 2005;34:443-445.
14. Oaklander AL, Siegel SM. Cutaneous innervation: form and function. J Am Acad Dermatol. 2005;53:1027-1037.
Practice Points
- Clinicians should remember to include trigeminal trophic syndrome in the differential diagnosis of patients with facial ulcers.
- Trigeminal trophic syndrome is a rare syndrome with a variety of treatment options, though no gold standard for treatment exists.
Glatiramer Acetate–Induced Lobular Panniculitis and Skin Necrosis
Glatiramer acetate (GA), a synthetic polypeptide that is injected subcutaneously, has proven effective in the treatment of relapsing-remitting multiple sclerosis (RRMS) and is now considered a first-line agent in the treatment of this condition. Adverse effects associated with GA primarily include local injection-site reactions (LISRs)(eg, erythema, pruritus, burning, pain, inflammation). Transient acute systemic reactions such as flushing and dyspnea also are commonly reported. Lipoatrophy at the injection site frequently has been reported in the literature as a cutaneous adverse effect of GA, but lobular panniculitis and necrosis at the site of injection rarely have been noted.
We report the case of a 36-year-old woman who experienced a severe adverse reaction to a single injection of GA after nearly 1 year of daily use to control symptoms of RRMS. Review of the current literature revealed few reports of the severe reaction of panniculitis and necrosis occurring at the injection site of GA.
Case Report
A 36-year-old woman was referred by her neurologist to the emergency department of our institution’s allergy and immunology clinic for treatment of an allergic reaction to a 20-mg GA injection, which she had been receiving daily for nearly 1 year as therapy for RRMS. A nodule immediately formed at the injection site and eventually became ulcerated. The patient also reported intense chest tightness, shortness of breath, and flushing following the injection. Physical examination revealed a large 8- to 9-cm erythematous area at the injection site on the left buttock. Necrosis and eschar formation also were evident (Figure 1).
Figure 1. Panniculitis with central ulceration and necrosis at the site of a glatiramer acetate injection on the left buttock (A). Closer view of an irregularly shaped necrotic lesion with surrounding erythema (B). |
A punch biopsy from the edge of the lesion revealed predominantly lobular panniculitis (Figure 2A) with fat necrosis and numerous foamy macrophages (Figures 2B and 2C). Scattered lymphocytes also were present but no neutrophils or eosinophils were noted (Figure 2B). Interlobular septa were widened secondary to fibrosis (Figure 2A). No lymphoid follicles were identified. A subcutaneous artery was sampled but was negative for vasculitis (Figure 2D).
|
The necrotic lesion on the left buttock was present for more than 2 months before complete healing occurred. The patient had a history of intolerance or unresponsiveness to all prior medications for RRMS. Several years prior she responded well to treatment with GA for a few months and had been responding well to the injections over the last year. Incremental challenge testing with GA for desensitization was offered to the patient, but she declined treatment out of fear of a recurrent episode, particularly the severe systemic symptoms she had experienced. Unfortunately, she was lost to follow-up.
Comment
Glatiramer acetate, formerly known as copolymer-1, is a first-line treatment of patients with RRMS.1 Daily administration of subcutaneous injections of GA (20 mg/mL) has proven effective in relapse rate reduction and reduced morbidity in patients with RRMS.2 Long-term studies support a relapse rate reduction of more than 50% in patients using GA.3 The most common adverse effects are LISRs.2 Systemic reactions following GA injection also are common. A much less common reaction is panniculitis followed by lipoatrophy and/or skin necrosis. Only a few instances of panniculitis-associated necrosis have been reported.
The occurrence of LISRs was reported in 20% to 90% of patients using GA to control RRMS.2,4 Local injection-site reactions typically resolve within hours to days and have been reported to decrease in frequency over time.5 Acute systemic reactions (eg, anxiety, flushing, palpitations, dyspnea) to GA injection are described in approximately 15% of patients.6 Systemic reactions usually resolve in 5 to 15 minutes but can last for more than 1 hour.5 These reactions are mostly benign and generally are not considered to be allergic or anaphylactic in nature. True systemic anaphylaxis associated with administration of GA is extremely rare.7
Lipoatrophy, or localized loss of subcutaneous adipose tissue without evidence of inflammation, has been reported fairly frequently in association with GA (up to 45% of patients receiving GA injections).2,6,8,9 Lipoatrophy also has been seen following subcutaneous injection of many other drugs, including steroids and insulin. Unlike LISRs, the likelihood of developing lipoatrophy at the injection site increases with longer durations of GA injections.5 Lipoatrophy also develops following panniculitis at the site of GA injection.
Based on a search of the MeSH (Medical Subject Headings) database using the terms panniculitis and glatiramer acetate, there only are 10 reported cases of panniculitis as an adverse effect of GA injections.2,6,10 Lesions were described as either subcutaneous erythematous nodules or atrophic areas that demonstrated panniculitis on histologic examination. Injections preceding the development of panniculitis often were described as remarkably painful.4 Residual lipoatrophy and/or hyperpigmentation at the site of panniculitis development is common.2 It has been suggested that GA-induced panniculitis may be an early underlying mechanism for the development of lipoatrophy, and thus may be more common than originally suspected.10
Histopathologic examination of GA-induced panniculitis typically reveals a localized, mostly lobular, panniculitis with lipophagic granulomas, lymphocytes, and thickened septa. The lipophagic granulomas (a characteristic finding in panniculitis) form from local macrophages that engulf the lipids released from necrotic adipocytes.11 A large, pale, granular or vacuolated cytoplasm typically can be observed on microscopic examination of the macrophages (Figure 2C). Connective tissue septa typically are widened with cell infiltrates, usually lymphocytes. Other cell types, including macrophages, eosinophils, and neutrophils, also have been identified in both the septa and fat lobules. These histologic elements may change and evolve over time.
Necrosis in association with panniculitis, as seen in our patient, rarely has been reported.4,12 All of the necrotic reactions described occurred after at least 1 year of GA therapy and took several weeks to resolve.4,12 When presented with the development of skin necrosis at the site of GA injection, it is essential to distinguish between an adverse effect associated with the drug itself and Nicolau syndrome (embolia cutis medicamentosa).13 Necrosis at multiple injection sites or recurrence with later injections supports a GA-specific effect.12
Nicolau syndrome is a well-known traumatic reaction that leads to microembolization and resultant vasospasm as well as necrosis throughout the skin and possibly the underlying muscular layer.14 Although more commonly associated with intramuscular injections, Nicolau syndrome has been described with subcutaneous injections of GA in a few rare instances.13,15 Because of the associated severe systemic reaction as well as the histologic examination (Figure 2D), we believe the skin necrosis seen in our patient was from a reaction to GA rather than Nicolau syndrome. Our patient was not interested in restarting GA therapy; therefore, it is unknown if this reaction would have recurred, but we suspect high probability of recurrence without desensitization attempts.
Preventative measures can be taken to decrease the risk for LISRs, and patients should be educated on these techniques. Applying ice to the injection site for at least 30 seconds before cleaning the skin for injection may reduce local adverse effects.4 Proper instruction on injection techniques should be provided by a knowledgeable health care professional and topical anesthetics and/or steroids may be offered to reduce pain associated with injection. There have been no proven measures for prevention of lipoatrophy, panniculitis, or necrosis, and these adverse effects are not thought to be attributed to improper injection techniques.14 Rotation of injection sites is the only suggested means of decreasing the potential risk for more severely and permanently disfiguring local reactions.
