User login
ACE inhibitors and ARBs: Managing potassium and renal function
A highly active, water- and alcohol-soluble, basic pressor substance is formed when renin and renin-activator interact, for which we suggest the name “angiotonin.”
—Irvine H. Page and O.M. Helmer, 1940.1
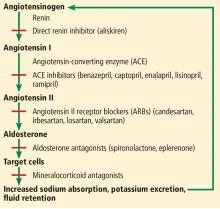
The renin-angiotensin-aldosterone system regulates salt and, in part, water homeostasis, and therefore blood pressure and fluid balance through its actions on the heart, kidneys, and blood vessels.2 Drugs that target this system—angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs)—are used primarily to treat hypertension and also to treat chronic kidney disease and heart failure with reduced ejection fraction.
Controlling blood pressure is important, as hypertension increases the risk of myocardial infarction, cerebrovascular events, and progression of chronic kidney disease, which itself is a risk factor for cardiovascular disease. However, the benefit of these drugs is only partly due to their effect on blood pressure. They also reduce proteinuria, which is a graded risk factor for progression of kidney disease as well as morbidity and death from vascular events.3
Despite the benefits of ACE inhibitors and ARBs, concern about their adverse effects—especially hyperkalemia and a decline in renal function—has led to their underuse in patients likely to derive the greatest benefit.3
ACE INHIBITORS AND ARBs
ACE inhibitors, as their name indicates, inhibit conversion of angiotensin I to angiotensin II by ACE, resulting in vasodilation of the efferent arteriole and a drop in blood pressure. Inhibition of ACE, a kininase, also results in a rise in kinins. One of these, bradykinin, is associated with some of the side effects of this class of drugs such as cough, which affects 5% to 20% of patients.4 Elevation of bradykinin is also believed to account for ACE inhibitor-induced angioedema, an uncommon but potentially serious side effect. Kinins are also associated with desirable effects such as lowering blood pressure, increasing insulin sensitivity, and dilating blood vessels.
ARBs were developed as an alternative for patients unable to tolerate the adverse effects of ACE inhibitors. While ACE inhibitors reduce the activity of angiotensin II at both the AT1 and AT2 receptors, ARBs block only the AT1 receptors, thereby inhibiting their vasoconstricting activity on smooth muscle. ARBs also raise the levels of renin, angiotensin I, and angiotensin II as a result of feedback inhibition. Angiotensin II is associated with release of inflammatory mediators such as tumor necrosis factor alpha, cytokines, and chemokines, the consequences of which are also inhibited by ARBs, further preventing renal fibrosis and scarring from chronic inflammation.3
What is the evidence supporting the use of ACE inhibitors and ARBs?
ACE inhibitors and ARBs, used singly, reduce blood pressure and proteinuria, slow progression of kidney disease, and improve outcomes in patients who have heart failure, diabetes mellitus, or a history of myocardial infarction.5–11
While dual blockade with the combination of an ACE inhibitor and an ARB lowers blood pressure and proteinuria to a greater degree than monotherapy, dual blockade has been associated with higher rates of complications, including hyperkalemia.12–17
RISK FACTORS FOR HYPERKALEMIA
ACE inhibitors and ARBs raise potassium, especially when used in combination. Other risk factors for hyperkalemia include the following—and note that some of them are also indications for ACE inhibitors and ARBs:
Renal insufficiency. The kidneys are responsible for over 90% of potassium removal in healthy individuals,18,19 and the lower the GFR, the higher the risk of hyperkalemia.3,20,21
Heart failure
Diabetes mellitus6,21–23
Endogenous potassium load due to hemolysis, rhabdomyolysis, insulin deficiency, lactic acidosis, or gastrointestinal bleeding
Exogenous potassium load due to dietary consumption or blood products
Other medications, eg, sacubitril-valsartan, aldosterone antagonists, mineralocorticoid receptor antagonists, potassium-sparing diuretics, beta-adrenergic antagonists, nonsteroidal anti-inflammatory drugs, heparin, cyclosporine, trimethoprim, digoxin
Hypertension
Hypoaldosteronism (including type 4 renal tubular acidosis)
Addison disease
Advanced age
Lower body mass index.
Both hypokalemia and hyperkalemia are associated with a higher risk of death,20,21,24 but in patients with heart failure, the survival benefit from ACE inhibitors, ARBs, and mineralocorticoid receptor antagonists outweighs the risk of hyperkalemia.25–27 Weir and Rolfe28 concluded that patients with heart failure and chronic kidney disease are at greatest risk of hyperkalemia from renin-angiotensin-aldosterone system inhibition, but the increases in potassium levels are small (about 0.1 to 0.3 mmol/L) and unlikely to be clinically significant.
Hyperkalemia tends to recur. Einhorn et al20 found that nearly half of patients with chronic kidney disease who had an episode of hyperkalemia had 1 or more recurrent episodes within a year.
ACE INHIBITORS, ARBs, ABD RENAL FUNCTION
Another concern about using ACE inhibitors and ARBs, especially in patients with chronic kidney disease, is that the serum creatinine level tends to rise when starting these drugs,29 although several studies have shown that an acute rise in creatinine may demonstrate that the drug is actually protecting the kidney.30,31 Hirsch32 described this phenomenon as “prerenal success,” proposing that the decline in GFR is hemodynamic, secondary to a fall in intraglomerular pressure as a result of efferent vasodilation, and therefore should not be reversed.
Schmidt et al,33,34 in a study in 122,363 patients who began ACE inhibitor or ARB therapy, found that cardiorenal outcomes were worse, with higher rates of end-stage renal disease, myocardial infarction, heart failure, and death, in those in whom creatinine rose by 30% or more since starting treatment. This trend was also seen, to a lesser degree, in those with a smaller increase in creatinine, suggesting that even this group of patients should receive close monitoring.
Whether renin-angiotensin-aldosterone system inhibitors provide a benefit in advanced progressive chronic kidney disease remains unclear.35–37 The Angiotensin Converting Enzyme Inhibitor (ACEi)/Angiotensin Receptor Blocker (ARB) Withdrawal in Advanced Renal Disease trial (STOP-ACEi),38 currently under way, will provide valuable data to help close this gap in our knowledge. This open-label randomized controlled trial is testing the hypothesis that stopping ACE inhibitor or ARB treatment, or a combination of both, compared with continuing these treatments, will improve or stabilize renal function in patients with progressive stage 4 or 5 chronic kidney disease.
NEED FOR MONITORING
Taken together, the above data suggest close and regular monitoring is required in patients receiving these drugs. However, monitoring tends to be lax.34,37,39 A 2017 study of adherence to the guidelines for monitoring serum creatinine and potassium after starting an ACE inhibitor or ARB and subsequent discontinuation found that fewer than 10% of patients had follow-up within the recommended 2 weeks after starting these drugs.34 Most patients with a creatinine rise of 30% or more or a potassium level higher than 6.0 mmol/L continued treatment. There was also no evidence of increased monitoring in those deemed at higher risk of these complications.
WHAT DO THE GUIDELINES SUGGEST?
ACE inhibitors and ARBs in chronic kidney disease and hypertension
Target blood pressures vary in guidelines from different organizations.4,40–45 The 2017 joint guidelines of the American College of Cardiology and American Heart Association (ACC/AHA)40 recommend a target blood pressure of 130/80 mm Hg or less in all patients irrespective of the level of proteinuria and whether they have diabetes mellitus, based on several studies.46–48 In the elderly, other factors such as the risk of hypotension and falls must be taken into consideration in establishing the most appropriate blood pressure target.
In general, a renin-angiotensin-aldosterone system inhibitor is recommended if the patient has diabetes, stage 1, 2, or 3 chronic kidney disease, or proteinuria. For example, the guidelines recommend a renin-angiotensin-aldosterone system inhibitor in diabetic patients with albuminuria.
None of the guidelines recommend routine use of combination therapy.
ACE inhibitors and ARBs in heart failure
The 2017 ACC/AHA and Heart Failure Society of America (HFSA) guidelines for heart failure49 recommend an ACE inhibitor or ARB for patients with stage C (symptomatic) heart failure with reduced ejection fraction, in view of the known cardiovascular morbidity and mortality benefits.
The European Society of Cardiology50 recommends ACE inhibitors for patients with symptomatic heart failure with reduced ejection fraction, as well as those with asymptomatic left ventricular systolic dysfunction. In patients with stable coronary artery disease, an ACE inhibitor should be considered even with normal left ventricular function.
ARBs should be used as alternatives in those unable to tolerate ACE inhibitors.
Combination therapy should be avoided due to the increased risk of renal impairment and hyperkalemia but may be considered in patients with heart failure and reduced ejection fraction in whom other treatments are unsuitable. These include patients on beta-blockers who cannot tolerate mineralocorticoid receptor antagonists such as spironolactone. Combination therapy should be done only under strict supervision.50
Starting ACE or ARB therapy
Close monitoring of serum potassium is recommended during ACE inhibitor or ARB use. Those at greatest risk of hyperkalemia include elderly patients, those taking other medications associated with hyperkalemia, and diabetic patients, because of their higher risk of renovascular disease.
Caution is advised when starting ACE inhibitor or ARB therapy in these high-risk groups as well as in patients with potassium levels higher than 5.0 mmol/L at baseline, at high risk of prerenal acute kidney injury, with known renal insufficiency, and with previous deterioration in renal function on these medications.3,41,51
Before starting therapy, ensure that patients are volume-replete and measure baseline serum electrolytes and creatinine.41,51
The ACC/AHA and HFSA recommend starting at a low dose and titrating upward slowly. If maximal doses are not tolerated, then a lower dose should be maintained.49 The European Society of Cardiology guidelines52 suggest increasing the dose at no less than every 2 weeks unless in an inpatient setting. Blood testing should be done 7 to 14 days after starting therapy, after any titration in dosage, and every 4 months thereafter.53
The guidelines generally agree that a rise in creatinine of up to 30% and a fall in eGFR of up to 25% is acceptable, with the need for regular monitoring, particularly in high-risk groups.40–42,51,52
What if serum potassium or creatinine rises during treatment?
If hyperkalemia arises or renal function declines by a significant amount, one should first address contributing factors. If no improvement is seen, then the dose of the ACE inhibitor or ARB should be reduced by 50% and blood work repeated in 1 to 2 weeks. If the laboratory values do not return to an acceptable level, reducing the dose further or stopping the drug is advised.
Give dietary advice to all patients with chronic kidney disease being considered for a renin-angiotensin-aldosterone system inhibitor or for an increase in dose with a potassium level higher than 4.5 mmol/L. A low-potassium diet should aim for potassium intake of less than 50 or 75 mmol/day and sodium intake of less than 60 mmol/day for hypertensive patients with chronic kidney disease.
Review the patient’s medications if the baseline potassium level is higher than 5.0 mmol/L. Consider stopping potassium-sparing agents, digoxin, trimethoprim, and nonsteroidal anti-inflammatory drugs. Also think about starting a non–potassium-sparing diuretic as well as sodium bicarbonate to reduce potassium levels. Blood work should be repeated within 2 weeks after these changes.
Do not start a renin-angiotensin-aldosterone system inhibitor, or do not increase the dose, if the potassium level is elevated until measures have been taken to reduce the degree of hyperkalemia.51
In renal transplant recipients, renin-angiotensin-aldosterone system inhibitors are often preferred to manage hypertension in those who have proteinuria or cardiovascular disease. However, the risk of hyperkalemia is also greater with concomitant use of immunosuppressive drugs such as tacrolimus and cyclosporine. Management of complications should be approached according to guidelines discussed above.51
Monitor renal function, potassium. The National Institute for Health and Care Excellence guideline54 advocates that baseline renal function testing should be followed by repeat blood testing 1 to 2 weeks after starting renin-angiotensin-aldosterone system inhibitors in patients with ischemic heart disease. The advice is similar when starting therapy in patients with chronic heart failure, emphasizing the need to monitor after each dose increment and to use clinical judgment when deciding to start treatment. The AHA advises caution in patients with renal insufficiency or a potassium level above 5.0 mmol/L.49
Sick day rules. The National Institute for Health and Care Excellence encourages discussing “sick day rules” with patients starting renin-angiotensin-aldosterone system inhibitors. This means patients should be advised to temporarily stop taking nephrotoxic medications, including over-the-counter nonsteroidal anti-inflammatory drugs, in any potential state of illness or dehydration, such as diarrhea and vomiting. There is, however, little evidence that this advice can actually reduce the incidence of acute kidney injury.55,56
OUR RECOMMENDATIONS
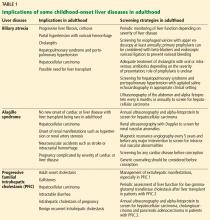
Our advice for managing patients receiving ACE inhibitors or ARBs is summarized in Table 1.
- Page IH, Helmer OM. A crystalline pressor substance (angiotonin) resulting from the reaction between renin and renin-activator. Exp Med 1940; 71(1):29–42. doi:10.1084/jem.71.1.29
- Steddon S, Ashman N, Chesser A, Cunningham J. Oxford Handbook of Nephrology and Hypertension. 2nd ed. Oxford: Oxford University Press; 2016:203–206, 508–509.
- Barratt J, Topham P, Harris K. Oxford Desk Reference. 1st ed. Oxford: Oxford University Press; 2008.
- International Kidney Foundation. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. http://www.kdigo.org/clinical_practice_guidelines/pdf/KDIGO_BP_GL.pdf. Accessed April 3, 2019.
- Heart Outcomes Prevention Evaluation Study Investigators; Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med 2000; 342(3):145–153. doi:10.1056/NEJM200001203420301
- Swedberg K, Kjekshus J. Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). Am J Cardiol 1988; 62(2):60A–66A. pmid:2839019
- Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345(12):861–869. doi:10.1056/NEJMoa011161
- Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003; 349(20):1893–1906. doi:10.1056/NEJMoa032292
- Epstein M. Reduction of cardiovascular risk in chronic kidney disease by mineralocorticoid receptor antagonism. Lancet Diabetes Endocrinol 2015; 3(12):993–1003. doi:10.1016/S2213-8587(15)00289-2
- SOLVD Investigators; Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325(5):293–302. doi:10.1056/NEJM199108013250501
- Jafar TH, Stark PC, Schmid CH, et al; AIPRD Study Group; Angiotensin-Converting Enzymne Inhibition and Progression of Renal Disease. Proteinuria as a modifiable risk factor for the progression of non-diabetic renal disease. Kidney Int 2001; 60(3):1131–1140. doi:10.1046/j.1523-1755.2001.0600031131.x
- Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet 2015; 385(9982):2047–2056. doi:10.1016/S0140-6736(14)62459-4
- Ruggenenti P, Perticucci E, Cravedi P, et al. Role of remission clinics in the longitudinal treatment of CKD. J Am Soc Nephrol 2008; 19(6):1213–1224. doi:10.1681/ASN.2007090970
- Makani H, Bangalore S, Desouza KA, Shah A, Messerli FH. Efficacy and safety of dual blockade of the renin-angiotensin system: meta-analysis of randomised trials. BMJ 2013; 346:f360. doi:10.1136/bmj.f360
- ONTARGET Investigators; Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358(15):1547–1559. doi:10.1056/NEJMoa0801317
- Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013; 369(20):1892–1903.
doi:10.1056/NEJMoa1303154 - Catalá-López F, Macías Saint-Gerons D, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med 2016; 13(3):e1001971. doi:10.1371/journal.pmed.1001971
- Agarwal R, Afzalpurkar R, Fordtran JS. Pathophysiology of potassium absorption and secretion by the human intestine. Gastroenterology 1994; 107(2):548–571. pmid:8039632
- Palmer BF. Regulation of potassium homeostasis. Clin J Am Soc Nephrol 2015; 10(6):1050–1060. doi:10.2215/CJN.08580813
- Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med 2009; 169(12):1156–1162. doi:10.1001/archinternmed.2009.132
- Nakhoul GN, Huang H, Arrigain S, et al. Serum potassium, end-stage renal disease and mortality in chronic kidney disease. Am J Nephrol 2015; 41(6):456–463. doi:10.1159/000437151
- Acker CG, Johnson JP, Palevsky PM, Greenberg A. Hyperkalemia in hospitalized patients: causes, adequacy of treatment, and results of an attempt to improve physician compliance with published therapy guidelines. Arch Intern Med 1998; 158(8):917–924. pmid:9570179
- Desai AS, Swedberg K, McMurray JJ, et al; CHARM Program Investigators. Incidence and predictors of hyperkalemia in patients with heart failure: an analysis of the CHARM Program. J Am Coll Cardiol 2007; 50(20):1959–1966. doi:10.1016/j.jacc.2007.07.067
- Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, Sakhuja A, Mao MA, Erickson SB. Impact of admission serum potassium on mortality in patients with chronic kidney disease and cardiovascular disease. QJM 2017; 110(11):713–719. doi:10.1093/qjmed/hcx118
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364(1):11–21. doi:10.1056/NEJMoa1009492
- Rossignol P, Dobre D, McMurray JJ, et al. Incidence, determinants, and prognostic significance of hyperkalemia and worsening renal function in patients with heart failure receiving the mineralocorticoid receptor antagonist eplerenone or placebo in addition to optimal medical therapy: results from the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF). Circ Heart Fail 2014; 7(1):51–58. doi:10.1161/CIRCHEARTFAILURE.113.000792
- Testani JM, Kimmel SE, Dries DL, Coca SG. Prognostic importance of early worsening renal function after initiation of angiotensin-converting enzyme inhibitor therapy in patients with cardiac dysfunction. Circ Heart Fail 2011; 4(6):685–691. doi:10.1161/CIRCHEARTFAILURE.111.963256
- Weir M, Rolfe M. Potassium homeostasis and renin-angiotensin-aldosterone system inhibitors. Clin J Am Soc Nephrol 2010; 5(3):531–548. doi:10.2215/CJN.07821109
- Valente M, Bhandari S. Renal function after new treatment with renin-angiotensin system blockers. BMJ 2017; 356:j1122. doi:10.1136/bmj.j1122
- Bakris G, Weir M. Angiotensin-converting enzyme inhibitor–associated elevations in serum creatinine. Arch Intern Med 2000; 160(5):685–693. pmid:10724055
- Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345(12):861–869. doi:10.1056/NEJMoa011161
- Hirsch S. Pre-renal success. Kidney Int 2012; 81(6):596. doi:10.1038/ki.2011.418
- Schmidt M, Mansfield KE, Bhaskaran K, et al. Serum creatinine elevation after renin-angiotensin system blockade and long term cardiorenal risks: cohort study. BMJ 2017; 356:j791. doi:10.1136/bmj.j791
- Schmidt M, Mansfield KE, Bhaskaran K, et al. Adherence to guidelines for creatinine and potassium monitoring and discontinuation following renin–angiotensin system blockade: a UK general practice-based cohort study. BMJ Open 2017; 7(1):e012818. doi:10.1136/bmjopen-2016-012818
- Lund LH, Carrero JJ, Farahmand B, et al. Association between enrollment in a heart failure quality registry and subsequent mortality—a nationwide cohort study. Eur J Heart Fail 2017; 19(9):1107–1116. doi:10.1002/ejhf.762
- Edner M, Benson L, Dahlstrom U, Lund LH. Association between renin-angiotensin system antagonist use and mortality in heart failure with severe renal insuffuciency: a prospective propensity score-matched cohort study. Eur Heart J 2015; 36(34):2318–2326. doi:10.1093/eurheartj/ehv268
- Epstein M, Reaven NL, Funk SE, McGaughey KJ, Oestreicher N, Knispel J. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care 2015; 21(suppl 11):S212–S220. pmid:26619183
- Bhandari S, Ives N, Brettell EA, et al. Multicentre randomized controlled trial of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker withdrawal in advanced renal disease: the STOP-ACEi trial. Nephrol Dial Transplant 2016; 31(2):255–261. doi:10.1093/ndt/gfv346
- Raebel MA, Ross C, Xu S, et al. Diabetes and drug-associated hyperkalemia: effect of potassium monitoring. J Gen Intern Med 2010; 25(4):326–333. doi:10.1007/s11606-009-1228-x
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018; 71(6):e13–e115. doi:10.1161/HYP.0000000000000065
- The Renal Association. The UK eCKD Guide. https://renal.org/information-resources/the-uk-eckd-guide. Accessed August 12, 2019.
- National Institute for Health and Care Excellence (NICE). Chronic kidney disease in adults: assessment and management. https://www.nice.org.uk/guidance/cg182. Accessed August 12, 2019.
- National Institute for Health and Care Excellence (NICE). Hypertension in adults: diagnosis and management. https://www.nice.org.uk/Guidance/CG127. Accessed August 12, 2019.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34(28):2159–2219. doi:10.1093/eurheartj/eht151
- International Kidney Foundation. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. https://www.sciencedirect.com/journal/kidney-international-supplements/vol/3/issue/1. Accessed August 12, 2019.
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373(22):2103–2116. doi:10.1056/NEJMoa1511939
- Wright J, Bakris G, Greene T. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease. Results from the AASK trial. ACC Current Journal Review 2003; 12(2):37–38. doi:10.1016/s1062-1458(03)00035-7
- Ku E, Bakris G, Johansen K, et al. Acute declines in renal function during intensive BP lowering: implications for future ESRD risk. J Am Soc Nephrol 2017; 28(9):2794–2801. doi:10.1681/ASN.2017010040
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017; 136(6):e137–e161. doi:10.1161/CIR.0000000000000509
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37(27):2129–2200. doi:10.1093/eurheartj/ehw128
- Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 2004; 43(suppl 51):S1–S290. pmid:15114537
- Asenjo RM, Bueno H, Mcintosh M. Angiotensin converting enzyme inhibitors (ACE inhibitors) and angiotensin II receptor blockers (ARBs). ACE inhibitors and ARBs, a cornerstone in the prevention and treatment of cardiovascular disease. www.escardio.org/Education/ESC-Prevention-of-CVD-Programme/Treatment-goals/Cardio-Protective-drugs/angiotensin-converting-enzyme-inhibitors-ace-inhibitors-and-angiotensin-ii-rec. Accessed August 12, 2019.
- López-Sendón J, Swedberg K, McMurray J, et al; Task Force on ACE-inhibitors of the European Society of Cardiology. Expert consensus document on angiotensin converting enzyme inhibitors in cardiovascular disease. The Task Force on ACE-inhibitors of the European Society of Cardiology. Eur Heart J 2004; 25(16):1454–1470. doi:10.1016/j.ehj.2004.06.003
- National Institute for Health and Care Excellence (NICE). Myocardial infarction: cardiac rehabilitation and prevention of further cardiovascular disease. https://www.nice.org.uk/Guidance/CG172. Accessed April 3, 2019.
- National Institute for Health and Care Excellence (NICE). Acute kidney injury: prevention, detection and management. https://www.nice.org.uk/Guidance/CG169. Accessed August 12, 2019.
- Think Kidneys. “Sick day” guidance in patients at risk of acute kidney injury: a position statement from the Think Kidneys Board. https://www.thinkkidneys.nhs.uk/aki/wp-content/uploads/sites/2/2018/01/Think-Kidneys-Sick-Day-Guidance-2018.pdf. Accessed August 12, 2019.
- Meaney CJ, Beccari MV, Yang Y, Zhao J. Systematic review and meta-analysis of patiromer and sodium zirconium cyclosilicate: a new armamentarium for the treatment of hyperkalemia. Pharmacotherapy 2017; 37(4):401–411. doi:10.1002/phar.1906
A highly active, water- and alcohol-soluble, basic pressor substance is formed when renin and renin-activator interact, for which we suggest the name “angiotonin.”
—Irvine H. Page and O.M. Helmer, 1940.1
The renin-angiotensin-aldosterone system regulates salt and, in part, water homeostasis, and therefore blood pressure and fluid balance through its actions on the heart, kidneys, and blood vessels.2 Drugs that target this system—angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs)—are used primarily to treat hypertension and also to treat chronic kidney disease and heart failure with reduced ejection fraction.
Controlling blood pressure is important, as hypertension increases the risk of myocardial infarction, cerebrovascular events, and progression of chronic kidney disease, which itself is a risk factor for cardiovascular disease. However, the benefit of these drugs is only partly due to their effect on blood pressure. They also reduce proteinuria, which is a graded risk factor for progression of kidney disease as well as morbidity and death from vascular events.3
Despite the benefits of ACE inhibitors and ARBs, concern about their adverse effects—especially hyperkalemia and a decline in renal function—has led to their underuse in patients likely to derive the greatest benefit.3
ACE INHIBITORS AND ARBs
ACE inhibitors, as their name indicates, inhibit conversion of angiotensin I to angiotensin II by ACE, resulting in vasodilation of the efferent arteriole and a drop in blood pressure. Inhibition of ACE, a kininase, also results in a rise in kinins. One of these, bradykinin, is associated with some of the side effects of this class of drugs such as cough, which affects 5% to 20% of patients.4 Elevation of bradykinin is also believed to account for ACE inhibitor-induced angioedema, an uncommon but potentially serious side effect. Kinins are also associated with desirable effects such as lowering blood pressure, increasing insulin sensitivity, and dilating blood vessels.
ARBs were developed as an alternative for patients unable to tolerate the adverse effects of ACE inhibitors. While ACE inhibitors reduce the activity of angiotensin II at both the AT1 and AT2 receptors, ARBs block only the AT1 receptors, thereby inhibiting their vasoconstricting activity on smooth muscle. ARBs also raise the levels of renin, angiotensin I, and angiotensin II as a result of feedback inhibition. Angiotensin II is associated with release of inflammatory mediators such as tumor necrosis factor alpha, cytokines, and chemokines, the consequences of which are also inhibited by ARBs, further preventing renal fibrosis and scarring from chronic inflammation.3
What is the evidence supporting the use of ACE inhibitors and ARBs?
ACE inhibitors and ARBs, used singly, reduce blood pressure and proteinuria, slow progression of kidney disease, and improve outcomes in patients who have heart failure, diabetes mellitus, or a history of myocardial infarction.5–11
While dual blockade with the combination of an ACE inhibitor and an ARB lowers blood pressure and proteinuria to a greater degree than monotherapy, dual blockade has been associated with higher rates of complications, including hyperkalemia.12–17
RISK FACTORS FOR HYPERKALEMIA
ACE inhibitors and ARBs raise potassium, especially when used in combination. Other risk factors for hyperkalemia include the following—and note that some of them are also indications for ACE inhibitors and ARBs:
Renal insufficiency. The kidneys are responsible for over 90% of potassium removal in healthy individuals,18,19 and the lower the GFR, the higher the risk of hyperkalemia.3,20,21
Heart failure
Diabetes mellitus6,21–23
Endogenous potassium load due to hemolysis, rhabdomyolysis, insulin deficiency, lactic acidosis, or gastrointestinal bleeding
Exogenous potassium load due to dietary consumption or blood products
Other medications, eg, sacubitril-valsartan, aldosterone antagonists, mineralocorticoid receptor antagonists, potassium-sparing diuretics, beta-adrenergic antagonists, nonsteroidal anti-inflammatory drugs, heparin, cyclosporine, trimethoprim, digoxin
Hypertension
Hypoaldosteronism (including type 4 renal tubular acidosis)
Addison disease
Advanced age
Lower body mass index.
Both hypokalemia and hyperkalemia are associated with a higher risk of death,20,21,24 but in patients with heart failure, the survival benefit from ACE inhibitors, ARBs, and mineralocorticoid receptor antagonists outweighs the risk of hyperkalemia.25–27 Weir and Rolfe28 concluded that patients with heart failure and chronic kidney disease are at greatest risk of hyperkalemia from renin-angiotensin-aldosterone system inhibition, but the increases in potassium levels are small (about 0.1 to 0.3 mmol/L) and unlikely to be clinically significant.
Hyperkalemia tends to recur. Einhorn et al20 found that nearly half of patients with chronic kidney disease who had an episode of hyperkalemia had 1 or more recurrent episodes within a year.
ACE INHIBITORS, ARBs, ABD RENAL FUNCTION
Another concern about using ACE inhibitors and ARBs, especially in patients with chronic kidney disease, is that the serum creatinine level tends to rise when starting these drugs,29 although several studies have shown that an acute rise in creatinine may demonstrate that the drug is actually protecting the kidney.30,31 Hirsch32 described this phenomenon as “prerenal success,” proposing that the decline in GFR is hemodynamic, secondary to a fall in intraglomerular pressure as a result of efferent vasodilation, and therefore should not be reversed.
Schmidt et al,33,34 in a study in 122,363 patients who began ACE inhibitor or ARB therapy, found that cardiorenal outcomes were worse, with higher rates of end-stage renal disease, myocardial infarction, heart failure, and death, in those in whom creatinine rose by 30% or more since starting treatment. This trend was also seen, to a lesser degree, in those with a smaller increase in creatinine, suggesting that even this group of patients should receive close monitoring.
Whether renin-angiotensin-aldosterone system inhibitors provide a benefit in advanced progressive chronic kidney disease remains unclear.35–37 The Angiotensin Converting Enzyme Inhibitor (ACEi)/Angiotensin Receptor Blocker (ARB) Withdrawal in Advanced Renal Disease trial (STOP-ACEi),38 currently under way, will provide valuable data to help close this gap in our knowledge. This open-label randomized controlled trial is testing the hypothesis that stopping ACE inhibitor or ARB treatment, or a combination of both, compared with continuing these treatments, will improve or stabilize renal function in patients with progressive stage 4 or 5 chronic kidney disease.
NEED FOR MONITORING
Taken together, the above data suggest close and regular monitoring is required in patients receiving these drugs. However, monitoring tends to be lax.34,37,39 A 2017 study of adherence to the guidelines for monitoring serum creatinine and potassium after starting an ACE inhibitor or ARB and subsequent discontinuation found that fewer than 10% of patients had follow-up within the recommended 2 weeks after starting these drugs.34 Most patients with a creatinine rise of 30% or more or a potassium level higher than 6.0 mmol/L continued treatment. There was also no evidence of increased monitoring in those deemed at higher risk of these complications.
WHAT DO THE GUIDELINES SUGGEST?
ACE inhibitors and ARBs in chronic kidney disease and hypertension
Target blood pressures vary in guidelines from different organizations.4,40–45 The 2017 joint guidelines of the American College of Cardiology and American Heart Association (ACC/AHA)40 recommend a target blood pressure of 130/80 mm Hg or less in all patients irrespective of the level of proteinuria and whether they have diabetes mellitus, based on several studies.46–48 In the elderly, other factors such as the risk of hypotension and falls must be taken into consideration in establishing the most appropriate blood pressure target.
In general, a renin-angiotensin-aldosterone system inhibitor is recommended if the patient has diabetes, stage 1, 2, or 3 chronic kidney disease, or proteinuria. For example, the guidelines recommend a renin-angiotensin-aldosterone system inhibitor in diabetic patients with albuminuria.
None of the guidelines recommend routine use of combination therapy.
ACE inhibitors and ARBs in heart failure
The 2017 ACC/AHA and Heart Failure Society of America (HFSA) guidelines for heart failure49 recommend an ACE inhibitor or ARB for patients with stage C (symptomatic) heart failure with reduced ejection fraction, in view of the known cardiovascular morbidity and mortality benefits.
The European Society of Cardiology50 recommends ACE inhibitors for patients with symptomatic heart failure with reduced ejection fraction, as well as those with asymptomatic left ventricular systolic dysfunction. In patients with stable coronary artery disease, an ACE inhibitor should be considered even with normal left ventricular function.
ARBs should be used as alternatives in those unable to tolerate ACE inhibitors.
Combination therapy should be avoided due to the increased risk of renal impairment and hyperkalemia but may be considered in patients with heart failure and reduced ejection fraction in whom other treatments are unsuitable. These include patients on beta-blockers who cannot tolerate mineralocorticoid receptor antagonists such as spironolactone. Combination therapy should be done only under strict supervision.50
Starting ACE or ARB therapy
Close monitoring of serum potassium is recommended during ACE inhibitor or ARB use. Those at greatest risk of hyperkalemia include elderly patients, those taking other medications associated with hyperkalemia, and diabetic patients, because of their higher risk of renovascular disease.
Caution is advised when starting ACE inhibitor or ARB therapy in these high-risk groups as well as in patients with potassium levels higher than 5.0 mmol/L at baseline, at high risk of prerenal acute kidney injury, with known renal insufficiency, and with previous deterioration in renal function on these medications.3,41,51
Before starting therapy, ensure that patients are volume-replete and measure baseline serum electrolytes and creatinine.41,51
The ACC/AHA and HFSA recommend starting at a low dose and titrating upward slowly. If maximal doses are not tolerated, then a lower dose should be maintained.49 The European Society of Cardiology guidelines52 suggest increasing the dose at no less than every 2 weeks unless in an inpatient setting. Blood testing should be done 7 to 14 days after starting therapy, after any titration in dosage, and every 4 months thereafter.53
The guidelines generally agree that a rise in creatinine of up to 30% and a fall in eGFR of up to 25% is acceptable, with the need for regular monitoring, particularly in high-risk groups.40–42,51,52
What if serum potassium or creatinine rises during treatment?
If hyperkalemia arises or renal function declines by a significant amount, one should first address contributing factors. If no improvement is seen, then the dose of the ACE inhibitor or ARB should be reduced by 50% and blood work repeated in 1 to 2 weeks. If the laboratory values do not return to an acceptable level, reducing the dose further or stopping the drug is advised.
Give dietary advice to all patients with chronic kidney disease being considered for a renin-angiotensin-aldosterone system inhibitor or for an increase in dose with a potassium level higher than 4.5 mmol/L. A low-potassium diet should aim for potassium intake of less than 50 or 75 mmol/day and sodium intake of less than 60 mmol/day for hypertensive patients with chronic kidney disease.
Review the patient’s medications if the baseline potassium level is higher than 5.0 mmol/L. Consider stopping potassium-sparing agents, digoxin, trimethoprim, and nonsteroidal anti-inflammatory drugs. Also think about starting a non–potassium-sparing diuretic as well as sodium bicarbonate to reduce potassium levels. Blood work should be repeated within 2 weeks after these changes.
Do not start a renin-angiotensin-aldosterone system inhibitor, or do not increase the dose, if the potassium level is elevated until measures have been taken to reduce the degree of hyperkalemia.51
In renal transplant recipients, renin-angiotensin-aldosterone system inhibitors are often preferred to manage hypertension in those who have proteinuria or cardiovascular disease. However, the risk of hyperkalemia is also greater with concomitant use of immunosuppressive drugs such as tacrolimus and cyclosporine. Management of complications should be approached according to guidelines discussed above.51
Monitor renal function, potassium. The National Institute for Health and Care Excellence guideline54 advocates that baseline renal function testing should be followed by repeat blood testing 1 to 2 weeks after starting renin-angiotensin-aldosterone system inhibitors in patients with ischemic heart disease. The advice is similar when starting therapy in patients with chronic heart failure, emphasizing the need to monitor after each dose increment and to use clinical judgment when deciding to start treatment. The AHA advises caution in patients with renal insufficiency or a potassium level above 5.0 mmol/L.49
Sick day rules. The National Institute for Health and Care Excellence encourages discussing “sick day rules” with patients starting renin-angiotensin-aldosterone system inhibitors. This means patients should be advised to temporarily stop taking nephrotoxic medications, including over-the-counter nonsteroidal anti-inflammatory drugs, in any potential state of illness or dehydration, such as diarrhea and vomiting. There is, however, little evidence that this advice can actually reduce the incidence of acute kidney injury.55,56
OUR RECOMMENDATIONS
Our advice for managing patients receiving ACE inhibitors or ARBs is summarized in Table 1.
A highly active, water- and alcohol-soluble, basic pressor substance is formed when renin and renin-activator interact, for which we suggest the name “angiotonin.”
—Irvine H. Page and O.M. Helmer, 1940.1
The renin-angiotensin-aldosterone system regulates salt and, in part, water homeostasis, and therefore blood pressure and fluid balance through its actions on the heart, kidneys, and blood vessels.2 Drugs that target this system—angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs)—are used primarily to treat hypertension and also to treat chronic kidney disease and heart failure with reduced ejection fraction.
Controlling blood pressure is important, as hypertension increases the risk of myocardial infarction, cerebrovascular events, and progression of chronic kidney disease, which itself is a risk factor for cardiovascular disease. However, the benefit of these drugs is only partly due to their effect on blood pressure. They also reduce proteinuria, which is a graded risk factor for progression of kidney disease as well as morbidity and death from vascular events.3
Despite the benefits of ACE inhibitors and ARBs, concern about their adverse effects—especially hyperkalemia and a decline in renal function—has led to their underuse in patients likely to derive the greatest benefit.3
ACE INHIBITORS AND ARBs
ACE inhibitors, as their name indicates, inhibit conversion of angiotensin I to angiotensin II by ACE, resulting in vasodilation of the efferent arteriole and a drop in blood pressure. Inhibition of ACE, a kininase, also results in a rise in kinins. One of these, bradykinin, is associated with some of the side effects of this class of drugs such as cough, which affects 5% to 20% of patients.4 Elevation of bradykinin is also believed to account for ACE inhibitor-induced angioedema, an uncommon but potentially serious side effect. Kinins are also associated with desirable effects such as lowering blood pressure, increasing insulin sensitivity, and dilating blood vessels.
ARBs were developed as an alternative for patients unable to tolerate the adverse effects of ACE inhibitors. While ACE inhibitors reduce the activity of angiotensin II at both the AT1 and AT2 receptors, ARBs block only the AT1 receptors, thereby inhibiting their vasoconstricting activity on smooth muscle. ARBs also raise the levels of renin, angiotensin I, and angiotensin II as a result of feedback inhibition. Angiotensin II is associated with release of inflammatory mediators such as tumor necrosis factor alpha, cytokines, and chemokines, the consequences of which are also inhibited by ARBs, further preventing renal fibrosis and scarring from chronic inflammation.3
What is the evidence supporting the use of ACE inhibitors and ARBs?
ACE inhibitors and ARBs, used singly, reduce blood pressure and proteinuria, slow progression of kidney disease, and improve outcomes in patients who have heart failure, diabetes mellitus, or a history of myocardial infarction.5–11
While dual blockade with the combination of an ACE inhibitor and an ARB lowers blood pressure and proteinuria to a greater degree than monotherapy, dual blockade has been associated with higher rates of complications, including hyperkalemia.12–17
RISK FACTORS FOR HYPERKALEMIA
ACE inhibitors and ARBs raise potassium, especially when used in combination. Other risk factors for hyperkalemia include the following—and note that some of them are also indications for ACE inhibitors and ARBs:
Renal insufficiency. The kidneys are responsible for over 90% of potassium removal in healthy individuals,18,19 and the lower the GFR, the higher the risk of hyperkalemia.3,20,21
Heart failure
Diabetes mellitus6,21–23
Endogenous potassium load due to hemolysis, rhabdomyolysis, insulin deficiency, lactic acidosis, or gastrointestinal bleeding
Exogenous potassium load due to dietary consumption or blood products
Other medications, eg, sacubitril-valsartan, aldosterone antagonists, mineralocorticoid receptor antagonists, potassium-sparing diuretics, beta-adrenergic antagonists, nonsteroidal anti-inflammatory drugs, heparin, cyclosporine, trimethoprim, digoxin
Hypertension
Hypoaldosteronism (including type 4 renal tubular acidosis)
Addison disease
Advanced age
Lower body mass index.
Both hypokalemia and hyperkalemia are associated with a higher risk of death,20,21,24 but in patients with heart failure, the survival benefit from ACE inhibitors, ARBs, and mineralocorticoid receptor antagonists outweighs the risk of hyperkalemia.25–27 Weir and Rolfe28 concluded that patients with heart failure and chronic kidney disease are at greatest risk of hyperkalemia from renin-angiotensin-aldosterone system inhibition, but the increases in potassium levels are small (about 0.1 to 0.3 mmol/L) and unlikely to be clinically significant.
Hyperkalemia tends to recur. Einhorn et al20 found that nearly half of patients with chronic kidney disease who had an episode of hyperkalemia had 1 or more recurrent episodes within a year.
ACE INHIBITORS, ARBs, ABD RENAL FUNCTION
Another concern about using ACE inhibitors and ARBs, especially in patients with chronic kidney disease, is that the serum creatinine level tends to rise when starting these drugs,29 although several studies have shown that an acute rise in creatinine may demonstrate that the drug is actually protecting the kidney.30,31 Hirsch32 described this phenomenon as “prerenal success,” proposing that the decline in GFR is hemodynamic, secondary to a fall in intraglomerular pressure as a result of efferent vasodilation, and therefore should not be reversed.
Schmidt et al,33,34 in a study in 122,363 patients who began ACE inhibitor or ARB therapy, found that cardiorenal outcomes were worse, with higher rates of end-stage renal disease, myocardial infarction, heart failure, and death, in those in whom creatinine rose by 30% or more since starting treatment. This trend was also seen, to a lesser degree, in those with a smaller increase in creatinine, suggesting that even this group of patients should receive close monitoring.
Whether renin-angiotensin-aldosterone system inhibitors provide a benefit in advanced progressive chronic kidney disease remains unclear.35–37 The Angiotensin Converting Enzyme Inhibitor (ACEi)/Angiotensin Receptor Blocker (ARB) Withdrawal in Advanced Renal Disease trial (STOP-ACEi),38 currently under way, will provide valuable data to help close this gap in our knowledge. This open-label randomized controlled trial is testing the hypothesis that stopping ACE inhibitor or ARB treatment, or a combination of both, compared with continuing these treatments, will improve or stabilize renal function in patients with progressive stage 4 or 5 chronic kidney disease.
NEED FOR MONITORING
Taken together, the above data suggest close and regular monitoring is required in patients receiving these drugs. However, monitoring tends to be lax.34,37,39 A 2017 study of adherence to the guidelines for monitoring serum creatinine and potassium after starting an ACE inhibitor or ARB and subsequent discontinuation found that fewer than 10% of patients had follow-up within the recommended 2 weeks after starting these drugs.34 Most patients with a creatinine rise of 30% or more or a potassium level higher than 6.0 mmol/L continued treatment. There was also no evidence of increased monitoring in those deemed at higher risk of these complications.
WHAT DO THE GUIDELINES SUGGEST?
ACE inhibitors and ARBs in chronic kidney disease and hypertension
Target blood pressures vary in guidelines from different organizations.4,40–45 The 2017 joint guidelines of the American College of Cardiology and American Heart Association (ACC/AHA)40 recommend a target blood pressure of 130/80 mm Hg or less in all patients irrespective of the level of proteinuria and whether they have diabetes mellitus, based on several studies.46–48 In the elderly, other factors such as the risk of hypotension and falls must be taken into consideration in establishing the most appropriate blood pressure target.
In general, a renin-angiotensin-aldosterone system inhibitor is recommended if the patient has diabetes, stage 1, 2, or 3 chronic kidney disease, or proteinuria. For example, the guidelines recommend a renin-angiotensin-aldosterone system inhibitor in diabetic patients with albuminuria.
None of the guidelines recommend routine use of combination therapy.
ACE inhibitors and ARBs in heart failure
The 2017 ACC/AHA and Heart Failure Society of America (HFSA) guidelines for heart failure49 recommend an ACE inhibitor or ARB for patients with stage C (symptomatic) heart failure with reduced ejection fraction, in view of the known cardiovascular morbidity and mortality benefits.
The European Society of Cardiology50 recommends ACE inhibitors for patients with symptomatic heart failure with reduced ejection fraction, as well as those with asymptomatic left ventricular systolic dysfunction. In patients with stable coronary artery disease, an ACE inhibitor should be considered even with normal left ventricular function.
ARBs should be used as alternatives in those unable to tolerate ACE inhibitors.
Combination therapy should be avoided due to the increased risk of renal impairment and hyperkalemia but may be considered in patients with heart failure and reduced ejection fraction in whom other treatments are unsuitable. These include patients on beta-blockers who cannot tolerate mineralocorticoid receptor antagonists such as spironolactone. Combination therapy should be done only under strict supervision.50
Starting ACE or ARB therapy
Close monitoring of serum potassium is recommended during ACE inhibitor or ARB use. Those at greatest risk of hyperkalemia include elderly patients, those taking other medications associated with hyperkalemia, and diabetic patients, because of their higher risk of renovascular disease.
Caution is advised when starting ACE inhibitor or ARB therapy in these high-risk groups as well as in patients with potassium levels higher than 5.0 mmol/L at baseline, at high risk of prerenal acute kidney injury, with known renal insufficiency, and with previous deterioration in renal function on these medications.3,41,51
Before starting therapy, ensure that patients are volume-replete and measure baseline serum electrolytes and creatinine.41,51
The ACC/AHA and HFSA recommend starting at a low dose and titrating upward slowly. If maximal doses are not tolerated, then a lower dose should be maintained.49 The European Society of Cardiology guidelines52 suggest increasing the dose at no less than every 2 weeks unless in an inpatient setting. Blood testing should be done 7 to 14 days after starting therapy, after any titration in dosage, and every 4 months thereafter.53
The guidelines generally agree that a rise in creatinine of up to 30% and a fall in eGFR of up to 25% is acceptable, with the need for regular monitoring, particularly in high-risk groups.40–42,51,52
What if serum potassium or creatinine rises during treatment?
If hyperkalemia arises or renal function declines by a significant amount, one should first address contributing factors. If no improvement is seen, then the dose of the ACE inhibitor or ARB should be reduced by 50% and blood work repeated in 1 to 2 weeks. If the laboratory values do not return to an acceptable level, reducing the dose further or stopping the drug is advised.
Give dietary advice to all patients with chronic kidney disease being considered for a renin-angiotensin-aldosterone system inhibitor or for an increase in dose with a potassium level higher than 4.5 mmol/L. A low-potassium diet should aim for potassium intake of less than 50 or 75 mmol/day and sodium intake of less than 60 mmol/day for hypertensive patients with chronic kidney disease.
Review the patient’s medications if the baseline potassium level is higher than 5.0 mmol/L. Consider stopping potassium-sparing agents, digoxin, trimethoprim, and nonsteroidal anti-inflammatory drugs. Also think about starting a non–potassium-sparing diuretic as well as sodium bicarbonate to reduce potassium levels. Blood work should be repeated within 2 weeks after these changes.
Do not start a renin-angiotensin-aldosterone system inhibitor, or do not increase the dose, if the potassium level is elevated until measures have been taken to reduce the degree of hyperkalemia.51
In renal transplant recipients, renin-angiotensin-aldosterone system inhibitors are often preferred to manage hypertension in those who have proteinuria or cardiovascular disease. However, the risk of hyperkalemia is also greater with concomitant use of immunosuppressive drugs such as tacrolimus and cyclosporine. Management of complications should be approached according to guidelines discussed above.51
Monitor renal function, potassium. The National Institute for Health and Care Excellence guideline54 advocates that baseline renal function testing should be followed by repeat blood testing 1 to 2 weeks after starting renin-angiotensin-aldosterone system inhibitors in patients with ischemic heart disease. The advice is similar when starting therapy in patients with chronic heart failure, emphasizing the need to monitor after each dose increment and to use clinical judgment when deciding to start treatment. The AHA advises caution in patients with renal insufficiency or a potassium level above 5.0 mmol/L.49
Sick day rules. The National Institute for Health and Care Excellence encourages discussing “sick day rules” with patients starting renin-angiotensin-aldosterone system inhibitors. This means patients should be advised to temporarily stop taking nephrotoxic medications, including over-the-counter nonsteroidal anti-inflammatory drugs, in any potential state of illness or dehydration, such as diarrhea and vomiting. There is, however, little evidence that this advice can actually reduce the incidence of acute kidney injury.55,56
OUR RECOMMENDATIONS
Our advice for managing patients receiving ACE inhibitors or ARBs is summarized in Table 1.
- Page IH, Helmer OM. A crystalline pressor substance (angiotonin) resulting from the reaction between renin and renin-activator. Exp Med 1940; 71(1):29–42. doi:10.1084/jem.71.1.29
- Steddon S, Ashman N, Chesser A, Cunningham J. Oxford Handbook of Nephrology and Hypertension. 2nd ed. Oxford: Oxford University Press; 2016:203–206, 508–509.
- Barratt J, Topham P, Harris K. Oxford Desk Reference. 1st ed. Oxford: Oxford University Press; 2008.
- International Kidney Foundation. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. http://www.kdigo.org/clinical_practice_guidelines/pdf/KDIGO_BP_GL.pdf. Accessed April 3, 2019.
- Heart Outcomes Prevention Evaluation Study Investigators; Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med 2000; 342(3):145–153. doi:10.1056/NEJM200001203420301
- Swedberg K, Kjekshus J. Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). Am J Cardiol 1988; 62(2):60A–66A. pmid:2839019
- Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345(12):861–869. doi:10.1056/NEJMoa011161
- Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003; 349(20):1893–1906. doi:10.1056/NEJMoa032292
- Epstein M. Reduction of cardiovascular risk in chronic kidney disease by mineralocorticoid receptor antagonism. Lancet Diabetes Endocrinol 2015; 3(12):993–1003. doi:10.1016/S2213-8587(15)00289-2
- SOLVD Investigators; Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325(5):293–302. doi:10.1056/NEJM199108013250501
- Jafar TH, Stark PC, Schmid CH, et al; AIPRD Study Group; Angiotensin-Converting Enzymne Inhibition and Progression of Renal Disease. Proteinuria as a modifiable risk factor for the progression of non-diabetic renal disease. Kidney Int 2001; 60(3):1131–1140. doi:10.1046/j.1523-1755.2001.0600031131.x
- Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet 2015; 385(9982):2047–2056. doi:10.1016/S0140-6736(14)62459-4
- Ruggenenti P, Perticucci E, Cravedi P, et al. Role of remission clinics in the longitudinal treatment of CKD. J Am Soc Nephrol 2008; 19(6):1213–1224. doi:10.1681/ASN.2007090970
- Makani H, Bangalore S, Desouza KA, Shah A, Messerli FH. Efficacy and safety of dual blockade of the renin-angiotensin system: meta-analysis of randomised trials. BMJ 2013; 346:f360. doi:10.1136/bmj.f360
- ONTARGET Investigators; Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358(15):1547–1559. doi:10.1056/NEJMoa0801317
- Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013; 369(20):1892–1903.
doi:10.1056/NEJMoa1303154 - Catalá-López F, Macías Saint-Gerons D, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med 2016; 13(3):e1001971. doi:10.1371/journal.pmed.1001971
- Agarwal R, Afzalpurkar R, Fordtran JS. Pathophysiology of potassium absorption and secretion by the human intestine. Gastroenterology 1994; 107(2):548–571. pmid:8039632
- Palmer BF. Regulation of potassium homeostasis. Clin J Am Soc Nephrol 2015; 10(6):1050–1060. doi:10.2215/CJN.08580813
- Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med 2009; 169(12):1156–1162. doi:10.1001/archinternmed.2009.132
- Nakhoul GN, Huang H, Arrigain S, et al. Serum potassium, end-stage renal disease and mortality in chronic kidney disease. Am J Nephrol 2015; 41(6):456–463. doi:10.1159/000437151
- Acker CG, Johnson JP, Palevsky PM, Greenberg A. Hyperkalemia in hospitalized patients: causes, adequacy of treatment, and results of an attempt to improve physician compliance with published therapy guidelines. Arch Intern Med 1998; 158(8):917–924. pmid:9570179
- Desai AS, Swedberg K, McMurray JJ, et al; CHARM Program Investigators. Incidence and predictors of hyperkalemia in patients with heart failure: an analysis of the CHARM Program. J Am Coll Cardiol 2007; 50(20):1959–1966. doi:10.1016/j.jacc.2007.07.067
- Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, Sakhuja A, Mao MA, Erickson SB. Impact of admission serum potassium on mortality in patients with chronic kidney disease and cardiovascular disease. QJM 2017; 110(11):713–719. doi:10.1093/qjmed/hcx118
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364(1):11–21. doi:10.1056/NEJMoa1009492
- Rossignol P, Dobre D, McMurray JJ, et al. Incidence, determinants, and prognostic significance of hyperkalemia and worsening renal function in patients with heart failure receiving the mineralocorticoid receptor antagonist eplerenone or placebo in addition to optimal medical therapy: results from the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF). Circ Heart Fail 2014; 7(1):51–58. doi:10.1161/CIRCHEARTFAILURE.113.000792
- Testani JM, Kimmel SE, Dries DL, Coca SG. Prognostic importance of early worsening renal function after initiation of angiotensin-converting enzyme inhibitor therapy in patients with cardiac dysfunction. Circ Heart Fail 2011; 4(6):685–691. doi:10.1161/CIRCHEARTFAILURE.111.963256
- Weir M, Rolfe M. Potassium homeostasis and renin-angiotensin-aldosterone system inhibitors. Clin J Am Soc Nephrol 2010; 5(3):531–548. doi:10.2215/CJN.07821109
- Valente M, Bhandari S. Renal function after new treatment with renin-angiotensin system blockers. BMJ 2017; 356:j1122. doi:10.1136/bmj.j1122
- Bakris G, Weir M. Angiotensin-converting enzyme inhibitor–associated elevations in serum creatinine. Arch Intern Med 2000; 160(5):685–693. pmid:10724055
- Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345(12):861–869. doi:10.1056/NEJMoa011161
- Hirsch S. Pre-renal success. Kidney Int 2012; 81(6):596. doi:10.1038/ki.2011.418
- Schmidt M, Mansfield KE, Bhaskaran K, et al. Serum creatinine elevation after renin-angiotensin system blockade and long term cardiorenal risks: cohort study. BMJ 2017; 356:j791. doi:10.1136/bmj.j791
- Schmidt M, Mansfield KE, Bhaskaran K, et al. Adherence to guidelines for creatinine and potassium monitoring and discontinuation following renin–angiotensin system blockade: a UK general practice-based cohort study. BMJ Open 2017; 7(1):e012818. doi:10.1136/bmjopen-2016-012818
- Lund LH, Carrero JJ, Farahmand B, et al. Association between enrollment in a heart failure quality registry and subsequent mortality—a nationwide cohort study. Eur J Heart Fail 2017; 19(9):1107–1116. doi:10.1002/ejhf.762
- Edner M, Benson L, Dahlstrom U, Lund LH. Association between renin-angiotensin system antagonist use and mortality in heart failure with severe renal insuffuciency: a prospective propensity score-matched cohort study. Eur Heart J 2015; 36(34):2318–2326. doi:10.1093/eurheartj/ehv268
- Epstein M, Reaven NL, Funk SE, McGaughey KJ, Oestreicher N, Knispel J. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care 2015; 21(suppl 11):S212–S220. pmid:26619183
- Bhandari S, Ives N, Brettell EA, et al. Multicentre randomized controlled trial of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker withdrawal in advanced renal disease: the STOP-ACEi trial. Nephrol Dial Transplant 2016; 31(2):255–261. doi:10.1093/ndt/gfv346
- Raebel MA, Ross C, Xu S, et al. Diabetes and drug-associated hyperkalemia: effect of potassium monitoring. J Gen Intern Med 2010; 25(4):326–333. doi:10.1007/s11606-009-1228-x
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018; 71(6):e13–e115. doi:10.1161/HYP.0000000000000065
- The Renal Association. The UK eCKD Guide. https://renal.org/information-resources/the-uk-eckd-guide. Accessed August 12, 2019.
- National Institute for Health and Care Excellence (NICE). Chronic kidney disease in adults: assessment and management. https://www.nice.org.uk/guidance/cg182. Accessed August 12, 2019.
- National Institute for Health and Care Excellence (NICE). Hypertension in adults: diagnosis and management. https://www.nice.org.uk/Guidance/CG127. Accessed August 12, 2019.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34(28):2159–2219. doi:10.1093/eurheartj/eht151
- International Kidney Foundation. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. https://www.sciencedirect.com/journal/kidney-international-supplements/vol/3/issue/1. Accessed August 12, 2019.
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373(22):2103–2116. doi:10.1056/NEJMoa1511939
- Wright J, Bakris G, Greene T. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease. Results from the AASK trial. ACC Current Journal Review 2003; 12(2):37–38. doi:10.1016/s1062-1458(03)00035-7
- Ku E, Bakris G, Johansen K, et al. Acute declines in renal function during intensive BP lowering: implications for future ESRD risk. J Am Soc Nephrol 2017; 28(9):2794–2801. doi:10.1681/ASN.2017010040
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017; 136(6):e137–e161. doi:10.1161/CIR.0000000000000509
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37(27):2129–2200. doi:10.1093/eurheartj/ehw128
- Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 2004; 43(suppl 51):S1–S290. pmid:15114537
- Asenjo RM, Bueno H, Mcintosh M. Angiotensin converting enzyme inhibitors (ACE inhibitors) and angiotensin II receptor blockers (ARBs). ACE inhibitors and ARBs, a cornerstone in the prevention and treatment of cardiovascular disease. www.escardio.org/Education/ESC-Prevention-of-CVD-Programme/Treatment-goals/Cardio-Protective-drugs/angiotensin-converting-enzyme-inhibitors-ace-inhibitors-and-angiotensin-ii-rec. Accessed August 12, 2019.
- López-Sendón J, Swedberg K, McMurray J, et al; Task Force on ACE-inhibitors of the European Society of Cardiology. Expert consensus document on angiotensin converting enzyme inhibitors in cardiovascular disease. The Task Force on ACE-inhibitors of the European Society of Cardiology. Eur Heart J 2004; 25(16):1454–1470. doi:10.1016/j.ehj.2004.06.003
- National Institute for Health and Care Excellence (NICE). Myocardial infarction: cardiac rehabilitation and prevention of further cardiovascular disease. https://www.nice.org.uk/Guidance/CG172. Accessed April 3, 2019.
- National Institute for Health and Care Excellence (NICE). Acute kidney injury: prevention, detection and management. https://www.nice.org.uk/Guidance/CG169. Accessed August 12, 2019.
- Think Kidneys. “Sick day” guidance in patients at risk of acute kidney injury: a position statement from the Think Kidneys Board. https://www.thinkkidneys.nhs.uk/aki/wp-content/uploads/sites/2/2018/01/Think-Kidneys-Sick-Day-Guidance-2018.pdf. Accessed August 12, 2019.
- Meaney CJ, Beccari MV, Yang Y, Zhao J. Systematic review and meta-analysis of patiromer and sodium zirconium cyclosilicate: a new armamentarium for the treatment of hyperkalemia. Pharmacotherapy 2017; 37(4):401–411. doi:10.1002/phar.1906
- Page IH, Helmer OM. A crystalline pressor substance (angiotonin) resulting from the reaction between renin and renin-activator. Exp Med 1940; 71(1):29–42. doi:10.1084/jem.71.1.29
- Steddon S, Ashman N, Chesser A, Cunningham J. Oxford Handbook of Nephrology and Hypertension. 2nd ed. Oxford: Oxford University Press; 2016:203–206, 508–509.
- Barratt J, Topham P, Harris K. Oxford Desk Reference. 1st ed. Oxford: Oxford University Press; 2008.
- International Kidney Foundation. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. http://www.kdigo.org/clinical_practice_guidelines/pdf/KDIGO_BP_GL.pdf. Accessed April 3, 2019.
- Heart Outcomes Prevention Evaluation Study Investigators; Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med 2000; 342(3):145–153. doi:10.1056/NEJM200001203420301
- Swedberg K, Kjekshus J. Effects of enalapril on mortality in severe congestive heart failure: results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). Am J Cardiol 1988; 62(2):60A–66A. pmid:2839019
- Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345(12):861–869. doi:10.1056/NEJMoa011161
- Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003; 349(20):1893–1906. doi:10.1056/NEJMoa032292
- Epstein M. Reduction of cardiovascular risk in chronic kidney disease by mineralocorticoid receptor antagonism. Lancet Diabetes Endocrinol 2015; 3(12):993–1003. doi:10.1016/S2213-8587(15)00289-2
- SOLVD Investigators; Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325(5):293–302. doi:10.1056/NEJM199108013250501
- Jafar TH, Stark PC, Schmid CH, et al; AIPRD Study Group; Angiotensin-Converting Enzymne Inhibition and Progression of Renal Disease. Proteinuria as a modifiable risk factor for the progression of non-diabetic renal disease. Kidney Int 2001; 60(3):1131–1140. doi:10.1046/j.1523-1755.2001.0600031131.x
- Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet 2015; 385(9982):2047–2056. doi:10.1016/S0140-6736(14)62459-4
- Ruggenenti P, Perticucci E, Cravedi P, et al. Role of remission clinics in the longitudinal treatment of CKD. J Am Soc Nephrol 2008; 19(6):1213–1224. doi:10.1681/ASN.2007090970
- Makani H, Bangalore S, Desouza KA, Shah A, Messerli FH. Efficacy and safety of dual blockade of the renin-angiotensin system: meta-analysis of randomised trials. BMJ 2013; 346:f360. doi:10.1136/bmj.f360
- ONTARGET Investigators; Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358(15):1547–1559. doi:10.1056/NEJMoa0801317
- Fried LF, Emanuele N, Zhang JH, et al; VA NEPHRON-D Investigators. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013; 369(20):1892–1903.
doi:10.1056/NEJMoa1303154 - Catalá-López F, Macías Saint-Gerons D, González-Bermejo D, et al. Cardiovascular and renal outcomes of renin-angiotensin system blockade in adult patients with diabetes mellitus: a systematic review with network meta-analyses. PLoS Med 2016; 13(3):e1001971. doi:10.1371/journal.pmed.1001971
- Agarwal R, Afzalpurkar R, Fordtran JS. Pathophysiology of potassium absorption and secretion by the human intestine. Gastroenterology 1994; 107(2):548–571. pmid:8039632
- Palmer BF. Regulation of potassium homeostasis. Clin J Am Soc Nephrol 2015; 10(6):1050–1060. doi:10.2215/CJN.08580813
- Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med 2009; 169(12):1156–1162. doi:10.1001/archinternmed.2009.132
- Nakhoul GN, Huang H, Arrigain S, et al. Serum potassium, end-stage renal disease and mortality in chronic kidney disease. Am J Nephrol 2015; 41(6):456–463. doi:10.1159/000437151
- Acker CG, Johnson JP, Palevsky PM, Greenberg A. Hyperkalemia in hospitalized patients: causes, adequacy of treatment, and results of an attempt to improve physician compliance with published therapy guidelines. Arch Intern Med 1998; 158(8):917–924. pmid:9570179
- Desai AS, Swedberg K, McMurray JJ, et al; CHARM Program Investigators. Incidence and predictors of hyperkalemia in patients with heart failure: an analysis of the CHARM Program. J Am Coll Cardiol 2007; 50(20):1959–1966. doi:10.1016/j.jacc.2007.07.067
- Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, Sakhuja A, Mao MA, Erickson SB. Impact of admission serum potassium on mortality in patients with chronic kidney disease and cardiovascular disease. QJM 2017; 110(11):713–719. doi:10.1093/qjmed/hcx118
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364(1):11–21. doi:10.1056/NEJMoa1009492
- Rossignol P, Dobre D, McMurray JJ, et al. Incidence, determinants, and prognostic significance of hyperkalemia and worsening renal function in patients with heart failure receiving the mineralocorticoid receptor antagonist eplerenone or placebo in addition to optimal medical therapy: results from the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF). Circ Heart Fail 2014; 7(1):51–58. doi:10.1161/CIRCHEARTFAILURE.113.000792
- Testani JM, Kimmel SE, Dries DL, Coca SG. Prognostic importance of early worsening renal function after initiation of angiotensin-converting enzyme inhibitor therapy in patients with cardiac dysfunction. Circ Heart Fail 2011; 4(6):685–691. doi:10.1161/CIRCHEARTFAILURE.111.963256
- Weir M, Rolfe M. Potassium homeostasis and renin-angiotensin-aldosterone system inhibitors. Clin J Am Soc Nephrol 2010; 5(3):531–548. doi:10.2215/CJN.07821109
- Valente M, Bhandari S. Renal function after new treatment with renin-angiotensin system blockers. BMJ 2017; 356:j1122. doi:10.1136/bmj.j1122
- Bakris G, Weir M. Angiotensin-converting enzyme inhibitor–associated elevations in serum creatinine. Arch Intern Med 2000; 160(5):685–693. pmid:10724055
- Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345(12):861–869. doi:10.1056/NEJMoa011161
- Hirsch S. Pre-renal success. Kidney Int 2012; 81(6):596. doi:10.1038/ki.2011.418
- Schmidt M, Mansfield KE, Bhaskaran K, et al. Serum creatinine elevation after renin-angiotensin system blockade and long term cardiorenal risks: cohort study. BMJ 2017; 356:j791. doi:10.1136/bmj.j791
- Schmidt M, Mansfield KE, Bhaskaran K, et al. Adherence to guidelines for creatinine and potassium monitoring and discontinuation following renin–angiotensin system blockade: a UK general practice-based cohort study. BMJ Open 2017; 7(1):e012818. doi:10.1136/bmjopen-2016-012818
- Lund LH, Carrero JJ, Farahmand B, et al. Association between enrollment in a heart failure quality registry and subsequent mortality—a nationwide cohort study. Eur J Heart Fail 2017; 19(9):1107–1116. doi:10.1002/ejhf.762
- Edner M, Benson L, Dahlstrom U, Lund LH. Association between renin-angiotensin system antagonist use and mortality in heart failure with severe renal insuffuciency: a prospective propensity score-matched cohort study. Eur Heart J 2015; 36(34):2318–2326. doi:10.1093/eurheartj/ehv268
- Epstein M, Reaven NL, Funk SE, McGaughey KJ, Oestreicher N, Knispel J. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care 2015; 21(suppl 11):S212–S220. pmid:26619183
- Bhandari S, Ives N, Brettell EA, et al. Multicentre randomized controlled trial of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker withdrawal in advanced renal disease: the STOP-ACEi trial. Nephrol Dial Transplant 2016; 31(2):255–261. doi:10.1093/ndt/gfv346
- Raebel MA, Ross C, Xu S, et al. Diabetes and drug-associated hyperkalemia: effect of potassium monitoring. J Gen Intern Med 2010; 25(4):326–333. doi:10.1007/s11606-009-1228-x
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018; 71(6):e13–e115. doi:10.1161/HYP.0000000000000065
- The Renal Association. The UK eCKD Guide. https://renal.org/information-resources/the-uk-eckd-guide. Accessed August 12, 2019.
- National Institute for Health and Care Excellence (NICE). Chronic kidney disease in adults: assessment and management. https://www.nice.org.uk/guidance/cg182. Accessed August 12, 2019.
- National Institute for Health and Care Excellence (NICE). Hypertension in adults: diagnosis and management. https://www.nice.org.uk/Guidance/CG127. Accessed August 12, 2019.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34(28):2159–2219. doi:10.1093/eurheartj/eht151
- International Kidney Foundation. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. https://www.sciencedirect.com/journal/kidney-international-supplements/vol/3/issue/1. Accessed August 12, 2019.
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373(22):2103–2116. doi:10.1056/NEJMoa1511939
- Wright J, Bakris G, Greene T. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease. Results from the AASK trial. ACC Current Journal Review 2003; 12(2):37–38. doi:10.1016/s1062-1458(03)00035-7
- Ku E, Bakris G, Johansen K, et al. Acute declines in renal function during intensive BP lowering: implications for future ESRD risk. J Am Soc Nephrol 2017; 28(9):2794–2801. doi:10.1681/ASN.2017010040
- Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017; 136(6):e137–e161. doi:10.1161/CIR.0000000000000509
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37(27):2129–2200. doi:10.1093/eurheartj/ehw128
- Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 2004; 43(suppl 51):S1–S290. pmid:15114537
- Asenjo RM, Bueno H, Mcintosh M. Angiotensin converting enzyme inhibitors (ACE inhibitors) and angiotensin II receptor blockers (ARBs). ACE inhibitors and ARBs, a cornerstone in the prevention and treatment of cardiovascular disease. www.escardio.org/Education/ESC-Prevention-of-CVD-Programme/Treatment-goals/Cardio-Protective-drugs/angiotensin-converting-enzyme-inhibitors-ace-inhibitors-and-angiotensin-ii-rec. Accessed August 12, 2019.
- López-Sendón J, Swedberg K, McMurray J, et al; Task Force on ACE-inhibitors of the European Society of Cardiology. Expert consensus document on angiotensin converting enzyme inhibitors in cardiovascular disease. The Task Force on ACE-inhibitors of the European Society of Cardiology. Eur Heart J 2004; 25(16):1454–1470. doi:10.1016/j.ehj.2004.06.003
- National Institute for Health and Care Excellence (NICE). Myocardial infarction: cardiac rehabilitation and prevention of further cardiovascular disease. https://www.nice.org.uk/Guidance/CG172. Accessed April 3, 2019.
- National Institute for Health and Care Excellence (NICE). Acute kidney injury: prevention, detection and management. https://www.nice.org.uk/Guidance/CG169. Accessed August 12, 2019.
- Think Kidneys. “Sick day” guidance in patients at risk of acute kidney injury: a position statement from the Think Kidneys Board. https://www.thinkkidneys.nhs.uk/aki/wp-content/uploads/sites/2/2018/01/Think-Kidneys-Sick-Day-Guidance-2018.pdf. Accessed August 12, 2019.
- Meaney CJ, Beccari MV, Yang Y, Zhao J. Systematic review and meta-analysis of patiromer and sodium zirconium cyclosilicate: a new armamentarium for the treatment of hyperkalemia. Pharmacotherapy 2017; 37(4):401–411. doi:10.1002/phar.1906
KEY POINTS
- ACE inhibitors and ARBs reduce proteinuria by lowering the intraglomerular pressure, reducing hyperfiltration.
- These drugs tend to raise the serum potassium level and reduce the glomerular filtration rate (GFR). Monitoring the serum potassium and creatinine levels and the GFR is therefore imperative.
- Despite the benefits, concern for adverse effects including hyperkalemia and a rise in serum creatinine has led to reluctance to prescribe these drugs, and they are underused in the patients who may derive the greatest benefit.
Infective endocarditis: Beyond the usual tests
Prompt diagnois of infective endocarditis is critical. Potential consequences of missed or delayed diagnosis, including heart failure, stroke, intracardiac abscess, conduction delays, prosthesis dysfunction, and cerebral emboli, are often catastrophic. Echocardiography is the test used most frequently to evaluate for infective endocarditis, but it misses the diagnosis in almost one-third of cases, and even more often if the patient has a prosthetic valve.
But now, several sophisticated imaging tests are available that complement echocardiography in diagnosing and assessing infective endocarditis; these include 4-dimensional computed tomography (4D CT), fluorodeoxyglucose positron emission tomography (FDG-PET), and leukocyte scintigraphy. These tests have greatly improved our ability not only to diagnose infective endocarditis, but also to determine the extent and spread of infection, and they aid in perioperative assessment. Abnormal findings on these tests have been incorporated into the European Society of Cardiology’s 2015 modified diagnostic criteria for infective endocarditis.1
This article details the indications, advantages, and limitations of the various imaging tests for diagnosing and evaluating infective endocarditis (Table 1).
INFECTIVE ENDOCARDITIS IS DIFFICULT TO DIAGNOSE AND TREAT
Infective endocarditis is difficult to diagnose and treat. Clinical and imaging clues can be subtle, and the diagnosis requires a high level of suspicion and visualization of cardiac structures.
Further, the incidence of infective endocarditis is on the rise in the United States, particularly in women and young adults, likely due to intravenous drug use.2,3
ECHOCARDIOGRAPHY HAS AN IMPORTANT ROLE, BUT IS LIMITED
Echocardiography remains the most commonly performed study for diagnosing infective endocarditis, as it is fast, widely accessible, and less expensive than other imaging tests.
Transthoracic echocardiography (TTE) is often the first choice for testing. However, its sensitivity is only about 70% for detecting vegetations on native valves and 50% for detecting vegetations on prosthetic valves.1 It is inherently constrained by the limited number of views by which a comprehensive external evaluation of the heart can be achieved. Using a 2-dimensional instrument to view a 3-dimensional object is difficult, and depending on several factors, it can be hard to see vegetations and abscesses that are associated with infective endocarditis. Further, TTE is impeded by obesity and by hyperinflated lungs from obstructive pulmonary disease or mechanical ventilation. It has poor sensitivity for detecting small vegetations and for detecting vegetations and paravalvular complications in patients who have a prosthetic valve or a cardiac implanted electronic device.
Transesophageal echocardiography (TEE) is the recommended first-line imaging test for patients with prosthetic valves and no contraindications to the test. Otherwise, it should be done after TTE if the results of TTE are negative but clinical suspicion for infective endocarditis remains high (eg, because the patient uses intravenous drugs). But although TEE has a higher sensitivity than TTE (up to 96% for vegetations on native valves and 92% for those on prosthetic valves, if performed by an experienced sonographer), it can still miss infective endocarditis. Also, TEE does not provide a significant advantage over TTE in patients who have a cardiac implanted electronic device.1,4,5
Regardless of whether TTE or TEE is used, they are estimated to miss up to 30% of cases of infective endocarditis and its sequelae.4 False-negative findings are likelier in patients who have preexisting severe valvular lesions, prosthetic valves, cardiac implanted electronic devices, small vegetations, or abscesses, or if a vegetation has already broken free and embolized. Furthermore, distinguishing between vegetations and thrombi, cardiac tumors, and myxomatous changes using echocardiography is difficult.
CARDIAC CT
For patients who have inconclusive results on echocardiography, contraindications to TEE, or poor sonic windows, cardiac CT can be an excellent alternative. It is especially useful in the setting of a prosthetic valve.
Synchronized (“gated”) with the patient’s heart rate and rhythm, CT machines can acquire images during diastole, reducing motion artifact, and can create 3D images of the heart. In addition, newer machines can acquire several images at different points in the heart cycle to add a fourth dimension—time. The resulting 4D images play like short video loops of the beating heart and allow noninvasive assessment of cardiac anatomy with remarkable detail and resolution.
4D CT is increasingly being used in infective endocarditis, and growing evidence indicates that its accuracy is similar to that of TEE in the preoperative evaluation of patients with aortic prosthetic valve endocarditis.6 In a study of 28 patients, complementary use of CT angiography led to a change in treatment strategy in 7 (25%) compared with routine clinical workup.7 Several studies have found no difference between 4D CT and preoperative TEE in detecting pseudoaneurysm, abscess, or valve dehiscence. TEE and 4D CT also have similar sensitivities for detecting infective endocarditis in native and prosthetic valves.8,9
Coupled with CT angiography, 4D CT is also an excellent noninvasive way to perioperatively evaluate the coronary arteries without the risks associated with catheterization in those requiring nonemergency surgery (Figure 1A, B, and C).
4D CT performs well for detecting abscess and pseudoaneurysm but has slightly lower sensitivity for vegetations than TEE (91% vs 99%).9
Gated CT, PET, or both may be useful in cases of suspected prosthetic aortic valve endocarditis when TEE is negative. Pseudoaneurysms are not well visualized with TEE, and the atrial mitral curtain area is often thickened on TEE in cases of aortic prosthetic valve infective endocarditis that do not definitely involve abscesses. Gated CT and PET show this area better.8 This information is important in cases in which a surgeon may be unconvinced that the patient has prosthetic valve endocarditis.
Limitations of 4D cardiac CT
4D CT with or without angiography has limitations. It requires a wide-volume scanner and an experienced reader.
Patients with irregular heart rhythms or uncontrolled tachycardia pose technical problems for image acquisition. Cardiac CT is typically gated (ie, images are obtained within a defined time period) to acquire images during diastole. Ideally, images are acquired when the heart is in mid to late diastole, a time of minimal cardiac motion, so that motion artifact is minimized. To estimate the timing of image acquisition, the cardiac cycle must be predictable, and its duration should be as long as possible. Tachycardia or irregular rhythms such as frequent ectopic beats or atrial fibrillation make acquisition timing difficult, and thus make it nearly impossible to accurately obtain images when the heart is at minimum motion, limiting assessment of cardiac structures or the coronary tree.4,10
Extensive coronary calcification can hinder assessment of the coronary tree by CT coronary angiography.
Contrast exposure may limit the use of CT in some patients (eg, those with contrast allergies or renal dysfunction). However, modern scanners allow for much smaller contrast boluses without decreasing sensitivity.
4D CT involves radiation exposure, especially when done with angiography, although modern scanners have greatly reduced exposure. The average radiation dose in CT coronary angiography is 2.9 to 5.9 mSv11 compared with 7 mSv in diagnostic cardiac catheterization (without angioplasty or stenting) or 16 mSv in routine CT of the abdomen and pelvis with contrast.12,13 In view of the morbidity and mortality risks associated with infective endocarditis, especially if the diagnosis is delayed, this small radiation exposure may be justifiable.
Bottom line for cardiac CT
4D CT is an excellent alternative to echocardiography for select patients. Clinicians should strongly consider this study in the following situations:
- Patients with a prosthetic valve
- Patients who are strongly suspected of having infective endocarditis but who have a poor sonic window on TTE or TEE, as can occur with chronic obstructive lung disease, morbid obesity, or previous thoracic or cardiovascular surgery
- Patients who meet clinical indications for TEE, such as having a prosthetic valve or a high suspicion for native valve infective endocarditis with negative TTE, but who have contraindications to TEE
- As an alternative to TEE for preoperative evaluation in patients with known infective endocarditis.
Patients with tachycardia or irregular heart rhythms are not good candidates for this test.
FDG-PET AND LEUKOCYTE SCINTIGRAPHY
FDG-PET and leukocyte scintigraphy are other options for diagnosing infective endocarditis and determining the presence and extent of intra- and extracardiac infection. They are more sensitive than echocardiography for detecting infection of cardiac implanted electronic devices such as ventricular assist devices, pacemakers, implanted cardiac defibrillators, and cardiac resynchronization therapy devices.14–16
The utility of FDG-PET is founded on the uptake of 18F-fluorodeoxyglucose by cells, with higher uptake taking place in cells with higher metabolic activity (such as in areas of inflammation). Similarly, leukocyte scintigraphy relies on the use of radiolabeled leukocytes (ie, leukocytes previously extracted from the patient, labelled, and re-introduced into the patient) to allow for localization of inflamed tissue.
The most significant contribution of FDG-PET may be the ability to detect infective endocarditis early, when echocardiography is initially negative. When abnormal FDG uptake was included in the modified Duke criteria, it increased the sensitivity to 97% for detecting infective endocarditis on admission, leading some to propose its incorporation as a major criterion.17 In patients with prosthetic valves and suspected infective endocarditis, FDG-PET was found in one study to have a sensitivity of up to 91% and a specificity of up to 95%.18
Both FDG-PET and leukocyte scintigraphy have a high sensitivity, specificity, and negative predictive value for cardiac implanted electronic device infection, and should be strongly considered in patients in whom it is suspected but who have negative or inconclusive findings on echocardiography.14,15
In addition, a common conundrum faced by clinicians with use of echocardiography is the difficulty of differentiating thrombus from infected vegetation on valves or device lead wires. Some evidence indicates that FDG-PET may help to discriminate between vegetation and thrombus, although more rigorous studies are needed before its use for that purpose can be recommended.19
Limitations of nuclear studies
Both FDG-PET and leukocyte scintigraphy perform poorly for detecting native-valve infective endocarditis. In a study in which 90% of the patients had native-valve infective endocarditis according to the Duke criteria, FDG-PET had a specificity of 93% but a sensitivity of only 39%.20
Both studies can be cumbersome, laborious, and time-consuming for patients. FDG-PET requires a fasting or glucose-restricted diet before testing, and the test itself can be complicated by development of hyperglycemia, although this is rare.
While FDG-PET is most effective in detecting infections of prosthetic valves and cardiac implanted electronic devices, the results can be falsely positive in patients with a history of recent cardiac surgery (due to ongoing tissue healing), as well as maladies other than infective endocarditis that lead to inflammation, such as vasculitis or malignancy. Similarly, for unclear reasons, leukocyte scintigraphy can yield false-negative results in patients with enterococcal or candidal infective endocarditis.21
FDG-PET and leukocyte scintigraphy are more expensive than TEE and cardiac CT22 and are not widely available.
Both tests entail radiation exposure, with the average dose ranging from 7 to 14 mSv. However, this is less than the average amount acquired during percutaneous coronary intervention (16 mSv), and overlaps with the amount in chest CT with contrast when assessing for pulmonary embolism (7 to 9 mSv). Lower doses are possible with optimized protocols.12,13,15,23
Bottom line for nuclear studies
FDG-PET and leukocyte scintigraphy are especially useful for patients with a prosthetic valve or cardiac implanted electronic device. However, limitations must be kept in mind.
A suggested algorithm for testing with nuclear imaging is shown in Figure 2.1,4
CEREBRAL MAGNETIC RESONANCE IMAGING
Cerebral magnetic resonance imaging (MRI) is more sensitive than cerebral CT for detecting emboli in the brain. According to American Heart Association guidelines, cerebral MRI should be done in patients with known or suspected infective endocarditis and neurologic impairment, defined as headaches, meningeal symptoms, or neurologic deficits. It is also often used in neurologically asymptomatic patients with infective endocarditis who have indications for valve surgery to assess for mycotic aneurysms, which are associated with increased intracranial bleeding during surgery.
MRI use in other asymptomatic patients remains controversial.24 In cases with high clinical suspicion for infective endocarditis and no findings on echocardiography, cerebral MRI can increase the sensitivity of the Duke criteria by adding a minor criterion. Some have argued that, in patients with definite infective endocarditis, detecting silent cerebral complications can lead to management changes. However, more studies are needed to determine if there is indeed a group of neurologically asymptomatic infective endocarditis patients for whom cerebral MRI leads to improved outcomes.
Limitations of cerebral MRI
Cerebral MRI cannot be used in patients with non-MRI-compatible implanted hardware.
Gadolinium, the contrast agent typically used, can cause nephrogenic systemic fibrosis in patients who have poor renal function. This rare but serious adverse effect is characterized by irreversible systemic fibrosis affecting skin, muscles, and even visceral tissue such as lungs. The American College of Radiology allows for gadolinium use in patients without acute kidney injury and patients with stable chronic kidney disease with a glomerular filtration rate of at least 30 mL/min/1.73 m2. Its use should be avoided in patients with renal failure on replacement therapy, with advanced chronic kidney disease (glomerular filtration rate < 30 mL/min/1.73 m2), or with acute kidney injury, even if they do not need renal replacement therapy.25
Concerns have also been raised about gadolinium retention in the brain, even in patients with normal renal function.26–28 Thus far, no conclusive clinical adverse effects of retention have been found, although more study is warranted. Nevertheless, the US Food and Drug Administration now requires a black-box warning about this possibility and advises clinicians to counsel patients appropriately.
Bottom line on cerebral MRI
Cerebral MRI should be obtained when a patient presents with definite or possible infective endocarditis with neurologic impairment, such as new headaches, meningismus, or focal neurologic deficits. Routine brain MRI in patients with confirmed infective endocarditis without neurologic symptoms, or those without definite infective endocarditis, is discouraged.
CARDIAC MRI
Cardiac MRI, typically obtained with gadolinium contrast, allows for better 3D assessment of cardiac structures and morphology than echocardiography or CT, and can detect infiltrative cardiac disease, myopericarditis, and much more. It is increasingly used in the field of structural cardiology, but its role for evaluating infective endocarditis remains unclear.
Cardiac MRI does not appear to be better than echocardiography for diagnosing infective endocarditis. However, it may prove helpful in the evaluation of patients known to have infective endocarditis but who cannot be properly evaluated for disease extent because of poor image quality on echocardiography and contraindications to CT.1,29 Its role is limited in patients with cardiac implanted electronic devices, as most devices are incompatible with MRI use, although newer devices obviate this concern. But even for devices that are MRI-compatible, results are diminished due to an eclipsing effect, wherein the device parts can make it hard to see structures clearly because the “brightness” basically eclipses the surrounding area.4
Concerns regarding use of gadolinium as described above need also be considered.
The role of cardiac MRI in diagnosing and managing infective endocarditis may evolve, but at present, the 2017 American College of Cardiology and American Heart Association appropriate-use criteria discourage its use for these purposes.16
Bottom line for cardiac MRI
Cardiac MRI to evaluate a patient for suspected infective endocarditis is not recommended due to lack of superiority compared with echocardiography or CT, and the risk of nephrogenic systemic fibrosis from gadolinium in patients with renal compromise.
- Habib G, Lancellotti P, Antunes MJ, et al; ESC Scientific Document Group. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36(44):3075–3128. doi:10.1093/eurheartj/ehv319
- Durante-Mangoni E, Bradley S, Selton-Suty C, et al; International Collaboration on Endocarditis Prospective Cohort Study Group. Current features of infective endocarditis in elderly patients: results of the International Collaboration on Endocarditis Prospective Cohort Study. Arch Intern Med 2008; 168(19):2095–2103. doi:10.1001/archinte.168.19.2095
- Wurcel AG, Anderson JE, Chui KK, et al. Increasing infectious endocarditis admissions among young people who inject drugs. Open Forum Infect Dis 2016; 3(3):ofw157. doi:10.1093/ofid/ofw157
- Gomes A, Glaudemans AW, Touw DJ, et al. Diagnostic value of imaging in infective endocarditis: a systematic review. Lancet Infect Dis 2017; 17(1):e1–e14. doi:10.1016/S1473-3099(16)30141-4
- Cahill TJ, Baddour LM, Habib G, et al. Challenges in infective endocarditis. J Am Coll Cardiol 2017; 69(3):325–344. doi:10.1016/j.jacc.2016.10.066
- Fagman E, Perrotta S, Bech-Hanssen O, et al. ECG-gated computed tomography: a new role for patients with suspected aortic prosthetic valve endocarditis. Eur Radiol 2012; 22(11):2407–2414. doi:10.1007/s00330-012-2491-5
- Habets J, Tanis W, van Herwerden LA, et al. Cardiac computed tomography angiography results in diagnostic and therapeutic change in prosthetic heart valve endocarditis. Int J Cardiovasc Imaging 2014; 30(2):377–387. doi:10.1007/s10554-013-0335-2
- Koneru S, Huang SS, Oldan J, et al. Role of preoperative cardiac CT in the evaluation of infective endocarditis: comparison with transesophageal echocardiography and surgical findings. Cardiovasc Diagn Ther 2018; 8(4):439–449. doi:10.21037/cdt.2018.07.07
- Koo HJ, Yang DH, Kang J, et al. Demonstration of infective endocarditis by cardiac CT and transoesophageal echocardiography: comparison with intra-operative findings. Eur Heart J Cardiovasc Imaging 2018; 19(2):199–207. doi:10.1093/ehjci/jex010
- Feuchtner GM, Stolzmann P, Dichtl W, et al. Multislice computed tomography in infective endocarditis: comparison with transesophageal echocardiography and intraoperative findings. J Am Coll Cardiol 2009; 53(5):436–444. doi:10.1016/j.jacc.2008.01.077
- Castellano IA, Nicol ED, Bull RK, Roobottom CA, Williams MC, Harden SP. A prospective national survey of coronary CT angiography radiation doses in the United Kingdom. J Cardiovasc Comput Tomogr 2017; 11(4):268–273. doi:10.1016/j.jcct.2017.05.002
- Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology 2008; 248(1):254–263. doi:10.1148/radiol.2481071451
- Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med 2009; 169(22):2078–2086. doi:10.1001/archinternmed.2009.427
- Ploux S, Riviere A, Amraoui S, et al. Positron emission tomography in patients with suspected pacing system infections may play a critical role in difficult cases. Heart Rhythm 2011; 8(9):1478–1481. doi:10.1016/j.hrthm.2011.03.062
- Sarrazin J, Philippon F, Tessier M, et al. Usefulness of fluorine-18 positron emission tomography/computed tomography for identification of cardiovascular implantable electronic device infections. J Am Coll Cardiol 2012; 59(18):1616–1625. doi:10.1016/j.jacc.2011.11.059
- Doherty JU, Kort S, Mehran R, Schoenhagen P, Soman P; Rating Panel Members; Appropriate Use Criteria Task Force. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2017 Appropriate use criteria for multimodality imaging in valvular heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Nucl Cardiol 2017; 24(6):2043–2063. doi:10.1007/s12350-017-1070-1
- Saby L, Laas O, Habib G, et al. Positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditis: increased valvular 18F-fluorodeoxyglucose uptake as a novel major criterion. J Am Coll Cardiol 2013; 61(23):2374–2382. doi:10.1016/j.jacc.2013.01.092
- Swart LE, Gomes A, Scholtens AM, et al. Improving the diagnostic performance of 18F-fluorodeoxyglucose positron-emission tomography/computed tomography in prosthetic heart valve endocarditis. Circulation 2018; 138(14):1412–1427. doi:10.1161/CIRCULATIONAHA.118.035032
- Graziosi M, Nanni C, Lorenzini M, et al. Role of 18F-FDG PET/CT in the diagnosis of infective endocarditis in patients with an implanted cardiac device: a prospective study. Eur J Nucl Med Mol Imaging 2014; 41(8):1617–1623. doi:10.1007/s00259-014-2773-z
- Kouijzer IJ, Vos FJ, Janssen MJ, van Dijk AP, Oyen WJ, Bleeker-Rovers CP. The value of 18F-FDG PET/CT in diagnosing infectious endocarditis. Eur J Nucl Med Mol Imaging 2013; 40(7):1102–1107. doi:10.1007/s00259-013-2376-0
- Wong D, Rubinshtein R, Keynan Y. Alternative cardiac imaging modalities to echocardiography for the diagnosis of infective endocarditis. Am J Cardiol 2016; 118(9):1410–1418. doi:10.1016/j.amjcard.2016.07.053
- Vos FJ, Bleeker-Rovers CP, Kullberg BJ, Adang EM, Oyen WJ. Cost-effectiveness of routine (18)F-FDG PET/CT in high-risk patients with gram-positive bacteremia. J Nucl Med 2011; 52(11):1673–1678. doi:10.2967/jnumed.111.089714
- McCollough CH, Bushberg JT, Fletcher JG, Eckel LJ. Answers to common questions about the use and safety of CT scans. Mayo Clin Proc 2015; 90(10):1380–1392. doi:10.1016/j.mayocp.2015.07.011
- Duval X, Iung B, Klein I, et al; IMAGE (Resonance Magnetic Imaging at the Acute Phase of Endocarditis) Study Group. Effect of early cerebral magnetic resonance imaging on clinical decisions in infective endocarditis: a prospective study. Ann Intern Med 2010; 152(8):497–504, W175. doi:10.7326/0003-4819-152-8-201004200-00006
- ACR Committee on Drugs and Contrast Media. ACR Manual on Contrast Media: 2018. www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf. Accessed July 19, 2019.
- Kanda T, Fukusato T, Matsuda M, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 2015; 276(1):228–232. doi:10.1148/radiol.2015142690
- McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 2015; 275(3):772–782. doi:10.1148/radiol.15150025
- Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014; 270(3):834–841. doi:10.1148/radiol.13131669
- Expert Panel on Pediatric Imaging; Hayes LL, Palasis S, Bartel TB, et al. ACR appropriateness criteria headache-child. J Am Coll Radiol 2018; 15(5S):S78–S90. doi:10.1016/j.jacr.2018.03.017
Prompt diagnois of infective endocarditis is critical. Potential consequences of missed or delayed diagnosis, including heart failure, stroke, intracardiac abscess, conduction delays, prosthesis dysfunction, and cerebral emboli, are often catastrophic. Echocardiography is the test used most frequently to evaluate for infective endocarditis, but it misses the diagnosis in almost one-third of cases, and even more often if the patient has a prosthetic valve.
But now, several sophisticated imaging tests are available that complement echocardiography in diagnosing and assessing infective endocarditis; these include 4-dimensional computed tomography (4D CT), fluorodeoxyglucose positron emission tomography (FDG-PET), and leukocyte scintigraphy. These tests have greatly improved our ability not only to diagnose infective endocarditis, but also to determine the extent and spread of infection, and they aid in perioperative assessment. Abnormal findings on these tests have been incorporated into the European Society of Cardiology’s 2015 modified diagnostic criteria for infective endocarditis.1
This article details the indications, advantages, and limitations of the various imaging tests for diagnosing and evaluating infective endocarditis (Table 1).
INFECTIVE ENDOCARDITIS IS DIFFICULT TO DIAGNOSE AND TREAT
Infective endocarditis is difficult to diagnose and treat. Clinical and imaging clues can be subtle, and the diagnosis requires a high level of suspicion and visualization of cardiac structures.
Further, the incidence of infective endocarditis is on the rise in the United States, particularly in women and young adults, likely due to intravenous drug use.2,3
ECHOCARDIOGRAPHY HAS AN IMPORTANT ROLE, BUT IS LIMITED
Echocardiography remains the most commonly performed study for diagnosing infective endocarditis, as it is fast, widely accessible, and less expensive than other imaging tests.
Transthoracic echocardiography (TTE) is often the first choice for testing. However, its sensitivity is only about 70% for detecting vegetations on native valves and 50% for detecting vegetations on prosthetic valves.1 It is inherently constrained by the limited number of views by which a comprehensive external evaluation of the heart can be achieved. Using a 2-dimensional instrument to view a 3-dimensional object is difficult, and depending on several factors, it can be hard to see vegetations and abscesses that are associated with infective endocarditis. Further, TTE is impeded by obesity and by hyperinflated lungs from obstructive pulmonary disease or mechanical ventilation. It has poor sensitivity for detecting small vegetations and for detecting vegetations and paravalvular complications in patients who have a prosthetic valve or a cardiac implanted electronic device.
Transesophageal echocardiography (TEE) is the recommended first-line imaging test for patients with prosthetic valves and no contraindications to the test. Otherwise, it should be done after TTE if the results of TTE are negative but clinical suspicion for infective endocarditis remains high (eg, because the patient uses intravenous drugs). But although TEE has a higher sensitivity than TTE (up to 96% for vegetations on native valves and 92% for those on prosthetic valves, if performed by an experienced sonographer), it can still miss infective endocarditis. Also, TEE does not provide a significant advantage over TTE in patients who have a cardiac implanted electronic device.1,4,5
Regardless of whether TTE or TEE is used, they are estimated to miss up to 30% of cases of infective endocarditis and its sequelae.4 False-negative findings are likelier in patients who have preexisting severe valvular lesions, prosthetic valves, cardiac implanted electronic devices, small vegetations, or abscesses, or if a vegetation has already broken free and embolized. Furthermore, distinguishing between vegetations and thrombi, cardiac tumors, and myxomatous changes using echocardiography is difficult.
CARDIAC CT
For patients who have inconclusive results on echocardiography, contraindications to TEE, or poor sonic windows, cardiac CT can be an excellent alternative. It is especially useful in the setting of a prosthetic valve.
Synchronized (“gated”) with the patient’s heart rate and rhythm, CT machines can acquire images during diastole, reducing motion artifact, and can create 3D images of the heart. In addition, newer machines can acquire several images at different points in the heart cycle to add a fourth dimension—time. The resulting 4D images play like short video loops of the beating heart and allow noninvasive assessment of cardiac anatomy with remarkable detail and resolution.
4D CT is increasingly being used in infective endocarditis, and growing evidence indicates that its accuracy is similar to that of TEE in the preoperative evaluation of patients with aortic prosthetic valve endocarditis.6 In a study of 28 patients, complementary use of CT angiography led to a change in treatment strategy in 7 (25%) compared with routine clinical workup.7 Several studies have found no difference between 4D CT and preoperative TEE in detecting pseudoaneurysm, abscess, or valve dehiscence. TEE and 4D CT also have similar sensitivities for detecting infective endocarditis in native and prosthetic valves.8,9
Coupled with CT angiography, 4D CT is also an excellent noninvasive way to perioperatively evaluate the coronary arteries without the risks associated with catheterization in those requiring nonemergency surgery (Figure 1A, B, and C).
4D CT performs well for detecting abscess and pseudoaneurysm but has slightly lower sensitivity for vegetations than TEE (91% vs 99%).9
Gated CT, PET, or both may be useful in cases of suspected prosthetic aortic valve endocarditis when TEE is negative. Pseudoaneurysms are not well visualized with TEE, and the atrial mitral curtain area is often thickened on TEE in cases of aortic prosthetic valve infective endocarditis that do not definitely involve abscesses. Gated CT and PET show this area better.8 This information is important in cases in which a surgeon may be unconvinced that the patient has prosthetic valve endocarditis.
Limitations of 4D cardiac CT
4D CT with or without angiography has limitations. It requires a wide-volume scanner and an experienced reader.
Patients with irregular heart rhythms or uncontrolled tachycardia pose technical problems for image acquisition. Cardiac CT is typically gated (ie, images are obtained within a defined time period) to acquire images during diastole. Ideally, images are acquired when the heart is in mid to late diastole, a time of minimal cardiac motion, so that motion artifact is minimized. To estimate the timing of image acquisition, the cardiac cycle must be predictable, and its duration should be as long as possible. Tachycardia or irregular rhythms such as frequent ectopic beats or atrial fibrillation make acquisition timing difficult, and thus make it nearly impossible to accurately obtain images when the heart is at minimum motion, limiting assessment of cardiac structures or the coronary tree.4,10
Extensive coronary calcification can hinder assessment of the coronary tree by CT coronary angiography.
Contrast exposure may limit the use of CT in some patients (eg, those with contrast allergies or renal dysfunction). However, modern scanners allow for much smaller contrast boluses without decreasing sensitivity.
4D CT involves radiation exposure, especially when done with angiography, although modern scanners have greatly reduced exposure. The average radiation dose in CT coronary angiography is 2.9 to 5.9 mSv11 compared with 7 mSv in diagnostic cardiac catheterization (without angioplasty or stenting) or 16 mSv in routine CT of the abdomen and pelvis with contrast.12,13 In view of the morbidity and mortality risks associated with infective endocarditis, especially if the diagnosis is delayed, this small radiation exposure may be justifiable.
Bottom line for cardiac CT
4D CT is an excellent alternative to echocardiography for select patients. Clinicians should strongly consider this study in the following situations:
- Patients with a prosthetic valve
- Patients who are strongly suspected of having infective endocarditis but who have a poor sonic window on TTE or TEE, as can occur with chronic obstructive lung disease, morbid obesity, or previous thoracic or cardiovascular surgery
- Patients who meet clinical indications for TEE, such as having a prosthetic valve or a high suspicion for native valve infective endocarditis with negative TTE, but who have contraindications to TEE
- As an alternative to TEE for preoperative evaluation in patients with known infective endocarditis.
Patients with tachycardia or irregular heart rhythms are not good candidates for this test.
FDG-PET AND LEUKOCYTE SCINTIGRAPHY
FDG-PET and leukocyte scintigraphy are other options for diagnosing infective endocarditis and determining the presence and extent of intra- and extracardiac infection. They are more sensitive than echocardiography for detecting infection of cardiac implanted electronic devices such as ventricular assist devices, pacemakers, implanted cardiac defibrillators, and cardiac resynchronization therapy devices.14–16
The utility of FDG-PET is founded on the uptake of 18F-fluorodeoxyglucose by cells, with higher uptake taking place in cells with higher metabolic activity (such as in areas of inflammation). Similarly, leukocyte scintigraphy relies on the use of radiolabeled leukocytes (ie, leukocytes previously extracted from the patient, labelled, and re-introduced into the patient) to allow for localization of inflamed tissue.
The most significant contribution of FDG-PET may be the ability to detect infective endocarditis early, when echocardiography is initially negative. When abnormal FDG uptake was included in the modified Duke criteria, it increased the sensitivity to 97% for detecting infective endocarditis on admission, leading some to propose its incorporation as a major criterion.17 In patients with prosthetic valves and suspected infective endocarditis, FDG-PET was found in one study to have a sensitivity of up to 91% and a specificity of up to 95%.18
Both FDG-PET and leukocyte scintigraphy have a high sensitivity, specificity, and negative predictive value for cardiac implanted electronic device infection, and should be strongly considered in patients in whom it is suspected but who have negative or inconclusive findings on echocardiography.14,15
In addition, a common conundrum faced by clinicians with use of echocardiography is the difficulty of differentiating thrombus from infected vegetation on valves or device lead wires. Some evidence indicates that FDG-PET may help to discriminate between vegetation and thrombus, although more rigorous studies are needed before its use for that purpose can be recommended.19
Limitations of nuclear studies
Both FDG-PET and leukocyte scintigraphy perform poorly for detecting native-valve infective endocarditis. In a study in which 90% of the patients had native-valve infective endocarditis according to the Duke criteria, FDG-PET had a specificity of 93% but a sensitivity of only 39%.20
Both studies can be cumbersome, laborious, and time-consuming for patients. FDG-PET requires a fasting or glucose-restricted diet before testing, and the test itself can be complicated by development of hyperglycemia, although this is rare.
