User login
Imiquimod Induces Sustained Remission of Actinic Damage: A Case Report Spanning One Decade of Observation
Sun damage and chronic exposure to UV radiation have been recognized as causative factors for the development of squamous cell carcinoma (SCC), its precursor actinic keratosis (AK), and basal cell carcinoma (BCC). Although surgical treatment is necessary for most advanced cases of skin cancer, several other therapeutic approaches have been described including the use of topical chemotherapy agents such as 5-fluorouracil (5-FU) and topical immunomodulators such as imiquimod. Unlike surgery, these agents provide the added benefit of treating larger fields of photodamaged skin. With the increasing prevalence of nonmelanoma skin cancers (NMSCs), the use of multiple topical agents for treatment will continue to become more common.
We present the case of a patient who underwent field therapy with topical 5-FU for diffuse actinic damage and AKs. There was no subsequent inflammatory response within the perimeter of a BCC that had been treated with imiquimod 10 years prior.
Case Report
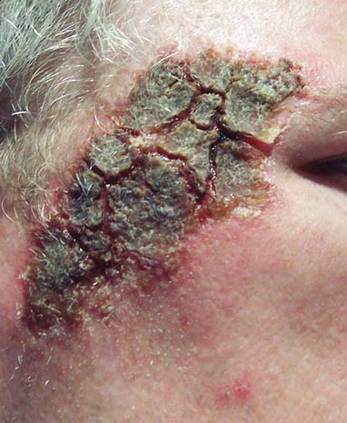
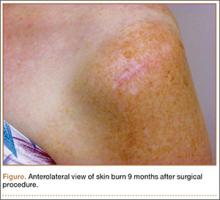
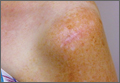
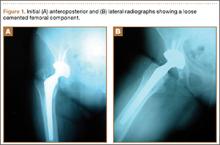
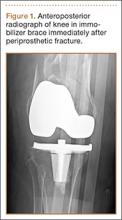
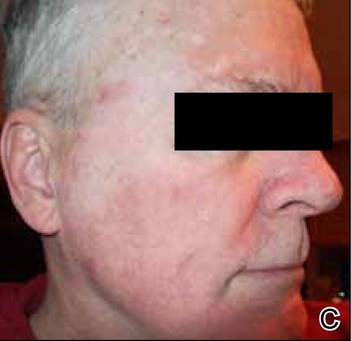
An otherwise healthy 58-year-old man with a history of long-standing diffuse sun damage and multiple prior NMSCs presented for treatment of a recurrent BCC on the right cheek. The patient reported that the BCC had initially been biopsied and excised by his primary care physician. Two months later local recurrence was noted by the primary care physician and the patient was subsequently referred to our dermatology office. A 2-month treatment course with daily imiquimod cream 5% was initiated. This treatment caused extensive inflammation of the right cheek but was otherwise well tolerated (Figure 1).
 |
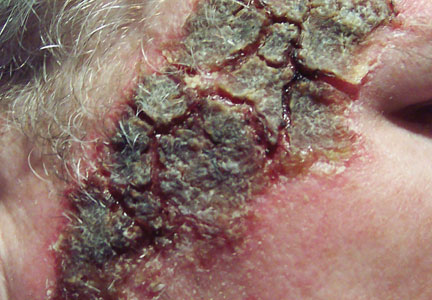
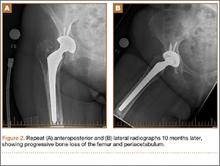
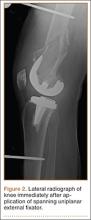
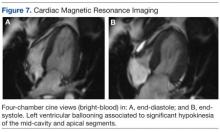
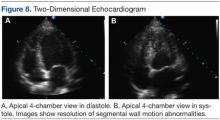
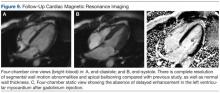
During a routine skin cancer screening 10 years later, no recurrence of the BCC was noted on the right cheek; however, the patient had developed multiple AKs on the face. Therapeutic options were discussed with the patient; he agreed to topical field therapy with 5-FU cream 0.5%. The patient applied the 5-FU cream to the entire face nightly for 1 month. During this time he experienced a brisk inflammatory response with painful cracking and redness of the skin. On follow-up, it was noted that the area on the right cheek that had been treated with imiquimod 10 years prior showed no inflammatory response despite nightly application of 5-FU cream to the area (Figure 2). The patient denied any routine use of sunscreen or other sun-protective practices.
Comment
Basal cell carcinoma is the most common skin cancer in the United States with an incidence of 1.4% to 2% per year. It has become more prevalent in recent decades, likely due to genetic predisposition and increasing cumulative sun exposure.1-4 A variety of treatment options are available. Surgical interventions include destruction via electrodesiccation and curettage, local excision, and Mohs micrographic surgery. One of the challenges in the management of BCC, as was the case in our patient, is the treatment of tumors that arise in cosmetically or functionally sensitive areas. Approaches that minimize the amount of tissue removed while ensuring the highest possible cure rate are favorable. In addition to surgery, topical imiquimod has been established as a potential treatment of BCC. Imiquimod, a nucleoside analogue of the imidazoquinoline family, is an agonist of toll-like receptors 7 and 8 that promotes cytokine-induced cell death via nuclear factor kB and a helper T cell TH1-weighted antitumor inflammatory response.5,6 Although clearance rates with imiquimod vary by drug regimen, success rates of 43% to 100% for superficial BCCs, 42% to 100% for nodular BCCs, and 56% to 63% for infiltrative BCCs have been reported.7 In a 2007 randomized study of imiquimod cream 5%, 5-FU ointment 5%, or cryosurgery for the treatment of AK, imiquimod resulted in superior and more reliable clearance with lower recurrence rates.8
Similar to BCC, AK is closely linked to lifetime cumulative sun exposure.9 Actinic keratoses have been well established as precursors to SCC, and some researchers advocate for their reclassification as early SCC in situ.10 The incidence of malignant conversion of AK to SCC has been estimated at 0.025% to 16% annually, with an estimated lifetime risk for malignant transformation of 8% per individual AK.11,12 Cryotherapy has been a mainstay for the treatment of isolated AK, and alternative therapies including curettage, photodynamic therapy, and laser therapy have been employed. Field-directed therapy has become a popular alternative that targets multiple lesions and field cancerization.8,13,14 Field cancerization implies that if one cell in the patient’s epidermis has been exposed to enough UV radiation to develop into a precancerous lesion or early skin cancer, then many other cells in the same environment likely have some degree of UV radiation–induced atypia.15 5-Fluorouacil is a pyrimidine analogue chemotherapeutic agent that inhibits thymidylate synthase and interferes with DNA synthesis.16 This mechanism of 5-FU commonly causes an inflammatory response characterized by burning, dryness, and redness, but these effects rarely force early discontinuation of treatment. A randomized controlled trial comparing 5-FU cream 0.5% to a placebo found that complete clearance rates at 4 weeks posttreatment were significantly higher in the treatment group (47.5%) versus placebo (3.4%)(P<.001).13 Additional trials have established no significant superiority of 5-FU cream 5% over 5-FU cream 0.5%, with a decrease in side effects noted in patients treated with the lower concentration.17
Our patient had a history of a recurrent BCC and was previously treated with imiquimod. He showed no inflammatory response to field therapy with 5-FU within the perimeter of prior immunomodulatory therapy. Although no frank scaling or crusting papules consistent with AK were observed in the previously treated area prior to 5-FU therapy, subclinical field damage in that area was expected because 10 years of additional sun exposure had accumulated since imiquimod therapy was completed. Several conclusions can be drawn from this observation. Primarily, no new clinically significant actinic lesions occurred on the previously treated skin. This observation is consistent with 12-month follow-up data on AKs treated with either 5-FU, imiquimod, or cryosurgery that identified imiquimod as having the lowest recurrence rate.8 Thus, a photoprotective effect may be ascribed to imiquimod therapy that extends beyond its drug effects on atypical keratinocytes. It has been one author’s personal experience (M.Q.) that patients treated with 5-FU experience recurrence of AKs within 3 to 5 years versus 10 years of remission with imiquimod. In our patient, imiquimod therapy seemed to reset the patient’s skin at the location of the prior BCC and surrounding field cancerization.
Studies with long-term follow-up are needed to investigate the need for re-treatment with imiquimod or 5-FU. The longevity of imiquimod treatment may be of importance beyond the treatment of AKs or NMSCs. For instance, during the treatment of lentigo maligna with imiquimod, Metcalf et al18 found a significant reduction in solar elastosis (P=.0036), normalization of epidermal thickness (P=.0073), and increased papillary dermal fibroplasia in pre- and posttreatment biopsies (P<.0001), which have been described as antiaging effects in the laypress. Some of these mechanisms appear to be implicated in the observations noted in our patient. The 10-year period between the 2 courses of therapy in our patient suggests that imiquimod may cause sustained healing of skin that was previously classified both clinically and microscopically as UV damaged.
Conclusion
Both topical immunomodulators such as imiquimod and topical chemotherapeutic agents such as 5-FU have a role in the field treatment of AK and the focal treatment of superficial BCC and SCC. As multiple topical immunomodulators continue to be evaluated, long-term studies assessing the need for re-treatment as well as the degree of sustained remission of sun damage will be necessary. We expect that their individual roles will continue to become more precisely defined and distinct in the coming years.
1. Flohil SC, de Vries E, Neumann HA, et al. Incidence, prevalence and future trends of primary basal cell carcinoma in the Netherlands. Acta Derm Venereol. 2011;91:24-30.
2. Donaldson MR, Coldiron BM. No end in sight: the skin cancer epidemic continues. Semin Cutan Med Surg. 2011;30:3-5.
3. Gallagher RP, Hill GB, Bajdik CD, et al. Sunlight exposure, pigmentary factors, and risk of nonmelanocytic skin cancer. I. Basal cell carcinoma. Arch Dermatol. 1995;131:157-163.
4. Gailani MR, Leffell DJ, Ziegler A, et al. Relationship between sunlight exposure and a key genetic alteration in basal cell carcinoma. J Natl Cancer Inst. 1996;88:349-354.
5. Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway [published online ahead of print January 22, 2002]. Nat Immunol. 2002;3:196-200.
6. Schön MP, Schön M. Imiquimod: mode of action. Br J Dermatol. 2007;157(suppl 2):8-13.
7. Love WE, Bernhard JD, Bordeaux JS. Topical imiquimod or fluorouracil therapy for basal and squamous cell carcinoma: a systematic review. Arch Dermatol. 2009;145:1431-1438.
8. Krawtchenko N, Roewert-Huber J, Ulrich M, et al. A randomised study of topical 5% imiquimod vs. topical5-fluorouracil vs. cryosurgery in immunocompetent patients with actinic keratoses: a comparison of clinical and histological outcomes including 1-year follow-up. Br J Dermatol. 2007;157(suppl 2):34-40.
9. Feldman SR, Fleischer AB Jr. Progression of actinic keratosis to squamous cell carcinoma revisited: clinical and treatment implications. Cutis. 2011;87:201-207.
10. Röwert-Huber J, Patel MJ, Forschner T, et al. Actinic keratosis is an early in situ squamous cell carcinoma: a proposal for reclassification. Br J Dermatol. 2007;156(suppl 3):8-12.
11. Glogau RG. The risk of progression to invasive disease. J Am Acad Dermatol. 2000;42(1 pt 2):23-24.
12. Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
13. Weiss J, Menter A, Hevia O, et al. Effective treatment of actinic keratosis with 0.5% fluorouracil cream for 1, 2, or 4 weeks. Cutis. 2002;70(2 suppl):22-29.
14. Almeida Gonçalves JC, De Noronha T. 5-fluouracil (5-FU) ointment in the treatment of skin tumours and keratoses. Dermatologica. 1970;140(suppl 1):97+.
15. Vanharanta S, Massagué J. Field cancerization: something new under the sun. Cell. 2012;149:1179-1181.
16. Robins P, Gupta AK. The use of topical fluorouracil to treat actinic keratosis. Cutis. 2002;70(2 suppl):4-7.
17. Kaur R, Alikhan A, Maibach H. Comparison of topical 5-fluorouracil formulations in actinic keratosis treatment. J Dermatolog Treat. 2010;2:267-271.
18. Metcalf S, Crowson AN, Naylor M, et al. Imiquimod as an antiaging agent [published online ahead of print December 20, 2006]. J Am Acad Dermatol. 2007;56:422-425.
Sun damage and chronic exposure to UV radiation have been recognized as causative factors for the development of squamous cell carcinoma (SCC), its precursor actinic keratosis (AK), and basal cell carcinoma (BCC). Although surgical treatment is necessary for most advanced cases of skin cancer, several other therapeutic approaches have been described including the use of topical chemotherapy agents such as 5-fluorouracil (5-FU) and topical immunomodulators such as imiquimod. Unlike surgery, these agents provide the added benefit of treating larger fields of photodamaged skin. With the increasing prevalence of nonmelanoma skin cancers (NMSCs), the use of multiple topical agents for treatment will continue to become more common.
We present the case of a patient who underwent field therapy with topical 5-FU for diffuse actinic damage and AKs. There was no subsequent inflammatory response within the perimeter of a BCC that had been treated with imiquimod 10 years prior.
Case Report
An otherwise healthy 58-year-old man with a history of long-standing diffuse sun damage and multiple prior NMSCs presented for treatment of a recurrent BCC on the right cheek. The patient reported that the BCC had initially been biopsied and excised by his primary care physician. Two months later local recurrence was noted by the primary care physician and the patient was subsequently referred to our dermatology office. A 2-month treatment course with daily imiquimod cream 5% was initiated. This treatment caused extensive inflammation of the right cheek but was otherwise well tolerated (Figure 1).
 |
During a routine skin cancer screening 10 years later, no recurrence of the BCC was noted on the right cheek; however, the patient had developed multiple AKs on the face. Therapeutic options were discussed with the patient; he agreed to topical field therapy with 5-FU cream 0.5%. The patient applied the 5-FU cream to the entire face nightly for 1 month. During this time he experienced a brisk inflammatory response with painful cracking and redness of the skin. On follow-up, it was noted that the area on the right cheek that had been treated with imiquimod 10 years prior showed no inflammatory response despite nightly application of 5-FU cream to the area (Figure 2). The patient denied any routine use of sunscreen or other sun-protective practices.
Comment
Basal cell carcinoma is the most common skin cancer in the United States with an incidence of 1.4% to 2% per year. It has become more prevalent in recent decades, likely due to genetic predisposition and increasing cumulative sun exposure.1-4 A variety of treatment options are available. Surgical interventions include destruction via electrodesiccation and curettage, local excision, and Mohs micrographic surgery. One of the challenges in the management of BCC, as was the case in our patient, is the treatment of tumors that arise in cosmetically or functionally sensitive areas. Approaches that minimize the amount of tissue removed while ensuring the highest possible cure rate are favorable. In addition to surgery, topical imiquimod has been established as a potential treatment of BCC. Imiquimod, a nucleoside analogue of the imidazoquinoline family, is an agonist of toll-like receptors 7 and 8 that promotes cytokine-induced cell death via nuclear factor kB and a helper T cell TH1-weighted antitumor inflammatory response.5,6 Although clearance rates with imiquimod vary by drug regimen, success rates of 43% to 100% for superficial BCCs, 42% to 100% for nodular BCCs, and 56% to 63% for infiltrative BCCs have been reported.7 In a 2007 randomized study of imiquimod cream 5%, 5-FU ointment 5%, or cryosurgery for the treatment of AK, imiquimod resulted in superior and more reliable clearance with lower recurrence rates.8
Similar to BCC, AK is closely linked to lifetime cumulative sun exposure.9 Actinic keratoses have been well established as precursors to SCC, and some researchers advocate for their reclassification as early SCC in situ.10 The incidence of malignant conversion of AK to SCC has been estimated at 0.025% to 16% annually, with an estimated lifetime risk for malignant transformation of 8% per individual AK.11,12 Cryotherapy has been a mainstay for the treatment of isolated AK, and alternative therapies including curettage, photodynamic therapy, and laser therapy have been employed. Field-directed therapy has become a popular alternative that targets multiple lesions and field cancerization.8,13,14 Field cancerization implies that if one cell in the patient’s epidermis has been exposed to enough UV radiation to develop into a precancerous lesion or early skin cancer, then many other cells in the same environment likely have some degree of UV radiation–induced atypia.15 5-Fluorouacil is a pyrimidine analogue chemotherapeutic agent that inhibits thymidylate synthase and interferes with DNA synthesis.16 This mechanism of 5-FU commonly causes an inflammatory response characterized by burning, dryness, and redness, but these effects rarely force early discontinuation of treatment. A randomized controlled trial comparing 5-FU cream 0.5% to a placebo found that complete clearance rates at 4 weeks posttreatment were significantly higher in the treatment group (47.5%) versus placebo (3.4%)(P<.001).13 Additional trials have established no significant superiority of 5-FU cream 5% over 5-FU cream 0.5%, with a decrease in side effects noted in patients treated with the lower concentration.17
Our patient had a history of a recurrent BCC and was previously treated with imiquimod. He showed no inflammatory response to field therapy with 5-FU within the perimeter of prior immunomodulatory therapy. Although no frank scaling or crusting papules consistent with AK were observed in the previously treated area prior to 5-FU therapy, subclinical field damage in that area was expected because 10 years of additional sun exposure had accumulated since imiquimod therapy was completed. Several conclusions can be drawn from this observation. Primarily, no new clinically significant actinic lesions occurred on the previously treated skin. This observation is consistent with 12-month follow-up data on AKs treated with either 5-FU, imiquimod, or cryosurgery that identified imiquimod as having the lowest recurrence rate.8 Thus, a photoprotective effect may be ascribed to imiquimod therapy that extends beyond its drug effects on atypical keratinocytes. It has been one author’s personal experience (M.Q.) that patients treated with 5-FU experience recurrence of AKs within 3 to 5 years versus 10 years of remission with imiquimod. In our patient, imiquimod therapy seemed to reset the patient’s skin at the location of the prior BCC and surrounding field cancerization.
Studies with long-term follow-up are needed to investigate the need for re-treatment with imiquimod or 5-FU. The longevity of imiquimod treatment may be of importance beyond the treatment of AKs or NMSCs. For instance, during the treatment of lentigo maligna with imiquimod, Metcalf et al18 found a significant reduction in solar elastosis (P=.0036), normalization of epidermal thickness (P=.0073), and increased papillary dermal fibroplasia in pre- and posttreatment biopsies (P<.0001), which have been described as antiaging effects in the laypress. Some of these mechanisms appear to be implicated in the observations noted in our patient. The 10-year period between the 2 courses of therapy in our patient suggests that imiquimod may cause sustained healing of skin that was previously classified both clinically and microscopically as UV damaged.
Conclusion
Both topical immunomodulators such as imiquimod and topical chemotherapeutic agents such as 5-FU have a role in the field treatment of AK and the focal treatment of superficial BCC and SCC. As multiple topical immunomodulators continue to be evaluated, long-term studies assessing the need for re-treatment as well as the degree of sustained remission of sun damage will be necessary. We expect that their individual roles will continue to become more precisely defined and distinct in the coming years.
Sun damage and chronic exposure to UV radiation have been recognized as causative factors for the development of squamous cell carcinoma (SCC), its precursor actinic keratosis (AK), and basal cell carcinoma (BCC). Although surgical treatment is necessary for most advanced cases of skin cancer, several other therapeutic approaches have been described including the use of topical chemotherapy agents such as 5-fluorouracil (5-FU) and topical immunomodulators such as imiquimod. Unlike surgery, these agents provide the added benefit of treating larger fields of photodamaged skin. With the increasing prevalence of nonmelanoma skin cancers (NMSCs), the use of multiple topical agents for treatment will continue to become more common.
We present the case of a patient who underwent field therapy with topical 5-FU for diffuse actinic damage and AKs. There was no subsequent inflammatory response within the perimeter of a BCC that had been treated with imiquimod 10 years prior.
Case Report
An otherwise healthy 58-year-old man with a history of long-standing diffuse sun damage and multiple prior NMSCs presented for treatment of a recurrent BCC on the right cheek. The patient reported that the BCC had initially been biopsied and excised by his primary care physician. Two months later local recurrence was noted by the primary care physician and the patient was subsequently referred to our dermatology office. A 2-month treatment course with daily imiquimod cream 5% was initiated. This treatment caused extensive inflammation of the right cheek but was otherwise well tolerated (Figure 1).
 |
During a routine skin cancer screening 10 years later, no recurrence of the BCC was noted on the right cheek; however, the patient had developed multiple AKs on the face. Therapeutic options were discussed with the patient; he agreed to topical field therapy with 5-FU cream 0.5%. The patient applied the 5-FU cream to the entire face nightly for 1 month. During this time he experienced a brisk inflammatory response with painful cracking and redness of the skin. On follow-up, it was noted that the area on the right cheek that had been treated with imiquimod 10 years prior showed no inflammatory response despite nightly application of 5-FU cream to the area (Figure 2). The patient denied any routine use of sunscreen or other sun-protective practices.
Comment
Basal cell carcinoma is the most common skin cancer in the United States with an incidence of 1.4% to 2% per year. It has become more prevalent in recent decades, likely due to genetic predisposition and increasing cumulative sun exposure.1-4 A variety of treatment options are available. Surgical interventions include destruction via electrodesiccation and curettage, local excision, and Mohs micrographic surgery. One of the challenges in the management of BCC, as was the case in our patient, is the treatment of tumors that arise in cosmetically or functionally sensitive areas. Approaches that minimize the amount of tissue removed while ensuring the highest possible cure rate are favorable. In addition to surgery, topical imiquimod has been established as a potential treatment of BCC. Imiquimod, a nucleoside analogue of the imidazoquinoline family, is an agonist of toll-like receptors 7 and 8 that promotes cytokine-induced cell death via nuclear factor kB and a helper T cell TH1-weighted antitumor inflammatory response.5,6 Although clearance rates with imiquimod vary by drug regimen, success rates of 43% to 100% for superficial BCCs, 42% to 100% for nodular BCCs, and 56% to 63% for infiltrative BCCs have been reported.7 In a 2007 randomized study of imiquimod cream 5%, 5-FU ointment 5%, or cryosurgery for the treatment of AK, imiquimod resulted in superior and more reliable clearance with lower recurrence rates.8
Similar to BCC, AK is closely linked to lifetime cumulative sun exposure.9 Actinic keratoses have been well established as precursors to SCC, and some researchers advocate for their reclassification as early SCC in situ.10 The incidence of malignant conversion of AK to SCC has been estimated at 0.025% to 16% annually, with an estimated lifetime risk for malignant transformation of 8% per individual AK.11,12 Cryotherapy has been a mainstay for the treatment of isolated AK, and alternative therapies including curettage, photodynamic therapy, and laser therapy have been employed. Field-directed therapy has become a popular alternative that targets multiple lesions and field cancerization.8,13,14 Field cancerization implies that if one cell in the patient’s epidermis has been exposed to enough UV radiation to develop into a precancerous lesion or early skin cancer, then many other cells in the same environment likely have some degree of UV radiation–induced atypia.15 5-Fluorouacil is a pyrimidine analogue chemotherapeutic agent that inhibits thymidylate synthase and interferes with DNA synthesis.16 This mechanism of 5-FU commonly causes an inflammatory response characterized by burning, dryness, and redness, but these effects rarely force early discontinuation of treatment. A randomized controlled trial comparing 5-FU cream 0.5% to a placebo found that complete clearance rates at 4 weeks posttreatment were significantly higher in the treatment group (47.5%) versus placebo (3.4%)(P<.001).13 Additional trials have established no significant superiority of 5-FU cream 5% over 5-FU cream 0.5%, with a decrease in side effects noted in patients treated with the lower concentration.17
Our patient had a history of a recurrent BCC and was previously treated with imiquimod. He showed no inflammatory response to field therapy with 5-FU within the perimeter of prior immunomodulatory therapy. Although no frank scaling or crusting papules consistent with AK were observed in the previously treated area prior to 5-FU therapy, subclinical field damage in that area was expected because 10 years of additional sun exposure had accumulated since imiquimod therapy was completed. Several conclusions can be drawn from this observation. Primarily, no new clinically significant actinic lesions occurred on the previously treated skin. This observation is consistent with 12-month follow-up data on AKs treated with either 5-FU, imiquimod, or cryosurgery that identified imiquimod as having the lowest recurrence rate.8 Thus, a photoprotective effect may be ascribed to imiquimod therapy that extends beyond its drug effects on atypical keratinocytes. It has been one author’s personal experience (M.Q.) that patients treated with 5-FU experience recurrence of AKs within 3 to 5 years versus 10 years of remission with imiquimod. In our patient, imiquimod therapy seemed to reset the patient’s skin at the location of the prior BCC and surrounding field cancerization.
Studies with long-term follow-up are needed to investigate the need for re-treatment with imiquimod or 5-FU. The longevity of imiquimod treatment may be of importance beyond the treatment of AKs or NMSCs. For instance, during the treatment of lentigo maligna with imiquimod, Metcalf et al18 found a significant reduction in solar elastosis (P=.0036), normalization of epidermal thickness (P=.0073), and increased papillary dermal fibroplasia in pre- and posttreatment biopsies (P<.0001), which have been described as antiaging effects in the laypress. Some of these mechanisms appear to be implicated in the observations noted in our patient. The 10-year period between the 2 courses of therapy in our patient suggests that imiquimod may cause sustained healing of skin that was previously classified both clinically and microscopically as UV damaged.
Conclusion
Both topical immunomodulators such as imiquimod and topical chemotherapeutic agents such as 5-FU have a role in the field treatment of AK and the focal treatment of superficial BCC and SCC. As multiple topical immunomodulators continue to be evaluated, long-term studies assessing the need for re-treatment as well as the degree of sustained remission of sun damage will be necessary. We expect that their individual roles will continue to become more precisely defined and distinct in the coming years.
1. Flohil SC, de Vries E, Neumann HA, et al. Incidence, prevalence and future trends of primary basal cell carcinoma in the Netherlands. Acta Derm Venereol. 2011;91:24-30.
2. Donaldson MR, Coldiron BM. No end in sight: the skin cancer epidemic continues. Semin Cutan Med Surg. 2011;30:3-5.
3. Gallagher RP, Hill GB, Bajdik CD, et al. Sunlight exposure, pigmentary factors, and risk of nonmelanocytic skin cancer. I. Basal cell carcinoma. Arch Dermatol. 1995;131:157-163.
4. Gailani MR, Leffell DJ, Ziegler A, et al. Relationship between sunlight exposure and a key genetic alteration in basal cell carcinoma. J Natl Cancer Inst. 1996;88:349-354.
5. Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway [published online ahead of print January 22, 2002]. Nat Immunol. 2002;3:196-200.
6. Schön MP, Schön M. Imiquimod: mode of action. Br J Dermatol. 2007;157(suppl 2):8-13.
7. Love WE, Bernhard JD, Bordeaux JS. Topical imiquimod or fluorouracil therapy for basal and squamous cell carcinoma: a systematic review. Arch Dermatol. 2009;145:1431-1438.
8. Krawtchenko N, Roewert-Huber J, Ulrich M, et al. A randomised study of topical 5% imiquimod vs. topical5-fluorouracil vs. cryosurgery in immunocompetent patients with actinic keratoses: a comparison of clinical and histological outcomes including 1-year follow-up. Br J Dermatol. 2007;157(suppl 2):34-40.
9. Feldman SR, Fleischer AB Jr. Progression of actinic keratosis to squamous cell carcinoma revisited: clinical and treatment implications. Cutis. 2011;87:201-207.
10. Röwert-Huber J, Patel MJ, Forschner T, et al. Actinic keratosis is an early in situ squamous cell carcinoma: a proposal for reclassification. Br J Dermatol. 2007;156(suppl 3):8-12.
11. Glogau RG. The risk of progression to invasive disease. J Am Acad Dermatol. 2000;42(1 pt 2):23-24.
12. Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
13. Weiss J, Menter A, Hevia O, et al. Effective treatment of actinic keratosis with 0.5% fluorouracil cream for 1, 2, or 4 weeks. Cutis. 2002;70(2 suppl):22-29.
14. Almeida Gonçalves JC, De Noronha T. 5-fluouracil (5-FU) ointment in the treatment of skin tumours and keratoses. Dermatologica. 1970;140(suppl 1):97+.
15. Vanharanta S, Massagué J. Field cancerization: something new under the sun. Cell. 2012;149:1179-1181.
16. Robins P, Gupta AK. The use of topical fluorouracil to treat actinic keratosis. Cutis. 2002;70(2 suppl):4-7.
17. Kaur R, Alikhan A, Maibach H. Comparison of topical 5-fluorouracil formulations in actinic keratosis treatment. J Dermatolog Treat. 2010;2:267-271.
18. Metcalf S, Crowson AN, Naylor M, et al. Imiquimod as an antiaging agent [published online ahead of print December 20, 2006]. J Am Acad Dermatol. 2007;56:422-425.
1. Flohil SC, de Vries E, Neumann HA, et al. Incidence, prevalence and future trends of primary basal cell carcinoma in the Netherlands. Acta Derm Venereol. 2011;91:24-30.
2. Donaldson MR, Coldiron BM. No end in sight: the skin cancer epidemic continues. Semin Cutan Med Surg. 2011;30:3-5.
3. Gallagher RP, Hill GB, Bajdik CD, et al. Sunlight exposure, pigmentary factors, and risk of nonmelanocytic skin cancer. I. Basal cell carcinoma. Arch Dermatol. 1995;131:157-163.
4. Gailani MR, Leffell DJ, Ziegler A, et al. Relationship between sunlight exposure and a key genetic alteration in basal cell carcinoma. J Natl Cancer Inst. 1996;88:349-354.
5. Hemmi H, Kaisho T, Takeuchi O, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway [published online ahead of print January 22, 2002]. Nat Immunol. 2002;3:196-200.
6. Schön MP, Schön M. Imiquimod: mode of action. Br J Dermatol. 2007;157(suppl 2):8-13.
7. Love WE, Bernhard JD, Bordeaux JS. Topical imiquimod or fluorouracil therapy for basal and squamous cell carcinoma: a systematic review. Arch Dermatol. 2009;145:1431-1438.
8. Krawtchenko N, Roewert-Huber J, Ulrich M, et al. A randomised study of topical 5% imiquimod vs. topical5-fluorouracil vs. cryosurgery in immunocompetent patients with actinic keratoses: a comparison of clinical and histological outcomes including 1-year follow-up. Br J Dermatol. 2007;157(suppl 2):34-40.
9. Feldman SR, Fleischer AB Jr. Progression of actinic keratosis to squamous cell carcinoma revisited: clinical and treatment implications. Cutis. 2011;87:201-207.
10. Röwert-Huber J, Patel MJ, Forschner T, et al. Actinic keratosis is an early in situ squamous cell carcinoma: a proposal for reclassification. Br J Dermatol. 2007;156(suppl 3):8-12.
11. Glogau RG. The risk of progression to invasive disease. J Am Acad Dermatol. 2000;42(1 pt 2):23-24.
12. Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
13. Weiss J, Menter A, Hevia O, et al. Effective treatment of actinic keratosis with 0.5% fluorouracil cream for 1, 2, or 4 weeks. Cutis. 2002;70(2 suppl):22-29.
14. Almeida Gonçalves JC, De Noronha T. 5-fluouracil (5-FU) ointment in the treatment of skin tumours and keratoses. Dermatologica. 1970;140(suppl 1):97+.
15. Vanharanta S, Massagué J. Field cancerization: something new under the sun. Cell. 2012;149:1179-1181.
16. Robins P, Gupta AK. The use of topical fluorouracil to treat actinic keratosis. Cutis. 2002;70(2 suppl):4-7.
17. Kaur R, Alikhan A, Maibach H. Comparison of topical 5-fluorouracil formulations in actinic keratosis treatment. J Dermatolog Treat. 2010;2:267-271.
18. Metcalf S, Crowson AN, Naylor M, et al. Imiquimod as an antiaging agent [published online ahead of print December 20, 2006]. J Am Acad Dermatol. 2007;56:422-425.
Practice Points
- Topical immunomodulators such as imiquimod and topical chemotherapeutics such as 5-fluorouracil are effective in the field treatment of actinic keratoses.
- Prior topical immunomodulator use for nonmelanoma skin cancer may induce a sustained remission of actinic damage.
- The field effect of imiquimod treatment in actinically damaged skin may persist for several years.
Superficial Acral Fibromyxoma and Other Slow-Growing Tumors in Acral Areas
First described by Fetsch et al1 in 2001, superficial acral fibromyxoma (SAFM) is a rare fibromyxoid mesenchymal tumor that typically affects the fingers and toes with frequent involvement of the nail unit. It is not widely recognized and remains poorly understood. We describe a series of 3 cases of SAFM encountered at our institution and provide a review of the literature on this unique tumor.
Case Reports
Patient 1
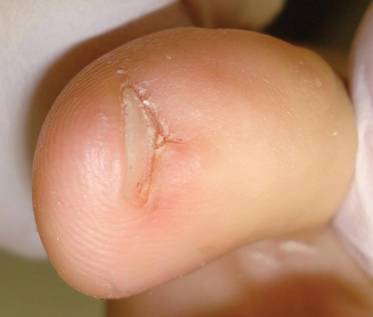
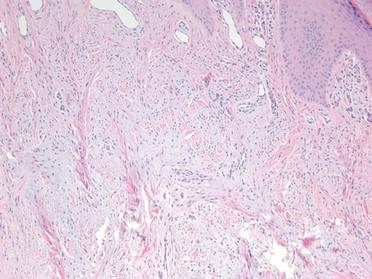
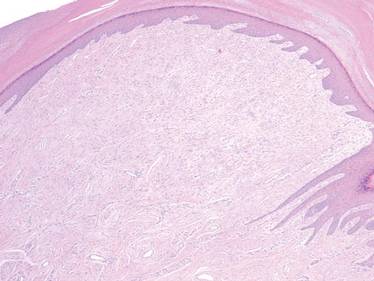
A 35-year-old man presented for treatment of a “wart” on the right fifth toe that had increased in size over the last year. He reported that the lesion was mildly painful and occasionally bled or drained clear fluid. He also noted cracking of the nail plate on the same toe. Physical examination revealed a firm, flesh-colored, 3-mm dermal papule on the proximal nail fold of the right fifth toe with subtle flattening of the underlying nail plate (Figure 1). The patient underwent biopsy of the involved proximal nail fold. Histopathology revealed a proliferation of small oval and spindle cells arranged in fascicles and bundles in the dermis (Figure 2). There was extensive mucin deposition associated with the spindle cell proliferation. Additionally, spindle cells and mucin surrounded and entrapped collagen bundles on the periphery of the lesion. Lesional cells were diffusely positive for CD34 and extended to the deep surgical margin (Figure 3). S-100 and factor XIIIa stains were negative. The diagnosis of SAFM was made based on the acral location, histopathologic appearance, and immunohistochemical profile of the tumor.
|
Patient 2
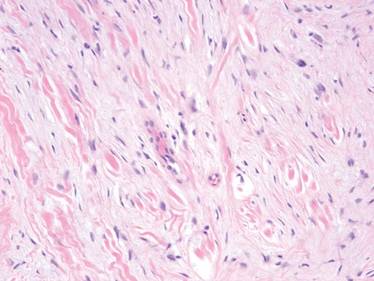
A 47-year-old man presented with an asymptomatic growth on the left fourth toe that had increased in size over the last year. Physical examination revealed an 8-mm, firm, fleshy, flesh-colored, smooth and slightly pedunculated papule on the distal aspect of the left fourth toe. The nail plate and periungual region were not involved. A shave biopsy of the papule was obtained. Histopathology demonstrated dermal stellate spindle cells arranged in a loose fascicular pattern with marked mucin deposition throughout the dermis (Figure 4). Lesional cells were positive for CD34. An S-100 stain highlighted dermal dendritic cells, but lesional cells were negative. No further excision was undertaken, and there was no evidence of recurrence at 1-year follow-up. The diagnosis of SAFM was made based on the acral location, histopathologic appearance, and immunohistochemical profile of the tumor.