If panniculitis following GA injection is suspected, a large biopsy that encompasses the entire subcutaneous fat layer is necessary for proper dermatopathologic classification.11 Glatiramer acetate injections should be stopped immediately. These reactions disappear when the injections are stopped but recur when restarting treatment.2 The efficacy of GA in the treatment of RRMS has led to the possible use of this drug in the treatment of other autoimmune diseases.16 Thus, it is important for clinicians to be aware of all adverse effects of subcutaneous injections of GA, including the rare occurrence of panniculitis and necrosis, and when discontinuation of therapy is indicated.
Conclusion
Daily subcutaneous injection of GA for the treatment of RRMS can result in the rare but characteristic development of localized panniculitis and necrosis. Glatiramer acetate is a common and highly effective therapy used for the treatment of RRMS. Common adverse effects include LISRs and transient acute systemic reactions. Less commonly observed but characteristic of GA injections is localized lipoatrophy and mostly lobular panniculitis. Necrosis rarely can develop in association with these cutaneous reactions. It is essential to differentiate between necrosis secondary to Nicolau syndrome and skin necrosis as a unique reaction to GA; the latter is an indication for discontinuation of GA injections. Dermatologists should be made aware of adverse cutaneous reactions seen with GA therapy, especially with the potential for expansion of the use of GA to treat other autoimmune processes. Further research is needed regarding the histopathologic evolution and mechanisms behind the development of lipoatrophy, panniculitis, and necrosis at the site of GA injection.
1. Anderson G, Meyer D, Herrman CE, et al. Tolerability and safety of novel half milliliter formulation of glatiramer acetate for subcutaneous injection: an open-label, multicenter, randomized comparative study. J Neurol. 2010;257:1917-1923.
2. Soares Almeida LM, Requena L, Kutzner H, et al. Localized panniculitis secondary to subcutaneous glatiramer acetate injections for the treatment of multiple sclerosis: a clinicopathologic and immunohistochemical study. J Am Acad Dermatol. 2006;55:968-974.
3. Ford CC, Johnson KP, Lisak RP, et al. A prospective open-label study of glatiramer acetate: over a decade of continuous use in multiple sclerosis patients. Mult Scler. 2006;12:309-320.
4. Frohman EM, Brannon K, Alexander S, et al. Disease modifying agent related skin reactions in multiple sclerosis: prevention, assessment, and management. Mult Scler. 2004;10:302-307.
5. Ziemssen T, Neuhaus O, Hohlfeld R. Risk-benefit assessment of glatiramer acetate in multiple sclerosis. Drug Saf. 2001;24:979-990.
6. Ball NJ, Cowan BJ, Moore GR, et al. Lobular panniculitis at the site of glatiramer acetate injections for the treatment of relapsing-remitting multiple sclerosis. a report of two cases. J Cutan Pathol. 2008;35:407-410.
7. Rauschka H, Farina C, Sator P, et al. Severe anaphylactic reaction to glatiramer acetate with specific IgE. Neurology. 2005;64:1481-1482.
8. Hwang L, Orengo I. Lipoatrophy associated with glatiramer acetate injections for the treatment of multiple sclerosis. Cutis. 2001;68:287-288.
9. Edgar CM, Brunet DG, Fenton P, et al. Lipoatrophy in patients with multiple sclerosis on glatiramer acetate. Can J Neurol Sci. 2004;31:58-63.
10. Soós N, Shakery K, Mrowietz U. Localized panniculitis and subsequent lipoatrophy with subcutaneous glatiramer acetate (Copaxone) injection for the treatment of multiple sclerosis. Am J Clin Dermatol. 2004;5:357-359.
11. Segura S, Requena L. Anatomy and histology of normal subcutaneous fat, necrosis of adipocytes, and classification of the panniculitides. Dermatol Clin. 2008;26:419-424, v.
12. Bosca I, Bosca M, Belenguer A, et al. Necrotising cutaneous lesions as a side effect of glatiramer acetate. J Neurol. 2006;253:1370-1371.
13. Feldmann R, Schierl M, Rauschka H, et al. Necrotizing skin lesions with involvement of muscle tissue after subcutaneous injection of glatiramer acetate. Eur J Dermatol. 2009;19:385.
14. Kluger N, Thouvenot E, Camu W, et al. Cutaneous adverse events related to glatiramer acetate injection (copolymer-1, Copaxone). J Eur Acad Dermatol Venereol. 2009;23:1332-1333.
15. Harde V, Schwarz T. Embolia cutis medicamentosa following subcutaneous injection of glatiramer acetate [in English, German]. J Dtsch Dermatol Ges. 2007;5:1122-1123.
16. Racke MK, Lovett-Racke AE. Glatiramer acetate treatment of multiple sclerosis: an immunological perspective. J Immunol. 2011;186:1887-1890.
Glatiramer acetate (GA), a synthetic polypeptide that is injected subcutaneously, has proven effective in the treatment of relapsing-remitting multiple sclerosis (RRMS) and is now considered a first-line agent in the treatment of this condition. Adverse effects associated with GA primarily include local injection-site reactions (LISRs)(eg, erythema, pruritus, burning, pain, inflammation). Transient acute systemic reactions such as flushing and dyspnea also are commonly reported. Lipoatrophy at the injection site frequently has been reported in the literature as a cutaneous adverse effect of GA, but lobular panniculitis and necrosis at the site of injection rarely have been noted.
We report the case of a 36-year-old woman who experienced a severe adverse reaction to a single injection of GA after nearly 1 year of daily use to control symptoms of RRMS. Review of the current literature revealed few reports of the severe reaction of panniculitis and necrosis occurring at the injection site of GA.
Case Report
A 36-year-old woman was referred by her neurologist to the emergency department of our institution’s allergy and immunology clinic for treatment of an allergic reaction to a 20-mg GA injection, which she had been receiving daily for nearly 1 year as therapy for RRMS. A nodule immediately formed at the injection site and eventually became ulcerated. The patient also reported intense chest tightness, shortness of breath, and flushing following the injection. Physical examination revealed a large 8- to 9-cm erythematous area at the injection site on the left buttock. Necrosis and eschar formation also were evident (Figure 1).
Figure 1. Panniculitis with central ulceration and necrosis at the site of a glatiramer acetate injection on the left buttock (A). Closer view of an irregularly shaped necrotic lesion with surrounding erythema (B). |
A punch biopsy from the edge of the lesion revealed predominantly lobular panniculitis (Figure 2A) with fat necrosis and numerous foamy macrophages (Figures 2B and 2C). Scattered lymphocytes also were present but no neutrophils or eosinophils were noted (Figure 2B). Interlobular septa were widened secondary to fibrosis (Figure 2A). No lymphoid follicles were identified. A subcutaneous artery was sampled but was negative for vasculitis (Figure 2D).
|
The necrotic lesion on the left buttock was present for more than 2 months before complete healing occurred. The patient had a history of intolerance or unresponsiveness to all prior medications for RRMS. Several years prior she responded well to treatment with GA for a few months and had been responding well to the injections over the last year. Incremental challenge testing with GA for desensitization was offered to the patient, but she declined treatment out of fear of a recurrent episode, particularly the severe systemic symptoms she had experienced. Unfortunately, she was lost to follow-up.
Comment
Glatiramer acetate, formerly known as copolymer-1, is a first-line treatment of patients with RRMS.1 Daily administration of subcutaneous injections of GA (20 mg/mL) has proven effective in relapse rate reduction and reduced morbidity in patients with RRMS.2 Long-term studies support a relapse rate reduction of more than 50% in patients using GA.3 The most common adverse effects are LISRs.2 Systemic reactions following GA injection also are common. A much less common reaction is panniculitis followed by lipoatrophy and/or skin necrosis. Only a few instances of panniculitis-associated necrosis have been reported.