While FDG-PET is most effective in detecting infections of prosthetic valves and cardiac implanted electronic devices, the results can be falsely positive in patients with a history of recent cardiac surgery (due to ongoing tissue healing), as well as maladies other than infective endocarditis that lead to inflammation, such as vasculitis or malignancy. Similarly, for unclear reasons, leukocyte scintigraphy can yield false-negative results in patients with enterococcal or candidal infective endocarditis.21
FDG-PET and leukocyte scintigraphy are more expensive than TEE and cardiac CT22 and are not widely available.
Both tests entail radiation exposure, with the average dose ranging from 7 to 14 mSv. However, this is less than the average amount acquired during percutaneous coronary intervention (16 mSv), and overlaps with the amount in chest CT with contrast when assessing for pulmonary embolism (7 to 9 mSv). Lower doses are possible with optimized protocols.12,13,15,23
Bottom line for nuclear studies
FDG-PET and leukocyte scintigraphy are especially useful for patients with a prosthetic valve or cardiac implanted electronic device. However, limitations must be kept in mind.
A suggested algorithm for testing with nuclear imaging is shown in Figure 2.1,4
CEREBRAL MAGNETIC RESONANCE IMAGING
Cerebral magnetic resonance imaging (MRI) is more sensitive than cerebral CT for detecting emboli in the brain. According to American Heart Association guidelines, cerebral MRI should be done in patients with known or suspected infective endocarditis and neurologic impairment, defined as headaches, meningeal symptoms, or neurologic deficits. It is also often used in neurologically asymptomatic patients with infective endocarditis who have indications for valve surgery to assess for mycotic aneurysms, which are associated with increased intracranial bleeding during surgery.
MRI use in other asymptomatic patients remains controversial.24 In cases with high clinical suspicion for infective endocarditis and no findings on echocardiography, cerebral MRI can increase the sensitivity of the Duke criteria by adding a minor criterion. Some have argued that, in patients with definite infective endocarditis, detecting silent cerebral complications can lead to management changes. However, more studies are needed to determine if there is indeed a group of neurologically asymptomatic infective endocarditis patients for whom cerebral MRI leads to improved outcomes.
Limitations of cerebral MRI
Cerebral MRI cannot be used in patients with non-MRI-compatible implanted hardware.
Gadolinium, the contrast agent typically used, can cause nephrogenic systemic fibrosis in patients who have poor renal function. This rare but serious adverse effect is characterized by irreversible systemic fibrosis affecting skin, muscles, and even visceral tissue such as lungs. The American College of Radiology allows for gadolinium use in patients without acute kidney injury and patients with stable chronic kidney disease with a glomerular filtration rate of at least 30 mL/min/1.73 m2. Its use should be avoided in patients with renal failure on replacement therapy, with advanced chronic kidney disease (glomerular filtration rate < 30 mL/min/1.73 m2), or with acute kidney injury, even if they do not need renal replacement therapy.25
Concerns have also been raised about gadolinium retention in the brain, even in patients with normal renal function.26–28 Thus far, no conclusive clinical adverse effects of retention have been found, although more study is warranted. Nevertheless, the US Food and Drug Administration now requires a black-box warning about this possibility and advises clinicians to counsel patients appropriately.
Bottom line on cerebral MRI
Cerebral MRI should be obtained when a patient presents with definite or possible infective endocarditis with neurologic impairment, such as new headaches, meningismus, or focal neurologic deficits. Routine brain MRI in patients with confirmed infective endocarditis without neurologic symptoms, or those without definite infective endocarditis, is discouraged.
CARDIAC MRI
Cardiac MRI, typically obtained with gadolinium contrast, allows for better 3D assessment of cardiac structures and morphology than echocardiography or CT, and can detect infiltrative cardiac disease, myopericarditis, and much more. It is increasingly used in the field of structural cardiology, but its role for evaluating infective endocarditis remains unclear.
Cardiac MRI does not appear to be better than echocardiography for diagnosing infective endocarditis. However, it may prove helpful in the evaluation of patients known to have infective endocarditis but who cannot be properly evaluated for disease extent because of poor image quality on echocardiography and contraindications to CT.1,29 Its role is limited in patients with cardiac implanted electronic devices, as most devices are incompatible with MRI use, although newer devices obviate this concern. But even for devices that are MRI-compatible, results are diminished due to an eclipsing effect, wherein the device parts can make it hard to see structures clearly because the “brightness” basically eclipses the surrounding area.4
Concerns regarding use of gadolinium as described above need also be considered.
The role of cardiac MRI in diagnosing and managing infective endocarditis may evolve, but at present, the 2017 American College of Cardiology and American Heart Association appropriate-use criteria discourage its use for these purposes.16
Bottom line for cardiac MRI
Cardiac MRI to evaluate a patient for suspected infective endocarditis is not recommended due to lack of superiority compared with echocardiography or CT, and the risk of nephrogenic systemic fibrosis from gadolinium in patients with renal compromise.
Prompt diagnois of infective endocarditis is critical. Potential consequences of missed or delayed diagnosis, including heart failure, stroke, intracardiac abscess, conduction delays, prosthesis dysfunction, and cerebral emboli, are often catastrophic. Echocardiography is the test used most frequently to evaluate for infective endocarditis, but it misses the diagnosis in almost one-third of cases, and even more often if the patient has a prosthetic valve.
But now, several sophisticated imaging tests are available that complement echocardiography in diagnosing and assessing infective endocarditis; these include 4-dimensional computed tomography (4D CT), fluorodeoxyglucose positron emission tomography (FDG-PET), and leukocyte scintigraphy. These tests have greatly improved our ability not only to diagnose infective endocarditis, but also to determine the extent and spread of infection, and they aid in perioperative assessment. Abnormal findings on these tests have been incorporated into the European Society of Cardiology’s 2015 modified diagnostic criteria for infective endocarditis.1
This article details the indications, advantages, and limitations of the various imaging tests for diagnosing and evaluating infective endocarditis (Table 1).
INFECTIVE ENDOCARDITIS IS DIFFICULT TO DIAGNOSE AND TREAT
Infective endocarditis is difficult to diagnose and treat. Clinical and imaging clues can be subtle, and the diagnosis requires a high level of suspicion and visualization of cardiac structures.
Further, the incidence of infective endocarditis is on the rise in the United States, particularly in women and young adults, likely due to intravenous drug use.2,3
ECHOCARDIOGRAPHY HAS AN IMPORTANT ROLE, BUT IS LIMITED
Echocardiography remains the most commonly performed study for diagnosing infective endocarditis, as it is fast, widely accessible, and less expensive than other imaging tests.
Transthoracic echocardiography (TTE) is often the first choice for testing. However, its sensitivity is only about 70% for detecting vegetations on native valves and 50% for detecting vegetations on prosthetic valves.1 It is inherently constrained by the limited number of views by which a comprehensive external evaluation of the heart can be achieved. Using a 2-dimensional instrument to view a 3-dimensional object is difficult, and depending on several factors, it can be hard to see vegetations and abscesses that are associated with infective endocarditis. Further, TTE is impeded by obesity and by hyperinflated lungs from obstructive pulmonary disease or mechanical ventilation. It has poor sensitivity for detecting small vegetations and for detecting vegetations and paravalvular complications in patients who have a prosthetic valve or a cardiac implanted electronic device.
Transesophageal echocardiography (TEE) is the recommended first-line imaging test for patients with prosthetic valves and no contraindications to the test. Otherwise, it should be done after TTE if the results of TTE are negative but clinical suspicion for infective endocarditis remains high (eg, because the patient uses intravenous drugs). But although TEE has a higher sensitivity than TTE (up to 96% for vegetations on native valves and 92% for those on prosthetic valves, if performed by an experienced sonographer), it can still miss infective endocarditis. Also, TEE does not provide a significant advantage over TTE in patients who have a cardiac implanted electronic device.1,4,5
Regardless of whether TTE or TEE is used, they are estimated to miss up to 30% of cases of infective endocarditis and its sequelae.4 False-negative findings are likelier in patients who have preexisting severe valvular lesions, prosthetic valves, cardiac implanted electronic devices, small vegetations, or abscesses, or if a vegetation has already broken free and embolized. Furthermore, distinguishing between vegetations and thrombi, cardiac tumors, and myxomatous changes using echocardiography is difficult.
CARDIAC CT
For patients who have inconclusive results on echocardiography, contraindications to TEE, or poor sonic windows, cardiac CT can be an excellent alternative. It is especially useful in the setting of a prosthetic valve.
Synchronized (“gated”) with the patient’s heart rate and rhythm, CT machines can acquire images during diastole, reducing motion artifact, and can create 3D images of the heart. In addition, newer machines can acquire several images at different points in the heart cycle to add a fourth dimension—time. The resulting 4D images play like short video loops of the beating heart and allow noninvasive assessment of cardiac anatomy with remarkable detail and resolution.
4D CT is increasingly being used in infective endocarditis, and growing evidence indicates that its accuracy is similar to that of TEE in the preoperative evaluation of patients with aortic prosthetic valve endocarditis.6 In a study of 28 patients, complementary use of CT angiography led to a change in treatment strategy in 7 (25%) compared with routine clinical workup.7 Several studies have found no difference between 4D CT and preoperative TEE in detecting pseudoaneurysm, abscess, or valve dehiscence. TEE and 4D CT also have similar sensitivities for detecting infective endocarditis in native and prosthetic valves.8,9
Coupled with CT angiography, 4D CT is also an excellent noninvasive way to perioperatively evaluate the coronary arteries without the risks associated with catheterization in those requiring nonemergency surgery (Figure 1A, B, and C).
4D CT performs well for detecting abscess and pseudoaneurysm but has slightly lower sensitivity for vegetations than TEE (91% vs 99%).9
Gated CT, PET, or both may be useful in cases of suspected prosthetic aortic valve endocarditis when TEE is negative. Pseudoaneurysms are not well visualized with TEE, and the atrial mitral curtain area is often thickened on TEE in cases of aortic prosthetic valve infective endocarditis that do not definitely involve abscesses. Gated CT and PET show this area better.8 This information is important in cases in which a surgeon may be unconvinced that the patient has prosthetic valve endocarditis.
Limitations of 4D cardiac CT
4D CT with or without angiography has limitations. It requires a wide-volume scanner and an experienced reader.
Patients with irregular heart rhythms or uncontrolled tachycardia pose technical problems for image acquisition. Cardiac CT is typically gated (ie, images are obtained within a defined time period) to acquire images during diastole. Ideally, images are acquired when the heart is in mid to late diastole, a time of minimal cardiac motion, so that motion artifact is minimized. To estimate the timing of image acquisition, the cardiac cycle must be predictable, and its duration should be as long as possible. Tachycardia or irregular rhythms such as frequent ectopic beats or atrial fibrillation make acquisition timing difficult, and thus make it nearly impossible to accurately obtain images when the heart is at minimum motion, limiting assessment of cardiac structures or the coronary tree.4,10
Extensive coronary calcification can hinder assessment of the coronary tree by CT coronary angiography.
Contrast exposure may limit the use of CT in some patients (eg, those with contrast allergies or renal dysfunction). However, modern scanners allow for much smaller contrast boluses without decreasing sensitivity.
4D CT involves radiation exposure, especially when done with angiography, although modern scanners have greatly reduced exposure. The average radiation dose in CT coronary angiography is 2.9 to 5.9 mSv11 compared with 7 mSv in diagnostic cardiac catheterization (without angioplasty or stenting) or 16 mSv in routine CT of the abdomen and pelvis with contrast.12,13 In view of the morbidity and mortality risks associated with infective endocarditis, especially if the diagnosis is delayed, this small radiation exposure may be justifiable.
Bottom line for cardiac CT
4D CT is an excellent alternative to echocardiography for select patients. Clinicians should strongly consider this study in the following situations:
- Patients with a prosthetic valve
- Patients who are strongly suspected of having infective endocarditis but who have a poor sonic window on TTE or TEE, as can occur with chronic obstructive lung disease, morbid obesity, or previous thoracic or cardiovascular surgery
- Patients who meet clinical indications for TEE, such as having a prosthetic valve or a high suspicion for native valve infective endocarditis with negative TTE, but who have contraindications to TEE
- As an alternative to TEE for preoperative evaluation in patients with known infective endocarditis.
Patients with tachycardia or irregular heart rhythms are not good candidates for this test.
FDG-PET AND LEUKOCYTE SCINTIGRAPHY
FDG-PET and leukocyte scintigraphy are other options for diagnosing infective endocarditis and determining the presence and extent of intra- and extracardiac infection. They are more sensitive than echocardiography for detecting infection of cardiac implanted electronic devices such as ventricular assist devices, pacemakers, implanted cardiac defibrillators, and cardiac resynchronization therapy devices.14–16
The utility of FDG-PET is founded on the uptake of 18F-fluorodeoxyglucose by cells, with higher uptake taking place in cells with higher metabolic activity (such as in areas of inflammation). Similarly, leukocyte scintigraphy relies on the use of radiolabeled leukocytes (ie, leukocytes previously extracted from the patient, labelled, and re-introduced into the patient) to allow for localization of inflamed tissue.
The most significant contribution of FDG-PET may be the ability to detect infective endocarditis early, when echocardiography is initially negative. When abnormal FDG uptake was included in the modified Duke criteria, it increased the sensitivity to 97% for detecting infective endocarditis on admission, leading some to propose its incorporation as a major criterion.17 In patients with prosthetic valves and suspected infective endocarditis, FDG-PET was found in one study to have a sensitivity of up to 91% and a specificity of up to 95%.18
Both FDG-PET and leukocyte scintigraphy have a high sensitivity, specificity, and negative predictive value for cardiac implanted electronic device infection, and should be strongly considered in patients in whom it is suspected but who have negative or inconclusive findings on echocardiography.14,15
In addition, a common conundrum faced by clinicians with use of echocardiography is the difficulty of differentiating thrombus from infected vegetation on valves or device lead wires. Some evidence indicates that FDG-PET may help to discriminate between vegetation and thrombus, although more rigorous studies are needed before its use for that purpose can be recommended.19
Limitations of nuclear studies
Both FDG-PET and leukocyte scintigraphy perform poorly for detecting native-valve infective endocarditis. In a study in which 90% of the patients had native-valve infective endocarditis according to the Duke criteria, FDG-PET had a specificity of 93% but a sensitivity of only 39%.20
Both studies can be cumbersome, laborious, and time-consuming for patients. FDG-PET requires a fasting or glucose-restricted diet before testing, and the test itself can be complicated by development of hyperglycemia, although this is rare.
While FDG-PET is most effective in detecting infections of prosthetic valves and cardiac implanted electronic devices, the results can be falsely positive in patients with a history of recent cardiac surgery (due to ongoing tissue healing), as well as maladies other than infective endocarditis that lead to inflammation, such as vasculitis or malignancy. Similarly, for unclear reasons, leukocyte scintigraphy can yield false-negative results in patients with enterococcal or candidal infective endocarditis.21
FDG-PET and leukocyte scintigraphy are more expensive than TEE and cardiac CT22 and are not widely available.
Both tests entail radiation exposure, with the average dose ranging from 7 to 14 mSv. However, this is less than the average amount acquired during percutaneous coronary intervention (16 mSv), and overlaps with the amount in chest CT with contrast when assessing for pulmonary embolism (7 to 9 mSv). Lower doses are possible with optimized protocols.12,13,15,23
Bottom line for nuclear studies
FDG-PET and leukocyte scintigraphy are especially useful for patients with a prosthetic valve or cardiac implanted electronic device. However, limitations must be kept in mind.
A suggested algorithm for testing with nuclear imaging is shown in Figure 2.1,4
CEREBRAL MAGNETIC RESONANCE IMAGING
Cerebral magnetic resonance imaging (MRI) is more sensitive than cerebral CT for detecting emboli in the brain. According to American Heart Association guidelines, cerebral MRI should be done in patients with known or suspected infective endocarditis and neurologic impairment, defined as headaches, meningeal symptoms, or neurologic deficits. It is also often used in neurologically asymptomatic patients with infective endocarditis who have indications for valve surgery to assess for mycotic aneurysms, which are associated with increased intracranial bleeding during surgery.
MRI use in other asymptomatic patients remains controversial.24 In cases with high clinical suspicion for infective endocarditis and no findings on echocardiography, cerebral MRI can increase the sensitivity of the Duke criteria by adding a minor criterion. Some have argued that, in patients with definite infective endocarditis, detecting silent cerebral complications can lead to management changes. However, more studies are needed to determine if there is indeed a group of neurologically asymptomatic infective endocarditis patients for whom cerebral MRI leads to improved outcomes.
Limitations of cerebral MRI
Cerebral MRI cannot be used in patients with non-MRI-compatible implanted hardware.
Gadolinium, the contrast agent typically used, can cause nephrogenic systemic fibrosis in patients who have poor renal function. This rare but serious adverse effect is characterized by irreversible systemic fibrosis affecting skin, muscles, and even visceral tissue such as lungs. The American College of Radiology allows for gadolinium use in patients without acute kidney injury and patients with stable chronic kidney disease with a glomerular filtration rate of at least 30 mL/min/1.73 m2. Its use should be avoided in patients with renal failure on replacement therapy, with advanced chronic kidney disease (glomerular filtration rate < 30 mL/min/1.73 m2), or with acute kidney injury, even if they do not need renal replacement therapy.25
Concerns have also been raised about gadolinium retention in the brain, even in patients with normal renal function.26–28 Thus far, no conclusive clinical adverse effects of retention have been found, although more study is warranted. Nevertheless, the US Food and Drug Administration now requires a black-box warning about this possibility and advises clinicians to counsel patients appropriately.
Bottom line on cerebral MRI
Cerebral MRI should be obtained when a patient presents with definite or possible infective endocarditis with neurologic impairment, such as new headaches, meningismus, or focal neurologic deficits. Routine brain MRI in patients with confirmed infective endocarditis without neurologic symptoms, or those without definite infective endocarditis, is discouraged.
CARDIAC MRI
Cardiac MRI, typically obtained with gadolinium contrast, allows for better 3D assessment of cardiac structures and morphology than echocardiography or CT, and can detect infiltrative cardiac disease, myopericarditis, and much more. It is increasingly used in the field of structural cardiology, but its role for evaluating infective endocarditis remains unclear.
Cardiac MRI does not appear to be better than echocardiography for diagnosing infective endocarditis. However, it may prove helpful in the evaluation of patients known to have infective endocarditis but who cannot be properly evaluated for disease extent because of poor image quality on echocardiography and contraindications to CT.1,29 Its role is limited in patients with cardiac implanted electronic devices, as most devices are incompatible with MRI use, although newer devices obviate this concern. But even for devices that are MRI-compatible, results are diminished due to an eclipsing effect, wherein the device parts can make it hard to see structures clearly because the “brightness” basically eclipses the surrounding area.4
Concerns regarding use of gadolinium as described above need also be considered.
The role of cardiac MRI in diagnosing and managing infective endocarditis may evolve, but at present, the 2017 American College of Cardiology and American Heart Association appropriate-use criteria discourage its use for these purposes.16
Bottom line for cardiac MRI
Cardiac MRI to evaluate a patient for suspected infective endocarditis is not recommended due to lack of superiority compared with echocardiography or CT, and the risk of nephrogenic systemic fibrosis from gadolinium in patients with renal compromise.
- Habib G, Lancellotti P, Antunes MJ, et al; ESC Scientific Document Group. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36(44):3075–3128. doi:10.1093/eurheartj/ehv319
- Durante-Mangoni E, Bradley S, Selton-Suty C, et al; International Collaboration on Endocarditis Prospective Cohort Study Group. Current features of infective endocarditis in elderly patients: results of the International Collaboration on Endocarditis Prospective Cohort Study. Arch Intern Med 2008; 168(19):2095–2103. doi:10.1001/archinte.168.19.2095
- Wurcel AG, Anderson JE, Chui KK, et al. Increasing infectious endocarditis admissions among young people who inject drugs. Open Forum Infect Dis 2016; 3(3):ofw157. doi:10.1093/ofid/ofw157
- Gomes A, Glaudemans AW, Touw DJ, et al. Diagnostic value of imaging in infective endocarditis: a systematic review. Lancet Infect Dis 2017; 17(1):e1–e14. doi:10.1016/S1473-3099(16)30141-4
- Cahill TJ, Baddour LM, Habib G, et al. Challenges in infective endocarditis. J Am Coll Cardiol 2017; 69(3):325–344. doi:10.1016/j.jacc.2016.10.066
- Fagman E, Perrotta S, Bech-Hanssen O, et al. ECG-gated computed tomography: a new role for patients with suspected aortic prosthetic valve endocarditis. Eur Radiol 2012; 22(11):2407–2414. doi:10.1007/s00330-012-2491-5
- Habets J, Tanis W, van Herwerden LA, et al. Cardiac computed tomography angiography results in diagnostic and therapeutic change in prosthetic heart valve endocarditis. Int J Cardiovasc Imaging 2014; 30(2):377–387. doi:10.1007/s10554-013-0335-2
- Koneru S, Huang SS, Oldan J, et al. Role of preoperative cardiac CT in the evaluation of infective endocarditis: comparison with transesophageal echocardiography and surgical findings. Cardiovasc Diagn Ther 2018; 8(4):439–449. doi:10.21037/cdt.2018.07.07
- Koo HJ, Yang DH, Kang J, et al. Demonstration of infective endocarditis by cardiac CT and transoesophageal echocardiography: comparison with intra-operative findings. Eur Heart J Cardiovasc Imaging 2018; 19(2):199–207. doi:10.1093/ehjci/jex010
- Feuchtner GM, Stolzmann P, Dichtl W, et al. Multislice computed tomography in infective endocarditis: comparison with transesophageal echocardiography and intraoperative findings. J Am Coll Cardiol 2009; 53(5):436–444. doi:10.1016/j.jacc.2008.01.077
- Castellano IA, Nicol ED, Bull RK, Roobottom CA, Williams MC, Harden SP. A prospective national survey of coronary CT angiography radiation doses in the United Kingdom. J Cardiovasc Comput Tomogr 2017; 11(4):268–273. doi:10.1016/j.jcct.2017.05.002
- Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology 2008; 248(1):254–263. doi:10.1148/radiol.2481071451
- Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med 2009; 169(22):2078–2086. doi:10.1001/archinternmed.2009.427
- Ploux S, Riviere A, Amraoui S, et al. Positron emission tomography in patients with suspected pacing system infections may play a critical role in difficult cases. Heart Rhythm 2011; 8(9):1478–1481. doi:10.1016/j.hrthm.2011.03.062
- Sarrazin J, Philippon F, Tessier M, et al. Usefulness of fluorine-18 positron emission tomography/computed tomography for identification of cardiovascular implantable electronic device infections. J Am Coll Cardiol 2012; 59(18):1616–1625. doi:10.1016/j.jacc.2011.11.059
- Doherty JU, Kort S, Mehran R, Schoenhagen P, Soman P; Rating Panel Members; Appropriate Use Criteria Task Force. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2017 Appropriate use criteria for multimodality imaging in valvular heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Nucl Cardiol 2017; 24(6):2043–2063. doi:10.1007/s12350-017-1070-1
- Saby L, Laas O, Habib G, et al. Positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditis: increased valvular 18F-fluorodeoxyglucose uptake as a novel major criterion. J Am Coll Cardiol 2013; 61(23):2374–2382. doi:10.1016/j.jacc.2013.01.092
- Swart LE, Gomes A, Scholtens AM, et al. Improving the diagnostic performance of 18F-fluorodeoxyglucose positron-emission tomography/computed tomography in prosthetic heart valve endocarditis. Circulation 2018; 138(14):1412–1427. doi:10.1161/CIRCULATIONAHA.118.035032
- Graziosi M, Nanni C, Lorenzini M, et al. Role of 18F-FDG PET/CT in the diagnosis of infective endocarditis in patients with an implanted cardiac device: a prospective study. Eur J Nucl Med Mol Imaging 2014; 41(8):1617–1623. doi:10.1007/s00259-014-2773-z
- Kouijzer IJ, Vos FJ, Janssen MJ, van Dijk AP, Oyen WJ, Bleeker-Rovers CP. The value of 18F-FDG PET/CT in diagnosing infectious endocarditis. Eur J Nucl Med Mol Imaging 2013; 40(7):1102–1107. doi:10.1007/s00259-013-2376-0
- Wong D, Rubinshtein R, Keynan Y. Alternative cardiac imaging modalities to echocardiography for the diagnosis of infective endocarditis. Am J Cardiol 2016; 118(9):1410–1418. doi:10.1016/j.amjcard.2016.07.053
- Vos FJ, Bleeker-Rovers CP, Kullberg BJ, Adang EM, Oyen WJ. Cost-effectiveness of routine (18)F-FDG PET/CT in high-risk patients with gram-positive bacteremia. J Nucl Med 2011; 52(11):1673–1678. doi:10.2967/jnumed.111.089714
- McCollough CH, Bushberg JT, Fletcher JG, Eckel LJ. Answers to common questions about the use and safety of CT scans. Mayo Clin Proc 2015; 90(10):1380–1392. doi:10.1016/j.mayocp.2015.07.011
- Duval X, Iung B, Klein I, et al; IMAGE (Resonance Magnetic Imaging at the Acute Phase of Endocarditis) Study Group. Effect of early cerebral magnetic resonance imaging on clinical decisions in infective endocarditis: a prospective study. Ann Intern Med 2010; 152(8):497–504, W175. doi:10.7326/0003-4819-152-8-201004200-00006
- ACR Committee on Drugs and Contrast Media. ACR Manual on Contrast Media: 2018. www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf. Accessed July 19, 2019.
- Kanda T, Fukusato T, Matsuda M, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 2015; 276(1):228–232. doi:10.1148/radiol.2015142690
- McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 2015; 275(3):772–782. doi:10.1148/radiol.15150025
- Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014; 270(3):834–841. doi:10.1148/radiol.13131669
- Expert Panel on Pediatric Imaging; Hayes LL, Palasis S, Bartel TB, et al. ACR appropriateness criteria headache-child. J Am Coll Radiol 2018; 15(5S):S78–S90. doi:10.1016/j.jacr.2018.03.017
- Habib G, Lancellotti P, Antunes MJ, et al; ESC Scientific Document Group. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36(44):3075–3128. doi:10.1093/eurheartj/ehv319
- Durante-Mangoni E, Bradley S, Selton-Suty C, et al; International Collaboration on Endocarditis Prospective Cohort Study Group. Current features of infective endocarditis in elderly patients: results of the International Collaboration on Endocarditis Prospective Cohort Study. Arch Intern Med 2008; 168(19):2095–2103. doi:10.1001/archinte.168.19.2095
- Wurcel AG, Anderson JE, Chui KK, et al. Increasing infectious endocarditis admissions among young people who inject drugs. Open Forum Infect Dis 2016; 3(3):ofw157. doi:10.1093/ofid/ofw157
- Gomes A, Glaudemans AW, Touw DJ, et al. Diagnostic value of imaging in infective endocarditis: a systematic review. Lancet Infect Dis 2017; 17(1):e1–e14. doi:10.1016/S1473-3099(16)30141-4
- Cahill TJ, Baddour LM, Habib G, et al. Challenges in infective endocarditis. J Am Coll Cardiol 2017; 69(3):325–344. doi:10.1016/j.jacc.2016.10.066
- Fagman E, Perrotta S, Bech-Hanssen O, et al. ECG-gated computed tomography: a new role for patients with suspected aortic prosthetic valve endocarditis. Eur Radiol 2012; 22(11):2407–2414. doi:10.1007/s00330-012-2491-5
- Habets J, Tanis W, van Herwerden LA, et al. Cardiac computed tomography angiography results in diagnostic and therapeutic change in prosthetic heart valve endocarditis. Int J Cardiovasc Imaging 2014; 30(2):377–387. doi:10.1007/s10554-013-0335-2
- Koneru S, Huang SS, Oldan J, et al. Role of preoperative cardiac CT in the evaluation of infective endocarditis: comparison with transesophageal echocardiography and surgical findings. Cardiovasc Diagn Ther 2018; 8(4):439–449. doi:10.21037/cdt.2018.07.07
- Koo HJ, Yang DH, Kang J, et al. Demonstration of infective endocarditis by cardiac CT and transoesophageal echocardiography: comparison with intra-operative findings. Eur Heart J Cardiovasc Imaging 2018; 19(2):199–207. doi:10.1093/ehjci/jex010
- Feuchtner GM, Stolzmann P, Dichtl W, et al. Multislice computed tomography in infective endocarditis: comparison with transesophageal echocardiography and intraoperative findings. J Am Coll Cardiol 2009; 53(5):436–444. doi:10.1016/j.jacc.2008.01.077
- Castellano IA, Nicol ED, Bull RK, Roobottom CA, Williams MC, Harden SP. A prospective national survey of coronary CT angiography radiation doses in the United Kingdom. J Cardiovasc Comput Tomogr 2017; 11(4):268–273. doi:10.1016/j.jcct.2017.05.002
- Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology 2008; 248(1):254–263. doi:10.1148/radiol.2481071451
- Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med 2009; 169(22):2078–2086. doi:10.1001/archinternmed.2009.427
- Ploux S, Riviere A, Amraoui S, et al. Positron emission tomography in patients with suspected pacing system infections may play a critical role in difficult cases. Heart Rhythm 2011; 8(9):1478–1481. doi:10.1016/j.hrthm.2011.03.062
- Sarrazin J, Philippon F, Tessier M, et al. Usefulness of fluorine-18 positron emission tomography/computed tomography for identification of cardiovascular implantable electronic device infections. J Am Coll Cardiol 2012; 59(18):1616–1625. doi:10.1016/j.jacc.2011.11.059
- Doherty JU, Kort S, Mehran R, Schoenhagen P, Soman P; Rating Panel Members; Appropriate Use Criteria Task Force. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2017 Appropriate use criteria for multimodality imaging in valvular heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Nucl Cardiol 2017; 24(6):2043–2063. doi:10.1007/s12350-017-1070-1
- Saby L, Laas O, Habib G, et al. Positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditis: increased valvular 18F-fluorodeoxyglucose uptake as a novel major criterion. J Am Coll Cardiol 2013; 61(23):2374–2382. doi:10.1016/j.jacc.2013.01.092
- Swart LE, Gomes A, Scholtens AM, et al. Improving the diagnostic performance of 18F-fluorodeoxyglucose positron-emission tomography/computed tomography in prosthetic heart valve endocarditis. Circulation 2018; 138(14):1412–1427. doi:10.1161/CIRCULATIONAHA.118.035032
- Graziosi M, Nanni C, Lorenzini M, et al. Role of 18F-FDG PET/CT in the diagnosis of infective endocarditis in patients with an implanted cardiac device: a prospective study. Eur J Nucl Med Mol Imaging 2014; 41(8):1617–1623. doi:10.1007/s00259-014-2773-z
- Kouijzer IJ, Vos FJ, Janssen MJ, van Dijk AP, Oyen WJ, Bleeker-Rovers CP. The value of 18F-FDG PET/CT in diagnosing infectious endocarditis. Eur J Nucl Med Mol Imaging 2013; 40(7):1102–1107. doi:10.1007/s00259-013-2376-0
- Wong D, Rubinshtein R, Keynan Y. Alternative cardiac imaging modalities to echocardiography for the diagnosis of infective endocarditis. Am J Cardiol 2016; 118(9):1410–1418. doi:10.1016/j.amjcard.2016.07.053
- Vos FJ, Bleeker-Rovers CP, Kullberg BJ, Adang EM, Oyen WJ. Cost-effectiveness of routine (18)F-FDG PET/CT in high-risk patients with gram-positive bacteremia. J Nucl Med 2011; 52(11):1673–1678. doi:10.2967/jnumed.111.089714
- McCollough CH, Bushberg JT, Fletcher JG, Eckel LJ. Answers to common questions about the use and safety of CT scans. Mayo Clin Proc 2015; 90(10):1380–1392. doi:10.1016/j.mayocp.2015.07.011
- Duval X, Iung B, Klein I, et al; IMAGE (Resonance Magnetic Imaging at the Acute Phase of Endocarditis) Study Group. Effect of early cerebral magnetic resonance imaging on clinical decisions in infective endocarditis: a prospective study. Ann Intern Med 2010; 152(8):497–504, W175. doi:10.7326/0003-4819-152-8-201004200-00006
- ACR Committee on Drugs and Contrast Media. ACR Manual on Contrast Media: 2018. www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf. Accessed July 19, 2019.
- Kanda T, Fukusato T, Matsuda M, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 2015; 276(1):228–232. doi:10.1148/radiol.2015142690
- McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 2015; 275(3):772–782. doi:10.1148/radiol.15150025
- Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014; 270(3):834–841. doi:10.1148/radiol.13131669
- Expert Panel on Pediatric Imaging; Hayes LL, Palasis S, Bartel TB, et al. ACR appropriateness criteria headache-child. J Am Coll Radiol 2018; 15(5S):S78–S90. doi:10.1016/j.jacr.2018.03.017
KEY POINTS
- Echocardiography can produce false-negative results in native-valve infective endocarditis and is even less sensitive in patients with a prosthetic valve or cardiac implanted electronic device.
- 4D CT is a reasonable alternative to transesophageal echocardiography. It can also be used as a second test if echocardiography is inconclusive. Coupled with angiography, it also provides a noninvasive method to evaluate coronary arteries perioperatively.
- Nuclear imaging tests—FDG-PET and leukocyte scintigraphy—increase the sensitivity of the Duke criteria for diagnosing infective endocarditis. They should be considered for evaluating suspected infective endocarditis in all patients who have a prosthetic valve or cardiac implanted electronic device, and whenever echocardiography is inconclusive and clinical suspicion remains high.
Adults with autism spectrum disorder: Updated considerations for healthcare providers
Autism spectrum disorder (ASD) has increased significantly over the past 40 years. Even in the past 2 decades, the prevalence increased from 6.7 per 1,000 in 20001 to 14.6 per 1,000 in 2012—1 in 59 people.2 Of those with ASD, 46% have an intelligence quotient (IQ) greater than 85, meaning they are of average or above-average intelligence.1
As more children with autism become adults, understanding this condition across the life span grows paramount. While many studies have focused on understanding how diagnosis and treatment can help young children, few have focused on adults with autism and how primary care teams can better assist these individuals. However, this is changing, with studies of the benefits of employment programs and pharmacologic treatment, and reproductive health needs of adults with ASD. Here we provide an updated review of ASD in adult patients.
NO MORE ASPERGER SYNDROME— IT’S ON THE SPECTRUM NOW
As the scientific understanding of autism has expanded, revisions in the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5),3 published in 2013, have paralleled these advances. For many adult patients with autism who were evaluated as children, these revisions have led to changes in diagnosis and available services.
In the previous edition (DSM-IV-TR, published in 2000),4 autistic disorder and Asperger syndrome were separate (Table 1). However, DSM-5 lumped autistic disorder and Asperger disorder together under the diagnosis of ASD; this leaves it to the clinician to specify whether the patient with ASD has accompanying intellectual or language impairment and to assign a level of severity based on communication deficits and restrictive behaviors.
The shift in diagnosis was worrisome for some, particularly for clinicians treating patients with DSM-IV Asperger syndrome, who lost this diagnostic label. Concerns that patients with Asperger syndrome may not meet the DSM-5 criteria for ASD were validated by a systematic review showing that only 50% to 75% of patients with DSM-IV autistic disorder, Asperger syndrome, or pervasive developmental disorder not otherwise specified (PDD-NOS) met the DSM-5 criteria for ASD.5 Most of those who no longer met the criteria for ASD carried a DSM-IV diagnosis of Asperger syndrome or PDD-NOS or had an IQ over 70.5 Nevertheless, these individuals may struggle with impairing symptoms related to repetitive behaviors or communication or may be affected by learning or social-emotional disabilities. Additionally, even if they meet the criteria for ASD, some may identify with the Asperger syndrome label and fear they will be stigmatized should they be classified as having the more general ASD.6,7
Although future revisions to the DSM may include further changes in classification, grouping adults with ASD according to their functional and cognitive ability may allow for pragmatic characterization of their needs. At least 3 informal groupings of autistic adults have been described that integrate cognitive ability and independence8:
- Those with low cognitive and social abilities, who need lifelong support
- Those with midrange cognitive and social limitations but who can complete their work in special education classes; they often find employment in supervised workshops or other work with repetitive tasks
- Those who have greater cognitive ability and some social skills; they may proceed to college and employment and live independently.
UNCERTAIN PROGNOSIS
Prognostication for people with ASD remains an area of research. Some adults experience a reduction in symptoms as they age, with significant improvements in speech and, sometimes, modest improvements in restrictive and repetitive behaviors.9,10
Nevertheless, autism remains a lifelong disorder for many. Adults may still require significant support and may experience impairment, particularly in social interaction.10 In longitudinal studies, only 15% to 27% of patients with ASD are characterized as having a positive outcome (often defined as variables related to independent function, near-normal relationships, employment, or a quantified reduction in core symptoms), and many experience significant dependency into adulthood.10–13
IQ has been cited as a possible prognostic factor,10,13 with an IQ below 70 associated with poorer outcome, although an IQ above 70 does not necessarily confer a positive outcome. Less-severe impairment in speech at baseline in early childhood also suggests better outcomes in adulthood.10
As we see more adults with autism, studies that include both children and adults, such as the Longitudinal European Autism Cohort, will be important to characterize the natural history, comorbidities, and genetics of ASD and may help provide more specific predictors of disease course into adulthood.14
ACHIEVING A DIAGNOSIS FOR ADULT PATIENTS WITH SUSPECTED AUTISM
While many patients are recognized as having autism in early to mid-childhood, some adults may not receive a formal diagnosis until much later in life. Those with fluent language and normal-range IQ are likely to be overlooked.15 People with ASD may have had mild symptoms during childhood that did not impair their functioning until demands of daily life exceeded their capacities in adulthood. Alternatively, parents of a child with newly diagnosed ASD may realize that they themselves or another adult family member also show signs of it.
The UK National Institute of Health and Care Excellence suggests that assessment should be considered if the patient meets psychiatric diagnostic criteria and one of the following:
- Difficulty obtaining or sustaining employment or education
- Difficulty initiating or sustaining social relationships
- Past or current contact with mental health or learning disability services
- History of a neurodevelopmental or mental health disorder.15,16
Currently, diagnosis typically involves a multidisciplinary approach, with psychiatric assessment, neuropsychological testing, and speech and language evaluation.17 Providers may need to refer patients for these services, sometimes at the patient’s request, if previous mental health misdiagnoses are suspected, if patients report symptoms or impairment consistent with ASD, or if benefits, services, or accommodations, such as a coach in the workplace, are needed.
Diagnosing ASD in adults can be difficult, given that the gold-standard diagnostic tests such as the Autism Diagnostic Observation Schedule-2 (ADOS-2)18 and the Autism Diagnostic Interview-Revised (ADI-R)19 are typically used to diagnose autism in children. However, Module 4 in the ADOS-2 was developed for adolescents and older patients with fluent language and has shown at least moderate power to distinguish adults with ASD from those without ASD.18,20
An initial psychiatric assessment should include a thorough history taken from the patient and, if applicable, the patient’s caregiver, as well as a psychiatric interview of the patient. Neuropsychological testing should include evaluation of cognitive function, social functioning (using the ADOS-2 for adults without intellectual disability, the ADI-R, or both), and adaptive functioning (using the Vineland Adaptive Behavior Scales, second edition21).
Evaluation of speech and language is particularly important in patients with limited language ability and should include both expressive and receptive language abilities. Serial testing every few years, as is often recommended in childhood, may help establish the pattern of impairment over time.
Comorbid psychiatric disorders are common
Many people with ASD also have other psychiatric disorders,17,22 which clinicians should keep in mind when seeing an adult seeking evaluation for ASD.
Attention-deficit/hyperactivity disorder is present at higher rates in patients of average intellectual function with ASD than in the general population.23
Anxiety disorders, including obsessive-compulsive disorder, were found to often coexist with autism in a sample of adults with autism without intellectual disability,24,25 and approximately 40% of youths with ASD have at least 1 comorbid anxiety disorder.26
Mood disorders are also prevalent in adults with ASD, with a small study showing that 70% of adults with DSM-IV Asperger syndrome had at least 1 depressive episode in their lifetime.27
BEHAVIORAL AND PHARMACOLOGIC THERAPIES FOR THE ADULT PATIENT
Services and medications for adults with ASD are discussed below. These will vary by individual, and services available may vary by region.
Historically, vocational and social outcomes have been poor for adults with ASD. It is estimated that most larger universities may be home to 100 to 300 students with ASD. To combat isolation, the University of California, Los Angeles, the University of Alabama, and others provide special support services, including group social activities such as board games and individual coaching.8 Nevertheless, half of the students with autism who attend institutions of higher learning leave without completing their intended degree.29 Many still struggle to establish meaningful friendships or romantic relationships.29
Planning for a transition of care
Healthcare transition planning is important but is strikingly underused.30 Individual providers, including adult psychiatrists, vary in their level of training and comfort in diagnosing, treating, and monitoring adults with autism. Youths with ASD are half as likely to receive healthcare transition services as other youths with special healthcare needs.31
Pediatric providers, including pediatric psychiatrists, developmental behavioral specialists, and pediatric neurologists, may be best equipped to treat young adult patients or to refer patients to appropriate generalists and specialists comfortable with autism-specific transition of care. The question of eligibility for services is important to patients and families during the transition period, with many parents and professionals unaware of services available to them.32 Receiving adequate transition services is enabled by having a medical home during childhood—that is, a comprehensive, centralized medical record, culturally competent care, interaction with schools, and patient access to clear, unbiased information.31
Ideally, in our experience, transitioning should be discussed well before the child ages out of the pediatric provider’s practice. If necessary, healthcare transition services should include 4 components:
- Discussing the switch to a new physician who treats adults
- Discussing changing healthcare needs as an adult
- Planning insurance coverage as an adult
- Encouragement by the physician for the child to take age-appropriate responsibility for his or her healthcare.31,33
Tools such as the Got Transition checklist from the National Health Care Transition Center can provide support during this process.34
Other services
Other services provided as an extension or adjunct to the medical home in early adulthood may include customized vocational or employment training, specialized mentorship or support in a college setting, housing support, and psychological services.35
Community-based programs that emphasize leisure have been shown to improve participants’ independence and quality of life.36 Similarly, participants in programs that emphasized supported employment, with a job coach, on-the-job support, collaboration with the participant’s larger social support network, and selection of tasks to match an individual’s abilities and strengths, demonstrated improved cognitive performance, particularly executive functioning,37 and employment.38,39 These programs work best for patients who have mild to moderate symptoms.37,39
Patients with symptoms that are more severe may do better in a residential program. Many of these programs maintain an emphasis on vocational and social skills development. One such long-standing program is Bittersweet Farms, a rural farming community in Ohio for adults with ASD, where individuals with moderate to low function live in a group setting, with emphasis on scheduled, meaningful work including horticulture, animal care, carpentry; and activities of daily living.40
Studies of patients across the autism spectrum have generally found better outcomes when vocational support is given, but larger and randomized studies are needed to characterize how to best support these individuals after they leave high school.41
Psychological services such as applied behavioral therapy, social cognition training, cognitive behavioral therapy, and mindfulness training may be particularly useful in adults.42–44
Some versions of applied behavioral therapy, such as the Early Start Denver Model,45 have been found to be cost-effective and offset some expenses in the care of children with autism, using play-based and relationship-based interventions to promote development across domains while reducing symptoms.
In randomized controlled trials, modified cognitive behavioral therapy43 and mindfulness44 were shown to reduce symptoms of anxiety, obsessive-compulsive disorder, and depression.
Dialectical behavior therapy, used to find a balance between accepting oneself and desiring to change, may help in some circumstances to regulate emotions and reduce reactivity and lability, although large randomized clinical trials have not been conducted in the ASD population.46
Drug therapy
Medications may be appropriate to manage symptoms or comorbid conditions in adults with ASD. Over 75% adults with ASD have been found to use psychotropic medications.47 However, although these drugs have been approved for treating behaviors commonly associated with ASD, none of them provide definitive treatment for this disorder, and they have not been rigorously tested or approved for use in adults with ASD.48
Irritability and aggression associated with ASD can be treated with risperidone (approved for children over age 5), aripiprazole (approved for children ages 6–17), clozapine, or haloperidol.49
Aberrant social behavior can be treated with risperidone.50 Treatments under investigation include oxytocin and secretin.49
While no approved drug has been shown to improve social communication,51 balovaptan, a vasopressin V1a agonist, has shown potential and has been granted breakthrough status by the US Food and Drug Administration for treating challenging behaviors in adults, with additional studies ongoing in children.52,53
Repetitive behaviors, if the patient finds them impairing, can be managed with selective serotonin reuptake inhibitors.49
Much more study of drug therapy in adults with ASD is needed to fully understand the best approaches to psychotropic medication use, including appropriate classes and effective dosage, in this population.
SEX: UNEXPLORED TERRITORY
The reproductive health needs of people with autism remain largely underexplored.54 Historically, individuals with ASD were thought to have little interest in sexual activity or parenthood, owing to the nature of the core symptoms of the disorder. This has been shown to be untrue, particularly as studies on this topic began to engage in direct interviews with people with ASD, rather than solely gathering information from caregivers or parents. The findings reinforce the importance of broaching this component of health in this population, for the following reasons:
Adults with ASD are at increased risk of sexual victimization, with nearly 4 out of 5 reporting unwanted sexual advances, coercion, or rape.55
They have a smaller pool of knowledge with respect to sexual health. They report56 that they learned about sex from television and from “making mistakes.” They use fewer sources. They are less likely to speak to peers and figures of authority to gain knowledge about sexually transmitted infections, sexual behaviors, and contraception. And they are more likely to use forms of nonsocial media, such as television, for information.55
They report more concerns about the future with respect to sexual behavior, suggesting the need for targeted sexual education programs.56
College-age young adults with ASD who misread communication may be particularly affected by Title IX, which requires schools to promptly investigate reports of sexual harassment and sexual assault, should they struggle to comport themselves appropriately.57 Early and frank conversations about issues of consent and appropriate displays of interest and affection may better equip youth to navigate new social scenarios as they plan to leave a supervised home environment for college or the workforce.
Gender identification: Male, female, other
In one study, 77.8% of birth-sex males with ASD said they identified as men, and 67.1% of birth-sex females identified as women, compared with 93.1% of birth-sex males and 87.3% of birth-sex females without ASD. Many of the remaining individuals with ASD reported a transgender, genderqueer, or other gender identity.58 Some studies have found females with ASD report a gay or bisexual orientation more often than males with ASD.59–61
Adolescents and young adults may be exploring their changing bodies, sexual preferences, and gender roles, and as for all people at this age, these roles emerge against a backdrop of familial and societal expectations that may or may not be concordant with their own projected path regarding sexuality and reproductive health.62
Having the conversation
As with non-ASD patients, a thorough sexual history should be collected via open-ended questions when possible to determine types of sexual activity and partners.
Education of the patient, alongside caregivers and parents, about healthy and safe sexual practices, screening for sexual violence, and hormonal and nonhormonal contraception options are important components of care for this population.
CAREGIVER STRESS MAY PERSIST INTO PATIENT’S ADULTHOOD
Caregiver burden is a monumental concern for parents or others who may have lifelong primary responsibility for these neurodiverse adults.63 Family members may feel isolated and may feel they have encountered many barriers to services.64 Remaining sensitive, knowledgeable, and inquisitive about the types of support that are needed may help forge a trusting relationship between the provider and the family.
Parents of children with ASD have been reported to experience worse physical and emotional health than parents whose children do not have developmental disabilities.63,65 These disparities have been found to persist as their children enter adolescence and young adulthood.66,67 Parents of children with ASD report more anxiety, depression, and distress compared with parents of children without ASD,63 and parents themselves may be affected by ASD symptoms, which has been linked to increased parenting stress.68 Some studies have found blunted cortisol responses,63,69,70 and some,71 but not all,63 have found elevated blood pressure in caregivers of children with developmental disabilities. Headache, backache, muscle soreness, and fatigue may also be commonly reported.67
In our experience, caregivers are tremendously appreciative when provided connections to adult ASD services and support systems as their child ages. The school system and other formal support systems often assist until the time of transition into adulthood. This transition can be stressful for the adolescent and family alike, and informal support systems such as friends and family may become increasingly crucial, particularly if the adolescent still lives at home.72,73
The affected young adult’s unmet needs, as perceived by the caregiver, have been found to be significantly associated with caregiver burden, whereas the severity of the adult patient’s ASD symptoms has not.66 Therefore, it may be helpful to ask caregivers whether they perceive any unmet needs, regardless of the clinician’s perception of the severity of the patient’s ASD symptoms. Providing support to address these needs, particularly those relating to the child’s mood disorders, communication, social needs, safety, and daytime activities, may be the domains of support that most effectively reduce the caregiver burden in this population.66
Caregiver positivity, lower stress levels, and increased social support, particularly in the form of friends and family members providing no-cost assistance to caregivers whose children do not live independently,74 have been linked to better outcomes for caregivers.70,74,75 Rigorous studies that examine caregiver burden as individuals with ASD enter mid- and late-adulthood are limited.
THE ROLE OF THE INTERNIST IN CARING FOR ADULTS WITH AUTISM
A major challenge for many adults with ASD is the transition from services provided during childhood to those provided in adulthood. While children with autism have subspecialty providers who diagnose and manage their condition, including developmental-behavioral pediatricians, pediatric neurologists, and child psychiatrists, adults with autism may have fewer options.
Autism centers are becoming more available across the nation, and many provide care across the life span. However, depending on a patient’s needs, the primary care provider may need to manage residual symptoms as the patient transitions from pediatric to adult care, ultimately deciding when and where to refer the patient.
The patient’s family should pay close attention to function and mood around the time the patient leaves the structure of high school, and they should build rapport with a primary care provider they can turn to if problems persist or arise. Referrals for behavioral therapy and for social work, job training, and vocational support can greatly benefit patients as they transition to young adulthood. Referrals and suggestions for social support can also help caregivers.
Medical care
Deciding when and how to medicate the patient for symptoms of autism and related behaviors necessitates consideration of the patient’s impairment, side effects of the medication, and the impact medications may have on the patient’s other conditions. Disordered eating, mood problems, anxiety, and attention-deficit/hyperactivity disorder should be considered, and, as in all patients, regular screenings of mental health status should be conducted.76,77
Comorbid medical conditions may cause worsening of a patient’s known behavioral symptoms or may precipitate new behaviors or aggression as a result of pain or discomfort, particularly in patients with limited speech. A change in stereotypes or increased irritability warrants a thoughtful investigation for a cause other than ASD before adding or increasing behavioral medications. Common comorbid conditions include gastrointestinal distress, most commonly constipation and diarrhea in an idiopathic ASD population, with increasing ASD symptom severity correlating with increased odds of a gastrointestinal problem.78 Allergies, sleep disorders, seizures, and other psychiatric conditions are also frequent.79
Preventive care, including vaccinations, should be given as scheduled. Caregivers and patients can be reminded if needed that vaccines do not cause or worsen autism, and vaccination is intended to improve the safety of the patient and those around them, protecting against potentially life-threatening disease. Regular dental care visits, particularly for patients who are using medications that may affect tooth or gingival health,80 and regular visits to an optometrist or ophthalmologist for screening of vision are also advised.
Adverse effects. Weight gain and metabolic syndrome are common adverse effects of medications used for behavioral management, and the primary care physician may uncover diabetes, cardiac disorders, and hyperlipidemia. Patients with ASD may be particularly sensitive to the effects of medications and therefore may require a lower dose or a slower titration than other patients. Working with a behavioral team, careful weaning of psychiatric medications to the minimum needed is strongly recommended whenever possible.81
TAKE-HOME POINTS
As more adults with autism enter society, they may require varying levels of support from the healthcare community to ensure that therapeutic gains from childhood persist, allowing them to achieve maximal functional potential.
Adults with ASD may have a high, normal, or low IQ and intellectual capability. Knowledge of this and of the patient’s symptom severity and presence of comorbid psychiatric and other health conditions can help the clinician guide the patient to appropriate social services and pharmacologic treatments.
Individualized support in the workplace, as well as education regarding sexual health, can help improve outcomes for affected individuals.
Caregiver burden for individuals with autism can be high, but it can be mitigated by social support.
Further research regarding appropriate diagnostic instruments in adulthood and appropriate treatments for impairing autism-related symptoms across the life span may be particularly helpful in supporting this patient population.
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2000 Principal Investigators; Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders—autism and developmental disabilities monitoring network, six sites, United States, 2000. MMWR Surveill Summ 2007; 56(1):1–11. pmid:17287714
- Christensen DL. Prevalence and characteristics of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2012. MMWR Surveill Summ 2016; 65(13):1–23. doi:10.15585/mmwr.ss6503a1
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edition. Washington, D.C: American Psychiatric Association; 2013.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision. Washington, DC: American Psychiatric Association; 2000.
- Smith IC, Reichow B, Volkmar FR. The effects of DSM-5 criteria on number of individuals diagnosed with autism spectrum disorder: a systematic review. J Autism Dev Disord 2015; 45(8):2541–2552. doi:10.1007/s10803-015-2423-8
- Barahona-Corrêa JB, Filipe CN. A concise history of Asperger syndrome: the short reign of a troublesome diagnosis. Front Psychol 2015; 6:2024. doi:10.3389/fpsyg.2015.02024
- Kite DM, Gullifer J, Tyson GA. Views on the diagnostic labels of autism and Asperger’s disorder and the proposed changes in the DSM. J Autism Dev Disord 2013; 43(7):1692–1700. doi:10.1007/s10803-012-1718-2
- Kuo AA. Autism in adults: an update. Presented at the: American College of Physicians Internal Medicine Meeting, New Orleans, LA, April 17–21, 2018.
- Shattuck PT, Seltzer MM, Greenberg JS, et al. Change in autism symptoms and maladaptive behaviors in adolescents and adults with an autism spectrum disorder. J Autism Dev Disord 2007; 37(9):1735–1747. doi:10.1007/s10803-006-0307-7
- Seltzer MM, Shattuck P, Abbeduto L, Greenberg JS. Trajectory of development in adolescents and adults with autism. Ment Retard Dev Disabil Res Rev 2004; 10(4):234–247. doi:10.1002/mrdd.20038
- Billstedt E, Carina Gillberg I, Gillberg C. Autism in adults: symptom patterns and early childhood predictors. Use of the DISCO in a community sample followed from childhood. J Child Psychol Psychiatry 2007; 48(11):1102–1110. doi:10.1111/j.1469-7610.2007.01774.x
- Howlin P, Goode S, Hutton J, Rutter M. Adult outcome for children with autism. J Child Psychol Psychiatry 2004; 45(2):212–229. pmid:14982237
- Marriage S, Wolverton A, Marriage K. Autism spectrum disorder grown up: a chart review of adult functioning. J Can Acad Child Adolesc Psychiatry 2009; 18(4):322–328. pmid: 19881941
- Isaksson J, Tammimies K, Neufeld J, et al. EU-AIMS Longitudinal European Autism Project (LEAP): the autism twin cohort. Mol Autism 2018; 9(1):26. doi:10.1186/s13229-018-0212-x
- Lai M-C, Baron-Cohen S. Identifying the lost generation of adults with autism spectrum conditions. Lancet Psychiatry 2015; 2(11):1013–1027. doi:10.1016/S2215-0366(15)00277-1
- National Institute for Health and Clinical Excellence. Autism: recognition, referral, diagnosis and management of adults on the autism spectrum. NICE clinical guideline 142. June 2012. https://grand.tghn.org/site_media/media/medialibrary/2015/03/ASD_NICE_3_.pdf. Accessed July 9, 2019.
- Wolf JM, Ventola P. Assessment and treatment planning in adults with autism spectrum disorders. In: Adolescents and Adults with Autism Spectrum Disorders. Springer, New York, NY; 2014:283–298.
- Lord C, Rutter M, DiLavore P, Risi S, Gotham K, Bishop SL. Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) manual. Torrance, CA: Western Psychological Services, 2012.
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 1994; 24(5):659–685.
- Hus V, Lord C. The autism diagnostic observation schedule, module 4: revised algorithm and standardized severity scores. J Autism Dev Disord 2014; 44(8):1996–2012. doi:10.1007/s10803-014-2080-3>
- Sparrow S, Balla D, Cicchetti D, Harrison P, Doll E. Vineland Adaptive Behavior Scales. Circle Pines, MN: American Guidance Service, 1984.
- Happé FG, Mansour H, Barrett P, Brown T, Abbott P, Charlton RA. Demographic and cognitive profile of individuals seeking a diagnosis of autism spectrum disorder in adulthood. J Autism Dev Disord 2016; 46(11):3469–3480. doi:10.1007/s10803-016-2886-2
- Johnston K, Dittner A, Bramham J, Murphy C, Knight A, Russell A. Attention deficit hyperactivity disorder symptoms in adults with autism spectrum disorders. Autism Res Off J Int Soc Autism Res 2013; 6(4):225–236. doi:10.1002/aur.1283
- Cadman T, Spain D, Johnston P, et al. Obsessive-compulsive disorder in adults with high-functioning autism spectrum disorder: what does self-report with the OCI-R tell us? Autism Res Off J Int Soc Autism Res 2015; 8(5):477–485. doi:10.1002/aur.1461
- Russell AJ, Mataix-Cols D, Anson M, Murphy DGM. Obsessions and compulsions in Asperger syndrome and high-functioning autism. Br J Psychiatry J Ment Sci 2005; 186:525–528. doi:10.1192/bjp.186.6.525
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry 2008; 47(8):921–929. doi:10.1097/CHI.0b013e318179964f
- Lugnegård T, Hallerbäck MU, Gillberg C. Psychiatric comorbidity in young adults with a clinical diagnosis of Asperger syndrome. Res Dev Disabil 2011; 32(5):1910–1917. doi:10.1016/j.ridd.2011.03.025
- Howlin P, Moss P. Adults with autism spectrum disorders. Can J Psychiatry 2012; 57(5):275–283. doi:10.1177/070674371205700502
- Levy A, Perry A. Outcomes in adolescents and adults with autism: a review of the literature. Res Autism Spectr Disord 2011; 5(4):1271–1282. doi:10.1016/J.RASD.2011.01.023
- Cheak-Zamora NC, Yang X, Farmer JE, Clark M. Disparities in transition planning for youth with autism spectrum disorder. Pediatrics 2013; 131(3):447–454. doi:10.1542/peds.2012-1572
- Rast JE, Shattuck PT, Roux AM, Anderson KA, Kuo A. The medical home and health care transition for youth with autism. Pediatrics 2018; 141(suppl 4):S328–S334. doi:10.1542/peds.2016-4300J
- Belling R, McLaren S, Paul M, et al. The effect of organisational resources and eligibility issues on transition from child and adolescent to adult mental health services. J Health Serv Res Policy 2014; 19(3):169–176. doi:10.1177/1355819614527439
- Data Resource Center for Child & Adolescent Health. 2009–2010 National Survey of Children with Special Health Care Needs. www.childhealthdata.org/docs/drc/200910-cshcn-spss-codebook_final_051012.pdf?sfvrsn=1. Accessed July 9, 2019.
- Got Transition Center for Health Care Transition Improvement. Six core elements of health care transition 2.0. Transitioning youth to an adult health care provider. For use by pediatric, family medicine, and med-peds providers. www.gottransition.org/resourceGet.cfm?id=208. Accessed July 9, 2019.
- Murphy CM, Wilson CE, Robertson DM, et al. Autism spectrum disorder in adults: diagnosis, management, and health services development. Neuropsychiatr Dis Treat 2016; 12:1669–1686. doi:10.2147/NDT.S65455
- García-Villamisar DA, Dattilo J. Effects of a leisure programme on quality of life and stress of individuals with ASD. J Intellect Disabil Res 2010; 54(7):611–619. doi:10.1111/j.1365-2788.2010.01289.x
- García-Villamisar D, Hughes C. Supported employment improves cognitive performance in adults with autism. J Intellect Disabil Res 2007; 51(pt 2):142–150. doi:10.1111/j.1365-2788.2006.00854.x
- Lawer L, Brusilovskiy E, Salzer MS, Mandell DS. Use of vocational rehabilitative services among adults with autism. J Autism Dev Disord 2009; 39(3):487–494. doi:10.1007/s10803-008-0649-4
- Howlin P, Alcock J, Burkin C. An 8 year follow-up of a specialist supported employment service for high-ability adults with autism or Asperger syndrome. Autism 2005; 9(5):533–549. doi:10.1177/1362361305057871
- Kay BR. Bittersweet Farms. J Autism Dev Disord 1990; 20(3):309–321. http://www.ncbi.nlm.nih.gov/pubmed/2228914. Accessed July 9, 2019.
- Taylor JL, McPheeters ML, Sathe NA, Dove D, Veenstra-Vanderweele J, Warren Z. A systematic review of vocational interventions for young adults with autism spectrum disorders. Pediatrics 2012; 130(3):531–538. doi:10.1542/peds.2012-0682
- Bishop-Fitzpatrick L, Minshew NJ, Eack SM. A systematic review of psychosocial interventions for adults with autism spectrum disorders. J Autism Dev Disord 2013; 43(3):687–694. doi:10.1007/s10803-012-1615-8
- Russell AJ, Jassi A, Fullana MA, et al. Cognitive behavior therapy for comorbid obsessive-compulsive disorder in high-functioning autism spectrum disorders: a randomized controlled trial. Depress Anxiety 2013; 30(8):697–708. doi:10.1002/da.22053
- Spek AA, van Ham NC, Nyklícek I. Mindfulness-based therapy in adults with an autism spectrum disorder: a randomized controlled trial. Res Dev Disabil 2013; 34(1):246–253. doi:10.1016/j.ridd.2012.08.009
- Eapen V, Crncec R, Walter A. Clinical outcomes of an early intervention program for preschool children with autism spectrum disorder in a community group setting. BMC Pediatr 2013; 13(1):3. doi:10.1186/1471-2431-13-3
- Mazefsky CA, White SW. Emotion regulation: concepts & practice in autism spectrum disorder. Child Adolesc Psychiatr Clin North Am 2014; 23(1):15–24. doi:10.1016/J.CHC.2013.07.002
- Esbensen AJ, Greenberg JS, Seltzer MM, Aman MG. A longitudinal investigation of psychotropic and non-psychotropic medication use among adolescents and adults with autism spectrum disorders. J Autism Dev Disord 2009; 39(9):1339–1349. doi:10.1007/s10803-009-0750-3
- Dove D, Warren Z, McPheeters ML, Taylor JL, Sathe NA, Veenstra-VanderWeele J. Medications for adolescents and young adults with autism spectrum disorders: a systematic review. Pediatrics 2012; 130(4):717–726. doi:10.1542/peds.2012-0683
- LeClerc S, Easley D. Pharmacological therapies for autism spectrum disorder: a review. Pharm Ther 2015; 40(6):389–397.
- Miral S, Gencer O, Inal-Emiroglu FN, Baykara B, Baykara A, Dirik E. Risperidone versus haloperidol in children and adolescents with AD: a randomized, controlled, double-blind trial. Eur Child Adolesc Psychiatry 2008; 17(1):1–8. doi:10.1007/s00787-007-0620-5
- Lai M-C, Lombardo MV, Baron-Cohen S. Autism. Lancet 2014; 383(9920):896–910. doi:10.1016/S0140-6736(13)61539-1
- Ratni H, Rogers-Evans M, Bissantz C, et al. Discovery of highly selective brain-penetrant vasopressin 1a antagonists for the potential treatment of autism via a chemogenomic and scaffold hopping approach. J Med Chem 2015; 58(5):2275–2289. doi:10.1021/jm501745f
- Umbricht D, Del Valle Rubido M, Hollander E, et al. A single dose, randomized, controlled proof-of-mechanism study of a novel vasopressin 1a receptor antagonist (RG7713) in high-functioning adults with autism spectrum disorder. Neuropsychopharmacology 2017; 42(9):1914–1923. doi:10.1038/npp.2016.232>
- Kellaher DC. Sexual behavior and autism spectrum disorders: an update and discussion. Curr Psychiatry Rep 2015; 17(4):25. doi:10.1007/s11920-015-0562-4
- Brown-Lavoie SM, Viecili MA, Weiss JA. Sexual knowledge and victimization in adults with autism spectrum disorders. J Autism Dev Disord 2014; 44(9):2185–2196. doi:10.1007/s10803-014-2093-y
- Mehzabin P, Stokes MA. Self-assessed sexuality in young adults with high-functioning autism. Res Autism Spectr Disord 2011; 5(1):614–621. doi:10.1016/J.RASD.2010.07.006>
- Brown KR. Accessibility for students with ASD: legal perspectives in the United States. In: Alphin HC Jr. Exploring the Future of Accessibility in Higher Education. Hershey, PA: IGI Global; 2017.
- George R, Stokes MA. Gender identity and sexual orientation in autism spectrum disorder. Autism 2018; 22(8):970–982. doi:10.1177/1362361317714587
- Byers ES, Nichols S, Voyer SD. Challenging stereotypes: sexual functioning of single adults with high functioning autism spectrum disorder. J Autism Dev Disord 2013; 43(11):2617–2627. doi:10.1007/s10803-013-1813-z
- Gilmour L, Schalomon PM, Smith V. Sexuality in a community based sample of adults with autism spectrum disorder. Res Autism Spectr Disord 2012; 6(1):313–318. doi:10.1016/J.RASD.2011.06.003
- Bejerot S, Eriksson JM. Sexuality and gender role in autism spectrum disorder: a case control study. Schmitz C, ed. PLoS One 2014; 9(1):e87961. doi:10.1371/journal.pone.0087961>
- Navot N, Jorgenson AG, Webb SJ. Maternal experience raising girls with autism spectrum disorder: a qualitative study. Child Care Health Dev 2017; 43(4):536–545. doi:10.1111/cch.12470
- Padden C, James JE. Stress among parents of children with and without autism spectrum disorder: a comparison involving physiological indicators and parent self-reports. J Dev Phys Disabil 2017; 29(4):567–586. doi:10.1007/s10882-017-9547-z
- Woodgate RL, Ateah C, Secco L. Living in a world of our own: the experience of parents who have a child with autism. Qual Health Res 2008; 18(8):1075–1083. doi:10.1177/1049732308320112
- Hayes SA, Watson SL. The impact of parenting stress: a meta-analysis of studies comparing the experience of parenting stress in parents of children with and without autism spectrum disorder. J Autism Dev Disord 2013; 43(3):629–642. doi:10.1007/s10803-012-1604-y
- Cadman T, Eklund H, Howley D, et al. Caregiver burden as people with autism spectrum disorder and attention-deficit/hyperactivity disorder transition into adolescence and adulthood in the United Kingdom. J Am Acad Child Adolesc Psychiatry 2012; 51(9):879–888. doi:10.1016/j.jaac.2012.06.017
- Smith LE, Seltzer MM, Greenberg JS. Daily health symptoms of mothers of adolescents and adults with fragile x syndrome and mothers of adolescents and adults with autism spectrum disorder. J Autism Dev Disord 2012; 42(9):1836–1846. doi:10.1007/s10803-011-1422-7
- van Steijn DJ, Oerlemans AM, van Aken MAG, Buitelaar JK, Rommelse NNJ. The reciprocal relationship of ASD, ADHD, depressive symptoms and stress in parents of children with ASD and/or ADHD. J Autism Dev Disord 2014; 44(5):1064–1076. doi:10.1007/s10803-013-1958-9
- Seltzer MM, Greenberg JS, Hong J, et al. Maternal cortisol levels and behavior problems in adolescents and adults with ASD. J Autism Dev Disord 2010; 40(4):457–469. doi:10.1007/S10803-009-0887-0
- Lovell B, Moss M, Wetherell MA. With a little help from my friends: psychological, endocrine and health corollaries of social support in parental caregivers of children with autism or ADHD. Res Dev Disabil 2012; 33(2):682–687. doi:10.1016/j.ridd.2011.11.014
- Gallagher S, Whiteley J. Social support is associated with blood pressure responses in parents caring for children with developmental disabilities. Res Dev Disabil 2012; 33(6):2099–2105. doi:10.1016/j.ridd.2012.06.007
- Baker JK, Smith LE, Greenberg JS, Seltzer MM, Taylor JL. Change in maternal criticism and behavior problems in adolescents and adults with autism across a 7-year period. J Abnorm Psychol 2011; 120(2):465–475. doi:10.1037/a0021900
- Marsack CN, Samuel PS. Mediating effects of social support on quality of life for parents of adults with autism. J Autism Dev Disord 2017; 47(8):2378–2389. doi:10.1007/s10803-017-3157-6
- Trute B, Benzies KM, Worthington C, Reddon JR, Moore M. Accentuate the positive to mitigate the negative: mother psychological coping resources and family adjustment in childhood disability. J Intellect Dev Disabil 2010; 35(1):36–43. doi:10.3109/13668250903496328
- Cantwell J, Muldoon OT, Gallagher S. Social support and mastery influence the association between stress and poor physical health in parents caring for children with developmental disabilities. Res Dev Disabil 2014; 35(9):2215–2223. doi:10.1016/j.ridd.2014.05.012
- Carton AM, Smith AD. Assessing the relationship between eating disorder psychopathology and autistic traits in a non-clinical adult population. Eat Weight Disord - Stud Anorexia, Bulim Obes 2014; 19(3):285–293. doi:10.1007/s40519-013-0086-z
- De Alwis D, Agrawal A, Reiersen AM, et al. ADHD symptoms, autistic traits, and substance use and misuse in adult Australian twins. J Stud Alcohol Drugs 2014; 75(2):211–221. doi:10.15288/jsad.2014.75.211
- Wang LW, Tancredi DJ, Thomas DW. The prevalence of gastrointestinal problems in children across the United States with autism spectrum disorders from families with multiple affected members. J Dev Behav Pediatr 2011; 32(5):351–360. doi:10.1097/DBP.0b013e31821bd06a
- Croen LA, Zerbo O, Qian Y, et al. The health status of adults on the autism spectrum. Autism 2015; 19(7):814–823. doi:10.1177/1362361315577517
- Kalyoncu IÖ, Tanboga I. Oral health status of children with autistic spectrum disorder compared with non-authentic peers. Iran J Public Health 2017; 46(11):1591–1593. www.ncbi.nlm.nih.gov/pmc/articles/PMC5696703. Accessed July 9, 2019.
- McGuire K, Fung LK, Hagopian L, et al. Irritability and problem behavior in autism spectrum disorder: a practice pathway for pediatric primary care. Pediatrics 2016; 137(suppl 2):S136–S148. doi:10.1542/peds.2015-2851L
Autism spectrum disorder (ASD) has increased significantly over the past 40 years. Even in the past 2 decades, the prevalence increased from 6.7 per 1,000 in 20001 to 14.6 per 1,000 in 2012—1 in 59 people.2 Of those with ASD, 46% have an intelligence quotient (IQ) greater than 85, meaning they are of average or above-average intelligence.1
As more children with autism become adults, understanding this condition across the life span grows paramount. While many studies have focused on understanding how diagnosis and treatment can help young children, few have focused on adults with autism and how primary care teams can better assist these individuals. However, this is changing, with studies of the benefits of employment programs and pharmacologic treatment, and reproductive health needs of adults with ASD. Here we provide an updated review of ASD in adult patients.
NO MORE ASPERGER SYNDROME— IT’S ON THE SPECTRUM NOW
As the scientific understanding of autism has expanded, revisions in the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5),3 published in 2013, have paralleled these advances. For many adult patients with autism who were evaluated as children, these revisions have led to changes in diagnosis and available services.
In the previous edition (DSM-IV-TR, published in 2000),4 autistic disorder and Asperger syndrome were separate (Table 1). However, DSM-5 lumped autistic disorder and Asperger disorder together under the diagnosis of ASD; this leaves it to the clinician to specify whether the patient with ASD has accompanying intellectual or language impairment and to assign a level of severity based on communication deficits and restrictive behaviors.
The shift in diagnosis was worrisome for some, particularly for clinicians treating patients with DSM-IV Asperger syndrome, who lost this diagnostic label. Concerns that patients with Asperger syndrome may not meet the DSM-5 criteria for ASD were validated by a systematic review showing that only 50% to 75% of patients with DSM-IV autistic disorder, Asperger syndrome, or pervasive developmental disorder not otherwise specified (PDD-NOS) met the DSM-5 criteria for ASD.5 Most of those who no longer met the criteria for ASD carried a DSM-IV diagnosis of Asperger syndrome or PDD-NOS or had an IQ over 70.5 Nevertheless, these individuals may struggle with impairing symptoms related to repetitive behaviors or communication or may be affected by learning or social-emotional disabilities. Additionally, even if they meet the criteria for ASD, some may identify with the Asperger syndrome label and fear they will be stigmatized should they be classified as having the more general ASD.6,7
Although future revisions to the DSM may include further changes in classification, grouping adults with ASD according to their functional and cognitive ability may allow for pragmatic characterization of their needs. At least 3 informal groupings of autistic adults have been described that integrate cognitive ability and independence8:
- Those with low cognitive and social abilities, who need lifelong support
- Those with midrange cognitive and social limitations but who can complete their work in special education classes; they often find employment in supervised workshops or other work with repetitive tasks
- Those who have greater cognitive ability and some social skills; they may proceed to college and employment and live independently.
UNCERTAIN PROGNOSIS
Prognostication for people with ASD remains an area of research. Some adults experience a reduction in symptoms as they age, with significant improvements in speech and, sometimes, modest improvements in restrictive and repetitive behaviors.9,10
Nevertheless, autism remains a lifelong disorder for many. Adults may still require significant support and may experience impairment, particularly in social interaction.10 In longitudinal studies, only 15% to 27% of patients with ASD are characterized as having a positive outcome (often defined as variables related to independent function, near-normal relationships, employment, or a quantified reduction in core symptoms), and many experience significant dependency into adulthood.10–13
IQ has been cited as a possible prognostic factor,10,13 with an IQ below 70 associated with poorer outcome, although an IQ above 70 does not necessarily confer a positive outcome. Less-severe impairment in speech at baseline in early childhood also suggests better outcomes in adulthood.10
As we see more adults with autism, studies that include both children and adults, such as the Longitudinal European Autism Cohort, will be important to characterize the natural history, comorbidities, and genetics of ASD and may help provide more specific predictors of disease course into adulthood.14
ACHIEVING A DIAGNOSIS FOR ADULT PATIENTS WITH SUSPECTED AUTISM
While many patients are recognized as having autism in early to mid-childhood, some adults may not receive a formal diagnosis until much later in life. Those with fluent language and normal-range IQ are likely to be overlooked.15 People with ASD may have had mild symptoms during childhood that did not impair their functioning until demands of daily life exceeded their capacities in adulthood. Alternatively, parents of a child with newly diagnosed ASD may realize that they themselves or another adult family member also show signs of it.
The UK National Institute of Health and Care Excellence suggests that assessment should be considered if the patient meets psychiatric diagnostic criteria and one of the following:
- Difficulty obtaining or sustaining employment or education
- Difficulty initiating or sustaining social relationships
- Past or current contact with mental health or learning disability services
- History of a neurodevelopmental or mental health disorder.15,16
Currently, diagnosis typically involves a multidisciplinary approach, with psychiatric assessment, neuropsychological testing, and speech and language evaluation.17 Providers may need to refer patients for these services, sometimes at the patient’s request, if previous mental health misdiagnoses are suspected, if patients report symptoms or impairment consistent with ASD, or if benefits, services, or accommodations, such as a coach in the workplace, are needed.
Diagnosing ASD in adults can be difficult, given that the gold-standard diagnostic tests such as the Autism Diagnostic Observation Schedule-2 (ADOS-2)18 and the Autism Diagnostic Interview-Revised (ADI-R)19 are typically used to diagnose autism in children. However, Module 4 in the ADOS-2 was developed for adolescents and older patients with fluent language and has shown at least moderate power to distinguish adults with ASD from those without ASD.18,20
An initial psychiatric assessment should include a thorough history taken from the patient and, if applicable, the patient’s caregiver, as well as a psychiatric interview of the patient. Neuropsychological testing should include evaluation of cognitive function, social functioning (using the ADOS-2 for adults without intellectual disability, the ADI-R, or both), and adaptive functioning (using the Vineland Adaptive Behavior Scales, second edition21).
Evaluation of speech and language is particularly important in patients with limited language ability and should include both expressive and receptive language abilities. Serial testing every few years, as is often recommended in childhood, may help establish the pattern of impairment over time.
Comorbid psychiatric disorders are common
Many people with ASD also have other psychiatric disorders,17,22 which clinicians should keep in mind when seeing an adult seeking evaluation for ASD.
Attention-deficit/hyperactivity disorder is present at higher rates in patients of average intellectual function with ASD than in the general population.23
Anxiety disorders, including obsessive-compulsive disorder, were found to often coexist with autism in a sample of adults with autism without intellectual disability,24,25 and approximately 40% of youths with ASD have at least 1 comorbid anxiety disorder.26
Mood disorders are also prevalent in adults with ASD, with a small study showing that 70% of adults with DSM-IV Asperger syndrome had at least 1 depressive episode in their lifetime.27
BEHAVIORAL AND PHARMACOLOGIC THERAPIES FOR THE ADULT PATIENT
Services and medications for adults with ASD are discussed below. These will vary by individual, and services available may vary by region.
Historically, vocational and social outcomes have been poor for adults with ASD. It is estimated that most larger universities may be home to 100 to 300 students with ASD. To combat isolation, the University of California, Los Angeles, the University of Alabama, and others provide special support services, including group social activities such as board games and individual coaching.8 Nevertheless, half of the students with autism who attend institutions of higher learning leave without completing their intended degree.29 Many still struggle to establish meaningful friendships or romantic relationships.29
Planning for a transition of care
Healthcare transition planning is important but is strikingly underused.30 Individual providers, including adult psychiatrists, vary in their level of training and comfort in diagnosing, treating, and monitoring adults with autism. Youths with ASD are half as likely to receive healthcare transition services as other youths with special healthcare needs.31
Pediatric providers, including pediatric psychiatrists, developmental behavioral specialists, and pediatric neurologists, may be best equipped to treat young adult patients or to refer patients to appropriate generalists and specialists comfortable with autism-specific transition of care. The question of eligibility for services is important to patients and families during the transition period, with many parents and professionals unaware of services available to them.32 Receiving adequate transition services is enabled by having a medical home during childhood—that is, a comprehensive, centralized medical record, culturally competent care, interaction with schools, and patient access to clear, unbiased information.31
Ideally, in our experience, transitioning should be discussed well before the child ages out of the pediatric provider’s practice. If necessary, healthcare transition services should include 4 components:
- Discussing the switch to a new physician who treats adults
- Discussing changing healthcare needs as an adult
- Planning insurance coverage as an adult
- Encouragement by the physician for the child to take age-appropriate responsibility for his or her healthcare.31,33
Tools such as the Got Transition checklist from the National Health Care Transition Center can provide support during this process.34
Other services
Other services provided as an extension or adjunct to the medical home in early adulthood may include customized vocational or employment training, specialized mentorship or support in a college setting, housing support, and psychological services.35
Community-based programs that emphasize leisure have been shown to improve participants’ independence and quality of life.36 Similarly, participants in programs that emphasized supported employment, with a job coach, on-the-job support, collaboration with the participant’s larger social support network, and selection of tasks to match an individual’s abilities and strengths, demonstrated improved cognitive performance, particularly executive functioning,37 and employment.38,39 These programs work best for patients who have mild to moderate symptoms.37,39
Patients with symptoms that are more severe may do better in a residential program. Many of these programs maintain an emphasis on vocational and social skills development. One such long-standing program is Bittersweet Farms, a rural farming community in Ohio for adults with ASD, where individuals with moderate to low function live in a group setting, with emphasis on scheduled, meaningful work including horticulture, animal care, carpentry; and activities of daily living.40
Studies of patients across the autism spectrum have generally found better outcomes when vocational support is given, but larger and randomized studies are needed to characterize how to best support these individuals after they leave high school.41
Psychological services such as applied behavioral therapy, social cognition training, cognitive behavioral therapy, and mindfulness training may be particularly useful in adults.42–44
Some versions of applied behavioral therapy, such as the Early Start Denver Model,45 have been found to be cost-effective and offset some expenses in the care of children with autism, using play-based and relationship-based interventions to promote development across domains while reducing symptoms.
In randomized controlled trials, modified cognitive behavioral therapy43 and mindfulness44 were shown to reduce symptoms of anxiety, obsessive-compulsive disorder, and depression.
Dialectical behavior therapy, used to find a balance between accepting oneself and desiring to change, may help in some circumstances to regulate emotions and reduce reactivity and lability, although large randomized clinical trials have not been conducted in the ASD population.46
Drug therapy
Medications may be appropriate to manage symptoms or comorbid conditions in adults with ASD. Over 75% adults with ASD have been found to use psychotropic medications.47 However, although these drugs have been approved for treating behaviors commonly associated with ASD, none of them provide definitive treatment for this disorder, and they have not been rigorously tested or approved for use in adults with ASD.48
Irritability and aggression associated with ASD can be treated with risperidone (approved for children over age 5), aripiprazole (approved for children ages 6–17), clozapine, or haloperidol.49
Aberrant social behavior can be treated with risperidone.50 Treatments under investigation include oxytocin and secretin.49
While no approved drug has been shown to improve social communication,51 balovaptan, a vasopressin V1a agonist, has shown potential and has been granted breakthrough status by the US Food and Drug Administration for treating challenging behaviors in adults, with additional studies ongoing in children.52,53
Repetitive behaviors, if the patient finds them impairing, can be managed with selective serotonin reuptake inhibitors.49
Much more study of drug therapy in adults with ASD is needed to fully understand the best approaches to psychotropic medication use, including appropriate classes and effective dosage, in this population.
SEX: UNEXPLORED TERRITORY
The reproductive health needs of people with autism remain largely underexplored.54 Historically, individuals with ASD were thought to have little interest in sexual activity or parenthood, owing to the nature of the core symptoms of the disorder. This has been shown to be untrue, particularly as studies on this topic began to engage in direct interviews with people with ASD, rather than solely gathering information from caregivers or parents. The findings reinforce the importance of broaching this component of health in this population, for the following reasons:
Adults with ASD are at increased risk of sexual victimization, with nearly 4 out of 5 reporting unwanted sexual advances, coercion, or rape.55
They have a smaller pool of knowledge with respect to sexual health. They report56 that they learned about sex from television and from “making mistakes.” They use fewer sources. They are less likely to speak to peers and figures of authority to gain knowledge about sexually transmitted infections, sexual behaviors, and contraception. And they are more likely to use forms of nonsocial media, such as television, for information.55
They report more concerns about the future with respect to sexual behavior, suggesting the need for targeted sexual education programs.56
College-age young adults with ASD who misread communication may be particularly affected by Title IX, which requires schools to promptly investigate reports of sexual harassment and sexual assault, should they struggle to comport themselves appropriately.57 Early and frank conversations about issues of consent and appropriate displays of interest and affection may better equip youth to navigate new social scenarios as they plan to leave a supervised home environment for college or the workforce.
Gender identification: Male, female, other
In one study, 77.8% of birth-sex males with ASD said they identified as men, and 67.1% of birth-sex females identified as women, compared with 93.1% of birth-sex males and 87.3% of birth-sex females without ASD. Many of the remaining individuals with ASD reported a transgender, genderqueer, or other gender identity.58 Some studies have found females with ASD report a gay or bisexual orientation more often than males with ASD.59–61
Adolescents and young adults may be exploring their changing bodies, sexual preferences, and gender roles, and as for all people at this age, these roles emerge against a backdrop of familial and societal expectations that may or may not be concordant with their own projected path regarding sexuality and reproductive health.62
Having the conversation
As with non-ASD patients, a thorough sexual history should be collected via open-ended questions when possible to determine types of sexual activity and partners.
Education of the patient, alongside caregivers and parents, about healthy and safe sexual practices, screening for sexual violence, and hormonal and nonhormonal contraception options are important components of care for this population.
CAREGIVER STRESS MAY PERSIST INTO PATIENT’S ADULTHOOD
Caregiver burden is a monumental concern for parents or others who may have lifelong primary responsibility for these neurodiverse adults.63 Family members may feel isolated and may feel they have encountered many barriers to services.64 Remaining sensitive, knowledgeable, and inquisitive about the types of support that are needed may help forge a trusting relationship between the provider and the family.
Parents of children with ASD have been reported to experience worse physical and emotional health than parents whose children do not have developmental disabilities.63,65 These disparities have been found to persist as their children enter adolescence and young adulthood.66,67 Parents of children with ASD report more anxiety, depression, and distress compared with parents of children without ASD,63 and parents themselves may be affected by ASD symptoms, which has been linked to increased parenting stress.68 Some studies have found blunted cortisol responses,63,69,70 and some,71 but not all,63 have found elevated blood pressure in caregivers of children with developmental disabilities. Headache, backache, muscle soreness, and fatigue may also be commonly reported.67
In our experience, caregivers are tremendously appreciative when provided connections to adult ASD services and support systems as their child ages. The school system and other formal support systems often assist until the time of transition into adulthood. This transition can be stressful for the adolescent and family alike, and informal support systems such as friends and family may become increasingly crucial, particularly if the adolescent still lives at home.72,73
The affected young adult’s unmet needs, as perceived by the caregiver, have been found to be significantly associated with caregiver burden, whereas the severity of the adult patient’s ASD symptoms has not.66 Therefore, it may be helpful to ask caregivers whether they perceive any unmet needs, regardless of the clinician’s perception of the severity of the patient’s ASD symptoms. Providing support to address these needs, particularly those relating to the child’s mood disorders, communication, social needs, safety, and daytime activities, may be the domains of support that most effectively reduce the caregiver burden in this population.66
Caregiver positivity, lower stress levels, and increased social support, particularly in the form of friends and family members providing no-cost assistance to caregivers whose children do not live independently,74 have been linked to better outcomes for caregivers.70,74,75 Rigorous studies that examine caregiver burden as individuals with ASD enter mid- and late-adulthood are limited.
THE ROLE OF THE INTERNIST IN CARING FOR ADULTS WITH AUTISM
A major challenge for many adults with ASD is the transition from services provided during childhood to those provided in adulthood. While children with autism have subspecialty providers who diagnose and manage their condition, including developmental-behavioral pediatricians, pediatric neurologists, and child psychiatrists, adults with autism may have fewer options.
Autism centers are becoming more available across the nation, and many provide care across the life span. However, depending on a patient’s needs, the primary care provider may need to manage residual symptoms as the patient transitions from pediatric to adult care, ultimately deciding when and where to refer the patient.
The patient’s family should pay close attention to function and mood around the time the patient leaves the structure of high school, and they should build rapport with a primary care provider they can turn to if problems persist or arise. Referrals for behavioral therapy and for social work, job training, and vocational support can greatly benefit patients as they transition to young adulthood. Referrals and suggestions for social support can also help caregivers.
Medical care
Deciding when and how to medicate the patient for symptoms of autism and related behaviors necessitates consideration of the patient’s impairment, side effects of the medication, and the impact medications may have on the patient’s other conditions. Disordered eating, mood problems, anxiety, and attention-deficit/hyperactivity disorder should be considered, and, as in all patients, regular screenings of mental health status should be conducted.76,77
Comorbid medical conditions may cause worsening of a patient’s known behavioral symptoms or may precipitate new behaviors or aggression as a result of pain or discomfort, particularly in patients with limited speech. A change in stereotypes or increased irritability warrants a thoughtful investigation for a cause other than ASD before adding or increasing behavioral medications. Common comorbid conditions include gastrointestinal distress, most commonly constipation and diarrhea in an idiopathic ASD population, with increasing ASD symptom severity correlating with increased odds of a gastrointestinal problem.78 Allergies, sleep disorders, seizures, and other psychiatric conditions are also frequent.79
Preventive care, including vaccinations, should be given as scheduled. Caregivers and patients can be reminded if needed that vaccines do not cause or worsen autism, and vaccination is intended to improve the safety of the patient and those around them, protecting against potentially life-threatening disease. Regular dental care visits, particularly for patients who are using medications that may affect tooth or gingival health,80 and regular visits to an optometrist or ophthalmologist for screening of vision are also advised.
Adverse effects. Weight gain and metabolic syndrome are common adverse effects of medications used for behavioral management, and the primary care physician may uncover diabetes, cardiac disorders, and hyperlipidemia. Patients with ASD may be particularly sensitive to the effects of medications and therefore may require a lower dose or a slower titration than other patients. Working with a behavioral team, careful weaning of psychiatric medications to the minimum needed is strongly recommended whenever possible.81
TAKE-HOME POINTS
As more adults with autism enter society, they may require varying levels of support from the healthcare community to ensure that therapeutic gains from childhood persist, allowing them to achieve maximal functional potential.
Adults with ASD may have a high, normal, or low IQ and intellectual capability. Knowledge of this and of the patient’s symptom severity and presence of comorbid psychiatric and other health conditions can help the clinician guide the patient to appropriate social services and pharmacologic treatments.
Individualized support in the workplace, as well as education regarding sexual health, can help improve outcomes for affected individuals.
Caregiver burden for individuals with autism can be high, but it can be mitigated by social support.
Further research regarding appropriate diagnostic instruments in adulthood and appropriate treatments for impairing autism-related symptoms across the life span may be particularly helpful in supporting this patient population.
Autism spectrum disorder (ASD) has increased significantly over the past 40 years. Even in the past 2 decades, the prevalence increased from 6.7 per 1,000 in 20001 to 14.6 per 1,000 in 2012—1 in 59 people.2 Of those with ASD, 46% have an intelligence quotient (IQ) greater than 85, meaning they are of average or above-average intelligence.1
As more children with autism become adults, understanding this condition across the life span grows paramount. While many studies have focused on understanding how diagnosis and treatment can help young children, few have focused on adults with autism and how primary care teams can better assist these individuals. However, this is changing, with studies of the benefits of employment programs and pharmacologic treatment, and reproductive health needs of adults with ASD. Here we provide an updated review of ASD in adult patients.
NO MORE ASPERGER SYNDROME— IT’S ON THE SPECTRUM NOW
As the scientific understanding of autism has expanded, revisions in the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5),3 published in 2013, have paralleled these advances. For many adult patients with autism who were evaluated as children, these revisions have led to changes in diagnosis and available services.
In the previous edition (DSM-IV-TR, published in 2000),4 autistic disorder and Asperger syndrome were separate (Table 1). However, DSM-5 lumped autistic disorder and Asperger disorder together under the diagnosis of ASD; this leaves it to the clinician to specify whether the patient with ASD has accompanying intellectual or language impairment and to assign a level of severity based on communication deficits and restrictive behaviors.
The shift in diagnosis was worrisome for some, particularly for clinicians treating patients with DSM-IV Asperger syndrome, who lost this diagnostic label. Concerns that patients with Asperger syndrome may not meet the DSM-5 criteria for ASD were validated by a systematic review showing that only 50% to 75% of patients with DSM-IV autistic disorder, Asperger syndrome, or pervasive developmental disorder not otherwise specified (PDD-NOS) met the DSM-5 criteria for ASD.5 Most of those who no longer met the criteria for ASD carried a DSM-IV diagnosis of Asperger syndrome or PDD-NOS or had an IQ over 70.5 Nevertheless, these individuals may struggle with impairing symptoms related to repetitive behaviors or communication or may be affected by learning or social-emotional disabilities. Additionally, even if they meet the criteria for ASD, some may identify with the Asperger syndrome label and fear they will be stigmatized should they be classified as having the more general ASD.6,7
Although future revisions to the DSM may include further changes in classification, grouping adults with ASD according to their functional and cognitive ability may allow for pragmatic characterization of their needs. At least 3 informal groupings of autistic adults have been described that integrate cognitive ability and independence8:
- Those with low cognitive and social abilities, who need lifelong support
- Those with midrange cognitive and social limitations but who can complete their work in special education classes; they often find employment in supervised workshops or other work with repetitive tasks
- Those who have greater cognitive ability and some social skills; they may proceed to college and employment and live independently.
UNCERTAIN PROGNOSIS
Prognostication for people with ASD remains an area of research. Some adults experience a reduction in symptoms as they age, with significant improvements in speech and, sometimes, modest improvements in restrictive and repetitive behaviors.9,10
Nevertheless, autism remains a lifelong disorder for many. Adults may still require significant support and may experience impairment, particularly in social interaction.10 In longitudinal studies, only 15% to 27% of patients with ASD are characterized as having a positive outcome (often defined as variables related to independent function, near-normal relationships, employment, or a quantified reduction in core symptoms), and many experience significant dependency into adulthood.10–13
IQ has been cited as a possible prognostic factor,10,13 with an IQ below 70 associated with poorer outcome, although an IQ above 70 does not necessarily confer a positive outcome. Less-severe impairment in speech at baseline in early childhood also suggests better outcomes in adulthood.10
As we see more adults with autism, studies that include both children and adults, such as the Longitudinal European Autism Cohort, will be important to characterize the natural history, comorbidities, and genetics of ASD and may help provide more specific predictors of disease course into adulthood.14
ACHIEVING A DIAGNOSIS FOR ADULT PATIENTS WITH SUSPECTED AUTISM
While many patients are recognized as having autism in early to mid-childhood, some adults may not receive a formal diagnosis until much later in life. Those with fluent language and normal-range IQ are likely to be overlooked.15 People with ASD may have had mild symptoms during childhood that did not impair their functioning until demands of daily life exceeded their capacities in adulthood. Alternatively, parents of a child with newly diagnosed ASD may realize that they themselves or another adult family member also show signs of it.
The UK National Institute of Health and Care Excellence suggests that assessment should be considered if the patient meets psychiatric diagnostic criteria and one of the following:
- Difficulty obtaining or sustaining employment or education
- Difficulty initiating or sustaining social relationships
- Past or current contact with mental health or learning disability services
- History of a neurodevelopmental or mental health disorder.15,16
Currently, diagnosis typically involves a multidisciplinary approach, with psychiatric assessment, neuropsychological testing, and speech and language evaluation.17 Providers may need to refer patients for these services, sometimes at the patient’s request, if previous mental health misdiagnoses are suspected, if patients report symptoms or impairment consistent with ASD, or if benefits, services, or accommodations, such as a coach in the workplace, are needed.
Diagnosing ASD in adults can be difficult, given that the gold-standard diagnostic tests such as the Autism Diagnostic Observation Schedule-2 (ADOS-2)18 and the Autism Diagnostic Interview-Revised (ADI-R)19 are typically used to diagnose autism in children. However, Module 4 in the ADOS-2 was developed for adolescents and older patients with fluent language and has shown at least moderate power to distinguish adults with ASD from those without ASD.18,20
An initial psychiatric assessment should include a thorough history taken from the patient and, if applicable, the patient’s caregiver, as well as a psychiatric interview of the patient. Neuropsychological testing should include evaluation of cognitive function, social functioning (using the ADOS-2 for adults without intellectual disability, the ADI-R, or both), and adaptive functioning (using the Vineland Adaptive Behavior Scales, second edition21).
Evaluation of speech and language is particularly important in patients with limited language ability and should include both expressive and receptive language abilities. Serial testing every few years, as is often recommended in childhood, may help establish the pattern of impairment over time.
Comorbid psychiatric disorders are common
Many people with ASD also have other psychiatric disorders,17,22 which clinicians should keep in mind when seeing an adult seeking evaluation for ASD.
Attention-deficit/hyperactivity disorder is present at higher rates in patients of average intellectual function with ASD than in the general population.23
Anxiety disorders, including obsessive-compulsive disorder, were found to often coexist with autism in a sample of adults with autism without intellectual disability,24,25 and approximately 40% of youths with ASD have at least 1 comorbid anxiety disorder.26
Mood disorders are also prevalent in adults with ASD, with a small study showing that 70% of adults with DSM-IV Asperger syndrome had at least 1 depressive episode in their lifetime.27
BEHAVIORAL AND PHARMACOLOGIC THERAPIES FOR THE ADULT PATIENT
Services and medications for adults with ASD are discussed below. These will vary by individual, and services available may vary by region.
Historically, vocational and social outcomes have been poor for adults with ASD. It is estimated that most larger universities may be home to 100 to 300 students with ASD. To combat isolation, the University of California, Los Angeles, the University of Alabama, and others provide special support services, including group social activities such as board games and individual coaching.8 Nevertheless, half of the students with autism who attend institutions of higher learning leave without completing their intended degree.29 Many still struggle to establish meaningful friendships or romantic relationships.29
Planning for a transition of care
Healthcare transition planning is important but is strikingly underused.30 Individual providers, including adult psychiatrists, vary in their level of training and comfort in diagnosing, treating, and monitoring adults with autism. Youths with ASD are half as likely to receive healthcare transition services as other youths with special healthcare needs.31
Pediatric providers, including pediatric psychiatrists, developmental behavioral specialists, and pediatric neurologists, may be best equipped to treat young adult patients or to refer patients to appropriate generalists and specialists comfortable with autism-specific transition of care. The question of eligibility for services is important to patients and families during the transition period, with many parents and professionals unaware of services available to them.32 Receiving adequate transition services is enabled by having a medical home during childhood—that is, a comprehensive, centralized medical record, culturally competent care, interaction with schools, and patient access to clear, unbiased information.31
Ideally, in our experience, transitioning should be discussed well before the child ages out of the pediatric provider’s practice. If necessary, healthcare transition services should include 4 components:
- Discussing the switch to a new physician who treats adults
- Discussing changing healthcare needs as an adult
- Planning insurance coverage as an adult
- Encouragement by the physician for the child to take age-appropriate responsibility for his or her healthcare.31,33
Tools such as the Got Transition checklist from the National Health Care Transition Center can provide support during this process.34
Other services
Other services provided as an extension or adjunct to the medical home in early adulthood may include customized vocational or employment training, specialized mentorship or support in a college setting, housing support, and psychological services.35
Community-based programs that emphasize leisure have been shown to improve participants’ independence and quality of life.36 Similarly, participants in programs that emphasized supported employment, with a job coach, on-the-job support, collaboration with the participant’s larger social support network, and selection of tasks to match an individual’s abilities and strengths, demonstrated improved cognitive performance, particularly executive functioning,37 and employment.38,39 These programs work best for patients who have mild to moderate symptoms.37,39
Patients with symptoms that are more severe may do better in a residential program. Many of these programs maintain an emphasis on vocational and social skills development. One such long-standing program is Bittersweet Farms, a rural farming community in Ohio for adults with ASD, where individuals with moderate to low function live in a group setting, with emphasis on scheduled, meaningful work including horticulture, animal care, carpentry; and activities of daily living.40
Studies of patients across the autism spectrum have generally found better outcomes when vocational support is given, but larger and randomized studies are needed to characterize how to best support these individuals after they leave high school.41
Psychological services such as applied behavioral therapy, social cognition training, cognitive behavioral therapy, and mindfulness training may be particularly useful in adults.42–44
Some versions of applied behavioral therapy, such as the Early Start Denver Model,45 have been found to be cost-effective and offset some expenses in the care of children with autism, using play-based and relationship-based interventions to promote development across domains while reducing symptoms.
In randomized controlled trials, modified cognitive behavioral therapy43 and mindfulness44 were shown to reduce symptoms of anxiety, obsessive-compulsive disorder, and depression.
