User login
Initiatives Aim at Improving HIV and Mental Health Services
Two new HHS initiatives will expand health services for people with HIV and for people with mental health and substance use disorders.
A new 3-year multi-agency project, Partnerships for Care: Health Departments and Health Centers Collaborating to Improve HIV Health Outcomes, is putting $11 million toward integrating high-quality HIV services into primary care. Run jointly by the CDC and the Health Resources and Services Administration (HRSA), and funded through the Affordable Care Act and HHS Minority AIDS Initiative Fund, the program will develop innovative partnerships between health centers and state health departments in Florida, Massachusetts, Maryland, and New York. The HRSA-funded health centers will work with CDC-funded state health departments to expand the provision of HIV prevention, testing, care, and treatment services, especially among racial and ethnic minorities.
In June 2014, CDC awarded cooperative agreements to the 4 state health departments to begin putting the program into practice in communities most affected by HIV. Those health departments identified 22 health centers as their partners; the health centers are eligible to apply for funding to support workforce development, infrastructure development, HIV service delivery, partnership building, and quality improvement activities.
The HHS also announced $54.6 million in funding to support 221 health centers in 47 states and Puerto Rico to establish or expand behavioral health services for over 450,000 patients. The funds will be used for hiring new mental health professionals, adding mental health and substance use disorder health services, and employing integrated models of primary care.
In 2013, more than 1.2 million patients visited health centers for behavioral health services. The new grant funding, said HHS Secretary Sylvia M. Burwell, will “further reduce the barriers that too often prevent people from getting the help they need for mental health problems. Health centers with these awards are on the front lines of better integrating mental health into primary care and improving access to care through the Affordable Care Act.”
For more information on the projects and their funding, visit http://www.hrsa.gov/grants/apply/assistance/bphchiv and http://www.hrsa.gov/about/news/2014tables/behavioralhealth.
Two new HHS initiatives will expand health services for people with HIV and for people with mental health and substance use disorders.
A new 3-year multi-agency project, Partnerships for Care: Health Departments and Health Centers Collaborating to Improve HIV Health Outcomes, is putting $11 million toward integrating high-quality HIV services into primary care. Run jointly by the CDC and the Health Resources and Services Administration (HRSA), and funded through the Affordable Care Act and HHS Minority AIDS Initiative Fund, the program will develop innovative partnerships between health centers and state health departments in Florida, Massachusetts, Maryland, and New York. The HRSA-funded health centers will work with CDC-funded state health departments to expand the provision of HIV prevention, testing, care, and treatment services, especially among racial and ethnic minorities.
In June 2014, CDC awarded cooperative agreements to the 4 state health departments to begin putting the program into practice in communities most affected by HIV. Those health departments identified 22 health centers as their partners; the health centers are eligible to apply for funding to support workforce development, infrastructure development, HIV service delivery, partnership building, and quality improvement activities.
The HHS also announced $54.6 million in funding to support 221 health centers in 47 states and Puerto Rico to establish or expand behavioral health services for over 450,000 patients. The funds will be used for hiring new mental health professionals, adding mental health and substance use disorder health services, and employing integrated models of primary care.
In 2013, more than 1.2 million patients visited health centers for behavioral health services. The new grant funding, said HHS Secretary Sylvia M. Burwell, will “further reduce the barriers that too often prevent people from getting the help they need for mental health problems. Health centers with these awards are on the front lines of better integrating mental health into primary care and improving access to care through the Affordable Care Act.”
For more information on the projects and their funding, visit http://www.hrsa.gov/grants/apply/assistance/bphchiv and http://www.hrsa.gov/about/news/2014tables/behavioralhealth.
Two new HHS initiatives will expand health services for people with HIV and for people with mental health and substance use disorders.
A new 3-year multi-agency project, Partnerships for Care: Health Departments and Health Centers Collaborating to Improve HIV Health Outcomes, is putting $11 million toward integrating high-quality HIV services into primary care. Run jointly by the CDC and the Health Resources and Services Administration (HRSA), and funded through the Affordable Care Act and HHS Minority AIDS Initiative Fund, the program will develop innovative partnerships between health centers and state health departments in Florida, Massachusetts, Maryland, and New York. The HRSA-funded health centers will work with CDC-funded state health departments to expand the provision of HIV prevention, testing, care, and treatment services, especially among racial and ethnic minorities.
In June 2014, CDC awarded cooperative agreements to the 4 state health departments to begin putting the program into practice in communities most affected by HIV. Those health departments identified 22 health centers as their partners; the health centers are eligible to apply for funding to support workforce development, infrastructure development, HIV service delivery, partnership building, and quality improvement activities.
The HHS also announced $54.6 million in funding to support 221 health centers in 47 states and Puerto Rico to establish or expand behavioral health services for over 450,000 patients. The funds will be used for hiring new mental health professionals, adding mental health and substance use disorder health services, and employing integrated models of primary care.
In 2013, more than 1.2 million patients visited health centers for behavioral health services. The new grant funding, said HHS Secretary Sylvia M. Burwell, will “further reduce the barriers that too often prevent people from getting the help they need for mental health problems. Health centers with these awards are on the front lines of better integrating mental health into primary care and improving access to care through the Affordable Care Act.”
For more information on the projects and their funding, visit http://www.hrsa.gov/grants/apply/assistance/bphchiv and http://www.hrsa.gov/about/news/2014tables/behavioralhealth.
CDC confirms Ebola case in New York City
The Centers for Disease Control and Prevention confirmed Thursday that a medical aid worker who was hospitalized in New York City less than a week after returning from the West African nation of Guinea has tested positive for Ebola, marking the first case of the disease in the United States’ most populous city.
According to the CDC, the patient returned from Guinea on Oct. 17 and went through rigorous entry screening at JFK International Airport, at which time the patient did not exhibit any symptoms or signs of infection. On Thursday, the patient reported having a fever and was transported via a specially trained Hazardous Material Tactical (HAZ TAC) Unit to New York’s Bellevue Hospital, one of eight hospitals in the city designated to treat potential Ebola cases, where the patient currently remains in isolation.
The “CDC is in close communications with the New York City Health Department and Bellevue Hospital, and is providing technical assistance and resources,” the agency said in a statement, adding that a three-member CDC Ebola Response Team arrived in New York on Thursday as doctors conduct further testing and await lab results. This team will supplement an existing team of Ebola experts that CDC already had in place in New York and will work with Bellevue and other local hospitals to ensure that their Ebola protocols meet CDC standards.
The news of New York City’s first Ebola case comes shortly after the state of New York was designated as one of six that will launch CDC’s active monitoring program, which will require everyone coming to the United States from the Ebola-stricken nations of Guinea, Liberia, and Sierra Leone to remain in close daily contact with local health officials to ensure that they do not have or spread the disease. The other five states are Pennsylvania, Maryland, Virginia, New Jersey, and Georgia; together, these states account for 70% of incoming traffic from the afflicted West African nations.
The CDC and the New York City Health Department have interviewed the patient to gather information on any “close contacts and activities” since returning to the United States last week, according to the statement. To contract the disease, a person must come into contact with the bodily fluids of an infected individual who is symptomatic. People who come into contact with an Ebola patient but show no symptoms for 21 days are no longer at risk.
The Centers for Disease Control and Prevention confirmed Thursday that a medical aid worker who was hospitalized in New York City less than a week after returning from the West African nation of Guinea has tested positive for Ebola, marking the first case of the disease in the United States’ most populous city.