The occurrence of LISRs was reported in 20% to 90% of patients using GA to control RRMS.2,4 Local injection-site reactions typically resolve within hours to days and have been reported to decrease in frequency over time.5 Acute systemic reactions (eg, anxiety, flushing, palpitations, dyspnea) to GA injection are described in approximately 15% of patients.6 Systemic reactions usually resolve in 5 to 15 minutes but can last for more than 1 hour.5 These reactions are mostly benign and generally are not considered to be allergic or anaphylactic in nature. True systemic anaphylaxis associated with administration of GA is extremely rare.7
Lipoatrophy, or localized loss of subcutaneous adipose tissue without evidence of inflammation, has been reported fairly frequently in association with GA (up to 45% of patients receiving GA injections).2,6,8,9 Lipoatrophy also has been seen following subcutaneous injection of many other drugs, including steroids and insulin. Unlike LISRs, the likelihood of developing lipoatrophy at the injection site increases with longer durations of GA injections.5 Lipoatrophy also develops following panniculitis at the site of GA injection.
Based on a search of the MeSH (Medical Subject Headings) database using the terms panniculitis and glatiramer acetate, there only are 10 reported cases of panniculitis as an adverse effect of GA injections.2,6,10 Lesions were described as either subcutaneous erythematous nodules or atrophic areas that demonstrated panniculitis on histologic examination. Injections preceding the development of panniculitis often were described as remarkably painful.4 Residual lipoatrophy and/or hyperpigmentation at the site of panniculitis development is common.2 It has been suggested that GA-induced panniculitis may be an early underlying mechanism for the development of lipoatrophy, and thus may be more common than originally suspected.10
Histopathologic examination of GA-induced panniculitis typically reveals a localized, mostly lobular, panniculitis with lipophagic granulomas, lymphocytes, and thickened septa. The lipophagic granulomas (a characteristic finding in panniculitis) form from local macrophages that engulf the lipids released from necrotic adipocytes.11 A large, pale, granular or vacuolated cytoplasm typically can be observed on microscopic examination of the macrophages (Figure 2C). Connective tissue septa typically are widened with cell infiltrates, usually lymphocytes. Other cell types, including macrophages, eosinophils, and neutrophils, also have been identified in both the septa and fat lobules. These histologic elements may change and evolve over time.
Necrosis in association with panniculitis, as seen in our patient, rarely has been reported.4,12 All of the necrotic reactions described occurred after at least 1 year of GA therapy and took several weeks to resolve.4,12 When presented with the development of skin necrosis at the site of GA injection, it is essential to distinguish between an adverse effect associated with the drug itself and Nicolau syndrome (embolia cutis medicamentosa).13 Necrosis at multiple injection sites or recurrence with later injections supports a GA-specific effect.12
Nicolau syndrome is a well-known traumatic reaction that leads to microembolization and resultant vasospasm as well as necrosis throughout the skin and possibly the underlying muscular layer.14 Although more commonly associated with intramuscular injections, Nicolau syndrome has been described with subcutaneous injections of GA in a few rare instances.13,15 Because of the associated severe systemic reaction as well as the histologic examination (Figure 2D), we believe the skin necrosis seen in our patient was from a reaction to GA rather than Nicolau syndrome. Our patient was not interested in restarting GA therapy; therefore, it is unknown if this reaction would have recurred, but we suspect high probability of recurrence without desensitization attempts.
Preventative measures can be taken to decrease the risk for LISRs, and patients should be educated on these techniques. Applying ice to the injection site for at least 30 seconds before cleaning the skin for injection may reduce local adverse effects.4 Proper instruction on injection techniques should be provided by a knowledgeable health care professional and topical anesthetics and/or steroids may be offered to reduce pain associated with injection. There have been no proven measures for prevention of lipoatrophy, panniculitis, or necrosis, and these adverse effects are not thought to be attributed to improper injection techniques.14 Rotation of injection sites is the only suggested means of decreasing the potential risk for more severely and permanently disfiguring local reactions.
If panniculitis following GA injection is suspected, a large biopsy that encompasses the entire subcutaneous fat layer is necessary for proper dermatopathologic classification.11 Glatiramer acetate injections should be stopped immediately. These reactions disappear when the injections are stopped but recur when restarting treatment.2 The efficacy of GA in the treatment of RRMS has led to the possible use of this drug in the treatment of other autoimmune diseases.16 Thus, it is important for clinicians to be aware of all adverse effects of subcutaneous injections of GA, including the rare occurrence of panniculitis and necrosis, and when discontinuation of therapy is indicated.
Conclusion
Daily subcutaneous injection of GA for the treatment of RRMS can result in the rare but characteristic development of localized panniculitis and necrosis. Glatiramer acetate is a common and highly effective therapy used for the treatment of RRMS. Common adverse effects include LISRs and transient acute systemic reactions. Less commonly observed but characteristic of GA injections is localized lipoatrophy and mostly lobular panniculitis. Necrosis rarely can develop in association with these cutaneous reactions. It is essential to differentiate between necrosis secondary to Nicolau syndrome and skin necrosis as a unique reaction to GA; the latter is an indication for discontinuation of GA injections. Dermatologists should be made aware of adverse cutaneous reactions seen with GA therapy, especially with the potential for expansion of the use of GA to treat other autoimmune processes. Further research is needed regarding the histopathologic evolution and mechanisms behind the development of lipoatrophy, panniculitis, and necrosis at the site of GA injection.
Glatiramer acetate (GA), a synthetic polypeptide that is injected subcutaneously, has proven effective in the treatment of relapsing-remitting multiple sclerosis (RRMS) and is now considered a first-line agent in the treatment of this condition. Adverse effects associated with GA primarily include local injection-site reactions (LISRs)(eg, erythema, pruritus, burning, pain, inflammation). Transient acute systemic reactions such as flushing and dyspnea also are commonly reported. Lipoatrophy at the injection site frequently has been reported in the literature as a cutaneous adverse effect of GA, but lobular panniculitis and necrosis at the site of injection rarely have been noted.
We report the case of a 36-year-old woman who experienced a severe adverse reaction to a single injection of GA after nearly 1 year of daily use to control symptoms of RRMS. Review of the current literature revealed few reports of the severe reaction of panniculitis and necrosis occurring at the injection site of GA.
Case Report
A 36-year-old woman was referred by her neurologist to the emergency department of our institution’s allergy and immunology clinic for treatment of an allergic reaction to a 20-mg GA injection, which she had been receiving daily for nearly 1 year as therapy for RRMS. A nodule immediately formed at the injection site and eventually became ulcerated. The patient also reported intense chest tightness, shortness of breath, and flushing following the injection. Physical examination revealed a large 8- to 9-cm erythematous area at the injection site on the left buttock. Necrosis and eschar formation also were evident (Figure 1).
Figure 1. Panniculitis with central ulceration and necrosis at the site of a glatiramer acetate injection on the left buttock (A). Closer view of an irregularly shaped necrotic lesion with surrounding erythema (B). |
A punch biopsy from the edge of the lesion revealed predominantly lobular panniculitis (Figure 2A) with fat necrosis and numerous foamy macrophages (Figures 2B and 2C). Scattered lymphocytes also were present but no neutrophils or eosinophils were noted (Figure 2B). Interlobular septa were widened secondary to fibrosis (Figure 2A). No lymphoid follicles were identified. A subcutaneous artery was sampled but was negative for vasculitis (Figure 2D).
|
The necrotic lesion on the left buttock was present for more than 2 months before complete healing occurred. The patient had a history of intolerance or unresponsiveness to all prior medications for RRMS. Several years prior she responded well to treatment with GA for a few months and had been responding well to the injections over the last year. Incremental challenge testing with GA for desensitization was offered to the patient, but she declined treatment out of fear of a recurrent episode, particularly the severe systemic symptoms she had experienced. Unfortunately, she was lost to follow-up.
