User login
Ebola in the office: What to do?
While plentiful guidance has been published on how hospitals should diagnose, isolate, transfer, and treat patients with Ebola virus infection or suspected infection, little information is available for the office-based physician.
The Centers for Disease Control and Prevention says that it is developing on a broader set of guidelines that will include primary care physicians and other clinicians working in outpatient practices. But these guidelines have yet to be published, and physicians at both standalone and hospital-affiliated practices are grappling with tough questions regarding isolation, transport to hospitals, and even whom to call first.
In the event of a patient presenting in an office with a travel history and symptoms suggestive of Ebola, "you would certainly do as much contact isolation as you can," said Dr. Kevin Powell, a pediatrician in St. Louis. "Most offices are prepared to do some, and it would reduce the risk markedly."
At that point, "I think the presumption of most doctors was, you call the CDC and say you need help," Dr. Powell added.
"Then we found out that the CDC isn't prepared to give that answer back."
Because an office-based physician will not be able to diagnose Ebola, regardless, Dr. Powell added, there should be “a strong emphasis toward saying that a person who thinks they have Ebola should stay away from an outpatient office and go to a hospital emergency room.”
Health officials in Ohio have advised primary care physicians to consult with any patient with suspected Ebola symptoms by phone, taking information about travel and also about potential exposures within the United States.
Dr. Jack T. Swanson, described a similar approach at the McFarland Clinic in Ames, Iowa. “If a patient calls for a sick appointment, our triage phone nurse will ask if they have been in western Africa or have had any contact with someone with Ebola in the past 21 days. If the answer is yes, they are to go to our emergency room rather than come to our office,” said Dr. Swanson, a pediatrician in private practice.
Some patients with suspected Ebola may arrive at outpatient clinics anyway. One way to establish a front-line response is to have nonclinical registration desk staff ask the first screening question, regarding travel history, Dr. Amy Gottlieb, said in an interview. At her hospital-linked outpatient practice, “If we [identified] someone with travel exposure, we could isolate them in a room and bring in someone who can do the clinical assessment in a private, controlled setting,” said Dr. Gottlieb of the departments of medicine and ob.gyn. at Brown University in Providence, R.I.
Dr. George DeVito, a pediatrician* in Concord, N.H., treats a large number of resettled and refugee families from all around the world. His outpatient practice has been working closely with infectious disease and infection control specialists at their local hospital to craft a clear and careful strategy, he said, that includes screening for any child presenting with fever.
“As we enter flu season, we’re going to be seeing lots and lots more kids with fever, and many of our refugees don’t speak English, so we’re talking through a translator. Many people here may be very fearful of expressing that someone in their family may have had Ebola-like symptoms or a travel history. There are huge issues for us here,” said Dr. DeVito. Nonetheless, as a hospital-affiliated practice, “at least we know who to call. In an isolated private practice, it would be a different story.”
Should a patient present to a clinic, Dr. Carolyn Lopez, president of the Chicago Board of Health said, “the travel history should be obtained and appropriate precautions implemented. The local and/or state health department should be contacted. They in turn should have a plan in place as to which hospital will be involved and how to transport the patient there. Bottom line is that everyone should have a plan, everyone should know their role in the plan and practice the plan so that if and when it is needed, people can implement the plan.”
Some physicians acknowledge that they currently do not have a strategy in place.
“I will be awaiting the recommendations from the CDC,” said Dr. Karalyn Kinsella, a pediatrician in private practice in Cheshire, Conn. “My questions would be how do we transport patients to the hospital? Will the ambulance companies be prepared?”
Dr. William E. Golden, professor of medicine and public health at the University of Arkansas, Little Rock, said that diagnosis and triage might be the easiest task in an office setting. It’s what to do next that’s the hard part. “Offices need guidance on the aftermath,” Dr. Golden said. “Current policy is erratic at best. On the one hand, we are told that asymptomatic patients are not contagious, but now health workers in Dallas are told to stay away from public places for 3 weeks after contact with an Ebola patient. We need consistent clinical leadership, stat.”
*An earlier version of this story misidentified Dr. DeVito's medical specialty.
While plentiful guidance has been published on how hospitals should diagnose, isolate, transfer, and treat patients with Ebola virus infection or suspected infection, little information is available for the office-based physician.
The Centers for Disease Control and Prevention says that it is developing on a broader set of guidelines that will include primary care physicians and other clinicians working in outpatient practices. But these guidelines have yet to be published, and physicians at both standalone and hospital-affiliated practices are grappling with tough questions regarding isolation, transport to hospitals, and even whom to call first.
In the event of a patient presenting in an office with a travel history and symptoms suggestive of Ebola, "you would certainly do as much contact isolation as you can," said Dr. Kevin Powell, a pediatrician in St. Louis. "Most offices are prepared to do some, and it would reduce the risk markedly."
At that point, "I think the presumption of most doctors was, you call the CDC and say you need help," Dr. Powell added.
"Then we found out that the CDC isn't prepared to give that answer back."
Because an office-based physician will not be able to diagnose Ebola, regardless, Dr. Powell added, there should be “a strong emphasis toward saying that a person who thinks they have Ebola should stay away from an outpatient office and go to a hospital emergency room.”
Health officials in Ohio have advised primary care physicians to consult with any patient with suspected Ebola symptoms by phone, taking information about travel and also about potential exposures within the United States.
Dr. Jack T. Swanson, described a similar approach at the McFarland Clinic in Ames, Iowa. “If a patient calls for a sick appointment, our triage phone nurse will ask if they have been in western Africa or have had any contact with someone with Ebola in the past 21 days. If the answer is yes, they are to go to our emergency room rather than come to our office,” said Dr. Swanson, a pediatrician in private practice.
Some patients with suspected Ebola may arrive at outpatient clinics anyway. One way to establish a front-line response is to have nonclinical registration desk staff ask the first screening question, regarding travel history, Dr. Amy Gottlieb, said in an interview. At her hospital-linked outpatient practice, “If we [identified] someone with travel exposure, we could isolate them in a room and bring in someone who can do the clinical assessment in a private, controlled setting,” said Dr. Gottlieb of the departments of medicine and ob.gyn. at Brown University in Providence, R.I.
Dr. George DeVito, a pediatrician* in Concord, N.H., treats a large number of resettled and refugee families from all around the world. His outpatient practice has been working closely with infectious disease and infection control specialists at their local hospital to craft a clear and careful strategy, he said, that includes screening for any child presenting with fever.
“As we enter flu season, we’re going to be seeing lots and lots more kids with fever, and many of our refugees don’t speak English, so we’re talking through a translator. Many people here may be very fearful of expressing that someone in their family may have had Ebola-like symptoms or a travel history. There are huge issues for us here,” said Dr. DeVito. Nonetheless, as a hospital-affiliated practice, “at least we know who to call. In an isolated private practice, it would be a different story.”
Should a patient present to a clinic, Dr. Carolyn Lopez, president of the Chicago Board of Health said, “the travel history should be obtained and appropriate precautions implemented. The local and/or state health department should be contacted. They in turn should have a plan in place as to which hospital will be involved and how to transport the patient there. Bottom line is that everyone should have a plan, everyone should know their role in the plan and practice the plan so that if and when it is needed, people can implement the plan.”
Some physicians acknowledge that they currently do not have a strategy in place.
“I will be awaiting the recommendations from the CDC,” said Dr. Karalyn Kinsella, a pediatrician in private practice in Cheshire, Conn. “My questions would be how do we transport patients to the hospital? Will the ambulance companies be prepared?”
Dr. William E. Golden, professor of medicine and public health at the University of Arkansas, Little Rock, said that diagnosis and triage might be the easiest task in an office setting. It’s what to do next that’s the hard part. “Offices need guidance on the aftermath,” Dr. Golden said. “Current policy is erratic at best. On the one hand, we are told that asymptomatic patients are not contagious, but now health workers in Dallas are told to stay away from public places for 3 weeks after contact with an Ebola patient. We need consistent clinical leadership, stat.”
*An earlier version of this story misidentified Dr. DeVito's medical specialty.
While plentiful guidance has been published on how hospitals should diagnose, isolate, transfer, and treat patients with Ebola virus infection or suspected infection, little information is available for the office-based physician.
The Centers for Disease Control and Prevention says that it is developing on a broader set of guidelines that will include primary care physicians and other clinicians working in outpatient practices. But these guidelines have yet to be published, and physicians at both standalone and hospital-affiliated practices are grappling with tough questions regarding isolation, transport to hospitals, and even whom to call first.
In the event of a patient presenting in an office with a travel history and symptoms suggestive of Ebola, "you would certainly do as much contact isolation as you can," said Dr. Kevin Powell, a pediatrician in St. Louis. "Most offices are prepared to do some, and it would reduce the risk markedly."
At that point, "I think the presumption of most doctors was, you call the CDC and say you need help," Dr. Powell added.
"Then we found out that the CDC isn't prepared to give that answer back."
Because an office-based physician will not be able to diagnose Ebola, regardless, Dr. Powell added, there should be “a strong emphasis toward saying that a person who thinks they have Ebola should stay away from an outpatient office and go to a hospital emergency room.”