According to the CDC, the patient returned from Guinea on Oct. 17 and went through rigorous entry screening at JFK International Airport, at which time the patient did not exhibit any symptoms or signs of infection. On Thursday, the patient reported having a fever and was transported via a specially trained Hazardous Material Tactical (HAZ TAC) Unit to New York’s Bellevue Hospital, one of eight hospitals in the city designated to treat potential Ebola cases, where the patient currently remains in isolation.
The “CDC is in close communications with the New York City Health Department and Bellevue Hospital, and is providing technical assistance and resources,” the agency said in a statement, adding that a three-member CDC Ebola Response Team arrived in New York on Thursday as doctors conduct further testing and await lab results. This team will supplement an existing team of Ebola experts that CDC already had in place in New York and will work with Bellevue and other local hospitals to ensure that their Ebola protocols meet CDC standards.
The news of New York City’s first Ebola case comes shortly after the state of New York was designated as one of six that will launch CDC’s active monitoring program, which will require everyone coming to the United States from the Ebola-stricken nations of Guinea, Liberia, and Sierra Leone to remain in close daily contact with local health officials to ensure that they do not have or spread the disease. The other five states are Pennsylvania, Maryland, Virginia, New Jersey, and Georgia; together, these states account for 70% of incoming traffic from the afflicted West African nations.
The CDC and the New York City Health Department have interviewed the patient to gather information on any “close contacts and activities” since returning to the United States last week, according to the statement. To contract the disease, a person must come into contact with the bodily fluids of an infected individual who is symptomatic. People who come into contact with an Ebola patient but show no symptoms for 21 days are no longer at risk.
The Centers for Disease Control and Prevention confirmed Thursday that a medical aid worker who was hospitalized in New York City less than a week after returning from the West African nation of Guinea has tested positive for Ebola, marking the first case of the disease in the United States’ most populous city.
According to the CDC, the patient returned from Guinea on Oct. 17 and went through rigorous entry screening at JFK International Airport, at which time the patient did not exhibit any symptoms or signs of infection. On Thursday, the patient reported having a fever and was transported via a specially trained Hazardous Material Tactical (HAZ TAC) Unit to New York’s Bellevue Hospital, one of eight hospitals in the city designated to treat potential Ebola cases, where the patient currently remains in isolation.
The “CDC is in close communications with the New York City Health Department and Bellevue Hospital, and is providing technical assistance and resources,” the agency said in a statement, adding that a three-member CDC Ebola Response Team arrived in New York on Thursday as doctors conduct further testing and await lab results. This team will supplement an existing team of Ebola experts that CDC already had in place in New York and will work with Bellevue and other local hospitals to ensure that their Ebola protocols meet CDC standards.
The news of New York City’s first Ebola case comes shortly after the state of New York was designated as one of six that will launch CDC’s active monitoring program, which will require everyone coming to the United States from the Ebola-stricken nations of Guinea, Liberia, and Sierra Leone to remain in close daily contact with local health officials to ensure that they do not have or spread the disease. The other five states are Pennsylvania, Maryland, Virginia, New Jersey, and Georgia; together, these states account for 70% of incoming traffic from the afflicted West African nations.
The CDC and the New York City Health Department have interviewed the patient to gather information on any “close contacts and activities” since returning to the United States last week, according to the statement. To contract the disease, a person must come into contact with the bodily fluids of an infected individual who is symptomatic. People who come into contact with an Ebola patient but show no symptoms for 21 days are no longer at risk.
Health Care Is Coming to a “Crossing”
Between 2015 and 2020, for the first time, globally, the percentage of people aged ≥ 65 years will surpass the percentage of the very young (aged ≤ 4 years). It is an unprecedented event called “the crossing,” discussed in a special report, 65+ in the United States: 2010, commissioned by the NIH. Drawing on data from the 2010 U.S. census and other nationally representative surveys, the report covers a range of topics, from health to economic characteristics, to where most of the older people are living and moving to (the West and the South remain the most popular), down to their use of the Internet and voting habits.
What will that momentous shift mean for health care? The report aims to both anticipate and answer that question. Here are some of the highlights—and implications—of the data.
Rates of smoking and drinking among adults aged ≥ 65 years have declined, but rates of overweight and obese have increased. Between 2003 and 2006, 72% of men and 67% of women aged ≥ 65 years were overweight or obese. That’s one reason why rates of a variety of chronic diseases have increased in this age group. High blood pressure, heart disease, chronic lung disease, and diabetes increased between 1998 and 2008. In 2008, 41% of the older population had ≥ 3 chronic conditions, 51% had < 3. Only 8% had no chronic conditions. Moreover, more than one-third of Americans had ≥ 1 disability, most commonly affecting walking, climbing stairs, and doing errands alone. According to census data, about 50% of people aged ≥ 65 years have physical limitations, mostly caused by arthritis and other musculoskeletal disorders.
Life expectancy is still on the rise, the report says, and death rates have declined “dramatically.” The report expands on previous reports by more thoroughly discussing long-term care, nursing homes, and in-home care, including the impact the recent 2007-2009 recession had on older Americans. Skilled nursing facilities have been losing their share of patients, down from 4.5% in 2000 to 3.1% in 2010, while other long-term care facilities, such as assisted living, have been growing. Medicaid funds for long-term care have been shifting away from nursing homes, while funding for home- and community-based services has risen from 13% of total funding in 1990 to 43% in 2007. As the report points out, Medicaid can provide home- and community-based services to 3 people for the same cost as 1 patient in a nursing home.
Getting older has changed, and health care will need to change as well. Although many older people have chronic diseases or disabilities, not all do. Most (61%) older adults living in the traditional community (eg, in their own home) have no functional limitations, compared with 68% of adults in long-term care facilities, who have ≥ 3 activities of daily living (ADL) limitations. And now there’s a new generation of products designed to make getting around during older age easier. Assistive devices can help people stay in their homes longer—canes to help with mobility, shower benches for independent bathing, and other tools reduce the need for outside help. In the 1990s, the report notes, the proportion of chronically disabled older adults with ADL or instrumental ADL problems who were able to manage with only assistive devices rose, while use of personal care assistants declined.
Technology also can be an assistive device. Computer programs and electronic devices make it possible for adults with vision problems to read, and modified phones and appliances can help people with cognitive impairment or arthritis. And the report adds, the field of telemedicine can help patients receive health care in their homes.
Still, no matter how healthy baby boomers are as they age, there are a lot of them. An estimated 83.7 million people will be aged ≥ 65 years by 2050—one-fifth the U.S. population. And as in so many areas, they’re breaking new ground, including in how they’re cared for. Richard Suzman, director of the Division of Behavioral and Social Research at the National Institute on Aging, predicts “an approaching crisis in caregiving,” because the baby boomers had fewer children compared with their parents. Less than one-fifth of older people have the personal financial resources to live in a nursing home for > 3 years. Almost two-thirds cannot afford even 1 year. “Most of the long-term care provided to older people today comes from unpaid family members and friends,” Mr. Suzman said. As people live longer with conditions such as cancer, heart disease, and Alzheimer disease, that could become a “serious” issue.
Thus, the report discusses raising awareness of the issue of increasing populations of older adults. “We hope this report will serve as a useful resource to policymakers, researchers, educators, students, and the public at large,” said Enrique Lamas, the Census Bureau’s associate director for demographic programs. If nothing else, it should serve as a warning bell.
Between 2015 and 2020, for the first time, globally, the percentage of people aged ≥ 65 years will surpass the percentage of the very young (aged ≤ 4 years). It is an unprecedented event called “the crossing,” discussed in a special report, 65+ in the United States: 2010, commissioned by the NIH. Drawing on data from the 2010 U.S. census and other nationally representative surveys, the report covers a range of topics, from health to economic characteristics, to where most of the older people are living and moving to (the West and the South remain the most popular), down to their use of the Internet and voting habits.