Comment
Glatiramer acetate, formerly known as copolymer-1, is a first-line treatment of patients with RRMS.1 Daily administration of subcutaneous injections of GA (20 mg/mL) has proven effective in relapse rate reduction and reduced morbidity in patients with RRMS.2 Long-term studies support a relapse rate reduction of more than 50% in patients using GA.3 The most common adverse effects are LISRs.2 Systemic reactions following GA injection also are common. A much less common reaction is panniculitis followed by lipoatrophy and/or skin necrosis. Only a few instances of panniculitis-associated necrosis have been reported.
The occurrence of LISRs was reported in 20% to 90% of patients using GA to control RRMS.2,4 Local injection-site reactions typically resolve within hours to days and have been reported to decrease in frequency over time.5 Acute systemic reactions (eg, anxiety, flushing, palpitations, dyspnea) to GA injection are described in approximately 15% of patients.6 Systemic reactions usually resolve in 5 to 15 minutes but can last for more than 1 hour.5 These reactions are mostly benign and generally are not considered to be allergic or anaphylactic in nature. True systemic anaphylaxis associated with administration of GA is extremely rare.7
Lipoatrophy, or localized loss of subcutaneous adipose tissue without evidence of inflammation, has been reported fairly frequently in association with GA (up to 45% of patients receiving GA injections).2,6,8,9 Lipoatrophy also has been seen following subcutaneous injection of many other drugs, including steroids and insulin. Unlike LISRs, the likelihood of developing lipoatrophy at the injection site increases with longer durations of GA injections.5 Lipoatrophy also develops following panniculitis at the site of GA injection.
Based on a search of the MeSH (Medical Subject Headings) database using the terms panniculitis and glatiramer acetate, there only are 10 reported cases of panniculitis as an adverse effect of GA injections.2,6,10 Lesions were described as either subcutaneous erythematous nodules or atrophic areas that demonstrated panniculitis on histologic examination. Injections preceding the development of panniculitis often were described as remarkably painful.4 Residual lipoatrophy and/or hyperpigmentation at the site of panniculitis development is common.2 It has been suggested that GA-induced panniculitis may be an early underlying mechanism for the development of lipoatrophy, and thus may be more common than originally suspected.10
Histopathologic examination of GA-induced panniculitis typically reveals a localized, mostly lobular, panniculitis with lipophagic granulomas, lymphocytes, and thickened septa. The lipophagic granulomas (a characteristic finding in panniculitis) form from local macrophages that engulf the lipids released from necrotic adipocytes.11 A large, pale, granular or vacuolated cytoplasm typically can be observed on microscopic examination of the macrophages (Figure 2C). Connective tissue septa typically are widened with cell infiltrates, usually lymphocytes. Other cell types, including macrophages, eosinophils, and neutrophils, also have been identified in both the septa and fat lobules. These histologic elements may change and evolve over time.
Necrosis in association with panniculitis, as seen in our patient, rarely has been reported.4,12 All of the necrotic reactions described occurred after at least 1 year of GA therapy and took several weeks to resolve.4,12 When presented with the development of skin necrosis at the site of GA injection, it is essential to distinguish between an adverse effect associated with the drug itself and Nicolau syndrome (embolia cutis medicamentosa).13 Necrosis at multiple injection sites or recurrence with later injections supports a GA-specific effect.12
Nicolau syndrome is a well-known traumatic reaction that leads to microembolization and resultant vasospasm as well as necrosis throughout the skin and possibly the underlying muscular layer.14 Although more commonly associated with intramuscular injections, Nicolau syndrome has been described with subcutaneous injections of GA in a few rare instances.13,15 Because of the associated severe systemic reaction as well as the histologic examination (Figure 2D), we believe the skin necrosis seen in our patient was from a reaction to GA rather than Nicolau syndrome. Our patient was not interested in restarting GA therapy; therefore, it is unknown if this reaction would have recurred, but we suspect high probability of recurrence without desensitization attempts.
Preventative measures can be taken to decrease the risk for LISRs, and patients should be educated on these techniques. Applying ice to the injection site for at least 30 seconds before cleaning the skin for injection may reduce local adverse effects.4 Proper instruction on injection techniques should be provided by a knowledgeable health care professional and topical anesthetics and/or steroids may be offered to reduce pain associated with injection. There have been no proven measures for prevention of lipoatrophy, panniculitis, or necrosis, and these adverse effects are not thought to be attributed to improper injection techniques.14 Rotation of injection sites is the only suggested means of decreasing the potential risk for more severely and permanently disfiguring local reactions.
If panniculitis following GA injection is suspected, a large biopsy that encompasses the entire subcutaneous fat layer is necessary for proper dermatopathologic classification.11 Glatiramer acetate injections should be stopped immediately. These reactions disappear when the injections are stopped but recur when restarting treatment.2 The efficacy of GA in the treatment of RRMS has led to the possible use of this drug in the treatment of other autoimmune diseases.16 Thus, it is important for clinicians to be aware of all adverse effects of subcutaneous injections of GA, including the rare occurrence of panniculitis and necrosis, and when discontinuation of therapy is indicated.
Conclusion
Daily subcutaneous injection of GA for the treatment of RRMS can result in the rare but characteristic development of localized panniculitis and necrosis. Glatiramer acetate is a common and highly effective therapy used for the treatment of RRMS. Common adverse effects include LISRs and transient acute systemic reactions. Less commonly observed but characteristic of GA injections is localized lipoatrophy and mostly lobular panniculitis. Necrosis rarely can develop in association with these cutaneous reactions. It is essential to differentiate between necrosis secondary to Nicolau syndrome and skin necrosis as a unique reaction to GA; the latter is an indication for discontinuation of GA injections. Dermatologists should be made aware of adverse cutaneous reactions seen with GA therapy, especially with the potential for expansion of the use of GA to treat other autoimmune processes. Further research is needed regarding the histopathologic evolution and mechanisms behind the development of lipoatrophy, panniculitis, and necrosis at the site of GA injection.
1. Anderson G, Meyer D, Herrman CE, et al. Tolerability and safety of novel half milliliter formulation of glatiramer acetate for subcutaneous injection: an open-label, multicenter, randomized comparative study. J Neurol. 2010;257:1917-1923.
2. Soares Almeida LM, Requena L, Kutzner H, et al. Localized panniculitis secondary to subcutaneous glatiramer acetate injections for the treatment of multiple sclerosis: a clinicopathologic and immunohistochemical study. J Am Acad Dermatol. 2006;55:968-974.
3. Ford CC, Johnson KP, Lisak RP, et al. A prospective open-label study of glatiramer acetate: over a decade of continuous use in multiple sclerosis patients. Mult Scler. 2006;12:309-320.
4. Frohman EM, Brannon K, Alexander S, et al. Disease modifying agent related skin reactions in multiple sclerosis: prevention, assessment, and management. Mult Scler. 2004;10:302-307.
5. Ziemssen T, Neuhaus O, Hohlfeld R. Risk-benefit assessment of glatiramer acetate in multiple sclerosis. Drug Saf. 2001;24:979-990.