Dialectical behavior therapy, used to find a balance between accepting oneself and desiring to change, may help in some circumstances to regulate emotions and reduce reactivity and lability, although large randomized clinical trials have not been conducted in the ASD population.46
Drug therapy
Medications may be appropriate to manage symptoms or comorbid conditions in adults with ASD. Over 75% adults with ASD have been found to use psychotropic medications.47 However, although these drugs have been approved for treating behaviors commonly associated with ASD, none of them provide definitive treatment for this disorder, and they have not been rigorously tested or approved for use in adults with ASD.48
Irritability and aggression associated with ASD can be treated with risperidone (approved for children over age 5), aripiprazole (approved for children ages 6–17), clozapine, or haloperidol.49
Aberrant social behavior can be treated with risperidone.50 Treatments under investigation include oxytocin and secretin.49
While no approved drug has been shown to improve social communication,51 balovaptan, a vasopressin V1a agonist, has shown potential and has been granted breakthrough status by the US Food and Drug Administration for treating challenging behaviors in adults, with additional studies ongoing in children.52,53
Repetitive behaviors, if the patient finds them impairing, can be managed with selective serotonin reuptake inhibitors.49
Much more study of drug therapy in adults with ASD is needed to fully understand the best approaches to psychotropic medication use, including appropriate classes and effective dosage, in this population.
SEX: UNEXPLORED TERRITORY
The reproductive health needs of people with autism remain largely underexplored.54 Historically, individuals with ASD were thought to have little interest in sexual activity or parenthood, owing to the nature of the core symptoms of the disorder. This has been shown to be untrue, particularly as studies on this topic began to engage in direct interviews with people with ASD, rather than solely gathering information from caregivers or parents. The findings reinforce the importance of broaching this component of health in this population, for the following reasons:
Adults with ASD are at increased risk of sexual victimization, with nearly 4 out of 5 reporting unwanted sexual advances, coercion, or rape.55
They have a smaller pool of knowledge with respect to sexual health. They report56 that they learned about sex from television and from “making mistakes.” They use fewer sources. They are less likely to speak to peers and figures of authority to gain knowledge about sexually transmitted infections, sexual behaviors, and contraception. And they are more likely to use forms of nonsocial media, such as television, for information.55
They report more concerns about the future with respect to sexual behavior, suggesting the need for targeted sexual education programs.56
College-age young adults with ASD who misread communication may be particularly affected by Title IX, which requires schools to promptly investigate reports of sexual harassment and sexual assault, should they struggle to comport themselves appropriately.57 Early and frank conversations about issues of consent and appropriate displays of interest and affection may better equip youth to navigate new social scenarios as they plan to leave a supervised home environment for college or the workforce.
Gender identification: Male, female, other
In one study, 77.8% of birth-sex males with ASD said they identified as men, and 67.1% of birth-sex females identified as women, compared with 93.1% of birth-sex males and 87.3% of birth-sex females without ASD. Many of the remaining individuals with ASD reported a transgender, genderqueer, or other gender identity.58 Some studies have found females with ASD report a gay or bisexual orientation more often than males with ASD.59–61
Adolescents and young adults may be exploring their changing bodies, sexual preferences, and gender roles, and as for all people at this age, these roles emerge against a backdrop of familial and societal expectations that may or may not be concordant with their own projected path regarding sexuality and reproductive health.62
Having the conversation
As with non-ASD patients, a thorough sexual history should be collected via open-ended questions when possible to determine types of sexual activity and partners.
Education of the patient, alongside caregivers and parents, about healthy and safe sexual practices, screening for sexual violence, and hormonal and nonhormonal contraception options are important components of care for this population.
CAREGIVER STRESS MAY PERSIST INTO PATIENT’S ADULTHOOD
Caregiver burden is a monumental concern for parents or others who may have lifelong primary responsibility for these neurodiverse adults.63 Family members may feel isolated and may feel they have encountered many barriers to services.64 Remaining sensitive, knowledgeable, and inquisitive about the types of support that are needed may help forge a trusting relationship between the provider and the family.
Parents of children with ASD have been reported to experience worse physical and emotional health than parents whose children do not have developmental disabilities.63,65 These disparities have been found to persist as their children enter adolescence and young adulthood.66,67 Parents of children with ASD report more anxiety, depression, and distress compared with parents of children without ASD,63 and parents themselves may be affected by ASD symptoms, which has been linked to increased parenting stress.68 Some studies have found blunted cortisol responses,63,69,70 and some,71 but not all,63 have found elevated blood pressure in caregivers of children with developmental disabilities. Headache, backache, muscle soreness, and fatigue may also be commonly reported.67
In our experience, caregivers are tremendously appreciative when provided connections to adult ASD services and support systems as their child ages. The school system and other formal support systems often assist until the time of transition into adulthood. This transition can be stressful for the adolescent and family alike, and informal support systems such as friends and family may become increasingly crucial, particularly if the adolescent still lives at home.72,73
The affected young adult’s unmet needs, as perceived by the caregiver, have been found to be significantly associated with caregiver burden, whereas the severity of the adult patient’s ASD symptoms has not.66 Therefore, it may be helpful to ask caregivers whether they perceive any unmet needs, regardless of the clinician’s perception of the severity of the patient’s ASD symptoms. Providing support to address these needs, particularly those relating to the child’s mood disorders, communication, social needs, safety, and daytime activities, may be the domains of support that most effectively reduce the caregiver burden in this population.66
Caregiver positivity, lower stress levels, and increased social support, particularly in the form of friends and family members providing no-cost assistance to caregivers whose children do not live independently,74 have been linked to better outcomes for caregivers.70,74,75 Rigorous studies that examine caregiver burden as individuals with ASD enter mid- and late-adulthood are limited.
THE ROLE OF THE INTERNIST IN CARING FOR ADULTS WITH AUTISM
A major challenge for many adults with ASD is the transition from services provided during childhood to those provided in adulthood. While children with autism have subspecialty providers who diagnose and manage their condition, including developmental-behavioral pediatricians, pediatric neurologists, and child psychiatrists, adults with autism may have fewer options.
Autism centers are becoming more available across the nation, and many provide care across the life span. However, depending on a patient’s needs, the primary care provider may need to manage residual symptoms as the patient transitions from pediatric to adult care, ultimately deciding when and where to refer the patient.
The patient’s family should pay close attention to function and mood around the time the patient leaves the structure of high school, and they should build rapport with a primary care provider they can turn to if problems persist or arise. Referrals for behavioral therapy and for social work, job training, and vocational support can greatly benefit patients as they transition to young adulthood. Referrals and suggestions for social support can also help caregivers.
Medical care
Deciding when and how to medicate the patient for symptoms of autism and related behaviors necessitates consideration of the patient’s impairment, side effects of the medication, and the impact medications may have on the patient’s other conditions. Disordered eating, mood problems, anxiety, and attention-deficit/hyperactivity disorder should be considered, and, as in all patients, regular screenings of mental health status should be conducted.76,77
Comorbid medical conditions may cause worsening of a patient’s known behavioral symptoms or may precipitate new behaviors or aggression as a result of pain or discomfort, particularly in patients with limited speech. A change in stereotypes or increased irritability warrants a thoughtful investigation for a cause other than ASD before adding or increasing behavioral medications. Common comorbid conditions include gastrointestinal distress, most commonly constipation and diarrhea in an idiopathic ASD population, with increasing ASD symptom severity correlating with increased odds of a gastrointestinal problem.78 Allergies, sleep disorders, seizures, and other psychiatric conditions are also frequent.79
Preventive care, including vaccinations, should be given as scheduled. Caregivers and patients can be reminded if needed that vaccines do not cause or worsen autism, and vaccination is intended to improve the safety of the patient and those around them, protecting against potentially life-threatening disease. Regular dental care visits, particularly for patients who are using medications that may affect tooth or gingival health,80 and regular visits to an optometrist or ophthalmologist for screening of vision are also advised.
Adverse effects. Weight gain and metabolic syndrome are common adverse effects of medications used for behavioral management, and the primary care physician may uncover diabetes, cardiac disorders, and hyperlipidemia. Patients with ASD may be particularly sensitive to the effects of medications and therefore may require a lower dose or a slower titration than other patients. Working with a behavioral team, careful weaning of psychiatric medications to the minimum needed is strongly recommended whenever possible.81
TAKE-HOME POINTS
As more adults with autism enter society, they may require varying levels of support from the healthcare community to ensure that therapeutic gains from childhood persist, allowing them to achieve maximal functional potential.
Adults with ASD may have a high, normal, or low IQ and intellectual capability. Knowledge of this and of the patient’s symptom severity and presence of comorbid psychiatric and other health conditions can help the clinician guide the patient to appropriate social services and pharmacologic treatments.
Individualized support in the workplace, as well as education regarding sexual health, can help improve outcomes for affected individuals.
Caregiver burden for individuals with autism can be high, but it can be mitigated by social support.
Further research regarding appropriate diagnostic instruments in adulthood and appropriate treatments for impairing autism-related symptoms across the life span may be particularly helpful in supporting this patient population.
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2000 Principal Investigators; Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders—autism and developmental disabilities monitoring network, six sites, United States, 2000. MMWR Surveill Summ 2007; 56(1):1–11. pmid:17287714
- Christensen DL. Prevalence and characteristics of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2012. MMWR Surveill Summ 2016; 65(13):1–23. doi:10.15585/mmwr.ss6503a1
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edition. Washington, D.C: American Psychiatric Association; 2013.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision. Washington, DC: American Psychiatric Association; 2000.
- Smith IC, Reichow B, Volkmar FR. The effects of DSM-5 criteria on number of individuals diagnosed with autism spectrum disorder: a systematic review. J Autism Dev Disord 2015; 45(8):2541–2552. doi:10.1007/s10803-015-2423-8
- Barahona-Corrêa JB, Filipe CN. A concise history of Asperger syndrome: the short reign of a troublesome diagnosis. Front Psychol 2015; 6:2024. doi:10.3389/fpsyg.2015.02024
- Kite DM, Gullifer J, Tyson GA. Views on the diagnostic labels of autism and Asperger’s disorder and the proposed changes in the DSM. J Autism Dev Disord 2013; 43(7):1692–1700. doi:10.1007/s10803-012-1718-2
- Kuo AA. Autism in adults: an update. Presented at the: American College of Physicians Internal Medicine Meeting, New Orleans, LA, April 17–21, 2018.
- Shattuck PT, Seltzer MM, Greenberg JS, et al. Change in autism symptoms and maladaptive behaviors in adolescents and adults with an autism spectrum disorder. J Autism Dev Disord 2007; 37(9):1735–1747. doi:10.1007/s10803-006-0307-7
- Seltzer MM, Shattuck P, Abbeduto L, Greenberg JS. Trajectory of development in adolescents and adults with autism. Ment Retard Dev Disabil Res Rev 2004; 10(4):234–247. doi:10.1002/mrdd.20038
- Billstedt E, Carina Gillberg I, Gillberg C. Autism in adults: symptom patterns and early childhood predictors. Use of the DISCO in a community sample followed from childhood. J Child Psychol Psychiatry 2007; 48(11):1102–1110. doi:10.1111/j.1469-7610.2007.01774.x
- Howlin P, Goode S, Hutton J, Rutter M. Adult outcome for children with autism. J Child Psychol Psychiatry 2004; 45(2):212–229. pmid:14982237
- Marriage S, Wolverton A, Marriage K. Autism spectrum disorder grown up: a chart review of adult functioning. J Can Acad Child Adolesc Psychiatry 2009; 18(4):322–328. pmid: 19881941
- Isaksson J, Tammimies K, Neufeld J, et al. EU-AIMS Longitudinal European Autism Project (LEAP): the autism twin cohort. Mol Autism 2018; 9(1):26. doi:10.1186/s13229-018-0212-x
- Lai M-C, Baron-Cohen S. Identifying the lost generation of adults with autism spectrum conditions. Lancet Psychiatry 2015; 2(11):1013–1027. doi:10.1016/S2215-0366(15)00277-1
- National Institute for Health and Clinical Excellence. Autism: recognition, referral, diagnosis and management of adults on the autism spectrum. NICE clinical guideline 142. June 2012. https://grand.tghn.org/site_media/media/medialibrary/2015/03/ASD_NICE_3_.pdf. Accessed July 9, 2019.
- Wolf JM, Ventola P. Assessment and treatment planning in adults with autism spectrum disorders. In: Adolescents and Adults with Autism Spectrum Disorders. Springer, New York, NY; 2014:283–298.
- Lord C, Rutter M, DiLavore P, Risi S, Gotham K, Bishop SL. Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) manual. Torrance, CA: Western Psychological Services, 2012.
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 1994; 24(5):659–685.
- Hus V, Lord C. The autism diagnostic observation schedule, module 4: revised algorithm and standardized severity scores. J Autism Dev Disord 2014; 44(8):1996–2012. doi:10.1007/s10803-014-2080-3>
- Sparrow S, Balla D, Cicchetti D, Harrison P, Doll E. Vineland Adaptive Behavior Scales. Circle Pines, MN: American Guidance Service, 1984.
- Happé FG, Mansour H, Barrett P, Brown T, Abbott P, Charlton RA. Demographic and cognitive profile of individuals seeking a diagnosis of autism spectrum disorder in adulthood. J Autism Dev Disord 2016; 46(11):3469–3480. doi:10.1007/s10803-016-2886-2
- Johnston K, Dittner A, Bramham J, Murphy C, Knight A, Russell A. Attention deficit hyperactivity disorder symptoms in adults with autism spectrum disorders. Autism Res Off J Int Soc Autism Res 2013; 6(4):225–236. doi:10.1002/aur.1283
- Cadman T, Spain D, Johnston P, et al. Obsessive-compulsive disorder in adults with high-functioning autism spectrum disorder: what does self-report with the OCI-R tell us? Autism Res Off J Int Soc Autism Res 2015; 8(5):477–485. doi:10.1002/aur.1461
- Russell AJ, Mataix-Cols D, Anson M, Murphy DGM. Obsessions and compulsions in Asperger syndrome and high-functioning autism. Br J Psychiatry J Ment Sci 2005; 186:525–528. doi:10.1192/bjp.186.6.525
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry 2008; 47(8):921–929. doi:10.1097/CHI.0b013e318179964f
- Lugnegård T, Hallerbäck MU, Gillberg C. Psychiatric comorbidity in young adults with a clinical diagnosis of Asperger syndrome. Res Dev Disabil 2011; 32(5):1910–1917. doi:10.1016/j.ridd.2011.03.025
- Howlin P, Moss P. Adults with autism spectrum disorders. Can J Psychiatry 2012; 57(5):275–283. doi:10.1177/070674371205700502
- Levy A, Perry A. Outcomes in adolescents and adults with autism: a review of the literature. Res Autism Spectr Disord 2011; 5(4):1271–1282. doi:10.1016/J.RASD.2011.01.023
- Cheak-Zamora NC, Yang X, Farmer JE, Clark M. Disparities in transition planning for youth with autism spectrum disorder. Pediatrics 2013; 131(3):447–454. doi:10.1542/peds.2012-1572
- Rast JE, Shattuck PT, Roux AM, Anderson KA, Kuo A. The medical home and health care transition for youth with autism. Pediatrics 2018; 141(suppl 4):S328–S334. doi:10.1542/peds.2016-4300J
- Belling R, McLaren S, Paul M, et al. The effect of organisational resources and eligibility issues on transition from child and adolescent to adult mental health services. J Health Serv Res Policy 2014; 19(3):169–176. doi:10.1177/1355819614527439
- Data Resource Center for Child & Adolescent Health. 2009–2010 National Survey of Children with Special Health Care Needs. www.childhealthdata.org/docs/drc/200910-cshcn-spss-codebook_final_051012.pdf?sfvrsn=1. Accessed July 9, 2019.
- Got Transition Center for Health Care Transition Improvement. Six core elements of health care transition 2.0. Transitioning youth to an adult health care provider. For use by pediatric, family medicine, and med-peds providers. www.gottransition.org/resourceGet.cfm?id=208. Accessed July 9, 2019.
- Murphy CM, Wilson CE, Robertson DM, et al. Autism spectrum disorder in adults: diagnosis, management, and health services development. Neuropsychiatr Dis Treat 2016; 12:1669–1686. doi:10.2147/NDT.S65455
- García-Villamisar DA, Dattilo J. Effects of a leisure programme on quality of life and stress of individuals with ASD. J Intellect Disabil Res 2010; 54(7):611–619. doi:10.1111/j.1365-2788.2010.01289.x
- García-Villamisar D, Hughes C. Supported employment improves cognitive performance in adults with autism. J Intellect Disabil Res 2007; 51(pt 2):142–150. doi:10.1111/j.1365-2788.2006.00854.x
- Lawer L, Brusilovskiy E, Salzer MS, Mandell DS. Use of vocational rehabilitative services among adults with autism. J Autism Dev Disord 2009; 39(3):487–494. doi:10.1007/s10803-008-0649-4
- Howlin P, Alcock J, Burkin C. An 8 year follow-up of a specialist supported employment service for high-ability adults with autism or Asperger syndrome. Autism 2005; 9(5):533–549. doi:10.1177/1362361305057871
- Kay BR. Bittersweet Farms. J Autism Dev Disord 1990; 20(3):309–321. http://www.ncbi.nlm.nih.gov/pubmed/2228914. Accessed July 9, 2019.
- Taylor JL, McPheeters ML, Sathe NA, Dove D, Veenstra-Vanderweele J, Warren Z. A systematic review of vocational interventions for young adults with autism spectrum disorders. Pediatrics 2012; 130(3):531–538. doi:10.1542/peds.2012-0682
- Bishop-Fitzpatrick L, Minshew NJ, Eack SM. A systematic review of psychosocial interventions for adults with autism spectrum disorders. J Autism Dev Disord 2013; 43(3):687–694. doi:10.1007/s10803-012-1615-8
- Russell AJ, Jassi A, Fullana MA, et al. Cognitive behavior therapy for comorbid obsessive-compulsive disorder in high-functioning autism spectrum disorders: a randomized controlled trial. Depress Anxiety 2013; 30(8):697–708. doi:10.1002/da.22053
- Spek AA, van Ham NC, Nyklícek I. Mindfulness-based therapy in adults with an autism spectrum disorder: a randomized controlled trial. Res Dev Disabil 2013; 34(1):246–253. doi:10.1016/j.ridd.2012.08.009
- Eapen V, Crncec R, Walter A. Clinical outcomes of an early intervention program for preschool children with autism spectrum disorder in a community group setting. BMC Pediatr 2013; 13(1):3. doi:10.1186/1471-2431-13-3
- Mazefsky CA, White SW. Emotion regulation: concepts & practice in autism spectrum disorder. Child Adolesc Psychiatr Clin North Am 2014; 23(1):15–24. doi:10.1016/J.CHC.2013.07.002
- Esbensen AJ, Greenberg JS, Seltzer MM, Aman MG. A longitudinal investigation of psychotropic and non-psychotropic medication use among adolescents and adults with autism spectrum disorders. J Autism Dev Disord 2009; 39(9):1339–1349. doi:10.1007/s10803-009-0750-3
- Dove D, Warren Z, McPheeters ML, Taylor JL, Sathe NA, Veenstra-VanderWeele J. Medications for adolescents and young adults with autism spectrum disorders: a systematic review. Pediatrics 2012; 130(4):717–726. doi:10.1542/peds.2012-0683
- LeClerc S, Easley D. Pharmacological therapies for autism spectrum disorder: a review. Pharm Ther 2015; 40(6):389–397.
- Miral S, Gencer O, Inal-Emiroglu FN, Baykara B, Baykara A, Dirik E. Risperidone versus haloperidol in children and adolescents with AD: a randomized, controlled, double-blind trial. Eur Child Adolesc Psychiatry 2008; 17(1):1–8. doi:10.1007/s00787-007-0620-5
- Lai M-C, Lombardo MV, Baron-Cohen S. Autism. Lancet 2014; 383(9920):896–910. doi:10.1016/S0140-6736(13)61539-1
- Ratni H, Rogers-Evans M, Bissantz C, et al. Discovery of highly selective brain-penetrant vasopressin 1a antagonists for the potential treatment of autism via a chemogenomic and scaffold hopping approach. J Med Chem 2015; 58(5):2275–2289. doi:10.1021/jm501745f
- Umbricht D, Del Valle Rubido M, Hollander E, et al. A single dose, randomized, controlled proof-of-mechanism study of a novel vasopressin 1a receptor antagonist (RG7713) in high-functioning adults with autism spectrum disorder. Neuropsychopharmacology 2017; 42(9):1914–1923. doi:10.1038/npp.2016.232>
- Kellaher DC. Sexual behavior and autism spectrum disorders: an update and discussion. Curr Psychiatry Rep 2015; 17(4):25. doi:10.1007/s11920-015-0562-4
- Brown-Lavoie SM, Viecili MA, Weiss JA. Sexual knowledge and victimization in adults with autism spectrum disorders. J Autism Dev Disord 2014; 44(9):2185–2196. doi:10.1007/s10803-014-2093-y
- Mehzabin P, Stokes MA. Self-assessed sexuality in young adults with high-functioning autism. Res Autism Spectr Disord 2011; 5(1):614–621. doi:10.1016/J.RASD.2010.07.006>
- Brown KR. Accessibility for students with ASD: legal perspectives in the United States. In: Alphin HC Jr. Exploring the Future of Accessibility in Higher Education. Hershey, PA: IGI Global; 2017.
- George R, Stokes MA. Gender identity and sexual orientation in autism spectrum disorder. Autism 2018; 22(8):970–982. doi:10.1177/1362361317714587
- Byers ES, Nichols S, Voyer SD. Challenging stereotypes: sexual functioning of single adults with high functioning autism spectrum disorder. J Autism Dev Disord 2013; 43(11):2617–2627. doi:10.1007/s10803-013-1813-z
- Gilmour L, Schalomon PM, Smith V. Sexuality in a community based sample of adults with autism spectrum disorder. Res Autism Spectr Disord 2012; 6(1):313–318. doi:10.1016/J.RASD.2011.06.003
- Bejerot S, Eriksson JM. Sexuality and gender role in autism spectrum disorder: a case control study. Schmitz C, ed. PLoS One 2014; 9(1):e87961. doi:10.1371/journal.pone.0087961>
- Navot N, Jorgenson AG, Webb SJ. Maternal experience raising girls with autism spectrum disorder: a qualitative study. Child Care Health Dev 2017; 43(4):536–545. doi:10.1111/cch.12470
- Padden C, James JE. Stress among parents of children with and without autism spectrum disorder: a comparison involving physiological indicators and parent self-reports. J Dev Phys Disabil 2017; 29(4):567–586. doi:10.1007/s10882-017-9547-z
- Woodgate RL, Ateah C, Secco L. Living in a world of our own: the experience of parents who have a child with autism. Qual Health Res 2008; 18(8):1075–1083. doi:10.1177/1049732308320112
- Hayes SA, Watson SL. The impact of parenting stress: a meta-analysis of studies comparing the experience of parenting stress in parents of children with and without autism spectrum disorder. J Autism Dev Disord 2013; 43(3):629–642. doi:10.1007/s10803-012-1604-y
- Cadman T, Eklund H, Howley D, et al. Caregiver burden as people with autism spectrum disorder and attention-deficit/hyperactivity disorder transition into adolescence and adulthood in the United Kingdom. J Am Acad Child Adolesc Psychiatry 2012; 51(9):879–888. doi:10.1016/j.jaac.2012.06.017
- Smith LE, Seltzer MM, Greenberg JS. Daily health symptoms of mothers of adolescents and adults with fragile x syndrome and mothers of adolescents and adults with autism spectrum disorder. J Autism Dev Disord 2012; 42(9):1836–1846. doi:10.1007/s10803-011-1422-7
- van Steijn DJ, Oerlemans AM, van Aken MAG, Buitelaar JK, Rommelse NNJ. The reciprocal relationship of ASD, ADHD, depressive symptoms and stress in parents of children with ASD and/or ADHD. J Autism Dev Disord 2014; 44(5):1064–1076. doi:10.1007/s10803-013-1958-9
- Seltzer MM, Greenberg JS, Hong J, et al. Maternal cortisol levels and behavior problems in adolescents and adults with ASD. J Autism Dev Disord 2010; 40(4):457–469. doi:10.1007/S10803-009-0887-0
- Lovell B, Moss M, Wetherell MA. With a little help from my friends: psychological, endocrine and health corollaries of social support in parental caregivers of children with autism or ADHD. Res Dev Disabil 2012; 33(2):682–687. doi:10.1016/j.ridd.2011.11.014
- Gallagher S, Whiteley J. Social support is associated with blood pressure responses in parents caring for children with developmental disabilities. Res Dev Disabil 2012; 33(6):2099–2105. doi:10.1016/j.ridd.2012.06.007
- Baker JK, Smith LE, Greenberg JS, Seltzer MM, Taylor JL. Change in maternal criticism and behavior problems in adolescents and adults with autism across a 7-year period. J Abnorm Psychol 2011; 120(2):465–475. doi:10.1037/a0021900
- Marsack CN, Samuel PS. Mediating effects of social support on quality of life for parents of adults with autism. J Autism Dev Disord 2017; 47(8):2378–2389. doi:10.1007/s10803-017-3157-6
- Trute B, Benzies KM, Worthington C, Reddon JR, Moore M. Accentuate the positive to mitigate the negative: mother psychological coping resources and family adjustment in childhood disability. J Intellect Dev Disabil 2010; 35(1):36–43. doi:10.3109/13668250903496328
- Cantwell J, Muldoon OT, Gallagher S. Social support and mastery influence the association between stress and poor physical health in parents caring for children with developmental disabilities. Res Dev Disabil 2014; 35(9):2215–2223. doi:10.1016/j.ridd.2014.05.012
- Carton AM, Smith AD. Assessing the relationship between eating disorder psychopathology and autistic traits in a non-clinical adult population. Eat Weight Disord - Stud Anorexia, Bulim Obes 2014; 19(3):285–293. doi:10.1007/s40519-013-0086-z
- De Alwis D, Agrawal A, Reiersen AM, et al. ADHD symptoms, autistic traits, and substance use and misuse in adult Australian twins. J Stud Alcohol Drugs 2014; 75(2):211–221. doi:10.15288/jsad.2014.75.211
- Wang LW, Tancredi DJ, Thomas DW. The prevalence of gastrointestinal problems in children across the United States with autism spectrum disorders from families with multiple affected members. J Dev Behav Pediatr 2011; 32(5):351–360. doi:10.1097/DBP.0b013e31821bd06a
- Croen LA, Zerbo O, Qian Y, et al. The health status of adults on the autism spectrum. Autism 2015; 19(7):814–823. doi:10.1177/1362361315577517
- Kalyoncu IÖ, Tanboga I. Oral health status of children with autistic spectrum disorder compared with non-authentic peers. Iran J Public Health 2017; 46(11):1591–1593. www.ncbi.nlm.nih.gov/pmc/articles/PMC5696703. Accessed July 9, 2019.
- McGuire K, Fung LK, Hagopian L, et al. Irritability and problem behavior in autism spectrum disorder: a practice pathway for pediatric primary care. Pediatrics 2016; 137(suppl 2):S136–S148. doi:10.1542/peds.2015-2851L
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2000 Principal Investigators; Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders—autism and developmental disabilities monitoring network, six sites, United States, 2000. MMWR Surveill Summ 2007; 56(1):1–11. pmid:17287714
- Christensen DL. Prevalence and characteristics of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2012. MMWR Surveill Summ 2016; 65(13):1–23. doi:10.15585/mmwr.ss6503a1
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edition. Washington, D.C: American Psychiatric Association; 2013.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision. Washington, DC: American Psychiatric Association; 2000.
- Smith IC, Reichow B, Volkmar FR. The effects of DSM-5 criteria on number of individuals diagnosed with autism spectrum disorder: a systematic review. J Autism Dev Disord 2015; 45(8):2541–2552. doi:10.1007/s10803-015-2423-8
- Barahona-Corrêa JB, Filipe CN. A concise history of Asperger syndrome: the short reign of a troublesome diagnosis. Front Psychol 2015; 6:2024. doi:10.3389/fpsyg.2015.02024
- Kite DM, Gullifer J, Tyson GA. Views on the diagnostic labels of autism and Asperger’s disorder and the proposed changes in the DSM. J Autism Dev Disord 2013; 43(7):1692–1700. doi:10.1007/s10803-012-1718-2
- Kuo AA. Autism in adults: an update. Presented at the: American College of Physicians Internal Medicine Meeting, New Orleans, LA, April 17–21, 2018.
- Shattuck PT, Seltzer MM, Greenberg JS, et al. Change in autism symptoms and maladaptive behaviors in adolescents and adults with an autism spectrum disorder. J Autism Dev Disord 2007; 37(9):1735–1747. doi:10.1007/s10803-006-0307-7
- Seltzer MM, Shattuck P, Abbeduto L, Greenberg JS. Trajectory of development in adolescents and adults with autism. Ment Retard Dev Disabil Res Rev 2004; 10(4):234–247. doi:10.1002/mrdd.20038
- Billstedt E, Carina Gillberg I, Gillberg C. Autism in adults: symptom patterns and early childhood predictors. Use of the DISCO in a community sample followed from childhood. J Child Psychol Psychiatry 2007; 48(11):1102–1110. doi:10.1111/j.1469-7610.2007.01774.x
- Howlin P, Goode S, Hutton J, Rutter M. Adult outcome for children with autism. J Child Psychol Psychiatry 2004; 45(2):212–229. pmid:14982237
- Marriage S, Wolverton A, Marriage K. Autism spectrum disorder grown up: a chart review of adult functioning. J Can Acad Child Adolesc Psychiatry 2009; 18(4):322–328. pmid: 19881941
- Isaksson J, Tammimies K, Neufeld J, et al. EU-AIMS Longitudinal European Autism Project (LEAP): the autism twin cohort. Mol Autism 2018; 9(1):26. doi:10.1186/s13229-018-0212-x
- Lai M-C, Baron-Cohen S. Identifying the lost generation of adults with autism spectrum conditions. Lancet Psychiatry 2015; 2(11):1013–1027. doi:10.1016/S2215-0366(15)00277-1
- National Institute for Health and Clinical Excellence. Autism: recognition, referral, diagnosis and management of adults on the autism spectrum. NICE clinical guideline 142. June 2012. https://grand.tghn.org/site_media/media/medialibrary/2015/03/ASD_NICE_3_.pdf. Accessed July 9, 2019.
- Wolf JM, Ventola P. Assessment and treatment planning in adults with autism spectrum disorders. In: Adolescents and Adults with Autism Spectrum Disorders. Springer, New York, NY; 2014:283–298.
- Lord C, Rutter M, DiLavore P, Risi S, Gotham K, Bishop SL. Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) manual. Torrance, CA: Western Psychological Services, 2012.
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 1994; 24(5):659–685.
- Hus V, Lord C. The autism diagnostic observation schedule, module 4: revised algorithm and standardized severity scores. J Autism Dev Disord 2014; 44(8):1996–2012. doi:10.1007/s10803-014-2080-3>
- Sparrow S, Balla D, Cicchetti D, Harrison P, Doll E. Vineland Adaptive Behavior Scales. Circle Pines, MN: American Guidance Service, 1984.
- Happé FG, Mansour H, Barrett P, Brown T, Abbott P, Charlton RA. Demographic and cognitive profile of individuals seeking a diagnosis of autism spectrum disorder in adulthood. J Autism Dev Disord 2016; 46(11):3469–3480. doi:10.1007/s10803-016-2886-2
- Johnston K, Dittner A, Bramham J, Murphy C, Knight A, Russell A. Attention deficit hyperactivity disorder symptoms in adults with autism spectrum disorders. Autism Res Off J Int Soc Autism Res 2013; 6(4):225–236. doi:10.1002/aur.1283
- Cadman T, Spain D, Johnston P, et al. Obsessive-compulsive disorder in adults with high-functioning autism spectrum disorder: what does self-report with the OCI-R tell us? Autism Res Off J Int Soc Autism Res 2015; 8(5):477–485. doi:10.1002/aur.1461
- Russell AJ, Mataix-Cols D, Anson M, Murphy DGM. Obsessions and compulsions in Asperger syndrome and high-functioning autism. Br J Psychiatry J Ment Sci 2005; 186:525–528. doi:10.1192/bjp.186.6.525
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry 2008; 47(8):921–929. doi:10.1097/CHI.0b013e318179964f
- Lugnegård T, Hallerbäck MU, Gillberg C. Psychiatric comorbidity in young adults with a clinical diagnosis of Asperger syndrome. Res Dev Disabil 2011; 32(5):1910–1917. doi:10.1016/j.ridd.2011.03.025
- Howlin P, Moss P. Adults with autism spectrum disorders. Can J Psychiatry 2012; 57(5):275–283. doi:10.1177/070674371205700502
- Levy A, Perry A. Outcomes in adolescents and adults with autism: a review of the literature. Res Autism Spectr Disord 2011; 5(4):1271–1282. doi:10.1016/J.RASD.2011.01.023
- Cheak-Zamora NC, Yang X, Farmer JE, Clark M. Disparities in transition planning for youth with autism spectrum disorder. Pediatrics 2013; 131(3):447–454. doi:10.1542/peds.2012-1572
- Rast JE, Shattuck PT, Roux AM, Anderson KA, Kuo A. The medical home and health care transition for youth with autism. Pediatrics 2018; 141(suppl 4):S328–S334. doi:10.1542/peds.2016-4300J
- Belling R, McLaren S, Paul M, et al. The effect of organisational resources and eligibility issues on transition from child and adolescent to adult mental health services. J Health Serv Res Policy 2014; 19(3):169–176. doi:10.1177/1355819614527439
- Data Resource Center for Child & Adolescent Health. 2009–2010 National Survey of Children with Special Health Care Needs. www.childhealthdata.org/docs/drc/200910-cshcn-spss-codebook_final_051012.pdf?sfvrsn=1. Accessed July 9, 2019.
- Got Transition Center for Health Care Transition Improvement. Six core elements of health care transition 2.0. Transitioning youth to an adult health care provider. For use by pediatric, family medicine, and med-peds providers. www.gottransition.org/resourceGet.cfm?id=208. Accessed July 9, 2019.
- Murphy CM, Wilson CE, Robertson DM, et al. Autism spectrum disorder in adults: diagnosis, management, and health services development. Neuropsychiatr Dis Treat 2016; 12:1669–1686. doi:10.2147/NDT.S65455
- García-Villamisar DA, Dattilo J. Effects of a leisure programme on quality of life and stress of individuals with ASD. J Intellect Disabil Res 2010; 54(7):611–619. doi:10.1111/j.1365-2788.2010.01289.x
- García-Villamisar D, Hughes C. Supported employment improves cognitive performance in adults with autism. J Intellect Disabil Res 2007; 51(pt 2):142–150. doi:10.1111/j.1365-2788.2006.00854.x
- Lawer L, Brusilovskiy E, Salzer MS, Mandell DS. Use of vocational rehabilitative services among adults with autism. J Autism Dev Disord 2009; 39(3):487–494. doi:10.1007/s10803-008-0649-4
- Howlin P, Alcock J, Burkin C. An 8 year follow-up of a specialist supported employment service for high-ability adults with autism or Asperger syndrome. Autism 2005; 9(5):533–549. doi:10.1177/1362361305057871
- Kay BR. Bittersweet Farms. J Autism Dev Disord 1990; 20(3):309–321. http://www.ncbi.nlm.nih.gov/pubmed/2228914. Accessed July 9, 2019.
- Taylor JL, McPheeters ML, Sathe NA, Dove D, Veenstra-Vanderweele J, Warren Z. A systematic review of vocational interventions for young adults with autism spectrum disorders. Pediatrics 2012; 130(3):531–538. doi:10.1542/peds.2012-0682
- Bishop-Fitzpatrick L, Minshew NJ, Eack SM. A systematic review of psychosocial interventions for adults with autism spectrum disorders. J Autism Dev Disord 2013; 43(3):687–694. doi:10.1007/s10803-012-1615-8
- Russell AJ, Jassi A, Fullana MA, et al. Cognitive behavior therapy for comorbid obsessive-compulsive disorder in high-functioning autism spectrum disorders: a randomized controlled trial. Depress Anxiety 2013; 30(8):697–708. doi:10.1002/da.22053
- Spek AA, van Ham NC, Nyklícek I. Mindfulness-based therapy in adults with an autism spectrum disorder: a randomized controlled trial. Res Dev Disabil 2013; 34(1):246–253. doi:10.1016/j.ridd.2012.08.009
- Eapen V, Crncec R, Walter A. Clinical outcomes of an early intervention program for preschool children with autism spectrum disorder in a community group setting. BMC Pediatr 2013; 13(1):3. doi:10.1186/1471-2431-13-3
- Mazefsky CA, White SW. Emotion regulation: concepts & practice in autism spectrum disorder. Child Adolesc Psychiatr Clin North Am 2014; 23(1):15–24. doi:10.1016/J.CHC.2013.07.002
- Esbensen AJ, Greenberg JS, Seltzer MM, Aman MG. A longitudinal investigation of psychotropic and non-psychotropic medication use among adolescents and adults with autism spectrum disorders. J Autism Dev Disord 2009; 39(9):1339–1349. doi:10.1007/s10803-009-0750-3
- Dove D, Warren Z, McPheeters ML, Taylor JL, Sathe NA, Veenstra-VanderWeele J. Medications for adolescents and young adults with autism spectrum disorders: a systematic review. Pediatrics 2012; 130(4):717–726. doi:10.1542/peds.2012-0683
- LeClerc S, Easley D. Pharmacological therapies for autism spectrum disorder: a review. Pharm Ther 2015; 40(6):389–397.
- Miral S, Gencer O, Inal-Emiroglu FN, Baykara B, Baykara A, Dirik E. Risperidone versus haloperidol in children and adolescents with AD: a randomized, controlled, double-blind trial. Eur Child Adolesc Psychiatry 2008; 17(1):1–8. doi:10.1007/s00787-007-0620-5
- Lai M-C, Lombardo MV, Baron-Cohen S. Autism. Lancet 2014; 383(9920):896–910. doi:10.1016/S0140-6736(13)61539-1
- Ratni H, Rogers-Evans M, Bissantz C, et al. Discovery of highly selective brain-penetrant vasopressin 1a antagonists for the potential treatment of autism via a chemogenomic and scaffold hopping approach. J Med Chem 2015; 58(5):2275–2289. doi:10.1021/jm501745f
- Umbricht D, Del Valle Rubido M, Hollander E, et al. A single dose, randomized, controlled proof-of-mechanism study of a novel vasopressin 1a receptor antagonist (RG7713) in high-functioning adults with autism spectrum disorder. Neuropsychopharmacology 2017; 42(9):1914–1923. doi:10.1038/npp.2016.232>
- Kellaher DC. Sexual behavior and autism spectrum disorders: an update and discussion. Curr Psychiatry Rep 2015; 17(4):25. doi:10.1007/s11920-015-0562-4
- Brown-Lavoie SM, Viecili MA, Weiss JA. Sexual knowledge and victimization in adults with autism spectrum disorders. J Autism Dev Disord 2014; 44(9):2185–2196. doi:10.1007/s10803-014-2093-y
- Mehzabin P, Stokes MA. Self-assessed sexuality in young adults with high-functioning autism. Res Autism Spectr Disord 2011; 5(1):614–621. doi:10.1016/J.RASD.2010.07.006>
- Brown KR. Accessibility for students with ASD: legal perspectives in the United States. In: Alphin HC Jr. Exploring the Future of Accessibility in Higher Education. Hershey, PA: IGI Global; 2017.
- George R, Stokes MA. Gender identity and sexual orientation in autism spectrum disorder. Autism 2018; 22(8):970–982. doi:10.1177/1362361317714587
- Byers ES, Nichols S, Voyer SD. Challenging stereotypes: sexual functioning of single adults with high functioning autism spectrum disorder. J Autism Dev Disord 2013; 43(11):2617–2627. doi:10.1007/s10803-013-1813-z
- Gilmour L, Schalomon PM, Smith V. Sexuality in a community based sample of adults with autism spectrum disorder. Res Autism Spectr Disord 2012; 6(1):313–318. doi:10.1016/J.RASD.2011.06.003
- Bejerot S, Eriksson JM. Sexuality and gender role in autism spectrum disorder: a case control study. Schmitz C, ed. PLoS One 2014; 9(1):e87961. doi:10.1371/journal.pone.0087961>
- Navot N, Jorgenson AG, Webb SJ. Maternal experience raising girls with autism spectrum disorder: a qualitative study. Child Care Health Dev 2017; 43(4):536–545. doi:10.1111/cch.12470
- Padden C, James JE. Stress among parents of children with and without autism spectrum disorder: a comparison involving physiological indicators and parent self-reports. J Dev Phys Disabil 2017; 29(4):567–586. doi:10.1007/s10882-017-9547-z
- Woodgate RL, Ateah C, Secco L. Living in a world of our own: the experience of parents who have a child with autism. Qual Health Res 2008; 18(8):1075–1083. doi:10.1177/1049732308320112
- Hayes SA, Watson SL. The impact of parenting stress: a meta-analysis of studies comparing the experience of parenting stress in parents of children with and without autism spectrum disorder. J Autism Dev Disord 2013; 43(3):629–642. doi:10.1007/s10803-012-1604-y
- Cadman T, Eklund H, Howley D, et al. Caregiver burden as people with autism spectrum disorder and attention-deficit/hyperactivity disorder transition into adolescence and adulthood in the United Kingdom. J Am Acad Child Adolesc Psychiatry 2012; 51(9):879–888. doi:10.1016/j.jaac.2012.06.017
- Smith LE, Seltzer MM, Greenberg JS. Daily health symptoms of mothers of adolescents and adults with fragile x syndrome and mothers of adolescents and adults with autism spectrum disorder. J Autism Dev Disord 2012; 42(9):1836–1846. doi:10.1007/s10803-011-1422-7
- van Steijn DJ, Oerlemans AM, van Aken MAG, Buitelaar JK, Rommelse NNJ. The reciprocal relationship of ASD, ADHD, depressive symptoms and stress in parents of children with ASD and/or ADHD. J Autism Dev Disord 2014; 44(5):1064–1076. doi:10.1007/s10803-013-1958-9
- Seltzer MM, Greenberg JS, Hong J, et al. Maternal cortisol levels and behavior problems in adolescents and adults with ASD. J Autism Dev Disord 2010; 40(4):457–469. doi:10.1007/S10803-009-0887-0
- Lovell B, Moss M, Wetherell MA. With a little help from my friends: psychological, endocrine and health corollaries of social support in parental caregivers of children with autism or ADHD. Res Dev Disabil 2012; 33(2):682–687. doi:10.1016/j.ridd.2011.11.014
- Gallagher S, Whiteley J. Social support is associated with blood pressure responses in parents caring for children with developmental disabilities. Res Dev Disabil 2012; 33(6):2099–2105. doi:10.1016/j.ridd.2012.06.007
- Baker JK, Smith LE, Greenberg JS, Seltzer MM, Taylor JL. Change in maternal criticism and behavior problems in adolescents and adults with autism across a 7-year period. J Abnorm Psychol 2011; 120(2):465–475. doi:10.1037/a0021900
- Marsack CN, Samuel PS. Mediating effects of social support on quality of life for parents of adults with autism. J Autism Dev Disord 2017; 47(8):2378–2389. doi:10.1007/s10803-017-3157-6
- Trute B, Benzies KM, Worthington C, Reddon JR, Moore M. Accentuate the positive to mitigate the negative: mother psychological coping resources and family adjustment in childhood disability. J Intellect Dev Disabil 2010; 35(1):36–43. doi:10.3109/13668250903496328
- Cantwell J, Muldoon OT, Gallagher S. Social support and mastery influence the association between stress and poor physical health in parents caring for children with developmental disabilities. Res Dev Disabil 2014; 35(9):2215–2223. doi:10.1016/j.ridd.2014.05.012
- Carton AM, Smith AD. Assessing the relationship between eating disorder psychopathology and autistic traits in a non-clinical adult population. Eat Weight Disord - Stud Anorexia, Bulim Obes 2014; 19(3):285–293. doi:10.1007/s40519-013-0086-z
- De Alwis D, Agrawal A, Reiersen AM, et al. ADHD symptoms, autistic traits, and substance use and misuse in adult Australian twins. J Stud Alcohol Drugs 2014; 75(2):211–221. doi:10.15288/jsad.2014.75.211
- Wang LW, Tancredi DJ, Thomas DW. The prevalence of gastrointestinal problems in children across the United States with autism spectrum disorders from families with multiple affected members. J Dev Behav Pediatr 2011; 32(5):351–360. doi:10.1097/DBP.0b013e31821bd06a
- Croen LA, Zerbo O, Qian Y, et al. The health status of adults on the autism spectrum. Autism 2015; 19(7):814–823. doi:10.1177/1362361315577517
- Kalyoncu IÖ, Tanboga I. Oral health status of children with autistic spectrum disorder compared with non-authentic peers. Iran J Public Health 2017; 46(11):1591–1593. www.ncbi.nlm.nih.gov/pmc/articles/PMC5696703. Accessed July 9, 2019.
- McGuire K, Fung LK, Hagopian L, et al. Irritability and problem behavior in autism spectrum disorder: a practice pathway for pediatric primary care. Pediatrics 2016; 137(suppl 2):S136–S148. doi:10.1542/peds.2015-2851L
KEY POINTS
- Autism is becoming more common, with most recent statistics showing at least 1 in 59 children affected.
- Asperger syndrome is now included in the category of ASD, with possible implications for coverage of care.
- Some children with ASD get better as they get older, but many do not, and some do not receive a diagnosis until adulthood.
- Diagnosing ASD in adults can be difficult and involves specialists from multiple disciplines.
- Social support is important. Community programs and behavioral therapies can help. Drug therapy has not been rigorously tested and is not approved for use in adults with ASD. Caregivers may also need support.
An unusual cause of bruising
A 61-year-old woman presented to our hematology clinic for evaluation of multiple episodes of bruising. The first episode occurred 8 months earlier, when she developed a large bruise after water skiing. Two months before coming to us, she went to her local emergency room because of new bruising and was found to have a prolonged activated partial thromboplastin time (aPTT) of 60 seconds (reference range 23.3–34.9), but she underwent no further testing at that time.
At presentation to our clinic, she reported having no fevers, night sweats, unintentional weight loss, swollen lymph nodes, joint pain, rashes, mouth sores, nosebleeds, or blood in the urine or stool. Her history was notable only for hypothyroidism, which was diagnosed in the previous year. Her medications included levothyroxine, vitamin D3, and vitamin C. She had been taking a baby aspirin daily for the past 10 years but had stopped 1 month earlier because of the bruising.
Ten years earlier she had been evaluated for a possible transient ischemic attack; laboratory results at that time included a normal aPTT of 25.1 seconds and a normal factor VIII level of 153% (reference range 50%–173%).
EVALUATION FOR AN ISOLATED PROLONGED aPTT
1. What is the appropriate next test to evaluate this patient’s prolonged aPTT?
- Lupus anticoagulant panel
- Coagulation factor levels
- Mixing studies
- Bethesda assay
Mixing studies
Once a prolonged aPTT is confirmed, the appropriate next step is a mixing study. This involves mixing the patient’s plasma with pooled normal plasma in a 1-to-1 ratio, then repeating the aPTT test immediately, and again after 1 hour of incubation at 37°C. If the patient does not have enough of one of the coagulation factors, the aPTT immediately returns to the normal range when plasma is mixed with the pooled plasma because the pooled plasma contains the factor that is lacking. If this happens, then factor assays should be performed to identify the deficient factor.1
Various antibodies that inhibit coagulation factors can also affect the aPTT. There are 2 general types: immediate-acting and delayed.
With an immediate-acting inhibitor, the aPTT does not correct into the normal range with initial mixing. Immediate-acting inhibitors are often seen together with lupus anticoagulants, which are nonspecific phospholipid antibodies. If an immediate-acting inhibitor is detected, further testing should focus on evaluation for lupus anticoagulant, including phospholipid-dependency studies.
With a delayed inhibitor, the aPTT initially comes down, but subsequently goes back up after incubation. Acquired factor VIII inhibitor is a classic delayed-type inhibitor and is also the most common factor inhibitor.1 If a delayed-acting inhibitor is found, specific intrinsic factor levels should be measured (factors VIII, IX, XI, and XII),2 and testing should also be done for lupus anticoagulant, as these inhibitors may occur together.
Bethesda assay
Case continued: Results of mixing and Bethesda studies
FACTOR VIII INHIBITOR EVALUATION
2. What is the most likely underlying condition associated with this patient’s factor VIII inhibitor?
- Autoimmune disease
- Malignancy
- A medication
- Unknown (idiopathic)
Acquired hemophilia A (AHA) is a rare disorder caused by autoantibodies against factor VIII. Its estimated incidence is about 1 person per million per year.4 It usually presents as unexplained bruising or bleeding and is only rarely diagnosed by an incidentally noted prolonged aPTT. The severity of bleeding is variable and can include subcutaneous, soft-tissue, retroperitoneal, gastrointestinal, and intracranial hemorrhage.5
AHA is considered idiopathic in more than half of cases. A study based on a European registry5 of 501 patients with AHA and a UK study6 of 172 patients found no underlying disease in 52% and 65% of patients, respectively. For patients with an identified cause, the most common causes were malignancy (12%5 and 15%6) and autoimmune disease (12%5 and 17%6).
Drugs have rarely been associated with factor VIII inhibitors. Such occurrences have been reported with interferon, blood thinners, antibiotics, and psychiatric medications, but no study yet has indicated causation. However, patients with congenital hemophilia A treated with factor VIII preparations have about a 15% chance of developing factor VIII inhibitors. In this setting, inhibitors develop in response to recombinant factor VIII exposure, unlike the autoimmune phenomena seen in AHA.
TREATMENT OF ACQUIRED HEMOPHILIA A
3. What is the most appropriate treatment for AHA?
- Desmopressin and prednisone
- Recombinant porcine factor VIII and prednisone plus cyclophosphamide
- Recombinant factor VIIa and rituximab
- Any of the above
Any of the above regimens can be used. In general, treatment of AHA has two purposes: to stop acute hemorrhage, and to reduce the level of factor VIII inhibitor. No standard treatment guidelines are available; evidence of the effectiveness of different drugs is based largely on data on congenital hemophilia A.3
Acute treatment to stop bleeding
Initial treatment of AHA often focuses on stopping an acute hemorrhage by either raising circulating levels of factor VIII or bypassing it in the coagulation cascade.
Desmopressin can temporarily raise factor VIII levels, but it is often ineffective in AHA unless the patient has very low inhibitor titers.3
Factor VIII concentrate (human or recombinant porcine factor VIII) may be effective in patients with low inhibitor titers (< 5 BU). Higher doses are often required than those used in congenital hemophilia A. Factor VIII concentrate is usually combined with immunosuppressive treatment to lower the factor VIII inhibitor level (described below).3
If these methods are ineffective or the patient has high inhibitor titers (> 5 BU), activated prothrombin complex concentrates, known as FEIBA (factor eight inhibitor bypassing activity), or recombinant factor VIIa is available. These agents bypass factor VIII in the clotting cascade.
Immunosuppression to reduce factor VIII inhibitor
Immunosuppressive agents are the mainstay of AHA treatment to lower the inhibitor level.
Regimens vary. A 2003 meta-analysis4 including 249 patients found that prednisone alone resulted in complete response in about 30% of patients, and the addition of cyclophosphamide increased the response rate to 60% to 100%. High-dose intravenous immunoglobulin led to conflicting results. Conclusions were limited by the variability of dosing and duration in treatment regimens among the 20 different studies included.
An analysis of 331 patients in the European Acquired Hemophilia Registry (EACH2)7 found that steroids alone produced remission in 48% of patients, while steroids combined with cyclophosphamide raised the rate to 70%. Rituximab-based regimens were successful in 59% but required twice as long to achieve remission as steroid or cyclophosphamide-based regimens. No benefit was noted from intravenous immunoglobulin.
Risks of disease and treatment
AHA is associated with significant risk of morbidity and death related to bleeding, complications of treatment, and underlying disease.
In EACH2, 16 of the 331 patients died of bleeding, 16 died of causes related to immunosuppression, and 45 died of causes related to the underlying condition.5 In the UK registry of 172 patients, 13 patients died of bleeding, and 12 died of sepsis related to immunosuppression.6
The factor VIII level and inhibitor titer are not necessarily useful in stratifying bleeding risk, as severe and fatal bleeding can occur at variable levels and patients remain at risk of bleeding as long as the inhibitor persists.6,7
CASE CONTINUED: TREATMENT, LYMPHOCYTOSIS
The patient was started on 60 mg daily of prednisone, resulting in a decrease in her aPTT, increase in factor VIII level, and lower Bethesda titer. On a return visit, her absolute lymphocyte count was 7.04 × 109/L (reference range 1.0–4.0). She reported no fevers, chills, or recent infections.
EVALUATING LYMPHOCYTOSIS
Lymphocytosis is defined in most laboratories as an absolute lymphocyte count greater than 4.0 × 109/L for adults. Normally, T cells (CD3+) make up 60% to 80% of lymphocytes, B cells (CD20+) 10% to 20%, and natural killer (NK) cells (CD3–, CD56+) 5% to 10%. Lymphocytosis is usually caused by infection, but it can have other causes, including malignancy.
Peripheral blood smear. If there is no clear cause of lymphocytosis, a peripheral blood smear can be used to assess lymphocyte morphology, providing clues to the underlying etiology. For example, atypical lymphocytes are often seen in infectious mononucleosis, while “smudge” lymphocytes are characteristic of chronic lymphocytic leukemia. If a peripheral smear shows abnormal morphology, further workup should include establishing whether the lymphocytes are polyclonal or clonal.8
CASE CONTINUED: LARGE GRANULAR LYMPHOCYTES
4. What is the next step to evaluate the patient’s lymphocytosis?
- Bone marrow biopsy
- Karyotype analysis
- Flow cytometry
- Fluorescence in situ hybridization
Flow cytometry with V-beta analysis is the best first test to determine the cause of lymphocytosis after review of the peripheral smear. For persistent lymphocytosis, flow cytometry should be done even if a peripheral smear shows normal lymphocyte morphology.
Most T cells possess receptors composed of alpha and beta chains, each encoded by variable (V), diversity (D), joining (J), and constant (C) gene segments. The V, D, and J segments undergo rearrangement during T-cell development in the thymus based on antigen exposure, producing a diverse T-cell receptor population.
In a polyclonal population of lymphocytes, the T-cell receptors have a variety of gene segment arrangements, indicating normal T-cell development. But in a clonal population of lymphocytes, the T-cell receptors have a single identical gene segment arrangement, indicating they all originated from a single clone.9 Lymphocytosis in response to an infection is typically polyclonal, while malignant lymphocytosis is clonal.
Monoclonal antibodies against many of the variable regions of the beta chain (V-beta) of T-cell receptors have been developed, enabling flow cytometry to establish clonality.
T-cell receptor gene rearrangement studies can also be performed using polymerase chain reaction and Southern blot techniques.9
Karyotype analysis is usually not performed for the finding of LGLs, because most leukemias (eg, T-cell and NK-cell leukemias) have cells with a normal karyotype.
Bone marrow biopsy is invasive and usually not required to evaluate LGLs. It can be especially risky for a patient with a bleeding disorder such as a factor VIII inhibitor.10
Case continued: Flow cytometry confirms clonality
Subsequent flow cytometry found that more than 50% of the patient’s lymphocytes were LGLs that co-expressed CD3+, CD8+, CD56+, and CD57+, with aberrantly decreased CD7 expression. T-cell V-beta analysis demonstrated an expansion of the V-beta 17 family, and T-cell receptor gene analysis with polymerase chain reaction confirmed the presence of a clonal rearrangement.
LGL LEUKEMIA: CLASSIFICATION AND MANAGEMENT
LGLs normally account for 10% to 15% of peripheral mononuclear cells.11 LGL leukemia is caused by a clonal population of cytotoxic T cells or NK cells and involves an increased number of LGLs (usually > 2 × 109/L).10
LGL leukemia is divided into 3 categories according to the most recent World Health Organization classification10,12:
T-cell LGL leukemia (about 85% of cases) is considered indolent but can cause significant cytopenias and is often associated with autoimmune disease.13 Cells usually express a CD3+, CD8+, CD16+, and CD57+ phenotype. Survival is about 70% at 10 years.
Chronic NK-cell lymphocytosis (about 10%) also tends to have an indolent course with cytopenia and an autoimmune association, and with a similar prognosis to T-cell LGL leukemia. Cells express a CD3–, CD16+, and CD56+ phenotype.
Aggressive NK-cell LGL leukemia (about 5%) is associated with Epstein-Barr virus infection and occurs in younger patients. It is characterized by severe cytopenias, “B symptoms” (ie, fever, night sweats, weight loss), and has a very poor prognosis. Like chronic NK-cell lymphocytosis, cells express a CD3–, CD16+, and CD56+ phenotype. Fas (CD95) and Fas-ligand (CD178) are strongly expressed.10,13
Most cases of LGL leukemia can be diagnosed on the basis of classic morphology on peripheral blood smear and evidence of clonality on flow cytometry or gene rearrangement studies. T-cell receptor gene studies cannot be used to establish clonality in the NK subtypes, as NK cells do not express T-cell receptors.11
Case continued: Diagnosis, continued course
In our patient, T-cell LGL leukemia was diagnosed on the basis of the peripheral smear, flow cytometry results, and positive T-cell receptor gene studies for clonal rearrangement in the T-cell receptor beta region.
While her corticosteroid therapy was being tapered, her factor III inhibitor level increased, and she had a small episode of bleeding, prompting the start of cyclophosphamide 50 mg daily with lower doses of prednisone.
LGL LEUKEMIA AND AUTOIMMUNE DISEASE
Patients with LGL leukemia commonly have or develop autoimmune conditions. Immune-mediated cytopenias including pure red cell aplasia, aplastic anemia, and autoimmune hemolytic anemias can occur. Neutropenia, the most common cytopenia in LGL leukemia, is thought to be at least partly autoimmune, as the degree of neutropenia is often worse than would be expected solely from bone-marrow infiltration of LGL cells.10,14,15
Rheumatoid arthritis is the most common autoimmune condition associated with LGL leukemia, with a reported incidence between 11% and 36%.13–15
Felty syndrome (rheumatoid arthritis, splenomegaly, and neutropenia) is often associated with LGL leukemia and is thought by some to be part of the same disease process.15
Treat with immunosuppressives if needed
Indications for treating LGL leukemia include the development of cytopenias and associated autoimmune diseases. Immunosuppressive agents, such as methotrexate, cyclophosphamide, and cyclosporine, are commonly used.10,11,14 Most evidence of treatment efficacy is from retrospective studies and case reports, with widely variable response rates that overall are around 50%.10
ACQUIRED HEMOPHILIA A AND HEMATOLOGIC MALIGNANCY
A systematic review found 30 cases of AHA associated with hematologic malignancies.16 The largest case series17 in this analysis had 8 patients, and included diagnoses of chronic lymphocytic leukemia, erythroleukemia, myelofibrosis, multiple myeloma, and myelodysplastic syndrome. In 3 of these patients, the appearance of the inhibitor preceded the diagnosis of the underlying malignancy by an average of 3.5 months. In 1 patient with erythroleukemia and another with multiple myeloma, the activity of the inhibitor could be clearly correlated with the underlying malignancy. In the other 6 patients, no association between the two could be made.
In the same series, complete resolution of the inhibitor was related only to the level of Bethesda titer present at diagnosis, with those who achieved resolution having lower mean Bethesda titers.17 Similarly, in EACH2, lower inhibitor Bethesda titers and higher factor VIII levels at presentation were associated with faster inhibitor eradication and normalization of factor VIII levels.7
Murphy et al18 described a 62-year-old woman with Felty syndrome who developed a factor VIII inhibitor and was subsequently given a diagnosis of LGL leukemia. Treatment with immunosuppressive agents, including cyclophosphamide, azathioprine, and rituximab, successfully eradicated her factor VIII inhibitor, although the LGL leukemia persisted.
Case conclusion: Eradication of factor VIII inhibitor
Our patient, similar to the patient described by Murphy et al18 above, had eradication of the factor VIII inhibitor despite persistence of LGL leukemia. Between the time of diagnosis at our clinic, when she had 54% LGLs, and eradication of the inhibitor 3 months later, the LGL percentage ranged from 45% to 89%. No clear direct correlation between LGL and factor VIII inhibitor levels could be detected.
Given the strong association of LGL leukemia with autoimmune disease, it is tempting to believe that her factor VIII inhibitor was somehow related to her malignancy, although the exact mechanism remained unclear. The average age at diagnosis is 60 for LGL leukemia11 and over 70 for AHA,5,6 so advanced age may be the common denominator. Whether or not our patient will have recurrence of her factor VIII inhibitor or the development of other autoimmune diseases with the persistence of her LGL leukemia remains to be seen.
At last follow-up, our patient was off all therapy and continued to have normal aPTT and factor VIII levels. Repeat flow cytometry after treatment of her factor VIII inhibitor showed persistence of a clonal T-cell population, although reduced from 72% to 60%. It may be that the 2 entities were unrelated, and the clonal T-cell population was simply fluctuating over time. This can be determined only with further observation. As the patient had no symptoms from her LGL leukemia, she continued to be observed without treatment.
TAKE-HOME POINTS
- The coagulation assay is key to initially assessing a bleeding abnormality; whether the prothrombin time and aPTT are normal or prolonged narrows the differential diagnosis and determines next steps in evaluation.
- Mixing studies can help pinpoint the responsible deficient factor.
- Acquired factor VIII deficiency, also known as AHA, may be caused by autoimmune disease, malignancy, or medications, but it is usually idiopathic.
- AHA treatment is focused on achieving hemostasis and reducing factor VIII inhibitor.
- Lymphocytosis should be evaluated with a peripheral blood smear and flow cytometry to determine if the population is polyclonal (associated with infection) or clonal (associated with malignancy).
- LGL leukemia is usually a chronic, indolent disease, although an uncommon subtype has an aggressive course.
- The association between AHA and LGL leukemia is unclear, and both conditions must be monitored and managed.
- Kamal AH, Tefferi A, Pruthi RK. How to interpret and pursue an abnormal prothrombin time, activated partial thromboplastin time, and bleeding time in adults. Mayo Clin Proc 2007; 82(7):864–873. doi:10.4065/82.7.864
- Tcherniantchouk O, Laposata M, Marques MB. The isolated prolonged PTT. Am J Hematol 2013; 88(1):82–85. doi:10.1002/ajh.23285
- Ma AD, Carrizosa D. Acquired factor VIII inhibitors: pathophysiology and treatment. Hematology Am Soc Hematol Educ Program 2006:432–437. doi:10.1182/asheducation-2006.1.432
- Delgado J, Jimenez-Yuste V, Hernandez-Navarro F, Villar A. Acquired haemophilia: review and meta-analysis focused on therapy and prognostic factors. Br J Haematol 2003; 121(1):21–35. pmid:12670328
- Knoebl P, Marco P, Baudo F, et al; EACH2 Registry Contributors. Demographic and clinical data in acquired hemophilia A: results from the European Acquired Haemophilia Registry (EACH2). J Thromb Haemost 2012; 10(4):622–631. doi:10.1111/j.1538-7836.2012.04654.x
- Collins PW, Hirsch S, Baglin TP, et al; UK Haemophilia Centre Doctors’ Organisation. Acquired hemophilia A in the United Kingdom: a 2-year national surveillance study by the United Kingdom Haemophilia Centre Doctors’ Organisation. Blood 2007; 109(5):1870–1877. doi:10.1182/blood-2006-06-029850
- Collins P, Baudo F, Knoebl P, et al; EACH2 Registry Collaborators. Immunosuppression for acquired hemophilia A: results from the European Acquired Haemophilia Registry (EACH2). Blood 2012; 120(1):47–55. doi:10.1182/blood-2012-02-409185
- George TI. Malignant or benign leukocytosis. Hematology Am Soc Hematol Educ Program 2012; 2012:475–484. doi:10.1182/asheducation-2012.1.475
- Watters RJ, Liu X, Loughran TP Jr. T-cell and natural killer-cell large granular lymphocyte leukemia neoplasias. Leuk Lymphoma 2011; 52(12):2217–2225. doi:10.3109/10428194.2011.593276
- Lamy T, Moignet A, Loughran TP Jr. LGL leukemia: from pathogenesis to treatment. Blood 2017; 129(9):1082–1094. doi:10.1182/blood-2016-08-692590
- Zhang D, Loughran TP Jr. Large granular lymphocytic leukemia: molecular pathogenesis, clinical manifestations, and treatment. Hematology Am Soc Hematol Educ Program 2012; 2012:652–659. doi:10.1182/asheducation-2012.1.652
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016; 127(20):2375–2390. doi:10.1182/blood-2016-01-643569
- Rose MG, Berliner N. T-cell large granular lymphocyte leukemia and related disorders. Oncologist 2004; 9(3):247–258. pmid:15169980
- Bockorny B, Dasanu CA. Autoimmune manifestations in large granular lymphocyte leukemia. Clin Lymphoma Myeloma Leuk 2012; 12(6):400–405. doi:10.1016/j.clml.2012.06.006
- Liu X, Loughran TP Jr. The spectrum of large granular lymphocyte leukemia and Felty’s syndrome. Curr Opin Hematol 2011; 18(4):254–259. doi:10.1097/MOH.0b013e32834760fb
- Franchini M, Lippi G. Acquired factor V inhibitors: a systematic review. J Thromb Thrombolysis 2011; 31(4):449–457. doi:10.1007/s11239-010-0529-6
- Sallah S, Nguyen NP, Abdallah JM, Hanrahan LR. Acquired hemophilia in patients with hematologic malignancies. Arch Pathol Lab Med 2000; 124(5):730–734.
- Murphy PW, Brett LK, Verla-Tebit E, Macik BG, Loughran TP Jr. Acquired inhibitors to factor VIII and fibrinogen in the setting of T-cell large granular lymphocyte leukemia: a case report and review of the literature. Blood Coagul Fibrinolysis 2015; 26(2):211–213. doi:10.1097/MBC.0000000000000209
A 61-year-old woman presented to our hematology clinic for evaluation of multiple episodes of bruising. The first episode occurred 8 months earlier, when she developed a large bruise after water skiing. Two months before coming to us, she went to her local emergency room because of new bruising and was found to have a prolonged activated partial thromboplastin time (aPTT) of 60 seconds (reference range 23.3–34.9), but she underwent no further testing at that time.
At presentation to our clinic, she reported having no fevers, night sweats, unintentional weight loss, swollen lymph nodes, joint pain, rashes, mouth sores, nosebleeds, or blood in the urine or stool. Her history was notable only for hypothyroidism, which was diagnosed in the previous year. Her medications included levothyroxine, vitamin D3, and vitamin C. She had been taking a baby aspirin daily for the past 10 years but had stopped 1 month earlier because of the bruising.
Ten years earlier she had been evaluated for a possible transient ischemic attack; laboratory results at that time included a normal aPTT of 25.1 seconds and a normal factor VIII level of 153% (reference range 50%–173%).
EVALUATION FOR AN ISOLATED PROLONGED aPTT
1. What is the appropriate next test to evaluate this patient’s prolonged aPTT?
- Lupus anticoagulant panel
- Coagulation factor levels
- Mixing studies
- Bethesda assay
Mixing studies
Once a prolonged aPTT is confirmed, the appropriate next step is a mixing study. This involves mixing the patient’s plasma with pooled normal plasma in a 1-to-1 ratio, then repeating the aPTT test immediately, and again after 1 hour of incubation at 37°C. If the patient does not have enough of one of the coagulation factors, the aPTT immediately returns to the normal range when plasma is mixed with the pooled plasma because the pooled plasma contains the factor that is lacking. If this happens, then factor assays should be performed to identify the deficient factor.1
Various antibodies that inhibit coagulation factors can also affect the aPTT. There are 2 general types: immediate-acting and delayed.
With an immediate-acting inhibitor, the aPTT does not correct into the normal range with initial mixing. Immediate-acting inhibitors are often seen together with lupus anticoagulants, which are nonspecific phospholipid antibodies. If an immediate-acting inhibitor is detected, further testing should focus on evaluation for lupus anticoagulant, including phospholipid-dependency studies.
With a delayed inhibitor, the aPTT initially comes down, but subsequently goes back up after incubation. Acquired factor VIII inhibitor is a classic delayed-type inhibitor and is also the most common factor inhibitor.1 If a delayed-acting inhibitor is found, specific intrinsic factor levels should be measured (factors VIII, IX, XI, and XII),2 and testing should also be done for lupus anticoagulant, as these inhibitors may occur together.
Bethesda assay
Case continued: Results of mixing and Bethesda studies
FACTOR VIII INHIBITOR EVALUATION
2. What is the most likely underlying condition associated with this patient’s factor VIII inhibitor?
- Autoimmune disease
- Malignancy
- A medication
- Unknown (idiopathic)
Acquired hemophilia A (AHA) is a rare disorder caused by autoantibodies against factor VIII. Its estimated incidence is about 1 person per million per year.4 It usually presents as unexplained bruising or bleeding and is only rarely diagnosed by an incidentally noted prolonged aPTT. The severity of bleeding is variable and can include subcutaneous, soft-tissue, retroperitoneal, gastrointestinal, and intracranial hemorrhage.5
AHA is considered idiopathic in more than half of cases. A study based on a European registry5 of 501 patients with AHA and a UK study6 of 172 patients found no underlying disease in 52% and 65% of patients, respectively. For patients with an identified cause, the most common causes were malignancy (12%5 and 15%6) and autoimmune disease (12%5 and 17%6).
Drugs have rarely been associated with factor VIII inhibitors. Such occurrences have been reported with interferon, blood thinners, antibiotics, and psychiatric medications, but no study yet has indicated causation. However, patients with congenital hemophilia A treated with factor VIII preparations have about a 15% chance of developing factor VIII inhibitors. In this setting, inhibitors develop in response to recombinant factor VIII exposure, unlike the autoimmune phenomena seen in AHA.
TREATMENT OF ACQUIRED HEMOPHILIA A
3. What is the most appropriate treatment for AHA?
- Desmopressin and prednisone
- Recombinant porcine factor VIII and prednisone plus cyclophosphamide
- Recombinant factor VIIa and rituximab
- Any of the above
Any of the above regimens can be used. In general, treatment of AHA has two purposes: to stop acute hemorrhage, and to reduce the level of factor VIII inhibitor. No standard treatment guidelines are available; evidence of the effectiveness of different drugs is based largely on data on congenital hemophilia A.3
Acute treatment to stop bleeding
Initial treatment of AHA often focuses on stopping an acute hemorrhage by either raising circulating levels of factor VIII or bypassing it in the coagulation cascade.
Desmopressin can temporarily raise factor VIII levels, but it is often ineffective in AHA unless the patient has very low inhibitor titers.3
Factor VIII concentrate (human or recombinant porcine factor VIII) may be effective in patients with low inhibitor titers (< 5 BU). Higher doses are often required than those used in congenital hemophilia A. Factor VIII concentrate is usually combined with immunosuppressive treatment to lower the factor VIII inhibitor level (described below).3
If these methods are ineffective or the patient has high inhibitor titers (> 5 BU), activated prothrombin complex concentrates, known as FEIBA (factor eight inhibitor bypassing activity), or recombinant factor VIIa is available. These agents bypass factor VIII in the clotting cascade.
Immunosuppression to reduce factor VIII inhibitor
Immunosuppressive agents are the mainstay of AHA treatment to lower the inhibitor level.
Regimens vary. A 2003 meta-analysis4 including 249 patients found that prednisone alone resulted in complete response in about 30% of patients, and the addition of cyclophosphamide increased the response rate to 60% to 100%. High-dose intravenous immunoglobulin led to conflicting results. Conclusions were limited by the variability of dosing and duration in treatment regimens among the 20 different studies included.
An analysis of 331 patients in the European Acquired Hemophilia Registry (EACH2)7 found that steroids alone produced remission in 48% of patients, while steroids combined with cyclophosphamide raised the rate to 70%. Rituximab-based regimens were successful in 59% but required twice as long to achieve remission as steroid or cyclophosphamide-based regimens. No benefit was noted from intravenous immunoglobulin.
Risks of disease and treatment
AHA is associated with significant risk of morbidity and death related to bleeding, complications of treatment, and underlying disease.
In EACH2, 16 of the 331 patients died of bleeding, 16 died of causes related to immunosuppression, and 45 died of causes related to the underlying condition.5 In the UK registry of 172 patients, 13 patients died of bleeding, and 12 died of sepsis related to immunosuppression.6
The factor VIII level and inhibitor titer are not necessarily useful in stratifying bleeding risk, as severe and fatal bleeding can occur at variable levels and patients remain at risk of bleeding as long as the inhibitor persists.6,7
CASE CONTINUED: TREATMENT, LYMPHOCYTOSIS
The patient was started on 60 mg daily of prednisone, resulting in a decrease in her aPTT, increase in factor VIII level, and lower Bethesda titer. On a return visit, her absolute lymphocyte count was 7.04 × 109/L (reference range 1.0–4.0). She reported no fevers, chills, or recent infections.
EVALUATING LYMPHOCYTOSIS
Lymphocytosis is defined in most laboratories as an absolute lymphocyte count greater than 4.0 × 109/L for adults. Normally, T cells (CD3+) make up 60% to 80% of lymphocytes, B cells (CD20+) 10% to 20%, and natural killer (NK) cells (CD3–, CD56+) 5% to 10%. Lymphocytosis is usually caused by infection, but it can have other causes, including malignancy.
Peripheral blood smear. If there is no clear cause of lymphocytosis, a peripheral blood smear can be used to assess lymphocyte morphology, providing clues to the underlying etiology. For example, atypical lymphocytes are often seen in infectious mononucleosis, while “smudge” lymphocytes are characteristic of chronic lymphocytic leukemia. If a peripheral smear shows abnormal morphology, further workup should include establishing whether the lymphocytes are polyclonal or clonal.8
CASE CONTINUED: LARGE GRANULAR LYMPHOCYTES
4. What is the next step to evaluate the patient’s lymphocytosis?
- Bone marrow biopsy
- Karyotype analysis
- Flow cytometry
- Fluorescence in situ hybridization
Flow cytometry with V-beta analysis is the best first test to determine the cause of lymphocytosis after review of the peripheral smear. For persistent lymphocytosis, flow cytometry should be done even if a peripheral smear shows normal lymphocyte morphology.
Most T cells possess receptors composed of alpha and beta chains, each encoded by variable (V), diversity (D), joining (J), and constant (C) gene segments. The V, D, and J segments undergo rearrangement during T-cell development in the thymus based on antigen exposure, producing a diverse T-cell receptor population.
In a polyclonal population of lymphocytes, the T-cell receptors have a variety of gene segment arrangements, indicating normal T-cell development. But in a clonal population of lymphocytes, the T-cell receptors have a single identical gene segment arrangement, indicating they all originated from a single clone.9 Lymphocytosis in response to an infection is typically polyclonal, while malignant lymphocytosis is clonal.
Monoclonal antibodies against many of the variable regions of the beta chain (V-beta) of T-cell receptors have been developed, enabling flow cytometry to establish clonality.
T-cell receptor gene rearrangement studies can also be performed using polymerase chain reaction and Southern blot techniques.9
Karyotype analysis is usually not performed for the finding of LGLs, because most leukemias (eg, T-cell and NK-cell leukemias) have cells with a normal karyotype.
Bone marrow biopsy is invasive and usually not required to evaluate LGLs. It can be especially risky for a patient with a bleeding disorder such as a factor VIII inhibitor.10
Case continued: Flow cytometry confirms clonality
Subsequent flow cytometry found that more than 50% of the patient’s lymphocytes were LGLs that co-expressed CD3+, CD8+, CD56+, and CD57+, with aberrantly decreased CD7 expression. T-cell V-beta analysis demonstrated an expansion of the V-beta 17 family, and T-cell receptor gene analysis with polymerase chain reaction confirmed the presence of a clonal rearrangement.
LGL LEUKEMIA: CLASSIFICATION AND MANAGEMENT
LGLs normally account for 10% to 15% of peripheral mononuclear cells.11 LGL leukemia is caused by a clonal population of cytotoxic T cells or NK cells and involves an increased number of LGLs (usually > 2 × 109/L).10
LGL leukemia is divided into 3 categories according to the most recent World Health Organization classification10,12:
T-cell LGL leukemia (about 85% of cases) is considered indolent but can cause significant cytopenias and is often associated with autoimmune disease.13 Cells usually express a CD3+, CD8+, CD16+, and CD57+ phenotype. Survival is about 70% at 10 years.
Chronic NK-cell lymphocytosis (about 10%) also tends to have an indolent course with cytopenia and an autoimmune association, and with a similar prognosis to T-cell LGL leukemia. Cells express a CD3–, CD16+, and CD56+ phenotype.
Aggressive NK-cell LGL leukemia (about 5%) is associated with Epstein-Barr virus infection and occurs in younger patients. It is characterized by severe cytopenias, “B symptoms” (ie, fever, night sweats, weight loss), and has a very poor prognosis. Like chronic NK-cell lymphocytosis, cells express a CD3–, CD16+, and CD56+ phenotype. Fas (CD95) and Fas-ligand (CD178) are strongly expressed.10,13
Most cases of LGL leukemia can be diagnosed on the basis of classic morphology on peripheral blood smear and evidence of clonality on flow cytometry or gene rearrangement studies. T-cell receptor gene studies cannot be used to establish clonality in the NK subtypes, as NK cells do not express T-cell receptors.11
Case continued: Diagnosis, continued course
In our patient, T-cell LGL leukemia was diagnosed on the basis of the peripheral smear, flow cytometry results, and positive T-cell receptor gene studies for clonal rearrangement in the T-cell receptor beta region.
While her corticosteroid therapy was being tapered, her factor III inhibitor level increased, and she had a small episode of bleeding, prompting the start of cyclophosphamide 50 mg daily with lower doses of prednisone.
LGL LEUKEMIA AND AUTOIMMUNE DISEASE
Patients with LGL leukemia commonly have or develop autoimmune conditions. Immune-mediated cytopenias including pure red cell aplasia, aplastic anemia, and autoimmune hemolytic anemias can occur. Neutropenia, the most common cytopenia in LGL leukemia, is thought to be at least partly autoimmune, as the degree of neutropenia is often worse than would be expected solely from bone-marrow infiltration of LGL cells.10,14,15
Rheumatoid arthritis is the most common autoimmune condition associated with LGL leukemia, with a reported incidence between 11% and 36%.13–15
Felty syndrome (rheumatoid arthritis, splenomegaly, and neutropenia) is often associated with LGL leukemia and is thought by some to be part of the same disease process.15
Treat with immunosuppressives if needed
Indications for treating LGL leukemia include the development of cytopenias and associated autoimmune diseases. Immunosuppressive agents, such as methotrexate, cyclophosphamide, and cyclosporine, are commonly used.10,11,14 Most evidence of treatment efficacy is from retrospective studies and case reports, with widely variable response rates that overall are around 50%.10
ACQUIRED HEMOPHILIA A AND HEMATOLOGIC MALIGNANCY
A systematic review found 30 cases of AHA associated with hematologic malignancies.16 The largest case series17 in this analysis had 8 patients, and included diagnoses of chronic lymphocytic leukemia, erythroleukemia, myelofibrosis, multiple myeloma, and myelodysplastic syndrome. In 3 of these patients, the appearance of the inhibitor preceded the diagnosis of the underlying malignancy by an average of 3.5 months. In 1 patient with erythroleukemia and another with multiple myeloma, the activity of the inhibitor could be clearly correlated with the underlying malignancy. In the other 6 patients, no association between the two could be made.
In the same series, complete resolution of the inhibitor was related only to the level of Bethesda titer present at diagnosis, with those who achieved resolution having lower mean Bethesda titers.17 Similarly, in EACH2, lower inhibitor Bethesda titers and higher factor VIII levels at presentation were associated with faster inhibitor eradication and normalization of factor VIII levels.7
Murphy et al18 described a 62-year-old woman with Felty syndrome who developed a factor VIII inhibitor and was subsequently given a diagnosis of LGL leukemia. Treatment with immunosuppressive agents, including cyclophosphamide, azathioprine, and rituximab, successfully eradicated her factor VIII inhibitor, although the LGL leukemia persisted.
Case conclusion: Eradication of factor VIII inhibitor
Our patient, similar to the patient described by Murphy et al18 above, had eradication of the factor VIII inhibitor despite persistence of LGL leukemia. Between the time of diagnosis at our clinic, when she had 54% LGLs, and eradication of the inhibitor 3 months later, the LGL percentage ranged from 45% to 89%. No clear direct correlation between LGL and factor VIII inhibitor levels could be detected.
Given the strong association of LGL leukemia with autoimmune disease, it is tempting to believe that her factor VIII inhibitor was somehow related to her malignancy, although the exact mechanism remained unclear. The average age at diagnosis is 60 for LGL leukemia11 and over 70 for AHA,5,6 so advanced age may be the common denominator. Whether or not our patient will have recurrence of her factor VIII inhibitor or the development of other autoimmune diseases with the persistence of her LGL leukemia remains to be seen.
At last follow-up, our patient was off all therapy and continued to have normal aPTT and factor VIII levels. Repeat flow cytometry after treatment of her factor VIII inhibitor showed persistence of a clonal T-cell population, although reduced from 72% to 60%. It may be that the 2 entities were unrelated, and the clonal T-cell population was simply fluctuating over time. This can be determined only with further observation. As the patient had no symptoms from her LGL leukemia, she continued to be observed without treatment.
TAKE-HOME POINTS
- The coagulation assay is key to initially assessing a bleeding abnormality; whether the prothrombin time and aPTT are normal or prolonged narrows the differential diagnosis and determines next steps in evaluation.
- Mixing studies can help pinpoint the responsible deficient factor.
- Acquired factor VIII deficiency, also known as AHA, may be caused by autoimmune disease, malignancy, or medications, but it is usually idiopathic.
- AHA treatment is focused on achieving hemostasis and reducing factor VIII inhibitor.
- Lymphocytosis should be evaluated with a peripheral blood smear and flow cytometry to determine if the population is polyclonal (associated with infection) or clonal (associated with malignancy).
- LGL leukemia is usually a chronic, indolent disease, although an uncommon subtype has an aggressive course.
- The association between AHA and LGL leukemia is unclear, and both conditions must be monitored and managed.
A 61-year-old woman presented to our hematology clinic for evaluation of multiple episodes of bruising. The first episode occurred 8 months earlier, when she developed a large bruise after water skiing. Two months before coming to us, she went to her local emergency room because of new bruising and was found to have a prolonged activated partial thromboplastin time (aPTT) of 60 seconds (reference range 23.3–34.9), but she underwent no further testing at that time.
At presentation to our clinic, she reported having no fevers, night sweats, unintentional weight loss, swollen lymph nodes, joint pain, rashes, mouth sores, nosebleeds, or blood in the urine or stool. Her history was notable only for hypothyroidism, which was diagnosed in the previous year. Her medications included levothyroxine, vitamin D3, and vitamin C. She had been taking a baby aspirin daily for the past 10 years but had stopped 1 month earlier because of the bruising.
Ten years earlier she had been evaluated for a possible transient ischemic attack; laboratory results at that time included a normal aPTT of 25.1 seconds and a normal factor VIII level of 153% (reference range 50%–173%).
EVALUATION FOR AN ISOLATED PROLONGED aPTT
1. What is the appropriate next test to evaluate this patient’s prolonged aPTT?
- Lupus anticoagulant panel
- Coagulation factor levels
- Mixing studies
- Bethesda assay
Mixing studies
Once a prolonged aPTT is confirmed, the appropriate next step is a mixing study. This involves mixing the patient’s plasma with pooled normal plasma in a 1-to-1 ratio, then repeating the aPTT test immediately, and again after 1 hour of incubation at 37°C. If the patient does not have enough of one of the coagulation factors, the aPTT immediately returns to the normal range when plasma is mixed with the pooled plasma because the pooled plasma contains the factor that is lacking. If this happens, then factor assays should be performed to identify the deficient factor.1
Various antibodies that inhibit coagulation factors can also affect the aPTT. There are 2 general types: immediate-acting and delayed.
With an immediate-acting inhibitor, the aPTT does not correct into the normal range with initial mixing. Immediate-acting inhibitors are often seen together with lupus anticoagulants, which are nonspecific phospholipid antibodies. If an immediate-acting inhibitor is detected, further testing should focus on evaluation for lupus anticoagulant, including phospholipid-dependency studies.
With a delayed inhibitor, the aPTT initially comes down, but subsequently goes back up after incubation. Acquired factor VIII inhibitor is a classic delayed-type inhibitor and is also the most common factor inhibitor.1 If a delayed-acting inhibitor is found, specific intrinsic factor levels should be measured (factors VIII, IX, XI, and XII),2 and testing should also be done for lupus anticoagulant, as these inhibitors may occur together.
Bethesda assay
Case continued: Results of mixing and Bethesda studies
FACTOR VIII INHIBITOR EVALUATION
2. What is the most likely underlying condition associated with this patient’s factor VIII inhibitor?
- Autoimmune disease
- Malignancy
- A medication
- Unknown (idiopathic)
Acquired hemophilia A (AHA) is a rare disorder caused by autoantibodies against factor VIII. Its estimated incidence is about 1 person per million per year.4 It usually presents as unexplained bruising or bleeding and is only rarely diagnosed by an incidentally noted prolonged aPTT. The severity of bleeding is variable and can include subcutaneous, soft-tissue, retroperitoneal, gastrointestinal, and intracranial hemorrhage.5
AHA is considered idiopathic in more than half of cases. A study based on a European registry5 of 501 patients with AHA and a UK study6 of 172 patients found no underlying disease in 52% and 65% of patients, respectively. For patients with an identified cause, the most common causes were malignancy (12%5 and 15%6) and autoimmune disease (12%5 and 17%6).
Drugs have rarely been associated with factor VIII inhibitors. Such occurrences have been reported with interferon, blood thinners, antibiotics, and psychiatric medications, but no study yet has indicated causation. However, patients with congenital hemophilia A treated with factor VIII preparations have about a 15% chance of developing factor VIII inhibitors. In this setting, inhibitors develop in response to recombinant factor VIII exposure, unlike the autoimmune phenomena seen in AHA.
TREATMENT OF ACQUIRED HEMOPHILIA A
3. What is the most appropriate treatment for AHA?
- Desmopressin and prednisone
- Recombinant porcine factor VIII and prednisone plus cyclophosphamide
- Recombinant factor VIIa and rituximab
- Any of the above
Any of the above regimens can be used. In general, treatment of AHA has two purposes: to stop acute hemorrhage, and to reduce the level of factor VIII inhibitor. No standard treatment guidelines are available; evidence of the effectiveness of different drugs is based largely on data on congenital hemophilia A.3
Acute treatment to stop bleeding
Initial treatment of AHA often focuses on stopping an acute hemorrhage by either raising circulating levels of factor VIII or bypassing it in the coagulation cascade.
Desmopressin can temporarily raise factor VIII levels, but it is often ineffective in AHA unless the patient has very low inhibitor titers.3
Factor VIII concentrate (human or recombinant porcine factor VIII) may be effective in patients with low inhibitor titers (< 5 BU). Higher doses are often required than those used in congenital hemophilia A. Factor VIII concentrate is usually combined with immunosuppressive treatment to lower the factor VIII inhibitor level (described below).3
If these methods are ineffective or the patient has high inhibitor titers (> 5 BU), activated prothrombin complex concentrates, known as FEIBA (factor eight inhibitor bypassing activity), or recombinant factor VIIa is available. These agents bypass factor VIII in the clotting cascade.
Immunosuppression to reduce factor VIII inhibitor
Immunosuppressive agents are the mainstay of AHA treatment to lower the inhibitor level.
Regimens vary. A 2003 meta-analysis4 including 249 patients found that prednisone alone resulted in complete response in about 30% of patients, and the addition of cyclophosphamide increased the response rate to 60% to 100%. High-dose intravenous immunoglobulin led to conflicting results. Conclusions were limited by the variability of dosing and duration in treatment regimens among the 20 different studies included.
An analysis of 331 patients in the European Acquired Hemophilia Registry (EACH2)7 found that steroids alone produced remission in 48% of patients, while steroids combined with cyclophosphamide raised the rate to 70%. Rituximab-based regimens were successful in 59% but required twice as long to achieve remission as steroid or cyclophosphamide-based regimens. No benefit was noted from intravenous immunoglobulin.
Risks of disease and treatment
AHA is associated with significant risk of morbidity and death related to bleeding, complications of treatment, and underlying disease.
In EACH2, 16 of the 331 patients died of bleeding, 16 died of causes related to immunosuppression, and 45 died of causes related to the underlying condition.5 In the UK registry of 172 patients, 13 patients died of bleeding, and 12 died of sepsis related to immunosuppression.6
The factor VIII level and inhibitor titer are not necessarily useful in stratifying bleeding risk, as severe and fatal bleeding can occur at variable levels and patients remain at risk of bleeding as long as the inhibitor persists.6,7
CASE CONTINUED: TREATMENT, LYMPHOCYTOSIS
The patient was started on 60 mg daily of prednisone, resulting in a decrease in her aPTT, increase in factor VIII level, and lower Bethesda titer. On a return visit, her absolute lymphocyte count was 7.04 × 109/L (reference range 1.0–4.0). She reported no fevers, chills, or recent infections.
EVALUATING LYMPHOCYTOSIS
Lymphocytosis is defined in most laboratories as an absolute lymphocyte count greater than 4.0 × 109/L for adults. Normally, T cells (CD3+) make up 60% to 80% of lymphocytes, B cells (CD20+) 10% to 20%, and natural killer (NK) cells (CD3–, CD56+) 5% to 10%. Lymphocytosis is usually caused by infection, but it can have other causes, including malignancy.
Peripheral blood smear. If there is no clear cause of lymphocytosis, a peripheral blood smear can be used to assess lymphocyte morphology, providing clues to the underlying etiology. For example, atypical lymphocytes are often seen in infectious mononucleosis, while “smudge” lymphocytes are characteristic of chronic lymphocytic leukemia. If a peripheral smear shows abnormal morphology, further workup should include establishing whether the lymphocytes are polyclonal or clonal.8
CASE CONTINUED: LARGE GRANULAR LYMPHOCYTES
4. What is the next step to evaluate the patient’s lymphocytosis?
- Bone marrow biopsy
- Karyotype analysis
- Flow cytometry
- Fluorescence in situ hybridization
Flow cytometry with V-beta analysis is the best first test to determine the cause of lymphocytosis after review of the peripheral smear. For persistent lymphocytosis, flow cytometry should be done even if a peripheral smear shows normal lymphocyte morphology.
Most T cells possess receptors composed of alpha and beta chains, each encoded by variable (V), diversity (D), joining (J), and constant (C) gene segments. The V, D, and J segments undergo rearrangement during T-cell development in the thymus based on antigen exposure, producing a diverse T-cell receptor population.
In a polyclonal population of lymphocytes, the T-cell receptors have a variety of gene segment arrangements, indicating normal T-cell development. But in a clonal population of lymphocytes, the T-cell receptors have a single identical gene segment arrangement, indicating they all originated from a single clone.9 Lymphocytosis in response to an infection is typically polyclonal, while malignant lymphocytosis is clonal.
Monoclonal antibodies against many of the variable regions of the beta chain (V-beta) of T-cell receptors have been developed, enabling flow cytometry to establish clonality.
T-cell receptor gene rearrangement studies can also be performed using polymerase chain reaction and Southern blot techniques.9
Karyotype analysis is usually not performed for the finding of LGLs, because most leukemias (eg, T-cell and NK-cell leukemias) have cells with a normal karyotype.
Bone marrow biopsy is invasive and usually not required to evaluate LGLs. It can be especially risky for a patient with a bleeding disorder such as a factor VIII inhibitor.10
Case continued: Flow cytometry confirms clonality
Subsequent flow cytometry found that more than 50% of the patient’s lymphocytes were LGLs that co-expressed CD3+, CD8+, CD56+, and CD57+, with aberrantly decreased CD7 expression. T-cell V-beta analysis demonstrated an expansion of the V-beta 17 family, and T-cell receptor gene analysis with polymerase chain reaction confirmed the presence of a clonal rearrangement.
LGL LEUKEMIA: CLASSIFICATION AND MANAGEMENT
LGLs normally account for 10% to 15% of peripheral mononuclear cells.11 LGL leukemia is caused by a clonal population of cytotoxic T cells or NK cells and involves an increased number of LGLs (usually > 2 × 109/L).10
LGL leukemia is divided into 3 categories according to the most recent World Health Organization classification10,12:
T-cell LGL leukemia (about 85% of cases) is considered indolent but can cause significant cytopenias and is often associated with autoimmune disease.13 Cells usually express a CD3+, CD8+, CD16+, and CD57+ phenotype. Survival is about 70% at 10 years.
Chronic NK-cell lymphocytosis (about 10%) also tends to have an indolent course with cytopenia and an autoimmune association, and with a similar prognosis to T-cell LGL leukemia. Cells express a CD3–, CD16+, and CD56+ phenotype.
Aggressive NK-cell LGL leukemia (about 5%) is associated with Epstein-Barr virus infection and occurs in younger patients. It is characterized by severe cytopenias, “B symptoms” (ie, fever, night sweats, weight loss), and has a very poor prognosis. Like chronic NK-cell lymphocytosis, cells express a CD3–, CD16+, and CD56+ phenotype. Fas (CD95) and Fas-ligand (CD178) are strongly expressed.10,13
Most cases of LGL leukemia can be diagnosed on the basis of classic morphology on peripheral blood smear and evidence of clonality on flow cytometry or gene rearrangement studies. T-cell receptor gene studies cannot be used to establish clonality in the NK subtypes, as NK cells do not express T-cell receptors.11
Case continued: Diagnosis, continued course
In our patient, T-cell LGL leukemia was diagnosed on the basis of the peripheral smear, flow cytometry results, and positive T-cell receptor gene studies for clonal rearrangement in the T-cell receptor beta region.
While her corticosteroid therapy was being tapered, her factor III inhibitor level increased, and she had a small episode of bleeding, prompting the start of cyclophosphamide 50 mg daily with lower doses of prednisone.
LGL LEUKEMIA AND AUTOIMMUNE DISEASE
Patients with LGL leukemia commonly have or develop autoimmune conditions. Immune-mediated cytopenias including pure red cell aplasia, aplastic anemia, and autoimmune hemolytic anemias can occur. Neutropenia, the most common cytopenia in LGL leukemia, is thought to be at least partly autoimmune, as the degree of neutropenia is often worse than would be expected solely from bone-marrow infiltration of LGL cells.10,14,15
Rheumatoid arthritis is the most common autoimmune condition associated with LGL leukemia, with a reported incidence between 11% and 36%.13–15
Felty syndrome (rheumatoid arthritis, splenomegaly, and neutropenia) is often associated with LGL leukemia and is thought by some to be part of the same disease process.15
Treat with immunosuppressives if needed
Indications for treating LGL leukemia include the development of cytopenias and associated autoimmune diseases. Immunosuppressive agents, such as methotrexate, cyclophosphamide, and cyclosporine, are commonly used.10,11,14 Most evidence of treatment efficacy is from retrospective studies and case reports, with widely variable response rates that overall are around 50%.10
ACQUIRED HEMOPHILIA A AND HEMATOLOGIC MALIGNANCY
A systematic review found 30 cases of AHA associated with hematologic malignancies.16 The largest case series17 in this analysis had 8 patients, and included diagnoses of chronic lymphocytic leukemia, erythroleukemia, myelofibrosis, multiple myeloma, and myelodysplastic syndrome. In 3 of these patients, the appearance of the inhibitor preceded the diagnosis of the underlying malignancy by an average of 3.5 months. In 1 patient with erythroleukemia and another with multiple myeloma, the activity of the inhibitor could be clearly correlated with the underlying malignancy. In the other 6 patients, no association between the two could be made.
In the same series, complete resolution of the inhibitor was related only to the level of Bethesda titer present at diagnosis, with those who achieved resolution having lower mean Bethesda titers.17 Similarly, in EACH2, lower inhibitor Bethesda titers and higher factor VIII levels at presentation were associated with faster inhibitor eradication and normalization of factor VIII levels.7
Murphy et al18 described a 62-year-old woman with Felty syndrome who developed a factor VIII inhibitor and was subsequently given a diagnosis of LGL leukemia. Treatment with immunosuppressive agents, including cyclophosphamide, azathioprine, and rituximab, successfully eradicated her factor VIII inhibitor, although the LGL leukemia persisted.
Case conclusion: Eradication of factor VIII inhibitor
Our patient, similar to the patient described by Murphy et al18 above, had eradication of the factor VIII inhibitor despite persistence of LGL leukemia. Between the time of diagnosis at our clinic, when she had 54% LGLs, and eradication of the inhibitor 3 months later, the LGL percentage ranged from 45% to 89%. No clear direct correlation between LGL and factor VIII inhibitor levels could be detected.
Given the strong association of LGL leukemia with autoimmune disease, it is tempting to believe that her factor VIII inhibitor was somehow related to her malignancy, although the exact mechanism remained unclear. The average age at diagnosis is 60 for LGL leukemia11 and over 70 for AHA,5,6 so advanced age may be the common denominator. Whether or not our patient will have recurrence of her factor VIII inhibitor or the development of other autoimmune diseases with the persistence of her LGL leukemia remains to be seen.
At last follow-up, our patient was off all therapy and continued to have normal aPTT and factor VIII levels. Repeat flow cytometry after treatment of her factor VIII inhibitor showed persistence of a clonal T-cell population, although reduced from 72% to 60%. It may be that the 2 entities were unrelated, and the clonal T-cell population was simply fluctuating over time. This can be determined only with further observation. As the patient had no symptoms from her LGL leukemia, she continued to be observed without treatment.
TAKE-HOME POINTS
- The coagulation assay is key to initially assessing a bleeding abnormality; whether the prothrombin time and aPTT are normal or prolonged narrows the differential diagnosis and determines next steps in evaluation.
- Mixing studies can help pinpoint the responsible deficient factor.
- Acquired factor VIII deficiency, also known as AHA, may be caused by autoimmune disease, malignancy, or medications, but it is usually idiopathic.
- AHA treatment is focused on achieving hemostasis and reducing factor VIII inhibitor.
- Lymphocytosis should be evaluated with a peripheral blood smear and flow cytometry to determine if the population is polyclonal (associated with infection) or clonal (associated with malignancy).
- LGL leukemia is usually a chronic, indolent disease, although an uncommon subtype has an aggressive course.
- The association between AHA and LGL leukemia is unclear, and both conditions must be monitored and managed.