Health officials in Ohio have advised primary care physicians to consult with any patient with suspected Ebola symptoms by phone, taking information about travel and also about potential exposures within the United States.
Dr. Jack T. Swanson, described a similar approach at the McFarland Clinic in Ames, Iowa. “If a patient calls for a sick appointment, our triage phone nurse will ask if they have been in western Africa or have had any contact with someone with Ebola in the past 21 days. If the answer is yes, they are to go to our emergency room rather than come to our office,” said Dr. Swanson, a pediatrician in private practice.
Some patients with suspected Ebola may arrive at outpatient clinics anyway. One way to establish a front-line response is to have nonclinical registration desk staff ask the first screening question, regarding travel history, Dr. Amy Gottlieb, said in an interview. At her hospital-linked outpatient practice, “If we [identified] someone with travel exposure, we could isolate them in a room and bring in someone who can do the clinical assessment in a private, controlled setting,” said Dr. Gottlieb of the departments of medicine and ob.gyn. at Brown University in Providence, R.I.
Dr. George DeVito, a pediatrician* in Concord, N.H., treats a large number of resettled and refugee families from all around the world. His outpatient practice has been working closely with infectious disease and infection control specialists at their local hospital to craft a clear and careful strategy, he said, that includes screening for any child presenting with fever.
“As we enter flu season, we’re going to be seeing lots and lots more kids with fever, and many of our refugees don’t speak English, so we’re talking through a translator. Many people here may be very fearful of expressing that someone in their family may have had Ebola-like symptoms or a travel history. There are huge issues for us here,” said Dr. DeVito. Nonetheless, as a hospital-affiliated practice, “at least we know who to call. In an isolated private practice, it would be a different story.”
Should a patient present to a clinic, Dr. Carolyn Lopez, president of the Chicago Board of Health said, “the travel history should be obtained and appropriate precautions implemented. The local and/or state health department should be contacted. They in turn should have a plan in place as to which hospital will be involved and how to transport the patient there. Bottom line is that everyone should have a plan, everyone should know their role in the plan and practice the plan so that if and when it is needed, people can implement the plan.”
Some physicians acknowledge that they currently do not have a strategy in place.
“I will be awaiting the recommendations from the CDC,” said Dr. Karalyn Kinsella, a pediatrician in private practice in Cheshire, Conn. “My questions would be how do we transport patients to the hospital? Will the ambulance companies be prepared?”
Dr. William E. Golden, professor of medicine and public health at the University of Arkansas, Little Rock, said that diagnosis and triage might be the easiest task in an office setting. It’s what to do next that’s the hard part. “Offices need guidance on the aftermath,” Dr. Golden said. “Current policy is erratic at best. On the one hand, we are told that asymptomatic patients are not contagious, but now health workers in Dallas are told to stay away from public places for 3 weeks after contact with an Ebola patient. We need consistent clinical leadership, stat.”
*An earlier version of this story misidentified Dr. DeVito's medical specialty.
President Obama names Ebola czar
President Obama has named attorney Ronald Klain its Ebola czar to oversee the federal goverment’s response to the Ebola crisis.
Mr. Klain, 53, has extensive management experience but no background in medicine. He is a long-time Democratic political operative and chief of staff to two vice presidents, Al Gore and Joe Biden. Mr. Klain left the Obama administration in January 2011 to work at a private law firm.
Mr. Klain’s career has been divided among work in the private sector, politics, and government. In addition to his service as vice-presidential chief of staff, he has played a key role in presidential election campaigns, including Al Gore’s 2000 campaign. He clerked for U.S. Supreme Court Justice Byron R. White, served as chief counsel to the Senate Judiciary Committee, and lobbied the federal government on behalf of corporate clients, according to the biography page published by his current firm, Revolution.
“Klain, an attorney, comes to the job with strong management credentials, extensive federal government experience overseeing complex operations, and good working relationships with leading members of Congress, as well as senior Obama administration officials, including the president,” said a senior administration official in a statement emailed to reporters on Friday.
President Obama has named attorney Ronald Klain its Ebola czar to oversee the federal goverment’s response to the Ebola crisis.
Mr. Klain, 53, has extensive management experience but no background in medicine. He is a long-time Democratic political operative and chief of staff to two vice presidents, Al Gore and Joe Biden. Mr. Klain left the Obama administration in January 2011 to work at a private law firm.
Mr. Klain’s career has been divided among work in the private sector, politics, and government. In addition to his service as vice-presidential chief of staff, he has played a key role in presidential election campaigns, including Al Gore’s 2000 campaign. He clerked for U.S. Supreme Court Justice Byron R. White, served as chief counsel to the Senate Judiciary Committee, and lobbied the federal government on behalf of corporate clients, according to the biography page published by his current firm, Revolution.
“Klain, an attorney, comes to the job with strong management credentials, extensive federal government experience overseeing complex operations, and good working relationships with leading members of Congress, as well as senior Obama administration officials, including the president,” said a senior administration official in a statement emailed to reporters on Friday.
President Obama has named attorney Ronald Klain its Ebola czar to oversee the federal goverment’s response to the Ebola crisis.
Mr. Klain, 53, has extensive management experience but no background in medicine. He is a long-time Democratic political operative and chief of staff to two vice presidents, Al Gore and Joe Biden. Mr. Klain left the Obama administration in January 2011 to work at a private law firm.
Mr. Klain’s career has been divided among work in the private sector, politics, and government. In addition to his service as vice-presidential chief of staff, he has played a key role in presidential election campaigns, including Al Gore’s 2000 campaign. He clerked for U.S. Supreme Court Justice Byron R. White, served as chief counsel to the Senate Judiciary Committee, and lobbied the federal government on behalf of corporate clients, according to the biography page published by his current firm, Revolution.
“Klain, an attorney, comes to the job with strong management credentials, extensive federal government experience overseeing complex operations, and good working relationships with leading members of Congress, as well as senior Obama administration officials, including the president,” said a senior administration official in a statement emailed to reporters on Friday.
Ebola: CDC director cautions against proposal to ban flights from West Africa
WASHINGTON – The director of the Centers for Disease Control and Prevention cautioned against proposals to ban flights from West African countries affected by the Ebola epidemic during a hearing held by the House Energy & Commerce Committee’s Subcommittee on Oversight and Investigations Oct. 16.
At the hearing, subcommittee chairman Tim Murphy (R-Penn.) proposed an immediate ban on commercial, non-essential travel from three West African nations to the United States. Additionally, he called for a mandatory quarantine for any American who treats an Ebola patient in those countries.
Dr. Tom Frieden told the subcommittee that a flight ban might drive people to come into the country by other means, which could lead to potentially more infections because those people would not be efficiently tracked. He added that the CDC continues to be “confident that Ebola is not a significant public health threat to the United States.”
Starting today, passengers from Sierra Leone, Guinea, and Liberia are screened upon exiting those countries, he said. They are having their temperatures taken and are being subjected to additional screening upon entering the United States at five major airports: Atlanta’s Hartsfield; Dulles, near Washington, D.C.; New York’s J.F.K.; Newark, N.J.; and Chicago’s O’Hare.
“Your protocol depends on everyone being honest,” Rep Murphy countered. It depends on thermometers being accurate, and it still does not catch people who are asymptomatic but carrying the virus.
House subcommittee members also wanted to know whether officials at the CDC and Texas Health Presbyterian Hospital had determined how two nurses involved in the care of an Ebola patient became infected, despite theoretically following CDC protocols. Nancy Pham and Amber Vinson provided care for Thomas Eric Duncan, who developed the infection after flying from Liberia to Dallas, where he died on Oct. 8.
“We’ve learned frontline hospital workers were not fully trained in those procedures, do not have proper equipment, do not know how to properly put on and remove safety gear,” said subcommittee chairman Tim Murphy (R-Penn.). “Educating, training and assisting our public health workforce on the frontlines across the country must be a priority.” Dr. Daniel Varga, chief clinical officer and senior vice president for Texas Health Resources, the parent company of Texas Health Presbyterian Hospital, said the investigation continues. Both nurses were “using full protective measures under the CDC protocols,” and officials still don’t know how or when the nurses were infected.
Dr. Frieden said that “more than 20 of the world’s top disease detectives” are at the Dallas hospital trying to determine what went wrong and that they are also conducting extensive contact tracing.
Dr. Varga acknowledged that there had been no actual training rehearsal of the hospital’s emergency room workers in handling a potential Ebola patient before Mr. Duncan arrived at the facility in late September.
“Unfortunately, in our initial treatment of Mr. Duncan, despite our best intentions and a highly skilled medical team, we made mistakes,” said Dr. Varga.
Rep. Murphy called for immediate and thorough training of U.S. hospital personnel in the proper use of personal protective equipment. Specialized medical centers should be identified for treatment of Ebola patients, and those centers need to be expanded, he said.
Currently, there are four specialized centers and they have a limited bed capacity. They are located at the National Institutes of Health in Bethesda, Md. (2 beds), at Emory University (2 beds), the Nebraska Medical Center (10 beds), and St. Patrick Hospital in Missoula, Mont. (3 beds).