6. Ball NJ, Cowan BJ, Moore GR, et al. Lobular panniculitis at the site of glatiramer acetate injections for the treatment of relapsing-remitting multiple sclerosis. a report of two cases. J Cutan Pathol. 2008;35:407-410.
7. Rauschka H, Farina C, Sator P, et al. Severe anaphylactic reaction to glatiramer acetate with specific IgE. Neurology. 2005;64:1481-1482.
8. Hwang L, Orengo I. Lipoatrophy associated with glatiramer acetate injections for the treatment of multiple sclerosis. Cutis. 2001;68:287-288.
9. Edgar CM, Brunet DG, Fenton P, et al. Lipoatrophy in patients with multiple sclerosis on glatiramer acetate. Can J Neurol Sci. 2004;31:58-63.
10. Soós N, Shakery K, Mrowietz U. Localized panniculitis and subsequent lipoatrophy with subcutaneous glatiramer acetate (Copaxone) injection for the treatment of multiple sclerosis. Am J Clin Dermatol. 2004;5:357-359.
11. Segura S, Requena L. Anatomy and histology of normal subcutaneous fat, necrosis of adipocytes, and classification of the panniculitides. Dermatol Clin. 2008;26:419-424, v.
12. Bosca I, Bosca M, Belenguer A, et al. Necrotising cutaneous lesions as a side effect of glatiramer acetate. J Neurol. 2006;253:1370-1371.
13. Feldmann R, Schierl M, Rauschka H, et al. Necrotizing skin lesions with involvement of muscle tissue after subcutaneous injection of glatiramer acetate. Eur J Dermatol. 2009;19:385.
14. Kluger N, Thouvenot E, Camu W, et al. Cutaneous adverse events related to glatiramer acetate injection (copolymer-1, Copaxone). J Eur Acad Dermatol Venereol. 2009;23:1332-1333.
15. Harde V, Schwarz T. Embolia cutis medicamentosa following subcutaneous injection of glatiramer acetate [in English, German]. J Dtsch Dermatol Ges. 2007;5:1122-1123.
16. Racke MK, Lovett-Racke AE. Glatiramer acetate treatment of multiple sclerosis: an immunological perspective. J Immunol. 2011;186:1887-1890.
1. Anderson G, Meyer D, Herrman CE, et al. Tolerability and safety of novel half milliliter formulation of glatiramer acetate for subcutaneous injection: an open-label, multicenter, randomized comparative study. J Neurol. 2010;257:1917-1923.
2. Soares Almeida LM, Requena L, Kutzner H, et al. Localized panniculitis secondary to subcutaneous glatiramer acetate injections for the treatment of multiple sclerosis: a clinicopathologic and immunohistochemical study. J Am Acad Dermatol. 2006;55:968-974.
3. Ford CC, Johnson KP, Lisak RP, et al. A prospective open-label study of glatiramer acetate: over a decade of continuous use in multiple sclerosis patients. Mult Scler. 2006;12:309-320.
4. Frohman EM, Brannon K, Alexander S, et al. Disease modifying agent related skin reactions in multiple sclerosis: prevention, assessment, and management. Mult Scler. 2004;10:302-307.
5. Ziemssen T, Neuhaus O, Hohlfeld R. Risk-benefit assessment of glatiramer acetate in multiple sclerosis. Drug Saf. 2001;24:979-990.
6. Ball NJ, Cowan BJ, Moore GR, et al. Lobular panniculitis at the site of glatiramer acetate injections for the treatment of relapsing-remitting multiple sclerosis. a report of two cases. J Cutan Pathol. 2008;35:407-410.
7. Rauschka H, Farina C, Sator P, et al. Severe anaphylactic reaction to glatiramer acetate with specific IgE. Neurology. 2005;64:1481-1482.
8. Hwang L, Orengo I. Lipoatrophy associated with glatiramer acetate injections for the treatment of multiple sclerosis. Cutis. 2001;68:287-288.
9. Edgar CM, Brunet DG, Fenton P, et al. Lipoatrophy in patients with multiple sclerosis on glatiramer acetate. Can J Neurol Sci. 2004;31:58-63.
10. Soós N, Shakery K, Mrowietz U. Localized panniculitis and subsequent lipoatrophy with subcutaneous glatiramer acetate (Copaxone) injection for the treatment of multiple sclerosis. Am J Clin Dermatol. 2004;5:357-359.
11. Segura S, Requena L. Anatomy and histology of normal subcutaneous fat, necrosis of adipocytes, and classification of the panniculitides. Dermatol Clin. 2008;26:419-424, v.
12. Bosca I, Bosca M, Belenguer A, et al. Necrotising cutaneous lesions as a side effect of glatiramer acetate. J Neurol. 2006;253:1370-1371.
13. Feldmann R, Schierl M, Rauschka H, et al. Necrotizing skin lesions with involvement of muscle tissue after subcutaneous injection of glatiramer acetate. Eur J Dermatol. 2009;19:385.
14. Kluger N, Thouvenot E, Camu W, et al. Cutaneous adverse events related to glatiramer acetate injection (copolymer-1, Copaxone). J Eur Acad Dermatol Venereol. 2009;23:1332-1333.
15. Harde V, Schwarz T. Embolia cutis medicamentosa following subcutaneous injection of glatiramer acetate [in English, German]. J Dtsch Dermatol Ges. 2007;5:1122-1123.
16. Racke MK, Lovett-Racke AE. Glatiramer acetate treatment of multiple sclerosis: an immunological perspective. J Immunol. 2011;186:1887-1890.
Practice Points
- Glatiramer acetate is a common and highly effective therapy administered subcutaneously for the treatment of relapsing-remitting multiple sclerosis.
- Common adverse effects include local injection-site reactions and transient acute systemic reactions.
- Rarely, localized lipoatrophy and mostly lobular panniculitis with occasional necrosis can be observed at the site of glatiramer acetate injections. This reaction is specific to the medication and can recur with subsequent injections.
Weight loss • diarrhea • mild eosinophilia • Dx?
THE CASE
A 31-year-old man came to an internal medicine clinic because he’d been losing weight over the past 2 years and hadn’t been able to regain any weight despite eating properly. Our patient was born in Ethiopia, but had been living in Canada for 6 years. He reported a remote history of 2 episodes of diarrhea.
His physical exam was normal and laboratory results revealed mild eosinophilia of 0.6 × 109/L (normal range, <0.45 × 109/L). Additional tests (including complete blood count, electrolytes, liver panel, thyrotropin, and blood smear) revealed no apparent metabolic causes of the patient’s weight loss. Stool analysis (3 exams) was negative for ova and parasites.
THE DIAGNOSIS
Because our patient was born in Ethiopia, we did serologic testing for Strongyloides, which was positive (enzyme-linked immunosorbent assay for immunoglobulin G antibodies [IgG-ELISA] was 2.9; positive is >2.1). We diagnosed strongyloidiasis in this patient.