- Kamal AH, Tefferi A, Pruthi RK. How to interpret and pursue an abnormal prothrombin time, activated partial thromboplastin time, and bleeding time in adults. Mayo Clin Proc 2007; 82(7):864–873. doi:10.4065/82.7.864
- Tcherniantchouk O, Laposata M, Marques MB. The isolated prolonged PTT. Am J Hematol 2013; 88(1):82–85. doi:10.1002/ajh.23285
- Ma AD, Carrizosa D. Acquired factor VIII inhibitors: pathophysiology and treatment. Hematology Am Soc Hematol Educ Program 2006:432–437. doi:10.1182/asheducation-2006.1.432
- Delgado J, Jimenez-Yuste V, Hernandez-Navarro F, Villar A. Acquired haemophilia: review and meta-analysis focused on therapy and prognostic factors. Br J Haematol 2003; 121(1):21–35. pmid:12670328
- Knoebl P, Marco P, Baudo F, et al; EACH2 Registry Contributors. Demographic and clinical data in acquired hemophilia A: results from the European Acquired Haemophilia Registry (EACH2). J Thromb Haemost 2012; 10(4):622–631. doi:10.1111/j.1538-7836.2012.04654.x
- Collins PW, Hirsch S, Baglin TP, et al; UK Haemophilia Centre Doctors’ Organisation. Acquired hemophilia A in the United Kingdom: a 2-year national surveillance study by the United Kingdom Haemophilia Centre Doctors’ Organisation. Blood 2007; 109(5):1870–1877. doi:10.1182/blood-2006-06-029850
- Collins P, Baudo F, Knoebl P, et al; EACH2 Registry Collaborators. Immunosuppression for acquired hemophilia A: results from the European Acquired Haemophilia Registry (EACH2). Blood 2012; 120(1):47–55. doi:10.1182/blood-2012-02-409185
- George TI. Malignant or benign leukocytosis. Hematology Am Soc Hematol Educ Program 2012; 2012:475–484. doi:10.1182/asheducation-2012.1.475
- Watters RJ, Liu X, Loughran TP Jr. T-cell and natural killer-cell large granular lymphocyte leukemia neoplasias. Leuk Lymphoma 2011; 52(12):2217–2225. doi:10.3109/10428194.2011.593276
- Lamy T, Moignet A, Loughran TP Jr. LGL leukemia: from pathogenesis to treatment. Blood 2017; 129(9):1082–1094. doi:10.1182/blood-2016-08-692590
- Zhang D, Loughran TP Jr. Large granular lymphocytic leukemia: molecular pathogenesis, clinical manifestations, and treatment. Hematology Am Soc Hematol Educ Program 2012; 2012:652–659. doi:10.1182/asheducation-2012.1.652
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016; 127(20):2375–2390. doi:10.1182/blood-2016-01-643569
- Rose MG, Berliner N. T-cell large granular lymphocyte leukemia and related disorders. Oncologist 2004; 9(3):247–258. pmid:15169980
- Bockorny B, Dasanu CA. Autoimmune manifestations in large granular lymphocyte leukemia. Clin Lymphoma Myeloma Leuk 2012; 12(6):400–405. doi:10.1016/j.clml.2012.06.006
- Liu X, Loughran TP Jr. The spectrum of large granular lymphocyte leukemia and Felty’s syndrome. Curr Opin Hematol 2011; 18(4):254–259. doi:10.1097/MOH.0b013e32834760fb
- Franchini M, Lippi G. Acquired factor V inhibitors: a systematic review. J Thromb Thrombolysis 2011; 31(4):449–457. doi:10.1007/s11239-010-0529-6
- Sallah S, Nguyen NP, Abdallah JM, Hanrahan LR. Acquired hemophilia in patients with hematologic malignancies. Arch Pathol Lab Med 2000; 124(5):730–734.
- Murphy PW, Brett LK, Verla-Tebit E, Macik BG, Loughran TP Jr. Acquired inhibitors to factor VIII and fibrinogen in the setting of T-cell large granular lymphocyte leukemia: a case report and review of the literature. Blood Coagul Fibrinolysis 2015; 26(2):211–213. doi:10.1097/MBC.0000000000000209
- Kamal AH, Tefferi A, Pruthi RK. How to interpret and pursue an abnormal prothrombin time, activated partial thromboplastin time, and bleeding time in adults. Mayo Clin Proc 2007; 82(7):864–873. doi:10.4065/82.7.864
- Tcherniantchouk O, Laposata M, Marques MB. The isolated prolonged PTT. Am J Hematol 2013; 88(1):82–85. doi:10.1002/ajh.23285
- Ma AD, Carrizosa D. Acquired factor VIII inhibitors: pathophysiology and treatment. Hematology Am Soc Hematol Educ Program 2006:432–437. doi:10.1182/asheducation-2006.1.432
- Delgado J, Jimenez-Yuste V, Hernandez-Navarro F, Villar A. Acquired haemophilia: review and meta-analysis focused on therapy and prognostic factors. Br J Haematol 2003; 121(1):21–35. pmid:12670328
- Knoebl P, Marco P, Baudo F, et al; EACH2 Registry Contributors. Demographic and clinical data in acquired hemophilia A: results from the European Acquired Haemophilia Registry (EACH2). J Thromb Haemost 2012; 10(4):622–631. doi:10.1111/j.1538-7836.2012.04654.x
- Collins PW, Hirsch S, Baglin TP, et al; UK Haemophilia Centre Doctors’ Organisation. Acquired hemophilia A in the United Kingdom: a 2-year national surveillance study by the United Kingdom Haemophilia Centre Doctors’ Organisation. Blood 2007; 109(5):1870–1877. doi:10.1182/blood-2006-06-029850
- Collins P, Baudo F, Knoebl P, et al; EACH2 Registry Collaborators. Immunosuppression for acquired hemophilia A: results from the European Acquired Haemophilia Registry (EACH2). Blood 2012; 120(1):47–55. doi:10.1182/blood-2012-02-409185
- George TI. Malignant or benign leukocytosis. Hematology Am Soc Hematol Educ Program 2012; 2012:475–484. doi:10.1182/asheducation-2012.1.475
- Watters RJ, Liu X, Loughran TP Jr. T-cell and natural killer-cell large granular lymphocyte leukemia neoplasias. Leuk Lymphoma 2011; 52(12):2217–2225. doi:10.3109/10428194.2011.593276
- Lamy T, Moignet A, Loughran TP Jr. LGL leukemia: from pathogenesis to treatment. Blood 2017; 129(9):1082–1094. doi:10.1182/blood-2016-08-692590
- Zhang D, Loughran TP Jr. Large granular lymphocytic leukemia: molecular pathogenesis, clinical manifestations, and treatment. Hematology Am Soc Hematol Educ Program 2012; 2012:652–659. doi:10.1182/asheducation-2012.1.652
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016; 127(20):2375–2390. doi:10.1182/blood-2016-01-643569
- Rose MG, Berliner N. T-cell large granular lymphocyte leukemia and related disorders. Oncologist 2004; 9(3):247–258. pmid:15169980
- Bockorny B, Dasanu CA. Autoimmune manifestations in large granular lymphocyte leukemia. Clin Lymphoma Myeloma Leuk 2012; 12(6):400–405. doi:10.1016/j.clml.2012.06.006
- Liu X, Loughran TP Jr. The spectrum of large granular lymphocyte leukemia and Felty’s syndrome. Curr Opin Hematol 2011; 18(4):254–259. doi:10.1097/MOH.0b013e32834760fb
- Franchini M, Lippi G. Acquired factor V inhibitors: a systematic review. J Thromb Thrombolysis 2011; 31(4):449–457. doi:10.1007/s11239-010-0529-6
- Sallah S, Nguyen NP, Abdallah JM, Hanrahan LR. Acquired hemophilia in patients with hematologic malignancies. Arch Pathol Lab Med 2000; 124(5):730–734.
- Murphy PW, Brett LK, Verla-Tebit E, Macik BG, Loughran TP Jr. Acquired inhibitors to factor VIII and fibrinogen in the setting of T-cell large granular lymphocyte leukemia: a case report and review of the literature. Blood Coagul Fibrinolysis 2015; 26(2):211–213. doi:10.1097/MBC.0000000000000209
Pediatric cholestatic liver disease: Successful transition of care
Thanks to advances in medical science and our understanding of inherited and acquired liver disease, many more children with acquired or congenital liver disease survive into adulthood than they did 2 decades ago. Improvements in immunosuppression and surgery have increased the chances of pediatric liver transplant recipients reaching adulthood, with a survival rate of 75% at 15 to 20 years.1
With the growing number of adult patients with pediatric-onset liver disease, internists and adult hepatologists need to be aware of these liver diseases and develop expertise to manage this challenging group of patients. Moreover, young adults with pediatric-onset chronic liver disease pose distinct challenges such as pregnancy, adherence to medical regimens, and psychosocial changes in life.
These patients need a “transition of care” rather than a “transfer of care.” Transition of care is a multifaceted process that takes the medical, educational, and psychosocial needs of the patient into consideration before switching their care to adult care physicians, whereas transfer of care is simply an administrative process of change to adult care without previous knowledge of the patients.2
BILIARY ATRESIA
Biliary atresia is a progressive inflammatory fibrosclerosing cholangiopathy of unknown cause. Its prevalence varies with geographic location, ranging from 1 in 6,000 to 1 in 19,000, with the highest prevalence in Taiwan.3
Biliary atresia usually presents within the first few weeks of life, with progressive cholestasis leading to failure to thrive and to fat-soluble vitamin deficiency. Approximately 20% of patients have congenital splenic, gastrointestinal, genitourinary, cardiac, and venous malformations.4,5 Untreated, biliary atresia progresses to end-stage liver disease and death within 2 years.
The first-line treatment for biliary atresia is to establish biliary outflow with the Kasai procedure (hepatic portoenterostomy), in which a jejunal limb is anastomosed in a Roux-en-Y with the liver. The outcomes of the Kasai procedure depend on the timing of surgery, so timely diagnosis of biliary atresia is crucial. When the Kasai procedure is performed within 60 days of birth, biliary flow is achieved in up to 70% of patients; but if performed after 90 days, biliary flow is achieved in fewer than 25%.6
Long-term outcomes of biliary atresia in patients with their native liver have been reported in a few studies.
In a French study,7 743 patients with biliary atresia underwent the Kasai procedure at a median age of 60 days. Survival rates were 57.1% at 2 years, 37.9% at 5 years, 32.4% at 10 years, and 28.5% at 15 years. In other studies,4–9 the 20-year transplant-free survival rate ranged from 23% to 46%. Therefore, at least one-third of children with biliary atresia survive to adulthood with their native liver.
Implications of biliary atresia in adulthood
Although the Kasai procedure improves biliary outflow, up to 70% of patients develop complications of biliary atresia such as progressive fibrosis, cirrhosis, portal hypertension, cholangitis, and hepatocellular carcinoma, even after a successful Kasai procedure.10
Portal hypertension with evidence of splenomegaly, thrombocytopenia, or ascites is found in two-thirds of long-term survivors of biliary atresia with a native liver, with variceal hemorrhage occurring in 30%.11 Therefore, patients with biliary atresia who have evidence of portal hypertension should be screened for varices with upper endoscopy on an annual basis. Management of variceal hemorrhage in these patients includes the use of octreotide, antibiotics, variceal ligation, and sclerotherapy; primary prophylaxis can be achieved with beta-blockers and endoscopic variceal ligation.12
Cholangitis is frequent, occurring in 40% to 60% of biliary atresia patients after the Kasai procedure, and about one-fourth of these patients have multiple episodes.13 The number of episodes of cholangitis negatively affects transplant-free survival.14 Patients with cholangitis should be adequately treated with oral or intravenous antibiotics depending on the severity of presentation. The role of prophylaxis with antibiotics remains unclear.15
Pulmonary complications such as hepatopulmonary syndrome and portopulmonary hypertension can also occur in biliary atresia patients with a native liver. It is important for physicians to be aware of these complications and to screen for them, for example, with agitated saline echocardiography for hepatopulmonary syndrome and with echocardiography for portopulmonary hypertension. Timely screening is crucial, as the outcome of liver transplant depends on the severity at the time of transplant in these conditions, especially portopulmonary hypertension.
Hepatocellular carcinoma has been rarely reported in children with biliary atresia,16 so well-defined guidelines for screening in young adults with biliary atresia are lacking. Most centers recommend screening with ultrasonography of the abdomen and alpha-fetoprotein measurement every 6 months or annually starting soon after the Kasai procedure, since hepatocellular carcinoma has been reported in children as young as age 2.16
Transplant. Adult hepatologists are faced with the challenging task of deciding when it is time for transplant, balancing the long-term complications of biliary atresia with the risk of long-term immunosuppression after transplant. In addition, young adults with these complications may have preserved synthetic function, resulting in low Model for End-Stage Liver Disease (MELD) scores, which may complicate the process of listing for transplant.
Neurocognitive deficits are reported in children with biliary atresia,17 but young adults with biliary atresia generally have reasonable cognitive function and prospects for education and employment.
Pregnancy with successful outcomes has been reported.8
ALAGILLE SYNDROME
Alagille syndrome is an autosomal-dominant multisystemic disease caused by mutations in the JAG1 gene (accounting for > 95% of cases) and the NOTCH2 gene, with highly variable expression.18
Extrahepatic manifestations include butterfly vertebral defects, facial dysmorphism (eg, deep-set and low-set eyes, with characteristic “triangular” facies), posterior embryotoxon (a congenital defect of the eye characterized by an opaque ring around the margin of the cornea), peripheral pulmonary stenosis, renal abnormalities, and vascular malformations.
Hepatic manifestations vary from asymptomatic laboratory abnormalities to progressive cholestasis starting in early infancy with intractable pruritus, xanthomas, failure to thrive, and end-stage liver disease requiring liver transplant in childhood in 15% to 20% of patients.19
Implications of Alagille syndrome in adulthood
Transplant. Interestingly, the phenotype of hepatic disease is already established in childhood, with minimal or no progression in adulthood. Most children with minimal liver disease experience spontaneous resolution, whereas those with significant cholestasis might ultimately develop progressive liver fibrosis or cirrhosis requiring liver transplant in childhood. Only a small subset of children with minimal cholestasis progress to end-stage liver disease in late childhood or early adulthood.20 Therefore, liver transplant for progressive liver disease from significant cholestasis almost always occurs in childhood, usually between ages 1 and 4.21
In a retrospective study comparing posttransplant outcomes in children with Alagille syndrome and biliary atresia, 1-year patient survival was excellent overall in children with Alagille syndrome, although slightly lower than in children with biliary atresia, most likely owing to extrahepatic morbidities of Alagille syndrome and especially the use of immunosuppression in those with renal disease.21 Similarly, 1- and 5-year patient and graft survival outcomes of liver transplant in adults with Alagille syndrome were also excellent compared with those who received a liver transplant in childhood for Alagille syndrome or in adulthood for biliary atresia.22
Hepatocellular carcinoma has occurred in these patients in the absence of cirrhosis, which makes implementation of prognostic and surveillance strategies almost impossible to design for them. Annual ultrasonography with alpha-fetoprotein testing might be applicable in Alagille syndrome patients. However, deciding which patients should undergo this testing and when it should start will be challenging, given the paucity of data.
Cardiovascular disease. Cardiac phenotype is also mostly established in childhood, with the pulmonary vasculature being most commonly involved.19 In contrast, renal and other vascular abnormalities can manifest in adulthood. Renal manifestations vary and include structural anomalies such as hyperechoic kidneys or renal cysts, which can manifest in childhood, and some abnormalities such as hypertension and renal artery stenosis that can manifest in adulthood.23,24
Vasculopathy is reported to involve the intracranial, renal, and intra-abdominal blood vessels.25 Neurovascular accidents such as stroke and intracranial hemorrhage can occur at any age, with significant rates of morbidity and death.26 Therefore, some experts recommend magnetic resonance angiography every 5 years and before any major intervention to prevent these devastating complications.20
Pregnancy. Successful pregnancies have been reported. Preexisting cardiac and hepatic disease can complicate pregnancy depending on the severity of the disease. Because of the autosomal-dominant pattern of inheritance, infants have a 50% risk of the disease, so genetic counseling should be seriously considered before conception.27 Prenatal diagnosis is possible, but the lack of genotype-phenotype correlation precludes its use in clinical practice.
PROGRESSIVE FAMILIAL INTRAHEPATIC CHOLESTASIS
Progressive familial intrahepatic cholestasis (PFIC) is a heterogeneous group of autosomal-recessive conditions associated with disruption of bile formation causing cholestatic liver disease in infants and young children. Three types have been described, depending on the genetic mutation in the hepatobiliary transport pathway:
- PFIC 1 (Byler disease) is caused by impaired bile salt secretion due to mutations in the ATP8B1 gene encoding for the familial intrahepatic cholestasis 1 (FIC 1) protein
- PFIC 2 is caused by impaired bile salt secretion due to mutations in the ABCB11 gene encoding for the bile salt export pump (BSEP) protein
- PFIC 3 is caused by impaired biliary phospholipid secretion due to a defect in ABCB4 encoding for multidrug resistance 3 (MDR3) protein.28
PFIC 1 and 2 manifest with low gamma-glutamyl transferase (GGT) cholestasis, whereas PFIC 3 presents with high GGT cholestasis.
PFIC 1 and PFIC 2 usually cause cholestasis in early infancy, but PFIC 3 can cause cholestasis in late infancy, childhood, and even adulthood.
Because ATP8B1 is expressed in other tissues, PFIC 1 is characterized by extrahepatic manifestations such as sensorineural hearing loss, growth failure, severe diarrhea, and pancreatic insufficiency.
Implications of PFIC in adulthood
PFIC 1 and 2 (low-GGT cholestasis) are usually progressive and often lead to end-stage liver disease and cirrhosis before adulthood. Therefore, almost all patients with PFIC 1 and 2 undergo liver transplant or at least a biliary diversion procedure before reaching adulthood. Intractable pruritus is one of the most challenging clinical manifestations in patients with PFIC.
First-line management is pharmacologic and includes ursodeoxycholic acid, antihistamines (eg, hydroxyzine), bile acid sequestrants (eg, cholestyramine, colestipol), naltrexone, and rifampin, but these have limited efficacy.10
Most patients, especially those with PFIC 1 and 2, undergo a biliary diversion procedure such as partial external biliary diversion (cholecystojejunocutaneostomy), ileal exclusion, or partial internal biliary diversion (cholecystojejunocolic anastomosis) to decrease enterohepatic circulation of bile salts. The efficacy of these procedures is very limited in patients with established cirrhosis. Excessive losses of bile can occur through the biliary stoma, leading to dehydration in patients with external biliary diversion. In patients who are not candidates for biliary diversion, endoscopic nasobiliary drainage of pancreatobiliary secretions could be achieved by placing a catheter in the common bile duct; this has been reported to be effective in relieving cholestasis in a few cases.29
Liver transplant is needed in patients with progressive liver disease and intractable pruritus despite medical management and biliary diversion. Unlike in biliary atresia, liver transplant is not curative in PFIC 1, due to extrahepatic manifestations: patients with PFIC 1 can still have intractable diarrhea and pancreatitis after liver transplant. More importantly, allograft steatohepatitis with further progression to cirrhosis can occur after liver transplant in patients with PFIC 1. Interestingly, biliary diversion has been reported to improve graft steatosis and diarrhea after liver transplant.30
Although graft survival after transplant is good in patients with PFIC 2, recurrence of low-GGT cholestasis has been reported and is believed to be due to the formation of anti-bile salt export pump (anti-BSEP) antibodies by the host immune system in response to exposure to new proteins from the transplant graft.31
Cancer. The risk of malignancy, especially hepatocellular carcinoma, is also increased in PFIC 2, affecting nearly 15% of patients. Therefore, standard hepatocellular carcinoma surveillance with ultrasonography or alpha-fetoprotein testing or both is recommended in patients with PFIC 2. Cholangiocarcinoma and pancreatic adenocarcinoma have also been reported in patients with PFIC 2.20
Incomplete penetrance of mutations in ATP8B1 and ABCB11 can cause recurrent episodes of cholestasis and pruritus with asymptomatic periods between episodes, referred to as benign recurrent intrahepatic cholestasis. Prognosis is usually good, with no progression to cirrhosis.32
Pregnancy. In contrast to FIC 1 and BSEP deficiency, MDR3 defects lead to a wide phenotypic spectrum depending on the type of mutation. Heterozygous mutation is associated with increased risk of development of cholestasis during pregnancy, which typically presents with generalized pruritus in the third trimester and is associated with adverse fetal outcomes. Intrahepatic cholestasis of pregnancy is usually treated with ursodeoxycholic acid, with reported improvement in pruritus, liver function, and pregnancy outcomes.33
In adults, drug-induced liver injury and idiopathic cirrhosis have also been described with MDR3 defects. Intrahepatic lithiasis and cholesterol gallstones can also occur with MDR3 defects as a result of impaired secretion of biliary phospholipid.32 Despite intrahepatic cholestasis of pregnancy, successful outcomes have been reported in women with PFIC.20
OTHER CHILDHOOD-ONSET INHERITED CHOLESTATIC DISEASES
Cystic fibrosis-associated liver disease
Nearly 40% of patients with cystic fibrosis develop liver disease.34 Cystic fibrosis-associated liver disease encompasses a broad clinical spectrum including asymptomatic elevation of aminotransferases, neonatal cholestasis, hepatic steatosis, focal biliary cirrhosis, and multilobar cirrhosis. Cirrhosis and portal hypertension can occur in 5% to 10% of patients and is the third-leading cause of death in patients with cystic fibrosis.35
Risk factors for cystic fibrosis-associated liver disease include male sex, meconium ileus, and severe CFTR gene mutation (class I–III) with pancreatic insufficiency. Cystic fibrosis-related cirrhosis is more frequent in children and adolescents, whereas noncirrhotic portal hypertension and intrahepatic cholangiopathies are more common in adults.36
Limited available studies support treatment with ursodeoxycholic acid in patients with cholestasis to delay the progression of liver disease, but the impact of this drug on long-term outcome is unknown.29
Most patients remain in compensated cirrhosis for many years before progressing to decompensated cirrhosis requiring liver transplant. Other indications for liver transplant include recurrent intractable variceal bleeding, hepatopulmonary syndrome, and portopulmonary hypertension. Combined liver and lung transplant may be considered in patients with advanced liver and lung disease. Outcomes after isolated liver or liver-lung transplant in cystic fibrosis patients have been comparable to those in patients with other liver diseases.37
Defects in bile acid synthesis
Inherited defects of enzymes required for the synthesis of primary bile acids from cholesterol can cause cholestasis from impaired bile flow and production of hepatotoxic aberrant bile acids. The clinical presentation varies depending on the enzymatic defect and can range from liver disease of varying severity to neurologic manifestations. Idiopathic late-onset cholestasis and cirrhosis of unknown etiology have been reported in adults with bile acid synthesis defects.38,39 Therefore, this diagnosis should be considered in cases of cryptogenic cirrhosis and other cholestatic features.
Treatment with primary bile acids (cholic acid) has been effective in most patients with defective bile acid synthesis.
Primary sclerosing cholangitis
Primary sclerosing cholangitis is characterized by progressive obliteration of intrahepatic and extrahepatic bile ducts and is most commonly seen in patients with inflammatory bowel disease. Sclerosing cholangitis can also be secondary to other diseases in children such as immunodeficiency syndromes, Langerhans cell histiocytosis, cystic fibrosis, or sickle cell anemia.40 Neonatal sclerosing cholangitis is a rare autosomal-recessive disease characterized by a severe form of cholangiopathy in neonates and young infants requiring transplant. It can be associated with Kabuki syndrome and neonatal ichthyosis-sclerosing cholangitis syndrome.
Treatment options are limited. Ursodeoxycholic acid and oral vancomycin have variable efficacy. Liver transplant is needed in patients with decompensated cirrhosis. Patients with primary sclerosing cholangitis, especially adults, are at higher risk of developing cholangiocarcinoma, and therefore screening with ultrasonography or magnetic resonance imaging every 6 to 12 months is recommended.
The risk of preterm and cesarean deliveries may be elevated in women with primary sclerosing cholangitis, though data are limited.33
PEDIATRIC LIVER TRANSPLANT RECIPIENTS WHO SURVIVE INTO ADULTHOOD
Adolescent rebellion poses risks
Outcomes of liver transplant in children and adolescents have improved tremendously in the past 2 decades with advances in surgical techniques, pre- and postoperative management, organ preservation, and immunosuppression. Now, most pediatric liver transplant recipients survive into adulthood, creating a unique challenge for internists and adult care hepatologists.41
In rebellious adolescents and young adults, risk-taking behavior, nonadherence to immunosuppressive medications, alcohol intake, and substance abuse increase the risk of graft rejection and loss. Current immunosuppressive drugs such as calcineurin inhibitors (tacrolimus, cyclosporine), mycophenolate mofetil, sirolimus, and corticosteroids have drastically decreased rejection rates in compliant patients.41 Educating patients on the importance of taking their medications and avoiding alcohol and drug abuse is especially important for adolescents and young adults, as rates of nonadherence are high in these age groups.
Although pregnancy is usually successful after liver transplant, it should be considered high-risk due to reported complications such as graft rejection, diabetes, preeclampsia, sepsis, prematurity, and low birth weight. Conception should be avoided for at least 1 year after transplant.42 Appropriate counseling with regard to pregnancy and contraception is important.
There is no consensus on breastfeeding, but it is considered safe in women on low-dose calcineurin inhibitors.43
Life is better with a new liver, but patients have special needs
Liver transplant is life-saving and improves quality of life. However, long-term pediatric liver transplant recipients face challenges such as strict adherence to medications and follow-up visits, avoiding exposure to infections, and fear of graft rejection.
Chronic liver disease in children leads to failure to thrive, growth failure, and even delayed puberty, which resolve in most patients after liver transplant before adulthood in the absence of other comorbidities.44 However, these patients are reported to have lower psychosocial functioning and more psychiatric disorders such as anxiety or posttraumatic disorder.41,44
Therefore, a psychologist or other mental health professional should be part of the management team from the time of pretransplant assessment to identify mental health problems and the need for adjustments before liver transplant. Ongoing psychosocial assessment after liver transplant is equally important to identify risks such as drug or alcohol abuse, depression, posttraumatic stress disorder, and medication nonadherence, all of which can negatively affect posttransplant outcome.45
In addition, assessment of family functioning and structure is important for good long-term outcomes posttransplant; therefore, a social worker should also be a part of the transplant team. Psyschosocial assessment tools can identify high-risk candidates who would benefit from earlier intervention to avoid any negative impact posttransplant.
Neurocognitive development can be delayed in children with chronic liver disease, and the delay may persist even after liver transplant, with reported impairments in intellectual ability, language, verbal, and visuospatial functioning skills.41 In spite of this, a recent study found that more than half the study patients were employed at a median follow-up of 24 years from liver transplant and a median age of 27.46
Remarkably, pediatric liver transplant recipients have reported quality of life comparable to that in the general population,47 and even better than in patients with other chronic illnesses.48
Long-term medical comorbidities in pediatric liver transplant recipients
Favorable outcomes such as long-term survival and good quality of life in pediatric liver transplant recipients are lessened by late complications such as portal vein thrombosis or biliary strictures needing interventions, chronic graft rejection, adverse effects of immunosuppression, and recurrence of the disease.
Split-liver transplant—splitting a deceased-donor allograft to provide grafts for 2 recipients—has revolutionized liver transplant by increasing the donor pool and thereby decreasing waitlist mortality rates, especially in pediatric candidates. Despite this advantage, split-liver transplant is technically challenging and associated with increased perioperative complications compared with whole-liver transplant, especially in adult recipients. Recently, experienced centers have reported favorable outcomes with split-liver transplant comparable to those with whole-liver transplant; therefore, split-liver transplant should be considered after careful evaluation of donor organ and recipient clinical status.49
Old age in the recipient can also adversely affect liver transplant outcomes.50
Interestingly, even in patients whose clinical course is unremarkable and biochemical values are normal, graft hepatitis or fibrosis of unknown cause with progression to cirrhosis has been described in the decade after transplant.41
Chronic rejection with eventual graft loss may be related to nonadherence in adolescents and can be reduced with use of an additional immunosuppressant such as sirolimus or mycophenolate. Chronic kidney disease can occur in about one-third of liver transplant recipients secondary to renal disease associated with primary disease (like Alagille syndrome), hepatorenal syndrome, and most importantly, use of calcineurin inhibitors.45
Components of the metabolic syndrome such as type 2 diabetes, obesity, nonalcoholic fatty liver disease, hypertension, and dyslipidemia are also seen in long-term pediatric liver transplant survivors. Internists are advised to screen for these comorbidities so that interventions can be applied early to improve long-term health outcomes and graft survival.
Of importance, multiple studies have shown a 2-fold increase in the rates of de novo malignancy in liver transplant recipients, including solid-organ and lymphoproliferative cancers, probably due to long-term immunosuppression. Posttransplant lymphoproliferative disorder occurs at lower rates than with other solid-organ transplants; its incidence is greatest in pediatric patients and in the first 12 to 18 months after transplant.51
TRANSITION TO ADULT CARE
While the number of patients with childhood-onset liver disease and pediatric liver transplant recipients who survive into adulthood is increasing, there are no established guidelines or formal models for transitioning these patients into adult care. Consequently, studies on transitional process have examined various issues such as patient and parent frustration, poor medical knowledge among patients during transition, lack of parental facilitation, and inadequate knowledge on disease process among adult-care hepatologists.52–54
A prolonged period of transition up to age 25 is preferred in complicated cases. Distinctive consideration for transition should include those with neurocognitive developmental delay from underlying disease or hepatic encephalopathy before transplant. These patients need additional support and time to achieve independence in health management before transition.57 Validated questionnaires are available to assess readiness to transition into adult care,58 implying that the decision to transition should not be based solely on age.
- Kelly DA, Bucuvalas JC, Alonso EM, et al; American Association for the Study of Liver Diseases; American Society of Transplantation. Long-term medical management of the pediatric patient after liver transplantation: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl 2013; 19(8):798–825. doi:10.1002/lt.23697
- Rosen DS, Blum RW, Britto M, Sawyer SM, Siegel DM; Society for Adolescent Medicine. Transition to adult health care for adolescents and young adults with chronic conditions: position paper of the Society for Adolescent Medicine. J Adolesc Health 2003; 33(4):309–311. pmid:14519573
- Fawaz R, Baumann U, Ekong U, et al. Guideline for the evaluation of cholestatic jaundice in infants: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr 2017; 64(1):154–168. doi:10.1097/MPG.0000000000001334
- Vajro P, Ferrante L, Lenta S, Mandato C, Persico M. Management of adults with paediatric-onset chronic liver disease: strategic issues for transition care. Dig Liver Dis 2014; 46(4):295–301. doi:10.1016/j.dld.2013.10.018
- Davenport M, Tizzard SA, Underhill J, Mieli-Vergani G, Portmann B, Hadzic N. The biliary atresia splenic malformation syndrome: a 28-year single-center retrospective study. J Pediatr 2006; 149(3):393–400. doi:10.1016/j.jpeds.2006.05.030
- Balistreri WF, Bezerra JA. Whatever happened to “neonatal hepatitis?” Clin Liver Dis 2006; 10(1):27–53. doi:10.1016/j.cld.2005.10.008
- Serinet MO, Wildhaber BE, Broué P, et al. Impact of age at Kasai operation on its results in late childhood and adolescence: a rational basis for biliary atresia screening. Pediatrics 2009; 123(5):1280–1286. doi:10.1542/peds.2008-1949
- de Vries W, Homan-Van der Veen J, Hulscher JB, Hoekstra-Weebers JE, Houwen RH, Verkade HJ; Netherlands Study Group of Biliary Atresia Registry. Twenty-year transplant-free survival rate among patients with biliary atresia. Clin Gastroenterol Hepatol 2011; 9(12):1086–1091. doi:10.1016/j.cgh.2011.07.024
- Lykavieris P, Chardot C, Sokhn M, Gauthier F, Valayer J, Bernard O. Outcome in adulthood of biliary atresia: a study of 63 patients who survived for over 20 years with their native liver. Hepatology 2005; 41(2):366–371. doi:10.1002/hep.20547
- Joshi D, Gupta N, Samyn M, Deheragoda M, Dobbels F, Heneghan MA. The management of childhood liver diseases in adulthood. J Hepatol 2017; 66(3):631–644. doi:10.1016/j.jhep.2016.11.013
- Shneider BL, Abel B, Haber B, et al; Childhood Liver Disease Research and Education Network. Portal hypertension in children and young adults with biliary atresia. J Pediatr Gastroenterol Nutr 2012; 55(5):567–573. doi:10.1097/MPG.0b013e31826eb0cf
- Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2017; 65(1):310–335. doi:10.1002/hep.28906
- Shneider BL, Brown MB, Haber B, et al; Biliary Atresia Research Consortium. A multicenter study of the outcome of biliary atresia in the United States, 1997 to 2000. J Pediatr 2006; 148(4):467–474. doi:10.1016/j.jpeds.2005.12.054
- Hung PY, Chen CC, Chen WJ, et al. Long-term prognosis of patients with biliary atresia: a 25 year summary. J Pediatr Gastroenterol Nutr 2006; 42(2):190–195. doi:10.1097/01.mpg.0000189339.92891.64
- Verkade HJ, Bezerra JA, Davenport M, et al. Biliary atresia and other cholestatic childhood diseases: advances and future challenges. J Hepatol 2016; 65(3):631–642. doi:10.1016/j.jhep.2016.04.032
- Hadžic N, Quaglia A, Portmann B, et al. Hepatocellular carcinoma in biliary atresia: King’s College Hospital experience. J Pediatr 2011; 159(4):617–622.e1. doi:10.1016/j.jpeds.2011.03.004
- Sokol RJ, Shepherd RW, Superina R, Bezerra JA, Robuck P, Hoofnagle JH. Screening and outcomes in biliary atresia: summary of a National Institutes of Health workshop. Hepatology 2007; 46(2):566–581. doi:10.1002/hep.21790
- Li L, Krantz ID, Deng Y, et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet 1997; 16(3):243–251. doi:10.1038/ng0797-243
- Saleh M, Kamath BM, Chitayat D. Alagille syndrome: clinical perspectives. Appl Clin Genet 2016; 9:75–82. doi:10.2147/TACG.S86420
- Bass LM, Kamath BM. Inherited disorders of cholestasis in adulthood. Clinical Liver Disease 2013; 2(5):200–203. doi:10.1002/cld.245
- Kamath BM, Yin W, Miller H, Anand R, Rand EB, Alonso E, Bucuvalas J; Studies of Pediatric Liver Transplantation. Outcomes of liver transplantation for patients with Alagille syndrome: the studies of pediatric liver transplantation experience. Liver Transpl 2012; 18(8):940–948. doi:10.1002/lt.23437
- Arnon R, Annunziato R, Schiano T, et al. Orthotopic liver transplantation for adults with Alagille syndrome. Clin Transplant 2012; 26(2):E94–E100. doi:10.1111/j.1399-0012.2011.01574.x
- Salem JE, Bruguiere E, Iserin L, Guiochon-Mantel A, Plouin PF. Hypertension and aortorenal disease in Alagille syndrome. J Hypertens 2012; 30(7):1300–1306. doi:10.1097/HJH.0b013e3283531e1f
- Kamath BM, Podkameni G, Hutchinson AL, et al. Renal anomalies in Alagille syndrome: a disease-defining feature. Am J Med Genet A 2012; 158A(1):85–89. doi:10.1002/ajmg.a.34369
- Kamath BM, Bason L, Piccoli DA, Krantz ID, Spinner NB. Consequences of JAG1 mutations. J Med Genet 2003; 40(12):891–895. pmid:14684686
- Emerick KM, Krantz ID, Kamath BM, et al. Intracranial vascular abnormalities in patients with Alagille syndrome. J Pediatr Gastroenterol Nutr 2005; 41(1):99–107. pmid:15990638
- Ferrarese A, Senzolo M, Burra P. Successful pregnancy in Alagille syndrome. Dig Liver Dis 2015; 47(1):86–87. doi:10.1016/j.dld.2014.08.047
- Davit-Spraul A, Fabre M, Branchereau S, et al. ATP8B1 and ABCB11 analysis in 62 children with normal gamma-glutamyl transferase progressive familial intrahepatic cholestasis (PFIC): phenotypic differences between PFIC1 and PFIC2 and natural history. Hepatology 2010; 51(5):1645–1655. doi:10.1002/hep.23539
- Zellos A, Lykopoulou L, Polydorou A, et al. Nasobiliary drainage in an episode of intrahepatic cholestasis in a child with mild ABCB11 disease. J Pediatr Gastroenterol Nutr 2012; 55(1):88–90. doi:10.1097/MPG.0b013e31822f2bda
- Alrabadi LS, Morotti RA, Valentino PL, Rodriguez-Davalos MI, Ekong UD, Emre SH. Biliary drainage as treatment for allograft steatosis following liver transplantation for PFIC-1 disease: a single-center experience. Pediatr Transplant 2018; 22(4):e13184. doi:10.1111/petr.13184
- Kubitz R, Dröge C, Kluge S, et al. Autoimmune BSEP disease: disease recurrence after liver transplantation for progressive familial intrahepatic cholestasis. Clin Rev Allergy Immunol 2015; 48(2–3):273–284. doi:10.1007/s12016-014-8457-4
- Jacquemin E. Progressive familial intrahepatic cholestasis. Clin Res Hepatol Gastroenterol 2012; 36(suppl 1):S26–S35. doi:10.1016/S2210-7401(12)70018-9
- Pataia V, Dixon PH, Williamson C. Pregnancy and bile acid disorders. Am J Physiol Gastrointest Liver Physiol 2017; 313(1):G1–G6. doi:10.1152/ajpgi.00028.2017
- Lamireau T, Monnereau S, Martin S, Marcotte JE, Winnock M, Alvarez F. Epidemiology of liver disease in cystic fibrosis: a longitudinal study. J Hepatol 2004; 41(6):920–925. doi:10.1016/j.jhep.2004.08.006
- Bolia R, Ooi CY, Lewindon P, et al. Practical approach to the gastrointestinal manifestations of cystic fibrosis. J Paediatr Child Health 2018; 54(6):609–619. doi:10.1111/jpc.13921
- Debray D, Kelly D, Houwen R, Strandvik B, Colombo C. Best practice guidance for the diagnosis and management of cystic fibrosis-associated liver disease. J Cyst Fibros 2011; 10(suppl 2):S29–S36. doi:10.1016/S1569-1993(11)60006-4
- Fridell JA, Bond GJ, Mazariegos G V, et al. Liver transplantation in children with cystic fibrosis: a long-term longitudinal review of a single center’s experience. J Pediatr Surg 2003; 38(8):1152–1156. pmid:12891484
- Fischler B, Bodin K, Stjernman H, et al. Cholestatic liver disease in adults may be due to an inherited defect in bile acid biosynthesis. J Intern Med 2007; 262(2):254–262. doi:10.1111/j.1365-2796.2007.01814.x
- Molho-Pessach V, Rios JJ, Xing C, Setchell KD, Cohen JC, Hobbs HH. Homozygosity mapping identifies a bile acid biosynthetic defect in an adult with cirrhosis of unknown etiology. Hepatology 2012; 55(4):1139–1145. doi:10.1002/hep.24781
- Mieli-Vergani G, Vergani D. Sclerosing cholangitis in children and adolescents. Clin Liver Dis 2016; 20(1):99–111. doi:10.1016/j.cld.2015.08.008
- Kelly D, Wray J. The adolescent liver transplant patient. Clin Liver Dis 2014; 18(3):613–632. doi:10.1016/j.cld.2014.05.006
- Westbrook RH, Yeoman AD, Agarwal K, et al. Outcomes of pregnancy following liver transplantation: the King’s College Hospital experience. Liver Transpl. 2015; 21(9):1153–1159. doi:10.1002/lt.24182
- Hammoud GM, Almashhrawi AA, Ahmed KT, Rahman R, Ibdah JA. Liver diseases in pregnancy: liver transplantation in pregnancy. World J Gastroenterol 2013; 19(43):7647–7651. doi:10.3748/wjg.v19.i43.7647
- Codoner-Franch P, Bernard O, Alvarez F. Long-term follow-up of growth in height after successful liver transplantation. J Pediatr 1994; 124(3):368–373. pmid:8120704
- Shemesh E. Assessment and management of psychosocial challenges in pediatric liver transplantation. Liver Transpl 2008; 14(9):1229–1236. doi:10.1002/lt.21582
- Martinelli J, Habes D, Majed L, et al. Long-term outcome of liver transplantation in childhood: a study of 20-year survivors. Am J Transplant 2018; 18(7):1680–1689. doi:10.1111/ajt.14626
- Roblin E, Audhuy F, Boillot O, Rivet C, Lachaux A. Long-term quality of life after pediatric liver transplantation. Arch Pediatr 2012; 19(10):1039–1052. French. doi:10.1016/j.arcped.2012.06.020
- Duffy JP, Kao K, Ko CY, et al. Long-term patient outcome and quality of life after liver transplantation: analysis of 20-year survivors. Ann Surg 2010; 252(4):652–661. doi:10.1097/SLA.0b013e3181f5f23a
- Hackl C, Schmidt KM, Süsal C, Döhler B, Zidek M, Schlitt HJ. Split liver transplantation: Current developments. World J Gastroenterol 2018; 24(47):5312–5321. doi:10.3748/wjg.v24.i47.5312
- Durand F, Levitsky J, Cauchy F, Gilgenkrantz H, Soubrane O, Francoz C. Age and liver transplantation. J Hepatol 2019; 70(4):745–758. doi:10.1016/j.jhep.2018.12.009
- Chandok N, Watt KD. Burden of de novo malignancy in the liver transplant recipient. Liver Transpl 2012; 18(11):1277–1289. doi:10.1002/lt.23531
- Ferrarese A, Germani G, Lazzaro S, et al. Short-term outcomes of paediatric liver transplant recipients after transition to Adult Healthcare Service. Liver Int 2018; 38(7):1316–1321. doi:10.1111/liv.13655
- Wright J, Elwell L, McDonagh JE, Kelly DA, Wray J. “Are these adult doctors gonna know me?” Experiences of transition for young people with a liver transplant. Pediatr Transplant 2016; 20(7):912–920. doi:10.1111/petr.12777
- Heldman MR, Sohn MW, Gordon EJ, et al. National survey of adult transplant hepatologists on the pediatric-to-adult care transition after liver transplantation. Liver Transpl 2015; 21(2):213–223. doi:10.1002/lt.24044
- Vajro P, Fischler B, Burra P, et al. The health care transition of youth with liver disease into the adult health system. J Pediatr Gastroenterol Nutr 2018; 66(6):976–990. doi:10.1097/MPG.0000000000001965
- Fredericks EM, Lopez MJ. Transition of the adolescent transplant patient to adult care. Clin Liver Dis (Hoboken) 2013; 2(5):223–226. doi:10.1002/cld.243
- Kaufman M. Transition of cognitively delayed adolescent organ transplant recipients to adult care. Pediatr Transplant 2006; 10(4):413–417. doi:10.1111/j.1399-3046.2006.00491.x
- Sawicki GS, Lukens-Bull K, Yin X, et al. Measuring the transition readiness of youth with special healthcare needs: validation of the TRAQ—Transition Readiness Assessment Questionnaire. J Pediatr Psychol 2011; 36(2):160–171. doi:10.1093/jpepsy/jsp128
Thanks to advances in medical science and our understanding of inherited and acquired liver disease, many more children with acquired or congenital liver disease survive into adulthood than they did 2 decades ago. Improvements in immunosuppression and surgery have increased the chances of pediatric liver transplant recipients reaching adulthood, with a survival rate of 75% at 15 to 20 years.1
With the growing number of adult patients with pediatric-onset liver disease, internists and adult hepatologists need to be aware of these liver diseases and develop expertise to manage this challenging group of patients. Moreover, young adults with pediatric-onset chronic liver disease pose distinct challenges such as pregnancy, adherence to medical regimens, and psychosocial changes in life.
These patients need a “transition of care” rather than a “transfer of care.” Transition of care is a multifaceted process that takes the medical, educational, and psychosocial needs of the patient into consideration before switching their care to adult care physicians, whereas transfer of care is simply an administrative process of change to adult care without previous knowledge of the patients.2
BILIARY ATRESIA
Biliary atresia is a progressive inflammatory fibrosclerosing cholangiopathy of unknown cause. Its prevalence varies with geographic location, ranging from 1 in 6,000 to 1 in 19,000, with the highest prevalence in Taiwan.3
Biliary atresia usually presents within the first few weeks of life, with progressive cholestasis leading to failure to thrive and to fat-soluble vitamin deficiency. Approximately 20% of patients have congenital splenic, gastrointestinal, genitourinary, cardiac, and venous malformations.4,5 Untreated, biliary atresia progresses to end-stage liver disease and death within 2 years.
The first-line treatment for biliary atresia is to establish biliary outflow with the Kasai procedure (hepatic portoenterostomy), in which a jejunal limb is anastomosed in a Roux-en-Y with the liver. The outcomes of the Kasai procedure depend on the timing of surgery, so timely diagnosis of biliary atresia is crucial. When the Kasai procedure is performed within 60 days of birth, biliary flow is achieved in up to 70% of patients; but if performed after 90 days, biliary flow is achieved in fewer than 25%.6
Long-term outcomes of biliary atresia in patients with their native liver have been reported in a few studies.
In a French study,7 743 patients with biliary atresia underwent the Kasai procedure at a median age of 60 days. Survival rates were 57.1% at 2 years, 37.9% at 5 years, 32.4% at 10 years, and 28.5% at 15 years. In other studies,4–9 the 20-year transplant-free survival rate ranged from 23% to 46%. Therefore, at least one-third of children with biliary atresia survive to adulthood with their native liver.
Implications of biliary atresia in adulthood
Although the Kasai procedure improves biliary outflow, up to 70% of patients develop complications of biliary atresia such as progressive fibrosis, cirrhosis, portal hypertension, cholangitis, and hepatocellular carcinoma, even after a successful Kasai procedure.10
Portal hypertension with evidence of splenomegaly, thrombocytopenia, or ascites is found in two-thirds of long-term survivors of biliary atresia with a native liver, with variceal hemorrhage occurring in 30%.11 Therefore, patients with biliary atresia who have evidence of portal hypertension should be screened for varices with upper endoscopy on an annual basis. Management of variceal hemorrhage in these patients includes the use of octreotide, antibiotics, variceal ligation, and sclerotherapy; primary prophylaxis can be achieved with beta-blockers and endoscopic variceal ligation.12
Cholangitis is frequent, occurring in 40% to 60% of biliary atresia patients after the Kasai procedure, and about one-fourth of these patients have multiple episodes.13 The number of episodes of cholangitis negatively affects transplant-free survival.14 Patients with cholangitis should be adequately treated with oral or intravenous antibiotics depending on the severity of presentation. The role of prophylaxis with antibiotics remains unclear.15
Pulmonary complications such as hepatopulmonary syndrome and portopulmonary hypertension can also occur in biliary atresia patients with a native liver. It is important for physicians to be aware of these complications and to screen for them, for example, with agitated saline echocardiography for hepatopulmonary syndrome and with echocardiography for portopulmonary hypertension. Timely screening is crucial, as the outcome of liver transplant depends on the severity at the time of transplant in these conditions, especially portopulmonary hypertension.
Hepatocellular carcinoma has been rarely reported in children with biliary atresia,16 so well-defined guidelines for screening in young adults with biliary atresia are lacking. Most centers recommend screening with ultrasonography of the abdomen and alpha-fetoprotein measurement every 6 months or annually starting soon after the Kasai procedure, since hepatocellular carcinoma has been reported in children as young as age 2.16
Transplant. Adult hepatologists are faced with the challenging task of deciding when it is time for transplant, balancing the long-term complications of biliary atresia with the risk of long-term immunosuppression after transplant. In addition, young adults with these complications may have preserved synthetic function, resulting in low Model for End-Stage Liver Disease (MELD) scores, which may complicate the process of listing for transplant.
Neurocognitive deficits are reported in children with biliary atresia,17 but young adults with biliary atresia generally have reasonable cognitive function and prospects for education and employment.
Pregnancy with successful outcomes has been reported.8
ALAGILLE SYNDROME
Alagille syndrome is an autosomal-dominant multisystemic disease caused by mutations in the JAG1 gene (accounting for > 95% of cases) and the NOTCH2 gene, with highly variable expression.18
Extrahepatic manifestations include butterfly vertebral defects, facial dysmorphism (eg, deep-set and low-set eyes, with characteristic “triangular” facies), posterior embryotoxon (a congenital defect of the eye characterized by an opaque ring around the margin of the cornea), peripheral pulmonary stenosis, renal abnormalities, and vascular malformations.
Hepatic manifestations vary from asymptomatic laboratory abnormalities to progressive cholestasis starting in early infancy with intractable pruritus, xanthomas, failure to thrive, and end-stage liver disease requiring liver transplant in childhood in 15% to 20% of patients.19
Implications of Alagille syndrome in adulthood
Transplant. Interestingly, the phenotype of hepatic disease is already established in childhood, with minimal or no progression in adulthood. Most children with minimal liver disease experience spontaneous resolution, whereas those with significant cholestasis might ultimately develop progressive liver fibrosis or cirrhosis requiring liver transplant in childhood. Only a small subset of children with minimal cholestasis progress to end-stage liver disease in late childhood or early adulthood.20 Therefore, liver transplant for progressive liver disease from significant cholestasis almost always occurs in childhood, usually between ages 1 and 4.21
In a retrospective study comparing posttransplant outcomes in children with Alagille syndrome and biliary atresia, 1-year patient survival was excellent overall in children with Alagille syndrome, although slightly lower than in children with biliary atresia, most likely owing to extrahepatic morbidities of Alagille syndrome and especially the use of immunosuppression in those with renal disease.21 Similarly, 1- and 5-year patient and graft survival outcomes of liver transplant in adults with Alagille syndrome were also excellent compared with those who received a liver transplant in childhood for Alagille syndrome or in adulthood for biliary atresia.22
Hepatocellular carcinoma has occurred in these patients in the absence of cirrhosis, which makes implementation of prognostic and surveillance strategies almost impossible to design for them. Annual ultrasonography with alpha-fetoprotein testing might be applicable in Alagille syndrome patients. However, deciding which patients should undergo this testing and when it should start will be challenging, given the paucity of data.
Cardiovascular disease. Cardiac phenotype is also mostly established in childhood, with the pulmonary vasculature being most commonly involved.19 In contrast, renal and other vascular abnormalities can manifest in adulthood. Renal manifestations vary and include structural anomalies such as hyperechoic kidneys or renal cysts, which can manifest in childhood, and some abnormalities such as hypertension and renal artery stenosis that can manifest in adulthood.23,24
Vasculopathy is reported to involve the intracranial, renal, and intra-abdominal blood vessels.25 Neurovascular accidents such as stroke and intracranial hemorrhage can occur at any age, with significant rates of morbidity and death.26 Therefore, some experts recommend magnetic resonance angiography every 5 years and before any major intervention to prevent these devastating complications.20
Pregnancy. Successful pregnancies have been reported. Preexisting cardiac and hepatic disease can complicate pregnancy depending on the severity of the disease. Because of the autosomal-dominant pattern of inheritance, infants have a 50% risk of the disease, so genetic counseling should be seriously considered before conception.27 Prenatal diagnosis is possible, but the lack of genotype-phenotype correlation precludes its use in clinical practice.
PROGRESSIVE FAMILIAL INTRAHEPATIC CHOLESTASIS
Progressive familial intrahepatic cholestasis (PFIC) is a heterogeneous group of autosomal-recessive conditions associated with disruption of bile formation causing cholestatic liver disease in infants and young children. Three types have been described, depending on the genetic mutation in the hepatobiliary transport pathway:
- PFIC 1 (Byler disease) is caused by impaired bile salt secretion due to mutations in the ATP8B1 gene encoding for the familial intrahepatic cholestasis 1 (FIC 1) protein
- PFIC 2 is caused by impaired bile salt secretion due to mutations in the ABCB11 gene encoding for the bile salt export pump (BSEP) protein
- PFIC 3 is caused by impaired biliary phospholipid secretion due to a defect in ABCB4 encoding for multidrug resistance 3 (MDR3) protein.28
PFIC 1 and 2 manifest with low gamma-glutamyl transferase (GGT) cholestasis, whereas PFIC 3 presents with high GGT cholestasis.
PFIC 1 and PFIC 2 usually cause cholestasis in early infancy, but PFIC 3 can cause cholestasis in late infancy, childhood, and even adulthood.
Because ATP8B1 is expressed in other tissues, PFIC 1 is characterized by extrahepatic manifestations such as sensorineural hearing loss, growth failure, severe diarrhea, and pancreatic insufficiency.
Implications of PFIC in adulthood
PFIC 1 and 2 (low-GGT cholestasis) are usually progressive and often lead to end-stage liver disease and cirrhosis before adulthood. Therefore, almost all patients with PFIC 1 and 2 undergo liver transplant or at least a biliary diversion procedure before reaching adulthood. Intractable pruritus is one of the most challenging clinical manifestations in patients with PFIC.
First-line management is pharmacologic and includes ursodeoxycholic acid, antihistamines (eg, hydroxyzine), bile acid sequestrants (eg, cholestyramine, colestipol), naltrexone, and rifampin, but these have limited efficacy.10
Most patients, especially those with PFIC 1 and 2, undergo a biliary diversion procedure such as partial external biliary diversion (cholecystojejunocutaneostomy), ileal exclusion, or partial internal biliary diversion (cholecystojejunocolic anastomosis) to decrease enterohepatic circulation of bile salts. The efficacy of these procedures is very limited in patients with established cirrhosis. Excessive losses of bile can occur through the biliary stoma, leading to dehydration in patients with external biliary diversion. In patients who are not candidates for biliary diversion, endoscopic nasobiliary drainage of pancreatobiliary secretions could be achieved by placing a catheter in the common bile duct; this has been reported to be effective in relieving cholestasis in a few cases.29
Liver transplant is needed in patients with progressive liver disease and intractable pruritus despite medical management and biliary diversion. Unlike in biliary atresia, liver transplant is not curative in PFIC 1, due to extrahepatic manifestations: patients with PFIC 1 can still have intractable diarrhea and pancreatitis after liver transplant. More importantly, allograft steatohepatitis with further progression to cirrhosis can occur after liver transplant in patients with PFIC 1. Interestingly, biliary diversion has been reported to improve graft steatosis and diarrhea after liver transplant.30
Although graft survival after transplant is good in patients with PFIC 2, recurrence of low-GGT cholestasis has been reported and is believed to be due to the formation of anti-bile salt export pump (anti-BSEP) antibodies by the host immune system in response to exposure to new proteins from the transplant graft.31
Cancer. The risk of malignancy, especially hepatocellular carcinoma, is also increased in PFIC 2, affecting nearly 15% of patients. Therefore, standard hepatocellular carcinoma surveillance with ultrasonography or alpha-fetoprotein testing or both is recommended in patients with PFIC 2. Cholangiocarcinoma and pancreatic adenocarcinoma have also been reported in patients with PFIC 2.20
Incomplete penetrance of mutations in ATP8B1 and ABCB11 can cause recurrent episodes of cholestasis and pruritus with asymptomatic periods between episodes, referred to as benign recurrent intrahepatic cholestasis. Prognosis is usually good, with no progression to cirrhosis.32
Pregnancy. In contrast to FIC 1 and BSEP deficiency, MDR3 defects lead to a wide phenotypic spectrum depending on the type of mutation. Heterozygous mutation is associated with increased risk of development of cholestasis during pregnancy, which typically presents with generalized pruritus in the third trimester and is associated with adverse fetal outcomes. Intrahepatic cholestasis of pregnancy is usually treated with ursodeoxycholic acid, with reported improvement in pruritus, liver function, and pregnancy outcomes.33
In adults, drug-induced liver injury and idiopathic cirrhosis have also been described with MDR3 defects. Intrahepatic lithiasis and cholesterol gallstones can also occur with MDR3 defects as a result of impaired secretion of biliary phospholipid.32 Despite intrahepatic cholestasis of pregnancy, successful outcomes have been reported in women with PFIC.20
OTHER CHILDHOOD-ONSET INHERITED CHOLESTATIC DISEASES
Cystic fibrosis-associated liver disease
Nearly 40% of patients with cystic fibrosis develop liver disease.34 Cystic fibrosis-associated liver disease encompasses a broad clinical spectrum including asymptomatic elevation of aminotransferases, neonatal cholestasis, hepatic steatosis, focal biliary cirrhosis, and multilobar cirrhosis. Cirrhosis and portal hypertension can occur in 5% to 10% of patients and is the third-leading cause of death in patients with cystic fibrosis.35
Risk factors for cystic fibrosis-associated liver disease include male sex, meconium ileus, and severe CFTR gene mutation (class I–III) with pancreatic insufficiency. Cystic fibrosis-related cirrhosis is more frequent in children and adolescents, whereas noncirrhotic portal hypertension and intrahepatic cholangiopathies are more common in adults.36
Limited available studies support treatment with ursodeoxycholic acid in patients with cholestasis to delay the progression of liver disease, but the impact of this drug on long-term outcome is unknown.29
Most patients remain in compensated cirrhosis for many years before progressing to decompensated cirrhosis requiring liver transplant. Other indications for liver transplant include recurrent intractable variceal bleeding, hepatopulmonary syndrome, and portopulmonary hypertension. Combined liver and lung transplant may be considered in patients with advanced liver and lung disease. Outcomes after isolated liver or liver-lung transplant in cystic fibrosis patients have been comparable to those in patients with other liver diseases.37
Defects in bile acid synthesis
Inherited defects of enzymes required for the synthesis of primary bile acids from cholesterol can cause cholestasis from impaired bile flow and production of hepatotoxic aberrant bile acids. The clinical presentation varies depending on the enzymatic defect and can range from liver disease of varying severity to neurologic manifestations. Idiopathic late-onset cholestasis and cirrhosis of unknown etiology have been reported in adults with bile acid synthesis defects.38,39 Therefore, this diagnosis should be considered in cases of cryptogenic cirrhosis and other cholestatic features.
Treatment with primary bile acids (cholic acid) has been effective in most patients with defective bile acid synthesis.
Primary sclerosing cholangitis
Primary sclerosing cholangitis is characterized by progressive obliteration of intrahepatic and extrahepatic bile ducts and is most commonly seen in patients with inflammatory bowel disease. Sclerosing cholangitis can also be secondary to other diseases in children such as immunodeficiency syndromes, Langerhans cell histiocytosis, cystic fibrosis, or sickle cell anemia.40 Neonatal sclerosing cholangitis is a rare autosomal-recessive disease characterized by a severe form of cholangiopathy in neonates and young infants requiring transplant. It can be associated with Kabuki syndrome and neonatal ichthyosis-sclerosing cholangitis syndrome.
Treatment options are limited. Ursodeoxycholic acid and oral vancomycin have variable efficacy. Liver transplant is needed in patients with decompensated cirrhosis. Patients with primary sclerosing cholangitis, especially adults, are at higher risk of developing cholangiocarcinoma, and therefore screening with ultrasonography or magnetic resonance imaging every 6 to 12 months is recommended.
The risk of preterm and cesarean deliveries may be elevated in women with primary sclerosing cholangitis, though data are limited.33
PEDIATRIC LIVER TRANSPLANT RECIPIENTS WHO SURVIVE INTO ADULTHOOD
Adolescent rebellion poses risks
Outcomes of liver transplant in children and adolescents have improved tremendously in the past 2 decades with advances in surgical techniques, pre- and postoperative management, organ preservation, and immunosuppression. Now, most pediatric liver transplant recipients survive into adulthood, creating a unique challenge for internists and adult care hepatologists.41
In rebellious adolescents and young adults, risk-taking behavior, nonadherence to immunosuppressive medications, alcohol intake, and substance abuse increase the risk of graft rejection and loss. Current immunosuppressive drugs such as calcineurin inhibitors (tacrolimus, cyclosporine), mycophenolate mofetil, sirolimus, and corticosteroids have drastically decreased rejection rates in compliant patients.41 Educating patients on the importance of taking their medications and avoiding alcohol and drug abuse is especially important for adolescents and young adults, as rates of nonadherence are high in these age groups.
Although pregnancy is usually successful after liver transplant, it should be considered high-risk due to reported complications such as graft rejection, diabetes, preeclampsia, sepsis, prematurity, and low birth weight. Conception should be avoided for at least 1 year after transplant.42 Appropriate counseling with regard to pregnancy and contraception is important.
There is no consensus on breastfeeding, but it is considered safe in women on low-dose calcineurin inhibitors.43
Life is better with a new liver, but patients have special needs
Liver transplant is life-saving and improves quality of life. However, long-term pediatric liver transplant recipients face challenges such as strict adherence to medications and follow-up visits, avoiding exposure to infections, and fear of graft rejection.
Chronic liver disease in children leads to failure to thrive, growth failure, and even delayed puberty, which resolve in most patients after liver transplant before adulthood in the absence of other comorbidities.44 However, these patients are reported to have lower psychosocial functioning and more psychiatric disorders such as anxiety or posttraumatic disorder.41,44
Therefore, a psychologist or other mental health professional should be part of the management team from the time of pretransplant assessment to identify mental health problems and the need for adjustments before liver transplant. Ongoing psychosocial assessment after liver transplant is equally important to identify risks such as drug or alcohol abuse, depression, posttraumatic stress disorder, and medication nonadherence, all of which can negatively affect posttransplant outcome.45
In addition, assessment of family functioning and structure is important for good long-term outcomes posttransplant; therefore, a social worker should also be a part of the transplant team. Psyschosocial assessment tools can identify high-risk candidates who would benefit from earlier intervention to avoid any negative impact posttransplant.
Neurocognitive development can be delayed in children with chronic liver disease, and the delay may persist even after liver transplant, with reported impairments in intellectual ability, language, verbal, and visuospatial functioning skills.41 In spite of this, a recent study found that more than half the study patients were employed at a median follow-up of 24 years from liver transplant and a median age of 27.46
Remarkably, pediatric liver transplant recipients have reported quality of life comparable to that in the general population,47 and even better than in patients with other chronic illnesses.48
Long-term medical comorbidities in pediatric liver transplant recipients
Favorable outcomes such as long-term survival and good quality of life in pediatric liver transplant recipients are lessened by late complications such as portal vein thrombosis or biliary strictures needing interventions, chronic graft rejection, adverse effects of immunosuppression, and recurrence of the disease.
Split-liver transplant—splitting a deceased-donor allograft to provide grafts for 2 recipients—has revolutionized liver transplant by increasing the donor pool and thereby decreasing waitlist mortality rates, especially in pediatric candidates. Despite this advantage, split-liver transplant is technically challenging and associated with increased perioperative complications compared with whole-liver transplant, especially in adult recipients. Recently, experienced centers have reported favorable outcomes with split-liver transplant comparable to those with whole-liver transplant; therefore, split-liver transplant should be considered after careful evaluation of donor organ and recipient clinical status.49
Old age in the recipient can also adversely affect liver transplant outcomes.50
Interestingly, even in patients whose clinical course is unremarkable and biochemical values are normal, graft hepatitis or fibrosis of unknown cause with progression to cirrhosis has been described in the decade after transplant.41
Chronic rejection with eventual graft loss may be related to nonadherence in adolescents and can be reduced with use of an additional immunosuppressant such as sirolimus or mycophenolate. Chronic kidney disease can occur in about one-third of liver transplant recipients secondary to renal disease associated with primary disease (like Alagille syndrome), hepatorenal syndrome, and most importantly, use of calcineurin inhibitors.45
Components of the metabolic syndrome such as type 2 diabetes, obesity, nonalcoholic fatty liver disease, hypertension, and dyslipidemia are also seen in long-term pediatric liver transplant survivors. Internists are advised to screen for these comorbidities so that interventions can be applied early to improve long-term health outcomes and graft survival.
Of importance, multiple studies have shown a 2-fold increase in the rates of de novo malignancy in liver transplant recipients, including solid-organ and lymphoproliferative cancers, probably due to long-term immunosuppression. Posttransplant lymphoproliferative disorder occurs at lower rates than with other solid-organ transplants; its incidence is greatest in pediatric patients and in the first 12 to 18 months after transplant.51
TRANSITION TO ADULT CARE
While the number of patients with childhood-onset liver disease and pediatric liver transplant recipients who survive into adulthood is increasing, there are no established guidelines or formal models for transitioning these patients into adult care. Consequently, studies on transitional process have examined various issues such as patient and parent frustration, poor medical knowledge among patients during transition, lack of parental facilitation, and inadequate knowledge on disease process among adult-care hepatologists.52–54
A prolonged period of transition up to age 25 is preferred in complicated cases. Distinctive consideration for transition should include those with neurocognitive developmental delay from underlying disease or hepatic encephalopathy before transplant. These patients need additional support and time to achieve independence in health management before transition.57 Validated questionnaires are available to assess readiness to transition into adult care,58 implying that the decision to transition should not be based solely on age.
Thanks to advances in medical science and our understanding of inherited and acquired liver disease, many more children with acquired or congenital liver disease survive into adulthood than they did 2 decades ago. Improvements in immunosuppression and surgery have increased the chances of pediatric liver transplant recipients reaching adulthood, with a survival rate of 75% at 15 to 20 years.1
With the growing number of adult patients with pediatric-onset liver disease, internists and adult hepatologists need to be aware of these liver diseases and develop expertise to manage this challenging group of patients. Moreover, young adults with pediatric-onset chronic liver disease pose distinct challenges such as pregnancy, adherence to medical regimens, and psychosocial changes in life.
These patients need a “transition of care” rather than a “transfer of care.” Transition of care is a multifaceted process that takes the medical, educational, and psychosocial needs of the patient into consideration before switching their care to adult care physicians, whereas transfer of care is simply an administrative process of change to adult care without previous knowledge of the patients.2
BILIARY ATRESIA
Biliary atresia is a progressive inflammatory fibrosclerosing cholangiopathy of unknown cause. Its prevalence varies with geographic location, ranging from 1 in 6,000 to 1 in 19,000, with the highest prevalence in Taiwan.3
Biliary atresia usually presents within the first few weeks of life, with progressive cholestasis leading to failure to thrive and to fat-soluble vitamin deficiency. Approximately 20% of patients have congenital splenic, gastrointestinal, genitourinary, cardiac, and venous malformations.4,5 Untreated, biliary atresia progresses to end-stage liver disease and death within 2 years.
The first-line treatment for biliary atresia is to establish biliary outflow with the Kasai procedure (hepatic portoenterostomy), in which a jejunal limb is anastomosed in a Roux-en-Y with the liver. The outcomes of the Kasai procedure depend on the timing of surgery, so timely diagnosis of biliary atresia is crucial. When the Kasai procedure is performed within 60 days of birth, biliary flow is achieved in up to 70% of patients; but if performed after 90 days, biliary flow is achieved in fewer than 25%.6
Long-term outcomes of biliary atresia in patients with their native liver have been reported in a few studies.
In a French study,7 743 patients with biliary atresia underwent the Kasai procedure at a median age of 60 days. Survival rates were 57.1% at 2 years, 37.9% at 5 years, 32.4% at 10 years, and 28.5% at 15 years. In other studies,4–9 the 20-year transplant-free survival rate ranged from 23% to 46%. Therefore, at least one-third of children with biliary atresia survive to adulthood with their native liver.
Implications of biliary atresia in adulthood
Although the Kasai procedure improves biliary outflow, up to 70% of patients develop complications of biliary atresia such as progressive fibrosis, cirrhosis, portal hypertension, cholangitis, and hepatocellular carcinoma, even after a successful Kasai procedure.10
Portal hypertension with evidence of splenomegaly, thrombocytopenia, or ascites is found in two-thirds of long-term survivors of biliary atresia with a native liver, with variceal hemorrhage occurring in 30%.11 Therefore, patients with biliary atresia who have evidence of portal hypertension should be screened for varices with upper endoscopy on an annual basis. Management of variceal hemorrhage in these patients includes the use of octreotide, antibiotics, variceal ligation, and sclerotherapy; primary prophylaxis can be achieved with beta-blockers and endoscopic variceal ligation.12
Cholangitis is frequent, occurring in 40% to 60% of biliary atresia patients after the Kasai procedure, and about one-fourth of these patients have multiple episodes.13 The number of episodes of cholangitis negatively affects transplant-free survival.14 Patients with cholangitis should be adequately treated with oral or intravenous antibiotics depending on the severity of presentation. The role of prophylaxis with antibiotics remains unclear.15
Pulmonary complications such as hepatopulmonary syndrome and portopulmonary hypertension can also occur in biliary atresia patients with a native liver. It is important for physicians to be aware of these complications and to screen for them, for example, with agitated saline echocardiography for hepatopulmonary syndrome and with echocardiography for portopulmonary hypertension. Timely screening is crucial, as the outcome of liver transplant depends on the severity at the time of transplant in these conditions, especially portopulmonary hypertension.
Hepatocellular carcinoma has been rarely reported in children with biliary atresia,16 so well-defined guidelines for screening in young adults with biliary atresia are lacking. Most centers recommend screening with ultrasonography of the abdomen and alpha-fetoprotein measurement every 6 months or annually starting soon after the Kasai procedure, since hepatocellular carcinoma has been reported in children as young as age 2.16
Transplant. Adult hepatologists are faced with the challenging task of deciding when it is time for transplant, balancing the long-term complications of biliary atresia with the risk of long-term immunosuppression after transplant. In addition, young adults with these complications may have preserved synthetic function, resulting in low Model for End-Stage Liver Disease (MELD) scores, which may complicate the process of listing for transplant.
Neurocognitive deficits are reported in children with biliary atresia,17 but young adults with biliary atresia generally have reasonable cognitive function and prospects for education and employment.
Pregnancy with successful outcomes has been reported.8
ALAGILLE SYNDROME
Alagille syndrome is an autosomal-dominant multisystemic disease caused by mutations in the JAG1 gene (accounting for > 95% of cases) and the NOTCH2 gene, with highly variable expression.18
Extrahepatic manifestations include butterfly vertebral defects, facial dysmorphism (eg, deep-set and low-set eyes, with characteristic “triangular” facies), posterior embryotoxon (a congenital defect of the eye characterized by an opaque ring around the margin of the cornea), peripheral pulmonary stenosis, renal abnormalities, and vascular malformations.
Hepatic manifestations vary from asymptomatic laboratory abnormalities to progressive cholestasis starting in early infancy with intractable pruritus, xanthomas, failure to thrive, and end-stage liver disease requiring liver transplant in childhood in 15% to 20% of patients.19
Implications of Alagille syndrome in adulthood
Transplant. Interestingly, the phenotype of hepatic disease is already established in childhood, with minimal or no progression in adulthood. Most children with minimal liver disease experience spontaneous resolution, whereas those with significant cholestasis might ultimately develop progressive liver fibrosis or cirrhosis requiring liver transplant in childhood. Only a small subset of children with minimal cholestasis progress to end-stage liver disease in late childhood or early adulthood.20 Therefore, liver transplant for progressive liver disease from significant cholestasis almost always occurs in childhood, usually between ages 1 and 4.21
In a retrospective study comparing posttransplant outcomes in children with Alagille syndrome and biliary atresia, 1-year patient survival was excellent overall in children with Alagille syndrome, although slightly lower than in children with biliary atresia, most likely owing to extrahepatic morbidities of Alagille syndrome and especially the use of immunosuppression in those with renal disease.21 Similarly, 1- and 5-year patient and graft survival outcomes of liver transplant in adults with Alagille syndrome were also excellent compared with those who received a liver transplant in childhood for Alagille syndrome or in adulthood for biliary atresia.22
Hepatocellular carcinoma has occurred in these patients in the absence of cirrhosis, which makes implementation of prognostic and surveillance strategies almost impossible to design for them. Annual ultrasonography with alpha-fetoprotein testing might be applicable in Alagille syndrome patients. However, deciding which patients should undergo this testing and when it should start will be challenging, given the paucity of data.
Cardiovascular disease. Cardiac phenotype is also mostly established in childhood, with the pulmonary vasculature being most commonly involved.19 In contrast, renal and other vascular abnormalities can manifest in adulthood. Renal manifestations vary and include structural anomalies such as hyperechoic kidneys or renal cysts, which can manifest in childhood, and some abnormalities such as hypertension and renal artery stenosis that can manifest in adulthood.23,24
Vasculopathy is reported to involve the intracranial, renal, and intra-abdominal blood vessels.25 Neurovascular accidents such as stroke and intracranial hemorrhage can occur at any age, with significant rates of morbidity and death.26 Therefore, some experts recommend magnetic resonance angiography every 5 years and before any major intervention to prevent these devastating complications.20
Pregnancy. Successful pregnancies have been reported. Preexisting cardiac and hepatic disease can complicate pregnancy depending on the severity of the disease. Because of the autosomal-dominant pattern of inheritance, infants have a 50% risk of the disease, so genetic counseling should be seriously considered before conception.27 Prenatal diagnosis is possible, but the lack of genotype-phenotype correlation precludes its use in clinical practice.
PROGRESSIVE FAMILIAL INTRAHEPATIC CHOLESTASIS
Progressive familial intrahepatic cholestasis (PFIC) is a heterogeneous group of autosomal-recessive conditions associated with disruption of bile formation causing cholestatic liver disease in infants and young children. Three types have been described, depending on the genetic mutation in the hepatobiliary transport pathway:
- PFIC 1 (Byler disease) is caused by impaired bile salt secretion due to mutations in the ATP8B1 gene encoding for the familial intrahepatic cholestasis 1 (FIC 1) protein
- PFIC 2 is caused by impaired bile salt secretion due to mutations in the ABCB11 gene encoding for the bile salt export pump (BSEP) protein
- PFIC 3 is caused by impaired biliary phospholipid secretion due to a defect in ABCB4 encoding for multidrug resistance 3 (MDR3) protein.28
PFIC 1 and 2 manifest with low gamma-glutamyl transferase (GGT) cholestasis, whereas PFIC 3 presents with high GGT cholestasis.
PFIC 1 and PFIC 2 usually cause cholestasis in early infancy, but PFIC 3 can cause cholestasis in late infancy, childhood, and even adulthood.
Because ATP8B1 is expressed in other tissues, PFIC 1 is characterized by extrahepatic manifestations such as sensorineural hearing loss, growth failure, severe diarrhea, and pancreatic insufficiency.
Implications of PFIC in adulthood
PFIC 1 and 2 (low-GGT cholestasis) are usually progressive and often lead to end-stage liver disease and cirrhosis before adulthood. Therefore, almost all patients with PFIC 1 and 2 undergo liver transplant or at least a biliary diversion procedure before reaching adulthood. Intractable pruritus is one of the most challenging clinical manifestations in patients with PFIC.
First-line management is pharmacologic and includes ursodeoxycholic acid, antihistamines (eg, hydroxyzine), bile acid sequestrants (eg, cholestyramine, colestipol), naltrexone, and rifampin, but these have limited efficacy.10
Most patients, especially those with PFIC 1 and 2, undergo a biliary diversion procedure such as partial external biliary diversion (cholecystojejunocutaneostomy), ileal exclusion, or partial internal biliary diversion (cholecystojejunocolic anastomosis) to decrease enterohepatic circulation of bile salts. The efficacy of these procedures is very limited in patients with established cirrhosis. Excessive losses of bile can occur through the biliary stoma, leading to dehydration in patients with external biliary diversion. In patients who are not candidates for biliary diversion, endoscopic nasobiliary drainage of pancreatobiliary secretions could be achieved by placing a catheter in the common bile duct; this has been reported to be effective in relieving cholestasis in a few cases.29
Liver transplant is needed in patients with progressive liver disease and intractable pruritus despite medical management and biliary diversion. Unlike in biliary atresia, liver transplant is not curative in PFIC 1, due to extrahepatic manifestations: patients with PFIC 1 can still have intractable diarrhea and pancreatitis after liver transplant. More importantly, allograft steatohepatitis with further progression to cirrhosis can occur after liver transplant in patients with PFIC 1. Interestingly, biliary diversion has been reported to improve graft steatosis and diarrhea after liver transplant.30
Although graft survival after transplant is good in patients with PFIC 2, recurrence of low-GGT cholestasis has been reported and is believed to be due to the formation of anti-bile salt export pump (anti-BSEP) antibodies by the host immune system in response to exposure to new proteins from the transplant graft.31
Cancer. The risk of malignancy, especially hepatocellular carcinoma, is also increased in PFIC 2, affecting nearly 15% of patients. Therefore, standard hepatocellular carcinoma surveillance with ultrasonography or alpha-fetoprotein testing or both is recommended in patients with PFIC 2. Cholangiocarcinoma and pancreatic adenocarcinoma have also been reported in patients with PFIC 2.20
Incomplete penetrance of mutations in ATP8B1 and ABCB11 can cause recurrent episodes of cholestasis and pruritus with asymptomatic periods between episodes, referred to as benign recurrent intrahepatic cholestasis. Prognosis is usually good, with no progression to cirrhosis.32
Pregnancy. In contrast to FIC 1 and BSEP deficiency, MDR3 defects lead to a wide phenotypic spectrum depending on the type of mutation. Heterozygous mutation is associated with increased risk of development of cholestasis during pregnancy, which typically presents with generalized pruritus in the third trimester and is associated with adverse fetal outcomes. Intrahepatic cholestasis of pregnancy is usually treated with ursodeoxycholic acid, with reported improvement in pruritus, liver function, and pregnancy outcomes.33
In adults, drug-induced liver injury and idiopathic cirrhosis have also been described with MDR3 defects. Intrahepatic lithiasis and cholesterol gallstones can also occur with MDR3 defects as a result of impaired secretion of biliary phospholipid.32 Despite intrahepatic cholestasis of pregnancy, successful outcomes have been reported in women with PFIC.20
OTHER CHILDHOOD-ONSET INHERITED CHOLESTATIC DISEASES
Cystic fibrosis-associated liver disease
Nearly 40% of patients with cystic fibrosis develop liver disease.34 Cystic fibrosis-associated liver disease encompasses a broad clinical spectrum including asymptomatic elevation of aminotransferases, neonatal cholestasis, hepatic steatosis, focal biliary cirrhosis, and multilobar cirrhosis. Cirrhosis and portal hypertension can occur in 5% to 10% of patients and is the third-leading cause of death in patients with cystic fibrosis.35
Risk factors for cystic fibrosis-associated liver disease include male sex, meconium ileus, and severe CFTR gene mutation (class I–III) with pancreatic insufficiency. Cystic fibrosis-related cirrhosis is more frequent in children and adolescents, whereas noncirrhotic portal hypertension and intrahepatic cholangiopathies are more common in adults.36
Limited available studies support treatment with ursodeoxycholic acid in patients with cholestasis to delay the progression of liver disease, but the impact of this drug on long-term outcome is unknown.29
Most patients remain in compensated cirrhosis for many years before progressing to decompensated cirrhosis requiring liver transplant. Other indications for liver transplant include recurrent intractable variceal bleeding, hepatopulmonary syndrome, and portopulmonary hypertension. Combined liver and lung transplant may be considered in patients with advanced liver and lung disease. Outcomes after isolated liver or liver-lung transplant in cystic fibrosis patients have been comparable to those in patients with other liver diseases.37
Defects in bile acid synthesis
Inherited defects of enzymes required for the synthesis of primary bile acids from cholesterol can cause cholestasis from impaired bile flow and production of hepatotoxic aberrant bile acids. The clinical presentation varies depending on the enzymatic defect and can range from liver disease of varying severity to neurologic manifestations. Idiopathic late-onset cholestasis and cirrhosis of unknown etiology have been reported in adults with bile acid synthesis defects.38,39 Therefore, this diagnosis should be considered in cases of cryptogenic cirrhosis and other cholestatic features.
Treatment with primary bile acids (cholic acid) has been effective in most patients with defective bile acid synthesis.
Primary sclerosing cholangitis
Primary sclerosing cholangitis is characterized by progressive obliteration of intrahepatic and extrahepatic bile ducts and is most commonly seen in patients with inflammatory bowel disease. Sclerosing cholangitis can also be secondary to other diseases in children such as immunodeficiency syndromes, Langerhans cell histiocytosis, cystic fibrosis, or sickle cell anemia.40 Neonatal sclerosing cholangitis is a rare autosomal-recessive disease characterized by a severe form of cholangiopathy in neonates and young infants requiring transplant. It can be associated with Kabuki syndrome and neonatal ichthyosis-sclerosing cholangitis syndrome.
Treatment options are limited. Ursodeoxycholic acid and oral vancomycin have variable efficacy. Liver transplant is needed in patients with decompensated cirrhosis. Patients with primary sclerosing cholangitis, especially adults, are at higher risk of developing cholangiocarcinoma, and therefore screening with ultrasonography or magnetic resonance imaging every 6 to 12 months is recommended.
The risk of preterm and cesarean deliveries may be elevated in women with primary sclerosing cholangitis, though data are limited.33
PEDIATRIC LIVER TRANSPLANT RECIPIENTS WHO SURVIVE INTO ADULTHOOD
Adolescent rebellion poses risks
Outcomes of liver transplant in children and adolescents have improved tremendously in the past 2 decades with advances in surgical techniques, pre- and postoperative management, organ preservation, and immunosuppression. Now, most pediatric liver transplant recipients survive into adulthood, creating a unique challenge for internists and adult care hepatologists.41
In rebellious adolescents and young adults, risk-taking behavior, nonadherence to immunosuppressive medications, alcohol intake, and substance abuse increase the risk of graft rejection and loss. Current immunosuppressive drugs such as calcineurin inhibitors (tacrolimus, cyclosporine), mycophenolate mofetil, sirolimus, and corticosteroids have drastically decreased rejection rates in compliant patients.41 Educating patients on the importance of taking their medications and avoiding alcohol and drug abuse is especially important for adolescents and young adults, as rates of nonadherence are high in these age groups.
Although pregnancy is usually successful after liver transplant, it should be considered high-risk due to reported complications such as graft rejection, diabetes, preeclampsia, sepsis, prematurity, and low birth weight. Conception should be avoided for at least 1 year after transplant.42 Appropriate counseling with regard to pregnancy and contraception is important.
There is no consensus on breastfeeding, but it is considered safe in women on low-dose calcineurin inhibitors.43
Life is better with a new liver, but patients have special needs
Liver transplant is life-saving and improves quality of life. However, long-term pediatric liver transplant recipients face challenges such as strict adherence to medications and follow-up visits, avoiding exposure to infections, and fear of graft rejection.
Chronic liver disease in children leads to failure to thrive, growth failure, and even delayed puberty, which resolve in most patients after liver transplant before adulthood in the absence of other comorbidities.44 However, these patients are reported to have lower psychosocial functioning and more psychiatric disorders such as anxiety or posttraumatic disorder.41,44
Therefore, a psychologist or other mental health professional should be part of the management team from the time of pretransplant assessment to identify mental health problems and the need for adjustments before liver transplant. Ongoing psychosocial assessment after liver transplant is equally important to identify risks such as drug or alcohol abuse, depression, posttraumatic stress disorder, and medication nonadherence, all of which can negatively affect posttransplant outcome.45
In addition, assessment of family functioning and structure is important for good long-term outcomes posttransplant; therefore, a social worker should also be a part of the transplant team. Psyschosocial assessment tools can identify high-risk candidates who would benefit from earlier intervention to avoid any negative impact posttransplant.
Neurocognitive development can be delayed in children with chronic liver disease, and the delay may persist even after liver transplant, with reported impairments in intellectual ability, language, verbal, and visuospatial functioning skills.41 In spite of this, a recent study found that more than half the study patients were employed at a median follow-up of 24 years from liver transplant and a median age of 27.46
Remarkably, pediatric liver transplant recipients have reported quality of life comparable to that in the general population,47 and even better than in patients with other chronic illnesses.48
Long-term medical comorbidities in pediatric liver transplant recipients
Favorable outcomes such as long-term survival and good quality of life in pediatric liver transplant recipients are lessened by late complications such as portal vein thrombosis or biliary strictures needing interventions, chronic graft rejection, adverse effects of immunosuppression, and recurrence of the disease.
Split-liver transplant—splitting a deceased-donor allograft to provide grafts for 2 recipients—has revolutionized liver transplant by increasing the donor pool and thereby decreasing waitlist mortality rates, especially in pediatric candidates. Despite this advantage, split-liver transplant is technically challenging and associated with increased perioperative complications compared with whole-liver transplant, especially in adult recipients. Recently, experienced centers have reported favorable outcomes with split-liver transplant comparable to those with whole-liver transplant; therefore, split-liver transplant should be considered after careful evaluation of donor organ and recipient clinical status.49
Old age in the recipient can also adversely affect liver transplant outcomes.50
Interestingly, even in patients whose clinical course is unremarkable and biochemical values are normal, graft hepatitis or fibrosis of unknown cause with progression to cirrhosis has been described in the decade after transplant.41
Chronic rejection with eventual graft loss may be related to nonadherence in adolescents and can be reduced with use of an additional immunosuppressant such as sirolimus or mycophenolate. Chronic kidney disease can occur in about one-third of liver transplant recipients secondary to renal disease associated with primary disease (like Alagille syndrome), hepatorenal syndrome, and most importantly, use of calcineurin inhibitors.45
Components of the metabolic syndrome such as type 2 diabetes, obesity, nonalcoholic fatty liver disease, hypertension, and dyslipidemia are also seen in long-term pediatric liver transplant survivors. Internists are advised to screen for these comorbidities so that interventions can be applied early to improve long-term health outcomes and graft survival.
Of importance, multiple studies have shown a 2-fold increase in the rates of de novo malignancy in liver transplant recipients, including solid-organ and lymphoproliferative cancers, probably due to long-term immunosuppression. Posttransplant lymphoproliferative disorder occurs at lower rates than with other solid-organ transplants; its incidence is greatest in pediatric patients and in the first 12 to 18 months after transplant.51
TRANSITION TO ADULT CARE
While the number of patients with childhood-onset liver disease and pediatric liver transplant recipients who survive into adulthood is increasing, there are no established guidelines or formal models for transitioning these patients into adult care. Consequently, studies on transitional process have examined various issues such as patient and parent frustration, poor medical knowledge among patients during transition, lack of parental facilitation, and inadequate knowledge on disease process among adult-care hepatologists.52–54
A prolonged period of transition up to age 25 is preferred in complicated cases. Distinctive consideration for transition should include those with neurocognitive developmental delay from underlying disease or hepatic encephalopathy before transplant. These patients need additional support and time to achieve independence in health management before transition.57 Validated questionnaires are available to assess readiness to transition into adult care,58 implying that the decision to transition should not be based solely on age.
- Kelly DA, Bucuvalas JC, Alonso EM, et al; American Association for the Study of Liver Diseases; American Society of Transplantation. Long-term medical management of the pediatric patient after liver transplantation: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl 2013; 19(8):798–825. doi:10.1002/lt.23697
- Rosen DS, Blum RW, Britto M, Sawyer SM, Siegel DM; Society for Adolescent Medicine. Transition to adult health care for adolescents and young adults with chronic conditions: position paper of the Society for Adolescent Medicine. J Adolesc Health 2003; 33(4):309–311. pmid:14519573
- Fawaz R, Baumann U, Ekong U, et al. Guideline for the evaluation of cholestatic jaundice in infants: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr 2017; 64(1):154–168. doi:10.1097/MPG.0000000000001334
- Vajro P, Ferrante L, Lenta S, Mandato C, Persico M. Management of adults with paediatric-onset chronic liver disease: strategic issues for transition care. Dig Liver Dis 2014; 46(4):295–301. doi:10.1016/j.dld.2013.10.018
- Davenport M, Tizzard SA, Underhill J, Mieli-Vergani G, Portmann B, Hadzic N. The biliary atresia splenic malformation syndrome: a 28-year single-center retrospective study. J Pediatr 2006; 149(3):393–400. doi:10.1016/j.jpeds.2006.05.030
- Balistreri WF, Bezerra JA. Whatever happened to “neonatal hepatitis?” Clin Liver Dis 2006; 10(1):27–53. doi:10.1016/j.cld.2005.10.008
- Serinet MO, Wildhaber BE, Broué P, et al. Impact of age at Kasai operation on its results in late childhood and adolescence: a rational basis for biliary atresia screening. Pediatrics 2009; 123(5):1280–1286. doi:10.1542/peds.2008-1949
- de Vries W, Homan-Van der Veen J, Hulscher JB, Hoekstra-Weebers JE, Houwen RH, Verkade HJ; Netherlands Study Group of Biliary Atresia Registry. Twenty-year transplant-free survival rate among patients with biliary atresia. Clin Gastroenterol Hepatol 2011; 9(12):1086–1091. doi:10.1016/j.cgh.2011.07.024
- Lykavieris P, Chardot C, Sokhn M, Gauthier F, Valayer J, Bernard O. Outcome in adulthood of biliary atresia: a study of 63 patients who survived for over 20 years with their native liver. Hepatology 2005; 41(2):366–371. doi:10.1002/hep.20547
- Joshi D, Gupta N, Samyn M, Deheragoda M, Dobbels F, Heneghan MA. The management of childhood liver diseases in adulthood. J Hepatol 2017; 66(3):631–644. doi:10.1016/j.jhep.2016.11.013
- Shneider BL, Abel B, Haber B, et al; Childhood Liver Disease Research and Education Network. Portal hypertension in children and young adults with biliary atresia. J Pediatr Gastroenterol Nutr 2012; 55(5):567–573. doi:10.1097/MPG.0b013e31826eb0cf
- Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2017; 65(1):310–335. doi:10.1002/hep.28906
- Shneider BL, Brown MB, Haber B, et al; Biliary Atresia Research Consortium. A multicenter study of the outcome of biliary atresia in the United States, 1997 to 2000. J Pediatr 2006; 148(4):467–474. doi:10.1016/j.jpeds.2005.12.054
- Hung PY, Chen CC, Chen WJ, et al. Long-term prognosis of patients with biliary atresia: a 25 year summary. J Pediatr Gastroenterol Nutr 2006; 42(2):190–195. doi:10.1097/01.mpg.0000189339.92891.64
- Verkade HJ, Bezerra JA, Davenport M, et al. Biliary atresia and other cholestatic childhood diseases: advances and future challenges. J Hepatol 2016; 65(3):631–642. doi:10.1016/j.jhep.2016.04.032
- Hadžic N, Quaglia A, Portmann B, et al. Hepatocellular carcinoma in biliary atresia: King’s College Hospital experience. J Pediatr 2011; 159(4):617–622.e1. doi:10.1016/j.jpeds.2011.03.004
- Sokol RJ, Shepherd RW, Superina R, Bezerra JA, Robuck P, Hoofnagle JH. Screening and outcomes in biliary atresia: summary of a National Institutes of Health workshop. Hepatology 2007; 46(2):566–581. doi:10.1002/hep.21790
- Li L, Krantz ID, Deng Y, et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet 1997; 16(3):243–251. doi:10.1038/ng0797-243
- Saleh M, Kamath BM, Chitayat D. Alagille syndrome: clinical perspectives. Appl Clin Genet 2016; 9:75–82. doi:10.2147/TACG.S86420
- Bass LM, Kamath BM. Inherited disorders of cholestasis in adulthood. Clinical Liver Disease 2013; 2(5):200–203. doi:10.1002/cld.245
- Kamath BM, Yin W, Miller H, Anand R, Rand EB, Alonso E, Bucuvalas J; Studies of Pediatric Liver Transplantation. Outcomes of liver transplantation for patients with Alagille syndrome: the studies of pediatric liver transplantation experience. Liver Transpl 2012; 18(8):940–948. doi:10.1002/lt.23437
- Arnon R, Annunziato R, Schiano T, et al. Orthotopic liver transplantation for adults with Alagille syndrome. Clin Transplant 2012; 26(2):E94–E100. doi:10.1111/j.1399-0012.2011.01574.x
- Salem JE, Bruguiere E, Iserin L, Guiochon-Mantel A, Plouin PF. Hypertension and aortorenal disease in Alagille syndrome. J Hypertens 2012; 30(7):1300–1306. doi:10.1097/HJH.0b013e3283531e1f
- Kamath BM, Podkameni G, Hutchinson AL, et al. Renal anomalies in Alagille syndrome: a disease-defining feature. Am J Med Genet A 2012; 158A(1):85–89. doi:10.1002/ajmg.a.34369
- Kamath BM, Bason L, Piccoli DA, Krantz ID, Spinner NB. Consequences of JAG1 mutations. J Med Genet 2003; 40(12):891–895. pmid:14684686
- Emerick KM, Krantz ID, Kamath BM, et al. Intracranial vascular abnormalities in patients with Alagille syndrome. J Pediatr Gastroenterol Nutr 2005; 41(1):99–107. pmid:15990638
- Ferrarese A, Senzolo M, Burra P. Successful pregnancy in Alagille syndrome. Dig Liver Dis 2015; 47(1):86–87. doi:10.1016/j.dld.2014.08.047
- Davit-Spraul A, Fabre M, Branchereau S, et al. ATP8B1 and ABCB11 analysis in 62 children with normal gamma-glutamyl transferase progressive familial intrahepatic cholestasis (PFIC): phenotypic differences between PFIC1 and PFIC2 and natural history. Hepatology 2010; 51(5):1645–1655. doi:10.1002/hep.23539
- Zellos A, Lykopoulou L, Polydorou A, et al. Nasobiliary drainage in an episode of intrahepatic cholestasis in a child with mild ABCB11 disease. J Pediatr Gastroenterol Nutr 2012; 55(1):88–90. doi:10.1097/MPG.0b013e31822f2bda
- Alrabadi LS, Morotti RA, Valentino PL, Rodriguez-Davalos MI, Ekong UD, Emre SH. Biliary drainage as treatment for allograft steatosis following liver transplantation for PFIC-1 disease: a single-center experience. Pediatr Transplant 2018; 22(4):e13184. doi:10.1111/petr.13184
- Kubitz R, Dröge C, Kluge S, et al. Autoimmune BSEP disease: disease recurrence after liver transplantation for progressive familial intrahepatic cholestasis. Clin Rev Allergy Immunol 2015; 48(2–3):273–284. doi:10.1007/s12016-014-8457-4
- Jacquemin E. Progressive familial intrahepatic cholestasis. Clin Res Hepatol Gastroenterol 2012; 36(suppl 1):S26–S35. doi:10.1016/S2210-7401(12)70018-9
- Pataia V, Dixon PH, Williamson C. Pregnancy and bile acid disorders. Am J Physiol Gastrointest Liver Physiol 2017; 313(1):G1–G6. doi:10.1152/ajpgi.00028.2017
- Lamireau T, Monnereau S, Martin S, Marcotte JE, Winnock M, Alvarez F. Epidemiology of liver disease in cystic fibrosis: a longitudinal study. J Hepatol 2004; 41(6):920–925. doi:10.1016/j.jhep.2004.08.006
- Bolia R, Ooi CY, Lewindon P, et al. Practical approach to the gastrointestinal manifestations of cystic fibrosis. J Paediatr Child Health 2018; 54(6):609–619. doi:10.1111/jpc.13921
- Debray D, Kelly D, Houwen R, Strandvik B, Colombo C. Best practice guidance for the diagnosis and management of cystic fibrosis-associated liver disease. J Cyst Fibros 2011; 10(suppl 2):S29–S36. doi:10.1016/S1569-1993(11)60006-4
- Fridell JA, Bond GJ, Mazariegos G V, et al. Liver transplantation in children with cystic fibrosis: a long-term longitudinal review of a single center’s experience. J Pediatr Surg 2003; 38(8):1152–1156. pmid:12891484
- Fischler B, Bodin K, Stjernman H, et al. Cholestatic liver disease in adults may be due to an inherited defect in bile acid biosynthesis. J Intern Med 2007; 262(2):254–262. doi:10.1111/j.1365-2796.2007.01814.x
- Molho-Pessach V, Rios JJ, Xing C, Setchell KD, Cohen JC, Hobbs HH. Homozygosity mapping identifies a bile acid biosynthetic defect in an adult with cirrhosis of unknown etiology. Hepatology 2012; 55(4):1139–1145. doi:10.1002/hep.24781
- Mieli-Vergani G, Vergani D. Sclerosing cholangitis in children and adolescents. Clin Liver Dis 2016; 20(1):99–111. doi:10.1016/j.cld.2015.08.008
- Kelly D, Wray J. The adolescent liver transplant patient. Clin Liver Dis 2014; 18(3):613–632. doi:10.1016/j.cld.2014.05.006
- Westbrook RH, Yeoman AD, Agarwal K, et al. Outcomes of pregnancy following liver transplantation: the King’s College Hospital experience. Liver Transpl. 2015; 21(9):1153–1159. doi:10.1002/lt.24182
- Hammoud GM, Almashhrawi AA, Ahmed KT, Rahman R, Ibdah JA. Liver diseases in pregnancy: liver transplantation in pregnancy. World J Gastroenterol 2013; 19(43):7647–7651. doi:10.3748/wjg.v19.i43.7647
- Codoner-Franch P, Bernard O, Alvarez F. Long-term follow-up of growth in height after successful liver transplantation. J Pediatr 1994; 124(3):368–373. pmid:8120704
- Shemesh E. Assessment and management of psychosocial challenges in pediatric liver transplantation. Liver Transpl 2008; 14(9):1229–1236. doi:10.1002/lt.21582
- Martinelli J, Habes D, Majed L, et al. Long-term outcome of liver transplantation in childhood: a study of 20-year survivors. Am J Transplant 2018; 18(7):1680–1689. doi:10.1111/ajt.14626
- Roblin E, Audhuy F, Boillot O, Rivet C, Lachaux A. Long-term quality of life after pediatric liver transplantation. Arch Pediatr 2012; 19(10):1039–1052. French. doi:10.1016/j.arcped.2012.06.020
- Duffy JP, Kao K, Ko CY, et al. Long-term patient outcome and quality of life after liver transplantation: analysis of 20-year survivors. Ann Surg 2010; 252(4):652–661. doi:10.1097/SLA.0b013e3181f5f23a
- Hackl C, Schmidt KM, Süsal C, Döhler B, Zidek M, Schlitt HJ. Split liver transplantation: Current developments. World J Gastroenterol 2018; 24(47):5312–5321. doi:10.3748/wjg.v24.i47.5312
- Durand F, Levitsky J, Cauchy F, Gilgenkrantz H, Soubrane O, Francoz C. Age and liver transplantation. J Hepatol 2019; 70(4):745–758. doi:10.1016/j.jhep.2018.12.009
- Chandok N, Watt KD. Burden of de novo malignancy in the liver transplant recipient. Liver Transpl 2012; 18(11):1277–1289. doi:10.1002/lt.23531
- Ferrarese A, Germani G, Lazzaro S, et al. Short-term outcomes of paediatric liver transplant recipients after transition to Adult Healthcare Service. Liver Int 2018; 38(7):1316–1321. doi:10.1111/liv.13655
- Wright J, Elwell L, McDonagh JE, Kelly DA, Wray J. “Are these adult doctors gonna know me?” Experiences of transition for young people with a liver transplant. Pediatr Transplant 2016; 20(7):912–920. doi:10.1111/petr.12777
- Heldman MR, Sohn MW, Gordon EJ, et al. National survey of adult transplant hepatologists on the pediatric-to-adult care transition after liver transplantation. Liver Transpl 2015; 21(2):213–223. doi:10.1002/lt.24044
- Vajro P, Fischler B, Burra P, et al. The health care transition of youth with liver disease into the adult health system. J Pediatr Gastroenterol Nutr 2018; 66(6):976–990. doi:10.1097/MPG.0000000000001965
- Fredericks EM, Lopez MJ. Transition of the adolescent transplant patient to adult care. Clin Liver Dis (Hoboken) 2013; 2(5):223–226. doi:10.1002/cld.243
- Kaufman M. Transition of cognitively delayed adolescent organ transplant recipients to adult care. Pediatr Transplant 2006; 10(4):413–417. doi:10.1111/j.1399-3046.2006.00491.x
- Sawicki GS, Lukens-Bull K, Yin X, et al. Measuring the transition readiness of youth with special healthcare needs: validation of the TRAQ—Transition Readiness Assessment Questionnaire. J Pediatr Psychol 2011; 36(2):160–171. doi:10.1093/jpepsy/jsp128
- Kelly DA, Bucuvalas JC, Alonso EM, et al; American Association for the Study of Liver Diseases; American Society of Transplantation. Long-term medical management of the pediatric patient after liver transplantation: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl 2013; 19(8):798–825. doi:10.1002/lt.23697
- Rosen DS, Blum RW, Britto M, Sawyer SM, Siegel DM; Society for Adolescent Medicine. Transition to adult health care for adolescents and young adults with chronic conditions: position paper of the Society for Adolescent Medicine. J Adolesc Health 2003; 33(4):309–311. pmid:14519573
- Fawaz R, Baumann U, Ekong U, et al. Guideline for the evaluation of cholestatic jaundice in infants: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr 2017; 64(1):154–168. doi:10.1097/MPG.0000000000001334
- Vajro P, Ferrante L, Lenta S, Mandato C, Persico M. Management of adults with paediatric-onset chronic liver disease: strategic issues for transition care. Dig Liver Dis 2014; 46(4):295–301. doi:10.1016/j.dld.2013.10.018
- Davenport M, Tizzard SA, Underhill J, Mieli-Vergani G, Portmann B, Hadzic N. The biliary atresia splenic malformation syndrome: a 28-year single-center retrospective study. J Pediatr 2006; 149(3):393–400. doi:10.1016/j.jpeds.2006.05.030
- Balistreri WF, Bezerra JA. Whatever happened to “neonatal hepatitis?” Clin Liver Dis 2006; 10(1):27–53. doi:10.1016/j.cld.2005.10.008
- Serinet MO, Wildhaber BE, Broué P, et al. Impact of age at Kasai operation on its results in late childhood and adolescence: a rational basis for biliary atresia screening. Pediatrics 2009; 123(5):1280–1286. doi:10.1542/peds.2008-1949
- de Vries W, Homan-Van der Veen J, Hulscher JB, Hoekstra-Weebers JE, Houwen RH, Verkade HJ; Netherlands Study Group of Biliary Atresia Registry. Twenty-year transplant-free survival rate among patients with biliary atresia. Clin Gastroenterol Hepatol 2011; 9(12):1086–1091. doi:10.1016/j.cgh.2011.07.024
- Lykavieris P, Chardot C, Sokhn M, Gauthier F, Valayer J, Bernard O. Outcome in adulthood of biliary atresia: a study of 63 patients who survived for over 20 years with their native liver. Hepatology 2005; 41(2):366–371. doi:10.1002/hep.20547
- Joshi D, Gupta N, Samyn M, Deheragoda M, Dobbels F, Heneghan MA. The management of childhood liver diseases in adulthood. J Hepatol 2017; 66(3):631–644. doi:10.1016/j.jhep.2016.11.013
- Shneider BL, Abel B, Haber B, et al; Childhood Liver Disease Research and Education Network. Portal hypertension in children and young adults with biliary atresia. J Pediatr Gastroenterol Nutr 2012; 55(5):567–573. doi:10.1097/MPG.0b013e31826eb0cf
- Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2017; 65(1):310–335. doi:10.1002/hep.28906
- Shneider BL, Brown MB, Haber B, et al; Biliary Atresia Research Consortium. A multicenter study of the outcome of biliary atresia in the United States, 1997 to 2000. J Pediatr 2006; 148(4):467–474. doi:10.1016/j.jpeds.2005.12.054
- Hung PY, Chen CC, Chen WJ, et al. Long-term prognosis of patients with biliary atresia: a 25 year summary. J Pediatr Gastroenterol Nutr 2006; 42(2):190–195. doi:10.1097/01.mpg.0000189339.92891.64
- Verkade HJ, Bezerra JA, Davenport M, et al. Biliary atresia and other cholestatic childhood diseases: advances and future challenges. J Hepatol 2016; 65(3):631–642. doi:10.1016/j.jhep.2016.04.032
- Hadžic N, Quaglia A, Portmann B, et al. Hepatocellular carcinoma in biliary atresia: King’s College Hospital experience. J Pediatr 2011; 159(4):617–622.e1. doi:10.1016/j.jpeds.2011.03.004
- Sokol RJ, Shepherd RW, Superina R, Bezerra JA, Robuck P, Hoofnagle JH. Screening and outcomes in biliary atresia: summary of a National Institutes of Health workshop. Hepatology 2007; 46(2):566–581. doi:10.1002/hep.21790
- Li L, Krantz ID, Deng Y, et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet 1997; 16(3):243–251. doi:10.1038/ng0797-243
- Saleh M, Kamath BM, Chitayat D. Alagille syndrome: clinical perspectives. Appl Clin Genet 2016; 9:75–82. doi:10.2147/TACG.S86420
- Bass LM, Kamath BM. Inherited disorders of cholestasis in adulthood. Clinical Liver Disease 2013; 2(5):200–203. doi:10.1002/cld.245
- Kamath BM, Yin W, Miller H, Anand R, Rand EB, Alonso E, Bucuvalas J; Studies of Pediatric Liver Transplantation. Outcomes of liver transplantation for patients with Alagille syndrome: the studies of pediatric liver transplantation experience. Liver Transpl 2012; 18(8):940–948. doi:10.1002/lt.23437
- Arnon R, Annunziato R, Schiano T, et al. Orthotopic liver transplantation for adults with Alagille syndrome. Clin Transplant 2012; 26(2):E94–E100. doi:10.1111/j.1399-0012.2011.01574.x
- Salem JE, Bruguiere E, Iserin L, Guiochon-Mantel A, Plouin PF. Hypertension and aortorenal disease in Alagille syndrome. J Hypertens 2012; 30(7):1300–1306. doi:10.1097/HJH.0b013e3283531e1f
- Kamath BM, Podkameni G, Hutchinson AL, et al. Renal anomalies in Alagille syndrome: a disease-defining feature. Am J Med Genet A 2012; 158A(1):85–89. doi:10.1002/ajmg.a.34369
- Kamath BM, Bason L, Piccoli DA, Krantz ID, Spinner NB. Consequences of JAG1 mutations. J Med Genet 2003; 40(12):891–895. pmid:14684686
- Emerick KM, Krantz ID, Kamath BM, et al. Intracranial vascular abnormalities in patients with Alagille syndrome. J Pediatr Gastroenterol Nutr 2005; 41(1):99–107. pmid:15990638
- Ferrarese A, Senzolo M, Burra P. Successful pregnancy in Alagille syndrome. Dig Liver Dis 2015; 47(1):86–87. doi:10.1016/j.dld.2014.08.047
- Davit-Spraul A, Fabre M, Branchereau S, et al. ATP8B1 and ABCB11 analysis in 62 children with normal gamma-glutamyl transferase progressive familial intrahepatic cholestasis (PFIC): phenotypic differences between PFIC1 and PFIC2 and natural history. Hepatology 2010; 51(5):1645–1655. doi:10.1002/hep.23539
- Zellos A, Lykopoulou L, Polydorou A, et al. Nasobiliary drainage in an episode of intrahepatic cholestasis in a child with mild ABCB11 disease. J Pediatr Gastroenterol Nutr 2012; 55(1):88–90. doi:10.1097/MPG.0b013e31822f2bda
- Alrabadi LS, Morotti RA, Valentino PL, Rodriguez-Davalos MI, Ekong UD, Emre SH. Biliary drainage as treatment for allograft steatosis following liver transplantation for PFIC-1 disease: a single-center experience. Pediatr Transplant 2018; 22(4):e13184. doi:10.1111/petr.13184
- Kubitz R, Dröge C, Kluge S, et al. Autoimmune BSEP disease: disease recurrence after liver transplantation for progressive familial intrahepatic cholestasis. Clin Rev Allergy Immunol 2015; 48(2–3):273–284. doi:10.1007/s12016-014-8457-4
- Jacquemin E. Progressive familial intrahepatic cholestasis. Clin Res Hepatol Gastroenterol 2012; 36(suppl 1):S26–S35. doi:10.1016/S2210-7401(12)70018-9
- Pataia V, Dixon PH, Williamson C. Pregnancy and bile acid disorders. Am J Physiol Gastrointest Liver Physiol 2017; 313(1):G1–G6. doi:10.1152/ajpgi.00028.2017
- Lamireau T, Monnereau S, Martin S, Marcotte JE, Winnock M, Alvarez F. Epidemiology of liver disease in cystic fibrosis: a longitudinal study. J Hepatol 2004; 41(6):920–925. doi:10.1016/j.jhep.2004.08.006
- Bolia R, Ooi CY, Lewindon P, et al. Practical approach to the gastrointestinal manifestations of cystic fibrosis. J Paediatr Child Health 2018; 54(6):609–619. doi:10.1111/jpc.13921
- Debray D, Kelly D, Houwen R, Strandvik B, Colombo C. Best practice guidance for the diagnosis and management of cystic fibrosis-associated liver disease. J Cyst Fibros 2011; 10(suppl 2):S29–S36. doi:10.1016/S1569-1993(11)60006-4
- Fridell JA, Bond GJ, Mazariegos G V, et al. Liver transplantation in children with cystic fibrosis: a long-term longitudinal review of a single center’s experience. J Pediatr Surg 2003; 38(8):1152–1156. pmid:12891484
- Fischler B, Bodin K, Stjernman H, et al. Cholestatic liver disease in adults may be due to an inherited defect in bile acid biosynthesis. J Intern Med 2007; 262(2):254–262. doi:10.1111/j.1365-2796.2007.01814.x
- Molho-Pessach V, Rios JJ, Xing C, Setchell KD, Cohen JC, Hobbs HH. Homozygosity mapping identifies a bile acid biosynthetic defect in an adult with cirrhosis of unknown etiology. Hepatology 2012; 55(4):1139–1145. doi:10.1002/hep.24781
- Mieli-Vergani G, Vergani D. Sclerosing cholangitis in children and adolescents. Clin Liver Dis 2016; 20(1):99–111. doi:10.1016/j.cld.2015.08.008
- Kelly D, Wray J. The adolescent liver transplant patient. Clin Liver Dis 2014; 18(3):613–632. doi:10.1016/j.cld.2014.05.006
- Westbrook RH, Yeoman AD, Agarwal K, et al. Outcomes of pregnancy following liver transplantation: the King’s College Hospital experience. Liver Transpl. 2015; 21(9):1153–1159. doi:10.1002/lt.24182
- Hammoud GM, Almashhrawi AA, Ahmed KT, Rahman R, Ibdah JA. Liver diseases in pregnancy: liver transplantation in pregnancy. World J Gastroenterol 2013; 19(43):7647–7651. doi:10.3748/wjg.v19.i43.7647
- Codoner-Franch P, Bernard O, Alvarez F. Long-term follow-up of growth in height after successful liver transplantation. J Pediatr 1994; 124(3):368–373. pmid:8120704
- Shemesh E. Assessment and management of psychosocial challenges in pediatric liver transplantation. Liver Transpl 2008; 14(9):1229–1236. doi:10.1002/lt.21582
- Martinelli J, Habes D, Majed L, et al. Long-term outcome of liver transplantation in childhood: a study of 20-year survivors. Am J Transplant 2018; 18(7):1680–1689. doi:10.1111/ajt.14626
- Roblin E, Audhuy F, Boillot O, Rivet C, Lachaux A. Long-term quality of life after pediatric liver transplantation. Arch Pediatr 2012; 19(10):1039–1052. French. doi:10.1016/j.arcped.2012.06.020
- Duffy JP, Kao K, Ko CY, et al. Long-term patient outcome and quality of life after liver transplantation: analysis of 20-year survivors. Ann Surg 2010; 252(4):652–661. doi:10.1097/SLA.0b013e3181f5f23a
- Hackl C, Schmidt KM, Süsal C, Döhler B, Zidek M, Schlitt HJ. Split liver transplantation: Current developments. World J Gastroenterol 2018; 24(47):5312–5321. doi:10.3748/wjg.v24.i47.5312
- Durand F, Levitsky J, Cauchy F, Gilgenkrantz H, Soubrane O, Francoz C. Age and liver transplantation. J Hepatol 2019; 70(4):745–758. doi:10.1016/j.jhep.2018.12.009
- Chandok N, Watt KD. Burden of de novo malignancy in the liver transplant recipient. Liver Transpl 2012; 18(11):1277–1289. doi:10.1002/lt.23531
- Ferrarese A, Germani G, Lazzaro S, et al. Short-term outcomes of paediatric liver transplant recipients after transition to Adult Healthcare Service. Liver Int 2018; 38(7):1316–1321. doi:10.1111/liv.13655
- Wright J, Elwell L, McDonagh JE, Kelly DA, Wray J. “Are these adult doctors gonna know me?” Experiences of transition for young people with a liver transplant. Pediatr Transplant 2016; 20(7):912–920. doi:10.1111/petr.12777
- Heldman MR, Sohn MW, Gordon EJ, et al. National survey of adult transplant hepatologists on the pediatric-to-adult care transition after liver transplantation. Liver Transpl 2015; 21(2):213–223. doi:10.1002/lt.24044
- Vajro P, Fischler B, Burra P, et al. The health care transition of youth with liver disease into the adult health system. J Pediatr Gastroenterol Nutr 2018; 66(6):976–990. doi:10.1097/MPG.0000000000001965
- Fredericks EM, Lopez MJ. Transition of the adolescent transplant patient to adult care. Clin Liver Dis (Hoboken) 2013; 2(5):223–226. doi:10.1002/cld.243
- Kaufman M. Transition of cognitively delayed adolescent organ transplant recipients to adult care. Pediatr Transplant 2006; 10(4):413–417. doi:10.1111/j.1399-3046.2006.00491.x
- Sawicki GS, Lukens-Bull K, Yin X, et al. Measuring the transition readiness of youth with special healthcare needs: validation of the TRAQ—Transition Readiness Assessment Questionnaire. J Pediatr Psychol 2011; 36(2):160–171. doi:10.1093/jpepsy/jsp128
KEY POINTS
- The causes of cholestasis in children are different from those in adults, with genetic inherited causes more common in childhood.