The CDC is continuing to field calls from health care providers, and to offer training and education, Dr. Frieden said.
Hospitals and clinicians should keep Ebola in mind when treating any patient who presents with a fever or flu-like symptoms, and ask whether the patient has traveled to West Africa within the last 21 days.
Meanwhile, 45% of Americans say they are worried about contracting Ebola, according to a poll released on Oct. 16 by the Kaiser Family Foundation. A similar number thought, mistakenly, that they could get the virus by shaking hands with someone who is asymptomatic. A majority said they had confidence in the CDC, their local hospital, and state health officials to contain the virus.
The Oversight and Investigations Subcommittee plans to hold another hearing on Ebola preparedness in November, said Rep. Murphy.
On Twitter @aliciaault
WASHINGTON – The director of the Centers for Disease Control and Prevention cautioned against proposals to ban flights from West African countries affected by the Ebola epidemic during a hearing held by the House Energy & Commerce Committee’s Subcommittee on Oversight and Investigations Oct. 16.
At the hearing, subcommittee chairman Tim Murphy (R-Penn.) proposed an immediate ban on commercial, non-essential travel from three West African nations to the United States. Additionally, he called for a mandatory quarantine for any American who treats an Ebola patient in those countries.
Dr. Tom Frieden told the subcommittee that a flight ban might drive people to come into the country by other means, which could lead to potentially more infections because those people would not be efficiently tracked. He added that the CDC continues to be “confident that Ebola is not a significant public health threat to the United States.”
Starting today, passengers from Sierra Leone, Guinea, and Liberia are screened upon exiting those countries, he said. They are having their temperatures taken and are being subjected to additional screening upon entering the United States at five major airports: Atlanta’s Hartsfield; Dulles, near Washington, D.C.; New York’s J.F.K.; Newark, N.J.; and Chicago’s O’Hare.
“Your protocol depends on everyone being honest,” Rep Murphy countered. It depends on thermometers being accurate, and it still does not catch people who are asymptomatic but carrying the virus.
House subcommittee members also wanted to know whether officials at the CDC and Texas Health Presbyterian Hospital had determined how two nurses involved in the care of an Ebola patient became infected, despite theoretically following CDC protocols. Nancy Pham and Amber Vinson provided care for Thomas Eric Duncan, who developed the infection after flying from Liberia to Dallas, where he died on Oct. 8.
“We’ve learned frontline hospital workers were not fully trained in those procedures, do not have proper equipment, do not know how to properly put on and remove safety gear,” said subcommittee chairman Tim Murphy (R-Penn.). “Educating, training and assisting our public health workforce on the frontlines across the country must be a priority.” Dr. Daniel Varga, chief clinical officer and senior vice president for Texas Health Resources, the parent company of Texas Health Presbyterian Hospital, said the investigation continues. Both nurses were “using full protective measures under the CDC protocols,” and officials still don’t know how or when the nurses were infected.
Dr. Frieden said that “more than 20 of the world’s top disease detectives” are at the Dallas hospital trying to determine what went wrong and that they are also conducting extensive contact tracing.
Dr. Varga acknowledged that there had been no actual training rehearsal of the hospital’s emergency room workers in handling a potential Ebola patient before Mr. Duncan arrived at the facility in late September.
“Unfortunately, in our initial treatment of Mr. Duncan, despite our best intentions and a highly skilled medical team, we made mistakes,” said Dr. Varga.
Rep. Murphy called for immediate and thorough training of U.S. hospital personnel in the proper use of personal protective equipment. Specialized medical centers should be identified for treatment of Ebola patients, and those centers need to be expanded, he said.
Currently, there are four specialized centers and they have a limited bed capacity. They are located at the National Institutes of Health in Bethesda, Md. (2 beds), at Emory University (2 beds), the Nebraska Medical Center (10 beds), and St. Patrick Hospital in Missoula, Mont. (3 beds).
The CDC is continuing to field calls from health care providers, and to offer training and education, Dr. Frieden said.
Hospitals and clinicians should keep Ebola in mind when treating any patient who presents with a fever or flu-like symptoms, and ask whether the patient has traveled to West Africa within the last 21 days.
Meanwhile, 45% of Americans say they are worried about contracting Ebola, according to a poll released on Oct. 16 by the Kaiser Family Foundation. A similar number thought, mistakenly, that they could get the virus by shaking hands with someone who is asymptomatic. A majority said they had confidence in the CDC, their local hospital, and state health officials to contain the virus.
The Oversight and Investigations Subcommittee plans to hold another hearing on Ebola preparedness in November, said Rep. Murphy.
On Twitter @aliciaault
WASHINGTON – The director of the Centers for Disease Control and Prevention cautioned against proposals to ban flights from West African countries affected by the Ebola epidemic during a hearing held by the House Energy & Commerce Committee’s Subcommittee on Oversight and Investigations Oct. 16.
At the hearing, subcommittee chairman Tim Murphy (R-Penn.) proposed an immediate ban on commercial, non-essential travel from three West African nations to the United States. Additionally, he called for a mandatory quarantine for any American who treats an Ebola patient in those countries.
Dr. Tom Frieden told the subcommittee that a flight ban might drive people to come into the country by other means, which could lead to potentially more infections because those people would not be efficiently tracked. He added that the CDC continues to be “confident that Ebola is not a significant public health threat to the United States.”
Starting today, passengers from Sierra Leone, Guinea, and Liberia are screened upon exiting those countries, he said. They are having their temperatures taken and are being subjected to additional screening upon entering the United States at five major airports: Atlanta’s Hartsfield; Dulles, near Washington, D.C.; New York’s J.F.K.; Newark, N.J.; and Chicago’s O’Hare.
“Your protocol depends on everyone being honest,” Rep Murphy countered. It depends on thermometers being accurate, and it still does not catch people who are asymptomatic but carrying the virus.
House subcommittee members also wanted to know whether officials at the CDC and Texas Health Presbyterian Hospital had determined how two nurses involved in the care of an Ebola patient became infected, despite theoretically following CDC protocols. Nancy Pham and Amber Vinson provided care for Thomas Eric Duncan, who developed the infection after flying from Liberia to Dallas, where he died on Oct. 8.
“We’ve learned frontline hospital workers were not fully trained in those procedures, do not have proper equipment, do not know how to properly put on and remove safety gear,” said subcommittee chairman Tim Murphy (R-Penn.). “Educating, training and assisting our public health workforce on the frontlines across the country must be a priority.” Dr. Daniel Varga, chief clinical officer and senior vice president for Texas Health Resources, the parent company of Texas Health Presbyterian Hospital, said the investigation continues. Both nurses were “using full protective measures under the CDC protocols,” and officials still don’t know how or when the nurses were infected.
Dr. Frieden said that “more than 20 of the world’s top disease detectives” are at the Dallas hospital trying to determine what went wrong and that they are also conducting extensive contact tracing.
Dr. Varga acknowledged that there had been no actual training rehearsal of the hospital’s emergency room workers in handling a potential Ebola patient before Mr. Duncan arrived at the facility in late September.
“Unfortunately, in our initial treatment of Mr. Duncan, despite our best intentions and a highly skilled medical team, we made mistakes,” said Dr. Varga.
Rep. Murphy called for immediate and thorough training of U.S. hospital personnel in the proper use of personal protective equipment. Specialized medical centers should be identified for treatment of Ebola patients, and those centers need to be expanded, he said.
Currently, there are four specialized centers and they have a limited bed capacity. They are located at the National Institutes of Health in Bethesda, Md. (2 beds), at Emory University (2 beds), the Nebraska Medical Center (10 beds), and St. Patrick Hospital in Missoula, Mont. (3 beds).
The CDC is continuing to field calls from health care providers, and to offer training and education, Dr. Frieden said.
Hospitals and clinicians should keep Ebola in mind when treating any patient who presents with a fever or flu-like symptoms, and ask whether the patient has traveled to West Africa within the last 21 days.
Meanwhile, 45% of Americans say they are worried about contracting Ebola, according to a poll released on Oct. 16 by the Kaiser Family Foundation. A similar number thought, mistakenly, that they could get the virus by shaking hands with someone who is asymptomatic. A majority said they had confidence in the CDC, their local hospital, and state health officials to contain the virus.
The Oversight and Investigations Subcommittee plans to hold another hearing on Ebola preparedness in November, said Rep. Murphy.