What will that momentous shift mean for health care? The report aims to both anticipate and answer that question. Here are some of the highlights—and implications—of the data.
Rates of smoking and drinking among adults aged ≥ 65 years have declined, but rates of overweight and obese have increased. Between 2003 and 2006, 72% of men and 67% of women aged ≥ 65 years were overweight or obese. That’s one reason why rates of a variety of chronic diseases have increased in this age group. High blood pressure, heart disease, chronic lung disease, and diabetes increased between 1998 and 2008. In 2008, 41% of the older population had ≥ 3 chronic conditions, 51% had < 3. Only 8% had no chronic conditions. Moreover, more than one-third of Americans had ≥ 1 disability, most commonly affecting walking, climbing stairs, and doing errands alone. According to census data, about 50% of people aged ≥ 65 years have physical limitations, mostly caused by arthritis and other musculoskeletal disorders.
Life expectancy is still on the rise, the report says, and death rates have declined “dramatically.” The report expands on previous reports by more thoroughly discussing long-term care, nursing homes, and in-home care, including the impact the recent 2007-2009 recession had on older Americans. Skilled nursing facilities have been losing their share of patients, down from 4.5% in 2000 to 3.1% in 2010, while other long-term care facilities, such as assisted living, have been growing. Medicaid funds for long-term care have been shifting away from nursing homes, while funding for home- and community-based services has risen from 13% of total funding in 1990 to 43% in 2007. As the report points out, Medicaid can provide home- and community-based services to 3 people for the same cost as 1 patient in a nursing home.
Getting older has changed, and health care will need to change as well. Although many older people have chronic diseases or disabilities, not all do. Most (61%) older adults living in the traditional community (eg, in their own home) have no functional limitations, compared with 68% of adults in long-term care facilities, who have ≥ 3 activities of daily living (ADL) limitations. And now there’s a new generation of products designed to make getting around during older age easier. Assistive devices can help people stay in their homes longer—canes to help with mobility, shower benches for independent bathing, and other tools reduce the need for outside help. In the 1990s, the report notes, the proportion of chronically disabled older adults with ADL or instrumental ADL problems who were able to manage with only assistive devices rose, while use of personal care assistants declined.
Technology also can be an assistive device. Computer programs and electronic devices make it possible for adults with vision problems to read, and modified phones and appliances can help people with cognitive impairment or arthritis. And the report adds, the field of telemedicine can help patients receive health care in their homes.
Still, no matter how healthy baby boomers are as they age, there are a lot of them. An estimated 83.7 million people will be aged ≥ 65 years by 2050—one-fifth the U.S. population. And as in so many areas, they’re breaking new ground, including in how they’re cared for. Richard Suzman, director of the Division of Behavioral and Social Research at the National Institute on Aging, predicts “an approaching crisis in caregiving,” because the baby boomers had fewer children compared with their parents. Less than one-fifth of older people have the personal financial resources to live in a nursing home for > 3 years. Almost two-thirds cannot afford even 1 year. “Most of the long-term care provided to older people today comes from unpaid family members and friends,” Mr. Suzman said. As people live longer with conditions such as cancer, heart disease, and Alzheimer disease, that could become a “serious” issue.
Thus, the report discusses raising awareness of the issue of increasing populations of older adults. “We hope this report will serve as a useful resource to policymakers, researchers, educators, students, and the public at large,” said Enrique Lamas, the Census Bureau’s associate director for demographic programs. If nothing else, it should serve as a warning bell.
Between 2015 and 2020, for the first time, globally, the percentage of people aged ≥ 65 years will surpass the percentage of the very young (aged ≤ 4 years). It is an unprecedented event called “the crossing,” discussed in a special report, 65+ in the United States: 2010, commissioned by the NIH. Drawing on data from the 2010 U.S. census and other nationally representative surveys, the report covers a range of topics, from health to economic characteristics, to where most of the older people are living and moving to (the West and the South remain the most popular), down to their use of the Internet and voting habits.
What will that momentous shift mean for health care? The report aims to both anticipate and answer that question. Here are some of the highlights—and implications—of the data.
Rates of smoking and drinking among adults aged ≥ 65 years have declined, but rates of overweight and obese have increased. Between 2003 and 2006, 72% of men and 67% of women aged ≥ 65 years were overweight or obese. That’s one reason why rates of a variety of chronic diseases have increased in this age group. High blood pressure, heart disease, chronic lung disease, and diabetes increased between 1998 and 2008. In 2008, 41% of the older population had ≥ 3 chronic conditions, 51% had < 3. Only 8% had no chronic conditions. Moreover, more than one-third of Americans had ≥ 1 disability, most commonly affecting walking, climbing stairs, and doing errands alone. According to census data, about 50% of people aged ≥ 65 years have physical limitations, mostly caused by arthritis and other musculoskeletal disorders.
Life expectancy is still on the rise, the report says, and death rates have declined “dramatically.” The report expands on previous reports by more thoroughly discussing long-term care, nursing homes, and in-home care, including the impact the recent 2007-2009 recession had on older Americans. Skilled nursing facilities have been losing their share of patients, down from 4.5% in 2000 to 3.1% in 2010, while other long-term care facilities, such as assisted living, have been growing. Medicaid funds for long-term care have been shifting away from nursing homes, while funding for home- and community-based services has risen from 13% of total funding in 1990 to 43% in 2007. As the report points out, Medicaid can provide home- and community-based services to 3 people for the same cost as 1 patient in a nursing home.
Getting older has changed, and health care will need to change as well. Although many older people have chronic diseases or disabilities, not all do. Most (61%) older adults living in the traditional community (eg, in their own home) have no functional limitations, compared with 68% of adults in long-term care facilities, who have ≥ 3 activities of daily living (ADL) limitations. And now there’s a new generation of products designed to make getting around during older age easier. Assistive devices can help people stay in their homes longer—canes to help with mobility, shower benches for independent bathing, and other tools reduce the need for outside help. In the 1990s, the report notes, the proportion of chronically disabled older adults with ADL or instrumental ADL problems who were able to manage with only assistive devices rose, while use of personal care assistants declined.
Technology also can be an assistive device. Computer programs and electronic devices make it possible for adults with vision problems to read, and modified phones and appliances can help people with cognitive impairment or arthritis. And the report adds, the field of telemedicine can help patients receive health care in their homes.
Still, no matter how healthy baby boomers are as they age, there are a lot of them. An estimated 83.7 million people will be aged ≥ 65 years by 2050—one-fifth the U.S. population. And as in so many areas, they’re breaking new ground, including in how they’re cared for. Richard Suzman, director of the Division of Behavioral and Social Research at the National Institute on Aging, predicts “an approaching crisis in caregiving,” because the baby boomers had fewer children compared with their parents. Less than one-fifth of older people have the personal financial resources to live in a nursing home for > 3 years. Almost two-thirds cannot afford even 1 year. “Most of the long-term care provided to older people today comes from unpaid family members and friends,” Mr. Suzman said. As people live longer with conditions such as cancer, heart disease, and Alzheimer disease, that could become a “serious” issue.
Thus, the report discusses raising awareness of the issue of increasing populations of older adults. “We hope this report will serve as a useful resource to policymakers, researchers, educators, students, and the public at large,” said Enrique Lamas, the Census Bureau’s associate director for demographic programs. If nothing else, it should serve as a warning bell.