Patient 3
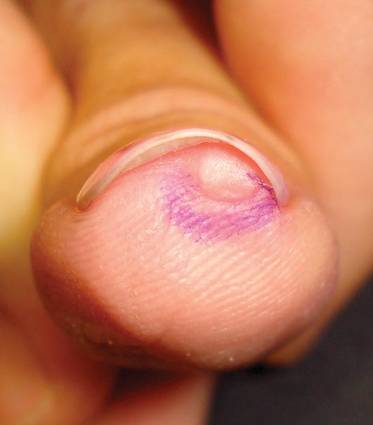
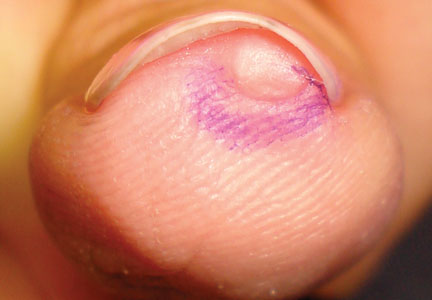
A 45-year-old woman presented with asymptomatic distal onycholysis of the right thumbnail of 1 year’s duration. She denied any history of trauma, and no bleeding or pigmentary changes were noted. Physical examination revealed a 5-mm flesh-colored papule on the hyponychium of the right thumb with focal onycholysis (Figure 5). A wedge biopsy of the lesion was performed. Histopathology showed an intradermal nodular proliferation of bland spindle cells arranged in loose fascicles and bundles and embedded in a myxoid stroma (Figure 6). CD34 staining strongly highlighted lesional cells. S-100 and neurofilament stains were negative. The diagnosis of SAFM was made based on the acral location, histopathologic appearance, and immunohistochemical profile of the tumor.
Comment
Clinically, SAFM typically presents as a slow-growing solitary nodule on the distal fingers or toes. The great toe is the most commonly affected digit, and the tumor may be subungual in up to two-thirds of cases.1 Unusual locations, such as the heel, also have been reported.2 Onset typically occurs in the fifth or sixth decade, and there is an approximately 2-fold higher incidence in men than women.1-3
Histopathologically, SAFM is a characteristically well-circumscribed but unencapsulated dermal tumor composed of spindle and stellate cells in a loose storiform or fascicular arrangement embedded in a myxoid, myxocollagenous, or collagenous stroma.4 The tumor often occupies the entire dermis and may extend into the subcutis or occasionally the underlying fascia and bone.4,5 Mast cells often are prominent, and microvascular accentuation also may be seen. Inflammatory infiltrates and multinucleated giant cells typically are not seen.6 Although 2 cases of atypical SAFM have been described,2 cellular atypia is not a characteristic feature of SAFM.
The immunohistochemical profile of SAFM is characterized by diffuse or focal expression of CD34, focal expression of epithelial membrane antigen (EMA), CD99 expression, and varying numbers of factor XIIIa–positive histiocytes.2,3 Positive staining for vimentin also is common. Staining typically is negative for S-100, human melanoma black 45, keratin, smooth muscle actin, and desmin.
The standard treatment of SAFM is complete local resection of the tumor, though some patients have been treated with partial excision or biopsy and partial or complete digital amputation.1 Local recurrence may occur in up to 20% of cases; however, approximately two-thirds of the reported recurrences in the literature occurred after incomplete tumor excision.1,2 It may be more appropriate to consider these cases as persistent rather than recurrent tumors. Superficial acral fibromyxoma is considered a benign tumor, with no known cases of metastases.4
|
A broad differential diagnosis exists for SAFM and it can be difficult to differentiate it from a wide variety of benign and malignant tumors that may be seen on the nail unit and distal extremities (Table). Myxoid neurofibromas typically present as solitary lesions on the hands and feet. Similar to SAFM, myxoid neurofibromas are unencapsulated dermal tumors composed of spindle-shaped cells in which mast cells often are conspicuous.2,7 However, tumor cells in myxoid neurofibromas are S-100 positive, and the lesions typically do not show vasculature accentuation.4,7
Sclerosing perineuriomas are benign fibrous tumors of the fingers and palms. Histopathologically, bland spindle cells arranged in fascicles and whorls are observed in a hyalinized collagen matrix.8 Immunohistochemically, sclerosing perineuriomas are positive for EMA and negative for S-100, but unlike SAFM, these tumors usually are CD34 negative.8
Superficial angiomyxomas typically are located on the head and neck but also may be found in other locations such as the trunk. They present as cutaneous papules or polypoid lesions. Histopathologically, superficial angiomyxomas are poorly circumscribed with a lobular pattern. Spindle-shaped fibroblasts exist in a myxoid matrix with neutrophils and thin-walled capillaries. The fibroblasts are variably positive for CD34 but also are S-100 positive.1,9
Myxoid dermatofibrosarcoma protuberans is a rare, locally aggressive, mesenchymal tumor of the skin and subcutis2 that typically presents on the trunk, proximal extremities, or head and neck; occurrence on the fingers or toes is exceedingly rare.2,10 Histopathologically, a myxoid stroma contains sheets of bland spindle-shaped cells with minimal to no atypia, sometimes arranged in a storiform pattern. The tumor characteristically invades deeply into the subcutaneous tissues. CD34 is characteristically positive and S-100 is negative.2,10
Low-grade myxofibrosarcoma is a soft tissue sarcoma easily confused with other spindle cell tumors. It is one of the most common sarcomas in adults but rarely arises in acral areas.2 It is characterized by a nodular growth pattern with marked nuclear atypia and perivascular clustering of tumor cells. CD34 staining may be positive in some cases.11
Similar to SAFM, myxoinflammatory fibroblastic sarcoma has a predilection for the extremities.4 However, it typically presents as a subcutaneous mass and has no documented tendency for nail bed involvement. Also unlike SAFM, it has a remarkable inflammatory infiltrate and characteristic virocyte or Reed-Sternberg cells.12
Acquired digital fibrokeratomas are benign neoplasms that occur on fingers and toes; the classic clinical presentation is a solitary smooth nodule or dome, often with a characteristic projecting configuration and horn shape.1 Histopathologically, these tumors are paucicellular with thick, vertically oriented, interwoven collagen bundles; cells may be positive for CD34 but are negative for EMA.1,13 Related to acquired digital fibrokeratomas are Koenen tumors, which share a similar histology but are distinguished by their clinical characteristics. For example, Koenen tumors tend to be multifocal and are strongly associated with tuberous sclerosis. These tumors also have a tendency to recur.1
Conclusion
Our report of 3 typical cases of SAFM highlights the need to keep this increasingly recognized and well-defined clinicopathological entity in the differential for slow-growing tumors in acral locations, particularly those in the periungual and subungual regions.
1. Fetsch JF, Laskin WB, Miettinen M. Superficial acral fibromyxoma: a clinicopathologic and immunohistochemical analysis of 37 cases of a distinctive soft tissue tumor with a predilection for the fingers and toes. Hum Pathol. 2001;32:704-714.
2. Al-Daraji WI, Miettinen M. Superficial acral fibromyxoma: a clinicopathological analysis of 32 tumors including 4 in the heel. J Cutan Pathol. 2008;35:1020-1026.
3. Hollmann TJ, Bovée JV, Fletcher CD. Digital fibromyxoma (superficial acral fibromyxoma): a detailed characterization of 124 cases. Am J Surg Pathol. 2012;36:789-798.
4. André J, Theunis A, Richert B, et al. Superficial acral fibromyxoma: clinical and pathological features. Am J Dermatopathol. 2004;26:472-474.
5. Kazakov DV, Mentzel T, Burg G, et al. Superficial acral fibromyxoma: report of two cases. Dermatology. 2002;205:285-288.
6. Meyerle JH, Keller RA, Krivda SJ. Superficial acral fibromyxoma of the index finger. J Am Acad Dermatol. 2004;50:134-136.
7. Graadt van Roggen JF, Hogendoorn PC, Fletcher CD. Myxoid tumours of soft tissue. Histopathology. 1999;35:291-312.
8. Fetsch JF, Miettinen M. Sclerosing perineurioma: a clinicopathologic study of 19 cases of a distinctive soft tissue lesion with a predilection for the fingers and palms of young adults. Am J Surg Pathol. 1997;21:1433-1442.
9. Calonje E, Guerin D, McCormick D, et al. Superficial angiomyxoma: clinicopathologic analysis of a series of distinctive but poorly recognized cutaneous tumors with tendency for recurrence. Am J Surg Pathol. 1999;23:910-917.
10. Taylor HB, Helwig EB. Dermatofibrosarcoma protuberans. a study of 115 cases. Cancer. 1962;15:717-725.
11. Wada T, Hasegawa T, Nagoya S, et al. Myxofibrosarcoma with an infiltrative growth pattern: a case report. Jpn J Clin Oncol. 2000;30:458-462.
12. Meis-Kindblom JM, Kindblom LG. Acral myxoinflammatory fibroblastic sarcoma: a low-grade tumor of the hands and feet. Am J Surg Pathol. 1998;22:911-924.
13. Bart RS, Andrade R, Kopf AW, et al. Acquired digital fibrokeratomas. Arch Dermatol. 1968;97:120-129.
First described by Fetsch et al1 in 2001, superficial acral fibromyxoma (SAFM) is a rare fibromyxoid mesenchymal tumor that typically affects the fingers and toes with frequent involvement of the nail unit. It is not widely recognized and remains poorly understood. We describe a series of 3 cases of SAFM encountered at our institution and provide a review of the literature on this unique tumor.
Case Reports
Patient 1
A 35-year-old man presented for treatment of a “wart” on the right fifth toe that had increased in size over the last year. He reported that the lesion was mildly painful and occasionally bled or drained clear fluid. He also noted cracking of the nail plate on the same toe. Physical examination revealed a firm, flesh-colored, 3-mm dermal papule on the proximal nail fold of the right fifth toe with subtle flattening of the underlying nail plate (Figure 1). The patient underwent biopsy of the involved proximal nail fold. Histopathology revealed a proliferation of small oval and spindle cells arranged in fascicles and bundles in the dermis (Figure 2). There was extensive mucin deposition associated with the spindle cell proliferation. Additionally, spindle cells and mucin surrounded and entrapped collagen bundles on the periphery of the lesion. Lesional cells were diffusely positive for CD34 and extended to the deep surgical margin (Figure 3). S-100 and factor XIIIa stains were negative. The diagnosis of SAFM was made based on the acral location, histopathologic appearance, and immunohistochemical profile of the tumor.
|
Patient 2
A 47-year-old man presented with an asymptomatic growth on the left fourth toe that had increased in size over the last year. Physical examination revealed an 8-mm, firm, fleshy, flesh-colored, smooth and slightly pedunculated papule on the distal aspect of the left fourth toe. The nail plate and periungual region were not involved. A shave biopsy of the papule was obtained. Histopathology demonstrated dermal stellate spindle cells arranged in a loose fascicular pattern with marked mucin deposition throughout the dermis (Figure 4). Lesional cells were positive for CD34. An S-100 stain highlighted dermal dendritic cells, but lesional cells were negative. No further excision was undertaken, and there was no evidence of recurrence at 1-year follow-up. The diagnosis of SAFM was made based on the acral location, histopathologic appearance, and immunohistochemical profile of the tumor.
Patient 3
A 45-year-old woman presented with asymptomatic distal onycholysis of the right thumbnail of 1 year’s duration. She denied any history of trauma, and no bleeding or pigmentary changes were noted. Physical examination revealed a 5-mm flesh-colored papule on the hyponychium of the right thumb with focal onycholysis (Figure 5). A wedge biopsy of the lesion was performed. Histopathology showed an intradermal nodular proliferation of bland spindle cells arranged in loose fascicles and bundles and embedded in a myxoid stroma (Figure 6). CD34 staining strongly highlighted lesional cells. S-100 and neurofilament stains were negative. The diagnosis of SAFM was made based on the acral location, histopathologic appearance, and immunohistochemical profile of the tumor.
Comment
Clinically, SAFM typically presents as a slow-growing solitary nodule on the distal fingers or toes. The great toe is the most commonly affected digit, and the tumor may be subungual in up to two-thirds of cases.1 Unusual locations, such as the heel, also have been reported.2 Onset typically occurs in the fifth or sixth decade, and there is an approximately 2-fold higher incidence in men than women.1-3
Histopathologically, SAFM is a characteristically well-circumscribed but unencapsulated dermal tumor composed of spindle and stellate cells in a loose storiform or fascicular arrangement embedded in a myxoid, myxocollagenous, or collagenous stroma.4 The tumor often occupies the entire dermis and may extend into the subcutis or occasionally the underlying fascia and bone.4,5 Mast cells often are prominent, and microvascular accentuation also may be seen. Inflammatory infiltrates and multinucleated giant cells typically are not seen.6 Although 2 cases of atypical SAFM have been described,2 cellular atypia is not a characteristic feature of SAFM.
The immunohistochemical profile of SAFM is characterized by diffuse or focal expression of CD34, focal expression of epithelial membrane antigen (EMA), CD99 expression, and varying numbers of factor XIIIa–positive histiocytes.2,3 Positive staining for vimentin also is common. Staining typically is negative for S-100, human melanoma black 45, keratin, smooth muscle actin, and desmin.
The standard treatment of SAFM is complete local resection of the tumor, though some patients have been treated with partial excision or biopsy and partial or complete digital amputation.1 Local recurrence may occur in up to 20% of cases; however, approximately two-thirds of the reported recurrences in the literature occurred after incomplete tumor excision.1,2 It may be more appropriate to consider these cases as persistent rather than recurrent tumors. Superficial acral fibromyxoma is considered a benign tumor, with no known cases of metastases.4
|
A broad differential diagnosis exists for SAFM and it can be difficult to differentiate it from a wide variety of benign and malignant tumors that may be seen on the nail unit and distal extremities (Table). Myxoid neurofibromas typically present as solitary lesions on the hands and feet. Similar to SAFM, myxoid neurofibromas are unencapsulated dermal tumors composed of spindle-shaped cells in which mast cells often are conspicuous.2,7 However, tumor cells in myxoid neurofibromas are S-100 positive, and the lesions typically do not show vasculature accentuation.4,7
Sclerosing perineuriomas are benign fibrous tumors of the fingers and palms. Histopathologically, bland spindle cells arranged in fascicles and whorls are observed in a hyalinized collagen matrix.8 Immunohistochemically, sclerosing perineuriomas are positive for EMA and negative for S-100, but unlike SAFM, these tumors usually are CD34 negative.8
Superficial angiomyxomas typically are located on the head and neck but also may be found in other locations such as the trunk. They present as cutaneous papules or polypoid lesions. Histopathologically, superficial angiomyxomas are poorly circumscribed with a lobular pattern. Spindle-shaped fibroblasts exist in a myxoid matrix with neutrophils and thin-walled capillaries. The fibroblasts are variably positive for CD34 but also are S-100 positive.1,9
Myxoid dermatofibrosarcoma protuberans is a rare, locally aggressive, mesenchymal tumor of the skin and subcutis2 that typically presents on the trunk, proximal extremities, or head and neck; occurrence on the fingers or toes is exceedingly rare.2,10 Histopathologically, a myxoid stroma contains sheets of bland spindle-shaped cells with minimal to no atypia, sometimes arranged in a storiform pattern. The tumor characteristically invades deeply into the subcutaneous tissues. CD34 is characteristically positive and S-100 is negative.2,10
Low-grade myxofibrosarcoma is a soft tissue sarcoma easily confused with other spindle cell tumors. It is one of the most common sarcomas in adults but rarely arises in acral areas.2 It is characterized by a nodular growth pattern with marked nuclear atypia and perivascular clustering of tumor cells. CD34 staining may be positive in some cases.11
Similar to SAFM, myxoinflammatory fibroblastic sarcoma has a predilection for the extremities.4 However, it typically presents as a subcutaneous mass and has no documented tendency for nail bed involvement. Also unlike SAFM, it has a remarkable inflammatory infiltrate and characteristic virocyte or Reed-Sternberg cells.12
Acquired digital fibrokeratomas are benign neoplasms that occur on fingers and toes; the classic clinical presentation is a solitary smooth nodule or dome, often with a characteristic projecting configuration and horn shape.1 Histopathologically, these tumors are paucicellular with thick, vertically oriented, interwoven collagen bundles; cells may be positive for CD34 but are negative for EMA.1,13 Related to acquired digital fibrokeratomas are Koenen tumors, which share a similar histology but are distinguished by their clinical characteristics. For example, Koenen tumors tend to be multifocal and are strongly associated with tuberous sclerosis. These tumors also have a tendency to recur.1
Conclusion
Our report of 3 typical cases of SAFM highlights the need to keep this increasingly recognized and well-defined clinicopathological entity in the differential for slow-growing tumors in acral locations, particularly those in the periungual and subungual regions.
First described by Fetsch et al1 in 2001, superficial acral fibromyxoma (SAFM) is a rare fibromyxoid mesenchymal tumor that typically affects the fingers and toes with frequent involvement of the nail unit. It is not widely recognized and remains poorly understood. We describe a series of 3 cases of SAFM encountered at our institution and provide a review of the literature on this unique tumor.
Case Reports
Patient 1
A 35-year-old man presented for treatment of a “wart” on the right fifth toe that had increased in size over the last year. He reported that the lesion was mildly painful and occasionally bled or drained clear fluid. He also noted cracking of the nail plate on the same toe. Physical examination revealed a firm, flesh-colored, 3-mm dermal papule on the proximal nail fold of the right fifth toe with subtle flattening of the underlying nail plate (Figure 1). The patient underwent biopsy of the involved proximal nail fold. Histopathology revealed a proliferation of small oval and spindle cells arranged in fascicles and bundles in the dermis (Figure 2). There was extensive mucin deposition associated with the spindle cell proliferation. Additionally, spindle cells and mucin surrounded and entrapped collagen bundles on the periphery of the lesion. Lesional cells were diffusely positive for CD34 and extended to the deep surgical margin (Figure 3). S-100 and factor XIIIa stains were negative. The diagnosis of SAFM was made based on the acral location, histopathologic appearance, and immunohistochemical profile of the tumor.
|
Patient 2
A 47-year-old man presented with an asymptomatic growth on the left fourth toe that had increased in size over the last year. Physical examination revealed an 8-mm, firm, fleshy, flesh-colored, smooth and slightly pedunculated papule on the distal aspect of the left fourth toe. The nail plate and periungual region were not involved. A shave biopsy of the papule was obtained. Histopathology demonstrated dermal stellate spindle cells arranged in a loose fascicular pattern with marked mucin deposition throughout the dermis (Figure 4). Lesional cells were positive for CD34. An S-100 stain highlighted dermal dendritic cells, but lesional cells were negative. No further excision was undertaken, and there was no evidence of recurrence at 1-year follow-up. The diagnosis of SAFM was made based on the acral location, histopathologic appearance, and immunohistochemical profile of the tumor.
Patient 3
A 45-year-old woman presented with asymptomatic distal onycholysis of the right thumbnail of 1 year’s duration. She denied any history of trauma, and no bleeding or pigmentary changes were noted. Physical examination revealed a 5-mm flesh-colored papule on the hyponychium of the right thumb with focal onycholysis (Figure 5). A wedge biopsy of the lesion was performed. Histopathology showed an intradermal nodular proliferation of bland spindle cells arranged in loose fascicles and bundles and embedded in a myxoid stroma (Figure 6). CD34 staining strongly highlighted lesional cells. S-100 and neurofilament stains were negative. The diagnosis of SAFM was made based on the acral location, histopathologic appearance, and immunohistochemical profile of the tumor.
Comment
Clinically, SAFM typically presents as a slow-growing solitary nodule on the distal fingers or toes. The great toe is the most commonly affected digit, and the tumor may be subungual in up to two-thirds of cases.1 Unusual locations, such as the heel, also have been reported.2 Onset typically occurs in the fifth or sixth decade, and there is an approximately 2-fold higher incidence in men than women.1-3
Histopathologically, SAFM is a characteristically well-circumscribed but unencapsulated dermal tumor composed of spindle and stellate cells in a loose storiform or fascicular arrangement embedded in a myxoid, myxocollagenous, or collagenous stroma.4 The tumor often occupies the entire dermis and may extend into the subcutis or occasionally the underlying fascia and bone.4,5 Mast cells often are prominent, and microvascular accentuation also may be seen. Inflammatory infiltrates and multinucleated giant cells typically are not seen.6 Although 2 cases of atypical SAFM have been described,2 cellular atypia is not a characteristic feature of SAFM.
The immunohistochemical profile of SAFM is characterized by diffuse or focal expression of CD34, focal expression of epithelial membrane antigen (EMA), CD99 expression, and varying numbers of factor XIIIa–positive histiocytes.2,3 Positive staining for vimentin also is common. Staining typically is negative for S-100, human melanoma black 45, keratin, smooth muscle actin, and desmin.
The standard treatment of SAFM is complete local resection of the tumor, though some patients have been treated with partial excision or biopsy and partial or complete digital amputation.1 Local recurrence may occur in up to 20% of cases; however, approximately two-thirds of the reported recurrences in the literature occurred after incomplete tumor excision.1,2 It may be more appropriate to consider these cases as persistent rather than recurrent tumors. Superficial acral fibromyxoma is considered a benign tumor, with no known cases of metastases.4
|
A broad differential diagnosis exists for SAFM and it can be difficult to differentiate it from a wide variety of benign and malignant tumors that may be seen on the nail unit and distal extremities (Table). Myxoid neurofibromas typically present as solitary lesions on the hands and feet. Similar to SAFM, myxoid neurofibromas are unencapsulated dermal tumors composed of spindle-shaped cells in which mast cells often are conspicuous.2,7 However, tumor cells in myxoid neurofibromas are S-100 positive, and the lesions typically do not show vasculature accentuation.4,7
Sclerosing perineuriomas are benign fibrous tumors of the fingers and palms. Histopathologically, bland spindle cells arranged in fascicles and whorls are observed in a hyalinized collagen matrix.8 Immunohistochemically, sclerosing perineuriomas are positive for EMA and negative for S-100, but unlike SAFM, these tumors usually are CD34 negative.8
Superficial angiomyxomas typically are located on the head and neck but also may be found in other locations such as the trunk. They present as cutaneous papules or polypoid lesions. Histopathologically, superficial angiomyxomas are poorly circumscribed with a lobular pattern. Spindle-shaped fibroblasts exist in a myxoid matrix with neutrophils and thin-walled capillaries. The fibroblasts are variably positive for CD34 but also are S-100 positive.1,9
Myxoid dermatofibrosarcoma protuberans is a rare, locally aggressive, mesenchymal tumor of the skin and subcutis2 that typically presents on the trunk, proximal extremities, or head and neck; occurrence on the fingers or toes is exceedingly rare.2,10 Histopathologically, a myxoid stroma contains sheets of bland spindle-shaped cells with minimal to no atypia, sometimes arranged in a storiform pattern. The tumor characteristically invades deeply into the subcutaneous tissues. CD34 is characteristically positive and S-100 is negative.2,10
Low-grade myxofibrosarcoma is a soft tissue sarcoma easily confused with other spindle cell tumors. It is one of the most common sarcomas in adults but rarely arises in acral areas.2 It is characterized by a nodular growth pattern with marked nuclear atypia and perivascular clustering of tumor cells. CD34 staining may be positive in some cases.11
Similar to SAFM, myxoinflammatory fibroblastic sarcoma has a predilection for the extremities.4 However, it typically presents as a subcutaneous mass and has no documented tendency for nail bed involvement. Also unlike SAFM, it has a remarkable inflammatory infiltrate and characteristic virocyte or Reed-Sternberg cells.12
Acquired digital fibrokeratomas are benign neoplasms that occur on fingers and toes; the classic clinical presentation is a solitary smooth nodule or dome, often with a characteristic projecting configuration and horn shape.1 Histopathologically, these tumors are paucicellular with thick, vertically oriented, interwoven collagen bundles; cells may be positive for CD34 but are negative for EMA.1,13 Related to acquired digital fibrokeratomas are Koenen tumors, which share a similar histology but are distinguished by their clinical characteristics. For example, Koenen tumors tend to be multifocal and are strongly associated with tuberous sclerosis. These tumors also have a tendency to recur.1
Conclusion
Our report of 3 typical cases of SAFM highlights the need to keep this increasingly recognized and well-defined clinicopathological entity in the differential for slow-growing tumors in acral locations, particularly those in the periungual and subungual regions.
1. Fetsch JF, Laskin WB, Miettinen M. Superficial acral fibromyxoma: a clinicopathologic and immunohistochemical analysis of 37 cases of a distinctive soft tissue tumor with a predilection for the fingers and toes. Hum Pathol. 2001;32:704-714.
2. Al-Daraji WI, Miettinen M. Superficial acral fibromyxoma: a clinicopathological analysis of 32 tumors including 4 in the heel. J Cutan Pathol. 2008;35:1020-1026.
3. Hollmann TJ, Bovée JV, Fletcher CD. Digital fibromyxoma (superficial acral fibromyxoma): a detailed characterization of 124 cases. Am J Surg Pathol. 2012;36:789-798.
4. André J, Theunis A, Richert B, et al. Superficial acral fibromyxoma: clinical and pathological features. Am J Dermatopathol. 2004;26:472-474.
5. Kazakov DV, Mentzel T, Burg G, et al. Superficial acral fibromyxoma: report of two cases. Dermatology. 2002;205:285-288.
6. Meyerle JH, Keller RA, Krivda SJ. Superficial acral fibromyxoma of the index finger. J Am Acad Dermatol. 2004;50:134-136.
7. Graadt van Roggen JF, Hogendoorn PC, Fletcher CD. Myxoid tumours of soft tissue. Histopathology. 1999;35:291-312.
8. Fetsch JF, Miettinen M. Sclerosing perineurioma: a clinicopathologic study of 19 cases of a distinctive soft tissue lesion with a predilection for the fingers and palms of young adults. Am J Surg Pathol. 1997;21:1433-1442.
9. Calonje E, Guerin D, McCormick D, et al. Superficial angiomyxoma: clinicopathologic analysis of a series of distinctive but poorly recognized cutaneous tumors with tendency for recurrence. Am J Surg Pathol. 1999;23:910-917.
10. Taylor HB, Helwig EB. Dermatofibrosarcoma protuberans. a study of 115 cases. Cancer. 1962;15:717-725.
11. Wada T, Hasegawa T, Nagoya S, et al. Myxofibrosarcoma with an infiltrative growth pattern: a case report. Jpn J Clin Oncol. 2000;30:458-462.
12. Meis-Kindblom JM, Kindblom LG. Acral myxoinflammatory fibroblastic sarcoma: a low-grade tumor of the hands and feet. Am J Surg Pathol. 1998;22:911-924.
13. Bart RS, Andrade R, Kopf AW, et al. Acquired digital fibrokeratomas. Arch Dermatol. 1968;97:120-129.
1. Fetsch JF, Laskin WB, Miettinen M. Superficial acral fibromyxoma: a clinicopathologic and immunohistochemical analysis of 37 cases of a distinctive soft tissue tumor with a predilection for the fingers and toes. Hum Pathol. 2001;32:704-714.
2. Al-Daraji WI, Miettinen M. Superficial acral fibromyxoma: a clinicopathological analysis of 32 tumors including 4 in the heel. J Cutan Pathol. 2008;35:1020-1026.
3. Hollmann TJ, Bovée JV, Fletcher CD. Digital fibromyxoma (superficial acral fibromyxoma): a detailed characterization of 124 cases. Am J Surg Pathol. 2012;36:789-798.
4. André J, Theunis A, Richert B, et al. Superficial acral fibromyxoma: clinical and pathological features. Am J Dermatopathol. 2004;26:472-474.
5. Kazakov DV, Mentzel T, Burg G, et al. Superficial acral fibromyxoma: report of two cases. Dermatology. 2002;205:285-288.
6. Meyerle JH, Keller RA, Krivda SJ. Superficial acral fibromyxoma of the index finger. J Am Acad Dermatol. 2004;50:134-136.
7. Graadt van Roggen JF, Hogendoorn PC, Fletcher CD. Myxoid tumours of soft tissue. Histopathology. 1999;35:291-312.
8. Fetsch JF, Miettinen M. Sclerosing perineurioma: a clinicopathologic study of 19 cases of a distinctive soft tissue lesion with a predilection for the fingers and palms of young adults. Am J Surg Pathol. 1997;21:1433-1442.
9. Calonje E, Guerin D, McCormick D, et al. Superficial angiomyxoma: clinicopathologic analysis of a series of distinctive but poorly recognized cutaneous tumors with tendency for recurrence. Am J Surg Pathol. 1999;23:910-917.
10. Taylor HB, Helwig EB. Dermatofibrosarcoma protuberans. a study of 115 cases. Cancer. 1962;15:717-725.
11. Wada T, Hasegawa T, Nagoya S, et al. Myxofibrosarcoma with an infiltrative growth pattern: a case report. Jpn J Clin Oncol. 2000;30:458-462.
12. Meis-Kindblom JM, Kindblom LG. Acral myxoinflammatory fibroblastic sarcoma: a low-grade tumor of the hands and feet. Am J Surg Pathol. 1998;22:911-924.
13. Bart RS, Andrade R, Kopf AW, et al. Acquired digital fibrokeratomas. Arch Dermatol. 1968;97:120-129.
Practice Points
- Superficial acral fibromyxoma (SAFM) is a rare but distinct tumor that may affect the nail bed and nail plate, and it may clinically or histopathologically mimic other tumors of the distal extremities.
- Although SAFM is considered a benign tumor, it frequently persists or recurs after incomplete excision, and therefore complete local resection may be recommended, particularly for symptomatic lesions.
Sharp, left-sided back pain • bilateral leg weakness • degenerative disc disease • Dx?
THE CASE
An 84-year old woman came to the emergency department (ED) with sharp back pain on her left side that she’d had for 4 days. The pain radiated to her posterior hips when standing. She said her whole body felt achy and she was experiencing weakness in both legs.
The patient had a history of hypertension, coronary artery disease, and aortic stenosis; she’d received a bioprosthetic aortic valve 7 years ago. She was not immunocompromised or receiving steroids but was taking docusate, oxybutynin, carvedilol, amlodipine, atorvastatin, furosemide, rivaroxaban, and a multivitamin. Her physical exam, vital signs, and complete blood count (CBC) were normal. An x-ray of the lumbar spine showed degenerative joint/disc disease and spondylosis at L4-L5 and L5-S1. The patient was sent home with oxycodone/acetaminophen 5 mg/325 mg every 6 hours as needed for pain and told to follow up with her family physician (FP).
Six days later, the patient went to see her FP and told her that her symptoms hadn’t improved. She was afebrile and her blood pressure was 150/80 mm Hg. Her muscle strength was 4/5 with hip flexion bilaterally; the rest of her strength was 5/5. There was no lumbar paraspinal tenderness and she had a negative straight leg raise test. No other neurologic deficits were noted. The FP prescribed physical therapy at home with a licensed therapist, which consisted of stretching exercises and active, dynamic exercise to improve the patient’s range of motion. She also ordered outpatient lumbar magnetic resonance imaging (MRI).
THE DIAGNOSIS
Approximately 3 weeks later, the patient’s MRI revealed osteomyelitis/discitis at the L3-L4 level and severe tricompartmental stenosis from L2-L3 through L4-L5. A day after receiving the results—and about a month after having first gone to the ED—the patient was admitted to the hospital. She was afebrile and her blood pressure was 148/75 mm Hg. Her physical exam revealed no leukocytosis or neurologic deficits, but did show a systolic murmur from her aortic valve.
She had an erythrocyte sedimentation rate (ESR) of 77 mm/hr (normal range for women, <30 mm/hr) and her C-reactive protein (CRP) level was 5.88 mg/dL (<.50 mg/dL indicates average risk for cardiovascular disease). A transesophageal echocardiogram was performed and there was no sign of vegetation or thrombi. However, blood cultures were positive for Streptococcus salivarius—a bacterium found on human dental plaque—which we determined was the cause of the osteomyelitis.
To the best of our knowledge, there have been no other case reports that described S. salivarius as having caused osteomyelitis without concurrent endocarditis.
DISCUSSION
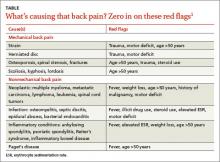
Back pain is a common and costly issue among primary care patients. More than two-thirds of adults suffer from low back pain at some point, primarily without underlying malignancy or neurologic deficits.1,2 Acute low back pain is often mechanical (97%); however, other causes, including infection, may be to blame (TABLE).1 Most acute back pain will improve with conservative treatment and patients need only reassurance of a favorable prognosis, but 20% of patients may develop chronic back pain.2
The diagnostic approach to low back pain varies widely.3 Some data indicate that early imaging of back pain can lead to unneeded follow-up testing, radiation exposure, unnecessary surgery, patient “labeling,” and increased health care costs, all of which suggest that routine imaging shouldn’t be pursued in acute low back pain.4
Red flags for acute low back pain that warrant imaging include age >50 years, fever, weight loss, elevated ESR, history of malignancy, trauma, motor deficits, steroid or illicit drug use, and litigation.1 If not already done, it’s also important to order a CBC, ESR, and CRP for patients with any of these red flags.
Imaging studies are important, but clinical correlation is crucial because imaging can reveal disk abnormalities even in healthy, asymptomatic patients.5 Computed tomography scans or MRI is indicated for patients with neurologic deficits or nerve root tension signs, but only if a patient is a potential candidate for surgery or epidural steroid injection.6,7 If you suspect an infection (such as spondylodiscitis or osteomyelitis), diagnosing the condition quickly is key.
Our patient had 2 red flags (age >50 years and elevated ESR) that helped us reach an unlikely diagnosis of lumbar osteomyelitis with S. salivarius as the cause. Degenerative spinal disease seen on x-ray may have delayed our patient’s diagnosis. If our patient had had an ESR or CRP test earlier, or if further imaging had been conducted sooner (given her proximal muscle weakness), the correct diagnosis would have been made more quickly and appropriate treatment provided sooner.
Our patient
The patient was started on a 6-week course of intravenous ceftriaxone 2g/d, which she continued to receive at home via a peripherally inserted central catheter. The patient was instructed at discharge (on Day 8) to follow up with her FP, which she did 12 days later. At that visit, her back pain was improved and her ESR and CRP levels were within normal ranges.
THE TAKEAWAY
When evaluating a patient who presents with low back pain, perform a focused history and be on the lookout for “red flags” that warrant further imaging and testing. Routine imaging is not recommended for patients with nonspecific low back pain, but imaging may be indicated for patients with neurologic deficits or nerve root tension signs.
A patient with low back pain caused by osteomyelitis may present with fever, elevated ESR, and/or motor deficits. Identifying the bacteria underlying the infection will help guide selection of appropriate antibiotics.
1. Deyo RA, Weinstein JN. Low back pain. N Engl J Med. 2001;344:363-370.
2. Deyo RA, Phillips WR. Low back pain. A primary care challenge. Spine (Phila Pa 1976). 1996;21:2826-2832.
3. Cherkin DC, Deyo RA, Wheeler K, et al. Physician variation in diagnostic testing for low back pain. Who you see is what you get. Arthritis Rheum. 1994;37:15-22.
4. Srinivas SV, Deyo RA, Berger ZD. Application of “less is more” to low back pain. Arch Intern Med. 2012;172:1016-1020.
5. Jensen MC, Brant-Zawadzki MN, Obuchowski N, et al. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331:69-73.
6. Wipf JE, Deyo RA. Low back pain. Med Clin North Am. 1995;79:231-246.
7. Chou R, Qaseem A, Snow V, et al; Clinical Efficacy Assessment Committee of the American College of Physicians; American College of Physicians; American Pain Society Low Back Pain Guidelines Panel. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147:478-493.
THE CASE
An 84-year old woman came to the emergency department (ED) with sharp back pain on her left side that she’d had for 4 days. The pain radiated to her posterior hips when standing. She said her whole body felt achy and she was experiencing weakness in both legs.
The patient had a history of hypertension, coronary artery disease, and aortic stenosis; she’d received a bioprosthetic aortic valve 7 years ago. She was not immunocompromised or receiving steroids but was taking docusate, oxybutynin, carvedilol, amlodipine, atorvastatin, furosemide, rivaroxaban, and a multivitamin. Her physical exam, vital signs, and complete blood count (CBC) were normal. An x-ray of the lumbar spine showed degenerative joint/disc disease and spondylosis at L4-L5 and L5-S1. The patient was sent home with oxycodone/acetaminophen 5 mg/325 mg every 6 hours as needed for pain and told to follow up with her family physician (FP).
Six days later, the patient went to see her FP and told her that her symptoms hadn’t improved. She was afebrile and her blood pressure was 150/80 mm Hg. Her muscle strength was 4/5 with hip flexion bilaterally; the rest of her strength was 5/5. There was no lumbar paraspinal tenderness and she had a negative straight leg raise test. No other neurologic deficits were noted. The FP prescribed physical therapy at home with a licensed therapist, which consisted of stretching exercises and active, dynamic exercise to improve the patient’s range of motion. She also ordered outpatient lumbar magnetic resonance imaging (MRI).