- Cholestasis in children can be caused by biliary tract obstruction such as in biliary atresia or defects in forming and excreting bile acids and other components of bile.
- With the growing number of people with childhood-onset liver disease surviving into adulthood, it is important for internists to be aware of unique problems and challenges in continuing management of this population.
- In addition to medical comorbidities, these patients may also have impaired psychosocial functioning and quality of life.
Giant cell arteritis: An updated review of an old disease
Giant cell arteritis (GCA) is a systemic vasculitis involving medium-sized and large arteries, most commonly the temporal, ophthalmic, occipital, vertebral, posterior ciliary, and proximal vertebral arteries. Moreover, involvement of the ophthalmic artery and its branches results in loss of vision. GCA can also involve the aorta and its proximal branches, especially in the upper extremities.
GCA is the most common systemic vasculitis in adults. It occurs almost exclusively in patients over age 50 and affects women more than men. It is most frequent in populations of northern European ancestry, especially Scandinavian. In a retrospective cohort study in Norway, the average annual cumulative incidence rate of GCA was 16.7 per 100,000 people over age 50.1 Risk factors include older age, history of smoking, current smoking, early menopause, and, possibly, stress-related disorders.2
PATHOGENESIS IS NOT COMPLETELY UNDERSTOOD
The pathogenesis of GCA is not completely understood, but there is evidence of immune activation in the arterial wall leading to activation of macrophages and formation of multinucleated giant cells (which may not always be present in biopsies).
The most relevant cytokines in the ongoing pathogenesis are still being defined, but the presence of interferon gamma and interleukin 6 (IL-6) seem to be critical for the expression of the disease. The primary immunogenic triggers for the elaboration of these cytokines and the arteritis remain elusive.
A SPECTRUM OF PRESENTATIONS
The initial symptoms of GCA may be vague, such as malaise, fever, and night sweats, and are likely due to systemic inflammation. Features of vascular involvement include headache, scalp tenderness, and jaw claudication (cramping pain in the jaw while chewing).
A less common but serious feature associated with GCA is partial or complete vision loss affecting 1 or both eyes.3 Some patients suddenly go completely blind without any visual prodrome.
Overlapping GCA phenotypes exist, with a spectrum of presentations that include classic cranial arteritis, extracranial GCA (also called large-vessel GCA), and polymyalgia rheumatica.2
Cranial GCA, the best-characterized clinical presentation, causes symptoms such as headache or signs such as tenderness of the temporal artery. On examination, the temporal arteries may be tender or nodular, and the pulses may be felt above the zygomatic arch, above and in front of the tragus of the ear. About two-thirds of patients with cranial GCA present with new-onset headache, most often in the temporal area, but possibly anywhere throughout the head.
Visual disturbance, jaw claudication, and tongue pain are less common but, if present, increase the likelihood of this diagnosis.2
Large-vessel involvement in GCA is common and refers to involvement of the aorta and its proximal branches. Imaging methods used in diagnosing large-vessel GCA include color Doppler ultrasonography, computed tomography with angiography, magnetic resonance imaging with angiography, and positron emission tomography. In some centers, such imaging is performed in all patients diagnosed with GCA to survey for large-vessel involvement.
Depending on the imaging study, large-vessel involvement has been found in 30% to 80% of cases of GCA.4,5 It is often associated with nonspecific symptoms such as fever, weight loss, chills, and malaise, but it can also cause more specific symptoms such as unilateral extremity claudication. In contrast to patients with cranial GCA, patients with large-vessel GCA were younger at onset, less likely to have headaches, and more likely to have arm claudication at presentation.6 Aortitis of the ascending aorta can occur with a histopathologic pattern of GCA but without the clinical stigmata of GCA.
The finding of aortitis should prompt the clinician to question the patient about other symptoms of GCA and to order imaging of the whole vascular tree. Ultrasonography and biopsy of the temporal arteries can be considered. Whether idiopathic aortitis is part of the GCA spectrum remains to be seen.
Laboratory tests often show anemia, leukocytosis, and thrombocytosis. Acute-phase reactants such as C-reactive protein and the erythrocyte sedimentation rate are often elevated. The sedimentation rate often exceeds 50 mm/hour and sometimes 100 mm/hour.
In 2 retrospective studies, the number of patients with GCA whose sedimentation rate was less than 50 mm/hour ranged between 5% and 11%.7,8 However, a small percentage of patients with GCA have normal inflammatory markers. Therefore, if the suspicion for GCA is high, treatment should be started and biopsy pursued.9 In patients with paraproteinemia or other causes of a spuriously elevated or low erythrocyte sedimentation rate, C-reactive protein is a more reliable test.
Polymyalgia rheumatica is another rheumatologic condition that can occur independently or in conjunction with GCA. It is characterized by stiffness and pain in the proximal joints such as the hips and shoulders, typically worse in the morning and better with activity. Although the patient may subjectively feel weak, a close neurologic examination will reveal normal muscle strength.
Polymyalgia rheumatica is observed in 40% to 60% of patients with GCA at the time of diagnosis; 16% to 21% of patients with polymyalgia rheumatica may develop GCA, especially if untreated.2,10
Differential diagnosis
Other vasculitides (eg, Takayasu arteritis) can also present with unexplained fever, anemia, and constitutional symptoms.
Infection should be considered if fever is present. An infectious disease accompanied by fever, headache, and elevated inflammatory markers can mimic GCA.
Nonarteritic anterior ischemic optic neuropathy can present with sudden vision loss, prompting concern for underlying GCA. Risk factors include hypertension and diabetes mellitus; other features of GCA, including elevated inflammatory markers, are generally absent.
TEMPORAL ARTERY BIOPSY: THE GOLD STANDARD FOR DIAGNOSIS
Temporal artery biopsy remains the standard to confirm the diagnosis. However, because inflammation in the temporal arteries can affect some segments but not others, biopsy results on conventional hematoxylin and eosin staining can be falsely negative in patients with GCA. In one study,11 the mean sensitivity of unilateral temporal artery biopsy was 86.9%.
Typical positive histologic findings are inflammation with panarteritis, CD4-positive lymphocytes, macrophages, giant cells, and fragmentation of the internal elastic lamina.12
When GCA is suspected, treatment with glucocorticoids should be started immediately and biopsy performed as soon as possible. Delaying biopsy for 14 days or more may not affect the accuracy of biopsy study.13 Treatment should never be withheld while awaiting the results of biopsy study.
Biopsy is usually performed unilaterally, on the same side as the symptoms or abnormal findings on examination. Bilateral temporal artery biopsy is also performed and compared with unilateral biopsy; this approach increases the diagnostic yield by about 5%.14
IMAGING
In patients with suspected GCA, imaging is recommended early to complement the clinical criteria for the diagnosis of GCA.15 Positron emission tomography, computed tomography angiography, magnetic resonance angiography, or Doppler ultrasonography can reveal inflammation of the arteries in the proximal upper or lower limbs or the aorta.2
In patients with suspected cranial GCA, ultrasonography of the temporal and axillary arteries is recommended first. If ultrasonography is not available or is inconclusive, high-resolution magnetic resonance imaging of the cranial arteries can be used as an alternative. Computed tomography and positron emission tomography of the cranial arteries are not recommended.
In patients with suspected large-vessel GCA, ultrasonography, positron emission tomography, computed tomography, and magnetic resonance imaging may be used to screen for vessel wall inflammation, edema, and luminal narrowing in extracranial arteries. Ultrasonography is of limited value in assessing aortitis.
Color duplex ultrasonography can be applied to assess for vascular inflammation of the temporal or large arteries. The typical finding of the “halo” sign, a hypoechoic ring around the arterial lumen, represents the inflammation-induced thickening of the arterial wall. The “compression sign,” the persistence of the “halo” during compression of the vessel lumen by the ultrasound probe, has high specificity for the diagnosis.16
Ultrasonography of suspected GCA has yielded sensitivities of 55% to 100% and specificities of 78% to 100%. However, its sensitivity depends on the user’s level of expertise, so it should be done only in medical centers with a high number of GCA cases and with highly experienced sonographers. High-resolution magnetic resonance imaging is an alternative to ultrasonography and has shown similar sensitivity and specificity.3
TREATMENT WITH GLUCOCORTICOIDS
Glucocorticoids remain the standard for treatment of GCA. The therapeutic effect of glucocorticoids in GCA has been established by years of clinical experience, but has never been proven in a placebo-controlled trial. When started appropriately and expeditiously, glucocorticoids produce exquisite resolution of signs and symptoms and prevent the serious complication of vision loss. Rapid resolution of symptoms is so typical of GCA that if the patient’s symptoms persist more than a few days after starting a glucocorticoid, the diagnosis of GCA should be reconsidered.
In a retrospective study of 245 patients with biopsy-proven GCA treated with glucocorticoids, 34 had permanent loss of sight.17 In 32 (94%) of the 34, the vision loss occurred before glucocorticoids were started. Of the remaining 2 patients, 1 lost vision 8 days into treatment, and the other lost vision 3 years after diagnosis and 1 year after discontinuation of glucocorticoids.
In a series of 144 patients with biopsy-proven GCA, 51 had no vision loss at presentation and no vision loss after starting glucocorticoids, and 93 had vision loss at presentation. In the latter group, symptoms worsened within 5 days of starting glucocorticoids in 9 patients.18 If vision was intact at the time of presentation, prompt initiation of glucocorticoids reduced the risk of vision loss to less than 1%.
High doses, slowly tapered
The European League Against Rheumatism recommends early initiation of high-dose glucocorticoids for patients with large-vessel vasculitis,19 and it also recommends glucocorticoids for patients with polymyalgia rheumatica.20 The optimal initial and tapering dosage has never been formally evaluated, but regimens have been devised on the basis of expert opinion.21
For patients with GCA who do not have vision loss at the time of diagnosis, the initial dose is prednisone 1 mg/kg or its equivalent daily for 2 to 4 weeks, after which it is tapered.21 If the initial dosage is prednisone 60 mg orally daily for 2 to 4 weeks, our practice is to taper it to 50 mg daily for 2 weeks, then 40 mg daily for 2 weeks. Then, it is decreased by 5 mg every 2 weeks until it is 20 mg daily, and then by 2.5 mg every 2 weeks until it is 10 mg orally daily. Thereafter, the dosage is decreased by 1 mg every 2 to 4 weeks.
For patients with GCA who experience transient vision loss or diplopia at the time of diagnosis, intravenous pulse glucocorticoid therapy should be initiated to reduce the risk of vision loss as rapidly as possible.22 A typical pulse regimen is methylprednisolone 1 g intravenously daily for 3 days. Though not rigorously validated in studies, such an approach is used to avoid vision impairment due to GCA, which is rarely reversible.
RELAPSE OF DISEASE
Suspect a relapse of GCA if the patient’s initial symptoms recur, if inflammatory markers become elevated, or if classic symptoms of GCA or polymyalgia rheumatica occur. Elevations in inflammatory markers do not definitely indicate a flare of GCA, but they should trigger close monitoring of the patient’s symptoms.
Relapse is treated by increasing the glucocorticoid dosage as appropriate to the nature of the relapse. If vision is affected or the patient has symptoms of GCA, then increments of 30 to 60 mg of prednisone are warranted, whereas if the patient has symptoms of polymyalgia rheumatica, then increments of 5 to 10 mg of prednisone are usually used.
The incidence of relapses of GCA in multiple tertiary care centers has been reported to vary between 34% and 75%.23,24 Most relapses occur at prednisone dosages of less than 20 mg orally daily and within the first year after diagnosis. The most common symptoms are limb ischemia, jaw claudication, constitutional symptoms, headaches, and polymyalgia rheumatica. In a review of 286 patients,25 213 (74%) had at least 1 relapse. The first relapse occurred in the first year in 50%, by 2 years in 68%, and by 5 years in 79%.
ADVERSE EFFECTS OF GLUCOCORTICOIDS
In high doses, glucocorticoids have well-known adverse effects. In a population-based study of 120 patients, each patient treated with glucocorticoids experienced at least 1 adverse effect (cataract, fracture, infection, osteonecrosis, diabetes, hypertension, weight gain, capillary fragility, or hair loss).26 The effects were related to aging and cumulative dosage of prednisone but not to the initial dosage.
Glucocorticoids can affect many organs and systems:
- Eyes (cataracts, increased intraocular pressure, exophthalmos)
- Heart (premature atherosclerotic disease, hypertension, fluid retention, hyperlipidemia, arrhythmias)
- Gastrointestinal system (ulcer, gastrointestinal bleeding, gastritis, visceral perforation, hepatic steatosis, acute pancreatitis)
- Bone and muscle (osteopenia, osteoporosis, osteonecrosis, myopathy)
- Brain (mood disorder, psychosis, memory impairment)
- Endocrine system (hyperglycemia, hypothalamic-pituitary-adrenal axis suppression)
- Immune system (immunosuppression, leading to infection and leukocytosis).
Patients receiving a glucocorticoid dose equivalent to 20 mg or more of prednisone daily for 1 month or more who also have another cause of immunocompromise need prophylaxis against Pneumocystis jirovecii pneumonia.27 They should also receive appropriate immunizations before starting glucocorticoids. Live-virus vaccines should not be given to these patients until they have been off glucocorticoids for 1 month.
Glucocorticoids and bone loss
Glucocorticoids are associated with bone loss and fracture, which can occur within the first few months of use and with dosages as low as 2.5 to 7.5 mg orally daily.28 Therefore, glucocorticoid-induced bone loss has to be treated aggressively, particularly in patients who are older and have a history of fragility fracture.
For patients with GCA who need glucocorticoids in doses greater than 5 mg orally daily for more than 3 months, the following measures are advised to decrease the risk of bone loss:
- Weight-bearing exercise
- Smoking cessation
- Moderation in alcohol intake
- Measures to prevent falls29
- Supplementation with 1,200 mg of calcium and 800 IU of vitamin D.30
Pharmacologic therapy should be initiated in men over age 50 who have established osteoporosis and in postmenopausal women with established osteoporosis or osteopenia. For men over age 50 with established osteopenia, risk assessment with the glucocorticoid-corrected FRAX score (www.sheffield.ac.uk/FRAX) should be performed to identify those at high risk in whom pharmacologic therapy is warranted.31
Bisphosphonates are the first-line therapy for glucocorticoid-induced osteoporosis.32
Teriparatide is the second-line therapy and is used in patients who cannot tolerate bisphosphonates or other osteoporosis therapies, and in those who have severe osteoporosis, with T scores of –3.5 and below if they have not had a fracture, and –2.5 and below if they have had a fragility fracture.33
Denosumab, a monoclonal antibody to an osteoclast differentiating factor, may be beneficial for some patients with glucocorticoid-induced osteoporosis.34
To assess the efficacy of therapy, measuring bone mineral density at baseline and at 1 year of therapy is recommended. If density is stable or improved, then repeating the measurement at 2- to 3-year intervals is suggested.
TOCILIZUMAB: A STEROID-SPARING MEDICATION
Due to the adverse effects of long-term use of glucocorticoids and high rates of relapse, there is a pressing need for medications that are more efficacious and less toxic to treat GCA.
The European League Against Rheumatism, in its 2009 management guidelines for large-vessel vasculitis, recommend using an adjunctive immunosuppressant agent.19 In the case of GCA, they recommend using methotrexate 10 to 15 mg/week, which has shown modest evidence of reducing the relapse rate and lowering the cumulative doses of glucocorticoids needed.35,36
Studies of tumor necrosis factor inhibitors and abatacept have not yielded significant reductions in the relapse rate or decreased cumulative doses of prednisone.37,38
Advances in treatment for GCA have stagnated, but recent trials39,40 have evaluated the IL-6 receptor alpha inhibitor tocilizumab, given the central role of IL-6 in the pathogenesis of GCA. Case reports have revealed rapid induction and maintenance of remission in GCA using tocilizumab.41,42
Villiger et al39 performed a randomized, placebo-controlled trial to study the efficacy and safety of tocilizumab in induction and maintenance of disease remission in 30 patients with newly diagnosed GCA. The primary outcome, complete remission at 12 weeks, was achieved in 85% of patients who received tocilizumab plus tapered prednisolone, compared with 40% of patients who received placebo plus tapering prednisolone. The tocilizumab group also had favorable results in secondary outcomes including relapse-free survival at 52 weeks, time to first relapse after induction of remission, and cumulative dose of prednisolone.
The GiACTA trial. Stone et al40 studied the effect of tocilizumab on rates of relapse during glucocorticoid tapering in 251 GCA patients over the course of 52 weeks. Patients were randomized in a 2:1:1:1 ratio to 4 treatment groups:
- Tocilizumab weekly plus prednisone, with prednisone tapered over 26 weeks
- Tocilizumab every other week plus prednisone tapered over 26 weeks
- Placebo plus prednisone tapered over 26 weeks
- Placebo plus prednisone tapered over 52 weeks.
The primary outcome was the rate of sustained glucocorticoid-free remission at 52 weeks. Secondary outcomes included the remission rate, the cumulative glucocorticoid dose, and safety measures. At 52 weeks, the rates of sustained remission were:
- 56% with tocilizumab weekly
- 53% with tocilizumab every other week
- 14% with placebo plus 26-week prednisone taper
- 18% with placebo plus 52-week taper.
Differences between the active treatment groups and the placebo groups were statistically significant (P < .001).
The cumulative dose of prednisone in tocilizumab recipients was significantly less than in placebo recipients. Rates of adverse events were similar. Ultimately, the study showed that tocilizumab, either weekly or every other week, was more effective than prednisone alone at sustaining glucocorticoid-free remission in patients with GCA.
However, the study also raised questions about tocilizumab’s toxic effect profile and its long-term efficacy, as well as who are the optimal candidates for this therapy. Data on long-term use of tocilizumab are primarily taken from its use in rheumatoid arthritis.43 As of this writing, Stone et al are conducting an open-label trial to help provide long-term safety and efficacy data in patients with GCA. In the meantime, we must extrapolate data from the long-term use of tocilizumab in rheumatoid arthritis.
Tocilizumab and lower gastrointestinal tract perforation
One of the major adverse effects of long-term use of tocilizumab is lower gastrointestinal tract perforation.
Xie et al,44 in 2016, reported that the risk of perforation in patients on tocilizumab for rheumatoid arthritis was more than 2 times higher than in patients taking a tumor necrosis factor inhibitor. However, the absolute rates of perforation were low overall, roughly 1 to 3 per 1,000 patient-years in the tocilizumab group. Risk factors for perforation included older age, history of diverticulitis or other gastrointestinal tract condition, and prednisone doses of 7.5 mg or more a day.
Does tocilizumab prevent blindness?
Another consideration is that tocilizumab may not prevent optic neuropathy. In the GiACTA trial, 1 patient in the group receiving tocilizumab every other week developed optic neuropathy.40 Prednisone had been completely tapered off at the time, and the condition resolved when glucocorticoids were restarted. Thus, it is unknown if tocilizumab would be effective on its own without concomitant use of glucocorticoids.
Vision loss is one of the most severe complications of GCA, and it is still unclear whether tocilizumab can prevent vision loss in GCA. Also, we still have no data on the effect of tocilizumab on histopathologic findings, and whether biopsy yield diminishes over time. We hope future studies will help guide us in this regard.
No guidelines on tocilizumab yet
Clinical guidelines on the appropriate use of tocilizumab in GCA are lacking. The American College of Rheumatology and the European League Against Rheumatism have yet to publish updated guidelines with comments on use of tocilizumab. Therefore, it is unclear if tocilizumab is a first-line treatment in GCA, as its efficacy alone without glucocorticoids and its long-term safety in GCA patients have not been studied.
Treatment with tocilizumab should be individualized; it should be considered in patients who have had adverse effects from glucocorticoids, and in patients who experience a flare or cannot have their glucocorticoid dose lowered to an appropriate range.
The optimal duration of tocilizumab therapy is also unknown. However, using the GiACTA study as a rough guide, we try to limit its use to 1 year until additional data are available.
Patients on IL-6 inhibition may have suppressed C-reactive protein regardless of disease activity.43 Therefore, this laboratory value may not be reliable in determining active disease in patients on tocilizumab.
The GiACTA trial has shown an impressive improvement in the relapse-free remission period in patients with GCA taking tocilizumab. However, much work needs to be done to define the safety of this medication and determine which patients should be started on it. In the meantime, we recommend starting high-dose glucocorticoid therapy as soon as the diagnosis of GCA is suspected. In patients who do not tolerate glucocorticoids or whose disease flares during glucocorticoid taper, we recommend starting treatment with tocilizumab either once a week or every other week for at least 1 year.
- Brekke LK, Diamantopoulos AP, Fevang BT, Aßmus J, Esperø E, Gjesdal CG. Incidence of giant cell arteritis in Western Norway 1972–2012: a retrospective cohort study. Arthritis Res Ther 2017; 19(1):278. doi:10.1186/s13075-017-1479-6
- Dejaco C, Duftner C, Buttgereit F, Matteson EL, Dasgupta B. The spectrum of giant cell arteritis and polymyalgia rheumatica: revisiting the concept of the disease. Rheumatology (Oxford) 2017; 56(4):506–515. doi:10.1093/rheumatology/kew273
- Weyand CM, Goronzy JJ. Giant-cell arteritis and polymyalgia rheumatica. N Engl J Med 2014; 371(17):1653. doi:10.1056/NEJMc1409206
- Ghinoi A, Pipitone N, Nicolini A, et al. Large-vessel involvement in recent-onset giant cell arteritis: a case-control colour-Doppler sonography study. Rheumatology (Oxford) 2012; 51(4):730–734. doi:10.1093/rheumatology/ker329
- Prieto-González S, Depetris M, García-Martínez A, et al. Positron emission tomography assessment of large vessel inflammation in patients with newly diagnosed, biopsy-proven giant cell arteritis: a prospective, case-control study. Ann Rheum Dis 2014; 73(7):1388–1392. doi:10.1136/annrheumdis-2013-204572
- Brack A, Martinez-Taboada V, Stanson A, Goronzy JJ, Weyand CM. Disease pattern in cranial and large-vessel giant cell arteritis. Arthritis Rheum 1999; 42(2):311–317. doi:10.1002/1529-0131(199902)42:2<311::AID-ANR14>3.0.CO;2-F
- Salvarani C, Hunder GG. Giant cell arteritis with low erythrocyte sedimentation rate: frequency of occurence in a population-based study. Arthritis Rheum 2001; 45(2):140–145. doi:10.1002/1529-0131(200104)45:2<140::AID-ANR166>3.0.CO;2-2
- Liozon E, Jauberteau-Marchan MO, Ly K, Loustaud-Ratti V, Soria P, Vidal E. Giant cell arteritis with a low erythrocyte sedimentation rate: comments on the article by Salvarani and Hunder. Arthritis Rheum 2002; 47(6):692–694. doi:10.1002/art.10809
- Yu-Wai-Man P, Dayan MR. Giant cell arteritis with normal inflammatory markers. Acta Ophthalmol Scand 2007; 85(4):460. doi:10.1111/j.1600-0420.2006.00864.x
- Buttgereit F, Dejaco C, Matteson EL, Dasgupta B. Polymyalgia rheumatica and giant cell arteritis: a systematic review. JAMA 2016; 315(22):2442–2458. doi:10.1001/jama.2016.5444
- Niederkohr RD, Levin LA. Management of the patient with suspected temporal arteritis a decision-analytic approach. Ophthalmology 2005; 112(5):744–756. doi:10.1016/j.ophtha.2005.01.031
- Bowling K, Rait J, Atkinson J, Srinivas G. Temporal artery biopsy in the diagnosis of giant cell arteritis: does the end justify the means? Ann Med Surg (Lond) 2017; 20:1–5. doi:10.1016/j.amsu.2017.06.020
- Daily B, Dassow P, Haynes J, Nashelsky J. Giant cell arteritis: biopsy after corticosteroid initiation. Am Fam Physician 2017; 95(2):116–117. pmid:28084703
- Durling B, Toren A, Patel V, Gilberg S, Weis E, Jordan D. Incidence of discordant temporal artery biopsy in the diagnosis of giant cell arteritis. Can J Ophthalmol 2014; 49(2):157–161. doi:10.1016/j.jcjo.2013.12.008
- Dejaco C, Ramiro S, Duftner C, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis 2018; 77(5):636–643. doi:10.1136/annrheumdis-2017-212649
- Aschwanden M, Imfeld S, Staub D, et al. The ultrasound compression sign to diagnose temporal giant cell arteritis shows an excellent interobserver agreement. Clin Exp Rheumatol 2015; 33(2 suppl 89):S-113–S-115. pmid:26016760
- Aiello PD, Trautmann JC, McPhee TJ, Kunselman AR, Hunder GG. Visual prognosis in giant cell arteritis. Ophthalmology 1993; 100(4):550–555. pmid:8479714
- Hayreh SS, Zimmerman B. Visual deterioration in giant cell arteritis patients while on high doses of corticosteroid therapy. Ophthalmology 2003; 110(6):1204–1215. doi:10.1016/S0161-6420(03)00228-8
- Mukhtyar C, Guillevin L, Cid MC, et al; European Vasculitis Study Group. EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis 2009; 68(3):318–323. doi:10.1136/ard.2008.088351
- Dejaco C, Singh YP, Perel P, et al; European League Against Rheumatism; American College of Rheumatology. 2015 recommendations for the management of polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann Rheum Dis 2015; 74(10):1799–1807. doi:10.1136/annrheumdis-2015-207492
- Bienvenu B, Ly KH, Lambert M, et al; Groupe d’Étude Français des Artérites des gros Vaisseaux, under the Aegis of the Filière des Maladies Auto-Immunes et Auto-Inflammatoires Rares. Management of giant cell arteritis: recommendations of the French Study Group for Large Vessel Vasculitis (GEFA). Rev Med Interne 2016; 37(3):154–165. doi:10.1016/j.revmed.2015.12.015
- Hayreh SS, Biousse V. Treatment of acute visual loss in giant cell arteritis: should we prescribe high-dose intravenous steroids or just oral steroids? J Neuroophthalmol 2012; 32(3):278–287. doi:10.1097/WNO.0b013e3182688218
- Restuccia G, Boiardi L, Cavazza A, et al. Flares in biopsy-proven giant cell arteritis in Northern Italy: characteristics and predictors in a long-term follow-up study. Medicine (Baltimore) 2016; 95(19):e3524. doi:10.1097/MD.0000000000003524
- Kermani TA, Warrington KJ, Cuthbertson D, et al; Vasculitis Clinical Research Consortium. Disease relapses among patients with giant cell arteritis: a prospective, longitudinal cohort study. J Rheumatol 2015; 42(7):1213–1217. doi:10.3899/jrheum.141347
- Labarca C, Koster MJ, Crowson CS, et al. Predictors of relapse and treatment outcomes in biopsy-proven giant cell arteritis: a retrospective cohort study. Rheumatology (Oxford) 2016; 55(2):347–356. doi:10.1093/rheumatology/kev348
- Proven A, Gabriel SE, Orces C, O’Fallon WM, Hunder GG. Glucocorticoid therapy in giant cell arteritis: duration and adverse outcomes. Arthritis Rheum 2003; 49(5):703–708. doi:10.1002/art.11388
- Sepkowitz KA. Opportunistic infections in patients with and patients without acquired immunodeficiency syndrome. Clin Infect Dis 2002; 34(8):1098–1107. doi:10.1086/339548
- van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int 2002; 13(10):777–787. doi:10.1007/s001980200108
- Heffernan MP, Saag KG, Robinson JK, Callen JP. Prevention of osteoporosis associated with chronic glucocorticoid therapy. JAMA 2006; 295(11):1300–1303. pmid:16541489
- Buckley L, Guyatt G, Fink HA, et al. 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken) 2017; 69(8):1095–1110. doi:10.1002/acr.23279
- Grossman JM, Gordon R, Ranganath VK, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res 201; 62(11):1515–1526. doi:10.1002/acr.20295
- Allen CS, Yeung JH, Vandermeer B, Homik J. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst Rev 2016; 10:CD001347. doi:10.1002/14651858.CD001347.pub2
- Carpinteri R, Porcelli T, Mejia C, et al. Glucocorticoid-induced osteoporosis and parathyroid hormone. J Endocrinol Invest 2010; 33(suppl 7):16–21. pmid:20938221
- Saag KG, Wagman RB, Geusens P, et al. Denosumab versus risedronate in glucocorticoid-induced osteoporosis: a multicentre, randomised, double-blind, active-controlled, double-dummy, non-inferiority study. Lancet Diabetes Endocrinol 2018; 6(6):445–454. doi:10.1016/S2213-8587(18)30075-5
- Hoffman GS, Cid MC, Hellmann DB, et al; International Network for the Study of Systemic Vasculitides. A multicenter, randomized, double-blind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis Rheum 2002; 46(5):1309–1318. doi:10.1002/art.10262
- Spiera RF, Mitnick HJ, Kupersmith M, et al. A prospective, double-blind, randomized, placebo controlled trial of methotrexate in the treatment of giant cell arteritis (GCA). Clin Exp Rheumatol 2001; 19(5):495–501. pmid:11579707
- Hoffman GS, Cid MC, Rendt-Zagar KE, et al; Infliximab-GCA Study Group. Infliximab for maintenance of glucocorticosteroid-induced remission of giant cell arteritis: a randomized trial. Ann Intern Med 2007; 146(9):621–630. pmid:17470830
- Langford CA, Cuthbertson D, Ytterberg SR, et al; Vasculitis Clinical Research Consortium. A randomized, double-blind trial of abatacept (CTLA-4Ig) for the treatment of giant cell arteritis. Arthritis Rheumatol 2017; 69(4):837–845. doi:10.1002/art.40044
- Villiger PM, Adler S, Kuchen S, et al. Tocilizumab for induction and maintenance of remission in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2016; 387(10031):1921–1927. doi:10.1016/S0140-6736(16)00560-2
- Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017; 377(4):317–328. doi:10.1056/NEJMoa1613849
- Oliveira F, Butendieck RR, Ginsburg WW, Parikh K, Abril A. Tocilizumab, an effective treatment for relapsing giant cell arteritis. Clin Exp Rheumatol 2014; 32(3 suppl 82):S76–S78. pmid:24854376
- Loricera J, Blanco R, Hernández JL, et al. Tocilizumab in giant cell arteritis: multicenter open-label study of 22 patients. Semin Arthritis Rheum 2015; 44(6):717–723. doi:10.1016/j.semarthrit.2014.12.005
- Tamaki H, Hajj-Ali RA. Tocilizumab for giant cell arteritis—a new giant step in an old disease. JAMA Neurol 2018; 75(2):145–146. doi:10.1001/jamaneurol.2017.3811
- Xie F, Yun H, Bernatsky S, Curtis JR. Risk for gastrointestinal perforation among rheumatoid arthritis patients receiving tofacitinib, tocilizumab, or other biologics. Arthritis Rheumatol 2016; 68(11):2612–2617. doi:10.1002/art.39761
Giant cell arteritis (GCA) is a systemic vasculitis involving medium-sized and large arteries, most commonly the temporal, ophthalmic, occipital, vertebral, posterior ciliary, and proximal vertebral arteries. Moreover, involvement of the ophthalmic artery and its branches results in loss of vision. GCA can also involve the aorta and its proximal branches, especially in the upper extremities.
GCA is the most common systemic vasculitis in adults. It occurs almost exclusively in patients over age 50 and affects women more than men. It is most frequent in populations of northern European ancestry, especially Scandinavian. In a retrospective cohort study in Norway, the average annual cumulative incidence rate of GCA was 16.7 per 100,000 people over age 50.1 Risk factors include older age, history of smoking, current smoking, early menopause, and, possibly, stress-related disorders.2
PATHOGENESIS IS NOT COMPLETELY UNDERSTOOD
The pathogenesis of GCA is not completely understood, but there is evidence of immune activation in the arterial wall leading to activation of macrophages and formation of multinucleated giant cells (which may not always be present in biopsies).
The most relevant cytokines in the ongoing pathogenesis are still being defined, but the presence of interferon gamma and interleukin 6 (IL-6) seem to be critical for the expression of the disease. The primary immunogenic triggers for the elaboration of these cytokines and the arteritis remain elusive.
A SPECTRUM OF PRESENTATIONS
The initial symptoms of GCA may be vague, such as malaise, fever, and night sweats, and are likely due to systemic inflammation. Features of vascular involvement include headache, scalp tenderness, and jaw claudication (cramping pain in the jaw while chewing).
A less common but serious feature associated with GCA is partial or complete vision loss affecting 1 or both eyes.3 Some patients suddenly go completely blind without any visual prodrome.
Overlapping GCA phenotypes exist, with a spectrum of presentations that include classic cranial arteritis, extracranial GCA (also called large-vessel GCA), and polymyalgia rheumatica.2
Cranial GCA, the best-characterized clinical presentation, causes symptoms such as headache or signs such as tenderness of the temporal artery. On examination, the temporal arteries may be tender or nodular, and the pulses may be felt above the zygomatic arch, above and in front of the tragus of the ear. About two-thirds of patients with cranial GCA present with new-onset headache, most often in the temporal area, but possibly anywhere throughout the head.
Visual disturbance, jaw claudication, and tongue pain are less common but, if present, increase the likelihood of this diagnosis.2
Large-vessel involvement in GCA is common and refers to involvement of the aorta and its proximal branches. Imaging methods used in diagnosing large-vessel GCA include color Doppler ultrasonography, computed tomography with angiography, magnetic resonance imaging with angiography, and positron emission tomography. In some centers, such imaging is performed in all patients diagnosed with GCA to survey for large-vessel involvement.
Depending on the imaging study, large-vessel involvement has been found in 30% to 80% of cases of GCA.4,5 It is often associated with nonspecific symptoms such as fever, weight loss, chills, and malaise, but it can also cause more specific symptoms such as unilateral extremity claudication. In contrast to patients with cranial GCA, patients with large-vessel GCA were younger at onset, less likely to have headaches, and more likely to have arm claudication at presentation.6 Aortitis of the ascending aorta can occur with a histopathologic pattern of GCA but without the clinical stigmata of GCA.
The finding of aortitis should prompt the clinician to question the patient about other symptoms of GCA and to order imaging of the whole vascular tree. Ultrasonography and biopsy of the temporal arteries can be considered. Whether idiopathic aortitis is part of the GCA spectrum remains to be seen.
Laboratory tests often show anemia, leukocytosis, and thrombocytosis. Acute-phase reactants such as C-reactive protein and the erythrocyte sedimentation rate are often elevated. The sedimentation rate often exceeds 50 mm/hour and sometimes 100 mm/hour.
In 2 retrospective studies, the number of patients with GCA whose sedimentation rate was less than 50 mm/hour ranged between 5% and 11%.7,8 However, a small percentage of patients with GCA have normal inflammatory markers. Therefore, if the suspicion for GCA is high, treatment should be started and biopsy pursued.9 In patients with paraproteinemia or other causes of a spuriously elevated or low erythrocyte sedimentation rate, C-reactive protein is a more reliable test.
Polymyalgia rheumatica is another rheumatologic condition that can occur independently or in conjunction with GCA. It is characterized by stiffness and pain in the proximal joints such as the hips and shoulders, typically worse in the morning and better with activity. Although the patient may subjectively feel weak, a close neurologic examination will reveal normal muscle strength.
Polymyalgia rheumatica is observed in 40% to 60% of patients with GCA at the time of diagnosis; 16% to 21% of patients with polymyalgia rheumatica may develop GCA, especially if untreated.2,10
Differential diagnosis
Other vasculitides (eg, Takayasu arteritis) can also present with unexplained fever, anemia, and constitutional symptoms.
Infection should be considered if fever is present. An infectious disease accompanied by fever, headache, and elevated inflammatory markers can mimic GCA.
Nonarteritic anterior ischemic optic neuropathy can present with sudden vision loss, prompting concern for underlying GCA. Risk factors include hypertension and diabetes mellitus; other features of GCA, including elevated inflammatory markers, are generally absent.
TEMPORAL ARTERY BIOPSY: THE GOLD STANDARD FOR DIAGNOSIS
Temporal artery biopsy remains the standard to confirm the diagnosis. However, because inflammation in the temporal arteries can affect some segments but not others, biopsy results on conventional hematoxylin and eosin staining can be falsely negative in patients with GCA. In one study,11 the mean sensitivity of unilateral temporal artery biopsy was 86.9%.
Typical positive histologic findings are inflammation with panarteritis, CD4-positive lymphocytes, macrophages, giant cells, and fragmentation of the internal elastic lamina.12
When GCA is suspected, treatment with glucocorticoids should be started immediately and biopsy performed as soon as possible. Delaying biopsy for 14 days or more may not affect the accuracy of biopsy study.13 Treatment should never be withheld while awaiting the results of biopsy study.
Biopsy is usually performed unilaterally, on the same side as the symptoms or abnormal findings on examination. Bilateral temporal artery biopsy is also performed and compared with unilateral biopsy; this approach increases the diagnostic yield by about 5%.14
IMAGING
In patients with suspected GCA, imaging is recommended early to complement the clinical criteria for the diagnosis of GCA.15 Positron emission tomography, computed tomography angiography, magnetic resonance angiography, or Doppler ultrasonography can reveal inflammation of the arteries in the proximal upper or lower limbs or the aorta.2
In patients with suspected cranial GCA, ultrasonography of the temporal and axillary arteries is recommended first. If ultrasonography is not available or is inconclusive, high-resolution magnetic resonance imaging of the cranial arteries can be used as an alternative. Computed tomography and positron emission tomography of the cranial arteries are not recommended.
In patients with suspected large-vessel GCA, ultrasonography, positron emission tomography, computed tomography, and magnetic resonance imaging may be used to screen for vessel wall inflammation, edema, and luminal narrowing in extracranial arteries. Ultrasonography is of limited value in assessing aortitis.
Color duplex ultrasonography can be applied to assess for vascular inflammation of the temporal or large arteries. The typical finding of the “halo” sign, a hypoechoic ring around the arterial lumen, represents the inflammation-induced thickening of the arterial wall. The “compression sign,” the persistence of the “halo” during compression of the vessel lumen by the ultrasound probe, has high specificity for the diagnosis.16
Ultrasonography of suspected GCA has yielded sensitivities of 55% to 100% and specificities of 78% to 100%. However, its sensitivity depends on the user’s level of expertise, so it should be done only in medical centers with a high number of GCA cases and with highly experienced sonographers. High-resolution magnetic resonance imaging is an alternative to ultrasonography and has shown similar sensitivity and specificity.3
TREATMENT WITH GLUCOCORTICOIDS
Glucocorticoids remain the standard for treatment of GCA. The therapeutic effect of glucocorticoids in GCA has been established by years of clinical experience, but has never been proven in a placebo-controlled trial. When started appropriately and expeditiously, glucocorticoids produce exquisite resolution of signs and symptoms and prevent the serious complication of vision loss. Rapid resolution of symptoms is so typical of GCA that if the patient’s symptoms persist more than a few days after starting a glucocorticoid, the diagnosis of GCA should be reconsidered.
In a retrospective study of 245 patients with biopsy-proven GCA treated with glucocorticoids, 34 had permanent loss of sight.17 In 32 (94%) of the 34, the vision loss occurred before glucocorticoids were started. Of the remaining 2 patients, 1 lost vision 8 days into treatment, and the other lost vision 3 years after diagnosis and 1 year after discontinuation of glucocorticoids.
In a series of 144 patients with biopsy-proven GCA, 51 had no vision loss at presentation and no vision loss after starting glucocorticoids, and 93 had vision loss at presentation. In the latter group, symptoms worsened within 5 days of starting glucocorticoids in 9 patients.18 If vision was intact at the time of presentation, prompt initiation of glucocorticoids reduced the risk of vision loss to less than 1%.
High doses, slowly tapered
The European League Against Rheumatism recommends early initiation of high-dose glucocorticoids for patients with large-vessel vasculitis,19 and it also recommends glucocorticoids for patients with polymyalgia rheumatica.20 The optimal initial and tapering dosage has never been formally evaluated, but regimens have been devised on the basis of expert opinion.21
For patients with GCA who do not have vision loss at the time of diagnosis, the initial dose is prednisone 1 mg/kg or its equivalent daily for 2 to 4 weeks, after which it is tapered.21 If the initial dosage is prednisone 60 mg orally daily for 2 to 4 weeks, our practice is to taper it to 50 mg daily for 2 weeks, then 40 mg daily for 2 weeks. Then, it is decreased by 5 mg every 2 weeks until it is 20 mg daily, and then by 2.5 mg every 2 weeks until it is 10 mg orally daily. Thereafter, the dosage is decreased by 1 mg every 2 to 4 weeks.
For patients with GCA who experience transient vision loss or diplopia at the time of diagnosis, intravenous pulse glucocorticoid therapy should be initiated to reduce the risk of vision loss as rapidly as possible.22 A typical pulse regimen is methylprednisolone 1 g intravenously daily for 3 days. Though not rigorously validated in studies, such an approach is used to avoid vision impairment due to GCA, which is rarely reversible.
RELAPSE OF DISEASE
Suspect a relapse of GCA if the patient’s initial symptoms recur, if inflammatory markers become elevated, or if classic symptoms of GCA or polymyalgia rheumatica occur. Elevations in inflammatory markers do not definitely indicate a flare of GCA, but they should trigger close monitoring of the patient’s symptoms.
Relapse is treated by increasing the glucocorticoid dosage as appropriate to the nature of the relapse. If vision is affected or the patient has symptoms of GCA, then increments of 30 to 60 mg of prednisone are warranted, whereas if the patient has symptoms of polymyalgia rheumatica, then increments of 5 to 10 mg of prednisone are usually used.
The incidence of relapses of GCA in multiple tertiary care centers has been reported to vary between 34% and 75%.23,24 Most relapses occur at prednisone dosages of less than 20 mg orally daily and within the first year after diagnosis. The most common symptoms are limb ischemia, jaw claudication, constitutional symptoms, headaches, and polymyalgia rheumatica. In a review of 286 patients,25 213 (74%) had at least 1 relapse. The first relapse occurred in the first year in 50%, by 2 years in 68%, and by 5 years in 79%.
ADVERSE EFFECTS OF GLUCOCORTICOIDS
In high doses, glucocorticoids have well-known adverse effects. In a population-based study of 120 patients, each patient treated with glucocorticoids experienced at least 1 adverse effect (cataract, fracture, infection, osteonecrosis, diabetes, hypertension, weight gain, capillary fragility, or hair loss).26 The effects were related to aging and cumulative dosage of prednisone but not to the initial dosage.
Glucocorticoids can affect many organs and systems:
- Eyes (cataracts, increased intraocular pressure, exophthalmos)
- Heart (premature atherosclerotic disease, hypertension, fluid retention, hyperlipidemia, arrhythmias)
- Gastrointestinal system (ulcer, gastrointestinal bleeding, gastritis, visceral perforation, hepatic steatosis, acute pancreatitis)
- Bone and muscle (osteopenia, osteoporosis, osteonecrosis, myopathy)
- Brain (mood disorder, psychosis, memory impairment)
- Endocrine system (hyperglycemia, hypothalamic-pituitary-adrenal axis suppression)
- Immune system (immunosuppression, leading to infection and leukocytosis).
Patients receiving a glucocorticoid dose equivalent to 20 mg or more of prednisone daily for 1 month or more who also have another cause of immunocompromise need prophylaxis against Pneumocystis jirovecii pneumonia.27 They should also receive appropriate immunizations before starting glucocorticoids. Live-virus vaccines should not be given to these patients until they have been off glucocorticoids for 1 month.
Glucocorticoids and bone loss
Glucocorticoids are associated with bone loss and fracture, which can occur within the first few months of use and with dosages as low as 2.5 to 7.5 mg orally daily.28 Therefore, glucocorticoid-induced bone loss has to be treated aggressively, particularly in patients who are older and have a history of fragility fracture.
For patients with GCA who need glucocorticoids in doses greater than 5 mg orally daily for more than 3 months, the following measures are advised to decrease the risk of bone loss:
- Weight-bearing exercise
- Smoking cessation
- Moderation in alcohol intake
- Measures to prevent falls29
- Supplementation with 1,200 mg of calcium and 800 IU of vitamin D.30
Pharmacologic therapy should be initiated in men over age 50 who have established osteoporosis and in postmenopausal women with established osteoporosis or osteopenia. For men over age 50 with established osteopenia, risk assessment with the glucocorticoid-corrected FRAX score (www.sheffield.ac.uk/FRAX) should be performed to identify those at high risk in whom pharmacologic therapy is warranted.31
Bisphosphonates are the first-line therapy for glucocorticoid-induced osteoporosis.32
Teriparatide is the second-line therapy and is used in patients who cannot tolerate bisphosphonates or other osteoporosis therapies, and in those who have severe osteoporosis, with T scores of –3.5 and below if they have not had a fracture, and –2.5 and below if they have had a fragility fracture.33
Denosumab, a monoclonal antibody to an osteoclast differentiating factor, may be beneficial for some patients with glucocorticoid-induced osteoporosis.34
To assess the efficacy of therapy, measuring bone mineral density at baseline and at 1 year of therapy is recommended. If density is stable or improved, then repeating the measurement at 2- to 3-year intervals is suggested.
TOCILIZUMAB: A STEROID-SPARING MEDICATION
Due to the adverse effects of long-term use of glucocorticoids and high rates of relapse, there is a pressing need for medications that are more efficacious and less toxic to treat GCA.
The European League Against Rheumatism, in its 2009 management guidelines for large-vessel vasculitis, recommend using an adjunctive immunosuppressant agent.19 In the case of GCA, they recommend using methotrexate 10 to 15 mg/week, which has shown modest evidence of reducing the relapse rate and lowering the cumulative doses of glucocorticoids needed.35,36
Studies of tumor necrosis factor inhibitors and abatacept have not yielded significant reductions in the relapse rate or decreased cumulative doses of prednisone.37,38
Advances in treatment for GCA have stagnated, but recent trials39,40 have evaluated the IL-6 receptor alpha inhibitor tocilizumab, given the central role of IL-6 in the pathogenesis of GCA. Case reports have revealed rapid induction and maintenance of remission in GCA using tocilizumab.41,42
Villiger et al39 performed a randomized, placebo-controlled trial to study the efficacy and safety of tocilizumab in induction and maintenance of disease remission in 30 patients with newly diagnosed GCA. The primary outcome, complete remission at 12 weeks, was achieved in 85% of patients who received tocilizumab plus tapered prednisolone, compared with 40% of patients who received placebo plus tapering prednisolone. The tocilizumab group also had favorable results in secondary outcomes including relapse-free survival at 52 weeks, time to first relapse after induction of remission, and cumulative dose of prednisolone.
The GiACTA trial. Stone et al40 studied the effect of tocilizumab on rates of relapse during glucocorticoid tapering in 251 GCA patients over the course of 52 weeks. Patients were randomized in a 2:1:1:1 ratio to 4 treatment groups:
- Tocilizumab weekly plus prednisone, with prednisone tapered over 26 weeks
- Tocilizumab every other week plus prednisone tapered over 26 weeks
- Placebo plus prednisone tapered over 26 weeks
- Placebo plus prednisone tapered over 52 weeks.
The primary outcome was the rate of sustained glucocorticoid-free remission at 52 weeks. Secondary outcomes included the remission rate, the cumulative glucocorticoid dose, and safety measures. At 52 weeks, the rates of sustained remission were:
- 56% with tocilizumab weekly
- 53% with tocilizumab every other week
- 14% with placebo plus 26-week prednisone taper
- 18% with placebo plus 52-week taper.
Differences between the active treatment groups and the placebo groups were statistically significant (P < .001).
The cumulative dose of prednisone in tocilizumab recipients was significantly less than in placebo recipients. Rates of adverse events were similar. Ultimately, the study showed that tocilizumab, either weekly or every other week, was more effective than prednisone alone at sustaining glucocorticoid-free remission in patients with GCA.
However, the study also raised questions about tocilizumab’s toxic effect profile and its long-term efficacy, as well as who are the optimal candidates for this therapy. Data on long-term use of tocilizumab are primarily taken from its use in rheumatoid arthritis.43 As of this writing, Stone et al are conducting an open-label trial to help provide long-term safety and efficacy data in patients with GCA. In the meantime, we must extrapolate data from the long-term use of tocilizumab in rheumatoid arthritis.
Tocilizumab and lower gastrointestinal tract perforation
One of the major adverse effects of long-term use of tocilizumab is lower gastrointestinal tract perforation.
Xie et al,44 in 2016, reported that the risk of perforation in patients on tocilizumab for rheumatoid arthritis was more than 2 times higher than in patients taking a tumor necrosis factor inhibitor. However, the absolute rates of perforation were low overall, roughly 1 to 3 per 1,000 patient-years in the tocilizumab group. Risk factors for perforation included older age, history of diverticulitis or other gastrointestinal tract condition, and prednisone doses of 7.5 mg or more a day.
Does tocilizumab prevent blindness?
Another consideration is that tocilizumab may not prevent optic neuropathy. In the GiACTA trial, 1 patient in the group receiving tocilizumab every other week developed optic neuropathy.40 Prednisone had been completely tapered off at the time, and the condition resolved when glucocorticoids were restarted. Thus, it is unknown if tocilizumab would be effective on its own without concomitant use of glucocorticoids.
Vision loss is one of the most severe complications of GCA, and it is still unclear whether tocilizumab can prevent vision loss in GCA. Also, we still have no data on the effect of tocilizumab on histopathologic findings, and whether biopsy yield diminishes over time. We hope future studies will help guide us in this regard.
No guidelines on tocilizumab yet
Clinical guidelines on the appropriate use of tocilizumab in GCA are lacking. The American College of Rheumatology and the European League Against Rheumatism have yet to publish updated guidelines with comments on use of tocilizumab. Therefore, it is unclear if tocilizumab is a first-line treatment in GCA, as its efficacy alone without glucocorticoids and its long-term safety in GCA patients have not been studied.
Treatment with tocilizumab should be individualized; it should be considered in patients who have had adverse effects from glucocorticoids, and in patients who experience a flare or cannot have their glucocorticoid dose lowered to an appropriate range.
The optimal duration of tocilizumab therapy is also unknown. However, using the GiACTA study as a rough guide, we try to limit its use to 1 year until additional data are available.
Patients on IL-6 inhibition may have suppressed C-reactive protein regardless of disease activity.43 Therefore, this laboratory value may not be reliable in determining active disease in patients on tocilizumab.
The GiACTA trial has shown an impressive improvement in the relapse-free remission period in patients with GCA taking tocilizumab. However, much work needs to be done to define the safety of this medication and determine which patients should be started on it. In the meantime, we recommend starting high-dose glucocorticoid therapy as soon as the diagnosis of GCA is suspected. In patients who do not tolerate glucocorticoids or whose disease flares during glucocorticoid taper, we recommend starting treatment with tocilizumab either once a week or every other week for at least 1 year.
Giant cell arteritis (GCA) is a systemic vasculitis involving medium-sized and large arteries, most commonly the temporal, ophthalmic, occipital, vertebral, posterior ciliary, and proximal vertebral arteries. Moreover, involvement of the ophthalmic artery and its branches results in loss of vision. GCA can also involve the aorta and its proximal branches, especially in the upper extremities.
GCA is the most common systemic vasculitis in adults. It occurs almost exclusively in patients over age 50 and affects women more than men. It is most frequent in populations of northern European ancestry, especially Scandinavian. In a retrospective cohort study in Norway, the average annual cumulative incidence rate of GCA was 16.7 per 100,000 people over age 50.1 Risk factors include older age, history of smoking, current smoking, early menopause, and, possibly, stress-related disorders.2
PATHOGENESIS IS NOT COMPLETELY UNDERSTOOD
The pathogenesis of GCA is not completely understood, but there is evidence of immune activation in the arterial wall leading to activation of macrophages and formation of multinucleated giant cells (which may not always be present in biopsies).
The most relevant cytokines in the ongoing pathogenesis are still being defined, but the presence of interferon gamma and interleukin 6 (IL-6) seem to be critical for the expression of the disease. The primary immunogenic triggers for the elaboration of these cytokines and the arteritis remain elusive.
A SPECTRUM OF PRESENTATIONS
The initial symptoms of GCA may be vague, such as malaise, fever, and night sweats, and are likely due to systemic inflammation. Features of vascular involvement include headache, scalp tenderness, and jaw claudication (cramping pain in the jaw while chewing).
A less common but serious feature associated with GCA is partial or complete vision loss affecting 1 or both eyes.3 Some patients suddenly go completely blind without any visual prodrome.
Overlapping GCA phenotypes exist, with a spectrum of presentations that include classic cranial arteritis, extracranial GCA (also called large-vessel GCA), and polymyalgia rheumatica.2
Cranial GCA, the best-characterized clinical presentation, causes symptoms such as headache or signs such as tenderness of the temporal artery. On examination, the temporal arteries may be tender or nodular, and the pulses may be felt above the zygomatic arch, above and in front of the tragus of the ear. About two-thirds of patients with cranial GCA present with new-onset headache, most often in the temporal area, but possibly anywhere throughout the head.
Visual disturbance, jaw claudication, and tongue pain are less common but, if present, increase the likelihood of this diagnosis.2
Large-vessel involvement in GCA is common and refers to involvement of the aorta and its proximal branches. Imaging methods used in diagnosing large-vessel GCA include color Doppler ultrasonography, computed tomography with angiography, magnetic resonance imaging with angiography, and positron emission tomography. In some centers, such imaging is performed in all patients diagnosed with GCA to survey for large-vessel involvement.
Depending on the imaging study, large-vessel involvement has been found in 30% to 80% of cases of GCA.4,5 It is often associated with nonspecific symptoms such as fever, weight loss, chills, and malaise, but it can also cause more specific symptoms such as unilateral extremity claudication. In contrast to patients with cranial GCA, patients with large-vessel GCA were younger at onset, less likely to have headaches, and more likely to have arm claudication at presentation.6 Aortitis of the ascending aorta can occur with a histopathologic pattern of GCA but without the clinical stigmata of GCA.
The finding of aortitis should prompt the clinician to question the patient about other symptoms of GCA and to order imaging of the whole vascular tree. Ultrasonography and biopsy of the temporal arteries can be considered. Whether idiopathic aortitis is part of the GCA spectrum remains to be seen.
Laboratory tests often show anemia, leukocytosis, and thrombocytosis. Acute-phase reactants such as C-reactive protein and the erythrocyte sedimentation rate are often elevated. The sedimentation rate often exceeds 50 mm/hour and sometimes 100 mm/hour.
In 2 retrospective studies, the number of patients with GCA whose sedimentation rate was less than 50 mm/hour ranged between 5% and 11%.7,8 However, a small percentage of patients with GCA have normal inflammatory markers. Therefore, if the suspicion for GCA is high, treatment should be started and biopsy pursued.9 In patients with paraproteinemia or other causes of a spuriously elevated or low erythrocyte sedimentation rate, C-reactive protein is a more reliable test.
Polymyalgia rheumatica is another rheumatologic condition that can occur independently or in conjunction with GCA. It is characterized by stiffness and pain in the proximal joints such as the hips and shoulders, typically worse in the morning and better with activity. Although the patient may subjectively feel weak, a close neurologic examination will reveal normal muscle strength.
Polymyalgia rheumatica is observed in 40% to 60% of patients with GCA at the time of diagnosis; 16% to 21% of patients with polymyalgia rheumatica may develop GCA, especially if untreated.2,10
Differential diagnosis
Other vasculitides (eg, Takayasu arteritis) can also present with unexplained fever, anemia, and constitutional symptoms.
Infection should be considered if fever is present. An infectious disease accompanied by fever, headache, and elevated inflammatory markers can mimic GCA.
Nonarteritic anterior ischemic optic neuropathy can present with sudden vision loss, prompting concern for underlying GCA. Risk factors include hypertension and diabetes mellitus; other features of GCA, including elevated inflammatory markers, are generally absent.
TEMPORAL ARTERY BIOPSY: THE GOLD STANDARD FOR DIAGNOSIS
Temporal artery biopsy remains the standard to confirm the diagnosis. However, because inflammation in the temporal arteries can affect some segments but not others, biopsy results on conventional hematoxylin and eosin staining can be falsely negative in patients with GCA. In one study,11 the mean sensitivity of unilateral temporal artery biopsy was 86.9%.
Typical positive histologic findings are inflammation with panarteritis, CD4-positive lymphocytes, macrophages, giant cells, and fragmentation of the internal elastic lamina.12
When GCA is suspected, treatment with glucocorticoids should be started immediately and biopsy performed as soon as possible. Delaying biopsy for 14 days or more may not affect the accuracy of biopsy study.13 Treatment should never be withheld while awaiting the results of biopsy study.
Biopsy is usually performed unilaterally, on the same side as the symptoms or abnormal findings on examination. Bilateral temporal artery biopsy is also performed and compared with unilateral biopsy; this approach increases the diagnostic yield by about 5%.14
IMAGING
In patients with suspected GCA, imaging is recommended early to complement the clinical criteria for the diagnosis of GCA.15 Positron emission tomography, computed tomography angiography, magnetic resonance angiography, or Doppler ultrasonography can reveal inflammation of the arteries in the proximal upper or lower limbs or the aorta.2
In patients with suspected cranial GCA, ultrasonography of the temporal and axillary arteries is recommended first. If ultrasonography is not available or is inconclusive, high-resolution magnetic resonance imaging of the cranial arteries can be used as an alternative. Computed tomography and positron emission tomography of the cranial arteries are not recommended.
In patients with suspected large-vessel GCA, ultrasonography, positron emission tomography, computed tomography, and magnetic resonance imaging may be used to screen for vessel wall inflammation, edema, and luminal narrowing in extracranial arteries. Ultrasonography is of limited value in assessing aortitis.
Color duplex ultrasonography can be applied to assess for vascular inflammation of the temporal or large arteries. The typical finding of the “halo” sign, a hypoechoic ring around the arterial lumen, represents the inflammation-induced thickening of the arterial wall. The “compression sign,” the persistence of the “halo” during compression of the vessel lumen by the ultrasound probe, has high specificity for the diagnosis.16
Ultrasonography of suspected GCA has yielded sensitivities of 55% to 100% and specificities of 78% to 100%. However, its sensitivity depends on the user’s level of expertise, so it should be done only in medical centers with a high number of GCA cases and with highly experienced sonographers. High-resolution magnetic resonance imaging is an alternative to ultrasonography and has shown similar sensitivity and specificity.3
TREATMENT WITH GLUCOCORTICOIDS
Glucocorticoids remain the standard for treatment of GCA. The therapeutic effect of glucocorticoids in GCA has been established by years of clinical experience, but has never been proven in a placebo-controlled trial. When started appropriately and expeditiously, glucocorticoids produce exquisite resolution of signs and symptoms and prevent the serious complication of vision loss. Rapid resolution of symptoms is so typical of GCA that if the patient’s symptoms persist more than a few days after starting a glucocorticoid, the diagnosis of GCA should be reconsidered.
In a retrospective study of 245 patients with biopsy-proven GCA treated with glucocorticoids, 34 had permanent loss of sight.17 In 32 (94%) of the 34, the vision loss occurred before glucocorticoids were started. Of the remaining 2 patients, 1 lost vision 8 days into treatment, and the other lost vision 3 years after diagnosis and 1 year after discontinuation of glucocorticoids.
In a series of 144 patients with biopsy-proven GCA, 51 had no vision loss at presentation and no vision loss after starting glucocorticoids, and 93 had vision loss at presentation. In the latter group, symptoms worsened within 5 days of starting glucocorticoids in 9 patients.18 If vision was intact at the time of presentation, prompt initiation of glucocorticoids reduced the risk of vision loss to less than 1%.
High doses, slowly tapered
The European League Against Rheumatism recommends early initiation of high-dose glucocorticoids for patients with large-vessel vasculitis,19 and it also recommends glucocorticoids for patients with polymyalgia rheumatica.20 The optimal initial and tapering dosage has never been formally evaluated, but regimens have been devised on the basis of expert opinion.21
For patients with GCA who do not have vision loss at the time of diagnosis, the initial dose is prednisone 1 mg/kg or its equivalent daily for 2 to 4 weeks, after which it is tapered.21 If the initial dosage is prednisone 60 mg orally daily for 2 to 4 weeks, our practice is to taper it to 50 mg daily for 2 weeks, then 40 mg daily for 2 weeks. Then, it is decreased by 5 mg every 2 weeks until it is 20 mg daily, and then by 2.5 mg every 2 weeks until it is 10 mg orally daily. Thereafter, the dosage is decreased by 1 mg every 2 to 4 weeks.
For patients with GCA who experience transient vision loss or diplopia at the time of diagnosis, intravenous pulse glucocorticoid therapy should be initiated to reduce the risk of vision loss as rapidly as possible.22 A typical pulse regimen is methylprednisolone 1 g intravenously daily for 3 days. Though not rigorously validated in studies, such an approach is used to avoid vision impairment due to GCA, which is rarely reversible.
RELAPSE OF DISEASE
Suspect a relapse of GCA if the patient’s initial symptoms recur, if inflammatory markers become elevated, or if classic symptoms of GCA or polymyalgia rheumatica occur. Elevations in inflammatory markers do not definitely indicate a flare of GCA, but they should trigger close monitoring of the patient’s symptoms.
Relapse is treated by increasing the glucocorticoid dosage as appropriate to the nature of the relapse. If vision is affected or the patient has symptoms of GCA, then increments of 30 to 60 mg of prednisone are warranted, whereas if the patient has symptoms of polymyalgia rheumatica, then increments of 5 to 10 mg of prednisone are usually used.
The incidence of relapses of GCA in multiple tertiary care centers has been reported to vary between 34% and 75%.23,24 Most relapses occur at prednisone dosages of less than 20 mg orally daily and within the first year after diagnosis. The most common symptoms are limb ischemia, jaw claudication, constitutional symptoms, headaches, and polymyalgia rheumatica. In a review of 286 patients,25 213 (74%) had at least 1 relapse. The first relapse occurred in the first year in 50%, by 2 years in 68%, and by 5 years in 79%.
ADVERSE EFFECTS OF GLUCOCORTICOIDS
In high doses, glucocorticoids have well-known adverse effects. In a population-based study of 120 patients, each patient treated with glucocorticoids experienced at least 1 adverse effect (cataract, fracture, infection, osteonecrosis, diabetes, hypertension, weight gain, capillary fragility, or hair loss).26 The effects were related to aging and cumulative dosage of prednisone but not to the initial dosage.
Glucocorticoids can affect many organs and systems:
- Eyes (cataracts, increased intraocular pressure, exophthalmos)
- Heart (premature atherosclerotic disease, hypertension, fluid retention, hyperlipidemia, arrhythmias)
- Gastrointestinal system (ulcer, gastrointestinal bleeding, gastritis, visceral perforation, hepatic steatosis, acute pancreatitis)
- Bone and muscle (osteopenia, osteoporosis, osteonecrosis, myopathy)
- Brain (mood disorder, psychosis, memory impairment)
- Endocrine system (hyperglycemia, hypothalamic-pituitary-adrenal axis suppression)
- Immune system (immunosuppression, leading to infection and leukocytosis).
Patients receiving a glucocorticoid dose equivalent to 20 mg or more of prednisone daily for 1 month or more who also have another cause of immunocompromise need prophylaxis against Pneumocystis jirovecii pneumonia.27 They should also receive appropriate immunizations before starting glucocorticoids. Live-virus vaccines should not be given to these patients until they have been off glucocorticoids for 1 month.
Glucocorticoids and bone loss
Glucocorticoids are associated with bone loss and fracture, which can occur within the first few months of use and with dosages as low as 2.5 to 7.5 mg orally daily.28 Therefore, glucocorticoid-induced bone loss has to be treated aggressively, particularly in patients who are older and have a history of fragility fracture.
For patients with GCA who need glucocorticoids in doses greater than 5 mg orally daily for more than 3 months, the following measures are advised to decrease the risk of bone loss:
- Weight-bearing exercise
- Smoking cessation
- Moderation in alcohol intake
- Measures to prevent falls29
- Supplementation with 1,200 mg of calcium and 800 IU of vitamin D.30
Pharmacologic therapy should be initiated in men over age 50 who have established osteoporosis and in postmenopausal women with established osteoporosis or osteopenia. For men over age 50 with established osteopenia, risk assessment with the glucocorticoid-corrected FRAX score (www.sheffield.ac.uk/FRAX) should be performed to identify those at high risk in whom pharmacologic therapy is warranted.31
Bisphosphonates are the first-line therapy for glucocorticoid-induced osteoporosis.32
Teriparatide is the second-line therapy and is used in patients who cannot tolerate bisphosphonates or other osteoporosis therapies, and in those who have severe osteoporosis, with T scores of –3.5 and below if they have not had a fracture, and –2.5 and below if they have had a fragility fracture.33
Denosumab, a monoclonal antibody to an osteoclast differentiating factor, may be beneficial for some patients with glucocorticoid-induced osteoporosis.34
To assess the efficacy of therapy, measuring bone mineral density at baseline and at 1 year of therapy is recommended. If density is stable or improved, then repeating the measurement at 2- to 3-year intervals is suggested.
TOCILIZUMAB: A STEROID-SPARING MEDICATION
Due to the adverse effects of long-term use of glucocorticoids and high rates of relapse, there is a pressing need for medications that are more efficacious and less toxic to treat GCA.
The European League Against Rheumatism, in its 2009 management guidelines for large-vessel vasculitis, recommend using an adjunctive immunosuppressant agent.19 In the case of GCA, they recommend using methotrexate 10 to 15 mg/week, which has shown modest evidence of reducing the relapse rate and lowering the cumulative doses of glucocorticoids needed.35,36
Studies of tumor necrosis factor inhibitors and abatacept have not yielded significant reductions in the relapse rate or decreased cumulative doses of prednisone.37,38
Advances in treatment for GCA have stagnated, but recent trials39,40 have evaluated the IL-6 receptor alpha inhibitor tocilizumab, given the central role of IL-6 in the pathogenesis of GCA. Case reports have revealed rapid induction and maintenance of remission in GCA using tocilizumab.41,42
Villiger et al39 performed a randomized, placebo-controlled trial to study the efficacy and safety of tocilizumab in induction and maintenance of disease remission in 30 patients with newly diagnosed GCA. The primary outcome, complete remission at 12 weeks, was achieved in 85% of patients who received tocilizumab plus tapered prednisolone, compared with 40% of patients who received placebo plus tapering prednisolone. The tocilizumab group also had favorable results in secondary outcomes including relapse-free survival at 52 weeks, time to first relapse after induction of remission, and cumulative dose of prednisolone.
The GiACTA trial. Stone et al40 studied the effect of tocilizumab on rates of relapse during glucocorticoid tapering in 251 GCA patients over the course of 52 weeks. Patients were randomized in a 2:1:1:1 ratio to 4 treatment groups:
- Tocilizumab weekly plus prednisone, with prednisone tapered over 26 weeks
- Tocilizumab every other week plus prednisone tapered over 26 weeks
- Placebo plus prednisone tapered over 26 weeks
- Placebo plus prednisone tapered over 52 weeks.
The primary outcome was the rate of sustained glucocorticoid-free remission at 52 weeks. Secondary outcomes included the remission rate, the cumulative glucocorticoid dose, and safety measures. At 52 weeks, the rates of sustained remission were:
- 56% with tocilizumab weekly
- 53% with tocilizumab every other week
- 14% with placebo plus 26-week prednisone taper
- 18% with placebo plus 52-week taper.
Differences between the active treatment groups and the placebo groups were statistically significant (P < .001).
The cumulative dose of prednisone in tocilizumab recipients was significantly less than in placebo recipients. Rates of adverse events were similar. Ultimately, the study showed that tocilizumab, either weekly or every other week, was more effective than prednisone alone at sustaining glucocorticoid-free remission in patients with GCA.
However, the study also raised questions about tocilizumab’s toxic effect profile and its long-term efficacy, as well as who are the optimal candidates for this therapy. Data on long-term use of tocilizumab are primarily taken from its use in rheumatoid arthritis.43 As of this writing, Stone et al are conducting an open-label trial to help provide long-term safety and efficacy data in patients with GCA. In the meantime, we must extrapolate data from the long-term use of tocilizumab in rheumatoid arthritis.
Tocilizumab and lower gastrointestinal tract perforation
One of the major adverse effects of long-term use of tocilizumab is lower gastrointestinal tract perforation.
Xie et al,44 in 2016, reported that the risk of perforation in patients on tocilizumab for rheumatoid arthritis was more than 2 times higher than in patients taking a tumor necrosis factor inhibitor. However, the absolute rates of perforation were low overall, roughly 1 to 3 per 1,000 patient-years in the tocilizumab group. Risk factors for perforation included older age, history of diverticulitis or other gastrointestinal tract condition, and prednisone doses of 7.5 mg or more a day.
Does tocilizumab prevent blindness?
Another consideration is that tocilizumab may not prevent optic neuropathy. In the GiACTA trial, 1 patient in the group receiving tocilizumab every other week developed optic neuropathy.40 Prednisone had been completely tapered off at the time, and the condition resolved when glucocorticoids were restarted. Thus, it is unknown if tocilizumab would be effective on its own without concomitant use of glucocorticoids.
Vision loss is one of the most severe complications of GCA, and it is still unclear whether tocilizumab can prevent vision loss in GCA. Also, we still have no data on the effect of tocilizumab on histopathologic findings, and whether biopsy yield diminishes over time. We hope future studies will help guide us in this regard.
No guidelines on tocilizumab yet
Clinical guidelines on the appropriate use of tocilizumab in GCA are lacking. The American College of Rheumatology and the European League Against Rheumatism have yet to publish updated guidelines with comments on use of tocilizumab. Therefore, it is unclear if tocilizumab is a first-line treatment in GCA, as its efficacy alone without glucocorticoids and its long-term safety in GCA patients have not been studied.
Treatment with tocilizumab should be individualized; it should be considered in patients who have had adverse effects from glucocorticoids, and in patients who experience a flare or cannot have their glucocorticoid dose lowered to an appropriate range.
The optimal duration of tocilizumab therapy is also unknown. However, using the GiACTA study as a rough guide, we try to limit its use to 1 year until additional data are available.
Patients on IL-6 inhibition may have suppressed C-reactive protein regardless of disease activity.43 Therefore, this laboratory value may not be reliable in determining active disease in patients on tocilizumab.
The GiACTA trial has shown an impressive improvement in the relapse-free remission period in patients with GCA taking tocilizumab. However, much work needs to be done to define the safety of this medication and determine which patients should be started on it. In the meantime, we recommend starting high-dose glucocorticoid therapy as soon as the diagnosis of GCA is suspected. In patients who do not tolerate glucocorticoids or whose disease flares during glucocorticoid taper, we recommend starting treatment with tocilizumab either once a week or every other week for at least 1 year.
- Brekke LK, Diamantopoulos AP, Fevang BT, Aßmus J, Esperø E, Gjesdal CG. Incidence of giant cell arteritis in Western Norway 1972–2012: a retrospective cohort study. Arthritis Res Ther 2017; 19(1):278. doi:10.1186/s13075-017-1479-6
- Dejaco C, Duftner C, Buttgereit F, Matteson EL, Dasgupta B. The spectrum of giant cell arteritis and polymyalgia rheumatica: revisiting the concept of the disease. Rheumatology (Oxford) 2017; 56(4):506–515. doi:10.1093/rheumatology/kew273
- Weyand CM, Goronzy JJ. Giant-cell arteritis and polymyalgia rheumatica. N Engl J Med 2014; 371(17):1653. doi:10.1056/NEJMc1409206
- Ghinoi A, Pipitone N, Nicolini A, et al. Large-vessel involvement in recent-onset giant cell arteritis: a case-control colour-Doppler sonography study. Rheumatology (Oxford) 2012; 51(4):730–734. doi:10.1093/rheumatology/ker329
- Prieto-González S, Depetris M, García-Martínez A, et al. Positron emission tomography assessment of large vessel inflammation in patients with newly diagnosed, biopsy-proven giant cell arteritis: a prospective, case-control study. Ann Rheum Dis 2014; 73(7):1388–1392. doi:10.1136/annrheumdis-2013-204572
- Brack A, Martinez-Taboada V, Stanson A, Goronzy JJ, Weyand CM. Disease pattern in cranial and large-vessel giant cell arteritis. Arthritis Rheum 1999; 42(2):311–317. doi:10.1002/1529-0131(199902)42:2<311::AID-ANR14>3.0.CO;2-F
- Salvarani C, Hunder GG. Giant cell arteritis with low erythrocyte sedimentation rate: frequency of occurence in a population-based study. Arthritis Rheum 2001; 45(2):140–145. doi:10.1002/1529-0131(200104)45:2<140::AID-ANR166>3.0.CO;2-2
- Liozon E, Jauberteau-Marchan MO, Ly K, Loustaud-Ratti V, Soria P, Vidal E. Giant cell arteritis with a low erythrocyte sedimentation rate: comments on the article by Salvarani and Hunder. Arthritis Rheum 2002; 47(6):692–694. doi:10.1002/art.10809
- Yu-Wai-Man P, Dayan MR. Giant cell arteritis with normal inflammatory markers. Acta Ophthalmol Scand 2007; 85(4):460. doi:10.1111/j.1600-0420.2006.00864.x
- Buttgereit F, Dejaco C, Matteson EL, Dasgupta B. Polymyalgia rheumatica and giant cell arteritis: a systematic review. JAMA 2016; 315(22):2442–2458. doi:10.1001/jama.2016.5444
- Niederkohr RD, Levin LA. Management of the patient with suspected temporal arteritis a decision-analytic approach. Ophthalmology 2005; 112(5):744–756. doi:10.1016/j.ophtha.2005.01.031
- Bowling K, Rait J, Atkinson J, Srinivas G. Temporal artery biopsy in the diagnosis of giant cell arteritis: does the end justify the means? Ann Med Surg (Lond) 2017; 20:1–5. doi:10.1016/j.amsu.2017.06.020
- Daily B, Dassow P, Haynes J, Nashelsky J. Giant cell arteritis: biopsy after corticosteroid initiation. Am Fam Physician 2017; 95(2):116–117. pmid:28084703
- Durling B, Toren A, Patel V, Gilberg S, Weis E, Jordan D. Incidence of discordant temporal artery biopsy in the diagnosis of giant cell arteritis. Can J Ophthalmol 2014; 49(2):157–161. doi:10.1016/j.jcjo.2013.12.008
- Dejaco C, Ramiro S, Duftner C, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis 2018; 77(5):636–643. doi:10.1136/annrheumdis-2017-212649
- Aschwanden M, Imfeld S, Staub D, et al. The ultrasound compression sign to diagnose temporal giant cell arteritis shows an excellent interobserver agreement. Clin Exp Rheumatol 2015; 33(2 suppl 89):S-113–S-115. pmid:26016760
- Aiello PD, Trautmann JC, McPhee TJ, Kunselman AR, Hunder GG. Visual prognosis in giant cell arteritis. Ophthalmology 1993; 100(4):550–555. pmid:8479714
- Hayreh SS, Zimmerman B. Visual deterioration in giant cell arteritis patients while on high doses of corticosteroid therapy. Ophthalmology 2003; 110(6):1204–1215. doi:10.1016/S0161-6420(03)00228-8
- Mukhtyar C, Guillevin L, Cid MC, et al; European Vasculitis Study Group. EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis 2009; 68(3):318–323. doi:10.1136/ard.2008.088351
- Dejaco C, Singh YP, Perel P, et al; European League Against Rheumatism; American College of Rheumatology. 2015 recommendations for the management of polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann Rheum Dis 2015; 74(10):1799–1807. doi:10.1136/annrheumdis-2015-207492
- Bienvenu B, Ly KH, Lambert M, et al; Groupe d’Étude Français des Artérites des gros Vaisseaux, under the Aegis of the Filière des Maladies Auto-Immunes et Auto-Inflammatoires Rares. Management of giant cell arteritis: recommendations of the French Study Group for Large Vessel Vasculitis (GEFA). Rev Med Interne 2016; 37(3):154–165. doi:10.1016/j.revmed.2015.12.015
- Hayreh SS, Biousse V. Treatment of acute visual loss in giant cell arteritis: should we prescribe high-dose intravenous steroids or just oral steroids? J Neuroophthalmol 2012; 32(3):278–287. doi:10.1097/WNO.0b013e3182688218
- Restuccia G, Boiardi L, Cavazza A, et al. Flares in biopsy-proven giant cell arteritis in Northern Italy: characteristics and predictors in a long-term follow-up study. Medicine (Baltimore) 2016; 95(19):e3524. doi:10.1097/MD.0000000000003524
- Kermani TA, Warrington KJ, Cuthbertson D, et al; Vasculitis Clinical Research Consortium. Disease relapses among patients with giant cell arteritis: a prospective, longitudinal cohort study. J Rheumatol 2015; 42(7):1213–1217. doi:10.3899/jrheum.141347
- Labarca C, Koster MJ, Crowson CS, et al. Predictors of relapse and treatment outcomes in biopsy-proven giant cell arteritis: a retrospective cohort study. Rheumatology (Oxford) 2016; 55(2):347–356. doi:10.1093/rheumatology/kev348
- Proven A, Gabriel SE, Orces C, O’Fallon WM, Hunder GG. Glucocorticoid therapy in giant cell arteritis: duration and adverse outcomes. Arthritis Rheum 2003; 49(5):703–708. doi:10.1002/art.11388
- Sepkowitz KA. Opportunistic infections in patients with and patients without acquired immunodeficiency syndrome. Clin Infect Dis 2002; 34(8):1098–1107. doi:10.1086/339548
- van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int 2002; 13(10):777–787. doi:10.1007/s001980200108
- Heffernan MP, Saag KG, Robinson JK, Callen JP. Prevention of osteoporosis associated with chronic glucocorticoid therapy. JAMA 2006; 295(11):1300–1303. pmid:16541489
- Buckley L, Guyatt G, Fink HA, et al. 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken) 2017; 69(8):1095–1110. doi:10.1002/acr.23279
- Grossman JM, Gordon R, Ranganath VK, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res 201; 62(11):1515–1526. doi:10.1002/acr.20295
- Allen CS, Yeung JH, Vandermeer B, Homik J. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst Rev 2016; 10:CD001347. doi:10.1002/14651858.CD001347.pub2
- Carpinteri R, Porcelli T, Mejia C, et al. Glucocorticoid-induced osteoporosis and parathyroid hormone. J Endocrinol Invest 2010; 33(suppl 7):16–21. pmid:20938221
- Saag KG, Wagman RB, Geusens P, et al. Denosumab versus risedronate in glucocorticoid-induced osteoporosis: a multicentre, randomised, double-blind, active-controlled, double-dummy, non-inferiority study. Lancet Diabetes Endocrinol 2018; 6(6):445–454. doi:10.1016/S2213-8587(18)30075-5
- Hoffman GS, Cid MC, Hellmann DB, et al; International Network for the Study of Systemic Vasculitides. A multicenter, randomized, double-blind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis Rheum 2002; 46(5):1309–1318. doi:10.1002/art.10262
- Spiera RF, Mitnick HJ, Kupersmith M, et al. A prospective, double-blind, randomized, placebo controlled trial of methotrexate in the treatment of giant cell arteritis (GCA). Clin Exp Rheumatol 2001; 19(5):495–501. pmid:11579707
- Hoffman GS, Cid MC, Rendt-Zagar KE, et al; Infliximab-GCA Study Group. Infliximab for maintenance of glucocorticosteroid-induced remission of giant cell arteritis: a randomized trial. Ann Intern Med 2007; 146(9):621–630. pmid:17470830
- Langford CA, Cuthbertson D, Ytterberg SR, et al; Vasculitis Clinical Research Consortium. A randomized, double-blind trial of abatacept (CTLA-4Ig) for the treatment of giant cell arteritis. Arthritis Rheumatol 2017; 69(4):837–845. doi:10.1002/art.40044
- Villiger PM, Adler S, Kuchen S, et al. Tocilizumab for induction and maintenance of remission in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2016; 387(10031):1921–1927. doi:10.1016/S0140-6736(16)00560-2
- Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017; 377(4):317–328. doi:10.1056/NEJMoa1613849
- Oliveira F, Butendieck RR, Ginsburg WW, Parikh K, Abril A. Tocilizumab, an effective treatment for relapsing giant cell arteritis. Clin Exp Rheumatol 2014; 32(3 suppl 82):S76–S78. pmid:24854376
- Loricera J, Blanco R, Hernández JL, et al. Tocilizumab in giant cell arteritis: multicenter open-label study of 22 patients. Semin Arthritis Rheum 2015; 44(6):717–723. doi:10.1016/j.semarthrit.2014.12.005
- Tamaki H, Hajj-Ali RA. Tocilizumab for giant cell arteritis—a new giant step in an old disease. JAMA Neurol 2018; 75(2):145–146. doi:10.1001/jamaneurol.2017.3811
- Xie F, Yun H, Bernatsky S, Curtis JR. Risk for gastrointestinal perforation among rheumatoid arthritis patients receiving tofacitinib, tocilizumab, or other biologics. Arthritis Rheumatol 2016; 68(11):2612–2617. doi:10.1002/art.39761
- Brekke LK, Diamantopoulos AP, Fevang BT, Aßmus J, Esperø E, Gjesdal CG. Incidence of giant cell arteritis in Western Norway 1972–2012: a retrospective cohort study. Arthritis Res Ther 2017; 19(1):278. doi:10.1186/s13075-017-1479-6
- Dejaco C, Duftner C, Buttgereit F, Matteson EL, Dasgupta B. The spectrum of giant cell arteritis and polymyalgia rheumatica: revisiting the concept of the disease. Rheumatology (Oxford) 2017; 56(4):506–515. doi:10.1093/rheumatology/kew273
- Weyand CM, Goronzy JJ. Giant-cell arteritis and polymyalgia rheumatica. N Engl J Med 2014; 371(17):1653. doi:10.1056/NEJMc1409206
- Ghinoi A, Pipitone N, Nicolini A, et al. Large-vessel involvement in recent-onset giant cell arteritis: a case-control colour-Doppler sonography study. Rheumatology (Oxford) 2012; 51(4):730–734. doi:10.1093/rheumatology/ker329
- Prieto-González S, Depetris M, García-Martínez A, et al. Positron emission tomography assessment of large vessel inflammation in patients with newly diagnosed, biopsy-proven giant cell arteritis: a prospective, case-control study. Ann Rheum Dis 2014; 73(7):1388–1392. doi:10.1136/annrheumdis-2013-204572
- Brack A, Martinez-Taboada V, Stanson A, Goronzy JJ, Weyand CM. Disease pattern in cranial and large-vessel giant cell arteritis. Arthritis Rheum 1999; 42(2):311–317. doi:10.1002/1529-0131(199902)42:2<311::AID-ANR14>3.0.CO;2-F
- Salvarani C, Hunder GG. Giant cell arteritis with low erythrocyte sedimentation rate: frequency of occurence in a population-based study. Arthritis Rheum 2001; 45(2):140–145. doi:10.1002/1529-0131(200104)45:2<140::AID-ANR166>3.0.CO;2-2
- Liozon E, Jauberteau-Marchan MO, Ly K, Loustaud-Ratti V, Soria P, Vidal E. Giant cell arteritis with a low erythrocyte sedimentation rate: comments on the article by Salvarani and Hunder. Arthritis Rheum 2002; 47(6):692–694. doi:10.1002/art.10809
- Yu-Wai-Man P, Dayan MR. Giant cell arteritis with normal inflammatory markers. Acta Ophthalmol Scand 2007; 85(4):460. doi:10.1111/j.1600-0420.2006.00864.x
- Buttgereit F, Dejaco C, Matteson EL, Dasgupta B. Polymyalgia rheumatica and giant cell arteritis: a systematic review. JAMA 2016; 315(22):2442–2458. doi:10.1001/jama.2016.5444
- Niederkohr RD, Levin LA. Management of the patient with suspected temporal arteritis a decision-analytic approach. Ophthalmology 2005; 112(5):744–756. doi:10.1016/j.ophtha.2005.01.031
- Bowling K, Rait J, Atkinson J, Srinivas G. Temporal artery biopsy in the diagnosis of giant cell arteritis: does the end justify the means? Ann Med Surg (Lond) 2017; 20:1–5. doi:10.1016/j.amsu.2017.06.020
- Daily B, Dassow P, Haynes J, Nashelsky J. Giant cell arteritis: biopsy after corticosteroid initiation. Am Fam Physician 2017; 95(2):116–117. pmid:28084703
- Durling B, Toren A, Patel V, Gilberg S, Weis E, Jordan D. Incidence of discordant temporal artery biopsy in the diagnosis of giant cell arteritis. Can J Ophthalmol 2014; 49(2):157–161. doi:10.1016/j.jcjo.2013.12.008
- Dejaco C, Ramiro S, Duftner C, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis 2018; 77(5):636–643. doi:10.1136/annrheumdis-2017-212649
- Aschwanden M, Imfeld S, Staub D, et al. The ultrasound compression sign to diagnose temporal giant cell arteritis shows an excellent interobserver agreement. Clin Exp Rheumatol 2015; 33(2 suppl 89):S-113–S-115. pmid:26016760
- Aiello PD, Trautmann JC, McPhee TJ, Kunselman AR, Hunder GG. Visual prognosis in giant cell arteritis. Ophthalmology 1993; 100(4):550–555. pmid:8479714
- Hayreh SS, Zimmerman B. Visual deterioration in giant cell arteritis patients while on high doses of corticosteroid therapy. Ophthalmology 2003; 110(6):1204–1215. doi:10.1016/S0161-6420(03)00228-8
- Mukhtyar C, Guillevin L, Cid MC, et al; European Vasculitis Study Group. EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis 2009; 68(3):318–323. doi:10.1136/ard.2008.088351
- Dejaco C, Singh YP, Perel P, et al; European League Against Rheumatism; American College of Rheumatology. 2015 recommendations for the management of polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann Rheum Dis 2015; 74(10):1799–1807. doi:10.1136/annrheumdis-2015-207492
- Bienvenu B, Ly KH, Lambert M, et al; Groupe d’Étude Français des Artérites des gros Vaisseaux, under the Aegis of the Filière des Maladies Auto-Immunes et Auto-Inflammatoires Rares. Management of giant cell arteritis: recommendations of the French Study Group for Large Vessel Vasculitis (GEFA). Rev Med Interne 2016; 37(3):154–165. doi:10.1016/j.revmed.2015.12.015
- Hayreh SS, Biousse V. Treatment of acute visual loss in giant cell arteritis: should we prescribe high-dose intravenous steroids or just oral steroids? J Neuroophthalmol 2012; 32(3):278–287. doi:10.1097/WNO.0b013e3182688218
- Restuccia G, Boiardi L, Cavazza A, et al. Flares in biopsy-proven giant cell arteritis in Northern Italy: characteristics and predictors in a long-term follow-up study. Medicine (Baltimore) 2016; 95(19):e3524. doi:10.1097/MD.0000000000003524
- Kermani TA, Warrington KJ, Cuthbertson D, et al; Vasculitis Clinical Research Consortium. Disease relapses among patients with giant cell arteritis: a prospective, longitudinal cohort study. J Rheumatol 2015; 42(7):1213–1217. doi:10.3899/jrheum.141347
- Labarca C, Koster MJ, Crowson CS, et al. Predictors of relapse and treatment outcomes in biopsy-proven giant cell arteritis: a retrospective cohort study. Rheumatology (Oxford) 2016; 55(2):347–356. doi:10.1093/rheumatology/kev348
- Proven A, Gabriel SE, Orces C, O’Fallon WM, Hunder GG. Glucocorticoid therapy in giant cell arteritis: duration and adverse outcomes. Arthritis Rheum 2003; 49(5):703–708. doi:10.1002/art.11388
- Sepkowitz KA. Opportunistic infections in patients with and patients without acquired immunodeficiency syndrome. Clin Infect Dis 2002; 34(8):1098–1107. doi:10.1086/339548
- van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int 2002; 13(10):777–787. doi:10.1007/s001980200108
- Heffernan MP, Saag KG, Robinson JK, Callen JP. Prevention of osteoporosis associated with chronic glucocorticoid therapy. JAMA 2006; 295(11):1300–1303. pmid:16541489
- Buckley L, Guyatt G, Fink HA, et al. 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken) 2017; 69(8):1095–1110. doi:10.1002/acr.23279
- Grossman JM, Gordon R, Ranganath VK, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res 201; 62(11):1515–1526. doi:10.1002/acr.20295
- Allen CS, Yeung JH, Vandermeer B, Homik J. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst Rev 2016; 10:CD001347. doi:10.1002/14651858.CD001347.pub2
- Carpinteri R, Porcelli T, Mejia C, et al. Glucocorticoid-induced osteoporosis and parathyroid hormone. J Endocrinol Invest 2010; 33(suppl 7):16–21. pmid:20938221
- Saag KG, Wagman RB, Geusens P, et al. Denosumab versus risedronate in glucocorticoid-induced osteoporosis: a multicentre, randomised, double-blind, active-controlled, double-dummy, non-inferiority study. Lancet Diabetes Endocrinol 2018; 6(6):445–454. doi:10.1016/S2213-8587(18)30075-5
- Hoffman GS, Cid MC, Hellmann DB, et al; International Network for the Study of Systemic Vasculitides. A multicenter, randomized, double-blind, placebo-controlled trial of adjuvant methotrexate treatment for giant cell arteritis. Arthritis Rheum 2002; 46(5):1309–1318. doi:10.1002/art.10262
- Spiera RF, Mitnick HJ, Kupersmith M, et al. A prospective, double-blind, randomized, placebo controlled trial of methotrexate in the treatment of giant cell arteritis (GCA). Clin Exp Rheumatol 2001; 19(5):495–501. pmid:11579707
- Hoffman GS, Cid MC, Rendt-Zagar KE, et al; Infliximab-GCA Study Group. Infliximab for maintenance of glucocorticosteroid-induced remission of giant cell arteritis: a randomized trial. Ann Intern Med 2007; 146(9):621–630. pmid:17470830
- Langford CA, Cuthbertson D, Ytterberg SR, et al; Vasculitis Clinical Research Consortium. A randomized, double-blind trial of abatacept (CTLA-4Ig) for the treatment of giant cell arteritis. Arthritis Rheumatol 2017; 69(4):837–845. doi:10.1002/art.40044
- Villiger PM, Adler S, Kuchen S, et al. Tocilizumab for induction and maintenance of remission in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2016; 387(10031):1921–1927. doi:10.1016/S0140-6736(16)00560-2
- Stone JH, Tuckwell K, Dimonaco S, et al. Trial of tocilizumab in giant-cell arteritis. N Engl J Med 2017; 377(4):317–328. doi:10.1056/NEJMoa1613849
- Oliveira F, Butendieck RR, Ginsburg WW, Parikh K, Abril A. Tocilizumab, an effective treatment for relapsing giant cell arteritis. Clin Exp Rheumatol 2014; 32(3 suppl 82):S76–S78. pmid:24854376
- Loricera J, Blanco R, Hernández JL, et al. Tocilizumab in giant cell arteritis: multicenter open-label study of 22 patients. Semin Arthritis Rheum 2015; 44(6):717–723. doi:10.1016/j.semarthrit.2014.12.005
- Tamaki H, Hajj-Ali RA. Tocilizumab for giant cell arteritis—a new giant step in an old disease. JAMA Neurol 2018; 75(2):145–146. doi:10.1001/jamaneurol.2017.3811
- Xie F, Yun H, Bernatsky S, Curtis JR. Risk for gastrointestinal perforation among rheumatoid arthritis patients receiving tofacitinib, tocilizumab, or other biologics. Arthritis Rheumatol 2016; 68(11):2612–2617. doi:10.1002/art.39761
KEY POINTS
- Giant cell arteritis can present with cranial symptoms, extracranial large-vessel involvement, or polymyalgia rheumatica.