On Twitter @aliciaault
AT A U.S. HOUSE HEARING
Ebola deaths near 4,500 in West Africa
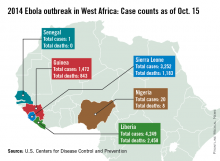
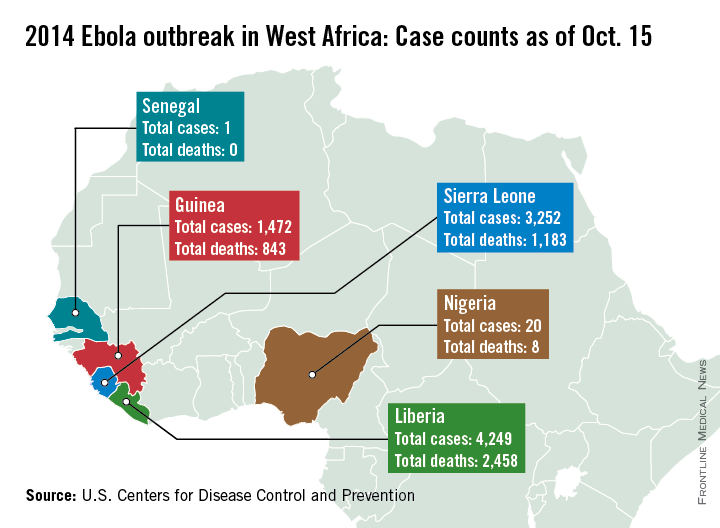
The Ebola outbreak continues to worsen in West Africa, with almost 9,000 cases and nearly 4,500 deaths in Liberia, Guinea, and Sierra Leone as of Oct. 15, according to the Centers for Disease Control and Prevention.
With nearly 4,250 reported cases and more than 2,450 deaths, Liberia accounts for almost half of the known Ebola cases, and more than half of Ebola-related deaths, according to the CDC data.
More than 3,250 cases have been reported in Sierra Leone, but with fewer than 1,200 deaths, mortality is lower there than in the other affected countries. With almost 1,500 official cases, Guinea has the fewest Ebola cases of the three nations, but with more than 840 deaths, it has the highest mortality among the afflicted countries.
Nigeria and Senegal also have reported Ebola cases related to the outbreak in West Africa. Nigeria has reported 20 cases and 8 deaths, with the last case reported on Sept. 5, according to the CDC. Senegal has reported one case of Ebola, with no deaths. Both countries have completed the required 21-day follow-up with no additional Ebola cases, the CDC reported.
Spain and the United States have reported locally transmitted Ebola cases, one in Spain and two in the United States. All three cases involve health care workers who attended to Ebola patients.
The Democratic Republic of the Congo reported 70 cases of Ebola as of Oct. 5 in an isolated area of the country, the seventh outbreak in the Congo since the discovery of Ebola there in 1976. That outbreak is considered to be separate from the one in West Africa, according to the CDC, and seems to be contained, with no new cases reported since Sept. 24.
The Ebola outbreak continues to worsen in West Africa, with almost 9,000 cases and nearly 4,500 deaths in Liberia, Guinea, and Sierra Leone as of Oct. 15, according to the Centers for Disease Control and Prevention.
With nearly 4,250 reported cases and more than 2,450 deaths, Liberia accounts for almost half of the known Ebola cases, and more than half of Ebola-related deaths, according to the CDC data.
More than 3,250 cases have been reported in Sierra Leone, but with fewer than 1,200 deaths, mortality is lower there than in the other affected countries. With almost 1,500 official cases, Guinea has the fewest Ebola cases of the three nations, but with more than 840 deaths, it has the highest mortality among the afflicted countries.
Nigeria and Senegal also have reported Ebola cases related to the outbreak in West Africa. Nigeria has reported 20 cases and 8 deaths, with the last case reported on Sept. 5, according to the CDC. Senegal has reported one case of Ebola, with no deaths. Both countries have completed the required 21-day follow-up with no additional Ebola cases, the CDC reported.
Spain and the United States have reported locally transmitted Ebola cases, one in Spain and two in the United States. All three cases involve health care workers who attended to Ebola patients.
The Democratic Republic of the Congo reported 70 cases of Ebola as of Oct. 5 in an isolated area of the country, the seventh outbreak in the Congo since the discovery of Ebola there in 1976. That outbreak is considered to be separate from the one in West Africa, according to the CDC, and seems to be contained, with no new cases reported since Sept. 24.
The Ebola outbreak continues to worsen in West Africa, with almost 9,000 cases and nearly 4,500 deaths in Liberia, Guinea, and Sierra Leone as of Oct. 15, according to the Centers for Disease Control and Prevention.
With nearly 4,250 reported cases and more than 2,450 deaths, Liberia accounts for almost half of the known Ebola cases, and more than half of Ebola-related deaths, according to the CDC data.
More than 3,250 cases have been reported in Sierra Leone, but with fewer than 1,200 deaths, mortality is lower there than in the other affected countries. With almost 1,500 official cases, Guinea has the fewest Ebola cases of the three nations, but with more than 840 deaths, it has the highest mortality among the afflicted countries.
Nigeria and Senegal also have reported Ebola cases related to the outbreak in West Africa. Nigeria has reported 20 cases and 8 deaths, with the last case reported on Sept. 5, according to the CDC. Senegal has reported one case of Ebola, with no deaths. Both countries have completed the required 21-day follow-up with no additional Ebola cases, the CDC reported.
Spain and the United States have reported locally transmitted Ebola cases, one in Spain and two in the United States. All three cases involve health care workers who attended to Ebola patients.
The Democratic Republic of the Congo reported 70 cases of Ebola as of Oct. 5 in an isolated area of the country, the seventh outbreak in the Congo since the discovery of Ebola there in 1976. That outbreak is considered to be separate from the one in West Africa, according to the CDC, and seems to be contained, with no new cases reported since Sept. 24.
CDC: Second nurse diagnosed with Ebola being transferred
A second health care worker infected with Ebola will be transferred to Emory University Hospital in Atlanta for care, according to the U.S. Department of Health and Human Services and the Centers for Disease Control and Prevention.
The first health care worker to be infected, nurse Nina Pham, remains at Texas Health Presbyterian in Dallas and is in good condition, top officials from both agencies said in a joint press conference Oct. 15.
The second health care worker, also said to be a nurse, was isolated in the morning of Oct. 14 and was diagnosed in preliminary testing that night. Officials revealed that she had traveled on a commercial airline Oct. 13 from Ohio to Dallas. The CDC said it was working with the airline to notify fellow passengers.
Facing increasing criticism over poor preparedness at Texas Health Presbyterian and concern that only certain U.S. hospitals may be ready to treat Ebola patients safely, CDC director Tom Frieden told reporters that “we’ll assess each day whether this is the right place” for Ms. Pham or whether she would be moved as well.
Both nurses had contact with Thomas Eric Duncan, the first patient in the United States to be diagnosed with Ebola, during times when the patient “had extensive production of body fluids because of vomiting and diarrhea,” Dr. Frieden said. Mr. Duncan died last week at Texas Health Presbyterian.
Dr. Frieden acknowledged the possibility or even likelihood of more Ebola cases “in the coming days” among the 76 health care workers at Texas Health Presbyterian who had been exposed to Mr. Duncan or his blood during his illness.
In the early days of Mr. Duncan’s care, “a variety of forms of [personal protective equipment] were used at the hospital” and that these were used in “several ways,” some of which may have led to infection, he said.
Three people have been identified as having had close contact with newest Ebola case before her isolation Oct. 14.
Dr. Frieden said that the nurse “should not have been allowed to travel by plane” per CDC guidelines, because she was being monitored as part of a group potentially exposed to Ebola and because she already had a temperature of 99.5°, though she was without further symptoms of illness.
Dr. Frieden said that health officials “think there is an extremely low risk” of other passengers on the plane having been exposed to Ebola.
A second health care worker infected with Ebola will be transferred to Emory University Hospital in Atlanta for care, according to the U.S. Department of Health and Human Services and the Centers for Disease Control and Prevention.
The first health care worker to be infected, nurse Nina Pham, remains at Texas Health Presbyterian in Dallas and is in good condition, top officials from both agencies said in a joint press conference Oct. 15.
The second health care worker, also said to be a nurse, was isolated in the morning of Oct. 14 and was diagnosed in preliminary testing that night. Officials revealed that she had traveled on a commercial airline Oct. 13 from Ohio to Dallas. The CDC said it was working with the airline to notify fellow passengers.
Facing increasing criticism over poor preparedness at Texas Health Presbyterian and concern that only certain U.S. hospitals may be ready to treat Ebola patients safely, CDC director Tom Frieden told reporters that “we’ll assess each day whether this is the right place” for Ms. Pham or whether she would be moved as well.
Both nurses had contact with Thomas Eric Duncan, the first patient in the United States to be diagnosed with Ebola, during times when the patient “had extensive production of body fluids because of vomiting and diarrhea,” Dr. Frieden said. Mr. Duncan died last week at Texas Health Presbyterian.
Dr. Frieden acknowledged the possibility or even likelihood of more Ebola cases “in the coming days” among the 76 health care workers at Texas Health Presbyterian who had been exposed to Mr. Duncan or his blood during his illness.
In the early days of Mr. Duncan’s care, “a variety of forms of [personal protective equipment] were used at the hospital” and that these were used in “several ways,” some of which may have led to infection, he said.
Three people have been identified as having had close contact with newest Ebola case before her isolation Oct. 14.
Dr. Frieden said that the nurse “should not have been allowed to travel by plane” per CDC guidelines, because she was being monitored as part of a group potentially exposed to Ebola and because she already had a temperature of 99.5°, though she was without further symptoms of illness.