THE DIAGNOSIS
Approximately 3 weeks later, the patient’s MRI revealed osteomyelitis/discitis at the L3-L4 level and severe tricompartmental stenosis from L2-L3 through L4-L5. A day after receiving the results—and about a month after having first gone to the ED—the patient was admitted to the hospital. She was afebrile and her blood pressure was 148/75 mm Hg. Her physical exam revealed no leukocytosis or neurologic deficits, but did show a systolic murmur from her aortic valve.
She had an erythrocyte sedimentation rate (ESR) of 77 mm/hr (normal range for women, <30 mm/hr) and her C-reactive protein (CRP) level was 5.88 mg/dL (<.50 mg/dL indicates average risk for cardiovascular disease). A transesophageal echocardiogram was performed and there was no sign of vegetation or thrombi. However, blood cultures were positive for Streptococcus salivarius—a bacterium found on human dental plaque—which we determined was the cause of the osteomyelitis.
To the best of our knowledge, there have been no other case reports that described S. salivarius as having caused osteomyelitis without concurrent endocarditis.
DISCUSSION
Back pain is a common and costly issue among primary care patients. More than two-thirds of adults suffer from low back pain at some point, primarily without underlying malignancy or neurologic deficits.1,2 Acute low back pain is often mechanical (97%); however, other causes, including infection, may be to blame (TABLE).1 Most acute back pain will improve with conservative treatment and patients need only reassurance of a favorable prognosis, but 20% of patients may develop chronic back pain.2
The diagnostic approach to low back pain varies widely.3 Some data indicate that early imaging of back pain can lead to unneeded follow-up testing, radiation exposure, unnecessary surgery, patient “labeling,” and increased health care costs, all of which suggest that routine imaging shouldn’t be pursued in acute low back pain.4
Red flags for acute low back pain that warrant imaging include age >50 years, fever, weight loss, elevated ESR, history of malignancy, trauma, motor deficits, steroid or illicit drug use, and litigation.1 If not already done, it’s also important to order a CBC, ESR, and CRP for patients with any of these red flags.
Imaging studies are important, but clinical correlation is crucial because imaging can reveal disk abnormalities even in healthy, asymptomatic patients.5 Computed tomography scans or MRI is indicated for patients with neurologic deficits or nerve root tension signs, but only if a patient is a potential candidate for surgery or epidural steroid injection.6,7 If you suspect an infection (such as spondylodiscitis or osteomyelitis), diagnosing the condition quickly is key.
Our patient had 2 red flags (age >50 years and elevated ESR) that helped us reach an unlikely diagnosis of lumbar osteomyelitis with S. salivarius as the cause. Degenerative spinal disease seen on x-ray may have delayed our patient’s diagnosis. If our patient had had an ESR or CRP test earlier, or if further imaging had been conducted sooner (given her proximal muscle weakness), the correct diagnosis would have been made more quickly and appropriate treatment provided sooner.
Our patient
The patient was started on a 6-week course of intravenous ceftriaxone 2g/d, which she continued to receive at home via a peripherally inserted central catheter. The patient was instructed at discharge (on Day 8) to follow up with her FP, which she did 12 days later. At that visit, her back pain was improved and her ESR and CRP levels were within normal ranges.
THE TAKEAWAY
When evaluating a patient who presents with low back pain, perform a focused history and be on the lookout for “red flags” that warrant further imaging and testing. Routine imaging is not recommended for patients with nonspecific low back pain, but imaging may be indicated for patients with neurologic deficits or nerve root tension signs.
A patient with low back pain caused by osteomyelitis may present with fever, elevated ESR, and/or motor deficits. Identifying the bacteria underlying the infection will help guide selection of appropriate antibiotics.
THE CASE
An 84-year old woman came to the emergency department (ED) with sharp back pain on her left side that she’d had for 4 days. The pain radiated to her posterior hips when standing. She said her whole body felt achy and she was experiencing weakness in both legs.
The patient had a history of hypertension, coronary artery disease, and aortic stenosis; she’d received a bioprosthetic aortic valve 7 years ago. She was not immunocompromised or receiving steroids but was taking docusate, oxybutynin, carvedilol, amlodipine, atorvastatin, furosemide, rivaroxaban, and a multivitamin. Her physical exam, vital signs, and complete blood count (CBC) were normal. An x-ray of the lumbar spine showed degenerative joint/disc disease and spondylosis at L4-L5 and L5-S1. The patient was sent home with oxycodone/acetaminophen 5 mg/325 mg every 6 hours as needed for pain and told to follow up with her family physician (FP).
Six days later, the patient went to see her FP and told her that her symptoms hadn’t improved. She was afebrile and her blood pressure was 150/80 mm Hg. Her muscle strength was 4/5 with hip flexion bilaterally; the rest of her strength was 5/5. There was no lumbar paraspinal tenderness and she had a negative straight leg raise test. No other neurologic deficits were noted. The FP prescribed physical therapy at home with a licensed therapist, which consisted of stretching exercises and active, dynamic exercise to improve the patient’s range of motion. She also ordered outpatient lumbar magnetic resonance imaging (MRI).
THE DIAGNOSIS
Approximately 3 weeks later, the patient’s MRI revealed osteomyelitis/discitis at the L3-L4 level and severe tricompartmental stenosis from L2-L3 through L4-L5. A day after receiving the results—and about a month after having first gone to the ED—the patient was admitted to the hospital. She was afebrile and her blood pressure was 148/75 mm Hg. Her physical exam revealed no leukocytosis or neurologic deficits, but did show a systolic murmur from her aortic valve.
She had an erythrocyte sedimentation rate (ESR) of 77 mm/hr (normal range for women, <30 mm/hr) and her C-reactive protein (CRP) level was 5.88 mg/dL (<.50 mg/dL indicates average risk for cardiovascular disease). A transesophageal echocardiogram was performed and there was no sign of vegetation or thrombi. However, blood cultures were positive for Streptococcus salivarius—a bacterium found on human dental plaque—which we determined was the cause of the osteomyelitis.
To the best of our knowledge, there have been no other case reports that described S. salivarius as having caused osteomyelitis without concurrent endocarditis.
DISCUSSION
Back pain is a common and costly issue among primary care patients. More than two-thirds of adults suffer from low back pain at some point, primarily without underlying malignancy or neurologic deficits.1,2 Acute low back pain is often mechanical (97%); however, other causes, including infection, may be to blame (TABLE).1 Most acute back pain will improve with conservative treatment and patients need only reassurance of a favorable prognosis, but 20% of patients may develop chronic back pain.2
The diagnostic approach to low back pain varies widely.3 Some data indicate that early imaging of back pain can lead to unneeded follow-up testing, radiation exposure, unnecessary surgery, patient “labeling,” and increased health care costs, all of which suggest that routine imaging shouldn’t be pursued in acute low back pain.4
Red flags for acute low back pain that warrant imaging include age >50 years, fever, weight loss, elevated ESR, history of malignancy, trauma, motor deficits, steroid or illicit drug use, and litigation.1 If not already done, it’s also important to order a CBC, ESR, and CRP for patients with any of these red flags.
Imaging studies are important, but clinical correlation is crucial because imaging can reveal disk abnormalities even in healthy, asymptomatic patients.5 Computed tomography scans or MRI is indicated for patients with neurologic deficits or nerve root tension signs, but only if a patient is a potential candidate for surgery or epidural steroid injection.6,7 If you suspect an infection (such as spondylodiscitis or osteomyelitis), diagnosing the condition quickly is key.
Our patient had 2 red flags (age >50 years and elevated ESR) that helped us reach an unlikely diagnosis of lumbar osteomyelitis with S. salivarius as the cause. Degenerative spinal disease seen on x-ray may have delayed our patient’s diagnosis. If our patient had had an ESR or CRP test earlier, or if further imaging had been conducted sooner (given her proximal muscle weakness), the correct diagnosis would have been made more quickly and appropriate treatment provided sooner.
Our patient
The patient was started on a 6-week course of intravenous ceftriaxone 2g/d, which she continued to receive at home via a peripherally inserted central catheter. The patient was instructed at discharge (on Day 8) to follow up with her FP, which she did 12 days later. At that visit, her back pain was improved and her ESR and CRP levels were within normal ranges.
THE TAKEAWAY
When evaluating a patient who presents with low back pain, perform a focused history and be on the lookout for “red flags” that warrant further imaging and testing. Routine imaging is not recommended for patients with nonspecific low back pain, but imaging may be indicated for patients with neurologic deficits or nerve root tension signs.
A patient with low back pain caused by osteomyelitis may present with fever, elevated ESR, and/or motor deficits. Identifying the bacteria underlying the infection will help guide selection of appropriate antibiotics.
1. Deyo RA, Weinstein JN. Low back pain. N Engl J Med. 2001;344:363-370.
2. Deyo RA, Phillips WR. Low back pain. A primary care challenge. Spine (Phila Pa 1976). 1996;21:2826-2832.
3. Cherkin DC, Deyo RA, Wheeler K, et al. Physician variation in diagnostic testing for low back pain. Who you see is what you get. Arthritis Rheum. 1994;37:15-22.
4. Srinivas SV, Deyo RA, Berger ZD. Application of “less is more” to low back pain. Arch Intern Med. 2012;172:1016-1020.
5. Jensen MC, Brant-Zawadzki MN, Obuchowski N, et al. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331:69-73.
6. Wipf JE, Deyo RA. Low back pain. Med Clin North Am. 1995;79:231-246.
7. Chou R, Qaseem A, Snow V, et al; Clinical Efficacy Assessment Committee of the American College of Physicians; American College of Physicians; American Pain Society Low Back Pain Guidelines Panel. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147:478-493.
1. Deyo RA, Weinstein JN. Low back pain. N Engl J Med. 2001;344:363-370.
2. Deyo RA, Phillips WR. Low back pain. A primary care challenge. Spine (Phila Pa 1976). 1996;21:2826-2832.
3. Cherkin DC, Deyo RA, Wheeler K, et al. Physician variation in diagnostic testing for low back pain. Who you see is what you get. Arthritis Rheum. 1994;37:15-22.
4. Srinivas SV, Deyo RA, Berger ZD. Application of “less is more” to low back pain. Arch Intern Med. 2012;172:1016-1020.
5. Jensen MC, Brant-Zawadzki MN, Obuchowski N, et al. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331:69-73.
6. Wipf JE, Deyo RA. Low back pain. Med Clin North Am. 1995;79:231-246.
7. Chou R, Qaseem A, Snow V, et al; Clinical Efficacy Assessment Committee of the American College of Physicians; American College of Physicians; American Pain Society Low Back Pain Guidelines Panel. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147:478-493.
Daily episodes of confusion • altered behavior • chronic sleep deprivation • Dx?
THE CASE
A 60-year-old man with hypertension, gout, hyperlipidemia, and chronic sleep deprivation was referred to our neurology department for evaluation because he’d recently developed episodes of confusion and altered behavior that occurred daily. According to the patient’s wife, these episodes had started 4 weeks earlier while the patient was driving. He drove off the road while staring ahead with a “Joker-like” smile on his face. He was unable to utter more than a few words or respond to his wife, who was able to safely bring the car to a stop. The patient had spotty memory of this 40-minute episode.
Since then, he’d had similar but shorter episodes each morning, 20 to 75 minutes after taking his prescribed medications (lisinopril, simvastatin, and allopurinol). According to the patient’s wife, during these episodes, the patient would “act childish.” He would develop a voracious appetite and experience double or distorted vision, an unsteady gait, and poor muscle tone. These episodes were always followed by a long nap.
The man denied drinking, head trauma, acute illness, or taking illicit substances or any medications other than lisinopril, simvastatin, and allopurinol. Computed tomography, magnetic resonance imaging/magnetic resonance angiography, carotid Doppler ultrasound, and routine and 24-hour ambulatory electroencephalography (EEG) were normal.
Before the patient was referred to our neurology department, he had been prescribed a short course of the antiepileptic/mood stabilizer valproate and the wakefulness agent armodafinil, but neither medication had helped. The patient’s episodes continued daily, usually 20 to 75 minutes after taking his regular medications. When he decided to take them at night, the episodes began to occur at night.
His neurologic exam was normal. Family history was positive for a cousin with narcolepsy but negative for seizures and obstructive sleep apnea (OSA). Polysomnography revealed moderate OSA with minimal oxygen desaturation. Inpatient video EEG monitoring captured several of the events that the patient and his wife had described; the patient seemed “uninhibited” in his behavior. His EEG, cardiac telemetry, oxygen saturation, blood pressure, and serum glucose level remained normal.
The episodes’ sudden onset, peculiar symptoms, and duration—and the fact that they occurred after he took his usual medications—made complex partial seizures unlikely. The patient’s chronic sleep deprivation and family history of narcolepsy raised the possibility of “sleep attacks,” but the sudden onset and age of onset of his symptoms made those conditions less likely to explain the complete clinical picture. No particular hormonal disturbance could explain his presentation, and blood work was normal.
THE DIAGNOSIS
Because the patient’s episodes had been occurring shortly after the patient took his lisinopril, simvastatin, and allopurinol, and because his blood pressure and lipid levels were normal and his gout was asymptomatic, we decided to stop these medications. Later that day, the patient reported that he had discovered that his vial of lisinopril, which he had obtained from his regular pharmacy the day before his first episode, contained a different medication. He consulted a pharmacist, who determined that the vial contained extended release zolpidem 12.5 mg, and not his antihypertensive.
DISCUSSION
Although the true incidence of medication errors is difficult to determine, a 2006 Institute of Medicine report estimated that there are at least 1.5 million cases of preventable adverse drug events in the United States each year.1 In light of these statistics, medication errors need to be near the top of our differential diagnosis when patients suddenly develop symptoms for which there is no obvious cause.
Cause to pause? If you observe a temporal association between the onset of a patient’s symptoms and medication administration, consider possible adverse effects of the medication before ordering tests.
In this case … Our patient’s peculiar presentation correlated with regular ingestion of a high dose of zolpidem, a short-acting non-benzodiazepine gamma-aminobutyric acid (GABA) agonist. Zolpidem binds to the same GABAA receptor as benzodiazepines and therefore acts as a hypnotic by increasing GABA transmission.2 Neuropsychiatric adverse events associated with zolpidem include hallucinations, amnesia, parasomnia, psychomotor impairment, and complex behaviors (eg, sleepwalking or sleep-driving).2 Higher doses may cause coma or (rarely) death.2 One case report describes a patient who heard command hallucinations and stabbed himself after ingesting a large dose of zolpidem.3
Our patient
The patient’s episodes stopped after he discontinued the zolpidem. He subsequently received a correct prescription for lisinopril, and did not experience any additional episodes.
THE TAKEAWAY
Consider medication errors and adverse drug events in the differential diagnosis for patients who develop symptoms for which there is no obvious etiology. Educate patients, as well, to question their pharmacist if a recently filled prescription doesn’t look like the pill they usually take or makes them feel different than usual when they take it.
Of course, patients should be reminded that a generic medication may not always look the same as a brand-name drug or a previous generic prescription. But it can’t hurt for the patient to ask whether that medication that “looks different” is just a different generic—or a sign of a more worrisome mix-up.
1. Institute of Medicine. Preventing medication errors. Report Brief. July 2006. Institute of Medicine Web site. Available at: http://iom.edu/~/media/Files/Report%20Files/2006/Preventing-Medication-Errors-Quality-Chasm-Series/medicationerrorsnew.pdf. Accessed January 13, 2015.
2. Gunja N. The clinical and forensic toxicology of Z-drugs. J Med Toxicol. 2013;9:155-162.
3. Manfredi G, Kotzalidis GD, Lazanio S, et al. Command hallucinations with self-stabbing associated with zolpidem overdose. J Clin Psychiatry. 2010;71:92-93.
THE CASE
A 60-year-old man with hypertension, gout, hyperlipidemia, and chronic sleep deprivation was referred to our neurology department for evaluation because he’d recently developed episodes of confusion and altered behavior that occurred daily. According to the patient’s wife, these episodes had started 4 weeks earlier while the patient was driving. He drove off the road while staring ahead with a “Joker-like” smile on his face. He was unable to utter more than a few words or respond to his wife, who was able to safely bring the car to a stop. The patient had spotty memory of this 40-minute episode.
Since then, he’d had similar but shorter episodes each morning, 20 to 75 minutes after taking his prescribed medications (lisinopril, simvastatin, and allopurinol). According to the patient’s wife, during these episodes, the patient would “act childish.” He would develop a voracious appetite and experience double or distorted vision, an unsteady gait, and poor muscle tone. These episodes were always followed by a long nap.
The man denied drinking, head trauma, acute illness, or taking illicit substances or any medications other than lisinopril, simvastatin, and allopurinol. Computed tomography, magnetic resonance imaging/magnetic resonance angiography, carotid Doppler ultrasound, and routine and 24-hour ambulatory electroencephalography (EEG) were normal.
Before the patient was referred to our neurology department, he had been prescribed a short course of the antiepileptic/mood stabilizer valproate and the wakefulness agent armodafinil, but neither medication had helped. The patient’s episodes continued daily, usually 20 to 75 minutes after taking his regular medications. When he decided to take them at night, the episodes began to occur at night.
His neurologic exam was normal. Family history was positive for a cousin with narcolepsy but negative for seizures and obstructive sleep apnea (OSA). Polysomnography revealed moderate OSA with minimal oxygen desaturation. Inpatient video EEG monitoring captured several of the events that the patient and his wife had described; the patient seemed “uninhibited” in his behavior. His EEG, cardiac telemetry, oxygen saturation, blood pressure, and serum glucose level remained normal.
The episodes’ sudden onset, peculiar symptoms, and duration—and the fact that they occurred after he took his usual medications—made complex partial seizures unlikely. The patient’s chronic sleep deprivation and family history of narcolepsy raised the possibility of “sleep attacks,” but the sudden onset and age of onset of his symptoms made those conditions less likely to explain the complete clinical picture. No particular hormonal disturbance could explain his presentation, and blood work was normal.
THE DIAGNOSIS
Because the patient’s episodes had been occurring shortly after the patient took his lisinopril, simvastatin, and allopurinol, and because his blood pressure and lipid levels were normal and his gout was asymptomatic, we decided to stop these medications. Later that day, the patient reported that he had discovered that his vial of lisinopril, which he had obtained from his regular pharmacy the day before his first episode, contained a different medication. He consulted a pharmacist, who determined that the vial contained extended release zolpidem 12.5 mg, and not his antihypertensive.
DISCUSSION
Although the true incidence of medication errors is difficult to determine, a 2006 Institute of Medicine report estimated that there are at least 1.5 million cases of preventable adverse drug events in the United States each year.1 In light of these statistics, medication errors need to be near the top of our differential diagnosis when patients suddenly develop symptoms for which there is no obvious cause.
Cause to pause? If you observe a temporal association between the onset of a patient’s symptoms and medication administration, consider possible adverse effects of the medication before ordering tests.
In this case … Our patient’s peculiar presentation correlated with regular ingestion of a high dose of zolpidem, a short-acting non-benzodiazepine gamma-aminobutyric acid (GABA) agonist. Zolpidem binds to the same GABAA receptor as benzodiazepines and therefore acts as a hypnotic by increasing GABA transmission.2 Neuropsychiatric adverse events associated with zolpidem include hallucinations, amnesia, parasomnia, psychomotor impairment, and complex behaviors (eg, sleepwalking or sleep-driving).2 Higher doses may cause coma or (rarely) death.2 One case report describes a patient who heard command hallucinations and stabbed himself after ingesting a large dose of zolpidem.3
Our patient
The patient’s episodes stopped after he discontinued the zolpidem. He subsequently received a correct prescription for lisinopril, and did not experience any additional episodes.
THE TAKEAWAY
Consider medication errors and adverse drug events in the differential diagnosis for patients who develop symptoms for which there is no obvious etiology. Educate patients, as well, to question their pharmacist if a recently filled prescription doesn’t look like the pill they usually take or makes them feel different than usual when they take it.
Of course, patients should be reminded that a generic medication may not always look the same as a brand-name drug or a previous generic prescription. But it can’t hurt for the patient to ask whether that medication that “looks different” is just a different generic—or a sign of a more worrisome mix-up.
THE CASE
A 60-year-old man with hypertension, gout, hyperlipidemia, and chronic sleep deprivation was referred to our neurology department for evaluation because he’d recently developed episodes of confusion and altered behavior that occurred daily. According to the patient’s wife, these episodes had started 4 weeks earlier while the patient was driving. He drove off the road while staring ahead with a “Joker-like” smile on his face. He was unable to utter more than a few words or respond to his wife, who was able to safely bring the car to a stop. The patient had spotty memory of this 40-minute episode.
Since then, he’d had similar but shorter episodes each morning, 20 to 75 minutes after taking his prescribed medications (lisinopril, simvastatin, and allopurinol). According to the patient’s wife, during these episodes, the patient would “act childish.” He would develop a voracious appetite and experience double or distorted vision, an unsteady gait, and poor muscle tone. These episodes were always followed by a long nap.
The man denied drinking, head trauma, acute illness, or taking illicit substances or any medications other than lisinopril, simvastatin, and allopurinol. Computed tomography, magnetic resonance imaging/magnetic resonance angiography, carotid Doppler ultrasound, and routine and 24-hour ambulatory electroencephalography (EEG) were normal.
Before the patient was referred to our neurology department, he had been prescribed a short course of the antiepileptic/mood stabilizer valproate and the wakefulness agent armodafinil, but neither medication had helped. The patient’s episodes continued daily, usually 20 to 75 minutes after taking his regular medications. When he decided to take them at night, the episodes began to occur at night.
His neurologic exam was normal. Family history was positive for a cousin with narcolepsy but negative for seizures and obstructive sleep apnea (OSA). Polysomnography revealed moderate OSA with minimal oxygen desaturation. Inpatient video EEG monitoring captured several of the events that the patient and his wife had described; the patient seemed “uninhibited” in his behavior. His EEG, cardiac telemetry, oxygen saturation, blood pressure, and serum glucose level remained normal.
The episodes’ sudden onset, peculiar symptoms, and duration—and the fact that they occurred after he took his usual medications—made complex partial seizures unlikely. The patient’s chronic sleep deprivation and family history of narcolepsy raised the possibility of “sleep attacks,” but the sudden onset and age of onset of his symptoms made those conditions less likely to explain the complete clinical picture. No particular hormonal disturbance could explain his presentation, and blood work was normal.
THE DIAGNOSIS
Because the patient’s episodes had been occurring shortly after the patient took his lisinopril, simvastatin, and allopurinol, and because his blood pressure and lipid levels were normal and his gout was asymptomatic, we decided to stop these medications. Later that day, the patient reported that he had discovered that his vial of lisinopril, which he had obtained from his regular pharmacy the day before his first episode, contained a different medication. He consulted a pharmacist, who determined that the vial contained extended release zolpidem 12.5 mg, and not his antihypertensive.
DISCUSSION
Although the true incidence of medication errors is difficult to determine, a 2006 Institute of Medicine report estimated that there are at least 1.5 million cases of preventable adverse drug events in the United States each year.1 In light of these statistics, medication errors need to be near the top of our differential diagnosis when patients suddenly develop symptoms for which there is no obvious cause.
Cause to pause? If you observe a temporal association between the onset of a patient’s symptoms and medication administration, consider possible adverse effects of the medication before ordering tests.
In this case … Our patient’s peculiar presentation correlated with regular ingestion of a high dose of zolpidem, a short-acting non-benzodiazepine gamma-aminobutyric acid (GABA) agonist. Zolpidem binds to the same GABAA receptor as benzodiazepines and therefore acts as a hypnotic by increasing GABA transmission.2 Neuropsychiatric adverse events associated with zolpidem include hallucinations, amnesia, parasomnia, psychomotor impairment, and complex behaviors (eg, sleepwalking or sleep-driving).2 Higher doses may cause coma or (rarely) death.2 One case report describes a patient who heard command hallucinations and stabbed himself after ingesting a large dose of zolpidem.3
Our patient
The patient’s episodes stopped after he discontinued the zolpidem. He subsequently received a correct prescription for lisinopril, and did not experience any additional episodes.
THE TAKEAWAY
Consider medication errors and adverse drug events in the differential diagnosis for patients who develop symptoms for which there is no obvious etiology. Educate patients, as well, to question their pharmacist if a recently filled prescription doesn’t look like the pill they usually take or makes them feel different than usual when they take it.
Of course, patients should be reminded that a generic medication may not always look the same as a brand-name drug or a previous generic prescription. But it can’t hurt for the patient to ask whether that medication that “looks different” is just a different generic—or a sign of a more worrisome mix-up.
1. Institute of Medicine. Preventing medication errors. Report Brief. July 2006. Institute of Medicine Web site. Available at: http://iom.edu/~/media/Files/Report%20Files/2006/Preventing-Medication-Errors-Quality-Chasm-Series/medicationerrorsnew.pdf. Accessed January 13, 2015.
2. Gunja N. The clinical and forensic toxicology of Z-drugs. J Med Toxicol. 2013;9:155-162.
3. Manfredi G, Kotzalidis GD, Lazanio S, et al. Command hallucinations with self-stabbing associated with zolpidem overdose. J Clin Psychiatry. 2010;71:92-93.
1. Institute of Medicine. Preventing medication errors. Report Brief. July 2006. Institute of Medicine Web site. Available at: http://iom.edu/~/media/Files/Report%20Files/2006/Preventing-Medication-Errors-Quality-Chasm-Series/medicationerrorsnew.pdf. Accessed January 13, 2015.
2. Gunja N. The clinical and forensic toxicology of Z-drugs. J Med Toxicol. 2013;9:155-162.
3. Manfredi G, Kotzalidis GD, Lazanio S, et al. Command hallucinations with self-stabbing associated with zolpidem overdose. J Clin Psychiatry. 2010;71:92-93.
Case Report: Recurrent Sagittal Sinus Thrombosis
Case
The patient’s past medical history included one miscarriage, as well as a papillary thyroid carcinoma with resection, which was discovered a few months before her presentation to the ED and after diagnosis of the initial SSS thrombosis.
Physical examination revealed a well-developed, mildly obese female. On arrival at the ED, the patient’s National Institutes of Health Stroke Scale score was 0. Her vital signs and ocular, neurological, and psychiatric examinations were all normal. The social history was negative for tobacco or alcohol use, and she had no family history of deep vein thrombosis (DVT) or pulmonary embolism.
A noncontrast computed tomography (CT) of the head demonstrated a hemorrhagic venous infarction involving the posterior right parietal lobe. Intracranial magnetic resonance venography (MRV) and brain magnetic resonance imaging (MRI) revealed thrombosis of the posterior third of the SSS as the source of the infarction. This sinus had been patent during the patient’s previous hospital admissions.
The patient’s international normalized ratio (INR) was therapeutic on presentation. Warfarin was discontinued, and she was started on an intravenous (IV) heparin drip. For anticoagulation, she was prescribed 20 mg rivaroxaban daily and 2,000 mg levetiracetam daily.
One week after discharge, the patient again presented to the ED with a recurrence of symptoms, including confusion, slurred speech, and headache, which she rated a “5” on a pain scale of 0 to 10. Similar to the previous ED visit, the slurred speech had resolved by the time of examination. The patient did not exhibit facial asymmetry but did complain of bilateral numbness and tingling in both hands. A noncontrast CT of the head showed no changes in the right parietal hemorrhagic venous infarct and intraparenchymal hemorrhage; however, there was an interval increase in edema compared to the prior CT. Rivaroxaban and levetiracetam were continued, and 20 mg simvastatin daily was prescribed.
Overview
Cerebral venous sinus thrombosis is a rare condition with an often varied clinical presentation—the symptoms of which can take hours to weeks to evolve, thus making the diagnosis challenging. In 70% of cases, the SSS and lateral sinuses are individually involved, and in 30% of cases, both regions are affected simultaneously.1 Only recently have clinicians been able to diagnose this condition antemortem.
Risk Factors and Etiology
Inherited and Acquired hypercoagulable states
Cerebral venous sinus thrombosis (CVST) and cerebrovascular accident (CVA) often result from a hypercoagulable state (HCS), and both acquired and inherited factors place patients at risk. Inherited factors are the most common cause of venous thromboembolism in patients younger than age 40 years. Acquired factors have a combined effect with inherited ones, leading to increased risk of CVST or CVA.2
The patient in this case possessed both acquired and inherited factors of an HCS. Inherited factors can be found through a thrombophilia evaluation. In general, acquired factors of thrombophilia include obesity, a prior history of thrombosis, pregnancy, and cancer and its treatment. A thrombophilia evaluation revealed the patient was homozygous for the 4G allele, which has been shown to increase concentration of plasminogen activator inhibitor (PAI-1) by 30%. An inhibitor to the pathway of fibrinolysis, PAI-1 is a major factor preventing the excessive presence and magnitude of blood clots.3
Pregnancy and the Puerperium
Cerebral vascular sinus thrombosis is most commonly seen in young to middle-aged women. High risk factors include pregnancy and the puerperium due to increased HCS during these periods.4 The incidence of CVST in this population is approximately 10 per 100,000 women.4
Oral Hormonal Contraceptives
In approximately 10% of CVST cases, oral hormonal contraceptive use in the presence of a coagulation disorder are frequently the cause—as observed in the incidence of DVT in this patient population.
Septic Cerebral Venous Sinus Thrombosis
Septic CVST occurs mainly in children and up to 18% of adult cases in developing countries. It is associated with localized infections (eg, mastoiditis, otitis media, sinusitis, meningitis).
Other Causes
Although rare, other causes of CVST include intracranial hypotension, hydrocephalus, and the use of certain drugs and supplements (eg, corticosteroids, high doses of vitamin A). Each of these potential causes also should be considered when evaluating for CVST.4
Symptoms and Signs
Common symptoms and signs of CVST include headache, nausea, vomiting, seizure, and focal neurological deficit. Papilledema is present in 40% of cases, primarily in patients with delayed diagnosis or a chronic course.
Neurological Deficits
Cerebral venous sinus thrombosis may not necessarily cause focal neurological deficits due to numerous pathways of venous drainage and the possibility of reversal of venous blood flow. However, the condition can lead to impaired resorption of CSF causing intracranial hypertension.
Headache
In 70% of cases, headache is the initial symptom of CVST, and it is the only symptom in 16% of cases. With respect to headache presentation, it is important to remember that thunderclap headache is not exclusive to the diagnosis of subarachnoid hemorrhage (SAH). The absence of findings on workup to support the diagnosis of SAH should prompt investigation with MRV and evaluation of CVST.4
Seizure
Focal or generalized seizure on initial presentation occurs in 30% to 40% of cases of CVST. When smaller cerebral veins are involved, this can lead to focal edema, neurological deficits, venous infarction, and seizure. Focal deficits are determined by the localization of CVST and associated lesions. Other symptoms may include migraine headache, transient ischemic attack, cranial nerve palsies, and subarachnoid hemorrhage.4
Complications
Cerebral venous sinus thrombosis is a rare condition with an often varied clinical presentation—the symptoms of which can take hours to weeks to evolve, thus making the diagnosis challenging.
Complications in patients with CVST occur when venous congestion increases and raises dural venous sinus and cerebral spinal fluid (CSF) pressure. Parenchymal edema with venous infarction and hemorrhage complicates up to 50% of venous sinus thromboses (as seen in this patient).1 Unfortunately, little is known about long-term risk outcomes or recurrence of CVST.1
As previously noted, the patient presented with transient slurred speech, mild headache, and bilateral hand tingling. On workup, she was found to have an SSS thrombosis with an associated right intraparenchymal hemorrhage that occurred despite therapeutic INR levels and the initiation of coumadin therapy prior to admission.
Evaluation and Diagnosis
D-Dimer Evaluation
There is a strong association between D-dimer levels above 500 ng/mL and acute CVST. Nevertheless, lower levels do not rule out the diagnosis in a patient presenting with headache.4
Imaging Techniques
Important imaging techniques in the evaluation of CVST include CT, MRV, MRI, and magnetic resonance angiography (MRA). The first imaging modality in evaluating a patient with neurological symptoms and headache in the ED is CT, which can show evidence of an infarction that does not respond to an arterial distribution. In the absence of a hemorrhagic component, however, infarct demonstration may be delayed for up to 72 hours.5 On contrast CT, an empty delta sign may be apparent due to enhancement of the collateral veins in the SSS walls surrounding a nonenhanced thrombus. The delta sign is not frequently present and may be false due to early division of the SSS.5
Computed tomography venography, CT angiography, and MRI can also be utilized to evaluate for CVST. Computed tomography venography is especially useful in identifying the cerebral veins and dural sinuses,6 and MRV is an excellent method for visualizing the dural venous sinuses and larger cerebral veins. Single-slice phase-contrast angiography is also a rapid and reliable test for CVST.7 Conventional angiography and direct venography should be considered if MR studies are nondiagnostic; however, this test is invasive with associated risks.5
Treatment
Heparin therapy should be initiated in patients presenting with dural sinus thrombosis even if pre-existing hemorrhage exists. Patients failing to respond to therapy with worsening neurological deficits may warrant local thrombolysis with tissue plasminogen activator. Identifying those in the acute state of disease is essential as they may have poor prognostic outcomes that may warrant more invasive intervention.1
Conclusion
Cerebral venous sinus thrombosis is a rare condition with a diverse clinical presentation. As demonstrated in this case, some patients present with stroke-like symptoms of nontraumatic headache, slurred speech, and bilateral hand tingling, which, on workup, reveal SSS thrombosis associated right intraparenchymal hemorrhage.
This case draws attention to the importance of risk stratification in patients with a history of HCS and neurological complaints presenting to the ED. Dural sinus thrombosis may have a vague initial neurological presentation; therefore, early recognition and initiation of therapy will assist in reducing morbidity and mortality.
Dr Orlik is a resident, department of emergency medicine, Akron General Medical Center, Ohio. Mr Kovacs is a student and summer research fellow, department of emergency medicine, Akron General Medical Center, Ohio. Dr Simon is the emergency medicine research director, department of emergency medicine, Akron General Medical Center, Northeast Ohio Medical University.
- Kimber J. Cerebral venous sinus thrombosis. QJM. 2002;95(3):137-142.
- Anderson JA, Weitz JI. Hypercoagulable states. In: Hoffman R, Benz EJ, Jr, Silberstein LE, Heslop HE, Weitz JI, Anastasi J, eds. Hematology: Basic Principles and Practice. 6th ed. Philadelphia, PA: Elsevier Saunders; 2013:2013-2024.
- Humphries SE, Panahloo A, Montgomery HE, Green F, Yudkin J. Gene-environment interaction in the determination of levels of haemostatic variables involved in thrombosis and fibrinolysis. Thromb Haemost. 1997;78(1):457-461.
- Weimar C. Diagnosis and treatment of cerebral venous and sinus thrombosis. Curr Neurol Neurosci Rep. 2014;14(1):417.
- Masuhr F, Mehraein S, Einhäupl K. Cerebral venous and sinus thrombosis. J Neurol. 2004;251(1):11-23.
- Ozsvath RR, Casey SO, Lustrin ES, et al. Cerebral venography: comparison of CT and MR projection venography. AJR Am J Roentgenol. 1997;169(6):1699-1707.
- Adams WM, Laitt RD, Beards SC, Kassner A, Jackson A. Use of single-slice thick slab phase-contrast angiography for the diagnosis of dural venous sinus thrombosis. Eur Radiol. 1999;9(8):1614-1619.
Case
The patient’s past medical history included one miscarriage, as well as a papillary thyroid carcinoma with resection, which was discovered a few months before her presentation to the ED and after diagnosis of the initial SSS thrombosis.
Physical examination revealed a well-developed, mildly obese female. On arrival at the ED, the patient’s National Institutes of Health Stroke Scale score was 0. Her vital signs and ocular, neurological, and psychiatric examinations were all normal. The social history was negative for tobacco or alcohol use, and she had no family history of deep vein thrombosis (DVT) or pulmonary embolism.
A noncontrast computed tomography (CT) of the head demonstrated a hemorrhagic venous infarction involving the posterior right parietal lobe. Intracranial magnetic resonance venography (MRV) and brain magnetic resonance imaging (MRI) revealed thrombosis of the posterior third of the SSS as the source of the infarction. This sinus had been patent during the patient’s previous hospital admissions.
The patient’s international normalized ratio (INR) was therapeutic on presentation. Warfarin was discontinued, and she was started on an intravenous (IV) heparin drip. For anticoagulation, she was prescribed 20 mg rivaroxaban daily and 2,000 mg levetiracetam daily.