DISCUSSION
Strongyloidiasis is an infection caused by the parasite Strongyloides stercoralis.1 It affects an estimated 30 to 100 million people worldwide, mainly in Africa, Southeast Asia, Central America, and South America, but it also can occur in temperate climates.2Strongyloides is a soil-transmitted helminth (parasitic worm). The prevalence of Strongyloides infection among refugee groups in the United States is 1% to 4.3%.3-5
Although patients with strongyloidiasis are often asymptomatic, they can present with a wide range of nonspecific symptoms. In the acute stage, patients may develop signs and symptoms including cough, wheeze, abdominal pain, weight loss, diarrhea, pruritus ani, and larva currens.2 Respiratory symptoms, including tracheal irritation and a dry cough, are often confused with asthma. In the generally asymptomatic chronic stage, patients may develop gastrointestinal complaints, such as epigastric pain and heartburn.6
Hyperinfection syndrome can occur when patients with subclinical infection receive high doses of corticosteroids for asthma or chronic obstructive pulmonary disease exacerbations. Risk of hyperinfection is increased among immunocompromised patients with human T lymphotropic virus type-1 (HTLV-1),7 as well as in patients with malignancies, malnutrition, and alcohol use disorder. Eosinophilia is often absent in patients with hyperinfection, and stool examination results are almost always positive.8
Who to screen, how to make the diagnosis
The presence of eosinophilia in immigrants, refugees, and travelers from endemic regions should alert clinicians to the possibility of an underlying helminth infection. However, because eosinophilia occurs intermittently in response to tissue invasion, absence of eosinophilia does not exclude strongyloidiasis.
The Canadian Collaboration for Immigrants and Refugee Health (CCIRH) recommends using serologic testing to screen for Strongyloides in all newly arrived refugees from low-income countries in Southeast Asia and Africa.9 The CCIRH also advises that while data on the burden of strongyloidiasis in non-refugee immigrant populations is limited, you should consider screening foreign-born individuals who have lived in endemic areas, have symptoms and/or signs of Strongyloides infection, and/or have evidence of eosinophilia.9 Because the risk of hyperinfection is increased in immunocompromised individuals, screening should be done to detect Strongyloides infection before starting chemotherapy and before initiating corticosteroids in patients from endemic areas.10
Diagnostic methods. Stool examination6 and IgG-ELISA2 are the main methods used to diagnose strongyloidiasis. However, traditional stool examinations have low sensitivity, and it may require up to 7 stool exams to reach a sensitivity of 100%,6 which could explain why our patient’s stool analysis was negative for parasites. In our experience, a positive serology result should always be assumed to indicate an active infection unless there is a well documented history of prior therapy. (In such cases, a positive serology result could represent persistent antibodies following therapy.)
First-line therapy and alternative treatment
All patients with strongyloidiasis, regardless of whether they are symptomatic, must be treated to prevent possible late-onset disseminated disease and hyperinfection.9 The Centers for Disease Control and Prevention recommends one to 2 doses of ivermectin 200 mcg/kg as first-line therapy or albendazole 400 mg twice daily for 3 days as an alternative treatment (TABLE).11 Ivermectin cures more than 95% of cases.12 Albendazole has lower efficacy (78%).13 Some experts recommend administering the 2 doses of ivermectin 2 weeks apart to allow enough time for the parasite to migrate to the gut.4
Coinfection with HTLV-1 (which is endemic in areas where Strongyloides also is
endemic) modifies patients’ immune response and can complicate treatment.9 Clinicians should screen strongyloidiasis patients for HTLV-1 if they come from high-prevalence areas and/or have persistent strongyloidiasis that responds poorly to antiparasitic treatment.9
Consider referral to an infectious disease specialist for patients coinfected with
HTLV-1, as well as those who are immunocompromised. Such referral also may be appropriate for patients from countries where loa loa is endemic, because encephalopathy has occurred in patients coinfected with loa loa who were treated with ivermectin.10
Our patient was treated with 2 doses of ivermectin 200 mcg/kg, 2 weeks apart. Four months later, his eosinophilia had resolved, his IgG-ELISA dropped to 0.37, and he had gained 2.5 pounds.
THE TAKEAWAY
Strongyloidiasis is an infection caused by the parasitic worm Strongyloides stercoralis that is most common in tropical or subtropical areas. It can be asymptomatic or present with a wide range of nonspecific signs and symptoms, such as eosinophilia, cough, wheeze, abdominal pain, weight loss, diarrhea, pruritus ani, and larva currens. It is diagnosed by stool examination and serologic testing. Ivermectin is first-line therapy; albendazole is an alternative.
1. World Health Organization. Strongyloidiasis. World Health Organization Web site. Available at: http://www.who.int/neglected_diseases/diseases/strongyloidiasis/en/. Accessed January 29, 2015.
2. Lim S, Katz K, Krajden S, et al. Complicated and fatal Strongyloides infection in Canadians: risk factors, diagnosis and management. CMAJ. 2004;171:479-484.
3. Lifson AR, Thai D, O’Fallon A, et al. Prevalence of tuberculosis, hepatitis B virus, and intestinal parasitic infections among refugees to Minnesota. Public Health Rep. 2002;117:69-77.
4. Miller JM, Boyd HA, Ostrowski SR, et al. Malaria, intestinal parasites, and schistosomiasis among Barawan Somali refugees resettling to the United States: a strategy to reduce morbidity and decrease the risk of imported infections. Am J Trop Med Hyg. 2000;62:115-121.
5. Molina CD, Molina MM, Molina JM. Intestinal parasites in southeast Asian refugees two years after immigration. West J Med. 1988;149:422-425.
6. Centers for Disease Control and Prevention. Parasites – strongyloides. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/parasites/strongyloides/health_professionals/index.html. Accessed February 3, 2015.
7. Requena-Méndez A, Chiodini P, Bisoffi Z, et al. The laboratory diagnosis and follow up of strongyloidiasis: a systemic review. PLoS Negl Trop Dis. 2013;7:e2002.
8. Mirdha BR. Human strogyloidiasis: often brushed under the carpet. Trop Gastroenterol. 2009;30:1-4.
9. Pottie K, Greenaway C, Feightner J, et al; Canadian Collaboration for Immigrant and Refugee Health. Evidence-based clinical guidelines for immigrants and refugees. CMAJ. 2011;183:E824-E925.
10. Lagacé-Wiens PR, Harding GK. A Canadian immigrant with coinfection of Strongyloides stercoralis and human T-lymphotropic virus 1. CMAJ. 2007;177:451-453.
11. Centers for Disease Control and Prevention. Guidelines for overseas presumptive treatment of strongyloidiasis, schistosomiasis, and soil-transmitted helminth infections. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/overseas/intestinal-parasites-overseas.html. Accessed January 29, 2015.
12. Igual-Adell R, Oltra-Alcaraz C, Soler-Company E, et al. Efficacy and safety of ivermectin and thiabendazole in the treatment of strongyloidiasis. Expert Opin Pharmacother. 2004;5:2615-2619.
13. Horton J. Albendazole: a review of antihelmintic efficacy and safety in humans. Parasitology. 2000;121:S113-S132.
THE CASE
A 31-year-old man came to an internal medicine clinic because he’d been losing weight over the past 2 years and hadn’t been able to regain any weight despite eating properly. Our patient was born in Ethiopia, but had been living in Canada for 6 years. He reported a remote history of 2 episodes of diarrhea.
His physical exam was normal and laboratory results revealed mild eosinophilia of 0.6 × 109/L (normal range, <0.45 × 109/L). Additional tests (including complete blood count, electrolytes, liver panel, thyrotropin, and blood smear) revealed no apparent metabolic causes of the patient’s weight loss. Stool analysis (3 exams) was negative for ova and parasites.
THE DIAGNOSIS
Because our patient was born in Ethiopia, we did serologic testing for Strongyloides, which was positive (enzyme-linked immunosorbent assay for immunoglobulin G antibodies [IgG-ELISA] was 2.9; positive is >2.1). We diagnosed strongyloidiasis in this patient.