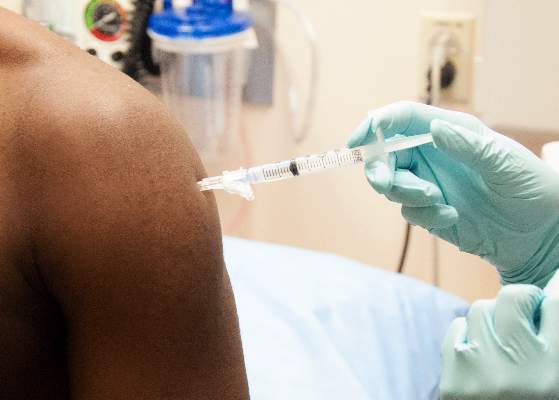
Nigeria and Senegal now free of Ebola, averting potential urban crisis
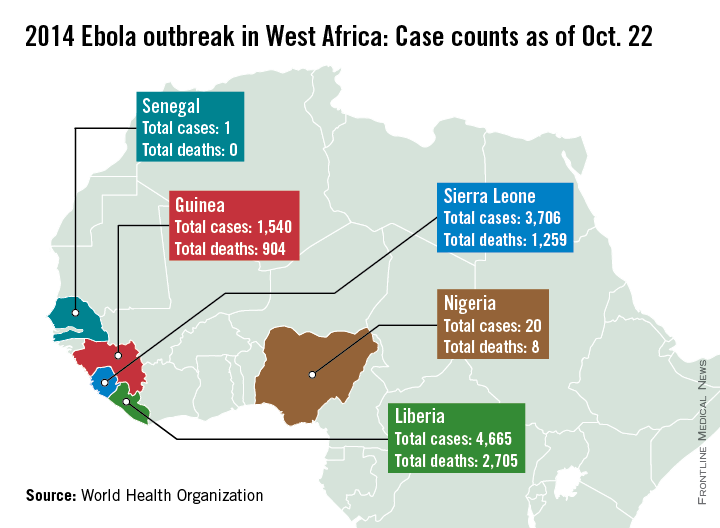
Nigeria and Senegal have officially been declared Ebola-free, according to the World Health Organization.
In Nigeria, there were 20 cases of Ebola and eight deaths, with the last case testing negative on Sept. 8. Nigeria represents a remarkable success story for containment of the disease, after cases were confirmed in Lagos, Africa’s largest city, the WHO said. With a population of 21 million, nearly the combined population of Liberia, Sierra Leone, and Guinea, and a large number of people living in slums, an outbreak in Lagos could have been catastrophic. But 100% of contacts in Lagos were reached and monitored, along with 99.8% of contacts in Port Harcourt, an important oil city and the second potential flash point. Nigeria was declared to be free of Ebola Oct. 20.
On Sept. 5, the man who was Senegal’s lone Ebola case tested negative for the disease, and after 42 days, no additional cases were reported in any of the monitored contacts, so on Oct. 17, the WHO officiallydeclaredSenegal Ebola free.
However, the outbreak continues to worsen in Guinea, Sierra Leone, and Liberia, with more than 9,900 cases and nearly 4,900 confirmed Ebola-related deaths as of Oct. 13. With more than 4,650 reported cases and more than 2,700 deaths, Liberia remains the hardest hit of the three nations. Sierra Leone has more than 3,700 cases and more than 1,250 deaths, and Guinea has reported nearly 1,550 cases and more than 900 deaths, the WHO reports.
No additional cases have been reported in the United States or in Spain, the other two countries with local transmission. Spain has had one case with no deaths, and the United States has had three cases with one death. On Oct. 21, the patient in Spain tested negative for the disease, and if no new cases appear, Spain will be declared free of Ebola 42 days later, according to the WHO.
The outbreak in the Democratic Republic of the Congo also seems to be under control, after extensive laboratory tests, 68 cases of Ebola have been confirmed with 49 deaths. The last confirmed case was isolated on Oct. 4, according to the WHO. More than 850 people have completed a 21-day follow-up, and about 270 are still being monitored. The outbreak in that country is unrelated to the outbreak in West Africa, according to the CDC.
Nigeria and Senegal have officially been declared Ebola-free, according to the World Health Organization.
In Nigeria, there were 20 cases of Ebola and eight deaths, with the last case testing negative on Sept. 8. Nigeria represents a remarkable success story for containment of the disease, after cases were confirmed in Lagos, Africa’s largest city, the WHO said. With a population of 21 million, nearly the combined population of Liberia, Sierra Leone, and Guinea, and a large number of people living in slums, an outbreak in Lagos could have been catastrophic. But 100% of contacts in Lagos were reached and monitored, along with 99.8% of contacts in Port Harcourt, an important oil city and the second potential flash point. Nigeria was declared to be free of Ebola Oct. 20.
On Sept. 5, the man who was Senegal’s lone Ebola case tested negative for the disease, and after 42 days, no additional cases were reported in any of the monitored contacts, so on Oct. 17, the WHO officiallydeclaredSenegal Ebola free.
However, the outbreak continues to worsen in Guinea, Sierra Leone, and Liberia, with more than 9,900 cases and nearly 4,900 confirmed Ebola-related deaths as of Oct. 13. With more than 4,650 reported cases and more than 2,700 deaths, Liberia remains the hardest hit of the three nations. Sierra Leone has more than 3,700 cases and more than 1,250 deaths, and Guinea has reported nearly 1,550 cases and more than 900 deaths, the WHO reports.
No additional cases have been reported in the United States or in Spain, the other two countries with local transmission. Spain has had one case with no deaths, and the United States has had three cases with one death. On Oct. 21, the patient in Spain tested negative for the disease, and if no new cases appear, Spain will be declared free of Ebola 42 days later, according to the WHO.
The outbreak in the Democratic Republic of the Congo also seems to be under control, after extensive laboratory tests, 68 cases of Ebola have been confirmed with 49 deaths. The last confirmed case was isolated on Oct. 4, according to the WHO. More than 850 people have completed a 21-day follow-up, and about 270 are still being monitored. The outbreak in that country is unrelated to the outbreak in West Africa, according to the CDC.

Nigeria and Senegal have officially been declared Ebola-free, according to the World Health Organization.
In Nigeria, there were 20 cases of Ebola and eight deaths, with the last case testing negative on Sept. 8. Nigeria represents a remarkable success story for containment of the disease, after cases were confirmed in Lagos, Africa’s largest city, the WHO said. With a population of 21 million, nearly the combined population of Liberia, Sierra Leone, and Guinea, and a large number of people living in slums, an outbreak in Lagos could have been catastrophic. But 100% of contacts in Lagos were reached and monitored, along with 99.8% of contacts in Port Harcourt, an important oil city and the second potential flash point. Nigeria was declared to be free of Ebola Oct. 20.
On Sept. 5, the man who was Senegal’s lone Ebola case tested negative for the disease, and after 42 days, no additional cases were reported in any of the monitored contacts, so on Oct. 17, the WHO officiallydeclaredSenegal Ebola free.
However, the outbreak continues to worsen in Guinea, Sierra Leone, and Liberia, with more than 9,900 cases and nearly 4,900 confirmed Ebola-related deaths as of Oct. 13. With more than 4,650 reported cases and more than 2,700 deaths, Liberia remains the hardest hit of the three nations. Sierra Leone has more than 3,700 cases and more than 1,250 deaths, and Guinea has reported nearly 1,550 cases and more than 900 deaths, the WHO reports.
No additional cases have been reported in the United States or in Spain, the other two countries with local transmission. Spain has had one case with no deaths, and the United States has had three cases with one death. On Oct. 21, the patient in Spain tested negative for the disease, and if no new cases appear, Spain will be declared free of Ebola 42 days later, according to the WHO.