One week after discharge, the patient again presented to the ED with a recurrence of symptoms, including confusion, slurred speech, and headache, which she rated a “5” on a pain scale of 0 to 10. Similar to the previous ED visit, the slurred speech had resolved by the time of examination. The patient did not exhibit facial asymmetry but did complain of bilateral numbness and tingling in both hands. A noncontrast CT of the head showed no changes in the right parietal hemorrhagic venous infarct and intraparenchymal hemorrhage; however, there was an interval increase in edema compared to the prior CT. Rivaroxaban and levetiracetam were continued, and 20 mg simvastatin daily was prescribed.
Overview
Cerebral venous sinus thrombosis is a rare condition with an often varied clinical presentation—the symptoms of which can take hours to weeks to evolve, thus making the diagnosis challenging. In 70% of cases, the SSS and lateral sinuses are individually involved, and in 30% of cases, both regions are affected simultaneously.1 Only recently have clinicians been able to diagnose this condition antemortem.
Risk Factors and Etiology
Inherited and Acquired hypercoagulable states
Cerebral venous sinus thrombosis (CVST) and cerebrovascular accident (CVA) often result from a hypercoagulable state (HCS), and both acquired and inherited factors place patients at risk. Inherited factors are the most common cause of venous thromboembolism in patients younger than age 40 years. Acquired factors have a combined effect with inherited ones, leading to increased risk of CVST or CVA.2
The patient in this case possessed both acquired and inherited factors of an HCS. Inherited factors can be found through a thrombophilia evaluation. In general, acquired factors of thrombophilia include obesity, a prior history of thrombosis, pregnancy, and cancer and its treatment. A thrombophilia evaluation revealed the patient was homozygous for the 4G allele, which has been shown to increase concentration of plasminogen activator inhibitor (PAI-1) by 30%. An inhibitor to the pathway of fibrinolysis, PAI-1 is a major factor preventing the excessive presence and magnitude of blood clots.3
Pregnancy and the Puerperium
Cerebral vascular sinus thrombosis is most commonly seen in young to middle-aged women. High risk factors include pregnancy and the puerperium due to increased HCS during these periods.4 The incidence of CVST in this population is approximately 10 per 100,000 women.4
Oral Hormonal Contraceptives
In approximately 10% of CVST cases, oral hormonal contraceptive use in the presence of a coagulation disorder are frequently the cause—as observed in the incidence of DVT in this patient population.
Septic Cerebral Venous Sinus Thrombosis
Septic CVST occurs mainly in children and up to 18% of adult cases in developing countries. It is associated with localized infections (eg, mastoiditis, otitis media, sinusitis, meningitis).
Other Causes
Although rare, other causes of CVST include intracranial hypotension, hydrocephalus, and the use of certain drugs and supplements (eg, corticosteroids, high doses of vitamin A). Each of these potential causes also should be considered when evaluating for CVST.4
Symptoms and Signs
Common symptoms and signs of CVST include headache, nausea, vomiting, seizure, and focal neurological deficit. Papilledema is present in 40% of cases, primarily in patients with delayed diagnosis or a chronic course.
Neurological Deficits
Cerebral venous sinus thrombosis may not necessarily cause focal neurological deficits due to numerous pathways of venous drainage and the possibility of reversal of venous blood flow. However, the condition can lead to impaired resorption of CSF causing intracranial hypertension.
Headache
In 70% of cases, headache is the initial symptom of CVST, and it is the only symptom in 16% of cases. With respect to headache presentation, it is important to remember that thunderclap headache is not exclusive to the diagnosis of subarachnoid hemorrhage (SAH). The absence of findings on workup to support the diagnosis of SAH should prompt investigation with MRV and evaluation of CVST.4
Seizure
Focal or generalized seizure on initial presentation occurs in 30% to 40% of cases of CVST. When smaller cerebral veins are involved, this can lead to focal edema, neurological deficits, venous infarction, and seizure. Focal deficits are determined by the localization of CVST and associated lesions. Other symptoms may include migraine headache, transient ischemic attack, cranial nerve palsies, and subarachnoid hemorrhage.4
Complications
Cerebral venous sinus thrombosis is a rare condition with an often varied clinical presentation—the symptoms of which can take hours to weeks to evolve, thus making the diagnosis challenging.
Complications in patients with CVST occur when venous congestion increases and raises dural venous sinus and cerebral spinal fluid (CSF) pressure. Parenchymal edema with venous infarction and hemorrhage complicates up to 50% of venous sinus thromboses (as seen in this patient).1 Unfortunately, little is known about long-term risk outcomes or recurrence of CVST.1
As previously noted, the patient presented with transient slurred speech, mild headache, and bilateral hand tingling. On workup, she was found to have an SSS thrombosis with an associated right intraparenchymal hemorrhage that occurred despite therapeutic INR levels and the initiation of coumadin therapy prior to admission.
Evaluation and Diagnosis
D-Dimer Evaluation
There is a strong association between D-dimer levels above 500 ng/mL and acute CVST. Nevertheless, lower levels do not rule out the diagnosis in a patient presenting with headache.4
Imaging Techniques
Important imaging techniques in the evaluation of CVST include CT, MRV, MRI, and magnetic resonance angiography (MRA). The first imaging modality in evaluating a patient with neurological symptoms and headache in the ED is CT, which can show evidence of an infarction that does not respond to an arterial distribution. In the absence of a hemorrhagic component, however, infarct demonstration may be delayed for up to 72 hours.5 On contrast CT, an empty delta sign may be apparent due to enhancement of the collateral veins in the SSS walls surrounding a nonenhanced thrombus. The delta sign is not frequently present and may be false due to early division of the SSS.5
Computed tomography venography, CT angiography, and MRI can also be utilized to evaluate for CVST. Computed tomography venography is especially useful in identifying the cerebral veins and dural sinuses,6 and MRV is an excellent method for visualizing the dural venous sinuses and larger cerebral veins. Single-slice phase-contrast angiography is also a rapid and reliable test for CVST.7 Conventional angiography and direct venography should be considered if MR studies are nondiagnostic; however, this test is invasive with associated risks.5
Treatment
Heparin therapy should be initiated in patients presenting with dural sinus thrombosis even if pre-existing hemorrhage exists. Patients failing to respond to therapy with worsening neurological deficits may warrant local thrombolysis with tissue plasminogen activator. Identifying those in the acute state of disease is essential as they may have poor prognostic outcomes that may warrant more invasive intervention.1
Conclusion
Cerebral venous sinus thrombosis is a rare condition with a diverse clinical presentation. As demonstrated in this case, some patients present with stroke-like symptoms of nontraumatic headache, slurred speech, and bilateral hand tingling, which, on workup, reveal SSS thrombosis associated right intraparenchymal hemorrhage.
This case draws attention to the importance of risk stratification in patients with a history of HCS and neurological complaints presenting to the ED. Dural sinus thrombosis may have a vague initial neurological presentation; therefore, early recognition and initiation of therapy will assist in reducing morbidity and mortality.
Dr Orlik is a resident, department of emergency medicine, Akron General Medical Center, Ohio. Mr Kovacs is a student and summer research fellow, department of emergency medicine, Akron General Medical Center, Ohio. Dr Simon is the emergency medicine research director, department of emergency medicine, Akron General Medical Center, Northeast Ohio Medical University.
Case
The patient’s past medical history included one miscarriage, as well as a papillary thyroid carcinoma with resection, which was discovered a few months before her presentation to the ED and after diagnosis of the initial SSS thrombosis.
Physical examination revealed a well-developed, mildly obese female. On arrival at the ED, the patient’s National Institutes of Health Stroke Scale score was 0. Her vital signs and ocular, neurological, and psychiatric examinations were all normal. The social history was negative for tobacco or alcohol use, and she had no family history of deep vein thrombosis (DVT) or pulmonary embolism.
A noncontrast computed tomography (CT) of the head demonstrated a hemorrhagic venous infarction involving the posterior right parietal lobe. Intracranial magnetic resonance venography (MRV) and brain magnetic resonance imaging (MRI) revealed thrombosis of the posterior third of the SSS as the source of the infarction. This sinus had been patent during the patient’s previous hospital admissions.
The patient’s international normalized ratio (INR) was therapeutic on presentation. Warfarin was discontinued, and she was started on an intravenous (IV) heparin drip. For anticoagulation, she was prescribed 20 mg rivaroxaban daily and 2,000 mg levetiracetam daily.
One week after discharge, the patient again presented to the ED with a recurrence of symptoms, including confusion, slurred speech, and headache, which she rated a “5” on a pain scale of 0 to 10. Similar to the previous ED visit, the slurred speech had resolved by the time of examination. The patient did not exhibit facial asymmetry but did complain of bilateral numbness and tingling in both hands. A noncontrast CT of the head showed no changes in the right parietal hemorrhagic venous infarct and intraparenchymal hemorrhage; however, there was an interval increase in edema compared to the prior CT. Rivaroxaban and levetiracetam were continued, and 20 mg simvastatin daily was prescribed.
Overview
Cerebral venous sinus thrombosis is a rare condition with an often varied clinical presentation—the symptoms of which can take hours to weeks to evolve, thus making the diagnosis challenging. In 70% of cases, the SSS and lateral sinuses are individually involved, and in 30% of cases, both regions are affected simultaneously.1 Only recently have clinicians been able to diagnose this condition antemortem.
Risk Factors and Etiology
Inherited and Acquired hypercoagulable states
Cerebral venous sinus thrombosis (CVST) and cerebrovascular accident (CVA) often result from a hypercoagulable state (HCS), and both acquired and inherited factors place patients at risk. Inherited factors are the most common cause of venous thromboembolism in patients younger than age 40 years. Acquired factors have a combined effect with inherited ones, leading to increased risk of CVST or CVA.2
The patient in this case possessed both acquired and inherited factors of an HCS. Inherited factors can be found through a thrombophilia evaluation. In general, acquired factors of thrombophilia include obesity, a prior history of thrombosis, pregnancy, and cancer and its treatment. A thrombophilia evaluation revealed the patient was homozygous for the 4G allele, which has been shown to increase concentration of plasminogen activator inhibitor (PAI-1) by 30%. An inhibitor to the pathway of fibrinolysis, PAI-1 is a major factor preventing the excessive presence and magnitude of blood clots.3
Pregnancy and the Puerperium
Cerebral vascular sinus thrombosis is most commonly seen in young to middle-aged women. High risk factors include pregnancy and the puerperium due to increased HCS during these periods.4 The incidence of CVST in this population is approximately 10 per 100,000 women.4
Oral Hormonal Contraceptives
In approximately 10% of CVST cases, oral hormonal contraceptive use in the presence of a coagulation disorder are frequently the cause—as observed in the incidence of DVT in this patient population.
Septic Cerebral Venous Sinus Thrombosis
Septic CVST occurs mainly in children and up to 18% of adult cases in developing countries. It is associated with localized infections (eg, mastoiditis, otitis media, sinusitis, meningitis).
Other Causes
Although rare, other causes of CVST include intracranial hypotension, hydrocephalus, and the use of certain drugs and supplements (eg, corticosteroids, high doses of vitamin A). Each of these potential causes also should be considered when evaluating for CVST.4
Symptoms and Signs
Common symptoms and signs of CVST include headache, nausea, vomiting, seizure, and focal neurological deficit. Papilledema is present in 40% of cases, primarily in patients with delayed diagnosis or a chronic course.
Neurological Deficits
Cerebral venous sinus thrombosis may not necessarily cause focal neurological deficits due to numerous pathways of venous drainage and the possibility of reversal of venous blood flow. However, the condition can lead to impaired resorption of CSF causing intracranial hypertension.
Headache
In 70% of cases, headache is the initial symptom of CVST, and it is the only symptom in 16% of cases. With respect to headache presentation, it is important to remember that thunderclap headache is not exclusive to the diagnosis of subarachnoid hemorrhage (SAH). The absence of findings on workup to support the diagnosis of SAH should prompt investigation with MRV and evaluation of CVST.4
Seizure
Focal or generalized seizure on initial presentation occurs in 30% to 40% of cases of CVST. When smaller cerebral veins are involved, this can lead to focal edema, neurological deficits, venous infarction, and seizure. Focal deficits are determined by the localization of CVST and associated lesions. Other symptoms may include migraine headache, transient ischemic attack, cranial nerve palsies, and subarachnoid hemorrhage.4
Complications
Cerebral venous sinus thrombosis is a rare condition with an often varied clinical presentation—the symptoms of which can take hours to weeks to evolve, thus making the diagnosis challenging.
Complications in patients with CVST occur when venous congestion increases and raises dural venous sinus and cerebral spinal fluid (CSF) pressure. Parenchymal edema with venous infarction and hemorrhage complicates up to 50% of venous sinus thromboses (as seen in this patient).1 Unfortunately, little is known about long-term risk outcomes or recurrence of CVST.1
As previously noted, the patient presented with transient slurred speech, mild headache, and bilateral hand tingling. On workup, she was found to have an SSS thrombosis with an associated right intraparenchymal hemorrhage that occurred despite therapeutic INR levels and the initiation of coumadin therapy prior to admission.
Evaluation and Diagnosis
D-Dimer Evaluation
There is a strong association between D-dimer levels above 500 ng/mL and acute CVST. Nevertheless, lower levels do not rule out the diagnosis in a patient presenting with headache.4
Imaging Techniques
Important imaging techniques in the evaluation of CVST include CT, MRV, MRI, and magnetic resonance angiography (MRA). The first imaging modality in evaluating a patient with neurological symptoms and headache in the ED is CT, which can show evidence of an infarction that does not respond to an arterial distribution. In the absence of a hemorrhagic component, however, infarct demonstration may be delayed for up to 72 hours.5 On contrast CT, an empty delta sign may be apparent due to enhancement of the collateral veins in the SSS walls surrounding a nonenhanced thrombus. The delta sign is not frequently present and may be false due to early division of the SSS.5
Computed tomography venography, CT angiography, and MRI can also be utilized to evaluate for CVST. Computed tomography venography is especially useful in identifying the cerebral veins and dural sinuses,6 and MRV is an excellent method for visualizing the dural venous sinuses and larger cerebral veins. Single-slice phase-contrast angiography is also a rapid and reliable test for CVST.7 Conventional angiography and direct venography should be considered if MR studies are nondiagnostic; however, this test is invasive with associated risks.5
Treatment
Heparin therapy should be initiated in patients presenting with dural sinus thrombosis even if pre-existing hemorrhage exists. Patients failing to respond to therapy with worsening neurological deficits may warrant local thrombolysis with tissue plasminogen activator. Identifying those in the acute state of disease is essential as they may have poor prognostic outcomes that may warrant more invasive intervention.1
Conclusion
Cerebral venous sinus thrombosis is a rare condition with a diverse clinical presentation. As demonstrated in this case, some patients present with stroke-like symptoms of nontraumatic headache, slurred speech, and bilateral hand tingling, which, on workup, reveal SSS thrombosis associated right intraparenchymal hemorrhage.
This case draws attention to the importance of risk stratification in patients with a history of HCS and neurological complaints presenting to the ED. Dural sinus thrombosis may have a vague initial neurological presentation; therefore, early recognition and initiation of therapy will assist in reducing morbidity and mortality.
Dr Orlik is a resident, department of emergency medicine, Akron General Medical Center, Ohio. Mr Kovacs is a student and summer research fellow, department of emergency medicine, Akron General Medical Center, Ohio. Dr Simon is the emergency medicine research director, department of emergency medicine, Akron General Medical Center, Northeast Ohio Medical University.
- Kimber J. Cerebral venous sinus thrombosis. QJM. 2002;95(3):137-142.
- Anderson JA, Weitz JI. Hypercoagulable states. In: Hoffman R, Benz EJ, Jr, Silberstein LE, Heslop HE, Weitz JI, Anastasi J, eds. Hematology: Basic Principles and Practice. 6th ed. Philadelphia, PA: Elsevier Saunders; 2013:2013-2024.
- Humphries SE, Panahloo A, Montgomery HE, Green F, Yudkin J. Gene-environment interaction in the determination of levels of haemostatic variables involved in thrombosis and fibrinolysis. Thromb Haemost. 1997;78(1):457-461.
- Weimar C. Diagnosis and treatment of cerebral venous and sinus thrombosis. Curr Neurol Neurosci Rep. 2014;14(1):417.
- Masuhr F, Mehraein S, Einhäupl K. Cerebral venous and sinus thrombosis. J Neurol. 2004;251(1):11-23.
- Ozsvath RR, Casey SO, Lustrin ES, et al. Cerebral venography: comparison of CT and MR projection venography. AJR Am J Roentgenol. 1997;169(6):1699-1707.
- Adams WM, Laitt RD, Beards SC, Kassner A, Jackson A. Use of single-slice thick slab phase-contrast angiography for the diagnosis of dural venous sinus thrombosis. Eur Radiol. 1999;9(8):1614-1619.
- Kimber J. Cerebral venous sinus thrombosis. QJM. 2002;95(3):137-142.
- Anderson JA, Weitz JI. Hypercoagulable states. In: Hoffman R, Benz EJ, Jr, Silberstein LE, Heslop HE, Weitz JI, Anastasi J, eds. Hematology: Basic Principles and Practice. 6th ed. Philadelphia, PA: Elsevier Saunders; 2013:2013-2024.
- Humphries SE, Panahloo A, Montgomery HE, Green F, Yudkin J. Gene-environment interaction in the determination of levels of haemostatic variables involved in thrombosis and fibrinolysis. Thromb Haemost. 1997;78(1):457-461.
- Weimar C. Diagnosis and treatment of cerebral venous and sinus thrombosis. Curr Neurol Neurosci Rep. 2014;14(1):417.
- Masuhr F, Mehraein S, Einhäupl K. Cerebral venous and sinus thrombosis. J Neurol. 2004;251(1):11-23.
- Ozsvath RR, Casey SO, Lustrin ES, et al. Cerebral venography: comparison of CT and MR projection venography. AJR Am J Roentgenol. 1997;169(6):1699-1707.
- Adams WM, Laitt RD, Beards SC, Kassner A, Jackson A. Use of single-slice thick slab phase-contrast angiography for the diagnosis of dural venous sinus thrombosis. Eur Radiol. 1999;9(8):1614-1619.
Cutaneous Burn Caused by Radiofrequency Ablation Probe During Shoulder Arthroscopy
Cautery and radiofrequency ablation (RFA) devices are commonly used in shoulder arthroscopic surgery for hemostasis and ablation of soft tissue. Although these devices are easily used and applied, complications (eg, extensive release of deltoid muscle,1 nerve damage,2 tendon damage,3 cartilage damage from heat transfer4) can occur during arthroscopic surgery. Radiofrequency devices can elevate fluid temperatures to unsafe levels and directly or indirectly injure surrounding tissue.5,6 Skin complications from using these devices include direct burns to the subcutaneous tissues from the joint to the skin surface7 and skin burns related to overheated arthroscopic fluid.8
In our English-language literature review, however, we found no report of a skin burn secondary to contact between a RFA device and a spinal needle used in identifying structures during an arthroscopic acromioplasty. We report such a case here. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old woman injured her left, nondominant shoulder when a descending garage door hit her directly on the superior aspect of the shoulder. She had immediate onset of pain on the top and lateral side of the shoulder and was evaluated by a primary care physician. Radiographs and magnetic resonance imaging (MRI) were normal. The patient was referred to an orthopedic surgeon for further evaluation.
The orthopedic surgeon found her to be in good health, with no history of diabetes, vascular conditions, or skin disorders. The initial diagnosis after history taking and physical examination was impingement syndrome with subacromial bursitis. The surgeon recommended nonoperative treatment: ice, nonsteroidal anti-inflammatory drugs, and physical therapy. After 3 months, the patient’s examination was unchanged, and there was no improvement in pain. Cortisone injected into the subacromial space helped for a few weeks, but the pain returned. After 2 more cortisone injections over 9 months failed, repeat MRI showed no tears of the rotator cuff or any other salient abnormalities. The treatment options were discussed with the patient, and, because the physical examination findings were consistent with impingement syndrome and nonoperative measures had failed, she consented to arthroscopic evaluation of the shoulder and arthroscopic partial anterior-lateral acromioplasty.
The procedure was performed 8 months after initial injury. With the patient under general anesthesia and in a lateral decubitus position, her arm was placed in an arm holder. Before the partial acromioplasty, two 18-gauge spinal needles were inserted from the skin surface into the subacromial space to help localize the anterolateral acromion and the acromioclavicular joint. The procedure was performed with a pump using saline bags kept at room temperature. A bipolar radiofrequency device (Stryker Energy Radiofrequency Ablation System; Stryker, Mahwah, New Jersey) was used to débride the subacromial bursa and the periosteum of the undersurface of the acromion. While the bursa was being débrided, the radiofrequency device inadvertently touched the anterior lateral needle probe, and a small skin burn formed around the needle on the surface of the shoulder (Figure). The radiofrequency device did not directly contact the skin, and the deltoid fascia was intact. The spinal needle was removed, and the skin around the burn was excised; the muscle beneath the skin was intact and showed no signs of thermal damage. The skin was mobilized and closed with interrupted simple sutures using a 4-0 nylon suture. The procedure was then completed with no other complications.
After surgery, the patient recovered without complications, and the skin lesion healed with no signs of infection and no skin or muscle defects. Some stiffness was treated with medication and physical therapy. Nine months after surgery, the patient reported mild shoulder stiffness and remained dissatisfied with the appearance of the skin in the area of the burn.
Discussion
Our patient’s case is a reminder that contact between a radiofrequency device and metal needles can transfer heat to tissues and cause skin burns. When using a radiofrequency device around metal needles or cannulas, surgeons should be sure to avoid prolonged contact with the metal. Our patient’s case is the first reported case of a thermal skin injury occurring when a spinal needle was heated by an arthroscopic ablater.
Other authors have reported indirect thermal skin injuries caused by radiofrequency devices during arthroscopic surgery, but the causes were postulated to be direct contact between device and skin7 and overheating of the arthroscopy fluid.5,6,8 Huang and colleagues8 reported that full-thickness skin burns occurred when normal saline used during routine knee arthroscopy overheated from use of a radiofrequency device. Burn lesions, noted on their patient’s leg within 1 day after surgery, required subsequent débridement, a muscle flap, and split-skin grafting. Skin burns caused by overheated fluid have occurred irrespective of type of fluid used (eg, 1.5% glycine or lactated Ringer solution).6 There was no evidence that our patient’s burn resulted from extravasated overheated fluid, as the lesion was localized to the area immediately around the needle and was not geographic, as was described by Huang and colleagues.8
Other possible causes of skin burns during arthroscopic surgery have been described, but none applies in our patient’s case. Segami and colleagues7 described a burn resulting from direct transfer of heat from the radiofrequency device to the skin because of their proximity. This mechanism was not the cause in our patient’s case; there was no evidence of a defect or burned deltoid muscle at time of surgery. Lau and Dao9 reported 2 small full-thickness skin burns caused by a fiberoptic-light cable tip placed on a patient’s leg; in addition, the hot (>170°C) cables caused the paper drapes to combust.9 Skin burns secondary to use of skin antiseptics have been reported,10 but such lesions typically are located beneath tourniquets or in areas of friction from surgical drapes. In some cases, lesions described as skin burns may actually have been pressure lesions secondary to moist skin and friction.11
Whether type of radiofrequency device contributes to the occurrence of heat-related lesions during arthroscopic surgery is unknown. Some investigators have suggested there is more potential for harm with bipolar RFA devices than with monopolar devices.12,13 Monopolar devices pass energy between a probe and a grounding plate, whereas bipolar devices pass energy through 2 points on the probe.14 Because the heat for the monopolar probe derives from the frictional resistance of tissues to each other rather than from the probe itself, the bipolar probe theoretically allows for better temperature control. In addition, bipolar probes require less current to achieve the same heating effect. However, recent studies have suggested that, compared with monopolar radiofrequency devices, bipolar radiofrequency devices are associated with larger increases in temperature at equal depths after an equal number of applications.12,13
To our knowledge, no one has specifically investigated the type of bipolar device used in the present case. This case report, the first to describe a thermal skin injury caused by direct contact between a radiofrequency device and a metal needle inserted in the skin, is a reminder that contact between radiofrequency devices and spinal needles or other metal cannulas used in arthroscopic surgery should be avoided.
1. Bonsell S. Detached deltoid during arthroscopic subacromial decompression. Arthroscopy. 2000;16(7):745-748.
2. Mohammed KD, Hayes MG, Saies AD. Unusual complications of shoulder arthroscopy. J Shoulder Elbow Surg. 2000;9(4):350-353.
3. Pell RF 4th, Uhl RL. Complications of thermal ablation in wrist arthroscopy. Arthroscopy. 2004;20(suppl 2):84-86.
4. Lu Y, Hayashi K, Hecht P, et al. The effect of monopolar radiofrequency energy on partial-thickness defects of articular cartilage. Arthroscopy. 2000;16(5):527-536.
5. Kouk SN, Zoric B, Stetson WB. Complication of the use of a radiofrequency device in arthroscopic shoulder surgery: second-degree burn of the shoulder girdle. Arthroscopy. 2011;27(1):136-141.
6. Lord MJ, Maltry JA, Shall LM. Thermal injury resulting from arthroscopic lateral retinacular release by electrocautery: report of three cases and a review of the literature. Arthroscopy. 1991;7(1):33-37.
7. Segami N, Yamada T, Nishimura M. Thermal injury during temporomandibular joint arthroscopy: a case report. J Oral Maxillofac Surg. 2004;62(4):508-510.
8. Huang S, Gateley D, Moss ALH. Accidental burn injury during knee arthroscopy. Arthroscopy. 2007;23(12):1363.e1-e3.
9. Lau YJ, Dao Q. Cutaneous burns from a fiberoptic cable tip during arthroscopy of the knee. Knee. 2008;15(4):333-335.
10. Sanders TH, Hawken SM. Chlorhexidine burns after shoulder arthroscopy. Am J Orthop. 2012;41(4):172-174.
11. Keyurapan E, Hu SJ, Redett R, McCarthy EF, McFarland EG. Pressure ulcers of the thorax after shoulder surgery. Knee Surg Sports Traumatol Arthrosc. 2007;15(12):1489-1493.
12. Edwards RB 3rd, Lu Y, Rodriguez E, Markel MD. Thermometric determination of cartilage matrix temperatures during thermal chondroplasty: comparison of bipolar and monopolar radiofrequency devices. Arthroscopy. 2002;18(4):339-346.
13. Figueroa D, Calvo R, Vaisman A, et al. Bipolar radiofrequency in the human meniscus. Comparative study between patients younger and older than 40 years of age. Knee. 2007;14(5):357-360.
14. Sahasrabudhe A, McMahon PJ. Thermal probes: what’s available in 2004. Oper Tech Sports Med. 2004;12:206-209.
Cautery and radiofrequency ablation (RFA) devices are commonly used in shoulder arthroscopic surgery for hemostasis and ablation of soft tissue. Although these devices are easily used and applied, complications (eg, extensive release of deltoid muscle,1 nerve damage,2 tendon damage,3 cartilage damage from heat transfer4) can occur during arthroscopic surgery. Radiofrequency devices can elevate fluid temperatures to unsafe levels and directly or indirectly injure surrounding tissue.5,6 Skin complications from using these devices include direct burns to the subcutaneous tissues from the joint to the skin surface7 and skin burns related to overheated arthroscopic fluid.8
In our English-language literature review, however, we found no report of a skin burn secondary to contact between a RFA device and a spinal needle used in identifying structures during an arthroscopic acromioplasty. We report such a case here. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old woman injured her left, nondominant shoulder when a descending garage door hit her directly on the superior aspect of the shoulder. She had immediate onset of pain on the top and lateral side of the shoulder and was evaluated by a primary care physician. Radiographs and magnetic resonance imaging (MRI) were normal. The patient was referred to an orthopedic surgeon for further evaluation.
The orthopedic surgeon found her to be in good health, with no history of diabetes, vascular conditions, or skin disorders. The initial diagnosis after history taking and physical examination was impingement syndrome with subacromial bursitis. The surgeon recommended nonoperative treatment: ice, nonsteroidal anti-inflammatory drugs, and physical therapy. After 3 months, the patient’s examination was unchanged, and there was no improvement in pain. Cortisone injected into the subacromial space helped for a few weeks, but the pain returned. After 2 more cortisone injections over 9 months failed, repeat MRI showed no tears of the rotator cuff or any other salient abnormalities. The treatment options were discussed with the patient, and, because the physical examination findings were consistent with impingement syndrome and nonoperative measures had failed, she consented to arthroscopic evaluation of the shoulder and arthroscopic partial anterior-lateral acromioplasty.
The procedure was performed 8 months after initial injury. With the patient under general anesthesia and in a lateral decubitus position, her arm was placed in an arm holder. Before the partial acromioplasty, two 18-gauge spinal needles were inserted from the skin surface into the subacromial space to help localize the anterolateral acromion and the acromioclavicular joint. The procedure was performed with a pump using saline bags kept at room temperature. A bipolar radiofrequency device (Stryker Energy Radiofrequency Ablation System; Stryker, Mahwah, New Jersey) was used to débride the subacromial bursa and the periosteum of the undersurface of the acromion. While the bursa was being débrided, the radiofrequency device inadvertently touched the anterior lateral needle probe, and a small skin burn formed around the needle on the surface of the shoulder (Figure). The radiofrequency device did not directly contact the skin, and the deltoid fascia was intact. The spinal needle was removed, and the skin around the burn was excised; the muscle beneath the skin was intact and showed no signs of thermal damage. The skin was mobilized and closed with interrupted simple sutures using a 4-0 nylon suture. The procedure was then completed with no other complications.
After surgery, the patient recovered without complications, and the skin lesion healed with no signs of infection and no skin or muscle defects. Some stiffness was treated with medication and physical therapy. Nine months after surgery, the patient reported mild shoulder stiffness and remained dissatisfied with the appearance of the skin in the area of the burn.
Discussion
Our patient’s case is a reminder that contact between a radiofrequency device and metal needles can transfer heat to tissues and cause skin burns. When using a radiofrequency device around metal needles or cannulas, surgeons should be sure to avoid prolonged contact with the metal. Our patient’s case is the first reported case of a thermal skin injury occurring when a spinal needle was heated by an arthroscopic ablater.
Other authors have reported indirect thermal skin injuries caused by radiofrequency devices during arthroscopic surgery, but the causes were postulated to be direct contact between device and skin7 and overheating of the arthroscopy fluid.5,6,8 Huang and colleagues8 reported that full-thickness skin burns occurred when normal saline used during routine knee arthroscopy overheated from use of a radiofrequency device. Burn lesions, noted on their patient’s leg within 1 day after surgery, required subsequent débridement, a muscle flap, and split-skin grafting. Skin burns caused by overheated fluid have occurred irrespective of type of fluid used (eg, 1.5% glycine or lactated Ringer solution).6 There was no evidence that our patient’s burn resulted from extravasated overheated fluid, as the lesion was localized to the area immediately around the needle and was not geographic, as was described by Huang and colleagues.8
Other possible causes of skin burns during arthroscopic surgery have been described, but none applies in our patient’s case. Segami and colleagues7 described a burn resulting from direct transfer of heat from the radiofrequency device to the skin because of their proximity. This mechanism was not the cause in our patient’s case; there was no evidence of a defect or burned deltoid muscle at time of surgery. Lau and Dao9 reported 2 small full-thickness skin burns caused by a fiberoptic-light cable tip placed on a patient’s leg; in addition, the hot (>170°C) cables caused the paper drapes to combust.9 Skin burns secondary to use of skin antiseptics have been reported,10 but such lesions typically are located beneath tourniquets or in areas of friction from surgical drapes. In some cases, lesions described as skin burns may actually have been pressure lesions secondary to moist skin and friction.11
Whether type of radiofrequency device contributes to the occurrence of heat-related lesions during arthroscopic surgery is unknown. Some investigators have suggested there is more potential for harm with bipolar RFA devices than with monopolar devices.12,13 Monopolar devices pass energy between a probe and a grounding plate, whereas bipolar devices pass energy through 2 points on the probe.14 Because the heat for the monopolar probe derives from the frictional resistance of tissues to each other rather than from the probe itself, the bipolar probe theoretically allows for better temperature control. In addition, bipolar probes require less current to achieve the same heating effect. However, recent studies have suggested that, compared with monopolar radiofrequency devices, bipolar radiofrequency devices are associated with larger increases in temperature at equal depths after an equal number of applications.12,13
To our knowledge, no one has specifically investigated the type of bipolar device used in the present case. This case report, the first to describe a thermal skin injury caused by direct contact between a radiofrequency device and a metal needle inserted in the skin, is a reminder that contact between radiofrequency devices and spinal needles or other metal cannulas used in arthroscopic surgery should be avoided.
Cautery and radiofrequency ablation (RFA) devices are commonly used in shoulder arthroscopic surgery for hemostasis and ablation of soft tissue. Although these devices are easily used and applied, complications (eg, extensive release of deltoid muscle,1 nerve damage,2 tendon damage,3 cartilage damage from heat transfer4) can occur during arthroscopic surgery. Radiofrequency devices can elevate fluid temperatures to unsafe levels and directly or indirectly injure surrounding tissue.5,6 Skin complications from using these devices include direct burns to the subcutaneous tissues from the joint to the skin surface7 and skin burns related to overheated arthroscopic fluid.8
In our English-language literature review, however, we found no report of a skin burn secondary to contact between a RFA device and a spinal needle used in identifying structures during an arthroscopic acromioplasty. We report such a case here. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 51-year-old woman injured her left, nondominant shoulder when a descending garage door hit her directly on the superior aspect of the shoulder. She had immediate onset of pain on the top and lateral side of the shoulder and was evaluated by a primary care physician. Radiographs and magnetic resonance imaging (MRI) were normal. The patient was referred to an orthopedic surgeon for further evaluation.
The orthopedic surgeon found her to be in good health, with no history of diabetes, vascular conditions, or skin disorders. The initial diagnosis after history taking and physical examination was impingement syndrome with subacromial bursitis. The surgeon recommended nonoperative treatment: ice, nonsteroidal anti-inflammatory drugs, and physical therapy. After 3 months, the patient’s examination was unchanged, and there was no improvement in pain. Cortisone injected into the subacromial space helped for a few weeks, but the pain returned. After 2 more cortisone injections over 9 months failed, repeat MRI showed no tears of the rotator cuff or any other salient abnormalities. The treatment options were discussed with the patient, and, because the physical examination findings were consistent with impingement syndrome and nonoperative measures had failed, she consented to arthroscopic evaluation of the shoulder and arthroscopic partial anterior-lateral acromioplasty.
The procedure was performed 8 months after initial injury. With the patient under general anesthesia and in a lateral decubitus position, her arm was placed in an arm holder. Before the partial acromioplasty, two 18-gauge spinal needles were inserted from the skin surface into the subacromial space to help localize the anterolateral acromion and the acromioclavicular joint. The procedure was performed with a pump using saline bags kept at room temperature. A bipolar radiofrequency device (Stryker Energy Radiofrequency Ablation System; Stryker, Mahwah, New Jersey) was used to débride the subacromial bursa and the periosteum of the undersurface of the acromion. While the bursa was being débrided, the radiofrequency device inadvertently touched the anterior lateral needle probe, and a small skin burn formed around the needle on the surface of the shoulder (Figure). The radiofrequency device did not directly contact the skin, and the deltoid fascia was intact. The spinal needle was removed, and the skin around the burn was excised; the muscle beneath the skin was intact and showed no signs of thermal damage. The skin was mobilized and closed with interrupted simple sutures using a 4-0 nylon suture. The procedure was then completed with no other complications.
After surgery, the patient recovered without complications, and the skin lesion healed with no signs of infection and no skin or muscle defects. Some stiffness was treated with medication and physical therapy. Nine months after surgery, the patient reported mild shoulder stiffness and remained dissatisfied with the appearance of the skin in the area of the burn.
Discussion
Our patient’s case is a reminder that contact between a radiofrequency device and metal needles can transfer heat to tissues and cause skin burns. When using a radiofrequency device around metal needles or cannulas, surgeons should be sure to avoid prolonged contact with the metal. Our patient’s case is the first reported case of a thermal skin injury occurring when a spinal needle was heated by an arthroscopic ablater.