- Temporal artery biopsy is the standard for diagnosis.
- Adverse effects of glucocorticoid treatment, particularly bone loss, need to be managed.
- In patients treated with glucocorticoids alone, the relapse rate is high when the drugs are tapered; thus, prolonged treatment is required.
Infertility: A practical framework
For millions of couples, a primary care physician may be the first point of contact for fertility concerns. Statistics from the US Centers for Disease Control and Prevention indicate that 12% of women ages 15 to 44 received fertility services from 2006 to 2010.1 Despite seeking services, most couples requested only advice or testing rather than treatments such as ovulation-inducing medications, surgery, or, rarely, assisted reproductive technologies including in vitro fertilization. Based on these data, primary care physicians are in a unique position to offer guidance and provide fertility services in most circumstances without the need for referral.
This article reviews the answers to questions patients frequently ask, and outlines a practical framework for the evaluation and management of the infertile couple.
MANY PATIENTS SEEK INFORMATION
At least 1 million medical visits per year are for women seeking help in becoming pregnant, with the number increasing over the last several decades.1 Reasons for the increase include delayed childbearing and the effects of aging on the female reproductive system (“female reproductive aging”), as well as the availability of increasingly effective treatments for infertility.
While the prevalence of infertility in US couples is widely quoted as 10% to 15%,2 there is no estimate for the number of fertility-related questions patients routinely pose to care providers. These questions often relate to coital timing, use of lubricants, positioning, and the use of fertility trackers and ovulation predictors.
A 2017 study of women with 12 months of infertility found that only 8% sought subspecialist care vs care from a general physician or provider, indicating that generalists are most often the first point of contact.3 The majority (92%) of women responding to a survey regarding fertility-awareness education indicated a preference for immediate counseling from their general practitioner.4
Although some healthcare providers may consider infertility simply a quality-of-life issue, the World Health Organization classifies it as a disease, and as such it warrants identification, assessment, and intervention.5 Further, patients with infertility are known to experience considerable psychological distress related to their condition. In a comparison study, women with infertility experienced levels of psychological distress similar to the level in patients with cancer and patients with chronic medical illness.6
In the current era, general practitioners and women’s health specialists may also now address patients’ questions about reproductive aging and egg-freezing, which is now an established technology.7
FAILURE TO CONCEIVE AFTER 1 YEAR
As women approach age 40, the potential for fertility decreases rapidly and significantly. Women in their later 30s have only half the fertility of women in their early 20s.10 Misperceptions of aging and female fertility have been fueled by widely publicized celebrity births from women in their 40s and even 50s, without disclosing the use of frozen or donor eggs. This unfortunate fact affects women actively trying to conceive as well as women who wish to delay childbearing due to lack of a partner or for personal or professional reasons. Primary care physicians should be able to provide counseling relevant to female reproductive aging and make suitable and timely referrals for fertility preservation if indicated.
AN EMOTIONAL ISSUE
In approaching the couple with infertility, it is important to proceed with great sensitivity for the socioemotional context of this diagnosis. For both the male and female partner, infertility can be highly stigmatizing, and can be viewed as a personal or relationship failure.
Couples should be encouraged to ask embarrassing or uncomfortable questions. Although this may not be feasible in many circumstances, interviews should ideally be conducted with both partners individually as well as together, to allow sensitive issues to be shared. In some cases, a partner may be unaware of a history of a sexually transmitted infection, a prior abortion, the use of testosterone supplements or medications to enhance male sexual performance, or a vasectomy or tubal ligation during a previous relationship.
It is not unusual that the anxiety of infertility can cause decreased libido and sexual and erectile dysfunction. These issues can further complicate the problem of conceiving, and couples counseling is not uncommonly required.11 Patients are often reassured to know that they are not alone in their diagnosis.
LOOK FOR CLUES
Before embarking on a series of tests, the primary care physician can carefully evaluate for clues that may guide the diagnostic evaluation. The approach can be individualized based on the patient’s age, duration of subfertility (ie, how long they have been trying to become pregnant), and risk factors. But as a general rule, regardless of age, couples who have been trying to conceive for more than 1 year should be encouraged to pursue additional testing.
Because each month presents a new cycle of hope (often followed by intense disappointment), the prevailing sentiment to “just give it a little more time” must be countered by education and counseling. The primary care physician must increase awareness that lack of pregnancy in the stated time periods is a compelling reason for evaluation.
History-taking in the infertile couple should include a complete gynecologic and menstrual history. A history of sexually transmitted diseases that can cause tubal disease, such as gonorrhea and Chlamydia, is significant. Both partners should be assessed for a history of prior conceptions, past medical or surgical problems, medications, and exposures to environmental toxins including alcohol, tobacco, and drugs.
A detailed physical examination can provide clues to the cause of subfertility, especially if signs of obesity, androgen excess, or insulin resistance are present.
QUESTIONS OFTEN ASKED BY COUPLES TRYING TO CONCEIVE
Clinicians are frequently asked questions related to sexual practices and lifestyle in relation to fertility and should be comfortable responding to questions in these areas.
Does frequent ejaculation ‘use up’ my sperm?
Men should be reassured that frequent ejaculations do not decrease sperm counts; even daily ejaculation does not deplete the concentration of sperm. Male partners can be reassured that “saving up” is not an effective strategy; in fact, abstinence periods of greater than 5 days can adversely affect semen parameters.12
How often should we have sex?
Infrequent intercourse (< 1 time per week) reduces the monthly chance of conceiving.13 There does not seem to be a significant improvement in fecundity with daily intercourse vs intercourse on alternate days. Strict schedules surrounding intercourse may increase stress, and reassurance should be offered that intercourse need not be regimented. Every 1 to 2 days should suffice.
Are any sexual positions better for conception?
There is no evidence that particular coital positioning or remaining supine after intercourse improves fertility. Sperm can be found within the endocervix within seconds of ejaculation, irrespective of sexual position.
What is the window of fertility?
There is good evidence that the fertile window lasts approximately 6 days and closes after ovulation.13,14 Women with regular cycles can determine their typical day of ovulation based on menstrual tracking. Intercourse should begin about 6 days before ovulation and should continue every 1 to 2 days for 1 week to fully capture this window.
Should we change our lifestyle?
Couples seeking pregnancy should be advised to limit alcohol and caffeine use, completely abstain from cigarette smoking or illicit drug use, and maintain a healthy body mass index.
Very few data exist to support particular diets or supplements to promote fertility, including antioxidants and herbal remedies. Folic acid supplementation is recommended in all women attempting to conceive to reduce the incidence of birth defects.
Do lubricants reduce fertility?
Although there seem to be no differences in fecundity rates in couples using commercial lubricants, most water-based lubricants are best avoided in couples with infertility, as adverse effects on sperm have been demonstrated in vitro.15 If lubrication is needed, couples may try mineral oil, canola oil, or hydroxyetylcellulose-based lubricants (eg, Pre-seed).
Do fertility trackers work?
Many couples with primary infertility perceive that coital timing is critical and worry that their infertility is due to poorly timed intercourse; in fact, this is seldom the case.
Despite widespread marketing of urinary luteinizing hormone (LH) detection kits and electronic trackers and monitors, there is no clear evidence that these methods improve monthly rates of conception.
Women with a regular menstrual cycle should be encouraged to take notice when their cervical mucus appears clear and slippery (a sign of ovulation). Not all women are able to detect these fluctuations; however, for those who can, observing cervical mucus changes appears to be equivalent or superior to predictor kits in predicting conception.16
A PRACTICAL FRAMEWORK FOR EVALUATING THE INFERTILE COUPLE
To assess for the common factors identified in Table 1, the essential investigation of the infertile couple includes:
- Semen analysis
- Confirmation of ovulation
- Hysterosalpingography.
Consideration can also be given to ovarian reserve testing in women at risk of diminished ovarian reserve. The above investigation can be performed simultaneously to allow for prompt identification of any issues. Further, infertility is often a combination of problems (eg, anovulation in the woman together with a problem in the man), so an incomplete evaluation may overlook a coexisting diagnosis and lead to delays in treatment and pregnancy.
Tests that are no longer typically used in clinical practice are outlined in Table 2.
OVARIAN RESERVE TESTING AND FEMALE REPRODUCTIVE AGING
Ovarian reserve refers to the number of fertilizable oocytes that remain in the ovary. This reserve changes over time, and changes occur rapidly as women approach and enter their 30s. Though not the case in men, the age of the female partner is an independent risk factor for infertility. This discrepancy is due to loss of ovarian reserve, chromosome abnormalities in embryos, and the development of medical conditions with age that affect fertility.
Testing for ovarian reserve does not necessarily predict an overall inability to achieve a live birth,17 but it can predict response to exogenous gonadotropins and, to some degree, the chance for successful pregnancy with assisted reproductive technology.18
The ASRM states that testing for diminished ovarian reserve may provide useful information in women who have had a previous poor response to gonadotropins and in women planning assisted reproductive technology.19 The ASRM also indicates that the following are risk factors for diminished ovarian reserve, and clinicians may target the assessment accordingly19:
- Age 35 or older
- History of exposure to chemotherapy or pelvic radiation
- Family history of early menopause (age < 40)
- History of ovarian surgery
- Unexplained or idiopathic fertility.
Although several tests of ovarian reserve exist, either an antimullerian hormone (AMH) test or a combined cycle day-3 follicle-stimulating hormone (FSH) and estradiol level are the 2 tests commonly used in clinical practice. Antral follicle counts are an ultrasonographic measure used by infertility specialists but rarely by primary care physicians. Assays such as inhibin are rarely ordered and have limited clinical utility.
The AMH test
Many reproductive endocrinologists rely on the AMH level as a single test of ovarian reserve as it is easy to obtain, has a relatively low cost, and offers stable results. AMH is produced by the granulosa cells of the ovarian antral follicles and is readily detected in serum samples.
Conveniently for the clinician, levels of this hormone remain stable throughout the menstrual cycle and therefore can be tested on any day and at any time of day. Lower serum AMH levels (< 1 ng/mL) have been shown to correspond to diminished ovarian stimulation with gonadotropins as well as decreased embryo quality and poor pregnancy outcomes with assisted reproductive technology.19
Nevertheless, despite overall stability, AMH levels can be falsely lowered in women using exogenous hormones or with a diagnosis of hypogonadotropic hypogonadism. Levels may be higher than expected in women with polycystic ovary syndrome due to higher numbers of antral and preantral follicles in the polycystic ovary.
The day-3 follicle-stimulating hormone test
FSH and 17-beta estradiol testing can be ordered in combination to assess function of the hypothalamic-pituitary-ovarian axis on day 3 of the menstrual cycle. There is some flexibility, however, and testing obtained on cycle day 2, 3, or 4 yields equivalent results.
Although there are no strict cutoffs, FSH levels that appear elevated (> 10–20 IU/L) are associated with lower chances of conceiving with in vitro fertilization in multiple studies.20
The test is limited by levels that may fluctuate cycle to cycle, and reassuring test results do not necessarily indicate that a woman will achieve a pregnancy. Although a serum estradiol value alone is not a useful test, it can be used in combination with day-3 FSH to screen for diminished ovarian reserve.
As premature recruitment of a follicle can cause an early follicular rise in estradiol, FSH may be falsely suppressed on day 3. For example, a “normal” day-3 FSH combined with an elevated day-3 17-beta estradiol level of 60 to 80 pg/mL is associated with a poor response to medical treatments for infertility.
Female reproductive aging
Aging of the female reproductive system is a central threat to fertility, and prompt assessment and referral are warranted for women age 35 or older who have been trying to conceive for more than 6 months. The ASRM recommends that women over age 40 be evaluated immediately.21
A prevailing misconception is that regular menstrual cycles correspond with normal fertility. In reality, women lose their ability to achieve a healthy live birth in the 5 to 10 years preceding menopause. Although all women who do not desire pregnancy should still use appropriate contraception to avoid unintended pregnancy, women who do desire pregnancy should be aware of these physiologic changes.
Classic age-related changes in ovarian reserve are accompanied by a steep rise in aneuploidy and miscarriage risk.22 This is particularly relevant as women increasingly delay childbearing in modern society. Loss of fertility begins at 32 and abruptly accelerates at age 3721; this fact is poorly communicated to and understood by patients. In a 2018 study of highly educated women, most respondents failed to identify that 45-year-old women can only rarely achieve a successful pregnancy.23
In recent decades, the percentage of women who delay childbearing until after age 35 has steadily increased. There is a widespread misconception that fertility treatments and assisted reproductive technology can compensate for female reproductive aging. Primary care physicians can play a central role in reminding couples that age remains the single greatest predictor of natural fertility and the chance of success with assisted reproduction.
Further, for women who desire future fertility and are without a partner, primary care physicians can counsel them regarding the availability of donor insemination or egg freezing. Studies confirm that women want clinicians to initiate information on reproductive health, and 80% of women undergoing elective egg-freezing for fertility preservation wished that they had done so at an earlier age.24,25
FEMALE PERITONEAL AND STRUCTURAL CAUSES
Women with endometriosis, fibroids, or a history of tubal disease have impaired fecundity. Pelvic imaging is an essential component of their evaluation. Although hysterosalpingography is the mainstay of tubal assessment, in select cases ultrasonography or hysteroscopy may be indicated.
Tubal disease and hysterosalpingography
Tubal disease remains one of the most common causes of infertility in the US females. In most cases, tubal damage is secondary to pelvic inflammatory disease from infection with gonorrhea or Chlamydia, or both.
Rates of confirmed tubal-factor infertility have been shown to increase with both the severity of the infection and the number of past infections.26 In a landmark study, 1 episode of pelvic inflammatory disease was associated with a 12% risk of tubal-factor infertility, whereas 3 infections carried a risk as high as 54%. Pelvic inflammatory disease is also known to increase the risk of ectopic pregnancy.
To assess tubal patency, hysterosalpingography, a radiographic procedure, is typically performed using fluoroscopy and injected contrast material. Some centers may offer sonohysterography as a radiation-free alternative, depending on sonographic skill and experience. Both tests are best scheduled in the window between the end of menstrual bleeding and ovulation. In practice, patients with regular cycles can typically schedule hysterosalpingography between cycle days 5 and 12.
In patients with known hydrosalpinx (a distended fallopian tube due to blockage) or a history of pelvic infection, doxycycline should be given before the procedure.27 Patients with demonstrated hydrosalpinx on hysterosalpingography should receive doxycycline 100 mg twice daily for 5 days to prevent posthysterosalpingography pelvic inflammatory disease.27 Patients with active pelvic or cervical infection should not undergo hysterosalpingography .
Women with confirmed hydrosalpinx or tubal obstruction can be referred for laparoscopy. Gynecologic surgeons will plan their approach based on whether the obstruction is proximal (near the uterus) or distal (near the ovary) as well as whether hydrosalpinx, abnormal tubal architecture, salpingitis isthmica nodosa, or peritubal adhesions are noted. Tubal surgery can be effective in mild cases of tubal disease; however, as in vitro fertilization is becoming more effective, patients with moderate or severe tubal disease are increasingly being referred directly for assisted reproductive technology. Before undergoing assisted reproductive technology, hydrosalpinx will need to be addressed, as it can decrease clinical pregnancy rates with in vitro fertilization.
Endometriosis
Endometriosis is found in 21% to 47% of women with subfertility28 and commonly causes pain, ovarian cysts, and tubal disease. There is often a delay of 7 to 8 years for diagnosis due to the misapprehension that severe dysmenorrhea is normal. Women with an affected first-degree family member are at substantially increased risk.
Although endometriosis is commonly thought to result from reflux of endometrial tissue into the peritoneal cavity with menses, there are multiple proposed mechanisms for the disease.29 The pathogenesis of endometriosis is enigmatic, and there are likely as yet undetermined immunologic and genetic predispositions that confer increased risk.
Common symptoms of endometriosis are dysmenorrhea, dyspareunia, and pelvic pain, and these are sometimes accompanied by bowel and bladder symptoms. Pelvic examination classically demonstrates an immobile uterus and uterosacral nodularity; palpation of these nodules can elicit pain. On laparoscopy, endometriosis can range from minimal to severe; however, stage of endometriosis correlates poorly with reported symptoms.30
Consideration of surgery is based on clinical history, results of the pelvic examination, and possible findings on ultrasonography or hysterosalpingography. Although positive findings on imaging can support a plan for intervention, endometriosis is largely a peritoneal disease, and evidence of tubal damage or ovarian cysts is rarely evident on ultrasonography. In women with menstrual complaints (eg, dysmenorrhea, heavy menstrual bleeding, abnormal uterine bleeding) and a history of infertility, ultrasonography may be useful in determining the presence of uterine pathology such as ovarian cyst or endometrioma, large hydrosalpinx, polyp, or substantial fibroid burden—any of which may have a significant impact on female fertility.
In the absence of a reliable blood test or imaging study, the gold standard for the diagnosis of endometriosis continues to be laparoscopic surgery. Hormonal treatments for endometriosis symptoms are not effective in improving infertility and will preclude pregnancy. Laparoscopic surgery is more successful in improving pregnancy rates in women with advanced disease: pregnancy rates after surgery can be as high as 60% in women with ovarian endometriomas but are significantly lower in women with removal of minimal to mild disease.30,31 Women over age 35 or who present with low ovarian reserve and whose male partner has semen abnormalities should consider moving directly to assisted reproductive technology rather than pursuing endometriosis surgery.
MALE FACTOR INFERTILITY
Although male partners are often highly engaged in and supportive of the fertility evaluation, some are reluctant to undergo testing, and some wish to undergo semen analysis only after female factors have been ruled out. Our practice is to evaluate male factors immediately, due to the high contribution of male factors (up to 40% of cases) either alone or in combination with female factors.32
Men at particularly increased risk of semen abnormalities include those with a history of chemotherapy or radiation or exposure to toxins (eg, environmental exposures, alcohol, tobacco, illicit substances) and prescribed medications.
At a minimum, for the male partner, a reproductive history should be taken and a semen analysis ordered. Men should be directly queried about testosterone use, as this often-used anabolic steroid hormone can severely impair sperm production.
Men who have low sperm counts, motility, or morphology scores based on World Health Organization criteria should not be deemed “infertile,” as there is significant variation from one analysis to the next, and normal fertility has been reported in men with notably low sperm counts. Particular caution should be exercised in interpreting low morphology scores in men with normal counts and motility, as this parameter appears to have the least prognostic value in this context. Men with abnormal semen analyses should be referred to a specialist for further urologic evaluation and treatment.
Treatments for male factor infertility include surgery, steroid hormones, and possibly intrauterine insemination or assisted reproductive technology. In even the most challenging cases, male infertility is now largely treatable with intracytoplasmic sperm injection with assisted reproductive technology. While most advances in in vitro fertilization have been evolutionary, intracytoplasmic sperm injection was revolutionary. This breakthrough technology allows a single sperm to be injected directly into the oocyte. Sperm for this procedure can be obtained either from the ejaculate or from microsurgical testicular sperm extraction.
ANOVULATION
A thorough menstrual history can be informative, as most females of reproductive age have a fairly predictable 25-to-35-day monthly menstrual cycle. Women presenting with menstrual charting with this pattern do not require laboratory confirmation of ovulation. Basal body temperatures are rarely used currently, as they are time-consuming, can induce stress, and are confirmatory rather than predictive of ovulation. Endometrial biopsy for endometrial “dating” is no longer performed in infertile women.
If laboratory confirmation is desired, LH kit testing with a commercially available test or a luteal phase serum progesterone obtained 7 days after suspected ovulation can be obtained. A serum progesterone level higher than 3 ng/mL is indicative of ovulation.19 Due to the notable fluctuations in ovulatory-appearing progesterone levels over several hours, caution must be taken in interpreting a lower-normal level as indicative of a luteal phase insufficiency.
Polycystic ovary syndrome
Polycystic ovary syndrome is important to understand because it is a metabolic condition that predisposes patients to a variety of health risks. Along with gynecologic consequences such as infertility, abnormal uterine bleeding, and endometrial pathology, it is often accompanied by alterations in glucose and lipid metabolism, obesity, hypertension, and cardiovascular disease.35
Despite its name, the syndrome does not involve the presence of classic ovarian cysts. In fact, the cysts associated with polycystic ovary syndrome are dense accumulations of antral follicles arranged peripherally in the ovarian cortex; they should not be removed surgically as they represent the ovarian reserve.
Although ovaries that appear polycystic on transvaginal ultrasonography are often associated with the syndrome, they are not invariably present and are not absolutely required for the diagnosis of polycystic ovary syndrome based on the most commonly used criteria.35 Several diagnostic criteria have been proposed for polycystic ovary syndrome and its phenotypes. The 2003 revised Rotterdam criteria require 2 out of the following 3 features:
- Oligo-ovulation or anovulation
- Evidence of hyperandrogenism, whether clinical (eg, acne or hirsutism) or based on laboratory testing
- Polycystic-appearing ovaries on ultrasonography.
There is no single test that can diagnose the disease. Although polycystic ovary syndrome is often characterized by elevated LH levels, LH–FSH ratios, and fasting insulin levels, these are not diagnostic criteria. The diagnosis hinges on excluding other causes of anovulation such as thyroid disease, hyperprolactinemia, 21-hydroxylase deficiency, androgen-producing neoplasms, and Cushing syndrome. In addition to checking serum testosterone levels, irregular menstrual cycles and infertility should be assessed at minimum with measurement of TSH, prolactin, and day-3 FSH. Obese women should be screened for metabolic syndrome, which should include an assessment of impaired glucose tolerance with a 2-hour oral glucose tolerance test.36
Women with polycystic ovary syndrome are known to have insulin resistance, which is difficult to assess and is independent of their body mass index.37 They often report a family history of diabetes or a personal history of gestational diabetes or giving birth to infants who are large for gestational age. Although most women diagnosed with insulin resistance and anovulatory infertility will not yet have a diagnosis of diabetes, women with polycystic ovary syndrome are 3 to 7 times more likely to develop type 2 diabetes later in life37 and are at increased risk of lipid abnormalities, cardiovascular disease, and stroke. Therefore, interventions to address the compounding influences of polycystic ovary syndrome and obesity can improve fertility outcomes and help prevent long-term sequelae that accompany the syndrome.
Treatment for women with polycystic ovary syndrome attempting conception includes lifestyle modifications, medications for ovulation induction, and possible use of insulin sensitizers. Metformin alone is not effective as a single agent for achieving pregnancy.38 Diet, weight loss, and exercise can have dramatic effects on ovulation and pregnancy and should be highly encouraged.
Ovulation induction is often required in anovulatory women, either in combination with lifestyle modifications or used subsequently if modifications are not successful. Letrozole is advised as the initial agent in women with obesity and anovulatory infertility rather than clomiphene citrate; a side-by-side comparison demonstrated increased rates of ovulation and live birth with letrozole.39
Once-daily letrozole 2.5 mg or clomiphene 50 mg can be prescribed for 5 days, from cycle days 3 through 7 to cycle days 5 through 9. If this initial dosing fails to result in ovulation, the dose can be increased. Known adverse effects are hot flashes, headaches, ovarian cysts, and increased risk of multiple gestation.
Metformin should be considered as an adjunct to fertility treatments in women with polycystic ovary syndrome, especially those with obesity or impaired glucose tolerance, or if there is no response to standard ovulation induction.
Ovarian hyperstimulation syndrome (cystic enlargement of the ovaries with potentially dangerous fluid and electrolyte imbalances) can occur in women with polycystic ovary syndrome; however, it rarely occurs with oral medications.
- Chandra A, Copen CE, Stephen EH. Infertility service use in the United States: data from the National Survey of Family Growth, 1982–2010. Natl Health Stat Report 2014; (73):1–21. pmid:24467919
- Mosher WD, Pratt WF. Fecundity and infertility in the United States: incidence and trends. Fertil Steril 1991; 56(2):192–193. pmid:2070846
- Boltz MW, Sanders JN, Simonsen SE, Stanford JB. Fertility treatment, use of in vitro fertilization, and time to live birth based on initial provider type. J Am Board Fam Med 2017; 30(2):230–238. doi:10.3122/jabfm.2017.02.160184
- Hampton K, Mazza D. Fertility-awareness knowledge, attitudes and practices of women attending general practice. Aust Fam Physician 2015; 44(11):840–845. pmid:26590626
- Zegers-Hochschild F, Adamson GD, de Mouzon J, et al; International Committee for Monitoring Assisted Reproductive Technology; World Health Organization. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril 2009; 92(5):1520–1524. doi:10.1016/j.fertnstert.2009.09.009
- Domar AD, Zuttermeister PC, Friedman R. The psychological impact of infertility: a comparison with patients with other medical conditions. J Psychosom Obstet Gynaecol 1993; 14(suppl):45–52. pmid:8142988
- Argyle CE, Harper JC, Davies MC. Oocyte cryopreservation: where are we now? Hum Reprod Update 2016; 22(4):440–449. doi:10.1093/humupd/dmw007
- Practice Committee of American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril 2013; 99(1):63. doi:10.1016/j.fertnstert.2012.09.023
- Guttmacher AF. Factors affecting normal expectancy of conception. J Am Med Assoc 1956; 161(9):855–860. pmid:13319020
- Dunson DB, Baird DD, Colombo B. Increased infertility with age in men and women. Obstet Gynecol 2004; 103(1):51–56. doi:10.1097/01.AOG.0000100153.24061.45
- National Collaborating Centre for Women’s and Children’s Health (UK). Fertility: assessment and treatment for people with fertility problems. London: Royal College of Obstetricians & Gynaecologists; 2013. www.ncbi.nlm.nih.gov/books/NBK247932. Accessed May 6, 2019.
- Elzanaty S, Malm J, Giwercman A. Duration of sexual abstinence: epididymal and accessory sex gland secretions and their relationship to sperm motility. Hum Reprod 2005; 20(1):221–225. doi:10.1093/humrep/deh586
- Wilcox AJ, Weinberg CR, Baird DD. Timing of sexual intercourse in relation to ovulation. Effects on the probability of conception, survival of the pregnancy, and sex of the baby. N Engl J Med 1995; 333(23):1517–1521. doi:10.1056/NEJM199512073332301
- Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society for Reproductive Endocrinology and Infertility. Optimizing natural fertility: a committee opinion. Fertil Steril 2017; 107(1):52–58. doi:10.1016/j.fertnstert.2016.09.029
- Kutteh WH, Chao CH, Ritter JO, Byrd W. Vaginal lubricants for the infertile couple: effect on sperm activity. Int J Fertil Menopausal Stud 1996; 41(4):400–404. pmid:8894797
- Bigelow JL, Dunson DB, Stanford JB, Ecochard R, Gnoth C, Colombo B. Mucus observations in the fertile window: a better predictor of conception than timing of intercourse. Hum Reprod 2004; 19(4):889–892. doi:10.1093/humrep/deh173
- Steiner AZ, Pritchard D, Stanczyk FZ, et al. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. JAMA 2017; 318(14):1367–1376. doi:10.1001/jama.2017.14588
- Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update 2006; 12(6):685–718. doi:10.1093/humupd/dml034
- Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile female: a committee opinion. Fertil Steril 2015; 103(6):e44–e50. doi:10.1016/j.fertnstert.2015.03.019
- Sharara FI, Scott RT Jr, Seifer DB. The detection of diminished ovarian reserve in infertile women. Am J Obstet Gynecol 1998; 179(3 Pt 1):804–812. pmid:9757994
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline. Committee Opinion No. 589. Fertil Steril 2014; 101(3):633–634. doi:10.1016/j.fertnstert.2013.12.032
- Balasch J, Gratacós E. Delayed childbearing: effects on fertility and the outcome of pregnancy. Curr Opin Obstet Gynecol 2012; 24(3):187–193. doi:10.1097/GCO.0b013e3283517908
- Hickman LC, Fortin C, Goodman L, Liu X, Flyckt R. Fertility and fertility preservation: knowledge, awareness and attitudes of female graduate students. Eur J Contracept Reprod Health Care 2018; 23(2):130–138. doi:10.1080/13625187.2018.1455085
- Lundsberg LS, Pal L, Gariepy AM, Xu X, Chu MC, Illuzzi JL. Knowledge, attitudes, and practices regarding conception and fertility: a population-based survey among reproductive-age United States women. Fertil Steril 2014; 101(3):767–774. doi:10.1016/j.fertnstert.2013.12.006
- Hodes-Wertz B, Druckenmiller S, Smith M, Noyes N. What do reproductive-age women who undergo oocyte cryopreservation think about the process as a means to preserve fertility? Fertil Steril 2013; 100(5):1343–1349. doi:10.1016/j.fertnstert.2013.07.201
- Weström L, Joesoef R, Reynolds G, Hagdu A, Thompson SE. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis 1992; 19(4):185–192. pmid:1411832
- ACOG Practice Bulletin No. 195: prevention of infection after gynecologic procedures. Obstet Gynecol 2018; 131(6):e172–e189. doi:10.1097/AOG.0000000000002670
- Balasch J, Creus M, Fábregues F, et al. Visible and non-visible endometriosis at laparoscopy in fertile and infertile women and in patients with chronic pelvic pain: a prospective study. Hum Reprod 1996; 11(2):387–391. pmid:8671229
- Falcone T, Flyckt R. Clinical management of endometriosis. Obstet Gynecol 2018; 131(3):557–571. doi:10.1097/AOG.0000000000002469
- Flyckt R, Kim S, Falcone T. Surgical management of endometriosis in patients with chronic pelvic pain. Semin Reprod Med 2017; 35(1):54–64. doi:10.1055/s-0036-1597306
- Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: a committee opinion. Fertil Steril 2012; 98(3):591–598. doi:10.1016/j.fertnstert.2012.05.031
- Thonneau P, Marchand S, Tallec A, et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988–1989). Hum Reprod 1991; 6(6):811–816. pmid:1757519
- Cooper TG, Noonan E, von Eckardstein S, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update 2010; 16(3):231–245. doi:10.1093/humupd/dmp048
- Practice Committee of American Society for Reproductive Medicine. Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril 2012; 98(2):294–301. doi:10.1016/j.fertnstert.2012.05.033
- Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004; 19(1):41–47. pmid:14688154
- Falcone T, Finegood DT, Fantus IG, Morris D. Androgen response to endogenous insulin secretion during the frequently sampled intravenous glucose tolerance test in normal and hyperandrogenic women. J Clin Endocrinol Metab 1990; 71(6):1653–1657. doi:10.1210/jcem-71-6-1653
- Daniilidis A, Dinas K. Long term health consequences of polycystic ovarian syndrome: a review analysis. Hippokratia 2009; 13(2):90–92. pmid:19561777
- Legro RS, Barnhart HX, Schlaff WD, et al; Cooperative Multicenter Reproductive Medicine Network. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med 2007; 356(6):551–566. doi:10.1056/NEJMoa063971
- Legro RS, Brzyski RG, Diamond MP, et al; NICHD Reproductive Medicine Network. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med 2014; 371(2):119–129. doi:10.1056/NEJMoa1313517
For millions of couples, a primary care physician may be the first point of contact for fertility concerns. Statistics from the US Centers for Disease Control and Prevention indicate that 12% of women ages 15 to 44 received fertility services from 2006 to 2010.1 Despite seeking services, most couples requested only advice or testing rather than treatments such as ovulation-inducing medications, surgery, or, rarely, assisted reproductive technologies including in vitro fertilization. Based on these data, primary care physicians are in a unique position to offer guidance and provide fertility services in most circumstances without the need for referral.
This article reviews the answers to questions patients frequently ask, and outlines a practical framework for the evaluation and management of the infertile couple.
MANY PATIENTS SEEK INFORMATION
At least 1 million medical visits per year are for women seeking help in becoming pregnant, with the number increasing over the last several decades.1 Reasons for the increase include delayed childbearing and the effects of aging on the female reproductive system (“female reproductive aging”), as well as the availability of increasingly effective treatments for infertility.
While the prevalence of infertility in US couples is widely quoted as 10% to 15%,2 there is no estimate for the number of fertility-related questions patients routinely pose to care providers. These questions often relate to coital timing, use of lubricants, positioning, and the use of fertility trackers and ovulation predictors.
A 2017 study of women with 12 months of infertility found that only 8% sought subspecialist care vs care from a general physician or provider, indicating that generalists are most often the first point of contact.3 The majority (92%) of women responding to a survey regarding fertility-awareness education indicated a preference for immediate counseling from their general practitioner.4
Although some healthcare providers may consider infertility simply a quality-of-life issue, the World Health Organization classifies it as a disease, and as such it warrants identification, assessment, and intervention.5 Further, patients with infertility are known to experience considerable psychological distress related to their condition. In a comparison study, women with infertility experienced levels of psychological distress similar to the level in patients with cancer and patients with chronic medical illness.6
In the current era, general practitioners and women’s health specialists may also now address patients’ questions about reproductive aging and egg-freezing, which is now an established technology.7
FAILURE TO CONCEIVE AFTER 1 YEAR
As women approach age 40, the potential for fertility decreases rapidly and significantly. Women in their later 30s have only half the fertility of women in their early 20s.10 Misperceptions of aging and female fertility have been fueled by widely publicized celebrity births from women in their 40s and even 50s, without disclosing the use of frozen or donor eggs. This unfortunate fact affects women actively trying to conceive as well as women who wish to delay childbearing due to lack of a partner or for personal or professional reasons. Primary care physicians should be able to provide counseling relevant to female reproductive aging and make suitable and timely referrals for fertility preservation if indicated.
AN EMOTIONAL ISSUE
In approaching the couple with infertility, it is important to proceed with great sensitivity for the socioemotional context of this diagnosis. For both the male and female partner, infertility can be highly stigmatizing, and can be viewed as a personal or relationship failure.
Couples should be encouraged to ask embarrassing or uncomfortable questions. Although this may not be feasible in many circumstances, interviews should ideally be conducted with both partners individually as well as together, to allow sensitive issues to be shared. In some cases, a partner may be unaware of a history of a sexually transmitted infection, a prior abortion, the use of testosterone supplements or medications to enhance male sexual performance, or a vasectomy or tubal ligation during a previous relationship.
It is not unusual that the anxiety of infertility can cause decreased libido and sexual and erectile dysfunction. These issues can further complicate the problem of conceiving, and couples counseling is not uncommonly required.11 Patients are often reassured to know that they are not alone in their diagnosis.
LOOK FOR CLUES
Before embarking on a series of tests, the primary care physician can carefully evaluate for clues that may guide the diagnostic evaluation. The approach can be individualized based on the patient’s age, duration of subfertility (ie, how long they have been trying to become pregnant), and risk factors. But as a general rule, regardless of age, couples who have been trying to conceive for more than 1 year should be encouraged to pursue additional testing.
Because each month presents a new cycle of hope (often followed by intense disappointment), the prevailing sentiment to “just give it a little more time” must be countered by education and counseling. The primary care physician must increase awareness that lack of pregnancy in the stated time periods is a compelling reason for evaluation.
History-taking in the infertile couple should include a complete gynecologic and menstrual history. A history of sexually transmitted diseases that can cause tubal disease, such as gonorrhea and Chlamydia, is significant. Both partners should be assessed for a history of prior conceptions, past medical or surgical problems, medications, and exposures to environmental toxins including alcohol, tobacco, and drugs.
A detailed physical examination can provide clues to the cause of subfertility, especially if signs of obesity, androgen excess, or insulin resistance are present.
QUESTIONS OFTEN ASKED BY COUPLES TRYING TO CONCEIVE
Clinicians are frequently asked questions related to sexual practices and lifestyle in relation to fertility and should be comfortable responding to questions in these areas.
Does frequent ejaculation ‘use up’ my sperm?
Men should be reassured that frequent ejaculations do not decrease sperm counts; even daily ejaculation does not deplete the concentration of sperm. Male partners can be reassured that “saving up” is not an effective strategy; in fact, abstinence periods of greater than 5 days can adversely affect semen parameters.12
How often should we have sex?
Infrequent intercourse (< 1 time per week) reduces the monthly chance of conceiving.13 There does not seem to be a significant improvement in fecundity with daily intercourse vs intercourse on alternate days. Strict schedules surrounding intercourse may increase stress, and reassurance should be offered that intercourse need not be regimented. Every 1 to 2 days should suffice.
Are any sexual positions better for conception?
There is no evidence that particular coital positioning or remaining supine after intercourse improves fertility. Sperm can be found within the endocervix within seconds of ejaculation, irrespective of sexual position.
What is the window of fertility?
There is good evidence that the fertile window lasts approximately 6 days and closes after ovulation.13,14 Women with regular cycles can determine their typical day of ovulation based on menstrual tracking. Intercourse should begin about 6 days before ovulation and should continue every 1 to 2 days for 1 week to fully capture this window.
Should we change our lifestyle?
Couples seeking pregnancy should be advised to limit alcohol and caffeine use, completely abstain from cigarette smoking or illicit drug use, and maintain a healthy body mass index.
Very few data exist to support particular diets or supplements to promote fertility, including antioxidants and herbal remedies. Folic acid supplementation is recommended in all women attempting to conceive to reduce the incidence of birth defects.
Do lubricants reduce fertility?
Although there seem to be no differences in fecundity rates in couples using commercial lubricants, most water-based lubricants are best avoided in couples with infertility, as adverse effects on sperm have been demonstrated in vitro.15 If lubrication is needed, couples may try mineral oil, canola oil, or hydroxyetylcellulose-based lubricants (eg, Pre-seed).
Do fertility trackers work?
Many couples with primary infertility perceive that coital timing is critical and worry that their infertility is due to poorly timed intercourse; in fact, this is seldom the case.
Despite widespread marketing of urinary luteinizing hormone (LH) detection kits and electronic trackers and monitors, there is no clear evidence that these methods improve monthly rates of conception.
Women with a regular menstrual cycle should be encouraged to take notice when their cervical mucus appears clear and slippery (a sign of ovulation). Not all women are able to detect these fluctuations; however, for those who can, observing cervical mucus changes appears to be equivalent or superior to predictor kits in predicting conception.16
A PRACTICAL FRAMEWORK FOR EVALUATING THE INFERTILE COUPLE
To assess for the common factors identified in Table 1, the essential investigation of the infertile couple includes:
- Semen analysis
- Confirmation of ovulation
- Hysterosalpingography.
Consideration can also be given to ovarian reserve testing in women at risk of diminished ovarian reserve. The above investigation can be performed simultaneously to allow for prompt identification of any issues. Further, infertility is often a combination of problems (eg, anovulation in the woman together with a problem in the man), so an incomplete evaluation may overlook a coexisting diagnosis and lead to delays in treatment and pregnancy.
Tests that are no longer typically used in clinical practice are outlined in Table 2.
OVARIAN RESERVE TESTING AND FEMALE REPRODUCTIVE AGING
Ovarian reserve refers to the number of fertilizable oocytes that remain in the ovary. This reserve changes over time, and changes occur rapidly as women approach and enter their 30s. Though not the case in men, the age of the female partner is an independent risk factor for infertility. This discrepancy is due to loss of ovarian reserve, chromosome abnormalities in embryos, and the development of medical conditions with age that affect fertility.
Testing for ovarian reserve does not necessarily predict an overall inability to achieve a live birth,17 but it can predict response to exogenous gonadotropins and, to some degree, the chance for successful pregnancy with assisted reproductive technology.18
The ASRM states that testing for diminished ovarian reserve may provide useful information in women who have had a previous poor response to gonadotropins and in women planning assisted reproductive technology.19 The ASRM also indicates that the following are risk factors for diminished ovarian reserve, and clinicians may target the assessment accordingly19:
- Age 35 or older
- History of exposure to chemotherapy or pelvic radiation
- Family history of early menopause (age < 40)
- History of ovarian surgery
- Unexplained or idiopathic fertility.
Although several tests of ovarian reserve exist, either an antimullerian hormone (AMH) test or a combined cycle day-3 follicle-stimulating hormone (FSH) and estradiol level are the 2 tests commonly used in clinical practice. Antral follicle counts are an ultrasonographic measure used by infertility specialists but rarely by primary care physicians. Assays such as inhibin are rarely ordered and have limited clinical utility.
The AMH test
Many reproductive endocrinologists rely on the AMH level as a single test of ovarian reserve as it is easy to obtain, has a relatively low cost, and offers stable results. AMH is produced by the granulosa cells of the ovarian antral follicles and is readily detected in serum samples.
Conveniently for the clinician, levels of this hormone remain stable throughout the menstrual cycle and therefore can be tested on any day and at any time of day. Lower serum AMH levels (< 1 ng/mL) have been shown to correspond to diminished ovarian stimulation with gonadotropins as well as decreased embryo quality and poor pregnancy outcomes with assisted reproductive technology.19
Nevertheless, despite overall stability, AMH levels can be falsely lowered in women using exogenous hormones or with a diagnosis of hypogonadotropic hypogonadism. Levels may be higher than expected in women with polycystic ovary syndrome due to higher numbers of antral and preantral follicles in the polycystic ovary.
The day-3 follicle-stimulating hormone test
FSH and 17-beta estradiol testing can be ordered in combination to assess function of the hypothalamic-pituitary-ovarian axis on day 3 of the menstrual cycle. There is some flexibility, however, and testing obtained on cycle day 2, 3, or 4 yields equivalent results.
Although there are no strict cutoffs, FSH levels that appear elevated (> 10–20 IU/L) are associated with lower chances of conceiving with in vitro fertilization in multiple studies.20
The test is limited by levels that may fluctuate cycle to cycle, and reassuring test results do not necessarily indicate that a woman will achieve a pregnancy. Although a serum estradiol value alone is not a useful test, it can be used in combination with day-3 FSH to screen for diminished ovarian reserve.
As premature recruitment of a follicle can cause an early follicular rise in estradiol, FSH may be falsely suppressed on day 3. For example, a “normal” day-3 FSH combined with an elevated day-3 17-beta estradiol level of 60 to 80 pg/mL is associated with a poor response to medical treatments for infertility.
Female reproductive aging
Aging of the female reproductive system is a central threat to fertility, and prompt assessment and referral are warranted for women age 35 or older who have been trying to conceive for more than 6 months. The ASRM recommends that women over age 40 be evaluated immediately.21
A prevailing misconception is that regular menstrual cycles correspond with normal fertility. In reality, women lose their ability to achieve a healthy live birth in the 5 to 10 years preceding menopause. Although all women who do not desire pregnancy should still use appropriate contraception to avoid unintended pregnancy, women who do desire pregnancy should be aware of these physiologic changes.
Classic age-related changes in ovarian reserve are accompanied by a steep rise in aneuploidy and miscarriage risk.22 This is particularly relevant as women increasingly delay childbearing in modern society. Loss of fertility begins at 32 and abruptly accelerates at age 3721; this fact is poorly communicated to and understood by patients. In a 2018 study of highly educated women, most respondents failed to identify that 45-year-old women can only rarely achieve a successful pregnancy.23
In recent decades, the percentage of women who delay childbearing until after age 35 has steadily increased. There is a widespread misconception that fertility treatments and assisted reproductive technology can compensate for female reproductive aging. Primary care physicians can play a central role in reminding couples that age remains the single greatest predictor of natural fertility and the chance of success with assisted reproduction.
Further, for women who desire future fertility and are without a partner, primary care physicians can counsel them regarding the availability of donor insemination or egg freezing. Studies confirm that women want clinicians to initiate information on reproductive health, and 80% of women undergoing elective egg-freezing for fertility preservation wished that they had done so at an earlier age.24,25
FEMALE PERITONEAL AND STRUCTURAL CAUSES
Women with endometriosis, fibroids, or a history of tubal disease have impaired fecundity. Pelvic imaging is an essential component of their evaluation. Although hysterosalpingography is the mainstay of tubal assessment, in select cases ultrasonography or hysteroscopy may be indicated.
Tubal disease and hysterosalpingography
Tubal disease remains one of the most common causes of infertility in the US females. In most cases, tubal damage is secondary to pelvic inflammatory disease from infection with gonorrhea or Chlamydia, or both.
Rates of confirmed tubal-factor infertility have been shown to increase with both the severity of the infection and the number of past infections.26 In a landmark study, 1 episode of pelvic inflammatory disease was associated with a 12% risk of tubal-factor infertility, whereas 3 infections carried a risk as high as 54%. Pelvic inflammatory disease is also known to increase the risk of ectopic pregnancy.
To assess tubal patency, hysterosalpingography, a radiographic procedure, is typically performed using fluoroscopy and injected contrast material. Some centers may offer sonohysterography as a radiation-free alternative, depending on sonographic skill and experience. Both tests are best scheduled in the window between the end of menstrual bleeding and ovulation. In practice, patients with regular cycles can typically schedule hysterosalpingography between cycle days 5 and 12.
In patients with known hydrosalpinx (a distended fallopian tube due to blockage) or a history of pelvic infection, doxycycline should be given before the procedure.27 Patients with demonstrated hydrosalpinx on hysterosalpingography should receive doxycycline 100 mg twice daily for 5 days to prevent posthysterosalpingography pelvic inflammatory disease.27 Patients with active pelvic or cervical infection should not undergo hysterosalpingography .
Women with confirmed hydrosalpinx or tubal obstruction can be referred for laparoscopy. Gynecologic surgeons will plan their approach based on whether the obstruction is proximal (near the uterus) or distal (near the ovary) as well as whether hydrosalpinx, abnormal tubal architecture, salpingitis isthmica nodosa, or peritubal adhesions are noted. Tubal surgery can be effective in mild cases of tubal disease; however, as in vitro fertilization is becoming more effective, patients with moderate or severe tubal disease are increasingly being referred directly for assisted reproductive technology. Before undergoing assisted reproductive technology, hydrosalpinx will need to be addressed, as it can decrease clinical pregnancy rates with in vitro fertilization.
Endometriosis
Endometriosis is found in 21% to 47% of women with subfertility28 and commonly causes pain, ovarian cysts, and tubal disease. There is often a delay of 7 to 8 years for diagnosis due to the misapprehension that severe dysmenorrhea is normal. Women with an affected first-degree family member are at substantially increased risk.
Although endometriosis is commonly thought to result from reflux of endometrial tissue into the peritoneal cavity with menses, there are multiple proposed mechanisms for the disease.29 The pathogenesis of endometriosis is enigmatic, and there are likely as yet undetermined immunologic and genetic predispositions that confer increased risk.
Common symptoms of endometriosis are dysmenorrhea, dyspareunia, and pelvic pain, and these are sometimes accompanied by bowel and bladder symptoms. Pelvic examination classically demonstrates an immobile uterus and uterosacral nodularity; palpation of these nodules can elicit pain. On laparoscopy, endometriosis can range from minimal to severe; however, stage of endometriosis correlates poorly with reported symptoms.30
Consideration of surgery is based on clinical history, results of the pelvic examination, and possible findings on ultrasonography or hysterosalpingography. Although positive findings on imaging can support a plan for intervention, endometriosis is largely a peritoneal disease, and evidence of tubal damage or ovarian cysts is rarely evident on ultrasonography. In women with menstrual complaints (eg, dysmenorrhea, heavy menstrual bleeding, abnormal uterine bleeding) and a history of infertility, ultrasonography may be useful in determining the presence of uterine pathology such as ovarian cyst or endometrioma, large hydrosalpinx, polyp, or substantial fibroid burden—any of which may have a significant impact on female fertility.
In the absence of a reliable blood test or imaging study, the gold standard for the diagnosis of endometriosis continues to be laparoscopic surgery. Hormonal treatments for endometriosis symptoms are not effective in improving infertility and will preclude pregnancy. Laparoscopic surgery is more successful in improving pregnancy rates in women with advanced disease: pregnancy rates after surgery can be as high as 60% in women with ovarian endometriomas but are significantly lower in women with removal of minimal to mild disease.30,31 Women over age 35 or who present with low ovarian reserve and whose male partner has semen abnormalities should consider moving directly to assisted reproductive technology rather than pursuing endometriosis surgery.
MALE FACTOR INFERTILITY
Although male partners are often highly engaged in and supportive of the fertility evaluation, some are reluctant to undergo testing, and some wish to undergo semen analysis only after female factors have been ruled out. Our practice is to evaluate male factors immediately, due to the high contribution of male factors (up to 40% of cases) either alone or in combination with female factors.32
Men at particularly increased risk of semen abnormalities include those with a history of chemotherapy or radiation or exposure to toxins (eg, environmental exposures, alcohol, tobacco, illicit substances) and prescribed medications.
At a minimum, for the male partner, a reproductive history should be taken and a semen analysis ordered. Men should be directly queried about testosterone use, as this often-used anabolic steroid hormone can severely impair sperm production.
Men who have low sperm counts, motility, or morphology scores based on World Health Organization criteria should not be deemed “infertile,” as there is significant variation from one analysis to the next, and normal fertility has been reported in men with notably low sperm counts. Particular caution should be exercised in interpreting low morphology scores in men with normal counts and motility, as this parameter appears to have the least prognostic value in this context. Men with abnormal semen analyses should be referred to a specialist for further urologic evaluation and treatment.
Treatments for male factor infertility include surgery, steroid hormones, and possibly intrauterine insemination or assisted reproductive technology. In even the most challenging cases, male infertility is now largely treatable with intracytoplasmic sperm injection with assisted reproductive technology. While most advances in in vitro fertilization have been evolutionary, intracytoplasmic sperm injection was revolutionary. This breakthrough technology allows a single sperm to be injected directly into the oocyte. Sperm for this procedure can be obtained either from the ejaculate or from microsurgical testicular sperm extraction.
ANOVULATION
A thorough menstrual history can be informative, as most females of reproductive age have a fairly predictable 25-to-35-day monthly menstrual cycle. Women presenting with menstrual charting with this pattern do not require laboratory confirmation of ovulation. Basal body temperatures are rarely used currently, as they are time-consuming, can induce stress, and are confirmatory rather than predictive of ovulation. Endometrial biopsy for endometrial “dating” is no longer performed in infertile women.
If laboratory confirmation is desired, LH kit testing with a commercially available test or a luteal phase serum progesterone obtained 7 days after suspected ovulation can be obtained. A serum progesterone level higher than 3 ng/mL is indicative of ovulation.19 Due to the notable fluctuations in ovulatory-appearing progesterone levels over several hours, caution must be taken in interpreting a lower-normal level as indicative of a luteal phase insufficiency.
Polycystic ovary syndrome
Polycystic ovary syndrome is important to understand because it is a metabolic condition that predisposes patients to a variety of health risks. Along with gynecologic consequences such as infertility, abnormal uterine bleeding, and endometrial pathology, it is often accompanied by alterations in glucose and lipid metabolism, obesity, hypertension, and cardiovascular disease.35
Despite its name, the syndrome does not involve the presence of classic ovarian cysts. In fact, the cysts associated with polycystic ovary syndrome are dense accumulations of antral follicles arranged peripherally in the ovarian cortex; they should not be removed surgically as they represent the ovarian reserve.
Although ovaries that appear polycystic on transvaginal ultrasonography are often associated with the syndrome, they are not invariably present and are not absolutely required for the diagnosis of polycystic ovary syndrome based on the most commonly used criteria.35 Several diagnostic criteria have been proposed for polycystic ovary syndrome and its phenotypes. The 2003 revised Rotterdam criteria require 2 out of the following 3 features:
- Oligo-ovulation or anovulation
- Evidence of hyperandrogenism, whether clinical (eg, acne or hirsutism) or based on laboratory testing
- Polycystic-appearing ovaries on ultrasonography.
There is no single test that can diagnose the disease. Although polycystic ovary syndrome is often characterized by elevated LH levels, LH–FSH ratios, and fasting insulin levels, these are not diagnostic criteria. The diagnosis hinges on excluding other causes of anovulation such as thyroid disease, hyperprolactinemia, 21-hydroxylase deficiency, androgen-producing neoplasms, and Cushing syndrome. In addition to checking serum testosterone levels, irregular menstrual cycles and infertility should be assessed at minimum with measurement of TSH, prolactin, and day-3 FSH. Obese women should be screened for metabolic syndrome, which should include an assessment of impaired glucose tolerance with a 2-hour oral glucose tolerance test.36
Women with polycystic ovary syndrome are known to have insulin resistance, which is difficult to assess and is independent of their body mass index.37 They often report a family history of diabetes or a personal history of gestational diabetes or giving birth to infants who are large for gestational age. Although most women diagnosed with insulin resistance and anovulatory infertility will not yet have a diagnosis of diabetes, women with polycystic ovary syndrome are 3 to 7 times more likely to develop type 2 diabetes later in life37 and are at increased risk of lipid abnormalities, cardiovascular disease, and stroke. Therefore, interventions to address the compounding influences of polycystic ovary syndrome and obesity can improve fertility outcomes and help prevent long-term sequelae that accompany the syndrome.
Treatment for women with polycystic ovary syndrome attempting conception includes lifestyle modifications, medications for ovulation induction, and possible use of insulin sensitizers. Metformin alone is not effective as a single agent for achieving pregnancy.38 Diet, weight loss, and exercise can have dramatic effects on ovulation and pregnancy and should be highly encouraged.
Ovulation induction is often required in anovulatory women, either in combination with lifestyle modifications or used subsequently if modifications are not successful. Letrozole is advised as the initial agent in women with obesity and anovulatory infertility rather than clomiphene citrate; a side-by-side comparison demonstrated increased rates of ovulation and live birth with letrozole.39
Once-daily letrozole 2.5 mg or clomiphene 50 mg can be prescribed for 5 days, from cycle days 3 through 7 to cycle days 5 through 9. If this initial dosing fails to result in ovulation, the dose can be increased. Known adverse effects are hot flashes, headaches, ovarian cysts, and increased risk of multiple gestation.
Metformin should be considered as an adjunct to fertility treatments in women with polycystic ovary syndrome, especially those with obesity or impaired glucose tolerance, or if there is no response to standard ovulation induction.
Ovarian hyperstimulation syndrome (cystic enlargement of the ovaries with potentially dangerous fluid and electrolyte imbalances) can occur in women with polycystic ovary syndrome; however, it rarely occurs with oral medications.
For millions of couples, a primary care physician may be the first point of contact for fertility concerns. Statistics from the US Centers for Disease Control and Prevention indicate that 12% of women ages 15 to 44 received fertility services from 2006 to 2010.1 Despite seeking services, most couples requested only advice or testing rather than treatments such as ovulation-inducing medications, surgery, or, rarely, assisted reproductive technologies including in vitro fertilization. Based on these data, primary care physicians are in a unique position to offer guidance and provide fertility services in most circumstances without the need for referral.
This article reviews the answers to questions patients frequently ask, and outlines a practical framework for the evaluation and management of the infertile couple.
MANY PATIENTS SEEK INFORMATION
At least 1 million medical visits per year are for women seeking help in becoming pregnant, with the number increasing over the last several decades.1 Reasons for the increase include delayed childbearing and the effects of aging on the female reproductive system (“female reproductive aging”), as well as the availability of increasingly effective treatments for infertility.
While the prevalence of infertility in US couples is widely quoted as 10% to 15%,2 there is no estimate for the number of fertility-related questions patients routinely pose to care providers. These questions often relate to coital timing, use of lubricants, positioning, and the use of fertility trackers and ovulation predictors.
A 2017 study of women with 12 months of infertility found that only 8% sought subspecialist care vs care from a general physician or provider, indicating that generalists are most often the first point of contact.3 The majority (92%) of women responding to a survey regarding fertility-awareness education indicated a preference for immediate counseling from their general practitioner.4
Although some healthcare providers may consider infertility simply a quality-of-life issue, the World Health Organization classifies it as a disease, and as such it warrants identification, assessment, and intervention.5 Further, patients with infertility are known to experience considerable psychological distress related to their condition. In a comparison study, women with infertility experienced levels of psychological distress similar to the level in patients with cancer and patients with chronic medical illness.6
In the current era, general practitioners and women’s health specialists may also now address patients’ questions about reproductive aging and egg-freezing, which is now an established technology.7
FAILURE TO CONCEIVE AFTER 1 YEAR
As women approach age 40, the potential for fertility decreases rapidly and significantly. Women in their later 30s have only half the fertility of women in their early 20s.10 Misperceptions of aging and female fertility have been fueled by widely publicized celebrity births from women in their 40s and even 50s, without disclosing the use of frozen or donor eggs. This unfortunate fact affects women actively trying to conceive as well as women who wish to delay childbearing due to lack of a partner or for personal or professional reasons. Primary care physicians should be able to provide counseling relevant to female reproductive aging and make suitable and timely referrals for fertility preservation if indicated.
AN EMOTIONAL ISSUE
In approaching the couple with infertility, it is important to proceed with great sensitivity for the socioemotional context of this diagnosis. For both the male and female partner, infertility can be highly stigmatizing, and can be viewed as a personal or relationship failure.
Couples should be encouraged to ask embarrassing or uncomfortable questions. Although this may not be feasible in many circumstances, interviews should ideally be conducted with both partners individually as well as together, to allow sensitive issues to be shared. In some cases, a partner may be unaware of a history of a sexually transmitted infection, a prior abortion, the use of testosterone supplements or medications to enhance male sexual performance, or a vasectomy or tubal ligation during a previous relationship.
It is not unusual that the anxiety of infertility can cause decreased libido and sexual and erectile dysfunction. These issues can further complicate the problem of conceiving, and couples counseling is not uncommonly required.11 Patients are often reassured to know that they are not alone in their diagnosis.
LOOK FOR CLUES
Before embarking on a series of tests, the primary care physician can carefully evaluate for clues that may guide the diagnostic evaluation. The approach can be individualized based on the patient’s age, duration of subfertility (ie, how long they have been trying to become pregnant), and risk factors. But as a general rule, regardless of age, couples who have been trying to conceive for more than 1 year should be encouraged to pursue additional testing.
Because each month presents a new cycle of hope (often followed by intense disappointment), the prevailing sentiment to “just give it a little more time” must be countered by education and counseling. The primary care physician must increase awareness that lack of pregnancy in the stated time periods is a compelling reason for evaluation.
History-taking in the infertile couple should include a complete gynecologic and menstrual history. A history of sexually transmitted diseases that can cause tubal disease, such as gonorrhea and Chlamydia, is significant. Both partners should be assessed for a history of prior conceptions, past medical or surgical problems, medications, and exposures to environmental toxins including alcohol, tobacco, and drugs.
A detailed physical examination can provide clues to the cause of subfertility, especially if signs of obesity, androgen excess, or insulin resistance are present.
QUESTIONS OFTEN ASKED BY COUPLES TRYING TO CONCEIVE
Clinicians are frequently asked questions related to sexual practices and lifestyle in relation to fertility and should be comfortable responding to questions in these areas.
Does frequent ejaculation ‘use up’ my sperm?
Men should be reassured that frequent ejaculations do not decrease sperm counts; even daily ejaculation does not deplete the concentration of sperm. Male partners can be reassured that “saving up” is not an effective strategy; in fact, abstinence periods of greater than 5 days can adversely affect semen parameters.12
How often should we have sex?
Infrequent intercourse (< 1 time per week) reduces the monthly chance of conceiving.13 There does not seem to be a significant improvement in fecundity with daily intercourse vs intercourse on alternate days. Strict schedules surrounding intercourse may increase stress, and reassurance should be offered that intercourse need not be regimented. Every 1 to 2 days should suffice.
Are any sexual positions better for conception?
There is no evidence that particular coital positioning or remaining supine after intercourse improves fertility. Sperm can be found within the endocervix within seconds of ejaculation, irrespective of sexual position.
What is the window of fertility?
There is good evidence that the fertile window lasts approximately 6 days and closes after ovulation.13,14 Women with regular cycles can determine their typical day of ovulation based on menstrual tracking. Intercourse should begin about 6 days before ovulation and should continue every 1 to 2 days for 1 week to fully capture this window.
Should we change our lifestyle?
Couples seeking pregnancy should be advised to limit alcohol and caffeine use, completely abstain from cigarette smoking or illicit drug use, and maintain a healthy body mass index.
Very few data exist to support particular diets or supplements to promote fertility, including antioxidants and herbal remedies. Folic acid supplementation is recommended in all women attempting to conceive to reduce the incidence of birth defects.
Do lubricants reduce fertility?
Although there seem to be no differences in fecundity rates in couples using commercial lubricants, most water-based lubricants are best avoided in couples with infertility, as adverse effects on sperm have been demonstrated in vitro.15 If lubrication is needed, couples may try mineral oil, canola oil, or hydroxyetylcellulose-based lubricants (eg, Pre-seed).
Do fertility trackers work?
Many couples with primary infertility perceive that coital timing is critical and worry that their infertility is due to poorly timed intercourse; in fact, this is seldom the case.
Despite widespread marketing of urinary luteinizing hormone (LH) detection kits and electronic trackers and monitors, there is no clear evidence that these methods improve monthly rates of conception.
Women with a regular menstrual cycle should be encouraged to take notice when their cervical mucus appears clear and slippery (a sign of ovulation). Not all women are able to detect these fluctuations; however, for those who can, observing cervical mucus changes appears to be equivalent or superior to predictor kits in predicting conception.16
A PRACTICAL FRAMEWORK FOR EVALUATING THE INFERTILE COUPLE
To assess for the common factors identified in Table 1, the essential investigation of the infertile couple includes:
- Semen analysis
- Confirmation of ovulation
- Hysterosalpingography.
Consideration can also be given to ovarian reserve testing in women at risk of diminished ovarian reserve. The above investigation can be performed simultaneously to allow for prompt identification of any issues. Further, infertility is often a combination of problems (eg, anovulation in the woman together with a problem in the man), so an incomplete evaluation may overlook a coexisting diagnosis and lead to delays in treatment and pregnancy.
Tests that are no longer typically used in clinical practice are outlined in Table 2.
OVARIAN RESERVE TESTING AND FEMALE REPRODUCTIVE AGING
Ovarian reserve refers to the number of fertilizable oocytes that remain in the ovary. This reserve changes over time, and changes occur rapidly as women approach and enter their 30s. Though not the case in men, the age of the female partner is an independent risk factor for infertility. This discrepancy is due to loss of ovarian reserve, chromosome abnormalities in embryos, and the development of medical conditions with age that affect fertility.
Testing for ovarian reserve does not necessarily predict an overall inability to achieve a live birth,17 but it can predict response to exogenous gonadotropins and, to some degree, the chance for successful pregnancy with assisted reproductive technology.18
The ASRM states that testing for diminished ovarian reserve may provide useful information in women who have had a previous poor response to gonadotropins and in women planning assisted reproductive technology.19 The ASRM also indicates that the following are risk factors for diminished ovarian reserve, and clinicians may target the assessment accordingly19:
- Age 35 or older
- History of exposure to chemotherapy or pelvic radiation
- Family history of early menopause (age < 40)
- History of ovarian surgery
- Unexplained or idiopathic fertility.
Although several tests of ovarian reserve exist, either an antimullerian hormone (AMH) test or a combined cycle day-3 follicle-stimulating hormone (FSH) and estradiol level are the 2 tests commonly used in clinical practice. Antral follicle counts are an ultrasonographic measure used by infertility specialists but rarely by primary care physicians. Assays such as inhibin are rarely ordered and have limited clinical utility.
The AMH test
Many reproductive endocrinologists rely on the AMH level as a single test of ovarian reserve as it is easy to obtain, has a relatively low cost, and offers stable results. AMH is produced by the granulosa cells of the ovarian antral follicles and is readily detected in serum samples.
Conveniently for the clinician, levels of this hormone remain stable throughout the menstrual cycle and therefore can be tested on any day and at any time of day. Lower serum AMH levels (< 1 ng/mL) have been shown to correspond to diminished ovarian stimulation with gonadotropins as well as decreased embryo quality and poor pregnancy outcomes with assisted reproductive technology.19
Nevertheless, despite overall stability, AMH levels can be falsely lowered in women using exogenous hormones or with a diagnosis of hypogonadotropic hypogonadism. Levels may be higher than expected in women with polycystic ovary syndrome due to higher numbers of antral and preantral follicles in the polycystic ovary.
The day-3 follicle-stimulating hormone test
FSH and 17-beta estradiol testing can be ordered in combination to assess function of the hypothalamic-pituitary-ovarian axis on day 3 of the menstrual cycle. There is some flexibility, however, and testing obtained on cycle day 2, 3, or 4 yields equivalent results.
Although there are no strict cutoffs, FSH levels that appear elevated (> 10–20 IU/L) are associated with lower chances of conceiving with in vitro fertilization in multiple studies.20
The test is limited by levels that may fluctuate cycle to cycle, and reassuring test results do not necessarily indicate that a woman will achieve a pregnancy. Although a serum estradiol value alone is not a useful test, it can be used in combination with day-3 FSH to screen for diminished ovarian reserve.
As premature recruitment of a follicle can cause an early follicular rise in estradiol, FSH may be falsely suppressed on day 3. For example, a “normal” day-3 FSH combined with an elevated day-3 17-beta estradiol level of 60 to 80 pg/mL is associated with a poor response to medical treatments for infertility.
Female reproductive aging
Aging of the female reproductive system is a central threat to fertility, and prompt assessment and referral are warranted for women age 35 or older who have been trying to conceive for more than 6 months. The ASRM recommends that women over age 40 be evaluated immediately.21
A prevailing misconception is that regular menstrual cycles correspond with normal fertility. In reality, women lose their ability to achieve a healthy live birth in the 5 to 10 years preceding menopause. Although all women who do not desire pregnancy should still use appropriate contraception to avoid unintended pregnancy, women who do desire pregnancy should be aware of these physiologic changes.
Classic age-related changes in ovarian reserve are accompanied by a steep rise in aneuploidy and miscarriage risk.22 This is particularly relevant as women increasingly delay childbearing in modern society. Loss of fertility begins at 32 and abruptly accelerates at age 3721; this fact is poorly communicated to and understood by patients. In a 2018 study of highly educated women, most respondents failed to identify that 45-year-old women can only rarely achieve a successful pregnancy.23
In recent decades, the percentage of women who delay childbearing until after age 35 has steadily increased. There is a widespread misconception that fertility treatments and assisted reproductive technology can compensate for female reproductive aging. Primary care physicians can play a central role in reminding couples that age remains the single greatest predictor of natural fertility and the chance of success with assisted reproduction.
Further, for women who desire future fertility and are without a partner, primary care physicians can counsel them regarding the availability of donor insemination or egg freezing. Studies confirm that women want clinicians to initiate information on reproductive health, and 80% of women undergoing elective egg-freezing for fertility preservation wished that they had done so at an earlier age.24,25
FEMALE PERITONEAL AND STRUCTURAL CAUSES
Women with endometriosis, fibroids, or a history of tubal disease have impaired fecundity. Pelvic imaging is an essential component of their evaluation. Although hysterosalpingography is the mainstay of tubal assessment, in select cases ultrasonography or hysteroscopy may be indicated.
Tubal disease and hysterosalpingography
Tubal disease remains one of the most common causes of infertility in the US females. In most cases, tubal damage is secondary to pelvic inflammatory disease from infection with gonorrhea or Chlamydia, or both.
Rates of confirmed tubal-factor infertility have been shown to increase with both the severity of the infection and the number of past infections.26 In a landmark study, 1 episode of pelvic inflammatory disease was associated with a 12% risk of tubal-factor infertility, whereas 3 infections carried a risk as high as 54%. Pelvic inflammatory disease is also known to increase the risk of ectopic pregnancy.
To assess tubal patency, hysterosalpingography, a radiographic procedure, is typically performed using fluoroscopy and injected contrast material. Some centers may offer sonohysterography as a radiation-free alternative, depending on sonographic skill and experience. Both tests are best scheduled in the window between the end of menstrual bleeding and ovulation. In practice, patients with regular cycles can typically schedule hysterosalpingography between cycle days 5 and 12.
In patients with known hydrosalpinx (a distended fallopian tube due to blockage) or a history of pelvic infection, doxycycline should be given before the procedure.27 Patients with demonstrated hydrosalpinx on hysterosalpingography should receive doxycycline 100 mg twice daily for 5 days to prevent posthysterosalpingography pelvic inflammatory disease.27 Patients with active pelvic or cervical infection should not undergo hysterosalpingography .
Women with confirmed hydrosalpinx or tubal obstruction can be referred for laparoscopy. Gynecologic surgeons will plan their approach based on whether the obstruction is proximal (near the uterus) or distal (near the ovary) as well as whether hydrosalpinx, abnormal tubal architecture, salpingitis isthmica nodosa, or peritubal adhesions are noted. Tubal surgery can be effective in mild cases of tubal disease; however, as in vitro fertilization is becoming more effective, patients with moderate or severe tubal disease are increasingly being referred directly for assisted reproductive technology. Before undergoing assisted reproductive technology, hydrosalpinx will need to be addressed, as it can decrease clinical pregnancy rates with in vitro fertilization.
Endometriosis
Endometriosis is found in 21% to 47% of women with subfertility28 and commonly causes pain, ovarian cysts, and tubal disease. There is often a delay of 7 to 8 years for diagnosis due to the misapprehension that severe dysmenorrhea is normal. Women with an affected first-degree family member are at substantially increased risk.
Although endometriosis is commonly thought to result from reflux of endometrial tissue into the peritoneal cavity with menses, there are multiple proposed mechanisms for the disease.29 The pathogenesis of endometriosis is enigmatic, and there are likely as yet undetermined immunologic and genetic predispositions that confer increased risk.
Common symptoms of endometriosis are dysmenorrhea, dyspareunia, and pelvic pain, and these are sometimes accompanied by bowel and bladder symptoms. Pelvic examination classically demonstrates an immobile uterus and uterosacral nodularity; palpation of these nodules can elicit pain. On laparoscopy, endometriosis can range from minimal to severe; however, stage of endometriosis correlates poorly with reported symptoms.30
Consideration of surgery is based on clinical history, results of the pelvic examination, and possible findings on ultrasonography or hysterosalpingography. Although positive findings on imaging can support a plan for intervention, endometriosis is largely a peritoneal disease, and evidence of tubal damage or ovarian cysts is rarely evident on ultrasonography. In women with menstrual complaints (eg, dysmenorrhea, heavy menstrual bleeding, abnormal uterine bleeding) and a history of infertility, ultrasonography may be useful in determining the presence of uterine pathology such as ovarian cyst or endometrioma, large hydrosalpinx, polyp, or substantial fibroid burden—any of which may have a significant impact on female fertility.
In the absence of a reliable blood test or imaging study, the gold standard for the diagnosis of endometriosis continues to be laparoscopic surgery. Hormonal treatments for endometriosis symptoms are not effective in improving infertility and will preclude pregnancy. Laparoscopic surgery is more successful in improving pregnancy rates in women with advanced disease: pregnancy rates after surgery can be as high as 60% in women with ovarian endometriomas but are significantly lower in women with removal of minimal to mild disease.30,31 Women over age 35 or who present with low ovarian reserve and whose male partner has semen abnormalities should consider moving directly to assisted reproductive technology rather than pursuing endometriosis surgery.
MALE FACTOR INFERTILITY
Although male partners are often highly engaged in and supportive of the fertility evaluation, some are reluctant to undergo testing, and some wish to undergo semen analysis only after female factors have been ruled out. Our practice is to evaluate male factors immediately, due to the high contribution of male factors (up to 40% of cases) either alone or in combination with female factors.32
Men at particularly increased risk of semen abnormalities include those with a history of chemotherapy or radiation or exposure to toxins (eg, environmental exposures, alcohol, tobacco, illicit substances) and prescribed medications.
At a minimum, for the male partner, a reproductive history should be taken and a semen analysis ordered. Men should be directly queried about testosterone use, as this often-used anabolic steroid hormone can severely impair sperm production.
Men who have low sperm counts, motility, or morphology scores based on World Health Organization criteria should not be deemed “infertile,” as there is significant variation from one analysis to the next, and normal fertility has been reported in men with notably low sperm counts. Particular caution should be exercised in interpreting low morphology scores in men with normal counts and motility, as this parameter appears to have the least prognostic value in this context. Men with abnormal semen analyses should be referred to a specialist for further urologic evaluation and treatment.
Treatments for male factor infertility include surgery, steroid hormones, and possibly intrauterine insemination or assisted reproductive technology. In even the most challenging cases, male infertility is now largely treatable with intracytoplasmic sperm injection with assisted reproductive technology. While most advances in in vitro fertilization have been evolutionary, intracytoplasmic sperm injection was revolutionary. This breakthrough technology allows a single sperm to be injected directly into the oocyte. Sperm for this procedure can be obtained either from the ejaculate or from microsurgical testicular sperm extraction.
ANOVULATION
A thorough menstrual history can be informative, as most females of reproductive age have a fairly predictable 25-to-35-day monthly menstrual cycle. Women presenting with menstrual charting with this pattern do not require laboratory confirmation of ovulation. Basal body temperatures are rarely used currently, as they are time-consuming, can induce stress, and are confirmatory rather than predictive of ovulation. Endometrial biopsy for endometrial “dating” is no longer performed in infertile women.
If laboratory confirmation is desired, LH kit testing with a commercially available test or a luteal phase serum progesterone obtained 7 days after suspected ovulation can be obtained. A serum progesterone level higher than 3 ng/mL is indicative of ovulation.19 Due to the notable fluctuations in ovulatory-appearing progesterone levels over several hours, caution must be taken in interpreting a lower-normal level as indicative of a luteal phase insufficiency.
Polycystic ovary syndrome
Polycystic ovary syndrome is important to understand because it is a metabolic condition that predisposes patients to a variety of health risks. Along with gynecologic consequences such as infertility, abnormal uterine bleeding, and endometrial pathology, it is often accompanied by alterations in glucose and lipid metabolism, obesity, hypertension, and cardiovascular disease.35
Despite its name, the syndrome does not involve the presence of classic ovarian cysts. In fact, the cysts associated with polycystic ovary syndrome are dense accumulations of antral follicles arranged peripherally in the ovarian cortex; they should not be removed surgically as they represent the ovarian reserve.
Although ovaries that appear polycystic on transvaginal ultrasonography are often associated with the syndrome, they are not invariably present and are not absolutely required for the diagnosis of polycystic ovary syndrome based on the most commonly used criteria.35 Several diagnostic criteria have been proposed for polycystic ovary syndrome and its phenotypes. The 2003 revised Rotterdam criteria require 2 out of the following 3 features:
- Oligo-ovulation or anovulation
- Evidence of hyperandrogenism, whether clinical (eg, acne or hirsutism) or based on laboratory testing
- Polycystic-appearing ovaries on ultrasonography.
There is no single test that can diagnose the disease. Although polycystic ovary syndrome is often characterized by elevated LH levels, LH–FSH ratios, and fasting insulin levels, these are not diagnostic criteria. The diagnosis hinges on excluding other causes of anovulation such as thyroid disease, hyperprolactinemia, 21-hydroxylase deficiency, androgen-producing neoplasms, and Cushing syndrome. In addition to checking serum testosterone levels, irregular menstrual cycles and infertility should be assessed at minimum with measurement of TSH, prolactin, and day-3 FSH. Obese women should be screened for metabolic syndrome, which should include an assessment of impaired glucose tolerance with a 2-hour oral glucose tolerance test.36
Women with polycystic ovary syndrome are known to have insulin resistance, which is difficult to assess and is independent of their body mass index.37 They often report a family history of diabetes or a personal history of gestational diabetes or giving birth to infants who are large for gestational age. Although most women diagnosed with insulin resistance and anovulatory infertility will not yet have a diagnosis of diabetes, women with polycystic ovary syndrome are 3 to 7 times more likely to develop type 2 diabetes later in life37 and are at increased risk of lipid abnormalities, cardiovascular disease, and stroke. Therefore, interventions to address the compounding influences of polycystic ovary syndrome and obesity can improve fertility outcomes and help prevent long-term sequelae that accompany the syndrome.
Treatment for women with polycystic ovary syndrome attempting conception includes lifestyle modifications, medications for ovulation induction, and possible use of insulin sensitizers. Metformin alone is not effective as a single agent for achieving pregnancy.38 Diet, weight loss, and exercise can have dramatic effects on ovulation and pregnancy and should be highly encouraged.
Ovulation induction is often required in anovulatory women, either in combination with lifestyle modifications or used subsequently if modifications are not successful. Letrozole is advised as the initial agent in women with obesity and anovulatory infertility rather than clomiphene citrate; a side-by-side comparison demonstrated increased rates of ovulation and live birth with letrozole.39
Once-daily letrozole 2.5 mg or clomiphene 50 mg can be prescribed for 5 days, from cycle days 3 through 7 to cycle days 5 through 9. If this initial dosing fails to result in ovulation, the dose can be increased. Known adverse effects are hot flashes, headaches, ovarian cysts, and increased risk of multiple gestation.
Metformin should be considered as an adjunct to fertility treatments in women with polycystic ovary syndrome, especially those with obesity or impaired glucose tolerance, or if there is no response to standard ovulation induction.
Ovarian hyperstimulation syndrome (cystic enlargement of the ovaries with potentially dangerous fluid and electrolyte imbalances) can occur in women with polycystic ovary syndrome; however, it rarely occurs with oral medications.
- Chandra A, Copen CE, Stephen EH. Infertility service use in the United States: data from the National Survey of Family Growth, 1982–2010. Natl Health Stat Report 2014; (73):1–21. pmid:24467919
- Mosher WD, Pratt WF. Fecundity and infertility in the United States: incidence and trends. Fertil Steril 1991; 56(2):192–193. pmid:2070846
- Boltz MW, Sanders JN, Simonsen SE, Stanford JB. Fertility treatment, use of in vitro fertilization, and time to live birth based on initial provider type. J Am Board Fam Med 2017; 30(2):230–238. doi:10.3122/jabfm.2017.02.160184
- Hampton K, Mazza D. Fertility-awareness knowledge, attitudes and practices of women attending general practice. Aust Fam Physician 2015; 44(11):840–845. pmid:26590626
- Zegers-Hochschild F, Adamson GD, de Mouzon J, et al; International Committee for Monitoring Assisted Reproductive Technology; World Health Organization. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril 2009; 92(5):1520–1524. doi:10.1016/j.fertnstert.2009.09.009
- Domar AD, Zuttermeister PC, Friedman R. The psychological impact of infertility: a comparison with patients with other medical conditions. J Psychosom Obstet Gynaecol 1993; 14(suppl):45–52. pmid:8142988
- Argyle CE, Harper JC, Davies MC. Oocyte cryopreservation: where are we now? Hum Reprod Update 2016; 22(4):440–449. doi:10.1093/humupd/dmw007
- Practice Committee of American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril 2013; 99(1):63. doi:10.1016/j.fertnstert.2012.09.023
- Guttmacher AF. Factors affecting normal expectancy of conception. J Am Med Assoc 1956; 161(9):855–860. pmid:13319020
- Dunson DB, Baird DD, Colombo B. Increased infertility with age in men and women. Obstet Gynecol 2004; 103(1):51–56. doi:10.1097/01.AOG.0000100153.24061.45
- National Collaborating Centre for Women’s and Children’s Health (UK). Fertility: assessment and treatment for people with fertility problems. London: Royal College of Obstetricians & Gynaecologists; 2013. www.ncbi.nlm.nih.gov/books/NBK247932. Accessed May 6, 2019.
- Elzanaty S, Malm J, Giwercman A. Duration of sexual abstinence: epididymal and accessory sex gland secretions and their relationship to sperm motility. Hum Reprod 2005; 20(1):221–225. doi:10.1093/humrep/deh586
- Wilcox AJ, Weinberg CR, Baird DD. Timing of sexual intercourse in relation to ovulation. Effects on the probability of conception, survival of the pregnancy, and sex of the baby. N Engl J Med 1995; 333(23):1517–1521. doi:10.1056/NEJM199512073332301
- Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society for Reproductive Endocrinology and Infertility. Optimizing natural fertility: a committee opinion. Fertil Steril 2017; 107(1):52–58. doi:10.1016/j.fertnstert.2016.09.029
- Kutteh WH, Chao CH, Ritter JO, Byrd W. Vaginal lubricants for the infertile couple: effect on sperm activity. Int J Fertil Menopausal Stud 1996; 41(4):400–404. pmid:8894797
- Bigelow JL, Dunson DB, Stanford JB, Ecochard R, Gnoth C, Colombo B. Mucus observations in the fertile window: a better predictor of conception than timing of intercourse. Hum Reprod 2004; 19(4):889–892. doi:10.1093/humrep/deh173
- Steiner AZ, Pritchard D, Stanczyk FZ, et al. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. JAMA 2017; 318(14):1367–1376. doi:10.1001/jama.2017.14588
- Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update 2006; 12(6):685–718. doi:10.1093/humupd/dml034
- Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile female: a committee opinion. Fertil Steril 2015; 103(6):e44–e50. doi:10.1016/j.fertnstert.2015.03.019
- Sharara FI, Scott RT Jr, Seifer DB. The detection of diminished ovarian reserve in infertile women. Am J Obstet Gynecol 1998; 179(3 Pt 1):804–812. pmid:9757994
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline. Committee Opinion No. 589. Fertil Steril 2014; 101(3):633–634. doi:10.1016/j.fertnstert.2013.12.032
- Balasch J, Gratacós E. Delayed childbearing: effects on fertility and the outcome of pregnancy. Curr Opin Obstet Gynecol 2012; 24(3):187–193. doi:10.1097/GCO.0b013e3283517908
- Hickman LC, Fortin C, Goodman L, Liu X, Flyckt R. Fertility and fertility preservation: knowledge, awareness and attitudes of female graduate students. Eur J Contracept Reprod Health Care 2018; 23(2):130–138. doi:10.1080/13625187.2018.1455085
- Lundsberg LS, Pal L, Gariepy AM, Xu X, Chu MC, Illuzzi JL. Knowledge, attitudes, and practices regarding conception and fertility: a population-based survey among reproductive-age United States women. Fertil Steril 2014; 101(3):767–774. doi:10.1016/j.fertnstert.2013.12.006
- Hodes-Wertz B, Druckenmiller S, Smith M, Noyes N. What do reproductive-age women who undergo oocyte cryopreservation think about the process as a means to preserve fertility? Fertil Steril 2013; 100(5):1343–1349. doi:10.1016/j.fertnstert.2013.07.201
- Weström L, Joesoef R, Reynolds G, Hagdu A, Thompson SE. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis 1992; 19(4):185–192. pmid:1411832
- ACOG Practice Bulletin No. 195: prevention of infection after gynecologic procedures. Obstet Gynecol 2018; 131(6):e172–e189. doi:10.1097/AOG.0000000000002670
- Balasch J, Creus M, Fábregues F, et al. Visible and non-visible endometriosis at laparoscopy in fertile and infertile women and in patients with chronic pelvic pain: a prospective study. Hum Reprod 1996; 11(2):387–391. pmid:8671229
- Falcone T, Flyckt R. Clinical management of endometriosis. Obstet Gynecol 2018; 131(3):557–571. doi:10.1097/AOG.0000000000002469
- Flyckt R, Kim S, Falcone T. Surgical management of endometriosis in patients with chronic pelvic pain. Semin Reprod Med 2017; 35(1):54–64. doi:10.1055/s-0036-1597306
- Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: a committee opinion. Fertil Steril 2012; 98(3):591–598. doi:10.1016/j.fertnstert.2012.05.031
- Thonneau P, Marchand S, Tallec A, et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988–1989). Hum Reprod 1991; 6(6):811–816. pmid:1757519
- Cooper TG, Noonan E, von Eckardstein S, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update 2010; 16(3):231–245. doi:10.1093/humupd/dmp048
- Practice Committee of American Society for Reproductive Medicine. Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril 2012; 98(2):294–301. doi:10.1016/j.fertnstert.2012.05.033
- Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004; 19(1):41–47. pmid:14688154
- Falcone T, Finegood DT, Fantus IG, Morris D. Androgen response to endogenous insulin secretion during the frequently sampled intravenous glucose tolerance test in normal and hyperandrogenic women. J Clin Endocrinol Metab 1990; 71(6):1653–1657. doi:10.1210/jcem-71-6-1653
- Daniilidis A, Dinas K. Long term health consequences of polycystic ovarian syndrome: a review analysis. Hippokratia 2009; 13(2):90–92. pmid:19561777
- Legro RS, Barnhart HX, Schlaff WD, et al; Cooperative Multicenter Reproductive Medicine Network. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med 2007; 356(6):551–566. doi:10.1056/NEJMoa063971
- Legro RS, Brzyski RG, Diamond MP, et al; NICHD Reproductive Medicine Network. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med 2014; 371(2):119–129. doi:10.1056/NEJMoa1313517
- Chandra A, Copen CE, Stephen EH. Infertility service use in the United States: data from the National Survey of Family Growth, 1982–2010. Natl Health Stat Report 2014; (73):1–21. pmid:24467919
- Mosher WD, Pratt WF. Fecundity and infertility in the United States: incidence and trends. Fertil Steril 1991; 56(2):192–193. pmid:2070846
- Boltz MW, Sanders JN, Simonsen SE, Stanford JB. Fertility treatment, use of in vitro fertilization, and time to live birth based on initial provider type. J Am Board Fam Med 2017; 30(2):230–238. doi:10.3122/jabfm.2017.02.160184
- Hampton K, Mazza D. Fertility-awareness knowledge, attitudes and practices of women attending general practice. Aust Fam Physician 2015; 44(11):840–845. pmid:26590626
- Zegers-Hochschild F, Adamson GD, de Mouzon J, et al; International Committee for Monitoring Assisted Reproductive Technology; World Health Organization. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril 2009; 92(5):1520–1524. doi:10.1016/j.fertnstert.2009.09.009
- Domar AD, Zuttermeister PC, Friedman R. The psychological impact of infertility: a comparison with patients with other medical conditions. J Psychosom Obstet Gynaecol 1993; 14(suppl):45–52. pmid:8142988
- Argyle CE, Harper JC, Davies MC. Oocyte cryopreservation: where are we now? Hum Reprod Update 2016; 22(4):440–449. doi:10.1093/humupd/dmw007
- Practice Committee of American Society for Reproductive Medicine. Definitions of infertility and recurrent pregnancy loss: a committee opinion. Fertil Steril 2013; 99(1):63. doi:10.1016/j.fertnstert.2012.09.023
- Guttmacher AF. Factors affecting normal expectancy of conception. J Am Med Assoc 1956; 161(9):855–860. pmid:13319020
- Dunson DB, Baird DD, Colombo B. Increased infertility with age in men and women. Obstet Gynecol 2004; 103(1):51–56. doi:10.1097/01.AOG.0000100153.24061.45
- National Collaborating Centre for Women’s and Children’s Health (UK). Fertility: assessment and treatment for people with fertility problems. London: Royal College of Obstetricians & Gynaecologists; 2013. www.ncbi.nlm.nih.gov/books/NBK247932. Accessed May 6, 2019.
- Elzanaty S, Malm J, Giwercman A. Duration of sexual abstinence: epididymal and accessory sex gland secretions and their relationship to sperm motility. Hum Reprod 2005; 20(1):221–225. doi:10.1093/humrep/deh586
- Wilcox AJ, Weinberg CR, Baird DD. Timing of sexual intercourse in relation to ovulation. Effects on the probability of conception, survival of the pregnancy, and sex of the baby. N Engl J Med 1995; 333(23):1517–1521. doi:10.1056/NEJM199512073332301
- Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society for Reproductive Endocrinology and Infertility. Optimizing natural fertility: a committee opinion. Fertil Steril 2017; 107(1):52–58. doi:10.1016/j.fertnstert.2016.09.029
- Kutteh WH, Chao CH, Ritter JO, Byrd W. Vaginal lubricants for the infertile couple: effect on sperm activity. Int J Fertil Menopausal Stud 1996; 41(4):400–404. pmid:8894797
- Bigelow JL, Dunson DB, Stanford JB, Ecochard R, Gnoth C, Colombo B. Mucus observations in the fertile window: a better predictor of conception than timing of intercourse. Hum Reprod 2004; 19(4):889–892. doi:10.1093/humrep/deh173
- Steiner AZ, Pritchard D, Stanczyk FZ, et al. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. JAMA 2017; 318(14):1367–1376. doi:10.1001/jama.2017.14588
- Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update 2006; 12(6):685–718. doi:10.1093/humupd/dml034
- Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile female: a committee opinion. Fertil Steril 2015; 103(6):e44–e50. doi:10.1016/j.fertnstert.2015.03.019
- Sharara FI, Scott RT Jr, Seifer DB. The detection of diminished ovarian reserve in infertile women. Am J Obstet Gynecol 1998; 179(3 Pt 1):804–812. pmid:9757994
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice and Practice Committee. Female age-related fertility decline. Committee Opinion No. 589. Fertil Steril 2014; 101(3):633–634. doi:10.1016/j.fertnstert.2013.12.032
- Balasch J, Gratacós E. Delayed childbearing: effects on fertility and the outcome of pregnancy. Curr Opin Obstet Gynecol 2012; 24(3):187–193. doi:10.1097/GCO.0b013e3283517908
- Hickman LC, Fortin C, Goodman L, Liu X, Flyckt R. Fertility and fertility preservation: knowledge, awareness and attitudes of female graduate students. Eur J Contracept Reprod Health Care 2018; 23(2):130–138. doi:10.1080/13625187.2018.1455085
- Lundsberg LS, Pal L, Gariepy AM, Xu X, Chu MC, Illuzzi JL. Knowledge, attitudes, and practices regarding conception and fertility: a population-based survey among reproductive-age United States women. Fertil Steril 2014; 101(3):767–774. doi:10.1016/j.fertnstert.2013.12.006
- Hodes-Wertz B, Druckenmiller S, Smith M, Noyes N. What do reproductive-age women who undergo oocyte cryopreservation think about the process as a means to preserve fertility? Fertil Steril 2013; 100(5):1343–1349. doi:10.1016/j.fertnstert.2013.07.201
- Weström L, Joesoef R, Reynolds G, Hagdu A, Thompson SE. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis 1992; 19(4):185–192. pmid:1411832
- ACOG Practice Bulletin No. 195: prevention of infection after gynecologic procedures. Obstet Gynecol 2018; 131(6):e172–e189. doi:10.1097/AOG.0000000000002670
- Balasch J, Creus M, Fábregues F, et al. Visible and non-visible endometriosis at laparoscopy in fertile and infertile women and in patients with chronic pelvic pain: a prospective study. Hum Reprod 1996; 11(2):387–391. pmid:8671229
- Falcone T, Flyckt R. Clinical management of endometriosis. Obstet Gynecol 2018; 131(3):557–571. doi:10.1097/AOG.0000000000002469
- Flyckt R, Kim S, Falcone T. Surgical management of endometriosis in patients with chronic pelvic pain. Semin Reprod Med 2017; 35(1):54–64. doi:10.1055/s-0036-1597306
- Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: a committee opinion. Fertil Steril 2012; 98(3):591–598. doi:10.1016/j.fertnstert.2012.05.031
- Thonneau P, Marchand S, Tallec A, et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988–1989). Hum Reprod 1991; 6(6):811–816. pmid:1757519
- Cooper TG, Noonan E, von Eckardstein S, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update 2010; 16(3):231–245. doi:10.1093/humupd/dmp048
- Practice Committee of American Society for Reproductive Medicine. Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril 2012; 98(2):294–301. doi:10.1016/j.fertnstert.2012.05.033
- Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004; 19(1):41–47. pmid:14688154
- Falcone T, Finegood DT, Fantus IG, Morris D. Androgen response to endogenous insulin secretion during the frequently sampled intravenous glucose tolerance test in normal and hyperandrogenic women. J Clin Endocrinol Metab 1990; 71(6):1653–1657. doi:10.1210/jcem-71-6-1653
- Daniilidis A, Dinas K. Long term health consequences of polycystic ovarian syndrome: a review analysis. Hippokratia 2009; 13(2):90–92. pmid:19561777
- Legro RS, Barnhart HX, Schlaff WD, et al; Cooperative Multicenter Reproductive Medicine Network. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med 2007; 356(6):551–566. doi:10.1056/NEJMoa063971
- Legro RS, Brzyski RG, Diamond MP, et al; NICHD Reproductive Medicine Network. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med 2014; 371(2):119–129. doi:10.1056/NEJMoa1313517
KEY POINTS
- A primary care physician can provide advice and testing regarding most fertility concerns.
- Female reproductive aging is a central threat to fertility, and prompt assessment and referral are warranted for women age 35 and older.
- Male factor infertility can now often be overcome with assisted reproductive technologies.
- Polycystic ovary syndrome can cause anovulation and has metabolic effects that can evolve into metabolic syndrome, with serious health consequences.
Ambulatory ECG monitoring in the age of smartphones
A mbulatory electrocardiography (ECG) began in 1949 when Norman “Jeff” Holter developed a monitor that could wirelessly transmit electrophysiologic data.1 His original device used vacuum tubes, weighed 85 pounds, and had to be carried in a backpack. Furthermore, it could send a signal a distance of only 1 block.2
At the time, it was uncertain if this technology would have any clinical utility. However, in 1952, Holter published the first tracing of abnormal cardiac electrical activity in a patient who had suffered a posterior myocardial infarction.3 By the 1960s, Holter monitoring systems were in full production and use.4
Since then, advances in technology have led to small, lightweight devices that enable clinicians to evaluate patients for arrhythmias in a real-world context for extended times, often with the ability to respond in real time.
Many ambulatory devices are available, and choosing the optimal one requires an understanding of which features they have and which are the most appropriate for the specific clinical context. This article reviews the features, indications, advantages, and disadvantages of current devices, and their best use in clinical practice.
INDICATIONS FOR AMBULATORY ECG MONITORING
Diagnosis
The most common diagnostic role of monitoring is to correlate unexplained symptoms, including palpitations, presyncope, and syncope, with a transient cardiac arrhythmia. Monitoring can be considered successful if findings on ECG identify risks for serious arrhythmia and either correlate symptoms with those findings or demonstrate no arrhythmia when symptoms occur.
A range of arrhythmias can cause symptoms. Some, such as premature atrial contractions and premature ventricular contractions, may be benign in many clinical contexts. Others, such as atrial fibrillation, are more serious, and some, such as third-degree heart block and ventricular tachycardia, can be lethal.
Arrhythmia symptoms can vary in frequency and cause differing degrees of debility. The patient’s symptoms, family history, and baseline ECG findings can suggest a more serious or a less serious underlying rhythm. These factors are important when determining which device is most appropriate.
Ambulatory ECG can also be useful in looking for a cause of cryptogenic stroke, ie, an ischemic stroke with an unexplained cause, even after a thorough initial workup. Paroxysmal atrial fibrillation is a frequent cause of cryptogenic stroke, and because it is transient, short-term inpatient telemetry may not be sufficient to detect it. Extended cardiac monitoring, lasting weeks or even months, is often needed for clinicians to make this diagnosis and initiate appropriate secondary prevention.
Prognosis: Identifying patients at risk
In a patient with known structural or electrical heart disease, ambulatory ECG can be used to stratify risk. This is particularly true in evaluating conditions associated with sudden cardiac death.
For example, hypertrophic cardiomyopathy and arrhythmogenic right ventricular dysplasia or cardiomyopathy are 2 cardiomyopathies that can manifest clinically with ventricular arrhythmias and sudden cardiac death. Ambulatory ECG can detect premature ventricular contractions and ventricular tachycardia and identify their frequency, duration, and anatomic origin. This information is useful in assessing risk of sudden cardiac death and determining the need for an implantable cardioverter-defibrillator.
Similarly, Wolff-Parkinson-White syndrome, involving rapid conduction through an accessory pathway, is associated with increased risk of ventricular fibrillation and sudden cardiac death. Ambulatory ECG monitoring can identify patients who have electrical features that portend the development of ventricular fibrillation.
Also associated with sudden cardiac death are the inherited channelopathies, a heterogeneous group of primary arrhythmic disorders without accompanying structural pathology. Ambulatory ECG monitoring can detect transient electrical changes and nonsustained ventricular arrhythmias that would indicate the patient is at high risk of these disorders.
Assessing arrhythmia treatment
Arrhythmia monitoring using an ambulatory ECG device can also provide data to assess the efficacy of treatment under several circumstances.
The “pill-in-the-pocket” approach to treating atrial fibrillation, for example, involves self-administering a single dose of an antiarrhythmic drug when symptoms occur. Patients with infrequent but bothersome episodes can use an ambulatory ECG device to detect when they are having atrial fibrillation, take their prescribed drug, and see whether it terminates the arrhythmia, all without going to the hospital.