Dr. Frieden said that health officials “think there is an extremely low risk” of other passengers on the plane having been exposed to Ebola.
A second health care worker infected with Ebola will be transferred to Emory University Hospital in Atlanta for care, according to the U.S. Department of Health and Human Services and the Centers for Disease Control and Prevention.
The first health care worker to be infected, nurse Nina Pham, remains at Texas Health Presbyterian in Dallas and is in good condition, top officials from both agencies said in a joint press conference Oct. 15.
The second health care worker, also said to be a nurse, was isolated in the morning of Oct. 14 and was diagnosed in preliminary testing that night. Officials revealed that she had traveled on a commercial airline Oct. 13 from Ohio to Dallas. The CDC said it was working with the airline to notify fellow passengers.
Facing increasing criticism over poor preparedness at Texas Health Presbyterian and concern that only certain U.S. hospitals may be ready to treat Ebola patients safely, CDC director Tom Frieden told reporters that “we’ll assess each day whether this is the right place” for Ms. Pham or whether she would be moved as well.
Both nurses had contact with Thomas Eric Duncan, the first patient in the United States to be diagnosed with Ebola, during times when the patient “had extensive production of body fluids because of vomiting and diarrhea,” Dr. Frieden said. Mr. Duncan died last week at Texas Health Presbyterian.
Dr. Frieden acknowledged the possibility or even likelihood of more Ebola cases “in the coming days” among the 76 health care workers at Texas Health Presbyterian who had been exposed to Mr. Duncan or his blood during his illness.
In the early days of Mr. Duncan’s care, “a variety of forms of [personal protective equipment] were used at the hospital” and that these were used in “several ways,” some of which may have led to infection, he said.
Three people have been identified as having had close contact with newest Ebola case before her isolation Oct. 14.
Dr. Frieden said that the nurse “should not have been allowed to travel by plane” per CDC guidelines, because she was being monitored as part of a group potentially exposed to Ebola and because she already had a temperature of 99.5°, though she was without further symptoms of illness.
Dr. Frieden said that health officials “think there is an extremely low risk” of other passengers on the plane having been exposed to Ebola.
FROM A CDC TELEBRIEFING
Comparative effectiveness research results alone don’t change clinical practice
Practice patterns shifted little in the 12 months following the publishing of study results and in the 12 months following incorporation into clinical practice guidelines, based on an analysis of four comparative effectiveness research case studies, funded by the National Pharmaceutical Council (NPC).
The case studies, published in the American Journal of Managed Care (Am. J. Manag. Care 2014;20:e208-e220), looked at practice changes after the 2004 PROVE-IT study on statin therapies, the 2004 Mammography With MRI study on breast cancer surveillance methods in women with BRCA1 or BRCA2 mutations, the 2006 SPORT study comparing standard open diskectomy versus nonoperative treatments for patients with intervertebal disk herniation, and the 2007 COURAGE trial comparing optimal medical therapy and percutaneous coronary intervention versus optimal medical therapy alone.
"In some cases, we might have expected an uptick or change in what was happening," said report author and NPC Director of Comparative Effectiveness Research Jennifer Graff, Pharm.D., speaking in an interview. "For instance, in the PROVE-IT study, we would have expected, based upon the results, that you would have seen many more providers and patients using intensive statin therapy, ... [but it took] 3 years after the study’s publication before you started to see the change in which types of statins were being used."
The report authors developed suggestions on how to get clinicians to more quickly incorporate the results of comparative effectiveness research.
Involve clinicians, payers, policy makers, and patients in the research design "to make sure we are asking the right questions," said Dr. Graff. This approach is now being taken at the Food and Drug Administration to spur drug development and at the Patient-Centered Outcomes Research Institute, a comparative effectiveness research body created as part of the Affordable Care Act.
Perform more confirmatory studies. "One single study probably won’t change the mind of a provider who is seeing many patients and has in their mind what treatments work," Dr. Graff said. "Similarly, we need to fund studies when clinical opinion is shifting."
Align financial incentives with study results. This can be accomplished through value-based insurance design or incentivized provider pay based on outcomes. Bundled payments as well as incentivizing the use of clinical pathways, such as WellPoint’s recently announced program to provide bonus payments in oncology, are examples of such options, Dr. Graff said.
The study was funded by the NPC. Authors Teresa Gibson, Emily Ehrlich, and Amanda Farr reported employment with Truven Health Analytics, which received consulting fees from NPC. Authors Dr. Robert Dubois and Dr. Jennifer Graff are employees of the NPC. The remaining authors reported no financial conflicts of interest.
I disagree with the first sentence of this article. The gist of the article and the interview is that clinicians are guilty of not adopting important new changes in evidence into their practices soon enough.
 |
| Dr. Larry Kraiss |
What this report does not make clear is that the four comparative effectiveness research (CER) studies cited in the American Journal Managed of Care paper were specifically chosen for analysis because no subsequent studies had been published to contradict the major findings. The period of analysis extended to the end of 2009, so in the case of the two 2004 studies, the waiting period to see if contradictory results were published lasted as long as 5 years.
To retrospectively criticize practitioners for not responding to the major findings in these studies sooner is logically fallacious because it presumes that we all should have known that no conflicting information was going to later appear. This is hard to swallow by those of us smarting from being duped by the discredited DECREASE trials regarding perioperative beta-blockade usage.
The article also claims that clinician behavior is not influenced by clinical practice guidelines. This generalization is inaccurate because the American Journal of Managed Care article does indicate that clinical practice guidelines do influence practice.
Finally, in the interview, one of the authors bemoans the 3-year lag between the publication of PROVE-IT and more widespread use of statins. In my opinion, this is not an excessively long lag period.
A healthy sense of skepticism regarding the results of individual CER trials, especially randomized controlled trials where generalization can be suspect, is still warranted. Waiting for the appearance of clinical practice guidelines is prudent. This is what happened with PROVE-IT and what should still happen going forward.
Dr. Larry Kraiss is a professor and chief of the Division of Vascular Surgery and medical director of the Noninvasive Vascular Laboratory at the University of Utah School of Medicine and an associate medical editor of Vascular Specialist.
I disagree with the first sentence of this article. The gist of the article and the interview is that clinicians are guilty of not adopting important new changes in evidence into their practices soon enough.
 |
| Dr. Larry Kraiss |
What this report does not make clear is that the four comparative effectiveness research (CER) studies cited in the American Journal Managed of Care paper were specifically chosen for analysis because no subsequent studies had been published to contradict the major findings. The period of analysis extended to the end of 2009, so in the case of the two 2004 studies, the waiting period to see if contradictory results were published lasted as long as 5 years.
To retrospectively criticize practitioners for not responding to the major findings in these studies sooner is logically fallacious because it presumes that we all should have known that no conflicting information was going to later appear. This is hard to swallow by those of us smarting from being duped by the discredited DECREASE trials regarding perioperative beta-blockade usage.
The article also claims that clinician behavior is not influenced by clinical practice guidelines. This generalization is inaccurate because the American Journal of Managed Care article does indicate that clinical practice guidelines do influence practice.
Finally, in the interview, one of the authors bemoans the 3-year lag between the publication of PROVE-IT and more widespread use of statins. In my opinion, this is not an excessively long lag period.
A healthy sense of skepticism regarding the results of individual CER trials, especially randomized controlled trials where generalization can be suspect, is still warranted. Waiting for the appearance of clinical practice guidelines is prudent. This is what happened with PROVE-IT and what should still happen going forward.
Dr. Larry Kraiss is a professor and chief of the Division of Vascular Surgery and medical director of the Noninvasive Vascular Laboratory at the University of Utah School of Medicine and an associate medical editor of Vascular Specialist.
I disagree with the first sentence of this article. The gist of the article and the interview is that clinicians are guilty of not adopting important new changes in evidence into their practices soon enough.
 |
| Dr. Larry Kraiss |
What this report does not make clear is that the four comparative effectiveness research (CER) studies cited in the American Journal Managed of Care paper were specifically chosen for analysis because no subsequent studies had been published to contradict the major findings. The period of analysis extended to the end of 2009, so in the case of the two 2004 studies, the waiting period to see if contradictory results were published lasted as long as 5 years.
To retrospectively criticize practitioners for not responding to the major findings in these studies sooner is logically fallacious because it presumes that we all should have known that no conflicting information was going to later appear. This is hard to swallow by those of us smarting from being duped by the discredited DECREASE trials regarding perioperative beta-blockade usage.
The article also claims that clinician behavior is not influenced by clinical practice guidelines. This generalization is inaccurate because the American Journal of Managed Care article does indicate that clinical practice guidelines do influence practice.
Finally, in the interview, one of the authors bemoans the 3-year lag between the publication of PROVE-IT and more widespread use of statins. In my opinion, this is not an excessively long lag period.
A healthy sense of skepticism regarding the results of individual CER trials, especially randomized controlled trials where generalization can be suspect, is still warranted. Waiting for the appearance of clinical practice guidelines is prudent. This is what happened with PROVE-IT and what should still happen going forward.