Other authors have reported indirect thermal skin injuries caused by radiofrequency devices during arthroscopic surgery, but the causes were postulated to be direct contact between device and skin7 and overheating of the arthroscopy fluid.5,6,8 Huang and colleagues8 reported that full-thickness skin burns occurred when normal saline used during routine knee arthroscopy overheated from use of a radiofrequency device. Burn lesions, noted on their patient’s leg within 1 day after surgery, required subsequent débridement, a muscle flap, and split-skin grafting. Skin burns caused by overheated fluid have occurred irrespective of type of fluid used (eg, 1.5% glycine or lactated Ringer solution).6 There was no evidence that our patient’s burn resulted from extravasated overheated fluid, as the lesion was localized to the area immediately around the needle and was not geographic, as was described by Huang and colleagues.8
Other possible causes of skin burns during arthroscopic surgery have been described, but none applies in our patient’s case. Segami and colleagues7 described a burn resulting from direct transfer of heat from the radiofrequency device to the skin because of their proximity. This mechanism was not the cause in our patient’s case; there was no evidence of a defect or burned deltoid muscle at time of surgery. Lau and Dao9 reported 2 small full-thickness skin burns caused by a fiberoptic-light cable tip placed on a patient’s leg; in addition, the hot (>170°C) cables caused the paper drapes to combust.9 Skin burns secondary to use of skin antiseptics have been reported,10 but such lesions typically are located beneath tourniquets or in areas of friction from surgical drapes. In some cases, lesions described as skin burns may actually have been pressure lesions secondary to moist skin and friction.11
Whether type of radiofrequency device contributes to the occurrence of heat-related lesions during arthroscopic surgery is unknown. Some investigators have suggested there is more potential for harm with bipolar RFA devices than with monopolar devices.12,13 Monopolar devices pass energy between a probe and a grounding plate, whereas bipolar devices pass energy through 2 points on the probe.14 Because the heat for the monopolar probe derives from the frictional resistance of tissues to each other rather than from the probe itself, the bipolar probe theoretically allows for better temperature control. In addition, bipolar probes require less current to achieve the same heating effect. However, recent studies have suggested that, compared with monopolar radiofrequency devices, bipolar radiofrequency devices are associated with larger increases in temperature at equal depths after an equal number of applications.12,13
To our knowledge, no one has specifically investigated the type of bipolar device used in the present case. This case report, the first to describe a thermal skin injury caused by direct contact between a radiofrequency device and a metal needle inserted in the skin, is a reminder that contact between radiofrequency devices and spinal needles or other metal cannulas used in arthroscopic surgery should be avoided.
1. Bonsell S. Detached deltoid during arthroscopic subacromial decompression. Arthroscopy. 2000;16(7):745-748.
2. Mohammed KD, Hayes MG, Saies AD. Unusual complications of shoulder arthroscopy. J Shoulder Elbow Surg. 2000;9(4):350-353.
3. Pell RF 4th, Uhl RL. Complications of thermal ablation in wrist arthroscopy. Arthroscopy. 2004;20(suppl 2):84-86.
4. Lu Y, Hayashi K, Hecht P, et al. The effect of monopolar radiofrequency energy on partial-thickness defects of articular cartilage. Arthroscopy. 2000;16(5):527-536.
5. Kouk SN, Zoric B, Stetson WB. Complication of the use of a radiofrequency device in arthroscopic shoulder surgery: second-degree burn of the shoulder girdle. Arthroscopy. 2011;27(1):136-141.
6. Lord MJ, Maltry JA, Shall LM. Thermal injury resulting from arthroscopic lateral retinacular release by electrocautery: report of three cases and a review of the literature. Arthroscopy. 1991;7(1):33-37.
7. Segami N, Yamada T, Nishimura M. Thermal injury during temporomandibular joint arthroscopy: a case report. J Oral Maxillofac Surg. 2004;62(4):508-510.
8. Huang S, Gateley D, Moss ALH. Accidental burn injury during knee arthroscopy. Arthroscopy. 2007;23(12):1363.e1-e3.
9. Lau YJ, Dao Q. Cutaneous burns from a fiberoptic cable tip during arthroscopy of the knee. Knee. 2008;15(4):333-335.
10. Sanders TH, Hawken SM. Chlorhexidine burns after shoulder arthroscopy. Am J Orthop. 2012;41(4):172-174.
11. Keyurapan E, Hu SJ, Redett R, McCarthy EF, McFarland EG. Pressure ulcers of the thorax after shoulder surgery. Knee Surg Sports Traumatol Arthrosc. 2007;15(12):1489-1493.
12. Edwards RB 3rd, Lu Y, Rodriguez E, Markel MD. Thermometric determination of cartilage matrix temperatures during thermal chondroplasty: comparison of bipolar and monopolar radiofrequency devices. Arthroscopy. 2002;18(4):339-346.
13. Figueroa D, Calvo R, Vaisman A, et al. Bipolar radiofrequency in the human meniscus. Comparative study between patients younger and older than 40 years of age. Knee. 2007;14(5):357-360.
14. Sahasrabudhe A, McMahon PJ. Thermal probes: what’s available in 2004. Oper Tech Sports Med. 2004;12:206-209.
1. Bonsell S. Detached deltoid during arthroscopic subacromial decompression. Arthroscopy. 2000;16(7):745-748.
2. Mohammed KD, Hayes MG, Saies AD. Unusual complications of shoulder arthroscopy. J Shoulder Elbow Surg. 2000;9(4):350-353.
3. Pell RF 4th, Uhl RL. Complications of thermal ablation in wrist arthroscopy. Arthroscopy. 2004;20(suppl 2):84-86.
4. Lu Y, Hayashi K, Hecht P, et al. The effect of monopolar radiofrequency energy on partial-thickness defects of articular cartilage. Arthroscopy. 2000;16(5):527-536.
5. Kouk SN, Zoric B, Stetson WB. Complication of the use of a radiofrequency device in arthroscopic shoulder surgery: second-degree burn of the shoulder girdle. Arthroscopy. 2011;27(1):136-141.
6. Lord MJ, Maltry JA, Shall LM. Thermal injury resulting from arthroscopic lateral retinacular release by electrocautery: report of three cases and a review of the literature. Arthroscopy. 1991;7(1):33-37.
7. Segami N, Yamada T, Nishimura M. Thermal injury during temporomandibular joint arthroscopy: a case report. J Oral Maxillofac Surg. 2004;62(4):508-510.
8. Huang S, Gateley D, Moss ALH. Accidental burn injury during knee arthroscopy. Arthroscopy. 2007;23(12):1363.e1-e3.
9. Lau YJ, Dao Q. Cutaneous burns from a fiberoptic cable tip during arthroscopy of the knee. Knee. 2008;15(4):333-335.
10. Sanders TH, Hawken SM. Chlorhexidine burns after shoulder arthroscopy. Am J Orthop. 2012;41(4):172-174.
11. Keyurapan E, Hu SJ, Redett R, McCarthy EF, McFarland EG. Pressure ulcers of the thorax after shoulder surgery. Knee Surg Sports Traumatol Arthrosc. 2007;15(12):1489-1493.
12. Edwards RB 3rd, Lu Y, Rodriguez E, Markel MD. Thermometric determination of cartilage matrix temperatures during thermal chondroplasty: comparison of bipolar and monopolar radiofrequency devices. Arthroscopy. 2002;18(4):339-346.
13. Figueroa D, Calvo R, Vaisman A, et al. Bipolar radiofrequency in the human meniscus. Comparative study between patients younger and older than 40 years of age. Knee. 2007;14(5):357-360.
14. Sahasrabudhe A, McMahon PJ. Thermal probes: what’s available in 2004. Oper Tech Sports Med. 2004;12:206-209.
Mycobacterium bovis Infection of Total Knee Arthroplasty After Bacillus Calmette-Guérin Therapy for Bladder Cancer
Intravesicular instillation of bacillus Calmette-Guérin (BCG), an attenuated form of Mycobacterium bovis, is the most effective treatment for superficial bladder cancer.1,2 Minor local reactions to this treatment, such as cystitis and hematuria, are common, but more severe systemic complications3,4 have also been documented, including sepsis, pneumonitis, granulomatous hepatitis, vertebral osteomyelitis,5,6 and rarely, total joint infection.7-11
We present a case of M bovis infection of a total knee arthroplasty (TKA) after BCG immunotherapy for bladder cancer that was successfully treated with antitubercular chemotherapy and retention of implants. We include a review of the literature addressing this rare mode of infection. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 66-year-old man presented with a chief complaint of progressive left knee stiffness over several months. Five years earlier, he underwent uncemented left TKA. His knee was functioning well with active range of motion from 0° to 126°, and he had returned to strenuous cycling. One year after his TKA and 4 years prior to the onset of stiffness, he had been diagnosed with superficial transitional cell carcinoma of the bladder. His treatment included intravesicular BCG therapy weekly for 6 weeks followed by semi-annual maintenance therapy.
Initial examination upon presentation with left knee stiffness showed a significant effusion and diminished range of motion but little discomfort. The patient denied fever, chills, night sweats, and weight loss. Radiographs were normal with good component positioning and normal-appearing bone-implant interfaces (Figures A, B). Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and white blood cell count (WBC) were within normal limits, and aspirate of the knee revealed no organisms. Based on these findings, the presumptive diagnosis was an adverse reaction to polyethylene wear. Because of persistent stiffness, the patient underwent an examination under anesthesia, arthroscopy, and major synovectomy with biopsy. Intraoperative findings included normal polyethylene but a marked hypertrophic synovitis and abnormal, semi-turbid fluid. The fluid WBC count was 5.35×109/L but no organisms were isolated initially. Histologic samples showed chronic inflammation with patches of acute inflammation. Approximately 6 weeks after surgery, cultures became positive for acid-fast bacillus, which was identified as M bovis.
Maintenance BCG therapy was discontinued, and antitubercular chemotherapy was initiated, consisting of 12 months of rifampin 600 mg daily and isoniazid 300 mg daily. Because symptoms significantly improved after arthroscopic incision and drainage and synovectomy, the TKA implants were maintained and symptoms closely monitored. Subsequent cultures and biopsies remained negative, and the patient continued to do well clinically with no residual stiffness.
At 7½-year follow-up, there is no clinical evidence of infection, and the patient continues to enjoy a high level of function with no pain and no recurrent stiffness. He has returned to cycling, logging more than 40,000 miles. However, a recurrence of bladder cancer is being treated with mitomycin C and gemcitabine, alternative to BCG.
Discussion
Mycobacterial infection in total joint arthroplasty (TJA) is uncommon;12M bovis infection of joint arthroplasty after intravesicular BCG therapy is exceedingly rare. Joint infection is thought to be the result of dissemination of BCG throughout the bloodstream.13
A review of the literature of BCG infection of TJA after intravesicular therapy for bladder cancer revealed only 5 case reports (Table). The average age on presentation was 77 years, and all patients were men, with 4 total hip arthroplasties (THAs) and 1 TKA. The average time from index procedure to initial presentation was 7.8 years, and the average time from cancer diagnosis to initial presentation was 20 months. Patients received an average of 8.6 consecutive weeks of BCG treatments, and maintenance therapy was not noted in any of the published reports. The average duration of antitubercular therapy was 13 months, and it comprised either 2- or 3-agent therapy. All reported cases were treated with removal of primary implants in either a 1- or 2-stage fashion. To our knowledge, this is only the second case of BCG infection of TKA reported in the literature and the first report of successful treatment with retention of primary implants.
There are several possible explanations for the success of a more conservative treatment approach in our patient. First, this TKA was uncemented. Second, BCG is an attenuated form of M bovis, which is itself a relatively less virulent species than M tuberculosis. Finally, mycobacterial species do not produce the biofilm that is seen in other bacterial arthroplasty infections, which typically necessitate removal of implants in cases of chronic infection.14
This case was unique because the patient lacked signs of infectious symptoms, there were normal inflammatory markers, and arthroscopy was necessary to aid in the diagnosis. The definitive diagnosis in this case was significantly delayed to attain a positive M bovis culture. Definitive treatment was provided by arthroscopy, implant salvage, and antitubercular chemotherapy only. The standard of care for an infected modular TKA normally involves revision of the polyethylene tibial insert with irrigation and débridement, or removal of components and insertion of new implants in a 1- or 2-stage procedure. Despite the unusual algorithm to reach a definitive diagnosis of an infected joint arthroplasty in this case, we do not recommend arthroscopic biopsy, washout, and antimicrobial therapy as definitive treatment for infected joint arthroplasty, and we continue to support the removal of infected components in a staged manner.
Conclusion
Joint replacement patients with bladder cancer represent a relatively small cohort. Based on current demographics and the increasing demand for joint arthroplasty, it is likely that this unique subset of patients will grow. No current standard of care exists for the treatment of these patients. One preventative measure is to consider alternative types of chemotherapy for bladder cancer treatment, such as mitomycin. Another potential solution would be administration of prophylactic doses of antitubercular agents concomitantly with intravesicular BCG, which would allow for the local effects of BCG immunotherapy while controlling the potential for systemic dissemination. The optimal dose range to achieve this dual effect is not known and is an area for research.
It is important for both arthroplasty surgeons and urologists to be aware of this potential complication in order to appropriately counsel this unique subset of patients. Our case report is the first to demonstrate that a successful outcome can be obtained with retention of primary components. Through research and continued data acquisition, a more concrete standard of care can be established. Until then, we recommend a collaborative approach between informed parties to devise a patient-specific plan of care.
1. Herr HW, Morales A. History of bacillus Calmette-Guérin and bladder cancer: an immunotherapy success story. J Urol. 2008;179(1):53-56.
2. Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guérin in the treatment of superficial bladder tumors. J Urol. 1976;116(2):180-183.
3. Lamm DL. Complications of bacillus Calmette-Guérin immunotherapy. Urol Clin North Am. 1992;19(3):565-572.
4. Lamm DL, van der Meijden PM, Morales A, et al. Incidence and treatment of complications of bacillus Calmette-Guérin intravesical therapy in superficial bladder cancer. J Urol. 1992;147(3):596-600.
5. Rozenblit A, Wasserman E, Marin ML, Veith FJ, Cynamon J, Rosenblit G. Infected aortic aneurysm and vertebral osteomyelitis after intravesical bacillus Calmette-Guérin therapy. AJR Am J Roentgenol. 1996;167(3):711-713.
6. Aljada IS, Crane JK, Corriere N, Wagle DG, Amsterdam D. Mycobacterium bovis BCG causing vertebral osteomyelitis (Pott’s disease) following intravesical BCG therapy. J Clin Microbiol. 1999;37(6):2106-2108.
7. Chazerain P, Desplaces N, Mamoudy P, Leonard P, Ziza JM. Prosthetic total knee infection with a bacillus Calmette-Guerin (BCG) strain after BCG therapy for bladder cancer. J Rheum. 1993;20(12):2171-2172.
8. Guerra CE, Betts RF, O’Keefe RJ, Shilling JW. Mycobacterium bovis osteomyelitis involving a hip arthroplasty after intravesicular bacille Calmette-Guérin for bladder cancer. Clin Infect Dis. 1998;27(3):639-640.
9. Segal A, Krauss ES. Infected total hip arthroplasty after intravesical bacillus Calmette-Guérin therapy. J Arthroplasty. 2007;22(5):759-762.
10. Reigstad O, Siewers P. A total hip replacement infected with mycobacterium bovis after intravesicular treatment with Bacille Calmette-Guérin for bladder cancer. J Bone Joint Surg Br. 2008;90(2):225-227.
11. Gomez E, Chiang T, Louie T, Ponnapalli M, Eng R, Huang DB. Prosthetic joint infection due to Mycobacterium bovis after intravesical instillation of Bacillus Calmette-Guerin (BCG). International J Microbiol. 2009;2009:527208. doi: 10.1155/2009/527208. Epub 2009 Dec 16.
12. Buchholz HW, Elson RA, Engelbrecht E, Lodenkämper H, Röttger J, Siegel A. Management of deep infection of total hip replacement. J Bone Joint Surg Br. 1981;63(3):342-353.
13. Xerri B, Chrétien Y, Le Parc JM. Reactive polyarthritis induced by intravesical BCG therapy for carcinoma of the bladder. Eur J Med. 1993;2(8):503-505.
14. Ha KY, Chung YG, Ryoo SJ. Adherence and biofilm formation of Staphylococcus epidermidis and Mycobacterium tuberculosis on various spinal implants. Spine (Phila Pa 1976). 2005;30(1):38-43.
Intravesicular instillation of bacillus Calmette-Guérin (BCG), an attenuated form of Mycobacterium bovis, is the most effective treatment for superficial bladder cancer.1,2 Minor local reactions to this treatment, such as cystitis and hematuria, are common, but more severe systemic complications3,4 have also been documented, including sepsis, pneumonitis, granulomatous hepatitis, vertebral osteomyelitis,5,6 and rarely, total joint infection.7-11
We present a case of M bovis infection of a total knee arthroplasty (TKA) after BCG immunotherapy for bladder cancer that was successfully treated with antitubercular chemotherapy and retention of implants. We include a review of the literature addressing this rare mode of infection. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 66-year-old man presented with a chief complaint of progressive left knee stiffness over several months. Five years earlier, he underwent uncemented left TKA. His knee was functioning well with active range of motion from 0° to 126°, and he had returned to strenuous cycling. One year after his TKA and 4 years prior to the onset of stiffness, he had been diagnosed with superficial transitional cell carcinoma of the bladder. His treatment included intravesicular BCG therapy weekly for 6 weeks followed by semi-annual maintenance therapy.
Initial examination upon presentation with left knee stiffness showed a significant effusion and diminished range of motion but little discomfort. The patient denied fever, chills, night sweats, and weight loss. Radiographs were normal with good component positioning and normal-appearing bone-implant interfaces (Figures A, B). Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and white blood cell count (WBC) were within normal limits, and aspirate of the knee revealed no organisms. Based on these findings, the presumptive diagnosis was an adverse reaction to polyethylene wear. Because of persistent stiffness, the patient underwent an examination under anesthesia, arthroscopy, and major synovectomy with biopsy. Intraoperative findings included normal polyethylene but a marked hypertrophic synovitis and abnormal, semi-turbid fluid. The fluid WBC count was 5.35×109/L but no organisms were isolated initially. Histologic samples showed chronic inflammation with patches of acute inflammation. Approximately 6 weeks after surgery, cultures became positive for acid-fast bacillus, which was identified as M bovis.
Maintenance BCG therapy was discontinued, and antitubercular chemotherapy was initiated, consisting of 12 months of rifampin 600 mg daily and isoniazid 300 mg daily. Because symptoms significantly improved after arthroscopic incision and drainage and synovectomy, the TKA implants were maintained and symptoms closely monitored. Subsequent cultures and biopsies remained negative, and the patient continued to do well clinically with no residual stiffness.
At 7½-year follow-up, there is no clinical evidence of infection, and the patient continues to enjoy a high level of function with no pain and no recurrent stiffness. He has returned to cycling, logging more than 40,000 miles. However, a recurrence of bladder cancer is being treated with mitomycin C and gemcitabine, alternative to BCG.
Discussion
Mycobacterial infection in total joint arthroplasty (TJA) is uncommon;12M bovis infection of joint arthroplasty after intravesicular BCG therapy is exceedingly rare. Joint infection is thought to be the result of dissemination of BCG throughout the bloodstream.13
A review of the literature of BCG infection of TJA after intravesicular therapy for bladder cancer revealed only 5 case reports (Table). The average age on presentation was 77 years, and all patients were men, with 4 total hip arthroplasties (THAs) and 1 TKA. The average time from index procedure to initial presentation was 7.8 years, and the average time from cancer diagnosis to initial presentation was 20 months. Patients received an average of 8.6 consecutive weeks of BCG treatments, and maintenance therapy was not noted in any of the published reports. The average duration of antitubercular therapy was 13 months, and it comprised either 2- or 3-agent therapy. All reported cases were treated with removal of primary implants in either a 1- or 2-stage fashion. To our knowledge, this is only the second case of BCG infection of TKA reported in the literature and the first report of successful treatment with retention of primary implants.
There are several possible explanations for the success of a more conservative treatment approach in our patient. First, this TKA was uncemented. Second, BCG is an attenuated form of M bovis, which is itself a relatively less virulent species than M tuberculosis. Finally, mycobacterial species do not produce the biofilm that is seen in other bacterial arthroplasty infections, which typically necessitate removal of implants in cases of chronic infection.14
This case was unique because the patient lacked signs of infectious symptoms, there were normal inflammatory markers, and arthroscopy was necessary to aid in the diagnosis. The definitive diagnosis in this case was significantly delayed to attain a positive M bovis culture. Definitive treatment was provided by arthroscopy, implant salvage, and antitubercular chemotherapy only. The standard of care for an infected modular TKA normally involves revision of the polyethylene tibial insert with irrigation and débridement, or removal of components and insertion of new implants in a 1- or 2-stage procedure. Despite the unusual algorithm to reach a definitive diagnosis of an infected joint arthroplasty in this case, we do not recommend arthroscopic biopsy, washout, and antimicrobial therapy as definitive treatment for infected joint arthroplasty, and we continue to support the removal of infected components in a staged manner.
Conclusion
Joint replacement patients with bladder cancer represent a relatively small cohort. Based on current demographics and the increasing demand for joint arthroplasty, it is likely that this unique subset of patients will grow. No current standard of care exists for the treatment of these patients. One preventative measure is to consider alternative types of chemotherapy for bladder cancer treatment, such as mitomycin. Another potential solution would be administration of prophylactic doses of antitubercular agents concomitantly with intravesicular BCG, which would allow for the local effects of BCG immunotherapy while controlling the potential for systemic dissemination. The optimal dose range to achieve this dual effect is not known and is an area for research.
It is important for both arthroplasty surgeons and urologists to be aware of this potential complication in order to appropriately counsel this unique subset of patients. Our case report is the first to demonstrate that a successful outcome can be obtained with retention of primary components. Through research and continued data acquisition, a more concrete standard of care can be established. Until then, we recommend a collaborative approach between informed parties to devise a patient-specific plan of care.
Intravesicular instillation of bacillus Calmette-Guérin (BCG), an attenuated form of Mycobacterium bovis, is the most effective treatment for superficial bladder cancer.1,2 Minor local reactions to this treatment, such as cystitis and hematuria, are common, but more severe systemic complications3,4 have also been documented, including sepsis, pneumonitis, granulomatous hepatitis, vertebral osteomyelitis,5,6 and rarely, total joint infection.7-11
We present a case of M bovis infection of a total knee arthroplasty (TKA) after BCG immunotherapy for bladder cancer that was successfully treated with antitubercular chemotherapy and retention of implants. We include a review of the literature addressing this rare mode of infection. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 66-year-old man presented with a chief complaint of progressive left knee stiffness over several months. Five years earlier, he underwent uncemented left TKA. His knee was functioning well with active range of motion from 0° to 126°, and he had returned to strenuous cycling. One year after his TKA and 4 years prior to the onset of stiffness, he had been diagnosed with superficial transitional cell carcinoma of the bladder. His treatment included intravesicular BCG therapy weekly for 6 weeks followed by semi-annual maintenance therapy.
Initial examination upon presentation with left knee stiffness showed a significant effusion and diminished range of motion but little discomfort. The patient denied fever, chills, night sweats, and weight loss. Radiographs were normal with good component positioning and normal-appearing bone-implant interfaces (Figures A, B). Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and white blood cell count (WBC) were within normal limits, and aspirate of the knee revealed no organisms. Based on these findings, the presumptive diagnosis was an adverse reaction to polyethylene wear. Because of persistent stiffness, the patient underwent an examination under anesthesia, arthroscopy, and major synovectomy with biopsy. Intraoperative findings included normal polyethylene but a marked hypertrophic synovitis and abnormal, semi-turbid fluid. The fluid WBC count was 5.35×109/L but no organisms were isolated initially. Histologic samples showed chronic inflammation with patches of acute inflammation. Approximately 6 weeks after surgery, cultures became positive for acid-fast bacillus, which was identified as M bovis.
Maintenance BCG therapy was discontinued, and antitubercular chemotherapy was initiated, consisting of 12 months of rifampin 600 mg daily and isoniazid 300 mg daily. Because symptoms significantly improved after arthroscopic incision and drainage and synovectomy, the TKA implants were maintained and symptoms closely monitored. Subsequent cultures and biopsies remained negative, and the patient continued to do well clinically with no residual stiffness.
At 7½-year follow-up, there is no clinical evidence of infection, and the patient continues to enjoy a high level of function with no pain and no recurrent stiffness. He has returned to cycling, logging more than 40,000 miles. However, a recurrence of bladder cancer is being treated with mitomycin C and gemcitabine, alternative to BCG.
Discussion
Mycobacterial infection in total joint arthroplasty (TJA) is uncommon;12M bovis infection of joint arthroplasty after intravesicular BCG therapy is exceedingly rare. Joint infection is thought to be the result of dissemination of BCG throughout the bloodstream.13
A review of the literature of BCG infection of TJA after intravesicular therapy for bladder cancer revealed only 5 case reports (Table). The average age on presentation was 77 years, and all patients were men, with 4 total hip arthroplasties (THAs) and 1 TKA. The average time from index procedure to initial presentation was 7.8 years, and the average time from cancer diagnosis to initial presentation was 20 months. Patients received an average of 8.6 consecutive weeks of BCG treatments, and maintenance therapy was not noted in any of the published reports. The average duration of antitubercular therapy was 13 months, and it comprised either 2- or 3-agent therapy. All reported cases were treated with removal of primary implants in either a 1- or 2-stage fashion. To our knowledge, this is only the second case of BCG infection of TKA reported in the literature and the first report of successful treatment with retention of primary implants.
There are several possible explanations for the success of a more conservative treatment approach in our patient. First, this TKA was uncemented. Second, BCG is an attenuated form of M bovis, which is itself a relatively less virulent species than M tuberculosis. Finally, mycobacterial species do not produce the biofilm that is seen in other bacterial arthroplasty infections, which typically necessitate removal of implants in cases of chronic infection.14
This case was unique because the patient lacked signs of infectious symptoms, there were normal inflammatory markers, and arthroscopy was necessary to aid in the diagnosis. The definitive diagnosis in this case was significantly delayed to attain a positive M bovis culture. Definitive treatment was provided by arthroscopy, implant salvage, and antitubercular chemotherapy only. The standard of care for an infected modular TKA normally involves revision of the polyethylene tibial insert with irrigation and débridement, or removal of components and insertion of new implants in a 1- or 2-stage procedure. Despite the unusual algorithm to reach a definitive diagnosis of an infected joint arthroplasty in this case, we do not recommend arthroscopic biopsy, washout, and antimicrobial therapy as definitive treatment for infected joint arthroplasty, and we continue to support the removal of infected components in a staged manner.
Conclusion
Joint replacement patients with bladder cancer represent a relatively small cohort. Based on current demographics and the increasing demand for joint arthroplasty, it is likely that this unique subset of patients will grow. No current standard of care exists for the treatment of these patients. One preventative measure is to consider alternative types of chemotherapy for bladder cancer treatment, such as mitomycin. Another potential solution would be administration of prophylactic doses of antitubercular agents concomitantly with intravesicular BCG, which would allow for the local effects of BCG immunotherapy while controlling the potential for systemic dissemination. The optimal dose range to achieve this dual effect is not known and is an area for research.
It is important for both arthroplasty surgeons and urologists to be aware of this potential complication in order to appropriately counsel this unique subset of patients. Our case report is the first to demonstrate that a successful outcome can be obtained with retention of primary components. Through research and continued data acquisition, a more concrete standard of care can be established. Until then, we recommend a collaborative approach between informed parties to devise a patient-specific plan of care.
1. Herr HW, Morales A. History of bacillus Calmette-Guérin and bladder cancer: an immunotherapy success story. J Urol. 2008;179(1):53-56.
2. Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guérin in the treatment of superficial bladder tumors. J Urol. 1976;116(2):180-183.
3. Lamm DL. Complications of bacillus Calmette-Guérin immunotherapy. Urol Clin North Am. 1992;19(3):565-572.
4. Lamm DL, van der Meijden PM, Morales A, et al. Incidence and treatment of complications of bacillus Calmette-Guérin intravesical therapy in superficial bladder cancer. J Urol. 1992;147(3):596-600.
5. Rozenblit A, Wasserman E, Marin ML, Veith FJ, Cynamon J, Rosenblit G. Infected aortic aneurysm and vertebral osteomyelitis after intravesical bacillus Calmette-Guérin therapy. AJR Am J Roentgenol. 1996;167(3):711-713.
6. Aljada IS, Crane JK, Corriere N, Wagle DG, Amsterdam D. Mycobacterium bovis BCG causing vertebral osteomyelitis (Pott’s disease) following intravesical BCG therapy. J Clin Microbiol. 1999;37(6):2106-2108.
7. Chazerain P, Desplaces N, Mamoudy P, Leonard P, Ziza JM. Prosthetic total knee infection with a bacillus Calmette-Guerin (BCG) strain after BCG therapy for bladder cancer. J Rheum. 1993;20(12):2171-2172.
8. Guerra CE, Betts RF, O’Keefe RJ, Shilling JW. Mycobacterium bovis osteomyelitis involving a hip arthroplasty after intravesicular bacille Calmette-Guérin for bladder cancer. Clin Infect Dis. 1998;27(3):639-640.
9. Segal A, Krauss ES. Infected total hip arthroplasty after intravesical bacillus Calmette-Guérin therapy. J Arthroplasty. 2007;22(5):759-762.
10. Reigstad O, Siewers P. A total hip replacement infected with mycobacterium bovis after intravesicular treatment with Bacille Calmette-Guérin for bladder cancer. J Bone Joint Surg Br. 2008;90(2):225-227.
11. Gomez E, Chiang T, Louie T, Ponnapalli M, Eng R, Huang DB. Prosthetic joint infection due to Mycobacterium bovis after intravesical instillation of Bacillus Calmette-Guerin (BCG). International J Microbiol. 2009;2009:527208. doi: 10.1155/2009/527208. Epub 2009 Dec 16.
12. Buchholz HW, Elson RA, Engelbrecht E, Lodenkämper H, Röttger J, Siegel A. Management of deep infection of total hip replacement. J Bone Joint Surg Br. 1981;63(3):342-353.
13. Xerri B, Chrétien Y, Le Parc JM. Reactive polyarthritis induced by intravesical BCG therapy for carcinoma of the bladder. Eur J Med. 1993;2(8):503-505.
14. Ha KY, Chung YG, Ryoo SJ. Adherence and biofilm formation of Staphylococcus epidermidis and Mycobacterium tuberculosis on various spinal implants. Spine (Phila Pa 1976). 2005;30(1):38-43.
1. Herr HW, Morales A. History of bacillus Calmette-Guérin and bladder cancer: an immunotherapy success story. J Urol. 2008;179(1):53-56.
2. Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guérin in the treatment of superficial bladder tumors. J Urol. 1976;116(2):180-183.
3. Lamm DL. Complications of bacillus Calmette-Guérin immunotherapy. Urol Clin North Am. 1992;19(3):565-572.
4. Lamm DL, van der Meijden PM, Morales A, et al. Incidence and treatment of complications of bacillus Calmette-Guérin intravesical therapy in superficial bladder cancer. J Urol. 1992;147(3):596-600.
5. Rozenblit A, Wasserman E, Marin ML, Veith FJ, Cynamon J, Rosenblit G. Infected aortic aneurysm and vertebral osteomyelitis after intravesical bacillus Calmette-Guérin therapy. AJR Am J Roentgenol. 1996;167(3):711-713.
6. Aljada IS, Crane JK, Corriere N, Wagle DG, Amsterdam D. Mycobacterium bovis BCG causing vertebral osteomyelitis (Pott’s disease) following intravesical BCG therapy. J Clin Microbiol. 1999;37(6):2106-2108.
7. Chazerain P, Desplaces N, Mamoudy P, Leonard P, Ziza JM. Prosthetic total knee infection with a bacillus Calmette-Guerin (BCG) strain after BCG therapy for bladder cancer. J Rheum. 1993;20(12):2171-2172.
8. Guerra CE, Betts RF, O’Keefe RJ, Shilling JW. Mycobacterium bovis osteomyelitis involving a hip arthroplasty after intravesicular bacille Calmette-Guérin for bladder cancer. Clin Infect Dis. 1998;27(3):639-640.
9. Segal A, Krauss ES. Infected total hip arthroplasty after intravesical bacillus Calmette-Guérin therapy. J Arthroplasty. 2007;22(5):759-762.
10. Reigstad O, Siewers P. A total hip replacement infected with mycobacterium bovis after intravesicular treatment with Bacille Calmette-Guérin for bladder cancer. J Bone Joint Surg Br. 2008;90(2):225-227.
11. Gomez E, Chiang T, Louie T, Ponnapalli M, Eng R, Huang DB. Prosthetic joint infection due to Mycobacterium bovis after intravesical instillation of Bacillus Calmette-Guerin (BCG). International J Microbiol. 2009;2009:527208. doi: 10.1155/2009/527208. Epub 2009 Dec 16.
12. Buchholz HW, Elson RA, Engelbrecht E, Lodenkämper H, Röttger J, Siegel A. Management of deep infection of total hip replacement. J Bone Joint Surg Br. 1981;63(3):342-353.
13. Xerri B, Chrétien Y, Le Parc JM. Reactive polyarthritis induced by intravesical BCG therapy for carcinoma of the bladder. Eur J Med. 1993;2(8):503-505.
14. Ha KY, Chung YG, Ryoo SJ. Adherence and biofilm formation of Staphylococcus epidermidis and Mycobacterium tuberculosis on various spinal implants. Spine (Phila Pa 1976). 2005;30(1):38-43.
Failure of Total Hip Arthroplasty Secondary to Infection Caused by Brucella abortus and the Risk of Transmission to Operative Staff
Brucellosis is a zoonotic disease transmitted to humans through contact with animal hosts or animal products. Infection of total knee or hip arthroplasty by Brucella species is a rare complication with only 18 cases reported in the English literature.1-12 We describe a case of an infected total hip replacement, its treatment, and 2-year follow-up and review the available literature. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 67-year-old Spanish-speaking woman, a native of Mexico, presented with a painful right total hip arthroplasty (THA) 2 years after implantation in Chihuahua, Mexico. The patient reported 1 year of increasing thigh pain with recent onset of start-up pain, and also mild groin pain. The patient reported an uneventful postoperative course without wound drainage and denied any history of fevers, chills, or night sweats after the procedure. Preoperative notes and radiographs were unavailable for review. Radiographic evaluation showed a hybrid construct with a well-fixed–appearing, uncemented acetabular component but a failed cemented femoral stem (Figures 1A, 1B). Although we discussed revision surgery, the patient elected not to proceed with surgery or to undergo evaluation to rule out infection. Nine months later, she returned with worsening pain and requested revision surgery; radiographs showed progressive bone loss around the cement mantle (Figures 2A, 2B).
Hematologic evaluation showed an erythrocyte sedimentation rate (ESR) of 54 mm/h (normal, 0-27 mm/h) and C-reactive protein (CRP) level of 0.24 mg/L (normal, <0.8). An aspiration of the hip with fluoroscopic guidance produced a small sample (0.2 mL) of yellow synovial fluid. There was not enough fluid for cell count, but fluid culture was negative.
The patient was taken to the operating room for revision THA. Because of concern about progressive bone loss and elevated infectious indices, the administration of antibiotics was delayed until we obtained sufficient deep-tissue specimens. Before opening the capsule, we introduced a syringe into the joint and aspirated 10 mL of cloudy yellow synovial fluid that was sent for cell count. Additional findings at surgery included a grossly loose stem with a fragmented cement mantle surrounded by poor bone stock with anterior cortical bone loss and a loose acetabular component with pockets of cavitary bone loss. Frozen section showed up to 5 nucleated cells per high power field, and the cell count showed 1480 nucleated cells/µL (50% polymorphonuclear cells). The equivocal intraoperative findings (cell count and frozen section) and the loose femoral and acetabular components with significant bone loss were sufficiently concerning that we removed the components and placed a cement spacer rather than proceed with revision arthroplasty (Figures 3A, 3B). The surgeon, first assistant, and scrub technician wore body exhaust suits. We performed irrigation of the wound bed with pulse lavage.
Intraoperative cultures (synovial fluid, joint capsule synovium, and femur pseudocapsule) were positive after 8 days and growing B abortus. Infectious disease consultants prescribed rifampin 300 mg twice daily and doxycycline 100 mg twice daily for 5 months. Follow-up ESR and CRP returned to normal range. A preoperative aspiration of the hip was negative as well. The patient returned to the operating room at 6 months for re-implantation using uncemented components; synovial fluid and tissue cultures taken at this time were negative. Two years after re-implantation, the patient is doing well without evidence of infection (Figures 4A, 4B). Additional follow-up will be required to monitor for infection and implant survival. Additional history taken from the patient after the culture results revealed that her development of hip pain was preceded by a febrile illness consistent with brucellosis.