Ambulatory ECG also is useful for assessing pharmacologic or ablative therapy in patients with atrial fibrillation or ventricular tachycardia. Monitoring for several weeks can help clinicians assess the burden of atrial fibrillation when using a rhythm-control strategy; assessing the ventricular rate in real-world situations is useful to determine the success of a rate-control strategy. Shortly after ablation of either atrial fibrillation or ventricular tachycardia, ECG home monitoring for 24 to 48 hours can detect asymptomatic recurrence and treatment failure.
Some antiarrhythmic drugs can prolong the QT interval. Ambulatory ECG devices that feature real-time monitoring can be used during drug initiation, enabling the clinician to monitor the QT interval without admitting the patient to the hospital.
Ultimately, ambulatory ECG monitoring is most commonly used to evaluate symptoms. Because arrhythmias and specific symptoms are unpredictable and transient, extended monitoring in a real-world setting allows for a more comprehensive evaluation than a standard 10-second ECG recording.
AMBULATORY ECG DEVICES
Continuous external monitoring: The Holter monitor
Recording is typically done continuously for 24 to 48 hours, although some newer devices can record for longer. Patients can press a button to note when they are experiencing symptoms, allowing for potential correlation with ECG abnormalities. The data are stored on a flash drive that can be uploaded for analysis after recording is complete.
What is its best use? Given its relatively short duration of monitoring, the Holter device is typically used to evaluate symptoms that occur daily or nearly daily. An advantage of the Holter monitor is its ability to record continuously, without requiring the patient to interact with the device. This feature provides “full disclosure,” which is the ability to see arrhythmia data from the entire recording period.
These features make Holter monitoring useful to identify suspected frequently occurring silent arrhythmias or to assess the overall arrhythmia burden. A typical Holter report can contain information on the heart rate (maximum, minimum, and average), ectopic beats, and tachy- and bradyarrhythmias, as well as representative samples.
The Holter device is familiar to most practitioners and remains an effective choice for ambulatory ECG monitoring. However, its use has largely been replaced by newer devices that overcome the Holter’s drawbacks, particularly its short duration of monitoring and the need for postmonitoring analysis. Additionally, although newer Holter devices are more ergonomic, some patients find the wires and gel electrodes uncomfortable or inconvenient.
Intermittent monitoring: Event recorders
Unlike the continuous monitors, intermittent recording devices (also called event recorders), capture and store tracings only during an event.
Intermittent recording monitors are of 2 general types: post-event recorders and loop recorders. These devices can extend the overall duration of observation, which can be especially useful for those whose symptoms and arrhythmias are infrequent.
Post-event recorders are small and self-contained, not requiring electrodes (Figure 1). The device is carried by the patient but not worn continuously. When the patient experiences symptoms, he or she places the device against the chest and presses a button to begin recording. These tracings are stored on the device and can be transmitted by telephone to a data center for analysis. Although post-event recorders allow for monitoring periods typically up to 30 days, they are limited by requiring the patient to act to record an event.
What is its best use? These devices are best used in patients who have infrequent symptoms and are at low risk. Transient or debilitating symptoms, including syncope, can limit the possibility of capturing an event.
Intermittent monitoring: Loop recorders
Loop recorders monitor continuously but record only intermittently. The name refers to the device’s looping memory: ie, to extend how long it can be used and make the most of its limited storage, the device records over previously captured data, saving only the most important data. The device saves the data whenever it detects an abnormal rhythm or the patient experiences symptoms and pushes a button. Data are recorded for a specified time before and after the activation, typically 30 seconds.
Loop recorders come in 2 types: external and implantable.
External loop recorders
External loop recorders look like Holter monitors (Figure 1), but they have the advantage of a much longer observation period—typically up to 1 month. The newest devices have even greater storage capacity and can provide “backward” memory, saving data that were captured just before the patient pushed the button.
In studies of patients with palpitations, presyncope, or syncope, external loop recorders had greater diagnostic yield than traditional 24-hour Holter monitors.7,8 This finding was supported by a clinical trial that found 30-day monitoring with an external loop recorder led to a 5-fold increase in detecting atrial fibrillation in patients with cryptogenic stroke.9
Disadvantages of external loop recorders are limited memory storage, a considerable reliance on patient activation of the device, and wires and electrodes that need to be worn continuously.
What is their best use? External loop recorders are most effective when used to detect an arrhythmia or to correlate infrequent symptoms with an arrhythmia. They are most appropriately used in patients whose symptoms occur more often than every 4 weeks. They are less useful in assessing very infrequent symptoms, overall arrhythmia burden, or responsiveness to therapy.10
Implantable loop recorders
Implantable loop recorders are small devices that contain a pair of sensing electrodes housed within an outer shell (Figure 1). They are implanted subcutaneously, usually in the left parasternal region, using local anesthesia. The subcutaneous location eliminates many of the drawbacks of the skin-electrode interface of external loop recorders.
Similar to the external loop recorder, this device monitors continuously and can be activated to record either by the patient by pressing a button on a separate device, or automatically when an arrhythmia is detected using a preprogrammed algorithm.
In contrast to external devices, many internal loop recorders have a battery life and monitoring capability of up to 3 years. This extended monitoring period has been shown to increase the likelihood of diagnosing syncope or infrequent palpitations.11,12 Given that paroxysmal atrial fibrillation can be sporadic and reveal itself months after a stroke, internal loop recorders may also have a role in evaluating cryptogenic stroke.13,14
The most important drawbacks of internal loop recorders are the surgical procedure for insertion, their limited memory storage, and high upfront cost.15 Furthermore, even though they allow for extended monitoring, there may be diminishing returns for prolonged observation.
What is their best use? For patients with palpitations, intermittent event monitoring has been shown to be cost-effective for the first 2 weeks, but after 3 weeks, the cost per diagnosis increases dramatically.16 As a result, internal loop recorders are reserved primarily for scenarios in which prolonged external monitoring has not revealed a source of arrhythmia despite a high degree of suspicion.
Mobile cardiac telemetry
Mobile cardiac telemetry builds on other ECG monitoring systems by adding real-time communication and technician evaluation.
Physically, these devices resemble either hand-held event records, with a single-channel sensing unit embedded in the case, or a traditional Holter monitor, with 3 channels, wires, and electrodes (Figure 1).
The sensor wirelessly communicates with a nearby portable monitor, which continuously observes and analyzes the patient’s heart rhythm. When an abnormal rhythm is detected or when the patient marks the presence of symptoms, data are recorded and sent in real time via a cellular network to a monitoring center; the newest monitors can send data via any Wi-Fi system. The rhythm is then either evaluated by a trained technician or relayed to a physician. If necessary, the patient can be contacted immediately.
Mobile cardiac telemetry is typically used for up to 30 days, which allows for evaluation of less-frequent symptoms. As a result, it may have a higher diagnostic yield for palpitations, syncope, and presyncope than the 24-hour Holter monitor.17
Further, perhaps because mobile cardiac telemetry relies less on stored information and requires less patient-device interaction than external loop recorders, it is more effective at symptom evaluation.18
Mobile cardiac telemetry also has a diagnostic role in evaluating patients with cryptogenic stroke. This is based on studies showing it has a high rate of atrial fibrillation detection in this patient population and is more effective at determining overall atrial fibrillation burden than loop recorders.18,19
What is its best use? The key advantage of mobile cardiac telemetry is its ability to make rhythm assessments and communicate with technicians in real time. This allows high-risk patients to be immediately alerted to a life-threatening arrhythmia. It also gives providers an opportunity to initiate anticoagulation or titrate antiarrhythmic therapy in the outpatient setting without a delay in obtaining information. This intensive monitoring, however, requires significant manpower, which translates to higher cost, averaging 3 times that of other standard external monitors.15
Patch monitors
These ultraportable devices are a relatively unobtrusive and easy-to-use alternative for short-term ambulatory ECG monitoring. They monitor continuously with full disclosure, outpatient telemetry, and post-event recording features.
Patch monitors are small, leadless, wireless, and water-resistant (Figure 1). They are affixed to the left pectoral region with a waterproof adhesive and can be worn for 14 to 28 days. Recording is usually done continuously; however, these devices have an event marker button that can be pressed when the user experiences symptoms. They acquire a single channel of data, and each manufacturer has a proprietary algorithm for automated rhythm detection and analysis.20
Several manufacturers produce ECG patch monitors. Two notable devices are the Zio patch (iRhythm Technologies, San Francisco, CA) and the Mobile Cardiac Outpatient Telemetry patch (BioTelemetry, Inc, Malvern, PA).
The Zio patch is a continuous external monitor with full disclosure. It is comparable to the Holter monitor, but has a longer recording period. After completing a 2-week monitoring period, the device is returned for comprehensive rhythm analysis. A typical Zio report contains information on atrial fibrillation burden, ectopic rhythm burden, symptom and rhythm correlation, heart rate trends, and relevant rhythm strips.
The Mobile Cardiac Outpatient Telemetry patch collects data continuously and communicates wirelessly by Bluetooth to send its ECG data to a monitoring center for evaluation.
A principal advantage of patch monitors—and a major selling point for manufacturers—is their low-profile, ergonomic, and patient-friendly design. Patients do not have to manage wires or batteries and are able to shower with their devices. Studies show that these features increase patient satisfaction and compliance, resulting in increased diagnostic yield.21,22 Additionally, patch monitors have the advantage of a longer continuous monitoring period than traditional Holter devices (2 weeks vs 1 or 2 days), affording an opportunity to capture events that occur less frequently.
Validation studies have reinforced their efficacy and utility in clinical scenarios.22,23 In large part because of the extended monitoring period, patch monitors have been shown to have greater diagnostic yield than the 24-hour Holter monitor in symptomatic patients undergoing workup for suspected arrhythmia.
The role of patch monitors in evaluating atrial fibrillation is also being established. For patients with cryptogenic stroke, patch monitors have shown better atrial fibrillation detection than the 24-hour Holter monitor.24 Compared with traditional loop monitors, patch monitors have the added advantage of assessing total atrial fibrillation burden. Further, although screening for atrial fibrillation with a traditional 12-lead ECG monitor has not been shown to be effective, clinical studies have found that the patch monitor may be a useful screening tool for high-risk patients.25,26
Nevertheless, patch monitors have drawbacks. They are not capable of long-term monitoring, owing to battery and adhesive limitations.20 More important, they have been able to offer only single-channel acquisition, which makes it more difficult to detect an arrhythmia that is characterized by a change in QRS axis or change in QRS width, or to distinguish an arrhythmia from an artifact. This appears to be changing, however, as several manufacturers have recently developed multilead ECG patch monitors or attachments and are attempting to merge this technology with fully capable remote telemetry.
CHOOSING THE RIGHT DEVICE
Recent improvements in battery life, memory, detection algorithms, wireless transmission, cellular communication, and adhesives have enabled multiple features to be combined into a single device. Patch monitors, for example, are small devices that now offer full-disclosure recording, extended monitoring, and telemetry transmitting. Automated arrhythmia recognition that triggers recording is central to all modern devices, regardless of type.
As a result of these trends, the traditional features used to differentiate devices may become less applicable. The classic Holter monitor may become obsolete as its advantages (full disclosure, continuous recording) are being incorporated into smaller devices that can record longer. Similarly, external monitors that have the capacity for full disclosure and continuous recording are no longer loop recorders in that they do not record into a circular memory.
It may be preferable to describe all non-Holter devices as event monitors or ambulatory monitors, with the main distinguishing features being the ability to transmit data (telemetry), full disclosure vs patient- or arrhythmia-activated recording, and single-channel or multichannel recording (single-lead or 3-lead ECG).
The following are the main distinguishing features that should influence the choice of device for a given clinical context.
Real-time data evaluation provided by mobile telemetry makes this feature ideal to monitor patients with suspected high-risk arrhythmias and their response to antiarrhythmic therapy.
Full-disclosure recording is necessary to assess the overall burden of an arrhythmia, which is frequently important in making treatment decisions, risk-stratifying, and assessing response to therapy. In contrast, patient- or arrhythmia-activated devices are best used when the goal is simply to establish the presence of an arrhythmia.
Multichannel recording may be better than single-channel recording, as it is needed to determine the anatomic origin of an arrhythmia, as might be the case in risk-stratification in a patient with a ventricular tachycardia.
Long duration. The clinician must have a reasonable estimate of how often the symptoms or arrhythmia occur to determine which device will offer a monitoring duration sufficient to detect an arrhythmia.
NEWER TECHNOLOGIES
The newest ambulatory ECG devices build on the foundational concepts of the older ones. However, with miniaturized electronic circuits, Bluetooth, Wi-Fi, and smartphones, these new devices can capture ECG tracings and diagnose offending arrhythmias on more consumer-friendly devices.
Smartphones and smartwatches have become increasingly powerful. Some have the ability to capture, display, and record the cardiac waveform. One manufacturer to capitalize on these technologies, AliveCor (Mountain View, CA), has developed 2 products capable of generating a single-lead ECG recording using either a smartphone (KardiaMobile) or an Apple watch (KardiaBand).
KardiaMobile has a 2-electrode band that can be carried in a pocket or attached to the back of a smartphone (Figure 1). The user places 1 or 2 fingers from each hand on the electrodes, and the device sends an ultrasound signal that is picked up by the smartphone’s microphone. The signal is digitized to produce a 30-second ECG tracing on the phone’s screen. A proprietary algorithm analyzes the rhythm and generates a description of “normal” or “possible atrial fibrillation.” The ECG is then uploaded to a cloud-based storage system for later access or transmission. KardiaMobile is compatible with both iOS and Android devices.
The KardiaBand is a specialized Apple watch band that has an electrode embedded in it. The user places a thumb on the electrode for 30 seconds, and an ECG tracing is displayed on the watch screen.
The Kardia devices were developed (and advertised) predominantly to assess atrial fibrillation. Studies have validated the accuracy of their algorithm. One study showed that, compared with physician-interpreted ECGs, the algorithm had a 96.6% sensitivity and 94.1% specificity for detecting atrial fibrillation.27 They have been found useful for detecting and evaluating atrial fibrillation in several clinical scenarios, including discharge monitoring in patients after ablation or cardiac surgery.28,29 In a longer study of patients at risk of stroke, twice-weekly ECG screening using a Kardia device for 1 year was more likely to detect incident atrial fibrillation than routine care alone.30
Also, the Kardia devices can effectively function as post-event recorders when activated by patients when they experience symptoms. In a small study of outpatients with palpitations and a prior nondiagnostic workup, the KardiaMobile device was found to be noninferior to external loop recorders for detecting arrhythmias.31 Additional studies are assessing Kardia’s utility in other scenarios, including the evaluation of ST-segment elevation myocardial infarction32,33 and QT interval for patients receiving antiarrhythmic therapy.34
Cardiio Inc. (Cambridge, MA) has developed technology to screen for atrial fibrillation using an app that requires no additional external hardware. Instead, the app uses a smartphone’s camera and flashlight to perform photoplethysmography to detect pulsatile changes in blood volume and generate a waveform. Based on waveform variability, a proprietary algorithm attempts to determine whether the user is in atrial fibrillation. It does not produce an ECG tracing. Initial studies suggest it has good diagnostic accuracy and potential utility as a population-based screening tool,35,36 but it has not been fully validated.
Recently, Apple entered the arena of ambulatory cardiac monitoring with the release of its fourth-generation watch (Apple Watch Series 4 model). This watch has built-in electrodes that can generate a single-lead ECG on the watch screen. Its algorithm can discriminate between atrial fibrillation and sinus rhythm, but it has not been assessed for its ability to evaluate other arrhythmias. Even though it has been “cleared” by the US Food and Drug Administration, it is approved only for informational use, not to make a medical diagnosis.
Integration of ambulatory ECG technology with smartphone and watch technology is an exciting new wearable option for arrhythmia detection. The patient-centered and controlled nature of these devices have the potential to help patients with palpitations or other symptoms determine if their cardiac rhythms are normal.
This technology, however, is still in its infancy and has many limitations. For example, even though these devices can function as post-event recorders, they depend on user-device interactions. Plus, they cannot yet perform continuous arrhythmia monitoring like modern loop recorders.
Additionally, automated analysis has largely been limited to distinguishing atrial fibrillation from normal sinus rhythm. It is uncertain how effective the devices may be in evaluating other arrhythmias. Single-lead ECG recordings, as discussed, have limited interpretability and value. And even though studies have shown utility in certain clinical scenarios, large-scale validation studies are lacking. This technology will likely continue to be developed and its clinical value improved; however, its clinical use requires careful consideration and collaborative physician-patient decision-making.
DISRUPTIVE TECHNOLOGY AND DIRECT-TO-CONSUMER MARKETING
The development of smartphone and watch ECG technology has led to a rise in direct-to-consumer healthcare delivery. By devising technology that is appealing, useful, and affordable, companies can bypass the insurer and practitioner by targeting increasingly health-literate consumers. For many companies, there is great motivation to enter this healthcare space. Wearable devices are immensely popular and, as a result, generate substantial revenue. One analysis estimates that 1 in 10 Americans (nearly 30 million) owns a wearable, smart-technology device.37
This direct-to-consumer approach has specific implications for cardiology and, more broadly, for healthcare overall. By directly selling to consumers, companies have an opportunity to reach many more people. The Apple Watch Series 4 has taken this a step further: by including this technology in the watch, consumers not necessarily seeking an ambulatory cardiac monitor will have one with a watch purchase. This could lead to increases in monitoring and could alert people to previously undiagnosed disorders.
For consumers, this technology can empower them to choose how and when to be monitored. Further, it gives them personal control of their healthcare data, and helps move the point of care out of hospitals and clinics and into the home.
But wearable medical technology and direct-to-consumer healthcare have risks. First, in the absence of appropriate regulation, patients have to distinguish between products that are well validated and those that are unproven. Consumers also may inappropriately use devices for indications or in scenarios for which the value is uncertain.
Also, there is potential for confusion and misunderstanding of results, including false-positive readings, which could lead to excessive and costly use of unnecessary diagnostic workups. Instead of providing peace of mind, these devices could cause greater worry. This may be especially true with the newest Apple watch, as this product will introduce ambulatory ECG to a younger and healthier segment of the population who are less likely to have true disease.
Further, these devices have algorithms that detect atrial fibrillation, but is it the same as that detected by traditional methods? Sometimes termed “subclinical” atrial fibrillation, it poses uncertainties: ie, Do patients need anticoagulation, pharmacologic therapy, and ablation? The optimal management of subclinical atrial fibrillation, as well as its similarities to and differences from atrial fibrillation diagnosed by traditional methods, are topics that need further study.
Wearable technology is still developing and will continue to do so. Medical practice will have to adapt to it.
FUTURE DIRECTIONS
Changes in technology have led to remarkable advances in the convenience and accuracy of ambulatory ECG monitoring. Ongoing research is expected to lead to even more improvements. Devices will become more ergonomic and technically capable, and they may expand monitoring to include other biologic parameters beyond ECG.
Comfort is important to ensure patient adherence. Newer, flexible electronics embedded in ultrathin materials can potentially improve the wearability of devices that require gel electrodes or adhesive patches.38 Wireless technology may obviate the need for on-skin attachments. Future recording systems may be embedded into clothing or incorporated into wearable vests capable of wirelessly transmitting ECG signals to separate recording stations.39
In addition to becoming smaller and more comfortable, future devices will be more technically capable, leading to a merging of technologies that will further blur the distinctions among devices. Eventually, the features of full disclosure, extended monitoring duration, and telemetric communication will all be present together. Perhaps more important is that ambulatory ECG devices may become fully capable biosensor monitors. These devices would have the potential to monitor respiratory frequency, peripheral oxygen saturation, potassium levels, and arterial pulse pressure.39,40
- Holter NJ, Gengerelli JA. Remote recording of physiological data by radio. Rocky Mt Med J 1949; 46(9):747–751. pmid:18137532
- Kennedy HL. The history, science, and innovation of Holter technology. Ann Noninvasive Elecrocardiol 2006; 11(1):85–94. doi:10.1111/j.1542-474X.2006.00067.x
- MacInnis HF. The clinical application of radioelectrocardiography. Can Med Assoc J 1954; 70(5):574– 576. pmid:13160894
- Del Mar B. The history of clinical Holter monitoring. Ann Noninvasive Elecrocardiol. 2005; 10(2):226–230. doi:10.1111/j.1542-474X.2005.10202.x
- Crawford MH, Bernstein SJ, Deedwania PC, et al. ACC/AHA guidelines for ambulatory electrocardiography. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the Guidelines for Ambulatory Electrocardiography). Developed in collaboration with the North American Society for Pacing and Electrophysiology. J Am Coll Cardiol 1999; 34(3):912–948. pmid:10483977
- Steinberg JS, Varma N, Cygankiewicz I, et al. 2017 ISHNE-HRS expert consensus statement on ambulatory ECG and external cardiac monitoring/telemetry. Heart Rhythm 2017; 14(7):e55–e96. doi:10.1016/j.hrthm.2017.03.038
- Locati ET, Vecchi AM, Vargiu S, Cattafi G, Lunati M. Role of extended external loop recorders for the diagnosis of unexplained syncope, pre-syncope, and sustained palpitations. Europace 2014; 16(6):914–922. doi:10.1093/europace/eut337
- Locati ET, Moya A, Oliveira, et al. External prolonged electrocardiogram monitoring in unexplained syncope and palpitations: results of the SYNARR-Flash study. Europace 2016; 18(8):1265–1272. doi:10.1093/europace/euv311
- Gladstone DJ, Spring M, Dorian P, et al; EMBRACE Investigators and Coordinators. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014; 370(26):2467–2477. doi:10.1056/NEJMoa1311376
- Brignole M, Vardas P, Hoffman E, et al; EHRA Scientific Documents Committee. Indications for the use of diagnostic implantable and external ECG loop recorders. Europace 2009; 11(5):671–687. doi:10.1093/europace/eup097
- Edvardsson N, Frykman V, van Mechelen R, et al; PICTURE Study Investigators. Use of an implantable loop recorder to increase the diagnostic yield in unexplained syncope: results from the PICTURE registry. Europace 2011; 13(2):262–269. doi:10.1093/europace/euq418
- Giada F, Gulizia M, Francese M, et al. Recurrent unexplained palpitations (RUP) study comparison of implantable loop recorder versus conventional diagnostic strategy. J Am Coll Cardiol 2007; 49(19):1951–1956. doi:10.1016/j.jacc.2007.02.036
- Christensen LM, Krieger DW, Hojberg S, et al. Paroxysmal atrial fibrillation occurs often in cryptogenic ischaemic stroke. Final results from the SURPRISE study. Eur J Neurol 2014; 21(6):884–889. doi:10.1111/ene.12400
- Cotter PE, Martin PJ, Ring L, Warburton EA, Belham M, Pugh PJ. Incidence of atrial fibrillation detected by implantable loop recorders in unexplained stroke. Neurology 2013; 80(17):1546–1550. doi:10.1212/WNL.0b013e31828f1828
- Zimetbaum P, Goldman A. Ambulatory arrhythmia monitoring: choosing the right device. Circulation 2010; 122(16):1629–1636. doi:10.1161/CIRCULATIONAHA.109.925610
- Zimetbaum PJ, Kim KY, Josephson ME, Goldberger AL, Cohen DJ. Diagnostic yield and optimal duration of continuous-loop event monitoring for the diagnosis of palpitations: a cost-effectiveness analysis. Ann Intern Med 1998; 128(11):890–895. pmid:9634426
- Joshi AK, Kowey PR, Prystowksy EN, et al. First experience with a mobile cardiac outpatient telemetry (MCOT) system for the diagnosis and management of cardiac arrhythmia. Am J Cardiol 2005; 95(7):878–881. doi:10.1016/j.amjcard.2004.12.015
- Rothman SA, Laughlin JC, Seltzer J, et al., The diagnosis of cardiac arrhythmias: a prospective multi-center randomized study comparing mobile cardiac outpatient telemetry versus standard loop event monitoring. J Cardiovasc Electrophysiol 2007; 18(3):241–247. pmid:17318994
- Tayal AH, Tian M, Kelly KM, et al. Atrial fibrillation detected by mobile cardiac outpatient telemetry in cryptogenic TIA or stroke. Neurology 2008; 71(21):1696–1701. doi:10.1212/01.wnl.0000325059.86313.31
- Lobodzinski SS. ECG patch monitors for assessment of cardiac rhythm abnormalities. Prog Cardiovasc Dis 2013; 56(2):224–229. doi:10.1016/j.pcad.2013.08.006
- Fung E, Jarvelin MR, Doshi RN, et al. Electrocardiographic patch devices and contemporary wireless cardiac monitoring. Front Physiol 2015; 6:149. doi:10.3389/fphys.2015.00149
- Barrett PM, Komatireddy R, Haaser S, et al. Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am J Med 2014; 127(1):95.e11–95.e17. doi:10.1016/j.amjmed.2013.10.003
- Schreiber D, Sattar A, Drigalla D, Higgins S. Ambulatory cardiac monitoring for discharged emergency department patients with possible cardiac arrhythmias. West J Emerg Med 2014; 15(2):194–198. doi:10.5811/westjem.2013.11.18973
- Tung CE, Su D, Turakhia MP, Lansberg MG. Diagnostic yield of extended cardiac patch monitoring in patients with stroke or TIA. Front Neurol 2015; 5:266. doi:10.3389/fneur.2014.00266
- Turakhia MP, Ullal AJ, Hoang DD, et al. Feasibility of extended ambulatory electrocardiogram monitoring to identify silent atrial fibrillation in high-risk patients: the Screening Study for Undiagnosed Atrial Fibrillation (STUDY-AF). Clin Cardiol 2015; 38(5):285–292. doi:10.1002/clc.22387
- Steinhubl SR, Waalen J, Edwards AM, et al. Effect of a home-based wearable continuous ECG monitoring patch on detection of undiagnosed atrial fibrillation: the mSToPS randomized clinical trial. JAMA 2018; 320(2):146–155. doi:10.1001/jama.2018.8102
- William AD, Kanbour M, Callahan T, et al. Assessing the accuracy of an automated atrial fibrillation detection algorithm using smartphone technology: the iREAD study. Heart Rhythm 2018; 15(10):1561–1565. doi:10.1016/j.hrthm.2018.06.037
- Tarakji KG, Wazni OM, Callahan T, et al. Using a novel wireless system for monitoring patients after the atrial fibrillation ablation procedure: the iTransmit study. Heart Rhythm 2015; 12(3):554–559. doi:10.1016/j.hrthm.2014.11.015
- Lowres N, Mulcahy G, Gallagher R, et al. Self-monitoring for atrial fibrillation recurrence in the discharge period post-cardiac surgery using an iPhone electrocardiogram. Eur J Cardiothorac Surg 2016; 50(1):44–51. doi:10.1093/ejcts/ezv486
- Halcox JPJ, Wareham K, Cardew A, et al. Assessment of remote heart rhythm sampling using the AliveCor heart monitor to screen for atrial fibrillation: the REHEARSE-AF study. Circulation 2017; 136(19):1784–1794. doi:10.1161/CIRCULATIONAHA.117.030583
- Narasimha D, Hanna N, Beck H, et al. Validation of a smartphone-based event recorder for arrhythmia detection. Pacing Clin Electrophysiol 2018; 41(5):487–494. doi:10.1111/pace.13317
- Muhlestein JB, Le V, Albert D, et al. Smartphone ECG for evaluation of STEMI: results of the ST LEUIS pilot study. J Electrocardiol 2015; 48(2):249–259. doi:10.1016/j.jelectrocard.2014.11.005
- Barbagelata A, Bethea CF, Severance HW, et al. Smartphone ECG for evaluation of ST-segment elevation myocardial infarction (STEMI): design of the ST LEUIS international multicenter study. J Electrocardiol 2018; 51(2):260–264. doi:10.1016/j.jelectrocard.2017.10.011
- Garabelli P, Stavrakis S, Albert M, et al. Comparison of QT interval readings in normal sinus rhythm between a smartphone heart monitor and a 12-lead ECG for healthy volunteers and inpatients receiving sotalol or dofetilide. J Cardiovasc Electrophysiol 2016; 27(7):827–832. doi:10.1111/jce.12976
- Rozen G, Vai J, Hosseini SM, et al. Diagnostic accuracy of a novel mobile phone application in monitoring atrial fibrillation. Am J Cardiol 2018; 121(10):1187–1191. doi:10.1016/j.amjcard.2018.01.035
- Chan PH, Wong CK, Poh YC, et al. Diagnostic performance of a smartphone-based photoplethysmographic application for atrial fibrillation screening in a primary care setting. J Am Heart Assoc 2016; 5(7). pii:e003428. doi:10.1161/JAHA.116.003428
- Mitchell ARJ, Le Page P. Living with the handheld ECG. BMJ Innov 2015; 1:46–48.
- Lee SP, Ha G, Wright DE, et al. Highly flexible, wearable, and disposable cardiac biosensors for remote and ambulatory monitoring. npj Digital Medicine 2018. doi:10.1038/s41746-017-0009-x
- Locati ET. New directions for ambulatory monitoring following the 2017 HRS-ISHNE expert consensus. J Electrocardiol 2017; 50(6):828–832. doi:10.1016/j.jelectrocard.2017.08.009
- Dillon JJ, DeSimone CV, Sapir Y, et al. Noninvasive potassium determination using a mathematically processed ECG: proof of concept for a novel “blood-less, blood test”. J Electrocardiol 2015; 48(1):12–18. doi:10.1016/j.jelectrocard.2014.10.002
A mbulatory electrocardiography (ECG) began in 1949 when Norman “Jeff” Holter developed a monitor that could wirelessly transmit electrophysiologic data.1 His original device used vacuum tubes, weighed 85 pounds, and had to be carried in a backpack. Furthermore, it could send a signal a distance of only 1 block.2
At the time, it was uncertain if this technology would have any clinical utility. However, in 1952, Holter published the first tracing of abnormal cardiac electrical activity in a patient who had suffered a posterior myocardial infarction.3 By the 1960s, Holter monitoring systems were in full production and use.4
Since then, advances in technology have led to small, lightweight devices that enable clinicians to evaluate patients for arrhythmias in a real-world context for extended times, often with the ability to respond in real time.
Many ambulatory devices are available, and choosing the optimal one requires an understanding of which features they have and which are the most appropriate for the specific clinical context. This article reviews the features, indications, advantages, and disadvantages of current devices, and their best use in clinical practice.
INDICATIONS FOR AMBULATORY ECG MONITORING
Diagnosis
The most common diagnostic role of monitoring is to correlate unexplained symptoms, including palpitations, presyncope, and syncope, with a transient cardiac arrhythmia. Monitoring can be considered successful if findings on ECG identify risks for serious arrhythmia and either correlate symptoms with those findings or demonstrate no arrhythmia when symptoms occur.
A range of arrhythmias can cause symptoms. Some, such as premature atrial contractions and premature ventricular contractions, may be benign in many clinical contexts. Others, such as atrial fibrillation, are more serious, and some, such as third-degree heart block and ventricular tachycardia, can be lethal.
Arrhythmia symptoms can vary in frequency and cause differing degrees of debility. The patient’s symptoms, family history, and baseline ECG findings can suggest a more serious or a less serious underlying rhythm. These factors are important when determining which device is most appropriate.
Ambulatory ECG can also be useful in looking for a cause of cryptogenic stroke, ie, an ischemic stroke with an unexplained cause, even after a thorough initial workup. Paroxysmal atrial fibrillation is a frequent cause of cryptogenic stroke, and because it is transient, short-term inpatient telemetry may not be sufficient to detect it. Extended cardiac monitoring, lasting weeks or even months, is often needed for clinicians to make this diagnosis and initiate appropriate secondary prevention.
Prognosis: Identifying patients at risk
In a patient with known structural or electrical heart disease, ambulatory ECG can be used to stratify risk. This is particularly true in evaluating conditions associated with sudden cardiac death.
For example, hypertrophic cardiomyopathy and arrhythmogenic right ventricular dysplasia or cardiomyopathy are 2 cardiomyopathies that can manifest clinically with ventricular arrhythmias and sudden cardiac death. Ambulatory ECG can detect premature ventricular contractions and ventricular tachycardia and identify their frequency, duration, and anatomic origin. This information is useful in assessing risk of sudden cardiac death and determining the need for an implantable cardioverter-defibrillator.
Similarly, Wolff-Parkinson-White syndrome, involving rapid conduction through an accessory pathway, is associated with increased risk of ventricular fibrillation and sudden cardiac death. Ambulatory ECG monitoring can identify patients who have electrical features that portend the development of ventricular fibrillation.
Also associated with sudden cardiac death are the inherited channelopathies, a heterogeneous group of primary arrhythmic disorders without accompanying structural pathology. Ambulatory ECG monitoring can detect transient electrical changes and nonsustained ventricular arrhythmias that would indicate the patient is at high risk of these disorders.
Assessing arrhythmia treatment
Arrhythmia monitoring using an ambulatory ECG device can also provide data to assess the efficacy of treatment under several circumstances.
The “pill-in-the-pocket” approach to treating atrial fibrillation, for example, involves self-administering a single dose of an antiarrhythmic drug when symptoms occur. Patients with infrequent but bothersome episodes can use an ambulatory ECG device to detect when they are having atrial fibrillation, take their prescribed drug, and see whether it terminates the arrhythmia, all without going to the hospital.
Ambulatory ECG also is useful for assessing pharmacologic or ablative therapy in patients with atrial fibrillation or ventricular tachycardia. Monitoring for several weeks can help clinicians assess the burden of atrial fibrillation when using a rhythm-control strategy; assessing the ventricular rate in real-world situations is useful to determine the success of a rate-control strategy. Shortly after ablation of either atrial fibrillation or ventricular tachycardia, ECG home monitoring for 24 to 48 hours can detect asymptomatic recurrence and treatment failure.
Some antiarrhythmic drugs can prolong the QT interval. Ambulatory ECG devices that feature real-time monitoring can be used during drug initiation, enabling the clinician to monitor the QT interval without admitting the patient to the hospital.
Ultimately, ambulatory ECG monitoring is most commonly used to evaluate symptoms. Because arrhythmias and specific symptoms are unpredictable and transient, extended monitoring in a real-world setting allows for a more comprehensive evaluation than a standard 10-second ECG recording.
AMBULATORY ECG DEVICES
Continuous external monitoring: The Holter monitor
Recording is typically done continuously for 24 to 48 hours, although some newer devices can record for longer. Patients can press a button to note when they are experiencing symptoms, allowing for potential correlation with ECG abnormalities. The data are stored on a flash drive that can be uploaded for analysis after recording is complete.
What is its best use? Given its relatively short duration of monitoring, the Holter device is typically used to evaluate symptoms that occur daily or nearly daily. An advantage of the Holter monitor is its ability to record continuously, without requiring the patient to interact with the device. This feature provides “full disclosure,” which is the ability to see arrhythmia data from the entire recording period.
These features make Holter monitoring useful to identify suspected frequently occurring silent arrhythmias or to assess the overall arrhythmia burden. A typical Holter report can contain information on the heart rate (maximum, minimum, and average), ectopic beats, and tachy- and bradyarrhythmias, as well as representative samples.
The Holter device is familiar to most practitioners and remains an effective choice for ambulatory ECG monitoring. However, its use has largely been replaced by newer devices that overcome the Holter’s drawbacks, particularly its short duration of monitoring and the need for postmonitoring analysis. Additionally, although newer Holter devices are more ergonomic, some patients find the wires and gel electrodes uncomfortable or inconvenient.
Intermittent monitoring: Event recorders
Unlike the continuous monitors, intermittent recording devices (also called event recorders), capture and store tracings only during an event.
Intermittent recording monitors are of 2 general types: post-event recorders and loop recorders. These devices can extend the overall duration of observation, which can be especially useful for those whose symptoms and arrhythmias are infrequent.
Post-event recorders are small and self-contained, not requiring electrodes (Figure 1). The device is carried by the patient but not worn continuously. When the patient experiences symptoms, he or she places the device against the chest and presses a button to begin recording. These tracings are stored on the device and can be transmitted by telephone to a data center for analysis. Although post-event recorders allow for monitoring periods typically up to 30 days, they are limited by requiring the patient to act to record an event.
What is its best use? These devices are best used in patients who have infrequent symptoms and are at low risk. Transient or debilitating symptoms, including syncope, can limit the possibility of capturing an event.
Intermittent monitoring: Loop recorders
Loop recorders monitor continuously but record only intermittently. The name refers to the device’s looping memory: ie, to extend how long it can be used and make the most of its limited storage, the device records over previously captured data, saving only the most important data. The device saves the data whenever it detects an abnormal rhythm or the patient experiences symptoms and pushes a button. Data are recorded for a specified time before and after the activation, typically 30 seconds.
Loop recorders come in 2 types: external and implantable.
External loop recorders
External loop recorders look like Holter monitors (Figure 1), but they have the advantage of a much longer observation period—typically up to 1 month. The newest devices have even greater storage capacity and can provide “backward” memory, saving data that were captured just before the patient pushed the button.
In studies of patients with palpitations, presyncope, or syncope, external loop recorders had greater diagnostic yield than traditional 24-hour Holter monitors.7,8 This finding was supported by a clinical trial that found 30-day monitoring with an external loop recorder led to a 5-fold increase in detecting atrial fibrillation in patients with cryptogenic stroke.9
Disadvantages of external loop recorders are limited memory storage, a considerable reliance on patient activation of the device, and wires and electrodes that need to be worn continuously.
What is their best use? External loop recorders are most effective when used to detect an arrhythmia or to correlate infrequent symptoms with an arrhythmia. They are most appropriately used in patients whose symptoms occur more often than every 4 weeks. They are less useful in assessing very infrequent symptoms, overall arrhythmia burden, or responsiveness to therapy.10
Implantable loop recorders
Implantable loop recorders are small devices that contain a pair of sensing electrodes housed within an outer shell (Figure 1). They are implanted subcutaneously, usually in the left parasternal region, using local anesthesia. The subcutaneous location eliminates many of the drawbacks of the skin-electrode interface of external loop recorders.
Similar to the external loop recorder, this device monitors continuously and can be activated to record either by the patient by pressing a button on a separate device, or automatically when an arrhythmia is detected using a preprogrammed algorithm.
In contrast to external devices, many internal loop recorders have a battery life and monitoring capability of up to 3 years. This extended monitoring period has been shown to increase the likelihood of diagnosing syncope or infrequent palpitations.11,12 Given that paroxysmal atrial fibrillation can be sporadic and reveal itself months after a stroke, internal loop recorders may also have a role in evaluating cryptogenic stroke.13,14
The most important drawbacks of internal loop recorders are the surgical procedure for insertion, their limited memory storage, and high upfront cost.15 Furthermore, even though they allow for extended monitoring, there may be diminishing returns for prolonged observation.
What is their best use? For patients with palpitations, intermittent event monitoring has been shown to be cost-effective for the first 2 weeks, but after 3 weeks, the cost per diagnosis increases dramatically.16 As a result, internal loop recorders are reserved primarily for scenarios in which prolonged external monitoring has not revealed a source of arrhythmia despite a high degree of suspicion.
Mobile cardiac telemetry
Mobile cardiac telemetry builds on other ECG monitoring systems by adding real-time communication and technician evaluation.
Physically, these devices resemble either hand-held event records, with a single-channel sensing unit embedded in the case, or a traditional Holter monitor, with 3 channels, wires, and electrodes (Figure 1).
The sensor wirelessly communicates with a nearby portable monitor, which continuously observes and analyzes the patient’s heart rhythm. When an abnormal rhythm is detected or when the patient marks the presence of symptoms, data are recorded and sent in real time via a cellular network to a monitoring center; the newest monitors can send data via any Wi-Fi system. The rhythm is then either evaluated by a trained technician or relayed to a physician. If necessary, the patient can be contacted immediately.
Mobile cardiac telemetry is typically used for up to 30 days, which allows for evaluation of less-frequent symptoms. As a result, it may have a higher diagnostic yield for palpitations, syncope, and presyncope than the 24-hour Holter monitor.17
Further, perhaps because mobile cardiac telemetry relies less on stored information and requires less patient-device interaction than external loop recorders, it is more effective at symptom evaluation.18
Mobile cardiac telemetry also has a diagnostic role in evaluating patients with cryptogenic stroke. This is based on studies showing it has a high rate of atrial fibrillation detection in this patient population and is more effective at determining overall atrial fibrillation burden than loop recorders.18,19
What is its best use? The key advantage of mobile cardiac telemetry is its ability to make rhythm assessments and communicate with technicians in real time. This allows high-risk patients to be immediately alerted to a life-threatening arrhythmia. It also gives providers an opportunity to initiate anticoagulation or titrate antiarrhythmic therapy in the outpatient setting without a delay in obtaining information. This intensive monitoring, however, requires significant manpower, which translates to higher cost, averaging 3 times that of other standard external monitors.15
Patch monitors
These ultraportable devices are a relatively unobtrusive and easy-to-use alternative for short-term ambulatory ECG monitoring. They monitor continuously with full disclosure, outpatient telemetry, and post-event recording features.
Patch monitors are small, leadless, wireless, and water-resistant (Figure 1). They are affixed to the left pectoral region with a waterproof adhesive and can be worn for 14 to 28 days. Recording is usually done continuously; however, these devices have an event marker button that can be pressed when the user experiences symptoms. They acquire a single channel of data, and each manufacturer has a proprietary algorithm for automated rhythm detection and analysis.20
Several manufacturers produce ECG patch monitors. Two notable devices are the Zio patch (iRhythm Technologies, San Francisco, CA) and the Mobile Cardiac Outpatient Telemetry patch (BioTelemetry, Inc, Malvern, PA).
The Zio patch is a continuous external monitor with full disclosure. It is comparable to the Holter monitor, but has a longer recording period. After completing a 2-week monitoring period, the device is returned for comprehensive rhythm analysis. A typical Zio report contains information on atrial fibrillation burden, ectopic rhythm burden, symptom and rhythm correlation, heart rate trends, and relevant rhythm strips.
The Mobile Cardiac Outpatient Telemetry patch collects data continuously and communicates wirelessly by Bluetooth to send its ECG data to a monitoring center for evaluation.
A principal advantage of patch monitors—and a major selling point for manufacturers—is their low-profile, ergonomic, and patient-friendly design. Patients do not have to manage wires or batteries and are able to shower with their devices. Studies show that these features increase patient satisfaction and compliance, resulting in increased diagnostic yield.21,22 Additionally, patch monitors have the advantage of a longer continuous monitoring period than traditional Holter devices (2 weeks vs 1 or 2 days), affording an opportunity to capture events that occur less frequently.
Validation studies have reinforced their efficacy and utility in clinical scenarios.22,23 In large part because of the extended monitoring period, patch monitors have been shown to have greater diagnostic yield than the 24-hour Holter monitor in symptomatic patients undergoing workup for suspected arrhythmia.
The role of patch monitors in evaluating atrial fibrillation is also being established. For patients with cryptogenic stroke, patch monitors have shown better atrial fibrillation detection than the 24-hour Holter monitor.24 Compared with traditional loop monitors, patch monitors have the added advantage of assessing total atrial fibrillation burden. Further, although screening for atrial fibrillation with a traditional 12-lead ECG monitor has not been shown to be effective, clinical studies have found that the patch monitor may be a useful screening tool for high-risk patients.25,26
Nevertheless, patch monitors have drawbacks. They are not capable of long-term monitoring, owing to battery and adhesive limitations.20 More important, they have been able to offer only single-channel acquisition, which makes it more difficult to detect an arrhythmia that is characterized by a change in QRS axis or change in QRS width, or to distinguish an arrhythmia from an artifact. This appears to be changing, however, as several manufacturers have recently developed multilead ECG patch monitors or attachments and are attempting to merge this technology with fully capable remote telemetry.
CHOOSING THE RIGHT DEVICE
Recent improvements in battery life, memory, detection algorithms, wireless transmission, cellular communication, and adhesives have enabled multiple features to be combined into a single device. Patch monitors, for example, are small devices that now offer full-disclosure recording, extended monitoring, and telemetry transmitting. Automated arrhythmia recognition that triggers recording is central to all modern devices, regardless of type.
As a result of these trends, the traditional features used to differentiate devices may become less applicable. The classic Holter monitor may become obsolete as its advantages (full disclosure, continuous recording) are being incorporated into smaller devices that can record longer. Similarly, external monitors that have the capacity for full disclosure and continuous recording are no longer loop recorders in that they do not record into a circular memory.
It may be preferable to describe all non-Holter devices as event monitors or ambulatory monitors, with the main distinguishing features being the ability to transmit data (telemetry), full disclosure vs patient- or arrhythmia-activated recording, and single-channel or multichannel recording (single-lead or 3-lead ECG).
The following are the main distinguishing features that should influence the choice of device for a given clinical context.
Real-time data evaluation provided by mobile telemetry makes this feature ideal to monitor patients with suspected high-risk arrhythmias and their response to antiarrhythmic therapy.
Full-disclosure recording is necessary to assess the overall burden of an arrhythmia, which is frequently important in making treatment decisions, risk-stratifying, and assessing response to therapy. In contrast, patient- or arrhythmia-activated devices are best used when the goal is simply to establish the presence of an arrhythmia.
Multichannel recording may be better than single-channel recording, as it is needed to determine the anatomic origin of an arrhythmia, as might be the case in risk-stratification in a patient with a ventricular tachycardia.
Long duration. The clinician must have a reasonable estimate of how often the symptoms or arrhythmia occur to determine which device will offer a monitoring duration sufficient to detect an arrhythmia.
NEWER TECHNOLOGIES
The newest ambulatory ECG devices build on the foundational concepts of the older ones. However, with miniaturized electronic circuits, Bluetooth, Wi-Fi, and smartphones, these new devices can capture ECG tracings and diagnose offending arrhythmias on more consumer-friendly devices.
Smartphones and smartwatches have become increasingly powerful. Some have the ability to capture, display, and record the cardiac waveform. One manufacturer to capitalize on these technologies, AliveCor (Mountain View, CA), has developed 2 products capable of generating a single-lead ECG recording using either a smartphone (KardiaMobile) or an Apple watch (KardiaBand).
KardiaMobile has a 2-electrode band that can be carried in a pocket or attached to the back of a smartphone (Figure 1). The user places 1 or 2 fingers from each hand on the electrodes, and the device sends an ultrasound signal that is picked up by the smartphone’s microphone. The signal is digitized to produce a 30-second ECG tracing on the phone’s screen. A proprietary algorithm analyzes the rhythm and generates a description of “normal” or “possible atrial fibrillation.” The ECG is then uploaded to a cloud-based storage system for later access or transmission. KardiaMobile is compatible with both iOS and Android devices.
The KardiaBand is a specialized Apple watch band that has an electrode embedded in it. The user places a thumb on the electrode for 30 seconds, and an ECG tracing is displayed on the watch screen.
The Kardia devices were developed (and advertised) predominantly to assess atrial fibrillation. Studies have validated the accuracy of their algorithm. One study showed that, compared with physician-interpreted ECGs, the algorithm had a 96.6% sensitivity and 94.1% specificity for detecting atrial fibrillation.27 They have been found useful for detecting and evaluating atrial fibrillation in several clinical scenarios, including discharge monitoring in patients after ablation or cardiac surgery.28,29 In a longer study of patients at risk of stroke, twice-weekly ECG screening using a Kardia device for 1 year was more likely to detect incident atrial fibrillation than routine care alone.30
Also, the Kardia devices can effectively function as post-event recorders when activated by patients when they experience symptoms. In a small study of outpatients with palpitations and a prior nondiagnostic workup, the KardiaMobile device was found to be noninferior to external loop recorders for detecting arrhythmias.31 Additional studies are assessing Kardia’s utility in other scenarios, including the evaluation of ST-segment elevation myocardial infarction32,33 and QT interval for patients receiving antiarrhythmic therapy.34
Cardiio Inc. (Cambridge, MA) has developed technology to screen for atrial fibrillation using an app that requires no additional external hardware. Instead, the app uses a smartphone’s camera and flashlight to perform photoplethysmography to detect pulsatile changes in blood volume and generate a waveform. Based on waveform variability, a proprietary algorithm attempts to determine whether the user is in atrial fibrillation. It does not produce an ECG tracing. Initial studies suggest it has good diagnostic accuracy and potential utility as a population-based screening tool,35,36 but it has not been fully validated.
Recently, Apple entered the arena of ambulatory cardiac monitoring with the release of its fourth-generation watch (Apple Watch Series 4 model). This watch has built-in electrodes that can generate a single-lead ECG on the watch screen. Its algorithm can discriminate between atrial fibrillation and sinus rhythm, but it has not been assessed for its ability to evaluate other arrhythmias. Even though it has been “cleared” by the US Food and Drug Administration, it is approved only for informational use, not to make a medical diagnosis.
Integration of ambulatory ECG technology with smartphone and watch technology is an exciting new wearable option for arrhythmia detection. The patient-centered and controlled nature of these devices have the potential to help patients with palpitations or other symptoms determine if their cardiac rhythms are normal.
This technology, however, is still in its infancy and has many limitations. For example, even though these devices can function as post-event recorders, they depend on user-device interactions. Plus, they cannot yet perform continuous arrhythmia monitoring like modern loop recorders.
Additionally, automated analysis has largely been limited to distinguishing atrial fibrillation from normal sinus rhythm. It is uncertain how effective the devices may be in evaluating other arrhythmias. Single-lead ECG recordings, as discussed, have limited interpretability and value. And even though studies have shown utility in certain clinical scenarios, large-scale validation studies are lacking. This technology will likely continue to be developed and its clinical value improved; however, its clinical use requires careful consideration and collaborative physician-patient decision-making.
DISRUPTIVE TECHNOLOGY AND DIRECT-TO-CONSUMER MARKETING
The development of smartphone and watch ECG technology has led to a rise in direct-to-consumer healthcare delivery. By devising technology that is appealing, useful, and affordable, companies can bypass the insurer and practitioner by targeting increasingly health-literate consumers. For many companies, there is great motivation to enter this healthcare space. Wearable devices are immensely popular and, as a result, generate substantial revenue. One analysis estimates that 1 in 10 Americans (nearly 30 million) owns a wearable, smart-technology device.37
This direct-to-consumer approach has specific implications for cardiology and, more broadly, for healthcare overall. By directly selling to consumers, companies have an opportunity to reach many more people. The Apple Watch Series 4 has taken this a step further: by including this technology in the watch, consumers not necessarily seeking an ambulatory cardiac monitor will have one with a watch purchase. This could lead to increases in monitoring and could alert people to previously undiagnosed disorders.
For consumers, this technology can empower them to choose how and when to be monitored. Further, it gives them personal control of their healthcare data, and helps move the point of care out of hospitals and clinics and into the home.
But wearable medical technology and direct-to-consumer healthcare have risks. First, in the absence of appropriate regulation, patients have to distinguish between products that are well validated and those that are unproven. Consumers also may inappropriately use devices for indications or in scenarios for which the value is uncertain.
Also, there is potential for confusion and misunderstanding of results, including false-positive readings, which could lead to excessive and costly use of unnecessary diagnostic workups. Instead of providing peace of mind, these devices could cause greater worry. This may be especially true with the newest Apple watch, as this product will introduce ambulatory ECG to a younger and healthier segment of the population who are less likely to have true disease.
Further, these devices have algorithms that detect atrial fibrillation, but is it the same as that detected by traditional methods? Sometimes termed “subclinical” atrial fibrillation, it poses uncertainties: ie, Do patients need anticoagulation, pharmacologic therapy, and ablation? The optimal management of subclinical atrial fibrillation, as well as its similarities to and differences from atrial fibrillation diagnosed by traditional methods, are topics that need further study.
Wearable technology is still developing and will continue to do so. Medical practice will have to adapt to it.
FUTURE DIRECTIONS
Changes in technology have led to remarkable advances in the convenience and accuracy of ambulatory ECG monitoring. Ongoing research is expected to lead to even more improvements. Devices will become more ergonomic and technically capable, and they may expand monitoring to include other biologic parameters beyond ECG.
Comfort is important to ensure patient adherence. Newer, flexible electronics embedded in ultrathin materials can potentially improve the wearability of devices that require gel electrodes or adhesive patches.38 Wireless technology may obviate the need for on-skin attachments. Future recording systems may be embedded into clothing or incorporated into wearable vests capable of wirelessly transmitting ECG signals to separate recording stations.39
In addition to becoming smaller and more comfortable, future devices will be more technically capable, leading to a merging of technologies that will further blur the distinctions among devices. Eventually, the features of full disclosure, extended monitoring duration, and telemetric communication will all be present together. Perhaps more important is that ambulatory ECG devices may become fully capable biosensor monitors. These devices would have the potential to monitor respiratory frequency, peripheral oxygen saturation, potassium levels, and arterial pulse pressure.39,40
A mbulatory electrocardiography (ECG) began in 1949 when Norman “Jeff” Holter developed a monitor that could wirelessly transmit electrophysiologic data.1 His original device used vacuum tubes, weighed 85 pounds, and had to be carried in a backpack. Furthermore, it could send a signal a distance of only 1 block.2
At the time, it was uncertain if this technology would have any clinical utility. However, in 1952, Holter published the first tracing of abnormal cardiac electrical activity in a patient who had suffered a posterior myocardial infarction.3 By the 1960s, Holter monitoring systems were in full production and use.4
Since then, advances in technology have led to small, lightweight devices that enable clinicians to evaluate patients for arrhythmias in a real-world context for extended times, often with the ability to respond in real time.
Many ambulatory devices are available, and choosing the optimal one requires an understanding of which features they have and which are the most appropriate for the specific clinical context. This article reviews the features, indications, advantages, and disadvantages of current devices, and their best use in clinical practice.
INDICATIONS FOR AMBULATORY ECG MONITORING
Diagnosis
The most common diagnostic role of monitoring is to correlate unexplained symptoms, including palpitations, presyncope, and syncope, with a transient cardiac arrhythmia. Monitoring can be considered successful if findings on ECG identify risks for serious arrhythmia and either correlate symptoms with those findings or demonstrate no arrhythmia when symptoms occur.
A range of arrhythmias can cause symptoms. Some, such as premature atrial contractions and premature ventricular contractions, may be benign in many clinical contexts. Others, such as atrial fibrillation, are more serious, and some, such as third-degree heart block and ventricular tachycardia, can be lethal.
Arrhythmia symptoms can vary in frequency and cause differing degrees of debility. The patient’s symptoms, family history, and baseline ECG findings can suggest a more serious or a less serious underlying rhythm. These factors are important when determining which device is most appropriate.
Ambulatory ECG can also be useful in looking for a cause of cryptogenic stroke, ie, an ischemic stroke with an unexplained cause, even after a thorough initial workup. Paroxysmal atrial fibrillation is a frequent cause of cryptogenic stroke, and because it is transient, short-term inpatient telemetry may not be sufficient to detect it. Extended cardiac monitoring, lasting weeks or even months, is often needed for clinicians to make this diagnosis and initiate appropriate secondary prevention.
Prognosis: Identifying patients at risk
In a patient with known structural or electrical heart disease, ambulatory ECG can be used to stratify risk. This is particularly true in evaluating conditions associated with sudden cardiac death.
For example, hypertrophic cardiomyopathy and arrhythmogenic right ventricular dysplasia or cardiomyopathy are 2 cardiomyopathies that can manifest clinically with ventricular arrhythmias and sudden cardiac death. Ambulatory ECG can detect premature ventricular contractions and ventricular tachycardia and identify their frequency, duration, and anatomic origin. This information is useful in assessing risk of sudden cardiac death and determining the need for an implantable cardioverter-defibrillator.
Similarly, Wolff-Parkinson-White syndrome, involving rapid conduction through an accessory pathway, is associated with increased risk of ventricular fibrillation and sudden cardiac death. Ambulatory ECG monitoring can identify patients who have electrical features that portend the development of ventricular fibrillation.
Also associated with sudden cardiac death are the inherited channelopathies, a heterogeneous group of primary arrhythmic disorders without accompanying structural pathology. Ambulatory ECG monitoring can detect transient electrical changes and nonsustained ventricular arrhythmias that would indicate the patient is at high risk of these disorders.
Assessing arrhythmia treatment
Arrhythmia monitoring using an ambulatory ECG device can also provide data to assess the efficacy of treatment under several circumstances.
The “pill-in-the-pocket” approach to treating atrial fibrillation, for example, involves self-administering a single dose of an antiarrhythmic drug when symptoms occur. Patients with infrequent but bothersome episodes can use an ambulatory ECG device to detect when they are having atrial fibrillation, take their prescribed drug, and see whether it terminates the arrhythmia, all without going to the hospital.
Ambulatory ECG also is useful for assessing pharmacologic or ablative therapy in patients with atrial fibrillation or ventricular tachycardia. Monitoring for several weeks can help clinicians assess the burden of atrial fibrillation when using a rhythm-control strategy; assessing the ventricular rate in real-world situations is useful to determine the success of a rate-control strategy. Shortly after ablation of either atrial fibrillation or ventricular tachycardia, ECG home monitoring for 24 to 48 hours can detect asymptomatic recurrence and treatment failure.
Some antiarrhythmic drugs can prolong the QT interval. Ambulatory ECG devices that feature real-time monitoring can be used during drug initiation, enabling the clinician to monitor the QT interval without admitting the patient to the hospital.
Ultimately, ambulatory ECG monitoring is most commonly used to evaluate symptoms. Because arrhythmias and specific symptoms are unpredictable and transient, extended monitoring in a real-world setting allows for a more comprehensive evaluation than a standard 10-second ECG recording.
AMBULATORY ECG DEVICES
Continuous external monitoring: The Holter monitor
Recording is typically done continuously for 24 to 48 hours, although some newer devices can record for longer. Patients can press a button to note when they are experiencing symptoms, allowing for potential correlation with ECG abnormalities. The data are stored on a flash drive that can be uploaded for analysis after recording is complete.
What is its best use? Given its relatively short duration of monitoring, the Holter device is typically used to evaluate symptoms that occur daily or nearly daily. An advantage of the Holter monitor is its ability to record continuously, without requiring the patient to interact with the device. This feature provides “full disclosure,” which is the ability to see arrhythmia data from the entire recording period.
These features make Holter monitoring useful to identify suspected frequently occurring silent arrhythmias or to assess the overall arrhythmia burden. A typical Holter report can contain information on the heart rate (maximum, minimum, and average), ectopic beats, and tachy- and bradyarrhythmias, as well as representative samples.
The Holter device is familiar to most practitioners and remains an effective choice for ambulatory ECG monitoring. However, its use has largely been replaced by newer devices that overcome the Holter’s drawbacks, particularly its short duration of monitoring and the need for postmonitoring analysis. Additionally, although newer Holter devices are more ergonomic, some patients find the wires and gel electrodes uncomfortable or inconvenient.
Intermittent monitoring: Event recorders
Unlike the continuous monitors, intermittent recording devices (also called event recorders), capture and store tracings only during an event.
Intermittent recording monitors are of 2 general types: post-event recorders and loop recorders. These devices can extend the overall duration of observation, which can be especially useful for those whose symptoms and arrhythmias are infrequent.
Post-event recorders are small and self-contained, not requiring electrodes (Figure 1). The device is carried by the patient but not worn continuously. When the patient experiences symptoms, he or she places the device against the chest and presses a button to begin recording. These tracings are stored on the device and can be transmitted by telephone to a data center for analysis. Although post-event recorders allow for monitoring periods typically up to 30 days, they are limited by requiring the patient to act to record an event.
What is its best use? These devices are best used in patients who have infrequent symptoms and are at low risk. Transient or debilitating symptoms, including syncope, can limit the possibility of capturing an event.
Intermittent monitoring: Loop recorders
Loop recorders monitor continuously but record only intermittently. The name refers to the device’s looping memory: ie, to extend how long it can be used and make the most of its limited storage, the device records over previously captured data, saving only the most important data. The device saves the data whenever it detects an abnormal rhythm or the patient experiences symptoms and pushes a button. Data are recorded for a specified time before and after the activation, typically 30 seconds.
Loop recorders come in 2 types: external and implantable.
External loop recorders
External loop recorders look like Holter monitors (Figure 1), but they have the advantage of a much longer observation period—typically up to 1 month. The newest devices have even greater storage capacity and can provide “backward” memory, saving data that were captured just before the patient pushed the button.
In studies of patients with palpitations, presyncope, or syncope, external loop recorders had greater diagnostic yield than traditional 24-hour Holter monitors.7,8 This finding was supported by a clinical trial that found 30-day monitoring with an external loop recorder led to a 5-fold increase in detecting atrial fibrillation in patients with cryptogenic stroke.9
Disadvantages of external loop recorders are limited memory storage, a considerable reliance on patient activation of the device, and wires and electrodes that need to be worn continuously.
What is their best use? External loop recorders are most effective when used to detect an arrhythmia or to correlate infrequent symptoms with an arrhythmia. They are most appropriately used in patients whose symptoms occur more often than every 4 weeks. They are less useful in assessing very infrequent symptoms, overall arrhythmia burden, or responsiveness to therapy.10
Implantable loop recorders
Implantable loop recorders are small devices that contain a pair of sensing electrodes housed within an outer shell (Figure 1). They are implanted subcutaneously, usually in the left parasternal region, using local anesthesia. The subcutaneous location eliminates many of the drawbacks of the skin-electrode interface of external loop recorders.
Similar to the external loop recorder, this device monitors continuously and can be activated to record either by the patient by pressing a button on a separate device, or automatically when an arrhythmia is detected using a preprogrammed algorithm.
In contrast to external devices, many internal loop recorders have a battery life and monitoring capability of up to 3 years. This extended monitoring period has been shown to increase the likelihood of diagnosing syncope or infrequent palpitations.11,12 Given that paroxysmal atrial fibrillation can be sporadic and reveal itself months after a stroke, internal loop recorders may also have a role in evaluating cryptogenic stroke.13,14
The most important drawbacks of internal loop recorders are the surgical procedure for insertion, their limited memory storage, and high upfront cost.15 Furthermore, even though they allow for extended monitoring, there may be diminishing returns for prolonged observation.
What is their best use? For patients with palpitations, intermittent event monitoring has been shown to be cost-effective for the first 2 weeks, but after 3 weeks, the cost per diagnosis increases dramatically.16 As a result, internal loop recorders are reserved primarily for scenarios in which prolonged external monitoring has not revealed a source of arrhythmia despite a high degree of suspicion.
Mobile cardiac telemetry
Mobile cardiac telemetry builds on other ECG monitoring systems by adding real-time communication and technician evaluation.
Physically, these devices resemble either hand-held event records, with a single-channel sensing unit embedded in the case, or a traditional Holter monitor, with 3 channels, wires, and electrodes (Figure 1).
The sensor wirelessly communicates with a nearby portable monitor, which continuously observes and analyzes the patient’s heart rhythm. When an abnormal rhythm is detected or when the patient marks the presence of symptoms, data are recorded and sent in real time via a cellular network to a monitoring center; the newest monitors can send data via any Wi-Fi system. The rhythm is then either evaluated by a trained technician or relayed to a physician. If necessary, the patient can be contacted immediately.
Mobile cardiac telemetry is typically used for up to 30 days, which allows for evaluation of less-frequent symptoms. As a result, it may have a higher diagnostic yield for palpitations, syncope, and presyncope than the 24-hour Holter monitor.17
Further, perhaps because mobile cardiac telemetry relies less on stored information and requires less patient-device interaction than external loop recorders, it is more effective at symptom evaluation.18
Mobile cardiac telemetry also has a diagnostic role in evaluating patients with cryptogenic stroke. This is based on studies showing it has a high rate of atrial fibrillation detection in this patient population and is more effective at determining overall atrial fibrillation burden than loop recorders.18,19
What is its best use? The key advantage of mobile cardiac telemetry is its ability to make rhythm assessments and communicate with technicians in real time. This allows high-risk patients to be immediately alerted to a life-threatening arrhythmia. It also gives providers an opportunity to initiate anticoagulation or titrate antiarrhythmic therapy in the outpatient setting without a delay in obtaining information. This intensive monitoring, however, requires significant manpower, which translates to higher cost, averaging 3 times that of other standard external monitors.15
Patch monitors
These ultraportable devices are a relatively unobtrusive and easy-to-use alternative for short-term ambulatory ECG monitoring. They monitor continuously with full disclosure, outpatient telemetry, and post-event recording features.
Patch monitors are small, leadless, wireless, and water-resistant (Figure 1). They are affixed to the left pectoral region with a waterproof adhesive and can be worn for 14 to 28 days. Recording is usually done continuously; however, these devices have an event marker button that can be pressed when the user experiences symptoms. They acquire a single channel of data, and each manufacturer has a proprietary algorithm for automated rhythm detection and analysis.20
Several manufacturers produce ECG patch monitors. Two notable devices are the Zio patch (iRhythm Technologies, San Francisco, CA) and the Mobile Cardiac Outpatient Telemetry patch (BioTelemetry, Inc, Malvern, PA).
The Zio patch is a continuous external monitor with full disclosure. It is comparable to the Holter monitor, but has a longer recording period. After completing a 2-week monitoring period, the device is returned for comprehensive rhythm analysis. A typical Zio report contains information on atrial fibrillation burden, ectopic rhythm burden, symptom and rhythm correlation, heart rate trends, and relevant rhythm strips.
The Mobile Cardiac Outpatient Telemetry patch collects data continuously and communicates wirelessly by Bluetooth to send its ECG data to a monitoring center for evaluation.
A principal advantage of patch monitors—and a major selling point for manufacturers—is their low-profile, ergonomic, and patient-friendly design. Patients do not have to manage wires or batteries and are able to shower with their devices. Studies show that these features increase patient satisfaction and compliance, resulting in increased diagnostic yield.21,22 Additionally, patch monitors have the advantage of a longer continuous monitoring period than traditional Holter devices (2 weeks vs 1 or 2 days), affording an opportunity to capture events that occur less frequently.
Validation studies have reinforced their efficacy and utility in clinical scenarios.22,23 In large part because of the extended monitoring period, patch monitors have been shown to have greater diagnostic yield than the 24-hour Holter monitor in symptomatic patients undergoing workup for suspected arrhythmia.
The role of patch monitors in evaluating atrial fibrillation is also being established. For patients with cryptogenic stroke, patch monitors have shown better atrial fibrillation detection than the 24-hour Holter monitor.24 Compared with traditional loop monitors, patch monitors have the added advantage of assessing total atrial fibrillation burden. Further, although screening for atrial fibrillation with a traditional 12-lead ECG monitor has not been shown to be effective, clinical studies have found that the patch monitor may be a useful screening tool for high-risk patients.25,26
Nevertheless, patch monitors have drawbacks. They are not capable of long-term monitoring, owing to battery and adhesive limitations.20 More important, they have been able to offer only single-channel acquisition, which makes it more difficult to detect an arrhythmia that is characterized by a change in QRS axis or change in QRS width, or to distinguish an arrhythmia from an artifact. This appears to be changing, however, as several manufacturers have recently developed multilead ECG patch monitors or attachments and are attempting to merge this technology with fully capable remote telemetry.
CHOOSING THE RIGHT DEVICE
Recent improvements in battery life, memory, detection algorithms, wireless transmission, cellular communication, and adhesives have enabled multiple features to be combined into a single device. Patch monitors, for example, are small devices that now offer full-disclosure recording, extended monitoring, and telemetry transmitting. Automated arrhythmia recognition that triggers recording is central to all modern devices, regardless of type.
As a result of these trends, the traditional features used to differentiate devices may become less applicable. The classic Holter monitor may become obsolete as its advantages (full disclosure, continuous recording) are being incorporated into smaller devices that can record longer. Similarly, external monitors that have the capacity for full disclosure and continuous recording are no longer loop recorders in that they do not record into a circular memory.
It may be preferable to describe all non-Holter devices as event monitors or ambulatory monitors, with the main distinguishing features being the ability to transmit data (telemetry), full disclosure vs patient- or arrhythmia-activated recording, and single-channel or multichannel recording (single-lead or 3-lead ECG).
The following are the main distinguishing features that should influence the choice of device for a given clinical context.
Real-time data evaluation provided by mobile telemetry makes this feature ideal to monitor patients with suspected high-risk arrhythmias and their response to antiarrhythmic therapy.
Full-disclosure recording is necessary to assess the overall burden of an arrhythmia, which is frequently important in making treatment decisions, risk-stratifying, and assessing response to therapy. In contrast, patient- or arrhythmia-activated devices are best used when the goal is simply to establish the presence of an arrhythmia.
Multichannel recording may be better than single-channel recording, as it is needed to determine the anatomic origin of an arrhythmia, as might be the case in risk-stratification in a patient with a ventricular tachycardia.
Long duration. The clinician must have a reasonable estimate of how often the symptoms or arrhythmia occur to determine which device will offer a monitoring duration sufficient to detect an arrhythmia.
NEWER TECHNOLOGIES
The newest ambulatory ECG devices build on the foundational concepts of the older ones. However, with miniaturized electronic circuits, Bluetooth, Wi-Fi, and smartphones, these new devices can capture ECG tracings and diagnose offending arrhythmias on more consumer-friendly devices.
Smartphones and smartwatches have become increasingly powerful. Some have the ability to capture, display, and record the cardiac waveform. One manufacturer to capitalize on these technologies, AliveCor (Mountain View, CA), has developed 2 products capable of generating a single-lead ECG recording using either a smartphone (KardiaMobile) or an Apple watch (KardiaBand).
KardiaMobile has a 2-electrode band that can be carried in a pocket or attached to the back of a smartphone (Figure 1). The user places 1 or 2 fingers from each hand on the electrodes, and the device sends an ultrasound signal that is picked up by the smartphone’s microphone. The signal is digitized to produce a 30-second ECG tracing on the phone’s screen. A proprietary algorithm analyzes the rhythm and generates a description of “normal” or “possible atrial fibrillation.” The ECG is then uploaded to a cloud-based storage system for later access or transmission. KardiaMobile is compatible with both iOS and Android devices.
The KardiaBand is a specialized Apple watch band that has an electrode embedded in it. The user places a thumb on the electrode for 30 seconds, and an ECG tracing is displayed on the watch screen.
The Kardia devices were developed (and advertised) predominantly to assess atrial fibrillation. Studies have validated the accuracy of their algorithm. One study showed that, compared with physician-interpreted ECGs, the algorithm had a 96.6% sensitivity and 94.1% specificity for detecting atrial fibrillation.27 They have been found useful for detecting and evaluating atrial fibrillation in several clinical scenarios, including discharge monitoring in patients after ablation or cardiac surgery.28,29 In a longer study of patients at risk of stroke, twice-weekly ECG screening using a Kardia device for 1 year was more likely to detect incident atrial fibrillation than routine care alone.30
Also, the Kardia devices can effectively function as post-event recorders when activated by patients when they experience symptoms. In a small study of outpatients with palpitations and a prior nondiagnostic workup, the KardiaMobile device was found to be noninferior to external loop recorders for detecting arrhythmias.31 Additional studies are assessing Kardia’s utility in other scenarios, including the evaluation of ST-segment elevation myocardial infarction32,33 and QT interval for patients receiving antiarrhythmic therapy.34
Cardiio Inc. (Cambridge, MA) has developed technology to screen for atrial fibrillation using an app that requires no additional external hardware. Instead, the app uses a smartphone’s camera and flashlight to perform photoplethysmography to detect pulsatile changes in blood volume and generate a waveform. Based on waveform variability, a proprietary algorithm attempts to determine whether the user is in atrial fibrillation. It does not produce an ECG tracing. Initial studies suggest it has good diagnostic accuracy and potential utility as a population-based screening tool,35,36 but it has not been fully validated.
Recently, Apple entered the arena of ambulatory cardiac monitoring with the release of its fourth-generation watch (Apple Watch Series 4 model). This watch has built-in electrodes that can generate a single-lead ECG on the watch screen. Its algorithm can discriminate between atrial fibrillation and sinus rhythm, but it has not been assessed for its ability to evaluate other arrhythmias. Even though it has been “cleared” by the US Food and Drug Administration, it is approved only for informational use, not to make a medical diagnosis.
Integration of ambulatory ECG technology with smartphone and watch technology is an exciting new wearable option for arrhythmia detection. The patient-centered and controlled nature of these devices have the potential to help patients with palpitations or other symptoms determine if their cardiac rhythms are normal.
This technology, however, is still in its infancy and has many limitations. For example, even though these devices can function as post-event recorders, they depend on user-device interactions. Plus, they cannot yet perform continuous arrhythmia monitoring like modern loop recorders.
Additionally, automated analysis has largely been limited to distinguishing atrial fibrillation from normal sinus rhythm. It is uncertain how effective the devices may be in evaluating other arrhythmias. Single-lead ECG recordings, as discussed, have limited interpretability and value. And even though studies have shown utility in certain clinical scenarios, large-scale validation studies are lacking. This technology will likely continue to be developed and its clinical value improved; however, its clinical use requires careful consideration and collaborative physician-patient decision-making.
DISRUPTIVE TECHNOLOGY AND DIRECT-TO-CONSUMER MARKETING
The development of smartphone and watch ECG technology has led to a rise in direct-to-consumer healthcare delivery. By devising technology that is appealing, useful, and affordable, companies can bypass the insurer and practitioner by targeting increasingly health-literate consumers. For many companies, there is great motivation to enter this healthcare space. Wearable devices are immensely popular and, as a result, generate substantial revenue. One analysis estimates that 1 in 10 Americans (nearly 30 million) owns a wearable, smart-technology device.37
This direct-to-consumer approach has specific implications for cardiology and, more broadly, for healthcare overall. By directly selling to consumers, companies have an opportunity to reach many more people. The Apple Watch Series 4 has taken this a step further: by including this technology in the watch, consumers not necessarily seeking an ambulatory cardiac monitor will have one with a watch purchase. This could lead to increases in monitoring and could alert people to previously undiagnosed disorders.
For consumers, this technology can empower them to choose how and when to be monitored. Further, it gives them personal control of their healthcare data, and helps move the point of care out of hospitals and clinics and into the home.
But wearable medical technology and direct-to-consumer healthcare have risks. First, in the absence of appropriate regulation, patients have to distinguish between products that are well validated and those that are unproven. Consumers also may inappropriately use devices for indications or in scenarios for which the value is uncertain.
Also, there is potential for confusion and misunderstanding of results, including false-positive readings, which could lead to excessive and costly use of unnecessary diagnostic workups. Instead of providing peace of mind, these devices could cause greater worry. This may be especially true with the newest Apple watch, as this product will introduce ambulatory ECG to a younger and healthier segment of the population who are less likely to have true disease.
Further, these devices have algorithms that detect atrial fibrillation, but is it the same as that detected by traditional methods? Sometimes termed “subclinical” atrial fibrillation, it poses uncertainties: ie, Do patients need anticoagulation, pharmacologic therapy, and ablation? The optimal management of subclinical atrial fibrillation, as well as its similarities to and differences from atrial fibrillation diagnosed by traditional methods, are topics that need further study.
Wearable technology is still developing and will continue to do so. Medical practice will have to adapt to it.
FUTURE DIRECTIONS
Changes in technology have led to remarkable advances in the convenience and accuracy of ambulatory ECG monitoring. Ongoing research is expected to lead to even more improvements. Devices will become more ergonomic and technically capable, and they may expand monitoring to include other biologic parameters beyond ECG.
Comfort is important to ensure patient adherence. Newer, flexible electronics embedded in ultrathin materials can potentially improve the wearability of devices that require gel electrodes or adhesive patches.38 Wireless technology may obviate the need for on-skin attachments. Future recording systems may be embedded into clothing or incorporated into wearable vests capable of wirelessly transmitting ECG signals to separate recording stations.39
In addition to becoming smaller and more comfortable, future devices will be more technically capable, leading to a merging of technologies that will further blur the distinctions among devices. Eventually, the features of full disclosure, extended monitoring duration, and telemetric communication will all be present together. Perhaps more important is that ambulatory ECG devices may become fully capable biosensor monitors. These devices would have the potential to monitor respiratory frequency, peripheral oxygen saturation, potassium levels, and arterial pulse pressure.39,40
- Holter NJ, Gengerelli JA. Remote recording of physiological data by radio. Rocky Mt Med J 1949; 46(9):747–751. pmid:18137532
- Kennedy HL. The history, science, and innovation of Holter technology. Ann Noninvasive Elecrocardiol 2006; 11(1):85–94. doi:10.1111/j.1542-474X.2006.00067.x
- MacInnis HF. The clinical application of radioelectrocardiography. Can Med Assoc J 1954; 70(5):574– 576. pmid:13160894
- Del Mar B. The history of clinical Holter monitoring. Ann Noninvasive Elecrocardiol. 2005; 10(2):226–230. doi:10.1111/j.1542-474X.2005.10202.x
- Crawford MH, Bernstein SJ, Deedwania PC, et al. ACC/AHA guidelines for ambulatory electrocardiography. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the Guidelines for Ambulatory Electrocardiography). Developed in collaboration with the North American Society for Pacing and Electrophysiology. J Am Coll Cardiol 1999; 34(3):912–948. pmid:10483977
- Steinberg JS, Varma N, Cygankiewicz I, et al. 2017 ISHNE-HRS expert consensus statement on ambulatory ECG and external cardiac monitoring/telemetry. Heart Rhythm 2017; 14(7):e55–e96. doi:10.1016/j.hrthm.2017.03.038
- Locati ET, Vecchi AM, Vargiu S, Cattafi G, Lunati M. Role of extended external loop recorders for the diagnosis of unexplained syncope, pre-syncope, and sustained palpitations. Europace 2014; 16(6):914–922. doi:10.1093/europace/eut337
- Locati ET, Moya A, Oliveira, et al. External prolonged electrocardiogram monitoring in unexplained syncope and palpitations: results of the SYNARR-Flash study. Europace 2016; 18(8):1265–1272. doi:10.1093/europace/euv311
- Gladstone DJ, Spring M, Dorian P, et al; EMBRACE Investigators and Coordinators. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014; 370(26):2467–2477. doi:10.1056/NEJMoa1311376
- Brignole M, Vardas P, Hoffman E, et al; EHRA Scientific Documents Committee. Indications for the use of diagnostic implantable and external ECG loop recorders. Europace 2009; 11(5):671–687. doi:10.1093/europace/eup097
- Edvardsson N, Frykman V, van Mechelen R, et al; PICTURE Study Investigators. Use of an implantable loop recorder to increase the diagnostic yield in unexplained syncope: results from the PICTURE registry. Europace 2011; 13(2):262–269. doi:10.1093/europace/euq418
- Giada F, Gulizia M, Francese M, et al. Recurrent unexplained palpitations (RUP) study comparison of implantable loop recorder versus conventional diagnostic strategy. J Am Coll Cardiol 2007; 49(19):1951–1956. doi:10.1016/j.jacc.2007.02.036
- Christensen LM, Krieger DW, Hojberg S, et al. Paroxysmal atrial fibrillation occurs often in cryptogenic ischaemic stroke. Final results from the SURPRISE study. Eur J Neurol 2014; 21(6):884–889. doi:10.1111/ene.12400
- Cotter PE, Martin PJ, Ring L, Warburton EA, Belham M, Pugh PJ. Incidence of atrial fibrillation detected by implantable loop recorders in unexplained stroke. Neurology 2013; 80(17):1546–1550. doi:10.1212/WNL.0b013e31828f1828
- Zimetbaum P, Goldman A. Ambulatory arrhythmia monitoring: choosing the right device. Circulation 2010; 122(16):1629–1636. doi:10.1161/CIRCULATIONAHA.109.925610
- Zimetbaum PJ, Kim KY, Josephson ME, Goldberger AL, Cohen DJ. Diagnostic yield and optimal duration of continuous-loop event monitoring for the diagnosis of palpitations: a cost-effectiveness analysis. Ann Intern Med 1998; 128(11):890–895. pmid:9634426
- Joshi AK, Kowey PR, Prystowksy EN, et al. First experience with a mobile cardiac outpatient telemetry (MCOT) system for the diagnosis and management of cardiac arrhythmia. Am J Cardiol 2005; 95(7):878–881. doi:10.1016/j.amjcard.2004.12.015
- Rothman SA, Laughlin JC, Seltzer J, et al., The diagnosis of cardiac arrhythmias: a prospective multi-center randomized study comparing mobile cardiac outpatient telemetry versus standard loop event monitoring. J Cardiovasc Electrophysiol 2007; 18(3):241–247. pmid:17318994
- Tayal AH, Tian M, Kelly KM, et al. Atrial fibrillation detected by mobile cardiac outpatient telemetry in cryptogenic TIA or stroke. Neurology 2008; 71(21):1696–1701. doi:10.1212/01.wnl.0000325059.86313.31
- Lobodzinski SS. ECG patch monitors for assessment of cardiac rhythm abnormalities. Prog Cardiovasc Dis 2013; 56(2):224–229. doi:10.1016/j.pcad.2013.08.006
- Fung E, Jarvelin MR, Doshi RN, et al. Electrocardiographic patch devices and contemporary wireless cardiac monitoring. Front Physiol 2015; 6:149. doi:10.3389/fphys.2015.00149
- Barrett PM, Komatireddy R, Haaser S, et al. Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am J Med 2014; 127(1):95.e11–95.e17. doi:10.1016/j.amjmed.2013.10.003
- Schreiber D, Sattar A, Drigalla D, Higgins S. Ambulatory cardiac monitoring for discharged emergency department patients with possible cardiac arrhythmias. West J Emerg Med 2014; 15(2):194–198. doi:10.5811/westjem.2013.11.18973
- Tung CE, Su D, Turakhia MP, Lansberg MG. Diagnostic yield of extended cardiac patch monitoring in patients with stroke or TIA. Front Neurol 2015; 5:266. doi:10.3389/fneur.2014.00266
- Turakhia MP, Ullal AJ, Hoang DD, et al. Feasibility of extended ambulatory electrocardiogram monitoring to identify silent atrial fibrillation in high-risk patients: the Screening Study for Undiagnosed Atrial Fibrillation (STUDY-AF). Clin Cardiol 2015; 38(5):285–292. doi:10.1002/clc.22387
- Steinhubl SR, Waalen J, Edwards AM, et al. Effect of a home-based wearable continuous ECG monitoring patch on detection of undiagnosed atrial fibrillation: the mSToPS randomized clinical trial. JAMA 2018; 320(2):146–155. doi:10.1001/jama.2018.8102
- William AD, Kanbour M, Callahan T, et al. Assessing the accuracy of an automated atrial fibrillation detection algorithm using smartphone technology: the iREAD study. Heart Rhythm 2018; 15(10):1561–1565. doi:10.1016/j.hrthm.2018.06.037
- Tarakji KG, Wazni OM, Callahan T, et al. Using a novel wireless system for monitoring patients after the atrial fibrillation ablation procedure: the iTransmit study. Heart Rhythm 2015; 12(3):554–559. doi:10.1016/j.hrthm.2014.11.015
- Lowres N, Mulcahy G, Gallagher R, et al. Self-monitoring for atrial fibrillation recurrence in the discharge period post-cardiac surgery using an iPhone electrocardiogram. Eur J Cardiothorac Surg 2016; 50(1):44–51. doi:10.1093/ejcts/ezv486
- Halcox JPJ, Wareham K, Cardew A, et al. Assessment of remote heart rhythm sampling using the AliveCor heart monitor to screen for atrial fibrillation: the REHEARSE-AF study. Circulation 2017; 136(19):1784–1794. doi:10.1161/CIRCULATIONAHA.117.030583
- Narasimha D, Hanna N, Beck H, et al. Validation of a smartphone-based event recorder for arrhythmia detection. Pacing Clin Electrophysiol 2018; 41(5):487–494. doi:10.1111/pace.13317
- Muhlestein JB, Le V, Albert D, et al. Smartphone ECG for evaluation of STEMI: results of the ST LEUIS pilot study. J Electrocardiol 2015; 48(2):249–259. doi:10.1016/j.jelectrocard.2014.11.005
- Barbagelata A, Bethea CF, Severance HW, et al. Smartphone ECG for evaluation of ST-segment elevation myocardial infarction (STEMI): design of the ST LEUIS international multicenter study. J Electrocardiol 2018; 51(2):260–264. doi:10.1016/j.jelectrocard.2017.10.011
- Garabelli P, Stavrakis S, Albert M, et al. Comparison of QT interval readings in normal sinus rhythm between a smartphone heart monitor and a 12-lead ECG for healthy volunteers and inpatients receiving sotalol or dofetilide. J Cardiovasc Electrophysiol 2016; 27(7):827–832. doi:10.1111/jce.12976
- Rozen G, Vai J, Hosseini SM, et al. Diagnostic accuracy of a novel mobile phone application in monitoring atrial fibrillation. Am J Cardiol 2018; 121(10):1187–1191. doi:10.1016/j.amjcard.2018.01.035
- Chan PH, Wong CK, Poh YC, et al. Diagnostic performance of a smartphone-based photoplethysmographic application for atrial fibrillation screening in a primary care setting. J Am Heart Assoc 2016; 5(7). pii:e003428. doi:10.1161/JAHA.116.003428
- Mitchell ARJ, Le Page P. Living with the handheld ECG. BMJ Innov 2015; 1:46–48.
- Lee SP, Ha G, Wright DE, et al. Highly flexible, wearable, and disposable cardiac biosensors for remote and ambulatory monitoring. npj Digital Medicine 2018. doi:10.1038/s41746-017-0009-x
- Locati ET. New directions for ambulatory monitoring following the 2017 HRS-ISHNE expert consensus. J Electrocardiol 2017; 50(6):828–832. doi:10.1016/j.jelectrocard.2017.08.009
- Dillon JJ, DeSimone CV, Sapir Y, et al. Noninvasive potassium determination using a mathematically processed ECG: proof of concept for a novel “blood-less, blood test”. J Electrocardiol 2015; 48(1):12–18. doi:10.1016/j.jelectrocard.2014.10.002
- Holter NJ, Gengerelli JA. Remote recording of physiological data by radio. Rocky Mt Med J 1949; 46(9):747–751. pmid:18137532
- Kennedy HL. The history, science, and innovation of Holter technology. Ann Noninvasive Elecrocardiol 2006; 11(1):85–94. doi:10.1111/j.1542-474X.2006.00067.x
- MacInnis HF. The clinical application of radioelectrocardiography. Can Med Assoc J 1954; 70(5):574– 576. pmid:13160894
- Del Mar B. The history of clinical Holter monitoring. Ann Noninvasive Elecrocardiol. 2005; 10(2):226–230. doi:10.1111/j.1542-474X.2005.10202.x
- Crawford MH, Bernstein SJ, Deedwania PC, et al. ACC/AHA guidelines for ambulatory electrocardiography. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the Guidelines for Ambulatory Electrocardiography). Developed in collaboration with the North American Society for Pacing and Electrophysiology. J Am Coll Cardiol 1999; 34(3):912–948. pmid:10483977
- Steinberg JS, Varma N, Cygankiewicz I, et al. 2017 ISHNE-HRS expert consensus statement on ambulatory ECG and external cardiac monitoring/telemetry. Heart Rhythm 2017; 14(7):e55–e96. doi:10.1016/j.hrthm.2017.03.038
- Locati ET, Vecchi AM, Vargiu S, Cattafi G, Lunati M. Role of extended external loop recorders for the diagnosis of unexplained syncope, pre-syncope, and sustained palpitations. Europace 2014; 16(6):914–922. doi:10.1093/europace/eut337
- Locati ET, Moya A, Oliveira, et al. External prolonged electrocardiogram monitoring in unexplained syncope and palpitations: results of the SYNARR-Flash study. Europace 2016; 18(8):1265–1272. doi:10.1093/europace/euv311
- Gladstone DJ, Spring M, Dorian P, et al; EMBRACE Investigators and Coordinators. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014; 370(26):2467–2477. doi:10.1056/NEJMoa1311376
- Brignole M, Vardas P, Hoffman E, et al; EHRA Scientific Documents Committee. Indications for the use of diagnostic implantable and external ECG loop recorders. Europace 2009; 11(5):671–687. doi:10.1093/europace/eup097
- Edvardsson N, Frykman V, van Mechelen R, et al; PICTURE Study Investigators. Use of an implantable loop recorder to increase the diagnostic yield in unexplained syncope: results from the PICTURE registry. Europace 2011; 13(2):262–269. doi:10.1093/europace/euq418
- Giada F, Gulizia M, Francese M, et al. Recurrent unexplained palpitations (RUP) study comparison of implantable loop recorder versus conventional diagnostic strategy. J Am Coll Cardiol 2007; 49(19):1951–1956. doi:10.1016/j.jacc.2007.02.036
- Christensen LM, Krieger DW, Hojberg S, et al. Paroxysmal atrial fibrillation occurs often in cryptogenic ischaemic stroke. Final results from the SURPRISE study. Eur J Neurol 2014; 21(6):884–889. doi:10.1111/ene.12400
- Cotter PE, Martin PJ, Ring L, Warburton EA, Belham M, Pugh PJ. Incidence of atrial fibrillation detected by implantable loop recorders in unexplained stroke. Neurology 2013; 80(17):1546–1550. doi:10.1212/WNL.0b013e31828f1828
- Zimetbaum P, Goldman A. Ambulatory arrhythmia monitoring: choosing the right device. Circulation 2010; 122(16):1629–1636. doi:10.1161/CIRCULATIONAHA.109.925610
- Zimetbaum PJ, Kim KY, Josephson ME, Goldberger AL, Cohen DJ. Diagnostic yield and optimal duration of continuous-loop event monitoring for the diagnosis of palpitations: a cost-effectiveness analysis. Ann Intern Med 1998; 128(11):890–895. pmid:9634426
- Joshi AK, Kowey PR, Prystowksy EN, et al. First experience with a mobile cardiac outpatient telemetry (MCOT) system for the diagnosis and management of cardiac arrhythmia. Am J Cardiol 2005; 95(7):878–881. doi:10.1016/j.amjcard.2004.12.015
- Rothman SA, Laughlin JC, Seltzer J, et al., The diagnosis of cardiac arrhythmias: a prospective multi-center randomized study comparing mobile cardiac outpatient telemetry versus standard loop event monitoring. J Cardiovasc Electrophysiol 2007; 18(3):241–247. pmid:17318994
- Tayal AH, Tian M, Kelly KM, et al. Atrial fibrillation detected by mobile cardiac outpatient telemetry in cryptogenic TIA or stroke. Neurology 2008; 71(21):1696–1701. doi:10.1212/01.wnl.0000325059.86313.31
- Lobodzinski SS. ECG patch monitors for assessment of cardiac rhythm abnormalities. Prog Cardiovasc Dis 2013; 56(2):224–229. doi:10.1016/j.pcad.2013.08.006
- Fung E, Jarvelin MR, Doshi RN, et al. Electrocardiographic patch devices and contemporary wireless cardiac monitoring. Front Physiol 2015; 6:149. doi:10.3389/fphys.2015.00149
- Barrett PM, Komatireddy R, Haaser S, et al. Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am J Med 2014; 127(1):95.e11–95.e17. doi:10.1016/j.amjmed.2013.10.003
- Schreiber D, Sattar A, Drigalla D, Higgins S. Ambulatory cardiac monitoring for discharged emergency department patients with possible cardiac arrhythmias. West J Emerg Med 2014; 15(2):194–198. doi:10.5811/westjem.2013.11.18973
- Tung CE, Su D, Turakhia MP, Lansberg MG. Diagnostic yield of extended cardiac patch monitoring in patients with stroke or TIA. Front Neurol 2015; 5:266. doi:10.3389/fneur.2014.00266
- Turakhia MP, Ullal AJ, Hoang DD, et al. Feasibility of extended ambulatory electrocardiogram monitoring to identify silent atrial fibrillation in high-risk patients: the Screening Study for Undiagnosed Atrial Fibrillation (STUDY-AF). Clin Cardiol 2015; 38(5):285–292. doi:10.1002/clc.22387
- Steinhubl SR, Waalen J, Edwards AM, et al. Effect of a home-based wearable continuous ECG monitoring patch on detection of undiagnosed atrial fibrillation: the mSToPS randomized clinical trial. JAMA 2018; 320(2):146–155. doi:10.1001/jama.2018.8102
- William AD, Kanbour M, Callahan T, et al. Assessing the accuracy of an automated atrial fibrillation detection algorithm using smartphone technology: the iREAD study. Heart Rhythm 2018; 15(10):1561–1565. doi:10.1016/j.hrthm.2018.06.037
- Tarakji KG, Wazni OM, Callahan T, et al. Using a novel wireless system for monitoring patients after the atrial fibrillation ablation procedure: the iTransmit study. Heart Rhythm 2015; 12(3):554–559. doi:10.1016/j.hrthm.2014.11.015
- Lowres N, Mulcahy G, Gallagher R, et al. Self-monitoring for atrial fibrillation recurrence in the discharge period post-cardiac surgery using an iPhone electrocardiogram. Eur J Cardiothorac Surg 2016; 50(1):44–51. doi:10.1093/ejcts/ezv486
- Halcox JPJ, Wareham K, Cardew A, et al. Assessment of remote heart rhythm sampling using the AliveCor heart monitor to screen for atrial fibrillation: the REHEARSE-AF study. Circulation 2017; 136(19):1784–1794. doi:10.1161/CIRCULATIONAHA.117.030583
- Narasimha D, Hanna N, Beck H, et al. Validation of a smartphone-based event recorder for arrhythmia detection. Pacing Clin Electrophysiol 2018; 41(5):487–494. doi:10.1111/pace.13317
- Muhlestein JB, Le V, Albert D, et al. Smartphone ECG for evaluation of STEMI: results of the ST LEUIS pilot study. J Electrocardiol 2015; 48(2):249–259. doi:10.1016/j.jelectrocard.2014.11.005
- Barbagelata A, Bethea CF, Severance HW, et al. Smartphone ECG for evaluation of ST-segment elevation myocardial infarction (STEMI): design of the ST LEUIS international multicenter study. J Electrocardiol 2018; 51(2):260–264. doi:10.1016/j.jelectrocard.2017.10.011
- Garabelli P, Stavrakis S, Albert M, et al. Comparison of QT interval readings in normal sinus rhythm between a smartphone heart monitor and a 12-lead ECG for healthy volunteers and inpatients receiving sotalol or dofetilide. J Cardiovasc Electrophysiol 2016; 27(7):827–832. doi:10.1111/jce.12976
- Rozen G, Vai J, Hosseini SM, et al. Diagnostic accuracy of a novel mobile phone application in monitoring atrial fibrillation. Am J Cardiol 2018; 121(10):1187–1191. doi:10.1016/j.amjcard.2018.01.035
- Chan PH, Wong CK, Poh YC, et al. Diagnostic performance of a smartphone-based photoplethysmographic application for atrial fibrillation screening in a primary care setting. J Am Heart Assoc 2016; 5(7). pii:e003428. doi:10.1161/JAHA.116.003428
- Mitchell ARJ, Le Page P. Living with the handheld ECG. BMJ Innov 2015; 1:46–48.
- Lee SP, Ha G, Wright DE, et al. Highly flexible, wearable, and disposable cardiac biosensors for remote and ambulatory monitoring. npj Digital Medicine 2018. doi:10.1038/s41746-017-0009-x
- Locati ET. New directions for ambulatory monitoring following the 2017 HRS-ISHNE expert consensus. J Electrocardiol 2017; 50(6):828–832. doi:10.1016/j.jelectrocard.2017.08.009
- Dillon JJ, DeSimone CV, Sapir Y, et al. Noninvasive potassium determination using a mathematically processed ECG: proof of concept for a novel “blood-less, blood test”. J Electrocardiol 2015; 48(1):12–18. doi:10.1016/j.jelectrocard.2014.10.002
KEY POINTS
- Ambulatory ECG monitoring is commonly used to correlate symptoms with arrhythmia, confirm occult atrial fibrillation, and assess the efficacy of antiarrhythmic therapy.
- Devices have features such as access to the full monitoring time (“full disclosure”), extended monitoring, and telemetry, each with advantages and limitations.
- Consumer-oriented wearable devices are aimed at arrhythmia monitoring, which could lead to increased arrhythmia detection, but at the risk of more false-positive results and excessive use of healthcare resources.
Measles: A dangerous vaccine-preventable disease returns
Measles, an ancient, highly contagious disease with a history of successful control by vaccination, is now threatening to have an epidemic resurgence. Until recently, measles vaccination largely controlled outbreaks in the United States. The Global Vaccine Action Plan under the World Health Organization aimed to eliminate measles worldwide. Nonetheless, the vaccine refusal movement and slow rollout of vaccine programs globally have interfered with control of the virus. A record number of measles cases have emerged in recent months: more than 700 since January 2019.1 Approximately 70% of recent cases were in unvaccinated patients, and almost all were in US residents.
This update reviews the history, presentation and diagnosis, complications, management, contagion control, and emerging threat of a measles epidemic. It concludes with recommendations for clinical practice in the context of the current measles outbreaks.
FROM UBIQUITOUS TO ERADICATED—AND BACK
Before the measles vaccine was developed and became available in the 1960s, outbreaks of measles occurred predictably every year in the United States and other temperate regions. During yearly outbreaks, measles was so contagious that household contacts had attack rates above 95%. Most cases occurred in very young children, and because infection with the virus causes lifelong immunity, it could be safely assumed that by adulthood, everyone was immune. In an outbreak in the Faroe Islands in 1846, no one who had been alive in the last major outbreak 65 years earlier became ill, but everyone under age 65 was at high risk with “high attack rates,” estimated as 99% from other outbreaks (reviewed by Krugman et al2).
In isolated regions previously free of measles, adults did not have immunity, and when exposed, they often developed severe disease. When European settlers brought measles and smallpox to the Americas beginning in the late 15th century, these diseases decimated whole populations of native peoples who had never been exposed to them.
That was premature. A number of outbreaks have occurred since then; the largest in the United States (before 2019) was in 2000. Over half of the 667 cases reported during that outbreak were in an underimmunized Amish community in Ohio.3
Now it emerges again.
PRESENTATION CAN VARY
The presentation varies somewhat among certain groups.
Nonimmune pregnant women have an especially severe course, likely related to the relative immune suppression of pregnancy.
In immune-suppressed states, measles is not only more severe, it is also difficult to diagnose because the rash can be absent.
In partially vaccinated children and adults, the disease may present atypically, without cough, conjunctivitis, and coryza, and it may be milder, lacking some of the extreme malaise typical of measles and with a shortened course. Measles infection in people who received the inactivated measles vaccine that was briefly available from 1963 to 1967 is also associated with atypical measles syndrome, a severe hypersensitivity reaction to the measles virus. Atypical measles can be prevented by revaccination with a live-virus vaccine.
DIAGNOSIS MAY NEED TO BE CONFIRMED
The diagnosis of measles is straightforward when all of the signs and symptoms are present. In partially vaccinated populations, however, the diagnosis may need to be confirmed by serologic or polymerase chain reaction (PCR) testing.
Differential diagnosis
The differential diagnosis of the fever and a rash typical of measles in children, especially when accompanied by severe malaise, includes the following:
Kawasaki disease. However, the red eyes of Kawasaki are an injection of the bulbar conjunctivae with sparing of the limbus. No eye exudate is present, and respiratory illness is not part of the disease.
Drug eruptions can present with a morbilliform rash and sometimes fever, but not the other signs of measles in either adults or children.
Scarlet fever has a different rash, the sandpaper rash typical of toxin-mediated disease.
Rubella tends to cause mild respiratory symptoms and illness rather than the severe disease of measles and other rash-causing viral infections in children and infants.
Confirmation in confusing cases
To confirm a diagnosis of measles, samples from throat, nasal, and posterior nasopharyngeal swabs should be collected with a blood specimen for serology and sent to the state public health laboratory.4 The US Centers for Disease Control and Prevention gives instructions on who should be tested and with which tests.4
Most testing now uses PCR for viral RNA, as viral culture is more costly and takes longer. For accurate diagnosis, samples for PCR must be obtained during the acute illness.
The serologic gold standard for diagnosis is a 4-fold rise or fall in immunoglobulin G (IgG) titer of paired serum samples sent 10 days to 2 weeks apart around the illness. The IgM test may be negative initially, and a negative test cannot be used to rule out the diagnosis. Confirmed cases should be reported to public health authorities.