DISCUSSION
Strongyloidiasis is an infection caused by the parasite Strongyloides stercoralis.1 It affects an estimated 30 to 100 million people worldwide, mainly in Africa, Southeast Asia, Central America, and South America, but it also can occur in temperate climates.2Strongyloides is a soil-transmitted helminth (parasitic worm). The prevalence of Strongyloides infection among refugee groups in the United States is 1% to 4.3%.3-5
Although patients with strongyloidiasis are often asymptomatic, they can present with a wide range of nonspecific symptoms. In the acute stage, patients may develop signs and symptoms including cough, wheeze, abdominal pain, weight loss, diarrhea, pruritus ani, and larva currens.2 Respiratory symptoms, including tracheal irritation and a dry cough, are often confused with asthma. In the generally asymptomatic chronic stage, patients may develop gastrointestinal complaints, such as epigastric pain and heartburn.6
Hyperinfection syndrome can occur when patients with subclinical infection receive high doses of corticosteroids for asthma or chronic obstructive pulmonary disease exacerbations. Risk of hyperinfection is increased among immunocompromised patients with human T lymphotropic virus type-1 (HTLV-1),7 as well as in patients with malignancies, malnutrition, and alcohol use disorder. Eosinophilia is often absent in patients with hyperinfection, and stool examination results are almost always positive.8
Who to screen, how to make the diagnosis
The presence of eosinophilia in immigrants, refugees, and travelers from endemic regions should alert clinicians to the possibility of an underlying helminth infection. However, because eosinophilia occurs intermittently in response to tissue invasion, absence of eosinophilia does not exclude strongyloidiasis.
The Canadian Collaboration for Immigrants and Refugee Health (CCIRH) recommends using serologic testing to screen for Strongyloides in all newly arrived refugees from low-income countries in Southeast Asia and Africa.9 The CCIRH also advises that while data on the burden of strongyloidiasis in non-refugee immigrant populations is limited, you should consider screening foreign-born individuals who have lived in endemic areas, have symptoms and/or signs of Strongyloides infection, and/or have evidence of eosinophilia.9 Because the risk of hyperinfection is increased in immunocompromised individuals, screening should be done to detect Strongyloides infection before starting chemotherapy and before initiating corticosteroids in patients from endemic areas.10
Diagnostic methods. Stool examination6 and IgG-ELISA2 are the main methods used to diagnose strongyloidiasis. However, traditional stool examinations have low sensitivity, and it may require up to 7 stool exams to reach a sensitivity of 100%,6 which could explain why our patient’s stool analysis was negative for parasites. In our experience, a positive serology result should always be assumed to indicate an active infection unless there is a well documented history of prior therapy. (In such cases, a positive serology result could represent persistent antibodies following therapy.)
First-line therapy and alternative treatment
All patients with strongyloidiasis, regardless of whether they are symptomatic, must be treated to prevent possible late-onset disseminated disease and hyperinfection.9 The Centers for Disease Control and Prevention recommends one to 2 doses of ivermectin 200 mcg/kg as first-line therapy or albendazole 400 mg twice daily for 3 days as an alternative treatment (TABLE).11 Ivermectin cures more than 95% of cases.12 Albendazole has lower efficacy (78%).13 Some experts recommend administering the 2 doses of ivermectin 2 weeks apart to allow enough time for the parasite to migrate to the gut.4
Coinfection with HTLV-1 (which is endemic in areas where Strongyloides also is
endemic) modifies patients’ immune response and can complicate treatment.9 Clinicians should screen strongyloidiasis patients for HTLV-1 if they come from high-prevalence areas and/or have persistent strongyloidiasis that responds poorly to antiparasitic treatment.9
Consider referral to an infectious disease specialist for patients coinfected with
HTLV-1, as well as those who are immunocompromised. Such referral also may be appropriate for patients from countries where loa loa is endemic, because encephalopathy has occurred in patients coinfected with loa loa who were treated with ivermectin.10
Our patient was treated with 2 doses of ivermectin 200 mcg/kg, 2 weeks apart. Four months later, his eosinophilia had resolved, his IgG-ELISA dropped to 0.37, and he had gained 2.5 pounds.
THE TAKEAWAY
Strongyloidiasis is an infection caused by the parasitic worm Strongyloides stercoralis that is most common in tropical or subtropical areas. It can be asymptomatic or present with a wide range of nonspecific signs and symptoms, such as eosinophilia, cough, wheeze, abdominal pain, weight loss, diarrhea, pruritus ani, and larva currens. It is diagnosed by stool examination and serologic testing. Ivermectin is first-line therapy; albendazole is an alternative.
THE CASE
A 31-year-old man came to an internal medicine clinic because he’d been losing weight over the past 2 years and hadn’t been able to regain any weight despite eating properly. Our patient was born in Ethiopia, but had been living in Canada for 6 years. He reported a remote history of 2 episodes of diarrhea.
His physical exam was normal and laboratory results revealed mild eosinophilia of 0.6 × 109/L (normal range, <0.45 × 109/L). Additional tests (including complete blood count, electrolytes, liver panel, thyrotropin, and blood smear) revealed no apparent metabolic causes of the patient’s weight loss. Stool analysis (3 exams) was negative for ova and parasites.
THE DIAGNOSIS
Because our patient was born in Ethiopia, we did serologic testing for Strongyloides, which was positive (enzyme-linked immunosorbent assay for immunoglobulin G antibodies [IgG-ELISA] was 2.9; positive is >2.1). We diagnosed strongyloidiasis in this patient.
DISCUSSION
Strongyloidiasis is an infection caused by the parasite Strongyloides stercoralis.1 It affects an estimated 30 to 100 million people worldwide, mainly in Africa, Southeast Asia, Central America, and South America, but it also can occur in temperate climates.2Strongyloides is a soil-transmitted helminth (parasitic worm). The prevalence of Strongyloides infection among refugee groups in the United States is 1% to 4.3%.3-5
Although patients with strongyloidiasis are often asymptomatic, they can present with a wide range of nonspecific symptoms. In the acute stage, patients may develop signs and symptoms including cough, wheeze, abdominal pain, weight loss, diarrhea, pruritus ani, and larva currens.2 Respiratory symptoms, including tracheal irritation and a dry cough, are often confused with asthma. In the generally asymptomatic chronic stage, patients may develop gastrointestinal complaints, such as epigastric pain and heartburn.6
Hyperinfection syndrome can occur when patients with subclinical infection receive high doses of corticosteroids for asthma or chronic obstructive pulmonary disease exacerbations. Risk of hyperinfection is increased among immunocompromised patients with human T lymphotropic virus type-1 (HTLV-1),7 as well as in patients with malignancies, malnutrition, and alcohol use disorder. Eosinophilia is often absent in patients with hyperinfection, and stool examination results are almost always positive.8
Who to screen, how to make the diagnosis
The presence of eosinophilia in immigrants, refugees, and travelers from endemic regions should alert clinicians to the possibility of an underlying helminth infection. However, because eosinophilia occurs intermittently in response to tissue invasion, absence of eosinophilia does not exclude strongyloidiasis.
The Canadian Collaboration for Immigrants and Refugee Health (CCIRH) recommends using serologic testing to screen for Strongyloides in all newly arrived refugees from low-income countries in Southeast Asia and Africa.9 The CCIRH also advises that while data on the burden of strongyloidiasis in non-refugee immigrant populations is limited, you should consider screening foreign-born individuals who have lived in endemic areas, have symptoms and/or signs of Strongyloides infection, and/or have evidence of eosinophilia.9 Because the risk of hyperinfection is increased in immunocompromised individuals, screening should be done to detect Strongyloides infection before starting chemotherapy and before initiating corticosteroids in patients from endemic areas.10
Diagnostic methods. Stool examination6 and IgG-ELISA2 are the main methods used to diagnose strongyloidiasis. However, traditional stool examinations have low sensitivity, and it may require up to 7 stool exams to reach a sensitivity of 100%,6 which could explain why our patient’s stool analysis was negative for parasites. In our experience, a positive serology result should always be assumed to indicate an active infection unless there is a well documented history of prior therapy. (In such cases, a positive serology result could represent persistent antibodies following therapy.)