Because of the nature of the procedure (irrigation and débridement using pulse lavage), we were concerned about aerosolization of Brucella bacteria and possible transmission to all staff present during the procedure. After consulting with the New Mexico Department of Health (NMDOH) and the Centers for Disease Control and Prevention (CDC), all surgical, anesthesia, and support personnel present in the operative suite and staff who cleaned the room after the procedure were treated prophylactically (rifampin 600 mg daily, doxycycline 100 mg twice daily for 3 weeks) to prevent development of brucellosis.13 All 15 operating room personnel who were exposed elected to proceed with antibiotic prophylaxis. In addition to prophylactic antibiotics, serial serologic testing for anti-Brucella antibodies was conducted at baseline and 2, 4, 6, and 24 weeks postexposure to monitor for the development of Brucella infection. There were no conversions to positive antibody status. No personnel complained of symptoms that would indicate development of brucellosis. At the recommendation of NMDOH and CDC, all staff in the operating room during and immediately after the re-implantation procedure wore properly fitting N-95 disposable respiratory masks (3M, St. Paul, Minnesota) to guard against the potential risk of further exposure.
Discussion
Brucellosis is a zoonotic disease transmitted to humans through contact with animal hosts. Transmission can occur via breaks in the skin in direct contact, through the ingestion of unpasteurized dairy products or raw meat, or through ingestion of aerosolized bacteria. Transmission via aerosolization has been described during medical procedures.
Brucella is endemic in India, Middle Eastern and Mediterranean countries, Central Asia, and South America. Brucella species are gram-negative coccobacilli that are capable of surviving within phagocytic cells, making antibiotic treatment difficult. Brucellosis is a febrile illness that occurs after a 1- to 3-week incubation period and is often accompanied by headache, arthralgias, and hepatosplenomegaly. Osteoarticular infection is the most common complication, occurring in 10% to 85% of cases and usually involves the sacroiliac joint and the large joints of the lower extremity. Spondylitis, bursitis, tenosynovitis, endocarditis, colitis, meningitis, and osteomyelitis have also been described.7,14-17
As mentioned previously, 18 cases of infected THAs and total knee arthroplasties (TKAs) in 16 patients were identified in the English literature: 9 THAs and 9 TKAs.1-12 With the exception of 1 case reported in Texas, all others were from the Middle East or the Mediterranean region. In these patients, symptom onset occurred from 2 months to 14 years from the time of the index surgery, and symptom duration ranged from 1 month to 2 years prior to presentation. The exposure was not reported in 2 cases, but the remaining patients either ingested unpasteurized dairy products or worked closely with livestock. Laboratory evaluation revealed elevated ESR or CRP in 8 cases. In 7 cases, no laboratory results were reported, although 1 had a draining sinus. In 1 case, the ESR was normal, but a bone scan was positive. Joint aspiration yielded Brucella species in 8 cases, was negative in 3, and not reported in 5 cases (one aspirate yielded Acinetobacter baumanii). Only 3 cases reported a time-to-culture positivity (1 “prolonged” and 2 took 7 days).
Eight cases presented with loose components, while 1 case was not reported, and the remaining were presumed to be well-fixed. In cases that were identified as loose, 5 underwent a 2-stage revision and 2 underwent a 1-stage revision (in one of the 1-stage revisions, the infection was identified only after the revision from intra-operative cultures). Of those with well-fixed components, 7 patients with 9 infected joints (including the case where no preoperative description of the components was reported) were treated with oral antibiotics only (range, 6 weeks to 26 months) and 1 with irrigation and débridement and oral antibiotics. Among those treated only with antibiotics, there were 2 failures (2 joints) leading to revision surgery. The other 5 cases were reportedly doing well between 8 months and 5 years after treatment. There were no reports of transmission to hospital or laboratory personnel in any of these cases nor were there reports of precautions to limit exposure for operating room staff or hospital personnel.
Failure of TKA or THA secondary to periprosthetic infection by Brucella species is rare, and this represents only the second reported case in the United States.4 This case highlights several important principles. Maintaining a high level of suspicion for infection in cases of failed joint arthroplasty is important. In addition, as more international travel occurs and patients are seen from areas where Brucella is endemic, the possibility of this infectious etiology should be considered. Based on reported cases, patients will usually have elevated ESR or CRP; all (except 2 cases in which no exposure was reported) had known exposure to unpasteurized dairy products or livestock. Joint aspiration yielded Brucella species in 8 cases, was negative in 3, and not reported in 5 cases (1 aspirate yielded Acinetobacter baumanii). In this case, ESR and CRP were elevated, and infection was suspected but joint aspiration was negative. The initial aspiration was cultured for 5 days and previous data, as well as that presented here, suggest that prolonged culture may provide diagnostic value.18 The patient had resided in an endemic area and had exposure to unpasteurized dairy products, but Brucella infection was not considered and, therefore, no precautions were taken.
Of the reported cases, only 1 met major criteria for periprosthetic joint infection (draining sinus) while 10 of the remaining 15 cases were positive for minor criteria of periprosthetic joint infection (elevated ESR or CRP, or positive culture from joint aspiration).19 Unfortunately, the available case reports did not detail the extent to which preoperative periprosthetic joint infection could be established based on minor criteria for periprosthetic joint infection (elevated joint synovial white blood cell count or neutrophil percentage, intra-articular purulence, or elevated neutrophil count on periprosthetic tissue histologic analysis).19
Periprosthetic joint infection by Brucella species is so rare that specific recommendations for this infectious etiology based on 18 reported cases would be overreaching. However, Brucella should be considered when evaluating a potentially infected joint replacement where the possibility of exposure exists (eg, travel to or previous residence in endemic areas, close contact with livestock, or ingestion of unpasteurized dairy products in endemic regions), with the potential for transmission to operating room and hospital personnel also considered. If there is concern about Brucella involvement, tissue and fluid specimens should be labeled so that laboratory personnel can take appropriate precautions. Brucella can be cultured using routine techniques on standard, nonselective media, but the culture time-to-growth may be prolonged. Culture plates should be held for 14 days before reporting no growth of Brucella if it is suspected; the New Mexico Department of Health Microbiology Laboratory holds routine cultures for 1 week after a report of no growth. Thus, a suspicion of Brucella should be communicated in order for culture time to be adjusted if the holding of culture plates after an initial report of no growth is not standard practice. If operative intervention is planned and brucellosis is known, personnel should be notified of the possibility of exposure and appropriate measures taken (ie, wearing N-95 respiratory masks during the procedure and considering other methods of irrigation less likely to aerosolize particulates). It is not known if preoperative antibiotic therapy can sufficiently lower the bacterial load to make aerosolization less likely. If brucellosis is suspected but not identified preoperatively, wearing N-95 respiratory masks should be considered during any open procedures.
Conclusion
In cases of Brucella infection and loose components, 1- or 2-stage revision with appropriate antibiotic therapy is indicated. (There is not enough data to recommend either 1- or 2-stage revision.) Several reports comment on the ability to treat periprosthetic joint infection in the setting of well-fixed components with antibiotic therapy alone. While this appears to have been successful in 7 of 9 infected joints reported in the literature, length of follow-up ranged from 8 months to 5 years, with no report of length of follow-up in some cases. Antibiotic therapy duration ranged from 6 weeks to 26 months, and the antibiotic treatment involved combination therapy with multiple agents reported but, most commonly, doxycycline, rifampin, and streptomycin. With 2 of 9 (22%) joints failing antibiotic therapy alone and those reported to be successful having relatively short-term follow-up, this treatment strategy should be approached with caution.
1. Agarwal S, Kadhi SK, Rooney RJ. Brucellosis complicating bilateral total knee arthroplasty. Clin Orthop. 1991;267:179-181.
2. Cairó M, Calbo E, Gomez L, et al. Foreign-body osteoarticular infection by Brucella melitensis: A report of three cases. J Bone Joint Surg Am. 2006; 88(1):202-204.
3. Erdogan H, Cakmak G, Erdogan A, Arslan H. Brucella melitensis infection in total knee arthroplasty: a case report. Knee Surg Sports Traumatol Arthrosc. 2010;18(7):908-910.
4. Jones RE, Berryhill WH, Smith J, Hofman A, Rogers D. Secondary infection of a total hip replacement with Brucella abortus. Orthopedics. 1983; 6(2):184-186.
5. Kasim RA, Araj GF, Afeiche NE, Tabbarah ZA. Brucella infection in total hip replacement: case report and review of the literature. Scand J Infect Dis. 2004;36(1):65-67.
6. Malizos KN, Makris CA, Soucacos PN. Total knee arthroplasties infected by Brucella melitensis: a case report. Am J Orthop. 1997;26(4):283-285.
7. Ortega-Andreu M, Rodriguez-Merchan EC, Aguera-Gavalda M. Brucellosis as a cause of septic loosening of total hip arthroplasty. J Arthroplasty. 2002;17(3):384-387.
8. Orti A, Alcala R, Navarro V, et al. Brucellar arthritis in a total knee replacement. Eur J Clin Microbiol Infect Dis. 1997;16(11):843-845.
9. Ruiz-Iban MA, Crespo P, Diaz-Peletier R, Rozado AM, Lopez-Pardo A. Total hip arthroplasty infected by Brucella: a report of two cases. J Orthop Surg (Hong Kong). 2006;14(1):99-103.
10. Tassinari E, Di Motta D, Giardina F, Traina F, Fine MD, Toni A. Brucella infection in total knee arthroplasty. Case report and revision of the literature. Chir Organi Mov. 2008;92(1):55-59.
11. Tena D, Romanillos O, Rodriguez-Zapata M, et al. Prosthetic hip infection due to Brucella melitensis: case report and literature review. Diagn Microbiol Infect Dis. 2007;58(4):481-485.
12. Weil Y, Mattan Y, Liebergall M, Rahav G. Brucella prosthetic joint infection: a report of 3 cases and a review of the literature. Clin Infect Dis. 2003;36(7):e81-e86.
13. Brucellosis. Centers for Disease Control and Prevention website. http://www.cdc.gov/nczved/divisions/dfbmd/diseases/brucellosis/recommendations.html. Updated November 12, 2012. Accessed December 22, 2014.
14. Franco MP, Mulder M, Gilman RH, Smits HL. Human brucellosis. Lancet Infect Dis. 2007;7(12):775-786.
15. Khateeb MI, Araj GF, Majeed SA, Lulu AR. Brucella arthritis: a study of 96 cases in Kuwait. Ann Rheum Dis. 1990;49(12):994-998.
16. Luna-Martinez JE, Mejía-Terán C. Brucellosis in Mexico: current status and trends. Vet Microbiol. 2002;90(1-4):19-30.
17. Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect Dis. 2006;6(2):91-99.
18. Schafer P, Fink B, Margull A, Berger I, Frommelt L. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Disease. 2008;47(11):1403-1409.
19. Parvizi J, Zmistowski B, Berbari EF, et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop. 2011;469(11):2992-2994.
Brucellosis is a zoonotic disease transmitted to humans through contact with animal hosts or animal products. Infection of total knee or hip arthroplasty by Brucella species is a rare complication with only 18 cases reported in the English literature.1-12 We describe a case of an infected total hip replacement, its treatment, and 2-year follow-up and review the available literature. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 67-year-old Spanish-speaking woman, a native of Mexico, presented with a painful right total hip arthroplasty (THA) 2 years after implantation in Chihuahua, Mexico. The patient reported 1 year of increasing thigh pain with recent onset of start-up pain, and also mild groin pain. The patient reported an uneventful postoperative course without wound drainage and denied any history of fevers, chills, or night sweats after the procedure. Preoperative notes and radiographs were unavailable for review. Radiographic evaluation showed a hybrid construct with a well-fixed–appearing, uncemented acetabular component but a failed cemented femoral stem (Figures 1A, 1B). Although we discussed revision surgery, the patient elected not to proceed with surgery or to undergo evaluation to rule out infection. Nine months later, she returned with worsening pain and requested revision surgery; radiographs showed progressive bone loss around the cement mantle (Figures 2A, 2B).
Hematologic evaluation showed an erythrocyte sedimentation rate (ESR) of 54 mm/h (normal, 0-27 mm/h) and C-reactive protein (CRP) level of 0.24 mg/L (normal, <0.8). An aspiration of the hip with fluoroscopic guidance produced a small sample (0.2 mL) of yellow synovial fluid. There was not enough fluid for cell count, but fluid culture was negative.
The patient was taken to the operating room for revision THA. Because of concern about progressive bone loss and elevated infectious indices, the administration of antibiotics was delayed until we obtained sufficient deep-tissue specimens. Before opening the capsule, we introduced a syringe into the joint and aspirated 10 mL of cloudy yellow synovial fluid that was sent for cell count. Additional findings at surgery included a grossly loose stem with a fragmented cement mantle surrounded by poor bone stock with anterior cortical bone loss and a loose acetabular component with pockets of cavitary bone loss. Frozen section showed up to 5 nucleated cells per high power field, and the cell count showed 1480 nucleated cells/µL (50% polymorphonuclear cells). The equivocal intraoperative findings (cell count and frozen section) and the loose femoral and acetabular components with significant bone loss were sufficiently concerning that we removed the components and placed a cement spacer rather than proceed with revision arthroplasty (Figures 3A, 3B). The surgeon, first assistant, and scrub technician wore body exhaust suits. We performed irrigation of the wound bed with pulse lavage.
Intraoperative cultures (synovial fluid, joint capsule synovium, and femur pseudocapsule) were positive after 8 days and growing B abortus. Infectious disease consultants prescribed rifampin 300 mg twice daily and doxycycline 100 mg twice daily for 5 months. Follow-up ESR and CRP returned to normal range. A preoperative aspiration of the hip was negative as well. The patient returned to the operating room at 6 months for re-implantation using uncemented components; synovial fluid and tissue cultures taken at this time were negative. Two years after re-implantation, the patient is doing well without evidence of infection (Figures 4A, 4B). Additional follow-up will be required to monitor for infection and implant survival. Additional history taken from the patient after the culture results revealed that her development of hip pain was preceded by a febrile illness consistent with brucellosis.
Because of the nature of the procedure (irrigation and débridement using pulse lavage), we were concerned about aerosolization of Brucella bacteria and possible transmission to all staff present during the procedure. After consulting with the New Mexico Department of Health (NMDOH) and the Centers for Disease Control and Prevention (CDC), all surgical, anesthesia, and support personnel present in the operative suite and staff who cleaned the room after the procedure were treated prophylactically (rifampin 600 mg daily, doxycycline 100 mg twice daily for 3 weeks) to prevent development of brucellosis.13 All 15 operating room personnel who were exposed elected to proceed with antibiotic prophylaxis. In addition to prophylactic antibiotics, serial serologic testing for anti-Brucella antibodies was conducted at baseline and 2, 4, 6, and 24 weeks postexposure to monitor for the development of Brucella infection. There were no conversions to positive antibody status. No personnel complained of symptoms that would indicate development of brucellosis. At the recommendation of NMDOH and CDC, all staff in the operating room during and immediately after the re-implantation procedure wore properly fitting N-95 disposable respiratory masks (3M, St. Paul, Minnesota) to guard against the potential risk of further exposure.
Discussion
Brucellosis is a zoonotic disease transmitted to humans through contact with animal hosts. Transmission can occur via breaks in the skin in direct contact, through the ingestion of unpasteurized dairy products or raw meat, or through ingestion of aerosolized bacteria. Transmission via aerosolization has been described during medical procedures.
Brucella is endemic in India, Middle Eastern and Mediterranean countries, Central Asia, and South America. Brucella species are gram-negative coccobacilli that are capable of surviving within phagocytic cells, making antibiotic treatment difficult. Brucellosis is a febrile illness that occurs after a 1- to 3-week incubation period and is often accompanied by headache, arthralgias, and hepatosplenomegaly. Osteoarticular infection is the most common complication, occurring in 10% to 85% of cases and usually involves the sacroiliac joint and the large joints of the lower extremity. Spondylitis, bursitis, tenosynovitis, endocarditis, colitis, meningitis, and osteomyelitis have also been described.7,14-17
As mentioned previously, 18 cases of infected THAs and total knee arthroplasties (TKAs) in 16 patients were identified in the English literature: 9 THAs and 9 TKAs.1-12 With the exception of 1 case reported in Texas, all others were from the Middle East or the Mediterranean region. In these patients, symptom onset occurred from 2 months to 14 years from the time of the index surgery, and symptom duration ranged from 1 month to 2 years prior to presentation. The exposure was not reported in 2 cases, but the remaining patients either ingested unpasteurized dairy products or worked closely with livestock. Laboratory evaluation revealed elevated ESR or CRP in 8 cases. In 7 cases, no laboratory results were reported, although 1 had a draining sinus. In 1 case, the ESR was normal, but a bone scan was positive. Joint aspiration yielded Brucella species in 8 cases, was negative in 3, and not reported in 5 cases (one aspirate yielded Acinetobacter baumanii). Only 3 cases reported a time-to-culture positivity (1 “prolonged” and 2 took 7 days).
Eight cases presented with loose components, while 1 case was not reported, and the remaining were presumed to be well-fixed. In cases that were identified as loose, 5 underwent a 2-stage revision and 2 underwent a 1-stage revision (in one of the 1-stage revisions, the infection was identified only after the revision from intra-operative cultures). Of those with well-fixed components, 7 patients with 9 infected joints (including the case where no preoperative description of the components was reported) were treated with oral antibiotics only (range, 6 weeks to 26 months) and 1 with irrigation and débridement and oral antibiotics. Among those treated only with antibiotics, there were 2 failures (2 joints) leading to revision surgery. The other 5 cases were reportedly doing well between 8 months and 5 years after treatment. There were no reports of transmission to hospital or laboratory personnel in any of these cases nor were there reports of precautions to limit exposure for operating room staff or hospital personnel.
Failure of TKA or THA secondary to periprosthetic infection by Brucella species is rare, and this represents only the second reported case in the United States.4 This case highlights several important principles. Maintaining a high level of suspicion for infection in cases of failed joint arthroplasty is important. In addition, as more international travel occurs and patients are seen from areas where Brucella is endemic, the possibility of this infectious etiology should be considered. Based on reported cases, patients will usually have elevated ESR or CRP; all (except 2 cases in which no exposure was reported) had known exposure to unpasteurized dairy products or livestock. Joint aspiration yielded Brucella species in 8 cases, was negative in 3, and not reported in 5 cases (1 aspirate yielded Acinetobacter baumanii). In this case, ESR and CRP were elevated, and infection was suspected but joint aspiration was negative. The initial aspiration was cultured for 5 days and previous data, as well as that presented here, suggest that prolonged culture may provide diagnostic value.18 The patient had resided in an endemic area and had exposure to unpasteurized dairy products, but Brucella infection was not considered and, therefore, no precautions were taken.
Of the reported cases, only 1 met major criteria for periprosthetic joint infection (draining sinus) while 10 of the remaining 15 cases were positive for minor criteria of periprosthetic joint infection (elevated ESR or CRP, or positive culture from joint aspiration).19 Unfortunately, the available case reports did not detail the extent to which preoperative periprosthetic joint infection could be established based on minor criteria for periprosthetic joint infection (elevated joint synovial white blood cell count or neutrophil percentage, intra-articular purulence, or elevated neutrophil count on periprosthetic tissue histologic analysis).19
Periprosthetic joint infection by Brucella species is so rare that specific recommendations for this infectious etiology based on 18 reported cases would be overreaching. However, Brucella should be considered when evaluating a potentially infected joint replacement where the possibility of exposure exists (eg, travel to or previous residence in endemic areas, close contact with livestock, or ingestion of unpasteurized dairy products in endemic regions), with the potential for transmission to operating room and hospital personnel also considered. If there is concern about Brucella involvement, tissue and fluid specimens should be labeled so that laboratory personnel can take appropriate precautions. Brucella can be cultured using routine techniques on standard, nonselective media, but the culture time-to-growth may be prolonged. Culture plates should be held for 14 days before reporting no growth of Brucella if it is suspected; the New Mexico Department of Health Microbiology Laboratory holds routine cultures for 1 week after a report of no growth. Thus, a suspicion of Brucella should be communicated in order for culture time to be adjusted if the holding of culture plates after an initial report of no growth is not standard practice. If operative intervention is planned and brucellosis is known, personnel should be notified of the possibility of exposure and appropriate measures taken (ie, wearing N-95 respiratory masks during the procedure and considering other methods of irrigation less likely to aerosolize particulates). It is not known if preoperative antibiotic therapy can sufficiently lower the bacterial load to make aerosolization less likely. If brucellosis is suspected but not identified preoperatively, wearing N-95 respiratory masks should be considered during any open procedures.
Conclusion
In cases of Brucella infection and loose components, 1- or 2-stage revision with appropriate antibiotic therapy is indicated. (There is not enough data to recommend either 1- or 2-stage revision.) Several reports comment on the ability to treat periprosthetic joint infection in the setting of well-fixed components with antibiotic therapy alone. While this appears to have been successful in 7 of 9 infected joints reported in the literature, length of follow-up ranged from 8 months to 5 years, with no report of length of follow-up in some cases. Antibiotic therapy duration ranged from 6 weeks to 26 months, and the antibiotic treatment involved combination therapy with multiple agents reported but, most commonly, doxycycline, rifampin, and streptomycin. With 2 of 9 (22%) joints failing antibiotic therapy alone and those reported to be successful having relatively short-term follow-up, this treatment strategy should be approached with caution.
Brucellosis is a zoonotic disease transmitted to humans through contact with animal hosts or animal products. Infection of total knee or hip arthroplasty by Brucella species is a rare complication with only 18 cases reported in the English literature.1-12 We describe a case of an infected total hip replacement, its treatment, and 2-year follow-up and review the available literature. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 67-year-old Spanish-speaking woman, a native of Mexico, presented with a painful right total hip arthroplasty (THA) 2 years after implantation in Chihuahua, Mexico. The patient reported 1 year of increasing thigh pain with recent onset of start-up pain, and also mild groin pain. The patient reported an uneventful postoperative course without wound drainage and denied any history of fevers, chills, or night sweats after the procedure. Preoperative notes and radiographs were unavailable for review. Radiographic evaluation showed a hybrid construct with a well-fixed–appearing, uncemented acetabular component but a failed cemented femoral stem (Figures 1A, 1B). Although we discussed revision surgery, the patient elected not to proceed with surgery or to undergo evaluation to rule out infection. Nine months later, she returned with worsening pain and requested revision surgery; radiographs showed progressive bone loss around the cement mantle (Figures 2A, 2B).
Hematologic evaluation showed an erythrocyte sedimentation rate (ESR) of 54 mm/h (normal, 0-27 mm/h) and C-reactive protein (CRP) level of 0.24 mg/L (normal, <0.8). An aspiration of the hip with fluoroscopic guidance produced a small sample (0.2 mL) of yellow synovial fluid. There was not enough fluid for cell count, but fluid culture was negative.
The patient was taken to the operating room for revision THA. Because of concern about progressive bone loss and elevated infectious indices, the administration of antibiotics was delayed until we obtained sufficient deep-tissue specimens. Before opening the capsule, we introduced a syringe into the joint and aspirated 10 mL of cloudy yellow synovial fluid that was sent for cell count. Additional findings at surgery included a grossly loose stem with a fragmented cement mantle surrounded by poor bone stock with anterior cortical bone loss and a loose acetabular component with pockets of cavitary bone loss. Frozen section showed up to 5 nucleated cells per high power field, and the cell count showed 1480 nucleated cells/µL (50% polymorphonuclear cells). The equivocal intraoperative findings (cell count and frozen section) and the loose femoral and acetabular components with significant bone loss were sufficiently concerning that we removed the components and placed a cement spacer rather than proceed with revision arthroplasty (Figures 3A, 3B). The surgeon, first assistant, and scrub technician wore body exhaust suits. We performed irrigation of the wound bed with pulse lavage.
Intraoperative cultures (synovial fluid, joint capsule synovium, and femur pseudocapsule) were positive after 8 days and growing B abortus. Infectious disease consultants prescribed rifampin 300 mg twice daily and doxycycline 100 mg twice daily for 5 months. Follow-up ESR and CRP returned to normal range. A preoperative aspiration of the hip was negative as well. The patient returned to the operating room at 6 months for re-implantation using uncemented components; synovial fluid and tissue cultures taken at this time were negative. Two years after re-implantation, the patient is doing well without evidence of infection (Figures 4A, 4B). Additional follow-up will be required to monitor for infection and implant survival. Additional history taken from the patient after the culture results revealed that her development of hip pain was preceded by a febrile illness consistent with brucellosis.
Because of the nature of the procedure (irrigation and débridement using pulse lavage), we were concerned about aerosolization of Brucella bacteria and possible transmission to all staff present during the procedure. After consulting with the New Mexico Department of Health (NMDOH) and the Centers for Disease Control and Prevention (CDC), all surgical, anesthesia, and support personnel present in the operative suite and staff who cleaned the room after the procedure were treated prophylactically (rifampin 600 mg daily, doxycycline 100 mg twice daily for 3 weeks) to prevent development of brucellosis.13 All 15 operating room personnel who were exposed elected to proceed with antibiotic prophylaxis. In addition to prophylactic antibiotics, serial serologic testing for anti-Brucella antibodies was conducted at baseline and 2, 4, 6, and 24 weeks postexposure to monitor for the development of Brucella infection. There were no conversions to positive antibody status. No personnel complained of symptoms that would indicate development of brucellosis. At the recommendation of NMDOH and CDC, all staff in the operating room during and immediately after the re-implantation procedure wore properly fitting N-95 disposable respiratory masks (3M, St. Paul, Minnesota) to guard against the potential risk of further exposure.
Discussion
Brucellosis is a zoonotic disease transmitted to humans through contact with animal hosts. Transmission can occur via breaks in the skin in direct contact, through the ingestion of unpasteurized dairy products or raw meat, or through ingestion of aerosolized bacteria. Transmission via aerosolization has been described during medical procedures.
Brucella is endemic in India, Middle Eastern and Mediterranean countries, Central Asia, and South America. Brucella species are gram-negative coccobacilli that are capable of surviving within phagocytic cells, making antibiotic treatment difficult. Brucellosis is a febrile illness that occurs after a 1- to 3-week incubation period and is often accompanied by headache, arthralgias, and hepatosplenomegaly. Osteoarticular infection is the most common complication, occurring in 10% to 85% of cases and usually involves the sacroiliac joint and the large joints of the lower extremity. Spondylitis, bursitis, tenosynovitis, endocarditis, colitis, meningitis, and osteomyelitis have also been described.7,14-17
As mentioned previously, 18 cases of infected THAs and total knee arthroplasties (TKAs) in 16 patients were identified in the English literature: 9 THAs and 9 TKAs.1-12 With the exception of 1 case reported in Texas, all others were from the Middle East or the Mediterranean region. In these patients, symptom onset occurred from 2 months to 14 years from the time of the index surgery, and symptom duration ranged from 1 month to 2 years prior to presentation. The exposure was not reported in 2 cases, but the remaining patients either ingested unpasteurized dairy products or worked closely with livestock. Laboratory evaluation revealed elevated ESR or CRP in 8 cases. In 7 cases, no laboratory results were reported, although 1 had a draining sinus. In 1 case, the ESR was normal, but a bone scan was positive. Joint aspiration yielded Brucella species in 8 cases, was negative in 3, and not reported in 5 cases (one aspirate yielded Acinetobacter baumanii). Only 3 cases reported a time-to-culture positivity (1 “prolonged” and 2 took 7 days).
Eight cases presented with loose components, while 1 case was not reported, and the remaining were presumed to be well-fixed. In cases that were identified as loose, 5 underwent a 2-stage revision and 2 underwent a 1-stage revision (in one of the 1-stage revisions, the infection was identified only after the revision from intra-operative cultures). Of those with well-fixed components, 7 patients with 9 infected joints (including the case where no preoperative description of the components was reported) were treated with oral antibiotics only (range, 6 weeks to 26 months) and 1 with irrigation and débridement and oral antibiotics. Among those treated only with antibiotics, there were 2 failures (2 joints) leading to revision surgery. The other 5 cases were reportedly doing well between 8 months and 5 years after treatment. There were no reports of transmission to hospital or laboratory personnel in any of these cases nor were there reports of precautions to limit exposure for operating room staff or hospital personnel.
Failure of TKA or THA secondary to periprosthetic infection by Brucella species is rare, and this represents only the second reported case in the United States.4 This case highlights several important principles. Maintaining a high level of suspicion for infection in cases of failed joint arthroplasty is important. In addition, as more international travel occurs and patients are seen from areas where Brucella is endemic, the possibility of this infectious etiology should be considered. Based on reported cases, patients will usually have elevated ESR or CRP; all (except 2 cases in which no exposure was reported) had known exposure to unpasteurized dairy products or livestock. Joint aspiration yielded Brucella species in 8 cases, was negative in 3, and not reported in 5 cases (1 aspirate yielded Acinetobacter baumanii). In this case, ESR and CRP were elevated, and infection was suspected but joint aspiration was negative. The initial aspiration was cultured for 5 days and previous data, as well as that presented here, suggest that prolonged culture may provide diagnostic value.18 The patient had resided in an endemic area and had exposure to unpasteurized dairy products, but Brucella infection was not considered and, therefore, no precautions were taken.
Of the reported cases, only 1 met major criteria for periprosthetic joint infection (draining sinus) while 10 of the remaining 15 cases were positive for minor criteria of periprosthetic joint infection (elevated ESR or CRP, or positive culture from joint aspiration).19 Unfortunately, the available case reports did not detail the extent to which preoperative periprosthetic joint infection could be established based on minor criteria for periprosthetic joint infection (elevated joint synovial white blood cell count or neutrophil percentage, intra-articular purulence, or elevated neutrophil count on periprosthetic tissue histologic analysis).19
Periprosthetic joint infection by Brucella species is so rare that specific recommendations for this infectious etiology based on 18 reported cases would be overreaching. However, Brucella should be considered when evaluating a potentially infected joint replacement where the possibility of exposure exists (eg, travel to or previous residence in endemic areas, close contact with livestock, or ingestion of unpasteurized dairy products in endemic regions), with the potential for transmission to operating room and hospital personnel also considered. If there is concern about Brucella involvement, tissue and fluid specimens should be labeled so that laboratory personnel can take appropriate precautions. Brucella can be cultured using routine techniques on standard, nonselective media, but the culture time-to-growth may be prolonged. Culture plates should be held for 14 days before reporting no growth of Brucella if it is suspected; the New Mexico Department of Health Microbiology Laboratory holds routine cultures for 1 week after a report of no growth. Thus, a suspicion of Brucella should be communicated in order for culture time to be adjusted if the holding of culture plates after an initial report of no growth is not standard practice. If operative intervention is planned and brucellosis is known, personnel should be notified of the possibility of exposure and appropriate measures taken (ie, wearing N-95 respiratory masks during the procedure and considering other methods of irrigation less likely to aerosolize particulates). It is not known if preoperative antibiotic therapy can sufficiently lower the bacterial load to make aerosolization less likely. If brucellosis is suspected but not identified preoperatively, wearing N-95 respiratory masks should be considered during any open procedures.
Conclusion
In cases of Brucella infection and loose components, 1- or 2-stage revision with appropriate antibiotic therapy is indicated. (There is not enough data to recommend either 1- or 2-stage revision.) Several reports comment on the ability to treat periprosthetic joint infection in the setting of well-fixed components with antibiotic therapy alone. While this appears to have been successful in 7 of 9 infected joints reported in the literature, length of follow-up ranged from 8 months to 5 years, with no report of length of follow-up in some cases. Antibiotic therapy duration ranged from 6 weeks to 26 months, and the antibiotic treatment involved combination therapy with multiple agents reported but, most commonly, doxycycline, rifampin, and streptomycin. With 2 of 9 (22%) joints failing antibiotic therapy alone and those reported to be successful having relatively short-term follow-up, this treatment strategy should be approached with caution.
1. Agarwal S, Kadhi SK, Rooney RJ. Brucellosis complicating bilateral total knee arthroplasty. Clin Orthop. 1991;267:179-181.
2. Cairó M, Calbo E, Gomez L, et al. Foreign-body osteoarticular infection by Brucella melitensis: A report of three cases. J Bone Joint Surg Am. 2006; 88(1):202-204.
3. Erdogan H, Cakmak G, Erdogan A, Arslan H. Brucella melitensis infection in total knee arthroplasty: a case report. Knee Surg Sports Traumatol Arthrosc. 2010;18(7):908-910.
4. Jones RE, Berryhill WH, Smith J, Hofman A, Rogers D. Secondary infection of a total hip replacement with Brucella abortus. Orthopedics. 1983; 6(2):184-186.
5. Kasim RA, Araj GF, Afeiche NE, Tabbarah ZA. Brucella infection in total hip replacement: case report and review of the literature. Scand J Infect Dis. 2004;36(1):65-67.
6. Malizos KN, Makris CA, Soucacos PN. Total knee arthroplasties infected by Brucella melitensis: a case report. Am J Orthop. 1997;26(4):283-285.
7. Ortega-Andreu M, Rodriguez-Merchan EC, Aguera-Gavalda M. Brucellosis as a cause of septic loosening of total hip arthroplasty. J Arthroplasty. 2002;17(3):384-387.
8. Orti A, Alcala R, Navarro V, et al. Brucellar arthritis in a total knee replacement. Eur J Clin Microbiol Infect Dis. 1997;16(11):843-845.
9. Ruiz-Iban MA, Crespo P, Diaz-Peletier R, Rozado AM, Lopez-Pardo A. Total hip arthroplasty infected by Brucella: a report of two cases. J Orthop Surg (Hong Kong). 2006;14(1):99-103.
10. Tassinari E, Di Motta D, Giardina F, Traina F, Fine MD, Toni A. Brucella infection in total knee arthroplasty. Case report and revision of the literature. Chir Organi Mov. 2008;92(1):55-59.
11. Tena D, Romanillos O, Rodriguez-Zapata M, et al. Prosthetic hip infection due to Brucella melitensis: case report and literature review. Diagn Microbiol Infect Dis. 2007;58(4):481-485.
12. Weil Y, Mattan Y, Liebergall M, Rahav G. Brucella prosthetic joint infection: a report of 3 cases and a review of the literature. Clin Infect Dis. 2003;36(7):e81-e86.
13. Brucellosis. Centers for Disease Control and Prevention website. http://www.cdc.gov/nczved/divisions/dfbmd/diseases/brucellosis/recommendations.html. Updated November 12, 2012. Accessed December 22, 2014.
14. Franco MP, Mulder M, Gilman RH, Smits HL. Human brucellosis. Lancet Infect Dis. 2007;7(12):775-786.
15. Khateeb MI, Araj GF, Majeed SA, Lulu AR. Brucella arthritis: a study of 96 cases in Kuwait. Ann Rheum Dis. 1990;49(12):994-998.
16. Luna-Martinez JE, Mejía-Terán C. Brucellosis in Mexico: current status and trends. Vet Microbiol. 2002;90(1-4):19-30.
17. Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect Dis. 2006;6(2):91-99.
18. Schafer P, Fink B, Margull A, Berger I, Frommelt L. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Disease. 2008;47(11):1403-1409.
19. Parvizi J, Zmistowski B, Berbari EF, et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop. 2011;469(11):2992-2994.
1. Agarwal S, Kadhi SK, Rooney RJ. Brucellosis complicating bilateral total knee arthroplasty. Clin Orthop. 1991;267:179-181.
2. Cairó M, Calbo E, Gomez L, et al. Foreign-body osteoarticular infection by Brucella melitensis: A report of three cases. J Bone Joint Surg Am. 2006; 88(1):202-204.
3. Erdogan H, Cakmak G, Erdogan A, Arslan H. Brucella melitensis infection in total knee arthroplasty: a case report. Knee Surg Sports Traumatol Arthrosc. 2010;18(7):908-910.
4. Jones RE, Berryhill WH, Smith J, Hofman A, Rogers D. Secondary infection of a total hip replacement with Brucella abortus. Orthopedics. 1983; 6(2):184-186.
5. Kasim RA, Araj GF, Afeiche NE, Tabbarah ZA. Brucella infection in total hip replacement: case report and review of the literature. Scand J Infect Dis. 2004;36(1):65-67.
6. Malizos KN, Makris CA, Soucacos PN. Total knee arthroplasties infected by Brucella melitensis: a case report. Am J Orthop. 1997;26(4):283-285.
7. Ortega-Andreu M, Rodriguez-Merchan EC, Aguera-Gavalda M. Brucellosis as a cause of septic loosening of total hip arthroplasty. J Arthroplasty. 2002;17(3):384-387.
8. Orti A, Alcala R, Navarro V, et al. Brucellar arthritis in a total knee replacement. Eur J Clin Microbiol Infect Dis. 1997;16(11):843-845.
9. Ruiz-Iban MA, Crespo P, Diaz-Peletier R, Rozado AM, Lopez-Pardo A. Total hip arthroplasty infected by Brucella: a report of two cases. J Orthop Surg (Hong Kong). 2006;14(1):99-103.