COMPLICATIONS: EARLY AND LATE
Frequent complications of measles infection include those related to the primary viral infection of respiratory tract mucosal surfaces, as well as bacterial superinfections. Complications are most likely in children under age 5, nonimmune adults, pregnant women, and immunocompromised people. Typical complications include otitis media, laryngotracheobronchitis (presenting as a croupy cough), pneumonia, and diarrhea.
Late sequelae of measles infection are related in part to serious mucosal damage and generalized immune suppression caused by the virus. Even after recovery from acute infection, children can have persistent diarrhea and failure to thrive, with increased mortality risk in the months after infection. Tuberculosis can reactivate in patients already infected, and new tuberculosis infection can be especially severe. Further, tuberculosis skin tests become less reliable immediately after measles infection. Severe disease and fatalities are increased in populations that have baseline vitamin A deficiency and malnutrition.
Death from measles is most often caused by viral pneumonia, secondary bacterial pneumonia, and postviral encephalitis. Before the vaccine era, measles encephalitis occurred in the United States in about 1 in 1,000 measles cases.
Subacute sclerosing panencephalitis is a rare, late, and often fatal complication of measles that presents 7 to 10 years after acute measles infection, usually in adolescence. Beginning with myoclonic jerks, stiffening, and slow mental deterioration, it progresses over 1 to 3 years, with a relentless degenerative course leading to death. Since the introduction of the measles vaccine in 1957, this disease has essentially disappeared in the United States.
SUPPORTIVE CARE, INFECTION CONTROL
Management of measles and its complications is primarily supportive.
Preventing contagion
Measles infection has an incubation period of 8 to 12 days. Individuals are contagious 4 days before to 4 days after rash onset in the normal host but longer in those lacking immune function. Cases can occur up to 21 days after exposure during the contagious period.
The disease is highly contagious, so hospitalized patients should be cared for with airborne precautions. It is crucial that caretakers be vaccinated properly, so that they can care for patients safely. Recommendations for preventing secondary cases by prompt vaccination and giving immune globulin are detailed below, including specific recommendations for individuals with immune system suppression.
The current US public health policy regarding measles vaccine booster doses began in response to the widespread measles outbreak in the United States from 1989 to 1991. Cases occurred more commonly in unvaccinated individuals and in young adults who had received only 1 dose of vaccine.
Today, the policy in areas where measles has been controlled is to vaccinate between 12 and 15 months of age and to boost with a second dose before starting kindergarten. In outbreak situations, the first dose should be given at 6 months of age, with a repeat dose at 12 to 15 months of age and the usual booster before starting kindergarten.
Those born before 1957 can be presumed to have had natural measles, which confers lifelong immunity (Table 3).7
CURRENT THREAT
In 2000, measles was considered controlled in the United States, thanks to the national vaccination policy. But despite overall control, small numbers of cases continued to occur each year, related to exposure to cases imported from areas of the world endemic with measles.
Within the last year, however, major outbreaks have emerged. Incompletely vaccinated populations and unvaccinated individuals are the reason for the progression of current outbreaks.8
Until there is broader acceptance of the vaccine and better adherence to vaccine policies nationally and globally, measles cannot be completely eradicated. But with high vaccination rates, it is predicted that this infection can be controlled and ultimately eradicated.
RECOMMENDATIONS
In the midst of an outbreak and with rising public awareness of the threat of measles, it is important to recognize that MMR vaccination is the most effective way to prevent spread of the virus and maintain measles elimination in the United States. With this in mind, there are several key facts and recommendations regarding vaccination:
Recommendations on vaccination
- In measles-controlled populations, all children should be vaccinated between 12 and 15 months of age and again before kindergarten.
- In outbreak settings, children should receive a first vaccine dose at 6 months of age, a second at 12 to 15 months of age, and a third before kindergarten.
- Children who have received 2 measles vaccine doses can be assumed to be fully vaccinated and thus protected as long as the first dose was after 12 months of age. If the first dose was before 12 months of age, a child needs 3 doses.
- Adults born before 1957 can be assumed to have had measles infection and to be immune.
- Adults who were immunized with the inactivated measles vaccine available between 1963 and 1967 should receive 1 dose of live virus vaccine.
- Boosters are recommended for young adults who did not receive a second dose of vaccine and for adults with an uncertain history of immunization. There is no need to check titers before giving a booster, but if a positive titer is available in an adult, a booster is not needed.
- Heathcare providers should vaccinate unvaccinated or undervaccinated US residents traveling internationally (as long as they do not have contraindications) or traveling within the country to areas with outbreaks of measles.
Recommendations on vaccination after exposure to measles
- Vaccine is recommended for a nonimmune contact, including anyone with a history of only a single dose of vaccine.
- If a child got a first dose of vaccine before 12 months of age, give the second dose as soon as he or she turns 1 year old, or at least 28 days after the first dose.
- Vaccine must be given within 72 hours of exposure to confer protection (or at least decrease disease severity).
- The second dose of vaccine should be given at least 28 days after the first dose.
Recommendations on immune globulin after exposure to measles
- Immune globulin is recommended for anyone with exposure and no history of vaccination or immunity.
- Immune globulin can be given up to 6 days after exposure to prevent or decrease the severity of measles in immunocompromised hosts who have not been previously vaccinated. It is best to give it as early as possible.
- Immune globulin is given intramuscularly at 0.5 mL/kg, up to a to maximum dose of 15 mL.
- Pregnant women and immunocompromised hosts without immunity should receive immunoglobulin intravenously. Children and adults who have had a recent bone marrow transplant and likely do not yet have a reconstituted immune system should be treated with immune globulin to prevent infection, as vaccine cannot be given immediately after transplant. This is also true for other immunocompromised individuals who have not been vaccinated and who are not candidates for vaccine because of the severity of their immune suppression.
- Children with human immunodeficiency virus infection are routinely vaccinated. As long as they have evidence of serologic immunity, they do not need additional treatment.
- Kimberlin DW, Brady MT, Jackson MA, Long SS, editors. Measles. In Red Book: 2018 Report of the Committee on Infectious Diseases. American Academy of Pediatrics 2018; 537–550.
- Krugman S, Giles JP, Friedman H, Stone S. Studies on immunity to measles. J Pediatr 1965; 66:471–488. pmid:14264306.
- Gastañaduy PA, Budd J, Fisher N, et al. A measles outbreak in an underimmunized Amish community in Ohio. N Engl J Med 2016; 375(14):1343–1354. doi:10.1056/NEJMoa1602295
- Centers for Disease Control and Prevention. Measles (rubeola). www.cdc.gov/measles/index.html. Accessed May 16, 2019.
- World Health Organization. Measles vaccines: WHO position paper—April 2017. Wkly Epidemiol Rec 2017; 92(17):205–227. pmid:28459148
- McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS; Centers for Disease Control and Prevention. Prevention of measles, rubella, congenital rubella syndrome and mumps, 2013; summary: recommendations of the Advisory Committee on Immunization Practices ACIP. MMWR Recomm Rep 2013 Jun 14; 62(RR-4)1–34. pmid:23760231
- Advisory Committee on Immunization Practices; Centers for Disease Control and Prevention. Immunization for health-care personnel: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011 Nov 25; 60(RR-7)1–45. pmid:22108587
- Patel M, Lee AD, Redd SB, et al. Increase in measles cases—United States, January 1–April 26, 2019. MMWR Morb Mortal Wkly Rep 2019; May 3; 68(17):402–404. doi:10.15585/mmwr.mm6817e1
Measles, an ancient, highly contagious disease with a history of successful control by vaccination, is now threatening to have an epidemic resurgence. Until recently, measles vaccination largely controlled outbreaks in the United States. The Global Vaccine Action Plan under the World Health Organization aimed to eliminate measles worldwide. Nonetheless, the vaccine refusal movement and slow rollout of vaccine programs globally have interfered with control of the virus. A record number of measles cases have emerged in recent months: more than 700 since January 2019.1 Approximately 70% of recent cases were in unvaccinated patients, and almost all were in US residents.
This update reviews the history, presentation and diagnosis, complications, management, contagion control, and emerging threat of a measles epidemic. It concludes with recommendations for clinical practice in the context of the current measles outbreaks.
FROM UBIQUITOUS TO ERADICATED—AND BACK
Before the measles vaccine was developed and became available in the 1960s, outbreaks of measles occurred predictably every year in the United States and other temperate regions. During yearly outbreaks, measles was so contagious that household contacts had attack rates above 95%. Most cases occurred in very young children, and because infection with the virus causes lifelong immunity, it could be safely assumed that by adulthood, everyone was immune. In an outbreak in the Faroe Islands in 1846, no one who had been alive in the last major outbreak 65 years earlier became ill, but everyone under age 65 was at high risk with “high attack rates,” estimated as 99% from other outbreaks (reviewed by Krugman et al2).
In isolated regions previously free of measles, adults did not have immunity, and when exposed, they often developed severe disease. When European settlers brought measles and smallpox to the Americas beginning in the late 15th century, these diseases decimated whole populations of native peoples who had never been exposed to them.
That was premature. A number of outbreaks have occurred since then; the largest in the United States (before 2019) was in 2000. Over half of the 667 cases reported during that outbreak were in an underimmunized Amish community in Ohio.3
Now it emerges again.
PRESENTATION CAN VARY
The presentation varies somewhat among certain groups.
Nonimmune pregnant women have an especially severe course, likely related to the relative immune suppression of pregnancy.
In immune-suppressed states, measles is not only more severe, it is also difficult to diagnose because the rash can be absent.
In partially vaccinated children and adults, the disease may present atypically, without cough, conjunctivitis, and coryza, and it may be milder, lacking some of the extreme malaise typical of measles and with a shortened course. Measles infection in people who received the inactivated measles vaccine that was briefly available from 1963 to 1967 is also associated with atypical measles syndrome, a severe hypersensitivity reaction to the measles virus. Atypical measles can be prevented by revaccination with a live-virus vaccine.
DIAGNOSIS MAY NEED TO BE CONFIRMED
The diagnosis of measles is straightforward when all of the signs and symptoms are present. In partially vaccinated populations, however, the diagnosis may need to be confirmed by serologic or polymerase chain reaction (PCR) testing.
Differential diagnosis
The differential diagnosis of the fever and a rash typical of measles in children, especially when accompanied by severe malaise, includes the following:
Kawasaki disease. However, the red eyes of Kawasaki are an injection of the bulbar conjunctivae with sparing of the limbus. No eye exudate is present, and respiratory illness is not part of the disease.
Drug eruptions can present with a morbilliform rash and sometimes fever, but not the other signs of measles in either adults or children.
Scarlet fever has a different rash, the sandpaper rash typical of toxin-mediated disease.
Rubella tends to cause mild respiratory symptoms and illness rather than the severe disease of measles and other rash-causing viral infections in children and infants.
Confirmation in confusing cases
To confirm a diagnosis of measles, samples from throat, nasal, and posterior nasopharyngeal swabs should be collected with a blood specimen for serology and sent to the state public health laboratory.4 The US Centers for Disease Control and Prevention gives instructions on who should be tested and with which tests.4
Most testing now uses PCR for viral RNA, as viral culture is more costly and takes longer. For accurate diagnosis, samples for PCR must be obtained during the acute illness.
The serologic gold standard for diagnosis is a 4-fold rise or fall in immunoglobulin G (IgG) titer of paired serum samples sent 10 days to 2 weeks apart around the illness. The IgM test may be negative initially, and a negative test cannot be used to rule out the diagnosis. Confirmed cases should be reported to public health authorities.
COMPLICATIONS: EARLY AND LATE
Frequent complications of measles infection include those related to the primary viral infection of respiratory tract mucosal surfaces, as well as bacterial superinfections. Complications are most likely in children under age 5, nonimmune adults, pregnant women, and immunocompromised people. Typical complications include otitis media, laryngotracheobronchitis (presenting as a croupy cough), pneumonia, and diarrhea.
Late sequelae of measles infection are related in part to serious mucosal damage and generalized immune suppression caused by the virus. Even after recovery from acute infection, children can have persistent diarrhea and failure to thrive, with increased mortality risk in the months after infection. Tuberculosis can reactivate in patients already infected, and new tuberculosis infection can be especially severe. Further, tuberculosis skin tests become less reliable immediately after measles infection. Severe disease and fatalities are increased in populations that have baseline vitamin A deficiency and malnutrition.
Death from measles is most often caused by viral pneumonia, secondary bacterial pneumonia, and postviral encephalitis. Before the vaccine era, measles encephalitis occurred in the United States in about 1 in 1,000 measles cases.
Subacute sclerosing panencephalitis is a rare, late, and often fatal complication of measles that presents 7 to 10 years after acute measles infection, usually in adolescence. Beginning with myoclonic jerks, stiffening, and slow mental deterioration, it progresses over 1 to 3 years, with a relentless degenerative course leading to death. Since the introduction of the measles vaccine in 1957, this disease has essentially disappeared in the United States.
SUPPORTIVE CARE, INFECTION CONTROL
Management of measles and its complications is primarily supportive.
Preventing contagion
Measles infection has an incubation period of 8 to 12 days. Individuals are contagious 4 days before to 4 days after rash onset in the normal host but longer in those lacking immune function. Cases can occur up to 21 days after exposure during the contagious period.
The disease is highly contagious, so hospitalized patients should be cared for with airborne precautions. It is crucial that caretakers be vaccinated properly, so that they can care for patients safely. Recommendations for preventing secondary cases by prompt vaccination and giving immune globulin are detailed below, including specific recommendations for individuals with immune system suppression.
The current US public health policy regarding measles vaccine booster doses began in response to the widespread measles outbreak in the United States from 1989 to 1991. Cases occurred more commonly in unvaccinated individuals and in young adults who had received only 1 dose of vaccine.
Today, the policy in areas where measles has been controlled is to vaccinate between 12 and 15 months of age and to boost with a second dose before starting kindergarten. In outbreak situations, the first dose should be given at 6 months of age, with a repeat dose at 12 to 15 months of age and the usual booster before starting kindergarten.
Those born before 1957 can be presumed to have had natural measles, which confers lifelong immunity (Table 3).7
CURRENT THREAT
In 2000, measles was considered controlled in the United States, thanks to the national vaccination policy. But despite overall control, small numbers of cases continued to occur each year, related to exposure to cases imported from areas of the world endemic with measles.
Within the last year, however, major outbreaks have emerged. Incompletely vaccinated populations and unvaccinated individuals are the reason for the progression of current outbreaks.8
Until there is broader acceptance of the vaccine and better adherence to vaccine policies nationally and globally, measles cannot be completely eradicated. But with high vaccination rates, it is predicted that this infection can be controlled and ultimately eradicated.
RECOMMENDATIONS
In the midst of an outbreak and with rising public awareness of the threat of measles, it is important to recognize that MMR vaccination is the most effective way to prevent spread of the virus and maintain measles elimination in the United States. With this in mind, there are several key facts and recommendations regarding vaccination:
Recommendations on vaccination
- In measles-controlled populations, all children should be vaccinated between 12 and 15 months of age and again before kindergarten.
- In outbreak settings, children should receive a first vaccine dose at 6 months of age, a second at 12 to 15 months of age, and a third before kindergarten.
- Children who have received 2 measles vaccine doses can be assumed to be fully vaccinated and thus protected as long as the first dose was after 12 months of age. If the first dose was before 12 months of age, a child needs 3 doses.
- Adults born before 1957 can be assumed to have had measles infection and to be immune.
- Adults who were immunized with the inactivated measles vaccine available between 1963 and 1967 should receive 1 dose of live virus vaccine.
- Boosters are recommended for young adults who did not receive a second dose of vaccine and for adults with an uncertain history of immunization. There is no need to check titers before giving a booster, but if a positive titer is available in an adult, a booster is not needed.
- Heathcare providers should vaccinate unvaccinated or undervaccinated US residents traveling internationally (as long as they do not have contraindications) or traveling within the country to areas with outbreaks of measles.
Recommendations on vaccination after exposure to measles
- Vaccine is recommended for a nonimmune contact, including anyone with a history of only a single dose of vaccine.
- If a child got a first dose of vaccine before 12 months of age, give the second dose as soon as he or she turns 1 year old, or at least 28 days after the first dose.
- Vaccine must be given within 72 hours of exposure to confer protection (or at least decrease disease severity).
- The second dose of vaccine should be given at least 28 days after the first dose.
Recommendations on immune globulin after exposure to measles
- Immune globulin is recommended for anyone with exposure and no history of vaccination or immunity.
- Immune globulin can be given up to 6 days after exposure to prevent or decrease the severity of measles in immunocompromised hosts who have not been previously vaccinated. It is best to give it as early as possible.
- Immune globulin is given intramuscularly at 0.5 mL/kg, up to a to maximum dose of 15 mL.
- Pregnant women and immunocompromised hosts without immunity should receive immunoglobulin intravenously. Children and adults who have had a recent bone marrow transplant and likely do not yet have a reconstituted immune system should be treated with immune globulin to prevent infection, as vaccine cannot be given immediately after transplant. This is also true for other immunocompromised individuals who have not been vaccinated and who are not candidates for vaccine because of the severity of their immune suppression.
- Children with human immunodeficiency virus infection are routinely vaccinated. As long as they have evidence of serologic immunity, they do not need additional treatment.
Measles, an ancient, highly contagious disease with a history of successful control by vaccination, is now threatening to have an epidemic resurgence. Until recently, measles vaccination largely controlled outbreaks in the United States. The Global Vaccine Action Plan under the World Health Organization aimed to eliminate measles worldwide. Nonetheless, the vaccine refusal movement and slow rollout of vaccine programs globally have interfered with control of the virus. A record number of measles cases have emerged in recent months: more than 700 since January 2019.1 Approximately 70% of recent cases were in unvaccinated patients, and almost all were in US residents.
This update reviews the history, presentation and diagnosis, complications, management, contagion control, and emerging threat of a measles epidemic. It concludes with recommendations for clinical practice in the context of the current measles outbreaks.
FROM UBIQUITOUS TO ERADICATED—AND BACK
Before the measles vaccine was developed and became available in the 1960s, outbreaks of measles occurred predictably every year in the United States and other temperate regions. During yearly outbreaks, measles was so contagious that household contacts had attack rates above 95%. Most cases occurred in very young children, and because infection with the virus causes lifelong immunity, it could be safely assumed that by adulthood, everyone was immune. In an outbreak in the Faroe Islands in 1846, no one who had been alive in the last major outbreak 65 years earlier became ill, but everyone under age 65 was at high risk with “high attack rates,” estimated as 99% from other outbreaks (reviewed by Krugman et al2).
In isolated regions previously free of measles, adults did not have immunity, and when exposed, they often developed severe disease. When European settlers brought measles and smallpox to the Americas beginning in the late 15th century, these diseases decimated whole populations of native peoples who had never been exposed to them.
That was premature. A number of outbreaks have occurred since then; the largest in the United States (before 2019) was in 2000. Over half of the 667 cases reported during that outbreak were in an underimmunized Amish community in Ohio.3
Now it emerges again.
PRESENTATION CAN VARY
The presentation varies somewhat among certain groups.
Nonimmune pregnant women have an especially severe course, likely related to the relative immune suppression of pregnancy.
In immune-suppressed states, measles is not only more severe, it is also difficult to diagnose because the rash can be absent.
In partially vaccinated children and adults, the disease may present atypically, without cough, conjunctivitis, and coryza, and it may be milder, lacking some of the extreme malaise typical of measles and with a shortened course. Measles infection in people who received the inactivated measles vaccine that was briefly available from 1963 to 1967 is also associated with atypical measles syndrome, a severe hypersensitivity reaction to the measles virus. Atypical measles can be prevented by revaccination with a live-virus vaccine.
DIAGNOSIS MAY NEED TO BE CONFIRMED
The diagnosis of measles is straightforward when all of the signs and symptoms are present. In partially vaccinated populations, however, the diagnosis may need to be confirmed by serologic or polymerase chain reaction (PCR) testing.
Differential diagnosis
The differential diagnosis of the fever and a rash typical of measles in children, especially when accompanied by severe malaise, includes the following:
Kawasaki disease. However, the red eyes of Kawasaki are an injection of the bulbar conjunctivae with sparing of the limbus. No eye exudate is present, and respiratory illness is not part of the disease.
Drug eruptions can present with a morbilliform rash and sometimes fever, but not the other signs of measles in either adults or children.
Scarlet fever has a different rash, the sandpaper rash typical of toxin-mediated disease.
Rubella tends to cause mild respiratory symptoms and illness rather than the severe disease of measles and other rash-causing viral infections in children and infants.
Confirmation in confusing cases
To confirm a diagnosis of measles, samples from throat, nasal, and posterior nasopharyngeal swabs should be collected with a blood specimen for serology and sent to the state public health laboratory.4 The US Centers for Disease Control and Prevention gives instructions on who should be tested and with which tests.4
Most testing now uses PCR for viral RNA, as viral culture is more costly and takes longer. For accurate diagnosis, samples for PCR must be obtained during the acute illness.
The serologic gold standard for diagnosis is a 4-fold rise or fall in immunoglobulin G (IgG) titer of paired serum samples sent 10 days to 2 weeks apart around the illness. The IgM test may be negative initially, and a negative test cannot be used to rule out the diagnosis. Confirmed cases should be reported to public health authorities.
COMPLICATIONS: EARLY AND LATE
Frequent complications of measles infection include those related to the primary viral infection of respiratory tract mucosal surfaces, as well as bacterial superinfections. Complications are most likely in children under age 5, nonimmune adults, pregnant women, and immunocompromised people. Typical complications include otitis media, laryngotracheobronchitis (presenting as a croupy cough), pneumonia, and diarrhea.
Late sequelae of measles infection are related in part to serious mucosal damage and generalized immune suppression caused by the virus. Even after recovery from acute infection, children can have persistent diarrhea and failure to thrive, with increased mortality risk in the months after infection. Tuberculosis can reactivate in patients already infected, and new tuberculosis infection can be especially severe. Further, tuberculosis skin tests become less reliable immediately after measles infection. Severe disease and fatalities are increased in populations that have baseline vitamin A deficiency and malnutrition.
Death from measles is most often caused by viral pneumonia, secondary bacterial pneumonia, and postviral encephalitis. Before the vaccine era, measles encephalitis occurred in the United States in about 1 in 1,000 measles cases.
Subacute sclerosing panencephalitis is a rare, late, and often fatal complication of measles that presents 7 to 10 years after acute measles infection, usually in adolescence. Beginning with myoclonic jerks, stiffening, and slow mental deterioration, it progresses over 1 to 3 years, with a relentless degenerative course leading to death. Since the introduction of the measles vaccine in 1957, this disease has essentially disappeared in the United States.
SUPPORTIVE CARE, INFECTION CONTROL
Management of measles and its complications is primarily supportive.
Preventing contagion
Measles infection has an incubation period of 8 to 12 days. Individuals are contagious 4 days before to 4 days after rash onset in the normal host but longer in those lacking immune function. Cases can occur up to 21 days after exposure during the contagious period.
The disease is highly contagious, so hospitalized patients should be cared for with airborne precautions. It is crucial that caretakers be vaccinated properly, so that they can care for patients safely. Recommendations for preventing secondary cases by prompt vaccination and giving immune globulin are detailed below, including specific recommendations for individuals with immune system suppression.
The current US public health policy regarding measles vaccine booster doses began in response to the widespread measles outbreak in the United States from 1989 to 1991. Cases occurred more commonly in unvaccinated individuals and in young adults who had received only 1 dose of vaccine.
Today, the policy in areas where measles has been controlled is to vaccinate between 12 and 15 months of age and to boost with a second dose before starting kindergarten. In outbreak situations, the first dose should be given at 6 months of age, with a repeat dose at 12 to 15 months of age and the usual booster before starting kindergarten.
Those born before 1957 can be presumed to have had natural measles, which confers lifelong immunity (Table 3).7
CURRENT THREAT
In 2000, measles was considered controlled in the United States, thanks to the national vaccination policy. But despite overall control, small numbers of cases continued to occur each year, related to exposure to cases imported from areas of the world endemic with measles.
Within the last year, however, major outbreaks have emerged. Incompletely vaccinated populations and unvaccinated individuals are the reason for the progression of current outbreaks.8
Until there is broader acceptance of the vaccine and better adherence to vaccine policies nationally and globally, measles cannot be completely eradicated. But with high vaccination rates, it is predicted that this infection can be controlled and ultimately eradicated.
RECOMMENDATIONS
In the midst of an outbreak and with rising public awareness of the threat of measles, it is important to recognize that MMR vaccination is the most effective way to prevent spread of the virus and maintain measles elimination in the United States. With this in mind, there are several key facts and recommendations regarding vaccination:
Recommendations on vaccination
- In measles-controlled populations, all children should be vaccinated between 12 and 15 months of age and again before kindergarten.
- In outbreak settings, children should receive a first vaccine dose at 6 months of age, a second at 12 to 15 months of age, and a third before kindergarten.
- Children who have received 2 measles vaccine doses can be assumed to be fully vaccinated and thus protected as long as the first dose was after 12 months of age. If the first dose was before 12 months of age, a child needs 3 doses.
- Adults born before 1957 can be assumed to have had measles infection and to be immune.
- Adults who were immunized with the inactivated measles vaccine available between 1963 and 1967 should receive 1 dose of live virus vaccine.
- Boosters are recommended for young adults who did not receive a second dose of vaccine and for adults with an uncertain history of immunization. There is no need to check titers before giving a booster, but if a positive titer is available in an adult, a booster is not needed.
- Heathcare providers should vaccinate unvaccinated or undervaccinated US residents traveling internationally (as long as they do not have contraindications) or traveling within the country to areas with outbreaks of measles.
Recommendations on vaccination after exposure to measles
- Vaccine is recommended for a nonimmune contact, including anyone with a history of only a single dose of vaccine.
- If a child got a first dose of vaccine before 12 months of age, give the second dose as soon as he or she turns 1 year old, or at least 28 days after the first dose.
- Vaccine must be given within 72 hours of exposure to confer protection (or at least decrease disease severity).
- The second dose of vaccine should be given at least 28 days after the first dose.
Recommendations on immune globulin after exposure to measles
- Immune globulin is recommended for anyone with exposure and no history of vaccination or immunity.
- Immune globulin can be given up to 6 days after exposure to prevent or decrease the severity of measles in immunocompromised hosts who have not been previously vaccinated. It is best to give it as early as possible.
- Immune globulin is given intramuscularly at 0.5 mL/kg, up to a to maximum dose of 15 mL.
- Pregnant women and immunocompromised hosts without immunity should receive immunoglobulin intravenously. Children and adults who have had a recent bone marrow transplant and likely do not yet have a reconstituted immune system should be treated with immune globulin to prevent infection, as vaccine cannot be given immediately after transplant. This is also true for other immunocompromised individuals who have not been vaccinated and who are not candidates for vaccine because of the severity of their immune suppression.
- Children with human immunodeficiency virus infection are routinely vaccinated. As long as they have evidence of serologic immunity, they do not need additional treatment.
- Kimberlin DW, Brady MT, Jackson MA, Long SS, editors. Measles. In Red Book: 2018 Report of the Committee on Infectious Diseases. American Academy of Pediatrics 2018; 537–550.
- Krugman S, Giles JP, Friedman H, Stone S. Studies on immunity to measles. J Pediatr 1965; 66:471–488. pmid:14264306.
- Gastañaduy PA, Budd J, Fisher N, et al. A measles outbreak in an underimmunized Amish community in Ohio. N Engl J Med 2016; 375(14):1343–1354. doi:10.1056/NEJMoa1602295
- Centers for Disease Control and Prevention. Measles (rubeola). www.cdc.gov/measles/index.html. Accessed May 16, 2019.
- World Health Organization. Measles vaccines: WHO position paper—April 2017. Wkly Epidemiol Rec 2017; 92(17):205–227. pmid:28459148
- McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS; Centers for Disease Control and Prevention. Prevention of measles, rubella, congenital rubella syndrome and mumps, 2013; summary: recommendations of the Advisory Committee on Immunization Practices ACIP. MMWR Recomm Rep 2013 Jun 14; 62(RR-4)1–34. pmid:23760231
- Advisory Committee on Immunization Practices; Centers for Disease Control and Prevention. Immunization for health-care personnel: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011 Nov 25; 60(RR-7)1–45. pmid:22108587
- Patel M, Lee AD, Redd SB, et al. Increase in measles cases—United States, January 1–April 26, 2019. MMWR Morb Mortal Wkly Rep 2019; May 3; 68(17):402–404. doi:10.15585/mmwr.mm6817e1
- Kimberlin DW, Brady MT, Jackson MA, Long SS, editors. Measles. In Red Book: 2018 Report of the Committee on Infectious Diseases. American Academy of Pediatrics 2018; 537–550.
- Krugman S, Giles JP, Friedman H, Stone S. Studies on immunity to measles. J Pediatr 1965; 66:471–488. pmid:14264306.
- Gastañaduy PA, Budd J, Fisher N, et al. A measles outbreak in an underimmunized Amish community in Ohio. N Engl J Med 2016; 375(14):1343–1354. doi:10.1056/NEJMoa1602295
- Centers for Disease Control and Prevention. Measles (rubeola). www.cdc.gov/measles/index.html. Accessed May 16, 2019.
- World Health Organization. Measles vaccines: WHO position paper—April 2017. Wkly Epidemiol Rec 2017; 92(17):205–227. pmid:28459148
- McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS; Centers for Disease Control and Prevention. Prevention of measles, rubella, congenital rubella syndrome and mumps, 2013; summary: recommendations of the Advisory Committee on Immunization Practices ACIP. MMWR Recomm Rep 2013 Jun 14; 62(RR-4)1–34. pmid:23760231
- Advisory Committee on Immunization Practices; Centers for Disease Control and Prevention. Immunization for health-care personnel: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2011 Nov 25; 60(RR-7)1–45. pmid:22108587
- Patel M, Lee AD, Redd SB, et al. Increase in measles cases—United States, January 1–April 26, 2019. MMWR Morb Mortal Wkly Rep 2019; May 3; 68(17):402–404. doi:10.15585/mmwr.mm6817e1
KEY POINTS
- Measles is highly contagious and can have serious complications, including death.
- Measles vaccine is given in a 2-dose series. People who have received only 1 dose should receive either 1 or 2 more doses, depending on the situation, so that they are protected.
- The diagnosis of measles is straightforward when classic signs and symptoms are present—fever, cough, conjunctivitis, runny nose, and rash—especially after a known exposure or in the setting of outbreak. On the other hand, in partially vaccinated or immunosuppressed people, the illness presents atypically, and confirmation of diagnosis requires laboratory testing.
- Management is mostly supportive. Children—and probably also adults—should receive vitamin A.
- Since disease can be severe in the unvaccinated, immune globulin and vaccine are given to the normal host with an exposure and no history of vaccine or immunity.
Women’s health 2019: Osteoporosis, breast cancer, contraception, and hormone therapy
Keeping up with current evidence-based healthcare practices is key to providing good clinical care to patients. This review presents 5 vignettes that highlight key issues in women’s health: osteoporosis screening, hormonal contraceptive interactions with antibiotics, hormone replacement therapy in carriers of the BRCA1 gene mutation, risks associated with hormonal contraception, and breast cancer diagnosis using digital tomosynthesis in addition to digital mammography. Supporting articles, all published in 2017 and 2018, were selected from high-impact medical and women’s health journals.
OSTEOPOROSIS SCREENING FOR FRACTURE PREVENTION
A 60-year-old woman reports that her last menstrual period was 7 years ago. She has no history of falls or fractures, and she takes no medications. She smokes 10 cigarettes per day and drinks 3 to 4 alcoholic beverages on most days of the week. She is 5 feet 6 inches (170 cm) tall and weighs 107 lb. Should she be screened for osteoporosis?
Osteoporosis is underdiagnosed
It is estimated that, in the United States, 12.3 million individuals older than 50 will develop osteoporosis by 2020. Missed opportunities to screen high-risk individuals can lead to fractures, including fractures of the hip.1
Updated screening recommendations
In 2018, the US Preventive Services Task Force (USPSTF) developed and published evidence-based recommendations for osteoporosis screening to help providers identify and treat osteoporosis early to prevent fractures.2 Available evidence on screening and treatment in women and men were reviewed with the intention of updating the 2011 USPSTF recommendations. The review also evaluated risk assessment tools, screening intervals, and efficacy of screening and treatment in various subpopulations.
Since the 2011 recommendations, more data have become available on fracture risk assessment with or without bone mineral density measurements. In its 2018 report, the USPSTF recommends that postmenopausal women younger than 65 should undergo screening with a bone density test if their 10-year risk of major osteoporotic fracture is more than 8.4%. This is equivalent to the fracture risk of a 65-year-old white woman with no major risk factors for fracture (grade B recommendation—high certainty that the benefit is moderate, or moderate certainty that the benefit is moderate to substantial).2
Assessment of fracture risk
For postmenopausal women who are under age 65 and who have at least 1 risk factor for fracture, it is reasonable to use a clinical risk assessment tool to determine who should undergo screening with bone mineral density measurement. Risk factors associated with an increased risk of osteoporotic fractures include a parental history of hip fracture, smoking, intake of 3 or more alcoholic drinks per day, low body weight, malabsorption, rheumatoid arthritis, diabetes, and postmenopausal status (not using estrogen replacement). Medications should be carefully reviewed for those that can increase the risk of fractures, including steroids and antiestrogen treatments.
The 10-year risk of a major osteoporotic or hip fracture can be assessed using the Fractional Risk Assessment Tool (FRAX), available at www.sheffield.ac.uk/FRAX/. Other acceptable tools that perform similarly to FRAX include the Osteoporosis Risk Assessment Instrument (ORAI) (10 studies; N = 16,780), Osteoporosis Index of Risk (OSIRIS) (5 studies; N = 5,649), Osteoporosis Self-Assessment Tool (OST) (13 studies; N = 44,323), and Simple Calculated Osteoporosis Risk Estimation (SCORE) (8 studies; N = 15,362).
Should this patient be screened for osteoporosis?
Based on the FRAX, this patient’s 10-year risk of major osteoporosis fracture is 9.2%. She would benefit from osteoporosis screening with a bone density test.
DO ANTIBIOTICS REDUCE EFFECTIVENESS OF HORMONAL CONTRACEPTION?
A 27-year-old woman presents with a dog bite on her right hand and is started on oral antibiotics. She takes an oral contraceptive that contains 35 µg of ethinyl estradiol and 0.25 mg of norgestimate. She asks if she should use condoms while taking antibiotics.
The antibiotics rifampin and rifabutin are known inducers of the hepatic enzymes required for contraceptive steroid metabolism, whereas other antibiotics are not. Despite the lack of compelling evidence that broad-spectrum antibiotics interfere with the efficacy of hormonal contraception, most pharmacists recommend backup contraception for women who use concomitant antibiotics.3 This practice could lead to poor compliance with the contraceptive regimen, the antibiotic regimen, or both.3
Simmons et al3 conducted a systematic review of randomized and nonrandomized studies that assessed pregnancy rates, breakthrough bleeding, ovulation suppression, and hormone pharmacokinetics in women taking oral or vaginal hormonal contraceptives in combination with nonrifamycin antibiotics, including oral, intramuscular, and intravenous forms. Oral contraceptives used in the studies included a range of doses and progestins, but lowest-dose pills, such as those containing less than 30 µg ethinyl estradiol or less than 150 µg levonorgestrel, were not included.
The contraceptive formulations in this systematic review3 included oral contraceptive pills, emergency contraception pills, and the contraceptive vaginal ring. The effect of antibiotics on other nonoral contraceptives, such as the transdermal patch, injectables, and progestin implants was not studied.
Four observational studies3 evaluated pregnancy rates or hormonal contraception failure with any antibiotic use. In 2 of these 4 studies, there was no difference in pregnancy rates in women who used oral contraceptives with and without nonrifamycin antibiotics. However, ethinyl estradiol was shown to have increased clearance when administered with dirithromycin (a macrolide).3 Twenty-five of the studies reported measures of contraceptive effectiveness (ovulation) and pharmacokinetic outcomes.
There were no observed differences in ovulation suppression or breakthrough bleeding in any study that combined hormonal contraceptives with an antibiotic. Furthermore, there was no significant decrease in progestin pharmacokinetic parameters during coadministration with an antibiotic.3 Study limitations included small sample sizes and the observational nature of the data.
How would you counsel this patient?
Available evidence suggests that nonrifamycin antibiotics do not diminish the effectiveness of the vaginal contraceptive ring or an oral hormonal contraceptive that contains at least 30 µg of ethinyl estradiol or 150 µg of levonorgestrel. Current guidelines do not recommend the use of additional backup contraception, regardless of hormonal contraception dose or formulation.4 Likewise, the most recent guidance for dental practitioners (ie, from 2012) no longer advises women to use additional contraceptive protection when taking nonrifamycin antibiotics.5
In our practice, we discuss the option of additional protection when prescribing formulations with lower estrogen doses (< 30 µg), not only because of the limitations of the available data, but also because of the high rates of unintended pregnancy with typical use of combined hormonal contraceptives (9% per year, unrelated to use of antibiotics).4 However, if our patient would rather not use additional barrier methods, she can be reassured that concomitant nonrifamycin antibiotic use is unlikely to affect contraceptive effectiveness.
HORMONE REPLACEMENT THERAPY IN CARRIERS OF THE BRCA1 MUTATION
A 41-year-old healthy mother of 3 was recently found to be a carrier of the BRCA1 mutation. She is planning to undergo prophylactic bilateral salpingo-oophorectomy for ovarian cancer prevention. However, she is apprehensive about undergoing surgical menopause. Should she be started on hormone replacement therapy after oophorectomy? How would hormone replacement therapy affect her risk of breast cancer?
In females who carry the BRCA1 mutation, the cumulative risk of both ovarian and breast cancer approaches 44% (95% confidence interval [CI] 36%–53%) and 72% (95% CI 65%–79%) by age 80.6 Prophylactic salpingo-oophorectomy reduces the risk of breast cancer by 50% and the risk of ovarian cancer by 90%. Unfortunately, premature withdrawal of ovarian hormones has been associated with long-term adverse effects including significant vasomotor symptoms, decreased quality of life, sexual dysfunction, early mortality, bone loss, decline in mood and cognition, and poor cardiovascular outcomes.7 Many of these effects can be avoided or lessened with hormone replacement therapy.
Kotsopoulos et al8 conducted a longitudinal, prospective analysis of BRCA1 mutation carriers in a multicenter study between 1995 and 2017. The mean follow-up period was 7.6 years (range 0.4–22.1). The study assessed associations between the use of hormone replacement therapy and breast cancer risk in carriers of the BRCA1 mutation who underwent prophylactic salpingo-oophorectomy. Study participants did not have a personal history of cancer. Those with a history of prophylactic mastectomy were excluded.
Participants completed a series of questionnaires every 2 years, disclosing updates in personal medical, cancer, and reproductive history. The questionnaires also inquired about the use of hormone replacement therapy, including the type used (estrogen only, progestin only, estrogen plus progestin, other), brand name, duration of use, and dose and route of administration (pill, patch, suppository).
Of the 13,087 BRCA1 mutation carriers identified, 872 met the study criteria. Of those, 377 (43%) reported using some form of hormone replacement therapy after salpingo-oophorectomy, and 495 (57%) did not. The average duration of use was 3.9 years (range 0.5–19), with most (69%) using estrogen alone; 18% used other regimens, including estrogen plus progestin and progestin only. A small percentage of participants did not indicate which formulation they used. On average, women using hormone replacement therapy underwent prophylactic oophorectomy earlier than nonusers (age 43.0 vs 48.4; absolute difference 5.5 years, P < .001).
During follow-up, there was no significant difference noted in the proportion of women diagnosed with breast cancer between hormone replacement therapy users and nonusers (10.3 vs 10.7%; absolute difference 0.4%; P = .86). In fact, for each year of estrogen-containing hormone replacement therapy, there was an 18% reduction in breast cancer risk when oophorectomy was performed before age 45 (95% CI 0.69–0.97). The authors also noted a nonsignificant 14% trend toward an increase in breast cancer risk for each year of progestin use after oophorectomy when surgery was performed before age 45 (95% CI 0.9–1.46).
Although prophylactic hysterectomy was not recommended, the authors noted that hysterectomy would eliminate the need for progestin-containing hormone replacement therapy. For those who underwent oophorectomy after age 45, hormone replacement therapy did not increase or decrease the risk of breast cancer.7
A meta-analysis by Marchetti et al9 also supports the safety of hormone replacement therapy after risk-reducing salpingo-oophorectomy. Three studies that included 1,100 patients were analyzed (including the Kotsopoulos study8 noted above). There was a nonsignificant decrease in breast cancer risk in women on estrogen-only hormone replacement therapy compared with women on estrogen-plus-progestin therapy (odds ratio 0.53, 95% CI 0.25–1.15). Overall, the authors regarded hormone replacement therapy as a safe therapeutic option after prophylactic salpingo-oophorectomy in carriers of the BRCA1 and BRCA2 mutations.9
In a case-control study published in 2016,10 hormone replacement therapy was assessed in 432 postmenopausal BRCA1 mutation carriers with invasive breast cancer (cases) and in 432 BRCA1 mutation carriers without a history of breast cancer (controls). Results showed no difference in breast cancer risk between hormone replacement therapy users and nonusers.10
Rebbeck et al11 evaluated short-term hormone replacement therapy in BRCA1 and BRCA2 gene-mutation carriers after they underwent prophylactic salpingo-oophorectomy. The results showed that hormone replacement did not affect the breast cancer risk-reduction conferred with prophylactic bilateral salpingo-oophorectomy.
Johansen et al12 evaluated hormone replacement therapy in premenopausal women after prophylactic salpingo-oophorectomy. They studied 324 carriers of BRCA gene mutations after they underwent prophylactic salpingo-oophorectomy and a subset of 950 controls who had bilateral salpingo-oophorectomy for reasons unrelated to cancer. In both groups, hormone replacement therapy was underutilized. The authors recommended using it when clinically indicated.
Should your patient start hormone replacement therapy?
This patient is healthy, and in the absence of contraindications, systemic hormone replacement therapy after prophylactic oophorectomy could mitigate the potential adverse effects of surgically induced menopause. The patient can be reassured that estrogen-containing short-term hormone replacement therapy is unlikely to increase her breast cancer risk.
HORMONAL CONTRACEPTION AND THE RISK OF BREAST CANCER
A 44-year-old woman presents to your office for an annual visit. She is sexually active but does not wish to become pregnant. She has a family history of breast cancer: her mother was diagnosed at age 53. She is interested in an oral contraceptive to prevent pregnancy and acne. However, she is nervous about being on any contraceptive that may increase her risk of breast cancer.
To date, studies assessing the effect of hormonal contraception on the risk of breast cancer have produced inconsistent results. Although most studies have shown no associated risk, a few have shown a temporary 20% to 30% increased risk of breast cancer during use.13,14 Case-controlled studies that reported an association between hormonal contraception and breast cancer included populations taking higher-dose combination pills, which are no longer prescribed. Most studies do not evaluate specific formulations of hormonal contraception, and little is known about effects associated with intrauterine devices or progestin-only contraception.
A prospective study performed by Mørch et al13 followed more than 1 million reproductive-aged women for a mean of 10.9 years. The Danish Cancer Registry was used to identify cases of invasive breast cancer. Women who used hormonal contraceptives had a relative risk of breast cancer of 1.20 compared with women not on hormonal contraception (95% CI 1.14–1.26). The study suggested that those who had been on contraceptive agents for more than 5 years had an increased risk and that this risk remained for 5 years after the agents were discontinued. Conversely, no increased risk of cancer was noted in those who used hormonal contraception for less than 5 years. No notable differences were seen among various formulations.
For women using the levonorgestrel-containing intrauterine device, the relative risk of breast cancer was 1.21 (95% CI 1.11–1.33). A few cancers were noted in those who used the progestin-only implant or those using depot medroxyprogesterone acetate. While the study showed an increased relative risk of breast cancer, the absolute risk was low—13 cases per 100,000, or approximately 1 additional case of breast cancer per 7,690 per year.13
This study had several important limitations. The authors did not adjust for common breast cancer risk factors including age at menarche, alcohol use, or breastfeeding. Additionally, the study did not account for the use of hormonal contraception before the study period and conversely, did not account for women who may have stopped taking their contraceptive despite their prescribed duration. The frequency of mammography was not explicitly noted, which could have shifted results for women who had more aggressive screening.
It is also noteworthy that the use of high-dose systemic progestins was not associated with an increased risk, whereas the levonorgestrel intrauterine device, which contains only 1/20th the dose of a low-dose oral contraceptive pill, was associated with an increased risk. This discrepancy in risk warrants further investigation, and clinicians should be aware that this inconsistency needs validation before changing clinical practice.
In an observational cohort study,15 more than 100,000 women ages 50 to 71 were followed prospectively for 15 years to evaluate the association between hormonal contraceptive use and the risk of gynecologic and breast cancers. In this study, the duration of hormonal contraceptive use, smoking status, alcohol use, body mass index, physical activity, and family history of cancer were recorded. Long-term hormonal contraceptive use reduced ovarian and endometrial cancer risks by 40% and 34%, respectively, with no increase in breast cancer risk regardless of family history.
How would you counsel the patient?
The patient should be educated on the benefits of hormonal contraception that extend beyond pregnancy prevention, including regulation of menses, improved acne, decreased risk of endometrial and ovarian cancer, and likely reductions in colorectal cancer and overall mortality risk.13–16 Further, after their own systematic review of the data assessing risk of breast cancer with hormonal contraception, the US Centers for Disease Control and Prevention state in their guidelines that all contraceptives may be used without limitation in those who have a family history of breast cancer.4 Any potential increased risk of breast cancer in women using hormonal contraception is small and would not outweigh the benefits associated with use.
One must consider the impact of an unintended pregnancy in such women, including effects on the health of the fetus and mother. Recent reports on the increasing rates of maternal death in the US (23.8 of 100,000 live births) serve as a reminder of the complications that can arise with pregnancy, especially if a mother’s health is not optimized before conception.17
MAMMOGRAPHY PLUS TOMOSYNTHESIS VS MAMMOGRAPHY ALONE
The same 44-year-old patient now inquires about screening for breast cancer. She is curious about 3-dimensional mammography and whether it would be a better screening test for her.
Digital breast tomosynthesis (DBT) is a newer imaging modality that provides a 3-dimensional reconstruction of the breast using low-dose x-ray imaging. Some studies have shown that combining DBT with digital mammography may be superior to digital mammography alone in detecting cancers.18 However, digital mammography is currently the gold standard for breast cancer screening and is the only test proven to reduce mortality.18,19
In a retrospective US study of 13 medical centers,20 breast cancer detection rates increased by 41% the year after DBT was introduced, from 2.9 to 4.1 per 1,000 cases. DBT was associated with 16 fewer patients recalled for repeat imaging out of 1,000 women screened (as opposed to mammography alone). Two European studies similarly suggested an increase in cancer detection with lower recall rates.21,22
Is 3-D mammography a better option?
In a 2-arm study by Pattacini et al,18 nearly 20,000 women ages 45 to 70 were randomized to undergo either digital mammography or digital mammography plus DBT for primary breast cancer screening. Women were enrolled over a 2-year period and were followed for 4.5 years, and the development of a primary invasive cancer was the primary end point. Recall rates, reading times, and radiation doses were also compared between the 2 groups.
Overall, the cancer detection rate was higher in the digital mammography plus DBT arm compared with digital mammography alone (8.6 vs 4.5 per 1,000). The detection rates were higher in the combined screening group among all age subgroups, with relative risks ranging from 1.83 to 2.04 (P = .93). The recall rate was 3.5% in the 2 arms, with relative risks ranging from 0.93 to 1.11 (P = .52). There was a reduction in the number of false positives seen in women undergoing digital mammography plus DBT when compared with digital mammography alone, from 30 per 1,000 to 27 per 1,000.
Detection of ductal carcinoma in situ increased in the experimental arm (relative detection 2.80, 95% CI 1.01–7.65) compared with invasive cancers. Comparing radiation, the dose was 2.3 times higher in those who underwent digital mammography plus DBT. The average reading times for digital mammography alone were 20 to 85 seconds; adding DBT added 35 to 81 seconds.19
Should you advise 3-D mammography?
The patient should be educated on the benefits of both digital mammography alone and digital mammography plus DBT. The use of digital mammography plus DBT has been supported in various studies and has been shown to increase cancer detection rates, although data are still conflicting regarding recall rates.19,20 More studies are needed to determine its effect on breast cancer morality.
Routine use of DBT in women with or without dense breast tissue has not been recommended by organizations such as the USPSTF and the American College of Obstetricians and Gynecologists.23,24 While there is an increased dose of radiation, it still falls below the US Food and Drug Administration limits and should not be the sole barrier to use.
- Cauley JA. Screening for osteoporosis. JAMA 2018; 319(24):2483–2485. doi:10.1001/jama.2018.5722
- US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA 2018; 319(24):2521–2531. doi:10.1001/jama.2018.7498
- Simmons KB, Haddad LB, Nanda K, Curtis KM. Drug interactions between non-rifamycin antibiotics and hormonal contraception: a systematic review. Am J Obstet Gynecol 2018; 218(1):88–97.e14. doi:10.1016/j.ajog.2017.07.003
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US Medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep 2016; 65(3):1–103. doi:10.15585/mmwr.rr6503a1
- Taylor J, Pemberton MN. Antibiotics and oral contraceptives: new considerations for dental practice. Br Dent J 2012; 212(10):481–483. doi:10.1038/sj.bdj.2012.414
- Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 2017; 317(23):2402–2416. doi:10.1001/jama.2017.7112
- Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long-term health consequences of premature or early menopause and considerations for management. Climacteric 2015; 18(4):483–491. doi:10.3109/13697137.2015.1020484
- Kotsopoulos J, Gronwald J, Karlan BY, et al; Hereditary Breast Cancer Clinical Study Group. Hormone replacement therapy after oophorectomy and breast cancer risk among BRCA1 mutation carriers. JAMA Oncol 2018; 4(8):1059–1065. doi:10.1001/jamaoncol.2018.0211
- Marchetti C, De Felice F, Boccia S, et al. Hormone replacement therapy after prophylactic risk reducing salpingo-oophorectomy and breast cancer risk in BRCA1 and BRCA2 mutation carriers: a meta-analysis. Crit Rev Oncol Hematol 2018; 132:111–115. doi:10.1016/j.critrevonc.2018.09.018
- Kotsopoulos J, Huzarski T, Gronwald J, et al. Hormone replacement therapy after menopause and risk of breast cancer in BRCA1 mutation carriers: a case-control study. Breast Cancer Res Treat 2016; 155(2):365–373. doi:10.1007/s10549-016-3685-3
- Rebbeck TR, Friebel T, Wagner T, et al; PROSE Study Group. Effect of short-term hormone replacement therapy on breast cancer risk reduction after bilateral prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol 2005; 23(31):7804–7810. doi:10.1200/JCO.2004.00.8151
- Johansen N, Liavaag AH, Iversen OE, Dørum A, Braaten T, Michelsen TM. Use of hormone replacement therapy after risk-reducing salpingo-oophorectomy. Acta Obstet Gynecol Scand 2017; 96(5):547–555. doi:10.1111/aogs.13120
- Mørch LS, Skovlund CW, Hannaford PC, Iversen L, Fielding S, Lidegaard Ø. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med 2017; 377(23):2228–2239. doi:10.1056/NEJMoa1700732
- Batur P, Sikka S, McNamara M. Contraception update: extended use of long acting methods, hormonal contraception risks, and over the counter access. J Womens Health (Larchmt) 2018. doi:10.1089/jwh.2018.7391. [Epub ahead of print]
- Michels KA, Pfeiffer RM, Brinton LA, Trabert B. Modification of the associations between duration of oral contraceptive use and ovarian, endometrial, breast, and colorectal cancers. JAMA Oncol 2018; 4(4):516–521. doi:10.1001/jamaoncol.2017.4942
- Iversen L, Fielding S, Lidegaard Ø, Mørch LS, Skovlund CW, Hannaford PC. Association between contemporary hormonal contraception and ovarian cancer in women of reproductive age in Denmark: prospective, nationwide cohort study. BMJ 2018; 362:k3609. doi:10.1136/bmj.k3609
- MacDorman MF, Declercq E, Cabral H, Morton C. Recent increases in the US maternal mortality rate: disentangling trends from measurement issues. Obstet Gynecol 2016; 128(3):447–455. doi:10.1097/AOG.0000000000001556
- Pattacini P, Nitrosi A, Giorgi Rossi P, et al; RETomo Working Group. Digital mammography versus digital mammography plus tomosynthesis for breast cancer screening: the Reggio Emilia tomosynthesis randomized trial. Radiology 2018; 288(2):375–385. doi:10.1148/radiol.2018172119
- Pace L, Keating NL. A systematic assessment of benefits and risks to guide breast cancer screening decisions. JAMA 2014; 311(13):1327–1335. doi:10.1001/jama.2014.1398
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014; 311(24):2499–2507. doi:10.1001/jama.2014.6095
- Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology 2013; 267(1):47–56. doi:10.1148/radiol.12121373
- Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol 2013; 14(7):583–589. doi:10.1016/S1470-2045(13)70134-7
- US Preventive Services Task Force. Final recommendation statement: breast cancer: screening. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/breast-cancer-screening1. Accessed May 13, 2019.
- American College of Obstetricians and Gynecologists. Breast cancer risk assessment and screening in average-risk women. www.acog.org/Clinical-Guidance-and-Publications/Practice-Bulletins/Committee-on-Practice-Bulletins-Gynecology/Breast-Cancer-Risk-Assessment-and-Screening-in-Average-Risk-Women?IsMobileSet=false#5. Accessed May 13, 2019.
Keeping up with current evidence-based healthcare practices is key to providing good clinical care to patients. This review presents 5 vignettes that highlight key issues in women’s health: osteoporosis screening, hormonal contraceptive interactions with antibiotics, hormone replacement therapy in carriers of the BRCA1 gene mutation, risks associated with hormonal contraception, and breast cancer diagnosis using digital tomosynthesis in addition to digital mammography. Supporting articles, all published in 2017 and 2018, were selected from high-impact medical and women’s health journals.
OSTEOPOROSIS SCREENING FOR FRACTURE PREVENTION
A 60-year-old woman reports that her last menstrual period was 7 years ago. She has no history of falls or fractures, and she takes no medications. She smokes 10 cigarettes per day and drinks 3 to 4 alcoholic beverages on most days of the week. She is 5 feet 6 inches (170 cm) tall and weighs 107 lb. Should she be screened for osteoporosis?
Osteoporosis is underdiagnosed
It is estimated that, in the United States, 12.3 million individuals older than 50 will develop osteoporosis by 2020. Missed opportunities to screen high-risk individuals can lead to fractures, including fractures of the hip.1
Updated screening recommendations
In 2018, the US Preventive Services Task Force (USPSTF) developed and published evidence-based recommendations for osteoporosis screening to help providers identify and treat osteoporosis early to prevent fractures.2 Available evidence on screening and treatment in women and men were reviewed with the intention of updating the 2011 USPSTF recommendations. The review also evaluated risk assessment tools, screening intervals, and efficacy of screening and treatment in various subpopulations.
Since the 2011 recommendations, more data have become available on fracture risk assessment with or without bone mineral density measurements. In its 2018 report, the USPSTF recommends that postmenopausal women younger than 65 should undergo screening with a bone density test if their 10-year risk of major osteoporotic fracture is more than 8.4%. This is equivalent to the fracture risk of a 65-year-old white woman with no major risk factors for fracture (grade B recommendation—high certainty that the benefit is moderate, or moderate certainty that the benefit is moderate to substantial).2
Assessment of fracture risk
For postmenopausal women who are under age 65 and who have at least 1 risk factor for fracture, it is reasonable to use a clinical risk assessment tool to determine who should undergo screening with bone mineral density measurement. Risk factors associated with an increased risk of osteoporotic fractures include a parental history of hip fracture, smoking, intake of 3 or more alcoholic drinks per day, low body weight, malabsorption, rheumatoid arthritis, diabetes, and postmenopausal status (not using estrogen replacement). Medications should be carefully reviewed for those that can increase the risk of fractures, including steroids and antiestrogen treatments.
The 10-year risk of a major osteoporotic or hip fracture can be assessed using the Fractional Risk Assessment Tool (FRAX), available at www.sheffield.ac.uk/FRAX/. Other acceptable tools that perform similarly to FRAX include the Osteoporosis Risk Assessment Instrument (ORAI) (10 studies; N = 16,780), Osteoporosis Index of Risk (OSIRIS) (5 studies; N = 5,649), Osteoporosis Self-Assessment Tool (OST) (13 studies; N = 44,323), and Simple Calculated Osteoporosis Risk Estimation (SCORE) (8 studies; N = 15,362).
Should this patient be screened for osteoporosis?
Based on the FRAX, this patient’s 10-year risk of major osteoporosis fracture is 9.2%. She would benefit from osteoporosis screening with a bone density test.
DO ANTIBIOTICS REDUCE EFFECTIVENESS OF HORMONAL CONTRACEPTION?
A 27-year-old woman presents with a dog bite on her right hand and is started on oral antibiotics. She takes an oral contraceptive that contains 35 µg of ethinyl estradiol and 0.25 mg of norgestimate. She asks if she should use condoms while taking antibiotics.
The antibiotics rifampin and rifabutin are known inducers of the hepatic enzymes required for contraceptive steroid metabolism, whereas other antibiotics are not. Despite the lack of compelling evidence that broad-spectrum antibiotics interfere with the efficacy of hormonal contraception, most pharmacists recommend backup contraception for women who use concomitant antibiotics.3 This practice could lead to poor compliance with the contraceptive regimen, the antibiotic regimen, or both.3
Simmons et al3 conducted a systematic review of randomized and nonrandomized studies that assessed pregnancy rates, breakthrough bleeding, ovulation suppression, and hormone pharmacokinetics in women taking oral or vaginal hormonal contraceptives in combination with nonrifamycin antibiotics, including oral, intramuscular, and intravenous forms. Oral contraceptives used in the studies included a range of doses and progestins, but lowest-dose pills, such as those containing less than 30 µg ethinyl estradiol or less than 150 µg levonorgestrel, were not included.
The contraceptive formulations in this systematic review3 included oral contraceptive pills, emergency contraception pills, and the contraceptive vaginal ring. The effect of antibiotics on other nonoral contraceptives, such as the transdermal patch, injectables, and progestin implants was not studied.
Four observational studies3 evaluated pregnancy rates or hormonal contraception failure with any antibiotic use. In 2 of these 4 studies, there was no difference in pregnancy rates in women who used oral contraceptives with and without nonrifamycin antibiotics. However, ethinyl estradiol was shown to have increased clearance when administered with dirithromycin (a macrolide).3 Twenty-five of the studies reported measures of contraceptive effectiveness (ovulation) and pharmacokinetic outcomes.
There were no observed differences in ovulation suppression or breakthrough bleeding in any study that combined hormonal contraceptives with an antibiotic. Furthermore, there was no significant decrease in progestin pharmacokinetic parameters during coadministration with an antibiotic.3 Study limitations included small sample sizes and the observational nature of the data.
How would you counsel this patient?
Available evidence suggests that nonrifamycin antibiotics do not diminish the effectiveness of the vaginal contraceptive ring or an oral hormonal contraceptive that contains at least 30 µg of ethinyl estradiol or 150 µg of levonorgestrel. Current guidelines do not recommend the use of additional backup contraception, regardless of hormonal contraception dose or formulation.4 Likewise, the most recent guidance for dental practitioners (ie, from 2012) no longer advises women to use additional contraceptive protection when taking nonrifamycin antibiotics.5
In our practice, we discuss the option of additional protection when prescribing formulations with lower estrogen doses (< 30 µg), not only because of the limitations of the available data, but also because of the high rates of unintended pregnancy with typical use of combined hormonal contraceptives (9% per year, unrelated to use of antibiotics).4 However, if our patient would rather not use additional barrier methods, she can be reassured that concomitant nonrifamycin antibiotic use is unlikely to affect contraceptive effectiveness.
HORMONE REPLACEMENT THERAPY IN CARRIERS OF THE BRCA1 MUTATION
A 41-year-old healthy mother of 3 was recently found to be a carrier of the BRCA1 mutation. She is planning to undergo prophylactic bilateral salpingo-oophorectomy for ovarian cancer prevention. However, she is apprehensive about undergoing surgical menopause. Should she be started on hormone replacement therapy after oophorectomy? How would hormone replacement therapy affect her risk of breast cancer?
In females who carry the BRCA1 mutation, the cumulative risk of both ovarian and breast cancer approaches 44% (95% confidence interval [CI] 36%–53%) and 72% (95% CI 65%–79%) by age 80.6 Prophylactic salpingo-oophorectomy reduces the risk of breast cancer by 50% and the risk of ovarian cancer by 90%. Unfortunately, premature withdrawal of ovarian hormones has been associated with long-term adverse effects including significant vasomotor symptoms, decreased quality of life, sexual dysfunction, early mortality, bone loss, decline in mood and cognition, and poor cardiovascular outcomes.7 Many of these effects can be avoided or lessened with hormone replacement therapy.
Kotsopoulos et al8 conducted a longitudinal, prospective analysis of BRCA1 mutation carriers in a multicenter study between 1995 and 2017. The mean follow-up period was 7.6 years (range 0.4–22.1). The study assessed associations between the use of hormone replacement therapy and breast cancer risk in carriers of the BRCA1 mutation who underwent prophylactic salpingo-oophorectomy. Study participants did not have a personal history of cancer. Those with a history of prophylactic mastectomy were excluded.
Participants completed a series of questionnaires every 2 years, disclosing updates in personal medical, cancer, and reproductive history. The questionnaires also inquired about the use of hormone replacement therapy, including the type used (estrogen only, progestin only, estrogen plus progestin, other), brand name, duration of use, and dose and route of administration (pill, patch, suppository).
Of the 13,087 BRCA1 mutation carriers identified, 872 met the study criteria. Of those, 377 (43%) reported using some form of hormone replacement therapy after salpingo-oophorectomy, and 495 (57%) did not. The average duration of use was 3.9 years (range 0.5–19), with most (69%) using estrogen alone; 18% used other regimens, including estrogen plus progestin and progestin only. A small percentage of participants did not indicate which formulation they used. On average, women using hormone replacement therapy underwent prophylactic oophorectomy earlier than nonusers (age 43.0 vs 48.4; absolute difference 5.5 years, P < .001).
During follow-up, there was no significant difference noted in the proportion of women diagnosed with breast cancer between hormone replacement therapy users and nonusers (10.3 vs 10.7%; absolute difference 0.4%; P = .86). In fact, for each year of estrogen-containing hormone replacement therapy, there was an 18% reduction in breast cancer risk when oophorectomy was performed before age 45 (95% CI 0.69–0.97). The authors also noted a nonsignificant 14% trend toward an increase in breast cancer risk for each year of progestin use after oophorectomy when surgery was performed before age 45 (95% CI 0.9–1.46).
Although prophylactic hysterectomy was not recommended, the authors noted that hysterectomy would eliminate the need for progestin-containing hormone replacement therapy. For those who underwent oophorectomy after age 45, hormone replacement therapy did not increase or decrease the risk of breast cancer.7
A meta-analysis by Marchetti et al9 also supports the safety of hormone replacement therapy after risk-reducing salpingo-oophorectomy. Three studies that included 1,100 patients were analyzed (including the Kotsopoulos study8 noted above). There was a nonsignificant decrease in breast cancer risk in women on estrogen-only hormone replacement therapy compared with women on estrogen-plus-progestin therapy (odds ratio 0.53, 95% CI 0.25–1.15). Overall, the authors regarded hormone replacement therapy as a safe therapeutic option after prophylactic salpingo-oophorectomy in carriers of the BRCA1 and BRCA2 mutations.9
In a case-control study published in 2016,10 hormone replacement therapy was assessed in 432 postmenopausal BRCA1 mutation carriers with invasive breast cancer (cases) and in 432 BRCA1 mutation carriers without a history of breast cancer (controls). Results showed no difference in breast cancer risk between hormone replacement therapy users and nonusers.10
Rebbeck et al11 evaluated short-term hormone replacement therapy in BRCA1 and BRCA2 gene-mutation carriers after they underwent prophylactic salpingo-oophorectomy. The results showed that hormone replacement did not affect the breast cancer risk-reduction conferred with prophylactic bilateral salpingo-oophorectomy.
Johansen et al12 evaluated hormone replacement therapy in premenopausal women after prophylactic salpingo-oophorectomy. They studied 324 carriers of BRCA gene mutations after they underwent prophylactic salpingo-oophorectomy and a subset of 950 controls who had bilateral salpingo-oophorectomy for reasons unrelated to cancer. In both groups, hormone replacement therapy was underutilized. The authors recommended using it when clinically indicated.
Should your patient start hormone replacement therapy?
This patient is healthy, and in the absence of contraindications, systemic hormone replacement therapy after prophylactic oophorectomy could mitigate the potential adverse effects of surgically induced menopause. The patient can be reassured that estrogen-containing short-term hormone replacement therapy is unlikely to increase her breast cancer risk.
HORMONAL CONTRACEPTION AND THE RISK OF BREAST CANCER
A 44-year-old woman presents to your office for an annual visit. She is sexually active but does not wish to become pregnant. She has a family history of breast cancer: her mother was diagnosed at age 53. She is interested in an oral contraceptive to prevent pregnancy and acne. However, she is nervous about being on any contraceptive that may increase her risk of breast cancer.
To date, studies assessing the effect of hormonal contraception on the risk of breast cancer have produced inconsistent results. Although most studies have shown no associated risk, a few have shown a temporary 20% to 30% increased risk of breast cancer during use.13,14 Case-controlled studies that reported an association between hormonal contraception and breast cancer included populations taking higher-dose combination pills, which are no longer prescribed. Most studies do not evaluate specific formulations of hormonal contraception, and little is known about effects associated with intrauterine devices or progestin-only contraception.
A prospective study performed by Mørch et al13 followed more than 1 million reproductive-aged women for a mean of 10.9 years. The Danish Cancer Registry was used to identify cases of invasive breast cancer. Women who used hormonal contraceptives had a relative risk of breast cancer of 1.20 compared with women not on hormonal contraception (95% CI 1.14–1.26). The study suggested that those who had been on contraceptive agents for more than 5 years had an increased risk and that this risk remained for 5 years after the agents were discontinued. Conversely, no increased risk of cancer was noted in those who used hormonal contraception for less than 5 years. No notable differences were seen among various formulations.
For women using the levonorgestrel-containing intrauterine device, the relative risk of breast cancer was 1.21 (95% CI 1.11–1.33). A few cancers were noted in those who used the progestin-only implant or those using depot medroxyprogesterone acetate. While the study showed an increased relative risk of breast cancer, the absolute risk was low—13 cases per 100,000, or approximately 1 additional case of breast cancer per 7,690 per year.13
This study had several important limitations. The authors did not adjust for common breast cancer risk factors including age at menarche, alcohol use, or breastfeeding. Additionally, the study did not account for the use of hormonal contraception before the study period and conversely, did not account for women who may have stopped taking their contraceptive despite their prescribed duration. The frequency of mammography was not explicitly noted, which could have shifted results for women who had more aggressive screening.
It is also noteworthy that the use of high-dose systemic progestins was not associated with an increased risk, whereas the levonorgestrel intrauterine device, which contains only 1/20th the dose of a low-dose oral contraceptive pill, was associated with an increased risk. This discrepancy in risk warrants further investigation, and clinicians should be aware that this inconsistency needs validation before changing clinical practice.
In an observational cohort study,15 more than 100,000 women ages 50 to 71 were followed prospectively for 15 years to evaluate the association between hormonal contraceptive use and the risk of gynecologic and breast cancers. In this study, the duration of hormonal contraceptive use, smoking status, alcohol use, body mass index, physical activity, and family history of cancer were recorded. Long-term hormonal contraceptive use reduced ovarian and endometrial cancer risks by 40% and 34%, respectively, with no increase in breast cancer risk regardless of family history.
How would you counsel the patient?
The patient should be educated on the benefits of hormonal contraception that extend beyond pregnancy prevention, including regulation of menses, improved acne, decreased risk of endometrial and ovarian cancer, and likely reductions in colorectal cancer and overall mortality risk.13–16 Further, after their own systematic review of the data assessing risk of breast cancer with hormonal contraception, the US Centers for Disease Control and Prevention state in their guidelines that all contraceptives may be used without limitation in those who have a family history of breast cancer.4 Any potential increased risk of breast cancer in women using hormonal contraception is small and would not outweigh the benefits associated with use.
One must consider the impact of an unintended pregnancy in such women, including effects on the health of the fetus and mother. Recent reports on the increasing rates of maternal death in the US (23.8 of 100,000 live births) serve as a reminder of the complications that can arise with pregnancy, especially if a mother’s health is not optimized before conception.17
MAMMOGRAPHY PLUS TOMOSYNTHESIS VS MAMMOGRAPHY ALONE
The same 44-year-old patient now inquires about screening for breast cancer. She is curious about 3-dimensional mammography and whether it would be a better screening test for her.
Digital breast tomosynthesis (DBT) is a newer imaging modality that provides a 3-dimensional reconstruction of the breast using low-dose x-ray imaging. Some studies have shown that combining DBT with digital mammography may be superior to digital mammography alone in detecting cancers.18 However, digital mammography is currently the gold standard for breast cancer screening and is the only test proven to reduce mortality.18,19
In a retrospective US study of 13 medical centers,20 breast cancer detection rates increased by 41% the year after DBT was introduced, from 2.9 to 4.1 per 1,000 cases. DBT was associated with 16 fewer patients recalled for repeat imaging out of 1,000 women screened (as opposed to mammography alone). Two European studies similarly suggested an increase in cancer detection with lower recall rates.21,22
Is 3-D mammography a better option?
In a 2-arm study by Pattacini et al,18 nearly 20,000 women ages 45 to 70 were randomized to undergo either digital mammography or digital mammography plus DBT for primary breast cancer screening. Women were enrolled over a 2-year period and were followed for 4.5 years, and the development of a primary invasive cancer was the primary end point. Recall rates, reading times, and radiation doses were also compared between the 2 groups.
Overall, the cancer detection rate was higher in the digital mammography plus DBT arm compared with digital mammography alone (8.6 vs 4.5 per 1,000). The detection rates were higher in the combined screening group among all age subgroups, with relative risks ranging from 1.83 to 2.04 (P = .93). The recall rate was 3.5% in the 2 arms, with relative risks ranging from 0.93 to 1.11 (P = .52). There was a reduction in the number of false positives seen in women undergoing digital mammography plus DBT when compared with digital mammography alone, from 30 per 1,000 to 27 per 1,000.
Detection of ductal carcinoma in situ increased in the experimental arm (relative detection 2.80, 95% CI 1.01–7.65) compared with invasive cancers. Comparing radiation, the dose was 2.3 times higher in those who underwent digital mammography plus DBT. The average reading times for digital mammography alone were 20 to 85 seconds; adding DBT added 35 to 81 seconds.19
Should you advise 3-D mammography?
The patient should be educated on the benefits of both digital mammography alone and digital mammography plus DBT. The use of digital mammography plus DBT has been supported in various studies and has been shown to increase cancer detection rates, although data are still conflicting regarding recall rates.19,20 More studies are needed to determine its effect on breast cancer morality.
Routine use of DBT in women with or without dense breast tissue has not been recommended by organizations such as the USPSTF and the American College of Obstetricians and Gynecologists.23,24 While there is an increased dose of radiation, it still falls below the US Food and Drug Administration limits and should not be the sole barrier to use.
Keeping up with current evidence-based healthcare practices is key to providing good clinical care to patients. This review presents 5 vignettes that highlight key issues in women’s health: osteoporosis screening, hormonal contraceptive interactions with antibiotics, hormone replacement therapy in carriers of the BRCA1 gene mutation, risks associated with hormonal contraception, and breast cancer diagnosis using digital tomosynthesis in addition to digital mammography. Supporting articles, all published in 2017 and 2018, were selected from high-impact medical and women’s health journals.
OSTEOPOROSIS SCREENING FOR FRACTURE PREVENTION
A 60-year-old woman reports that her last menstrual period was 7 years ago. She has no history of falls or fractures, and she takes no medications. She smokes 10 cigarettes per day and drinks 3 to 4 alcoholic beverages on most days of the week. She is 5 feet 6 inches (170 cm) tall and weighs 107 lb. Should she be screened for osteoporosis?
Osteoporosis is underdiagnosed
It is estimated that, in the United States, 12.3 million individuals older than 50 will develop osteoporosis by 2020. Missed opportunities to screen high-risk individuals can lead to fractures, including fractures of the hip.1
Updated screening recommendations
In 2018, the US Preventive Services Task Force (USPSTF) developed and published evidence-based recommendations for osteoporosis screening to help providers identify and treat osteoporosis early to prevent fractures.2 Available evidence on screening and treatment in women and men were reviewed with the intention of updating the 2011 USPSTF recommendations. The review also evaluated risk assessment tools, screening intervals, and efficacy of screening and treatment in various subpopulations.
Since the 2011 recommendations, more data have become available on fracture risk assessment with or without bone mineral density measurements. In its 2018 report, the USPSTF recommends that postmenopausal women younger than 65 should undergo screening with a bone density test if their 10-year risk of major osteoporotic fracture is more than 8.4%. This is equivalent to the fracture risk of a 65-year-old white woman with no major risk factors for fracture (grade B recommendation—high certainty that the benefit is moderate, or moderate certainty that the benefit is moderate to substantial).2
Assessment of fracture risk
For postmenopausal women who are under age 65 and who have at least 1 risk factor for fracture, it is reasonable to use a clinical risk assessment tool to determine who should undergo screening with bone mineral density measurement. Risk factors associated with an increased risk of osteoporotic fractures include a parental history of hip fracture, smoking, intake of 3 or more alcoholic drinks per day, low body weight, malabsorption, rheumatoid arthritis, diabetes, and postmenopausal status (not using estrogen replacement). Medications should be carefully reviewed for those that can increase the risk of fractures, including steroids and antiestrogen treatments.
The 10-year risk of a major osteoporotic or hip fracture can be assessed using the Fractional Risk Assessment Tool (FRAX), available at www.sheffield.ac.uk/FRAX/. Other acceptable tools that perform similarly to FRAX include the Osteoporosis Risk Assessment Instrument (ORAI) (10 studies; N = 16,780), Osteoporosis Index of Risk (OSIRIS) (5 studies; N = 5,649), Osteoporosis Self-Assessment Tool (OST) (13 studies; N = 44,323), and Simple Calculated Osteoporosis Risk Estimation (SCORE) (8 studies; N = 15,362).
Should this patient be screened for osteoporosis?
Based on the FRAX, this patient’s 10-year risk of major osteoporosis fracture is 9.2%. She would benefit from osteoporosis screening with a bone density test.
DO ANTIBIOTICS REDUCE EFFECTIVENESS OF HORMONAL CONTRACEPTION?
A 27-year-old woman presents with a dog bite on her right hand and is started on oral antibiotics. She takes an oral contraceptive that contains 35 µg of ethinyl estradiol and 0.25 mg of norgestimate. She asks if she should use condoms while taking antibiotics.
The antibiotics rifampin and rifabutin are known inducers of the hepatic enzymes required for contraceptive steroid metabolism, whereas other antibiotics are not. Despite the lack of compelling evidence that broad-spectrum antibiotics interfere with the efficacy of hormonal contraception, most pharmacists recommend backup contraception for women who use concomitant antibiotics.3 This practice could lead to poor compliance with the contraceptive regimen, the antibiotic regimen, or both.3
Simmons et al3 conducted a systematic review of randomized and nonrandomized studies that assessed pregnancy rates, breakthrough bleeding, ovulation suppression, and hormone pharmacokinetics in women taking oral or vaginal hormonal contraceptives in combination with nonrifamycin antibiotics, including oral, intramuscular, and intravenous forms. Oral contraceptives used in the studies included a range of doses and progestins, but lowest-dose pills, such as those containing less than 30 µg ethinyl estradiol or less than 150 µg levonorgestrel, were not included.
The contraceptive formulations in this systematic review3 included oral contraceptive pills, emergency contraception pills, and the contraceptive vaginal ring. The effect of antibiotics on other nonoral contraceptives, such as the transdermal patch, injectables, and progestin implants was not studied.
Four observational studies3 evaluated pregnancy rates or hormonal contraception failure with any antibiotic use. In 2 of these 4 studies, there was no difference in pregnancy rates in women who used oral contraceptives with and without nonrifamycin antibiotics. However, ethinyl estradiol was shown to have increased clearance when administered with dirithromycin (a macrolide).3 Twenty-five of the studies reported measures of contraceptive effectiveness (ovulation) and pharmacokinetic outcomes.
There were no observed differences in ovulation suppression or breakthrough bleeding in any study that combined hormonal contraceptives with an antibiotic. Furthermore, there was no significant decrease in progestin pharmacokinetic parameters during coadministration with an antibiotic.3 Study limitations included small sample sizes and the observational nature of the data.
How would you counsel this patient?
Available evidence suggests that nonrifamycin antibiotics do not diminish the effectiveness of the vaginal contraceptive ring or an oral hormonal contraceptive that contains at least 30 µg of ethinyl estradiol or 150 µg of levonorgestrel. Current guidelines do not recommend the use of additional backup contraception, regardless of hormonal contraception dose or formulation.4 Likewise, the most recent guidance for dental practitioners (ie, from 2012) no longer advises women to use additional contraceptive protection when taking nonrifamycin antibiotics.5
In our practice, we discuss the option of additional protection when prescribing formulations with lower estrogen doses (< 30 µg), not only because of the limitations of the available data, but also because of the high rates of unintended pregnancy with typical use of combined hormonal contraceptives (9% per year, unrelated to use of antibiotics).4 However, if our patient would rather not use additional barrier methods, she can be reassured that concomitant nonrifamycin antibiotic use is unlikely to affect contraceptive effectiveness.
HORMONE REPLACEMENT THERAPY IN CARRIERS OF THE BRCA1 MUTATION
A 41-year-old healthy mother of 3 was recently found to be a carrier of the BRCA1 mutation. She is planning to undergo prophylactic bilateral salpingo-oophorectomy for ovarian cancer prevention. However, she is apprehensive about undergoing surgical menopause. Should she be started on hormone replacement therapy after oophorectomy? How would hormone replacement therapy affect her risk of breast cancer?
In females who carry the BRCA1 mutation, the cumulative risk of both ovarian and breast cancer approaches 44% (95% confidence interval [CI] 36%–53%) and 72% (95% CI 65%–79%) by age 80.6 Prophylactic salpingo-oophorectomy reduces the risk of breast cancer by 50% and the risk of ovarian cancer by 90%. Unfortunately, premature withdrawal of ovarian hormones has been associated with long-term adverse effects including significant vasomotor symptoms, decreased quality of life, sexual dysfunction, early mortality, bone loss, decline in mood and cognition, and poor cardiovascular outcomes.7 Many of these effects can be avoided or lessened with hormone replacement therapy.
Kotsopoulos et al8 conducted a longitudinal, prospective analysis of BRCA1 mutation carriers in a multicenter study between 1995 and 2017. The mean follow-up period was 7.6 years (range 0.4–22.1). The study assessed associations between the use of hormone replacement therapy and breast cancer risk in carriers of the BRCA1 mutation who underwent prophylactic salpingo-oophorectomy. Study participants did not have a personal history of cancer. Those with a history of prophylactic mastectomy were excluded.
Participants completed a series of questionnaires every 2 years, disclosing updates in personal medical, cancer, and reproductive history. The questionnaires also inquired about the use of hormone replacement therapy, including the type used (estrogen only, progestin only, estrogen plus progestin, other), brand name, duration of use, and dose and route of administration (pill, patch, suppository).
Of the 13,087 BRCA1 mutation carriers identified, 872 met the study criteria. Of those, 377 (43%) reported using some form of hormone replacement therapy after salpingo-oophorectomy, and 495 (57%) did not. The average duration of use was 3.9 years (range 0.5–19), with most (69%) using estrogen alone; 18% used other regimens, including estrogen plus progestin and progestin only. A small percentage of participants did not indicate which formulation they used. On average, women using hormone replacement therapy underwent prophylactic oophorectomy earlier than nonusers (age 43.0 vs 48.4; absolute difference 5.5 years, P < .001).
During follow-up, there was no significant difference noted in the proportion of women diagnosed with breast cancer between hormone replacement therapy users and nonusers (10.3 vs 10.7%; absolute difference 0.4%; P = .86). In fact, for each year of estrogen-containing hormone replacement therapy, there was an 18% reduction in breast cancer risk when oophorectomy was performed before age 45 (95% CI 0.69–0.97). The authors also noted a nonsignificant 14% trend toward an increase in breast cancer risk for each year of progestin use after oophorectomy when surgery was performed before age 45 (95% CI 0.9–1.46).
Although prophylactic hysterectomy was not recommended, the authors noted that hysterectomy would eliminate the need for progestin-containing hormone replacement therapy. For those who underwent oophorectomy after age 45, hormone replacement therapy did not increase or decrease the risk of breast cancer.7
A meta-analysis by Marchetti et al9 also supports the safety of hormone replacement therapy after risk-reducing salpingo-oophorectomy. Three studies that included 1,100 patients were analyzed (including the Kotsopoulos study8 noted above). There was a nonsignificant decrease in breast cancer risk in women on estrogen-only hormone replacement therapy compared with women on estrogen-plus-progestin therapy (odds ratio 0.53, 95% CI 0.25–1.15). Overall, the authors regarded hormone replacement therapy as a safe therapeutic option after prophylactic salpingo-oophorectomy in carriers of the BRCA1 and BRCA2 mutations.9
In a case-control study published in 2016,10 hormone replacement therapy was assessed in 432 postmenopausal BRCA1 mutation carriers with invasive breast cancer (cases) and in 432 BRCA1 mutation carriers without a history of breast cancer (controls). Results showed no difference in breast cancer risk between hormone replacement therapy users and nonusers.10
Rebbeck et al11 evaluated short-term hormone replacement therapy in BRCA1 and BRCA2 gene-mutation carriers after they underwent prophylactic salpingo-oophorectomy. The results showed that hormone replacement did not affect the breast cancer risk-reduction conferred with prophylactic bilateral salpingo-oophorectomy.
Johansen et al12 evaluated hormone replacement therapy in premenopausal women after prophylactic salpingo-oophorectomy. They studied 324 carriers of BRCA gene mutations after they underwent prophylactic salpingo-oophorectomy and a subset of 950 controls who had bilateral salpingo-oophorectomy for reasons unrelated to cancer. In both groups, hormone replacement therapy was underutilized. The authors recommended using it when clinically indicated.
Should your patient start hormone replacement therapy?
This patient is healthy, and in the absence of contraindications, systemic hormone replacement therapy after prophylactic oophorectomy could mitigate the potential adverse effects of surgically induced menopause. The patient can be reassured that estrogen-containing short-term hormone replacement therapy is unlikely to increase her breast cancer risk.
HORMONAL CONTRACEPTION AND THE RISK OF BREAST CANCER
A 44-year-old woman presents to your office for an annual visit. She is sexually active but does not wish to become pregnant. She has a family history of breast cancer: her mother was diagnosed at age 53. She is interested in an oral contraceptive to prevent pregnancy and acne. However, she is nervous about being on any contraceptive that may increase her risk of breast cancer.
To date, studies assessing the effect of hormonal contraception on the risk of breast cancer have produced inconsistent results. Although most studies have shown no associated risk, a few have shown a temporary 20% to 30% increased risk of breast cancer during use.13,14 Case-controlled studies that reported an association between hormonal contraception and breast cancer included populations taking higher-dose combination pills, which are no longer prescribed. Most studies do not evaluate specific formulations of hormonal contraception, and little is known about effects associated with intrauterine devices or progestin-only contraception.
A prospective study performed by Mørch et al13 followed more than 1 million reproductive-aged women for a mean of 10.9 years. The Danish Cancer Registry was used to identify cases of invasive breast cancer. Women who used hormonal contraceptives had a relative risk of breast cancer of 1.20 compared with women not on hormonal contraception (95% CI 1.14–1.26). The study suggested that those who had been on contraceptive agents for more than 5 years had an increased risk and that this risk remained for 5 years after the agents were discontinued. Conversely, no increased risk of cancer was noted in those who used hormonal contraception for less than 5 years. No notable differences were seen among various formulations.
For women using the levonorgestrel-containing intrauterine device, the relative risk of breast cancer was 1.21 (95% CI 1.11–1.33). A few cancers were noted in those who used the progestin-only implant or those using depot medroxyprogesterone acetate. While the study showed an increased relative risk of breast cancer, the absolute risk was low—13 cases per 100,000, or approximately 1 additional case of breast cancer per 7,690 per year.13
This study had several important limitations. The authors did not adjust for common breast cancer risk factors including age at menarche, alcohol use, or breastfeeding. Additionally, the study did not account for the use of hormonal contraception before the study period and conversely, did not account for women who may have stopped taking their contraceptive despite their prescribed duration. The frequency of mammography was not explicitly noted, which could have shifted results for women who had more aggressive screening.
It is also noteworthy that the use of high-dose systemic progestins was not associated with an increased risk, whereas the levonorgestrel intrauterine device, which contains only 1/20th the dose of a low-dose oral contraceptive pill, was associated with an increased risk. This discrepancy in risk warrants further investigation, and clinicians should be aware that this inconsistency needs validation before changing clinical practice.
In an observational cohort study,15 more than 100,000 women ages 50 to 71 were followed prospectively for 15 years to evaluate the association between hormonal contraceptive use and the risk of gynecologic and breast cancers. In this study, the duration of hormonal contraceptive use, smoking status, alcohol use, body mass index, physical activity, and family history of cancer were recorded. Long-term hormonal contraceptive use reduced ovarian and endometrial cancer risks by 40% and 34%, respectively, with no increase in breast cancer risk regardless of family history.
How would you counsel the patient?
The patient should be educated on the benefits of hormonal contraception that extend beyond pregnancy prevention, including regulation of menses, improved acne, decreased risk of endometrial and ovarian cancer, and likely reductions in colorectal cancer and overall mortality risk.13–16 Further, after their own systematic review of the data assessing risk of breast cancer with hormonal contraception, the US Centers for Disease Control and Prevention state in their guidelines that all contraceptives may be used without limitation in those who have a family history of breast cancer.4 Any potential increased risk of breast cancer in women using hormonal contraception is small and would not outweigh the benefits associated with use.
One must consider the impact of an unintended pregnancy in such women, including effects on the health of the fetus and mother. Recent reports on the increasing rates of maternal death in the US (23.8 of 100,000 live births) serve as a reminder of the complications that can arise with pregnancy, especially if a mother’s health is not optimized before conception.17
MAMMOGRAPHY PLUS TOMOSYNTHESIS VS MAMMOGRAPHY ALONE
The same 44-year-old patient now inquires about screening for breast cancer. She is curious about 3-dimensional mammography and whether it would be a better screening test for her.
Digital breast tomosynthesis (DBT) is a newer imaging modality that provides a 3-dimensional reconstruction of the breast using low-dose x-ray imaging. Some studies have shown that combining DBT with digital mammography may be superior to digital mammography alone in detecting cancers.18 However, digital mammography is currently the gold standard for breast cancer screening and is the only test proven to reduce mortality.18,19
In a retrospective US study of 13 medical centers,20 breast cancer detection rates increased by 41% the year after DBT was introduced, from 2.9 to 4.1 per 1,000 cases. DBT was associated with 16 fewer patients recalled for repeat imaging out of 1,000 women screened (as opposed to mammography alone). Two European studies similarly suggested an increase in cancer detection with lower recall rates.21,22
Is 3-D mammography a better option?
In a 2-arm study by Pattacini et al,18 nearly 20,000 women ages 45 to 70 were randomized to undergo either digital mammography or digital mammography plus DBT for primary breast cancer screening. Women were enrolled over a 2-year period and were followed for 4.5 years, and the development of a primary invasive cancer was the primary end point. Recall rates, reading times, and radiation doses were also compared between the 2 groups.
Overall, the cancer detection rate was higher in the digital mammography plus DBT arm compared with digital mammography alone (8.6 vs 4.5 per 1,000). The detection rates were higher in the combined screening group among all age subgroups, with relative risks ranging from 1.83 to 2.04 (P = .93). The recall rate was 3.5% in the 2 arms, with relative risks ranging from 0.93 to 1.11 (P = .52). There was a reduction in the number of false positives seen in women undergoing digital mammography plus DBT when compared with digital mammography alone, from 30 per 1,000 to 27 per 1,000.
Detection of ductal carcinoma in situ increased in the experimental arm (relative detection 2.80, 95% CI 1.01–7.65) compared with invasive cancers. Comparing radiation, the dose was 2.3 times higher in those who underwent digital mammography plus DBT. The average reading times for digital mammography alone were 20 to 85 seconds; adding DBT added 35 to 81 seconds.19
Should you advise 3-D mammography?
The patient should be educated on the benefits of both digital mammography alone and digital mammography plus DBT. The use of digital mammography plus DBT has been supported in various studies and has been shown to increase cancer detection rates, although data are still conflicting regarding recall rates.19,20 More studies are needed to determine its effect on breast cancer morality.
Routine use of DBT in women with or without dense breast tissue has not been recommended by organizations such as the USPSTF and the American College of Obstetricians and Gynecologists.23,24 While there is an increased dose of radiation, it still falls below the US Food and Drug Administration limits and should not be the sole barrier to use.
- Cauley JA. Screening for osteoporosis. JAMA 2018; 319(24):2483–2485. doi:10.1001/jama.2018.5722
- US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA 2018; 319(24):2521–2531. doi:10.1001/jama.2018.7498
- Simmons KB, Haddad LB, Nanda K, Curtis KM. Drug interactions between non-rifamycin antibiotics and hormonal contraception: a systematic review. Am J Obstet Gynecol 2018; 218(1):88–97.e14. doi:10.1016/j.ajog.2017.07.003
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US Medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep 2016; 65(3):1–103. doi:10.15585/mmwr.rr6503a1
- Taylor J, Pemberton MN. Antibiotics and oral contraceptives: new considerations for dental practice. Br Dent J 2012; 212(10):481–483. doi:10.1038/sj.bdj.2012.414
- Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 2017; 317(23):2402–2416. doi:10.1001/jama.2017.7112
- Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long-term health consequences of premature or early menopause and considerations for management. Climacteric 2015; 18(4):483–491. doi:10.3109/13697137.2015.1020484
- Kotsopoulos J, Gronwald J, Karlan BY, et al; Hereditary Breast Cancer Clinical Study Group. Hormone replacement therapy after oophorectomy and breast cancer risk among BRCA1 mutation carriers. JAMA Oncol 2018; 4(8):1059–1065. doi:10.1001/jamaoncol.2018.0211
- Marchetti C, De Felice F, Boccia S, et al. Hormone replacement therapy after prophylactic risk reducing salpingo-oophorectomy and breast cancer risk in BRCA1 and BRCA2 mutation carriers: a meta-analysis. Crit Rev Oncol Hematol 2018; 132:111–115. doi:10.1016/j.critrevonc.2018.09.018
- Kotsopoulos J, Huzarski T, Gronwald J, et al. Hormone replacement therapy after menopause and risk of breast cancer in BRCA1 mutation carriers: a case-control study. Breast Cancer Res Treat 2016; 155(2):365–373. doi:10.1007/s10549-016-3685-3
- Rebbeck TR, Friebel T, Wagner T, et al; PROSE Study Group. Effect of short-term hormone replacement therapy on breast cancer risk reduction after bilateral prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol 2005; 23(31):7804–7810. doi:10.1200/JCO.2004.00.8151
- Johansen N, Liavaag AH, Iversen OE, Dørum A, Braaten T, Michelsen TM. Use of hormone replacement therapy after risk-reducing salpingo-oophorectomy. Acta Obstet Gynecol Scand 2017; 96(5):547–555. doi:10.1111/aogs.13120
- Mørch LS, Skovlund CW, Hannaford PC, Iversen L, Fielding S, Lidegaard Ø. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med 2017; 377(23):2228–2239. doi:10.1056/NEJMoa1700732
- Batur P, Sikka S, McNamara M. Contraception update: extended use of long acting methods, hormonal contraception risks, and over the counter access. J Womens Health (Larchmt) 2018. doi:10.1089/jwh.2018.7391. [Epub ahead of print]
- Michels KA, Pfeiffer RM, Brinton LA, Trabert B. Modification of the associations between duration of oral contraceptive use and ovarian, endometrial, breast, and colorectal cancers. JAMA Oncol 2018; 4(4):516–521. doi:10.1001/jamaoncol.2017.4942
- Iversen L, Fielding S, Lidegaard Ø, Mørch LS, Skovlund CW, Hannaford PC. Association between contemporary hormonal contraception and ovarian cancer in women of reproductive age in Denmark: prospective, nationwide cohort study. BMJ 2018; 362:k3609. doi:10.1136/bmj.k3609
- MacDorman MF, Declercq E, Cabral H, Morton C. Recent increases in the US maternal mortality rate: disentangling trends from measurement issues. Obstet Gynecol 2016; 128(3):447–455. doi:10.1097/AOG.0000000000001556
- Pattacini P, Nitrosi A, Giorgi Rossi P, et al; RETomo Working Group. Digital mammography versus digital mammography plus tomosynthesis for breast cancer screening: the Reggio Emilia tomosynthesis randomized trial. Radiology 2018; 288(2):375–385. doi:10.1148/radiol.2018172119
- Pace L, Keating NL. A systematic assessment of benefits and risks to guide breast cancer screening decisions. JAMA 2014; 311(13):1327–1335. doi:10.1001/jama.2014.1398
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014; 311(24):2499–2507. doi:10.1001/jama.2014.6095
- Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology 2013; 267(1):47–56. doi:10.1148/radiol.12121373
- Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol 2013; 14(7):583–589. doi:10.1016/S1470-2045(13)70134-7
- US Preventive Services Task Force. Final recommendation statement: breast cancer: screening. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/breast-cancer-screening1. Accessed May 13, 2019.
- American College of Obstetricians and Gynecologists. Breast cancer risk assessment and screening in average-risk women. www.acog.org/Clinical-Guidance-and-Publications/Practice-Bulletins/Committee-on-Practice-Bulletins-Gynecology/Breast-Cancer-Risk-Assessment-and-Screening-in-Average-Risk-Women?IsMobileSet=false#5. Accessed May 13, 2019.
- Cauley JA. Screening for osteoporosis. JAMA 2018; 319(24):2483–2485. doi:10.1001/jama.2018.5722
- US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, et al. Screening for osteoporosis to prevent fractures: US Preventive Services Task Force recommendation statement. JAMA 2018; 319(24):2521–2531. doi:10.1001/jama.2018.7498
- Simmons KB, Haddad LB, Nanda K, Curtis KM. Drug interactions between non-rifamycin antibiotics and hormonal contraception: a systematic review. Am J Obstet Gynecol 2018; 218(1):88–97.e14. doi:10.1016/j.ajog.2017.07.003
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US Medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep 2016; 65(3):1–103. doi:10.15585/mmwr.rr6503a1
- Taylor J, Pemberton MN. Antibiotics and oral contraceptives: new considerations for dental practice. Br Dent J 2012; 212(10):481–483. doi:10.1038/sj.bdj.2012.414
- Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 2017; 317(23):2402–2416. doi:10.1001/jama.2017.7112
- Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long-term health consequences of premature or early menopause and considerations for management. Climacteric 2015; 18(4):483–491. doi:10.3109/13697137.2015.1020484
- Kotsopoulos J, Gronwald J, Karlan BY, et al; Hereditary Breast Cancer Clinical Study Group. Hormone replacement therapy after oophorectomy and breast cancer risk among BRCA1 mutation carriers. JAMA Oncol 2018; 4(8):1059–1065. doi:10.1001/jamaoncol.2018.0211
- Marchetti C, De Felice F, Boccia S, et al. Hormone replacement therapy after prophylactic risk reducing salpingo-oophorectomy and breast cancer risk in BRCA1 and BRCA2 mutation carriers: a meta-analysis. Crit Rev Oncol Hematol 2018; 132:111–115. doi:10.1016/j.critrevonc.2018.09.018
- Kotsopoulos J, Huzarski T, Gronwald J, et al. Hormone replacement therapy after menopause and risk of breast cancer in BRCA1 mutation carriers: a case-control study. Breast Cancer Res Treat 2016; 155(2):365–373. doi:10.1007/s10549-016-3685-3
- Rebbeck TR, Friebel T, Wagner T, et al; PROSE Study Group. Effect of short-term hormone replacement therapy on breast cancer risk reduction after bilateral prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol 2005; 23(31):7804–7810. doi:10.1200/JCO.2004.00.8151
- Johansen N, Liavaag AH, Iversen OE, Dørum A, Braaten T, Michelsen TM. Use of hormone replacement therapy after risk-reducing salpingo-oophorectomy. Acta Obstet Gynecol Scand 2017; 96(5):547–555. doi:10.1111/aogs.13120
- Mørch LS, Skovlund CW, Hannaford PC, Iversen L, Fielding S, Lidegaard Ø. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med 2017; 377(23):2228–2239. doi:10.1056/NEJMoa1700732
- Batur P, Sikka S, McNamara M. Contraception update: extended use of long acting methods, hormonal contraception risks, and over the counter access. J Womens Health (Larchmt) 2018. doi:10.1089/jwh.2018.7391. [Epub ahead of print]
- Michels KA, Pfeiffer RM, Brinton LA, Trabert B. Modification of the associations between duration of oral contraceptive use and ovarian, endometrial, breast, and colorectal cancers. JAMA Oncol 2018; 4(4):516–521. doi:10.1001/jamaoncol.2017.4942
- Iversen L, Fielding S, Lidegaard Ø, Mørch LS, Skovlund CW, Hannaford PC. Association between contemporary hormonal contraception and ovarian cancer in women of reproductive age in Denmark: prospective, nationwide cohort study. BMJ 2018; 362:k3609. doi:10.1136/bmj.k3609
- MacDorman MF, Declercq E, Cabral H, Morton C. Recent increases in the US maternal mortality rate: disentangling trends from measurement issues. Obstet Gynecol 2016; 128(3):447–455. doi:10.1097/AOG.0000000000001556
- Pattacini P, Nitrosi A, Giorgi Rossi P, et al; RETomo Working Group. Digital mammography versus digital mammography plus tomosynthesis for breast cancer screening: the Reggio Emilia tomosynthesis randomized trial. Radiology 2018; 288(2):375–385. doi:10.1148/radiol.2018172119
- Pace L, Keating NL. A systematic assessment of benefits and risks to guide breast cancer screening decisions. JAMA 2014; 311(13):1327–1335. doi:10.1001/jama.2014.1398
- Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA 2014; 311(24):2499–2507. doi:10.1001/jama.2014.6095
- Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology 2013; 267(1):47–56. doi:10.1148/radiol.12121373
- Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol 2013; 14(7):583–589. doi:10.1016/S1470-2045(13)70134-7
- US Preventive Services Task Force. Final recommendation statement: breast cancer: screening. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/breast-cancer-screening1. Accessed May 13, 2019.
- American College of Obstetricians and Gynecologists. Breast cancer risk assessment and screening in average-risk women. www.acog.org/Clinical-Guidance-and-Publications/Practice-Bulletins/Committee-on-Practice-Bulletins-Gynecology/Breast-Cancer-Risk-Assessment-and-Screening-in-Average-Risk-Women?IsMobileSet=false#5. Accessed May 13, 2019.
KEY POINTS
- The US Preventive Services Task Force recommends screening bone density when the 10-year risk of major osteoporotic fracture is more than 8.4%.
- Women can be reassured that nonrifamycin antibiotics are unlikely to reduce efficacy of hormonal contraception.
- Hormone replacement therapy after prophylactic bilateral salpingo-oophorectomy does not increase breast cancer risk in women who carry the BRCA1 gene mutation.
- Hormonal contraception may increase the risk of breast cancer by 1 extra case per 7,690 women, although most studies suggest there is no increased risk.
- The use of digital breast tomosynthesis along with digital mammography can increase cancer detection in women with dense breast tissue, but it is not yet routinely recommended by most professional societies.