The outbreak in the Democratic Republic of the Congo also seems to be under control, after extensive laboratory tests, 68 cases of Ebola have been confirmed with 49 deaths. The last confirmed case was isolated on Oct. 4, according to the WHO. More than 850 people have completed a 21-day follow-up, and about 270 are still being monitored. The outbreak in that country is unrelated to the outbreak in West Africa, according to the CDC.

CDC announces monitoring of travelers from Ebola-stricken countries
Travelers arriving in the United States from any of three Ebola-stricken African countries will be monitored for symptoms for 21 days, the Centers for Disease Control and Prevention has announced.
The program, which will commence on Oct. 27, will require travelers from Liberia, Guinea, and Sierra Leone to check in with local public health officials on a daily basis to ensure that they have not contracted the Ebola virus. If Ebola symptoms are absent at the end of the 21-day monitoring period, the traveler is free of the virus and poses no threat to the general public.
“The bottom line here is that we have to keep up our guard against Ebola,” said Dr. Tom Frieden, director of the Centers for Disease Control and Prevention (CDC), during a teleconference with members of the media. “These additional steps will protect families, communities, and health care workers.”
The CDC and local health agencies will collect e-mail addresses, telephone numbers, and addresses from travelers to monitor them when they enter the United States. Similar information for a friend or relative in the United States also will be noted. Once within the country, passengers will be required to check in with local health agencies every day to report their temperature and any flulike symptoms, and will have to coordinate with the relevant public health officials if they plan to do any additional traveling within the United States.
Hospitals and other medical facilities are continuing to be instructed on the best ways to transport patients with suspected Ebola and care for those who are infected. A 24/7 CDC hotline also will be available for anyone who needs more information, and all travelers from the affected West African nations will be given Ebola “care kits” as well. Each kit includes a thermometer, temperature tracking log, and instructions on whom to contact if symptoms or fever develops.
The active monitoring programs will initially launch in just six states: New York, Pennsylvania, Maryland, Virginia, New Jersey, and Georgia. These states, according to the CDC, are where roughly 70% of travelers from West Africa end up, although the CDC will continue to work with all other states to set up active monitoring programs.
The announcement comes just 1 day after the CDC released from a 21-day monitoring period those individuals who came into contact with Thomas Eric Duncan, the first Ebola patient in the United States. Mr. Duncan died Oct. 8.
Travelers arriving in the United States from any of three Ebola-stricken African countries will be monitored for symptoms for 21 days, the Centers for Disease Control and Prevention has announced.
The program, which will commence on Oct. 27, will require travelers from Liberia, Guinea, and Sierra Leone to check in with local public health officials on a daily basis to ensure that they have not contracted the Ebola virus. If Ebola symptoms are absent at the end of the 21-day monitoring period, the traveler is free of the virus and poses no threat to the general public.
“The bottom line here is that we have to keep up our guard against Ebola,” said Dr. Tom Frieden, director of the Centers for Disease Control and Prevention (CDC), during a teleconference with members of the media. “These additional steps will protect families, communities, and health care workers.”
The CDC and local health agencies will collect e-mail addresses, telephone numbers, and addresses from travelers to monitor them when they enter the United States. Similar information for a friend or relative in the United States also will be noted. Once within the country, passengers will be required to check in with local health agencies every day to report their temperature and any flulike symptoms, and will have to coordinate with the relevant public health officials if they plan to do any additional traveling within the United States.
Hospitals and other medical facilities are continuing to be instructed on the best ways to transport patients with suspected Ebola and care for those who are infected. A 24/7 CDC hotline also will be available for anyone who needs more information, and all travelers from the affected West African nations will be given Ebola “care kits” as well. Each kit includes a thermometer, temperature tracking log, and instructions on whom to contact if symptoms or fever develops.
The active monitoring programs will initially launch in just six states: New York, Pennsylvania, Maryland, Virginia, New Jersey, and Georgia. These states, according to the CDC, are where roughly 70% of travelers from West Africa end up, although the CDC will continue to work with all other states to set up active monitoring programs.
The announcement comes just 1 day after the CDC released from a 21-day monitoring period those individuals who came into contact with Thomas Eric Duncan, the first Ebola patient in the United States. Mr. Duncan died Oct. 8.
Travelers arriving in the United States from any of three Ebola-stricken African countries will be monitored for symptoms for 21 days, the Centers for Disease Control and Prevention has announced.
The program, which will commence on Oct. 27, will require travelers from Liberia, Guinea, and Sierra Leone to check in with local public health officials on a daily basis to ensure that they have not contracted the Ebola virus. If Ebola symptoms are absent at the end of the 21-day monitoring period, the traveler is free of the virus and poses no threat to the general public.
“The bottom line here is that we have to keep up our guard against Ebola,” said Dr. Tom Frieden, director of the Centers for Disease Control and Prevention (CDC), during a teleconference with members of the media. “These additional steps will protect families, communities, and health care workers.”
The CDC and local health agencies will collect e-mail addresses, telephone numbers, and addresses from travelers to monitor them when they enter the United States. Similar information for a friend or relative in the United States also will be noted. Once within the country, passengers will be required to check in with local health agencies every day to report their temperature and any flulike symptoms, and will have to coordinate with the relevant public health officials if they plan to do any additional traveling within the United States.
Hospitals and other medical facilities are continuing to be instructed on the best ways to transport patients with suspected Ebola and care for those who are infected. A 24/7 CDC hotline also will be available for anyone who needs more information, and all travelers from the affected West African nations will be given Ebola “care kits” as well. Each kit includes a thermometer, temperature tracking log, and instructions on whom to contact if symptoms or fever develops.
The active monitoring programs will initially launch in just six states: New York, Pennsylvania, Maryland, Virginia, New Jersey, and Georgia. These states, according to the CDC, are where roughly 70% of travelers from West Africa end up, although the CDC will continue to work with all other states to set up active monitoring programs.
The announcement comes just 1 day after the CDC released from a 21-day monitoring period those individuals who came into contact with Thomas Eric Duncan, the first Ebola patient in the United States. Mr. Duncan died Oct. 8.
FROM A CDC TELECONFERENCE
Funding for Adaptive Sports
The VA has announced $8 million in grants for adaptive sports programs for disabled veterans and service members. The money can be used for development, training, equipment, recreation therapists, coaches, sports equipment, supplies, and other program components. The amount of funding available for each eligible organization varies between calendar years, but organizations may apply for more than 1 grant annually.
Studies have shown that adaptive sports and recreation can help with the transition from active duty to being home with a disability. One study, for instance, found that sports programs can help participants develop a sense of competence and “may also lead to a feeling of coherence between life prior to acquiring a disability and their current situation.” A study of 18 Iraq and Afghanistan War veterans who participated in weeklong therapeutic and adaptive sports programs found significant pre- and posttest differences in mood states (eg, tension, depression, anger) and vigor. The researchers also found a “promising trend” regarding improvement in quality of life and psychological health.
Risk and physical exertion are part of many service members’ daily routines, and adaptive sports can help them by calling on the same skills after they have returned home. In announcing the grant, then VA Acting Secretary Sloan Gibson said, “Adaptive sports can help veterans confront challenges and redefine their capabilities, which is critical to successful rehabilitation.”
For more information about the VA’s adaptive sports initiatives, visit http://www.va.gov/adaptivesports.
The VA has announced $8 million in grants for adaptive sports programs for disabled veterans and service members. The money can be used for development, training, equipment, recreation therapists, coaches, sports equipment, supplies, and other program components. The amount of funding available for each eligible organization varies between calendar years, but organizations may apply for more than 1 grant annually.