10. Tassinari E, Di Motta D, Giardina F, Traina F, Fine MD, Toni A. Brucella infection in total knee arthroplasty. Case report and revision of the literature. Chir Organi Mov. 2008;92(1):55-59.
11. Tena D, Romanillos O, Rodriguez-Zapata M, et al. Prosthetic hip infection due to Brucella melitensis: case report and literature review. Diagn Microbiol Infect Dis. 2007;58(4):481-485.
12. Weil Y, Mattan Y, Liebergall M, Rahav G. Brucella prosthetic joint infection: a report of 3 cases and a review of the literature. Clin Infect Dis. 2003;36(7):e81-e86.
13. Brucellosis. Centers for Disease Control and Prevention website. http://www.cdc.gov/nczved/divisions/dfbmd/diseases/brucellosis/recommendations.html. Updated November 12, 2012. Accessed December 22, 2014.
14. Franco MP, Mulder M, Gilman RH, Smits HL. Human brucellosis. Lancet Infect Dis. 2007;7(12):775-786.
15. Khateeb MI, Araj GF, Majeed SA, Lulu AR. Brucella arthritis: a study of 96 cases in Kuwait. Ann Rheum Dis. 1990;49(12):994-998.
16. Luna-Martinez JE, Mejía-Terán C. Brucellosis in Mexico: current status and trends. Vet Microbiol. 2002;90(1-4):19-30.
17. Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect Dis. 2006;6(2):91-99.
18. Schafer P, Fink B, Margull A, Berger I, Frommelt L. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin Infect Disease. 2008;47(11):1403-1409.
19. Parvizi J, Zmistowski B, Berbari EF, et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop. 2011;469(11):2992-2994.
Periprosthetic Supracondylar Femur Fracture Treated With Spanning External Fixation
The incidence of periprosthetic supracondylar fractures of the femur after total knee arthroplasty (TKA) ranges from 0.6% to 2.5%.1 Treatment of periprosthetic fractures is often complicated by advanced patient age and osteoporosis, which frequently accompanies these fractures. Management of a periprosthetic fracture depends on the relation between the fracture site and the prosthesis, displacement of the prosthesis, integrity of the fixation of the prosthesis, extent of the bone loss caused by fracture comminution or preexisting osteolysis, general health of the patient, and surgeon expertise.2,3 The aim is to achieve fracture union around a stable, well-aligned arthroplasty with preserved or restored bone stock and therefore to return the patient to previous level of function. Although nonoperative treatments have been shown to be successful,4,5 in the great majority of cases surgical treatment is advised for these fractures.6-10 In cases in which bone stock is adequate for fixation rather than replacement of the distal femur, 2 modalities are commonly used: retrograde intramedullary nailing and locking plates. Each has its drawbacks and advantages.11,12
Although external fixation has been used in the treatment of distal femoral fractures,13 it is seldom considered in the treatment of periprosthetic fractures. Several authors have described cases that used external fixators, occasionally spanning the knee. The specific types of external fixators discussed in the literature have included ring fixators,14-17 hybrid fixators,18 and uniplanar nonspanning fixators14,19 (Table). Use of a simple anterior spanning external fixator in treating a periprosthetic femoral fracture has received little attention in the literature.
The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 54-year-old woman with previous total hip arthroplasty (THA) and ipsilateral TKA tripped on a carpet and sustained a comminuted fracture of the distal femur just above the TKA prosthesis (Figure 1). She was a Jehovah’s Witness and thus refused all blood products. She had an extensive history of osteoporosis, morbid obesity (5 feet tall, 250 pounds; body mass index, 49), diabetes, and rheumatoid arthritis. Evaluation by the internal medicine service revealed severe coronary artery disease on a stress thallium test and anemia with hematocrit of 24%. Given the patient’s medical comorbidities and religious status, and the location of the comminuted distal femur fracture, several treatment options were considered. First was nonoperative treatment with a cast or cast-brace (hinged cast). Because of her body habitus, however, we thought she would very likely experience skin complications, inadequate immobilization of the bone, and significant discomfort. Ultimately, use of a spanning external fixator was chosen as the safest course, given the significant medical risks accompanying a more extensive surgical reconstruction. With the spanning external fixator, the main risks were the inability to fully control fracture alignment and the potential introduction of infection into the functional THA. We thought that, by limiting the amount of time in the fixator and managing the pin site aggressively, we could minimize the risk for infection in this setting.
The procedure was performed with the patient under general anesthesia. During surgery, a lateral image of the femur was used to identify the distal end of the THA prosthesis. A level was marked 2 to 3 cm distal to the tip of this prosthesis, and another about 1 cm above the fracture (noted to be above the most proximal extent of the knee joint). These planned pin-entry sites were prepared from an anterior approach with incisions (using a No. 11 blade) of about 1 cm each. Blunt dissection was carried down to the femur. Each planned pin site was predrilled with a 3.5-mm drill; then, a 5-mm Shanz pin was placed. This process was repeated immediately distal to the tibial component and at the junction of the mid and distal thirds of the tibia (Figure 2). The preliminary external fixator frame was then applied. Once the reduction was satisfactory in the anteroposterior and lateral planes, the fixator clamps were tightened. A second row of bars was then incorporated.
Six weeks after surgery, radiographs showed early callus formation. Removing the external fixator and examining the knee under anesthesia confirmed there was no significant motion through the fracture site. A cast-brace (fiberglass thigh segment, fiberglass lower leg cast with hinged knee segment) was then applied. We remained concerned about skin complications but were encouraged by the early healing achieved with the fixator. The patient was started on a physical therapy program of gait training with a walker and toe-touch weight-bearing on the injured extremity. She also started a limited lower-extremity strengthening program. Three months after surgery, she was tolerating weight-bearing on the injured extremity with no pain. At 6 months, knee radiographs showed fracture consolidation with active range of motion of 10° to 120° and no pain (Figures 3A, 3B). Distal sensation, motor function, and vascular examination were normal. Two years after surgery, radiographs of the right knee showed minor malalignment in the coronal and sagittal planes (Figures 4A, 4B) and complete consolidation of the fracture.
Discussion
Periprosthetic fractures of the femur after TKA often occur in the setting of osteopenia, and some are associated with concurrent implant loosening. In most cases, these fractures require surgical stabilization. Nevertheless, the goals of treatment are to obtain and maintain anatomical alignment and stability to allow early range of motion. Nonoperative options include skeletal traction, cast, pins and plaster, and cast-brace.3-5,20 Operative options include intramedullary fixation,12,21 stabilization with various plates,21-23 revision knee arthroplasty, and arthrodesis.1 Treatment selection should be based on patient health, fracture displacement, comminution, osteopenia severity, and status of the prosthetic components.
The present case exemplifies some of the highest degrees of medical and surgical risk factors in people with a periprosthetic femoral fracture after TKA. Patients with rheumatoid arthritis, patients having corticosteroid treatment, patients of advanced age, and female patients are all at higher risk for supracondylar femoral fracture.9 Our patient had these risk factors on a background of anemia and extensive coronary artery disease. Given her past medical history and refusal of blood products out of religious belief, we thought she was too high risk for extensive surgical treatment for her fracture. In addition, she was not an ideal candidate for nonoperative treatment, as a periprosthetic fracture typically is treated with surgical revision or open reduction and internal fixation. Therefore, we selected an unconventional treatment modality, typically used as a temporizing measure in severe fractures around the knee—a spanning external fixator worn for 6 weeks and a cast-brace for an additional 6 weeks. This led to successful clinical and radiographic outcomes. We consider spanning external fixation a viable option for periprosthetic fractures after TKA in morbidly obese patients with relatively well-aligned fractures and extremely high risk for medical complications associated with traditional open surgery.
1. Figgie MP, Goldberg VM, Figgie HE 3rd, Sobel M. The results of treatment of supracondylar fracture above total knee arthroplasty. J Arthroplasty. 1990;5(3):267-276.
2. Su ET, Kubiak EN, Dewal H, Hiebert R, Di Cesare PE. A proposed classification of supracondylar femur fractures above total knee arthroplasties. J Arthroplasty. 2006;21(3):405-408.
3. Kim KI, Egol KA, Hozack WJ, Parvizi J. Periprosthetic fractures after total knee arthroplasties. Clin Orthop. 2006;(446):167-175.
4. Sochart DH, Hardinge K. Nonsurgical management of supracondylar fracture above total knee arthroplasty. Still the nineties option. J Arthroplasty. 1997;12(7):830-834.
5. Delport PH, Van Audekercke R, Martens M, Mulier JC. Conservative treatment of ipsilateral supracondylar femoral fracture after total knee arthroplasty. J Trauma. 1984;24(9):846-849.
6. Frigg R, Appenzeller A, Christensen R, Frenk A, Gilbert S, Schavan R. The development of the distal femur less invasive stabilization system (LISS). Injury. 2001;32(suppl 3):SC24-SC31.
7. Goesling T, Frenk A, Appenzeller A, Garapati R, Marti A, Krettek C. LISS PLT: design, mechanical and biomechanical characteristics. Injury. 2003;34(suppl 1):A11-A15.
8. Huang HT, Huang PJ, Su JY, Lin SY. Indirect reduction and bridge plating of supracondylar fractures of the femur. Injury. 2003;34(2):135-140.
9. Dennis DA. Periprosthetic fractures following total knee arthroplasty. Instr Course Lect. 2001;50:379-389.
10. Jamali AA, Lee MA, Donthineni R, Meehan JP. Minimally invasive management of a floating prosthesis injury with locking plates. J Arthroplasty. 2007;22(6):928-933.
11. Bong MR, Egol KA, Koval KJ, et al. Comparison of the LISS and a retrograde-inserted supracondylar intramedullary nail for fixation of a periprosthetic distal femur fracture proximal to a total knee arthroplasty. J Arthroplasty. 2002;17(7):876-881.
12. Firoozbakhsh K, Behzadi K, DeCoster TA, Moneim MS, Naraghi FF. Mechanics of retrograde nail versus plate fixation for supracondylar femur fractures. J Orthop Trauma. 1995;9(2):152-157.
13. Arazi M, Memik R, Ogun TC, Yel M. Ilizarov external fixation for severely comminuted supracondylar and intercondylar fractures of the distal femur. J Bone Joint Surg Br. 2001;83(5):663-667.
14. Pleva L, Sir M, Madeja R. Our experiences with the treatment of periprosthetic fractures of femur. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2004;148(1):75-79.
15. Simon RG, Brinker MR. Use of Ilizarov external fixation for a periprosthetic supracondylar femur fracture. J Arthroplasty. 1999;14(1):118-121.
16. Hurson C, Synnott K, McCormack D. Above-knee Ilizarov external fixation for early periprosthetic supracondylar femoral fracture—a case report. Knee. 2005;12(2):145-147.
17. Beris AE, Lykissas MG, Sioros V, Mavrodontidis AN, Korompilias AV. Femoral periprosthetic fracture in osteoporotic bone after a total knee replacement: treatment with Ilizarov external fixation. J Arthroplasty. 2010;25(7):1168.e9-e12.
18. Pafilas D, Kourtzis N. Hybrid external fixation as a new treatment method for periprosthetic femoral fracture. A case report. J Bone Joint Surg Am. 2006;88(1):188-192.
19. Merkel KD, Johnson EW Jr. Supracondylar fracture of the femur after total knee arthroplasty. J Bone Joint Surg Am. 1986;68(1):29-43.
20. Cordeiro EN, Costa RC, Carazzato JG, Silva Jdos S. Periprosthetic fractures in patients with total knee arthroplasties. Clin Orthop. 1990;(252):182-189.
21. Riemer BL, Butterfield SL, Burke CJ 3rd, Mathews D. Immediate plate fixation of highly comminuted femoral diaphyseal fractures in blunt polytrauma patients. Orthopedics. 1992;15(8):907-916.
22. Kregor PJ, Hughes JL, Cole PA. Fixation of distal femoral fractures above total knee arthroplasty utilizing the less invasive stabilization system (L.I.S.S.). Injury. 2001;32(suppl 3):SC64-SC75.
23. Althausen PL, Lee MA, Finkemeier CG, Meehan JP, Rodrigo JJ. Operative stabilization of supracondylar femur fractures above total knee arthroplasty: a comparison of four treatment methods. J Arthroplasty. 2003;18(7):834-839.
The incidence of periprosthetic supracondylar fractures of the femur after total knee arthroplasty (TKA) ranges from 0.6% to 2.5%.1 Treatment of periprosthetic fractures is often complicated by advanced patient age and osteoporosis, which frequently accompanies these fractures. Management of a periprosthetic fracture depends on the relation between the fracture site and the prosthesis, displacement of the prosthesis, integrity of the fixation of the prosthesis, extent of the bone loss caused by fracture comminution or preexisting osteolysis, general health of the patient, and surgeon expertise.2,3 The aim is to achieve fracture union around a stable, well-aligned arthroplasty with preserved or restored bone stock and therefore to return the patient to previous level of function. Although nonoperative treatments have been shown to be successful,4,5 in the great majority of cases surgical treatment is advised for these fractures.6-10 In cases in which bone stock is adequate for fixation rather than replacement of the distal femur, 2 modalities are commonly used: retrograde intramedullary nailing and locking plates. Each has its drawbacks and advantages.11,12
Although external fixation has been used in the treatment of distal femoral fractures,13 it is seldom considered in the treatment of periprosthetic fractures. Several authors have described cases that used external fixators, occasionally spanning the knee. The specific types of external fixators discussed in the literature have included ring fixators,14-17 hybrid fixators,18 and uniplanar nonspanning fixators14,19 (Table). Use of a simple anterior spanning external fixator in treating a periprosthetic femoral fracture has received little attention in the literature.
The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 54-year-old woman with previous total hip arthroplasty (THA) and ipsilateral TKA tripped on a carpet and sustained a comminuted fracture of the distal femur just above the TKA prosthesis (Figure 1). She was a Jehovah’s Witness and thus refused all blood products. She had an extensive history of osteoporosis, morbid obesity (5 feet tall, 250 pounds; body mass index, 49), diabetes, and rheumatoid arthritis. Evaluation by the internal medicine service revealed severe coronary artery disease on a stress thallium test and anemia with hematocrit of 24%. Given the patient’s medical comorbidities and religious status, and the location of the comminuted distal femur fracture, several treatment options were considered. First was nonoperative treatment with a cast or cast-brace (hinged cast). Because of her body habitus, however, we thought she would very likely experience skin complications, inadequate immobilization of the bone, and significant discomfort. Ultimately, use of a spanning external fixator was chosen as the safest course, given the significant medical risks accompanying a more extensive surgical reconstruction. With the spanning external fixator, the main risks were the inability to fully control fracture alignment and the potential introduction of infection into the functional THA. We thought that, by limiting the amount of time in the fixator and managing the pin site aggressively, we could minimize the risk for infection in this setting.
The procedure was performed with the patient under general anesthesia. During surgery, a lateral image of the femur was used to identify the distal end of the THA prosthesis. A level was marked 2 to 3 cm distal to the tip of this prosthesis, and another about 1 cm above the fracture (noted to be above the most proximal extent of the knee joint). These planned pin-entry sites were prepared from an anterior approach with incisions (using a No. 11 blade) of about 1 cm each. Blunt dissection was carried down to the femur. Each planned pin site was predrilled with a 3.5-mm drill; then, a 5-mm Shanz pin was placed. This process was repeated immediately distal to the tibial component and at the junction of the mid and distal thirds of the tibia (Figure 2). The preliminary external fixator frame was then applied. Once the reduction was satisfactory in the anteroposterior and lateral planes, the fixator clamps were tightened. A second row of bars was then incorporated.
Six weeks after surgery, radiographs showed early callus formation. Removing the external fixator and examining the knee under anesthesia confirmed there was no significant motion through the fracture site. A cast-brace (fiberglass thigh segment, fiberglass lower leg cast with hinged knee segment) was then applied. We remained concerned about skin complications but were encouraged by the early healing achieved with the fixator. The patient was started on a physical therapy program of gait training with a walker and toe-touch weight-bearing on the injured extremity. She also started a limited lower-extremity strengthening program. Three months after surgery, she was tolerating weight-bearing on the injured extremity with no pain. At 6 months, knee radiographs showed fracture consolidation with active range of motion of 10° to 120° and no pain (Figures 3A, 3B). Distal sensation, motor function, and vascular examination were normal. Two years after surgery, radiographs of the right knee showed minor malalignment in the coronal and sagittal planes (Figures 4A, 4B) and complete consolidation of the fracture.
Discussion
Periprosthetic fractures of the femur after TKA often occur in the setting of osteopenia, and some are associated with concurrent implant loosening. In most cases, these fractures require surgical stabilization. Nevertheless, the goals of treatment are to obtain and maintain anatomical alignment and stability to allow early range of motion. Nonoperative options include skeletal traction, cast, pins and plaster, and cast-brace.3-5,20 Operative options include intramedullary fixation,12,21 stabilization with various plates,21-23 revision knee arthroplasty, and arthrodesis.1 Treatment selection should be based on patient health, fracture displacement, comminution, osteopenia severity, and status of the prosthetic components.
The present case exemplifies some of the highest degrees of medical and surgical risk factors in people with a periprosthetic femoral fracture after TKA. Patients with rheumatoid arthritis, patients having corticosteroid treatment, patients of advanced age, and female patients are all at higher risk for supracondylar femoral fracture.9 Our patient had these risk factors on a background of anemia and extensive coronary artery disease. Given her past medical history and refusal of blood products out of religious belief, we thought she was too high risk for extensive surgical treatment for her fracture. In addition, she was not an ideal candidate for nonoperative treatment, as a periprosthetic fracture typically is treated with surgical revision or open reduction and internal fixation. Therefore, we selected an unconventional treatment modality, typically used as a temporizing measure in severe fractures around the knee—a spanning external fixator worn for 6 weeks and a cast-brace for an additional 6 weeks. This led to successful clinical and radiographic outcomes. We consider spanning external fixation a viable option for periprosthetic fractures after TKA in morbidly obese patients with relatively well-aligned fractures and extremely high risk for medical complications associated with traditional open surgery.
The incidence of periprosthetic supracondylar fractures of the femur after total knee arthroplasty (TKA) ranges from 0.6% to 2.5%.1 Treatment of periprosthetic fractures is often complicated by advanced patient age and osteoporosis, which frequently accompanies these fractures. Management of a periprosthetic fracture depends on the relation between the fracture site and the prosthesis, displacement of the prosthesis, integrity of the fixation of the prosthesis, extent of the bone loss caused by fracture comminution or preexisting osteolysis, general health of the patient, and surgeon expertise.2,3 The aim is to achieve fracture union around a stable, well-aligned arthroplasty with preserved or restored bone stock and therefore to return the patient to previous level of function. Although nonoperative treatments have been shown to be successful,4,5 in the great majority of cases surgical treatment is advised for these fractures.6-10 In cases in which bone stock is adequate for fixation rather than replacement of the distal femur, 2 modalities are commonly used: retrograde intramedullary nailing and locking plates. Each has its drawbacks and advantages.11,12
Although external fixation has been used in the treatment of distal femoral fractures,13 it is seldom considered in the treatment of periprosthetic fractures. Several authors have described cases that used external fixators, occasionally spanning the knee. The specific types of external fixators discussed in the literature have included ring fixators,14-17 hybrid fixators,18 and uniplanar nonspanning fixators14,19 (Table). Use of a simple anterior spanning external fixator in treating a periprosthetic femoral fracture has received little attention in the literature.
The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 54-year-old woman with previous total hip arthroplasty (THA) and ipsilateral TKA tripped on a carpet and sustained a comminuted fracture of the distal femur just above the TKA prosthesis (Figure 1). She was a Jehovah’s Witness and thus refused all blood products. She had an extensive history of osteoporosis, morbid obesity (5 feet tall, 250 pounds; body mass index, 49), diabetes, and rheumatoid arthritis. Evaluation by the internal medicine service revealed severe coronary artery disease on a stress thallium test and anemia with hematocrit of 24%. Given the patient’s medical comorbidities and religious status, and the location of the comminuted distal femur fracture, several treatment options were considered. First was nonoperative treatment with a cast or cast-brace (hinged cast). Because of her body habitus, however, we thought she would very likely experience skin complications, inadequate immobilization of the bone, and significant discomfort. Ultimately, use of a spanning external fixator was chosen as the safest course, given the significant medical risks accompanying a more extensive surgical reconstruction. With the spanning external fixator, the main risks were the inability to fully control fracture alignment and the potential introduction of infection into the functional THA. We thought that, by limiting the amount of time in the fixator and managing the pin site aggressively, we could minimize the risk for infection in this setting.
The procedure was performed with the patient under general anesthesia. During surgery, a lateral image of the femur was used to identify the distal end of the THA prosthesis. A level was marked 2 to 3 cm distal to the tip of this prosthesis, and another about 1 cm above the fracture (noted to be above the most proximal extent of the knee joint). These planned pin-entry sites were prepared from an anterior approach with incisions (using a No. 11 blade) of about 1 cm each. Blunt dissection was carried down to the femur. Each planned pin site was predrilled with a 3.5-mm drill; then, a 5-mm Shanz pin was placed. This process was repeated immediately distal to the tibial component and at the junction of the mid and distal thirds of the tibia (Figure 2). The preliminary external fixator frame was then applied. Once the reduction was satisfactory in the anteroposterior and lateral planes, the fixator clamps were tightened. A second row of bars was then incorporated.
Six weeks after surgery, radiographs showed early callus formation. Removing the external fixator and examining the knee under anesthesia confirmed there was no significant motion through the fracture site. A cast-brace (fiberglass thigh segment, fiberglass lower leg cast with hinged knee segment) was then applied. We remained concerned about skin complications but were encouraged by the early healing achieved with the fixator. The patient was started on a physical therapy program of gait training with a walker and toe-touch weight-bearing on the injured extremity. She also started a limited lower-extremity strengthening program. Three months after surgery, she was tolerating weight-bearing on the injured extremity with no pain. At 6 months, knee radiographs showed fracture consolidation with active range of motion of 10° to 120° and no pain (Figures 3A, 3B). Distal sensation, motor function, and vascular examination were normal. Two years after surgery, radiographs of the right knee showed minor malalignment in the coronal and sagittal planes (Figures 4A, 4B) and complete consolidation of the fracture.
Discussion
Periprosthetic fractures of the femur after TKA often occur in the setting of osteopenia, and some are associated with concurrent implant loosening. In most cases, these fractures require surgical stabilization. Nevertheless, the goals of treatment are to obtain and maintain anatomical alignment and stability to allow early range of motion. Nonoperative options include skeletal traction, cast, pins and plaster, and cast-brace.3-5,20 Operative options include intramedullary fixation,12,21 stabilization with various plates,21-23 revision knee arthroplasty, and arthrodesis.1 Treatment selection should be based on patient health, fracture displacement, comminution, osteopenia severity, and status of the prosthetic components.
The present case exemplifies some of the highest degrees of medical and surgical risk factors in people with a periprosthetic femoral fracture after TKA. Patients with rheumatoid arthritis, patients having corticosteroid treatment, patients of advanced age, and female patients are all at higher risk for supracondylar femoral fracture.9 Our patient had these risk factors on a background of anemia and extensive coronary artery disease. Given her past medical history and refusal of blood products out of religious belief, we thought she was too high risk for extensive surgical treatment for her fracture. In addition, she was not an ideal candidate for nonoperative treatment, as a periprosthetic fracture typically is treated with surgical revision or open reduction and internal fixation. Therefore, we selected an unconventional treatment modality, typically used as a temporizing measure in severe fractures around the knee—a spanning external fixator worn for 6 weeks and a cast-brace for an additional 6 weeks. This led to successful clinical and radiographic outcomes. We consider spanning external fixation a viable option for periprosthetic fractures after TKA in morbidly obese patients with relatively well-aligned fractures and extremely high risk for medical complications associated with traditional open surgery.
1. Figgie MP, Goldberg VM, Figgie HE 3rd, Sobel M. The results of treatment of supracondylar fracture above total knee arthroplasty. J Arthroplasty. 1990;5(3):267-276.
2. Su ET, Kubiak EN, Dewal H, Hiebert R, Di Cesare PE. A proposed classification of supracondylar femur fractures above total knee arthroplasties. J Arthroplasty. 2006;21(3):405-408.
3. Kim KI, Egol KA, Hozack WJ, Parvizi J. Periprosthetic fractures after total knee arthroplasties. Clin Orthop. 2006;(446):167-175.
4. Sochart DH, Hardinge K. Nonsurgical management of supracondylar fracture above total knee arthroplasty. Still the nineties option. J Arthroplasty. 1997;12(7):830-834.
5. Delport PH, Van Audekercke R, Martens M, Mulier JC. Conservative treatment of ipsilateral supracondylar femoral fracture after total knee arthroplasty. J Trauma. 1984;24(9):846-849.
6. Frigg R, Appenzeller A, Christensen R, Frenk A, Gilbert S, Schavan R. The development of the distal femur less invasive stabilization system (LISS). Injury. 2001;32(suppl 3):SC24-SC31.
7. Goesling T, Frenk A, Appenzeller A, Garapati R, Marti A, Krettek C. LISS PLT: design, mechanical and biomechanical characteristics. Injury. 2003;34(suppl 1):A11-A15.
8. Huang HT, Huang PJ, Su JY, Lin SY. Indirect reduction and bridge plating of supracondylar fractures of the femur. Injury. 2003;34(2):135-140.
9. Dennis DA. Periprosthetic fractures following total knee arthroplasty. Instr Course Lect. 2001;50:379-389.
10. Jamali AA, Lee MA, Donthineni R, Meehan JP. Minimally invasive management of a floating prosthesis injury with locking plates. J Arthroplasty. 2007;22(6):928-933.
11. Bong MR, Egol KA, Koval KJ, et al. Comparison of the LISS and a retrograde-inserted supracondylar intramedullary nail for fixation of a periprosthetic distal femur fracture proximal to a total knee arthroplasty. J Arthroplasty. 2002;17(7):876-881.
12. Firoozbakhsh K, Behzadi K, DeCoster TA, Moneim MS, Naraghi FF. Mechanics of retrograde nail versus plate fixation for supracondylar femur fractures. J Orthop Trauma. 1995;9(2):152-157.
13. Arazi M, Memik R, Ogun TC, Yel M. Ilizarov external fixation for severely comminuted supracondylar and intercondylar fractures of the distal femur. J Bone Joint Surg Br. 2001;83(5):663-667.
14. Pleva L, Sir M, Madeja R. Our experiences with the treatment of periprosthetic fractures of femur. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2004;148(1):75-79.
15. Simon RG, Brinker MR. Use of Ilizarov external fixation for a periprosthetic supracondylar femur fracture. J Arthroplasty. 1999;14(1):118-121.
16. Hurson C, Synnott K, McCormack D. Above-knee Ilizarov external fixation for early periprosthetic supracondylar femoral fracture—a case report. Knee. 2005;12(2):145-147.
17. Beris AE, Lykissas MG, Sioros V, Mavrodontidis AN, Korompilias AV. Femoral periprosthetic fracture in osteoporotic bone after a total knee replacement: treatment with Ilizarov external fixation. J Arthroplasty. 2010;25(7):1168.e9-e12.
18. Pafilas D, Kourtzis N. Hybrid external fixation as a new treatment method for periprosthetic femoral fracture. A case report. J Bone Joint Surg Am. 2006;88(1):188-192.
19. Merkel KD, Johnson EW Jr. Supracondylar fracture of the femur after total knee arthroplasty. J Bone Joint Surg Am. 1986;68(1):29-43.
20. Cordeiro EN, Costa RC, Carazzato JG, Silva Jdos S. Periprosthetic fractures in patients with total knee arthroplasties. Clin Orthop. 1990;(252):182-189.
21. Riemer BL, Butterfield SL, Burke CJ 3rd, Mathews D. Immediate plate fixation of highly comminuted femoral diaphyseal fractures in blunt polytrauma patients. Orthopedics. 1992;15(8):907-916.
22. Kregor PJ, Hughes JL, Cole PA. Fixation of distal femoral fractures above total knee arthroplasty utilizing the less invasive stabilization system (L.I.S.S.). Injury. 2001;32(suppl 3):SC64-SC75.
23. Althausen PL, Lee MA, Finkemeier CG, Meehan JP, Rodrigo JJ. Operative stabilization of supracondylar femur fractures above total knee arthroplasty: a comparison of four treatment methods. J Arthroplasty. 2003;18(7):834-839.
1. Figgie MP, Goldberg VM, Figgie HE 3rd, Sobel M. The results of treatment of supracondylar fracture above total knee arthroplasty. J Arthroplasty. 1990;5(3):267-276.
2. Su ET, Kubiak EN, Dewal H, Hiebert R, Di Cesare PE. A proposed classification of supracondylar femur fractures above total knee arthroplasties. J Arthroplasty. 2006;21(3):405-408.
3. Kim KI, Egol KA, Hozack WJ, Parvizi J. Periprosthetic fractures after total knee arthroplasties. Clin Orthop. 2006;(446):167-175.
4. Sochart DH, Hardinge K. Nonsurgical management of supracondylar fracture above total knee arthroplasty. Still the nineties option. J Arthroplasty. 1997;12(7):830-834.
5. Delport PH, Van Audekercke R, Martens M, Mulier JC. Conservative treatment of ipsilateral supracondylar femoral fracture after total knee arthroplasty. J Trauma. 1984;24(9):846-849.
6. Frigg R, Appenzeller A, Christensen R, Frenk A, Gilbert S, Schavan R. The development of the distal femur less invasive stabilization system (LISS). Injury. 2001;32(suppl 3):SC24-SC31.
7. Goesling T, Frenk A, Appenzeller A, Garapati R, Marti A, Krettek C. LISS PLT: design, mechanical and biomechanical characteristics. Injury. 2003;34(suppl 1):A11-A15.
8. Huang HT, Huang PJ, Su JY, Lin SY. Indirect reduction and bridge plating of supracondylar fractures of the femur. Injury. 2003;34(2):135-140.
9. Dennis DA. Periprosthetic fractures following total knee arthroplasty. Instr Course Lect. 2001;50:379-389.
10. Jamali AA, Lee MA, Donthineni R, Meehan JP. Minimally invasive management of a floating prosthesis injury with locking plates. J Arthroplasty. 2007;22(6):928-933.
11. Bong MR, Egol KA, Koval KJ, et al. Comparison of the LISS and a retrograde-inserted supracondylar intramedullary nail for fixation of a periprosthetic distal femur fracture proximal to a total knee arthroplasty. J Arthroplasty. 2002;17(7):876-881.
12. Firoozbakhsh K, Behzadi K, DeCoster TA, Moneim MS, Naraghi FF. Mechanics of retrograde nail versus plate fixation for supracondylar femur fractures. J Orthop Trauma. 1995;9(2):152-157.
13. Arazi M, Memik R, Ogun TC, Yel M. Ilizarov external fixation for severely comminuted supracondylar and intercondylar fractures of the distal femur. J Bone Joint Surg Br. 2001;83(5):663-667.
14. Pleva L, Sir M, Madeja R. Our experiences with the treatment of periprosthetic fractures of femur. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2004;148(1):75-79.
15. Simon RG, Brinker MR. Use of Ilizarov external fixation for a periprosthetic supracondylar femur fracture. J Arthroplasty. 1999;14(1):118-121.
16. Hurson C, Synnott K, McCormack D. Above-knee Ilizarov external fixation for early periprosthetic supracondylar femoral fracture—a case report. Knee. 2005;12(2):145-147.
17. Beris AE, Lykissas MG, Sioros V, Mavrodontidis AN, Korompilias AV. Femoral periprosthetic fracture in osteoporotic bone after a total knee replacement: treatment with Ilizarov external fixation. J Arthroplasty. 2010;25(7):1168.e9-e12.
18. Pafilas D, Kourtzis N. Hybrid external fixation as a new treatment method for periprosthetic femoral fracture. A case report. J Bone Joint Surg Am. 2006;88(1):188-192.
19. Merkel KD, Johnson EW Jr. Supracondylar fracture of the femur after total knee arthroplasty. J Bone Joint Surg Am. 1986;68(1):29-43.
20. Cordeiro EN, Costa RC, Carazzato JG, Silva Jdos S. Periprosthetic fractures in patients with total knee arthroplasties. Clin Orthop. 1990;(252):182-189.
21. Riemer BL, Butterfield SL, Burke CJ 3rd, Mathews D. Immediate plate fixation of highly comminuted femoral diaphyseal fractures in blunt polytrauma patients. Orthopedics. 1992;15(8):907-916.
22. Kregor PJ, Hughes JL, Cole PA. Fixation of distal femoral fractures above total knee arthroplasty utilizing the less invasive stabilization system (L.I.S.S.). Injury. 2001;32(suppl 3):SC64-SC75.
23. Althausen PL, Lee MA, Finkemeier CG, Meehan JP, Rodrigo JJ. Operative stabilization of supracondylar femur fractures above total knee arthroplasty: a comparison of four treatment methods. J Arthroplasty. 2003;18(7):834-839.
49-Year-Old Woman With a Broken Heart
Emotional stress can induce different responses in the body, particularly in the cardiovascular system. Apical ballooning syndrome (ABS), also known as takotsubo cardiomyopathy and broken heart syndrome, is a transient cardiomyopathy that mimics an acute myocardial infarction (AMI). Dote and colleagues first described this transient entity in Japan in the early 1990s.1 A case review series reported that 57.2% of patients were Asian, 40% were white.2 Mean patient age was 67 years, although cases of ABS have occurred in children and young adults.3,4
The term tako-tsubo means “octopus trap,” which is the morphology that the left ventricle resembles during systole in patients with this syndrome.5 The pathophysiology of ABS is thought to be mediated by a catecholamine surge. The presentation of ABS is indistinguishable from an AMI. The majority of patients present with angina-like chest pain, ischemic changes on an electrocardiogram (ECG), pulmonary edema, and elevation of cardiac enzymes. Apical ballooning syndrome is accompanied by reversible left ventricular apical ballooning in the absence of angiographically significant coronary artery disease.
Typically, echocardiographic findings show a left ventricle with preserved function in the basal segments, moderate-to-severe dysfunction in the mid portion of the left ventricle, and hypokinesis, akinesis, or dyskinesis in the apex. A unique but not exclusive feature of this syndrome is the occurrence of a preceding emotional trigger, usually sudden or unexpected. Most patients are initially treated for an AMI until angiography can rule out coronary obstruction. After several weeks, the left ventricular systolic function usually returns to normal.
Case Presentation
A 49-year-old woman with a history of arterial hypertension, fibromyalgia, peptic ulcer disease, and major depressive disorder with multiple admissions to the psychiatric ward (last admission was 4 weeks prior to the current presentation) presented to the emergency department, reporting severe retrosternal, oppressive chest pain with 9/10 intensity and 3 hours’ duration. The pain was associated with nausea, vomiting, diaphoresis, and palpitations. She reported no previous episodes of exertional angina, fever, illicit drug use, recent illness, or travel. She also reported no prodromal symptoms.
Her initial vital signs were essentially unremarkable, except for mild hypertension (148/84 mm Hg). The physical examination showed an anxious patient in acute distress due to chest pain. A cardiovascular examination revealed a regular heart rate and rhythm, no audible murmurs or gallops, no jugular vein distention, clear breath sounds, and no peripheral edema. The rest of the examination was otherwise unremarkable. An initial 12-lead ECG showed a normal sinus rhythm without any ST-T changes (Figure 1).
The initial cardiac markers were elevated (troponin T 0.36 ng/mL, CK-MB 4.51 ng/mL), as were NT-proBNP levels (1,057 pg/mL). The rest of the laboratory results were essentially unremarkable. The patient was started on aspirin, clopidogrel, enoxaparin, eptifibatide, and IV nitrates. She was admitted to the coronary care unit with a diagnostic impression of non-ST elevation MI. Despite medical management, the patient’s chest pain persisted for several hours from her initial presentation. A repeated 12-lead ECG revealed new borderline (1-1.5 mm) ST segment elevation in V2-V3, suggestive of possible myocardial injury (Figure 2).
A bedside echocardiogram revealed severe wall motion abnor malities, ranging from hypokinesia to dyskinesia of all mid-to-distal left ventricular wall segments with sparing of the basal segment (Figure 3). The estimated left ventricular ejection fraction was 40% to 45%.
In view of these findings, the patient was taken to the catheterization laboratory for emergent coronary angiography, which ruled out significant obstructive coronary disease (Figure 4).
Left ventriculography in right and left anterior oblique projections revealed significant wall motion abnormalities of the mid-to-distal anterolateral and inferior wall segments, sparing the basal and apical segments, giving the appearance of ballooning in systole (Figure 5). The diagnosis of ABS involving the mid ventricular walls was explored.