Dr. Larry Kraiss is a professor and chief of the Division of Vascular Surgery and medical director of the Noninvasive Vascular Laboratory at the University of Utah School of Medicine and an associate medical editor of Vascular Specialist.
Practice patterns shifted little in the 12 months following the publishing of study results and in the 12 months following incorporation into clinical practice guidelines, based on an analysis of four comparative effectiveness research case studies, funded by the National Pharmaceutical Council (NPC).
The case studies, published in the American Journal of Managed Care (Am. J. Manag. Care 2014;20:e208-e220), looked at practice changes after the 2004 PROVE-IT study on statin therapies, the 2004 Mammography With MRI study on breast cancer surveillance methods in women with BRCA1 or BRCA2 mutations, the 2006 SPORT study comparing standard open diskectomy versus nonoperative treatments for patients with intervertebal disk herniation, and the 2007 COURAGE trial comparing optimal medical therapy and percutaneous coronary intervention versus optimal medical therapy alone.
"In some cases, we might have expected an uptick or change in what was happening," said report author and NPC Director of Comparative Effectiveness Research Jennifer Graff, Pharm.D., speaking in an interview. "For instance, in the PROVE-IT study, we would have expected, based upon the results, that you would have seen many more providers and patients using intensive statin therapy, ... [but it took] 3 years after the study’s publication before you started to see the change in which types of statins were being used."
The report authors developed suggestions on how to get clinicians to more quickly incorporate the results of comparative effectiveness research.
Involve clinicians, payers, policy makers, and patients in the research design "to make sure we are asking the right questions," said Dr. Graff. This approach is now being taken at the Food and Drug Administration to spur drug development and at the Patient-Centered Outcomes Research Institute, a comparative effectiveness research body created as part of the Affordable Care Act.
Perform more confirmatory studies. "One single study probably won’t change the mind of a provider who is seeing many patients and has in their mind what treatments work," Dr. Graff said. "Similarly, we need to fund studies when clinical opinion is shifting."
Align financial incentives with study results. This can be accomplished through value-based insurance design or incentivized provider pay based on outcomes. Bundled payments as well as incentivizing the use of clinical pathways, such as WellPoint’s recently announced program to provide bonus payments in oncology, are examples of such options, Dr. Graff said.
The study was funded by the NPC. Authors Teresa Gibson, Emily Ehrlich, and Amanda Farr reported employment with Truven Health Analytics, which received consulting fees from NPC. Authors Dr. Robert Dubois and Dr. Jennifer Graff are employees of the NPC. The remaining authors reported no financial conflicts of interest.
Practice patterns shifted little in the 12 months following the publishing of study results and in the 12 months following incorporation into clinical practice guidelines, based on an analysis of four comparative effectiveness research case studies, funded by the National Pharmaceutical Council (NPC).
The case studies, published in the American Journal of Managed Care (Am. J. Manag. Care 2014;20:e208-e220), looked at practice changes after the 2004 PROVE-IT study on statin therapies, the 2004 Mammography With MRI study on breast cancer surveillance methods in women with BRCA1 or BRCA2 mutations, the 2006 SPORT study comparing standard open diskectomy versus nonoperative treatments for patients with intervertebal disk herniation, and the 2007 COURAGE trial comparing optimal medical therapy and percutaneous coronary intervention versus optimal medical therapy alone.
"In some cases, we might have expected an uptick or change in what was happening," said report author and NPC Director of Comparative Effectiveness Research Jennifer Graff, Pharm.D., speaking in an interview. "For instance, in the PROVE-IT study, we would have expected, based upon the results, that you would have seen many more providers and patients using intensive statin therapy, ... [but it took] 3 years after the study’s publication before you started to see the change in which types of statins were being used."
The report authors developed suggestions on how to get clinicians to more quickly incorporate the results of comparative effectiveness research.
Involve clinicians, payers, policy makers, and patients in the research design "to make sure we are asking the right questions," said Dr. Graff. This approach is now being taken at the Food and Drug Administration to spur drug development and at the Patient-Centered Outcomes Research Institute, a comparative effectiveness research body created as part of the Affordable Care Act.
Perform more confirmatory studies. "One single study probably won’t change the mind of a provider who is seeing many patients and has in their mind what treatments work," Dr. Graff said. "Similarly, we need to fund studies when clinical opinion is shifting."
Align financial incentives with study results. This can be accomplished through value-based insurance design or incentivized provider pay based on outcomes. Bundled payments as well as incentivizing the use of clinical pathways, such as WellPoint’s recently announced program to provide bonus payments in oncology, are examples of such options, Dr. Graff said.
The study was funded by the NPC. Authors Teresa Gibson, Emily Ehrlich, and Amanda Farr reported employment with Truven Health Analytics, which received consulting fees from NPC. Authors Dr. Robert Dubois and Dr. Jennifer Graff are employees of the NPC. The remaining authors reported no financial conflicts of interest.
FROM THE AMERICAN JOURNAL OF MANAGED CARE
News From Washington: SVS comments on CMS 2015
Recently, SVS submitted comments on the Centers for Medicare & Medicaid Services’ (CMS) Calendar Year (CY) 2015 Medicare Physician Fee Schedule (MPFS) Proposed Rule. The following are some of the issues that SVS addressed:
Improving the valuation and coding of the global package
The most controversial issue that SVS commented on was the proposal to transition all 10- and 90-day global bundles to 0-day global codes by 2018, with medically reasonable and necessary visits billed separately during the pre- and postoperative periods outside the day for surgical procedures. SVS opposed this proposal along with many other surgical societies.
Some of the reasons for opposition included:
▶ CMS has no idea how to implement this change in policy;
▶ The agency’s concerns regarding the accuracy of payments would not be addressed by this proposal;
▶ Patients would be subject to extra co-pays, particularly for postoperative care and additional administrative costs would be incurred for the extra billing;
▶ New values would need to be created for postoperative care because not all these procedures have separate codes;
▶ Many other services are being bundled – this creates unbundling for surgical care which is inconsistent with what CMS is proposing in other areas.
Using hospital outpatient prospective payment system (OPPS) and ambulatory surgery center (ASC) data in developing practice expense (PE) relative value units (RVU)
SVS thanked CMS for withdrawing the nonfacility cap proposal that was in the CY 2014 MPFS Proposed Rule and for acknowledging that “the comparison of OPPS or ASC payment amounts to PFS payment amounts for particular procedures is not the most appropriate or effective approach to ensuring that PFS payment rates are based on accurate cost assumptions.”
SVS continues to oppose using OPPS cost data for potential revisions of PFS PE methodology as there are many differences, which include using an averaging mechanism that undervalues high-cost items and overvalues low-cost items versus creating codes on actual costs. This would be a disservice to patients on many levels and not provide true costs for the work provided.
Abdominal aortic aneurysm (AAA) ultrasound screening – G0389
SVS members and staff met with CMS staff in May regarding AAA ultrasound screening reimbursement. G0389 had an undervalued Technical Component of $36.90, which was equivalent to retroperitoneal ultrasound that uses different equipment and takes less time to perform.
The low reimbursement also created a disincentive to provide this life-saving screening. CMS proposed maintaining the work RVU and reverting back to the same PE RVU used in 2013, which is now $80.24; SVS strongly agreed with this proposal.
Valuing new, revised and potentially misvalued codes
CMS proposed a new timeline and process for the publication and implementation of changes to physician codes which SVS supports as a more rational approach.
The current process announces proposed changes at the beginning of November and implements these changes on Jan. 1 of the following year. This does not allow adequate public comment or sufficient time for physicians to prepare for the changes, including a reasonable evaluation of how the revisions might impact their practices and patients.
To accommodate the proposed process, SVS recommended that the meeting structure for both the Current Procedural Terminology (CPT) Panel and Relative Value Update Committee (RUC) be maintained, but the workflow should be shifted to review the commonly performed services at the May CPT/October RUC and the October CPT/January RUC in order to publish the proposed values for new, revised, and misvalued codes in the July MPFS Proposed Rules.
In addition, SVS recommended that the new timeline and process be delayed until 2017 to allow sufficient time to implement the revised process.
To download a PDF of additional comments on these and other issues contained in the 2015 MPFS Proposed rule, please visit vsweb.org/2015MPFS.
Recently, SVS submitted comments on the Centers for Medicare & Medicaid Services’ (CMS) Calendar Year (CY) 2015 Medicare Physician Fee Schedule (MPFS) Proposed Rule. The following are some of the issues that SVS addressed:
Improving the valuation and coding of the global package
The most controversial issue that SVS commented on was the proposal to transition all 10- and 90-day global bundles to 0-day global codes by 2018, with medically reasonable and necessary visits billed separately during the pre- and postoperative periods outside the day for surgical procedures. SVS opposed this proposal along with many other surgical societies.
Some of the reasons for opposition included:
▶ CMS has no idea how to implement this change in policy;
▶ The agency’s concerns regarding the accuracy of payments would not be addressed by this proposal;
▶ Patients would be subject to extra co-pays, particularly for postoperative care and additional administrative costs would be incurred for the extra billing;
▶ New values would need to be created for postoperative care because not all these procedures have separate codes;
▶ Many other services are being bundled – this creates unbundling for surgical care which is inconsistent with what CMS is proposing in other areas.