Studies have shown that adaptive sports and recreation can help with the transition from active duty to being home with a disability. One study, for instance, found that sports programs can help participants develop a sense of competence and “may also lead to a feeling of coherence between life prior to acquiring a disability and their current situation.” A study of 18 Iraq and Afghanistan War veterans who participated in weeklong therapeutic and adaptive sports programs found significant pre- and posttest differences in mood states (eg, tension, depression, anger) and vigor. The researchers also found a “promising trend” regarding improvement in quality of life and psychological health.
Risk and physical exertion are part of many service members’ daily routines, and adaptive sports can help them by calling on the same skills after they have returned home. In announcing the grant, then VA Acting Secretary Sloan Gibson said, “Adaptive sports can help veterans confront challenges and redefine their capabilities, which is critical to successful rehabilitation.”
For more information about the VA’s adaptive sports initiatives, visit http://www.va.gov/adaptivesports.
The VA has announced $8 million in grants for adaptive sports programs for disabled veterans and service members. The money can be used for development, training, equipment, recreation therapists, coaches, sports equipment, supplies, and other program components. The amount of funding available for each eligible organization varies between calendar years, but organizations may apply for more than 1 grant annually.
Studies have shown that adaptive sports and recreation can help with the transition from active duty to being home with a disability. One study, for instance, found that sports programs can help participants develop a sense of competence and “may also lead to a feeling of coherence between life prior to acquiring a disability and their current situation.” A study of 18 Iraq and Afghanistan War veterans who participated in weeklong therapeutic and adaptive sports programs found significant pre- and posttest differences in mood states (eg, tension, depression, anger) and vigor. The researchers also found a “promising trend” regarding improvement in quality of life and psychological health.
Risk and physical exertion are part of many service members’ daily routines, and adaptive sports can help them by calling on the same skills after they have returned home. In announcing the grant, then VA Acting Secretary Sloan Gibson said, “Adaptive sports can help veterans confront challenges and redefine their capabilities, which is critical to successful rehabilitation.”
For more information about the VA’s adaptive sports initiatives, visit http://www.va.gov/adaptivesports.
WHO: Ebola vaccines may reach Africa in January
A top scientist with the World Health Organization says that the two leading Ebola vaccine candidates were close to being tested in healthy volunteers, and that if an ideal dosage can be successfully established in the coming weeks, clinical efficacy testing could begin in Africa in January.
Marie-Paule Kieny, Ph.D., WHO’s assistant director-general for health systems and innovation, told reporters at a press conference Oct. 21 that the two experimental live attenuated vaccines, one developed by GlaxoSmithKline and the other by the Canadian government, would be tested in hundreds of healthy volunteers, most of them in Europe, starting in the next 2 weeks. This round of testing involves a range of doses to determine the ideal dose for safety and immunogenicity.
Some 800 vials of the Canadian vaccine have been sent to WHO’s offices in Geneva for distribution, Dr. Kieny said, and will be forwarded to the testing sites in Germany and Switzerland within days, along with sites in Kenya and Gabon.
With both vaccines, “we should know dose levels by December,” Dr. Kieny said, “and these data will be absolutely crucial to allow decision-making in determining what dose level should go in efficacy testing in Africa allowing for trials of the vaccines in Africa to begin as soon as January.”
Clinical efficacy testing would most likely begin with people at highest risk: health care workers, burial teams, family members, and contacts of known Ebola cases. “These possible targets are being discussed now,” Dr. Kieny said.
Dr. Kieny also noted that there were additional vaccine candidates in advanced stages of development in the United States and Russia.
One Ebola treatment strategy currently being investigated involves transfusions of blood and serum of convalescent patients. In Liberia, progress in collecting and processing blood “is moving quickly,” Dr. Kieny reported. WHO is also in discussions with facilities in Guinea and Sierra Leone, the other two countries where Ebola transmission is intense, to set up blood-processing centers.
On the drug front, Dr. Kieny said that an antiviral agent developed in Japan is about to undergo efficacy testing in Guinea, which would represent the first formal clinical drug trial in this Ebola outbreak, though experimental agents have been used ad-hoc to date.
A top scientist with the World Health Organization says that the two leading Ebola vaccine candidates were close to being tested in healthy volunteers, and that if an ideal dosage can be successfully established in the coming weeks, clinical efficacy testing could begin in Africa in January.
Marie-Paule Kieny, Ph.D., WHO’s assistant director-general for health systems and innovation, told reporters at a press conference Oct. 21 that the two experimental live attenuated vaccines, one developed by GlaxoSmithKline and the other by the Canadian government, would be tested in hundreds of healthy volunteers, most of them in Europe, starting in the next 2 weeks. This round of testing involves a range of doses to determine the ideal dose for safety and immunogenicity.
Some 800 vials of the Canadian vaccine have been sent to WHO’s offices in Geneva for distribution, Dr. Kieny said, and will be forwarded to the testing sites in Germany and Switzerland within days, along with sites in Kenya and Gabon.
With both vaccines, “we should know dose levels by December,” Dr. Kieny said, “and these data will be absolutely crucial to allow decision-making in determining what dose level should go in efficacy testing in Africa allowing for trials of the vaccines in Africa to begin as soon as January.”
Clinical efficacy testing would most likely begin with people at highest risk: health care workers, burial teams, family members, and contacts of known Ebola cases. “These possible targets are being discussed now,” Dr. Kieny said.
Dr. Kieny also noted that there were additional vaccine candidates in advanced stages of development in the United States and Russia.
One Ebola treatment strategy currently being investigated involves transfusions of blood and serum of convalescent patients. In Liberia, progress in collecting and processing blood “is moving quickly,” Dr. Kieny reported. WHO is also in discussions with facilities in Guinea and Sierra Leone, the other two countries where Ebola transmission is intense, to set up blood-processing centers.
On the drug front, Dr. Kieny said that an antiviral agent developed in Japan is about to undergo efficacy testing in Guinea, which would represent the first formal clinical drug trial in this Ebola outbreak, though experimental agents have been used ad-hoc to date.
A top scientist with the World Health Organization says that the two leading Ebola vaccine candidates were close to being tested in healthy volunteers, and that if an ideal dosage can be successfully established in the coming weeks, clinical efficacy testing could begin in Africa in January.
Marie-Paule Kieny, Ph.D., WHO’s assistant director-general for health systems and innovation, told reporters at a press conference Oct. 21 that the two experimental live attenuated vaccines, one developed by GlaxoSmithKline and the other by the Canadian government, would be tested in hundreds of healthy volunteers, most of them in Europe, starting in the next 2 weeks. This round of testing involves a range of doses to determine the ideal dose for safety and immunogenicity.
Some 800 vials of the Canadian vaccine have been sent to WHO’s offices in Geneva for distribution, Dr. Kieny said, and will be forwarded to the testing sites in Germany and Switzerland within days, along with sites in Kenya and Gabon.
With both vaccines, “we should know dose levels by December,” Dr. Kieny said, “and these data will be absolutely crucial to allow decision-making in determining what dose level should go in efficacy testing in Africa allowing for trials of the vaccines in Africa to begin as soon as January.”
Clinical efficacy testing would most likely begin with people at highest risk: health care workers, burial teams, family members, and contacts of known Ebola cases. “These possible targets are being discussed now,” Dr. Kieny said.
Dr. Kieny also noted that there were additional vaccine candidates in advanced stages of development in the United States and Russia.
One Ebola treatment strategy currently being investigated involves transfusions of blood and serum of convalescent patients. In Liberia, progress in collecting and processing blood “is moving quickly,” Dr. Kieny reported. WHO is also in discussions with facilities in Guinea and Sierra Leone, the other two countries where Ebola transmission is intense, to set up blood-processing centers.