First-line therapy and alternative treatment
All patients with strongyloidiasis, regardless of whether they are symptomatic, must be treated to prevent possible late-onset disseminated disease and hyperinfection.9 The Centers for Disease Control and Prevention recommends one to 2 doses of ivermectin 200 mcg/kg as first-line therapy or albendazole 400 mg twice daily for 3 days as an alternative treatment (TABLE).11 Ivermectin cures more than 95% of cases.12 Albendazole has lower efficacy (78%).13 Some experts recommend administering the 2 doses of ivermectin 2 weeks apart to allow enough time for the parasite to migrate to the gut.4
Coinfection with HTLV-1 (which is endemic in areas where Strongyloides also is
endemic) modifies patients’ immune response and can complicate treatment.9 Clinicians should screen strongyloidiasis patients for HTLV-1 if they come from high-prevalence areas and/or have persistent strongyloidiasis that responds poorly to antiparasitic treatment.9
Consider referral to an infectious disease specialist for patients coinfected with
HTLV-1, as well as those who are immunocompromised. Such referral also may be appropriate for patients from countries where loa loa is endemic, because encephalopathy has occurred in patients coinfected with loa loa who were treated with ivermectin.10
Our patient was treated with 2 doses of ivermectin 200 mcg/kg, 2 weeks apart. Four months later, his eosinophilia had resolved, his IgG-ELISA dropped to 0.37, and he had gained 2.5 pounds.
THE TAKEAWAY
Strongyloidiasis is an infection caused by the parasitic worm Strongyloides stercoralis that is most common in tropical or subtropical areas. It can be asymptomatic or present with a wide range of nonspecific signs and symptoms, such as eosinophilia, cough, wheeze, abdominal pain, weight loss, diarrhea, pruritus ani, and larva currens. It is diagnosed by stool examination and serologic testing. Ivermectin is first-line therapy; albendazole is an alternative.
1. World Health Organization. Strongyloidiasis. World Health Organization Web site. Available at: http://www.who.int/neglected_diseases/diseases/strongyloidiasis/en/. Accessed January 29, 2015.
2. Lim S, Katz K, Krajden S, et al. Complicated and fatal Strongyloides infection in Canadians: risk factors, diagnosis and management. CMAJ. 2004;171:479-484.
3. Lifson AR, Thai D, O’Fallon A, et al. Prevalence of tuberculosis, hepatitis B virus, and intestinal parasitic infections among refugees to Minnesota. Public Health Rep. 2002;117:69-77.
4. Miller JM, Boyd HA, Ostrowski SR, et al. Malaria, intestinal parasites, and schistosomiasis among Barawan Somali refugees resettling to the United States: a strategy to reduce morbidity and decrease the risk of imported infections. Am J Trop Med Hyg. 2000;62:115-121.
5. Molina CD, Molina MM, Molina JM. Intestinal parasites in southeast Asian refugees two years after immigration. West J Med. 1988;149:422-425.
6. Centers for Disease Control and Prevention. Parasites – strongyloides. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/parasites/strongyloides/health_professionals/index.html. Accessed February 3, 2015.
7. Requena-Méndez A, Chiodini P, Bisoffi Z, et al. The laboratory diagnosis and follow up of strongyloidiasis: a systemic review. PLoS Negl Trop Dis. 2013;7:e2002.
8. Mirdha BR. Human strogyloidiasis: often brushed under the carpet. Trop Gastroenterol. 2009;30:1-4.
9. Pottie K, Greenaway C, Feightner J, et al; Canadian Collaboration for Immigrant and Refugee Health. Evidence-based clinical guidelines for immigrants and refugees. CMAJ. 2011;183:E824-E925.
10. Lagacé-Wiens PR, Harding GK. A Canadian immigrant with coinfection of Strongyloides stercoralis and human T-lymphotropic virus 1. CMAJ. 2007;177:451-453.
11. Centers for Disease Control and Prevention. Guidelines for overseas presumptive treatment of strongyloidiasis, schistosomiasis, and soil-transmitted helminth infections. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/overseas/intestinal-parasites-overseas.html. Accessed January 29, 2015.
12. Igual-Adell R, Oltra-Alcaraz C, Soler-Company E, et al. Efficacy and safety of ivermectin and thiabendazole in the treatment of strongyloidiasis. Expert Opin Pharmacother. 2004;5:2615-2619.
13. Horton J. Albendazole: a review of antihelmintic efficacy and safety in humans. Parasitology. 2000;121:S113-S132.
1. World Health Organization. Strongyloidiasis. World Health Organization Web site. Available at: http://www.who.int/neglected_diseases/diseases/strongyloidiasis/en/. Accessed January 29, 2015.
2. Lim S, Katz K, Krajden S, et al. Complicated and fatal Strongyloides infection in Canadians: risk factors, diagnosis and management. CMAJ. 2004;171:479-484.
3. Lifson AR, Thai D, O’Fallon A, et al. Prevalence of tuberculosis, hepatitis B virus, and intestinal parasitic infections among refugees to Minnesota. Public Health Rep. 2002;117:69-77.
4. Miller JM, Boyd HA, Ostrowski SR, et al. Malaria, intestinal parasites, and schistosomiasis among Barawan Somali refugees resettling to the United States: a strategy to reduce morbidity and decrease the risk of imported infections. Am J Trop Med Hyg. 2000;62:115-121.
5. Molina CD, Molina MM, Molina JM. Intestinal parasites in southeast Asian refugees two years after immigration. West J Med. 1988;149:422-425.
6. Centers for Disease Control and Prevention. Parasites – strongyloides. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/parasites/strongyloides/health_professionals/index.html. Accessed February 3, 2015.
7. Requena-Méndez A, Chiodini P, Bisoffi Z, et al. The laboratory diagnosis and follow up of strongyloidiasis: a systemic review. PLoS Negl Trop Dis. 2013;7:e2002.
8. Mirdha BR. Human strogyloidiasis: often brushed under the carpet. Trop Gastroenterol. 2009;30:1-4.
9. Pottie K, Greenaway C, Feightner J, et al; Canadian Collaboration for Immigrant and Refugee Health. Evidence-based clinical guidelines for immigrants and refugees. CMAJ. 2011;183:E824-E925.
10. Lagacé-Wiens PR, Harding GK. A Canadian immigrant with coinfection of Strongyloides stercoralis and human T-lymphotropic virus 1. CMAJ. 2007;177:451-453.
11. Centers for Disease Control and Prevention. Guidelines for overseas presumptive treatment of strongyloidiasis, schistosomiasis, and soil-transmitted helminth infections. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/immigrantrefugeehealth/guidelines/overseas/intestinal-parasites-overseas.html. Accessed January 29, 2015.
12. Igual-Adell R, Oltra-Alcaraz C, Soler-Company E, et al. Efficacy and safety of ivermectin and thiabendazole in the treatment of strongyloidiasis. Expert Opin Pharmacother. 2004;5:2615-2619.
13. Horton J. Albendazole: a review of antihelmintic efficacy and safety in humans. Parasitology. 2000;121:S113-S132.