Using hospital outpatient prospective payment system (OPPS) and ambulatory surgery center (ASC) data in developing practice expense (PE) relative value units (RVU)
SVS thanked CMS for withdrawing the nonfacility cap proposal that was in the CY 2014 MPFS Proposed Rule and for acknowledging that “the comparison of OPPS or ASC payment amounts to PFS payment amounts for particular procedures is not the most appropriate or effective approach to ensuring that PFS payment rates are based on accurate cost assumptions.”
SVS continues to oppose using OPPS cost data for potential revisions of PFS PE methodology as there are many differences, which include using an averaging mechanism that undervalues high-cost items and overvalues low-cost items versus creating codes on actual costs. This would be a disservice to patients on many levels and not provide true costs for the work provided.
Abdominal aortic aneurysm (AAA) ultrasound screening – G0389
SVS members and staff met with CMS staff in May regarding AAA ultrasound screening reimbursement. G0389 had an undervalued Technical Component of $36.90, which was equivalent to retroperitoneal ultrasound that uses different equipment and takes less time to perform.
The low reimbursement also created a disincentive to provide this life-saving screening. CMS proposed maintaining the work RVU and reverting back to the same PE RVU used in 2013, which is now $80.24; SVS strongly agreed with this proposal.
Valuing new, revised and potentially misvalued codes
CMS proposed a new timeline and process for the publication and implementation of changes to physician codes which SVS supports as a more rational approach.
The current process announces proposed changes at the beginning of November and implements these changes on Jan. 1 of the following year. This does not allow adequate public comment or sufficient time for physicians to prepare for the changes, including a reasonable evaluation of how the revisions might impact their practices and patients.
To accommodate the proposed process, SVS recommended that the meeting structure for both the Current Procedural Terminology (CPT) Panel and Relative Value Update Committee (RUC) be maintained, but the workflow should be shifted to review the commonly performed services at the May CPT/October RUC and the October CPT/January RUC in order to publish the proposed values for new, revised, and misvalued codes in the July MPFS Proposed Rules.
In addition, SVS recommended that the new timeline and process be delayed until 2017 to allow sufficient time to implement the revised process.
To download a PDF of additional comments on these and other issues contained in the 2015 MPFS Proposed rule, please visit vsweb.org/2015MPFS.
Recently, SVS submitted comments on the Centers for Medicare & Medicaid Services’ (CMS) Calendar Year (CY) 2015 Medicare Physician Fee Schedule (MPFS) Proposed Rule. The following are some of the issues that SVS addressed:
Improving the valuation and coding of the global package
The most controversial issue that SVS commented on was the proposal to transition all 10- and 90-day global bundles to 0-day global codes by 2018, with medically reasonable and necessary visits billed separately during the pre- and postoperative periods outside the day for surgical procedures. SVS opposed this proposal along with many other surgical societies.
Some of the reasons for opposition included:
▶ CMS has no idea how to implement this change in policy;
▶ The agency’s concerns regarding the accuracy of payments would not be addressed by this proposal;
▶ Patients would be subject to extra co-pays, particularly for postoperative care and additional administrative costs would be incurred for the extra billing;
▶ New values would need to be created for postoperative care because not all these procedures have separate codes;
▶ Many other services are being bundled – this creates unbundling for surgical care which is inconsistent with what CMS is proposing in other areas.
Using hospital outpatient prospective payment system (OPPS) and ambulatory surgery center (ASC) data in developing practice expense (PE) relative value units (RVU)
SVS thanked CMS for withdrawing the nonfacility cap proposal that was in the CY 2014 MPFS Proposed Rule and for acknowledging that “the comparison of OPPS or ASC payment amounts to PFS payment amounts for particular procedures is not the most appropriate or effective approach to ensuring that PFS payment rates are based on accurate cost assumptions.”
SVS continues to oppose using OPPS cost data for potential revisions of PFS PE methodology as there are many differences, which include using an averaging mechanism that undervalues high-cost items and overvalues low-cost items versus creating codes on actual costs. This would be a disservice to patients on many levels and not provide true costs for the work provided.
Abdominal aortic aneurysm (AAA) ultrasound screening – G0389
SVS members and staff met with CMS staff in May regarding AAA ultrasound screening reimbursement. G0389 had an undervalued Technical Component of $36.90, which was equivalent to retroperitoneal ultrasound that uses different equipment and takes less time to perform.
The low reimbursement also created a disincentive to provide this life-saving screening. CMS proposed maintaining the work RVU and reverting back to the same PE RVU used in 2013, which is now $80.24; SVS strongly agreed with this proposal.
Valuing new, revised and potentially misvalued codes
CMS proposed a new timeline and process for the publication and implementation of changes to physician codes which SVS supports as a more rational approach.
The current process announces proposed changes at the beginning of November and implements these changes on Jan. 1 of the following year. This does not allow adequate public comment or sufficient time for physicians to prepare for the changes, including a reasonable evaluation of how the revisions might impact their practices and patients.
To accommodate the proposed process, SVS recommended that the meeting structure for both the Current Procedural Terminology (CPT) Panel and Relative Value Update Committee (RUC) be maintained, but the workflow should be shifted to review the commonly performed services at the May CPT/October RUC and the October CPT/January RUC in order to publish the proposed values for new, revised, and misvalued codes in the July MPFS Proposed Rules.
In addition, SVS recommended that the new timeline and process be delayed until 2017 to allow sufficient time to implement the revised process.
To download a PDF of additional comments on these and other issues contained in the 2015 MPFS Proposed rule, please visit vsweb.org/2015MPFS.
CDC program supplies guidance on Ebola patient management
Drawing upon the experience of Emory Healthcare in treating Ebola patients, the Centers for Disease Control and Prevention has issued a Clinician Outreach and Communication Activity program.
As hospitals in the United States begin to treat Ebola patients, a wide variety of processes including biological waste management, shipping processes, and lab testing are coming under scrutiny. At Emory, patients’ liquid wastes were disinfected with bleach or quaternary disinfecting detergents for more than 5 minutes prior to flushing or disposal. Linens and disposable material exposed to patients were handled as regulated medical waste and placed in leakproof containers. Additionally, Emory’s contractor called for a certification that materials were Ebola-free before transporting the waste to its incinerator.
Because sending a possible Ebola-contaminated specimen to the main hospital laboratory could cause that lab to be shut down for hours to address decontamination, Emory put in place a small point-of-care testing lab dedicated solely to specimens related to patients suspected of having Ebola. The dedicated lab included a chemistry analyzer, hematology analyzer, blood-gas analyzer, automated urinalysis analyzer, coagulation analyzer, and a malaria point-of-care device.
The Centers for Disease Control and Prevention has posted several online resources related to Ebola treatment and is expected to have a new web-based on-demand training video posted on Oct. 14.
Drawing upon the experience of Emory Healthcare in treating Ebola patients, the Centers for Disease Control and Prevention has issued a Clinician Outreach and Communication Activity program.
As hospitals in the United States begin to treat Ebola patients, a wide variety of processes including biological waste management, shipping processes, and lab testing are coming under scrutiny. At Emory, patients’ liquid wastes were disinfected with bleach or quaternary disinfecting detergents for more than 5 minutes prior to flushing or disposal. Linens and disposable material exposed to patients were handled as regulated medical waste and placed in leakproof containers. Additionally, Emory’s contractor called for a certification that materials were Ebola-free before transporting the waste to its incinerator.
Because sending a possible Ebola-contaminated specimen to the main hospital laboratory could cause that lab to be shut down for hours to address decontamination, Emory put in place a small point-of-care testing lab dedicated solely to specimens related to patients suspected of having Ebola. The dedicated lab included a chemistry analyzer, hematology analyzer, blood-gas analyzer, automated urinalysis analyzer, coagulation analyzer, and a malaria point-of-care device.
The Centers for Disease Control and Prevention has posted several online resources related to Ebola treatment and is expected to have a new web-based on-demand training video posted on Oct. 14.
Drawing upon the experience of Emory Healthcare in treating Ebola patients, the Centers for Disease Control and Prevention has issued a Clinician Outreach and Communication Activity program.
As hospitals in the United States begin to treat Ebola patients, a wide variety of processes including biological waste management, shipping processes, and lab testing are coming under scrutiny. At Emory, patients’ liquid wastes were disinfected with bleach or quaternary disinfecting detergents for more than 5 minutes prior to flushing or disposal. Linens and disposable material exposed to patients were handled as regulated medical waste and placed in leakproof containers. Additionally, Emory’s contractor called for a certification that materials were Ebola-free before transporting the waste to its incinerator.
Because sending a possible Ebola-contaminated specimen to the main hospital laboratory could cause that lab to be shut down for hours to address decontamination, Emory put in place a small point-of-care testing lab dedicated solely to specimens related to patients suspected of having Ebola. The dedicated lab included a chemistry analyzer, hematology analyzer, blood-gas analyzer, automated urinalysis analyzer, coagulation analyzer, and a malaria point-of-care device.