On the drug front, Dr. Kieny said that an antiviral agent developed in Japan is about to undergo efficacy testing in Guinea, which would represent the first formal clinical drug trial in this Ebola outbreak, though experimental agents have been used ad-hoc to date.
CDC updates guidance on protecting health care workers from Ebola
The Centers for Disease Control and Prevention has put forward a revised series of recommendations on the use of personal protective equipment in treating Ebola patients.
The new guidance, issued Oct. 20, closely mirrors the Ebola personal protective equipment (PPE) guidance issued by MSF (Doctors Without Borders).
The CDC’s recommendations update the PPE guidance issued Aug. 1 that is now widely considered inadequate. The new recommendations advise, among other things, that health care workers have no skin exposed, that they be supervised while donning and removing PPE, and that rigorous training and practice accompany any use of PPE in the treatment of patients with Ebola.
Specific equipment recommendations include double gloves, waterproof boot covers to mid-calf or higher, and a disposable fluid-resistant or impermeable gown that extends to at least mid-calf, or a coverall without hood. The agency also recommends the use of respirators (N95 or powered air purifying), disposable single-use full-face shields in lieu of goggles, surgical hoods for complete coverage of the head and neck, and a waterproof apron extending from torso to mid-calf if patients have vomiting or diarrhea.
The guidance specifies that hospitals must have designated areas for putting on and taking off PPE and that trained observers monitor all donning and removal. The guidance also contains instructions for disinfecting PPE prior to its removal.
The agency emphasized that training, not merely having the correct equipment in place, was key to the successful use of PPE. “Focusing only on PPE gives a false sense of security of safe care and worker safety,” the CDC stated. “Training is a critical aspect of ensuring infection control.”
Facilities must make sure all health care providers practice “numerous times” until they understand how to properly and safely use the equipment, the CDC advised.
The Centers for Disease Control and Prevention has put forward a revised series of recommendations on the use of personal protective equipment in treating Ebola patients.
The new guidance, issued Oct. 20, closely mirrors the Ebola personal protective equipment (PPE) guidance issued by MSF (Doctors Without Borders).
The CDC’s recommendations update the PPE guidance issued Aug. 1 that is now widely considered inadequate. The new recommendations advise, among other things, that health care workers have no skin exposed, that they be supervised while donning and removing PPE, and that rigorous training and practice accompany any use of PPE in the treatment of patients with Ebola.
Specific equipment recommendations include double gloves, waterproof boot covers to mid-calf or higher, and a disposable fluid-resistant or impermeable gown that extends to at least mid-calf, or a coverall without hood. The agency also recommends the use of respirators (N95 or powered air purifying), disposable single-use full-face shields in lieu of goggles, surgical hoods for complete coverage of the head and neck, and a waterproof apron extending from torso to mid-calf if patients have vomiting or diarrhea.
The guidance specifies that hospitals must have designated areas for putting on and taking off PPE and that trained observers monitor all donning and removal. The guidance also contains instructions for disinfecting PPE prior to its removal.
The agency emphasized that training, not merely having the correct equipment in place, was key to the successful use of PPE. “Focusing only on PPE gives a false sense of security of safe care and worker safety,” the CDC stated. “Training is a critical aspect of ensuring infection control.”
Facilities must make sure all health care providers practice “numerous times” until they understand how to properly and safely use the equipment, the CDC advised.
The Centers for Disease Control and Prevention has put forward a revised series of recommendations on the use of personal protective equipment in treating Ebola patients.
The new guidance, issued Oct. 20, closely mirrors the Ebola personal protective equipment (PPE) guidance issued by MSF (Doctors Without Borders).
The CDC’s recommendations update the PPE guidance issued Aug. 1 that is now widely considered inadequate. The new recommendations advise, among other things, that health care workers have no skin exposed, that they be supervised while donning and removing PPE, and that rigorous training and practice accompany any use of PPE in the treatment of patients with Ebola.
Specific equipment recommendations include double gloves, waterproof boot covers to mid-calf or higher, and a disposable fluid-resistant or impermeable gown that extends to at least mid-calf, or a coverall without hood. The agency also recommends the use of respirators (N95 or powered air purifying), disposable single-use full-face shields in lieu of goggles, surgical hoods for complete coverage of the head and neck, and a waterproof apron extending from torso to mid-calf if patients have vomiting or diarrhea.
The guidance specifies that hospitals must have designated areas for putting on and taking off PPE and that trained observers monitor all donning and removal. The guidance also contains instructions for disinfecting PPE prior to its removal.
The agency emphasized that training, not merely having the correct equipment in place, was key to the successful use of PPE. “Focusing only on PPE gives a false sense of security of safe care and worker safety,” the CDC stated. “Training is a critical aspect of ensuring infection control.”
Facilities must make sure all health care providers practice “numerous times” until they understand how to properly and safely use the equipment, the CDC advised.
Bioengineered Brain Tissue: A Research Breakthrough
Bioengineers at Tufts University in Boston, Massachusetts have created 3-dimensional (3D), functional brainlike tissue that can be kept alive in a laboratory for more than 2 months. It is a major research achievement that promises to advance research into brain injury and disease.
The tissue was developed at the Tufts Tissue Engineering Resource Center, which is funded by the National Institute of Biomedical Imaging and Bioengineering (NIBIB). The researchers generated the brainlike tissue by creating a novel composite structure of 2 biomaterials: a spongy scaffold of silk protein that neurons can attach to and a softer, collagen-based gel to encourage axon growth.
The 3D aspect of the new tissue represents a step beyond the current research situation, in which scientists grow neurons in petri dishes. Neurons grown that way can’t duplicate the compartmentalization of gray and white matter in the brain, which is critical to research into brain injuries and diseases that affect those areas differently. Moreover, attempts to grow neurons in 3D gel environments have produced tissue models that don’t allow for tissue-level function, according to a NIBIB release. By contrast, neurons in the 3D tissue act more like those seen in a rat brain, with similar electrical activity and responsiveness to stimuli such as neurotoxins. The gel-based neurons begin to deteriorate within 24 hours.
The longevity and functionality of the new tissue allow researchers to track tissue response and repair in real time, over longer periods. David Kaplan, PhD, director of the Tufts Tissue Engineering Resource Center and lead investigator, said, “The fact that we can maintain this tissue for months in the lab means we can start to look at neurological diseases in ways that you can’t otherwise because you need long timeframes to study some of the key brain diseases.”
The discovery could bring new treatments for veterans with brain injuries. In early experiments, the researchers studied chemical and electrical changes that immediately follow traumatic brain injury and changes in the brain as it responds to a drug. Calling the work “an exceptional feat,” Rosemarie Hunziker, PhD, program director of Tissue Engineering at NIBIB, said, “The hope is that use of this model could lead to an acceleration of therapies for brain dysfunction.”
Bioengineers at Tufts University in Boston, Massachusetts have created 3-dimensional (3D), functional brainlike tissue that can be kept alive in a laboratory for more than 2 months. It is a major research achievement that promises to advance research into brain injury and disease.
The tissue was developed at the Tufts Tissue Engineering Resource Center, which is funded by the National Institute of Biomedical Imaging and Bioengineering (NIBIB). The researchers generated the brainlike tissue by creating a novel composite structure of 2 biomaterials: a spongy scaffold of silk protein that neurons can attach to and a softer, collagen-based gel to encourage axon growth.