Subsequent sets of cardiac enzymes at 4 and 8 hours after arrival remained elevated, with a maximum troponin T 0.55 and CK-MB of 11.19. Repeated 12-lead ECG 24 hours post coronary angiography revealed anterolateral T wave inversion (Figure 6).
Noncontrast enhanced cardiac magnetic resonance imaging (MRI) (Figure 7) performed 5 days later revealed wall motion abnormalities highly suggestive of ABS, supporting previous echocardiographic and ventriculography findings. Unfortunately, contrast-enhanced phase for evaluation of delayed enhancement could not be completed, because the patient did not continue the study.
Toxicology tests were negative for sympathomimetic drugs. Metanephrine levels were within the normal range. Viral titers for cytomegalovirus and coxsackie virus also were negative. Inflammatory markers were mildly elevated (erythrocyte sedimentation rate, 22 mm/h; C-reactive protein, 4.2 mg/L).
The patient was treated with supportive care, psychotropic therapy, angiotensin-converting enzyme inhibitor (ACE-I), and beta blocker therapy. Within 9 days, NT-proBNP levels normalized (from peak 8,834 pg/mL to 191.5 pg/mL).
Six weeks later, an echocardiogram confirmed resolution of wall motion abnormalities (Figure 8). Follow-up cardiac MRI showed complete resolution of segmental wall motion abnormalities and the apical ballooning, normal wall thickness, and absent delayed enhancement (Figure 9). These findings further supported the diagnosis of ABS and excluded MI and myocarditis.
Discussion
What is striking about takotsubo cardiomyopathy is that the clinical presentation resembles an AMI. Several studies have reported that 1.7% to 2.2% of patients who had suspected acute coronary syndrome were subsequently diagnosed with takotsubo cardiomyopathy.6-8 Nearly 90% of reported cases involved postmenopausal women, and this may be related to loss of the cardioprotective effect of estrogen.5,9
A preceding stressful emotional or physical event is usually identified in about two-thirds of the patients with ABS.9 Most common emotional triggers are death of a relative or friend, broken relationships, assaults, and rapes, among others. Physical triggers include severe sepsis, shock, acute respiratory failure, seizures, and intracranial bleeds. Sometimes a specific trigger cannot be identified from the history, but the absence of an emotional or physical trigger does not exclude the diagnosis.
Although the exact pathogenesis of ABS remains unclear, it is likely that multiple factors are involved. Some of the suggested mechanisms are high levels of catecholamines, multivessel epicardial spasm, or coronary microvascular dysfunction.4 The catecholamine hypothesis has been supported by the finding that several patients with pheochromocytoma and subarachnoid hemorrhage also present with high levels of catecholamine and a cardiomyopathy resembling ABS. Furthermore, ABS has been reported in patients on catecholamine infusions and those treated with agents that inhibit reuptake of catecholamines.5
The presence of multivessel coronary spasm was suggested by early small studies in Japan, but more recent case series have not validated this hypothesis.5 The microvascular dysfunction hypothesis is supported by the presence of myocardial ischemia, diagnosed by ECG changes and elevated troponins, in the absence of significant coronary disease. However, it remains unclear whether this is a primary mechanism or a manifestation of a primary process.4 Microvascular dysfunction may be more likely related to impairment of myocardial relaxation with extramural coronary compression.
Signs and symptoms of ABS mimic those of AMI, with angina-like chest pain as the main presenting symptom in about 50% of cases.10 Other symptoms include dyspnea and less commonly, syncope or sudden cardiac death. Decompensated left heart failure occurs in 50% of patients, with severe hemodynamic compromise and cardiogenic shock not being uncommon. Other complications that may occur are tachyarrhythmias (atrial or ventricular) and ventricular thromboembolism.4
Common ECG changes in ABS include precordial ST segment elevations, symmetric T wave inversions, and nonspecific T wave changes.4,10 QT interval prolongation may be seen during the first days. Transient pathologic Q waves may be seen at presentation or afterward. These ECG changes tend to revert after weeks or months of presentation.
Elevation of cardiac biomarkers is usually present in laboratory data. Levels peak at 24 hours, and the degree of elevation is usually less than that seen in patients with an AMI.10 Most important, the degree of cardiac biomarker elevation is disproportionately low for the extent of involved coronary territory and left ventricular dysfunction. Other laboratory tests that are frequently altered are the BNP and pro-BNP levels, which are usually elevated due to transient left ventricular dysfunction. C-reactive protein elevates in most patients and indicates the presence of an acute inflammatory response.
Early coronary angiography should be performed in all patients with ABS to rule out the presence of a significant obstructive coronary lesion. Patients with ABS often have luminal irregularities or normal coronary vessels. However, concomitant obstructive coronary lesions may be found, especially in elderly patients.
The hallmark of ABS is a characteristic transient contractility abnormality of the left ventricle causing ballooning of the apex, which can be detected on left ventricular angiography or echocardiography. There are 3 distinct variants of ABS, according to the left ventricular myocardial wall segments involved.10 The classic form of takotsubo is characterized by hypokinesis, dyskinesis, or akinesis of the middle and apical segments of the left ventricle. The basal segment is usually spared and may be hyperdynamic. In the midventricular or apical sparing variant, the wall motion abnormalities are restricted to the midventricular segments, and apical contraction is preserved. This case resembles the atypical variant, because the midventricular segments were affected, whereas apical and basal regions were preserved. A rare variant of takotsubo exists with hypokinesis or akinesis of the base and preserved apical function.
Besides ABS and AMI, an important entity to consider in the differential diagnosis of transient wall motion abnormalities is regional myocarditis. Viral titers are helpful in excluding this condition. Furthermore, prolonged recovery is more commonly seen in myocarditis compared with ABS. Imaging studies are particularly helpful in this scenario.
Cardiac MRI demonstrates the wall motion abnormalities or apical ballooning typical for this condition and can differentiate ABS from myocarditis or MI. It is known that delayed myocardial enhancement is seen with myocardial fibrosis. Typically in ischemic cardiomyopathy, there is wall thinning with associated delayed enhancement that extends from the subendocardium to the epicardium (from 0%-90% of wall thickness) of a particular vascular territory. In myocarditis, the enhancement is usually seen in the involved intramyocardial (mesocardium) region, and the pattern is patchy. In ABS, the delayed enhancement is absent, because there is no fibrosis in the area of regional wall motion abnormalities, and wall thickness is usually normal.9,10
No evidence-based guidelines for treating ABS are currently available. Most patients are initially treated with antiplatelets/anticoagulant therapy, nitrates, and diuretics if the patient presents with heart failure. Patients should be admitted to an intensive care unit for close cardiac monitoring. Once ABS is diagnosed and significant coronary stenosis is excluded, patients should receive standard supportive care and optimal neurohormonal therapy. This should include beta blocker or combined alpha/beta blocker agents, an ACE-I or angiotensin receptor blocker, and diuretics if appropriate. Once left ventricular function (LVF) is recovered, therapy with inhibitors of the renin-angiotensin system may be discontinued, but patients should remain on long-term alpha or beta blocker therapy, because the sympathetic blockade provided by these agents may prevent recurrences of this disease.10
Prognosis is generally favorable, and most patients recover to normal LVF over weeks to months. It is important to assess the LVF 4 to 6 weeks after the patient is discharged to confirm the diagnosis of ABS. Recurrence may occur in up to 9% of cases.10 Long-term mortality is similar compared with the age-matched general population.
Conclusion
Apical ballooning syndrome is a relatively novel cardiomyopathy that has gained important attention among the cardiovascular community, mostly because its clinical presentation mimics that of an acute coronary syndrome. Awareness of this entity will result in a more focused diagnosis and appropriate treatment. Managing both cardiac and emotional components of this disease will have a permanent impact in the reversibility and secondary prevention of this cardiomyopathy.
Acknowledgments
Special thanks to the Radiology Service at the VA Caribbean Healthcare System, in particular Dr. Frances Aulet for interpretation of the cardiac MRI results and assistance with MRI images.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Dote K, Sato H, Tateishi H, Uchida T, Ishihara M. Myocardial stunning due to simultaneous multivessel coronary spasms: A review of 5 cases [in Japanese]. J Cardiol. 1991;21(2):203-214.
2. Donohue D, Movahed MR. Clinical characteristics, demographics and prognosis of transient left ventricular apical ballooning syndrome. Heart Fail Rev. 2005;9(4):311-316.
3. Afonso L, Bachour K, Awad K, Sandidge G. Takotsubo cardiomyopathy: Pathogenetic insights and myocardial perfusion kinetics using myocardial contrast echocardiography. Eur J Echocardiogr. 2008;9(6):849-854.
4. Buchholz S, Rudan G. Tako-tsubo syndrome on the rise: A review of the current literature. Postgrad Med J. 2007;83(978):261-264.
5. Hare J. The dilated, restrictive and infiltrative cardiomyopathies. Braunwald’s Heart Disease, A Textbook of Cardiovascular Medicine. 9th ed. Philadelphia, PA: Saunders; 2012:1562-1580.
6. Bybee KA, Prasad A, Barsness GW, et al. Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular apical ballooning syndrome. Am J Cardiol. 2004;94(3):343-346.
7. Ito K, Sugihara H, Katoh S, Azuma A, Nakagawa M. Assessment of Takotsubo (ampulla) cardiomyopathy using 99mTc-tetrofosmin myocardial SPECT—Comparison with acute coronary syndrome. Ann Nucl Med. 2003;17(2):115-122.
8. Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): A mimic of acute myocardial infarction. Am Heart J. 2008;155(3):408-417.
9. Lange R, Hills D. Chemical cardiomyopathies. Braunwald’s Heart Disease, A Textbook of Cardiovascular Medicine. 10th ed. Philadelphia, PA: Saunders; 2014:1609-1611.
10. Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E. Apical ballooning syndrome or takotsubo cardiomyopathy: A systematic review. Eur Heart J. 2006;27(13):1523-1529.
Emotional stress can induce different responses in the body, particularly in the cardiovascular system. Apical ballooning syndrome (ABS), also known as takotsubo cardiomyopathy and broken heart syndrome, is a transient cardiomyopathy that mimics an acute myocardial infarction (AMI). Dote and colleagues first described this transient entity in Japan in the early 1990s.1 A case review series reported that 57.2% of patients were Asian, 40% were white.2 Mean patient age was 67 years, although cases of ABS have occurred in children and young adults.3,4
The term tako-tsubo means “octopus trap,” which is the morphology that the left ventricle resembles during systole in patients with this syndrome.5 The pathophysiology of ABS is thought to be mediated by a catecholamine surge. The presentation of ABS is indistinguishable from an AMI. The majority of patients present with angina-like chest pain, ischemic changes on an electrocardiogram (ECG), pulmonary edema, and elevation of cardiac enzymes. Apical ballooning syndrome is accompanied by reversible left ventricular apical ballooning in the absence of angiographically significant coronary artery disease.
Typically, echocardiographic findings show a left ventricle with preserved function in the basal segments, moderate-to-severe dysfunction in the mid portion of the left ventricle, and hypokinesis, akinesis, or dyskinesis in the apex. A unique but not exclusive feature of this syndrome is the occurrence of a preceding emotional trigger, usually sudden or unexpected. Most patients are initially treated for an AMI until angiography can rule out coronary obstruction. After several weeks, the left ventricular systolic function usually returns to normal.
Case Presentation
A 49-year-old woman with a history of arterial hypertension, fibromyalgia, peptic ulcer disease, and major depressive disorder with multiple admissions to the psychiatric ward (last admission was 4 weeks prior to the current presentation) presented to the emergency department, reporting severe retrosternal, oppressive chest pain with 9/10 intensity and 3 hours’ duration. The pain was associated with nausea, vomiting, diaphoresis, and palpitations. She reported no previous episodes of exertional angina, fever, illicit drug use, recent illness, or travel. She also reported no prodromal symptoms.
Her initial vital signs were essentially unremarkable, except for mild hypertension (148/84 mm Hg). The physical examination showed an anxious patient in acute distress due to chest pain. A cardiovascular examination revealed a regular heart rate and rhythm, no audible murmurs or gallops, no jugular vein distention, clear breath sounds, and no peripheral edema. The rest of the examination was otherwise unremarkable. An initial 12-lead ECG showed a normal sinus rhythm without any ST-T changes (Figure 1).
The initial cardiac markers were elevated (troponin T 0.36 ng/mL, CK-MB 4.51 ng/mL), as were NT-proBNP levels (1,057 pg/mL). The rest of the laboratory results were essentially unremarkable. The patient was started on aspirin, clopidogrel, enoxaparin, eptifibatide, and IV nitrates. She was admitted to the coronary care unit with a diagnostic impression of non-ST elevation MI. Despite medical management, the patient’s chest pain persisted for several hours from her initial presentation. A repeated 12-lead ECG revealed new borderline (1-1.5 mm) ST segment elevation in V2-V3, suggestive of possible myocardial injury (Figure 2).
A bedside echocardiogram revealed severe wall motion abnor malities, ranging from hypokinesia to dyskinesia of all mid-to-distal left ventricular wall segments with sparing of the basal segment (Figure 3). The estimated left ventricular ejection fraction was 40% to 45%.
In view of these findings, the patient was taken to the catheterization laboratory for emergent coronary angiography, which ruled out significant obstructive coronary disease (Figure 4).
Left ventriculography in right and left anterior oblique projections revealed significant wall motion abnormalities of the mid-to-distal anterolateral and inferior wall segments, sparing the basal and apical segments, giving the appearance of ballooning in systole (Figure 5). The diagnosis of ABS involving the mid ventricular walls was explored.
Subsequent sets of cardiac enzymes at 4 and 8 hours after arrival remained elevated, with a maximum troponin T 0.55 and CK-MB of 11.19. Repeated 12-lead ECG 24 hours post coronary angiography revealed anterolateral T wave inversion (Figure 6).
Noncontrast enhanced cardiac magnetic resonance imaging (MRI) (Figure 7) performed 5 days later revealed wall motion abnormalities highly suggestive of ABS, supporting previous echocardiographic and ventriculography findings. Unfortunately, contrast-enhanced phase for evaluation of delayed enhancement could not be completed, because the patient did not continue the study.
Toxicology tests were negative for sympathomimetic drugs. Metanephrine levels were within the normal range. Viral titers for cytomegalovirus and coxsackie virus also were negative. Inflammatory markers were mildly elevated (erythrocyte sedimentation rate, 22 mm/h; C-reactive protein, 4.2 mg/L).
The patient was treated with supportive care, psychotropic therapy, angiotensin-converting enzyme inhibitor (ACE-I), and beta blocker therapy. Within 9 days, NT-proBNP levels normalized (from peak 8,834 pg/mL to 191.5 pg/mL).
Six weeks later, an echocardiogram confirmed resolution of wall motion abnormalities (Figure 8). Follow-up cardiac MRI showed complete resolution of segmental wall motion abnormalities and the apical ballooning, normal wall thickness, and absent delayed enhancement (Figure 9). These findings further supported the diagnosis of ABS and excluded MI and myocarditis.
Discussion
What is striking about takotsubo cardiomyopathy is that the clinical presentation resembles an AMI. Several studies have reported that 1.7% to 2.2% of patients who had suspected acute coronary syndrome were subsequently diagnosed with takotsubo cardiomyopathy.6-8 Nearly 90% of reported cases involved postmenopausal women, and this may be related to loss of the cardioprotective effect of estrogen.5,9
A preceding stressful emotional or physical event is usually identified in about two-thirds of the patients with ABS.9 Most common emotional triggers are death of a relative or friend, broken relationships, assaults, and rapes, among others. Physical triggers include severe sepsis, shock, acute respiratory failure, seizures, and intracranial bleeds. Sometimes a specific trigger cannot be identified from the history, but the absence of an emotional or physical trigger does not exclude the diagnosis.
Although the exact pathogenesis of ABS remains unclear, it is likely that multiple factors are involved. Some of the suggested mechanisms are high levels of catecholamines, multivessel epicardial spasm, or coronary microvascular dysfunction.4 The catecholamine hypothesis has been supported by the finding that several patients with pheochromocytoma and subarachnoid hemorrhage also present with high levels of catecholamine and a cardiomyopathy resembling ABS. Furthermore, ABS has been reported in patients on catecholamine infusions and those treated with agents that inhibit reuptake of catecholamines.5
The presence of multivessel coronary spasm was suggested by early small studies in Japan, but more recent case series have not validated this hypothesis.5 The microvascular dysfunction hypothesis is supported by the presence of myocardial ischemia, diagnosed by ECG changes and elevated troponins, in the absence of significant coronary disease. However, it remains unclear whether this is a primary mechanism or a manifestation of a primary process.4 Microvascular dysfunction may be more likely related to impairment of myocardial relaxation with extramural coronary compression.
Signs and symptoms of ABS mimic those of AMI, with angina-like chest pain as the main presenting symptom in about 50% of cases.10 Other symptoms include dyspnea and less commonly, syncope or sudden cardiac death. Decompensated left heart failure occurs in 50% of patients, with severe hemodynamic compromise and cardiogenic shock not being uncommon. Other complications that may occur are tachyarrhythmias (atrial or ventricular) and ventricular thromboembolism.4
Common ECG changes in ABS include precordial ST segment elevations, symmetric T wave inversions, and nonspecific T wave changes.4,10 QT interval prolongation may be seen during the first days. Transient pathologic Q waves may be seen at presentation or afterward. These ECG changes tend to revert after weeks or months of presentation.
Elevation of cardiac biomarkers is usually present in laboratory data. Levels peak at 24 hours, and the degree of elevation is usually less than that seen in patients with an AMI.10 Most important, the degree of cardiac biomarker elevation is disproportionately low for the extent of involved coronary territory and left ventricular dysfunction. Other laboratory tests that are frequently altered are the BNP and pro-BNP levels, which are usually elevated due to transient left ventricular dysfunction. C-reactive protein elevates in most patients and indicates the presence of an acute inflammatory response.
Early coronary angiography should be performed in all patients with ABS to rule out the presence of a significant obstructive coronary lesion. Patients with ABS often have luminal irregularities or normal coronary vessels. However, concomitant obstructive coronary lesions may be found, especially in elderly patients.
The hallmark of ABS is a characteristic transient contractility abnormality of the left ventricle causing ballooning of the apex, which can be detected on left ventricular angiography or echocardiography. There are 3 distinct variants of ABS, according to the left ventricular myocardial wall segments involved.10 The classic form of takotsubo is characterized by hypokinesis, dyskinesis, or akinesis of the middle and apical segments of the left ventricle. The basal segment is usually spared and may be hyperdynamic. In the midventricular or apical sparing variant, the wall motion abnormalities are restricted to the midventricular segments, and apical contraction is preserved. This case resembles the atypical variant, because the midventricular segments were affected, whereas apical and basal regions were preserved. A rare variant of takotsubo exists with hypokinesis or akinesis of the base and preserved apical function.
Besides ABS and AMI, an important entity to consider in the differential diagnosis of transient wall motion abnormalities is regional myocarditis. Viral titers are helpful in excluding this condition. Furthermore, prolonged recovery is more commonly seen in myocarditis compared with ABS. Imaging studies are particularly helpful in this scenario.
Cardiac MRI demonstrates the wall motion abnormalities or apical ballooning typical for this condition and can differentiate ABS from myocarditis or MI. It is known that delayed myocardial enhancement is seen with myocardial fibrosis. Typically in ischemic cardiomyopathy, there is wall thinning with associated delayed enhancement that extends from the subendocardium to the epicardium (from 0%-90% of wall thickness) of a particular vascular territory. In myocarditis, the enhancement is usually seen in the involved intramyocardial (mesocardium) region, and the pattern is patchy. In ABS, the delayed enhancement is absent, because there is no fibrosis in the area of regional wall motion abnormalities, and wall thickness is usually normal.9,10
No evidence-based guidelines for treating ABS are currently available. Most patients are initially treated with antiplatelets/anticoagulant therapy, nitrates, and diuretics if the patient presents with heart failure. Patients should be admitted to an intensive care unit for close cardiac monitoring. Once ABS is diagnosed and significant coronary stenosis is excluded, patients should receive standard supportive care and optimal neurohormonal therapy. This should include beta blocker or combined alpha/beta blocker agents, an ACE-I or angiotensin receptor blocker, and diuretics if appropriate. Once left ventricular function (LVF) is recovered, therapy with inhibitors of the renin-angiotensin system may be discontinued, but patients should remain on long-term alpha or beta blocker therapy, because the sympathetic blockade provided by these agents may prevent recurrences of this disease.10
Prognosis is generally favorable, and most patients recover to normal LVF over weeks to months. It is important to assess the LVF 4 to 6 weeks after the patient is discharged to confirm the diagnosis of ABS. Recurrence may occur in up to 9% of cases.10 Long-term mortality is similar compared with the age-matched general population.
Conclusion
Apical ballooning syndrome is a relatively novel cardiomyopathy that has gained important attention among the cardiovascular community, mostly because its clinical presentation mimics that of an acute coronary syndrome. Awareness of this entity will result in a more focused diagnosis and appropriate treatment. Managing both cardiac and emotional components of this disease will have a permanent impact in the reversibility and secondary prevention of this cardiomyopathy.
Acknowledgments
Special thanks to the Radiology Service at the VA Caribbean Healthcare System, in particular Dr. Frances Aulet for interpretation of the cardiac MRI results and assistance with MRI images.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Emotional stress can induce different responses in the body, particularly in the cardiovascular system. Apical ballooning syndrome (ABS), also known as takotsubo cardiomyopathy and broken heart syndrome, is a transient cardiomyopathy that mimics an acute myocardial infarction (AMI). Dote and colleagues first described this transient entity in Japan in the early 1990s.1 A case review series reported that 57.2% of patients were Asian, 40% were white.2 Mean patient age was 67 years, although cases of ABS have occurred in children and young adults.3,4
The term tako-tsubo means “octopus trap,” which is the morphology that the left ventricle resembles during systole in patients with this syndrome.5 The pathophysiology of ABS is thought to be mediated by a catecholamine surge. The presentation of ABS is indistinguishable from an AMI. The majority of patients present with angina-like chest pain, ischemic changes on an electrocardiogram (ECG), pulmonary edema, and elevation of cardiac enzymes. Apical ballooning syndrome is accompanied by reversible left ventricular apical ballooning in the absence of angiographically significant coronary artery disease.
Typically, echocardiographic findings show a left ventricle with preserved function in the basal segments, moderate-to-severe dysfunction in the mid portion of the left ventricle, and hypokinesis, akinesis, or dyskinesis in the apex. A unique but not exclusive feature of this syndrome is the occurrence of a preceding emotional trigger, usually sudden or unexpected. Most patients are initially treated for an AMI until angiography can rule out coronary obstruction. After several weeks, the left ventricular systolic function usually returns to normal.
Case Presentation
A 49-year-old woman with a history of arterial hypertension, fibromyalgia, peptic ulcer disease, and major depressive disorder with multiple admissions to the psychiatric ward (last admission was 4 weeks prior to the current presentation) presented to the emergency department, reporting severe retrosternal, oppressive chest pain with 9/10 intensity and 3 hours’ duration. The pain was associated with nausea, vomiting, diaphoresis, and palpitations. She reported no previous episodes of exertional angina, fever, illicit drug use, recent illness, or travel. She also reported no prodromal symptoms.
Her initial vital signs were essentially unremarkable, except for mild hypertension (148/84 mm Hg). The physical examination showed an anxious patient in acute distress due to chest pain. A cardiovascular examination revealed a regular heart rate and rhythm, no audible murmurs or gallops, no jugular vein distention, clear breath sounds, and no peripheral edema. The rest of the examination was otherwise unremarkable. An initial 12-lead ECG showed a normal sinus rhythm without any ST-T changes (Figure 1).
The initial cardiac markers were elevated (troponin T 0.36 ng/mL, CK-MB 4.51 ng/mL), as were NT-proBNP levels (1,057 pg/mL). The rest of the laboratory results were essentially unremarkable. The patient was started on aspirin, clopidogrel, enoxaparin, eptifibatide, and IV nitrates. She was admitted to the coronary care unit with a diagnostic impression of non-ST elevation MI. Despite medical management, the patient’s chest pain persisted for several hours from her initial presentation. A repeated 12-lead ECG revealed new borderline (1-1.5 mm) ST segment elevation in V2-V3, suggestive of possible myocardial injury (Figure 2).
A bedside echocardiogram revealed severe wall motion abnor malities, ranging from hypokinesia to dyskinesia of all mid-to-distal left ventricular wall segments with sparing of the basal segment (Figure 3). The estimated left ventricular ejection fraction was 40% to 45%.
In view of these findings, the patient was taken to the catheterization laboratory for emergent coronary angiography, which ruled out significant obstructive coronary disease (Figure 4).
Left ventriculography in right and left anterior oblique projections revealed significant wall motion abnormalities of the mid-to-distal anterolateral and inferior wall segments, sparing the basal and apical segments, giving the appearance of ballooning in systole (Figure 5). The diagnosis of ABS involving the mid ventricular walls was explored.
Subsequent sets of cardiac enzymes at 4 and 8 hours after arrival remained elevated, with a maximum troponin T 0.55 and CK-MB of 11.19. Repeated 12-lead ECG 24 hours post coronary angiography revealed anterolateral T wave inversion (Figure 6).
Noncontrast enhanced cardiac magnetic resonance imaging (MRI) (Figure 7) performed 5 days later revealed wall motion abnormalities highly suggestive of ABS, supporting previous echocardiographic and ventriculography findings. Unfortunately, contrast-enhanced phase for evaluation of delayed enhancement could not be completed, because the patient did not continue the study.
Toxicology tests were negative for sympathomimetic drugs. Metanephrine levels were within the normal range. Viral titers for cytomegalovirus and coxsackie virus also were negative. Inflammatory markers were mildly elevated (erythrocyte sedimentation rate, 22 mm/h; C-reactive protein, 4.2 mg/L).
The patient was treated with supportive care, psychotropic therapy, angiotensin-converting enzyme inhibitor (ACE-I), and beta blocker therapy. Within 9 days, NT-proBNP levels normalized (from peak 8,834 pg/mL to 191.5 pg/mL).
Six weeks later, an echocardiogram confirmed resolution of wall motion abnormalities (Figure 8). Follow-up cardiac MRI showed complete resolution of segmental wall motion abnormalities and the apical ballooning, normal wall thickness, and absent delayed enhancement (Figure 9). These findings further supported the diagnosis of ABS and excluded MI and myocarditis.
Discussion
What is striking about takotsubo cardiomyopathy is that the clinical presentation resembles an AMI. Several studies have reported that 1.7% to 2.2% of patients who had suspected acute coronary syndrome were subsequently diagnosed with takotsubo cardiomyopathy.6-8 Nearly 90% of reported cases involved postmenopausal women, and this may be related to loss of the cardioprotective effect of estrogen.5,9
A preceding stressful emotional or physical event is usually identified in about two-thirds of the patients with ABS.9 Most common emotional triggers are death of a relative or friend, broken relationships, assaults, and rapes, among others. Physical triggers include severe sepsis, shock, acute respiratory failure, seizures, and intracranial bleeds. Sometimes a specific trigger cannot be identified from the history, but the absence of an emotional or physical trigger does not exclude the diagnosis.
Although the exact pathogenesis of ABS remains unclear, it is likely that multiple factors are involved. Some of the suggested mechanisms are high levels of catecholamines, multivessel epicardial spasm, or coronary microvascular dysfunction.4 The catecholamine hypothesis has been supported by the finding that several patients with pheochromocytoma and subarachnoid hemorrhage also present with high levels of catecholamine and a cardiomyopathy resembling ABS. Furthermore, ABS has been reported in patients on catecholamine infusions and those treated with agents that inhibit reuptake of catecholamines.5
The presence of multivessel coronary spasm was suggested by early small studies in Japan, but more recent case series have not validated this hypothesis.5 The microvascular dysfunction hypothesis is supported by the presence of myocardial ischemia, diagnosed by ECG changes and elevated troponins, in the absence of significant coronary disease. However, it remains unclear whether this is a primary mechanism or a manifestation of a primary process.4 Microvascular dysfunction may be more likely related to impairment of myocardial relaxation with extramural coronary compression.
Signs and symptoms of ABS mimic those of AMI, with angina-like chest pain as the main presenting symptom in about 50% of cases.10 Other symptoms include dyspnea and less commonly, syncope or sudden cardiac death. Decompensated left heart failure occurs in 50% of patients, with severe hemodynamic compromise and cardiogenic shock not being uncommon. Other complications that may occur are tachyarrhythmias (atrial or ventricular) and ventricular thromboembolism.4
Common ECG changes in ABS include precordial ST segment elevations, symmetric T wave inversions, and nonspecific T wave changes.4,10 QT interval prolongation may be seen during the first days. Transient pathologic Q waves may be seen at presentation or afterward. These ECG changes tend to revert after weeks or months of presentation.
Elevation of cardiac biomarkers is usually present in laboratory data. Levels peak at 24 hours, and the degree of elevation is usually less than that seen in patients with an AMI.10 Most important, the degree of cardiac biomarker elevation is disproportionately low for the extent of involved coronary territory and left ventricular dysfunction. Other laboratory tests that are frequently altered are the BNP and pro-BNP levels, which are usually elevated due to transient left ventricular dysfunction. C-reactive protein elevates in most patients and indicates the presence of an acute inflammatory response.
Early coronary angiography should be performed in all patients with ABS to rule out the presence of a significant obstructive coronary lesion. Patients with ABS often have luminal irregularities or normal coronary vessels. However, concomitant obstructive coronary lesions may be found, especially in elderly patients.
The hallmark of ABS is a characteristic transient contractility abnormality of the left ventricle causing ballooning of the apex, which can be detected on left ventricular angiography or echocardiography. There are 3 distinct variants of ABS, according to the left ventricular myocardial wall segments involved.10 The classic form of takotsubo is characterized by hypokinesis, dyskinesis, or akinesis of the middle and apical segments of the left ventricle. The basal segment is usually spared and may be hyperdynamic. In the midventricular or apical sparing variant, the wall motion abnormalities are restricted to the midventricular segments, and apical contraction is preserved. This case resembles the atypical variant, because the midventricular segments were affected, whereas apical and basal regions were preserved. A rare variant of takotsubo exists with hypokinesis or akinesis of the base and preserved apical function.
Besides ABS and AMI, an important entity to consider in the differential diagnosis of transient wall motion abnormalities is regional myocarditis. Viral titers are helpful in excluding this condition. Furthermore, prolonged recovery is more commonly seen in myocarditis compared with ABS. Imaging studies are particularly helpful in this scenario.
Cardiac MRI demonstrates the wall motion abnormalities or apical ballooning typical for this condition and can differentiate ABS from myocarditis or MI. It is known that delayed myocardial enhancement is seen with myocardial fibrosis. Typically in ischemic cardiomyopathy, there is wall thinning with associated delayed enhancement that extends from the subendocardium to the epicardium (from 0%-90% of wall thickness) of a particular vascular territory. In myocarditis, the enhancement is usually seen in the involved intramyocardial (mesocardium) region, and the pattern is patchy. In ABS, the delayed enhancement is absent, because there is no fibrosis in the area of regional wall motion abnormalities, and wall thickness is usually normal.9,10
No evidence-based guidelines for treating ABS are currently available. Most patients are initially treated with antiplatelets/anticoagulant therapy, nitrates, and diuretics if the patient presents with heart failure. Patients should be admitted to an intensive care unit for close cardiac monitoring. Once ABS is diagnosed and significant coronary stenosis is excluded, patients should receive standard supportive care and optimal neurohormonal therapy. This should include beta blocker or combined alpha/beta blocker agents, an ACE-I or angiotensin receptor blocker, and diuretics if appropriate. Once left ventricular function (LVF) is recovered, therapy with inhibitors of the renin-angiotensin system may be discontinued, but patients should remain on long-term alpha or beta blocker therapy, because the sympathetic blockade provided by these agents may prevent recurrences of this disease.10
Prognosis is generally favorable, and most patients recover to normal LVF over weeks to months. It is important to assess the LVF 4 to 6 weeks after the patient is discharged to confirm the diagnosis of ABS. Recurrence may occur in up to 9% of cases.10 Long-term mortality is similar compared with the age-matched general population.
Conclusion
Apical ballooning syndrome is a relatively novel cardiomyopathy that has gained important attention among the cardiovascular community, mostly because its clinical presentation mimics that of an acute coronary syndrome. Awareness of this entity will result in a more focused diagnosis and appropriate treatment. Managing both cardiac and emotional components of this disease will have a permanent impact in the reversibility and secondary prevention of this cardiomyopathy.
Acknowledgments
Special thanks to the Radiology Service at the VA Caribbean Healthcare System, in particular Dr. Frances Aulet for interpretation of the cardiac MRI results and assistance with MRI images.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Dote K, Sato H, Tateishi H, Uchida T, Ishihara M. Myocardial stunning due to simultaneous multivessel coronary spasms: A review of 5 cases [in Japanese]. J Cardiol. 1991;21(2):203-214.
2. Donohue D, Movahed MR. Clinical characteristics, demographics and prognosis of transient left ventricular apical ballooning syndrome. Heart Fail Rev. 2005;9(4):311-316.
3. Afonso L, Bachour K, Awad K, Sandidge G. Takotsubo cardiomyopathy: Pathogenetic insights and myocardial perfusion kinetics using myocardial contrast echocardiography. Eur J Echocardiogr. 2008;9(6):849-854.
4. Buchholz S, Rudan G. Tako-tsubo syndrome on the rise: A review of the current literature. Postgrad Med J. 2007;83(978):261-264.
5. Hare J. The dilated, restrictive and infiltrative cardiomyopathies. Braunwald’s Heart Disease, A Textbook of Cardiovascular Medicine. 9th ed. Philadelphia, PA: Saunders; 2012:1562-1580.
6. Bybee KA, Prasad A, Barsness GW, et al. Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular apical ballooning syndrome. Am J Cardiol. 2004;94(3):343-346.
7. Ito K, Sugihara H, Katoh S, Azuma A, Nakagawa M. Assessment of Takotsubo (ampulla) cardiomyopathy using 99mTc-tetrofosmin myocardial SPECT—Comparison with acute coronary syndrome. Ann Nucl Med. 2003;17(2):115-122.
8. Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): A mimic of acute myocardial infarction. Am Heart J. 2008;155(3):408-417.
9. Lange R, Hills D. Chemical cardiomyopathies. Braunwald’s Heart Disease, A Textbook of Cardiovascular Medicine. 10th ed. Philadelphia, PA: Saunders; 2014:1609-1611.
10. Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E. Apical ballooning syndrome or takotsubo cardiomyopathy: A systematic review. Eur Heart J. 2006;27(13):1523-1529.
1. Dote K, Sato H, Tateishi H, Uchida T, Ishihara M. Myocardial stunning due to simultaneous multivessel coronary spasms: A review of 5 cases [in Japanese]. J Cardiol. 1991;21(2):203-214.
2. Donohue D, Movahed MR. Clinical characteristics, demographics and prognosis of transient left ventricular apical ballooning syndrome. Heart Fail Rev. 2005;9(4):311-316.
3. Afonso L, Bachour K, Awad K, Sandidge G. Takotsubo cardiomyopathy: Pathogenetic insights and myocardial perfusion kinetics using myocardial contrast echocardiography. Eur J Echocardiogr. 2008;9(6):849-854.
4. Buchholz S, Rudan G. Tako-tsubo syndrome on the rise: A review of the current literature. Postgrad Med J. 2007;83(978):261-264.
5. Hare J. The dilated, restrictive and infiltrative cardiomyopathies. Braunwald’s Heart Disease, A Textbook of Cardiovascular Medicine. 9th ed. Philadelphia, PA: Saunders; 2012:1562-1580.
6. Bybee KA, Prasad A, Barsness GW, et al. Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular apical ballooning syndrome. Am J Cardiol. 2004;94(3):343-346.
7. Ito K, Sugihara H, Katoh S, Azuma A, Nakagawa M. Assessment of Takotsubo (ampulla) cardiomyopathy using 99mTc-tetrofosmin myocardial SPECT—Comparison with acute coronary syndrome. Ann Nucl Med. 2003;17(2):115-122.
8. Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): A mimic of acute myocardial infarction. Am Heart J. 2008;155(3):408-417.
9. Lange R, Hills D. Chemical cardiomyopathies. Braunwald’s Heart Disease, A Textbook of Cardiovascular Medicine. 10th ed. Philadelphia, PA: Saunders; 2014:1609-1611.
10. Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E. Apical ballooning syndrome or takotsubo cardiomyopathy: A systematic review. Eur Heart J. 2006;27(13):1523-1529.