The Centers for Disease Control and Prevention has posted several online resources related to Ebola treatment and is expected to have a new web-based on-demand training video posted on Oct. 14.
CDC: 76 HCWs being monitored for Ebola in Dallas
The Centers for Disease Control and Prevention will now send response teams that include specialized nurse trainers to any hospital with an Ebola diagnosis.
In a press conference on Oct.14, CDC director Thomas Frieden said the response teams would consist of experts in infection control, nursing, laboratory science, management of Ebola units, infectious waste management, and personal protective equipment. Teams would deploy “within hours” of an Ebola diagnosis and assist with transfer of patients if necessary, Dr. Frieden said.
The announcement comes as concerns grow among health care workers that hospitals are neither prepared nor equipped to protect them from Ebola infection.
“We wish we had put a team like this on the ground the minute the [index] patient was diagnosed, but we will going forward,” Dr. Frieden said, referring to Thomas Eric Duncan, the first Ebola patient diagnosed in the United States, who died last week at Texas Health Presbyterian Hospital in Dallas. A nurse who treated Mr. Duncan, Nina Pham, became infected with Ebola and is now being treated at the same hospital. She is in stable condition.
Dr. Frieden said that 76 health care workers at Texas Health Presbyterian were now being actively monitored for symptoms of Ebola infection because of possible exposure to Mr. Duncan or his blood. This is in addition to 48 contacts of Mr. Duncan prior to his admission, and one contact of Ms. Pham’s, none of whom have shown evidence of infection.
Dr. Frieden acknowledged that the agency has not ruled out the possibility of transferring any newly diagnosed Ebola patients to one of four U.S. hospitals with special biocontainment units and Ebola expertise, such as Emory University Hospital in Atlanta. Expert nurses from Emory have recently arrived at Texas Health Presbyterian to perform Ebola-specific training.
The breach in infection control that led to Ms. Pham’s infection still has not been identified and may never be, Dr. Frieden said, but he highlighted the use of additional or excessive layers of protective gear and clothing as an area of concern. He also identified the proper use and removal of these as an intense focus of the CDC-led training efforts ongoing at Texas Health Presbyterian.
The Centers for Disease Control and Prevention will now send response teams that include specialized nurse trainers to any hospital with an Ebola diagnosis.
In a press conference on Oct.14, CDC director Thomas Frieden said the response teams would consist of experts in infection control, nursing, laboratory science, management of Ebola units, infectious waste management, and personal protective equipment. Teams would deploy “within hours” of an Ebola diagnosis and assist with transfer of patients if necessary, Dr. Frieden said.
The announcement comes as concerns grow among health care workers that hospitals are neither prepared nor equipped to protect them from Ebola infection.
“We wish we had put a team like this on the ground the minute the [index] patient was diagnosed, but we will going forward,” Dr. Frieden said, referring to Thomas Eric Duncan, the first Ebola patient diagnosed in the United States, who died last week at Texas Health Presbyterian Hospital in Dallas. A nurse who treated Mr. Duncan, Nina Pham, became infected with Ebola and is now being treated at the same hospital. She is in stable condition.
Dr. Frieden said that 76 health care workers at Texas Health Presbyterian were now being actively monitored for symptoms of Ebola infection because of possible exposure to Mr. Duncan or his blood. This is in addition to 48 contacts of Mr. Duncan prior to his admission, and one contact of Ms. Pham’s, none of whom have shown evidence of infection.
Dr. Frieden acknowledged that the agency has not ruled out the possibility of transferring any newly diagnosed Ebola patients to one of four U.S. hospitals with special biocontainment units and Ebola expertise, such as Emory University Hospital in Atlanta. Expert nurses from Emory have recently arrived at Texas Health Presbyterian to perform Ebola-specific training.
The breach in infection control that led to Ms. Pham’s infection still has not been identified and may never be, Dr. Frieden said, but he highlighted the use of additional or excessive layers of protective gear and clothing as an area of concern. He also identified the proper use and removal of these as an intense focus of the CDC-led training efforts ongoing at Texas Health Presbyterian.
The Centers for Disease Control and Prevention will now send response teams that include specialized nurse trainers to any hospital with an Ebola diagnosis.
In a press conference on Oct.14, CDC director Thomas Frieden said the response teams would consist of experts in infection control, nursing, laboratory science, management of Ebola units, infectious waste management, and personal protective equipment. Teams would deploy “within hours” of an Ebola diagnosis and assist with transfer of patients if necessary, Dr. Frieden said.
The announcement comes as concerns grow among health care workers that hospitals are neither prepared nor equipped to protect them from Ebola infection.
“We wish we had put a team like this on the ground the minute the [index] patient was diagnosed, but we will going forward,” Dr. Frieden said, referring to Thomas Eric Duncan, the first Ebola patient diagnosed in the United States, who died last week at Texas Health Presbyterian Hospital in Dallas. A nurse who treated Mr. Duncan, Nina Pham, became infected with Ebola and is now being treated at the same hospital. She is in stable condition.
Dr. Frieden said that 76 health care workers at Texas Health Presbyterian were now being actively monitored for symptoms of Ebola infection because of possible exposure to Mr. Duncan or his blood. This is in addition to 48 contacts of Mr. Duncan prior to his admission, and one contact of Ms. Pham’s, none of whom have shown evidence of infection.
Dr. Frieden acknowledged that the agency has not ruled out the possibility of transferring any newly diagnosed Ebola patients to one of four U.S. hospitals with special biocontainment units and Ebola expertise, such as Emory University Hospital in Atlanta. Expert nurses from Emory have recently arrived at Texas Health Presbyterian to perform Ebola-specific training.
The breach in infection control that led to Ms. Pham’s infection still has not been identified and may never be, Dr. Frieden said, but he highlighted the use of additional or excessive layers of protective gear and clothing as an area of concern. He also identified the proper use and removal of these as an intense focus of the CDC-led training efforts ongoing at Texas Health Presbyterian.
New test will speed enterovirus D68 case confirmation from weeks to days
A new lab test for enterovirus D68 is expected to speed up testing and confirmation of cases, according to a press release from the Centers for Disease Control and Prevention.
The new test is a “real-time” reverse transcription polymerase chain reaction and can identify all strains of EV-D68. The previous test could be used to detect almost any enterovirus, but was labor intensive to perform and not conducive to large-scale testing.
Of the 1,200 samples from 45 states sent to the CDC for EV-D68 testing between Aug. 1 to Oct. 10, less than 200 have been tested and about half have tested positive. The CDC now expects to be able to test around 180 samples a day and complete in 7-10 days the testing on samples received since mid-September, The new method will “reduce what would normally take several weeks to get results to a few days,” Dr. Anne Schuchat, assistant surgeon general and director of the CDC’s National Center for Immunization and Respiratory Diseases, said in the press release.
As with other enteroviruses, the CDC expects new cases of EV-D68 will decrease in the fall, but faster testing will more accurately show trends of the disease and will help to monitor changes in the outbreak as it winds down, according to the CDC press release.
A new lab test for enterovirus D68 is expected to speed up testing and confirmation of cases, according to a press release from the Centers for Disease Control and Prevention.
The new test is a “real-time” reverse transcription polymerase chain reaction and can identify all strains of EV-D68. The previous test could be used to detect almost any enterovirus, but was labor intensive to perform and not conducive to large-scale testing.
Of the 1,200 samples from 45 states sent to the CDC for EV-D68 testing between Aug. 1 to Oct. 10, less than 200 have been tested and about half have tested positive. The CDC now expects to be able to test around 180 samples a day and complete in 7-10 days the testing on samples received since mid-September, The new method will “reduce what would normally take several weeks to get results to a few days,” Dr. Anne Schuchat, assistant surgeon general and director of the CDC’s National Center for Immunization and Respiratory Diseases, said in the press release.
As with other enteroviruses, the CDC expects new cases of EV-D68 will decrease in the fall, but faster testing will more accurately show trends of the disease and will help to monitor changes in the outbreak as it winds down, according to the CDC press release.
A new lab test for enterovirus D68 is expected to speed up testing and confirmation of cases, according to a press release from the Centers for Disease Control and Prevention.
The new test is a “real-time” reverse transcription polymerase chain reaction and can identify all strains of EV-D68. The previous test could be used to detect almost any enterovirus, but was labor intensive to perform and not conducive to large-scale testing.
Of the 1,200 samples from 45 states sent to the CDC for EV-D68 testing between Aug. 1 to Oct. 10, less than 200 have been tested and about half have tested positive. The CDC now expects to be able to test around 180 samples a day and complete in 7-10 days the testing on samples received since mid-September, The new method will “reduce what would normally take several weeks to get results to a few days,” Dr. Anne Schuchat, assistant surgeon general and director of the CDC’s National Center for Immunization and Respiratory Diseases, said in the press release.
As with other enteroviruses, the CDC expects new cases of EV-D68 will decrease in the fall, but faster testing will more accurately show trends of the disease and will help to monitor changes in the outbreak as it winds down, according to the CDC press release.