The 3D aspect of the new tissue represents a step beyond the current research situation, in which scientists grow neurons in petri dishes. Neurons grown that way can’t duplicate the compartmentalization of gray and white matter in the brain, which is critical to research into brain injuries and diseases that affect those areas differently. Moreover, attempts to grow neurons in 3D gel environments have produced tissue models that don’t allow for tissue-level function, according to a NIBIB release. By contrast, neurons in the 3D tissue act more like those seen in a rat brain, with similar electrical activity and responsiveness to stimuli such as neurotoxins. The gel-based neurons begin to deteriorate within 24 hours.
The longevity and functionality of the new tissue allow researchers to track tissue response and repair in real time, over longer periods. David Kaplan, PhD, director of the Tufts Tissue Engineering Resource Center and lead investigator, said, “The fact that we can maintain this tissue for months in the lab means we can start to look at neurological diseases in ways that you can’t otherwise because you need long timeframes to study some of the key brain diseases.”
The discovery could bring new treatments for veterans with brain injuries. In early experiments, the researchers studied chemical and electrical changes that immediately follow traumatic brain injury and changes in the brain as it responds to a drug. Calling the work “an exceptional feat,” Rosemarie Hunziker, PhD, program director of Tissue Engineering at NIBIB, said, “The hope is that use of this model could lead to an acceleration of therapies for brain dysfunction.”
Bioengineers at Tufts University in Boston, Massachusetts have created 3-dimensional (3D), functional brainlike tissue that can be kept alive in a laboratory for more than 2 months. It is a major research achievement that promises to advance research into brain injury and disease.
The tissue was developed at the Tufts Tissue Engineering Resource Center, which is funded by the National Institute of Biomedical Imaging and Bioengineering (NIBIB). The researchers generated the brainlike tissue by creating a novel composite structure of 2 biomaterials: a spongy scaffold of silk protein that neurons can attach to and a softer, collagen-based gel to encourage axon growth.
The 3D aspect of the new tissue represents a step beyond the current research situation, in which scientists grow neurons in petri dishes. Neurons grown that way can’t duplicate the compartmentalization of gray and white matter in the brain, which is critical to research into brain injuries and diseases that affect those areas differently. Moreover, attempts to grow neurons in 3D gel environments have produced tissue models that don’t allow for tissue-level function, according to a NIBIB release. By contrast, neurons in the 3D tissue act more like those seen in a rat brain, with similar electrical activity and responsiveness to stimuli such as neurotoxins. The gel-based neurons begin to deteriorate within 24 hours.
The longevity and functionality of the new tissue allow researchers to track tissue response and repair in real time, over longer periods. David Kaplan, PhD, director of the Tufts Tissue Engineering Resource Center and lead investigator, said, “The fact that we can maintain this tissue for months in the lab means we can start to look at neurological diseases in ways that you can’t otherwise because you need long timeframes to study some of the key brain diseases.”
The discovery could bring new treatments for veterans with brain injuries. In early experiments, the researchers studied chemical and electrical changes that immediately follow traumatic brain injury and changes in the brain as it responds to a drug. Calling the work “an exceptional feat,” Rosemarie Hunziker, PhD, program director of Tissue Engineering at NIBIB, said, “The hope is that use of this model could lead to an acceleration of therapies for brain dysfunction.”
FDA approves new abuse-prevention labeling for extended-release Embeda
The Food and Drug Administration has approved new labeling for the opioid analgesic Embeda to include information about the drug’s abuse-preventive properties, the agency announced Oct. 17.
The extended-release morphine sulfate and naltrexone hydrochloride tablets are used to treat severe, long-term pain resistant to alternative treatment options. Embeda acts as an abuse deterrent by releasing morphine only when taken properly as a whole capsule, and not when crushed or snorted, the FDA said in a statement.
“When crushed, the naltrexone in Embeda blocks some of the euphoric effects of the morphine and can precipitate withdrawal in persons dependent on opioids,” according to the announcement.
However, the drug can still be abused when taken as a whole capsule, because the naltrexone does not effectively block the euphoric morphine effects when consumed properly, the FDA said. Such misuse could result in overdose or death, FDA officials added. It is still unknown whether intravenous administration also would have abuse-deterrent properties.
Embeda was first approved in 2009, but was temporarily withdrawn from the market in 2011 because of “stability concerns” found in the manufacturing process. According to the FDA, that issue was resolved in November 2013 with the approval of a manufacturing supplement.
Because of its highly addictive nature, Embeda is not approved for as-needed pain relief, and should only be used in severe cases when pain cannot be effectively managed by other means, the agency said in the statement.
The new labeling is consistent with the FDA’s 2013 draft guidance. Embeda is the third extended-release opioid analgesic to include a label describing its abuse-deterrent properties.
Embeda is marketed by Pfizer.
For more information on opioid medications, please visit www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm337066.htm.
The Food and Drug Administration has approved new labeling for the opioid analgesic Embeda to include information about the drug’s abuse-preventive properties, the agency announced Oct. 17.
The extended-release morphine sulfate and naltrexone hydrochloride tablets are used to treat severe, long-term pain resistant to alternative treatment options. Embeda acts as an abuse deterrent by releasing morphine only when taken properly as a whole capsule, and not when crushed or snorted, the FDA said in a statement.
“When crushed, the naltrexone in Embeda blocks some of the euphoric effects of the morphine and can precipitate withdrawal in persons dependent on opioids,” according to the announcement.
However, the drug can still be abused when taken as a whole capsule, because the naltrexone does not effectively block the euphoric morphine effects when consumed properly, the FDA said. Such misuse could result in overdose or death, FDA officials added. It is still unknown whether intravenous administration also would have abuse-deterrent properties.
Embeda was first approved in 2009, but was temporarily withdrawn from the market in 2011 because of “stability concerns” found in the manufacturing process. According to the FDA, that issue was resolved in November 2013 with the approval of a manufacturing supplement.
Because of its highly addictive nature, Embeda is not approved for as-needed pain relief, and should only be used in severe cases when pain cannot be effectively managed by other means, the agency said in the statement.
The new labeling is consistent with the FDA’s 2013 draft guidance. Embeda is the third extended-release opioid analgesic to include a label describing its abuse-deterrent properties.
Embeda is marketed by Pfizer.
For more information on opioid medications, please visit www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm337066.htm.
The Food and Drug Administration has approved new labeling for the opioid analgesic Embeda to include information about the drug’s abuse-preventive properties, the agency announced Oct. 17.
The extended-release morphine sulfate and naltrexone hydrochloride tablets are used to treat severe, long-term pain resistant to alternative treatment options. Embeda acts as an abuse deterrent by releasing morphine only when taken properly as a whole capsule, and not when crushed or snorted, the FDA said in a statement.
“When crushed, the naltrexone in Embeda blocks some of the euphoric effects of the morphine and can precipitate withdrawal in persons dependent on opioids,” according to the announcement.
However, the drug can still be abused when taken as a whole capsule, because the naltrexone does not effectively block the euphoric morphine effects when consumed properly, the FDA said. Such misuse could result in overdose or death, FDA officials added. It is still unknown whether intravenous administration also would have abuse-deterrent properties.
Embeda was first approved in 2009, but was temporarily withdrawn from the market in 2011 because of “stability concerns” found in the manufacturing process. According to the FDA, that issue was resolved in November 2013 with the approval of a manufacturing supplement.
Because of its highly addictive nature, Embeda is not approved for as-needed pain relief, and should only be used in severe cases when pain cannot be effectively managed by other means, the agency said in the statement.
The new labeling is consistent with the FDA’s 2013 draft guidance. Embeda is the third extended-release opioid analgesic to include a label describing its abuse-deterrent properties.
Embeda is marketed by Pfizer.
For more information on opioid medications, please visit www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm337066.htm.